User login
Isolated ocular metastases from lung cancer
Non–small cell lung cancer constitutes 80%-85% of lung cancers, and 40% of NSCLC are adenocarcinoma. It is rare to find intraocular metastasis from lung cancer. In this article, we present the case of a patient who presented with complaints of diminished vision redness of the eye and was found to have intra-ocular metastases from lung cancer.
Case presentation and summary
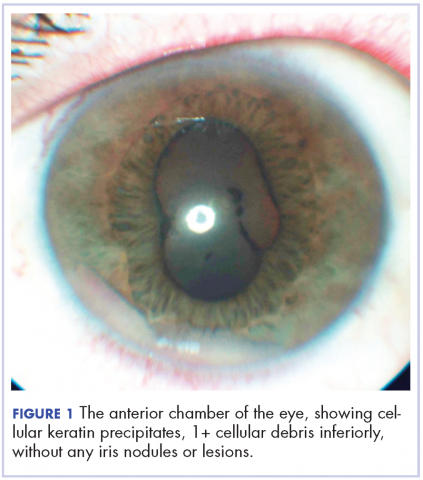
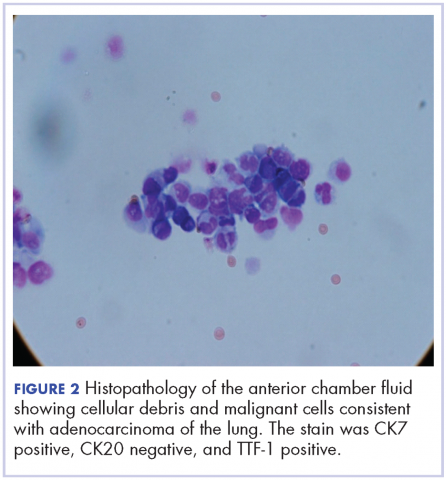
A 60-year-old man with a 40-pack per year history of smoking presented to multiple ophthalmologists with complaints of decreased vision and redness of the left eye. He was eventually evaluated by an ophthalmologist who performed a biopsy of the anterior chamber of the eye. Histologic findings were consistent with adenocarcinoma of lung primary (Figures 1 and 2).
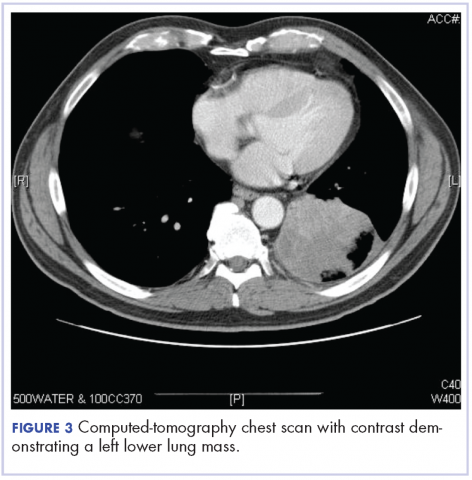
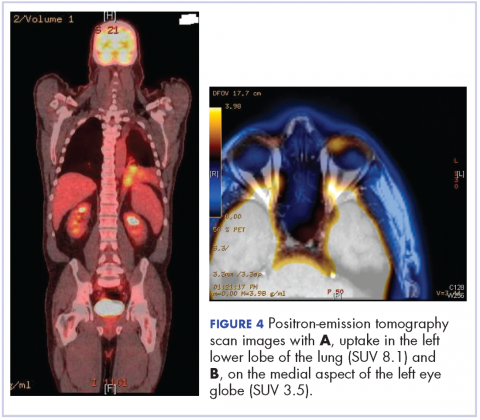
After the diagnosis, a chest X-ray showed that the patient had a left lower lung mass. The results of his physical exam were all within normal limits, with the exception of decreased visual acuity in the left eye. The results of his laboratory studies, including complete blood count and serum chemistries, were also within normal limits. Imaging studies – including a computed-tomography (CT) scan of the chest, abdomen, and pelvis and a full-body positron-emission tomography–CT scan – showed a hypermetabolic left lower lobe mass 4.5 cm and right lower paratracheal lymph node metastasis 2 cm with a small focus of increased uptake alone the medial aspect of the left globe (Figures 3 and 4).
An MRI orbit was performed in an attempt to better characterize the left eye mass, but no optic lesion was identified. A biopsy of the left lower lung mass was consistent with non–small-cell lung cancer (NSCLC). Aside from the isolated left eye metastases, the patient did not have evidence of other distant metastatic involvement.
He was started on palliative chemotherapy on a clinical trial and received intravenous carboplatin AUC 6, pemetrexed 500 mg/m2, and bevacizumab 15 mg/kg every 3 weeks. He received 1 dose intraocular bevacizumab injection before initiation of systemic chemotherapy as he was symptomatic from the intraocular metastases. Within 2 weeks after intravitreal bevacizumab was administered, the patient had subjective improvement in vision. Mutational analysis to identify if the patient would benefit from targeted therapy showed no presence of EGFR mutation and ALK gene rearrangement, and that the patient was K-RAS mutant.
After treatment initiation, interval imaging studies (a computed-tomography scan of the chest, abdomen, pelvis; and magnetic-resonance imaging of the brain) after 3 cycles showed no evidence of disease progression, and after 4 cycles of chemotherapy with these drugs, the patient was started on maintenance chemotherapy with bevacizumab 15 mg/kg and pemetrexed 500 mg/m2.
Discussion
Choroidal metastasis is the most common site of intraocular tumor. In an autopsy study of 230 patients with carcinoma, 12% of cases demonstrated histologic evidence of ocular metastasis.1 A retrospective series of patients with malignant involvement of the eye, 66% of patients had a known history of primary cancer and in 34% of patients the ocular tumor was the first sign of cancer.2 The most common cancers that were found to have ocular metastasis were lung and breast cancer.2 Adenocarcinoma was the most common histologic type of lung cancer to result in ocular metastases and was seen in 41% of patients.3
Decreased or blurred vision with redness as the primary complaint of NSCLC is rare. Only a few case reports are available. Abundo and colleagues reported that 0.7%-12% of patients with lung cancer develop ocular metastases.4 Therefore, routine ophthalmologic screening for ocular metastases in patients with cancer has not been pursued in asymptomatic patients.5 Ophthalmological evaluation is recommended in symptomatic patients.
Metastatic involvement of two or more other organs was found to be a risk factor for development of choroidal metastasis in patients with lung cancer though in our patient no evidence of other organ involvement was found.5 The most common site of metastases in patients with NSCLC with ocular metastases was found to be the liver. Choroidal metastases was reported to be the sixth common site of metastases in patients with lung cancer.5
Treatment of ocular manifestations has been generally confined to surgical resection or radiation therapy, but advances in chemotherapy and development of novel targeted agents have shown promising results.7 Median life expectancy after a diagnosis of uveal metastases was reported to be 12 months in a retrospective study, which is similar to the reported median survival in metastatic NSCLC.8
Our patient was enrolled in a clinical trial and was treated with a regimen of carboplatin, paclitaxel, and bevacizumab. On presentation, he had significant impairment of vision with pain. He was treated with intravitreal bevacizumab yielding improvement in his visual symptoms. Bevacizumab is a vascular endothelial growth factor receptor monoclonal antibody approved for use in patients with metastatic lung cancer. Other pathways that have been reported in development of lung cancer involve the ALK gene translocation, and EGFR and K-RAS mutations, and targeted therapy has shown good results in cancer patients with these molecular defects. Randomized clinical trials in patients with advanced NSCLC and an EGFR mutation have shown significant improvement in overall survival with the use of erlotinib, a tyrosine kinase inhibitor targeting the epidermal growth factor receptor.9 Similarly, crizotinib has shown promising results in patients with metastatic NSCLC who have ELM-ALK rearrangement.10 As our patient’s tumor did not have either of these mutations, he was initiated on chemotherapy with bevacizumab. The presence of a K-RAS mutation in this patient further supported the use of front-line chemotherapy given that it may confer resistance against agents that target the EGFR pathway.
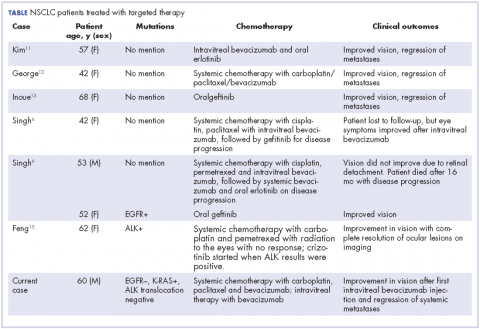
In our review of the literature, we found cases of patients with ocular metastases who responded well to therapy with targeted agents (Table).
Singh and colleagues did a systematic review of 55 cases of patients with lung cancer and choroidal metastases and found that the type of therapy depended on when the diagnosis had been made in relation to the advent of targeted therapy: cases diagnosed before targeted therapy had received radiation therapy or enucleation.6 As far as we could ascertain, there have been no randomized studies evaluating the impact of various targeted therapies or systemic chemotherapy on ocular metastases, although case reports have documented improvement in vision and regression of metastases with such therapy.
Conclusion
The goal of therapy in metastatic lung cancer is palliation of symptoms and improvement in patient quality of life with prolongation in overall survival. The newer targeted chemotherapeutic agents assist in achieving these goals and may decrease the morbidity associated from radiation or surgery with improvement in vision and regression of ocular metastatic lesions. Targeted therapies should be considered in the treatment of patients with ocular metastases from NSCLC.
1. Bloch RS, Gartner S. The incidence of ocular metastatic carcinoma. Arch Ophthalmol-Chic. 1971;85(6):673-675.
2. Shields CL, Shields JA, Gross NE, Schwartz GP, Lally SE. Survey of 520 eyes with uveal metastases. Ophthalmology. 1997;104(8):1265-1276.
3. Kreusel KM, Bechrakis NE, Wiegel T, Krause L, Foerster MH. Incidence and clinical characteristics of symptomatic choroidal metastasis from lung cancer. Acta Ophthalmol. 2008;86(5):515-519.
4. Abundo RE, Orenic CJ, Anderson SF, Townsend JC. Choroidal metastases resulting from carcinoma of the lung. J Am Optom Assoc. 1997;68(2):95-108.
5. Kreusel KM, Wiegel T, Stange M, Bornfeld N, Hinkelbein W, Foerster MH. Choroidal metastasis in disseminated lung cancer: frequency and risk factors. Am J Ophthalmol. 2002;134(3):445-447.
6. Singh N, Kulkarni P, Aggarwal AN, et al. Choroidal metastasis as a presenting manifestation of lung cancer: a report of 3 cases and systematic review of the literature. Medicine (Baltimore). 2012;91(4):179-194.
7. Chen CJ, McCoy AN, Brahmer J, Handa JT. Emerging treatments for choroidal metastases. Surv Ophthalmol. 2011;56(6):511-521.
8. Shah SU, Mashayekhi A, Shields CL, et al. Uveal metastasis from lung cancer: clinical features, treatment, and outcome in 194 patients. Ophthalmology. 2014;121(1):352-357.
9. Shepherd FA, Rodrigues Pereira J, Ciuleanu T, et al. Erlotinib in previously treated non-small-cell lung cancer. N Engl J Med. 2005;353(2):123-132.
10. Shaw AT, Kim DW, Nakagawa K, et al. Crizotinib versus chemotherapy in advanced ALK-positive lung cancer. N Engl J Med. 2013;368(25):2385-2394.
11. Kim SW, Kim MJ, Huh K, Oh J. Complete regression of choroidal metastasis secondary to non-small-cell lung cancer with intravitreal bevacizumab and oral erlotinib combination therapy. Ophthalmologica. 2009;223(6):411-413.
12. George B, Wirostko WJ, Connor TB, Choong NW. Complete and durable response of choroid metastasis from non-small cell lung cancer with systemic bevacizumab and chemotherapy. J Thorac Oncol. 2009;4(5):661-662.
13. Inoue M, Watanabe Y, Yamane S, et al. Choroidal metastasis with adenocarcinoma of the lung treated with gefitinib. Eur J Ophthalmol. 2010;20(5):963-965.
14. Shimomura I, Tada Y, Miura G, et al. Choroidal metastasis of non-small cell lung cancer that responded to gefitinib. https://www.hindawi.com/journals/criopm/2013/213124/. Published 2013. Accessed May 4, 2017.
15. Feng Y, Singh AD, Lanigan C, Tubbs RR, Ma PC. Choroidal metastases responsive to crizotinib therapy in a lung adenocarcinoma patient with ALK 2p23 fusion identified by ALK immunohistochemistry. J Thorac Oncol. 2013;8(12):e109-111.
Non–small cell lung cancer constitutes 80%-85% of lung cancers, and 40% of NSCLC are adenocarcinoma. It is rare to find intraocular metastasis from lung cancer. In this article, we present the case of a patient who presented with complaints of diminished vision redness of the eye and was found to have intra-ocular metastases from lung cancer.
Case presentation and summary
A 60-year-old man with a 40-pack per year history of smoking presented to multiple ophthalmologists with complaints of decreased vision and redness of the left eye. He was eventually evaluated by an ophthalmologist who performed a biopsy of the anterior chamber of the eye. Histologic findings were consistent with adenocarcinoma of lung primary (Figures 1 and 2).
After the diagnosis, a chest X-ray showed that the patient had a left lower lung mass. The results of his physical exam were all within normal limits, with the exception of decreased visual acuity in the left eye. The results of his laboratory studies, including complete blood count and serum chemistries, were also within normal limits. Imaging studies – including a computed-tomography (CT) scan of the chest, abdomen, and pelvis and a full-body positron-emission tomography–CT scan – showed a hypermetabolic left lower lobe mass 4.5 cm and right lower paratracheal lymph node metastasis 2 cm with a small focus of increased uptake alone the medial aspect of the left globe (Figures 3 and 4).
An MRI orbit was performed in an attempt to better characterize the left eye mass, but no optic lesion was identified. A biopsy of the left lower lung mass was consistent with non–small-cell lung cancer (NSCLC). Aside from the isolated left eye metastases, the patient did not have evidence of other distant metastatic involvement.
He was started on palliative chemotherapy on a clinical trial and received intravenous carboplatin AUC 6, pemetrexed 500 mg/m2, and bevacizumab 15 mg/kg every 3 weeks. He received 1 dose intraocular bevacizumab injection before initiation of systemic chemotherapy as he was symptomatic from the intraocular metastases. Within 2 weeks after intravitreal bevacizumab was administered, the patient had subjective improvement in vision. Mutational analysis to identify if the patient would benefit from targeted therapy showed no presence of EGFR mutation and ALK gene rearrangement, and that the patient was K-RAS mutant.
After treatment initiation, interval imaging studies (a computed-tomography scan of the chest, abdomen, pelvis; and magnetic-resonance imaging of the brain) after 3 cycles showed no evidence of disease progression, and after 4 cycles of chemotherapy with these drugs, the patient was started on maintenance chemotherapy with bevacizumab 15 mg/kg and pemetrexed 500 mg/m2.
Discussion
Choroidal metastasis is the most common site of intraocular tumor. In an autopsy study of 230 patients with carcinoma, 12% of cases demonstrated histologic evidence of ocular metastasis.1 A retrospective series of patients with malignant involvement of the eye, 66% of patients had a known history of primary cancer and in 34% of patients the ocular tumor was the first sign of cancer.2 The most common cancers that were found to have ocular metastasis were lung and breast cancer.2 Adenocarcinoma was the most common histologic type of lung cancer to result in ocular metastases and was seen in 41% of patients.3
Decreased or blurred vision with redness as the primary complaint of NSCLC is rare. Only a few case reports are available. Abundo and colleagues reported that 0.7%-12% of patients with lung cancer develop ocular metastases.4 Therefore, routine ophthalmologic screening for ocular metastases in patients with cancer has not been pursued in asymptomatic patients.5 Ophthalmological evaluation is recommended in symptomatic patients.
Metastatic involvement of two or more other organs was found to be a risk factor for development of choroidal metastasis in patients with lung cancer though in our patient no evidence of other organ involvement was found.5 The most common site of metastases in patients with NSCLC with ocular metastases was found to be the liver. Choroidal metastases was reported to be the sixth common site of metastases in patients with lung cancer.5
Treatment of ocular manifestations has been generally confined to surgical resection or radiation therapy, but advances in chemotherapy and development of novel targeted agents have shown promising results.7 Median life expectancy after a diagnosis of uveal metastases was reported to be 12 months in a retrospective study, which is similar to the reported median survival in metastatic NSCLC.8
Our patient was enrolled in a clinical trial and was treated with a regimen of carboplatin, paclitaxel, and bevacizumab. On presentation, he had significant impairment of vision with pain. He was treated with intravitreal bevacizumab yielding improvement in his visual symptoms. Bevacizumab is a vascular endothelial growth factor receptor monoclonal antibody approved for use in patients with metastatic lung cancer. Other pathways that have been reported in development of lung cancer involve the ALK gene translocation, and EGFR and K-RAS mutations, and targeted therapy has shown good results in cancer patients with these molecular defects. Randomized clinical trials in patients with advanced NSCLC and an EGFR mutation have shown significant improvement in overall survival with the use of erlotinib, a tyrosine kinase inhibitor targeting the epidermal growth factor receptor.9 Similarly, crizotinib has shown promising results in patients with metastatic NSCLC who have ELM-ALK rearrangement.10 As our patient’s tumor did not have either of these mutations, he was initiated on chemotherapy with bevacizumab. The presence of a K-RAS mutation in this patient further supported the use of front-line chemotherapy given that it may confer resistance against agents that target the EGFR pathway.
In our review of the literature, we found cases of patients with ocular metastases who responded well to therapy with targeted agents (Table).
Singh and colleagues did a systematic review of 55 cases of patients with lung cancer and choroidal metastases and found that the type of therapy depended on when the diagnosis had been made in relation to the advent of targeted therapy: cases diagnosed before targeted therapy had received radiation therapy or enucleation.6 As far as we could ascertain, there have been no randomized studies evaluating the impact of various targeted therapies or systemic chemotherapy on ocular metastases, although case reports have documented improvement in vision and regression of metastases with such therapy.
Conclusion
The goal of therapy in metastatic lung cancer is palliation of symptoms and improvement in patient quality of life with prolongation in overall survival. The newer targeted chemotherapeutic agents assist in achieving these goals and may decrease the morbidity associated from radiation or surgery with improvement in vision and regression of ocular metastatic lesions. Targeted therapies should be considered in the treatment of patients with ocular metastases from NSCLC.
Non–small cell lung cancer constitutes 80%-85% of lung cancers, and 40% of NSCLC are adenocarcinoma. It is rare to find intraocular metastasis from lung cancer. In this article, we present the case of a patient who presented with complaints of diminished vision redness of the eye and was found to have intra-ocular metastases from lung cancer.
Case presentation and summary
A 60-year-old man with a 40-pack per year history of smoking presented to multiple ophthalmologists with complaints of decreased vision and redness of the left eye. He was eventually evaluated by an ophthalmologist who performed a biopsy of the anterior chamber of the eye. Histologic findings were consistent with adenocarcinoma of lung primary (Figures 1 and 2).
After the diagnosis, a chest X-ray showed that the patient had a left lower lung mass. The results of his physical exam were all within normal limits, with the exception of decreased visual acuity in the left eye. The results of his laboratory studies, including complete blood count and serum chemistries, were also within normal limits. Imaging studies – including a computed-tomography (CT) scan of the chest, abdomen, and pelvis and a full-body positron-emission tomography–CT scan – showed a hypermetabolic left lower lobe mass 4.5 cm and right lower paratracheal lymph node metastasis 2 cm with a small focus of increased uptake alone the medial aspect of the left globe (Figures 3 and 4).
An MRI orbit was performed in an attempt to better characterize the left eye mass, but no optic lesion was identified. A biopsy of the left lower lung mass was consistent with non–small-cell lung cancer (NSCLC). Aside from the isolated left eye metastases, the patient did not have evidence of other distant metastatic involvement.
He was started on palliative chemotherapy on a clinical trial and received intravenous carboplatin AUC 6, pemetrexed 500 mg/m2, and bevacizumab 15 mg/kg every 3 weeks. He received 1 dose intraocular bevacizumab injection before initiation of systemic chemotherapy as he was symptomatic from the intraocular metastases. Within 2 weeks after intravitreal bevacizumab was administered, the patient had subjective improvement in vision. Mutational analysis to identify if the patient would benefit from targeted therapy showed no presence of EGFR mutation and ALK gene rearrangement, and that the patient was K-RAS mutant.
After treatment initiation, interval imaging studies (a computed-tomography scan of the chest, abdomen, pelvis; and magnetic-resonance imaging of the brain) after 3 cycles showed no evidence of disease progression, and after 4 cycles of chemotherapy with these drugs, the patient was started on maintenance chemotherapy with bevacizumab 15 mg/kg and pemetrexed 500 mg/m2.
Discussion
Choroidal metastasis is the most common site of intraocular tumor. In an autopsy study of 230 patients with carcinoma, 12% of cases demonstrated histologic evidence of ocular metastasis.1 A retrospective series of patients with malignant involvement of the eye, 66% of patients had a known history of primary cancer and in 34% of patients the ocular tumor was the first sign of cancer.2 The most common cancers that were found to have ocular metastasis were lung and breast cancer.2 Adenocarcinoma was the most common histologic type of lung cancer to result in ocular metastases and was seen in 41% of patients.3
Decreased or blurred vision with redness as the primary complaint of NSCLC is rare. Only a few case reports are available. Abundo and colleagues reported that 0.7%-12% of patients with lung cancer develop ocular metastases.4 Therefore, routine ophthalmologic screening for ocular metastases in patients with cancer has not been pursued in asymptomatic patients.5 Ophthalmological evaluation is recommended in symptomatic patients.
Metastatic involvement of two or more other organs was found to be a risk factor for development of choroidal metastasis in patients with lung cancer though in our patient no evidence of other organ involvement was found.5 The most common site of metastases in patients with NSCLC with ocular metastases was found to be the liver. Choroidal metastases was reported to be the sixth common site of metastases in patients with lung cancer.5
Treatment of ocular manifestations has been generally confined to surgical resection or radiation therapy, but advances in chemotherapy and development of novel targeted agents have shown promising results.7 Median life expectancy after a diagnosis of uveal metastases was reported to be 12 months in a retrospective study, which is similar to the reported median survival in metastatic NSCLC.8
Our patient was enrolled in a clinical trial and was treated with a regimen of carboplatin, paclitaxel, and bevacizumab. On presentation, he had significant impairment of vision with pain. He was treated with intravitreal bevacizumab yielding improvement in his visual symptoms. Bevacizumab is a vascular endothelial growth factor receptor monoclonal antibody approved for use in patients with metastatic lung cancer. Other pathways that have been reported in development of lung cancer involve the ALK gene translocation, and EGFR and K-RAS mutations, and targeted therapy has shown good results in cancer patients with these molecular defects. Randomized clinical trials in patients with advanced NSCLC and an EGFR mutation have shown significant improvement in overall survival with the use of erlotinib, a tyrosine kinase inhibitor targeting the epidermal growth factor receptor.9 Similarly, crizotinib has shown promising results in patients with metastatic NSCLC who have ELM-ALK rearrangement.10 As our patient’s tumor did not have either of these mutations, he was initiated on chemotherapy with bevacizumab. The presence of a K-RAS mutation in this patient further supported the use of front-line chemotherapy given that it may confer resistance against agents that target the EGFR pathway.
