User login
CMS sticks with E/M pay plan over some objections
The Trump administration is sticking with a plan to boost certain Medicare pay for many primary care and other specialties focused heavily on office visits while lowering that for other groups to balance these increased costs.
On Aug. 4, the Centers for Medicare & Medicaid Services posted on the Federal Register draft versions of two of its major annual payment measures: the physician fee schedule and the payment rule for hospital outpatient and ambulatory surgery center services. On Aug. 3, the CMS informally posted a copy of the physician fee schedule on its own website, allowing medical groups to begin reading the more than 1,300-page rule.
Federal officials normally use annual Medicare payment rules to make many revisions to policies as well as adjust reimbursement.
The draft 2021 physician fee schedule, for example, calls for broadening the authority of clinicians other than physicians to authorize testing of people enrolled in Medicare.
The CMS intends to allow nurse practitioners, physician assistants, and certain other health care professionals to more widely supervise diagnostic psychological and neuropsychological tests.
The draft 2021 hospital outpatient rule proposes a gradual changeover to allow more procedures to be performed on an outpatient basis. This shift could save money for Medicare as well as for the people enrolled in the giant federal health program who need these services, the CMS explained.
Medicare would begin with a change in status for almost 300 musculoskeletal-related services, making them eligible for payment in the hospital outpatient setting when appropriate, CMS wrote in a fact sheet.
The initial reaction to Medicare’s proposed 2021 rules centered on its planned redistribution of funds among medical specialties. The CMS had outlined this plan last year. It is part of longstanding efforts to boost pay for primary care specialists and other physicians whose practice centers more around office visits than procedures.
There is broad support in health policy circles for raising pay for these specialties, but there also are strong objections to the cuts the CMS plans to offset the cost of rising pay for some fields.
Susan R. Bailey, MD, president of the American Medical Association, addressed both of these ideas in an AMA news release on the proposed 2021 physician fee schedule. The increase in pay for office visits, covered under evaluation and management services (E/M), stems from recommendations on resource costs from the AMA/Specialty Society RVS Update Committee, Dr. Bailey said.
“Unfortunately, these office visit payment increases, and a multitude of other new CMS proposed payment increases, are required by statute to be offset by payment reductions to other services, through an unsustainable reduction of nearly 11% to the Medicare conversion factor,” Dr. Bailey explained.
In the news release, Dr. Bailey asked Congress to waive Medicare’s budget-neutrality requirements to allow increases without the cuts.
“Physicians are already experiencing substantial economic hardships due to COVID-19, so these pay cuts could not come at a worse time,” she said.
Winners and losers
The CMS details the possible winners and losers in its payment reshuffle in Table 90 of the proposed 2021 physician fee schedule. In the proposed rule, CMS notes in the draft that these figures are based upon estimates of aggregate allowed charges across all services furnished by physicians and other clinicians.
Specialties in line for increases under the 2021 draft rule include allergy/immunology (9%), endocrinology (17%), family practice (13%), geriatrics (4%), hematology/oncology (14%), internal medicine (4%), physician assistants (8%), psychiatry (8%), rheumatology (16%), and urology (8%).
In line for cuts would be anesthesiology (–8%), cardiac surgery (–9%), emergency medicine (–6%), gastroenterology (–5%), general surgery (–7%), infectious disease (–4%), neurosurgery (–7%), physical/occupational therapy (–9%), plastic surgery (–7%), and radiology (–11%).
An umbrella group, the Surgical Care Coalition, had a quick statement ready about the CMS proposal. Writing on behalf of the group was David B. Hoyt, MD, executive director of the American College of Surgeons.
“Today’s proposed rule ignores both patients and the surgeons who care for them. The middle of a pandemic is no time for cuts to any form of health care, but today’s announcement moves ahead as if nothing has changed,” Dr. Hoyt said in the statement. “The Surgical Care Coalition believes no physician should see payment cuts that will reduce patients’ access to care.”
Making a similar request Aug. 4 in a unified statement were the American Physical Therapy Association (APTA), the American Occupational Therapy Association (AOTA), and the American Speech-Language-Hearing Association (ASHA).
“Our organizations call on Congress and CMS to advance well-reasoned fee schedule payment policies and waive budget neutrality,” the groups said.
A version of this article originally appeared on Medscape.com.
We all agree that E/M services have been undercompensated for many years and applaud CMS for increasing their reimbursements, but this does not mean that endoscopic services are suddenly less valuable as a result. Nor does it mean that the work required to perform endoscopic services has declined.
Lawrence R. Kosinski, MD, MBA, AGAF, is the chief medical officer at SonarMD, Chicago. He is also an associate editor for GI & Hepatology News.
We all agree that E/M services have been undercompensated for many years and applaud CMS for increasing their reimbursements, but this does not mean that endoscopic services are suddenly less valuable as a result. Nor does it mean that the work required to perform endoscopic services has declined.
Lawrence R. Kosinski, MD, MBA, AGAF, is the chief medical officer at SonarMD, Chicago. He is also an associate editor for GI & Hepatology News.
We all agree that E/M services have been undercompensated for many years and applaud CMS for increasing their reimbursements, but this does not mean that endoscopic services are suddenly less valuable as a result. Nor does it mean that the work required to perform endoscopic services has declined.
Lawrence R. Kosinski, MD, MBA, AGAF, is the chief medical officer at SonarMD, Chicago. He is also an associate editor for GI & Hepatology News.
The Trump administration is sticking with a plan to boost certain Medicare pay for many primary care and other specialties focused heavily on office visits while lowering that for other groups to balance these increased costs.
On Aug. 4, the Centers for Medicare & Medicaid Services posted on the Federal Register draft versions of two of its major annual payment measures: the physician fee schedule and the payment rule for hospital outpatient and ambulatory surgery center services. On Aug. 3, the CMS informally posted a copy of the physician fee schedule on its own website, allowing medical groups to begin reading the more than 1,300-page rule.
Federal officials normally use annual Medicare payment rules to make many revisions to policies as well as adjust reimbursement.
The draft 2021 physician fee schedule, for example, calls for broadening the authority of clinicians other than physicians to authorize testing of people enrolled in Medicare.
The CMS intends to allow nurse practitioners, physician assistants, and certain other health care professionals to more widely supervise diagnostic psychological and neuropsychological tests.
The draft 2021 hospital outpatient rule proposes a gradual changeover to allow more procedures to be performed on an outpatient basis. This shift could save money for Medicare as well as for the people enrolled in the giant federal health program who need these services, the CMS explained.
Medicare would begin with a change in status for almost 300 musculoskeletal-related services, making them eligible for payment in the hospital outpatient setting when appropriate, CMS wrote in a fact sheet.
The initial reaction to Medicare’s proposed 2021 rules centered on its planned redistribution of funds among medical specialties. The CMS had outlined this plan last year. It is part of longstanding efforts to boost pay for primary care specialists and other physicians whose practice centers more around office visits than procedures.
There is broad support in health policy circles for raising pay for these specialties, but there also are strong objections to the cuts the CMS plans to offset the cost of rising pay for some fields.
Susan R. Bailey, MD, president of the American Medical Association, addressed both of these ideas in an AMA news release on the proposed 2021 physician fee schedule. The increase in pay for office visits, covered under evaluation and management services (E/M), stems from recommendations on resource costs from the AMA/Specialty Society RVS Update Committee, Dr. Bailey said.
“Unfortunately, these office visit payment increases, and a multitude of other new CMS proposed payment increases, are required by statute to be offset by payment reductions to other services, through an unsustainable reduction of nearly 11% to the Medicare conversion factor,” Dr. Bailey explained.
In the news release, Dr. Bailey asked Congress to waive Medicare’s budget-neutrality requirements to allow increases without the cuts.
“Physicians are already experiencing substantial economic hardships due to COVID-19, so these pay cuts could not come at a worse time,” she said.
Winners and losers
The CMS details the possible winners and losers in its payment reshuffle in Table 90 of the proposed 2021 physician fee schedule. In the proposed rule, CMS notes in the draft that these figures are based upon estimates of aggregate allowed charges across all services furnished by physicians and other clinicians.
Specialties in line for increases under the 2021 draft rule include allergy/immunology (9%), endocrinology (17%), family practice (13%), geriatrics (4%), hematology/oncology (14%), internal medicine (4%), physician assistants (8%), psychiatry (8%), rheumatology (16%), and urology (8%).
In line for cuts would be anesthesiology (–8%), cardiac surgery (–9%), emergency medicine (–6%), gastroenterology (–5%), general surgery (–7%), infectious disease (–4%), neurosurgery (–7%), physical/occupational therapy (–9%), plastic surgery (–7%), and radiology (–11%).
An umbrella group, the Surgical Care Coalition, had a quick statement ready about the CMS proposal. Writing on behalf of the group was David B. Hoyt, MD, executive director of the American College of Surgeons.
“Today’s proposed rule ignores both patients and the surgeons who care for them. The middle of a pandemic is no time for cuts to any form of health care, but today’s announcement moves ahead as if nothing has changed,” Dr. Hoyt said in the statement. “The Surgical Care Coalition believes no physician should see payment cuts that will reduce patients’ access to care.”
Making a similar request Aug. 4 in a unified statement were the American Physical Therapy Association (APTA), the American Occupational Therapy Association (AOTA), and the American Speech-Language-Hearing Association (ASHA).
“Our organizations call on Congress and CMS to advance well-reasoned fee schedule payment policies and waive budget neutrality,” the groups said.
A version of this article originally appeared on Medscape.com.
The Trump administration is sticking with a plan to boost certain Medicare pay for many primary care and other specialties focused heavily on office visits while lowering that for other groups to balance these increased costs.
On Aug. 4, the Centers for Medicare & Medicaid Services posted on the Federal Register draft versions of two of its major annual payment measures: the physician fee schedule and the payment rule for hospital outpatient and ambulatory surgery center services. On Aug. 3, the CMS informally posted a copy of the physician fee schedule on its own website, allowing medical groups to begin reading the more than 1,300-page rule.
Federal officials normally use annual Medicare payment rules to make many revisions to policies as well as adjust reimbursement.
The draft 2021 physician fee schedule, for example, calls for broadening the authority of clinicians other than physicians to authorize testing of people enrolled in Medicare.
The CMS intends to allow nurse practitioners, physician assistants, and certain other health care professionals to more widely supervise diagnostic psychological and neuropsychological tests.
The draft 2021 hospital outpatient rule proposes a gradual changeover to allow more procedures to be performed on an outpatient basis. This shift could save money for Medicare as well as for the people enrolled in the giant federal health program who need these services, the CMS explained.
Medicare would begin with a change in status for almost 300 musculoskeletal-related services, making them eligible for payment in the hospital outpatient setting when appropriate, CMS wrote in a fact sheet.
The initial reaction to Medicare’s proposed 2021 rules centered on its planned redistribution of funds among medical specialties. The CMS had outlined this plan last year. It is part of longstanding efforts to boost pay for primary care specialists and other physicians whose practice centers more around office visits than procedures.
There is broad support in health policy circles for raising pay for these specialties, but there also are strong objections to the cuts the CMS plans to offset the cost of rising pay for some fields.
Susan R. Bailey, MD, president of the American Medical Association, addressed both of these ideas in an AMA news release on the proposed 2021 physician fee schedule. The increase in pay for office visits, covered under evaluation and management services (E/M), stems from recommendations on resource costs from the AMA/Specialty Society RVS Update Committee, Dr. Bailey said.
“Unfortunately, these office visit payment increases, and a multitude of other new CMS proposed payment increases, are required by statute to be offset by payment reductions to other services, through an unsustainable reduction of nearly 11% to the Medicare conversion factor,” Dr. Bailey explained.
In the news release, Dr. Bailey asked Congress to waive Medicare’s budget-neutrality requirements to allow increases without the cuts.
“Physicians are already experiencing substantial economic hardships due to COVID-19, so these pay cuts could not come at a worse time,” she said.
Winners and losers
The CMS details the possible winners and losers in its payment reshuffle in Table 90 of the proposed 2021 physician fee schedule. In the proposed rule, CMS notes in the draft that these figures are based upon estimates of aggregate allowed charges across all services furnished by physicians and other clinicians.
Specialties in line for increases under the 2021 draft rule include allergy/immunology (9%), endocrinology (17%), family practice (13%), geriatrics (4%), hematology/oncology (14%), internal medicine (4%), physician assistants (8%), psychiatry (8%), rheumatology (16%), and urology (8%).
In line for cuts would be anesthesiology (–8%), cardiac surgery (–9%), emergency medicine (–6%), gastroenterology (–5%), general surgery (–7%), infectious disease (–4%), neurosurgery (–7%), physical/occupational therapy (–9%), plastic surgery (–7%), and radiology (–11%).
An umbrella group, the Surgical Care Coalition, had a quick statement ready about the CMS proposal. Writing on behalf of the group was David B. Hoyt, MD, executive director of the American College of Surgeons.
“Today’s proposed rule ignores both patients and the surgeons who care for them. The middle of a pandemic is no time for cuts to any form of health care, but today’s announcement moves ahead as if nothing has changed,” Dr. Hoyt said in the statement. “The Surgical Care Coalition believes no physician should see payment cuts that will reduce patients’ access to care.”
Making a similar request Aug. 4 in a unified statement were the American Physical Therapy Association (APTA), the American Occupational Therapy Association (AOTA), and the American Speech-Language-Hearing Association (ASHA).
“Our organizations call on Congress and CMS to advance well-reasoned fee schedule payment policies and waive budget neutrality,” the groups said.
A version of this article originally appeared on Medscape.com.
Patient visits post COVID-19
Has telemedicine found its footing?
When Alexander Graham Bell invented the telephone, he accomplished something that many telegraph devotees never thought possible: the synchronous, bidirectional transmission of voice over electrical lines.
This was an incredible milestone in the advancement of mankind and enabled true revolutions in commerce, scientific collaboration, and human interaction. But Mr. Bell knew his invention didn’t represent the final advancement in telecommunication; he was quite prescient in imagining a day when individuals could see each other while speaking on the phone.
Many years later, what was once only a dream is now commonplace, and children growing up today can’t imagine a world where apps such as FaceTime and Skype don’t exist. Until recently, however, the medical community has been slow to adopt the idea of video interactions. This has dramatically changed because of the pandemic and the need for social distancing. It appears that telemedicine has found its footing, but whether it will remain popular once patients feel safe going to see their doctors in person again remains to be seen. This month, we’ll examine a few key issues that will determine the future of virtual medical visits.
Collect calling
The pandemic has wrought both human and economic casualties. With fear, job loss, and regulations leading to decreased spending, many large and small businesses have been and will continue to be unable to survive. Companies, including Brooks Brothers, Hertz, Lord and Taylor, GNC, and J.C. Penney, have declared bankruptcy.1 Medical practices and hospitals have taken cuts to their bottom line, and we’ve heard of many physician groups that have had to enact substantial salary cuts or even lay off providers – something previously unheard of. Recent months have demonstrated the health care community’s commitment to put patients first, but we simply cannot survive if we aren’t adequately reimbursed. Traditionally, this has been a significant roadblock toward the widespread adoption of telemedicine.
In most cases, these visits were not reimbursed at all. Thankfully, shortly after the coronavirus hit our shores, Medicare and Medicaid changed their policies, offering equal payment for video and in-person patient encounters. Most private insurers have followed suit, but the commitment to this payment parity appears – thus far – to be temporary. It is unclear that the financial support of telemedicine will continue post COVID-19, and this has many physicians feeling uncomfortable. In the meantime, many patients have come to prefer virtual visits, appreciating the convenience and efficiency.
Physicians don’t always have the same experience. Telemedicine can be technically challenging and take just as much – or sometimes more – time to navigate and document. Unless they are reimbursed equitably, providers will be forced to limit their use of virtual visits or not offer them at all. This leads to another issue: reliability.
‘Can you hear me now?’
Over the past several months, we have had the opportunity to use telemedicine firsthand and have spoken to many other physicians and patients about their experiences with it. The reports are all quite consistent: Most have had generally positive things to say. Still, some common concerns emerge when diving a bit deeper. Most notably are complaints about usability and reliability of the software.
While there are large telemedicine companies that have developed world-class cross-platform products, many in use today are proprietary and EHR dependent. As a result, the quality varies widely. Many EHR vendors were caught completely off guard by the sudden demand for telemedicine and are playing catch-up as they develop their own virtual visit platforms. While these vendor-developed platforms promise tight integration with patient records, some have significant shortcomings in stability when taxed under high utilization, including choppy video and garbled voice. This simply won’t do if telemedicine is to survive. It is incumbent on software developers and health care providers to invest in high-quality, reliable platforms on which to build their virtual visit offerings. This will ensure a more rapid adoption and the “staying power” of the new technology.
Dialing ‘0’ for the operator
Once seen as a “novelty” offered by only a small number of medical providers, virtual visits now represent a significant and ever-increasing percentage of patient encounters. The technology therefore must be easy to use. Given confidentiality and documentation requirements, along with the broad variety of available computing platforms and devices (e.g., PC, Mac, iOS, and Android), the process is often far from problem free. Patients may need help downloading apps, setting up webcams, or registering for the service. Providers may face issues with Internet connectivity or EHR-related delays.
It is critical that help be available to make the connection seamless and the experience a positive one. We are fortunate to work for a health care institution that has made this a priority, dedicating a team of individuals to provide real-time support to patients and clinicians. Small independent practices may not have this luxury, but we would encourage all providers to engage with their telemedicine or EHR vendors to determine what resources are available when problems arise, as they undoubtedly will.
Answering the call
Like the invention of the telephone, the advent of telemedicine is another milestone on the journey toward better communication with our patients, and it appears to be here to stay. Virtual visits won’t completely replace in-person care, nor minimize the benefit of human interaction, but they will continue to play an important role in the care continuum. By addressing the above concerns, we’ll lay a solid foundation for success and create a positive experience for physicians and patients alike.
Dr. Notte is a family physician and chief medical officer of Abington (Pa.) Hospital–Jefferson Health. Follow him on Twitter (@doctornotte). Dr. Skolnik is professor of family and community medicine at Sidney Kimmel Medical College, Philadelphia, and associate director of the family medicine residency program at Abington (Pa.) Hospital–Jefferson Health. They have no conflicts related to the content of this piece.
Reference
1. A running list of companies that have filed for bankruptcy during the coronavirus pandemic. Fortune.
Has telemedicine found its footing?
Has telemedicine found its footing?
When Alexander Graham Bell invented the telephone, he accomplished something that many telegraph devotees never thought possible: the synchronous, bidirectional transmission of voice over electrical lines.
This was an incredible milestone in the advancement of mankind and enabled true revolutions in commerce, scientific collaboration, and human interaction. But Mr. Bell knew his invention didn’t represent the final advancement in telecommunication; he was quite prescient in imagining a day when individuals could see each other while speaking on the phone.
Many years later, what was once only a dream is now commonplace, and children growing up today can’t imagine a world where apps such as FaceTime and Skype don’t exist. Until recently, however, the medical community has been slow to adopt the idea of video interactions. This has dramatically changed because of the pandemic and the need for social distancing. It appears that telemedicine has found its footing, but whether it will remain popular once patients feel safe going to see their doctors in person again remains to be seen. This month, we’ll examine a few key issues that will determine the future of virtual medical visits.
Collect calling
The pandemic has wrought both human and economic casualties. With fear, job loss, and regulations leading to decreased spending, many large and small businesses have been and will continue to be unable to survive. Companies, including Brooks Brothers, Hertz, Lord and Taylor, GNC, and J.C. Penney, have declared bankruptcy.1 Medical practices and hospitals have taken cuts to their bottom line, and we’ve heard of many physician groups that have had to enact substantial salary cuts or even lay off providers – something previously unheard of. Recent months have demonstrated the health care community’s commitment to put patients first, but we simply cannot survive if we aren’t adequately reimbursed. Traditionally, this has been a significant roadblock toward the widespread adoption of telemedicine.
In most cases, these visits were not reimbursed at all. Thankfully, shortly after the coronavirus hit our shores, Medicare and Medicaid changed their policies, offering equal payment for video and in-person patient encounters. Most private insurers have followed suit, but the commitment to this payment parity appears – thus far – to be temporary. It is unclear that the financial support of telemedicine will continue post COVID-19, and this has many physicians feeling uncomfortable. In the meantime, many patients have come to prefer virtual visits, appreciating the convenience and efficiency.
Physicians don’t always have the same experience. Telemedicine can be technically challenging and take just as much – or sometimes more – time to navigate and document. Unless they are reimbursed equitably, providers will be forced to limit their use of virtual visits or not offer them at all. This leads to another issue: reliability.
‘Can you hear me now?’
Over the past several months, we have had the opportunity to use telemedicine firsthand and have spoken to many other physicians and patients about their experiences with it. The reports are all quite consistent: Most have had generally positive things to say. Still, some common concerns emerge when diving a bit deeper. Most notably are complaints about usability and reliability of the software.
While there are large telemedicine companies that have developed world-class cross-platform products, many in use today are proprietary and EHR dependent. As a result, the quality varies widely. Many EHR vendors were caught completely off guard by the sudden demand for telemedicine and are playing catch-up as they develop their own virtual visit platforms. While these vendor-developed platforms promise tight integration with patient records, some have significant shortcomings in stability when taxed under high utilization, including choppy video and garbled voice. This simply won’t do if telemedicine is to survive. It is incumbent on software developers and health care providers to invest in high-quality, reliable platforms on which to build their virtual visit offerings. This will ensure a more rapid adoption and the “staying power” of the new technology.
Dialing ‘0’ for the operator
Once seen as a “novelty” offered by only a small number of medical providers, virtual visits now represent a significant and ever-increasing percentage of patient encounters. The technology therefore must be easy to use. Given confidentiality and documentation requirements, along with the broad variety of available computing platforms and devices (e.g., PC, Mac, iOS, and Android), the process is often far from problem free. Patients may need help downloading apps, setting up webcams, or registering for the service. Providers may face issues with Internet connectivity or EHR-related delays.
It is critical that help be available to make the connection seamless and the experience a positive one. We are fortunate to work for a health care institution that has made this a priority, dedicating a team of individuals to provide real-time support to patients and clinicians. Small independent practices may not have this luxury, but we would encourage all providers to engage with their telemedicine or EHR vendors to determine what resources are available when problems arise, as they undoubtedly will.
Answering the call
Like the invention of the telephone, the advent of telemedicine is another milestone on the journey toward better communication with our patients, and it appears to be here to stay. Virtual visits won’t completely replace in-person care, nor minimize the benefit of human interaction, but they will continue to play an important role in the care continuum. By addressing the above concerns, we’ll lay a solid foundation for success and create a positive experience for physicians and patients alike.
Dr. Notte is a family physician and chief medical officer of Abington (Pa.) Hospital–Jefferson Health. Follow him on Twitter (@doctornotte). Dr. Skolnik is professor of family and community medicine at Sidney Kimmel Medical College, Philadelphia, and associate director of the family medicine residency program at Abington (Pa.) Hospital–Jefferson Health. They have no conflicts related to the content of this piece.
Reference
1. A running list of companies that have filed for bankruptcy during the coronavirus pandemic. Fortune.
When Alexander Graham Bell invented the telephone, he accomplished something that many telegraph devotees never thought possible: the synchronous, bidirectional transmission of voice over electrical lines.
This was an incredible milestone in the advancement of mankind and enabled true revolutions in commerce, scientific collaboration, and human interaction. But Mr. Bell knew his invention didn’t represent the final advancement in telecommunication; he was quite prescient in imagining a day when individuals could see each other while speaking on the phone.
Many years later, what was once only a dream is now commonplace, and children growing up today can’t imagine a world where apps such as FaceTime and Skype don’t exist. Until recently, however, the medical community has been slow to adopt the idea of video interactions. This has dramatically changed because of the pandemic and the need for social distancing. It appears that telemedicine has found its footing, but whether it will remain popular once patients feel safe going to see their doctors in person again remains to be seen. This month, we’ll examine a few key issues that will determine the future of virtual medical visits.
Collect calling
The pandemic has wrought both human and economic casualties. With fear, job loss, and regulations leading to decreased spending, many large and small businesses have been and will continue to be unable to survive. Companies, including Brooks Brothers, Hertz, Lord and Taylor, GNC, and J.C. Penney, have declared bankruptcy.1 Medical practices and hospitals have taken cuts to their bottom line, and we’ve heard of many physician groups that have had to enact substantial salary cuts or even lay off providers – something previously unheard of. Recent months have demonstrated the health care community’s commitment to put patients first, but we simply cannot survive if we aren’t adequately reimbursed. Traditionally, this has been a significant roadblock toward the widespread adoption of telemedicine.
In most cases, these visits were not reimbursed at all. Thankfully, shortly after the coronavirus hit our shores, Medicare and Medicaid changed their policies, offering equal payment for video and in-person patient encounters. Most private insurers have followed suit, but the commitment to this payment parity appears – thus far – to be temporary. It is unclear that the financial support of telemedicine will continue post COVID-19, and this has many physicians feeling uncomfortable. In the meantime, many patients have come to prefer virtual visits, appreciating the convenience and efficiency.
Physicians don’t always have the same experience. Telemedicine can be technically challenging and take just as much – or sometimes more – time to navigate and document. Unless they are reimbursed equitably, providers will be forced to limit their use of virtual visits or not offer them at all. This leads to another issue: reliability.
‘Can you hear me now?’
Over the past several months, we have had the opportunity to use telemedicine firsthand and have spoken to many other physicians and patients about their experiences with it. The reports are all quite consistent: Most have had generally positive things to say. Still, some common concerns emerge when diving a bit deeper. Most notably are complaints about usability and reliability of the software.
While there are large telemedicine companies that have developed world-class cross-platform products, many in use today are proprietary and EHR dependent. As a result, the quality varies widely. Many EHR vendors were caught completely off guard by the sudden demand for telemedicine and are playing catch-up as they develop their own virtual visit platforms. While these vendor-developed platforms promise tight integration with patient records, some have significant shortcomings in stability when taxed under high utilization, including choppy video and garbled voice. This simply won’t do if telemedicine is to survive. It is incumbent on software developers and health care providers to invest in high-quality, reliable platforms on which to build their virtual visit offerings. This will ensure a more rapid adoption and the “staying power” of the new technology.
Dialing ‘0’ for the operator
Once seen as a “novelty” offered by only a small number of medical providers, virtual visits now represent a significant and ever-increasing percentage of patient encounters. The technology therefore must be easy to use. Given confidentiality and documentation requirements, along with the broad variety of available computing platforms and devices (e.g., PC, Mac, iOS, and Android), the process is often far from problem free. Patients may need help downloading apps, setting up webcams, or registering for the service. Providers may face issues with Internet connectivity or EHR-related delays.
It is critical that help be available to make the connection seamless and the experience a positive one. We are fortunate to work for a health care institution that has made this a priority, dedicating a team of individuals to provide real-time support to patients and clinicians. Small independent practices may not have this luxury, but we would encourage all providers to engage with their telemedicine or EHR vendors to determine what resources are available when problems arise, as they undoubtedly will.
Answering the call
Like the invention of the telephone, the advent of telemedicine is another milestone on the journey toward better communication with our patients, and it appears to be here to stay. Virtual visits won’t completely replace in-person care, nor minimize the benefit of human interaction, but they will continue to play an important role in the care continuum. By addressing the above concerns, we’ll lay a solid foundation for success and create a positive experience for physicians and patients alike.
Dr. Notte is a family physician and chief medical officer of Abington (Pa.) Hospital–Jefferson Health. Follow him on Twitter (@doctornotte). Dr. Skolnik is professor of family and community medicine at Sidney Kimmel Medical College, Philadelphia, and associate director of the family medicine residency program at Abington (Pa.) Hospital–Jefferson Health. They have no conflicts related to the content of this piece.
Reference
1. A running list of companies that have filed for bankruptcy during the coronavirus pandemic. Fortune.
COVID-19 child case count now over 400,000
according to a new report from the American Academy of Pediatrics and the Children’s Hospital Association.
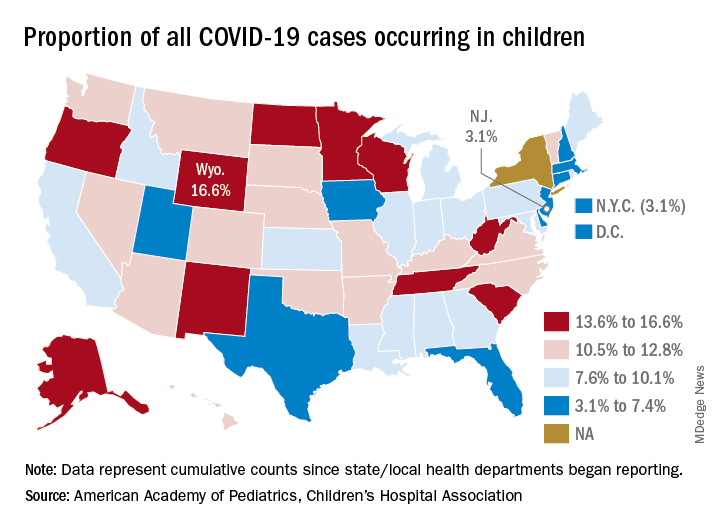
The 406,000 children who have tested positive for COVID-19 represent 9.1% of all cases reported so far by 49 states (New York does not provide age distribution), New York City, the District of Columbia, Puerto Rico, and Guam. Since the proportion of child cases also was 9.1% on Aug. 6, the most recent week is the first without an increase since tracking began in mid-April, the report shows.
State-level data show that Wyoming has the highest percentage of child cases (16.6%) after Alabama changed its “definition of child case from 0-24 to 0-17 years, resulting in a downward revision of cumulative child cases,” the AAP and the CHA said. Alabama’s proportion of such cases dropped from 22.5% to 9.0%.
New Jersey had the lowest rate (3.1%) again this week, along with New York City, but both were up slightly from the week before, when New Jersey was at 2.9% and N.Y.C. was 3.0%. The only states, other than Alabama, that saw declines over the last week were Arkansas, Massachusetts, Mississippi, South Dakota, Texas, and West Virginia. Texas, however, has reported age for only 8% of its confirmed cases, the report noted.
The overall rate of child COVID-19 cases as of Aug. 13 was 538 per 100,000 children, up from 500.7 per 100,000 a week earlier. Arizona was again highest among the states with a rate of 1,254 per 100,000 (up from 1,206) and Vermont was lowest at 121, although Puerto Rico (114) and Guam (88) were lower still, the AAP/CHA data indicate.
For the nine states that report testing information for children, Arizona has the highest positivity rate at 18.3% and West Virginia has the lowest at 3.6%. Data on hospitalizations – available from 21 states and N.Y.C. – show that 3,849 children have been admitted, with rates varying from 0.2% of children in Hawaii to 8.8% in the Big Apple, according to the report.
More specific information on child cases, such as symptoms or underlying conditions, is not being provided by states at this time, the AAP and CHA pointed out.
according to a new report from the American Academy of Pediatrics and the Children’s Hospital Association.

The 406,000 children who have tested positive for COVID-19 represent 9.1% of all cases reported so far by 49 states (New York does not provide age distribution), New York City, the District of Columbia, Puerto Rico, and Guam. Since the proportion of child cases also was 9.1% on Aug. 6, the most recent week is the first without an increase since tracking began in mid-April, the report shows.
