User login
Evidence for medical marijuana largely up in smoke
SAN DIEGO – Despite the popularity of medical marijuana, robust evidence for its use is limited or nonexistent for most medical conditions.
“This is a tough subject to study,” Ellie Grossman, MD, said at the annual meeting of the American College of Physicians. “There is federal money that can only be used in very limited ways to study it. Our science is way behind the times in terms of what our patients are doing and using.”
Typical limitations of marijuana studies include self-report of quantity/duration used and the fact that biochemical/quantifiable measures are lacking. “For inhaled marijuana, there is variability in how much is inhaled and how deeply it’s being inhaled,” said Dr. Grossman, an internist who practices in Somerville, Mass.
Then there’s the issue of recall bias and the question as to whether oral cannabinoids equate to the plant-derived forms of medical marijuana that patients obtain from their local dispensaries.
That matters, because the majority of published studies on the topic have evaluated oral cannabinoids, not the plant form. “So, what we’re studying is vastly different from what our patients are using,” she said.
The most solid indication clinicians have for recommending medical marijuana is for chronic pain, and the most common condition studied has been neuropathy.
“Most evidence compares cannabinoid to placebo,” said Dr. Grossman, primary care lead for behavioral health integration at Cambridge (Mass.) Health Alliance. “There’s almost nothing out there comparing cannabinoid to any other pain-relieving agent that a patient might choose to use.
“A lot of the literature comes from oral synthesized agents,” she continued. “There’s a little bit of science about inhaled forms, but a lot of this is very different from what my patient got last week in a medical marijuana dispensary in Massachusetts.”
Results from a systematic review of 79 studies of cannabinoids for medical use in 6,462 study participants showed that, compared with placebo, cannabinoids were associated with a greater average number of patients showing a complete nausea and vomiting response (47% vs. 20%; odds ratio, 3.82), reduction in pain (37% vs. 31%; OR, 1.41), a greater average reduction in numerical rating scale pain assessment (on a 0- to 10-point scale; weighted mean difference of –0.46), and average reduction in the Ashworth spasticity scale (–0.36) (JAMA 2015 Jun 23-30;313[24]:2456-73).
A separate meta-analysis of studies compared inhaled cannabis sativa to placebo for chronic painful neuropathy. The researchers found that those patients who used inhaled cannabis sativa were 3.2 times more likely to achieve a 30% or greater reduction in pain, compared with those in the placebo group (J. Pain 2015 Dec;16[12]:1221-32).
However, Dr. Grossman cautioned that the number of patients studied was fewer than 200, “so, you could argue that this is a body of knowledge where the jury is still out.”
According to a 2017 report from the National Academy of Sciences titled, “The Health Effects of Cannabis and Cannabinoids,” another area in which the knowledge base is less solid is the use of oral cannabinoids for chemotherapy-induced nausea and vomiting.
“There’s a reasonable amount of evidence showing that some of these are better than placebo for relief of these symptoms,” Dr. Grossman said. “That said, the jury’s out as to whether they are any better than our other antiemetic agents. And there are no studies comparing them to neurokinin-1 inhibitors, which are the newest class of drug often used by oncologists for this indication. There is also no good evidence about inhaled plant cannabis.”
Studies of oral cannabinoids for multiple-sclerosis–related spasticity have demonstrated a small improvement on patient-reported spasticity (less than 1 point on a 10-point scale), but there was no improvement in clinician-reported outcomes. At the same time, their use for weight loss/anorexia in HIV “is very limited, and there are no studies of plant-derived cannabis,” Dr. Grossman said.
According to the National Academy of Sciences report, some evidence supports the use of oral cannabinoids for short-term sleep outcomes in patients with chronic diseases such as fibromyalgia and MS. One small study of oral cannabinoid for anxiety found that it improved social anxiety symptoms on the public speaking test, but there have been no studies using inhaled cannabinoids/marijuana.
The health risks of medical marijuana are largely unknown, Dr. Grossman said, noting that most evidence on longer‐term health risks comes from epidemiologic studies of recreational cannabis users.
“Medical marijuana users tend to be older and tend to be sicker,” she said. “We don’t know anything about the long-term effects in that sicker population.”
Among healthier people, Dr. Grossman continued, cannabis use is associated with increased risk of cough, wheeze, and sputum/phlegm. “There’s also an increased risk of motor vehicle accidents,” she said. “That is certainly a concern in places where they’re legalizing marijuana.”
Cannabis use is associated with lower neonatal birth weight, case reports/series of unintentional pediatric ingestions, and a possible increase in suicidal ideation, suicide attempts, and completed suicides.
“The evidence is very limited regarding associations with myocardial infarction, stroke, COPD, and mortality,” she added. “We don’t really know.”
Dr. Grossman reported having no financial disclosures.
SAN DIEGO – Despite the popularity of medical marijuana, robust evidence for its use is limited or nonexistent for most medical conditions.
“This is a tough subject to study,” Ellie Grossman, MD, said at the annual meeting of the American College of Physicians. “There is federal money that can only be used in very limited ways to study it. Our science is way behind the times in terms of what our patients are doing and using.”
Typical limitations of marijuana studies include self-report of quantity/duration used and the fact that biochemical/quantifiable measures are lacking. “For inhaled marijuana, there is variability in how much is inhaled and how deeply it’s being inhaled,” said Dr. Grossman, an internist who practices in Somerville, Mass.
Then there’s the issue of recall bias and the question as to whether oral cannabinoids equate to the plant-derived forms of medical marijuana that patients obtain from their local dispensaries.
That matters, because the majority of published studies on the topic have evaluated oral cannabinoids, not the plant form. “So, what we’re studying is vastly different from what our patients are using,” she said.
The most solid indication clinicians have for recommending medical marijuana is for chronic pain, and the most common condition studied has been neuropathy.
“Most evidence compares cannabinoid to placebo,” said Dr. Grossman, primary care lead for behavioral health integration at Cambridge (Mass.) Health Alliance. “There’s almost nothing out there comparing cannabinoid to any other pain-relieving agent that a patient might choose to use.
“A lot of the literature comes from oral synthesized agents,” she continued. “There’s a little bit of science about inhaled forms, but a lot of this is very different from what my patient got last week in a medical marijuana dispensary in Massachusetts.”
Results from a systematic review of 79 studies of cannabinoids for medical use in 6,462 study participants showed that, compared with placebo, cannabinoids were associated with a greater average number of patients showing a complete nausea and vomiting response (47% vs. 20%; odds ratio, 3.82), reduction in pain (37% vs. 31%; OR, 1.41), a greater average reduction in numerical rating scale pain assessment (on a 0- to 10-point scale; weighted mean difference of –0.46), and average reduction in the Ashworth spasticity scale (–0.36) (JAMA 2015 Jun 23-30;313[24]:2456-73).
A separate meta-analysis of studies compared inhaled cannabis sativa to placebo for chronic painful neuropathy. The researchers found that those patients who used inhaled cannabis sativa were 3.2 times more likely to achieve a 30% or greater reduction in pain, compared with those in the placebo group (J. Pain 2015 Dec;16[12]:1221-32).
However, Dr. Grossman cautioned that the number of patients studied was fewer than 200, “so, you could argue that this is a body of knowledge where the jury is still out.”
According to a 2017 report from the National Academy of Sciences titled, “The Health Effects of Cannabis and Cannabinoids,” another area in which the knowledge base is less solid is the use of oral cannabinoids for chemotherapy-induced nausea and vomiting.
“There’s a reasonable amount of evidence showing that some of these are better than placebo for relief of these symptoms,” Dr. Grossman said. “That said, the jury’s out as to whether they are any better than our other antiemetic agents. And there are no studies comparing them to neurokinin-1 inhibitors, which are the newest class of drug often used by oncologists for this indication. There is also no good evidence about inhaled plant cannabis.”
Studies of oral cannabinoids for multiple-sclerosis–related spasticity have demonstrated a small improvement on patient-reported spasticity (less than 1 point on a 10-point scale), but there was no improvement in clinician-reported outcomes. At the same time, their use for weight loss/anorexia in HIV “is very limited, and there are no studies of plant-derived cannabis,” Dr. Grossman said.
According to the National Academy of Sciences report, some evidence supports the use of oral cannabinoids for short-term sleep outcomes in patients with chronic diseases such as fibromyalgia and MS. One small study of oral cannabinoid for anxiety found that it improved social anxiety symptoms on the public speaking test, but there have been no studies using inhaled cannabinoids/marijuana.
The health risks of medical marijuana are largely unknown, Dr. Grossman said, noting that most evidence on longer‐term health risks comes from epidemiologic studies of recreational cannabis users.
“Medical marijuana users tend to be older and tend to be sicker,” she said. “We don’t know anything about the long-term effects in that sicker population.”
Among healthier people, Dr. Grossman continued, cannabis use is associated with increased risk of cough, wheeze, and sputum/phlegm. “There’s also an increased risk of motor vehicle accidents,” she said. “That is certainly a concern in places where they’re legalizing marijuana.”
Cannabis use is associated with lower neonatal birth weight, case reports/series of unintentional pediatric ingestions, and a possible increase in suicidal ideation, suicide attempts, and completed suicides.
“The evidence is very limited regarding associations with myocardial infarction, stroke, COPD, and mortality,” she added. “We don’t really know.”
Dr. Grossman reported having no financial disclosures.
SAN DIEGO – Despite the popularity of medical marijuana, robust evidence for its use is limited or nonexistent for most medical conditions.
“This is a tough subject to study,” Ellie Grossman, MD, said at the annual meeting of the American College of Physicians. “There is federal money that can only be used in very limited ways to study it. Our science is way behind the times in terms of what our patients are doing and using.”
Typical limitations of marijuana studies include self-report of quantity/duration used and the fact that biochemical/quantifiable measures are lacking. “For inhaled marijuana, there is variability in how much is inhaled and how deeply it’s being inhaled,” said Dr. Grossman, an internist who practices in Somerville, Mass.
Then there’s the issue of recall bias and the question as to whether oral cannabinoids equate to the plant-derived forms of medical marijuana that patients obtain from their local dispensaries.
That matters, because the majority of published studies on the topic have evaluated oral cannabinoids, not the plant form. “So, what we’re studying is vastly different from what our patients are using,” she said.
The most solid indication clinicians have for recommending medical marijuana is for chronic pain, and the most common condition studied has been neuropathy.
“Most evidence compares cannabinoid to placebo,” said Dr. Grossman, primary care lead for behavioral health integration at Cambridge (Mass.) Health Alliance. “There’s almost nothing out there comparing cannabinoid to any other pain-relieving agent that a patient might choose to use.
“A lot of the literature comes from oral synthesized agents,” she continued. “There’s a little bit of science about inhaled forms, but a lot of this is very different from what my patient got last week in a medical marijuana dispensary in Massachusetts.”
Results from a systematic review of 79 studies of cannabinoids for medical use in 6,462 study participants showed that, compared with placebo, cannabinoids were associated with a greater average number of patients showing a complete nausea and vomiting response (47% vs. 20%; odds ratio, 3.82), reduction in pain (37% vs. 31%; OR, 1.41), a greater average reduction in numerical rating scale pain assessment (on a 0- to 10-point scale; weighted mean difference of –0.46), and average reduction in the Ashworth spasticity scale (–0.36) (JAMA 2015 Jun 23-30;313[24]:2456-73).
A separate meta-analysis of studies compared inhaled cannabis sativa to placebo for chronic painful neuropathy. The researchers found that those patients who used inhaled cannabis sativa were 3.2 times more likely to achieve a 30% or greater reduction in pain, compared with those in the placebo group (J. Pain 2015 Dec;16[12]:1221-32).
However, Dr. Grossman cautioned that the number of patients studied was fewer than 200, “so, you could argue that this is a body of knowledge where the jury is still out.”
According to a 2017 report from the National Academy of Sciences titled, “The Health Effects of Cannabis and Cannabinoids,” another area in which the knowledge base is less solid is the use of oral cannabinoids for chemotherapy-induced nausea and vomiting.
“There’s a reasonable amount of evidence showing that some of these are better than placebo for relief of these symptoms,” Dr. Grossman said. “That said, the jury’s out as to whether they are any better than our other antiemetic agents. And there are no studies comparing them to neurokinin-1 inhibitors, which are the newest class of drug often used by oncologists for this indication. There is also no good evidence about inhaled plant cannabis.”
Studies of oral cannabinoids for multiple-sclerosis–related spasticity have demonstrated a small improvement on patient-reported spasticity (less than 1 point on a 10-point scale), but there was no improvement in clinician-reported outcomes. At the same time, their use for weight loss/anorexia in HIV “is very limited, and there are no studies of plant-derived cannabis,” Dr. Grossman said.
According to the National Academy of Sciences report, some evidence supports the use of oral cannabinoids for short-term sleep outcomes in patients with chronic diseases such as fibromyalgia and MS. One small study of oral cannabinoid for anxiety found that it improved social anxiety symptoms on the public speaking test, but there have been no studies using inhaled cannabinoids/marijuana.
The health risks of medical marijuana are largely unknown, Dr. Grossman said, noting that most evidence on longer‐term health risks comes from epidemiologic studies of recreational cannabis users.
“Medical marijuana users tend to be older and tend to be sicker,” she said. “We don’t know anything about the long-term effects in that sicker population.”
Among healthier people, Dr. Grossman continued, cannabis use is associated with increased risk of cough, wheeze, and sputum/phlegm. “There’s also an increased risk of motor vehicle accidents,” she said. “That is certainly a concern in places where they’re legalizing marijuana.”
Cannabis use is associated with lower neonatal birth weight, case reports/series of unintentional pediatric ingestions, and a possible increase in suicidal ideation, suicide attempts, and completed suicides.
“The evidence is very limited regarding associations with myocardial infarction, stroke, COPD, and mortality,” she added. “We don’t really know.”
Dr. Grossman reported having no financial disclosures.
EXPERT ANALYSIS AT ACP INTERNAL MEDICINE
Off-the-shelf T cells an option for post-HCT viral infections
ORLANDO – Infusions of banked multivirus-specific T lymphocytes were associated with complete or partial responses in 93% of 42 patients who had undergone hematopoietic cell transplants and had drug-refractory viral illnesses. Further, these patients experienced minimal new or reactivated graft-versus-host disease (GVHD).
Viral infections cause nearly 40% of deaths after alternative donor hematopoietic cell transfer (HCT), Ifigeneia Tzannou, MD, said at the combined annual meetings of the Center for International Blood & Marrow Transplant Research and the American Society of Blood and Marrow Transplantation. Banked, “off-the-shelf” donor virus-resistant T cells can be an alternative to antiviral drugs, which are far from universally effective and may have serious side effects.
“Traditionally, we have generated T cells for infusion from the stem cell donor” by isolating and then stimulating and expanding the peripheral blood mononuclear cells for about 10 days ex vivo, said Dr. Tzannou. At that point, the clonal multivirus-resistant T cells can then be transferred to the recipient.
Donor-derived T cells have been used to prevent and treat Epstein-Barr virus (EBV), cytomegalovirus (CMV), adenovirus (AdV), BK virus (BKV), and human herpes virus 6 (HHV6) infections. The approach has been safe, reconstituting antiviral immunity and clearing disease effectively, with a 94% response rate reported in one recent study. However, said Dr. Tzannou, donor-derived virus-specific T cells (VSTs) have their limitations. Donors are increasingly younger and cord blood is being used more commonly, so there are growing numbers of donors who are seronegative for pathogenic viruses. In addition, the 10 days of production time and the additional week or 10 days required for release means that donor-derived VSTs can’t be urgently used.
The concept of banked third party VST therapy came about to address those limitations, said Dr. Tzannou of Baylor College of Medicine, Houston.
In a banked VST scenario, donor T cells with specific multiviral immunity are human leukocyte antigen (HLA) typed, expanded, and cryopreserved. A post-HCT patient with drug-refractory viral illness can receive T cells that are partially matched at HLA –A, HLA-B, or HLA-DR. Dr. Tzannou said that her group has now generated a bank of 59 VST lines to use in clinical testing of the third party approach.
In the study, Dr. Tzannou and her colleagues included both pediatric and adult post-allo-HCT patients with refractory EBV, CMV, AdV, BKV, and/or HHV6 infections. All had either failed a 14-day trial of antiviral therapy or could not tolerate antivirals. Patients could not be on more than 0.5 mg/kg per day of prednisone; they had to have an absolute neutrophil count above 500 per microliter and hemoglobin greater than 8 g/dL. Patients were excluded if they had acute GVHD of grade 2 or higher. There had to be a compatible VST line available that matched both the patient’s illness and HLA typing.
Patients initially received 20,000,000 VST cells per square meter of body surface area. If the investigators saw a partial response, patients could receive additional VST doses every 2 weeks.
Of the 42 patients infused, 23 received one infusion and 19 required two or more infusions. Seven study participants had two viral infections; 18 had CMV, 2 had EBV, 9 had AdV, 17 had BKV, and 3 had HHV6.
Dr. Tzannou and her colleagues tracked the virus-specific T cells and viral load for particular viruses. Virus-specific peripheral T cell counts also rose measurably and viral load plummeted within 2 weeks of VST infusions for most patients.
Overall, 93% of patients met the primary outcome measure of achieving complete or partial response; a partial response was defined as a 50% or better decrease in the viral load and/or clinical improvement.
All of the 17 BKV patients treated to date had tissue disease; 15 had hemorrhagic cystitis and 2 had nephritis. All responded to VSTs, and all of those with hemorrhagic cystitis had symptomatic improvement or resolution.
Overall, the safety profile for VST was good, said Dr. Tzannou. Four patients developed grade 1 acute cutaneous GVHD within 45 days of infusion; one of these developed de novo, but resolved with topical steroids. Another patient had a flare of gastrointestinal GVHD when immunosuppresion was being tapered. One more patient had a transient fever post infusion that resolved spontaneously, said Dr. Tzannou.
Next steps include a multicenter registration study, said Dr. Tzannou, who reports being a consultant for ViraCyte, which helped fund the study.
[email protected]
On Twitter @karioakes
ORLANDO – Infusions of banked multivirus-specific T lymphocytes were associated with complete or partial responses in 93% of 42 patients who had undergone hematopoietic cell transplants and had drug-refractory viral illnesses. Further, these patients experienced minimal new or reactivated graft-versus-host disease (GVHD).
Viral infections cause nearly 40% of deaths after alternative donor hematopoietic cell transfer (HCT), Ifigeneia Tzannou, MD, said at the combined annual meetings of the Center for International Blood & Marrow Transplant Research and the American Society of Blood and Marrow Transplantation. Banked, “off-the-shelf” donor virus-resistant T cells can be an alternative to antiviral drugs, which are far from universally effective and may have serious side effects.
“Traditionally, we have generated T cells for infusion from the stem cell donor” by isolating and then stimulating and expanding the peripheral blood mononuclear cells for about 10 days ex vivo, said Dr. Tzannou. At that point, the clonal multivirus-resistant T cells can then be transferred to the recipient.
Donor-derived T cells have been used to prevent and treat Epstein-Barr virus (EBV), cytomegalovirus (CMV), adenovirus (AdV), BK virus (BKV), and human herpes virus 6 (HHV6) infections. The approach has been safe, reconstituting antiviral immunity and clearing disease effectively, with a 94% response rate reported in one recent study. However, said Dr. Tzannou, donor-derived virus-specific T cells (VSTs) have their limitations. Donors are increasingly younger and cord blood is being used more commonly, so there are growing numbers of donors who are seronegative for pathogenic viruses. In addition, the 10 days of production time and the additional week or 10 days required for release means that donor-derived VSTs can’t be urgently used.
The concept of banked third party VST therapy came about to address those limitations, said Dr. Tzannou of Baylor College of Medicine, Houston.
In a banked VST scenario, donor T cells with specific multiviral immunity are human leukocyte antigen (HLA) typed, expanded, and cryopreserved. A post-HCT patient with drug-refractory viral illness can receive T cells that are partially matched at HLA –A, HLA-B, or HLA-DR. Dr. Tzannou said that her group has now generated a bank of 59 VST lines to use in clinical testing of the third party approach.
In the study, Dr. Tzannou and her colleagues included both pediatric and adult post-allo-HCT patients with refractory EBV, CMV, AdV, BKV, and/or HHV6 infections. All had either failed a 14-day trial of antiviral therapy or could not tolerate antivirals. Patients could not be on more than 0.5 mg/kg per day of prednisone; they had to have an absolute neutrophil count above 500 per microliter and hemoglobin greater than 8 g/dL. Patients were excluded if they had acute GVHD of grade 2 or higher. There had to be a compatible VST line available that matched both the patient’s illness and HLA typing.
Patients initially received 20,000,000 VST cells per square meter of body surface area. If the investigators saw a partial response, patients could receive additional VST doses every 2 weeks.
Of the 42 patients infused, 23 received one infusion and 19 required two or more infusions. Seven study participants had two viral infections; 18 had CMV, 2 had EBV, 9 had AdV, 17 had BKV, and 3 had HHV6.
Dr. Tzannou and her colleagues tracked the virus-specific T cells and viral load for particular viruses. Virus-specific peripheral T cell counts also rose measurably and viral load plummeted within 2 weeks of VST infusions for most patients.
Overall, 93% of patients met the primary outcome measure of achieving complete or partial response; a partial response was defined as a 50% or better decrease in the viral load and/or clinical improvement.
All of the 17 BKV patients treated to date had tissue disease; 15 had hemorrhagic cystitis and 2 had nephritis. All responded to VSTs, and all of those with hemorrhagic cystitis had symptomatic improvement or resolution.
Overall, the safety profile for VST was good, said Dr. Tzannou. Four patients developed grade 1 acute cutaneous GVHD within 45 days of infusion; one of these developed de novo, but resolved with topical steroids. Another patient had a flare of gastrointestinal GVHD when immunosuppresion was being tapered. One more patient had a transient fever post infusion that resolved spontaneously, said Dr. Tzannou.
Next steps include a multicenter registration study, said Dr. Tzannou, who reports being a consultant for ViraCyte, which helped fund the study.
[email protected]
On Twitter @karioakes
ORLANDO – Infusions of banked multivirus-specific T lymphocytes were associated with complete or partial responses in 93% of 42 patients who had undergone hematopoietic cell transplants and had drug-refractory viral illnesses. Further, these patients experienced minimal new or reactivated graft-versus-host disease (GVHD).
Viral infections cause nearly 40% of deaths after alternative donor hematopoietic cell transfer (HCT), Ifigeneia Tzannou, MD, said at the combined annual meetings of the Center for International Blood & Marrow Transplant Research and the American Society of Blood and Marrow Transplantation. Banked, “off-the-shelf” donor virus-resistant T cells can be an alternative to antiviral drugs, which are far from universally effective and may have serious side effects.
“Traditionally, we have generated T cells for infusion from the stem cell donor” by isolating and then stimulating and expanding the peripheral blood mononuclear cells for about 10 days ex vivo, said Dr. Tzannou. At that point, the clonal multivirus-resistant T cells can then be transferred to the recipient.
