User login
Black Children With Vitiligo at Increased Risk for Psychiatric Disorders: Study
TOPLINE:
Black children with vitiligo are significantly more likely to be diagnosed with psychiatric disorders, including depression, suicidal ideation, and disruptive behavior disorders, than matched controls who did not have vitiligo, according to a case-control study.
METHODOLOGY:
- Researchers conducted a retrospective, single-center, case-control study at Texas Children’s Hospital in Houston on 327 Black children with vitiligo and 981 matched controls without vitiligo.
- The average age of participants was 11.7 years, and 62% were girls.
- The study outcome was the prevalence of psychiatric conditions and rates of treatment (pharmacotherapy and/or psychotherapy) initiation for those conditions.
TAKEAWAY:
- Black children with vitiligo were more likely to be diagnosed with depression (odds ratio [OR], 3.63; P < .001), suicidal ideation (OR, 2.88; P = .005), disruptive behavior disorders (OR, 7.68; P < .001), eating disorders (OR, 15.22; P = .013), generalized anxiety disorder (OR, 2.61; P < .001), and substance abuse (OR, 2.67; P = .011).
- The likelihood of having a psychiatric comorbidity was not significantly different between children with segmental vitiligo and those with generalized vitiligo or between girls and boys.
- Among the patients with vitiligo and psychiatric comorbidities, treatment initiation rates were higher for depression (76.5%), disruptive behavior disorders (82.1%), and eating disorders (100%).
- Treatment initiation rates were lower in patients with vitiligo diagnosed with generalized anxiety disorder (55.3%) and substance abuse (61.5%). Treatment was not initiated in 14% patients with suicidal ideation.
IN PRACTICE:
“Pediatric dermatologists have an important role in screening for psychiatric comorbidities, and implementation of appropriate screening tools while treating vitiligo is likely to have a bidirectional positive impact,” the authors wrote, adding: “By better understanding psychiatric comorbidities of African American children with vitiligo, dermatologists can be more aware of pediatric mental health needs and provide appropriate referrals.”
SOURCE:
This study was led by Emily Strouphauer, BSA, Baylor College of Medicine, Houston, and was published online in JAAD International.
LIMITATIONS:
The study limitations were the retrospective design, small sample size, and heterogeneity in the control group.
DISCLOSURES:
The study did not receive any funding. The authors declared no competing interests.
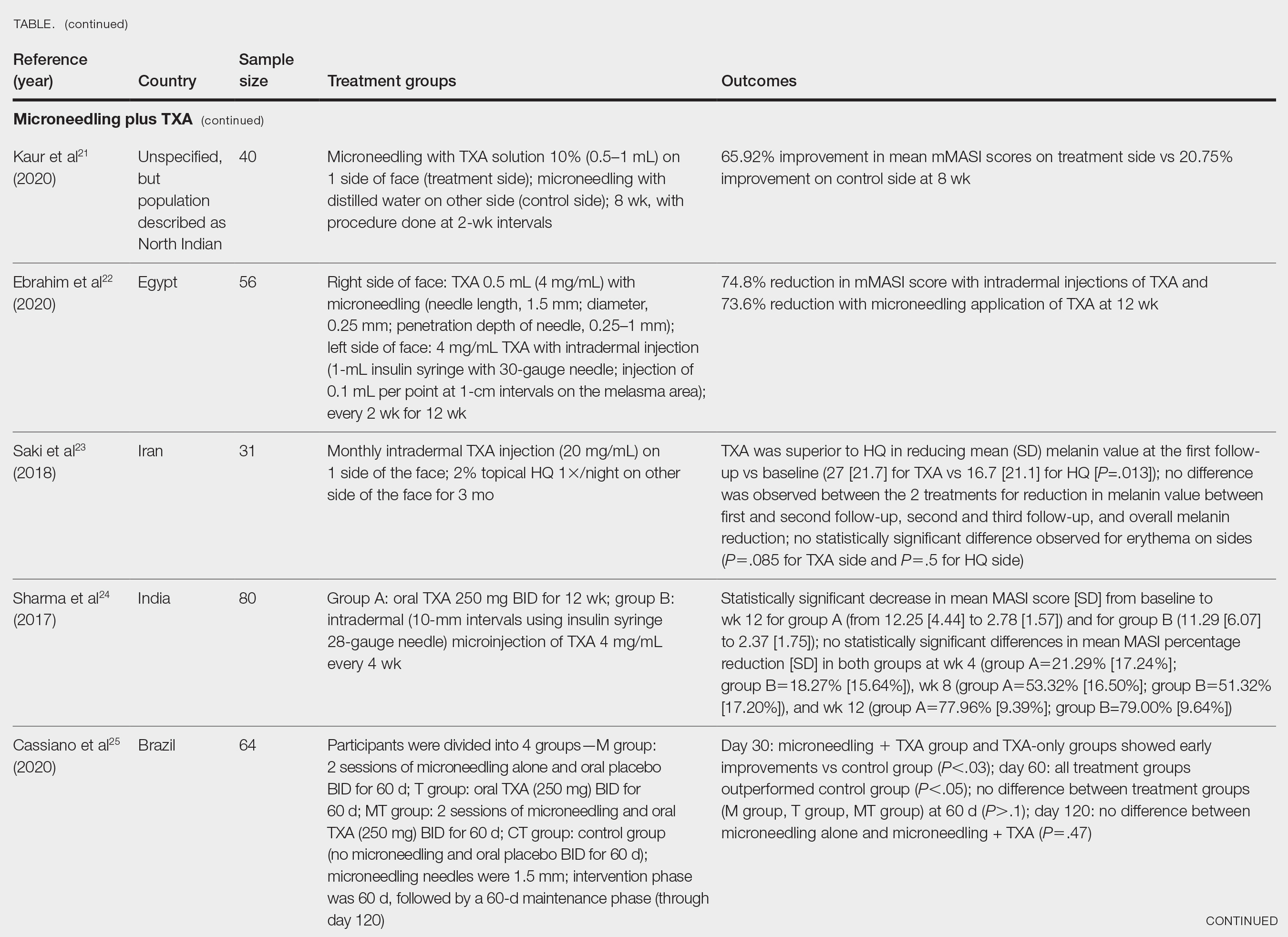
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
TOPLINE:
Black children with vitiligo are significantly more likely to be diagnosed with psychiatric disorders, including depression, suicidal ideation, and disruptive behavior disorders, than matched controls who did not have vitiligo, according to a case-control study.
METHODOLOGY:
- Researchers conducted a retrospective, single-center, case-control study at Texas Children’s Hospital in Houston on 327 Black children with vitiligo and 981 matched controls without vitiligo.
- The average age of participants was 11.7 years, and 62% were girls.
- The study outcome was the prevalence of psychiatric conditions and rates of treatment (pharmacotherapy and/or psychotherapy) initiation for those conditions.
TAKEAWAY:
- Black children with vitiligo were more likely to be diagnosed with depression (odds ratio [OR], 3.63; P < .001), suicidal ideation (OR, 2.88; P = .005), disruptive behavior disorders (OR, 7.68; P < .001), eating disorders (OR, 15.22; P = .013), generalized anxiety disorder (OR, 2.61; P < .001), and substance abuse (OR, 2.67; P = .011).
- The likelihood of having a psychiatric comorbidity was not significantly different between children with segmental vitiligo and those with generalized vitiligo or between girls and boys.
- Among the patients with vitiligo and psychiatric comorbidities, treatment initiation rates were higher for depression (76.5%), disruptive behavior disorders (82.1%), and eating disorders (100%).
- Treatment initiation rates were lower in patients with vitiligo diagnosed with generalized anxiety disorder (55.3%) and substance abuse (61.5%). Treatment was not initiated in 14% patients with suicidal ideation.
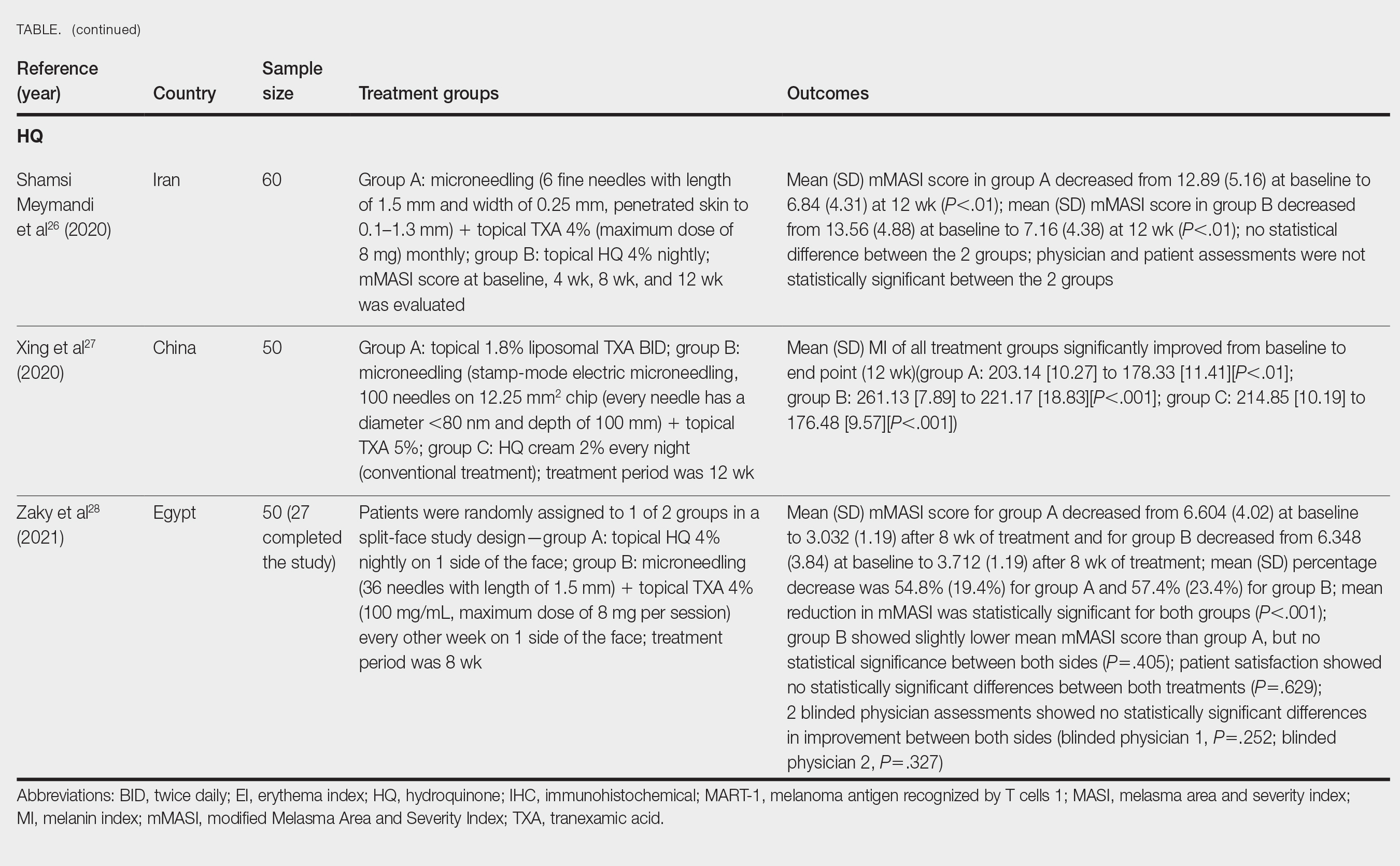
IN PRACTICE:
“Pediatric dermatologists have an important role in screening for psychiatric comorbidities, and implementation of appropriate screening tools while treating vitiligo is likely to have a bidirectional positive impact,” the authors wrote, adding: “By better understanding psychiatric comorbidities of African American children with vitiligo, dermatologists can be more aware of pediatric mental health needs and provide appropriate referrals.”
SOURCE:
This study was led by Emily Strouphauer, BSA, Baylor College of Medicine, Houston, and was published online in JAAD International.
LIMITATIONS:
The study limitations were the retrospective design, small sample size, and heterogeneity in the control group.
DISCLOSURES:
The study did not receive any funding. The authors declared no competing interests.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
TOPLINE:
Black children with vitiligo are significantly more likely to be diagnosed with psychiatric disorders, including depression, suicidal ideation, and disruptive behavior disorders, than matched controls who did not have vitiligo, according to a case-control study.
METHODOLOGY:
- Researchers conducted a retrospective, single-center, case-control study at Texas Children’s Hospital in Houston on 327 Black children with vitiligo and 981 matched controls without vitiligo.
- The average age of participants was 11.7 years, and 62% were girls.
- The study outcome was the prevalence of psychiatric conditions and rates of treatment (pharmacotherapy and/or psychotherapy) initiation for those conditions.
TAKEAWAY:
- Black children with vitiligo were more likely to be diagnosed with depression (odds ratio [OR], 3.63; P < .001), suicidal ideation (OR, 2.88; P = .005), disruptive behavior disorders (OR, 7.68; P < .001), eating disorders (OR, 15.22; P = .013), generalized anxiety disorder (OR, 2.61; P < .001), and substance abuse (OR, 2.67; P = .011).
- The likelihood of having a psychiatric comorbidity was not significantly different between children with segmental vitiligo and those with generalized vitiligo or between girls and boys.
- Among the patients with vitiligo and psychiatric comorbidities, treatment initiation rates were higher for depression (76.5%), disruptive behavior disorders (82.1%), and eating disorders (100%).
- Treatment initiation rates were lower in patients with vitiligo diagnosed with generalized anxiety disorder (55.3%) and substance abuse (61.5%). Treatment was not initiated in 14% patients with suicidal ideation.
IN PRACTICE:
“Pediatric dermatologists have an important role in screening for psychiatric comorbidities, and implementation of appropriate screening tools while treating vitiligo is likely to have a bidirectional positive impact,” the authors wrote, adding: “By better understanding psychiatric comorbidities of African American children with vitiligo, dermatologists can be more aware of pediatric mental health needs and provide appropriate referrals.”
SOURCE:
This study was led by Emily Strouphauer, BSA, Baylor College of Medicine, Houston, and was published online in JAAD International.
LIMITATIONS:
The study limitations were the retrospective design, small sample size, and heterogeneity in the control group.
DISCLOSURES:
The study did not receive any funding. The authors declared no competing interests.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
Storybooks Can Help Children Deal with Skin Conditions
TORONTO —
So far, “the study demonstrates that these books have value to patients and families,” one of the study authors, Sonia Havele, MD, a pediatrician and dermatology resident at Children’s Mercy Hospital Kansas City, Kansas City, Missouri, said in an interview.
“There are tools to help kids cope with their skin conditions, but we’re underutilizing them,” she added. “And part of the reason we’re underutilizing storybooks is that we just don’t know what’s out there.” For the study, the researchers received funding to purchase 18 “creative and thoughtful” storybooks related to pediatric skin conditions, reviewed by at least two pediatric dermatologists before being selected, which are just a sample of related books that are available.
The study results were presented as a poster at the annual meeting of the Society for Pediatric Dermatology.
Children with visible skin conditions, which can include port-wine stains, capillary malformations, and congenital moles, may be subjected to teasing or bullying at school, and the conditions can also affect their quality of life.
Beauty and the Birthmark
The books include one titled “Beauty with a Birthmark” and another, “My Hair Went on Vacation.” An illustrated book, “Just Ask: Be Different, Be Brave, Be You,” by US Supreme Court Justice Sonia Sotomayor, offers tips on how to answer common questions about someone’s appearance.
Dr. Havele said that Justice Sotomayor’s book “empowers kids, their siblings, their classmates ... to ask questions, and it teaches patients not to be afraid of those questions, and to really lean into educating their peers, and their family members.”
“Kids are really just curious,” she added. “They’ll make comments like: ‘Hey, what’s that spot on your face?’ Or, they’ll ask about vitiligo because they’ve never seen somebody with it before.”
To evaluate the psychosocial impact of these types of books for children with visible skin conditions, Dr. Havele and colleagues designed a study that includes patients aged 2-12 years dealing with issues related to self-esteem, acceptance, coping, or bullying. Parents are provided with a relevant storybook to read at home with their child in a “safe and comfortable space” and “at their own pace and their own time,” said Dr. Havele.
Inside the book is a QR code to access the validated Children’s Dermatology Life Quality Index (CDLQI). Families complete the survey at baseline and provide feedback after reading the book. Researchers collect information about demographics, age, gender, and skin conditions, which included atopic dermatitis, alopecia areata, vitiligo, hemangioma, and port-wine stain.
The response rate so far is 34%, and close to 80 parents have completed the survey with their child, Dr. Havele said.
At baseline, many of the children were either moderately or severely affected in terms of their quality of life (45% scored ≥ 6 on the CDLQI).
After reading the book, about 80% of parents reported it had a positive impact, and about 20% said it had a somewhat positive impact on their child’s self-image or confidence. Almost 80% agreed, and the remainder somewhat agreed it encouraged their child to embrace differences.
Most respondents also said the book helped the parent and child cope with the child’s condition. “So really, it was overall a positive response,” said Dr. Havele. “We are able to demonstrate that these books have value in a more scientific or objective way.”
This may not be surprising. Dr. Havele referred to more formal bibliotherapy (book therapy), which has been studied in other pediatric populations, including patients with cancer and those who have experienced trauma.
Awesome Space
Pediatric dermatologists are perfectly positioned to play a role in improving the lives of their patients with skin issues. “We see the impact of visible skin disease on children all the time,” said Dr. Havele. “The dermatology visit is an awesome space and opportunity to introduce these books to families and potentially help them talk about the skin condition with their child.”
In addition to prescribing therapies, “we’re also with these kids through an emotional journey, and I think giving them tools for that emotional journey is very helpful,” she added.
Such books would have been a great help to Dr. Havele herself. Growing up, she had severe atopic dermatitis covering much of her body. “Having such a resource would have helped me better cope with my reality of being different than everyone else.”
She hopes a database will be established to house these resources so other providers can refer patients to the list of books. Other books include “The Itchy-saurus: The Dino with an itch that can’t be scratched,” “Hair in My Brush,” and “I am Unique!”
Dr. Havele had no relevant disclosures.
A version of this article first appeared on Medscape.com.
TORONTO —
So far, “the study demonstrates that these books have value to patients and families,” one of the study authors, Sonia Havele, MD, a pediatrician and dermatology resident at Children’s Mercy Hospital Kansas City, Kansas City, Missouri, said in an interview.
“There are tools to help kids cope with their skin conditions, but we’re underutilizing them,” she added. “And part of the reason we’re underutilizing storybooks is that we just don’t know what’s out there.” For the study, the researchers received funding to purchase 18 “creative and thoughtful” storybooks related to pediatric skin conditions, reviewed by at least two pediatric dermatologists before being selected, which are just a sample of related books that are available.
The study results were presented as a poster at the annual meeting of the Society for Pediatric Dermatology.
Children with visible skin conditions, which can include port-wine stains, capillary malformations, and congenital moles, may be subjected to teasing or bullying at school, and the conditions can also affect their quality of life.
Beauty and the Birthmark
The books include one titled “Beauty with a Birthmark” and another, “My Hair Went on Vacation.” An illustrated book, “Just Ask: Be Different, Be Brave, Be You,” by US Supreme Court Justice Sonia Sotomayor, offers tips on how to answer common questions about someone’s appearance.
Dr. Havele said that Justice Sotomayor’s book “empowers kids, their siblings, their classmates ... to ask questions, and it teaches patients not to be afraid of those questions, and to really lean into educating their peers, and their family members.”
“Kids are really just curious,” she added. “They’ll make comments like: ‘Hey, what’s that spot on your face?’ Or, they’ll ask about vitiligo because they’ve never seen somebody with it before.”
To evaluate the psychosocial impact of these types of books for children with visible skin conditions, Dr. Havele and colleagues designed a study that includes patients aged 2-12 years dealing with issues related to self-esteem, acceptance, coping, or bullying. Parents are provided with a relevant storybook to read at home with their child in a “safe and comfortable space” and “at their own pace and their own time,” said Dr. Havele.
Inside the book is a QR code to access the validated Children’s Dermatology Life Quality Index (CDLQI). Families complete the survey at baseline and provide feedback after reading the book. Researchers collect information about demographics, age, gender, and skin conditions, which included atopic dermatitis, alopecia areata, vitiligo, hemangioma, and port-wine stain.
The response rate so far is 34%, and close to 80 parents have completed the survey with their child, Dr. Havele said.
At baseline, many of the children were either moderately or severely affected in terms of their quality of life (45% scored ≥ 6 on the CDLQI).
After reading the book, about 80% of parents reported it had a positive impact, and about 20% said it had a somewhat positive impact on their child’s self-image or confidence. Almost 80% agreed, and the remainder somewhat agreed it encouraged their child to embrace differences.
Most respondents also said the book helped the parent and child cope with the child’s condition. “So really, it was overall a positive response,” said Dr. Havele. “We are able to demonstrate that these books have value in a more scientific or objective way.”
This may not be surprising. Dr. Havele referred to more formal bibliotherapy (book therapy), which has been studied in other pediatric populations, including patients with cancer and those who have experienced trauma.
Awesome Space
Pediatric dermatologists are perfectly positioned to play a role in improving the lives of their patients with skin issues. “We see the impact of visible skin disease on children all the time,” said Dr. Havele. “The dermatology visit is an awesome space and opportunity to introduce these books to families and potentially help them talk about the skin condition with their child.”
In addition to prescribing therapies, “we’re also with these kids through an emotional journey, and I think giving them tools for that emotional journey is very helpful,” she added.
Such books would have been a great help to Dr. Havele herself. Growing up, she had severe atopic dermatitis covering much of her body. “Having such a resource would have helped me better cope with my reality of being different than everyone else.”
She hopes a database will be established to house these resources so other providers can refer patients to the list of books. Other books include “The Itchy-saurus: The Dino with an itch that can’t be scratched,” “Hair in My Brush,” and “I am Unique!”
Dr. Havele had no relevant disclosures.
A version of this article first appeared on Medscape.com.
TORONTO —
So far, “the study demonstrates that these books have value to patients and families,” one of the study authors, Sonia Havele, MD, a pediatrician and dermatology resident at Children’s Mercy Hospital Kansas City, Kansas City, Missouri, said in an interview.
“There are tools to help kids cope with their skin conditions, but we’re underutilizing them,” she added. “And part of the reason we’re underutilizing storybooks is that we just don’t know what’s out there.” For the study, the researchers received funding to purchase 18 “creative and thoughtful” storybooks related to pediatric skin conditions, reviewed by at least two pediatric dermatologists before being selected, which are just a sample of related books that are available.
The study results were presented as a poster at the annual meeting of the Society for Pediatric Dermatology.
Children with visible skin conditions, which can include port-wine stains, capillary malformations, and congenital moles, may be subjected to teasing or bullying at school, and the conditions can also affect their quality of life.
Beauty and the Birthmark
The books include one titled “Beauty with a Birthmark” and another, “My Hair Went on Vacation.” An illustrated book, “Just Ask: Be Different, Be Brave, Be You,” by US Supreme Court Justice Sonia Sotomayor, offers tips on how to answer common questions about someone’s appearance.
Dr. Havele said that Justice Sotomayor’s book “empowers kids, their siblings, their classmates ... to ask questions, and it teaches patients not to be afraid of those questions, and to really lean into educating their peers, and their family members.”
“Kids are really just curious,” she added. “They’ll make comments like: ‘Hey, what’s that spot on your face?’ Or, they’ll ask about vitiligo because they’ve never seen somebody with it before.”
To evaluate the psychosocial impact of these types of books for children with visible skin conditions, Dr. Havele and colleagues designed a study that includes patients aged 2-12 years dealing with issues related to self-esteem, acceptance, coping, or bullying. Parents are provided with a relevant storybook to read at home with their child in a “safe and comfortable space” and “at their own pace and their own time,” said Dr. Havele.
