User login
Osteonecrosis of jaw in mRCC higher with denosumab/antiangiogenics
The combination of a targeted antiangiogenic agent and denosumab (Xgeva) appears to significantly increase the risk for osteonecrosis of the jaw in patients with metastatic renal cell carcinoma (mRCC), French investigators cautioned.
Among 41 patients with RCC metastatic to bone treated with an antiangiogenic agent and denosumab, 7 (17%) developed osteonecrosis of the jaw (ONJ). All seven received the combination in the first line, reported Aline Guillot, MD, from the Institut de cancérologie Lucien Neuwirth in Saint-Priest-en-Jarez, France, and her colleagues.
“The incidence of ONJ was high in this real-life population of patients with mRCC treated with antiangiogenic therapies combined with denosumab. This toxicity signal should warn physicians about this combination in the mRCC population,” they wrote in Clinical Genitourinary Cancer.
Previous studies have shown that the combination of another bone-targeted therapy – zoledronic acid – with antiangiogenic tyrosine kinase inhibitors (TKIs) such as sunitinib (Sutent) is associated with increased risk for ONJ. In addition, ONJ with the combination of denosumab and antiangiogenic therapies “has been noticed but never estimated,” the authors noted.
To evaluate the incidence of hypocalcemia and ONJ in patients with RCC metastatic to bone treated with denosumab and a TKI or mammalian target of rapamycin inhibitor, the investigators gathered data retrospectively from 10 French centers.
They identified 25 men and 16 women with a median age of 62 years (range, 54-68 years). Of this group, 40 received denosumab in the first line in combination with either sunitinib (31 patients), pazopanib (6), everolimus (Afinitor and generics; 2), and temsirolimus (Torisel and generics; 1). The median duration of first-line therapy was 12 months.
Denosumab was given in the second line with axitinib (Inlyta) to nine patients, with everolimus to nine, and with sunitinib to three patients. The median duration of second-line therapy was 3 months.
Skeletal-related events occurred in 41% of patients prior to receiving denosumab and in 61% (25 patients) after denosumab. The events included clinical fracture, bone pain requiring radiation, and spinal compression.
Of the seven patients who developed ONJ, six received denosumab and sunitinib in the first line and one received denosumab plus temsirolimus, with a median duration of treatment of 11.8 months. Three of these patients also received denosumab in the second line with either axitinib, everolimus, or sunitinib, with a median duration of 9.8 months. Data on second-line therapy for the remaining four patients with ONJ were not available.
The investigators noted that the 17% incidence of ONJ was higher than the 10% rate seen in a study of patients with mRCC treated with a TKI and a bisphosphonate (Acta Clin Belg. 2018 Apr;73(2):100-9), and the 1.8% incidence seen in an analysis of three phase 3 trials in cancer patients with bone metastases (Ann Oncol. 2012 May;23(5):1341-7).
Although the etiology of ONJ is unknown, patient education and oral hygiene may reduce the risk in patients treated with denosumab and a TKI.
“The benefit of denosumab in this setting in regard [to] toxicity needs to be demonstrated in a prospective trial,” they wrote.
No conflicts of interest or disclosures were reported.
SOURCE: Guillot A et al. Clin Genitourin Cancer. 2018 Sep 6. doi: 10.1016/j.clgc.2018.08.006.
The combination of a targeted antiangiogenic agent and denosumab (Xgeva) appears to significantly increase the risk for osteonecrosis of the jaw in patients with metastatic renal cell carcinoma (mRCC), French investigators cautioned.
Among 41 patients with RCC metastatic to bone treated with an antiangiogenic agent and denosumab, 7 (17%) developed osteonecrosis of the jaw (ONJ). All seven received the combination in the first line, reported Aline Guillot, MD, from the Institut de cancérologie Lucien Neuwirth in Saint-Priest-en-Jarez, France, and her colleagues.
“The incidence of ONJ was high in this real-life population of patients with mRCC treated with antiangiogenic therapies combined with denosumab. This toxicity signal should warn physicians about this combination in the mRCC population,” they wrote in Clinical Genitourinary Cancer.
Previous studies have shown that the combination of another bone-targeted therapy – zoledronic acid – with antiangiogenic tyrosine kinase inhibitors (TKIs) such as sunitinib (Sutent) is associated with increased risk for ONJ. In addition, ONJ with the combination of denosumab and antiangiogenic therapies “has been noticed but never estimated,” the authors noted.
To evaluate the incidence of hypocalcemia and ONJ in patients with RCC metastatic to bone treated with denosumab and a TKI or mammalian target of rapamycin inhibitor, the investigators gathered data retrospectively from 10 French centers.
They identified 25 men and 16 women with a median age of 62 years (range, 54-68 years). Of this group, 40 received denosumab in the first line in combination with either sunitinib (31 patients), pazopanib (6), everolimus (Afinitor and generics; 2), and temsirolimus (Torisel and generics; 1). The median duration of first-line therapy was 12 months.
Denosumab was given in the second line with axitinib (Inlyta) to nine patients, with everolimus to nine, and with sunitinib to three patients. The median duration of second-line therapy was 3 months.
Skeletal-related events occurred in 41% of patients prior to receiving denosumab and in 61% (25 patients) after denosumab. The events included clinical fracture, bone pain requiring radiation, and spinal compression.
Of the seven patients who developed ONJ, six received denosumab and sunitinib in the first line and one received denosumab plus temsirolimus, with a median duration of treatment of 11.8 months. Three of these patients also received denosumab in the second line with either axitinib, everolimus, or sunitinib, with a median duration of 9.8 months. Data on second-line therapy for the remaining four patients with ONJ were not available.
The investigators noted that the 17% incidence of ONJ was higher than the 10% rate seen in a study of patients with mRCC treated with a TKI and a bisphosphonate (Acta Clin Belg. 2018 Apr;73(2):100-9), and the 1.8% incidence seen in an analysis of three phase 3 trials in cancer patients with bone metastases (Ann Oncol. 2012 May;23(5):1341-7).
Although the etiology of ONJ is unknown, patient education and oral hygiene may reduce the risk in patients treated with denosumab and a TKI.
“The benefit of denosumab in this setting in regard [to] toxicity needs to be demonstrated in a prospective trial,” they wrote.
No conflicts of interest or disclosures were reported.
SOURCE: Guillot A et al. Clin Genitourin Cancer. 2018 Sep 6. doi: 10.1016/j.clgc.2018.08.006.
The combination of a targeted antiangiogenic agent and denosumab (Xgeva) appears to significantly increase the risk for osteonecrosis of the jaw in patients with metastatic renal cell carcinoma (mRCC), French investigators cautioned.
Among 41 patients with RCC metastatic to bone treated with an antiangiogenic agent and denosumab, 7 (17%) developed osteonecrosis of the jaw (ONJ). All seven received the combination in the first line, reported Aline Guillot, MD, from the Institut de cancérologie Lucien Neuwirth in Saint-Priest-en-Jarez, France, and her colleagues.
“The incidence of ONJ was high in this real-life population of patients with mRCC treated with antiangiogenic therapies combined with denosumab. This toxicity signal should warn physicians about this combination in the mRCC population,” they wrote in Clinical Genitourinary Cancer.
Previous studies have shown that the combination of another bone-targeted therapy – zoledronic acid – with antiangiogenic tyrosine kinase inhibitors (TKIs) such as sunitinib (Sutent) is associated with increased risk for ONJ. In addition, ONJ with the combination of denosumab and antiangiogenic therapies “has been noticed but never estimated,” the authors noted.
To evaluate the incidence of hypocalcemia and ONJ in patients with RCC metastatic to bone treated with denosumab and a TKI or mammalian target of rapamycin inhibitor, the investigators gathered data retrospectively from 10 French centers.
They identified 25 men and 16 women with a median age of 62 years (range, 54-68 years). Of this group, 40 received denosumab in the first line in combination with either sunitinib (31 patients), pazopanib (6), everolimus (Afinitor and generics; 2), and temsirolimus (Torisel and generics; 1). The median duration of first-line therapy was 12 months.
Denosumab was given in the second line with axitinib (Inlyta) to nine patients, with everolimus to nine, and with sunitinib to three patients. The median duration of second-line therapy was 3 months.
Skeletal-related events occurred in 41% of patients prior to receiving denosumab and in 61% (25 patients) after denosumab. The events included clinical fracture, bone pain requiring radiation, and spinal compression.
Of the seven patients who developed ONJ, six received denosumab and sunitinib in the first line and one received denosumab plus temsirolimus, with a median duration of treatment of 11.8 months. Three of these patients also received denosumab in the second line with either axitinib, everolimus, or sunitinib, with a median duration of 9.8 months. Data on second-line therapy for the remaining four patients with ONJ were not available.
The investigators noted that the 17% incidence of ONJ was higher than the 10% rate seen in a study of patients with mRCC treated with a TKI and a bisphosphonate (Acta Clin Belg. 2018 Apr;73(2):100-9), and the 1.8% incidence seen in an analysis of three phase 3 trials in cancer patients with bone metastases (Ann Oncol. 2012 May;23(5):1341-7).
Although the etiology of ONJ is unknown, patient education and oral hygiene may reduce the risk in patients treated with denosumab and a TKI.
“The benefit of denosumab in this setting in regard [to] toxicity needs to be demonstrated in a prospective trial,” they wrote.
No conflicts of interest or disclosures were reported.
SOURCE: Guillot A et al. Clin Genitourin Cancer. 2018 Sep 6. doi: 10.1016/j.clgc.2018.08.006.
FROM CLINICAL GENITOURINARY CANCER
Key clinical point: The combination of denosumab and an antiangiogenic agent is associated with increased risk for osteonecrosis of the jaw (ONJ), a serious and debilitating side effect.
Major finding: Of 41 patients treated with denosumab and a tyrosine kinase inhibitor in the front line, 7 developed ONJ.
Study details: A retrospective analysis of data on 41 patients with mRCC treated with denosumab and an antiangiogenic agent at 10 cancer centers in France.
Disclosures: No conflicts of interest or disclosures were reported.
Source: Guillot A et al. Clin Genitourin Cancer. 2018 Sep 6. doi: 10.1016/j.clgc.2018.08.006.
Skin signs may be good omens during cancer therapy
Signs of efficacy of anti-cancer therapies may be only skin deep, results of a retrospective review indicate.
Cutaneous toxicities such as vitiligo, rash, alopecia, and nail toxicities may be early signs of efficacy of targeted therapies, immunotherapy, or cytotoxic chemotherapy, according to Alexandra K. Rzepecki, of the University of Michigan, and her coauthors from Albert Einstein Medical College in the Bronx, New York.
“Because cutaneous toxicities are a clinically visible parameter, they may alert clinicians to the possibility of treatment success or failure in a rapid, cost-effective, and noninvasive manner,” they wrote. The report is in the Journal of the American Academy of Dermatology.
The investigators reviewed the medical literature for clinical studies of three major classes of anti-cancer therapies that included data on associations between cutaneous toxicities and clinical outcomes such progression-free survival (PFS) overall survival (OS).
The drug classes and their associations with cutaneous toxicities and clinical outcomes were as follows:
- Targeted therapies, including tyrosine kinase inhibitors targeting the epidermal growth factor receptor (EGFR) such as cetuximab (Erbitux) and erlotinib (Tarceva), and multikinase targeted agents such as sorafenib (Nexavar) and sunitinib (Sutent). Toxicities associated with clinical benefit from EGFR inhibitors include rash, xerosis, leukocytoclastic vasculitis, paronychia, and pruritus, whereas skin toxicities associated with the multikinase inhibitors trended toward the hand-foot syndrome and hand-foot skin reaction.
- Immunotherapies included blockers of cytotoxic T-lymphocyte associated protein 4 (CTLA4) such as ipilimumab (Yervoy) and inhibitors of programmed death 1 protein (PD-1) and its ligand 1 (PD-L1) such as nivolumab (Opdivo), pembrolizumab (Keytruda), and atezolizumab (Tecentriq). In studies of pembrolizumab for various malignancies, rash or vitiligo was an independent prognostic factor for longer OS, a higher proportion of objective responses, and longer PFS. Similar associations were seen with nivolumab, with the additional association of hair repigmentation among patients with non–small-cell lung cancer being associated with stable disease responses or better. Among patients with melanoma treated with ipilimumab, hair depigmentation correlated with durable responses.
- Cytotoxic chemotherapy agents included the anthracycline doxorubicin, taxanes such as paclitaxel and docetaxel, platinum agents (cisplatin and carboplatin), and fluoropyrimidines such as capecitabine. Patients treated for various cancers with doxorubicin who had alopecia were significantly more likely to have clinical remissions than were patients who did not lose their hair, and patients treated with this agent who developed hand-foot syndrome had significantly longer PFS. For patients treated with docetaxel, severity of nail changes and/or development of nail alterations were associated with both improved OS and PFS. Patients treated with the combination of paclitaxel and a platinum agent who developed grade 2 or greater alopecia up to cycle 3 had significantly longer OS than did patients who had hair loss later in the course of therapy. Patients treated with capecitabine who developed had hand-foot skin reactions had improved progression-free and disease-free survival.
“Although further studies are needed to better evaluate these promising associations, vigilant monitoring of cutaneous toxicities should be a priority, as their development may indicate a favorable response to treatment. Dermatologists have a unique opportunity to collaborate with oncologists to help identify and manage these toxicities, thereby allowing patients to receive life-prolonging anticancer therapy while minimizing dose reduction or interruption of their treatment,” the authors wrote.
They reported no study funding source and no conflicts of interest.
SOURCE: Rzepecki A, et al. J Am Acad Dermatol. 2018;79:545-555.
Signs of efficacy of anti-cancer therapies may be only skin deep, results of a retrospective review indicate.
Cutaneous toxicities such as vitiligo, rash, alopecia, and nail toxicities may be early signs of efficacy of targeted therapies, immunotherapy, or cytotoxic chemotherapy, according to Alexandra K. Rzepecki, of the University of Michigan, and her coauthors from Albert Einstein Medical College in the Bronx, New York.
“Because cutaneous toxicities are a clinically visible parameter, they may alert clinicians to the possibility of treatment success or failure in a rapid, cost-effective, and noninvasive manner,” they wrote. The report is in the Journal of the American Academy of Dermatology.
The investigators reviewed the medical literature for clinical studies of three major classes of anti-cancer therapies that included data on associations between cutaneous toxicities and clinical outcomes such progression-free survival (PFS) overall survival (OS).
The drug classes and their associations with cutaneous toxicities and clinical outcomes were as follows:
- Targeted therapies, including tyrosine kinase inhibitors targeting the epidermal growth factor receptor (EGFR) such as cetuximab (Erbitux) and erlotinib (Tarceva), and multikinase targeted agents such as sorafenib (Nexavar) and sunitinib (Sutent). Toxicities associated with clinical benefit from EGFR inhibitors include rash, xerosis, leukocytoclastic vasculitis, paronychia, and pruritus, whereas skin toxicities associated with the multikinase inhibitors trended toward the hand-foot syndrome and hand-foot skin reaction.
- Immunotherapies included blockers of cytotoxic T-lymphocyte associated protein 4 (CTLA4) such as ipilimumab (Yervoy) and inhibitors of programmed death 1 protein (PD-1) and its ligand 1 (PD-L1) such as nivolumab (Opdivo), pembrolizumab (Keytruda), and atezolizumab (Tecentriq). In studies of pembrolizumab for various malignancies, rash or vitiligo was an independent prognostic factor for longer OS, a higher proportion of objective responses, and longer PFS. Similar associations were seen with nivolumab, with the additional association of hair repigmentation among patients with non–small-cell lung cancer being associated with stable disease responses or better. Among patients with melanoma treated with ipilimumab, hair depigmentation correlated with durable responses.
- Cytotoxic chemotherapy agents included the anthracycline doxorubicin, taxanes such as paclitaxel and docetaxel, platinum agents (cisplatin and carboplatin), and fluoropyrimidines such as capecitabine. Patients treated for various cancers with doxorubicin who had alopecia were significantly more likely to have clinical remissions than were patients who did not lose their hair, and patients treated with this agent who developed hand-foot syndrome had significantly longer PFS. For patients treated with docetaxel, severity of nail changes and/or development of nail alterations were associated with both improved OS and PFS. Patients treated with the combination of paclitaxel and a platinum agent who developed grade 2 or greater alopecia up to cycle 3 had significantly longer OS than did patients who had hair loss later in the course of therapy. Patients treated with capecitabine who developed had hand-foot skin reactions had improved progression-free and disease-free survival.
“Although further studies are needed to better evaluate these promising associations, vigilant monitoring of cutaneous toxicities should be a priority, as their development may indicate a favorable response to treatment. Dermatologists have a unique opportunity to collaborate with oncologists to help identify and manage these toxicities, thereby allowing patients to receive life-prolonging anticancer therapy while minimizing dose reduction or interruption of their treatment,” the authors wrote.
They reported no study funding source and no conflicts of interest.
SOURCE: Rzepecki A, et al. J Am Acad Dermatol. 2018;79:545-555.
Signs of efficacy of anti-cancer therapies may be only skin deep, results of a retrospective review indicate.
Cutaneous toxicities such as vitiligo, rash, alopecia, and nail toxicities may be early signs of efficacy of targeted therapies, immunotherapy, or cytotoxic chemotherapy, according to Alexandra K. Rzepecki, of the University of Michigan, and her coauthors from Albert Einstein Medical College in the Bronx, New York.
“Because cutaneous toxicities are a clinically visible parameter, they may alert clinicians to the possibility of treatment success or failure in a rapid, cost-effective, and noninvasive manner,” they wrote. The report is in the Journal of the American Academy of Dermatology.
The investigators reviewed the medical literature for clinical studies of three major classes of anti-cancer therapies that included data on associations between cutaneous toxicities and clinical outcomes such progression-free survival (PFS) overall survival (OS).
The drug classes and their associations with cutaneous toxicities and clinical outcomes were as follows:
- Targeted therapies, including tyrosine kinase inhibitors targeting the epidermal growth factor receptor (EGFR) such as cetuximab (Erbitux) and erlotinib (Tarceva), and multikinase targeted agents such as sorafenib (Nexavar) and sunitinib (Sutent). Toxicities associated with clinical benefit from EGFR inhibitors include rash, xerosis, leukocytoclastic vasculitis, paronychia, and pruritus, whereas skin toxicities associated with the multikinase inhibitors trended toward the hand-foot syndrome and hand-foot skin reaction.
- Immunotherapies included blockers of cytotoxic T-lymphocyte associated protein 4 (CTLA4) such as ipilimumab (Yervoy) and inhibitors of programmed death 1 protein (PD-1) and its ligand 1 (PD-L1) such as nivolumab (Opdivo), pembrolizumab (Keytruda), and atezolizumab (Tecentriq). In studies of pembrolizumab for various malignancies, rash or vitiligo was an independent prognostic factor for longer OS, a higher proportion of objective responses, and longer PFS. Similar associations were seen with nivolumab, with the additional association of hair repigmentation among patients with non–small-cell lung cancer being associated with stable disease responses or better. Among patients with melanoma treated with ipilimumab, hair depigmentation correlated with durable responses.
- Cytotoxic chemotherapy agents included the anthracycline doxorubicin, taxanes such as paclitaxel and docetaxel, platinum agents (cisplatin and carboplatin), and fluoropyrimidines such as capecitabine. Patients treated for various cancers with doxorubicin who had alopecia were significantly more likely to have clinical remissions than were patients who did not lose their hair, and patients treated with this agent who developed hand-foot syndrome had significantly longer PFS. For patients treated with docetaxel, severity of nail changes and/or development of nail alterations were associated with both improved OS and PFS. Patients treated with the combination of paclitaxel and a platinum agent who developed grade 2 or greater alopecia up to cycle 3 had significantly longer OS than did patients who had hair loss later in the course of therapy. Patients treated with capecitabine who developed had hand-foot skin reactions had improved progression-free and disease-free survival.
“Although further studies are needed to better evaluate these promising associations, vigilant monitoring of cutaneous toxicities should be a priority, as their development may indicate a favorable response to treatment. Dermatologists have a unique opportunity to collaborate with oncologists to help identify and manage these toxicities, thereby allowing patients to receive life-prolonging anticancer therapy while minimizing dose reduction or interruption of their treatment,” the authors wrote.
They reported no study funding source and no conflicts of interest.
SOURCE: Rzepecki A, et al. J Am Acad Dermatol. 2018;79:545-555.
FROM JOURNAL OF THE AMERICAN ACADEMY OF DERMATOLOGY
Key clinical point: Cutaneous adverse events may be early signs of drug efficacy in patients treated for various cancers.
Major finding: Cutaneous toxicities with targeted therapies, immunotherapy, and cytotoxic drugs were associated in multiple studies with improved outcomes, including progression-free and overall survival.
Study details: Retrospective review of medical literature for clinical studies reporting associations between cutaneous toxicities and clinical outcomes of cancer therapy.
Disclosures: The authors reported no study funding source and no conflicts of interest.
Source: Rzepecki A et al. J Am Acad Dermatol. 2018 Sep;79[3]:545-55.
AACR: New cancer cases predicted to rise above 2.3 million by 2035
during the last 12 months.
Among the advances outlined in the AACR Cancer Progress Report 2018 are “revolutionary new immunotherapeutics called CAR T–cell therapies, exciting new targeted radiotherapeutics, and numerous new targeted therapeutics that are expanding the scope of precision medicine,” the AACR said in a written statement.
Despite this progress, however, cancer continues to pose immense public health challenges.
The number of new cancer cases in the United States is predicted to increase from more than 1.7 million in 2018 to almost 2.4 million in 2035, due in large part to the rising number of people age 65 and older, according to the report.
AACR calls on elected officials to:
- Maintain “robust, sustained, and predictable growth” of the National Institutes of Health budget, increasing it at least $2 billion in fiscal year (FY) 2019, for a total funding level of at least $39.1 billion.
- Make sure that the $711 million in funding provided through the 21st Century Cures Act for targeted initiatives – including the National Cancer Moonshot – “is fully appropriated in FY 2019 and is supplemental to the healthy increase for the NIH’s base budget.”
- Raise the Food and Drug Administration’s base budget in FY 2019 to $3.1 billion – a $308 million increase above its FY 2018 level – to secure support for regulatory science and speed the development of medical products, ones that are safe and effective. Particularly, in FY 2019, the AACR backs a funding level of $20 million for the FDA Oncology Center of Excellence.
- Provide the Centers for Disease Control and Prevention Cancer Prevention and Control Programs with total funding of at least $517 million. This would include funding for “comprehensive cancer control, cancer registries, and screening and awareness programs for specific cancers.”
Read the full report and watch video stories from patients here.
during the last 12 months.
Among the advances outlined in the AACR Cancer Progress Report 2018 are “revolutionary new immunotherapeutics called CAR T–cell therapies, exciting new targeted radiotherapeutics, and numerous new targeted therapeutics that are expanding the scope of precision medicine,” the AACR said in a written statement.
Despite this progress, however, cancer continues to pose immense public health challenges.
The number of new cancer cases in the United States is predicted to increase from more than 1.7 million in 2018 to almost 2.4 million in 2035, due in large part to the rising number of people age 65 and older, according to the report.
AACR calls on elected officials to:
- Maintain “robust, sustained, and predictable growth” of the National Institutes of Health budget, increasing it at least $2 billion in fiscal year (FY) 2019, for a total funding level of at least $39.1 billion.
- Make sure that the $711 million in funding provided through the 21st Century Cures Act for targeted initiatives – including the National Cancer Moonshot – “is fully appropriated in FY 2019 and is supplemental to the healthy increase for the NIH’s base budget.”
- Raise the Food and Drug Administration’s base budget in FY 2019 to $3.1 billion – a $308 million increase above its FY 2018 level – to secure support for regulatory science and speed the development of medical products, ones that are safe and effective. Particularly, in FY 2019, the AACR backs a funding level of $20 million for the FDA Oncology Center of Excellence.
- Provide the Centers for Disease Control and Prevention Cancer Prevention and Control Programs with total funding of at least $517 million. This would include funding for “comprehensive cancer control, cancer registries, and screening and awareness programs for specific cancers.”
Read the full report and watch video stories from patients here.
during the last 12 months.
Among the advances outlined in the AACR Cancer Progress Report 2018 are “revolutionary new immunotherapeutics called CAR T–cell therapies, exciting new targeted radiotherapeutics, and numerous new targeted therapeutics that are expanding the scope of precision medicine,” the AACR said in a written statement.
Despite this progress, however, cancer continues to pose immense public health challenges.
The number of new cancer cases in the United States is predicted to increase from more than 1.7 million in 2018 to almost 2.4 million in 2035, due in large part to the rising number of people age 65 and older, according to the report.
AACR calls on elected officials to:
- Maintain “robust, sustained, and predictable growth” of the National Institutes of Health budget, increasing it at least $2 billion in fiscal year (FY) 2019, for a total funding level of at least $39.1 billion.
- Make sure that the $711 million in funding provided through the 21st Century Cures Act for targeted initiatives – including the National Cancer Moonshot – “is fully appropriated in FY 2019 and is supplemental to the healthy increase for the NIH’s base budget.”
- Raise the Food and Drug Administration’s base budget in FY 2019 to $3.1 billion – a $308 million increase above its FY 2018 level – to secure support for regulatory science and speed the development of medical products, ones that are safe and effective. Particularly, in FY 2019, the AACR backs a funding level of $20 million for the FDA Oncology Center of Excellence.
- Provide the Centers for Disease Control and Prevention Cancer Prevention and Control Programs with total funding of at least $517 million. This would include funding for “comprehensive cancer control, cancer registries, and screening and awareness programs for specific cancers.”
Read the full report and watch video stories from patients here.
ASCO updates guidance on prophylaxis for adults with cancer-related immunosuppression
Fluoroquinolones are recommended for adults with cancer-related immunosuppression if they are at high risk of infection, according to an updated clinical practice guideline on antimicrobial prophylaxis.
