User login
Poor lung function linked to risk for sudden cardiac death
Poor lung function appears to be a stronger marker of risk for sudden cardiac death than for a survivable first coronary event, results of a prospective population-based study suggest.
Among 28,584 adults with no history of acute coronary events who were followed over 4 decades, every standard deviation decrease in forced expiratory volume in 1 second (FEV1) was associated with a 23% increase in risk for sudden cardiac death, reported Suneela Zaigham, PhD, a cardiovascular epidemiology fellow at the University of Lund, Sweden, and colleagues.
“Our main findings and subsequent conclusions are that low FEV1 is associated with both sudden cardiac death and nonfatal coronary events but is consistently more strongly associated with future sudden cardiac death,” Dr. Zaigham said in a narrated poster presented at the European Respiratory Society (ERS) 2021 International Congress, which was held online.
“We propose that measurement with spirometry in early life could aid in the risk stratification of future sudden cardiac death, and our results support the use of spirometry for cardiovascular risk assessment,” she said.
Marc Humbert, MD, PhD, professor of respiratory medicine at Université Paris–Saclay, who was not involved in the study, said that “this is something we can measure fairly easily, meaning that lung function could be used as part of a screening tool.
“We need to do more research to understand the links between lung function and sudden cardiac death and to investigate whether we can use lung function tests to help prevent deaths in the future,” he said.
Fatal vs. nonfatal events
It is well known that poor lung function is a strong predictor of future coronary events, but it was unknown whether patterns of lung impairment differ in their ability to predict future nonfatal coronary events or sudden cardiac death, Dr. Zaigham said.
To see whether measurable differences in lung function could predict risk for both fatal and nonfatal coronary events, the investigators studied 28,584 middle-aged residents of Malmö, Sweden. Baseline spirometry test results were available for all study participants.
The patients were followed for approximately 40 years for sudden cardiac death, defined as death on the day of a coronary event, or nonfatal events, defined as survival for at least 24 hours after an event.
Dr. Zaigham and colleagues used a modified version of Lunn McNeil’s competing risks method to create Cox regression models.
Results of an analysis that was adjusted for potential confounding factors indicated that one standard deviation reduction in FEV1 was associated with a hazard ratio (HR) for sudden cardiac death of 1.23 (95% confidence interval, 1.15-1.31). In contrast, one standard deviation in FEV1 was associated with a lower but still significant risk for nonfatal events, with an HR of 1.08 (95% CI, 1.04-1.13; P for equal associations = .002).
The results remained significant among participants who had never smoked, with an HR for sudden cardiac death of 1.34 (95% CI, 1.15-1.55) and for nonfatal events of 1.11 (95% CI, 1.02-1.21; P for equal associations = .038).
“This study suggests a link between lung health and sudden cardiac death. It shows a higher risk of fatal than nonfatal coronary events even in people whose lung function is moderately lower but may still be within a normal range,” Dr. Humbert said.
The study was supported by the Swedish Heart-Lung Foundation. Dr. Zaigham and Dr. Humbert reported having no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Poor lung function appears to be a stronger marker of risk for sudden cardiac death than for a survivable first coronary event, results of a prospective population-based study suggest.
Among 28,584 adults with no history of acute coronary events who were followed over 4 decades, every standard deviation decrease in forced expiratory volume in 1 second (FEV1) was associated with a 23% increase in risk for sudden cardiac death, reported Suneela Zaigham, PhD, a cardiovascular epidemiology fellow at the University of Lund, Sweden, and colleagues.
“Our main findings and subsequent conclusions are that low FEV1 is associated with both sudden cardiac death and nonfatal coronary events but is consistently more strongly associated with future sudden cardiac death,” Dr. Zaigham said in a narrated poster presented at the European Respiratory Society (ERS) 2021 International Congress, which was held online.
“We propose that measurement with spirometry in early life could aid in the risk stratification of future sudden cardiac death, and our results support the use of spirometry for cardiovascular risk assessment,” she said.
Marc Humbert, MD, PhD, professor of respiratory medicine at Université Paris–Saclay, who was not involved in the study, said that “this is something we can measure fairly easily, meaning that lung function could be used as part of a screening tool.
“We need to do more research to understand the links between lung function and sudden cardiac death and to investigate whether we can use lung function tests to help prevent deaths in the future,” he said.
Fatal vs. nonfatal events
It is well known that poor lung function is a strong predictor of future coronary events, but it was unknown whether patterns of lung impairment differ in their ability to predict future nonfatal coronary events or sudden cardiac death, Dr. Zaigham said.
To see whether measurable differences in lung function could predict risk for both fatal and nonfatal coronary events, the investigators studied 28,584 middle-aged residents of Malmö, Sweden. Baseline spirometry test results were available for all study participants.
The patients were followed for approximately 40 years for sudden cardiac death, defined as death on the day of a coronary event, or nonfatal events, defined as survival for at least 24 hours after an event.
Dr. Zaigham and colleagues used a modified version of Lunn McNeil’s competing risks method to create Cox regression models.
Results of an analysis that was adjusted for potential confounding factors indicated that one standard deviation reduction in FEV1 was associated with a hazard ratio (HR) for sudden cardiac death of 1.23 (95% confidence interval, 1.15-1.31). In contrast, one standard deviation in FEV1 was associated with a lower but still significant risk for nonfatal events, with an HR of 1.08 (95% CI, 1.04-1.13; P for equal associations = .002).
The results remained significant among participants who had never smoked, with an HR for sudden cardiac death of 1.34 (95% CI, 1.15-1.55) and for nonfatal events of 1.11 (95% CI, 1.02-1.21; P for equal associations = .038).
“This study suggests a link between lung health and sudden cardiac death. It shows a higher risk of fatal than nonfatal coronary events even in people whose lung function is moderately lower but may still be within a normal range,” Dr. Humbert said.
The study was supported by the Swedish Heart-Lung Foundation. Dr. Zaigham and Dr. Humbert reported having no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Poor lung function appears to be a stronger marker of risk for sudden cardiac death than for a survivable first coronary event, results of a prospective population-based study suggest.
Among 28,584 adults with no history of acute coronary events who were followed over 4 decades, every standard deviation decrease in forced expiratory volume in 1 second (FEV1) was associated with a 23% increase in risk for sudden cardiac death, reported Suneela Zaigham, PhD, a cardiovascular epidemiology fellow at the University of Lund, Sweden, and colleagues.
“Our main findings and subsequent conclusions are that low FEV1 is associated with both sudden cardiac death and nonfatal coronary events but is consistently more strongly associated with future sudden cardiac death,” Dr. Zaigham said in a narrated poster presented at the European Respiratory Society (ERS) 2021 International Congress, which was held online.
“We propose that measurement with spirometry in early life could aid in the risk stratification of future sudden cardiac death, and our results support the use of spirometry for cardiovascular risk assessment,” she said.
Marc Humbert, MD, PhD, professor of respiratory medicine at Université Paris–Saclay, who was not involved in the study, said that “this is something we can measure fairly easily, meaning that lung function could be used as part of a screening tool.
“We need to do more research to understand the links between lung function and sudden cardiac death and to investigate whether we can use lung function tests to help prevent deaths in the future,” he said.
Fatal vs. nonfatal events
It is well known that poor lung function is a strong predictor of future coronary events, but it was unknown whether patterns of lung impairment differ in their ability to predict future nonfatal coronary events or sudden cardiac death, Dr. Zaigham said.
To see whether measurable differences in lung function could predict risk for both fatal and nonfatal coronary events, the investigators studied 28,584 middle-aged residents of Malmö, Sweden. Baseline spirometry test results were available for all study participants.
The patients were followed for approximately 40 years for sudden cardiac death, defined as death on the day of a coronary event, or nonfatal events, defined as survival for at least 24 hours after an event.
Dr. Zaigham and colleagues used a modified version of Lunn McNeil’s competing risks method to create Cox regression models.
Results of an analysis that was adjusted for potential confounding factors indicated that one standard deviation reduction in FEV1 was associated with a hazard ratio (HR) for sudden cardiac death of 1.23 (95% confidence interval, 1.15-1.31). In contrast, one standard deviation in FEV1 was associated with a lower but still significant risk for nonfatal events, with an HR of 1.08 (95% CI, 1.04-1.13; P for equal associations = .002).
The results remained significant among participants who had never smoked, with an HR for sudden cardiac death of 1.34 (95% CI, 1.15-1.55) and for nonfatal events of 1.11 (95% CI, 1.02-1.21; P for equal associations = .038).
“This study suggests a link between lung health and sudden cardiac death. It shows a higher risk of fatal than nonfatal coronary events even in people whose lung function is moderately lower but may still be within a normal range,” Dr. Humbert said.
The study was supported by the Swedish Heart-Lung Foundation. Dr. Zaigham and Dr. Humbert reported having no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Fibrosis progression flies below the radar in subclinical ILD
Subclinical or preclinical interstitial lung disease in patients with connective tissue diseases is not a benign entity, and many patients may experience progression of lung fibrosis before a diagnosis of ILD is made, investigators caution.
Among patients with connective tissue disease assessed with baseline and follow-up high-resolution CT scans for ILD, nearly one-fourth had evidence of ILD progression over a median of 4.5 years, reported Anna-Maria Hoffmann-Vold, MD, PhD, from Oslo University Hospital.
“Subclinical ILD is frequently present across all connective tissue diseases. It progresses over time in a substantial subgroup of people comparable to patients with clinical ILD, and our findings really question the terms ‘subclinical/preclinical ILD,’ which may potentially lead to a suboptimal watchful waiting management,” she said in an oral abstract presentation during the European Respiratory Society International Congress.
Jesse Roman, MD, CEO at the Jane & Leonard Korman Respiratory Institute at Thomas Jefferson University, Philadelphia, who was not involved in the study, commented that the findings regarding subclinical disease come as no surprise.
“The connective tissue disorders are linked to interstitial lung disease, and we believe that they are the primary causes of interstitial lung diseases in most countries,” he said in an interview.
“Basically, what you’re detecting is that if you can identify these people early, then you can see that they behave like any other patients with interstitial lung disease with progression, so most experts recommend that patients with any kind of connective tissue disorder be followed with either CT scans or pulmonary function tests, or carefully interviewed every time they come to identify any kind of very early interstitial lung disease – particularly in patients with rheumatoid arthritis, in patients with systemic sclerosis, and in patients with dermatomyositis,” Dr. Roman said.
He noted that when patients present with an idiopathic or undiagnosed condition suggestive of ILD, clinicians at his center will order serology tests to detect potential cases of subclinical connective tissue disorders.
Observational study
Dr. Hoffmann-Vold and colleagues looked at 525 patients with connective tissue diseases assessed for ILD at their center, including 296 with systemic sclerosis, 94 with anti-synthetase syndrome, and 135 with mixed connective tissue disease.
They used semiquantitative assessment to determine the prevalence of ILD, defining subclinical disease as ILD extent of less than 5% on high-resolution CT, preserved lung function with forced vital capacity (FVC) greater than 80% of predicted, and no respiratory symptoms.
Clinical ILD was defined as either ILD extent greater than 5%, or ILD extent below 5% but with respiratory symptoms and FVC below 80% of predicted.
They found that 44% of the patients had ILD on high-resolution CT, 43% had no evidence of ILD, and 13% had subclinical ILD.
In a comparison of patients without ILD and those with either clinical or subclinical ILD, they found that, while the mean patient age was about 51 in all three groups, men were more likely than women to have clinical ILD. A higher proportion of patients with clinical ILD (39%) died during the total observation period of about 13 years, compared with 22% of patients without ILD, and 18% of those with subclinical ILD.
As noted before, of 395 patients with baseline and follow-up high-resolution CT, 95 (24%) had evidence of lung fibrosis progression, with 38% of patients with subclinical ILD and 51% of patients with clinical ILD having progression during follow-up.
“In our connective tissue disease patients with ILD, the symptoms-define-disease argument would clearly lead to [the idea] that ILD is not a disease until patients become symptomatic, which we all know is frequently appearing in advanced stages of ILD,” Dr. Hoffmann-Vold said.
The study was funded by Oslo University Hospital. Dr. Hoffmann-Vold and Dr. Roman reported no relevant conflicts of interest to disclose.
Subclinical or preclinical interstitial lung disease in patients with connective tissue diseases is not a benign entity, and many patients may experience progression of lung fibrosis before a diagnosis of ILD is made, investigators caution.
Among patients with connective tissue disease assessed with baseline and follow-up high-resolution CT scans for ILD, nearly one-fourth had evidence of ILD progression over a median of 4.5 years, reported Anna-Maria Hoffmann-Vold, MD, PhD, from Oslo University Hospital.
“Subclinical ILD is frequently present across all connective tissue diseases. It progresses over time in a substantial subgroup of people comparable to patients with clinical ILD, and our findings really question the terms ‘subclinical/preclinical ILD,’ which may potentially lead to a suboptimal watchful waiting management,” she said in an oral abstract presentation during the European Respiratory Society International Congress.
Jesse Roman, MD, CEO at the Jane & Leonard Korman Respiratory Institute at Thomas Jefferson University, Philadelphia, who was not involved in the study, commented that the findings regarding subclinical disease come as no surprise.
“The connective tissue disorders are linked to interstitial lung disease, and we believe that they are the primary causes of interstitial lung diseases in most countries,” he said in an interview.
“Basically, what you’re detecting is that if you can identify these people early, then you can see that they behave like any other patients with interstitial lung disease with progression, so most experts recommend that patients with any kind of connective tissue disorder be followed with either CT scans or pulmonary function tests, or carefully interviewed every time they come to identify any kind of very early interstitial lung disease – particularly in patients with rheumatoid arthritis, in patients with systemic sclerosis, and in patients with dermatomyositis,” Dr. Roman said.
He noted that when patients present with an idiopathic or undiagnosed condition suggestive of ILD, clinicians at his center will order serology tests to detect potential cases of subclinical connective tissue disorders.
Observational study
Dr. Hoffmann-Vold and colleagues looked at 525 patients with connective tissue diseases assessed for ILD at their center, including 296 with systemic sclerosis, 94 with anti-synthetase syndrome, and 135 with mixed connective tissue disease.
They used semiquantitative assessment to determine the prevalence of ILD, defining subclinical disease as ILD extent of less than 5% on high-resolution CT, preserved lung function with forced vital capacity (FVC) greater than 80% of predicted, and no respiratory symptoms.
Clinical ILD was defined as either ILD extent greater than 5%, or ILD extent below 5% but with respiratory symptoms and FVC below 80% of predicted.
They found that 44% of the patients had ILD on high-resolution CT, 43% had no evidence of ILD, and 13% had subclinical ILD.
In a comparison of patients without ILD and those with either clinical or subclinical ILD, they found that, while the mean patient age was about 51 in all three groups, men were more likely than women to have clinical ILD. A higher proportion of patients with clinical ILD (39%) died during the total observation period of about 13 years, compared with 22% of patients without ILD, and 18% of those with subclinical ILD.
As noted before, of 395 patients with baseline and follow-up high-resolution CT, 95 (24%) had evidence of lung fibrosis progression, with 38% of patients with subclinical ILD and 51% of patients with clinical ILD having progression during follow-up.
“In our connective tissue disease patients with ILD, the symptoms-define-disease argument would clearly lead to [the idea] that ILD is not a disease until patients become symptomatic, which we all know is frequently appearing in advanced stages of ILD,” Dr. Hoffmann-Vold said.
The study was funded by Oslo University Hospital. Dr. Hoffmann-Vold and Dr. Roman reported no relevant conflicts of interest to disclose.
Subclinical or preclinical interstitial lung disease in patients with connective tissue diseases is not a benign entity, and many patients may experience progression of lung fibrosis before a diagnosis of ILD is made, investigators caution.
Among patients with connective tissue disease assessed with baseline and follow-up high-resolution CT scans for ILD, nearly one-fourth had evidence of ILD progression over a median of 4.5 years, reported Anna-Maria Hoffmann-Vold, MD, PhD, from Oslo University Hospital.
“Subclinical ILD is frequently present across all connective tissue diseases. It progresses over time in a substantial subgroup of people comparable to patients with clinical ILD, and our findings really question the terms ‘subclinical/preclinical ILD,’ which may potentially lead to a suboptimal watchful waiting management,” she said in an oral abstract presentation during the European Respiratory Society International Congress.
Jesse Roman, MD, CEO at the Jane & Leonard Korman Respiratory Institute at Thomas Jefferson University, Philadelphia, who was not involved in the study, commented that the findings regarding subclinical disease come as no surprise.
“The connective tissue disorders are linked to interstitial lung disease, and we believe that they are the primary causes of interstitial lung diseases in most countries,” he said in an interview.
“Basically, what you’re detecting is that if you can identify these people early, then you can see that they behave like any other patients with interstitial lung disease with progression, so most experts recommend that patients with any kind of connective tissue disorder be followed with either CT scans or pulmonary function tests, or carefully interviewed every time they come to identify any kind of very early interstitial lung disease – particularly in patients with rheumatoid arthritis, in patients with systemic sclerosis, and in patients with dermatomyositis,” Dr. Roman said.
He noted that when patients present with an idiopathic or undiagnosed condition suggestive of ILD, clinicians at his center will order serology tests to detect potential cases of subclinical connective tissue disorders.
Observational study
Dr. Hoffmann-Vold and colleagues looked at 525 patients with connective tissue diseases assessed for ILD at their center, including 296 with systemic sclerosis, 94 with anti-synthetase syndrome, and 135 with mixed connective tissue disease.
They used semiquantitative assessment to determine the prevalence of ILD, defining subclinical disease as ILD extent of less than 5% on high-resolution CT, preserved lung function with forced vital capacity (FVC) greater than 80% of predicted, and no respiratory symptoms.
Clinical ILD was defined as either ILD extent greater than 5%, or ILD extent below 5% but with respiratory symptoms and FVC below 80% of predicted.
They found that 44% of the patients had ILD on high-resolution CT, 43% had no evidence of ILD, and 13% had subclinical ILD.
In a comparison of patients without ILD and those with either clinical or subclinical ILD, they found that, while the mean patient age was about 51 in all three groups, men were more likely than women to have clinical ILD. A higher proportion of patients with clinical ILD (39%) died during the total observation period of about 13 years, compared with 22% of patients without ILD, and 18% of those with subclinical ILD.
As noted before, of 395 patients with baseline and follow-up high-resolution CT, 95 (24%) had evidence of lung fibrosis progression, with 38% of patients with subclinical ILD and 51% of patients with clinical ILD having progression during follow-up.
“In our connective tissue disease patients with ILD, the symptoms-define-disease argument would clearly lead to [the idea] that ILD is not a disease until patients become symptomatic, which we all know is frequently appearing in advanced stages of ILD,” Dr. Hoffmann-Vold said.
The study was funded by Oslo University Hospital. Dr. Hoffmann-Vold and Dr. Roman reported no relevant conflicts of interest to disclose.
FROM ERS 2021
Air pollution – second leading cause of lung cancer
The new data show that the rate of lung cancer deaths attributable to air pollution varies widely between countries. Serbia, Poland, China, Mongolia, and Turkey are among the worst affected. The analysis shows an association between deaths from lung cancer and the proportion of national energy that is produced from coal.
“Both smoking and air pollution are important causes of lung cancer,” said study presenter Christine D. Berg, MD, former codirector of the National Lung Screening Trial, and “both need to be eliminated to help prevent lung cancer and save lives.
“As lung cancer professionals, we can mitigate the effects of air pollution on causing lung cancer by speaking out for clean energy standards,” she said.
Dr. Berg presented the new analysis on Sept. 9 at the 2021 World Conference on Lung Cancer, which was organized by the International Association for the Study of Lung Cancer.
She welcomed the recent statement issued by the IASLC in support of the International Day of Clean Air for Blue Skies, which took place on Sept. 7. It was a call for action that emphasized the need for further efforts to improve air quality to protect human health.
The findings from the new analysis are “depressing,” commented Joachim G. J. V. Aerts, MD. PhD, department of pulmonary diseases, Erasmus University Medical Center, Rotterdam, the Netherlands.
It is now clear that air pollution has an impact not only on the incidence of lung cancer but also on its outcome, he added.
Indeed, previous research showed that each 10 mcg/m3 increase in particular matter of 2.5 mcg in size was associated with a 15%-27% increase in lung cancer mortality. There was no difference in rates between women and men.
A key question, Dr. Aerts said, is whether reducing air pollution would be beneficial.
Efforts to reduce air pollution over recent decades in the United Kingdom have not led to a reduction in lung cancer deaths. This is because of the increase in life expectancy – individuals have been exposed to pollution for longer, albeit at lower levels, he pointed out.
Because of lockdowns during the COVID pandemic, travel has been greatly reduced. This has resulted in a dramatic reduction in air pollution, “and this led to a decrease in the number of children born with low birth weight,” said Dr. Aerts.
Hopefully, that benefit will also be seen regarding other diseases, he added.
The call to action to reduce air pollution is of the “utmost importance,” he said. He noted that the focus should be on global, national, local, and personal preventive measures.
“It is time to join forces,” he added, “to ‘clean the air.’ ”
Dr. Berg’s presentation was warmly received on social media.
It was “fabulous,” commented Eric H. Bernicker, MD, director of medical thoracic oncology at Houston Methodist Cancer Center.
“Thoracic oncologists need to add air pollution to things they advocate about; we have an important voice here,” he added.
It is “so important to understand that air pollution is a human carcinogen,” commented Ivy Elkins, a lung cancer survivor and advocate and cofounder of the EGFR Resisters Lung Cancer Patient Group. “All you need are lungs to get lung cancer!”
Contribution of air pollution to lung cancer
In her presentation, Dr. Berg emphasized that lung cancer is the leading cause of cancer death worldwide, although the distribution between countries “depends on historical and current smoking patterns and the demographics of the population.”
Overall, data from GLOBOCAN 2018 indicate that annually there are approximately 2.1 million incident cases of lung cancer and almost 1.8 million lung cancer deaths around the globe.
A recent study estimated that, worldwide, 14.1% of all lung cancer deaths, including in never-smokers, are directly linked to air pollution.
Dr. Berg said that this makes it the “second-leading cause of lung cancer” behind smoking.
The figure is somewhat lower for the United States, where around 4.7% of lung cancer deaths each year are directly attributable to pollution. However, with “the wildfires out West, we’re going to be seeing more of a toll from air pollution,” she predicted.
She pointed out that the International Agency for Research on Cancer classifies outdoor air pollution, especially particulate matter, as a human carcinogen on the basis of evidence of an association with lung cancer.
It is thought that direct deposits and local effects of particulate matter lead to oxidative damage and low-grade chronic inflammation. These in turn result in molecular changes that affect DNA and gene transcription and inhibit apoptosis, all of which lead to the development of cancerous lesions, she explained.
Synthesizing various estimates on global burden of disease, Dr. Berg and colleagues calculated that in 2019 the rate of lung cancer deaths attributable to particular matter in people aged 50-69 years was highest in Serbia, at 36.88 attributable deaths per 100,000.
Next was Poland, with a rate of 27.97 per 100,000, followed by China at 24.63 per 100,000, Mongolia at 19.71 per 100,000, and Turkey at 19.2 per 100,000.
The major sources of air pollution in the most affected countries were transportation, indoor cooking, and energy sources, she said.
In Serbia, 70% of energy production was from coal. It was 74% in Poland, 65% in China, 80% in Mongolia, 35% in Turkey, and 19% in the United States.
At the time of the analysis, only 17.3% of U.S. adults were smokers, and the air concentration of particular matter of 2.5 mcm was 9.6% mcg/m3. Both of these rates are far below those seen in more severely affected countries.
“But 40% of our energy now comes from natural gas,” noted Dr. Berg, “which is still a pollutant and a source of methane. It’s a very potent greenhouse gas.”
No funding for the study has been reported. Dr. Berg has relationships with GRAIL and Mercy BioAnalytics. Dr. Aerts has relationships with Amphera, AstraZeneca, Bayer, BIOCAD, Bristol-Myers Squibb, Eli Lilly, and Roche.
A version of this article first appeared on Medscape.com.
The new data show that the rate of lung cancer deaths attributable to air pollution varies widely between countries. Serbia, Poland, China, Mongolia, and Turkey are among the worst affected. The analysis shows an association between deaths from lung cancer and the proportion of national energy that is produced from coal.