Inside the book is a QR code to access the validated Children’s Dermatology Life Quality Index (CDLQI). Families complete the survey at baseline and provide feedback after reading the book. Researchers collect information about demographics, age, gender, and skin conditions, which included atopic dermatitis, alopecia areata, vitiligo, hemangioma, and port-wine stain.
The response rate so far is 34%, and close to 80 parents have completed the survey with their child, Dr. Havele said.
At baseline, many of the children were either moderately or severely affected in terms of their quality of life (45% scored ≥ 6 on the CDLQI).
After reading the book, about 80% of parents reported it had a positive impact, and about 20% said it had a somewhat positive impact on their child’s self-image or confidence. Almost 80% agreed, and the remainder somewhat agreed it encouraged their child to embrace differences.
Most respondents also said the book helped the parent and child cope with the child’s condition. “So really, it was overall a positive response,” said Dr. Havele. “We are able to demonstrate that these books have value in a more scientific or objective way.”
This may not be surprising. Dr. Havele referred to more formal bibliotherapy (book therapy), which has been studied in other pediatric populations, including patients with cancer and those who have experienced trauma.
Awesome Space
Pediatric dermatologists are perfectly positioned to play a role in improving the lives of their patients with skin issues. “We see the impact of visible skin disease on children all the time,” said Dr. Havele. “The dermatology visit is an awesome space and opportunity to introduce these books to families and potentially help them talk about the skin condition with their child.”
In addition to prescribing therapies, “we’re also with these kids through an emotional journey, and I think giving them tools for that emotional journey is very helpful,” she added.
Such books would have been a great help to Dr. Havele herself. Growing up, she had severe atopic dermatitis covering much of her body. “Having such a resource would have helped me better cope with my reality of being different than everyone else.”
She hopes a database will be established to house these resources so other providers can refer patients to the list of books. Other books include “The Itchy-saurus: The Dino with an itch that can’t be scratched,” “Hair in My Brush,” and “I am Unique!”
Dr. Havele had no relevant disclosures.
A version of this article first appeared on Medscape.com.
FROM SPD 2024
The Use of Tranexamic Acid and Microneedling in the Treatment of Melasma: A Systematic Review
Melasma (also known as chloasma faciei) is a common chronic skin disorder that results in well-demarcated, hyperpigmented, tan to dark patches that mostly appear in sun-exposed areas such as the face and neck and sometimes the arms. The exact prevalence or incidence is not known but is estimated to be 1% to 50% overall depending on the ethnic population and geographic location.1,2 Melasma predominantly affects women, but research has shown that approximately 10% to 20% of men are affected by this condition.3,4 Although melasma can affect patients of all skin types, it primarily affects those with darker skin tones.5 The groups most often affected are women of Black, Hispanic, Middle Eastern, and Southeast Asian ethnicity. Although the pathogenesis is complex and not fully understood, multiple pathways and etiologies have been theorized to cause melasma. Potential causes include exposure to UV radiation, oral contraceptives, hormonal changes, medications, thyroid dysfunction, genetics, and pregnancy.6,7 Cytokines and growth factors, including adipokine and angiopoietin, synthesized by sebaceous glands play a role in the pathogenic mechanism of melasma. Cytokines and growth factors are hypothesized to modulate the function of melanocytes.8 Both melanocytes and sebocytes are controlled by α–melanocyte-stimulating hormone. Therefore, overexpression of α–melanocyte-stimulating hormone will result in overproduction of these 2 cell types, resulting in melasma. Melasma can be classified into 4 subtypes using Wood lamp examination: epidermal, dermal, mixed, or indeterminate.3 Furthermore, melasma is divided into subgroups based on the location: malar region, mandibular region, and centrofacial patch pattern.9,10 The involvement of sebaceous glands in the pathogenesis of melasma may explain the predilection for the centrofacial region, which is the most common pattern.
The severity of melasma can be assessed using the melasma area and severity index (MASI), which is calculated by subjective assessment of 3 main factors: (1) facial area of involvement; (2) darkness of affected region; and (3) homogeneity, with the extent of melasma indicated by a score ranging from 0 to 48.11 The modified MASI (mMASI) subsequently was introduced to assist with assessing the severity of melasma and creating distinct ranges for mild, moderate, and severe cases, ranging from 0 (mild) to 24 (severe).12 Both indices are used in research to assess the improvement of melasma with treatment.
Patients with melasma report a decrease in quality of life, increased emotional stress, and lower self-esteem due to cosmesis.13 Treatment of melasma can be highly challenging and often is complicated by relapsing. Historically, the treatment of melasma has included the use of chemical lightening agents. Additional treatment options include the use of lasers and complex chemical peels,9,10 but these interventions may result in adverse outcomes for individuals with darker skin tones. The current gold-standard treatment is topical hydroquinone and broad-spectrum sunscreen. Although hydroquinone is effective in the treatment of melasma, relapse is common. The goal of melasma management is not only to treat acute hyperpigmentation but also to prevent relapse. Other therapies that currently are being explored for the clinically sustained treatment of melasma include tranexamic acid (TXA)(trans-4-[aminomethyl]cyclohexanecarboxylic acid),9,10 an antifibrinolytic agent routinely used to prevent blood loss during surgery and in the management of menorrhagia. It is a synthetic derivative of lysine and serves as a potent plasmin inhibitor by blocking the lysine-binding sites of plasminogen molecules, thus preventing the conversion of plasminogen to plasmin. It also prevents fibrinolysis and blood loss.
In addition to its hemostatic properties, TXA has been found to have hypopigmentation properties.14,15 Plasminogen also can be found in human epidermal basal cells and human keratinocytes, and it is postulated that TXA’s interaction with these cells explains its hypopigmentation properties. Both UV radiation and hormones activate plasminogen into plasmin, resulting in the activation of tyrosinase and melanogenesis.14,15 Tranexamic acid is postulated to inhibit the keratinocyte-plasminogen pathway, thus leading to the inhibition of UV-induced and hormone-induced pigmentation. Also, TXA serves as a competitive inhibitor for tyrosinase due to its structural similarity to tyrosine.15 The combination of these 2 mechanisms contributes to the skin-lightening effects of TXA, making it a potential treatment for melasma.
Furthermore, the use of microneedling is being explored as a treatment option for melasma. Microneedling creates microscopic punctures in the skin using tiny needles, resulting in a wound-healing response and skin resurfacing. The microneedling technique is utilized to create small holes in the skin, with needle depths that can be adjusted from 0.5 to 3.5 mm to target different layers of the dermis and allow for discreet application of TXA.16 We sought to look at the current literature on the use and effectiveness of microneedling in combination with TXA to treat melasma and prevent relapse.
Methods
A systematic review was performed of PubMed articles indexed for MEDLINE and Embase in November 2021 to compile available articles that studied TXA and microneedling as a treatment for melasma. The PubMed search terms were (melasma) AND (microneedling* OR ‘tranexamic acid’ OR TXA or TA). The Embase search terms were (cholasma OR melasma) AND (tranexamic acid OR TXA) AND (microneedling)(Figure). The search was then limited to ”randomized controlled trial” and ”clinical trial” in English-language journals. Duplicates were excluded. After thorough evaluation, articles that discussed the use of TXA in combination with treatment options other than microneedling also were excluded.
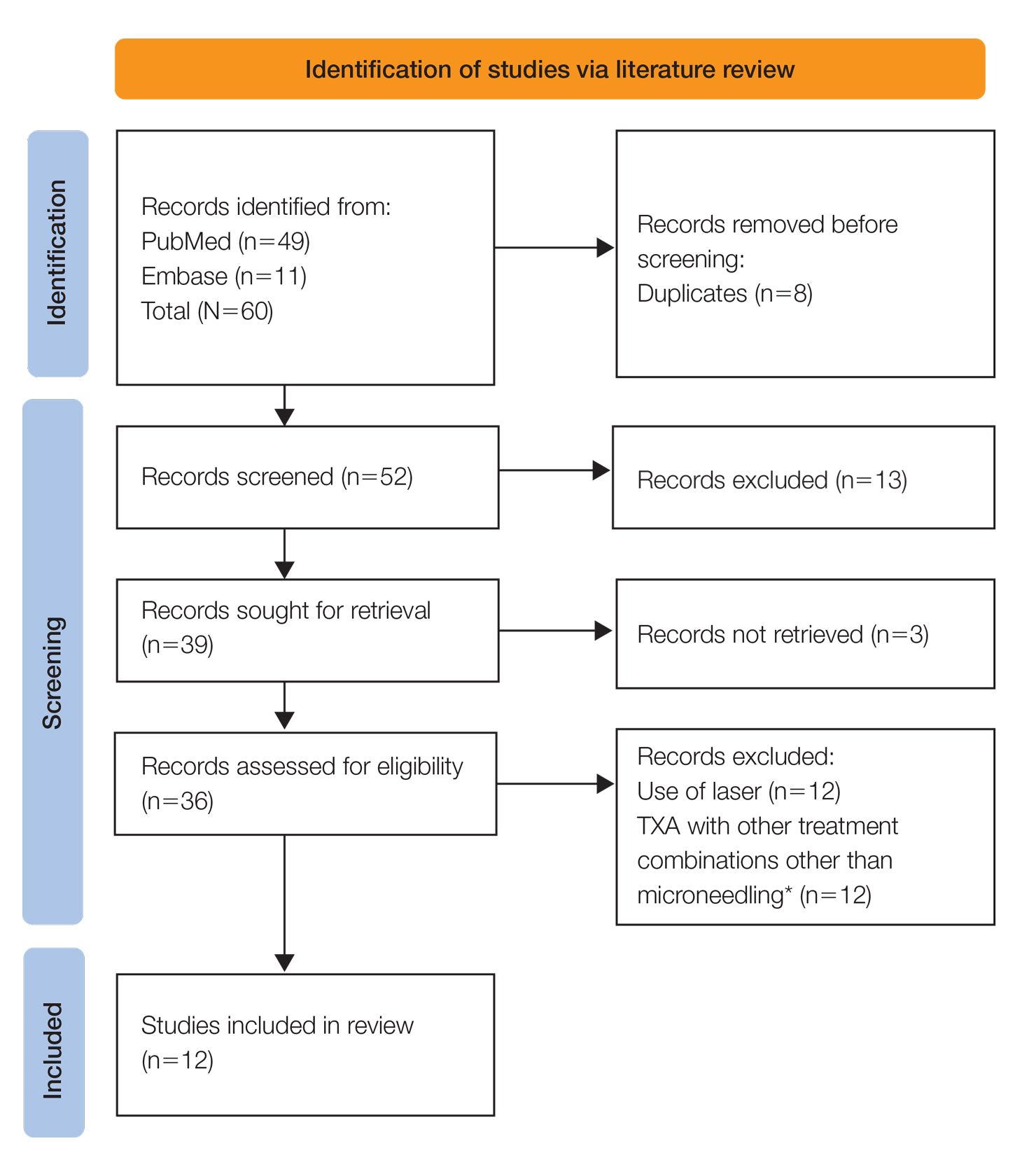
Results
The literature search yielded a total of 12 articles that assessed the effectiveness of TXA and microneedling for the treatment of melasma (Table).17-28 Several articles concluded that TXA was equally effective at reducing melasma lesions when compared with the standard treatment of hydroquinone. Some of the reviewed articles also demonstrated the effectiveness of microneedling in improving melasma lesions as a stand-alone treatment. These studies highlighted the enhanced efficacy of the combined treatment of TXA and microneedling compared with their individual uses.17-28
Comment
Melasma is a common chronic hyperpigmentation disorder, making its treatment clinically challenging. Many patients experience symptom relapses, and limited effective treatment options make achieving complete clearance difficult, underscoring the need for improved therapeutic approaches. Recently, researchers have explored alternative treatments to address the challenges of melasma management. Tranexamic acid is an antifibrinolytic used to prevent blood loss and has emerged as a potential treatment for melasma. Similarly, microneedling—a technique in which multiple punctures are made in the skin to activate and stimulate wound healing and skin rejuvenation—shows promise for melasma.
Oral TXA for Melasma—Oral TXA has been shown to reduce melasma lesions. Del Rosario et al17 recruited 44 women (39 of whom completed the study) with moderate to severe melasma and randomized them into 2 groups: oral TXA and placebo. This study demonstrated a 49% reduction in the mMASI score in all participants taking oral TXA (250 mg twice daily [BID]) compared with an 18% reduction in the control group (placebo capsule BID) after 3 months of treatment. In patients with moderate and severe melasma, 45% and 51% mMASI score reductions were reported in the treatment group, respectively, vs 16% and 19% score reductions in placebo group, respectively. These researchers concluded that oral TXA may be effective at treating moderate to severe melasma. Although patients with severe melasma had a better response to treatment, their improvement was not sustained compared with patients with moderate melasma after a 3-month posttreatment follow-up.17
Microneedling Plus TXA for Melasma—Microneedling alone has been shown to be effective for melasma. El Attar et al18 conducted a split-face study of microneedling (1.5-mm depth) plus topical TXA (0.5 mL)(right side of the face[treatment arm]) compared with microneedling (1.5-mm depth) plus topical vitamin C (0.5 mL)(left side of the face [control group]) in 20 women with melasma. The sessions were repeated every 2 weeks for a total of 6 sessions. Although researchers found no statistically significant differences between the 2 treatment sides, microneedling plus TXA showed a slight advantage over microneedling plus vitamin C in dermoscopic examination. Both sides showed improvement in pigmented lesions, but vitamin C–treated lesions did not show an improvement in vascularity vs TXA.18
Saleh et al19 further showed that combination treatment with microneedling and TXA may improve clinical outcomes better than microneedling alone. Their study demonstrated a reduction in MASI score that was significantly higher in the combination treatment group compared with the microneedling alone group (P=.001). There was a significant reduction in melanoma antigen recognized by T cells 1 (MART-1)–positive cells in the combination treatment group compared with the microneedling alone group (P=.001). Lastly, combined therapy improved melasma patches better than microneedling alone.19
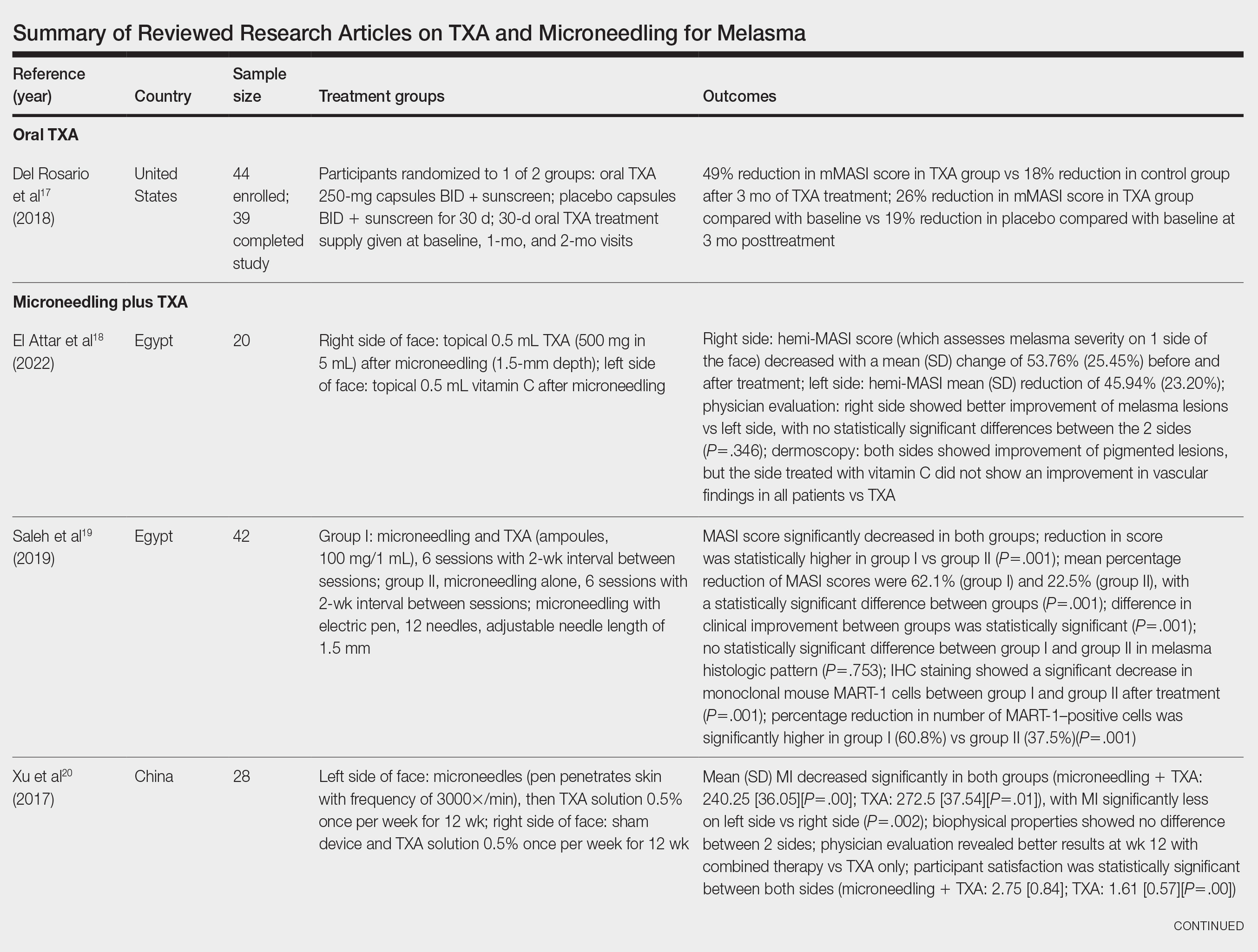
Xu et al20 conducted a split-face study (N=28) exploring the effectiveness of transdermal application of topical TXA using a microarray pen with microneedles (vibration at 3000×/min) plus topical TXA on one side of the face, while the other side received only topical TXA as a control. After 12 weeks of treatment, combination therapy with microneedling and TXA decreased brown spot scores, lowered melanin index (MI) values, improved blinded physician assessment, and improved patient satisfaction vs TXA therapy alone.20
Kaur et al21 conducted a split-face, randomized, controlled trial of microneedling (1-mm depth) with TXA solution 10% vs microneedling (1-mm depth) with distilled water alone for 8 weeks (N=40). They graded participant responses to treatment using reductions in mMASI scores12 at every 2 weeks of follow-up (no response, minimal or poor response=0%–25%; partial or fair response=26%–50%; good response=51%–75%; and excellent response=>75%). They reported an overall reduction in mMASI scores for both the treatment side and the control side in all participants, showing a 65.92% improvement in mean mMASI scores on the treatment side vs 20.75% improvement on the control side at week 8. Both sides showed statistically significant reductions in mean mMASI scores (P<.05). Clinically, 40% (16/40) of participants showed an excellent response to combined treatment compared with 0% (0/40) to microneedling alone. Overall, patient satisfaction was similar across both groups. This study demonstrated that microneedling alone improves melasma, but a combination of microneedling plus TXA showed a better clinical reduction in melasma. However, the researchers did not follow up with participants posttreatment, so it remains unclear if the improved clinical outcomes were sustained long-term.21
Ebrahim et al22 reported that the combination of 0.5 mL TXA (4 mg/mL) and microneedling (0.25- to 1-mm depth) was effective for melasma. Although there was improvement within microneedling and TXA, the study also showed that intradermal injection of TXA was significant in reducing mean mMASI scores and improving melasma (P<.001). The reduction in mMASI scores for the group receiving intradermal injections of TXA (left side; 74.8% reduction in mean mMASI score) vs the group receiving microneedling application of TXA (right side; 73.6% reduction in mean mMASI score) was not statistically significant. These findings suggest that the mode of TXA application may not be critical in determining clinical responses to TXA treatment. Although there was no reported statistically significant difference in clinical outcomes between the 2 treatments, patient satisfaction was higher on the microneedling side. Only 8 of 50 participants (16%) experienced recurrence 3 months posttreatment.22
Saki et al23 compared the efficacy of topical hydroquinone (2%) to intradermal TXA injections in treating melasma. They found intradermal TXA injections to be a clinically effective mode of treatment.23
Sharma et al24 explored the efficacy and safety of oral TXA by randomly assigning 100 Indian patients (20 of whom withdrew before study completion) with melasma into 2 groups: group A received TXA 250 mg twice daily, and group B received intradermal microinjections of TXA (4 mg/mL) every 4 weeks. The MASI scores were assessed at 4-week intervals for a total of 12 weeks. There was a decrease in MASI scores in both groups, and there was no statistically significant difference in mean percentage reduction in MASI scores between the 2 routes of drug administration, further suggesting the effectiveness of TXA independent of administration route. Two patients in group A relapsed at 24 weeks, and there were no relapses in group B, which may suggest a minimal superiority of TXA plus microneedling at providing more sustainable results compared with oral TXA alone. A notable limitation of this study was a high dropout rate as well as lack of long-term follow-up with participants, limiting the generalizability of the conclusions.24
Cassiano et al25 assigned 64 women with melasma to 1 of 3 treatment groups or a control group to compare the effectiveness of microneedling (M group: 1.5 mm; 2 sessions), oral TXA (T group: 250 mg/d twice daily for 60 days), and a combination of microneedling (2 sessions) and oral TXA (MT group: 250 mg/d twice daily for 60 days)with placebo for clinically reducing melasma lesions. The intervention period was 60 days followed by a 60-day maintenance phase for a total study period of 120 days. The researchers evaluated mMASI scores, quality of life, and difference in colorimetric luminosity. All treatment groups showed a reduction in mMASI scores at both 30 days and 60 days, indicating improved melasma severity. The MT and T groups had more significant improvement at 30 days compared with the control group (P<.03), suggesting that microneedling plus TXA and TXA alone promote faster improvement in melasma lesions. By 60 days, the M, T, and MT groups outperformed the control group, with no significant differences between the M, T, and MT groups. However, at the 120-day maintenance follow-up, the T group did not maintain its improvement compared with the control group. The M and MT groups showed no significance difference in effectiveness at 120 days, suggesting that microneedling may promote less frequent relapse and sustained remission compared to TXA alone.25
Hydroquinone for Melasma—Additional studies on the use of TXA treatments show that TXA may be an equally effective alternative to the standard use of hydroquinone treatment. Shamsi Meymandi et al26 did not find a statistically significant difference in treatment with TXA plus microneedling vs the standard regimen of hydroquinone. More importantly, patient and physician satisfaction assessments were similar between the 2 groups. Compared to hydroquinone, nightly treatment is not necessary with microneedling and TXA.26
Xing et al27 supported these conclusions with their study. They compared 3 study arms for a duration of 12 weeks: group A received topical 1.8% liposomal TXA BID, group B received stamp-mode electric microneedling with 5% TXA weekly, and group C applied 2% hydroquinone cream nightly. The study concluded that all 3 groups showed a significant reduction in mean MI by the end of the study, but a better MI improvement was observed in groups B and C (both P<.001) compared with group A (P<.01).27
Zaky et al28 showed that both hydroquinone and combination treatment of TXA plus microneedling are effective at improving melasma lesions. Further studies are needed to definitively conclude if combination treatment is more efficacious than hydroquinone; if the combination is more effective, it provides a treatment option for patients with melasma who may not be good candidates for hydroquinone treatment.
Study Limitations—One limitation in all the studies evaluated is the sample size. Because they all had small sample sizes, it is difficult to definitively conclude that the combination TXA and microneedling is an effective and appropriate treatment for patients with melasma. Furthermore, the quality of these studies was mostly dependent on subjectivity of the mMASI scores. Future large randomized controlled trials with a diverse participant population are needed to assess the effectiveness of TXA and microneedling in melasma treatment.