State-level data show that Wyoming has the highest percentage of child cases (16.6%) after Alabama changed its “definition of child case from 0-24 to 0-17 years, resulting in a downward revision of cumulative child cases,” the AAP and the CHA said. Alabama’s proportion of such cases dropped from 22.5% to 9.0%.
New Jersey had the lowest rate (3.1%) again this week, along with New York City, but both were up slightly from the week before, when New Jersey was at 2.9% and N.Y.C. was 3.0%. The only states, other than Alabama, that saw declines over the last week were Arkansas, Massachusetts, Mississippi, South Dakota, Texas, and West Virginia. Texas, however, has reported age for only 8% of its confirmed cases, the report noted.
The overall rate of child COVID-19 cases as of Aug. 13 was 538 per 100,000 children, up from 500.7 per 100,000 a week earlier. Arizona was again highest among the states with a rate of 1,254 per 100,000 (up from 1,206) and Vermont was lowest at 121, although Puerto Rico (114) and Guam (88) were lower still, the AAP/CHA data indicate.
For the nine states that report testing information for children, Arizona has the highest positivity rate at 18.3% and West Virginia has the lowest at 3.6%. Data on hospitalizations – available from 21 states and N.Y.C. – show that 3,849 children have been admitted, with rates varying from 0.2% of children in Hawaii to 8.8% in the Big Apple, according to the report.
More specific information on child cases, such as symptoms or underlying conditions, is not being provided by states at this time, the AAP and CHA pointed out.
according to a new report from the American Academy of Pediatrics and the Children’s Hospital Association.

The 406,000 children who have tested positive for COVID-19 represent 9.1% of all cases reported so far by 49 states (New York does not provide age distribution), New York City, the District of Columbia, Puerto Rico, and Guam. Since the proportion of child cases also was 9.1% on Aug. 6, the most recent week is the first without an increase since tracking began in mid-April, the report shows.
State-level data show that Wyoming has the highest percentage of child cases (16.6%) after Alabama changed its “definition of child case from 0-24 to 0-17 years, resulting in a downward revision of cumulative child cases,” the AAP and the CHA said. Alabama’s proportion of such cases dropped from 22.5% to 9.0%.
New Jersey had the lowest rate (3.1%) again this week, along with New York City, but both were up slightly from the week before, when New Jersey was at 2.9% and N.Y.C. was 3.0%. The only states, other than Alabama, that saw declines over the last week were Arkansas, Massachusetts, Mississippi, South Dakota, Texas, and West Virginia. Texas, however, has reported age for only 8% of its confirmed cases, the report noted.
The overall rate of child COVID-19 cases as of Aug. 13 was 538 per 100,000 children, up from 500.7 per 100,000 a week earlier. Arizona was again highest among the states with a rate of 1,254 per 100,000 (up from 1,206) and Vermont was lowest at 121, although Puerto Rico (114) and Guam (88) were lower still, the AAP/CHA data indicate.
For the nine states that report testing information for children, Arizona has the highest positivity rate at 18.3% and West Virginia has the lowest at 3.6%. Data on hospitalizations – available from 21 states and N.Y.C. – show that 3,849 children have been admitted, with rates varying from 0.2% of children in Hawaii to 8.8% in the Big Apple, according to the report.
More specific information on child cases, such as symptoms or underlying conditions, is not being provided by states at this time, the AAP and CHA pointed out.
Are aging physicians a burden?
The evaluation of physicians with alleged cognitive decline
As forensic evaluators, we are often asked to review and assess the cognition of aging colleagues. The premise often involves a minor mistake, a poor choice of words, or a lapse in judgment. A physician gets reported for having difficulty using a new electronic form, forgetting the dose of a brand new medication, or getting upset in a public setting. Those behaviors often lead to mandatory psychiatric evaluations. Those requirements are often perceived by the provider as an insult, and betrayal by peers despite many years of dedicated work.
Interestingly, we have noticed many independent evaluators and hospital administrators using this opportunity to send many of our colleagues to pasture. There seems to be an unspoken rule among some forensic evaluators that physicians should represent some form of apex of humanity, beyond reproach, and beyond any fault. Those evaluators will point to any mistake on cognitive scales as proof that the aging physician is no longer safe to practice.1 Forgetting that Jill is from Illinois in the Saint Louis University Mental Status Examination test or how to copy a three-dimensional cube on the Montreal Cognitive Assessment can cost someone their license.2 We are also aware of some evaluators even taking the step further and opining that physicians not only need to score adequately but also demonstrate cognition significantly above average to maintain their privileges.
There is certainly significant appeal in setting a high bar for physicians. In many ways, physicians are characterized in society by their astuteness, intelligence, and high ethical standards. Patients place their lives in the hands of physicians and should trust that those physicians have the cognitive tools to heal them. It could almost seem evident that physicians should have high IQs, score perfectly on screening tools for dementia, and complete a mandatory psychiatric evaluation without any reproach. Yet the reality is often more complex.
We have two main concerns about the idea that we should be intransigent with aging physicians. The first one is the vast differential diagnosis for minor mistakes. An aging physician refusing to comply with a new form or yelling at a clerk once when asked to learn a new electronic medical record are inappropriate though not specific assessments for dementia. Similarly, having significant difficulty learning a new electronic medical record system more often is a sign of ageism rather than cognitive impairment. Subsequently, when arriving for their evaluation, forgetting the date is a common sign of anxiety. A relatable analogy would be to compare the mistake with a medical student forgetting part of the anatomy while questioning by an attending during surgery. Imagine such medical students being referred to mandatory psychiatric evaluation when failing to answer a question during rounds.
In our practice, the most common reason for those minor mistakes during our clinical evaluation is anxiety. After all, patients who present for problems completely unrelated to cognitive decline make similar mistakes. Psychological stressors in physicians require no introduction. The concept is so prevalent and pervasive that it has its own name, “burnout.” Imagine having dedicated most of one’s life to a profession then being enumerated a list of complaints, having one’s privileges put on hold, then being told to complete an independent psychiatric evaluation. If burnout is in part caused by a lack of control, unclear job expectations, rapidly changing models of health care, and dysfunctional workplace dynamics, imagine the consequence of such a referral.
The militant evaluator will use jargon to vilify the reviewed physician. If the physician complains too voraciously, he will be described as having signs of frontotemporal dementia. If the physician comes with a written list of rebuttals, he will be described as having memory problems requiring aids. If the physician is demoralized and quiet, he will be described as being withdrawn and apathetic. If the physician refuses to use or has difficulty with new forms or electronic systems, he will be described as having “impaired executive function,” an ominous term that surely should not be associated with a practicing physician.
The second concern arises from problems with the validity and use of diagnoses like mild cognitive impairment (MCI). MCI is considered to be a transition stage when one maintains “normal activities of daily living, and normal general cognitive function.”3 The American Psychiatric Association Textbook of Psychiatry mentions that there are “however, many cases of nonprogressive MCI.” Should a disorder with generally normal cognition and unclear progression to a more severe disorder require one to be dispensed of their privileges? Should any disorder trump an assessment of functioning?
It is our experience that many if not most physicians’ practice of medicine is not a job but a profession that defines who they are. As such, their occupational habits are an overly repeated and ingrained series of maneuvers analogous to so-called muscle memory. This kind of ritualistic pattern is precisely the kind of cognition that may persist as one starts to have some deficits. This requires the evaluator to be particularly sensitive and cognizant that one may still be able to perform professionally despite some mild but notable deficits. While it is facile to diagnose someone with MCI and justify removing their license, a review of their actual clinical skills is, despite being more time consuming, more pertinent to the evaluation.
In practice, we find that many cases lie in a gray area, which is hard to define. Physicians may come to our office for an evaluation after having said something odd at work. Maybe they misdosed a medication on one occasion. Maybe they wrote the wrong year on a chart. However, if the physician was 30 years old, would we consider any one of those incidents significant? As a psychiatrist rather than a physician practicing the specialty in review, it is particularly hard and sometimes unwise to condone or sanction individual incidents.
Evaluators find solace in neuropsychological testing. However the relevance to the safety of patients is unclear. Many of those tests end up being a simple proxy for age. A physicians’ ability to sort words or cards at a certain speed might correlate to cognitive performance but has unclear significance to the ability to care for patients. Using such tests becomes a de facto age limit on the practice of medicine. It seems essential to expand and refine our repertoire of evaluation tools for the assessment of physicians. As when we perform capacity evaluation in the hospital, we enlist the assistance of the treating team in understanding the questions being asked for a patient, medical boards could consider creating independent multidisciplinary teams where psychiatry has a seat along with the relevant specialties of the evaluee. Likewise, the assessment would benefit from a broad review of the physicians’ general practice rather than the more typical review of one or two incidents.
We are promoting a more individualized approach by medical boards to the many issues of the aging physician. Retiring is no longer the dream of older physicians, but rather working in the suitable position where their contributions, clinical experience, and wisdom are positive contributions to patient care. Furthermore, we encourage medical boards to consider more nuanced decisions. A binary approach fits few cases that we see. Surgeons are a prime example of this. A surgeon in the early stages of Parkinsonism may be unfit to perform surgery but very capable of continuing to contribute to the well-being of patients in other forms of clinical work, including postsurgical care that doesn’t involve physical dexterity. Similarly, medical boards could consider other forms of partial restrictions, including a ban on procedures, a ban on hospital privileges, as well as required supervision or working in teams. Accumulated clinical wisdom allows older physicians to be excellent mentors and educators for younger doctors. There is no simple method to predict which physicians may have the early stages of a progressive dementia, and which may have a stable MCI. A yearly reevaluation if there are no further complaints, is the best approach to determine progression of cognitive problems.
Few crises like the current COVID-19 pandemic can better remind us of the importance of the place of medicine in society. Many states have encouraged retired physicians to contribute their knowledge and expertise, putting themselves in particular risk because of their age. It is a good time to be reminded that we owe them significant respect and care when deciding to remove their license. We are encouraged by the diligent efforts of medical boards in supervising our colleagues but warn against zealot evaluators who use this opportunity to force physicians into retirement. We also encourage medical boards to expand their tools and approaches when facing such cases, as mislabeled cognitive diagnoses can be an easy scapegoat of a poor understanding of the more important psychological and biological factors in the evaluation.
References
1. Tariq SH et al. Am J Geriatr Psychiatry. 2006;14:900-10.
2. Nasreddine Z. mocatest.org. Version 2004 Nov 7.
3. Hales RE et al. The American Psychiatric Publishing Textbook of Psychiatry. Washington: American Psychiatric Association Publishing, 2014.
Dr. Badre is a forensic psychiatrist in San Diego and an expert in correctional mental health. He holds teaching positions at the University of California, San Diego, and the University of San Diego. He teaches medical education, psychopharmacology, ethics in psychiatry, and correctional care. Among his writings in chapter 7 in the book “Critical Psychiatry: Controversies and Clinical Implications” (Cham, Switzerland: Springer, 2019). He has no disclosures.
Dr. Abrams is a forensic psychiatrist and attorney in San Diego. He is an expert in addictionology, behavioral toxicology, psychopharmacology and correctional mental health. He holds a teaching positions at the University of California, San Diego. Among his writings are chapters about competency in national textbooks. Dr. Abrams has no disclosures.
The evaluation of physicians with alleged cognitive decline
The evaluation of physicians with alleged cognitive decline
As forensic evaluators, we are often asked to review and assess the cognition of aging colleagues. The premise often involves a minor mistake, a poor choice of words, or a lapse in judgment. A physician gets reported for having difficulty using a new electronic form, forgetting the dose of a brand new medication, or getting upset in a public setting. Those behaviors often lead to mandatory psychiatric evaluations. Those requirements are often perceived by the provider as an insult, and betrayal by peers despite many years of dedicated work.
Interestingly, we have noticed many independent evaluators and hospital administrators using this opportunity to send many of our colleagues to pasture. There seems to be an unspoken rule among some forensic evaluators that physicians should represent some form of apex of humanity, beyond reproach, and beyond any fault. Those evaluators will point to any mistake on cognitive scales as proof that the aging physician is no longer safe to practice.1 Forgetting that Jill is from Illinois in the Saint Louis University Mental Status Examination test or how to copy a three-dimensional cube on the Montreal Cognitive Assessment can cost someone their license.2 We are also aware of some evaluators even taking the step further and opining that physicians not only need to score adequately but also demonstrate cognition significantly above average to maintain their privileges.
There is certainly significant appeal in setting a high bar for physicians. In many ways, physicians are characterized in society by their astuteness, intelligence, and high ethical standards. Patients place their lives in the hands of physicians and should trust that those physicians have the cognitive tools to heal them. It could almost seem evident that physicians should have high IQs, score perfectly on screening tools for dementia, and complete a mandatory psychiatric evaluation without any reproach. Yet the reality is often more complex.
We have two main concerns about the idea that we should be intransigent with aging physicians. The first one is the vast differential diagnosis for minor mistakes. An aging physician refusing to comply with a new form or yelling at a clerk once when asked to learn a new electronic medical record are inappropriate though not specific assessments for dementia. Similarly, having significant difficulty learning a new electronic medical record system more often is a sign of ageism rather than cognitive impairment. Subsequently, when arriving for their evaluation, forgetting the date is a common sign of anxiety. A relatable analogy would be to compare the mistake with a medical student forgetting part of the anatomy while questioning by an attending during surgery. Imagine such medical students being referred to mandatory psychiatric evaluation when failing to answer a question during rounds.
In our practice, the most common reason for those minor mistakes during our clinical evaluation is anxiety. After all, patients who present for problems completely unrelated to cognitive decline make similar mistakes. Psychological stressors in physicians require no introduction. The concept is so prevalent and pervasive that it has its own name, “burnout.” Imagine having dedicated most of one’s life to a profession then being enumerated a list of complaints, having one’s privileges put on hold, then being told to complete an independent psychiatric evaluation. If burnout is in part caused by a lack of control, unclear job expectations, rapidly changing models of health care, and dysfunctional workplace dynamics, imagine the consequence of such a referral.
The militant evaluator will use jargon to vilify the reviewed physician. If the physician complains too voraciously, he will be described as having signs of frontotemporal dementia. If the physician comes with a written list of rebuttals, he will be described as having memory problems requiring aids. If the physician is demoralized and quiet, he will be described as being withdrawn and apathetic. If the physician refuses to use or has difficulty with new forms or electronic systems, he will be described as having “impaired executive function,” an ominous term that surely should not be associated with a practicing physician.
The second concern arises from problems with the validity and use of diagnoses like mild cognitive impairment (MCI). MCI is considered to be a transition stage when one maintains “normal activities of daily living, and normal general cognitive function.”3 The American Psychiatric Association Textbook of Psychiatry mentions that there are “however, many cases of nonprogressive MCI.” Should a disorder with generally normal cognition and unclear progression to a more severe disorder require one to be dispensed of their privileges? Should any disorder trump an assessment of functioning?
It is our experience that many if not most physicians’ practice of medicine is not a job but a profession that defines who they are. As such, their occupational habits are an overly repeated and ingrained series of maneuvers analogous to so-called muscle memory. This kind of ritualistic pattern is precisely the kind of cognition that may persist as one starts to have some deficits. This requires the evaluator to be particularly sensitive and cognizant that one may still be able to perform professionally despite some mild but notable deficits. While it is facile to diagnose someone with MCI and justify removing their license, a review of their actual clinical skills is, despite being more time consuming, more pertinent to the evaluation.
In practice, we find that many cases lie in a gray area, which is hard to define. Physicians may come to our office for an evaluation after having said something odd at work. Maybe they misdosed a medication on one occasion. Maybe they wrote the wrong year on a chart. However, if the physician was 30 years old, would we consider any one of those incidents significant? As a psychiatrist rather than a physician practicing the specialty in review, it is particularly hard and sometimes unwise to condone or sanction individual incidents.
Evaluators find solace in neuropsychological testing. However the relevance to the safety of patients is unclear. Many of those tests end up being a simple proxy for age. A physicians’ ability to sort words or cards at a certain speed might correlate to cognitive performance but has unclear significance to the ability to care for patients. Using such tests becomes a de facto age limit on the practice of medicine. It seems essential to expand and refine our repertoire of evaluation tools for the assessment of physicians. As when we perform capacity evaluation in the hospital, we enlist the assistance of the treating team in understanding the questions being asked for a patient, medical boards could consider creating independent multidisciplinary teams where psychiatry has a seat along with the relevant specialties of the evaluee. Likewise, the assessment would benefit from a broad review of the physicians’ general practice rather than the more typical review of one or two incidents.
We are promoting a more individualized approach by medical boards to the many issues of the aging physician. Retiring is no longer the dream of older physicians, but rather working in the suitable position where their contributions, clinical experience, and wisdom are positive contributions to patient care. Furthermore, we encourage medical boards to consider more nuanced decisions. A binary approach fits few cases that we see. Surgeons are a prime example of this. A surgeon in the early stages of Parkinsonism may be unfit to perform surgery but very capable of continuing to contribute to the well-being of patients in other forms of clinical work, including postsurgical care that doesn’t involve physical dexterity. Similarly, medical boards could consider other forms of partial restrictions, including a ban on procedures, a ban on hospital privileges, as well as required supervision or working in teams. Accumulated clinical wisdom allows older physicians to be excellent mentors and educators for younger doctors. There is no simple method to predict which physicians may have the early stages of a progressive dementia, and which may have a stable MCI. A yearly reevaluation if there are no further complaints, is the best approach to determine progression of cognitive problems.
Few crises like the current COVID-19 pandemic can better remind us of the importance of the place of medicine in society. Many states have encouraged retired physicians to contribute their knowledge and expertise, putting themselves in particular risk because of their age. It is a good time to be reminded that we owe them significant respect and care when deciding to remove their license. We are encouraged by the diligent efforts of medical boards in supervising our colleagues but warn against zealot evaluators who use this opportunity to force physicians into retirement. We also encourage medical boards to expand their tools and approaches when facing such cases, as mislabeled cognitive diagnoses can be an easy scapegoat of a poor understanding of the more important psychological and biological factors in the evaluation.
References
1. Tariq SH et al. Am J Geriatr Psychiatry. 2006;14:900-10.
2. Nasreddine Z. mocatest.org. Version 2004 Nov 7.
3. Hales RE et al. The American Psychiatric Publishing Textbook of Psychiatry. Washington: American Psychiatric Association Publishing, 2014.
Dr. Badre is a forensic psychiatrist in San Diego and an expert in correctional mental health. He holds teaching positions at the University of California, San Diego, and the University of San Diego. He teaches medical education, psychopharmacology, ethics in psychiatry, and correctional care. Among his writings in chapter 7 in the book “Critical Psychiatry: Controversies and Clinical Implications” (Cham, Switzerland: Springer, 2019). He has no disclosures.
Dr. Abrams is a forensic psychiatrist and attorney in San Diego. He is an expert in addictionology, behavioral toxicology, psychopharmacology and correctional mental health. He holds a teaching positions at the University of California, San Diego. Among his writings are chapters about competency in national textbooks. Dr. Abrams has no disclosures.
As forensic evaluators, we are often asked to review and assess the cognition of aging colleagues. The premise often involves a minor mistake, a poor choice of words, or a lapse in judgment. A physician gets reported for having difficulty using a new electronic form, forgetting the dose of a brand new medication, or getting upset in a public setting. Those behaviors often lead to mandatory psychiatric evaluations. Those requirements are often perceived by the provider as an insult, and betrayal by peers despite many years of dedicated work.
Interestingly, we have noticed many independent evaluators and hospital administrators using this opportunity to send many of our colleagues to pasture. There seems to be an unspoken rule among some forensic evaluators that physicians should represent some form of apex of humanity, beyond reproach, and beyond any fault. Those evaluators will point to any mistake on cognitive scales as proof that the aging physician is no longer safe to practice.1 Forgetting that Jill is from Illinois in the Saint Louis University Mental Status Examination test or how to copy a three-dimensional cube on the Montreal Cognitive Assessment can cost someone their license.2 We are also aware of some evaluators even taking the step further and opining that physicians not only need to score adequately but also demonstrate cognition significantly above average to maintain their privileges.
There is certainly significant appeal in setting a high bar for physicians. In many ways, physicians are characterized in society by their astuteness, intelligence, and high ethical standards. Patients place their lives in the hands of physicians and should trust that those physicians have the cognitive tools to heal them. It could almost seem evident that physicians should have high IQs, score perfectly on screening tools for dementia, and complete a mandatory psychiatric evaluation without any reproach. Yet the reality is often more complex.
We have two main concerns about the idea that we should be intransigent with aging physicians. The first one is the vast differential diagnosis for minor mistakes. An aging physician refusing to comply with a new form or yelling at a clerk once when asked to learn a new electronic medical record are inappropriate though not specific assessments for dementia. Similarly, having significant difficulty learning a new electronic medical record system more often is a sign of ageism rather than cognitive impairment. Subsequently, when arriving for their evaluation, forgetting the date is a common sign of anxiety. A relatable analogy would be to compare the mistake with a medical student forgetting part of the anatomy while questioning by an attending during surgery. Imagine such medical students being referred to mandatory psychiatric evaluation when failing to answer a question during rounds.
In our practice, the most common reason for those minor mistakes during our clinical evaluation is anxiety. After all, patients who present for problems completely unrelated to cognitive decline make similar mistakes. Psychological stressors in physicians require no introduction. The concept is so prevalent and pervasive that it has its own name, “burnout.” Imagine having dedicated most of one’s life to a profession then being enumerated a list of complaints, having one’s privileges put on hold, then being told to complete an independent psychiatric evaluation. If burnout is in part caused by a lack of control, unclear job expectations, rapidly changing models of health care, and dysfunctional workplace dynamics, imagine the consequence of such a referral.
The militant evaluator will use jargon to vilify the reviewed physician. If the physician complains too voraciously, he will be described as having signs of frontotemporal dementia. If the physician comes with a written list of rebuttals, he will be described as having memory problems requiring aids. If the physician is demoralized and quiet, he will be described as being withdrawn and apathetic. If the physician refuses to use or has difficulty with new forms or electronic systems, he will be described as having “impaired executive function,” an ominous term that surely should not be associated with a practicing physician.
The second concern arises from problems with the validity and use of diagnoses like mild cognitive impairment (MCI). MCI is considered to be a transition stage when one maintains “normal activities of daily living, and normal general cognitive function.”3 The American Psychiatric Association Textbook of Psychiatry mentions that there are “however, many cases of nonprogressive MCI.” Should a disorder with generally normal cognition and unclear progression to a more severe disorder require one to be dispensed of their privileges? Should any disorder trump an assessment of functioning?
It is our experience that many if not most physicians’ practice of medicine is not a job but a profession that defines who they are. As such, their occupational habits are an overly repeated and ingrained series of maneuvers analogous to so-called muscle memory. This kind of ritualistic pattern is precisely the kind of cognition that may persist as one starts to have some deficits. This requires the evaluator to be particularly sensitive and cognizant that one may still be able to perform professionally despite some mild but notable deficits. While it is facile to diagnose someone with MCI and justify removing their license, a review of their actual clinical skills is, despite being more time consuming, more pertinent to the evaluation.
In practice, we find that many cases lie in a gray area, which is hard to define. Physicians may come to our office for an evaluation after having said something odd at work. Maybe they misdosed a medication on one occasion. Maybe they wrote the wrong year on a chart. However, if the physician was 30 years old, would we consider any one of those incidents significant? As a psychiatrist rather than a physician practicing the specialty in review, it is particularly hard and sometimes unwise to condone or sanction individual incidents.
Evaluators find solace in neuropsychological testing. However the relevance to the safety of patients is unclear. Many of those tests end up being a simple proxy for age. A physicians’ ability to sort words or cards at a certain speed might correlate to cognitive performance but has unclear significance to the ability to care for patients. Using such tests becomes a de facto age limit on the practice of medicine. It seems essential to expand and refine our repertoire of evaluation tools for the assessment of physicians. As when we perform capacity evaluation in the hospital, we enlist the assistance of the treating team in understanding the questions being asked for a patient, medical boards could consider creating independent multidisciplinary teams where psychiatry has a seat along with the relevant specialties of the evaluee. Likewise, the assessment would benefit from a broad review of the physicians’ general practice rather than the more typical review of one or two incidents.
We are promoting a more individualized approach by medical boards to the many issues of the aging physician. Retiring is no longer the dream of older physicians, but rather working in the suitable position where their contributions, clinical experience, and wisdom are positive contributions to patient care. Furthermore, we encourage medical boards to consider more nuanced decisions. A binary approach fits few cases that we see. Surgeons are a prime example of this. A surgeon in the early stages of Parkinsonism may be unfit to perform surgery but very capable of continuing to contribute to the well-being of patients in other forms of clinical work, including postsurgical care that doesn’t involve physical dexterity. Similarly, medical boards could consider other forms of partial restrictions, including a ban on procedures, a ban on hospital privileges, as well as required supervision or working in teams. Accumulated clinical wisdom allows older physicians to be excellent mentors and educators for younger doctors. There is no simple method to predict which physicians may have the early stages of a progressive dementia, and which may have a stable MCI. A yearly reevaluation if there are no further complaints, is the best approach to determine progression of cognitive problems.
Few crises like the current COVID-19 pandemic can better remind us of the importance of the place of medicine in society. Many states have encouraged retired physicians to contribute their knowledge and expertise, putting themselves in particular risk because of their age. It is a good time to be reminded that we owe them significant respect and care when deciding to remove their license. We are encouraged by the diligent efforts of medical boards in supervising our colleagues but warn against zealot evaluators who use this opportunity to force physicians into retirement. We also encourage medical boards to expand their tools and approaches when facing such cases, as mislabeled cognitive diagnoses can be an easy scapegoat of a poor understanding of the more important psychological and biological factors in the evaluation.
References
1. Tariq SH et al. Am J Geriatr Psychiatry. 2006;14:900-10.
2. Nasreddine Z. mocatest.org. Version 2004 Nov 7.
3. Hales RE et al. The American Psychiatric Publishing Textbook of Psychiatry. Washington: American Psychiatric Association Publishing, 2014.
Dr. Badre is a forensic psychiatrist in San Diego and an expert in correctional mental health. He holds teaching positions at the University of California, San Diego, and the University of San Diego. He teaches medical education, psychopharmacology, ethics in psychiatry, and correctional care. Among his writings in chapter 7 in the book “Critical Psychiatry: Controversies and Clinical Implications” (Cham, Switzerland: Springer, 2019). He has no disclosures.
Dr. Abrams is a forensic psychiatrist and attorney in San Diego. He is an expert in addictionology, behavioral toxicology, psychopharmacology and correctional mental health. He holds a teaching positions at the University of California, San Diego. Among his writings are chapters about competency in national textbooks. Dr. Abrams has no disclosures.
COVID-19: A Dermatologist’s Experience From the US Epicenter
The 1918 H1N1 influenza pandemic was the most severe pandemic in recent history. Fifty to 100 million individuals died worldwide, with approximately 675,000 deaths in the United States.1-3 The fatality rate was approximately 2% and was highest during the second and third waves of the disease.4 At that time, there were no diagnostic tests for influenza infection, influenza vaccines, antiviral drugs, antibiotics to treat secondary bacterial infections, or mechanical ventilation. Some cities decided to close schools, limit public gatherings, self-isolate, and issue quarantine orders; the federal government took no central role.
The 1918 influenza pandemic seems far away in history, but my mother often tells me stories about her own grandmother who disliked shaking anyone’s hands and would worry when people coughed or sneezed around her. It sounded like she was overreacting. Now, we can better relate to her concerns. Life has changed dramatically.
In mid-February 2020, news spread that the coronavirus disease 2019 (COVID-19) had spread from Wuhan, China, to a number of countries in Asia and the Middle East. I was following the news with great sadness for those affected countries, especially for Iran, my country of origin, which had become an epicenter of COVID-19. We were not worried for ourselves in the United States. These infections seemed far away. However, once Italy became the new epicenter of COVID-19 with alarmingly high death rates, I grasped the inevitable reality: The novel coronavirus would not spare the United States and would not spare New York.
Then the virus arrived in New York City. On March 10, 2020, our hospital recommended using teledermatology instead of in-person visits in an attempt to keep patients safe in their own homes. Cases of COVID-19 were escalating, hospitals were filling up, health care workers were falling ill, and there was a shortage of health care staff and personal protective equipment (PPE). Dermatologists at various hospitals were asked to retrain to help care for COVID-19 patients.
On March 13, flights from Europe to the United States were suspended. A statewide stay-at-home order subsequently went into effect on March 22. It felt surreal. From March 23 on, various specialty physicians and nurses in our hospital volunteered to work as frontline staff in the newly prepared annex where patients with possible COVID-19 would arrive. My dermatology co-residents and I started working as frontline physicians. Everything we had heard from the countries affected first had become our reality. Our hospital, part of the largest public health care system in the nation, became a dedicated COVID-19 treatment center.
Large numbers of scared patients with symptoms of COVID-19 flooded the annex. We sent the majority of them home, unable to offer them even a diagnostic test, and advised them to stay isolated. We only had the capacity to test those who required hospital admission.
It broke my heart even more when my colleagues became patients. We often felt helpless, not being able to help every patient and not being able to help our infected colleagues.
Elective surgeries were suspended. Inpatient beds, including specialized intensive care unit beds, rapidly filled up with COVID-19 patients. To help with the surge of patients, our hospital added medical and intensive care unit beds. The hospital became surreal, the corridors eerily empty and silent while every bed was filled, and health care workers were rushing around the inpatient units.
Life quickly became filled with fears—worries about how sick the patients would be, how much we would be able to help them, whether we would have enough PPE, who among our friends or family might be infected next, and whether we might ourselves be next. As PPE became scarce, I desperately searched for some form of protective equipment. I hunted for protective masks, face shields, eye protection, and gowns. We had to reuse disposable N95 masks and face shields multiple times and disinfect them as best we could. Our attendings ordered any protective gear they could find for us. Nearly everything was sold out; the very few items remaining would not for arrive for months. I could have never imagined that I would be afraid of going to work, of not having the appropriate protective gear, and that any day might be my last because of my profession.
New York City had become the epicenter of COVID-19. The city, the country, and the world were in chaos. Hospitals were overflowing, and makeshift morgues were appearing outside of hospitals. Those who could fled the city. Despite warnings from experts, we were not prepared. The number of deaths was climbing rapidly. There was no clarity on who could be tested or how to get it done. It felt like a nightmare.
Social distancing was in place, nonessential businesses were shut down, street vendors disappeared, and people were advised to wear face coverings. People were afraid of each other, afraid of getting too close and catching the virus. New York City—The City That Never Sleeps—went into deep sleep. Every day brought ever greater numbers of infected patients and more deaths.
Every day at 7:00
After around 2 months of lockdown, New York City passed its peak, and the epicenter moved on. The current death toll (ie, confirmed deaths due to COVID-19) in New York stands at 18,836, while the reported death toll in the United States is 143,868, according to the Centers for Disease Control and Prevention. New York City has started a phased reopening to a new normal. Elective care has resumed, and people are leaving their homes again, eager to bring some sense of normalcy back into their lives.
