User login
New CDC opioid guideline targets overprescribing for chronic pain
Nonopioid therapy is the preferred approach for managing chronic pain outside of active cancer, palliative, and end-of-life care, according to a new guideline released today by the Centers for Disease Control and Prevention.
The 12 recommendations included in the guideline center around this principle and two others: using the lowest possible effective dosage when opioids are used, and exercising caution and monitoring patients closely when prescribing opioids.
Specifically, the guideline states that “clinicians should consider opioid therapy only if expected benefits for both pain and function are anticipated to outweigh risks to the patient,” and that “treatment should be combined with nonpharmacologic and nonopioid therapy, as appropriate.”
The guideline also addresses steps to take before starting or continuing opioid therapy, and drug selection, dosage, duration, follow-up, and discontinuation. Recommendations for assessing risk and addressing harms of opioid use are also included.
The CDC developed the guideline as part of the U.S. government’s urgent response to the epidemic of overdose deaths, which has been fueled by a quadrupling of the prescribing and sales of opioids since 1999, according to a CDC press statement. The guideline’s purpose is to help prevent opioid misuse and overdose.
“The CDC Guideline for Prescribing Opioids for Chronic Pain, United States, 2016 will help primary care providers ensure the safest and most effective treatment for their patients,” according to the statement. The CDC’s director, Dr. Tom Frieden, noted that “overprescribing opioids – largely for chronic pain – is a key driver of America’s drug-overdose epidemic.”
In a CDC teleconference marking the release of the guideline, Dr. Frieden said it has become increasingly clear that opioids “carry substantial risks but only uncertain benefits, especially compared with other treatments for chronic pain.
“Beginning treatment with an opioid is a momentous decision, and it should only be done with full understanding by both the clinician and the patient of the substantial risks and uncertain benefits involved,” Dr. Frieden said. He added that he knows of no other medication “that’s routinely used for a nonfatal condition [and] that kills patients so frequently.
“With more than 250 million prescriptions written each year, it’s so important that doctors understand that any one of those prescriptions could potentially end a patient’s life,” he cautioned.
A 2015 study showed that 1 of every 550 patients treated with opioids for noncancer pain – and 1 of 32 who received the highest doses (more than 200 morphine milligram equivalents per day) – died within 2.5 years of the first prescription.
Dr. Frieden noted that opioids do have a place when the potential benefits outweigh the potential harms. “But for most patients – the vast majority of patients – the risks will outweigh the benefits,” he said.
The opioid epidemic is one of the most pressing public health issues in the United States today, said Sylvia M. Burwell, secretary of the Department of Health & Human Services. A year ago, she announced an HHS initiative to reduce prescription opioid and heroin-related drug overdose, death, and dependence.
“Last year, more Americans died from drug overdoses than car crashes,” Ms. Burwell said during the teleconference, noting that families across the nation and from all walks of life have been affected.
Combating the opioid epidemic is a national priority, she said, and the CDC guideline will help in that effort.
“We believe this guideline will help health care professionals provide safer and more effective care for patients dealing with chronic pain, and we also believe it will help these providers drive down the rates of opioid use disorder, overdose, and ... death,” she said.
The American Medical Association greeted the guideline with cautious support.
“While we are largely supportive of the guidelines, we remain concerned about the evidence base informing some of the recommendations,” noted Dr. Patrice A. Harris, chair-elect of the AMA board and chair of the AMA Task Force to Reduce Opioid Abuse, in a statement.
The AMA also cited potential conflicts between the guideline and product labeling and state laws, as well as obstacles such as insurance coverage limits on nonpharmacologic treatments.
“If these guidelines help reduce the deaths resulting from opioids, they will prove to be valuable,” Dr. Harris said in the statement. “If they produce unintended consequences, we will need to mitigate them.”
Of note, the guideline stresses the right of patients with chronic pain to receive safe and effective pain management, and focuses on giving primary care providers – who account for about half of all opioid prescriptions – a road map for providing such pain management by increasing the use of effective nonopioid and nonpharmacologic therapies.
It was developed through a “rigorous scientific process using the best available scientific evidence, consulting with experts, and listening to comments from the public and partner organizations,” according to the CDC statement. The organization “is dedicated to working with partners to improve the evidence base and will refine the recommendations as new research becomes available.
”In conjunction with the release of the guideline, the CDC has provided a checklist for prescribing opioids for chronic pain, and a website with additional tools for implementing the recommendations within the guideline.
The CDC's opioid recommendations
The Centers for Disease Control and Prevention’s new opioid prescription guideline includes 12 recommendations. Here they are, modified slightly for style:
1. Nonpharmacologic therapy and nonopioid pharmacologic therapy are preferred for chronic pain. Providers should only consider adding opioid therapy if expected benefits for both pain and function are anticipated to outweigh risks.
2. Before starting opioid therapy for chronic pain, providers should establish treatment goals with all patients, including realistic goals for pain and function. Providers should not initiate opioid therapy without consideration of how therapy will be discontinued if unsuccessful. Providers should continue opioid therapy only if there is clinically meaningful improvement in pain and function.
3. Before starting and periodically during opioid therapy, providers should discuss with patients known risks and realistic benefits of opioid therapy, and patient and provider responsibilities for managing therapy.
4. When starting opioid therapy for chronic pain, providers should prescribe immediate-release opioids instead of extended-release/long-acting opioids.
5. When opioids are started, providers should prescribe the lowest effective dosage. Providers should use caution when prescribing opioids at any dosage, should implement additional precautions when increasing dosage to 50 or more morphine milligram equivalents (MME) per day, and generally should avoid increasing dosage to 90 or more MME per day.
6. When opioids are used for acute pain, providers should prescribe the lowest effective dose of immediate-release opioids. Three or fewer days often will be sufficient.
7. Providers should evaluate the benefits and harms with patients within 1-4 weeks of starting opioid therapy for chronic pain or of dose escalation. They should reevaluate continued therapy’s benefits and harms every 3 months or more frequently. If continued therapy’s benefits do not outweigh harms, providers should work with patients to reduce dosages or discontinue opioids.
8. During therapy, providers should evaluate risk factors for opioid-related harm. Providers should incorporate into the management plan strategies to mitigate risk, including considering offering naloxone when factors that increase risk for opioid overdose – such as history of overdose, history of substance use disorder, or higher opioid dosage (50 MME or more) – are present.
9. Providers should review the patient’s history of controlled substance prescriptions using state prescription drug monitoring program (PDMP) data to determine whether the patient is receiving high opioid dosages or dangerous combinations that put him or her at high risk for overdose. Providers should review PDMP data when starting opioid therapy for chronic pain and periodically during opioid therapy for chronic pain, ranging from every prescription to every 3 months.
10. When prescribing opioids for chronic pain, providers should use urine drug testing before starting opioid therapy and consider urine drug testing at least annually to assess for prescribed medications, as well as other controlled prescription drugs and illicit drugs.
11. Providers should avoid concurrent prescriptions of opioid pain medication and benzodiazepines whenever possible.
12. Providers should offer or arrange evidence-based treatment (usually medication-assisted treatment with buprenorphine or methadone in combination with behavioral therapies) for patients with opioid use disorder.
M. Alexander Otto contributed to this article.
Nonopioid therapy is the preferred approach for managing chronic pain outside of active cancer, palliative, and end-of-life care, according to a new guideline released today by the Centers for Disease Control and Prevention.
The 12 recommendations included in the guideline center around this principle and two others: using the lowest possible effective dosage when opioids are used, and exercising caution and monitoring patients closely when prescribing opioids.
Specifically, the guideline states that “clinicians should consider opioid therapy only if expected benefits for both pain and function are anticipated to outweigh risks to the patient,” and that “treatment should be combined with nonpharmacologic and nonopioid therapy, as appropriate.”
The guideline also addresses steps to take before starting or continuing opioid therapy, and drug selection, dosage, duration, follow-up, and discontinuation. Recommendations for assessing risk and addressing harms of opioid use are also included.
The CDC developed the guideline as part of the U.S. government’s urgent response to the epidemic of overdose deaths, which has been fueled by a quadrupling of the prescribing and sales of opioids since 1999, according to a CDC press statement. The guideline’s purpose is to help prevent opioid misuse and overdose.
“The CDC Guideline for Prescribing Opioids for Chronic Pain, United States, 2016 will help primary care providers ensure the safest and most effective treatment for their patients,” according to the statement. The CDC’s director, Dr. Tom Frieden, noted that “overprescribing opioids – largely for chronic pain – is a key driver of America’s drug-overdose epidemic.”
In a CDC teleconference marking the release of the guideline, Dr. Frieden said it has become increasingly clear that opioids “carry substantial risks but only uncertain benefits, especially compared with other treatments for chronic pain.
“Beginning treatment with an opioid is a momentous decision, and it should only be done with full understanding by both the clinician and the patient of the substantial risks and uncertain benefits involved,” Dr. Frieden said. He added that he knows of no other medication “that’s routinely used for a nonfatal condition [and] that kills patients so frequently.
“With more than 250 million prescriptions written each year, it’s so important that doctors understand that any one of those prescriptions could potentially end a patient’s life,” he cautioned.
A 2015 study showed that 1 of every 550 patients treated with opioids for noncancer pain – and 1 of 32 who received the highest doses (more than 200 morphine milligram equivalents per day) – died within 2.5 years of the first prescription.
Dr. Frieden noted that opioids do have a place when the potential benefits outweigh the potential harms. “But for most patients – the vast majority of patients – the risks will outweigh the benefits,” he said.
The opioid epidemic is one of the most pressing public health issues in the United States today, said Sylvia M. Burwell, secretary of the Department of Health & Human Services. A year ago, she announced an HHS initiative to reduce prescription opioid and heroin-related drug overdose, death, and dependence.
“Last year, more Americans died from drug overdoses than car crashes,” Ms. Burwell said during the teleconference, noting that families across the nation and from all walks of life have been affected.
Combating the opioid epidemic is a national priority, she said, and the CDC guideline will help in that effort.
“We believe this guideline will help health care professionals provide safer and more effective care for patients dealing with chronic pain, and we also believe it will help these providers drive down the rates of opioid use disorder, overdose, and ... death,” she said.
The American Medical Association greeted the guideline with cautious support.
“While we are largely supportive of the guidelines, we remain concerned about the evidence base informing some of the recommendations,” noted Dr. Patrice A. Harris, chair-elect of the AMA board and chair of the AMA Task Force to Reduce Opioid Abuse, in a statement.
The AMA also cited potential conflicts between the guideline and product labeling and state laws, as well as obstacles such as insurance coverage limits on nonpharmacologic treatments.
“If these guidelines help reduce the deaths resulting from opioids, they will prove to be valuable,” Dr. Harris said in the statement. “If they produce unintended consequences, we will need to mitigate them.”
Of note, the guideline stresses the right of patients with chronic pain to receive safe and effective pain management, and focuses on giving primary care providers – who account for about half of all opioid prescriptions – a road map for providing such pain management by increasing the use of effective nonopioid and nonpharmacologic therapies.
It was developed through a “rigorous scientific process using the best available scientific evidence, consulting with experts, and listening to comments from the public and partner organizations,” according to the CDC statement. The organization “is dedicated to working with partners to improve the evidence base and will refine the recommendations as new research becomes available.
”In conjunction with the release of the guideline, the CDC has provided a checklist for prescribing opioids for chronic pain, and a website with additional tools for implementing the recommendations within the guideline.
The CDC's opioid recommendations
The Centers for Disease Control and Prevention’s new opioid prescription guideline includes 12 recommendations. Here they are, modified slightly for style:
1. Nonpharmacologic therapy and nonopioid pharmacologic therapy are preferred for chronic pain. Providers should only consider adding opioid therapy if expected benefits for both pain and function are anticipated to outweigh risks.
2. Before starting opioid therapy for chronic pain, providers should establish treatment goals with all patients, including realistic goals for pain and function. Providers should not initiate opioid therapy without consideration of how therapy will be discontinued if unsuccessful. Providers should continue opioid therapy only if there is clinically meaningful improvement in pain and function.
3. Before starting and periodically during opioid therapy, providers should discuss with patients known risks and realistic benefits of opioid therapy, and patient and provider responsibilities for managing therapy.
4. When starting opioid therapy for chronic pain, providers should prescribe immediate-release opioids instead of extended-release/long-acting opioids.
5. When opioids are started, providers should prescribe the lowest effective dosage. Providers should use caution when prescribing opioids at any dosage, should implement additional precautions when increasing dosage to 50 or more morphine milligram equivalents (MME) per day, and generally should avoid increasing dosage to 90 or more MME per day.