Another limitation is that many of the studies did not follow the patients longitudinally, which did not allow for an evaluation of whether patients had a relapse of melasma. Due to the chronic nature of melasma and frequent disease recurrence, future longitudinal studies are needed to monitor for disease recurrence.
Conclusion
Tranexamic acid and microneedling are potential treatment options for patients with melasma, and combination therapy appears more effective than either TXA or microneedling alone at providing sustained improvement of melasma lesions. Combination therapy appears safe and well tolerated, but its effect on reducing long-term disease recurrence is yet to be established.
- Neagu N, Conforti C, Agozzino M, et al. Melasma treatment: a systematic review. J Dermatolog Treat. 2022;33:1816-1837. doi:10.1080/09546634.2021.1914313
- Ogbechie-Godec OA, Elbuluk N. Melasma: an up-to-date comprehensive review. Dermatol Ther (Heidelb). 2017;7:305-318. doi:10.1007/s13555-017-0194-1
- Mahajan VK, Patil A, Blicharz L, et al. Medical therapies for melasma. J Cosmet Dermatol. 2022;21:3707-3728. doi:10.1111/jocd.15242
- Rigopoulos D, Gregoriou S, Katsambas A. Hyperpigmentation and melasma. J Cosmet Dermatol. 2007;6:195-202. doi:10.1111/j.1473-2165.2007.00321.x
- Kagha K, Fabi S, Goldman M. Melasma’s impact on quality of life. J Drugs Dermatol. 2020;19:184-187. doi:10.36849/JDD.2020.4663
- Lutfi RJ, Fridmanis M, Misiunas AL, et al. Association of melasma with thyroid autoimmunity and other thyroidal abnormalities and their relationship to the origin of the melasma. J Clin Endocrinol Metab. 1985;61:28-31. doi:10.1210/jcem-61-1-28
- Handel AC, Lima PB, Tonolli VM, et al. Risk factors for facial melasma in women: a case-control study. Br J Dermatol. 2014;171:588-594. doi:10.1111/bjd.13059
- Filoni A, Mariano M, Cameli N. Melasma: how hormones can modulate skin pigmentation. J Cosmet Dermatol. 2019;18:458-463. doi:10.1111/jocd.12877
- Rodrigues M, Pandya AG. Melasma: clinical diagnosis and management options. Australasian J Dermatol. 2015;56:151-163.
- Huerth KA, Hassan S, Callender VD. Therapeutic insights in melasma and hyperpigmentation management. J Drugs Dermatol. 2019;18:718-727.
- Pandya AG, Hynan LS, Bhore R, et al. Reliability assessment and validation of the Melasma Area and Severity Index (MASI) and a new modified MASI scoring method. J Am Acad Dermatol. 2011;64:78-83.e832. doi:10.1016/j.jaad.2009.10.051
- Rodrigues M, Ayala-Cortés AS, Rodríguez-Arámbula A, et al. Interpretability of the modified Melasma Area and Severity Index (mMASI). JAMA Dermatol. 2016;152:1051-1052. doi:10.1001/jamadermatol.2016.1006
- Ikino JK, Nunes DH, da Silva VPM, et al. Melasma and assessment of the quality of life in Brazilian women. An Bras Dermatol. 2015;90:196-200. doi:10.1590/abd1806-4841.20152771
- Taraz M, Niknam S, Ehsani AH. Tranexamic acid in treatment of melasma: a comprehensive review of clinical studies. Dermatolog Ther. 2017;30:E12465. doi:10.1111/dth.12465
- Bala HR, Lee S, Wong C, et al. Oral tranexamic acid for the treatment of melasma: a review. Dermatol Surg. 2018;44:814-825. doi:10.1097/DSS.0000000000001518
- Singh A, Yadav S. Microneedling: advances and widening horizons. Indian Dermatol Online J. 2016;7:244-254. doi:10.4103/2229-5178.185468
- Del Rosario E, Florez-Pollack S, Zapata L, et al. Randomized, placebo-controlled, double-blind study of oral tranexamic acid in the treatment of moderate-to-severe melasma. J Am Acad Dermatol. 2018;78:363-369. doi:10.1016/j.jaad.2017.09.053
- El Attar Y, Doghaim N, El Far N, et al. Efficacy and safety of tranexamic acid versus vitamin C after microneedling in treatment of melasma: clinical and dermoscopic study. J Cosmet Dermatol. 2022;21:2817-2825. doi:10.1111/jocd.14538
- Saleh FY, Abdel-Azim ES, Ragaie MH, et al. Topical tranexamic acid with microneedling versus microneedling alone in treatment of melasma: clinical, histopathologic, and immunohistochemical study. J Egyptian Womens Dermatolog Soc. 2019;16:89-96. doi:10.4103/jewd.jewd_25_19
- Xu Y, Ma R, Juliandri J, et al. Efficacy of functional microarray of microneedles combined with topical tranexamic acid for melasma: a randomized, self-controlled, split-face study. Medicine (Baltimore). 2017;96:e6897. doi:10.1097/MD.0000000000006897
- Kaur A, Bhalla M, Pal Thami G, et al. Clinical efficacy of topical tranexamic acid with microneedling in melasma. Dermatol Surg. 2020;46:E96-E101. doi:10.1097/DSS.0000000000002520
- Ebrahim HM, Said Abdelshafy A, Khattab F, et al. Tranexamic acid for melasma treatment: a split-face study. Dermatol Surg. 2020;46:E102-E107. doi:10.1097/DSS.0000000000002449
- Saki N, Darayesh M, Heiran A. Comparing the efficacy of topical hydroquinone 2% versus intradermal tranexamic acid microinjections in treating melasma: a split-face controlled trial. J Dermatolog Treat. 2018;29:405-410. doi:10.1080/09546634.2017.1392476
- Sharma R, Mahajan VK, Mehta KS, et al. Therapeutic efficacy and safety of oral tranexamic acid and that of tranexamic acid local infiltration with microinjections in patients with melasma: a comparative study. Clin Exp Dermatol. 2017;42:728-734. doi:10.1111/ced.13164
- Cassiano D, Esposito ACC, Hassun K, et al. Efficacy and safety of microneedling and oral tranexamic acid in the treatment of facial melasma in women: an open, evaluator-blinded, randomized clinical trial. J Am Acad Dermatol. 2020;83:1176-1178. doi:10.1016/j.jaad.2020.02.002
- Shamsi Meymandi S, Mozayyeni A, Shamsi Meymandi M, et al. Efficacy of microneedling plus topical 4% tranexamic acid solution vs 4% hydroquinone in the treatment of melasma: a single-blind randomized clinical trial. J Cosmet Dermatol. 2020;19:2906-2911. doi:10.1111/jocd.13392
- Xing X, Chen L, Xu Z, et al. The efficacy and safety of topical tranexamic acid (liposomal or lotion with microneedling) versus conventional hydroquinone in the treatment of melasma. J Cosmet Dermatol. 2020;19:3238-3244. doi:10.1111/jocd.13810
- Zaky MS, Obaid ZM, Khalil EA, et al. Microneedling-assisted topical tranexamic acid solution versus 4% hydroquinone for treating melasma: a split-face randomized study. J Cosmet Dermatol. 2021;20:4011-4016. doi:10.1111/jocd.14440
Melasma (also known as chloasma faciei) is a common chronic skin disorder that results in well-demarcated, hyperpigmented, tan to dark patches that mostly appear in sun-exposed areas such as the face and neck and sometimes the arms. The exact prevalence or incidence is not known but is estimated to be 1% to 50% overall depending on the ethnic population and geographic location.1,2 Melasma predominantly affects women, but research has shown that approximately 10% to 20% of men are affected by this condition.3,4 Although melasma can affect patients of all skin types, it primarily affects those with darker skin tones.5 The groups most often affected are women of Black, Hispanic, Middle Eastern, and Southeast Asian ethnicity. Although the pathogenesis is complex and not fully understood, multiple pathways and etiologies have been theorized to cause melasma. Potential causes include exposure to UV radiation, oral contraceptives, hormonal changes, medications, thyroid dysfunction, genetics, and pregnancy.6,7 Cytokines and growth factors, including adipokine and angiopoietin, synthesized by sebaceous glands play a role in the pathogenic mechanism of melasma. Cytokines and growth factors are hypothesized to modulate the function of melanocytes.8 Both melanocytes and sebocytes are controlled by α–melanocyte-stimulating hormone. Therefore, overexpression of α–melanocyte-stimulating hormone will result in overproduction of these 2 cell types, resulting in melasma. Melasma can be classified into 4 subtypes using Wood lamp examination: epidermal, dermal, mixed, or indeterminate.3 Furthermore, melasma is divided into subgroups based on the location: malar region, mandibular region, and centrofacial patch pattern.9,10 The involvement of sebaceous glands in the pathogenesis of melasma may explain the predilection for the centrofacial region, which is the most common pattern.
The severity of melasma can be assessed using the melasma area and severity index (MASI), which is calculated by subjective assessment of 3 main factors: (1) facial area of involvement; (2) darkness of affected region; and (3) homogeneity, with the extent of melasma indicated by a score ranging from 0 to 48.11 The modified MASI (mMASI) subsequently was introduced to assist with assessing the severity of melasma and creating distinct ranges for mild, moderate, and severe cases, ranging from 0 (mild) to 24 (severe).12 Both indices are used in research to assess the improvement of melasma with treatment.
Patients with melasma report a decrease in quality of life, increased emotional stress, and lower self-esteem due to cosmesis.13 Treatment of melasma can be highly challenging and often is complicated by relapsing. Historically, the treatment of melasma has included the use of chemical lightening agents. Additional treatment options include the use of lasers and complex chemical peels,9,10 but these interventions may result in adverse outcomes for individuals with darker skin tones. The current gold-standard treatment is topical hydroquinone and broad-spectrum sunscreen. Although hydroquinone is effective in the treatment of melasma, relapse is common. The goal of melasma management is not only to treat acute hyperpigmentation but also to prevent relapse. Other therapies that currently are being explored for the clinically sustained treatment of melasma include tranexamic acid (TXA)(trans-4-[aminomethyl]cyclohexanecarboxylic acid),9,10 an antifibrinolytic agent routinely used to prevent blood loss during surgery and in the management of menorrhagia. It is a synthetic derivative of lysine and serves as a potent plasmin inhibitor by blocking the lysine-binding sites of plasminogen molecules, thus preventing the conversion of plasminogen to plasmin. It also prevents fibrinolysis and blood loss.
In addition to its hemostatic properties, TXA has been found to have hypopigmentation properties.14,15 Plasminogen also can be found in human epidermal basal cells and human keratinocytes, and it is postulated that TXA’s interaction with these cells explains its hypopigmentation properties. Both UV radiation and hormones activate plasminogen into plasmin, resulting in the activation of tyrosinase and melanogenesis.14,15 Tranexamic acid is postulated to inhibit the keratinocyte-plasminogen pathway, thus leading to the inhibition of UV-induced and hormone-induced pigmentation. Also, TXA serves as a competitive inhibitor for tyrosinase due to its structural similarity to tyrosine.15 The combination of these 2 mechanisms contributes to the skin-lightening effects of TXA, making it a potential treatment for melasma.
Furthermore, the use of microneedling is being explored as a treatment option for melasma. Microneedling creates microscopic punctures in the skin using tiny needles, resulting in a wound-healing response and skin resurfacing. The microneedling technique is utilized to create small holes in the skin, with needle depths that can be adjusted from 0.5 to 3.5 mm to target different layers of the dermis and allow for discreet application of TXA.16 We sought to look at the current literature on the use and effectiveness of microneedling in combination with TXA to treat melasma and prevent relapse.
Methods
A systematic review was performed of PubMed articles indexed for MEDLINE and Embase in November 2021 to compile available articles that studied TXA and microneedling as a treatment for melasma. The PubMed search terms were (melasma) AND (microneedling* OR ‘tranexamic acid’ OR TXA or TA). The Embase search terms were (cholasma OR melasma) AND (tranexamic acid OR TXA) AND (microneedling)(Figure). The search was then limited to ”randomized controlled trial” and ”clinical trial” in English-language journals. Duplicates were excluded. After thorough evaluation, articles that discussed the use of TXA in combination with treatment options other than microneedling also were excluded.

Results
The literature search yielded a total of 12 articles that assessed the effectiveness of TXA and microneedling for the treatment of melasma (Table).17-28 Several articles concluded that TXA was equally effective at reducing melasma lesions when compared with the standard treatment of hydroquinone. Some of the reviewed articles also demonstrated the effectiveness of microneedling in improving melasma lesions as a stand-alone treatment. These studies highlighted the enhanced efficacy of the combined treatment of TXA and microneedling compared with their individual uses.17-28
Comment
Melasma is a common chronic hyperpigmentation disorder, making its treatment clinically challenging. Many patients experience symptom relapses, and limited effective treatment options make achieving complete clearance difficult, underscoring the need for improved therapeutic approaches. Recently, researchers have explored alternative treatments to address the challenges of melasma management. Tranexamic acid is an antifibrinolytic used to prevent blood loss and has emerged as a potential treatment for melasma. Similarly, microneedling—a technique in which multiple punctures are made in the skin to activate and stimulate wound healing and skin rejuvenation—shows promise for melasma.
Oral TXA for Melasma—Oral TXA has been shown to reduce melasma lesions. Del Rosario et al17 recruited 44 women (39 of whom completed the study) with moderate to severe melasma and randomized them into 2 groups: oral TXA and placebo. This study demonstrated a 49% reduction in the mMASI score in all participants taking oral TXA (250 mg twice daily [BID]) compared with an 18% reduction in the control group (placebo capsule BID) after 3 months of treatment. In patients with moderate and severe melasma, 45% and 51% mMASI score reductions were reported in the treatment group, respectively, vs 16% and 19% score reductions in placebo group, respectively. These researchers concluded that oral TXA may be effective at treating moderate to severe melasma. Although patients with severe melasma had a better response to treatment, their improvement was not sustained compared with patients with moderate melasma after a 3-month posttreatment follow-up.17
Microneedling Plus TXA for Melasma—Microneedling alone has been shown to be effective for melasma. El Attar et al18 conducted a split-face study of microneedling (1.5-mm depth) plus topical TXA (0.5 mL)(right side of the face[treatment arm]) compared with microneedling (1.5-mm depth) plus topical vitamin C (0.5 mL)(left side of the face [control group]) in 20 women with melasma. The sessions were repeated every 2 weeks for a total of 6 sessions. Although researchers found no statistically significant differences between the 2 treatment sides, microneedling plus TXA showed a slight advantage over microneedling plus vitamin C in dermoscopic examination. Both sides showed improvement in pigmented lesions, but vitamin C–treated lesions did not show an improvement in vascularity vs TXA.18
Saleh et al19 further showed that combination treatment with microneedling and TXA may improve clinical outcomes better than microneedling alone. Their study demonstrated a reduction in MASI score that was significantly higher in the combination treatment group compared with the microneedling alone group (P=.001). There was a significant reduction in melanoma antigen recognized by T cells 1 (MART-1)–positive cells in the combination treatment group compared with the microneedling alone group (P=.001). Lastly, combined therapy improved melasma patches better than microneedling alone.19



Xu et al20 conducted a split-face study (N=28) exploring the effectiveness of transdermal application of topical TXA using a microarray pen with microneedles (vibration at 3000×/min) plus topical TXA on one side of the face, while the other side received only topical TXA as a control. After 12 weeks of treatment, combination therapy with microneedling and TXA decreased brown spot scores, lowered melanin index (MI) values, improved blinded physician assessment, and improved patient satisfaction vs TXA therapy alone.20
Kaur et al21 conducted a split-face, randomized, controlled trial of microneedling (1-mm depth) with TXA solution 10% vs microneedling (1-mm depth) with distilled water alone for 8 weeks (N=40). They graded participant responses to treatment using reductions in mMASI scores12 at every 2 weeks of follow-up (no response, minimal or poor response=0%–25%; partial or fair response=26%–50%; good response=51%–75%; and excellent response=>75%). They reported an overall reduction in mMASI scores for both the treatment side and the control side in all participants, showing a 65.92% improvement in mean mMASI scores on the treatment side vs 20.75% improvement on the control side at week 8. Both sides showed statistically significant reductions in mean mMASI scores (P<.05). Clinically, 40% (16/40) of participants showed an excellent response to combined treatment compared with 0% (0/40) to microneedling alone. Overall, patient satisfaction was similar across both groups. This study demonstrated that microneedling alone improves melasma, but a combination of microneedling plus TXA showed a better clinical reduction in melasma. However, the researchers did not follow up with participants posttreatment, so it remains unclear if the improved clinical outcomes were sustained long-term.21
Ebrahim et al22 reported that the combination of 0.5 mL TXA (4 mg/mL) and microneedling (0.25- to 1-mm depth) was effective for melasma. Although there was improvement within microneedling and TXA, the study also showed that intradermal injection of TXA was significant in reducing mean mMASI scores and improving melasma (P<.001). The reduction in mMASI scores for the group receiving intradermal injections of TXA (left side; 74.8% reduction in mean mMASI score) vs the group receiving microneedling application of TXA (right side; 73.6% reduction in mean mMASI score) was not statistically significant. These findings suggest that the mode of TXA application may not be critical in determining clinical responses to TXA treatment. Although there was no reported statistically significant difference in clinical outcomes between the 2 treatments, patient satisfaction was higher on the microneedling side. Only 8 of 50 participants (16%) experienced recurrence 3 months posttreatment.22
Saki et al23 compared the efficacy of topical hydroquinone (2%) to intradermal TXA injections in treating melasma. They found intradermal TXA injections to be a clinically effective mode of treatment.23
Sharma et al24 explored the efficacy and safety of oral TXA by randomly assigning 100 Indian patients (20 of whom withdrew before study completion) with melasma into 2 groups: group A received TXA 250 mg twice daily, and group B received intradermal microinjections of TXA (4 mg/mL) every 4 weeks. The MASI scores were assessed at 4-week intervals for a total of 12 weeks. There was a decrease in MASI scores in both groups, and there was no statistically significant difference in mean percentage reduction in MASI scores between the 2 routes of drug administration, further suggesting the effectiveness of TXA independent of administration route. Two patients in group A relapsed at 24 weeks, and there were no relapses in group B, which may suggest a minimal superiority of TXA plus microneedling at providing more sustainable results compared with oral TXA alone. A notable limitation of this study was a high dropout rate as well as lack of long-term follow-up with participants, limiting the generalizability of the conclusions.24
Cassiano et al25 assigned 64 women with melasma to 1 of 3 treatment groups or a control group to compare the effectiveness of microneedling (M group: 1.5 mm; 2 sessions), oral TXA (T group: 250 mg/d twice daily for 60 days), and a combination of microneedling (2 sessions) and oral TXA (MT group: 250 mg/d twice daily for 60 days)with placebo for clinically reducing melasma lesions. The intervention period was 60 days followed by a 60-day maintenance phase for a total study period of 120 days. The researchers evaluated mMASI scores, quality of life, and difference in colorimetric luminosity. All treatment groups showed a reduction in mMASI scores at both 30 days and 60 days, indicating improved melasma severity. The MT and T groups had more significant improvement at 30 days compared with the control group (P<.03), suggesting that microneedling plus TXA and TXA alone promote faster improvement in melasma lesions. By 60 days, the M, T, and MT groups outperformed the control group, with no significant differences between the M, T, and MT groups. However, at the 120-day maintenance follow-up, the T group did not maintain its improvement compared with the control group. The M and MT groups showed no significance difference in effectiveness at 120 days, suggesting that microneedling may promote less frequent relapse and sustained remission compared to TXA alone.25
Hydroquinone for Melasma—Additional studies on the use of TXA treatments show that TXA may be an equally effective alternative to the standard use of hydroquinone treatment. Shamsi Meymandi et al26 did not find a statistically significant difference in treatment with TXA plus microneedling vs the standard regimen of hydroquinone. More importantly, patient and physician satisfaction assessments were similar between the 2 groups. Compared to hydroquinone, nightly treatment is not necessary with microneedling and TXA.26
Xing et al27 supported these conclusions with their study. They compared 3 study arms for a duration of 12 weeks: group A received topical 1.8% liposomal TXA BID, group B received stamp-mode electric microneedling with 5% TXA weekly, and group C applied 2% hydroquinone cream nightly. The study concluded that all 3 groups showed a significant reduction in mean MI by the end of the study, but a better MI improvement was observed in groups B and C (both P<.001) compared with group A (P<.01).27
Zaky et al28 showed that both hydroquinone and combination treatment of TXA plus microneedling are effective at improving melasma lesions. Further studies are needed to definitively conclude if combination treatment is more efficacious than hydroquinone; if the combination is more effective, it provides a treatment option for patients with melasma who may not be good candidates for hydroquinone treatment.
Study Limitations—One limitation in all the studies evaluated is the sample size. Because they all had small sample sizes, it is difficult to definitively conclude that the combination TXA and microneedling is an effective and appropriate treatment for patients with melasma. Furthermore, the quality of these studies was mostly dependent on subjectivity of the mMASI scores. Future large randomized controlled trials with a diverse participant population are needed to assess the effectiveness of TXA and microneedling in melasma treatment.
Another limitation is that many of the studies did not follow the patients longitudinally, which did not allow for an evaluation of whether patients had a relapse of melasma. Due to the chronic nature of melasma and frequent disease recurrence, future longitudinal studies are needed to monitor for disease recurrence.
Conclusion
Tranexamic acid and microneedling are potential treatment options for patients with melasma, and combination therapy appears more effective than either TXA or microneedling alone at providing sustained improvement of melasma lesions. Combination therapy appears safe and well tolerated, but its effect on reducing long-term disease recurrence is yet to be established.
Melasma (also known as chloasma faciei) is a common chronic skin disorder that results in well-demarcated, hyperpigmented, tan to dark patches that mostly appear in sun-exposed areas such as the face and neck and sometimes the arms. The exact prevalence or incidence is not known but is estimated to be 1% to 50% overall depending on the ethnic population and geographic location.1,2 Melasma predominantly affects women, but research has shown that approximately 10% to 20% of men are affected by this condition.3,4 Although melasma can affect patients of all skin types, it primarily affects those with darker skin tones.5 The groups most often affected are women of Black, Hispanic, Middle Eastern, and Southeast Asian ethnicity. Although the pathogenesis is complex and not fully understood, multiple pathways and etiologies have been theorized to cause melasma. Potential causes include exposure to UV radiation, oral contraceptives, hormonal changes, medications, thyroid dysfunction, genetics, and pregnancy.6,7 Cytokines and growth factors, including adipokine and angiopoietin, synthesized by sebaceous glands play a role in the pathogenic mechanism of melasma. Cytokines and growth factors are hypothesized to modulate the function of melanocytes.8 Both melanocytes and sebocytes are controlled by α–melanocyte-stimulating hormone. Therefore, overexpression of α–melanocyte-stimulating hormone will result in overproduction of these 2 cell types, resulting in melasma. Melasma can be classified into 4 subtypes using Wood lamp examination: epidermal, dermal, mixed, or indeterminate.3 Furthermore, melasma is divided into subgroups based on the location: malar region, mandibular region, and centrofacial patch pattern.9,10 The involvement of sebaceous glands in the pathogenesis of melasma may explain the predilection for the centrofacial region, which is the most common pattern.
The severity of melasma can be assessed using the melasma area and severity index (MASI), which is calculated by subjective assessment of 3 main factors: (1) facial area of involvement; (2) darkness of affected region; and (3) homogeneity, with the extent of melasma indicated by a score ranging from 0 to 48.11 The modified MASI (mMASI) subsequently was introduced to assist with assessing the severity of melasma and creating distinct ranges for mild, moderate, and severe cases, ranging from 0 (mild) to 24 (severe).12 Both indices are used in research to assess the improvement of melasma with treatment.