I fear for those who will contract the virus in the next wave. I wonder what we will have learned.
Acknowledgment
The author wishes to thank Steven R. Feldman, MD, PhD (Winston-Salem, North Carolina), for his friendship and invaluable assistance with the conception and editing of this manuscript.
- Taubenberger JK. The origin and virulence of the 1918 “Spanish” influenza virus. Proc Am Philos Soc. 2006;150:86-112.
- Morens DM, Taubenberger JK. The mother of all pandemics is 100 years old (and going strong)! Am J Public Health. 2018;108:1449-1454.
- Johnson NPAS, Mueller J. Updating the accounts: global mortality of the 1918-1920 “Spanish” influenza pandemic. Bull Hist Med. 2002;76:105-115.
- Morens DM, Fauci AS. The 1918 influenza pandemic: insights for the 21st century. J Infect Dis. 2007;195:1018-1028.
The 1918 H1N1 influenza pandemic was the most severe pandemic in recent history. Fifty to 100 million individuals died worldwide, with approximately 675,000 deaths in the United States.1-3 The fatality rate was approximately 2% and was highest during the second and third waves of the disease.4 At that time, there were no diagnostic tests for influenza infection, influenza vaccines, antiviral drugs, antibiotics to treat secondary bacterial infections, or mechanical ventilation. Some cities decided to close schools, limit public gatherings, self-isolate, and issue quarantine orders; the federal government took no central role.
The 1918 influenza pandemic seems far away in history, but my mother often tells me stories about her own grandmother who disliked shaking anyone’s hands and would worry when people coughed or sneezed around her. It sounded like she was overreacting. Now, we can better relate to her concerns. Life has changed dramatically.
In mid-February 2020, news spread that the coronavirus disease 2019 (COVID-19) had spread from Wuhan, China, to a number of countries in Asia and the Middle East. I was following the news with great sadness for those affected countries, especially for Iran, my country of origin, which had become an epicenter of COVID-19. We were not worried for ourselves in the United States. These infections seemed far away. However, once Italy became the new epicenter of COVID-19 with alarmingly high death rates, I grasped the inevitable reality: The novel coronavirus would not spare the United States and would not spare New York.
Then the virus arrived in New York City. On March 10, 2020, our hospital recommended using teledermatology instead of in-person visits in an attempt to keep patients safe in their own homes. Cases of COVID-19 were escalating, hospitals were filling up, health care workers were falling ill, and there was a shortage of health care staff and personal protective equipment (PPE). Dermatologists at various hospitals were asked to retrain to help care for COVID-19 patients.
On March 13, flights from Europe to the United States were suspended. A statewide stay-at-home order subsequently went into effect on March 22. It felt surreal. From March 23 on, various specialty physicians and nurses in our hospital volunteered to work as frontline staff in the newly prepared annex where patients with possible COVID-19 would arrive. My dermatology co-residents and I started working as frontline physicians. Everything we had heard from the countries affected first had become our reality. Our hospital, part of the largest public health care system in the nation, became a dedicated COVID-19 treatment center.
Large numbers of scared patients with symptoms of COVID-19 flooded the annex. We sent the majority of them home, unable to offer them even a diagnostic test, and advised them to stay isolated. We only had the capacity to test those who required hospital admission.
It broke my heart even more when my colleagues became patients. We often felt helpless, not being able to help every patient and not being able to help our infected colleagues.
Elective surgeries were suspended. Inpatient beds, including specialized intensive care unit beds, rapidly filled up with COVID-19 patients. To help with the surge of patients, our hospital added medical and intensive care unit beds. The hospital became surreal, the corridors eerily empty and silent while every bed was filled, and health care workers were rushing around the inpatient units.
Life quickly became filled with fears—worries about how sick the patients would be, how much we would be able to help them, whether we would have enough PPE, who among our friends or family might be infected next, and whether we might ourselves be next. As PPE became scarce, I desperately searched for some form of protective equipment. I hunted for protective masks, face shields, eye protection, and gowns. We had to reuse disposable N95 masks and face shields multiple times and disinfect them as best we could. Our attendings ordered any protective gear they could find for us. Nearly everything was sold out; the very few items remaining would not for arrive for months. I could have never imagined that I would be afraid of going to work, of not having the appropriate protective gear, and that any day might be my last because of my profession.
New York City had become the epicenter of COVID-19. The city, the country, and the world were in chaos. Hospitals were overflowing, and makeshift morgues were appearing outside of hospitals. Those who could fled the city. Despite warnings from experts, we were not prepared. The number of deaths was climbing rapidly. There was no clarity on who could be tested or how to get it done. It felt like a nightmare.
Social distancing was in place, nonessential businesses were shut down, street vendors disappeared, and people were advised to wear face coverings. People were afraid of each other, afraid of getting too close and catching the virus. New York City—The City That Never Sleeps—went into deep sleep. Every day brought ever greater numbers of infected patients and more deaths.
Every day at 7:00
After around 2 months of lockdown, New York City passed its peak, and the epicenter moved on. The current death toll (ie, confirmed deaths due to COVID-19) in New York stands at 18,836, while the reported death toll in the United States is 143,868, according to the Centers for Disease Control and Prevention. New York City has started a phased reopening to a new normal. Elective care has resumed, and people are leaving their homes again, eager to bring some sense of normalcy back into their lives.
I fear for those who will contract the virus in the next wave. I wonder what we will have learned.
Acknowledgment
The author wishes to thank Steven R. Feldman, MD, PhD (Winston-Salem, North Carolina), for his friendship and invaluable assistance with the conception and editing of this manuscript.
The 1918 H1N1 influenza pandemic was the most severe pandemic in recent history. Fifty to 100 million individuals died worldwide, with approximately 675,000 deaths in the United States.1-3 The fatality rate was approximately 2% and was highest during the second and third waves of the disease.4 At that time, there were no diagnostic tests for influenza infection, influenza vaccines, antiviral drugs, antibiotics to treat secondary bacterial infections, or mechanical ventilation. Some cities decided to close schools, limit public gatherings, self-isolate, and issue quarantine orders; the federal government took no central role.
The 1918 influenza pandemic seems far away in history, but my mother often tells me stories about her own grandmother who disliked shaking anyone’s hands and would worry when people coughed or sneezed around her. It sounded like she was overreacting. Now, we can better relate to her concerns. Life has changed dramatically.
In mid-February 2020, news spread that the coronavirus disease 2019 (COVID-19) had spread from Wuhan, China, to a number of countries in Asia and the Middle East. I was following the news with great sadness for those affected countries, especially for Iran, my country of origin, which had become an epicenter of COVID-19. We were not worried for ourselves in the United States. These infections seemed far away. However, once Italy became the new epicenter of COVID-19 with alarmingly high death rates, I grasped the inevitable reality: The novel coronavirus would not spare the United States and would not spare New York.
Then the virus arrived in New York City. On March 10, 2020, our hospital recommended using teledermatology instead of in-person visits in an attempt to keep patients safe in their own homes. Cases of COVID-19 were escalating, hospitals were filling up, health care workers were falling ill, and there was a shortage of health care staff and personal protective equipment (PPE). Dermatologists at various hospitals were asked to retrain to help care for COVID-19 patients.
On March 13, flights from Europe to the United States were suspended. A statewide stay-at-home order subsequently went into effect on March 22. It felt surreal. From March 23 on, various specialty physicians and nurses in our hospital volunteered to work as frontline staff in the newly prepared annex where patients with possible COVID-19 would arrive. My dermatology co-residents and I started working as frontline physicians. Everything we had heard from the countries affected first had become our reality. Our hospital, part of the largest public health care system in the nation, became a dedicated COVID-19 treatment center.
Large numbers of scared patients with symptoms of COVID-19 flooded the annex. We sent the majority of them home, unable to offer them even a diagnostic test, and advised them to stay isolated. We only had the capacity to test those who required hospital admission.
It broke my heart even more when my colleagues became patients. We often felt helpless, not being able to help every patient and not being able to help our infected colleagues.
Elective surgeries were suspended. Inpatient beds, including specialized intensive care unit beds, rapidly filled up with COVID-19 patients. To help with the surge of patients, our hospital added medical and intensive care unit beds. The hospital became surreal, the corridors eerily empty and silent while every bed was filled, and health care workers were rushing around the inpatient units.
Life quickly became filled with fears—worries about how sick the patients would be, how much we would be able to help them, whether we would have enough PPE, who among our friends or family might be infected next, and whether we might ourselves be next. As PPE became scarce, I desperately searched for some form of protective equipment. I hunted for protective masks, face shields, eye protection, and gowns. We had to reuse disposable N95 masks and face shields multiple times and disinfect them as best we could. Our attendings ordered any protective gear they could find for us. Nearly everything was sold out; the very few items remaining would not for arrive for months. I could have never imagined that I would be afraid of going to work, of not having the appropriate protective gear, and that any day might be my last because of my profession.
New York City had become the epicenter of COVID-19. The city, the country, and the world were in chaos. Hospitals were overflowing, and makeshift morgues were appearing outside of hospitals. Those who could fled the city. Despite warnings from experts, we were not prepared. The number of deaths was climbing rapidly. There was no clarity on who could be tested or how to get it done. It felt like a nightmare.
Social distancing was in place, nonessential businesses were shut down, street vendors disappeared, and people were advised to wear face coverings. People were afraid of each other, afraid of getting too close and catching the virus. New York City—The City That Never Sleeps—went into deep sleep. Every day brought ever greater numbers of infected patients and more deaths.
Every day at 7:00
After around 2 months of lockdown, New York City passed its peak, and the epicenter moved on. The current death toll (ie, confirmed deaths due to COVID-19) in New York stands at 18,836, while the reported death toll in the United States is 143,868, according to the Centers for Disease Control and Prevention. New York City has started a phased reopening to a new normal. Elective care has resumed, and people are leaving their homes again, eager to bring some sense of normalcy back into their lives.
I fear for those who will contract the virus in the next wave. I wonder what we will have learned.
Acknowledgment
The author wishes to thank Steven R. Feldman, MD, PhD (Winston-Salem, North Carolina), for his friendship and invaluable assistance with the conception and editing of this manuscript.
- Taubenberger JK. The origin and virulence of the 1918 “Spanish” influenza virus. Proc Am Philos Soc. 2006;150:86-112.
- Morens DM, Taubenberger JK. The mother of all pandemics is 100 years old (and going strong)! Am J Public Health. 2018;108:1449-1454.
- Johnson NPAS, Mueller J. Updating the accounts: global mortality of the 1918-1920 “Spanish” influenza pandemic. Bull Hist Med. 2002;76:105-115.
- Morens DM, Fauci AS. The 1918 influenza pandemic: insights for the 21st century. J Infect Dis. 2007;195:1018-1028.
- Taubenberger JK. The origin and virulence of the 1918 “Spanish” influenza virus. Proc Am Philos Soc. 2006;150:86-112.
- Morens DM, Taubenberger JK. The mother of all pandemics is 100 years old (and going strong)! Am J Public Health. 2018;108:1449-1454.
- Johnson NPAS, Mueller J. Updating the accounts: global mortality of the 1918-1920 “Spanish” influenza pandemic. Bull Hist Med. 2002;76:105-115.
- Morens DM, Fauci AS. The 1918 influenza pandemic: insights for the 21st century. J Infect Dis. 2007;195:1018-1028.
Practice Points
- Coronavirus disease 2019 (COVID-19) can spread quickly, creating chaos in the health care system and leading to critical supply shortages within a short amount of time.
- Social distancing, quarantine, and isolation appear to be powerful tools in reducing the spread of COVID-19.
Restructuring health care delivery for the future: What we need to do post–COVID-19
Recently,
Barbara Levy, MD: The disruption of the COVID-19 pandemic has given us an opportunity to consider how we would recraft the delivery of health care for women if we could. My goal for this discussion is to talk about that and see if we can incentivize people to make changes.
Cindy, what are women looking for in health care that they are not getting now?
What women want in health care
Cynthia A. Pearson: Women, like men, want a sense of assurance that health care can be provided in a safe way, and that can’t be given completely right now.
Aside from that, women want a personal connection, ideally with the same provider. Many women are embracing telehealth, which came about because of this disruptive time, and that has potential that we can possibly mobilize around. One thing women don’t always find is consistency and contact, and they would like that.
Scott D. Hayworth, MD: Women want to be listened to, and they want their doctors to take a holistic and individualized approach to their care. In-person visits are the ideal setting for this, but during the pandemic we have had to adapt to new modalities for delivering care: government regulations restricting services, and the necessity to limit the flow of patients into offices, has meant that we have had to rely on remote visits. CareMount Medical has been in the forefront of telehealth with our “Virtual Visit” technology, so we were well prepared, and our patients have embraced this truly vital option. We’ve ramped up capabilities significantly to deal with the surge in volume.
While our practice has been able to provide consistent and convenient access to care, this isn’t the case in all areas of the country. Even before the pandemic, the cost of malpractice insurance has led to shortages of ObGyns; this deficit has been compounded by the closing of hospitals due to restrictions on services imposed to try to stem the spread of COVID-19. The affordability of care has also been jeopardized by job losses and therefore of employer-provided insurance, following months of lockdowns.
Continue to: Dr. Levy...
Dr. Levy: To balance that long-term relationship with access and cost, clearly we are not delivering what is needed. Janice, at UnitedHealth you have experimented with some products and some different ways of delivering care. What are beneficiaries looking for?
Janice Huckaby, MD: There is a real thirst for digital content—everybody consults with Dr. Google. They are looking for reliable sources of clinical content. Ideally, that comes from their physician, but people access it in other ways as well.
I agree that women desire a personalized relationship. That is why we are seeing more communities of women, such as virtual pregnancy support groups, that have cropped up in the age of COVID-19. Women are not content with the idea of “I’m going to see my doctor, get my tummy measured, listen to the heartbeat, and go home.” That model is done. Patients will look for practices that are accessible at convenient times and that can give them the personalized experience to make them feel well cared for and that offer them a long-term relationship.
One concern is that as more obstetric groups use laborists to do their deliveries at the hospital, I wonder whether we do a good job of forming that relationship on the front end, and when it comes to the delivery, will we drop the ball? The jury is out, but it’s worth watching.
Dr. Levy: How do we as obstetrician-gynecologists get patients to consider that we are providing reliable information? There is so much disinformation out there.
Errol R. Norwitz, MD, PhD, MBA: I echo the sentiments discussed and I’ll add that many women want care that is convenient, close to home, coordinated, and integrated—not fragmented. They want their providers and their office to anticipate and know who they are even before they arrive, to be prepared for the visit. And it’s not only care for them, but also care for their families. Women are the gatekeepers to the health care system. They want a health care system in place that will care not just for each member separately but also for the family as an integrated whole.
To answer your question, Barbara, we have all been overwhelmed with the amount of data coming at us, both providers and patients. Teaching providers how to synthesize and integrate the data and then present it to patients is quite a challenge. We have to instill this skill in our trainees, teach them how to absorb and present the data.
Consensus bodies can help in this regard, and ACOG (American College of Obstetricians and Gynecologists) has led the way in providing guidance around the management of pregnancy in the setting of COVID-19. Another reliable site for my trainees is UpToDate, which is easy to access. If a scientific paper comes out today, it will be covered in UpToDate tomorrow. Patients need someone who can synthesize the data and give it to them in little pieces, and keep it current.
Dr. Levy: We need to be a reliable source not only for medical information but also for referral to resources in the community for families and for women.
Continue to: ObGyn services...
ObGyn services: Primary care or specialty?
Dr. Norwitz: That begs the question, who are we? Are we primary care providers or are we a subspecialty, or are we both?
Ms. Pearson: Women, particularly in their younger, middle reproductive years, see their ObGyn as a primary care provider. The way forward for the profession is to embrace the call that Barbara articulated, to know what other referral sources are available beyond other clinicians. We need to be aware of the social determinants of health—that there are times when the primary care provider needs to know the community well enough to know what is available that would make a difference for that person and her family.
Dr. Levy: Scott, how do you manage that?
Dr. Hayworth: As reimbursement models move rapidly toward value, practices that can undertake risk are in the best position to thrive; specialty providers relying solely on fee-for-service may well be unable to survive. The key for any ObGyn practice is to be of sufficient size and scope that it can manage the primary care for a panel of patients, the more numerous the better; being in charge of those dollars allows maximum control. ObGyns who subspecialize should seek to become members of larger groups, whether comprehensive women’s health practices or multispecialty groups like ours at CareMount Medical, that manage the spectrum of care for their patients.
Dr. Levy: Janice, fill us in on some of the structures that exist now for ObGyns that they may be able to participate in—payment structures like the Women’s Medical Home. Does UnitedHealth have anything like that?
Dr. Huckaby: Probably 3 or 4 exist now, but I agree that risk arrangements are perhaps a wave of the future. Right now, UnitedHealth has accountable care organizations (ACOs) that include ObGyns, a number of them in the Northeast. We also rolled out bundled payment programs.
Our hospital contracts have always had metrics around infection rates and elective deliveries before 39 weeks, and we will probably start seeing some of that put into the provider contracts as well.
There is a desire to move people into a risk-sharing model for payment, but part of the concern there is the infrastructure, because if you are going to manage risk, you need to have staff that can do care coordination. Care coordinators can ensure, for example, that people have transportation to their appointments, and thus address some of the social determinants in ways that historically have not been done in obstetrics.
The ACOs sometimes have given seed money for practices to hire additional staff to do those kinds of things, and that can help get practices started. Probably the people best positioned are in large multispecialty groups that can leverage case management and maybe support other specialties.
I do think we are going to see a move to risk in the future. Obstetrics has moved at a slower pace than we have seen in internal medicine and some other specialties.
Dr. Hayworth: The value model for reimbursement can only be managed via care coordination, maximizing efficacy and efficiency at every level for every patient. Fortunately for ObGyns, we are familiar with the value concept via bundling for obstetrical services covering prenatal to postpartum, including delivery. ObGyn practices need to prepare for a future in which insurers will pay for patient panels in which providers take on the risk for the entirety of care.
At CareMount Medical, we have embraced the value model as one of 40 Next Generation Medicare Accountable Care Organizations across the country. We’ve put in place the infrastructure, from front desk through back office, to optimize resource utilization. Our team approach includes both patient advocates and care coordinators who extend the capabilities of our physicians and ensure that our patients’ needs, including well care, are met comprehensively.
Dr. Huckaby: One area that we sometimes leave out, whether we are talking about payment or a patient-centered medical home, is integration with behavioral health. Anxiety and depression are fairly rampant, fairly underdiagnosed, and woefully undertreated. I hope that our ObGyn practices of the future—and maybe this is the broadening into primary care—will engage and take the lead in addressing some of those issues, because women suffer. We need to embrace the behavioral aspect of care for the whole person more than we have.
Continue to: Physician training issues...
Physician training issues
Dr. Levy: I could not agree more. We have trained physicians to do illness care, not wellness care, and to be physician and practice centered, not patient centered. While we train medical students in hospital settings and in acute care, there’s not much training in how to manage people or in the factors that determine whether someone is truly well, such as housing security and food security. We are not training physicians in nutrition or in mental health.
Errol, how do we help an ObGyn or women’s health trainee to prepare for the ideal world we are trying to create?
Dr. Norwitz: It’s a challenging question. I like to reference a remarkable piece by Atul Gawande in The New Yorker, in which he interviewed the CEO of the Cheesecake Factory restaurant chain, who in effect said that we’ve got it all wrong; there’s no health in health care.1 We don’t manage health; we wait until people get sick and then we treat them. We have to put the health back into health care.
It has always been my passion to focus on preventative care. We need to reclaim our identity—I have never particularly liked the name “ObGyn,” the term “women’s health” may be more appropriate and help us focus on disease prevention—and we need to stand up for training programs that separate the O from the G.
Low-volume surgeons, who may do only 1 or 2 hysterectomies per year, can’t maintain their proficiency, and many don’t do enough cases to maintain their robotics privileges. I can foresee a time where labor and delivery units are like ICUs, where the people who work there do nothing but manage labor and perform deliveries using standardized bundles of practice. Such an approach will decrease variability in management and lead to improved outcomes.
We need to completely reframe how we train our pipeline providers to provide care in women’s health. It would be difficult, take a lot of effort, and there would be pushback, I suspect, but that’s where the field needs to go.
The ideal system redesign
Dr. Levy: Cindy, if you could start from scratch and design an ideal comprehensive system to better deliver care for women of all ages, what would that look like?
Ms. Pearson: I would design a system in which people at any life stage met with providers who were less trained in dealing with disease and more trained in the holistic approach to maintaining health. That might be a nurse practitioner or maybe a version of what Errol describes as a new way of training ObGyns. That’s the initial interaction, and the person could be with someone for decades and deepen the relationship in that wonderful way. It would also have an avenue for the times when disease needed to be treated or when more specialized care would be provided. And the financing would be worked out to support consistency.
Dr. Norwitz: We can learn from other countries. Singapore, with only 5.5 million people, has the best health care system in the world. They have a great model. Costa Rica and Cuba have completely redesigned their health care systems. You go through medical school in 2 or 3 years, and then you get embedded in the community. So you have doctors living in the community responsible for the health of their neighbors. They get to know people in the context in which they live and refer them on only when they need more than basic care. These countries have vastly superior outcome measures, and they spend less money on health care.
Dr. Levy: My dream, as we reinvent things, is that we could create a comprehensive Women’s Medical Home where there’s a hub and an opportunity to be centered on patients so they could reach us when needed.
Ideally we could create a structure with a central contact person—a nurse practitioner, a midwife, someone in family medicine or internal medicine—someone focused on women’s health who has researched how inequities apply to women and women’s health and the areas where research doesn’t necessarily apply to women as just “smaller men.” Then we would have the hub, and the spokes—those would be mental health care providers, surgeons, and people to provide additional services when needed.
The only way I can figure how to make that work from a payment perspective is with a prospective payment system, a per member, per month capitated payment structure. That way, ancillary and other services would be available, and overtesting and such would be disincentivized.
Continue to: The question of payment...
The question of payment
Dr. Hayworth: I agree. For every practice, the two key considerations in addressing the challenges of capitation are, first, that the team approach is essential, and, second, that providers appreciate that everything they do for their patients is reimbursed in a global payment.
At CareMount Medical, our team system embeds advanced practice professionals in our primary care and ObGyn offices. Everyone—physicians, midwives, nurse practitioners—practices at the top of their license. Our care coordinators ensure that our patients’ health journeys are optimized from well care through specialized needs, engaging every member of the care team effectively.
To optimize our success in a risk model, we recognize that tasks and services that went without direct reimbursement in a fee-for-service arrangement are integral to producing the best outcomes for our patients. We examine everything we do from the perspective of how to provide the most advanced care in the most efficient manner. For example, we drive toward moving procedures from the hospital to the outpatient setting, and from the ambulatory surgical center to the office. This allows us maximal control of both quality and cost, with savings benefiting our group as well as the payers with whom we have contracts.
Dr. Norwitz: I have been fortunate to have trained and worked in 5 different countries on 3 continents. There’s no question there are better health care systems out there. Some form of capitation is needed, whether it’s value-based care or a risk-sharing arrangement. But how do you do it without a single payer? I don’t think you can, but I’m ready to listen.
Dr. Hayworth: You can have capitation without a single payer; in fact, it’s far better to have many payers compete to offer the greatest flexibility to both patients and providers. CareMount Medical has 650,000 patients who rely on us to provide their care with the utmost quality and affordability. In our Next Generation ACO, our Medicare patients have the benefit of care coordination in a team approach that saves our government money, and we are incentivized to do our best because some of those savings return to us.
The needs of Medicare patients, of course, are different from those in other age groups, and our contracts with other payers will reflect that distinction. There’s no inherent reason why capitation has to equal “single payer.” The benefits of the risk model are magnified by incentivizing all participants to provide maximum value.
Continue to: Ms. Pearson...
Ms. Pearson: I am going to comment on capitated care because I think educated consumers are well aware of the benefits of moving away from fee-for-service and bringing in some more sensible system. However, given the historical racial inequities and injustices, and lack of access and disparate treatment, capitation raises fear in the hearts of people whose communities have not gotten the care that they need.
The answer is not to avoid capitation, but to find a way for the profession to be seen more visibly as reflecting who they serve, and we know we can’t change the profession’s racial makeup overnight. That’s a generation-long effort.
Dr. Levy: For capitation to work, there has to be value, you have to meet the quality metrics. Having served on the National Quality Forum on multiple different committees, I am convinced that we measure what is easy to measure, and we are not measuring what really matters to people. My thought is to embrace the communities that have been underserved to help us design the metrics for a capitated system that is meaningful to the people that we serve.
Ms. Pearson: On the West Coast, some people are leading efforts to create patient-centered metrics for respectful maternity care led by Black, indigenous, and people of color communities that are validated with solid research tools.
Algorithms for care
Dr. Norwitz: Artificial intelligence (AI) may have a role to play. For example, I think we do a terrible job of caring for women in the postpartum period. We focus almost all of our care in the antepartum period and not postpartum. I am working with a group with a finance and banking background to try and risk-adjust patients in the antepartum, intrapartum, and postpartum period. We are developing algorithms using AI and deep learning technologies to risk-stratify patients and say, “This patient is low risk so can safely get obstetric care with a family medicine doctor or midwife. That patient requires consultation with a maternal-fetal medicine subspecialist or a general internist,” and so on.
Ms. Pearson: As policy advocates, we are trying to get Medicaid postpartum coverage expanded to 12 months. Too many women fall into a coverage gap shortly after delivery; continued coverage would help improve postpartum outcomes. I am curious how an algorithm might help take better care of women postpartum.
Dr. Norwitz: Postpartum care is one of the greatest areas of need. I love the Dutch model. In the Netherlands, when a woman goes home after giving birth, a designated nurse comes home with her, teaches her how to breastfeed and how to bathe the baby, and assists with routine activities such as cooking and washing. And the nurse remains engaged for a prolonged period of time, paid for by the government. There are also other social welfare packages, such as a full 4-year or more maternity leave.
The solution is part political and part medical. We need to rethink our care model, and I don’t think we provide enough postpartum care.
Continue to: Dr. Hayworth...
Dr. Hayworth: Errol made an excellent point about AI. There is a product that’s being used in Europe and in some other parts of the world that can provide 85% of care through an algorithm without a patient even having to speak to a nurse or doctor. The company that offers the product claims a high level of patient satisfaction and a very low error rate.
We are a long way from the point at which—and I don’t anticipate that we’ll ever get there—AI fully replaces human providers, but there’s enormous and growing potential for data aggregation and machine learning to enhance, exponentially, the capabilities and capacity of care teams.
The most immediate applications for AI in the United States are in diagnostics, pathology, and the mapping of protocols for patients with cancer who will benefit from access to investigational interventions and clinical trials. As we gain experience in those areas, acceptance and confidence will lead steadily to broader deployment of AI, enhancing the quality of care and the efficiency of delivery and saving costs.
Dr. Norwitz: AI is a tool to assist providers. It is not going to replace us, which is the fear.
Ms. Pearson: From the consumer perspective, again, there is concern that if not enough data are available from Black, indigenous, and people of color, the levels won’t start out in a good place. The criticism over mammography randomized controlled trials (RCTs) has existed for a long time. The big trials that got all the way out to mortality did not include enough women of color; and so women of color rightly say, “Why should we believe these guidelines developed on results of the RCTs?” My point is that because of historical inequity, logical solutions such as algorithms do not always work for communities that were previously excluded or mistreated.
Dr. Levy: Your point is incredibly well taken. That means that those of us researching and working with AI need to ensure that the data going in are representative, that we are not embedding implicit biases into the AI algorithms, which clearly has sometimes already happened. We have to be careful to embrace input from multiple sources that we have not thought of before.
As we look at an algorithm for managing a postpartum patient or a postoperative patient, have we thought about how she’s managing her children at home after she goes home? What else is happening in her life? How can we impact her recovery in a positive way? We need to hear the voices of the people that we are trying to serve as we develop those algorithms.
Perspectives on future health care delivery
Dr. Levy: To summarize so far, we are thinking about a Woman’s Medical Home, a capitated model of comprehensive care for women that includes mental health, social determinants, and home care. There are different models, but a payment structure where we would have the capital to invest in community services and in things that we think may make a difference.
Dr. Norwitz: I think the health care system of the future is not going to be based in large academic medical centers. It’s going to be in community hospitals close to home. It’s going to be in the home. And it will be provided by different types of practitioners, whose performances are tracked using more appropriate outcome metrics.
Dr. Levy: I also think we will have community health workers. While we haven’t talked about rural health and access to care, there are some structural things we can do to reach rural communities with really excellent care, such as training community health workers and using telemedicine. It does require thinking through a different payment structure, though, because there really isn’t money in the system to do that currently, at least to my knowledge.
Janice, do we have enough motivation to take care of women? Women are so underrepresented when we look at care models.
Dr. Huckaby: I do think there is hope, but it will truly take a village. While CMS (Centers for Medicare and Medicaid Services) has its innovation center in the Medicaid space, it’s almost like we have to have the payers, the government, the specialty societies, and so on say that we need to do something better. I mention the government because it is not only a payer but also a regulator. They can help create some of these things.
There are opportunities with payers to say, “Let’s move to this kind of model for that.” But still, we are implementing change but on a fairly minor scale.
We could have the people who care about issues, help deliver the care, pay the bills, and so on say, “This is what we want to do,” and then we could pilot them. It may be one type of pilot in a rural area and one type of pilot in an urban area, because they are going to differ, and do it that way and then scale it.
Telemedicine, or telehealth, is part of creating access. Even some nontraditional settings, such as retail store clinics, may work.
Continue to: Dr. Levy...
Dr. Levy: Cindy, is there any last thing you wanted to comment on?
Ms. Pearson: All the changes we have talked about require public policy change. Physicians become physicians to take care of people, not because they want to be policy wonks like us. We love policy because we see how it can benefit. To our readers I say be part of making this generational change in the profession and women’s health care, get involved in policy, because these things can’t happen without the policy changes.
Dr. Norwitz: That is so important. In most developed countries around the world, you get trained in medical school, the cost of training is subsidized, and in return you owe 2 years of service. In this country, if we subsidized the training of doctors and in return they owed us 2 years of primary care service based in the community or in an underserved area, they would get valuable clinical experience and wouldn’t have so many loans to pay back. I think it is a policy that could work and could profoundly change the health care landscape in time.
Dr. Levy: And it would save a great deal of money. The reality is that if we subsidize medical education and in return required service in a national public health service, we would move providers out into rural areas. That would to some extent solve our rural problem. We would train people to think about diagnostic options when the resources are not unlimited, so that they will perhaps not order quite so many tests.
That policy change would foundationally allow for more minority students to become physicians and health care workers. If there were one thing we could do to begin to drive this change, that would be it.
Who would have thought a disruptive pandemic could affect the way people receive care, in bad and good ways? Some carriers, for example, are now paying for telehealth visits who previously did not.
Final thoughts
Dr. Hayworth: It’s an exciting time to be in medicine and women’s health: We are ushering in a new era in which we can fulfill the vision of comprehensive care, patient-focused and seamlessly delivered by teams whose capabilities are optimized by ever-improving technology. ObGyns, with our foundation in the continuum of care, have the experience and the sensibilities to adapt to the challenges of the value model, in which our success will depend on fully embracing our role as primary care providers.