Donor-derived T cells have been used to prevent and treat Epstein-Barr virus (EBV), cytomegalovirus (CMV), adenovirus (AdV), BK virus (BKV), and human herpes virus 6 (HHV6) infections. The approach has been safe, reconstituting antiviral immunity and clearing disease effectively, with a 94% response rate reported in one recent study. However, said Dr. Tzannou, donor-derived virus-specific T cells (VSTs) have their limitations. Donors are increasingly younger and cord blood is being used more commonly, so there are growing numbers of donors who are seronegative for pathogenic viruses. In addition, the 10 days of production time and the additional week or 10 days required for release means that donor-derived VSTs can’t be urgently used.
The concept of banked third party VST therapy came about to address those limitations, said Dr. Tzannou of Baylor College of Medicine, Houston.
In a banked VST scenario, donor T cells with specific multiviral immunity are human leukocyte antigen (HLA) typed, expanded, and cryopreserved. A post-HCT patient with drug-refractory viral illness can receive T cells that are partially matched at HLA –A, HLA-B, or HLA-DR. Dr. Tzannou said that her group has now generated a bank of 59 VST lines to use in clinical testing of the third party approach.
In the study, Dr. Tzannou and her colleagues included both pediatric and adult post-allo-HCT patients with refractory EBV, CMV, AdV, BKV, and/or HHV6 infections. All had either failed a 14-day trial of antiviral therapy or could not tolerate antivirals. Patients could not be on more than 0.5 mg/kg per day of prednisone; they had to have an absolute neutrophil count above 500 per microliter and hemoglobin greater than 8 g/dL. Patients were excluded if they had acute GVHD of grade 2 or higher. There had to be a compatible VST line available that matched both the patient’s illness and HLA typing.
Patients initially received 20,000,000 VST cells per square meter of body surface area. If the investigators saw a partial response, patients could receive additional VST doses every 2 weeks.
Of the 42 patients infused, 23 received one infusion and 19 required two or more infusions. Seven study participants had two viral infections; 18 had CMV, 2 had EBV, 9 had AdV, 17 had BKV, and 3 had HHV6.
Dr. Tzannou and her colleagues tracked the virus-specific T cells and viral load for particular viruses. Virus-specific peripheral T cell counts also rose measurably and viral load plummeted within 2 weeks of VST infusions for most patients.
Overall, 93% of patients met the primary outcome measure of achieving complete or partial response; a partial response was defined as a 50% or better decrease in the viral load and/or clinical improvement.
All of the 17 BKV patients treated to date had tissue disease; 15 had hemorrhagic cystitis and 2 had nephritis. All responded to VSTs, and all of those with hemorrhagic cystitis had symptomatic improvement or resolution.
Overall, the safety profile for VST was good, said Dr. Tzannou. Four patients developed grade 1 acute cutaneous GVHD within 45 days of infusion; one of these developed de novo, but resolved with topical steroids. Another patient had a flare of gastrointestinal GVHD when immunosuppresion was being tapered. One more patient had a transient fever post infusion that resolved spontaneously, said Dr. Tzannou.
Next steps include a multicenter registration study, said Dr. Tzannou, who reports being a consultant for ViraCyte, which helped fund the study.
[email protected]
On Twitter @karioakes
AT THE 2017 BMT TANDEM MEETINGS
Key clinical point:
Major finding: With banked multivirus-specific T cells, viral illnesses either improved or resolved in 93% of 42 patients.
Data source: Clinical trial of 42 postallogeneic hematopoietic cell transfer patients who had any of five viral illnesses and had either failed a 14-day trial of antiviral therapy or could not tolerate antivirals.
Disclosures: Dr. Tzannou is a consultant for Incyte, which partially funded the trial and is developing third-party VSTs.
Tocilizumab shows promise for GVHD prevention
ORLANDO – Tocilizumab plus standard immune suppression appears to drive down the risk for graft-versus-host disease (GVHD), according to results from a phase II study of 35 adults undergoing allogeneic stem cell transplants.
The effect was particularly pronounced for prevention of GVHD in the colon, William Drobyski, MD, reported at the combined annual meetings of the Center for International Blood & Marrow Transplant Research and the American Society for Blood and Marrow Transplantation.
The incidence rate of grades II-IV and III-IV acute GVHD was 12% at day 100 in patients given standard prophylaxis of tacrolimus/methotrexate (Tac/MTX) and 3% in patients given Tac/MTX plus 8 mg/kg of tocilizumab (Toc, capped at 800 mg), said Dr. Drobyski of the Medical College of Wisconsin, Milwaukee.
To provide further context to the results, Dr. Drobyski and his colleagues performed a matched case-control analysis using contemporary controls in the Center for International Blood & Marrow Transplant Research from 2000 to 2014. The same eligibility criteria used for the trial were applied to the matched controls except for the use of Tac/MTX as GVHD prophylaxis. Patients were otherwise matched based on age, performance score, disease, and donor type.
The incidence of grades II-IV acute GVHD at day 100 was significantly lower in the Toc/Tac/MTX group than in the Tac/MTX control population (12% vs. 41%). The incidence of grades III-IV acute GVHD was slightly lower with tocilizumab, but the difference between the groups was not statistically significant, Dr. Drobyski said.
The probability of grade II-IV acute GVHD–free survival, which was the primary endpoint of the study, was significantly higher in the Toc/Tac/MTX group (79% vs 52%), he said.
Five patients developed grade 2 acute GVHD of the skin or upper GI tract, and one patient died of grade 4 acute GVHD of the skin in the first 100 days. Notably, there were no cases of acute GVHD of the lower GI tract during that time, although two cases did occur between days 130 and 180, he said.
“There was no difference in transplant-related mortality, relapse, disease-free survival, or overall survival,” he said, adding that preliminary data suggest there were no differences in chronic GVHD between the groups.
Causes of death also were similar between the two cohorts with respect to disease- and transplant-related complications.
Patients in the tocilizumab study were enrolled between January 2015 and June 2016; the median age was 66 years. Diseases represented in the cohort included de novo acute myeloid leukemia (13 patients), AML (6 patients), chronic myelomonocytic leukemia (6 patients), acute lymphoblastic leukemia (4 patients), myelodysplastic syndrome (3 patients), and T-cell lymphoma, chronic myeloid leukemia, and NK/T cell lymphoma (in 1 patient each). Most patients were classified as high risk (9 patients) or intermediate risk (22 patients) by the disease risk index.
Conditioning was entirely busulfan based. Myeloablative conditioning was with busulfan and cyclophosphamide (Cytoxan) in 5 patients, or fludarabine and 4 days of busulfan in 10 patients, and reduced-intensity conditioning was with fludarabine and 2 days of busulfan in 18 patients. Transplants were with either HLA-matched related or unrelated donor grafts. Most patients (29 of 35) received peripheral stem cell grafts.
Tocilizumab, an interleuken-6 receptor blocker that is approved for treatment of rheumatoid arthritis, was administered after completion of conditioning and on the day prior to stem cell infusion.
In a pilot clinical trial of tocilizumab for the treatment of steroid-resistant acute GVHD in patients who had primarily had lower GI tract disease, “we were able to demonstrate responses in a majority of these patients,” Dr. Drobyski said, noting that a recent study presented at the 2016 annual meeting of the American Society of Hematology also showed efficacy in the treatment of lower tract GI GVHD, “providing evidence that tocilizumab had activity in acute GVHD, and perhaps in the treatment of steroid-refractory lower GI GVHD.”
Elevated IL-6 levels in the peripheral blood are correlated with an increased incidence and severity of GVHD; administration of an anti-IL-6 receptor antibody has been shown in preclinical studies to protect mice from lethal GVHD. The current open-label study was performed to “try to advance this concept” by assessing whether inhibition of IL-6 signaling could also prevent acute GVHD.
The findings confirm those of a 2014 study by Kennedy et al. in Lancet Oncology (2014;15:1451-9), and imply that tocilizumab warrants a randomized trial as prophylaxis for acute GVHD, he concluded.
Dr. Drobyski reported having no disclosures.
ORLANDO – Tocilizumab plus standard immune suppression appears to drive down the risk for graft-versus-host disease (GVHD), according to results from a phase II study of 35 adults undergoing allogeneic stem cell transplants.
The effect was particularly pronounced for prevention of GVHD in the colon, William Drobyski, MD, reported at the combined annual meetings of the Center for International Blood & Marrow Transplant Research and the American Society for Blood and Marrow Transplantation.
The incidence rate of grades II-IV and III-IV acute GVHD was 12% at day 100 in patients given standard prophylaxis of tacrolimus/methotrexate (Tac/MTX) and 3% in patients given Tac/MTX plus 8 mg/kg of tocilizumab (Toc, capped at 800 mg), said Dr. Drobyski of the Medical College of Wisconsin, Milwaukee.
To provide further context to the results, Dr. Drobyski and his colleagues performed a matched case-control analysis using contemporary controls in the Center for International Blood & Marrow Transplant Research from 2000 to 2014. The same eligibility criteria used for the trial were applied to the matched controls except for the use of Tac/MTX as GVHD prophylaxis. Patients were otherwise matched based on age, performance score, disease, and donor type.
The incidence of grades II-IV acute GVHD at day 100 was significantly lower in the Toc/Tac/MTX group than in the Tac/MTX control population (12% vs. 41%). The incidence of grades III-IV acute GVHD was slightly lower with tocilizumab, but the difference between the groups was not statistically significant, Dr. Drobyski said.
The probability of grade II-IV acute GVHD–free survival, which was the primary endpoint of the study, was significantly higher in the Toc/Tac/MTX group (79% vs 52%), he said.
Five patients developed grade 2 acute GVHD of the skin or upper GI tract, and one patient died of grade 4 acute GVHD of the skin in the first 100 days. Notably, there were no cases of acute GVHD of the lower GI tract during that time, although two cases did occur between days 130 and 180, he said.
“There was no difference in transplant-related mortality, relapse, disease-free survival, or overall survival,” he said, adding that preliminary data suggest there were no differences in chronic GVHD between the groups.
Causes of death also were similar between the two cohorts with respect to disease- and transplant-related complications.
Patients in the tocilizumab study were enrolled between January 2015 and June 2016; the median age was 66 years. Diseases represented in the cohort included de novo acute myeloid leukemia (13 patients), AML (6 patients), chronic myelomonocytic leukemia (6 patients), acute lymphoblastic leukemia (4 patients), myelodysplastic syndrome (3 patients), and T-cell lymphoma, chronic myeloid leukemia, and NK/T cell lymphoma (in 1 patient each). Most patients were classified as high risk (9 patients) or intermediate risk (22 patients) by the disease risk index.
Conditioning was entirely busulfan based. Myeloablative conditioning was with busulfan and cyclophosphamide (Cytoxan) in 5 patients, or fludarabine and 4 days of busulfan in 10 patients, and reduced-intensity conditioning was with fludarabine and 2 days of busulfan in 18 patients. Transplants were with either HLA-matched related or unrelated donor grafts. Most patients (29 of 35) received peripheral stem cell grafts.
Tocilizumab, an interleuken-6 receptor blocker that is approved for treatment of rheumatoid arthritis, was administered after completion of conditioning and on the day prior to stem cell infusion.
In a pilot clinical trial of tocilizumab for the treatment of steroid-resistant acute GVHD in patients who had primarily had lower GI tract disease, “we were able to demonstrate responses in a majority of these patients,” Dr. Drobyski said, noting that a recent study presented at the 2016 annual meeting of the American Society of Hematology also showed efficacy in the treatment of lower tract GI GVHD, “providing evidence that tocilizumab had activity in acute GVHD, and perhaps in the treatment of steroid-refractory lower GI GVHD.”
Elevated IL-6 levels in the peripheral blood are correlated with an increased incidence and severity of GVHD; administration of an anti-IL-6 receptor antibody has been shown in preclinical studies to protect mice from lethal GVHD. The current open-label study was performed to “try to advance this concept” by assessing whether inhibition of IL-6 signaling could also prevent acute GVHD.
The findings confirm those of a 2014 study by Kennedy et al. in Lancet Oncology (2014;15:1451-9), and imply that tocilizumab warrants a randomized trial as prophylaxis for acute GVHD, he concluded.
Dr. Drobyski reported having no disclosures.
ORLANDO – Tocilizumab plus standard immune suppression appears to drive down the risk for graft-versus-host disease (GVHD), according to results from a phase II study of 35 adults undergoing allogeneic stem cell transplants.
The effect was particularly pronounced for prevention of GVHD in the colon, William Drobyski, MD, reported at the combined annual meetings of the Center for International Blood & Marrow Transplant Research and the American Society for Blood and Marrow Transplantation.
The incidence rate of grades II-IV and III-IV acute GVHD was 12% at day 100 in patients given standard prophylaxis of tacrolimus/methotrexate (Tac/MTX) and 3% in patients given Tac/MTX plus 8 mg/kg of tocilizumab (Toc, capped at 800 mg), said Dr. Drobyski of the Medical College of Wisconsin, Milwaukee.
To provide further context to the results, Dr. Drobyski and his colleagues performed a matched case-control analysis using contemporary controls in the Center for International Blood & Marrow Transplant Research from 2000 to 2014. The same eligibility criteria used for the trial were applied to the matched controls except for the use of Tac/MTX as GVHD prophylaxis. Patients were otherwise matched based on age, performance score, disease, and donor type.
The incidence of grades II-IV acute GVHD at day 100 was significantly lower in the Toc/Tac/MTX group than in the Tac/MTX control population (12% vs. 41%). The incidence of grades III-IV acute GVHD was slightly lower with tocilizumab, but the difference between the groups was not statistically significant, Dr. Drobyski said.
The probability of grade II-IV acute GVHD–free survival, which was the primary endpoint of the study, was significantly higher in the Toc/Tac/MTX group (79% vs 52%), he said.
Five patients developed grade 2 acute GVHD of the skin or upper GI tract, and one patient died of grade 4 acute GVHD of the skin in the first 100 days. Notably, there were no cases of acute GVHD of the lower GI tract during that time, although two cases did occur between days 130 and 180, he said.
“There was no difference in transplant-related mortality, relapse, disease-free survival, or overall survival,” he said, adding that preliminary data suggest there were no differences in chronic GVHD between the groups.
Causes of death also were similar between the two cohorts with respect to disease- and transplant-related complications.
Patients in the tocilizumab study were enrolled between January 2015 and June 2016; the median age was 66 years. Diseases represented in the cohort included de novo acute myeloid leukemia (13 patients), AML (6 patients), chronic myelomonocytic leukemia (6 patients), acute lymphoblastic leukemia (4 patients), myelodysplastic syndrome (3 patients), and T-cell lymphoma, chronic myeloid leukemia, and NK/T cell lymphoma (in 1 patient each). Most patients were classified as high risk (9 patients) or intermediate risk (22 patients) by the disease risk index.
Conditioning was entirely busulfan based. Myeloablative conditioning was with busulfan and cyclophosphamide (Cytoxan) in 5 patients, or fludarabine and 4 days of busulfan in 10 patients, and reduced-intensity conditioning was with fludarabine and 2 days of busulfan in 18 patients. Transplants were with either HLA-matched related or unrelated donor grafts. Most patients (29 of 35) received peripheral stem cell grafts.
Tocilizumab, an interleuken-6 receptor blocker that is approved for treatment of rheumatoid arthritis, was administered after completion of conditioning and on the day prior to stem cell infusion.
In a pilot clinical trial of tocilizumab for the treatment of steroid-resistant acute GVHD in patients who had primarily had lower GI tract disease, “we were able to demonstrate responses in a majority of these patients,” Dr. Drobyski said, noting that a recent study presented at the 2016 annual meeting of the American Society of Hematology also showed efficacy in the treatment of lower tract GI GVHD, “providing evidence that tocilizumab had activity in acute GVHD, and perhaps in the treatment of steroid-refractory lower GI GVHD.”
Elevated IL-6 levels in the peripheral blood are correlated with an increased incidence and severity of GVHD; administration of an anti-IL-6 receptor antibody has been shown in preclinical studies to protect mice from lethal GVHD. The current open-label study was performed to “try to advance this concept” by assessing whether inhibition of IL-6 signaling could also prevent acute GVHD.
The findings confirm those of a 2014 study by Kennedy et al. in Lancet Oncology (2014;15:1451-9), and imply that tocilizumab warrants a randomized trial as prophylaxis for acute GVHD, he concluded.
Dr. Drobyski reported having no disclosures.
Key clinical point:
Major finding: The probability of grade II-IV acute GVHD-free survival was 79% vs. 52% in the tocilizumab group vs. age-matched controls.
Data source: An open-label phase II study of 35 patients.
Disclosures: Dr. Drobyski reported having no disclosures.
Demystifying the diagnosis and classification of lymphoma: a guide to the hematopathologist’s galaxy
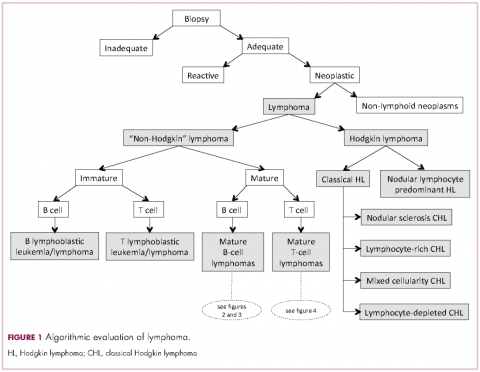
Lymphomas constitute a very heterogeneous group of neoplasms with diverse clinical presentations, prognoses, and responses to therapy. Approximately 80,500 new cases of lymphoma are expected to be diagnosed in the United States in 2017, of which about one quarter will lead to the death of the patient.1 Perhaps more so than any other group of neoplasms, the diagnosis of lymphoma involves the integration of a multiplicity of clinical, histologic and immunophenotypic findings and, on occasion, cytogenetic and molecular results as well. An accurate diagnosis of lymphoma, usually rendered by hematopathologists, allows hematologists/oncologists to treat patients appropriately. Herein we will describe a simplified approach to the diagnosis and classification of lymphomas (Figure 1).
Lymphoma classification
Lymphomas are clonal neoplasms characterized by the expansion of abnormal lymphoid cells that may develop in any organ but commonly involve lymph nodes. The fourth edition of the World Health Organization (WHO) Classification of Tumours of Haematopoietic and Lymphoid tissues, published in 2008, is the official and most current guideline used for diagnosis of lymphoid neoplasms.2 The WHO scheme classifies lymphomas according to the type of cell from which they are derived (mature and immature B cells, T cells, or natural killer (NK) cells, findings determined by their morphology and immunophenotype) and their clinical, cytogenetic, and/or molecular features. This official classification is currently being updated3 and is expected to be published in full in 2017, at which time it is anticipated to include definitions for more than 70 distinct neoplasms.
Lymphomas are broadly and informally classified as Hodgkin lymphomas (HLs) and non-Hodgkin lymphomas (NHLs), based on the differences these two groups show in their clinical presentation, treatment, prognosis, and proportion of neoplastic cells, among others. NHLs are by far the most common type of lymphomas, accounting for approximately 90% of all new cases of lymphoma in the United States and 70% worldwide.1,2 NHLs are a very heterogeneous group of B-, T-, or NK-cell neoplasms that, in turn, can also be informally subclassified as low-grade (or indolent) or high-grade (or aggressive) according to their predicted clinical behavior. HLs are comparatively rare, less heterogeneous, uniformly of B-cell origin and, in the case of classical Hodgkin lymphoma, highly curable.1,2 It is beyond the scope of this manuscript to outline the features of each of the >70 specific entities, but the reader is referred elsewhere for more detail and encouraged to become familiarized with the complexity, challenges, and beauty of lymphoma diagnosis.2,3
Biopsy procedure
A correct diagnosis begins with an adequate biopsy procedure. It is essential that biopsy specimens for lymphoma evaluation be submitted fresh and unfixed, because some crucial analyses such as flow cytometry or conventional cytogenetics can only be performed on fresh tissue. Indeed, it is important for the hematologist/oncologist and/or surgeon and/or interventional radiologist to converse with the hematopathologist prior to and even during some procedures to ensure the correct processing of the specimen. Also, it is important to limit the compression of the specimen and the excessive use of cauterization during the biopsy procedure, both of which cause artifacts that may render impossible the interpretation of the histopathologic findings.
Given that the diagnosis of lymphoma is based not only on the cytologic details of the lymphoma cells but also on the architectural pattern with which they infiltrate an organ, the larger the biopsy specimen, the easier it will be for a hematopathologist to identify the pattern. In addition, excisional biopsies frequently contain more diagnostic tissue than needle core biopsies and this provides pathologists with the option to submit tissue fragments for ancillary tests that require unfixed tissue as noted above. Needle core biopsies of lymph nodes are increasingly being used because of their association with fewer complications and lower cost than excisional biopsies. However, needle core biopsies provide only a glimpse of the pattern of infiltration and may not be completely representative of the architecture. Therefore, excisional lymph node biopsies of lymph nodes are preferred over needle core biopsies, recognizing that in the setting of deeply seated lymph nodes, needle core biopsies may be the only or the best surgical option.
Clinical presentation
Accurate diagnosis of lymphoma cannot take place in a vacuum. The hematopathologist’s initial approach to the diagnosis of lymphoid processes in tissue biopsies should begin with a thorough review of the clinical history, although some pathology laboratories may not have immediate access to this information. The hematopathologist should evaluate factors such as age, gender, location of the tumor, symptomatology, medications, serology, and prior history of malignancy, immunosuppression or immunodeficiency in every case. Other important but frequently omitted parts of the clinical history are the patient’s occupation, history of exposure to animals, and the presence of tattoos, which may be associated with certain reactive lymphadenopathies.
Histomorphologic evaluation
Despite the plethora of new and increasingly sophisticated tools, histologic and morphologic analysis still remains the cornerstone of diagnosis in hematopathology. However, for the characterization of an increasing number of reactive and neoplastic lymphoid processes, hematopathologists may also require immunophenotypic, molecular, and cytogenetic tests for an accurate diagnosis. Upon review of the clinical information, a microscopic evaluation of the tissue submitted for processing by the histology laboratory will be performed. The results of concurrent flow cytometric evaluation (performed on fresh unfixed material) should also be available in most if not all cases before the H&E-stained slides are available for review. Upon receipt of H&E-stained slides, the hematopathologist will evaluate the quality of the submitted specimen, since many diagnostic difficulties stem from suboptimal techniques related to the biopsy procedure, fixation, processing, cutting, or staining (Figure 1). If deemed suitable for accurate diagnosis, a search for signs of preservation or disruption of the organ that was biopsied will follow. The identification of certain morphologic patterns aids the hematopathologist in answering the first question: “what organ is this and is this consistent with what is indicated on the requisition?” This is usually immediately followed by “is this sufficient and adequate material for a diagnosis?” and “is there any normal architecture?” If the architecture is not normal, “is this alteration due to a reactive or a neoplastic process?” If neoplastic, “is it lymphoma or a non-hematolymphoid neoplasm?”
