User login
FDA approves first tocilizumab biosimilar
The Food and Drug Administration has approved the biosimilar tocilizumab-bavi (Tofidence), Biogen, the drug’s manufacturer, announced on Sept. 29.
It is the first tocilizumab biosimilar approved by the FDA. The reference product, Actemra (Genentech), was first approved by the agency in 2010.
“The approval of Tofidence in the U.S. marks another positive step toward helping more people with chronic autoimmune conditions gain access to leading therapies,” Ian Henshaw, global head of biosimilars at Biogen, said in a statement. “With the increasing numbers of approved biosimilars, we expect increased savings and sustainability for health care systems and an increase in physician choice and patient access to biologics.”
Biogen’s pricing for tocilizumab-bavi will be available closer to the product’s launch date, which has yet to be determined, a company spokesman said. The U.S. average monthly cost of Actemra for rheumatoid arthritis, administered intravenously, is $2,134-$4,268 depending on dosage, according to a Genentech spokesperson.
Tocilizumab-bavi is an intravenous formulation (20 mg/mL) indicated for treatment of moderately to severely active RA, polyarticular juvenile idiopathic arthritis (PJIA), and systemic juvenile idiopathic arthritis (SJIA). The medication is administered every 4 weeks in RA and PJIA and every 8 weeks in SJIA as a single intravenous drip infusion over 1 hour.
The European Commission approved its first tocilizumab biosimilar, Tyenne (Fresenius Kabi), earlier in 2023 in both subcutaneous and intravenous formulations. Biogen did not comment on whether the company is working on a subcutaneous formulation for tocilizumab-bavi.
A version of this article appeared on Medscape.com.
The Food and Drug Administration has approved the biosimilar tocilizumab-bavi (Tofidence), Biogen, the drug’s manufacturer, announced on Sept. 29.
It is the first tocilizumab biosimilar approved by the FDA. The reference product, Actemra (Genentech), was first approved by the agency in 2010.
“The approval of Tofidence in the U.S. marks another positive step toward helping more people with chronic autoimmune conditions gain access to leading therapies,” Ian Henshaw, global head of biosimilars at Biogen, said in a statement. “With the increasing numbers of approved biosimilars, we expect increased savings and sustainability for health care systems and an increase in physician choice and patient access to biologics.”
Biogen’s pricing for tocilizumab-bavi will be available closer to the product’s launch date, which has yet to be determined, a company spokesman said. The U.S. average monthly cost of Actemra for rheumatoid arthritis, administered intravenously, is $2,134-$4,268 depending on dosage, according to a Genentech spokesperson.
Tocilizumab-bavi is an intravenous formulation (20 mg/mL) indicated for treatment of moderately to severely active RA, polyarticular juvenile idiopathic arthritis (PJIA), and systemic juvenile idiopathic arthritis (SJIA). The medication is administered every 4 weeks in RA and PJIA and every 8 weeks in SJIA as a single intravenous drip infusion over 1 hour.
The European Commission approved its first tocilizumab biosimilar, Tyenne (Fresenius Kabi), earlier in 2023 in both subcutaneous and intravenous formulations. Biogen did not comment on whether the company is working on a subcutaneous formulation for tocilizumab-bavi.
A version of this article appeared on Medscape.com.
The Food and Drug Administration has approved the biosimilar tocilizumab-bavi (Tofidence), Biogen, the drug’s manufacturer, announced on Sept. 29.
It is the first tocilizumab biosimilar approved by the FDA. The reference product, Actemra (Genentech), was first approved by the agency in 2010.
“The approval of Tofidence in the U.S. marks another positive step toward helping more people with chronic autoimmune conditions gain access to leading therapies,” Ian Henshaw, global head of biosimilars at Biogen, said in a statement. “With the increasing numbers of approved biosimilars, we expect increased savings and sustainability for health care systems and an increase in physician choice and patient access to biologics.”
Biogen’s pricing for tocilizumab-bavi will be available closer to the product’s launch date, which has yet to be determined, a company spokesman said. The U.S. average monthly cost of Actemra for rheumatoid arthritis, administered intravenously, is $2,134-$4,268 depending on dosage, according to a Genentech spokesperson.
Tocilizumab-bavi is an intravenous formulation (20 mg/mL) indicated for treatment of moderately to severely active RA, polyarticular juvenile idiopathic arthritis (PJIA), and systemic juvenile idiopathic arthritis (SJIA). The medication is administered every 4 weeks in RA and PJIA and every 8 weeks in SJIA as a single intravenous drip infusion over 1 hour.
The European Commission approved its first tocilizumab biosimilar, Tyenne (Fresenius Kabi), earlier in 2023 in both subcutaneous and intravenous formulations. Biogen did not comment on whether the company is working on a subcutaneous formulation for tocilizumab-bavi.
A version of this article appeared on Medscape.com.
AHA updates CPR guidelines on cardiac arrest after poisoning
The update reflects treatment advances and new knowledge, including the use of venoarterial extracorporeal membrane oxygenation (VA-ECMO) for patients whose condition is refractory to poison antidotes and other therapies.
The new guidelines are designed primarily for North American health care professionals who treat adults and children who are critically ill because of poisoning, including intentional and unintentional drug overdose, chemical exposure, and drug-drug interactions, the authors note.
Published online in Circulation, the update was endorsed by the American Academy of Pediatrics.
‘Nearly miraculous’
“It’s been 13 years since the poisoning treatment guidelines had a comprehensive update,” lead author Eric J. Lavonas, MD, professor of emergency medicine at Denver Health and the Rocky Mountain Poison and Drug Center, Colo., told this news organization. “In that time, we’ve learned a lot about how to best use antidotes and other treatments to save the most critically poisoned patients.”
Highlighting a few key points from the update, he said, “For those rare situations when antidotes aren’t enough, the new guidelines include the use of heart-lung machines (VA-ECMO) for patients with beta-blocker, calcium channel blocker, or sodium channel blocker poisoning causing cardiogenic shock.”
Furthermore, he said, “High-dose insulin treatment for patients with beta-blocker and calcium channel blocker poisoning [also recommended in the update] has really become mainstream. The doses are up to 10 times higher than the amount used to treat diabetic emergencies.
“Some excellent science has shown that giving IV lipid emulsion can save the life of someone with an accidental overdose of local anesthetic medications, particularly bupivacaine,” he added. “The result is sometimes nearly miraculous.
“But when this treatment is extended to poisoning from other medications, it often doesn’t work as well, and in some situations may make things worse,” he said. “The issue may be that giving lipids increases absorption of drug from the stomach and intestines, which can be dangerous when the patient took an overdose of pills.”
Low level of evidence
The guidelines were compiled by the Critical Poisoning Writing Group, which includes experts from emergency medicine, pediatrics, medical toxicology, pharmacology, critical care, emergency medical services, education, research, and nursing. Group members were appointed by the AHA Emergency Cardiovascular Care Science Subcommittee and were approved by the AHA Manuscript Oversight Committee.
First and foremost, the group recommends timely consultation with a medical toxicologist, a clinical toxicologist, or a regional poison center to facilitate rapid, effective therapy, because treatment of cardiac arrest and toxicity from poisoning often requires treatments that most clinicians don’t use frequently.
Other key points include the following:
- Naloxone administration may reverse respiratory arrest due to opioid overdose, preventing progression to cardiac arrest.
- Give high-dose insulin therapy early in the treatment of patients with beta-blocker and calcium channel blocker poisoning, Dr. Lavonas noted.
- Standard advanced life support plus sodium bicarbonate is appropriate for life-threatening dysrhythmias caused by cocaine or other sodium channel blockers.
- If cyanide poisoning is suspected, clinicians should not wait for confirmatory testing; treatment should begin immediately with hydroxocobalamin (preferred) or sodium nitrite plus sodium thiosulfate.
- Digoxin-specific immune antibody fragments can reverse life-threatening dysrhythmias from digoxin poisoning.
- Use of 20% intravenous lipid emulsion can be efficacious in the resuscitation of life-threatening local anesthetic toxicity, especially from bupivacaine, Dr. Lavonas indicated.
- Sedation is recommended for patients with severe agitation from sympathomimetic poisoning to manage hyperthermia and acidosis, prevent rhabdomyolysis and injury, and allow evaluation for other life-threatening conditions.
- Although flumazenil reverses central nervous system and respiratory depression from benzodiazepine poisoning, risks and contraindications, provided in the guidelines, limit its use.
- VA-ECMO can be lifesaving for patients with cardiogenic shock or dysrhythmias that are refractory to other treatments.
“Unfortunately, despite improvements in the design and funding support for resuscitation research, the overall certainty of the evidence base for resuscitation science and management of critical poisoning is low,” the group acknowledges.
Of the 73 guideline recommendations, only 2 are supported by level A evidence; 3 are supported by level B-randomized evidence, 12 by level B-nonrandomized evidence, and the rest by level C evidence.
“Accordingly, the strength of recommendations is weaker than optimal,” they write. “Clinical trials in resuscitation and the management of critical poisoning are sorely needed.”
‘Don’t go it alone!’
“Most critical poisonings are pretty uncommon, and each patient is different,” Dr. Lavonas said. “Even in the emergency department or ICU, most physicians will treat a patient who is critically ill with any given poison less than once a year. The antidotes and medication doses needed to effectively treat these patients are often very different than everyday medical practice.
“Don’t try to go it alone!” he urges. “Poisoning cases are complex, and the treatments work best when they are implemented quickly and assertively. A toxicologist can help sort through complex situations and get effective treatment started without delay.”
Every certified poison center has a medical toxicologist or clinical toxicologist on call 24/7 to give advice to physicians and hospitals about patients who are critically ill after being poisoned, he added. “Everyone in the U.S. has access to a poison center by calling one number: 1-800-222-1222.”
Dr. Lavonas has disclosed no relevant financial relationships.
A version of this article appeared on Medscape.com.
The update reflects treatment advances and new knowledge, including the use of venoarterial extracorporeal membrane oxygenation (VA-ECMO) for patients whose condition is refractory to poison antidotes and other therapies.
The new guidelines are designed primarily for North American health care professionals who treat adults and children who are critically ill because of poisoning, including intentional and unintentional drug overdose, chemical exposure, and drug-drug interactions, the authors note.
Published online in Circulation, the update was endorsed by the American Academy of Pediatrics.
‘Nearly miraculous’
“It’s been 13 years since the poisoning treatment guidelines had a comprehensive update,” lead author Eric J. Lavonas, MD, professor of emergency medicine at Denver Health and the Rocky Mountain Poison and Drug Center, Colo., told this news organization. “In that time, we’ve learned a lot about how to best use antidotes and other treatments to save the most critically poisoned patients.”
Highlighting a few key points from the update, he said, “For those rare situations when antidotes aren’t enough, the new guidelines include the use of heart-lung machines (VA-ECMO) for patients with beta-blocker, calcium channel blocker, or sodium channel blocker poisoning causing cardiogenic shock.”
Furthermore, he said, “High-dose insulin treatment for patients with beta-blocker and calcium channel blocker poisoning [also recommended in the update] has really become mainstream. The doses are up to 10 times higher than the amount used to treat diabetic emergencies.
“Some excellent science has shown that giving IV lipid emulsion can save the life of someone with an accidental overdose of local anesthetic medications, particularly bupivacaine,” he added. “The result is sometimes nearly miraculous.
“But when this treatment is extended to poisoning from other medications, it often doesn’t work as well, and in some situations may make things worse,” he said. “The issue may be that giving lipids increases absorption of drug from the stomach and intestines, which can be dangerous when the patient took an overdose of pills.”
Low level of evidence
The guidelines were compiled by the Critical Poisoning Writing Group, which includes experts from emergency medicine, pediatrics, medical toxicology, pharmacology, critical care, emergency medical services, education, research, and nursing. Group members were appointed by the AHA Emergency Cardiovascular Care Science Subcommittee and were approved by the AHA Manuscript Oversight Committee.
First and foremost, the group recommends timely consultation with a medical toxicologist, a clinical toxicologist, or a regional poison center to facilitate rapid, effective therapy, because treatment of cardiac arrest and toxicity from poisoning often requires treatments that most clinicians don’t use frequently.
Other key points include the following:
- Naloxone administration may reverse respiratory arrest due to opioid overdose, preventing progression to cardiac arrest.
- Give high-dose insulin therapy early in the treatment of patients with beta-blocker and calcium channel blocker poisoning, Dr. Lavonas noted.
- Standard advanced life support plus sodium bicarbonate is appropriate for life-threatening dysrhythmias caused by cocaine or other sodium channel blockers.
- If cyanide poisoning is suspected, clinicians should not wait for confirmatory testing; treatment should begin immediately with hydroxocobalamin (preferred) or sodium nitrite plus sodium thiosulfate.
- Digoxin-specific immune antibody fragments can reverse life-threatening dysrhythmias from digoxin poisoning.
- Use of 20% intravenous lipid emulsion can be efficacious in the resuscitation of life-threatening local anesthetic toxicity, especially from bupivacaine, Dr. Lavonas indicated.
- Sedation is recommended for patients with severe agitation from sympathomimetic poisoning to manage hyperthermia and acidosis, prevent rhabdomyolysis and injury, and allow evaluation for other life-threatening conditions.
- Although flumazenil reverses central nervous system and respiratory depression from benzodiazepine poisoning, risks and contraindications, provided in the guidelines, limit its use.
- VA-ECMO can be lifesaving for patients with cardiogenic shock or dysrhythmias that are refractory to other treatments.
“Unfortunately, despite improvements in the design and funding support for resuscitation research, the overall certainty of the evidence base for resuscitation science and management of critical poisoning is low,” the group acknowledges.
Of the 73 guideline recommendations, only 2 are supported by level A evidence; 3 are supported by level B-randomized evidence, 12 by level B-nonrandomized evidence, and the rest by level C evidence.
“Accordingly, the strength of recommendations is weaker than optimal,” they write. “Clinical trials in resuscitation and the management of critical poisoning are sorely needed.”
‘Don’t go it alone!’
“Most critical poisonings are pretty uncommon, and each patient is different,” Dr. Lavonas said. “Even in the emergency department or ICU, most physicians will treat a patient who is critically ill with any given poison less than once a year. The antidotes and medication doses needed to effectively treat these patients are often very different than everyday medical practice.
“Don’t try to go it alone!” he urges. “Poisoning cases are complex, and the treatments work best when they are implemented quickly and assertively. A toxicologist can help sort through complex situations and get effective treatment started without delay.”
Every certified poison center has a medical toxicologist or clinical toxicologist on call 24/7 to give advice to physicians and hospitals about patients who are critically ill after being poisoned, he added. “Everyone in the U.S. has access to a poison center by calling one number: 1-800-222-1222.”
Dr. Lavonas has disclosed no relevant financial relationships.
A version of this article appeared on Medscape.com.
The update reflects treatment advances and new knowledge, including the use of venoarterial extracorporeal membrane oxygenation (VA-ECMO) for patients whose condition is refractory to poison antidotes and other therapies.
The new guidelines are designed primarily for North American health care professionals who treat adults and children who are critically ill because of poisoning, including intentional and unintentional drug overdose, chemical exposure, and drug-drug interactions, the authors note.
Published online in Circulation, the update was endorsed by the American Academy of Pediatrics.
‘Nearly miraculous’
“It’s been 13 years since the poisoning treatment guidelines had a comprehensive update,” lead author Eric J. Lavonas, MD, professor of emergency medicine at Denver Health and the Rocky Mountain Poison and Drug Center, Colo., told this news organization. “In that time, we’ve learned a lot about how to best use antidotes and other treatments to save the most critically poisoned patients.”
Highlighting a few key points from the update, he said, “For those rare situations when antidotes aren’t enough, the new guidelines include the use of heart-lung machines (VA-ECMO) for patients with beta-blocker, calcium channel blocker, or sodium channel blocker poisoning causing cardiogenic shock.”
Furthermore, he said, “High-dose insulin treatment for patients with beta-blocker and calcium channel blocker poisoning [also recommended in the update] has really become mainstream. The doses are up to 10 times higher than the amount used to treat diabetic emergencies.
“Some excellent science has shown that giving IV lipid emulsion can save the life of someone with an accidental overdose of local anesthetic medications, particularly bupivacaine,” he added. “The result is sometimes nearly miraculous.
“But when this treatment is extended to poisoning from other medications, it often doesn’t work as well, and in some situations may make things worse,” he said. “The issue may be that giving lipids increases absorption of drug from the stomach and intestines, which can be dangerous when the patient took an overdose of pills.”
Low level of evidence
The guidelines were compiled by the Critical Poisoning Writing Group, which includes experts from emergency medicine, pediatrics, medical toxicology, pharmacology, critical care, emergency medical services, education, research, and nursing. Group members were appointed by the AHA Emergency Cardiovascular Care Science Subcommittee and were approved by the AHA Manuscript Oversight Committee.
First and foremost, the group recommends timely consultation with a medical toxicologist, a clinical toxicologist, or a regional poison center to facilitate rapid, effective therapy, because treatment of cardiac arrest and toxicity from poisoning often requires treatments that most clinicians don’t use frequently.
Other key points include the following:
- Naloxone administration may reverse respiratory arrest due to opioid overdose, preventing progression to cardiac arrest.