Patients with melasma report a decrease in quality of life, increased emotional stress, and lower self-esteem due to cosmesis.13 Treatment of melasma can be highly challenging and often is complicated by relapsing. Historically, the treatment of melasma has included the use of chemical lightening agents. Additional treatment options include the use of lasers and complex chemical peels,9,10 but these interventions may result in adverse outcomes for individuals with darker skin tones. The current gold-standard treatment is topical hydroquinone and broad-spectrum sunscreen. Although hydroquinone is effective in the treatment of melasma, relapse is common. The goal of melasma management is not only to treat acute hyperpigmentation but also to prevent relapse. Other therapies that currently are being explored for the clinically sustained treatment of melasma include tranexamic acid (TXA)(trans-4-[aminomethyl]cyclohexanecarboxylic acid),9,10 an antifibrinolytic agent routinely used to prevent blood loss during surgery and in the management of menorrhagia. It is a synthetic derivative of lysine and serves as a potent plasmin inhibitor by blocking the lysine-binding sites of plasminogen molecules, thus preventing the conversion of plasminogen to plasmin. It also prevents fibrinolysis and blood loss.
In addition to its hemostatic properties, TXA has been found to have hypopigmentation properties.14,15 Plasminogen also can be found in human epidermal basal cells and human keratinocytes, and it is postulated that TXA’s interaction with these cells explains its hypopigmentation properties. Both UV radiation and hormones activate plasminogen into plasmin, resulting in the activation of tyrosinase and melanogenesis.14,15 Tranexamic acid is postulated to inhibit the keratinocyte-plasminogen pathway, thus leading to the inhibition of UV-induced and hormone-induced pigmentation. Also, TXA serves as a competitive inhibitor for tyrosinase due to its structural similarity to tyrosine.15 The combination of these 2 mechanisms contributes to the skin-lightening effects of TXA, making it a potential treatment for melasma.
Furthermore, the use of microneedling is being explored as a treatment option for melasma. Microneedling creates microscopic punctures in the skin using tiny needles, resulting in a wound-healing response and skin resurfacing. The microneedling technique is utilized to create small holes in the skin, with needle depths that can be adjusted from 0.5 to 3.5 mm to target different layers of the dermis and allow for discreet application of TXA.16 We sought to look at the current literature on the use and effectiveness of microneedling in combination with TXA to treat melasma and prevent relapse.
Methods
A systematic review was performed of PubMed articles indexed for MEDLINE and Embase in November 2021 to compile available articles that studied TXA and microneedling as a treatment for melasma. The PubMed search terms were (melasma) AND (microneedling* OR ‘tranexamic acid’ OR TXA or TA). The Embase search terms were (cholasma OR melasma) AND (tranexamic acid OR TXA) AND (microneedling)(Figure). The search was then limited to ”randomized controlled trial” and ”clinical trial” in English-language journals. Duplicates were excluded. After thorough evaluation, articles that discussed the use of TXA in combination with treatment options other than microneedling also were excluded.

Results
The literature search yielded a total of 12 articles that assessed the effectiveness of TXA and microneedling for the treatment of melasma (Table).17-28 Several articles concluded that TXA was equally effective at reducing melasma lesions when compared with the standard treatment of hydroquinone. Some of the reviewed articles also demonstrated the effectiveness of microneedling in improving melasma lesions as a stand-alone treatment. These studies highlighted the enhanced efficacy of the combined treatment of TXA and microneedling compared with their individual uses.17-28
Comment
Melasma is a common chronic hyperpigmentation disorder, making its treatment clinically challenging. Many patients experience symptom relapses, and limited effective treatment options make achieving complete clearance difficult, underscoring the need for improved therapeutic approaches. Recently, researchers have explored alternative treatments to address the challenges of melasma management. Tranexamic acid is an antifibrinolytic used to prevent blood loss and has emerged as a potential treatment for melasma. Similarly, microneedling—a technique in which multiple punctures are made in the skin to activate and stimulate wound healing and skin rejuvenation—shows promise for melasma.
Oral TXA for Melasma—Oral TXA has been shown to reduce melasma lesions. Del Rosario et al17 recruited 44 women (39 of whom completed the study) with moderate to severe melasma and randomized them into 2 groups: oral TXA and placebo. This study demonstrated a 49% reduction in the mMASI score in all participants taking oral TXA (250 mg twice daily [BID]) compared with an 18% reduction in the control group (placebo capsule BID) after 3 months of treatment. In patients with moderate and severe melasma, 45% and 51% mMASI score reductions were reported in the treatment group, respectively, vs 16% and 19% score reductions in placebo group, respectively. These researchers concluded that oral TXA may be effective at treating moderate to severe melasma. Although patients with severe melasma had a better response to treatment, their improvement was not sustained compared with patients with moderate melasma after a 3-month posttreatment follow-up.17
Microneedling Plus TXA for Melasma—Microneedling alone has been shown to be effective for melasma. El Attar et al18 conducted a split-face study of microneedling (1.5-mm depth) plus topical TXA (0.5 mL)(right side of the face[treatment arm]) compared with microneedling (1.5-mm depth) plus topical vitamin C (0.5 mL)(left side of the face [control group]) in 20 women with melasma. The sessions were repeated every 2 weeks for a total of 6 sessions. Although researchers found no statistically significant differences between the 2 treatment sides, microneedling plus TXA showed a slight advantage over microneedling plus vitamin C in dermoscopic examination. Both sides showed improvement in pigmented lesions, but vitamin C–treated lesions did not show an improvement in vascularity vs TXA.18
Saleh et al19 further showed that combination treatment with microneedling and TXA may improve clinical outcomes better than microneedling alone. Their study demonstrated a reduction in MASI score that was significantly higher in the combination treatment group compared with the microneedling alone group (P=.001). There was a significant reduction in melanoma antigen recognized by T cells 1 (MART-1)–positive cells in the combination treatment group compared with the microneedling alone group (P=.001). Lastly, combined therapy improved melasma patches better than microneedling alone.19



Xu et al20 conducted a split-face study (N=28) exploring the effectiveness of transdermal application of topical TXA using a microarray pen with microneedles (vibration at 3000×/min) plus topical TXA on one side of the face, while the other side received only topical TXA as a control. After 12 weeks of treatment, combination therapy with microneedling and TXA decreased brown spot scores, lowered melanin index (MI) values, improved blinded physician assessment, and improved patient satisfaction vs TXA therapy alone.20
Kaur et al21 conducted a split-face, randomized, controlled trial of microneedling (1-mm depth) with TXA solution 10% vs microneedling (1-mm depth) with distilled water alone for 8 weeks (N=40). They graded participant responses to treatment using reductions in mMASI scores12 at every 2 weeks of follow-up (no response, minimal or poor response=0%–25%; partial or fair response=26%–50%; good response=51%–75%; and excellent response=>75%). They reported an overall reduction in mMASI scores for both the treatment side and the control side in all participants, showing a 65.92% improvement in mean mMASI scores on the treatment side vs 20.75% improvement on the control side at week 8. Both sides showed statistically significant reductions in mean mMASI scores (P<.05). Clinically, 40% (16/40) of participants showed an excellent response to combined treatment compared with 0% (0/40) to microneedling alone. Overall, patient satisfaction was similar across both groups. This study demonstrated that microneedling alone improves melasma, but a combination of microneedling plus TXA showed a better clinical reduction in melasma. However, the researchers did not follow up with participants posttreatment, so it remains unclear if the improved clinical outcomes were sustained long-term.21
Ebrahim et al22 reported that the combination of 0.5 mL TXA (4 mg/mL) and microneedling (0.25- to 1-mm depth) was effective for melasma. Although there was improvement within microneedling and TXA, the study also showed that intradermal injection of TXA was significant in reducing mean mMASI scores and improving melasma (P<.001). The reduction in mMASI scores for the group receiving intradermal injections of TXA (left side; 74.8% reduction in mean mMASI score) vs the group receiving microneedling application of TXA (right side; 73.6% reduction in mean mMASI score) was not statistically significant. These findings suggest that the mode of TXA application may not be critical in determining clinical responses to TXA treatment. Although there was no reported statistically significant difference in clinical outcomes between the 2 treatments, patient satisfaction was higher on the microneedling side. Only 8 of 50 participants (16%) experienced recurrence 3 months posttreatment.22
Saki et al23 compared the efficacy of topical hydroquinone (2%) to intradermal TXA injections in treating melasma. They found intradermal TXA injections to be a clinically effective mode of treatment.23
Sharma et al24 explored the efficacy and safety of oral TXA by randomly assigning 100 Indian patients (20 of whom withdrew before study completion) with melasma into 2 groups: group A received TXA 250 mg twice daily, and group B received intradermal microinjections of TXA (4 mg/mL) every 4 weeks. The MASI scores were assessed at 4-week intervals for a total of 12 weeks. There was a decrease in MASI scores in both groups, and there was no statistically significant difference in mean percentage reduction in MASI scores between the 2 routes of drug administration, further suggesting the effectiveness of TXA independent of administration route. Two patients in group A relapsed at 24 weeks, and there were no relapses in group B, which may suggest a minimal superiority of TXA plus microneedling at providing more sustainable results compared with oral TXA alone. A notable limitation of this study was a high dropout rate as well as lack of long-term follow-up with participants, limiting the generalizability of the conclusions.24
Cassiano et al25 assigned 64 women with melasma to 1 of 3 treatment groups or a control group to compare the effectiveness of microneedling (M group: 1.5 mm; 2 sessions), oral TXA (T group: 250 mg/d twice daily for 60 days), and a combination of microneedling (2 sessions) and oral TXA (MT group: 250 mg/d twice daily for 60 days)with placebo for clinically reducing melasma lesions. The intervention period was 60 days followed by a 60-day maintenance phase for a total study period of 120 days. The researchers evaluated mMASI scores, quality of life, and difference in colorimetric luminosity. All treatment groups showed a reduction in mMASI scores at both 30 days and 60 days, indicating improved melasma severity. The MT and T groups had more significant improvement at 30 days compared with the control group (P<.03), suggesting that microneedling plus TXA and TXA alone promote faster improvement in melasma lesions. By 60 days, the M, T, and MT groups outperformed the control group, with no significant differences between the M, T, and MT groups. However, at the 120-day maintenance follow-up, the T group did not maintain its improvement compared with the control group. The M and MT groups showed no significance difference in effectiveness at 120 days, suggesting that microneedling may promote less frequent relapse and sustained remission compared to TXA alone.25
Hydroquinone for Melasma—Additional studies on the use of TXA treatments show that TXA may be an equally effective alternative to the standard use of hydroquinone treatment. Shamsi Meymandi et al26 did not find a statistically significant difference in treatment with TXA plus microneedling vs the standard regimen of hydroquinone. More importantly, patient and physician satisfaction assessments were similar between the 2 groups. Compared to hydroquinone, nightly treatment is not necessary with microneedling and TXA.26
Xing et al27 supported these conclusions with their study. They compared 3 study arms for a duration of 12 weeks: group A received topical 1.8% liposomal TXA BID, group B received stamp-mode electric microneedling with 5% TXA weekly, and group C applied 2% hydroquinone cream nightly. The study concluded that all 3 groups showed a significant reduction in mean MI by the end of the study, but a better MI improvement was observed in groups B and C (both P<.001) compared with group A (P<.01).27
Zaky et al28 showed that both hydroquinone and combination treatment of TXA plus microneedling are effective at improving melasma lesions. Further studies are needed to definitively conclude if combination treatment is more efficacious than hydroquinone; if the combination is more effective, it provides a treatment option for patients with melasma who may not be good candidates for hydroquinone treatment.
Study Limitations—One limitation in all the studies evaluated is the sample size. Because they all had small sample sizes, it is difficult to definitively conclude that the combination TXA and microneedling is an effective and appropriate treatment for patients with melasma. Furthermore, the quality of these studies was mostly dependent on subjectivity of the mMASI scores. Future large randomized controlled trials with a diverse participant population are needed to assess the effectiveness of TXA and microneedling in melasma treatment.
Another limitation is that many of the studies did not follow the patients longitudinally, which did not allow for an evaluation of whether patients had a relapse of melasma. Due to the chronic nature of melasma and frequent disease recurrence, future longitudinal studies are needed to monitor for disease recurrence.
Conclusion
Tranexamic acid and microneedling are potential treatment options for patients with melasma, and combination therapy appears more effective than either TXA or microneedling alone at providing sustained improvement of melasma lesions. Combination therapy appears safe and well tolerated, but its effect on reducing long-term disease recurrence is yet to be established.
- Neagu N, Conforti C, Agozzino M, et al. Melasma treatment: a systematic review. J Dermatolog Treat. 2022;33:1816-1837. doi:10.1080/09546634.2021.1914313
- Ogbechie-Godec OA, Elbuluk N. Melasma: an up-to-date comprehensive review. Dermatol Ther (Heidelb). 2017;7:305-318. doi:10.1007/s13555-017-0194-1
- Mahajan VK, Patil A, Blicharz L, et al. Medical therapies for melasma. J Cosmet Dermatol. 2022;21:3707-3728. doi:10.1111/jocd.15242
- Rigopoulos D, Gregoriou S, Katsambas A. Hyperpigmentation and melasma. J Cosmet Dermatol. 2007;6:195-202. doi:10.1111/j.1473-2165.2007.00321.x
- Kagha K, Fabi S, Goldman M. Melasma’s impact on quality of life. J Drugs Dermatol. 2020;19:184-187. doi:10.36849/JDD.2020.4663
- Lutfi RJ, Fridmanis M, Misiunas AL, et al. Association of melasma with thyroid autoimmunity and other thyroidal abnormalities and their relationship to the origin of the melasma. J Clin Endocrinol Metab. 1985;61:28-31. doi:10.1210/jcem-61-1-28
- Handel AC, Lima PB, Tonolli VM, et al. Risk factors for facial melasma in women: a case-control study. Br J Dermatol. 2014;171:588-594. doi:10.1111/bjd.13059
- Filoni A, Mariano M, Cameli N. Melasma: how hormones can modulate skin pigmentation. J Cosmet Dermatol. 2019;18:458-463. doi:10.1111/jocd.12877
- Rodrigues M, Pandya AG. Melasma: clinical diagnosis and management options. Australasian J Dermatol. 2015;56:151-163.
- Huerth KA, Hassan S, Callender VD. Therapeutic insights in melasma and hyperpigmentation management. J Drugs Dermatol. 2019;18:718-727.
- Pandya AG, Hynan LS, Bhore R, et al. Reliability assessment and validation of the Melasma Area and Severity Index (MASI) and a new modified MASI scoring method. J Am Acad Dermatol. 2011;64:78-83.e832. doi:10.1016/j.jaad.2009.10.051
- Rodrigues M, Ayala-Cortés AS, Rodríguez-Arámbula A, et al. Interpretability of the modified Melasma Area and Severity Index (mMASI). JAMA Dermatol. 2016;152:1051-1052. doi:10.1001/jamadermatol.2016.1006
- Ikino JK, Nunes DH, da Silva VPM, et al. Melasma and assessment of the quality of life in Brazilian women. An Bras Dermatol. 2015;90:196-200. doi:10.1590/abd1806-4841.20152771
- Taraz M, Niknam S, Ehsani AH. Tranexamic acid in treatment of melasma: a comprehensive review of clinical studies. Dermatolog Ther. 2017;30:E12465. doi:10.1111/dth.12465
- Bala HR, Lee S, Wong C, et al. Oral tranexamic acid for the treatment of melasma: a review. Dermatol Surg. 2018;44:814-825. doi:10.1097/DSS.0000000000001518
- Singh A, Yadav S. Microneedling: advances and widening horizons. Indian Dermatol Online J. 2016;7:244-254. doi:10.4103/2229-5178.185468
- Del Rosario E, Florez-Pollack S, Zapata L, et al. Randomized, placebo-controlled, double-blind study of oral tranexamic acid in the treatment of moderate-to-severe melasma. J Am Acad Dermatol. 2018;78:363-369. doi:10.1016/j.jaad.2017.09.053
- El Attar Y, Doghaim N, El Far N, et al. Efficacy and safety of tranexamic acid versus vitamin C after microneedling in treatment of melasma: clinical and dermoscopic study. J Cosmet Dermatol. 2022;21:2817-2825. doi:10.1111/jocd.14538
- Saleh FY, Abdel-Azim ES, Ragaie MH, et al. Topical tranexamic acid with microneedling versus microneedling alone in treatment of melasma: clinical, histopathologic, and immunohistochemical study. J Egyptian Womens Dermatolog Soc. 2019;16:89-96. doi:10.4103/jewd.jewd_25_19
- Xu Y, Ma R, Juliandri J, et al. Efficacy of functional microarray of microneedles combined with topical tranexamic acid for melasma: a randomized, self-controlled, split-face study. Medicine (Baltimore). 2017;96:e6897. doi:10.1097/MD.0000000000006897
- Kaur A, Bhalla M, Pal Thami G, et al. Clinical efficacy of topical tranexamic acid with microneedling in melasma. Dermatol Surg. 2020;46:E96-E101. doi:10.1097/DSS.0000000000002520
- Ebrahim HM, Said Abdelshafy A, Khattab F, et al. Tranexamic acid for melasma treatment: a split-face study. Dermatol Surg. 2020;46:E102-E107. doi:10.1097/DSS.0000000000002449
- Saki N, Darayesh M, Heiran A. Comparing the efficacy of topical hydroquinone 2% versus intradermal tranexamic acid microinjections in treating melasma: a split-face controlled trial. J Dermatolog Treat. 2018;29:405-410. doi:10.1080/09546634.2017.1392476
- Sharma R, Mahajan VK, Mehta KS, et al. Therapeutic efficacy and safety of oral tranexamic acid and that of tranexamic acid local infiltration with microinjections in patients with melasma: a comparative study. Clin Exp Dermatol. 2017;42:728-734. doi:10.1111/ced.13164
- Cassiano D, Esposito ACC, Hassun K, et al. Efficacy and safety of microneedling and oral tranexamic acid in the treatment of facial melasma in women: an open, evaluator-blinded, randomized clinical trial. J Am Acad Dermatol. 2020;83:1176-1178. doi:10.1016/j.jaad.2020.02.002
- Shamsi Meymandi S, Mozayyeni A, Shamsi Meymandi M, et al. Efficacy of microneedling plus topical 4% tranexamic acid solution vs 4% hydroquinone in the treatment of melasma: a single-blind randomized clinical trial. J Cosmet Dermatol. 2020;19:2906-2911. doi:10.1111/jocd.13392
- Xing X, Chen L, Xu Z, et al. The efficacy and safety of topical tranexamic acid (liposomal or lotion with microneedling) versus conventional hydroquinone in the treatment of melasma. J Cosmet Dermatol. 2020;19:3238-3244. doi:10.1111/jocd.13810
- Zaky MS, Obaid ZM, Khalil EA, et al. Microneedling-assisted topical tranexamic acid solution versus 4% hydroquinone for treating melasma: a split-face randomized study. J Cosmet Dermatol. 2021;20:4011-4016. doi:10.1111/jocd.14440
- Neagu N, Conforti C, Agozzino M, et al. Melasma treatment: a systematic review. J Dermatolog Treat. 2022;33:1816-1837. doi:10.1080/09546634.2021.1914313
- Ogbechie-Godec OA, Elbuluk N. Melasma: an up-to-date comprehensive review. Dermatol Ther (Heidelb). 2017;7:305-318. doi:10.1007/s13555-017-0194-1
- Mahajan VK, Patil A, Blicharz L, et al. Medical therapies for melasma. J Cosmet Dermatol. 2022;21:3707-3728. doi:10.1111/jocd.15242
- Rigopoulos D, Gregoriou S, Katsambas A. Hyperpigmentation and melasma. J Cosmet Dermatol. 2007;6:195-202. doi:10.1111/j.1473-2165.2007.00321.x
- Kagha K, Fabi S, Goldman M. Melasma’s impact on quality of life. J Drugs Dermatol. 2020;19:184-187. doi:10.36849/JDD.2020.4663
- Lutfi RJ, Fridmanis M, Misiunas AL, et al. Association of melasma with thyroid autoimmunity and other thyroidal abnormalities and their relationship to the origin of the melasma. J Clin Endocrinol Metab. 1985;61:28-31. doi:10.1210/jcem-61-1-28
- Handel AC, Lima PB, Tonolli VM, et al. Risk factors for facial melasma in women: a case-control study. Br J Dermatol. 2014;171:588-594. doi:10.1111/bjd.13059
- Filoni A, Mariano M, Cameli N. Melasma: how hormones can modulate skin pigmentation. J Cosmet Dermatol. 2019;18:458-463. doi:10.1111/jocd.12877
- Rodrigues M, Pandya AG. Melasma: clinical diagnosis and management options. Australasian J Dermatol. 2015;56:151-163.
- Huerth KA, Hassan S, Callender VD. Therapeutic insights in melasma and hyperpigmentation management. J Drugs Dermatol. 2019;18:718-727.
- Pandya AG, Hynan LS, Bhore R, et al. Reliability assessment and validation of the Melasma Area and Severity Index (MASI) and a new modified MASI scoring method. J Am Acad Dermatol. 2011;64:78-83.e832. doi:10.1016/j.jaad.2009.10.051
- Rodrigues M, Ayala-Cortés AS, Rodríguez-Arámbula A, et al. Interpretability of the modified Melasma Area and Severity Index (mMASI). JAMA Dermatol. 2016;152:1051-1052. doi:10.1001/jamadermatol.2016.1006
- Ikino JK, Nunes DH, da Silva VPM, et al. Melasma and assessment of the quality of life in Brazilian women. An Bras Dermatol. 2015;90:196-200. doi:10.1590/abd1806-4841.20152771
- Taraz M, Niknam S, Ehsani AH. Tranexamic acid in treatment of melasma: a comprehensive review of clinical studies. Dermatolog Ther. 2017;30:E12465. doi:10.1111/dth.12465
- Bala HR, Lee S, Wong C, et al. Oral tranexamic acid for the treatment of melasma: a review. Dermatol Surg. 2018;44:814-825. doi:10.1097/DSS.0000000000001518
- Singh A, Yadav S. Microneedling: advances and widening horizons. Indian Dermatol Online J. 2016;7:244-254. doi:10.4103/2229-5178.185468
- Del Rosario E, Florez-Pollack S, Zapata L, et al. Randomized, placebo-controlled, double-blind study of oral tranexamic acid in the treatment of moderate-to-severe melasma. J Am Acad Dermatol. 2018;78:363-369. doi:10.1016/j.jaad.2017.09.053
- El Attar Y, Doghaim N, El Far N, et al. Efficacy and safety of tranexamic acid versus vitamin C after microneedling in treatment of melasma: clinical and dermoscopic study. J Cosmet Dermatol. 2022;21:2817-2825. doi:10.1111/jocd.14538
- Saleh FY, Abdel-Azim ES, Ragaie MH, et al. Topical tranexamic acid with microneedling versus microneedling alone in treatment of melasma: clinical, histopathologic, and immunohistochemical study. J Egyptian Womens Dermatolog Soc. 2019;16:89-96. doi:10.4103/jewd.jewd_25_19
- Xu Y, Ma R, Juliandri J, et al. Efficacy of functional microarray of microneedles combined with topical tranexamic acid for melasma: a randomized, self-controlled, split-face study. Medicine (Baltimore). 2017;96:e6897. doi:10.1097/MD.0000000000006897
- Kaur A, Bhalla M, Pal Thami G, et al. Clinical efficacy of topical tranexamic acid with microneedling in melasma. Dermatol Surg. 2020;46:E96-E101. doi:10.1097/DSS.0000000000002520
- Ebrahim HM, Said Abdelshafy A, Khattab F, et al. Tranexamic acid for melasma treatment: a split-face study. Dermatol Surg. 2020;46:E102-E107. doi:10.1097/DSS.0000000000002449
- Saki N, Darayesh M, Heiran A. Comparing the efficacy of topical hydroquinone 2% versus intradermal tranexamic acid microinjections in treating melasma: a split-face controlled trial. J Dermatolog Treat. 2018;29:405-410. doi:10.1080/09546634.2017.1392476
- Sharma R, Mahajan VK, Mehta KS, et al. Therapeutic efficacy and safety of oral tranexamic acid and that of tranexamic acid local infiltration with microinjections in patients with melasma: a comparative study. Clin Exp Dermatol. 2017;42:728-734. doi:10.1111/ced.13164
- Cassiano D, Esposito ACC, Hassun K, et al. Efficacy and safety of microneedling and oral tranexamic acid in the treatment of facial melasma in women: an open, evaluator-blinded, randomized clinical trial. J Am Acad Dermatol. 2020;83:1176-1178. doi:10.1016/j.jaad.2020.02.002
- Shamsi Meymandi S, Mozayyeni A, Shamsi Meymandi M, et al. Efficacy of microneedling plus topical 4% tranexamic acid solution vs 4% hydroquinone in the treatment of melasma: a single-blind randomized clinical trial. J Cosmet Dermatol. 2020;19:2906-2911. doi:10.1111/jocd.13392
- Xing X, Chen L, Xu Z, et al. The efficacy and safety of topical tranexamic acid (liposomal or lotion with microneedling) versus conventional hydroquinone in the treatment of melasma. J Cosmet Dermatol. 2020;19:3238-3244. doi:10.1111/jocd.13810
- Zaky MS, Obaid ZM, Khalil EA, et al. Microneedling-assisted topical tranexamic acid solution versus 4% hydroquinone for treating melasma: a split-face randomized study. J Cosmet Dermatol. 2021;20:4011-4016. doi:10.1111/jocd.14440
Practice Points
- Combination therapy with tranexamic acid (TXA) and microneedling is a safe and effective treatment for melasma.
