User login
Unavoidable, random DNA replication errors are the most common cancer drivers
Up to two-thirds of the mutations that drive human cancers may be due to DNA replication errors in normally dividing stem cells, not by inherited or environmentally induced mutations, according to a mathematical modeling study.
The proportion of replication error-driven mutations varied widely among 17 cancers analyzed, but the overall attributable risk of these errors was remarkably consistent among 69 countries included in the study, said Cristian Tomasetti, PhD, a coauthor of the paper and a biostatistician at Johns Hopkins University, Baltimore.
The findings should be a game-changer in the cancer field, Dr. Tomasetti said during a press briefing sponsored by the American Association for the Advancement of Science. Research dogma has long held that most cancers are related to lifestyle and environmental exposure, with a few primarily due to genetic factors.
“We have now determined that there is a third factor, and that it causes most of the mutations that drive cancer,” Dr. Tomasetti said. “We cannot ignore it and pretend it doesn’t exist. This is a complete paradigm shift in how we think of cancer and what causes it.”
The finding that 66% of cancer-driving mutations are based on unavoidable replication errors doesn’t challenge well-established epidemiology, said Dr. Tomasetti and his coauthor, Bert Vogelstein, MD. Rather, it fits perfectly with several key understandings of cancer: that about 40% of cases are preventable, that rapidly dividing tissues are more prone to develop cancers, and that cancer incidence rises exponentially as humans age.
“If we have as our starting point the assumption that 42% of cancers are preventable, we are completely consistent with that,” in finding that about 60% of cancers are unavoidable, Dr. Tomasetti said. “Those two numbers go perfectly together.”
The study also found that replication-error mutations (R) were most likely to drive cancers in tissues with rapid turnover, such as colorectal tissue. This makes intuitive sense, given that basal mutation rates hover at about three errors per cell replication cycle regardless of tissue type.
“The basal mutation rate in all cells is pretty even,” said Dr. Vogelstein, the Clayton Professor of Oncology and Pathology at John Hopkins University, Baltimore. “The difference is the number of stem cells. The more cells, the more divisions, and the more mistakes.”
R-mutations also contribute to age-related cancer incidence. As a person ages, more cell divisions accumulate, thus increasing the risk of a cancer-driving R-error. But these mutations also occur in children, who have rapid cell division in all their tissues. In fact, the colleagues suspect that R-errors are the main drivers of almost all pediatric cancers.
The new study bolsters the duo’s controversial 2015 work.
The theory sparked controversy among scholars and researchers. They challenged it on a number of technical fronts, from stem cell counts and division rates to charges that it didn’t adequately assess the interaction between R-mutations and environmental risks.
Some commentators, perceiving nihilism in the paper, expressed concern that clinicians and patients would get the idea that cancer prevention strategies were useless, since most cancers were simply a case of “bad luck.”
A pervading theme of these counter arguments was one familiar to any researcher: Correlation does not equal causation. The new study was an attempt to expand upon and strengthen the original findings, Dr. Tomasetti said.
“There are well-known environmental risk variations across the world, and there was a question of how our findings might change if we did this analysis in a different country. This paper is also the very first time that someone has ever looked at the proportions of mutations in each cancer type and assigned them to these factors.”
The new study employed a similar mathematical model, but comprised data from 423 cancer registries in 69 countries. The researchers examined the relationship between the lifetime risk of 17 cancers (including breast and prostate, which were not included in the 2015 study) and lifetime stem cell divisions for each tissue. The median correlation coefficient was 0.80; 89% of the countries examined had a correlation of greater than 0.70. This was “remarkably similar” to the correlation determined in the 2015 U.S.-only study.
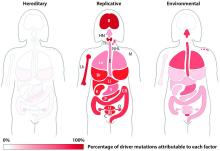
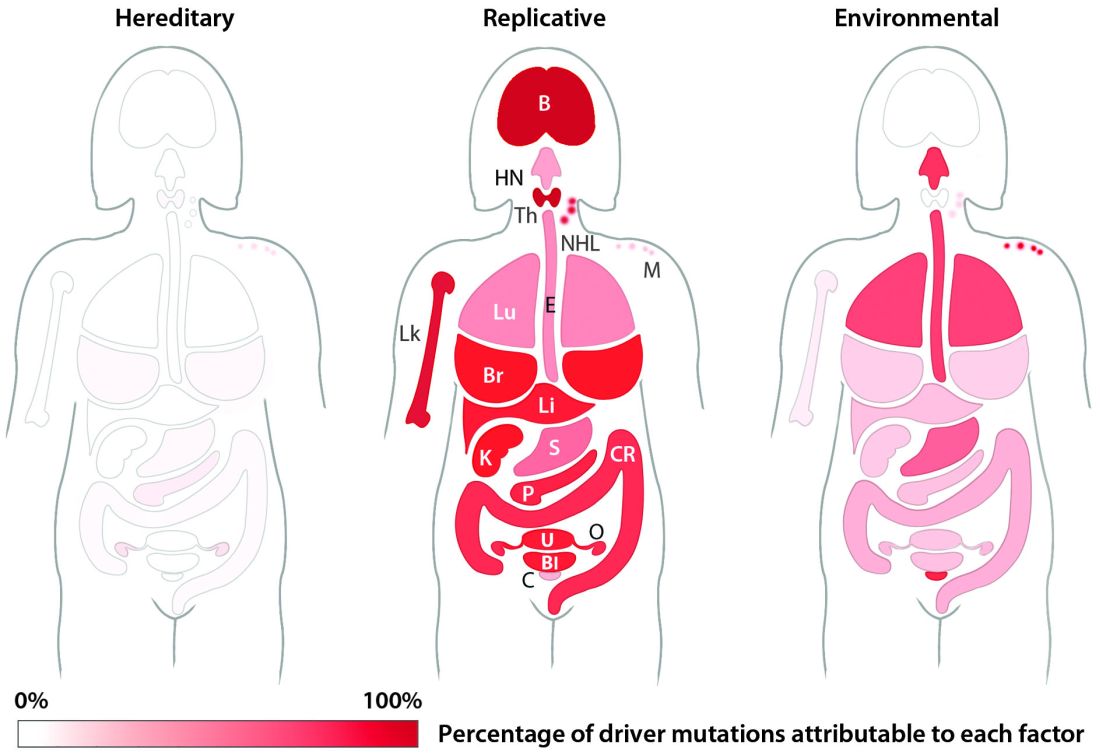
The team’s next step was to determine what fraction of cancer-driving mutations arose from R-errors, from environmental factors (E), and from hereditary factors (H). They examined these proportions in 32 different cancers in which environmental, lifestyle, and genetic factors have been thoroughly studied. Overall, 29% of the driver mutations were due to environment, 5% to heredity, and 66% to R-errors.
The proportions of these drivers did vary widely between the cancer types, the team noted. For example, lung and esophageal cancers and melanoma were primarily driven by environmental factors (more than 60% each). However, they wrote, “even in lung adenocarcinomas, R contributes a third of the total mutations, with tobacco smoke [including secondhand smoke], diet, radiation, and occupational exposures contributing the remainder. In cancers that are less strongly associated with environmental factors, such as those of the pancreas, brain, bone, or prostate, the majority of the mutations are attributable to R.”
During the press briefing, Dr. Tomasetti and Dr. Vogelstein stressed that most of the inevitable R-errors don’t precipitate cancer – and that even if they do increase risk, that risk may not ever trip the disease process.
“Most of the time these replicative mutations do no harm,” Dr Vogelstein said. “They occur in junk DNA genes, or in areas that are unimportant with respect to cancer. That’s the good luck. Occasionally, they occur in a cancer driver gene, and that is bad luck.”
But even a dose of bad luck isn’t enough to cause cancer. Most cancers require multiple hits to develop – which makes primary prevention strategies more important than ever, Dr. Tomasetti said.
“In the case of lung cancer, for instance, three or more mutations are needed. We showed that these mutations are caused by a combination of environment and R-errors. In theory, then, all of these cancers are preventable because if we can prevent even one of the environmentally caused mutations, then that patient won’t develop cancer.”
However, he said, some cancers do appear to be entirely driven by E-errors and, thus, appear entirely unavoidable. This is an extremely difficult area for clinicians and patients to navigate, said Dr. Vogelstein, a former pediatrician.
“We hope that understanding this will offer some comfort to the literally millions of patients who develop cancer despite having lead a near-perfect life,” in terms of managing risk factors. “Cancer develops in people who haven’t smoked, who avoided the sun and wore sunscreen, who eat perfectly healthy diets and exercise regularly. This is a particularly important concept for parents of children who have cancer, who think ‘I either transmitted a bad gene or unknowingly exposed my child to an environmental agent that caused their cancer.’ They need to understand that these cancers would have occurred no matter what they did.”
Dr. Tomasetti had no disclosures. Dr. Vogelstein is on the scientific advisory boards of Morphotek, Exelixis GP, and Sysmex Inostics, and is a founder of PapGene and Personal Genome Diagnostics.
[email protected]
On Twitter @Alz_gal
Up to two-thirds of the mutations that drive human cancers may be due to DNA replication errors in normally dividing stem cells, not by inherited or environmentally induced mutations, according to a mathematical modeling study.
The proportion of replication error-driven mutations varied widely among 17 cancers analyzed, but the overall attributable risk of these errors was remarkably consistent among 69 countries included in the study, said Cristian Tomasetti, PhD, a coauthor of the paper and a biostatistician at Johns Hopkins University, Baltimore.
The findings should be a game-changer in the cancer field, Dr. Tomasetti said during a press briefing sponsored by the American Association for the Advancement of Science. Research dogma has long held that most cancers are related to lifestyle and environmental exposure, with a few primarily due to genetic factors.
“We have now determined that there is a third factor, and that it causes most of the mutations that drive cancer,” Dr. Tomasetti said. “We cannot ignore it and pretend it doesn’t exist. This is a complete paradigm shift in how we think of cancer and what causes it.”
The finding that 66% of cancer-driving mutations are based on unavoidable replication errors doesn’t challenge well-established epidemiology, said Dr. Tomasetti and his coauthor, Bert Vogelstein, MD. Rather, it fits perfectly with several key understandings of cancer: that about 40% of cases are preventable, that rapidly dividing tissues are more prone to develop cancers, and that cancer incidence rises exponentially as humans age.
“If we have as our starting point the assumption that 42% of cancers are preventable, we are completely consistent with that,” in finding that about 60% of cancers are unavoidable, Dr. Tomasetti said. “Those two numbers go perfectly together.”
The study also found that replication-error mutations (R) were most likely to drive cancers in tissues with rapid turnover, such as colorectal tissue. This makes intuitive sense, given that basal mutation rates hover at about three errors per cell replication cycle regardless of tissue type.
“The basal mutation rate in all cells is pretty even,” said Dr. Vogelstein, the Clayton Professor of Oncology and Pathology at John Hopkins University, Baltimore. “The difference is the number of stem cells. The more cells, the more divisions, and the more mistakes.”
R-mutations also contribute to age-related cancer incidence. As a person ages, more cell divisions accumulate, thus increasing the risk of a cancer-driving R-error. But these mutations also occur in children, who have rapid cell division in all their tissues. In fact, the colleagues suspect that R-errors are the main drivers of almost all pediatric cancers.
The new study bolsters the duo’s controversial 2015 work.
The theory sparked controversy among scholars and researchers. They challenged it on a number of technical fronts, from stem cell counts and division rates to charges that it didn’t adequately assess the interaction between R-mutations and environmental risks.
Some commentators, perceiving nihilism in the paper, expressed concern that clinicians and patients would get the idea that cancer prevention strategies were useless, since most cancers were simply a case of “bad luck.”
A pervading theme of these counter arguments was one familiar to any researcher: Correlation does not equal causation. The new study was an attempt to expand upon and strengthen the original findings, Dr. Tomasetti said.
“There are well-known environmental risk variations across the world, and there was a question of how our findings might change if we did this analysis in a different country. This paper is also the very first time that someone has ever looked at the proportions of mutations in each cancer type and assigned them to these factors.”
The new study employed a similar mathematical model, but comprised data from 423 cancer registries in 69 countries. The researchers examined the relationship between the lifetime risk of 17 cancers (including breast and prostate, which were not included in the 2015 study) and lifetime stem cell divisions for each tissue. The median correlation coefficient was 0.80; 89% of the countries examined had a correlation of greater than 0.70. This was “remarkably similar” to the correlation determined in the 2015 U.S.-only study.
The team’s next step was to determine what fraction of cancer-driving mutations arose from R-errors, from environmental factors (E), and from hereditary factors (H). They examined these proportions in 32 different cancers in which environmental, lifestyle, and genetic factors have been thoroughly studied. Overall, 29% of the driver mutations were due to environment, 5% to heredity, and 66% to R-errors.
The proportions of these drivers did vary widely between the cancer types, the team noted. For example, lung and esophageal cancers and melanoma were primarily driven by environmental factors (more than 60% each). However, they wrote, “even in lung adenocarcinomas, R contributes a third of the total mutations, with tobacco smoke [including secondhand smoke], diet, radiation, and occupational exposures contributing the remainder. In cancers that are less strongly associated with environmental factors, such as those of the pancreas, brain, bone, or prostate, the majority of the mutations are attributable to R.”
During the press briefing, Dr. Tomasetti and Dr. Vogelstein stressed that most of the inevitable R-errors don’t precipitate cancer – and that even if they do increase risk, that risk may not ever trip the disease process.
“Most of the time these replicative mutations do no harm,” Dr Vogelstein said. “They occur in junk DNA genes, or in areas that are unimportant with respect to cancer. That’s the good luck. Occasionally, they occur in a cancer driver gene, and that is bad luck.”
But even a dose of bad luck isn’t enough to cause cancer. Most cancers require multiple hits to develop – which makes primary prevention strategies more important than ever, Dr. Tomasetti said.
“In the case of lung cancer, for instance, three or more mutations are needed. We showed that these mutations are caused by a combination of environment and R-errors. In theory, then, all of these cancers are preventable because if we can prevent even one of the environmentally caused mutations, then that patient won’t develop cancer.”
However, he said, some cancers do appear to be entirely driven by E-errors and, thus, appear entirely unavoidable. This is an extremely difficult area for clinicians and patients to navigate, said Dr. Vogelstein, a former pediatrician.
“We hope that understanding this will offer some comfort to the literally millions of patients who develop cancer despite having lead a near-perfect life,” in terms of managing risk factors. “Cancer develops in people who haven’t smoked, who avoided the sun and wore sunscreen, who eat perfectly healthy diets and exercise regularly. This is a particularly important concept for parents of children who have cancer, who think ‘I either transmitted a bad gene or unknowingly exposed my child to an environmental agent that caused their cancer.’ They need to understand that these cancers would have occurred no matter what they did.”
Dr. Tomasetti had no disclosures. Dr. Vogelstein is on the scientific advisory boards of Morphotek, Exelixis GP, and Sysmex Inostics, and is a founder of PapGene and Personal Genome Diagnostics.
[email protected]
On Twitter @Alz_gal
Up to two-thirds of the mutations that drive human cancers may be due to DNA replication errors in normally dividing stem cells, not by inherited or environmentally induced mutations, according to a mathematical modeling study.
The proportion of replication error-driven mutations varied widely among 17 cancers analyzed, but the overall attributable risk of these errors was remarkably consistent among 69 countries included in the study, said Cristian Tomasetti, PhD, a coauthor of the paper and a biostatistician at Johns Hopkins University, Baltimore.
The findings should be a game-changer in the cancer field, Dr. Tomasetti said during a press briefing sponsored by the American Association for the Advancement of Science. Research dogma has long held that most cancers are related to lifestyle and environmental exposure, with a few primarily due to genetic factors.
“We have now determined that there is a third factor, and that it causes most of the mutations that drive cancer,” Dr. Tomasetti said. “We cannot ignore it and pretend it doesn’t exist. This is a complete paradigm shift in how we think of cancer and what causes it.”
The finding that 66% of cancer-driving mutations are based on unavoidable replication errors doesn’t challenge well-established epidemiology, said Dr. Tomasetti and his coauthor, Bert Vogelstein, MD. Rather, it fits perfectly with several key understandings of cancer: that about 40% of cases are preventable, that rapidly dividing tissues are more prone to develop cancers, and that cancer incidence rises exponentially as humans age.
“If we have as our starting point the assumption that 42% of cancers are preventable, we are completely consistent with that,” in finding that about 60% of cancers are unavoidable, Dr. Tomasetti said. “Those two numbers go perfectly together.”
The study also found that replication-error mutations (R) were most likely to drive cancers in tissues with rapid turnover, such as colorectal tissue. This makes intuitive sense, given that basal mutation rates hover at about three errors per cell replication cycle regardless of tissue type.
“The basal mutation rate in all cells is pretty even,” said Dr. Vogelstein, the Clayton Professor of Oncology and Pathology at John Hopkins University, Baltimore. “The difference is the number of stem cells. The more cells, the more divisions, and the more mistakes.”
R-mutations also contribute to age-related cancer incidence. As a person ages, more cell divisions accumulate, thus increasing the risk of a cancer-driving R-error. But these mutations also occur in children, who have rapid cell division in all their tissues. In fact, the colleagues suspect that R-errors are the main drivers of almost all pediatric cancers.
The new study bolsters the duo’s controversial 2015 work.
The theory sparked controversy among scholars and researchers. They challenged it on a number of technical fronts, from stem cell counts and division rates to charges that it didn’t adequately assess the interaction between R-mutations and environmental risks.
Some commentators, perceiving nihilism in the paper, expressed concern that clinicians and patients would get the idea that cancer prevention strategies were useless, since most cancers were simply a case of “bad luck.”
A pervading theme of these counter arguments was one familiar to any researcher: Correlation does not equal causation. The new study was an attempt to expand upon and strengthen the original findings, Dr. Tomasetti said.
“There are well-known environmental risk variations across the world, and there was a question of how our findings might change if we did this analysis in a different country. This paper is also the very first time that someone has ever looked at the proportions of mutations in each cancer type and assigned them to these factors.”
The new study employed a similar mathematical model, but comprised data from 423 cancer registries in 69 countries. The researchers examined the relationship between the lifetime risk of 17 cancers (including breast and prostate, which were not included in the 2015 study) and lifetime stem cell divisions for each tissue. The median correlation coefficient was 0.80; 89% of the countries examined had a correlation of greater than 0.70. This was “remarkably similar” to the correlation determined in the 2015 U.S.-only study.
The team’s next step was to determine what fraction of cancer-driving mutations arose from R-errors, from environmental factors (E), and from hereditary factors (H). They examined these proportions in 32 different cancers in which environmental, lifestyle, and genetic factors have been thoroughly studied. Overall, 29% of the driver mutations were due to environment, 5% to heredity, and 66% to R-errors.
The proportions of these drivers did vary widely between the cancer types, the team noted. For example, lung and esophageal cancers and melanoma were primarily driven by environmental factors (more than 60% each). However, they wrote, “even in lung adenocarcinomas, R contributes a third of the total mutations, with tobacco smoke [including secondhand smoke], diet, radiation, and occupational exposures contributing the remainder. In cancers that are less strongly associated with environmental factors, such as those of the pancreas, brain, bone, or prostate, the majority of the mutations are attributable to R.”
During the press briefing, Dr. Tomasetti and Dr. Vogelstein stressed that most of the inevitable R-errors don’t precipitate cancer – and that even if they do increase risk, that risk may not ever trip the disease process.
“Most of the time these replicative mutations do no harm,” Dr Vogelstein said. “They occur in junk DNA genes, or in areas that are unimportant with respect to cancer. That’s the good luck. Occasionally, they occur in a cancer driver gene, and that is bad luck.”
But even a dose of bad luck isn’t enough to cause cancer. Most cancers require multiple hits to develop – which makes primary prevention strategies more important than ever, Dr. Tomasetti said.
“In the case of lung cancer, for instance, three or more mutations are needed. We showed that these mutations are caused by a combination of environment and R-errors. In theory, then, all of these cancers are preventable because if we can prevent even one of the environmentally caused mutations, then that patient won’t develop cancer.”
However, he said, some cancers do appear to be entirely driven by E-errors and, thus, appear entirely unavoidable. This is an extremely difficult area for clinicians and patients to navigate, said Dr. Vogelstein, a former pediatrician.
“We hope that understanding this will offer some comfort to the literally millions of patients who develop cancer despite having lead a near-perfect life,” in terms of managing risk factors. “Cancer develops in people who haven’t smoked, who avoided the sun and wore sunscreen, who eat perfectly healthy diets and exercise regularly. This is a particularly important concept for parents of children who have cancer, who think ‘I either transmitted a bad gene or unknowingly exposed my child to an environmental agent that caused their cancer.’ They need to understand that these cancers would have occurred no matter what they did.”
Dr. Tomasetti had no disclosures. Dr. Vogelstein is on the scientific advisory boards of Morphotek, Exelixis GP, and Sysmex Inostics, and is a founder of PapGene and Personal Genome Diagnostics.
[email protected]
On Twitter @Alz_gal
Key clinical point:
Major finding: Two-thirds (66%) of cancer drivers are replication errors, 29% are environmentally induced, and 5% are hereditary.
Data source: The researchers examined cancer mutation drivers in two cohorts that spanned 69 countries.
Disclosures: Dr. Tomasetti had no disclosures. Dr. Vogelstein is on the scientific advisory boards of Morphotek, Exelixis GP, and Sysmex Inostics, and is a founder of PapGene and Personal Genome Diagnostics.
Cardiovascular disease most common cause of death in CRC survivors
SEATTLE – Improvements in diagnosis and treatment have lengthened the survival time of patients with colorectal cancer, but the majority of deaths from CRC occur within the first 5 years.
According to new findings presented at the annual Society of Surgical Oncology Cancer Symposium, CRC as a cause of death is surpassed by cardiovascular disease (CVD) and second primary cancers as time goes on.
Dr. Lewis explained that CRC as a cause of death begins to plateau over time and other causes become more important.
“As time goes on, colorectal cancer becomes less prominent, and by year 8, cardiovascular death surpasses it. By year 10, colorectal cancer is surpassed by second cancers and neurologic diseases.”
Information about long-term health problems in long-term colorectal cancer survivors is limited. To address this, Dr. Lewis and his colleagues sought to understand the trends and causes of death over time.
They analyzed causes of death in CRC patients who have survived 5 years and longer using the California Cancer Registry (2000-2011) that is linked to inpatient records. From this database, 139,743 patients with CRC were identified, with 97,604 (69.8%) having been treated for disease originating from the colon and 42,139 (30.2%) from the rectum.
The median age of the patients at the time of presentation was 68 years; at 5 years after diagnosis, 70 years; and at 10 years, 74 years. The 5-year overall survival was 59.1%, and it was during that 5 years that 95% of cancer-specific deaths occurred.
During the first 5 years, the major cause of death was CRC, accounting for nearly two-thirds of the mortality (n = 38,992, 65.4%). This was followed by cardiovascular disease (n = 7,140, 12.0%), second primary cancer (n = 3,775, 6.3%), neurologic disease (n = 2,329, 3.9%), and pulmonary disease (n = 2,307, 3.9%).
The most common second primary malignancies affecting CRC survivors were lung and hematologic cancers, followed by pancreatic and liver cancers.
Overall, in long-term survivors, cardiovascular disease was the major cause of death (n = 2,163, 24.0%) although nearly as many deaths were due to CRC (2,094, 23.2%). This was followed by neurologic disease (n = 1,174, 13.0%), secondary primary cancer (n = 1,146, 12.7%), and pulmonary disease (n = 765, 8.5%).
There was no funding source disclosed in the abstract. Dr. Lewis had no disclosures.
SEATTLE – Improvements in diagnosis and treatment have lengthened the survival time of patients with colorectal cancer, but the majority of deaths from CRC occur within the first 5 years.
According to new findings presented at the annual Society of Surgical Oncology Cancer Symposium, CRC as a cause of death is surpassed by cardiovascular disease (CVD) and second primary cancers as time goes on.
Dr. Lewis explained that CRC as a cause of death begins to plateau over time and other causes become more important.
“As time goes on, colorectal cancer becomes less prominent, and by year 8, cardiovascular death surpasses it. By year 10, colorectal cancer is surpassed by second cancers and neurologic diseases.”
Information about long-term health problems in long-term colorectal cancer survivors is limited. To address this, Dr. Lewis and his colleagues sought to understand the trends and causes of death over time.
They analyzed causes of death in CRC patients who have survived 5 years and longer using the California Cancer Registry (2000-2011) that is linked to inpatient records. From this database, 139,743 patients with CRC were identified, with 97,604 (69.8%) having been treated for disease originating from the colon and 42,139 (30.2%) from the rectum.
The median age of the patients at the time of presentation was 68 years; at 5 years after diagnosis, 70 years; and at 10 years, 74 years. The 5-year overall survival was 59.1%, and it was during that 5 years that 95% of cancer-specific deaths occurred.
During the first 5 years, the major cause of death was CRC, accounting for nearly two-thirds of the mortality (n = 38,992, 65.4%). This was followed by cardiovascular disease (n = 7,140, 12.0%), second primary cancer (n = 3,775, 6.3%), neurologic disease (n = 2,329, 3.9%), and pulmonary disease (n = 2,307, 3.9%).
The most common second primary malignancies affecting CRC survivors were lung and hematologic cancers, followed by pancreatic and liver cancers.
Overall, in long-term survivors, cardiovascular disease was the major cause of death (n = 2,163, 24.0%) although nearly as many deaths were due to CRC (2,094, 23.2%). This was followed by neurologic disease (n = 1,174, 13.0%), secondary primary cancer (n = 1,146, 12.7%), and pulmonary disease (n = 765, 8.5%).
There was no funding source disclosed in the abstract. Dr. Lewis had no disclosures.
SEATTLE – Improvements in diagnosis and treatment have lengthened the survival time of patients with colorectal cancer, but the majority of deaths from CRC occur within the first 5 years.
According to new findings presented at the annual Society of Surgical Oncology Cancer Symposium, CRC as a cause of death is surpassed by cardiovascular disease (CVD) and second primary cancers as time goes on.
Dr. Lewis explained that CRC as a cause of death begins to plateau over time and other causes become more important.
“As time goes on, colorectal cancer becomes less prominent, and by year 8, cardiovascular death surpasses it. By year 10, colorectal cancer is surpassed by second cancers and neurologic diseases.”
Information about long-term health problems in long-term colorectal cancer survivors is limited. To address this, Dr. Lewis and his colleagues sought to understand the trends and causes of death over time.
They analyzed causes of death in CRC patients who have survived 5 years and longer using the California Cancer Registry (2000-2011) that is linked to inpatient records. From this database, 139,743 patients with CRC were identified, with 97,604 (69.8%) having been treated for disease originating from the colon and 42,139 (30.2%) from the rectum.
The median age of the patients at the time of presentation was 68 years; at 5 years after diagnosis, 70 years; and at 10 years, 74 years. The 5-year overall survival was 59.1%, and it was during that 5 years that 95% of cancer-specific deaths occurred.
During the first 5 years, the major cause of death was CRC, accounting for nearly two-thirds of the mortality (n = 38,992, 65.4%). This was followed by cardiovascular disease (n = 7,140, 12.0%), second primary cancer (n = 3,775, 6.3%), neurologic disease (n = 2,329, 3.9%), and pulmonary disease (n = 2,307, 3.9%).
The most common second primary malignancies affecting CRC survivors were lung and hematologic cancers, followed by pancreatic and liver cancers.
Overall, in long-term survivors, cardiovascular disease was the major cause of death (n = 2,163, 24.0%) although nearly as many deaths were due to CRC (2,094, 23.2%). This was followed by neurologic disease (n = 1,174, 13.0%), secondary primary cancer (n = 1,146, 12.7%), and pulmonary disease (n = 765, 8.5%).
There was no funding source disclosed in the abstract. Dr. Lewis had no disclosures.
AT SSO 2017
Key clinical point: Long-term colorectal cancer survivors generally will die from other causes.
Major finding: By year 8, cardiovascular disease surpasses colorectal cancer in survivors, as the leading cause of death.
Data source: Large cancer registry with almost 140,000 colorectal cancer patients.
Disclosures: There was no funding source disclosed in the abstract. Dr. Lewis had no disclosures.
Portfolio of physician-led measures nets better quality of care
ORLANDO – A multifaceted portfolio of physician-led measures with feedback and financial incentives can dramatically improve the quality of care provided at cancer centers, suggests the experience of Stanford (Calif.) Health Care.
Physician leaders of 13 disease-specific cancer care programs (CCPs) identified measures of care that were meaningful to their team and patients, spanning the spectrum from new diagnosis through end of life and survivorship care. Quality and analytics teams developed 16 corresponding metrics and performance reports used for feedback. Programs were also given a financial incentive to meet jointly set targets.
After a year, the CCPs had improved on 12 of the metrics and maintained high baseline levels of performance on the other 4 metrics, investigators reported at a symposium on quality care sponsored by the American Society of Clinical Oncology. For example, they got better at entering staging information in a dedicated field in the electronic health record (+50% absolute increase), recording hand and foot pain (+34%), performing hepatitis B testing before rituximab use (+17%), and referring patients with ovarian cancer for genetic counseling (+43%).
“The main drivers, I would argue, besides the Hawthorne effect, were a high level of physician engagement in the selection, management, and improvement of the metrics, and these metrics excited the care teams, which also provided some motivation,” she said. “We provided real-time, high-quality feedback of performance. And last but probably not least was a financial incentive for the CCP as a team, not part of any individual compensation.”
The investigators plan to continue measuring the metrics, to expand them to other sites in their network, and to add new metrics that are common across the programs to minimize measurement burden, according to Ms. Porter. “We also plan to build cohorts for value-based care and unplanned care like ED visits and unplanned admissions. Finally, we want to keep momentum going and capitalize upon a provider engagement in value measurement and improvement,” she said.
“Based on this work and prior abstracts, … there are many validated metrics to be used. So, to choose those metrics and to choose them through local leadership support, most importantly, engaging frontline staff and having their buy-in of the measures that you are collecting are important,” commented invited discussant Jessica A. Zerillo, MD, MPH, of the Beth Israel Deaconess Medical Center in Boston. “And this can include using incentives that drive such stakeholders, whether they be financial or simply pride with public reporting.”
Study details
“In the summer of 2015, we were starting to feel a lot of pressure to prepare for evolving reimbursement models,” Ms. Porter said, explaining the initiative’s genesis. “Mainly, how do we define our value, and how can we measure and improve on that value of the care we deliver? One answer, of course, is to measure and reduce unnecessary variation. And we knew, to be successful, we had to increase our physician engagement and leadership in the selection and improvement of our metrics.”
Physician leaders of the CCPs were asked to choose quality measures that met three criteria: they were meaningful and important to both the care team and patients, they had pertinent data elements already available in existing databases (to reduce documentation burden), and they were multidisciplinary in nature, reflecting the care provided by the whole program. The measures ultimately selected included a variety of those put forth by American Society of Clinical Oncology’s Quality Oncology Practice Initiative and the American Society for Radiation Oncology. CCPs were offered a financial incentive for meeting targets ranging from $75,000 to $125,000 that was based on number of providers and patient volume, rather than on the impact of improved metric. “This was really meant for reinvestment back into their quality programs,” Ms. Porter said. “I would argue this was really a culture-building year for us, and we hope that next year there might be a little bit more tangible value with the metrics.”
The quality team gave CCPs monthly or quarterly performance reports with unblinded physician- and patient-level details that were ultimately disseminated to all the other CCPs. They also investigated any missing data for individual metrics.
Study results showed that half of the 16 measures the physician leaders chose pertained to the diagnosis and treatment planning phase of care, according to Ms. Porter. “It was important to many of our CCPs to ensure that specific testing was done, which would then, in turn, drive treatment planning decisions,” she commented.
At the end of the year, each metric was assessed among 13 to 2,406 patients. “All CCPs met their predetermined target and earned their financial incentive award for the year,” Ms. Porter reported.
Improvement was most marked, with a 50% absolute increase, for the metric of completing a staging module, which required conversion of staging information (historically embedded in progress notes) into a structured format in a dedicated field in the electronic health record within 45 days of a patient’s first cancer treatment. This practice enables ready identification of stage cohorts in which value of care can be assessed, she noted.
There were also sizable absolute increases in relevant CCPs in the proportion of blood and marrow transplant recipients referred to survivorship care by day 100 (+20%) and visiting that service by day 180 (+13%), recording of hand and foot pain (+34%) and radiation dermatitis (+21%), mismatch repair testing in patients with newly diagnosed colorectal cancer (+10%), referral of patients with newly diagnosed ovarian cancer for genetic counseling (+43%), cytogenetic testing in patients with newly diagnosed hematologic malignancies (+17%), hepatitis B testing before rituximab administration (+17%), and allowance of at least 2 nights for treatment plan physics–quality assurance before the start of a nonemergent radiation oncology treatment (+14%).
Meanwhile, there were decreases, considered favorable changes, in chemotherapy use in the last 2 weeks of life among neuro-oncology patients (–9%) and in patients’ receipt of more than 10 fractions of radiation therapy for palliation of bone metastases (–9%).
Finally, there was no change in several metrics of quality that were already at very high or low levels, as appropriate, at baseline: molecular testing in patients with newly diagnosed acute myeloid leukemia (stable at 95%), hospice enrollment at the time of death for neuro-oncology patients (stable at 100%), chemotherapy in the last 2 weeks of life for patients with sarcoma (stable at 0%), and epidermal growth factor receptor testing in patients with newly diagnosed lung adenocarcinoma (stable at 98%).
ORLANDO – A multifaceted portfolio of physician-led measures with feedback and financial incentives can dramatically improve the quality of care provided at cancer centers, suggests the experience of Stanford (Calif.) Health Care.
Physician leaders of 13 disease-specific cancer care programs (CCPs) identified measures of care that were meaningful to their team and patients, spanning the spectrum from new diagnosis through end of life and survivorship care. Quality and analytics teams developed 16 corresponding metrics and performance reports used for feedback. Programs were also given a financial incentive to meet jointly set targets.