- Give high-dose insulin therapy early in the treatment of patients with beta-blocker and calcium channel blocker poisoning, Dr. Lavonas noted.
- Standard advanced life support plus sodium bicarbonate is appropriate for life-threatening dysrhythmias caused by cocaine or other sodium channel blockers.
- If cyanide poisoning is suspected, clinicians should not wait for confirmatory testing; treatment should begin immediately with hydroxocobalamin (preferred) or sodium nitrite plus sodium thiosulfate.
- Digoxin-specific immune antibody fragments can reverse life-threatening dysrhythmias from digoxin poisoning.
- Use of 20% intravenous lipid emulsion can be efficacious in the resuscitation of life-threatening local anesthetic toxicity, especially from bupivacaine, Dr. Lavonas indicated.
- Sedation is recommended for patients with severe agitation from sympathomimetic poisoning to manage hyperthermia and acidosis, prevent rhabdomyolysis and injury, and allow evaluation for other life-threatening conditions.
- Although flumazenil reverses central nervous system and respiratory depression from benzodiazepine poisoning, risks and contraindications, provided in the guidelines, limit its use.
- VA-ECMO can be lifesaving for patients with cardiogenic shock or dysrhythmias that are refractory to other treatments.
“Unfortunately, despite improvements in the design and funding support for resuscitation research, the overall certainty of the evidence base for resuscitation science and management of critical poisoning is low,” the group acknowledges.
Of the 73 guideline recommendations, only 2 are supported by level A evidence; 3 are supported by level B-randomized evidence, 12 by level B-nonrandomized evidence, and the rest by level C evidence.
“Accordingly, the strength of recommendations is weaker than optimal,” they write. “Clinical trials in resuscitation and the management of critical poisoning are sorely needed.”
‘Don’t go it alone!’
“Most critical poisonings are pretty uncommon, and each patient is different,” Dr. Lavonas said. “Even in the emergency department or ICU, most physicians will treat a patient who is critically ill with any given poison less than once a year. The antidotes and medication doses needed to effectively treat these patients are often very different than everyday medical practice.
“Don’t try to go it alone!” he urges. “Poisoning cases are complex, and the treatments work best when they are implemented quickly and assertively. A toxicologist can help sort through complex situations and get effective treatment started without delay.”
Every certified poison center has a medical toxicologist or clinical toxicologist on call 24/7 to give advice to physicians and hospitals about patients who are critically ill after being poisoned, he added. “Everyone in the U.S. has access to a poison center by calling one number: 1-800-222-1222.”
Dr. Lavonas has disclosed no relevant financial relationships.
A version of this article appeared on Medscape.com.
Study finds inflammatory bowel disease risk higher in children, adults with atopic dermatitis
The published recently in JAMA Dermatology.
The study also found an increased risk for Crohn’s disease (CD) in adults and children with AD, as well as an increased risk for ulcerative colitis (UC) in adults with AD and in children with severe AD, researchers reported.
“It is imperative for clinicians to understand atopic dermatitis and the trajectory of our patients with it in order to provide the best standard of care,” senior author Joel M. Gelfand, MD, MSCE, professor in clinical investigation with the department of dermatology at the University of Pennsylvania, Philadelphia, said in a news release.
“There are new and better treatments for AD today, and there will likely continue to be more,” continued Dr. Gelfand. “But providers have to understand how those treatments could impact other autoimmune diseases. For patients with AD and another autoimmune disease, some currently available medications can exacerbate symptoms of their other disease or can help treat two immune diseases at the same time.”
The study results support the idea that AD and IBD may have some common underlying causes, said Sheilagh Maguiness, MD, pediatric dermatologist and associate professor in the department of dermatology at the University of Minnesota, Minneapolis, who was asked to comment on the findings.
“As the pathogenesis of AD is becoming better understood, we are recognizing that, rather than simply a cutaneous disease, the underlying inflammation and immune dysregulation that leads to AD best categorizes it as a systemic inflammatory disease with significant comorbidities,” she told this news organization. “I will be more likely to ask patients and families about GI symptoms, and if positive, may plan to refer to GI more readily than in the past,” added Dr. Maguiness, who was not involved in the study.
UK general practice cohort
AD has been associated with an increasing number of comorbidities, including IBD, but studies linking AD with IBD, including UC, have had mixed results, the authors wrote. And few studies have separately examined how AD or AD severity may be linked with UC or CD risk.
To examine the risk for new-onset IBD, UC, and CD in children and adults with atopic dermatitis, the researchers conducted a population-based cohort study using the THIN (The Health Improvement Network) electronic medical record database of patients registered with United Kingdom general practices. They used 21 years of data collected from January 1994 to February 2015.
The researchers matched each patient who had AD with up to five controls based on age, practice, and index date. Because THIN does not capture AD severity, they used treatment exposure assessed by dermatologic referrals and treatments patients received as proxy for severity. The authors used logistic regression to examine the risks for IBD, UC, and CD in children (aged 1-10) with AD, and in adults (aged 30-68) with AD, and they compared their outcomes with the outcomes for controls.
In the pediatric cohort, the team compared 409,431 children who had AD with 1.8 million children without AD. Slightly more than half were boys. In the adult cohort, they compared 625,083 people who had AD with 2.68 million controls, and slightly more than half were women. Data on race or ethnicity were not available, the authors wrote, but the THIN database is considered to be representative of the UK population.
AD severity linked with IBD risk
The risk for new-onset inflammatory bowel disease appears to be higher in children and adults with AD, and the risk varies based on age, AD severity, and subtype of inflammatory bowel disease, the authors reported.
Overall, AD in children was associated with a 44% increased risk for IBD (adjusted hazard ratio (HR), 1.44; 95% confidence interval [CI], 1.31-1.58) compared with controls, the authors reported. They found a 74% increased risk for CD in children with AD compared with controls (HR, 1.74; 95% CI, 1.54-1.97). More severe AD was linked with increased risk for both IBD and CD.
AD did not appear to increase risk for UC in children, except those with severe AD (HR, 1.65; 95% CI, 1.02-2.67).
Overall, adults with AD had a 34% (HR, 1.34; 95% CI, 1.27-1.40) increased risk for IBD, a 36% (HR, 1.36; 95% CI, 1.26-1.47) increased risk for CD, and a 32% (HR, 1.32; 95% CI, 1.24-1.41) increased risk for UC, with risk increasing with increased AD severity.
Robust data with cautionary note
“This study provides the most robust data to date on the association between IBD and AD. It provides clear evidence for an association that most dermatologists or primary care providers are not typically taught in training,” Kelly Scarberry, MD, assistant professor of dermatology at Case Western Reserve University in Cleveland, told this news organization. “I will be much more likely to pursue diagnostic workup in my AD patients who have GI complaints.”
However, AD severity was measured by proxy, added Dr. Scarberry, who was not involved in the study, and the study lacked important racial and ethnic data.
Lindsay C. Strowd, MD, associate professor of dermatology at Wake Forest University, Winston-Salem, N.C., also not involved in the study, said in an interview that she found the size of the cohort and the longitudinal data to be strengths of the study.
But, she added, the “lack of family IBD history, race and ethnicity, and comorbidities, are limitations, as is treatment exposure used as a proxy for disease severity, given that physician treatment practices differ.”
For Steven R. Feldman, MD, PhD, professor of dermatology at Wake Forest, “the most important conclusion, and it is a definitive finding, [is] that IBD is uncommon, even in patients with AD.
“The findings could be misinterpreted,” cautioned Dr. Feldman, who was not involved in the study. “While there is an increased relative risk, the absolute risk is small.” The study found that “the highest relative risk group is children with severe AD, who have a roughly fivefold increased risk for CD.” However, he added, the incidence rates of CD were 0.68 per 1,000 person-years in children with severe AD and 0.08 per 1,000 person-years in controls.
“Basically, because Crohn’s disease and IBD don’t happen very often, the modest increase in relative risk the investigators found doesn’t amount to much we’d have to worry about,” he said. “The findings do not show any need to screen patients with atopic dermatitis for IBD any more than we’d need to screen patients without atopic dermatitis.”
The increased relative risk “could be a clue to possible genetic connections between diseases,” he added. “But when we’re making clinical decisions, those decisions should be based on the absolute risk that some event may occur.”
Susan Massick, MD, dermatologist and associate professor at The Ohio State University in Columbus, who was not involved with the study, said in an interview, “We are still scratching the surface of the complexity of the immune and inflammatory pathways in AD and IBD.
“It is important to remember that correlation does not mean causation,” Dr. Massick said. “It would be premature to draw direct conclusions based on this study alone.”
The authors recommend future related studies in more diverse populations.
Dr. Gelfand and two coauthors reported ties with Pfizer, which supported the study. Dr. Gelfand and three coauthors reported ties with other pharmaceutical companies. Dr. Maguiness, Dr. Scarberry, Dr. Strowd, and Dr. Massick reported having no relevant disclosures. Dr. Feldman reported ties with Pfizer and other pharmaceutical companies.
A version of this article appeared on Medscape.com.
The published recently in JAMA Dermatology.
The study also found an increased risk for Crohn’s disease (CD) in adults and children with AD, as well as an increased risk for ulcerative colitis (UC) in adults with AD and in children with severe AD, researchers reported.
“It is imperative for clinicians to understand atopic dermatitis and the trajectory of our patients with it in order to provide the best standard of care,” senior author Joel M. Gelfand, MD, MSCE, professor in clinical investigation with the department of dermatology at the University of Pennsylvania, Philadelphia, said in a news release.
“There are new and better treatments for AD today, and there will likely continue to be more,” continued Dr. Gelfand. “But providers have to understand how those treatments could impact other autoimmune diseases. For patients with AD and another autoimmune disease, some currently available medications can exacerbate symptoms of their other disease or can help treat two immune diseases at the same time.”
The study results support the idea that AD and IBD may have some common underlying causes, said Sheilagh Maguiness, MD, pediatric dermatologist and associate professor in the department of dermatology at the University of Minnesota, Minneapolis, who was asked to comment on the findings.
“As the pathogenesis of AD is becoming better understood, we are recognizing that, rather than simply a cutaneous disease, the underlying inflammation and immune dysregulation that leads to AD best categorizes it as a systemic inflammatory disease with significant comorbidities,” she told this news organization. “I will be more likely to ask patients and families about GI symptoms, and if positive, may plan to refer to GI more readily than in the past,” added Dr. Maguiness, who was not involved in the study.
UK general practice cohort
AD has been associated with an increasing number of comorbidities, including IBD, but studies linking AD with IBD, including UC, have had mixed results, the authors wrote. And few studies have separately examined how AD or AD severity may be linked with UC or CD risk.
To examine the risk for new-onset IBD, UC, and CD in children and adults with atopic dermatitis, the researchers conducted a population-based cohort study using the THIN (The Health Improvement Network) electronic medical record database of patients registered with United Kingdom general practices. They used 21 years of data collected from January 1994 to February 2015.
The researchers matched each patient who had AD with up to five controls based on age, practice, and index date. Because THIN does not capture AD severity, they used treatment exposure assessed by dermatologic referrals and treatments patients received as proxy for severity. The authors used logistic regression to examine the risks for IBD, UC, and CD in children (aged 1-10) with AD, and in adults (aged 30-68) with AD, and they compared their outcomes with the outcomes for controls.
In the pediatric cohort, the team compared 409,431 children who had AD with 1.8 million children without AD. Slightly more than half were boys. In the adult cohort, they compared 625,083 people who had AD with 2.68 million controls, and slightly more than half were women. Data on race or ethnicity were not available, the authors wrote, but the THIN database is considered to be representative of the UK population.
AD severity linked with IBD risk
The risk for new-onset inflammatory bowel disease appears to be higher in children and adults with AD, and the risk varies based on age, AD severity, and subtype of inflammatory bowel disease, the authors reported.
Overall, AD in children was associated with a 44% increased risk for IBD (adjusted hazard ratio (HR), 1.44; 95% confidence interval [CI], 1.31-1.58) compared with controls, the authors reported. They found a 74% increased risk for CD in children with AD compared with controls (HR, 1.74; 95% CI, 1.54-1.97). More severe AD was linked with increased risk for both IBD and CD.
AD did not appear to increase risk for UC in children, except those with severe AD (HR, 1.65; 95% CI, 1.02-2.67).
Overall, adults with AD had a 34% (HR, 1.34; 95% CI, 1.27-1.40) increased risk for IBD, a 36% (HR, 1.36; 95% CI, 1.26-1.47) increased risk for CD, and a 32% (HR, 1.32; 95% CI, 1.24-1.41) increased risk for UC, with risk increasing with increased AD severity.
Robust data with cautionary note
“This study provides the most robust data to date on the association between IBD and AD. It provides clear evidence for an association that most dermatologists or primary care providers are not typically taught in training,” Kelly Scarberry, MD, assistant professor of dermatology at Case Western Reserve University in Cleveland, told this news organization. “I will be much more likely to pursue diagnostic workup in my AD patients who have GI complaints.”
However, AD severity was measured by proxy, added Dr. Scarberry, who was not involved in the study, and the study lacked important racial and ethnic data.
Lindsay C. Strowd, MD, associate professor of dermatology at Wake Forest University, Winston-Salem, N.C., also not involved in the study, said in an interview that she found the size of the cohort and the longitudinal data to be strengths of the study.
But, she added, the “lack of family IBD history, race and ethnicity, and comorbidities, are limitations, as is treatment exposure used as a proxy for disease severity, given that physician treatment practices differ.”
For Steven R. Feldman, MD, PhD, professor of dermatology at Wake Forest, “the most important conclusion, and it is a definitive finding, [is] that IBD is uncommon, even in patients with AD.
“The findings could be misinterpreted,” cautioned Dr. Feldman, who was not involved in the study. “While there is an increased relative risk, the absolute risk is small.” The study found that “the highest relative risk group is children with severe AD, who have a roughly fivefold increased risk for CD.” However, he added, the incidence rates of CD were 0.68 per 1,000 person-years in children with severe AD and 0.08 per 1,000 person-years in controls.
“Basically, because Crohn’s disease and IBD don’t happen very often, the modest increase in relative risk the investigators found doesn’t amount to much we’d have to worry about,” he said. “The findings do not show any need to screen patients with atopic dermatitis for IBD any more than we’d need to screen patients without atopic dermatitis.”
The increased relative risk “could be a clue to possible genetic connections between diseases,” he added. “But when we’re making clinical decisions, those decisions should be based on the absolute risk that some event may occur.”
Susan Massick, MD, dermatologist and associate professor at The Ohio State University in Columbus, who was not involved with the study, said in an interview, “We are still scratching the surface of the complexity of the immune and inflammatory pathways in AD and IBD.
“It is important to remember that correlation does not mean causation,” Dr. Massick said. “It would be premature to draw direct conclusions based on this study alone.”
The authors recommend future related studies in more diverse populations.
Dr. Gelfand and two coauthors reported ties with Pfizer, which supported the study. Dr. Gelfand and three coauthors reported ties with other pharmaceutical companies. Dr. Maguiness, Dr. Scarberry, Dr. Strowd, and Dr. Massick reported having no relevant disclosures. Dr. Feldman reported ties with Pfizer and other pharmaceutical companies.
A version of this article appeared on Medscape.com.
The published recently in JAMA Dermatology.
The study also found an increased risk for Crohn’s disease (CD) in adults and children with AD, as well as an increased risk for ulcerative colitis (UC) in adults with AD and in children with severe AD, researchers reported.
“It is imperative for clinicians to understand atopic dermatitis and the trajectory of our patients with it in order to provide the best standard of care,” senior author Joel M. Gelfand, MD, MSCE, professor in clinical investigation with the department of dermatology at the University of Pennsylvania, Philadelphia, said in a news release.
“There are new and better treatments for AD today, and there will likely continue to be more,” continued Dr. Gelfand. “But providers have to understand how those treatments could impact other autoimmune diseases. For patients with AD and another autoimmune disease, some currently available medications can exacerbate symptoms of their other disease or can help treat two immune diseases at the same time.”
The study results support the idea that AD and IBD may have some common underlying causes, said Sheilagh Maguiness, MD, pediatric dermatologist and associate professor in the department of dermatology at the University of Minnesota, Minneapolis, who was asked to comment on the findings.
“As the pathogenesis of AD is becoming better understood, we are recognizing that, rather than simply a cutaneous disease, the underlying inflammation and immune dysregulation that leads to AD best categorizes it as a systemic inflammatory disease with significant comorbidities,” she told this news organization. “I will be more likely to ask patients and families about GI symptoms, and if positive, may plan to refer to GI more readily than in the past,” added Dr. Maguiness, who was not involved in the study.
UK general practice cohort
AD has been associated with an increasing number of comorbidities, including IBD, but studies linking AD with IBD, including UC, have had mixed results, the authors wrote. And few studies have separately examined how AD or AD severity may be linked with UC or CD risk.
To examine the risk for new-onset IBD, UC, and CD in children and adults with atopic dermatitis, the researchers conducted a population-based cohort study using the THIN (The Health Improvement Network) electronic medical record database of patients registered with United Kingdom general practices. They used 21 years of data collected from January 1994 to February 2015.
The researchers matched each patient who had AD with up to five controls based on age, practice, and index date. Because THIN does not capture AD severity, they used treatment exposure assessed by dermatologic referrals and treatments patients received as proxy for severity. The authors used logistic regression to examine the risks for IBD, UC, and CD in children (aged 1-10) with AD, and in adults (aged 30-68) with AD, and they compared their outcomes with the outcomes for controls.