“Both smoking and air pollution are important causes of lung cancer,” said study presenter Christine D. Berg, MD, former codirector of the National Lung Screening Trial, and “both need to be eliminated to help prevent lung cancer and save lives.
“As lung cancer professionals, we can mitigate the effects of air pollution on causing lung cancer by speaking out for clean energy standards,” she said.
Dr. Berg presented the new analysis on Sept. 9 at the 2021 World Conference on Lung Cancer, which was organized by the International Association for the Study of Lung Cancer.
She welcomed the recent statement issued by the IASLC in support of the International Day of Clean Air for Blue Skies, which took place on Sept. 7. It was a call for action that emphasized the need for further efforts to improve air quality to protect human health.
The findings from the new analysis are “depressing,” commented Joachim G. J. V. Aerts, MD. PhD, department of pulmonary diseases, Erasmus University Medical Center, Rotterdam, the Netherlands.
It is now clear that air pollution has an impact not only on the incidence of lung cancer but also on its outcome, he added.
Indeed, previous research showed that each 10 mcg/m3 increase in particular matter of 2.5 mcg in size was associated with a 15%-27% increase in lung cancer mortality. There was no difference in rates between women and men.
A key question, Dr. Aerts said, is whether reducing air pollution would be beneficial.
Efforts to reduce air pollution over recent decades in the United Kingdom have not led to a reduction in lung cancer deaths. This is because of the increase in life expectancy – individuals have been exposed to pollution for longer, albeit at lower levels, he pointed out.
Because of lockdowns during the COVID pandemic, travel has been greatly reduced. This has resulted in a dramatic reduction in air pollution, “and this led to a decrease in the number of children born with low birth weight,” said Dr. Aerts.
Hopefully, that benefit will also be seen regarding other diseases, he added.
The call to action to reduce air pollution is of the “utmost importance,” he said. He noted that the focus should be on global, national, local, and personal preventive measures.
“It is time to join forces,” he added, “to ‘clean the air.’ ”
Dr. Berg’s presentation was warmly received on social media.
It was “fabulous,” commented Eric H. Bernicker, MD, director of medical thoracic oncology at Houston Methodist Cancer Center.
“Thoracic oncologists need to add air pollution to things they advocate about; we have an important voice here,” he added.
It is “so important to understand that air pollution is a human carcinogen,” commented Ivy Elkins, a lung cancer survivor and advocate and cofounder of the EGFR Resisters Lung Cancer Patient Group. “All you need are lungs to get lung cancer!”
Contribution of air pollution to lung cancer
In her presentation, Dr. Berg emphasized that lung cancer is the leading cause of cancer death worldwide, although the distribution between countries “depends on historical and current smoking patterns and the demographics of the population.”
Overall, data from GLOBOCAN 2018 indicate that annually there are approximately 2.1 million incident cases of lung cancer and almost 1.8 million lung cancer deaths around the globe.
A recent study estimated that, worldwide, 14.1% of all lung cancer deaths, including in never-smokers, are directly linked to air pollution.
Dr. Berg said that this makes it the “second-leading cause of lung cancer” behind smoking.
The figure is somewhat lower for the United States, where around 4.7% of lung cancer deaths each year are directly attributable to pollution. However, with “the wildfires out West, we’re going to be seeing more of a toll from air pollution,” she predicted.
She pointed out that the International Agency for Research on Cancer classifies outdoor air pollution, especially particulate matter, as a human carcinogen on the basis of evidence of an association with lung cancer.
It is thought that direct deposits and local effects of particulate matter lead to oxidative damage and low-grade chronic inflammation. These in turn result in molecular changes that affect DNA and gene transcription and inhibit apoptosis, all of which lead to the development of cancerous lesions, she explained.
Synthesizing various estimates on global burden of disease, Dr. Berg and colleagues calculated that in 2019 the rate of lung cancer deaths attributable to particular matter in people aged 50-69 years was highest in Serbia, at 36.88 attributable deaths per 100,000.
Next was Poland, with a rate of 27.97 per 100,000, followed by China at 24.63 per 100,000, Mongolia at 19.71 per 100,000, and Turkey at 19.2 per 100,000.
The major sources of air pollution in the most affected countries were transportation, indoor cooking, and energy sources, she said.
In Serbia, 70% of energy production was from coal. It was 74% in Poland, 65% in China, 80% in Mongolia, 35% in Turkey, and 19% in the United States.
At the time of the analysis, only 17.3% of U.S. adults were smokers, and the air concentration of particular matter of 2.5 mcm was 9.6% mcg/m3. Both of these rates are far below those seen in more severely affected countries.
“But 40% of our energy now comes from natural gas,” noted Dr. Berg, “which is still a pollutant and a source of methane. It’s a very potent greenhouse gas.”
No funding for the study has been reported. Dr. Berg has relationships with GRAIL and Mercy BioAnalytics. Dr. Aerts has relationships with Amphera, AstraZeneca, Bayer, BIOCAD, Bristol-Myers Squibb, Eli Lilly, and Roche.
A version of this article first appeared on Medscape.com.
The new data show that the rate of lung cancer deaths attributable to air pollution varies widely between countries. Serbia, Poland, China, Mongolia, and Turkey are among the worst affected. The analysis shows an association between deaths from lung cancer and the proportion of national energy that is produced from coal.
“Both smoking and air pollution are important causes of lung cancer,” said study presenter Christine D. Berg, MD, former codirector of the National Lung Screening Trial, and “both need to be eliminated to help prevent lung cancer and save lives.
“As lung cancer professionals, we can mitigate the effects of air pollution on causing lung cancer by speaking out for clean energy standards,” she said.
Dr. Berg presented the new analysis on Sept. 9 at the 2021 World Conference on Lung Cancer, which was organized by the International Association for the Study of Lung Cancer.
She welcomed the recent statement issued by the IASLC in support of the International Day of Clean Air for Blue Skies, which took place on Sept. 7. It was a call for action that emphasized the need for further efforts to improve air quality to protect human health.
The findings from the new analysis are “depressing,” commented Joachim G. J. V. Aerts, MD. PhD, department of pulmonary diseases, Erasmus University Medical Center, Rotterdam, the Netherlands.
It is now clear that air pollution has an impact not only on the incidence of lung cancer but also on its outcome, he added.
Indeed, previous research showed that each 10 mcg/m3 increase in particular matter of 2.5 mcg in size was associated with a 15%-27% increase in lung cancer mortality. There was no difference in rates between women and men.
A key question, Dr. Aerts said, is whether reducing air pollution would be beneficial.
Efforts to reduce air pollution over recent decades in the United Kingdom have not led to a reduction in lung cancer deaths. This is because of the increase in life expectancy – individuals have been exposed to pollution for longer, albeit at lower levels, he pointed out.
Because of lockdowns during the COVID pandemic, travel has been greatly reduced. This has resulted in a dramatic reduction in air pollution, “and this led to a decrease in the number of children born with low birth weight,” said Dr. Aerts.
Hopefully, that benefit will also be seen regarding other diseases, he added.
The call to action to reduce air pollution is of the “utmost importance,” he said. He noted that the focus should be on global, national, local, and personal preventive measures.
“It is time to join forces,” he added, “to ‘clean the air.’ ”
Dr. Berg’s presentation was warmly received on social media.
It was “fabulous,” commented Eric H. Bernicker, MD, director of medical thoracic oncology at Houston Methodist Cancer Center.
“Thoracic oncologists need to add air pollution to things they advocate about; we have an important voice here,” he added.
It is “so important to understand that air pollution is a human carcinogen,” commented Ivy Elkins, a lung cancer survivor and advocate and cofounder of the EGFR Resisters Lung Cancer Patient Group. “All you need are lungs to get lung cancer!”
Contribution of air pollution to lung cancer
In her presentation, Dr. Berg emphasized that lung cancer is the leading cause of cancer death worldwide, although the distribution between countries “depends on historical and current smoking patterns and the demographics of the population.”
Overall, data from GLOBOCAN 2018 indicate that annually there are approximately 2.1 million incident cases of lung cancer and almost 1.8 million lung cancer deaths around the globe.
A recent study estimated that, worldwide, 14.1% of all lung cancer deaths, including in never-smokers, are directly linked to air pollution.
Dr. Berg said that this makes it the “second-leading cause of lung cancer” behind smoking.
The figure is somewhat lower for the United States, where around 4.7% of lung cancer deaths each year are directly attributable to pollution. However, with “the wildfires out West, we’re going to be seeing more of a toll from air pollution,” she predicted.
She pointed out that the International Agency for Research on Cancer classifies outdoor air pollution, especially particulate matter, as a human carcinogen on the basis of evidence of an association with lung cancer.
It is thought that direct deposits and local effects of particulate matter lead to oxidative damage and low-grade chronic inflammation. These in turn result in molecular changes that affect DNA and gene transcription and inhibit apoptosis, all of which lead to the development of cancerous lesions, she explained.
Synthesizing various estimates on global burden of disease, Dr. Berg and colleagues calculated that in 2019 the rate of lung cancer deaths attributable to particular matter in people aged 50-69 years was highest in Serbia, at 36.88 attributable deaths per 100,000.
Next was Poland, with a rate of 27.97 per 100,000, followed by China at 24.63 per 100,000, Mongolia at 19.71 per 100,000, and Turkey at 19.2 per 100,000.
The major sources of air pollution in the most affected countries were transportation, indoor cooking, and energy sources, she said.
In Serbia, 70% of energy production was from coal. It was 74% in Poland, 65% in China, 80% in Mongolia, 35% in Turkey, and 19% in the United States.
At the time of the analysis, only 17.3% of U.S. adults were smokers, and the air concentration of particular matter of 2.5 mcm was 9.6% mcg/m3. Both of these rates are far below those seen in more severely affected countries.
“But 40% of our energy now comes from natural gas,” noted Dr. Berg, “which is still a pollutant and a source of methane. It’s a very potent greenhouse gas.”
No funding for the study has been reported. Dr. Berg has relationships with GRAIL and Mercy BioAnalytics. Dr. Aerts has relationships with Amphera, AstraZeneca, Bayer, BIOCAD, Bristol-Myers Squibb, Eli Lilly, and Roche.
A version of this article first appeared on Medscape.com.
Virtual Respiratory Urgent Clinics for COVID-19 Symptoms
Virtual care (VC) has emerged as an effective mode of health care delivery especially in settings where significant barriers to traditional in-person visits exist; a large systematic review supports feasibility of telemedicine in primary care and suggests that telemedicine is at least as effective as traditional care.1 Nevertheless, broad adoption of VC into practice has lagged, impeded by government and private insurance reimbursement requirements as well as the persistent belief that care can best be delivered in person.2-4 Before the COVID-19 pandemic, states that enacted parity legislation that required private insurance companies to provide reimbursement coverage for telehealth services saw a significant increase in the number of outpatient telehealth visits (about ≥ 30% odds compared with nonparity states).3
With the onset of the COVID-19 pandemic, in-person medical appointments were converted to VC visits to reduce increased exposure risks to patients and health care workers.5 Prior government and private sector policies were suspended, and payment restrictions lifted, enabling adoption of VC modalities to rapidly accommodate the emergent need and Centers for Disease Control and Prevention (CDC) recommendations for virtual care.6-11
The CDC guidelines on managing operations during the COVID-19 pandemic highlighted the need to provide care in the safest way for patients and health care personnel and emphasized the importance of optimizing telehealth services. The federal government facilitated telehealth during the COVID-19 pandemic via temporary measures under the COVID-19 public health emergency declaration. This included Health Insurance Portability and Accountability Act flexibility to use everyday technology for VC visits, regulatory changes to deliver services to Medicare and Medicaid patients, permission of telehealth services across state lines, and prescribing of controlled substances via telehealth without an in-person medical evaluation.7
In response, health care providers (HCPs) and health care organizations created or expanded on existing telehealth infrastructure, developing virtual urgent care centers and telephone-based programs to evaluate patients remotely via screening questions that triaged them to a correct level of response, with possible subsequent virtual physician evaluation if indicated.12,13
The Veterans Health Administration (VHA) also shifted to a VC model in response to COVID-19 guided by a unique perspective from a well-developed prior VC experience.14-16 As a federally funded system, the VHA depends on workload documentation for budgeting. Since 2015, the VHA has provided workload credit and incentivized HCPs (via pay for performance) for the use of VC, including telephone visits, video visits, and secure messaging. These incentives resulted in higher rates of telehealth utilization before the COVID-19 pandemic compared with the private sector (with 4.2% and 0.7% of visits within the VHA being telephone and video visits, respectively, compared with telehealth utilization rates of 1.0% for Medicare recipients and 1.1% in an all-payer database).16
Historically, VHA care has successfully transitioned from in-person care models to exclusively virtual modalities to prevent suspension of medical services during natural disasters. Studies performed during these periods, specifically during the 2017 hurricane season (during which multiple VHA hospitals were closed or had limited in-person service available), supported telehealth as an efficient health care delivery method, and even recommended expanding telehealth services within non-VHA environments to accommodate needs of the general public during crises and postdisaster health care delivery.17
Armed with both a well-established telehealth infrastructure and prior knowledge gained from successful systemwide implementation of virtual care during times of disaster, US Department of Veterans Affairs (VA) Connecticut Healthcare System (VACHS) primary care quickly transitioned to a VC model in response to COVID-19.16 Early in the pandemic, a rapid transition to virtual care (RTVC) model was developed, including implementation of virtual respiratory urgent clinics (VRUCs), defined as virtual respiratory symptom triage clinics, staffed by primary care providers (PCPs) aimed at minimizing patient and health care worker exposure risk.
Methods
VACHS consists of 8 primary care sites, including a major tertiary care center, a smaller medical center with full ambulatory services, and 6 community-based outpatient clinics with only primary care and mental health. There are 80 individual PCPs delivering care to 58,058 veterans. VRUCs were established during the COVID-19 pandemic to cover patients across the entire health care system, using a rotational schedule of VA PCPs.
COVID-19 Urgent Clinics Program
Within the first few weeks of the pandemic, VACHS primary care established VRUCS to provide expeditious virtual assessment of respiratory or flu-like symptoms. Using the established telehealth system, the intervention aimed to provide emergent screening, testing, and care to those with potential COVID-19 infections. The model also was designed to minimize exposures to the health care workforce and patients.
Retrospective analysis was performed using information obtained from the electronic health record (EHR) database to describe the characteristics of patients who received care through the VRUCs, such as demographics, era of military service, COVID-19 testing rates and results, as well as subsequent emergency department (ED) visits and hospital admissions. A secondary aim included collection of additional qualitative data via a random sample chart review.
Virtual clinics were established January 22, 2020, and data were analyzed over the next 3 months. Data were retrieved and analyzed from the EHR, and codes were used to categorize the VRUCs.
Results
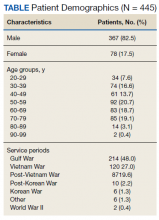
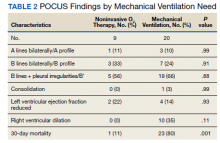
A total of 445 unique patients used these clinics during this period. Unique patients were defined as individual patients (some may have used a clinic more than once but were counted only once). Of this group, 82% were male, and 48% served in the Gulf War era (1990 to present). A total of 51% of patients received a COVID-19 test (clinics began before wide testing availability), and 10% tested positive. Of all patients using the clinics, approximately 5% were admitted to the hospital, and 18% had at least 1 subsequent ED visit (Table).
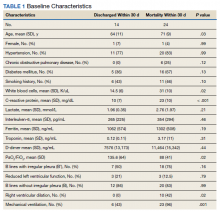
A secondary aim included review of a random sample of 99 patient charts to gain additional information regarding whether the patient was given appropriate isolation precautions, was in a high-exposure occupation (eg, could expose a large number of people), and whether there was appropriate documentation of goals of care, health care proxy or referral to social work to discuss advance directives. In addition, we calculated the average length of time between patients’ initial contact with the health care system call center and the return call by the PCP (wait time).Of charts reviewed, the majority (71%) had documentation of appropriate isolation precautions. Although 25% of patients had documentation of a high-risk profession with potential to expose many people, more than half of the patients had no documentation of occupation. Most patients (86%) had no updated documentation regarding goals of care, health care proxy, or advance directives in their urgent care VC visit. The average time between the patient initiating contact with the health care system call center and a return call to the patient from a PCP was 104 minutes (excluding calls received after 3:30
Discussion
This analysis adds to the growing literature on use of VC during the COVID-19 pandemic. Specifically, we describe the population of patients who used VRUCs within a large health care system in a RTVC. This analysis was limited by lack of available testing during the initial phase of the pandemic, which contributed to the lower than expected rates of testing and test positivity in patients managed via VRUCs. In addition, chart review data are limited as the data includes only what was documented during the visit and not the entire discussion during the encounter.
Several important outcomes from this analysis can be applied to interventions in the future, which may have large public health implications: Several hundred patients who reported respiratory symptoms were expeditiously evaluated by a PCP using VC. The average wait time to full clinical assessment was about 1.5 hours. This short duration between contact and evaluation permitted early education about isolation precautions, which may have minimized spread. In addition, this innovation kept patients out of the medical center, eliminating chains of transmission to other vulnerable patients and health care workers.
Our retrospective chart review also revealed that more than half the patients were not queried about their occupation, but of those that were asked, a significant number were in high-risk professions potentially exposing large numbers of people. This would be an important aspect to add to future templated notes to minimize work-related exposures. Also, we identified that few HCPs discussed goals of care with patients. Given the nature of COVID-19 and potential for rapid decompensation especially in vulnerable patients, this also would be important to include in the future.
Conclusions
VC urgent care clinics to address possible COVID-19 symptoms facilitated expeditious PCP assessment while keeping potentially contagious patients outside of high-risk health care environments. Streamlining and optimizing clinical VC assessments will be imperative to future management of COVID-19 and potentially to other future infectious pandemics. This includes development of templated notes incorporating counseling regarding appropriate isolation, questions about high-contact occupations, and goals of care discussions.
Acknowledgment
The authors thank Robert F. Walsh, MHA.
1. Bashshur RL, Howell JD, Krupinski EA, Harms KM, Bashshur N, Doarn CR. The empirical foundations of telemedicine interventions in primary care. Telemed J E Health. 2016;22(5):342-375. doi:10.1089/tmj.2016.0045
2. Centers for Disease Control and Prevention. Using telehealth to expand access to essential health services during the COVID-19 pandemic. Updated June 10, 2020. Accessed August 20, 2021. https://www.cdc.gov/coronavirus/2019-ncov/hcp/telehealth.html
3. Harvey JB, Valenta S, Simpson K, Lyles M, McElligott J. Utilization of outpatient telehealth services in parity and nonparity states 2010-2015. Telemed J E Health. 2019;25(2):132-136. doi:10.1089/tmj.2017.0265
4. Dorsey ER, Topol EJ. State of telehealth. N Engl J Med. 2016;375(2):154-161. doi:10.1056/NEJMra1601705
5. Rockwell KL, Gilroy AS. Incorporating telemedicine as part of COVID-19 outbreak response systems. Am J Manag Care. 2020;26(4):147-148. doi:10.37765/ajmc.2020.42784
6. Centers for Disease Control and Prevention. Healthcare facility guidance. Updated April 17, 2021. Accessed August 20, 2021. https://www.cdc.gov/coronavirus/2019-ncov/hcp/clinical-care.html
7. US Department of Health and Human Services, Health Resources and Services Administration. Policy changes during COVID-19. Accessed August 20, 2021. https://telehealth.hhs.gov/providers/policy-changes-during-the-covid-19-public-health-emergency
8. Coronavirus Preparedness and Response Supplemental Appropriation Act of 2020. 134 Stat. 146. Published February 2, 2021. Accessed August 20, 2021. https://www.govinfo.gov/content/pkg/CREC-2021-02-02/html/CREC-2021-02-02-pt1-PgS226.htm
9. US Department of Health and Human Services. Notification of enforcement discretion for telehealth remote communications during the COVID-19 nationwide public health emergency. Updated January 20, 2021. Accessed August 20, 2021. https://www.hhs.gov/hipaa/for-professionals/special-topics/emergency-preparedness/notification-enforcement-discretion-telehealth/index.html
10. Centers for Medicare and Medicaid Services. Coverage and payment related to COVID-19 Medicare. 2020. Published March 23, 2020. Accessed August 20, 2021. https://www.cms.gov/files/document/03052020-medicare-covid-19-fact-sheet.pdf
11. American Telemedicine Association. ATA commends 2020 Congress for giving HHS authority to waive restrictions on telehealth for Medicare beneficiaries in response to the COVID-19 outbreak [press release]. Published March 5, 2020. Accessed August 20, 2021. https://www.americantelemed.org/press-releases/ata-commends-congress-for-waiving-restrictions-on-telehealth-for-medicare-beneficiaries-in-response-to-the-covid-19-outbreak
12. Hollander JE, Carr BG. Virtually perfect? Telemedicine for Covid-19. N Engl J Med. 2020;382(18):1679-1681. doi:10.1056/NEJMp2003539
13. Khairat S, Meng C, Xu Y, Edson B, Gianforcaro R. Interpreting COVID-19 and Virtual Care Trends: Cohort Study. JMIR Public Health Surveill. 2020;6(2):e18811. Published 2020 Apr 15. doi:10.2196/18811
14. Ferguson JM, Jacobs J, Yefimova M, Greene L, Heyworth L, Zulman DM. Virtual care expansion in the Veterans Health Administration during the COVID-19 pandemic: clinical services and patient characteristics associated with utilization. J Am Med Inform Assoc. 2021;28(3):453-462. doi:10.1093/jamia/ocaa284
15. Baum A, Kaboli PJ, Schwartz MD. Reduced in-person and increased telehealth outpatient visits during the COVID-19 Pandemic. Ann Intern Med. 2021;174(1):129-131. doi:10.7326/M20-3026
16. Spelman JF, Brienza R, Walsh RF, et al. A model for rapid transition to virtual care, VA Connecticut primary care response to COVID-19. J Gen Intern Med. 2020;35(10):3073-3076. doi:10.1007/s11606-020-06041-4
17. Der-Martirosian C, Chu K, Dobalian A. Use of telehealth to improve access to care at the United States Department of Veterans Affairs during the 2017 Atlantic hurricane season [published online ahead of print, 2020 Apr 13]. Disaster Med Public Health Prep. 2020;1-5. doi:10.1017/dmp.2020.88
Virtual care (VC) has emerged as an effective mode of health care delivery especially in settings where significant barriers to traditional in-person visits exist; a large systematic review supports feasibility of telemedicine in primary care and suggests that telemedicine is at least as effective as traditional care.1 Nevertheless, broad adoption of VC into practice has lagged, impeded by government and private insurance reimbursement requirements as well as the persistent belief that care can best be delivered in person.2-4 Before the COVID-19 pandemic, states that enacted parity legislation that required private insurance companies to provide reimbursement coverage for telehealth services saw a significant increase in the number of outpatient telehealth visits (about ≥ 30% odds compared with nonparity states).3
With the onset of the COVID-19 pandemic, in-person medical appointments were converted to VC visits to reduce increased exposure risks to patients and health care workers.5 Prior government and private sector policies were suspended, and payment restrictions lifted, enabling adoption of VC modalities to rapidly accommodate the emergent need and Centers for Disease Control and Prevention (CDC) recommendations for virtual care.6-11
The CDC guidelines on managing operations during the COVID-19 pandemic highlighted the need to provide care in the safest way for patients and health care personnel and emphasized the importance of optimizing telehealth services. The federal government facilitated telehealth during the COVID-19 pandemic via temporary measures under the COVID-19 public health emergency declaration. This included Health Insurance Portability and Accountability Act flexibility to use everyday technology for VC visits, regulatory changes to deliver services to Medicare and Medicaid patients, permission of telehealth services across state lines, and prescribing of controlled substances via telehealth without an in-person medical evaluation.7
In response, health care providers (HCPs) and health care organizations created or expanded on existing telehealth infrastructure, developing virtual urgent care centers and telephone-based programs to evaluate patients remotely via screening questions that triaged them to a correct level of response, with possible subsequent virtual physician evaluation if indicated.12,13
The Veterans Health Administration (VHA) also shifted to a VC model in response to COVID-19 guided by a unique perspective from a well-developed prior VC experience.14-16 As a federally funded system, the VHA depends on workload documentation for budgeting. Since 2015, the VHA has provided workload credit and incentivized HCPs (via pay for performance) for the use of VC, including telephone visits, video visits, and secure messaging. These incentives resulted in higher rates of telehealth utilization before the COVID-19 pandemic compared with the private sector (with 4.2% and 0.7% of visits within the VHA being telephone and video visits, respectively, compared with telehealth utilization rates of 1.0% for Medicare recipients and 1.1% in an all-payer database).16
Historically, VHA care has successfully transitioned from in-person care models to exclusively virtual modalities to prevent suspension of medical services during natural disasters. Studies performed during these periods, specifically during the 2017 hurricane season (during which multiple VHA hospitals were closed or had limited in-person service available), supported telehealth as an efficient health care delivery method, and even recommended expanding telehealth services within non-VHA environments to accommodate needs of the general public during crises and postdisaster health care delivery.17
Armed with both a well-established telehealth infrastructure and prior knowledge gained from successful systemwide implementation of virtual care during times of disaster, US Department of Veterans Affairs (VA) Connecticut Healthcare System (VACHS) primary care quickly transitioned to a VC model in response to COVID-19.16 Early in the pandemic, a rapid transition to virtual care (RTVC) model was developed, including implementation of virtual respiratory urgent clinics (VRUCs), defined as virtual respiratory symptom triage clinics, staffed by primary care providers (PCPs) aimed at minimizing patient and health care worker exposure risk.
