User login
Are you SARS-CoV-2 vaccine hesitant?
When the pandemic was just emerging from its infancy and we were just beginning to think about social distancing, I was sitting around enjoying an adult beverage and some gluten free (not my choice) snacks with some friends. A retired nurse who had just celebrated her 80th birthday said, “I can’t wait until they’ve developed a vaccine.” A former electrical engineer sitting just short of 2 meters to her left responded, “Don’t save me a place near the front of the line for something that is being developed in a program called Warp Speed.”
How do you feel about the potential SARS-CoV-2 vaccine? Are you going to roll up your sleeve as soon as the vaccine becomes available in your community? What are you going to suggest to your patients, your children? I suspect many of you will answer, “It depends.”
Will it make any difference to you which biochemical-immune-bending strategy is being used to make the vaccine? All of them will probably be the result of a clever sounding but novel technique, all of them with a track record that is measured in months and not years. Will you be swayed by how large the trials were? Or how long the follow-up lasted? How effective must the vaccine be to convince you that it is worth receiving or recommending? Do you have the tools and experience to make a decision like that? I know I don’t. And should you and I even be put in a position to make that decision?
In the past, you and I may have relied on the Centers for Disease Control and Prevention for advice. But given the somewhat murky and stormy relationship between the CDC and the president, the vaccine recommendation may be issued by the White House and not the CDC.
For those of us who were practicing medicine during the Swine Flu fiasco of 1976, the pace and the politics surrounding the development of a SARS-CoV-2 vaccine has a discomforting déjà vu quality about it. The fact that like this year 1976 was an election year that infused the development process with a sense of urgency above and beyond any of the concerns about the pandemic that never happened. Although causality was never proven, there was a surge in Guillain-Barré syndrome cases that had been linked temporally to the vaccine.
Of course, our pandemic is real, and it would be imprudent to wait a year or more to watch for long-term vaccine sequelae. However, I am more than a little concerned that fast tracking the development process may result in unfortunate consequences in the short term that could have been avoided with a more measured approach to trialing the vaccines.
The sad reality is that as a nation we tend to be impatient. We are drawn to quick fixes that come in a vial or a capsule. We are learning that simple measures like mask wearing and social distancing can make a difference in slowing the spread of the virus. It would be tragic to rush a vaccine into production that at best turns out to simply be an expensive alternative to the measures that we know work or at worst injures more of us than it saves.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Email him at [email protected].
When the pandemic was just emerging from its infancy and we were just beginning to think about social distancing, I was sitting around enjoying an adult beverage and some gluten free (not my choice) snacks with some friends. A retired nurse who had just celebrated her 80th birthday said, “I can’t wait until they’ve developed a vaccine.” A former electrical engineer sitting just short of 2 meters to her left responded, “Don’t save me a place near the front of the line for something that is being developed in a program called Warp Speed.”
How do you feel about the potential SARS-CoV-2 vaccine? Are you going to roll up your sleeve as soon as the vaccine becomes available in your community? What are you going to suggest to your patients, your children? I suspect many of you will answer, “It depends.”
Will it make any difference to you which biochemical-immune-bending strategy is being used to make the vaccine? All of them will probably be the result of a clever sounding but novel technique, all of them with a track record that is measured in months and not years. Will you be swayed by how large the trials were? Or how long the follow-up lasted? How effective must the vaccine be to convince you that it is worth receiving or recommending? Do you have the tools and experience to make a decision like that? I know I don’t. And should you and I even be put in a position to make that decision?
In the past, you and I may have relied on the Centers for Disease Control and Prevention for advice. But given the somewhat murky and stormy relationship between the CDC and the president, the vaccine recommendation may be issued by the White House and not the CDC.
For those of us who were practicing medicine during the Swine Flu fiasco of 1976, the pace and the politics surrounding the development of a SARS-CoV-2 vaccine has a discomforting déjà vu quality about it. The fact that like this year 1976 was an election year that infused the development process with a sense of urgency above and beyond any of the concerns about the pandemic that never happened. Although causality was never proven, there was a surge in Guillain-Barré syndrome cases that had been linked temporally to the vaccine.
Of course, our pandemic is real, and it would be imprudent to wait a year or more to watch for long-term vaccine sequelae. However, I am more than a little concerned that fast tracking the development process may result in unfortunate consequences in the short term that could have been avoided with a more measured approach to trialing the vaccines.
The sad reality is that as a nation we tend to be impatient. We are drawn to quick fixes that come in a vial or a capsule. We are learning that simple measures like mask wearing and social distancing can make a difference in slowing the spread of the virus. It would be tragic to rush a vaccine into production that at best turns out to simply be an expensive alternative to the measures that we know work or at worst injures more of us than it saves.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Email him at [email protected].
When the pandemic was just emerging from its infancy and we were just beginning to think about social distancing, I was sitting around enjoying an adult beverage and some gluten free (not my choice) snacks with some friends. A retired nurse who had just celebrated her 80th birthday said, “I can’t wait until they’ve developed a vaccine.” A former electrical engineer sitting just short of 2 meters to her left responded, “Don’t save me a place near the front of the line for something that is being developed in a program called Warp Speed.”
How do you feel about the potential SARS-CoV-2 vaccine? Are you going to roll up your sleeve as soon as the vaccine becomes available in your community? What are you going to suggest to your patients, your children? I suspect many of you will answer, “It depends.”
Will it make any difference to you which biochemical-immune-bending strategy is being used to make the vaccine? All of them will probably be the result of a clever sounding but novel technique, all of them with a track record that is measured in months and not years. Will you be swayed by how large the trials were? Or how long the follow-up lasted? How effective must the vaccine be to convince you that it is worth receiving or recommending? Do you have the tools and experience to make a decision like that? I know I don’t. And should you and I even be put in a position to make that decision?
In the past, you and I may have relied on the Centers for Disease Control and Prevention for advice. But given the somewhat murky and stormy relationship between the CDC and the president, the vaccine recommendation may be issued by the White House and not the CDC.
For those of us who were practicing medicine during the Swine Flu fiasco of 1976, the pace and the politics surrounding the development of a SARS-CoV-2 vaccine has a discomforting déjà vu quality about it. The fact that like this year 1976 was an election year that infused the development process with a sense of urgency above and beyond any of the concerns about the pandemic that never happened. Although causality was never proven, there was a surge in Guillain-Barré syndrome cases that had been linked temporally to the vaccine.
Of course, our pandemic is real, and it would be imprudent to wait a year or more to watch for long-term vaccine sequelae. However, I am more than a little concerned that fast tracking the development process may result in unfortunate consequences in the short term that could have been avoided with a more measured approach to trialing the vaccines.
The sad reality is that as a nation we tend to be impatient. We are drawn to quick fixes that come in a vial or a capsule. We are learning that simple measures like mask wearing and social distancing can make a difference in slowing the spread of the virus. It would be tragic to rush a vaccine into production that at best turns out to simply be an expensive alternative to the measures that we know work or at worst injures more of us than it saves.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Email him at [email protected].
MIS-C is a serious immune-mediated response to COVID-19 infection
One of the take-away messages from a review of multisystem inflammatory syndrome in children (MIS-C) is that clinicians treating this condition “need to be comfortable with uncertainty,” Melissa Hazen, MD, said at a synthesis of multiple published case series and personal experience summarized at the virtual Pediatric Hospital Medicine meeting.
She emphasized MIS-C patient care “requires flexibility,” and she advised clinicians managing these patients to open the lines of communication with the many specialists who often are required to deal with complications affecting an array of organ systems.
MIS-C might best be understood as the most serious manifestation of an immune-mediated response to COVID-19 infection that ranges from transient mild symptoms to the life-threatening multiple organ involvement that characterizes this newly recognized threat. Although “most children who encounter this pathogen only develop mild disease,” the spectrum of the disease can move in a subset of patients to a “Kawasaki-like illness” without hemodynamic instability and then to MIS-C “with highly elevated systemic inflammatory markers and multiple organ involvement,” explained Dr. Hazen, an attending physician in the rheumatology program at Boston Children’s Hospital.
most of which have only recently reached publication, according to Dr. Hazen. In general, the description of the most common symptoms and their course has been relatively consistent.
In 186 cases of MIS-C collected in a study funded by the Centers for Disease Control and Prevention, 148 (80%) were admitted to intensive care, 90 patients (48%) received vasoactive support, 37 (20%) received mechanical ventilation, and 4 (2%) died.1 The median age was 8 years (range, 3-13 years) in this study. The case definition was fever for at least 24 hours, laboratory evidence of inflammation, multisystem organ involvement, and evidence of COVID-19 infection. In this cohort of 186 children, 92% had gastrointestinal, 80% had cardiovascular, 76% had hematologic, and 70% had respiratory system involvement.
In a different series of 95 cases collected in New York State, 79 (80%) were admitted to intensive care, 61 (62%) received vasoactive support, 10 (10%) received mechanical ventilation, 4 (4%) received extracorporeal membrane oxygenation (ECMO), and 2 (2%) died. 2 Thirty-one percent patients were aged 0-5 years, 42% were 6-12 years, and 26% were 13-20 years of age. In that series, for which the case definition was elevation of two or more inflammatory markers, virologic evidence of COVID-19 infection, 80% had gastrointestinal system involvement, and 53% had evidence of myocarditis.
In both of these series, as well as others published and unpublished, the peak in MIS-C cases has occurred about 3 to 4 weeks after peak COVID-19 activity, according to Diana Lee, MD, a pediatrician at Icahn School of Medicine at Mount Sinai, New York. This pattern, reported by others, was observed in New York State, where 230 cases of MIS-C were collected from the beginning of May until the end of June, which reflected this 3- to 4-week delay in peak incidence.
“This does seem to be a rare syndrome since this [group of] 230 cases is amongst the entire population of children in New York State. So, yes, we should be keeping this in mind in our differential, but we should not forget all the other reasons that children can have a fever,” she said.
Both Dr. Hazen and Dr. Lee cautioned that MIS-C, despite a general consistency among published studies, remains a moving target in regard to how it is being characterized. In a 2-day period in May, the CDC, the World Health Organization, and New York State all issued descriptions of MIS-C, employing compatible but slightly different terminology and diagnostic criteria. Many questions regarding optimal methods of diagnosis, treatment, and follow-up remain unanswered.
Questions regarding the risk to the cardiovascular system, one of the organs most commonly affected in MIS-C, are among the most urgent. It is not now clear how best to monitor cardiovascular involvement, how to intervene, and how to follow patients in the postinfection period, according to Kevin G. Friedman, MD, a pediatrician at Harvard Medical School, Boston, and an attending physician in the department of cardiology at Boston Children’s Hospital.
“The most frequent complication we have seen is ventricular dysfunction, which occurs in about half of these patients,” he reported. “Usually it is in the mild to moderate range, but occasionally patients have an ejection fraction of less than 40%.”
Coronary abnormalities, typically in the form of dilations or small aneurysms, occur in 10%-20% of children with MIS-C, according to Dr. Friedman. Giant aneurysms have been reported.
“Some of these findings can progress including in both the acute phase and, particularly for the coronary aneurysms, in the subacute phase. We recommend echocardiograms and EKGs at diagnosis and at 1-2 weeks to recheck coronary size or sooner if there are clinical indications,” Dr. Friedman advised.
Protocols like these are constantly under review as more information becomes available. There are as yet no guidelines, and practice differs across institutions, according to the investigators summarizing this information.
None of the speakers had any relevant financial disclosures.
References
1. Feldstein LR et al. Multisystem inflammatory syndrome in U.S. children and adolescents. N Engl J Med. 2020;383:334-46.
2. Dufort EM et al. Multisystem inflammatory syndrome in children in New York State. N Engl J Med 2020;383:347-58.
One of the take-away messages from a review of multisystem inflammatory syndrome in children (MIS-C) is that clinicians treating this condition “need to be comfortable with uncertainty,” Melissa Hazen, MD, said at a synthesis of multiple published case series and personal experience summarized at the virtual Pediatric Hospital Medicine meeting.
She emphasized MIS-C patient care “requires flexibility,” and she advised clinicians managing these patients to open the lines of communication with the many specialists who often are required to deal with complications affecting an array of organ systems.
MIS-C might best be understood as the most serious manifestation of an immune-mediated response to COVID-19 infection that ranges from transient mild symptoms to the life-threatening multiple organ involvement that characterizes this newly recognized threat. Although “most children who encounter this pathogen only develop mild disease,” the spectrum of the disease can move in a subset of patients to a “Kawasaki-like illness” without hemodynamic instability and then to MIS-C “with highly elevated systemic inflammatory markers and multiple organ involvement,” explained Dr. Hazen, an attending physician in the rheumatology program at Boston Children’s Hospital.
most of which have only recently reached publication, according to Dr. Hazen. In general, the description of the most common symptoms and their course has been relatively consistent.
In 186 cases of MIS-C collected in a study funded by the Centers for Disease Control and Prevention, 148 (80%) were admitted to intensive care, 90 patients (48%) received vasoactive support, 37 (20%) received mechanical ventilation, and 4 (2%) died.1 The median age was 8 years (range, 3-13 years) in this study. The case definition was fever for at least 24 hours, laboratory evidence of inflammation, multisystem organ involvement, and evidence of COVID-19 infection. In this cohort of 186 children, 92% had gastrointestinal, 80% had cardiovascular, 76% had hematologic, and 70% had respiratory system involvement.
In a different series of 95 cases collected in New York State, 79 (80%) were admitted to intensive care, 61 (62%) received vasoactive support, 10 (10%) received mechanical ventilation, 4 (4%) received extracorporeal membrane oxygenation (ECMO), and 2 (2%) died. 2 Thirty-one percent patients were aged 0-5 years, 42% were 6-12 years, and 26% were 13-20 years of age. In that series, for which the case definition was elevation of two or more inflammatory markers, virologic evidence of COVID-19 infection, 80% had gastrointestinal system involvement, and 53% had evidence of myocarditis.
In both of these series, as well as others published and unpublished, the peak in MIS-C cases has occurred about 3 to 4 weeks after peak COVID-19 activity, according to Diana Lee, MD, a pediatrician at Icahn School of Medicine at Mount Sinai, New York. This pattern, reported by others, was observed in New York State, where 230 cases of MIS-C were collected from the beginning of May until the end of June, which reflected this 3- to 4-week delay in peak incidence.
“This does seem to be a rare syndrome since this [group of] 230 cases is amongst the entire population of children in New York State. So, yes, we should be keeping this in mind in our differential, but we should not forget all the other reasons that children can have a fever,” she said.
Both Dr. Hazen and Dr. Lee cautioned that MIS-C, despite a general consistency among published studies, remains a moving target in regard to how it is being characterized. In a 2-day period in May, the CDC, the World Health Organization, and New York State all issued descriptions of MIS-C, employing compatible but slightly different terminology and diagnostic criteria. Many questions regarding optimal methods of diagnosis, treatment, and follow-up remain unanswered.
Questions regarding the risk to the cardiovascular system, one of the organs most commonly affected in MIS-C, are among the most urgent. It is not now clear how best to monitor cardiovascular involvement, how to intervene, and how to follow patients in the postinfection period, according to Kevin G. Friedman, MD, a pediatrician at Harvard Medical School, Boston, and an attending physician in the department of cardiology at Boston Children’s Hospital.
“The most frequent complication we have seen is ventricular dysfunction, which occurs in about half of these patients,” he reported. “Usually it is in the mild to moderate range, but occasionally patients have an ejection fraction of less than 40%.”
Coronary abnormalities, typically in the form of dilations or small aneurysms, occur in 10%-20% of children with MIS-C, according to Dr. Friedman. Giant aneurysms have been reported.
“Some of these findings can progress including in both the acute phase and, particularly for the coronary aneurysms, in the subacute phase. We recommend echocardiograms and EKGs at diagnosis and at 1-2 weeks to recheck coronary size or sooner if there are clinical indications,” Dr. Friedman advised.
Protocols like these are constantly under review as more information becomes available. There are as yet no guidelines, and practice differs across institutions, according to the investigators summarizing this information.
None of the speakers had any relevant financial disclosures.
References
1. Feldstein LR et al. Multisystem inflammatory syndrome in U.S. children and adolescents. N Engl J Med. 2020;383:334-46.
2. Dufort EM et al. Multisystem inflammatory syndrome in children in New York State. N Engl J Med 2020;383:347-58.
One of the take-away messages from a review of multisystem inflammatory syndrome in children (MIS-C) is that clinicians treating this condition “need to be comfortable with uncertainty,” Melissa Hazen, MD, said at a synthesis of multiple published case series and personal experience summarized at the virtual Pediatric Hospital Medicine meeting.