In our review of the literature, we found cases of patients with ocular metastases who responded well to therapy with targeted agents (Table).
Singh and colleagues did a systematic review of 55 cases of patients with lung cancer and choroidal metastases and found that the type of therapy depended on when the diagnosis had been made in relation to the advent of targeted therapy: cases diagnosed before targeted therapy had received radiation therapy or enucleation.6 As far as we could ascertain, there have been no randomized studies evaluating the impact of various targeted therapies or systemic chemotherapy on ocular metastases, although case reports have documented improvement in vision and regression of metastases with such therapy.
Conclusion
The goal of therapy in metastatic lung cancer is palliation of symptoms and improvement in patient quality of life with prolongation in overall survival. The newer targeted chemotherapeutic agents assist in achieving these goals and may decrease the morbidity associated from radiation or surgery with improvement in vision and regression of ocular metastatic lesions. Targeted therapies should be considered in the treatment of patients with ocular metastases from NSCLC.
1. Bloch RS, Gartner S. The incidence of ocular metastatic carcinoma. Arch Ophthalmol-Chic. 1971;85(6):673-675.
2. Shields CL, Shields JA, Gross NE, Schwartz GP, Lally SE. Survey of 520 eyes with uveal metastases. Ophthalmology. 1997;104(8):1265-1276.
3. Kreusel KM, Bechrakis NE, Wiegel T, Krause L, Foerster MH. Incidence and clinical characteristics of symptomatic choroidal metastasis from lung cancer. Acta Ophthalmol. 2008;86(5):515-519.
4. Abundo RE, Orenic CJ, Anderson SF, Townsend JC. Choroidal metastases resulting from carcinoma of the lung. J Am Optom Assoc. 1997;68(2):95-108.
5. Kreusel KM, Wiegel T, Stange M, Bornfeld N, Hinkelbein W, Foerster MH. Choroidal metastasis in disseminated lung cancer: frequency and risk factors. Am J Ophthalmol. 2002;134(3):445-447.
6. Singh N, Kulkarni P, Aggarwal AN, et al. Choroidal metastasis as a presenting manifestation of lung cancer: a report of 3 cases and systematic review of the literature. Medicine (Baltimore). 2012;91(4):179-194.
7. Chen CJ, McCoy AN, Brahmer J, Handa JT. Emerging treatments for choroidal metastases. Surv Ophthalmol. 2011;56(6):511-521.
8. Shah SU, Mashayekhi A, Shields CL, et al. Uveal metastasis from lung cancer: clinical features, treatment, and outcome in 194 patients. Ophthalmology. 2014;121(1):352-357.
9. Shepherd FA, Rodrigues Pereira J, Ciuleanu T, et al. Erlotinib in previously treated non-small-cell lung cancer. N Engl J Med. 2005;353(2):123-132.
10. Shaw AT, Kim DW, Nakagawa K, et al. Crizotinib versus chemotherapy in advanced ALK-positive lung cancer. N Engl J Med. 2013;368(25):2385-2394.
11. Kim SW, Kim MJ, Huh K, Oh J. Complete regression of choroidal metastasis secondary to non-small-cell lung cancer with intravitreal bevacizumab and oral erlotinib combination therapy. Ophthalmologica. 2009;223(6):411-413.
12. George B, Wirostko WJ, Connor TB, Choong NW. Complete and durable response of choroid metastasis from non-small cell lung cancer with systemic bevacizumab and chemotherapy. J Thorac Oncol. 2009;4(5):661-662.
13. Inoue M, Watanabe Y, Yamane S, et al. Choroidal metastasis with adenocarcinoma of the lung treated with gefitinib. Eur J Ophthalmol. 2010;20(5):963-965.
14. Shimomura I, Tada Y, Miura G, et al. Choroidal metastasis of non-small cell lung cancer that responded to gefitinib. https://www.hindawi.com/journals/criopm/2013/213124/. Published 2013. Accessed May 4, 2017.
15. Feng Y, Singh AD, Lanigan C, Tubbs RR, Ma PC. Choroidal metastases responsive to crizotinib therapy in a lung adenocarcinoma patient with ALK 2p23 fusion identified by ALK immunohistochemistry. J Thorac Oncol. 2013;8(12):e109-111.
1. Bloch RS, Gartner S. The incidence of ocular metastatic carcinoma. Arch Ophthalmol-Chic. 1971;85(6):673-675.
2. Shields CL, Shields JA, Gross NE, Schwartz GP, Lally SE. Survey of 520 eyes with uveal metastases. Ophthalmology. 1997;104(8):1265-1276.
3. Kreusel KM, Bechrakis NE, Wiegel T, Krause L, Foerster MH. Incidence and clinical characteristics of symptomatic choroidal metastasis from lung cancer. Acta Ophthalmol. 2008;86(5):515-519.
4. Abundo RE, Orenic CJ, Anderson SF, Townsend JC. Choroidal metastases resulting from carcinoma of the lung. J Am Optom Assoc. 1997;68(2):95-108.
5. Kreusel KM, Wiegel T, Stange M, Bornfeld N, Hinkelbein W, Foerster MH. Choroidal metastasis in disseminated lung cancer: frequency and risk factors. Am J Ophthalmol. 2002;134(3):445-447.
6. Singh N, Kulkarni P, Aggarwal AN, et al. Choroidal metastasis as a presenting manifestation of lung cancer: a report of 3 cases and systematic review of the literature. Medicine (Baltimore). 2012;91(4):179-194.
7. Chen CJ, McCoy AN, Brahmer J, Handa JT. Emerging treatments for choroidal metastases. Surv Ophthalmol. 2011;56(6):511-521.
8. Shah SU, Mashayekhi A, Shields CL, et al. Uveal metastasis from lung cancer: clinical features, treatment, and outcome in 194 patients. Ophthalmology. 2014;121(1):352-357.
9. Shepherd FA, Rodrigues Pereira J, Ciuleanu T, et al. Erlotinib in previously treated non-small-cell lung cancer. N Engl J Med. 2005;353(2):123-132.
10. Shaw AT, Kim DW, Nakagawa K, et al. Crizotinib versus chemotherapy in advanced ALK-positive lung cancer. N Engl J Med. 2013;368(25):2385-2394.
11. Kim SW, Kim MJ, Huh K, Oh J. Complete regression of choroidal metastasis secondary to non-small-cell lung cancer with intravitreal bevacizumab and oral erlotinib combination therapy. Ophthalmologica. 2009;223(6):411-413.
12. George B, Wirostko WJ, Connor TB, Choong NW. Complete and durable response of choroid metastasis from non-small cell lung cancer with systemic bevacizumab and chemotherapy. J Thorac Oncol. 2009;4(5):661-662.
13. Inoue M, Watanabe Y, Yamane S, et al. Choroidal metastasis with adenocarcinoma of the lung treated with gefitinib. Eur J Ophthalmol. 2010;20(5):963-965.
14. Shimomura I, Tada Y, Miura G, et al. Choroidal metastasis of non-small cell lung cancer that responded to gefitinib. https://www.hindawi.com/journals/criopm/2013/213124/. Published 2013. Accessed May 4, 2017.
15. Feng Y, Singh AD, Lanigan C, Tubbs RR, Ma PC. Choroidal metastases responsive to crizotinib therapy in a lung adenocarcinoma patient with ALK 2p23 fusion identified by ALK immunohistochemistry. J Thorac Oncol. 2013;8(12):e109-111.
Resolution of refractory pruritus with aprepitant in a patient with microcystic adnexal carcinoma
Substance P is an important neurotransmitter implicated in itch pathways.1 After binding to its receptor, neurokinin-1 (NK-1), substance P induces release of factors including histamine, which may cause pruritus.2 Recent literature has reported successful use of aprepitant, an NK-1 antagonist that has been approved by the US Food and Drug Administration for the treatment of chemotherapy-induced nausea and vomiting, for treatment of pruritus. We report here the case of a patient with microcystic adnexal carcinoma (MAC) who presented with refractory pruritus and who had rapid and complete resolution of itch after administration of aprepitant.
Case presentation and summary
A 73-year-old man presented with a 12-year history of a small nodule on his philtrum, which had been increasing in size. He subsequently developed upper-lip numbness and nasal induration. He complained of 2.5 months of severe, debilitating, full-body pruritus. His symptoms were refractory to treatment with prednisone, gabapentin, doxycycline, doxepin, antihistamines, and topical steroids. At the time of consultation, he was being treated with hydroxyzine and topical pramocaine lotion with minimal relief.
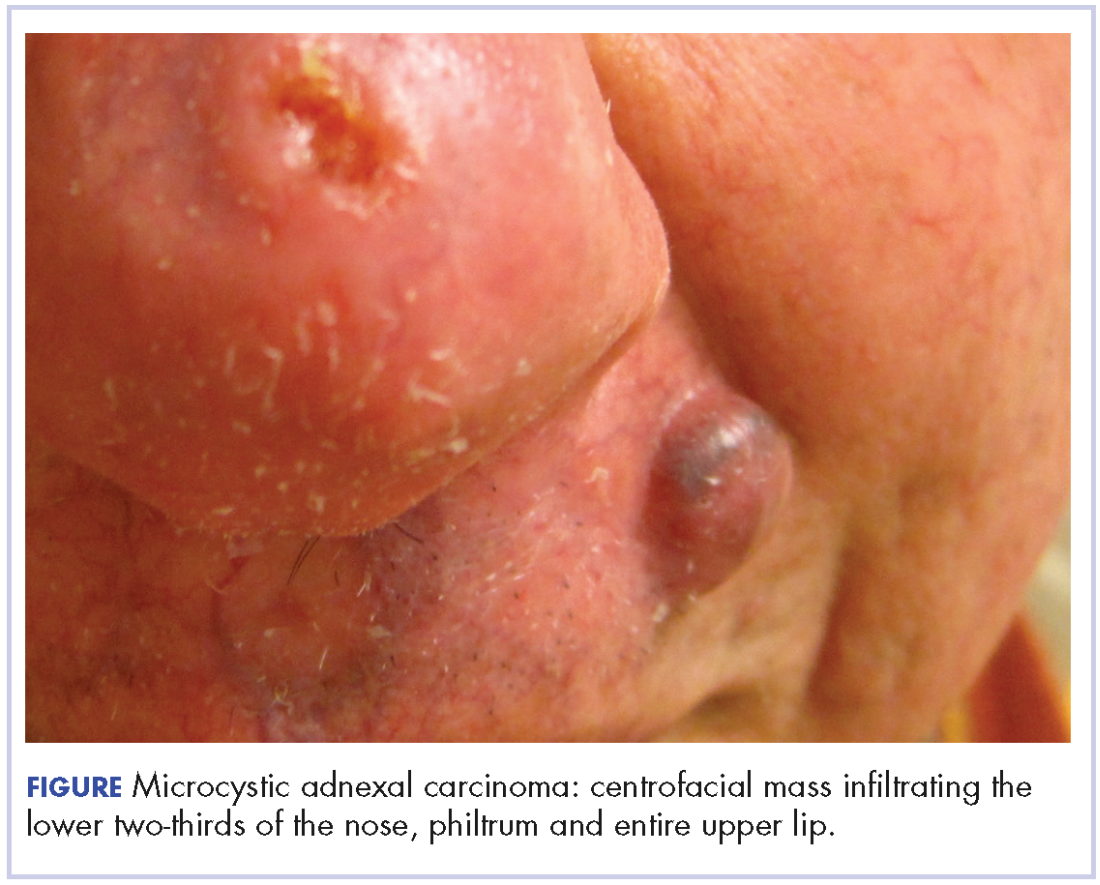
At initial dermatologic evaluation, his tumor involved the lower two-thirds of the nose and entire upper cutaneous lip. There was a 4-mm rolled ulcer on the nasal tip and a 1-cm exophytic, smooth nodule on the left upper lip with palpable 4-cm submandibular adenopathy (Figure). Skin examination otherwise revealed linear excoriations on the upper back with no additional primary lesions. The nodule was biopsied, and the patient was diagnosed with MAC with gross nodal involvement. Laboratory findings including serum chemistries, blood urea nitrogen, complete blood cell count, thyroid, and liver function were normal. Positron emission tomography-computed tomography (PET-CT) imaging was negative for distant metastases.
Treatment was initiated with oral aprepitant – 125 mg on day 1, 80 mg on day 2, and 80 mg on day 3 –with concomitant weekly carboplatin (AUC 1.5) and paclitaxel (30 mg/m2) as well as radiation. Within hours after the first dose of aprepitant, the patient reported a notable cessation in his pruritus. He reported that after 5 hours, his skin “finally turned off” and over the hour that followed, he had complete resolution of symptoms. He completed chemoradiation with a significant disease response. Despite persistent MAC confined to the philtrum, he has been followed for over 2 years without recurrence of itch.
Discussion
MAC is an uncommon cutaneous malignancy of sweat and eccrine gland differentiation. In all, 700 cases of MAC have been described in the literature; a 2008 review estimated the incidence of metastasis at around 2.1%.3 Though metastasis is exceedingly rare, the tumor is locally aggressive and there are reports of invasion into the muscle, perichondrium, periosteum, bone marrow, as well as perineural spaces and vascular adventitia.4
The clinical presentation of MAC includes smooth, flesh-colored or yellow papules, nodules, or plaques.3 Patients often present with numbness, paresthesia, and burning in the area of involvement because of neural infiltration with tumor. Despite the rarity of MAC, pruritus has been reported as a presenting symptom in 1 other case in the literature.4 Our case represents the first report of MAC presenting with a grossly enlarging centrofacial mass, lymph node involvement, and severe full-body pruritus. Our patient responded completely, and within hours, to treatment with aprepitant after experiencing months of failure with conventional antipruritus treatments and without recurrence in symptoms in more than 2 years of follow-up.
Aprepitant blocks the binding of substance P to its receptor NK-1 and has been approved as an anti-emetic for chemotherapy patients. Substance P has been shown to be important in both nausea and itch pathways. The largest prospective study to date on aprepitant for the indication of pruritus in 45 patients with metastatic solid tumors demonstrated a 91% response rate, defined by >50% reduction in pruritus intensity, and 13% recurrence rate that occurred at a median of 7 weeks after initial treatment.5 Aprepitant treatment has been used with success for pruritus associated with both malignant and nonmalignant conditions in at least 74 patients,6 among whom the malignant conditions included cutaneous T-cell lymphoma, Hodgkin lymphoma, and metastatic solid tumors.5-7 Aprepitant has also been used for erlotinib- and nivolumab-induced pruritus in non–small cell lung cancer, which suggests a possible future role for aprepitant in the treatment of pruritus secondary to novel cancer therapies, perhaps including immune checkpoint inhibitors.8-10
However, despite those reports, and likely owing to the multifactorial nature of pruritus, aprepitant is not unviversally effective. Mechanisms of malignancy-associated itch are yet to be elucidated, and optimal patient selection for aprepitant use needs to be determined. However, our patient’s notable response supports the increasing evidence that substance P is a key mediator of pruritus and that disruption of binding to its receptor may result in significant improvement in symptoms in certain patients. It remains to be seen whether the cell type or the tendency toward neural invasion plays a role. Large, randomized studies are needed to guide patient selection and confirm the findings reported here and in the literature, with careful documentation of and close attention paid to timing of pruritus relief and improvement in patient quality of life. Aprepitant might be an important therapeutic tool for refractory, malignancy-associated pruritus, in which patient quality of life is especially critical.
Acknowledgments
This work was presented at the Multinational Association of Supportive Care and Cancer Meeting, in Miami Florida, June 26-28, 2014. The authors are indebted to Saajar Jadeja for his assistance preparing the manuscript.
1. Wallengren J. Neuroanatomy and neurophysiology of itch. Dermatol Ther. 2005;18(4):292-303.
2. Kulka M, Sheen CH, Tancowny BP, Grammer LC, Schleimer RP. Neuropeptides activate human mast cell degranulation and chemokine production. Immunology. 2008;123(3):398-410.
3. Wetter R, Goldstein GD. Microcystic adnexal carcinoma: a diagnostic and therapeutic challenge. Dermatol Ther. 2008;21(6):452-458.
4. Adamson T. Microcystic adnexal carcinoma. Dermatol Nurs. 2004;16(4):365.
5. Santini D, Vincenzi B, Guida FM, et al. Aprepitant for management of severe pruritus related to biological cancer treatments: a pilot study. Lancet Oncol. 2012;13(10):1020-1024.
6. Song JS, Tawa M, Chau NG, Kupper TS, LeBoeuf NR. Aprepitant for refractory cutaneous T-cell lymphoma-associated pruritus: 4 cases and a review of the literature. BMC Cancer. 2017;17.
7. Villafranca JJA, Siles MG, Casanova M, Goitia BT, Domínguez AR. Paraneoplastic pruritus presenting with Hodgkin’s lymphoma: a case report. J Med Case Reports. 2014;8:300.
8. Ito J, Fujimoto D, Nakamura A, et al. Aprepitant for refractory nivolumab-induced pruritus. Lung Cancer Amst Neth. 2017;109:58-61.
9. Levêque D. Aprepitant for erlotinib-induced pruritus. N Engl J Med. 2010;363(17):1680-1681; author reply 1681.
10. Gerber PA, Buhren BA, Homey B. More on aprepitant for erlotinib-induced pruritus. N Engl J Med. 2011;364(5):486-487.
Substance P is an important neurotransmitter implicated in itch pathways.1 After binding to its receptor, neurokinin-1 (NK-1), substance P induces release of factors including histamine, which may cause pruritus.2 Recent literature has reported successful use of aprepitant, an NK-1 antagonist that has been approved by the US Food and Drug Administration for the treatment of chemotherapy-induced nausea and vomiting, for treatment of pruritus. We report here the case of a patient with microcystic adnexal carcinoma (MAC) who presented with refractory pruritus and who had rapid and complete resolution of itch after administration of aprepitant.
Case presentation and summary
A 73-year-old man presented with a 12-year history of a small nodule on his philtrum, which had been increasing in size. He subsequently developed upper-lip numbness and nasal induration. He complained of 2.5 months of severe, debilitating, full-body pruritus. His symptoms were refractory to treatment with prednisone, gabapentin, doxycycline, doxepin, antihistamines, and topical steroids. At the time of consultation, he was being treated with hydroxyzine and topical pramocaine lotion with minimal relief.
At initial dermatologic evaluation, his tumor involved the lower two-thirds of the nose and entire upper cutaneous lip. There was a 4-mm rolled ulcer on the nasal tip and a 1-cm exophytic, smooth nodule on the left upper lip with palpable 4-cm submandibular adenopathy (Figure). Skin examination otherwise revealed linear excoriations on the upper back with no additional primary lesions. The nodule was biopsied, and the patient was diagnosed with MAC with gross nodal involvement. Laboratory findings including serum chemistries, blood urea nitrogen, complete blood cell count, thyroid, and liver function were normal. Positron emission tomography-computed tomography (PET-CT) imaging was negative for distant metastases.
Treatment was initiated with oral aprepitant – 125 mg on day 1, 80 mg on day 2, and 80 mg on day 3 –with concomitant weekly carboplatin (AUC 1.5) and paclitaxel (30 mg/m2) as well as radiation. Within hours after the first dose of aprepitant, the patient reported a notable cessation in his pruritus. He reported that after 5 hours, his skin “finally turned off” and over the hour that followed, he had complete resolution of symptoms. He completed chemoradiation with a significant disease response. Despite persistent MAC confined to the philtrum, he has been followed for over 2 years without recurrence of itch.
Discussion
MAC is an uncommon cutaneous malignancy of sweat and eccrine gland differentiation. In all, 700 cases of MAC have been described in the literature; a 2008 review estimated the incidence of metastasis at around 2.1%.3 Though metastasis is exceedingly rare, the tumor is locally aggressive and there are reports of invasion into the muscle, perichondrium, periosteum, bone marrow, as well as perineural spaces and vascular adventitia.4
The clinical presentation of MAC includes smooth, flesh-colored or yellow papules, nodules, or plaques.3 Patients often present with numbness, paresthesia, and burning in the area of involvement because of neural infiltration with tumor. Despite the rarity of MAC, pruritus has been reported as a presenting symptom in 1 other case in the literature.4 Our case represents the first report of MAC presenting with a grossly enlarging centrofacial mass, lymph node involvement, and severe full-body pruritus. Our patient responded completely, and within hours, to treatment with aprepitant after experiencing months of failure with conventional antipruritus treatments and without recurrence in symptoms in more than 2 years of follow-up.
Aprepitant blocks the binding of substance P to its receptor NK-1 and has been approved as an anti-emetic for chemotherapy patients. Substance P has been shown to be important in both nausea and itch pathways. The largest prospective study to date on aprepitant for the indication of pruritus in 45 patients with metastatic solid tumors demonstrated a 91% response rate, defined by >50% reduction in pruritus intensity, and 13% recurrence rate that occurred at a median of 7 weeks after initial treatment.5 Aprepitant treatment has been used with success for pruritus associated with both malignant and nonmalignant conditions in at least 74 patients,6 among whom the malignant conditions included cutaneous T-cell lymphoma, Hodgkin lymphoma, and metastatic solid tumors.5-7 Aprepitant has also been used for erlotinib- and nivolumab-induced pruritus in non–small cell lung cancer, which suggests a possible future role for aprepitant in the treatment of pruritus secondary to novel cancer therapies, perhaps including immune checkpoint inhibitors.8-10
However, despite those reports, and likely owing to the multifactorial nature of pruritus, aprepitant is not unviversally effective. Mechanisms of malignancy-associated itch are yet to be elucidated, and optimal patient selection for aprepitant use needs to be determined. However, our patient’s notable response supports the increasing evidence that substance P is a key mediator of pruritus and that disruption of binding to its receptor may result in significant improvement in symptoms in certain patients. It remains to be seen whether the cell type or the tendency toward neural invasion plays a role. Large, randomized studies are needed to guide patient selection and confirm the findings reported here and in the literature, with careful documentation of and close attention paid to timing of pruritus relief and improvement in patient quality of life. Aprepitant might be an important therapeutic tool for refractory, malignancy-associated pruritus, in which patient quality of life is especially critical.
Acknowledgments
This work was presented at the Multinational Association of Supportive Care and Cancer Meeting, in Miami Florida, June 26-28, 2014. The authors are indebted to Saajar Jadeja for his assistance preparing the manuscript.
Substance P is an important neurotransmitter implicated in itch pathways.1 After binding to its receptor, neurokinin-1 (NK-1), substance P induces release of factors including histamine, which may cause pruritus.2 Recent literature has reported successful use of aprepitant, an NK-1 antagonist that has been approved by the US Food and Drug Administration for the treatment of chemotherapy-induced nausea and vomiting, for treatment of pruritus. We report here the case of a patient with microcystic adnexal carcinoma (MAC) who presented with refractory pruritus and who had rapid and complete resolution of itch after administration of aprepitant.