Dr. Levy: Circling back to the beginning of our discussion, we talked about relationships, and developing deep relationships with patients is the internal reward and the piece that prevents us from burnout. It makes you feel good at the end of the day—or sometimes bad at the end of the day when something didn’t go well. Restructuring the system in a way that gets us back to personalized relationship-centered care will benefit ObGyns and our patients.
I thank you all for participating in this thoughtful discussion. ●
- Gawande A. Big med. The New Yorker. August 13, 2012. https://www.newyorker.com/magazine/2012/08/13/big-med. Accessed July 24, 2020.
Recently,
Barbara Levy, MD: The disruption of the COVID-19 pandemic has given us an opportunity to consider how we would recraft the delivery of health care for women if we could. My goal for this discussion is to talk about that and see if we can incentivize people to make changes.
Cindy, what are women looking for in health care that they are not getting now?
What women want in health care
Cynthia A. Pearson: Women, like men, want a sense of assurance that health care can be provided in a safe way, and that can’t be given completely right now.
Aside from that, women want a personal connection, ideally with the same provider. Many women are embracing telehealth, which came about because of this disruptive time, and that has potential that we can possibly mobilize around. One thing women don’t always find is consistency and contact, and they would like that.
Scott D. Hayworth, MD: Women want to be listened to, and they want their doctors to take a holistic and individualized approach to their care. In-person visits are the ideal setting for this, but during the pandemic we have had to adapt to new modalities for delivering care: government regulations restricting services, and the necessity to limit the flow of patients into offices, has meant that we have had to rely on remote visits. CareMount Medical has been in the forefront of telehealth with our “Virtual Visit” technology, so we were well prepared, and our patients have embraced this truly vital option. We’ve ramped up capabilities significantly to deal with the surge in volume.
While our practice has been able to provide consistent and convenient access to care, this isn’t the case in all areas of the country. Even before the pandemic, the cost of malpractice insurance has led to shortages of ObGyns; this deficit has been compounded by the closing of hospitals due to restrictions on services imposed to try to stem the spread of COVID-19. The affordability of care has also been jeopardized by job losses and therefore of employer-provided insurance, following months of lockdowns.
Continue to: Dr. Levy...
Dr. Levy: To balance that long-term relationship with access and cost, clearly we are not delivering what is needed. Janice, at UnitedHealth you have experimented with some products and some different ways of delivering care. What are beneficiaries looking for?
Janice Huckaby, MD: There is a real thirst for digital content—everybody consults with Dr. Google. They are looking for reliable sources of clinical content. Ideally, that comes from their physician, but people access it in other ways as well.
I agree that women desire a personalized relationship. That is why we are seeing more communities of women, such as virtual pregnancy support groups, that have cropped up in the age of COVID-19. Women are not content with the idea of “I’m going to see my doctor, get my tummy measured, listen to the heartbeat, and go home.” That model is done. Patients will look for practices that are accessible at convenient times and that can give them the personalized experience to make them feel well cared for and that offer them a long-term relationship.
One concern is that as more obstetric groups use laborists to do their deliveries at the hospital, I wonder whether we do a good job of forming that relationship on the front end, and when it comes to the delivery, will we drop the ball? The jury is out, but it’s worth watching.
Dr. Levy: How do we as obstetrician-gynecologists get patients to consider that we are providing reliable information? There is so much disinformation out there.
Errol R. Norwitz, MD, PhD, MBA: I echo the sentiments discussed and I’ll add that many women want care that is convenient, close to home, coordinated, and integrated—not fragmented. They want their providers and their office to anticipate and know who they are even before they arrive, to be prepared for the visit. And it’s not only care for them, but also care for their families. Women are the gatekeepers to the health care system. They want a health care system in place that will care not just for each member separately but also for the family as an integrated whole.
To answer your question, Barbara, we have all been overwhelmed with the amount of data coming at us, both providers and patients. Teaching providers how to synthesize and integrate the data and then present it to patients is quite a challenge. We have to instill this skill in our trainees, teach them how to absorb and present the data.
Consensus bodies can help in this regard, and ACOG (American College of Obstetricians and Gynecologists) has led the way in providing guidance around the management of pregnancy in the setting of COVID-19. Another reliable site for my trainees is UpToDate, which is easy to access. If a scientific paper comes out today, it will be covered in UpToDate tomorrow. Patients need someone who can synthesize the data and give it to them in little pieces, and keep it current.
Dr. Levy: We need to be a reliable source not only for medical information but also for referral to resources in the community for families and for women.
Continue to: ObGyn services...
ObGyn services: Primary care or specialty?
Dr. Norwitz: That begs the question, who are we? Are we primary care providers or are we a subspecialty, or are we both?
Ms. Pearson: Women, particularly in their younger, middle reproductive years, see their ObGyn as a primary care provider. The way forward for the profession is to embrace the call that Barbara articulated, to know what other referral sources are available beyond other clinicians. We need to be aware of the social determinants of health—that there are times when the primary care provider needs to know the community well enough to know what is available that would make a difference for that person and her family.
Dr. Levy: Scott, how do you manage that?
Dr. Hayworth: As reimbursement models move rapidly toward value, practices that can undertake risk are in the best position to thrive; specialty providers relying solely on fee-for-service may well be unable to survive. The key for any ObGyn practice is to be of sufficient size and scope that it can manage the primary care for a panel of patients, the more numerous the better; being in charge of those dollars allows maximum control. ObGyns who subspecialize should seek to become members of larger groups, whether comprehensive women’s health practices or multispecialty groups like ours at CareMount Medical, that manage the spectrum of care for their patients.
Dr. Levy: Janice, fill us in on some of the structures that exist now for ObGyns that they may be able to participate in—payment structures like the Women’s Medical Home. Does UnitedHealth have anything like that?
Dr. Huckaby: Probably 3 or 4 exist now, but I agree that risk arrangements are perhaps a wave of the future. Right now, UnitedHealth has accountable care organizations (ACOs) that include ObGyns, a number of them in the Northeast. We also rolled out bundled payment programs.
Our hospital contracts have always had metrics around infection rates and elective deliveries before 39 weeks, and we will probably start seeing some of that put into the provider contracts as well.
There is a desire to move people into a risk-sharing model for payment, but part of the concern there is the infrastructure, because if you are going to manage risk, you need to have staff that can do care coordination. Care coordinators can ensure, for example, that people have transportation to their appointments, and thus address some of the social determinants in ways that historically have not been done in obstetrics.
The ACOs sometimes have given seed money for practices to hire additional staff to do those kinds of things, and that can help get practices started. Probably the people best positioned are in large multispecialty groups that can leverage case management and maybe support other specialties.
I do think we are going to see a move to risk in the future. Obstetrics has moved at a slower pace than we have seen in internal medicine and some other specialties.
Dr. Hayworth: The value model for reimbursement can only be managed via care coordination, maximizing efficacy and efficiency at every level for every patient. Fortunately for ObGyns, we are familiar with the value concept via bundling for obstetrical services covering prenatal to postpartum, including delivery. ObGyn practices need to prepare for a future in which insurers will pay for patient panels in which providers take on the risk for the entirety of care.
At CareMount Medical, we have embraced the value model as one of 40 Next Generation Medicare Accountable Care Organizations across the country. We’ve put in place the infrastructure, from front desk through back office, to optimize resource utilization. Our team approach includes both patient advocates and care coordinators who extend the capabilities of our physicians and ensure that our patients’ needs, including well care, are met comprehensively.
Dr. Huckaby: One area that we sometimes leave out, whether we are talking about payment or a patient-centered medical home, is integration with behavioral health. Anxiety and depression are fairly rampant, fairly underdiagnosed, and woefully undertreated. I hope that our ObGyn practices of the future—and maybe this is the broadening into primary care—will engage and take the lead in addressing some of those issues, because women suffer. We need to embrace the behavioral aspect of care for the whole person more than we have.
Continue to: Physician training issues...
Physician training issues
Dr. Levy: I could not agree more. We have trained physicians to do illness care, not wellness care, and to be physician and practice centered, not patient centered. While we train medical students in hospital settings and in acute care, there’s not much training in how to manage people or in the factors that determine whether someone is truly well, such as housing security and food security. We are not training physicians in nutrition or in mental health.
Errol, how do we help an ObGyn or women’s health trainee to prepare for the ideal world we are trying to create?
Dr. Norwitz: It’s a challenging question. I like to reference a remarkable piece by Atul Gawande in The New Yorker, in which he interviewed the CEO of the Cheesecake Factory restaurant chain, who in effect said that we’ve got it all wrong; there’s no health in health care.1 We don’t manage health; we wait until people get sick and then we treat them. We have to put the health back into health care.
It has always been my passion to focus on preventative care. We need to reclaim our identity—I have never particularly liked the name “ObGyn,” the term “women’s health” may be more appropriate and help us focus on disease prevention—and we need to stand up for training programs that separate the O from the G.
Low-volume surgeons, who may do only 1 or 2 hysterectomies per year, can’t maintain their proficiency, and many don’t do enough cases to maintain their robotics privileges. I can foresee a time where labor and delivery units are like ICUs, where the people who work there do nothing but manage labor and perform deliveries using standardized bundles of practice. Such an approach will decrease variability in management and lead to improved outcomes.
We need to completely reframe how we train our pipeline providers to provide care in women’s health. It would be difficult, take a lot of effort, and there would be pushback, I suspect, but that’s where the field needs to go.
The ideal system redesign
Dr. Levy: Cindy, if you could start from scratch and design an ideal comprehensive system to better deliver care for women of all ages, what would that look like?
Ms. Pearson: I would design a system in which people at any life stage met with providers who were less trained in dealing with disease and more trained in the holistic approach to maintaining health. That might be a nurse practitioner or maybe a version of what Errol describes as a new way of training ObGyns. That’s the initial interaction, and the person could be with someone for decades and deepen the relationship in that wonderful way. It would also have an avenue for the times when disease needed to be treated or when more specialized care would be provided. And the financing would be worked out to support consistency.
Dr. Norwitz: We can learn from other countries. Singapore, with only 5.5 million people, has the best health care system in the world. They have a great model. Costa Rica and Cuba have completely redesigned their health care systems. You go through medical school in 2 or 3 years, and then you get embedded in the community. So you have doctors living in the community responsible for the health of their neighbors. They get to know people in the context in which they live and refer them on only when they need more than basic care. These countries have vastly superior outcome measures, and they spend less money on health care.
Dr. Levy: My dream, as we reinvent things, is that we could create a comprehensive Women’s Medical Home where there’s a hub and an opportunity to be centered on patients so they could reach us when needed.
Ideally we could create a structure with a central contact person—a nurse practitioner, a midwife, someone in family medicine or internal medicine—someone focused on women’s health who has researched how inequities apply to women and women’s health and the areas where research doesn’t necessarily apply to women as just “smaller men.” Then we would have the hub, and the spokes—those would be mental health care providers, surgeons, and people to provide additional services when needed.
The only way I can figure how to make that work from a payment perspective is with a prospective payment system, a per member, per month capitated payment structure. That way, ancillary and other services would be available, and overtesting and such would be disincentivized.
Continue to: The question of payment...
The question of payment
Dr. Hayworth: I agree. For every practice, the two key considerations in addressing the challenges of capitation are, first, that the team approach is essential, and, second, that providers appreciate that everything they do for their patients is reimbursed in a global payment.
At CareMount Medical, our team system embeds advanced practice professionals in our primary care and ObGyn offices. Everyone—physicians, midwives, nurse practitioners—practices at the top of their license. Our care coordinators ensure that our patients’ health journeys are optimized from well care through specialized needs, engaging every member of the care team effectively.
To optimize our success in a risk model, we recognize that tasks and services that went without direct reimbursement in a fee-for-service arrangement are integral to producing the best outcomes for our patients. We examine everything we do from the perspective of how to provide the most advanced care in the most efficient manner. For example, we drive toward moving procedures from the hospital to the outpatient setting, and from the ambulatory surgical center to the office. This allows us maximal control of both quality and cost, with savings benefiting our group as well as the payers with whom we have contracts.
Dr. Norwitz: I have been fortunate to have trained and worked in 5 different countries on 3 continents. There’s no question there are better health care systems out there. Some form of capitation is needed, whether it’s value-based care or a risk-sharing arrangement. But how do you do it without a single payer? I don’t think you can, but I’m ready to listen.
Dr. Hayworth: You can have capitation without a single payer; in fact, it’s far better to have many payers compete to offer the greatest flexibility to both patients and providers. CareMount Medical has 650,000 patients who rely on us to provide their care with the utmost quality and affordability. In our Next Generation ACO, our Medicare patients have the benefit of care coordination in a team approach that saves our government money, and we are incentivized to do our best because some of those savings return to us.
The needs of Medicare patients, of course, are different from those in other age groups, and our contracts with other payers will reflect that distinction. There’s no inherent reason why capitation has to equal “single payer.” The benefits of the risk model are magnified by incentivizing all participants to provide maximum value.
Continue to: Ms. Pearson...
Ms. Pearson: I am going to comment on capitated care because I think educated consumers are well aware of the benefits of moving away from fee-for-service and bringing in some more sensible system. However, given the historical racial inequities and injustices, and lack of access and disparate treatment, capitation raises fear in the hearts of people whose communities have not gotten the care that they need.
The answer is not to avoid capitation, but to find a way for the profession to be seen more visibly as reflecting who they serve, and we know we can’t change the profession’s racial makeup overnight. That’s a generation-long effort.
Dr. Levy: For capitation to work, there has to be value, you have to meet the quality metrics. Having served on the National Quality Forum on multiple different committees, I am convinced that we measure what is easy to measure, and we are not measuring what really matters to people. My thought is to embrace the communities that have been underserved to help us design the metrics for a capitated system that is meaningful to the people that we serve.
Ms. Pearson: On the West Coast, some people are leading efforts to create patient-centered metrics for respectful maternity care led by Black, indigenous, and people of color communities that are validated with solid research tools.
Algorithms for care
Dr. Norwitz: Artificial intelligence (AI) may have a role to play. For example, I think we do a terrible job of caring for women in the postpartum period. We focus almost all of our care in the antepartum period and not postpartum. I am working with a group with a finance and banking background to try and risk-adjust patients in the antepartum, intrapartum, and postpartum period. We are developing algorithms using AI and deep learning technologies to risk-stratify patients and say, “This patient is low risk so can safely get obstetric care with a family medicine doctor or midwife. That patient requires consultation with a maternal-fetal medicine subspecialist or a general internist,” and so on.
Ms. Pearson: As policy advocates, we are trying to get Medicaid postpartum coverage expanded to 12 months. Too many women fall into a coverage gap shortly after delivery; continued coverage would help improve postpartum outcomes. I am curious how an algorithm might help take better care of women postpartum.
Dr. Norwitz: Postpartum care is one of the greatest areas of need. I love the Dutch model. In the Netherlands, when a woman goes home after giving birth, a designated nurse comes home with her, teaches her how to breastfeed and how to bathe the baby, and assists with routine activities such as cooking and washing. And the nurse remains engaged for a prolonged period of time, paid for by the government. There are also other social welfare packages, such as a full 4-year or more maternity leave.
The solution is part political and part medical. We need to rethink our care model, and I don’t think we provide enough postpartum care.
Continue to: Dr. Hayworth...
Dr. Hayworth: Errol made an excellent point about AI. There is a product that’s being used in Europe and in some other parts of the world that can provide 85% of care through an algorithm without a patient even having to speak to a nurse or doctor. The company that offers the product claims a high level of patient satisfaction and a very low error rate.
We are a long way from the point at which—and I don’t anticipate that we’ll ever get there—AI fully replaces human providers, but there’s enormous and growing potential for data aggregation and machine learning to enhance, exponentially, the capabilities and capacity of care teams.
The most immediate applications for AI in the United States are in diagnostics, pathology, and the mapping of protocols for patients with cancer who will benefit from access to investigational interventions and clinical trials. As we gain experience in those areas, acceptance and confidence will lead steadily to broader deployment of AI, enhancing the quality of care and the efficiency of delivery and saving costs.
Dr. Norwitz: AI is a tool to assist providers. It is not going to replace us, which is the fear.
Ms. Pearson: From the consumer perspective, again, there is concern that if not enough data are available from Black, indigenous, and people of color, the levels won’t start out in a good place. The criticism over mammography randomized controlled trials (RCTs) has existed for a long time. The big trials that got all the way out to mortality did not include enough women of color; and so women of color rightly say, “Why should we believe these guidelines developed on results of the RCTs?” My point is that because of historical inequity, logical solutions such as algorithms do not always work for communities that were previously excluded or mistreated.
Dr. Levy: Your point is incredibly well taken. That means that those of us researching and working with AI need to ensure that the data going in are representative, that we are not embedding implicit biases into the AI algorithms, which clearly has sometimes already happened. We have to be careful to embrace input from multiple sources that we have not thought of before.
As we look at an algorithm for managing a postpartum patient or a postoperative patient, have we thought about how she’s managing her children at home after she goes home? What else is happening in her life? How can we impact her recovery in a positive way? We need to hear the voices of the people that we are trying to serve as we develop those algorithms.
Perspectives on future health care delivery
Dr. Levy: To summarize so far, we are thinking about a Woman’s Medical Home, a capitated model of comprehensive care for women that includes mental health, social determinants, and home care. There are different models, but a payment structure where we would have the capital to invest in community services and in things that we think may make a difference.
Dr. Norwitz: I think the health care system of the future is not going to be based in large academic medical centers. It’s going to be in community hospitals close to home. It’s going to be in the home. And it will be provided by different types of practitioners, whose performances are tracked using more appropriate outcome metrics.
Dr. Levy: I also think we will have community health workers. While we haven’t talked about rural health and access to care, there are some structural things we can do to reach rural communities with really excellent care, such as training community health workers and using telemedicine. It does require thinking through a different payment structure, though, because there really isn’t money in the system to do that currently, at least to my knowledge.
Janice, do we have enough motivation to take care of women? Women are so underrepresented when we look at care models.
Dr. Huckaby: I do think there is hope, but it will truly take a village. While CMS (Centers for Medicare and Medicaid Services) has its innovation center in the Medicaid space, it’s almost like we have to have the payers, the government, the specialty societies, and so on say that we need to do something better. I mention the government because it is not only a payer but also a regulator. They can help create some of these things.
There are opportunities with payers to say, “Let’s move to this kind of model for that.” But still, we are implementing change but on a fairly minor scale.
We could have the people who care about issues, help deliver the care, pay the bills, and so on say, “This is what we want to do,” and then we could pilot them. It may be one type of pilot in a rural area and one type of pilot in an urban area, because they are going to differ, and do it that way and then scale it.
Telemedicine, or telehealth, is part of creating access. Even some nontraditional settings, such as retail store clinics, may work.
Continue to: Dr. Levy...
Dr. Levy: Cindy, is there any last thing you wanted to comment on?
Ms. Pearson: All the changes we have talked about require public policy change. Physicians become physicians to take care of people, not because they want to be policy wonks like us. We love policy because we see how it can benefit. To our readers I say be part of making this generational change in the profession and women’s health care, get involved in policy, because these things can’t happen without the policy changes.
Dr. Norwitz: That is so important. In most developed countries around the world, you get trained in medical school, the cost of training is subsidized, and in return you owe 2 years of service. In this country, if we subsidized the training of doctors and in return they owed us 2 years of primary care service based in the community or in an underserved area, they would get valuable clinical experience and wouldn’t have so many loans to pay back. I think it is a policy that could work and could profoundly change the health care landscape in time.
Dr. Levy: And it would save a great deal of money. The reality is that if we subsidize medical education and in return required service in a national public health service, we would move providers out into rural areas. That would to some extent solve our rural problem. We would train people to think about diagnostic options when the resources are not unlimited, so that they will perhaps not order quite so many tests.
That policy change would foundationally allow for more minority students to become physicians and health care workers. If there were one thing we could do to begin to drive this change, that would be it.
Who would have thought a disruptive pandemic could affect the way people receive care, in bad and good ways? Some carriers, for example, are now paying for telehealth visits who previously did not.
Final thoughts
Dr. Hayworth: It’s an exciting time to be in medicine and women’s health: We are ushering in a new era in which we can fulfill the vision of comprehensive care, patient-focused and seamlessly delivered by teams whose capabilities are optimized by ever-improving technology. ObGyns, with our foundation in the continuum of care, have the experience and the sensibilities to adapt to the challenges of the value model, in which our success will depend on fully embracing our role as primary care providers.
Dr. Levy: Circling back to the beginning of our discussion, we talked about relationships, and developing deep relationships with patients is the internal reward and the piece that prevents us from burnout. It makes you feel good at the end of the day—or sometimes bad at the end of the day when something didn’t go well. Restructuring the system in a way that gets us back to personalized relationship-centered care will benefit ObGyns and our patients.
I thank you all for participating in this thoughtful discussion. ●
Recently,
Barbara Levy, MD: The disruption of the COVID-19 pandemic has given us an opportunity to consider how we would recraft the delivery of health care for women if we could. My goal for this discussion is to talk about that and see if we can incentivize people to make changes.
Cindy, what are women looking for in health care that they are not getting now?
What women want in health care
Cynthia A. Pearson: Women, like men, want a sense of assurance that health care can be provided in a safe way, and that can’t be given completely right now.
Aside from that, women want a personal connection, ideally with the same provider. Many women are embracing telehealth, which came about because of this disruptive time, and that has potential that we can possibly mobilize around. One thing women don’t always find is consistency and contact, and they would like that.
Scott D. Hayworth, MD: Women want to be listened to, and they want their doctors to take a holistic and individualized approach to their care. In-person visits are the ideal setting for this, but during the pandemic we have had to adapt to new modalities for delivering care: government regulations restricting services, and the necessity to limit the flow of patients into offices, has meant that we have had to rely on remote visits. CareMount Medical has been in the forefront of telehealth with our “Virtual Visit” technology, so we were well prepared, and our patients have embraced this truly vital option. We’ve ramped up capabilities significantly to deal with the surge in volume.
While our practice has been able to provide consistent and convenient access to care, this isn’t the case in all areas of the country. Even before the pandemic, the cost of malpractice insurance has led to shortages of ObGyns; this deficit has been compounded by the closing of hospitals due to restrictions on services imposed to try to stem the spread of COVID-19. The affordability of care has also been jeopardized by job losses and therefore of employer-provided insurance, following months of lockdowns.
Continue to: Dr. Levy...
Dr. Levy: To balance that long-term relationship with access and cost, clearly we are not delivering what is needed. Janice, at UnitedHealth you have experimented with some products and some different ways of delivering care. What are beneficiaries looking for?
Janice Huckaby, MD: There is a real thirst for digital content—everybody consults with Dr. Google. They are looking for reliable sources of clinical content. Ideally, that comes from their physician, but people access it in other ways as well.
I agree that women desire a personalized relationship. That is why we are seeing more communities of women, such as virtual pregnancy support groups, that have cropped up in the age of COVID-19. Women are not content with the idea of “I’m going to see my doctor, get my tummy measured, listen to the heartbeat, and go home.” That model is done. Patients will look for practices that are accessible at convenient times and that can give them the personalized experience to make them feel well cared for and that offer them a long-term relationship.
One concern is that as more obstetric groups use laborists to do their deliveries at the hospital, I wonder whether we do a good job of forming that relationship on the front end, and when it comes to the delivery, will we drop the ball? The jury is out, but it’s worth watching.
Dr. Levy: How do we as obstetrician-gynecologists get patients to consider that we are providing reliable information? There is so much disinformation out there.
Errol R. Norwitz, MD, PhD, MBA: I echo the sentiments discussed and I’ll add that many women want care that is convenient, close to home, coordinated, and integrated—not fragmented. They want their providers and their office to anticipate and know who they are even before they arrive, to be prepared for the visit. And it’s not only care for them, but also care for their families. Women are the gatekeepers to the health care system. They want a health care system in place that will care not just for each member separately but also for the family as an integrated whole.
To answer your question, Barbara, we have all been overwhelmed with the amount of data coming at us, both providers and patients. Teaching providers how to synthesize and integrate the data and then present it to patients is quite a challenge. We have to instill this skill in our trainees, teach them how to absorb and present the data.
Consensus bodies can help in this regard, and ACOG (American College of Obstetricians and Gynecologists) has led the way in providing guidance around the management of pregnancy in the setting of COVID-19. Another reliable site for my trainees is UpToDate, which is easy to access. If a scientific paper comes out today, it will be covered in UpToDate tomorrow. Patients need someone who can synthesize the data and give it to them in little pieces, and keep it current.
Dr. Levy: We need to be a reliable source not only for medical information but also for referral to resources in the community for families and for women.
Continue to: ObGyn services...
ObGyn services: Primary care or specialty?
Dr. Norwitz: That begs the question, who are we? Are we primary care providers or are we a subspecialty, or are we both?
Ms. Pearson: Women, particularly in their younger, middle reproductive years, see their ObGyn as a primary care provider. The way forward for the profession is to embrace the call that Barbara articulated, to know what other referral sources are available beyond other clinicians. We need to be aware of the social determinants of health—that there are times when the primary care provider needs to know the community well enough to know what is available that would make a difference for that person and her family.
Dr. Levy: Scott, how do you manage that?
Dr. Hayworth: As reimbursement models move rapidly toward value, practices that can undertake risk are in the best position to thrive; specialty providers relying solely on fee-for-service may well be unable to survive. The key for any ObGyn practice is to be of sufficient size and scope that it can manage the primary care for a panel of patients, the more numerous the better; being in charge of those dollars allows maximum control. ObGyns who subspecialize should seek to become members of larger groups, whether comprehensive women’s health practices or multispecialty groups like ours at CareMount Medical, that manage the spectrum of care for their patients.
Dr. Levy: Janice, fill us in on some of the structures that exist now for ObGyns that they may be able to participate in—payment structures like the Women’s Medical Home. Does UnitedHealth have anything like that?
Dr. Huckaby: Probably 3 or 4 exist now, but I agree that risk arrangements are perhaps a wave of the future. Right now, UnitedHealth has accountable care organizations (ACOs) that include ObGyns, a number of them in the Northeast. We also rolled out bundled payment programs.
Our hospital contracts have always had metrics around infection rates and elective deliveries before 39 weeks, and we will probably start seeing some of that put into the provider contracts as well.
There is a desire to move people into a risk-sharing model for payment, but part of the concern there is the infrastructure, because if you are going to manage risk, you need to have staff that can do care coordination. Care coordinators can ensure, for example, that people have transportation to their appointments, and thus address some of the social determinants in ways that historically have not been done in obstetrics.
The ACOs sometimes have given seed money for practices to hire additional staff to do those kinds of things, and that can help get practices started. Probably the people best positioned are in large multispecialty groups that can leverage case management and maybe support other specialties.
I do think we are going to see a move to risk in the future. Obstetrics has moved at a slower pace than we have seen in internal medicine and some other specialties.
Dr. Hayworth: The value model for reimbursement can only be managed via care coordination, maximizing efficacy and efficiency at every level for every patient. Fortunately for ObGyns, we are familiar with the value concept via bundling for obstetrical services covering prenatal to postpartum, including delivery. ObGyn practices need to prepare for a future in which insurers will pay for patient panels in which providers take on the risk for the entirety of care.
At CareMount Medical, we have embraced the value model as one of 40 Next Generation Medicare Accountable Care Organizations across the country. We’ve put in place the infrastructure, from front desk through back office, to optimize resource utilization. Our team approach includes both patient advocates and care coordinators who extend the capabilities of our physicians and ensure that our patients’ needs, including well care, are met comprehensively.
Dr. Huckaby: One area that we sometimes leave out, whether we are talking about payment or a patient-centered medical home, is integration with behavioral health. Anxiety and depression are fairly rampant, fairly underdiagnosed, and woefully undertreated. I hope that our ObGyn practices of the future—and maybe this is the broadening into primary care—will engage and take the lead in addressing some of those issues, because women suffer. We need to embrace the behavioral aspect of care for the whole person more than we have.
Continue to: Physician training issues...
Physician training issues
Dr. Levy: I could not agree more. We have trained physicians to do illness care, not wellness care, and to be physician and practice centered, not patient centered. While we train medical students in hospital settings and in acute care, there’s not much training in how to manage people or in the factors that determine whether someone is truly well, such as housing security and food security. We are not training physicians in nutrition or in mental health.
Errol, how do we help an ObGyn or women’s health trainee to prepare for the ideal world we are trying to create?
Dr. Norwitz: It’s a challenging question. I like to reference a remarkable piece by Atul Gawande in The New Yorker, in which he interviewed the CEO of the Cheesecake Factory restaurant chain, who in effect said that we’ve got it all wrong; there’s no health in health care.1 We don’t manage health; we wait until people get sick and then we treat them. We have to put the health back into health care.
It has always been my passion to focus on preventative care. We need to reclaim our identity—I have never particularly liked the name “ObGyn,” the term “women’s health” may be more appropriate and help us focus on disease prevention—and we need to stand up for training programs that separate the O from the G.
Low-volume surgeons, who may do only 1 or 2 hysterectomies per year, can’t maintain their proficiency, and many don’t do enough cases to maintain their robotics privileges. I can foresee a time where labor and delivery units are like ICUs, where the people who work there do nothing but manage labor and perform deliveries using standardized bundles of practice. Such an approach will decrease variability in management and lead to improved outcomes.
We need to completely reframe how we train our pipeline providers to provide care in women’s health. It would be difficult, take a lot of effort, and there would be pushback, I suspect, but that’s where the field needs to go.
The ideal system redesign
Dr. Levy: Cindy, if you could start from scratch and design an ideal comprehensive system to better deliver care for women of all ages, what would that look like?
Ms. Pearson: I would design a system in which people at any life stage met with providers who were less trained in dealing with disease and more trained in the holistic approach to maintaining health. That might be a nurse practitioner or maybe a version of what Errol describes as a new way of training ObGyns. That’s the initial interaction, and the person could be with someone for decades and deepen the relationship in that wonderful way. It would also have an avenue for the times when disease needed to be treated or when more specialized care would be provided. And the financing would be worked out to support consistency.
Dr. Norwitz: We can learn from other countries. Singapore, with only 5.5 million people, has the best health care system in the world. They have a great model. Costa Rica and Cuba have completely redesigned their health care systems. You go through medical school in 2 or 3 years, and then you get embedded in the community. So you have doctors living in the community responsible for the health of their neighbors. They get to know people in the context in which they live and refer them on only when they need more than basic care. These countries have vastly superior outcome measures, and they spend less money on health care.
Dr. Levy: My dream, as we reinvent things, is that we could create a comprehensive Women’s Medical Home where there’s a hub and an opportunity to be centered on patients so they could reach us when needed.
Ideally we could create a structure with a central contact person—a nurse practitioner, a midwife, someone in family medicine or internal medicine—someone focused on women’s health who has researched how inequities apply to women and women’s health and the areas where research doesn’t necessarily apply to women as just “smaller men.” Then we would have the hub, and the spokes—those would be mental health care providers, surgeons, and people to provide additional services when needed.