6. When opioids are used for acute pain, providers should prescribe the lowest effective dose of immediate-release opioids. Three or fewer days often will be sufficient.
7. Providers should evaluate the benefits and harms with patients within 1-4 weeks of starting opioid therapy for chronic pain or of dose escalation. They should reevaluate continued therapy’s benefits and harms every 3 months or more frequently. If continued therapy’s benefits do not outweigh harms, providers should work with patients to reduce dosages or discontinue opioids.
8. During therapy, providers should evaluate risk factors for opioid-related harm. Providers should incorporate into the management plan strategies to mitigate risk, including considering offering naloxone when factors that increase risk for opioid overdose – such as history of overdose, history of substance use disorder, or higher opioid dosage (50 MME or more) – are present.
9. Providers should review the patient’s history of controlled substance prescriptions using state prescription drug monitoring program (PDMP) data to determine whether the patient is receiving high opioid dosages or dangerous combinations that put him or her at high risk for overdose. Providers should review PDMP data when starting opioid therapy for chronic pain and periodically during opioid therapy for chronic pain, ranging from every prescription to every 3 months.
10. When prescribing opioids for chronic pain, providers should use urine drug testing before starting opioid therapy and consider urine drug testing at least annually to assess for prescribed medications, as well as other controlled prescription drugs and illicit drugs.
11. Providers should avoid concurrent prescriptions of opioid pain medication and benzodiazepines whenever possible.
12. Providers should offer or arrange evidence-based treatment (usually medication-assisted treatment with buprenorphine or methadone in combination with behavioral therapies) for patients with opioid use disorder.
M. Alexander Otto contributed to this article.
Nonopioid therapy is the preferred approach for managing chronic pain outside of active cancer, palliative, and end-of-life care, according to a new guideline released today by the Centers for Disease Control and Prevention.
The 12 recommendations included in the guideline center around this principle and two others: using the lowest possible effective dosage when opioids are used, and exercising caution and monitoring patients closely when prescribing opioids.
Specifically, the guideline states that “clinicians should consider opioid therapy only if expected benefits for both pain and function are anticipated to outweigh risks to the patient,” and that “treatment should be combined with nonpharmacologic and nonopioid therapy, as appropriate.”
The guideline also addresses steps to take before starting or continuing opioid therapy, and drug selection, dosage, duration, follow-up, and discontinuation. Recommendations for assessing risk and addressing harms of opioid use are also included.
The CDC developed the guideline as part of the U.S. government’s urgent response to the epidemic of overdose deaths, which has been fueled by a quadrupling of the prescribing and sales of opioids since 1999, according to a CDC press statement. The guideline’s purpose is to help prevent opioid misuse and overdose.
“The CDC Guideline for Prescribing Opioids for Chronic Pain, United States, 2016 will help primary care providers ensure the safest and most effective treatment for their patients,” according to the statement. The CDC’s director, Dr. Tom Frieden, noted that “overprescribing opioids – largely for chronic pain – is a key driver of America’s drug-overdose epidemic.”
In a CDC teleconference marking the release of the guideline, Dr. Frieden said it has become increasingly clear that opioids “carry substantial risks but only uncertain benefits, especially compared with other treatments for chronic pain.
“Beginning treatment with an opioid is a momentous decision, and it should only be done with full understanding by both the clinician and the patient of the substantial risks and uncertain benefits involved,” Dr. Frieden said. He added that he knows of no other medication “that’s routinely used for a nonfatal condition [and] that kills patients so frequently.
“With more than 250 million prescriptions written each year, it’s so important that doctors understand that any one of those prescriptions could potentially end a patient’s life,” he cautioned.
A 2015 study showed that 1 of every 550 patients treated with opioids for noncancer pain – and 1 of 32 who received the highest doses (more than 200 morphine milligram equivalents per day) – died within 2.5 years of the first prescription.
Dr. Frieden noted that opioids do have a place when the potential benefits outweigh the potential harms. “But for most patients – the vast majority of patients – the risks will outweigh the benefits,” he said.
The opioid epidemic is one of the most pressing public health issues in the United States today, said Sylvia M. Burwell, secretary of the Department of Health & Human Services. A year ago, she announced an HHS initiative to reduce prescription opioid and heroin-related drug overdose, death, and dependence.
“Last year, more Americans died from drug overdoses than car crashes,” Ms. Burwell said during the teleconference, noting that families across the nation and from all walks of life have been affected.
Combating the opioid epidemic is a national priority, she said, and the CDC guideline will help in that effort.
“We believe this guideline will help health care professionals provide safer and more effective care for patients dealing with chronic pain, and we also believe it will help these providers drive down the rates of opioid use disorder, overdose, and ... death,” she said.
The American Medical Association greeted the guideline with cautious support.
“While we are largely supportive of the guidelines, we remain concerned about the evidence base informing some of the recommendations,” noted Dr. Patrice A. Harris, chair-elect of the AMA board and chair of the AMA Task Force to Reduce Opioid Abuse, in a statement.
The AMA also cited potential conflicts between the guideline and product labeling and state laws, as well as obstacles such as insurance coverage limits on nonpharmacologic treatments.
“If these guidelines help reduce the deaths resulting from opioids, they will prove to be valuable,” Dr. Harris said in the statement. “If they produce unintended consequences, we will need to mitigate them.”
Of note, the guideline stresses the right of patients with chronic pain to receive safe and effective pain management, and focuses on giving primary care providers – who account for about half of all opioid prescriptions – a road map for providing such pain management by increasing the use of effective nonopioid and nonpharmacologic therapies.
It was developed through a “rigorous scientific process using the best available scientific evidence, consulting with experts, and listening to comments from the public and partner organizations,” according to the CDC statement. The organization “is dedicated to working with partners to improve the evidence base and will refine the recommendations as new research becomes available.
”In conjunction with the release of the guideline, the CDC has provided a checklist for prescribing opioids for chronic pain, and a website with additional tools for implementing the recommendations within the guideline.
The CDC's opioid recommendations
The Centers for Disease Control and Prevention’s new opioid prescription guideline includes 12 recommendations. Here they are, modified slightly for style:
1. Nonpharmacologic therapy and nonopioid pharmacologic therapy are preferred for chronic pain. Providers should only consider adding opioid therapy if expected benefits for both pain and function are anticipated to outweigh risks.
2. Before starting opioid therapy for chronic pain, providers should establish treatment goals with all patients, including realistic goals for pain and function. Providers should not initiate opioid therapy without consideration of how therapy will be discontinued if unsuccessful. Providers should continue opioid therapy only if there is clinically meaningful improvement in pain and function.
3. Before starting and periodically during opioid therapy, providers should discuss with patients known risks and realistic benefits of opioid therapy, and patient and provider responsibilities for managing therapy.
4. When starting opioid therapy for chronic pain, providers should prescribe immediate-release opioids instead of extended-release/long-acting opioids.
5. When opioids are started, providers should prescribe the lowest effective dosage. Providers should use caution when prescribing opioids at any dosage, should implement additional precautions when increasing dosage to 50 or more morphine milligram equivalents (MME) per day, and generally should avoid increasing dosage to 90 or more MME per day.
6. When opioids are used for acute pain, providers should prescribe the lowest effective dose of immediate-release opioids. Three or fewer days often will be sufficient.
7. Providers should evaluate the benefits and harms with patients within 1-4 weeks of starting opioid therapy for chronic pain or of dose escalation. They should reevaluate continued therapy’s benefits and harms every 3 months or more frequently. If continued therapy’s benefits do not outweigh harms, providers should work with patients to reduce dosages or discontinue opioids.
8. During therapy, providers should evaluate risk factors for opioid-related harm. Providers should incorporate into the management plan strategies to mitigate risk, including considering offering naloxone when factors that increase risk for opioid overdose – such as history of overdose, history of substance use disorder, or higher opioid dosage (50 MME or more) – are present.
9. Providers should review the patient’s history of controlled substance prescriptions using state prescription drug monitoring program (PDMP) data to determine whether the patient is receiving high opioid dosages or dangerous combinations that put him or her at high risk for overdose. Providers should review PDMP data when starting opioid therapy for chronic pain and periodically during opioid therapy for chronic pain, ranging from every prescription to every 3 months.
10. When prescribing opioids for chronic pain, providers should use urine drug testing before starting opioid therapy and consider urine drug testing at least annually to assess for prescribed medications, as well as other controlled prescription drugs and illicit drugs.
11. Providers should avoid concurrent prescriptions of opioid pain medication and benzodiazepines whenever possible.
12. Providers should offer or arrange evidence-based treatment (usually medication-assisted treatment with buprenorphine or methadone in combination with behavioral therapies) for patients with opioid use disorder.
M. Alexander Otto contributed to this article.
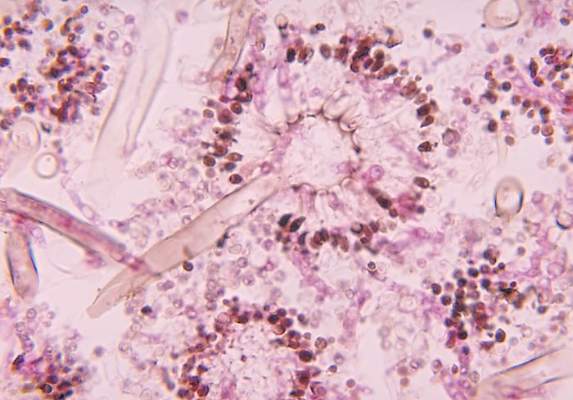
When toenail onychomycosis can turn deadly
WAIKOLOA, HAWAII – Toenail onychomycosis is a common condition in the general population, but it’s three- to fourfold more prevalent in certain at risk populations where it can have serious and even life-threatening consequences, Dr. Theodore Rosen observed at the Hawaii Dermatology Seminar provided by the Global Academy for Medical Education/Skin Disease Education Foundation.
He cited a recent systematic review led by Dr. Aditya K. Gupta, professor of dermatology at the University of Toronto, whom Dr. Rosen hailed as one of the world’s great fungal disease authorities. Dr. Gupta and coworkers concluded that while the prevalence of dermatophyte toenail onychomycosis is 3.2% worldwide in the general population, it climbs to 8.8% in diabetics, 10.2% in psoriatics, 10.3% in the elderly, 11.9% in dialysis patients, 5.2% in renal transplant recipients, and 10.4% in HIV-positive individuals. The highest prevalence of onychomycosis due to non-dermatophyte molds was seen in psoriasis patients, at 2.5%, while elderly patients had the highest prevalence of onychomycosis caused by yeasts, at 6.1% (J Eur Acad Dermatol Venereol. 2015 Jun;29[6]:1039-44).
“Onychomycosis is especially important in those who are immunocompromised and immunosuppressed, for two reasons. One is that really odd organisms that aren’t Trichophyton rubrum or T. interdigitale can be involved: saprophytes like Scopulariopsis, Acremonium, Aspergillus, and Paecilomyces. And some of these saprophytes, like Fusarium, can get from the nail and nail bed into the bloodstream and can kill,” explained Dr. Rosen, professor of dermatology at Baylor College of Medicine in Houston.
“Onychomycosis, aside from the fact that it looks bad and often leads to pain, can also lead to breaks in the skin which then result in secondary bacterial infections. In fact, after motor vehicle accidents, onychomycosis and tinea pedis combined are the most common cause of lower extremity cellulitis leading to hospitalization in the United States,” he continued.
The go-to treatments for onychomycosis in patients with a bad prognostic factor are oral itraconazole (Sporanox) and terbinafine. Don’t be unduly swayed by the complete cure rates reported in clinical trials and cited in the product package inserts; they don’t tell the full story because of important differences in study design, according to Dr. Rosen.
He recommended that physicians familiarize themselves with posaconazole (Noxafil) as an antifungal to consider for second-line therapy in difficult-to-cure cases of onychomycosis in immunosuppressed patients. This is off-label therapy. The approved indications for this triazole antifungal agent are prophylaxis of invasive Aspergillus and Candida infections in severely immunocompromised patients, as well as treatment of oropharyngeal candidiasis. But this is a potent agent that provides broad-spectrum coverage coupled with a favorable safety profile. It performed well in a phase IIb randomized, placebo- and active-controlled, multicenter, investigator-blinded study of 218 adults with toenail onychomycosis (Br J Dermatol. 2012 Feb;166[2]:389-98).