Case presentation and summary
A 73-year-old man presented with a 12-year history of a small nodule on his philtrum, which had been increasing in size. He subsequently developed upper-lip numbness and nasal induration. He complained of 2.5 months of severe, debilitating, full-body pruritus. His symptoms were refractory to treatment with prednisone, gabapentin, doxycycline, doxepin, antihistamines, and topical steroids. At the time of consultation, he was being treated with hydroxyzine and topical pramocaine lotion with minimal relief.
At initial dermatologic evaluation, his tumor involved the lower two-thirds of the nose and entire upper cutaneous lip. There was a 4-mm rolled ulcer on the nasal tip and a 1-cm exophytic, smooth nodule on the left upper lip with palpable 4-cm submandibular adenopathy (Figure). Skin examination otherwise revealed linear excoriations on the upper back with no additional primary lesions. The nodule was biopsied, and the patient was diagnosed with MAC with gross nodal involvement. Laboratory findings including serum chemistries, blood urea nitrogen, complete blood cell count, thyroid, and liver function were normal. Positron emission tomography-computed tomography (PET-CT) imaging was negative for distant metastases.
Treatment was initiated with oral aprepitant – 125 mg on day 1, 80 mg on day 2, and 80 mg on day 3 –with concomitant weekly carboplatin (AUC 1.5) and paclitaxel (30 mg/m2) as well as radiation. Within hours after the first dose of aprepitant, the patient reported a notable cessation in his pruritus. He reported that after 5 hours, his skin “finally turned off” and over the hour that followed, he had complete resolution of symptoms. He completed chemoradiation with a significant disease response. Despite persistent MAC confined to the philtrum, he has been followed for over 2 years without recurrence of itch.
Discussion
MAC is an uncommon cutaneous malignancy of sweat and eccrine gland differentiation. In all, 700 cases of MAC have been described in the literature; a 2008 review estimated the incidence of metastasis at around 2.1%.3 Though metastasis is exceedingly rare, the tumor is locally aggressive and there are reports of invasion into the muscle, perichondrium, periosteum, bone marrow, as well as perineural spaces and vascular adventitia.4
The clinical presentation of MAC includes smooth, flesh-colored or yellow papules, nodules, or plaques.3 Patients often present with numbness, paresthesia, and burning in the area of involvement because of neural infiltration with tumor. Despite the rarity of MAC, pruritus has been reported as a presenting symptom in 1 other case in the literature.4 Our case represents the first report of MAC presenting with a grossly enlarging centrofacial mass, lymph node involvement, and severe full-body pruritus. Our patient responded completely, and within hours, to treatment with aprepitant after experiencing months of failure with conventional antipruritus treatments and without recurrence in symptoms in more than 2 years of follow-up.
Aprepitant blocks the binding of substance P to its receptor NK-1 and has been approved as an anti-emetic for chemotherapy patients. Substance P has been shown to be important in both nausea and itch pathways. The largest prospective study to date on aprepitant for the indication of pruritus in 45 patients with metastatic solid tumors demonstrated a 91% response rate, defined by >50% reduction in pruritus intensity, and 13% recurrence rate that occurred at a median of 7 weeks after initial treatment.5 Aprepitant treatment has been used with success for pruritus associated with both malignant and nonmalignant conditions in at least 74 patients,6 among whom the malignant conditions included cutaneous T-cell lymphoma, Hodgkin lymphoma, and metastatic solid tumors.5-7 Aprepitant has also been used for erlotinib- and nivolumab-induced pruritus in non–small cell lung cancer, which suggests a possible future role for aprepitant in the treatment of pruritus secondary to novel cancer therapies, perhaps including immune checkpoint inhibitors.8-10
However, despite those reports, and likely owing to the multifactorial nature of pruritus, aprepitant is not unviversally effective. Mechanisms of malignancy-associated itch are yet to be elucidated, and optimal patient selection for aprepitant use needs to be determined. However, our patient’s notable response supports the increasing evidence that substance P is a key mediator of pruritus and that disruption of binding to its receptor may result in significant improvement in symptoms in certain patients. It remains to be seen whether the cell type or the tendency toward neural invasion plays a role. Large, randomized studies are needed to guide patient selection and confirm the findings reported here and in the literature, with careful documentation of and close attention paid to timing of pruritus relief and improvement in patient quality of life. Aprepitant might be an important therapeutic tool for refractory, malignancy-associated pruritus, in which patient quality of life is especially critical.
Acknowledgments
This work was presented at the Multinational Association of Supportive Care and Cancer Meeting, in Miami Florida, June 26-28, 2014. The authors are indebted to Saajar Jadeja for his assistance preparing the manuscript.
1. Wallengren J. Neuroanatomy and neurophysiology of itch. Dermatol Ther. 2005;18(4):292-303.
2. Kulka M, Sheen CH, Tancowny BP, Grammer LC, Schleimer RP. Neuropeptides activate human mast cell degranulation and chemokine production. Immunology. 2008;123(3):398-410.
3. Wetter R, Goldstein GD. Microcystic adnexal carcinoma: a diagnostic and therapeutic challenge. Dermatol Ther. 2008;21(6):452-458.
4. Adamson T. Microcystic adnexal carcinoma. Dermatol Nurs. 2004;16(4):365.
5. Santini D, Vincenzi B, Guida FM, et al. Aprepitant for management of severe pruritus related to biological cancer treatments: a pilot study. Lancet Oncol. 2012;13(10):1020-1024.
6. Song JS, Tawa M, Chau NG, Kupper TS, LeBoeuf NR. Aprepitant for refractory cutaneous T-cell lymphoma-associated pruritus: 4 cases and a review of the literature. BMC Cancer. 2017;17.
7. Villafranca JJA, Siles MG, Casanova M, Goitia BT, Domínguez AR. Paraneoplastic pruritus presenting with Hodgkin’s lymphoma: a case report. J Med Case Reports. 2014;8:300.
8. Ito J, Fujimoto D, Nakamura A, et al. Aprepitant for refractory nivolumab-induced pruritus. Lung Cancer Amst Neth. 2017;109:58-61.
9. Levêque D. Aprepitant for erlotinib-induced pruritus. N Engl J Med. 2010;363(17):1680-1681; author reply 1681.
10. Gerber PA, Buhren BA, Homey B. More on aprepitant for erlotinib-induced pruritus. N Engl J Med. 2011;364(5):486-487.
1. Wallengren J. Neuroanatomy and neurophysiology of itch. Dermatol Ther. 2005;18(4):292-303.
2. Kulka M, Sheen CH, Tancowny BP, Grammer LC, Schleimer RP. Neuropeptides activate human mast cell degranulation and chemokine production. Immunology. 2008;123(3):398-410.
3. Wetter R, Goldstein GD. Microcystic adnexal carcinoma: a diagnostic and therapeutic challenge. Dermatol Ther. 2008;21(6):452-458.
4. Adamson T. Microcystic adnexal carcinoma. Dermatol Nurs. 2004;16(4):365.
5. Santini D, Vincenzi B, Guida FM, et al. Aprepitant for management of severe pruritus related to biological cancer treatments: a pilot study. Lancet Oncol. 2012;13(10):1020-1024.
6. Song JS, Tawa M, Chau NG, Kupper TS, LeBoeuf NR. Aprepitant for refractory cutaneous T-cell lymphoma-associated pruritus: 4 cases and a review of the literature. BMC Cancer. 2017;17.
7. Villafranca JJA, Siles MG, Casanova M, Goitia BT, Domínguez AR. Paraneoplastic pruritus presenting with Hodgkin’s lymphoma: a case report. J Med Case Reports. 2014;8:300.
8. Ito J, Fujimoto D, Nakamura A, et al. Aprepitant for refractory nivolumab-induced pruritus. Lung Cancer Amst Neth. 2017;109:58-61.
9. Levêque D. Aprepitant for erlotinib-induced pruritus. N Engl J Med. 2010;363(17):1680-1681; author reply 1681.
10. Gerber PA, Buhren BA, Homey B. More on aprepitant for erlotinib-induced pruritus. N Engl J Med. 2011;364(5):486-487.
Rare paraneoplastic dermatomyositis secondary to high-grade bladder cancer
The clinical presentation of bladder cancer typically presents with hematuria; changes in voiding habits such as urgency, frequency, and pain; or less commonly, obstructive symptoms. Rarely does bladder cancer first present as part of a paraneoplastic syndrome with an inflammatory myopathy. Inflammatory myopathies such as dermatomyositis have been known to be associated with malignancy, however, in a meta-analysis by Yang and colleagues of 449 patients with dermatomyositis and malignancy there were only 8 cases reported of bladder cancer.1 Herein, we report a paraneoplastic dermatomyositis in the setting of a bladder cancer.
Case presentation and summary
A 65-year-old man with a medical history of hypertension and alcohol use presented to the emergency department with worsening pain, stiffness in the neck, shoulders, and inability to lift his arms above his shoulders. During the physical exam, an erythematous purple rash was noted over his chest, neck, and arms. Upon further evaluation, his creatine phosphokinase was 3,500 U/L (reference range 52-336 U/L) suggesting muscle breakdown and possible inflammatory myopathy. A biopsy of the left deltoid and quadriceps muscles was performed and yielded a diagnosis of dermatomyositis. He was treated with prednisone 60 mg daily for his inflammatory myopathy. The patient also reported an unintentional weight loss of 20 lbs. and increasing weakness and inability to swallow, which caused aspiration events without developing pneumonia.
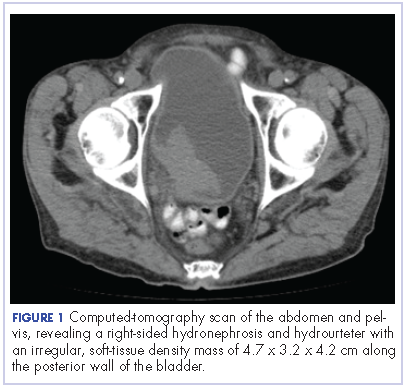
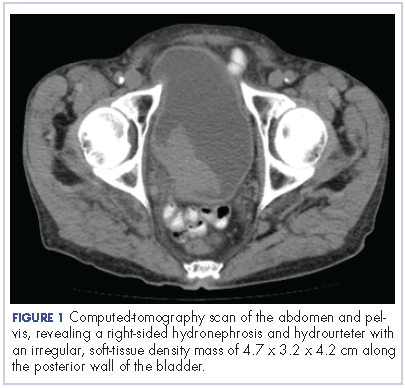
The patient’s symptoms worsened while he was on steroids, and we became concerned about the possibility of a primary malignancy, which led to further work-up. The results of a computed-tomography (CT) scan of the abdomen and pelvis showed right-sided hydronephrosis and hydrourteter with an irregular, soft-tissue density mass of 4.7 x 3.2 x 4.2 cm along the posterior wall of the bladder (Figure 1).
A cystoscopy was performed with transurethral resection of a bladder tumor that was more than 8 cm in diameter. Because the mass was not fully resectable, only 25% of the tumor burden was removed. The pathology report revealed an invasive, high-grade urothelial cell carcinoma (Figure 2, see PDF). Further imaging ruled out metastatic spread. The patient was continued on steroids. He was not a candidate for neoadjuvant chemotherapy because of his comorbidities and cisplatin ineligibility owing to his significant bilateral hearing deficiencies. Members of a multidisciplinary tumor board decided to move forward with definitive surgery. The patient underwent a robotic-assisted laparoscoptic cystoprostatectomy with bilateral pelvic lymph node dissection and open ileal conduit urinary diversion. Staging of tumor was determined as pT3b N1 (1/30) M0, LVI+. After the surgery, the patient had resolution of his rash and significant improvement in his muscle weakness with the ability to raise his arms over his head and climb stairs. Adjuvant chemotherapy was not given since he was cisplatin ineligible as a result of his hearing loss. Active surveillance was preferred.
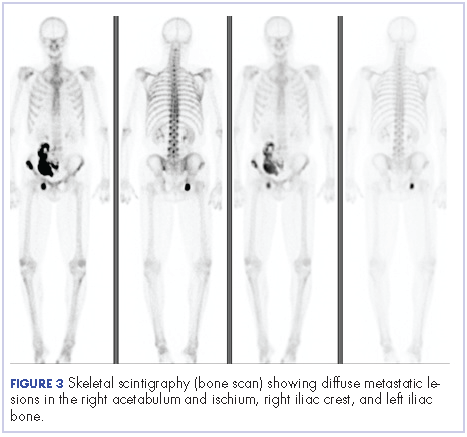
Four months after his cystoprostatectomy, he experienced new-onset hip pain and further imaging, including a bone scan, was performed. It showed metastatic disease in the ischium and iliac crest (Figure 3).
The patient decided to forgo any palliative chemotherapy and to have palliative radiation for pain and enroll in hospice. He died nine months after the initial diagnosis of urothelial cell carcinoma.
Discussion
Dermatomyositis is one of the inflammatory myopathies with a clinical presentation of proximal muscle weakness and characteristic skin findings of Gottron papules and heliotrope eruption. The most common subgroups of inflammatory myopathies are dermatomyositis, polymyositis, necrotizing autoimmune myopathy, and inclusion body myopathy. The pathogenesis of inflammatory myopathies is not well understood; however, some theories have been described, including: type 1 interferon signaling causing myofiber injury and antibody-complement mediated processes causing ischemia resulting in myofiber injury. 2,3 The diagnoses of inflammatory myopathies may be suggested based on history, physical examination findings, laboratory values showing muscle injury (creatine kinase, aldolase, ALT, AST, LDH), myositis-specific antibodies (antisynthetase autoantibodies), electromyogram, and magnetic-resonance imaging. However, muscle biopsy remains the gold standard.4
The initial treatment of inflammatory myopathies begins with glucocorticoid therapy at 0.5-1.0 mg/kg. This regimen may be titrated down over 6 weeks to a level adequate to control symptoms. Even while on glucocorticoid therapy, this patient’s symptoms continued, along with the development of dysphagia. Dysphagia is another notable symptom of dermatomyositis that may result in aspiration pneumonia with fatal outcomes.5,6,7 Not only did this patient initially respond poorly to corticosteroids, but the unintentional weight loss was another alarming feature prompting further evaluation. That led to the diagnosis of urothelial cell carcinoma, which was causing the paraneoplastic syndrome.
A paraneoplastic syndrome is a collection of symptoms that are observed in organ systems separate from the primary disease. This process is mostly caused by an autoimmune response to the tumor and nervous system.8 Inflammatory myopathies, such as dermatomyositis, have been shown to be associated with a variety of malignancies as part of a paraneoplastic syndrome. The most common cancers associated with dermatomyositis are ovarian, lung, pancreatic, stomach, colorectal, and non-Hodgkin lymphoma.9 Although an association between dermatomyositis and bladder cancer has been established, very few cases have been reported in the literature. In the Yang meta-analysis, the relative risk of malignancy for patients with dermatomyositis was 5.5%, and of the 449 patients with dermatomyositis who had malignancy, only 8 cases of bladder cancer were reported.1
After a patient has been diagnosed with an inflammatory myopathy, there should be further evaluation for an underling malignancy causing a paraneoplastic process. The risk of these patients having a malignancy overall is 4.5 times higher than patients without dermatomyositis.1 Definite screening recommendations have not been established, but screening should be based on patient’s age, gender, and clinical scenario. The European Federation of Neurological Societies formed a task force to focus on malignancy screening of paraneoplastic neurological syndromes and included dermatomyositis as one of the signs.10 Patients should have a CT scan of the chest, abdomen, and pelvis. Women should have a mammogram and a pelvis ultrasound. Men younger than 50 years should consider testes ultrasound, and patients older than 50 years should undergo usual colonoscopy screening.
The risk of malignancy is highest in the first year after diagnosis, but may extend to 5 years after the diagnosis, so repeat screening should be performed 3-6 months after diagnosis, followed with biannual testing for 4 years. If a malignancy is present, then treatment should be tailored to the neoplasm to improve symptoms of myositis; however, response is generally worse than it would be with dermatomyositis in the absence of malignancy. In the present case with bladder cancer, therapies may include platinum-based-chemotherapy, resection, and radiation. Dermatomyositis as a result of a bladder cancer paraneoplastic syndrome is associated with a poor prognosis as demonstrated in the case of this patient and others reported in the literature.11
Even though dermatomyositis is usually a chronic disease process, 87% of patients respond initially to corticosteroid treatment.12 Therefore, treatment should be escalated with an agent such as azathioprine or methotrexate, or, like in this case, an underlying malignancy should be suspected. This case emphasizes the importance of screening patients appropriately for malignancy in patients with an inflammatory myopathy and reveals the poor prognosis associated with this disease.
1. Yang Z, Lin F, Qin B, Liang Y, Zhong R. Polymyositis/dermatomyositis and malignancy risk: a metaanalysis study. J Rheumatol. 2015;42(2):282-291.
2. Greenberg, SA. Dermatomyositis and type 1 interferons. Curr Rheumatol Rep. 2010;12(3):198-203.
3. Dalakas, MC, Hohlfeld, R. Polymyositis and dermatomyositis. Lancet. 2003;362(9388):971-982.
4. Malik A, Hayat G, Kalia JS, Guzman MA. Idiopathic inflammatory myopathies: clinical approach and management. Front Neurol. 2016;7:64.
5. Sabio JM, Vargas-Hitos JA, Jiménez-Alonso J. Paraneoplastic dermatomyositis associated with bladder cancer. Lupus. 2006;15(9):619-620.
6. Mallon E, Osborne G, Dinneen M, Lane RJ, Glaser M, Bunker CB. Dermatomyositis in association with transitional cell carcinoma of the bladder. Clin Exp Dermatol. 1999;24(2):94-96.
7. Hafejee A, Coulson IH. Dysphagia in dermatomyositis secondary to bladder cancer: rapid response to combined immunoglobulin and methylprednisolone. Clin Exp Dermatol. 2005;30(1):93-94.
8. Dalmau J, Gultekin HS, Posner JB. Paraneoplastic neurologic syndromes: pathogenesis and physiopathology. Brain Pathol. 1999;9(2):275-284.
9. Hill CL, Zhang Y, Sigureirsson B, et al. Frequency of specific cancer types in dermatomyositis and polymyositis: a population-based study. Lancet. 2001;357(9250):96-100.
10. Titulaer, MJ, Soffietti R, Dalmau J, et al. Screening for tumours in paraneoplastic syndromes: report of an EFNS Task Force. Eur J Neurol. 2011;18(1):19-e3.
11. Xu R, Zhong Z, Jiang H, Zhang L, Zhao X. A rare paraneoplastic dermatomyositis in bladder cancer with fatal outcome. Urol J. 2013;10(1):815-817.
12. Troyanov Y, Targoff IN, Tremblay JL, Goulet JR, Raymond Y, Senecal JL. Novel classification of idiopathic inflammatory myopathies based on overlap syndrome features and autoantibodies: analysis of 100 French Canadian patients. Medicine (Baltimore), 2005;84(4):231-249.
The clinical presentation of bladder cancer typically presents with hematuria; changes in voiding habits such as urgency, frequency, and pain; or less commonly, obstructive symptoms. Rarely does bladder cancer first present as part of a paraneoplastic syndrome with an inflammatory myopathy. Inflammatory myopathies such as dermatomyositis have been known to be associated with malignancy, however, in a meta-analysis by Yang and colleagues of 449 patients with dermatomyositis and malignancy there were only 8 cases reported of bladder cancer.1 Herein, we report a paraneoplastic dermatomyositis in the setting of a bladder cancer.
Case presentation and summary
A 65-year-old man with a medical history of hypertension and alcohol use presented to the emergency department with worsening pain, stiffness in the neck, shoulders, and inability to lift his arms above his shoulders. During the physical exam, an erythematous purple rash was noted over his chest, neck, and arms. Upon further evaluation, his creatine phosphokinase was 3,500 U/L (reference range 52-336 U/L) suggesting muscle breakdown and possible inflammatory myopathy. A biopsy of the left deltoid and quadriceps muscles was performed and yielded a diagnosis of dermatomyositis. He was treated with prednisone 60 mg daily for his inflammatory myopathy. The patient also reported an unintentional weight loss of 20 lbs. and increasing weakness and inability to swallow, which caused aspiration events without developing pneumonia.
The patient’s symptoms worsened while he was on steroids, and we became concerned about the possibility of a primary malignancy, which led to further work-up. The results of a computed-tomography (CT) scan of the abdomen and pelvis showed right-sided hydronephrosis and hydrourteter with an irregular, soft-tissue density mass of 4.7 x 3.2 x 4.2 cm along the posterior wall of the bladder (Figure 1).
A cystoscopy was performed with transurethral resection of a bladder tumor that was more than 8 cm in diameter. Because the mass was not fully resectable, only 25% of the tumor burden was removed. The pathology report revealed an invasive, high-grade urothelial cell carcinoma (Figure 2, see PDF). Further imaging ruled out metastatic spread. The patient was continued on steroids. He was not a candidate for neoadjuvant chemotherapy because of his comorbidities and cisplatin ineligibility owing to his significant bilateral hearing deficiencies. Members of a multidisciplinary tumor board decided to move forward with definitive surgery. The patient underwent a robotic-assisted laparoscoptic cystoprostatectomy with bilateral pelvic lymph node dissection and open ileal conduit urinary diversion. Staging of tumor was determined as pT3b N1 (1/30) M0, LVI+. After the surgery, the patient had resolution of his rash and significant improvement in his muscle weakness with the ability to raise his arms over his head and climb stairs. Adjuvant chemotherapy was not given since he was cisplatin ineligible as a result of his hearing loss. Active surveillance was preferred.
Four months after his cystoprostatectomy, he experienced new-onset hip pain and further imaging, including a bone scan, was performed. It showed metastatic disease in the ischium and iliac crest (Figure 3).
The patient decided to forgo any palliative chemotherapy and to have palliative radiation for pain and enroll in hospice. He died nine months after the initial diagnosis of urothelial cell carcinoma.
Discussion
Dermatomyositis is one of the inflammatory myopathies with a clinical presentation of proximal muscle weakness and characteristic skin findings of Gottron papules and heliotrope eruption. The most common subgroups of inflammatory myopathies are dermatomyositis, polymyositis, necrotizing autoimmune myopathy, and inclusion body myopathy. The pathogenesis of inflammatory myopathies is not well understood; however, some theories have been described, including: type 1 interferon signaling causing myofiber injury and antibody-complement mediated processes causing ischemia resulting in myofiber injury. 2,3 The diagnoses of inflammatory myopathies may be suggested based on history, physical examination findings, laboratory values showing muscle injury (creatine kinase, aldolase, ALT, AST, LDH), myositis-specific antibodies (antisynthetase autoantibodies), electromyogram, and magnetic-resonance imaging. However, muscle biopsy remains the gold standard.4
The initial treatment of inflammatory myopathies begins with glucocorticoid therapy at 0.5-1.0 mg/kg. This regimen may be titrated down over 6 weeks to a level adequate to control symptoms. Even while on glucocorticoid therapy, this patient’s symptoms continued, along with the development of dysphagia. Dysphagia is another notable symptom of dermatomyositis that may result in aspiration pneumonia with fatal outcomes.5,6,7 Not only did this patient initially respond poorly to corticosteroids, but the unintentional weight loss was another alarming feature prompting further evaluation. That led to the diagnosis of urothelial cell carcinoma, which was causing the paraneoplastic syndrome.