After a year, the CCPs had improved on 12 of the metrics and maintained high baseline levels of performance on the other 4 metrics, investigators reported at a symposium on quality care sponsored by the American Society of Clinical Oncology. For example, they got better at entering staging information in a dedicated field in the electronic health record (+50% absolute increase), recording hand and foot pain (+34%), performing hepatitis B testing before rituximab use (+17%), and referring patients with ovarian cancer for genetic counseling (+43%).
“The main drivers, I would argue, besides the Hawthorne effect, were a high level of physician engagement in the selection, management, and improvement of the metrics, and these metrics excited the care teams, which also provided some motivation,” she said. “We provided real-time, high-quality feedback of performance. And last but probably not least was a financial incentive for the CCP as a team, not part of any individual compensation.”
The investigators plan to continue measuring the metrics, to expand them to other sites in their network, and to add new metrics that are common across the programs to minimize measurement burden, according to Ms. Porter. “We also plan to build cohorts for value-based care and unplanned care like ED visits and unplanned admissions. Finally, we want to keep momentum going and capitalize upon a provider engagement in value measurement and improvement,” she said.
“Based on this work and prior abstracts, … there are many validated metrics to be used. So, to choose those metrics and to choose them through local leadership support, most importantly, engaging frontline staff and having their buy-in of the measures that you are collecting are important,” commented invited discussant Jessica A. Zerillo, MD, MPH, of the Beth Israel Deaconess Medical Center in Boston. “And this can include using incentives that drive such stakeholders, whether they be financial or simply pride with public reporting.”
Study details
“In the summer of 2015, we were starting to feel a lot of pressure to prepare for evolving reimbursement models,” Ms. Porter said, explaining the initiative’s genesis. “Mainly, how do we define our value, and how can we measure and improve on that value of the care we deliver? One answer, of course, is to measure and reduce unnecessary variation. And we knew, to be successful, we had to increase our physician engagement and leadership in the selection and improvement of our metrics.”
Physician leaders of the CCPs were asked to choose quality measures that met three criteria: they were meaningful and important to both the care team and patients, they had pertinent data elements already available in existing databases (to reduce documentation burden), and they were multidisciplinary in nature, reflecting the care provided by the whole program. The measures ultimately selected included a variety of those put forth by American Society of Clinical Oncology’s Quality Oncology Practice Initiative and the American Society for Radiation Oncology. CCPs were offered a financial incentive for meeting targets ranging from $75,000 to $125,000 that was based on number of providers and patient volume, rather than on the impact of improved metric. “This was really meant for reinvestment back into their quality programs,” Ms. Porter said. “I would argue this was really a culture-building year for us, and we hope that next year there might be a little bit more tangible value with the metrics.”
The quality team gave CCPs monthly or quarterly performance reports with unblinded physician- and patient-level details that were ultimately disseminated to all the other CCPs. They also investigated any missing data for individual metrics.
Study results showed that half of the 16 measures the physician leaders chose pertained to the diagnosis and treatment planning phase of care, according to Ms. Porter. “It was important to many of our CCPs to ensure that specific testing was done, which would then, in turn, drive treatment planning decisions,” she commented.
At the end of the year, each metric was assessed among 13 to 2,406 patients. “All CCPs met their predetermined target and earned their financial incentive award for the year,” Ms. Porter reported.
Improvement was most marked, with a 50% absolute increase, for the metric of completing a staging module, which required conversion of staging information (historically embedded in progress notes) into a structured format in a dedicated field in the electronic health record within 45 days of a patient’s first cancer treatment. This practice enables ready identification of stage cohorts in which value of care can be assessed, she noted.
There were also sizable absolute increases in relevant CCPs in the proportion of blood and marrow transplant recipients referred to survivorship care by day 100 (+20%) and visiting that service by day 180 (+13%), recording of hand and foot pain (+34%) and radiation dermatitis (+21%), mismatch repair testing in patients with newly diagnosed colorectal cancer (+10%), referral of patients with newly diagnosed ovarian cancer for genetic counseling (+43%), cytogenetic testing in patients with newly diagnosed hematologic malignancies (+17%), hepatitis B testing before rituximab administration (+17%), and allowance of at least 2 nights for treatment plan physics–quality assurance before the start of a nonemergent radiation oncology treatment (+14%).
Meanwhile, there were decreases, considered favorable changes, in chemotherapy use in the last 2 weeks of life among neuro-oncology patients (–9%) and in patients’ receipt of more than 10 fractions of radiation therapy for palliation of bone metastases (–9%).
Finally, there was no change in several metrics of quality that were already at very high or low levels, as appropriate, at baseline: molecular testing in patients with newly diagnosed acute myeloid leukemia (stable at 95%), hospice enrollment at the time of death for neuro-oncology patients (stable at 100%), chemotherapy in the last 2 weeks of life for patients with sarcoma (stable at 0%), and epidermal growth factor receptor testing in patients with newly diagnosed lung adenocarcinoma (stable at 98%).
ORLANDO – A multifaceted portfolio of physician-led measures with feedback and financial incentives can dramatically improve the quality of care provided at cancer centers, suggests the experience of Stanford (Calif.) Health Care.
Physician leaders of 13 disease-specific cancer care programs (CCPs) identified measures of care that were meaningful to their team and patients, spanning the spectrum from new diagnosis through end of life and survivorship care. Quality and analytics teams developed 16 corresponding metrics and performance reports used for feedback. Programs were also given a financial incentive to meet jointly set targets.
After a year, the CCPs had improved on 12 of the metrics and maintained high baseline levels of performance on the other 4 metrics, investigators reported at a symposium on quality care sponsored by the American Society of Clinical Oncology. For example, they got better at entering staging information in a dedicated field in the electronic health record (+50% absolute increase), recording hand and foot pain (+34%), performing hepatitis B testing before rituximab use (+17%), and referring patients with ovarian cancer for genetic counseling (+43%).
“The main drivers, I would argue, besides the Hawthorne effect, were a high level of physician engagement in the selection, management, and improvement of the metrics, and these metrics excited the care teams, which also provided some motivation,” she said. “We provided real-time, high-quality feedback of performance. And last but probably not least was a financial incentive for the CCP as a team, not part of any individual compensation.”
The investigators plan to continue measuring the metrics, to expand them to other sites in their network, and to add new metrics that are common across the programs to minimize measurement burden, according to Ms. Porter. “We also plan to build cohorts for value-based care and unplanned care like ED visits and unplanned admissions. Finally, we want to keep momentum going and capitalize upon a provider engagement in value measurement and improvement,” she said.
“Based on this work and prior abstracts, … there are many validated metrics to be used. So, to choose those metrics and to choose them through local leadership support, most importantly, engaging frontline staff and having their buy-in of the measures that you are collecting are important,” commented invited discussant Jessica A. Zerillo, MD, MPH, of the Beth Israel Deaconess Medical Center in Boston. “And this can include using incentives that drive such stakeholders, whether they be financial or simply pride with public reporting.”
Study details
“In the summer of 2015, we were starting to feel a lot of pressure to prepare for evolving reimbursement models,” Ms. Porter said, explaining the initiative’s genesis. “Mainly, how do we define our value, and how can we measure and improve on that value of the care we deliver? One answer, of course, is to measure and reduce unnecessary variation. And we knew, to be successful, we had to increase our physician engagement and leadership in the selection and improvement of our metrics.”
Physician leaders of the CCPs were asked to choose quality measures that met three criteria: they were meaningful and important to both the care team and patients, they had pertinent data elements already available in existing databases (to reduce documentation burden), and they were multidisciplinary in nature, reflecting the care provided by the whole program. The measures ultimately selected included a variety of those put forth by American Society of Clinical Oncology’s Quality Oncology Practice Initiative and the American Society for Radiation Oncology. CCPs were offered a financial incentive for meeting targets ranging from $75,000 to $125,000 that was based on number of providers and patient volume, rather than on the impact of improved metric. “This was really meant for reinvestment back into their quality programs,” Ms. Porter said. “I would argue this was really a culture-building year for us, and we hope that next year there might be a little bit more tangible value with the metrics.”
The quality team gave CCPs monthly or quarterly performance reports with unblinded physician- and patient-level details that were ultimately disseminated to all the other CCPs. They also investigated any missing data for individual metrics.
Study results showed that half of the 16 measures the physician leaders chose pertained to the diagnosis and treatment planning phase of care, according to Ms. Porter. “It was important to many of our CCPs to ensure that specific testing was done, which would then, in turn, drive treatment planning decisions,” she commented.
At the end of the year, each metric was assessed among 13 to 2,406 patients. “All CCPs met their predetermined target and earned their financial incentive award for the year,” Ms. Porter reported.
Improvement was most marked, with a 50% absolute increase, for the metric of completing a staging module, which required conversion of staging information (historically embedded in progress notes) into a structured format in a dedicated field in the electronic health record within 45 days of a patient’s first cancer treatment. This practice enables ready identification of stage cohorts in which value of care can be assessed, she noted.
There were also sizable absolute increases in relevant CCPs in the proportion of blood and marrow transplant recipients referred to survivorship care by day 100 (+20%) and visiting that service by day 180 (+13%), recording of hand and foot pain (+34%) and radiation dermatitis (+21%), mismatch repair testing in patients with newly diagnosed colorectal cancer (+10%), referral of patients with newly diagnosed ovarian cancer for genetic counseling (+43%), cytogenetic testing in patients with newly diagnosed hematologic malignancies (+17%), hepatitis B testing before rituximab administration (+17%), and allowance of at least 2 nights for treatment plan physics–quality assurance before the start of a nonemergent radiation oncology treatment (+14%).
Meanwhile, there were decreases, considered favorable changes, in chemotherapy use in the last 2 weeks of life among neuro-oncology patients (–9%) and in patients’ receipt of more than 10 fractions of radiation therapy for palliation of bone metastases (–9%).
Finally, there was no change in several metrics of quality that were already at very high or low levels, as appropriate, at baseline: molecular testing in patients with newly diagnosed acute myeloid leukemia (stable at 95%), hospice enrollment at the time of death for neuro-oncology patients (stable at 100%), chemotherapy in the last 2 weeks of life for patients with sarcoma (stable at 0%), and epidermal growth factor receptor testing in patients with newly diagnosed lung adenocarcinoma (stable at 98%).
AT THE QUALITY CARE SYMPOSIUM
Key clinical point:
Major finding: Over a 1-year period, the center saw improvements in practices such as completion of staging modules (+50%), recording of hand and foot pain (+34%), hepatitis B testing before rituximab use (+17%), and referral of patients with ovarian cancer for genetic counseling (+43%).
Data source: An initiative targeting 16 quality metrics undertaken by 13 cancer care programs at Stanford Health Care.
Disclosures: Ms. Porter disclosed that she had no relevant conflicts of interest.
Decision support tool appears to safely reduce CSF use
ORLANDO – A decision support tool safely reduces use of colony-stimulating factors (CSFs) in patients undergoing chemotherapy for lung cancer, suggests a retrospective claims-based cohort study of nearly 3,500 patients across the country.
The rate of CSF use fell among patients treated in the nine states that implemented the tool – a library of chemotherapy regimens and their expected FN risk that uses preauthorization and an algorithm to promote risk-appropriate, guideline-adherent use – but it remained unchanged in the 39 states and the District of Columbia, where usual practice continued, investigators reported at a symposium on quality care sponsored by the American Society of Clinical Oncology and simultaneously published (J Oncol Pract. 2017 March 4. doi: 10.1200/JOP.2017.020867). The adjusted difference was nearly 9%.
“Decision support programs like the one highlighted here could be one way, definitely not the only way, of achieving guideline-adherent CSF use and reducing practice variation across the country,” commented coinvestigator Abiy Agiro, PhD, associate director of payer and provider research at HealthCore, a subsidiary of Anthem, in Wilmington, Delaware.
“Such efforts could also have unintended consequences, so it’s important to study relevant patient outcomes,” he added. “In this case, although it appears that the incidence of febrile neutropenia rising does not seem to relate with the program, the study does not establish the safety of CSF use reduction in lung cancer patients receiving chemotherapy. So, we should take the results with that caveat.”
Parsing the findings
Although the United States makes up just 4% of the world’s population, it uses nearly 80% of CSFs sold by a leading manufacturer, according to invited discussant Thomas J. Smith, MD, a professor of oncology and palliative medicine at Johns Hopkins University in Baltimore.
“When we rewrote the ASCO [American Society of Clinical Oncology] guidelines on CSF use in 2015, there were some specific indications: dose-intense chemo for adjuvant breast cancer and uroepithelial cancer and ... when the risk of febrile neutropenia is about 20% and dose reduction is not an appropriate strategy. We were quick to point out that most regimens have a risk of febrile neutropenia much less than that,” he noted.
Dr. Agiro and his colleagues’ findings are valid, real, and reproducible, Dr. Smith maintained. However, it is unclear to what extent the observed levels of CSF use represented overuse.
“In lung cancer, there are very few regimens that have a febrile neutropenia rate close to 20%,” he elaborated. “What we don’t know is how much of this [use] was actually justified. I would suspect it is 10% or 15%, rather than 40%.”
CSF use, as guided by the new tool, “might not support increased dose density [of chemotherapy], but I would challenge anybody in the audience to show me data in normal solid tumor patients that [show that] dose density maintained by CSFs makes a difference in overall survival,” he said.
Questions yet to be addressed include the difficulty and cost of using the decision support tool and the possible negative impact on practices’ finances, according to Dr. Smith.
“When ESAs [erythropoiesis-stimulating agents] came off being used so much, some of my friends’ practices took a 15% to 20% drop in their revenue, and this is an important source of revenue for a lot of practices,” he explained. “So, I hope that when we take this revenue away, that we are cognizant of that and realize that it’s just another stress on practices, many of which are under significant stress already.”
Study details
An estimated 26% of uses of CSFs in patients with lung cancer are not in accordance with the ASCO practice guidelines, according to Dr. Agiro. “Such variations from recommendations are sometimes the reason why different stakeholders take actions” to improve care, such as ASCO’s Quality Oncology Practice Initiative (QOPI) and the American Board of Internal Medicine’s Choosing Wisely initiative (J Oncol Pract. 2015;11:338-43).
The decision support tool evaluated in the study uses preauthorization before delivery of care and, therefore, differs from point-of-care interventions, he noted.
“The tool allows access to a library of chemotherapy regimens and their associated, expected febrile neutropenia risk based on the myelotoxicity of the planned regimens as indicated in published trials. The tool is accessible online and provides real-time recommendations that are tailored based on disease- and patient-specific factors for either the use of CSF or not,” he elaborated.
According to the tool’s algorithm, use is recommended for patients who are given a regimen with a high risk of febrile neutropenia (greater than 20%) and is not recommended for those given a low-risk regimen (less than 10%). It is tailored according to the presence of additional risk factors for the intermediate-risk group.
Oncologists use the tool only for patients starting a new chemotherapy and only in the first cycle, when the risk of febrile neutropenia is highest, according to Dr. Agiro. “Once the approval is given in the first cycle, it remains in effect for the next 6 months, so they don’t have to use it again and again in additional cycles,” he explained.
The decision support tool was implemented in nine states starting in July 2014. In the study, which was funded by Anthem, the investigators analyzed administrative claims data from commercially insured adult patients starting chemotherapy for lung cancer, assessing changes in outcomes between a preimplementation period (April 2013 to Dec. 2013) and a postimplementation period (July 2014 to March 2015).
Analyses were based on 1,857 patients in the states that implemented the tool and 1,610 patients in the states that did not.
The percentage of patients receiving CSFs in the 6 months after starting chemotherapy fell in states that implemented the decision support tool (from 48.4% to 35.6%) but remained stable in states that did not (43.2% and 44.4%), Dr. Agiro reported. The adjusted difference in differences was –8.7% (P less than .001).
Meanwhile, the percentage of patients admitted for febrile neutropenia or experiencing this outcome while hospitalized increased in both states implementing the tool (from 2.8% to 4.3%) and those not implementing it (from 3.1% to 5.1%). Although the magnitude of increase was smaller in the former (+1.5% vs. +2.0%), the difference was not significant. Findings were essentially the same among the subset of patients aged 65 years and older.
“It’s important to study both intended and unintended consequences of such interventions,” Dr. Agiro noted. “Our study goes beyond financial considerations by looking at unintended outcomes: in this case, focusing on the incidence of febrile neutropenia, an outcome that is of prime interest to patients and oncologists and payers alike.”
The study may have missed some cases of febrile neutropenia, he acknowledged. “Also, there are other important outcomes of concern. For example, were there any delays in chemotherapy administration or immune recovery that could have been triggered by the implementation of the decision support program?”
The impact, both intended and unintended, on practices warrants evaluation as well, he further noted. “An important question could be, ‘Does it take less time to use this decision support tool compared to the time taken with normal care processes?’ ”
Dr. Agiro disclosed that he is employed by, has stock or other ownership interests in, and receives research funding from Anthem.
ORLANDO – A decision support tool safely reduces use of colony-stimulating factors (CSFs) in patients undergoing chemotherapy for lung cancer, suggests a retrospective claims-based cohort study of nearly 3,500 patients across the country.
The rate of CSF use fell among patients treated in the nine states that implemented the tool – a library of chemotherapy regimens and their expected FN risk that uses preauthorization and an algorithm to promote risk-appropriate, guideline-adherent use – but it remained unchanged in the 39 states and the District of Columbia, where usual practice continued, investigators reported at a symposium on quality care sponsored by the American Society of Clinical Oncology and simultaneously published (J Oncol Pract. 2017 March 4. doi: 10.1200/JOP.2017.020867). The adjusted difference was nearly 9%.
“Decision support programs like the one highlighted here could be one way, definitely not the only way, of achieving guideline-adherent CSF use and reducing practice variation across the country,” commented coinvestigator Abiy Agiro, PhD, associate director of payer and provider research at HealthCore, a subsidiary of Anthem, in Wilmington, Delaware.
“Such efforts could also have unintended consequences, so it’s important to study relevant patient outcomes,” he added. “In this case, although it appears that the incidence of febrile neutropenia rising does not seem to relate with the program, the study does not establish the safety of CSF use reduction in lung cancer patients receiving chemotherapy. So, we should take the results with that caveat.”
Parsing the findings
Although the United States makes up just 4% of the world’s population, it uses nearly 80% of CSFs sold by a leading manufacturer, according to invited discussant Thomas J. Smith, MD, a professor of oncology and palliative medicine at Johns Hopkins University in Baltimore.
“When we rewrote the ASCO [American Society of Clinical Oncology] guidelines on CSF use in 2015, there were some specific indications: dose-intense chemo for adjuvant breast cancer and uroepithelial cancer and ... when the risk of febrile neutropenia is about 20% and dose reduction is not an appropriate strategy. We were quick to point out that most regimens have a risk of febrile neutropenia much less than that,” he noted.
Dr. Agiro and his colleagues’ findings are valid, real, and reproducible, Dr. Smith maintained. However, it is unclear to what extent the observed levels of CSF use represented overuse.
“In lung cancer, there are very few regimens that have a febrile neutropenia rate close to 20%,” he elaborated. “What we don’t know is how much of this [use] was actually justified. I would suspect it is 10% or 15%, rather than 40%.”
CSF use, as guided by the new tool, “might not support increased dose density [of chemotherapy], but I would challenge anybody in the audience to show me data in normal solid tumor patients that [show that] dose density maintained by CSFs makes a difference in overall survival,” he said.
Questions yet to be addressed include the difficulty and cost of using the decision support tool and the possible negative impact on practices’ finances, according to Dr. Smith.
“When ESAs [erythropoiesis-stimulating agents] came off being used so much, some of my friends’ practices took a 15% to 20% drop in their revenue, and this is an important source of revenue for a lot of practices,” he explained. “So, I hope that when we take this revenue away, that we are cognizant of that and realize that it’s just another stress on practices, many of which are under significant stress already.”
Study details
An estimated 26% of uses of CSFs in patients with lung cancer are not in accordance with the ASCO practice guidelines, according to Dr. Agiro. “Such variations from recommendations are sometimes the reason why different stakeholders take actions” to improve care, such as ASCO’s Quality Oncology Practice Initiative (QOPI) and the American Board of Internal Medicine’s Choosing Wisely initiative (J Oncol Pract. 2015;11:338-43).
The decision support tool evaluated in the study uses preauthorization before delivery of care and, therefore, differs from point-of-care interventions, he noted.
“The tool allows access to a library of chemotherapy regimens and their associated, expected febrile neutropenia risk based on the myelotoxicity of the planned regimens as indicated in published trials. The tool is accessible online and provides real-time recommendations that are tailored based on disease- and patient-specific factors for either the use of CSF or not,” he elaborated.
According to the tool’s algorithm, use is recommended for patients who are given a regimen with a high risk of febrile neutropenia (greater than 20%) and is not recommended for those given a low-risk regimen (less than 10%). It is tailored according to the presence of additional risk factors for the intermediate-risk group.
Oncologists use the tool only for patients starting a new chemotherapy and only in the first cycle, when the risk of febrile neutropenia is highest, according to Dr. Agiro. “Once the approval is given in the first cycle, it remains in effect for the next 6 months, so they don’t have to use it again and again in additional cycles,” he explained.
The decision support tool was implemented in nine states starting in July 2014. In the study, which was funded by Anthem, the investigators analyzed administrative claims data from commercially insured adult patients starting chemotherapy for lung cancer, assessing changes in outcomes between a preimplementation period (April 2013 to Dec. 2013) and a postimplementation period (July 2014 to March 2015).
Analyses were based on 1,857 patients in the states that implemented the tool and 1,610 patients in the states that did not.
The percentage of patients receiving CSFs in the 6 months after starting chemotherapy fell in states that implemented the decision support tool (from 48.4% to 35.6%) but remained stable in states that did not (43.2% and 44.4%), Dr. Agiro reported. The adjusted difference in differences was –8.7% (P less than .001).
Meanwhile, the percentage of patients admitted for febrile neutropenia or experiencing this outcome while hospitalized increased in both states implementing the tool (from 2.8% to 4.3%) and those not implementing it (from 3.1% to 5.1%). Although the magnitude of increase was smaller in the former (+1.5% vs. +2.0%), the difference was not significant. Findings were essentially the same among the subset of patients aged 65 years and older.
“It’s important to study both intended and unintended consequences of such interventions,” Dr. Agiro noted. “Our study goes beyond financial considerations by looking at unintended outcomes: in this case, focusing on the incidence of febrile neutropenia, an outcome that is of prime interest to patients and oncologists and payers alike.”
The study may have missed some cases of febrile neutropenia, he acknowledged. “Also, there are other important outcomes of concern. For example, were there any delays in chemotherapy administration or immune recovery that could have been triggered by the implementation of the decision support program?”
The impact, both intended and unintended, on practices warrants evaluation as well, he further noted. “An important question could be, ‘Does it take less time to use this decision support tool compared to the time taken with normal care processes?’ ”
Dr. Agiro disclosed that he is employed by, has stock or other ownership interests in, and receives research funding from Anthem.
ORLANDO – A decision support tool safely reduces use of colony-stimulating factors (CSFs) in patients undergoing chemotherapy for lung cancer, suggests a retrospective claims-based cohort study of nearly 3,500 patients across the country.
The rate of CSF use fell among patients treated in the nine states that implemented the tool – a library of chemotherapy regimens and their expected FN risk that uses preauthorization and an algorithm to promote risk-appropriate, guideline-adherent use – but it remained unchanged in the 39 states and the District of Columbia, where usual practice continued, investigators reported at a symposium on quality care sponsored by the American Society of Clinical Oncology and simultaneously published (J Oncol Pract. 2017 March 4. doi: 10.1200/JOP.2017.020867). The adjusted difference was nearly 9%.
“Decision support programs like the one highlighted here could be one way, definitely not the only way, of achieving guideline-adherent CSF use and reducing practice variation across the country,” commented coinvestigator Abiy Agiro, PhD, associate director of payer and provider research at HealthCore, a subsidiary of Anthem, in Wilmington, Delaware.
“Such efforts could also have unintended consequences, so it’s important to study relevant patient outcomes,” he added. “In this case, although it appears that the incidence of febrile neutropenia rising does not seem to relate with the program, the study does not establish the safety of CSF use reduction in lung cancer patients receiving chemotherapy. So, we should take the results with that caveat.”
Parsing the findings
Although the United States makes up just 4% of the world’s population, it uses nearly 80% of CSFs sold by a leading manufacturer, according to invited discussant Thomas J. Smith, MD, a professor of oncology and palliative medicine at Johns Hopkins University in Baltimore.
“When we rewrote the ASCO [American Society of Clinical Oncology] guidelines on CSF use in 2015, there were some specific indications: dose-intense chemo for adjuvant breast cancer and uroepithelial cancer and ... when the risk of febrile neutropenia is about 20% and dose reduction is not an appropriate strategy. We were quick to point out that most regimens have a risk of febrile neutropenia much less than that,” he noted.
Dr. Agiro and his colleagues’ findings are valid, real, and reproducible, Dr. Smith maintained. However, it is unclear to what extent the observed levels of CSF use represented overuse.
“In lung cancer, there are very few regimens that have a febrile neutropenia rate close to 20%,” he elaborated. “What we don’t know is how much of this [use] was actually justified. I would suspect it is 10% or 15%, rather than 40%.”
CSF use, as guided by the new tool, “might not support increased dose density [of chemotherapy], but I would challenge anybody in the audience to show me data in normal solid tumor patients that [show that] dose density maintained by CSFs makes a difference in overall survival,” he said.
Questions yet to be addressed include the difficulty and cost of using the decision support tool and the possible negative impact on practices’ finances, according to Dr. Smith.
“When ESAs [erythropoiesis-stimulating agents] came off being used so much, some of my friends’ practices took a 15% to 20% drop in their revenue, and this is an important source of revenue for a lot of practices,” he explained. “So, I hope that when we take this revenue away, that we are cognizant of that and realize that it’s just another stress on practices, many of which are under significant stress already.”
Study details
An estimated 26% of uses of CSFs in patients with lung cancer are not in accordance with the ASCO practice guidelines, according to Dr. Agiro. “Such variations from recommendations are sometimes the reason why different stakeholders take actions” to improve care, such as ASCO’s Quality Oncology Practice Initiative (QOPI) and the American Board of Internal Medicine’s Choosing Wisely initiative (J Oncol Pract. 2015;11:338-43).
The decision support tool evaluated in the study uses preauthorization before delivery of care and, therefore, differs from point-of-care interventions, he noted.
“The tool allows access to a library of chemotherapy regimens and their associated, expected febrile neutropenia risk based on the myelotoxicity of the planned regimens as indicated in published trials. The tool is accessible online and provides real-time recommendations that are tailored based on disease- and patient-specific factors for either the use of CSF or not,” he elaborated.
According to the tool’s algorithm, use is recommended for patients who are given a regimen with a high risk of febrile neutropenia (greater than 20%) and is not recommended for those given a low-risk regimen (less than 10%). It is tailored according to the presence of additional risk factors for the intermediate-risk group.
Oncologists use the tool only for patients starting a new chemotherapy and only in the first cycle, when the risk of febrile neutropenia is highest, according to Dr. Agiro. “Once the approval is given in the first cycle, it remains in effect for the next 6 months, so they don’t have to use it again and again in additional cycles,” he explained.
The decision support tool was implemented in nine states starting in July 2014. In the study, which was funded by Anthem, the investigators analyzed administrative claims data from commercially insured adult patients starting chemotherapy for lung cancer, assessing changes in outcomes between a preimplementation period (April 2013 to Dec. 2013) and a postimplementation period (July 2014 to March 2015).
Analyses were based on 1,857 patients in the states that implemented the tool and 1,610 patients in the states that did not.
The percentage of patients receiving CSFs in the 6 months after starting chemotherapy fell in states that implemented the decision support tool (from 48.4% to 35.6%) but remained stable in states that did not (43.2% and 44.4%), Dr. Agiro reported. The adjusted difference in differences was –8.7% (P less than .001).
Meanwhile, the percentage of patients admitted for febrile neutropenia or experiencing this outcome while hospitalized increased in both states implementing the tool (from 2.8% to 4.3%) and those not implementing it (from 3.1% to 5.1%). Although the magnitude of increase was smaller in the former (+1.5% vs. +2.0%), the difference was not significant. Findings were essentially the same among the subset of patients aged 65 years and older.
“It’s important to study both intended and unintended consequences of such interventions,” Dr. Agiro noted. “Our study goes beyond financial considerations by looking at unintended outcomes: in this case, focusing on the incidence of febrile neutropenia, an outcome that is of prime interest to patients and oncologists and payers alike.”
The study may have missed some cases of febrile neutropenia, he acknowledged. “Also, there are other important outcomes of concern. For example, were there any delays in chemotherapy administration or immune recovery that could have been triggered by the implementation of the decision support program?”
The impact, both intended and unintended, on practices warrants evaluation as well, he further noted. “An important question could be, ‘Does it take less time to use this decision support tool compared to the time taken with normal care processes?’ ”
Dr. Agiro disclosed that he is employed by, has stock or other ownership interests in, and receives research funding from Anthem.
AT THE QUALITY CARE SYMPOSIUM
Key clinical point:
Major finding: The percentage of patients receiving CSFs fell in states that used the tool, versus those that did not (difference in differences, –8.7%), but changes in admissions for febrile neutropenia did not differ significantly.
Data source: A retrospective cohort study of 3,467 patients from 48 states starting chemotherapy for lung cancer.
Disclosures: Dr. Agiro disclosed that he is employed by, has stock or other ownership interests in, and receives research funding from Anthem. The study was funded by Anthem.
VIDEO: Sexuality, fertility are focus of cancer education website
MIAMI BEACH – Often people living with cancer hesitate to ask their providers about sensitive and important issues surrounding sexuality and fertility. At the same time, some clinicians remain uncomfortable raising questions regarding sexual function or simply lack the time to appropriately address the issues during a patient encounter.
A new online resource aims to solve both problems simultaneously, giving both patients and providers the tools to meaningfully address sexuality and fertility issues, Leslie R. Schover, PhD, said in a video interview at the annual Miami Breast Cancer Conference, held by Physicians’ Education Resource.
The Will2love.com site features first-person patient account videos from women and men who faced similar concerns, said Dr. Schover, founder of the Will2Love digital health company based in Houston. In addition, vignettes with actors inform patients and also model how oncologists, oncology nurses, and other staff could effectively communicate with concerned patients. A professional portal offers online skills training for clinicians.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
MIAMI BEACH – Often people living with cancer hesitate to ask their providers about sensitive and important issues surrounding sexuality and fertility. At the same time, some clinicians remain uncomfortable raising questions regarding sexual function or simply lack the time to appropriately address the issues during a patient encounter.
A new online resource aims to solve both problems simultaneously, giving both patients and providers the tools to meaningfully address sexuality and fertility issues, Leslie R. Schover, PhD, said in a video interview at the annual Miami Breast Cancer Conference, held by Physicians’ Education Resource.
The Will2love.com site features first-person patient account videos from women and men who faced similar concerns, said Dr. Schover, founder of the Will2Love digital health company based in Houston. In addition, vignettes with actors inform patients and also model how oncologists, oncology nurses, and other staff could effectively communicate with concerned patients. A professional portal offers online skills training for clinicians.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
MIAMI BEACH – Often people living with cancer hesitate to ask their providers about sensitive and important issues surrounding sexuality and fertility. At the same time, some clinicians remain uncomfortable raising questions regarding sexual function or simply lack the time to appropriately address the issues during a patient encounter.
A new online resource aims to solve both problems simultaneously, giving both patients and providers the tools to meaningfully address sexuality and fertility issues, Leslie R. Schover, PhD, said in a video interview at the annual Miami Breast Cancer Conference, held by Physicians’ Education Resource.
The Will2love.com site features first-person patient account videos from women and men who faced similar concerns, said Dr. Schover, founder of the Will2Love digital health company based in Houston. In addition, vignettes with actors inform patients and also model how oncologists, oncology nurses, and other staff could effectively communicate with concerned patients. A professional portal offers online skills training for clinicians.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
Lung cancer pathways reduce cost of care without compromising outcomes
ORLANDO – Implementation of clinical pathways aimed at improving appropriate, evidence-based care for patients with metastatic non–small-cell lung cancer (NSCLC) reduces costs without negatively affecting survival, the Dana-Farber Cancer Institute’s experience suggests.
“At Dana-Farber ... we have looked toward pathways as a potential tool to help manage complexity and resource utilization,” senior author David M. Jackman, MD, explained at a symposium on quality care sponsored by the American Society of Clinical Oncology. “We see pathways as a patient-centered platform that provides real-time decision-making support across the continuum of cancer care. We think that these should be based on preemptive decision making, reflect current standards of care, incorporate feedback from which we can learn from our practice patterns, and support clinical research.”
After the customized Dana-Farber Lung Pathways were implemented in 2014, the cost of outpatient care per patient in the first year after diagnosis fell by about $17,000, or 25%, primarily driven by reduced use of antineoplastic agents, according to data reported at the symposium and simultaneously published (J Oncol Pract. 2017 Mar 4. doi: 10.1200/JOP.2017.021741). Meanwhile, median survival remained at about 11 months, even trending slightly upward.
“Frankly, I’d like to think that we were delivering reasonable and expert care prior to 2014, so I did not anticipate that we were going to see a major change in terms of improvement in survival. But it is important for us to make sure that as we implemented Pathways, there was certainly no decrease in such care,”said Dr. Jackman, medical director of Clinical Pathways at Dana-Farber and an assistant professor of medicine, Harvard Medical School, Boston.
He and his colleagues plan to expand Pathways to cover the full spectrum of cancer care at their center, encompassing medical, radiation, and surgical oncology, he said.
“We also think that pathways can have a major impact on things like symptom management and survivorship care,” he added. “And as we work to embed all of our trials within our Pathways system, and as we push to have our trials in our satellites and in our network affiliates, we hope that this combination of activity can help move us from being not just a good care network, but also a research network.”