In the pediatric cohort, the team compared 409,431 children who had AD with 1.8 million children without AD. Slightly more than half were boys. In the adult cohort, they compared 625,083 people who had AD with 2.68 million controls, and slightly more than half were women. Data on race or ethnicity were not available, the authors wrote, but the THIN database is considered to be representative of the UK population.
AD severity linked with IBD risk
The risk for new-onset inflammatory bowel disease appears to be higher in children and adults with AD, and the risk varies based on age, AD severity, and subtype of inflammatory bowel disease, the authors reported.
Overall, AD in children was associated with a 44% increased risk for IBD (adjusted hazard ratio (HR), 1.44; 95% confidence interval [CI], 1.31-1.58) compared with controls, the authors reported. They found a 74% increased risk for CD in children with AD compared with controls (HR, 1.74; 95% CI, 1.54-1.97). More severe AD was linked with increased risk for both IBD and CD.
AD did not appear to increase risk for UC in children, except those with severe AD (HR, 1.65; 95% CI, 1.02-2.67).
Overall, adults with AD had a 34% (HR, 1.34; 95% CI, 1.27-1.40) increased risk for IBD, a 36% (HR, 1.36; 95% CI, 1.26-1.47) increased risk for CD, and a 32% (HR, 1.32; 95% CI, 1.24-1.41) increased risk for UC, with risk increasing with increased AD severity.
Robust data with cautionary note
“This study provides the most robust data to date on the association between IBD and AD. It provides clear evidence for an association that most dermatologists or primary care providers are not typically taught in training,” Kelly Scarberry, MD, assistant professor of dermatology at Case Western Reserve University in Cleveland, told this news organization. “I will be much more likely to pursue diagnostic workup in my AD patients who have GI complaints.”
However, AD severity was measured by proxy, added Dr. Scarberry, who was not involved in the study, and the study lacked important racial and ethnic data.
Lindsay C. Strowd, MD, associate professor of dermatology at Wake Forest University, Winston-Salem, N.C., also not involved in the study, said in an interview that she found the size of the cohort and the longitudinal data to be strengths of the study.
But, she added, the “lack of family IBD history, race and ethnicity, and comorbidities, are limitations, as is treatment exposure used as a proxy for disease severity, given that physician treatment practices differ.”
For Steven R. Feldman, MD, PhD, professor of dermatology at Wake Forest, “the most important conclusion, and it is a definitive finding, [is] that IBD is uncommon, even in patients with AD.
“The findings could be misinterpreted,” cautioned Dr. Feldman, who was not involved in the study. “While there is an increased relative risk, the absolute risk is small.” The study found that “the highest relative risk group is children with severe AD, who have a roughly fivefold increased risk for CD.” However, he added, the incidence rates of CD were 0.68 per 1,000 person-years in children with severe AD and 0.08 per 1,000 person-years in controls.
“Basically, because Crohn’s disease and IBD don’t happen very often, the modest increase in relative risk the investigators found doesn’t amount to much we’d have to worry about,” he said. “The findings do not show any need to screen patients with atopic dermatitis for IBD any more than we’d need to screen patients without atopic dermatitis.”
The increased relative risk “could be a clue to possible genetic connections between diseases,” he added. “But when we’re making clinical decisions, those decisions should be based on the absolute risk that some event may occur.”
Susan Massick, MD, dermatologist and associate professor at The Ohio State University in Columbus, who was not involved with the study, said in an interview, “We are still scratching the surface of the complexity of the immune and inflammatory pathways in AD and IBD.
“It is important to remember that correlation does not mean causation,” Dr. Massick said. “It would be premature to draw direct conclusions based on this study alone.”
The authors recommend future related studies in more diverse populations.
Dr. Gelfand and two coauthors reported ties with Pfizer, which supported the study. Dr. Gelfand and three coauthors reported ties with other pharmaceutical companies. Dr. Maguiness, Dr. Scarberry, Dr. Strowd, and Dr. Massick reported having no relevant disclosures. Dr. Feldman reported ties with Pfizer and other pharmaceutical companies.
A version of this article appeared on Medscape.com.
FROM JAMA DERMATOLOGY
FDA approves bosutinib for children with CML
The agency also approved new 50-mg and 100-mg capsules to help treat children.
For newly diagnosed disease, the dose is 300 mg/m2 once daily with food. For resistant/intolerant disease, the dose is 400 mg/m2 once daily. For children who cannot swallow capsules, the contents can be mixed into applesauce or yogurt, the FDA said in a press release announcing the approval.
The tyrosine kinase inhibitor (TKI) was previously approved for adults. Three other TKIs were previously approved for pediatric CML.
The approval was based on the BCHILD trial, a pediatric dose-finding study involving patients aged 1 year or older. Among the 21 children with newly diagnosed chronic phase, Ph+ CML treated with 300 mg/m2, the rate of major cytogenetic response was 76.2%, the rate of complete cytogenetic response was 71.4%, and the rate of major molecular response rate was 28.6% over a median duration of 14.2 months.
Among the 28 children with relapsed/intolerant disease treated with up to 400 mg/m2, the rate of major cytogenetic response was 82.1%, the rate of complete cytogenetic response was 78.6%, and the rate of major molecular response was 50% over a median duration of 23.2 months. Among the 14 patients who had a major molecular response, two lost it – one after 13.6 months of treatment, and the other after 24.7 months of treatment.
Adverse events that occurred in 20% or more of children included diarrhea, abdominal pain, vomiting, nausea, rash, fatigue, hepatic dysfunction, headache, pyrexia, decreased appetite, and constipation. Overall, 45% or more of patients experienced an increase in creatinine, alanine aminotransferase, or aspartate aminotransferase levels, or a decrease in white blood cell count or platelet count.
The full labeling information is available online.
A version of this article first appeared on Medscape.com.
The agency also approved new 50-mg and 100-mg capsules to help treat children.
For newly diagnosed disease, the dose is 300 mg/m2 once daily with food. For resistant/intolerant disease, the dose is 400 mg/m2 once daily. For children who cannot swallow capsules, the contents can be mixed into applesauce or yogurt, the FDA said in a press release announcing the approval.
The tyrosine kinase inhibitor (TKI) was previously approved for adults. Three other TKIs were previously approved for pediatric CML.
The approval was based on the BCHILD trial, a pediatric dose-finding study involving patients aged 1 year or older. Among the 21 children with newly diagnosed chronic phase, Ph+ CML treated with 300 mg/m2, the rate of major cytogenetic response was 76.2%, the rate of complete cytogenetic response was 71.4%, and the rate of major molecular response rate was 28.6% over a median duration of 14.2 months.
Among the 28 children with relapsed/intolerant disease treated with up to 400 mg/m2, the rate of major cytogenetic response was 82.1%, the rate of complete cytogenetic response was 78.6%, and the rate of major molecular response was 50% over a median duration of 23.2 months. Among the 14 patients who had a major molecular response, two lost it – one after 13.6 months of treatment, and the other after 24.7 months of treatment.
Adverse events that occurred in 20% or more of children included diarrhea, abdominal pain, vomiting, nausea, rash, fatigue, hepatic dysfunction, headache, pyrexia, decreased appetite, and constipation. Overall, 45% or more of patients experienced an increase in creatinine, alanine aminotransferase, or aspartate aminotransferase levels, or a decrease in white blood cell count or platelet count.
The full labeling information is available online.
A version of this article first appeared on Medscape.com.
The agency also approved new 50-mg and 100-mg capsules to help treat children.
For newly diagnosed disease, the dose is 300 mg/m2 once daily with food. For resistant/intolerant disease, the dose is 400 mg/m2 once daily. For children who cannot swallow capsules, the contents can be mixed into applesauce or yogurt, the FDA said in a press release announcing the approval.
The tyrosine kinase inhibitor (TKI) was previously approved for adults. Three other TKIs were previously approved for pediatric CML.
The approval was based on the BCHILD trial, a pediatric dose-finding study involving patients aged 1 year or older. Among the 21 children with newly diagnosed chronic phase, Ph+ CML treated with 300 mg/m2, the rate of major cytogenetic response was 76.2%, the rate of complete cytogenetic response was 71.4%, and the rate of major molecular response rate was 28.6% over a median duration of 14.2 months.
Among the 28 children with relapsed/intolerant disease treated with up to 400 mg/m2, the rate of major cytogenetic response was 82.1%, the rate of complete cytogenetic response was 78.6%, and the rate of major molecular response was 50% over a median duration of 23.2 months. Among the 14 patients who had a major molecular response, two lost it – one after 13.6 months of treatment, and the other after 24.7 months of treatment.
Adverse events that occurred in 20% or more of children included diarrhea, abdominal pain, vomiting, nausea, rash, fatigue, hepatic dysfunction, headache, pyrexia, decreased appetite, and constipation. Overall, 45% or more of patients experienced an increase in creatinine, alanine aminotransferase, or aspartate aminotransferase levels, or a decrease in white blood cell count or platelet count.
The full labeling information is available online.
A version of this article first appeared on Medscape.com.
Diagnosing pediatric forearm fractures: Radiograph or ultrasound?
TOPLINE:
as well as their waiting time in ED.
METHODOLOGY:
- After the World Health Organization reported a lack of access to any diagnostic imaging in approximately two-thirds of the world population in 2010, ultrasonography has gained popularity in low- and middle-income countries.
- The initial use of ultrasonography is in accordance with the principle of maintaining radiation levels as low as reasonably achievable.
- The BUCKLED trial was conducted, including 270 pediatric patients (age, 5-15 years) who presented to the ED with isolated, acute, clinically nondeformed distal forearm fractures.
- The participants were randomly assigned to receive initial point-of-care ultrasonography (n = 135) or radiography (n = 135) in the ED.
- The primary outcome was the physical function of the affected arm at 4 weeks evaluated using the Pediatric Upper Extremity Short Patient-Reported Outcomes Measurement Information System (PROMIS) tool.
TAKEAWAY:
- At 4 weeks, mean PROMIS scores were 36.4 and 36.3 points in ultrasonography and radiography groups, respectively (mean difference, 0.1 point; 95% confidence interval, − 1.3 to 1.4), indicating noninferiority of ultrasonography over radiography.
- Ultrasonography and radiography groups showed similar efficacy in terms of PROMIS scores at 1 week (MD, 0.7 points; 95% CI, − 1.4 to 2.8) and 8 weeks (MD, 0.1 points; 95% CI, − 0.5 to 0.7).
- Participants in the ultrasonography group had a shorter length of stay in the ED (median difference, 15 minutes; 95% CI, 1-29) and a shorter treatment time (median difference, 28 minutes; 95% CI, 17-40) than those in the radiography group.
- No important fractures were missed with ultrasonography, and no significant difference was observed in the frequency of adverse events or unplanned returns to the ED between the two groups.
IN PRACTICE:
Noting the benefit-risk profile of an ultrasound-first approach in an ED setting, the lead author, Peter J. Snelling, MB, BS, MPH&TM, from Menzies Health Institute Queensland, Gold Coast, Australia, said: “It is highly unlikely that any important fractures would be missed using the protocol that we trained clinicians. The risk is low and the benefit is moderate, such as reducing length of stay and increased level of patient satisfaction.”
He further added that, “with an ultrasound-first approach, clinicians can scan the patient at time of review and may even be able to discharge them immediately (two-thirds of instances in our NEJM trial). This places the patient at the center of care being provided.”
SOURCE:
Authors from the BUCKLED Trial Group published their study in the New England Journal of Medicine.
LIMITATIONS:
PROMIS scores may have been affected by variations in subsequent therapeutic interventions rather than the initial diagnostic method. PROMIS tool was not validated in children younger than 5 years of age.
DISCLOSURES:
The study was funded by the Emergency Medicine Foundation and others. The authors have declared no relevant interests to disclose.
A version of this article first appeared on Medscape.com.
TOPLINE:
as well as their waiting time in ED.
METHODOLOGY:
- After the World Health Organization reported a lack of access to any diagnostic imaging in approximately two-thirds of the world population in 2010, ultrasonography has gained popularity in low- and middle-income countries.
- The initial use of ultrasonography is in accordance with the principle of maintaining radiation levels as low as reasonably achievable.
- The BUCKLED trial was conducted, including 270 pediatric patients (age, 5-15 years) who presented to the ED with isolated, acute, clinically nondeformed distal forearm fractures.
- The participants were randomly assigned to receive initial point-of-care ultrasonography (n = 135) or radiography (n = 135) in the ED.
- The primary outcome was the physical function of the affected arm at 4 weeks evaluated using the Pediatric Upper Extremity Short Patient-Reported Outcomes Measurement Information System (PROMIS) tool.
TAKEAWAY:
- At 4 weeks, mean PROMIS scores were 36.4 and 36.3 points in ultrasonography and radiography groups, respectively (mean difference, 0.1 point; 95% confidence interval, − 1.3 to 1.4), indicating noninferiority of ultrasonography over radiography.
- Ultrasonography and radiography groups showed similar efficacy in terms of PROMIS scores at 1 week (MD, 0.7 points; 95% CI, − 1.4 to 2.8) and 8 weeks (MD, 0.1 points; 95% CI, − 0.5 to 0.7).
- Participants in the ultrasonography group had a shorter length of stay in the ED (median difference, 15 minutes; 95% CI, 1-29) and a shorter treatment time (median difference, 28 minutes; 95% CI, 17-40) than those in the radiography group.
- No important fractures were missed with ultrasonography, and no significant difference was observed in the frequency of adverse events or unplanned returns to the ED between the two groups.
IN PRACTICE:
Noting the benefit-risk profile of an ultrasound-first approach in an ED setting, the lead author, Peter J. Snelling, MB, BS, MPH&TM, from Menzies Health Institute Queensland, Gold Coast, Australia, said: “It is highly unlikely that any important fractures would be missed using the protocol that we trained clinicians. The risk is low and the benefit is moderate, such as reducing length of stay and increased level of patient satisfaction.”
He further added that, “with an ultrasound-first approach, clinicians can scan the patient at time of review and may even be able to discharge them immediately (two-thirds of instances in our NEJM trial). This places the patient at the center of care being provided.”
SOURCE:
Authors from the BUCKLED Trial Group published their study in the New England Journal of Medicine.
LIMITATIONS:
PROMIS scores may have been affected by variations in subsequent therapeutic interventions rather than the initial diagnostic method. PROMIS tool was not validated in children younger than 5 years of age.
DISCLOSURES:
The study was funded by the Emergency Medicine Foundation and others. The authors have declared no relevant interests to disclose.
A version of this article first appeared on Medscape.com.
TOPLINE:
as well as their waiting time in ED.
METHODOLOGY:
- After the World Health Organization reported a lack of access to any diagnostic imaging in approximately two-thirds of the world population in 2010, ultrasonography has gained popularity in low- and middle-income countries.
- The initial use of ultrasonography is in accordance with the principle of maintaining radiation levels as low as reasonably achievable.
- The BUCKLED trial was conducted, including 270 pediatric patients (age, 5-15 years) who presented to the ED with isolated, acute, clinically nondeformed distal forearm fractures.
- The participants were randomly assigned to receive initial point-of-care ultrasonography (n = 135) or radiography (n = 135) in the ED.
- The primary outcome was the physical function of the affected arm at 4 weeks evaluated using the Pediatric Upper Extremity Short Patient-Reported Outcomes Measurement Information System (PROMIS) tool.
TAKEAWAY:
- At 4 weeks, mean PROMIS scores were 36.4 and 36.3 points in ultrasonography and radiography groups, respectively (mean difference, 0.1 point; 95% confidence interval, − 1.3 to 1.4), indicating noninferiority of ultrasonography over radiography.
- Ultrasonography and radiography groups showed similar efficacy in terms of PROMIS scores at 1 week (MD, 0.7 points; 95% CI, − 1.4 to 2.8) and 8 weeks (MD, 0.1 points; 95% CI, − 0.5 to 0.7).
- Participants in the ultrasonography group had a shorter length of stay in the ED (median difference, 15 minutes; 95% CI, 1-29) and a shorter treatment time (median difference, 28 minutes; 95% CI, 17-40) than those in the radiography group.
- No important fractures were missed with ultrasonography, and no significant difference was observed in the frequency of adverse events or unplanned returns to the ED between the two groups.
IN PRACTICE:
Noting the benefit-risk profile of an ultrasound-first approach in an ED setting, the lead author, Peter J. Snelling, MB, BS, MPH&TM, from Menzies Health Institute Queensland, Gold Coast, Australia, said: “It is highly unlikely that any important fractures would be missed using the protocol that we trained clinicians. The risk is low and the benefit is moderate, such as reducing length of stay and increased level of patient satisfaction.”
He further added that, “with an ultrasound-first approach, clinicians can scan the patient at time of review and may even be able to discharge them immediately (two-thirds of instances in our NEJM trial). This places the patient at the center of care being provided.”
SOURCE:
Authors from the BUCKLED Trial Group published their study in the New England Journal of Medicine.
LIMITATIONS:
PROMIS scores may have been affected by variations in subsequent therapeutic interventions rather than the initial diagnostic method. PROMIS tool was not validated in children younger than 5 years of age.