Dr. Rosen reported serving on scientific advisory boards for Anacor, Merz, and Valeant.
SDEF and this news organization are owned by the same parent company.
WAIKOLOA, HAWAII – Toenail onychomycosis is a common condition in the general population, but it’s three- to fourfold more prevalent in certain at risk populations where it can have serious and even life-threatening consequences, Dr. Theodore Rosen observed at the Hawaii Dermatology Seminar provided by the Global Academy for Medical Education/Skin Disease Education Foundation.
He cited a recent systematic review led by Dr. Aditya K. Gupta, professor of dermatology at the University of Toronto, whom Dr. Rosen hailed as one of the world’s great fungal disease authorities. Dr. Gupta and coworkers concluded that while the prevalence of dermatophyte toenail onychomycosis is 3.2% worldwide in the general population, it climbs to 8.8% in diabetics, 10.2% in psoriatics, 10.3% in the elderly, 11.9% in dialysis patients, 5.2% in renal transplant recipients, and 10.4% in HIV-positive individuals. The highest prevalence of onychomycosis due to non-dermatophyte molds was seen in psoriasis patients, at 2.5%, while elderly patients had the highest prevalence of onychomycosis caused by yeasts, at 6.1% (J Eur Acad Dermatol Venereol. 2015 Jun;29[6]:1039-44).
“Onychomycosis is especially important in those who are immunocompromised and immunosuppressed, for two reasons. One is that really odd organisms that aren’t Trichophyton rubrum or T. interdigitale can be involved: saprophytes like Scopulariopsis, Acremonium, Aspergillus, and Paecilomyces. And some of these saprophytes, like Fusarium, can get from the nail and nail bed into the bloodstream and can kill,” explained Dr. Rosen, professor of dermatology at Baylor College of Medicine in Houston.
“Onychomycosis, aside from the fact that it looks bad and often leads to pain, can also lead to breaks in the skin which then result in secondary bacterial infections. In fact, after motor vehicle accidents, onychomycosis and tinea pedis combined are the most common cause of lower extremity cellulitis leading to hospitalization in the United States,” he continued.
The go-to treatments for onychomycosis in patients with a bad prognostic factor are oral itraconazole (Sporanox) and terbinafine. Don’t be unduly swayed by the complete cure rates reported in clinical trials and cited in the product package inserts; they don’t tell the full story because of important differences in study design, according to Dr. Rosen.
He recommended that physicians familiarize themselves with posaconazole (Noxafil) as an antifungal to consider for second-line therapy in difficult-to-cure cases of onychomycosis in immunosuppressed patients. This is off-label therapy. The approved indications for this triazole antifungal agent are prophylaxis of invasive Aspergillus and Candida infections in severely immunocompromised patients, as well as treatment of oropharyngeal candidiasis. But this is a potent agent that provides broad-spectrum coverage coupled with a favorable safety profile. It performed well in a phase IIb randomized, placebo- and active-controlled, multicenter, investigator-blinded study of 218 adults with toenail onychomycosis (Br J Dermatol. 2012 Feb;166[2]:389-98).
Dr. Rosen reported serving on scientific advisory boards for Anacor, Merz, and Valeant.
SDEF and this news organization are owned by the same parent company.
WAIKOLOA, HAWAII – Toenail onychomycosis is a common condition in the general population, but it’s three- to fourfold more prevalent in certain at risk populations where it can have serious and even life-threatening consequences, Dr. Theodore Rosen observed at the Hawaii Dermatology Seminar provided by the Global Academy for Medical Education/Skin Disease Education Foundation.
He cited a recent systematic review led by Dr. Aditya K. Gupta, professor of dermatology at the University of Toronto, whom Dr. Rosen hailed as one of the world’s great fungal disease authorities. Dr. Gupta and coworkers concluded that while the prevalence of dermatophyte toenail onychomycosis is 3.2% worldwide in the general population, it climbs to 8.8% in diabetics, 10.2% in psoriatics, 10.3% in the elderly, 11.9% in dialysis patients, 5.2% in renal transplant recipients, and 10.4% in HIV-positive individuals. The highest prevalence of onychomycosis due to non-dermatophyte molds was seen in psoriasis patients, at 2.5%, while elderly patients had the highest prevalence of onychomycosis caused by yeasts, at 6.1% (J Eur Acad Dermatol Venereol. 2015 Jun;29[6]:1039-44).
“Onychomycosis is especially important in those who are immunocompromised and immunosuppressed, for two reasons. One is that really odd organisms that aren’t Trichophyton rubrum or T. interdigitale can be involved: saprophytes like Scopulariopsis, Acremonium, Aspergillus, and Paecilomyces. And some of these saprophytes, like Fusarium, can get from the nail and nail bed into the bloodstream and can kill,” explained Dr. Rosen, professor of dermatology at Baylor College of Medicine in Houston.
“Onychomycosis, aside from the fact that it looks bad and often leads to pain, can also lead to breaks in the skin which then result in secondary bacterial infections. In fact, after motor vehicle accidents, onychomycosis and tinea pedis combined are the most common cause of lower extremity cellulitis leading to hospitalization in the United States,” he continued.
The go-to treatments for onychomycosis in patients with a bad prognostic factor are oral itraconazole (Sporanox) and terbinafine. Don’t be unduly swayed by the complete cure rates reported in clinical trials and cited in the product package inserts; they don’t tell the full story because of important differences in study design, according to Dr. Rosen.
He recommended that physicians familiarize themselves with posaconazole (Noxafil) as an antifungal to consider for second-line therapy in difficult-to-cure cases of onychomycosis in immunosuppressed patients. This is off-label therapy. The approved indications for this triazole antifungal agent are prophylaxis of invasive Aspergillus and Candida infections in severely immunocompromised patients, as well as treatment of oropharyngeal candidiasis. But this is a potent agent that provides broad-spectrum coverage coupled with a favorable safety profile. It performed well in a phase IIb randomized, placebo- and active-controlled, multicenter, investigator-blinded study of 218 adults with toenail onychomycosis (Br J Dermatol. 2012 Feb;166[2]:389-98).
Dr. Rosen reported serving on scientific advisory boards for Anacor, Merz, and Valeant.
SDEF and this news organization are owned by the same parent company.
EXPERT ANALYSIS FROM SDEF HAWAII DERMATOLOGY SEMINAR
New prediction tool forecasts long-term ICU outcomes for very elderly
ORLANDO – A new clinical prediction rule correlates well with performance status at 1 year after ICU hospitalization in patients over age 80.
Illness severity, comorbidities, baseline frailty, a primary diagnosis of stroke, and being male were all predictors of poor performance status at 1 year. A primary diagnosis of emergency coronary artery bypass grafting or valve replacement, a high baseline performance status, and being married were associated with good performance status at 1 year. The c-statistic for the model, a standard indicator of predictive power, was 0.811, a figure that indicates good predictive ability.
The findings from the REALISTIC 80 (Realities, Expectations ,and Attitudes to Life Support Technologies in Intensive Care for Octogenarians) study of 17 patient and illness characteristics allowed Dr. Daren Heyland, professor of medicine and epidemiology at Queen’s University, Kingston, Ont., and his coinvestigators in the Canadian Critical Care Trials Group (CCCTG), to conclude that eight factors were most predictive of performance status at 12 months for ICU patients aged 80 and over. REALISTIC 80 is a CCCTG project.
The values for the predictors are derived from responses to an online guided questionnaire called the ICU Workbook. The questionnaire is completed by patients’ family members or surrogates, and the responses are used to calculate the values that constitute the clinical prediction rule’s components.
Gathering this information may help health care providers and family members in end-of-life decision making, said Dr. Heyland. “For the very elderly, it is plausible that poor communication and decision making lead to overutilization of ICU resources and poor-quality end-of-life care,” he said. “Hopefully, with these strategies, we’ll improve clinical decision making and improve the quality of end-of-life care we provide for our older patients,” said Dr. Heyland, who is also director of CARENET, which hosts the online guided questionnaire and is an affiliation of Canadian researchers focused on end-of-life care. He spoke at the Society of Critical Care Medicine’s Critical Care Congress.
REALISTIC 80 enrolled 434 patients, aged 80-100 years (mean age, 84.6) who were admitted to ICUs at participating institutions.
Previous European and U.S. studies have shown an ICU mortality of 30%-35%, and an overall mortality rate of 60%-70% in the 12 months following ICU admission. In those studies, illness severity most strongly predicted short-term survival, and comorbidities best predicted long-term survival.
The primary outcome measure of REALISTIC 80 was the 12-month survival and health-related quality of life; “recovery from critical illness” was defined as a Palliative Performance Scale (PPS) score of greater than or equal to 60% at 12 months. Patients scoring at 60% on the 0%-100% scale of this functional status measure may have reduced ambulation, be unable to engage in housework or hobbies, have significant disease, need assistance, and be confused at times. An advantage of this scale, said Dr. Heyland, is that it eliminates survivorship bias in analyzing data, since a score of 0 is assigned to individuals who die.
About 50% of patients had died by 12 months; about 21% were alive, with a reduced health status below the threshold of 60 on the PPS; and about 29% were alive, with a PPS score above the predetermined quality of life threshold.
Dr. Heyland acknowledged that the study lost a significant number of participants – about 17% – to follow-up. The predictive model was derived from completed cases, and a sensitivity analysis using imputed data for missing patients showed that it retained its predictive value.
Dr. Heyland said the presence of advance directives didn’t appear to affect outcomes. “We subsequently in a different analysis showed that whether they had [a directive] or not, did not affect subsequent process or outcome of care in the ICU,” he said.
Dying in the ICU after days of mechanical ventilation or surviving with very low performance status “doesn’t sound like good quality of life to me, and it illustrates the challenge we have as clinicians in getting to what’s best for patients,” said Dr. Heyland. “Hopefully, prediction models will help, as well as better elicitation of authentic values and preferences from patients.”
The study was funded by the Canadian Institutes of Health Research and conducted under the auspices of the CCCTG and CARENET. The study investigators reported no other relevant financial disclosures.
On Twitter @karioakes
ORLANDO – A new clinical prediction rule correlates well with performance status at 1 year after ICU hospitalization in patients over age 80.
Illness severity, comorbidities, baseline frailty, a primary diagnosis of stroke, and being male were all predictors of poor performance status at 1 year. A primary diagnosis of emergency coronary artery bypass grafting or valve replacement, a high baseline performance status, and being married were associated with good performance status at 1 year. The c-statistic for the model, a standard indicator of predictive power, was 0.811, a figure that indicates good predictive ability.
The findings from the REALISTIC 80 (Realities, Expectations ,and Attitudes to Life Support Technologies in Intensive Care for Octogenarians) study of 17 patient and illness characteristics allowed Dr. Daren Heyland, professor of medicine and epidemiology at Queen’s University, Kingston, Ont., and his coinvestigators in the Canadian Critical Care Trials Group (CCCTG), to conclude that eight factors were most predictive of performance status at 12 months for ICU patients aged 80 and over. REALISTIC 80 is a CCCTG project.
The values for the predictors are derived from responses to an online guided questionnaire called the ICU Workbook. The questionnaire is completed by patients’ family members or surrogates, and the responses are used to calculate the values that constitute the clinical prediction rule’s components.
Gathering this information may help health care providers and family members in end-of-life decision making, said Dr. Heyland. “For the very elderly, it is plausible that poor communication and decision making lead to overutilization of ICU resources and poor-quality end-of-life care,” he said. “Hopefully, with these strategies, we’ll improve clinical decision making and improve the quality of end-of-life care we provide for our older patients,” said Dr. Heyland, who is also director of CARENET, which hosts the online guided questionnaire and is an affiliation of Canadian researchers focused on end-of-life care. He spoke at the Society of Critical Care Medicine’s Critical Care Congress.
REALISTIC 80 enrolled 434 patients, aged 80-100 years (mean age, 84.6) who were admitted to ICUs at participating institutions.
Previous European and U.S. studies have shown an ICU mortality of 30%-35%, and an overall mortality rate of 60%-70% in the 12 months following ICU admission. In those studies, illness severity most strongly predicted short-term survival, and comorbidities best predicted long-term survival.
The primary outcome measure of REALISTIC 80 was the 12-month survival and health-related quality of life; “recovery from critical illness” was defined as a Palliative Performance Scale (PPS) score of greater than or equal to 60% at 12 months. Patients scoring at 60% on the 0%-100% scale of this functional status measure may have reduced ambulation, be unable to engage in housework or hobbies, have significant disease, need assistance, and be confused at times. An advantage of this scale, said Dr. Heyland, is that it eliminates survivorship bias in analyzing data, since a score of 0 is assigned to individuals who die.