A paraneoplastic syndrome is a collection of symptoms that are observed in organ systems separate from the primary disease. This process is mostly caused by an autoimmune response to the tumor and nervous system.8 Inflammatory myopathies, such as dermatomyositis, have been shown to be associated with a variety of malignancies as part of a paraneoplastic syndrome. The most common cancers associated with dermatomyositis are ovarian, lung, pancreatic, stomach, colorectal, and non-Hodgkin lymphoma.9 Although an association between dermatomyositis and bladder cancer has been established, very few cases have been reported in the literature. In the Yang meta-analysis, the relative risk of malignancy for patients with dermatomyositis was 5.5%, and of the 449 patients with dermatomyositis who had malignancy, only 8 cases of bladder cancer were reported.1
After a patient has been diagnosed with an inflammatory myopathy, there should be further evaluation for an underling malignancy causing a paraneoplastic process. The risk of these patients having a malignancy overall is 4.5 times higher than patients without dermatomyositis.1 Definite screening recommendations have not been established, but screening should be based on patient’s age, gender, and clinical scenario. The European Federation of Neurological Societies formed a task force to focus on malignancy screening of paraneoplastic neurological syndromes and included dermatomyositis as one of the signs.10 Patients should have a CT scan of the chest, abdomen, and pelvis. Women should have a mammogram and a pelvis ultrasound. Men younger than 50 years should consider testes ultrasound, and patients older than 50 years should undergo usual colonoscopy screening.
The risk of malignancy is highest in the first year after diagnosis, but may extend to 5 years after the diagnosis, so repeat screening should be performed 3-6 months after diagnosis, followed with biannual testing for 4 years. If a malignancy is present, then treatment should be tailored to the neoplasm to improve symptoms of myositis; however, response is generally worse than it would be with dermatomyositis in the absence of malignancy. In the present case with bladder cancer, therapies may include platinum-based-chemotherapy, resection, and radiation. Dermatomyositis as a result of a bladder cancer paraneoplastic syndrome is associated with a poor prognosis as demonstrated in the case of this patient and others reported in the literature.11
Even though dermatomyositis is usually a chronic disease process, 87% of patients respond initially to corticosteroid treatment.12 Therefore, treatment should be escalated with an agent such as azathioprine or methotrexate, or, like in this case, an underlying malignancy should be suspected. This case emphasizes the importance of screening patients appropriately for malignancy in patients with an inflammatory myopathy and reveals the poor prognosis associated with this disease.
The clinical presentation of bladder cancer typically presents with hematuria; changes in voiding habits such as urgency, frequency, and pain; or less commonly, obstructive symptoms. Rarely does bladder cancer first present as part of a paraneoplastic syndrome with an inflammatory myopathy. Inflammatory myopathies such as dermatomyositis have been known to be associated with malignancy, however, in a meta-analysis by Yang and colleagues of 449 patients with dermatomyositis and malignancy there were only 8 cases reported of bladder cancer.1 Herein, we report a paraneoplastic dermatomyositis in the setting of a bladder cancer.
Case presentation and summary
A 65-year-old man with a medical history of hypertension and alcohol use presented to the emergency department with worsening pain, stiffness in the neck, shoulders, and inability to lift his arms above his shoulders. During the physical exam, an erythematous purple rash was noted over his chest, neck, and arms. Upon further evaluation, his creatine phosphokinase was 3,500 U/L (reference range 52-336 U/L) suggesting muscle breakdown and possible inflammatory myopathy. A biopsy of the left deltoid and quadriceps muscles was performed and yielded a diagnosis of dermatomyositis. He was treated with prednisone 60 mg daily for his inflammatory myopathy. The patient also reported an unintentional weight loss of 20 lbs. and increasing weakness and inability to swallow, which caused aspiration events without developing pneumonia.
The patient’s symptoms worsened while he was on steroids, and we became concerned about the possibility of a primary malignancy, which led to further work-up. The results of a computed-tomography (CT) scan of the abdomen and pelvis showed right-sided hydronephrosis and hydrourteter with an irregular, soft-tissue density mass of 4.7 x 3.2 x 4.2 cm along the posterior wall of the bladder (Figure 1).
A cystoscopy was performed with transurethral resection of a bladder tumor that was more than 8 cm in diameter. Because the mass was not fully resectable, only 25% of the tumor burden was removed. The pathology report revealed an invasive, high-grade urothelial cell carcinoma (Figure 2, see PDF). Further imaging ruled out metastatic spread. The patient was continued on steroids. He was not a candidate for neoadjuvant chemotherapy because of his comorbidities and cisplatin ineligibility owing to his significant bilateral hearing deficiencies. Members of a multidisciplinary tumor board decided to move forward with definitive surgery. The patient underwent a robotic-assisted laparoscoptic cystoprostatectomy with bilateral pelvic lymph node dissection and open ileal conduit urinary diversion. Staging of tumor was determined as pT3b N1 (1/30) M0, LVI+. After the surgery, the patient had resolution of his rash and significant improvement in his muscle weakness with the ability to raise his arms over his head and climb stairs. Adjuvant chemotherapy was not given since he was cisplatin ineligible as a result of his hearing loss. Active surveillance was preferred.
Four months after his cystoprostatectomy, he experienced new-onset hip pain and further imaging, including a bone scan, was performed. It showed metastatic disease in the ischium and iliac crest (Figure 3).
The patient decided to forgo any palliative chemotherapy and to have palliative radiation for pain and enroll in hospice. He died nine months after the initial diagnosis of urothelial cell carcinoma.
Discussion
Dermatomyositis is one of the inflammatory myopathies with a clinical presentation of proximal muscle weakness and characteristic skin findings of Gottron papules and heliotrope eruption. The most common subgroups of inflammatory myopathies are dermatomyositis, polymyositis, necrotizing autoimmune myopathy, and inclusion body myopathy. The pathogenesis of inflammatory myopathies is not well understood; however, some theories have been described, including: type 1 interferon signaling causing myofiber injury and antibody-complement mediated processes causing ischemia resulting in myofiber injury. 2,3 The diagnoses of inflammatory myopathies may be suggested based on history, physical examination findings, laboratory values showing muscle injury (creatine kinase, aldolase, ALT, AST, LDH), myositis-specific antibodies (antisynthetase autoantibodies), electromyogram, and magnetic-resonance imaging. However, muscle biopsy remains the gold standard.4
The initial treatment of inflammatory myopathies begins with glucocorticoid therapy at 0.5-1.0 mg/kg. This regimen may be titrated down over 6 weeks to a level adequate to control symptoms. Even while on glucocorticoid therapy, this patient’s symptoms continued, along with the development of dysphagia. Dysphagia is another notable symptom of dermatomyositis that may result in aspiration pneumonia with fatal outcomes.5,6,7 Not only did this patient initially respond poorly to corticosteroids, but the unintentional weight loss was another alarming feature prompting further evaluation. That led to the diagnosis of urothelial cell carcinoma, which was causing the paraneoplastic syndrome.
A paraneoplastic syndrome is a collection of symptoms that are observed in organ systems separate from the primary disease. This process is mostly caused by an autoimmune response to the tumor and nervous system.8 Inflammatory myopathies, such as dermatomyositis, have been shown to be associated with a variety of malignancies as part of a paraneoplastic syndrome. The most common cancers associated with dermatomyositis are ovarian, lung, pancreatic, stomach, colorectal, and non-Hodgkin lymphoma.9 Although an association between dermatomyositis and bladder cancer has been established, very few cases have been reported in the literature. In the Yang meta-analysis, the relative risk of malignancy for patients with dermatomyositis was 5.5%, and of the 449 patients with dermatomyositis who had malignancy, only 8 cases of bladder cancer were reported.1
After a patient has been diagnosed with an inflammatory myopathy, there should be further evaluation for an underling malignancy causing a paraneoplastic process. The risk of these patients having a malignancy overall is 4.5 times higher than patients without dermatomyositis.1 Definite screening recommendations have not been established, but screening should be based on patient’s age, gender, and clinical scenario. The European Federation of Neurological Societies formed a task force to focus on malignancy screening of paraneoplastic neurological syndromes and included dermatomyositis as one of the signs.10 Patients should have a CT scan of the chest, abdomen, and pelvis. Women should have a mammogram and a pelvis ultrasound. Men younger than 50 years should consider testes ultrasound, and patients older than 50 years should undergo usual colonoscopy screening.
The risk of malignancy is highest in the first year after diagnosis, but may extend to 5 years after the diagnosis, so repeat screening should be performed 3-6 months after diagnosis, followed with biannual testing for 4 years. If a malignancy is present, then treatment should be tailored to the neoplasm to improve symptoms of myositis; however, response is generally worse than it would be with dermatomyositis in the absence of malignancy. In the present case with bladder cancer, therapies may include platinum-based-chemotherapy, resection, and radiation. Dermatomyositis as a result of a bladder cancer paraneoplastic syndrome is associated with a poor prognosis as demonstrated in the case of this patient and others reported in the literature.11
Even though dermatomyositis is usually a chronic disease process, 87% of patients respond initially to corticosteroid treatment.12 Therefore, treatment should be escalated with an agent such as azathioprine or methotrexate, or, like in this case, an underlying malignancy should be suspected. This case emphasizes the importance of screening patients appropriately for malignancy in patients with an inflammatory myopathy and reveals the poor prognosis associated with this disease.
1. Yang Z, Lin F, Qin B, Liang Y, Zhong R. Polymyositis/dermatomyositis and malignancy risk: a metaanalysis study. J Rheumatol. 2015;42(2):282-291.
2. Greenberg, SA. Dermatomyositis and type 1 interferons. Curr Rheumatol Rep. 2010;12(3):198-203.
3. Dalakas, MC, Hohlfeld, R. Polymyositis and dermatomyositis. Lancet. 2003;362(9388):971-982.
4. Malik A, Hayat G, Kalia JS, Guzman MA. Idiopathic inflammatory myopathies: clinical approach and management. Front Neurol. 2016;7:64.
5. Sabio JM, Vargas-Hitos JA, Jiménez-Alonso J. Paraneoplastic dermatomyositis associated with bladder cancer. Lupus. 2006;15(9):619-620.
6. Mallon E, Osborne G, Dinneen M, Lane RJ, Glaser M, Bunker CB. Dermatomyositis in association with transitional cell carcinoma of the bladder. Clin Exp Dermatol. 1999;24(2):94-96.
7. Hafejee A, Coulson IH. Dysphagia in dermatomyositis secondary to bladder cancer: rapid response to combined immunoglobulin and methylprednisolone. Clin Exp Dermatol. 2005;30(1):93-94.
8. Dalmau J, Gultekin HS, Posner JB. Paraneoplastic neurologic syndromes: pathogenesis and physiopathology. Brain Pathol. 1999;9(2):275-284.
9. Hill CL, Zhang Y, Sigureirsson B, et al. Frequency of specific cancer types in dermatomyositis and polymyositis: a population-based study. Lancet. 2001;357(9250):96-100.
10. Titulaer, MJ, Soffietti R, Dalmau J, et al. Screening for tumours in paraneoplastic syndromes: report of an EFNS Task Force. Eur J Neurol. 2011;18(1):19-e3.
11. Xu R, Zhong Z, Jiang H, Zhang L, Zhao X. A rare paraneoplastic dermatomyositis in bladder cancer with fatal outcome. Urol J. 2013;10(1):815-817.
12. Troyanov Y, Targoff IN, Tremblay JL, Goulet JR, Raymond Y, Senecal JL. Novel classification of idiopathic inflammatory myopathies based on overlap syndrome features and autoantibodies: analysis of 100 French Canadian patients. Medicine (Baltimore), 2005;84(4):231-249.
1. Yang Z, Lin F, Qin B, Liang Y, Zhong R. Polymyositis/dermatomyositis and malignancy risk: a metaanalysis study. J Rheumatol. 2015;42(2):282-291.
2. Greenberg, SA. Dermatomyositis and type 1 interferons. Curr Rheumatol Rep. 2010;12(3):198-203.
3. Dalakas, MC, Hohlfeld, R. Polymyositis and dermatomyositis. Lancet. 2003;362(9388):971-982.
4. Malik A, Hayat G, Kalia JS, Guzman MA. Idiopathic inflammatory myopathies: clinical approach and management. Front Neurol. 2016;7:64.
5. Sabio JM, Vargas-Hitos JA, Jiménez-Alonso J. Paraneoplastic dermatomyositis associated with bladder cancer. Lupus. 2006;15(9):619-620.
6. Mallon E, Osborne G, Dinneen M, Lane RJ, Glaser M, Bunker CB. Dermatomyositis in association with transitional cell carcinoma of the bladder. Clin Exp Dermatol. 1999;24(2):94-96.
7. Hafejee A, Coulson IH. Dysphagia in dermatomyositis secondary to bladder cancer: rapid response to combined immunoglobulin and methylprednisolone. Clin Exp Dermatol. 2005;30(1):93-94.
8. Dalmau J, Gultekin HS, Posner JB. Paraneoplastic neurologic syndromes: pathogenesis and physiopathology. Brain Pathol. 1999;9(2):275-284.
9. Hill CL, Zhang Y, Sigureirsson B, et al. Frequency of specific cancer types in dermatomyositis and polymyositis: a population-based study. Lancet. 2001;357(9250):96-100.
10. Titulaer, MJ, Soffietti R, Dalmau J, et al. Screening for tumours in paraneoplastic syndromes: report of an EFNS Task Force. Eur J Neurol. 2011;18(1):19-e3.
11. Xu R, Zhong Z, Jiang H, Zhang L, Zhao X. A rare paraneoplastic dermatomyositis in bladder cancer with fatal outcome. Urol J. 2013;10(1):815-817.
12. Troyanov Y, Targoff IN, Tremblay JL, Goulet JR, Raymond Y, Senecal JL. Novel classification of idiopathic inflammatory myopathies based on overlap syndrome features and autoantibodies: analysis of 100 French Canadian patients. Medicine (Baltimore), 2005;84(4):231-249.
World Trade Center responders face greater cancer burden, including greater risk of multiple myeloma
Rescue and recovery workers who were involved in the aftermath of the World Trade Center disaster may face a greater cancer burden than the general population, according to two studies published in JAMA Oncology.
In particular, they may be at risk of developing multiple myeloma at an earlier age.
The first study was a closed-cohort study of 14,474 employees of the Fire Department of the City of New York (FDNY) who were exposed to the World Trade Center disaster but were cancer-free as of Jan. 1, 2012. The aim was to project cancer incidence from 2012 through 2031, based on data from the FDNY World Trade Center Health Program, and compare those rates with age-, race-, and sex-specific New York cancer rates from the general population.
The modeling projected a “modestly” higher number of cancer cases in the white male subgroup of rescue and recovery workers exposed to the World Trade Center (2,714 vs. 2,596 for the general population of New York; P less than .001). Specifically, the investigators projected significantly higher case counts of prostate cancer (1,437 vs. 863), thyroid cancer (73 vs. 57), and melanoma (201 vs. 131), compared with the general population in New York, but fewer lung (237 vs. 373), colorectal (172 vs. 267), and kidney cancers (66 vs. 132) (P less than .001 for all).
“Our findings suggest that the FDNY WTC-exposed cohort may experience a greater burden of cancer than would be expected from a population with similar demographic characteristics,” wrote Rachel Zeig-Owens, DrPH, from the Montefiore Medical Center and Albert Einstein College of Medicine, both in New York, and coauthors, highlighting prostate cancer as a particular concern.
However, they also acknowledged that the elevated rates observed in people exposed to the World Trade Center disaster could be a result of increased surveillance, even though they did attempt to correct for that, and that firefighters in general might face higher risks.
“It is possible that firefighters have a higher risk of cancer than the general population owing to exposures associated with the occupation,” they wrote. However occupation could also have the opposite effect, as rescue and recovery workers tend to have lower smoking rates, which may explain the relatively low rates of certain cancers such as lung cancer, they said.
A second study examined the effect of the World Trade Center disaster on the risk of multiple myeloma and monoclonal gammopathies in exposed firefighters.
The seroprevalence study of monoclonal gammopathies of undetermined significance (MGUS) in 781 exposed firefighters revealed that the age-standardized prevalence of these was 76% higher in this population than it was in a white male reference population living in Minnesota.
In particular, the age-standardized prevalence of light-chain MGUS was more than threefold higher in exposed firefighters, compared with the reference population.
Researchers also analyzed a case series of 16 exposed white male firefighters who received a diagnosis of multiple myeloma after Sept. 11, 2001. Of the 14 patients for whom data on the monoclonal protein isotype was available, half had light-chain multiple myeloma.
“These findings are of interest due to previously observed associations between light-chain multiple myeloma and light-chain MGUS and exposure to toxins, and chronic immune stimulation,” wrote Ola Landgren, MD, PhD, from the Memorial Sloan Kettering Cancer Center and his coauthors.
Seven patients were also assessed for CD20 expression – a marker of poorer prognosis – and 71% were found to be CD20-positive, a prevalence around 3.5-fold higher than that seen in the general population.
The cohort with multiple myeloma was diagnosed on average 12 years younger than those in the general population. The authors commented that this was unlikely to be caused by lead-time bias because the time from first symptoms to clinical manifestation of the disease is usually around 1 year.
“Taken together, our results show that environmental exposure due to the WTC attacks is associated with myeloma precursor disease (MGUS and light-chain MGUS) and may be a risk factor for the development of multiple myeloma at an earlier age, particularly the light-chain subtype,” the authors wrote.
The first study was supported by the National Institute of Occupational Safety and Health; no conflicts of interest were declared.
The second study was supported by the V Foundation for Cancer Research, the Byrne Fund for the benefit of Memorial Sloan-Kettering Cancer Center, the National Cancer Institute, the Albert Einstein Cancer Center, and the National Institute for Occupational Safety and Health; no conflicts of interest were declared.
SOURCE: Zeig-Owens R et al. JAMA Oncology. 2018 April 26. doi: 10.1001/jamaoncol.2018.0504. Landgren O et al. JAMA Oncology. 2018 April 16. doi: 10.1001/jamaoncol.2018.0509.
When the heroes of the World Trade Center are diagnosed with even a common cancer, there is a natural tendency to assume that the diagnosis is the result of their service during the disaster. However, it is important to appreciate that the firefighting profession is known to be associated with higher risks of monoclonal gammopathy of undetermined significance and multiple myelomas, compared with the general population.
Given that, it would have been preferable to compare the World Trade Center–exposed populations with an equally intensively screened, age-matched cohort of firefighters from another major city.
If we apply Sir Richard Doll’s rule that a single epidemiologic study cannot be persuasive until the lower bound of the 95% confidence interval is greater than three, the relative risks in the study by Landgren and colleagues are too small to be persuasive.
The predicted increases in cancers of the prostate, thyroid, and myeloma are interesting, but these have also been previously reported in firefighters from other cities.
Despite this, we owe it to these men and women to find the truth and determine the illnesses that are associated with their service.
Otis W. Brawley, MD, is chief medical and scientific officer and executive vice president of the American Cancer Society and a professor at Emory University, Atlanta. These comments are taken from an accompanying editorial (JAMA Oncology. 2018 April 26. doi: 10.1001/jamaoncol.2018.0498.) No conflicts of interest were declared.
When the heroes of the World Trade Center are diagnosed with even a common cancer, there is a natural tendency to assume that the diagnosis is the result of their service during the disaster. However, it is important to appreciate that the firefighting profession is known to be associated with higher risks of monoclonal gammopathy of undetermined significance and multiple myelomas, compared with the general population.
Given that, it would have been preferable to compare the World Trade Center–exposed populations with an equally intensively screened, age-matched cohort of firefighters from another major city.
If we apply Sir Richard Doll’s rule that a single epidemiologic study cannot be persuasive until the lower bound of the 95% confidence interval is greater than three, the relative risks in the study by Landgren and colleagues are too small to be persuasive.
The predicted increases in cancers of the prostate, thyroid, and myeloma are interesting, but these have also been previously reported in firefighters from other cities.
Despite this, we owe it to these men and women to find the truth and determine the illnesses that are associated with their service.
Otis W. Brawley, MD, is chief medical and scientific officer and executive vice president of the American Cancer Society and a professor at Emory University, Atlanta. These comments are taken from an accompanying editorial (JAMA Oncology. 2018 April 26. doi: 10.1001/jamaoncol.2018.0498.) No conflicts of interest were declared.
When the heroes of the World Trade Center are diagnosed with even a common cancer, there is a natural tendency to assume that the diagnosis is the result of their service during the disaster. However, it is important to appreciate that the firefighting profession is known to be associated with higher risks of monoclonal gammopathy of undetermined significance and multiple myelomas, compared with the general population.
Given that, it would have been preferable to compare the World Trade Center–exposed populations with an equally intensively screened, age-matched cohort of firefighters from another major city.
If we apply Sir Richard Doll’s rule that a single epidemiologic study cannot be persuasive until the lower bound of the 95% confidence interval is greater than three, the relative risks in the study by Landgren and colleagues are too small to be persuasive.
The predicted increases in cancers of the prostate, thyroid, and myeloma are interesting, but these have also been previously reported in firefighters from other cities.
Despite this, we owe it to these men and women to find the truth and determine the illnesses that are associated with their service.
Otis W. Brawley, MD, is chief medical and scientific officer and executive vice president of the American Cancer Society and a professor at Emory University, Atlanta. These comments are taken from an accompanying editorial (JAMA Oncology. 2018 April 26. doi: 10.1001/jamaoncol.2018.0498.) No conflicts of interest were declared.
Rescue and recovery workers who were involved in the aftermath of the World Trade Center disaster may face a greater cancer burden than the general population, according to two studies published in JAMA Oncology.
In particular, they may be at risk of developing multiple myeloma at an earlier age.
The first study was a closed-cohort study of 14,474 employees of the Fire Department of the City of New York (FDNY) who were exposed to the World Trade Center disaster but were cancer-free as of Jan. 1, 2012. The aim was to project cancer incidence from 2012 through 2031, based on data from the FDNY World Trade Center Health Program, and compare those rates with age-, race-, and sex-specific New York cancer rates from the general population.
The modeling projected a “modestly” higher number of cancer cases in the white male subgroup of rescue and recovery workers exposed to the World Trade Center (2,714 vs. 2,596 for the general population of New York; P less than .001). Specifically, the investigators projected significantly higher case counts of prostate cancer (1,437 vs. 863), thyroid cancer (73 vs. 57), and melanoma (201 vs. 131), compared with the general population in New York, but fewer lung (237 vs. 373), colorectal (172 vs. 267), and kidney cancers (66 vs. 132) (P less than .001 for all).
“Our findings suggest that the FDNY WTC-exposed cohort may experience a greater burden of cancer than would be expected from a population with similar demographic characteristics,” wrote Rachel Zeig-Owens, DrPH, from the Montefiore Medical Center and Albert Einstein College of Medicine, both in New York, and coauthors, highlighting prostate cancer as a particular concern.