The only way I can figure how to make that work from a payment perspective is with a prospective payment system, a per member, per month capitated payment structure. That way, ancillary and other services would be available, and overtesting and such would be disincentivized.
Continue to: The question of payment...
The question of payment
Dr. Hayworth: I agree. For every practice, the two key considerations in addressing the challenges of capitation are, first, that the team approach is essential, and, second, that providers appreciate that everything they do for their patients is reimbursed in a global payment.
At CareMount Medical, our team system embeds advanced practice professionals in our primary care and ObGyn offices. Everyone—physicians, midwives, nurse practitioners—practices at the top of their license. Our care coordinators ensure that our patients’ health journeys are optimized from well care through specialized needs, engaging every member of the care team effectively.
To optimize our success in a risk model, we recognize that tasks and services that went without direct reimbursement in a fee-for-service arrangement are integral to producing the best outcomes for our patients. We examine everything we do from the perspective of how to provide the most advanced care in the most efficient manner. For example, we drive toward moving procedures from the hospital to the outpatient setting, and from the ambulatory surgical center to the office. This allows us maximal control of both quality and cost, with savings benefiting our group as well as the payers with whom we have contracts.
Dr. Norwitz: I have been fortunate to have trained and worked in 5 different countries on 3 continents. There’s no question there are better health care systems out there. Some form of capitation is needed, whether it’s value-based care or a risk-sharing arrangement. But how do you do it without a single payer? I don’t think you can, but I’m ready to listen.
Dr. Hayworth: You can have capitation without a single payer; in fact, it’s far better to have many payers compete to offer the greatest flexibility to both patients and providers. CareMount Medical has 650,000 patients who rely on us to provide their care with the utmost quality and affordability. In our Next Generation ACO, our Medicare patients have the benefit of care coordination in a team approach that saves our government money, and we are incentivized to do our best because some of those savings return to us.
The needs of Medicare patients, of course, are different from those in other age groups, and our contracts with other payers will reflect that distinction. There’s no inherent reason why capitation has to equal “single payer.” The benefits of the risk model are magnified by incentivizing all participants to provide maximum value.
Continue to: Ms. Pearson...
Ms. Pearson: I am going to comment on capitated care because I think educated consumers are well aware of the benefits of moving away from fee-for-service and bringing in some more sensible system. However, given the historical racial inequities and injustices, and lack of access and disparate treatment, capitation raises fear in the hearts of people whose communities have not gotten the care that they need.
The answer is not to avoid capitation, but to find a way for the profession to be seen more visibly as reflecting who they serve, and we know we can’t change the profession’s racial makeup overnight. That’s a generation-long effort.
Dr. Levy: For capitation to work, there has to be value, you have to meet the quality metrics. Having served on the National Quality Forum on multiple different committees, I am convinced that we measure what is easy to measure, and we are not measuring what really matters to people. My thought is to embrace the communities that have been underserved to help us design the metrics for a capitated system that is meaningful to the people that we serve.
Ms. Pearson: On the West Coast, some people are leading efforts to create patient-centered metrics for respectful maternity care led by Black, indigenous, and people of color communities that are validated with solid research tools.
Algorithms for care
Dr. Norwitz: Artificial intelligence (AI) may have a role to play. For example, I think we do a terrible job of caring for women in the postpartum period. We focus almost all of our care in the antepartum period and not postpartum. I am working with a group with a finance and banking background to try and risk-adjust patients in the antepartum, intrapartum, and postpartum period. We are developing algorithms using AI and deep learning technologies to risk-stratify patients and say, “This patient is low risk so can safely get obstetric care with a family medicine doctor or midwife. That patient requires consultation with a maternal-fetal medicine subspecialist or a general internist,” and so on.
Ms. Pearson: As policy advocates, we are trying to get Medicaid postpartum coverage expanded to 12 months. Too many women fall into a coverage gap shortly after delivery; continued coverage would help improve postpartum outcomes. I am curious how an algorithm might help take better care of women postpartum.
Dr. Norwitz: Postpartum care is one of the greatest areas of need. I love the Dutch model. In the Netherlands, when a woman goes home after giving birth, a designated nurse comes home with her, teaches her how to breastfeed and how to bathe the baby, and assists with routine activities such as cooking and washing. And the nurse remains engaged for a prolonged period of time, paid for by the government. There are also other social welfare packages, such as a full 4-year or more maternity leave.
The solution is part political and part medical. We need to rethink our care model, and I don’t think we provide enough postpartum care.
Continue to: Dr. Hayworth...
Dr. Hayworth: Errol made an excellent point about AI. There is a product that’s being used in Europe and in some other parts of the world that can provide 85% of care through an algorithm without a patient even having to speak to a nurse or doctor. The company that offers the product claims a high level of patient satisfaction and a very low error rate.
We are a long way from the point at which—and I don’t anticipate that we’ll ever get there—AI fully replaces human providers, but there’s enormous and growing potential for data aggregation and machine learning to enhance, exponentially, the capabilities and capacity of care teams.
The most immediate applications for AI in the United States are in diagnostics, pathology, and the mapping of protocols for patients with cancer who will benefit from access to investigational interventions and clinical trials. As we gain experience in those areas, acceptance and confidence will lead steadily to broader deployment of AI, enhancing the quality of care and the efficiency of delivery and saving costs.
Dr. Norwitz: AI is a tool to assist providers. It is not going to replace us, which is the fear.
Ms. Pearson: From the consumer perspective, again, there is concern that if not enough data are available from Black, indigenous, and people of color, the levels won’t start out in a good place. The criticism over mammography randomized controlled trials (RCTs) has existed for a long time. The big trials that got all the way out to mortality did not include enough women of color; and so women of color rightly say, “Why should we believe these guidelines developed on results of the RCTs?” My point is that because of historical inequity, logical solutions such as algorithms do not always work for communities that were previously excluded or mistreated.
Dr. Levy: Your point is incredibly well taken. That means that those of us researching and working with AI need to ensure that the data going in are representative, that we are not embedding implicit biases into the AI algorithms, which clearly has sometimes already happened. We have to be careful to embrace input from multiple sources that we have not thought of before.
As we look at an algorithm for managing a postpartum patient or a postoperative patient, have we thought about how she’s managing her children at home after she goes home? What else is happening in her life? How can we impact her recovery in a positive way? We need to hear the voices of the people that we are trying to serve as we develop those algorithms.
Perspectives on future health care delivery
Dr. Levy: To summarize so far, we are thinking about a Woman’s Medical Home, a capitated model of comprehensive care for women that includes mental health, social determinants, and home care. There are different models, but a payment structure where we would have the capital to invest in community services and in things that we think may make a difference.
Dr. Norwitz: I think the health care system of the future is not going to be based in large academic medical centers. It’s going to be in community hospitals close to home. It’s going to be in the home. And it will be provided by different types of practitioners, whose performances are tracked using more appropriate outcome metrics.
Dr. Levy: I also think we will have community health workers. While we haven’t talked about rural health and access to care, there are some structural things we can do to reach rural communities with really excellent care, such as training community health workers and using telemedicine. It does require thinking through a different payment structure, though, because there really isn’t money in the system to do that currently, at least to my knowledge.
Janice, do we have enough motivation to take care of women? Women are so underrepresented when we look at care models.
Dr. Huckaby: I do think there is hope, but it will truly take a village. While CMS (Centers for Medicare and Medicaid Services) has its innovation center in the Medicaid space, it’s almost like we have to have the payers, the government, the specialty societies, and so on say that we need to do something better. I mention the government because it is not only a payer but also a regulator. They can help create some of these things.
There are opportunities with payers to say, “Let’s move to this kind of model for that.” But still, we are implementing change but on a fairly minor scale.
We could have the people who care about issues, help deliver the care, pay the bills, and so on say, “This is what we want to do,” and then we could pilot them. It may be one type of pilot in a rural area and one type of pilot in an urban area, because they are going to differ, and do it that way and then scale it.
Telemedicine, or telehealth, is part of creating access. Even some nontraditional settings, such as retail store clinics, may work.
Continue to: Dr. Levy...
Dr. Levy: Cindy, is there any last thing you wanted to comment on?
Ms. Pearson: All the changes we have talked about require public policy change. Physicians become physicians to take care of people, not because they want to be policy wonks like us. We love policy because we see how it can benefit. To our readers I say be part of making this generational change in the profession and women’s health care, get involved in policy, because these things can’t happen without the policy changes.
Dr. Norwitz: That is so important. In most developed countries around the world, you get trained in medical school, the cost of training is subsidized, and in return you owe 2 years of service. In this country, if we subsidized the training of doctors and in return they owed us 2 years of primary care service based in the community or in an underserved area, they would get valuable clinical experience and wouldn’t have so many loans to pay back. I think it is a policy that could work and could profoundly change the health care landscape in time.
Dr. Levy: And it would save a great deal of money. The reality is that if we subsidize medical education and in return required service in a national public health service, we would move providers out into rural areas. That would to some extent solve our rural problem. We would train people to think about diagnostic options when the resources are not unlimited, so that they will perhaps not order quite so many tests.
That policy change would foundationally allow for more minority students to become physicians and health care workers. If there were one thing we could do to begin to drive this change, that would be it.
Who would have thought a disruptive pandemic could affect the way people receive care, in bad and good ways? Some carriers, for example, are now paying for telehealth visits who previously did not.
Final thoughts
Dr. Hayworth: It’s an exciting time to be in medicine and women’s health: We are ushering in a new era in which we can fulfill the vision of comprehensive care, patient-focused and seamlessly delivered by teams whose capabilities are optimized by ever-improving technology. ObGyns, with our foundation in the continuum of care, have the experience and the sensibilities to adapt to the challenges of the value model, in which our success will depend on fully embracing our role as primary care providers.
Dr. Levy: Circling back to the beginning of our discussion, we talked about relationships, and developing deep relationships with patients is the internal reward and the piece that prevents us from burnout. It makes you feel good at the end of the day—or sometimes bad at the end of the day when something didn’t go well. Restructuring the system in a way that gets us back to personalized relationship-centered care will benefit ObGyns and our patients.
I thank you all for participating in this thoughtful discussion. ●
- Gawande A. Big med. The New Yorker. August 13, 2012. https://www.newyorker.com/magazine/2012/08/13/big-med. Accessed July 24, 2020.
- Gawande A. Big med. The New Yorker. August 13, 2012. https://www.newyorker.com/magazine/2012/08/13/big-med. Accessed July 24, 2020.
Only 40% of residents said training prepped them for COVID-19
Most residents who were asked whether their training prepared them for COVID-19 in a Medscape survey said it had not or they weren’t sure.
Whereas 40% said they felt prepared, 30% said they did not feel prepared and 31% answered they were unsure. (Numbers were rounded, so some answers pushed above 100%.)
One quarter have $300,000 or more in student debt
The Medscape Residents Salary & Debt Report 2020, with data collected April 3 to June 1, found that nearly one in four residents (24%) had medical school debt of more than $300,000. Half (49%) had more than $200,000.
The data include answers from 1,659 U.S. medical residents.
For the sixth straight year, female residents were more satisfied with their pay than were their male colleagues. This year the satisfaction gap was 45% female compared with 42% male. That imbalance came despite their making nearly the same pay overall ($63,700 for men and $63,000 for women).
Among practicing physicians, the pay gap is much wider: Men make 25% more in primary care and 31% more in specialties.
Ten percent thought they should earn 76%-100% more.
For those not satisfied with pay, the top reasons were feeling the pay was too low for the hours worked (81%) or too low compared with other medical staff, such as physician assistants (PAs) or nurses (77% chose that answer).
As for hours worked, 31% of residents reported they spend more than 60 hours/week seeing patients.
The top-paying specialties, averaging $69,500, were allergy and immunology, hematology, plastic surgery, aesthetic medicine, rheumatology, and specialized surgery. The lowest paid were family medicine residents at $58,500.
In primary care, overall, most residents said they planned to specialize. Only 47% planned to continue to work in primary care. Male residents were much more likely to say they will subspecialize than were their female colleagues (52% vs. 35%).
More than 90% of residents say future pay has influenced their choice of specialty, though more men than women felt that way (93% vs. 86%).
Good relationships with others
Overall, residents reported good relationships with attending physicians and nurses.
Most (88%) said they had good or very good relationships with attending physicians, 10% said the relationships were fair, and 2% said they were poor.
In addition, 89% of residents said the amount of supervision was appropriate, 4% said there was too much, and 7% said there was too little.
Relationships with nurses/PAs were slightly less positive overall: Eighty-two percent reported good or very good relationships with nurses/PAs, 15% said those relationships were fair, and 3% said they were poor.
One respondent said: “Our relationships could be better, but I think everyone is just overwhelmed with COVID-19, so emotions are heightened.”
Another said: “It takes time to earn the respect from nurses.”
Seventy-seven percent said they were satisfied with their learning experience overall, 12% were neutral on the question, and 11% said they were dissatisfied or very dissatisfied.
Work-life balance is the top concern
Work-life balance continues to be the top concern for residents. More than one-quarter (27%) in residency years 1 through 4 listed that as the top concern, and even more (32%) of those in years 5 through 8 agreed.
That was followed by demands on time and fear of failure or making a serious mistake.
The survey indicates that benefit packages for residents have stayed much the same over the past 2 years with health insurance and paid time off for sick leave, vacation, and personal time most commonly reported at 89% and 87%, respectively.
Much less common were benefits including commuter assistance (parking, public transportation) at 24%, housing allowance (8%), and child care (4%).
The vast majority of residents reported doing scut work (unskilled tasks): More than half (54%) reported doing 1-10 hours/week and 22% did 11-20 hours/week. Regardless of the number of hours, however, 62% said the time spent performing these tasks was appropriate.
A version of this article originally appeared on Medscape.com.
Most residents who were asked whether their training prepared them for COVID-19 in a Medscape survey said it had not or they weren’t sure.
Whereas 40% said they felt prepared, 30% said they did not feel prepared and 31% answered they were unsure. (Numbers were rounded, so some answers pushed above 100%.)
One quarter have $300,000 or more in student debt
The Medscape Residents Salary & Debt Report 2020, with data collected April 3 to June 1, found that nearly one in four residents (24%) had medical school debt of more than $300,000. Half (49%) had more than $200,000.
The data include answers from 1,659 U.S. medical residents.
For the sixth straight year, female residents were more satisfied with their pay than were their male colleagues. This year the satisfaction gap was 45% female compared with 42% male. That imbalance came despite their making nearly the same pay overall ($63,700 for men and $63,000 for women).
Among practicing physicians, the pay gap is much wider: Men make 25% more in primary care and 31% more in specialties.
Ten percent thought they should earn 76%-100% more.
For those not satisfied with pay, the top reasons were feeling the pay was too low for the hours worked (81%) or too low compared with other medical staff, such as physician assistants (PAs) or nurses (77% chose that answer).
As for hours worked, 31% of residents reported they spend more than 60 hours/week seeing patients.
The top-paying specialties, averaging $69,500, were allergy and immunology, hematology, plastic surgery, aesthetic medicine, rheumatology, and specialized surgery. The lowest paid were family medicine residents at $58,500.
In primary care, overall, most residents said they planned to specialize. Only 47% planned to continue to work in primary care. Male residents were much more likely to say they will subspecialize than were their female colleagues (52% vs. 35%).
More than 90% of residents say future pay has influenced their choice of specialty, though more men than women felt that way (93% vs. 86%).
Good relationships with others
Overall, residents reported good relationships with attending physicians and nurses.
Most (88%) said they had good or very good relationships with attending physicians, 10% said the relationships were fair, and 2% said they were poor.
In addition, 89% of residents said the amount of supervision was appropriate, 4% said there was too much, and 7% said there was too little.
Relationships with nurses/PAs were slightly less positive overall: Eighty-two percent reported good or very good relationships with nurses/PAs, 15% said those relationships were fair, and 3% said they were poor.
One respondent said: “Our relationships could be better, but I think everyone is just overwhelmed with COVID-19, so emotions are heightened.”
Another said: “It takes time to earn the respect from nurses.”
Seventy-seven percent said they were satisfied with their learning experience overall, 12% were neutral on the question, and 11% said they were dissatisfied or very dissatisfied.
Work-life balance is the top concern
Work-life balance continues to be the top concern for residents. More than one-quarter (27%) in residency years 1 through 4 listed that as the top concern, and even more (32%) of those in years 5 through 8 agreed.
That was followed by demands on time and fear of failure or making a serious mistake.
The survey indicates that benefit packages for residents have stayed much the same over the past 2 years with health insurance and paid time off for sick leave, vacation, and personal time most commonly reported at 89% and 87%, respectively.
Much less common were benefits including commuter assistance (parking, public transportation) at 24%, housing allowance (8%), and child care (4%).
The vast majority of residents reported doing scut work (unskilled tasks): More than half (54%) reported doing 1-10 hours/week and 22% did 11-20 hours/week. Regardless of the number of hours, however, 62% said the time spent performing these tasks was appropriate.
A version of this article originally appeared on Medscape.com.
Most residents who were asked whether their training prepared them for COVID-19 in a Medscape survey said it had not or they weren’t sure.
Whereas 40% said they felt prepared, 30% said they did not feel prepared and 31% answered they were unsure. (Numbers were rounded, so some answers pushed above 100%.)
One quarter have $300,000 or more in student debt
The Medscape Residents Salary & Debt Report 2020, with data collected April 3 to June 1, found that nearly one in four residents (24%) had medical school debt of more than $300,000. Half (49%) had more than $200,000.
The data include answers from 1,659 U.S. medical residents.
For the sixth straight year, female residents were more satisfied with their pay than were their male colleagues. This year the satisfaction gap was 45% female compared with 42% male. That imbalance came despite their making nearly the same pay overall ($63,700 for men and $63,000 for women).
Among practicing physicians, the pay gap is much wider: Men make 25% more in primary care and 31% more in specialties.
Ten percent thought they should earn 76%-100% more.
For those not satisfied with pay, the top reasons were feeling the pay was too low for the hours worked (81%) or too low compared with other medical staff, such as physician assistants (PAs) or nurses (77% chose that answer).
As for hours worked, 31% of residents reported they spend more than 60 hours/week seeing patients.
The top-paying specialties, averaging $69,500, were allergy and immunology, hematology, plastic surgery, aesthetic medicine, rheumatology, and specialized surgery. The lowest paid were family medicine residents at $58,500.
In primary care, overall, most residents said they planned to specialize. Only 47% planned to continue to work in primary care. Male residents were much more likely to say they will subspecialize than were their female colleagues (52% vs. 35%).
More than 90% of residents say future pay has influenced their choice of specialty, though more men than women felt that way (93% vs. 86%).
Good relationships with others
Overall, residents reported good relationships with attending physicians and nurses.
Most (88%) said they had good or very good relationships with attending physicians, 10% said the relationships were fair, and 2% said they were poor.
In addition, 89% of residents said the amount of supervision was appropriate, 4% said there was too much, and 7% said there was too little.
Relationships with nurses/PAs were slightly less positive overall: Eighty-two percent reported good or very good relationships with nurses/PAs, 15% said those relationships were fair, and 3% said they were poor.
One respondent said: “Our relationships could be better, but I think everyone is just overwhelmed with COVID-19, so emotions are heightened.”
Another said: “It takes time to earn the respect from nurses.”
Seventy-seven percent said they were satisfied with their learning experience overall, 12% were neutral on the question, and 11% said they were dissatisfied or very dissatisfied.
Work-life balance is the top concern
Work-life balance continues to be the top concern for residents. More than one-quarter (27%) in residency years 1 through 4 listed that as the top concern, and even more (32%) of those in years 5 through 8 agreed.
That was followed by demands on time and fear of failure or making a serious mistake.
The survey indicates that benefit packages for residents have stayed much the same over the past 2 years with health insurance and paid time off for sick leave, vacation, and personal time most commonly reported at 89% and 87%, respectively.
Much less common were benefits including commuter assistance (parking, public transportation) at 24%, housing allowance (8%), and child care (4%).
The vast majority of residents reported doing scut work (unskilled tasks): More than half (54%) reported doing 1-10 hours/week and 22% did 11-20 hours/week. Regardless of the number of hours, however, 62% said the time spent performing these tasks was appropriate.
A version of this article originally appeared on Medscape.com.
Telemedicine checklist may smooth visits with older patients
During the pandemic, physicians have raced to set up or expand telemedicine, uncovering both advantages and shortcomings.
Although many of the suggestions, published online in Annals of Internal Medicine, are useful for all patients, Carrie Nieman, MD, MPH, and Esther S. Oh, MD, PhD, developed the list with older patients in mind.
“I have a number of patients into their 90s and with hearing loss, and we have had very successful video-based telemedicine visits,” Dr. Nieman, with the Cochlear Center for Hearing and Public Health at Johns Hopkins Bloomberg School of Public Health in Baltimore said in an interview. “Age should not be considered synonymous with inability or unwillingness to use technology.”
Their recommendations included the following:
- Assume some degree of hearing loss, which affects about two-thirds of adults aged 70 years and older.
- Ask patients to wear headphones or a headset or confirm that they are wearing their hearing aids and are in a quiet location.
- Use a headset.
- When possible, use video and have the camera focused on your face.
- Use captioning when available and provide a written summary of key points and instructions.
- Pay attention to cues, such as nodding along or looking to a loved one, that suggest a patient may not be following the conversation.
“If cognitive impairment is suspected, several screening tools can be used over the telephone to identify individuals who may need more comprehensive, in-person assessment,” wrote Dr. Nieman and Dr. Oh, who is with the division of geriatric medicine and gerontology at Johns Hopkins University School of Medicine. For example, data suggest that a modified version of the Mini–Mental State Examination and the Delirium Symptom Interview could be useful tools. “A formal diagnosis of dementia is not recommended solely based on a telephone-based cognitive screening,” however, Dr. Nieman and Dr. Oh said.
For patients with hearing loss, video visits avoid a current limitation of in-person visits: face masks that hinder patients’ ability to read lips and other visual cues. “For many of us, we rely on these types of cues more than we think,” Dr. Nieman said in an interview.
“When you have doubts about whether you and your patient are on the same page, check in with the patient,” Dr. Nieman said. “When appropriate, having a loved one or a care partner join an encounter, or at least a portion of the encounter, can be helpful to both the patient and the provider.”
Many older patients unprepared
Millions of older patients may not have been ready for the rapid shift to telemedicine brought on by COVID-19, a recent study in JAMA Internal Medicine suggests. Between 32% and 38% of older adults in the United States may not have been ready for video visits, largely because of inexperience with technology. Approximately 20% could have difficulty with telephone visits because of problems hearing or communicating or because of dementia.
Kenneth Lam, MD, of the division of geriatrics at the University of California, San Francisco (UCSF), and colleagues arrived at these estimates after analyzing data from more than 4,500 participants in the National Health and Aging Trends Study that was conducted in 2018. The study is nationally representative of Medicare beneficiaries 65 years or older.
The aim of the study “was to call attention to what clinicians were already experiencing on the front lines,” Dr. Lam said. In an interview, he imagined two scenarios based on his colleagues’ accounts of telemedicine visits.
In one case, a 72-year-old woman logs into Zoom Health on her iPad without any trouble. “She explains she just pushed on the URL and everything loaded up and you have a great visit,” Dr. Lam said. “This is likely to be the case for over 50% of the older people you see; I share this picture to combat ageism, which is, truthfully, just inaccurate stereotyping of older people and gets in the way of actionable, data-driven policies.
“However, for around one in three older adults (and closer to three out of every four of those over the age of 85), you will book an appointment and they will say they don’t have an email address or a computer or know how to go online,” Dr. Lam said. “Or suppose they decide to try it out. ... Come appointment time, you log on and they pick up, but now their sound doesn’t work. They keep saying they can see you but they can’t hear you. ... They accidentally hang up. You place another call, and they ask if you can switch to a phone conversation instead.”
By phone, the physician can address concerns about the patient’s blood pressure, which the patient has been measuring daily. “But when it comes to looking at the swelling in their legs, you’re out of luck, and you’ve been on this call for 45 minutes,” Dr. Lam said.
Have a backup plan
Making sure patients are prepared and having a backup plan can help, said Kaitlin Willham, MD, of UCSF and the San Francisco VA Medical Center.
She says older patients fall into a wide range of categories in terms of skills and access to equipment. Knowing which category a patient falls into and having relevant support available to troubleshoot are important.
During the pandemic, Dr. Willham has conducted many more telemedicine visits with patients who are at their place of residence, whether a private home or a residential care facility. “Even outside of the current crisis, there are benefits to home video visits,” Dr. Willham said. “A home video visit can provide a more holistic view of the patient than an office visit, allowing the clinician to see how the person lives, what they might be challenged by. It allows the clinician to identify areas of intervention and, if there is a care partner, involving that person in the plan. If the visit starts without major technical or communication barriers, they are generally very well received.”
For patients with problems hearing for whom headphones or amplification devices are not available, “using a landline for the audio portion of the visit can help, as can having someone with the patient reiterate what was said,” Dr. Willham suggested. “Many video platforms also enable the clinician to type messages or share a screen with a live document. These options can work well when there is very severe or complete lack of hearing.”
Sometimes an in-person visit is the right way to go, even when technical hurdles can be overcome.
“Although many older adults are willing and able to learn to use telemedicine, an equitable health system should recognize that for some, such as those with dementia and social isolation, in-person visits are already difficult and telemedicine may be impossible,” Dr. Lam and coauthors wrote. “For these patients, clinics and geriatric models of care such as home visits are essential.”
Dr. Nieman, Dr. Oh, and one of Dr. Lam’s coauthors have received funding from the National Institutes of Health. Dr. Oh also has received funding from the Roberts Family Fund. Dr. Nieman serves as a board member of the nonprofit organization Access HEARS and is on the board of trustees of the Hearing Loss Association of America.
A version of this article originally appeared on Medscape.com.
During the pandemic, physicians have raced to set up or expand telemedicine, uncovering both advantages and shortcomings.
Although many of the suggestions, published online in Annals of Internal Medicine, are useful for all patients, Carrie Nieman, MD, MPH, and Esther S. Oh, MD, PhD, developed the list with older patients in mind.
“I have a number of patients into their 90s and with hearing loss, and we have had very successful video-based telemedicine visits,” Dr. Nieman, with the Cochlear Center for Hearing and Public Health at Johns Hopkins Bloomberg School of Public Health in Baltimore said in an interview. “Age should not be considered synonymous with inability or unwillingness to use technology.”
Their recommendations included the following:
- Assume some degree of hearing loss, which affects about two-thirds of adults aged 70 years and older.
- Ask patients to wear headphones or a headset or confirm that they are wearing their hearing aids and are in a quiet location.
- Use a headset.
- When possible, use video and have the camera focused on your face.
- Use captioning when available and provide a written summary of key points and instructions.
- Pay attention to cues, such as nodding along or looking to a loved one, that suggest a patient may not be following the conversation.
“If cognitive impairment is suspected, several screening tools can be used over the telephone to identify individuals who may need more comprehensive, in-person assessment,” wrote Dr. Nieman and Dr. Oh, who is with the division of geriatric medicine and gerontology at Johns Hopkins University School of Medicine. For example, data suggest that a modified version of the Mini–Mental State Examination and the Delirium Symptom Interview could be useful tools. “A formal diagnosis of dementia is not recommended solely based on a telephone-based cognitive screening,” however, Dr. Nieman and Dr. Oh said.
For patients with hearing loss, video visits avoid a current limitation of in-person visits: face masks that hinder patients’ ability to read lips and other visual cues. “For many of us, we rely on these types of cues more than we think,” Dr. Nieman said in an interview.
“When you have doubts about whether you and your patient are on the same page, check in with the patient,” Dr. Nieman said. “When appropriate, having a loved one or a care partner join an encounter, or at least a portion of the encounter, can be helpful to both the patient and the provider.”
Many older patients unprepared
Millions of older patients may not have been ready for the rapid shift to telemedicine brought on by COVID-19, a recent study in JAMA Internal Medicine suggests. Between 32% and 38% of older adults in the United States may not have been ready for video visits, largely because of inexperience with technology. Approximately 20% could have difficulty with telephone visits because of problems hearing or communicating or because of dementia.
Kenneth Lam, MD, of the division of geriatrics at the University of California, San Francisco (UCSF), and colleagues arrived at these estimates after analyzing data from more than 4,500 participants in the National Health and Aging Trends Study that was conducted in 2018. The study is nationally representative of Medicare beneficiaries 65 years or older.
The aim of the study “was to call attention to what clinicians were already experiencing on the front lines,” Dr. Lam said. In an interview, he imagined two scenarios based on his colleagues’ accounts of telemedicine visits.
In one case, a 72-year-old woman logs into Zoom Health on her iPad without any trouble. “She explains she just pushed on the URL and everything loaded up and you have a great visit,” Dr. Lam said. “This is likely to be the case for over 50% of the older people you see; I share this picture to combat ageism, which is, truthfully, just inaccurate stereotyping of older people and gets in the way of actionable, data-driven policies.
“However, for around one in three older adults (and closer to three out of every four of those over the age of 85), you will book an appointment and they will say they don’t have an email address or a computer or know how to go online,” Dr. Lam said. “Or suppose they decide to try it out. ... Come appointment time, you log on and they pick up, but now their sound doesn’t work. They keep saying they can see you but they can’t hear you. ... They accidentally hang up. You place another call, and they ask if you can switch to a phone conversation instead.”
By phone, the physician can address concerns about the patient’s blood pressure, which the patient has been measuring daily. “But when it comes to looking at the swelling in their legs, you’re out of luck, and you’ve been on this call for 45 minutes,” Dr. Lam said.
Have a backup plan
Making sure patients are prepared and having a backup plan can help, said Kaitlin Willham, MD, of UCSF and the San Francisco VA Medical Center.
She says older patients fall into a wide range of categories in terms of skills and access to equipment. Knowing which category a patient falls into and having relevant support available to troubleshoot are important.
During the pandemic, Dr. Willham has conducted many more telemedicine visits with patients who are at their place of residence, whether a private home or a residential care facility. “Even outside of the current crisis, there are benefits to home video visits,” Dr. Willham said. “A home video visit can provide a more holistic view of the patient than an office visit, allowing the clinician to see how the person lives, what they might be challenged by. It allows the clinician to identify areas of intervention and, if there is a care partner, involving that person in the plan. If the visit starts without major technical or communication barriers, they are generally very well received.”
For patients with problems hearing for whom headphones or amplification devices are not available, “using a landline for the audio portion of the visit can help, as can having someone with the patient reiterate what was said,” Dr. Willham suggested. “Many video platforms also enable the clinician to type messages or share a screen with a live document. These options can work well when there is very severe or complete lack of hearing.”
Sometimes an in-person visit is the right way to go, even when technical hurdles can be overcome.
“Although many older adults are willing and able to learn to use telemedicine, an equitable health system should recognize that for some, such as those with dementia and social isolation, in-person visits are already difficult and telemedicine may be impossible,” Dr. Lam and coauthors wrote. “For these patients, clinics and geriatric models of care such as home visits are essential.”
Dr. Nieman, Dr. Oh, and one of Dr. Lam’s coauthors have received funding from the National Institutes of Health. Dr. Oh also has received funding from the Roberts Family Fund. Dr. Nieman serves as a board member of the nonprofit organization Access HEARS and is on the board of trustees of the Hearing Loss Association of America.