Methods
VACHS consists of 8 primary care sites, including a major tertiary care center, a smaller medical center with full ambulatory services, and 6 community-based outpatient clinics with only primary care and mental health. There are 80 individual PCPs delivering care to 58,058 veterans. VRUCs were established during the COVID-19 pandemic to cover patients across the entire health care system, using a rotational schedule of VA PCPs.
COVID-19 Urgent Clinics Program
Within the first few weeks of the pandemic, VACHS primary care established VRUCS to provide expeditious virtual assessment of respiratory or flu-like symptoms. Using the established telehealth system, the intervention aimed to provide emergent screening, testing, and care to those with potential COVID-19 infections. The model also was designed to minimize exposures to the health care workforce and patients.
Retrospective analysis was performed using information obtained from the electronic health record (EHR) database to describe the characteristics of patients who received care through the VRUCs, such as demographics, era of military service, COVID-19 testing rates and results, as well as subsequent emergency department (ED) visits and hospital admissions. A secondary aim included collection of additional qualitative data via a random sample chart review.
Virtual clinics were established January 22, 2020, and data were analyzed over the next 3 months. Data were retrieved and analyzed from the EHR, and codes were used to categorize the VRUCs.
Results
A total of 445 unique patients used these clinics during this period. Unique patients were defined as individual patients (some may have used a clinic more than once but were counted only once). Of this group, 82% were male, and 48% served in the Gulf War era (1990 to present). A total of 51% of patients received a COVID-19 test (clinics began before wide testing availability), and 10% tested positive. Of all patients using the clinics, approximately 5% were admitted to the hospital, and 18% had at least 1 subsequent ED visit (Table).
A secondary aim included review of a random sample of 99 patient charts to gain additional information regarding whether the patient was given appropriate isolation precautions, was in a high-exposure occupation (eg, could expose a large number of people), and whether there was appropriate documentation of goals of care, health care proxy or referral to social work to discuss advance directives. In addition, we calculated the average length of time between patients’ initial contact with the health care system call center and the return call by the PCP (wait time).Of charts reviewed, the majority (71%) had documentation of appropriate isolation precautions. Although 25% of patients had documentation of a high-risk profession with potential to expose many people, more than half of the patients had no documentation of occupation. Most patients (86%) had no updated documentation regarding goals of care, health care proxy, or advance directives in their urgent care VC visit. The average time between the patient initiating contact with the health care system call center and a return call to the patient from a PCP was 104 minutes (excluding calls received after 3:30
Discussion
This analysis adds to the growing literature on use of VC during the COVID-19 pandemic. Specifically, we describe the population of patients who used VRUCs within a large health care system in a RTVC. This analysis was limited by lack of available testing during the initial phase of the pandemic, which contributed to the lower than expected rates of testing and test positivity in patients managed via VRUCs. In addition, chart review data are limited as the data includes only what was documented during the visit and not the entire discussion during the encounter.
Several important outcomes from this analysis can be applied to interventions in the future, which may have large public health implications: Several hundred patients who reported respiratory symptoms were expeditiously evaluated by a PCP using VC. The average wait time to full clinical assessment was about 1.5 hours. This short duration between contact and evaluation permitted early education about isolation precautions, which may have minimized spread. In addition, this innovation kept patients out of the medical center, eliminating chains of transmission to other vulnerable patients and health care workers.
Our retrospective chart review also revealed that more than half the patients were not queried about their occupation, but of those that were asked, a significant number were in high-risk professions potentially exposing large numbers of people. This would be an important aspect to add to future templated notes to minimize work-related exposures. Also, we identified that few HCPs discussed goals of care with patients. Given the nature of COVID-19 and potential for rapid decompensation especially in vulnerable patients, this also would be important to include in the future.
Conclusions
VC urgent care clinics to address possible COVID-19 symptoms facilitated expeditious PCP assessment while keeping potentially contagious patients outside of high-risk health care environments. Streamlining and optimizing clinical VC assessments will be imperative to future management of COVID-19 and potentially to other future infectious pandemics. This includes development of templated notes incorporating counseling regarding appropriate isolation, questions about high-contact occupations, and goals of care discussions.
Acknowledgment
The authors thank Robert F. Walsh, MHA.
Virtual care (VC) has emerged as an effective mode of health care delivery especially in settings where significant barriers to traditional in-person visits exist; a large systematic review supports feasibility of telemedicine in primary care and suggests that telemedicine is at least as effective as traditional care.1 Nevertheless, broad adoption of VC into practice has lagged, impeded by government and private insurance reimbursement requirements as well as the persistent belief that care can best be delivered in person.2-4 Before the COVID-19 pandemic, states that enacted parity legislation that required private insurance companies to provide reimbursement coverage for telehealth services saw a significant increase in the number of outpatient telehealth visits (about ≥ 30% odds compared with nonparity states).3
With the onset of the COVID-19 pandemic, in-person medical appointments were converted to VC visits to reduce increased exposure risks to patients and health care workers.5 Prior government and private sector policies were suspended, and payment restrictions lifted, enabling adoption of VC modalities to rapidly accommodate the emergent need and Centers for Disease Control and Prevention (CDC) recommendations for virtual care.6-11
The CDC guidelines on managing operations during the COVID-19 pandemic highlighted the need to provide care in the safest way for patients and health care personnel and emphasized the importance of optimizing telehealth services. The federal government facilitated telehealth during the COVID-19 pandemic via temporary measures under the COVID-19 public health emergency declaration. This included Health Insurance Portability and Accountability Act flexibility to use everyday technology for VC visits, regulatory changes to deliver services to Medicare and Medicaid patients, permission of telehealth services across state lines, and prescribing of controlled substances via telehealth without an in-person medical evaluation.7
In response, health care providers (HCPs) and health care organizations created or expanded on existing telehealth infrastructure, developing virtual urgent care centers and telephone-based programs to evaluate patients remotely via screening questions that triaged them to a correct level of response, with possible subsequent virtual physician evaluation if indicated.12,13
The Veterans Health Administration (VHA) also shifted to a VC model in response to COVID-19 guided by a unique perspective from a well-developed prior VC experience.14-16 As a federally funded system, the VHA depends on workload documentation for budgeting. Since 2015, the VHA has provided workload credit and incentivized HCPs (via pay for performance) for the use of VC, including telephone visits, video visits, and secure messaging. These incentives resulted in higher rates of telehealth utilization before the COVID-19 pandemic compared with the private sector (with 4.2% and 0.7% of visits within the VHA being telephone and video visits, respectively, compared with telehealth utilization rates of 1.0% for Medicare recipients and 1.1% in an all-payer database).16
Historically, VHA care has successfully transitioned from in-person care models to exclusively virtual modalities to prevent suspension of medical services during natural disasters. Studies performed during these periods, specifically during the 2017 hurricane season (during which multiple VHA hospitals were closed or had limited in-person service available), supported telehealth as an efficient health care delivery method, and even recommended expanding telehealth services within non-VHA environments to accommodate needs of the general public during crises and postdisaster health care delivery.17
Armed with both a well-established telehealth infrastructure and prior knowledge gained from successful systemwide implementation of virtual care during times of disaster, US Department of Veterans Affairs (VA) Connecticut Healthcare System (VACHS) primary care quickly transitioned to a VC model in response to COVID-19.16 Early in the pandemic, a rapid transition to virtual care (RTVC) model was developed, including implementation of virtual respiratory urgent clinics (VRUCs), defined as virtual respiratory symptom triage clinics, staffed by primary care providers (PCPs) aimed at minimizing patient and health care worker exposure risk.
Methods
VACHS consists of 8 primary care sites, including a major tertiary care center, a smaller medical center with full ambulatory services, and 6 community-based outpatient clinics with only primary care and mental health. There are 80 individual PCPs delivering care to 58,058 veterans. VRUCs were established during the COVID-19 pandemic to cover patients across the entire health care system, using a rotational schedule of VA PCPs.
COVID-19 Urgent Clinics Program
Within the first few weeks of the pandemic, VACHS primary care established VRUCS to provide expeditious virtual assessment of respiratory or flu-like symptoms. Using the established telehealth system, the intervention aimed to provide emergent screening, testing, and care to those with potential COVID-19 infections. The model also was designed to minimize exposures to the health care workforce and patients.
Retrospective analysis was performed using information obtained from the electronic health record (EHR) database to describe the characteristics of patients who received care through the VRUCs, such as demographics, era of military service, COVID-19 testing rates and results, as well as subsequent emergency department (ED) visits and hospital admissions. A secondary aim included collection of additional qualitative data via a random sample chart review.
Virtual clinics were established January 22, 2020, and data were analyzed over the next 3 months. Data were retrieved and analyzed from the EHR, and codes were used to categorize the VRUCs.
Results
A total of 445 unique patients used these clinics during this period. Unique patients were defined as individual patients (some may have used a clinic more than once but were counted only once). Of this group, 82% were male, and 48% served in the Gulf War era (1990 to present). A total of 51% of patients received a COVID-19 test (clinics began before wide testing availability), and 10% tested positive. Of all patients using the clinics, approximately 5% were admitted to the hospital, and 18% had at least 1 subsequent ED visit (Table).
A secondary aim included review of a random sample of 99 patient charts to gain additional information regarding whether the patient was given appropriate isolation precautions, was in a high-exposure occupation (eg, could expose a large number of people), and whether there was appropriate documentation of goals of care, health care proxy or referral to social work to discuss advance directives. In addition, we calculated the average length of time between patients’ initial contact with the health care system call center and the return call by the PCP (wait time).Of charts reviewed, the majority (71%) had documentation of appropriate isolation precautions. Although 25% of patients had documentation of a high-risk profession with potential to expose many people, more than half of the patients had no documentation of occupation. Most patients (86%) had no updated documentation regarding goals of care, health care proxy, or advance directives in their urgent care VC visit. The average time between the patient initiating contact with the health care system call center and a return call to the patient from a PCP was 104 minutes (excluding calls received after 3:30
Discussion
This analysis adds to the growing literature on use of VC during the COVID-19 pandemic. Specifically, we describe the population of patients who used VRUCs within a large health care system in a RTVC. This analysis was limited by lack of available testing during the initial phase of the pandemic, which contributed to the lower than expected rates of testing and test positivity in patients managed via VRUCs. In addition, chart review data are limited as the data includes only what was documented during the visit and not the entire discussion during the encounter.
Several important outcomes from this analysis can be applied to interventions in the future, which may have large public health implications: Several hundred patients who reported respiratory symptoms were expeditiously evaluated by a PCP using VC. The average wait time to full clinical assessment was about 1.5 hours. This short duration between contact and evaluation permitted early education about isolation precautions, which may have minimized spread. In addition, this innovation kept patients out of the medical center, eliminating chains of transmission to other vulnerable patients and health care workers.
Our retrospective chart review also revealed that more than half the patients were not queried about their occupation, but of those that were asked, a significant number were in high-risk professions potentially exposing large numbers of people. This would be an important aspect to add to future templated notes to minimize work-related exposures. Also, we identified that few HCPs discussed goals of care with patients. Given the nature of COVID-19 and potential for rapid decompensation especially in vulnerable patients, this also would be important to include in the future.
Conclusions
VC urgent care clinics to address possible COVID-19 symptoms facilitated expeditious PCP assessment while keeping potentially contagious patients outside of high-risk health care environments. Streamlining and optimizing clinical VC assessments will be imperative to future management of COVID-19 and potentially to other future infectious pandemics. This includes development of templated notes incorporating counseling regarding appropriate isolation, questions about high-contact occupations, and goals of care discussions.
Acknowledgment
The authors thank Robert F. Walsh, MHA.
1. Bashshur RL, Howell JD, Krupinski EA, Harms KM, Bashshur N, Doarn CR. The empirical foundations of telemedicine interventions in primary care. Telemed J E Health. 2016;22(5):342-375. doi:10.1089/tmj.2016.0045
2. Centers for Disease Control and Prevention. Using telehealth to expand access to essential health services during the COVID-19 pandemic. Updated June 10, 2020. Accessed August 20, 2021. https://www.cdc.gov/coronavirus/2019-ncov/hcp/telehealth.html
3. Harvey JB, Valenta S, Simpson K, Lyles M, McElligott J. Utilization of outpatient telehealth services in parity and nonparity states 2010-2015. Telemed J E Health. 2019;25(2):132-136. doi:10.1089/tmj.2017.0265
4. Dorsey ER, Topol EJ. State of telehealth. N Engl J Med. 2016;375(2):154-161. doi:10.1056/NEJMra1601705
5. Rockwell KL, Gilroy AS. Incorporating telemedicine as part of COVID-19 outbreak response systems. Am J Manag Care. 2020;26(4):147-148. doi:10.37765/ajmc.2020.42784
6. Centers for Disease Control and Prevention. Healthcare facility guidance. Updated April 17, 2021. Accessed August 20, 2021. https://www.cdc.gov/coronavirus/2019-ncov/hcp/clinical-care.html
7. US Department of Health and Human Services, Health Resources and Services Administration. Policy changes during COVID-19. Accessed August 20, 2021. https://telehealth.hhs.gov/providers/policy-changes-during-the-covid-19-public-health-emergency
8. Coronavirus Preparedness and Response Supplemental Appropriation Act of 2020. 134 Stat. 146. Published February 2, 2021. Accessed August 20, 2021. https://www.govinfo.gov/content/pkg/CREC-2021-02-02/html/CREC-2021-02-02-pt1-PgS226.htm
9. US Department of Health and Human Services. Notification of enforcement discretion for telehealth remote communications during the COVID-19 nationwide public health emergency. Updated January 20, 2021. Accessed August 20, 2021. https://www.hhs.gov/hipaa/for-professionals/special-topics/emergency-preparedness/notification-enforcement-discretion-telehealth/index.html
10. Centers for Medicare and Medicaid Services. Coverage and payment related to COVID-19 Medicare. 2020. Published March 23, 2020. Accessed August 20, 2021. https://www.cms.gov/files/document/03052020-medicare-covid-19-fact-sheet.pdf
11. American Telemedicine Association. ATA commends 2020 Congress for giving HHS authority to waive restrictions on telehealth for Medicare beneficiaries in response to the COVID-19 outbreak [press release]. Published March 5, 2020. Accessed August 20, 2021. https://www.americantelemed.org/press-releases/ata-commends-congress-for-waiving-restrictions-on-telehealth-for-medicare-beneficiaries-in-response-to-the-covid-19-outbreak
12. Hollander JE, Carr BG. Virtually perfect? Telemedicine for Covid-19. N Engl J Med. 2020;382(18):1679-1681. doi:10.1056/NEJMp2003539
13. Khairat S, Meng C, Xu Y, Edson B, Gianforcaro R. Interpreting COVID-19 and Virtual Care Trends: Cohort Study. JMIR Public Health Surveill. 2020;6(2):e18811. Published 2020 Apr 15. doi:10.2196/18811
14. Ferguson JM, Jacobs J, Yefimova M, Greene L, Heyworth L, Zulman DM. Virtual care expansion in the Veterans Health Administration during the COVID-19 pandemic: clinical services and patient characteristics associated with utilization. J Am Med Inform Assoc. 2021;28(3):453-462. doi:10.1093/jamia/ocaa284
15. Baum A, Kaboli PJ, Schwartz MD. Reduced in-person and increased telehealth outpatient visits during the COVID-19 Pandemic. Ann Intern Med. 2021;174(1):129-131. doi:10.7326/M20-3026
16. Spelman JF, Brienza R, Walsh RF, et al. A model for rapid transition to virtual care, VA Connecticut primary care response to COVID-19. J Gen Intern Med. 2020;35(10):3073-3076. doi:10.1007/s11606-020-06041-4
17. Der-Martirosian C, Chu K, Dobalian A. Use of telehealth to improve access to care at the United States Department of Veterans Affairs during the 2017 Atlantic hurricane season [published online ahead of print, 2020 Apr 13]. Disaster Med Public Health Prep. 2020;1-5. doi:10.1017/dmp.2020.88
1. Bashshur RL, Howell JD, Krupinski EA, Harms KM, Bashshur N, Doarn CR. The empirical foundations of telemedicine interventions in primary care. Telemed J E Health. 2016;22(5):342-375. doi:10.1089/tmj.2016.0045
2. Centers for Disease Control and Prevention. Using telehealth to expand access to essential health services during the COVID-19 pandemic. Updated June 10, 2020. Accessed August 20, 2021. https://www.cdc.gov/coronavirus/2019-ncov/hcp/telehealth.html
3. Harvey JB, Valenta S, Simpson K, Lyles M, McElligott J. Utilization of outpatient telehealth services in parity and nonparity states 2010-2015. Telemed J E Health. 2019;25(2):132-136. doi:10.1089/tmj.2017.0265
4. Dorsey ER, Topol EJ. State of telehealth. N Engl J Med. 2016;375(2):154-161. doi:10.1056/NEJMra1601705
5. Rockwell KL, Gilroy AS. Incorporating telemedicine as part of COVID-19 outbreak response systems. Am J Manag Care. 2020;26(4):147-148. doi:10.37765/ajmc.2020.42784
6. Centers for Disease Control and Prevention. Healthcare facility guidance. Updated April 17, 2021. Accessed August 20, 2021. https://www.cdc.gov/coronavirus/2019-ncov/hcp/clinical-care.html
7. US Department of Health and Human Services, Health Resources and Services Administration. Policy changes during COVID-19. Accessed August 20, 2021. https://telehealth.hhs.gov/providers/policy-changes-during-the-covid-19-public-health-emergency
8. Coronavirus Preparedness and Response Supplemental Appropriation Act of 2020. 134 Stat. 146. Published February 2, 2021. Accessed August 20, 2021. https://www.govinfo.gov/content/pkg/CREC-2021-02-02/html/CREC-2021-02-02-pt1-PgS226.htm
9. US Department of Health and Human Services. Notification of enforcement discretion for telehealth remote communications during the COVID-19 nationwide public health emergency. Updated January 20, 2021. Accessed August 20, 2021. https://www.hhs.gov/hipaa/for-professionals/special-topics/emergency-preparedness/notification-enforcement-discretion-telehealth/index.html
10. Centers for Medicare and Medicaid Services. Coverage and payment related to COVID-19 Medicare. 2020. Published March 23, 2020. Accessed August 20, 2021. https://www.cms.gov/files/document/03052020-medicare-covid-19-fact-sheet.pdf
11. American Telemedicine Association. ATA commends 2020 Congress for giving HHS authority to waive restrictions on telehealth for Medicare beneficiaries in response to the COVID-19 outbreak [press release]. Published March 5, 2020. Accessed August 20, 2021. https://www.americantelemed.org/press-releases/ata-commends-congress-for-waiving-restrictions-on-telehealth-for-medicare-beneficiaries-in-response-to-the-covid-19-outbreak
12. Hollander JE, Carr BG. Virtually perfect? Telemedicine for Covid-19. N Engl J Med. 2020;382(18):1679-1681. doi:10.1056/NEJMp2003539
13. Khairat S, Meng C, Xu Y, Edson B, Gianforcaro R. Interpreting COVID-19 and Virtual Care Trends: Cohort Study. JMIR Public Health Surveill. 2020;6(2):e18811. Published 2020 Apr 15. doi:10.2196/18811
14. Ferguson JM, Jacobs J, Yefimova M, Greene L, Heyworth L, Zulman DM. Virtual care expansion in the Veterans Health Administration during the COVID-19 pandemic: clinical services and patient characteristics associated with utilization. J Am Med Inform Assoc. 2021;28(3):453-462. doi:10.1093/jamia/ocaa284
15. Baum A, Kaboli PJ, Schwartz MD. Reduced in-person and increased telehealth outpatient visits during the COVID-19 Pandemic. Ann Intern Med. 2021;174(1):129-131. doi:10.7326/M20-3026
16. Spelman JF, Brienza R, Walsh RF, et al. A model for rapid transition to virtual care, VA Connecticut primary care response to COVID-19. J Gen Intern Med. 2020;35(10):3073-3076. doi:10.1007/s11606-020-06041-4
17. Der-Martirosian C, Chu K, Dobalian A. Use of telehealth to improve access to care at the United States Department of Veterans Affairs during the 2017 Atlantic hurricane season [published online ahead of print, 2020 Apr 13]. Disaster Med Public Health Prep. 2020;1-5. doi:10.1017/dmp.2020.88
Right Ventricle Dilation Detected on Point-of-Care Ultrasound Is a Predictor of Poor Outcomes in Critically Ill Patients With COVID-19
Point-of-care ultrasound (POCUS) is increasingly being used by critical care physicians to augment the physical examination and guide clinical decision making, and several protocols have been established to standardize the POCUS evaluation.1 During the COVID-19 pandemic, POCUS has been a valuable tool as standard imaging techniques were used judiciously to minimize exposure of personnel and use of personal protective equipment (PPE).2
In the US Department of Veterans Affairs (VA) New York Harbor Healthcare System (VANYHHS) intensive care unit (ICU) on initial clinical examination included POCUS, which was helpful to examine deep vein thromboses, cardiac function, and the presence and extent of pneumonia. An international expert consensus on the use of POCUS for COVID-19 published in December 2020 called for further studies defining the role of lung and cardiac ultrasound in risk stratification, outcomes, and clinical management.3
The objective of this study was to review POCUS findings and correlate them with severity of illness and 30-day outcomes in critically ill patients with COVID-19.
Methods
The study was submitted to and reviewed by the VANYHHS Research and Development committee and study approval and informed consent waiver was granted. The study was a retrospective chart review of patients admitted to the VANYHHS ICU between March and April 2020, a tertiary health care center designated as a COVID-19 hospital.
Patients admitted to the ICU aged > 18 years with a diagnosis of acute hypoxemic respiratory failure, diagnosis of COVID-19, and documentation of POCUS findings in the chart were included in the study. A patient was considered to have a COVID-19 diagnosis following a positive SARS-CoV-2 polymerase chain reaction test documented in the electronic health record (EHR). Acute respiratory failure was defined as hypoxemia < 94% and the need for either supplemental oxygen by nasal cannula > 2 L/min, high flow nasal cannula, noninvasive ventilation, or mechanical ventilation.
To minimize personnel exposure, initial patient evaluations and POCUS examinations were performed by the most senior personnel (ie, fellowship trained, board-certified pulmonary critical care attending physicians or pulmonary and critical care fellowship trainees). Three members of the team had certification in advanced critical care echocardiography by the National Board of Echocardiography and oversaw POCUS imaging. POCUS examinations were performed with a GE Heathcare Venue POCUS or handheld unit. After use, ultrasound probes and ultrasound units were disinfected with wipes designated by the manufacturer and US Environmental Protection Agency for use during the COVID-19 pandemic.
The POCUS protocol used by members of the team was as follows: POCUS lung—at least 2 anterior fields and 1 posterior/lateral field looking at the costophrenic angle on each hemithorax with a phased array or curvilinear probe. A linear probe was used to look for subpleural changes per physician discretion.4,5 Lung ultrasound findings in anterior lung fields were documented as A lines, B lines (as defined by the bedside lung ultrasound in emergency [BLUE] protocol)anterior pleural abnormalities or consolidations.4,5 The costophrenic point findings were documented as presence of consolidation or pleural effusion.