She emphasized MIS-C patient care “requires flexibility,” and she advised clinicians managing these patients to open the lines of communication with the many specialists who often are required to deal with complications affecting an array of organ systems.
MIS-C might best be understood as the most serious manifestation of an immune-mediated response to COVID-19 infection that ranges from transient mild symptoms to the life-threatening multiple organ involvement that characterizes this newly recognized threat. Although “most children who encounter this pathogen only develop mild disease,” the spectrum of the disease can move in a subset of patients to a “Kawasaki-like illness” without hemodynamic instability and then to MIS-C “with highly elevated systemic inflammatory markers and multiple organ involvement,” explained Dr. Hazen, an attending physician in the rheumatology program at Boston Children’s Hospital.
most of which have only recently reached publication, according to Dr. Hazen. In general, the description of the most common symptoms and their course has been relatively consistent.
In 186 cases of MIS-C collected in a study funded by the Centers for Disease Control and Prevention, 148 (80%) were admitted to intensive care, 90 patients (48%) received vasoactive support, 37 (20%) received mechanical ventilation, and 4 (2%) died.1 The median age was 8 years (range, 3-13 years) in this study. The case definition was fever for at least 24 hours, laboratory evidence of inflammation, multisystem organ involvement, and evidence of COVID-19 infection. In this cohort of 186 children, 92% had gastrointestinal, 80% had cardiovascular, 76% had hematologic, and 70% had respiratory system involvement.
In a different series of 95 cases collected in New York State, 79 (80%) were admitted to intensive care, 61 (62%) received vasoactive support, 10 (10%) received mechanical ventilation, 4 (4%) received extracorporeal membrane oxygenation (ECMO), and 2 (2%) died. 2 Thirty-one percent patients were aged 0-5 years, 42% were 6-12 years, and 26% were 13-20 years of age. In that series, for which the case definition was elevation of two or more inflammatory markers, virologic evidence of COVID-19 infection, 80% had gastrointestinal system involvement, and 53% had evidence of myocarditis.
In both of these series, as well as others published and unpublished, the peak in MIS-C cases has occurred about 3 to 4 weeks after peak COVID-19 activity, according to Diana Lee, MD, a pediatrician at Icahn School of Medicine at Mount Sinai, New York. This pattern, reported by others, was observed in New York State, where 230 cases of MIS-C were collected from the beginning of May until the end of June, which reflected this 3- to 4-week delay in peak incidence.
“This does seem to be a rare syndrome since this [group of] 230 cases is amongst the entire population of children in New York State. So, yes, we should be keeping this in mind in our differential, but we should not forget all the other reasons that children can have a fever,” she said.
Both Dr. Hazen and Dr. Lee cautioned that MIS-C, despite a general consistency among published studies, remains a moving target in regard to how it is being characterized. In a 2-day period in May, the CDC, the World Health Organization, and New York State all issued descriptions of MIS-C, employing compatible but slightly different terminology and diagnostic criteria. Many questions regarding optimal methods of diagnosis, treatment, and follow-up remain unanswered.
Questions regarding the risk to the cardiovascular system, one of the organs most commonly affected in MIS-C, are among the most urgent. It is not now clear how best to monitor cardiovascular involvement, how to intervene, and how to follow patients in the postinfection period, according to Kevin G. Friedman, MD, a pediatrician at Harvard Medical School, Boston, and an attending physician in the department of cardiology at Boston Children’s Hospital.
“The most frequent complication we have seen is ventricular dysfunction, which occurs in about half of these patients,” he reported. “Usually it is in the mild to moderate range, but occasionally patients have an ejection fraction of less than 40%.”
Coronary abnormalities, typically in the form of dilations or small aneurysms, occur in 10%-20% of children with MIS-C, according to Dr. Friedman. Giant aneurysms have been reported.
“Some of these findings can progress including in both the acute phase and, particularly for the coronary aneurysms, in the subacute phase. We recommend echocardiograms and EKGs at diagnosis and at 1-2 weeks to recheck coronary size or sooner if there are clinical indications,” Dr. Friedman advised.
Protocols like these are constantly under review as more information becomes available. There are as yet no guidelines, and practice differs across institutions, according to the investigators summarizing this information.
None of the speakers had any relevant financial disclosures.
References
1. Feldstein LR et al. Multisystem inflammatory syndrome in U.S. children and adolescents. N Engl J Med. 2020;383:334-46.
2. Dufort EM et al. Multisystem inflammatory syndrome in children in New York State. N Engl J Med 2020;383:347-58.
FROM PHM20 VIRTUAL
Small NY study: Mother-baby transmission of COVID-19 not seen
according to a study out of New York-Presbyterian Hospital.
“It is suggested in the cumulative data that the virus does not confer additional risk to the fetus during labor or during the early postnatal period in both preterm and term infants,” concluded Jeffrey Perlman, MB ChB, and colleagues in Pediatrics.
But other experts suggest substantial gaps remain in our understanding of maternal transmission of SARS-CoV-2.
“Much more needs to be known,” Munish Gupta, MD, and colleagues from Beth Israel Deaconess Medical Center and Harvard Medical School, Boston, said in an accompanying editorial.
The prospective study is the first to describe a cohort of U.S. COVID-19–related deliveries, with the prior neonatal impact of COVID-19 “almost exclusively” reported from China, noted the authors. They included a cohort of 326 women who were tested for SARS-CoV-2 on admission to labor and delivery at New York-Presbyterian Hospital between March 22 and April 15th, 2020. Of the 31 (10%) mothers who tested positive, 15 (48%) were asymptomatic and 16 (52%) were symptomatic.
Two babies were born prematurely (one by Cesarean) and were isolated in negative pressure rooms with continuous positive airway pressure. Both were moved out of isolation after two negative test results and “have exhibited an unremarkable clinical course,” the authors reported.
The other 29 term babies were cared for in their mothers’ rooms, with breastfeeding allowed, if desired. These babies and their mothers were discharged from the hospital between 24 and 48 hours after delivery.
“Visitor restriction for mothers who were positive for COVID-19 included 14 days of no visitation from the start of symptoms,” noted the team.
They added “since the prepublication release there have been a total of 47 mothers positive for COVID-19, resulting in 47 infants; 4 have been admitted to neonatal intensive care. In addition, 32 other infants have been tested for a variety of indications within the unit. All infants test results have been negative.”
The brief report outlined the institution’s checklist for delivery preparedness in either the operating room or labor delivery room, including personal protective equipment, resuscitation, transportation to the neonatal intensive care unit, and early postresuscitation care. “Suspected or confirmed COVID-19 alone in an otherwise uncomplicated pregnancy is not an indication for the resuscitation team or the neonatal fellow,” they noted, adding delivery room preparation and management should include contact precautions. “With scrupulous attention to infectious precautions, horizontal viral transmission should be minimized,” they advised.
Dr. Perlman and associates emphasized that rapid turnaround SARSCoV-2 testing is “crucial to minimize the likelihood of a provider becoming infected and/or infecting the infant.”
Although the findings are “clearly reassuring,” Dr. Gupta and colleagues have reservations. “To what extent does this report address concerns for infection risk with a rooming-in approach to care?” they asked in their accompanying editorial. “The answer is likely some, but not much.”
Many questions remain, they said, including: “What precautions were used to minimize infection risk during the postbirth hospital course? What was the approach to skin-to-skin care and direct mother-newborn contact? Were restrictions placed on family members? Were changes made to routine interventions such as hearing screens or circumcisions? What practices were in place around environmental cleaning? Most important, how did the newborns do after discharge?”
The current uncertainty around neonatal COVID-19 infection risk has led to “disparate” variations in care recommendations, they pointed out. Whereas China’s consensus guidelines recommend a 14-day separation of COVID-19–positive mothers from their healthy infants, a practice supported by the American Academy of Pediatrics “when possible,” the Italian Society of Neonatology, the Royal College of Paediatrics and Child Health, and the Canadian Paediatric Society advise “rooming-in and breastfeeding with appropriate infection prevention measures.”
Dr. Gupta and colleagues pointed to the following as at least three “critical and time-sensitive needs for research around neonatal care and outcomes related to COVID-19”:
- Studies need to have much larger sample sizes and include diverse populations. This will allow for reliable measurement of outcomes.
- Descriptions of care practices must be in detail, especially about infection prevention; these should be presented in a way to compare the efficacy of different approaches.
- There needs to be follow-up information on outcomes of both the mother and the neonate after the birth hospitalization.
Asked to comment, Lillian Beard, MD, of George Washington University in Washington welcomed the data as “good news.”
“Although small, the study was done during a 3-week peak period at the hottest spot of the pandemic in the United States during that period. It illustrates how delivery room preparedness, adequate personal protective equipment, and carefully planned infection control precautions can positively impact outcomes even during a seemingly impossible period,” she said.
“Although there are many uncertainties about maternal COVID-19 transmission and neonatal infection risks ... in my opinion, during the after birth hospitalization, the inherent benefits of rooming in for breast feeding and the opportunities for the demonstration and teaching of infection prevention practices for the family home, far outweigh the risks of disease transmission,” said Dr. Beard, who was not involved with the study.
The study and the commentary emphasize the likely low risk of vertical transmission of the virus, with horizontal transmission being the greater risk. However, cases of transplacental transmission have been reported, and the lead investigator of one recent placental study cautions against complacency.
“Neonates can get infected in both ways. The majority of cases seem to be horizontal, but those who have been infected or highly suspected to be vertically infected are not a small percentage either,” said Daniele de Luca, MD, PhD, president-elect of the European Society for Pediatric and Neonatal Intensive Care (ESPNIC) and a neonatologist at Antoine Béclère Hospital in Clamart, France.
“Perlman’s data are interesting and consistent with other reports around the world. However, two things must be remembered,” he said in an interview. “First, newborn infants are at relatively low risk from SARS-CoV-2 infections, but this is very far from zero risk. Neonatal SARS-CoV-2 infections do exist and have been described around the world. While they have a mild course in the majority of cases, neonatologists should not forget them and should be prepared to offer the best care to these babies.”
“Second, how this can be balanced with the need to promote breastfeeding and avoid overtreatment or separation from the mother is a question far from being answered. Gupta et al. in their commentary are right in saying that we have more questions than answers. While waiting for the results of large initiatives (such as the ESPNIC EPICENTRE Registry that they cite) to answer these open points, the best we can do is to provide a personalised case by case approach, transparent information to parents, and an open counselling informing clinical decisions.”
The study received no external funding. Dr. Perlman and associates had no financial disclosures. Dr. Gupta and colleagues had no relevant financial disclosures. Neither Dr. Beard nor Dr. de Luca had any relevant financial disclosures.
SOURCE: Perlman J et al. Pediatrics. 2020;146(2):e20201567.
according to a study out of New York-Presbyterian Hospital.
“It is suggested in the cumulative data that the virus does not confer additional risk to the fetus during labor or during the early postnatal period in both preterm and term infants,” concluded Jeffrey Perlman, MB ChB, and colleagues in Pediatrics.
But other experts suggest substantial gaps remain in our understanding of maternal transmission of SARS-CoV-2.
“Much more needs to be known,” Munish Gupta, MD, and colleagues from Beth Israel Deaconess Medical Center and Harvard Medical School, Boston, said in an accompanying editorial.
The prospective study is the first to describe a cohort of U.S. COVID-19–related deliveries, with the prior neonatal impact of COVID-19 “almost exclusively” reported from China, noted the authors. They included a cohort of 326 women who were tested for SARS-CoV-2 on admission to labor and delivery at New York-Presbyterian Hospital between March 22 and April 15th, 2020. Of the 31 (10%) mothers who tested positive, 15 (48%) were asymptomatic and 16 (52%) were symptomatic.
Two babies were born prematurely (one by Cesarean) and were isolated in negative pressure rooms with continuous positive airway pressure. Both were moved out of isolation after two negative test results and “have exhibited an unremarkable clinical course,” the authors reported.
The other 29 term babies were cared for in their mothers’ rooms, with breastfeeding allowed, if desired. These babies and their mothers were discharged from the hospital between 24 and 48 hours after delivery.
“Visitor restriction for mothers who were positive for COVID-19 included 14 days of no visitation from the start of symptoms,” noted the team.
They added “since the prepublication release there have been a total of 47 mothers positive for COVID-19, resulting in 47 infants; 4 have been admitted to neonatal intensive care. In addition, 32 other infants have been tested for a variety of indications within the unit. All infants test results have been negative.”
The brief report outlined the institution’s checklist for delivery preparedness in either the operating room or labor delivery room, including personal protective equipment, resuscitation, transportation to the neonatal intensive care unit, and early postresuscitation care. “Suspected or confirmed COVID-19 alone in an otherwise uncomplicated pregnancy is not an indication for the resuscitation team or the neonatal fellow,” they noted, adding delivery room preparation and management should include contact precautions. “With scrupulous attention to infectious precautions, horizontal viral transmission should be minimized,” they advised.
Dr. Perlman and associates emphasized that rapid turnaround SARSCoV-2 testing is “crucial to minimize the likelihood of a provider becoming infected and/or infecting the infant.”
Although the findings are “clearly reassuring,” Dr. Gupta and colleagues have reservations. “To what extent does this report address concerns for infection risk with a rooming-in approach to care?” they asked in their accompanying editorial. “The answer is likely some, but not much.”
Many questions remain, they said, including: “What precautions were used to minimize infection risk during the postbirth hospital course? What was the approach to skin-to-skin care and direct mother-newborn contact? Were restrictions placed on family members? Were changes made to routine interventions such as hearing screens or circumcisions? What practices were in place around environmental cleaning? Most important, how did the newborns do after discharge?”
The current uncertainty around neonatal COVID-19 infection risk has led to “disparate” variations in care recommendations, they pointed out. Whereas China’s consensus guidelines recommend a 14-day separation of COVID-19–positive mothers from their healthy infants, a practice supported by the American Academy of Pediatrics “when possible,” the Italian Society of Neonatology, the Royal College of Paediatrics and Child Health, and the Canadian Paediatric Society advise “rooming-in and breastfeeding with appropriate infection prevention measures.”
Dr. Gupta and colleagues pointed to the following as at least three “critical and time-sensitive needs for research around neonatal care and outcomes related to COVID-19”:
- Studies need to have much larger sample sizes and include diverse populations. This will allow for reliable measurement of outcomes.
- Descriptions of care practices must be in detail, especially about infection prevention; these should be presented in a way to compare the efficacy of different approaches.
- There needs to be follow-up information on outcomes of both the mother and the neonate after the birth hospitalization.
Asked to comment, Lillian Beard, MD, of George Washington University in Washington welcomed the data as “good news.”
“Although small, the study was done during a 3-week peak period at the hottest spot of the pandemic in the United States during that period. It illustrates how delivery room preparedness, adequate personal protective equipment, and carefully planned infection control precautions can positively impact outcomes even during a seemingly impossible period,” she said.
“Although there are many uncertainties about maternal COVID-19 transmission and neonatal infection risks ... in my opinion, during the after birth hospitalization, the inherent benefits of rooming in for breast feeding and the opportunities for the demonstration and teaching of infection prevention practices for the family home, far outweigh the risks of disease transmission,” said Dr. Beard, who was not involved with the study.
The study and the commentary emphasize the likely low risk of vertical transmission of the virus, with horizontal transmission being the greater risk. However, cases of transplacental transmission have been reported, and the lead investigator of one recent placental study cautions against complacency.
“Neonates can get infected in both ways. The majority of cases seem to be horizontal, but those who have been infected or highly suspected to be vertically infected are not a small percentage either,” said Daniele de Luca, MD, PhD, president-elect of the European Society for Pediatric and Neonatal Intensive Care (ESPNIC) and a neonatologist at Antoine Béclère Hospital in Clamart, France.
“Perlman’s data are interesting and consistent with other reports around the world. However, two things must be remembered,” he said in an interview. “First, newborn infants are at relatively low risk from SARS-CoV-2 infections, but this is very far from zero risk. Neonatal SARS-CoV-2 infections do exist and have been described around the world. While they have a mild course in the majority of cases, neonatologists should not forget them and should be prepared to offer the best care to these babies.”
“Second, how this can be balanced with the need to promote breastfeeding and avoid overtreatment or separation from the mother is a question far from being answered. Gupta et al. in their commentary are right in saying that we have more questions than answers. While waiting for the results of large initiatives (such as the ESPNIC EPICENTRE Registry that they cite) to answer these open points, the best we can do is to provide a personalised case by case approach, transparent information to parents, and an open counselling informing clinical decisions.”
The study received no external funding. Dr. Perlman and associates had no financial disclosures. Dr. Gupta and colleagues had no relevant financial disclosures. Neither Dr. Beard nor Dr. de Luca had any relevant financial disclosures.