By contrast, patients with solid tumors are not routinely recommended to receive antibiotic prophylaxis, according to the guideline, developed by the American Society of Clinical Oncology (ASCO) with the Infectious Diseases Society of America (IDSA).
The guideline includes antibacterial, antifungal, and antiviral prophylaxis recommendations, along with additional precautions such as hand hygiene that may reduce infection risk.
Released in the Journal of Clinical Oncology, the updated guidelines were developed by an expert panel cochaired by Christopher R. Flowers, MD of Emory University, Atlanta, and Randy A. Taplitz, MD of the University of California, San Diego, Health.
For the most part, the panel endorsed the previous ASCO recommendations, published in 2013. However, the panel considered six new high-quality studies and six new or updated meta-analyses to make modifications and add some new recommendations.
Fluoroquinolones, in the 2013 guideline, were recommended over trimethoprim-sulfamethoxazole because of fewer adverse events leading to treatment discontinuation. Panelists for the new guidelines said they continued to support that recommendation, based on an updated literature review.
That review showed significant reductions in both febrile neutropenia incidence and all-cause mortality, not only for patients at high risk of febrile neutropenia or profound, protracted neutropenia but also for lower-risk patients with solid tumors, they said.
However, the benefits did not sufficiently outweigh the harms to justify recommending fluoroquinolone prophylaxis for all patients with solid tumors or lymphoma, according to the report from the expert panel.
Those harms could include antibiotic-associated adverse effects, emergence of resistance, and Clostridium difficile infections, they said.
Accordingly, they recommended fluoroquinolone prophylaxis for the high-risk patients, including most patients with acute myeloid leukemia/myelodysplastic syndromes (AML/MDS) or those undergoing hematopoietic stem-cell transplantation (HSCT).
Similarly, the panel recommended that high-risk patients should receive antifungal prophylaxis with an oral triazole or parenteral echinocandin, while prophylaxis would not be routinely recommended for solid tumor patients.
By contrast, all patients undergoing chemotherapy for malignancy should receive yearly influenza vaccination with an inactivated quadrivalent vaccine, the panel said in its antiviral prophylaxis recommendations.
Family members, household contacts, and health care providers also should receive influenza vaccinations, said the panel, endorsing recommendations from the Centers for Disease Control and Prevention that were also cited in the 2013 ASCO guidelines.
Health care workers should follow hand hygiene and respiratory hygiene/cough etiquette to reduce risk of pathogen transmission, the panel said, endorsing CDC recommendations cited in the previous guideline.
However, the panel said they recommend against interventions such as neutropenic diet, footwear exchange, nutritional supplements, and surgical masks.
“Evidence of clinical benefit is lacking” for those interventions, they said.
Participants in the expert panel disclosed potential conflicts of interest related to Merck, Chimerix, GlyPharma Therapeutic, Pfizer, Cidara Therapeutics, Celgene, Astellas Pharma, Gilead Sciences, and Allergan, among other entities.
SOURCE: Taplitz RA et al. J Clin Oncol. 2018 Sept 4. doi: 10.1200/JCO.18.00374.
Fluoroquinolones are recommended for adults with cancer-related immunosuppression if they are at high risk of infection, according to an updated clinical practice guideline on antimicrobial prophylaxis.
By contrast, patients with solid tumors are not routinely recommended to receive antibiotic prophylaxis, according to the guideline, developed by the American Society of Clinical Oncology (ASCO) with the Infectious Diseases Society of America (IDSA).
The guideline includes antibacterial, antifungal, and antiviral prophylaxis recommendations, along with additional precautions such as hand hygiene that may reduce infection risk.
Released in the Journal of Clinical Oncology, the updated guidelines were developed by an expert panel cochaired by Christopher R. Flowers, MD of Emory University, Atlanta, and Randy A. Taplitz, MD of the University of California, San Diego, Health.
For the most part, the panel endorsed the previous ASCO recommendations, published in 2013. However, the panel considered six new high-quality studies and six new or updated meta-analyses to make modifications and add some new recommendations.
Fluoroquinolones, in the 2013 guideline, were recommended over trimethoprim-sulfamethoxazole because of fewer adverse events leading to treatment discontinuation. Panelists for the new guidelines said they continued to support that recommendation, based on an updated literature review.
That review showed significant reductions in both febrile neutropenia incidence and all-cause mortality, not only for patients at high risk of febrile neutropenia or profound, protracted neutropenia but also for lower-risk patients with solid tumors, they said.
However, the benefits did not sufficiently outweigh the harms to justify recommending fluoroquinolone prophylaxis for all patients with solid tumors or lymphoma, according to the report from the expert panel.
Those harms could include antibiotic-associated adverse effects, emergence of resistance, and Clostridium difficile infections, they said.
Accordingly, they recommended fluoroquinolone prophylaxis for the high-risk patients, including most patients with acute myeloid leukemia/myelodysplastic syndromes (AML/MDS) or those undergoing hematopoietic stem-cell transplantation (HSCT).
Similarly, the panel recommended that high-risk patients should receive antifungal prophylaxis with an oral triazole or parenteral echinocandin, while prophylaxis would not be routinely recommended for solid tumor patients.
By contrast, all patients undergoing chemotherapy for malignancy should receive yearly influenza vaccination with an inactivated quadrivalent vaccine, the panel said in its antiviral prophylaxis recommendations.
Family members, household contacts, and health care providers also should receive influenza vaccinations, said the panel, endorsing recommendations from the Centers for Disease Control and Prevention that were also cited in the 2013 ASCO guidelines.
Health care workers should follow hand hygiene and respiratory hygiene/cough etiquette to reduce risk of pathogen transmission, the panel said, endorsing CDC recommendations cited in the previous guideline.
However, the panel said they recommend against interventions such as neutropenic diet, footwear exchange, nutritional supplements, and surgical masks.
“Evidence of clinical benefit is lacking” for those interventions, they said.
Participants in the expert panel disclosed potential conflicts of interest related to Merck, Chimerix, GlyPharma Therapeutic, Pfizer, Cidara Therapeutics, Celgene, Astellas Pharma, Gilead Sciences, and Allergan, among other entities.
SOURCE: Taplitz RA et al. J Clin Oncol. 2018 Sept 4. doi: 10.1200/JCO.18.00374.
Fluoroquinolones are recommended for adults with cancer-related immunosuppression if they are at high risk of infection, according to an updated clinical practice guideline on antimicrobial prophylaxis.
By contrast, patients with solid tumors are not routinely recommended to receive antibiotic prophylaxis, according to the guideline, developed by the American Society of Clinical Oncology (ASCO) with the Infectious Diseases Society of America (IDSA).
The guideline includes antibacterial, antifungal, and antiviral prophylaxis recommendations, along with additional precautions such as hand hygiene that may reduce infection risk.
Released in the Journal of Clinical Oncology, the updated guidelines were developed by an expert panel cochaired by Christopher R. Flowers, MD of Emory University, Atlanta, and Randy A. Taplitz, MD of the University of California, San Diego, Health.
For the most part, the panel endorsed the previous ASCO recommendations, published in 2013. However, the panel considered six new high-quality studies and six new or updated meta-analyses to make modifications and add some new recommendations.
Fluoroquinolones, in the 2013 guideline, were recommended over trimethoprim-sulfamethoxazole because of fewer adverse events leading to treatment discontinuation. Panelists for the new guidelines said they continued to support that recommendation, based on an updated literature review.
That review showed significant reductions in both febrile neutropenia incidence and all-cause mortality, not only for patients at high risk of febrile neutropenia or profound, protracted neutropenia but also for lower-risk patients with solid tumors, they said.
However, the benefits did not sufficiently outweigh the harms to justify recommending fluoroquinolone prophylaxis for all patients with solid tumors or lymphoma, according to the report from the expert panel.
Those harms could include antibiotic-associated adverse effects, emergence of resistance, and Clostridium difficile infections, they said.
Accordingly, they recommended fluoroquinolone prophylaxis for the high-risk patients, including most patients with acute myeloid leukemia/myelodysplastic syndromes (AML/MDS) or those undergoing hematopoietic stem-cell transplantation (HSCT).
Similarly, the panel recommended that high-risk patients should receive antifungal prophylaxis with an oral triazole or parenteral echinocandin, while prophylaxis would not be routinely recommended for solid tumor patients.
By contrast, all patients undergoing chemotherapy for malignancy should receive yearly influenza vaccination with an inactivated quadrivalent vaccine, the panel said in its antiviral prophylaxis recommendations.
Family members, household contacts, and health care providers also should receive influenza vaccinations, said the panel, endorsing recommendations from the Centers for Disease Control and Prevention that were also cited in the 2013 ASCO guidelines.
Health care workers should follow hand hygiene and respiratory hygiene/cough etiquette to reduce risk of pathogen transmission, the panel said, endorsing CDC recommendations cited in the previous guideline.
However, the panel said they recommend against interventions such as neutropenic diet, footwear exchange, nutritional supplements, and surgical masks.
“Evidence of clinical benefit is lacking” for those interventions, they said.
Participants in the expert panel disclosed potential conflicts of interest related to Merck, Chimerix, GlyPharma Therapeutic, Pfizer, Cidara Therapeutics, Celgene, Astellas Pharma, Gilead Sciences, and Allergan, among other entities.
SOURCE: Taplitz RA et al. J Clin Oncol. 2018 Sept 4. doi: 10.1200/JCO.18.00374.
FROM THE JOURNAL OF CLINICAL ONCOLOGY
ESMO scale offers guidance on cancer targets
The European Society for Medical Oncology (ESMO) has published a proposed scale that would rank molecular targets for various cancers by how well they can be treated with new or emerging drugs.
The ESMO Scale of Clinical Actionability for Molecular Targets is designed to “harmonize and standardize the reporting and interpretation of clinically relevant genomics data,” according to Joaquin Mateo, MD, PhD, from the Vall d’Hebron Institute of Oncology in Barcelona, Spain, and his fellow members of the ESMO Translational Research and Precision Medicine Working Group.
“A major challenge for oncologists in the clinic is to distinguish between findings that represent proven clinical value or potential value based on preliminary clinical or preclinical evidence from hypothetical gene-drug matches and findings that are currently irrelevant for clinical practice,” they wrote in Annals of Oncology.
The scale groups targets into one of six tiers based on levels of evidence ranging from the gold standard of prospective, randomized clinical trials to targets for which there are no evidence and only hypothetical actionability. The primary goal is to help oncologists assign priority to potential targets when they review results of gene-sequencing panels for individual patients, according to the developers.
Briefly, the six tiers are:
Tier I includes targets that are agreed to be suitable for routine use and a recommended specific drug when a specific molecular alteration is detected. Examples include trastuzumab for human epidermal growth factor receptor 2 (HER2)–positive breast cancer, and inhibitors of epidermal growth factor receptor (EGFR) in patients with non–small cell lung cancer positive for EGFR mutations.
Tier II includes “investigational targets that likely define a patient population that benefits from a targeted drug but additional data are needed.” This tier includes agents that work in the phosphatidylinostiol 3-kinase pathway.
Tier III is similar to Tier II, in that it includes investigational targets that define a patient population with proven benefit from a targeted therapy, but in this case the target is detected in a different tumor type that has not previously been studied. For example, the targeted agent vemurafenib (Zelboraf), which extends survival of patients with metastatic melanomas carrying the BRAF V600E mutation, has only limited activity against BRAF-mutated colorectal cancers.
Tier IV includes targets with preclinical evidence of actionability.
Tier V includes targets with “evidence of relevant antitumor activity, not resulting in clinical meaningful benefit as single treatment but supporting development of cotargeting approaches.” The authors cite the example of PIK3CA inhibitors in patients with estrogen receptor–positive, HER2-negative breast cancers who also have PIK3CA activating mutations. In clinical trials, this strategy led to objective responses but not change outcomes.
The final tier is not Tier VI, as might be expected, but Tier X, with the X in this case being the unknown – that is, alterations/mutations for which there is neither preclinical nor clinical evidence to support their hypothetical use as a drug target.
“This clinical benefit–centered classification system offers a common language for all the actors involved in clinical cancer drug development. Its implementation in sequencing reports, tumor boards, and scientific communication can enable precise treatment decisions and facilitate discussions with patients about novel therapeutic options,” Dr. Mateo and his associates wrote in their conclusion.
The development process was supported by ESMO. Multiple coauthors reported financial relationships with various companies as well as grants/support from other foundations or charities.
SOURCE: Mateo J et al. Ann Oncol. 2018 Aug 21. doi: 10.1093/annonc/mdy263.
The European Society for Medical Oncology (ESMO) has published a proposed scale that would rank molecular targets for various cancers by how well they can be treated with new or emerging drugs.
The ESMO Scale of Clinical Actionability for Molecular Targets is designed to “harmonize and standardize the reporting and interpretation of clinically relevant genomics data,” according to Joaquin Mateo, MD, PhD, from the Vall d’Hebron Institute of Oncology in Barcelona, Spain, and his fellow members of the ESMO Translational Research and Precision Medicine Working Group.
“A major challenge for oncologists in the clinic is to distinguish between findings that represent proven clinical value or potential value based on preliminary clinical or preclinical evidence from hypothetical gene-drug matches and findings that are currently irrelevant for clinical practice,” they wrote in Annals of Oncology.
The scale groups targets into one of six tiers based on levels of evidence ranging from the gold standard of prospective, randomized clinical trials to targets for which there are no evidence and only hypothetical actionability. The primary goal is to help oncologists assign priority to potential targets when they review results of gene-sequencing panels for individual patients, according to the developers.
Briefly, the six tiers are:
Tier I includes targets that are agreed to be suitable for routine use and a recommended specific drug when a specific molecular alteration is detected. Examples include trastuzumab for human epidermal growth factor receptor 2 (HER2)–positive breast cancer, and inhibitors of epidermal growth factor receptor (EGFR) in patients with non–small cell lung cancer positive for EGFR mutations.
Tier II includes “investigational targets that likely define a patient population that benefits from a targeted drug but additional data are needed.” This tier includes agents that work in the phosphatidylinostiol 3-kinase pathway.
Tier III is similar to Tier II, in that it includes investigational targets that define a patient population with proven benefit from a targeted therapy, but in this case the target is detected in a different tumor type that has not previously been studied. For example, the targeted agent vemurafenib (Zelboraf), which extends survival of patients with metastatic melanomas carrying the BRAF V600E mutation, has only limited activity against BRAF-mutated colorectal cancers.
Tier IV includes targets with preclinical evidence of actionability.
Tier V includes targets with “evidence of relevant antitumor activity, not resulting in clinical meaningful benefit as single treatment but supporting development of cotargeting approaches.” The authors cite the example of PIK3CA inhibitors in patients with estrogen receptor–positive, HER2-negative breast cancers who also have PIK3CA activating mutations. In clinical trials, this strategy led to objective responses but not change outcomes.
The final tier is not Tier VI, as might be expected, but Tier X, with the X in this case being the unknown – that is, alterations/mutations for which there is neither preclinical nor clinical evidence to support their hypothetical use as a drug target.
“This clinical benefit–centered classification system offers a common language for all the actors involved in clinical cancer drug development. Its implementation in sequencing reports, tumor boards, and scientific communication can enable precise treatment decisions and facilitate discussions with patients about novel therapeutic options,” Dr. Mateo and his associates wrote in their conclusion.
The development process was supported by ESMO. Multiple coauthors reported financial relationships with various companies as well as grants/support from other foundations or charities.
SOURCE: Mateo J et al. Ann Oncol. 2018 Aug 21. doi: 10.1093/annonc/mdy263.
The European Society for Medical Oncology (ESMO) has published a proposed scale that would rank molecular targets for various cancers by how well they can be treated with new or emerging drugs.
The ESMO Scale of Clinical Actionability for Molecular Targets is designed to “harmonize and standardize the reporting and interpretation of clinically relevant genomics data,” according to Joaquin Mateo, MD, PhD, from the Vall d’Hebron Institute of Oncology in Barcelona, Spain, and his fellow members of the ESMO Translational Research and Precision Medicine Working Group.
“A major challenge for oncologists in the clinic is to distinguish between findings that represent proven clinical value or potential value based on preliminary clinical or preclinical evidence from hypothetical gene-drug matches and findings that are currently irrelevant for clinical practice,” they wrote in Annals of Oncology.
The scale groups targets into one of six tiers based on levels of evidence ranging from the gold standard of prospective, randomized clinical trials to targets for which there are no evidence and only hypothetical actionability. The primary goal is to help oncologists assign priority to potential targets when they review results of gene-sequencing panels for individual patients, according to the developers.
Briefly, the six tiers are:
Tier I includes targets that are agreed to be suitable for routine use and a recommended specific drug when a specific molecular alteration is detected. Examples include trastuzumab for human epidermal growth factor receptor 2 (HER2)–positive breast cancer, and inhibitors of epidermal growth factor receptor (EGFR) in patients with non–small cell lung cancer positive for EGFR mutations.
Tier II includes “investigational targets that likely define a patient population that benefits from a targeted drug but additional data are needed.” This tier includes agents that work in the phosphatidylinostiol 3-kinase pathway.
Tier III is similar to Tier II, in that it includes investigational targets that define a patient population with proven benefit from a targeted therapy, but in this case the target is detected in a different tumor type that has not previously been studied. For example, the targeted agent vemurafenib (Zelboraf), which extends survival of patients with metastatic melanomas carrying the BRAF V600E mutation, has only limited activity against BRAF-mutated colorectal cancers.
Tier IV includes targets with preclinical evidence of actionability.
Tier V includes targets with “evidence of relevant antitumor activity, not resulting in clinical meaningful benefit as single treatment but supporting development of cotargeting approaches.” The authors cite the example of PIK3CA inhibitors in patients with estrogen receptor–positive, HER2-negative breast cancers who also have PIK3CA activating mutations. In clinical trials, this strategy led to objective responses but not change outcomes.
The final tier is not Tier VI, as might be expected, but Tier X, with the X in this case being the unknown – that is, alterations/mutations for which there is neither preclinical nor clinical evidence to support their hypothetical use as a drug target.
“This clinical benefit–centered classification system offers a common language for all the actors involved in clinical cancer drug development. Its implementation in sequencing reports, tumor boards, and scientific communication can enable precise treatment decisions and facilitate discussions with patients about novel therapeutic options,” Dr. Mateo and his associates wrote in their conclusion.
The development process was supported by ESMO. Multiple coauthors reported financial relationships with various companies as well as grants/support from other foundations or charities.
SOURCE: Mateo J et al. Ann Oncol. 2018 Aug 21. doi: 10.1093/annonc/mdy263.
FROM ANNALS OF ONCOLOGY
Key clinical point: The scale is intended to standardize reporting and interpretation of cancer gene panel results to help oncologists plan treatment.
Major finding: The scale divides current and future therapeutic targets into tiers based on levels of clinical and preclinical evidence.
Study details: Proposed guiding principles for a classification system developed by the Translational Research and Precision Medicine Working Group of the European Society of Medical Oncology.
Disclosures: The development process was supported by ESMO. Multiple coauthors reported financial relationships with various companies as well as grants/support from other foundations or charities.
Source: Mateo J et al. Ann Oncol. 2018 Aug 21. doi: 10.1093/annonc/mdy263.
When is the right time to stop treatment?
Martha Boxer (not her real name) had mentally prepared herself that this could happen, but the news still hit hard. Her doctors on the leukemia service broke the facts as gently as we could: The chemotherapy she had been suffering through for the last 2 weeks hadn’t worked. The results of her latest bone marrow biopsy showed it remained packed with cancer cells.
As Martha absorbed the news quietly, her son, sitting next to her bedside with his hand on hers, spoke first. “What now?”
I looked at my attending and nodded, as we were fully ready to answer this question. From the outset, we knew that Martha’s leukemia carried a genetic mutation that unfortunately put her in a high-risk category. The chances of her cancer responding to the first round of chemotherapy were low. When this happens, what we typically do next is reinduction, we explained. It’s a different combination of chemotherapy drugs, with a somewhat different side effect profile. But it would give her the best chance of response, we believed. We could start the new chemotherapy as early as today, we said.
Martha took this in. “Okay,” she said pensively. “I’ve been thinking. And I think maybe … I won’t do chemotherapy anymore.”
Her words caught me off guard because, frankly, they seemed premature. Her leukemia had not budged with the first round of treatment. But we still had an option B, and then an option C. It was usually at a later, more dire stage – when multiple lines of treatment had not worked, and instead had only caused harm – or, when the decision was forced by the medical system’s admission that we had nothing left to offer – that I’d heard patients express similar preferences. It was then that I’d seen patients and their loved ones flip a mental switch and choose to focus the time they had left on what really mattered to them.
It didn’t feel like we were at that point.
And so, as we debriefed outside her room, my first instinct was to convince her otherwise.
However, Martha had other priorities, as I would come to learn. Above all else, she hated the hospital. She hated feeling trapped in a strange room that wasn’t hers; she hated how the chemotherapy stole her energy and made her feel too weak to even shower. She wanted to be in her own home. She wanted to eat her own food, sleep in her own bed, and be surrounded by what she recognized.
But, she also wanted to live. Two paths lay ahead of her. It was a trade-off of more suffering with a small chance at remission, versus accepting no chance of cure but feeling well for as long as she could. She soon clarified that she wasn’t definitely against chemotherapy. She couldn’t decide. She needed more information from us to make this decision, the hardest of her life.
Over the next few days, I watched as our attending physician expertly provided just that. There were actually three options, she laid out. There was aggressive chemotherapy, entailing at least 3 more weeks in the hospital and coming with significant risk of infection, nausea, vomiting, and fatigue. The chances of inducing a remission were about one in three to one in two, and that remission would likely last between several months and 2 years before the leukemia would relapse. The second option was a chemotherapy pill she could take at home, an option with fewer side effects but no longer aimed at cure. The third option was home hospice support, focused on symptoms, without any anticancer medication.
I noticed a few things during those conversations. I noticed how my attending took a navigator role, not pushing Martha in one direction or another, but rather imparting all the relevant information to empower Martha to decide for herself. I noticed how she provided realistic estimates, not hedging away from numbers, but giving the honest, nitty-gritty facts, as best as she could predict. I noticed how she took the time and never rushed, even in spite of external pressures to discharge the patient from the hospital.
There was no right or wrong answer. I no longer felt that we had something in our grasp – a clear-cut, best decision – to persuade Martha toward. Is one in three good odds, or bad odds? Is 2 years a long period of time, or a short one? Of course, there is no actual answer to these questions; the answer is as elusive and personal as if we had asked Martha: What do you think?
What I learned from Martha is that, with a devastating diagnosis, there isn’t a right time to make this decision. There isn’t one defining moment where we flip a switch and change course. It isn’t only when we run out of treatment options that the choice to forgo it makes sense. That option is on a flexible line, different for every person and priority. As my attending later said, with Martha’s diagnosis and her values, it wouldn’t have been unreasonable to decide against chemotherapy from the start.
The language we use can sometimes mask that reality. As doctors, we may casually slip in words like “need” and “have to” in response to patients’ questions about what to do next. “You need more chemotherapy,” we might say. “We’d have to treat it.” We have “treatment,” after all, and so we go down the line of offering what’s next in the medical algorithm. That word, too, can be deceivingly tempting, enticing down a road that makes it seem like the obvious answer – or the only one. If the choices are treatment versus not, who wouldn’t want the treatment? But the details are where things get murky. What does that treatment involve? What are the chances it will work, and for how long?
Surreptitiously missing from this language is the fact that there’s a choice. There’s always a choice, and it’s on the table at any point. You can start chemotherapy without committing to stick it out until the end. You can go home, if that is what’s important to you. The best treatment option is the one the patient wants.
After 4 days, Martha decided to go home with palliative chemotherapy and a bridge to hospice. Each member of our team hugged her goodbye and wished her luck. She was nervous. But she packed her hospital room, and she left.
I recently pulled up her medical chart, bracing myself for bad news. But the interesting thing about hospice is that even though the focus is no longer on prolonging life, people sometimes live longer.
She felt well, the most recent palliative note said. She was spending her time writing, getting her finances in order, and finishing a legacy project for her grandchildren.
For Martha, it seemed to be the right choice.
Dr. Yurkiewicz is a fellow in hematology and oncology at Stanford (Calif.) University. Follow her on Twitter @ilanayurkiewicz.
Martha Boxer (not her real name) had mentally prepared herself that this could happen, but the news still hit hard. Her doctors on the leukemia service broke the facts as gently as we could: The chemotherapy she had been suffering through for the last 2 weeks hadn’t worked. The results of her latest bone marrow biopsy showed it remained packed with cancer cells.
As Martha absorbed the news quietly, her son, sitting next to her bedside with his hand on hers, spoke first. “What now?”
I looked at my attending and nodded, as we were fully ready to answer this question. From the outset, we knew that Martha’s leukemia carried a genetic mutation that unfortunately put her in a high-risk category. The chances of her cancer responding to the first round of chemotherapy were low. When this happens, what we typically do next is reinduction, we explained. It’s a different combination of chemotherapy drugs, with a somewhat different side effect profile. But it would give her the best chance of response, we believed. We could start the new chemotherapy as early as today, we said.
Martha took this in. “Okay,” she said pensively. “I’ve been thinking. And I think maybe … I won’t do chemotherapy anymore.”
Her words caught me off guard because, frankly, they seemed premature. Her leukemia had not budged with the first round of treatment. But we still had an option B, and then an option C. It was usually at a later, more dire stage – when multiple lines of treatment had not worked, and instead had only caused harm – or, when the decision was forced by the medical system’s admission that we had nothing left to offer – that I’d heard patients express similar preferences. It was then that I’d seen patients and their loved ones flip a mental switch and choose to focus the time they had left on what really mattered to them.
It didn’t feel like we were at that point.
And so, as we debriefed outside her room, my first instinct was to convince her otherwise.
However, Martha had other priorities, as I would come to learn. Above all else, she hated the hospital. She hated feeling trapped in a strange room that wasn’t hers; she hated how the chemotherapy stole her energy and made her feel too weak to even shower. She wanted to be in her own home. She wanted to eat her own food, sleep in her own bed, and be surrounded by what she recognized.