Both reactive and neoplastic processes have variably unique morphologic features that if properly recognized, guide the subsequent testing. However, some reactive and neoplastic processes can present with overlapping features, and even after extensive immunophenotypic evaluation and the performance of ancillary studies, it may not be possible to conclusively determine its nature. If the lymph node architecture is altered or effaced, the predominant pattern of infiltration (eg, nodular, diffuse, interfollicular, intrasinusoidal) and the degree of alteration of the normal architecture is evaluated, usually at low magnification. When the presence of an infiltrate is recognized, its components must be characterized. If the infiltrate is composed of a homogeneous expansion of lymphoid cells that disrupts or replaces the normal lymphoid architecture, a lymphoma will be suspected or diagnosed. The pattern of distribution of the cells along with their individual morphologic characteristics (ie, size, nuclear shape, chromatin configuration, nucleoli, amount and hue of cytoplasm) are key factors for the diagnosis and classification of the lymphoma that will guide subsequent testing. The immunophenotypic analysis (by immunohistochemistry, flow cytometry or a combination of both) may confirm the reactive or neoplastic nature of the process, and its subclassification. B-cell lymphomas, in particular have variable and distinctive histologic features: as a diffuse infiltrate of large mature lymphoid cells (eg, diffuse large B-cell lymphoma), an expansion of immature lymphoid cells (lymphoblastic lymphoma), and a nodular infiltrate of small, intermediate and/or mature large B cells (eg, follicular lymphoma).
Mature T-cell lymphomas may display similar histologic, features but they can be quite heterogeneous with an infiltrate composed of one predominant cell type or a mixture of small, medium-sized, and large atypical lymphoid cells (on occasion with abundant clear cytoplasm) and a variable number of eosinophils, plasma cells, macrophages (including granulomas), and B cells. HLs most commonly efface the lymph node architecture with a nodular or diffuse infiltrate variably composed of reactive lymphocytes, granulocytes, macrophages, and plasma cells and usually a minority of large neoplastic cells (Hodgkin/Reed-Sternberg cells and/or lymphocyte predominant cells).
Once the H&E-stained slides are evaluated and a diagnosis of lymphoma is suspected or established, the hematopathologist will attempt to determine whether it has mature or immature features, and whether low- or high-grade morphologic characteristics are present. The maturity of lymphoid cells is generally determined by the nature of the chromatin, which if “fine” and homogeneous (with or without a conspicuous nucleolus) will usually, but not always, be considered immature, whereas clumped, vesicular or hyperchromatic chromatin is generally, but not always, associated with maturity. If the chromatin displays immature features, the differential diagnosis will mainly include B- and T-lymphoblastic lymphomas, but also blastoid variants of mature neoplasm such as mantle cell lymphoma, and follicular lymphoma, as well as high-grade B-cell lymphomas. Features associated with low-grade lymphomas (eg, follicular lymphoma, small lymphocytic lymphoma/chronic lymphocytic leukemia, marginal zone lymphoma, lymphoplasmacytic lymphoma) include small cell morphology, mature chromatin, absence of a significant number of mitoses or apoptotic cells, and a low proliferation index as shown by immunohistochemistry for Ki67. High-grade lymphomas, such as lymphoblastic lymphoma, Burkitt lymphoma, or certain large B-cell lymphomas tend to show opposite features, and some of the mature entities are frequently associated with MYC rearrangements. Of note, immature lymphomas tend to be clinically high grade, but not all clinically high-grade lymphomas are immature. Conversely, the majority of low-grade lymphomas are usually mature.
Immunophenotypic evaluation
Immunophenotypic evaluation is essential because the lineage of lymphoma cells cannot be determined by morphology alone. The immunophenotype is the combination of proteins/markers (eg, CD20, CD3, TdT) expressed by cells. Usually, it is evaluated by immunohistochemistry and/or flow cytometry, which help determine the proportion of lymphoid cells that express a certain marker and its location and intensity within the cells. While immunohistochemistry is normally performed on formalin-fixed and paraffin-embedded tissue, flow cytometry can be evaluated only on fresh unfixed tissue. Flow cytometry has the advantage over immunohistochemistry of being faster and better at simultaneously identifying coexpression of multiple markers on multiple cell populations. However, certain markers can only be evaluated by immunohistochemistry.
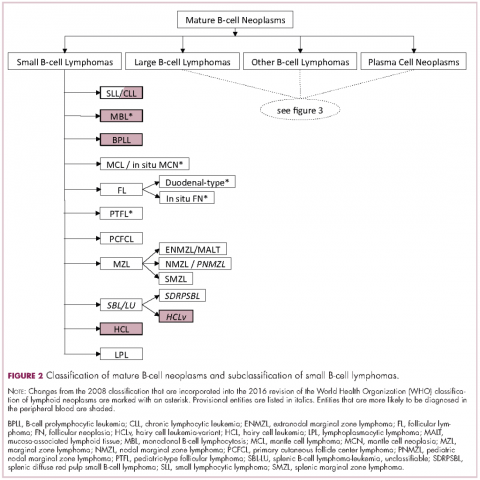
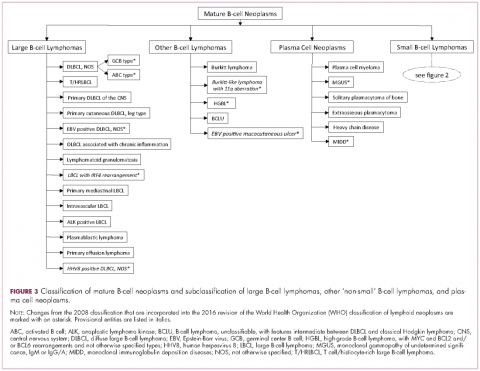
The immunophenotypic analysis will in most cases reveal whether the lymphomas is of B-, T- or NK-cell origin, and whether a lymphoma subtype associated immunophenotype is present. Typical pan B-cell antigens include PAX5, CD19, and CD79a (CD20 is less broadly expressed throughout B-cell differentiation, although it is usually evident in most mature B-cell lymphomas), and typical pan T-cell antigens include CD2, CD5, and CD7. The immature or mature nature of a lymphoma can also be confirmed by evaluation of the immunophenotype. Immature lymphomas commonly express one or more of TdT, CD10, or CD34; T-lymphoblastic lymphoma cells may also coexpress CD1a. The majority of NHLs and all HLs are derived from (or reflect) B cells at different stages of maturation. Mature B-cell lymphomas are the most common type of lymphoma and typically, but not always, express pan B-cell markers as well as surface membrane immunoglobulin, with the latter also most useful in assessing clonality via a determination of light chain restriction. Some mature B-cell lymphomas tend to acquire markers that are either never physiologically expressed by normal mature B cells (eg, cyclin D1 in mantle cell lymphoma, or BCL2 in germinal center B cells in follicular lymphoma) or only expressed in a minor fraction (eg, CD5 that is characteristically expressed in small lymphocytic and mantle cell lymphoma). The most common mature B-cell lymphomas include diffuse large B-cell lymphoma, follicular lymphoma, small lymphocytic lymphoma, mantle cell lymphoma, marginal zone lymphoma, Burkitt lymphoma, and lymphoplasmacytic lymphoma (Figures 2 and 3). Classical HLs are also lymphomas of B-cell origin that demonstrate diminished preservation of their B-cell immunophenotype (as evidenced by the dim expression of PAX5 but absence of most other pan B-cell antigens), expression of CD30, variable expression of CD15, and loss of CD45 (Figure 1). In contrast, nodular lymphocyte predominant HL shows a preserved B-cell immunophenotypic program and expression of CD45, typically without CD30 and CD15. Of note, the evaluation of the immunophenotype of the neoplastic cells in HL is routinely assessed by immunohistochemistry because most flow cytometry laboratories cannot reliably detect and characterize the low numbers of these cells.
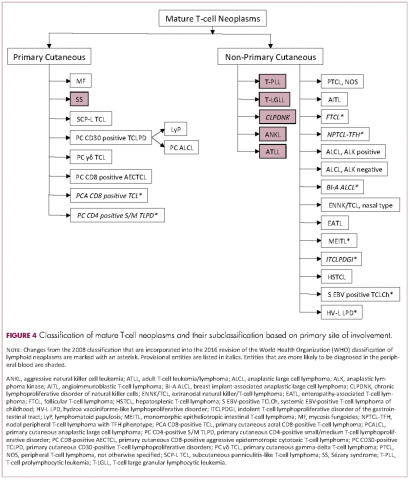
Mature T-cell lymphomas generally express one or more T-cell markers, and tend to display a T-helper (CD4-positive) or cytotoxic (CD8-positive) immunophenotype and may show loss of markers expressed by most normal T-cells (eg, CD5, CD7; Figure 4). However, a subset of them may express markers not commonly detected in normal T cells, such as ALK. NK-cell lymphomas lack surface CD3 (expressing only cytoplasmic CD3) and CD5 but express some pan T-cell antigens (such as CD2 and CD7) as well as CD16 and/or CD56.
Patients with primary or acquired immune dysfunction are at risk for development of lymphoma and other less clearly defined lymphoproliferative disorders, the majority of which are associated with infection of the lymphoid cells with Epstein-Barr virus (EBV). Therefore, evaluation with chromogenic in situ hybridization for an EBV-encoded early RNA (EBER1) is routinely performed in these cases; it is thus essential that the hematopathologist be informed of the altered immune system of the patient. If lymphoma develops, they may be morphologically similar to those that appear in immunocompetent patients, which specifically in the post-transplant setting are known as monomorphic post-transplant lymphoproliferative disorders (PTLD). If the PTLD does not meet the criteria for any of the recognized types of lymphoma, it may be best characterized as a polymorphic PTLD.
Once the lineage (B-, T-, or NK-cell) of the mature lymphoma has been established, the sum (and on occasion the gestalt) of the clinical, morphologic, immunophenotypic and other findings will be considered for the subclassification of the neoplasm.
Cytogenetic and molecular evaluation
If the morphologic and immunophenotypic analysis is inconclusive or nondiagnostic, then molecular and/or cytogenetic testing may further aid in the characterization of the process. Some of available molecular tests include analyses for the rearrangements of the variable region of the immunoglobulin (IG) or T-cell receptor (TCR) genes and for mutations on specific genes. The identification of specific mutations not only confirms the clonal nature of the process but, on occasion, it may also help subclassify the lymphoma, whereas IG or TCR rearrangement studies are used to establish whether a lymphoid expansion is polyclonal or monoclonal. The molecular findings should not be evaluated in isolation, because not all monoclonal rearrangements are diagnostic of lymphoma, and not all lymphomas will show a monoclonal rearrangement. Other methodologies that can aid in the identification of a clonal process or specific genetic abnormalities include metaphase cytogenetics (karyotyping) and fluorescence in situ hybridization (FISH). If any cytogenetic abnormalities are found in sufficient numbers (and constitutional abnormalities are excluded), their identification indicates the presence of a clonal process. Also, some cytogenetic abnormalities are characteristic of certain lymphomas. However, they may be neither 100% diagnostically sensitive nor diagnostically specific, for example, the hallmark t(14;18)/IGH-BCL2 is not present in all follicular lymphomas and not all lymphomas with this translocation are follicular lymphomas. Whereas FISH is generally performed on a minimum of 200 cells, compared with typically 20 metaphase by “conventional” karyotyping, and is therefore considered to have higher analytical sensitivity, it evaluates only for the presence or absence of the abnormality being investigated with a given set of probes, and therefore other abnormalities, if present, will not be identified. The value of FISH cytogenetic studies is perhaps best illustrated in the need to diagnose double hit lymphomas, amongst other scenarios. The detection of certain mutations can aid in the diagnosis of certain lymphomas, such as MYD88 in lymphoplasmacytic lymphoma, prognosis of others, such as in follicular lymphoma and identify pathways that may be precisely therapeutically targeted.
Final remarks
The diagnosis of lymphoma can be complex and usually requires the hematopathologist to integrate multiple parameters. The classification of lymphomas is not static, and new entities or variants are continuously described, and the facets of well-known ones refined. While such changes are often to the chagrin of hematologists/oncologists and hematopathologists alike, we should embrace the incorporation of nascent and typically cool data into our practice, as more therapeutically relevant entities are molded.
1. Siegel RL, Miller KD, Jemal A. Cancer Statistics, 2017. CA Cancer J Clin. 2017 ;67(1):7-30.
2. Swerdlow SH, Campo E, Harris NL, et al, eds. WHO classification of tumours of haematopoietic and lymphoid tissues. In: Bosman FT, Jaffe ES, Lakhani SR, Ohgaki H, eds. World Health Organization Classification of Tumours. Lyon, France: IARC; 2008.
3. Swerdlow SH, Campo E, Pileri SA, et al. The 2016 revision of the World Health Organization classification of lymphoid neoplasms. Blood. 2016 ;127(20):2375-2390.
Lymphomas constitute a very heterogeneous group of neoplasms with diverse clinical presentations, prognoses, and responses to therapy. Approximately 80,500 new cases of lymphoma are expected to be diagnosed in the United States in 2017, of which about one quarter will lead to the death of the patient.1 Perhaps more so than any other group of neoplasms, the diagnosis of lymphoma involves the integration of a multiplicity of clinical, histologic and immunophenotypic findings and, on occasion, cytogenetic and molecular results as well. An accurate diagnosis of lymphoma, usually rendered by hematopathologists, allows hematologists/oncologists to treat patients appropriately. Herein we will describe a simplified approach to the diagnosis and classification of lymphomas (Figure 1).
Lymphoma classification
Lymphomas are clonal neoplasms characterized by the expansion of abnormal lymphoid cells that may develop in any organ but commonly involve lymph nodes. The fourth edition of the World Health Organization (WHO) Classification of Tumours of Haematopoietic and Lymphoid tissues, published in 2008, is the official and most current guideline used for diagnosis of lymphoid neoplasms.2 The WHO scheme classifies lymphomas according to the type of cell from which they are derived (mature and immature B cells, T cells, or natural killer (NK) cells, findings determined by their morphology and immunophenotype) and their clinical, cytogenetic, and/or molecular features. This official classification is currently being updated3 and is expected to be published in full in 2017, at which time it is anticipated to include definitions for more than 70 distinct neoplasms.
Lymphomas are broadly and informally classified as Hodgkin lymphomas (HLs) and non-Hodgkin lymphomas (NHLs), based on the differences these two groups show in their clinical presentation, treatment, prognosis, and proportion of neoplastic cells, among others. NHLs are by far the most common type of lymphomas, accounting for approximately 90% of all new cases of lymphoma in the United States and 70% worldwide.1,2 NHLs are a very heterogeneous group of B-, T-, or NK-cell neoplasms that, in turn, can also be informally subclassified as low-grade (or indolent) or high-grade (or aggressive) according to their predicted clinical behavior. HLs are comparatively rare, less heterogeneous, uniformly of B-cell origin and, in the case of classical Hodgkin lymphoma, highly curable.1,2 It is beyond the scope of this manuscript to outline the features of each of the >70 specific entities, but the reader is referred elsewhere for more detail and encouraged to become familiarized with the complexity, challenges, and beauty of lymphoma diagnosis.2,3
Biopsy procedure
A correct diagnosis begins with an adequate biopsy procedure. It is essential that biopsy specimens for lymphoma evaluation be submitted fresh and unfixed, because some crucial analyses such as flow cytometry or conventional cytogenetics can only be performed on fresh tissue. Indeed, it is important for the hematologist/oncologist and/or surgeon and/or interventional radiologist to converse with the hematopathologist prior to and even during some procedures to ensure the correct processing of the specimen. Also, it is important to limit the compression of the specimen and the excessive use of cauterization during the biopsy procedure, both of which cause artifacts that may render impossible the interpretation of the histopathologic findings.
Given that the diagnosis of lymphoma is based not only on the cytologic details of the lymphoma cells but also on the architectural pattern with which they infiltrate an organ, the larger the biopsy specimen, the easier it will be for a hematopathologist to identify the pattern. In addition, excisional biopsies frequently contain more diagnostic tissue than needle core biopsies and this provides pathologists with the option to submit tissue fragments for ancillary tests that require unfixed tissue as noted above. Needle core biopsies of lymph nodes are increasingly being used because of their association with fewer complications and lower cost than excisional biopsies. However, needle core biopsies provide only a glimpse of the pattern of infiltration and may not be completely representative of the architecture. Therefore, excisional lymph node biopsies of lymph nodes are preferred over needle core biopsies, recognizing that in the setting of deeply seated lymph nodes, needle core biopsies may be the only or the best surgical option.
Clinical presentation
Accurate diagnosis of lymphoma cannot take place in a vacuum. The hematopathologist’s initial approach to the diagnosis of lymphoid processes in tissue biopsies should begin with a thorough review of the clinical history, although some pathology laboratories may not have immediate access to this information. The hematopathologist should evaluate factors such as age, gender, location of the tumor, symptomatology, medications, serology, and prior history of malignancy, immunosuppression or immunodeficiency in every case. Other important but frequently omitted parts of the clinical history are the patient’s occupation, history of exposure to animals, and the presence of tattoos, which may be associated with certain reactive lymphadenopathies.
Histomorphologic evaluation
Despite the plethora of new and increasingly sophisticated tools, histologic and morphologic analysis still remains the cornerstone of diagnosis in hematopathology. However, for the characterization of an increasing number of reactive and neoplastic lymphoid processes, hematopathologists may also require immunophenotypic, molecular, and cytogenetic tests for an accurate diagnosis. Upon review of the clinical information, a microscopic evaluation of the tissue submitted for processing by the histology laboratory will be performed. The results of concurrent flow cytometric evaluation (performed on fresh unfixed material) should also be available in most if not all cases before the H&E-stained slides are available for review. Upon receipt of H&E-stained slides, the hematopathologist will evaluate the quality of the submitted specimen, since many diagnostic difficulties stem from suboptimal techniques related to the biopsy procedure, fixation, processing, cutting, or staining (Figure 1). If deemed suitable for accurate diagnosis, a search for signs of preservation or disruption of the organ that was biopsied will follow. The identification of certain morphologic patterns aids the hematopathologist in answering the first question: “what organ is this and is this consistent with what is indicated on the requisition?” This is usually immediately followed by “is this sufficient and adequate material for a diagnosis?” and “is there any normal architecture?” If the architecture is not normal, “is this alteration due to a reactive or a neoplastic process?” If neoplastic, “is it lymphoma or a non-hematolymphoid neoplasm?”
Both reactive and neoplastic processes have variably unique morphologic features that if properly recognized, guide the subsequent testing. However, some reactive and neoplastic processes can present with overlapping features, and even after extensive immunophenotypic evaluation and the performance of ancillary studies, it may not be possible to conclusively determine its nature. If the lymph node architecture is altered or effaced, the predominant pattern of infiltration (eg, nodular, diffuse, interfollicular, intrasinusoidal) and the degree of alteration of the normal architecture is evaluated, usually at low magnification. When the presence of an infiltrate is recognized, its components must be characterized. If the infiltrate is composed of a homogeneous expansion of lymphoid cells that disrupts or replaces the normal lymphoid architecture, a lymphoma will be suspected or diagnosed. The pattern of distribution of the cells along with their individual morphologic characteristics (ie, size, nuclear shape, chromatin configuration, nucleoli, amount and hue of cytoplasm) are key factors for the diagnosis and classification of the lymphoma that will guide subsequent testing. The immunophenotypic analysis (by immunohistochemistry, flow cytometry or a combination of both) may confirm the reactive or neoplastic nature of the process, and its subclassification. B-cell lymphomas, in particular have variable and distinctive histologic features: as a diffuse infiltrate of large mature lymphoid cells (eg, diffuse large B-cell lymphoma), an expansion of immature lymphoid cells (lymphoblastic lymphoma), and a nodular infiltrate of small, intermediate and/or mature large B cells (eg, follicular lymphoma).
Mature T-cell lymphomas may display similar histologic, features but they can be quite heterogeneous with an infiltrate composed of one predominant cell type or a mixture of small, medium-sized, and large atypical lymphoid cells (on occasion with abundant clear cytoplasm) and a variable number of eosinophils, plasma cells, macrophages (including granulomas), and B cells. HLs most commonly efface the lymph node architecture with a nodular or diffuse infiltrate variably composed of reactive lymphocytes, granulocytes, macrophages, and plasma cells and usually a minority of large neoplastic cells (Hodgkin/Reed-Sternberg cells and/or lymphocyte predominant cells).
Once the H&E-stained slides are evaluated and a diagnosis of lymphoma is suspected or established, the hematopathologist will attempt to determine whether it has mature or immature features, and whether low- or high-grade morphologic characteristics are present. The maturity of lymphoid cells is generally determined by the nature of the chromatin, which if “fine” and homogeneous (with or without a conspicuous nucleolus) will usually, but not always, be considered immature, whereas clumped, vesicular or hyperchromatic chromatin is generally, but not always, associated with maturity. If the chromatin displays immature features, the differential diagnosis will mainly include B- and T-lymphoblastic lymphomas, but also blastoid variants of mature neoplasm such as mantle cell lymphoma, and follicular lymphoma, as well as high-grade B-cell lymphomas. Features associated with low-grade lymphomas (eg, follicular lymphoma, small lymphocytic lymphoma/chronic lymphocytic leukemia, marginal zone lymphoma, lymphoplasmacytic lymphoma) include small cell morphology, mature chromatin, absence of a significant number of mitoses or apoptotic cells, and a low proliferation index as shown by immunohistochemistry for Ki67. High-grade lymphomas, such as lymphoblastic lymphoma, Burkitt lymphoma, or certain large B-cell lymphomas tend to show opposite features, and some of the mature entities are frequently associated with MYC rearrangements. Of note, immature lymphomas tend to be clinically high grade, but not all clinically high-grade lymphomas are immature. Conversely, the majority of low-grade lymphomas are usually mature.
Immunophenotypic evaluation
Immunophenotypic evaluation is essential because the lineage of lymphoma cells cannot be determined by morphology alone. The immunophenotype is the combination of proteins/markers (eg, CD20, CD3, TdT) expressed by cells. Usually, it is evaluated by immunohistochemistry and/or flow cytometry, which help determine the proportion of lymphoid cells that express a certain marker and its location and intensity within the cells. While immunohistochemistry is normally performed on formalin-fixed and paraffin-embedded tissue, flow cytometry can be evaluated only on fresh unfixed tissue. Flow cytometry has the advantage over immunohistochemistry of being faster and better at simultaneously identifying coexpression of multiple markers on multiple cell populations. However, certain markers can only be evaluated by immunohistochemistry.