The pathways will still have to address some of the thornier issues related to the value of care, Dr. Jackman acknowledged “It’s incredibly easy for us to look at two equivalent therapies in terms of toxicity and efficacy and pick the cheaper one. The harder conversations are to come, that is, what if something is x dollars more expensive and only improves things by a small number of months, is it really worth it?
“Finally, we hope that pathways can be an area for innovation, not used solely to manage costs and to make decisions based on yesteryear, but also to help us move forward and to be the watering hole where everybody comes, as we build out our system that is looking granularly at genomics in order to help match patients with trial opportunities, and for researchers, to help them find specific patients for their trials,” he said. “Pathways can potentially be the nexus where everyone comes and where doctors are informed in real time about opportunities for their patients.”
More evidence of benefit
The Dana-Farber study adds to others showing that the benefits of pathways are real and reproducible, according to invited discussant Thomas J. Smith, MD, professor of oncology and palliative medicine at Johns Hopkins Medicine in Baltimore.
“More importantly, I think, for patients, who are getting hit with these bills and might have a 20% copay, it’s going to reduce their copays and for all the right reasons,” Dr. Smith concluded.
Pathways development
In developing the pathways, Dana-Farber began with lung cancer in part because the center sees a high volume of patients with the disease. In addition, decision making for this malignancy is complex, and there was considerable variation in oncologists’ practices.
“Our platform exists as an independent web-based system that currently lives outside of our EMR. Physicians can access this in real time, in the clinic room with the patient if they so choose,” Dr. Jackman explained. “From our EMR, we are flagged every time a provider orders a new start [of therapy], whether it’s IV chemo, oral chemo, or hormonal therapy. From our vendor, we receive granular treatment decision information made within the pathways system – information about the provider and site, information about the patients, their disease, and the line of therapy, as well as other important factors that drive decision making. Finally, from our clinical trials system interface, we can confirm trial enrollment data.”
Oncologists are free to leave the suggested pathway if their clinical judgment favors an alternate course, according to Dr. Jackman.
“We always want our physicians to feel comfortable treating the patients in front of them however they see best fit. If that means an off-pathway therapy, we want them to have the freedom to do that,” he said. “But we think one of the major tools of the pathways is to help capture the reasons why. So if they think it’s warranted and appropriate, go ahead, go off pathway, but tell us why you are doing it so we can learn from it.”
Using Pathways has not proved burdensome, according to Dr. Jackman. Navigating through the system requires about a minute or two, and use is required only when a patient is starting a new therapy, which typically occurs less than once per half-day clinic session.
Study details
In the study, he and colleagues compared costs of care in the first year after diagnosis of stage IV NSCLC between 160 patients treated at Dana-Farber in 2012 (before Pathways implementation) and 210 patients treated there in 2014 (after Pathways implementation).
“It should be noted that because we are a free-standing outpatient cancer center, all of the costs that we were able to gather are intramural and therefore related only to outpatient activities,” he pointed out.
The total annual costs of care per patient, adjusted for potential confounders (age, sex, race, distance to the institute, clinical trial enrollment, and EGFR and ALK status) fell by $17,085 after implementation of Pathways, from $69,122 to $52,037 (P = .01), he reported.
The largest source of cost savings by far, accounting for 73% of the total, was reduced use of antineoplastic agents (chemotherapy, biologics, and other anticancer agents). Cost for this component fell from $44,237 per patient to $31,846 (P less than .01).
“The majority of this savings came through a reduction in the use of what we considered unwarranted use of combination chemotherapy,” Dr. Jackman said. “In the first-line setting, we specifically went after the regimen of carboplatin, pemetrexed, and bevacizumab; based on our interpretation of the PointBreak study, we felt that that regimen did not bring additional efficacy but did essentially double drug costs. In going after that, we reduced not only use of that but also the subsequent use of pemetrexed plus bevacizumab maintenance. In the second-line setting, with the implementation of Pathways, we saw a decrease in the use of inappropriate platinum-based doublet therapy in those patients who had previously progressed on a platinum-based doublet.”
Median overall survival did not decrease and in fact increased slightly, from 10.7 months before Pathways implementation to 11.2 months afterward (P = .08). Corresponding 1-year rates of survival were 52% and 64%.
“We stand on the shoulders of those who came before us, who have also shown savings associated with implementation of pathways,” concluded Dr. Jackman. “But we hope that we add our voice and our data to this argument that pathways, I think, are a reasonable tool as we try to manage complexity and resource utilization. In addition, we do so without impinging upon clinical outcomes.”
The study was limited by its inclusion of only outpatient costs at Dana-Farber, he acknowledged. “You and we would be very interested in being able to know whether our Pathways implementation affected ED [emergency department] visits or hospitalizations. To that end, we are working with some of our regional payers to try to transparently share data around outcomes, costs, and usage, so that we can learn more in this regard.”
Dr. Jackman disclosed that he is an adviser or consultant to Bayer, Celgene, CVS Caremark, Genentech, and Lilly.
ORLANDO – Implementation of clinical pathways aimed at improving appropriate, evidence-based care for patients with metastatic non–small-cell lung cancer (NSCLC) reduces costs without negatively affecting survival, the Dana-Farber Cancer Institute’s experience suggests.
“At Dana-Farber ... we have looked toward pathways as a potential tool to help manage complexity and resource utilization,” senior author David M. Jackman, MD, explained at a symposium on quality care sponsored by the American Society of Clinical Oncology. “We see pathways as a patient-centered platform that provides real-time decision-making support across the continuum of cancer care. We think that these should be based on preemptive decision making, reflect current standards of care, incorporate feedback from which we can learn from our practice patterns, and support clinical research.”
After the customized Dana-Farber Lung Pathways were implemented in 2014, the cost of outpatient care per patient in the first year after diagnosis fell by about $17,000, or 25%, primarily driven by reduced use of antineoplastic agents, according to data reported at the symposium and simultaneously published (J Oncol Pract. 2017 Mar 4. doi: 10.1200/JOP.2017.021741). Meanwhile, median survival remained at about 11 months, even trending slightly upward.
“Frankly, I’d like to think that we were delivering reasonable and expert care prior to 2014, so I did not anticipate that we were going to see a major change in terms of improvement in survival. But it is important for us to make sure that as we implemented Pathways, there was certainly no decrease in such care,”said Dr. Jackman, medical director of Clinical Pathways at Dana-Farber and an assistant professor of medicine, Harvard Medical School, Boston.
He and his colleagues plan to expand Pathways to cover the full spectrum of cancer care at their center, encompassing medical, radiation, and surgical oncology, he said.
“We also think that pathways can have a major impact on things like symptom management and survivorship care,” he added. “And as we work to embed all of our trials within our Pathways system, and as we push to have our trials in our satellites and in our network affiliates, we hope that this combination of activity can help move us from being not just a good care network, but also a research network.”
The pathways will still have to address some of the thornier issues related to the value of care, Dr. Jackman acknowledged “It’s incredibly easy for us to look at two equivalent therapies in terms of toxicity and efficacy and pick the cheaper one. The harder conversations are to come, that is, what if something is x dollars more expensive and only improves things by a small number of months, is it really worth it?
“Finally, we hope that pathways can be an area for innovation, not used solely to manage costs and to make decisions based on yesteryear, but also to help us move forward and to be the watering hole where everybody comes, as we build out our system that is looking granularly at genomics in order to help match patients with trial opportunities, and for researchers, to help them find specific patients for their trials,” he said. “Pathways can potentially be the nexus where everyone comes and where doctors are informed in real time about opportunities for their patients.”
More evidence of benefit
The Dana-Farber study adds to others showing that the benefits of pathways are real and reproducible, according to invited discussant Thomas J. Smith, MD, professor of oncology and palliative medicine at Johns Hopkins Medicine in Baltimore.
“More importantly, I think, for patients, who are getting hit with these bills and might have a 20% copay, it’s going to reduce their copays and for all the right reasons,” Dr. Smith concluded.
Pathways development
In developing the pathways, Dana-Farber began with lung cancer in part because the center sees a high volume of patients with the disease. In addition, decision making for this malignancy is complex, and there was considerable variation in oncologists’ practices.
“Our platform exists as an independent web-based system that currently lives outside of our EMR. Physicians can access this in real time, in the clinic room with the patient if they so choose,” Dr. Jackman explained. “From our EMR, we are flagged every time a provider orders a new start [of therapy], whether it’s IV chemo, oral chemo, or hormonal therapy. From our vendor, we receive granular treatment decision information made within the pathways system – information about the provider and site, information about the patients, their disease, and the line of therapy, as well as other important factors that drive decision making. Finally, from our clinical trials system interface, we can confirm trial enrollment data.”
Oncologists are free to leave the suggested pathway if their clinical judgment favors an alternate course, according to Dr. Jackman.
“We always want our physicians to feel comfortable treating the patients in front of them however they see best fit. If that means an off-pathway therapy, we want them to have the freedom to do that,” he said. “But we think one of the major tools of the pathways is to help capture the reasons why. So if they think it’s warranted and appropriate, go ahead, go off pathway, but tell us why you are doing it so we can learn from it.”
Using Pathways has not proved burdensome, according to Dr. Jackman. Navigating through the system requires about a minute or two, and use is required only when a patient is starting a new therapy, which typically occurs less than once per half-day clinic session.
Study details
In the study, he and colleagues compared costs of care in the first year after diagnosis of stage IV NSCLC between 160 patients treated at Dana-Farber in 2012 (before Pathways implementation) and 210 patients treated there in 2014 (after Pathways implementation).
“It should be noted that because we are a free-standing outpatient cancer center, all of the costs that we were able to gather are intramural and therefore related only to outpatient activities,” he pointed out.
The total annual costs of care per patient, adjusted for potential confounders (age, sex, race, distance to the institute, clinical trial enrollment, and EGFR and ALK status) fell by $17,085 after implementation of Pathways, from $69,122 to $52,037 (P = .01), he reported.
The largest source of cost savings by far, accounting for 73% of the total, was reduced use of antineoplastic agents (chemotherapy, biologics, and other anticancer agents). Cost for this component fell from $44,237 per patient to $31,846 (P less than .01).
“The majority of this savings came through a reduction in the use of what we considered unwarranted use of combination chemotherapy,” Dr. Jackman said. “In the first-line setting, we specifically went after the regimen of carboplatin, pemetrexed, and bevacizumab; based on our interpretation of the PointBreak study, we felt that that regimen did not bring additional efficacy but did essentially double drug costs. In going after that, we reduced not only use of that but also the subsequent use of pemetrexed plus bevacizumab maintenance. In the second-line setting, with the implementation of Pathways, we saw a decrease in the use of inappropriate platinum-based doublet therapy in those patients who had previously progressed on a platinum-based doublet.”
Median overall survival did not decrease and in fact increased slightly, from 10.7 months before Pathways implementation to 11.2 months afterward (P = .08). Corresponding 1-year rates of survival were 52% and 64%.
“We stand on the shoulders of those who came before us, who have also shown savings associated with implementation of pathways,” concluded Dr. Jackman. “But we hope that we add our voice and our data to this argument that pathways, I think, are a reasonable tool as we try to manage complexity and resource utilization. In addition, we do so without impinging upon clinical outcomes.”
The study was limited by its inclusion of only outpatient costs at Dana-Farber, he acknowledged. “You and we would be very interested in being able to know whether our Pathways implementation affected ED [emergency department] visits or hospitalizations. To that end, we are working with some of our regional payers to try to transparently share data around outcomes, costs, and usage, so that we can learn more in this regard.”
Dr. Jackman disclosed that he is an adviser or consultant to Bayer, Celgene, CVS Caremark, Genentech, and Lilly.
ORLANDO – Implementation of clinical pathways aimed at improving appropriate, evidence-based care for patients with metastatic non–small-cell lung cancer (NSCLC) reduces costs without negatively affecting survival, the Dana-Farber Cancer Institute’s experience suggests.
“At Dana-Farber ... we have looked toward pathways as a potential tool to help manage complexity and resource utilization,” senior author David M. Jackman, MD, explained at a symposium on quality care sponsored by the American Society of Clinical Oncology. “We see pathways as a patient-centered platform that provides real-time decision-making support across the continuum of cancer care. We think that these should be based on preemptive decision making, reflect current standards of care, incorporate feedback from which we can learn from our practice patterns, and support clinical research.”
After the customized Dana-Farber Lung Pathways were implemented in 2014, the cost of outpatient care per patient in the first year after diagnosis fell by about $17,000, or 25%, primarily driven by reduced use of antineoplastic agents, according to data reported at the symposium and simultaneously published (J Oncol Pract. 2017 Mar 4. doi: 10.1200/JOP.2017.021741). Meanwhile, median survival remained at about 11 months, even trending slightly upward.
“Frankly, I’d like to think that we were delivering reasonable and expert care prior to 2014, so I did not anticipate that we were going to see a major change in terms of improvement in survival. But it is important for us to make sure that as we implemented Pathways, there was certainly no decrease in such care,”said Dr. Jackman, medical director of Clinical Pathways at Dana-Farber and an assistant professor of medicine, Harvard Medical School, Boston.
He and his colleagues plan to expand Pathways to cover the full spectrum of cancer care at their center, encompassing medical, radiation, and surgical oncology, he said.
“We also think that pathways can have a major impact on things like symptom management and survivorship care,” he added. “And as we work to embed all of our trials within our Pathways system, and as we push to have our trials in our satellites and in our network affiliates, we hope that this combination of activity can help move us from being not just a good care network, but also a research network.”
The pathways will still have to address some of the thornier issues related to the value of care, Dr. Jackman acknowledged “It’s incredibly easy for us to look at two equivalent therapies in terms of toxicity and efficacy and pick the cheaper one. The harder conversations are to come, that is, what if something is x dollars more expensive and only improves things by a small number of months, is it really worth it?
“Finally, we hope that pathways can be an area for innovation, not used solely to manage costs and to make decisions based on yesteryear, but also to help us move forward and to be the watering hole where everybody comes, as we build out our system that is looking granularly at genomics in order to help match patients with trial opportunities, and for researchers, to help them find specific patients for their trials,” he said. “Pathways can potentially be the nexus where everyone comes and where doctors are informed in real time about opportunities for their patients.”
More evidence of benefit
The Dana-Farber study adds to others showing that the benefits of pathways are real and reproducible, according to invited discussant Thomas J. Smith, MD, professor of oncology and palliative medicine at Johns Hopkins Medicine in Baltimore.
“More importantly, I think, for patients, who are getting hit with these bills and might have a 20% copay, it’s going to reduce their copays and for all the right reasons,” Dr. Smith concluded.
Pathways development
In developing the pathways, Dana-Farber began with lung cancer in part because the center sees a high volume of patients with the disease. In addition, decision making for this malignancy is complex, and there was considerable variation in oncologists’ practices.
“Our platform exists as an independent web-based system that currently lives outside of our EMR. Physicians can access this in real time, in the clinic room with the patient if they so choose,” Dr. Jackman explained. “From our EMR, we are flagged every time a provider orders a new start [of therapy], whether it’s IV chemo, oral chemo, or hormonal therapy. From our vendor, we receive granular treatment decision information made within the pathways system – information about the provider and site, information about the patients, their disease, and the line of therapy, as well as other important factors that drive decision making. Finally, from our clinical trials system interface, we can confirm trial enrollment data.”
Oncologists are free to leave the suggested pathway if their clinical judgment favors an alternate course, according to Dr. Jackman.
“We always want our physicians to feel comfortable treating the patients in front of them however they see best fit. If that means an off-pathway therapy, we want them to have the freedom to do that,” he said. “But we think one of the major tools of the pathways is to help capture the reasons why. So if they think it’s warranted and appropriate, go ahead, go off pathway, but tell us why you are doing it so we can learn from it.”
Using Pathways has not proved burdensome, according to Dr. Jackman. Navigating through the system requires about a minute or two, and use is required only when a patient is starting a new therapy, which typically occurs less than once per half-day clinic session.
Study details
In the study, he and colleagues compared costs of care in the first year after diagnosis of stage IV NSCLC between 160 patients treated at Dana-Farber in 2012 (before Pathways implementation) and 210 patients treated there in 2014 (after Pathways implementation).
“It should be noted that because we are a free-standing outpatient cancer center, all of the costs that we were able to gather are intramural and therefore related only to outpatient activities,” he pointed out.
The total annual costs of care per patient, adjusted for potential confounders (age, sex, race, distance to the institute, clinical trial enrollment, and EGFR and ALK status) fell by $17,085 after implementation of Pathways, from $69,122 to $52,037 (P = .01), he reported.
The largest source of cost savings by far, accounting for 73% of the total, was reduced use of antineoplastic agents (chemotherapy, biologics, and other anticancer agents). Cost for this component fell from $44,237 per patient to $31,846 (P less than .01).
“The majority of this savings came through a reduction in the use of what we considered unwarranted use of combination chemotherapy,” Dr. Jackman said. “In the first-line setting, we specifically went after the regimen of carboplatin, pemetrexed, and bevacizumab; based on our interpretation of the PointBreak study, we felt that that regimen did not bring additional efficacy but did essentially double drug costs. In going after that, we reduced not only use of that but also the subsequent use of pemetrexed plus bevacizumab maintenance. In the second-line setting, with the implementation of Pathways, we saw a decrease in the use of inappropriate platinum-based doublet therapy in those patients who had previously progressed on a platinum-based doublet.”
Median overall survival did not decrease and in fact increased slightly, from 10.7 months before Pathways implementation to 11.2 months afterward (P = .08). Corresponding 1-year rates of survival were 52% and 64%.
“We stand on the shoulders of those who came before us, who have also shown savings associated with implementation of pathways,” concluded Dr. Jackman. “But we hope that we add our voice and our data to this argument that pathways, I think, are a reasonable tool as we try to manage complexity and resource utilization. In addition, we do so without impinging upon clinical outcomes.”
The study was limited by its inclusion of only outpatient costs at Dana-Farber, he acknowledged. “You and we would be very interested in being able to know whether our Pathways implementation affected ED [emergency department] visits or hospitalizations. To that end, we are working with some of our regional payers to try to transparently share data around outcomes, costs, and usage, so that we can learn more in this regard.”
Dr. Jackman disclosed that he is an adviser or consultant to Bayer, Celgene, CVS Caremark, Genentech, and Lilly.
AT THE QUALITY CARE SYMPOSIUM
Key clinical point:
Major finding: The annual cost of outpatient care per patient fell by $17,085, mainly because of reduced use of antineoplastic agents, whereas median survival remained at about 11 months.
Data source: A cohort study among patients with newly diagnosed metastatic NSCLC, comparing 160 treated before and 210 treated after pathways implementation.
Disclosures: Dr. Jackman disclosed that he is an adviser or consultant to Bayer, Celgene, CVS Caremark, Genentech, and Lilly.
Posttreatment survivorship care needs of Spanish-speaking Latinas with breast cancer
After treatment, cancer patients transition to a survivorship phase, often with little information or support. Cancer survivors are at increased risk of recurrence, secondary cancers, comorbid conditions, and late treatment effects.1,2 However, many remain unaware of these risks and the options for managing them3 and face numerous unmet medical, psychosocial, and informational needs that can be addressed through survivorship care programs.4 Anxiety may increase as they lose their treatment team’s support while attempting to reestablish their lives.2 Patients need to know the long-term risks of cancer treatments, probabilities of recurrence and second cancers, effectiveness of surveillance and interventions for managing late effects and psychosocial concerns, and benefits of healthy lifestyles.2
Due to sociocultural and economic factors, Spanish-speaking Latina breast cancer survivors (SSBCS) suffer worse posttreatment health-related quality of life and more pain, fatigue, depressive symptoms, body image issues, and distress than their white counterparts.5-7 However, they are less likely to receive necessary cancer treatment, symptom management, and surveillance. For example, compared with whites, Latina breast cancer survivors receive less guideline-adherent treatment8 and follow-up care, including survivorship information.3,9 SSBCS, in particular have less access to survivorship information.10 Consequently, SSBCS are more likely to report unmet symptom management needs.11
Several breast cancer survivorship program trials have included Latinas,12,13 but their effectiveness has been demonstrated only for depressive symptoms or health worry. A comprehensive assessment of the posttreatment needs of SSBCS would provide a foundation for designing tailored survivorship interventions for this vulnerable group. This study aimed to identify the symptom management, psychosocial, and informational needs of SSBCS during the transition to survivorship from the perspectives of SSBCS and their cancer support providers and cancer physicians.
Methods
We sampled respondents within a 5-county area in Northern California to obtain multiple perspectives of the survivorship care needs of SSBCS using structured and in-depth methods: a telephone survey of SSBCS; semistructured interviews with SSBCS; semistructured interviews with cancer support providers serving SSBCS; and semistructured interviews with physicians providing cancer care for SSBCS. The study protocol was approved by the University of California San Francisco Committee on Human Research.
Sample and procedures
Structured telephone survey with SSBCS. The sample was drawn evenly from San Francisco General Hospital-University of California San Francisco primary care practices and SSBCS from a previous study who agreed to be re-contacted.14 The inclusion criteria were: completed active treatment (except adjuvant hormonal therapy) for nonmetastatic breast cancer within 10 years; living in one of the five counties; primarily Spanish-speaking; and self-identified as Latina. The exclusion criteria were: previous cancer except nonmelanoma skin cancer; terminal illness; or metastatic breast cancer. Study staff mailed potential participants a bilingual letter and information sheet, and bilingual opt-out postcard (6th grade reading level assessed by Flesch-Kincaid grade level statistic). Female bilingual-bicultural research associates conducted interviews of 20-30 minutes in Spanish after obtaining verbal consent. Participants were mailed $20. Surveys were conducted during March-November 2014.
Semistructured in-person interviews with SSBCS. Four community-based organizations (CBOs) in the targeted area providing cancer support services to Latinos agreed to recruit SSBCS for interviews. Inclusion criteria were identical to the survey. Patient navigators or support providers from CBOs contacted women by phone or in-person to invite them to an interview to assess their cancer survivorship needs. Women could choose a focus group or individual interview. With permission, names and contact information were given to study interviewers who called, explained the study, screened for eligibility, and scheduled an interview.
Recruitment was stratified by age (under or over age 50). We sampled women until saturation was achieved (no new themes emerged). Focus groups (90 minutes) were conducted at the CBOs. Individual interviews (45 minutes) were conducted in participants’ homes. Written informed consent was obtained. Participants were paid $50. Interviews were conducted during August-November, 2014, audiotaped, and transcribed.
Semistructured in-person interviews with cancer support providers and physicians. Investigators invited five cancer support providers (three patient navigators from three county hospitals, and two CBO directors of cancer psychosocial support services) and four physicians (three oncologists and one breast cancer surgeon from three county hospitals) to an in-person interview to identify SSBCS’ survivorship care needs. All agreed to participate. No further candidates were approached because saturation was achieved. We obtained written informed consent and 30-minute interviews were conducted in participants’ offices during August-October, 2014. Interviews were audiotaped and transcribed. Participants were paid $50.
Ethical approval. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and the 1964 Helsinki declaration and its later amendments or comparable ethical standards. This article does not contain any studies with animals performed by any of the authors.
Informed consent. Informed consent was obtained from all individual participants included in the study.
Measures
Structured telephone survey. Based on cancer survivor needs assessments,15 we assessed: physical and emotional symptoms; problems with sleep and memory/concentration; concerns about mortality, family, social isolation, intimacy, appearance; and healthy lifestyles. Items were adapted and translated into Spanish if needed, using forward/backward translation with team reconciliation. These questions used the introduction, “Now I am going to ask you if you have had any problems because of your cancer. In the past month, how much have you been bothered by …” with responses rated on a scale of 1-5 (1, Not at all; 5, A lot). For example, we asked, “In the past month, how much have you been bothered by fatigue?”
Regarding healthy lifestyles, we used the introduction, “Here are some changes women sometimes want to make after cancer. Would you like help with …?” For example, we asked, “Would you like help with getting more exercise?” We asked if they wanted help getting more exercise, eating healthier, managing stress, and doing meditation or yoga (Yes/No).
Semistructured interview guide for SSBCS. Participants were asked about their emotional and physical concerns when treatment ended, current cancer needs, symptoms or late effects, and issues related to relationships, family, employment, insurance, financial hardships, barriers to follow-up care, health behaviors, and survivorship program content. Sample questions are, “Have you had any symptoms or side effects related to your cancer or treatment?” and “What kinds of information do you feel you need now about your cancer or treatment?” A brief questionnaire assessed demographics.
Semistructured interview guide for cancer support providers and physicians. Support providers and physicians were asked about informational, psychosocial, and symptom management needs of SSBCS and recommended self-management content and formats. Sample questions are, “What kinds of information and support do you wish was available to help Spanish-speaking women take care of their health after treatment ends?” and “What do you think are the most pressing emotional needs of Spanish-speaking women after breast cancer treatment ends?” A brief questionnaire assessed demographics.
Analysis
Frequencies are reported for survey items. For questions about symptoms/concerns, we report the frequency of responding that they were bothered Somewhat/Quite a bit/A lot. For healthy lifestyles, we present the frequency answering Yes.
Verbatim semistructured interview transcripts were verified against audiotapes. Using QSR NVIVO software, transcripts were coded independently by two bilingual-bicultural investigators using a constant comparative method to generate coding categories for cancer survivorship needs.16 Coders started with themes specified by the interview guides, expanded them to represent the data, and discussed and reconciled coding discrepancies. Coding was compared by type of interview participant (survivor, support provider, or physician). Triangulation of survey and semistructured interviews occurred through team discussions to verify codes, themes, and implications for interventions.
Results
Telephone survey of SSBCS
Of the telephone survey sampling frame (N = 231), 118 individuals (51%) completed the interview, 37 (16%) were ineligible, 31 (13%) could not be reached, 22 (10%) had incorrect contact information, 19 (8%) refused to participate, and 4 (2%) were deceased. Mean age of the participants was 54.9 years (SD, 12.3); all were foreign-born, with more than half of Mexican origin; and most had less than a high-school education (Table 1). All had completed active treatment, and most (68%) were within 2 years of diagnosis.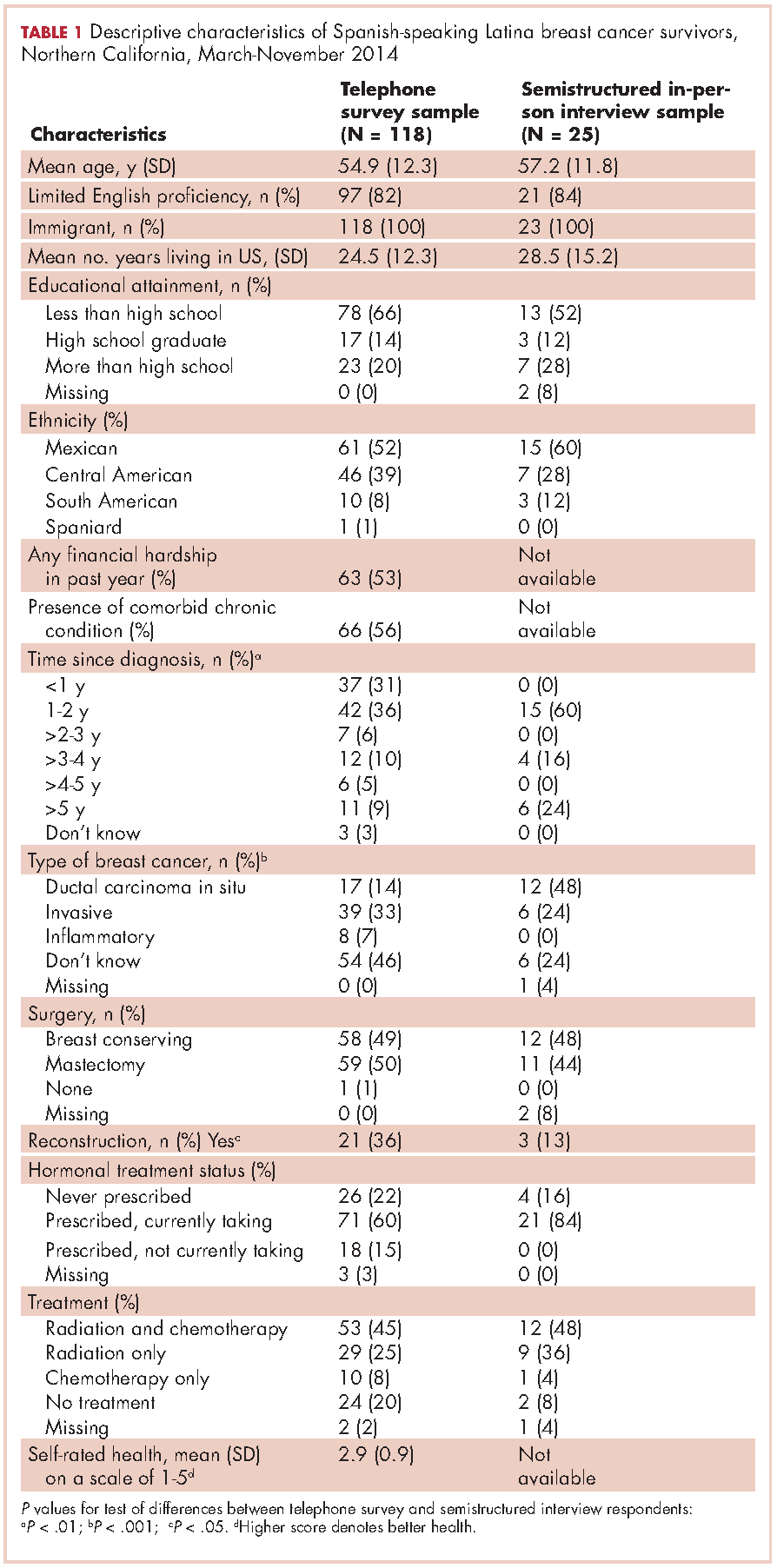
For symptom management needs (Table 2), the most prevalent (bothersome) symptoms (reported by more than 30%, in rank order) were joint pain, sleep problems, fatigue, hot flashes, numbness/tingling of extremities, and memory. Next most prevalent (reported by 20%-30%) were vaginal dryness, dry/itchy skin, dry nose/mouth, inability to concentrate, constipation, changes in urination, and shortness of breath.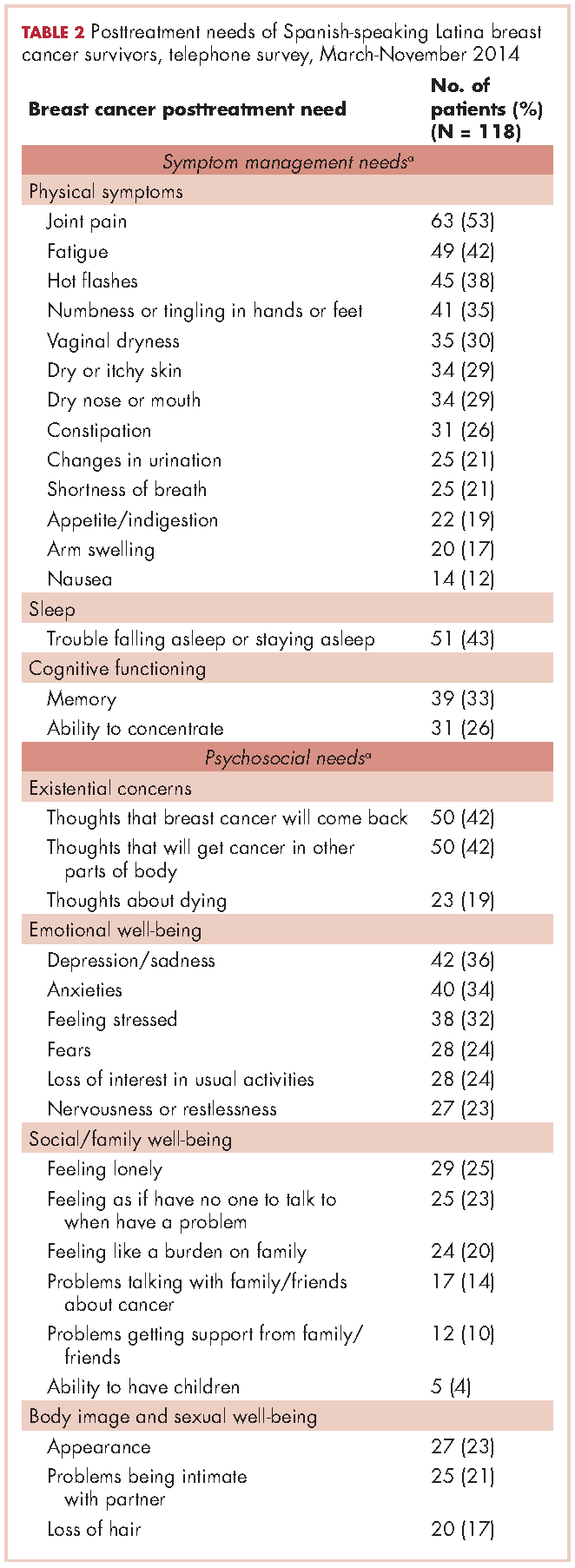

For psychosocial needs, fears of recurrence or new cancers were reported by 42%. Emotional symptoms reported by more than 30% were depression/sadness, anxieties, and feeling stressed. Next most prevalent (20%-30%) were fears, loss of interest in usual activities, and nervousness/restlessness. Social well-being concerns reported by 20%-30% of survivors were loneliness, having no one to talk to, and being a burden to their families. Body image and sexual problems reported by 20%-30% of survivors included appearance and problems being intimate with partners.
Regarding lifestyle, most of the participants said they wanted help with eating a healthier diet (74%), getting more exercise (69%), managing stress (63%), and doing yoga or meditation (55%).