The POCUS cardiac examination consisted of parasternal long and short axis views, apical 4 chamber view, subcostal and inferior vena cava (IVC) view. Left ventricular (LV) ejection fraction was visually estimated as reduced or normal. Right ventricular (RV) dilation was considered present if RV size approached or exceeded LV size in the apical 4 chamber view. RV dysfunction was considered present if in addition there was flattening of interventricular septum, RV free wall hypokinesis or reduced tricuspid annular plane systolic excursion (TAPSE).6 IVC was documented as collapsible or plethoric by size and respirophasic variability (2 cm and 50%). Other POCUS examinations including venous compression were done at the discretion of the treating physician.7 POCUS was also used for the placement of central and arterial lines and to guide fluid management.8
The VA EHR and Venue image local archives were reviewed for patient demographics, laboratory findings, imaging studies and outcomes. All ICU attending physician and fellow notes were reviewed for POCUS lung, cardiac and vascular findings. The chart was also reviewed for management changes as a result of POCUS findings. Patients who had at minimum a POCUS lung or cardiac examination documented in the EHR were included in the study. For patients with serial POCUS the most severe findings were included.
Patients were divided into 2 groups based on 30-day outcome: discharge home vs mortality for comparison. POCUS findings were also compared by need for mechanical ventilation. Patients still hospitalized or transferred to other facilities were excluded from the analysis. A Student t test was used for comparison between the groups for continuous normally distributed variables. Linear and stepwise regression models were used to evaluate univariate and multivariate associations of baseline characteristics, biomarker, and ultrasound findings with patient outcomes. Analyses were performed using R 4.0.2 statistical software.
Results
Eighty-two patients were admitted to the VANYHHS ICU in March and April 2020, including 12 nonveterans. Sixty-four had COVID-19 and acute respiratory failure. POCUS findings were documented in 43 (67%) patients. Thirty-nine patients had documented lung examinations, and 25 patients had documented cardiac examinations. Patients were divided into 2 groups by 30-day outcome (discharge home vs mortality) for statistical analysis. Five patients who were either still hospitalized or had been transferred to another facility were excluded.
Baseline characteristics of patients included in the study stratified by 30-day outcomes are shown in Table 1. The study group was predominantly male (95%). Patients with poor 30-day outcomes were older, had higher white blood cell counts, more severe hypoxemia, higher rates of mechanical ventilation and RV dilation (Figures 1, 2, 3, 4, and 5). RV dilation was an independent predictor of mortality (odds ratio [OR], 12.0; P = .048).
Serial POCUS documented development or progression of RV dilation and dysfunction from the time of ICU admission in 4 of the patients. The presence of B lines with irregular pleura was predictive of a lower arterial pressure of oxygen to fraction of inspired oxygen ratio (PaO2/FiO2) by a value of 71 compared with those without B lines with irregular pleura (P = .005, adjusted R2 = 0.238). All patients with RV dilation had bilateral B lines with pleural irregularities on lung ultrasound. Vascular POCUS detected 4 deep vein thromboses (DVT).7 An arterial thrombus was also detected on focused examination. There was a higher mortality in patients who required mechanical ventilation; however, there was no difference in POCUS characteristics between the groups (Table 2).
Two severely hypoxemic patients received systemic tissue plasminogen activator (TPA) after findings of massive RV dilation with signs of volume and pressure overload and clinical suspicion of pulmonary embolism (PE). One of these patients also had a popliteal DVT. Both patients were too unstable to transport for additional imaging or therapies. Therapeutic anticoagulation was initiated on 4 patients with positive DVT examinations. In a fifth case an arterial thrombectomy and anticoagulation was required after diminished pulses led to the finding of an occlusive brachial artery thrombus on vascular POCUS.
Discussion
POCUS identified both lung and cardiac features that were associated with worse outcomes. While lung ultrasound abnormalities were very prevalent and associated with worse PaO2 to FiO2 ratios, the presence of RV dilation was associated most clearly with mortality and poor 30-day outcomes in the critical care setting.
Lung ultrasound abnormalities were pervasive in patients with acute respiratory failure and COVID-19. On linear regression we found that presence with bilateral B lines and pleural thickening was predictive of a lower PaO2/FiO2 (coefficient, -70; P = .005). Our study found that B lines with pleural irregularities, otherwise known as a B’ profile per the BLUE protocol, was seen in patients with severe COVID-19. Thus severe acute respiratory failure secondary to COVID-19 has similar lung ultrasound findings as non-COVID-19 acute respiratory distress syndrome (ARDS).4,5 Based on prior lung ultrasound studies in ARDS, lung ultrasound findings can be used as an alternate to chest radiography for the diagnosis of ARDS in COVID-19 and predict the severity of ARDS.9 This has particular implications in overwhelmed and resource poor health care settings.
We found no difference in 30-day mortality based on lung ultrasound findings or profile, probably because of small sample size or because the findings were tabulated as profiles and not differentiated further with lung ultrasound scores.10,11 However, there was a significant difference in RV dilation between the 2 groups by 30 days and its presence was found to be a predictor of mortality even when controlled for hypertension and diabetes mellitus (P = .048) with an OR of 12. RV dysfunction in patients with ARDS on mechanical ventilation ranges from 22 to 25% and is typically associated with high driving pressures.12-14 The mechanism is thought to be multifactorial including hypoxemic vasoconstriction in the pulmonary vasculature in addition to the increased transpulmonary pressure.15 While all of the above are at play in COVID-19 infection, there is reported damage to the pulmonary vascular endothelium and resultant hypercoagulability and thrombosis that further increases the RV afterload.16
While RV strain and dysfunction indices done by an echocardiographer would be ideal, given the surge in infections and hospitalizations and strain on health care resources, POCUS by the treating or examining clinician was considered the only feasible way to screen a large number of patients.17 Identification of RV dilation could influence clinical management including workup for venous thromboembolic disease and optimization of lung protective strategies. Further studies are needed to understand the particular etiology and pathophysiology of COVID-19 associated RV dilation. Given increased thrombosis events in COVID-19 infection we believe a POCUS vascular examination should be included as part of evaluation especially in the presence of increased D-dimers and has been discussed above for its important role in working up RV dilation.18
Limitations
Our study has several limitations. It was retrospective in nature and involved a small group of individuals. There was some variation in POCUS examinations done at the discretion of the examining physician. We did not have a blinded observer independently review all images. Since RV dilation was documented only when RV size approached or exceeded LV size in the apical 4 chamber view representing moderate or severe dilation, we may be underreporting the prevalence in critically ill patients.
Conclusions
POCUS is an invaluable adjunct to clinical evaluation and procedures in patients with severe COVID-19 with the ability to identity patients at risk for worse outcomes. B lines with pleural thickening is a sign of severe ARDS and RV dilatation is predictive of mortality. POCUS should be made available to the treating physician for monitoring and risk stratification and can be incorporated into management algorithms.
Additional point-of-care ultrasound videos.
Acknowledgments
We thank frontline healthcare workers and intensive care unit staff of the US Department of Veterans Affairs New York Harbor Healthcare System (NYHHS) for their dedication to the care of veterans and civilians during the COVID-19 pandemic in New York City. The authors acknowledge the NYHHS research and development committee and administration for their support.
1. Cardenas-Garcia J, Mayo PH. Bedside ultrasonography for the intensivist. Crit Care Clin. 2015;31(1):43-66. doi:10.1016/j.ccc.2014.08.003
2. Vetrugno L, Baciarello M, Bignami E, et al. The “pandemic” increase in lung ultrasound use in response to Covid-19: can we complement computed tomography findings? A narrative review. Ultrasound J. 2020;12(1):39. Published 2020 Aug 17. doi:10.1186/s13089-020-00185-4
3. Hussain A, Via G, Melniker L, et al. Multi-organ point-of-care ultrasound for COVID-19 (PoCUS4COVID): international expert consensus. Crit Care. 2020;24(1):702. Published 2020 Dec 24. doi:10.1186/s13054-020-03369-5
4. Lichtenstein DA, Mezière GA. Relevance of lung ultrasound in the diagnosis of acute respiratory failure: the BLUE protocol [published correction appears in Chest. 2013 Aug;144(2):721]. Chest. 2008;134(1):117-125. doi:10.1378/chest.07-2800
5. Volpicelli G, Elbarbary M, Blaivas M, et al. International evidence-based recommendations for point-of-care lung ultrasound. Intensive Care Med. 2012;38(4):577-591. doi:10.1007/s00134-012-2513-4
6. Narasimhan M, Koenig SJ, Mayo PH. Advanced echocardiography for the critical care physician: part 1. Chest. 2014;145(1):129-134. doi:10.1378/chest.12-2441
7. Kory PD, Pellecchia CM, Shiloh AL, Mayo PH, DiBello C, Koenig S. Accuracy of ultrasonography performed by critical care physicians for the diagnosis of DVT. Chest. 2011;139(3):538-542. doi:10.1378/chest.10-1479
8. Bentzer P, Griesdale DE, Boyd J, MacLean K, Sirounis D, Ayas NT. Will this hemodynamically unstable patient respond to a bolus of intravenous fluids? JAMA. 2016;316(12):1298-1309. doi:10.1001/jama.2016.12310
9. See KC, Ong V, Tan YL, Sahagun J, Taculod J. Chest radiography versus lung ultrasound for identification of acute respiratory distress syndrome: a retrospective observational study. Crit Care. 2018;22(1):203. Published 2018 Aug 18. doi:10.1186/s13054-018-2105-y
10. Deng Q, Zhang Y, Wang H, et al. Semiquantitative lung ultrasound scores in the evaluation and follow-up of critically ill patients with COVID-19: a single-center study. Acad Radiol. 2020;27(10):1363-1372. doi:10.1016/j.acra.2020.07.002
11. Brahier T, Meuwly JY, Pantet O, et al. Lung ultrasonography for risk stratification in patients with COVID-19: a prospective observational cohort study [published online ahead of print, 2020 Sep 17]. Clin Infect Dis. 2020;ciaa1408. doi:10.1093/cid/ciaa1408
12. Vieillard-Baron A, Schmitt JM, Augarde R, et al. Acute cor pulmonale in acute respiratory distress syndrome submitted to protective ventilation: incidence, clinical implications, and prognosis [published correction appears in Crit Care Med. 2002 Mar;30(3):726]. Crit Care Med. 2001;29(8):1551-1555. doi:10.1097/00003246-200108000-00009
13. Boissier F, Katsahian S, Razazi K, et al. Prevalence and prognosis of cor pulmonale during protective ventilation for acute respiratory distress syndrome. Intensive Care Med. 2013;39(10):1725-1733. doi:10.1007/s00134-013-2941-9
14. Jardin F, Vieillard-Baron A. Is there a safe plateau pressure in ARDS? The right heart only knows. Intensive Care Med. 2007;33(3):444-447. doi:10.1007/s00134-007-0552-z
15. Repessé X, Vieillard-Baron A. Right heart function during acute respiratory distress syndrome. Ann Transl Med 2017;5(14):295. doi:10.21037/atm.2017.06.66
16. Abou-Ismail MY, Diamond A, Kapoor S, Arafah Y, Nayak L. The hypercoagulable state in COVID-19: Incidence, pathophysiology, and management [published correction appears in Thromb Res. 2020 Nov 26]. Thromb Res. 2020;194:101-115. doi:10.1016/j.thromres.2020.06.029
17. Kim J, Volodarskiy A, Sultana R, et al. Prognostic utility of right ventricular remodeling over conventional risk stratification in patients with COVID-19. J Am Coll Cardiol. 2020;76(17):1965-1977. doi:10.1016/j.jacc.2020.08.066
18. Al-Samkari H, Karp Leaf RS, Dzik WH, et al. COVID-19 and coagulation: bleeding and thrombotic manifestations of SARS-CoV-2 infection. Blood. 2020;136(4):489-500. doi:10.1182/blood.2020006520
Point-of-care ultrasound (POCUS) is increasingly being used by critical care physicians to augment the physical examination and guide clinical decision making, and several protocols have been established to standardize the POCUS evaluation.1 During the COVID-19 pandemic, POCUS has been a valuable tool as standard imaging techniques were used judiciously to minimize exposure of personnel and use of personal protective equipment (PPE).2
In the US Department of Veterans Affairs (VA) New York Harbor Healthcare System (VANYHHS) intensive care unit (ICU) on initial clinical examination included POCUS, which was helpful to examine deep vein thromboses, cardiac function, and the presence and extent of pneumonia. An international expert consensus on the use of POCUS for COVID-19 published in December 2020 called for further studies defining the role of lung and cardiac ultrasound in risk stratification, outcomes, and clinical management.3
The objective of this study was to review POCUS findings and correlate them with severity of illness and 30-day outcomes in critically ill patients with COVID-19.
Methods
The study was submitted to and reviewed by the VANYHHS Research and Development committee and study approval and informed consent waiver was granted. The study was a retrospective chart review of patients admitted to the VANYHHS ICU between March and April 2020, a tertiary health care center designated as a COVID-19 hospital.
Patients admitted to the ICU aged > 18 years with a diagnosis of acute hypoxemic respiratory failure, diagnosis of COVID-19, and documentation of POCUS findings in the chart were included in the study. A patient was considered to have a COVID-19 diagnosis following a positive SARS-CoV-2 polymerase chain reaction test documented in the electronic health record (EHR). Acute respiratory failure was defined as hypoxemia < 94% and the need for either supplemental oxygen by nasal cannula > 2 L/min, high flow nasal cannula, noninvasive ventilation, or mechanical ventilation.
To minimize personnel exposure, initial patient evaluations and POCUS examinations were performed by the most senior personnel (ie, fellowship trained, board-certified pulmonary critical care attending physicians or pulmonary and critical care fellowship trainees). Three members of the team had certification in advanced critical care echocardiography by the National Board of Echocardiography and oversaw POCUS imaging. POCUS examinations were performed with a GE Heathcare Venue POCUS or handheld unit. After use, ultrasound probes and ultrasound units were disinfected with wipes designated by the manufacturer and US Environmental Protection Agency for use during the COVID-19 pandemic.
The POCUS protocol used by members of the team was as follows: POCUS lung—at least 2 anterior fields and 1 posterior/lateral field looking at the costophrenic angle on each hemithorax with a phased array or curvilinear probe. A linear probe was used to look for subpleural changes per physician discretion.4,5 Lung ultrasound findings in anterior lung fields were documented as A lines, B lines (as defined by the bedside lung ultrasound in emergency [BLUE] protocol)anterior pleural abnormalities or consolidations.4,5 The costophrenic point findings were documented as presence of consolidation or pleural effusion.
The POCUS cardiac examination consisted of parasternal long and short axis views, apical 4 chamber view, subcostal and inferior vena cava (IVC) view. Left ventricular (LV) ejection fraction was visually estimated as reduced or normal. Right ventricular (RV) dilation was considered present if RV size approached or exceeded LV size in the apical 4 chamber view. RV dysfunction was considered present if in addition there was flattening of interventricular septum, RV free wall hypokinesis or reduced tricuspid annular plane systolic excursion (TAPSE).6 IVC was documented as collapsible or plethoric by size and respirophasic variability (2 cm and 50%). Other POCUS examinations including venous compression were done at the discretion of the treating physician.7 POCUS was also used for the placement of central and arterial lines and to guide fluid management.8
The VA EHR and Venue image local archives were reviewed for patient demographics, laboratory findings, imaging studies and outcomes. All ICU attending physician and fellow notes were reviewed for POCUS lung, cardiac and vascular findings. The chart was also reviewed for management changes as a result of POCUS findings. Patients who had at minimum a POCUS lung or cardiac examination documented in the EHR were included in the study. For patients with serial POCUS the most severe findings were included.
Patients were divided into 2 groups based on 30-day outcome: discharge home vs mortality for comparison. POCUS findings were also compared by need for mechanical ventilation. Patients still hospitalized or transferred to other facilities were excluded from the analysis. A Student t test was used for comparison between the groups for continuous normally distributed variables. Linear and stepwise regression models were used to evaluate univariate and multivariate associations of baseline characteristics, biomarker, and ultrasound findings with patient outcomes. Analyses were performed using R 4.0.2 statistical software.
Results
Eighty-two patients were admitted to the VANYHHS ICU in March and April 2020, including 12 nonveterans. Sixty-four had COVID-19 and acute respiratory failure. POCUS findings were documented in 43 (67%) patients. Thirty-nine patients had documented lung examinations, and 25 patients had documented cardiac examinations. Patients were divided into 2 groups by 30-day outcome (discharge home vs mortality) for statistical analysis. Five patients who were either still hospitalized or had been transferred to another facility were excluded.
Baseline characteristics of patients included in the study stratified by 30-day outcomes are shown in Table 1. The study group was predominantly male (95%). Patients with poor 30-day outcomes were older, had higher white blood cell counts, more severe hypoxemia, higher rates of mechanical ventilation and RV dilation (Figures 1, 2, 3, 4, and 5). RV dilation was an independent predictor of mortality (odds ratio [OR], 12.0; P = .048).
Serial POCUS documented development or progression of RV dilation and dysfunction from the time of ICU admission in 4 of the patients. The presence of B lines with irregular pleura was predictive of a lower arterial pressure of oxygen to fraction of inspired oxygen ratio (PaO2/FiO2) by a value of 71 compared with those without B lines with irregular pleura (P = .005, adjusted R2 = 0.238). All patients with RV dilation had bilateral B lines with pleural irregularities on lung ultrasound. Vascular POCUS detected 4 deep vein thromboses (DVT).7 An arterial thrombus was also detected on focused examination. There was a higher mortality in patients who required mechanical ventilation; however, there was no difference in POCUS characteristics between the groups (Table 2).
Two severely hypoxemic patients received systemic tissue plasminogen activator (TPA) after findings of massive RV dilation with signs of volume and pressure overload and clinical suspicion of pulmonary embolism (PE). One of these patients also had a popliteal DVT. Both patients were too unstable to transport for additional imaging or therapies. Therapeutic anticoagulation was initiated on 4 patients with positive DVT examinations. In a fifth case an arterial thrombectomy and anticoagulation was required after diminished pulses led to the finding of an occlusive brachial artery thrombus on vascular POCUS.
Discussion
POCUS identified both lung and cardiac features that were associated with worse outcomes. While lung ultrasound abnormalities were very prevalent and associated with worse PaO2 to FiO2 ratios, the presence of RV dilation was associated most clearly with mortality and poor 30-day outcomes in the critical care setting.
Lung ultrasound abnormalities were pervasive in patients with acute respiratory failure and COVID-19. On linear regression we found that presence with bilateral B lines and pleural thickening was predictive of a lower PaO2/FiO2 (coefficient, -70; P = .005). Our study found that B lines with pleural irregularities, otherwise known as a B’ profile per the BLUE protocol, was seen in patients with severe COVID-19. Thus severe acute respiratory failure secondary to COVID-19 has similar lung ultrasound findings as non-COVID-19 acute respiratory distress syndrome (ARDS).4,5 Based on prior lung ultrasound studies in ARDS, lung ultrasound findings can be used as an alternate to chest radiography for the diagnosis of ARDS in COVID-19 and predict the severity of ARDS.9 This has particular implications in overwhelmed and resource poor health care settings.
We found no difference in 30-day mortality based on lung ultrasound findings or profile, probably because of small sample size or because the findings were tabulated as profiles and not differentiated further with lung ultrasound scores.10,11 However, there was a significant difference in RV dilation between the 2 groups by 30 days and its presence was found to be a predictor of mortality even when controlled for hypertension and diabetes mellitus (P = .048) with an OR of 12. RV dysfunction in patients with ARDS on mechanical ventilation ranges from 22 to 25% and is typically associated with high driving pressures.12-14 The mechanism is thought to be multifactorial including hypoxemic vasoconstriction in the pulmonary vasculature in addition to the increased transpulmonary pressure.15 While all of the above are at play in COVID-19 infection, there is reported damage to the pulmonary vascular endothelium and resultant hypercoagulability and thrombosis that further increases the RV afterload.16
While RV strain and dysfunction indices done by an echocardiographer would be ideal, given the surge in infections and hospitalizations and strain on health care resources, POCUS by the treating or examining clinician was considered the only feasible way to screen a large number of patients.17 Identification of RV dilation could influence clinical management including workup for venous thromboembolic disease and optimization of lung protective strategies. Further studies are needed to understand the particular etiology and pathophysiology of COVID-19 associated RV dilation. Given increased thrombosis events in COVID-19 infection we believe a POCUS vascular examination should be included as part of evaluation especially in the presence of increased D-dimers and has been discussed above for its important role in working up RV dilation.18
Limitations
Our study has several limitations. It was retrospective in nature and involved a small group of individuals. There was some variation in POCUS examinations done at the discretion of the examining physician. We did not have a blinded observer independently review all images. Since RV dilation was documented only when RV size approached or exceeded LV size in the apical 4 chamber view representing moderate or severe dilation, we may be underreporting the prevalence in critically ill patients.
Conclusions
POCUS is an invaluable adjunct to clinical evaluation and procedures in patients with severe COVID-19 with the ability to identity patients at risk for worse outcomes. B lines with pleural thickening is a sign of severe ARDS and RV dilatation is predictive of mortality. POCUS should be made available to the treating physician for monitoring and risk stratification and can be incorporated into management algorithms.
Additional point-of-care ultrasound videos.
Acknowledgments
We thank frontline healthcare workers and intensive care unit staff of the US Department of Veterans Affairs New York Harbor Healthcare System (NYHHS) for their dedication to the care of veterans and civilians during the COVID-19 pandemic in New York City. The authors acknowledge the NYHHS research and development committee and administration for their support.
Point-of-care ultrasound (POCUS) is increasingly being used by critical care physicians to augment the physical examination and guide clinical decision making, and several protocols have been established to standardize the POCUS evaluation.1 During the COVID-19 pandemic, POCUS has been a valuable tool as standard imaging techniques were used judiciously to minimize exposure of personnel and use of personal protective equipment (PPE).2
In the US Department of Veterans Affairs (VA) New York Harbor Healthcare System (VANYHHS) intensive care unit (ICU) on initial clinical examination included POCUS, which was helpful to examine deep vein thromboses, cardiac function, and the presence and extent of pneumonia. An international expert consensus on the use of POCUS for COVID-19 published in December 2020 called for further studies defining the role of lung and cardiac ultrasound in risk stratification, outcomes, and clinical management.3
The objective of this study was to review POCUS findings and correlate them with severity of illness and 30-day outcomes in critically ill patients with COVID-19.
Methods
The study was submitted to and reviewed by the VANYHHS Research and Development committee and study approval and informed consent waiver was granted. The study was a retrospective chart review of patients admitted to the VANYHHS ICU between March and April 2020, a tertiary health care center designated as a COVID-19 hospital.
Patients admitted to the ICU aged > 18 years with a diagnosis of acute hypoxemic respiratory failure, diagnosis of COVID-19, and documentation of POCUS findings in the chart were included in the study. A patient was considered to have a COVID-19 diagnosis following a positive SARS-CoV-2 polymerase chain reaction test documented in the electronic health record (EHR). Acute respiratory failure was defined as hypoxemia < 94% and the need for either supplemental oxygen by nasal cannula > 2 L/min, high flow nasal cannula, noninvasive ventilation, or mechanical ventilation.
To minimize personnel exposure, initial patient evaluations and POCUS examinations were performed by the most senior personnel (ie, fellowship trained, board-certified pulmonary critical care attending physicians or pulmonary and critical care fellowship trainees). Three members of the team had certification in advanced critical care echocardiography by the National Board of Echocardiography and oversaw POCUS imaging. POCUS examinations were performed with a GE Heathcare Venue POCUS or handheld unit. After use, ultrasound probes and ultrasound units were disinfected with wipes designated by the manufacturer and US Environmental Protection Agency for use during the COVID-19 pandemic.
The POCUS protocol used by members of the team was as follows: POCUS lung—at least 2 anterior fields and 1 posterior/lateral field looking at the costophrenic angle on each hemithorax with a phased array or curvilinear probe. A linear probe was used to look for subpleural changes per physician discretion.4,5 Lung ultrasound findings in anterior lung fields were documented as A lines, B lines (as defined by the bedside lung ultrasound in emergency [BLUE] protocol)anterior pleural abnormalities or consolidations.4,5 The costophrenic point findings were documented as presence of consolidation or pleural effusion.