The immunophenotypic analysis will in most cases reveal whether the lymphomas is of B-, T- or NK-cell origin, and whether a lymphoma subtype associated immunophenotype is present. Typical pan B-cell antigens include PAX5, CD19, and CD79a (CD20 is less broadly expressed throughout B-cell differentiation, although it is usually evident in most mature B-cell lymphomas), and typical pan T-cell antigens include CD2, CD5, and CD7. The immature or mature nature of a lymphoma can also be confirmed by evaluation of the immunophenotype. Immature lymphomas commonly express one or more of TdT, CD10, or CD34; T-lymphoblastic lymphoma cells may also coexpress CD1a. The majority of NHLs and all HLs are derived from (or reflect) B cells at different stages of maturation. Mature B-cell lymphomas are the most common type of lymphoma and typically, but not always, express pan B-cell markers as well as surface membrane immunoglobulin, with the latter also most useful in assessing clonality via a determination of light chain restriction. Some mature B-cell lymphomas tend to acquire markers that are either never physiologically expressed by normal mature B cells (eg, cyclin D1 in mantle cell lymphoma, or BCL2 in germinal center B cells in follicular lymphoma) or only expressed in a minor fraction (eg, CD5 that is characteristically expressed in small lymphocytic and mantle cell lymphoma). The most common mature B-cell lymphomas include diffuse large B-cell lymphoma, follicular lymphoma, small lymphocytic lymphoma, mantle cell lymphoma, marginal zone lymphoma, Burkitt lymphoma, and lymphoplasmacytic lymphoma (Figures 2 and 3). Classical HLs are also lymphomas of B-cell origin that demonstrate diminished preservation of their B-cell immunophenotype (as evidenced by the dim expression of PAX5 but absence of most other pan B-cell antigens), expression of CD30, variable expression of CD15, and loss of CD45 (Figure 1). In contrast, nodular lymphocyte predominant HL shows a preserved B-cell immunophenotypic program and expression of CD45, typically without CD30 and CD15. Of note, the evaluation of the immunophenotype of the neoplastic cells in HL is routinely assessed by immunohistochemistry because most flow cytometry laboratories cannot reliably detect and characterize the low numbers of these cells.
Mature T-cell lymphomas generally express one or more T-cell markers, and tend to display a T-helper (CD4-positive) or cytotoxic (CD8-positive) immunophenotype and may show loss of markers expressed by most normal T-cells (eg, CD5, CD7; Figure 4). However, a subset of them may express markers not commonly detected in normal T cells, such as ALK. NK-cell lymphomas lack surface CD3 (expressing only cytoplasmic CD3) and CD5 but express some pan T-cell antigens (such as CD2 and CD7) as well as CD16 and/or CD56.
Patients with primary or acquired immune dysfunction are at risk for development of lymphoma and other less clearly defined lymphoproliferative disorders, the majority of which are associated with infection of the lymphoid cells with Epstein-Barr virus (EBV). Therefore, evaluation with chromogenic in situ hybridization for an EBV-encoded early RNA (EBER1) is routinely performed in these cases; it is thus essential that the hematopathologist be informed of the altered immune system of the patient. If lymphoma develops, they may be morphologically similar to those that appear in immunocompetent patients, which specifically in the post-transplant setting are known as monomorphic post-transplant lymphoproliferative disorders (PTLD). If the PTLD does not meet the criteria for any of the recognized types of lymphoma, it may be best characterized as a polymorphic PTLD.
Once the lineage (B-, T-, or NK-cell) of the mature lymphoma has been established, the sum (and on occasion the gestalt) of the clinical, morphologic, immunophenotypic and other findings will be considered for the subclassification of the neoplasm.
Cytogenetic and molecular evaluation
If the morphologic and immunophenotypic analysis is inconclusive or nondiagnostic, then molecular and/or cytogenetic testing may further aid in the characterization of the process. Some of available molecular tests include analyses for the rearrangements of the variable region of the immunoglobulin (IG) or T-cell receptor (TCR) genes and for mutations on specific genes. The identification of specific mutations not only confirms the clonal nature of the process but, on occasion, it may also help subclassify the lymphoma, whereas IG or TCR rearrangement studies are used to establish whether a lymphoid expansion is polyclonal or monoclonal. The molecular findings should not be evaluated in isolation, because not all monoclonal rearrangements are diagnostic of lymphoma, and not all lymphomas will show a monoclonal rearrangement. Other methodologies that can aid in the identification of a clonal process or specific genetic abnormalities include metaphase cytogenetics (karyotyping) and fluorescence in situ hybridization (FISH). If any cytogenetic abnormalities are found in sufficient numbers (and constitutional abnormalities are excluded), their identification indicates the presence of a clonal process. Also, some cytogenetic abnormalities are characteristic of certain lymphomas. However, they may be neither 100% diagnostically sensitive nor diagnostically specific, for example, the hallmark t(14;18)/IGH-BCL2 is not present in all follicular lymphomas and not all lymphomas with this translocation are follicular lymphomas. Whereas FISH is generally performed on a minimum of 200 cells, compared with typically 20 metaphase by “conventional” karyotyping, and is therefore considered to have higher analytical sensitivity, it evaluates only for the presence or absence of the abnormality being investigated with a given set of probes, and therefore other abnormalities, if present, will not be identified. The value of FISH cytogenetic studies is perhaps best illustrated in the need to diagnose double hit lymphomas, amongst other scenarios. The detection of certain mutations can aid in the diagnosis of certain lymphomas, such as MYD88 in lymphoplasmacytic lymphoma, prognosis of others, such as in follicular lymphoma and identify pathways that may be precisely therapeutically targeted.
Final remarks
The diagnosis of lymphoma can be complex and usually requires the hematopathologist to integrate multiple parameters. The classification of lymphomas is not static, and new entities or variants are continuously described, and the facets of well-known ones refined. While such changes are often to the chagrin of hematologists/oncologists and hematopathologists alike, we should embrace the incorporation of nascent and typically cool data into our practice, as more therapeutically relevant entities are molded.
Lymphomas constitute a very heterogeneous group of neoplasms with diverse clinical presentations, prognoses, and responses to therapy. Approximately 80,500 new cases of lymphoma are expected to be diagnosed in the United States in 2017, of which about one quarter will lead to the death of the patient.1 Perhaps more so than any other group of neoplasms, the diagnosis of lymphoma involves the integration of a multiplicity of clinical, histologic and immunophenotypic findings and, on occasion, cytogenetic and molecular results as well. An accurate diagnosis of lymphoma, usually rendered by hematopathologists, allows hematologists/oncologists to treat patients appropriately. Herein we will describe a simplified approach to the diagnosis and classification of lymphomas (Figure 1).
Lymphoma classification
Lymphomas are clonal neoplasms characterized by the expansion of abnormal lymphoid cells that may develop in any organ but commonly involve lymph nodes. The fourth edition of the World Health Organization (WHO) Classification of Tumours of Haematopoietic and Lymphoid tissues, published in 2008, is the official and most current guideline used for diagnosis of lymphoid neoplasms.2 The WHO scheme classifies lymphomas according to the type of cell from which they are derived (mature and immature B cells, T cells, or natural killer (NK) cells, findings determined by their morphology and immunophenotype) and their clinical, cytogenetic, and/or molecular features. This official classification is currently being updated3 and is expected to be published in full in 2017, at which time it is anticipated to include definitions for more than 70 distinct neoplasms.
Lymphomas are broadly and informally classified as Hodgkin lymphomas (HLs) and non-Hodgkin lymphomas (NHLs), based on the differences these two groups show in their clinical presentation, treatment, prognosis, and proportion of neoplastic cells, among others. NHLs are by far the most common type of lymphomas, accounting for approximately 90% of all new cases of lymphoma in the United States and 70% worldwide.1,2 NHLs are a very heterogeneous group of B-, T-, or NK-cell neoplasms that, in turn, can also be informally subclassified as low-grade (or indolent) or high-grade (or aggressive) according to their predicted clinical behavior. HLs are comparatively rare, less heterogeneous, uniformly of B-cell origin and, in the case of classical Hodgkin lymphoma, highly curable.1,2 It is beyond the scope of this manuscript to outline the features of each of the >70 specific entities, but the reader is referred elsewhere for more detail and encouraged to become familiarized with the complexity, challenges, and beauty of lymphoma diagnosis.2,3
Biopsy procedure
A correct diagnosis begins with an adequate biopsy procedure. It is essential that biopsy specimens for lymphoma evaluation be submitted fresh and unfixed, because some crucial analyses such as flow cytometry or conventional cytogenetics can only be performed on fresh tissue. Indeed, it is important for the hematologist/oncologist and/or surgeon and/or interventional radiologist to converse with the hematopathologist prior to and even during some procedures to ensure the correct processing of the specimen. Also, it is important to limit the compression of the specimen and the excessive use of cauterization during the biopsy procedure, both of which cause artifacts that may render impossible the interpretation of the histopathologic findings.
Given that the diagnosis of lymphoma is based not only on the cytologic details of the lymphoma cells but also on the architectural pattern with which they infiltrate an organ, the larger the biopsy specimen, the easier it will be for a hematopathologist to identify the pattern. In addition, excisional biopsies frequently contain more diagnostic tissue than needle core biopsies and this provides pathologists with the option to submit tissue fragments for ancillary tests that require unfixed tissue as noted above. Needle core biopsies of lymph nodes are increasingly being used because of their association with fewer complications and lower cost than excisional biopsies. However, needle core biopsies provide only a glimpse of the pattern of infiltration and may not be completely representative of the architecture. Therefore, excisional lymph node biopsies of lymph nodes are preferred over needle core biopsies, recognizing that in the setting of deeply seated lymph nodes, needle core biopsies may be the only or the best surgical option.
Clinical presentation
Accurate diagnosis of lymphoma cannot take place in a vacuum. The hematopathologist’s initial approach to the diagnosis of lymphoid processes in tissue biopsies should begin with a thorough review of the clinical history, although some pathology laboratories may not have immediate access to this information. The hematopathologist should evaluate factors such as age, gender, location of the tumor, symptomatology, medications, serology, and prior history of malignancy, immunosuppression or immunodeficiency in every case. Other important but frequently omitted parts of the clinical history are the patient’s occupation, history of exposure to animals, and the presence of tattoos, which may be associated with certain reactive lymphadenopathies.
Histomorphologic evaluation
Despite the plethora of new and increasingly sophisticated tools, histologic and morphologic analysis still remains the cornerstone of diagnosis in hematopathology. However, for the characterization of an increasing number of reactive and neoplastic lymphoid processes, hematopathologists may also require immunophenotypic, molecular, and cytogenetic tests for an accurate diagnosis. Upon review of the clinical information, a microscopic evaluation of the tissue submitted for processing by the histology laboratory will be performed. The results of concurrent flow cytometric evaluation (performed on fresh unfixed material) should also be available in most if not all cases before the H&E-stained slides are available for review. Upon receipt of H&E-stained slides, the hematopathologist will evaluate the quality of the submitted specimen, since many diagnostic difficulties stem from suboptimal techniques related to the biopsy procedure, fixation, processing, cutting, or staining (Figure 1). If deemed suitable for accurate diagnosis, a search for signs of preservation or disruption of the organ that was biopsied will follow. The identification of certain morphologic patterns aids the hematopathologist in answering the first question: “what organ is this and is this consistent with what is indicated on the requisition?” This is usually immediately followed by “is this sufficient and adequate material for a diagnosis?” and “is there any normal architecture?” If the architecture is not normal, “is this alteration due to a reactive or a neoplastic process?” If neoplastic, “is it lymphoma or a non-hematolymphoid neoplasm?”
Both reactive and neoplastic processes have variably unique morphologic features that if properly recognized, guide the subsequent testing. However, some reactive and neoplastic processes can present with overlapping features, and even after extensive immunophenotypic evaluation and the performance of ancillary studies, it may not be possible to conclusively determine its nature. If the lymph node architecture is altered or effaced, the predominant pattern of infiltration (eg, nodular, diffuse, interfollicular, intrasinusoidal) and the degree of alteration of the normal architecture is evaluated, usually at low magnification. When the presence of an infiltrate is recognized, its components must be characterized. If the infiltrate is composed of a homogeneous expansion of lymphoid cells that disrupts or replaces the normal lymphoid architecture, a lymphoma will be suspected or diagnosed. The pattern of distribution of the cells along with their individual morphologic characteristics (ie, size, nuclear shape, chromatin configuration, nucleoli, amount and hue of cytoplasm) are key factors for the diagnosis and classification of the lymphoma that will guide subsequent testing. The immunophenotypic analysis (by immunohistochemistry, flow cytometry or a combination of both) may confirm the reactive or neoplastic nature of the process, and its subclassification. B-cell lymphomas, in particular have variable and distinctive histologic features: as a diffuse infiltrate of large mature lymphoid cells (eg, diffuse large B-cell lymphoma), an expansion of immature lymphoid cells (lymphoblastic lymphoma), and a nodular infiltrate of small, intermediate and/or mature large B cells (eg, follicular lymphoma).
Mature T-cell lymphomas may display similar histologic, features but they can be quite heterogeneous with an infiltrate composed of one predominant cell type or a mixture of small, medium-sized, and large atypical lymphoid cells (on occasion with abundant clear cytoplasm) and a variable number of eosinophils, plasma cells, macrophages (including granulomas), and B cells. HLs most commonly efface the lymph node architecture with a nodular or diffuse infiltrate variably composed of reactive lymphocytes, granulocytes, macrophages, and plasma cells and usually a minority of large neoplastic cells (Hodgkin/Reed-Sternberg cells and/or lymphocyte predominant cells).
Once the H&E-stained slides are evaluated and a diagnosis of lymphoma is suspected or established, the hematopathologist will attempt to determine whether it has mature or immature features, and whether low- or high-grade morphologic characteristics are present. The maturity of lymphoid cells is generally determined by the nature of the chromatin, which if “fine” and homogeneous (with or without a conspicuous nucleolus) will usually, but not always, be considered immature, whereas clumped, vesicular or hyperchromatic chromatin is generally, but not always, associated with maturity. If the chromatin displays immature features, the differential diagnosis will mainly include B- and T-lymphoblastic lymphomas, but also blastoid variants of mature neoplasm such as mantle cell lymphoma, and follicular lymphoma, as well as high-grade B-cell lymphomas. Features associated with low-grade lymphomas (eg, follicular lymphoma, small lymphocytic lymphoma/chronic lymphocytic leukemia, marginal zone lymphoma, lymphoplasmacytic lymphoma) include small cell morphology, mature chromatin, absence of a significant number of mitoses or apoptotic cells, and a low proliferation index as shown by immunohistochemistry for Ki67. High-grade lymphomas, such as lymphoblastic lymphoma, Burkitt lymphoma, or certain large B-cell lymphomas tend to show opposite features, and some of the mature entities are frequently associated with MYC rearrangements. Of note, immature lymphomas tend to be clinically high grade, but not all clinically high-grade lymphomas are immature. Conversely, the majority of low-grade lymphomas are usually mature.
Immunophenotypic evaluation
Immunophenotypic evaluation is essential because the lineage of lymphoma cells cannot be determined by morphology alone. The immunophenotype is the combination of proteins/markers (eg, CD20, CD3, TdT) expressed by cells. Usually, it is evaluated by immunohistochemistry and/or flow cytometry, which help determine the proportion of lymphoid cells that express a certain marker and its location and intensity within the cells. While immunohistochemistry is normally performed on formalin-fixed and paraffin-embedded tissue, flow cytometry can be evaluated only on fresh unfixed tissue. Flow cytometry has the advantage over immunohistochemistry of being faster and better at simultaneously identifying coexpression of multiple markers on multiple cell populations. However, certain markers can only be evaluated by immunohistochemistry.
The immunophenotypic analysis will in most cases reveal whether the lymphomas is of B-, T- or NK-cell origin, and whether a lymphoma subtype associated immunophenotype is present. Typical pan B-cell antigens include PAX5, CD19, and CD79a (CD20 is less broadly expressed throughout B-cell differentiation, although it is usually evident in most mature B-cell lymphomas), and typical pan T-cell antigens include CD2, CD5, and CD7. The immature or mature nature of a lymphoma can also be confirmed by evaluation of the immunophenotype. Immature lymphomas commonly express one or more of TdT, CD10, or CD34; T-lymphoblastic lymphoma cells may also coexpress CD1a. The majority of NHLs and all HLs are derived from (or reflect) B cells at different stages of maturation. Mature B-cell lymphomas are the most common type of lymphoma and typically, but not always, express pan B-cell markers as well as surface membrane immunoglobulin, with the latter also most useful in assessing clonality via a determination of light chain restriction. Some mature B-cell lymphomas tend to acquire markers that are either never physiologically expressed by normal mature B cells (eg, cyclin D1 in mantle cell lymphoma, or BCL2 in germinal center B cells in follicular lymphoma) or only expressed in a minor fraction (eg, CD5 that is characteristically expressed in small lymphocytic and mantle cell lymphoma). The most common mature B-cell lymphomas include diffuse large B-cell lymphoma, follicular lymphoma, small lymphocytic lymphoma, mantle cell lymphoma, marginal zone lymphoma, Burkitt lymphoma, and lymphoplasmacytic lymphoma (Figures 2 and 3). Classical HLs are also lymphomas of B-cell origin that demonstrate diminished preservation of their B-cell immunophenotype (as evidenced by the dim expression of PAX5 but absence of most other pan B-cell antigens), expression of CD30, variable expression of CD15, and loss of CD45 (Figure 1). In contrast, nodular lymphocyte predominant HL shows a preserved B-cell immunophenotypic program and expression of CD45, typically without CD30 and CD15. Of note, the evaluation of the immunophenotype of the neoplastic cells in HL is routinely assessed by immunohistochemistry because most flow cytometry laboratories cannot reliably detect and characterize the low numbers of these cells.
Mature T-cell lymphomas generally express one or more T-cell markers, and tend to display a T-helper (CD4-positive) or cytotoxic (CD8-positive) immunophenotype and may show loss of markers expressed by most normal T-cells (eg, CD5, CD7; Figure 4). However, a subset of them may express markers not commonly detected in normal T cells, such as ALK. NK-cell lymphomas lack surface CD3 (expressing only cytoplasmic CD3) and CD5 but express some pan T-cell antigens (such as CD2 and CD7) as well as CD16 and/or CD56.
Patients with primary or acquired immune dysfunction are at risk for development of lymphoma and other less clearly defined lymphoproliferative disorders, the majority of which are associated with infection of the lymphoid cells with Epstein-Barr virus (EBV). Therefore, evaluation with chromogenic in situ hybridization for an EBV-encoded early RNA (EBER1) is routinely performed in these cases; it is thus essential that the hematopathologist be informed of the altered immune system of the patient. If lymphoma develops, they may be morphologically similar to those that appear in immunocompetent patients, which specifically in the post-transplant setting are known as monomorphic post-transplant lymphoproliferative disorders (PTLD). If the PTLD does not meet the criteria for any of the recognized types of lymphoma, it may be best characterized as a polymorphic PTLD.
Once the lineage (B-, T-, or NK-cell) of the mature lymphoma has been established, the sum (and on occasion the gestalt) of the clinical, morphologic, immunophenotypic and other findings will be considered for the subclassification of the neoplasm.
Cytogenetic and molecular evaluation
If the morphologic and immunophenotypic analysis is inconclusive or nondiagnostic, then molecular and/or cytogenetic testing may further aid in the characterization of the process. Some of available molecular tests include analyses for the rearrangements of the variable region of the immunoglobulin (IG) or T-cell receptor (TCR) genes and for mutations on specific genes. The identification of specific mutations not only confirms the clonal nature of the process but, on occasion, it may also help subclassify the lymphoma, whereas IG or TCR rearrangement studies are used to establish whether a lymphoid expansion is polyclonal or monoclonal. The molecular findings should not be evaluated in isolation, because not all monoclonal rearrangements are diagnostic of lymphoma, and not all lymphomas will show a monoclonal rearrangement. Other methodologies that can aid in the identification of a clonal process or specific genetic abnormalities include metaphase cytogenetics (karyotyping) and fluorescence in situ hybridization (FISH). If any cytogenetic abnormalities are found in sufficient numbers (and constitutional abnormalities are excluded), their identification indicates the presence of a clonal process. Also, some cytogenetic abnormalities are characteristic of certain lymphomas. However, they may be neither 100% diagnostically sensitive nor diagnostically specific, for example, the hallmark t(14;18)/IGH-BCL2 is not present in all follicular lymphomas and not all lymphomas with this translocation are follicular lymphomas. Whereas FISH is generally performed on a minimum of 200 cells, compared with typically 20 metaphase by “conventional” karyotyping, and is therefore considered to have higher analytical sensitivity, it evaluates only for the presence or absence of the abnormality being investigated with a given set of probes, and therefore other abnormalities, if present, will not be identified. The value of FISH cytogenetic studies is perhaps best illustrated in the need to diagnose double hit lymphomas, amongst other scenarios. The detection of certain mutations can aid in the diagnosis of certain lymphomas, such as MYD88 in lymphoplasmacytic lymphoma, prognosis of others, such as in follicular lymphoma and identify pathways that may be precisely therapeutically targeted.
Final remarks
The diagnosis of lymphoma can be complex and usually requires the hematopathologist to integrate multiple parameters. The classification of lymphomas is not static, and new entities or variants are continuously described, and the facets of well-known ones refined. While such changes are often to the chagrin of hematologists/oncologists and hematopathologists alike, we should embrace the incorporation of nascent and typically cool data into our practice, as more therapeutically relevant entities are molded.
1. Siegel RL, Miller KD, Jemal A. Cancer Statistics, 2017. CA Cancer J Clin. 2017 ;67(1):7-30.
2. Swerdlow SH, Campo E, Harris NL, et al, eds. WHO classification of tumours of haematopoietic and lymphoid tissues. In: Bosman FT, Jaffe ES, Lakhani SR, Ohgaki H, eds. World Health Organization Classification of Tumours. Lyon, France: IARC; 2008.
3. Swerdlow SH, Campo E, Pileri SA, et al. The 2016 revision of the World Health Organization classification of lymphoid neoplasms. Blood. 2016 ;127(20):2375-2390.
1. Siegel RL, Miller KD, Jemal A. Cancer Statistics, 2017. CA Cancer J Clin. 2017 ;67(1):7-30.
2. Swerdlow SH, Campo E, Harris NL, et al, eds. WHO classification of tumours of haematopoietic and lymphoid tissues. In: Bosman FT, Jaffe ES, Lakhani SR, Ohgaki H, eds. World Health Organization Classification of Tumours. Lyon, France: IARC; 2008.
3. Swerdlow SH, Campo E, Pileri SA, et al. The 2016 revision of the World Health Organization classification of lymphoid neoplasms. Blood. 2016 ;127(20):2375-2390.
Liquid gold: blood-based biopsies make headway
Pathologic and, increasingly, molecular analysis of tumor tissue biopsies is the gold standard in initial diagnosis of cancer, but liquid biopsies, which analyze tumor-derived material circulating in the bloodstream are gaining traction. Here, we discuss the current state of development of this complementary and potentially alternative approach to tumor analysis.
Liquid biopsy gaining traction
Biopsies enable oncologists to gather information about a potential or established tumor, including confirmation of the presence of cancerous tissue and determination of its histological characteristics, such as tumor grade and stage, as well as its molecular features, such as the presence of certain gene mutations. Ultimately, this information can be put to use in determining the most appropriate course of treatment.
The current gold standard is a tissue biopsy that typically involves an invasive procedure to permit the collection of a piece of tumor tissue. Yet, tissue biopsies are not always feasible because of the location of the tumor or the poor performance status of many patients with advanced disease. They also provide only a snapshot of the disease at the time at which they were taken and don’t necessarily reflect the genetic heterogeneity or evolution of a tumor over time.