But, she also wanted to live. Two paths lay ahead of her. It was a trade-off of more suffering with a small chance at remission, versus accepting no chance of cure but feeling well for as long as she could. She soon clarified that she wasn’t definitely against chemotherapy. She couldn’t decide. She needed more information from us to make this decision, the hardest of her life.
Over the next few days, I watched as our attending physician expertly provided just that. There were actually three options, she laid out. There was aggressive chemotherapy, entailing at least 3 more weeks in the hospital and coming with significant risk of infection, nausea, vomiting, and fatigue. The chances of inducing a remission were about one in three to one in two, and that remission would likely last between several months and 2 years before the leukemia would relapse. The second option was a chemotherapy pill she could take at home, an option with fewer side effects but no longer aimed at cure. The third option was home hospice support, focused on symptoms, without any anticancer medication.
I noticed a few things during those conversations. I noticed how my attending took a navigator role, not pushing Martha in one direction or another, but rather imparting all the relevant information to empower Martha to decide for herself. I noticed how she provided realistic estimates, not hedging away from numbers, but giving the honest, nitty-gritty facts, as best as she could predict. I noticed how she took the time and never rushed, even in spite of external pressures to discharge the patient from the hospital.
There was no right or wrong answer. I no longer felt that we had something in our grasp – a clear-cut, best decision – to persuade Martha toward. Is one in three good odds, or bad odds? Is 2 years a long period of time, or a short one? Of course, there is no actual answer to these questions; the answer is as elusive and personal as if we had asked Martha: What do you think?
What I learned from Martha is that, with a devastating diagnosis, there isn’t a right time to make this decision. There isn’t one defining moment where we flip a switch and change course. It isn’t only when we run out of treatment options that the choice to forgo it makes sense. That option is on a flexible line, different for every person and priority. As my attending later said, with Martha’s diagnosis and her values, it wouldn’t have been unreasonable to decide against chemotherapy from the start.
The language we use can sometimes mask that reality. As doctors, we may casually slip in words like “need” and “have to” in response to patients’ questions about what to do next. “You need more chemotherapy,” we might say. “We’d have to treat it.” We have “treatment,” after all, and so we go down the line of offering what’s next in the medical algorithm. That word, too, can be deceivingly tempting, enticing down a road that makes it seem like the obvious answer – or the only one. If the choices are treatment versus not, who wouldn’t want the treatment? But the details are where things get murky. What does that treatment involve? What are the chances it will work, and for how long?
Surreptitiously missing from this language is the fact that there’s a choice. There’s always a choice, and it’s on the table at any point. You can start chemotherapy without committing to stick it out until the end. You can go home, if that is what’s important to you. The best treatment option is the one the patient wants.
After 4 days, Martha decided to go home with palliative chemotherapy and a bridge to hospice. Each member of our team hugged her goodbye and wished her luck. She was nervous. But she packed her hospital room, and she left.
I recently pulled up her medical chart, bracing myself for bad news. But the interesting thing about hospice is that even though the focus is no longer on prolonging life, people sometimes live longer.
She felt well, the most recent palliative note said. She was spending her time writing, getting her finances in order, and finishing a legacy project for her grandchildren.
For Martha, it seemed to be the right choice.
Dr. Yurkiewicz is a fellow in hematology and oncology at Stanford (Calif.) University. Follow her on Twitter @ilanayurkiewicz.
Martha Boxer (not her real name) had mentally prepared herself that this could happen, but the news still hit hard. Her doctors on the leukemia service broke the facts as gently as we could: The chemotherapy she had been suffering through for the last 2 weeks hadn’t worked. The results of her latest bone marrow biopsy showed it remained packed with cancer cells.
As Martha absorbed the news quietly, her son, sitting next to her bedside with his hand on hers, spoke first. “What now?”
I looked at my attending and nodded, as we were fully ready to answer this question. From the outset, we knew that Martha’s leukemia carried a genetic mutation that unfortunately put her in a high-risk category. The chances of her cancer responding to the first round of chemotherapy were low. When this happens, what we typically do next is reinduction, we explained. It’s a different combination of chemotherapy drugs, with a somewhat different side effect profile. But it would give her the best chance of response, we believed. We could start the new chemotherapy as early as today, we said.
Martha took this in. “Okay,” she said pensively. “I’ve been thinking. And I think maybe … I won’t do chemotherapy anymore.”
Her words caught me off guard because, frankly, they seemed premature. Her leukemia had not budged with the first round of treatment. But we still had an option B, and then an option C. It was usually at a later, more dire stage – when multiple lines of treatment had not worked, and instead had only caused harm – or, when the decision was forced by the medical system’s admission that we had nothing left to offer – that I’d heard patients express similar preferences. It was then that I’d seen patients and their loved ones flip a mental switch and choose to focus the time they had left on what really mattered to them.
It didn’t feel like we were at that point.
And so, as we debriefed outside her room, my first instinct was to convince her otherwise.
However, Martha had other priorities, as I would come to learn. Above all else, she hated the hospital. She hated feeling trapped in a strange room that wasn’t hers; she hated how the chemotherapy stole her energy and made her feel too weak to even shower. She wanted to be in her own home. She wanted to eat her own food, sleep in her own bed, and be surrounded by what she recognized.
But, she also wanted to live. Two paths lay ahead of her. It was a trade-off of more suffering with a small chance at remission, versus accepting no chance of cure but feeling well for as long as she could. She soon clarified that she wasn’t definitely against chemotherapy. She couldn’t decide. She needed more information from us to make this decision, the hardest of her life.
Over the next few days, I watched as our attending physician expertly provided just that. There were actually three options, she laid out. There was aggressive chemotherapy, entailing at least 3 more weeks in the hospital and coming with significant risk of infection, nausea, vomiting, and fatigue. The chances of inducing a remission were about one in three to one in two, and that remission would likely last between several months and 2 years before the leukemia would relapse. The second option was a chemotherapy pill she could take at home, an option with fewer side effects but no longer aimed at cure. The third option was home hospice support, focused on symptoms, without any anticancer medication.
I noticed a few things during those conversations. I noticed how my attending took a navigator role, not pushing Martha in one direction or another, but rather imparting all the relevant information to empower Martha to decide for herself. I noticed how she provided realistic estimates, not hedging away from numbers, but giving the honest, nitty-gritty facts, as best as she could predict. I noticed how she took the time and never rushed, even in spite of external pressures to discharge the patient from the hospital.
There was no right or wrong answer. I no longer felt that we had something in our grasp – a clear-cut, best decision – to persuade Martha toward. Is one in three good odds, or bad odds? Is 2 years a long period of time, or a short one? Of course, there is no actual answer to these questions; the answer is as elusive and personal as if we had asked Martha: What do you think?
What I learned from Martha is that, with a devastating diagnosis, there isn’t a right time to make this decision. There isn’t one defining moment where we flip a switch and change course. It isn’t only when we run out of treatment options that the choice to forgo it makes sense. That option is on a flexible line, different for every person and priority. As my attending later said, with Martha’s diagnosis and her values, it wouldn’t have been unreasonable to decide against chemotherapy from the start.
The language we use can sometimes mask that reality. As doctors, we may casually slip in words like “need” and “have to” in response to patients’ questions about what to do next. “You need more chemotherapy,” we might say. “We’d have to treat it.” We have “treatment,” after all, and so we go down the line of offering what’s next in the medical algorithm. That word, too, can be deceivingly tempting, enticing down a road that makes it seem like the obvious answer – or the only one. If the choices are treatment versus not, who wouldn’t want the treatment? But the details are where things get murky. What does that treatment involve? What are the chances it will work, and for how long?
Surreptitiously missing from this language is the fact that there’s a choice. There’s always a choice, and it’s on the table at any point. You can start chemotherapy without committing to stick it out until the end. You can go home, if that is what’s important to you. The best treatment option is the one the patient wants.
After 4 days, Martha decided to go home with palliative chemotherapy and a bridge to hospice. Each member of our team hugged her goodbye and wished her luck. She was nervous. But she packed her hospital room, and she left.
I recently pulled up her medical chart, bracing myself for bad news. But the interesting thing about hospice is that even though the focus is no longer on prolonging life, people sometimes live longer.
She felt well, the most recent palliative note said. She was spending her time writing, getting her finances in order, and finishing a legacy project for her grandchildren.
For Martha, it seemed to be the right choice.
Dr. Yurkiewicz is a fellow in hematology and oncology at Stanford (Calif.) University. Follow her on Twitter @ilanayurkiewicz.
Immunotherapy-related adverse effects: how to identify and treat them in the emergency department
DR HENRY I am pleased to be talking with Dr Maura Sammon, an emergency department (ED) physician, about identifying and treating immunotherapy-related side effects in the ED. This is a hot topic in oncology, and I was very interested in having an ED physician talk about what happens when treating oncologists send their patients to the ED, because a physician may think it is chemotherapy when it is immunotherapy. Let’s start with the example of an oncology patient going to the ED with some symptoms, and the ED physician asks the patient what they’re being treated with. The patient may or may not say the right thing – that is, inform you whether they are being treated with chemotherapy or immunotherapy. How do you morph over into knowing that they are not getting chemotherapy?
DR SAMMON Yes, that’s a big problem in the ED. Patients come to the ED and say they’re being treated for cancer. They say they’re on chemotherapy, when they’re actually on immunotherapy, and it can really send the treatment team down the wrong path. I have a metaphor to explain this. They say that Great Britain and the United States are two nations separated by a common language. For example, when a British person talks about football, they mean something very different than when an American talks about football. If someone in Great Britain asks you to come play football, you might show up with shoulder pads and a helmet rather than shin guards, and you’re left without having the right tools to participate in the game.
How this sometimes plays out with immunotherapy, unfortunately, is that a patient will present to the ED and say they’re having a cough and that they’re on chemotherapy for melanoma. Usually, this patient would be worked up for being in a potentially immunosuppressed state. You might get a white blood cell count. You might get a chest X-ray. You might see what looks to you like a new infiltrate on this chest X-ray and then start going down the path of treating someone whom you think is immunosuppressed with pneumonia and giving them antibiotics rather than what could be life-saving steroids, as would be the case if the patient were on immunotherapy.
It’s a real problem, because you have one word that patients may use meaning two very different things. It can get you into trouble if you are treating someone for potentially infectious causes rather than immunotherapy-related adverse reactions, which are much more similar to graft-versus-host disease than in the case of traditional chemotherapy.
DR HENRY That’s a very good point. I think, as we in oncology use these immunotherapies/checkpoint inhibitors more often, you will see them more often in the ED. Let’s get right into that. You’ve identified this patient as not getting a traditional chemotherapy – hopefully, all our records on these patients are available. You’ve decided to follow onto what might be a side effect of the immunotherapy, so I’m going to name the side effects that always occur to me: lung, gastrointestinal (GI) – which could be loose bowels or liver function – rash, endocrine problems. Let’s start with lung symptoms. You see the patient is short of breath and you identified immunotherapy. What’s your next step?
DR SAMMON That’s a great example, because the problem is that you see these patients with cough or shortness of breath and pulmonary complications, and pulmonary complications of immunotherapy, while rare, are potentially life threatening if they’re not identified quickly.
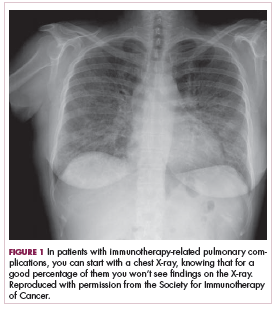
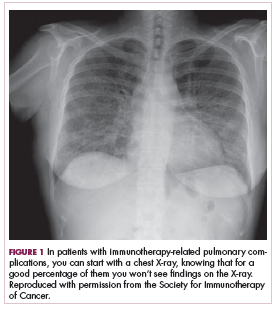
You can start with a chest X-ray on these patients knowing, however, that for a good percentage of them you won’t see findings on their chest X-ray (Figure 1, from Sammon M, Tobin T. Identification and management of immune-related adverse events in the emergency setting. Presented at: Advances in Cancer Immunotherapy – Society for Immunotherapy of Cancer (SITC); August 4, 2017; Philadelphia, PA). You need to proceed to computed tomography (CT), because the issue is that you can have protean findings on the CT related to immunotherapy treatment/adverse reactions. You need to have a very high index of suspicion regardless of what abnormal findings you’re seeing on this CT and erring toward withholding the drugs, starting treatment, and being more aggressive with this type of finding.
DR HENRY I’ve heard you talk at a conference about a patient with metastatic lung cancer, or some other tumor that may have existing disease in lung. The patient is aware of that, and the chart reflects that. Then you have this difficulty where the CT scan shows a pneumonitis, and it may not be tumor progression at all – it may be the drug. How do you work through that? Of course, your additional problem is you don’t have a whole lot of time. You must decide to whether you’re going to keep them in the ED, admit them, or send them home.
DR SAMMON Right. One of the first things is to get the oncologist involved at an early stage in treating this. We are all a team. We are all working together, and it’s very important to have that communication occur very early. I’m going to err on admitting those folks who have had symptoms and who have had findings on a chest CT, because they can progress. They can get much worse. I’m going to be getting the oncologist involved very early. We’re going to have the conversation about whether we should be starting steroids on them in the ED, getting them upstairs, and being aggressive in treating this.
DR HENRY So, time and therapy are very important.
DR SAMMON Yes.
DR HENRY You’ll get that steroid started as an antidote right away.
DR SAMMON Absolutely. For grade 2, you’re going to use methylprednisolone, 1 mg /kg daily. For anything higher than that – grade 3 or grade 4 – we’re going to start higher. We’re going to start at 2-4 mg/kg a day and get the patient upstairs and taper them slowly.
DR HENRY That’s worth empathizing. I, sadly, had a patient who ultimately did well, but had severe grade 4 pneumonitis. T
DR SAMMON Absolutely, but it’s very important to work together as a team to make these patients have good outcomes.
DR HENRY Agree. Let’s change over to the GI symptoms. The patient comes in and misidentifies him- or herself as having chemotherapy and diarrhea. We are used to causing nausea, vomiting, and diarrhea with some of our therapies. You realize, in talking to the oncologist, the patient is taking a checkpoint inhibitor. How would you approach the patient with diarrhea?
DR SAMMON First, I would talk to the patient. I would try to establish the baseline number of stools per day, because it’s not defined as a definite number of stools per day, it’s the number of stools above their baseline per day. If they’re having fewer than 4 stools above their baseline per day, I would send off some tests. We can send off a C diff (Clostridium difficile) test, we can send off a stool culture – all the parasites. Make sure the oncologist is going to be able to get these results and get them followed up, because these are results I’m not going to get back myself in the ED.
Then I’m going to talk to the patient about symptomatic treatment. I’m going to talk to them about oral hydration, a bland diet. Avoid using loperamide or any other antidiarrheal medicines, because that could decrease the frequency of stools but mask more severe symptoms that they may be having.
If I have a patient who is having more than 4 stools above their daily baseline and it’s been happening 4 to 6 stools a day for more than a week, I’m going to be sending those studies off, and I’m going to be having a conversation with the treating oncologist to find out if they want me to start the patient on steroids immediately, or if they want to wait for the test results to come back and have the steroids started as an outpatient.
These moderate patients can maybe wait a day until these test results come back. Those who are having more than 7 stools above baseline per day, peritoneal signs, ileus, or fever, are the patients you should worry about. You need to admit them for IV hydration. You need to do the stools, so you might need to keep them in the hospital until you find the results of the stool studies. You need to rule out perforation.
You may be starting steroids on these patients sooner rather than later. They’re going to be getting systemic corticosteroids at about 1-2 mg/kg of prednisone equivalent, assuming there is no perforation and their stool studies are negative. If they are unstable, though, they are really going to need high-dose corticosteroids. They are going to need methylprednisolone, 125 mg IV, to evaluate for their responsiveness. These folks really need to be treated as inpatients, and they need to have their oncologists involved early on with their treatment.
DR HENRY Yes. I couldn’t agree with you more. When I talk to the diarrhea side effect patients that we see, I tend to think it’s a curse. It’s volume. It’s calories. It’s electrolytes. The number of stools you’re mentioning, it is almost certainly going to need admission to rule out other causes. Then, if it’s the checkpoint inhibitor, the steroid antidotes.
Let’s move on to the rash. This is another organ system that can be affected by immunotherapy. What is your approach when you see a generalized body rash in a patient on one of these drugs who is sent to the ED?
DR SAMMON I am obviously going to be ruling out other causes first, but generally you’re going to see a maculopapular rash. It may be itchy. It may be burning. Patients will often describe it as just sort of having a tight sensation. I’m going to be looking at them a little bit like I look at a burn patient. What is their total body surface area that’s involved? If they’ve got less than 30% of their total body surface area involved, that’s considered a mild reaction.
For those folks, I’m not going to use systemic steroids, but I can give them some topical steroids, and I can give them some Benadryl, some diphenhydramine, and really treat them symptomatically as well as ensuring that they have early follow-up to make sure this isn’t progressing. Once we get between 30% and 50% body area, we’re talking about a moderate toxicity. If these patients are not improving rapidly with just withholding the drug, they need systemic corticosteroids.
We usually treat them at about 0.5-1 mg/kg body weight a day of prednisone equivalent. Just as with burn patients, these patients’ symptoms can become very severe. You can see signs of blistering, dermal ulceration, necrotic, bolus, hemorrhagic lesions (Figure 2, A-D).1 These folks can have very difficult-to-manage fluid balances, and they’re at very high risk for skin infections as well. They need to be treated as inpatients. If possible, you might want to consider sending these patients to a burn unit. They need systemic corticosteroids, 1-2 mg/kg per day, and they need careful monitoring for signs of dehydration, electrolytic abnormalities, and/or skin infections. They need excellent wound care.
DR HENRY That’s very well put and always difficult, because there are so many causes of rash. That takes me to an area that has always been difficult for me, which is therapy-related endocrine problems. It’s interesting to note that these drugs can cause endocrine problems. I’ve heard you speak about the pituitary affecting vision, as well as thyroid or adrenal issues. Let’s start with how you’d approach vision difficulty in a patient on these drugs.
DR SAMMON The endocrinopathies that you can get with these checkpoint inhibitors really have a myriad of symptoms. Your patient may present saying that they’re feeling tired, that they’re feeling weak, or they may have a headache. If your patient is having actual pituitary enlargement, they can present with headaches, visual field defects, or cranial nerve defects. The reason for those symptoms is that the pituitary sits in the cavernous sinus, and you have various cranial nerves passing through that area as well as the optic chiasm just above the pituitary gland (Figure 3).1 Your patient may present with a bitemporal hemianopia. Or with diplopia. You are going to want to very quickly get either a CT scan or an MRI to find out if that is what’s going on (Figure 4).1 These folks need to be treated aggressively as well.
DR HENRY You’ll get your CT scan or your MRI and rule out an enlargement or a change to the visual field. I haven’t seen this yet, but certainly exciting when you see it to treat it. Would you get the radialis brevis involved, steroids involved? How would you manage that?
DR SAMMON It’s interesting, because you do want to use corticosteroids. One of the questions here is, which corticosteroid do you want to use? If you’re talking about someone who may have adrenal insufficiency, you may want to be able to do a stimulation test. In these patients, you may want to choose using dexamethasone, because you can still do the corticotropin stimulation test. However, if your patient is in frank shock because of what you think is an adrenal crisis, you’re going to want to use hydrocortisone. If a patient is truly hypotensive and unstable, the testing is at that point less important than the treatment.
DR HENRY Very interesting. We have covered what I would consider the major aspects of these fascinating drugs. We haven’t covered all of what they do when they work well, which hopefully we’re seeing more and more often, but we have covered very well what can happen when things go wrong in side effects. Anything else that you would like to add from the ED perspective or other side effects worth mentioning?
DR SAMMON The thing that I would most like to share with the oncology office is the importance of communicating with your patients that, when they’re on these drugs, they need to tell emergency physicians that they’re on immunotherapy, not chemotherapy. It might be helpful to give these patients a card stating that they’re on immunotherapy, not chemotherapy, and outlining some of the side effects that ED physicians should be looking out for in these patients.
DR HENRY That’s a great point. I’ve seen that some of the manufacturers have little cheat cards that the patient can carry naming the drug and the side effects, because not all ED doctors are aware of the side effects of these drugs.
DR SAMMON Absolutely. We love those cards.
DR HENRY Yes. I’ve also given some to the ED doctors at Pennsylvania Hospital, and they love it. I think we’ve covered everything in quite a bit of detail. Thank you, Dr Sammon, for sharing this information from the frontlines of the ED.
DR HENRY I am pleased to be talking with Dr Maura Sammon, an emergency department (ED) physician, about identifying and treating immunotherapy-related side effects in the ED. This is a hot topic in oncology, and I was very interested in having an ED physician talk about what happens when treating oncologists send their patients to the ED, because a physician may think it is chemotherapy when it is immunotherapy. Let’s start with the example of an oncology patient going to the ED with some symptoms, and the ED physician asks the patient what they’re being treated with. The patient may or may not say the right thing – that is, inform you whether they are being treated with chemotherapy or immunotherapy. How do you morph over into knowing that they are not getting chemotherapy?
DR SAMMON Yes, that’s a big problem in the ED. Patients come to the ED and say they’re being treated for cancer. They say they’re on chemotherapy, when they’re actually on immunotherapy, and it can really send the treatment team down the wrong path. I have a metaphor to explain this. They say that Great Britain and the United States are two nations separated by a common language. For example, when a British person talks about football, they mean something very different than when an American talks about football. If someone in Great Britain asks you to come play football, you might show up with shoulder pads and a helmet rather than shin guards, and you’re left without having the right tools to participate in the game.
How this sometimes plays out with immunotherapy, unfortunately, is that a patient will present to the ED and say they’re having a cough and that they’re on chemotherapy for melanoma. Usually, this patient would be worked up for being in a potentially immunosuppressed state. You might get a white blood cell count. You might get a chest X-ray. You might see what looks to you like a new infiltrate on this chest X-ray and then start going down the path of treating someone whom you think is immunosuppressed with pneumonia and giving them antibiotics rather than what could be life-saving steroids, as would be the case if the patient were on immunotherapy.
It’s a real problem, because you have one word that patients may use meaning two very different things. It can get you into trouble if you are treating someone for potentially infectious causes rather than immunotherapy-related adverse reactions, which are much more similar to graft-versus-host disease than in the case of traditional chemotherapy.
DR HENRY That’s a very good point. I think, as we in oncology use these immunotherapies/checkpoint inhibitors more often, you will see them more often in the ED. Let’s get right into that. You’ve identified this patient as not getting a traditional chemotherapy – hopefully, all our records on these patients are available. You’ve decided to follow onto what might be a side effect of the immunotherapy, so I’m going to name the side effects that always occur to me: lung, gastrointestinal (GI) – which could be loose bowels or liver function – rash, endocrine problems. Let’s start with lung symptoms. You see the patient is short of breath and you identified immunotherapy. What’s your next step?
DR SAMMON That’s a great example, because the problem is that you see these patients with cough or shortness of breath and pulmonary complications, and pulmonary complications of immunotherapy, while rare, are potentially life threatening if they’re not identified quickly.
You can start with a chest X-ray on these patients knowing, however, that for a good percentage of them you won’t see findings on their chest X-ray (Figure 1, from Sammon M, Tobin T. Identification and management of immune-related adverse events in the emergency setting. Presented at: Advances in Cancer Immunotherapy – Society for Immunotherapy of Cancer (SITC); August 4, 2017; Philadelphia, PA). You need to proceed to computed tomography (CT), because the issue is that you can have protean findings on the CT related to immunotherapy treatment/adverse reactions. You need to have a very high index of suspicion regardless of what abnormal findings you’re seeing on this CT and erring toward withholding the drugs, starting treatment, and being more aggressive with this type of finding.
DR HENRY I’ve heard you talk at a conference about a patient with metastatic lung cancer, or some other tumor that may have existing disease in lung. The patient is aware of that, and the chart reflects that. Then you have this difficulty where the CT scan shows a pneumonitis, and it may not be tumor progression at all – it may be the drug. How do you work through that? Of course, your additional problem is you don’t have a whole lot of time. You must decide to whether you’re going to keep them in the ED, admit them, or send them home.
DR SAMMON Right. One of the first things is to get the oncologist involved at an early stage in treating this. We are all a team. We are all working together, and it’s very important to have that communication occur very early. I’m going to err on admitting those folks who have had symptoms and who have had findings on a chest CT, because they can progress. They can get much worse. I’m going to be getting the oncologist involved very early. We’re going to have the conversation about whether we should be starting steroids on them in the ED, getting them upstairs, and being aggressive in treating this.
DR HENRY So, time and therapy are very important.
DR SAMMON Yes.
DR HENRY You’ll get that steroid started as an antidote right away.
DR SAMMON Absolutely. For grade 2, you’re going to use methylprednisolone, 1 mg /kg daily. For anything higher than that – grade 3 or grade 4 – we’re going to start higher. We’re going to start at 2-4 mg/kg a day and get the patient upstairs and taper them slowly.
DR HENRY That’s worth empathizing. I, sadly, had a patient who ultimately did well, but had severe grade 4 pneumonitis. T
DR SAMMON Absolutely, but it’s very important to work together as a team to make these patients have good outcomes.
DR HENRY Agree. Let’s change over to the GI symptoms. The patient comes in and misidentifies him- or herself as having chemotherapy and diarrhea. We are used to causing nausea, vomiting, and diarrhea with some of our therapies. You realize, in talking to the oncologist, the patient is taking a checkpoint inhibitor. How would you approach the patient with diarrhea?
DR SAMMON First, I would talk to the patient. I would try to establish the baseline number of stools per day, because it’s not defined as a definite number of stools per day, it’s the number of stools above their baseline per day. If they’re having fewer than 4 stools above their baseline per day, I would send off some tests. We can send off a C diff (Clostridium difficile) test, we can send off a stool culture – all the parasites. Make sure the oncologist is going to be able to get these results and get them followed up, because these are results I’m not going to get back myself in the ED.