Semistructured interviews
Twenty-five SSBCS completed semistructured interviews, 10 in individual interviews and 15 in one of two focus groups (one of 9 women older than 50 years; one of 6 women younger than 50). The telephone survey respondents were similar to semistructured interview respondents on all sociodemographic characteristics, but differed slightly on some clinical characteristics (Table 1). The telephone survey women had been more recently diagnosed (P < .01), were less likely to have ductal carcinoma in situ (P < .001), and more likely to have had reconstructive surgery
(P < .05).
Five cancer support providers and 4 physicians were interviewed. All support providers were Spanish-speaking Latinas with at least some college education. Cancer physicians were board certified. Two were men; two were white and two Asian; one was a breast surgeon and three were hematologists/oncologists; three spoke Spanish poorly/not at all and one spoke it fairly well.
Seven themes emerged from interviews: unmet physical symptom management needs; social support often ends when treatment ends; challenges resuming roles; sense of abandonment by health care system when treatment ends; need for formal transition from active treatment to follow-up care; fear of recurrence especially when obtaining follow-up care; and desire for information on late effects of initial treatments and side effects of hormonal treatments. We summarize results according to these themes.
Unmet physical symptom management needs. The main physical symptoms reported by survivors and physicians in interviews were arthralgia, menopausal symptoms, and neuropathy. Fatigue was reported only by survivors. Many survivors and several support providers expressed that symptoms were poorly managed and often ignored. One stated,
I have a lot of pain where I had surgery, it burns. I worry a lot about my arm because I have sacs of fluid. My doctor only says, ‘They will dissolve over the years.’ So, I don’t feel any support. (FocGrp1#6)
Survivors reported side effects of hormonal therapies, and felt that physicians downplayed these to prevent them from discontinuing medications.
Social support from family and friends often ends when treatment ends. Many survivors described a loss of support from family and friends who expected them to get back to “normal” once treatment ended. One said,
My sisters have told me to my face that there’s nothing wrong with me. So now when people ask me, I say, ‘I’m fine, thank God, I have nothing,’ even though I’m dying of pain and have all these pills to take. (Survivor#1025)
A support provider related,
The client was telling me that as she was getting closer to finishing her treatment, her husband was upset because he felt like all she was doing was focusing on the cancer. I think caregivers, family, spouses, and children out of their own sort of selfishness want this person to be well. (SuppProv#104)
A few survivors said that family bonds were strengthened after cancer and several reported lacking support because their families were in their home countries.
Challenges resuming roles, especially returning to work. Survivors, support providers, and physicians described challenges and few resources as women transition back to their normal roles. Survivors questioned their ability to return to work due to physically demanding occupations. One stated,
I would like information on how to take care of myself, how working can affect this side if I don’t take care of it. I clean houses and I need both hands. (Survivor#3012)
Survivors described how changes in memory affected daily chores and work performance. Support providers and physicians described the need for resources to aid with return to work and household responsibilities. One physician noted,
There are usually questions about how to go ahead and live their lives from that point forward. It’s a sort of reverse shock: going back to life as they know it. (Physician#004)
Support providers and physicians mentioned that women needed help with resuming intimate partner relations.
Sense of abandonment by health care system once active treatment ends. Survivors, support providers, and physicians reported a loss of support and sense of abandonment by the patients’ oncology team at the end of active treatment. One survivor stated,
Once they tell you to stop the pills, ‘You’re cured, there’s nothing wrong with you,’ the truth is that one feels, ‘Now what do I do? I have no one to help me.’ I felt very abandoned. (FocGrp1#5)
A provider said,
The support system falls apart once women complete treatment. They lose their entire support system at the medical level. They no longer have nurses checking in about symptoms and addressing anything that’s come up. They won’t have access to doctors unless they’re doing their screening. (SuppProv#101)
An oncologist, noting that this loss of support occurs when women face pressures of transitioning back to work or family obligations, commented,
So here’s a woman whose marriage is in turmoil, whose husband may even have left her during this, and now her clinic is leaving her and she’s on her own … that must be scary as hell because there’s nobody out there to support her. (Physician#002)
Need for formal transition from active treatment to follow-up care. Two themes emerged about transitioning from active treatment: transferring care from oncologists to primary care physicians (PCP); and issues of follow-up care (with oncologists or PCPs). Survivors felt lost in transitioning from specialty to primary care, or expressed apprehension seeing a PCP rather than a cancer specialist. One stated,
I have my doctor but she is not a specialist. She does what I tell her to and orders a mammogram every year. But, I don’t go to the oncologist anymore, and so I worry. With the specialists, I feel protected. (FocGrp1#5)
Physicians acknowledged the lack of a formal transition to primary care such as a survivorship care program.
Follow-up care issues were common. Physicians stressed that women needed to know how often to return for follow-up once active treatment ends and about recommended examinations and tests, especially when receiving hormonal therapy. Physicians indicated the need for patient education materials specific to patients’ treatments, for example, elevated risk of heart disease with certain chemotherapy agents. An oncologist expressed concern that PCPs are not prepared adequately about late effects and hormonal treatment side effects, and suggested providing summary notes for PCPs detailing these.
Survivors identified several barriers to follow-up care: lacking information on which symptoms merited a call to physicians; financial burden/limited health insurance; lacking appointment reminders; fear of examinations; and limited English proficiency. A survivor stated,
If you have insurance, you can make your appointment, see the doctor, and have your mammogram. I stopped taking my pills because I didn’t have insurance. I tried to get them again but they told me they would cost me a thousand dollars. (FocGrp1#5)
One oncologist suggested scheduling a follow-up appointment before patients leave treatment and calling patients who miss appointments.
Facilitators of regular follow-up care identified by survivors were physicians informing them about symptom monitoring and reporting, having a clinic contact person/navigator, being given a follow-up appointment, being assertive about one’s care, and physicians’ reinforcement of adherence to hormonal treatment and follow-up. According to support providers, a key facilitator was having a clinic contact person/navigator. Once treatment ended, support providers often served as the liaison between the patient and the physician, making them the first point of contact for symptom reporting.
Fear of recurrence especially when obtaining follow-up care. Fear of recurrence dominated survivor interviews. This fear was heightened at the time of follow-up examinations or when they experienced unusual pain. A survivor commented,
Every time I’m due for my mammogram, I can’t sleep, worrying. I lose sleep until I get the letter with my results. Then I feel at peace again. (FocGrp1#9)
Support providers discussed the need to provide reassurance to SSBCS to help them cope with fears of recurrence. Physicians expressed challenges in allaying fears of recurrence among SSBCS, requiring a lot of time when recommending follow-up mammograms.
Desire for information on late effects of treatments and side effects of hormonal therapies. All survivors expressed receiving insufficient information on potential symptoms and side effects. One stated,
Doctors only have five minutes. There has never been someone who gave me guidance like, ‘From now on you have to do this or you might get these symptoms now or in the future. (Survivor#6019)
They indicated uncertainty about what symptoms were “normal” and when symptoms merited a call to the physician. Several survivors reported being unaware that fatigue, arthralgia and neuropathy were side effects of breast cancer treatments until they reported these to physicians.
Physicians stressed the importance of women knowing about the elevated risk of future cancers, symptoms of recurrence, and seeking follow-up care if they experience symptoms that are out of the ordinary. Support providers felt that it was important to provide SSBCS with information on signs of recurrence and when to report these. However, providers expressed concern that giving women too much information might elevate their anxiety. A physician suggested,
It’s probably better to have a symptom list that’s short and relevant for the most common and catastrophic things, same thing with side effects … short to avoid overwhelming the patient. (Physician#001)
Hormonal treatments were of special concern. Survivors expressed a need for information on hormonal treatments and support providers stressed that this information is needed in simple Spanish. Several survivors indicated they stopped taking hormonal treatments due to side effects. One woman experienced severe headaches and heart palpitations, stopped taking the hormonal medication, felt better, and did not inform her physician until her next appointment. A support provider stated,
What I hear from a lot of women is that if side effects are too uncomfortable, they just stop it (hormonal treatment) without saying anything to the doctor. So more information about why they have to take it and that there is a good chance of recurrence is really important. (SuppProv#101)
Likewise, physicians indicated that SSBCS’ lack of information on hormonal treatments often resulted in nonadherence, emphasizing the need to reinforce adherence to prevent recurrence.
Conceptual framework of interventions
Based on triangulation of survey and interview results, we compiled a conceptual framework that includes needs identified, suggested components of a survivorship care intervention to address these needs, potential mediators by which such interventions could improve outcomes, and relevant outcomes (Figure). Survivorship care needs fell into four categories: symptom management, psychosocial, sense of abandonment by health care team, and healthy lifestyles. Survivorship care programs would provide skills training in symptom and stress management, and communicating with providers, family, friends, and coworkers. Mediators include increased self-efficacy, knowledge and perceived social support, ultimately leading to reduced distress (anxiety and depressive symptoms) and stress, and improved health-related quality of life.
Conclusions
Our study aimed to identify the most critical needs of SSBCS in the posttreatment survivorship phase to facilitate the design of survivorship interventions for this vulnerable group. SSBCS, cancer support providers, and cancer physicians reported substantial symptom management, psychosocial, and informational needs among this population. Results from surveys and open-ended interviews were remarkably consistent. Survivors, physicians, and support providers viewed transition out of active treatment as a time of increased psychosocial need and heightened vulnerability.
Our findings are consistent with needs assessments conducted in other breast cancer survivors. Similar to a study of rural white women with breast cancer, fear of recurrence was among the most common psychosocial concerns.17 Results of two studies that included white, African American and Latina breast cancer survivors were consistent with ours in finding that pain and fatigue were among the most persistent symptoms; in both studies, Latinas were more likely to report pain and a higher number of symptoms.7,18 The prevalence of sleep problems in our sample was identical to that reported in a sample of African American breast cancer survivors.19 Our findings of a high need for symptom management information and support, social support from family and friends, and self-management resources were similar to studies of other vulnerable breast cancer survivors.18,20
Our results suggest that it is critical for health care professionals to provide assistance with managing side effects and information to alleviate fears, and reinforce behaviors of symptom monitoring and reporting, and adherence to follow-up care and hormonal therapies. Yet this information is not being conveyed effectively and is complicated by the need to balance women’s need for information with minimizing anxiety when providing such information.
A limitation of our study is that most of our sample was Mexican origin and may not reflect experiences of Spanish-speaking Latinas of other national origin groups or outside of Northern California. Another limitation is the lack of an English-speaking comparison group, which would have permitted the identification of similarities and differences across language groups. Finally, we did not interview radiation oncologists who may have had opinions that are not represented here.
Survivorship care programs offer great promise for meeting patients’ informational and symptom management needs and improving well-being and communication with clinicians.21 Due to limited access to survivorship care information, financial hardships, and pressures from their families to resume their social roles, concerted efforts are needed to develop appropriate survivorship programs for SSBCS.22 Unique language, cultural and socioeconomic factors of Spanish-speaking Latinas require tailoring of cancer survivorship programs to best meet their needs.23 These programs need to provide psychosocial stress and symptom management assistance, simple information on recommended follow-up care, and healthy lifestyle and role reintegration strategies that account for their unique sociocultural contexts.
1. Danese MD, O’Malley C, Lindquist K, Gleeson M, Griffiths RI. An observational study of the prevalence and incidence of comorbid conditions in older women with breast cancer. Ann Oncol. 2012;23(7):1756-1765.
2. Hewitt M, Greenfield S, Stovall E, eds. From cancer patient to cancer survivor: lost in transition. Washington, DC: National Academy of Sciences; 2006.
3. Beckjord EB, Arora NK, McLaughlin W, Oakley-Girvan I, Hamilton AS, Hesse BW. Health-related information needs in a large and diverse sample of adult cancer survivors: implications for cancer care. J Cancer Surviv. 2008;2(3):179-189.
4. Hewitt ME, Bamundo A, Day R, Harvey C. Perspectives on posttreatment cancer care: qualitative research with survivors, nurses, and physicians. J Clin Oncol. 2007;25(16):2270-2273.
5. Ashing-Giwa KT, Tejero JS, Kim J, Padilla GV, Hellemann G. Examining predictive models of HRQOL in a population-based, multiethnic sample of women with breast carcinoma. Qual Life Res. 2007;16(3):413-428.
6. Clauser SB, Arora NK, Bellizzi KM, Haffer SC, Topor M, Hays RD. Disparities in HRQOL of cancer survivors and non-cancer managed care enrollees. Health Care Financ Rev. 2008;29(4):23-40.
7. Eversley R, Estrin D, Dibble S, Wardlaw L, Pedrosa M, Favila-Penney W. Posttreatment symptoms among ethnic minority breast cancer survivors. Oncol Nurs Forum. 2005;32(2):250-256.
8. Bickell NA, Wang JJ, Oluwole S, et al. Missed opportunities: racial disparities in adjuvant breast cancer treatment. J Clin Oncol. 2006;24(9):1357-1362.
9. Arora NK, Reeve BB, Hays RD, Clauser SB, Oakley-Girvan I. Assessment of quality of cancer-related follow-up care from the cancer survivor’s perspective. J Clin Oncol. 2011;29(10):1280-1289.
10. Janz NK, Mujahid MS, Hawley ST, Griggs JJ, Hamilton AS, Katz SJ. Racial/ethnic differences in adequacy of information and support for women with breast cancer. Cancer. 2008;113(5):1058-1067.
11. Yoon J, Malin JL, Tisnado DM, et al. Symptom management after breast cancer treatment: is it influenced by patient characteristics? Breast Cancer Res Treat. 2008;108(1):69-77.
12. Ashing K, Rosales M. A telephonic-based trial to reduce depressive symptoms among Latina breast cancer survivors. Psychooncology. 2014;23(5):507-515.
13. Hershman DL, Greenlee H, Awad D, et al. Randomized controlled trial of a clinic-based survivorship intervention following adjuvant therapy in breast cancer survivors. Breast Cancer Res Treat. 2013;138(3):795-806.
14. Napoles AM, Ortiz C, Santoyo-Olsson J, et al. Nuevo Amanecer: results of a randomized controlled trial of a community-based, peer-delivered stress management intervention to improve quality of life in Latinas with breast cancer. Am J Public Health. 2015;105(suppl 3):e55-63.
15. Rechis R, Reynolds KA, Beckjord EB, Nutt S, Burns RM, Schaefer JS. ‘I learned to live with it’ is not good enough: challenges reported by posttreatment cancer survivors in the Livestrong surveys. Austin, TX: Livestrong;2011.
16. Glaser BG, Strauss AL. The discovery of grounded theory: strategies for qualitative research. Hawthorne: Aldine Publishing Company; 1967.
17. Befort CA, Klemp J. Sequelae of breast cancer and the influence of menopausal status at diagnosis among rural breast cancer survivors. J Womens Health (Larchmt). 2011;20(9):1307-1313.
18. Fu OS, Crew KD, Jacobson JS, et al. Ethnicity and persistent symptom burden in breast cancer survivors. J Cancer Surviv. 2009;3(4):241-250.
19. Taylor TR, Huntley ED, Makambi K, et al. Understanding sleep disturbances in African-American breast cancer survivors: a pilot study. Psychooncology. 2012;21(8):896-902.
20. Adams N, Gisiger-Camata S, Hardy CM, Thomas TF, Jukkala A, Meneses K. Evaluating survivorship experiences and needs among rural African American breast cancer survivors. J Cancer Educ. October 24, 2015 [Epub ahead of print].
21. Blinder VS, Patil S, Thind A, et al. Return to work in low-income Latina and non-Latina white breast cancer survivors: a 3-year longitudinal study. Cancer. 2012;118(6):1664-1674.
22. Lopez-Class M, Perret-Gentil M, Kreling B, Caicedo L, Mandelblatt J, Graves KD. Quality of life among immigrant Latina breast cancer survivors: realities of culture and enhancing cancer care. J Cancer Educ. 2011;26(4):724-733.
23. Napoles-Springer AM, Ortiz C, O’Brien H, Diaz-Mendez M. Developing a culturally competent peer support intervention for Spanish-speaking Latinas with breast cancer. J Immigr Minor Health. 2009;11(4):268-280
After treatment, cancer patients transition to a survivorship phase, often with little information or support. Cancer survivors are at increased risk of recurrence, secondary cancers, comorbid conditions, and late treatment effects.1,2 However, many remain unaware of these risks and the options for managing them3 and face numerous unmet medical, psychosocial, and informational needs that can be addressed through survivorship care programs.4 Anxiety may increase as they lose their treatment team’s support while attempting to reestablish their lives.2 Patients need to know the long-term risks of cancer treatments, probabilities of recurrence and second cancers, effectiveness of surveillance and interventions for managing late effects and psychosocial concerns, and benefits of healthy lifestyles.2
Due to sociocultural and economic factors, Spanish-speaking Latina breast cancer survivors (SSBCS) suffer worse posttreatment health-related quality of life and more pain, fatigue, depressive symptoms, body image issues, and distress than their white counterparts.5-7 However, they are less likely to receive necessary cancer treatment, symptom management, and surveillance. For example, compared with whites, Latina breast cancer survivors receive less guideline-adherent treatment8 and follow-up care, including survivorship information.3,9 SSBCS, in particular have less access to survivorship information.10 Consequently, SSBCS are more likely to report unmet symptom management needs.11
Several breast cancer survivorship program trials have included Latinas,12,13 but their effectiveness has been demonstrated only for depressive symptoms or health worry. A comprehensive assessment of the posttreatment needs of SSBCS would provide a foundation for designing tailored survivorship interventions for this vulnerable group. This study aimed to identify the symptom management, psychosocial, and informational needs of SSBCS during the transition to survivorship from the perspectives of SSBCS and their cancer support providers and cancer physicians.
Methods
We sampled respondents within a 5-county area in Northern California to obtain multiple perspectives of the survivorship care needs of SSBCS using structured and in-depth methods: a telephone survey of SSBCS; semistructured interviews with SSBCS; semistructured interviews with cancer support providers serving SSBCS; and semistructured interviews with physicians providing cancer care for SSBCS. The study protocol was approved by the University of California San Francisco Committee on Human Research.
Sample and procedures
Structured telephone survey with SSBCS. The sample was drawn evenly from San Francisco General Hospital-University of California San Francisco primary care practices and SSBCS from a previous study who agreed to be re-contacted.14 The inclusion criteria were: completed active treatment (except adjuvant hormonal therapy) for nonmetastatic breast cancer within 10 years; living in one of the five counties; primarily Spanish-speaking; and self-identified as Latina. The exclusion criteria were: previous cancer except nonmelanoma skin cancer; terminal illness; or metastatic breast cancer. Study staff mailed potential participants a bilingual letter and information sheet, and bilingual opt-out postcard (6th grade reading level assessed by Flesch-Kincaid grade level statistic). Female bilingual-bicultural research associates conducted interviews of 20-30 minutes in Spanish after obtaining verbal consent. Participants were mailed $20. Surveys were conducted during March-November 2014.
Semistructured in-person interviews with SSBCS. Four community-based organizations (CBOs) in the targeted area providing cancer support services to Latinos agreed to recruit SSBCS for interviews. Inclusion criteria were identical to the survey. Patient navigators or support providers from CBOs contacted women by phone or in-person to invite them to an interview to assess their cancer survivorship needs. Women could choose a focus group or individual interview. With permission, names and contact information were given to study interviewers who called, explained the study, screened for eligibility, and scheduled an interview.
Recruitment was stratified by age (under or over age 50). We sampled women until saturation was achieved (no new themes emerged). Focus groups (90 minutes) were conducted at the CBOs. Individual interviews (45 minutes) were conducted in participants’ homes. Written informed consent was obtained. Participants were paid $50. Interviews were conducted during August-November, 2014, audiotaped, and transcribed.
Semistructured in-person interviews with cancer support providers and physicians. Investigators invited five cancer support providers (three patient navigators from three county hospitals, and two CBO directors of cancer psychosocial support services) and four physicians (three oncologists and one breast cancer surgeon from three county hospitals) to an in-person interview to identify SSBCS’ survivorship care needs. All agreed to participate. No further candidates were approached because saturation was achieved. We obtained written informed consent and 30-minute interviews were conducted in participants’ offices during August-October, 2014. Interviews were audiotaped and transcribed. Participants were paid $50.
Ethical approval. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and the 1964 Helsinki declaration and its later amendments or comparable ethical standards. This article does not contain any studies with animals performed by any of the authors.
Informed consent. Informed consent was obtained from all individual participants included in the study.
Measures
Structured telephone survey. Based on cancer survivor needs assessments,15 we assessed: physical and emotional symptoms; problems with sleep and memory/concentration; concerns about mortality, family, social isolation, intimacy, appearance; and healthy lifestyles. Items were adapted and translated into Spanish if needed, using forward/backward translation with team reconciliation. These questions used the introduction, “Now I am going to ask you if you have had any problems because of your cancer. In the past month, how much have you been bothered by …” with responses rated on a scale of 1-5 (1, Not at all; 5, A lot). For example, we asked, “In the past month, how much have you been bothered by fatigue?”
Regarding healthy lifestyles, we used the introduction, “Here are some changes women sometimes want to make after cancer. Would you like help with …?” For example, we asked, “Would you like help with getting more exercise?” We asked if they wanted help getting more exercise, eating healthier, managing stress, and doing meditation or yoga (Yes/No).
Semistructured interview guide for SSBCS. Participants were asked about their emotional and physical concerns when treatment ended, current cancer needs, symptoms or late effects, and issues related to relationships, family, employment, insurance, financial hardships, barriers to follow-up care, health behaviors, and survivorship program content. Sample questions are, “Have you had any symptoms or side effects related to your cancer or treatment?” and “What kinds of information do you feel you need now about your cancer or treatment?” A brief questionnaire assessed demographics.
Semistructured interview guide for cancer support providers and physicians. Support providers and physicians were asked about informational, psychosocial, and symptom management needs of SSBCS and recommended self-management content and formats. Sample questions are, “What kinds of information and support do you wish was available to help Spanish-speaking women take care of their health after treatment ends?” and “What do you think are the most pressing emotional needs of Spanish-speaking women after breast cancer treatment ends?” A brief questionnaire assessed demographics.
Analysis
Frequencies are reported for survey items. For questions about symptoms/concerns, we report the frequency of responding that they were bothered Somewhat/Quite a bit/A lot. For healthy lifestyles, we present the frequency answering Yes.
Verbatim semistructured interview transcripts were verified against audiotapes. Using QSR NVIVO software, transcripts were coded independently by two bilingual-bicultural investigators using a constant comparative method to generate coding categories for cancer survivorship needs.16 Coders started with themes specified by the interview guides, expanded them to represent the data, and discussed and reconciled coding discrepancies. Coding was compared by type of interview participant (survivor, support provider, or physician). Triangulation of survey and semistructured interviews occurred through team discussions to verify codes, themes, and implications for interventions.
Results
Telephone survey of SSBCS
Of the telephone survey sampling frame (N = 231), 118 individuals (51%) completed the interview, 37 (16%) were ineligible, 31 (13%) could not be reached, 22 (10%) had incorrect contact information, 19 (8%) refused to participate, and 4 (2%) were deceased. Mean age of the participants was 54.9 years (SD, 12.3); all were foreign-born, with more than half of Mexican origin; and most had less than a high-school education (Table 1). All had completed active treatment, and most (68%) were within 2 years of diagnosis.
For symptom management needs (Table 2), the most prevalent (bothersome) symptoms (reported by more than 30%, in rank order) were joint pain, sleep problems, fatigue, hot flashes, numbness/tingling of extremities, and memory. Next most prevalent (reported by 20%-30%) were vaginal dryness, dry/itchy skin, dry nose/mouth, inability to concentrate, constipation, changes in urination, and shortness of breath.

For psychosocial needs, fears of recurrence or new cancers were reported by 42%. Emotional symptoms reported by more than 30% were depression/sadness, anxieties, and feeling stressed. Next most prevalent (20%-30%) were fears, loss of interest in usual activities, and nervousness/restlessness. Social well-being concerns reported by 20%-30% of survivors were loneliness, having no one to talk to, and being a burden to their families. Body image and sexual problems reported by 20%-30% of survivors included appearance and problems being intimate with partners.
Regarding lifestyle, most of the participants said they wanted help with eating a healthier diet (74%), getting more exercise (69%), managing stress (63%), and doing yoga or meditation (55%).
Semistructured interviews
Twenty-five SSBCS completed semistructured interviews, 10 in individual interviews and 15 in one of two focus groups (one of 9 women older than 50 years; one of 6 women younger than 50). The telephone survey respondents were similar to semistructured interview respondents on all sociodemographic characteristics, but differed slightly on some clinical characteristics (Table 1). The telephone survey women had been more recently diagnosed (P < .01), were less likely to have ductal carcinoma in situ (P < .001), and more likely to have had reconstructive surgery
(P < .05).
Five cancer support providers and 4 physicians were interviewed. All support providers were Spanish-speaking Latinas with at least some college education. Cancer physicians were board certified. Two were men; two were white and two Asian; one was a breast surgeon and three were hematologists/oncologists; three spoke Spanish poorly/not at all and one spoke it fairly well.
Seven themes emerged from interviews: unmet physical symptom management needs; social support often ends when treatment ends; challenges resuming roles; sense of abandonment by health care system when treatment ends; need for formal transition from active treatment to follow-up care; fear of recurrence especially when obtaining follow-up care; and desire for information on late effects of initial treatments and side effects of hormonal treatments. We summarize results according to these themes.
Unmet physical symptom management needs. The main physical symptoms reported by survivors and physicians in interviews were arthralgia, menopausal symptoms, and neuropathy. Fatigue was reported only by survivors. Many survivors and several support providers expressed that symptoms were poorly managed and often ignored. One stated,
I have a lot of pain where I had surgery, it burns. I worry a lot about my arm because I have sacs of fluid. My doctor only says, ‘They will dissolve over the years.’ So, I don’t feel any support. (FocGrp1#6)
Survivors reported side effects of hormonal therapies, and felt that physicians downplayed these to prevent them from discontinuing medications.
Social support from family and friends often ends when treatment ends. Many survivors described a loss of support from family and friends who expected them to get back to “normal” once treatment ended. One said,
My sisters have told me to my face that there’s nothing wrong with me. So now when people ask me, I say, ‘I’m fine, thank God, I have nothing,’ even though I’m dying of pain and have all these pills to take. (Survivor#1025)
A support provider related,
The client was telling me that as she was getting closer to finishing her treatment, her husband was upset because he felt like all she was doing was focusing on the cancer. I think caregivers, family, spouses, and children out of their own sort of selfishness want this person to be well. (SuppProv#104)
A few survivors said that family bonds were strengthened after cancer and several reported lacking support because their families were in their home countries.
Challenges resuming roles, especially returning to work. Survivors, support providers, and physicians described challenges and few resources as women transition back to their normal roles. Survivors questioned their ability to return to work due to physically demanding occupations. One stated,
I would like information on how to take care of myself, how working can affect this side if I don’t take care of it. I clean houses and I need both hands. (Survivor#3012)
Survivors described how changes in memory affected daily chores and work performance. Support providers and physicians described the need for resources to aid with return to work and household responsibilities. One physician noted,
There are usually questions about how to go ahead and live their lives from that point forward. It’s a sort of reverse shock: going back to life as they know it. (Physician#004)
Support providers and physicians mentioned that women needed help with resuming intimate partner relations.
Sense of abandonment by health care system once active treatment ends. Survivors, support providers, and physicians reported a loss of support and sense of abandonment by the patients’ oncology team at the end of active treatment. One survivor stated,
Once they tell you to stop the pills, ‘You’re cured, there’s nothing wrong with you,’ the truth is that one feels, ‘Now what do I do? I have no one to help me.’ I felt very abandoned. (FocGrp1#5)
A provider said,
The support system falls apart once women complete treatment. They lose their entire support system at the medical level. They no longer have nurses checking in about symptoms and addressing anything that’s come up. They won’t have access to doctors unless they’re doing their screening. (SuppProv#101)
An oncologist, noting that this loss of support occurs when women face pressures of transitioning back to work or family obligations, commented,
So here’s a woman whose marriage is in turmoil, whose husband may even have left her during this, and now her clinic is leaving her and she’s on her own … that must be scary as hell because there’s nobody out there to support her. (Physician#002)
Need for formal transition from active treatment to follow-up care. Two themes emerged about transitioning from active treatment: transferring care from oncologists to primary care physicians (PCP); and issues of follow-up care (with oncologists or PCPs). Survivors felt lost in transitioning from specialty to primary care, or expressed apprehension seeing a PCP rather than a cancer specialist. One stated,
I have my doctor but she is not a specialist. She does what I tell her to and orders a mammogram every year. But, I don’t go to the oncologist anymore, and so I worry. With the specialists, I feel protected. (FocGrp1#5)
Physicians acknowledged the lack of a formal transition to primary care such as a survivorship care program.
Follow-up care issues were common. Physicians stressed that women needed to know how often to return for follow-up once active treatment ends and about recommended examinations and tests, especially when receiving hormonal therapy. Physicians indicated the need for patient education materials specific to patients’ treatments, for example, elevated risk of heart disease with certain chemotherapy agents. An oncologist expressed concern that PCPs are not prepared adequately about late effects and hormonal treatment side effects, and suggested providing summary notes for PCPs detailing these.
Survivors identified several barriers to follow-up care: lacking information on which symptoms merited a call to physicians; financial burden/limited health insurance; lacking appointment reminders; fear of examinations; and limited English proficiency. A survivor stated,
If you have insurance, you can make your appointment, see the doctor, and have your mammogram. I stopped taking my pills because I didn’t have insurance. I tried to get them again but they told me they would cost me a thousand dollars. (FocGrp1#5)
One oncologist suggested scheduling a follow-up appointment before patients leave treatment and calling patients who miss appointments.
Facilitators of regular follow-up care identified by survivors were physicians informing them about symptom monitoring and reporting, having a clinic contact person/navigator, being given a follow-up appointment, being assertive about one’s care, and physicians’ reinforcement of adherence to hormonal treatment and follow-up. According to support providers, a key facilitator was having a clinic contact person/navigator. Once treatment ended, support providers often served as the liaison between the patient and the physician, making them the first point of contact for symptom reporting.
Fear of recurrence especially when obtaining follow-up care. Fear of recurrence dominated survivor interviews. This fear was heightened at the time of follow-up examinations or when they experienced unusual pain. A survivor commented,
Every time I’m due for my mammogram, I can’t sleep, worrying. I lose sleep until I get the letter with my results. Then I feel at peace again. (FocGrp1#9)
Support providers discussed the need to provide reassurance to SSBCS to help them cope with fears of recurrence. Physicians expressed challenges in allaying fears of recurrence among SSBCS, requiring a lot of time when recommending follow-up mammograms.
Desire for information on late effects of treatments and side effects of hormonal therapies. All survivors expressed receiving insufficient information on potential symptoms and side effects. One stated,
Doctors only have five minutes. There has never been someone who gave me guidance like, ‘From now on you have to do this or you might get these symptoms now or in the future. (Survivor#6019)
They indicated uncertainty about what symptoms were “normal” and when symptoms merited a call to the physician. Several survivors reported being unaware that fatigue, arthralgia and neuropathy were side effects of breast cancer treatments until they reported these to physicians.
Physicians stressed the importance of women knowing about the elevated risk of future cancers, symptoms of recurrence, and seeking follow-up care if they experience symptoms that are out of the ordinary. Support providers felt that it was important to provide SSBCS with information on signs of recurrence and when to report these. However, providers expressed concern that giving women too much information might elevate their anxiety. A physician suggested,
It’s probably better to have a symptom list that’s short and relevant for the most common and catastrophic things, same thing with side effects … short to avoid overwhelming the patient. (Physician#001)
Hormonal treatments were of special concern. Survivors expressed a need for information on hormonal treatments and support providers stressed that this information is needed in simple Spanish. Several survivors indicated they stopped taking hormonal treatments due to side effects. One woman experienced severe headaches and heart palpitations, stopped taking the hormonal medication, felt better, and did not inform her physician until her next appointment. A support provider stated,
What I hear from a lot of women is that if side effects are too uncomfortable, they just stop it (hormonal treatment) without saying anything to the doctor. So more information about why they have to take it and that there is a good chance of recurrence is really important. (SuppProv#101)
Likewise, physicians indicated that SSBCS’ lack of information on hormonal treatments often resulted in nonadherence, emphasizing the need to reinforce adherence to prevent recurrence.
Conceptual framework of interventions
Based on triangulation of survey and interview results, we compiled a conceptual framework that includes needs identified, suggested components of a survivorship care intervention to address these needs, potential mediators by which such interventions could improve outcomes, and relevant outcomes (Figure). Survivorship care needs fell into four categories: symptom management, psychosocial, sense of abandonment by health care team, and healthy lifestyles. Survivorship care programs would provide skills training in symptom and stress management, and communicating with providers, family, friends, and coworkers. Mediators include increased self-efficacy, knowledge and perceived social support, ultimately leading to reduced distress (anxiety and depressive symptoms) and stress, and improved health-related quality of life.
Conclusions
Our study aimed to identify the most critical needs of SSBCS in the posttreatment survivorship phase to facilitate the design of survivorship interventions for this vulnerable group. SSBCS, cancer support providers, and cancer physicians reported substantial symptom management, psychosocial, and informational needs among this population. Results from surveys and open-ended interviews were remarkably consistent. Survivors, physicians, and support providers viewed transition out of active treatment as a time of increased psychosocial need and heightened vulnerability.
Our findings are consistent with needs assessments conducted in other breast cancer survivors. Similar to a study of rural white women with breast cancer, fear of recurrence was among the most common psychosocial concerns.17 Results of two studies that included white, African American and Latina breast cancer survivors were consistent with ours in finding that pain and fatigue were among the most persistent symptoms; in both studies, Latinas were more likely to report pain and a higher number of symptoms.7,18 The prevalence of sleep problems in our sample was identical to that reported in a sample of African American breast cancer survivors.19 Our findings of a high need for symptom management information and support, social support from family and friends, and self-management resources were similar to studies of other vulnerable breast cancer survivors.18,20
Our results suggest that it is critical for health care professionals to provide assistance with managing side effects and information to alleviate fears, and reinforce behaviors of symptom monitoring and reporting, and adherence to follow-up care and hormonal therapies. Yet this information is not being conveyed effectively and is complicated by the need to balance women’s need for information with minimizing anxiety when providing such information.