SOURCE: Perlman J et al. Pediatrics. 2020;146(2):e20201567.
according to a study out of New York-Presbyterian Hospital.
“It is suggested in the cumulative data that the virus does not confer additional risk to the fetus during labor or during the early postnatal period in both preterm and term infants,” concluded Jeffrey Perlman, MB ChB, and colleagues in Pediatrics.
But other experts suggest substantial gaps remain in our understanding of maternal transmission of SARS-CoV-2.
“Much more needs to be known,” Munish Gupta, MD, and colleagues from Beth Israel Deaconess Medical Center and Harvard Medical School, Boston, said in an accompanying editorial.
The prospective study is the first to describe a cohort of U.S. COVID-19–related deliveries, with the prior neonatal impact of COVID-19 “almost exclusively” reported from China, noted the authors. They included a cohort of 326 women who were tested for SARS-CoV-2 on admission to labor and delivery at New York-Presbyterian Hospital between March 22 and April 15th, 2020. Of the 31 (10%) mothers who tested positive, 15 (48%) were asymptomatic and 16 (52%) were symptomatic.
Two babies were born prematurely (one by Cesarean) and were isolated in negative pressure rooms with continuous positive airway pressure. Both were moved out of isolation after two negative test results and “have exhibited an unremarkable clinical course,” the authors reported.
The other 29 term babies were cared for in their mothers’ rooms, with breastfeeding allowed, if desired. These babies and their mothers were discharged from the hospital between 24 and 48 hours after delivery.
“Visitor restriction for mothers who were positive for COVID-19 included 14 days of no visitation from the start of symptoms,” noted the team.
They added “since the prepublication release there have been a total of 47 mothers positive for COVID-19, resulting in 47 infants; 4 have been admitted to neonatal intensive care. In addition, 32 other infants have been tested for a variety of indications within the unit. All infants test results have been negative.”
The brief report outlined the institution’s checklist for delivery preparedness in either the operating room or labor delivery room, including personal protective equipment, resuscitation, transportation to the neonatal intensive care unit, and early postresuscitation care. “Suspected or confirmed COVID-19 alone in an otherwise uncomplicated pregnancy is not an indication for the resuscitation team or the neonatal fellow,” they noted, adding delivery room preparation and management should include contact precautions. “With scrupulous attention to infectious precautions, horizontal viral transmission should be minimized,” they advised.
Dr. Perlman and associates emphasized that rapid turnaround SARSCoV-2 testing is “crucial to minimize the likelihood of a provider becoming infected and/or infecting the infant.”
Although the findings are “clearly reassuring,” Dr. Gupta and colleagues have reservations. “To what extent does this report address concerns for infection risk with a rooming-in approach to care?” they asked in their accompanying editorial. “The answer is likely some, but not much.”
Many questions remain, they said, including: “What precautions were used to minimize infection risk during the postbirth hospital course? What was the approach to skin-to-skin care and direct mother-newborn contact? Were restrictions placed on family members? Were changes made to routine interventions such as hearing screens or circumcisions? What practices were in place around environmental cleaning? Most important, how did the newborns do after discharge?”
The current uncertainty around neonatal COVID-19 infection risk has led to “disparate” variations in care recommendations, they pointed out. Whereas China’s consensus guidelines recommend a 14-day separation of COVID-19–positive mothers from their healthy infants, a practice supported by the American Academy of Pediatrics “when possible,” the Italian Society of Neonatology, the Royal College of Paediatrics and Child Health, and the Canadian Paediatric Society advise “rooming-in and breastfeeding with appropriate infection prevention measures.”
Dr. Gupta and colleagues pointed to the following as at least three “critical and time-sensitive needs for research around neonatal care and outcomes related to COVID-19”:
- Studies need to have much larger sample sizes and include diverse populations. This will allow for reliable measurement of outcomes.
- Descriptions of care practices must be in detail, especially about infection prevention; these should be presented in a way to compare the efficacy of different approaches.
- There needs to be follow-up information on outcomes of both the mother and the neonate after the birth hospitalization.
Asked to comment, Lillian Beard, MD, of George Washington University in Washington welcomed the data as “good news.”
“Although small, the study was done during a 3-week peak period at the hottest spot of the pandemic in the United States during that period. It illustrates how delivery room preparedness, adequate personal protective equipment, and carefully planned infection control precautions can positively impact outcomes even during a seemingly impossible period,” she said.
“Although there are many uncertainties about maternal COVID-19 transmission and neonatal infection risks ... in my opinion, during the after birth hospitalization, the inherent benefits of rooming in for breast feeding and the opportunities for the demonstration and teaching of infection prevention practices for the family home, far outweigh the risks of disease transmission,” said Dr. Beard, who was not involved with the study.
The study and the commentary emphasize the likely low risk of vertical transmission of the virus, with horizontal transmission being the greater risk. However, cases of transplacental transmission have been reported, and the lead investigator of one recent placental study cautions against complacency.
“Neonates can get infected in both ways. The majority of cases seem to be horizontal, but those who have been infected or highly suspected to be vertically infected are not a small percentage either,” said Daniele de Luca, MD, PhD, president-elect of the European Society for Pediatric and Neonatal Intensive Care (ESPNIC) and a neonatologist at Antoine Béclère Hospital in Clamart, France.
“Perlman’s data are interesting and consistent with other reports around the world. However, two things must be remembered,” he said in an interview. “First, newborn infants are at relatively low risk from SARS-CoV-2 infections, but this is very far from zero risk. Neonatal SARS-CoV-2 infections do exist and have been described around the world. While they have a mild course in the majority of cases, neonatologists should not forget them and should be prepared to offer the best care to these babies.”
“Second, how this can be balanced with the need to promote breastfeeding and avoid overtreatment or separation from the mother is a question far from being answered. Gupta et al. in their commentary are right in saying that we have more questions than answers. While waiting for the results of large initiatives (such as the ESPNIC EPICENTRE Registry that they cite) to answer these open points, the best we can do is to provide a personalised case by case approach, transparent information to parents, and an open counselling informing clinical decisions.”
The study received no external funding. Dr. Perlman and associates had no financial disclosures. Dr. Gupta and colleagues had no relevant financial disclosures. Neither Dr. Beard nor Dr. de Luca had any relevant financial disclosures.
SOURCE: Perlman J et al. Pediatrics. 2020;146(2):e20201567.
FROM PEDIATRICS
Internists’ use of ultrasound can reduce radiology referrals
researchers say.
“It’s a safe and very useful tool,” Marco Barchiesi, MD, an internal medicine resident at Luigi Sacco Hospital in Milan, said in an interview. “We had a great reduction in chest x-rays because of the use of ultrasound.”
The finding addresses concerns that ultrasound used in primary care could consume more health care resources or put patients at risk.
Dr. Barchiesi and colleagues published their findings July 20 in the European Journal of Internal Medicine.
Point-of-care ultrasound has become increasingly common as miniaturization of devices has made them more portable. The approach has caught on particularly in emergency departments where quick decisions are of the essence.
Its use in internal medicine has been more controversial, with concerns raised that improperly trained practitioners may miss diagnoses or refer patients for unnecessary tests as a result of uncertainty about their findings.
To measure the effect of point-of-care ultrasound in an internal medicine hospital ward, Dr. Barchiesi and colleagues alternated months when point-of-care ultrasound was allowed with months when it was not allowed, for a total of 4 months each, on an internal medicine unit. They allowed the ultrasound to be used for invasive procedures and excluded patients whose critical condition made point-of-care ultrasound crucial.
The researchers analyzed data on 263 patients in the “on” months when point-of-care ultrasound was used, and 255 in the “off” months when it wasn’t used. The two groups were well balanced in age, sex, comorbidity, and clinical impairment.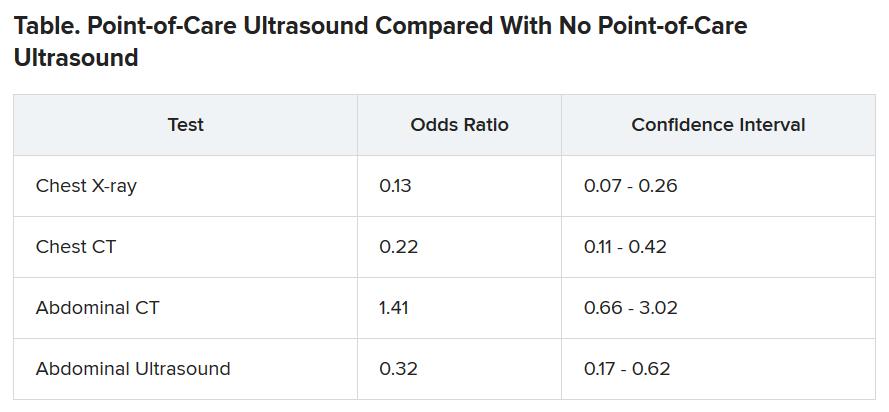
During the on months, the internists ordered 113 diagnostic tests (0.43 per patient). During the off months they ordered 329 tests (1.29 per patient).
The odds of being referred for a chest x-ray were 87% less in the “on” months, compared with the off months, a statistically significant finding (P < .001). The risk for a chest CT scan and abdominal ultrasound were also reduced during the on months, but the risk for an abdominal CT was increased.
Nineteen patients died during the o” months and 10 during the off months, a difference that was not statistically significant (P = .15). The median length of stay in the hospital was almost the same for the two groups: 9 days for the on months and 9 days for the off months. The difference was also not statistically significant (P = .094).
Point-of-care ultrasound is particularly accurate in identifying cardiac abnormalities and pleural fluid and pneumonia, and it can be used effectively for monitoring heart conditions, the researchers wrote. This could explain the reduction in chest x-rays and CT scans.
On the other hand, ultrasound cannot address such questions as staging in an abdominal malignancy, and unexpected findings are more common with abdominal than chest ultrasound. This could explain why the point-of-care ultrasound did not reduce the use of abdominal CT, the researchers speculated.
They acknowledged that the patients in their sample had an average age of 81 years, raising questions about how well their data could be applied to a younger population. And they noted that they used point-of-care ultrasound frequently, so they were particularly adept with it. “We use it almost every day in our clinical practice,” said Dr. Barchiesi.
Those factors may have played a key role in the success of point-of-care ultrasound in this study, said Michael Wagner, MD, an assistant professor of medicine at the University of South Carolina, Greenville, who has helped colleagues incorporate ultrasound into their practices.
Elderly patients often present with multiple comorbidities and atypical signs and symptoms, he said. “Sometimes they can be very confusing as to the underlying clinical picture. Ultrasound is being used frequently to better assess these complicated patients.”
Dr. Wagner said extensive training is required to use point-of-care ultrasound accurately.
Dr. Barchiesi also acknowledged that the devices used in this study were large portable machines, not the simpler and less expensive hand-held versions that are also available for similar purposes.
Point-of-care ultrasound is a promising innovation, said Thomas Melgar, MD, a professor of medicine at Western Michigan University, Kalamazoo. “The advantage is that the exam is being done by someone who knows the patient and specifically what they’re looking for. It’s done at the bedside so you don’t have to move the patient.”
The study could help address opposition to internal medicine residents being trained in the technique, he said, adding that “I think it’s very exciting.”
The study was partially supported by Philips, which provided the ultrasound devices. Dr. Barchiesi, Dr. Melgar, and Dr. Wagner disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
researchers say.
“It’s a safe and very useful tool,” Marco Barchiesi, MD, an internal medicine resident at Luigi Sacco Hospital in Milan, said in an interview. “We had a great reduction in chest x-rays because of the use of ultrasound.”
The finding addresses concerns that ultrasound used in primary care could consume more health care resources or put patients at risk.
Dr. Barchiesi and colleagues published their findings July 20 in the European Journal of Internal Medicine.
Point-of-care ultrasound has become increasingly common as miniaturization of devices has made them more portable. The approach has caught on particularly in emergency departments where quick decisions are of the essence.
Its use in internal medicine has been more controversial, with concerns raised that improperly trained practitioners may miss diagnoses or refer patients for unnecessary tests as a result of uncertainty about their findings.
To measure the effect of point-of-care ultrasound in an internal medicine hospital ward, Dr. Barchiesi and colleagues alternated months when point-of-care ultrasound was allowed with months when it was not allowed, for a total of 4 months each, on an internal medicine unit. They allowed the ultrasound to be used for invasive procedures and excluded patients whose critical condition made point-of-care ultrasound crucial.
The researchers analyzed data on 263 patients in the “on” months when point-of-care ultrasound was used, and 255 in the “off” months when it wasn’t used. The two groups were well balanced in age, sex, comorbidity, and clinical impairment.
During the on months, the internists ordered 113 diagnostic tests (0.43 per patient). During the off months they ordered 329 tests (1.29 per patient).
The odds of being referred for a chest x-ray were 87% less in the “on” months, compared with the off months, a statistically significant finding (P < .001). The risk for a chest CT scan and abdominal ultrasound were also reduced during the on months, but the risk for an abdominal CT was increased.
Nineteen patients died during the o” months and 10 during the off months, a difference that was not statistically significant (P = .15). The median length of stay in the hospital was almost the same for the two groups: 9 days for the on months and 9 days for the off months. The difference was also not statistically significant (P = .094).
Point-of-care ultrasound is particularly accurate in identifying cardiac abnormalities and pleural fluid and pneumonia, and it can be used effectively for monitoring heart conditions, the researchers wrote. This could explain the reduction in chest x-rays and CT scans.
On the other hand, ultrasound cannot address such questions as staging in an abdominal malignancy, and unexpected findings are more common with abdominal than chest ultrasound. This could explain why the point-of-care ultrasound did not reduce the use of abdominal CT, the researchers speculated.
They acknowledged that the patients in their sample had an average age of 81 years, raising questions about how well their data could be applied to a younger population. And they noted that they used point-of-care ultrasound frequently, so they were particularly adept with it. “We use it almost every day in our clinical practice,” said Dr. Barchiesi.
Those factors may have played a key role in the success of point-of-care ultrasound in this study, said Michael Wagner, MD, an assistant professor of medicine at the University of South Carolina, Greenville, who has helped colleagues incorporate ultrasound into their practices.
Elderly patients often present with multiple comorbidities and atypical signs and symptoms, he said. “Sometimes they can be very confusing as to the underlying clinical picture. Ultrasound is being used frequently to better assess these complicated patients.”
Dr. Wagner said extensive training is required to use point-of-care ultrasound accurately.
Dr. Barchiesi also acknowledged that the devices used in this study were large portable machines, not the simpler and less expensive hand-held versions that are also available for similar purposes.
Point-of-care ultrasound is a promising innovation, said Thomas Melgar, MD, a professor of medicine at Western Michigan University, Kalamazoo. “The advantage is that the exam is being done by someone who knows the patient and specifically what they’re looking for. It’s done at the bedside so you don’t have to move the patient.”
The study could help address opposition to internal medicine residents being trained in the technique, he said, adding that “I think it’s very exciting.”
The study was partially supported by Philips, which provided the ultrasound devices. Dr. Barchiesi, Dr. Melgar, and Dr. Wagner disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
researchers say.
“It’s a safe and very useful tool,” Marco Barchiesi, MD, an internal medicine resident at Luigi Sacco Hospital in Milan, said in an interview. “We had a great reduction in chest x-rays because of the use of ultrasound.”
The finding addresses concerns that ultrasound used in primary care could consume more health care resources or put patients at risk.
Dr. Barchiesi and colleagues published their findings July 20 in the European Journal of Internal Medicine.
Point-of-care ultrasound has become increasingly common as miniaturization of devices has made them more portable. The approach has caught on particularly in emergency departments where quick decisions are of the essence.
Its use in internal medicine has been more controversial, with concerns raised that improperly trained practitioners may miss diagnoses or refer patients for unnecessary tests as a result of uncertainty about their findings.
To measure the effect of point-of-care ultrasound in an internal medicine hospital ward, Dr. Barchiesi and colleagues alternated months when point-of-care ultrasound was allowed with months when it was not allowed, for a total of 4 months each, on an internal medicine unit. They allowed the ultrasound to be used for invasive procedures and excluded patients whose critical condition made point-of-care ultrasound crucial.
The researchers analyzed data on 263 patients in the “on” months when point-of-care ultrasound was used, and 255 in the “off” months when it wasn’t used. The two groups were well balanced in age, sex, comorbidity, and clinical impairment.
During the on months, the internists ordered 113 diagnostic tests (0.43 per patient). During the off months they ordered 329 tests (1.29 per patient).
The odds of being referred for a chest x-ray were 87% less in the “on” months, compared with the off months, a statistically significant finding (P < .001). The risk for a chest CT scan and abdominal ultrasound were also reduced during the on months, but the risk for an abdominal CT was increased.