About 50% of patients had died by 12 months; about 21% were alive, with a reduced health status below the threshold of 60 on the PPS; and about 29% were alive, with a PPS score above the predetermined quality of life threshold.
Dr. Heyland acknowledged that the study lost a significant number of participants – about 17% – to follow-up. The predictive model was derived from completed cases, and a sensitivity analysis using imputed data for missing patients showed that it retained its predictive value.
Dr. Heyland said the presence of advance directives didn’t appear to affect outcomes. “We subsequently in a different analysis showed that whether they had [a directive] or not, did not affect subsequent process or outcome of care in the ICU,” he said.
Dying in the ICU after days of mechanical ventilation or surviving with very low performance status “doesn’t sound like good quality of life to me, and it illustrates the challenge we have as clinicians in getting to what’s best for patients,” said Dr. Heyland. “Hopefully, prediction models will help, as well as better elicitation of authentic values and preferences from patients.”
The study was funded by the Canadian Institutes of Health Research and conducted under the auspices of the CCCTG and CARENET. The study investigators reported no other relevant financial disclosures.
On Twitter @karioakes
ORLANDO – A new clinical prediction rule correlates well with performance status at 1 year after ICU hospitalization in patients over age 80.
Illness severity, comorbidities, baseline frailty, a primary diagnosis of stroke, and being male were all predictors of poor performance status at 1 year. A primary diagnosis of emergency coronary artery bypass grafting or valve replacement, a high baseline performance status, and being married were associated with good performance status at 1 year. The c-statistic for the model, a standard indicator of predictive power, was 0.811, a figure that indicates good predictive ability.
The findings from the REALISTIC 80 (Realities, Expectations ,and Attitudes to Life Support Technologies in Intensive Care for Octogenarians) study of 17 patient and illness characteristics allowed Dr. Daren Heyland, professor of medicine and epidemiology at Queen’s University, Kingston, Ont., and his coinvestigators in the Canadian Critical Care Trials Group (CCCTG), to conclude that eight factors were most predictive of performance status at 12 months for ICU patients aged 80 and over. REALISTIC 80 is a CCCTG project.
The values for the predictors are derived from responses to an online guided questionnaire called the ICU Workbook. The questionnaire is completed by patients’ family members or surrogates, and the responses are used to calculate the values that constitute the clinical prediction rule’s components.
Gathering this information may help health care providers and family members in end-of-life decision making, said Dr. Heyland. “For the very elderly, it is plausible that poor communication and decision making lead to overutilization of ICU resources and poor-quality end-of-life care,” he said. “Hopefully, with these strategies, we’ll improve clinical decision making and improve the quality of end-of-life care we provide for our older patients,” said Dr. Heyland, who is also director of CARENET, which hosts the online guided questionnaire and is an affiliation of Canadian researchers focused on end-of-life care. He spoke at the Society of Critical Care Medicine’s Critical Care Congress.
REALISTIC 80 enrolled 434 patients, aged 80-100 years (mean age, 84.6) who were admitted to ICUs at participating institutions.
Previous European and U.S. studies have shown an ICU mortality of 30%-35%, and an overall mortality rate of 60%-70% in the 12 months following ICU admission. In those studies, illness severity most strongly predicted short-term survival, and comorbidities best predicted long-term survival.
The primary outcome measure of REALISTIC 80 was the 12-month survival and health-related quality of life; “recovery from critical illness” was defined as a Palliative Performance Scale (PPS) score of greater than or equal to 60% at 12 months. Patients scoring at 60% on the 0%-100% scale of this functional status measure may have reduced ambulation, be unable to engage in housework or hobbies, have significant disease, need assistance, and be confused at times. An advantage of this scale, said Dr. Heyland, is that it eliminates survivorship bias in analyzing data, since a score of 0 is assigned to individuals who die.
About 50% of patients had died by 12 months; about 21% were alive, with a reduced health status below the threshold of 60 on the PPS; and about 29% were alive, with a PPS score above the predetermined quality of life threshold.
Dr. Heyland acknowledged that the study lost a significant number of participants – about 17% – to follow-up. The predictive model was derived from completed cases, and a sensitivity analysis using imputed data for missing patients showed that it retained its predictive value.
Dr. Heyland said the presence of advance directives didn’t appear to affect outcomes. “We subsequently in a different analysis showed that whether they had [a directive] or not, did not affect subsequent process or outcome of care in the ICU,” he said.
Dying in the ICU after days of mechanical ventilation or surviving with very low performance status “doesn’t sound like good quality of life to me, and it illustrates the challenge we have as clinicians in getting to what’s best for patients,” said Dr. Heyland. “Hopefully, prediction models will help, as well as better elicitation of authentic values and preferences from patients.”
The study was funded by the Canadian Institutes of Health Research and conducted under the auspices of the CCCTG and CARENET. The study investigators reported no other relevant financial disclosures.
On Twitter @karioakes
AT THE CRITICAL CARE CONGRESS
Key clinical point: Eight patient and illness factors were associated with long-term outcomes for ICU patients over age 80.
Major finding: Illness severity, baseline frailty, and comorbidities were associated with lower performance status at 12 months.
Data source: A multicenter study examining 17 patient and illness factors for 434 patients aged 80 and older, admitted to ICUs and followed for 12 months.
Disclosures: The study was funded by the Canadian Institutes of Health Research and conducted under the auspices of the CCCTG and CARENET. The study investigators reported no relevant financial disclosures.
Children who have stem cell transplants need skin exams, sun protection
WASHINGTON – Children who have had a hematopoietic stem cell transplant (HSCT) have an increased risk of benign and atypical nevi, Dr. Johanna Sheu reported at the annual meeting of the American Academy of Dermatology.
These patients need to have routine skin exams and be educated about sun protection needs, she said. Based on her study, these needs are not routinely met.
At least 1 year after undergoing HSCT at Boston Children’s Hospital, 85 posttransplant patients had significantly more nevi and more atypical nevi than did 85 healthy controls who were matched by age, gender, and Fitzpatrick skin type. In addition, 41% of the transplant recipients had at least one actinic keratosis, a basal or squamous cell carcinoma, or a solar lentigo; 11% had at least one nevus spilus.
Moreover, “sun protection … and dermatology follow-up was poor” among the transplant recipients, said Dr. Sheu, of MassGeneral Hospital for Children, Boston. About 40% of the transplant recipients reported having a sunburn since their transplant, only 15% reported daily use of sunscreen, and 53% said they did not recall being told that sunburn could trigger graft-versus-host disease (GVHD).
About one-third of the patients had never seen a dermatologist; of those who had, two-thirds had only seen the dermatologist once, Dr. Sheu reported.
Late skin effects of HSCT are not as well described in children as they are in adults, she said. In adults, late skin effects include vitiligo, psoriasis, nonmelanoma skin cancers, and an increased nevi count.
The children in the study had undergone an HSCT between 1998 and 2013, at a median age of about 7 years (range was 1 month to 19 years). At the time of their skin exams, their mean age was 14 years, and they had been followed for a median of almost 4 years. Nevi were counted on the forearms, backs, legs, palms, and soles.
The median nevi count was 44 nevi, significantly more than the level seen in control subjects. Transplant recipients also had significantly more nevi in sun-exposed areas of the body, as well as on the palms and soles. Transplant recipients were more likely to have atypical nevi and to have nevi greater than 5 mm in diameter.
In addition to fair skin, factors associated with an increase in the overall nevi count included being older than age 10 at the time of the transplant and having total body irradiation, pretransplant chemotherapy, and myeloablative conditioning. Having had a sunburn since the transplant, reported by 40%, was also a risk factor.
Chronic GVHD and chronic GVHD of the skin were associated with the presence of atypical nevi; acute GVHD, the duration of immune suppression, and the use of topical steroids or calcineurin inhibitors were not associated with increased risk of atypical nevi.
She and her coinvestigators are currently analyzing the pathogenesis of these late effects in this population, and autoimmune skin conditions – vitiligo and alopecia – in 25% of the transplant recipients in the study.
In 2013, 1,100 children under aged 16 years in the United States underwent a bone marrow transplant, she noted.
Dr. Sheu had no disclosures.
WASHINGTON – Children who have had a hematopoietic stem cell transplant (HSCT) have an increased risk of benign and atypical nevi, Dr. Johanna Sheu reported at the annual meeting of the American Academy of Dermatology.
These patients need to have routine skin exams and be educated about sun protection needs, she said. Based on her study, these needs are not routinely met.
At least 1 year after undergoing HSCT at Boston Children’s Hospital, 85 posttransplant patients had significantly more nevi and more atypical nevi than did 85 healthy controls who were matched by age, gender, and Fitzpatrick skin type. In addition, 41% of the transplant recipients had at least one actinic keratosis, a basal or squamous cell carcinoma, or a solar lentigo; 11% had at least one nevus spilus.
Moreover, “sun protection … and dermatology follow-up was poor” among the transplant recipients, said Dr. Sheu, of MassGeneral Hospital for Children, Boston. About 40% of the transplant recipients reported having a sunburn since their transplant, only 15% reported daily use of sunscreen, and 53% said they did not recall being told that sunburn could trigger graft-versus-host disease (GVHD).
About one-third of the patients had never seen a dermatologist; of those who had, two-thirds had only seen the dermatologist once, Dr. Sheu reported.
Late skin effects of HSCT are not as well described in children as they are in adults, she said. In adults, late skin effects include vitiligo, psoriasis, nonmelanoma skin cancers, and an increased nevi count.
The children in the study had undergone an HSCT between 1998 and 2013, at a median age of about 7 years (range was 1 month to 19 years). At the time of their skin exams, their mean age was 14 years, and they had been followed for a median of almost 4 years. Nevi were counted on the forearms, backs, legs, palms, and soles.
The median nevi count was 44 nevi, significantly more than the level seen in control subjects. Transplant recipients also had significantly more nevi in sun-exposed areas of the body, as well as on the palms and soles. Transplant recipients were more likely to have atypical nevi and to have nevi greater than 5 mm in diameter.
In addition to fair skin, factors associated with an increase in the overall nevi count included being older than age 10 at the time of the transplant and having total body irradiation, pretransplant chemotherapy, and myeloablative conditioning. Having had a sunburn since the transplant, reported by 40%, was also a risk factor.
Chronic GVHD and chronic GVHD of the skin were associated with the presence of atypical nevi; acute GVHD, the duration of immune suppression, and the use of topical steroids or calcineurin inhibitors were not associated with increased risk of atypical nevi.
She and her coinvestigators are currently analyzing the pathogenesis of these late effects in this population, and autoimmune skin conditions – vitiligo and alopecia – in 25% of the transplant recipients in the study.
In 2013, 1,100 children under aged 16 years in the United States underwent a bone marrow transplant, she noted.
Dr. Sheu had no disclosures.
WASHINGTON – Children who have had a hematopoietic stem cell transplant (HSCT) have an increased risk of benign and atypical nevi, Dr. Johanna Sheu reported at the annual meeting of the American Academy of Dermatology.
These patients need to have routine skin exams and be educated about sun protection needs, she said. Based on her study, these needs are not routinely met.
At least 1 year after undergoing HSCT at Boston Children’s Hospital, 85 posttransplant patients had significantly more nevi and more atypical nevi than did 85 healthy controls who were matched by age, gender, and Fitzpatrick skin type. In addition, 41% of the transplant recipients had at least one actinic keratosis, a basal or squamous cell carcinoma, or a solar lentigo; 11% had at least one nevus spilus.
Moreover, “sun protection … and dermatology follow-up was poor” among the transplant recipients, said Dr. Sheu, of MassGeneral Hospital for Children, Boston. About 40% of the transplant recipients reported having a sunburn since their transplant, only 15% reported daily use of sunscreen, and 53% said they did not recall being told that sunburn could trigger graft-versus-host disease (GVHD).
About one-third of the patients had never seen a dermatologist; of those who had, two-thirds had only seen the dermatologist once, Dr. Sheu reported.
Late skin effects of HSCT are not as well described in children as they are in adults, she said. In adults, late skin effects include vitiligo, psoriasis, nonmelanoma skin cancers, and an increased nevi count.
The children in the study had undergone an HSCT between 1998 and 2013, at a median age of about 7 years (range was 1 month to 19 years). At the time of their skin exams, their mean age was 14 years, and they had been followed for a median of almost 4 years. Nevi were counted on the forearms, backs, legs, palms, and soles.