A limitation of our study is that most of our sample was Mexican origin and may not reflect experiences of Spanish-speaking Latinas of other national origin groups or outside of Northern California. Another limitation is the lack of an English-speaking comparison group, which would have permitted the identification of similarities and differences across language groups. Finally, we did not interview radiation oncologists who may have had opinions that are not represented here.
Survivorship care programs offer great promise for meeting patients’ informational and symptom management needs and improving well-being and communication with clinicians.21 Due to limited access to survivorship care information, financial hardships, and pressures from their families to resume their social roles, concerted efforts are needed to develop appropriate survivorship programs for SSBCS.22 Unique language, cultural and socioeconomic factors of Spanish-speaking Latinas require tailoring of cancer survivorship programs to best meet their needs.23 These programs need to provide psychosocial stress and symptom management assistance, simple information on recommended follow-up care, and healthy lifestyle and role reintegration strategies that account for their unique sociocultural contexts.
After treatment, cancer patients transition to a survivorship phase, often with little information or support. Cancer survivors are at increased risk of recurrence, secondary cancers, comorbid conditions, and late treatment effects.1,2 However, many remain unaware of these risks and the options for managing them3 and face numerous unmet medical, psychosocial, and informational needs that can be addressed through survivorship care programs.4 Anxiety may increase as they lose their treatment team’s support while attempting to reestablish their lives.2 Patients need to know the long-term risks of cancer treatments, probabilities of recurrence and second cancers, effectiveness of surveillance and interventions for managing late effects and psychosocial concerns, and benefits of healthy lifestyles.2
Due to sociocultural and economic factors, Spanish-speaking Latina breast cancer survivors (SSBCS) suffer worse posttreatment health-related quality of life and more pain, fatigue, depressive symptoms, body image issues, and distress than their white counterparts.5-7 However, they are less likely to receive necessary cancer treatment, symptom management, and surveillance. For example, compared with whites, Latina breast cancer survivors receive less guideline-adherent treatment8 and follow-up care, including survivorship information.3,9 SSBCS, in particular have less access to survivorship information.10 Consequently, SSBCS are more likely to report unmet symptom management needs.11
Several breast cancer survivorship program trials have included Latinas,12,13 but their effectiveness has been demonstrated only for depressive symptoms or health worry. A comprehensive assessment of the posttreatment needs of SSBCS would provide a foundation for designing tailored survivorship interventions for this vulnerable group. This study aimed to identify the symptom management, psychosocial, and informational needs of SSBCS during the transition to survivorship from the perspectives of SSBCS and their cancer support providers and cancer physicians.
Methods
We sampled respondents within a 5-county area in Northern California to obtain multiple perspectives of the survivorship care needs of SSBCS using structured and in-depth methods: a telephone survey of SSBCS; semistructured interviews with SSBCS; semistructured interviews with cancer support providers serving SSBCS; and semistructured interviews with physicians providing cancer care for SSBCS. The study protocol was approved by the University of California San Francisco Committee on Human Research.
Sample and procedures
Structured telephone survey with SSBCS. The sample was drawn evenly from San Francisco General Hospital-University of California San Francisco primary care practices and SSBCS from a previous study who agreed to be re-contacted.14 The inclusion criteria were: completed active treatment (except adjuvant hormonal therapy) for nonmetastatic breast cancer within 10 years; living in one of the five counties; primarily Spanish-speaking; and self-identified as Latina. The exclusion criteria were: previous cancer except nonmelanoma skin cancer; terminal illness; or metastatic breast cancer. Study staff mailed potential participants a bilingual letter and information sheet, and bilingual opt-out postcard (6th grade reading level assessed by Flesch-Kincaid grade level statistic). Female bilingual-bicultural research associates conducted interviews of 20-30 minutes in Spanish after obtaining verbal consent. Participants were mailed $20. Surveys were conducted during March-November 2014.
Semistructured in-person interviews with SSBCS. Four community-based organizations (CBOs) in the targeted area providing cancer support services to Latinos agreed to recruit SSBCS for interviews. Inclusion criteria were identical to the survey. Patient navigators or support providers from CBOs contacted women by phone or in-person to invite them to an interview to assess their cancer survivorship needs. Women could choose a focus group or individual interview. With permission, names and contact information were given to study interviewers who called, explained the study, screened for eligibility, and scheduled an interview.
Recruitment was stratified by age (under or over age 50). We sampled women until saturation was achieved (no new themes emerged). Focus groups (90 minutes) were conducted at the CBOs. Individual interviews (45 minutes) were conducted in participants’ homes. Written informed consent was obtained. Participants were paid $50. Interviews were conducted during August-November, 2014, audiotaped, and transcribed.
Semistructured in-person interviews with cancer support providers and physicians. Investigators invited five cancer support providers (three patient navigators from three county hospitals, and two CBO directors of cancer psychosocial support services) and four physicians (three oncologists and one breast cancer surgeon from three county hospitals) to an in-person interview to identify SSBCS’ survivorship care needs. All agreed to participate. No further candidates were approached because saturation was achieved. We obtained written informed consent and 30-minute interviews were conducted in participants’ offices during August-October, 2014. Interviews were audiotaped and transcribed. Participants were paid $50.
Ethical approval. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and the 1964 Helsinki declaration and its later amendments or comparable ethical standards. This article does not contain any studies with animals performed by any of the authors.
Informed consent. Informed consent was obtained from all individual participants included in the study.
Measures
Structured telephone survey. Based on cancer survivor needs assessments,15 we assessed: physical and emotional symptoms; problems with sleep and memory/concentration; concerns about mortality, family, social isolation, intimacy, appearance; and healthy lifestyles. Items were adapted and translated into Spanish if needed, using forward/backward translation with team reconciliation. These questions used the introduction, “Now I am going to ask you if you have had any problems because of your cancer. In the past month, how much have you been bothered by …” with responses rated on a scale of 1-5 (1, Not at all; 5, A lot). For example, we asked, “In the past month, how much have you been bothered by fatigue?”
Regarding healthy lifestyles, we used the introduction, “Here are some changes women sometimes want to make after cancer. Would you like help with …?” For example, we asked, “Would you like help with getting more exercise?” We asked if they wanted help getting more exercise, eating healthier, managing stress, and doing meditation or yoga (Yes/No).
Semistructured interview guide for SSBCS. Participants were asked about their emotional and physical concerns when treatment ended, current cancer needs, symptoms or late effects, and issues related to relationships, family, employment, insurance, financial hardships, barriers to follow-up care, health behaviors, and survivorship program content. Sample questions are, “Have you had any symptoms or side effects related to your cancer or treatment?” and “What kinds of information do you feel you need now about your cancer or treatment?” A brief questionnaire assessed demographics.
Semistructured interview guide for cancer support providers and physicians. Support providers and physicians were asked about informational, psychosocial, and symptom management needs of SSBCS and recommended self-management content and formats. Sample questions are, “What kinds of information and support do you wish was available to help Spanish-speaking women take care of their health after treatment ends?” and “What do you think are the most pressing emotional needs of Spanish-speaking women after breast cancer treatment ends?” A brief questionnaire assessed demographics.
Analysis
Frequencies are reported for survey items. For questions about symptoms/concerns, we report the frequency of responding that they were bothered Somewhat/Quite a bit/A lot. For healthy lifestyles, we present the frequency answering Yes.
Verbatim semistructured interview transcripts were verified against audiotapes. Using QSR NVIVO software, transcripts were coded independently by two bilingual-bicultural investigators using a constant comparative method to generate coding categories for cancer survivorship needs.16 Coders started with themes specified by the interview guides, expanded them to represent the data, and discussed and reconciled coding discrepancies. Coding was compared by type of interview participant (survivor, support provider, or physician). Triangulation of survey and semistructured interviews occurred through team discussions to verify codes, themes, and implications for interventions.
Results
Telephone survey of SSBCS
Of the telephone survey sampling frame (N = 231), 118 individuals (51%) completed the interview, 37 (16%) were ineligible, 31 (13%) could not be reached, 22 (10%) had incorrect contact information, 19 (8%) refused to participate, and 4 (2%) were deceased. Mean age of the participants was 54.9 years (SD, 12.3); all were foreign-born, with more than half of Mexican origin; and most had less than a high-school education (Table 1). All had completed active treatment, and most (68%) were within 2 years of diagnosis.
For symptom management needs (Table 2), the most prevalent (bothersome) symptoms (reported by more than 30%, in rank order) were joint pain, sleep problems, fatigue, hot flashes, numbness/tingling of extremities, and memory. Next most prevalent (reported by 20%-30%) were vaginal dryness, dry/itchy skin, dry nose/mouth, inability to concentrate, constipation, changes in urination, and shortness of breath.

For psychosocial needs, fears of recurrence or new cancers were reported by 42%. Emotional symptoms reported by more than 30% were depression/sadness, anxieties, and feeling stressed. Next most prevalent (20%-30%) were fears, loss of interest in usual activities, and nervousness/restlessness. Social well-being concerns reported by 20%-30% of survivors were loneliness, having no one to talk to, and being a burden to their families. Body image and sexual problems reported by 20%-30% of survivors included appearance and problems being intimate with partners.
Regarding lifestyle, most of the participants said they wanted help with eating a healthier diet (74%), getting more exercise (69%), managing stress (63%), and doing yoga or meditation (55%).
Semistructured interviews
Twenty-five SSBCS completed semistructured interviews, 10 in individual interviews and 15 in one of two focus groups (one of 9 women older than 50 years; one of 6 women younger than 50). The telephone survey respondents were similar to semistructured interview respondents on all sociodemographic characteristics, but differed slightly on some clinical characteristics (Table 1). The telephone survey women had been more recently diagnosed (P < .01), were less likely to have ductal carcinoma in situ (P < .001), and more likely to have had reconstructive surgery
(P < .05).
Five cancer support providers and 4 physicians were interviewed. All support providers were Spanish-speaking Latinas with at least some college education. Cancer physicians were board certified. Two were men; two were white and two Asian; one was a breast surgeon and three were hematologists/oncologists; three spoke Spanish poorly/not at all and one spoke it fairly well.
Seven themes emerged from interviews: unmet physical symptom management needs; social support often ends when treatment ends; challenges resuming roles; sense of abandonment by health care system when treatment ends; need for formal transition from active treatment to follow-up care; fear of recurrence especially when obtaining follow-up care; and desire for information on late effects of initial treatments and side effects of hormonal treatments. We summarize results according to these themes.
Unmet physical symptom management needs. The main physical symptoms reported by survivors and physicians in interviews were arthralgia, menopausal symptoms, and neuropathy. Fatigue was reported only by survivors. Many survivors and several support providers expressed that symptoms were poorly managed and often ignored. One stated,
I have a lot of pain where I had surgery, it burns. I worry a lot about my arm because I have sacs of fluid. My doctor only says, ‘They will dissolve over the years.’ So, I don’t feel any support. (FocGrp1#6)
Survivors reported side effects of hormonal therapies, and felt that physicians downplayed these to prevent them from discontinuing medications.
Social support from family and friends often ends when treatment ends. Many survivors described a loss of support from family and friends who expected them to get back to “normal” once treatment ended. One said,
My sisters have told me to my face that there’s nothing wrong with me. So now when people ask me, I say, ‘I’m fine, thank God, I have nothing,’ even though I’m dying of pain and have all these pills to take. (Survivor#1025)
A support provider related,
The client was telling me that as she was getting closer to finishing her treatment, her husband was upset because he felt like all she was doing was focusing on the cancer. I think caregivers, family, spouses, and children out of their own sort of selfishness want this person to be well. (SuppProv#104)
A few survivors said that family bonds were strengthened after cancer and several reported lacking support because their families were in their home countries.
Challenges resuming roles, especially returning to work. Survivors, support providers, and physicians described challenges and few resources as women transition back to their normal roles. Survivors questioned their ability to return to work due to physically demanding occupations. One stated,
I would like information on how to take care of myself, how working can affect this side if I don’t take care of it. I clean houses and I need both hands. (Survivor#3012)
Survivors described how changes in memory affected daily chores and work performance. Support providers and physicians described the need for resources to aid with return to work and household responsibilities. One physician noted,
There are usually questions about how to go ahead and live their lives from that point forward. It’s a sort of reverse shock: going back to life as they know it. (Physician#004)
Support providers and physicians mentioned that women needed help with resuming intimate partner relations.
Sense of abandonment by health care system once active treatment ends. Survivors, support providers, and physicians reported a loss of support and sense of abandonment by the patients’ oncology team at the end of active treatment. One survivor stated,
Once they tell you to stop the pills, ‘You’re cured, there’s nothing wrong with you,’ the truth is that one feels, ‘Now what do I do? I have no one to help me.’ I felt very abandoned. (FocGrp1#5)
A provider said,
The support system falls apart once women complete treatment. They lose their entire support system at the medical level. They no longer have nurses checking in about symptoms and addressing anything that’s come up. They won’t have access to doctors unless they’re doing their screening. (SuppProv#101)
An oncologist, noting that this loss of support occurs when women face pressures of transitioning back to work or family obligations, commented,
So here’s a woman whose marriage is in turmoil, whose husband may even have left her during this, and now her clinic is leaving her and she’s on her own … that must be scary as hell because there’s nobody out there to support her. (Physician#002)
Need for formal transition from active treatment to follow-up care. Two themes emerged about transitioning from active treatment: transferring care from oncologists to primary care physicians (PCP); and issues of follow-up care (with oncologists or PCPs). Survivors felt lost in transitioning from specialty to primary care, or expressed apprehension seeing a PCP rather than a cancer specialist. One stated,
I have my doctor but she is not a specialist. She does what I tell her to and orders a mammogram every year. But, I don’t go to the oncologist anymore, and so I worry. With the specialists, I feel protected. (FocGrp1#5)
Physicians acknowledged the lack of a formal transition to primary care such as a survivorship care program.
Follow-up care issues were common. Physicians stressed that women needed to know how often to return for follow-up once active treatment ends and about recommended examinations and tests, especially when receiving hormonal therapy. Physicians indicated the need for patient education materials specific to patients’ treatments, for example, elevated risk of heart disease with certain chemotherapy agents. An oncologist expressed concern that PCPs are not prepared adequately about late effects and hormonal treatment side effects, and suggested providing summary notes for PCPs detailing these.
Survivors identified several barriers to follow-up care: lacking information on which symptoms merited a call to physicians; financial burden/limited health insurance; lacking appointment reminders; fear of examinations; and limited English proficiency. A survivor stated,
If you have insurance, you can make your appointment, see the doctor, and have your mammogram. I stopped taking my pills because I didn’t have insurance. I tried to get them again but they told me they would cost me a thousand dollars. (FocGrp1#5)
One oncologist suggested scheduling a follow-up appointment before patients leave treatment and calling patients who miss appointments.
Facilitators of regular follow-up care identified by survivors were physicians informing them about symptom monitoring and reporting, having a clinic contact person/navigator, being given a follow-up appointment, being assertive about one’s care, and physicians’ reinforcement of adherence to hormonal treatment and follow-up. According to support providers, a key facilitator was having a clinic contact person/navigator. Once treatment ended, support providers often served as the liaison between the patient and the physician, making them the first point of contact for symptom reporting.
Fear of recurrence especially when obtaining follow-up care. Fear of recurrence dominated survivor interviews. This fear was heightened at the time of follow-up examinations or when they experienced unusual pain. A survivor commented,
Every time I’m due for my mammogram, I can’t sleep, worrying. I lose sleep until I get the letter with my results. Then I feel at peace again. (FocGrp1#9)
Support providers discussed the need to provide reassurance to SSBCS to help them cope with fears of recurrence. Physicians expressed challenges in allaying fears of recurrence among SSBCS, requiring a lot of time when recommending follow-up mammograms.
Desire for information on late effects of treatments and side effects of hormonal therapies. All survivors expressed receiving insufficient information on potential symptoms and side effects. One stated,
Doctors only have five minutes. There has never been someone who gave me guidance like, ‘From now on you have to do this or you might get these symptoms now or in the future. (Survivor#6019)
They indicated uncertainty about what symptoms were “normal” and when symptoms merited a call to the physician. Several survivors reported being unaware that fatigue, arthralgia and neuropathy were side effects of breast cancer treatments until they reported these to physicians.
Physicians stressed the importance of women knowing about the elevated risk of future cancers, symptoms of recurrence, and seeking follow-up care if they experience symptoms that are out of the ordinary. Support providers felt that it was important to provide SSBCS with information on signs of recurrence and when to report these. However, providers expressed concern that giving women too much information might elevate their anxiety. A physician suggested,
It’s probably better to have a symptom list that’s short and relevant for the most common and catastrophic things, same thing with side effects … short to avoid overwhelming the patient. (Physician#001)
Hormonal treatments were of special concern. Survivors expressed a need for information on hormonal treatments and support providers stressed that this information is needed in simple Spanish. Several survivors indicated they stopped taking hormonal treatments due to side effects. One woman experienced severe headaches and heart palpitations, stopped taking the hormonal medication, felt better, and did not inform her physician until her next appointment. A support provider stated,
What I hear from a lot of women is that if side effects are too uncomfortable, they just stop it (hormonal treatment) without saying anything to the doctor. So more information about why they have to take it and that there is a good chance of recurrence is really important. (SuppProv#101)
Likewise, physicians indicated that SSBCS’ lack of information on hormonal treatments often resulted in nonadherence, emphasizing the need to reinforce adherence to prevent recurrence.
Conceptual framework of interventions
Based on triangulation of survey and interview results, we compiled a conceptual framework that includes needs identified, suggested components of a survivorship care intervention to address these needs, potential mediators by which such interventions could improve outcomes, and relevant outcomes (Figure). Survivorship care needs fell into four categories: symptom management, psychosocial, sense of abandonment by health care team, and healthy lifestyles. Survivorship care programs would provide skills training in symptom and stress management, and communicating with providers, family, friends, and coworkers. Mediators include increased self-efficacy, knowledge and perceived social support, ultimately leading to reduced distress (anxiety and depressive symptoms) and stress, and improved health-related quality of life.
Conclusions
Our study aimed to identify the most critical needs of SSBCS in the posttreatment survivorship phase to facilitate the design of survivorship interventions for this vulnerable group. SSBCS, cancer support providers, and cancer physicians reported substantial symptom management, psychosocial, and informational needs among this population. Results from surveys and open-ended interviews were remarkably consistent. Survivors, physicians, and support providers viewed transition out of active treatment as a time of increased psychosocial need and heightened vulnerability.
Our findings are consistent with needs assessments conducted in other breast cancer survivors. Similar to a study of rural white women with breast cancer, fear of recurrence was among the most common psychosocial concerns.17 Results of two studies that included white, African American and Latina breast cancer survivors were consistent with ours in finding that pain and fatigue were among the most persistent symptoms; in both studies, Latinas were more likely to report pain and a higher number of symptoms.7,18 The prevalence of sleep problems in our sample was identical to that reported in a sample of African American breast cancer survivors.19 Our findings of a high need for symptom management information and support, social support from family and friends, and self-management resources were similar to studies of other vulnerable breast cancer survivors.18,20
Our results suggest that it is critical for health care professionals to provide assistance with managing side effects and information to alleviate fears, and reinforce behaviors of symptom monitoring and reporting, and adherence to follow-up care and hormonal therapies. Yet this information is not being conveyed effectively and is complicated by the need to balance women’s need for information with minimizing anxiety when providing such information.
A limitation of our study is that most of our sample was Mexican origin and may not reflect experiences of Spanish-speaking Latinas of other national origin groups or outside of Northern California. Another limitation is the lack of an English-speaking comparison group, which would have permitted the identification of similarities and differences across language groups. Finally, we did not interview radiation oncologists who may have had opinions that are not represented here.
Survivorship care programs offer great promise for meeting patients’ informational and symptom management needs and improving well-being and communication with clinicians.21 Due to limited access to survivorship care information, financial hardships, and pressures from their families to resume their social roles, concerted efforts are needed to develop appropriate survivorship programs for SSBCS.22 Unique language, cultural and socioeconomic factors of Spanish-speaking Latinas require tailoring of cancer survivorship programs to best meet their needs.23 These programs need to provide psychosocial stress and symptom management assistance, simple information on recommended follow-up care, and healthy lifestyle and role reintegration strategies that account for their unique sociocultural contexts.
1. Danese MD, O’Malley C, Lindquist K, Gleeson M, Griffiths RI. An observational study of the prevalence and incidence of comorbid conditions in older women with breast cancer. Ann Oncol. 2012;23(7):1756-1765.
2. Hewitt M, Greenfield S, Stovall E, eds. From cancer patient to cancer survivor: lost in transition. Washington, DC: National Academy of Sciences; 2006.
3. Beckjord EB, Arora NK, McLaughlin W, Oakley-Girvan I, Hamilton AS, Hesse BW. Health-related information needs in a large and diverse sample of adult cancer survivors: implications for cancer care. J Cancer Surviv. 2008;2(3):179-189.
4. Hewitt ME, Bamundo A, Day R, Harvey C. Perspectives on posttreatment cancer care: qualitative research with survivors, nurses, and physicians. J Clin Oncol. 2007;25(16):2270-2273.
5. Ashing-Giwa KT, Tejero JS, Kim J, Padilla GV, Hellemann G. Examining predictive models of HRQOL in a population-based, multiethnic sample of women with breast carcinoma. Qual Life Res. 2007;16(3):413-428.
6. Clauser SB, Arora NK, Bellizzi KM, Haffer SC, Topor M, Hays RD. Disparities in HRQOL of cancer survivors and non-cancer managed care enrollees. Health Care Financ Rev. 2008;29(4):23-40.
7. Eversley R, Estrin D, Dibble S, Wardlaw L, Pedrosa M, Favila-Penney W. Posttreatment symptoms among ethnic minority breast cancer survivors. Oncol Nurs Forum. 2005;32(2):250-256.
8. Bickell NA, Wang JJ, Oluwole S, et al. Missed opportunities: racial disparities in adjuvant breast cancer treatment. J Clin Oncol. 2006;24(9):1357-1362.
9. Arora NK, Reeve BB, Hays RD, Clauser SB, Oakley-Girvan I. Assessment of quality of cancer-related follow-up care from the cancer survivor’s perspective. J Clin Oncol. 2011;29(10):1280-1289.
10. Janz NK, Mujahid MS, Hawley ST, Griggs JJ, Hamilton AS, Katz SJ. Racial/ethnic differences in adequacy of information and support for women with breast cancer. Cancer. 2008;113(5):1058-1067.
11. Yoon J, Malin JL, Tisnado DM, et al. Symptom management after breast cancer treatment: is it influenced by patient characteristics? Breast Cancer Res Treat. 2008;108(1):69-77.
12. Ashing K, Rosales M. A telephonic-based trial to reduce depressive symptoms among Latina breast cancer survivors. Psychooncology. 2014;23(5):507-515.
13. Hershman DL, Greenlee H, Awad D, et al. Randomized controlled trial of a clinic-based survivorship intervention following adjuvant therapy in breast cancer survivors. Breast Cancer Res Treat. 2013;138(3):795-806.
14. Napoles AM, Ortiz C, Santoyo-Olsson J, et al. Nuevo Amanecer: results of a randomized controlled trial of a community-based, peer-delivered stress management intervention to improve quality of life in Latinas with breast cancer. Am J Public Health. 2015;105(suppl 3):e55-63.
15. Rechis R, Reynolds KA, Beckjord EB, Nutt S, Burns RM, Schaefer JS. ‘I learned to live with it’ is not good enough: challenges reported by posttreatment cancer survivors in the Livestrong surveys. Austin, TX: Livestrong;2011.
16. Glaser BG, Strauss AL. The discovery of grounded theory: strategies for qualitative research. Hawthorne: Aldine Publishing Company; 1967.
17. Befort CA, Klemp J. Sequelae of breast cancer and the influence of menopausal status at diagnosis among rural breast cancer survivors. J Womens Health (Larchmt). 2011;20(9):1307-1313.
18. Fu OS, Crew KD, Jacobson JS, et al. Ethnicity and persistent symptom burden in breast cancer survivors. J Cancer Surviv. 2009;3(4):241-250.
19. Taylor TR, Huntley ED, Makambi K, et al. Understanding sleep disturbances in African-American breast cancer survivors: a pilot study. Psychooncology. 2012;21(8):896-902.
20. Adams N, Gisiger-Camata S, Hardy CM, Thomas TF, Jukkala A, Meneses K. Evaluating survivorship experiences and needs among rural African American breast cancer survivors. J Cancer Educ. October 24, 2015 [Epub ahead of print].
21. Blinder VS, Patil S, Thind A, et al. Return to work in low-income Latina and non-Latina white breast cancer survivors: a 3-year longitudinal study. Cancer. 2012;118(6):1664-1674.
22. Lopez-Class M, Perret-Gentil M, Kreling B, Caicedo L, Mandelblatt J, Graves KD. Quality of life among immigrant Latina breast cancer survivors: realities of culture and enhancing cancer care. J Cancer Educ. 2011;26(4):724-733.
23. Napoles-Springer AM, Ortiz C, O’Brien H, Diaz-Mendez M. Developing a culturally competent peer support intervention for Spanish-speaking Latinas with breast cancer. J Immigr Minor Health. 2009;11(4):268-280
1. Danese MD, O’Malley C, Lindquist K, Gleeson M, Griffiths RI. An observational study of the prevalence and incidence of comorbid conditions in older women with breast cancer. Ann Oncol. 2012;23(7):1756-1765.
2. Hewitt M, Greenfield S, Stovall E, eds. From cancer patient to cancer survivor: lost in transition. Washington, DC: National Academy of Sciences; 2006.
3. Beckjord EB, Arora NK, McLaughlin W, Oakley-Girvan I, Hamilton AS, Hesse BW. Health-related information needs in a large and diverse sample of adult cancer survivors: implications for cancer care. J Cancer Surviv. 2008;2(3):179-189.
4. Hewitt ME, Bamundo A, Day R, Harvey C. Perspectives on posttreatment cancer care: qualitative research with survivors, nurses, and physicians. J Clin Oncol. 2007;25(16):2270-2273.
5. Ashing-Giwa KT, Tejero JS, Kim J, Padilla GV, Hellemann G. Examining predictive models of HRQOL in a population-based, multiethnic sample of women with breast carcinoma. Qual Life Res. 2007;16(3):413-428.
6. Clauser SB, Arora NK, Bellizzi KM, Haffer SC, Topor M, Hays RD. Disparities in HRQOL of cancer survivors and non-cancer managed care enrollees. Health Care Financ Rev. 2008;29(4):23-40.
7. Eversley R, Estrin D, Dibble S, Wardlaw L, Pedrosa M, Favila-Penney W. Posttreatment symptoms among ethnic minority breast cancer survivors. Oncol Nurs Forum. 2005;32(2):250-256.
8. Bickell NA, Wang JJ, Oluwole S, et al. Missed opportunities: racial disparities in adjuvant breast cancer treatment. J Clin Oncol. 2006;24(9):1357-1362.
9. Arora NK, Reeve BB, Hays RD, Clauser SB, Oakley-Girvan I. Assessment of quality of cancer-related follow-up care from the cancer survivor’s perspective. J Clin Oncol. 2011;29(10):1280-1289.
10. Janz NK, Mujahid MS, Hawley ST, Griggs JJ, Hamilton AS, Katz SJ. Racial/ethnic differences in adequacy of information and support for women with breast cancer. Cancer. 2008;113(5):1058-1067.
11. Yoon J, Malin JL, Tisnado DM, et al. Symptom management after breast cancer treatment: is it influenced by patient characteristics? Breast Cancer Res Treat. 2008;108(1):69-77.
12. Ashing K, Rosales M. A telephonic-based trial to reduce depressive symptoms among Latina breast cancer survivors. Psychooncology. 2014;23(5):507-515.
13. Hershman DL, Greenlee H, Awad D, et al. Randomized controlled trial of a clinic-based survivorship intervention following adjuvant therapy in breast cancer survivors. Breast Cancer Res Treat. 2013;138(3):795-806.
14. Napoles AM, Ortiz C, Santoyo-Olsson J, et al. Nuevo Amanecer: results of a randomized controlled trial of a community-based, peer-delivered stress management intervention to improve quality of life in Latinas with breast cancer. Am J Public Health. 2015;105(suppl 3):e55-63.
15. Rechis R, Reynolds KA, Beckjord EB, Nutt S, Burns RM, Schaefer JS. ‘I learned to live with it’ is not good enough: challenges reported by posttreatment cancer survivors in the Livestrong surveys. Austin, TX: Livestrong;2011.
16. Glaser BG, Strauss AL. The discovery of grounded theory: strategies for qualitative research. Hawthorne: Aldine Publishing Company; 1967.
17. Befort CA, Klemp J. Sequelae of breast cancer and the influence of menopausal status at diagnosis among rural breast cancer survivors. J Womens Health (Larchmt). 2011;20(9):1307-1313.
18. Fu OS, Crew KD, Jacobson JS, et al. Ethnicity and persistent symptom burden in breast cancer survivors. J Cancer Surviv. 2009;3(4):241-250.
19. Taylor TR, Huntley ED, Makambi K, et al. Understanding sleep disturbances in African-American breast cancer survivors: a pilot study. Psychooncology. 2012;21(8):896-902.
20. Adams N, Gisiger-Camata S, Hardy CM, Thomas TF, Jukkala A, Meneses K. Evaluating survivorship experiences and needs among rural African American breast cancer survivors. J Cancer Educ. October 24, 2015 [Epub ahead of print].
21. Blinder VS, Patil S, Thind A, et al. Return to work in low-income Latina and non-Latina white breast cancer survivors: a 3-year longitudinal study. Cancer. 2012;118(6):1664-1674.
22. Lopez-Class M, Perret-Gentil M, Kreling B, Caicedo L, Mandelblatt J, Graves KD. Quality of life among immigrant Latina breast cancer survivors: realities of culture and enhancing cancer care. J Cancer Educ. 2011;26(4):724-733.
23. Napoles-Springer AM, Ortiz C, O’Brien H, Diaz-Mendez M. Developing a culturally competent peer support intervention for Spanish-speaking Latinas with breast cancer. J Immigr Minor Health. 2009;11(4):268-280
Gap analysis: a strategy to improve the quality of care of head and neck cancer patients
In the United States, there will be an estimated 49,670 new cases of head and neck cancer for 2017.1 Head and neck cancer (HNC) is a term used to describe a range of tumors that originate in the area of the body spanning from the lower neck to the upper nasal cavity.2 Specifically, they are malignancies arising in the mouth, larynx, nasal cavity, sinuses, tongue, lips, and numerous glands such as the thyroid and salivary.2 To clarify, HNC, despite the encompassing name, does not include growths of the bones, teeth, skin, brain parenchyma, and eye; therefore, such tumors will not be addressed in this article.
Patients with HNC often experience fragmented and uncoordinated care that leads to delays in cancer treatment, severe distress in patients and families, and dissatisfaction with care. Literature reports that these patients face numerous stressors including aggressive cancer treatments, severe symptoms, body image concerns, loss of speech, difficulty swallowing, nutritional issues, and respiratory problems that affect their quality of life and ability to function on a day-to-day basis.3,4In addition, patients with HNC and their families are challenged to navigate the health care system and to overcome the difficulties of accessing services within the context of financial constraints. A multidisciplinary team (MDT) approach is the standard of care for HNC patients, as demonstrated in studies reporting better 5-year survival outcomes, increased completion of adjuvant therapy, and higher compliance with speech-language pathologist (SLP) recommendations.5, 6 Furthermore, a recent systematic review of cancer teams concluded that the MDT approach leads to improved clinical outcomes and enhanced communication between the patient and the team.7
The Institute of Medicine (IOM) stated in its 2013 report on cancer care that a high-quality care delivery system requires continuing measurement of cancer care and strategies to carry out performance improvement.8 Following the IOM premise, the cancer center at an academic medical center in Philadelphia made efforts to improve patient access to multidisciplinary services, first, by creating a multidisciplinary Cancer Appetite and Rehabilitation (CARE)clinic to address the symptoms and nutritional needs of HNC patients,9 and second, by using a gap analysis to conduct an assessment of the cancer care services provided to this cancer population. The need to conduct this assessment was generated by the desire to improve access to multidisciplinary care, with the goal of meeting standard benchmarks for completion of treatment while increasing the use of ancillary services. This article describes the process of conducting a gap analysis of cancer services for HNC patients, and includes discussion of the findings, recommendations for improving care, a description of the quality improvement interventions, and a report of the outcomes based on an interval re-assessment 18 months later.
Methods
Methods included a gap analysis, implementation of quality improvement recommendations, and re-assessment of indicators (Figure). A gap analysis “identifies differences between desired and actual practice conditions, including service delivery and quality patient outcomes as measured against evidence-based benchmarks while incorporating key stakeholder concerns and expectations.”10 The gap analysis of cancer care services offered to HNC patients was achieved through the step-by-step process described hereinafter. The implementation of quality improvement recommendations was accomplished by establishing two task force committees focused respectively on education and transitions in care coordination. Re-assessment of indicators related to timeliness of delivery of cancer treatments and collection of additional baseline data regarding supportive services.
Gap analysis
Identification of the scope of the problem. Members of the HNC multidisciplinary team raised concerns about unintended breaches in care for HNC patients that resulted not only in delay of the patients’ cancer treatments, but also in unnecessary distress for the patients and their families. As a result, the HNC team decided to conduct a gap analysis to identify the barriers in care for HNC patients, and by doing this, to determine possible solutions.