Then I’m going to talk to the patient about symptomatic treatment. I’m going to talk to them about oral hydration, a bland diet. Avoid using loperamide or any other antidiarrheal medicines, because that could decrease the frequency of stools but mask more severe symptoms that they may be having.
If I have a patient who is having more than 4 stools above their daily baseline and it’s been happening 4 to 6 stools a day for more than a week, I’m going to be sending those studies off, and I’m going to be having a conversation with the treating oncologist to find out if they want me to start the patient on steroids immediately, or if they want to wait for the test results to come back and have the steroids started as an outpatient.
These moderate patients can maybe wait a day until these test results come back. Those who are having more than 7 stools above baseline per day, peritoneal signs, ileus, or fever, are the patients you should worry about. You need to admit them for IV hydration. You need to do the stools, so you might need to keep them in the hospital until you find the results of the stool studies. You need to rule out perforation.
You may be starting steroids on these patients sooner rather than later. They’re going to be getting systemic corticosteroids at about 1-2 mg/kg of prednisone equivalent, assuming there is no perforation and their stool studies are negative. If they are unstable, though, they are really going to need high-dose corticosteroids. They are going to need methylprednisolone, 125 mg IV, to evaluate for their responsiveness. These folks really need to be treated as inpatients, and they need to have their oncologists involved early on with their treatment.
DR HENRY Yes. I couldn’t agree with you more. When I talk to the diarrhea side effect patients that we see, I tend to think it’s a curse. It’s volume. It’s calories. It’s electrolytes. The number of stools you’re mentioning, it is almost certainly going to need admission to rule out other causes. Then, if it’s the checkpoint inhibitor, the steroid antidotes.
Let’s move on to the rash. This is another organ system that can be affected by immunotherapy. What is your approach when you see a generalized body rash in a patient on one of these drugs who is sent to the ED?
DR SAMMON I am obviously going to be ruling out other causes first, but generally you’re going to see a maculopapular rash. It may be itchy. It may be burning. Patients will often describe it as just sort of having a tight sensation. I’m going to be looking at them a little bit like I look at a burn patient. What is their total body surface area that’s involved? If they’ve got less than 30% of their total body surface area involved, that’s considered a mild reaction.
For those folks, I’m not going to use systemic steroids, but I can give them some topical steroids, and I can give them some Benadryl, some diphenhydramine, and really treat them symptomatically as well as ensuring that they have early follow-up to make sure this isn’t progressing. Once we get between 30% and 50% body area, we’re talking about a moderate toxicity. If these patients are not improving rapidly with just withholding the drug, they need systemic corticosteroids.
We usually treat them at about 0.5-1 mg/kg body weight a day of prednisone equivalent. Just as with burn patients, these patients’ symptoms can become very severe. You can see signs of blistering, dermal ulceration, necrotic, bolus, hemorrhagic lesions (Figure 2, A-D).1 These folks can have very difficult-to-manage fluid balances, and they’re at very high risk for skin infections as well. They need to be treated as inpatients. If possible, you might want to consider sending these patients to a burn unit. They need systemic corticosteroids, 1-2 mg/kg per day, and they need careful monitoring for signs of dehydration, electrolytic abnormalities, and/or skin infections. They need excellent wound care.
DR HENRY That’s very well put and always difficult, because there are so many causes of rash. That takes me to an area that has always been difficult for me, which is therapy-related endocrine problems. It’s interesting to note that these drugs can cause endocrine problems. I’ve heard you speak about the pituitary affecting vision, as well as thyroid or adrenal issues. Let’s start with how you’d approach vision difficulty in a patient on these drugs.
DR SAMMON The endocrinopathies that you can get with these checkpoint inhibitors really have a myriad of symptoms. Your patient may present saying that they’re feeling tired, that they’re feeling weak, or they may have a headache. If your patient is having actual pituitary enlargement, they can present with headaches, visual field defects, or cranial nerve defects. The reason for those symptoms is that the pituitary sits in the cavernous sinus, and you have various cranial nerves passing through that area as well as the optic chiasm just above the pituitary gland (Figure 3).1 Your patient may present with a bitemporal hemianopia. Or with diplopia. You are going to want to very quickly get either a CT scan or an MRI to find out if that is what’s going on (Figure 4).1 These folks need to be treated aggressively as well.
DR HENRY You’ll get your CT scan or your MRI and rule out an enlargement or a change to the visual field. I haven’t seen this yet, but certainly exciting when you see it to treat it. Would you get the radialis brevis involved, steroids involved? How would you manage that?
DR SAMMON It’s interesting, because you do want to use corticosteroids. One of the questions here is, which corticosteroid do you want to use? If you’re talking about someone who may have adrenal insufficiency, you may want to be able to do a stimulation test. In these patients, you may want to choose using dexamethasone, because you can still do the corticotropin stimulation test. However, if your patient is in frank shock because of what you think is an adrenal crisis, you’re going to want to use hydrocortisone. If a patient is truly hypotensive and unstable, the testing is at that point less important than the treatment.
DR HENRY Very interesting. We have covered what I would consider the major aspects of these fascinating drugs. We haven’t covered all of what they do when they work well, which hopefully we’re seeing more and more often, but we have covered very well what can happen when things go wrong in side effects. Anything else that you would like to add from the ED perspective or other side effects worth mentioning?
DR SAMMON The thing that I would most like to share with the oncology office is the importance of communicating with your patients that, when they’re on these drugs, they need to tell emergency physicians that they’re on immunotherapy, not chemotherapy. It might be helpful to give these patients a card stating that they’re on immunotherapy, not chemotherapy, and outlining some of the side effects that ED physicians should be looking out for in these patients.
DR HENRY That’s a great point. I’ve seen that some of the manufacturers have little cheat cards that the patient can carry naming the drug and the side effects, because not all ED doctors are aware of the side effects of these drugs.
DR SAMMON Absolutely. We love those cards.
DR HENRY Yes. I’ve also given some to the ED doctors at Pennsylvania Hospital, and they love it. I think we’ve covered everything in quite a bit of detail. Thank you, Dr Sammon, for sharing this information from the frontlines of the ED.
DR HENRY I am pleased to be talking with Dr Maura Sammon, an emergency department (ED) physician, about identifying and treating immunotherapy-related side effects in the ED. This is a hot topic in oncology, and I was very interested in having an ED physician talk about what happens when treating oncologists send their patients to the ED, because a physician may think it is chemotherapy when it is immunotherapy. Let’s start with the example of an oncology patient going to the ED with some symptoms, and the ED physician asks the patient what they’re being treated with. The patient may or may not say the right thing – that is, inform you whether they are being treated with chemotherapy or immunotherapy. How do you morph over into knowing that they are not getting chemotherapy?
DR SAMMON Yes, that’s a big problem in the ED. Patients come to the ED and say they’re being treated for cancer. They say they’re on chemotherapy, when they’re actually on immunotherapy, and it can really send the treatment team down the wrong path. I have a metaphor to explain this. They say that Great Britain and the United States are two nations separated by a common language. For example, when a British person talks about football, they mean something very different than when an American talks about football. If someone in Great Britain asks you to come play football, you might show up with shoulder pads and a helmet rather than shin guards, and you’re left without having the right tools to participate in the game.
How this sometimes plays out with immunotherapy, unfortunately, is that a patient will present to the ED and say they’re having a cough and that they’re on chemotherapy for melanoma. Usually, this patient would be worked up for being in a potentially immunosuppressed state. You might get a white blood cell count. You might get a chest X-ray. You might see what looks to you like a new infiltrate on this chest X-ray and then start going down the path of treating someone whom you think is immunosuppressed with pneumonia and giving them antibiotics rather than what could be life-saving steroids, as would be the case if the patient were on immunotherapy.
It’s a real problem, because you have one word that patients may use meaning two very different things. It can get you into trouble if you are treating someone for potentially infectious causes rather than immunotherapy-related adverse reactions, which are much more similar to graft-versus-host disease than in the case of traditional chemotherapy.
DR HENRY That’s a very good point. I think, as we in oncology use these immunotherapies/checkpoint inhibitors more often, you will see them more often in the ED. Let’s get right into that. You’ve identified this patient as not getting a traditional chemotherapy – hopefully, all our records on these patients are available. You’ve decided to follow onto what might be a side effect of the immunotherapy, so I’m going to name the side effects that always occur to me: lung, gastrointestinal (GI) – which could be loose bowels or liver function – rash, endocrine problems. Let’s start with lung symptoms. You see the patient is short of breath and you identified immunotherapy. What’s your next step?
DR SAMMON That’s a great example, because the problem is that you see these patients with cough or shortness of breath and pulmonary complications, and pulmonary complications of immunotherapy, while rare, are potentially life threatening if they’re not identified quickly.
You can start with a chest X-ray on these patients knowing, however, that for a good percentage of them you won’t see findings on their chest X-ray (Figure 1, from Sammon M, Tobin T. Identification and management of immune-related adverse events in the emergency setting. Presented at: Advances in Cancer Immunotherapy – Society for Immunotherapy of Cancer (SITC); August 4, 2017; Philadelphia, PA). You need to proceed to computed tomography (CT), because the issue is that you can have protean findings on the CT related to immunotherapy treatment/adverse reactions. You need to have a very high index of suspicion regardless of what abnormal findings you’re seeing on this CT and erring toward withholding the drugs, starting treatment, and being more aggressive with this type of finding.
DR HENRY I’ve heard you talk at a conference about a patient with metastatic lung cancer, or some other tumor that may have existing disease in lung. The patient is aware of that, and the chart reflects that. Then you have this difficulty where the CT scan shows a pneumonitis, and it may not be tumor progression at all – it may be the drug. How do you work through that? Of course, your additional problem is you don’t have a whole lot of time. You must decide to whether you’re going to keep them in the ED, admit them, or send them home.
DR SAMMON Right. One of the first things is to get the oncologist involved at an early stage in treating this. We are all a team. We are all working together, and it’s very important to have that communication occur very early. I’m going to err on admitting those folks who have had symptoms and who have had findings on a chest CT, because they can progress. They can get much worse. I’m going to be getting the oncologist involved very early. We’re going to have the conversation about whether we should be starting steroids on them in the ED, getting them upstairs, and being aggressive in treating this.
DR HENRY So, time and therapy are very important.
DR SAMMON Yes.
DR HENRY You’ll get that steroid started as an antidote right away.
DR SAMMON Absolutely. For grade 2, you’re going to use methylprednisolone, 1 mg /kg daily. For anything higher than that – grade 3 or grade 4 – we’re going to start higher. We’re going to start at 2-4 mg/kg a day and get the patient upstairs and taper them slowly.
DR HENRY That’s worth empathizing. I, sadly, had a patient who ultimately did well, but had severe grade 4 pneumonitis. T
DR SAMMON Absolutely, but it’s very important to work together as a team to make these patients have good outcomes.
DR HENRY Agree. Let’s change over to the GI symptoms. The patient comes in and misidentifies him- or herself as having chemotherapy and diarrhea. We are used to causing nausea, vomiting, and diarrhea with some of our therapies. You realize, in talking to the oncologist, the patient is taking a checkpoint inhibitor. How would you approach the patient with diarrhea?
DR SAMMON First, I would talk to the patient. I would try to establish the baseline number of stools per day, because it’s not defined as a definite number of stools per day, it’s the number of stools above their baseline per day. If they’re having fewer than 4 stools above their baseline per day, I would send off some tests. We can send off a C diff (Clostridium difficile) test, we can send off a stool culture – all the parasites. Make sure the oncologist is going to be able to get these results and get them followed up, because these are results I’m not going to get back myself in the ED.
Then I’m going to talk to the patient about symptomatic treatment. I’m going to talk to them about oral hydration, a bland diet. Avoid using loperamide or any other antidiarrheal medicines, because that could decrease the frequency of stools but mask more severe symptoms that they may be having.
If I have a patient who is having more than 4 stools above their daily baseline and it’s been happening 4 to 6 stools a day for more than a week, I’m going to be sending those studies off, and I’m going to be having a conversation with the treating oncologist to find out if they want me to start the patient on steroids immediately, or if they want to wait for the test results to come back and have the steroids started as an outpatient.
These moderate patients can maybe wait a day until these test results come back. Those who are having more than 7 stools above baseline per day, peritoneal signs, ileus, or fever, are the patients you should worry about. You need to admit them for IV hydration. You need to do the stools, so you might need to keep them in the hospital until you find the results of the stool studies. You need to rule out perforation.
You may be starting steroids on these patients sooner rather than later. They’re going to be getting systemic corticosteroids at about 1-2 mg/kg of prednisone equivalent, assuming there is no perforation and their stool studies are negative. If they are unstable, though, they are really going to need high-dose corticosteroids. They are going to need methylprednisolone, 125 mg IV, to evaluate for their responsiveness. These folks really need to be treated as inpatients, and they need to have their oncologists involved early on with their treatment.
DR HENRY Yes. I couldn’t agree with you more. When I talk to the diarrhea side effect patients that we see, I tend to think it’s a curse. It’s volume. It’s calories. It’s electrolytes. The number of stools you’re mentioning, it is almost certainly going to need admission to rule out other causes. Then, if it’s the checkpoint inhibitor, the steroid antidotes.
Let’s move on to the rash. This is another organ system that can be affected by immunotherapy. What is your approach when you see a generalized body rash in a patient on one of these drugs who is sent to the ED?
DR SAMMON I am obviously going to be ruling out other causes first, but generally you’re going to see a maculopapular rash. It may be itchy. It may be burning. Patients will often describe it as just sort of having a tight sensation. I’m going to be looking at them a little bit like I look at a burn patient. What is their total body surface area that’s involved? If they’ve got less than 30% of their total body surface area involved, that’s considered a mild reaction.
For those folks, I’m not going to use systemic steroids, but I can give them some topical steroids, and I can give them some Benadryl, some diphenhydramine, and really treat them symptomatically as well as ensuring that they have early follow-up to make sure this isn’t progressing. Once we get between 30% and 50% body area, we’re talking about a moderate toxicity. If these patients are not improving rapidly with just withholding the drug, they need systemic corticosteroids.
We usually treat them at about 0.5-1 mg/kg body weight a day of prednisone equivalent. Just as with burn patients, these patients’ symptoms can become very severe. You can see signs of blistering, dermal ulceration, necrotic, bolus, hemorrhagic lesions (Figure 2, A-D).1 These folks can have very difficult-to-manage fluid balances, and they’re at very high risk for skin infections as well. They need to be treated as inpatients. If possible, you might want to consider sending these patients to a burn unit. They need systemic corticosteroids, 1-2 mg/kg per day, and they need careful monitoring for signs of dehydration, electrolytic abnormalities, and/or skin infections. They need excellent wound care.
DR HENRY That’s very well put and always difficult, because there are so many causes of rash. That takes me to an area that has always been difficult for me, which is therapy-related endocrine problems. It’s interesting to note that these drugs can cause endocrine problems. I’ve heard you speak about the pituitary affecting vision, as well as thyroid or adrenal issues. Let’s start with how you’d approach vision difficulty in a patient on these drugs.
DR SAMMON The endocrinopathies that you can get with these checkpoint inhibitors really have a myriad of symptoms. Your patient may present saying that they’re feeling tired, that they’re feeling weak, or they may have a headache. If your patient is having actual pituitary enlargement, they can present with headaches, visual field defects, or cranial nerve defects. The reason for those symptoms is that the pituitary sits in the cavernous sinus, and you have various cranial nerves passing through that area as well as the optic chiasm just above the pituitary gland (Figure 3).1 Your patient may present with a bitemporal hemianopia. Or with diplopia. You are going to want to very quickly get either a CT scan or an MRI to find out if that is what’s going on (Figure 4).1 These folks need to be treated aggressively as well.
DR HENRY You’ll get your CT scan or your MRI and rule out an enlargement or a change to the visual field. I haven’t seen this yet, but certainly exciting when you see it to treat it. Would you get the radialis brevis involved, steroids involved? How would you manage that?
DR SAMMON It’s interesting, because you do want to use corticosteroids. One of the questions here is, which corticosteroid do you want to use? If you’re talking about someone who may have adrenal insufficiency, you may want to be able to do a stimulation test. In these patients, you may want to choose using dexamethasone, because you can still do the corticotropin stimulation test. However, if your patient is in frank shock because of what you think is an adrenal crisis, you’re going to want to use hydrocortisone. If a patient is truly hypotensive and unstable, the testing is at that point less important than the treatment.
DR HENRY Very interesting. We have covered what I would consider the major aspects of these fascinating drugs. We haven’t covered all of what they do when they work well, which hopefully we’re seeing more and more often, but we have covered very well what can happen when things go wrong in side effects. Anything else that you would like to add from the ED perspective or other side effects worth mentioning?
DR SAMMON The thing that I would most like to share with the oncology office is the importance of communicating with your patients that, when they’re on these drugs, they need to tell emergency physicians that they’re on immunotherapy, not chemotherapy. It might be helpful to give these patients a card stating that they’re on immunotherapy, not chemotherapy, and outlining some of the side effects that ED physicians should be looking out for in these patients.
DR HENRY That’s a great point. I’ve seen that some of the manufacturers have little cheat cards that the patient can carry naming the drug and the side effects, because not all ED doctors are aware of the side effects of these drugs.
DR SAMMON Absolutely. We love those cards.
DR HENRY Yes. I’ve also given some to the ED doctors at Pennsylvania Hospital, and they love it. I think we’ve covered everything in quite a bit of detail. Thank you, Dr Sammon, for sharing this information from the frontlines of the ED.
Extramedullary plasmacytoma of the thyroid, refractory to radiation therapy and treated with bortezomib
Plasma cell neoplasms involving tissues other than the bone marrow are known as extramedullary plasmacytoma (EMP).1 EMPs mostly involve the head and neck region.2 Solitary EMP involving only the thyroid gland is very rare.3,4 Because of the limited knowledge about this condition and its rarity, its management can be challenging and is often extrapolated from plasma cell myeloma.5,6 In general, surgery or radiation are considered as front-line therapy.3,5 EMPs usually respond well to radiotherapy with almost complete remission. No definite guidelines outlining the treatment of radio-resistant EMP of the thyroid have yet been published. Data supporting the use of chemotherapy is particularly limited.4,7,8
Here, we describe the case of a 53-year-old woman with a long history of thyroiditis who presented with rapidly worsening symptomatic thyroid enlargement. She was diagnosed with EMP of the thyroid gland that was not amenable to surgery and was refractory to radiotherapy but responded to adjuvant chemotherapy with bortezomib. This report highlights 2 unique aspects of this condition: it focuses on a rare case of EMP and, as far as we know, it reports for the first time on EMP that was resistant to radiotherapy. It also highlights the need for guidelines for the treatment of EMPs.
Case presentation and summary
A 53-year-old woman presented to the emergency department with complaints of difficulty swallowing, hoarseness, and neck pain during the previous 1 month. She had a known history of Hashimoto’s thyroiditis, and an ultrasound scan of her neck 6 years previously had demonstrated diffuse thyromegaly without discrete nodules. On presentation, the patient’s vitals were stable, and a neck examination revealed a firm and enlarged thyroid without any cervical adenopathy. Laboratory investigations revealed a normal complete blood count and comprehensive metabolic panel. She had an elevated thyroid-stimulating hormone level of 13.40 mIU/L (reference range, 0.47-4.68 mIU/L) and normal thyroxine level of 4.5 pmol/L (reference range, 4.5-12.0 pmol/L). A computerized tomography (CT) scan of the neck revealed an enlarged thyroid gland (right lobe length, 10.3 cm; isthmus, 2 cm; left lobe, 8 cm) with a focal area of increased echogenicity in the midpole of the left lobe measuring 9.5 mm × 5.5 mm. The patient was discharged to home with pain medications, and urgent follow-up with an otolaryngologist was arranged. A flexible laryngoscopy was done in the otolaryngology clinic, which revealed retropharyngeal bulging that correlated with the thyromegaly evident on the CT scan.
Because of the patient’s significant symptoms, we decided to proceed with surgery with a clinical diagnosis of likely thyroiditis. A left subtotal thyroidectomy with extension to the superior mediastinum was performed, but a right thyroidectomy could not be done safely. On gross examination, a well-capsulated left lobe with a tan-white, lobulated, soft cut surface was seen. Microscopic examination revealed replacement of thyroid parenchyma with sheets of mature-appearing plasma cells with eccentric round nuclei, abundant eosinophilic cytoplasm without atypia, and few scattered thyroid follicles with lymphoepithelial lesions (Figure 1A). Immunohistochemistry confirmed plasma cells with expression of CD138 (Figure 1B).
Fluorescence in situ hybridization (FISH) showed that the neoplastic plasma cells contained monotypic kappa immunoglobulin light chain messenger RNA. Clonal immunoglobulin gene rearrangement was detected on polymerase chain reaction. A diagnosis of plasmacytoma of the thyroid gland in a background of thyroiditis was made on the basis of the aforementioned observations.
After that diagnosis, we performed an extensive work-up for plasma cell myeloma. Bone marrow biopsy showed normal maturing trilineage hematopoiesis with scattered mature-appearing plasma cells Figure 2A. Flow cytometry showed a rare (0.2%) population of polytypic plasma cells and was confirmed by CD138 immunohistochemistry. FISH showed proportionate distribution (2-5:1) of kappa and lambda light chains in plasma cells (Figure 2B).
Serum protein electrophoresis showed normal levels of serum proteins with no M spike. Serum total protein was 7.9 g/dL, albumin 5.0 g/dL, α1-globulin 0.3 g/dL, α2-globulin 0.8 g/dL, β-globulin 0.7 g/dL, and γ-globulin 1.6 g/dL, with an albumin–globulin ratio of 1.47. Calcium and β2-microglobulin were also in the normal ranges. Serum-free kappa light chain was found to be elevated (20.9 mg/L; reference range, 3.3-19.4 mg/L). The immunoglobulin G level was also elevated at 3,104 mg/dL (reference range, 700-1,600 mg/dL).
A positron-emission tomographic (PET) scan done 1 month after the surgery showed no other sites of disease except the thyroid. No lytic bone lesions were present. The patient was treated with 50.4 Gy of radiation by external beam radiotherapy to the thyroid in 28 fractions as definitive therapy. Despite treatment with surgery and radiation, she continued to have pain around the neck, and a repeat PET scan 3 months after completion of radiation showed persistent uptake in the thyroid. Because of her refractoriness to radiotherapy, she was started on systemic therapy with a weekly regimen of bortezomib and dexamethasone for 9 cycles. Her symptoms began to resolve, and a repeat PET scan done after completion of chemotherapy showed no evidence of uptake, suggesting adequate response to chemotherapy, and chemotherapy was therefore stopped. The patient was scheduled a regular follow-up in 3 months. Because of continued local symptoms in this follow-up period, the decision was made to perform surgical gland removal, and she underwent completion of
Discussion
Plasma cells are well-differentiated B-lymphocytes that secrete antibodies and provide protective immunity to the human body.9 Plasma cell neoplasms are clonal proliferation of plasma cells, producing monoclonal immunoglobulins. They are of the following different types: plasma cell myeloma, monoclonal gammopathy of unknown significance, immunoglobulin deposition disease, POEMS (polyneuropathy, organomegaly, endocrinopathy, monoclonal protein, skin changes) syndrome, and plasmacytomas, which are divided into 2 types – solitary plasmacytoma of the bone, and extramedullary plasmacytoma (EMP).10 EMP is a rare condition and encompasses 3% to 5% of all plasma cell neoplasms, depending on the study.1,2,5 It is more common in men than in women (2.6:1, respectively), with equal incidence among black and white patients. Median age at diagnosis is 62 years, and it is more common among those aged 40 to 70 years.2,11 The most common sites of occurrence are the respiratory tract, the mouth, and the pharynx, but other sites such as the eyes, brain, skin, and lymph nodes may also be involved.2
EMP involving the thyroid gland is a very rare occurrence, but plasma cell myeloma has been shown to secondarily involve the thyroid.4 Similar to our report, EMP of the thyroid in the setting of thyroiditis has been reported by other authors.3,4 The incidence of EMP occurring in the thyroid varies according to different authors. Wiltshaw found 7 cases involving the thyroid out of 272 cases of EMP.1 Galieni and colleagues reported only 1 case that involved the thyroid out of 46 described cases of EMP.12
El- Siemińska and colleagues showed that levels of interleukin (IL)-6 are elevated in thyroiditis.13 IL-6 promotes monoclonal as well as polyclonal proliferation of plasma cells. Kovalchuk and colleagues showed an increase in EMP in IL-6 transgenic mice, suggesting a pathophysiologic explanation.14
The diagnostic requirements of EMP include the following: histology showing monoclonal plasma cell infiltration in tissue; bone marrow biopsy with normal plasma cell aspirate and biopsy (plasma cells, <5%); no lytic lesions on skeletal survey; no anemia, renal impairment, or hypercalcemia; and absent or low serum M protein.12
Our case fulfilled those criteria.
The treatment options for EMP include surgery, radiotherapy, or a combined approach including both. Usually, EMPs are very sensitive to radiotherapy, and complete remission can be achieved by radiotherapy alone in 80% to 100% of cases.6,11,15 Surgery is considered if the tumor is diffuse or is causing symptoms secondary to pressure on surrounding structures. A combined approach is recommended in cases with incomplete surgical margin or lymph node involvement.5,6
There is limited evidence about and experience with the use of chemotherapy in the treatment of EMP. It has been recommended that chemotherapy be considered in patients with refractory or relapsed disease using the same regimen used in plasma cell myeloma.5 Katodritou and colleagues have reported using bortezomib and dexamethasone without surgery in a solitary gastric plasmacytoma to avoid the toxicity of gastrointestinal irradiation.7 Wei and colleagues treated a patient with EMP in the pancreas with bortezomib and achieved a near-complete remission.8 To our knowledge, there is no documented literature about the treatment of EMP of the thyroid with chemotherapy. In our patient, despite the treatment with surgery and radiation, there was persistent uptake on the PET scan, so we treated her with bortezomib and dexamethasone. Because there is an 11% to 30% risk of progression to multiple myeloma, long-term follow-up is recommended in EMP.11
Conclusions
Solitary EMP of the thyroid gland is a rare condition. Plasma cell myeloma must be ruled out to make a diagnosis. Data on the incidence of EMP and its clinicopathological features are sparse, and literature describing proper guidelines on treatment is limited. It can be treated with radiotherapy, surgery, or a combined approach. There is limited data on the role of chemotherapy; our case adds to the available literature on using myeloma-based therapy in refractory disease and, to our knowledge, is the only case report using this in the literature on cases of EMP of the thyroid. Regular follow-up, even after the disease is in remission, is necessary because of the high risk of progression to plasma cell myeloma.