The detection of components that are derived from the tumor circulating in the blood of cancer patients had fueled the idea of blood-based diagnostics in oncology – so-called liquid biopsies. These have rapidly gained traction in the past several decades as a less expensive (the cost of performing genomic analyses on blood samples is at least an order of magnitude less than on tissue samples), less invasive (requiring only a simple blood draw) alternative source of information about tumors.1
As researchers have refined the ability to exploit liquid biopsies, commercial interest has been piqued. More than 35 companies within the United States alone are developing liquid biopsies, and it’s easy to see why with a market projected to be in the many billions of dollars.2
Seeking out tumor clues in the blood
Liquid biopsies consist of a 10-15 mL blood sample drawn into a tube that contains an anticoagulant and it can contain several different types of tumor-associated material. Thus far, two components – circulating tumor cells (CTCs) and circulating tumor DNA (ctDNA) – have formed the cornerstone of liquid biopsies. At present, it is not clear whether these components are released randomly, as a by-product of tumor cell death or if they are released as part of a specific biologic process, such as for the colonization of metastatic sites. It reality, it may be a little of both, and active dissemination may be particularly relevant for CTCs, among which are postulated to be a population of cancer stem cells that can initiate distant metastases.3,4
The discovery of CTCs dates back to the 1860s, when cells that were morphologically identical to the tumor were identified in the blood of a patient with metastatic cancer. Their potential significance was not fully realized until a few decades ago, when they were found to exist from early on in the course of disease.3,4
CTCs, which can be either single cells or clusters of cells known as microemboli, have a short half-life in the bloodstream – less than 2 ½ hours – and are also extremely rare (1 mL of blood contains 1-10 CTCs) against a background of many millions of normal cells. Thus the detection and isolation of CTCs presents a significant challenge. More than 40 different platforms are being developed for the isolation and enrichment of CTCs. For the most part, these use a method called positive selection to pick out CTCs.1,3,4
Positive selection exploits the biological or physical properties that are specific to CTCs and absent in normal cells, for example, the presence of a specific tumor-associated antigen on their surface or differences in size, density or electric charge. The limitations of this method are that, not only do you need to know something about CTCs to begin to understand what makes them truly unique and ensure only isolation of CTCs, but their phenotype is also thought to be continually changing.1,3,4
In recent years, the focus has shifted toward technologies that use negative depletion, meaning that they target the other types of cells in the blood sample and filter those away until only the CTCs are left behind. The most advanced are devices that use microfluidic technology to sort the cells, such as the CTC-iChip system being developed by researchers at Massachusetts General Hospital in Boston.5
ctDNA consists of small fragments of nucleic acids that are not contained within a cell or associated with cell fragments and is thought to be present in 50%-90% of patients, depending on the type of cancer they have. ctDNA has a similarly short half-life in the circulation to CTCs and, like CTCs, ctDNA is present at very low levels in the bloodstream. Although levels of ctDNA have been shown to increase with increasing tumor burden, it is still often obscured by the presence of other cell-free DNA derived from non-tumor cells.
ctDNA can be distinguished from other cell-free DNA by the presence of somatic mutations and a number of highly sensitive methods have been developed to detect them, including the amplification-refractory mutation system (ARMS); digital polymerase chain reaction; and the beads, emulsification, amplification, and magnetics (BEAMing) system. Next-generation sequencing technologies, including tagged-amplicon deep sequencing (TAm-Seq), the Safe-Sequencing System (Safe-SeqS), and cancer personalized profiling by deep sequencing (CAPP-seq), can also be used and the race for ever more sensitive analytical tools is ongoing.1,3,4,6
Applying liquid biopsies now and in the future
There are a plethora of potential applications for liquid biopsies3,7 (Figure 1), and probably the most exciting among them is the potential for screening for and early detection of cancer. The fact that ctDNA and CTCs have both been shown to be present from the earliest stages of disease has sparked interest in the possibility of developing simple blood tests to identify tumors before they become detectable by other methods and at a point at which they may be curable.
Given that both are present at such low levels within the circulation and are particularly sparse at earlier stages of disease, current technologies may lack the specificity and sensitivity for this application at present. However, numerous clinical trials are ongoing.
For CTCs, simple enumeration has been the most extensively investigated application to date. Numerous studies have shown that the number of CTCs in the bloodstream has prognostic significance in various different tumor types. Three such studies led to the first regulatory approval for a CTC detection system (Table 1 and Table 2).8-10
One area in which liquid biopsies could really come into their own is in providing more real-time analysis of tumors. This is something that has proven particularly challenging with tissue biopsies because repeating these invasive procedures is problematic. But the ease of repeat blood draws means that serial liquid biopsies could be performed and might offer the possibility of monitoring disease progression and evolution over the course of disease and particularly in response to treatment.
Indeed, studies have shown that in addition to baseline CTC counts, changes in CTC number during treatment are also prognostic. There was improved survival among patients whose CTC counts decreased below a threshold value during treatment and vice versa. This is also an approved use for CellSearch though at present it is not widely clinically implemented.12
Clinical utility remains elusive
The ultimate goal would be for liquid biopsies to have an impact on treatment decisions, allowing oncologists to change management strategy based on predicted sensitivity or resistance to therapy, so-called clinical utility. Thus far, clinical utility has proved elusive, though liquid biopsies using ctDNA to evaluate tumor genotype have come closest.
The Cobas EGFR Mutation Test v2 recently became the first ctDNA-based liquid biopsy to receive regulatory approval. It was approved as a companion diagnostic to identify patients with advanced non–small-cell lung cancer (NSCLC) who have specific mutations in the epidermal growth factor receptor (EGFR) gene and are therefore eligible for treatment with the EGFR inhibitor erlotinib.13
Approval was based on comparison of EGFR mutation identification rates using plasma ctDNA samples and tumor tissue samples from patients enrolled in the phase 3 ENSURE trial, which compared the efficacy of erlotinib with chemotherapy as first-line therapy in patients with advanced NSCLC. Of the 217 patients enrolled in the trial, 98.6% of patients had both tumor biopsy and plasma ctDNA samples available for testing. Concordance between the two types of biopsy in identifying patients with EGFR mutations was high and patients with EGFR positivity according to liquid biopsy results demonstrated improved progression-free survival when treated with erlotinib.14
The results of a large-scale genomic analysis of various different types of tumors using ctDNA were also recently presented at the 2016 American Society of Clinical Oncology meeting. Blood samples from more than 15,000 patients with 50 different tumor types, including advanced lung cancer (37%), breast cancer (14%), and CRC (10%), were collected and compared with either available tumor biopsy samples from the same cases (n = 398) or, in the majority of cases, with The Cancer Genome Atlas database, which uses tumor biopsies to perform genome-wide sequencing studies. Both types of biopsy revealed very similar mutation patterns when the Guardant360 next-generation sequencing test, which targets 70 genes, was applied. In particular, when EGFR, BRAF, KRAS, ALK, RET, and ROS1 mutations were identified by tumor tissue biopsy, the same mutations were reported in 94%-100% of plasma samples.15
Studies of the clinical utility of ctDNA and CTCs are among ongoing clinical trials of liquid biopsies (Tables 2 and 3). The potential for using CTCs to guide treatment decisions has become particularly relevant in breast cancer in light of results showing that patients with primary tumors that are negative for human epidermal growth factor receptor 2 (HER2) amplification, an important biomarker in breast cancer, may have CTCs that are HER2-positive, in up to 30% of cases. These patients may therefore still benefit from HER2-targeted therapy.16
The DETECT studies are the first phase 3 trials in which treatment decisions are being based on the phenotypic characteristics of CTCs. DETECT III (NCT01619111) is comparing lapatinib in combination with standard therapy with standard therapy alone in patients with HER2-negative metastatic breast cancer who have HER2-positive CTCs, whereas DETECT IV (NCT02035813) is enrolling patients with HER2-negative, hormone receptor-positive metastatic breast cancer and persistent HER2-negative CTCs to receive standard endocrine therapy and the mammalian target of rapamycin inhibitor everolimus.
Other targets and sources for liquid biopsy
Another approach to liquid biopsies that is also beginning to take off is to collect tumor-derived exosomes from the bloodstream. Exosomes are tiny, fluid-filled, membrane-bound sacks that bud off from the surface of a cell to expel waste or to transport cargo from one cell to another. DNA, RNA, and protein can be extracted from tumor-derived exosomes and could also serve as molecular biomarkers relating to the cancer cells from which they came.6,7
Exosome Diagnostics is bringing the first exosome-based diagnostic tests to the market and recently teamed up with Amgen for the development of these liquid biopsies.17 In January 2016, they launched ExoDx Lung (ALK), for detection of EML4-ALK gene fusions in patients with NSCLC, using a proprietary platform for the isolation of RNA from exosomes. Data that was presented at several different conferences in 2015 demonstrated a sensitivity of 88% and specificity of 100% for this diagnostic when compared with tissue ALK status in NSCLC patients receiving a second-generation ALK inhibitor following progression on prior ALK inhibitor therapy.18
In September, they subsequently announced the launch of a test that analyses genetic information from exosomes collected from a urine sample taken from prostate cancer patients. Using a 3-gene signature, in combination with a proprietary algorithm, this diagnostic generates a score assessing a prostate cancer patient’s risk for higher grade, more aggressive disease. It is designed to complement the prostate-specific antigen score and has demonstrated accuracy in ruling out the presence of high-grade cancer before an initial biopsy in more than 1,
1. Lennon NK, Adalsteinsson VA, Gabriel SB. Technological considerations for genome-guided diagnosis and management of cancer. Genome Med. 2016;8:112.
2. MIT Technology Review website. Liquid biopsy: fast DNA-sequencing machines are leading to simple blood tests for cancer. https://www.technologyreview.com/s/534991/liquid-biopsy/. Published 2015. Accessed December 19, 2016.
3. Alix-Panabières C and Pantel K. Clinical applications of circulating tumor cells and circulating tumor DNA as liquid biopsy. Cancer Discov. 2016;6(5):479-491.
4. Calabuig-Farinãs S, Jantus-Lewintre E, Herreros-Pomares A, Camps C. Circulating tumor cells versus circulating tumor DNA in lung cancer – which one will win? Transl Lung Cancer Res. 2016;5(5):466-482.
5. Karabacak, NM, Spuhler PS, Fachin F, et al. Microfluidic, marker-free isolation of circulating tumor cells from blood samples. Nat Protoc. 2014;9:694-710.
6. Buder A, Tomuta C, and Filipits M. The potential of liquid biopsies. Curr Opin Oncol. 2016;28:130-134.
7. Hofman P, Popper HH. Pathologists and liquid biopsies: to be or not to be? Virchows Arch. 2016;469:601-609.
8. Bidard FC, Peeters DJ, Fehm T, et al. Clinical validity of circulating tumor cells in patients with metastatic breast cancer: a pooled analysis of individual patient data. Lancet Oncol. 2014;15(4):406-414.
9. de Bono JS, Scher HI, Montgomery RB, et al. Circulating tumor cells predict survival benefit from treatment in metastatic castration-resistant prostate cancer. Clin Cancer Res. 2008;14(19):6302-6309.
10. Cohen SJ, Punt CJ, Iannotti N, et al. Relationship of circulating tumor cells to tumor response, progression-free survival, and overall survival in patients with metastatic colorectal cancer. J Clin Oncol. 2008;26(19):3213-3221.
11. CellSearch Web site. What is the CELLSEARCH® System? https://www.cellsearchctc.com/product-systems-overview/cellsearch-system-overview. Last updated December 5th, 2016. Accessed online December 19th, 2016.
12. CellSearch Web site [advertisement]. https://www.cellsearchctc.com/clinical-applications/clinical-applications-overview. Last updated December 5, 2016. Accessed December 19, 2016.
13. US Food and Drug Administration. cobas EGFR Mutation Test v2 – P150047. http://www.fda.gov/MedicalDevices/ProductsandMedicalProcedures/DeviceApprovalsandClearances/Recently-ApprovedDevices/ucm519922.htm. Last updated September 9, 2016. Accessed December 19, 2016.
14. Wu YL, Zhou C, Liam CK, et al. First-line erlotinib versus gemcitabine/cisplatin in patients with advanced EGFR mutation-positive non-small cell lung cancer: analyses from the phase III, randomized, open-label, ENSURE study. Ann Oncol. 2015;26(9):1883-1889.
15. Zill OA, Mortimer S, Banks KC, et al. Somatic genomic landscape of over 15,000 patients with advanced-stage cancer from clinical next-generation sequencing analysis of circulating tumor DNA. J Clin Oncol. 2016;34(suppl;abstr LBA11501).
16. Jordan NV, Bardia A, Wittner BS, et al. HER2 expression identifies dynamic functional states within circulating breast cancer cells. Nature. 2016;537:102-106.
17. Exosome Diagnostics. Exosome diagnostics enters agreement with Amgen. http://www.exosomedx.com/news-events/press-releases/exosome-diagnostics-enters-agreement-amgen. Published October 3, 2016. Accessed December 19, 2016.
18. Brinkman K, Emenegger J, Tannous B, et al. Exosomal RNA-based liquid biopsy detection of EML4-ALK in plasma from NSCLC patients [2015 World Conference on Lung Cancer, Denver, CO; abstract 2591]. http://library.iaslc.org/search-speaker?search_speaker=30493. Accessed January 6, 2017.
19. Exosome Diagnostics website. Prostate cancer. http://www.exosomedx.com/prostate-cancer-0. Last updated 2017. Accessed online December 19, 2016.
Pathologic and, increasingly, molecular analysis of tumor tissue biopsies is the gold standard in initial diagnosis of cancer, but liquid biopsies, which analyze tumor-derived material circulating in the bloodstream are gaining traction. Here, we discuss the current state of development of this complementary and potentially alternative approach to tumor analysis.
Liquid biopsy gaining traction
Biopsies enable oncologists to gather information about a potential or established tumor, including confirmation of the presence of cancerous tissue and determination of its histological characteristics, such as tumor grade and stage, as well as its molecular features, such as the presence of certain gene mutations. Ultimately, this information can be put to use in determining the most appropriate course of treatment.
The current gold standard is a tissue biopsy that typically involves an invasive procedure to permit the collection of a piece of tumor tissue. Yet, tissue biopsies are not always feasible because of the location of the tumor or the poor performance status of many patients with advanced disease. They also provide only a snapshot of the disease at the time at which they were taken and don’t necessarily reflect the genetic heterogeneity or evolution of a tumor over time.
The detection of components that are derived from the tumor circulating in the blood of cancer patients had fueled the idea of blood-based diagnostics in oncology – so-called liquid biopsies. These have rapidly gained traction in the past several decades as a less expensive (the cost of performing genomic analyses on blood samples is at least an order of magnitude less than on tissue samples), less invasive (requiring only a simple blood draw) alternative source of information about tumors.1
As researchers have refined the ability to exploit liquid biopsies, commercial interest has been piqued. More than 35 companies within the United States alone are developing liquid biopsies, and it’s easy to see why with a market projected to be in the many billions of dollars.2
Seeking out tumor clues in the blood
Liquid biopsies consist of a 10-15 mL blood sample drawn into a tube that contains an anticoagulant and it can contain several different types of tumor-associated material. Thus far, two components – circulating tumor cells (CTCs) and circulating tumor DNA (ctDNA) – have formed the cornerstone of liquid biopsies. At present, it is not clear whether these components are released randomly, as a by-product of tumor cell death or if they are released as part of a specific biologic process, such as for the colonization of metastatic sites. It reality, it may be a little of both, and active dissemination may be particularly relevant for CTCs, among which are postulated to be a population of cancer stem cells that can initiate distant metastases.3,4
The discovery of CTCs dates back to the 1860s, when cells that were morphologically identical to the tumor were identified in the blood of a patient with metastatic cancer. Their potential significance was not fully realized until a few decades ago, when they were found to exist from early on in the course of disease.3,4
CTCs, which can be either single cells or clusters of cells known as microemboli, have a short half-life in the bloodstream – less than 2 ½ hours – and are also extremely rare (1 mL of blood contains 1-10 CTCs) against a background of many millions of normal cells. Thus the detection and isolation of CTCs presents a significant challenge. More than 40 different platforms are being developed for the isolation and enrichment of CTCs. For the most part, these use a method called positive selection to pick out CTCs.1,3,4
Positive selection exploits the biological or physical properties that are specific to CTCs and absent in normal cells, for example, the presence of a specific tumor-associated antigen on their surface or differences in size, density or electric charge. The limitations of this method are that, not only do you need to know something about CTCs to begin to understand what makes them truly unique and ensure only isolation of CTCs, but their phenotype is also thought to be continually changing.1,3,4
In recent years, the focus has shifted toward technologies that use negative depletion, meaning that they target the other types of cells in the blood sample and filter those away until only the CTCs are left behind. The most advanced are devices that use microfluidic technology to sort the cells, such as the CTC-iChip system being developed by researchers at Massachusetts General Hospital in Boston.5
ctDNA consists of small fragments of nucleic acids that are not contained within a cell or associated with cell fragments and is thought to be present in 50%-90% of patients, depending on the type of cancer they have. ctDNA has a similarly short half-life in the circulation to CTCs and, like CTCs, ctDNA is present at very low levels in the bloodstream. Although levels of ctDNA have been shown to increase with increasing tumor burden, it is still often obscured by the presence of other cell-free DNA derived from non-tumor cells.
ctDNA can be distinguished from other cell-free DNA by the presence of somatic mutations and a number of highly sensitive methods have been developed to detect them, including the amplification-refractory mutation system (ARMS); digital polymerase chain reaction; and the beads, emulsification, amplification, and magnetics (BEAMing) system. Next-generation sequencing technologies, including tagged-amplicon deep sequencing (TAm-Seq), the Safe-Sequencing System (Safe-SeqS), and cancer personalized profiling by deep sequencing (CAPP-seq), can also be used and the race for ever more sensitive analytical tools is ongoing.1,3,4,6
Applying liquid biopsies now and in the future
There are a plethora of potential applications for liquid biopsies3,7 (Figure 1), and probably the most exciting among them is the potential for screening for and early detection of cancer. The fact that ctDNA and CTCs have both been shown to be present from the earliest stages of disease has sparked interest in the possibility of developing simple blood tests to identify tumors before they become detectable by other methods and at a point at which they may be curable.
Given that both are present at such low levels within the circulation and are particularly sparse at earlier stages of disease, current technologies may lack the specificity and sensitivity for this application at present. However, numerous clinical trials are ongoing.
For CTCs, simple enumeration has been the most extensively investigated application to date. Numerous studies have shown that the number of CTCs in the bloodstream has prognostic significance in various different tumor types. Three such studies led to the first regulatory approval for a CTC detection system (Table 1 and Table 2).8-10
One area in which liquid biopsies could really come into their own is in providing more real-time analysis of tumors. This is something that has proven particularly challenging with tissue biopsies because repeating these invasive procedures is problematic. But the ease of repeat blood draws means that serial liquid biopsies could be performed and might offer the possibility of monitoring disease progression and evolution over the course of disease and particularly in response to treatment.
Indeed, studies have shown that in addition to baseline CTC counts, changes in CTC number during treatment are also prognostic. There was improved survival among patients whose CTC counts decreased below a threshold value during treatment and vice versa. This is also an approved use for CellSearch though at present it is not widely clinically implemented.12
Clinical utility remains elusive
The ultimate goal would be for liquid biopsies to have an impact on treatment decisions, allowing oncologists to change management strategy based on predicted sensitivity or resistance to therapy, so-called clinical utility. Thus far, clinical utility has proved elusive, though liquid biopsies using ctDNA to evaluate tumor genotype have come closest.
The Cobas EGFR Mutation Test v2 recently became the first ctDNA-based liquid biopsy to receive regulatory approval. It was approved as a companion diagnostic to identify patients with advanced non–small-cell lung cancer (NSCLC) who have specific mutations in the epidermal growth factor receptor (EGFR) gene and are therefore eligible for treatment with the EGFR inhibitor erlotinib.13
Approval was based on comparison of EGFR mutation identification rates using plasma ctDNA samples and tumor tissue samples from patients enrolled in the phase 3 ENSURE trial, which compared the efficacy of erlotinib with chemotherapy as first-line therapy in patients with advanced NSCLC. Of the 217 patients enrolled in the trial, 98.6% of patients had both tumor biopsy and plasma ctDNA samples available for testing. Concordance between the two types of biopsy in identifying patients with EGFR mutations was high and patients with EGFR positivity according to liquid biopsy results demonstrated improved progression-free survival when treated with erlotinib.14
The results of a large-scale genomic analysis of various different types of tumors using ctDNA were also recently presented at the 2016 American Society of Clinical Oncology meeting. Blood samples from more than 15,000 patients with 50 different tumor types, including advanced lung cancer (37%), breast cancer (14%), and CRC (10%), were collected and compared with either available tumor biopsy samples from the same cases (n = 398) or, in the majority of cases, with The Cancer Genome Atlas database, which uses tumor biopsies to perform genome-wide sequencing studies. Both types of biopsy revealed very similar mutation patterns when the Guardant360 next-generation sequencing test, which targets 70 genes, was applied. In particular, when EGFR, BRAF, KRAS, ALK, RET, and ROS1 mutations were identified by tumor tissue biopsy, the same mutations were reported in 94%-100% of plasma samples.15
Studies of the clinical utility of ctDNA and CTCs are among ongoing clinical trials of liquid biopsies (Tables 2 and 3). The potential for using CTCs to guide treatment decisions has become particularly relevant in breast cancer in light of results showing that patients with primary tumors that are negative for human epidermal growth factor receptor 2 (HER2) amplification, an important biomarker in breast cancer, may have CTCs that are HER2-positive, in up to 30% of cases. These patients may therefore still benefit from HER2-targeted therapy.16
The DETECT studies are the first phase 3 trials in which treatment decisions are being based on the phenotypic characteristics of CTCs. DETECT III (NCT01619111) is comparing lapatinib in combination with standard therapy with standard therapy alone in patients with HER2-negative metastatic breast cancer who have HER2-positive CTCs, whereas DETECT IV (NCT02035813) is enrolling patients with HER2-negative, hormone receptor-positive metastatic breast cancer and persistent HER2-negative CTCs to receive standard endocrine therapy and the mammalian target of rapamycin inhibitor everolimus.