Identification of best practice care indicators. The indicators of best practice care (benchmarks) for HNC patients were identified after exhaustive review of the literature11-22(Table 1). For this gap analysis, the indicators focused on waiting time to treatment (surgery, chemotherapy, radiation therapy) and to supportive care interventions (nutrition, speech and language pathology) as follows:
- 9.2Futura StdInitial ear-nose-throat (ENT) visit to surgery: <30 days
- Biopsy to start radiation therapy (RT) for nonsurgical patients: 40 days
- Surgery to RT start: 42 days
- Surgery to nutrition consultation (outpatient), start RT to nutrition: Pretreatment
- Surgery to outpatient SLP, initial ENT visit to SLP referral, surgery to SLP referral, RT start to outpatient SLP start: Pretreatment
Measure gaps against benchmarks. To Gap analysis of measure gaps against benchmarks, the authors used the Agency for Health Care Research and Quality tool that provides a systematic method to compare current practice with best practices and determine the barriers to best practice and the feasibility of implementing best practices by the institution23 (Table 1). For this project, a process map of waiting time to treatment and supportive care interventions was created, so that real-world conditions could be measured against benchmarks.
Process map. The authors identified 67 newly diagnosed HNC patients during January-July 2014 from the surgery, radiation therapy, and nutrition departments, but only 33 patients were able to be tracked from their initial visit at the cancer center until the completion of their treatment through the electronic medical record (EMR) system. Their information was compiled in a spreadsheet based on the EMR information. Data included patient access to supportive services and number of days between important treatment benchmarks. Tracking data was used to create a treatment flow chart and determine average treatment intervals.
To map the typical patient process, the patients were split into two groups: surgical (n = 22) and nonsurgical (n = 11). Surgical patients underwent surgery as their primary treatment and received adjuvant radiation therapy or concurrent chemotherapy. Nonsurgical patients did not require surgery other than biopsy as a part of their treatment. Most of the nonsurgical patients received chemotherapy, and 1 patient received palliative radiation therapy.
SWOT analysis. The SWOT analysis is used to chart institution performance in relation to benchmarks while describing stakeholders’ perceptions.24The stakeholder perspective for this project focused on the views of the health care providers from all disciplines regarding the quality of care provided to the HNC population. In addition, a patient survey was conducted to assess their perception of the care they received.
Clinician survey. We surveyed 25 clinicians, including physicians, advanced practice providers, nurses, and allied health professionals, from the surgical (n = 3), hospitalization (n = 6), radiation (n = 3), chemotherapy (n = 3) and supportive services teams (n = 10). The survey was conducted face to face and included 7 open-ended questions designed to gain insight about problems encountered with coordination of care, suggestions to improve coordination of care, factors in treatment delays, suggestions to decrease treatment delays, factors in excellent patient outcomes, rate overall patient care, and suggestions for improvement of service. Initial survey responses were filtered by recurring themes in each question among the different patient service teams.
Patient satisfaction survey. The sample of patients was obtained from the surgery, radiation therapy, and nutrition departments during January-July 2014. Sixty-seven initial patients were identified but only 43 were eligible for interview because they had a listed phone number. A six-question nonvalidated survey was developed by the authors to measure patient satisfaction with the scheduling process, waiting time, information provided about treatment and their medical status, emotional support, the coordination of care, and the payment process. Satisfaction was rated on a scale from 1 to 5 (1 = Poor, 2 = Fair, 3 = Satisfactory, 5 = Great).
Analysis and final report. See Results section.
Quality improvement implementation. The transitions and the education committees were created to address the gaps identified during the analysis. The transitions committee developed strategies to improve the coordination of care of HNC patients throughout their cancer treatment and the education committee elaborated new ways to enhance patient education while meeting treatment timeline standards. The implementation of the interventions was developed by the inpatient and outpatient MTD teams caring for the HNC population.
Re-assessment of indicators. During January-December 2015, a total of 58 patients diagnosed with HNC were identified. Of those, 40 patients with recurrent disease were eliminated, leaving 18 patients (10 surgical, 8 nonsurgical). Similar to the initial assessment for the gap analysis, data included patient access to supportive services and number of days between important treatment benchmarks. Tracking data was used to create a treatment flow chart and determine average treatment intervals.
Results
Most of the patients were men (70%), white (70%), and 60% were within the 50-69 years age range at the time of diagnosis.
Clinician survey
The clinicians were surveyed and their responses analyzed by two people, the project leader and the project assistant. The most commonly identified weaknesses in care that the clinicians identified were delayed access to dental referrals, insufficient preoperative patient education, and inefficient discharge planning and/or home care coordination. Dental referrals were identified as a major cause of delay in starting radiation therapy because of scheduling issues, a lack of patient motivation, limited insurance coverage, and difficulty identifying reliable dentists in the patient’s geographic area. Clinicians also identified problems coordinating smoking cessation referrals for patients.
In addition, they identified the hospitalization and/or home care phases as areas for potential improvement. During hospitalization, patients often expressed surprise upon learning that they had a feeding tube and/or tracheostomy despite having received pre-operative education. This misunderstanding by the patient was likely related to the clinicians’ assumptions about the best timing for patient education and the amount of time needed for education before the surgical procedure. The surgical team provided patient education based on individual needs, and it has not been standardized because they felt that patients’ education needs vary from person to person. In contrast, patient education prior radiation therapy is standardized, and all patients received a comprehensive package of information that is re-enforced by direct patient education by the clinicians.
Another gap in care identified by the inpatient team was a prolonged intensive care unit (ICU) stay for the HNC patients. These patients remained in the ICU for the entirety of their stay. Not only was this causing overuse of resources, but patients also felt unprepared for an independent discharge home given the high level of care received in the ICU.
A range of suggestions were made to solve these problems. The most prevalent suggestion was to use a nurse navigator to coordinate referrals, schedule appointments, facilitate interdisciplinary communication, and to address social, financial, and transport needs for HNC patients. Several other suggestions referred to standardizing treatment procedures and pre-operative patient education.
Patient survey
Forty-three patients were identified for the patient satisfaction survey. Each patient was contacted at least three times over the course of 3 weeks. Of the 43 patients, 20 had an invalid phone number, 10 were not available for participation, and 1 declined to participate. A total of 12 patients completed the survey.
Although the sample size was small, the patients surveyed were very satisfied with their care. Of the 12 patients, 5 patients rated all of the services relevant to their treatment as a 5 (Great). Areas of particular concern for the patients included the waiting time to see a physician in the ENT clinic, the explanation/collection of charges, and the accessibility of support groups. Services rated 3 (Satisfactory) included waiting time to schedule appointments; the amount of information and patient education provided by about radiation, nutrition, physical therapy (PT), occupational therapy (OT), and SLP; and overall satisfaction with care.
Surgical patients. The Danish Head and Neck Society guidelines state that the interval between the initial visit diagnosis and surgery should be within 30 days.12A comparison of the average intervals between important treatment points for the surgical sample patients with the benchmark timing recommended in the literature are shown in Table 2. The mean time from initial visit to surgery was 28 days in the cancer center sample; 67% of patients (n = 14) had surgery within 30 days, and 33% of patients (n = 7) had surgery beyond 30 days. The interval re-assessment showed improvements in this area: the mean time from initial visit to surgery went from 28 to 18 days, and 100% of patients
n = 10) had surgery within 30 days.
Huang and colleagues have indicated that postoperative radiation therapy should ideally occur within 42 days of surgery;13 however, in the present study, 79% (n = 11) of the sample surgical patients undergoing radiation began their therapy on average more than 63 days after surgery. The interval re-assessment found the same results with 80% of patients starting radiation over 42 days after surgery although the average time lag decreased from 68 days to 53 days.
Nonsurgical patients. Huang and colleagues have indicated that for patients undergoing radiation as their primary form of treatment, an interval of 40 days between biopsy and the start of radiation is ideal.13 The average intervals between important time points of treatment for patients who did not require surgery in their treatment are shown in Table 2. The cancer center met the benchmark at baseline with an average of 38 days (n = 11 patients). The re-assessment showed improvement in this area with 100% of cases (n = 10) meeting the benchmark with an average of 32 days. Likewise, the benchmark waiting time from RT consultation to RT start of less than 30 days11 was met by the cancer center for the nonsurgical group (n = 11).
Access to supportive services
Nutrition care. Studies have shown that standard nutritional care for HNC patients should start before treatment.18,19 In the present study, the waiting time from surgery to outpatient nutrition assessment improved from 61 days to 50 days (Table 2). For patients in the surgical group, the time interval between the initial ENT visit to the outpatient nutrition assessment decreased from 85 days at baseline to 66 days at reassessment, and 82 days to 35 days, respectively, for the nonsurgical group. The time interval from surgery to nutrition assessment has not reached the recommended pretreatment benchmark, but data showed a trend of improvement from 61 days at baseline to 50 days at reassessment for patients in the group.
Patients were typically referred to outpatient nutrition at the start of radiation therapy. In the initial assessment, all patients (n = 33) had access to nutrition services, but 21% (n = 7) never spoke to the nutritionist. The re-assessment found all but one (n = 7) of the patients had been seen by a nutritionist at some point during the treatment period. The benchmark of preradiation nutrition assessment was met by 2 postsurgical patients, with the remainder of the patients being seen within 3 days of the initiation of radiation.
Speech-language pathology management. The literature recommends that patients receive SLP management before the surgery.14-17 In this gap analysis, a difference in access to SLP services was identified between inpatient and outpatient settings. On average, patients within the sample were referred to outpatient SLP over a month after their surgery. In contrast, inpatient surgical patients had access to rapid consultations with SLP (eg, 1 day after surgery for total laryngectomy, and 4 days after surgery for oropharyngeal and oral surgery patients; T Hogan, unpublished data, June 2014). Overall, the benchmark was not met, as patients were not seen by the SPL prior to treatment.
New baseline data was collected about SLP services and showed that 70% of patients had contact with the outpatient SLP at some point during their treatment. Of those, only 29% of patients saw SLP before surgery, meeting the benchmark. The baseline waiting time was an average of 15 days before surgery and 43 days after surgery. Overall, the trend is moving toward the benchmark of care.
Similarly, studies determined that the gold standard of care for nonsurgical patients is that SLPs begin pretreatment management of HNC.16Patients in the baseline sample were typically referred to outpatient SLP about a month after biopsy (presumably diagnosis), but before the start of chemo-radiation. There were no data available for the number of patients who were actually seen by the outpatient SLP before the start of chemo-radiation.
The new baseline data found that 100% of nonsurgical patients were referred to SLP, but only25% (n = 2) were seen before they started chemo-radiation therapy (an average 5 days before) and 75% (n = 6) were seen after starting chemo-radiation therapy (an average 23 days after).
SWOT analysis
The SWOT analysis included strengths, weaknesses, opportunities and threats of the care provided to HNC patients at the cancer center. The gap analysis based on the results of the clinician surveys, process mapping, and patient satisfaction survey is summarized in Table 3. Three main gaps were identified: waiting time to treatment, education, and coordination of and transitions in care.
Quality improvement actions
Interventions by the outpatient MTD team included changing the process of scheduling dental appointments, creating a new approach to outpatient nutrition by proactively meeting patients in the ENT clinic, and conducting PT and SLP assessments to patients in the chemotherapy unit while receiving their treatment. A nurse navigator position for this patient population was approved and an expedited referral system was initiated. At the same time, the inpatient team implemented a specialized HNC unit in the medical-surgical floor, developed the protocols for the management of postsurgery HNC patients, educated nursing staff, and standardized patient education to facilitate transition to the next level of care (Table 3).
Discussion
The gap analysis of services provided to HNC patients at the cancer center identified three gaps in care: delay in treatment and supportive services, nonstandardized patient education, and lack of care coordination.
All patients should have access to a timely treatment initiation. In this analysis, surgical patients encountered a delay between surgery and the start of radiation therapy, about 3 weeks beyond the recommended in the literature.12 Clinicians mentioned delays in ensuring preradiation dental consultations as a significant issue affecting the patient treatment process. Re-assessment data reported that despite interventions for early dental referrals, 80% of patients still started radiation over 6 weeks after surgery; however, the average time lag decreased from 68 days to 53 days.
RT delays in HNC patients not only affect patients’ emotional state but may also impact clinical outcomes. Treatment delays have the potential to harm patients by: allowing tumor growth that impact on the curative outcomes of RT; postponing the benefits of palliative RT on symptom relief; and causing psychological distress.25 In addition, delay in starting treatment has shown to increase the risk for local recurrence,13,26 and decrease survival.27
Higher demand for advanced RT modalities has been linked to treatment delays. Waiting times from initial RT evaluation to start RT have increased over time, from <14 days in 1989 to 31 days in 1997.11 This is explained by the complexity of the pretreatment evaluations and the increasing demand of radiation services, especially in high volume institutions.25,27A fast-track program to reduce waiting time in the treatment of HNC patients reported to be effective.22 This program includes a patient coordinator, a hotline for referral procedures, prebooked slots for ENT and RT clinics, faster pathology and imaging reports, and the establishment of an MTD team.
The clinician survey identified patient needs classified in three categories: pre-operative education, hospitalization process, and access to support services. Regarding pre-operative education, clinicians acknowledged that although patients were educated about their surgical options and possible outcomes prior to hospitalization, they often could not fully understand this information at the time of the instruction. The high need for education particularly in the pretreatment phase was documented in a needs assessment survey for HNC patients conducted at the cancer center D DeMille, RD, unpublished data, August 2013).
Studies have looked at the effectiveness of education in cancer patients. The use of teaching interventions (written information, audiotapes, videotapes, and computer programs) has proven to be valuable for educating patients prior to experiencing cancer treatments.20Further, a systematic review of preparatory education for cancer patients undergoing surgery reported that face-to-face discussions appear to be effective at improving patient outcomes with regards to increasing knowledge and decreasing anxiety.21 However, it was stated that the timing of the delivery of education is critical to be efficient. For example, an education session provided one day prior the day of surgery is not useful as it may place additional stress on a patient who is already highly anxious and decreases the likelihood for the information to be managed. It is recommended to deliver education early enough prior surgery to allow time for the patient to process the information. Also, a study reported that presurgical education on potential side effects; the assessment of patients’ needs by an SLP, physical therapist, nutritionist, and social worker; and pre-operative nutritional support decrease postoperative complications.4
The education committee was created in response to the gap on patient education. The inpatient team took the lead and provided intense education on the care of HNC patients to the nursing staff and to HNC patients and their families about postoperative care at home. Education was also extended to rehabilitation facilities caring for this cancer population at discharge from the hospital.
Clinicians identified a gap during the hospitalization process. The gap included prolonged stay of patients in the ICU postsurgery, inefficient interclinician communication, lack of standardization of postsurgical care, and difficulty communicating with external home care teams. A major intervention was implemented that included the creation of a HNC specialized unit that offered a structured setting for standardized care and communication between patients and clinicians. Dedicated units for the management of HNC patients highly enhance the quality of care provided because it enables the MTD team to work properly by clearly defining roles and responsibilities, delineating evidence-based clinical interventions, and promoting expert care for this patient population.23In addition, several key steps have been recommended to reduce the fragmentation of care for hospital patients, including developing a referral/transition tracking system, organizing and training staff members to coordinate transition/referrals, and identifying and creating agreements with key care providers.28
Early patient access to supportive services was a concern to most clinicians. HNC providers were not consulting the CARE clinic about patients’ nutritional, physical and SLP needs until the patient was having serious problems. Patient tracking found that the minority of patients met the standard of having a presurgical speech referral. Most patients had access to outpatient nutrition services during radiation therapy but the majority of patients in the sample did not attend CARE clinic. The literature strongly supports early management of HNC patients by the SPL and nutrition counselor. Van der Molen and colleagues demonstrated that a pretreatment SPL rehabilitation program is feasible and offers reasonable patient compliance despite of the burden caused by ongoing chemo-radiation therapy for HNC patients.16Similarly, early nutrition counseling for HNC patients undergoing RT has reported to decrease unintended weight loss and malnutrition compared with late nutrition intervention.19
Although there are clear gaps in care for HNC patients from the clinicians’ perspective, the patients surveyed indicated a clear satisfaction with their care at the cancer center. Almost all patients were satisfied with their relationships with clinicians in the team. Some patients mentioned complaints of insufficient pre-operative education and waiting time, but there were not significant complaints about coordination, which clinicians had identified as a major issue. This is likely explained by the small sample size and the patients’ inability to see the background interclinician communication.
A crucial suggestion to address all of these gaps in care was the implementation of a nurse navigator. With the support of hospital and cancer center administration, a nurse navigator was hired to address the needs of HNC patients throughout their disease trajectory. The team agreed that the nurse navigator should make contact with HNC patients during their initial appointment at the surgical ENT office. This initial contact allows the nurse navigator to provide support and connection to resources. Thereafter, early contact with this patient population allows the nurse navigator to follow the patient through the continuum of care from biopsy and diagnosis to survivorship. The nurse navigator facilitates communication between clinicians, patients and their families; and provides emotional support to patients while helping to manage their financial and transportation needs.29
Limitations
This is a quality improvement project with a small sample size of HNC cases. Data from this gap analysis are not statistically significant; yet, are clinically relevant in the management of the HNC population at the cancer center. Likewise, the patient sample size was small, making definitive generalizations about patient experience difficult; however, the data are helpful in highlighting possible problems for patients.
1. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2017. CA Cancer J Clin. 2016;67:7-30.
2. National Cancer Institute. Head and neck cancers. https://www.cancer.gov/types/head-and-neck/head-neck-fact-sheet. Reviewed February 1, 2013. Accessed January 26, 2017
3. Weiderholt PA, Connor NP, Hartig GK, Harari PM. Bridging gaps in multidisciplinary head and neck cancer care: nursing coordination and case management. Int J Radiat Oncol Biol Phys. 2007;69(2 suppl):S88-S91.
4. Dingman C, Hegedus PD, Likes C, McDowell P, McCarthy E, Zwilling C. A coordinated, multidisciplinary approach to caring for the patient with head and neck cancer. J Support Oncol. 2008;6(3):125-131.
5. Liao C, Kang CJ, Lee LY, et al. Association between multidisciplinary team care approach and survival rates in patients with oral cavity squamous cell carcinoma. Head Neck. 2016;38(suppl 1):E1544-1553.
6. Starmer H, Sanguineti G, Marur S, Gourin CG. Multidisciplinary head and neck cancer clinic and adherence with speech pathology. Laryngoscope. 2011;121(10):2131-2135.
7. Prades J, Remue E, van Hoof E, Borras JM. Is it worth reorganizing cancer services on the basis of multidisciplinary teams (MDTs)? A systematic review of the objectives and organization of MDTs and their impact on patient outcomes. Health Pol. 2015;119(4):464-474.
8. Institute of Medicine. Delivering high-quality cancer care: charting a new course for a system in crisis. 2013. www.nationalacademies.org/hmd/Reports/2013/Delivering-High-Quality-Cancer-Care-Charting-a-New-Course-for-a-System-in-Crisis.aspx. Published September 10, 2013. Accessed May 29, 2016.
9. Granda-Cameron C, DeMille D, Lynch MP, et al. An interdisciplinary approach to manage cancer cachexia. Clin J Oncol Nurs. 2010;14(1):72-80.
10. Davis-Ajami ML, Costa L, Kulik S. Gap analysis: synergies and opportunities for effective nursing leadership. Nurs Econ. 2014;32(1):17-25.
11. Fortin A, Bairati I, Albert M, et al. Effect of treatment delay on outcome of patients with early-stage head-and-neck carcinoma receiving radical radiotherapy. Int J Radiat Oncol Biol Phys. 2002;52(4):929-936.
12. Van Harten MC, Ridder M, Hamming-Vrieze O, et al. The association of treatment delay and prognosis in head and neck squamous cell carcinoma (HNSCC) in a Dutch comprehensive cancer center. Oral Oncol. 2014;50:282-290.
13. Huang J, Barbera L, Brouwers M, et al. Does delay in starting treatment affect the outcomes of radiotherapy? A systematic review. J ClinOncol. 2003;21(3):555-563.
14. Lazarus CL. Management of swallowing disorders in head and neck cancer patients: optimal patterns of care. Sem Speech Lang. 2000;21(4):293-310.
15. Mayer KR. Learning to speak after laryngectomy. http://speech-language-pathology-audiology.advanceweb.com/Features/Articles/Learning-to-Speak-After-Laryngectomy.aspx. Posted October 27, 2014. Accessed January 17, 2017.
16. van der Molen L, van Rossum MA, Burkhead LM, et al. A randomized preventive rehabilitation trial in advanced head and neck cancer patients treated with chemo-radiotherapy: feasibility, compliance, and short-term effects. Dysphagia. 2011;26:155-170.
17. Starmer HM, Gourin CG. Is speech language pathologist evaluation necessary in the nonoperative treatment of head and neck cancer? Laryngoscope. 2013;123(7):1571-1572.
18. [Article in French] Meuric J, Garabige V, Blanc-Vincent MP, et al. Good clinical practice in nutritional management of head and neck cancer patients. Bull Cancer. 1999;86(10):843-854.
19. van den Berg MG, Rasmussen-Conrad EL, Wei KH, et al. Comparison of the effect of individual dietary counselling and of standard nutritional care on weight loss in patients with head and neck cancer undergoing radiotherapy. Br J Nutr. 2010;104:872-877.
20. Waller A, Forshaw K, Bryant J, Mair S. Interventions for preparing patients for chemotherapy and radiotherapy: a systematic review. Supp Care Ca. 2014;22(8):2297-2308.
21. Waller A, Forshaw K, Bryant J, et al. Preparatory education for cancer patients undergoing surgery: a systematic review of volume and quality of research output over time. Patient Educ Couns. 2015;98:1540-1549.
22. Toustrup K, Lambersten K, Birke-Sorensen H, et al. Reduction in waiting time for diagnosis and treatment of head and neck cancer – a fast track study. Acta Oncol. 2011;50:636-641.
23. Bergamini C, Locati L, Bossi P et al. Does a multidisciplinary team approach in a tertiary referral centre impact on the initial management of head and neck cancer? Oral Oncol. 2016;54:54-57.
24. AHRQ. Pediatric toolkit for using the AHRQ quality indicators. http://www.ahrq.gov/professionals/systems/hospital/qitoolkit/pediatrictoolkit.html . Reviewed July 2016. Accessed January 26, 2017.
25. Mackillop WJ. Killing time: the consequences of delays in radiotherapy. Radiother Oncol. 2007;84:1-4.
26.Chen Z, King, W, Pearcey R, Kerba M, Mackillop WJ. The relationship between waiting time for radiotherapy and clinical outcomes: a systematic review of the literature. Radiother Oncol. 2008;87:3-16.
27. Sharma S, Bekelman J, Lin A et al. Clinical impact of prolonged diagnosis to treatment interval (DTI) among patients with ororpharyngeal squamous cell carcinoma. Oral Oncol. 2016;56:17-24.
28. Improving chronic illness care. Reducing care fragmentation. Care coordination. http://www.improvingchroniccare.org/index.php?p=Care_Coordination&s=326. Published 2010. Accessed May 28, 2016.
29. Fillion L, de Serres M, Cook S, et al. Professional patient navigation in head and neck cancer. Sem Oncol Nurs. 2009;25(3):212-221.
In the United States, there will be an estimated 49,670 new cases of head and neck cancer for 2017.1 Head and neck cancer (HNC) is a term used to describe a range of tumors that originate in the area of the body spanning from the lower neck to the upper nasal cavity.2 Specifically, they are malignancies arising in the mouth, larynx, nasal cavity, sinuses, tongue, lips, and numerous glands such as the thyroid and salivary.2 To clarify, HNC, despite the encompassing name, does not include growths of the bones, teeth, skin, brain parenchyma, and eye; therefore, such tumors will not be addressed in this article.
Patients with HNC often experience fragmented and uncoordinated care that leads to delays in cancer treatment, severe distress in patients and families, and dissatisfaction with care. Literature reports that these patients face numerous stressors including aggressive cancer treatments, severe symptoms, body image concerns, loss of speech, difficulty swallowing, nutritional issues, and respiratory problems that affect their quality of life and ability to function on a day-to-day basis.3,4In addition, patients with HNC and their families are challenged to navigate the health care system and to overcome the difficulties of accessing services within the context of financial constraints. A multidisciplinary team (MDT) approach is the standard of care for HNC patients, as demonstrated in studies reporting better 5-year survival outcomes, increased completion of adjuvant therapy, and higher compliance with speech-language pathologist (SLP) recommendations.5, 6 Furthermore, a recent systematic review of cancer teams concluded that the MDT approach leads to improved clinical outcomes and enhanced communication between the patient and the team.7
The Institute of Medicine (IOM) stated in its 2013 report on cancer care that a high-quality care delivery system requires continuing measurement of cancer care and strategies to carry out performance improvement.8 Following the IOM premise, the cancer center at an academic medical center in Philadelphia made efforts to improve patient access to multidisciplinary services, first, by creating a multidisciplinary Cancer Appetite and Rehabilitation (CARE)clinic to address the symptoms and nutritional needs of HNC patients,9 and second, by using a gap analysis to conduct an assessment of the cancer care services provided to this cancer population. The need to conduct this assessment was generated by the desire to improve access to multidisciplinary care, with the goal of meeting standard benchmarks for completion of treatment while increasing the use of ancillary services. This article describes the process of conducting a gap analysis of cancer services for HNC patients, and includes discussion of the findings, recommendations for improving care, a description of the quality improvement interventions, and a report of the outcomes based on an interval re-assessment 18 months later.
Methods
Methods included a gap analysis, implementation of quality improvement recommendations, and re-assessment of indicators (Figure). A gap analysis “identifies differences between desired and actual practice conditions, including service delivery and quality patient outcomes as measured against evidence-based benchmarks while incorporating key stakeholder concerns and expectations.”10 The gap analysis of cancer care services offered to HNC patients was achieved through the step-by-step process described hereinafter. The implementation of quality improvement recommendations was accomplished by establishing two task force committees focused respectively on education and transitions in care coordination. Re-assessment of indicators related to timeliness of delivery of cancer treatments and collection of additional baseline data regarding supportive services.
Gap analysis
Identification of the scope of the problem. Members of the HNC multidisciplinary team raised concerns about unintended breaches in care for HNC patients that resulted not only in delay of the patients’ cancer treatments, but also in unnecessary distress for the patients and their families. As a result, the HNC team decided to conduct a gap analysis to identify the barriers in care for HNC patients, and by doing this, to determine possible solutions.
Identification of best practice care indicators. The indicators of best practice care (benchmarks) for HNC patients were identified after exhaustive review of the literature11-22(Table 1). For this gap analysis, the indicators focused on waiting time to treatment (surgery, chemotherapy, radiation therapy) and to supportive care interventions (nutrition, speech and language pathology) as follows:
- 9.2Futura StdInitial ear-nose-throat (ENT) visit to surgery: <30 days
- Biopsy to start radiation therapy (RT) for nonsurgical patients: 40 days
- Surgery to RT start: 42 days
- Surgery to nutrition consultation (outpatient), start RT to nutrition: Pretreatment
- Surgery to outpatient SLP, initial ENT visit to SLP referral, surgery to SLP referral, RT start to outpatient SLP start: Pretreatment
Measure gaps against benchmarks. To Gap analysis of measure gaps against benchmarks, the authors used the Agency for Health Care Research and Quality tool that provides a systematic method to compare current practice with best practices and determine the barriers to best practice and the feasibility of implementing best practices by the institution23 (Table 1). For this project, a process map of waiting time to treatment and supportive care interventions was created, so that real-world conditions could be measured against benchmarks.
Process map. The authors identified 67 newly diagnosed HNC patients during January-July 2014 from the surgery, radiation therapy, and nutrition departments, but only 33 patients were able to be tracked from their initial visit at the cancer center until the completion of their treatment through the electronic medical record (EMR) system. Their information was compiled in a spreadsheet based on the EMR information. Data included patient access to supportive services and number of days between important treatment benchmarks. Tracking data was used to create a treatment flow chart and determine average treatment intervals.
To map the typical patient process, the patients were split into two groups: surgical (n = 22) and nonsurgical (n = 11). Surgical patients underwent surgery as their primary treatment and received adjuvant radiation therapy or concurrent chemotherapy. Nonsurgical patients did not require surgery other than biopsy as a part of their treatment. Most of the nonsurgical patients received chemotherapy, and 1 patient received palliative radiation therapy.
SWOT analysis. The SWOT analysis is used to chart institution performance in relation to benchmarks while describing stakeholders’ perceptions.24The stakeholder perspective for this project focused on the views of the health care providers from all disciplines regarding the quality of care provided to the HNC population. In addition, a patient survey was conducted to assess their perception of the care they received.
Clinician survey. We surveyed 25 clinicians, including physicians, advanced practice providers, nurses, and allied health professionals, from the surgical (n = 3), hospitalization (n = 6), radiation (n = 3), chemotherapy (n = 3) and supportive services teams (n = 10). The survey was conducted face to face and included 7 open-ended questions designed to gain insight about problems encountered with coordination of care, suggestions to improve coordination of care, factors in treatment delays, suggestions to decrease treatment delays, factors in excellent patient outcomes, rate overall patient care, and suggestions for improvement of service. Initial survey responses were filtered by recurring themes in each question among the different patient service teams.
Patient satisfaction survey. The sample of patients was obtained from the surgery, radiation therapy, and nutrition departments during January-July 2014. Sixty-seven initial patients were identified but only 43 were eligible for interview because they had a listed phone number. A six-question nonvalidated survey was developed by the authors to measure patient satisfaction with the scheduling process, waiting time, information provided about treatment and their medical status, emotional support, the coordination of care, and the payment process. Satisfaction was rated on a scale from 1 to 5 (1 = Poor, 2 = Fair, 3 = Satisfactory, 5 = Great).
Analysis and final report. See Results section.
Quality improvement implementation. The transitions and the education committees were created to address the gaps identified during the analysis. The transitions committee developed strategies to improve the coordination of care of HNC patients throughout their cancer treatment and the education committee elaborated new ways to enhance patient education while meeting treatment timeline standards. The implementation of the interventions was developed by the inpatient and outpatient MTD teams caring for the HNC population.
Re-assessment of indicators. During January-December 2015, a total of 58 patients diagnosed with HNC were identified. Of those, 40 patients with recurrent disease were eliminated, leaving 18 patients (10 surgical, 8 nonsurgical). Similar to the initial assessment for the gap analysis, data included patient access to supportive services and number of days between important treatment benchmarks. Tracking data was used to create a treatment flow chart and determine average treatment intervals.
Results
Most of the patients were men (70%), white (70%), and 60% were within the 50-69 years age range at the time of diagnosis.
Clinician survey
The clinicians were surveyed and their responses analyzed by two people, the project leader and the project assistant. The most commonly identified weaknesses in care that the clinicians identified were delayed access to dental referrals, insufficient preoperative patient education, and inefficient discharge planning and/or home care coordination. Dental referrals were identified as a major cause of delay in starting radiation therapy because of scheduling issues, a lack of patient motivation, limited insurance coverage, and difficulty identifying reliable dentists in the patient’s geographic area. Clinicians also identified problems coordinating smoking cessation referrals for patients.
In addition, they identified the hospitalization and/or home care phases as areas for potential improvement. During hospitalization, patients often expressed surprise upon learning that they had a feeding tube and/or tracheostomy despite having received pre-operative education. This misunderstanding by the patient was likely related to the clinicians’ assumptions about the best timing for patient education and the amount of time needed for education before the surgical procedure. The surgical team provided patient education based on individual needs, and it has not been standardized because they felt that patients’ education needs vary from person to person. In contrast, patient education prior radiation therapy is standardized, and all patients received a comprehensive package of information that is re-enforced by direct patient education by the clinicians.
Another gap in care identified by the inpatient team was a prolonged intensive care unit (ICU) stay for the HNC patients. These patients remained in the ICU for the entirety of their stay. Not only was this causing overuse of resources, but patients also felt unprepared for an independent discharge home given the high level of care received in the ICU.
A range of suggestions were made to solve these problems. The most prevalent suggestion was to use a nurse navigator to coordinate referrals, schedule appointments, facilitate interdisciplinary communication, and to address social, financial, and transport needs for HNC patients. Several other suggestions referred to standardizing treatment procedures and pre-operative patient education.
Patient survey
Forty-three patients were identified for the patient satisfaction survey. Each patient was contacted at least three times over the course of 3 weeks. Of the 43 patients, 20 had an invalid phone number, 10 were not available for participation, and 1 declined to participate. A total of 12 patients completed the survey.
Although the sample size was small, the patients surveyed were very satisfied with their care. Of the 12 patients, 5 patients rated all of the services relevant to their treatment as a 5 (Great). Areas of particular concern for the patients included the waiting time to see a physician in the ENT clinic, the explanation/collection of charges, and the accessibility of support groups. Services rated 3 (Satisfactory) included waiting time to schedule appointments; the amount of information and patient education provided by about radiation, nutrition, physical therapy (PT), occupational therapy (OT), and SLP; and overall satisfaction with care.
Surgical patients. The Danish Head and Neck Society guidelines state that the interval between the initial visit diagnosis and surgery should be within 30 days.12A comparison of the average intervals between important treatment points for the surgical sample patients with the benchmark timing recommended in the literature are shown in Table 2. The mean time from initial visit to surgery was 28 days in the cancer center sample; 67% of patients (n = 14) had surgery within 30 days, and 33% of patients (n = 7) had surgery beyond 30 days. The interval re-assessment showed improvements in this area: the mean time from initial visit to surgery went from 28 to 18 days, and 100% of patients
n = 10) had surgery within 30 days.
Huang and colleagues have indicated that postoperative radiation therapy should ideally occur within 42 days of surgery;13 however, in the present study, 79% (n = 11) of the sample surgical patients undergoing radiation began their therapy on average more than 63 days after surgery. The interval re-assessment found the same results with 80% of patients starting radiation over 42 days after surgery although the average time lag decreased from 68 days to 53 days.