1. Wiltshaw E. The natural history of extramedullary plasmacytoma and its relation to solitary myeloma of bone and myelomatosis. Medicine (Baltimore). 1976;55(3):217-238.
2. Dores GM, Landgren O, McGlynn KA, Curtis RE, Linet MS, Devesa SS. Plasmacytoma of bone, extramedullary plasmacytoma, and multiple myeloma: incidence and survival in the United States, 1992-2004. Br J Haematol. 2009;144(1):86-94.
3. Kovacs CS, Mant MJ, Nguyen GK, Ginsberg J. Plasma cell lesions of the thyroid: report of a case of solitary plasmacytoma and a review of the literature. Thyroid. 1994;4(1):65-71.
4. Avila A, Villalpando A, Montoya G, Luna MA. Clinical features and differential diagnoses of solitary extramedullary plasmacytoma of the thyroid: a case report. Ann Diagn Pathol. 2009;13(2):119-123.
5. Hughes M, Soutar R, Lucraft H, Owen R, Bird J. Guidelines on the diagnosis and management of solitary plasmacytoma of bone, extramedullary plasmacytoma and multiple solitary plasmacytomas: 2009 update. London, United Kingdom: British Committee for Standards in Haematology; 2009.
6. Weber DM. Solitary bone and extramedullary plasmacytoma. Hematology Am Soc Hematol Educ Program. 2005;373-376.
7. Katodritou E, Kartsios C, Gastari V, et al. Successful treatment of extramedullary gastric plasmacytoma with the combination of bortezomib and dexamethasone: first reported case. Leuk Res. 2008;32(2):339-341.
8. Wei JY, Tong HY, Zhu WF, et al. Bortezomib in treatment of extramedullary plasmacytoma of the pancreas. Hepatobiliary Pancreat Dis Int. 2009;8(3):329-331.
9. Roth K, Oehme L, Zehentmeier S, Zhang Y, Niesner R, Hauser AE. Tracking plasma cell differentiation and survival. Cytometry A. 2014;85(1):15-24.
10. Swerdlow SH, Campo E, Harris NL, et al. WHO classification of tumours of haematopoietic and lymphoid tissues. 4th ed. Lyon, France: International Agency for Research on Cancer; 2008.
11. Alexiou C, Kau RJ, Dietzfelbinger H, et al. Extramedullary plasmacytoma: tumor occurrence and therapeutic concepts. Cancer. 1999;85(11):2305-2314.
12. Galieni P, Cavo M, Pulsoni A, et al. Clinical outcome of extramedullary plasmacytoma. Haematologica. 2000;85(1):47-51.
13. Siemińska L, Wojciechowska C, Kos-Kudła B, et al. Serum concentrations of leptin, adiponectin, and interleukin-6 in postmenopausal women with Hashimoto’s thyroiditis. Endokrynol Pol. 2010;61(1):112-116.
14. Kovalchuk AL, Kim JS, Park SS, et al. IL-6 transgenic mouse model for extraosseous plasmacytoma. Proc Natl Acad Sci US
15. Chao MW, Gibbs P, Wirth A, Quong G, Guiney MJ, Liew KH. Radiotherapy in the management of solitary extramedullary plasmacytoma. Intern Med J. 2005;35(4):211-215.
Plasma cell neoplasms involving tissues other than the bone marrow are known as extramedullary plasmacytoma (EMP).1 EMPs mostly involve the head and neck region.2 Solitary EMP involving only the thyroid gland is very rare.3,4 Because of the limited knowledge about this condition and its rarity, its management can be challenging and is often extrapolated from plasma cell myeloma.5,6 In general, surgery or radiation are considered as front-line therapy.3,5 EMPs usually respond well to radiotherapy with almost complete remission. No definite guidelines outlining the treatment of radio-resistant EMP of the thyroid have yet been published. Data supporting the use of chemotherapy is particularly limited.4,7,8
Here, we describe the case of a 53-year-old woman with a long history of thyroiditis who presented with rapidly worsening symptomatic thyroid enlargement. She was diagnosed with EMP of the thyroid gland that was not amenable to surgery and was refractory to radiotherapy but responded to adjuvant chemotherapy with bortezomib. This report highlights 2 unique aspects of this condition: it focuses on a rare case of EMP and, as far as we know, it reports for the first time on EMP that was resistant to radiotherapy. It also highlights the need for guidelines for the treatment of EMPs.
Case presentation and summary
A 53-year-old woman presented to the emergency department with complaints of difficulty swallowing, hoarseness, and neck pain during the previous 1 month. She had a known history of Hashimoto’s thyroiditis, and an ultrasound scan of her neck 6 years previously had demonstrated diffuse thyromegaly without discrete nodules. On presentation, the patient’s vitals were stable, and a neck examination revealed a firm and enlarged thyroid without any cervical adenopathy. Laboratory investigations revealed a normal complete blood count and comprehensive metabolic panel. She had an elevated thyroid-stimulating hormone level of 13.40 mIU/L (reference range, 0.47-4.68 mIU/L) and normal thyroxine level of 4.5 pmol/L (reference range, 4.5-12.0 pmol/L). A computerized tomography (CT) scan of the neck revealed an enlarged thyroid gland (right lobe length, 10.3 cm; isthmus, 2 cm; left lobe, 8 cm) with a focal area of increased echogenicity in the midpole of the left lobe measuring 9.5 mm × 5.5 mm. The patient was discharged to home with pain medications, and urgent follow-up with an otolaryngologist was arranged. A flexible laryngoscopy was done in the otolaryngology clinic, which revealed retropharyngeal bulging that correlated with the thyromegaly evident on the CT scan.
Because of the patient’s significant symptoms, we decided to proceed with surgery with a clinical diagnosis of likely thyroiditis. A left subtotal thyroidectomy with extension to the superior mediastinum was performed, but a right thyroidectomy could not be done safely. On gross examination, a well-capsulated left lobe with a tan-white, lobulated, soft cut surface was seen. Microscopic examination revealed replacement of thyroid parenchyma with sheets of mature-appearing plasma cells with eccentric round nuclei, abundant eosinophilic cytoplasm without atypia, and few scattered thyroid follicles with lymphoepithelial lesions (Figure 1A). Immunohistochemistry confirmed plasma cells with expression of CD138 (Figure 1B).
Fluorescence in situ hybridization (FISH) showed that the neoplastic plasma cells contained monotypic kappa immunoglobulin light chain messenger RNA. Clonal immunoglobulin gene rearrangement was detected on polymerase chain reaction. A diagnosis of plasmacytoma of the thyroid gland in a background of thyroiditis was made on the basis of the aforementioned observations.
After that diagnosis, we performed an extensive work-up for plasma cell myeloma. Bone marrow biopsy showed normal maturing trilineage hematopoiesis with scattered mature-appearing plasma cells Figure 2A. Flow cytometry showed a rare (0.2%) population of polytypic plasma cells and was confirmed by CD138 immunohistochemistry. FISH showed proportionate distribution (2-5:1) of kappa and lambda light chains in plasma cells (Figure 2B).
Serum protein electrophoresis showed normal levels of serum proteins with no M spike. Serum total protein was 7.9 g/dL, albumin 5.0 g/dL, α1-globulin 0.3 g/dL, α2-globulin 0.8 g/dL, β-globulin 0.7 g/dL, and γ-globulin 1.6 g/dL, with an albumin–globulin ratio of 1.47. Calcium and β2-microglobulin were also in the normal ranges. Serum-free kappa light chain was found to be elevated (20.9 mg/L; reference range, 3.3-19.4 mg/L). The immunoglobulin G level was also elevated at 3,104 mg/dL (reference range, 700-1,600 mg/dL).
A positron-emission tomographic (PET) scan done 1 month after the surgery showed no other sites of disease except the thyroid. No lytic bone lesions were present. The patient was treated with 50.4 Gy of radiation by external beam radiotherapy to the thyroid in 28 fractions as definitive therapy. Despite treatment with surgery and radiation, she continued to have pain around the neck, and a repeat PET scan 3 months after completion of radiation showed persistent uptake in the thyroid. Because of her refractoriness to radiotherapy, she was started on systemic therapy with a weekly regimen of bortezomib and dexamethasone for 9 cycles. Her symptoms began to resolve, and a repeat PET scan done after completion of chemotherapy showed no evidence of uptake, suggesting adequate response to chemotherapy, and chemotherapy was therefore stopped. The patient was scheduled a regular follow-up in 3 months. Because of continued local symptoms in this follow-up period, the decision was made to perform surgical gland removal, and she underwent completion of
Discussion
Plasma cells are well-differentiated B-lymphocytes that secrete antibodies and provide protective immunity to the human body.9 Plasma cell neoplasms are clonal proliferation of plasma cells, producing monoclonal immunoglobulins. They are of the following different types: plasma cell myeloma, monoclonal gammopathy of unknown significance, immunoglobulin deposition disease, POEMS (polyneuropathy, organomegaly, endocrinopathy, monoclonal protein, skin changes) syndrome, and plasmacytomas, which are divided into 2 types – solitary plasmacytoma of the bone, and extramedullary plasmacytoma (EMP).10 EMP is a rare condition and encompasses 3% to 5% of all plasma cell neoplasms, depending on the study.1,2,5 It is more common in men than in women (2.6:1, respectively), with equal incidence among black and white patients. Median age at diagnosis is 62 years, and it is more common among those aged 40 to 70 years.2,11 The most common sites of occurrence are the respiratory tract, the mouth, and the pharynx, but other sites such as the eyes, brain, skin, and lymph nodes may also be involved.2
EMP involving the thyroid gland is a very rare occurrence, but plasma cell myeloma has been shown to secondarily involve the thyroid.4 Similar to our report, EMP of the thyroid in the setting of thyroiditis has been reported by other authors.3,4 The incidence of EMP occurring in the thyroid varies according to different authors. Wiltshaw found 7 cases involving the thyroid out of 272 cases of EMP.1 Galieni and colleagues reported only 1 case that involved the thyroid out of 46 described cases of EMP.12
El- Siemińska and colleagues showed that levels of interleukin (IL)-6 are elevated in thyroiditis.13 IL-6 promotes monoclonal as well as polyclonal proliferation of plasma cells. Kovalchuk and colleagues showed an increase in EMP in IL-6 transgenic mice, suggesting a pathophysiologic explanation.14
The diagnostic requirements of EMP include the following: histology showing monoclonal plasma cell infiltration in tissue; bone marrow biopsy with normal plasma cell aspirate and biopsy (plasma cells, <5%); no lytic lesions on skeletal survey; no anemia, renal impairment, or hypercalcemia; and absent or low serum M protein.12
Our case fulfilled those criteria.
The treatment options for EMP include surgery, radiotherapy, or a combined approach including both. Usually, EMPs are very sensitive to radiotherapy, and complete remission can be achieved by radiotherapy alone in 80% to 100% of cases.6,11,15 Surgery is considered if the tumor is diffuse or is causing symptoms secondary to pressure on surrounding structures. A combined approach is recommended in cases with incomplete surgical margin or lymph node involvement.5,6
There is limited evidence about and experience with the use of chemotherapy in the treatment of EMP. It has been recommended that chemotherapy be considered in patients with refractory or relapsed disease using the same regimen used in plasma cell myeloma.5 Katodritou and colleagues have reported using bortezomib and dexamethasone without surgery in a solitary gastric plasmacytoma to avoid the toxicity of gastrointestinal irradiation.7 Wei and colleagues treated a patient with EMP in the pancreas with bortezomib and achieved a near-complete remission.8 To our knowledge, there is no documented literature about the treatment of EMP of the thyroid with chemotherapy. In our patient, despite the treatment with surgery and radiation, there was persistent uptake on the PET scan, so we treated her with bortezomib and dexamethasone. Because there is an 11% to 30% risk of progression to multiple myeloma, long-term follow-up is recommended in EMP.11
Conclusions
Solitary EMP of the thyroid gland is a rare condition. Plasma cell myeloma must be ruled out to make a diagnosis. Data on the incidence of EMP and its clinicopathological features are sparse, and literature describing proper guidelines on treatment is limited. It can be treated with radiotherapy, surgery, or a combined approach. There is limited data on the role of chemotherapy; our case adds to the available literature on using myeloma-based therapy in refractory disease and, to our knowledge, is the only case report using this in the literature on cases of EMP of the thyroid. Regular follow-up, even after the disease is in remission, is necessary because of the high risk of progression to plasma cell myeloma.
Plasma cell neoplasms involving tissues other than the bone marrow are known as extramedullary plasmacytoma (EMP).1 EMPs mostly involve the head and neck region.2 Solitary EMP involving only the thyroid gland is very rare.3,4 Because of the limited knowledge about this condition and its rarity, its management can be challenging and is often extrapolated from plasma cell myeloma.5,6 In general, surgery or radiation are considered as front-line therapy.3,5 EMPs usually respond well to radiotherapy with almost complete remission. No definite guidelines outlining the treatment of radio-resistant EMP of the thyroid have yet been published. Data supporting the use of chemotherapy is particularly limited.4,7,8
Here, we describe the case of a 53-year-old woman with a long history of thyroiditis who presented with rapidly worsening symptomatic thyroid enlargement. She was diagnosed with EMP of the thyroid gland that was not amenable to surgery and was refractory to radiotherapy but responded to adjuvant chemotherapy with bortezomib. This report highlights 2 unique aspects of this condition: it focuses on a rare case of EMP and, as far as we know, it reports for the first time on EMP that was resistant to radiotherapy. It also highlights the need for guidelines for the treatment of EMPs.
Case presentation and summary
A 53-year-old woman presented to the emergency department with complaints of difficulty swallowing, hoarseness, and neck pain during the previous 1 month. She had a known history of Hashimoto’s thyroiditis, and an ultrasound scan of her neck 6 years previously had demonstrated diffuse thyromegaly without discrete nodules. On presentation, the patient’s vitals were stable, and a neck examination revealed a firm and enlarged thyroid without any cervical adenopathy. Laboratory investigations revealed a normal complete blood count and comprehensive metabolic panel. She had an elevated thyroid-stimulating hormone level of 13.40 mIU/L (reference range, 0.47-4.68 mIU/L) and normal thyroxine level of 4.5 pmol/L (reference range, 4.5-12.0 pmol/L). A computerized tomography (CT) scan of the neck revealed an enlarged thyroid gland (right lobe length, 10.3 cm; isthmus, 2 cm; left lobe, 8 cm) with a focal area of increased echogenicity in the midpole of the left lobe measuring 9.5 mm × 5.5 mm. The patient was discharged to home with pain medications, and urgent follow-up with an otolaryngologist was arranged. A flexible laryngoscopy was done in the otolaryngology clinic, which revealed retropharyngeal bulging that correlated with the thyromegaly evident on the CT scan.
Because of the patient’s significant symptoms, we decided to proceed with surgery with a clinical diagnosis of likely thyroiditis. A left subtotal thyroidectomy with extension to the superior mediastinum was performed, but a right thyroidectomy could not be done safely. On gross examination, a well-capsulated left lobe with a tan-white, lobulated, soft cut surface was seen. Microscopic examination revealed replacement of thyroid parenchyma with sheets of mature-appearing plasma cells with eccentric round nuclei, abundant eosinophilic cytoplasm without atypia, and few scattered thyroid follicles with lymphoepithelial lesions (Figure 1A). Immunohistochemistry confirmed plasma cells with expression of CD138 (Figure 1B).
Fluorescence in situ hybridization (FISH) showed that the neoplastic plasma cells contained monotypic kappa immunoglobulin light chain messenger RNA. Clonal immunoglobulin gene rearrangement was detected on polymerase chain reaction. A diagnosis of plasmacytoma of the thyroid gland in a background of thyroiditis was made on the basis of the aforementioned observations.
After that diagnosis, we performed an extensive work-up for plasma cell myeloma. Bone marrow biopsy showed normal maturing trilineage hematopoiesis with scattered mature-appearing plasma cells Figure 2A. Flow cytometry showed a rare (0.2%) population of polytypic plasma cells and was confirmed by CD138 immunohistochemistry. FISH showed proportionate distribution (2-5:1) of kappa and lambda light chains in plasma cells (Figure 2B).
Serum protein electrophoresis showed normal levels of serum proteins with no M spike. Serum total protein was 7.9 g/dL, albumin 5.0 g/dL, α1-globulin 0.3 g/dL, α2-globulin 0.8 g/dL, β-globulin 0.7 g/dL, and γ-globulin 1.6 g/dL, with an albumin–globulin ratio of 1.47. Calcium and β2-microglobulin were also in the normal ranges. Serum-free kappa light chain was found to be elevated (20.9 mg/L; reference range, 3.3-19.4 mg/L). The immunoglobulin G level was also elevated at 3,104 mg/dL (reference range, 700-1,600 mg/dL).
A positron-emission tomographic (PET) scan done 1 month after the surgery showed no other sites of disease except the thyroid. No lytic bone lesions were present. The patient was treated with 50.4 Gy of radiation by external beam radiotherapy to the thyroid in 28 fractions as definitive therapy. Despite treatment with surgery and radiation, she continued to have pain around the neck, and a repeat PET scan 3 months after completion of radiation showed persistent uptake in the thyroid. Because of her refractoriness to radiotherapy, she was started on systemic therapy with a weekly regimen of bortezomib and dexamethasone for 9 cycles. Her symptoms began to resolve, and a repeat PET scan done after completion of chemotherapy showed no evidence of uptake, suggesting adequate response to chemotherapy, and chemotherapy was therefore stopped. The patient was scheduled a regular follow-up in 3 months. Because of continued local symptoms in this follow-up period, the decision was made to perform surgical gland removal, and she underwent completion of
Discussion
Plasma cells are well-differentiated B-lymphocytes that secrete antibodies and provide protective immunity to the human body.9 Plasma cell neoplasms are clonal proliferation of plasma cells, producing monoclonal immunoglobulins. They are of the following different types: plasma cell myeloma, monoclonal gammopathy of unknown significance, immunoglobulin deposition disease, POEMS (polyneuropathy, organomegaly, endocrinopathy, monoclonal protein, skin changes) syndrome, and plasmacytomas, which are divided into 2 types – solitary plasmacytoma of the bone, and extramedullary plasmacytoma (EMP).10 EMP is a rare condition and encompasses 3% to 5% of all plasma cell neoplasms, depending on the study.1,2,5 It is more common in men than in women (2.6:1, respectively), with equal incidence among black and white patients. Median age at diagnosis is 62 years, and it is more common among those aged 40 to 70 years.2,11 The most common sites of occurrence are the respiratory tract, the mouth, and the pharynx, but other sites such as the eyes, brain, skin, and lymph nodes may also be involved.2
EMP involving the thyroid gland is a very rare occurrence, but plasma cell myeloma has been shown to secondarily involve the thyroid.4 Similar to our report, EMP of the thyroid in the setting of thyroiditis has been reported by other authors.3,4 The incidence of EMP occurring in the thyroid varies according to different authors. Wiltshaw found 7 cases involving the thyroid out of 272 cases of EMP.1 Galieni and colleagues reported only 1 case that involved the thyroid out of 46 described cases of EMP.12
El- Siemińska and colleagues showed that levels of interleukin (IL)-6 are elevated in thyroiditis.13 IL-6 promotes monoclonal as well as polyclonal proliferation of plasma cells. Kovalchuk and colleagues showed an increase in EMP in IL-6 transgenic mice, suggesting a pathophysiologic explanation.14
The diagnostic requirements of EMP include the following: histology showing monoclonal plasma cell infiltration in tissue; bone marrow biopsy with normal plasma cell aspirate and biopsy (plasma cells, <5%); no lytic lesions on skeletal survey; no anemia, renal impairment, or hypercalcemia; and absent or low serum M protein.12
Our case fulfilled those criteria.
The treatment options for EMP include surgery, radiotherapy, or a combined approach including both. Usually, EMPs are very sensitive to radiotherapy, and complete remission can be achieved by radiotherapy alone in 80% to 100% of cases.6,11,15 Surgery is considered if the tumor is diffuse or is causing symptoms secondary to pressure on surrounding structures. A combined approach is recommended in cases with incomplete surgical margin or lymph node involvement.5,6
There is limited evidence about and experience with the use of chemotherapy in the treatment of EMP. It has been recommended that chemotherapy be considered in patients with refractory or relapsed disease using the same regimen used in plasma cell myeloma.5 Katodritou and colleagues have reported using bortezomib and dexamethasone without surgery in a solitary gastric plasmacytoma to avoid the toxicity of gastrointestinal irradiation.7 Wei and colleagues treated a patient with EMP in the pancreas with bortezomib and achieved a near-complete remission.8 To our knowledge, there is no documented literature about the treatment of EMP of the thyroid with chemotherapy. In our patient, despite the treatment with surgery and radiation, there was persistent uptake on the PET scan, so we treated her with bortezomib and dexamethasone. Because there is an 11% to 30% risk of progression to multiple myeloma, long-term follow-up is recommended in EMP.11
Conclusions
Solitary EMP of the thyroid gland is a rare condition. Plasma cell myeloma must be ruled out to make a diagnosis. Data on the incidence of EMP and its clinicopathological features are sparse, and literature describing proper guidelines on treatment is limited. It can be treated with radiotherapy, surgery, or a combined approach. There is limited data on the role of chemotherapy; our case adds to the available literature on using myeloma-based therapy in refractory disease and, to our knowledge, is the only case report using this in the literature on cases of EMP of the thyroid. Regular follow-up, even after the disease is in remission, is necessary because of the high risk of progression to plasma cell myeloma.
1. Wiltshaw E. The natural history of extramedullary plasmacytoma and its relation to solitary myeloma of bone and myelomatosis. Medicine (Baltimore). 1976;55(3):217-238.
2. Dores GM, Landgren O, McGlynn KA, Curtis RE, Linet MS, Devesa SS. Plasmacytoma of bone, extramedullary plasmacytoma, and multiple myeloma: incidence and survival in the United States, 1992-2004. Br J Haematol. 2009;144(1):86-94.
3. Kovacs CS, Mant MJ, Nguyen GK, Ginsberg J. Plasma cell lesions of the thyroid: report of a case of solitary plasmacytoma and a review of the literature. Thyroid. 1994;4(1):65-71.
4. Avila A, Villalpando A, Montoya G, Luna MA. Clinical features and differential diagnoses of solitary extramedullary plasmacytoma of the thyroid: a case report. Ann Diagn Pathol. 2009;13(2):119-123.
5. Hughes M, Soutar R, Lucraft H, Owen R, Bird J. Guidelines on the diagnosis and management of solitary plasmacytoma of bone, extramedullary plasmacytoma and multiple solitary plasmacytomas: 2009 update. London, United Kingdom: British Committee for Standards in Haematology; 2009.
6. Weber DM. Solitary bone and extramedullary plasmacytoma. Hematology Am Soc Hematol Educ Program. 2005;373-376.
7. Katodritou E, Kartsios C, Gastari V, et al. Successful treatment of extramedullary gastric plasmacytoma with the combination of bortezomib and dexamethasone: first reported case. Leuk Res. 2008;32(2):339-341.
8. Wei JY, Tong HY, Zhu WF, et al. Bortezomib in treatment of extramedullary plasmacytoma of the pancreas. Hepatobiliary Pancreat Dis Int. 2009;8(3):329-331.
9. Roth K, Oehme L, Zehentmeier S, Zhang Y, Niesner R, Hauser AE. Tracking plasma cell differentiation and survival. Cytometry A. 2014;85(1):15-24.
10. Swerdlow SH, Campo E, Harris NL, et al. WHO classification of tumours of haematopoietic and lymphoid tissues. 4th ed. Lyon, France: International Agency for Research on Cancer; 2008.
11. Alexiou C, Kau RJ, Dietzfelbinger H, et al. Extramedullary plasmacytoma: tumor occurrence and therapeutic concepts. Cancer. 1999;85(11):2305-2314.
12. Galieni P, Cavo M, Pulsoni A, et al. Clinical outcome of extramedullary plasmacytoma. Haematologica. 2000;85(1):47-51.
13. Siemińska L, Wojciechowska C, Kos-Kudła B, et al. Serum concentrations of leptin, adiponectin, and interleukin-6 in postmenopausal women with Hashimoto’s thyroiditis. Endokrynol Pol. 2010;61(1):112-116.
14. Kovalchuk AL, Kim JS, Park SS, et al. IL-6 transgenic mouse model for extraosseous plasmacytoma. Proc Natl Acad Sci US
15. Chao MW, Gibbs P, Wirth A, Quong G, Guiney MJ, Liew KH. Radiotherapy in the management of solitary extramedullary plasmacytoma. Intern Med J. 2005;35(4):211-215.
1. Wiltshaw E. The natural history of extramedullary plasmacytoma and its relation to solitary myeloma of bone and myelomatosis. Medicine (Baltimore). 1976;55(3):217-238.
2. Dores GM, Landgren O, McGlynn KA, Curtis RE, Linet MS, Devesa SS. Plasmacytoma of bone, extramedullary plasmacytoma, and multiple myeloma: incidence and survival in the United States, 1992-2004. Br J Haematol. 2009;144(1):86-94.
3. Kovacs CS, Mant MJ, Nguyen GK, Ginsberg J. Plasma cell lesions of the thyroid: report of a case of solitary plasmacytoma and a review of the literature. Thyroid. 1994;4(1):65-71.