The median nevi count was 44 nevi, significantly more than the level seen in control subjects. Transplant recipients also had significantly more nevi in sun-exposed areas of the body, as well as on the palms and soles. Transplant recipients were more likely to have atypical nevi and to have nevi greater than 5 mm in diameter.
In addition to fair skin, factors associated with an increase in the overall nevi count included being older than age 10 at the time of the transplant and having total body irradiation, pretransplant chemotherapy, and myeloablative conditioning. Having had a sunburn since the transplant, reported by 40%, was also a risk factor.
Chronic GVHD and chronic GVHD of the skin were associated with the presence of atypical nevi; acute GVHD, the duration of immune suppression, and the use of topical steroids or calcineurin inhibitors were not associated with increased risk of atypical nevi.
She and her coinvestigators are currently analyzing the pathogenesis of these late effects in this population, and autoimmune skin conditions – vitiligo and alopecia – in 25% of the transplant recipients in the study.
In 2013, 1,100 children under aged 16 years in the United States underwent a bone marrow transplant, she noted.
Dr. Sheu had no disclosures.
AT AAD 16
Key clinical point: Children who have had a hematopoietic stem cell transplant need to have routine skin exams and be educated about sun protection needs.
Major finding: 41% of the transplant recipients had at least one actinic keratosis, a basal or squamous cell carcinoma, or a solar lentigo; 11% had at least one nevus spilus.
Data source: A single-center study of 85 posttransplant patients and 85 healthy controls who were matched by age, gender, and Fitzpatrick skin type.
Disclosures: The study was not sponsored and Dr. Sheu had no disclosures.
New drug comparable to voriconazole for aspergillosis
The broad-spectrum triazole isavuconazole was as effective as voriconazole in patients with suspected invasive mold disease and caused significantly fewer drug-related adverse events, particularly those of the skin, eyes, and hepatobiliary system, a randomized double-blind study of 516 adults has shown.
The findings suggest that the newer agent “could allow safer therapy” for the primary treatment of invasive aspergillosis and other mold disease than standard therapy with voriconazole, researchers for the phase III, industry-sponsored SECURE trial say in a report published in the Lancet.
The researchers assessed the safety and efficacy of isavuconazole versus voriconazole in patients with invasive mold infection. Patients were recruited from 102 centers across 26 countries over a 7-year period and were randomized to receive either drug.
In the study group of 516 adults with suspected invasive mold infection who received at least one dose of either antifungal drug, isavuconazole proved to be noninferior to voriconazole, by the primary endpoint of all-cause mortality at 6 weeks.
All-cause mortality at 6 weeks in this intention-to-treat group, of whom more than 80% had hematologic malignant disease, was 19% in the isavuconazole group (48 of 258) and 20% (52 of 258) in the voriconazole group.
This primary endpoint was chosen because “it provides the most objective and reproducible effect of therapy, and approximates best the attributable mortality, because deaths due to competing causes occur increasingly after 6 weeks,” Dr. Johan A. Maertensof the UZ Leuven (Belgium), and his associates wrote.
Secondary endpoints included overall response at the end of treatment among patients who were determined by an independent review committee to have proven or probable invasive mold disease – the study’s modified intention-to-treat population – as well as all-cause mortality at day 42 and day 84.
All-cause mortality in this modified intention-to-treat group, as well as in the group of patients found to have proven or probable invasive aspergillosis, specifically, supported the study’s primary findings (Lancet 2016 Feb:387:760-9).
Nearly all patients in the study had at least one treatment-emergent adverse event, and the proportion with serious treatment-emergent adverse events was similar between the treatment groups. However, patients treated with isavuconazole had a significantly lower frequency of hepatobiliary disorders, eye disorders, and skin or subcutaneous disorders.
And overall, significantly fewer patients reported drug-related adverse events with isavuconazole (42% of patients) than with voriconazole (60% of patients). Discontinuation from adverse events, moreover, was significantly less common among isavuconazole-treated patients.
Of the 516 patients in the intention-to-treat group, approximately 53% were confirmed to have proven or probable invasive mold disease, and more than 80% of the mycologically documented cases were Aspergillus infections. Enrollment of patients with possible invasive mold disease at the start “reflects the real-life strategy of early initiation of antifungal treatment,” the investigators say.
Isavuconazonium sulfate was approved in 2015 by the FDA for the treatment of invasive aspergillosis and invasive mucormycosis.
Voriconazole is the current gold standard for the primary treatment of invasive aspergillosis and is recommended for some other mold infections as well, but it is not active against mucormycosis and has “highly variable nonlinear pharmacokinetics in adults,” which has triggered recommendations for drug monitoring, Dr. Maertens and his associates say.
Therapeutic monitoring aimed at individualizing dosage regimes in order to improve response and prevent adverse events became the standard of care in some institutions during the study period (2007-2013). The study used the labeled dose of voriconazole, however, and did not address the efficacy of either drug with therapeutic drug monitoring.
The study also excluded patients with AIDS, abnormal liver or renal function, and those receiving antifungal prophylaxis with a mold-active triazole – factors that may limit generalizability of the findings, the investigators note.
Funding for the study was provided by Astellas Pharma Global Development and Basilea Pharmaceutica International.
Dr. Maertens disclosed receiving grants and fees from Bio-Rad, personal fees and nonfinancial support from Astellas and Basilea, and grants, fees and support from Gilead Sciences, Merck Sharp and Dohme, and Pfizer during the study.
The advantages of isavuconazole over voriconazole include its broader spectrum of activity, linear pharmacokinetics, once-daily dosing after the loading dose, and fewer CYP enzyme-mediated drug-drug interactions. This trial represents important progress in widening the therapeutic options for mold infections.
Numerous issues require further evaluation, including the effectiveness of isavuconazole after mold-active triazole prophylaxis, which is a common practice in patients at risk for mold infection, and the agent’s effectiveness against molds other than Aspergillus.
In addition, experience in a more varied patient population will be required to be certain that therapeutic drug monitoring is unnecessary.
Cost-effectiveness must also be explored. Isavuconazole will probably achieve an equivalent recommendation as voriconazole for initial treatment of aspergillosis in clinical guidelines, but voriconazole will soon come off patent in many countries and new formulations of posaconazole are now available.
That the finding that 42-day mortality in both treatment groups (isavuconazole and voriconazole) was no different than the mortality seen in research done 15 years ago on voriconazole treatment of aspergillosis is disappointing and suggests that we need to do better with the prevention and early detection of mold infection in vulnerable patients.
Dr. Monica A. Slavin and Dr. Karin A. Thursky are affiliated with the Peter MacCallum Cancer Centre, East Melbourne, Australia. Their comments are excerpted from an accompanying editorial in the Lancet. Dr. Slavin reported receiving grants from Merck, Gilead, and Pfizer. Dr. Thursky reported no disclosures.
The advantages of isavuconazole over voriconazole include its broader spectrum of activity, linear pharmacokinetics, once-daily dosing after the loading dose, and fewer CYP enzyme-mediated drug-drug interactions. This trial represents important progress in widening the therapeutic options for mold infections.
Numerous issues require further evaluation, including the effectiveness of isavuconazole after mold-active triazole prophylaxis, which is a common practice in patients at risk for mold infection, and the agent’s effectiveness against molds other than Aspergillus.
In addition, experience in a more varied patient population will be required to be certain that therapeutic drug monitoring is unnecessary.
Cost-effectiveness must also be explored. Isavuconazole will probably achieve an equivalent recommendation as voriconazole for initial treatment of aspergillosis in clinical guidelines, but voriconazole will soon come off patent in many countries and new formulations of posaconazole are now available.
That the finding that 42-day mortality in both treatment groups (isavuconazole and voriconazole) was no different than the mortality seen in research done 15 years ago on voriconazole treatment of aspergillosis is disappointing and suggests that we need to do better with the prevention and early detection of mold infection in vulnerable patients.
Dr. Monica A. Slavin and Dr. Karin A. Thursky are affiliated with the Peter MacCallum Cancer Centre, East Melbourne, Australia. Their comments are excerpted from an accompanying editorial in the Lancet. Dr. Slavin reported receiving grants from Merck, Gilead, and Pfizer. Dr. Thursky reported no disclosures.
The advantages of isavuconazole over voriconazole include its broader spectrum of activity, linear pharmacokinetics, once-daily dosing after the loading dose, and fewer CYP enzyme-mediated drug-drug interactions. This trial represents important progress in widening the therapeutic options for mold infections.
Numerous issues require further evaluation, including the effectiveness of isavuconazole after mold-active triazole prophylaxis, which is a common practice in patients at risk for mold infection, and the agent’s effectiveness against molds other than Aspergillus.
In addition, experience in a more varied patient population will be required to be certain that therapeutic drug monitoring is unnecessary.
Cost-effectiveness must also be explored. Isavuconazole will probably achieve an equivalent recommendation as voriconazole for initial treatment of aspergillosis in clinical guidelines, but voriconazole will soon come off patent in many countries and new formulations of posaconazole are now available.
That the finding that 42-day mortality in both treatment groups (isavuconazole and voriconazole) was no different than the mortality seen in research done 15 years ago on voriconazole treatment of aspergillosis is disappointing and suggests that we need to do better with the prevention and early detection of mold infection in vulnerable patients.
Dr. Monica A. Slavin and Dr. Karin A. Thursky are affiliated with the Peter MacCallum Cancer Centre, East Melbourne, Australia. Their comments are excerpted from an accompanying editorial in the Lancet. Dr. Slavin reported receiving grants from Merck, Gilead, and Pfizer. Dr. Thursky reported no disclosures.
The broad-spectrum triazole isavuconazole was as effective as voriconazole in patients with suspected invasive mold disease and caused significantly fewer drug-related adverse events, particularly those of the skin, eyes, and hepatobiliary system, a randomized double-blind study of 516 adults has shown.
The findings suggest that the newer agent “could allow safer therapy” for the primary treatment of invasive aspergillosis and other mold disease than standard therapy with voriconazole, researchers for the phase III, industry-sponsored SECURE trial say in a report published in the Lancet.
The researchers assessed the safety and efficacy of isavuconazole versus voriconazole in patients with invasive mold infection. Patients were recruited from 102 centers across 26 countries over a 7-year period and were randomized to receive either drug.
In the study group of 516 adults with suspected invasive mold infection who received at least one dose of either antifungal drug, isavuconazole proved to be noninferior to voriconazole, by the primary endpoint of all-cause mortality at 6 weeks.
All-cause mortality at 6 weeks in this intention-to-treat group, of whom more than 80% had hematologic malignant disease, was 19% in the isavuconazole group (48 of 258) and 20% (52 of 258) in the voriconazole group.
This primary endpoint was chosen because “it provides the most objective and reproducible effect of therapy, and approximates best the attributable mortality, because deaths due to competing causes occur increasingly after 6 weeks,” Dr. Johan A. Maertensof the UZ Leuven (Belgium), and his associates wrote.
Secondary endpoints included overall response at the end of treatment among patients who were determined by an independent review committee to have proven or probable invasive mold disease – the study’s modified intention-to-treat population – as well as all-cause mortality at day 42 and day 84.
All-cause mortality in this modified intention-to-treat group, as well as in the group of patients found to have proven or probable invasive aspergillosis, specifically, supported the study’s primary findings (Lancet 2016 Feb:387:760-9).
Nearly all patients in the study had at least one treatment-emergent adverse event, and the proportion with serious treatment-emergent adverse events was similar between the treatment groups. However, patients treated with isavuconazole had a significantly lower frequency of hepatobiliary disorders, eye disorders, and skin or subcutaneous disorders.
And overall, significantly fewer patients reported drug-related adverse events with isavuconazole (42% of patients) than with voriconazole (60% of patients). Discontinuation from adverse events, moreover, was significantly less common among isavuconazole-treated patients.
Of the 516 patients in the intention-to-treat group, approximately 53% were confirmed to have proven or probable invasive mold disease, and more than 80% of the mycologically documented cases were Aspergillus infections. Enrollment of patients with possible invasive mold disease at the start “reflects the real-life strategy of early initiation of antifungal treatment,” the investigators say.
Isavuconazonium sulfate was approved in 2015 by the FDA for the treatment of invasive aspergillosis and invasive mucormycosis.
Voriconazole is the current gold standard for the primary treatment of invasive aspergillosis and is recommended for some other mold infections as well, but it is not active against mucormycosis and has “highly variable nonlinear pharmacokinetics in adults,” which has triggered recommendations for drug monitoring, Dr. Maertens and his associates say.