Nonsurgical patients. Huang and colleagues have indicated that for patients undergoing radiation as their primary form of treatment, an interval of 40 days between biopsy and the start of radiation is ideal.13 The average intervals between important time points of treatment for patients who did not require surgery in their treatment are shown in Table 2. The cancer center met the benchmark at baseline with an average of 38 days (n = 11 patients). The re-assessment showed improvement in this area with 100% of cases (n = 10) meeting the benchmark with an average of 32 days. Likewise, the benchmark waiting time from RT consultation to RT start of less than 30 days11 was met by the cancer center for the nonsurgical group (n = 11).
Access to supportive services
Nutrition care. Studies have shown that standard nutritional care for HNC patients should start before treatment.18,19 In the present study, the waiting time from surgery to outpatient nutrition assessment improved from 61 days to 50 days (Table 2). For patients in the surgical group, the time interval between the initial ENT visit to the outpatient nutrition assessment decreased from 85 days at baseline to 66 days at reassessment, and 82 days to 35 days, respectively, for the nonsurgical group. The time interval from surgery to nutrition assessment has not reached the recommended pretreatment benchmark, but data showed a trend of improvement from 61 days at baseline to 50 days at reassessment for patients in the group.
Patients were typically referred to outpatient nutrition at the start of radiation therapy. In the initial assessment, all patients (n = 33) had access to nutrition services, but 21% (n = 7) never spoke to the nutritionist. The re-assessment found all but one (n = 7) of the patients had been seen by a nutritionist at some point during the treatment period. The benchmark of preradiation nutrition assessment was met by 2 postsurgical patients, with the remainder of the patients being seen within 3 days of the initiation of radiation.
Speech-language pathology management. The literature recommends that patients receive SLP management before the surgery.14-17 In this gap analysis, a difference in access to SLP services was identified between inpatient and outpatient settings. On average, patients within the sample were referred to outpatient SLP over a month after their surgery. In contrast, inpatient surgical patients had access to rapid consultations with SLP (eg, 1 day after surgery for total laryngectomy, and 4 days after surgery for oropharyngeal and oral surgery patients; T Hogan, unpublished data, June 2014). Overall, the benchmark was not met, as patients were not seen by the SPL prior to treatment.
New baseline data was collected about SLP services and showed that 70% of patients had contact with the outpatient SLP at some point during their treatment. Of those, only 29% of patients saw SLP before surgery, meeting the benchmark. The baseline waiting time was an average of 15 days before surgery and 43 days after surgery. Overall, the trend is moving toward the benchmark of care.
Similarly, studies determined that the gold standard of care for nonsurgical patients is that SLPs begin pretreatment management of HNC.16Patients in the baseline sample were typically referred to outpatient SLP about a month after biopsy (presumably diagnosis), but before the start of chemo-radiation. There were no data available for the number of patients who were actually seen by the outpatient SLP before the start of chemo-radiation.
The new baseline data found that 100% of nonsurgical patients were referred to SLP, but only25% (n = 2) were seen before they started chemo-radiation therapy (an average 5 days before) and 75% (n = 6) were seen after starting chemo-radiation therapy (an average 23 days after).
SWOT analysis
The SWOT analysis included strengths, weaknesses, opportunities and threats of the care provided to HNC patients at the cancer center. The gap analysis based on the results of the clinician surveys, process mapping, and patient satisfaction survey is summarized in Table 3. Three main gaps were identified: waiting time to treatment, education, and coordination of and transitions in care.
Quality improvement actions
Interventions by the outpatient MTD team included changing the process of scheduling dental appointments, creating a new approach to outpatient nutrition by proactively meeting patients in the ENT clinic, and conducting PT and SLP assessments to patients in the chemotherapy unit while receiving their treatment. A nurse navigator position for this patient population was approved and an expedited referral system was initiated. At the same time, the inpatient team implemented a specialized HNC unit in the medical-surgical floor, developed the protocols for the management of postsurgery HNC patients, educated nursing staff, and standardized patient education to facilitate transition to the next level of care (Table 3).
Discussion
The gap analysis of services provided to HNC patients at the cancer center identified three gaps in care: delay in treatment and supportive services, nonstandardized patient education, and lack of care coordination.
All patients should have access to a timely treatment initiation. In this analysis, surgical patients encountered a delay between surgery and the start of radiation therapy, about 3 weeks beyond the recommended in the literature.12 Clinicians mentioned delays in ensuring preradiation dental consultations as a significant issue affecting the patient treatment process. Re-assessment data reported that despite interventions for early dental referrals, 80% of patients still started radiation over 6 weeks after surgery; however, the average time lag decreased from 68 days to 53 days.
RT delays in HNC patients not only affect patients’ emotional state but may also impact clinical outcomes. Treatment delays have the potential to harm patients by: allowing tumor growth that impact on the curative outcomes of RT; postponing the benefits of palliative RT on symptom relief; and causing psychological distress.25 In addition, delay in starting treatment has shown to increase the risk for local recurrence,13,26 and decrease survival.27
Higher demand for advanced RT modalities has been linked to treatment delays. Waiting times from initial RT evaluation to start RT have increased over time, from <14 days in 1989 to 31 days in 1997.11 This is explained by the complexity of the pretreatment evaluations and the increasing demand of radiation services, especially in high volume institutions.25,27A fast-track program to reduce waiting time in the treatment of HNC patients reported to be effective.22 This program includes a patient coordinator, a hotline for referral procedures, prebooked slots for ENT and RT clinics, faster pathology and imaging reports, and the establishment of an MTD team.
The clinician survey identified patient needs classified in three categories: pre-operative education, hospitalization process, and access to support services. Regarding pre-operative education, clinicians acknowledged that although patients were educated about their surgical options and possible outcomes prior to hospitalization, they often could not fully understand this information at the time of the instruction. The high need for education particularly in the pretreatment phase was documented in a needs assessment survey for HNC patients conducted at the cancer center D DeMille, RD, unpublished data, August 2013).
Studies have looked at the effectiveness of education in cancer patients. The use of teaching interventions (written information, audiotapes, videotapes, and computer programs) has proven to be valuable for educating patients prior to experiencing cancer treatments.20Further, a systematic review of preparatory education for cancer patients undergoing surgery reported that face-to-face discussions appear to be effective at improving patient outcomes with regards to increasing knowledge and decreasing anxiety.21 However, it was stated that the timing of the delivery of education is critical to be efficient. For example, an education session provided one day prior the day of surgery is not useful as it may place additional stress on a patient who is already highly anxious and decreases the likelihood for the information to be managed. It is recommended to deliver education early enough prior surgery to allow time for the patient to process the information. Also, a study reported that presurgical education on potential side effects; the assessment of patients’ needs by an SLP, physical therapist, nutritionist, and social worker; and pre-operative nutritional support decrease postoperative complications.4
The education committee was created in response to the gap on patient education. The inpatient team took the lead and provided intense education on the care of HNC patients to the nursing staff and to HNC patients and their families about postoperative care at home. Education was also extended to rehabilitation facilities caring for this cancer population at discharge from the hospital.
Clinicians identified a gap during the hospitalization process. The gap included prolonged stay of patients in the ICU postsurgery, inefficient interclinician communication, lack of standardization of postsurgical care, and difficulty communicating with external home care teams. A major intervention was implemented that included the creation of a HNC specialized unit that offered a structured setting for standardized care and communication between patients and clinicians. Dedicated units for the management of HNC patients highly enhance the quality of care provided because it enables the MTD team to work properly by clearly defining roles and responsibilities, delineating evidence-based clinical interventions, and promoting expert care for this patient population.23In addition, several key steps have been recommended to reduce the fragmentation of care for hospital patients, including developing a referral/transition tracking system, organizing and training staff members to coordinate transition/referrals, and identifying and creating agreements with key care providers.28
Early patient access to supportive services was a concern to most clinicians. HNC providers were not consulting the CARE clinic about patients’ nutritional, physical and SLP needs until the patient was having serious problems. Patient tracking found that the minority of patients met the standard of having a presurgical speech referral. Most patients had access to outpatient nutrition services during radiation therapy but the majority of patients in the sample did not attend CARE clinic. The literature strongly supports early management of HNC patients by the SPL and nutrition counselor. Van der Molen and colleagues demonstrated that a pretreatment SPL rehabilitation program is feasible and offers reasonable patient compliance despite of the burden caused by ongoing chemo-radiation therapy for HNC patients.16Similarly, early nutrition counseling for HNC patients undergoing RT has reported to decrease unintended weight loss and malnutrition compared with late nutrition intervention.19
Although there are clear gaps in care for HNC patients from the clinicians’ perspective, the patients surveyed indicated a clear satisfaction with their care at the cancer center. Almost all patients were satisfied with their relationships with clinicians in the team. Some patients mentioned complaints of insufficient pre-operative education and waiting time, but there were not significant complaints about coordination, which clinicians had identified as a major issue. This is likely explained by the small sample size and the patients’ inability to see the background interclinician communication.
A crucial suggestion to address all of these gaps in care was the implementation of a nurse navigator. With the support of hospital and cancer center administration, a nurse navigator was hired to address the needs of HNC patients throughout their disease trajectory. The team agreed that the nurse navigator should make contact with HNC patients during their initial appointment at the surgical ENT office. This initial contact allows the nurse navigator to provide support and connection to resources. Thereafter, early contact with this patient population allows the nurse navigator to follow the patient through the continuum of care from biopsy and diagnosis to survivorship. The nurse navigator facilitates communication between clinicians, patients and their families; and provides emotional support to patients while helping to manage their financial and transportation needs.29
Limitations
This is a quality improvement project with a small sample size of HNC cases. Data from this gap analysis are not statistically significant; yet, are clinically relevant in the management of the HNC population at the cancer center. Likewise, the patient sample size was small, making definitive generalizations about patient experience difficult; however, the data are helpful in highlighting possible problems for patients.
In the United States, there will be an estimated 49,670 new cases of head and neck cancer for 2017.1 Head and neck cancer (HNC) is a term used to describe a range of tumors that originate in the area of the body spanning from the lower neck to the upper nasal cavity.2 Specifically, they are malignancies arising in the mouth, larynx, nasal cavity, sinuses, tongue, lips, and numerous glands such as the thyroid and salivary.2 To clarify, HNC, despite the encompassing name, does not include growths of the bones, teeth, skin, brain parenchyma, and eye; therefore, such tumors will not be addressed in this article.
Patients with HNC often experience fragmented and uncoordinated care that leads to delays in cancer treatment, severe distress in patients and families, and dissatisfaction with care. Literature reports that these patients face numerous stressors including aggressive cancer treatments, severe symptoms, body image concerns, loss of speech, difficulty swallowing, nutritional issues, and respiratory problems that affect their quality of life and ability to function on a day-to-day basis.3,4In addition, patients with HNC and their families are challenged to navigate the health care system and to overcome the difficulties of accessing services within the context of financial constraints. A multidisciplinary team (MDT) approach is the standard of care for HNC patients, as demonstrated in studies reporting better 5-year survival outcomes, increased completion of adjuvant therapy, and higher compliance with speech-language pathologist (SLP) recommendations.5, 6 Furthermore, a recent systematic review of cancer teams concluded that the MDT approach leads to improved clinical outcomes and enhanced communication between the patient and the team.7
The Institute of Medicine (IOM) stated in its 2013 report on cancer care that a high-quality care delivery system requires continuing measurement of cancer care and strategies to carry out performance improvement.8 Following the IOM premise, the cancer center at an academic medical center in Philadelphia made efforts to improve patient access to multidisciplinary services, first, by creating a multidisciplinary Cancer Appetite and Rehabilitation (CARE)clinic to address the symptoms and nutritional needs of HNC patients,9 and second, by using a gap analysis to conduct an assessment of the cancer care services provided to this cancer population. The need to conduct this assessment was generated by the desire to improve access to multidisciplinary care, with the goal of meeting standard benchmarks for completion of treatment while increasing the use of ancillary services. This article describes the process of conducting a gap analysis of cancer services for HNC patients, and includes discussion of the findings, recommendations for improving care, a description of the quality improvement interventions, and a report of the outcomes based on an interval re-assessment 18 months later.
Methods
Methods included a gap analysis, implementation of quality improvement recommendations, and re-assessment of indicators (Figure). A gap analysis “identifies differences between desired and actual practice conditions, including service delivery and quality patient outcomes as measured against evidence-based benchmarks while incorporating key stakeholder concerns and expectations.”10 The gap analysis of cancer care services offered to HNC patients was achieved through the step-by-step process described hereinafter. The implementation of quality improvement recommendations was accomplished by establishing two task force committees focused respectively on education and transitions in care coordination. Re-assessment of indicators related to timeliness of delivery of cancer treatments and collection of additional baseline data regarding supportive services.
Gap analysis
Identification of the scope of the problem. Members of the HNC multidisciplinary team raised concerns about unintended breaches in care for HNC patients that resulted not only in delay of the patients’ cancer treatments, but also in unnecessary distress for the patients and their families. As a result, the HNC team decided to conduct a gap analysis to identify the barriers in care for HNC patients, and by doing this, to determine possible solutions.
Identification of best practice care indicators. The indicators of best practice care (benchmarks) for HNC patients were identified after exhaustive review of the literature11-22(Table 1). For this gap analysis, the indicators focused on waiting time to treatment (surgery, chemotherapy, radiation therapy) and to supportive care interventions (nutrition, speech and language pathology) as follows:
- 9.2Futura StdInitial ear-nose-throat (ENT) visit to surgery: <30 days
- Biopsy to start radiation therapy (RT) for nonsurgical patients: 40 days
- Surgery to RT start: 42 days
- Surgery to nutrition consultation (outpatient), start RT to nutrition: Pretreatment
- Surgery to outpatient SLP, initial ENT visit to SLP referral, surgery to SLP referral, RT start to outpatient SLP start: Pretreatment
Measure gaps against benchmarks. To Gap analysis of measure gaps against benchmarks, the authors used the Agency for Health Care Research and Quality tool that provides a systematic method to compare current practice with best practices and determine the barriers to best practice and the feasibility of implementing best practices by the institution23 (Table 1). For this project, a process map of waiting time to treatment and supportive care interventions was created, so that real-world conditions could be measured against benchmarks.
Process map. The authors identified 67 newly diagnosed HNC patients during January-July 2014 from the surgery, radiation therapy, and nutrition departments, but only 33 patients were able to be tracked from their initial visit at the cancer center until the completion of their treatment through the electronic medical record (EMR) system. Their information was compiled in a spreadsheet based on the EMR information. Data included patient access to supportive services and number of days between important treatment benchmarks. Tracking data was used to create a treatment flow chart and determine average treatment intervals.
To map the typical patient process, the patients were split into two groups: surgical (n = 22) and nonsurgical (n = 11). Surgical patients underwent surgery as their primary treatment and received adjuvant radiation therapy or concurrent chemotherapy. Nonsurgical patients did not require surgery other than biopsy as a part of their treatment. Most of the nonsurgical patients received chemotherapy, and 1 patient received palliative radiation therapy.
SWOT analysis. The SWOT analysis is used to chart institution performance in relation to benchmarks while describing stakeholders’ perceptions.24The stakeholder perspective for this project focused on the views of the health care providers from all disciplines regarding the quality of care provided to the HNC population. In addition, a patient survey was conducted to assess their perception of the care they received.
Clinician survey. We surveyed 25 clinicians, including physicians, advanced practice providers, nurses, and allied health professionals, from the surgical (n = 3), hospitalization (n = 6), radiation (n = 3), chemotherapy (n = 3) and supportive services teams (n = 10). The survey was conducted face to face and included 7 open-ended questions designed to gain insight about problems encountered with coordination of care, suggestions to improve coordination of care, factors in treatment delays, suggestions to decrease treatment delays, factors in excellent patient outcomes, rate overall patient care, and suggestions for improvement of service. Initial survey responses were filtered by recurring themes in each question among the different patient service teams.
Patient satisfaction survey. The sample of patients was obtained from the surgery, radiation therapy, and nutrition departments during January-July 2014. Sixty-seven initial patients were identified but only 43 were eligible for interview because they had a listed phone number. A six-question nonvalidated survey was developed by the authors to measure patient satisfaction with the scheduling process, waiting time, information provided about treatment and their medical status, emotional support, the coordination of care, and the payment process. Satisfaction was rated on a scale from 1 to 5 (1 = Poor, 2 = Fair, 3 = Satisfactory, 5 = Great).
Analysis and final report. See Results section.
Quality improvement implementation. The transitions and the education committees were created to address the gaps identified during the analysis. The transitions committee developed strategies to improve the coordination of care of HNC patients throughout their cancer treatment and the education committee elaborated new ways to enhance patient education while meeting treatment timeline standards. The implementation of the interventions was developed by the inpatient and outpatient MTD teams caring for the HNC population.
Re-assessment of indicators. During January-December 2015, a total of 58 patients diagnosed with HNC were identified. Of those, 40 patients with recurrent disease were eliminated, leaving 18 patients (10 surgical, 8 nonsurgical). Similar to the initial assessment for the gap analysis, data included patient access to supportive services and number of days between important treatment benchmarks. Tracking data was used to create a treatment flow chart and determine average treatment intervals.
Results
Most of the patients were men (70%), white (70%), and 60% were within the 50-69 years age range at the time of diagnosis.
Clinician survey
The clinicians were surveyed and their responses analyzed by two people, the project leader and the project assistant. The most commonly identified weaknesses in care that the clinicians identified were delayed access to dental referrals, insufficient preoperative patient education, and inefficient discharge planning and/or home care coordination. Dental referrals were identified as a major cause of delay in starting radiation therapy because of scheduling issues, a lack of patient motivation, limited insurance coverage, and difficulty identifying reliable dentists in the patient’s geographic area. Clinicians also identified problems coordinating smoking cessation referrals for patients.
In addition, they identified the hospitalization and/or home care phases as areas for potential improvement. During hospitalization, patients often expressed surprise upon learning that they had a feeding tube and/or tracheostomy despite having received pre-operative education. This misunderstanding by the patient was likely related to the clinicians’ assumptions about the best timing for patient education and the amount of time needed for education before the surgical procedure. The surgical team provided patient education based on individual needs, and it has not been standardized because they felt that patients’ education needs vary from person to person. In contrast, patient education prior radiation therapy is standardized, and all patients received a comprehensive package of information that is re-enforced by direct patient education by the clinicians.
Another gap in care identified by the inpatient team was a prolonged intensive care unit (ICU) stay for the HNC patients. These patients remained in the ICU for the entirety of their stay. Not only was this causing overuse of resources, but patients also felt unprepared for an independent discharge home given the high level of care received in the ICU.
A range of suggestions were made to solve these problems. The most prevalent suggestion was to use a nurse navigator to coordinate referrals, schedule appointments, facilitate interdisciplinary communication, and to address social, financial, and transport needs for HNC patients. Several other suggestions referred to standardizing treatment procedures and pre-operative patient education.
Patient survey
Forty-three patients were identified for the patient satisfaction survey. Each patient was contacted at least three times over the course of 3 weeks. Of the 43 patients, 20 had an invalid phone number, 10 were not available for participation, and 1 declined to participate. A total of 12 patients completed the survey.
Although the sample size was small, the patients surveyed were very satisfied with their care. Of the 12 patients, 5 patients rated all of the services relevant to their treatment as a 5 (Great). Areas of particular concern for the patients included the waiting time to see a physician in the ENT clinic, the explanation/collection of charges, and the accessibility of support groups. Services rated 3 (Satisfactory) included waiting time to schedule appointments; the amount of information and patient education provided by about radiation, nutrition, physical therapy (PT), occupational therapy (OT), and SLP; and overall satisfaction with care.
Surgical patients. The Danish Head and Neck Society guidelines state that the interval between the initial visit diagnosis and surgery should be within 30 days.12A comparison of the average intervals between important treatment points for the surgical sample patients with the benchmark timing recommended in the literature are shown in Table 2. The mean time from initial visit to surgery was 28 days in the cancer center sample; 67% of patients (n = 14) had surgery within 30 days, and 33% of patients (n = 7) had surgery beyond 30 days. The interval re-assessment showed improvements in this area: the mean time from initial visit to surgery went from 28 to 18 days, and 100% of patients
n = 10) had surgery within 30 days.
Huang and colleagues have indicated that postoperative radiation therapy should ideally occur within 42 days of surgery;13 however, in the present study, 79% (n = 11) of the sample surgical patients undergoing radiation began their therapy on average more than 63 days after surgery. The interval re-assessment found the same results with 80% of patients starting radiation over 42 days after surgery although the average time lag decreased from 68 days to 53 days.
Nonsurgical patients. Huang and colleagues have indicated that for patients undergoing radiation as their primary form of treatment, an interval of 40 days between biopsy and the start of radiation is ideal.13 The average intervals between important time points of treatment for patients who did not require surgery in their treatment are shown in Table 2. The cancer center met the benchmark at baseline with an average of 38 days (n = 11 patients). The re-assessment showed improvement in this area with 100% of cases (n = 10) meeting the benchmark with an average of 32 days. Likewise, the benchmark waiting time from RT consultation to RT start of less than 30 days11 was met by the cancer center for the nonsurgical group (n = 11).
Access to supportive services
Nutrition care. Studies have shown that standard nutritional care for HNC patients should start before treatment.18,19 In the present study, the waiting time from surgery to outpatient nutrition assessment improved from 61 days to 50 days (Table 2). For patients in the surgical group, the time interval between the initial ENT visit to the outpatient nutrition assessment decreased from 85 days at baseline to 66 days at reassessment, and 82 days to 35 days, respectively, for the nonsurgical group. The time interval from surgery to nutrition assessment has not reached the recommended pretreatment benchmark, but data showed a trend of improvement from 61 days at baseline to 50 days at reassessment for patients in the group.
Patients were typically referred to outpatient nutrition at the start of radiation therapy. In the initial assessment, all patients (n = 33) had access to nutrition services, but 21% (n = 7) never spoke to the nutritionist. The re-assessment found all but one (n = 7) of the patients had been seen by a nutritionist at some point during the treatment period. The benchmark of preradiation nutrition assessment was met by 2 postsurgical patients, with the remainder of the patients being seen within 3 days of the initiation of radiation.
Speech-language pathology management. The literature recommends that patients receive SLP management before the surgery.14-17 In this gap analysis, a difference in access to SLP services was identified between inpatient and outpatient settings. On average, patients within the sample were referred to outpatient SLP over a month after their surgery. In contrast, inpatient surgical patients had access to rapid consultations with SLP (eg, 1 day after surgery for total laryngectomy, and 4 days after surgery for oropharyngeal and oral surgery patients; T Hogan, unpublished data, June 2014). Overall, the benchmark was not met, as patients were not seen by the SPL prior to treatment.
New baseline data was collected about SLP services and showed that 70% of patients had contact with the outpatient SLP at some point during their treatment. Of those, only 29% of patients saw SLP before surgery, meeting the benchmark. The baseline waiting time was an average of 15 days before surgery and 43 days after surgery. Overall, the trend is moving toward the benchmark of care.
Similarly, studies determined that the gold standard of care for nonsurgical patients is that SLPs begin pretreatment management of HNC.16Patients in the baseline sample were typically referred to outpatient SLP about a month after biopsy (presumably diagnosis), but before the start of chemo-radiation. There were no data available for the number of patients who were actually seen by the outpatient SLP before the start of chemo-radiation.
The new baseline data found that 100% of nonsurgical patients were referred to SLP, but only25% (n = 2) were seen before they started chemo-radiation therapy (an average 5 days before) and 75% (n = 6) were seen after starting chemo-radiation therapy (an average 23 days after).
SWOT analysis
The SWOT analysis included strengths, weaknesses, opportunities and threats of the care provided to HNC patients at the cancer center. The gap analysis based on the results of the clinician surveys, process mapping, and patient satisfaction survey is summarized in Table 3. Three main gaps were identified: waiting time to treatment, education, and coordination of and transitions in care.
Quality improvement actions
Interventions by the outpatient MTD team included changing the process of scheduling dental appointments, creating a new approach to outpatient nutrition by proactively meeting patients in the ENT clinic, and conducting PT and SLP assessments to patients in the chemotherapy unit while receiving their treatment. A nurse navigator position for this patient population was approved and an expedited referral system was initiated. At the same time, the inpatient team implemented a specialized HNC unit in the medical-surgical floor, developed the protocols for the management of postsurgery HNC patients, educated nursing staff, and standardized patient education to facilitate transition to the next level of care (Table 3).
Discussion
The gap analysis of services provided to HNC patients at the cancer center identified three gaps in care: delay in treatment and supportive services, nonstandardized patient education, and lack of care coordination.
All patients should have access to a timely treatment initiation. In this analysis, surgical patients encountered a delay between surgery and the start of radiation therapy, about 3 weeks beyond the recommended in the literature.12 Clinicians mentioned delays in ensuring preradiation dental consultations as a significant issue affecting the patient treatment process. Re-assessment data reported that despite interventions for early dental referrals, 80% of patients still started radiation over 6 weeks after surgery; however, the average time lag decreased from 68 days to 53 days.
RT delays in HNC patients not only affect patients’ emotional state but may also impact clinical outcomes. Treatment delays have the potential to harm patients by: allowing tumor growth that impact on the curative outcomes of RT; postponing the benefits of palliative RT on symptom relief; and causing psychological distress.25 In addition, delay in starting treatment has shown to increase the risk for local recurrence,13,26 and decrease survival.27
Higher demand for advanced RT modalities has been linked to treatment delays. Waiting times from initial RT evaluation to start RT have increased over time, from <14 days in 1989 to 31 days in 1997.11 This is explained by the complexity of the pretreatment evaluations and the increasing demand of radiation services, especially in high volume institutions.25,27A fast-track program to reduce waiting time in the treatment of HNC patients reported to be effective.22 This program includes a patient coordinator, a hotline for referral procedures, prebooked slots for ENT and RT clinics, faster pathology and imaging reports, and the establishment of an MTD team.
The clinician survey identified patient needs classified in three categories: pre-operative education, hospitalization process, and access to support services. Regarding pre-operative education, clinicians acknowledged that although patients were educated about their surgical options and possible outcomes prior to hospitalization, they often could not fully understand this information at the time of the instruction. The high need for education particularly in the pretreatment phase was documented in a needs assessment survey for HNC patients conducted at the cancer center D DeMille, RD, unpublished data, August 2013).
Studies have looked at the effectiveness of education in cancer patients. The use of teaching interventions (written information, audiotapes, videotapes, and computer programs) has proven to be valuable for educating patients prior to experiencing cancer treatments.20Further, a systematic review of preparatory education for cancer patients undergoing surgery reported that face-to-face discussions appear to be effective at improving patient outcomes with regards to increasing knowledge and decreasing anxiety.21 However, it was stated that the timing of the delivery of education is critical to be efficient. For example, an education session provided one day prior the day of surgery is not useful as it may place additional stress on a patient who is already highly anxious and decreases the likelihood for the information to be managed. It is recommended to deliver education early enough prior surgery to allow time for the patient to process the information. Also, a study reported that presurgical education on potential side effects; the assessment of patients’ needs by an SLP, physical therapist, nutritionist, and social worker; and pre-operative nutritional support decrease postoperative complications.4
The education committee was created in response to the gap on patient education. The inpatient team took the lead and provided intense education on the care of HNC patients to the nursing staff and to HNC patients and their families about postoperative care at home. Education was also extended to rehabilitation facilities caring for this cancer population at discharge from the hospital.
Clinicians identified a gap during the hospitalization process. The gap included prolonged stay of patients in the ICU postsurgery, inefficient interclinician communication, lack of standardization of postsurgical care, and difficulty communicating with external home care teams. A major intervention was implemented that included the creation of a HNC specialized unit that offered a structured setting for standardized care and communication between patients and clinicians. Dedicated units for the management of HNC patients highly enhance the quality of care provided because it enables the MTD team to work properly by clearly defining roles and responsibilities, delineating evidence-based clinical interventions, and promoting expert care for this patient population.23In addition, several key steps have been recommended to reduce the fragmentation of care for hospital patients, including developing a referral/transition tracking system, organizing and training staff members to coordinate transition/referrals, and identifying and creating agreements with key care providers.28
Early patient access to supportive services was a concern to most clinicians. HNC providers were not consulting the CARE clinic about patients’ nutritional, physical and SLP needs until the patient was having serious problems. Patient tracking found that the minority of patients met the standard of having a presurgical speech referral. Most patients had access to outpatient nutrition services during radiation therapy but the majority of patients in the sample did not attend CARE clinic. The literature strongly supports early management of HNC patients by the SPL and nutrition counselor. Van der Molen and colleagues demonstrated that a pretreatment SPL rehabilitation program is feasible and offers reasonable patient compliance despite of the burden caused by ongoing chemo-radiation therapy for HNC patients.16Similarly, early nutrition counseling for HNC patients undergoing RT has reported to decrease unintended weight loss and malnutrition compared with late nutrition intervention.19
Although there are clear gaps in care for HNC patients from the clinicians’ perspective, the patients surveyed indicated a clear satisfaction with their care at the cancer center. Almost all patients were satisfied with their relationships with clinicians in the team. Some patients mentioned complaints of insufficient pre-operative education and waiting time, but there were not significant complaints about coordination, which clinicians had identified as a major issue. This is likely explained by the small sample size and the patients’ inability to see the background interclinician communication.
A crucial suggestion to address all of these gaps in care was the implementation of a nurse navigator. With the support of hospital and cancer center administration, a nurse navigator was hired to address the needs of HNC patients throughout their disease trajectory. The team agreed that the nurse navigator should make contact with HNC patients during their initial appointment at the surgical ENT office. This initial contact allows the nurse navigator to provide support and connection to resources. Thereafter, early contact with this patient population allows the nurse navigator to follow the patient through the continuum of care from biopsy and diagnosis to survivorship. The nurse navigator facilitates communication between clinicians, patients and their families; and provides emotional support to patients while helping to manage their financial and transportation needs.29
Limitations
This is a quality improvement project with a small sample size of HNC cases. Data from this gap analysis are not statistically significant; yet, are clinically relevant in the management of the HNC population at the cancer center. Likewise, the patient sample size was small, making definitive generalizations about patient experience difficult; however, the data are helpful in highlighting possible problems for patients.
1. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2017. CA Cancer J Clin. 2016;67:7-30.
2. National Cancer Institute. Head and neck cancers. https://www.cancer.gov/types/head-and-neck/head-neck-fact-sheet. Reviewed February 1, 2013. Accessed January 26, 2017
3. Weiderholt PA, Connor NP, Hartig GK, Harari PM. Bridging gaps in multidisciplinary head and neck cancer care: nursing coordination and case management. Int J Radiat Oncol Biol Phys. 2007;69(2 suppl):S88-S91.
4. Dingman C, Hegedus PD, Likes C, McDowell P, McCarthy E, Zwilling C. A coordinated, multidisciplinary approach to caring for the patient with head and neck cancer. J Support Oncol. 2008;6(3):125-131.
5. Liao C, Kang CJ, Lee LY, et al. Association between multidisciplinary team care approach and survival rates in patients with oral cavity squamous cell carcinoma. Head Neck. 2016;38(suppl 1):E1544-1553.
6. Starmer H, Sanguineti G, Marur S, Gourin CG. Multidisciplinary head and neck cancer clinic and adherence with speech pathology. Laryngoscope. 2011;121(10):2131-2135.
7. Prades J, Remue E, van Hoof E, Borras JM. Is it worth reorganizing cancer services on the basis of multidisciplinary teams (MDTs)? A systematic review of the objectives and organization of MDTs and their impact on patient outcomes. Health Pol. 2015;119(4):464-474.
8. Institute of Medicine. Delivering high-quality cancer care: charting a new course for a system in crisis. 2013. www.nationalacademies.org/hmd/Reports/2013/Delivering-High-Quality-Cancer-Care-Charting-a-New-Course-for-a-System-in-Crisis.aspx. Published September 10, 2013. Accessed May 29, 2016.
9. Granda-Cameron C, DeMille D, Lynch MP, et al. An interdisciplinary approach to manage cancer cachexia. Clin J Oncol Nurs. 2010;14(1):72-80.
10. Davis-Ajami ML, Costa L, Kulik S. Gap analysis: synergies and opportunities for effective nursing leadership. Nurs Econ. 2014;32(1):17-25.
11. Fortin A, Bairati I, Albert M, et al. Effect of treatment delay on outcome of patients with early-stage head-and-neck carcinoma receiving radical radiotherapy. Int J Radiat Oncol Biol Phys. 2002;52(4):929-936.
12. Van Harten MC, Ridder M, Hamming-Vrieze O, et al. The association of treatment delay and prognosis in head and neck squamous cell carcinoma (HNSCC) in a Dutch comprehensive cancer center. Oral Oncol. 2014;50:282-290.
13. Huang J, Barbera L, Brouwers M, et al. Does delay in starting treatment affect the outcomes of radiotherapy? A systematic review. J ClinOncol. 2003;21(3):555-563.
14. Lazarus CL. Management of swallowing disorders in head and neck cancer patients: optimal patterns of care. Sem Speech Lang. 2000;21(4):293-310.
15. Mayer KR. Learning to speak after laryngectomy. http://speech-language-pathology-audiology.advanceweb.com/Features/Articles/Learning-to-Speak-After-Laryngectomy.aspx. Posted October 27, 2014. Accessed January 17, 2017.
16. van der Molen L, van Rossum MA, Burkhead LM, et al. A randomized preventive rehabilitation trial in advanced head and neck cancer patients treated with chemo-radiotherapy: feasibility, compliance, and short-term effects. Dysphagia. 2011;26:155-170.
17. Starmer HM, Gourin CG. Is speech language pathologist evaluation necessary in the nonoperative treatment of head and neck cancer? Laryngoscope. 2013;123(7):1571-1572.
18. [Article in French] Meuric J, Garabige V, Blanc-Vincent MP, et al. Good clinical practice in nutritional management of head and neck cancer patients. Bull Cancer. 1999;86(10):843-854.
19. van den Berg MG, Rasmussen-Conrad EL, Wei KH, et al. Comparison of the effect of individual dietary counselling and of standard nutritional care on weight loss in patients with head and neck cancer undergoing radiotherapy. Br J Nutr. 2010;104:872-877.