However, they also acknowledged that the elevated rates observed in people exposed to the World Trade Center disaster could be a result of increased surveillance, even though they did attempt to correct for that, and that firefighters in general might face higher risks.
“It is possible that firefighters have a higher risk of cancer than the general population owing to exposures associated with the occupation,” they wrote. However occupation could also have the opposite effect, as rescue and recovery workers tend to have lower smoking rates, which may explain the relatively low rates of certain cancers such as lung cancer, they said.
A second study examined the effect of the World Trade Center disaster on the risk of multiple myeloma and monoclonal gammopathies in exposed firefighters.
The seroprevalence study of monoclonal gammopathies of undetermined significance (MGUS) in 781 exposed firefighters revealed that the age-standardized prevalence of these was 76% higher in this population than it was in a white male reference population living in Minnesota.
In particular, the age-standardized prevalence of light-chain MGUS was more than threefold higher in exposed firefighters, compared with the reference population.
Researchers also analyzed a case series of 16 exposed white male firefighters who received a diagnosis of multiple myeloma after Sept. 11, 2001. Of the 14 patients for whom data on the monoclonal protein isotype was available, half had light-chain multiple myeloma.
“These findings are of interest due to previously observed associations between light-chain multiple myeloma and light-chain MGUS and exposure to toxins, and chronic immune stimulation,” wrote Ola Landgren, MD, PhD, from the Memorial Sloan Kettering Cancer Center and his coauthors.
Seven patients were also assessed for CD20 expression – a marker of poorer prognosis – and 71% were found to be CD20-positive, a prevalence around 3.5-fold higher than that seen in the general population.
The cohort with multiple myeloma was diagnosed on average 12 years younger than those in the general population. The authors commented that this was unlikely to be caused by lead-time bias because the time from first symptoms to clinical manifestation of the disease is usually around 1 year.
“Taken together, our results show that environmental exposure due to the WTC attacks is associated with myeloma precursor disease (MGUS and light-chain MGUS) and may be a risk factor for the development of multiple myeloma at an earlier age, particularly the light-chain subtype,” the authors wrote.
The first study was supported by the National Institute of Occupational Safety and Health; no conflicts of interest were declared.
The second study was supported by the V Foundation for Cancer Research, the Byrne Fund for the benefit of Memorial Sloan-Kettering Cancer Center, the National Cancer Institute, the Albert Einstein Cancer Center, and the National Institute for Occupational Safety and Health; no conflicts of interest were declared.
SOURCE: Zeig-Owens R et al. JAMA Oncology. 2018 April 26. doi: 10.1001/jamaoncol.2018.0504. Landgren O et al. JAMA Oncology. 2018 April 16. doi: 10.1001/jamaoncol.2018.0509.
Rescue and recovery workers who were involved in the aftermath of the World Trade Center disaster may face a greater cancer burden than the general population, according to two studies published in JAMA Oncology.
In particular, they may be at risk of developing multiple myeloma at an earlier age.
The first study was a closed-cohort study of 14,474 employees of the Fire Department of the City of New York (FDNY) who were exposed to the World Trade Center disaster but were cancer-free as of Jan. 1, 2012. The aim was to project cancer incidence from 2012 through 2031, based on data from the FDNY World Trade Center Health Program, and compare those rates with age-, race-, and sex-specific New York cancer rates from the general population.
The modeling projected a “modestly” higher number of cancer cases in the white male subgroup of rescue and recovery workers exposed to the World Trade Center (2,714 vs. 2,596 for the general population of New York; P less than .001). Specifically, the investigators projected significantly higher case counts of prostate cancer (1,437 vs. 863), thyroid cancer (73 vs. 57), and melanoma (201 vs. 131), compared with the general population in New York, but fewer lung (237 vs. 373), colorectal (172 vs. 267), and kidney cancers (66 vs. 132) (P less than .001 for all).
“Our findings suggest that the FDNY WTC-exposed cohort may experience a greater burden of cancer than would be expected from a population with similar demographic characteristics,” wrote Rachel Zeig-Owens, DrPH, from the Montefiore Medical Center and Albert Einstein College of Medicine, both in New York, and coauthors, highlighting prostate cancer as a particular concern.
However, they also acknowledged that the elevated rates observed in people exposed to the World Trade Center disaster could be a result of increased surveillance, even though they did attempt to correct for that, and that firefighters in general might face higher risks.
“It is possible that firefighters have a higher risk of cancer than the general population owing to exposures associated with the occupation,” they wrote. However occupation could also have the opposite effect, as rescue and recovery workers tend to have lower smoking rates, which may explain the relatively low rates of certain cancers such as lung cancer, they said.
A second study examined the effect of the World Trade Center disaster on the risk of multiple myeloma and monoclonal gammopathies in exposed firefighters.
The seroprevalence study of monoclonal gammopathies of undetermined significance (MGUS) in 781 exposed firefighters revealed that the age-standardized prevalence of these was 76% higher in this population than it was in a white male reference population living in Minnesota.
In particular, the age-standardized prevalence of light-chain MGUS was more than threefold higher in exposed firefighters, compared with the reference population.
Researchers also analyzed a case series of 16 exposed white male firefighters who received a diagnosis of multiple myeloma after Sept. 11, 2001. Of the 14 patients for whom data on the monoclonal protein isotype was available, half had light-chain multiple myeloma.
“These findings are of interest due to previously observed associations between light-chain multiple myeloma and light-chain MGUS and exposure to toxins, and chronic immune stimulation,” wrote Ola Landgren, MD, PhD, from the Memorial Sloan Kettering Cancer Center and his coauthors.
Seven patients were also assessed for CD20 expression – a marker of poorer prognosis – and 71% were found to be CD20-positive, a prevalence around 3.5-fold higher than that seen in the general population.
The cohort with multiple myeloma was diagnosed on average 12 years younger than those in the general population. The authors commented that this was unlikely to be caused by lead-time bias because the time from first symptoms to clinical manifestation of the disease is usually around 1 year.
“Taken together, our results show that environmental exposure due to the WTC attacks is associated with myeloma precursor disease (MGUS and light-chain MGUS) and may be a risk factor for the development of multiple myeloma at an earlier age, particularly the light-chain subtype,” the authors wrote.
The first study was supported by the National Institute of Occupational Safety and Health; no conflicts of interest were declared.
The second study was supported by the V Foundation for Cancer Research, the Byrne Fund for the benefit of Memorial Sloan-Kettering Cancer Center, the National Cancer Institute, the Albert Einstein Cancer Center, and the National Institute for Occupational Safety and Health; no conflicts of interest were declared.
SOURCE: Zeig-Owens R et al. JAMA Oncology. 2018 April 26. doi: 10.1001/jamaoncol.2018.0504. Landgren O et al. JAMA Oncology. 2018 April 16. doi: 10.1001/jamaoncol.2018.0509.
FROM JAMA ONCOLOGY
Key clinical point: Monoclonal gammopathies and multiple myeloma may occur more often and earlier in World Trade Center rescue workers.
Major finding: Prevalence of light-chain monoclonal gammopathies is threefold higher in exposed firefighters than in a reference population of white males.
Study details: A cohort study in 14,474 employees of the Fire Department of the City of New York exposed to the Sept. 11, 2001, World Trade Center disaster, a case series of 16 exposed white male firefighters diagnosed with multiple myeloma, and a seroprevalence study of monoclonal gammopathies of undetermined significance in 781 exposed firefighters.
Disclosures: The first study was supported by the National Institute of Occupational Safety and Health; no conflicts of interest were declared. The second study was supported by the V Foundation for Cancer Research, the Byrne Fund for the benefit of Memorial Sloan-Kettering Cancer Center, the National Cancer Institute, the Albert Einstein Cancer Center, and the National Institute for Occupational Safety and Health; no conflicts of interest were declared.
Source: Zeig-Owens R et al. JAMA Oncology 2018, Apr 26. doi: 10.1001/jamaoncol.2018.0504. Landgren O et al. JAMA Oncology 2018, Apr 26. doi: 10.1001/jamaoncol.2018.0509.
Early breast cancer: Patients report favorable quality of life after partial breast irradiation
In women with breast cancer undergoing breast-conserving surgery, accelerated partial breast irradiation (APBI) using multicatheter brachytherapy does not negatively affect quality of life, compared with standard whole breast irradiation, investigators have reported.
Patients reported similar quality of life scores for multicatheter brachytherapy–based APBI and whole breast irradiation in the study by the Groupe Européen de Curiethérapie of European Society for Radiotherapy and Oncology (GEC-ESTRO).
Moreover, breast symptom scores were significantly worse for whole-breast radiation, Rebekka Schäfer, MD, of the department of radiation oncology at the University Hospital Würzburg (Germany) and colleagues reported in Lancet Oncology.
“This trial provides further clinical evidence that APBI with interstitial brachytherapy can be considered as an alternative treatment option after breast-conserving surgery for patients with low-risk breast cancer,” Dr. Schäfer and coauthors wrote.
In several previous studies, APBI has been shown to have clinical outcomes equivalent to those of whole breast irradiation in terms of disease recurrence, survival, and treatment side effects, they added.
The quality of life findings in the present report come from long-term follow-up of a randomized, controlled, phase 3 trial conducted at 16 European centers. This study included 1,184 women with early breast cancer randomly who, after receiving breast-conserving surgery, were assigned either to APBI that used multicatheter brachytherapy or to whole breast irradiation.
Women in the study completed validated quality of life questionnaires right before and right after radiotherapy, as well as during follow-up.
A little more than half of the women in each group completed the quality of life questionnaires after the treatment and again at follow-up, investigators said.
Global health status was stable over time in both groups, investigators reported. In the APBI group, global health status score on a scale of 0-100 was 65.6 right after the procedure and 66.2 at 5 years; similarly, scores in the whole breast irradiation group were 64.6 after radiotherapy and 66.0 at 5 years.
The only quality of life difference between arms that investigators characterized as moderately clinically relevant was in breast symptom scores, which were significantly worse in the whole breast radiation group right after radiotherapy (difference of means, 13.6; 95% CI, 9.7-17.5; P less than .0001) and at 3-month follow-up (difference of means, 12.7; 95% CI, 9.8-15.6; P less than .0001).
Emotional functioning, fatigue, and financial difficulty scores in the APBI group were “slightly better” than in the whole breast radiation group right after radiotherapy and at a 3-month follow-up, investigators reported; however, at 5 year follow-up, there were no significant differences between arms in those measures.
“Our findings show that APBI using multicatheter interstitial brachytherapy does not result in clinically significant deterioration of overall quality of life and that the different domains of quality of life after APBI were not worse in comparison with whole breast irradiation in terms of clinically relevant differences,” Dr. Schäfer and colleagues concluded in their report.
Dr. Schäfer reported no conflicts of interest. Coauthors reported disclosures outside of the submitted work including Nucletron Operations BV, Elekta Company, Merck Serono, Novocure, AstraZeneca, and Bristol-Myers Squibb, among others.
SOURCE: Schäfer R et al. Lancet Oncol. 2018 Apr 22. doi: 10.1016/S1470-2045(18)30195-5.
This study by Schäfer and colleagues supports results of earlier and smaller studies showing promising quality of life results following accelerated partial breast irradiation (APBI) using multicatheter brachytherapy, according to Reshma Jagsi, MD.
“The results suggest that for quality of life, multicatheter brachytherapy-based APBI does not adversely affect outcomes, compared with whole breast irradiation,” Dr. Jagsi wrote in an editorial accompanying the article.
In previous trials, APBI using external radiation beam techniques has likewise shown favorable and promising quality of life outcomes.
There are now eagerly anticipated studies of APBI delivered primarily using external beam techniques that have included rigorous collection of quality of life outcomes, Dr. Jagsi added.
Those trials, which include RAPID and RTOG 0413/NSABP B39, will provide additional evidence to consider alongside those of the trial reported by Schäfer and colleagues on behalf of the Groupe Européen de Curiethérapie of European Society for Radiotherapy and Oncology (GEC-ESTRO).
“Together with the results from the GEC-ESTRO trial, results from these trials will be meaningful to the many tens of thousands of women who undergo breast-conserving surgery and adjuvant radiotherapy each year,” Dr. Jagsi wrote.
Reshma Jagsi, MD, is with the department of radiation oncology at the University of Michigan, Ann Arbor. These comments are derived from editorial in Lancet Oncology . Dr. Jagsi reported receiving personal fees from Amgen.
This study by Schäfer and colleagues supports results of earlier and smaller studies showing promising quality of life results following accelerated partial breast irradiation (APBI) using multicatheter brachytherapy, according to Reshma Jagsi, MD.
“The results suggest that for quality of life, multicatheter brachytherapy-based APBI does not adversely affect outcomes, compared with whole breast irradiation,” Dr. Jagsi wrote in an editorial accompanying the article.
In previous trials, APBI using external radiation beam techniques has likewise shown favorable and promising quality of life outcomes.
There are now eagerly anticipated studies of APBI delivered primarily using external beam techniques that have included rigorous collection of quality of life outcomes, Dr. Jagsi added.
Those trials, which include RAPID and RTOG 0413/NSABP B39, will provide additional evidence to consider alongside those of the trial reported by Schäfer and colleagues on behalf of the Groupe Européen de Curiethérapie of European Society for Radiotherapy and Oncology (GEC-ESTRO).
“Together with the results from the GEC-ESTRO trial, results from these trials will be meaningful to the many tens of thousands of women who undergo breast-conserving surgery and adjuvant radiotherapy each year,” Dr. Jagsi wrote.
Reshma Jagsi, MD, is with the department of radiation oncology at the University of Michigan, Ann Arbor. These comments are derived from editorial in Lancet Oncology . Dr. Jagsi reported receiving personal fees from Amgen.
This study by Schäfer and colleagues supports results of earlier and smaller studies showing promising quality of life results following accelerated partial breast irradiation (APBI) using multicatheter brachytherapy, according to Reshma Jagsi, MD.
“The results suggest that for quality of life, multicatheter brachytherapy-based APBI does not adversely affect outcomes, compared with whole breast irradiation,” Dr. Jagsi wrote in an editorial accompanying the article.
In previous trials, APBI using external radiation beam techniques has likewise shown favorable and promising quality of life outcomes.
There are now eagerly anticipated studies of APBI delivered primarily using external beam techniques that have included rigorous collection of quality of life outcomes, Dr. Jagsi added.
Those trials, which include RAPID and RTOG 0413/NSABP B39, will provide additional evidence to consider alongside those of the trial reported by Schäfer and colleagues on behalf of the Groupe Européen de Curiethérapie of European Society for Radiotherapy and Oncology (GEC-ESTRO).
“Together with the results from the GEC-ESTRO trial, results from these trials will be meaningful to the many tens of thousands of women who undergo breast-conserving surgery and adjuvant radiotherapy each year,” Dr. Jagsi wrote.
Reshma Jagsi, MD, is with the department of radiation oncology at the University of Michigan, Ann Arbor. These comments are derived from editorial in Lancet Oncology . Dr. Jagsi reported receiving personal fees from Amgen.
In women with breast cancer undergoing breast-conserving surgery, accelerated partial breast irradiation (APBI) using multicatheter brachytherapy does not negatively affect quality of life, compared with standard whole breast irradiation, investigators have reported.
Patients reported similar quality of life scores for multicatheter brachytherapy–based APBI and whole breast irradiation in the study by the Groupe Européen de Curiethérapie of European Society for Radiotherapy and Oncology (GEC-ESTRO).
Moreover, breast symptom scores were significantly worse for whole-breast radiation, Rebekka Schäfer, MD, of the department of radiation oncology at the University Hospital Würzburg (Germany) and colleagues reported in Lancet Oncology.
“This trial provides further clinical evidence that APBI with interstitial brachytherapy can be considered as an alternative treatment option after breast-conserving surgery for patients with low-risk breast cancer,” Dr. Schäfer and coauthors wrote.
In several previous studies, APBI has been shown to have clinical outcomes equivalent to those of whole breast irradiation in terms of disease recurrence, survival, and treatment side effects, they added.
The quality of life findings in the present report come from long-term follow-up of a randomized, controlled, phase 3 trial conducted at 16 European centers. This study included 1,184 women with early breast cancer randomly who, after receiving breast-conserving surgery, were assigned either to APBI that used multicatheter brachytherapy or to whole breast irradiation.
Women in the study completed validated quality of life questionnaires right before and right after radiotherapy, as well as during follow-up.
A little more than half of the women in each group completed the quality of life questionnaires after the treatment and again at follow-up, investigators said.
Global health status was stable over time in both groups, investigators reported. In the APBI group, global health status score on a scale of 0-100 was 65.6 right after the procedure and 66.2 at 5 years; similarly, scores in the whole breast irradiation group were 64.6 after radiotherapy and 66.0 at 5 years.
The only quality of life difference between arms that investigators characterized as moderately clinically relevant was in breast symptom scores, which were significantly worse in the whole breast radiation group right after radiotherapy (difference of means, 13.6; 95% CI, 9.7-17.5; P less than .0001) and at 3-month follow-up (difference of means, 12.7; 95% CI, 9.8-15.6; P less than .0001).
Emotional functioning, fatigue, and financial difficulty scores in the APBI group were “slightly better” than in the whole breast radiation group right after radiotherapy and at a 3-month follow-up, investigators reported; however, at 5 year follow-up, there were no significant differences between arms in those measures.
“Our findings show that APBI using multicatheter interstitial brachytherapy does not result in clinically significant deterioration of overall quality of life and that the different domains of quality of life after APBI were not worse in comparison with whole breast irradiation in terms of clinically relevant differences,” Dr. Schäfer and colleagues concluded in their report.
Dr. Schäfer reported no conflicts of interest. Coauthors reported disclosures outside of the submitted work including Nucletron Operations BV, Elekta Company, Merck Serono, Novocure, AstraZeneca, and Bristol-Myers Squibb, among others.
SOURCE: Schäfer R et al. Lancet Oncol. 2018 Apr 22. doi: 10.1016/S1470-2045(18)30195-5.
In women with breast cancer undergoing breast-conserving surgery, accelerated partial breast irradiation (APBI) using multicatheter brachytherapy does not negatively affect quality of life, compared with standard whole breast irradiation, investigators have reported.
Patients reported similar quality of life scores for multicatheter brachytherapy–based APBI and whole breast irradiation in the study by the Groupe Européen de Curiethérapie of European Society for Radiotherapy and Oncology (GEC-ESTRO).
Moreover, breast symptom scores were significantly worse for whole-breast radiation, Rebekka Schäfer, MD, of the department of radiation oncology at the University Hospital Würzburg (Germany) and colleagues reported in Lancet Oncology.
“This trial provides further clinical evidence that APBI with interstitial brachytherapy can be considered as an alternative treatment option after breast-conserving surgery for patients with low-risk breast cancer,” Dr. Schäfer and coauthors wrote.
In several previous studies, APBI has been shown to have clinical outcomes equivalent to those of whole breast irradiation in terms of disease recurrence, survival, and treatment side effects, they added.
The quality of life findings in the present report come from long-term follow-up of a randomized, controlled, phase 3 trial conducted at 16 European centers. This study included 1,184 women with early breast cancer randomly who, after receiving breast-conserving surgery, were assigned either to APBI that used multicatheter brachytherapy or to whole breast irradiation.
Women in the study completed validated quality of life questionnaires right before and right after radiotherapy, as well as during follow-up.
A little more than half of the women in each group completed the quality of life questionnaires after the treatment and again at follow-up, investigators said.
Global health status was stable over time in both groups, investigators reported. In the APBI group, global health status score on a scale of 0-100 was 65.6 right after the procedure and 66.2 at 5 years; similarly, scores in the whole breast irradiation group were 64.6 after radiotherapy and 66.0 at 5 years.
The only quality of life difference between arms that investigators characterized as moderately clinically relevant was in breast symptom scores, which were significantly worse in the whole breast radiation group right after radiotherapy (difference of means, 13.6; 95% CI, 9.7-17.5; P less than .0001) and at 3-month follow-up (difference of means, 12.7; 95% CI, 9.8-15.6; P less than .0001).
Emotional functioning, fatigue, and financial difficulty scores in the APBI group were “slightly better” than in the whole breast radiation group right after radiotherapy and at a 3-month follow-up, investigators reported; however, at 5 year follow-up, there were no significant differences between arms in those measures.
“Our findings show that APBI using multicatheter interstitial brachytherapy does not result in clinically significant deterioration of overall quality of life and that the different domains of quality of life after APBI were not worse in comparison with whole breast irradiation in terms of clinically relevant differences,” Dr. Schäfer and colleagues concluded in their report.
Dr. Schäfer reported no conflicts of interest. Coauthors reported disclosures outside of the submitted work including Nucletron Operations BV, Elekta Company, Merck Serono, Novocure, AstraZeneca, and Bristol-Myers Squibb, among others.
SOURCE: Schäfer R et al. Lancet Oncol. 2018 Apr 22. doi: 10.1016/S1470-2045(18)30195-5.
FROM LANCET ONCOLOGY
Key clinical point: Quality of life results support the use of accelerated partial breast irradiation (APBI) using multicatheter brachytherapy as an alternative to whole breast radiation after breast-conserving surgery.
Major finding: Patients reported similar quality of life scores for the two modalities, while breast symptom scores for whole breast radiation were significantly worse right after radiotherapy (difference of means, 13.6; 95% confidence interval, 9.7-17.5; P less than .0001) and at 3-month follow-up (difference of means, 12.7; 95% CI, 9.8-15.6; P less than .0001), compared with those for APBI.
Study details: 5-year quality of life results from a European phase 3 trial including 1,184 women with early breast cancer who, after undergoing breast-conserving surgery, received either whole breast irradiation or APBI using multicatheter brachytherapy.
Disclosures: Authors reported disclosures outside of the submitted work including Nucletron Operations BV, Elekta Company, Merck Serono, Novocure, AstraZeneca, and Bristol-Myers Squibb, among others.
Source: Schäfer R et al. Lancet Oncol. 2018 Apr 22. doi: 10.1016/S1470-2045(18)30195-5.
Hormone therapy raises diabetes risk in breast cancer survivors
Hormone therapy for breast cancer more than doubles a woman’s risk for developing type 2 diabetes, results of a case-cohort study suggest.
Hormone therapy with tamoxifen was associated with a more than twofold increase in risk of diabetes, and aromatase inhibitors were associated with a more than fourfold increase, reported Hatem Hamood, MD, of Leumit Health Services in Karmiel, Israel, and colleagues.
Among 2,246 women with breast cancer and no diabetes at baseline, followed for a mean of 5.9 years (longest follow-up 13 years), the crude cumulative lifetime incidence rate of diabetes was 20.9%, the investigators wrote. The report was published in the Journal of Clinical Oncology.
“[Hormone therapy] is a significant risk factor of diabetes among breast cancer survivors. The underlying mechanism is unclear, and additional research is warranted. Although cessation of treatment is not recommended and progression of breast cancer often is inevitable, devised strategies aimed at lifestyle modifications in patients at high risk of diabetes could at least preserve the natural history of breast cancer,” they wrote.
Diabetes has previously been identified as a possible risk factor for breast cancer, but the potential for breast cancer therapy as a precipitating factor for diabetes is uncertain, the authors said.