The POCUS cardiac examination consisted of parasternal long and short axis views, apical 4 chamber view, subcostal and inferior vena cava (IVC) view. Left ventricular (LV) ejection fraction was visually estimated as reduced or normal. Right ventricular (RV) dilation was considered present if RV size approached or exceeded LV size in the apical 4 chamber view. RV dysfunction was considered present if in addition there was flattening of interventricular septum, RV free wall hypokinesis or reduced tricuspid annular plane systolic excursion (TAPSE).6 IVC was documented as collapsible or plethoric by size and respirophasic variability (2 cm and 50%). Other POCUS examinations including venous compression were done at the discretion of the treating physician.7 POCUS was also used for the placement of central and arterial lines and to guide fluid management.8
The VA EHR and Venue image local archives were reviewed for patient demographics, laboratory findings, imaging studies and outcomes. All ICU attending physician and fellow notes were reviewed for POCUS lung, cardiac and vascular findings. The chart was also reviewed for management changes as a result of POCUS findings. Patients who had at minimum a POCUS lung or cardiac examination documented in the EHR were included in the study. For patients with serial POCUS the most severe findings were included.
Patients were divided into 2 groups based on 30-day outcome: discharge home vs mortality for comparison. POCUS findings were also compared by need for mechanical ventilation. Patients still hospitalized or transferred to other facilities were excluded from the analysis. A Student t test was used for comparison between the groups for continuous normally distributed variables. Linear and stepwise regression models were used to evaluate univariate and multivariate associations of baseline characteristics, biomarker, and ultrasound findings with patient outcomes. Analyses were performed using R 4.0.2 statistical software.
Results
Eighty-two patients were admitted to the VANYHHS ICU in March and April 2020, including 12 nonveterans. Sixty-four had COVID-19 and acute respiratory failure. POCUS findings were documented in 43 (67%) patients. Thirty-nine patients had documented lung examinations, and 25 patients had documented cardiac examinations. Patients were divided into 2 groups by 30-day outcome (discharge home vs mortality) for statistical analysis. Five patients who were either still hospitalized or had been transferred to another facility were excluded.
Baseline characteristics of patients included in the study stratified by 30-day outcomes are shown in Table 1. The study group was predominantly male (95%). Patients with poor 30-day outcomes were older, had higher white blood cell counts, more severe hypoxemia, higher rates of mechanical ventilation and RV dilation (Figures 1, 2, 3, 4, and 5). RV dilation was an independent predictor of mortality (odds ratio [OR], 12.0; P = .048).
Serial POCUS documented development or progression of RV dilation and dysfunction from the time of ICU admission in 4 of the patients. The presence of B lines with irregular pleura was predictive of a lower arterial pressure of oxygen to fraction of inspired oxygen ratio (PaO2/FiO2) by a value of 71 compared with those without B lines with irregular pleura (P = .005, adjusted R2 = 0.238). All patients with RV dilation had bilateral B lines with pleural irregularities on lung ultrasound. Vascular POCUS detected 4 deep vein thromboses (DVT).7 An arterial thrombus was also detected on focused examination. There was a higher mortality in patients who required mechanical ventilation; however, there was no difference in POCUS characteristics between the groups (Table 2).
Two severely hypoxemic patients received systemic tissue plasminogen activator (TPA) after findings of massive RV dilation with signs of volume and pressure overload and clinical suspicion of pulmonary embolism (PE). One of these patients also had a popliteal DVT. Both patients were too unstable to transport for additional imaging or therapies. Therapeutic anticoagulation was initiated on 4 patients with positive DVT examinations. In a fifth case an arterial thrombectomy and anticoagulation was required after diminished pulses led to the finding of an occlusive brachial artery thrombus on vascular POCUS.
Discussion
POCUS identified both lung and cardiac features that were associated with worse outcomes. While lung ultrasound abnormalities were very prevalent and associated with worse PaO2 to FiO2 ratios, the presence of RV dilation was associated most clearly with mortality and poor 30-day outcomes in the critical care setting.
Lung ultrasound abnormalities were pervasive in patients with acute respiratory failure and COVID-19. On linear regression we found that presence with bilateral B lines and pleural thickening was predictive of a lower PaO2/FiO2 (coefficient, -70; P = .005). Our study found that B lines with pleural irregularities, otherwise known as a B’ profile per the BLUE protocol, was seen in patients with severe COVID-19. Thus severe acute respiratory failure secondary to COVID-19 has similar lung ultrasound findings as non-COVID-19 acute respiratory distress syndrome (ARDS).4,5 Based on prior lung ultrasound studies in ARDS, lung ultrasound findings can be used as an alternate to chest radiography for the diagnosis of ARDS in COVID-19 and predict the severity of ARDS.9 This has particular implications in overwhelmed and resource poor health care settings.
We found no difference in 30-day mortality based on lung ultrasound findings or profile, probably because of small sample size or because the findings were tabulated as profiles and not differentiated further with lung ultrasound scores.10,11 However, there was a significant difference in RV dilation between the 2 groups by 30 days and its presence was found to be a predictor of mortality even when controlled for hypertension and diabetes mellitus (P = .048) with an OR of 12. RV dysfunction in patients with ARDS on mechanical ventilation ranges from 22 to 25% and is typically associated with high driving pressures.12-14 The mechanism is thought to be multifactorial including hypoxemic vasoconstriction in the pulmonary vasculature in addition to the increased transpulmonary pressure.15 While all of the above are at play in COVID-19 infection, there is reported damage to the pulmonary vascular endothelium and resultant hypercoagulability and thrombosis that further increases the RV afterload.16
While RV strain and dysfunction indices done by an echocardiographer would be ideal, given the surge in infections and hospitalizations and strain on health care resources, POCUS by the treating or examining clinician was considered the only feasible way to screen a large number of patients.17 Identification of RV dilation could influence clinical management including workup for venous thromboembolic disease and optimization of lung protective strategies. Further studies are needed to understand the particular etiology and pathophysiology of COVID-19 associated RV dilation. Given increased thrombosis events in COVID-19 infection we believe a POCUS vascular examination should be included as part of evaluation especially in the presence of increased D-dimers and has been discussed above for its important role in working up RV dilation.18
Limitations
Our study has several limitations. It was retrospective in nature and involved a small group of individuals. There was some variation in POCUS examinations done at the discretion of the examining physician. We did not have a blinded observer independently review all images. Since RV dilation was documented only when RV size approached or exceeded LV size in the apical 4 chamber view representing moderate or severe dilation, we may be underreporting the prevalence in critically ill patients.
Conclusions
POCUS is an invaluable adjunct to clinical evaluation and procedures in patients with severe COVID-19 with the ability to identity patients at risk for worse outcomes. B lines with pleural thickening is a sign of severe ARDS and RV dilatation is predictive of mortality. POCUS should be made available to the treating physician for monitoring and risk stratification and can be incorporated into management algorithms.
Additional point-of-care ultrasound videos.
Acknowledgments
We thank frontline healthcare workers and intensive care unit staff of the US Department of Veterans Affairs New York Harbor Healthcare System (NYHHS) for their dedication to the care of veterans and civilians during the COVID-19 pandemic in New York City. The authors acknowledge the NYHHS research and development committee and administration for their support.
1. Cardenas-Garcia J, Mayo PH. Bedside ultrasonography for the intensivist. Crit Care Clin. 2015;31(1):43-66. doi:10.1016/j.ccc.2014.08.003
2. Vetrugno L, Baciarello M, Bignami E, et al. The “pandemic” increase in lung ultrasound use in response to Covid-19: can we complement computed tomography findings? A narrative review. Ultrasound J. 2020;12(1):39. Published 2020 Aug 17. doi:10.1186/s13089-020-00185-4
3. Hussain A, Via G, Melniker L, et al. Multi-organ point-of-care ultrasound for COVID-19 (PoCUS4COVID): international expert consensus. Crit Care. 2020;24(1):702. Published 2020 Dec 24. doi:10.1186/s13054-020-03369-5
4. Lichtenstein DA, Mezière GA. Relevance of lung ultrasound in the diagnosis of acute respiratory failure: the BLUE protocol [published correction appears in Chest. 2013 Aug;144(2):721]. Chest. 2008;134(1):117-125. doi:10.1378/chest.07-2800
5. Volpicelli G, Elbarbary M, Blaivas M, et al. International evidence-based recommendations for point-of-care lung ultrasound. Intensive Care Med. 2012;38(4):577-591. doi:10.1007/s00134-012-2513-4
6. Narasimhan M, Koenig SJ, Mayo PH. Advanced echocardiography for the critical care physician: part 1. Chest. 2014;145(1):129-134. doi:10.1378/chest.12-2441
7. Kory PD, Pellecchia CM, Shiloh AL, Mayo PH, DiBello C, Koenig S. Accuracy of ultrasonography performed by critical care physicians for the diagnosis of DVT. Chest. 2011;139(3):538-542. doi:10.1378/chest.10-1479
8. Bentzer P, Griesdale DE, Boyd J, MacLean K, Sirounis D, Ayas NT. Will this hemodynamically unstable patient respond to a bolus of intravenous fluids? JAMA. 2016;316(12):1298-1309. doi:10.1001/jama.2016.12310
9. See KC, Ong V, Tan YL, Sahagun J, Taculod J. Chest radiography versus lung ultrasound for identification of acute respiratory distress syndrome: a retrospective observational study. Crit Care. 2018;22(1):203. Published 2018 Aug 18. doi:10.1186/s13054-018-2105-y
10. Deng Q, Zhang Y, Wang H, et al. Semiquantitative lung ultrasound scores in the evaluation and follow-up of critically ill patients with COVID-19: a single-center study. Acad Radiol. 2020;27(10):1363-1372. doi:10.1016/j.acra.2020.07.002
11. Brahier T, Meuwly JY, Pantet O, et al. Lung ultrasonography for risk stratification in patients with COVID-19: a prospective observational cohort study [published online ahead of print, 2020 Sep 17]. Clin Infect Dis. 2020;ciaa1408. doi:10.1093/cid/ciaa1408
12. Vieillard-Baron A, Schmitt JM, Augarde R, et al. Acute cor pulmonale in acute respiratory distress syndrome submitted to protective ventilation: incidence, clinical implications, and prognosis [published correction appears in Crit Care Med. 2002 Mar;30(3):726]. Crit Care Med. 2001;29(8):1551-1555. doi:10.1097/00003246-200108000-00009
13. Boissier F, Katsahian S, Razazi K, et al. Prevalence and prognosis of cor pulmonale during protective ventilation for acute respiratory distress syndrome. Intensive Care Med. 2013;39(10):1725-1733. doi:10.1007/s00134-013-2941-9
14. Jardin F, Vieillard-Baron A. Is there a safe plateau pressure in ARDS? The right heart only knows. Intensive Care Med. 2007;33(3):444-447. doi:10.1007/s00134-007-0552-z
15. Repessé X, Vieillard-Baron A. Right heart function during acute respiratory distress syndrome. Ann Transl Med 2017;5(14):295. doi:10.21037/atm.2017.06.66
16. Abou-Ismail MY, Diamond A, Kapoor S, Arafah Y, Nayak L. The hypercoagulable state in COVID-19: Incidence, pathophysiology, and management [published correction appears in Thromb Res. 2020 Nov 26]. Thromb Res. 2020;194:101-115. doi:10.1016/j.thromres.2020.06.029
17. Kim J, Volodarskiy A, Sultana R, et al. Prognostic utility of right ventricular remodeling over conventional risk stratification in patients with COVID-19. J Am Coll Cardiol. 2020;76(17):1965-1977. doi:10.1016/j.jacc.2020.08.066
18. Al-Samkari H, Karp Leaf RS, Dzik WH, et al. COVID-19 and coagulation: bleeding and thrombotic manifestations of SARS-CoV-2 infection. Blood. 2020;136(4):489-500. doi:10.1182/blood.2020006520
1. Cardenas-Garcia J, Mayo PH. Bedside ultrasonography for the intensivist. Crit Care Clin. 2015;31(1):43-66. doi:10.1016/j.ccc.2014.08.003
2. Vetrugno L, Baciarello M, Bignami E, et al. The “pandemic” increase in lung ultrasound use in response to Covid-19: can we complement computed tomography findings? A narrative review. Ultrasound J. 2020;12(1):39. Published 2020 Aug 17. doi:10.1186/s13089-020-00185-4
3. Hussain A, Via G, Melniker L, et al. Multi-organ point-of-care ultrasound for COVID-19 (PoCUS4COVID): international expert consensus. Crit Care. 2020;24(1):702. Published 2020 Dec 24. doi:10.1186/s13054-020-03369-5
4. Lichtenstein DA, Mezière GA. Relevance of lung ultrasound in the diagnosis of acute respiratory failure: the BLUE protocol [published correction appears in Chest. 2013 Aug;144(2):721]. Chest. 2008;134(1):117-125. doi:10.1378/chest.07-2800
5. Volpicelli G, Elbarbary M, Blaivas M, et al. International evidence-based recommendations for point-of-care lung ultrasound. Intensive Care Med. 2012;38(4):577-591. doi:10.1007/s00134-012-2513-4
6. Narasimhan M, Koenig SJ, Mayo PH. Advanced echocardiography for the critical care physician: part 1. Chest. 2014;145(1):129-134. doi:10.1378/chest.12-2441
7. Kory PD, Pellecchia CM, Shiloh AL, Mayo PH, DiBello C, Koenig S. Accuracy of ultrasonography performed by critical care physicians for the diagnosis of DVT. Chest. 2011;139(3):538-542. doi:10.1378/chest.10-1479
8. Bentzer P, Griesdale DE, Boyd J, MacLean K, Sirounis D, Ayas NT. Will this hemodynamically unstable patient respond to a bolus of intravenous fluids? JAMA. 2016;316(12):1298-1309. doi:10.1001/jama.2016.12310
9. See KC, Ong V, Tan YL, Sahagun J, Taculod J. Chest radiography versus lung ultrasound for identification of acute respiratory distress syndrome: a retrospective observational study. Crit Care. 2018;22(1):203. Published 2018 Aug 18. doi:10.1186/s13054-018-2105-y
10. Deng Q, Zhang Y, Wang H, et al. Semiquantitative lung ultrasound scores in the evaluation and follow-up of critically ill patients with COVID-19: a single-center study. Acad Radiol. 2020;27(10):1363-1372. doi:10.1016/j.acra.2020.07.002
11. Brahier T, Meuwly JY, Pantet O, et al. Lung ultrasonography for risk stratification in patients with COVID-19: a prospective observational cohort study [published online ahead of print, 2020 Sep 17]. Clin Infect Dis. 2020;ciaa1408. doi:10.1093/cid/ciaa1408
12. Vieillard-Baron A, Schmitt JM, Augarde R, et al. Acute cor pulmonale in acute respiratory distress syndrome submitted to protective ventilation: incidence, clinical implications, and prognosis [published correction appears in Crit Care Med. 2002 Mar;30(3):726]. Crit Care Med. 2001;29(8):1551-1555. doi:10.1097/00003246-200108000-00009
13. Boissier F, Katsahian S, Razazi K, et al. Prevalence and prognosis of cor pulmonale during protective ventilation for acute respiratory distress syndrome. Intensive Care Med. 2013;39(10):1725-1733. doi:10.1007/s00134-013-2941-9
14. Jardin F, Vieillard-Baron A. Is there a safe plateau pressure in ARDS? The right heart only knows. Intensive Care Med. 2007;33(3):444-447. doi:10.1007/s00134-007-0552-z
15. Repessé X, Vieillard-Baron A. Right heart function during acute respiratory distress syndrome. Ann Transl Med 2017;5(14):295. doi:10.21037/atm.2017.06.66
16. Abou-Ismail MY, Diamond A, Kapoor S, Arafah Y, Nayak L. The hypercoagulable state in COVID-19: Incidence, pathophysiology, and management [published correction appears in Thromb Res. 2020 Nov 26]. Thromb Res. 2020;194:101-115. doi:10.1016/j.thromres.2020.06.029
17. Kim J, Volodarskiy A, Sultana R, et al. Prognostic utility of right ventricular remodeling over conventional risk stratification in patients with COVID-19. J Am Coll Cardiol. 2020;76(17):1965-1977. doi:10.1016/j.jacc.2020.08.066
18. Al-Samkari H, Karp Leaf RS, Dzik WH, et al. COVID-19 and coagulation: bleeding and thrombotic manifestations of SARS-CoV-2 infection. Blood. 2020;136(4):489-500. doi:10.1182/blood.2020006520
Infants breathe better when pregnant moms exercise
Lung function in early infancy may be influenced by the mother’s level of physical activity during pregnancy, results of a study from Sweden suggest.
Low-lung function at 3 months of age, as measured by the ratio of time to peak tidal expiratory flow to expiratory time (tPTEF/tE), was more frequent among children whose mothers were physically inactive during the first half of pregnancy compared with those who exercised either moderately or strenuously, reported Hrefna Katrin Gudmundsdottir, MD, a pediatrician and PhD candidate at the University of Oslo, Norway. The results were based on a prospective observational study of 841 mother-child pairs.
“The potential link between maternal inactivity and low lung function in infancy adds to the importance of advising pregnant women and women of childbearing age on physical activity,” she said in an oral abstract presented during the virtual European Respiratory Society (ERS) International Congress.
Jonathan Grigg, MD, professor of pediatric respiratory and environmental medicine at Queen Mary University of London, who was not involved in the study, commented that it “offers a fascinating hint that increased physical activity of mothers is associated with better lung function in their babies and, therefore, possibly their health in later life. More research is needed to confirm this link, but it is important that women feel supported by their health care providers to be active in a way that is comfortable and accessible to them.”
Impaired lung function in infancy is associated with wheezing and asthma in childhood, and lower lung function later in life, Dr. Gudmundsdottir said. She also noted that impaired lung function begins in utero and is related to fetal and infant size, family history of asthma, and/or maternal smoking.
Physical activity during pregnancy has been demonstrated to reduce the risk of preterm birth and cesarean birth and of children being born either abnormally small or abnormally large for their gestational age, she explained.
To see where physical inactivity in the first half of pregnancy is associated with lower lung function in otherwise healthy 3-month old infants, Dr. Gudmundsdottir and colleagues looked at data on a mother-child cohort from the prospective population-based PreventADALL study, which was designed to study prevention of atopic dermatitis and allergies in children in Norway and Sweden.
A total of 814 infants (49% female) had available measures of tidal flow volume in the awake state at 3 months, as well as mother-reported data on physical activity at 18 weeks of pregnancy.
The investigators categorized the mothers as inactive, with either no or only low-intensity physical activity, “fairly” active, or “very” active based on self reporting.
The average tPTEF/tE value among all infants in the study was 0.391. The average value for 290 infants born to inactive mothers was 0.387, compared with 0.394 for 299 infants born to very active mothers, a difference that was not statistically significant.
Maternal physical activity level was not significantly associated with continuous tPTEF/tE, but the investigators did find that the offspring of inactive mothers were significantly more likely than the children of fairly or very active mothers to have a tPTEF/tE below 0.25 in both univariate analysis (odds ratio, 2.15; P = .011), and in multivariate analysis controlling for maternal age, education, parity, prepregnancy body-mass index, parental atopy, and in-utero exposure to nicotine (OR, 2.18; P = .013).
In univariate but not multivariate analysis, children of inactive mothers were significantly more likely than infants of more active mothers to have tPTEF/tE values below the 50th percentile (OR, 1.35; P = .042).
“We observed a trend that adds to the importance of advising women of childbearing age and pregnant women about physical activity. However, there may be factors that affect both maternal physical activity and lung function in offspring that we have not accounted for and could affect the results, so more research is needed,” Dr. Gudmundsdottir said in a statement.
Dr. Grigg pointed out that “it’s also worth keeping in mind that the single most important thing that mothers can do for their own health and that of their baby is to ensure that they do not smoke or use other tobacco products before, during, and after pregnancy. A smoke-free home has the biggest impact on lung function and health in childhood and later life.”
The study was supported by the University of Oslo. Dr. Gudmundsdottir and Dr. Grigg have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Lung function in early infancy may be influenced by the mother’s level of physical activity during pregnancy, results of a study from Sweden suggest.
Low-lung function at 3 months of age, as measured by the ratio of time to peak tidal expiratory flow to expiratory time (tPTEF/tE), was more frequent among children whose mothers were physically inactive during the first half of pregnancy compared with those who exercised either moderately or strenuously, reported Hrefna Katrin Gudmundsdottir, MD, a pediatrician and PhD candidate at the University of Oslo, Norway. The results were based on a prospective observational study of 841 mother-child pairs.
“The potential link between maternal inactivity and low lung function in infancy adds to the importance of advising pregnant women and women of childbearing age on physical activity,” she said in an oral abstract presented during the virtual European Respiratory Society (ERS) International Congress.
Jonathan Grigg, MD, professor of pediatric respiratory and environmental medicine at Queen Mary University of London, who was not involved in the study, commented that it “offers a fascinating hint that increased physical activity of mothers is associated with better lung function in their babies and, therefore, possibly their health in later life. More research is needed to confirm this link, but it is important that women feel supported by their health care providers to be active in a way that is comfortable and accessible to them.”
Impaired lung function in infancy is associated with wheezing and asthma in childhood, and lower lung function later in life, Dr. Gudmundsdottir said. She also noted that impaired lung function begins in utero and is related to fetal and infant size, family history of asthma, and/or maternal smoking.
Physical activity during pregnancy has been demonstrated to reduce the risk of preterm birth and cesarean birth and of children being born either abnormally small or abnormally large for their gestational age, she explained.
To see where physical inactivity in the first half of pregnancy is associated with lower lung function in otherwise healthy 3-month old infants, Dr. Gudmundsdottir and colleagues looked at data on a mother-child cohort from the prospective population-based PreventADALL study, which was designed to study prevention of atopic dermatitis and allergies in children in Norway and Sweden.
A total of 814 infants (49% female) had available measures of tidal flow volume in the awake state at 3 months, as well as mother-reported data on physical activity at 18 weeks of pregnancy.
The investigators categorized the mothers as inactive, with either no or only low-intensity physical activity, “fairly” active, or “very” active based on self reporting.
The average tPTEF/tE value among all infants in the study was 0.391. The average value for 290 infants born to inactive mothers was 0.387, compared with 0.394 for 299 infants born to very active mothers, a difference that was not statistically significant.
Maternal physical activity level was not significantly associated with continuous tPTEF/tE, but the investigators did find that the offspring of inactive mothers were significantly more likely than the children of fairly or very active mothers to have a tPTEF/tE below 0.25 in both univariate analysis (odds ratio, 2.15; P = .011), and in multivariate analysis controlling for maternal age, education, parity, prepregnancy body-mass index, parental atopy, and in-utero exposure to nicotine (OR, 2.18; P = .013).
In univariate but not multivariate analysis, children of inactive mothers were significantly more likely than infants of more active mothers to have tPTEF/tE values below the 50th percentile (OR, 1.35; P = .042).
“We observed a trend that adds to the importance of advising women of childbearing age and pregnant women about physical activity. However, there may be factors that affect both maternal physical activity and lung function in offspring that we have not accounted for and could affect the results, so more research is needed,” Dr. Gudmundsdottir said in a statement.
Dr. Grigg pointed out that “it’s also worth keeping in mind that the single most important thing that mothers can do for their own health and that of their baby is to ensure that they do not smoke or use other tobacco products before, during, and after pregnancy. A smoke-free home has the biggest impact on lung function and health in childhood and later life.”
The study was supported by the University of Oslo. Dr. Gudmundsdottir and Dr. Grigg have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Lung function in early infancy may be influenced by the mother’s level of physical activity during pregnancy, results of a study from Sweden suggest.
Low-lung function at 3 months of age, as measured by the ratio of time to peak tidal expiratory flow to expiratory time (tPTEF/tE), was more frequent among children whose mothers were physically inactive during the first half of pregnancy compared with those who exercised either moderately or strenuously, reported Hrefna Katrin Gudmundsdottir, MD, a pediatrician and PhD candidate at the University of Oslo, Norway. The results were based on a prospective observational study of 841 mother-child pairs.