Nineteen patients died during the o” months and 10 during the off months, a difference that was not statistically significant (P = .15). The median length of stay in the hospital was almost the same for the two groups: 9 days for the on months and 9 days for the off months. The difference was also not statistically significant (P = .094).
Point-of-care ultrasound is particularly accurate in identifying cardiac abnormalities and pleural fluid and pneumonia, and it can be used effectively for monitoring heart conditions, the researchers wrote. This could explain the reduction in chest x-rays and CT scans.
On the other hand, ultrasound cannot address such questions as staging in an abdominal malignancy, and unexpected findings are more common with abdominal than chest ultrasound. This could explain why the point-of-care ultrasound did not reduce the use of abdominal CT, the researchers speculated.
They acknowledged that the patients in their sample had an average age of 81 years, raising questions about how well their data could be applied to a younger population. And they noted that they used point-of-care ultrasound frequently, so they were particularly adept with it. “We use it almost every day in our clinical practice,” said Dr. Barchiesi.
Those factors may have played a key role in the success of point-of-care ultrasound in this study, said Michael Wagner, MD, an assistant professor of medicine at the University of South Carolina, Greenville, who has helped colleagues incorporate ultrasound into their practices.
Elderly patients often present with multiple comorbidities and atypical signs and symptoms, he said. “Sometimes they can be very confusing as to the underlying clinical picture. Ultrasound is being used frequently to better assess these complicated patients.”
Dr. Wagner said extensive training is required to use point-of-care ultrasound accurately.
Dr. Barchiesi also acknowledged that the devices used in this study were large portable machines, not the simpler and less expensive hand-held versions that are also available for similar purposes.
Point-of-care ultrasound is a promising innovation, said Thomas Melgar, MD, a professor of medicine at Western Michigan University, Kalamazoo. “The advantage is that the exam is being done by someone who knows the patient and specifically what they’re looking for. It’s done at the bedside so you don’t have to move the patient.”
The study could help address opposition to internal medicine residents being trained in the technique, he said, adding that “I think it’s very exciting.”
The study was partially supported by Philips, which provided the ultrasound devices. Dr. Barchiesi, Dr. Melgar, and Dr. Wagner disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
Ob.gyns. struggle to keep pace with changing COVID-19 knowledge
In early April, Maura Quinlan, MD, was working nights on the labor and delivery unit at Northwestern Medicine Prentice Women’s Hospital in Chicago. At the time, hospital policy was to test only patients with known COVID-19 symptoms for SARS-CoV-2. Women in labor wore N95 masks, but only while pushing – and practitioners didn’t always don proper protection in time.
Babies came and families rejoiced. But Dr. Quinlan looks back on those weeks with a degree of horror. “We were laboring a bunch of patients that probably had COVID,” she said, and they were doing so without proper protection.
She’s probably right. According to one study in the New England Journal of Medicine, 13.7% of 211 women who came into the labor and delivery unit at one New York City hospital between March 22 and April 2 were asymptomatic but infected, potentially putting staff and doctors at risk.
Dr. Quinlan already knew she and her fellow ob.gyns. had been walking a thin line and, upon seeing that research, her heart sank. In the middle of a pandemic, they had been racing to keep up with the reality of delivering babies. But despite their efforts to protect both practitioners and patients, some aspects slipped through the cracks. Today, every laboring patient admitted to Northwestern is now tested for the novel coronavirus.
Across the country, hospital labor and delivery wards have been working to find a careful and informed balance among multiple competing interests: the safety of their health care workers, the health of tiny and vulnerable new humans, and the stability of a birthing mother. Each hospital has been making the best decisions it can based on available data. The result is a patchwork of policies, but all of them center around rapid testing and appropriate protection.
Shifting recommendations
One case study of women in a New York City hospital during the height of the city’s surge found that, of seven confirmed COVID-19–positive patients, two were asymptomatic upon admission to the obstetrical service, and these same two patients ultimately required unplanned ICU admission. The women’s care prior to their positive diagnosis had exposed multiple health care workers, all of whom lacked appropriate personal protective equipment (PPE), the study authors wrote. “Further, five of seven confirmed COVID-19–positive women were afebrile on initial screen, and four did not first report a cough. In some locations where testing availability remains limited, the minimal symptoms reported for some of these cases might have been insufficient to prompt COVID-19 testing.”
As studies like this pour in, societies continue to update their recommendations accordingly. The latest guidance from the American College of Obstetricians and Gynecologists came on July 1. The group suggests testing all labor and delivery patients, particularly in high-prevalence areas. If tests are in short supply, it recommends prioritizing testing pregnant women with suspected COVID-19 and those who develop symptoms during admission.
At Northwestern, the hospital requests patients stay home and quarantine for the weeks leading up to their delivery date. Then, they rapidly test every patient who comes in for delivery and aim to have results available within a few hours.
The hospital’s 30-room labor and delivery wing remains reserved for patients who test negative. Those with positive COVID-19 results are sent to a 6-bed COVID labor and delivery unit elsewhere in the hospital. “We were lucky we had the space to do that, because smaller community hospitals wouldn’t have a separate unused unit where they could put these women,” Dr. Quinlan said.
In the COVID unit, women deliver without a support person – no partner, doula, or family member can join. Doctors and nurses wear full PPE and work only in that ward. And because some research shows that pregnant women who are asymptomatic or presymptomatic may develop symptoms quickly after starting labor with no measurable illness, Dr. Quinlan must decide on a case-by-case basis what to do, if anything at all.
Delaying an induction could allow the infection to resolve or it could result in her patient moving from presymptomatic disease to full-blown pneumonia. Accelerating labor could bring on symptoms or it could allow a mother to deliver safely and get out of the hospital as quickly as possible. “There is an advantage to having the baby now if you feel okay – even if it’s alone – and getting home,” Dr. Quinlan said.
The hospital also tests the partners of women who are COVID-19 positive. Those with negative results can take the newborn home and try to maintain distance until the mother is no longer symptomatic.
In different parts of the country, hospitals have developed different approaches. Southern California is experiencing its own surge, but at the Ronald Reagan University of California, Los Angeles, Medical Center there still haven’t been enough COVID-19 patients to warrant a separate labor and delivery unit.
At UCLA, staff swab patients when they enter the labor and delivery ward — those who test positive have specific room designations. For both COVID-19–positive patients and women who progress faster than test results can be returned, the goals are the same, said Rashmi Rao, MD, an ob.gyn. at UCLA: Deliver in the safest way possible for both mother and baby.
All women, positive or negative, must wear masks during labor – as much as they can tolerate, at least. For patients who are only mildly ill or asymptomatic, the only difference is that everyone wears protective gear. But if a patient’s oxygen levels dip, or her baby is in distress, the team moves more quickly to a cesarean delivery than they’d do with a healthy patient.
Just as hospital policies have been evolving, rules for visitors have been constantly changing too. Initially, UCLA allowed a support person to be present during delivery but had to leave immediately following. Now, each new mother is allowed one visitor for the duration of their stay. And the hospital suggests that patients who are COVID-19 positive recover in separate rooms from their babies and encourages them to maintain distance from their infants, except when breastfeeding.
“We respect and understand that this is a joyous occasion and we’re trying to keep families together as much as possible,” Dr. Rao said.
Care conundrums
How hospitals protect their smallest charges keeps changing too. Reports have been circulating about newborns being taken away from COVID-19-positive mothers, especially in marginalized communities. The stories have led many to worry they’d be forcibly separated from their babies. Most hospitals, however, leave it up to the woman and her doctors to decide how much separation is needed. “After delivery, it depends on how someone is feeling,” Dr. Rao said.
The American Academy of Pediatrics recommends that mothers who are COVID-19–positive pump breast milk and have a healthy caregiver use that milk, or formula, to bottle-feed the baby, with the new mother remaining 6 feet away from the child as much as she can. If that’s not possible, she should wear gloves and a mask while breastfeeding until she has been naturally afebrile for 72 hours and at least 1 week removed from the first appearance of her symptoms.
“It’s tragically hard,” said Dr. Quinlan, to keep a COVID-19–positive mother even 6 feet away from her newborn baby. “If a mother declines separation, we ask the acting pediatric team to discuss the theoretical risks and paucity of data.”
Until recently, research indicated that SARS-CoV-2 wasn’t being transmitted through the uterus from mothers to their babies. And despite a recent case study reporting transplacental transmission between a mother and her fetus in France, researchers still say that the risk of transference is low. To ensure newborn risk remains as low as possible, UCLA’s policy is to swab the baby when he/she is 24 hours old and keep watch for signs of infection: increased lethargy, difficulty waking, or gastrointestinal symptoms like vomiting.
Transmission via breast milk has also, to date, proven relatively unlikely. One study in The Lancet detected the novel coronavirus in breast milk, although it’s not clear that the virus can be passed on in the fluid, says Christina Chambers, PhD, a professor of pediatrics at the University of California, San Diego. Dr. Chambers is studying breast milk to see if the virus or antibodies to it are present. She is also investigating how infection with SARS-CoV-2 impacts women at different times in pregnancy, something that’s still an open question.
“[In] pregnant women with a deteriorating infection, the decisions are the same you would make with any delivery: Save the mom and save the baby,” Dr. Chambers said. “Beyond that, I am encouraged to see that pregnant women are prioritized to being tested,” something that will help researchers understand prevalence of disease in order to better understand whether some symptoms are more dangerous than others.
The situation is evolving so quickly that hospitals and providers are simply trying to stay abreast of the flood of new research. In the absence of definitive answers, they are using the information available and adjusting on the fly. “We are cautiously waiting for more data,” said Dr. Rao. “With the information we have we are doing the best we can to keep our patients safe. And we’re just going to keep at it.”
A version of this article originally appeared on Medscape.com.
In early April, Maura Quinlan, MD, was working nights on the labor and delivery unit at Northwestern Medicine Prentice Women’s Hospital in Chicago. At the time, hospital policy was to test only patients with known COVID-19 symptoms for SARS-CoV-2. Women in labor wore N95 masks, but only while pushing – and practitioners didn’t always don proper protection in time.
Babies came and families rejoiced. But Dr. Quinlan looks back on those weeks with a degree of horror. “We were laboring a bunch of patients that probably had COVID,” she said, and they were doing so without proper protection.
She’s probably right. According to one study in the New England Journal of Medicine, 13.7% of 211 women who came into the labor and delivery unit at one New York City hospital between March 22 and April 2 were asymptomatic but infected, potentially putting staff and doctors at risk.
Dr. Quinlan already knew she and her fellow ob.gyns. had been walking a thin line and, upon seeing that research, her heart sank. In the middle of a pandemic, they had been racing to keep up with the reality of delivering babies. But despite their efforts to protect both practitioners and patients, some aspects slipped through the cracks. Today, every laboring patient admitted to Northwestern is now tested for the novel coronavirus.
Across the country, hospital labor and delivery wards have been working to find a careful and informed balance among multiple competing interests: the safety of their health care workers, the health of tiny and vulnerable new humans, and the stability of a birthing mother. Each hospital has been making the best decisions it can based on available data. The result is a patchwork of policies, but all of them center around rapid testing and appropriate protection.
Shifting recommendations
One case study of women in a New York City hospital during the height of the city’s surge found that, of seven confirmed COVID-19–positive patients, two were asymptomatic upon admission to the obstetrical service, and these same two patients ultimately required unplanned ICU admission. The women’s care prior to their positive diagnosis had exposed multiple health care workers, all of whom lacked appropriate personal protective equipment (PPE), the study authors wrote. “Further, five of seven confirmed COVID-19–positive women were afebrile on initial screen, and four did not first report a cough. In some locations where testing availability remains limited, the minimal symptoms reported for some of these cases might have been insufficient to prompt COVID-19 testing.”
As studies like this pour in, societies continue to update their recommendations accordingly. The latest guidance from the American College of Obstetricians and Gynecologists came on July 1. The group suggests testing all labor and delivery patients, particularly in high-prevalence areas. If tests are in short supply, it recommends prioritizing testing pregnant women with suspected COVID-19 and those who develop symptoms during admission.
At Northwestern, the hospital requests patients stay home and quarantine for the weeks leading up to their delivery date. Then, they rapidly test every patient who comes in for delivery and aim to have results available within a few hours.
The hospital’s 30-room labor and delivery wing remains reserved for patients who test negative. Those with positive COVID-19 results are sent to a 6-bed COVID labor and delivery unit elsewhere in the hospital. “We were lucky we had the space to do that, because smaller community hospitals wouldn’t have a separate unused unit where they could put these women,” Dr. Quinlan said.
In the COVID unit, women deliver without a support person – no partner, doula, or family member can join. Doctors and nurses wear full PPE and work only in that ward. And because some research shows that pregnant women who are asymptomatic or presymptomatic may develop symptoms quickly after starting labor with no measurable illness, Dr. Quinlan must decide on a case-by-case basis what to do, if anything at all.
Delaying an induction could allow the infection to resolve or it could result in her patient moving from presymptomatic disease to full-blown pneumonia. Accelerating labor could bring on symptoms or it could allow a mother to deliver safely and get out of the hospital as quickly as possible. “There is an advantage to having the baby now if you feel okay – even if it’s alone – and getting home,” Dr. Quinlan said.
The hospital also tests the partners of women who are COVID-19 positive. Those with negative results can take the newborn home and try to maintain distance until the mother is no longer symptomatic.
In different parts of the country, hospitals have developed different approaches. Southern California is experiencing its own surge, but at the Ronald Reagan University of California, Los Angeles, Medical Center there still haven’t been enough COVID-19 patients to warrant a separate labor and delivery unit.
At UCLA, staff swab patients when they enter the labor and delivery ward — those who test positive have specific room designations. For both COVID-19–positive patients and women who progress faster than test results can be returned, the goals are the same, said Rashmi Rao, MD, an ob.gyn. at UCLA: Deliver in the safest way possible for both mother and baby.
All women, positive or negative, must wear masks during labor – as much as they can tolerate, at least. For patients who are only mildly ill or asymptomatic, the only difference is that everyone wears protective gear. But if a patient’s oxygen levels dip, or her baby is in distress, the team moves more quickly to a cesarean delivery than they’d do with a healthy patient.
Just as hospital policies have been evolving, rules for visitors have been constantly changing too. Initially, UCLA allowed a support person to be present during delivery but had to leave immediately following. Now, each new mother is allowed one visitor for the duration of their stay. And the hospital suggests that patients who are COVID-19 positive recover in separate rooms from their babies and encourages them to maintain distance from their infants, except when breastfeeding.
“We respect and understand that this is a joyous occasion and we’re trying to keep families together as much as possible,” Dr. Rao said.
Care conundrums
How hospitals protect their smallest charges keeps changing too. Reports have been circulating about newborns being taken away from COVID-19-positive mothers, especially in marginalized communities. The stories have led many to worry they’d be forcibly separated from their babies. Most hospitals, however, leave it up to the woman and her doctors to decide how much separation is needed. “After delivery, it depends on how someone is feeling,” Dr. Rao said.
The American Academy of Pediatrics recommends that mothers who are COVID-19–positive pump breast milk and have a healthy caregiver use that milk, or formula, to bottle-feed the baby, with the new mother remaining 6 feet away from the child as much as she can. If that’s not possible, she should wear gloves and a mask while breastfeeding until she has been naturally afebrile for 72 hours and at least 1 week removed from the first appearance of her symptoms.
“It’s tragically hard,” said Dr. Quinlan, to keep a COVID-19–positive mother even 6 feet away from her newborn baby. “If a mother declines separation, we ask the acting pediatric team to discuss the theoretical risks and paucity of data.”
Until recently, research indicated that SARS-CoV-2 wasn’t being transmitted through the uterus from mothers to their babies. And despite a recent case study reporting transplacental transmission between a mother and her fetus in France, researchers still say that the risk of transference is low. To ensure newborn risk remains as low as possible, UCLA’s policy is to swab the baby when he/she is 24 hours old and keep watch for signs of infection: increased lethargy, difficulty waking, or gastrointestinal symptoms like vomiting.
Transmission via breast milk has also, to date, proven relatively unlikely. One study in The Lancet detected the novel coronavirus in breast milk, although it’s not clear that the virus can be passed on in the fluid, says Christina Chambers, PhD, a professor of pediatrics at the University of California, San Diego. Dr. Chambers is studying breast milk to see if the virus or antibodies to it are present. She is also investigating how infection with SARS-CoV-2 impacts women at different times in pregnancy, something that’s still an open question.