20. Waller A, Forshaw K, Bryant J, Mair S. Interventions for preparing patients for chemotherapy and radiotherapy: a systematic review. Supp Care Ca. 2014;22(8):2297-2308.
21. Waller A, Forshaw K, Bryant J, et al. Preparatory education for cancer patients undergoing surgery: a systematic review of volume and quality of research output over time. Patient Educ Couns. 2015;98:1540-1549.
22. Toustrup K, Lambersten K, Birke-Sorensen H, et al. Reduction in waiting time for diagnosis and treatment of head and neck cancer – a fast track study. Acta Oncol. 2011;50:636-641.
23. Bergamini C, Locati L, Bossi P et al. Does a multidisciplinary team approach in a tertiary referral centre impact on the initial management of head and neck cancer? Oral Oncol. 2016;54:54-57.
24. AHRQ. Pediatric toolkit for using the AHRQ quality indicators. http://www.ahrq.gov/professionals/systems/hospital/qitoolkit/pediatrictoolkit.html . Reviewed July 2016. Accessed January 26, 2017.
25. Mackillop WJ. Killing time: the consequences of delays in radiotherapy. Radiother Oncol. 2007;84:1-4.
26.Chen Z, King, W, Pearcey R, Kerba M, Mackillop WJ. The relationship between waiting time for radiotherapy and clinical outcomes: a systematic review of the literature. Radiother Oncol. 2008;87:3-16.
27. Sharma S, Bekelman J, Lin A et al. Clinical impact of prolonged diagnosis to treatment interval (DTI) among patients with ororpharyngeal squamous cell carcinoma. Oral Oncol. 2016;56:17-24.
28. Improving chronic illness care. Reducing care fragmentation. Care coordination. http://www.improvingchroniccare.org/index.php?p=Care_Coordination&s=326. Published 2010. Accessed May 28, 2016.
29. Fillion L, de Serres M, Cook S, et al. Professional patient navigation in head and neck cancer. Sem Oncol Nurs. 2009;25(3):212-221.
1. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2017. CA Cancer J Clin. 2016;67:7-30.
2. National Cancer Institute. Head and neck cancers. https://www.cancer.gov/types/head-and-neck/head-neck-fact-sheet. Reviewed February 1, 2013. Accessed January 26, 2017
3. Weiderholt PA, Connor NP, Hartig GK, Harari PM. Bridging gaps in multidisciplinary head and neck cancer care: nursing coordination and case management. Int J Radiat Oncol Biol Phys. 2007;69(2 suppl):S88-S91.
4. Dingman C, Hegedus PD, Likes C, McDowell P, McCarthy E, Zwilling C. A coordinated, multidisciplinary approach to caring for the patient with head and neck cancer. J Support Oncol. 2008;6(3):125-131.
5. Liao C, Kang CJ, Lee LY, et al. Association between multidisciplinary team care approach and survival rates in patients with oral cavity squamous cell carcinoma. Head Neck. 2016;38(suppl 1):E1544-1553.
6. Starmer H, Sanguineti G, Marur S, Gourin CG. Multidisciplinary head and neck cancer clinic and adherence with speech pathology. Laryngoscope. 2011;121(10):2131-2135.
7. Prades J, Remue E, van Hoof E, Borras JM. Is it worth reorganizing cancer services on the basis of multidisciplinary teams (MDTs)? A systematic review of the objectives and organization of MDTs and their impact on patient outcomes. Health Pol. 2015;119(4):464-474.
8. Institute of Medicine. Delivering high-quality cancer care: charting a new course for a system in crisis. 2013. www.nationalacademies.org/hmd/Reports/2013/Delivering-High-Quality-Cancer-Care-Charting-a-New-Course-for-a-System-in-Crisis.aspx. Published September 10, 2013. Accessed May 29, 2016.
9. Granda-Cameron C, DeMille D, Lynch MP, et al. An interdisciplinary approach to manage cancer cachexia. Clin J Oncol Nurs. 2010;14(1):72-80.
10. Davis-Ajami ML, Costa L, Kulik S. Gap analysis: synergies and opportunities for effective nursing leadership. Nurs Econ. 2014;32(1):17-25.
11. Fortin A, Bairati I, Albert M, et al. Effect of treatment delay on outcome of patients with early-stage head-and-neck carcinoma receiving radical radiotherapy. Int J Radiat Oncol Biol Phys. 2002;52(4):929-936.
12. Van Harten MC, Ridder M, Hamming-Vrieze O, et al. The association of treatment delay and prognosis in head and neck squamous cell carcinoma (HNSCC) in a Dutch comprehensive cancer center. Oral Oncol. 2014;50:282-290.
13. Huang J, Barbera L, Brouwers M, et al. Does delay in starting treatment affect the outcomes of radiotherapy? A systematic review. J ClinOncol. 2003;21(3):555-563.
14. Lazarus CL. Management of swallowing disorders in head and neck cancer patients: optimal patterns of care. Sem Speech Lang. 2000;21(4):293-310.
15. Mayer KR. Learning to speak after laryngectomy. http://speech-language-pathology-audiology.advanceweb.com/Features/Articles/Learning-to-Speak-After-Laryngectomy.aspx. Posted October 27, 2014. Accessed January 17, 2017.
16. van der Molen L, van Rossum MA, Burkhead LM, et al. A randomized preventive rehabilitation trial in advanced head and neck cancer patients treated with chemo-radiotherapy: feasibility, compliance, and short-term effects. Dysphagia. 2011;26:155-170.
17. Starmer HM, Gourin CG. Is speech language pathologist evaluation necessary in the nonoperative treatment of head and neck cancer? Laryngoscope. 2013;123(7):1571-1572.
18. [Article in French] Meuric J, Garabige V, Blanc-Vincent MP, et al. Good clinical practice in nutritional management of head and neck cancer patients. Bull Cancer. 1999;86(10):843-854.
19. van den Berg MG, Rasmussen-Conrad EL, Wei KH, et al. Comparison of the effect of individual dietary counselling and of standard nutritional care on weight loss in patients with head and neck cancer undergoing radiotherapy. Br J Nutr. 2010;104:872-877.
20. Waller A, Forshaw K, Bryant J, Mair S. Interventions for preparing patients for chemotherapy and radiotherapy: a systematic review. Supp Care Ca. 2014;22(8):2297-2308.
21. Waller A, Forshaw K, Bryant J, et al. Preparatory education for cancer patients undergoing surgery: a systematic review of volume and quality of research output over time. Patient Educ Couns. 2015;98:1540-1549.
22. Toustrup K, Lambersten K, Birke-Sorensen H, et al. Reduction in waiting time for diagnosis and treatment of head and neck cancer – a fast track study. Acta Oncol. 2011;50:636-641.
23. Bergamini C, Locati L, Bossi P et al. Does a multidisciplinary team approach in a tertiary referral centre impact on the initial management of head and neck cancer? Oral Oncol. 2016;54:54-57.
24. AHRQ. Pediatric toolkit for using the AHRQ quality indicators. http://www.ahrq.gov/professionals/systems/hospital/qitoolkit/pediatrictoolkit.html . Reviewed July 2016. Accessed January 26, 2017.
25. Mackillop WJ. Killing time: the consequences of delays in radiotherapy. Radiother Oncol. 2007;84:1-4.
26.Chen Z, King, W, Pearcey R, Kerba M, Mackillop WJ. The relationship between waiting time for radiotherapy and clinical outcomes: a systematic review of the literature. Radiother Oncol. 2008;87:3-16.
27. Sharma S, Bekelman J, Lin A et al. Clinical impact of prolonged diagnosis to treatment interval (DTI) among patients with ororpharyngeal squamous cell carcinoma. Oral Oncol. 2016;56:17-24.
28. Improving chronic illness care. Reducing care fragmentation. Care coordination. http://www.improvingchroniccare.org/index.php?p=Care_Coordination&s=326. Published 2010. Accessed May 28, 2016.
29. Fillion L, de Serres M, Cook S, et al. Professional patient navigation in head and neck cancer. Sem Oncol Nurs. 2009;25(3):212-221.
Connective tissue diseases reported in patients receiving immune checkpoint inhibitors
For the first time, new-onset connective tissue disease has been reported in patients who were treated with anti-PD1/PDL-1 agents, according to findings published in the Annals of the Rheumatic Diseases.
In a cohort of 447 cancer patients who received therapy with immune checkpoint inhibitors (ICIs), Sébastien Le Burel, MD, of the Bicêtre Hospital in Le Kremlin-Bicêtre, France, and his colleagues described four patients who developed a connective tissue disease (CTD). There were two cases of Sjögren’s syndrome in patients taking an anti–programmed cell death 1 (anti-PD1) drug, one case of cryoglobulinemic vasculitis as a complication of suspected Sjögren’s syndrome in a patient taking an anti–programmed cell death ligand 1 (PDL-1) agent, and a case of a patient with antinuclear antibody positive myositis who was taking an anti-PDL-1 drug (Ann Rheum Dis. 2017 Feb 27. doi: 10.1136/annrheumdis-2016-210820).
“While the onset of systemic autoimmune disease after ICI treatment remains uncommon, greater awareness of these conditions should enable physicians to provide more effective patient care,” the investigators wrote. “This underlines the need for close collaboration within a network of oncologists and other specialist physicians in the new era of immunotherapy.”
The investigators discovered the cases by screening the French prospective, multicenter, academic REISAMIC registry for reports of CTD among patients being treated with anti-PD1 or anti-PDL-1 agents.
All four of the patients who developed a CTD had metastatic cancer, and their mean age was 62 years. Two patients had been treated with anti-PD1 agents and two with anti-PDL-1 agents. None of the four patients had presented with symptoms of CTD before they began treatment.
The mean time interval between the first treatment dose and the first symptom of CTD was 60 days (range, 24-72), and the mean time interval between the first symptom and subsequent diagnosis of CTD was 40 days (range, 10-74).
Three patients discontinued the ICI agent, and two patients were treated with steroids (1 mg/kg/day).
The estimated prevalence of CTD was 0.7% in the REISAMIC registry, and the authors emphasize that the high proportion of cases of Sjögren’s syndrome is noteworthy, with two of the patients fulfilling the recent American College of Rheumatology/European League Against Rheumatism criteria for Sjögren’s syndrome.
A limitation of the study is that some patients presenting with milder symptoms might not have been investigated by their oncologist.
The findings raise the question of whether asymptomatic patients taking ICIs who are at risk for immune-related adverse events should be screened and monitored closely, the authors explained.
One of the study authors received research funding from Novartis and Pfizer for the current paper. Several authors report relationships with industry.
For the first time, new-onset connective tissue disease has been reported in patients who were treated with anti-PD1/PDL-1 agents, according to findings published in the Annals of the Rheumatic Diseases.
In a cohort of 447 cancer patients who received therapy with immune checkpoint inhibitors (ICIs), Sébastien Le Burel, MD, of the Bicêtre Hospital in Le Kremlin-Bicêtre, France, and his colleagues described four patients who developed a connective tissue disease (CTD). There were two cases of Sjögren’s syndrome in patients taking an anti–programmed cell death 1 (anti-PD1) drug, one case of cryoglobulinemic vasculitis as a complication of suspected Sjögren’s syndrome in a patient taking an anti–programmed cell death ligand 1 (PDL-1) agent, and a case of a patient with antinuclear antibody positive myositis who was taking an anti-PDL-1 drug (Ann Rheum Dis. 2017 Feb 27. doi: 10.1136/annrheumdis-2016-210820).
“While the onset of systemic autoimmune disease after ICI treatment remains uncommon, greater awareness of these conditions should enable physicians to provide more effective patient care,” the investigators wrote. “This underlines the need for close collaboration within a network of oncologists and other specialist physicians in the new era of immunotherapy.”
The investigators discovered the cases by screening the French prospective, multicenter, academic REISAMIC registry for reports of CTD among patients being treated with anti-PD1 or anti-PDL-1 agents.
All four of the patients who developed a CTD had metastatic cancer, and their mean age was 62 years. Two patients had been treated with anti-PD1 agents and two with anti-PDL-1 agents. None of the four patients had presented with symptoms of CTD before they began treatment.
The mean time interval between the first treatment dose and the first symptom of CTD was 60 days (range, 24-72), and the mean time interval between the first symptom and subsequent diagnosis of CTD was 40 days (range, 10-74).
Three patients discontinued the ICI agent, and two patients were treated with steroids (1 mg/kg/day).
The estimated prevalence of CTD was 0.7% in the REISAMIC registry, and the authors emphasize that the high proportion of cases of Sjögren’s syndrome is noteworthy, with two of the patients fulfilling the recent American College of Rheumatology/European League Against Rheumatism criteria for Sjögren’s syndrome.
A limitation of the study is that some patients presenting with milder symptoms might not have been investigated by their oncologist.
The findings raise the question of whether asymptomatic patients taking ICIs who are at risk for immune-related adverse events should be screened and monitored closely, the authors explained.
One of the study authors received research funding from Novartis and Pfizer for the current paper. Several authors report relationships with industry.
For the first time, new-onset connective tissue disease has been reported in patients who were treated with anti-PD1/PDL-1 agents, according to findings published in the Annals of the Rheumatic Diseases.
In a cohort of 447 cancer patients who received therapy with immune checkpoint inhibitors (ICIs), Sébastien Le Burel, MD, of the Bicêtre Hospital in Le Kremlin-Bicêtre, France, and his colleagues described four patients who developed a connective tissue disease (CTD). There were two cases of Sjögren’s syndrome in patients taking an anti–programmed cell death 1 (anti-PD1) drug, one case of cryoglobulinemic vasculitis as a complication of suspected Sjögren’s syndrome in a patient taking an anti–programmed cell death ligand 1 (PDL-1) agent, and a case of a patient with antinuclear antibody positive myositis who was taking an anti-PDL-1 drug (Ann Rheum Dis. 2017 Feb 27. doi: 10.1136/annrheumdis-2016-210820).
“While the onset of systemic autoimmune disease after ICI treatment remains uncommon, greater awareness of these conditions should enable physicians to provide more effective patient care,” the investigators wrote. “This underlines the need for close collaboration within a network of oncologists and other specialist physicians in the new era of immunotherapy.”
The investigators discovered the cases by screening the French prospective, multicenter, academic REISAMIC registry for reports of CTD among patients being treated with anti-PD1 or anti-PDL-1 agents.
All four of the patients who developed a CTD had metastatic cancer, and their mean age was 62 years. Two patients had been treated with anti-PD1 agents and two with anti-PDL-1 agents. None of the four patients had presented with symptoms of CTD before they began treatment.
The mean time interval between the first treatment dose and the first symptom of CTD was 60 days (range, 24-72), and the mean time interval between the first symptom and subsequent diagnosis of CTD was 40 days (range, 10-74).
Three patients discontinued the ICI agent, and two patients were treated with steroids (1 mg/kg/day).
The estimated prevalence of CTD was 0.7% in the REISAMIC registry, and the authors emphasize that the high proportion of cases of Sjögren’s syndrome is noteworthy, with two of the patients fulfilling the recent American College of Rheumatology/European League Against Rheumatism criteria for Sjögren’s syndrome.
A limitation of the study is that some patients presenting with milder symptoms might not have been investigated by their oncologist.
The findings raise the question of whether asymptomatic patients taking ICIs who are at risk for immune-related adverse events should be screened and monitored closely, the authors explained.
One of the study authors received research funding from Novartis and Pfizer for the current paper. Several authors report relationships with industry.
Key clinical point:
Major finding: In a cohort of 447 patients, 4 with metastatic cancer developed connective tissue disease following anti-PD-1/PDL-1 treatment.
Data source: A prospective, multicenter, academic registry was screened for reports of CTD among patients being treated with anti-PD1/PDL-1 agents.
Disclosures: One of the study authors received research funding from Novartis and Pfizer for the current paper. Several authors report relationships with industry.
Postoperative pain in women with preexisting chronic pain
Chronic pain disorders have reached epidemic levels in the United States, with the Institute of Medicine reporting more than 100 million Americans affected and health care costs more than $500 billion annually.1 Although many pain disorders are confined to the abdomen or pelvis (chronic pelvic pain, vulvodynia, irritable bowel syndrome, and bladder pain syndrome), others present with global symptoms (fibromyalgia and chronic fatigue syndrome). Women are more likely to be diagnosed with a chronic pain condition and more likely to seek treatment for chronic pain, including undergoing a surgical intervention. In fact, chronic pelvic pain alone affects upward of 20% of women in the United States, and, of the 400,000 hysterectomies performed each year (54.2%, abdominal; 16.7%, vaginal; and 16.8%, laparoscopic/robotic assisted), approximately 15% are for chronic pain.2
Neurobiology of pain
Perioperative pain control, specifically in women with preexisting pain disorders, can provide an additional challenge. Unlike acute pain, chronic pain (lasting more than 6 months) is associated with an amplified pain response of the central nervous system. This abnormal pain processing, known as centralization of pain, may result in a decrease of the inhibitory pain pathways and/or an increase of the amplification pathways, often augmenting the pain response of the original peripheral insult, specifically surgery. Because of these physiologic changes, a multimodal approach to perioperative pain should be offered, especially in women with preexisting pain. The approach ideally ought to target the different mechanisms of actions in both the peripheral and central nervous systems to provide an overall reduction in pain perception.
Preoperative visit
Perhaps the most underutilized opportunity to optimize postoperative pain is a proactive, preoperative approach. Preoperative education, including goal setting of postoperative pain expectations, has been associated with a significant reduction in postoperative opioid use, less preoperative anxiety, and a decreased length of surgical stay.3 While it is unknown exactly when this should be provided to the patient in the treatment course, it should occur prior to the day of surgery to allow for appropriate intervention.
The use of a shared decision-making model between the clinician and the chronic pain patient in the development of a pain management plan has been highly successful in improving pain outcomes in the outpatient setting.4 A similar method can be applied to the preoperative course as well. A detailed history (including the use of an opioid risk assessment tool) allows the clinician to identify patients at risk for opioid misuse and abuse. This is also an opportunity to review a plan for an opioid taper with the patient and the prescriber, if the postoperative plan includes opioid reduction/cessation. The preoperative visit may be an opportunity to adjust centrally acting medications (antidepressants, anticonvulsants) before surgery or to reduce the dose or frequency of high-risk medications, such as benzodiazepines.
Perioperative strategy
One of the most impactful ways for us, as surgeons, to reduce tissue injury and decrease pain from surgery is by offering a minimally invasive approach. The benefits of minimally invasive surgery are well established, resulting in improved perioperative pain control, decreased blood loss, lower infection rates, decreased length of hospital stay, and a faster recovery, compared with laparotomy. Because patients with chronic pain disorders are at increased risk of greater acute postoperative pain and have an elevated risk for the development of chronic postsurgical pain, a minimally invasive surgical approach should be prioritized, when available.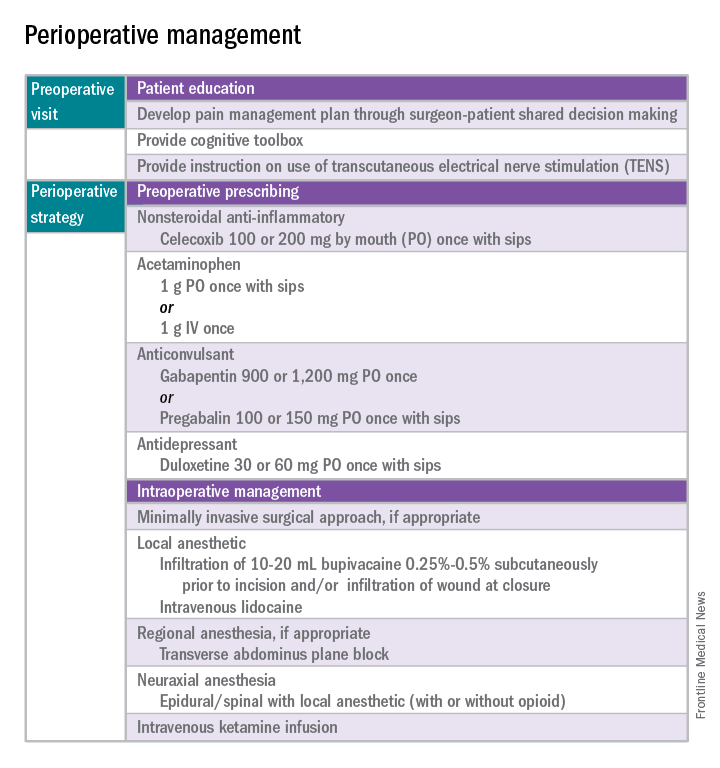
Perioperative multimodal drug therapy is associated with significant decreases in opioid consumption and reductions in acute postoperative pain.6 Recently, a multidisciplinary expert panel from the American Pain Society devised an evidence-based clinical practice guideline for postoperative pain.7 While there is no consensus as to the best regimen specific to gynecologic surgery, the general principles are similar across disciplines.
The postoperative period
Opioid-tolerant patients may experience greater pain during the first 24 hours postoperatively and require an increase in opioids, compared with opioid-naive patients.8 In the event that a postoperative patient does not respond as expected to the usual course, that patient should be evaluated for barriers to routine postoperative care, such as a surgical complication, opioid tolerance, or psychological distress. Surgeons should be aggressive with pain management immediately after surgery, even in the opioid-tolerant patient, and make short-term adjustments as needed based on the pain response. These patients will require pain medications beyond their baseline dose. Additionally, if an opioid taper is not planned in a chronic opioid user, work with the patient and the long-term opioid prescriber in restarting baseline opioid therapy outside of the acute surgical window. 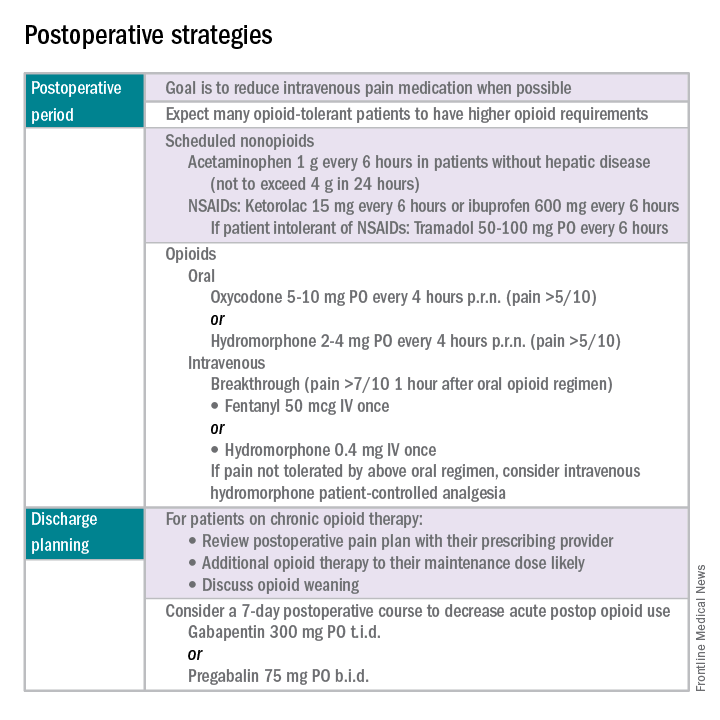
References
1. Institute of Medicine. Relieving Pain in America: A Blueprint for Transforming Prevention, Care, Education, and Research. Washington, DC: The National Academies Press, 2011.
2. Obstet Gynecol. 2013 Aug;122(2 Pt 1):233-41.
3. N Engl J Med. 1964 Apr 16;270:825-7.
4. J Pain Symptom Manage. 1999 Jul;18(1):38-48.
5. Pain. 2010 Dec;151(3):694-702.
6. Anesthesiology. 2005 Dec;103(6):1296-304.
7. J Pain. 2016 Feb;17(2):131-57.
8. Pharmacotherapy. 2008 Dec;28(12):1453-60.
Dr. Carey is the director of minimally invasive gynecologic surgery at the University of North Carolina at Chapel Hill, and specializes in the medical and surgical management of pelvic pain disorders. Dr. Rossi is an assistant professor in the division of gynecologic oncology at UNC–Chapel Hill. They reported having no relevant financial disclosures.
Chronic pain disorders have reached epidemic levels in the United States, with the Institute of Medicine reporting more than 100 million Americans affected and health care costs more than $500 billion annually.1 Although many pain disorders are confined to the abdomen or pelvis (chronic pelvic pain, vulvodynia, irritable bowel syndrome, and bladder pain syndrome), others present with global symptoms (fibromyalgia and chronic fatigue syndrome). Women are more likely to be diagnosed with a chronic pain condition and more likely to seek treatment for chronic pain, including undergoing a surgical intervention. In fact, chronic pelvic pain alone affects upward of 20% of women in the United States, and, of the 400,000 hysterectomies performed each year (54.2%, abdominal; 16.7%, vaginal; and 16.8%, laparoscopic/robotic assisted), approximately 15% are for chronic pain.2
Neurobiology of pain
Perioperative pain control, specifically in women with preexisting pain disorders, can provide an additional challenge. Unlike acute pain, chronic pain (lasting more than 6 months) is associated with an amplified pain response of the central nervous system. This abnormal pain processing, known as centralization of pain, may result in a decrease of the inhibitory pain pathways and/or an increase of the amplification pathways, often augmenting the pain response of the original peripheral insult, specifically surgery. Because of these physiologic changes, a multimodal approach to perioperative pain should be offered, especially in women with preexisting pain. The approach ideally ought to target the different mechanisms of actions in both the peripheral and central nervous systems to provide an overall reduction in pain perception.
Preoperative visit
Perhaps the most underutilized opportunity to optimize postoperative pain is a proactive, preoperative approach. Preoperative education, including goal setting of postoperative pain expectations, has been associated with a significant reduction in postoperative opioid use, less preoperative anxiety, and a decreased length of surgical stay.3 While it is unknown exactly when this should be provided to the patient in the treatment course, it should occur prior to the day of surgery to allow for appropriate intervention.
The use of a shared decision-making model between the clinician and the chronic pain patient in the development of a pain management plan has been highly successful in improving pain outcomes in the outpatient setting.4 A similar method can be applied to the preoperative course as well. A detailed history (including the use of an opioid risk assessment tool) allows the clinician to identify patients at risk for opioid misuse and abuse. This is also an opportunity to review a plan for an opioid taper with the patient and the prescriber, if the postoperative plan includes opioid reduction/cessation. The preoperative visit may be an opportunity to adjust centrally acting medications (antidepressants, anticonvulsants) before surgery or to reduce the dose or frequency of high-risk medications, such as benzodiazepines.
Perioperative strategy
One of the most impactful ways for us, as surgeons, to reduce tissue injury and decrease pain from surgery is by offering a minimally invasive approach. The benefits of minimally invasive surgery are well established, resulting in improved perioperative pain control, decreased blood loss, lower infection rates, decreased length of hospital stay, and a faster recovery, compared with laparotomy. Because patients with chronic pain disorders are at increased risk of greater acute postoperative pain and have an elevated risk for the development of chronic postsurgical pain, a minimally invasive surgical approach should be prioritized, when available.
Perioperative multimodal drug therapy is associated with significant decreases in opioid consumption and reductions in acute postoperative pain.6 Recently, a multidisciplinary expert panel from the American Pain Society devised an evidence-based clinical practice guideline for postoperative pain.7 While there is no consensus as to the best regimen specific to gynecologic surgery, the general principles are similar across disciplines.
The postoperative period
Opioid-tolerant patients may experience greater pain during the first 24 hours postoperatively and require an increase in opioids, compared with opioid-naive patients.8 In the event that a postoperative patient does not respond as expected to the usual course, that patient should be evaluated for barriers to routine postoperative care, such as a surgical complication, opioid tolerance, or psychological distress. Surgeons should be aggressive with pain management immediately after surgery, even in the opioid-tolerant patient, and make short-term adjustments as needed based on the pain response. These patients will require pain medications beyond their baseline dose. Additionally, if an opioid taper is not planned in a chronic opioid user, work with the patient and the long-term opioid prescriber in restarting baseline opioid therapy outside of the acute surgical window. 
References
1. Institute of Medicine. Relieving Pain in America: A Blueprint for Transforming Prevention, Care, Education, and Research. Washington, DC: The National Academies Press, 2011.
2. Obstet Gynecol. 2013 Aug;122(2 Pt 1):233-41.
3. N Engl J Med. 1964 Apr 16;270:825-7.
4. J Pain Symptom Manage. 1999 Jul;18(1):38-48.
5. Pain. 2010 Dec;151(3):694-702.
6. Anesthesiology. 2005 Dec;103(6):1296-304.
7. J Pain. 2016 Feb;17(2):131-57.
8. Pharmacotherapy. 2008 Dec;28(12):1453-60.
Dr. Carey is the director of minimally invasive gynecologic surgery at the University of North Carolina at Chapel Hill, and specializes in the medical and surgical management of pelvic pain disorders. Dr. Rossi is an assistant professor in the division of gynecologic oncology at UNC–Chapel Hill. They reported having no relevant financial disclosures.
Chronic pain disorders have reached epidemic levels in the United States, with the Institute of Medicine reporting more than 100 million Americans affected and health care costs more than $500 billion annually.1 Although many pain disorders are confined to the abdomen or pelvis (chronic pelvic pain, vulvodynia, irritable bowel syndrome, and bladder pain syndrome), others present with global symptoms (fibromyalgia and chronic fatigue syndrome). Women are more likely to be diagnosed with a chronic pain condition and more likely to seek treatment for chronic pain, including undergoing a surgical intervention. In fact, chronic pelvic pain alone affects upward of 20% of women in the United States, and, of the 400,000 hysterectomies performed each year (54.2%, abdominal; 16.7%, vaginal; and 16.8%, laparoscopic/robotic assisted), approximately 15% are for chronic pain.2
Neurobiology of pain
Perioperative pain control, specifically in women with preexisting pain disorders, can provide an additional challenge. Unlike acute pain, chronic pain (lasting more than 6 months) is associated with an amplified pain response of the central nervous system. This abnormal pain processing, known as centralization of pain, may result in a decrease of the inhibitory pain pathways and/or an increase of the amplification pathways, often augmenting the pain response of the original peripheral insult, specifically surgery. Because of these physiologic changes, a multimodal approach to perioperative pain should be offered, especially in women with preexisting pain. The approach ideally ought to target the different mechanisms of actions in both the peripheral and central nervous systems to provide an overall reduction in pain perception.
Preoperative visit
Perhaps the most underutilized opportunity to optimize postoperative pain is a proactive, preoperative approach. Preoperative education, including goal setting of postoperative pain expectations, has been associated with a significant reduction in postoperative opioid use, less preoperative anxiety, and a decreased length of surgical stay.3 While it is unknown exactly when this should be provided to the patient in the treatment course, it should occur prior to the day of surgery to allow for appropriate intervention.
The use of a shared decision-making model between the clinician and the chronic pain patient in the development of a pain management plan has been highly successful in improving pain outcomes in the outpatient setting.4 A similar method can be applied to the preoperative course as well. A detailed history (including the use of an opioid risk assessment tool) allows the clinician to identify patients at risk for opioid misuse and abuse. This is also an opportunity to review a plan for an opioid taper with the patient and the prescriber, if the postoperative plan includes opioid reduction/cessation. The preoperative visit may be an opportunity to adjust centrally acting medications (antidepressants, anticonvulsants) before surgery or to reduce the dose or frequency of high-risk medications, such as benzodiazepines.
Perioperative strategy
One of the most impactful ways for us, as surgeons, to reduce tissue injury and decrease pain from surgery is by offering a minimally invasive approach. The benefits of minimally invasive surgery are well established, resulting in improved perioperative pain control, decreased blood loss, lower infection rates, decreased length of hospital stay, and a faster recovery, compared with laparotomy. Because patients with chronic pain disorders are at increased risk of greater acute postoperative pain and have an elevated risk for the development of chronic postsurgical pain, a minimally invasive surgical approach should be prioritized, when available.
Perioperative multimodal drug therapy is associated with significant decreases in opioid consumption and reductions in acute postoperative pain.6 Recently, a multidisciplinary expert panel from the American Pain Society devised an evidence-based clinical practice guideline for postoperative pain.7 While there is no consensus as to the best regimen specific to gynecologic surgery, the general principles are similar across disciplines.
The postoperative period
Opioid-tolerant patients may experience greater pain during the first 24 hours postoperatively and require an increase in opioids, compared with opioid-naive patients.8 In the event that a postoperative patient does not respond as expected to the usual course, that patient should be evaluated for barriers to routine postoperative care, such as a surgical complication, opioid tolerance, or psychological distress. Surgeons should be aggressive with pain management immediately after surgery, even in the opioid-tolerant patient, and make short-term adjustments as needed based on the pain response. These patients will require pain medications beyond their baseline dose. Additionally, if an opioid taper is not planned in a chronic opioid user, work with the patient and the long-term opioid prescriber in restarting baseline opioid therapy outside of the acute surgical window. 
References
1. Institute of Medicine. Relieving Pain in America: A Blueprint for Transforming Prevention, Care, Education, and Research. Washington, DC: The National Academies Press, 2011.
2. Obstet Gynecol. 2013 Aug;122(2 Pt 1):233-41.
3. N Engl J Med. 1964 Apr 16;270:825-7.
4. J Pain Symptom Manage. 1999 Jul;18(1):38-48.
5. Pain. 2010 Dec;151(3):694-702.
6. Anesthesiology. 2005 Dec;103(6):1296-304.
7. J Pain. 2016 Feb;17(2):131-57.
8. Pharmacotherapy. 2008 Dec;28(12):1453-60.
Dr. Carey is the director of minimally invasive gynecologic surgery at the University of North Carolina at Chapel Hill, and specializes in the medical and surgical management of pelvic pain disorders. Dr. Rossi is an assistant professor in the division of gynecologic oncology at UNC–Chapel Hill. They reported having no relevant financial disclosures.