“The potential link between maternal inactivity and low lung function in infancy adds to the importance of advising pregnant women and women of childbearing age on physical activity,” she said in an oral abstract presented during the virtual European Respiratory Society (ERS) International Congress.
Jonathan Grigg, MD, professor of pediatric respiratory and environmental medicine at Queen Mary University of London, who was not involved in the study, commented that it “offers a fascinating hint that increased physical activity of mothers is associated with better lung function in their babies and, therefore, possibly their health in later life. More research is needed to confirm this link, but it is important that women feel supported by their health care providers to be active in a way that is comfortable and accessible to them.”
Impaired lung function in infancy is associated with wheezing and asthma in childhood, and lower lung function later in life, Dr. Gudmundsdottir said. She also noted that impaired lung function begins in utero and is related to fetal and infant size, family history of asthma, and/or maternal smoking.
Physical activity during pregnancy has been demonstrated to reduce the risk of preterm birth and cesarean birth and of children being born either abnormally small or abnormally large for their gestational age, she explained.
To see where physical inactivity in the first half of pregnancy is associated with lower lung function in otherwise healthy 3-month old infants, Dr. Gudmundsdottir and colleagues looked at data on a mother-child cohort from the prospective population-based PreventADALL study, which was designed to study prevention of atopic dermatitis and allergies in children in Norway and Sweden.
A total of 814 infants (49% female) had available measures of tidal flow volume in the awake state at 3 months, as well as mother-reported data on physical activity at 18 weeks of pregnancy.
The investigators categorized the mothers as inactive, with either no or only low-intensity physical activity, “fairly” active, or “very” active based on self reporting.
The average tPTEF/tE value among all infants in the study was 0.391. The average value for 290 infants born to inactive mothers was 0.387, compared with 0.394 for 299 infants born to very active mothers, a difference that was not statistically significant.
Maternal physical activity level was not significantly associated with continuous tPTEF/tE, but the investigators did find that the offspring of inactive mothers were significantly more likely than the children of fairly or very active mothers to have a tPTEF/tE below 0.25 in both univariate analysis (odds ratio, 2.15; P = .011), and in multivariate analysis controlling for maternal age, education, parity, prepregnancy body-mass index, parental atopy, and in-utero exposure to nicotine (OR, 2.18; P = .013).
In univariate but not multivariate analysis, children of inactive mothers were significantly more likely than infants of more active mothers to have tPTEF/tE values below the 50th percentile (OR, 1.35; P = .042).
“We observed a trend that adds to the importance of advising women of childbearing age and pregnant women about physical activity. However, there may be factors that affect both maternal physical activity and lung function in offspring that we have not accounted for and could affect the results, so more research is needed,” Dr. Gudmundsdottir said in a statement.
Dr. Grigg pointed out that “it’s also worth keeping in mind that the single most important thing that mothers can do for their own health and that of their baby is to ensure that they do not smoke or use other tobacco products before, during, and after pregnancy. A smoke-free home has the biggest impact on lung function and health in childhood and later life.”
The study was supported by the University of Oslo. Dr. Gudmundsdottir and Dr. Grigg have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
How an ‘ad hoc’ hospitalist model evolved during India’s COVID surge
Hospital administrators recognize the efficiencies
A year after the start of the COVID-19 pandemic, as the United States was getting a reprieve in new cases from its winter surge, the opposite was happening in the rest of the world. In India, a deadly second wave hit, crippling the health care system in the country for months.
Yugandhar Bhatt, MBBS, MD, a consultant pulmonologist with Yashoda Hospital–Malakpet in Hyderabad, India, told this news organization that someone looking at his hospital before the pandemic – a 400-bed multispecialty care unit – would see patients being treated for respiratory failure secondary to exacerbation of chronic obstructive pulmonary disease, bronchial asthma, community-acquired pneumonia, and heart failure. About 30-40 patients per day were treated on an outpatient basis, and more than 30 people were admitted as inpatients.
“After [the] COVID-19 surge, our hospital totally divided into COVID and non-COVID [wards], in which COVID patients occupied 70% of [the] total,” he said. About half of COVID-19 patients were in the ICU, with half of those patients requiring supplemental oxygen.
During the first wave in India, which lasted from May to December 2020, 50% of patients who were intubated were discharged. The percentage of extubated patients decreased to 20% in the second wave, Dr. Bhatt said.
The death toll during the second wave of COVID-19 cases was unlike anything India has seen previously. Between March 1 and June 29, 2021, an estimated 19.24 million individuals were newly infected with COVID-19 and 241,206 patients died, according to Our World in Data, a project of the Global Change Data Lab. When the second wave peaked on May 22, more than 4,000 people were dying each day.
“All hospitals [in India] were treating COVID-19 more than any other acute or chronic disease,” Ramesh Adhikari, MD, MS, SFHM, a hospitalist with Franciscan Health in Lafayette, Ind., said in an interview.
Challenges arose in treating COVID-19 in India that ran counter to how medicine was usually performed. Physicians were seeing more inpatient cases than usual – and more patients in general. The change, Dr. Adhikari said, forced health care providers to think outside the box.
An ‘on-the-fly’ hospitalist model
Patients in India access health care by visiting a hospital or primary health center and then are referred out to consultants – specialist doctors – if needed. While India has universal health coverage, it is a multi-payer system that includes approximately 37% of the population covered under the government plan, a large number of private health care facilities and no caps on cost-sharing for the patient. Initiatives like Rashtriya Swasthya Bima Yojana in 2008 and Ayushman Bharat-Pradhan Mantri Jan Arogya Yojana in 2018 have attempted to close the gap and raise the number of lower-income individuals in India covered under the government plan and reduce out-of-pocket spending. Out-of-pocket payments still consist of about 70% of total health expenditures, according to the Commonwealth Fund.
“There is not much scope for a hospitalist because it’s so cash driven,” Shyam Odeti, MD, SFHM, section chief, hospital medicine, at the Carilion Clinic in Roanoke, Va., said in an interview. “For a hospitalist, there is no urgency in getting them out of the hospital. There was no need for much efficiency before.”
The first issue during the second wave was figuring out which consultants would care for COVID-19 patients. As there is no dedicated specialty for infectious disease in India, the responsibilities fell to internists and critical care medicine consultants who volunteered. Both are considered small specialties in India. They became “makeshift hospitalists” who learned as they went and became the experts in COVID-19 care, treating their own patients while making themselves available for consultations, Dr. Odeti said.
While no official hospital medicine model in India exists like in the United States, the second COVID-19 surge caused these consultants to begin thinking like hospitalists. Tenets of hospital medicine – like team-based treatment across specialties – arose out of necessity during the crisis. “They were trying to implement a hospitalist model because that’s the only way they could treat COVID-19,” said Dr. Adhikari, an editorial advisory board member for the Hospitalist.
“Even in the U.S. when we started the hospitalist model, it started out of necessity. It’s a combination of creating efficiencies and improving quality,” Dr. Odeti said. “It’s the same thing in India. It’s borne of necessity, but it was [done] at a rapid pace.”
Problems with patient flow
The next issue was triaging patients in the hospital based on COVID-19 severity. When the second wave began, hospitals in India ran out of beds and experienced staff shortages like in many countries. But this situation “was unusual for the health system,” according to Dr. Odeti, who is also an editorial board member for the Hospitalist.
“We never had that issue. There were so many patients wanting to come to the hospital, and so there was this rush.” There was no process to triage patients to determine who needed to stay. “Everybody got put into the hospital,” he said.
Once it was determined who would take care of patients with COVID-19, access to supplies became the primary problem, Dr. Adhikari explained. Lack of oxygen, ventilators, and critical medicines like the antiviral drug remdesivir were and continue to be in short supply. “I had friends who [said] they could not admit patients because they were worried if their oxygen supply [went] low in the middle of the night. They will treat the patients who were already admitted versus taking new patients. That had caused problems for the administrators,” Dr. Adhikari added.
It is also a source of additional stress for the physicians. Where patients flow through a hospital medicine model in the United States, a system that might include case managers, social workers, pharmacists, physician advocates, and other professionals to keep a patient’s care on track, the physician is the go-to person in India for patient care. While physicians provide access to medications and remain available to a patient’s family, those duties become much harder when caring for a greater number of patients during the pandemic. “That has led to some unrealistic expectations among the patients,” Dr. Adhikari said.
Dr. Bhatt said “more than half” of a physician’s time in India is spent counseling patients on concerns about COVID-19. “Awareness about the disease is limited from the patient and patient’s family perspective, as [there is] too much apprehension toward the nature of [the] disease,” he added. “Theoretical discussions collected from social media” obstruct the physician from executing his or her duties.
Physicians in India have had to contend with physical violence from patients and individuals on the street, Dr. Adhikari added. Workplace violence was already a concern – for years, the Indian Medical Association has cited a statistic that 75% of doctors in India have experienced violence at work (Indian J Psychiatry. 2019 Apr;61[Suppl 4]:S782-5). But the threat of violence against physicians has sharply increased during the COVID-19 pandemic. Disruptions to daily life through lockdowns “made people fearful, anxious, and sometimes they have found it difficult to access emergency treatment,” according to a letter published by Karthikeyan Iyengar and colleagues in the Postgraduate Medical Journal. In response to the restlessness, irritation, and despair resulting from hospitals closing their doors, “people have shown their frustration by verbally abusing and threatening to physically assault doctors and other health care workers,” the authors wrote.
A telemedicine boon in India
Back in the United States, hospitalists with family and friends in India were trying to figure out how to help. Some were working through the day, only to answer calls and WhatsApp messages from loved ones at night. “Everyone knows a physician or someone who’s your colleague, who owns a hospital or runs a hospital, or one of the family members is sick,” Dr. Adhikari said.
These U.S.-based hospitalists were burning the candle at both ends, helping with the pandemic in both countries. Physicians in India were posing questions to U.S. colleagues who they saw as having the most recent evidence for COVID-19 treatment. Out of the 180 physicians he trained with in India, Dr. Odeti said 110 of the physicians were in a large WhatsApp group chat that was constantly exchanging messages and serving as “kind of a friendly support group.”
In Dr. Odeti’s group chat, physicians helped one another find hospital beds for patients who reached out to them. “The first couple of weeks, there was no proper way for people to know where [patients] were based. There was no way to find if this hospital had a bed, so they reached out to any doctors they knew,” he said.
While he said it was emotionally draining, “at the same time, we felt a responsibility toward colleagues in India,” Dr. Odeti said, noting that as COVID-19 cases have decreased in India, the requests have been less frequent.
Because of concerns about traveling to India during the pandemic while on a J-1, H-1B, or other visa with the United States, directly helping friends and family in India seemed out of reach. But many hospitalists of Indian origin instead turned to telemedicine to help their colleagues. Telemedicine had already been steadily growing in India, but was accelerated by the pandemic. The current ratio of doctors to patients in India is 0.62 to 1,000 – lower than recommendations from the World Health Organization. That makes telemedicine a unique opportunity for one physician in India to reach many patients regardless of location.
Dr. Adhikari said he helped out his colleagues in India by performing consults for their patients. “They were just worried because they did not ‘know where to go, or what to get,” he said. “I was treating more patients in India than I was actually treating here.”
In March 2020, the Indian Ministry of Health and Family Welfare released telemedicine practice guidelines for the country, which relaxed regulations on privacy requirements and has been credited in part for giving telemedicine an additional boost during the pandemic. “That makes it easy for people to reach out but also has its own problems,” Dr. Adhikari said.
Monitoring of milder COVID-19 cases that don’t require hospitalization can be performed by a nurse who calls every few hours to check on a patient, make recommendations, and text treatment plans. “The telemedicine platforms are being adopted really fast,” Dr. Adhikari said. “The platforms were built in no time.”
According to NewZoo, a games market data analytics company, India has 345.9 million smartphone users as of 2019 – the second highest number of users in the world after China. Dr. Odeti said he believes telemedicine will be widely adopted.
“In India, they are very proactive in accepting these kinds of methods, so I’m sure they will,” he said. “Governments were trying to do it before the pandemic, because access to care is a problem in India. There are villages which are very, very remote.”
Reversion to old systems
After the peak in late May, new COVID-19 cases in India began to decrease, and the second wave waned on a national level. Hospitals began to get the supplies they needed, beds are available, and patients aren’t as sick as before, according to Dr. Adhikari. The federal government has begun issuing supplies to patients in each state, including COVID-19 vaccines. “The peak for the second wave is gone,” he said.
What remains is a group of physicians trained in how to triage patients and create efficiencies in a hospital setting. Could those skills be put to use elsewhere in India after the pandemic?
According to Dr. Bhatt, the patient care model is likely to revert to the system that existed before. “Whatever the changes, interims of bed occupancy, cost of ICU will be temporary [and] will change to normal,” he said. “But awareness about masks [and] sanitizing methods will be permanent.”
Dr. Adhikari believes that not utilizing the skills of newly minted hospitalists in India would be a missed opportunity. “This is a silver lining from COVID-19, that hospital medicine plays a vital role in the sickest patients, whether it is in India or the U.S. or anywhere,” he said. “I think the model of hospital medicine should be adopted. It’s not: ‘Should it really be adopted or not?’ It should be. There is a huge potential in doing inpatient coordinated [care], having people dedicated in the hospital.”
There are tangible benefits to creating efficiencies in India’s health system, Dr. Odeti said. Length of stay for sicker patients “was much longer” at 10-14 days during the second wave, compared with the United States, before lowering to around 5 days. “These hospitals right now are learning the efficient ways of doing it: when to send [patients] out, how to send them out, how to [perform] service-based practices, creating processes which were nonexistent before.”
While he doesn’t personally believe physicians will adopt a full-fledged hospitalist model unless the payer structure in India changes, “these people are at an advantage with this extra set of skills,” he said. “I think all the knowledge that these people have are going to come in handy.”
Opportunities for growth
Dr. Odeti sees the potential for the hospitalist model to grow in India – if not into its own specialty, then in how critical care consultants handle sicker patients and handoffs.
“The critical care clinician cannot keep the patient from the time they are admitted to the ICU until the discharge, so there will be a need for the transition,” Dr. Odeti said. “In the past, there were not many capabilities in Indian health systems to take care of these extremely sick patients, and now it is evolving. I think that is one more thing that will help.”
Dr. Adhikari said hospital systems in India are beginning to realize how having dedicated hospital physicians could benefit them. In India, “if you’re sick, you go to your doctor, you get treated and you disappear,” he said. The next time, you may see the same doctor or a completely different doctor. “There’s no system there, so it’s really hard for hospital medicine as such because patients, when they are very sick, they just come to the ER. They’re not followed by their primary care.”
Anecdotally, Dr. Odeti sees patients already adapting to having access to a physician for asking questions normally answered by primary care physicians. “I think primary care will come into play,” he said. “When I was doing a Zoom call for patients, they were asking me questions about sciatica. I think they are getting comfortable with this technology.”
A hospitalist model could even be applied to specific diseases with a large population of patients. Hospital administrators “have seen this for the first time, how efficient it could be if they had their own hospitalists and actually run it. So that’s the part that has crossed their minds,” Dr. Adhikari said. “How they will apply it going forward, other than during the COVID-19 pandemic, depends on the size of the hospital and the volume of the patients for a particular disease.”
“You can see in certain areas there is large growth for hospital medicine. But to rise to the level of the United States and how we do it, India needs bigger health systems to adopt the model,” Dr. Adhikari said.
Hospital administrators recognize the efficiencies
Hospital administrators recognize the efficiencies
A year after the start of the COVID-19 pandemic, as the United States was getting a reprieve in new cases from its winter surge, the opposite was happening in the rest of the world. In India, a deadly second wave hit, crippling the health care system in the country for months.
Yugandhar Bhatt, MBBS, MD, a consultant pulmonologist with Yashoda Hospital–Malakpet in Hyderabad, India, told this news organization that someone looking at his hospital before the pandemic – a 400-bed multispecialty care unit – would see patients being treated for respiratory failure secondary to exacerbation of chronic obstructive pulmonary disease, bronchial asthma, community-acquired pneumonia, and heart failure. About 30-40 patients per day were treated on an outpatient basis, and more than 30 people were admitted as inpatients.
“After [the] COVID-19 surge, our hospital totally divided into COVID and non-COVID [wards], in which COVID patients occupied 70% of [the] total,” he said. About half of COVID-19 patients were in the ICU, with half of those patients requiring supplemental oxygen.
During the first wave in India, which lasted from May to December 2020, 50% of patients who were intubated were discharged. The percentage of extubated patients decreased to 20% in the second wave, Dr. Bhatt said.
The death toll during the second wave of COVID-19 cases was unlike anything India has seen previously. Between March 1 and June 29, 2021, an estimated 19.24 million individuals were newly infected with COVID-19 and 241,206 patients died, according to Our World in Data, a project of the Global Change Data Lab. When the second wave peaked on May 22, more than 4,000 people were dying each day.
“All hospitals [in India] were treating COVID-19 more than any other acute or chronic disease,” Ramesh Adhikari, MD, MS, SFHM, a hospitalist with Franciscan Health in Lafayette, Ind., said in an interview.
Challenges arose in treating COVID-19 in India that ran counter to how medicine was usually performed. Physicians were seeing more inpatient cases than usual – and more patients in general. The change, Dr. Adhikari said, forced health care providers to think outside the box.
An ‘on-the-fly’ hospitalist model
Patients in India access health care by visiting a hospital or primary health center and then are referred out to consultants – specialist doctors – if needed. While India has universal health coverage, it is a multi-payer system that includes approximately 37% of the population covered under the government plan, a large number of private health care facilities and no caps on cost-sharing for the patient. Initiatives like Rashtriya Swasthya Bima Yojana in 2008 and Ayushman Bharat-Pradhan Mantri Jan Arogya Yojana in 2018 have attempted to close the gap and raise the number of lower-income individuals in India covered under the government plan and reduce out-of-pocket spending. Out-of-pocket payments still consist of about 70% of total health expenditures, according to the Commonwealth Fund.
“There is not much scope for a hospitalist because it’s so cash driven,” Shyam Odeti, MD, SFHM, section chief, hospital medicine, at the Carilion Clinic in Roanoke, Va., said in an interview. “For a hospitalist, there is no urgency in getting them out of the hospital. There was no need for much efficiency before.”
The first issue during the second wave was figuring out which consultants would care for COVID-19 patients. As there is no dedicated specialty for infectious disease in India, the responsibilities fell to internists and critical care medicine consultants who volunteered. Both are considered small specialties in India. They became “makeshift hospitalists” who learned as they went and became the experts in COVID-19 care, treating their own patients while making themselves available for consultations, Dr. Odeti said.
While no official hospital medicine model in India exists like in the United States, the second COVID-19 surge caused these consultants to begin thinking like hospitalists. Tenets of hospital medicine – like team-based treatment across specialties – arose out of necessity during the crisis. “They were trying to implement a hospitalist model because that’s the only way they could treat COVID-19,” said Dr. Adhikari, an editorial advisory board member for the Hospitalist.
“Even in the U.S. when we started the hospitalist model, it started out of necessity. It’s a combination of creating efficiencies and improving quality,” Dr. Odeti said. “It’s the same thing in India. It’s borne of necessity, but it was [done] at a rapid pace.”
Problems with patient flow
The next issue was triaging patients in the hospital based on COVID-19 severity. When the second wave began, hospitals in India ran out of beds and experienced staff shortages like in many countries. But this situation “was unusual for the health system,” according to Dr. Odeti, who is also an editorial board member for the Hospitalist.
“We never had that issue. There were so many patients wanting to come to the hospital, and so there was this rush.” There was no process to triage patients to determine who needed to stay. “Everybody got put into the hospital,” he said.
Once it was determined who would take care of patients with COVID-19, access to supplies became the primary problem, Dr. Adhikari explained. Lack of oxygen, ventilators, and critical medicines like the antiviral drug remdesivir were and continue to be in short supply. “I had friends who [said] they could not admit patients because they were worried if their oxygen supply [went] low in the middle of the night. They will treat the patients who were already admitted versus taking new patients. That had caused problems for the administrators,” Dr. Adhikari added.
It is also a source of additional stress for the physicians. Where patients flow through a hospital medicine model in the United States, a system that might include case managers, social workers, pharmacists, physician advocates, and other professionals to keep a patient’s care on track, the physician is the go-to person in India for patient care. While physicians provide access to medications and remain available to a patient’s family, those duties become much harder when caring for a greater number of patients during the pandemic. “That has led to some unrealistic expectations among the patients,” Dr. Adhikari said.
Dr. Bhatt said “more than half” of a physician’s time in India is spent counseling patients on concerns about COVID-19. “Awareness about the disease is limited from the patient and patient’s family perspective, as [there is] too much apprehension toward the nature of [the] disease,” he added. “Theoretical discussions collected from social media” obstruct the physician from executing his or her duties.
Physicians in India have had to contend with physical violence from patients and individuals on the street, Dr. Adhikari added. Workplace violence was already a concern – for years, the Indian Medical Association has cited a statistic that 75% of doctors in India have experienced violence at work (Indian J Psychiatry. 2019 Apr;61[Suppl 4]:S782-5). But the threat of violence against physicians has sharply increased during the COVID-19 pandemic. Disruptions to daily life through lockdowns “made people fearful, anxious, and sometimes they have found it difficult to access emergency treatment,” according to a letter published by Karthikeyan Iyengar and colleagues in the Postgraduate Medical Journal. In response to the restlessness, irritation, and despair resulting from hospitals closing their doors, “people have shown their frustration by verbally abusing and threatening to physically assault doctors and other health care workers,” the authors wrote.
A telemedicine boon in India
Back in the United States, hospitalists with family and friends in India were trying to figure out how to help. Some were working through the day, only to answer calls and WhatsApp messages from loved ones at night. “Everyone knows a physician or someone who’s your colleague, who owns a hospital or runs a hospital, or one of the family members is sick,” Dr. Adhikari said.
These U.S.-based hospitalists were burning the candle at both ends, helping with the pandemic in both countries. Physicians in India were posing questions to U.S. colleagues who they saw as having the most recent evidence for COVID-19 treatment. Out of the 180 physicians he trained with in India, Dr. Odeti said 110 of the physicians were in a large WhatsApp group chat that was constantly exchanging messages and serving as “kind of a friendly support group.”
In Dr. Odeti’s group chat, physicians helped one another find hospital beds for patients who reached out to them. “The first couple of weeks, there was no proper way for people to know where [patients] were based. There was no way to find if this hospital had a bed, so they reached out to any doctors they knew,” he said.
While he said it was emotionally draining, “at the same time, we felt a responsibility toward colleagues in India,” Dr. Odeti said, noting that as COVID-19 cases have decreased in India, the requests have been less frequent.
Because of concerns about traveling to India during the pandemic while on a J-1, H-1B, or other visa with the United States, directly helping friends and family in India seemed out of reach. But many hospitalists of Indian origin instead turned to telemedicine to help their colleagues. Telemedicine had already been steadily growing in India, but was accelerated by the pandemic. The current ratio of doctors to patients in India is 0.62 to 1,000 – lower than recommendations from the World Health Organization. That makes telemedicine a unique opportunity for one physician in India to reach many patients regardless of location.
Dr. Adhikari said he helped out his colleagues in India by performing consults for their patients. “They were just worried because they did not ‘know where to go, or what to get,” he said. “I was treating more patients in India than I was actually treating here.”
In March 2020, the Indian Ministry of Health and Family Welfare released telemedicine practice guidelines for the country, which relaxed regulations on privacy requirements and has been credited in part for giving telemedicine an additional boost during the pandemic. “That makes it easy for people to reach out but also has its own problems,” Dr. Adhikari said.
Monitoring of milder COVID-19 cases that don’t require hospitalization can be performed by a nurse who calls every few hours to check on a patient, make recommendations, and text treatment plans. “The telemedicine platforms are being adopted really fast,” Dr. Adhikari said. “The platforms were built in no time.”
According to NewZoo, a games market data analytics company, India has 345.9 million smartphone users as of 2019 – the second highest number of users in the world after China. Dr. Odeti said he believes telemedicine will be widely adopted.
“In India, they are very proactive in accepting these kinds of methods, so I’m sure they will,” he said. “Governments were trying to do it before the pandemic, because access to care is a problem in India. There are villages which are very, very remote.”