DISCLOSURES:
The study was funded by the Emergency Medicine Foundation and others. The authors have declared no relevant interests to disclose.
A version of this article first appeared on Medscape.com.
FROM THE NEW ENGLAND JOURNAL OF MEDICINE
Hypotrichosis and Hair Loss on the Occipital Scalp
The Diagnosis: Monilethrix
A diagnosis of monilethrix was rendered based on the clinical and trichoscopic findings. Simple surveillance of the patient’s condition and prevention of further hair trauma were proposed as management options.
Monilethrix is a hair shaft disorder that is inherited in a predominantly autosomal-dominant pattern with variable expressiveness and penetrance resulting from heterozygous mutations in hair keratin genes KRT81, KRT83, and KRT86 in a region of chromosome 12q13.13.1,2 An autosomalrecessive form has been described with mutation in desmoglein 4, but it differs from the classical form by the variable periodicity of the region between the nodules.3,4
The morphologic alteration consists of the formation of fusiform nodules of normal structure alternated with narrow and dystrophic constrictions (Figure). These internodes are fragile areas that cause breakage at constricted points.5 Clinically, monilethrix presents as areas of focal or diffuse alopecia with frequent involvement of the terminal follicles, mainly in areas of friction. The hair is normal at birth due to the predominance of lanugo in the neonatal period, but it subsequently is replaced by abnormal hairs in the first months of life.6 Initial clinical signs begin to appear when the terminal hairs begin to form.7 Although rarer, the eyebrows and eyelashes, as well as the axillary, pubic, and body hair, may be involved.5
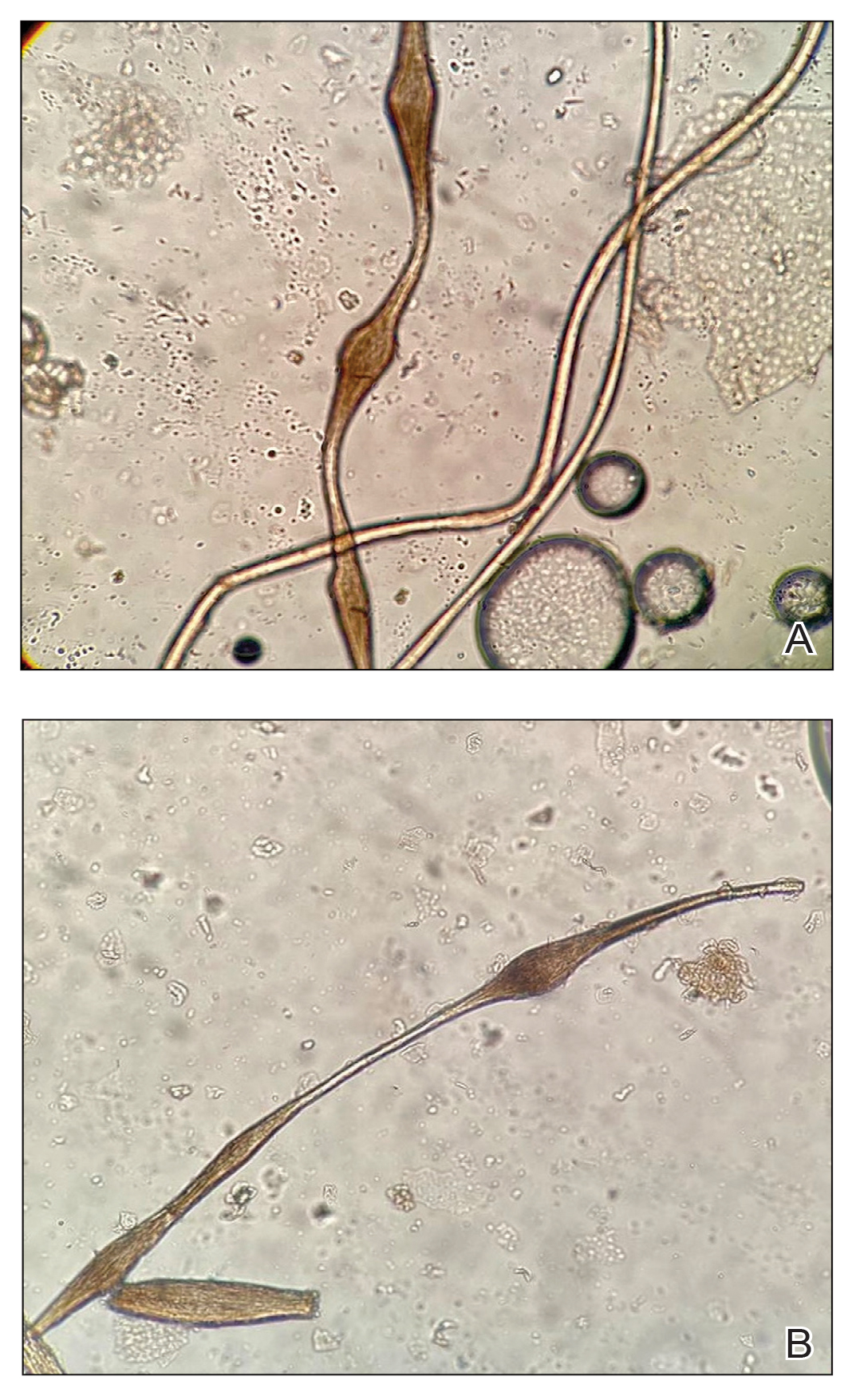
Other hair shaft anomalies merit consideration in the differential diagnosis of monilethrix, including pseudomonilethrix, pressure alopecia, trichorrhexis invaginata, ectodermal dysplasia, tinea capitis, and trichothiodystrophy.6 The diagnosis is reached by clinical history and physical examination. Trichoscopy and light microscopy are used to confirm the diagnosis. Trichoscopic examination shows markedly higher rates of anagen hair. The shafts examined in our patient revealed 0.7- to 1-mm intervals between nodes. Hair can be better visualized under a polarized microscope, and the condition can be distinguished from pseudomonilethrix using this approach.5,6 In our patient, the diagnosis was made based on light microscopy and trichoscopic findings with no genetic testing; however, genetic testing for the classic mutations of the keratin genes would be desirable to confirm the diagnosis but was not done in our patient.6 The prognosis of monilethrix is variable; most cases persist into adulthood, though spontaneous improvement may occur with advancing age, during summer, and during pregnancy.8
There is no definitive therapy for monilethrix. Although there have been reports of cases treated with systemic corticosteroids, oral retinoids, topical minoxidil, vitamins, and peeling ointments (desquamative oil), the cornerstone of management is protecting the hair against traumatic procedures such as excessive combing, brushing, and friction, as well as parent and patient education about the benign nature of the condition.9 Additionally, some cases have shown improvement with minoxidil solution at 2% and 5% concentrations, oral minoxidil, or acitretin.7-9
- Fontenelle de Oliveira E, Cotta de Alencar Araripe AL. Monilethrix: a typical case report with microscopic and dermatoscopic findings. An Bras Dermatol. 2015;90:126-127.
- de Cruz R, Horev L, Green J, et al. A novel monilethrix mutation in coil 2A of KRT86 causing autosomal dominant monilethrix with incomplete penetrance. Br J Dermatol. 2012;166(suppl 2):20-26.
- Baltazard T, Dhaille F, Chaby G, et al. Value of dermoscopy for the diagnosis of monilethrix. Dermatol Online J. 2017;23:13030 /qt9hf1p3xm.
- Kato M, Shimizu A, Yokoyama Y, et al. An autosomal recessive mutation of DSG4 causes monilethrix through the ER stress response. J Invest Dermatol. 2015;135:1253-1260.
- Gummer CL, Dawber RP, Swift JA. Monilethrix: an electron microscopic and electron histochemical study. Br J Dermatol. 1981;105:529-541.
- Sharma VK, Chiramel MJ, Rao A. Dermoscopy: a rapid bedside tool to assess monilethrix. Indian J Dermatol Venereol Leprol. 2016;82:73-74.
- Sinclair R. Treatment of monilethrix with oral minoxidil. JAAD Case Rep. 2016;2:212-215.
- Rakowska A, Slowinska M, Czuwara J, et al. Dermoscopy as a tool for rapid diagnosis of monilethrix. J Drugs Dermatol. 2007;6:222-224.
- Karincaoglu Y, Coskun BK, Seyhan ME, et al. Monilethrix. Am J Clin Dermatol. 2005;6:407-410.
The Diagnosis: Monilethrix
A diagnosis of monilethrix was rendered based on the clinical and trichoscopic findings. Simple surveillance of the patient’s condition and prevention of further hair trauma were proposed as management options.
Monilethrix is a hair shaft disorder that is inherited in a predominantly autosomal-dominant pattern with variable expressiveness and penetrance resulting from heterozygous mutations in hair keratin genes KRT81, KRT83, and KRT86 in a region of chromosome 12q13.13.1,2 An autosomalrecessive form has been described with mutation in desmoglein 4, but it differs from the classical form by the variable periodicity of the region between the nodules.3,4
The morphologic alteration consists of the formation of fusiform nodules of normal structure alternated with narrow and dystrophic constrictions (Figure). These internodes are fragile areas that cause breakage at constricted points.5 Clinically, monilethrix presents as areas of focal or diffuse alopecia with frequent involvement of the terminal follicles, mainly in areas of friction. The hair is normal at birth due to the predominance of lanugo in the neonatal period, but it subsequently is replaced by abnormal hairs in the first months of life.6 Initial clinical signs begin to appear when the terminal hairs begin to form.7 Although rarer, the eyebrows and eyelashes, as well as the axillary, pubic, and body hair, may be involved.5

Other hair shaft anomalies merit consideration in the differential diagnosis of monilethrix, including pseudomonilethrix, pressure alopecia, trichorrhexis invaginata, ectodermal dysplasia, tinea capitis, and trichothiodystrophy.6 The diagnosis is reached by clinical history and physical examination. Trichoscopy and light microscopy are used to confirm the diagnosis. Trichoscopic examination shows markedly higher rates of anagen hair. The shafts examined in our patient revealed 0.7- to 1-mm intervals between nodes. Hair can be better visualized under a polarized microscope, and the condition can be distinguished from pseudomonilethrix using this approach.5,6 In our patient, the diagnosis was made based on light microscopy and trichoscopic findings with no genetic testing; however, genetic testing for the classic mutations of the keratin genes would be desirable to confirm the diagnosis but was not done in our patient.6 The prognosis of monilethrix is variable; most cases persist into adulthood, though spontaneous improvement may occur with advancing age, during summer, and during pregnancy.8
There is no definitive therapy for monilethrix. Although there have been reports of cases treated with systemic corticosteroids, oral retinoids, topical minoxidil, vitamins, and peeling ointments (desquamative oil), the cornerstone of management is protecting the hair against traumatic procedures such as excessive combing, brushing, and friction, as well as parent and patient education about the benign nature of the condition.9 Additionally, some cases have shown improvement with minoxidil solution at 2% and 5% concentrations, oral minoxidil, or acitretin.7-9
The Diagnosis: Monilethrix
A diagnosis of monilethrix was rendered based on the clinical and trichoscopic findings. Simple surveillance of the patient’s condition and prevention of further hair trauma were proposed as management options.
Monilethrix is a hair shaft disorder that is inherited in a predominantly autosomal-dominant pattern with variable expressiveness and penetrance resulting from heterozygous mutations in hair keratin genes KRT81, KRT83, and KRT86 in a region of chromosome 12q13.13.1,2 An autosomalrecessive form has been described with mutation in desmoglein 4, but it differs from the classical form by the variable periodicity of the region between the nodules.3,4
The morphologic alteration consists of the formation of fusiform nodules of normal structure alternated with narrow and dystrophic constrictions (Figure). These internodes are fragile areas that cause breakage at constricted points.5 Clinically, monilethrix presents as areas of focal or diffuse alopecia with frequent involvement of the terminal follicles, mainly in areas of friction. The hair is normal at birth due to the predominance of lanugo in the neonatal period, but it subsequently is replaced by abnormal hairs in the first months of life.6 Initial clinical signs begin to appear when the terminal hairs begin to form.7 Although rarer, the eyebrows and eyelashes, as well as the axillary, pubic, and body hair, may be involved.5

Other hair shaft anomalies merit consideration in the differential diagnosis of monilethrix, including pseudomonilethrix, pressure alopecia, trichorrhexis invaginata, ectodermal dysplasia, tinea capitis, and trichothiodystrophy.6 The diagnosis is reached by clinical history and physical examination. Trichoscopy and light microscopy are used to confirm the diagnosis. Trichoscopic examination shows markedly higher rates of anagen hair. The shafts examined in our patient revealed 0.7- to 1-mm intervals between nodes. Hair can be better visualized under a polarized microscope, and the condition can be distinguished from pseudomonilethrix using this approach.5,6 In our patient, the diagnosis was made based on light microscopy and trichoscopic findings with no genetic testing; however, genetic testing for the classic mutations of the keratin genes would be desirable to confirm the diagnosis but was not done in our patient.6 The prognosis of monilethrix is variable; most cases persist into adulthood, though spontaneous improvement may occur with advancing age, during summer, and during pregnancy.8
There is no definitive therapy for monilethrix. Although there have been reports of cases treated with systemic corticosteroids, oral retinoids, topical minoxidil, vitamins, and peeling ointments (desquamative oil), the cornerstone of management is protecting the hair against traumatic procedures such as excessive combing, brushing, and friction, as well as parent and patient education about the benign nature of the condition.9 Additionally, some cases have shown improvement with minoxidil solution at 2% and 5% concentrations, oral minoxidil, or acitretin.7-9
- Fontenelle de Oliveira E, Cotta de Alencar Araripe AL. Monilethrix: a typical case report with microscopic and dermatoscopic findings. An Bras Dermatol. 2015;90:126-127.
- de Cruz R, Horev L, Green J, et al. A novel monilethrix mutation in coil 2A of KRT86 causing autosomal dominant monilethrix with incomplete penetrance. Br J Dermatol. 2012;166(suppl 2):20-26.
- Baltazard T, Dhaille F, Chaby G, et al. Value of dermoscopy for the diagnosis of monilethrix. Dermatol Online J. 2017;23:13030 /qt9hf1p3xm.
- Kato M, Shimizu A, Yokoyama Y, et al. An autosomal recessive mutation of DSG4 causes monilethrix through the ER stress response. J Invest Dermatol. 2015;135:1253-1260.
- Gummer CL, Dawber RP, Swift JA. Monilethrix: an electron microscopic and electron histochemical study. Br J Dermatol. 1981;105:529-541.
- Sharma VK, Chiramel MJ, Rao A. Dermoscopy: a rapid bedside tool to assess monilethrix. Indian J Dermatol Venereol Leprol. 2016;82:73-74.
- Sinclair R. Treatment of monilethrix with oral minoxidil. JAAD Case Rep. 2016;2:212-215.
- Rakowska A, Slowinska M, Czuwara J, et al. Dermoscopy as a tool for rapid diagnosis of monilethrix. J Drugs Dermatol. 2007;6:222-224.
- Karincaoglu Y, Coskun BK, Seyhan ME, et al. Monilethrix. Am J Clin Dermatol. 2005;6:407-410.
- Fontenelle de Oliveira E, Cotta de Alencar Araripe AL. Monilethrix: a typical case report with microscopic and dermatoscopic findings. An Bras Dermatol. 2015;90:126-127.
- de Cruz R, Horev L, Green J, et al. A novel monilethrix mutation in coil 2A of KRT86 causing autosomal dominant monilethrix with incomplete penetrance. Br J Dermatol. 2012;166(suppl 2):20-26.
- Baltazard T, Dhaille F, Chaby G, et al. Value of dermoscopy for the diagnosis of monilethrix. Dermatol Online J. 2017;23:13030 /qt9hf1p3xm.
- Kato M, Shimizu A, Yokoyama Y, et al. An autosomal recessive mutation of DSG4 causes monilethrix through the ER stress response. J Invest Dermatol. 2015;135:1253-1260.
- Gummer CL, Dawber RP, Swift JA. Monilethrix: an electron microscopic and electron histochemical study. Br J Dermatol. 1981;105:529-541.
- Sharma VK, Chiramel MJ, Rao A. Dermoscopy: a rapid bedside tool to assess monilethrix. Indian J Dermatol Venereol Leprol. 2016;82:73-74.
- Sinclair R. Treatment of monilethrix with oral minoxidil. JAAD Case Rep. 2016;2:212-215.
- Rakowska A, Slowinska M, Czuwara J, et al. Dermoscopy as a tool for rapid diagnosis of monilethrix. J Drugs Dermatol. 2007;6:222-224.
- Karincaoglu Y, Coskun BK, Seyhan ME, et al. Monilethrix. Am J Clin Dermatol. 2005;6:407-410.