“[In] pregnant women with a deteriorating infection, the decisions are the same you would make with any delivery: Save the mom and save the baby,” Dr. Chambers said. “Beyond that, I am encouraged to see that pregnant women are prioritized to being tested,” something that will help researchers understand prevalence of disease in order to better understand whether some symptoms are more dangerous than others.
The situation is evolving so quickly that hospitals and providers are simply trying to stay abreast of the flood of new research. In the absence of definitive answers, they are using the information available and adjusting on the fly. “We are cautiously waiting for more data,” said Dr. Rao. “With the information we have we are doing the best we can to keep our patients safe. And we’re just going to keep at it.”
A version of this article originally appeared on Medscape.com.
In early April, Maura Quinlan, MD, was working nights on the labor and delivery unit at Northwestern Medicine Prentice Women’s Hospital in Chicago. At the time, hospital policy was to test only patients with known COVID-19 symptoms for SARS-CoV-2. Women in labor wore N95 masks, but only while pushing – and practitioners didn’t always don proper protection in time.
Babies came and families rejoiced. But Dr. Quinlan looks back on those weeks with a degree of horror. “We were laboring a bunch of patients that probably had COVID,” she said, and they were doing so without proper protection.
She’s probably right. According to one study in the New England Journal of Medicine, 13.7% of 211 women who came into the labor and delivery unit at one New York City hospital between March 22 and April 2 were asymptomatic but infected, potentially putting staff and doctors at risk.
Dr. Quinlan already knew she and her fellow ob.gyns. had been walking a thin line and, upon seeing that research, her heart sank. In the middle of a pandemic, they had been racing to keep up with the reality of delivering babies. But despite their efforts to protect both practitioners and patients, some aspects slipped through the cracks. Today, every laboring patient admitted to Northwestern is now tested for the novel coronavirus.
Across the country, hospital labor and delivery wards have been working to find a careful and informed balance among multiple competing interests: the safety of their health care workers, the health of tiny and vulnerable new humans, and the stability of a birthing mother. Each hospital has been making the best decisions it can based on available data. The result is a patchwork of policies, but all of them center around rapid testing and appropriate protection.
Shifting recommendations
One case study of women in a New York City hospital during the height of the city’s surge found that, of seven confirmed COVID-19–positive patients, two were asymptomatic upon admission to the obstetrical service, and these same two patients ultimately required unplanned ICU admission. The women’s care prior to their positive diagnosis had exposed multiple health care workers, all of whom lacked appropriate personal protective equipment (PPE), the study authors wrote. “Further, five of seven confirmed COVID-19–positive women were afebrile on initial screen, and four did not first report a cough. In some locations where testing availability remains limited, the minimal symptoms reported for some of these cases might have been insufficient to prompt COVID-19 testing.”
As studies like this pour in, societies continue to update their recommendations accordingly. The latest guidance from the American College of Obstetricians and Gynecologists came on July 1. The group suggests testing all labor and delivery patients, particularly in high-prevalence areas. If tests are in short supply, it recommends prioritizing testing pregnant women with suspected COVID-19 and those who develop symptoms during admission.
At Northwestern, the hospital requests patients stay home and quarantine for the weeks leading up to their delivery date. Then, they rapidly test every patient who comes in for delivery and aim to have results available within a few hours.
The hospital’s 30-room labor and delivery wing remains reserved for patients who test negative. Those with positive COVID-19 results are sent to a 6-bed COVID labor and delivery unit elsewhere in the hospital. “We were lucky we had the space to do that, because smaller community hospitals wouldn’t have a separate unused unit where they could put these women,” Dr. Quinlan said.
In the COVID unit, women deliver without a support person – no partner, doula, or family member can join. Doctors and nurses wear full PPE and work only in that ward. And because some research shows that pregnant women who are asymptomatic or presymptomatic may develop symptoms quickly after starting labor with no measurable illness, Dr. Quinlan must decide on a case-by-case basis what to do, if anything at all.
Delaying an induction could allow the infection to resolve or it could result in her patient moving from presymptomatic disease to full-blown pneumonia. Accelerating labor could bring on symptoms or it could allow a mother to deliver safely and get out of the hospital as quickly as possible. “There is an advantage to having the baby now if you feel okay – even if it’s alone – and getting home,” Dr. Quinlan said.
The hospital also tests the partners of women who are COVID-19 positive. Those with negative results can take the newborn home and try to maintain distance until the mother is no longer symptomatic.
In different parts of the country, hospitals have developed different approaches. Southern California is experiencing its own surge, but at the Ronald Reagan University of California, Los Angeles, Medical Center there still haven’t been enough COVID-19 patients to warrant a separate labor and delivery unit.
At UCLA, staff swab patients when they enter the labor and delivery ward — those who test positive have specific room designations. For both COVID-19–positive patients and women who progress faster than test results can be returned, the goals are the same, said Rashmi Rao, MD, an ob.gyn. at UCLA: Deliver in the safest way possible for both mother and baby.
All women, positive or negative, must wear masks during labor – as much as they can tolerate, at least. For patients who are only mildly ill or asymptomatic, the only difference is that everyone wears protective gear. But if a patient’s oxygen levels dip, or her baby is in distress, the team moves more quickly to a cesarean delivery than they’d do with a healthy patient.
Just as hospital policies have been evolving, rules for visitors have been constantly changing too. Initially, UCLA allowed a support person to be present during delivery but had to leave immediately following. Now, each new mother is allowed one visitor for the duration of their stay. And the hospital suggests that patients who are COVID-19 positive recover in separate rooms from their babies and encourages them to maintain distance from their infants, except when breastfeeding.
“We respect and understand that this is a joyous occasion and we’re trying to keep families together as much as possible,” Dr. Rao said.
Care conundrums
How hospitals protect their smallest charges keeps changing too. Reports have been circulating about newborns being taken away from COVID-19-positive mothers, especially in marginalized communities. The stories have led many to worry they’d be forcibly separated from their babies. Most hospitals, however, leave it up to the woman and her doctors to decide how much separation is needed. “After delivery, it depends on how someone is feeling,” Dr. Rao said.
The American Academy of Pediatrics recommends that mothers who are COVID-19–positive pump breast milk and have a healthy caregiver use that milk, or formula, to bottle-feed the baby, with the new mother remaining 6 feet away from the child as much as she can. If that’s not possible, she should wear gloves and a mask while breastfeeding until she has been naturally afebrile for 72 hours and at least 1 week removed from the first appearance of her symptoms.
“It’s tragically hard,” said Dr. Quinlan, to keep a COVID-19–positive mother even 6 feet away from her newborn baby. “If a mother declines separation, we ask the acting pediatric team to discuss the theoretical risks and paucity of data.”
Until recently, research indicated that SARS-CoV-2 wasn’t being transmitted through the uterus from mothers to their babies. And despite a recent case study reporting transplacental transmission between a mother and her fetus in France, researchers still say that the risk of transference is low. To ensure newborn risk remains as low as possible, UCLA’s policy is to swab the baby when he/she is 24 hours old and keep watch for signs of infection: increased lethargy, difficulty waking, or gastrointestinal symptoms like vomiting.
Transmission via breast milk has also, to date, proven relatively unlikely. One study in The Lancet detected the novel coronavirus in breast milk, although it’s not clear that the virus can be passed on in the fluid, says Christina Chambers, PhD, a professor of pediatrics at the University of California, San Diego. Dr. Chambers is studying breast milk to see if the virus or antibodies to it are present. She is also investigating how infection with SARS-CoV-2 impacts women at different times in pregnancy, something that’s still an open question.
“[In] pregnant women with a deteriorating infection, the decisions are the same you would make with any delivery: Save the mom and save the baby,” Dr. Chambers said. “Beyond that, I am encouraged to see that pregnant women are prioritized to being tested,” something that will help researchers understand prevalence of disease in order to better understand whether some symptoms are more dangerous than others.
The situation is evolving so quickly that hospitals and providers are simply trying to stay abreast of the flood of new research. In the absence of definitive answers, they are using the information available and adjusting on the fly. “We are cautiously waiting for more data,” said Dr. Rao. “With the information we have we are doing the best we can to keep our patients safe. And we’re just going to keep at it.”
A version of this article originally appeared on Medscape.com.
Acute EVALI remains a diagnosis of exclusion
according to a synthesis of current information presented at the virtual Pediatric Hospital Medicine.
Respiratory symptoms, including cough, chest pain, and shortness of breath are common but so are constitutive symptoms, including fever, sore throat, muscle aches, nausea and vomiting, said Yamini Kuchipudi, MD, a staff physician at Cincinnati Children’s Hospital, during the session at the virtual meeting, sponsored by the Society of Hospital Medicine, the American Academy of Pediatrics, and the Academic Pediatric Association.
If EVALI is not considered across this broad array of symptoms, of which respiratory complaints might not be the most prominent at the time of presentation, the diagnosis might be delayed, Dr. Kuchipudi warned during the virtual meeting.
Teenagers and young adults are the most common users of e-cigarettes and vaping devices. In these patients or in any individual suspected of having EVALI, Dr. Kuchipudi recommended posing questions about vaping relatively early in the work-up “in a confidential and nonjudgmental way.”
Eliciting a truthful history will be particularly important, because the risk of EVALI appears to be largely related to vaping with tetrahydrocannabinol (THC)-containing products rather than with nicotine alone. Although the exact cause of EVALI is not yet completely clear, this condition is now strongly associated with additives to the THC, according to Issa Hanna, MD, of the department of pediatrics at the University of Florida, Jacksonville.
“E-liquid contains products like hydrocarbons, vitamin E acetate, and heavy metals that appear to damage the alveolar epithelium by direct cellular inflammation,” Dr. Hanna explained.
These products are not only found in THC processed for vaping but also for dabbing, a related but different form of inhalation that involves vaporization of highly concentrated THC waxes or resins. Dr. Hanna suggested that the decline in reported cases of EVALI, which has followed the peak incidence in September 2019, is likely to be related to a decline in THC additives as well as greater caution among users.
E-cigarettes were introduced in 2007, according to Dr. Hanna, but EVALI was not widely recognized until cases began accruing early in 2019. By June 2019, the growing number of case reports had attracted the attention of the media as well as public health officials, intensifying the effort to isolate the risks and causes.
Consistent with greater use of e-cigarettes and vaping among younger individuals, nearly 80% of the 2,807 patients hospitalized for EVALI in the United States by February of this year occurred in individuals aged less than 35 years, according to data released by the Centers for Disease Control and Prevention. The median age was less than 25 years. Of these hospitalizations, 68 deaths (2.5%) in 29 states and Washington, D.C., were attributed to EVALI.
Because of the nonspecific symptoms and lack of a definitive diagnostic test, EVALI is considered a diagnosis of exclusion, according to Abigail Musial, MD, who is completing a fellowship in hospital medicine at Cincinnati Children’s. She presented a case in which a patient suspected of EVALI went home after symptoms abated on steroids.
“Less than 24 hours later, she returned to the ED with tachypnea and hypoxemia,” Dr. Musial recounted. Although a chest x-ray at the initial evaluation showed lung opacities, a repeat chest x-ray when she returned to the ED showed bilateral worsening of these opacities and persistent elevation of inflammatory markers.
“She was started on steroids and also on antibiotics,” Dr. Musial said. “She was weaned quickly from oxygen once the steroids were started and was discharged on hospital day 3.”
For patients suspected of EVALI, COVID-19 testing should be part of the work-up, according to Dr. Kuchipudi. She also recommended an x-ray or CT scan of the lung as well as an evaluation of inflammatory markers.
Dr. Kuchipudi said that more invasive studies than lung function tests, such as bronchoalveolar lavage or lung biopsy, might be considered when severe symptoms make aggressive diagnostic studies attractive.
Steroids and antibiotics typically lead to control of acute symptoms, but patients should be clinically stable for 24-48 hours prior to hospital discharge, according to Dr. Kuchipudi. Follow-up after discharge should include lung function tests and imaging 2-4 weeks later to confirm resolution of abnormalities.
Dr. Kuchipudi stressed the opportunity that an episode of EVALI provides to induce patients to give up nicotine and vaping entirely. Such strategies, such as a nicotine patch, deserve consideration, but she also cautioned that e-cigarettes for smoking cessation should not be recommended to EVALI patients.
The speakers reported no potential conflicts of interest relevant to this study.
according to a synthesis of current information presented at the virtual Pediatric Hospital Medicine.
Respiratory symptoms, including cough, chest pain, and shortness of breath are common but so are constitutive symptoms, including fever, sore throat, muscle aches, nausea and vomiting, said Yamini Kuchipudi, MD, a staff physician at Cincinnati Children’s Hospital, during the session at the virtual meeting, sponsored by the Society of Hospital Medicine, the American Academy of Pediatrics, and the Academic Pediatric Association.
If EVALI is not considered across this broad array of symptoms, of which respiratory complaints might not be the most prominent at the time of presentation, the diagnosis might be delayed, Dr. Kuchipudi warned during the virtual meeting.
Teenagers and young adults are the most common users of e-cigarettes and vaping devices. In these patients or in any individual suspected of having EVALI, Dr. Kuchipudi recommended posing questions about vaping relatively early in the work-up “in a confidential and nonjudgmental way.”
Eliciting a truthful history will be particularly important, because the risk of EVALI appears to be largely related to vaping with tetrahydrocannabinol (THC)-containing products rather than with nicotine alone. Although the exact cause of EVALI is not yet completely clear, this condition is now strongly associated with additives to the THC, according to Issa Hanna, MD, of the department of pediatrics at the University of Florida, Jacksonville.
“E-liquid contains products like hydrocarbons, vitamin E acetate, and heavy metals that appear to damage the alveolar epithelium by direct cellular inflammation,” Dr. Hanna explained.
These products are not only found in THC processed for vaping but also for dabbing, a related but different form of inhalation that involves vaporization of highly concentrated THC waxes or resins. Dr. Hanna suggested that the decline in reported cases of EVALI, which has followed the peak incidence in September 2019, is likely to be related to a decline in THC additives as well as greater caution among users.
E-cigarettes were introduced in 2007, according to Dr. Hanna, but EVALI was not widely recognized until cases began accruing early in 2019. By June 2019, the growing number of case reports had attracted the attention of the media as well as public health officials, intensifying the effort to isolate the risks and causes.
Consistent with greater use of e-cigarettes and vaping among younger individuals, nearly 80% of the 2,807 patients hospitalized for EVALI in the United States by February of this year occurred in individuals aged less than 35 years, according to data released by the Centers for Disease Control and Prevention. The median age was less than 25 years. Of these hospitalizations, 68 deaths (2.5%) in 29 states and Washington, D.C., were attributed to EVALI.
Because of the nonspecific symptoms and lack of a definitive diagnostic test, EVALI is considered a diagnosis of exclusion, according to Abigail Musial, MD, who is completing a fellowship in hospital medicine at Cincinnati Children’s. She presented a case in which a patient suspected of EVALI went home after symptoms abated on steroids.
“Less than 24 hours later, she returned to the ED with tachypnea and hypoxemia,” Dr. Musial recounted. Although a chest x-ray at the initial evaluation showed lung opacities, a repeat chest x-ray when she returned to the ED showed bilateral worsening of these opacities and persistent elevation of inflammatory markers.
“She was started on steroids and also on antibiotics,” Dr. Musial said. “She was weaned quickly from oxygen once the steroids were started and was discharged on hospital day 3.”
For patients suspected of EVALI, COVID-19 testing should be part of the work-up, according to Dr. Kuchipudi. She also recommended an x-ray or CT scan of the lung as well as an evaluation of inflammatory markers.
Dr. Kuchipudi said that more invasive studies than lung function tests, such as bronchoalveolar lavage or lung biopsy, might be considered when severe symptoms make aggressive diagnostic studies attractive.
Steroids and antibiotics typically lead to control of acute symptoms, but patients should be clinically stable for 24-48 hours prior to hospital discharge, according to Dr. Kuchipudi. Follow-up after discharge should include lung function tests and imaging 2-4 weeks later to confirm resolution of abnormalities.
Dr. Kuchipudi stressed the opportunity that an episode of EVALI provides to induce patients to give up nicotine and vaping entirely. Such strategies, such as a nicotine patch, deserve consideration, but she also cautioned that e-cigarettes for smoking cessation should not be recommended to EVALI patients.
The speakers reported no potential conflicts of interest relevant to this study.
according to a synthesis of current information presented at the virtual Pediatric Hospital Medicine.
Respiratory symptoms, including cough, chest pain, and shortness of breath are common but so are constitutive symptoms, including fever, sore throat, muscle aches, nausea and vomiting, said Yamini Kuchipudi, MD, a staff physician at Cincinnati Children’s Hospital, during the session at the virtual meeting, sponsored by the Society of Hospital Medicine, the American Academy of Pediatrics, and the Academic Pediatric Association.