Reversion to old systems
After the peak in late May, new COVID-19 cases in India began to decrease, and the second wave waned on a national level. Hospitals began to get the supplies they needed, beds are available, and patients aren’t as sick as before, according to Dr. Adhikari. The federal government has begun issuing supplies to patients in each state, including COVID-19 vaccines. “The peak for the second wave is gone,” he said.
What remains is a group of physicians trained in how to triage patients and create efficiencies in a hospital setting. Could those skills be put to use elsewhere in India after the pandemic?
According to Dr. Bhatt, the patient care model is likely to revert to the system that existed before. “Whatever the changes, interims of bed occupancy, cost of ICU will be temporary [and] will change to normal,” he said. “But awareness about masks [and] sanitizing methods will be permanent.”
Dr. Adhikari believes that not utilizing the skills of newly minted hospitalists in India would be a missed opportunity. “This is a silver lining from COVID-19, that hospital medicine plays a vital role in the sickest patients, whether it is in India or the U.S. or anywhere,” he said. “I think the model of hospital medicine should be adopted. It’s not: ‘Should it really be adopted or not?’ It should be. There is a huge potential in doing inpatient coordinated [care], having people dedicated in the hospital.”
There are tangible benefits to creating efficiencies in India’s health system, Dr. Odeti said. Length of stay for sicker patients “was much longer” at 10-14 days during the second wave, compared with the United States, before lowering to around 5 days. “These hospitals right now are learning the efficient ways of doing it: when to send [patients] out, how to send them out, how to [perform] service-based practices, creating processes which were nonexistent before.”
While he doesn’t personally believe physicians will adopt a full-fledged hospitalist model unless the payer structure in India changes, “these people are at an advantage with this extra set of skills,” he said. “I think all the knowledge that these people have are going to come in handy.”
Opportunities for growth
Dr. Odeti sees the potential for the hospitalist model to grow in India – if not into its own specialty, then in how critical care consultants handle sicker patients and handoffs.
“The critical care clinician cannot keep the patient from the time they are admitted to the ICU until the discharge, so there will be a need for the transition,” Dr. Odeti said. “In the past, there were not many capabilities in Indian health systems to take care of these extremely sick patients, and now it is evolving. I think that is one more thing that will help.”
Dr. Adhikari said hospital systems in India are beginning to realize how having dedicated hospital physicians could benefit them. In India, “if you’re sick, you go to your doctor, you get treated and you disappear,” he said. The next time, you may see the same doctor or a completely different doctor. “There’s no system there, so it’s really hard for hospital medicine as such because patients, when they are very sick, they just come to the ER. They’re not followed by their primary care.”
Anecdotally, Dr. Odeti sees patients already adapting to having access to a physician for asking questions normally answered by primary care physicians. “I think primary care will come into play,” he said. “When I was doing a Zoom call for patients, they were asking me questions about sciatica. I think they are getting comfortable with this technology.”
A hospitalist model could even be applied to specific diseases with a large population of patients. Hospital administrators “have seen this for the first time, how efficient it could be if they had their own hospitalists and actually run it. So that’s the part that has crossed their minds,” Dr. Adhikari said. “How they will apply it going forward, other than during the COVID-19 pandemic, depends on the size of the hospital and the volume of the patients for a particular disease.”
“You can see in certain areas there is large growth for hospital medicine. But to rise to the level of the United States and how we do it, India needs bigger health systems to adopt the model,” Dr. Adhikari said.
A year after the start of the COVID-19 pandemic, as the United States was getting a reprieve in new cases from its winter surge, the opposite was happening in the rest of the world. In India, a deadly second wave hit, crippling the health care system in the country for months.
Yugandhar Bhatt, MBBS, MD, a consultant pulmonologist with Yashoda Hospital–Malakpet in Hyderabad, India, told this news organization that someone looking at his hospital before the pandemic – a 400-bed multispecialty care unit – would see patients being treated for respiratory failure secondary to exacerbation of chronic obstructive pulmonary disease, bronchial asthma, community-acquired pneumonia, and heart failure. About 30-40 patients per day were treated on an outpatient basis, and more than 30 people were admitted as inpatients.
“After [the] COVID-19 surge, our hospital totally divided into COVID and non-COVID [wards], in which COVID patients occupied 70% of [the] total,” he said. About half of COVID-19 patients were in the ICU, with half of those patients requiring supplemental oxygen.
During the first wave in India, which lasted from May to December 2020, 50% of patients who were intubated were discharged. The percentage of extubated patients decreased to 20% in the second wave, Dr. Bhatt said.
The death toll during the second wave of COVID-19 cases was unlike anything India has seen previously. Between March 1 and June 29, 2021, an estimated 19.24 million individuals were newly infected with COVID-19 and 241,206 patients died, according to Our World in Data, a project of the Global Change Data Lab. When the second wave peaked on May 22, more than 4,000 people were dying each day.
“All hospitals [in India] were treating COVID-19 more than any other acute or chronic disease,” Ramesh Adhikari, MD, MS, SFHM, a hospitalist with Franciscan Health in Lafayette, Ind., said in an interview.
Challenges arose in treating COVID-19 in India that ran counter to how medicine was usually performed. Physicians were seeing more inpatient cases than usual – and more patients in general. The change, Dr. Adhikari said, forced health care providers to think outside the box.
An ‘on-the-fly’ hospitalist model
Patients in India access health care by visiting a hospital or primary health center and then are referred out to consultants – specialist doctors – if needed. While India has universal health coverage, it is a multi-payer system that includes approximately 37% of the population covered under the government plan, a large number of private health care facilities and no caps on cost-sharing for the patient. Initiatives like Rashtriya Swasthya Bima Yojana in 2008 and Ayushman Bharat-Pradhan Mantri Jan Arogya Yojana in 2018 have attempted to close the gap and raise the number of lower-income individuals in India covered under the government plan and reduce out-of-pocket spending. Out-of-pocket payments still consist of about 70% of total health expenditures, according to the Commonwealth Fund.
“There is not much scope for a hospitalist because it’s so cash driven,” Shyam Odeti, MD, SFHM, section chief, hospital medicine, at the Carilion Clinic in Roanoke, Va., said in an interview. “For a hospitalist, there is no urgency in getting them out of the hospital. There was no need for much efficiency before.”
The first issue during the second wave was figuring out which consultants would care for COVID-19 patients. As there is no dedicated specialty for infectious disease in India, the responsibilities fell to internists and critical care medicine consultants who volunteered. Both are considered small specialties in India. They became “makeshift hospitalists” who learned as they went and became the experts in COVID-19 care, treating their own patients while making themselves available for consultations, Dr. Odeti said.
While no official hospital medicine model in India exists like in the United States, the second COVID-19 surge caused these consultants to begin thinking like hospitalists. Tenets of hospital medicine – like team-based treatment across specialties – arose out of necessity during the crisis. “They were trying to implement a hospitalist model because that’s the only way they could treat COVID-19,” said Dr. Adhikari, an editorial advisory board member for the Hospitalist.
“Even in the U.S. when we started the hospitalist model, it started out of necessity. It’s a combination of creating efficiencies and improving quality,” Dr. Odeti said. “It’s the same thing in India. It’s borne of necessity, but it was [done] at a rapid pace.”
Problems with patient flow
The next issue was triaging patients in the hospital based on COVID-19 severity. When the second wave began, hospitals in India ran out of beds and experienced staff shortages like in many countries. But this situation “was unusual for the health system,” according to Dr. Odeti, who is also an editorial board member for the Hospitalist.
“We never had that issue. There were so many patients wanting to come to the hospital, and so there was this rush.” There was no process to triage patients to determine who needed to stay. “Everybody got put into the hospital,” he said.
Once it was determined who would take care of patients with COVID-19, access to supplies became the primary problem, Dr. Adhikari explained. Lack of oxygen, ventilators, and critical medicines like the antiviral drug remdesivir were and continue to be in short supply. “I had friends who [said] they could not admit patients because they were worried if their oxygen supply [went] low in the middle of the night. They will treat the patients who were already admitted versus taking new patients. That had caused problems for the administrators,” Dr. Adhikari added.
It is also a source of additional stress for the physicians. Where patients flow through a hospital medicine model in the United States, a system that might include case managers, social workers, pharmacists, physician advocates, and other professionals to keep a patient’s care on track, the physician is the go-to person in India for patient care. While physicians provide access to medications and remain available to a patient’s family, those duties become much harder when caring for a greater number of patients during the pandemic. “That has led to some unrealistic expectations among the patients,” Dr. Adhikari said.
Dr. Bhatt said “more than half” of a physician’s time in India is spent counseling patients on concerns about COVID-19. “Awareness about the disease is limited from the patient and patient’s family perspective, as [there is] too much apprehension toward the nature of [the] disease,” he added. “Theoretical discussions collected from social media” obstruct the physician from executing his or her duties.
Physicians in India have had to contend with physical violence from patients and individuals on the street, Dr. Adhikari added. Workplace violence was already a concern – for years, the Indian Medical Association has cited a statistic that 75% of doctors in India have experienced violence at work (Indian J Psychiatry. 2019 Apr;61[Suppl 4]:S782-5). But the threat of violence against physicians has sharply increased during the COVID-19 pandemic. Disruptions to daily life through lockdowns “made people fearful, anxious, and sometimes they have found it difficult to access emergency treatment,” according to a letter published by Karthikeyan Iyengar and colleagues in the Postgraduate Medical Journal. In response to the restlessness, irritation, and despair resulting from hospitals closing their doors, “people have shown their frustration by verbally abusing and threatening to physically assault doctors and other health care workers,” the authors wrote.
A telemedicine boon in India
Back in the United States, hospitalists with family and friends in India were trying to figure out how to help. Some were working through the day, only to answer calls and WhatsApp messages from loved ones at night. “Everyone knows a physician or someone who’s your colleague, who owns a hospital or runs a hospital, or one of the family members is sick,” Dr. Adhikari said.
These U.S.-based hospitalists were burning the candle at both ends, helping with the pandemic in both countries. Physicians in India were posing questions to U.S. colleagues who they saw as having the most recent evidence for COVID-19 treatment. Out of the 180 physicians he trained with in India, Dr. Odeti said 110 of the physicians were in a large WhatsApp group chat that was constantly exchanging messages and serving as “kind of a friendly support group.”
In Dr. Odeti’s group chat, physicians helped one another find hospital beds for patients who reached out to them. “The first couple of weeks, there was no proper way for people to know where [patients] were based. There was no way to find if this hospital had a bed, so they reached out to any doctors they knew,” he said.
While he said it was emotionally draining, “at the same time, we felt a responsibility toward colleagues in India,” Dr. Odeti said, noting that as COVID-19 cases have decreased in India, the requests have been less frequent.
Because of concerns about traveling to India during the pandemic while on a J-1, H-1B, or other visa with the United States, directly helping friends and family in India seemed out of reach. But many hospitalists of Indian origin instead turned to telemedicine to help their colleagues. Telemedicine had already been steadily growing in India, but was accelerated by the pandemic. The current ratio of doctors to patients in India is 0.62 to 1,000 – lower than recommendations from the World Health Organization. That makes telemedicine a unique opportunity for one physician in India to reach many patients regardless of location.
Dr. Adhikari said he helped out his colleagues in India by performing consults for their patients. “They were just worried because they did not ‘know where to go, or what to get,” he said. “I was treating more patients in India than I was actually treating here.”
In March 2020, the Indian Ministry of Health and Family Welfare released telemedicine practice guidelines for the country, which relaxed regulations on privacy requirements and has been credited in part for giving telemedicine an additional boost during the pandemic. “That makes it easy for people to reach out but also has its own problems,” Dr. Adhikari said.
Monitoring of milder COVID-19 cases that don’t require hospitalization can be performed by a nurse who calls every few hours to check on a patient, make recommendations, and text treatment plans. “The telemedicine platforms are being adopted really fast,” Dr. Adhikari said. “The platforms were built in no time.”
According to NewZoo, a games market data analytics company, India has 345.9 million smartphone users as of 2019 – the second highest number of users in the world after China. Dr. Odeti said he believes telemedicine will be widely adopted.
“In India, they are very proactive in accepting these kinds of methods, so I’m sure they will,” he said. “Governments were trying to do it before the pandemic, because access to care is a problem in India. There are villages which are very, very remote.”
Reversion to old systems
After the peak in late May, new COVID-19 cases in India began to decrease, and the second wave waned on a national level. Hospitals began to get the supplies they needed, beds are available, and patients aren’t as sick as before, according to Dr. Adhikari. The federal government has begun issuing supplies to patients in each state, including COVID-19 vaccines. “The peak for the second wave is gone,” he said.
What remains is a group of physicians trained in how to triage patients and create efficiencies in a hospital setting. Could those skills be put to use elsewhere in India after the pandemic?
According to Dr. Bhatt, the patient care model is likely to revert to the system that existed before. “Whatever the changes, interims of bed occupancy, cost of ICU will be temporary [and] will change to normal,” he said. “But awareness about masks [and] sanitizing methods will be permanent.”
Dr. Adhikari believes that not utilizing the skills of newly minted hospitalists in India would be a missed opportunity. “This is a silver lining from COVID-19, that hospital medicine plays a vital role in the sickest patients, whether it is in India or the U.S. or anywhere,” he said. “I think the model of hospital medicine should be adopted. It’s not: ‘Should it really be adopted or not?’ It should be. There is a huge potential in doing inpatient coordinated [care], having people dedicated in the hospital.”
There are tangible benefits to creating efficiencies in India’s health system, Dr. Odeti said. Length of stay for sicker patients “was much longer” at 10-14 days during the second wave, compared with the United States, before lowering to around 5 days. “These hospitals right now are learning the efficient ways of doing it: when to send [patients] out, how to send them out, how to [perform] service-based practices, creating processes which were nonexistent before.”
While he doesn’t personally believe physicians will adopt a full-fledged hospitalist model unless the payer structure in India changes, “these people are at an advantage with this extra set of skills,” he said. “I think all the knowledge that these people have are going to come in handy.”
Opportunities for growth
Dr. Odeti sees the potential for the hospitalist model to grow in India – if not into its own specialty, then in how critical care consultants handle sicker patients and handoffs.
“The critical care clinician cannot keep the patient from the time they are admitted to the ICU until the discharge, so there will be a need for the transition,” Dr. Odeti said. “In the past, there were not many capabilities in Indian health systems to take care of these extremely sick patients, and now it is evolving. I think that is one more thing that will help.”
Dr. Adhikari said hospital systems in India are beginning to realize how having dedicated hospital physicians could benefit them. In India, “if you’re sick, you go to your doctor, you get treated and you disappear,” he said. The next time, you may see the same doctor or a completely different doctor. “There’s no system there, so it’s really hard for hospital medicine as such because patients, when they are very sick, they just come to the ER. They’re not followed by their primary care.”
Anecdotally, Dr. Odeti sees patients already adapting to having access to a physician for asking questions normally answered by primary care physicians. “I think primary care will come into play,” he said. “When I was doing a Zoom call for patients, they were asking me questions about sciatica. I think they are getting comfortable with this technology.”
A hospitalist model could even be applied to specific diseases with a large population of patients. Hospital administrators “have seen this for the first time, how efficient it could be if they had their own hospitalists and actually run it. So that’s the part that has crossed their minds,” Dr. Adhikari said. “How they will apply it going forward, other than during the COVID-19 pandemic, depends on the size of the hospital and the volume of the patients for a particular disease.”
“You can see in certain areas there is large growth for hospital medicine. But to rise to the level of the United States and how we do it, India needs bigger health systems to adopt the model,” Dr. Adhikari said.
FDA moves to block some vape products, delays action on Juul
The agency had a court-ordered deadline of Sept. 9 to review more than 6.5 million applications for approval of what are considered new tobacco products – the vast majority of which are e-cigarettes and liquids, none of which have gone through FDA review before.
The FDA reviewed 93% of those applications in the past year, acting FDA Commissioner Janet Woodcock, MD, and Mitch Zeller, director of the FDA’s Center for Tobacco Products, said in a statement.
Of those reviewed, the agency rejected more than 946,000 flavored vape products, “because their applications lacked sufficient evidence that they have a benefit to adult smokers sufficient to overcome the public health threat posed by the well-documented, alarming levels of youth use of such products,” Dr. Woodcock and Mr. Zeller said.
The pair said more work is needed to finish the reviews to “ensure that we continue taking appropriate action to protect our nation’s youth from the dangers of all tobacco products, including e-cigarettes, which remain the most commonly used tobacco product by youth in the United States.”
No e-cigarette product has been given official FDA approval to be sold, meaning all e-cigarette products technically are on the market illegally, the agency said in 2020, but federal officials decided only to begin enforcing rules against flavored products, which surveys show are more often used by children. Tobacco-flavored and menthol e-cigarette products – which some adults use to quit smoking cigarettes – were exempted.
The American Cancer Society and other advocacy groups slammed the FDA’s decision to withhold action on major e-cigarette manufacturers, including Juul.
“The FDA’s failure today to act on applications by Juul, the manufacturer with the single biggest e-cigarette market share, is extremely disappointing and will allow the industry to further endanger public health and hook more kids on their highly addictive products,” Lisa Lacasse, president of ACS CAN, said in a statement, according to CNN.
“The FDA has had ample time to review the applications and allowing additional delays is unconscionable. There is overwhelming data to demonstrate the negative impact these kinds of flavored products have had on public health and their role in the youth e-cigarette epidemic. The time to act is now,” Ms. Lacasse added.
E-cigarette use among high school students rose from 11.7% in 2017 to 19.6% in 2020, the American Cancer Society said. Nearly 5% of middle schoolers reported using them in 2020.
A version of this article first appeared on WebMD.com.
The agency had a court-ordered deadline of Sept. 9 to review more than 6.5 million applications for approval of what are considered new tobacco products – the vast majority of which are e-cigarettes and liquids, none of which have gone through FDA review before.
The FDA reviewed 93% of those applications in the past year, acting FDA Commissioner Janet Woodcock, MD, and Mitch Zeller, director of the FDA’s Center for Tobacco Products, said in a statement.
Of those reviewed, the agency rejected more than 946,000 flavored vape products, “because their applications lacked sufficient evidence that they have a benefit to adult smokers sufficient to overcome the public health threat posed by the well-documented, alarming levels of youth use of such products,” Dr. Woodcock and Mr. Zeller said.
The pair said more work is needed to finish the reviews to “ensure that we continue taking appropriate action to protect our nation’s youth from the dangers of all tobacco products, including e-cigarettes, which remain the most commonly used tobacco product by youth in the United States.”
No e-cigarette product has been given official FDA approval to be sold, meaning all e-cigarette products technically are on the market illegally, the agency said in 2020, but federal officials decided only to begin enforcing rules against flavored products, which surveys show are more often used by children. Tobacco-flavored and menthol e-cigarette products – which some adults use to quit smoking cigarettes – were exempted.
The American Cancer Society and other advocacy groups slammed the FDA’s decision to withhold action on major e-cigarette manufacturers, including Juul.
“The FDA’s failure today to act on applications by Juul, the manufacturer with the single biggest e-cigarette market share, is extremely disappointing and will allow the industry to further endanger public health and hook more kids on their highly addictive products,” Lisa Lacasse, president of ACS CAN, said in a statement, according to CNN.
“The FDA has had ample time to review the applications and allowing additional delays is unconscionable. There is overwhelming data to demonstrate the negative impact these kinds of flavored products have had on public health and their role in the youth e-cigarette epidemic. The time to act is now,” Ms. Lacasse added.
E-cigarette use among high school students rose from 11.7% in 2017 to 19.6% in 2020, the American Cancer Society said. Nearly 5% of middle schoolers reported using them in 2020.
A version of this article first appeared on WebMD.com.
The agency had a court-ordered deadline of Sept. 9 to review more than 6.5 million applications for approval of what are considered new tobacco products – the vast majority of which are e-cigarettes and liquids, none of which have gone through FDA review before.
The FDA reviewed 93% of those applications in the past year, acting FDA Commissioner Janet Woodcock, MD, and Mitch Zeller, director of the FDA’s Center for Tobacco Products, said in a statement.
Of those reviewed, the agency rejected more than 946,000 flavored vape products, “because their applications lacked sufficient evidence that they have a benefit to adult smokers sufficient to overcome the public health threat posed by the well-documented, alarming levels of youth use of such products,” Dr. Woodcock and Mr. Zeller said.
The pair said more work is needed to finish the reviews to “ensure that we continue taking appropriate action to protect our nation’s youth from the dangers of all tobacco products, including e-cigarettes, which remain the most commonly used tobacco product by youth in the United States.”
No e-cigarette product has been given official FDA approval to be sold, meaning all e-cigarette products technically are on the market illegally, the agency said in 2020, but federal officials decided only to begin enforcing rules against flavored products, which surveys show are more often used by children. Tobacco-flavored and menthol e-cigarette products – which some adults use to quit smoking cigarettes – were exempted.
The American Cancer Society and other advocacy groups slammed the FDA’s decision to withhold action on major e-cigarette manufacturers, including Juul.
“The FDA’s failure today to act on applications by Juul, the manufacturer with the single biggest e-cigarette market share, is extremely disappointing and will allow the industry to further endanger public health and hook more kids on their highly addictive products,” Lisa Lacasse, president of ACS CAN, said in a statement, according to CNN.
“The FDA has had ample time to review the applications and allowing additional delays is unconscionable. There is overwhelming data to demonstrate the negative impact these kinds of flavored products have had on public health and their role in the youth e-cigarette epidemic. The time to act is now,” Ms. Lacasse added.
E-cigarette use among high school students rose from 11.7% in 2017 to 19.6% in 2020, the American Cancer Society said. Nearly 5% of middle schoolers reported using them in 2020.
A version of this article first appeared on WebMD.com.
Politics or protection? What’s behind the push for boosters?
That plan, which was first announced on Aug. 18, has raised eyebrows because it comes in advance of regulatory reviews by the Food and Drug Administration and recommendations from the Centers for Disease Control and Prevention. Those reviews are needed to determine whether third doses of these vaccines are effective or even safe. The move could have important legal ramifications for doctors and patients, too.
On Aug. 31, two high-level officials in the FDA’s Office of Vaccines Research and Review abruptly resigned amid reports that they were angry that the Biden administration was making decisions that should be left up to that agency.
So far, data show that the vaccines are highly effective at preventing the most severe consequences of COVID-19 – hospitalization and death – even regarding the Delta variant. The World Health Organization has urged wealthy nations such as the United States not to offer boosters so that the limited supply of vaccines can be directed to countries with fewer resources.
White House supports boosters
In a recent press briefing, Jeff Zients, the White House COVID-19 response coordinator, defended the move.
“You know, the booster decision, which you referenced ... was made by and announced by the nation’s leading public health officials, including Dr. Walensky; Dr. Fauci; Surgeon General Vivek Murthy; Dr. Janet Woodcock; the FDA acting commissioner, Dr. Francis Collins; Dr. Kessler; and others,” Mr. Zients said.
“And as our medical experts laid out, having reviewed all of the available data, it is in their clinical judgment that it is time to prepare Americans for a booster shot.”
He said a target date of Sept. 20 was announced so as to give states and practitioners time to prepare. He also said the move to give boosters was meant to help the United States stay ahead of a rapidly changing virus. Mr. Zients added that whether boosters will be administered starting on Sept. 20 depends on the FDA’s and CDC’s giving the go-ahead.
“Booster doses are going to be handled the same way all vaccines are handled,” said Kristen Nordlund, a CDC spokesperson. “Companies will have to provide data to FDA. FDA will have to make a decision and authorize the use of those, and ACIP [the Advisory Committee on Immunization Practices] will have to look at the evidence as well and make recommendations on top of FDA’s regulatory action,” she said.
Ms. Nordlund agreed that the planned Sept. 20 start date for boosters was something to which they aspired and was not necessarily set.
Historically, the FDA has needed at least 4 months to review a change to a vaccine’s approval, even on an accelerated schedule. Reviewers use that time to assess data regarding individual patients in a study, to review raw data, and essentially to check a drug company’s math and conclusions. The Biden administration’s timeline would shorten that review period from months to just a few weeks.
‘FDA in a very difficult position’
After the FDA approves, the ACIP of the CDC must meet to review the evidence and make recommendations on the use of the boosters in the United States.