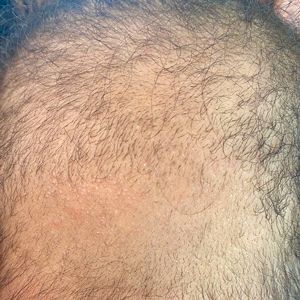
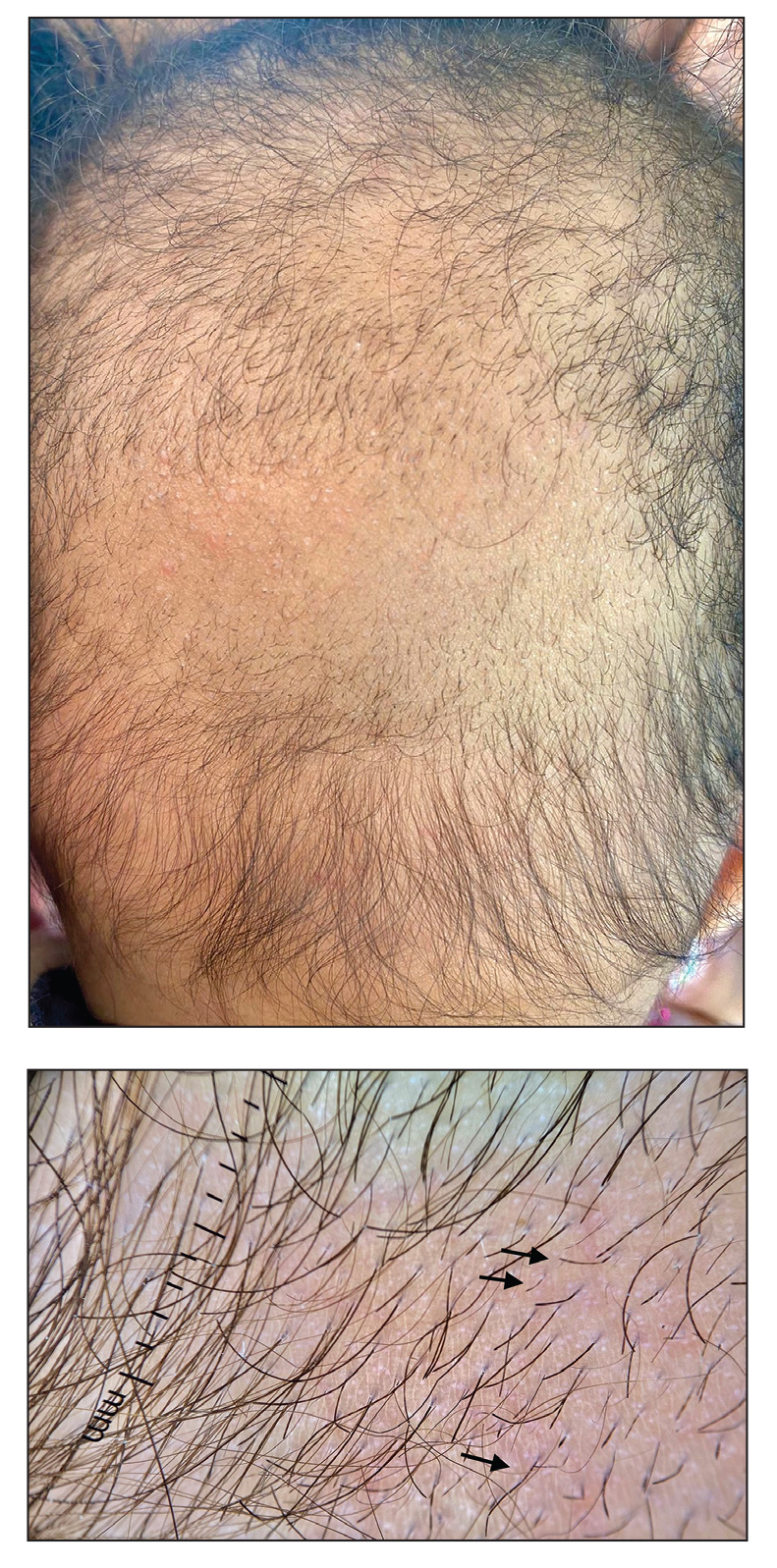
A 6-month-old infant girl was referred to the dermatology service with hypotrichosis and hair loss on the occipital region of the scalp of 4 months’ duration (top). The patient was born at full term by cesarean delivery without complications. There were no comorbidities or family history of alopecia. Clinical examination revealed an alopecic plaque in the occipital region with broken hairs and some dystrophic hairs associated with follicular papules and perifollicular hyperkeratosis. A hair pull test was positive for telogen hairs. Trichoscopy revealed black dots and broken hairs resembling Morse code (bottom). Hair microscopy showed regular alternation of constriction zones separated by intervals of normal thickness.
Cat Scratch Disease Presenting With Concurrent Pityriasis Rosea in a 10-Year-Old Girl
To the Editor:
Cat scratch disease (CSD) is caused by Bartonella henselae and Bartonella clarridgeiae bacteria transferred from cats to humans that results in an inflamed inoculation site and tender lymphadenopathy. Pityriasis rosea (PR) and PR-like eruptions are self-limited, acute exanthems that have been associated with infections, vaccinations, and medications. We report a case of PR occurring in a 10-year-old girl with CSD, which may suggest an association between the 2 diseases.

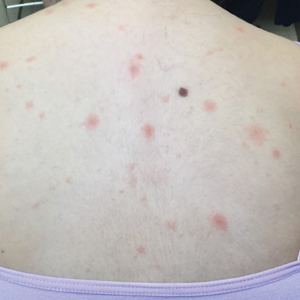
A 10-year-old girl who was otherwise healthy presented in the winter with a rash of 5 days’ duration. Fourteen days prior to the rash, the patient reported being scratched by a new kitten and noted a pinpoint “puncture” on the left forearm that developed into a red papule over the following week. Seven days after the cat scratch, the patient experienced pain and swelling in the left axilla. Approximately 1 week after the onset of lymphadenopathy, the patient developed an asymptomatic rash that started with a large spot on the left chest, followed by smaller spots appearing over the next 2 days and spreading to the rest of the trunk. Four days after the rash onset, the patient experienced a mild headache, low-grade subjective fever, and chills. She denied any recent travel, bug bites, sore throat, and diarrhea. She was up-to-date on all vaccinations and had not received any vaccines preceding the symptoms. Physical examination revealed a 2-cm pink, scaly, thin plaque with a collarette of scale on the left upper chest (herald patch), along with multiple thin pink papules and small plaques with central scale on the trunk (Figure 1). A pustule with adjacent linear erosion was present on the left ventral forearm (Figure 2). The patient had a tender subcutaneous nodule in the left axilla as well as bilateral anterior and posterior cervical-chain subcutaneous tender nodules. There was no involvement of the palms, soles, or mucosae.

The patient was empirically treated for CSD with azithromycin (200 mg/5 mL), 404 mg on day 1 followed by 202 mg daily for 4 days. The rash was treated with hydrocortisone cream 2.5% twice daily for 2 weeks. A wound culture of the pustule on the left forearm was negative for neutrophils and organisms. Antibody serologies obtained 4 weeks after presentation were notable for an elevated B henselae IgG titer of 1:640, confirming the diagnosis of CSD. Following treatment with azithromycin and hydrocortisone, all of the patient’s symptoms resolved after 1 to 2 weeks.
Cat scratch disease is a zoonotic infection caused by the bacteria B henselae and the more recently described pathogen B clarridgeiae. Cat fleas spread these bacteria among cats, which subsequently inoculate the bacteria into humans through bites and scratches. The incidence of CSD in the United States is estimated to be 4.5 to 9.3 per 100,000 individuals in the outpatient setting and 0.19 to 0.86 per 100,000 individuals in the inpatient setting.1 Geographic variance can occur based on flea populations, resulting in higher incidence in warm humid climates and lower incidence in mountainous arid climates. The incidence of CSD in the pediatric population is highest in children aged 5 to 9 years. A national representative survey (N=3011) from 2017 revealed that 37.2% of primary care providers had diagnosed CSD in the prior year.1
Classic CSD presents as an erythematous papule at the inoculation site lasting days to weeks, with progression to tender lymphadenopathy lasting weeks to months. Fever, malaise, and chills also can be seen. Atypical CSD occurs in up to 24% of cases in immunocompetent patients.1 Atypical and systemic presentations are varied and can include fever of unknown origin, neuroretinitis, uveitis, retinal vessel occlusion, encephalitis, hepatosplenic lesions, Parinaud oculoglandular syndrome, osteomyelitis, and endocarditis.1,2 Atypical dermatologic presentations of CSD include maculopapular rash in 7% of cases and erythema nodosum in 2.5% of cases, as well as rare reports of cutaneous vasculitis, urticaria, immune thrombocytopenic purpura, and papuloedematous eruption.3 Treatment guidelines for CSD vary widely depending on the clinical presentation as well as the immunocompetence of the infected individual. Our patient had limited regional lymphadenopathy with no signs of dissemination or neurologic involvement and was successfully treated with a 5-day course of oral azithromycin (weight based, 10 mg/kg). More extensive disease such as hepatosplenic or neurologic CSD may require multiple antibiotics for up to 6 weeks. Alternative or additional antibiotics used for CSD include rifampin, trimethoprim-sulfamethoxazole, ciprofloxacin, doxycycline, gentamicin, and clarithromycin. Opinions vary as to whether all patients or just those with complicated infections warrant antibiotic therapy.4-6
Pityriasis rosea is a self-limited acute exanthematous disease that is classically associated with a systemic reactivation of human herpesvirus (HHV) 6 and/or HHV-7. The incidence of PR is estimated to be 480 per 100,000 dermatologic patients. It is slightly more common in females and occurs most often in patients aged 10 to 35 years.7 Clinically, PR appears with the abrupt onset of a single erythematous scaly patch (termed the herald patch), followed by a secondary eruption of smaller erythematous scaly macules and patches along the trunk’s cleavage lines. The secondary eruption on the back is sometimes termed a Christmas or fir tree pattern.7,8
In addition to the classic presentation of PR, there have been reports of numerous atypical clinical presentations. The herald patch, which classically presents on the trunk, also has been reported to present on the extremities; PR of the extremities is defined by lesions that appear as large scaly plaques on the extremities only. Inverse PR presents with lesions occurring in flexural areas and acral surfaces but not on the trunk. There also is an acral PR variant in which lesions appear only on the palms, wrists, and soles. Purpuric or hemorrhagic PR has been described and presents with purpura and petechiae with or without collarettes of scale in diffuse locations, including the palate. Oral PR presents more commonly in patients of color as erosions, ulcers, hemorrhagic lesions, bullae, or geographic tongue. Erythema multiforme–like PR appears with targetoid lesions on the trunk, face, neck, and arms without a history of herpes simplex virus infection. A large pear-shaped herald patch has been reported and characterizes the gigantea PR of Darier variant. Irritated PR occurs with typical PR findings, but afflicted patients report severe pain and burning with diaphoresis. Relapsing PR can occur within 1 year of a prior episode of PR and presents without a herald patch. Persistent PR is defined by PR lasting more than 3 months, and most reported cases have included oral lesions. Finally, other PR variants that have been described include urticarial, papular, follicular, vesicular, and hypopigmented types.7-9
Furthermore, there have been reports of multiple atypical presentations occurring simultaneously in the same patient.10 Although PR classically has been associated with HHV-6 and/or HHV-7 reactivation, it has been reported with a few other clinical situations and conditions. Pityriasislike eruption specifically refers to an exanthem secondary to drugs or vaccination that resembles PR but shows clinical differences, including diffuse and confluent dusky-red macules and/or plaques with or without desquamation on the trunk, extremities, and face. Drugs that have been implicated as triggers include ACE inhibitors, gold, isotretinoin, nonsteroidal anti-inflammatory agents, omeprazole, terbinafine, and tyrosine kinase inhibitors. Smallpox, tuberculosis, poliomyelitis, influenza, diphtheria, tetanus, hepatitis B virus, pneumococcus, papillomavirus, yellow fever, and pertussis vaccinations also have been associated with PR.7,11,12 Additionally, PR has been reported to occur with active systemic infections, specifically H1N1 influenza, though it is rare.13 Because of its self-limited course, treatment of PR most often involves only reassurance. Topical corticosteroids may be appropriate for pruritus.7,8
Pediatric health care providers including dermatologists should be familiar with both CSD and PR because they are common diseases that more often are encountered in the pediatric population. We present a unique case of CSD presenting with concurrent PR, which highlights a potential new etiology for PR and a rare cutaneous manifestation of CSD. Further investigation into a possible relationship between CSD and PR may be warranted. Patients with any signs and symptoms of fever, tender lymphadenopathy, worsening rash, or exposure to cats warrant a thorough history and physical examination to ensure that neither entity is overlooked.
- Nelson CA, Moore AR, Perea AE, et al. Cat scratch disease: U.S. clinicians’ experience and knowledge [published online July 14, 2017]. Zoonoses Public Health. 2018;65:67-73. doi:10.1111/zph.12368
- Habot-Wilner Z, Trivizki O, Goldstein M, et al. Cat-scratch disease: ocular manifestations and treatment outcome. Acta Ophthalmol. 2018;96:E524-E532. doi:10.1111/aos.13684
- Schattner A, Uliel L, Dubin I. The cat did it: erythema nodosum and additional atypical presentations of Bartonella henselae infection in immunocompetent hosts [published online February 16, 2018]. BMJ Case Rep. doi:10.1136/bcr-2017-222511
- Shorbatli L, Koranyi K, Nahata M. Effectiveness of antibiotic therapy in pediatric patients with cat scratch disease. Int J Clin Pharm. 2018;40:1458-1461. doi: 10.1007/s11096-018-0746-1
- Bass JW, Freitas BC, Freitas AD, et al. Prospective randomized double blind placebo-controlled evaluation of azithromycin for treatment of cat-scratch disease. Pediatr Infect Dis J. 1998;17:447-452. doi:10.1097/00006454-199806000-00002
- Spach DH, Kaplan SL. Treatment of cat scratch disease. UpToDate. Updated December 9, 2021. Accessed September 12, 2023. https://www.uptodate.com/contents/treatment-of-cat-scratch-disease
- Drago F, Ciccarese G, Rebora A, et al. Pityriasis rosea: a comprehensive classification. Dermatology. 2016;232:431-437. doi:10.1159/000445375
- Urbina F, Das A, Sudy E. Clinical variants of pityriasis rosea. World J Clin Cases. 2017;5:203-211. doi:10.12998/wjcc.v5.i6.203
- Alzahrani NA, Al Jasser MI. Geographic tonguelike presentation in a child with pityriasis rosea: case report and review of oral manifestations of pityriasis rosea. Pediatr Dermatol. 2018;35:E124-E127. doi:10.1111/pde.13417
- Sinha S, Sardana K, Garg V. Coexistence of two atypical variants of pityriasis rosea: a case report and review of literature. Pediatr Dermatol. 2012;29:538-540. doi:10.1111/j.1525-1470.2011.01549.x
- Drago F, Ciccarese G, Parodi A. Pityriasis rosea and pityriasis rosea-like eruptions: how to distinguish them? JAAD Case Rep. 2018;4:800-801. doi:10.1016/j.jdcr.2018.04.002
- Drago F, Ciccarese G, Javor S, et al. Vaccine-induced pityriasis rosea and pityriasis rosea-like eruptions: a review of the literature. J Eur Acad Dermatol Venereol. 2016;30:544-545. doi:10.1111/jdv.12942
- Mubki TF, Bin Dayel SA, Kadry R. A case of pityriasis rosea concurrent with the novel influenza A (H1N1) infection. Pediatr Dermatol. 2011;28:341-342. doi:10.1111/j.1525-1470.2010.01090.x
To the Editor:
Cat scratch disease (CSD) is caused by Bartonella henselae and Bartonella clarridgeiae bacteria transferred from cats to humans that results in an inflamed inoculation site and tender lymphadenopathy. Pityriasis rosea (PR) and PR-like eruptions are self-limited, acute exanthems that have been associated with infections, vaccinations, and medications. We report a case of PR occurring in a 10-year-old girl with CSD, which may suggest an association between the 2 diseases.

A 10-year-old girl who was otherwise healthy presented in the winter with a rash of 5 days’ duration. Fourteen days prior to the rash, the patient reported being scratched by a new kitten and noted a pinpoint “puncture” on the left forearm that developed into a red papule over the following week. Seven days after the cat scratch, the patient experienced pain and swelling in the left axilla. Approximately 1 week after the onset of lymphadenopathy, the patient developed an asymptomatic rash that started with a large spot on the left chest, followed by smaller spots appearing over the next 2 days and spreading to the rest of the trunk. Four days after the rash onset, the patient experienced a mild headache, low-grade subjective fever, and chills. She denied any recent travel, bug bites, sore throat, and diarrhea. She was up-to-date on all vaccinations and had not received any vaccines preceding the symptoms. Physical examination revealed a 2-cm pink, scaly, thin plaque with a collarette of scale on the left upper chest (herald patch), along with multiple thin pink papules and small plaques with central scale on the trunk (Figure 1). A pustule with adjacent linear erosion was present on the left ventral forearm (Figure 2). The patient had a tender subcutaneous nodule in the left axilla as well as bilateral anterior and posterior cervical-chain subcutaneous tender nodules. There was no involvement of the palms, soles, or mucosae.

The patient was empirically treated for CSD with azithromycin (200 mg/5 mL), 404 mg on day 1 followed by 202 mg daily for 4 days. The rash was treated with hydrocortisone cream 2.5% twice daily for 2 weeks. A wound culture of the pustule on the left forearm was negative for neutrophils and organisms. Antibody serologies obtained 4 weeks after presentation were notable for an elevated B henselae IgG titer of 1:640, confirming the diagnosis of CSD. Following treatment with azithromycin and hydrocortisone, all of the patient’s symptoms resolved after 1 to 2 weeks.