“Given the detrimental impact of diabetes on breast cancer survival, additional exploration of the role of breast cancer treatment in the development of diabetes is important not only because it would add valuable information on the etiology of diabetes but also because it would help to identify high-risk patients in need of accentuated clinical care,” they wrote.
To explore the possible association between hormone therapy and diabetes risk, the investigators performed a retrospective case-cohort study of 2,246 women who had been diagnosed with primary nonmetastatic breast cancer treated with hormone therapy from 2002 through 2012.
They examined data on a randomly selected cohort of 448 breast cancer survivors and all patients in the parent (no diabetes at baseline) cohort who developed diabetes during the study period (324 patients).
They found that the prevalence of diabetes among their source population of 2,644 breast cancer survivors (including those with baseline diabetes) increased “drastically” from 6% in 2002 to 28% in 2015. The prevalence exceeded Israeli national norms from 2010 through 2013, with standardized prevalence ratios of 1.61 to 1.81 (P less than .001).
As noted, in the population without baseline diabetes, the crude cumulative incidence rate of diabetes in the presence of death as a competing risk factor was 20.9%.
In multivariate analyses controlling for demographic and socioeconomic factors, and for chemotherapy type, hypertension, outpatient visits, use of corticosteroids, thiazide diuretics, beta-blockers, statins, and year of breast cancer diagnosis, factors significantly associated with diabetes risk were use of hormone therapy (adjusted hazard ratio [HR] 2.40, P = .008), tamoxifen (aHR 2.25, P = .013), aromatase inhibitors (aHR 4.27, P = .013), therapy duration more than 1 year (aHR 2.36, P = .009), and 1 year or less (aHR 6.48, P = .004).
The investigators noted that although other reports have found no association between aromatase inhibitors and diabetes risk, those studies had small samples or offered no explanation of the lack of association.
In contrast, a 2016 joint ACS/ASCO breast cancer survivorship-care guideline notes that aromatase inhibitors may raise the risk of diabetes, the investigators noted.
The study was supported by grants from the Israeli Council for Higher Education. The investigators reported no conflicts of interest.
SOURCE: Hamood H et al. J Clin Oncol. 2018 Apr 24. doi: 10.1200/JCO.2017.76.3524.
Hormone therapy for breast cancer more than doubles a woman’s risk for developing type 2 diabetes, results of a case-cohort study suggest.
Hormone therapy with tamoxifen was associated with a more than twofold increase in risk of diabetes, and aromatase inhibitors were associated with a more than fourfold increase, reported Hatem Hamood, MD, of Leumit Health Services in Karmiel, Israel, and colleagues.
Among 2,246 women with breast cancer and no diabetes at baseline, followed for a mean of 5.9 years (longest follow-up 13 years), the crude cumulative lifetime incidence rate of diabetes was 20.9%, the investigators wrote. The report was published in the Journal of Clinical Oncology.
“[Hormone therapy] is a significant risk factor of diabetes among breast cancer survivors. The underlying mechanism is unclear, and additional research is warranted. Although cessation of treatment is not recommended and progression of breast cancer often is inevitable, devised strategies aimed at lifestyle modifications in patients at high risk of diabetes could at least preserve the natural history of breast cancer,” they wrote.
Diabetes has previously been identified as a possible risk factor for breast cancer, but the potential for breast cancer therapy as a precipitating factor for diabetes is uncertain, the authors said.
“Given the detrimental impact of diabetes on breast cancer survival, additional exploration of the role of breast cancer treatment in the development of diabetes is important not only because it would add valuable information on the etiology of diabetes but also because it would help to identify high-risk patients in need of accentuated clinical care,” they wrote.
To explore the possible association between hormone therapy and diabetes risk, the investigators performed a retrospective case-cohort study of 2,246 women who had been diagnosed with primary nonmetastatic breast cancer treated with hormone therapy from 2002 through 2012.
They examined data on a randomly selected cohort of 448 breast cancer survivors and all patients in the parent (no diabetes at baseline) cohort who developed diabetes during the study period (324 patients).
They found that the prevalence of diabetes among their source population of 2,644 breast cancer survivors (including those with baseline diabetes) increased “drastically” from 6% in 2002 to 28% in 2015. The prevalence exceeded Israeli national norms from 2010 through 2013, with standardized prevalence ratios of 1.61 to 1.81 (P less than .001).
As noted, in the population without baseline diabetes, the crude cumulative incidence rate of diabetes in the presence of death as a competing risk factor was 20.9%.
In multivariate analyses controlling for demographic and socioeconomic factors, and for chemotherapy type, hypertension, outpatient visits, use of corticosteroids, thiazide diuretics, beta-blockers, statins, and year of breast cancer diagnosis, factors significantly associated with diabetes risk were use of hormone therapy (adjusted hazard ratio [HR] 2.40, P = .008), tamoxifen (aHR 2.25, P = .013), aromatase inhibitors (aHR 4.27, P = .013), therapy duration more than 1 year (aHR 2.36, P = .009), and 1 year or less (aHR 6.48, P = .004).
The investigators noted that although other reports have found no association between aromatase inhibitors and diabetes risk, those studies had small samples or offered no explanation of the lack of association.
In contrast, a 2016 joint ACS/ASCO breast cancer survivorship-care guideline notes that aromatase inhibitors may raise the risk of diabetes, the investigators noted.
The study was supported by grants from the Israeli Council for Higher Education. The investigators reported no conflicts of interest.
SOURCE: Hamood H et al. J Clin Oncol. 2018 Apr 24. doi: 10.1200/JCO.2017.76.3524.
Hormone therapy for breast cancer more than doubles a woman’s risk for developing type 2 diabetes, results of a case-cohort study suggest.
Hormone therapy with tamoxifen was associated with a more than twofold increase in risk of diabetes, and aromatase inhibitors were associated with a more than fourfold increase, reported Hatem Hamood, MD, of Leumit Health Services in Karmiel, Israel, and colleagues.
Among 2,246 women with breast cancer and no diabetes at baseline, followed for a mean of 5.9 years (longest follow-up 13 years), the crude cumulative lifetime incidence rate of diabetes was 20.9%, the investigators wrote. The report was published in the Journal of Clinical Oncology.
“[Hormone therapy] is a significant risk factor of diabetes among breast cancer survivors. The underlying mechanism is unclear, and additional research is warranted. Although cessation of treatment is not recommended and progression of breast cancer often is inevitable, devised strategies aimed at lifestyle modifications in patients at high risk of diabetes could at least preserve the natural history of breast cancer,” they wrote.
Diabetes has previously been identified as a possible risk factor for breast cancer, but the potential for breast cancer therapy as a precipitating factor for diabetes is uncertain, the authors said.
“Given the detrimental impact of diabetes on breast cancer survival, additional exploration of the role of breast cancer treatment in the development of diabetes is important not only because it would add valuable information on the etiology of diabetes but also because it would help to identify high-risk patients in need of accentuated clinical care,” they wrote.
To explore the possible association between hormone therapy and diabetes risk, the investigators performed a retrospective case-cohort study of 2,246 women who had been diagnosed with primary nonmetastatic breast cancer treated with hormone therapy from 2002 through 2012.
They examined data on a randomly selected cohort of 448 breast cancer survivors and all patients in the parent (no diabetes at baseline) cohort who developed diabetes during the study period (324 patients).
They found that the prevalence of diabetes among their source population of 2,644 breast cancer survivors (including those with baseline diabetes) increased “drastically” from 6% in 2002 to 28% in 2015. The prevalence exceeded Israeli national norms from 2010 through 2013, with standardized prevalence ratios of 1.61 to 1.81 (P less than .001).
As noted, in the population without baseline diabetes, the crude cumulative incidence rate of diabetes in the presence of death as a competing risk factor was 20.9%.
In multivariate analyses controlling for demographic and socioeconomic factors, and for chemotherapy type, hypertension, outpatient visits, use of corticosteroids, thiazide diuretics, beta-blockers, statins, and year of breast cancer diagnosis, factors significantly associated with diabetes risk were use of hormone therapy (adjusted hazard ratio [HR] 2.40, P = .008), tamoxifen (aHR 2.25, P = .013), aromatase inhibitors (aHR 4.27, P = .013), therapy duration more than 1 year (aHR 2.36, P = .009), and 1 year or less (aHR 6.48, P = .004).
The investigators noted that although other reports have found no association between aromatase inhibitors and diabetes risk, those studies had small samples or offered no explanation of the lack of association.
In contrast, a 2016 joint ACS/ASCO breast cancer survivorship-care guideline notes that aromatase inhibitors may raise the risk of diabetes, the investigators noted.
The study was supported by grants from the Israeli Council for Higher Education. The investigators reported no conflicts of interest.
SOURCE: Hamood H et al. J Clin Oncol. 2018 Apr 24. doi: 10.1200/JCO.2017.76.3524.
FROM JOURNAL OF CLINICAL ONCOLOGY
Key clinical point: Consider screening survivors of nonmetastatic breast cancer for diabetes.
Major finding: The crude lifetime incidence of diabetes following hormone therapy for breast cancer was 20.9%.
Study details: Case-cohort study of 2,246 women with nonmetastatic breast cancer and no baseline diabetes treated with hormone therapy.
Disclosures: The study was supported by grants from the Israeli Council for Higher Education. The investigators reported no conflicts of interest.
Source: Hamood H et al. J Clin Oncol. 2018 Apr 24. doi: 10.1200/JCO.2017.76.3524.
Analgesic management in radiation oncology for painful bone metastases
Bone metastases are a common cause of pain in patients with advanced cancer, with about three-quarters of patients with bone metastases experiencing pain as the dominant symptom.1 Inadequately treated cancer pain impairs patient quality of life, and is associated with higher rates of depression, anxiety, and fatigue. Palliative radiotherapy (RT) is effective in alleviating pain from bone metastases.4 Local field external beam radiotherapy can provide some pain relief at the site of treated metastasis in 80%-90% of cases, with complete pain relief in 50%-60% of cases.5,6 However, maximal pain relief from RT is delayed, in some cases taking days to up to multiple weeks to attain.7,8 Therefore, optimal management of bone metastases pain may require the use of analgesics until RT takes adequate effect.
National Comprehensive Cancer Network (NCCN) Guidelines for Adult Cancer Pain (v. 2.2015) recommend that pain intensity rating (PIR; range, 0-10, where 0 denotes no pain and 10, worst pain imaginable) be used to quantify pain for all symptomatic patients. These guidelines also recommend the pain medication regimen be assessed for all symptomatic patients. For patients with moderate or severe pain (PIR of ≥4), NCCN guidelines recommend that analgesic regimen be intervened upon by alteration of the analgesic regimen (initiating, rotating, or titrating analgesic) or consideration of referral to pain/symptom management specialty.
Previous findings have demonstrated inadequate analgesic management for cancer pain,2,9 including within the radiation oncology (RO) clinic, suggesting that patients seen in consultation for palliative RT may experience uncontrolled pain for days to weeks before the onset of relief from RT. Possible reasons for inadequate acute pain intervention in the RO clinic may be provider discomfort with analgesic management and infrequent formal integration of palliative care within RO.10
Limited single-institution data from the few institutions with dedicated palliative RO services have suggested that these services improve the quality of palliative care delivery, as demonstrated by providers perceptions’ of the clinical impact of a dedicated service11 and the implementation of expedited palliative RT delivery for acute cancer pain.12,13 To our knowledge, the impact of a dedicated palliative RO service on analgesic management for cancer pain has not been assessed.
Here, we report how often patients with symptomatic bone metastases had assessments of existing analgesic regimens and interventions at RO consultation at 2 cancer centers. Center 1 had implemented a dedicated palliative RO service in 2011, consisting of rotating attending physicians and residents as well as dedicated palliative care trained nurse practitioners and a fellow, with the service structured around daily rounds,11 whereas Center 2 had not yet implemented a dedicated service. Using data from both centers, we assessed the impact of a palliative RO service on analgesic assessment and management in patients with bone metastases.
Methods
We searched our institutional databases for patients seen in RO consultation for bone metastases using ICD-9 code 198.5, and retrospectively reviewed consultation notes for those patients during June-July 2008, January-February 2010, January-February 2013, and June-July 2014. Those time periods were chosen as evenly spaced representative samples before and after implementation of a dedicated palliative RO service in 2011 at Center 1. Center 2 did not implement a dedicated palliative RO service in these time periods.
Within consultation notes, we recorded the following data from the History of the Present Illness section: symptoms from bone metastases (symptomatic was defined as any pain present); PIR (range, 0-10); and whether or not the preconsultation analgesic regimen was reported for symptomatic patients (including analgesic type, dosing, effectiveness, and adherence).
Documentation of the analgesic regimen in the history section of the notes was considered the proxy for analgesic regimen assessment at time of RO consultation. Analgesics within the Medications list, which were autopopulated in the consultation note by the electronic medical record, were recorded.
Whether or not pain was addressed with initiation or titration of analgesics for patients with a PIR of ≥4 was recorded from the Assessment and Plan portion of the notes, and that metric was considered the proxy for pain intervention. In addition, the case was coded as having had pain intervention if there was documentation of the patient declining recommended analgesic intervention, or the patient had been referred to a symptom management service for intervention (eg, referral to a specialty palliative care clinic), or there was recommendation for the patient to discuss uncontrolled pain with the original prescriber. A PIR of 4 was chosen as the threshold for analgesic intervention because at that level, NCCN guidelines for cancer pain state that the analgesic regimen should be titrated, whereas for a PIR of 3 or less, the guidelines recommend only consideration of titrating the analgesic. Only patients with a documented PIR were included in the pain intervention analysis.
Frequencies of analgesic assessment and analgesic intervention were compared using t tests (Wizard Pro, v1.8.5; Evan Miller, Chicago IL).
Results
A total of 271 patients with RO consultation notes were identified at the 2 centers within the 4 time periods (Table 1).
Among symptomatic patients, any component of the preconsultation analgesic regimen (including analgesic type, dosing, pain response, and adherence) was documented for 37.9% of the entire cohort at RO consultation (Table 3). At Centers 1 and 2, the frequencies of analgesic regimen assessment were documented for 41.3% and 28.1%, respectively (P = .06). Among symptomatic patients, 81.5% had an opioid or nonopioid analgesic listed in the Medications section in the electronic medical record at time of consultation.
Patients seen on the dedicated palliative RO service at Center 1 had an analgesic assessment documentation rate of 59.5%, whereas the patients not seen on a palliative RO service (ie, patients seen on a nonpalliative RO service at Center 1 plus all patients at Center 2) had an assessment documentation rate of 33.5% (P = .002; Figure 1). There was no significant difference between rates of analgesic regimen assessment between patients seen at Center 2 and patients seen within nondedicated palliative RO services at Center 1 (28.1% vs 35.9%, respectively; P = .27).
In patients seen at Center 1 only, those seen on the palliative RO service had a higher documentation rate of analgesic assessment compared with those seen by other services after implementation of the dedicated service (59.5% vs 38%, respectively; P = .018). Time period (after versus before 2011) was not significantly associated with the rate of documentation of analgesic assessment at either Center 1 (after vs before 2011: 44.4% vs 31%, P = .23) or Center 2 (31.4% vs 24.1%, P = .60).
Among patients with a PIR of ≥4, analgesic intervention was reported for 17.2% of patients within the entire cohort (20.8% at Center 1 and 0% at Center 2, P = .05). Among those with a PIR of ≥4, documentation of analgesic assessment noted in the History of the Present Illness section was associated with increased documentation of an analgesic intervention in the Assessment and Plan section (25% vs 7.3%; odds ratio [OR], 4.22; 95% confidence interval [CI], 1.1-16.0; P = .03).
Patients seen on the dedicated palliative RO service at Center 1 had a documented analgesic intervention rate of 31.6%, whereas the patients not seen on a palliative RO service (ie, those seen on a nonpalliative RO service at Center 1 plus all patients at Center 2) had a documented analgesic intervention rate of 9.2% (P = .01; Figure 2). There was no statistically significant difference between rates of documentation of an analgesic regimen intervention between patients seen at Center 2 and patients seen within nondedicated palliative RO services at Center 1 (0% vs 17.2%, respectively; P = .07).
Looking at only patients seen at Center 1, patients with a PIR of ≥4 seen on the dedicated palliative RO service had a nearly significant higher rate of documented analgesic interventions in the time period after implementation of the dedicate service (31.6% if seen on the dedicated service vs 12% if seen on a nondedicated service, P = .06).
Discussion
Multiple studies demonstrate the undertreatment of cancer pain in the outpatient setting.2,9,14,15 At 2 cancer centers, we found that about half of patients who present for consideration of palliative RT for bone metastases had a PIR of ≥4, yet only 17% of them had documentation of analgesic intervention as recommended by NCCN guidelines for cancer pain. Underlying this low rate of appropriate intervention may be the assumption of rapid pain relief by RT. However, RT often does not begin at time of consultation,16 and maximal pain relief may take days to weeks after commencement of RT.17 It is estimated that a quarter of all patients with cancer develop bone metastases during the course of their disease,12 and most of those patients suffer from pain. Thus, inherent delay in pain relief before, during, and after RT results in significant morbidity for the cancer patient population if adequate analgesic management is not provided.
The low rate of appropriate analgesic intervention at the time of RO consultation may also be related to the low incidence of proper analgesic assessment. In our cohort, 80% of symptomatic patients had an opioid or nonopioid analgesic listed in their medications within the electronic medical record at time of consultation, but only 38% had the analgesic regimen and/or its effectiveness described in the History of the Present Illness section of the record. Inattentiveness to analgesic type, dosing, and effectiveness during consultation may result in any inadequacies of the analgesic regimen going unnoticed. Consistent with this notion, we found that the rate of appropriate intervention for patients with a PIR of ≥4 was higher among patients who had analgesic regimen reported in the consultation note. Thus, interventions to implement routine review and documentation of the analgesic regimen, for example within the electronic medical record, may be one way to improve pain management.
Another possible reason for low rates of acute pain management within the RO clinic is low provider confidence in regard to analgesic management. In a recent national survey, 96% of radiation oncologists stated they were at least moderately confident with assessment of pain, yet only 77% were at least moderately confident with titrating opioids, and just 56% were at least moderately confident with rotating opioids.10 Educational interventions that improve providers’ facility with analgesic management may increase the frequency of pain management in the RO clinic.
Patients seen on the dedicated palliative RO service had significantly higher rates of documented analgesic regimen assessment and appropriate intervention during RO consultation, compared with patients seen at Center 2 and those not seen on the dedicated palliative RO service at Center 1. The improvements we observed in analgesic assessment and intervention at Center 1 for patients seen on the palliative RO service are likely owing to involvement of palliative RO and not to secular trends, because there were not similar improvements for patients at Center 1 who were not seen by the palliative RO service and those at Center 2, where there was no service.
At Center 1, the dedicated palliative RO service was created to provide specialized care to patients with metastatic disease undergoing palliative radiation. Within its structure, topics within palliative RO, such as technical aspects of palliative RT, symptom management, and communication are taught and reinforced in a case-based approach. Such palliative care awareness, integration, and education within RO achieved by the palliative RO service likely contribute to the improved rates of analgesic management we found in our study. We do note that rate of analgesic intervention in the palliative RO cohort, though higher than in the nonpalliative RO group, was still low, with only a third of patients receiving proper analgesic management. These findings highlight the importance of continued effort in increasing providers’ awareness of the need to assess pain and raise comfort with analgesic initiation and titration and of having dedicated palliative care clinicians embedded within the RO setting.
Since the data for this study was acquired, Center 2 has implemented a short palliative RO didactic course for residents, which improved their comfort levels in assessing analgesic effectiveness and intervening for uncontrolled pain.18 The impact of this intervention on clinical care will need to be evaluated, but the improved provider comfort levels may translate into better-quality care.
Limitations
An important limitation of this retrospective study is the reliance on the documentation provided in the consultation note for determining frequencies of analgesic regimen assessment and intervention. The actual rates of analgesic management that occurred in clinic may have been higher than reported in the documentation. However, such discrepancy in documentation of analgesic management would also be an area for quality improvement. Inadequate documentation limits the ability for proper follow-up of cancer pain as recommended by a joint guidance statement from the American Society of Clinical Oncology and the American Academy of Hospice and Palliative Medicine.19,20 The results of our study may also partly reflect a positive impact in documentation of analgesic management by a dedicated palliative RO service.
Given the multi-institutional nature of this study, it may be that general practice differences confound the impact of the dedicated palliative RO service at Center 1. However, with excluding Center 2, the dedicated service was still strongly associated with a higher rate of analgesic assessment within Center 1 and was almost significantly associated with appropriate analgesic intervention within Center 1.
We used a PIR of ≥4 as a threshold for appropriate analgesic regimen intervention because it is what is recommended by the NCCN guidelines. However, close attention should be paid to the impact that any amount of pain has on an individual patient. The functional, spiritual, and existential impact of pain is unique to each patient’s experience, and optimal symptom management should take those elements into account.
Conclusion
In conclusion, this study indicates that advanced cancer patient pain assessment and intervention according to NCCN cancer pain management guidelines is not common in the RO setting, and it is an area that should be targeted for quality improvement because of the positive implications for patient well-being. Pain assessment and intervention were greater in the setting of a dedicated structure for palliative care within RO, suggesting that the integration of palliative care within RO is a promising means of improving quality of pain management.
This work was presented at the 2016 ASCO Palliative Care in Oncology Symposium (September 9-10, 2016), where this work received a Conquer Cancer Foundation Merit Award.
1. Amichetti M, Orrù P, Madeddu A, et al. Comparative evaluation of two hypofractionated radiotherapy regimens for painful bone metastases. Tumori. 2004;90(1):91-95.
2. Vuong S, Pulenzas N, DeAngelis C, et al. Inadequate pain management in cancer patients attending an outpatient palliative radiotherapy clinic. Support Care Cancer. 2016;24(2):887-892.
3. Portenoy RK, Payne D, Jacobsen P. Breakthrough pain: characteristics and impact in patients with cancer pain. Pain. 1999;81(1-2):129-134.
4. Sze WM, Shelley M, Held I, Mason M. Palliation of metastatic bone pain: single fraction versus multifraction radiotherapy - a systematic review of the randomised trials. Sze WM, ed. Cochrane Database Syst Rev. 2004;(2):CD004721-CD004721.
5. Ratanatharathorn V, Powers WE, Moss WT, Perez CA. Bone metastasis: review and critical analysis of random allocation trials of local field treatment. Int J Radiat Oncol Biol Phys. 1999;44(1):1-18.
6. Kirou-Mauro A, Hird A, Wong J, et al. Is response to radiotherapy in patients related to the severity of pretreatment pain? Int J Radiat Oncol Biol Phys. 2008;71(4):1208-1212.
7. Frassica DA. General principles of external beam radiation therapy for skeletal metastases. Clin Orthop Relat Res. 2003;(415 Suppl):S158-S164.
8. McDonald R, Ding K, Brundage M, et al. Effect of radiotherapy on painful bone metastases: a secondary analysis of the NCIC Clinical Trials Group Symptom Control Trial SC.23. JAMA Oncol. 2017 Jul 1;3(7):953-959.