If EVALI is not considered across this broad array of symptoms, of which respiratory complaints might not be the most prominent at the time of presentation, the diagnosis might be delayed, Dr. Kuchipudi warned during the virtual meeting.
Teenagers and young adults are the most common users of e-cigarettes and vaping devices. In these patients or in any individual suspected of having EVALI, Dr. Kuchipudi recommended posing questions about vaping relatively early in the work-up “in a confidential and nonjudgmental way.”
Eliciting a truthful history will be particularly important, because the risk of EVALI appears to be largely related to vaping with tetrahydrocannabinol (THC)-containing products rather than with nicotine alone. Although the exact cause of EVALI is not yet completely clear, this condition is now strongly associated with additives to the THC, according to Issa Hanna, MD, of the department of pediatrics at the University of Florida, Jacksonville.
“E-liquid contains products like hydrocarbons, vitamin E acetate, and heavy metals that appear to damage the alveolar epithelium by direct cellular inflammation,” Dr. Hanna explained.
These products are not only found in THC processed for vaping but also for dabbing, a related but different form of inhalation that involves vaporization of highly concentrated THC waxes or resins. Dr. Hanna suggested that the decline in reported cases of EVALI, which has followed the peak incidence in September 2019, is likely to be related to a decline in THC additives as well as greater caution among users.
E-cigarettes were introduced in 2007, according to Dr. Hanna, but EVALI was not widely recognized until cases began accruing early in 2019. By June 2019, the growing number of case reports had attracted the attention of the media as well as public health officials, intensifying the effort to isolate the risks and causes.
Consistent with greater use of e-cigarettes and vaping among younger individuals, nearly 80% of the 2,807 patients hospitalized for EVALI in the United States by February of this year occurred in individuals aged less than 35 years, according to data released by the Centers for Disease Control and Prevention. The median age was less than 25 years. Of these hospitalizations, 68 deaths (2.5%) in 29 states and Washington, D.C., were attributed to EVALI.
Because of the nonspecific symptoms and lack of a definitive diagnostic test, EVALI is considered a diagnosis of exclusion, according to Abigail Musial, MD, who is completing a fellowship in hospital medicine at Cincinnati Children’s. She presented a case in which a patient suspected of EVALI went home after symptoms abated on steroids.
“Less than 24 hours later, she returned to the ED with tachypnea and hypoxemia,” Dr. Musial recounted. Although a chest x-ray at the initial evaluation showed lung opacities, a repeat chest x-ray when she returned to the ED showed bilateral worsening of these opacities and persistent elevation of inflammatory markers.
“She was started on steroids and also on antibiotics,” Dr. Musial said. “She was weaned quickly from oxygen once the steroids were started and was discharged on hospital day 3.”
For patients suspected of EVALI, COVID-19 testing should be part of the work-up, according to Dr. Kuchipudi. She also recommended an x-ray or CT scan of the lung as well as an evaluation of inflammatory markers.
Dr. Kuchipudi said that more invasive studies than lung function tests, such as bronchoalveolar lavage or lung biopsy, might be considered when severe symptoms make aggressive diagnostic studies attractive.
Steroids and antibiotics typically lead to control of acute symptoms, but patients should be clinically stable for 24-48 hours prior to hospital discharge, according to Dr. Kuchipudi. Follow-up after discharge should include lung function tests and imaging 2-4 weeks later to confirm resolution of abnormalities.
Dr. Kuchipudi stressed the opportunity that an episode of EVALI provides to induce patients to give up nicotine and vaping entirely. Such strategies, such as a nicotine patch, deserve consideration, but she also cautioned that e-cigarettes for smoking cessation should not be recommended to EVALI patients.
The speakers reported no potential conflicts of interest relevant to this study.
FROM PHM20
Behind the mask
Bicycling has always been part of who I am because it offered me the freedom to explore as a preteen. As an adult I have always been a bicycle commuter and a very visible part of the community as I pedal around town to do my errands. But, I didn’t always wear a helmet ... because well, I just didn’t. I saw the helmet as a nuisance with very little benefit to myself. Eventually, when bike races required helmets I bought one just for the competitions. Until one day about 30 years ago when the mother of a child I was seeing in the office said, “Dr. Wilkoff, you know as an influential member of this community, particularly its children, you should be wearing a helmet.” My wife had been badgering me for years but this woman’s courage to speak up embarrassed me into changing my ways.
For some, maybe many, people, wearing a mask during the COVID-19 pandemic is a nuisance and an assault on their independence just as I viewed a bicycle helmet. Initially there was some information being circulated that any mask less robust than a N-95 had very little if any effect, either as protection or as way to decrease spread. I certainly had my doubts about the value of mask other than as a statement of solidarity. However, we are now learning that masks can serve an important role along with social distancing in a comprehensive community effort to minimize contagion.
In light of this new information, why are there are still people who won’t wear a mask? It may be that they are receiving their news filtered through a lens that discredits science. But, it is more likely the result of the same mindset that permeates the anti-vaccine faction that the common good is less important than personal freedom to follow their beliefs.
Do we have any tools at our disposal to increase the number of folks wearing masks? Based on our experience with attempts to convince those who are anti-vaccine, education will be ineffective in shifting the focus from personal freedom to a commitment to the welfare of the community at large. Shaming might be effective, but it runs the risk of igniting conflicts and further widening the gaps in our society. Some establishments have been effective in simply saying “no mask, no entry,” but this runs the same risk of creating friction depending on the community and the situation.
The ship may have already sailed on our best opportunity to achieve community compliance when the leaders of our national government have chosen to ignore their obligation to set an example by refusing to wear masks. I fear that the wedge has already been set and the widening of the gap between those who see their responsibility to the community at large and those who do not will continue to grow.
I am fortunate to live in a town whose residents look out for each other and have relied on local leaders to set an example in the absence of leadership on a national level.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Email him at [email protected].
Bicycling has always been part of who I am because it offered me the freedom to explore as a preteen. As an adult I have always been a bicycle commuter and a very visible part of the community as I pedal around town to do my errands. But, I didn’t always wear a helmet ... because well, I just didn’t. I saw the helmet as a nuisance with very little benefit to myself. Eventually, when bike races required helmets I bought one just for the competitions. Until one day about 30 years ago when the mother of a child I was seeing in the office said, “Dr. Wilkoff, you know as an influential member of this community, particularly its children, you should be wearing a helmet.” My wife had been badgering me for years but this woman’s courage to speak up embarrassed me into changing my ways.
For some, maybe many, people, wearing a mask during the COVID-19 pandemic is a nuisance and an assault on their independence just as I viewed a bicycle helmet. Initially there was some information being circulated that any mask less robust than a N-95 had very little if any effect, either as protection or as way to decrease spread. I certainly had my doubts about the value of mask other than as a statement of solidarity. However, we are now learning that masks can serve an important role along with social distancing in a comprehensive community effort to minimize contagion.
In light of this new information, why are there are still people who won’t wear a mask? It may be that they are receiving their news filtered through a lens that discredits science. But, it is more likely the result of the same mindset that permeates the anti-vaccine faction that the common good is less important than personal freedom to follow their beliefs.
Do we have any tools at our disposal to increase the number of folks wearing masks? Based on our experience with attempts to convince those who are anti-vaccine, education will be ineffective in shifting the focus from personal freedom to a commitment to the welfare of the community at large. Shaming might be effective, but it runs the risk of igniting conflicts and further widening the gaps in our society. Some establishments have been effective in simply saying “no mask, no entry,” but this runs the same risk of creating friction depending on the community and the situation.
The ship may have already sailed on our best opportunity to achieve community compliance when the leaders of our national government have chosen to ignore their obligation to set an example by refusing to wear masks. I fear that the wedge has already been set and the widening of the gap between those who see their responsibility to the community at large and those who do not will continue to grow.
I am fortunate to live in a town whose residents look out for each other and have relied on local leaders to set an example in the absence of leadership on a national level.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Email him at [email protected].
Bicycling has always been part of who I am because it offered me the freedom to explore as a preteen. As an adult I have always been a bicycle commuter and a very visible part of the community as I pedal around town to do my errands. But, I didn’t always wear a helmet ... because well, I just didn’t. I saw the helmet as a nuisance with very little benefit to myself. Eventually, when bike races required helmets I bought one just for the competitions. Until one day about 30 years ago when the mother of a child I was seeing in the office said, “Dr. Wilkoff, you know as an influential member of this community, particularly its children, you should be wearing a helmet.” My wife had been badgering me for years but this woman’s courage to speak up embarrassed me into changing my ways.
For some, maybe many, people, wearing a mask during the COVID-19 pandemic is a nuisance and an assault on their independence just as I viewed a bicycle helmet. Initially there was some information being circulated that any mask less robust than a N-95 had very little if any effect, either as protection or as way to decrease spread. I certainly had my doubts about the value of mask other than as a statement of solidarity. However, we are now learning that masks can serve an important role along with social distancing in a comprehensive community effort to minimize contagion.
In light of this new information, why are there are still people who won’t wear a mask? It may be that they are receiving their news filtered through a lens that discredits science. But, it is more likely the result of the same mindset that permeates the anti-vaccine faction that the common good is less important than personal freedom to follow their beliefs.
Do we have any tools at our disposal to increase the number of folks wearing masks? Based on our experience with attempts to convince those who are anti-vaccine, education will be ineffective in shifting the focus from personal freedom to a commitment to the welfare of the community at large. Shaming might be effective, but it runs the risk of igniting conflicts and further widening the gaps in our society. Some establishments have been effective in simply saying “no mask, no entry,” but this runs the same risk of creating friction depending on the community and the situation.
The ship may have already sailed on our best opportunity to achieve community compliance when the leaders of our national government have chosen to ignore their obligation to set an example by refusing to wear masks. I fear that the wedge has already been set and the widening of the gap between those who see their responsibility to the community at large and those who do not will continue to grow.
I am fortunate to live in a town whose residents look out for each other and have relied on local leaders to set an example in the absence of leadership on a national level.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Email him at [email protected].
Quitting smoking after MI has huge benefits in young adults
Young adult smokers who stop smoking in the first year after an initial myocardial infarction are far less likely to die over the next 10 years than their peers who continue to smoke. Yet nearly two-thirds keep smoking after the event, according to new data from the Partners YOUNG-MI Registry.
“Smoking is one of the most common risk factors for developing an MI at a young age. ... This reinforces the need to have more young individuals avoid, or quit, the use of tobacco,” Ron Blankstein, MD, Brigham and Women’s Hospital and Harvard Medical School, Boston, said in an interview.
Yet, the finding that 62% of young adults continue to smoke 1 year after MI points to an “enormous need for better smoking cessation efforts following a heart attack,” he said.
“Powerful” message for clinicians
“This study joins an incredibly powerful body of evidence that says if you quit smoking, you’re going to live longer,” said Michael Fiore, MD, MPH, MBA, director of the University of Wisconsin Center for Tobacco Research and Intervention, Madison, who wasn’t involved in the study.
“As physicians, there is nothing we can do that will have a greater impact for our patients than quitting smoking. The study is a powerful call for clinicians to intervene with their patients that smoke – both if you have an MI or if you don’t,” Dr. Fiore told this news organization.
The study involved 2,072 individuals 50 years or younger (median age, 45 years; 81% male) who were hospitalized for an initial MI at two large academic medical centers in Boston. Of these, 33.9% were never-smokers, 13.6% were former smokers, and 52.5% were smokers at the time of their MI.
During a median follow-up of 10.2 years, those who quit smoking had a significantly lower rate of death from any cause (unadjusted hazard ratio, 0.35; 95% confidence interval, 0.19-0.63; P < .001) and a cardiovascular cause (HR, 0.29; 95% CI, 0.11-0.79; P = .02), relative to those who continued to smoke.
The results remained statistically significant in a propensity-matched analysis for both all-cause (HR, 0.30; 95% CI, 0.16-0.56; P < .001) and CV mortality (HR, 0.19; 95% CI, 0.06-0.56; P = .003).
“Although patients who quit smoking were similar to those who continued to smoke with respect to their baseline characteristics, smoking cessation was associated with an approximate 70%-80% reduction in all-cause and CV mortality,” the authors note in their article, published online July 8 in JAMA Network Open.
They say it’s also noteworthy that long-term death rates of never-smokers and former smokers who quit before the MI were nearly identical.
‘A failure of our health care system’
The bottom line, said Dr. Blankstein, is that it is “never too late to quit, and those who experience an MI should do so right away. Our health care system must help promote such efforts, as there is immense room for improvement.”
Dr. Fiore said: “When I see an article like this, it just reminds me that, if you’re really thinking about staying healthy, there is nothing better you can do to improve the quality and longevity of your life than quitting smoking.”
The observation that many patients continue to smoke after MI is a “failure of our health care system, and it’s an individual failure in that these individuals are not able to overcome their powerful nicotine dependence. It’s an unfortunate occurrence that’s resulting in unnecessary deaths,” said Dr. Fiore.
There is no “magic bullet” to overcome nicotine addiction, but there are approved treatments that can “substantially boost quit rates,” he noted.
The two most effective smoking-cessation treatments are varenicline (Chantix) and combination nicotine replacement therapy, a patch combined ideally with nicotine mini lozenges, particularly when combined with some brief counseling, said Fiore.
He encourages cardiologists to get their patients to commit to quitting and then link them to resources such as 1-800-QUIT-NOW or SmokeFree.gov.
Funding for the study was provided by grants from the National Heart, Lung, and Blood Institute. Dr. Blankstein reported receiving research support from Amgen and Astellas. Dr. Fiore had no relevant disclosures.
A version of this article originally appeared on Medscape.com.
Young adult smokers who stop smoking in the first year after an initial myocardial infarction are far less likely to die over the next 10 years than their peers who continue to smoke. Yet nearly two-thirds keep smoking after the event, according to new data from the Partners YOUNG-MI Registry.
“Smoking is one of the most common risk factors for developing an MI at a young age. ... This reinforces the need to have more young individuals avoid, or quit, the use of tobacco,” Ron Blankstein, MD, Brigham and Women’s Hospital and Harvard Medical School, Boston, said in an interview.
Yet, the finding that 62% of young adults continue to smoke 1 year after MI points to an “enormous need for better smoking cessation efforts following a heart attack,” he said.
“Powerful” message for clinicians
“This study joins an incredibly powerful body of evidence that says if you quit smoking, you’re going to live longer,” said Michael Fiore, MD, MPH, MBA, director of the University of Wisconsin Center for Tobacco Research and Intervention, Madison, who wasn’t involved in the study.
“As physicians, there is nothing we can do that will have a greater impact for our patients than quitting smoking. The study is a powerful call for clinicians to intervene with their patients that smoke – both if you have an MI or if you don’t,” Dr. Fiore told this news organization.
The study involved 2,072 individuals 50 years or younger (median age, 45 years; 81% male) who were hospitalized for an initial MI at two large academic medical centers in Boston. Of these, 33.9% were never-smokers, 13.6% were former smokers, and 52.5% were smokers at the time of their MI.
During a median follow-up of 10.2 years, those who quit smoking had a significantly lower rate of death from any cause (unadjusted hazard ratio, 0.35; 95% confidence interval, 0.19-0.63; P < .001) and a cardiovascular cause (HR, 0.29; 95% CI, 0.11-0.79; P = .02), relative to those who continued to smoke.
The results remained statistically significant in a propensity-matched analysis for both all-cause (HR, 0.30; 95% CI, 0.16-0.56; P < .001) and CV mortality (HR, 0.19; 95% CI, 0.06-0.56; P = .003).
“Although patients who quit smoking were similar to those who continued to smoke with respect to their baseline characteristics, smoking cessation was associated with an approximate 70%-80% reduction in all-cause and CV mortality,” the authors note in their article, published online July 8 in JAMA Network Open.
They say it’s also noteworthy that long-term death rates of never-smokers and former smokers who quit before the MI were nearly identical.
‘A failure of our health care system’
The bottom line, said Dr. Blankstein, is that it is “never too late to quit, and those who experience an MI should do so right away. Our health care system must help promote such efforts, as there is immense room for improvement.”
Dr. Fiore said: “When I see an article like this, it just reminds me that, if you’re really thinking about staying healthy, there is nothing better you can do to improve the quality and longevity of your life than quitting smoking.”
The observation that many patients continue to smoke after MI is a “failure of our health care system, and it’s an individual failure in that these individuals are not able to overcome their powerful nicotine dependence. It’s an unfortunate occurrence that’s resulting in unnecessary deaths,” said Dr. Fiore.
There is no “magic bullet” to overcome nicotine addiction, but there are approved treatments that can “substantially boost quit rates,” he noted.