Cat scratch disease is a zoonotic infection caused by the bacteria B henselae and the more recently described pathogen B clarridgeiae. Cat fleas spread these bacteria among cats, which subsequently inoculate the bacteria into humans through bites and scratches. The incidence of CSD in the United States is estimated to be 4.5 to 9.3 per 100,000 individuals in the outpatient setting and 0.19 to 0.86 per 100,000 individuals in the inpatient setting.1 Geographic variance can occur based on flea populations, resulting in higher incidence in warm humid climates and lower incidence in mountainous arid climates. The incidence of CSD in the pediatric population is highest in children aged 5 to 9 years. A national representative survey (N=3011) from 2017 revealed that 37.2% of primary care providers had diagnosed CSD in the prior year.1
Classic CSD presents as an erythematous papule at the inoculation site lasting days to weeks, with progression to tender lymphadenopathy lasting weeks to months. Fever, malaise, and chills also can be seen. Atypical CSD occurs in up to 24% of cases in immunocompetent patients.1 Atypical and systemic presentations are varied and can include fever of unknown origin, neuroretinitis, uveitis, retinal vessel occlusion, encephalitis, hepatosplenic lesions, Parinaud oculoglandular syndrome, osteomyelitis, and endocarditis.1,2 Atypical dermatologic presentations of CSD include maculopapular rash in 7% of cases and erythema nodosum in 2.5% of cases, as well as rare reports of cutaneous vasculitis, urticaria, immune thrombocytopenic purpura, and papuloedematous eruption.3 Treatment guidelines for CSD vary widely depending on the clinical presentation as well as the immunocompetence of the infected individual. Our patient had limited regional lymphadenopathy with no signs of dissemination or neurologic involvement and was successfully treated with a 5-day course of oral azithromycin (weight based, 10 mg/kg). More extensive disease such as hepatosplenic or neurologic CSD may require multiple antibiotics for up to 6 weeks. Alternative or additional antibiotics used for CSD include rifampin, trimethoprim-sulfamethoxazole, ciprofloxacin, doxycycline, gentamicin, and clarithromycin. Opinions vary as to whether all patients or just those with complicated infections warrant antibiotic therapy.4-6
Pityriasis rosea is a self-limited acute exanthematous disease that is classically associated with a systemic reactivation of human herpesvirus (HHV) 6 and/or HHV-7. The incidence of PR is estimated to be 480 per 100,000 dermatologic patients. It is slightly more common in females and occurs most often in patients aged 10 to 35 years.7 Clinically, PR appears with the abrupt onset of a single erythematous scaly patch (termed the herald patch), followed by a secondary eruption of smaller erythematous scaly macules and patches along the trunk’s cleavage lines. The secondary eruption on the back is sometimes termed a Christmas or fir tree pattern.7,8
In addition to the classic presentation of PR, there have been reports of numerous atypical clinical presentations. The herald patch, which classically presents on the trunk, also has been reported to present on the extremities; PR of the extremities is defined by lesions that appear as large scaly plaques on the extremities only. Inverse PR presents with lesions occurring in flexural areas and acral surfaces but not on the trunk. There also is an acral PR variant in which lesions appear only on the palms, wrists, and soles. Purpuric or hemorrhagic PR has been described and presents with purpura and petechiae with or without collarettes of scale in diffuse locations, including the palate. Oral PR presents more commonly in patients of color as erosions, ulcers, hemorrhagic lesions, bullae, or geographic tongue. Erythema multiforme–like PR appears with targetoid lesions on the trunk, face, neck, and arms without a history of herpes simplex virus infection. A large pear-shaped herald patch has been reported and characterizes the gigantea PR of Darier variant. Irritated PR occurs with typical PR findings, but afflicted patients report severe pain and burning with diaphoresis. Relapsing PR can occur within 1 year of a prior episode of PR and presents without a herald patch. Persistent PR is defined by PR lasting more than 3 months, and most reported cases have included oral lesions. Finally, other PR variants that have been described include urticarial, papular, follicular, vesicular, and hypopigmented types.7-9
Furthermore, there have been reports of multiple atypical presentations occurring simultaneously in the same patient.10 Although PR classically has been associated with HHV-6 and/or HHV-7 reactivation, it has been reported with a few other clinical situations and conditions. Pityriasislike eruption specifically refers to an exanthem secondary to drugs or vaccination that resembles PR but shows clinical differences, including diffuse and confluent dusky-red macules and/or plaques with or without desquamation on the trunk, extremities, and face. Drugs that have been implicated as triggers include ACE inhibitors, gold, isotretinoin, nonsteroidal anti-inflammatory agents, omeprazole, terbinafine, and tyrosine kinase inhibitors. Smallpox, tuberculosis, poliomyelitis, influenza, diphtheria, tetanus, hepatitis B virus, pneumococcus, papillomavirus, yellow fever, and pertussis vaccinations also have been associated with PR.7,11,12 Additionally, PR has been reported to occur with active systemic infections, specifically H1N1 influenza, though it is rare.13 Because of its self-limited course, treatment of PR most often involves only reassurance. Topical corticosteroids may be appropriate for pruritus.7,8
Pediatric health care providers including dermatologists should be familiar with both CSD and PR because they are common diseases that more often are encountered in the pediatric population. We present a unique case of CSD presenting with concurrent PR, which highlights a potential new etiology for PR and a rare cutaneous manifestation of CSD. Further investigation into a possible relationship between CSD and PR may be warranted. Patients with any signs and symptoms of fever, tender lymphadenopathy, worsening rash, or exposure to cats warrant a thorough history and physical examination to ensure that neither entity is overlooked.
To the Editor:
Cat scratch disease (CSD) is caused by Bartonella henselae and Bartonella clarridgeiae bacteria transferred from cats to humans that results in an inflamed inoculation site and tender lymphadenopathy. Pityriasis rosea (PR) and PR-like eruptions are self-limited, acute exanthems that have been associated with infections, vaccinations, and medications. We report a case of PR occurring in a 10-year-old girl with CSD, which may suggest an association between the 2 diseases.

A 10-year-old girl who was otherwise healthy presented in the winter with a rash of 5 days’ duration. Fourteen days prior to the rash, the patient reported being scratched by a new kitten and noted a pinpoint “puncture” on the left forearm that developed into a red papule over the following week. Seven days after the cat scratch, the patient experienced pain and swelling in the left axilla. Approximately 1 week after the onset of lymphadenopathy, the patient developed an asymptomatic rash that started with a large spot on the left chest, followed by smaller spots appearing over the next 2 days and spreading to the rest of the trunk. Four days after the rash onset, the patient experienced a mild headache, low-grade subjective fever, and chills. She denied any recent travel, bug bites, sore throat, and diarrhea. She was up-to-date on all vaccinations and had not received any vaccines preceding the symptoms. Physical examination revealed a 2-cm pink, scaly, thin plaque with a collarette of scale on the left upper chest (herald patch), along with multiple thin pink papules and small plaques with central scale on the trunk (Figure 1). A pustule with adjacent linear erosion was present on the left ventral forearm (Figure 2). The patient had a tender subcutaneous nodule in the left axilla as well as bilateral anterior and posterior cervical-chain subcutaneous tender nodules. There was no involvement of the palms, soles, or mucosae.

The patient was empirically treated for CSD with azithromycin (200 mg/5 mL), 404 mg on day 1 followed by 202 mg daily for 4 days. The rash was treated with hydrocortisone cream 2.5% twice daily for 2 weeks. A wound culture of the pustule on the left forearm was negative for neutrophils and organisms. Antibody serologies obtained 4 weeks after presentation were notable for an elevated B henselae IgG titer of 1:640, confirming the diagnosis of CSD. Following treatment with azithromycin and hydrocortisone, all of the patient’s symptoms resolved after 1 to 2 weeks.
Cat scratch disease is a zoonotic infection caused by the bacteria B henselae and the more recently described pathogen B clarridgeiae. Cat fleas spread these bacteria among cats, which subsequently inoculate the bacteria into humans through bites and scratches. The incidence of CSD in the United States is estimated to be 4.5 to 9.3 per 100,000 individuals in the outpatient setting and 0.19 to 0.86 per 100,000 individuals in the inpatient setting.1 Geographic variance can occur based on flea populations, resulting in higher incidence in warm humid climates and lower incidence in mountainous arid climates. The incidence of CSD in the pediatric population is highest in children aged 5 to 9 years. A national representative survey (N=3011) from 2017 revealed that 37.2% of primary care providers had diagnosed CSD in the prior year.1
Classic CSD presents as an erythematous papule at the inoculation site lasting days to weeks, with progression to tender lymphadenopathy lasting weeks to months. Fever, malaise, and chills also can be seen. Atypical CSD occurs in up to 24% of cases in immunocompetent patients.1 Atypical and systemic presentations are varied and can include fever of unknown origin, neuroretinitis, uveitis, retinal vessel occlusion, encephalitis, hepatosplenic lesions, Parinaud oculoglandular syndrome, osteomyelitis, and endocarditis.1,2 Atypical dermatologic presentations of CSD include maculopapular rash in 7% of cases and erythema nodosum in 2.5% of cases, as well as rare reports of cutaneous vasculitis, urticaria, immune thrombocytopenic purpura, and papuloedematous eruption.3 Treatment guidelines for CSD vary widely depending on the clinical presentation as well as the immunocompetence of the infected individual. Our patient had limited regional lymphadenopathy with no signs of dissemination or neurologic involvement and was successfully treated with a 5-day course of oral azithromycin (weight based, 10 mg/kg). More extensive disease such as hepatosplenic or neurologic CSD may require multiple antibiotics for up to 6 weeks. Alternative or additional antibiotics used for CSD include rifampin, trimethoprim-sulfamethoxazole, ciprofloxacin, doxycycline, gentamicin, and clarithromycin. Opinions vary as to whether all patients or just those with complicated infections warrant antibiotic therapy.4-6
Pityriasis rosea is a self-limited acute exanthematous disease that is classically associated with a systemic reactivation of human herpesvirus (HHV) 6 and/or HHV-7. The incidence of PR is estimated to be 480 per 100,000 dermatologic patients. It is slightly more common in females and occurs most often in patients aged 10 to 35 years.7 Clinically, PR appears with the abrupt onset of a single erythematous scaly patch (termed the herald patch), followed by a secondary eruption of smaller erythematous scaly macules and patches along the trunk’s cleavage lines. The secondary eruption on the back is sometimes termed a Christmas or fir tree pattern.7,8
In addition to the classic presentation of PR, there have been reports of numerous atypical clinical presentations. The herald patch, which classically presents on the trunk, also has been reported to present on the extremities; PR of the extremities is defined by lesions that appear as large scaly plaques on the extremities only. Inverse PR presents with lesions occurring in flexural areas and acral surfaces but not on the trunk. There also is an acral PR variant in which lesions appear only on the palms, wrists, and soles. Purpuric or hemorrhagic PR has been described and presents with purpura and petechiae with or without collarettes of scale in diffuse locations, including the palate. Oral PR presents more commonly in patients of color as erosions, ulcers, hemorrhagic lesions, bullae, or geographic tongue. Erythema multiforme–like PR appears with targetoid lesions on the trunk, face, neck, and arms without a history of herpes simplex virus infection. A large pear-shaped herald patch has been reported and characterizes the gigantea PR of Darier variant. Irritated PR occurs with typical PR findings, but afflicted patients report severe pain and burning with diaphoresis. Relapsing PR can occur within 1 year of a prior episode of PR and presents without a herald patch. Persistent PR is defined by PR lasting more than 3 months, and most reported cases have included oral lesions. Finally, other PR variants that have been described include urticarial, papular, follicular, vesicular, and hypopigmented types.7-9
Furthermore, there have been reports of multiple atypical presentations occurring simultaneously in the same patient.10 Although PR classically has been associated with HHV-6 and/or HHV-7 reactivation, it has been reported with a few other clinical situations and conditions. Pityriasislike eruption specifically refers to an exanthem secondary to drugs or vaccination that resembles PR but shows clinical differences, including diffuse and confluent dusky-red macules and/or plaques with or without desquamation on the trunk, extremities, and face. Drugs that have been implicated as triggers include ACE inhibitors, gold, isotretinoin, nonsteroidal anti-inflammatory agents, omeprazole, terbinafine, and tyrosine kinase inhibitors. Smallpox, tuberculosis, poliomyelitis, influenza, diphtheria, tetanus, hepatitis B virus, pneumococcus, papillomavirus, yellow fever, and pertussis vaccinations also have been associated with PR.7,11,12 Additionally, PR has been reported to occur with active systemic infections, specifically H1N1 influenza, though it is rare.13 Because of its self-limited course, treatment of PR most often involves only reassurance. Topical corticosteroids may be appropriate for pruritus.7,8
Pediatric health care providers including dermatologists should be familiar with both CSD and PR because they are common diseases that more often are encountered in the pediatric population. We present a unique case of CSD presenting with concurrent PR, which highlights a potential new etiology for PR and a rare cutaneous manifestation of CSD. Further investigation into a possible relationship between CSD and PR may be warranted. Patients with any signs and symptoms of fever, tender lymphadenopathy, worsening rash, or exposure to cats warrant a thorough history and physical examination to ensure that neither entity is overlooked.
- Nelson CA, Moore AR, Perea AE, et al. Cat scratch disease: U.S. clinicians’ experience and knowledge [published online July 14, 2017]. Zoonoses Public Health. 2018;65:67-73. doi:10.1111/zph.12368
- Habot-Wilner Z, Trivizki O, Goldstein M, et al. Cat-scratch disease: ocular manifestations and treatment outcome. Acta Ophthalmol. 2018;96:E524-E532. doi:10.1111/aos.13684
- Schattner A, Uliel L, Dubin I. The cat did it: erythema nodosum and additional atypical presentations of Bartonella henselae infection in immunocompetent hosts [published online February 16, 2018]. BMJ Case Rep. doi:10.1136/bcr-2017-222511
- Shorbatli L, Koranyi K, Nahata M. Effectiveness of antibiotic therapy in pediatric patients with cat scratch disease. Int J Clin Pharm. 2018;40:1458-1461. doi: 10.1007/s11096-018-0746-1
- Bass JW, Freitas BC, Freitas AD, et al. Prospective randomized double blind placebo-controlled evaluation of azithromycin for treatment of cat-scratch disease. Pediatr Infect Dis J. 1998;17:447-452. doi:10.1097/00006454-199806000-00002
- Spach DH, Kaplan SL. Treatment of cat scratch disease. UpToDate. Updated December 9, 2021. Accessed September 12, 2023. https://www.uptodate.com/contents/treatment-of-cat-scratch-disease
- Drago F, Ciccarese G, Rebora A, et al. Pityriasis rosea: a comprehensive classification. Dermatology. 2016;232:431-437. doi:10.1159/000445375
- Urbina F, Das A, Sudy E. Clinical variants of pityriasis rosea. World J Clin Cases. 2017;5:203-211. doi:10.12998/wjcc.v5.i6.203
- Alzahrani NA, Al Jasser MI. Geographic tonguelike presentation in a child with pityriasis rosea: case report and review of oral manifestations of pityriasis rosea. Pediatr Dermatol. 2018;35:E124-E127. doi:10.1111/pde.13417
- Sinha S, Sardana K, Garg V. Coexistence of two atypical variants of pityriasis rosea: a case report and review of literature. Pediatr Dermatol. 2012;29:538-540. doi:10.1111/j.1525-1470.2011.01549.x
- Drago F, Ciccarese G, Parodi A. Pityriasis rosea and pityriasis rosea-like eruptions: how to distinguish them? JAAD Case Rep. 2018;4:800-801. doi:10.1016/j.jdcr.2018.04.002
- Drago F, Ciccarese G, Javor S, et al. Vaccine-induced pityriasis rosea and pityriasis rosea-like eruptions: a review of the literature. J Eur Acad Dermatol Venereol. 2016;30:544-545. doi:10.1111/jdv.12942
- Mubki TF, Bin Dayel SA, Kadry R. A case of pityriasis rosea concurrent with the novel influenza A (H1N1) infection. Pediatr Dermatol. 2011;28:341-342. doi:10.1111/j.1525-1470.2010.01090.x
- Nelson CA, Moore AR, Perea AE, et al. Cat scratch disease: U.S. clinicians’ experience and knowledge [published online July 14, 2017]. Zoonoses Public Health. 2018;65:67-73. doi:10.1111/zph.12368
- Habot-Wilner Z, Trivizki O, Goldstein M, et al. Cat-scratch disease: ocular manifestations and treatment outcome. Acta Ophthalmol. 2018;96:E524-E532. doi:10.1111/aos.13684
- Schattner A, Uliel L, Dubin I. The cat did it: erythema nodosum and additional atypical presentations of Bartonella henselae infection in immunocompetent hosts [published online February 16, 2018]. BMJ Case Rep. doi:10.1136/bcr-2017-222511
- Shorbatli L, Koranyi K, Nahata M. Effectiveness of antibiotic therapy in pediatric patients with cat scratch disease. Int J Clin Pharm. 2018;40:1458-1461. doi: 10.1007/s11096-018-0746-1
- Bass JW, Freitas BC, Freitas AD, et al. Prospective randomized double blind placebo-controlled evaluation of azithromycin for treatment of cat-scratch disease. Pediatr Infect Dis J. 1998;17:447-452. doi:10.1097/00006454-199806000-00002
- Spach DH, Kaplan SL. Treatment of cat scratch disease. UpToDate. Updated December 9, 2021. Accessed September 12, 2023. https://www.uptodate.com/contents/treatment-of-cat-scratch-disease
- Drago F, Ciccarese G, Rebora A, et al. Pityriasis rosea: a comprehensive classification. Dermatology. 2016;232:431-437. doi:10.1159/000445375
- Urbina F, Das A, Sudy E. Clinical variants of pityriasis rosea. World J Clin Cases. 2017;5:203-211. doi:10.12998/wjcc.v5.i6.203
- Alzahrani NA, Al Jasser MI. Geographic tonguelike presentation in a child with pityriasis rosea: case report and review of oral manifestations of pityriasis rosea. Pediatr Dermatol. 2018;35:E124-E127. doi:10.1111/pde.13417
- Sinha S, Sardana K, Garg V. Coexistence of two atypical variants of pityriasis rosea: a case report and review of literature. Pediatr Dermatol. 2012;29:538-540. doi:10.1111/j.1525-1470.2011.01549.x
- Drago F, Ciccarese G, Parodi A. Pityriasis rosea and pityriasis rosea-like eruptions: how to distinguish them? JAAD Case Rep. 2018;4:800-801. doi:10.1016/j.jdcr.2018.04.002
- Drago F, Ciccarese G, Javor S, et al. Vaccine-induced pityriasis rosea and pityriasis rosea-like eruptions: a review of the literature. J Eur Acad Dermatol Venereol. 2016;30:544-545. doi:10.1111/jdv.12942
- Mubki TF, Bin Dayel SA, Kadry R. A case of pityriasis rosea concurrent with the novel influenza A (H1N1) infection. Pediatr Dermatol. 2011;28:341-342. doi:10.1111/j.1525-1470.2010.01090.x
Practice Points
- Dermatologists should familiarize themselves with the physical examination findings of cat scratch disease.