- Combining TXA with microneedling may result in decreased melasma relapse rates.
Could Targeting ‘Zombie Cells’ Extend a Healthy Lifespan?
What if a drug could help you live a longer, healthier life?
Scientists at the University of Connecticut are working on it. In a new study in Cell Metabolism, researchers described how to target specific cells to extend the lifespan and improve the health of mice late in life.
The study builds on a growing body of research, mostly in animals, testing interventions to slow aging and prolong health span, the length of time that one is not just alive but also healthy.
“Aging is the most important risk factor for every disease that we deal with in adult human beings,” said cardiologist Douglas Vaughan, MD, director of the Potocsnak Longevity Institute at Northwestern University’s Feinberg School of Medicine, Chicago. (Dr. Vaughan was not involved in the new study.) “So the big hypothesis is: If we could slow down aging just a little bit, we can push back the onset of disease.”
Senescent cells — or “zombie cells” — secrete harmful substances that disrupt tissue functioning. They’ve been linked to chronic inflammation, tissue damage, and the development of age-related diseases.
Senescence can be characterized by the accumulation of cells with high levels of specific markers like p21, or p21high cells. Almost any cell can become a p21high cell, and they accumulate with age, said Ming Xu, PhD, a professor at the UConn Center on Aging, UConn Health, Farmington, Connecticut, who led the study.
By targeting and eliminating p21high senescent cells, Dr. Xu hopes to develop novel therapies that might help people live longer and enjoy more years in good health.
Such a treatment could be ready for human trials in 2-5 years, Dr. Xu said.
What the Researchers Did
Xu and colleagues used genetic engineering to eliminate p21high cells in mice, introducing into their genome something they describe as an inducible “suicide gene.” Giving the mice a certain drug (a low dose of tamoxifen) activated the suicide gene in all p21high cells, causing them to die. Administering this treatment once a month, from age 20 months (older age) until the end of life, significantly extended the rodents’ lifespan, reduced inflammation, and decreased gene activity linked to aging.
Treated mice lived, on average, for 33 months — 3 months longer than the untreated mice. The oldest treated mouse lived to 43 months — roughly 130 in human years.
But the treated mice didn’t just live longer; they were also healthier. In humans, walking speed and grip strength can be clues of overall health and vitality. The old, treated mice were able to walk faster and grip objects with greater strength than untreated mice of the same age.
Dr. Xu’s lab is now testing drugs that target p21high cells in hopes of finding one that would work in humans. Leveraging immunotherapy technology to target these cells could be another option, Dr. Xu said.
The team also plans to test whether eliminating p21high cells could prevent or alleviate diabetes or Alzheimer’s disease.
Challenges and Criticisms
The research provides “important evidence that targeting senescence and the molecular components of that pathway might provide some benefit in the long term,” Dr. Vaughan said.
But killing senescent cells could come with downsides.
“Senescence protects us from hyperproliferative responses,” potentially blocking cells from becoming malignant, Dr. Vaughan said. “There’s this effect on aging that is desirable, but at the same time, you may enhance your risk of cancer or malignancy or excessive proliferation in some cells.”
And of course, we don’t necessarily need drugs to prolong healthy life, Dr. Vaughan pointed out.
For many people, a long healthy life is already within reach. Humans live longer on average than they used to, and simple lifestyle choices — nourishing your body well, staying active, and maintaining a healthy weight — can increase one’s chances of good health.
The most consistently demonstrated intervention for extending lifespan “in almost every animal species is caloric restriction,” Dr. Vaughan said. (Dr. Xu’s team is also investigating whether fasting and exercise can lead to a decrease in p21high cells.)
As for brain health, Dr. Vaughan and colleagues at Northwestern are studying “super agers,” people who are cognitively intact into their 90s.
“The one single thing that they found that contributes to that process, and contributes to that success, is really a social network and human bonds and interaction,” Dr. Vaughan said.
A version of this article appeared on Medscape.com.
What if a drug could help you live a longer, healthier life?
Scientists at the University of Connecticut are working on it. In a new study in Cell Metabolism, researchers described how to target specific cells to extend the lifespan and improve the health of mice late in life.
The study builds on a growing body of research, mostly in animals, testing interventions to slow aging and prolong health span, the length of time that one is not just alive but also healthy.
“Aging is the most important risk factor for every disease that we deal with in adult human beings,” said cardiologist Douglas Vaughan, MD, director of the Potocsnak Longevity Institute at Northwestern University’s Feinberg School of Medicine, Chicago. (Dr. Vaughan was not involved in the new study.) “So the big hypothesis is: If we could slow down aging just a little bit, we can push back the onset of disease.”
Senescent cells — or “zombie cells” — secrete harmful substances that disrupt tissue functioning. They’ve been linked to chronic inflammation, tissue damage, and the development of age-related diseases.
Senescence can be characterized by the accumulation of cells with high levels of specific markers like p21, or p21high cells. Almost any cell can become a p21high cell, and they accumulate with age, said Ming Xu, PhD, a professor at the UConn Center on Aging, UConn Health, Farmington, Connecticut, who led the study.
By targeting and eliminating p21high senescent cells, Dr. Xu hopes to develop novel therapies that might help people live longer and enjoy more years in good health.
Such a treatment could be ready for human trials in 2-5 years, Dr. Xu said.
What the Researchers Did
Xu and colleagues used genetic engineering to eliminate p21high cells in mice, introducing into their genome something they describe as an inducible “suicide gene.” Giving the mice a certain drug (a low dose of tamoxifen) activated the suicide gene in all p21high cells, causing them to die. Administering this treatment once a month, from age 20 months (older age) until the end of life, significantly extended the rodents’ lifespan, reduced inflammation, and decreased gene activity linked to aging.
Treated mice lived, on average, for 33 months — 3 months longer than the untreated mice. The oldest treated mouse lived to 43 months — roughly 130 in human years.
But the treated mice didn’t just live longer; they were also healthier. In humans, walking speed and grip strength can be clues of overall health and vitality. The old, treated mice were able to walk faster and grip objects with greater strength than untreated mice of the same age.
Dr. Xu’s lab is now testing drugs that target p21high cells in hopes of finding one that would work in humans. Leveraging immunotherapy technology to target these cells could be another option, Dr. Xu said.
The team also plans to test whether eliminating p21high cells could prevent or alleviate diabetes or Alzheimer’s disease.
Challenges and Criticisms
The research provides “important evidence that targeting senescence and the molecular components of that pathway might provide some benefit in the long term,” Dr. Vaughan said.
But killing senescent cells could come with downsides.
“Senescence protects us from hyperproliferative responses,” potentially blocking cells from becoming malignant, Dr. Vaughan said. “There’s this effect on aging that is desirable, but at the same time, you may enhance your risk of cancer or malignancy or excessive proliferation in some cells.”
And of course, we don’t necessarily need drugs to prolong healthy life, Dr. Vaughan pointed out.
For many people, a long healthy life is already within reach. Humans live longer on average than they used to, and simple lifestyle choices — nourishing your body well, staying active, and maintaining a healthy weight — can increase one’s chances of good health.
The most consistently demonstrated intervention for extending lifespan “in almost every animal species is caloric restriction,” Dr. Vaughan said. (Dr. Xu’s team is also investigating whether fasting and exercise can lead to a decrease in p21high cells.)
As for brain health, Dr. Vaughan and colleagues at Northwestern are studying “super agers,” people who are cognitively intact into their 90s.
“The one single thing that they found that contributes to that process, and contributes to that success, is really a social network and human bonds and interaction,” Dr. Vaughan said.
A version of this article appeared on Medscape.com.
What if a drug could help you live a longer, healthier life?
Scientists at the University of Connecticut are working on it. In a new study in Cell Metabolism, researchers described how to target specific cells to extend the lifespan and improve the health of mice late in life.
The study builds on a growing body of research, mostly in animals, testing interventions to slow aging and prolong health span, the length of time that one is not just alive but also healthy.
“Aging is the most important risk factor for every disease that we deal with in adult human beings,” said cardiologist Douglas Vaughan, MD, director of the Potocsnak Longevity Institute at Northwestern University’s Feinberg School of Medicine, Chicago. (Dr. Vaughan was not involved in the new study.) “So the big hypothesis is: If we could slow down aging just a little bit, we can push back the onset of disease.”
Senescent cells — or “zombie cells” — secrete harmful substances that disrupt tissue functioning. They’ve been linked to chronic inflammation, tissue damage, and the development of age-related diseases.
Senescence can be characterized by the accumulation of cells with high levels of specific markers like p21, or p21high cells. Almost any cell can become a p21high cell, and they accumulate with age, said Ming Xu, PhD, a professor at the UConn Center on Aging, UConn Health, Farmington, Connecticut, who led the study.
By targeting and eliminating p21high senescent cells, Dr. Xu hopes to develop novel therapies that might help people live longer and enjoy more years in good health.
Such a treatment could be ready for human trials in 2-5 years, Dr. Xu said.
What the Researchers Did
Xu and colleagues used genetic engineering to eliminate p21high cells in mice, introducing into their genome something they describe as an inducible “suicide gene.” Giving the mice a certain drug (a low dose of tamoxifen) activated the suicide gene in all p21high cells, causing them to die. Administering this treatment once a month, from age 20 months (older age) until the end of life, significantly extended the rodents’ lifespan, reduced inflammation, and decreased gene activity linked to aging.
Treated mice lived, on average, for 33 months — 3 months longer than the untreated mice. The oldest treated mouse lived to 43 months — roughly 130 in human years.
But the treated mice didn’t just live longer; they were also healthier. In humans, walking speed and grip strength can be clues of overall health and vitality. The old, treated mice were able to walk faster and grip objects with greater strength than untreated mice of the same age.
Dr. Xu’s lab is now testing drugs that target p21high cells in hopes of finding one that would work in humans. Leveraging immunotherapy technology to target these cells could be another option, Dr. Xu said.
The team also plans to test whether eliminating p21high cells could prevent or alleviate diabetes or Alzheimer’s disease.
Challenges and Criticisms
The research provides “important evidence that targeting senescence and the molecular components of that pathway might provide some benefit in the long term,” Dr. Vaughan said.
But killing senescent cells could come with downsides.
“Senescence protects us from hyperproliferative responses,” potentially blocking cells from becoming malignant, Dr. Vaughan said. “There’s this effect on aging that is desirable, but at the same time, you may enhance your risk of cancer or malignancy or excessive proliferation in some cells.”
And of course, we don’t necessarily need drugs to prolong healthy life, Dr. Vaughan pointed out.
For many people, a long healthy life is already within reach. Humans live longer on average than they used to, and simple lifestyle choices — nourishing your body well, staying active, and maintaining a healthy weight — can increase one’s chances of good health.
The most consistently demonstrated intervention for extending lifespan “in almost every animal species is caloric restriction,” Dr. Vaughan said. (Dr. Xu’s team is also investigating whether fasting and exercise can lead to a decrease in p21high cells.)
As for brain health, Dr. Vaughan and colleagues at Northwestern are studying “super agers,” people who are cognitively intact into their 90s.
“The one single thing that they found that contributes to that process, and contributes to that success, is really a social network and human bonds and interaction,” Dr. Vaughan said.
A version of this article appeared on Medscape.com.
Customized Dermal Curette: An Alternative and Effective Shaving Tool in Nail Surgery
Practice Gap
Longitudinal melanonychia (LM) is characterized by the presence of a dark brown, longitudinal, pigmented band on the nail unit, often caused by melanocytic activation or melanocytic hyperplasia in the nail matrix. Distinguishing between benign and early malignant LM is crucial due to their similar clinical presentations.1 Hence, surgical excision of the pigmented nail matrix followed by histopathologic examination is a common procedure aimed at managing LM and reducing the risk for delayed diagnosis of subungual melanoma.
Tangential matrix excision combined with the nail window technique has emerged as a common and favored surgical strategy for managing LM.2 This method is highly valued for its ability to minimize the risk for severe permanent nail dystrophy and effectively reduce postsurgical pigmentation recurrence.
The procedure begins with the creation of a matrix window along the lateral edge of the pigmented band followed by 1 lateral incision carefully made on each side of the nail fold. This meticulous approach allows for the complete exposure of the pigmented lesion. Subsequently, the nail fold is separated from the dorsal surface of the nail plate to facilitate access to the pigmented nail matrix. Finally, the target pigmented area is excised using a scalpel.
Despite the recognized efficacy of this procedure, challenges do arise, particularly when the width of the pigmented matrix lesion is narrow. Holding the scalpel horizontally to ensure precise excision can prove to be demanding, leading to difficulty achieving complete lesion removal and obtaining the desired cosmetic outcomes. As such, there is a clear need to explore alternative tools that can effectively address these challenges while ensuring optimal surgical outcomes for patients with LM. We propose the use of the customized dermal curette.
The Technique
An improved curette tool is a practical solution for complete removal of the pigmented nail matrix. This enhanced instrument is crafted from a sterile disposable dermal curette with its top flattened using a needle holder(Figure 1). Termed the customized dermal curette, this device is a simple yet accurate tool for the precise excision of pigmented lesions within the nail matrix. Importantly, it offers versatility by accommodating different widths of pigmented lesions through the availability of various sizes of dermal curettes (Figure 2).

Histopathologically, we have found that the scalpel technique may lead to variable tissue removal, resulting in differences in tissue thickness, fragility, and completeness (Figure 3A). Conversely, the customized dermal curette consistently provides more accurate tissue excision, resulting in uniform tissue thickness and integrity (Figure 3B).
Practice Implications
Compared to the traditional scalpel, this modified tool offers distinct advantages. Specifically, the customized dermal curette provides enhanced maneuverability and control during the procedure, thereby improving the overall efficacy of the excision process. It also offers a more accurate approach to completely remove pigmented bands, which reduces the risk for postoperative recurrence. The simplicity, affordability, and ease of operation associated with customized dermal curettes holds promise as an effective alternative for tissue shaving, especially in cases involving narrow pigmented matrix lesions, thereby addressing a notable practice gap and enhancing patient care.
- Tan WC, Wang DY, Seghers AC, et al. Should we biopsy melanonychia striata in Asian children? a retrospective observational study. Pediatr Dermatol. 2019;36:864-868. doi:10.1111/pde.13934
- Zhou Y, Chen W, Liu ZR, et al. Modified shave surgery combined with nail window technique for the treatment of longitudinal melanonychia: evaluation of the method on a series of 67 cases. J Am Acad Dermatol. 2019;81:717-722. doi:10.1016/j.jaad.2019.03.065
Practice Gap
Longitudinal melanonychia (LM) is characterized by the presence of a dark brown, longitudinal, pigmented band on the nail unit, often caused by melanocytic activation or melanocytic hyperplasia in the nail matrix. Distinguishing between benign and early malignant LM is crucial due to their similar clinical presentations.1 Hence, surgical excision of the pigmented nail matrix followed by histopathologic examination is a common procedure aimed at managing LM and reducing the risk for delayed diagnosis of subungual melanoma.
Tangential matrix excision combined with the nail window technique has emerged as a common and favored surgical strategy for managing LM.2 This method is highly valued for its ability to minimize the risk for severe permanent nail dystrophy and effectively reduce postsurgical pigmentation recurrence.
The procedure begins with the creation of a matrix window along the lateral edge of the pigmented band followed by 1 lateral incision carefully made on each side of the nail fold. This meticulous approach allows for the complete exposure of the pigmented lesion. Subsequently, the nail fold is separated from the dorsal surface of the nail plate to facilitate access to the pigmented nail matrix. Finally, the target pigmented area is excised using a scalpel.
Despite the recognized efficacy of this procedure, challenges do arise, particularly when the width of the pigmented matrix lesion is narrow. Holding the scalpel horizontally to ensure precise excision can prove to be demanding, leading to difficulty achieving complete lesion removal and obtaining the desired cosmetic outcomes. As such, there is a clear need to explore alternative tools that can effectively address these challenges while ensuring optimal surgical outcomes for patients with LM. We propose the use of the customized dermal curette.
The Technique
An improved curette tool is a practical solution for complete removal of the pigmented nail matrix. This enhanced instrument is crafted from a sterile disposable dermal curette with its top flattened using a needle holder(Figure 1). Termed the customized dermal curette, this device is a simple yet accurate tool for the precise excision of pigmented lesions within the nail matrix. Importantly, it offers versatility by accommodating different widths of pigmented lesions through the availability of various sizes of dermal curettes (Figure 2).


Histopathologically, we have found that the scalpel technique may lead to variable tissue removal, resulting in differences in tissue thickness, fragility, and completeness (Figure 3A). Conversely, the customized dermal curette consistently provides more accurate tissue excision, resulting in uniform tissue thickness and integrity (Figure 3B).

Practice Implications
Compared to the traditional scalpel, this modified tool offers distinct advantages. Specifically, the customized dermal curette provides enhanced maneuverability and control during the procedure, thereby improving the overall efficacy of the excision process. It also offers a more accurate approach to completely remove pigmented bands, which reduces the risk for postoperative recurrence. The simplicity, affordability, and ease of operation associated with customized dermal curettes holds promise as an effective alternative for tissue shaving, especially in cases involving narrow pigmented matrix lesions, thereby addressing a notable practice gap and enhancing patient care.
Practice Gap
Longitudinal melanonychia (LM) is characterized by the presence of a dark brown, longitudinal, pigmented band on the nail unit, often caused by melanocytic activation or melanocytic hyperplasia in the nail matrix. Distinguishing between benign and early malignant LM is crucial due to their similar clinical presentations.1 Hence, surgical excision of the pigmented nail matrix followed by histopathologic examination is a common procedure aimed at managing LM and reducing the risk for delayed diagnosis of subungual melanoma.
Tangential matrix excision combined with the nail window technique has emerged as a common and favored surgical strategy for managing LM.2 This method is highly valued for its ability to minimize the risk for severe permanent nail dystrophy and effectively reduce postsurgical pigmentation recurrence.
The procedure begins with the creation of a matrix window along the lateral edge of the pigmented band followed by 1 lateral incision carefully made on each side of the nail fold. This meticulous approach allows for the complete exposure of the pigmented lesion. Subsequently, the nail fold is separated from the dorsal surface of the nail plate to facilitate access to the pigmented nail matrix. Finally, the target pigmented area is excised using a scalpel.
Despite the recognized efficacy of this procedure, challenges do arise, particularly when the width of the pigmented matrix lesion is narrow. Holding the scalpel horizontally to ensure precise excision can prove to be demanding, leading to difficulty achieving complete lesion removal and obtaining the desired cosmetic outcomes. As such, there is a clear need to explore alternative tools that can effectively address these challenges while ensuring optimal surgical outcomes for patients with LM. We propose the use of the customized dermal curette.
The Technique
An improved curette tool is a practical solution for complete removal of the pigmented nail matrix. This enhanced instrument is crafted from a sterile disposable dermal curette with its top flattened using a needle holder(Figure 1). Termed the customized dermal curette, this device is a simple yet accurate tool for the precise excision of pigmented lesions within the nail matrix. Importantly, it offers versatility by accommodating different widths of pigmented lesions through the availability of various sizes of dermal curettes (Figure 2).


Histopathologically, we have found that the scalpel technique may lead to variable tissue removal, resulting in differences in tissue thickness, fragility, and completeness (Figure 3A). Conversely, the customized dermal curette consistently provides more accurate tissue excision, resulting in uniform tissue thickness and integrity (Figure 3B).

Practice Implications
Compared to the traditional scalpel, this modified tool offers distinct advantages. Specifically, the customized dermal curette provides enhanced maneuverability and control during the procedure, thereby improving the overall efficacy of the excision process. It also offers a more accurate approach to completely remove pigmented bands, which reduces the risk for postoperative recurrence. The simplicity, affordability, and ease of operation associated with customized dermal curettes holds promise as an effective alternative for tissue shaving, especially in cases involving narrow pigmented matrix lesions, thereby addressing a notable practice gap and enhancing patient care.
- Tan WC, Wang DY, Seghers AC, et al. Should we biopsy melanonychia striata in Asian children? a retrospective observational study. Pediatr Dermatol. 2019;36:864-868. doi:10.1111/pde.13934
- Zhou Y, Chen W, Liu ZR, et al. Modified shave surgery combined with nail window technique for the treatment of longitudinal melanonychia: evaluation of the method on a series of 67 cases. J Am Acad Dermatol. 2019;81:717-722. doi:10.1016/j.jaad.2019.03.065
- Tan WC, Wang DY, Seghers AC, et al. Should we biopsy melanonychia striata in Asian children? a retrospective observational study. Pediatr Dermatol. 2019;36:864-868. doi:10.1111/pde.13934
- Zhou Y, Chen W, Liu ZR, et al. Modified shave surgery combined with nail window technique for the treatment of longitudinal melanonychia: evaluation of the method on a series of 67 cases. J Am Acad Dermatol. 2019;81:717-722. doi:10.1016/j.jaad.2019.03.065
Study Links Melasma With Comorbidities, Races, Ethnicities
TOPLINE:
A study found significant associations between melasma and several comorbidities, including hypertension and hormonal contraception use, which were the most common.
METHODOLOGY:
- Melasma predominantly affects young women of color and often worsens in hyperestrogen states; understanding the association with comorbidities can improve surveillance and treatment strategies.
- Researchers evaluated 41,283 patients with melasma (mean age, 48.8 years; 93% women) from the TriNetX database and an equal number of matched control individuals.
- The main outcome was comorbidities including allergic rhinitis, atopic dermatitis, anticonvulsants, diabetes, hormonal contraceptives, hypothyroidism, hypertension, lupus, rosacea, skin cancer, and malignancy.