Other targets and sources for liquid biopsy
Another approach to liquid biopsies that is also beginning to take off is to collect tumor-derived exosomes from the bloodstream. Exosomes are tiny, fluid-filled, membrane-bound sacks that bud off from the surface of a cell to expel waste or to transport cargo from one cell to another. DNA, RNA, and protein can be extracted from tumor-derived exosomes and could also serve as molecular biomarkers relating to the cancer cells from which they came.6,7
Exosome Diagnostics is bringing the first exosome-based diagnostic tests to the market and recently teamed up with Amgen for the development of these liquid biopsies.17 In January 2016, they launched ExoDx Lung (ALK), for detection of EML4-ALK gene fusions in patients with NSCLC, using a proprietary platform for the isolation of RNA from exosomes. Data that was presented at several different conferences in 2015 demonstrated a sensitivity of 88% and specificity of 100% for this diagnostic when compared with tissue ALK status in NSCLC patients receiving a second-generation ALK inhibitor following progression on prior ALK inhibitor therapy.18
In September, they subsequently announced the launch of a test that analyses genetic information from exosomes collected from a urine sample taken from prostate cancer patients. Using a 3-gene signature, in combination with a proprietary algorithm, this diagnostic generates a score assessing a prostate cancer patient’s risk for higher grade, more aggressive disease. It is designed to complement the prostate-specific antigen score and has demonstrated accuracy in ruling out the presence of high-grade cancer before an initial biopsy in more than 1,
Pathologic and, increasingly, molecular analysis of tumor tissue biopsies is the gold standard in initial diagnosis of cancer, but liquid biopsies, which analyze tumor-derived material circulating in the bloodstream are gaining traction. Here, we discuss the current state of development of this complementary and potentially alternative approach to tumor analysis.
Liquid biopsy gaining traction
Biopsies enable oncologists to gather information about a potential or established tumor, including confirmation of the presence of cancerous tissue and determination of its histological characteristics, such as tumor grade and stage, as well as its molecular features, such as the presence of certain gene mutations. Ultimately, this information can be put to use in determining the most appropriate course of treatment.
The current gold standard is a tissue biopsy that typically involves an invasive procedure to permit the collection of a piece of tumor tissue. Yet, tissue biopsies are not always feasible because of the location of the tumor or the poor performance status of many patients with advanced disease. They also provide only a snapshot of the disease at the time at which they were taken and don’t necessarily reflect the genetic heterogeneity or evolution of a tumor over time.
The detection of components that are derived from the tumor circulating in the blood of cancer patients had fueled the idea of blood-based diagnostics in oncology – so-called liquid biopsies. These have rapidly gained traction in the past several decades as a less expensive (the cost of performing genomic analyses on blood samples is at least an order of magnitude less than on tissue samples), less invasive (requiring only a simple blood draw) alternative source of information about tumors.1
As researchers have refined the ability to exploit liquid biopsies, commercial interest has been piqued. More than 35 companies within the United States alone are developing liquid biopsies, and it’s easy to see why with a market projected to be in the many billions of dollars.2
Seeking out tumor clues in the blood
Liquid biopsies consist of a 10-15 mL blood sample drawn into a tube that contains an anticoagulant and it can contain several different types of tumor-associated material. Thus far, two components – circulating tumor cells (CTCs) and circulating tumor DNA (ctDNA) – have formed the cornerstone of liquid biopsies. At present, it is not clear whether these components are released randomly, as a by-product of tumor cell death or if they are released as part of a specific biologic process, such as for the colonization of metastatic sites. It reality, it may be a little of both, and active dissemination may be particularly relevant for CTCs, among which are postulated to be a population of cancer stem cells that can initiate distant metastases.3,4
The discovery of CTCs dates back to the 1860s, when cells that were morphologically identical to the tumor were identified in the blood of a patient with metastatic cancer. Their potential significance was not fully realized until a few decades ago, when they were found to exist from early on in the course of disease.3,4
CTCs, which can be either single cells or clusters of cells known as microemboli, have a short half-life in the bloodstream – less than 2 ½ hours – and are also extremely rare (1 mL of blood contains 1-10 CTCs) against a background of many millions of normal cells. Thus the detection and isolation of CTCs presents a significant challenge. More than 40 different platforms are being developed for the isolation and enrichment of CTCs. For the most part, these use a method called positive selection to pick out CTCs.1,3,4
Positive selection exploits the biological or physical properties that are specific to CTCs and absent in normal cells, for example, the presence of a specific tumor-associated antigen on their surface or differences in size, density or electric charge. The limitations of this method are that, not only do you need to know something about CTCs to begin to understand what makes them truly unique and ensure only isolation of CTCs, but their phenotype is also thought to be continually changing.1,3,4
In recent years, the focus has shifted toward technologies that use negative depletion, meaning that they target the other types of cells in the blood sample and filter those away until only the CTCs are left behind. The most advanced are devices that use microfluidic technology to sort the cells, such as the CTC-iChip system being developed by researchers at Massachusetts General Hospital in Boston.5
ctDNA consists of small fragments of nucleic acids that are not contained within a cell or associated with cell fragments and is thought to be present in 50%-90% of patients, depending on the type of cancer they have. ctDNA has a similarly short half-life in the circulation to CTCs and, like CTCs, ctDNA is present at very low levels in the bloodstream. Although levels of ctDNA have been shown to increase with increasing tumor burden, it is still often obscured by the presence of other cell-free DNA derived from non-tumor cells.
ctDNA can be distinguished from other cell-free DNA by the presence of somatic mutations and a number of highly sensitive methods have been developed to detect them, including the amplification-refractory mutation system (ARMS); digital polymerase chain reaction; and the beads, emulsification, amplification, and magnetics (BEAMing) system. Next-generation sequencing technologies, including tagged-amplicon deep sequencing (TAm-Seq), the Safe-Sequencing System (Safe-SeqS), and cancer personalized profiling by deep sequencing (CAPP-seq), can also be used and the race for ever more sensitive analytical tools is ongoing.1,3,4,6
Applying liquid biopsies now and in the future
There are a plethora of potential applications for liquid biopsies3,7 (Figure 1), and probably the most exciting among them is the potential for screening for and early detection of cancer. The fact that ctDNA and CTCs have both been shown to be present from the earliest stages of disease has sparked interest in the possibility of developing simple blood tests to identify tumors before they become detectable by other methods and at a point at which they may be curable.
Given that both are present at such low levels within the circulation and are particularly sparse at earlier stages of disease, current technologies may lack the specificity and sensitivity for this application at present. However, numerous clinical trials are ongoing.
For CTCs, simple enumeration has been the most extensively investigated application to date. Numerous studies have shown that the number of CTCs in the bloodstream has prognostic significance in various different tumor types. Three such studies led to the first regulatory approval for a CTC detection system (Table 1 and Table 2).8-10
One area in which liquid biopsies could really come into their own is in providing more real-time analysis of tumors. This is something that has proven particularly challenging with tissue biopsies because repeating these invasive procedures is problematic. But the ease of repeat blood draws means that serial liquid biopsies could be performed and might offer the possibility of monitoring disease progression and evolution over the course of disease and particularly in response to treatment.
Indeed, studies have shown that in addition to baseline CTC counts, changes in CTC number during treatment are also prognostic. There was improved survival among patients whose CTC counts decreased below a threshold value during treatment and vice versa. This is also an approved use for CellSearch though at present it is not widely clinically implemented.12
Clinical utility remains elusive
The ultimate goal would be for liquid biopsies to have an impact on treatment decisions, allowing oncologists to change management strategy based on predicted sensitivity or resistance to therapy, so-called clinical utility. Thus far, clinical utility has proved elusive, though liquid biopsies using ctDNA to evaluate tumor genotype have come closest.
The Cobas EGFR Mutation Test v2 recently became the first ctDNA-based liquid biopsy to receive regulatory approval. It was approved as a companion diagnostic to identify patients with advanced non–small-cell lung cancer (NSCLC) who have specific mutations in the epidermal growth factor receptor (EGFR) gene and are therefore eligible for treatment with the EGFR inhibitor erlotinib.13
Approval was based on comparison of EGFR mutation identification rates using plasma ctDNA samples and tumor tissue samples from patients enrolled in the phase 3 ENSURE trial, which compared the efficacy of erlotinib with chemotherapy as first-line therapy in patients with advanced NSCLC. Of the 217 patients enrolled in the trial, 98.6% of patients had both tumor biopsy and plasma ctDNA samples available for testing. Concordance between the two types of biopsy in identifying patients with EGFR mutations was high and patients with EGFR positivity according to liquid biopsy results demonstrated improved progression-free survival when treated with erlotinib.14
The results of a large-scale genomic analysis of various different types of tumors using ctDNA were also recently presented at the 2016 American Society of Clinical Oncology meeting. Blood samples from more than 15,000 patients with 50 different tumor types, including advanced lung cancer (37%), breast cancer (14%), and CRC (10%), were collected and compared with either available tumor biopsy samples from the same cases (n = 398) or, in the majority of cases, with The Cancer Genome Atlas database, which uses tumor biopsies to perform genome-wide sequencing studies. Both types of biopsy revealed very similar mutation patterns when the Guardant360 next-generation sequencing test, which targets 70 genes, was applied. In particular, when EGFR, BRAF, KRAS, ALK, RET, and ROS1 mutations were identified by tumor tissue biopsy, the same mutations were reported in 94%-100% of plasma samples.15
Studies of the clinical utility of ctDNA and CTCs are among ongoing clinical trials of liquid biopsies (Tables 2 and 3). The potential for using CTCs to guide treatment decisions has become particularly relevant in breast cancer in light of results showing that patients with primary tumors that are negative for human epidermal growth factor receptor 2 (HER2) amplification, an important biomarker in breast cancer, may have CTCs that are HER2-positive, in up to 30% of cases. These patients may therefore still benefit from HER2-targeted therapy.16
The DETECT studies are the first phase 3 trials in which treatment decisions are being based on the phenotypic characteristics of CTCs. DETECT III (NCT01619111) is comparing lapatinib in combination with standard therapy with standard therapy alone in patients with HER2-negative metastatic breast cancer who have HER2-positive CTCs, whereas DETECT IV (NCT02035813) is enrolling patients with HER2-negative, hormone receptor-positive metastatic breast cancer and persistent HER2-negative CTCs to receive standard endocrine therapy and the mammalian target of rapamycin inhibitor everolimus.
Other targets and sources for liquid biopsy
Another approach to liquid biopsies that is also beginning to take off is to collect tumor-derived exosomes from the bloodstream. Exosomes are tiny, fluid-filled, membrane-bound sacks that bud off from the surface of a cell to expel waste or to transport cargo from one cell to another. DNA, RNA, and protein can be extracted from tumor-derived exosomes and could also serve as molecular biomarkers relating to the cancer cells from which they came.6,7
Exosome Diagnostics is bringing the first exosome-based diagnostic tests to the market and recently teamed up with Amgen for the development of these liquid biopsies.17 In January 2016, they launched ExoDx Lung (ALK), for detection of EML4-ALK gene fusions in patients with NSCLC, using a proprietary platform for the isolation of RNA from exosomes. Data that was presented at several different conferences in 2015 demonstrated a sensitivity of 88% and specificity of 100% for this diagnostic when compared with tissue ALK status in NSCLC patients receiving a second-generation ALK inhibitor following progression on prior ALK inhibitor therapy.18
In September, they subsequently announced the launch of a test that analyses genetic information from exosomes collected from a urine sample taken from prostate cancer patients. Using a 3-gene signature, in combination with a proprietary algorithm, this diagnostic generates a score assessing a prostate cancer patient’s risk for higher grade, more aggressive disease. It is designed to complement the prostate-specific antigen score and has demonstrated accuracy in ruling out the presence of high-grade cancer before an initial biopsy in more than 1,
1. Lennon NK, Adalsteinsson VA, Gabriel SB. Technological considerations for genome-guided diagnosis and management of cancer. Genome Med. 2016;8:112.
2. MIT Technology Review website. Liquid biopsy: fast DNA-sequencing machines are leading to simple blood tests for cancer. https://www.technologyreview.com/s/534991/liquid-biopsy/. Published 2015. Accessed December 19, 2016.
3. Alix-Panabières C and Pantel K. Clinical applications of circulating tumor cells and circulating tumor DNA as liquid biopsy. Cancer Discov. 2016;6(5):479-491.
4. Calabuig-Farinãs S, Jantus-Lewintre E, Herreros-Pomares A, Camps C. Circulating tumor cells versus circulating tumor DNA in lung cancer – which one will win? Transl Lung Cancer Res. 2016;5(5):466-482.
5. Karabacak, NM, Spuhler PS, Fachin F, et al. Microfluidic, marker-free isolation of circulating tumor cells from blood samples. Nat Protoc. 2014;9:694-710.
6. Buder A, Tomuta C, and Filipits M. The potential of liquid biopsies. Curr Opin Oncol. 2016;28:130-134.
7. Hofman P, Popper HH. Pathologists and liquid biopsies: to be or not to be? Virchows Arch. 2016;469:601-609.
8. Bidard FC, Peeters DJ, Fehm T, et al. Clinical validity of circulating tumor cells in patients with metastatic breast cancer: a pooled analysis of individual patient data. Lancet Oncol. 2014;15(4):406-414.
9. de Bono JS, Scher HI, Montgomery RB, et al. Circulating tumor cells predict survival benefit from treatment in metastatic castration-resistant prostate cancer. Clin Cancer Res. 2008;14(19):6302-6309.
10. Cohen SJ, Punt CJ, Iannotti N, et al. Relationship of circulating tumor cells to tumor response, progression-free survival, and overall survival in patients with metastatic colorectal cancer. J Clin Oncol. 2008;26(19):3213-3221.
11. CellSearch Web site. What is the CELLSEARCH® System? https://www.cellsearchctc.com/product-systems-overview/cellsearch-system-overview. Last updated December 5th, 2016. Accessed online December 19th, 2016.
12. CellSearch Web site [advertisement]. https://www.cellsearchctc.com/clinical-applications/clinical-applications-overview. Last updated December 5, 2016. Accessed December 19, 2016.
13. US Food and Drug Administration. cobas EGFR Mutation Test v2 – P150047. http://www.fda.gov/MedicalDevices/ProductsandMedicalProcedures/DeviceApprovalsandClearances/Recently-ApprovedDevices/ucm519922.htm. Last updated September 9, 2016. Accessed December 19, 2016.
14. Wu YL, Zhou C, Liam CK, et al. First-line erlotinib versus gemcitabine/cisplatin in patients with advanced EGFR mutation-positive non-small cell lung cancer: analyses from the phase III, randomized, open-label, ENSURE study. Ann Oncol. 2015;26(9):1883-1889.
15. Zill OA, Mortimer S, Banks KC, et al. Somatic genomic landscape of over 15,000 patients with advanced-stage cancer from clinical next-generation sequencing analysis of circulating tumor DNA. J Clin Oncol. 2016;34(suppl;abstr LBA11501).
16. Jordan NV, Bardia A, Wittner BS, et al. HER2 expression identifies dynamic functional states within circulating breast cancer cells. Nature. 2016;537:102-106.
17. Exosome Diagnostics. Exosome diagnostics enters agreement with Amgen. http://www.exosomedx.com/news-events/press-releases/exosome-diagnostics-enters-agreement-amgen. Published October 3, 2016. Accessed December 19, 2016.
18. Brinkman K, Emenegger J, Tannous B, et al. Exosomal RNA-based liquid biopsy detection of EML4-ALK in plasma from NSCLC patients [2015 World Conference on Lung Cancer, Denver, CO; abstract 2591]. http://library.iaslc.org/search-speaker?search_speaker=30493. Accessed January 6, 2017.
19. Exosome Diagnostics website. Prostate cancer. http://www.exosomedx.com/prostate-cancer-0. Last updated 2017. Accessed online December 19, 2016.
1. Lennon NK, Adalsteinsson VA, Gabriel SB. Technological considerations for genome-guided diagnosis and management of cancer. Genome Med. 2016;8:112.
2. MIT Technology Review website. Liquid biopsy: fast DNA-sequencing machines are leading to simple blood tests for cancer. https://www.technologyreview.com/s/534991/liquid-biopsy/. Published 2015. Accessed December 19, 2016.
3. Alix-Panabières C and Pantel K. Clinical applications of circulating tumor cells and circulating tumor DNA as liquid biopsy. Cancer Discov. 2016;6(5):479-491.
4. Calabuig-Farinãs S, Jantus-Lewintre E, Herreros-Pomares A, Camps C. Circulating tumor cells versus circulating tumor DNA in lung cancer – which one will win? Transl Lung Cancer Res. 2016;5(5):466-482.
5. Karabacak, NM, Spuhler PS, Fachin F, et al. Microfluidic, marker-free isolation of circulating tumor cells from blood samples. Nat Protoc. 2014;9:694-710.
6. Buder A, Tomuta C, and Filipits M. The potential of liquid biopsies. Curr Opin Oncol. 2016;28:130-134.
7. Hofman P, Popper HH. Pathologists and liquid biopsies: to be or not to be? Virchows Arch. 2016;469:601-609.
8. Bidard FC, Peeters DJ, Fehm T, et al. Clinical validity of circulating tumor cells in patients with metastatic breast cancer: a pooled analysis of individual patient data. Lancet Oncol. 2014;15(4):406-414.
9. de Bono JS, Scher HI, Montgomery RB, et al. Circulating tumor cells predict survival benefit from treatment in metastatic castration-resistant prostate cancer. Clin Cancer Res. 2008;14(19):6302-6309.
10. Cohen SJ, Punt CJ, Iannotti N, et al. Relationship of circulating tumor cells to tumor response, progression-free survival, and overall survival in patients with metastatic colorectal cancer. J Clin Oncol. 2008;26(19):3213-3221.
11. CellSearch Web site. What is the CELLSEARCH® System? https://www.cellsearchctc.com/product-systems-overview/cellsearch-system-overview. Last updated December 5th, 2016. Accessed online December 19th, 2016.
12. CellSearch Web site [advertisement]. https://www.cellsearchctc.com/clinical-applications/clinical-applications-overview. Last updated December 5, 2016. Accessed December 19, 2016.
13. US Food and Drug Administration. cobas EGFR Mutation Test v2 – P150047. http://www.fda.gov/MedicalDevices/ProductsandMedicalProcedures/DeviceApprovalsandClearances/Recently-ApprovedDevices/ucm519922.htm. Last updated September 9, 2016. Accessed December 19, 2016.
14. Wu YL, Zhou C, Liam CK, et al. First-line erlotinib versus gemcitabine/cisplatin in patients with advanced EGFR mutation-positive non-small cell lung cancer: analyses from the phase III, randomized, open-label, ENSURE study. Ann Oncol. 2015;26(9):1883-1889.
15. Zill OA, Mortimer S, Banks KC, et al. Somatic genomic landscape of over 15,000 patients with advanced-stage cancer from clinical next-generation sequencing analysis of circulating tumor DNA. J Clin Oncol. 2016;34(suppl;abstr LBA11501).
16. Jordan NV, Bardia A, Wittner BS, et al. HER2 expression identifies dynamic functional states within circulating breast cancer cells. Nature. 2016;537:102-106.
17. Exosome Diagnostics. Exosome diagnostics enters agreement with Amgen. http://www.exosomedx.com/news-events/press-releases/exosome-diagnostics-enters-agreement-amgen. Published October 3, 2016. Accessed December 19, 2016.
18. Brinkman K, Emenegger J, Tannous B, et al. Exosomal RNA-based liquid biopsy detection of EML4-ALK in plasma from NSCLC patients [2015 World Conference on Lung Cancer, Denver, CO; abstract 2591]. http://library.iaslc.org/search-speaker?search_speaker=30493. Accessed January 6, 2017.
19. Exosome Diagnostics website. Prostate cancer. http://www.exosomedx.com/prostate-cancer-0. Last updated 2017. Accessed online December 19, 2016.
VIDEO: How to start an oncology sexual health clinic
NATIONAL HARBOR, MD – Start small, but anticipate growth. Engage your administration from the start. Be smart about resources, and consider using advanced practice providers to keep costs down. Above all, keep lines of communication open with physicians and other members of the care team.
In a video interview, Joanne Rash, PA-C, a certified physician assistant at the University of Wisconsin–Madison offers these and other tips. She explains her collaborative work with David Kushner, MD, director of the gynecologic oncology program and professor at the University of Wisconsin School of Medicine and Public Health, and a colleague to develop the Women’s Integrative Sexual Health (WISH) program.
WISH is modeled on the University of Chicago’s Program in Integrative Sex and Medicine for Women and Girls with Cancer (PRISM) and participates in the PRISM registry, which studies ways to prevent and treat sexual problems for women and girls with cancer.
“I think what makes the WISH program unique is that we carve time out,” said Ms. Rash. “Of course, we address some of these issues in my gynecologic oncology practice, but, when we do it in WISH, the format is different,” and there’s just more time for discussion.
Communication is key to the model’s success in safe integration of sexual health into cancer care, she said. “We certainly don’t want to do something that compromises cancer care, and so, it’s important that we have those conversations with that woman’s team. And now we get to be a part of that team, which is a real privilege.”
Ms. Rash reported no conflicts of interest.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
[email protected]
On Twitter @karioakes
NATIONAL HARBOR, MD – Start small, but anticipate growth. Engage your administration from the start. Be smart about resources, and consider using advanced practice providers to keep costs down. Above all, keep lines of communication open with physicians and other members of the care team.
In a video interview, Joanne Rash, PA-C, a certified physician assistant at the University of Wisconsin–Madison offers these and other tips. She explains her collaborative work with David Kushner, MD, director of the gynecologic oncology program and professor at the University of Wisconsin School of Medicine and Public Health, and a colleague to develop the Women’s Integrative Sexual Health (WISH) program.
WISH is modeled on the University of Chicago’s Program in Integrative Sex and Medicine for Women and Girls with Cancer (PRISM) and participates in the PRISM registry, which studies ways to prevent and treat sexual problems for women and girls with cancer.
“I think what makes the WISH program unique is that we carve time out,” said Ms. Rash. “Of course, we address some of these issues in my gynecologic oncology practice, but, when we do it in WISH, the format is different,” and there’s just more time for discussion.
Communication is key to the model’s success in safe integration of sexual health into cancer care, she said. “We certainly don’t want to do something that compromises cancer care, and so, it’s important that we have those conversations with that woman’s team. And now we get to be a part of that team, which is a real privilege.”
Ms. Rash reported no conflicts of interest.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
[email protected]
On Twitter @karioakes
NATIONAL HARBOR, MD – Start small, but anticipate growth. Engage your administration from the start. Be smart about resources, and consider using advanced practice providers to keep costs down. Above all, keep lines of communication open with physicians and other members of the care team.
In a video interview, Joanne Rash, PA-C, a certified physician assistant at the University of Wisconsin–Madison offers these and other tips. She explains her collaborative work with David Kushner, MD, director of the gynecologic oncology program and professor at the University of Wisconsin School of Medicine and Public Health, and a colleague to develop the Women’s Integrative Sexual Health (WISH) program.
WISH is modeled on the University of Chicago’s Program in Integrative Sex and Medicine for Women and Girls with Cancer (PRISM) and participates in the PRISM registry, which studies ways to prevent and treat sexual problems for women and girls with cancer.
“I think what makes the WISH program unique is that we carve time out,” said Ms. Rash. “Of course, we address some of these issues in my gynecologic oncology practice, but, when we do it in WISH, the format is different,” and there’s just more time for discussion.
Communication is key to the model’s success in safe integration of sexual health into cancer care, she said. “We certainly don’t want to do something that compromises cancer care, and so, it’s important that we have those conversations with that woman’s team. And now we get to be a part of that team, which is a real privilege.”