9. Greco MT, Roberto A, Corli O, et al. Quality of cancer pain management: an update of a systematic review of undertreatment of patients with cancer. J Clin Oncol. 2014;32(36):4149-4154.
10. Wei RL, Mattes MD, Yu J, et al. Attitudes of radiation oncologists toward palliative and supportive care in the united states: report on national membership survey by the American Society for Radiation Oncology (ASTRO). Pract Radiat Oncol. 2017;7(2):113-119.
11. Tseng YD, Krishnan MS, Jones JA, et al. Supportive and palliative radiation oncology service: impact of a dedicated service on palliative cancer care. Pract Radiat Oncol. 2014;4(4):247-253.
12. Fairchild A, Pituskin E, Rose B, et al. The rapid access palliative radiotherapy program: blueprint for initiation of a one-stop multidisciplinary bone metastases clinic. Support Care Cancer. 2009;17(2):163-170.
13. de Sa E, Sinclair E, Mitera G, et al. Continued success of the rapid response radiotherapy program: a review of 2004-2008. Support Care Cancer. 2009;17(7):757-762.
14. Deandrea S, Montanari M, Moja L, Apolone G. Prevalence of undertreatment in cancer pain. A review of published literature. Ann Oncol. 2008;19(12):1985-1991.
15. Mitera G, Zeiadin N, Kirou-Mauro A, et al. Retrospective assessment of cancer pain management in an outpatient palliative radiotherapy clinic using the Pain Management Index. J Pain Symptom Manage. 2010;39(2):259-267.
16. Danjoux C, Chow E, Drossos A, et al. An innovative rapid response radiotherapy program to reduce waiting time for palliative radiotherapy. Support Care Cancer. 2006;14(1):38-43.
17. Feyer PC, Steingraeber M. Radiotherapy of bone metastasis in breast cancer patients – current approaches. Breast Care (Basel). 2012;7(2):108-112.
18. Garcia MA, Braunstein SE, Anderson WG. Palliative Care Didactic Course for Radiation Oncology Residents. Int J Radiat Oncol Biol Phys. 2017;97(5):884-885.
19. Ferrell BR, Temel JS, Temin S, et al. Integration of palliative care into standard oncology care: American Society of Clinical Oncology clinical practice guideline update. J Clin Oncol. 2017;35(1):96-112.
20. Bickel KE, McNiff K, Buss MK, et al. Defining high-quality palliative care in oncology practice: an American Society of Clinical Oncology/American Academy of Hospice and Palliative Medicine guidance statement. J Oncol Pract. 2016;12(9):e828-e838.
Bone metastases are a common cause of pain in patients with advanced cancer, with about three-quarters of patients with bone metastases experiencing pain as the dominant symptom.1 Inadequately treated cancer pain impairs patient quality of life, and is associated with higher rates of depression, anxiety, and fatigue. Palliative radiotherapy (RT) is effective in alleviating pain from bone metastases.4 Local field external beam radiotherapy can provide some pain relief at the site of treated metastasis in 80%-90% of cases, with complete pain relief in 50%-60% of cases.5,6 However, maximal pain relief from RT is delayed, in some cases taking days to up to multiple weeks to attain.7,8 Therefore, optimal management of bone metastases pain may require the use of analgesics until RT takes adequate effect.
National Comprehensive Cancer Network (NCCN) Guidelines for Adult Cancer Pain (v. 2.2015) recommend that pain intensity rating (PIR; range, 0-10, where 0 denotes no pain and 10, worst pain imaginable) be used to quantify pain for all symptomatic patients. These guidelines also recommend the pain medication regimen be assessed for all symptomatic patients. For patients with moderate or severe pain (PIR of ≥4), NCCN guidelines recommend that analgesic regimen be intervened upon by alteration of the analgesic regimen (initiating, rotating, or titrating analgesic) or consideration of referral to pain/symptom management specialty.
Previous findings have demonstrated inadequate analgesic management for cancer pain,2,9 including within the radiation oncology (RO) clinic, suggesting that patients seen in consultation for palliative RT may experience uncontrolled pain for days to weeks before the onset of relief from RT. Possible reasons for inadequate acute pain intervention in the RO clinic may be provider discomfort with analgesic management and infrequent formal integration of palliative care within RO.10
Limited single-institution data from the few institutions with dedicated palliative RO services have suggested that these services improve the quality of palliative care delivery, as demonstrated by providers perceptions’ of the clinical impact of a dedicated service11 and the implementation of expedited palliative RT delivery for acute cancer pain.12,13 To our knowledge, the impact of a dedicated palliative RO service on analgesic management for cancer pain has not been assessed.
Here, we report how often patients with symptomatic bone metastases had assessments of existing analgesic regimens and interventions at RO consultation at 2 cancer centers. Center 1 had implemented a dedicated palliative RO service in 2011, consisting of rotating attending physicians and residents as well as dedicated palliative care trained nurse practitioners and a fellow, with the service structured around daily rounds,11 whereas Center 2 had not yet implemented a dedicated service. Using data from both centers, we assessed the impact of a palliative RO service on analgesic assessment and management in patients with bone metastases.
Methods
We searched our institutional databases for patients seen in RO consultation for bone metastases using ICD-9 code 198.5, and retrospectively reviewed consultation notes for those patients during June-July 2008, January-February 2010, January-February 2013, and June-July 2014. Those time periods were chosen as evenly spaced representative samples before and after implementation of a dedicated palliative RO service in 2011 at Center 1. Center 2 did not implement a dedicated palliative RO service in these time periods.
Within consultation notes, we recorded the following data from the History of the Present Illness section: symptoms from bone metastases (symptomatic was defined as any pain present); PIR (range, 0-10); and whether or not the preconsultation analgesic regimen was reported for symptomatic patients (including analgesic type, dosing, effectiveness, and adherence).
Documentation of the analgesic regimen in the history section of the notes was considered the proxy for analgesic regimen assessment at time of RO consultation. Analgesics within the Medications list, which were autopopulated in the consultation note by the electronic medical record, were recorded.
Whether or not pain was addressed with initiation or titration of analgesics for patients with a PIR of ≥4 was recorded from the Assessment and Plan portion of the notes, and that metric was considered the proxy for pain intervention. In addition, the case was coded as having had pain intervention if there was documentation of the patient declining recommended analgesic intervention, or the patient had been referred to a symptom management service for intervention (eg, referral to a specialty palliative care clinic), or there was recommendation for the patient to discuss uncontrolled pain with the original prescriber. A PIR of 4 was chosen as the threshold for analgesic intervention because at that level, NCCN guidelines for cancer pain state that the analgesic regimen should be titrated, whereas for a PIR of 3 or less, the guidelines recommend only consideration of titrating the analgesic. Only patients with a documented PIR were included in the pain intervention analysis.
Frequencies of analgesic assessment and analgesic intervention were compared using t tests (Wizard Pro, v1.8.5; Evan Miller, Chicago IL).
Results
A total of 271 patients with RO consultation notes were identified at the 2 centers within the 4 time periods (Table 1).
Among symptomatic patients, any component of the preconsultation analgesic regimen (including analgesic type, dosing, pain response, and adherence) was documented for 37.9% of the entire cohort at RO consultation (Table 3). At Centers 1 and 2, the frequencies of analgesic regimen assessment were documented for 41.3% and 28.1%, respectively (P = .06). Among symptomatic patients, 81.5% had an opioid or nonopioid analgesic listed in the Medications section in the electronic medical record at time of consultation.
Patients seen on the dedicated palliative RO service at Center 1 had an analgesic assessment documentation rate of 59.5%, whereas the patients not seen on a palliative RO service (ie, patients seen on a nonpalliative RO service at Center 1 plus all patients at Center 2) had an assessment documentation rate of 33.5% (P = .002; Figure 1). There was no significant difference between rates of analgesic regimen assessment between patients seen at Center 2 and patients seen within nondedicated palliative RO services at Center 1 (28.1% vs 35.9%, respectively; P = .27).
In patients seen at Center 1 only, those seen on the palliative RO service had a higher documentation rate of analgesic assessment compared with those seen by other services after implementation of the dedicated service (59.5% vs 38%, respectively; P = .018). Time period (after versus before 2011) was not significantly associated with the rate of documentation of analgesic assessment at either Center 1 (after vs before 2011: 44.4% vs 31%, P = .23) or Center 2 (31.4% vs 24.1%, P = .60).
Among patients with a PIR of ≥4, analgesic intervention was reported for 17.2% of patients within the entire cohort (20.8% at Center 1 and 0% at Center 2, P = .05). Among those with a PIR of ≥4, documentation of analgesic assessment noted in the History of the Present Illness section was associated with increased documentation of an analgesic intervention in the Assessment and Plan section (25% vs 7.3%; odds ratio [OR], 4.22; 95% confidence interval [CI], 1.1-16.0; P = .03).
Patients seen on the dedicated palliative RO service at Center 1 had a documented analgesic intervention rate of 31.6%, whereas the patients not seen on a palliative RO service (ie, those seen on a nonpalliative RO service at Center 1 plus all patients at Center 2) had a documented analgesic intervention rate of 9.2% (P = .01; Figure 2). There was no statistically significant difference between rates of documentation of an analgesic regimen intervention between patients seen at Center 2 and patients seen within nondedicated palliative RO services at Center 1 (0% vs 17.2%, respectively; P = .07).
Looking at only patients seen at Center 1, patients with a PIR of ≥4 seen on the dedicated palliative RO service had a nearly significant higher rate of documented analgesic interventions in the time period after implementation of the dedicate service (31.6% if seen on the dedicated service vs 12% if seen on a nondedicated service, P = .06).
Discussion
Multiple studies demonstrate the undertreatment of cancer pain in the outpatient setting.2,9,14,15 At 2 cancer centers, we found that about half of patients who present for consideration of palliative RT for bone metastases had a PIR of ≥4, yet only 17% of them had documentation of analgesic intervention as recommended by NCCN guidelines for cancer pain. Underlying this low rate of appropriate intervention may be the assumption of rapid pain relief by RT. However, RT often does not begin at time of consultation,16 and maximal pain relief may take days to weeks after commencement of RT.17 It is estimated that a quarter of all patients with cancer develop bone metastases during the course of their disease,12 and most of those patients suffer from pain. Thus, inherent delay in pain relief before, during, and after RT results in significant morbidity for the cancer patient population if adequate analgesic management is not provided.
The low rate of appropriate analgesic intervention at the time of RO consultation may also be related to the low incidence of proper analgesic assessment. In our cohort, 80% of symptomatic patients had an opioid or nonopioid analgesic listed in their medications within the electronic medical record at time of consultation, but only 38% had the analgesic regimen and/or its effectiveness described in the History of the Present Illness section of the record. Inattentiveness to analgesic type, dosing, and effectiveness during consultation may result in any inadequacies of the analgesic regimen going unnoticed. Consistent with this notion, we found that the rate of appropriate intervention for patients with a PIR of ≥4 was higher among patients who had analgesic regimen reported in the consultation note. Thus, interventions to implement routine review and documentation of the analgesic regimen, for example within the electronic medical record, may be one way to improve pain management.
Another possible reason for low rates of acute pain management within the RO clinic is low provider confidence in regard to analgesic management. In a recent national survey, 96% of radiation oncologists stated they were at least moderately confident with assessment of pain, yet only 77% were at least moderately confident with titrating opioids, and just 56% were at least moderately confident with rotating opioids.10 Educational interventions that improve providers’ facility with analgesic management may increase the frequency of pain management in the RO clinic.
Patients seen on the dedicated palliative RO service had significantly higher rates of documented analgesic regimen assessment and appropriate intervention during RO consultation, compared with patients seen at Center 2 and those not seen on the dedicated palliative RO service at Center 1. The improvements we observed in analgesic assessment and intervention at Center 1 for patients seen on the palliative RO service are likely owing to involvement of palliative RO and not to secular trends, because there were not similar improvements for patients at Center 1 who were not seen by the palliative RO service and those at Center 2, where there was no service.
At Center 1, the dedicated palliative RO service was created to provide specialized care to patients with metastatic disease undergoing palliative radiation. Within its structure, topics within palliative RO, such as technical aspects of palliative RT, symptom management, and communication are taught and reinforced in a case-based approach. Such palliative care awareness, integration, and education within RO achieved by the palliative RO service likely contribute to the improved rates of analgesic management we found in our study. We do note that rate of analgesic intervention in the palliative RO cohort, though higher than in the nonpalliative RO group, was still low, with only a third of patients receiving proper analgesic management. These findings highlight the importance of continued effort in increasing providers’ awareness of the need to assess pain and raise comfort with analgesic initiation and titration and of having dedicated palliative care clinicians embedded within the RO setting.
Since the data for this study was acquired, Center 2 has implemented a short palliative RO didactic course for residents, which improved their comfort levels in assessing analgesic effectiveness and intervening for uncontrolled pain.18 The impact of this intervention on clinical care will need to be evaluated, but the improved provider comfort levels may translate into better-quality care.
Limitations
An important limitation of this retrospective study is the reliance on the documentation provided in the consultation note for determining frequencies of analgesic regimen assessment and intervention. The actual rates of analgesic management that occurred in clinic may have been higher than reported in the documentation. However, such discrepancy in documentation of analgesic management would also be an area for quality improvement. Inadequate documentation limits the ability for proper follow-up of cancer pain as recommended by a joint guidance statement from the American Society of Clinical Oncology and the American Academy of Hospice and Palliative Medicine.19,20 The results of our study may also partly reflect a positive impact in documentation of analgesic management by a dedicated palliative RO service.
Given the multi-institutional nature of this study, it may be that general practice differences confound the impact of the dedicated palliative RO service at Center 1. However, with excluding Center 2, the dedicated service was still strongly associated with a higher rate of analgesic assessment within Center 1 and was almost significantly associated with appropriate analgesic intervention within Center 1.
We used a PIR of ≥4 as a threshold for appropriate analgesic regimen intervention because it is what is recommended by the NCCN guidelines. However, close attention should be paid to the impact that any amount of pain has on an individual patient. The functional, spiritual, and existential impact of pain is unique to each patient’s experience, and optimal symptom management should take those elements into account.
Conclusion
In conclusion, this study indicates that advanced cancer patient pain assessment and intervention according to NCCN cancer pain management guidelines is not common in the RO setting, and it is an area that should be targeted for quality improvement because of the positive implications for patient well-being. Pain assessment and intervention were greater in the setting of a dedicated structure for palliative care within RO, suggesting that the integration of palliative care within RO is a promising means of improving quality of pain management.
This work was presented at the 2016 ASCO Palliative Care in Oncology Symposium (September 9-10, 2016), where this work received a Conquer Cancer Foundation Merit Award.
Bone metastases are a common cause of pain in patients with advanced cancer, with about three-quarters of patients with bone metastases experiencing pain as the dominant symptom.1 Inadequately treated cancer pain impairs patient quality of life, and is associated with higher rates of depression, anxiety, and fatigue. Palliative radiotherapy (RT) is effective in alleviating pain from bone metastases.4 Local field external beam radiotherapy can provide some pain relief at the site of treated metastasis in 80%-90% of cases, with complete pain relief in 50%-60% of cases.5,6 However, maximal pain relief from RT is delayed, in some cases taking days to up to multiple weeks to attain.7,8 Therefore, optimal management of bone metastases pain may require the use of analgesics until RT takes adequate effect.
National Comprehensive Cancer Network (NCCN) Guidelines for Adult Cancer Pain (v. 2.2015) recommend that pain intensity rating (PIR; range, 0-10, where 0 denotes no pain and 10, worst pain imaginable) be used to quantify pain for all symptomatic patients. These guidelines also recommend the pain medication regimen be assessed for all symptomatic patients. For patients with moderate or severe pain (PIR of ≥4), NCCN guidelines recommend that analgesic regimen be intervened upon by alteration of the analgesic regimen (initiating, rotating, or titrating analgesic) or consideration of referral to pain/symptom management specialty.
Previous findings have demonstrated inadequate analgesic management for cancer pain,2,9 including within the radiation oncology (RO) clinic, suggesting that patients seen in consultation for palliative RT may experience uncontrolled pain for days to weeks before the onset of relief from RT. Possible reasons for inadequate acute pain intervention in the RO clinic may be provider discomfort with analgesic management and infrequent formal integration of palliative care within RO.10
Limited single-institution data from the few institutions with dedicated palliative RO services have suggested that these services improve the quality of palliative care delivery, as demonstrated by providers perceptions’ of the clinical impact of a dedicated service11 and the implementation of expedited palliative RT delivery for acute cancer pain.12,13 To our knowledge, the impact of a dedicated palliative RO service on analgesic management for cancer pain has not been assessed.
Here, we report how often patients with symptomatic bone metastases had assessments of existing analgesic regimens and interventions at RO consultation at 2 cancer centers. Center 1 had implemented a dedicated palliative RO service in 2011, consisting of rotating attending physicians and residents as well as dedicated palliative care trained nurse practitioners and a fellow, with the service structured around daily rounds,11 whereas Center 2 had not yet implemented a dedicated service. Using data from both centers, we assessed the impact of a palliative RO service on analgesic assessment and management in patients with bone metastases.
Methods
We searched our institutional databases for patients seen in RO consultation for bone metastases using ICD-9 code 198.5, and retrospectively reviewed consultation notes for those patients during June-July 2008, January-February 2010, January-February 2013, and June-July 2014. Those time periods were chosen as evenly spaced representative samples before and after implementation of a dedicated palliative RO service in 2011 at Center 1. Center 2 did not implement a dedicated palliative RO service in these time periods.
Within consultation notes, we recorded the following data from the History of the Present Illness section: symptoms from bone metastases (symptomatic was defined as any pain present); PIR (range, 0-10); and whether or not the preconsultation analgesic regimen was reported for symptomatic patients (including analgesic type, dosing, effectiveness, and adherence).
Documentation of the analgesic regimen in the history section of the notes was considered the proxy for analgesic regimen assessment at time of RO consultation. Analgesics within the Medications list, which were autopopulated in the consultation note by the electronic medical record, were recorded.
Whether or not pain was addressed with initiation or titration of analgesics for patients with a PIR of ≥4 was recorded from the Assessment and Plan portion of the notes, and that metric was considered the proxy for pain intervention. In addition, the case was coded as having had pain intervention if there was documentation of the patient declining recommended analgesic intervention, or the patient had been referred to a symptom management service for intervention (eg, referral to a specialty palliative care clinic), or there was recommendation for the patient to discuss uncontrolled pain with the original prescriber. A PIR of 4 was chosen as the threshold for analgesic intervention because at that level, NCCN guidelines for cancer pain state that the analgesic regimen should be titrated, whereas for a PIR of 3 or less, the guidelines recommend only consideration of titrating the analgesic. Only patients with a documented PIR were included in the pain intervention analysis.
Frequencies of analgesic assessment and analgesic intervention were compared using t tests (Wizard Pro, v1.8.5; Evan Miller, Chicago IL).
Results
A total of 271 patients with RO consultation notes were identified at the 2 centers within the 4 time periods (Table 1).
Among symptomatic patients, any component of the preconsultation analgesic regimen (including analgesic type, dosing, pain response, and adherence) was documented for 37.9% of the entire cohort at RO consultation (Table 3). At Centers 1 and 2, the frequencies of analgesic regimen assessment were documented for 41.3% and 28.1%, respectively (P = .06). Among symptomatic patients, 81.5% had an opioid or nonopioid analgesic listed in the Medications section in the electronic medical record at time of consultation.
Patients seen on the dedicated palliative RO service at Center 1 had an analgesic assessment documentation rate of 59.5%, whereas the patients not seen on a palliative RO service (ie, patients seen on a nonpalliative RO service at Center 1 plus all patients at Center 2) had an assessment documentation rate of 33.5% (P = .002; Figure 1). There was no significant difference between rates of analgesic regimen assessment between patients seen at Center 2 and patients seen within nondedicated palliative RO services at Center 1 (28.1% vs 35.9%, respectively; P = .27).
In patients seen at Center 1 only, those seen on the palliative RO service had a higher documentation rate of analgesic assessment compared with those seen by other services after implementation of the dedicated service (59.5% vs 38%, respectively; P = .018). Time period (after versus before 2011) was not significantly associated with the rate of documentation of analgesic assessment at either Center 1 (after vs before 2011: 44.4% vs 31%, P = .23) or Center 2 (31.4% vs 24.1%, P = .60).
Among patients with a PIR of ≥4, analgesic intervention was reported for 17.2% of patients within the entire cohort (20.8% at Center 1 and 0% at Center 2, P = .05). Among those with a PIR of ≥4, documentation of analgesic assessment noted in the History of the Present Illness section was associated with increased documentation of an analgesic intervention in the Assessment and Plan section (25% vs 7.3%; odds ratio [OR], 4.22; 95% confidence interval [CI], 1.1-16.0; P = .03).
Patients seen on the dedicated palliative RO service at Center 1 had a documented analgesic intervention rate of 31.6%, whereas the patients not seen on a palliative RO service (ie, those seen on a nonpalliative RO service at Center 1 plus all patients at Center 2) had a documented analgesic intervention rate of 9.2% (P = .01; Figure 2). There was no statistically significant difference between rates of documentation of an analgesic regimen intervention between patients seen at Center 2 and patients seen within nondedicated palliative RO services at Center 1 (0% vs 17.2%, respectively; P = .07).
Looking at only patients seen at Center 1, patients with a PIR of ≥4 seen on the dedicated palliative RO service had a nearly significant higher rate of documented analgesic interventions in the time period after implementation of the dedicate service (31.6% if seen on the dedicated service vs 12% if seen on a nondedicated service, P = .06).
Discussion
Multiple studies demonstrate the undertreatment of cancer pain in the outpatient setting.2,9,14,15 At 2 cancer centers, we found that about half of patients who present for consideration of palliative RT for bone metastases had a PIR of ≥4, yet only 17% of them had documentation of analgesic intervention as recommended by NCCN guidelines for cancer pain. Underlying this low rate of appropriate intervention may be the assumption of rapid pain relief by RT. However, RT often does not begin at time of consultation,16 and maximal pain relief may take days to weeks after commencement of RT.17 It is estimated that a quarter of all patients with cancer develop bone metastases during the course of their disease,12 and most of those patients suffer from pain. Thus, inherent delay in pain relief before, during, and after RT results in significant morbidity for the cancer patient population if adequate analgesic management is not provided.
The low rate of appropriate analgesic intervention at the time of RO consultation may also be related to the low incidence of proper analgesic assessment. In our cohort, 80% of symptomatic patients had an opioid or nonopioid analgesic listed in their medications within the electronic medical record at time of consultation, but only 38% had the analgesic regimen and/or its effectiveness described in the History of the Present Illness section of the record. Inattentiveness to analgesic type, dosing, and effectiveness during consultation may result in any inadequacies of the analgesic regimen going unnoticed. Consistent with this notion, we found that the rate of appropriate intervention for patients with a PIR of ≥4 was higher among patients who had analgesic regimen reported in the consultation note. Thus, interventions to implement routine review and documentation of the analgesic regimen, for example within the electronic medical record, may be one way to improve pain management.