TAKEAWAY:
- Among those with melasma, 25% had hypertension and 24% used hormonal contraception, the two most commonly associated risk factors identified.
- Rosacea (odds ratio [OR], 5.1), atopic dermatitis (OR, 3.3), lupus (OR, 2.5), history of skin cancer (OR, 2.5), and history of internal malignancy (OR, 2.1) were associated with the highest risk of developing melasma (P < .01 for all).
- Asian (OR, 2.0; P < .01) and “other/unknown” races (OR, 1.7; P < .01) and Hispanic ethnicity (OR, 1.3; P < .01) were also significantly associated with melasma, while the odds were slightly lower among White, Black/African American, and “not Hispanic” groups (ORs, 0.8; P < .01 for all groups).
IN PRACTICE:
the authors wrote.
SOURCE:
The study, led by Ajay N. Sharma, MD, MBA, of the Department of Dermatology at the University of California, Irvine, was published online in Journal of Drugs in Dermatology.
LIMITATIONS:
The study limitations included the retrospective design, potential misclassification of diagnoses, and the inability to establish causality.
DISCLOSURES:
The study did not disclose any funding sources. The authors declared no conflicts of interest.
A version of this article first appeared on Medscape.com.
TOPLINE:
A study found significant associations between melasma and several comorbidities, including hypertension and hormonal contraception use, which were the most common.
METHODOLOGY:
- Melasma predominantly affects young women of color and often worsens in hyperestrogen states; understanding the association with comorbidities can improve surveillance and treatment strategies.
- Researchers evaluated 41,283 patients with melasma (mean age, 48.8 years; 93% women) from the TriNetX database and an equal number of matched control individuals.
- The main outcome was comorbidities including allergic rhinitis, atopic dermatitis, anticonvulsants, diabetes, hormonal contraceptives, hypothyroidism, hypertension, lupus, rosacea, skin cancer, and malignancy.
TAKEAWAY:
- Among those with melasma, 25% had hypertension and 24% used hormonal contraception, the two most commonly associated risk factors identified.
- Rosacea (odds ratio [OR], 5.1), atopic dermatitis (OR, 3.3), lupus (OR, 2.5), history of skin cancer (OR, 2.5), and history of internal malignancy (OR, 2.1) were associated with the highest risk of developing melasma (P < .01 for all).
- Asian (OR, 2.0; P < .01) and “other/unknown” races (OR, 1.7; P < .01) and Hispanic ethnicity (OR, 1.3; P < .01) were also significantly associated with melasma, while the odds were slightly lower among White, Black/African American, and “not Hispanic” groups (ORs, 0.8; P < .01 for all groups).
IN PRACTICE:
the authors wrote.
SOURCE:
The study, led by Ajay N. Sharma, MD, MBA, of the Department of Dermatology at the University of California, Irvine, was published online in Journal of Drugs in Dermatology.
LIMITATIONS:
The study limitations included the retrospective design, potential misclassification of diagnoses, and the inability to establish causality.
DISCLOSURES:
The study did not disclose any funding sources. The authors declared no conflicts of interest.
A version of this article first appeared on Medscape.com.
TOPLINE:
A study found significant associations between melasma and several comorbidities, including hypertension and hormonal contraception use, which were the most common.
METHODOLOGY:
- Melasma predominantly affects young women of color and often worsens in hyperestrogen states; understanding the association with comorbidities can improve surveillance and treatment strategies.
- Researchers evaluated 41,283 patients with melasma (mean age, 48.8 years; 93% women) from the TriNetX database and an equal number of matched control individuals.
- The main outcome was comorbidities including allergic rhinitis, atopic dermatitis, anticonvulsants, diabetes, hormonal contraceptives, hypothyroidism, hypertension, lupus, rosacea, skin cancer, and malignancy.
TAKEAWAY:
- Among those with melasma, 25% had hypertension and 24% used hormonal contraception, the two most commonly associated risk factors identified.
- Rosacea (odds ratio [OR], 5.1), atopic dermatitis (OR, 3.3), lupus (OR, 2.5), history of skin cancer (OR, 2.5), and history of internal malignancy (OR, 2.1) were associated with the highest risk of developing melasma (P < .01 for all).
- Asian (OR, 2.0; P < .01) and “other/unknown” races (OR, 1.7; P < .01) and Hispanic ethnicity (OR, 1.3; P < .01) were also significantly associated with melasma, while the odds were slightly lower among White, Black/African American, and “not Hispanic” groups (ORs, 0.8; P < .01 for all groups).
IN PRACTICE:
the authors wrote.
SOURCE:
The study, led by Ajay N. Sharma, MD, MBA, of the Department of Dermatology at the University of California, Irvine, was published online in Journal of Drugs in Dermatology.
LIMITATIONS:
The study limitations included the retrospective design, potential misclassification of diagnoses, and the inability to establish causality.
DISCLOSURES:
The study did not disclose any funding sources. The authors declared no conflicts of interest.
A version of this article first appeared on Medscape.com.
Pruritic Rash on the Neck and Back
The Diagnosis: Prurigo Pigmentosa
A comprehensive metabolic panel collected from our patient 1 month earlier did not reveal any abnormalities. Serum methylmalonic acid and homocysteine were both elevated at 417 nmol/L (reference range [for those aged 2–59 years], 55–335 nmol/L) and 23 μmol/L (reference range, 5–15 μmol/L), respectively. Serum folate and 25-hydroxyvitamin D were low at 3.1 ng/mL (reference range, >4.8 ng/mL) and 5 ng/mL (reference range, 30–80 ng/mL), respectively. Vitamin B12 was within reference range. Two 4-mm punch biopsies collected from the upper back showed spongiotic dermatitis.
Our patient’s histopathology results along with the rash distribution and medical history of anorexia increased suspicion for prurigo pigmentosa. A trial of oral doxycycline 100 mg twice daily for 2 weeks was prescribed. At 2-week follow-up, the patient’s mother revealed a history of ketosis in her daughter, solidifying the diagnosis. The patient was counseled on maintaining a healthy diet to prevent future breakouts. The patient’s rash resolved with diet modification and doxycycline; however, it recurred upon relapse of anorexia 4 months later.
Prurigo pigmentosa, originally identified in Japan by Nagashima et al,1 is an uncommon recurrent inflammatory disorder predominantly observed in young adults of Asian descent. Subsequently, it was reported to occur among individuals from different ethnic backgrounds, indicating potential underdiagnosis or misdiagnosis in Western countries.2 Although a direct pathogenic cause for prurigo pigmentosa has not been identified, a strong association has been linked to diet, specifically when ketosis is induced, such as in ketogenic diets and anorexia nervosa.3-5 Other possible causes include sunlight exposure, clothing friction, and sweating.1,5 The disease course is characterized by intermittent flares and spontaneous resolution, with recurrence in most cases. During the active phase, intensely pruritic, papulovesicular or urticarial papules are predominant and most often are localized to the upper body and torso, including the back, shoulders, neck, and chest.5 These flares can persist for several days but eventually subside, leaving behind a characteristic reticular pigmentation that can persist for months.5 First-line treatment often involves the use of tetracycline antibiotics, such as minocycline or doxycycline. 2,4,5 Dapsone often is used with successful resolution. 6 Dietary modifications also have been found to be effective in treating prurigo pigmentosa, particularly in patients presenting with dietary insufficiency.6,7 Increased carbohydrate intake has been shown to promote resolution. 6 Topical corticosteroids demonstrate limited efficacy in controlling flares.6,8
Histopathology has been variably described, with initial findings reported as nonspecific.1 However, it was later described as a distinct inflammatory disease of the skin with histologically distinct stages.2,9 Early stages reveal scattered dermal, dermal papillary, and perivascular neutrophilic infiltration.9 The lesions then progress and become fully developed, at which point neutrophilic infiltration becomes more prominent, accompanied by the presence of intraepidermal neutrophils and spongiosis. As the lesions resolve, the infiltration transitions to lymphocytic, and lichenoid changes can sometimes be appreciated along with epidermal hyperplasia, hyperpigmentation, and dermal melanophages.9 Although these findings aid in the diagnosis of prurigo pigmentosa, a clinicopathologic correlation is necessary to establish a definitive diagnosis.
Because prurigo pigmentosa is rare, it often is misdiagnosed as another condition with a similar presentation and nonspecific biopsy findings.6 Allergic contact dermatitis is a common type IV delayed hypersensitivity reaction that manifests similar to prurigo pigmentosa with pruritus and a well-demarcated distribution10 that is related to the pattern of allergen exposure; in the case of allergic contact dermatitis related to textiles, a well-demarcated rash will appear in the distribution area of the associated clothing (eg, shirt, pants, shorts).11 Development of allergy involves exposure and sensitization to an allergen, followed by subsequent re-exposure that results in cutaneous T-cell activation and inflammation. 10 Histopathology shows nonspecific spongiotic inflammation, and the gold standard for diagnosis is patch testing to identify the causative substance(s). Definitive treatment includes avoidance of identified allergies; however, if patients are unable to avoid the allergen or the cause is unknown, then corticosteroids, antihistamines, and/or calcineurin inhibitors are beneficial in controlling symptoms and flares.10
Pityrosporum folliculitis (also known as Malassezia folliculitis) is a fungal acneform condition that arises from overgrowth of normal skin flora Malassezia yeast,12 which may be due to occlusion of follicles or disruption of the normal flora composition. Clinically, the manifestation may resemble prurigo pigmentosa in distribution and presence of intense pruritus. However, pustular lesions and involvement of the face can aid in differentiating Pityrosporum from prurigo pigmentosa, which can be confirmed via periodic acid–Schiff staining with numerous round yeasts within affected follicles. Oral antifungal therapy typically yields rapid improvement and resolution of symptoms.12
Urticaria and prurigo pigmentosa share similar clinical characteristics, with symptoms of intense pruritus and urticarial lesions on the trunk.2,13 Urticaria is an IgEmediated type I hypersensitivity reaction characterized by wheals (ie, edematous red or pink lesions of variable size and shape that typically resolve spontaneously within 24–48 hours).13 Notably, urticaria will improve and in some cases completely resolve with antihistamines or anti-IgE antibody treatment, which may aid in distinguishing it from prurigo pigmentosa, as the latter typically exhibits limited response to such treatment.2 Histopathology also can assist in the diagnosis by ruling out other causes of similar rash; however, biopsies are not routinely done unless other inflammatory conditions are of high suspicion.13
Bullous pemphigoid is an autoimmune, subepidermal, blistering dermatosis that is most common among the elderly.14 It is characterized by the presence of IgG antibodies that target BP180 and BP230, which initiate inflammatory cascades that lead to tissue damage and blister formation. It typically manifests as pruritic blistering eruptions, primarily on the limbs and trunk, but may involve the head, neck, or palmoplantar regions.14 Although blistering eruptions are the prodrome of the disease, some cases may present with nonspecific urticarial or eczematous lesions14,15 that may resemble prurigo pigmentosa. The diagnosis is confirmed through direct immunofluorescence microscopy of biopsied lesions, which reveals IgG and/or C3 deposits along the dermoepidermal junction.14 Management of bullous pemphigoid involves timely initiation of dapsone or systemic corticosteroids, which have demonstrated high efficacy in controlling the disease and its associated symptoms.15
Our patient achieved a favorable response to diet modification and doxycycline therapy consistent with the diagnosis of prurigo pigmentosa. Unfortunately, the condition recurred following a relapse of anorexia. Management of prurigo pigmentosa necessitates not only accurate diagnosis but also addressing any underlying factors that may contribute to disease exacerbation. We anticipate the eating disorder will pose a major challenge in achieving long-term control of prurigo pigmentosa.
- Nagashima M, Ohshiro A, Shimizu N. A peculiar pruriginous dermatosis with gross reticular pigmentation. Jpn J Dermatol. 1971;81:38-39.
- Boer A, Asgari M. Prurigo pigmentosa: an underdiagnosed disease? Indian J Dermatol Venereol Leprol. 2006;72:405-409. doi:10.4103/0378-6323.29334
- Michaels JD, Hoss E, DiCaudo DJ, et al. Prurigo pigmentosa after a strict ketogenic diet. Pediatr Dermatol. 2013;32:248-251. doi:10.1111/pde.12275
- Teraki Y, Teraki E, Kawashima M, et al. Ketosis is involved in the origin of prurigo pigmentosa. J Am Acad Dermatol. 1996;34:509-511. doi:10.1016/s0190-9622(96)90460-0
- Böer A, Misago N, Wolter M, et al. Prurigo pigmentosa: a distinctive inflammatory disease of the skin. Am J Dermatopathol. 2003;25:117-129. doi:10.1097/00000372-200304000-00005
- Mufti A, Mirali S, Abduelmula A, et al. Clinical manifestations and treatment outcomes in prurigo pigmentosa (Nagashima disease): a systematic review of the literature. JAAD Int. 2021;3:79-87. doi:10.1016/j.jdin.2021.03.003
- Wong M, Lee E, Wu Y, et al. Treatment of prurigo pigmentosa with diet modification: a medical case study. Hawaii J Med Public Health. 2018;77:114-117.
- Almaani N, Al-Tarawneh AH, Msallam H. Prurigo pigmentosa: a clinicopathological report of three Middle Eastern patients. Case Rep Dermatol Med. 2018;2018:9406797. doi:10.1155/2018/9406797
- Kim JK, Chung WK, Chang SE, et al. Prurigo pigmentosa: clinicopathological study and analysis of 50 cases in Korea. J Dermatol. 2012;39:891-897. doi:10.1111/j.1346-8138.2012.01640.x
- Mowad CM, Anderson B, Scheinman P, et al. Allergic contact dermatitis: patient diagnosis and evaluation. J Am Acad Dermatol. 2016;74:1029-1040. doi:10.1016/j.jaad.2015.02.1139
- Lazarov A, Cordoba M, Plosk N, et al. Atypical and unusual clinical manifestations of contact dermatitis to clothing (textile contact dermatitis)—case presentation and review of the literature. Dermatol Online J. 2003;9. doi:10.5070/d30kd1d259
- Rubenstein RM, Malerich SA. Malassezia (Pityrosporum) folliculitis. J Clin Aesthet Dermatol. 2014;7:37-41.
- Bernstein JA, Lang DM, Khan DA, et al. The diagnosis and management of acute and chronic urticaria: 2014 update. J Allergy Clin Immunol. 2014;133:1270-1277. doi:10.1016/j.jaci.2014.02.036
- della Torre R, Combescure C, Cortés B, et al. Clinical presentation and diagnostic delay in bullous pemphigoid: a prospective nationwide cohort. Br J Dermatol. 2012;167:1111-1117. doi:10.1111/j.1365-2133.2012.11108.x
- Alonso-Llamazares J, Rogers RS 3rd, Oursler JR, et al. Bullous pemphigoid presenting as generalized pruritus: observations in six patients. Int J Dermatol. 1998;37:508-514.
The Diagnosis: Prurigo Pigmentosa
A comprehensive metabolic panel collected from our patient 1 month earlier did not reveal any abnormalities. Serum methylmalonic acid and homocysteine were both elevated at 417 nmol/L (reference range [for those aged 2–59 years], 55–335 nmol/L) and 23 μmol/L (reference range, 5–15 μmol/L), respectively. Serum folate and 25-hydroxyvitamin D were low at 3.1 ng/mL (reference range, >4.8 ng/mL) and 5 ng/mL (reference range, 30–80 ng/mL), respectively. Vitamin B12 was within reference range. Two 4-mm punch biopsies collected from the upper back showed spongiotic dermatitis.
Our patient’s histopathology results along with the rash distribution and medical history of anorexia increased suspicion for prurigo pigmentosa. A trial of oral doxycycline 100 mg twice daily for 2 weeks was prescribed. At 2-week follow-up, the patient’s mother revealed a history of ketosis in her daughter, solidifying the diagnosis. The patient was counseled on maintaining a healthy diet to prevent future breakouts. The patient’s rash resolved with diet modification and doxycycline; however, it recurred upon relapse of anorexia 4 months later.
Prurigo pigmentosa, originally identified in Japan by Nagashima et al,1 is an uncommon recurrent inflammatory disorder predominantly observed in young adults of Asian descent. Subsequently, it was reported to occur among individuals from different ethnic backgrounds, indicating potential underdiagnosis or misdiagnosis in Western countries.2 Although a direct pathogenic cause for prurigo pigmentosa has not been identified, a strong association has been linked to diet, specifically when ketosis is induced, such as in ketogenic diets and anorexia nervosa.3-5 Other possible causes include sunlight exposure, clothing friction, and sweating.1,5 The disease course is characterized by intermittent flares and spontaneous resolution, with recurrence in most cases. During the active phase, intensely pruritic, papulovesicular or urticarial papules are predominant and most often are localized to the upper body and torso, including the back, shoulders, neck, and chest.5 These flares can persist for several days but eventually subside, leaving behind a characteristic reticular pigmentation that can persist for months.5 First-line treatment often involves the use of tetracycline antibiotics, such as minocycline or doxycycline. 2,4,5 Dapsone often is used with successful resolution. 6 Dietary modifications also have been found to be effective in treating prurigo pigmentosa, particularly in patients presenting with dietary insufficiency.6,7 Increased carbohydrate intake has been shown to promote resolution. 6 Topical corticosteroids demonstrate limited efficacy in controlling flares.6,8
Histopathology has been variably described, with initial findings reported as nonspecific.1 However, it was later described as a distinct inflammatory disease of the skin with histologically distinct stages.2,9 Early stages reveal scattered dermal, dermal papillary, and perivascular neutrophilic infiltration.9 The lesions then progress and become fully developed, at which point neutrophilic infiltration becomes more prominent, accompanied by the presence of intraepidermal neutrophils and spongiosis. As the lesions resolve, the infiltration transitions to lymphocytic, and lichenoid changes can sometimes be appreciated along with epidermal hyperplasia, hyperpigmentation, and dermal melanophages.9 Although these findings aid in the diagnosis of prurigo pigmentosa, a clinicopathologic correlation is necessary to establish a definitive diagnosis.
Because prurigo pigmentosa is rare, it often is misdiagnosed as another condition with a similar presentation and nonspecific biopsy findings.6 Allergic contact dermatitis is a common type IV delayed hypersensitivity reaction that manifests similar to prurigo pigmentosa with pruritus and a well-demarcated distribution10 that is related to the pattern of allergen exposure; in the case of allergic contact dermatitis related to textiles, a well-demarcated rash will appear in the distribution area of the associated clothing (eg, shirt, pants, shorts).11 Development of allergy involves exposure and sensitization to an allergen, followed by subsequent re-exposure that results in cutaneous T-cell activation and inflammation. 10 Histopathology shows nonspecific spongiotic inflammation, and the gold standard for diagnosis is patch testing to identify the causative substance(s). Definitive treatment includes avoidance of identified allergies; however, if patients are unable to avoid the allergen or the cause is unknown, then corticosteroids, antihistamines, and/or calcineurin inhibitors are beneficial in controlling symptoms and flares.10
Pityrosporum folliculitis (also known as Malassezia folliculitis) is a fungal acneform condition that arises from overgrowth of normal skin flora Malassezia yeast,12 which may be due to occlusion of follicles or disruption of the normal flora composition. Clinically, the manifestation may resemble prurigo pigmentosa in distribution and presence of intense pruritus. However, pustular lesions and involvement of the face can aid in differentiating Pityrosporum from prurigo pigmentosa, which can be confirmed via periodic acid–Schiff staining with numerous round yeasts within affected follicles. Oral antifungal therapy typically yields rapid improvement and resolution of symptoms.12
Urticaria and prurigo pigmentosa share similar clinical characteristics, with symptoms of intense pruritus and urticarial lesions on the trunk.2,13 Urticaria is an IgEmediated type I hypersensitivity reaction characterized by wheals (ie, edematous red or pink lesions of variable size and shape that typically resolve spontaneously within 24–48 hours).13 Notably, urticaria will improve and in some cases completely resolve with antihistamines or anti-IgE antibody treatment, which may aid in distinguishing it from prurigo pigmentosa, as the latter typically exhibits limited response to such treatment.2 Histopathology also can assist in the diagnosis by ruling out other causes of similar rash; however, biopsies are not routinely done unless other inflammatory conditions are of high suspicion.13
Bullous pemphigoid is an autoimmune, subepidermal, blistering dermatosis that is most common among the elderly.14 It is characterized by the presence of IgG antibodies that target BP180 and BP230, which initiate inflammatory cascades that lead to tissue damage and blister formation. It typically manifests as pruritic blistering eruptions, primarily on the limbs and trunk, but may involve the head, neck, or palmoplantar regions.14 Although blistering eruptions are the prodrome of the disease, some cases may present with nonspecific urticarial or eczematous lesions14,15 that may resemble prurigo pigmentosa. The diagnosis is confirmed through direct immunofluorescence microscopy of biopsied lesions, which reveals IgG and/or C3 deposits along the dermoepidermal junction.14 Management of bullous pemphigoid involves timely initiation of dapsone or systemic corticosteroids, which have demonstrated high efficacy in controlling the disease and its associated symptoms.15
Our patient achieved a favorable response to diet modification and doxycycline therapy consistent with the diagnosis of prurigo pigmentosa. Unfortunately, the condition recurred following a relapse of anorexia. Management of prurigo pigmentosa necessitates not only accurate diagnosis but also addressing any underlying factors that may contribute to disease exacerbation. We anticipate the eating disorder will pose a major challenge in achieving long-term control of prurigo pigmentosa.
The Diagnosis: Prurigo Pigmentosa
A comprehensive metabolic panel collected from our patient 1 month earlier did not reveal any abnormalities. Serum methylmalonic acid and homocysteine were both elevated at 417 nmol/L (reference range [for those aged 2–59 years], 55–335 nmol/L) and 23 μmol/L (reference range, 5–15 μmol/L), respectively. Serum folate and 25-hydroxyvitamin D were low at 3.1 ng/mL (reference range, >4.8 ng/mL) and 5 ng/mL (reference range, 30–80 ng/mL), respectively. Vitamin B12 was within reference range. Two 4-mm punch biopsies collected from the upper back showed spongiotic dermatitis.
Our patient’s histopathology results along with the rash distribution and medical history of anorexia increased suspicion for prurigo pigmentosa. A trial of oral doxycycline 100 mg twice daily for 2 weeks was prescribed. At 2-week follow-up, the patient’s mother revealed a history of ketosis in her daughter, solidifying the diagnosis. The patient was counseled on maintaining a healthy diet to prevent future breakouts. The patient’s rash resolved with diet modification and doxycycline; however, it recurred upon relapse of anorexia 4 months later.
Prurigo pigmentosa, originally identified in Japan by Nagashima et al,1 is an uncommon recurrent inflammatory disorder predominantly observed in young adults of Asian descent. Subsequently, it was reported to occur among individuals from different ethnic backgrounds, indicating potential underdiagnosis or misdiagnosis in Western countries.2 Although a direct pathogenic cause for prurigo pigmentosa has not been identified, a strong association has been linked to diet, specifically when ketosis is induced, such as in ketogenic diets and anorexia nervosa.3-5 Other possible causes include sunlight exposure, clothing friction, and sweating.1,5 The disease course is characterized by intermittent flares and spontaneous resolution, with recurrence in most cases. During the active phase, intensely pruritic, papulovesicular or urticarial papules are predominant and most often are localized to the upper body and torso, including the back, shoulders, neck, and chest.5 These flares can persist for several days but eventually subside, leaving behind a characteristic reticular pigmentation that can persist for months.5 First-line treatment often involves the use of tetracycline antibiotics, such as minocycline or doxycycline. 2,4,5 Dapsone often is used with successful resolution. 6 Dietary modifications also have been found to be effective in treating prurigo pigmentosa, particularly in patients presenting with dietary insufficiency.6,7 Increased carbohydrate intake has been shown to promote resolution. 6 Topical corticosteroids demonstrate limited efficacy in controlling flares.6,8
Histopathology has been variably described, with initial findings reported as nonspecific.1 However, it was later described as a distinct inflammatory disease of the skin with histologically distinct stages.2,9 Early stages reveal scattered dermal, dermal papillary, and perivascular neutrophilic infiltration.9 The lesions then progress and become fully developed, at which point neutrophilic infiltration becomes more prominent, accompanied by the presence of intraepidermal neutrophils and spongiosis. As the lesions resolve, the infiltration transitions to lymphocytic, and lichenoid changes can sometimes be appreciated along with epidermal hyperplasia, hyperpigmentation, and dermal melanophages.9 Although these findings aid in the diagnosis of prurigo pigmentosa, a clinicopathologic correlation is necessary to establish a definitive diagnosis.