- There are numerous clinical variants and triggers of pityriasis rosea (PR).
- There may be a new infectious trigger for PR, and exposure to cats prior to a classic PR eruption should raise one’s suspicion as a possible cause.
European Commission grants approval of ritlecitinib for severe alopecia areata
This makes ritlecitinib the first medicine authorized by the EC to treat individuals with severe alopecia areata as young as 12 years of age.
Taken as a once-daily pill, ritlecitinib is a dual inhibitor of the TEC family of tyrosine kinases and of Janus kinase 3. In June of 2023, the drug received FDA approval for the treatment of severe alopecia areata in people ages 12 and older in the United States.
According to a press release from Pfizer, which developed the drug, EC approval was based on the pivotal ALLEGRO clinical trial program, which included the ALLEGRO phase 2b/3 study that evaluated ritlecitinib in patients aged 12 years and older with alopecia areata with 50% or more scalp hair loss, including patients with alopecia totalis (total scalp hair loss) and alopecia universalis (total body hair loss). Results from this study showed that 13.4% of adults and adolescents achieved 90% or more scalp hair coverage (Severity of Alopecia Tool score of 10 or less) after 24 weeks of treatment with ritlecitinib 50 mg, compared with 1.5% of those on placebo.
The study also measured Patient Global Impression of Change (PGI-C). At week 24, 49.2% of participants treated with ritlecitinib reported a PGI-C response of “moderate” to “great” improvement in their alopecia areata, compared with 9.2% with placebo.
According to results from an ongoing, long-term phase 3 study of ritlecitinib known as ALLEGRO-LT, the most common adverse reactions reported from use of the drug included diarrhea (9.2%), acne (6.2%), upper respiratory tract infections (6.2%), urticaria (4.6%), rash (3.8%), folliculitis (3.1%), and dizziness (2.3%), the company press release said.
This makes ritlecitinib the first medicine authorized by the EC to treat individuals with severe alopecia areata as young as 12 years of age.
Taken as a once-daily pill, ritlecitinib is a dual inhibitor of the TEC family of tyrosine kinases and of Janus kinase 3. In June of 2023, the drug received FDA approval for the treatment of severe alopecia areata in people ages 12 and older in the United States.
According to a press release from Pfizer, which developed the drug, EC approval was based on the pivotal ALLEGRO clinical trial program, which included the ALLEGRO phase 2b/3 study that evaluated ritlecitinib in patients aged 12 years and older with alopecia areata with 50% or more scalp hair loss, including patients with alopecia totalis (total scalp hair loss) and alopecia universalis (total body hair loss). Results from this study showed that 13.4% of adults and adolescents achieved 90% or more scalp hair coverage (Severity of Alopecia Tool score of 10 or less) after 24 weeks of treatment with ritlecitinib 50 mg, compared with 1.5% of those on placebo.
The study also measured Patient Global Impression of Change (PGI-C). At week 24, 49.2% of participants treated with ritlecitinib reported a PGI-C response of “moderate” to “great” improvement in their alopecia areata, compared with 9.2% with placebo.
According to results from an ongoing, long-term phase 3 study of ritlecitinib known as ALLEGRO-LT, the most common adverse reactions reported from use of the drug included diarrhea (9.2%), acne (6.2%), upper respiratory tract infections (6.2%), urticaria (4.6%), rash (3.8%), folliculitis (3.1%), and dizziness (2.3%), the company press release said.
This makes ritlecitinib the first medicine authorized by the EC to treat individuals with severe alopecia areata as young as 12 years of age.
Taken as a once-daily pill, ritlecitinib is a dual inhibitor of the TEC family of tyrosine kinases and of Janus kinase 3. In June of 2023, the drug received FDA approval for the treatment of severe alopecia areata in people ages 12 and older in the United States.
According to a press release from Pfizer, which developed the drug, EC approval was based on the pivotal ALLEGRO clinical trial program, which included the ALLEGRO phase 2b/3 study that evaluated ritlecitinib in patients aged 12 years and older with alopecia areata with 50% or more scalp hair loss, including patients with alopecia totalis (total scalp hair loss) and alopecia universalis (total body hair loss). Results from this study showed that 13.4% of adults and adolescents achieved 90% or more scalp hair coverage (Severity of Alopecia Tool score of 10 or less) after 24 weeks of treatment with ritlecitinib 50 mg, compared with 1.5% of those on placebo.
The study also measured Patient Global Impression of Change (PGI-C). At week 24, 49.2% of participants treated with ritlecitinib reported a PGI-C response of “moderate” to “great” improvement in their alopecia areata, compared with 9.2% with placebo.
According to results from an ongoing, long-term phase 3 study of ritlecitinib known as ALLEGRO-LT, the most common adverse reactions reported from use of the drug included diarrhea (9.2%), acne (6.2%), upper respiratory tract infections (6.2%), urticaria (4.6%), rash (3.8%), folliculitis (3.1%), and dizziness (2.3%), the company press release said.
Dialectical behavior therapy decreased suicide attempts in bipolar teens
, based on data from 100 individuals aged 12-18 years.
Bipolar spectrum disorder (BP) is known to substantially increase the risk for suicide in youth, but no psychosocial intervention for this population has targeted suicidal behavior in particular, wrote Tina R. Goldstein, PhD, of the University of Pittsburgh, and colleagues.
Dialectical behavior therapy (DBT) had shown effectiveness for decreasing suicide attempts in adults with borderline personality disorder, and previous studies of DBT have shown reduced suicidal ideation, self-harm, and suicide attempts in suicidal adolescents, but these studies have mainly excluded BP teens, the researchers said.
In a study published in JAMA Psychiatry, the researchers recruited adolescents aged 12-18 years with a diagnosis of BP who were treated at an outpatient clinic between November 2014 and September 2019. Of these, 47 were randomized to 1 year of DBT (a total of 36 sessions) and 53 to standard of care (SOC) psychotherapy. All participants also received medication using a flexible algorithm.
The primary outcomes were suicide attempts over a 1-year period and measurements of mood symptoms and states, specifically depression and hypomania/mania. Secondary analyses included the effect of DBT on individuals with a history of suicide attempt and on improving emotion dysregulation. The mean age of the participants was 16.1 years; 85 were female, and 74% were White.
Participants in both DBT and SOC groups reported similar rates of suicide attempt rates at study enrollment based on the Adolescent Longitudinal Follow-Up Evaluation (ALIFE) with a mean of 2.0 and 1.8 attempts, respectively (P = .80). Based on the Columbia–Suicide Severity Rating Scale Pediatric Version (C-SSRS), participants in the DBT group had slightly more suicide attempts than the SOC group at study enrollment, with a mean of 1.4 and 0.6 attempts, respectively (P = .02).
Controlling for baseline attempts, participants in the DBT group had significantly fewer suicide attempts over the study period, compared with the SOC group as measured by both ALIFE (mean 0.2 vs. 1.1) and C-SSRS (mean 0.04 vs. 0.10, P = .03 for both measures). The incidence rate ratios for reduced suicide attempts were 0.32 for ALIFE and 0.13 for C-SSRS, both significant in favor of DBT, compared with SOC.
Overall, both groups showed similarly significant improvement on measures of mood symptoms and episodes over the study period. The standardized depression rating scale slope was –0.17 and the standardized mania rating scale slope was –0.24.
DBT was significantly more effective than SOC psychotherapy at decreasing suicide attempts over 1 year (ALIFE: incidence rate ratio, 0.32; 95% CI, 0.11-0.96; C-SSRS: IRR, 0.13; 95% CI, 0.02-0.78).
On further analysis, the decrease in suicide attempts in the DBT group was greater over time and among those with a lifetime history of suicide attempts (IRR, 0.23). “Decreased risk of suicide attempt in DBT was mediated by improvement in emotion dysregulation, particularly for those with high baseline emotion dysregulation,” the researchers wrote in their discussion.
The findings were limited by several factors including the mainly female, non-Hispanic White study population, and controlled clinical setting, the researchers noted. Data from a forthcoming community implementation field trial will address some generalizability issues, although more work is needed to address disparities in BP diagnosis and treatment, they added.
However, the results support the potential of DBT for mood management and for reducing suicide attempts in a high-risk adolescent population, especially those with high levels of emotional dysregulation, on par with other established psychosocial treatments, the researchers concluded.
More options needed to manage increased risk
“It was important to conduct this study at this time because, while still relatively rare, bipolar spectrum disorders in adolescents confer increased risk for suicide,” Peter L. Loper Jr., MD, of the University of South Carolina, Columbia, said in an interview. The complexity of BP and the increased risk of suicide in these patients challenge clinicians to identify robust evidence-based interventions beyond pharmacotherapy that mitigate this risk, said Dr. Loper, who is triple board certified in pediatrics, general psychiatry, and child & adolescent psychiatry, but was not involved in the study.
The current study findings were not surprising, because DBT has proven effective in decreasing suicidal ideation and suicide attempts in other high-risk adolescent patient populations, Dr. Loper said. “Given the therapeutic content of DBT, with emphasis on mindfulness, distress tolerance, social skills, and emotional regulation, I think it is reasonable to hypothesize that DBT might be a globally applicable intervention, independent of mental health diagnosis or etiology of suicidal ideation,” he said.
The take-home message for clinicians is that the results support the efficacy of DBT as an intervention for adolescents with BP and suicidal ideation, self-injurious behavior, or suicide attempts, said Dr. Loper. For these patients, given their increased suicide risk, “DBT should certainly be recommended as a component of their treatment plan,” he said.
However, barriers to the use of DBT in clinical practice exist, notably access and cost, Dr. Loper noted. “I think that the most prominent barrier in accessing DBT in clinical practice is the availability of certified, structured DBT treatment programs, and particularly those willing to provide services to adolescents,” he said. “Additionally, certified DBT programs, which are the gold standard, are often not covered by third-party payers, making cost yet another potential barrier.”
Looking ahead, Dr. Loper agreed with the study authors that additional research with a more diverse patient population representative of adolescents with bipolar spectrum disorder “is a crucial area of focus.”
The study was funded by the National Institutes of Mental Health through a grant to Dr. Goldstein, who also disclosed royalties from Guilford Press unrelated to the current study. Dr. Loper had no financial conflicts to disclose.
, based on data from 100 individuals aged 12-18 years.
Bipolar spectrum disorder (BP) is known to substantially increase the risk for suicide in youth, but no psychosocial intervention for this population has targeted suicidal behavior in particular, wrote Tina R. Goldstein, PhD, of the University of Pittsburgh, and colleagues.
Dialectical behavior therapy (DBT) had shown effectiveness for decreasing suicide attempts in adults with borderline personality disorder, and previous studies of DBT have shown reduced suicidal ideation, self-harm, and suicide attempts in suicidal adolescents, but these studies have mainly excluded BP teens, the researchers said.
In a study published in JAMA Psychiatry, the researchers recruited adolescents aged 12-18 years with a diagnosis of BP who were treated at an outpatient clinic between November 2014 and September 2019. Of these, 47 were randomized to 1 year of DBT (a total of 36 sessions) and 53 to standard of care (SOC) psychotherapy. All participants also received medication using a flexible algorithm.
The primary outcomes were suicide attempts over a 1-year period and measurements of mood symptoms and states, specifically depression and hypomania/mania. Secondary analyses included the effect of DBT on individuals with a history of suicide attempt and on improving emotion dysregulation. The mean age of the participants was 16.1 years; 85 were female, and 74% were White.
Participants in both DBT and SOC groups reported similar rates of suicide attempt rates at study enrollment based on the Adolescent Longitudinal Follow-Up Evaluation (ALIFE) with a mean of 2.0 and 1.8 attempts, respectively (P = .80). Based on the Columbia–Suicide Severity Rating Scale Pediatric Version (C-SSRS), participants in the DBT group had slightly more suicide attempts than the SOC group at study enrollment, with a mean of 1.4 and 0.6 attempts, respectively (P = .02).
Controlling for baseline attempts, participants in the DBT group had significantly fewer suicide attempts over the study period, compared with the SOC group as measured by both ALIFE (mean 0.2 vs. 1.1) and C-SSRS (mean 0.04 vs. 0.10, P = .03 for both measures). The incidence rate ratios for reduced suicide attempts were 0.32 for ALIFE and 0.13 for C-SSRS, both significant in favor of DBT, compared with SOC.
Overall, both groups showed similarly significant improvement on measures of mood symptoms and episodes over the study period. The standardized depression rating scale slope was –0.17 and the standardized mania rating scale slope was –0.24.
DBT was significantly more effective than SOC psychotherapy at decreasing suicide attempts over 1 year (ALIFE: incidence rate ratio, 0.32; 95% CI, 0.11-0.96; C-SSRS: IRR, 0.13; 95% CI, 0.02-0.78).
On further analysis, the decrease in suicide attempts in the DBT group was greater over time and among those with a lifetime history of suicide attempts (IRR, 0.23). “Decreased risk of suicide attempt in DBT was mediated by improvement in emotion dysregulation, particularly for those with high baseline emotion dysregulation,” the researchers wrote in their discussion.
The findings were limited by several factors including the mainly female, non-Hispanic White study population, and controlled clinical setting, the researchers noted. Data from a forthcoming community implementation field trial will address some generalizability issues, although more work is needed to address disparities in BP diagnosis and treatment, they added.
However, the results support the potential of DBT for mood management and for reducing suicide attempts in a high-risk adolescent population, especially those with high levels of emotional dysregulation, on par with other established psychosocial treatments, the researchers concluded.
More options needed to manage increased risk
“It was important to conduct this study at this time because, while still relatively rare, bipolar spectrum disorders in adolescents confer increased risk for suicide,” Peter L. Loper Jr., MD, of the University of South Carolina, Columbia, said in an interview. The complexity of BP and the increased risk of suicide in these patients challenge clinicians to identify robust evidence-based interventions beyond pharmacotherapy that mitigate this risk, said Dr. Loper, who is triple board certified in pediatrics, general psychiatry, and child & adolescent psychiatry, but was not involved in the study.
The current study findings were not surprising, because DBT has proven effective in decreasing suicidal ideation and suicide attempts in other high-risk adolescent patient populations, Dr. Loper said. “Given the therapeutic content of DBT, with emphasis on mindfulness, distress tolerance, social skills, and emotional regulation, I think it is reasonable to hypothesize that DBT might be a globally applicable intervention, independent of mental health diagnosis or etiology of suicidal ideation,” he said.
The take-home message for clinicians is that the results support the efficacy of DBT as an intervention for adolescents with BP and suicidal ideation, self-injurious behavior, or suicide attempts, said Dr. Loper. For these patients, given their increased suicide risk, “DBT should certainly be recommended as a component of their treatment plan,” he said.
However, barriers to the use of DBT in clinical practice exist, notably access and cost, Dr. Loper noted. “I think that the most prominent barrier in accessing DBT in clinical practice is the availability of certified, structured DBT treatment programs, and particularly those willing to provide services to adolescents,” he said. “Additionally, certified DBT programs, which are the gold standard, are often not covered by third-party payers, making cost yet another potential barrier.”
Looking ahead, Dr. Loper agreed with the study authors that additional research with a more diverse patient population representative of adolescents with bipolar spectrum disorder “is a crucial area of focus.”