A version of this article originally appeared on Medscape.com.
During the pandemic, physicians have raced to set up or expand telemedicine, uncovering both advantages and shortcomings.
Although many of the suggestions, published online in Annals of Internal Medicine, are useful for all patients, Carrie Nieman, MD, MPH, and Esther S. Oh, MD, PhD, developed the list with older patients in mind.
“I have a number of patients into their 90s and with hearing loss, and we have had very successful video-based telemedicine visits,” Dr. Nieman, with the Cochlear Center for Hearing and Public Health at Johns Hopkins Bloomberg School of Public Health in Baltimore said in an interview. “Age should not be considered synonymous with inability or unwillingness to use technology.”
Their recommendations included the following:
- Assume some degree of hearing loss, which affects about two-thirds of adults aged 70 years and older.
- Ask patients to wear headphones or a headset or confirm that they are wearing their hearing aids and are in a quiet location.
- Use a headset.
- When possible, use video and have the camera focused on your face.
- Use captioning when available and provide a written summary of key points and instructions.
- Pay attention to cues, such as nodding along or looking to a loved one, that suggest a patient may not be following the conversation.
“If cognitive impairment is suspected, several screening tools can be used over the telephone to identify individuals who may need more comprehensive, in-person assessment,” wrote Dr. Nieman and Dr. Oh, who is with the division of geriatric medicine and gerontology at Johns Hopkins University School of Medicine. For example, data suggest that a modified version of the Mini–Mental State Examination and the Delirium Symptom Interview could be useful tools. “A formal diagnosis of dementia is not recommended solely based on a telephone-based cognitive screening,” however, Dr. Nieman and Dr. Oh said.
For patients with hearing loss, video visits avoid a current limitation of in-person visits: face masks that hinder patients’ ability to read lips and other visual cues. “For many of us, we rely on these types of cues more than we think,” Dr. Nieman said in an interview.
“When you have doubts about whether you and your patient are on the same page, check in with the patient,” Dr. Nieman said. “When appropriate, having a loved one or a care partner join an encounter, or at least a portion of the encounter, can be helpful to both the patient and the provider.”
Many older patients unprepared
Millions of older patients may not have been ready for the rapid shift to telemedicine brought on by COVID-19, a recent study in JAMA Internal Medicine suggests. Between 32% and 38% of older adults in the United States may not have been ready for video visits, largely because of inexperience with technology. Approximately 20% could have difficulty with telephone visits because of problems hearing or communicating or because of dementia.
Kenneth Lam, MD, of the division of geriatrics at the University of California, San Francisco (UCSF), and colleagues arrived at these estimates after analyzing data from more than 4,500 participants in the National Health and Aging Trends Study that was conducted in 2018. The study is nationally representative of Medicare beneficiaries 65 years or older.
The aim of the study “was to call attention to what clinicians were already experiencing on the front lines,” Dr. Lam said. In an interview, he imagined two scenarios based on his colleagues’ accounts of telemedicine visits.
In one case, a 72-year-old woman logs into Zoom Health on her iPad without any trouble. “She explains she just pushed on the URL and everything loaded up and you have a great visit,” Dr. Lam said. “This is likely to be the case for over 50% of the older people you see; I share this picture to combat ageism, which is, truthfully, just inaccurate stereotyping of older people and gets in the way of actionable, data-driven policies.
“However, for around one in three older adults (and closer to three out of every four of those over the age of 85), you will book an appointment and they will say they don’t have an email address or a computer or know how to go online,” Dr. Lam said. “Or suppose they decide to try it out. ... Come appointment time, you log on and they pick up, but now their sound doesn’t work. They keep saying they can see you but they can’t hear you. ... They accidentally hang up. You place another call, and they ask if you can switch to a phone conversation instead.”
By phone, the physician can address concerns about the patient’s blood pressure, which the patient has been measuring daily. “But when it comes to looking at the swelling in their legs, you’re out of luck, and you’ve been on this call for 45 minutes,” Dr. Lam said.
Have a backup plan
Making sure patients are prepared and having a backup plan can help, said Kaitlin Willham, MD, of UCSF and the San Francisco VA Medical Center.
She says older patients fall into a wide range of categories in terms of skills and access to equipment. Knowing which category a patient falls into and having relevant support available to troubleshoot are important.
During the pandemic, Dr. Willham has conducted many more telemedicine visits with patients who are at their place of residence, whether a private home or a residential care facility. “Even outside of the current crisis, there are benefits to home video visits,” Dr. Willham said. “A home video visit can provide a more holistic view of the patient than an office visit, allowing the clinician to see how the person lives, what they might be challenged by. It allows the clinician to identify areas of intervention and, if there is a care partner, involving that person in the plan. If the visit starts without major technical or communication barriers, they are generally very well received.”
For patients with problems hearing for whom headphones or amplification devices are not available, “using a landline for the audio portion of the visit can help, as can having someone with the patient reiterate what was said,” Dr. Willham suggested. “Many video platforms also enable the clinician to type messages or share a screen with a live document. These options can work well when there is very severe or complete lack of hearing.”
Sometimes an in-person visit is the right way to go, even when technical hurdles can be overcome.
“Although many older adults are willing and able to learn to use telemedicine, an equitable health system should recognize that for some, such as those with dementia and social isolation, in-person visits are already difficult and telemedicine may be impossible,” Dr. Lam and coauthors wrote. “For these patients, clinics and geriatric models of care such as home visits are essential.”
Dr. Nieman, Dr. Oh, and one of Dr. Lam’s coauthors have received funding from the National Institutes of Health. Dr. Oh also has received funding from the Roberts Family Fund. Dr. Nieman serves as a board member of the nonprofit organization Access HEARS and is on the board of trustees of the Hearing Loss Association of America.
A version of this article originally appeared on Medscape.com.
Laser Safety: The Need for Protocols
The use of lasers in dermatology has evolved and expanded since their first cutaneous use in 1963.1 As the fundamental understanding of the interaction of laser energy with biological tissues increased, the need for laser safety became apparent. Since then, lasers of varying wavelengths have been developed, each with its specific chromophore target and specific safety need. Protocols, such as a checklist, that have been shown to reduce adverse events in surgery and in the intensive care unit can be borrowed to decrease risk from laser injury and optimize laser safety in dermatology.2 The safety of the patient, the laser operator, and the other health care providers involved in the delivery of laser therapy led to the first US Food and Drug Administration (FDA) guidelines for laser use in 1984.3
There are 4 regulatory organizations for laser safety in the United States: the American National Standards Institute (ANSI), the Occupational Health and Safety Administration (OSHA), the FDA’s Center for Devices and Radiological Health, and The Joint Commission.
Laser Principles
The basic principles of lasers include transmission, absorption, scatter, and reflection, all occurring when laser light is applied to biological tissues. The effects of the laser are a function of the target tissue (the chromophore) and the wavelength of light being used.4 In the skin, there are 3 main endogenous chromophores: water, hemoglobin, and melanin. Some experts consider collagen to be a fourth and separate entity as a chromophore. Tattoos are considered exogenous chromophores.3 The basic principles of lasers are important to understand and keep in mind when discussing laser safety, as they are the mechanisms through which unintended consequences can occur.
Laser Safety
Ocular Hazards
Ocular hazards are a notable concern in laser surgery. The eye is uniquely susceptible to laser light, and eye injuries represent a majority of reported injuries, which can occur through direct beam, mirror reflection by surgical instruments, and beam reflection off the skin (4%–7% of light that hits the skin is reflected because of the refractive index between air and the stratum corneum).3 The different wavelengths of lasers affect different parts of the eye. The 3 parts of the eye affected most are the retina, cornea, and lens. Not only is the lens primarily at risk for acute (lenticular burns) and chronic (cataracts) injury from the laser, but secondarily the lens also can concentrate a laser beam onto the retina by a factor of 100,000 (Table 1).3
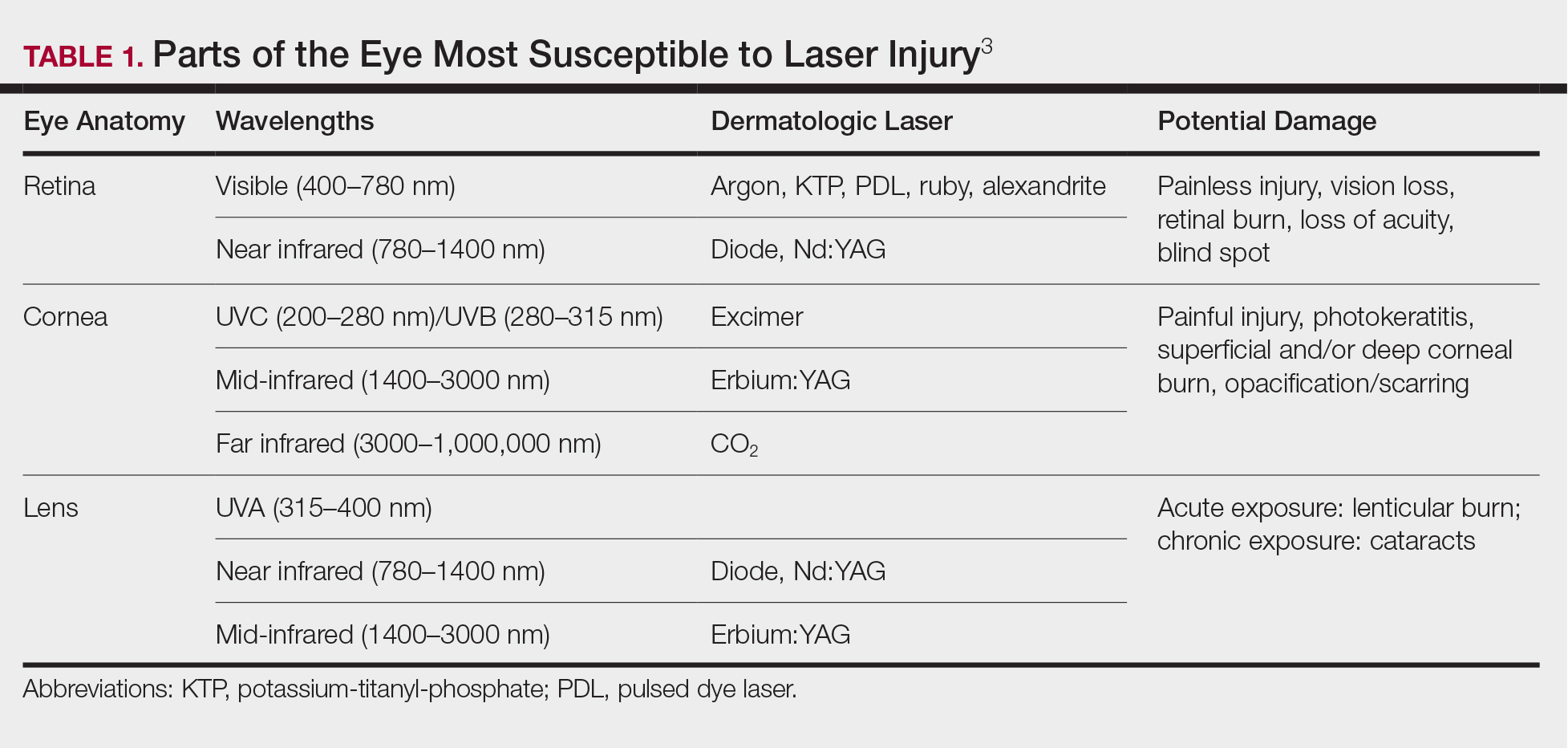
The use of ocular protective equipment, sometimes referred to as personal protective eyewear (PPE), is essential and is mandated by ANSI and OSHA for all class 3 and class 4 lasers. The eyewear must be labeled with the wavelength and the degree of optical protection—termed the optical density (OD) or filter factor—of each lens and should match the laser being used. Laser manufacturers, as required by ANSI, must provide the wavelength and OD of their lasers, and both can be found on each laser as well as in ANSI Z136.1.3
Vendors supplying PPE generally provide the material, usually glass or polycarbonate; color; visible light transmission, which is the actual amount of light that reaches one’s eye through the lens; filter specifications, which contain the OD at certain wavelengths; and the types of lasers for which each specific PPE is used. It is important to match the laser to the correct PPE. The use of multiple types of lasers in the same office or laser treatment area can present challenges regarding eye safety. Matching the PPE to the laser in use is critical, and therefore all steps to prevent error for patients and personnel should be employed. One recommendation is to place each laser in a separate room with the appropriate PPE hung outside on the door of that room.
When the treatment area is in the periocular region, protection of the patient’s cornea is essential. Leaded eye shields with nonreflective surfaces have been shown to offer the best protection.5 Prior to placement, anesthetic eye drops and lubrication are important for patient comfort and protection from corneal injury.
Laser-Generated Airborne Contaminants
Other hazards associated with laser use not directly related to the beam are laser-generated airborne contaminants (LGACs), including chemicals, viruses, bacteria, aerosolized blood products, and nanoparticles (<1 µm) known as ultrafine particles (UFPs). According to ANSI, electrosurgical devices and lasers generate the same smoke. The plume (surgical smoke) is known to contain as many as 60 chemicals, including but not limited to carbon monoxide, acrylonitrite, hydrocyanide, benzene, toluene, naphthalene, and formaldehyde. Several are known carcinogens, and others are environmental toxins.6,7
Smoke management is an important consideration for dermatologists and their patients and generally includes respiratory protection via masks and ventilation techniques. However, the practice is not universal, and oversight agencies such as OSHA and the National Institute for Occupational Safety and Health (NIOSH) provide guidelines only; they do not enforce. As such, smoke management is voluntary and not widely practiced. In a 2014 survey of 997 dermatologic surgeons who were asked if smoke management is used in their practice, 77% of respondents indicated no smoke management was used.6
The Surgical Plume: Composition
A 2014 study from the University of California, San Diego Department of Dermatology analyzed surgical smoke.6 The researchers placed the smoke collection probe 16 to 18 inches above the electrocautery site, which approximates the location of the surgeon’s head during the procedure. Assessing smoke composition, they found high levels of carcinogens and irritants. Two compounds found in their assay—1,3-butadiene and benzene—also are found in secondhand cigarette smoke. However, the concentrations in the plume were 17-fold higher for 1,3-butadiene and 10-fold higher for benzene than those found in secondhand cigarette smoke. The risk from chronic, long-term exposure to these airborne contaminants is notable, as benzene (a known carcinogen as determined by the US Department of Health and Human Services) is known to cause leukemia. For example, a busy Mohs surgeon can reach the equivalent of as many as 50 hours of continuous smoke exposure over the course of a year.6
The Surgical Plume: Particle Concentration
Ultrafine particles can bypass conventional filtering systems (surgical masks and N95 respirators) because of their extremely small size, which allows them to pass further into the lungs and all the way to the alveolar spaces. Geographic regions with high UFPs have been shown to have higher overall mortality rates, as well as higher rates of reactive airway disease, cardiovascular disease, and lung cancer. A 2016 study by Chuang et al7 published in JAMA Dermatology looked at the UFPs in the surgical plume from laser hair removal (LHR) procedures. The plume of LHR has a distinct odor and easily discernible particulates. The investigators measured the UFPs at the level of the laser practitioner and the patient’s face during LHR with a smoke evacuator turned on and again with it turned off for 30 seconds, and then compared them to UFPs measured in the treatment room, the waiting room, and outside the building. There were substantial increases in UFPs from the LHR procedure, especially for the laser practitioner, when the smoke evacuator was off. The ambient baseline particle count, as measured in the clinic waiting area, began at 15,300 particles per cubic centimeter (PPC), and once the LHR procedure began (smoke evacuator on), there was a greater than 8-fold PPC increase above baseline (15,300 PPC to 129,376 PPC) in UFPs measured for the laser practitioner. Importantly, during LHR when the smoke evacuator was turned off for 30 seconds, there was a more than 28-fold increase (15,300 PPC to 435,888 PPC) over baseline to the practitioner (Figure).7
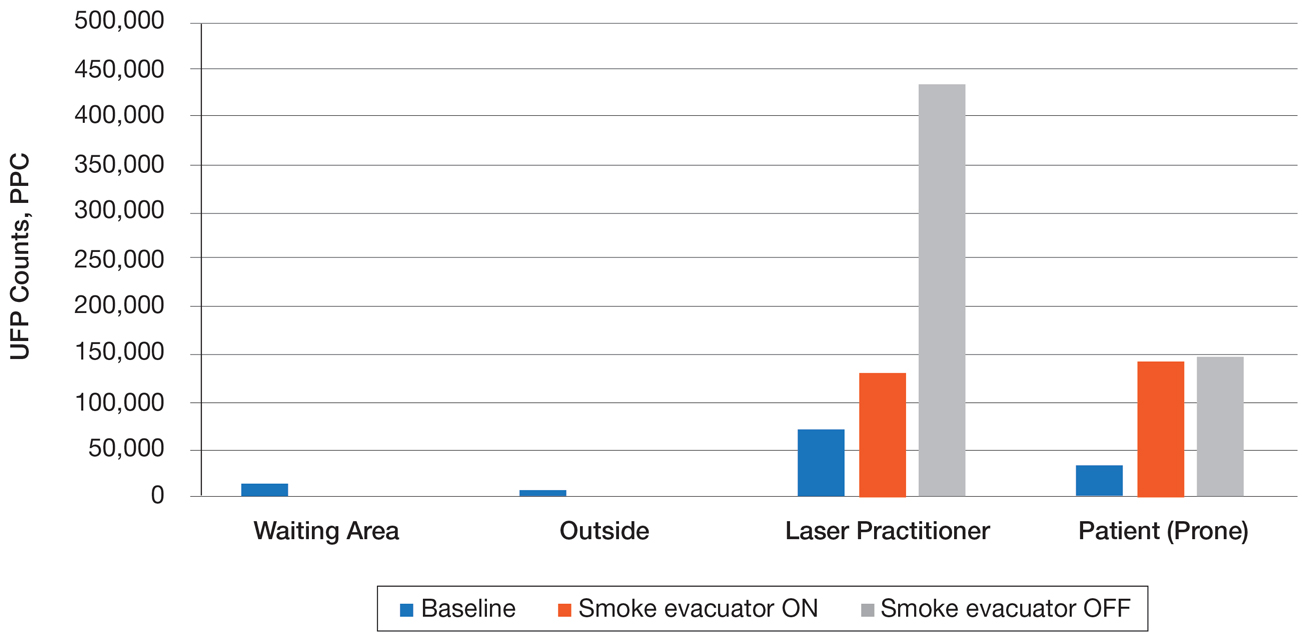
The Surgical Plume: Viruses, Bacteria, and Aerosolized Blood Products
Viruses and bacteria are thought to be transmissible via the plume, and proviral human immunodeficiency virus DNA has been found in the plume as well as evacuator equipment used to reduce plume exposure.8 A study from 1988 found that CO2 laser users treating verrucae had human papillomavirus in the laser plume.9 A comparison study of CO2 laser users treating verrucae had an increased incidence of nasopharyngeal human papillomavirus infection when compared to a control group, and the plume also contained aerosolized blood.10 The American National Standards Institute, OSHA, and NIOSH all agree that LGAC control from lasers is necessary through respiratory protection and ventilation, but none of these organizations provides specific equipment recommendations. The American Society for Laser Medicine and Surgery has published a position statement on laser plume.11
The Surgical Plume: Smoke Management
Many virus particles and UFPs are less than 0.1 µm in size. It is important to note that neither surgical masks nor high-filtration masks, such as the N95 respirator, filter particles smaller than 0.1 µm. The first line of defense in smoke management is the local exhaust ventilation (LEV) system, which includes wall suction and/or a smoke evacuator. The smoke evacuator is considered the more important of the two. General filtration, such as wall suction, is a low-flow system and is really used for liquids. It can be used as a supplement to the smoke evacuator to control small amounts of plume if fitted with an in-line filter. There are 2 types of LEV filters: ultralow particulate air filters filter particles larger than 0.1
Of utmost importance when using a smoke evacuator system is suction tip placement. Placing the suction tip 1 cm from the tissue damage site has been shown to be 98.6% effective at removing laser plume. If moved to 2 cm, effectiveness decreases to less than 50%.11 Proper management recommendations based on current evidence suggest that use of a smoke evacuator and an approved fit-tested N95 respirator might provide maximum protection.6 In addition to plume exposure, tissue splatter can occur, especially during ablative (CO2) and tattoo laser therapy, which should prompt consideration of a face shield.11 There are several vendors and models available online, and a simple Internet search for surgical tissue splatter face shields will provide multiple options.
The standard surgical mask is not NIOSH approved and only effectively (99%) filters particles larger than 5 µm (vs 25% efficacy for 0.3-µm particles). Its main purpose is to protect the patient from the wearer.12
High-filtration masks, which capture particles as small as 0.1 µm, should be used instead. The surgical N95 respirator is a NIOSH-certified respirator and is recommended for use in cases when smoke management is necessary. The FDA does not test or certify these masks; it only clears them after reviewing manufacturer test data. Technically, to be called a surgical mask, it must be cleared by the FDA.12 The 95 of N95 indicates filter efficiency ratings of 95% when testing the filter efficiency using particles of approximately 0.3 µm in diameter (Table 2).13 Because 77% of surgical smoke particles are smaller than 1.1 µm, surgical masks and N95 respirators are never sufficient as stand-alone protection.14 An LEV system is much more important for safe surgical smoke management. However, recommendations call for the use of a smoke evacuator and a high-filtration mask together to obtain the most protection available.14
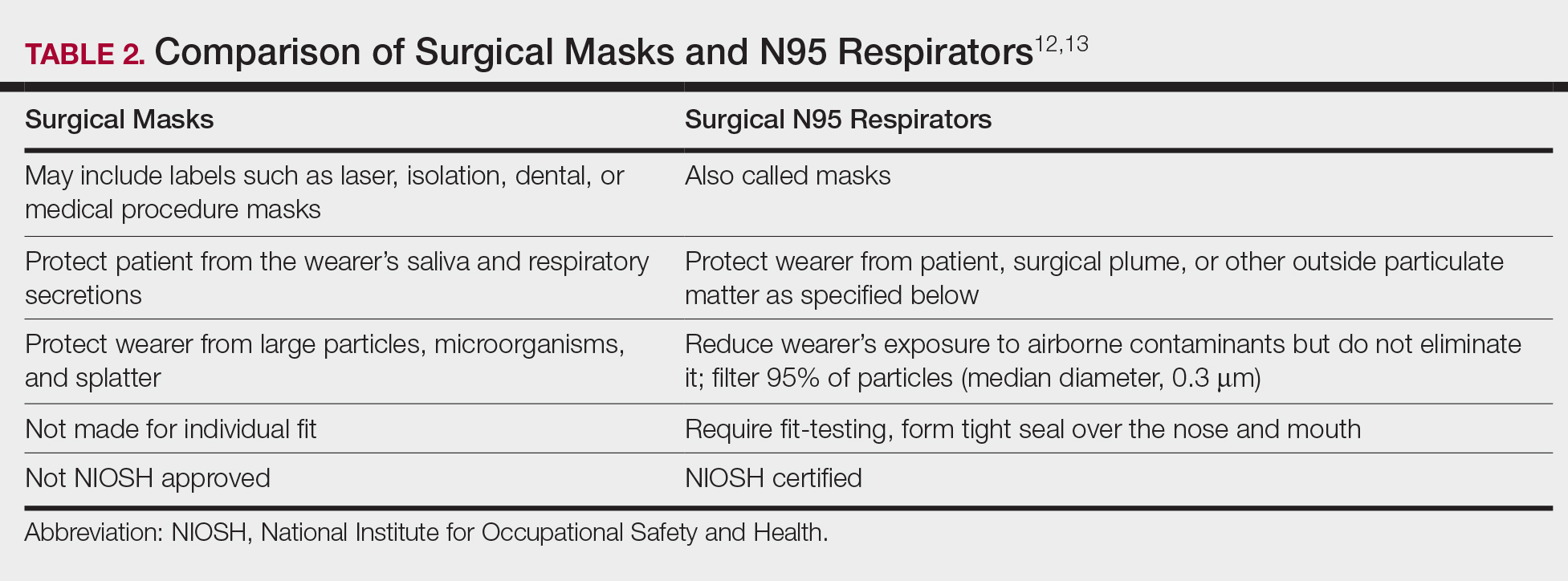
Fire Hazards
Fire hazards constitute another area of concern for the laser user and are seen with class 4 lasers. There usually are 2 types of fire hazards: electrical fires inside the laser (often faulty wiring) and flash fires (laser beam contacts flammable material). Flammable materials (eg, hair, hair products, makeup, fabrics, plastic, alcohol, chlorhexidine, aluminum chloride, elastic strap on safety goggles, gauze, drapes) should be identified and removed prior to laser use. CO2 and erbium:YAG lasers tend to pose the worst risk for flash fires.15
Precautions for fire control in the laser room should include fire extinguishers and/or fire extinguisher blankets, a water basin, and fire-resistant drapes available as needed. Flammable material such as gauze should be kept wet, or a nonflammable version should be used.3
Additional Safety Considerations
Whenever lasers are being used, it is important to cover any windows in the laser treatment area (LTA) to prevent the laser beam from passing through the glass window. Laser-blocking window covers are a requirement and are available from several vendors. Covers that block every laser class are available and come as a shade or a flat cover that is attached with Velcro or magnets. They also come with “Laser in Use” warning signs for additional safety. Access to the LTA when the laser is in use should be controlled and appropriate warning signs placed on the door to prevent inadvertent entry without proper PPE. Locking the door to the LTA while using the laser is an additional safety measure and can be included on a checklist.
For the dermatologist, the skin is a primary focus, and similar to the eye, can be at risk for injury. The most common type of injury resembles a sunburn, such as those seen in the UVB range, that appears as redness and sometimes blistering,15 which is an important consideration, and attention should be given to all those in the laser room.
Checklists
Checklists are ubiquitous throughout many occupations and many medical specialties. Their usefulness in preventing adverse events is well established. Any patient-provider encounter in which a series of sequential actions is required is a perfect situation for a checklist. In dermatologic laser surgery where the eye is uniquely susceptible to injury, a laser safety checklist is essential. Additionally, there are issues with LGACs and fire that are important to consider. Having protocols (ie, a checklist) in place that address these safety issues has been shown to reduce adverse outcomes.2 There are a number of templates available from various sources that can be customized to the laser treatment area. We provide a modifiable example (Table 3).
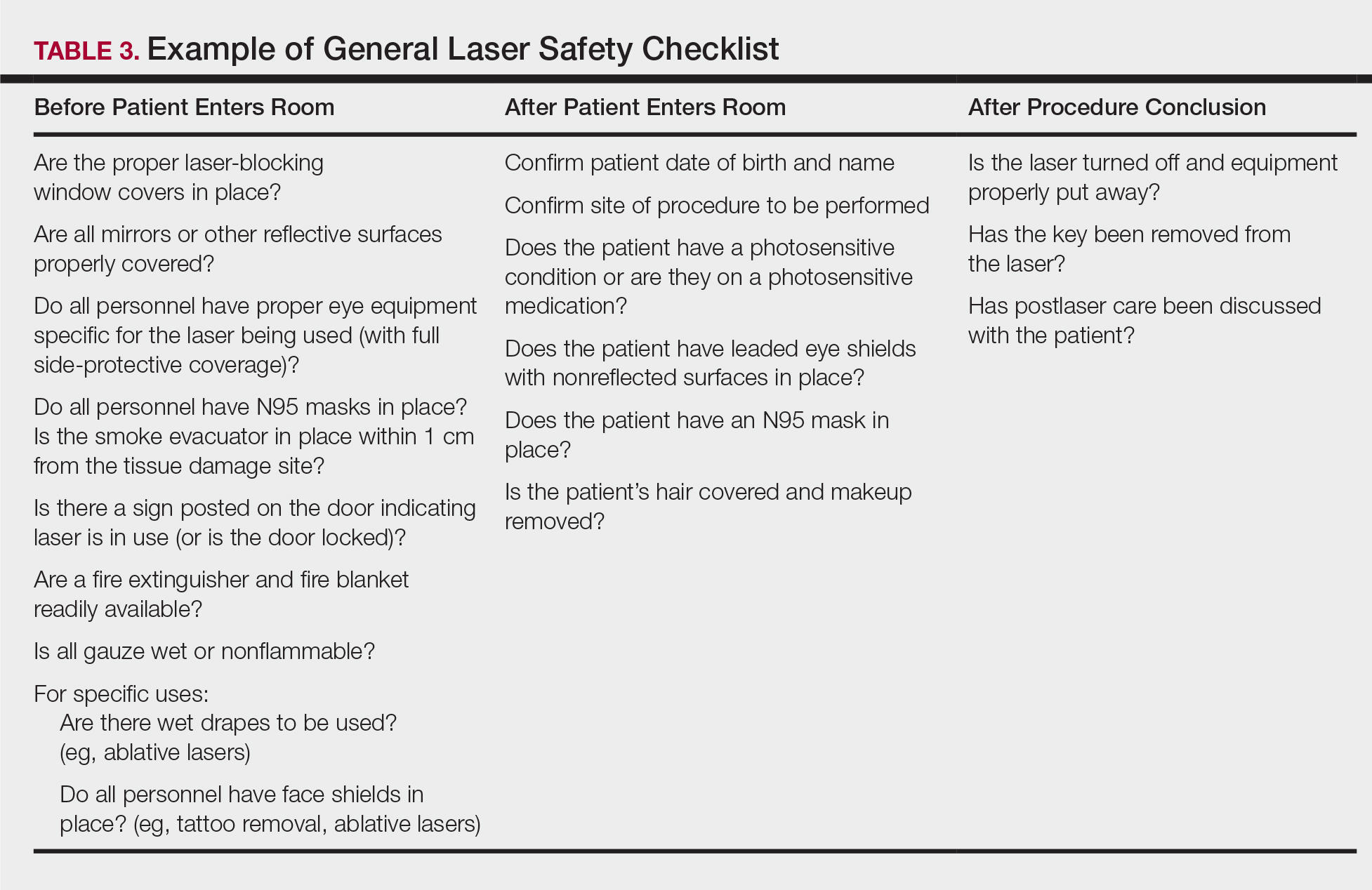
Conclusion
Laser usage in dermatologic surgery has increased. According to surveys from the American Society for Dermatologic Surgery, in 2012 there were approximately 2 million laser/light/energy-based procedures performed. By 2017, there were 3.27 million, up from 2.79 million in 2016, representing an approximate 1-year increase of 17%.16 Lasers have allowed interventions for skin, vascular, and aesthetic conditions that were once untreatable. As their use increases in number and broadens in scope, there also has been an increase in litigation alleging malpractice for misuse of the laser.17 Adverse events, which include photochemical or thermal injuries to the skin, pigmentation issues, scarring, plume-related issues, and fires, do occur. One solution to reduce the chance of an adverse outcome is to implement a checklist. Research using checklists has shown that adverse events are reduced when checklists are created and implemented properly. Improving checklist compliance also improves patient outcomes.17 The American National Standards Institute, in their ANSI Z136 series, and the World Health Organization provide checklist templates. We include our checklist for use in laser surgery (Table 3). Understanding that each laser treatment area is unique, the templates can serve as a starting point and can then be customized to suit the needs of each dermatologist.