The two most effective smoking-cessation treatments are varenicline (Chantix) and combination nicotine replacement therapy, a patch combined ideally with nicotine mini lozenges, particularly when combined with some brief counseling, said Fiore.
He encourages cardiologists to get their patients to commit to quitting and then link them to resources such as 1-800-QUIT-NOW or SmokeFree.gov.
Funding for the study was provided by grants from the National Heart, Lung, and Blood Institute. Dr. Blankstein reported receiving research support from Amgen and Astellas. Dr. Fiore had no relevant disclosures.
A version of this article originally appeared on Medscape.com.
Young adult smokers who stop smoking in the first year after an initial myocardial infarction are far less likely to die over the next 10 years than their peers who continue to smoke. Yet nearly two-thirds keep smoking after the event, according to new data from the Partners YOUNG-MI Registry.
“Smoking is one of the most common risk factors for developing an MI at a young age. ... This reinforces the need to have more young individuals avoid, or quit, the use of tobacco,” Ron Blankstein, MD, Brigham and Women’s Hospital and Harvard Medical School, Boston, said in an interview.
Yet, the finding that 62% of young adults continue to smoke 1 year after MI points to an “enormous need for better smoking cessation efforts following a heart attack,” he said.
“Powerful” message for clinicians
“This study joins an incredibly powerful body of evidence that says if you quit smoking, you’re going to live longer,” said Michael Fiore, MD, MPH, MBA, director of the University of Wisconsin Center for Tobacco Research and Intervention, Madison, who wasn’t involved in the study.
“As physicians, there is nothing we can do that will have a greater impact for our patients than quitting smoking. The study is a powerful call for clinicians to intervene with their patients that smoke – both if you have an MI or if you don’t,” Dr. Fiore told this news organization.
The study involved 2,072 individuals 50 years or younger (median age, 45 years; 81% male) who were hospitalized for an initial MI at two large academic medical centers in Boston. Of these, 33.9% were never-smokers, 13.6% were former smokers, and 52.5% were smokers at the time of their MI.
During a median follow-up of 10.2 years, those who quit smoking had a significantly lower rate of death from any cause (unadjusted hazard ratio, 0.35; 95% confidence interval, 0.19-0.63; P < .001) and a cardiovascular cause (HR, 0.29; 95% CI, 0.11-0.79; P = .02), relative to those who continued to smoke.
The results remained statistically significant in a propensity-matched analysis for both all-cause (HR, 0.30; 95% CI, 0.16-0.56; P < .001) and CV mortality (HR, 0.19; 95% CI, 0.06-0.56; P = .003).
“Although patients who quit smoking were similar to those who continued to smoke with respect to their baseline characteristics, smoking cessation was associated with an approximate 70%-80% reduction in all-cause and CV mortality,” the authors note in their article, published online July 8 in JAMA Network Open.
They say it’s also noteworthy that long-term death rates of never-smokers and former smokers who quit before the MI were nearly identical.
‘A failure of our health care system’
The bottom line, said Dr. Blankstein, is that it is “never too late to quit, and those who experience an MI should do so right away. Our health care system must help promote such efforts, as there is immense room for improvement.”
Dr. Fiore said: “When I see an article like this, it just reminds me that, if you’re really thinking about staying healthy, there is nothing better you can do to improve the quality and longevity of your life than quitting smoking.”
The observation that many patients continue to smoke after MI is a “failure of our health care system, and it’s an individual failure in that these individuals are not able to overcome their powerful nicotine dependence. It’s an unfortunate occurrence that’s resulting in unnecessary deaths,” said Dr. Fiore.
There is no “magic bullet” to overcome nicotine addiction, but there are approved treatments that can “substantially boost quit rates,” he noted.
The two most effective smoking-cessation treatments are varenicline (Chantix) and combination nicotine replacement therapy, a patch combined ideally with nicotine mini lozenges, particularly when combined with some brief counseling, said Fiore.
He encourages cardiologists to get their patients to commit to quitting and then link them to resources such as 1-800-QUIT-NOW or SmokeFree.gov.
Funding for the study was provided by grants from the National Heart, Lung, and Blood Institute. Dr. Blankstein reported receiving research support from Amgen and Astellas. Dr. Fiore had no relevant disclosures.
A version of this article originally appeared on Medscape.com.
Socioeconomic status key factor in CPAP adherence in older adults
The benefits of continuous positive airway pressure therapy for patients with obstructive sleep apnea are well documented, but it only works if patients can adhere to the therapy.
A large national study of older Medicare patients with obstructive sleep apnea (OSA) has identified lower socioeconomic status and comorbidities as independent risk factors for nonadherence to continuous positive airway pressure (CPAP) therapy.
“[The] present results represent the largest study to date of rates and predictors of CPAP adherence among older adults in the United States. In our national sample of Medicare beneficiaries, adherence rates were generally lower than previously reported in smaller, clinic-based studies,” Emerson M. Wickwire, PhD, of the Sleep Disorders Center and division of pulmonary and critical care medicine at the University of Maryland, Baltimore, and colleagues wrote in Sleep.
Dr. Wickwire and colleagues estimated CPAP machine adherence using a 5% sample of Medicare claims data, identifying 3,229 Medicare beneficiaries with OSA who began CPAP therapy between 2009 and 2011. Individuals in the sample were aged at least 65 years with a new diagnosis of OSA, 88.1% of beneficiaries were white, and 52.3% were male.
The researchers applied objective adherence criteria set by the Centers for Medicare & Medicaid Services, which defines CPAP adherence as a patient using CPAP for at least 4 hours on 70% of nights, or CPAP use for 21 of 30 consecutive days within 90 days after beginning therapy.
Using CPAP machine charges as a measure of who adhered to therapy, they found 1,420 of 3,229 individuals (44%) achieved adherence under these criteria, which included making 13 monthly payments during their CPAP machine’s “rent-to-own” period. Partial adherence was found in 997 individuals (30.9%) who made between 4-12 payments on their CPAP machine, while 812 individuals (25.2%) made 4 payments or fewer on their CPAP machines, which the researchers classified as nonadherence. Nonadherers tended to be slightly younger (mean, 72.5 years vs. 79.2 years; P < .001) and had a higher number of comorbidities (35.2% vs. 30.4%; P = .002), compared with individuals with high adherence. Anxiety (odds ratio, 1.34; 95% confidence interval, 1.12-1.61), anemia (OR, 1.16; 95% CI, 1.02-1.32), fibromyalgia (OR, 1.19; 95% CI, 1.03-1.38), traumatic brain injury (OR, 1.58; 95% CI, 1.21-2.07), and Medicaid eligibility (OR, 1.48; 95% CI, 1.24-1.75) were all independently associated with lower CPAP adherence. Medicaid eligibility was considered an indicator of lower socioeconomic status.
Krishna M. Sundar, MD, FCCP, director at the Sleep-Wake Center in the University of Utah pulmonary division in Salt Lake City and CHEST Physician editorial board member, said in an interview that studies have shown early signs of adherence within the first few weeks are an important indicator of overall adherence to CPAP therapy. However, the use of CPAP machine payments in the study by Dr. Wickwire and colleagues was a novel way to track adherence.
Some of the issues with nonadherence may be related to challenges in using the technology, but it is the clinician’s role to communicate with patients about the effectiveness of CPAP and identifying reasons for nonadherence while also attempting to tease out the subtle socioeconomic factors related to nonadherence, Dr. Sundar noted. “We need to alter our practice to make sure that we communicate with these patients and better understand what are the social factors in getting the CPAP or utilizing CPAP, and also following these patients more closely, especially in the first month of starting CPAP therapy.
“Just because somebody has severe sleep apnea and other comorbid conditions does not mean that they’re going to wear the CPAP,” he said. “So, the fact that socioeconomic factors play an equal if not more important role in terms of predicting CPAP adherence. That is an important takeaway.”
Octavian C. Ioachimescu, MD, FCCP, of Emory University, Atlanta, and the Atlanta Veteran Affairs Administration and CHEST Physician editorial board member, said in an interview that the study raises a major question of what is next. “What can we offer to these patients, and what is the real-world compliance to that ‘next-best’ modality?” Dr. Ioachimescu said. “What are the outcomes of these individuals in the point-of-care environment, or ‘real world?’ ”
The analysis by the authors adds the perspective of a “real-world depiction of clinical care for patients with OSA,” Dr. Ioachimescu said. “One major lesson of such an analysis is that the health care goal setting that is referential to initial, randomized, well-controlled studies on highly selected patient populations need to be reassessed periodically from the point of view of actual results in the clinics.”
Clinicians may need to borrow ideas from other therapeutic fields to help improve patient adherence, he said. “[W]e may be able to develop and implement in the future peer involvement, behavioral and cognitive approaches, motivational enhancement interventions, as well as elements of acceptance and commitment techniques, all in the larger context of more integrated and in the same time individualized approaches to therapy.”
The investigators concluded that, “relative to Medicare-only beneficiaries, those eligible for both Medicare and Medicaid were significantly less likely to adhere to CPAP. Future research should seek to develop a deeper understanding of the mechanisms through which [socioeconomic status] and other social determinants impact patient experience throughout the OSA diagnostic and treatment process, including receiving, acclimating, and adhering to CPAP therapy.”
Dr. Sundar concurred with this assessment and said more research is needed on factors impacting adherence such as poverty, homelessness, and home support systems. “It’s not just coordinating with the patient. Clearly, more work is needed in understanding the social aspects of CPAP adherence.”
This study was funded in part by an investigator-initiated grant provided by ResMed to Dr. Wickmire’s institution, the University of Maryland, Baltimore. Dr. Wickmire reported being a scientific consultant to DayZz, Eisai, Merck, and Purdue and holds shares in WellTap. Dr. Oldstone is a ResMed employee and shareholder. Dr. Sundar reported being a cofounder of Hypnoscure, which creates software for population management of sleep apnea, and an investigator in trials where ResMed and Respironics devices were used. Dr. Ioachimescu reported no relevant financial disclosures.
SOURCE: Wickwire EM et al. Sleep. 2020 Jun 23. doi: 10.1093/sleep/zsaa122.
The benefits of continuous positive airway pressure therapy for patients with obstructive sleep apnea are well documented, but it only works if patients can adhere to the therapy.
A large national study of older Medicare patients with obstructive sleep apnea (OSA) has identified lower socioeconomic status and comorbidities as independent risk factors for nonadherence to continuous positive airway pressure (CPAP) therapy.
“[The] present results represent the largest study to date of rates and predictors of CPAP adherence among older adults in the United States. In our national sample of Medicare beneficiaries, adherence rates were generally lower than previously reported in smaller, clinic-based studies,” Emerson M. Wickwire, PhD, of the Sleep Disorders Center and division of pulmonary and critical care medicine at the University of Maryland, Baltimore, and colleagues wrote in Sleep.
Dr. Wickwire and colleagues estimated CPAP machine adherence using a 5% sample of Medicare claims data, identifying 3,229 Medicare beneficiaries with OSA who began CPAP therapy between 2009 and 2011. Individuals in the sample were aged at least 65 years with a new diagnosis of OSA, 88.1% of beneficiaries were white, and 52.3% were male.
The researchers applied objective adherence criteria set by the Centers for Medicare & Medicaid Services, which defines CPAP adherence as a patient using CPAP for at least 4 hours on 70% of nights, or CPAP use for 21 of 30 consecutive days within 90 days after beginning therapy.
Using CPAP machine charges as a measure of who adhered to therapy, they found 1,420 of 3,229 individuals (44%) achieved adherence under these criteria, which included making 13 monthly payments during their CPAP machine’s “rent-to-own” period. Partial adherence was found in 997 individuals (30.9%) who made between 4-12 payments on their CPAP machine, while 812 individuals (25.2%) made 4 payments or fewer on their CPAP machines, which the researchers classified as nonadherence. Nonadherers tended to be slightly younger (mean, 72.5 years vs. 79.2 years; P < .001) and had a higher number of comorbidities (35.2% vs. 30.4%; P = .002), compared with individuals with high adherence. Anxiety (odds ratio, 1.34; 95% confidence interval, 1.12-1.61), anemia (OR, 1.16; 95% CI, 1.02-1.32), fibromyalgia (OR, 1.19; 95% CI, 1.03-1.38), traumatic brain injury (OR, 1.58; 95% CI, 1.21-2.07), and Medicaid eligibility (OR, 1.48; 95% CI, 1.24-1.75) were all independently associated with lower CPAP adherence. Medicaid eligibility was considered an indicator of lower socioeconomic status.
Krishna M. Sundar, MD, FCCP, director at the Sleep-Wake Center in the University of Utah pulmonary division in Salt Lake City and CHEST Physician editorial board member, said in an interview that studies have shown early signs of adherence within the first few weeks are an important indicator of overall adherence to CPAP therapy. However, the use of CPAP machine payments in the study by Dr. Wickwire and colleagues was a novel way to track adherence.
Some of the issues with nonadherence may be related to challenges in using the technology, but it is the clinician’s role to communicate with patients about the effectiveness of CPAP and identifying reasons for nonadherence while also attempting to tease out the subtle socioeconomic factors related to nonadherence, Dr. Sundar noted. “We need to alter our practice to make sure that we communicate with these patients and better understand what are the social factors in getting the CPAP or utilizing CPAP, and also following these patients more closely, especially in the first month of starting CPAP therapy.
“Just because somebody has severe sleep apnea and other comorbid conditions does not mean that they’re going to wear the CPAP,” he said. “So, the fact that socioeconomic factors play an equal if not more important role in terms of predicting CPAP adherence. That is an important takeaway.”
Octavian C. Ioachimescu, MD, FCCP, of Emory University, Atlanta, and the Atlanta Veteran Affairs Administration and CHEST Physician editorial board member, said in an interview that the study raises a major question of what is next. “What can we offer to these patients, and what is the real-world compliance to that ‘next-best’ modality?” Dr. Ioachimescu said. “What are the outcomes of these individuals in the point-of-care environment, or ‘real world?’ ”
The analysis by the authors adds the perspective of a “real-world depiction of clinical care for patients with OSA,” Dr. Ioachimescu said. “One major lesson of such an analysis is that the health care goal setting that is referential to initial, randomized, well-controlled studies on highly selected patient populations need to be reassessed periodically from the point of view of actual results in the clinics.”
Clinicians may need to borrow ideas from other therapeutic fields to help improve patient adherence, he said. “[W]e may be able to develop and implement in the future peer involvement, behavioral and cognitive approaches, motivational enhancement interventions, as well as elements of acceptance and commitment techniques, all in the larger context of more integrated and in the same time individualized approaches to therapy.”
The investigators concluded that, “relative to Medicare-only beneficiaries, those eligible for both Medicare and Medicaid were significantly less likely to adhere to CPAP. Future research should seek to develop a deeper understanding of the mechanisms through which [socioeconomic status] and other social determinants impact patient experience throughout the OSA diagnostic and treatment process, including receiving, acclimating, and adhering to CPAP therapy.”
Dr. Sundar concurred with this assessment and said more research is needed on factors impacting adherence such as poverty, homelessness, and home support systems. “It’s not just coordinating with the patient. Clearly, more work is needed in understanding the social aspects of CPAP adherence.”
This study was funded in part by an investigator-initiated grant provided by ResMed to Dr. Wickmire’s institution, the University of Maryland, Baltimore. Dr. Wickmire reported being a scientific consultant to DayZz, Eisai, Merck, and Purdue and holds shares in WellTap. Dr. Oldstone is a ResMed employee and shareholder. Dr. Sundar reported being a cofounder of Hypnoscure, which creates software for population management of sleep apnea, and an investigator in trials where ResMed and Respironics devices were used. Dr. Ioachimescu reported no relevant financial disclosures.
SOURCE: Wickwire EM et al. Sleep. 2020 Jun 23. doi: 10.1093/sleep/zsaa122.
The benefits of continuous positive airway pressure therapy for patients with obstructive sleep apnea are well documented, but it only works if patients can adhere to the therapy.
A large national study of older Medicare patients with obstructive sleep apnea (OSA) has identified lower socioeconomic status and comorbidities as independent risk factors for nonadherence to continuous positive airway pressure (CPAP) therapy.
“[The] present results represent the largest study to date of rates and predictors of CPAP adherence among older adults in the United States. In our national sample of Medicare beneficiaries, adherence rates were generally lower than previously reported in smaller, clinic-based studies,” Emerson M. Wickwire, PhD, of the Sleep Disorders Center and division of pulmonary and critical care medicine at the University of Maryland, Baltimore, and colleagues wrote in Sleep.