Because prurigo pigmentosa is rare, it often is misdiagnosed as another condition with a similar presentation and nonspecific biopsy findings.6 Allergic contact dermatitis is a common type IV delayed hypersensitivity reaction that manifests similar to prurigo pigmentosa with pruritus and a well-demarcated distribution10 that is related to the pattern of allergen exposure; in the case of allergic contact dermatitis related to textiles, a well-demarcated rash will appear in the distribution area of the associated clothing (eg, shirt, pants, shorts).11 Development of allergy involves exposure and sensitization to an allergen, followed by subsequent re-exposure that results in cutaneous T-cell activation and inflammation. 10 Histopathology shows nonspecific spongiotic inflammation, and the gold standard for diagnosis is patch testing to identify the causative substance(s). Definitive treatment includes avoidance of identified allergies; however, if patients are unable to avoid the allergen or the cause is unknown, then corticosteroids, antihistamines, and/or calcineurin inhibitors are beneficial in controlling symptoms and flares.10
Pityrosporum folliculitis (also known as Malassezia folliculitis) is a fungal acneform condition that arises from overgrowth of normal skin flora Malassezia yeast,12 which may be due to occlusion of follicles or disruption of the normal flora composition. Clinically, the manifestation may resemble prurigo pigmentosa in distribution and presence of intense pruritus. However, pustular lesions and involvement of the face can aid in differentiating Pityrosporum from prurigo pigmentosa, which can be confirmed via periodic acid–Schiff staining with numerous round yeasts within affected follicles. Oral antifungal therapy typically yields rapid improvement and resolution of symptoms.12
Urticaria and prurigo pigmentosa share similar clinical characteristics, with symptoms of intense pruritus and urticarial lesions on the trunk.2,13 Urticaria is an IgEmediated type I hypersensitivity reaction characterized by wheals (ie, edematous red or pink lesions of variable size and shape that typically resolve spontaneously within 24–48 hours).13 Notably, urticaria will improve and in some cases completely resolve with antihistamines or anti-IgE antibody treatment, which may aid in distinguishing it from prurigo pigmentosa, as the latter typically exhibits limited response to such treatment.2 Histopathology also can assist in the diagnosis by ruling out other causes of similar rash; however, biopsies are not routinely done unless other inflammatory conditions are of high suspicion.13
Bullous pemphigoid is an autoimmune, subepidermal, blistering dermatosis that is most common among the elderly.14 It is characterized by the presence of IgG antibodies that target BP180 and BP230, which initiate inflammatory cascades that lead to tissue damage and blister formation. It typically manifests as pruritic blistering eruptions, primarily on the limbs and trunk, but may involve the head, neck, or palmoplantar regions.14 Although blistering eruptions are the prodrome of the disease, some cases may present with nonspecific urticarial or eczematous lesions14,15 that may resemble prurigo pigmentosa. The diagnosis is confirmed through direct immunofluorescence microscopy of biopsied lesions, which reveals IgG and/or C3 deposits along the dermoepidermal junction.14 Management of bullous pemphigoid involves timely initiation of dapsone or systemic corticosteroids, which have demonstrated high efficacy in controlling the disease and its associated symptoms.15
Our patient achieved a favorable response to diet modification and doxycycline therapy consistent with the diagnosis of prurigo pigmentosa. Unfortunately, the condition recurred following a relapse of anorexia. Management of prurigo pigmentosa necessitates not only accurate diagnosis but also addressing any underlying factors that may contribute to disease exacerbation. We anticipate the eating disorder will pose a major challenge in achieving long-term control of prurigo pigmentosa.
- Nagashima M, Ohshiro A, Shimizu N. A peculiar pruriginous dermatosis with gross reticular pigmentation. Jpn J Dermatol. 1971;81:38-39.
- Boer A, Asgari M. Prurigo pigmentosa: an underdiagnosed disease? Indian J Dermatol Venereol Leprol. 2006;72:405-409. doi:10.4103/0378-6323.29334
- Michaels JD, Hoss E, DiCaudo DJ, et al. Prurigo pigmentosa after a strict ketogenic diet. Pediatr Dermatol. 2013;32:248-251. doi:10.1111/pde.12275
- Teraki Y, Teraki E, Kawashima M, et al. Ketosis is involved in the origin of prurigo pigmentosa. J Am Acad Dermatol. 1996;34:509-511. doi:10.1016/s0190-9622(96)90460-0
- Böer A, Misago N, Wolter M, et al. Prurigo pigmentosa: a distinctive inflammatory disease of the skin. Am J Dermatopathol. 2003;25:117-129. doi:10.1097/00000372-200304000-00005
- Mufti A, Mirali S, Abduelmula A, et al. Clinical manifestations and treatment outcomes in prurigo pigmentosa (Nagashima disease): a systematic review of the literature. JAAD Int. 2021;3:79-87. doi:10.1016/j.jdin.2021.03.003
- Wong M, Lee E, Wu Y, et al. Treatment of prurigo pigmentosa with diet modification: a medical case study. Hawaii J Med Public Health. 2018;77:114-117.
- Almaani N, Al-Tarawneh AH, Msallam H. Prurigo pigmentosa: a clinicopathological report of three Middle Eastern patients. Case Rep Dermatol Med. 2018;2018:9406797. doi:10.1155/2018/9406797
- Kim JK, Chung WK, Chang SE, et al. Prurigo pigmentosa: clinicopathological study and analysis of 50 cases in Korea. J Dermatol. 2012;39:891-897. doi:10.1111/j.1346-8138.2012.01640.x
- Mowad CM, Anderson B, Scheinman P, et al. Allergic contact dermatitis: patient diagnosis and evaluation. J Am Acad Dermatol. 2016;74:1029-1040. doi:10.1016/j.jaad.2015.02.1139
- Lazarov A, Cordoba M, Plosk N, et al. Atypical and unusual clinical manifestations of contact dermatitis to clothing (textile contact dermatitis)—case presentation and review of the literature. Dermatol Online J. 2003;9. doi:10.5070/d30kd1d259
- Rubenstein RM, Malerich SA. Malassezia (Pityrosporum) folliculitis. J Clin Aesthet Dermatol. 2014;7:37-41.
- Bernstein JA, Lang DM, Khan DA, et al. The diagnosis and management of acute and chronic urticaria: 2014 update. J Allergy Clin Immunol. 2014;133:1270-1277. doi:10.1016/j.jaci.2014.02.036
- della Torre R, Combescure C, Cortés B, et al. Clinical presentation and diagnostic delay in bullous pemphigoid: a prospective nationwide cohort. Br J Dermatol. 2012;167:1111-1117. doi:10.1111/j.1365-2133.2012.11108.x
- Alonso-Llamazares J, Rogers RS 3rd, Oursler JR, et al. Bullous pemphigoid presenting as generalized pruritus: observations in six patients. Int J Dermatol. 1998;37:508-514.
- Nagashima M, Ohshiro A, Shimizu N. A peculiar pruriginous dermatosis with gross reticular pigmentation. Jpn J Dermatol. 1971;81:38-39.
- Boer A, Asgari M. Prurigo pigmentosa: an underdiagnosed disease? Indian J Dermatol Venereol Leprol. 2006;72:405-409. doi:10.4103/0378-6323.29334
- Michaels JD, Hoss E, DiCaudo DJ, et al. Prurigo pigmentosa after a strict ketogenic diet. Pediatr Dermatol. 2013;32:248-251. doi:10.1111/pde.12275
- Teraki Y, Teraki E, Kawashima M, et al. Ketosis is involved in the origin of prurigo pigmentosa. J Am Acad Dermatol. 1996;34:509-511. doi:10.1016/s0190-9622(96)90460-0
- Böer A, Misago N, Wolter M, et al. Prurigo pigmentosa: a distinctive inflammatory disease of the skin. Am J Dermatopathol. 2003;25:117-129. doi:10.1097/00000372-200304000-00005
- Mufti A, Mirali S, Abduelmula A, et al. Clinical manifestations and treatment outcomes in prurigo pigmentosa (Nagashima disease): a systematic review of the literature. JAAD Int. 2021;3:79-87. doi:10.1016/j.jdin.2021.03.003
- Wong M, Lee E, Wu Y, et al. Treatment of prurigo pigmentosa with diet modification: a medical case study. Hawaii J Med Public Health. 2018;77:114-117.
- Almaani N, Al-Tarawneh AH, Msallam H. Prurigo pigmentosa: a clinicopathological report of three Middle Eastern patients. Case Rep Dermatol Med. 2018;2018:9406797. doi:10.1155/2018/9406797
- Kim JK, Chung WK, Chang SE, et al. Prurigo pigmentosa: clinicopathological study and analysis of 50 cases in Korea. J Dermatol. 2012;39:891-897. doi:10.1111/j.1346-8138.2012.01640.x
- Mowad CM, Anderson B, Scheinman P, et al. Allergic contact dermatitis: patient diagnosis and evaluation. J Am Acad Dermatol. 2016;74:1029-1040. doi:10.1016/j.jaad.2015.02.1139
- Lazarov A, Cordoba M, Plosk N, et al. Atypical and unusual clinical manifestations of contact dermatitis to clothing (textile contact dermatitis)—case presentation and review of the literature. Dermatol Online J. 2003;9. doi:10.5070/d30kd1d259
- Rubenstein RM, Malerich SA. Malassezia (Pityrosporum) folliculitis. J Clin Aesthet Dermatol. 2014;7:37-41.
- Bernstein JA, Lang DM, Khan DA, et al. The diagnosis and management of acute and chronic urticaria: 2014 update. J Allergy Clin Immunol. 2014;133:1270-1277. doi:10.1016/j.jaci.2014.02.036
- della Torre R, Combescure C, Cortés B, et al. Clinical presentation and diagnostic delay in bullous pemphigoid: a prospective nationwide cohort. Br J Dermatol. 2012;167:1111-1117. doi:10.1111/j.1365-2133.2012.11108.x
- Alonso-Llamazares J, Rogers RS 3rd, Oursler JR, et al. Bullous pemphigoid presenting as generalized pruritus: observations in six patients. Int J Dermatol. 1998;37:508-514.
A 43-year-old woman presented with a pruritic rash across the neck and back of 6 months’ duration that progressively worsened. She had a medical history of anorexia nervosa, herpes zoster with a recent flare, and peripheral neuropathy. Physical examination showed numerous red scaly papules across the upper back and shoulders that coalesced in a reticular pattern. No similar papules were seen elsewhere on the body.
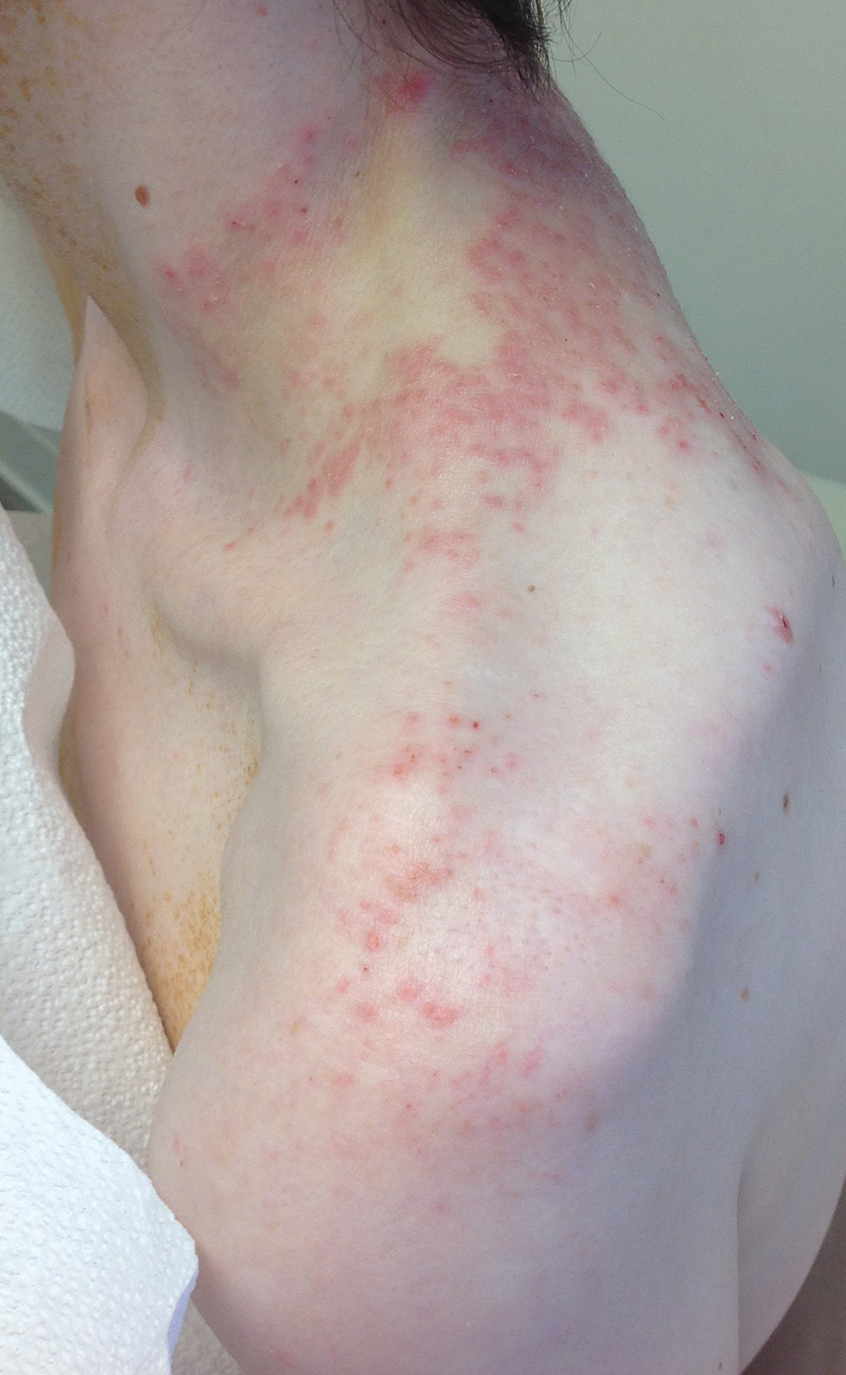
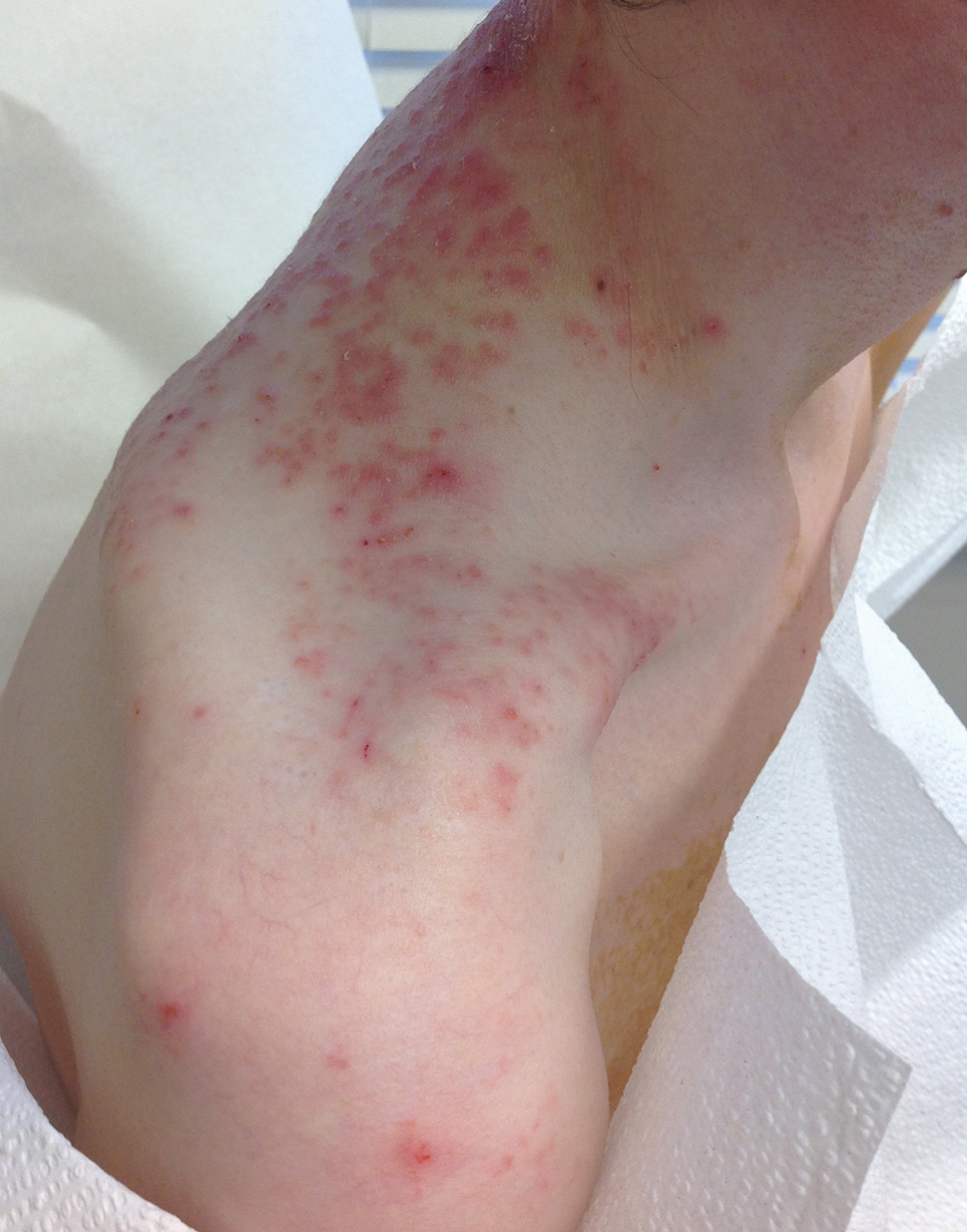
Topical Ruxolitinib: Analysis Finds Repigmentation Rates in Adolescents with Vitiligo
data showed.
“We consider repigmenting vitiligo a two-step process, where the overactive immune system needs to be calmed down and then the melanocytes need to repopulate to the white areas,” one of the study investigators, David Rosmarin, MD, chair of the Department of Dermatology at Indiana University School of Medicine, Indianapolis, said in an interview in advance of the annual meeting of the Society for Pediatric Dermatology, where the study results were presented during a poster session. “In younger patients, it may be that the melanocytes are more rapidly repigmenting the patches, which is why we see this effect.”
Ruxolitinib, 1.5% cream (Opzelura) is a Janus kinase inhibitor approved for the treatment of nonsegmental vitiligo in patients 12 years of age and older. Dr. Rosmarin and colleagues sought to evaluate differences in rates of complete or near-complete repigmentation and repigmentation by body region between adolescents 12-17 years of age and adults 18 years of age and older who applied ruxolitinib cream twice daily. The researchers evaluated patients who were initially randomized to ruxolitinib cream, 1.5% in the pivotal TRuE-V1 and TRuE-V2 studies and applied it for up to 104 weeks. Complete facial improvement was defined as 100% improvement on the Facial Vitiligo Area Scoring Index (F-VASI 100) from baseline, and near-total improvement was categorized as a ≥ 75% or ≥ 90% improvement from baseline on the Total body VASI (T-VASI). Responses for each of six body regions, excluding the face, were assessed by the proportion of patients who achieved at least a 50% improvement from baseline on the T-VASI.
Compared with adults, a greater proportion of adolescents achieved F-VASI 100 at week 24 (5.7% [3/53] vs 2.9% [10/341], respectively), but there were no differences between the two groups at week 52 (8.0% [4/50] vs 8.0% [24/300]). Response rates were greater among adolescents vs adults for T-VASI 75 at weeks 24 (13.2% [7/53] vs 5.6% [19/341]) and 52 (22.0% [11/50] vs 20.3% [61/300]), as well as T-VASI 90 at weeks 24 (3.8% [2/53] vs 0.3% [1/341]) and 52 (12.0% [6/50] vs 4.0% [12/300]).
The researchers observed that VASI 50 responses by body region were generally similar between adolescents and adults, but a greater proportion of adolescents achieved a VASI 50 in lower extremities (67.3% [33/49] vs 51.8% [118/228]) and feet (37.5% [12/32] vs 27.9% [51/183]) at week 52.
“Adolescents repigmented more rapidly than adults, so that at 24 weeks, more teens had complete facial repigmentation and T-VASI 75 and T-VASI 90 results,” Dr. Rosmarin said. “With continued use of ruxolitinib cream, both more adults and adolescents achieved greater repigmentation.” He acknowledged certain limitations of the study, including the fact that it was only vehicle controlled up through 24 weeks and that, after week 52, there were fewer patients who completed the long-term extension.
“The take-home message is that ruxolitinib cream can effectively and safely help many patients repigment, including adolescents,” he said.
The study was funded by topical ruxolitinib manufacturer Incyte. Dr. Rosmarin disclosed that he has consulted, spoken for, or conducted trials for AbbVie, Abcuro, Almirall, AltruBio, Amgen, Arena, Astria, Boehringer Ingelheim, Bristol Meyers Squibb, Celgene, Concert, CSL Behring, Dermavant Sciences, Dermira, Galderma, Incyte, Janssen, Kyowa Kirin, Lilly, Merck, Nektar, Novartis, Pfizer, RAPT, Regeneron, Recludix Pharma, Revolo Biotherapeutics, Sanofi, Sun Pharmaceuticals, UCB, Viela Bio, and Zura.
A version of this article first appeared on Medscape.com.
data showed.
“We consider repigmenting vitiligo a two-step process, where the overactive immune system needs to be calmed down and then the melanocytes need to repopulate to the white areas,” one of the study investigators, David Rosmarin, MD, chair of the Department of Dermatology at Indiana University School of Medicine, Indianapolis, said in an interview in advance of the annual meeting of the Society for Pediatric Dermatology, where the study results were presented during a poster session. “In younger patients, it may be that the melanocytes are more rapidly repigmenting the patches, which is why we see this effect.”
Ruxolitinib, 1.5% cream (Opzelura) is a Janus kinase inhibitor approved for the treatment of nonsegmental vitiligo in patients 12 years of age and older. Dr. Rosmarin and colleagues sought to evaluate differences in rates of complete or near-complete repigmentation and repigmentation by body region between adolescents 12-17 years of age and adults 18 years of age and older who applied ruxolitinib cream twice daily. The researchers evaluated patients who were initially randomized to ruxolitinib cream, 1.5% in the pivotal TRuE-V1 and TRuE-V2 studies and applied it for up to 104 weeks. Complete facial improvement was defined as 100% improvement on the Facial Vitiligo Area Scoring Index (F-VASI 100) from baseline, and near-total improvement was categorized as a ≥ 75% or ≥ 90% improvement from baseline on the Total body VASI (T-VASI). Responses for each of six body regions, excluding the face, were assessed by the proportion of patients who achieved at least a 50% improvement from baseline on the T-VASI.