4. Avila A, Villalpando A, Montoya G, Luna MA. Clinical features and differential diagnoses of solitary extramedullary plasmacytoma of the thyroid: a case report. Ann Diagn Pathol. 2009;13(2):119-123.
5. Hughes M, Soutar R, Lucraft H, Owen R, Bird J. Guidelines on the diagnosis and management of solitary plasmacytoma of bone, extramedullary plasmacytoma and multiple solitary plasmacytomas: 2009 update. London, United Kingdom: British Committee for Standards in Haematology; 2009.
6. Weber DM. Solitary bone and extramedullary plasmacytoma. Hematology Am Soc Hematol Educ Program. 2005;373-376.
7. Katodritou E, Kartsios C, Gastari V, et al. Successful treatment of extramedullary gastric plasmacytoma with the combination of bortezomib and dexamethasone: first reported case. Leuk Res. 2008;32(2):339-341.
8. Wei JY, Tong HY, Zhu WF, et al. Bortezomib in treatment of extramedullary plasmacytoma of the pancreas. Hepatobiliary Pancreat Dis Int. 2009;8(3):329-331.
9. Roth K, Oehme L, Zehentmeier S, Zhang Y, Niesner R, Hauser AE. Tracking plasma cell differentiation and survival. Cytometry A. 2014;85(1):15-24.
10. Swerdlow SH, Campo E, Harris NL, et al. WHO classification of tumours of haematopoietic and lymphoid tissues. 4th ed. Lyon, France: International Agency for Research on Cancer; 2008.
11. Alexiou C, Kau RJ, Dietzfelbinger H, et al. Extramedullary plasmacytoma: tumor occurrence and therapeutic concepts. Cancer. 1999;85(11):2305-2314.
12. Galieni P, Cavo M, Pulsoni A, et al. Clinical outcome of extramedullary plasmacytoma. Haematologica. 2000;85(1):47-51.
13. Siemińska L, Wojciechowska C, Kos-Kudła B, et al. Serum concentrations of leptin, adiponectin, and interleukin-6 in postmenopausal women with Hashimoto’s thyroiditis. Endokrynol Pol. 2010;61(1):112-116.
14. Kovalchuk AL, Kim JS, Park SS, et al. IL-6 transgenic mouse model for extraosseous plasmacytoma. Proc Natl Acad Sci US
15. Chao MW, Gibbs P, Wirth A, Quong G, Guiney MJ, Liew KH. Radiotherapy in the management of solitary extramedullary plasmacytoma. Intern Med J. 2005;35(4):211-215.
Salivary ductal adenocarcinoma with complete response to androgen blockade
Salivary ductal adenocarcinomas make up about 9% of malignant salivary gland tumors and occur mostly in men older than 50 years, with a peak incidence in the sixth and seventh decades. It is the most aggressive of salivary gland tumors and is histologically similar to high-grade, invasive ductal carcinoma of the breast. In all, 65% of patients will die of the disease, and most will experience skin ulceration and nerve palsy.1 With such an aggressive clinical picture, the temptation for many oncologists and patients is to use aggressive cytotoxic chemotherapies. Considering the lack of large trials exploring treatment options in this less-common subtype of salivary gland carcinoma, practice guidelines also recommend the use of aggressive chemotherapies. Unlike other types of malignant cancers of the salivary glands, 70% to 90% of ductal adenocarcinomas express the androgen receptor (AR) by immunohistochemistry.2 There are reported cases of androgen deprivation therapy (ADT) as a successful treatment for salivary ductal adenocarcinomas that express the AR (Table).In 2003, Locati and colleagues reported the case of a man with salivary ductal adenocarcinomas who had a complete response with ADT.3 In 2016, the same group of authors published a retrospective analysis of 17 patients with recurrent or metastatic AR-positive salivary gland cancers who were treated with ADT and reported a 64.7% overall response rate among the patients.4 A 10-patient case series in the Netherlands demonstrated a 50% response rate to ADT plus bicalutamide, including a palliative effect in the form of pain relief.5 A retrospective analysis by Price and colleagues of 5 patients with AR-positive metastatic salivary duct adenocarcinoma showed a 60% response rate to a combination of leuprolide and bicalutamide.6
Case presentation and summary
A 91-year-old man was diagnosed with salivary ductal adenocarcinoma of the left parotid gland in September 2013 and underwent left parotidectomy and lymph node dissection, which revealed AJCC stage IVA (pT2 pN3 M0) disease. The following year, in December 2014, he had an enlarging left neck mass that was pathologically confirmed to be recurrent disease, and he underwent left level V neck dissection in February 2015. Five months after surgery, in July 2015, he presented with left neck fullness and new skin nodules, and the results of a biopsy confirmed recurrent disease. Given his relatively asymptomatic state and advanced age, the oncology care team decided to follow the patient without any pharmacologic therapy.
The patient felt relatively well for 11 months but slowly developed increasing pain in the left neck in June 2016. The skin nodules also began to spread inferiorly from his left neck to his upper chest with the development of open sores that wept serous fluid with scab formation (Figure 1). He and his wife lived independently and managed all their own instrumental activities of daily living (IADL). Eventually, the pain in his neck became so severe that it began to interfere with his ability to drive. He declined radiation therapy because of side effects and transportation issues, but he desired something to alleviate the burden of the disease. During a multidisciplinary cancer conference, the staff pathologist and oncologist discussed AR immunohistochemistry to assist with management. In June 2016, the patient’s tumor was found to have AR immunostaining (nuclear pattern) in 100% of cells, and he was treated with combined androgen blockade, consisting of monthly 3.6 mg goserelin injections and daily bicalutamide 50 mg orally.
Within a week, the patient noticed that the skin lesions stopped weeping fluid. Within 2 weeks, the pain had begun to resolve. At his formal follow-up visit 11 weeks after starting treatment, he was not taking any pain medications and reported no pain. In addition, his visually apparent disease had almost completely resolved (Figure 2). He was fully able to manage his own IADL and reported a marked increase in satisfaction with the quality of his life.
Discussion
The oncology care team clearly defined the goal of care for this patient as palliative and conveyed as such to the patient. The team considered the risks and side effects of cytotoxic chemotherapy agents to be contrary to the patient’s stated primary goal of independence. We selected the combined androgen blockade because it has a low toxicity rate and thus met the primary goals of therapy.
The European Organization for Research and Treatment of Cancer is presently conducting a trial in which cytotoxic chemotherapy is being compared with ADT in AR-positive salivary duct tumors. Findings from a recent prospective, phase-2 trial conducted in Japan suggested that combined AR blockade has similar efficacy and less toxicity than conventional cytotoxic chemotherapy for recurrent and/or metastatic and unresectable locally advanced AR-positive salivary gland carcinoma.7 As more data become available from other studies, it is possible that practice guidelines will be revised to recommend this treatment approach for these cancers.
1. Eveson JW, Thompson LDR. Malignant neoplasms of the salivary glands. In: Thompson LDR, ed. Head and neck pathology. 2nd ed. Philadelphia, PA: Elsevier Inc; 2013:304-305.
2. Luk PP, Weston JD, Yu B, et al. Salivary duct carcinoma: clinicopathologic features, morphologic spectrum, and somatic mutations. Head Neck. 2016;38(suppl 1):E1838-E1847.
3. Locati LD, Quattrone P, Bossi P, Marchianò AV, Cantù G, Licitra L. A complete remission with androgen-deprivation therapy in a recurrent androgen receptor-expressing adenocarcinoma of the parotid gland. Ann Oncol. 2003;14(8):1327-1328.
4. Locati LD, Perrone F, Cortelazzi B, et al. Clinical activity of androgen deprivation therapy in patients with metastatic/relapsed androgen receptor-positive salivary gland cancers. Head Neck. 2016;38(5):724-731.
5. Jaspers HC, Verbist BM, Schoffelen R, et al. Androgen receptor-positive salivary duct carcinoma: a disease entity with promising new treatment options. J Clin Oncol. 2011;29(16):e473-e476.
6. Price KAR, Okuno SH, Molina JR, Garcia JJ. Treatment of metastatic salivary duct carcinoma with combined androgen blockade (CAB) with leuprolide acetate and bicalutamide. Int J Radiat Oncol Biol Phys. 2014;88(2):521-522.
7. Fushimi C, Tada Y, Takahashi H, et al. A prospective phase II study of combined androgen blockade in patients with androgen receptor-positive metastatic or locally advanced unresectable salivary gland carcinoma. Ann Oncol. 2018;29(4):979-984.
Salivary ductal adenocarcinomas make up about 9% of malignant salivary gland tumors and occur mostly in men older than 50 years, with a peak incidence in the sixth and seventh decades. It is the most aggressive of salivary gland tumors and is histologically similar to high-grade, invasive ductal carcinoma of the breast. In all, 65% of patients will die of the disease, and most will experience skin ulceration and nerve palsy.1 With such an aggressive clinical picture, the temptation for many oncologists and patients is to use aggressive cytotoxic chemotherapies. Considering the lack of large trials exploring treatment options in this less-common subtype of salivary gland carcinoma, practice guidelines also recommend the use of aggressive chemotherapies. Unlike other types of malignant cancers of the salivary glands, 70% to 90% of ductal adenocarcinomas express the androgen receptor (AR) by immunohistochemistry.2 There are reported cases of androgen deprivation therapy (ADT) as a successful treatment for salivary ductal adenocarcinomas that express the AR (Table).In 2003, Locati and colleagues reported the case of a man with salivary ductal adenocarcinomas who had a complete response with ADT.3 In 2016, the same group of authors published a retrospective analysis of 17 patients with recurrent or metastatic AR-positive salivary gland cancers who were treated with ADT and reported a 64.7% overall response rate among the patients.4 A 10-patient case series in the Netherlands demonstrated a 50% response rate to ADT plus bicalutamide, including a palliative effect in the form of pain relief.5 A retrospective analysis by Price and colleagues of 5 patients with AR-positive metastatic salivary duct adenocarcinoma showed a 60% response rate to a combination of leuprolide and bicalutamide.6
Case presentation and summary
A 91-year-old man was diagnosed with salivary ductal adenocarcinoma of the left parotid gland in September 2013 and underwent left parotidectomy and lymph node dissection, which revealed AJCC stage IVA (pT2 pN3 M0) disease. The following year, in December 2014, he had an enlarging left neck mass that was pathologically confirmed to be recurrent disease, and he underwent left level V neck dissection in February 2015. Five months after surgery, in July 2015, he presented with left neck fullness and new skin nodules, and the results of a biopsy confirmed recurrent disease. Given his relatively asymptomatic state and advanced age, the oncology care team decided to follow the patient without any pharmacologic therapy.
The patient felt relatively well for 11 months but slowly developed increasing pain in the left neck in June 2016. The skin nodules also began to spread inferiorly from his left neck to his upper chest with the development of open sores that wept serous fluid with scab formation (Figure 1). He and his wife lived independently and managed all their own instrumental activities of daily living (IADL). Eventually, the pain in his neck became so severe that it began to interfere with his ability to drive. He declined radiation therapy because of side effects and transportation issues, but he desired something to alleviate the burden of the disease. During a multidisciplinary cancer conference, the staff pathologist and oncologist discussed AR immunohistochemistry to assist with management. In June 2016, the patient’s tumor was found to have AR immunostaining (nuclear pattern) in 100% of cells, and he was treated with combined androgen blockade, consisting of monthly 3.6 mg goserelin injections and daily bicalutamide 50 mg orally.
Within a week, the patient noticed that the skin lesions stopped weeping fluid. Within 2 weeks, the pain had begun to resolve. At his formal follow-up visit 11 weeks after starting treatment, he was not taking any pain medications and reported no pain. In addition, his visually apparent disease had almost completely resolved (Figure 2). He was fully able to manage his own IADL and reported a marked increase in satisfaction with the quality of his life.
Discussion
The oncology care team clearly defined the goal of care for this patient as palliative and conveyed as such to the patient. The team considered the risks and side effects of cytotoxic chemotherapy agents to be contrary to the patient’s stated primary goal of independence. We selected the combined androgen blockade because it has a low toxicity rate and thus met the primary goals of therapy.
The European Organization for Research and Treatment of Cancer is presently conducting a trial in which cytotoxic chemotherapy is being compared with ADT in AR-positive salivary duct tumors. Findings from a recent prospective, phase-2 trial conducted in Japan suggested that combined AR blockade has similar efficacy and less toxicity than conventional cytotoxic chemotherapy for recurrent and/or metastatic and unresectable locally advanced AR-positive salivary gland carcinoma.7 As more data become available from other studies, it is possible that practice guidelines will be revised to recommend this treatment approach for these cancers.
Salivary ductal adenocarcinomas make up about 9% of malignant salivary gland tumors and occur mostly in men older than 50 years, with a peak incidence in the sixth and seventh decades. It is the most aggressive of salivary gland tumors and is histologically similar to high-grade, invasive ductal carcinoma of the breast. In all, 65% of patients will die of the disease, and most will experience skin ulceration and nerve palsy.1 With such an aggressive clinical picture, the temptation for many oncologists and patients is to use aggressive cytotoxic chemotherapies. Considering the lack of large trials exploring treatment options in this less-common subtype of salivary gland carcinoma, practice guidelines also recommend the use of aggressive chemotherapies. Unlike other types of malignant cancers of the salivary glands, 70% to 90% of ductal adenocarcinomas express the androgen receptor (AR) by immunohistochemistry.2 There are reported cases of androgen deprivation therapy (ADT) as a successful treatment for salivary ductal adenocarcinomas that express the AR (Table).In 2003, Locati and colleagues reported the case of a man with salivary ductal adenocarcinomas who had a complete response with ADT.3 In 2016, the same group of authors published a retrospective analysis of 17 patients with recurrent or metastatic AR-positive salivary gland cancers who were treated with ADT and reported a 64.7% overall response rate among the patients.4 A 10-patient case series in the Netherlands demonstrated a 50% response rate to ADT plus bicalutamide, including a palliative effect in the form of pain relief.5 A retrospective analysis by Price and colleagues of 5 patients with AR-positive metastatic salivary duct adenocarcinoma showed a 60% response rate to a combination of leuprolide and bicalutamide.6
Case presentation and summary
A 91-year-old man was diagnosed with salivary ductal adenocarcinoma of the left parotid gland in September 2013 and underwent left parotidectomy and lymph node dissection, which revealed AJCC stage IVA (pT2 pN3 M0) disease. The following year, in December 2014, he had an enlarging left neck mass that was pathologically confirmed to be recurrent disease, and he underwent left level V neck dissection in February 2015. Five months after surgery, in July 2015, he presented with left neck fullness and new skin nodules, and the results of a biopsy confirmed recurrent disease. Given his relatively asymptomatic state and advanced age, the oncology care team decided to follow the patient without any pharmacologic therapy.
The patient felt relatively well for 11 months but slowly developed increasing pain in the left neck in June 2016. The skin nodules also began to spread inferiorly from his left neck to his upper chest with the development of open sores that wept serous fluid with scab formation (Figure 1). He and his wife lived independently and managed all their own instrumental activities of daily living (IADL). Eventually, the pain in his neck became so severe that it began to interfere with his ability to drive. He declined radiation therapy because of side effects and transportation issues, but he desired something to alleviate the burden of the disease. During a multidisciplinary cancer conference, the staff pathologist and oncologist discussed AR immunohistochemistry to assist with management. In June 2016, the patient’s tumor was found to have AR immunostaining (nuclear pattern) in 100% of cells, and he was treated with combined androgen blockade, consisting of monthly 3.6 mg goserelin injections and daily bicalutamide 50 mg orally.
Within a week, the patient noticed that the skin lesions stopped weeping fluid. Within 2 weeks, the pain had begun to resolve. At his formal follow-up visit 11 weeks after starting treatment, he was not taking any pain medications and reported no pain. In addition, his visually apparent disease had almost completely resolved (Figure 2). He was fully able to manage his own IADL and reported a marked increase in satisfaction with the quality of his life.
Discussion
The oncology care team clearly defined the goal of care for this patient as palliative and conveyed as such to the patient. The team considered the risks and side effects of cytotoxic chemotherapy agents to be contrary to the patient’s stated primary goal of independence. We selected the combined androgen blockade because it has a low toxicity rate and thus met the primary goals of therapy.
The European Organization for Research and Treatment of Cancer is presently conducting a trial in which cytotoxic chemotherapy is being compared with ADT in AR-positive salivary duct tumors. Findings from a recent prospective, phase-2 trial conducted in Japan suggested that combined AR blockade has similar efficacy and less toxicity than conventional cytotoxic chemotherapy for recurrent and/or metastatic and unresectable locally advanced AR-positive salivary gland carcinoma.7 As more data become available from other studies, it is possible that practice guidelines will be revised to recommend this treatment approach for these cancers.
1. Eveson JW, Thompson LDR. Malignant neoplasms of the salivary glands. In: Thompson LDR, ed. Head and neck pathology. 2nd ed. Philadelphia, PA: Elsevier Inc; 2013:304-305.
2. Luk PP, Weston JD, Yu B, et al. Salivary duct carcinoma: clinicopathologic features, morphologic spectrum, and somatic mutations. Head Neck. 2016;38(suppl 1):E1838-E1847.
3. Locati LD, Quattrone P, Bossi P, Marchianò AV, Cantù G, Licitra L. A complete remission with androgen-deprivation therapy in a recurrent androgen receptor-expressing adenocarcinoma of the parotid gland. Ann Oncol. 2003;14(8):1327-1328.
4. Locati LD, Perrone F, Cortelazzi B, et al. Clinical activity of androgen deprivation therapy in patients with metastatic/relapsed androgen receptor-positive salivary gland cancers. Head Neck. 2016;38(5):724-731.
5. Jaspers HC, Verbist BM, Schoffelen R, et al. Androgen receptor-positive salivary duct carcinoma: a disease entity with promising new treatment options. J Clin Oncol. 2011;29(16):e473-e476.
6. Price KAR, Okuno SH, Molina JR, Garcia JJ. Treatment of metastatic salivary duct carcinoma with combined androgen blockade (CAB) with leuprolide acetate and bicalutamide. Int J Radiat Oncol Biol Phys. 2014;88(2):521-522.
7. Fushimi C, Tada Y, Takahashi H, et al. A prospective phase II study of combined androgen blockade in patients with androgen receptor-positive metastatic or locally advanced unresectable salivary gland carcinoma. Ann Oncol. 2018;29(4):979-984.
1. Eveson JW, Thompson LDR. Malignant neoplasms of the salivary glands. In: Thompson LDR, ed. Head and neck pathology. 2nd ed. Philadelphia, PA: Elsevier Inc; 2013:304-305.
2. Luk PP, Weston JD, Yu B, et al. Salivary duct carcinoma: clinicopathologic features, morphologic spectrum, and somatic mutations. Head Neck. 2016;38(suppl 1):E1838-E1847.
3. Locati LD, Quattrone P, Bossi P, Marchianò AV, Cantù G, Licitra L. A complete remission with androgen-deprivation therapy in a recurrent androgen receptor-expressing adenocarcinoma of the parotid gland. Ann Oncol. 2003;14(8):1327-1328.
4. Locati LD, Perrone F, Cortelazzi B, et al. Clinical activity of androgen deprivation therapy in patients with metastatic/relapsed androgen receptor-positive salivary gland cancers. Head Neck. 2016;38(5):724-731.
5. Jaspers HC, Verbist BM, Schoffelen R, et al. Androgen receptor-positive salivary duct carcinoma: a disease entity with promising new treatment options. J Clin Oncol. 2011;29(16):e473-e476.
6. Price KAR, Okuno SH, Molina JR, Garcia JJ. Treatment of metastatic salivary duct carcinoma with combined androgen blockade (CAB) with leuprolide acetate and bicalutamide. Int J Radiat Oncol Biol Phys. 2014;88(2):521-522.
7. Fushimi C, Tada Y, Takahashi H, et al. A prospective phase II study of combined androgen blockade in patients with androgen receptor-positive metastatic or locally advanced unresectable salivary gland carcinoma. Ann Oncol. 2018;29(4):979-984.
Characteristics of urgent palliative cancer care consultations encountered by radiation oncologists
Palliative radiation therapy (PRT) plays a major role in the management of incurable cancers. Study findings have demonstrated the efficacy of using PRT in treating tumor-related bone pain,1 brain metastases and related symptoms,2 thoracic disease-causing hemoptysis or obstruction,3 gastrointestinal involvement causing bleeding and/or obstruction,4 and genitourinary and/or gynecologic involvement causing bleeding.5,6
PRT accounts for between 30% and 50% of courses of radiotherapy delivered.3 These courses of RT typically require urgent evaluation since patients are seen because of new and/or progressive symptoms that give cause for concern. The urgency of presentation requires radiation oncologists and the departments receiving these patients to be equipped to manage these cases efficiently and effectively. Furthermore, the types of cases seen, including PRT indications and related symptoms requiring management, inform the training of radiation oncology physicians as well as nursing and other clinical staff. Finally, characterizing the types of urgent PRT cases that are seen can also guide research and quality improvement endeavors for advanced cancer care in radiation oncology settings.
There is currently a paucity of data characterizing the types and frequencies of urgent PRT indications in patients who present to radiation oncology departments, as well as a lack of data detailing the related symptoms radiation oncology clinicians are managing. The aim of this study was to characterize the types and frequencies of urgent PRT consultations and the related symptoms that radiation oncologists are managing as part of patient care.
Methods
Based on national palliative care practice and national oncology care practice guidelines,7,8 we designed a survey to categorize the cancer-related palliative care issues seen by radiation oncologists. Physical symptoms, psychosocial issues, cultural consideration, spiritual needs, care coordination, advanced-care planning, goals of care, and ethical and legal issues comprised the 8 palliative care domains that we evaluated. A survey was developed and critically evaluated by 3 investigators (MK, VL, TB). Each palliative care domain was ranked by clinicians by its relevance (5-point Likert scale [range, 1-5]: 1, Not Relevant, to 5, Extremely Relevant) to the patient’s care point in radiation oncology. In addition, 31 palliative care subissues related to the primary domains were identified by clinicians based on their presence (Yes, No, Not Assessed). Clinicians were also asked whether the consulted patient’s metastatic cancer diagnosis was established (longer than 1 month) or new (within the last 1 month). In addition, clinicians noted whether the patient was returning to active oncologic care (eg, chemotherapy) or to no further anticancer therapies (eg, hospice care) after RT consultation and intervention (if deemed necessary).
The survey’s face and content validity, ease of completion, and time of completion was assessed by a panel of 7 clinicians with expertise in medical oncology, radiation oncology, palliative care, and/or survey construction. The survey was then sent in a sequential manner to 1 member of the panel at a time after incorporating each panel member’s initial comments. After each panel member’s review, the survey was edited until 2 consecutive panel members had no further suggestions for improvement.
After receiving approval from the institutional review boards of participating radiation oncology centers, we electronically surveyed radiation oncology clinicians who were conducting PRT consultations. From May 19 to September 26, 2014, all consultations were evaluated prospectively for consideration of PRT performed by a dedicated PRT service at 3 centers (a large academic cancer center and 2 participating clinicians at affiliated regional hospitals). The consultations for patients aged 18 years or older with incurable, metastatic cancers were considered eligible. The consulting clinician was e-mailed a survey for completion within 5 business days immediately after each PRT consult. Three reminders to complete the survey were sent during the 5–business-day interval. Over the entire study period, 162 consecutive patients were identified, resulting in 162 surveys being sent to 15 radiation oncology clinicians, including nurse practitioners, resident physicians, and attending physicians. Each clinician received a $25 gift card for participating, regardless of the number of surveys completed. In total, 140 of the 162 surveys were returned, resulting in a response rate of 86%.
The investigators then collected patient demographics (age, gender, race, marital status) and disease characteristics (primary cancer type, Eastern Cooperative Oncology Group Performance Status, reasons for urgent RT consult, physical symptoms requiring management at presentation, patient’s place in illness trajectory, and RT recommendation) pertaining to each completed survey from the electronic medical record. Urgent consultations were defined as any patients who needed to be seen on the same day or within a few days of the consult request.
The descriptive statistics of all these data were calculated in terms of frequencies and percentage of categorical variables. Chi-squared statistics, Fisher exact test, and nonparametric rank sum tests were applied to various categories to determine any statistically significant differences between groups.
Results
In total, 162 patients were seen in consultation for PRT during the 19-week enrollment period, or an average of 8.7 consults a week. Of that total, surveys for 140 patients were returned (Table).
The median patient age was 63 years (range, 29-89 years). A sizeable minority (20%) was 50 years or younger. The most common cancer diagnosis was lung cancer (28%), followed by breast (13%) and prostate (10%) cancers, melanoma (10%), and sarcoma (7%). Other diagnoses accounted for the remaining 32%.
Timing of PRT consult in cancer trajectory
The points in the advanced cancer illness trajectory at which patients were seen for PRT evaluation are shown in Figure 1. Most patients (63%) were seen for a PRT evaluation at the time of an established diagnosis (>1 month after diagnosis of metastatic cancer) and were continuing to further cancer therapies. An additional 19% of patients with an established diagnosis proceeded to hospice or end-of-life care after the PRT evaluation. A notable minority of patients (18%) were seen for a PRT evaluation at the time of a new diagnosis (<1 month of diagnosis of metastatic cancer), and of those, 17% went on to receive anticancer therapy after the PRT evaluation and 1% proceeded to hospice or end-of-life care.
Characteristics of PRT consults and symptoms at presentation
The primary reasons for urgent consultation for PRT are shown in Figure 2. Cancer-related pain (57%), brain metastases (29%), and malignant spinal cord or cauda equina compression (13%) were the predominant reasons for consults. Notable minorities were seen for tumor-related dyspnea (10%), bleeding (8%), and bone fractures (4%).
PRT recommendations and targets
Recommendations regarding PRT are shown in Figure 4A. Of the total 140 patients, 18 (13%) were not recommended for RT. Of the 122 patients for whom PRT was recommended, 11 (9%) received RT at more than 1 site.