Ms. Rash reported no conflicts of interest.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
[email protected]
On Twitter @karioakes
AT THE ANNUAL MEETING ON WOMEN’S CANCER
VIDEO: Sex, intimacy part of history in gynecologic oncology care
NATIONAL HARBOR, MD. – Cancer care can feel like a sequence of crises, large and small; a comprehensive cancer care approach can keep the patient at the center of this flurry of events. But understanding a patient’s sexual history and assessing how a gynecologic malignancy has affected that patient’s sexual health can, too often, get lost in the shuffle, said Don Dizon, MD, of Massachusetts General Hospital, Boston.
“I think there are multiple reasons for that, but it has to be the job of the clinician – not necessarily the oncologist-physician – but somebody has to be able to say, ‘What is your sexual history? Can I ask about your sexual history?’ ” he said in a video interview at the annual meeting of the Society of Gynecologic Oncology.
Dr. Dizon, director of the Oncology Sexual Health Clinic at Massachusetts General Hospital*, emphasized the importance of providing sensitive sexual health care while staying true to one’s own framework of care. “I teach clinicians not to run away from their schema,” he said. “Having said that, the job of a clinician is to normalize the history, and to normalize that sex, intimacy, relationships are a part of someone’s history.”
Dr. Dizon sits on the board of the Patty Brisben Foundation and the Young Survival Coalition.
[email protected]
On Twitter @karioakes
*A previous version of this article misstated Dr. Dizon's affiliation.
NATIONAL HARBOR, MD. – Cancer care can feel like a sequence of crises, large and small; a comprehensive cancer care approach can keep the patient at the center of this flurry of events. But understanding a patient’s sexual history and assessing how a gynecologic malignancy has affected that patient’s sexual health can, too often, get lost in the shuffle, said Don Dizon, MD, of Massachusetts General Hospital, Boston.
“I think there are multiple reasons for that, but it has to be the job of the clinician – not necessarily the oncologist-physician – but somebody has to be able to say, ‘What is your sexual history? Can I ask about your sexual history?’ ” he said in a video interview at the annual meeting of the Society of Gynecologic Oncology.
Dr. Dizon, director of the Oncology Sexual Health Clinic at Massachusetts General Hospital*, emphasized the importance of providing sensitive sexual health care while staying true to one’s own framework of care. “I teach clinicians not to run away from their schema,” he said. “Having said that, the job of a clinician is to normalize the history, and to normalize that sex, intimacy, relationships are a part of someone’s history.”
Dr. Dizon sits on the board of the Patty Brisben Foundation and the Young Survival Coalition.
[email protected]
On Twitter @karioakes
*A previous version of this article misstated Dr. Dizon's affiliation.
NATIONAL HARBOR, MD. – Cancer care can feel like a sequence of crises, large and small; a comprehensive cancer care approach can keep the patient at the center of this flurry of events. But understanding a patient’s sexual history and assessing how a gynecologic malignancy has affected that patient’s sexual health can, too often, get lost in the shuffle, said Don Dizon, MD, of Massachusetts General Hospital, Boston.
“I think there are multiple reasons for that, but it has to be the job of the clinician – not necessarily the oncologist-physician – but somebody has to be able to say, ‘What is your sexual history? Can I ask about your sexual history?’ ” he said in a video interview at the annual meeting of the Society of Gynecologic Oncology.
Dr. Dizon, director of the Oncology Sexual Health Clinic at Massachusetts General Hospital*, emphasized the importance of providing sensitive sexual health care while staying true to one’s own framework of care. “I teach clinicians not to run away from their schema,” he said. “Having said that, the job of a clinician is to normalize the history, and to normalize that sex, intimacy, relationships are a part of someone’s history.”
Dr. Dizon sits on the board of the Patty Brisben Foundation and the Young Survival Coalition.
[email protected]
On Twitter @karioakes
*A previous version of this article misstated Dr. Dizon's affiliation.
AT THE ANNUAL MEETING ON WOMEN'S CANCER
Video: Try ‘PLISSIT’ to address postcancer sexual health
NATIONAL HARBOR, MD. – Comprehensive care of patients with gynecologic malignancies should include a sensitive and thorough assessment of sexual health.
In a video interview at the annual meeting of the Society of Gynecologic Oncology, Don Dizon, MD, of Massachusetts General Hospital, Boston, gives a series of practical tips to help physicians take a thorough sexual health history and provide information and guidance for patients and their partners.
Dr. Dizon, professor of gynecologic oncology and director of the oncology sexual health clinic at Brigham and Women’s Hospital, also in Boston, said that he likes to begin with the PLISSIT model, where patients are given permission (P) to talk about sexual problems. Then, the clinician gives the patient limited (LI) scientific or clinical information about the situation, followed by specific suggestions (SS) that might help. Finally, patients may be referred to mental health providers or sex counselors for intensive therapy (IT) if needed.
“It’s also important not to confuse terminology,” said Dr. Dizon. “Intimacy is experienced very differently between men and women. Women experience intimacy through arousal, desire, and, when desire is satisfied, that’s intimacy. Intercourse is not a part of that equation.” For men, he said, intimacy is more often experienced through intercourse. “So the disconnect is greater after cancer is diagnosed,” making it especially important to acknowledge problems sensitively, and to helps patients and partners find a way forward.
“The word I like to use is ‘play,’ ” said Dr. Dizon. When a renegotiation of an intimate relationship is framed in terms of play, the pressure is off, and “men can wrap their hands around that idea,” he said.
Dr. Dizon sits on the board of the Patty Brisben Foundation and the Young Survival Coalition.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
[email protected]
On Twitter @karioakes
NATIONAL HARBOR, MD. – Comprehensive care of patients with gynecologic malignancies should include a sensitive and thorough assessment of sexual health.
In a video interview at the annual meeting of the Society of Gynecologic Oncology, Don Dizon, MD, of Massachusetts General Hospital, Boston, gives a series of practical tips to help physicians take a thorough sexual health history and provide information and guidance for patients and their partners.
Dr. Dizon, professor of gynecologic oncology and director of the oncology sexual health clinic at Brigham and Women’s Hospital, also in Boston, said that he likes to begin with the PLISSIT model, where patients are given permission (P) to talk about sexual problems. Then, the clinician gives the patient limited (LI) scientific or clinical information about the situation, followed by specific suggestions (SS) that might help. Finally, patients may be referred to mental health providers or sex counselors for intensive therapy (IT) if needed.
“It’s also important not to confuse terminology,” said Dr. Dizon. “Intimacy is experienced very differently between men and women. Women experience intimacy through arousal, desire, and, when desire is satisfied, that’s intimacy. Intercourse is not a part of that equation.” For men, he said, intimacy is more often experienced through intercourse. “So the disconnect is greater after cancer is diagnosed,” making it especially important to acknowledge problems sensitively, and to helps patients and partners find a way forward.
“The word I like to use is ‘play,’ ” said Dr. Dizon. When a renegotiation of an intimate relationship is framed in terms of play, the pressure is off, and “men can wrap their hands around that idea,” he said.
Dr. Dizon sits on the board of the Patty Brisben Foundation and the Young Survival Coalition.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
[email protected]
On Twitter @karioakes
NATIONAL HARBOR, MD. – Comprehensive care of patients with gynecologic malignancies should include a sensitive and thorough assessment of sexual health.
In a video interview at the annual meeting of the Society of Gynecologic Oncology, Don Dizon, MD, of Massachusetts General Hospital, Boston, gives a series of practical tips to help physicians take a thorough sexual health history and provide information and guidance for patients and their partners.
Dr. Dizon, professor of gynecologic oncology and director of the oncology sexual health clinic at Brigham and Women’s Hospital, also in Boston, said that he likes to begin with the PLISSIT model, where patients are given permission (P) to talk about sexual problems. Then, the clinician gives the patient limited (LI) scientific or clinical information about the situation, followed by specific suggestions (SS) that might help. Finally, patients may be referred to mental health providers or sex counselors for intensive therapy (IT) if needed.
“It’s also important not to confuse terminology,” said Dr. Dizon. “Intimacy is experienced very differently between men and women. Women experience intimacy through arousal, desire, and, when desire is satisfied, that’s intimacy. Intercourse is not a part of that equation.” For men, he said, intimacy is more often experienced through intercourse. “So the disconnect is greater after cancer is diagnosed,” making it especially important to acknowledge problems sensitively, and to helps patients and partners find a way forward.
“The word I like to use is ‘play,’ ” said Dr. Dizon. When a renegotiation of an intimate relationship is framed in terms of play, the pressure is off, and “men can wrap their hands around that idea,” he said.
Dr. Dizon sits on the board of the Patty Brisben Foundation and the Young Survival Coalition.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
[email protected]
On Twitter @karioakes
AT THE ANNUAL MEETING ON WOMEN’S CANCER
Disease site determines QOL, pain in recurrent rectal cancer
SEATTLE – Disease anatomy is the main determinant of subsequent quality of life (QOL) in patients with locally recurrent rectal cancer at both base line and in the long term, according to findings presented at the annual Society of Surgical Oncology Cancer Symposium.
Posterior recurrences were associated with the worst QOL scores and the most severe pain.
Recurrent rectal cancer is a morbid disease state, leading to pain and disability. “Patients experience a multitude of symptoms, including disability as a result of tumor growth in a confined space in the pelvis and invasion of adjacent organs as well as complications from surgery and neoadjuvant treatment,” said lead author Dr. Tarik Sammour of the University of Texas MD Anderson Cancer Center in Houston.
He noted, however, that “it is not all doom and gloom.” Survival outcomes have been improving in this population, with the median 5-year survival now approaching 40%-50%.
“Decade by decade, the 5-year survival outcomes are increasing,” explained Dr. Sammour.
Increasing survival also begs the question: “Are we helping patients or affording them the option of living longer with pain and disability? In other words, we need to measure patient-centered outcomes,” he said.
Data for recurrent rectal cancer are very limited, particularly when it comes to measuring patient outcomes. Most studies have been retrospective in design, making it difficult to gauge symptoms and quality of life.
The majority of studies also have focused on surgery, have short follow-up times, and may be missing data.
“Very few measure baseline quality of life, so it makes it difficult to measure trajectories,” Dr. Sammour said. “So overall we don’t know very much about the quality of life of these patients, so we thought to remedy that with a prospective study.”
Dr. Sammour and his colleagues examined the longitudinal trajectory of cancer survivorship in recurrent rectal cancer over a 7-year period. A total of 104 patients diagnosed with recurrent rectal cancer were enrolled between 2008 and 2015, and they prospectively self reported QOL using the validated EORTC QLQ-C30 and EORTC QLQ-CR29. Pain was measured by the Brief Pain Inventory.
Symptoms were measured at baseline and then every 6 months for 5 years or until death.
Within this cohort, 73 (70.2%) patients were amenable to salvage surgery with curative intent. A variety of types of surgery were performed, and R0 resection was achieved in 75% of cases.
The 30-day complication rate was 49% (21% with grade 3/4), and 5-year disease-free survival was 40%. There was no immediate mortality from the surgery.
When looking at differences between patients who underwent surgery and those who didn’t, there was a significant difference in the location of the disease. The nonsurgical group was more likely to have posterior recurrences (26%) than was the surgical group (19%).
“This makes sense since these are more difficult to achieve a R0 result with, so it may be less likely that they were offered the procedure,” Dr. Sammour said.
There was also a significant difference in estimated 5-year survival. Overall survival was 7.7% in the nonsurgical group vs. 50.9% in the surgical group (P less than .0001), and cancer-specific survival was 11.5% vs. 59.6% (P less than .0001).
As for pain and QOL scores, there were no differences between groups at baseline.
“So when they arrived at clinic they had roughly equivalent quality of life,” he said.
At follow-up, male patients were more likely to experience severe pain, but “we felt it was due to the anatomical location. Men have a narrower pelvis and don’t have the luxury of a uterus to protect the genitourinary organs,” he explained. “We suspect this may have played a role in the severity of their pain.”
Patients with posterior recurrences also had worse pain, and this was true for both surgical and nonsurgical patients.
The only determinant for QOL in those who underwent surgery was a positive margin (global health score 70.4 for negative margin and 61.9 for positive margins [P = .024]), but otherwise there were no differences by type of surgery or postoperative complications.
At a median follow-up of 33 months, patients who underwent surgery showed gradual sustained improvement in QOL but not pain scores.
“We were encouraged to see improvement,” concluded Dr. Sammour. “Surgery does improve quality of life in resectable cases.”
No funding source was disclosed. Dr. Sammour and his coauthors had no disclosures.
SEATTLE – Disease anatomy is the main determinant of subsequent quality of life (QOL) in patients with locally recurrent rectal cancer at both base line and in the long term, according to findings presented at the annual Society of Surgical Oncology Cancer Symposium.
Posterior recurrences were associated with the worst QOL scores and the most severe pain.
Recurrent rectal cancer is a morbid disease state, leading to pain and disability. “Patients experience a multitude of symptoms, including disability as a result of tumor growth in a confined space in the pelvis and invasion of adjacent organs as well as complications from surgery and neoadjuvant treatment,” said lead author Dr. Tarik Sammour of the University of Texas MD Anderson Cancer Center in Houston.
He noted, however, that “it is not all doom and gloom.” Survival outcomes have been improving in this population, with the median 5-year survival now approaching 40%-50%.
“Decade by decade, the 5-year survival outcomes are increasing,” explained Dr. Sammour.
Increasing survival also begs the question: “Are we helping patients or affording them the option of living longer with pain and disability? In other words, we need to measure patient-centered outcomes,” he said.
Data for recurrent rectal cancer are very limited, particularly when it comes to measuring patient outcomes. Most studies have been retrospective in design, making it difficult to gauge symptoms and quality of life.
The majority of studies also have focused on surgery, have short follow-up times, and may be missing data.
“Very few measure baseline quality of life, so it makes it difficult to measure trajectories,” Dr. Sammour said. “So overall we don’t know very much about the quality of life of these patients, so we thought to remedy that with a prospective study.”
Dr. Sammour and his colleagues examined the longitudinal trajectory of cancer survivorship in recurrent rectal cancer over a 7-year period. A total of 104 patients diagnosed with recurrent rectal cancer were enrolled between 2008 and 2015, and they prospectively self reported QOL using the validated EORTC QLQ-C30 and EORTC QLQ-CR29. Pain was measured by the Brief Pain Inventory.
Symptoms were measured at baseline and then every 6 months for 5 years or until death.
Within this cohort, 73 (70.2%) patients were amenable to salvage surgery with curative intent. A variety of types of surgery were performed, and R0 resection was achieved in 75% of cases.
The 30-day complication rate was 49% (21% with grade 3/4), and 5-year disease-free survival was 40%. There was no immediate mortality from the surgery.
When looking at differences between patients who underwent surgery and those who didn’t, there was a significant difference in the location of the disease. The nonsurgical group was more likely to have posterior recurrences (26%) than was the surgical group (19%).
“This makes sense since these are more difficult to achieve a R0 result with, so it may be less likely that they were offered the procedure,” Dr. Sammour said.
There was also a significant difference in estimated 5-year survival. Overall survival was 7.7% in the nonsurgical group vs. 50.9% in the surgical group (P less than .0001), and cancer-specific survival was 11.5% vs. 59.6% (P less than .0001).
As for pain and QOL scores, there were no differences between groups at baseline.
“So when they arrived at clinic they had roughly equivalent quality of life,” he said.
At follow-up, male patients were more likely to experience severe pain, but “we felt it was due to the anatomical location. Men have a narrower pelvis and don’t have the luxury of a uterus to protect the genitourinary organs,” he explained. “We suspect this may have played a role in the severity of their pain.”
Patients with posterior recurrences also had worse pain, and this was true for both surgical and nonsurgical patients.
The only determinant for QOL in those who underwent surgery was a positive margin (global health score 70.4 for negative margin and 61.9 for positive margins [P = .024]), but otherwise there were no differences by type of surgery or postoperative complications.
At a median follow-up of 33 months, patients who underwent surgery showed gradual sustained improvement in QOL but not pain scores.
“We were encouraged to see improvement,” concluded Dr. Sammour. “Surgery does improve quality of life in resectable cases.”
No funding source was disclosed. Dr. Sammour and his coauthors had no disclosures.
SEATTLE – Disease anatomy is the main determinant of subsequent quality of life (QOL) in patients with locally recurrent rectal cancer at both base line and in the long term, according to findings presented at the annual Society of Surgical Oncology Cancer Symposium.
Posterior recurrences were associated with the worst QOL scores and the most severe pain.
Recurrent rectal cancer is a morbid disease state, leading to pain and disability. “Patients experience a multitude of symptoms, including disability as a result of tumor growth in a confined space in the pelvis and invasion of adjacent organs as well as complications from surgery and neoadjuvant treatment,” said lead author Dr. Tarik Sammour of the University of Texas MD Anderson Cancer Center in Houston.
He noted, however, that “it is not all doom and gloom.” Survival outcomes have been improving in this population, with the median 5-year survival now approaching 40%-50%.
“Decade by decade, the 5-year survival outcomes are increasing,” explained Dr. Sammour.
Increasing survival also begs the question: “Are we helping patients or affording them the option of living longer with pain and disability? In other words, we need to measure patient-centered outcomes,” he said.
Data for recurrent rectal cancer are very limited, particularly when it comes to measuring patient outcomes. Most studies have been retrospective in design, making it difficult to gauge symptoms and quality of life.
The majority of studies also have focused on surgery, have short follow-up times, and may be missing data.
“Very few measure baseline quality of life, so it makes it difficult to measure trajectories,” Dr. Sammour said. “So overall we don’t know very much about the quality of life of these patients, so we thought to remedy that with a prospective study.”
Dr. Sammour and his colleagues examined the longitudinal trajectory of cancer survivorship in recurrent rectal cancer over a 7-year period. A total of 104 patients diagnosed with recurrent rectal cancer were enrolled between 2008 and 2015, and they prospectively self reported QOL using the validated EORTC QLQ-C30 and EORTC QLQ-CR29. Pain was measured by the Brief Pain Inventory.
Symptoms were measured at baseline and then every 6 months for 5 years or until death.
Within this cohort, 73 (70.2%) patients were amenable to salvage surgery with curative intent. A variety of types of surgery were performed, and R0 resection was achieved in 75% of cases.
The 30-day complication rate was 49% (21% with grade 3/4), and 5-year disease-free survival was 40%. There was no immediate mortality from the surgery.
When looking at differences between patients who underwent surgery and those who didn’t, there was a significant difference in the location of the disease. The nonsurgical group was more likely to have posterior recurrences (26%) than was the surgical group (19%).
“This makes sense since these are more difficult to achieve a R0 result with, so it may be less likely that they were offered the procedure,” Dr. Sammour said.
There was also a significant difference in estimated 5-year survival. Overall survival was 7.7% in the nonsurgical group vs. 50.9% in the surgical group (P less than .0001), and cancer-specific survival was 11.5% vs. 59.6% (P less than .0001).
As for pain and QOL scores, there were no differences between groups at baseline.
“So when they arrived at clinic they had roughly equivalent quality of life,” he said.
At follow-up, male patients were more likely to experience severe pain, but “we felt it was due to the anatomical location. Men have a narrower pelvis and don’t have the luxury of a uterus to protect the genitourinary organs,” he explained. “We suspect this may have played a role in the severity of their pain.”
Patients with posterior recurrences also had worse pain, and this was true for both surgical and nonsurgical patients.
The only determinant for QOL in those who underwent surgery was a positive margin (global health score 70.4 for negative margin and 61.9 for positive margins [P = .024]), but otherwise there were no differences by type of surgery or postoperative complications.
At a median follow-up of 33 months, patients who underwent surgery showed gradual sustained improvement in QOL but not pain scores.
“We were encouraged to see improvement,” concluded Dr. Sammour. “Surgery does improve quality of life in resectable cases.”
No funding source was disclosed. Dr. Sammour and his coauthors had no disclosures.
AT SSO 2017
Key clinical point: Posterior recurrence in recurrent rectal cancer was associated with worst quality of life and pain.
Major finding: Surgery improved overall survival (7.7% in the nonsurgical group vs. 50.9% in the surgical group [P less than .0001]) and quality of life.
Data source: Prospective study involved 104 patients with recurrent rectal cancer.
Disclosures: No funding source was disclosed. Dr. Sammour and his coauthors had no disclosures.
Survivorship care models work, some better than others
ORLANDO – Accumulating experience is showing the benefits of various models of care for cancer survivors in terms of health care use and costs, while also suggesting that some provide higher-quality care than others, according to a pair of studies reported at a symposium on quality care sponsored by the American Society of Clinical Oncology.
Initiative for breast cancer survivors
“In 2011, Cancer Care Ontario did a quick environmental scan of our 14 regional cancer centers and found that the transition of breast cancer survivors from oncologists to primary care was very variable, and that centers often didn’t transition patients very frequently,” said Nicole Mittmann, PhD, first author on one of the studies, chief research officer for Cancer Care Ontario, and an investigator at Sunnybrook Research Institute, Toronto.
The advisory organization therefore implemented the Well Follow-Up Care Initiative to facilitate appropriate transition of breast cancer survivors. Each regional center was given a $100,000 incentive to roll out a model of the initiative.
Dr. Mittmann and her coinvestigators used provincial administrative databases to compare health care use and associated costs between 2,324 breast cancer survivors who were transitioned with the initiative and 2,324 propensity-matched control survivors who were not. The survivors were about 5 years out from their breast cancer diagnosis at baseline and had median follow-up of 2 years.
Study results reported at the symposium showed that the mean annual total cost of care per patient paid for by the provincial health ministry was $6,575 for the transitioned group and $10,832 for the nontransitioned group, a difference of $4,257 (39%). The main drivers were reduced costs of long-term care and cancer clinic visits.
Findings were similar for median annual costs, which amounted to $2,261 for the transitioned group and $2,903 for the control group, a difference of $638.
Compared with the nontransitioned group, the transitioned group had significantly fewer annual visits to medical oncologists (0.39 vs. 1.29) and radiation oncologists (0.16 vs. 0.36), while visits to general or family practitioners were statistically indistinguishable (7.35 and 7.91), Dr. Mittmann reported. There was also a trend toward fewer emergency department visits.
The transitioned group had fewer bone scans, CT and MRI scans, and radiographs annually, but differences were not significant.
Reassuringly, Dr. Mittmann said, survivors who were transitioned did not fare worse than their nontransitioned counterparts in overall survival; if anything, they tended to live longer. “We think that because the individual cancer centers enrolled patients that they thought were very well that this is a very well and highly selected and maybe a biased group,” Dr. Mittmann acknowledged. “But we certainly see that they are not doing worse than the control group.”
“About $1.4 million was distributed to the cancer centers” for the initiative, she noted. “That generated a savings for the health system of $1.5 million, if you are looking at median costs, to $9.9 million, if you are looking at mean costs.