- Goldman L, Blaney DJ, Kindel DJ, et al. Effect of the laser beam on the skin. J Invest Dermatol. 1963;40:121-122.
- Daggett C, Daggett A. The surgical check list revisited. Int J Surg Res Pract. 2017;4:051.
- Pritzker RN, Rohrer TE. Laser safety: standards and guidelines. In: Nouri K, ed. Handbook of Lasers in Dermatology. London, England: Springer; 2014:11-28.
- Husain Z, Alster TS. The role of lasers and intense pulsed light technology in dermatology. Clin Cosmet Investig Dermatol. 2016;9:29-40.
- Ries WR, Clymer MA, Reinisch L. Laser safety features of eye shields. Lasers Surg Med. 1996;18:309-315.
- Oganesyan G, Eimputh S, Kim SS, et al. Surgical smoke detection in dermatologic surgery. Dermatol Surg. 2014;40:1373-1377.
- Chuang GS, Farinelli W, Christiani DC, et al. Gaseous and particulate content of laser hair removal plume. JAMA Dermatol. 2016;152:1320-1326.
- Baggish MS, Poiesz BJ, Joret D, et al. Presence of human immunodeficiency DNA in laser smoke. Lasers Surg Med. 1991;11:197-203.
- Garden JM, O’Banion MK, Shelnitz LS, et al. Papilloma virus in the vapor of carbon dioxide laser-treated verrucae. JAMA. 1988;259:1199-1202.
- Gloster HM Jr, Roenigk RK. Risk of acquiring human papillomavirus from the plume produced by the carbon dioxide laser in the treatment of warts. J Am Acad Dermatol. 1995;32:436-441.
- American Society for Laser Medicine and Surgery. ASLMS laser and energy device plume position statement. http://www.aslms.org/for-professionals/professional-resources/safety-and-complications/aslms-laser-and-energy-device-plume-position-statement. Accessed October 4, 2019.
- A comparison of surgical masks, surgical N95 respirators, and industrial N95 respirators. OH&S website. https://ohsonline.com/Articles/2014/05/01/Comparison-Respiratory.aspx?Page=3. Published May 1, 2014. Accessed October 4, 2019.
- 3M Infection Prevention N95 particulate respirators, 1860/1860s and 1870. Frequently Asked Questions. http://multimedia.3m.com/mws/media/323208O/n95-particulate-respirators-1860-1860s-1870-faqs.pdf. Accessed October 4, 2019.
- Lewin JM, Brauer JA, Ostad A. Surgical smoke and the dermatologist. J Am Acad Dermatol. 2011;65:636-641.
- Arefiev K, Warycha M, Whiting D, et al. Flammability of topical preparations and surgical dressings in cutaneous and laser surgery: a controlled simulation study. J Am Acad Dermatol. 2012;67:700-705.
- ASDS survey on dermatologic procedures. American Society for Dermatologic Surgery website. https://www.asds.net/Medical-Professionals/Practice-Resources/ASDS-Survey-on-Dermatologic-Procedures. Accessed October 4, 2019.
- Jalian HR, Jalian CA, Avram MM. Common causes of injury and legal action in laser surgery. JAMA Dermatol. 2013;149:188-193.
The use of lasers in dermatology has evolved and expanded since their first cutaneous use in 1963.1 As the fundamental understanding of the interaction of laser energy with biological tissues increased, the need for laser safety became apparent. Since then, lasers of varying wavelengths have been developed, each with its specific chromophore target and specific safety need. Protocols, such as a checklist, that have been shown to reduce adverse events in surgery and in the intensive care unit can be borrowed to decrease risk from laser injury and optimize laser safety in dermatology.2 The safety of the patient, the laser operator, and the other health care providers involved in the delivery of laser therapy led to the first US Food and Drug Administration (FDA) guidelines for laser use in 1984.3
There are 4 regulatory organizations for laser safety in the United States: the American National Standards Institute (ANSI), the Occupational Health and Safety Administration (OSHA), the FDA’s Center for Devices and Radiological Health, and The Joint Commission.
Laser Principles
The basic principles of lasers include transmission, absorption, scatter, and reflection, all occurring when laser light is applied to biological tissues. The effects of the laser are a function of the target tissue (the chromophore) and the wavelength of light being used.4 In the skin, there are 3 main endogenous chromophores: water, hemoglobin, and melanin. Some experts consider collagen to be a fourth and separate entity as a chromophore. Tattoos are considered exogenous chromophores.3 The basic principles of lasers are important to understand and keep in mind when discussing laser safety, as they are the mechanisms through which unintended consequences can occur.
Laser Safety
Ocular Hazards
Ocular hazards are a notable concern in laser surgery. The eye is uniquely susceptible to laser light, and eye injuries represent a majority of reported injuries, which can occur through direct beam, mirror reflection by surgical instruments, and beam reflection off the skin (4%–7% of light that hits the skin is reflected because of the refractive index between air and the stratum corneum).3 The different wavelengths of lasers affect different parts of the eye. The 3 parts of the eye affected most are the retina, cornea, and lens. Not only is the lens primarily at risk for acute (lenticular burns) and chronic (cataracts) injury from the laser, but secondarily the lens also can concentrate a laser beam onto the retina by a factor of 100,000 (Table 1).3

The use of ocular protective equipment, sometimes referred to as personal protective eyewear (PPE), is essential and is mandated by ANSI and OSHA for all class 3 and class 4 lasers. The eyewear must be labeled with the wavelength and the degree of optical protection—termed the optical density (OD) or filter factor—of each lens and should match the laser being used. Laser manufacturers, as required by ANSI, must provide the wavelength and OD of their lasers, and both can be found on each laser as well as in ANSI Z136.1.3
Vendors supplying PPE generally provide the material, usually glass or polycarbonate; color; visible light transmission, which is the actual amount of light that reaches one’s eye through the lens; filter specifications, which contain the OD at certain wavelengths; and the types of lasers for which each specific PPE is used. It is important to match the laser to the correct PPE. The use of multiple types of lasers in the same office or laser treatment area can present challenges regarding eye safety. Matching the PPE to the laser in use is critical, and therefore all steps to prevent error for patients and personnel should be employed. One recommendation is to place each laser in a separate room with the appropriate PPE hung outside on the door of that room.
When the treatment area is in the periocular region, protection of the patient’s cornea is essential. Leaded eye shields with nonreflective surfaces have been shown to offer the best protection.5 Prior to placement, anesthetic eye drops and lubrication are important for patient comfort and protection from corneal injury.
Laser-Generated Airborne Contaminants
Other hazards associated with laser use not directly related to the beam are laser-generated airborne contaminants (LGACs), including chemicals, viruses, bacteria, aerosolized blood products, and nanoparticles (<1 µm) known as ultrafine particles (UFPs). According to ANSI, electrosurgical devices and lasers generate the same smoke. The plume (surgical smoke) is known to contain as many as 60 chemicals, including but not limited to carbon monoxide, acrylonitrite, hydrocyanide, benzene, toluene, naphthalene, and formaldehyde. Several are known carcinogens, and others are environmental toxins.6,7
Smoke management is an important consideration for dermatologists and their patients and generally includes respiratory protection via masks and ventilation techniques. However, the practice is not universal, and oversight agencies such as OSHA and the National Institute for Occupational Safety and Health (NIOSH) provide guidelines only; they do not enforce. As such, smoke management is voluntary and not widely practiced. In a 2014 survey of 997 dermatologic surgeons who were asked if smoke management is used in their practice, 77% of respondents indicated no smoke management was used.6
The Surgical Plume: Composition
A 2014 study from the University of California, San Diego Department of Dermatology analyzed surgical smoke.6 The researchers placed the smoke collection probe 16 to 18 inches above the electrocautery site, which approximates the location of the surgeon’s head during the procedure. Assessing smoke composition, they found high levels of carcinogens and irritants. Two compounds found in their assay—1,3-butadiene and benzene—also are found in secondhand cigarette smoke. However, the concentrations in the plume were 17-fold higher for 1,3-butadiene and 10-fold higher for benzene than those found in secondhand cigarette smoke. The risk from chronic, long-term exposure to these airborne contaminants is notable, as benzene (a known carcinogen as determined by the US Department of Health and Human Services) is known to cause leukemia. For example, a busy Mohs surgeon can reach the equivalent of as many as 50 hours of continuous smoke exposure over the course of a year.6
The Surgical Plume: Particle Concentration
Ultrafine particles can bypass conventional filtering systems (surgical masks and N95 respirators) because of their extremely small size, which allows them to pass further into the lungs and all the way to the alveolar spaces. Geographic regions with high UFPs have been shown to have higher overall mortality rates, as well as higher rates of reactive airway disease, cardiovascular disease, and lung cancer. A 2016 study by Chuang et al7 published in JAMA Dermatology looked at the UFPs in the surgical plume from laser hair removal (LHR) procedures. The plume of LHR has a distinct odor and easily discernible particulates. The investigators measured the UFPs at the level of the laser practitioner and the patient’s face during LHR with a smoke evacuator turned on and again with it turned off for 30 seconds, and then compared them to UFPs measured in the treatment room, the waiting room, and outside the building. There were substantial increases in UFPs from the LHR procedure, especially for the laser practitioner, when the smoke evacuator was off. The ambient baseline particle count, as measured in the clinic waiting area, began at 15,300 particles per cubic centimeter (PPC), and once the LHR procedure began (smoke evacuator on), there was a greater than 8-fold PPC increase above baseline (15,300 PPC to 129,376 PPC) in UFPs measured for the laser practitioner. Importantly, during LHR when the smoke evacuator was turned off for 30 seconds, there was a more than 28-fold increase (15,300 PPC to 435,888 PPC) over baseline to the practitioner (Figure).7

The Surgical Plume: Viruses, Bacteria, and Aerosolized Blood Products
Viruses and bacteria are thought to be transmissible via the plume, and proviral human immunodeficiency virus DNA has been found in the plume as well as evacuator equipment used to reduce plume exposure.8 A study from 1988 found that CO2 laser users treating verrucae had human papillomavirus in the laser plume.9 A comparison study of CO2 laser users treating verrucae had an increased incidence of nasopharyngeal human papillomavirus infection when compared to a control group, and the plume also contained aerosolized blood.10 The American National Standards Institute, OSHA, and NIOSH all agree that LGAC control from lasers is necessary through respiratory protection and ventilation, but none of these organizations provides specific equipment recommendations. The American Society for Laser Medicine and Surgery has published a position statement on laser plume.11
The Surgical Plume: Smoke Management
Many virus particles and UFPs are less than 0.1 µm in size. It is important to note that neither surgical masks nor high-filtration masks, such as the N95 respirator, filter particles smaller than 0.1 µm. The first line of defense in smoke management is the local exhaust ventilation (LEV) system, which includes wall suction and/or a smoke evacuator. The smoke evacuator is considered the more important of the two. General filtration, such as wall suction, is a low-flow system and is really used for liquids. It can be used as a supplement to the smoke evacuator to control small amounts of plume if fitted with an in-line filter. There are 2 types of LEV filters: ultralow particulate air filters filter particles larger than 0.1
Of utmost importance when using a smoke evacuator system is suction tip placement. Placing the suction tip 1 cm from the tissue damage site has been shown to be 98.6% effective at removing laser plume. If moved to 2 cm, effectiveness decreases to less than 50%.11 Proper management recommendations based on current evidence suggest that use of a smoke evacuator and an approved fit-tested N95 respirator might provide maximum protection.6 In addition to plume exposure, tissue splatter can occur, especially during ablative (CO2) and tattoo laser therapy, which should prompt consideration of a face shield.11 There are several vendors and models available online, and a simple Internet search for surgical tissue splatter face shields will provide multiple options.
The standard surgical mask is not NIOSH approved and only effectively (99%) filters particles larger than 5 µm (vs 25% efficacy for 0.3-µm particles). Its main purpose is to protect the patient from the wearer.12
High-filtration masks, which capture particles as small as 0.1 µm, should be used instead. The surgical N95 respirator is a NIOSH-certified respirator and is recommended for use in cases when smoke management is necessary. The FDA does not test or certify these masks; it only clears them after reviewing manufacturer test data. Technically, to be called a surgical mask, it must be cleared by the FDA.12 The 95 of N95 indicates filter efficiency ratings of 95% when testing the filter efficiency using particles of approximately 0.3 µm in diameter (Table 2).13 Because 77% of surgical smoke particles are smaller than 1.1 µm, surgical masks and N95 respirators are never sufficient as stand-alone protection.14 An LEV system is much more important for safe surgical smoke management. However, recommendations call for the use of a smoke evacuator and a high-filtration mask together to obtain the most protection available.14

Fire Hazards
Fire hazards constitute another area of concern for the laser user and are seen with class 4 lasers. There usually are 2 types of fire hazards: electrical fires inside the laser (often faulty wiring) and flash fires (laser beam contacts flammable material). Flammable materials (eg, hair, hair products, makeup, fabrics, plastic, alcohol, chlorhexidine, aluminum chloride, elastic strap on safety goggles, gauze, drapes) should be identified and removed prior to laser use. CO2 and erbium:YAG lasers tend to pose the worst risk for flash fires.15
Precautions for fire control in the laser room should include fire extinguishers and/or fire extinguisher blankets, a water basin, and fire-resistant drapes available as needed. Flammable material such as gauze should be kept wet, or a nonflammable version should be used.3
Additional Safety Considerations
Whenever lasers are being used, it is important to cover any windows in the laser treatment area (LTA) to prevent the laser beam from passing through the glass window. Laser-blocking window covers are a requirement and are available from several vendors. Covers that block every laser class are available and come as a shade or a flat cover that is attached with Velcro or magnets. They also come with “Laser in Use” warning signs for additional safety. Access to the LTA when the laser is in use should be controlled and appropriate warning signs placed on the door to prevent inadvertent entry without proper PPE. Locking the door to the LTA while using the laser is an additional safety measure and can be included on a checklist.
For the dermatologist, the skin is a primary focus, and similar to the eye, can be at risk for injury. The most common type of injury resembles a sunburn, such as those seen in the UVB range, that appears as redness and sometimes blistering,15 which is an important consideration, and attention should be given to all those in the laser room.
Checklists
Checklists are ubiquitous throughout many occupations and many medical specialties. Their usefulness in preventing adverse events is well established. Any patient-provider encounter in which a series of sequential actions is required is a perfect situation for a checklist. In dermatologic laser surgery where the eye is uniquely susceptible to injury, a laser safety checklist is essential. Additionally, there are issues with LGACs and fire that are important to consider. Having protocols (ie, a checklist) in place that address these safety issues has been shown to reduce adverse outcomes.2 There are a number of templates available from various sources that can be customized to the laser treatment area. We provide a modifiable example (Table 3).

Conclusion
Laser usage in dermatologic surgery has increased. According to surveys from the American Society for Dermatologic Surgery, in 2012 there were approximately 2 million laser/light/energy-based procedures performed. By 2017, there were 3.27 million, up from 2.79 million in 2016, representing an approximate 1-year increase of 17%.16 Lasers have allowed interventions for skin, vascular, and aesthetic conditions that were once untreatable. As their use increases in number and broadens in scope, there also has been an increase in litigation alleging malpractice for misuse of the laser.17 Adverse events, which include photochemical or thermal injuries to the skin, pigmentation issues, scarring, plume-related issues, and fires, do occur. One solution to reduce the chance of an adverse outcome is to implement a checklist. Research using checklists has shown that adverse events are reduced when checklists are created and implemented properly. Improving checklist compliance also improves patient outcomes.17 The American National Standards Institute, in their ANSI Z136 series, and the World Health Organization provide checklist templates. We include our checklist for use in laser surgery (Table 3). Understanding that each laser treatment area is unique, the templates can serve as a starting point and can then be customized to suit the needs of each dermatologist.
The use of lasers in dermatology has evolved and expanded since their first cutaneous use in 1963.1 As the fundamental understanding of the interaction of laser energy with biological tissues increased, the need for laser safety became apparent. Since then, lasers of varying wavelengths have been developed, each with its specific chromophore target and specific safety need. Protocols, such as a checklist, that have been shown to reduce adverse events in surgery and in the intensive care unit can be borrowed to decrease risk from laser injury and optimize laser safety in dermatology.2 The safety of the patient, the laser operator, and the other health care providers involved in the delivery of laser therapy led to the first US Food and Drug Administration (FDA) guidelines for laser use in 1984.3
There are 4 regulatory organizations for laser safety in the United States: the American National Standards Institute (ANSI), the Occupational Health and Safety Administration (OSHA), the FDA’s Center for Devices and Radiological Health, and The Joint Commission.
Laser Principles
The basic principles of lasers include transmission, absorption, scatter, and reflection, all occurring when laser light is applied to biological tissues. The effects of the laser are a function of the target tissue (the chromophore) and the wavelength of light being used.4 In the skin, there are 3 main endogenous chromophores: water, hemoglobin, and melanin. Some experts consider collagen to be a fourth and separate entity as a chromophore. Tattoos are considered exogenous chromophores.3 The basic principles of lasers are important to understand and keep in mind when discussing laser safety, as they are the mechanisms through which unintended consequences can occur.
Laser Safety
Ocular Hazards
Ocular hazards are a notable concern in laser surgery. The eye is uniquely susceptible to laser light, and eye injuries represent a majority of reported injuries, which can occur through direct beam, mirror reflection by surgical instruments, and beam reflection off the skin (4%–7% of light that hits the skin is reflected because of the refractive index between air and the stratum corneum).3 The different wavelengths of lasers affect different parts of the eye. The 3 parts of the eye affected most are the retina, cornea, and lens. Not only is the lens primarily at risk for acute (lenticular burns) and chronic (cataracts) injury from the laser, but secondarily the lens also can concentrate a laser beam onto the retina by a factor of 100,000 (Table 1).3

The use of ocular protective equipment, sometimes referred to as personal protective eyewear (PPE), is essential and is mandated by ANSI and OSHA for all class 3 and class 4 lasers. The eyewear must be labeled with the wavelength and the degree of optical protection—termed the optical density (OD) or filter factor—of each lens and should match the laser being used. Laser manufacturers, as required by ANSI, must provide the wavelength and OD of their lasers, and both can be found on each laser as well as in ANSI Z136.1.3
Vendors supplying PPE generally provide the material, usually glass or polycarbonate; color; visible light transmission, which is the actual amount of light that reaches one’s eye through the lens; filter specifications, which contain the OD at certain wavelengths; and the types of lasers for which each specific PPE is used. It is important to match the laser to the correct PPE. The use of multiple types of lasers in the same office or laser treatment area can present challenges regarding eye safety. Matching the PPE to the laser in use is critical, and therefore all steps to prevent error for patients and personnel should be employed. One recommendation is to place each laser in a separate room with the appropriate PPE hung outside on the door of that room.
When the treatment area is in the periocular region, protection of the patient’s cornea is essential. Leaded eye shields with nonreflective surfaces have been shown to offer the best protection.5 Prior to placement, anesthetic eye drops and lubrication are important for patient comfort and protection from corneal injury.
Laser-Generated Airborne Contaminants
Other hazards associated with laser use not directly related to the beam are laser-generated airborne contaminants (LGACs), including chemicals, viruses, bacteria, aerosolized blood products, and nanoparticles (<1 µm) known as ultrafine particles (UFPs). According to ANSI, electrosurgical devices and lasers generate the same smoke. The plume (surgical smoke) is known to contain as many as 60 chemicals, including but not limited to carbon monoxide, acrylonitrite, hydrocyanide, benzene, toluene, naphthalene, and formaldehyde. Several are known carcinogens, and others are environmental toxins.6,7
Smoke management is an important consideration for dermatologists and their patients and generally includes respiratory protection via masks and ventilation techniques. However, the practice is not universal, and oversight agencies such as OSHA and the National Institute for Occupational Safety and Health (NIOSH) provide guidelines only; they do not enforce. As such, smoke management is voluntary and not widely practiced. In a 2014 survey of 997 dermatologic surgeons who were asked if smoke management is used in their practice, 77% of respondents indicated no smoke management was used.6
The Surgical Plume: Composition
A 2014 study from the University of California, San Diego Department of Dermatology analyzed surgical smoke.6 The researchers placed the smoke collection probe 16 to 18 inches above the electrocautery site, which approximates the location of the surgeon’s head during the procedure. Assessing smoke composition, they found high levels of carcinogens and irritants. Two compounds found in their assay—1,3-butadiene and benzene—also are found in secondhand cigarette smoke. However, the concentrations in the plume were 17-fold higher for 1,3-butadiene and 10-fold higher for benzene than those found in secondhand cigarette smoke. The risk from chronic, long-term exposure to these airborne contaminants is notable, as benzene (a known carcinogen as determined by the US Department of Health and Human Services) is known to cause leukemia. For example, a busy Mohs surgeon can reach the equivalent of as many as 50 hours of continuous smoke exposure over the course of a year.6
The Surgical Plume: Particle Concentration
Ultrafine particles can bypass conventional filtering systems (surgical masks and N95 respirators) because of their extremely small size, which allows them to pass further into the lungs and all the way to the alveolar spaces. Geographic regions with high UFPs have been shown to have higher overall mortality rates, as well as higher rates of reactive airway disease, cardiovascular disease, and lung cancer. A 2016 study by Chuang et al7 published in JAMA Dermatology looked at the UFPs in the surgical plume from laser hair removal (LHR) procedures. The plume of LHR has a distinct odor and easily discernible particulates. The investigators measured the UFPs at the level of the laser practitioner and the patient’s face during LHR with a smoke evacuator turned on and again with it turned off for 30 seconds, and then compared them to UFPs measured in the treatment room, the waiting room, and outside the building. There were substantial increases in UFPs from the LHR procedure, especially for the laser practitioner, when the smoke evacuator was off. The ambient baseline particle count, as measured in the clinic waiting area, began at 15,300 particles per cubic centimeter (PPC), and once the LHR procedure began (smoke evacuator on), there was a greater than 8-fold PPC increase above baseline (15,300 PPC to 129,376 PPC) in UFPs measured for the laser practitioner. Importantly, during LHR when the smoke evacuator was turned off for 30 seconds, there was a more than 28-fold increase (15,300 PPC to 435,888 PPC) over baseline to the practitioner (Figure).7

The Surgical Plume: Viruses, Bacteria, and Aerosolized Blood Products
Viruses and bacteria are thought to be transmissible via the plume, and proviral human immunodeficiency virus DNA has been found in the plume as well as evacuator equipment used to reduce plume exposure.8 A study from 1988 found that CO2 laser users treating verrucae had human papillomavirus in the laser plume.9 A comparison study of CO2 laser users treating verrucae had an increased incidence of nasopharyngeal human papillomavirus infection when compared to a control group, and the plume also contained aerosolized blood.10 The American National Standards Institute, OSHA, and NIOSH all agree that LGAC control from lasers is necessary through respiratory protection and ventilation, but none of these organizations provides specific equipment recommendations. The American Society for Laser Medicine and Surgery has published a position statement on laser plume.11
The Surgical Plume: Smoke Management
Many virus particles and UFPs are less than 0.1 µm in size. It is important to note that neither surgical masks nor high-filtration masks, such as the N95 respirator, filter particles smaller than 0.1 µm. The first line of defense in smoke management is the local exhaust ventilation (LEV) system, which includes wall suction and/or a smoke evacuator. The smoke evacuator is considered the more important of the two. General filtration, such as wall suction, is a low-flow system and is really used for liquids. It can be used as a supplement to the smoke evacuator to control small amounts of plume if fitted with an in-line filter. There are 2 types of LEV filters: ultralow particulate air filters filter particles larger than 0.1
Of utmost importance when using a smoke evacuator system is suction tip placement. Placing the suction tip 1 cm from the tissue damage site has been shown to be 98.6% effective at removing laser plume. If moved to 2 cm, effectiveness decreases to less than 50%.11 Proper management recommendations based on current evidence suggest that use of a smoke evacuator and an approved fit-tested N95 respirator might provide maximum protection.6 In addition to plume exposure, tissue splatter can occur, especially during ablative (CO2) and tattoo laser therapy, which should prompt consideration of a face shield.11 There are several vendors and models available online, and a simple Internet search for surgical tissue splatter face shields will provide multiple options.
The standard surgical mask is not NIOSH approved and only effectively (99%) filters particles larger than 5 µm (vs 25% efficacy for 0.3-µm particles). Its main purpose is to protect the patient from the wearer.12
High-filtration masks, which capture particles as small as 0.1 µm, should be used instead. The surgical N95 respirator is a NIOSH-certified respirator and is recommended for use in cases when smoke management is necessary. The FDA does not test or certify these masks; it only clears them after reviewing manufacturer test data. Technically, to be called a surgical mask, it must be cleared by the FDA.12 The 95 of N95 indicates filter efficiency ratings of 95% when testing the filter efficiency using particles of approximately 0.3 µm in diameter (Table 2).13 Because 77% of surgical smoke particles are smaller than 1.1 µm, surgical masks and N95 respirators are never sufficient as stand-alone protection.14 An LEV system is much more important for safe surgical smoke management. However, recommendations call for the use of a smoke evacuator and a high-filtration mask together to obtain the most protection available.14

Fire Hazards
Fire hazards constitute another area of concern for the laser user and are seen with class 4 lasers. There usually are 2 types of fire hazards: electrical fires inside the laser (often faulty wiring) and flash fires (laser beam contacts flammable material). Flammable materials (eg, hair, hair products, makeup, fabrics, plastic, alcohol, chlorhexidine, aluminum chloride, elastic strap on safety goggles, gauze, drapes) should be identified and removed prior to laser use. CO2 and erbium:YAG lasers tend to pose the worst risk for flash fires.15
Precautions for fire control in the laser room should include fire extinguishers and/or fire extinguisher blankets, a water basin, and fire-resistant drapes available as needed. Flammable material such as gauze should be kept wet, or a nonflammable version should be used.3
Additional Safety Considerations
Whenever lasers are being used, it is important to cover any windows in the laser treatment area (LTA) to prevent the laser beam from passing through the glass window. Laser-blocking window covers are a requirement and are available from several vendors. Covers that block every laser class are available and come as a shade or a flat cover that is attached with Velcro or magnets. They also come with “Laser in Use” warning signs for additional safety. Access to the LTA when the laser is in use should be controlled and appropriate warning signs placed on the door to prevent inadvertent entry without proper PPE. Locking the door to the LTA while using the laser is an additional safety measure and can be included on a checklist.
For the dermatologist, the skin is a primary focus, and similar to the eye, can be at risk for injury. The most common type of injury resembles a sunburn, such as those seen in the UVB range, that appears as redness and sometimes blistering,15 which is an important consideration, and attention should be given to all those in the laser room.
Checklists
Checklists are ubiquitous throughout many occupations and many medical specialties. Their usefulness in preventing adverse events is well established. Any patient-provider encounter in which a series of sequential actions is required is a perfect situation for a checklist. In dermatologic laser surgery where the eye is uniquely susceptible to injury, a laser safety checklist is essential. Additionally, there are issues with LGACs and fire that are important to consider. Having protocols (ie, a checklist) in place that address these safety issues has been shown to reduce adverse outcomes.2 There are a number of templates available from various sources that can be customized to the laser treatment area. We provide a modifiable example (Table 3).

Conclusion
Laser usage in dermatologic surgery has increased. According to surveys from the American Society for Dermatologic Surgery, in 2012 there were approximately 2 million laser/light/energy-based procedures performed. By 2017, there were 3.27 million, up from 2.79 million in 2016, representing an approximate 1-year increase of 17%.16 Lasers have allowed interventions for skin, vascular, and aesthetic conditions that were once untreatable. As their use increases in number and broadens in scope, there also has been an increase in litigation alleging malpractice for misuse of the laser.17 Adverse events, which include photochemical or thermal injuries to the skin, pigmentation issues, scarring, plume-related issues, and fires, do occur. One solution to reduce the chance of an adverse outcome is to implement a checklist. Research using checklists has shown that adverse events are reduced when checklists are created and implemented properly. Improving checklist compliance also improves patient outcomes.17 The American National Standards Institute, in their ANSI Z136 series, and the World Health Organization provide checklist templates. We include our checklist for use in laser surgery (Table 3). Understanding that each laser treatment area is unique, the templates can serve as a starting point and can then be customized to suit the needs of each dermatologist.
- Goldman L, Blaney DJ, Kindel DJ, et al. Effect of the laser beam on the skin. J Invest Dermatol. 1963;40:121-122.
- Daggett C, Daggett A. The surgical check list revisited. Int J Surg Res Pract. 2017;4:051.
- Pritzker RN, Rohrer TE. Laser safety: standards and guidelines. In: Nouri K, ed. Handbook of Lasers in Dermatology. London, England: Springer; 2014:11-28.
- Husain Z, Alster TS. The role of lasers and intense pulsed light technology in dermatology. Clin Cosmet Investig Dermatol. 2016;9:29-40.
- Ries WR, Clymer MA, Reinisch L. Laser safety features of eye shields. Lasers Surg Med. 1996;18:309-315.
- Oganesyan G, Eimputh S, Kim SS, et al. Surgical smoke detection in dermatologic surgery. Dermatol Surg. 2014;40:1373-1377.
- Chuang GS, Farinelli W, Christiani DC, et al. Gaseous and particulate content of laser hair removal plume. JAMA Dermatol. 2016;152:1320-1326.
- Baggish MS, Poiesz BJ, Joret D, et al. Presence of human immunodeficiency DNA in laser smoke. Lasers Surg Med. 1991;11:197-203.
- Garden JM, O’Banion MK, Shelnitz LS, et al. Papilloma virus in the vapor of carbon dioxide laser-treated verrucae. JAMA. 1988;259:1199-1202.
- Gloster HM Jr, Roenigk RK. Risk of acquiring human papillomavirus from the plume produced by the carbon dioxide laser in the treatment of warts. J Am Acad Dermatol. 1995;32:436-441.
- American Society for Laser Medicine and Surgery. ASLMS laser and energy device plume position statement. http://www.aslms.org/for-professionals/professional-resources/safety-and-complications/aslms-laser-and-energy-device-plume-position-statement. Accessed October 4, 2019.
- A comparison of surgical masks, surgical N95 respirators, and industrial N95 respirators. OH&S website. https://ohsonline.com/Articles/2014/05/01/Comparison-Respiratory.aspx?Page=3. Published May 1, 2014. Accessed October 4, 2019.
- 3M Infection Prevention N95 particulate respirators, 1860/1860s and 1870. Frequently Asked Questions. http://multimedia.3m.com/mws/media/323208O/n95-particulate-respirators-1860-1860s-1870-faqs.pdf. Accessed October 4, 2019.
- Lewin JM, Brauer JA, Ostad A. Surgical smoke and the dermatologist. J Am Acad Dermatol. 2011;65:636-641.
- Arefiev K, Warycha M, Whiting D, et al. Flammability of topical preparations and surgical dressings in cutaneous and laser surgery: a controlled simulation study. J Am Acad Dermatol. 2012;67:700-705.