Therapeutic monitoring aimed at individualizing dosage regimes in order to improve response and prevent adverse events became the standard of care in some institutions during the study period (2007-2013). The study used the labeled dose of voriconazole, however, and did not address the efficacy of either drug with therapeutic drug monitoring.
The study also excluded patients with AIDS, abnormal liver or renal function, and those receiving antifungal prophylaxis with a mold-active triazole – factors that may limit generalizability of the findings, the investigators note.
Funding for the study was provided by Astellas Pharma Global Development and Basilea Pharmaceutica International.
Dr. Maertens disclosed receiving grants and fees from Bio-Rad, personal fees and nonfinancial support from Astellas and Basilea, and grants, fees and support from Gilead Sciences, Merck Sharp and Dohme, and Pfizer during the study.
The broad-spectrum triazole isavuconazole was as effective as voriconazole in patients with suspected invasive mold disease and caused significantly fewer drug-related adverse events, particularly those of the skin, eyes, and hepatobiliary system, a randomized double-blind study of 516 adults has shown.
The findings suggest that the newer agent “could allow safer therapy” for the primary treatment of invasive aspergillosis and other mold disease than standard therapy with voriconazole, researchers for the phase III, industry-sponsored SECURE trial say in a report published in the Lancet.
The researchers assessed the safety and efficacy of isavuconazole versus voriconazole in patients with invasive mold infection. Patients were recruited from 102 centers across 26 countries over a 7-year period and were randomized to receive either drug.
In the study group of 516 adults with suspected invasive mold infection who received at least one dose of either antifungal drug, isavuconazole proved to be noninferior to voriconazole, by the primary endpoint of all-cause mortality at 6 weeks.
All-cause mortality at 6 weeks in this intention-to-treat group, of whom more than 80% had hematologic malignant disease, was 19% in the isavuconazole group (48 of 258) and 20% (52 of 258) in the voriconazole group.
This primary endpoint was chosen because “it provides the most objective and reproducible effect of therapy, and approximates best the attributable mortality, because deaths due to competing causes occur increasingly after 6 weeks,” Dr. Johan A. Maertensof the UZ Leuven (Belgium), and his associates wrote.
Secondary endpoints included overall response at the end of treatment among patients who were determined by an independent review committee to have proven or probable invasive mold disease – the study’s modified intention-to-treat population – as well as all-cause mortality at day 42 and day 84.
All-cause mortality in this modified intention-to-treat group, as well as in the group of patients found to have proven or probable invasive aspergillosis, specifically, supported the study’s primary findings (Lancet 2016 Feb:387:760-9).
Nearly all patients in the study had at least one treatment-emergent adverse event, and the proportion with serious treatment-emergent adverse events was similar between the treatment groups. However, patients treated with isavuconazole had a significantly lower frequency of hepatobiliary disorders, eye disorders, and skin or subcutaneous disorders.
And overall, significantly fewer patients reported drug-related adverse events with isavuconazole (42% of patients) than with voriconazole (60% of patients). Discontinuation from adverse events, moreover, was significantly less common among isavuconazole-treated patients.
Of the 516 patients in the intention-to-treat group, approximately 53% were confirmed to have proven or probable invasive mold disease, and more than 80% of the mycologically documented cases were Aspergillus infections. Enrollment of patients with possible invasive mold disease at the start “reflects the real-life strategy of early initiation of antifungal treatment,” the investigators say.
Isavuconazonium sulfate was approved in 2015 by the FDA for the treatment of invasive aspergillosis and invasive mucormycosis.
Voriconazole is the current gold standard for the primary treatment of invasive aspergillosis and is recommended for some other mold infections as well, but it is not active against mucormycosis and has “highly variable nonlinear pharmacokinetics in adults,” which has triggered recommendations for drug monitoring, Dr. Maertens and his associates say.
Therapeutic monitoring aimed at individualizing dosage regimes in order to improve response and prevent adverse events became the standard of care in some institutions during the study period (2007-2013). The study used the labeled dose of voriconazole, however, and did not address the efficacy of either drug with therapeutic drug monitoring.
The study also excluded patients with AIDS, abnormal liver or renal function, and those receiving antifungal prophylaxis with a mold-active triazole – factors that may limit generalizability of the findings, the investigators note.
Funding for the study was provided by Astellas Pharma Global Development and Basilea Pharmaceutica International.
Dr. Maertens disclosed receiving grants and fees from Bio-Rad, personal fees and nonfinancial support from Astellas and Basilea, and grants, fees and support from Gilead Sciences, Merck Sharp and Dohme, and Pfizer during the study.
FROM THE LANCET
Key clinical point: Isavuconazole is an appropriate alternative for primary treatment of suspected invasive aspergillosis.
Major finding: All-cause mortality at 6 weeks in the intention-to-treat group of 516 patients was 19% with isavuconazole and 20% with voriconazole. Fewer drug-related adverse events were reported with isavuconazole, however (42% vs. 60% of patients).
Data source: A phase III randomized, double-blind noninferiority trial – the SECURE trial – comparing the safety and efficacy of intravenous and oral formulations of isavuconazole and voriconazole for the primary treatment of invasive aspergillosis and disease caused by other molds.
Disclosures: Funding for the study was provided by Astellas Pharma Global Development and Basilea Pharmaceutica International. Dr. Maertens disclosed receiving grants and fees from Bio-Rad, personal fees and nonfinancial support from Astellas and Basilea, and grants, fees, and support from Gilead Sciences, Merck Sharp and Dohme, and Pfizer, during the study.
Radiotherapy for prostate cancer linked to higher risk of secondary malignancies
Radiotherapy for prostate cancer may result in an increased risk of secondary malignancies of the bladder, colon, and rectum, according to findings of a systematic review and meta-analysis.
The unadjusted odds ratios for secondary bladder, colorectal, and rectal cancers in patients exposed to radiotherapy, compared with those not exposed, were 1.39 (95% CI, 1.12-1.71), 1.68 (1.33-2.12), and 1.62 (1.26-2.08), respectively. Differences in absolute risks for cases and controls were low, ranging from –0.9 to 1.9 cancers per 100 patients, and arose from variation in type of radiotherapy, comparator group, and lag time.
The findings, based on a relatively small number of studies with limited adjustments made for confounders, point to the need for future studies, according to the investigators. They described the implications of the findings for making treatment decisions with patients.
“In particular, for patients with a long life expectancy of 20 years or more, the possibility of secondary malignancy related to radiation needs to be included in management discussions. We acknowledge, however, that further studies are required before conclusive implication of the association between radiotherapy and secondary malignancy in these patients,” wrote Dr. Christopher J.D. Wallis of the University of Toronto and his colleagues (BMJ 2016 March 2. doi: 10.1136/bmj.i851).
Evidence suggests that irradiation of the prostate may contribute to carcinogenesis outside the irradiated area by radiation scatter, as well as the bystander effect, described as changes in gene expression due to an increase in reactive oxygen species. The researchers examined the association between radiotherapy and secondary malignancies of the bladder, colorectal tract, lung, and hematologic systems, and found no consistent evidence for associations with lung and hematologic cancers.
The systematic review and meta-analysis involved 9 studies (n = 555,873) for bladder cancer, 10 (n = 228,965) for colorectal cancer, and 8 (n = 157,239) for rectal cancer. Of the 21 reports total, 18 were multi-institutional and 3 were single-center studies. The lag period before outcome determination varied considerably, as did the comparator groups, with 13 studies (62%) designating patients treated with surgery as comparators, and 8 studies designating patients with no radiation or no radiation and no surgery as controls.
The results were similar when the analysis was restricted to studies with 5-year or 10-year lag periods. Notably, for bladder and rectal cancers, risk increased with longer lag time: from 1.3 to 1.89 for a 5-year and 10-year period, respectively, for bladder cancer and from 1.68 to 2.2 for rectal cancer.
Risks associated with brachytherapy were lower than for external beam radiotherapy.
The comprehensive systematic review and meta-analysis by Wallis et al. suggests an increased risk of bladder (odds ratio, 1.39), rectal (OR, 1.62), and colorectal (OR, 1.68) cancers subsequent to radiotherapy for prostate cancer. Despite the increased relative risks, absolute risk remains small, and discovered cancers, while still requiring treatment, might not be lethal. Indeed, there appears to be no survival difference between men with bladder cancers linked to previous prostate cancer treated with radiation vs. prostate cancer treated with surgery.

|
Dr. Anthony L. Zietman |
The current study found that brachytherapy (high radiation dose to a small volume of tissue) was not associated with an increased risk for secondary malignancies, which supports the move toward even more tightly targeted external radiation techniques.
As for real world implications of the findings, the observed increase in risk may have a bigger influence in treatment decisions for young patients with few comorbidities than it would for older patients or those with competing health risks.
“This study confirms our belief that second malignancy should be added to the already long list of avoidable hazards associated with treatment for those men with low-risk prostate cancer who simply need no treatment at all. Concern about second malignancies should not, however, stand in the way of an effective and well-studied treatment being given to men with higher-grade, lethal prostate cancer for whom the potential benefit simply dwarfs the risk,” wrote Dr. Christine E. Eyler and Dr. Anthony L. Zietman.
Dr. Eyler is a clinical fellow in radiation oncology at Massachusetts General Hospital, Boston. Dr. Zietman is professor of radiation oncology at Massachusetts General Hospital. These remarks were part of an editorial accompanying a report in the British Medical Journal (2016 March 2. doi: 10.1136/bmj.i1073).
The comprehensive systematic review and meta-analysis by Wallis et al. suggests an increased risk of bladder (odds ratio, 1.39), rectal (OR, 1.62), and colorectal (OR, 1.68) cancers subsequent to radiotherapy for prostate cancer. Despite the increased relative risks, absolute risk remains small, and discovered cancers, while still requiring treatment, might not be lethal. Indeed, there appears to be no survival difference between men with bladder cancers linked to previous prostate cancer treated with radiation vs. prostate cancer treated with surgery.

|
Dr. Anthony L. Zietman |
The current study found that brachytherapy (high radiation dose to a small volume of tissue) was not associated with an increased risk for secondary malignancies, which supports the move toward even more tightly targeted external radiation techniques.
As for real world implications of the findings, the observed increase in risk may have a bigger influence in treatment decisions for young patients with few comorbidities than it would for older patients or those with competing health risks.
“This study confirms our belief that second malignancy should be added to the already long list of avoidable hazards associated with treatment for those men with low-risk prostate cancer who simply need no treatment at all. Concern about second malignancies should not, however, stand in the way of an effective and well-studied treatment being given to men with higher-grade, lethal prostate cancer for whom the potential benefit simply dwarfs the risk,” wrote Dr. Christine E. Eyler and Dr. Anthony L. Zietman.
Dr. Eyler is a clinical fellow in radiation oncology at Massachusetts General Hospital, Boston. Dr. Zietman is professor of radiation oncology at Massachusetts General Hospital. These remarks were part of an editorial accompanying a report in the British Medical Journal (2016 March 2. doi: 10.1136/bmj.i1073).
The comprehensive systematic review and meta-analysis by Wallis et al. suggests an increased risk of bladder (odds ratio, 1.39), rectal (OR, 1.62), and colorectal (OR, 1.68) cancers subsequent to radiotherapy for prostate cancer. Despite the increased relative risks, absolute risk remains small, and discovered cancers, while still requiring treatment, might not be lethal. Indeed, there appears to be no survival difference between men with bladder cancers linked to previous prostate cancer treated with radiation vs. prostate cancer treated with surgery.

|
Dr. Anthony L. Zietman |
The current study found that brachytherapy (high radiation dose to a small volume of tissue) was not associated with an increased risk for secondary malignancies, which supports the move toward even more tightly targeted external radiation techniques.
As for real world implications of the findings, the observed increase in risk may have a bigger influence in treatment decisions for young patients with few comorbidities than it would for older patients or those with competing health risks.
“This study confirms our belief that second malignancy should be added to the already long list of avoidable hazards associated with treatment for those men with low-risk prostate cancer who simply need no treatment at all. Concern about second malignancies should not, however, stand in the way of an effective and well-studied treatment being given to men with higher-grade, lethal prostate cancer for whom the potential benefit simply dwarfs the risk,” wrote Dr. Christine E. Eyler and Dr. Anthony L. Zietman.