“The transition of appropriate breast cancer survivors to the community appears to be safe and effective outside of a clinical trial, at least based on this particular retrospective analysis using databases,” she said. “The overall costs are not increased, and they may actually be decreased based on our data, and certainly these results will inform policy.”
The investigators plan several next steps, such as encouraging senior leadership at Cancer Care Ontario and the Ministry of Health to endorse the findings, according to Dr. Mittmann. In addition, “[we plan to] engage with both oncology and primary care leadership and think about how we can potentially roll out a program like this, and develop tools, whether those are letters or information packages, and education, to … appropriately transition individuals.”
Considerations in interpreting the study’s findings include the quality of the matching of survivors, according to invited discussant Monika K. Krzyzanowska, MD, a medical oncologist at Princess Margaret Cancer Centre, an associate professor at the University of Toronto, and a clinical lead of Quality Care and Access, Systemic Treatment Program, at Cancer Care Ontario. “The quality of that match depends on what’s in the model, so there could be potential for residual confounding, and administrative data may not have all of the elements that you would need to get a perfect match.”
Additional considerations include costs not covered by the payer, impact of the initiative on delivery of guideline-recommended care and patient and provider satisfaction, generalizability of the findings, and long-term outcomes.
“This is a proof of concept, certainly, that transition of low-risk cancer survivors to primary care is feasible and potentially economically attractive,” Dr. Krzyzanowska concluded. “It would be useful to have a formal evaluation of effectiveness that would inform a comprehensive value assessment. And we do have data from a randomized trial about the safety of this particular approach, but it would be nice to see that following implementation in real practices, those safety considerations played out the same way.”
Comparison of survivorship care models
Two-thirds of the large and growing population of cancer survivors are at least 5 years out from diagnosis, stimulating considerable discussion in the oncology community about how to best address their needs, according to Sarah Raskin, PhD, senior author on the second study and a research scientist at the Institute for Patient-Centered Initiatives and Health Equity at George Washington University Cancer Center, Washington.
“Yet, for a lack of cancer survivorship–specific guidelines from research or practice, cancer centers are increasingly developing survivorship care in a variety of ways, many of which are ad hoc or unproven as yet,” she said.
Dr. Raskin and her colleagues compared three emerging models of survivorship care: a specialized consultative model and a specialized longitudinal model – whereby patients have a single or multiple formalized survivorship visits, respectively, with care typically led by an oncology nurse-practitioner – and an oncology-embedded model – whereby survivorship is addressed as a part of ongoing oncology follow-up care, typically by the oncologist.
The investigators worked with survivors to develop the Patient-Prioritized Measure of High-Quality Survivorship Care, a 46-question scale assessing nine components of survivorship care that capture the health care priorities and needs that matter most to patients. Each component is rated on a scale from 0 (not at all met) to 1 (somewhat met) to 2 (definitely met).
Analyses were based on responses of 827 survivors of breast, colorectal, and prostate cancer who received care at 28 U.S. institutions using one of the above models and who were surveyed by telephone about the care received 1 week after their initial survivorship visit.
Results showed that survivors cared for under the three models differed significantly with respect to scores for seven of the nine components of quality of care, Dr. Raskin reported. The exceptions were practical life support, where the mean score was about 0.6-0.8 across the board, and having a medical home, where the mean score was about 1.8-1.9 across the board.
The specialized consult model of care had the highest scores for mental health and social support, information and resources, and supportive and prepared clinicians. The specialized longitudinal model of care had the highest scores for empowered and engaged patients, open patient-clinician communication, care coordination and transitions, and access to full spectrum of care. The oncology-embedded model had the lowest scores. Analysis of the tool’s 46 individual questions showed that patients cared for at institutions using the oncology-embedded model were significantly less likely than were counterparts cared for at institutions using the specialized models to report that the institution performed various activities such as offering a treatment summary, inquiring about the patient’s biggest worries or problems, and explaining the reasons why tests were needed (P less than .05 for each).
For some metrics, the overall proportion reporting that an activity was performed was low, regardless of the model being used. For example, only 48% of all patients reported being helped to set goals or make short-term plans to manage follow-up care and improve health, merely 24% reported being provided emotional and social support to deal with changes in relationships, and just 19% reported being referred to special providers for other medical problems.
“Overall, all three models are performing highly in terms of providing survivors with a medical home and communicating with patients. However, all three are performing quite low in terms of providing mental health and social support, as well as practical life support,” said Dr. Raskin.
“By model, we see that the embedded ongoing care model is significantly underperforming compared with both specialized models on seven of nine components, and we have some hypotheses from our early work with [Commission on Cancer]–accredited centers to explain this,” she added. “Embedded survivorship models have a lot of variability – many are high performers but others are low performers as compared with specialized programs. Embedded survivorship care models are typically led by the treating oncologist, who historically has focused on treating sick patients and less so on providing social supports for follow-up of well patients or ‘well-er’ patients. At the same time, specialized models focus predominantly on survivorship care and providing services and referrals for survivors, which may explain their high scores.
“We know that the higher quality of care measures presented here do not necessarily translate to better patient outcomes, and that’s actually going to be the next phase of our analysis,” she concluded.
The study sample may have had some selection bias, and it is unclear how well validated the tool was, according to Dr. Krzyzanowska, the discussant. Another issue was its assessment of quality of care at only a single time point.
Nonetheless, the findings show “that measuring quality of survivorship care from a patient perspective is feasible and valuable. We have already heard about [need for] survivorship plans in survivorship care, so certainly the work that was just presented is extremely important to help to fill some of these gaps,” she said.
“I’m not sure that we yet know what the optimal model of survivorship care is without the information of the other outcomes. Furthermore, there’s different survivor populations and different ways that health care is organized, so perhaps there isn’t really one optimal model, but the model has to fit with the context,” Dr. Krzyzanowska concluded. “That being said … the tool that they have created can be a great tool for existing survivorship care programs to assess and improve the quality of their care.”
Dr. Mittmann and Dr. Raskin had no disclosures to report.
ORLANDO – Accumulating experience is showing the benefits of various models of care for cancer survivors in terms of health care use and costs, while also suggesting that some provide higher-quality care than others, according to a pair of studies reported at a symposium on quality care sponsored by the American Society of Clinical Oncology.
Initiative for breast cancer survivors
“In 2011, Cancer Care Ontario did a quick environmental scan of our 14 regional cancer centers and found that the transition of breast cancer survivors from oncologists to primary care was very variable, and that centers often didn’t transition patients very frequently,” said Nicole Mittmann, PhD, first author on one of the studies, chief research officer for Cancer Care Ontario, and an investigator at Sunnybrook Research Institute, Toronto.
The advisory organization therefore implemented the Well Follow-Up Care Initiative to facilitate appropriate transition of breast cancer survivors. Each regional center was given a $100,000 incentive to roll out a model of the initiative.
Dr. Mittmann and her coinvestigators used provincial administrative databases to compare health care use and associated costs between 2,324 breast cancer survivors who were transitioned with the initiative and 2,324 propensity-matched control survivors who were not. The survivors were about 5 years out from their breast cancer diagnosis at baseline and had median follow-up of 2 years.
Study results reported at the symposium showed that the mean annual total cost of care per patient paid for by the provincial health ministry was $6,575 for the transitioned group and $10,832 for the nontransitioned group, a difference of $4,257 (39%). The main drivers were reduced costs of long-term care and cancer clinic visits.
Findings were similar for median annual costs, which amounted to $2,261 for the transitioned group and $2,903 for the control group, a difference of $638.
Compared with the nontransitioned group, the transitioned group had significantly fewer annual visits to medical oncologists (0.39 vs. 1.29) and radiation oncologists (0.16 vs. 0.36), while visits to general or family practitioners were statistically indistinguishable (7.35 and 7.91), Dr. Mittmann reported. There was also a trend toward fewer emergency department visits.
The transitioned group had fewer bone scans, CT and MRI scans, and radiographs annually, but differences were not significant.
Reassuringly, Dr. Mittmann said, survivors who were transitioned did not fare worse than their nontransitioned counterparts in overall survival; if anything, they tended to live longer. “We think that because the individual cancer centers enrolled patients that they thought were very well that this is a very well and highly selected and maybe a biased group,” Dr. Mittmann acknowledged. “But we certainly see that they are not doing worse than the control group.”
“About $1.4 million was distributed to the cancer centers” for the initiative, she noted. “That generated a savings for the health system of $1.5 million, if you are looking at median costs, to $9.9 million, if you are looking at mean costs.
“The transition of appropriate breast cancer survivors to the community appears to be safe and effective outside of a clinical trial, at least based on this particular retrospective analysis using databases,” she said. “The overall costs are not increased, and they may actually be decreased based on our data, and certainly these results will inform policy.”
The investigators plan several next steps, such as encouraging senior leadership at Cancer Care Ontario and the Ministry of Health to endorse the findings, according to Dr. Mittmann. In addition, “[we plan to] engage with both oncology and primary care leadership and think about how we can potentially roll out a program like this, and develop tools, whether those are letters or information packages, and education, to … appropriately transition individuals.”
Considerations in interpreting the study’s findings include the quality of the matching of survivors, according to invited discussant Monika K. Krzyzanowska, MD, a medical oncologist at Princess Margaret Cancer Centre, an associate professor at the University of Toronto, and a clinical lead of Quality Care and Access, Systemic Treatment Program, at Cancer Care Ontario. “The quality of that match depends on what’s in the model, so there could be potential for residual confounding, and administrative data may not have all of the elements that you would need to get a perfect match.”
Additional considerations include costs not covered by the payer, impact of the initiative on delivery of guideline-recommended care and patient and provider satisfaction, generalizability of the findings, and long-term outcomes.
“This is a proof of concept, certainly, that transition of low-risk cancer survivors to primary care is feasible and potentially economically attractive,” Dr. Krzyzanowska concluded. “It would be useful to have a formal evaluation of effectiveness that would inform a comprehensive value assessment. And we do have data from a randomized trial about the safety of this particular approach, but it would be nice to see that following implementation in real practices, those safety considerations played out the same way.”
Comparison of survivorship care models
Two-thirds of the large and growing population of cancer survivors are at least 5 years out from diagnosis, stimulating considerable discussion in the oncology community about how to best address their needs, according to Sarah Raskin, PhD, senior author on the second study and a research scientist at the Institute for Patient-Centered Initiatives and Health Equity at George Washington University Cancer Center, Washington.
“Yet, for a lack of cancer survivorship–specific guidelines from research or practice, cancer centers are increasingly developing survivorship care in a variety of ways, many of which are ad hoc or unproven as yet,” she said.
Dr. Raskin and her colleagues compared three emerging models of survivorship care: a specialized consultative model and a specialized longitudinal model – whereby patients have a single or multiple formalized survivorship visits, respectively, with care typically led by an oncology nurse-practitioner – and an oncology-embedded model – whereby survivorship is addressed as a part of ongoing oncology follow-up care, typically by the oncologist.
The investigators worked with survivors to develop the Patient-Prioritized Measure of High-Quality Survivorship Care, a 46-question scale assessing nine components of survivorship care that capture the health care priorities and needs that matter most to patients. Each component is rated on a scale from 0 (not at all met) to 1 (somewhat met) to 2 (definitely met).
Analyses were based on responses of 827 survivors of breast, colorectal, and prostate cancer who received care at 28 U.S. institutions using one of the above models and who were surveyed by telephone about the care received 1 week after their initial survivorship visit.
Results showed that survivors cared for under the three models differed significantly with respect to scores for seven of the nine components of quality of care, Dr. Raskin reported. The exceptions were practical life support, where the mean score was about 0.6-0.8 across the board, and having a medical home, where the mean score was about 1.8-1.9 across the board.
The specialized consult model of care had the highest scores for mental health and social support, information and resources, and supportive and prepared clinicians. The specialized longitudinal model of care had the highest scores for empowered and engaged patients, open patient-clinician communication, care coordination and transitions, and access to full spectrum of care. The oncology-embedded model had the lowest scores. Analysis of the tool’s 46 individual questions showed that patients cared for at institutions using the oncology-embedded model were significantly less likely than were counterparts cared for at institutions using the specialized models to report that the institution performed various activities such as offering a treatment summary, inquiring about the patient’s biggest worries or problems, and explaining the reasons why tests were needed (P less than .05 for each).
For some metrics, the overall proportion reporting that an activity was performed was low, regardless of the model being used. For example, only 48% of all patients reported being helped to set goals or make short-term plans to manage follow-up care and improve health, merely 24% reported being provided emotional and social support to deal with changes in relationships, and just 19% reported being referred to special providers for other medical problems.
“Overall, all three models are performing highly in terms of providing survivors with a medical home and communicating with patients. However, all three are performing quite low in terms of providing mental health and social support, as well as practical life support,” said Dr. Raskin.
“By model, we see that the embedded ongoing care model is significantly underperforming compared with both specialized models on seven of nine components, and we have some hypotheses from our early work with [Commission on Cancer]–accredited centers to explain this,” she added. “Embedded survivorship models have a lot of variability – many are high performers but others are low performers as compared with specialized programs. Embedded survivorship care models are typically led by the treating oncologist, who historically has focused on treating sick patients and less so on providing social supports for follow-up of well patients or ‘well-er’ patients. At the same time, specialized models focus predominantly on survivorship care and providing services and referrals for survivors, which may explain their high scores.
“We know that the higher quality of care measures presented here do not necessarily translate to better patient outcomes, and that’s actually going to be the next phase of our analysis,” she concluded.
The study sample may have had some selection bias, and it is unclear how well validated the tool was, according to Dr. Krzyzanowska, the discussant. Another issue was its assessment of quality of care at only a single time point.
Nonetheless, the findings show “that measuring quality of survivorship care from a patient perspective is feasible and valuable. We have already heard about [need for] survivorship plans in survivorship care, so certainly the work that was just presented is extremely important to help to fill some of these gaps,” she said.
“I’m not sure that we yet know what the optimal model of survivorship care is without the information of the other outcomes. Furthermore, there’s different survivor populations and different ways that health care is organized, so perhaps there isn’t really one optimal model, but the model has to fit with the context,” Dr. Krzyzanowska concluded. “That being said … the tool that they have created can be a great tool for existing survivorship care programs to assess and improve the quality of their care.”
Dr. Mittmann and Dr. Raskin had no disclosures to report.
ORLANDO – Accumulating experience is showing the benefits of various models of care for cancer survivors in terms of health care use and costs, while also suggesting that some provide higher-quality care than others, according to a pair of studies reported at a symposium on quality care sponsored by the American Society of Clinical Oncology.
Initiative for breast cancer survivors
“In 2011, Cancer Care Ontario did a quick environmental scan of our 14 regional cancer centers and found that the transition of breast cancer survivors from oncologists to primary care was very variable, and that centers often didn’t transition patients very frequently,” said Nicole Mittmann, PhD, first author on one of the studies, chief research officer for Cancer Care Ontario, and an investigator at Sunnybrook Research Institute, Toronto.
The advisory organization therefore implemented the Well Follow-Up Care Initiative to facilitate appropriate transition of breast cancer survivors. Each regional center was given a $100,000 incentive to roll out a model of the initiative.
Dr. Mittmann and her coinvestigators used provincial administrative databases to compare health care use and associated costs between 2,324 breast cancer survivors who were transitioned with the initiative and 2,324 propensity-matched control survivors who were not. The survivors were about 5 years out from their breast cancer diagnosis at baseline and had median follow-up of 2 years.
Study results reported at the symposium showed that the mean annual total cost of care per patient paid for by the provincial health ministry was $6,575 for the transitioned group and $10,832 for the nontransitioned group, a difference of $4,257 (39%). The main drivers were reduced costs of long-term care and cancer clinic visits.
Findings were similar for median annual costs, which amounted to $2,261 for the transitioned group and $2,903 for the control group, a difference of $638.
Compared with the nontransitioned group, the transitioned group had significantly fewer annual visits to medical oncologists (0.39 vs. 1.29) and radiation oncologists (0.16 vs. 0.36), while visits to general or family practitioners were statistically indistinguishable (7.35 and 7.91), Dr. Mittmann reported. There was also a trend toward fewer emergency department visits.
The transitioned group had fewer bone scans, CT and MRI scans, and radiographs annually, but differences were not significant.
Reassuringly, Dr. Mittmann said, survivors who were transitioned did not fare worse than their nontransitioned counterparts in overall survival; if anything, they tended to live longer. “We think that because the individual cancer centers enrolled patients that they thought were very well that this is a very well and highly selected and maybe a biased group,” Dr. Mittmann acknowledged. “But we certainly see that they are not doing worse than the control group.”
“About $1.4 million was distributed to the cancer centers” for the initiative, she noted. “That generated a savings for the health system of $1.5 million, if you are looking at median costs, to $9.9 million, if you are looking at mean costs.
“The transition of appropriate breast cancer survivors to the community appears to be safe and effective outside of a clinical trial, at least based on this particular retrospective analysis using databases,” she said. “The overall costs are not increased, and they may actually be decreased based on our data, and certainly these results will inform policy.”
The investigators plan several next steps, such as encouraging senior leadership at Cancer Care Ontario and the Ministry of Health to endorse the findings, according to Dr. Mittmann. In addition, “[we plan to] engage with both oncology and primary care leadership and think about how we can potentially roll out a program like this, and develop tools, whether those are letters or information packages, and education, to … appropriately transition individuals.”
Considerations in interpreting the study’s findings include the quality of the matching of survivors, according to invited discussant Monika K. Krzyzanowska, MD, a medical oncologist at Princess Margaret Cancer Centre, an associate professor at the University of Toronto, and a clinical lead of Quality Care and Access, Systemic Treatment Program, at Cancer Care Ontario. “The quality of that match depends on what’s in the model, so there could be potential for residual confounding, and administrative data may not have all of the elements that you would need to get a perfect match.”
Additional considerations include costs not covered by the payer, impact of the initiative on delivery of guideline-recommended care and patient and provider satisfaction, generalizability of the findings, and long-term outcomes.
“This is a proof of concept, certainly, that transition of low-risk cancer survivors to primary care is feasible and potentially economically attractive,” Dr. Krzyzanowska concluded. “It would be useful to have a formal evaluation of effectiveness that would inform a comprehensive value assessment. And we do have data from a randomized trial about the safety of this particular approach, but it would be nice to see that following implementation in real practices, those safety considerations played out the same way.”
Comparison of survivorship care models
Two-thirds of the large and growing population of cancer survivors are at least 5 years out from diagnosis, stimulating considerable discussion in the oncology community about how to best address their needs, according to Sarah Raskin, PhD, senior author on the second study and a research scientist at the Institute for Patient-Centered Initiatives and Health Equity at George Washington University Cancer Center, Washington.
“Yet, for a lack of cancer survivorship–specific guidelines from research or practice, cancer centers are increasingly developing survivorship care in a variety of ways, many of which are ad hoc or unproven as yet,” she said.
Dr. Raskin and her colleagues compared three emerging models of survivorship care: a specialized consultative model and a specialized longitudinal model – whereby patients have a single or multiple formalized survivorship visits, respectively, with care typically led by an oncology nurse-practitioner – and an oncology-embedded model – whereby survivorship is addressed as a part of ongoing oncology follow-up care, typically by the oncologist.
The investigators worked with survivors to develop the Patient-Prioritized Measure of High-Quality Survivorship Care, a 46-question scale assessing nine components of survivorship care that capture the health care priorities and needs that matter most to patients. Each component is rated on a scale from 0 (not at all met) to 1 (somewhat met) to 2 (definitely met).
Analyses were based on responses of 827 survivors of breast, colorectal, and prostate cancer who received care at 28 U.S. institutions using one of the above models and who were surveyed by telephone about the care received 1 week after their initial survivorship visit.
Results showed that survivors cared for under the three models differed significantly with respect to scores for seven of the nine components of quality of care, Dr. Raskin reported. The exceptions were practical life support, where the mean score was about 0.6-0.8 across the board, and having a medical home, where the mean score was about 1.8-1.9 across the board.
The specialized consult model of care had the highest scores for mental health and social support, information and resources, and supportive and prepared clinicians. The specialized longitudinal model of care had the highest scores for empowered and engaged patients, open patient-clinician communication, care coordination and transitions, and access to full spectrum of care. The oncology-embedded model had the lowest scores. Analysis of the tool’s 46 individual questions showed that patients cared for at institutions using the oncology-embedded model were significantly less likely than were counterparts cared for at institutions using the specialized models to report that the institution performed various activities such as offering a treatment summary, inquiring about the patient’s biggest worries or problems, and explaining the reasons why tests were needed (P less than .05 for each).
For some metrics, the overall proportion reporting that an activity was performed was low, regardless of the model being used. For example, only 48% of all patients reported being helped to set goals or make short-term plans to manage follow-up care and improve health, merely 24% reported being provided emotional and social support to deal with changes in relationships, and just 19% reported being referred to special providers for other medical problems.
“Overall, all three models are performing highly in terms of providing survivors with a medical home and communicating with patients. However, all three are performing quite low in terms of providing mental health and social support, as well as practical life support,” said Dr. Raskin.
“By model, we see that the embedded ongoing care model is significantly underperforming compared with both specialized models on seven of nine components, and we have some hypotheses from our early work with [Commission on Cancer]–accredited centers to explain this,” she added. “Embedded survivorship models have a lot of variability – many are high performers but others are low performers as compared with specialized programs. Embedded survivorship care models are typically led by the treating oncologist, who historically has focused on treating sick patients and less so on providing social supports for follow-up of well patients or ‘well-er’ patients. At the same time, specialized models focus predominantly on survivorship care and providing services and referrals for survivors, which may explain their high scores.
“We know that the higher quality of care measures presented here do not necessarily translate to better patient outcomes, and that’s actually going to be the next phase of our analysis,” she concluded.
The study sample may have had some selection bias, and it is unclear how well validated the tool was, according to Dr. Krzyzanowska, the discussant. Another issue was its assessment of quality of care at only a single time point.
Nonetheless, the findings show “that measuring quality of survivorship care from a patient perspective is feasible and valuable. We have already heard about [need for] survivorship plans in survivorship care, so certainly the work that was just presented is extremely important to help to fill some of these gaps,” she said.
“I’m not sure that we yet know what the optimal model of survivorship care is without the information of the other outcomes. Furthermore, there’s different survivor populations and different ways that health care is organized, so perhaps there isn’t really one optimal model, but the model has to fit with the context,” Dr. Krzyzanowska concluded. “That being said … the tool that they have created can be a great tool for existing survivorship care programs to assess and improve the quality of their care.”
Dr. Mittmann and Dr. Raskin had no disclosures to report.
AT THE QUALITY CARE SYMPOSIUM
Key clinical point:
Major finding: Mean annual health care costs were $4,257 (39%) lower for breast cancer survivors actively transitioned to primary care versus control peers. Specialized consult and specialized longitudinal models outperformed an oncology-embedded model on seven quality metrics.
Data source: A cohort study of 2,324 breast cancer survivors transitioned to primary care and 2,324 not transitioned. A cohort study of 827 survivors of breast, colorectal, and prostate cancer receiving care under three differing models.
Disclosures: Dr. Mittmann and Dr. Raskin had no disclosures to report.