Discussion and conclusions
The present study provides a descriptive overview of urgent metastatic cancer patient presentations to radiation oncology clinicians through a comprehensive evaluation of 140 consults for PRT. The most common reasons for urgent evaluation were cancer-related pain (57%), but brain metastases (29%), spinal cord compression (13%), and respiratory symptoms (10%) were also common. Other less-common indications included cancer-related dysphagia, bleeding, and poststabilization management of bone fractures. The most common symptoms requiring management by radiation oncology clinicians were pain (69%), neurologic symptoms (51%), and fatigue (49%). The study also provides a comprehensive characterization of the timeframe of PRT consultation and the treatment recommendations in this cohort. Though most PRT consults occurred at the time of an established metastatic cancer diagnosis and before further anticancer therapies, sizeable minorities occurred at the time of a new diagnosis of metastatic cancer (18%) and before comfort-focused, end-of-life care and no further anticancer therapies (20%). Most patients (87%) were recommended PRT, and of those recommended RT, 11% received RT to more than 1 site. The most common PRT sites were to bone (46%), followed by brain (29%), nonlung soft-tissue sites (17%), and lung (8%). This comprehensive description of the day-to-day urgent, advanced cancer care issues seen and managed in radiation oncology practice can help guide PRT clinical structures, education, research, and quality improvement measures in clinical practice.
Our study provides an insight into urgent symptoms encountered by radiation oncology practitioners during their routine practice. Cancer-related pain remains the most common symptom requiring management. Given the frequency with which pain management is needed among PRT patients, this study highlights the need for radiation oncologists to be well trained in symptom management, particularly as the pain response to RT can often take several days. However, studies suggest that cancer-related pain is not frequently managed by radiation oncologists.9 For example, findings from an Italian study showed that the involvement of radiation oncologists in cancer pain management remains minimal compared with other medical professionals; during the treatment course, only half of the radiation oncologists implemented specific treatment for breakthrough pain.10 A nationwide survey in the United States implicated a number of barriers to adequate pain management, including poor assessment by the physician, reluctance in prescribing opioid analgesics, perceived excessive regulation, and patient reluctance to report pain.11 Notably, in a survey of the Radiation Therapy Oncology Group study physicians, 83% believed cancer patients with pain were undermedicated, and 40% reported that pain relief in their own practice setting was suboptimal. Furthermore, in the treatment plan, adjuvants and prophylactic side effect management were frequently not used properly.12 Education of radiation oncologists in pain assessment and management is key to overcome these barriers and to ensure adequate pain management and quality of life for patients in radiation oncology.
The next most common reason for which patients presented for palliative radiation oncology consultation was for central nervous system (CNS) metastatic disease, including brain metastases and spinal cord compression. Correspondingly, the next most common issue requiring management was neurologic symptoms. Management of CNS disease is becoming increasingly complex, and it benefits from multidisciplinary evaluation to guide optimal and personalized care for each patient, including medical oncology, radiation oncology, neurosurgery and/or orthopedic spine surgery, and palliative medicine. Treatment options include supportive care or corticosteroids alone, surgical resection, whole-brain RT, and/or radiosurgery or stereotactic RT alone. These treatment options are considered on the basis of global patient factors, such as prognosis, together with metastatic-site–specific factors, such as site-related symptoms and the number of metastatic diseases or the burden of the disease.13 For example, the use of the diagnosis-specific Graded Prognostic Assessment index to predict life expectancy can help tailor management of brain metastases based on performance status, age, number of brain metastases, extracranial metastases, and cancer type. Highlighting the complexity of this common PRT presentation, Tsao and colleagues showed that there was a lack of uniform agreement among radiation oncologists for common management issues in patients with brain metastatic disease.14
For metastatic spinal cord or cauda equina compression and the associated neurologic symptoms, initiation of immediate corticosteroids and implementation of local therapy within 24 hours of presentation is paramount,15 highlighting the need for rapid, comprehensive care decision-making for these patients. Treatment options that must be weighed include the potential benefit of upfront decompressive surgery, as supported by a randomized controlled trial by Patchell and colleagues16 for patients who are surgical candidates with true cord or cauda compression and have at least 1 neurologic symptom, a prognosis of ≥3 months, paraplegia of no longer than 48 hours, and no previous RT to the site or brain metastases. Compared with the RT alone, patients receiving surgery before RT had improved ambulatory status and overall survival. Hence, neurosurgical or orthopedic consultation should be standard in the evaluation of metastatic spinal cord or cauda equina compression patients. However, patients frequently do not meet these criteria, and corticosteroids and RT alone are considered. In addition to playing a role in surgical decision-making, prognosis also has a key role in decision-making about the RT fractionation. Short-course RT (8 Gy × 1) is as effectual as longer-course regimens (3 Gy × 10) in terms of motor function.17,18 However, more dose-intense or longer-course regimens, such as 3 Gy × 10, have been shown to have more durability beyond about 6 months and are therefore considered for intermediate to good prognosis.18 The common urgent presentation of CNS metastatic disease to radiation oncology clinics together with the complexity of management and urgency of care decision-making point to the need for dedicated structures of care for these patients in radiation oncology settings. For example, dedicated PRT programs, such as the Rapid Response Radiotherapy Program in Toronto and the Supportive and Palliative Radiation Oncology service in Boston, have demonstrated improved quality of care for patients being urgently evaluated for PRT.19
Following management of pain and neurologic symptoms, clinicians were faced with managing fatigue in nearly half of the patients (49%). The prevalence of fatigue among cancer patients and its impact on quality of life20 highlight the need for this key symptom to be addressed throughout the continuum of cancer care. National Comprehensive Cancer Network guidelines provide a comprehensive framework for addressing cancer-related fatigue.7 However, cancer-related fatigue is a largely underreported, underestimated, and thus undertreated problem.20 In a nationwide survey of members of the American Society for Radiation Oncology, radiation oncologists reported being significantly less confident in managing fatigue compared with managing other common symptoms.21 Furthermore, in a national survey of radiation oncology trainees, 67% of respondents indicated that they were not at all minimally or somewhat confident in their ability to manage fatigue symptoms. The frequency of this symptom together with the demonstrated need for improved education in fatigue management point to a need for radiation oncology palliative educational structures to include dedicated emphasis on managing fatigue in addition to other commonly encountered symptoms, such as pain.
Patients evaluated for PRT are seen across the trajectory of their metastatic cancer diagnosis. In our study, patients presented at all stages in their advanced cancers. These include patients seen at the time of initial diagnosis of cancer as well as those seen near the end of life when end-of-life care planning was underway. The broad spectrum of timing of PRT care underscores that radiation oncologists must be prepared to handle generalist palliative care issues encountered throughout the trajectory of advanced cancer care and hence need comprehensive education in generalist palliative care competencies. These include symptom management, end-of-life care coordination, and communication or goals-of-care discussions. Notably, a recent national survey of radiation oncology residents indicated that most residents, 77% on average, perceived their educational training as suboptimal across domains of generalist palliative care competencies needed in oncology practice.22 Furthermore, a majority (81%) desired greater palliative care education within training.
The most common sites treated in this study were bone, brain, and lung sites. These data provide guidance to both education and research initiatives aiming to advance PRT curriculum and care structures within departments. For example, a same-day simulation and radiation treatment program developed at Princess Margaret Hospital Palliative Radiation Oncology Program (Ontario, Canada) aids in providing streamlined care for patients with bone metastases, the most common presentation for PRT.23 Furthermore, education and research in the application of PRT techniques to bone, brain, and thoracic disease cover the majority of PRT presentations. It is notable, however, that 17% were other soft tissue body sites.
Limitations
There are a few limitations to this study. First, this is a survey-based study conducted at a single academic center within an urban setting and surrounding community regions, which affects its generalizability. Second, this study presents perspectives of radiation oncology practitioners evaluating patients and does not directly reflect patient perceptions or report of symptoms. Third, the data provided by this study are solely descriptive in nature. However, this can guide hypothesis-driven research regarding the evaluation and management of urgent palliative care issues encountered by radiation oncology clinicians and suggest educational objectives to address the needs of these patients.
Conclusions
Radiation oncologists are involved throughout the trajectory of care for advanced cancer patients. Furthermore, they manage a variety of urgent oncologic issues, most commonly metastases causing pain, brain metastases, and spinal cord or cauda equina compression. Radiation oncologists also manage many cancer-related symptoms, mostly pain, neurologic symptoms, fatigue, and gastrointestinal symptoms. These findings point toward the need for palliative care to be well integrated into radiation oncology training curricula and the need for dedicated care structures that enable rapid and multidisciplinary palliative oncology care within radiation oncology departments.
1. Chow E, Harris K, Fan G, Tsao M, Sze WM. Palliative radiotherapy trials for bone metastases: a systematic review. J Clin Oncol. 2007;25(11):1423-1436.
2. van Oorschot B, Rades D, Schulze W, Beckmann G, Feyer P. Palliative radiotherapy--new approaches. Semin Oncol. 2011;38(3):443-449.
3. Simone CB II, Jones JA. Palliative care for patients with locally advanced and metastatic non-small cell lung cancer. Ann Palliat Med. 2013;2(4):178-188.
4. Cihoric N, Crowe S, Eychmüller S, Aebersold DM, Ghadjar P. Clinically significant bleeding in incurable cancer patients: effectiveness of hemostatic radiotherapy. Radiat Oncol. 2012;7:132.
5. Duchesne GM, Bolger JJ, Griffiths GO, et al. A randomized trial of hypofractionated schedules of palliative radiotherapy in the management of bladder carcinoma: results of medical research council trial BA09. Int J Radiat Oncol Biol Phys. 2000;47(2):379-388.
6. Onsrud M, Hagen B, Strickert T. 10-Gy single-fraction pelvic irradiation for palliation and life prolongation in patients with cancer of the cervix and corpus uteri. Gynecol Oncol. 2001;82(1):167-171.
7. NCCN Guidelines(R) Updates. J Natl Compr Canc Netw. 2013;11(9):xxxii-xxxvi.
8. Colby WH, Dahlin C, Lantos J, Carney J, Christopher M. The National Consensus Project for Quality Palliative Care Clinical Practice Guidelines Domain 8: ethical and legal aspects of care. HEC Forum. 2010;22(2):117-131.
9. Stockler MR, Wilcken NR. Why is management of cancer pain still a problem? J Clin Oncol. 2012;30(16):1907-1908.
10. Caravatta L, Ramella S, Melano A, et al. Breakthrough pain management in patients undergoing radiotherapy: a national survey on behalf of the Palliative and Supportive Care Study Group. Tumori. 2015;101(6):603-608.
11. Breuer B, Fleishman SB, Cruciani RA, Portenoy RK. Medical oncologists’ attitudes and practice in cancer pain management: a national survey. J Clin Oncol. 2011;29(36):4769-4775.
12. Cleeland CS, Janjan NA, Scott CB, Seiferheld WF, Curran WJ. Cancer pain management by radiotherapists: a survey of radiation therapy oncology group physicians. Int J Radiat Oncol Biol Phys. 2000;47(1):203-208.
13. Tsao MN, Rades D, Wirth A, et al. Radiotherapeutic and surgical management for newly diagnosed brain metastasis(es): an American Society for Radiation Oncology evidence-based guideline. Pract Radiat Oncol. 2012;2(3):210-225.
14. Tsao MN, Rades D, Wirth A, et al. International practice survey on the management of brain metastases: third international consensus workshop on palliative radiotherapy and symptom control. Clin Oncol (R Coll Radiol). 2012;24(6):e81-e92.
15. Tang V, Harvey D, Park Dorsay J, Jiang S, Rathbone MP. Prognostic indicators in metastatic spinal cord compression: using functional independence measure and Tokuhashi scale to optimize rehabilitation planning. Spinal Cord. 2007;45(10):671-677.
16. Patchell RA, Tibbs PA, Regine WF, et al. Direct decompressive surgical resection in the treatment of spinal cord compression caused by metastatic cancer: a randomised trial. Lancet. 2005;366(9486):643-648.
17. Rades D, Stalpers LJ, Veninga T, et al. Evaluation of five radiation schedules and prognostic factors for metastatic spinal cord compression. J Clin Oncol. 2005;23(15):3366-3375.
18. Rades D, Stalpers LJ, Hulshof MC, et al. Comparison of 1 x 8 Gy and 10 x 3 Gy for functional outcome in patients with metastatic spinal cord compression. Int J Radiat Oncol Biol Phys. 2005;62(2):514-518.
19. Dennis K, Linden K, Balboni T, Chow E. Rapid access palliative radiation therapy programs: an efficient model of care. Future Oncol. 2015;11(17):2417-2426.
20. Kapoor A, Singhal MK, Bagri PK, Narayan S, Beniwal S, Kumar HS. Cancer related fatigue: a ubiquitous problem yet so under reported, under recognized and under treated. South Asian J Cancer. 2015;4(1):21-23.
21. Wei RL, Mattes MD, Yu J, et al. Attitudes of radiation oncologists toward palliative and supportive care in the United States: report on national membership survey by the American Society for Radiation Oncology (ASTRO). Pract Radiat Oncol. 2017;7(2):113-119.
22. Krishnan M, Racsa M, Jones J, et al. Radiation oncology resident palliative education. Pract Radiat Oncol. 2017;7(6):e439-e448.
23. McDonald R, Chow E, Lam H, Rowbottom L, Soliman H. International patterns of practice in radiotherapy for bone metastases: a review of the literature. J Bone Oncol. 2014;3(3-4):96-102.
Palliative radiation therapy (PRT) plays a major role in the management of incurable cancers. Study findings have demonstrated the efficacy of using PRT in treating tumor-related bone pain,1 brain metastases and related symptoms,2 thoracic disease-causing hemoptysis or obstruction,3 gastrointestinal involvement causing bleeding and/or obstruction,4 and genitourinary and/or gynecologic involvement causing bleeding.5,6
PRT accounts for between 30% and 50% of courses of radiotherapy delivered.3 These courses of RT typically require urgent evaluation since patients are seen because of new and/or progressive symptoms that give cause for concern. The urgency of presentation requires radiation oncologists and the departments receiving these patients to be equipped to manage these cases efficiently and effectively. Furthermore, the types of cases seen, including PRT indications and related symptoms requiring management, inform the training of radiation oncology physicians as well as nursing and other clinical staff. Finally, characterizing the types of urgent PRT cases that are seen can also guide research and quality improvement endeavors for advanced cancer care in radiation oncology settings.
There is currently a paucity of data characterizing the types and frequencies of urgent PRT indications in patients who present to radiation oncology departments, as well as a lack of data detailing the related symptoms radiation oncology clinicians are managing. The aim of this study was to characterize the types and frequencies of urgent PRT consultations and the related symptoms that radiation oncologists are managing as part of patient care.
Methods
Based on national palliative care practice and national oncology care practice guidelines,7,8 we designed a survey to categorize the cancer-related palliative care issues seen by radiation oncologists. Physical symptoms, psychosocial issues, cultural consideration, spiritual needs, care coordination, advanced-care planning, goals of care, and ethical and legal issues comprised the 8 palliative care domains that we evaluated. A survey was developed and critically evaluated by 3 investigators (MK, VL, TB). Each palliative care domain was ranked by clinicians by its relevance (5-point Likert scale [range, 1-5]: 1, Not Relevant, to 5, Extremely Relevant) to the patient’s care point in radiation oncology. In addition, 31 palliative care subissues related to the primary domains were identified by clinicians based on their presence (Yes, No, Not Assessed). Clinicians were also asked whether the consulted patient’s metastatic cancer diagnosis was established (longer than 1 month) or new (within the last 1 month). In addition, clinicians noted whether the patient was returning to active oncologic care (eg, chemotherapy) or to no further anticancer therapies (eg, hospice care) after RT consultation and intervention (if deemed necessary).
The survey’s face and content validity, ease of completion, and time of completion was assessed by a panel of 7 clinicians with expertise in medical oncology, radiation oncology, palliative care, and/or survey construction. The survey was then sent in a sequential manner to 1 member of the panel at a time after incorporating each panel member’s initial comments. After each panel member’s review, the survey was edited until 2 consecutive panel members had no further suggestions for improvement.
After receiving approval from the institutional review boards of participating radiation oncology centers, we electronically surveyed radiation oncology clinicians who were conducting PRT consultations. From May 19 to September 26, 2014, all consultations were evaluated prospectively for consideration of PRT performed by a dedicated PRT service at 3 centers (a large academic cancer center and 2 participating clinicians at affiliated regional hospitals). The consultations for patients aged 18 years or older with incurable, metastatic cancers were considered eligible. The consulting clinician was e-mailed a survey for completion within 5 business days immediately after each PRT consult. Three reminders to complete the survey were sent during the 5–business-day interval. Over the entire study period, 162 consecutive patients were identified, resulting in 162 surveys being sent to 15 radiation oncology clinicians, including nurse practitioners, resident physicians, and attending physicians. Each clinician received a $25 gift card for participating, regardless of the number of surveys completed. In total, 140 of the 162 surveys were returned, resulting in a response rate of 86%.
The investigators then collected patient demographics (age, gender, race, marital status) and disease characteristics (primary cancer type, Eastern Cooperative Oncology Group Performance Status, reasons for urgent RT consult, physical symptoms requiring management at presentation, patient’s place in illness trajectory, and RT recommendation) pertaining to each completed survey from the electronic medical record. Urgent consultations were defined as any patients who needed to be seen on the same day or within a few days of the consult request.
The descriptive statistics of all these data were calculated in terms of frequencies and percentage of categorical variables. Chi-squared statistics, Fisher exact test, and nonparametric rank sum tests were applied to various categories to determine any statistically significant differences between groups.
Results
In total, 162 patients were seen in consultation for PRT during the 19-week enrollment period, or an average of 8.7 consults a week. Of that total, surveys for 140 patients were returned (Table).
The median patient age was 63 years (range, 29-89 years). A sizeable minority (20%) was 50 years or younger. The most common cancer diagnosis was lung cancer (28%), followed by breast (13%) and prostate (10%) cancers, melanoma (10%), and sarcoma (7%). Other diagnoses accounted for the remaining 32%.
Timing of PRT consult in cancer trajectory
The points in the advanced cancer illness trajectory at which patients were seen for PRT evaluation are shown in Figure 1. Most patients (63%) were seen for a PRT evaluation at the time of an established diagnosis (>1 month after diagnosis of metastatic cancer) and were continuing to further cancer therapies. An additional 19% of patients with an established diagnosis proceeded to hospice or end-of-life care after the PRT evaluation. A notable minority of patients (18%) were seen for a PRT evaluation at the time of a new diagnosis (<1 month of diagnosis of metastatic cancer), and of those, 17% went on to receive anticancer therapy after the PRT evaluation and 1% proceeded to hospice or end-of-life care.
Characteristics of PRT consults and symptoms at presentation
The primary reasons for urgent consultation for PRT are shown in Figure 2. Cancer-related pain (57%), brain metastases (29%), and malignant spinal cord or cauda equina compression (13%) were the predominant reasons for consults. Notable minorities were seen for tumor-related dyspnea (10%), bleeding (8%), and bone fractures (4%).
PRT recommendations and targets
Recommendations regarding PRT are shown in Figure 4A. Of the total 140 patients, 18 (13%) were not recommended for RT. Of the 122 patients for whom PRT was recommended, 11 (9%) received RT at more than 1 site.
Discussion and conclusions
The present study provides a descriptive overview of urgent metastatic cancer patient presentations to radiation oncology clinicians through a comprehensive evaluation of 140 consults for PRT. The most common reasons for urgent evaluation were cancer-related pain (57%), but brain metastases (29%), spinal cord compression (13%), and respiratory symptoms (10%) were also common. Other less-common indications included cancer-related dysphagia, bleeding, and poststabilization management of bone fractures. The most common symptoms requiring management by radiation oncology clinicians were pain (69%), neurologic symptoms (51%), and fatigue (49%). The study also provides a comprehensive characterization of the timeframe of PRT consultation and the treatment recommendations in this cohort. Though most PRT consults occurred at the time of an established metastatic cancer diagnosis and before further anticancer therapies, sizeable minorities occurred at the time of a new diagnosis of metastatic cancer (18%) and before comfort-focused, end-of-life care and no further anticancer therapies (20%). Most patients (87%) were recommended PRT, and of those recommended RT, 11% received RT to more than 1 site. The most common PRT sites were to bone (46%), followed by brain (29%), nonlung soft-tissue sites (17%), and lung (8%). This comprehensive description of the day-to-day urgent, advanced cancer care issues seen and managed in radiation oncology practice can help guide PRT clinical structures, education, research, and quality improvement measures in clinical practice.
Our study provides an insight into urgent symptoms encountered by radiation oncology practitioners during their routine practice. Cancer-related pain remains the most common symptom requiring management. Given the frequency with which pain management is needed among PRT patients, this study highlights the need for radiation oncologists to be well trained in symptom management, particularly as the pain response to RT can often take several days. However, studies suggest that cancer-related pain is not frequently managed by radiation oncologists.9 For example, findings from an Italian study showed that the involvement of radiation oncologists in cancer pain management remains minimal compared with other medical professionals; during the treatment course, only half of the radiation oncologists implemented specific treatment for breakthrough pain.10 A nationwide survey in the United States implicated a number of barriers to adequate pain management, including poor assessment by the physician, reluctance in prescribing opioid analgesics, perceived excessive regulation, and patient reluctance to report pain.11 Notably, in a survey of the Radiation Therapy Oncology Group study physicians, 83% believed cancer patients with pain were undermedicated, and 40% reported that pain relief in their own practice setting was suboptimal. Furthermore, in the treatment plan, adjuvants and prophylactic side effect management were frequently not used properly.12 Education of radiation oncologists in pain assessment and management is key to overcome these barriers and to ensure adequate pain management and quality of life for patients in radiation oncology.
The next most common reason for which patients presented for palliative radiation oncology consultation was for central nervous system (CNS) metastatic disease, including brain metastases and spinal cord compression. Correspondingly, the next most common issue requiring management was neurologic symptoms. Management of CNS disease is becoming increasingly complex, and it benefits from multidisciplinary evaluation to guide optimal and personalized care for each patient, including medical oncology, radiation oncology, neurosurgery and/or orthopedic spine surgery, and palliative medicine. Treatment options include supportive care or corticosteroids alone, surgical resection, whole-brain RT, and/or radiosurgery or stereotactic RT alone. These treatment options are considered on the basis of global patient factors, such as prognosis, together with metastatic-site–specific factors, such as site-related symptoms and the number of metastatic diseases or the burden of the disease.13 For example, the use of the diagnosis-specific Graded Prognostic Assessment index to predict life expectancy can help tailor management of brain metastases based on performance status, age, number of brain metastases, extracranial metastases, and cancer type. Highlighting the complexity of this common PRT presentation, Tsao and colleagues showed that there was a lack of uniform agreement among radiation oncologists for common management issues in patients with brain metastatic disease.14
For metastatic spinal cord or cauda equina compression and the associated neurologic symptoms, initiation of immediate corticosteroids and implementation of local therapy within 24 hours of presentation is paramount,15 highlighting the need for rapid, comprehensive care decision-making for these patients. Treatment options that must be weighed include the potential benefit of upfront decompressive surgery, as supported by a randomized controlled trial by Patchell and colleagues16 for patients who are surgical candidates with true cord or cauda compression and have at least 1 neurologic symptom, a prognosis of ≥3 months, paraplegia of no longer than 48 hours, and no previous RT to the site or brain metastases. Compared with the RT alone, patients receiving surgery before RT had improved ambulatory status and overall survival. Hence, neurosurgical or orthopedic consultation should be standard in the evaluation of metastatic spinal cord or cauda equina compression patients. However, patients frequently do not meet these criteria, and corticosteroids and RT alone are considered. In addition to playing a role in surgical decision-making, prognosis also has a key role in decision-making about the RT fractionation. Short-course RT (8 Gy × 1) is as effectual as longer-course regimens (3 Gy × 10) in terms of motor function.17,18 However, more dose-intense or longer-course regimens, such as 3 Gy × 10, have been shown to have more durability beyond about 6 months and are therefore considered for intermediate to good prognosis.18 The common urgent presentation of CNS metastatic disease to radiation oncology clinics together with the complexity of management and urgency of care decision-making point to the need for dedicated structures of care for these patients in radiation oncology settings. For example, dedicated PRT programs, such as the Rapid Response Radiotherapy Program in Toronto and the Supportive and Palliative Radiation Oncology service in Boston, have demonstrated improved quality of care for patients being urgently evaluated for PRT.19
Following management of pain and neurologic symptoms, clinicians were faced with managing fatigue in nearly half of the patients (49%). The prevalence of fatigue among cancer patients and its impact on quality of life20 highlight the need for this key symptom to be addressed throughout the continuum of cancer care. National Comprehensive Cancer Network guidelines provide a comprehensive framework for addressing cancer-related fatigue.7 However, cancer-related fatigue is a largely underreported, underestimated, and thus undertreated problem.20 In a nationwide survey of members of the American Society for Radiation Oncology, radiation oncologists reported being significantly less confident in managing fatigue compared with managing other common symptoms.21 Furthermore, in a national survey of radiation oncology trainees, 67% of respondents indicated that they were not at all minimally or somewhat confident in their ability to manage fatigue symptoms. The frequency of this symptom together with the demonstrated need for improved education in fatigue management point to a need for radiation oncology palliative educational structures to include dedicated emphasis on managing fatigue in addition to other commonly encountered symptoms, such as pain.
Patients evaluated for PRT are seen across the trajectory of their metastatic cancer diagnosis. In our study, patients presented at all stages in their advanced cancers. These include patients seen at the time of initial diagnosis of cancer as well as those seen near the end of life when end-of-life care planning was underway. The broad spectrum of timing of PRT care underscores that radiation oncologists must be prepared to handle generalist palliative care issues encountered throughout the trajectory of advanced cancer care and hence need comprehensive education in generalist palliative care competencies. These include symptom management, end-of-life care coordination, and communication or goals-of-care discussions. Notably, a recent national survey of radiation oncology residents indicated that most residents, 77% on average, perceived their educational training as suboptimal across domains of generalist palliative care competencies needed in oncology practice.22 Furthermore, a majority (81%) desired greater palliative care education within training.