The study was funded by the National Institutes of Mental Health through a grant to Dr. Goldstein, who also disclosed royalties from Guilford Press unrelated to the current study. Dr. Loper had no financial conflicts to disclose.
, based on data from 100 individuals aged 12-18 years.
Bipolar spectrum disorder (BP) is known to substantially increase the risk for suicide in youth, but no psychosocial intervention for this population has targeted suicidal behavior in particular, wrote Tina R. Goldstein, PhD, of the University of Pittsburgh, and colleagues.
Dialectical behavior therapy (DBT) had shown effectiveness for decreasing suicide attempts in adults with borderline personality disorder, and previous studies of DBT have shown reduced suicidal ideation, self-harm, and suicide attempts in suicidal adolescents, but these studies have mainly excluded BP teens, the researchers said.
In a study published in JAMA Psychiatry, the researchers recruited adolescents aged 12-18 years with a diagnosis of BP who were treated at an outpatient clinic between November 2014 and September 2019. Of these, 47 were randomized to 1 year of DBT (a total of 36 sessions) and 53 to standard of care (SOC) psychotherapy. All participants also received medication using a flexible algorithm.
The primary outcomes were suicide attempts over a 1-year period and measurements of mood symptoms and states, specifically depression and hypomania/mania. Secondary analyses included the effect of DBT on individuals with a history of suicide attempt and on improving emotion dysregulation. The mean age of the participants was 16.1 years; 85 were female, and 74% were White.
Participants in both DBT and SOC groups reported similar rates of suicide attempt rates at study enrollment based on the Adolescent Longitudinal Follow-Up Evaluation (ALIFE) with a mean of 2.0 and 1.8 attempts, respectively (P = .80). Based on the Columbia–Suicide Severity Rating Scale Pediatric Version (C-SSRS), participants in the DBT group had slightly more suicide attempts than the SOC group at study enrollment, with a mean of 1.4 and 0.6 attempts, respectively (P = .02).
Controlling for baseline attempts, participants in the DBT group had significantly fewer suicide attempts over the study period, compared with the SOC group as measured by both ALIFE (mean 0.2 vs. 1.1) and C-SSRS (mean 0.04 vs. 0.10, P = .03 for both measures). The incidence rate ratios for reduced suicide attempts were 0.32 for ALIFE and 0.13 for C-SSRS, both significant in favor of DBT, compared with SOC.
Overall, both groups showed similarly significant improvement on measures of mood symptoms and episodes over the study period. The standardized depression rating scale slope was –0.17 and the standardized mania rating scale slope was –0.24.
DBT was significantly more effective than SOC psychotherapy at decreasing suicide attempts over 1 year (ALIFE: incidence rate ratio, 0.32; 95% CI, 0.11-0.96; C-SSRS: IRR, 0.13; 95% CI, 0.02-0.78).
On further analysis, the decrease in suicide attempts in the DBT group was greater over time and among those with a lifetime history of suicide attempts (IRR, 0.23). “Decreased risk of suicide attempt in DBT was mediated by improvement in emotion dysregulation, particularly for those with high baseline emotion dysregulation,” the researchers wrote in their discussion.
The findings were limited by several factors including the mainly female, non-Hispanic White study population, and controlled clinical setting, the researchers noted. Data from a forthcoming community implementation field trial will address some generalizability issues, although more work is needed to address disparities in BP diagnosis and treatment, they added.
However, the results support the potential of DBT for mood management and for reducing suicide attempts in a high-risk adolescent population, especially those with high levels of emotional dysregulation, on par with other established psychosocial treatments, the researchers concluded.
More options needed to manage increased risk
“It was important to conduct this study at this time because, while still relatively rare, bipolar spectrum disorders in adolescents confer increased risk for suicide,” Peter L. Loper Jr., MD, of the University of South Carolina, Columbia, said in an interview. The complexity of BP and the increased risk of suicide in these patients challenge clinicians to identify robust evidence-based interventions beyond pharmacotherapy that mitigate this risk, said Dr. Loper, who is triple board certified in pediatrics, general psychiatry, and child & adolescent psychiatry, but was not involved in the study.
The current study findings were not surprising, because DBT has proven effective in decreasing suicidal ideation and suicide attempts in other high-risk adolescent patient populations, Dr. Loper said. “Given the therapeutic content of DBT, with emphasis on mindfulness, distress tolerance, social skills, and emotional regulation, I think it is reasonable to hypothesize that DBT might be a globally applicable intervention, independent of mental health diagnosis or etiology of suicidal ideation,” he said.
The take-home message for clinicians is that the results support the efficacy of DBT as an intervention for adolescents with BP and suicidal ideation, self-injurious behavior, or suicide attempts, said Dr. Loper. For these patients, given their increased suicide risk, “DBT should certainly be recommended as a component of their treatment plan,” he said.
However, barriers to the use of DBT in clinical practice exist, notably access and cost, Dr. Loper noted. “I think that the most prominent barrier in accessing DBT in clinical practice is the availability of certified, structured DBT treatment programs, and particularly those willing to provide services to adolescents,” he said. “Additionally, certified DBT programs, which are the gold standard, are often not covered by third-party payers, making cost yet another potential barrier.”
Looking ahead, Dr. Loper agreed with the study authors that additional research with a more diverse patient population representative of adolescents with bipolar spectrum disorder “is a crucial area of focus.”
The study was funded by the National Institutes of Mental Health through a grant to Dr. Goldstein, who also disclosed royalties from Guilford Press unrelated to the current study. Dr. Loper had no financial conflicts to disclose.
FROM JAMA PSYCHIATRY
Surge in pediatric ADHD med errors prompts call for prevention
according to results of a study published in the journal Pediatrics.
The dramatic jump is likely attributable to an increase in the prescribing of ADHD medications for children. According to the study authors, in 2019, nearly 10% of children in the United States had been diagnosed with ADHD, and some 3.3 million – or about 5% of all children in the country – had received a prescription for an ADHD medication.
“Because therapeutic errors are preventable, more attention should be given to patient and caregiver education and development of improved child-resistant medication dispensing and tracking systems,” the authors commented.
The investigators analyzed data from the National Poison Data System from 2000 through 2021 for therapeutic errors associated with ADHD medication among patients younger than 20 years.
“As medicine changes, it’s nice to look back at some of these things and see how some of these problems have changed,” said Natalie I. Rine, PharmD, a coauthor of the study and director of the Central Ohio Poison Center at Nationwide Children’s Hospital in Columbus.
The researchers identified 124,383 such errors reported to U.S. poison centers during the study period. The frequency increased by 299%.
Two-thirds (66.6%) of the exposures involved children aged 6-12 years, three-fourths (76.4%) were among males, and half (50.5%) involved amphetamines and related compounds. Most (79.7%) therapeutic errors were linked to exposure to a single substance. Nearly 83% of patients did not receive treatment at a health care facility; however, 2.3% were admitted to the hospital, and 4.2% had a “serious medical outcome,” the researchers found.
The most common scenarios were “inadvertently took or given medication twice” (53.9%), followed by “inadvertently took or given someone else’s medication” (13.4%) and “wrong medication taken or given” (12.9%), according to the researchers. Two percent involved mistakes by a pharmacist or nurse.
Easily preventable
Dr. Rine attributed the errors to simple mistakes and said they were likely the product of busy households and distracted caregivers. She added that the errors are easily avoided by storing the medication properly, keeping a sheet with the medication to document what was taken and when, and using a pillbox or one of many apps that can assist in documenting the dispensing of medications.
“I think the biggest thing is that a lot of these errors are preventable, more than anything else,” Dr. Rine said.
The increase in ADHD diagnoses among children and the subsequent prescribing of medications are reasons for the nearly 300% increase in poison control calls. A 2018 study showed that the estimated prevalence of ADHD diagnoses among U.S. children and adolescents increased from 6.1% in 1997-1998 to 10.2% in 2015-2016. The Centers for Disease Control and Prevention states that 6 million children and adolescents aged 3-17 years have been diagnosed with ADHD, and 62% have received ADHD medication.
Colleen Kraft, MD, a pediatrician at Children’s Hospital Los Angeles, said she was not surprised by the reported increase in errors. In addition to the simple uptick in ADHD diagnoses and prescriptions in the past 2 decades, Dr. Kraft said the growing variety of ADHD medication is a cause for more errors.
“Because we have so many more different types of these medications, it’s easy to confuse them, and it’s easy to make an error when you give this to a child,” she said in an interview.
Dr. Kraft also hypothesized that because ADHD can have a genetic component, some parents with undiagnosed and untreated ADHD are responsible for their child’s medication, a scenario ripe for mistakes.
Potential dangers
Not all ADHD medicinal overdosing is created equal, Dr. Kraft pointed out. Doubling up on a stimulant such as methylphenidate (Ritalin) or the combination of amphetamine and dextroamphetamine (Adderall) may cause headaches, suppress appetite, and cause an upset stomach, although those symptoms usually clear up in a few hours.
However, she noted, the use of alpha-1 adrenergic blockers is more concerning. Also used to treat high blood pressure, medications such as guanfacine and clonidine cause sedation. A double dose can cause blood pressure to decrease to dangerous levels.
The study’s primary limitation was bias in self-reporting, which may have led to underreporting of incidences, according to the researchers. Not every case in which an error occurs that involves a child’s taking ADHD medication gets reported to poison control, because some will take a wait-and-see approach and may not call if their child is asymptomatic.
“Our data is only as good as what the callers report to us,” Dr. Rine said.
A version of this article appeared on Medscape.com.
according to results of a study published in the journal Pediatrics.
The dramatic jump is likely attributable to an increase in the prescribing of ADHD medications for children. According to the study authors, in 2019, nearly 10% of children in the United States had been diagnosed with ADHD, and some 3.3 million – or about 5% of all children in the country – had received a prescription for an ADHD medication.
“Because therapeutic errors are preventable, more attention should be given to patient and caregiver education and development of improved child-resistant medication dispensing and tracking systems,” the authors commented.
The investigators analyzed data from the National Poison Data System from 2000 through 2021 for therapeutic errors associated with ADHD medication among patients younger than 20 years.
“As medicine changes, it’s nice to look back at some of these things and see how some of these problems have changed,” said Natalie I. Rine, PharmD, a coauthor of the study and director of the Central Ohio Poison Center at Nationwide Children’s Hospital in Columbus.
The researchers identified 124,383 such errors reported to U.S. poison centers during the study period. The frequency increased by 299%.
Two-thirds (66.6%) of the exposures involved children aged 6-12 years, three-fourths (76.4%) were among males, and half (50.5%) involved amphetamines and related compounds. Most (79.7%) therapeutic errors were linked to exposure to a single substance. Nearly 83% of patients did not receive treatment at a health care facility; however, 2.3% were admitted to the hospital, and 4.2% had a “serious medical outcome,” the researchers found.
The most common scenarios were “inadvertently took or given medication twice” (53.9%), followed by “inadvertently took or given someone else’s medication” (13.4%) and “wrong medication taken or given” (12.9%), according to the researchers. Two percent involved mistakes by a pharmacist or nurse.
Easily preventable
Dr. Rine attributed the errors to simple mistakes and said they were likely the product of busy households and distracted caregivers. She added that the errors are easily avoided by storing the medication properly, keeping a sheet with the medication to document what was taken and when, and using a pillbox or one of many apps that can assist in documenting the dispensing of medications.
“I think the biggest thing is that a lot of these errors are preventable, more than anything else,” Dr. Rine said.
The increase in ADHD diagnoses among children and the subsequent prescribing of medications are reasons for the nearly 300% increase in poison control calls. A 2018 study showed that the estimated prevalence of ADHD diagnoses among U.S. children and adolescents increased from 6.1% in 1997-1998 to 10.2% in 2015-2016. The Centers for Disease Control and Prevention states that 6 million children and adolescents aged 3-17 years have been diagnosed with ADHD, and 62% have received ADHD medication.
Colleen Kraft, MD, a pediatrician at Children’s Hospital Los Angeles, said she was not surprised by the reported increase in errors. In addition to the simple uptick in ADHD diagnoses and prescriptions in the past 2 decades, Dr. Kraft said the growing variety of ADHD medication is a cause for more errors.
“Because we have so many more different types of these medications, it’s easy to confuse them, and it’s easy to make an error when you give this to a child,” she said in an interview.
Dr. Kraft also hypothesized that because ADHD can have a genetic component, some parents with undiagnosed and untreated ADHD are responsible for their child’s medication, a scenario ripe for mistakes.
Potential dangers
Not all ADHD medicinal overdosing is created equal, Dr. Kraft pointed out. Doubling up on a stimulant such as methylphenidate (Ritalin) or the combination of amphetamine and dextroamphetamine (Adderall) may cause headaches, suppress appetite, and cause an upset stomach, although those symptoms usually clear up in a few hours.
However, she noted, the use of alpha-1 adrenergic blockers is more concerning. Also used to treat high blood pressure, medications such as guanfacine and clonidine cause sedation. A double dose can cause blood pressure to decrease to dangerous levels.
The study’s primary limitation was bias in self-reporting, which may have led to underreporting of incidences, according to the researchers. Not every case in which an error occurs that involves a child’s taking ADHD medication gets reported to poison control, because some will take a wait-and-see approach and may not call if their child is asymptomatic.
“Our data is only as good as what the callers report to us,” Dr. Rine said.
A version of this article appeared on Medscape.com.
according to results of a study published in the journal Pediatrics.
The dramatic jump is likely attributable to an increase in the prescribing of ADHD medications for children. According to the study authors, in 2019, nearly 10% of children in the United States had been diagnosed with ADHD, and some 3.3 million – or about 5% of all children in the country – had received a prescription for an ADHD medication.
“Because therapeutic errors are preventable, more attention should be given to patient and caregiver education and development of improved child-resistant medication dispensing and tracking systems,” the authors commented.
The investigators analyzed data from the National Poison Data System from 2000 through 2021 for therapeutic errors associated with ADHD medication among patients younger than 20 years.
“As medicine changes, it’s nice to look back at some of these things and see how some of these problems have changed,” said Natalie I. Rine, PharmD, a coauthor of the study and director of the Central Ohio Poison Center at Nationwide Children’s Hospital in Columbus.
The researchers identified 124,383 such errors reported to U.S. poison centers during the study period. The frequency increased by 299%.
Two-thirds (66.6%) of the exposures involved children aged 6-12 years, three-fourths (76.4%) were among males, and half (50.5%) involved amphetamines and related compounds. Most (79.7%) therapeutic errors were linked to exposure to a single substance. Nearly 83% of patients did not receive treatment at a health care facility; however, 2.3% were admitted to the hospital, and 4.2% had a “serious medical outcome,” the researchers found.
The most common scenarios were “inadvertently took or given medication twice” (53.9%), followed by “inadvertently took or given someone else’s medication” (13.4%) and “wrong medication taken or given” (12.9%), according to the researchers. Two percent involved mistakes by a pharmacist or nurse.
Easily preventable
Dr. Rine attributed the errors to simple mistakes and said they were likely the product of busy households and distracted caregivers. She added that the errors are easily avoided by storing the medication properly, keeping a sheet with the medication to document what was taken and when, and using a pillbox or one of many apps that can assist in documenting the dispensing of medications.
“I think the biggest thing is that a lot of these errors are preventable, more than anything else,” Dr. Rine said.
The increase in ADHD diagnoses among children and the subsequent prescribing of medications are reasons for the nearly 300% increase in poison control calls. A 2018 study showed that the estimated prevalence of ADHD diagnoses among U.S. children and adolescents increased from 6.1% in 1997-1998 to 10.2% in 2015-2016. The Centers for Disease Control and Prevention states that 6 million children and adolescents aged 3-17 years have been diagnosed with ADHD, and 62% have received ADHD medication.
Colleen Kraft, MD, a pediatrician at Children’s Hospital Los Angeles, said she was not surprised by the reported increase in errors. In addition to the simple uptick in ADHD diagnoses and prescriptions in the past 2 decades, Dr. Kraft said the growing variety of ADHD medication is a cause for more errors.
“Because we have so many more different types of these medications, it’s easy to confuse them, and it’s easy to make an error when you give this to a child,” she said in an interview.
Dr. Kraft also hypothesized that because ADHD can have a genetic component, some parents with undiagnosed and untreated ADHD are responsible for their child’s medication, a scenario ripe for mistakes.
Potential dangers
Not all ADHD medicinal overdosing is created equal, Dr. Kraft pointed out. Doubling up on a stimulant such as methylphenidate (Ritalin) or the combination of amphetamine and dextroamphetamine (Adderall) may cause headaches, suppress appetite, and cause an upset stomach, although those symptoms usually clear up in a few hours.
However, she noted, the use of alpha-1 adrenergic blockers is more concerning. Also used to treat high blood pressure, medications such as guanfacine and clonidine cause sedation. A double dose can cause blood pressure to decrease to dangerous levels.
The study’s primary limitation was bias in self-reporting, which may have led to underreporting of incidences, according to the researchers. Not every case in which an error occurs that involves a child’s taking ADHD medication gets reported to poison control, because some will take a wait-and-see approach and may not call if their child is asymptomatic.
“Our data is only as good as what the callers report to us,” Dr. Rine said.
A version of this article appeared on Medscape.com.
FROM PEDIATRICS