Dr. Wickwire and colleagues estimated CPAP machine adherence using a 5% sample of Medicare claims data, identifying 3,229 Medicare beneficiaries with OSA who began CPAP therapy between 2009 and 2011. Individuals in the sample were aged at least 65 years with a new diagnosis of OSA, 88.1% of beneficiaries were white, and 52.3% were male.
The researchers applied objective adherence criteria set by the Centers for Medicare & Medicaid Services, which defines CPAP adherence as a patient using CPAP for at least 4 hours on 70% of nights, or CPAP use for 21 of 30 consecutive days within 90 days after beginning therapy.
Using CPAP machine charges as a measure of who adhered to therapy, they found 1,420 of 3,229 individuals (44%) achieved adherence under these criteria, which included making 13 monthly payments during their CPAP machine’s “rent-to-own” period. Partial adherence was found in 997 individuals (30.9%) who made between 4-12 payments on their CPAP machine, while 812 individuals (25.2%) made 4 payments or fewer on their CPAP machines, which the researchers classified as nonadherence. Nonadherers tended to be slightly younger (mean, 72.5 years vs. 79.2 years; P < .001) and had a higher number of comorbidities (35.2% vs. 30.4%; P = .002), compared with individuals with high adherence. Anxiety (odds ratio, 1.34; 95% confidence interval, 1.12-1.61), anemia (OR, 1.16; 95% CI, 1.02-1.32), fibromyalgia (OR, 1.19; 95% CI, 1.03-1.38), traumatic brain injury (OR, 1.58; 95% CI, 1.21-2.07), and Medicaid eligibility (OR, 1.48; 95% CI, 1.24-1.75) were all independently associated with lower CPAP adherence. Medicaid eligibility was considered an indicator of lower socioeconomic status.
Krishna M. Sundar, MD, FCCP, director at the Sleep-Wake Center in the University of Utah pulmonary division in Salt Lake City and CHEST Physician editorial board member, said in an interview that studies have shown early signs of adherence within the first few weeks are an important indicator of overall adherence to CPAP therapy. However, the use of CPAP machine payments in the study by Dr. Wickwire and colleagues was a novel way to track adherence.
Some of the issues with nonadherence may be related to challenges in using the technology, but it is the clinician’s role to communicate with patients about the effectiveness of CPAP and identifying reasons for nonadherence while also attempting to tease out the subtle socioeconomic factors related to nonadherence, Dr. Sundar noted. “We need to alter our practice to make sure that we communicate with these patients and better understand what are the social factors in getting the CPAP or utilizing CPAP, and also following these patients more closely, especially in the first month of starting CPAP therapy.
“Just because somebody has severe sleep apnea and other comorbid conditions does not mean that they’re going to wear the CPAP,” he said. “So, the fact that socioeconomic factors play an equal if not more important role in terms of predicting CPAP adherence. That is an important takeaway.”
Octavian C. Ioachimescu, MD, FCCP, of Emory University, Atlanta, and the Atlanta Veteran Affairs Administration and CHEST Physician editorial board member, said in an interview that the study raises a major question of what is next. “What can we offer to these patients, and what is the real-world compliance to that ‘next-best’ modality?” Dr. Ioachimescu said. “What are the outcomes of these individuals in the point-of-care environment, or ‘real world?’ ”
The analysis by the authors adds the perspective of a “real-world depiction of clinical care for patients with OSA,” Dr. Ioachimescu said. “One major lesson of such an analysis is that the health care goal setting that is referential to initial, randomized, well-controlled studies on highly selected patient populations need to be reassessed periodically from the point of view of actual results in the clinics.”
Clinicians may need to borrow ideas from other therapeutic fields to help improve patient adherence, he said. “[W]e may be able to develop and implement in the future peer involvement, behavioral and cognitive approaches, motivational enhancement interventions, as well as elements of acceptance and commitment techniques, all in the larger context of more integrated and in the same time individualized approaches to therapy.”
The investigators concluded that, “relative to Medicare-only beneficiaries, those eligible for both Medicare and Medicaid were significantly less likely to adhere to CPAP. Future research should seek to develop a deeper understanding of the mechanisms through which [socioeconomic status] and other social determinants impact patient experience throughout the OSA diagnostic and treatment process, including receiving, acclimating, and adhering to CPAP therapy.”
Dr. Sundar concurred with this assessment and said more research is needed on factors impacting adherence such as poverty, homelessness, and home support systems. “It’s not just coordinating with the patient. Clearly, more work is needed in understanding the social aspects of CPAP adherence.”
This study was funded in part by an investigator-initiated grant provided by ResMed to Dr. Wickmire’s institution, the University of Maryland, Baltimore. Dr. Wickmire reported being a scientific consultant to DayZz, Eisai, Merck, and Purdue and holds shares in WellTap. Dr. Oldstone is a ResMed employee and shareholder. Dr. Sundar reported being a cofounder of Hypnoscure, which creates software for population management of sleep apnea, and an investigator in trials where ResMed and Respironics devices were used. Dr. Ioachimescu reported no relevant financial disclosures.
SOURCE: Wickwire EM et al. Sleep. 2020 Jun 23. doi: 10.1093/sleep/zsaa122.
FROM SLEEP
Oxford coronavirus vaccine ‘triggers immune response’
The early stage results, published in The Lancet, found that the candidate vaccine, known as ChAdOx1 nCoV-19, provoked a T-cell response peaking 14 days after vaccination, and an antibody response within 28 days.
Andrew Pollard, chief investigator on the study, and professor of pediatric infection and immunity at Oxford University, described the results as “encouraging”. He told a briefing convened by the Science Media Centre on Monday that it was “a really important milestone on the path to the development of the vaccine”.
In the Commons, the Health Secretary, Matt Hancock, hailed the results for taking us “one step closer to finding a vaccine that can potentially save lives, all around the world”.
The trial, which has so far involved 1,077 healthy adults, caused minor side effects when compared with a control group given a meningitis vaccine. Fatigue and headache were the most commonly reported reactions.
However, there were no serious adverse events from the vaccine, the researchers said.
‘Still a long way to go’
Sarah Gilbert, lead researcher of the vaccine development program, and professor of vaccinology at Oxford, cautioned that there was still a long way to go before the team could confirm that the vaccine could protect against developing COVID-19.
“The difficulty that we have, and that all vaccine developers have in trying to make a vaccine against this particular virus, is that we don’t know how strong that immune response needs to be,” she said.
“So, we can’t say just by looking at immune responses whether this is going to protect people or not. And the only way we’re going to find out is by doing the large phase 3 trials and wait for people to be infected as part of that trial before we know if the vaccine can work.”
The authors noted some limitations to their findings. They said more research was needed to confirm their results in different groups of people – including older age groups, those with other health conditions, and in ethnically and geographically diverse populations.
A notable result of the trial was that participants given a second dose of the vaccine appeared to display a stronger immune response, a finding that had influenced plans to “look at two dose regimes as well as one dose regimes in the phase 3 trial”, Prof Adrian Hill, director of Oxford’s Jenner Institute, confirmed.
ChAdOx1 nCoV-19 is made from a weakened version of an adenovirus that causes infections in chimpanzees. The virus has been genetically modified so that it cannot grow in humans.
On Monday, the government announced that it had struck a deal with AstraZeneca for access to 100 million doses of the Oxford vaccine, in addition to millions of doses of other promising candidate vaccines.
Expert reaction to the findings
The Medical Research Council helped to fund the trial. Executive Chair Professor Fiona Watt commented: “It is truly remarkable how fast this vaccine has progressed, with our support, through early clinical trials, and it is very encouraging that it shows no safety concerns and evokes strong immune responses.
“There is a lot that we don’t yet know about immunity to the virus that causes COVID-19. However, it seems that both antibody and T cell immunity are important, and this vaccine triggers both responses. The much anticipated next milestone will be the results of the larger trials that are happening now to find out if the vaccine will protect people from the virus.”
Jonathan Ball, professor of molecular virology at the University of Nottingham, told the SMC: “The results of the Oxford chimp adenovirus vaccine candidate show that the vaccine is able to generate antibodies and T cells in humans and these persisted for several weeks. Whilst encouraging there is still a long way to go before we can herald the arrival of a successful coronavirus vaccine.
“It is unclear whether the levels of immunity can protect against infection – that’s what the larger ongoing phase III trials are designed to test. Nor do we know if this vaccine can protect those most vulnerable to severe COVID-19 disease.”
Stephen Evans, professor of pharmacoepidemiology at the London School of Hygiene and Tropical Medicine, commented: “For the vaccine to be really useful, we not only need the larger studies conducted where COVID-19 is still occurring at a high rate, but we need to be reasonably sure that the protection lasts for a considerable time.”
He said it was also vital that people older than 55 were included in later trials.
Richard Torbett, chief executive of the Association of the British Pharmaceutical Industry, said: “Developing a vaccine is an incredibly difficult challenge; the fact that there are multiple candidates in development is hopefully a sign that the hard work will ultimately pay off.
“But we must be patient. Proving that a vaccine is safe and effective is a long process and we could still be many months away.”
This article first appeared on Medscape.com.
The early stage results, published in The Lancet, found that the candidate vaccine, known as ChAdOx1 nCoV-19, provoked a T-cell response peaking 14 days after vaccination, and an antibody response within 28 days.
Andrew Pollard, chief investigator on the study, and professor of pediatric infection and immunity at Oxford University, described the results as “encouraging”. He told a briefing convened by the Science Media Centre on Monday that it was “a really important milestone on the path to the development of the vaccine”.
In the Commons, the Health Secretary, Matt Hancock, hailed the results for taking us “one step closer to finding a vaccine that can potentially save lives, all around the world”.
The trial, which has so far involved 1,077 healthy adults, caused minor side effects when compared with a control group given a meningitis vaccine. Fatigue and headache were the most commonly reported reactions.
However, there were no serious adverse events from the vaccine, the researchers said.
‘Still a long way to go’
Sarah Gilbert, lead researcher of the vaccine development program, and professor of vaccinology at Oxford, cautioned that there was still a long way to go before the team could confirm that the vaccine could protect against developing COVID-19.
“The difficulty that we have, and that all vaccine developers have in trying to make a vaccine against this particular virus, is that we don’t know how strong that immune response needs to be,” she said.
“So, we can’t say just by looking at immune responses whether this is going to protect people or not. And the only way we’re going to find out is by doing the large phase 3 trials and wait for people to be infected as part of that trial before we know if the vaccine can work.”
The authors noted some limitations to their findings. They said more research was needed to confirm their results in different groups of people – including older age groups, those with other health conditions, and in ethnically and geographically diverse populations.
A notable result of the trial was that participants given a second dose of the vaccine appeared to display a stronger immune response, a finding that had influenced plans to “look at two dose regimes as well as one dose regimes in the phase 3 trial”, Prof Adrian Hill, director of Oxford’s Jenner Institute, confirmed.
ChAdOx1 nCoV-19 is made from a weakened version of an adenovirus that causes infections in chimpanzees. The virus has been genetically modified so that it cannot grow in humans.
On Monday, the government announced that it had struck a deal with AstraZeneca for access to 100 million doses of the Oxford vaccine, in addition to millions of doses of other promising candidate vaccines.
Expert reaction to the findings
The Medical Research Council helped to fund the trial. Executive Chair Professor Fiona Watt commented: “It is truly remarkable how fast this vaccine has progressed, with our support, through early clinical trials, and it is very encouraging that it shows no safety concerns and evokes strong immune responses.
“There is a lot that we don’t yet know about immunity to the virus that causes COVID-19. However, it seems that both antibody and T cell immunity are important, and this vaccine triggers both responses. The much anticipated next milestone will be the results of the larger trials that are happening now to find out if the vaccine will protect people from the virus.”
Jonathan Ball, professor of molecular virology at the University of Nottingham, told the SMC: “The results of the Oxford chimp adenovirus vaccine candidate show that the vaccine is able to generate antibodies and T cells in humans and these persisted for several weeks. Whilst encouraging there is still a long way to go before we can herald the arrival of a successful coronavirus vaccine.
“It is unclear whether the levels of immunity can protect against infection – that’s what the larger ongoing phase III trials are designed to test. Nor do we know if this vaccine can protect those most vulnerable to severe COVID-19 disease.”
Stephen Evans, professor of pharmacoepidemiology at the London School of Hygiene and Tropical Medicine, commented: “For the vaccine to be really useful, we not only need the larger studies conducted where COVID-19 is still occurring at a high rate, but we need to be reasonably sure that the protection lasts for a considerable time.”
He said it was also vital that people older than 55 were included in later trials.
Richard Torbett, chief executive of the Association of the British Pharmaceutical Industry, said: “Developing a vaccine is an incredibly difficult challenge; the fact that there are multiple candidates in development is hopefully a sign that the hard work will ultimately pay off.
“But we must be patient. Proving that a vaccine is safe and effective is a long process and we could still be many months away.”
This article first appeared on Medscape.com.
The early stage results, published in The Lancet, found that the candidate vaccine, known as ChAdOx1 nCoV-19, provoked a T-cell response peaking 14 days after vaccination, and an antibody response within 28 days.
Andrew Pollard, chief investigator on the study, and professor of pediatric infection and immunity at Oxford University, described the results as “encouraging”. He told a briefing convened by the Science Media Centre on Monday that it was “a really important milestone on the path to the development of the vaccine”.
In the Commons, the Health Secretary, Matt Hancock, hailed the results for taking us “one step closer to finding a vaccine that can potentially save lives, all around the world”.
The trial, which has so far involved 1,077 healthy adults, caused minor side effects when compared with a control group given a meningitis vaccine. Fatigue and headache were the most commonly reported reactions.
However, there were no serious adverse events from the vaccine, the researchers said.
‘Still a long way to go’
Sarah Gilbert, lead researcher of the vaccine development program, and professor of vaccinology at Oxford, cautioned that there was still a long way to go before the team could confirm that the vaccine could protect against developing COVID-19.
“The difficulty that we have, and that all vaccine developers have in trying to make a vaccine against this particular virus, is that we don’t know how strong that immune response needs to be,” she said.
“So, we can’t say just by looking at immune responses whether this is going to protect people or not. And the only way we’re going to find out is by doing the large phase 3 trials and wait for people to be infected as part of that trial before we know if the vaccine can work.”
The authors noted some limitations to their findings. They said more research was needed to confirm their results in different groups of people – including older age groups, those with other health conditions, and in ethnically and geographically diverse populations.
A notable result of the trial was that participants given a second dose of the vaccine appeared to display a stronger immune response, a finding that had influenced plans to “look at two dose regimes as well as one dose regimes in the phase 3 trial”, Prof Adrian Hill, director of Oxford’s Jenner Institute, confirmed.
ChAdOx1 nCoV-19 is made from a weakened version of an adenovirus that causes infections in chimpanzees. The virus has been genetically modified so that it cannot grow in humans.
On Monday, the government announced that it had struck a deal with AstraZeneca for access to 100 million doses of the Oxford vaccine, in addition to millions of doses of other promising candidate vaccines.
Expert reaction to the findings
The Medical Research Council helped to fund the trial. Executive Chair Professor Fiona Watt commented: “It is truly remarkable how fast this vaccine has progressed, with our support, through early clinical trials, and it is very encouraging that it shows no safety concerns and evokes strong immune responses.
“There is a lot that we don’t yet know about immunity to the virus that causes COVID-19. However, it seems that both antibody and T cell immunity are important, and this vaccine triggers both responses. The much anticipated next milestone will be the results of the larger trials that are happening now to find out if the vaccine will protect people from the virus.”
Jonathan Ball, professor of molecular virology at the University of Nottingham, told the SMC: “The results of the Oxford chimp adenovirus vaccine candidate show that the vaccine is able to generate antibodies and T cells in humans and these persisted for several weeks. Whilst encouraging there is still a long way to go before we can herald the arrival of a successful coronavirus vaccine.
“It is unclear whether the levels of immunity can protect against infection – that’s what the larger ongoing phase III trials are designed to test. Nor do we know if this vaccine can protect those most vulnerable to severe COVID-19 disease.”
Stephen Evans, professor of pharmacoepidemiology at the London School of Hygiene and Tropical Medicine, commented: “For the vaccine to be really useful, we not only need the larger studies conducted where COVID-19 is still occurring at a high rate, but we need to be reasonably sure that the protection lasts for a considerable time.”
He said it was also vital that people older than 55 were included in later trials.
Richard Torbett, chief executive of the Association of the British Pharmaceutical Industry, said: “Developing a vaccine is an incredibly difficult challenge; the fact that there are multiple candidates in development is hopefully a sign that the hard work will ultimately pay off.
“But we must be patient. Proving that a vaccine is safe and effective is a long process and we could still be many months away.”
This article first appeared on Medscape.com.