Dr. Eyler is a clinical fellow in radiation oncology at Massachusetts General Hospital, Boston. Dr. Zietman is professor of radiation oncology at Massachusetts General Hospital. These remarks were part of an editorial accompanying a report in the British Medical Journal (2016 March 2. doi: 10.1136/bmj.i1073).
Radiotherapy for prostate cancer may result in an increased risk of secondary malignancies of the bladder, colon, and rectum, according to findings of a systematic review and meta-analysis.
The unadjusted odds ratios for secondary bladder, colorectal, and rectal cancers in patients exposed to radiotherapy, compared with those not exposed, were 1.39 (95% CI, 1.12-1.71), 1.68 (1.33-2.12), and 1.62 (1.26-2.08), respectively. Differences in absolute risks for cases and controls were low, ranging from –0.9 to 1.9 cancers per 100 patients, and arose from variation in type of radiotherapy, comparator group, and lag time.
The findings, based on a relatively small number of studies with limited adjustments made for confounders, point to the need for future studies, according to the investigators. They described the implications of the findings for making treatment decisions with patients.
“In particular, for patients with a long life expectancy of 20 years or more, the possibility of secondary malignancy related to radiation needs to be included in management discussions. We acknowledge, however, that further studies are required before conclusive implication of the association between radiotherapy and secondary malignancy in these patients,” wrote Dr. Christopher J.D. Wallis of the University of Toronto and his colleagues (BMJ 2016 March 2. doi: 10.1136/bmj.i851).
Evidence suggests that irradiation of the prostate may contribute to carcinogenesis outside the irradiated area by radiation scatter, as well as the bystander effect, described as changes in gene expression due to an increase in reactive oxygen species. The researchers examined the association between radiotherapy and secondary malignancies of the bladder, colorectal tract, lung, and hematologic systems, and found no consistent evidence for associations with lung and hematologic cancers.
The systematic review and meta-analysis involved 9 studies (n = 555,873) for bladder cancer, 10 (n = 228,965) for colorectal cancer, and 8 (n = 157,239) for rectal cancer. Of the 21 reports total, 18 were multi-institutional and 3 were single-center studies. The lag period before outcome determination varied considerably, as did the comparator groups, with 13 studies (62%) designating patients treated with surgery as comparators, and 8 studies designating patients with no radiation or no radiation and no surgery as controls.
The results were similar when the analysis was restricted to studies with 5-year or 10-year lag periods. Notably, for bladder and rectal cancers, risk increased with longer lag time: from 1.3 to 1.89 for a 5-year and 10-year period, respectively, for bladder cancer and from 1.68 to 2.2 for rectal cancer.
Risks associated with brachytherapy were lower than for external beam radiotherapy.
Radiotherapy for prostate cancer may result in an increased risk of secondary malignancies of the bladder, colon, and rectum, according to findings of a systematic review and meta-analysis.
The unadjusted odds ratios for secondary bladder, colorectal, and rectal cancers in patients exposed to radiotherapy, compared with those not exposed, were 1.39 (95% CI, 1.12-1.71), 1.68 (1.33-2.12), and 1.62 (1.26-2.08), respectively. Differences in absolute risks for cases and controls were low, ranging from –0.9 to 1.9 cancers per 100 patients, and arose from variation in type of radiotherapy, comparator group, and lag time.
The findings, based on a relatively small number of studies with limited adjustments made for confounders, point to the need for future studies, according to the investigators. They described the implications of the findings for making treatment decisions with patients.
“In particular, for patients with a long life expectancy of 20 years or more, the possibility of secondary malignancy related to radiation needs to be included in management discussions. We acknowledge, however, that further studies are required before conclusive implication of the association between radiotherapy and secondary malignancy in these patients,” wrote Dr. Christopher J.D. Wallis of the University of Toronto and his colleagues (BMJ 2016 March 2. doi: 10.1136/bmj.i851).
Evidence suggests that irradiation of the prostate may contribute to carcinogenesis outside the irradiated area by radiation scatter, as well as the bystander effect, described as changes in gene expression due to an increase in reactive oxygen species. The researchers examined the association between radiotherapy and secondary malignancies of the bladder, colorectal tract, lung, and hematologic systems, and found no consistent evidence for associations with lung and hematologic cancers.
The systematic review and meta-analysis involved 9 studies (n = 555,873) for bladder cancer, 10 (n = 228,965) for colorectal cancer, and 8 (n = 157,239) for rectal cancer. Of the 21 reports total, 18 were multi-institutional and 3 were single-center studies. The lag period before outcome determination varied considerably, as did the comparator groups, with 13 studies (62%) designating patients treated with surgery as comparators, and 8 studies designating patients with no radiation or no radiation and no surgery as controls.
The results were similar when the analysis was restricted to studies with 5-year or 10-year lag periods. Notably, for bladder and rectal cancers, risk increased with longer lag time: from 1.3 to 1.89 for a 5-year and 10-year period, respectively, for bladder cancer and from 1.68 to 2.2 for rectal cancer.
Risks associated with brachytherapy were lower than for external beam radiotherapy.
FROM BRITISH MEDICAL JOURNAL
Key clinical point: Radiotherapy for prostate cancer was associated with a higher risk of secondary malignancies of the bladder, colon, and rectum.
Major finding: The unadjusted odds ratios for secondary bladder, colorectal, and rectal cancers in patients exposed to radiotherapy, compared with those not exposed, were 1.39 (95% CI, 1.12-1.71), 1.68 (1.33-2.12), and 1.62 (1.26-2.08), respectively.
Data sources: The systematic review and meta-analysis of 21 reports involved 9 studies (n = 555,873) for bladder cancer, 10 (n = 228,965) for colorectal cancer, and 8 (n = 157,239) for rectal cancer.
Disclosures: Dr. Wallis and coauthors reported having no relevant financial disclosures.
Children’s cancer survival steadily increasing
The 5-year cancer survival rate for children younger than 15 years old is up by 43% since 1975, according to investigators from the American Cancer Society.
The 5-year survival rate for all cancers showed a statistically significant rise from 58% in 1975 to 83% in 2011, said Rebecca L. Siegel and her associates at the ACS (CA Cancer J Clin. 2016 Jan;66[1]:7-30).
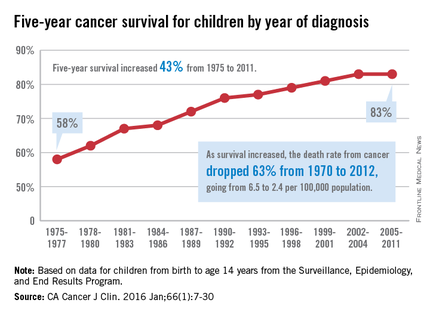
“The substantial progress for all of the major childhood cancers reflects both improvements in treatment and high levels of participation in clinical trials,” they wrote.
Survival for cancers of the brain and nervous system – now the leading cause of cancer death for those younger than 20 years old – increased from 57% in 1975 to 74% in 2011. The next-most-common cause of cancer death in children and adolescents is leukemia, and 5-year survival for acute myeloid leukemia went from 19% in 1975 to 67% in 2011, while 5-year survival for acute lymphocytic leukemia rose from 57% to 91% over that time period, the investigators reported.
The authors reported no conflicts of interest.
The 5-year cancer survival rate for children younger than 15 years old is up by 43% since 1975, according to investigators from the American Cancer Society.
The 5-year survival rate for all cancers showed a statistically significant rise from 58% in 1975 to 83% in 2011, said Rebecca L. Siegel and her associates at the ACS (CA Cancer J Clin. 2016 Jan;66[1]:7-30).

“The substantial progress for all of the major childhood cancers reflects both improvements in treatment and high levels of participation in clinical trials,” they wrote.
Survival for cancers of the brain and nervous system – now the leading cause of cancer death for those younger than 20 years old – increased from 57% in 1975 to 74% in 2011. The next-most-common cause of cancer death in children and adolescents is leukemia, and 5-year survival for acute myeloid leukemia went from 19% in 1975 to 67% in 2011, while 5-year survival for acute lymphocytic leukemia rose from 57% to 91% over that time period, the investigators reported.
The authors reported no conflicts of interest.
The 5-year cancer survival rate for children younger than 15 years old is up by 43% since 1975, according to investigators from the American Cancer Society.
The 5-year survival rate for all cancers showed a statistically significant rise from 58% in 1975 to 83% in 2011, said Rebecca L. Siegel and her associates at the ACS (CA Cancer J Clin. 2016 Jan;66[1]:7-30).

“The substantial progress for all of the major childhood cancers reflects both improvements in treatment and high levels of participation in clinical trials,” they wrote.
Survival for cancers of the brain and nervous system – now the leading cause of cancer death for those younger than 20 years old – increased from 57% in 1975 to 74% in 2011. The next-most-common cause of cancer death in children and adolescents is leukemia, and 5-year survival for acute myeloid leukemia went from 19% in 1975 to 67% in 2011, while 5-year survival for acute lymphocytic leukemia rose from 57% to 91% over that time period, the investigators reported.
The authors reported no conflicts of interest.
FROM CA: A CANCER JOURNAL FOR CLINICIANS
Prognostic value of complete remission with superior platelet counts in acute myeloid leukemia
Background Complete remission (CR) in acute myeloid leukemia (AML) is defined as having ≤5% leukemic blast cells in the bone marrow and return of normal hematopoiesis after the first induction cycle. There is a subset of patients, however, who achieve reduction of leukemic blast cells with a subnormal platelet count, designated as CR with incomplete platelet recovery (platelet count, ≤100,000/mcL; normal, 150,000-450,000/mcL), which is associated with inferior outcomes when compared with CR. Furthermore, there is another subset of patients with CR but superior platelet counts (≥400,000/mcL) whose prognostic significance is unclear.
Objective To establish whether CR with superior platelet counts is associated with better outcomes and can be used as a separate entity for prognostication.
Methods A retrospective chart review of 104 cases of AML was conducted. The highest platelet count during days 25-35 from initiation of induction chemotherapy (designated as day 30 platelet count) was documented. A multivariate analysis for other factors such as age, sex, risk categories, day 14+ plasma cell count (average plasma cell percentage at days 14-21), infections, allogeneic bone marrow transplant, and remission status was done.
Results Day 30 platelet count was found to be an independent predictor of survival in AML. On the multivariate analysis, the subgroup with superior platelet counts (≥400,000/mcL) was found to be associated with better outcomes.
Limitations Results need to be validated in a larger cohort.
Conclusions CR with superior platelet recovery (≥400,000/mcL) is a unique subcategory in itself and has prognostic significance. This may help better assess response to chemotherapeutic agents and aid in further decision-making regarding treatment.
Click on the PDF icon at the top of this introduction to read the full article.
Background Complete remission (CR) in acute myeloid leukemia (AML) is defined as having ≤5% leukemic blast cells in the bone marrow and return of normal hematopoiesis after the first induction cycle. There is a subset of patients, however, who achieve reduction of leukemic blast cells with a subnormal platelet count, designated as CR with incomplete platelet recovery (platelet count, ≤100,000/mcL; normal, 150,000-450,000/mcL), which is associated with inferior outcomes when compared with CR. Furthermore, there is another subset of patients with CR but superior platelet counts (≥400,000/mcL) whose prognostic significance is unclear.
Objective To establish whether CR with superior platelet counts is associated with better outcomes and can be used as a separate entity for prognostication.
Methods A retrospective chart review of 104 cases of AML was conducted. The highest platelet count during days 25-35 from initiation of induction chemotherapy (designated as day 30 platelet count) was documented. A multivariate analysis for other factors such as age, sex, risk categories, day 14+ plasma cell count (average plasma cell percentage at days 14-21), infections, allogeneic bone marrow transplant, and remission status was done.
Results Day 30 platelet count was found to be an independent predictor of survival in AML. On the multivariate analysis, the subgroup with superior platelet counts (≥400,000/mcL) was found to be associated with better outcomes.
Limitations Results need to be validated in a larger cohort.
Conclusions CR with superior platelet recovery (≥400,000/mcL) is a unique subcategory in itself and has prognostic significance. This may help better assess response to chemotherapeutic agents and aid in further decision-making regarding treatment.
Click on the PDF icon at the top of this introduction to read the full article.
Background Complete remission (CR) in acute myeloid leukemia (AML) is defined as having ≤5% leukemic blast cells in the bone marrow and return of normal hematopoiesis after the first induction cycle. There is a subset of patients, however, who achieve reduction of leukemic blast cells with a subnormal platelet count, designated as CR with incomplete platelet recovery (platelet count, ≤100,000/mcL; normal, 150,000-450,000/mcL), which is associated with inferior outcomes when compared with CR. Furthermore, there is another subset of patients with CR but superior platelet counts (≥400,000/mcL) whose prognostic significance is unclear.