Pfizer says it completed its submission for a supplemental biologics license application to the FDA on Aug. 27. To meet a Sept. 20 timeline, the entire process would have to be completed within 3 weeks.
“I don’t think that was handled, you know, ideally,” said Peter Lurie, MD, president of the Center for Science in the Public Interest and former associate commissioner of public health strategy and analysis at the FDA.
“It puts FDA in a very difficult position,” Dr. Lurie said. “It’s almost as if the decision has been made and they’re just checking a box, and that is, you know, contrary to the what FDA – at least the internal people at FDA – have been trying to do for ages.”
He said the agency took great pains with the emergency use authorizations and the full approvals of the vaccines to work as rapidly but thoroughly as possible. They did not skip steps.
“I think all of that reflected very well on the agency,” Dr. Lurie said. “And I think it worked out well in terms of trust in the vaccines.”
Although additional doses of vaccine are expected to be safe, little is known about side effects or adverse events after a third dose.
“It’s critical to wait for additional data and regulatory allowance for booster doses,” Sara Oliver, MD, a member of the CDC’s epidemic intelligence service, said in an Aug. 30 presentation to the ACIP, which is charged with making recommendations for use of all vaccines in the United States.
Boosters already being given
But after the White House announced that boosters were on the way, many people are not waiting.
Many health care practitioners and pharmacies have already been giving people third doses of vaccines, even if they are not among the immunocompromised – the group for which the shots are currently approved.
“You can walk into a pharmacy and ask for a third dose. Depending on which pharmacy you go to, you may get it,” said Helen Talbot, MD, associate professor of medicine at Vanderbilt University, Nashville, Tenn., and a member of the ACIP.
She says she has a friend who recently went for a checkup and was offered a third dose. His physician is already giving extra doses to everyone who is older than 65.
Dr. Talbot said that in fairness, pharmacies in the United States are throwing away doses of vaccine because they are expiring before they get used.
“Many of us may or may not be ready to give a third dose but would rather give someone a third dose than throw a vaccine away,” she said.
Consequences of a third shot
But giving or getting a third dose before approval by the FDA may have legal consequences.
In the ACIP meeting on Aug. 30, Demetre Daskalakis, MD, who leads vaccine equity efforts at the CDC, cautioned that physicians who give extra doses of the vaccine before the FDA and CDC have signed off may be in violation of practitioner agreements with the federal government and might not be covered by the federal PREP Act. The PREP Act provides immunity from lawsuits for people who administer COVID-19 vaccines and compensates patients in the event of injury. Patients who get a vaccine and suffer a rare but serious side effect may lose the ability to claim compensation offered by the act.
“Many of us gasped when he said that,” Dr. Talbot said, “because that’s a big deal.”
The ACIP signaled that it is considering recommending boosters for a much narrower slice of the American population than the Biden administration has suggested.
They said that so far, the data point only to the need for boosters for seniors, who are the patients most likely to experience breakthrough infections that require hospitalization, and health care workers, who are needed now more than ever and cannot work if they’re sick.
In a White House news briefing Aug. 31, CDC Director Rochelle Walensky, MD, was asked about the ACIP’s conclusions and whether she believed there were enough data to recommend booster shots for most Americans 8 months after their last dose.
“The ACIP did not review international data that actually has led us to be even more concerned about increased risk of vaccine effectiveness waning against hospitalization, severe disease, and death. They will be reviewing that as well,” she said.
A version of this article first appeared on Medscape.com.
That plan, which was first announced on Aug. 18, has raised eyebrows because it comes in advance of regulatory reviews by the Food and Drug Administration and recommendations from the Centers for Disease Control and Prevention. Those reviews are needed to determine whether third doses of these vaccines are effective or even safe. The move could have important legal ramifications for doctors and patients, too.
On Aug. 31, two high-level officials in the FDA’s Office of Vaccines Research and Review abruptly resigned amid reports that they were angry that the Biden administration was making decisions that should be left up to that agency.
So far, data show that the vaccines are highly effective at preventing the most severe consequences of COVID-19 – hospitalization and death – even regarding the Delta variant. The World Health Organization has urged wealthy nations such as the United States not to offer boosters so that the limited supply of vaccines can be directed to countries with fewer resources.
White House supports boosters
In a recent press briefing, Jeff Zients, the White House COVID-19 response coordinator, defended the move.
“You know, the booster decision, which you referenced ... was made by and announced by the nation’s leading public health officials, including Dr. Walensky; Dr. Fauci; Surgeon General Vivek Murthy; Dr. Janet Woodcock; the FDA acting commissioner, Dr. Francis Collins; Dr. Kessler; and others,” Mr. Zients said.
“And as our medical experts laid out, having reviewed all of the available data, it is in their clinical judgment that it is time to prepare Americans for a booster shot.”
He said a target date of Sept. 20 was announced so as to give states and practitioners time to prepare. He also said the move to give boosters was meant to help the United States stay ahead of a rapidly changing virus. Mr. Zients added that whether boosters will be administered starting on Sept. 20 depends on the FDA’s and CDC’s giving the go-ahead.
“Booster doses are going to be handled the same way all vaccines are handled,” said Kristen Nordlund, a CDC spokesperson. “Companies will have to provide data to FDA. FDA will have to make a decision and authorize the use of those, and ACIP [the Advisory Committee on Immunization Practices] will have to look at the evidence as well and make recommendations on top of FDA’s regulatory action,” she said.
Ms. Nordlund agreed that the planned Sept. 20 start date for boosters was something to which they aspired and was not necessarily set.
Historically, the FDA has needed at least 4 months to review a change to a vaccine’s approval, even on an accelerated schedule. Reviewers use that time to assess data regarding individual patients in a study, to review raw data, and essentially to check a drug company’s math and conclusions. The Biden administration’s timeline would shorten that review period from months to just a few weeks.
‘FDA in a very difficult position’
After the FDA approves, the ACIP of the CDC must meet to review the evidence and make recommendations on the use of the boosters in the United States.
Pfizer says it completed its submission for a supplemental biologics license application to the FDA on Aug. 27. To meet a Sept. 20 timeline, the entire process would have to be completed within 3 weeks.
“I don’t think that was handled, you know, ideally,” said Peter Lurie, MD, president of the Center for Science in the Public Interest and former associate commissioner of public health strategy and analysis at the FDA.
“It puts FDA in a very difficult position,” Dr. Lurie said. “It’s almost as if the decision has been made and they’re just checking a box, and that is, you know, contrary to the what FDA – at least the internal people at FDA – have been trying to do for ages.”
He said the agency took great pains with the emergency use authorizations and the full approvals of the vaccines to work as rapidly but thoroughly as possible. They did not skip steps.
“I think all of that reflected very well on the agency,” Dr. Lurie said. “And I think it worked out well in terms of trust in the vaccines.”
Although additional doses of vaccine are expected to be safe, little is known about side effects or adverse events after a third dose.
“It’s critical to wait for additional data and regulatory allowance for booster doses,” Sara Oliver, MD, a member of the CDC’s epidemic intelligence service, said in an Aug. 30 presentation to the ACIP, which is charged with making recommendations for use of all vaccines in the United States.
Boosters already being given
But after the White House announced that boosters were on the way, many people are not waiting.
Many health care practitioners and pharmacies have already been giving people third doses of vaccines, even if they are not among the immunocompromised – the group for which the shots are currently approved.
“You can walk into a pharmacy and ask for a third dose. Depending on which pharmacy you go to, you may get it,” said Helen Talbot, MD, associate professor of medicine at Vanderbilt University, Nashville, Tenn., and a member of the ACIP.
She says she has a friend who recently went for a checkup and was offered a third dose. His physician is already giving extra doses to everyone who is older than 65.
Dr. Talbot said that in fairness, pharmacies in the United States are throwing away doses of vaccine because they are expiring before they get used.
“Many of us may or may not be ready to give a third dose but would rather give someone a third dose than throw a vaccine away,” she said.
Consequences of a third shot
But giving or getting a third dose before approval by the FDA may have legal consequences.
In the ACIP meeting on Aug. 30, Demetre Daskalakis, MD, who leads vaccine equity efforts at the CDC, cautioned that physicians who give extra doses of the vaccine before the FDA and CDC have signed off may be in violation of practitioner agreements with the federal government and might not be covered by the federal PREP Act. The PREP Act provides immunity from lawsuits for people who administer COVID-19 vaccines and compensates patients in the event of injury. Patients who get a vaccine and suffer a rare but serious side effect may lose the ability to claim compensation offered by the act.
“Many of us gasped when he said that,” Dr. Talbot said, “because that’s a big deal.”
The ACIP signaled that it is considering recommending boosters for a much narrower slice of the American population than the Biden administration has suggested.
They said that so far, the data point only to the need for boosters for seniors, who are the patients most likely to experience breakthrough infections that require hospitalization, and health care workers, who are needed now more than ever and cannot work if they’re sick.
In a White House news briefing Aug. 31, CDC Director Rochelle Walensky, MD, was asked about the ACIP’s conclusions and whether she believed there were enough data to recommend booster shots for most Americans 8 months after their last dose.
“The ACIP did not review international data that actually has led us to be even more concerned about increased risk of vaccine effectiveness waning against hospitalization, severe disease, and death. They will be reviewing that as well,” she said.
A version of this article first appeared on Medscape.com.
That plan, which was first announced on Aug. 18, has raised eyebrows because it comes in advance of regulatory reviews by the Food and Drug Administration and recommendations from the Centers for Disease Control and Prevention. Those reviews are needed to determine whether third doses of these vaccines are effective or even safe. The move could have important legal ramifications for doctors and patients, too.
On Aug. 31, two high-level officials in the FDA’s Office of Vaccines Research and Review abruptly resigned amid reports that they were angry that the Biden administration was making decisions that should be left up to that agency.
So far, data show that the vaccines are highly effective at preventing the most severe consequences of COVID-19 – hospitalization and death – even regarding the Delta variant. The World Health Organization has urged wealthy nations such as the United States not to offer boosters so that the limited supply of vaccines can be directed to countries with fewer resources.
White House supports boosters
In a recent press briefing, Jeff Zients, the White House COVID-19 response coordinator, defended the move.
“You know, the booster decision, which you referenced ... was made by and announced by the nation’s leading public health officials, including Dr. Walensky; Dr. Fauci; Surgeon General Vivek Murthy; Dr. Janet Woodcock; the FDA acting commissioner, Dr. Francis Collins; Dr. Kessler; and others,” Mr. Zients said.
“And as our medical experts laid out, having reviewed all of the available data, it is in their clinical judgment that it is time to prepare Americans for a booster shot.”
He said a target date of Sept. 20 was announced so as to give states and practitioners time to prepare. He also said the move to give boosters was meant to help the United States stay ahead of a rapidly changing virus. Mr. Zients added that whether boosters will be administered starting on Sept. 20 depends on the FDA’s and CDC’s giving the go-ahead.
“Booster doses are going to be handled the same way all vaccines are handled,” said Kristen Nordlund, a CDC spokesperson. “Companies will have to provide data to FDA. FDA will have to make a decision and authorize the use of those, and ACIP [the Advisory Committee on Immunization Practices] will have to look at the evidence as well and make recommendations on top of FDA’s regulatory action,” she said.
Ms. Nordlund agreed that the planned Sept. 20 start date for boosters was something to which they aspired and was not necessarily set.
Historically, the FDA has needed at least 4 months to review a change to a vaccine’s approval, even on an accelerated schedule. Reviewers use that time to assess data regarding individual patients in a study, to review raw data, and essentially to check a drug company’s math and conclusions. The Biden administration’s timeline would shorten that review period from months to just a few weeks.
‘FDA in a very difficult position’
After the FDA approves, the ACIP of the CDC must meet to review the evidence and make recommendations on the use of the boosters in the United States.
Pfizer says it completed its submission for a supplemental biologics license application to the FDA on Aug. 27. To meet a Sept. 20 timeline, the entire process would have to be completed within 3 weeks.
“I don’t think that was handled, you know, ideally,” said Peter Lurie, MD, president of the Center for Science in the Public Interest and former associate commissioner of public health strategy and analysis at the FDA.
“It puts FDA in a very difficult position,” Dr. Lurie said. “It’s almost as if the decision has been made and they’re just checking a box, and that is, you know, contrary to the what FDA – at least the internal people at FDA – have been trying to do for ages.”
He said the agency took great pains with the emergency use authorizations and the full approvals of the vaccines to work as rapidly but thoroughly as possible. They did not skip steps.
“I think all of that reflected very well on the agency,” Dr. Lurie said. “And I think it worked out well in terms of trust in the vaccines.”
Although additional doses of vaccine are expected to be safe, little is known about side effects or adverse events after a third dose.
“It’s critical to wait for additional data and regulatory allowance for booster doses,” Sara Oliver, MD, a member of the CDC’s epidemic intelligence service, said in an Aug. 30 presentation to the ACIP, which is charged with making recommendations for use of all vaccines in the United States.
Boosters already being given
But after the White House announced that boosters were on the way, many people are not waiting.
Many health care practitioners and pharmacies have already been giving people third doses of vaccines, even if they are not among the immunocompromised – the group for which the shots are currently approved.
“You can walk into a pharmacy and ask for a third dose. Depending on which pharmacy you go to, you may get it,” said Helen Talbot, MD, associate professor of medicine at Vanderbilt University, Nashville, Tenn., and a member of the ACIP.
She says she has a friend who recently went for a checkup and was offered a third dose. His physician is already giving extra doses to everyone who is older than 65.
Dr. Talbot said that in fairness, pharmacies in the United States are throwing away doses of vaccine because they are expiring before they get used.
“Many of us may or may not be ready to give a third dose but would rather give someone a third dose than throw a vaccine away,” she said.
Consequences of a third shot
But giving or getting a third dose before approval by the FDA may have legal consequences.
In the ACIP meeting on Aug. 30, Demetre Daskalakis, MD, who leads vaccine equity efforts at the CDC, cautioned that physicians who give extra doses of the vaccine before the FDA and CDC have signed off may be in violation of practitioner agreements with the federal government and might not be covered by the federal PREP Act. The PREP Act provides immunity from lawsuits for people who administer COVID-19 vaccines and compensates patients in the event of injury. Patients who get a vaccine and suffer a rare but serious side effect may lose the ability to claim compensation offered by the act.
“Many of us gasped when he said that,” Dr. Talbot said, “because that’s a big deal.”
The ACIP signaled that it is considering recommending boosters for a much narrower slice of the American population than the Biden administration has suggested.
They said that so far, the data point only to the need for boosters for seniors, who are the patients most likely to experience breakthrough infections that require hospitalization, and health care workers, who are needed now more than ever and cannot work if they’re sick.
In a White House news briefing Aug. 31, CDC Director Rochelle Walensky, MD, was asked about the ACIP’s conclusions and whether she believed there were enough data to recommend booster shots for most Americans 8 months after their last dose.
“The ACIP did not review international data that actually has led us to be even more concerned about increased risk of vaccine effectiveness waning against hospitalization, severe disease, and death. They will be reviewing that as well,” she said.
A version of this article first appeared on Medscape.com.
Breakthrough infections twice as likely to be asymptomatic
Individuals infected with COVID-19 after receiving their first or second dose of either the Pfizer, Moderna, or AstraZeneca vaccine experienced a lower number of symptoms in the first week of infection, compared with those who did not receive a COVID-19 vaccine, reported the authors of the report in The Lancet Infectious Diseases. These patients also had a reduced need for hospitalization, compared with their unvaccinated peers. Those who received both doses of a vaccine were less likely to experience prolonged COVID - defined as at least 28 days of symptoms in this paper - compared with unvaccinated individuals.
“We are at a critical point in the pandemic as we see cases rising worldwide due to the delta variant,” study co–lead author Dr. Claire Steves, said in a statement. “Breakthrough infections are expected and don’t diminish the fact that these vaccines are doing exactly what they were designed to do – save lives and prevent serious illness.”
For the community-based, case-control study, Dr. Steves, who is a clinical senior lecturer at King’s College London, and her colleagues analyzed and presented self-reported data on demographics, geographical location, health risk factors, COVID-19 test results, symptoms, and vaccinations from more than 1.2 million UK-based adults through the COVID Symptom Study mobile phone app.
They found that, of the 1.2 million adults who received at least one dose of either the Pfizer, Moderna, or AstraZeneca vaccine, fewer than 0.5% tested positive for COVID-19 14 days after their first dose. Of those who received a second dose of a COVID-19 vaccine, 0.2% acquired the infection more than 7 days post vaccination.
Likelihood of severe symptoms dropped after one dose
After just one COVID-19 vaccine dose, the likelihood of experiencing severe symptoms from a COVID-19 infection dropped by a quarter. The odds of their infection being asymptomatic increased by 94% after the second dose. Researchers also found that vaccinated participants in the study were more likely to be completely asymptomatic, especially if they were 60 years or older.
Furthermore, the odds of those with breakthrough infections experiencing severe disease – which is characterized by having five or more symptoms within the first week of becoming ill – dropped by approximately one-third.
When evaluating risk factors, the researchers found that those most vulnerable to a breakthrough infection after receiving a first dose of Pfizer, Moderna, or Astrazeneca COVID-19 vaccine were older adults (ages 60 years or older) who are either frail or live with underlying conditions such as asthma, lung disease, and obesity.
The findings provide substantial evidence that there are benefits after just one dose of the vaccine, said Diego Hijano, MD, MSc, pediatric infectious disease specialist at St. Jude’s Children’s Research Hospital, Memphis. However, the report also supports caution around becoming lax on protective COVID-19 measures such as physical distancing and wearing masks, especially around vulnerable groups, he said.
Findings may have implications for health policies
“It’s also important for people who are fully vaccinated to understand that these infections are expected and are happening, especially now with the Delta variant” Dr. Hijano said. “While the outcomes are favorable, you need to still protect yourself to also protect your loved ones. You want to be very mindful that, if you are vaccinated and you get infected, you can pass it on to somebody that actually has not been vaccinated or has some of these risk factors.”
The authors of the new research paper believe their findings may have implications for health policies regarding the timing between vaccine doses, COVID-19 booster shots, and for continuing personal protective measures.
The authors of the paper and Dr. Hijano disclosed no conflicts.
Individuals infected with COVID-19 after receiving their first or second dose of either the Pfizer, Moderna, or AstraZeneca vaccine experienced a lower number of symptoms in the first week of infection, compared with those who did not receive a COVID-19 vaccine, reported the authors of the report in The Lancet Infectious Diseases. These patients also had a reduced need for hospitalization, compared with their unvaccinated peers. Those who received both doses of a vaccine were less likely to experience prolonged COVID - defined as at least 28 days of symptoms in this paper - compared with unvaccinated individuals.
“We are at a critical point in the pandemic as we see cases rising worldwide due to the delta variant,” study co–lead author Dr. Claire Steves, said in a statement. “Breakthrough infections are expected and don’t diminish the fact that these vaccines are doing exactly what they were designed to do – save lives and prevent serious illness.”
For the community-based, case-control study, Dr. Steves, who is a clinical senior lecturer at King’s College London, and her colleagues analyzed and presented self-reported data on demographics, geographical location, health risk factors, COVID-19 test results, symptoms, and vaccinations from more than 1.2 million UK-based adults through the COVID Symptom Study mobile phone app.
They found that, of the 1.2 million adults who received at least one dose of either the Pfizer, Moderna, or AstraZeneca vaccine, fewer than 0.5% tested positive for COVID-19 14 days after their first dose. Of those who received a second dose of a COVID-19 vaccine, 0.2% acquired the infection more than 7 days post vaccination.
Likelihood of severe symptoms dropped after one dose
After just one COVID-19 vaccine dose, the likelihood of experiencing severe symptoms from a COVID-19 infection dropped by a quarter. The odds of their infection being asymptomatic increased by 94% after the second dose. Researchers also found that vaccinated participants in the study were more likely to be completely asymptomatic, especially if they were 60 years or older.
Furthermore, the odds of those with breakthrough infections experiencing severe disease – which is characterized by having five or more symptoms within the first week of becoming ill – dropped by approximately one-third.
When evaluating risk factors, the researchers found that those most vulnerable to a breakthrough infection after receiving a first dose of Pfizer, Moderna, or Astrazeneca COVID-19 vaccine were older adults (ages 60 years or older) who are either frail or live with underlying conditions such as asthma, lung disease, and obesity.
The findings provide substantial evidence that there are benefits after just one dose of the vaccine, said Diego Hijano, MD, MSc, pediatric infectious disease specialist at St. Jude’s Children’s Research Hospital, Memphis. However, the report also supports caution around becoming lax on protective COVID-19 measures such as physical distancing and wearing masks, especially around vulnerable groups, he said.
Findings may have implications for health policies
“It’s also important for people who are fully vaccinated to understand that these infections are expected and are happening, especially now with the Delta variant” Dr. Hijano said. “While the outcomes are favorable, you need to still protect yourself to also protect your loved ones. You want to be very mindful that, if you are vaccinated and you get infected, you can pass it on to somebody that actually has not been vaccinated or has some of these risk factors.”
The authors of the new research paper believe their findings may have implications for health policies regarding the timing between vaccine doses, COVID-19 booster shots, and for continuing personal protective measures.
The authors of the paper and Dr. Hijano disclosed no conflicts.
Individuals infected with COVID-19 after receiving their first or second dose of either the Pfizer, Moderna, or AstraZeneca vaccine experienced a lower number of symptoms in the first week of infection, compared with those who did not receive a COVID-19 vaccine, reported the authors of the report in The Lancet Infectious Diseases. These patients also had a reduced need for hospitalization, compared with their unvaccinated peers. Those who received both doses of a vaccine were less likely to experience prolonged COVID - defined as at least 28 days of symptoms in this paper - compared with unvaccinated individuals.
“We are at a critical point in the pandemic as we see cases rising worldwide due to the delta variant,” study co–lead author Dr. Claire Steves, said in a statement. “Breakthrough infections are expected and don’t diminish the fact that these vaccines are doing exactly what they were designed to do – save lives and prevent serious illness.”
For the community-based, case-control study, Dr. Steves, who is a clinical senior lecturer at King’s College London, and her colleagues analyzed and presented self-reported data on demographics, geographical location, health risk factors, COVID-19 test results, symptoms, and vaccinations from more than 1.2 million UK-based adults through the COVID Symptom Study mobile phone app.
They found that, of the 1.2 million adults who received at least one dose of either the Pfizer, Moderna, or AstraZeneca vaccine, fewer than 0.5% tested positive for COVID-19 14 days after their first dose. Of those who received a second dose of a COVID-19 vaccine, 0.2% acquired the infection more than 7 days post vaccination.
Likelihood of severe symptoms dropped after one dose
After just one COVID-19 vaccine dose, the likelihood of experiencing severe symptoms from a COVID-19 infection dropped by a quarter. The odds of their infection being asymptomatic increased by 94% after the second dose. Researchers also found that vaccinated participants in the study were more likely to be completely asymptomatic, especially if they were 60 years or older.
Furthermore, the odds of those with breakthrough infections experiencing severe disease – which is characterized by having five or more symptoms within the first week of becoming ill – dropped by approximately one-third.
When evaluating risk factors, the researchers found that those most vulnerable to a breakthrough infection after receiving a first dose of Pfizer, Moderna, or Astrazeneca COVID-19 vaccine were older adults (ages 60 years or older) who are either frail or live with underlying conditions such as asthma, lung disease, and obesity.
The findings provide substantial evidence that there are benefits after just one dose of the vaccine, said Diego Hijano, MD, MSc, pediatric infectious disease specialist at St. Jude’s Children’s Research Hospital, Memphis. However, the report also supports caution around becoming lax on protective COVID-19 measures such as physical distancing and wearing masks, especially around vulnerable groups, he said.
Findings may have implications for health policies
“It’s also important for people who are fully vaccinated to understand that these infections are expected and are happening, especially now with the Delta variant” Dr. Hijano said. “While the outcomes are favorable, you need to still protect yourself to also protect your loved ones. You want to be very mindful that, if you are vaccinated and you get infected, you can pass it on to somebody that actually has not been vaccinated or has some of these risk factors.”
The authors of the new research paper believe their findings may have implications for health policies regarding the timing between vaccine doses, COVID-19 booster shots, and for continuing personal protective measures.
The authors of the paper and Dr. Hijano disclosed no conflicts.
FROM THE LANCET INFECTIOUS DISEASES