- ASDS survey on dermatologic procedures. American Society for Dermatologic Surgery website. https://www.asds.net/Medical-Professionals/Practice-Resources/ASDS-Survey-on-Dermatologic-Procedures. Accessed October 4, 2019.
- Jalian HR, Jalian CA, Avram MM. Common causes of injury and legal action in laser surgery. JAMA Dermatol. 2013;149:188-193.
- Goldman L, Blaney DJ, Kindel DJ, et al. Effect of the laser beam on the skin. J Invest Dermatol. 1963;40:121-122.
- Daggett C, Daggett A. The surgical check list revisited. Int J Surg Res Pract. 2017;4:051.
- Pritzker RN, Rohrer TE. Laser safety: standards and guidelines. In: Nouri K, ed. Handbook of Lasers in Dermatology. London, England: Springer; 2014:11-28.
- Husain Z, Alster TS. The role of lasers and intense pulsed light technology in dermatology. Clin Cosmet Investig Dermatol. 2016;9:29-40.
- Ries WR, Clymer MA, Reinisch L. Laser safety features of eye shields. Lasers Surg Med. 1996;18:309-315.
- Oganesyan G, Eimputh S, Kim SS, et al. Surgical smoke detection in dermatologic surgery. Dermatol Surg. 2014;40:1373-1377.
- Chuang GS, Farinelli W, Christiani DC, et al. Gaseous and particulate content of laser hair removal plume. JAMA Dermatol. 2016;152:1320-1326.
- Baggish MS, Poiesz BJ, Joret D, et al. Presence of human immunodeficiency DNA in laser smoke. Lasers Surg Med. 1991;11:197-203.
- Garden JM, O’Banion MK, Shelnitz LS, et al. Papilloma virus in the vapor of carbon dioxide laser-treated verrucae. JAMA. 1988;259:1199-1202.
- Gloster HM Jr, Roenigk RK. Risk of acquiring human papillomavirus from the plume produced by the carbon dioxide laser in the treatment of warts. J Am Acad Dermatol. 1995;32:436-441.
- American Society for Laser Medicine and Surgery. ASLMS laser and energy device plume position statement. http://www.aslms.org/for-professionals/professional-resources/safety-and-complications/aslms-laser-and-energy-device-plume-position-statement. Accessed October 4, 2019.
- A comparison of surgical masks, surgical N95 respirators, and industrial N95 respirators. OH&S website. https://ohsonline.com/Articles/2014/05/01/Comparison-Respiratory.aspx?Page=3. Published May 1, 2014. Accessed October 4, 2019.
- 3M Infection Prevention N95 particulate respirators, 1860/1860s and 1870. Frequently Asked Questions. http://multimedia.3m.com/mws/media/323208O/n95-particulate-respirators-1860-1860s-1870-faqs.pdf. Accessed October 4, 2019.
- Lewin JM, Brauer JA, Ostad A. Surgical smoke and the dermatologist. J Am Acad Dermatol. 2011;65:636-641.
- Arefiev K, Warycha M, Whiting D, et al. Flammability of topical preparations and surgical dressings in cutaneous and laser surgery: a controlled simulation study. J Am Acad Dermatol. 2012;67:700-705.
- ASDS survey on dermatologic procedures. American Society for Dermatologic Surgery website. https://www.asds.net/Medical-Professionals/Practice-Resources/ASDS-Survey-on-Dermatologic-Procedures. Accessed October 4, 2019.
- Jalian HR, Jalian CA, Avram MM. Common causes of injury and legal action in laser surgery. JAMA Dermatol. 2013;149:188-193.
Practice Points
- Laser therapy has evolved and expanded since its first cutaneous use in 1963.
- The 4 regulatory agencies for laser safety in the United States establish standards and guidelines, but implementation is voluntary.
- Ocular hazards, laser-generated airborne contaminants, fires, and unintended laser beam injuries constitute the main safety concerns.
- Safety protocols with a laser checklist can reduce adverse outcomes.
APPlying Knowledge: Evidence for and Regulation of Mobile Apps for Dermatologists
Since the first mobile application (app) was developed in the 1990s, apps have become increasingly integrated into medical practice and training. More than 5.5 million apps were downloadable in 2019,1 of which more than 300,000 were health related.2 In the United States, more than 80% of physicians reported using smartphones for professional purposes in 2016.3 As the complexity of apps and their purpose of use has evolved, regulatory bodies have not adapted adequately to monitor apps that have broad-reaching consequences in medicine.
We review the primary literature on PubMed behind health-related apps that impact dermatologists as well as the government regulation of these apps, with a focus on the 3 most prevalent dermatology-related apps used by dermatology residents in the United States: VisualDx, UpToDate, and Mohs Surgery Appropriate Use Criteria. This prevalence is according to a survey emailed to all dermatology residents in the United States by the American Academy of Dermatology (AAD) in 2019 (unpublished data).
VisualDx
VisualDx, which aims to improve diagnostic accuracy and patient safety, contains peer-reviewed data and more than 32,000 images of dermatologic conditions. The editorial board includes more than 50 physicians. It provides opportunities for continuing medical education credit, is used in more than 2300 medical settings, and costs $399.99 annually for a subscription with partial features. Prior to the launch of the app in 2010, some health science professionals noted that the website version lacked references to primary sources.4 The same issue carried over to the app, which has evolved to offer artificial intelligence (AI) analysis of photographed skin lesions. However, there are no peer-reviewed publications showing positive impact of the app on diagnostic skills among dermatology residents or on patient outcomes.
UpToDate
UpToDate is a web-based database created in the early 1990s. A corresponding app was created around 2010. Both internal and independent research has demonstrated improved outcomes, and the app is advertised as the only clinical decision support resource associated with improved outcomes, as shown in more than 80 publications.5 UpToDate covers more than 11,800 medical topics and contains more than 35,000 graphics. It cites primary sources and uses a published system for grading recommendation strength and evidence quality. The data are processed and produced by a team of more than 7100 physicians as authors, editors, and reviewers. The platform grants continuing medical education credit and is used by more than 1.9 million clinicians in more than 190 countries. A 1-year subscription for an individual US-based physician costs $559. An observational study assessed UpToDate articles for potential conflicts of interest between authors and their recommendations. Of the 6 articles that met inclusion criteria of discussing management of medical conditions that have controversial or mostly brand-name treatment options, all had conflicts of interest, such as naming drugs from companies with which the authors and/or editors had financial relationships.6
Mohs Surgery Appropriate Use Criteria
The Mohs Surgery Appropriate Use Criteria app is a free clinical decision-making tool based on a consensus statement published in 2012 by the AAD, American College of Mohs Surgery, American Society for Dermatologic Surgery Association, and American Society for Mohs Surgery.7 It helps guide management of more than 200 dermatologic scenarios. Critique has been made that the criteria are partly based on expert opinion and data largely from the United States and has not been revised to incorporate newer data.8 There are no publications regarding the app itself.
Regulation of Health-Related Apps
Health-related apps that are designed for utilization by health care providers can be a valuable tool. However, given their prevalence, cost, and potential impact on patient lives, these apps should be well regulated and researched. The general paucity of peer-reviewed literature demonstrating the utility, safety, quality, and accuracy of health-related apps commonly used by providers is a reflection of insufficient mobile health regulation in the United States.
There are 3 primary government agencies responsible for regulating mobile medical apps: the US Food and Drug Administration (FDA), Federal Trade Commission, and Office for Civil Rights.9 The FDA does not regulate all medical devices. Apps intended for use in the diagnosis, cure, mitigation, prevention, or treatment of a disease or condition are considered to be medical devices.10 The FDA regulates those apps only if they are judged to pose more than minimal risk. Apps that are designed only to provide easy access to information related to health conditions or treatment are considered to be minimal risk but can develop into a different risk level such as by offering AI.11 Although the FDA does update its approach to medical devices, including apps and AI- and machine learning–based software, the rate and direction of update has not kept pace with the rapid evolution of apps.12 In 2019, the FDA began piloting a precertification program that grants long-term approval to organizations that develop apps instead of reviewing each app product individually.13 This decrease in premarket oversight is intended to expedite innovation with the hopeful upside of improving patient outcomes but is inconsistent, with the FDA still reviewing other types of medical devices individually.
For apps that are already in use, the Federal Trade Commission only gets involved in response to deceptive or unfair acts or practices relating to privacy, data security, and false or misleading claims about safety or performance. It may be more beneficial for consumers if those apps had a more stringent initial approval process. The Office for Civil Rights enforces the Health Insurance Portability and Accountability Act when relevant to apps.
Nongovernment agencies also are involved in app regulation. The FDA believes sharing more regulatory responsibility with private industry would promote efficiency.14 Google does not allow apps that contain false or misleading health claims,15 and Apple may scrutinize medical apps that could provide inaccurate data or be used for diagnosing or treating patients.16 Xcertia, a nonprofit organization founded by the American Medical Association and others, develops standards for the security, privacy, content, and operability of health-related apps, but those standards have not been adopted by other parties. Ultimately, nongovernment agencies are not responsible for public health and do not boast the government’s ability to enforce rules or ensure public safety.
Final Thoughts
The AAD survey of US dermatology residents found that the top consideration when choosing apps was up-to-date and accurate information; however, the 3 most prevalent apps among those same respondents did not need government approval and are not required to contain up-to-date data or to improve clinical outcomes, similar to most other health-related apps. This discrepancy is concerning considering the increasing utilization of apps for physician education and health care delivery and the increasing complexity of those apps. In light of these results, the potential decrease in federal premarket regulation suggested by the FDA’s precertification program seems inappropriate. It is important for the government to take responsibility for regulating health-related apps and to find a balance between too much regulation delaying innovation and too little regulation hurting physician training and patient care. It also is important for providers to be aware of the evidence and oversight behind the technologies they use for professional purposes.
- Clement J. Number of apps available in leading app stores as of 1st quarter 2020. Statista website. https://www.statista.com/statistics/276623/number-of-apps-available-in-leading-app-stores/. Published May 4, 2020. Accessed July 23, 2020.
- mHealth App Economics 2017/2018. Current Status and Future Trends in Mobile Health. Berlin, Germany: Research 2 Guidance; 2018.
- Healthcare Client Services. Professional usage of smartphones by doctors. Kantar website. https://www.kantarmedia.com/us/thinking-and-resources/blog/professional-usage-of-smartphones-by-doctors-2016. Published November 16, 2016. Accessed July 23, 2020.
- Skhal KJ, Koffel J. VisualDx. J Med Libr Assoc. 2007;95:470-471.
- UpToDate is the only clinical decision support resource associated with improved outcomes. UpToDate website. https://www.uptodate.com/home/research. Accessed July 29, 2020.
- Connolly SM, Baker DR, Coldiron BM, et al. AAD/ACMS/ASDSA/ASMS 2012 appropriate use criteria for Mohs micrographic surgery: a report of the American Academy of Dermatology, American College of Mohs Surgery, American Society for Dermatologic Surgery Association, and the American Society for Mohs Surgery. J Am Acad Dermatol. 2012;67:531-550.
- Amber KT, Dhiman G, Goodman KW. Conflict of interest in online point-of-care clinical support websites. J Med Ethics. 2014;40:578-580.
- Croley JA, Joseph AK, Wagner RF Jr. Discrepancies in the Mohs micrographic surgery appropriate use criteria. J Am Acad Dermatol. 2020;82:E55.
- Mobile health apps interactive tool. Federal Trade Commission website. https://www.ftc.gov/tips-advice/business-center/guidance/mobile-health-apps-interactive-tool. Published April 2016. Accessed May 23, 2020.
- Federal Food, Drug, and Cosmetic Act, 21 USC §321 (2018).
- US Food and Drug Administration. Examples of software functions for which the FDA will exercise enforcement discretion. https://www.fda.gov/medical-devices/device-software-functions-including-mobile-medical-applications/examples-software-functions-which-fda-will-exercise-enforcement-discretion. Updated September 26, 2019. Accessed July 29, 2020.
- US Food and Drug Administration. Proposed regulatory framework for modifications to artificial intelligence/machine learning (AI/ML)‐based software as a medical device (SaMD). https://www.fda.gov/downloads/MedicalDevices/DigitalHealth/SoftwareasaMedicalDevice/UCM635052.pdf. Accessed July 23, 2020.
- US Food and Drug Administration. Digital health software precertification (pre-cert) program. https://www.fda.gov/medical-devices/digital-health/digital-health-software-precertification-pre-cert-program. Updated July 18, 2019. Accessed July 23, 2020.
- Gottlieb S. Fostering medical innovation: a plan for digital health devices. US Food and Drug Administration website. https://www.fda.gov/news-events/fda-voices/fostering-medical-innovation-plan-digital-health-devices. Published June 15, 2017. Accessed July 23, 2020.
- Restricted content: unapproved substances. Google Play website. https://play.google.com/about/restricted-content/unapproved-substances. Accessed July 23, 2020.
- App store review guidelines. Apple Developer website. https://developer.apple.com/app-store/review/guidelines. Updated March 4, 2020. Accessed July 23, 2020.
Since the first mobile application (app) was developed in the 1990s, apps have become increasingly integrated into medical practice and training. More than 5.5 million apps were downloadable in 2019,1 of which more than 300,000 were health related.2 In the United States, more than 80% of physicians reported using smartphones for professional purposes in 2016.3 As the complexity of apps and their purpose of use has evolved, regulatory bodies have not adapted adequately to monitor apps that have broad-reaching consequences in medicine.
We review the primary literature on PubMed behind health-related apps that impact dermatologists as well as the government regulation of these apps, with a focus on the 3 most prevalent dermatology-related apps used by dermatology residents in the United States: VisualDx, UpToDate, and Mohs Surgery Appropriate Use Criteria. This prevalence is according to a survey emailed to all dermatology residents in the United States by the American Academy of Dermatology (AAD) in 2019 (unpublished data).
VisualDx
VisualDx, which aims to improve diagnostic accuracy and patient safety, contains peer-reviewed data and more than 32,000 images of dermatologic conditions. The editorial board includes more than 50 physicians. It provides opportunities for continuing medical education credit, is used in more than 2300 medical settings, and costs $399.99 annually for a subscription with partial features. Prior to the launch of the app in 2010, some health science professionals noted that the website version lacked references to primary sources.4 The same issue carried over to the app, which has evolved to offer artificial intelligence (AI) analysis of photographed skin lesions. However, there are no peer-reviewed publications showing positive impact of the app on diagnostic skills among dermatology residents or on patient outcomes.
UpToDate
UpToDate is a web-based database created in the early 1990s. A corresponding app was created around 2010. Both internal and independent research has demonstrated improved outcomes, and the app is advertised as the only clinical decision support resource associated with improved outcomes, as shown in more than 80 publications.5 UpToDate covers more than 11,800 medical topics and contains more than 35,000 graphics. It cites primary sources and uses a published system for grading recommendation strength and evidence quality. The data are processed and produced by a team of more than 7100 physicians as authors, editors, and reviewers. The platform grants continuing medical education credit and is used by more than 1.9 million clinicians in more than 190 countries. A 1-year subscription for an individual US-based physician costs $559. An observational study assessed UpToDate articles for potential conflicts of interest between authors and their recommendations. Of the 6 articles that met inclusion criteria of discussing management of medical conditions that have controversial or mostly brand-name treatment options, all had conflicts of interest, such as naming drugs from companies with which the authors and/or editors had financial relationships.6
Mohs Surgery Appropriate Use Criteria
The Mohs Surgery Appropriate Use Criteria app is a free clinical decision-making tool based on a consensus statement published in 2012 by the AAD, American College of Mohs Surgery, American Society for Dermatologic Surgery Association, and American Society for Mohs Surgery.7 It helps guide management of more than 200 dermatologic scenarios. Critique has been made that the criteria are partly based on expert opinion and data largely from the United States and has not been revised to incorporate newer data.8 There are no publications regarding the app itself.
Regulation of Health-Related Apps
Health-related apps that are designed for utilization by health care providers can be a valuable tool. However, given their prevalence, cost, and potential impact on patient lives, these apps should be well regulated and researched. The general paucity of peer-reviewed literature demonstrating the utility, safety, quality, and accuracy of health-related apps commonly used by providers is a reflection of insufficient mobile health regulation in the United States.
There are 3 primary government agencies responsible for regulating mobile medical apps: the US Food and Drug Administration (FDA), Federal Trade Commission, and Office for Civil Rights.9 The FDA does not regulate all medical devices. Apps intended for use in the diagnosis, cure, mitigation, prevention, or treatment of a disease or condition are considered to be medical devices.10 The FDA regulates those apps only if they are judged to pose more than minimal risk. Apps that are designed only to provide easy access to information related to health conditions or treatment are considered to be minimal risk but can develop into a different risk level such as by offering AI.11 Although the FDA does update its approach to medical devices, including apps and AI- and machine learning–based software, the rate and direction of update has not kept pace with the rapid evolution of apps.12 In 2019, the FDA began piloting a precertification program that grants long-term approval to organizations that develop apps instead of reviewing each app product individually.13 This decrease in premarket oversight is intended to expedite innovation with the hopeful upside of improving patient outcomes but is inconsistent, with the FDA still reviewing other types of medical devices individually.
For apps that are already in use, the Federal Trade Commission only gets involved in response to deceptive or unfair acts or practices relating to privacy, data security, and false or misleading claims about safety or performance. It may be more beneficial for consumers if those apps had a more stringent initial approval process. The Office for Civil Rights enforces the Health Insurance Portability and Accountability Act when relevant to apps.
Nongovernment agencies also are involved in app regulation. The FDA believes sharing more regulatory responsibility with private industry would promote efficiency.14 Google does not allow apps that contain false or misleading health claims,15 and Apple may scrutinize medical apps that could provide inaccurate data or be used for diagnosing or treating patients.16 Xcertia, a nonprofit organization founded by the American Medical Association and others, develops standards for the security, privacy, content, and operability of health-related apps, but those standards have not been adopted by other parties. Ultimately, nongovernment agencies are not responsible for public health and do not boast the government’s ability to enforce rules or ensure public safety.
Final Thoughts
The AAD survey of US dermatology residents found that the top consideration when choosing apps was up-to-date and accurate information; however, the 3 most prevalent apps among those same respondents did not need government approval and are not required to contain up-to-date data or to improve clinical outcomes, similar to most other health-related apps. This discrepancy is concerning considering the increasing utilization of apps for physician education and health care delivery and the increasing complexity of those apps. In light of these results, the potential decrease in federal premarket regulation suggested by the FDA’s precertification program seems inappropriate. It is important for the government to take responsibility for regulating health-related apps and to find a balance between too much regulation delaying innovation and too little regulation hurting physician training and patient care. It also is important for providers to be aware of the evidence and oversight behind the technologies they use for professional purposes.
Since the first mobile application (app) was developed in the 1990s, apps have become increasingly integrated into medical practice and training. More than 5.5 million apps were downloadable in 2019,1 of which more than 300,000 were health related.2 In the United States, more than 80% of physicians reported using smartphones for professional purposes in 2016.3 As the complexity of apps and their purpose of use has evolved, regulatory bodies have not adapted adequately to monitor apps that have broad-reaching consequences in medicine.
We review the primary literature on PubMed behind health-related apps that impact dermatologists as well as the government regulation of these apps, with a focus on the 3 most prevalent dermatology-related apps used by dermatology residents in the United States: VisualDx, UpToDate, and Mohs Surgery Appropriate Use Criteria. This prevalence is according to a survey emailed to all dermatology residents in the United States by the American Academy of Dermatology (AAD) in 2019 (unpublished data).
VisualDx
VisualDx, which aims to improve diagnostic accuracy and patient safety, contains peer-reviewed data and more than 32,000 images of dermatologic conditions. The editorial board includes more than 50 physicians. It provides opportunities for continuing medical education credit, is used in more than 2300 medical settings, and costs $399.99 annually for a subscription with partial features. Prior to the launch of the app in 2010, some health science professionals noted that the website version lacked references to primary sources.4 The same issue carried over to the app, which has evolved to offer artificial intelligence (AI) analysis of photographed skin lesions. However, there are no peer-reviewed publications showing positive impact of the app on diagnostic skills among dermatology residents or on patient outcomes.
UpToDate
UpToDate is a web-based database created in the early 1990s. A corresponding app was created around 2010. Both internal and independent research has demonstrated improved outcomes, and the app is advertised as the only clinical decision support resource associated with improved outcomes, as shown in more than 80 publications.5 UpToDate covers more than 11,800 medical topics and contains more than 35,000 graphics. It cites primary sources and uses a published system for grading recommendation strength and evidence quality. The data are processed and produced by a team of more than 7100 physicians as authors, editors, and reviewers. The platform grants continuing medical education credit and is used by more than 1.9 million clinicians in more than 190 countries. A 1-year subscription for an individual US-based physician costs $559. An observational study assessed UpToDate articles for potential conflicts of interest between authors and their recommendations. Of the 6 articles that met inclusion criteria of discussing management of medical conditions that have controversial or mostly brand-name treatment options, all had conflicts of interest, such as naming drugs from companies with which the authors and/or editors had financial relationships.6
Mohs Surgery Appropriate Use Criteria
The Mohs Surgery Appropriate Use Criteria app is a free clinical decision-making tool based on a consensus statement published in 2012 by the AAD, American College of Mohs Surgery, American Society for Dermatologic Surgery Association, and American Society for Mohs Surgery.7 It helps guide management of more than 200 dermatologic scenarios. Critique has been made that the criteria are partly based on expert opinion and data largely from the United States and has not been revised to incorporate newer data.8 There are no publications regarding the app itself.
Regulation of Health-Related Apps
Health-related apps that are designed for utilization by health care providers can be a valuable tool. However, given their prevalence, cost, and potential impact on patient lives, these apps should be well regulated and researched. The general paucity of peer-reviewed literature demonstrating the utility, safety, quality, and accuracy of health-related apps commonly used by providers is a reflection of insufficient mobile health regulation in the United States.
There are 3 primary government agencies responsible for regulating mobile medical apps: the US Food and Drug Administration (FDA), Federal Trade Commission, and Office for Civil Rights.9 The FDA does not regulate all medical devices. Apps intended for use in the diagnosis, cure, mitigation, prevention, or treatment of a disease or condition are considered to be medical devices.10 The FDA regulates those apps only if they are judged to pose more than minimal risk. Apps that are designed only to provide easy access to information related to health conditions or treatment are considered to be minimal risk but can develop into a different risk level such as by offering AI.11 Although the FDA does update its approach to medical devices, including apps and AI- and machine learning–based software, the rate and direction of update has not kept pace with the rapid evolution of apps.12 In 2019, the FDA began piloting a precertification program that grants long-term approval to organizations that develop apps instead of reviewing each app product individually.13 This decrease in premarket oversight is intended to expedite innovation with the hopeful upside of improving patient outcomes but is inconsistent, with the FDA still reviewing other types of medical devices individually.
For apps that are already in use, the Federal Trade Commission only gets involved in response to deceptive or unfair acts or practices relating to privacy, data security, and false or misleading claims about safety or performance. It may be more beneficial for consumers if those apps had a more stringent initial approval process. The Office for Civil Rights enforces the Health Insurance Portability and Accountability Act when relevant to apps.
Nongovernment agencies also are involved in app regulation. The FDA believes sharing more regulatory responsibility with private industry would promote efficiency.14 Google does not allow apps that contain false or misleading health claims,15 and Apple may scrutinize medical apps that could provide inaccurate data or be used for diagnosing or treating patients.16 Xcertia, a nonprofit organization founded by the American Medical Association and others, develops standards for the security, privacy, content, and operability of health-related apps, but those standards have not been adopted by other parties. Ultimately, nongovernment agencies are not responsible for public health and do not boast the government’s ability to enforce rules or ensure public safety.
Final Thoughts
The AAD survey of US dermatology residents found that the top consideration when choosing apps was up-to-date and accurate information; however, the 3 most prevalent apps among those same respondents did not need government approval and are not required to contain up-to-date data or to improve clinical outcomes, similar to most other health-related apps. This discrepancy is concerning considering the increasing utilization of apps for physician education and health care delivery and the increasing complexity of those apps. In light of these results, the potential decrease in federal premarket regulation suggested by the FDA’s precertification program seems inappropriate. It is important for the government to take responsibility for regulating health-related apps and to find a balance between too much regulation delaying innovation and too little regulation hurting physician training and patient care. It also is important for providers to be aware of the evidence and oversight behind the technologies they use for professional purposes.
- Clement J. Number of apps available in leading app stores as of 1st quarter 2020. Statista website. https://www.statista.com/statistics/276623/number-of-apps-available-in-leading-app-stores/. Published May 4, 2020. Accessed July 23, 2020.
- mHealth App Economics 2017/2018. Current Status and Future Trends in Mobile Health. Berlin, Germany: Research 2 Guidance; 2018.
- Healthcare Client Services. Professional usage of smartphones by doctors. Kantar website. https://www.kantarmedia.com/us/thinking-and-resources/blog/professional-usage-of-smartphones-by-doctors-2016. Published November 16, 2016. Accessed July 23, 2020.
- Skhal KJ, Koffel J. VisualDx. J Med Libr Assoc. 2007;95:470-471.
- UpToDate is the only clinical decision support resource associated with improved outcomes. UpToDate website. https://www.uptodate.com/home/research. Accessed July 29, 2020.
- Connolly SM, Baker DR, Coldiron BM, et al. AAD/ACMS/ASDSA/ASMS 2012 appropriate use criteria for Mohs micrographic surgery: a report of the American Academy of Dermatology, American College of Mohs Surgery, American Society for Dermatologic Surgery Association, and the American Society for Mohs Surgery. J Am Acad Dermatol. 2012;67:531-550.
- Amber KT, Dhiman G, Goodman KW. Conflict of interest in online point-of-care clinical support websites. J Med Ethics. 2014;40:578-580.
- Croley JA, Joseph AK, Wagner RF Jr. Discrepancies in the Mohs micrographic surgery appropriate use criteria. J Am Acad Dermatol. 2020;82:E55.
- Mobile health apps interactive tool. Federal Trade Commission website. https://www.ftc.gov/tips-advice/business-center/guidance/mobile-health-apps-interactive-tool. Published April 2016. Accessed May 23, 2020.
- Federal Food, Drug, and Cosmetic Act, 21 USC §321 (2018).
- US Food and Drug Administration. Examples of software functions for which the FDA will exercise enforcement discretion. https://www.fda.gov/medical-devices/device-software-functions-including-mobile-medical-applications/examples-software-functions-which-fda-will-exercise-enforcement-discretion. Updated September 26, 2019. Accessed July 29, 2020.
- US Food and Drug Administration. Proposed regulatory framework for modifications to artificial intelligence/machine learning (AI/ML)‐based software as a medical device (SaMD). https://www.fda.gov/downloads/MedicalDevices/DigitalHealth/SoftwareasaMedicalDevice/UCM635052.pdf. Accessed July 23, 2020.
- US Food and Drug Administration. Digital health software precertification (pre-cert) program. https://www.fda.gov/medical-devices/digital-health/digital-health-software-precertification-pre-cert-program. Updated July 18, 2019. Accessed July 23, 2020.
- Gottlieb S. Fostering medical innovation: a plan for digital health devices. US Food and Drug Administration website. https://www.fda.gov/news-events/fda-voices/fostering-medical-innovation-plan-digital-health-devices. Published June 15, 2017. Accessed July 23, 2020.
- Restricted content: unapproved substances. Google Play website. https://play.google.com/about/restricted-content/unapproved-substances. Accessed July 23, 2020.
- App store review guidelines. Apple Developer website. https://developer.apple.com/app-store/review/guidelines. Updated March 4, 2020. Accessed July 23, 2020.
- Clement J. Number of apps available in leading app stores as of 1st quarter 2020. Statista website. https://www.statista.com/statistics/276623/number-of-apps-available-in-leading-app-stores/. Published May 4, 2020. Accessed July 23, 2020.
- mHealth App Economics 2017/2018. Current Status and Future Trends in Mobile Health. Berlin, Germany: Research 2 Guidance; 2018.
- Healthcare Client Services. Professional usage of smartphones by doctors. Kantar website. https://www.kantarmedia.com/us/thinking-and-resources/blog/professional-usage-of-smartphones-by-doctors-2016. Published November 16, 2016. Accessed July 23, 2020.
- Skhal KJ, Koffel J. VisualDx. J Med Libr Assoc. 2007;95:470-471.
- UpToDate is the only clinical decision support resource associated with improved outcomes. UpToDate website. https://www.uptodate.com/home/research. Accessed July 29, 2020.
- Connolly SM, Baker DR, Coldiron BM, et al. AAD/ACMS/ASDSA/ASMS 2012 appropriate use criteria for Mohs micrographic surgery: a report of the American Academy of Dermatology, American College of Mohs Surgery, American Society for Dermatologic Surgery Association, and the American Society for Mohs Surgery. J Am Acad Dermatol. 2012;67:531-550.
- Amber KT, Dhiman G, Goodman KW. Conflict of interest in online point-of-care clinical support websites. J Med Ethics. 2014;40:578-580.
- Croley JA, Joseph AK, Wagner RF Jr. Discrepancies in the Mohs micrographic surgery appropriate use criteria. J Am Acad Dermatol. 2020;82:E55.
- Mobile health apps interactive tool. Federal Trade Commission website. https://www.ftc.gov/tips-advice/business-center/guidance/mobile-health-apps-interactive-tool. Published April 2016. Accessed May 23, 2020.
- Federal Food, Drug, and Cosmetic Act, 21 USC §321 (2018).
- US Food and Drug Administration. Examples of software functions for which the FDA will exercise enforcement discretion. https://www.fda.gov/medical-devices/device-software-functions-including-mobile-medical-applications/examples-software-functions-which-fda-will-exercise-enforcement-discretion. Updated September 26, 2019. Accessed July 29, 2020.
- US Food and Drug Administration. Proposed regulatory framework for modifications to artificial intelligence/machine learning (AI/ML)‐based software as a medical device (SaMD). https://www.fda.gov/downloads/MedicalDevices/DigitalHealth/SoftwareasaMedicalDevice/UCM635052.pdf. Accessed July 23, 2020.
- US Food and Drug Administration. Digital health software precertification (pre-cert) program. https://www.fda.gov/medical-devices/digital-health/digital-health-software-precertification-pre-cert-program. Updated July 18, 2019. Accessed July 23, 2020.
- Gottlieb S. Fostering medical innovation: a plan for digital health devices. US Food and Drug Administration website. https://www.fda.gov/news-events/fda-voices/fostering-medical-innovation-plan-digital-health-devices. Published June 15, 2017. Accessed July 23, 2020.
- Restricted content: unapproved substances. Google Play website. https://play.google.com/about/restricted-content/unapproved-substances. Accessed July 23, 2020.
- App store review guidelines. Apple Developer website. https://developer.apple.com/app-store/review/guidelines. Updated March 4, 2020. Accessed July 23, 2020.
Practice Points
- Physicians who are selecting an app for self-education or patient care should take into consideration the strength of the evidence supporting the app as well as the rigor of any approval process the app had to undergo.
- Only a minority of health-related apps are regulated by the government. This regulation has not kept up with the evolution of app software and may become more indirect.