Compared with adults, a greater proportion of adolescents achieved F-VASI 100 at week 24 (5.7% [3/53] vs 2.9% [10/341], respectively), but there were no differences between the two groups at week 52 (8.0% [4/50] vs 8.0% [24/300]). Response rates were greater among adolescents vs adults for T-VASI 75 at weeks 24 (13.2% [7/53] vs 5.6% [19/341]) and 52 (22.0% [11/50] vs 20.3% [61/300]), as well as T-VASI 90 at weeks 24 (3.8% [2/53] vs 0.3% [1/341]) and 52 (12.0% [6/50] vs 4.0% [12/300]).
The researchers observed that VASI 50 responses by body region were generally similar between adolescents and adults, but a greater proportion of adolescents achieved a VASI 50 in lower extremities (67.3% [33/49] vs 51.8% [118/228]) and feet (37.5% [12/32] vs 27.9% [51/183]) at week 52.
“Adolescents repigmented more rapidly than adults, so that at 24 weeks, more teens had complete facial repigmentation and T-VASI 75 and T-VASI 90 results,” Dr. Rosmarin said. “With continued use of ruxolitinib cream, both more adults and adolescents achieved greater repigmentation.” He acknowledged certain limitations of the study, including the fact that it was only vehicle controlled up through 24 weeks and that, after week 52, there were fewer patients who completed the long-term extension.
“The take-home message is that ruxolitinib cream can effectively and safely help many patients repigment, including adolescents,” he said.
The study was funded by topical ruxolitinib manufacturer Incyte. Dr. Rosmarin disclosed that he has consulted, spoken for, or conducted trials for AbbVie, Abcuro, Almirall, AltruBio, Amgen, Arena, Astria, Boehringer Ingelheim, Bristol Meyers Squibb, Celgene, Concert, CSL Behring, Dermavant Sciences, Dermira, Galderma, Incyte, Janssen, Kyowa Kirin, Lilly, Merck, Nektar, Novartis, Pfizer, RAPT, Regeneron, Recludix Pharma, Revolo Biotherapeutics, Sanofi, Sun Pharmaceuticals, UCB, Viela Bio, and Zura.
A version of this article first appeared on Medscape.com.
data showed.
“We consider repigmenting vitiligo a two-step process, where the overactive immune system needs to be calmed down and then the melanocytes need to repopulate to the white areas,” one of the study investigators, David Rosmarin, MD, chair of the Department of Dermatology at Indiana University School of Medicine, Indianapolis, said in an interview in advance of the annual meeting of the Society for Pediatric Dermatology, where the study results were presented during a poster session. “In younger patients, it may be that the melanocytes are more rapidly repigmenting the patches, which is why we see this effect.”
Ruxolitinib, 1.5% cream (Opzelura) is a Janus kinase inhibitor approved for the treatment of nonsegmental vitiligo in patients 12 years of age and older. Dr. Rosmarin and colleagues sought to evaluate differences in rates of complete or near-complete repigmentation and repigmentation by body region between adolescents 12-17 years of age and adults 18 years of age and older who applied ruxolitinib cream twice daily. The researchers evaluated patients who were initially randomized to ruxolitinib cream, 1.5% in the pivotal TRuE-V1 and TRuE-V2 studies and applied it for up to 104 weeks. Complete facial improvement was defined as 100% improvement on the Facial Vitiligo Area Scoring Index (F-VASI 100) from baseline, and near-total improvement was categorized as a ≥ 75% or ≥ 90% improvement from baseline on the Total body VASI (T-VASI). Responses for each of six body regions, excluding the face, were assessed by the proportion of patients who achieved at least a 50% improvement from baseline on the T-VASI.
Compared with adults, a greater proportion of adolescents achieved F-VASI 100 at week 24 (5.7% [3/53] vs 2.9% [10/341], respectively), but there were no differences between the two groups at week 52 (8.0% [4/50] vs 8.0% [24/300]). Response rates were greater among adolescents vs adults for T-VASI 75 at weeks 24 (13.2% [7/53] vs 5.6% [19/341]) and 52 (22.0% [11/50] vs 20.3% [61/300]), as well as T-VASI 90 at weeks 24 (3.8% [2/53] vs 0.3% [1/341]) and 52 (12.0% [6/50] vs 4.0% [12/300]).
The researchers observed that VASI 50 responses by body region were generally similar between adolescents and adults, but a greater proportion of adolescents achieved a VASI 50 in lower extremities (67.3% [33/49] vs 51.8% [118/228]) and feet (37.5% [12/32] vs 27.9% [51/183]) at week 52.
“Adolescents repigmented more rapidly than adults, so that at 24 weeks, more teens had complete facial repigmentation and T-VASI 75 and T-VASI 90 results,” Dr. Rosmarin said. “With continued use of ruxolitinib cream, both more adults and adolescents achieved greater repigmentation.” He acknowledged certain limitations of the study, including the fact that it was only vehicle controlled up through 24 weeks and that, after week 52, there were fewer patients who completed the long-term extension.
“The take-home message is that ruxolitinib cream can effectively and safely help many patients repigment, including adolescents,” he said.
The study was funded by topical ruxolitinib manufacturer Incyte. Dr. Rosmarin disclosed that he has consulted, spoken for, or conducted trials for AbbVie, Abcuro, Almirall, AltruBio, Amgen, Arena, Astria, Boehringer Ingelheim, Bristol Meyers Squibb, Celgene, Concert, CSL Behring, Dermavant Sciences, Dermira, Galderma, Incyte, Janssen, Kyowa Kirin, Lilly, Merck, Nektar, Novartis, Pfizer, RAPT, Regeneron, Recludix Pharma, Revolo Biotherapeutics, Sanofi, Sun Pharmaceuticals, UCB, Viela Bio, and Zura.
A version of this article first appeared on Medscape.com.
FROM SPD 2024
Meta-Analysis Finds Combination Cream Plus Tranexamic Acid Effective for Melasma
TOPLINE:
A meta-analysis showed that .
METHODOLOGY:
- Current treatments for melasma focus on inducing remission and preventing relapse. Tranexamic acid, an antifibrinolytic drug, has shown promise in recent studies, but its optimal use, either alone or as an adjunct to TCC, remains unclear.
- Researchers conducted a meta-analysis of four randomized controlled trials patients that compared oral tranexamic acid plus TCC (hydroquinone, retinoic acid, and hydrocortisone) and TCC alone in 480 patients with melasma, divided almost evenly into the two treatment groups.
- The main outcome was the change in the Melasma Severity Area Index (MASI) score and recurrence rate from baseline.
TAKEAWAY:
- Patients treated with oral tranexamic acid plus TCC showed a greater reduction in MASI scores compared with those who received TCC alone (mean difference, −3.10; P = .03).
- The recurrence rate of melasma was significantly lower in the tranexamic acid plus TCC group (risk ratio [RR], 0.28; P < .001).
- There was no significant difference in the incidences of erythema (RR, 0.63; P = .147) and burning (RR, 0.59; P = .131).
IN PRACTICE:
“Evidence indicates that oral tranexamic acid confers clinical benefits, contributing to the enhancement of treatment outcomes in melasma when used in conjunction with TCC therapy,” and results are promising with regards to minimizing recurrence, the authors concluded.
SOURCE:
The study was led by Ocílio Ribeiro Gonçalves, MS, of the Federal University of Piauí, Teresina, Brazil, and was published online on June 8, 2024, in Clinical and Experimental Dermatology.
LIMITATIONS:
There was heterogeneity across studies, including different methods of administration, treatment protocols (including dosage), and timing of treatment.
DISCLOSURES:
The study reported receiving no funding. The authors declared no conflicts of interest.
A version of this article appeared on Medscape.com.
TOPLINE:
A meta-analysis showed that .
METHODOLOGY:
- Current treatments for melasma focus on inducing remission and preventing relapse. Tranexamic acid, an antifibrinolytic drug, has shown promise in recent studies, but its optimal use, either alone or as an adjunct to TCC, remains unclear.
- Researchers conducted a meta-analysis of four randomized controlled trials patients that compared oral tranexamic acid plus TCC (hydroquinone, retinoic acid, and hydrocortisone) and TCC alone in 480 patients with melasma, divided almost evenly into the two treatment groups.
- The main outcome was the change in the Melasma Severity Area Index (MASI) score and recurrence rate from baseline.
TAKEAWAY:
- Patients treated with oral tranexamic acid plus TCC showed a greater reduction in MASI scores compared with those who received TCC alone (mean difference, −3.10; P = .03).
- The recurrence rate of melasma was significantly lower in the tranexamic acid plus TCC group (risk ratio [RR], 0.28; P < .001).
- There was no significant difference in the incidences of erythema (RR, 0.63; P = .147) and burning (RR, 0.59; P = .131).
IN PRACTICE:
“Evidence indicates that oral tranexamic acid confers clinical benefits, contributing to the enhancement of treatment outcomes in melasma when used in conjunction with TCC therapy,” and results are promising with regards to minimizing recurrence, the authors concluded.
SOURCE:
The study was led by Ocílio Ribeiro Gonçalves, MS, of the Federal University of Piauí, Teresina, Brazil, and was published online on June 8, 2024, in Clinical and Experimental Dermatology.
LIMITATIONS:
There was heterogeneity across studies, including different methods of administration, treatment protocols (including dosage), and timing of treatment.
DISCLOSURES:
The study reported receiving no funding. The authors declared no conflicts of interest.
A version of this article appeared on Medscape.com.
TOPLINE:
A meta-analysis showed that .
METHODOLOGY:
- Current treatments for melasma focus on inducing remission and preventing relapse. Tranexamic acid, an antifibrinolytic drug, has shown promise in recent studies, but its optimal use, either alone or as an adjunct to TCC, remains unclear.
- Researchers conducted a meta-analysis of four randomized controlled trials patients that compared oral tranexamic acid plus TCC (hydroquinone, retinoic acid, and hydrocortisone) and TCC alone in 480 patients with melasma, divided almost evenly into the two treatment groups.
- The main outcome was the change in the Melasma Severity Area Index (MASI) score and recurrence rate from baseline.
TAKEAWAY:
- Patients treated with oral tranexamic acid plus TCC showed a greater reduction in MASI scores compared with those who received TCC alone (mean difference, −3.10; P = .03).
- The recurrence rate of melasma was significantly lower in the tranexamic acid plus TCC group (risk ratio [RR], 0.28; P < .001).
- There was no significant difference in the incidences of erythema (RR, 0.63; P = .147) and burning (RR, 0.59; P = .131).
IN PRACTICE:
“Evidence indicates that oral tranexamic acid confers clinical benefits, contributing to the enhancement of treatment outcomes in melasma when used in conjunction with TCC therapy,” and results are promising with regards to minimizing recurrence, the authors concluded.
SOURCE:
The study was led by Ocílio Ribeiro Gonçalves, MS, of the Federal University of Piauí, Teresina, Brazil, and was published online on June 8, 2024, in Clinical and Experimental Dermatology.
LIMITATIONS:
There was heterogeneity across studies, including different methods of administration, treatment protocols (including dosage), and timing of treatment.
DISCLOSURES:
The study reported receiving no funding. The authors declared no conflicts of interest.
A version of this article appeared on Medscape.com.
OTC Supplement Linked to Hyperpigmentation
CHICAGO —The .
“This is something we will see more and more,” Heather Woolery-Lloyd, MD, director of the Skin of Color Division at the University of Miami Department of Dermatology, said at the Pigmentary Disorders Exchange Symposium. The key marker of this hyperpigmentation, she said, is that “it’s strongly photoaccentuated,” affecting areas exposed to the sun — but it also tends to spare the knuckles on patients’ hands.
Used Like an Opioid, But It’s Not Regulated
Kratom is a plant common in southeast Asia and is used as an analgesic. It’s marketed as a “legal opioid” or “legal high” and is sold in 2- or 3-ounce containers of extract or sold as a powder, Dr. Woolery-Lloyd said. The leaves may be boiled into a tea, smoked, chewed, or put into capsules, according to a case report published in February in the Journal of Integrative Dermatology. It is used worldwide and is not regulated in the United States.
“Many of our patients think kratom is a safe, herbal supplement” but often don’t know it can have several side effects and can be addictive, Dr. Woolery-Lloyd said. Its popularity is increasing as reflected by the number of posts related to kratom on social media platforms.
In the February case report, Shaina Patel, BA, and Nathaniel Phelan, MD, from Kansas City University, Kansas City, Missouri, wrote that side effects of kratom include drowsiness, tachycardia, vomiting, respiratory depression, and cardiac arrest, in addition to confusion and hallucinations.
Kratom also has many different effects on the psyche, Dr. Woolery-Lloyd said at the meeting. At low doses, it blocks the reuptake of norepinephrine, serotonin, and dopamine, producing a motivational effect, and at high doses, it creates an analgesic, calming effect. And people who chronically consume high doses of kratom may be susceptible to hyperpigmentation.
Kratom-associated hyperpigmentation should be considered as a diagnosis when evaluating patients for other drug-associated pigmentary disorders, “especially if pigment is photodistributed,” she said. “If you see new-onset hyperpigmentation or onset over several months and it’s very photoaccentuated, definitely ask about use of kratom.”
Case Reports Show Patterns of Presentation
A 2022 report from Landon R. Powell, BS, with the department of biology, Whitworth University in Spokane, Washington, and coauthors, published in JAAD Case Reports, noted that kratom use in the United States has increased dramatically. “As measured by call reports to the United States National Poison Data System, in 2011, there were 11 reported kratom exposures, and in the first 7 months of 2018, there were 357 reported exposures,” they wrote.
An estimated 1.7 million Americans aged ≥ 12 years said they had used kratom in the previous year, according to the Substance Abuse and Mental Health Services Administration 2021 National Survey on Drug Use and Health.
In the case report, Mr. Powell and coauthors described a 54-year-old White male patient who had been using kratom for the previous four to five years to reduce opioid use. During this period, he consumed kratom powder mixed with orange juice three to four times a day. He presented with “diffuse hyperpigmented patches on his arms and face in a photodistributed manner, with notable sparing of the knuckles on both hands.”
Dark Gray-Blue Skin
In the more recent case report, Ms. Patel and Dr. Phelan described a 30-year-old White male patient who presented with dark gray-blue skin coloring on his cheeks, back of his neck, and the backs of his hands and forearms. He had no other medical conditions and did not take any medications or supplements that cause hyperpigmentation while using kratom.
The patient had been taking kratom for years in the wake of an opioid addiction following medications for a high school injury. He developed an opioid use disorder and tried to replace his pain medications with kratom.
“The patient stopped using kratom in May 2022, but the discoloration remains. It has not regressed in the following 16 months after discontinuing kratom use,” the authors wrote, noting that “whether or not the hyperpigmentation is able to regress is unknown.”
Dr. Woolery-Lloyd is a consultant for AbbVie, Incyte, Johnson & Johnson Consumer, LivDerm, and L’Oreal; a speaker for Eli Lilly, Incyte, L’Oreal, and Ortho Dermatologics; and a researcher/investigator for AbbVie, Allergan, Eirion Therapeutics, Galderma, Pfizer, Sanofi, and Vyne Therapeutics.
According to an information page on kratom on the Food and Drug Administration website, health care professionals and consumers can report adverse reactions associated with kratom to the FDA’s MedWatch program.
A version of this article appeared on Medscape.com.
CHICAGO —The .
“This is something we will see more and more,” Heather Woolery-Lloyd, MD, director of the Skin of Color Division at the University of Miami Department of Dermatology, said at the Pigmentary Disorders Exchange Symposium. The key marker of this hyperpigmentation, she said, is that “it’s strongly photoaccentuated,” affecting areas exposed to the sun — but it also tends to spare the knuckles on patients’ hands.
Used Like an Opioid, But It’s Not Regulated
Kratom is a plant common in southeast Asia and is used as an analgesic. It’s marketed as a “legal opioid” or “legal high” and is sold in 2- or 3-ounce containers of extract or sold as a powder, Dr. Woolery-Lloyd said. The leaves may be boiled into a tea, smoked, chewed, or put into capsules, according to a case report published in February in the Journal of Integrative Dermatology. It is used worldwide and is not regulated in the United States.
“Many of our patients think kratom is a safe, herbal supplement” but often don’t know it can have several side effects and can be addictive, Dr. Woolery-Lloyd said. Its popularity is increasing as reflected by the number of posts related to kratom on social media platforms.
In the February case report, Shaina Patel, BA, and Nathaniel Phelan, MD, from Kansas City University, Kansas City, Missouri, wrote that side effects of kratom include drowsiness, tachycardia, vomiting, respiratory depression, and cardiac arrest, in addition to confusion and hallucinations.
Kratom also has many different effects on the psyche, Dr. Woolery-Lloyd said at the meeting. At low doses, it blocks the reuptake of norepinephrine, serotonin, and dopamine, producing a motivational effect, and at high doses, it creates an analgesic, calming effect. And people who chronically consume high doses of kratom may be susceptible to hyperpigmentation.
Kratom-associated hyperpigmentation should be considered as a diagnosis when evaluating patients for other drug-associated pigmentary disorders, “especially if pigment is photodistributed,” she said. “If you see new-onset hyperpigmentation or onset over several months and it’s very photoaccentuated, definitely ask about use of kratom.”
Case Reports Show Patterns of Presentation
A 2022 report from Landon R. Powell, BS, with the department of biology, Whitworth University in Spokane, Washington, and coauthors, published in JAAD Case Reports, noted that kratom use in the United States has increased dramatically. “As measured by call reports to the United States National Poison Data System, in 2011, there were 11 reported kratom exposures, and in the first 7 months of 2018, there were 357 reported exposures,” they wrote.
An estimated 1.7 million Americans aged ≥ 12 years said they had used kratom in the previous year, according to the Substance Abuse and Mental Health Services Administration 2021 National Survey on Drug Use and Health.
In the case report, Mr. Powell and coauthors described a 54-year-old White male patient who had been using kratom for the previous four to five years to reduce opioid use. During this period, he consumed kratom powder mixed with orange juice three to four times a day. He presented with “diffuse hyperpigmented patches on his arms and face in a photodistributed manner, with notable sparing of the knuckles on both hands.”
Dark Gray-Blue Skin
In the more recent case report, Ms. Patel and Dr. Phelan described a 30-year-old White male patient who presented with dark gray-blue skin coloring on his cheeks, back of his neck, and the backs of his hands and forearms. He had no other medical conditions and did not take any medications or supplements that cause hyperpigmentation while using kratom.
The patient had been taking kratom for years in the wake of an opioid addiction following medications for a high school injury. He developed an opioid use disorder and tried to replace his pain medications with kratom.
“The patient stopped using kratom in May 2022, but the discoloration remains. It has not regressed in the following 16 months after discontinuing kratom use,” the authors wrote, noting that “whether or not the hyperpigmentation is able to regress is unknown.”
Dr. Woolery-Lloyd is a consultant for AbbVie, Incyte, Johnson & Johnson Consumer, LivDerm, and L’Oreal; a speaker for Eli Lilly, Incyte, L’Oreal, and Ortho Dermatologics; and a researcher/investigator for AbbVie, Allergan, Eirion Therapeutics, Galderma, Pfizer, Sanofi, and Vyne Therapeutics.
According to an information page on kratom on the Food and Drug Administration website, health care professionals and consumers can report adverse reactions associated with kratom to the FDA’s MedWatch program.
A version of this article appeared on Medscape.com.
CHICAGO —The .
“This is something we will see more and more,” Heather Woolery-Lloyd, MD, director of the Skin of Color Division at the University of Miami Department of Dermatology, said at the Pigmentary Disorders Exchange Symposium. The key marker of this hyperpigmentation, she said, is that “it’s strongly photoaccentuated,” affecting areas exposed to the sun — but it also tends to spare the knuckles on patients’ hands.
Used Like an Opioid, But It’s Not Regulated
Kratom is a plant common in southeast Asia and is used as an analgesic. It’s marketed as a “legal opioid” or “legal high” and is sold in 2- or 3-ounce containers of extract or sold as a powder, Dr. Woolery-Lloyd said. The leaves may be boiled into a tea, smoked, chewed, or put into capsules, according to a case report published in February in the Journal of Integrative Dermatology. It is used worldwide and is not regulated in the United States.
“Many of our patients think kratom is a safe, herbal supplement” but often don’t know it can have several side effects and can be addictive, Dr. Woolery-Lloyd said. Its popularity is increasing as reflected by the number of posts related to kratom on social media platforms.
In the February case report, Shaina Patel, BA, and Nathaniel Phelan, MD, from Kansas City University, Kansas City, Missouri, wrote that side effects of kratom include drowsiness, tachycardia, vomiting, respiratory depression, and cardiac arrest, in addition to confusion and hallucinations.
Kratom also has many different effects on the psyche, Dr. Woolery-Lloyd said at the meeting. At low doses, it blocks the reuptake of norepinephrine, serotonin, and dopamine, producing a motivational effect, and at high doses, it creates an analgesic, calming effect. And people who chronically consume high doses of kratom may be susceptible to hyperpigmentation.
Kratom-associated hyperpigmentation should be considered as a diagnosis when evaluating patients for other drug-associated pigmentary disorders, “especially if pigment is photodistributed,” she said. “If you see new-onset hyperpigmentation or onset over several months and it’s very photoaccentuated, definitely ask about use of kratom.”
Case Reports Show Patterns of Presentation
A 2022 report from Landon R. Powell, BS, with the department of biology, Whitworth University in Spokane, Washington, and coauthors, published in JAAD Case Reports, noted that kratom use in the United States has increased dramatically. “As measured by call reports to the United States National Poison Data System, in 2011, there were 11 reported kratom exposures, and in the first 7 months of 2018, there were 357 reported exposures,” they wrote.
An estimated 1.7 million Americans aged ≥ 12 years said they had used kratom in the previous year, according to the Substance Abuse and Mental Health Services Administration 2021 National Survey on Drug Use and Health.
In the case report, Mr. Powell and coauthors described a 54-year-old White male patient who had been using kratom for the previous four to five years to reduce opioid use. During this period, he consumed kratom powder mixed with orange juice three to four times a day. He presented with “diffuse hyperpigmented patches on his arms and face in a photodistributed manner, with notable sparing of the knuckles on both hands.”
Dark Gray-Blue Skin
In the more recent case report, Ms. Patel and Dr. Phelan described a 30-year-old White male patient who presented with dark gray-blue skin coloring on his cheeks, back of his neck, and the backs of his hands and forearms. He had no other medical conditions and did not take any medications or supplements that cause hyperpigmentation while using kratom.
The patient had been taking kratom for years in the wake of an opioid addiction following medications for a high school injury. He developed an opioid use disorder and tried to replace his pain medications with kratom.
“The patient stopped using kratom in May 2022, but the discoloration remains. It has not regressed in the following 16 months after discontinuing kratom use,” the authors wrote, noting that “whether or not the hyperpigmentation is able to regress is unknown.”
Dr. Woolery-Lloyd is a consultant for AbbVie, Incyte, Johnson & Johnson Consumer, LivDerm, and L’Oreal; a speaker for Eli Lilly, Incyte, L’Oreal, and Ortho Dermatologics; and a researcher/investigator for AbbVie, Allergan, Eirion Therapeutics, Galderma, Pfizer, Sanofi, and Vyne Therapeutics.
According to an information page on kratom on the Food and Drug Administration website, health care professionals and consumers can report adverse reactions associated with kratom to the FDA’s MedWatch program.
A version of this article appeared on Medscape.com.