Objective To establish whether CR with superior platelet counts is associated with better outcomes and can be used as a separate entity for prognostication.
Methods A retrospective chart review of 104 cases of AML was conducted. The highest platelet count during days 25-35 from initiation of induction chemotherapy (designated as day 30 platelet count) was documented. A multivariate analysis for other factors such as age, sex, risk categories, day 14+ plasma cell count (average plasma cell percentage at days 14-21), infections, allogeneic bone marrow transplant, and remission status was done.
Results Day 30 platelet count was found to be an independent predictor of survival in AML. On the multivariate analysis, the subgroup with superior platelet counts (≥400,000/mcL) was found to be associated with better outcomes.
Limitations Results need to be validated in a larger cohort.
Conclusions CR with superior platelet recovery (≥400,000/mcL) is a unique subcategory in itself and has prognostic significance. This may help better assess response to chemotherapeutic agents and aid in further decision-making regarding treatment.
Click on the PDF icon at the top of this introduction to read the full article.
Fluoroquinolone-related neuropsychiatric and mitochondrial toxicity: a collaborative investigation by scientists and members of a social network
Background The 3 fluoroquinolone (FQ) antibiotics – ciprofoxacin, levofoxacin, and moxifoxacin – are commonly administered to oncology patients. Although these oral antibiotics are approved by the US Food and Drug Administration (FDA) for treatment of urinary tract infections, acute bacterial sinusitis, or bacterial infection in patients with chronic obstructive pulmonary disease, they are commonly prescribed off-label to neutropenic cancer patients for the prevention and treatment of infections associated with febrile neutropenia. New serious FQ-associated safety concerns have been identified through novel collaborations between FQ-treated persons who have developed long-term neuropsychiatric (NP) toxicity, pharmacovigilance experts, and basic scientists.
Objective To conduct basic science and clinical investigations of a newly identified adverse drug reaction, termed FQ-associated disability.
Methods 5 groups of C57BL/6 mice receiving the antibiotic ciprofoxacin in 10-mg increments (10 mg/kg-50 mg/kg) and 1 group of control mice were evaluated. The Southern Network on Adverse Reactions (SONAR) and a social network of FQ-treated persons with long-term NP toxicity (the Floxed Network) conducted a web-based survey. The clinical toxicity manifestations reported by 94 respondents to the web-based survey of persons who had received 1 or more doses of an FQ prescribed for any indication (generally at FDA-approved dosages) and who subsequently experienced possible adverse drug reactions were compared with adverse event information included on the product label for levofoxacin and with FQ-associated adverse events reported to the FDA’s MedWatch program.
Results Mice treated with ciprofoxacin had lower grip strengths, reduced balance, and depressive behavior compared with the controls. For the survey, 93 of 94 respondents reported FQ-associated events including anxiety, depression, insomnia, panic attacks, clouded thinking, depersonalization, suicidal thoughts, psychosis, nightmares, and impaired memory beginning within days of FQ initiation or days to months of FQ discontinuation. The FDA Adverse Event Reporting System (FAERS) included 210,705 adverse events and 2,991 fatalities for FQs. Levofoxacin and ciprofoxacin toxicities were neurologic (30% and 26%, respectively), tendon damage (8% and 6%), and psychiatric (10% and 2%). In 2013, an FDA safety review reported that FQs affect mammalian topoisomerase II, especially in mitochondria. In 2013 and 2014, SONAR fled citizen petitions requesting black box revisions identifying neuropsychiatric toxicities and mitochrondrial toxicity as serious levofoxacin-associated adverse drug reactions. In 2015, FDA advisors recommended that FQ product labels be revised to include information about this newly identified disability syndrome termed “FQ-associated disability” (FQAD).
Limitations Basic science studies evaluated NP toxicity for only 1 FQ, ciprofoxacin.
Conclusion Pharmacovigilance investigators, a social network, and basic scientists can collaborate on pharmacovigilance investigations. Revised product labels describing a new serious adverse drug reaction, levofoxacin-associated long-term disability, as recommended by an FDA advisory committee, are advised.
Funding This work was funded partly by the National Cancer Institute (1R01CA165609-01A1), the American Cancer Society (IRG-13-043-01), the South Carolina SmartState Program, and an unrestricted from Doris Levkoff Meddin to the South Carolina College of Pharmacy Center for Medication Safety and Efficacy.
Click on the PDF icon at the top of this introduction to read the full article.
Background The 3 fluoroquinolone (FQ) antibiotics – ciprofoxacin, levofoxacin, and moxifoxacin – are commonly administered to oncology patients. Although these oral antibiotics are approved by the US Food and Drug Administration (FDA) for treatment of urinary tract infections, acute bacterial sinusitis, or bacterial infection in patients with chronic obstructive pulmonary disease, they are commonly prescribed off-label to neutropenic cancer patients for the prevention and treatment of infections associated with febrile neutropenia. New serious FQ-associated safety concerns have been identified through novel collaborations between FQ-treated persons who have developed long-term neuropsychiatric (NP) toxicity, pharmacovigilance experts, and basic scientists.
Objective To conduct basic science and clinical investigations of a newly identified adverse drug reaction, termed FQ-associated disability.
Methods 5 groups of C57BL/6 mice receiving the antibiotic ciprofoxacin in 10-mg increments (10 mg/kg-50 mg/kg) and 1 group of control mice were evaluated. The Southern Network on Adverse Reactions (SONAR) and a social network of FQ-treated persons with long-term NP toxicity (the Floxed Network) conducted a web-based survey. The clinical toxicity manifestations reported by 94 respondents to the web-based survey of persons who had received 1 or more doses of an FQ prescribed for any indication (generally at FDA-approved dosages) and who subsequently experienced possible adverse drug reactions were compared with adverse event information included on the product label for levofoxacin and with FQ-associated adverse events reported to the FDA’s MedWatch program.
Results Mice treated with ciprofoxacin had lower grip strengths, reduced balance, and depressive behavior compared with the controls. For the survey, 93 of 94 respondents reported FQ-associated events including anxiety, depression, insomnia, panic attacks, clouded thinking, depersonalization, suicidal thoughts, psychosis, nightmares, and impaired memory beginning within days of FQ initiation or days to months of FQ discontinuation. The FDA Adverse Event Reporting System (FAERS) included 210,705 adverse events and 2,991 fatalities for FQs. Levofoxacin and ciprofoxacin toxicities were neurologic (30% and 26%, respectively), tendon damage (8% and 6%), and psychiatric (10% and 2%). In 2013, an FDA safety review reported that FQs affect mammalian topoisomerase II, especially in mitochondria. In 2013 and 2014, SONAR fled citizen petitions requesting black box revisions identifying neuropsychiatric toxicities and mitochrondrial toxicity as serious levofoxacin-associated adverse drug reactions. In 2015, FDA advisors recommended that FQ product labels be revised to include information about this newly identified disability syndrome termed “FQ-associated disability” (FQAD).
Limitations Basic science studies evaluated NP toxicity for only 1 FQ, ciprofoxacin.
Conclusion Pharmacovigilance investigators, a social network, and basic scientists can collaborate on pharmacovigilance investigations. Revised product labels describing a new serious adverse drug reaction, levofoxacin-associated long-term disability, as recommended by an FDA advisory committee, are advised.
Funding This work was funded partly by the National Cancer Institute (1R01CA165609-01A1), the American Cancer Society (IRG-13-043-01), the South Carolina SmartState Program, and an unrestricted from Doris Levkoff Meddin to the South Carolina College of Pharmacy Center for Medication Safety and Efficacy.
Click on the PDF icon at the top of this introduction to read the full article.
Background The 3 fluoroquinolone (FQ) antibiotics – ciprofoxacin, levofoxacin, and moxifoxacin – are commonly administered to oncology patients. Although these oral antibiotics are approved by the US Food and Drug Administration (FDA) for treatment of urinary tract infections, acute bacterial sinusitis, or bacterial infection in patients with chronic obstructive pulmonary disease, they are commonly prescribed off-label to neutropenic cancer patients for the prevention and treatment of infections associated with febrile neutropenia. New serious FQ-associated safety concerns have been identified through novel collaborations between FQ-treated persons who have developed long-term neuropsychiatric (NP) toxicity, pharmacovigilance experts, and basic scientists.
Objective To conduct basic science and clinical investigations of a newly identified adverse drug reaction, termed FQ-associated disability.
Methods 5 groups of C57BL/6 mice receiving the antibiotic ciprofoxacin in 10-mg increments (10 mg/kg-50 mg/kg) and 1 group of control mice were evaluated. The Southern Network on Adverse Reactions (SONAR) and a social network of FQ-treated persons with long-term NP toxicity (the Floxed Network) conducted a web-based survey. The clinical toxicity manifestations reported by 94 respondents to the web-based survey of persons who had received 1 or more doses of an FQ prescribed for any indication (generally at FDA-approved dosages) and who subsequently experienced possible adverse drug reactions were compared with adverse event information included on the product label for levofoxacin and with FQ-associated adverse events reported to the FDA’s MedWatch program.
Results Mice treated with ciprofoxacin had lower grip strengths, reduced balance, and depressive behavior compared with the controls. For the survey, 93 of 94 respondents reported FQ-associated events including anxiety, depression, insomnia, panic attacks, clouded thinking, depersonalization, suicidal thoughts, psychosis, nightmares, and impaired memory beginning within days of FQ initiation or days to months of FQ discontinuation. The FDA Adverse Event Reporting System (FAERS) included 210,705 adverse events and 2,991 fatalities for FQs. Levofoxacin and ciprofoxacin toxicities were neurologic (30% and 26%, respectively), tendon damage (8% and 6%), and psychiatric (10% and 2%). In 2013, an FDA safety review reported that FQs affect mammalian topoisomerase II, especially in mitochondria. In 2013 and 2014, SONAR fled citizen petitions requesting black box revisions identifying neuropsychiatric toxicities and mitochrondrial toxicity as serious levofoxacin-associated adverse drug reactions. In 2015, FDA advisors recommended that FQ product labels be revised to include information about this newly identified disability syndrome termed “FQ-associated disability” (FQAD).
Limitations Basic science studies evaluated NP toxicity for only 1 FQ, ciprofoxacin.
Conclusion Pharmacovigilance investigators, a social network, and basic scientists can collaborate on pharmacovigilance investigations. Revised product labels describing a new serious adverse drug reaction, levofoxacin-associated long-term disability, as recommended by an FDA advisory committee, are advised.
Funding This work was funded partly by the National Cancer Institute (1R01CA165609-01A1), the American Cancer Society (IRG-13-043-01), the South Carolina SmartState Program, and an unrestricted from Doris Levkoff Meddin to the South Carolina College of Pharmacy Center for Medication Safety and Efficacy.
Click on the PDF icon at the top of this introduction to read the full article.
Making immunotherapy part of routine breast cancer treatment
Cancer treatment is evolving rapidly, and 2015 was no exception. It was the year of immunotherapy. Following the approval by the US Food and Drug Administration in 2014 of pembrolizumab for melanoma, 2015 saw approvals of nivolumab for melanoma, lung cancer, and kidney cancer; pembrolizumab for lung cancer; and the combination of ipilimumab and nivolimab for melanoma. That’s an impressive list of immunotherapy approvals for a single year.
Click on the PDF icon at the top of this introduction to read the full article.
Cancer treatment is evolving rapidly, and 2015 was no exception. It was the year of immunotherapy. Following the approval by the US Food and Drug Administration in 2014 of pembrolizumab for melanoma, 2015 saw approvals of nivolumab for melanoma, lung cancer, and kidney cancer; pembrolizumab for lung cancer; and the combination of ipilimumab and nivolimab for melanoma. That’s an impressive list of immunotherapy approvals for a single year.
Click on the PDF icon at the top of this introduction to read the full article.
Cancer treatment is evolving rapidly, and 2015 was no exception. It was the year of immunotherapy. Following the approval by the US Food and Drug Administration in 2014 of pembrolizumab for melanoma, 2015 saw approvals of nivolumab for melanoma, lung cancer, and kidney cancer; pembrolizumab for lung cancer; and the combination of ipilimumab and nivolimab for melanoma. That’s an impressive list of immunotherapy approvals for a single year.
Click on the PDF icon at the top of this introduction to read the full article.