The most common sites treated in this study were bone, brain, and lung sites. These data provide guidance to both education and research initiatives aiming to advance PRT curriculum and care structures within departments. For example, a same-day simulation and radiation treatment program developed at Princess Margaret Hospital Palliative Radiation Oncology Program (Ontario, Canada) aids in providing streamlined care for patients with bone metastases, the most common presentation for PRT.23 Furthermore, education and research in the application of PRT techniques to bone, brain, and thoracic disease cover the majority of PRT presentations. It is notable, however, that 17% were other soft tissue body sites.
Limitations
There are a few limitations to this study. First, this is a survey-based study conducted at a single academic center within an urban setting and surrounding community regions, which affects its generalizability. Second, this study presents perspectives of radiation oncology practitioners evaluating patients and does not directly reflect patient perceptions or report of symptoms. Third, the data provided by this study are solely descriptive in nature. However, this can guide hypothesis-driven research regarding the evaluation and management of urgent palliative care issues encountered by radiation oncology clinicians and suggest educational objectives to address the needs of these patients.
Conclusions
Radiation oncologists are involved throughout the trajectory of care for advanced cancer patients. Furthermore, they manage a variety of urgent oncologic issues, most commonly metastases causing pain, brain metastases, and spinal cord or cauda equina compression. Radiation oncologists also manage many cancer-related symptoms, mostly pain, neurologic symptoms, fatigue, and gastrointestinal symptoms. These findings point toward the need for palliative care to be well integrated into radiation oncology training curricula and the need for dedicated care structures that enable rapid and multidisciplinary palliative oncology care within radiation oncology departments.
Palliative radiation therapy (PRT) plays a major role in the management of incurable cancers. Study findings have demonstrated the efficacy of using PRT in treating tumor-related bone pain,1 brain metastases and related symptoms,2 thoracic disease-causing hemoptysis or obstruction,3 gastrointestinal involvement causing bleeding and/or obstruction,4 and genitourinary and/or gynecologic involvement causing bleeding.5,6
PRT accounts for between 30% and 50% of courses of radiotherapy delivered.3 These courses of RT typically require urgent evaluation since patients are seen because of new and/or progressive symptoms that give cause for concern. The urgency of presentation requires radiation oncologists and the departments receiving these patients to be equipped to manage these cases efficiently and effectively. Furthermore, the types of cases seen, including PRT indications and related symptoms requiring management, inform the training of radiation oncology physicians as well as nursing and other clinical staff. Finally, characterizing the types of urgent PRT cases that are seen can also guide research and quality improvement endeavors for advanced cancer care in radiation oncology settings.
There is currently a paucity of data characterizing the types and frequencies of urgent PRT indications in patients who present to radiation oncology departments, as well as a lack of data detailing the related symptoms radiation oncology clinicians are managing. The aim of this study was to characterize the types and frequencies of urgent PRT consultations and the related symptoms that radiation oncologists are managing as part of patient care.
Methods
Based on national palliative care practice and national oncology care practice guidelines,7,8 we designed a survey to categorize the cancer-related palliative care issues seen by radiation oncologists. Physical symptoms, psychosocial issues, cultural consideration, spiritual needs, care coordination, advanced-care planning, goals of care, and ethical and legal issues comprised the 8 palliative care domains that we evaluated. A survey was developed and critically evaluated by 3 investigators (MK, VL, TB). Each palliative care domain was ranked by clinicians by its relevance (5-point Likert scale [range, 1-5]: 1, Not Relevant, to 5, Extremely Relevant) to the patient’s care point in radiation oncology. In addition, 31 palliative care subissues related to the primary domains were identified by clinicians based on their presence (Yes, No, Not Assessed). Clinicians were also asked whether the consulted patient’s metastatic cancer diagnosis was established (longer than 1 month) or new (within the last 1 month). In addition, clinicians noted whether the patient was returning to active oncologic care (eg, chemotherapy) or to no further anticancer therapies (eg, hospice care) after RT consultation and intervention (if deemed necessary).
The survey’s face and content validity, ease of completion, and time of completion was assessed by a panel of 7 clinicians with expertise in medical oncology, radiation oncology, palliative care, and/or survey construction. The survey was then sent in a sequential manner to 1 member of the panel at a time after incorporating each panel member’s initial comments. After each panel member’s review, the survey was edited until 2 consecutive panel members had no further suggestions for improvement.
After receiving approval from the institutional review boards of participating radiation oncology centers, we electronically surveyed radiation oncology clinicians who were conducting PRT consultations. From May 19 to September 26, 2014, all consultations were evaluated prospectively for consideration of PRT performed by a dedicated PRT service at 3 centers (a large academic cancer center and 2 participating clinicians at affiliated regional hospitals). The consultations for patients aged 18 years or older with incurable, metastatic cancers were considered eligible. The consulting clinician was e-mailed a survey for completion within 5 business days immediately after each PRT consult. Three reminders to complete the survey were sent during the 5–business-day interval. Over the entire study period, 162 consecutive patients were identified, resulting in 162 surveys being sent to 15 radiation oncology clinicians, including nurse practitioners, resident physicians, and attending physicians. Each clinician received a $25 gift card for participating, regardless of the number of surveys completed. In total, 140 of the 162 surveys were returned, resulting in a response rate of 86%.
The investigators then collected patient demographics (age, gender, race, marital status) and disease characteristics (primary cancer type, Eastern Cooperative Oncology Group Performance Status, reasons for urgent RT consult, physical symptoms requiring management at presentation, patient’s place in illness trajectory, and RT recommendation) pertaining to each completed survey from the electronic medical record. Urgent consultations were defined as any patients who needed to be seen on the same day or within a few days of the consult request.
The descriptive statistics of all these data were calculated in terms of frequencies and percentage of categorical variables. Chi-squared statistics, Fisher exact test, and nonparametric rank sum tests were applied to various categories to determine any statistically significant differences between groups.
Results
In total, 162 patients were seen in consultation for PRT during the 19-week enrollment period, or an average of 8.7 consults a week. Of that total, surveys for 140 patients were returned (Table).
The median patient age was 63 years (range, 29-89 years). A sizeable minority (20%) was 50 years or younger. The most common cancer diagnosis was lung cancer (28%), followed by breast (13%) and prostate (10%) cancers, melanoma (10%), and sarcoma (7%). Other diagnoses accounted for the remaining 32%.
Timing of PRT consult in cancer trajectory
The points in the advanced cancer illness trajectory at which patients were seen for PRT evaluation are shown in Figure 1. Most patients (63%) were seen for a PRT evaluation at the time of an established diagnosis (>1 month after diagnosis of metastatic cancer) and were continuing to further cancer therapies. An additional 19% of patients with an established diagnosis proceeded to hospice or end-of-life care after the PRT evaluation. A notable minority of patients (18%) were seen for a PRT evaluation at the time of a new diagnosis (<1 month of diagnosis of metastatic cancer), and of those, 17% went on to receive anticancer therapy after the PRT evaluation and 1% proceeded to hospice or end-of-life care.
Characteristics of PRT consults and symptoms at presentation
The primary reasons for urgent consultation for PRT are shown in Figure 2. Cancer-related pain (57%), brain metastases (29%), and malignant spinal cord or cauda equina compression (13%) were the predominant reasons for consults. Notable minorities were seen for tumor-related dyspnea (10%), bleeding (8%), and bone fractures (4%).
PRT recommendations and targets
Recommendations regarding PRT are shown in Figure 4A. Of the total 140 patients, 18 (13%) were not recommended for RT. Of the 122 patients for whom PRT was recommended, 11 (9%) received RT at more than 1 site.
Discussion and conclusions
The present study provides a descriptive overview of urgent metastatic cancer patient presentations to radiation oncology clinicians through a comprehensive evaluation of 140 consults for PRT. The most common reasons for urgent evaluation were cancer-related pain (57%), but brain metastases (29%), spinal cord compression (13%), and respiratory symptoms (10%) were also common. Other less-common indications included cancer-related dysphagia, bleeding, and poststabilization management of bone fractures. The most common symptoms requiring management by radiation oncology clinicians were pain (69%), neurologic symptoms (51%), and fatigue (49%). The study also provides a comprehensive characterization of the timeframe of PRT consultation and the treatment recommendations in this cohort. Though most PRT consults occurred at the time of an established metastatic cancer diagnosis and before further anticancer therapies, sizeable minorities occurred at the time of a new diagnosis of metastatic cancer (18%) and before comfort-focused, end-of-life care and no further anticancer therapies (20%). Most patients (87%) were recommended PRT, and of those recommended RT, 11% received RT to more than 1 site. The most common PRT sites were to bone (46%), followed by brain (29%), nonlung soft-tissue sites (17%), and lung (8%). This comprehensive description of the day-to-day urgent, advanced cancer care issues seen and managed in radiation oncology practice can help guide PRT clinical structures, education, research, and quality improvement measures in clinical practice.
Our study provides an insight into urgent symptoms encountered by radiation oncology practitioners during their routine practice. Cancer-related pain remains the most common symptom requiring management. Given the frequency with which pain management is needed among PRT patients, this study highlights the need for radiation oncologists to be well trained in symptom management, particularly as the pain response to RT can often take several days. However, studies suggest that cancer-related pain is not frequently managed by radiation oncologists.9 For example, findings from an Italian study showed that the involvement of radiation oncologists in cancer pain management remains minimal compared with other medical professionals; during the treatment course, only half of the radiation oncologists implemented specific treatment for breakthrough pain.10 A nationwide survey in the United States implicated a number of barriers to adequate pain management, including poor assessment by the physician, reluctance in prescribing opioid analgesics, perceived excessive regulation, and patient reluctance to report pain.11 Notably, in a survey of the Radiation Therapy Oncology Group study physicians, 83% believed cancer patients with pain were undermedicated, and 40% reported that pain relief in their own practice setting was suboptimal. Furthermore, in the treatment plan, adjuvants and prophylactic side effect management were frequently not used properly.12 Education of radiation oncologists in pain assessment and management is key to overcome these barriers and to ensure adequate pain management and quality of life for patients in radiation oncology.
The next most common reason for which patients presented for palliative radiation oncology consultation was for central nervous system (CNS) metastatic disease, including brain metastases and spinal cord compression. Correspondingly, the next most common issue requiring management was neurologic symptoms. Management of CNS disease is becoming increasingly complex, and it benefits from multidisciplinary evaluation to guide optimal and personalized care for each patient, including medical oncology, radiation oncology, neurosurgery and/or orthopedic spine surgery, and palliative medicine. Treatment options include supportive care or corticosteroids alone, surgical resection, whole-brain RT, and/or radiosurgery or stereotactic RT alone. These treatment options are considered on the basis of global patient factors, such as prognosis, together with metastatic-site–specific factors, such as site-related symptoms and the number of metastatic diseases or the burden of the disease.13 For example, the use of the diagnosis-specific Graded Prognostic Assessment index to predict life expectancy can help tailor management of brain metastases based on performance status, age, number of brain metastases, extracranial metastases, and cancer type. Highlighting the complexity of this common PRT presentation, Tsao and colleagues showed that there was a lack of uniform agreement among radiation oncologists for common management issues in patients with brain metastatic disease.14
For metastatic spinal cord or cauda equina compression and the associated neurologic symptoms, initiation of immediate corticosteroids and implementation of local therapy within 24 hours of presentation is paramount,15 highlighting the need for rapid, comprehensive care decision-making for these patients. Treatment options that must be weighed include the potential benefit of upfront decompressive surgery, as supported by a randomized controlled trial by Patchell and colleagues16 for patients who are surgical candidates with true cord or cauda compression and have at least 1 neurologic symptom, a prognosis of ≥3 months, paraplegia of no longer than 48 hours, and no previous RT to the site or brain metastases. Compared with the RT alone, patients receiving surgery before RT had improved ambulatory status and overall survival. Hence, neurosurgical or orthopedic consultation should be standard in the evaluation of metastatic spinal cord or cauda equina compression patients. However, patients frequently do not meet these criteria, and corticosteroids and RT alone are considered. In addition to playing a role in surgical decision-making, prognosis also has a key role in decision-making about the RT fractionation. Short-course RT (8 Gy × 1) is as effectual as longer-course regimens (3 Gy × 10) in terms of motor function.17,18 However, more dose-intense or longer-course regimens, such as 3 Gy × 10, have been shown to have more durability beyond about 6 months and are therefore considered for intermediate to good prognosis.18 The common urgent presentation of CNS metastatic disease to radiation oncology clinics together with the complexity of management and urgency of care decision-making point to the need for dedicated structures of care for these patients in radiation oncology settings. For example, dedicated PRT programs, such as the Rapid Response Radiotherapy Program in Toronto and the Supportive and Palliative Radiation Oncology service in Boston, have demonstrated improved quality of care for patients being urgently evaluated for PRT.19
Following management of pain and neurologic symptoms, clinicians were faced with managing fatigue in nearly half of the patients (49%). The prevalence of fatigue among cancer patients and its impact on quality of life20 highlight the need for this key symptom to be addressed throughout the continuum of cancer care. National Comprehensive Cancer Network guidelines provide a comprehensive framework for addressing cancer-related fatigue.7 However, cancer-related fatigue is a largely underreported, underestimated, and thus undertreated problem.20 In a nationwide survey of members of the American Society for Radiation Oncology, radiation oncologists reported being significantly less confident in managing fatigue compared with managing other common symptoms.21 Furthermore, in a national survey of radiation oncology trainees, 67% of respondents indicated that they were not at all minimally or somewhat confident in their ability to manage fatigue symptoms. The frequency of this symptom together with the demonstrated need for improved education in fatigue management point to a need for radiation oncology palliative educational structures to include dedicated emphasis on managing fatigue in addition to other commonly encountered symptoms, such as pain.
Patients evaluated for PRT are seen across the trajectory of their metastatic cancer diagnosis. In our study, patients presented at all stages in their advanced cancers. These include patients seen at the time of initial diagnosis of cancer as well as those seen near the end of life when end-of-life care planning was underway. The broad spectrum of timing of PRT care underscores that radiation oncologists must be prepared to handle generalist palliative care issues encountered throughout the trajectory of advanced cancer care and hence need comprehensive education in generalist palliative care competencies. These include symptom management, end-of-life care coordination, and communication or goals-of-care discussions. Notably, a recent national survey of radiation oncology residents indicated that most residents, 77% on average, perceived their educational training as suboptimal across domains of generalist palliative care competencies needed in oncology practice.22 Furthermore, a majority (81%) desired greater palliative care education within training.
The most common sites treated in this study were bone, brain, and lung sites. These data provide guidance to both education and research initiatives aiming to advance PRT curriculum and care structures within departments. For example, a same-day simulation and radiation treatment program developed at Princess Margaret Hospital Palliative Radiation Oncology Program (Ontario, Canada) aids in providing streamlined care for patients with bone metastases, the most common presentation for PRT.23 Furthermore, education and research in the application of PRT techniques to bone, brain, and thoracic disease cover the majority of PRT presentations. It is notable, however, that 17% were other soft tissue body sites.
Limitations
There are a few limitations to this study. First, this is a survey-based study conducted at a single academic center within an urban setting and surrounding community regions, which affects its generalizability. Second, this study presents perspectives of radiation oncology practitioners evaluating patients and does not directly reflect patient perceptions or report of symptoms. Third, the data provided by this study are solely descriptive in nature. However, this can guide hypothesis-driven research regarding the evaluation and management of urgent palliative care issues encountered by radiation oncology clinicians and suggest educational objectives to address the needs of these patients.
Conclusions
Radiation oncologists are involved throughout the trajectory of care for advanced cancer patients. Furthermore, they manage a variety of urgent oncologic issues, most commonly metastases causing pain, brain metastases, and spinal cord or cauda equina compression. Radiation oncologists also manage many cancer-related symptoms, mostly pain, neurologic symptoms, fatigue, and gastrointestinal symptoms. These findings point toward the need for palliative care to be well integrated into radiation oncology training curricula and the need for dedicated care structures that enable rapid and multidisciplinary palliative oncology care within radiation oncology departments.
1. Chow E, Harris K, Fan G, Tsao M, Sze WM. Palliative radiotherapy trials for bone metastases: a systematic review. J Clin Oncol. 2007;25(11):1423-1436.
2. van Oorschot B, Rades D, Schulze W, Beckmann G, Feyer P. Palliative radiotherapy--new approaches. Semin Oncol. 2011;38(3):443-449.
3. Simone CB II, Jones JA. Palliative care for patients with locally advanced and metastatic non-small cell lung cancer. Ann Palliat Med. 2013;2(4):178-188.
4. Cihoric N, Crowe S, Eychmüller S, Aebersold DM, Ghadjar P. Clinically significant bleeding in incurable cancer patients: effectiveness of hemostatic radiotherapy. Radiat Oncol. 2012;7:132.
5. Duchesne GM, Bolger JJ, Griffiths GO, et al. A randomized trial of hypofractionated schedules of palliative radiotherapy in the management of bladder carcinoma: results of medical research council trial BA09. Int J Radiat Oncol Biol Phys. 2000;47(2):379-388.
6. Onsrud M, Hagen B, Strickert T. 10-Gy single-fraction pelvic irradiation for palliation and life prolongation in patients with cancer of the cervix and corpus uteri. Gynecol Oncol. 2001;82(1):167-171.
7. NCCN Guidelines(R) Updates. J Natl Compr Canc Netw. 2013;11(9):xxxii-xxxvi.
8. Colby WH, Dahlin C, Lantos J, Carney J, Christopher M. The National Consensus Project for Quality Palliative Care Clinical Practice Guidelines Domain 8: ethical and legal aspects of care. HEC Forum. 2010;22(2):117-131.
9. Stockler MR, Wilcken NR. Why is management of cancer pain still a problem? J Clin Oncol. 2012;30(16):1907-1908.
10. Caravatta L, Ramella S, Melano A, et al. Breakthrough pain management in patients undergoing radiotherapy: a national survey on behalf of the Palliative and Supportive Care Study Group. Tumori. 2015;101(6):603-608.
11. Breuer B, Fleishman SB, Cruciani RA, Portenoy RK. Medical oncologists’ attitudes and practice in cancer pain management: a national survey. J Clin Oncol. 2011;29(36):4769-4775.
12. Cleeland CS, Janjan NA, Scott CB, Seiferheld WF, Curran WJ. Cancer pain management by radiotherapists: a survey of radiation therapy oncology group physicians. Int J Radiat Oncol Biol Phys. 2000;47(1):203-208.
13. Tsao MN, Rades D, Wirth A, et al. Radiotherapeutic and surgical management for newly diagnosed brain metastasis(es): an American Society for Radiation Oncology evidence-based guideline. Pract Radiat Oncol. 2012;2(3):210-225.
14. Tsao MN, Rades D, Wirth A, et al. International practice survey on the management of brain metastases: third international consensus workshop on palliative radiotherapy and symptom control. Clin Oncol (R Coll Radiol). 2012;24(6):e81-e92.
15. Tang V, Harvey D, Park Dorsay J, Jiang S, Rathbone MP. Prognostic indicators in metastatic spinal cord compression: using functional independence measure and Tokuhashi scale to optimize rehabilitation planning. Spinal Cord. 2007;45(10):671-677.
16. Patchell RA, Tibbs PA, Regine WF, et al. Direct decompressive surgical resection in the treatment of spinal cord compression caused by metastatic cancer: a randomised trial. Lancet. 2005;366(9486):643-648.
17. Rades D, Stalpers LJ, Veninga T, et al. Evaluation of five radiation schedules and prognostic factors for metastatic spinal cord compression. J Clin Oncol. 2005;23(15):3366-3375.
18. Rades D, Stalpers LJ, Hulshof MC, et al. Comparison of 1 x 8 Gy and 10 x 3 Gy for functional outcome in patients with metastatic spinal cord compression. Int J Radiat Oncol Biol Phys. 2005;62(2):514-518.
19. Dennis K, Linden K, Balboni T, Chow E. Rapid access palliative radiation therapy programs: an efficient model of care. Future Oncol. 2015;11(17):2417-2426.
20. Kapoor A, Singhal MK, Bagri PK, Narayan S, Beniwal S, Kumar HS. Cancer related fatigue: a ubiquitous problem yet so under reported, under recognized and under treated. South Asian J Cancer. 2015;4(1):21-23.
21. Wei RL, Mattes MD, Yu J, et al. Attitudes of radiation oncologists toward palliative and supportive care in the United States: report on national membership survey by the American Society for Radiation Oncology (ASTRO). Pract Radiat Oncol. 2017;7(2):113-119.
22. Krishnan M, Racsa M, Jones J, et al. Radiation oncology resident palliative education. Pract Radiat Oncol. 2017;7(6):e439-e448.
23. McDonald R, Chow E, Lam H, Rowbottom L, Soliman H. International patterns of practice in radiotherapy for bone metastases: a review of the literature. J Bone Oncol. 2014;3(3-4):96-102.
1. Chow E, Harris K, Fan G, Tsao M, Sze WM. Palliative radiotherapy trials for bone metastases: a systematic review. J Clin Oncol. 2007;25(11):1423-1436.
2. van Oorschot B, Rades D, Schulze W, Beckmann G, Feyer P. Palliative radiotherapy--new approaches. Semin Oncol. 2011;38(3):443-449.
3. Simone CB II, Jones JA. Palliative care for patients with locally advanced and metastatic non-small cell lung cancer. Ann Palliat Med. 2013;2(4):178-188.
4. Cihoric N, Crowe S, Eychmüller S, Aebersold DM, Ghadjar P. Clinically significant bleeding in incurable cancer patients: effectiveness of hemostatic radiotherapy. Radiat Oncol. 2012;7:132.
5. Duchesne GM, Bolger JJ, Griffiths GO, et al. A randomized trial of hypofractionated schedules of palliative radiotherapy in the management of bladder carcinoma: results of medical research council trial BA09. Int J Radiat Oncol Biol Phys. 2000;47(2):379-388.
6. Onsrud M, Hagen B, Strickert T. 10-Gy single-fraction pelvic irradiation for palliation and life prolongation in patients with cancer of the cervix and corpus uteri. Gynecol Oncol. 2001;82(1):167-171.
7. NCCN Guidelines(R) Updates. J Natl Compr Canc Netw. 2013;11(9):xxxii-xxxvi.
8. Colby WH, Dahlin C, Lantos J, Carney J, Christopher M. The National Consensus Project for Quality Palliative Care Clinical Practice Guidelines Domain 8: ethical and legal aspects of care. HEC Forum. 2010;22(2):117-131.
9. Stockler MR, Wilcken NR. Why is management of cancer pain still a problem? J Clin Oncol. 2012;30(16):1907-1908.
10. Caravatta L, Ramella S, Melano A, et al. Breakthrough pain management in patients undergoing radiotherapy: a national survey on behalf of the Palliative and Supportive Care Study Group. Tumori. 2015;101(6):603-608.
11. Breuer B, Fleishman SB, Cruciani RA, Portenoy RK. Medical oncologists’ attitudes and practice in cancer pain management: a national survey. J Clin Oncol. 2011;29(36):4769-4775.
12. Cleeland CS, Janjan NA, Scott CB, Seiferheld WF, Curran WJ. Cancer pain management by radiotherapists: a survey of radiation therapy oncology group physicians. Int J Radiat Oncol Biol Phys. 2000;47(1):203-208.
13. Tsao MN, Rades D, Wirth A, et al. Radiotherapeutic and surgical management for newly diagnosed brain metastasis(es): an American Society for Radiation Oncology evidence-based guideline. Pract Radiat Oncol. 2012;2(3):210-225.
14. Tsao MN, Rades D, Wirth A, et al. International practice survey on the management of brain metastases: third international consensus workshop on palliative radiotherapy and symptom control. Clin Oncol (R Coll Radiol). 2012;24(6):e81-e92.
15. Tang V, Harvey D, Park Dorsay J, Jiang S, Rathbone MP. Prognostic indicators in metastatic spinal cord compression: using functional independence measure and Tokuhashi scale to optimize rehabilitation planning. Spinal Cord. 2007;45(10):671-677.
16. Patchell RA, Tibbs PA, Regine WF, et al. Direct decompressive surgical resection in the treatment of spinal cord compression caused by metastatic cancer: a randomised trial. Lancet. 2005;366(9486):643-648.
17. Rades D, Stalpers LJ, Veninga T, et al. Evaluation of five radiation schedules and prognostic factors for metastatic spinal cord compression. J Clin Oncol. 2005;23(15):3366-3375.
18. Rades D, Stalpers LJ, Hulshof MC, et al. Comparison of 1 x 8 Gy and 10 x 3 Gy for functional outcome in patients with metastatic spinal cord compression. Int J Radiat Oncol Biol Phys. 2005;62(2):514-518.
19. Dennis K, Linden K, Balboni T, Chow E. Rapid access palliative radiation therapy programs: an efficient model of care. Future Oncol. 2015;11(17):2417-2426.
20. Kapoor A, Singhal MK, Bagri PK, Narayan S, Beniwal S, Kumar HS. Cancer related fatigue: a ubiquitous problem yet so under reported, under recognized and under treated. South Asian J Cancer. 2015;4(1):21-23.
21. Wei RL, Mattes MD, Yu J, et al. Attitudes of radiation oncologists toward palliative and supportive care in the United States: report on national membership survey by the American Society for Radiation Oncology (ASTRO). Pract Radiat Oncol. 2017;7(2):113-119.
22. Krishnan M, Racsa M, Jones J, et al. Radiation oncology resident palliative education. Pract Radiat Oncol. 2017;7(6):e439-e448.
23. McDonald R, Chow E, Lam H, Rowbottom L, Soliman H. International patterns of practice in radiotherapy for bone metastases: a review of the literature. J Bone Oncol. 2014;3(3-4):96-102.