Another possible reason for low rates of acute pain management within the RO clinic is low provider confidence in regard to analgesic management. In a recent national survey, 96% of radiation oncologists stated they were at least moderately confident with assessment of pain, yet only 77% were at least moderately confident with titrating opioids, and just 56% were at least moderately confident with rotating opioids.10 Educational interventions that improve providers’ facility with analgesic management may increase the frequency of pain management in the RO clinic.
Patients seen on the dedicated palliative RO service had significantly higher rates of documented analgesic regimen assessment and appropriate intervention during RO consultation, compared with patients seen at Center 2 and those not seen on the dedicated palliative RO service at Center 1. The improvements we observed in analgesic assessment and intervention at Center 1 for patients seen on the palliative RO service are likely owing to involvement of palliative RO and not to secular trends, because there were not similar improvements for patients at Center 1 who were not seen by the palliative RO service and those at Center 2, where there was no service.
At Center 1, the dedicated palliative RO service was created to provide specialized care to patients with metastatic disease undergoing palliative radiation. Within its structure, topics within palliative RO, such as technical aspects of palliative RT, symptom management, and communication are taught and reinforced in a case-based approach. Such palliative care awareness, integration, and education within RO achieved by the palliative RO service likely contribute to the improved rates of analgesic management we found in our study. We do note that rate of analgesic intervention in the palliative RO cohort, though higher than in the nonpalliative RO group, was still low, with only a third of patients receiving proper analgesic management. These findings highlight the importance of continued effort in increasing providers’ awareness of the need to assess pain and raise comfort with analgesic initiation and titration and of having dedicated palliative care clinicians embedded within the RO setting.
Since the data for this study was acquired, Center 2 has implemented a short palliative RO didactic course for residents, which improved their comfort levels in assessing analgesic effectiveness and intervening for uncontrolled pain.18 The impact of this intervention on clinical care will need to be evaluated, but the improved provider comfort levels may translate into better-quality care.
Limitations
An important limitation of this retrospective study is the reliance on the documentation provided in the consultation note for determining frequencies of analgesic regimen assessment and intervention. The actual rates of analgesic management that occurred in clinic may have been higher than reported in the documentation. However, such discrepancy in documentation of analgesic management would also be an area for quality improvement. Inadequate documentation limits the ability for proper follow-up of cancer pain as recommended by a joint guidance statement from the American Society of Clinical Oncology and the American Academy of Hospice and Palliative Medicine.19,20 The results of our study may also partly reflect a positive impact in documentation of analgesic management by a dedicated palliative RO service.
Given the multi-institutional nature of this study, it may be that general practice differences confound the impact of the dedicated palliative RO service at Center 1. However, with excluding Center 2, the dedicated service was still strongly associated with a higher rate of analgesic assessment within Center 1 and was almost significantly associated with appropriate analgesic intervention within Center 1.
We used a PIR of ≥4 as a threshold for appropriate analgesic regimen intervention because it is what is recommended by the NCCN guidelines. However, close attention should be paid to the impact that any amount of pain has on an individual patient. The functional, spiritual, and existential impact of pain is unique to each patient’s experience, and optimal symptom management should take those elements into account.
Conclusion
In conclusion, this study indicates that advanced cancer patient pain assessment and intervention according to NCCN cancer pain management guidelines is not common in the RO setting, and it is an area that should be targeted for quality improvement because of the positive implications for patient well-being. Pain assessment and intervention were greater in the setting of a dedicated structure for palliative care within RO, suggesting that the integration of palliative care within RO is a promising means of improving quality of pain management.
This work was presented at the 2016 ASCO Palliative Care in Oncology Symposium (September 9-10, 2016), where this work received a Conquer Cancer Foundation Merit Award.
1. Amichetti M, Orrù P, Madeddu A, et al. Comparative evaluation of two hypofractionated radiotherapy regimens for painful bone metastases. Tumori. 2004;90(1):91-95.
2. Vuong S, Pulenzas N, DeAngelis C, et al. Inadequate pain management in cancer patients attending an outpatient palliative radiotherapy clinic. Support Care Cancer. 2016;24(2):887-892.
3. Portenoy RK, Payne D, Jacobsen P. Breakthrough pain: characteristics and impact in patients with cancer pain. Pain. 1999;81(1-2):129-134.
4. Sze WM, Shelley M, Held I, Mason M. Palliation of metastatic bone pain: single fraction versus multifraction radiotherapy - a systematic review of the randomised trials. Sze WM, ed. Cochrane Database Syst Rev. 2004;(2):CD004721-CD004721.
5. Ratanatharathorn V, Powers WE, Moss WT, Perez CA. Bone metastasis: review and critical analysis of random allocation trials of local field treatment. Int J Radiat Oncol Biol Phys. 1999;44(1):1-18.
6. Kirou-Mauro A, Hird A, Wong J, et al. Is response to radiotherapy in patients related to the severity of pretreatment pain? Int J Radiat Oncol Biol Phys. 2008;71(4):1208-1212.
7. Frassica DA. General principles of external beam radiation therapy for skeletal metastases. Clin Orthop Relat Res. 2003;(415 Suppl):S158-S164.
8. McDonald R, Ding K, Brundage M, et al. Effect of radiotherapy on painful bone metastases: a secondary analysis of the NCIC Clinical Trials Group Symptom Control Trial SC.23. JAMA Oncol. 2017 Jul 1;3(7):953-959.
9. Greco MT, Roberto A, Corli O, et al. Quality of cancer pain management: an update of a systematic review of undertreatment of patients with cancer. J Clin Oncol. 2014;32(36):4149-4154.
10. Wei RL, Mattes MD, Yu J, et al. Attitudes of radiation oncologists toward palliative and supportive care in the united states: report on national membership survey by the American Society for Radiation Oncology (ASTRO). Pract Radiat Oncol. 2017;7(2):113-119.
11. Tseng YD, Krishnan MS, Jones JA, et al. Supportive and palliative radiation oncology service: impact of a dedicated service on palliative cancer care. Pract Radiat Oncol. 2014;4(4):247-253.
12. Fairchild A, Pituskin E, Rose B, et al. The rapid access palliative radiotherapy program: blueprint for initiation of a one-stop multidisciplinary bone metastases clinic. Support Care Cancer. 2009;17(2):163-170.
13. de Sa E, Sinclair E, Mitera G, et al. Continued success of the rapid response radiotherapy program: a review of 2004-2008. Support Care Cancer. 2009;17(7):757-762.
14. Deandrea S, Montanari M, Moja L, Apolone G. Prevalence of undertreatment in cancer pain. A review of published literature. Ann Oncol. 2008;19(12):1985-1991.
15. Mitera G, Zeiadin N, Kirou-Mauro A, et al. Retrospective assessment of cancer pain management in an outpatient palliative radiotherapy clinic using the Pain Management Index. J Pain Symptom Manage. 2010;39(2):259-267.
16. Danjoux C, Chow E, Drossos A, et al. An innovative rapid response radiotherapy program to reduce waiting time for palliative radiotherapy. Support Care Cancer. 2006;14(1):38-43.
17. Feyer PC, Steingraeber M. Radiotherapy of bone metastasis in breast cancer patients – current approaches. Breast Care (Basel). 2012;7(2):108-112.
18. Garcia MA, Braunstein SE, Anderson WG. Palliative Care Didactic Course for Radiation Oncology Residents. Int J Radiat Oncol Biol Phys. 2017;97(5):884-885.
19. Ferrell BR, Temel JS, Temin S, et al. Integration of palliative care into standard oncology care: American Society of Clinical Oncology clinical practice guideline update. J Clin Oncol. 2017;35(1):96-112.
20. Bickel KE, McNiff K, Buss MK, et al. Defining high-quality palliative care in oncology practice: an American Society of Clinical Oncology/American Academy of Hospice and Palliative Medicine guidance statement. J Oncol Pract. 2016;12(9):e828-e838.
1. Amichetti M, Orrù P, Madeddu A, et al. Comparative evaluation of two hypofractionated radiotherapy regimens for painful bone metastases. Tumori. 2004;90(1):91-95.
2. Vuong S, Pulenzas N, DeAngelis C, et al. Inadequate pain management in cancer patients attending an outpatient palliative radiotherapy clinic. Support Care Cancer. 2016;24(2):887-892.
3. Portenoy RK, Payne D, Jacobsen P. Breakthrough pain: characteristics and impact in patients with cancer pain. Pain. 1999;81(1-2):129-134.
4. Sze WM, Shelley M, Held I, Mason M. Palliation of metastatic bone pain: single fraction versus multifraction radiotherapy - a systematic review of the randomised trials. Sze WM, ed. Cochrane Database Syst Rev. 2004;(2):CD004721-CD004721.
5. Ratanatharathorn V, Powers WE, Moss WT, Perez CA. Bone metastasis: review and critical analysis of random allocation trials of local field treatment. Int J Radiat Oncol Biol Phys. 1999;44(1):1-18.
6. Kirou-Mauro A, Hird A, Wong J, et al. Is response to radiotherapy in patients related to the severity of pretreatment pain? Int J Radiat Oncol Biol Phys. 2008;71(4):1208-1212.
7. Frassica DA. General principles of external beam radiation therapy for skeletal metastases. Clin Orthop Relat Res. 2003;(415 Suppl):S158-S164.
8. McDonald R, Ding K, Brundage M, et al. Effect of radiotherapy on painful bone metastases: a secondary analysis of the NCIC Clinical Trials Group Symptom Control Trial SC.23. JAMA Oncol. 2017 Jul 1;3(7):953-959.
9. Greco MT, Roberto A, Corli O, et al. Quality of cancer pain management: an update of a systematic review of undertreatment of patients with cancer. J Clin Oncol. 2014;32(36):4149-4154.
10. Wei RL, Mattes MD, Yu J, et al. Attitudes of radiation oncologists toward palliative and supportive care in the united states: report on national membership survey by the American Society for Radiation Oncology (ASTRO). Pract Radiat Oncol. 2017;7(2):113-119.
11. Tseng YD, Krishnan MS, Jones JA, et al. Supportive and palliative radiation oncology service: impact of a dedicated service on palliative cancer care. Pract Radiat Oncol. 2014;4(4):247-253.
12. Fairchild A, Pituskin E, Rose B, et al. The rapid access palliative radiotherapy program: blueprint for initiation of a one-stop multidisciplinary bone metastases clinic. Support Care Cancer. 2009;17(2):163-170.
13. de Sa E, Sinclair E, Mitera G, et al. Continued success of the rapid response radiotherapy program: a review of 2004-2008. Support Care Cancer. 2009;17(7):757-762.
14. Deandrea S, Montanari M, Moja L, Apolone G. Prevalence of undertreatment in cancer pain. A review of published literature. Ann Oncol. 2008;19(12):1985-1991.
15. Mitera G, Zeiadin N, Kirou-Mauro A, et al. Retrospective assessment of cancer pain management in an outpatient palliative radiotherapy clinic using the Pain Management Index. J Pain Symptom Manage. 2010;39(2):259-267.
16. Danjoux C, Chow E, Drossos A, et al. An innovative rapid response radiotherapy program to reduce waiting time for palliative radiotherapy. Support Care Cancer. 2006;14(1):38-43.
17. Feyer PC, Steingraeber M. Radiotherapy of bone metastasis in breast cancer patients – current approaches. Breast Care (Basel). 2012;7(2):108-112.
18. Garcia MA, Braunstein SE, Anderson WG. Palliative Care Didactic Course for Radiation Oncology Residents. Int J Radiat Oncol Biol Phys. 2017;97(5):884-885.
19. Ferrell BR, Temel JS, Temin S, et al. Integration of palliative care into standard oncology care: American Society of Clinical Oncology clinical practice guideline update. J Clin Oncol. 2017;35(1):96-112.
20. Bickel KE, McNiff K, Buss MK, et al. Defining high-quality palliative care in oncology practice: an American Society of Clinical Oncology/American Academy of Hospice and Palliative Medicine guidance statement. J Oncol Pract. 2016;12(9):e828-e838.
Outpatient talc administration improves malignant effusion outcomes
Patients with malignant pleural effusion treated with an indwelling pleural catheter have an improved chance of a positive outcome when talc administration is part of their procedure, suggest the results of a randomized, placebo-controlled study.
Malignant pleural effusion, which is usually caused by the spread of metastatic cancer, is typically treated by inducement of pleurodesis. Talc is probably the most effective agent for achieving this result, but there are drawbacks to using talc to induce pleurodesis. Patients who receive this treatment often need to stay in the hospital for 4-7 days, according to Rahul Bhatnagar, PhD, and the coauthors of a study published in the New England Journal of Medicine). Indwelling pleural catheters provide an “ambulatory alternative” for fluid management, they noted. In a noncomparative series of 22 patients, administering talc through such a catheter produced high rates of pleurodesis, they added.
In the new study, Dr. Bhatnagar of the Academic Respiratory Unit, University of Bristol, England, and his coauthors evaluated the use of an indwelling catheter, with or without talc, in patients with malignant pleural effusion recruited at 18 centers in the United Kingdom over 4 years.
“Our primary-outcome results, which were backed up by robust sensitivity analyses, strongly suggest that the administration of talc through an indwelling pleural catheter was significantly more efficacious than the use of an indwelling pleural catheter alone among patients without substantial lung entrapment,” the authors wrote.
A total of 154 patients underwent randomization to the talc or placebo group, and 139 had sufficient data to evaluate the primary outcome of successful pleurodesis at 35 days after randomization. The researchers excluded patients with evidence of lung entrapment, or nonexpandable lung, according to the study report.
In the talc group, pleurodesis was successful at day 35 in 30 of 69 patients (43%) versus 16 of 70 patients (23%) in the placebo group (P = .008).
At day 70, the success rate was 51% for the talc group vs. 27% for the placebo group, respectively.
The rate of pleurodesis was significantly higher when talc was administered through an indwelling pleural catheter, Dr. Bhatnagar and his colleagues noted.
“Success rates at day 70 suggested that pleurodesis was maintained to a point that is clinically relevant for patients with short median survival,” they added.
No excess of side effects or catheter blockages were associated with talc vs. placebo administration through a catheter. Additionally, no differences were seen between the talc and placebo groups in the number of adverse events, number of inpatient days, mortality, or other outcomes tracked by the researchers.
Dr. Bhatnagar reported he had no disclosures related to the study. Study coauthors reported disclosures related to Becton Dickinson – CareFusion, Rosetrees Trust, GE Medical, and Rocket Medical. Becton Dickinson supported the trial with an unrestricted research grant and supplied catheters and drainage bottles for the study’s participants.
SOURCE: Bhatnagar R et al. N Engl J Med. 2018;378:1313-22.
Patients with malignant pleural effusion treated with an indwelling pleural catheter have an improved chance of a positive outcome when talc administration is part of their procedure, suggest the results of a randomized, placebo-controlled study.
Malignant pleural effusion, which is usually caused by the spread of metastatic cancer, is typically treated by inducement of pleurodesis. Talc is probably the most effective agent for achieving this result, but there are drawbacks to using talc to induce pleurodesis. Patients who receive this treatment often need to stay in the hospital for 4-7 days, according to Rahul Bhatnagar, PhD, and the coauthors of a study published in the New England Journal of Medicine). Indwelling pleural catheters provide an “ambulatory alternative” for fluid management, they noted. In a noncomparative series of 22 patients, administering talc through such a catheter produced high rates of pleurodesis, they added.
In the new study, Dr. Bhatnagar of the Academic Respiratory Unit, University of Bristol, England, and his coauthors evaluated the use of an indwelling catheter, with or without talc, in patients with malignant pleural effusion recruited at 18 centers in the United Kingdom over 4 years.
“Our primary-outcome results, which were backed up by robust sensitivity analyses, strongly suggest that the administration of talc through an indwelling pleural catheter was significantly more efficacious than the use of an indwelling pleural catheter alone among patients without substantial lung entrapment,” the authors wrote.
A total of 154 patients underwent randomization to the talc or placebo group, and 139 had sufficient data to evaluate the primary outcome of successful pleurodesis at 35 days after randomization. The researchers excluded patients with evidence of lung entrapment, or nonexpandable lung, according to the study report.
In the talc group, pleurodesis was successful at day 35 in 30 of 69 patients (43%) versus 16 of 70 patients (23%) in the placebo group (P = .008).
At day 70, the success rate was 51% for the talc group vs. 27% for the placebo group, respectively.
The rate of pleurodesis was significantly higher when talc was administered through an indwelling pleural catheter, Dr. Bhatnagar and his colleagues noted.
“Success rates at day 70 suggested that pleurodesis was maintained to a point that is clinically relevant for patients with short median survival,” they added.
No excess of side effects or catheter blockages were associated with talc vs. placebo administration through a catheter. Additionally, no differences were seen between the talc and placebo groups in the number of adverse events, number of inpatient days, mortality, or other outcomes tracked by the researchers.
Dr. Bhatnagar reported he had no disclosures related to the study. Study coauthors reported disclosures related to Becton Dickinson – CareFusion, Rosetrees Trust, GE Medical, and Rocket Medical. Becton Dickinson supported the trial with an unrestricted research grant and supplied catheters and drainage bottles for the study’s participants.
SOURCE: Bhatnagar R et al. N Engl J Med. 2018;378:1313-22.
Patients with malignant pleural effusion treated with an indwelling pleural catheter have an improved chance of a positive outcome when talc administration is part of their procedure, suggest the results of a randomized, placebo-controlled study.
Malignant pleural effusion, which is usually caused by the spread of metastatic cancer, is typically treated by inducement of pleurodesis. Talc is probably the most effective agent for achieving this result, but there are drawbacks to using talc to induce pleurodesis. Patients who receive this treatment often need to stay in the hospital for 4-7 days, according to Rahul Bhatnagar, PhD, and the coauthors of a study published in the New England Journal of Medicine). Indwelling pleural catheters provide an “ambulatory alternative” for fluid management, they noted. In a noncomparative series of 22 patients, administering talc through such a catheter produced high rates of pleurodesis, they added.
In the new study, Dr. Bhatnagar of the Academic Respiratory Unit, University of Bristol, England, and his coauthors evaluated the use of an indwelling catheter, with or without talc, in patients with malignant pleural effusion recruited at 18 centers in the United Kingdom over 4 years.
“Our primary-outcome results, which were backed up by robust sensitivity analyses, strongly suggest that the administration of talc through an indwelling pleural catheter was significantly more efficacious than the use of an indwelling pleural catheter alone among patients without substantial lung entrapment,” the authors wrote.
A total of 154 patients underwent randomization to the talc or placebo group, and 139 had sufficient data to evaluate the primary outcome of successful pleurodesis at 35 days after randomization. The researchers excluded patients with evidence of lung entrapment, or nonexpandable lung, according to the study report.
In the talc group, pleurodesis was successful at day 35 in 30 of 69 patients (43%) versus 16 of 70 patients (23%) in the placebo group (P = .008).
At day 70, the success rate was 51% for the talc group vs. 27% for the placebo group, respectively.
The rate of pleurodesis was significantly higher when talc was administered through an indwelling pleural catheter, Dr. Bhatnagar and his colleagues noted.
“Success rates at day 70 suggested that pleurodesis was maintained to a point that is clinically relevant for patients with short median survival,” they added.
No excess of side effects or catheter blockages were associated with talc vs. placebo administration through a catheter. Additionally, no differences were seen between the talc and placebo groups in the number of adverse events, number of inpatient days, mortality, or other outcomes tracked by the researchers.
Dr. Bhatnagar reported he had no disclosures related to the study. Study coauthors reported disclosures related to Becton Dickinson – CareFusion, Rosetrees Trust, GE Medical, and Rocket Medical. Becton Dickinson supported the trial with an unrestricted research grant and supplied catheters and drainage bottles for the study’s participants.
SOURCE: Bhatnagar R et al. N Engl J Med. 2018;378:1313-22.
FROM NEW ENGLAND JOURNAL OF MEDICINE
Key clinical point: with no deleterious effects.
Major finding: At 35 days post randomization, pleurodesis was successful in 30 of 69 patients (43%) in the talc group versus 16 of 70 patients (23%) in the placebo group (P = .008).
Study details: A randomized, placebo-controlled, single-blind, parallel-group trial including 154 patients with malignant pleural effusion recruited at 18 U.K. centers over a period of 4 years.
Disclosures: Becton Dickinson supported the trial with an unrestricted research grant and supplied catheters and drainage bottles for participants. Study authors reported disclosures related to Becton Dickinson – CareFusion, Rosetrees Trust, GE Medical, and Rocket Medical.
Source: Bhatnagar R et al. N Engl J Med. 2018;378:1313-22.
Sarcopenia, body fat linked with mortality in nonmetastatic breast cancer
Among women with nonmetastatic breast cancer, low muscle mass and excess body fat are significantly associated with worse survival, investigators report.
An observational study of 3,241 women diagnosed with stage II or III breast cancer showed that low muscle mass (sarcopenia) was independently associated with a 41% increase in risk for overall mortality, and that total adipose tissue (TAT) measures were associated with a 35% increase in overall mortality.
Women with sarcopenia and high total TAT scores had a nearly twofold higher risk for death, reported Bette J. Caan, DrPH, of Kaiser Permanente in Oakland, Calif., and her colleagues.
Although low muscle mass was found to be a significant risk factor for death, neither poor muscle quality, measured by radiodensity, nor body mass index (BMI) were significantly associated with overall mortality, the investigators reported in a study published online in JAMA Oncology.
“Both muscle and adiposity represent modifiable risk factors in patients with breast cancer. In addition to weight loss, we should also consider interventions to improve muscle mass, such as resistance training or protein supplementation. In the era of precision medicine, the direct measurement of muscle and adiposity will help to guide treatment plans and interventions to optimize survival outcomes,” they wrote.
Although moderate to severe obesity measured by high BMI has been associated with worse outcomes for patients with breast cancer and other malignancies, the evidence is mixed for those who are merely overweight or have borderline obesity, the authors noted.
BMI is a simple ratio of height to weight, and does not measure body composition, and “low BMI can mask excess adiposity while high BMI can mask low muscularity,” they wrote.
To determine whether associations between measures of body composition could be prognostic for overall mortality, the investigators conducted a retrospective cohort study with patients from Kaiser Permanente Northern California and the Dana-Farber Cancer Institute in Boston.
The cohort included 3,241 women diagnosed with stage II or III invasive breast cancer during 2005-2013 in California and during 2000-2012 in Boston. All of the patients included had either abdominal or pelvic CT scans or PET-CT scans at the time of diagnosis.
The investigators looked at the associations between sarcopenia, TAT, and low muscle radiodensity, and created hazard ratio (HR) estimates of the effects of the various interactions on overall mortality, adjusted for sociodemographics, tumor characteristics, treatment, BMI, and other body composition measures.
They found that after a median follow-up of 6 years, patients with sarcopenia had a significantly greater risk for overall mortality than did patients without sarcopenia (HR, 1.41; 95% confidence interval, 1.18-1.69).
Additionally, patients in the highest tertile of TAT also had significantly higher overall mortality, compared with patients in the lowest tertile (HR, 1.35; CI, 1.08-1.69).
As noted before, poor muscle quality was not significantly associated with overall mortality.
Looking at both sarcopenia and TAT, the authors found that the highest risk for death was in those patients with both sarcopenia and high TAT (HR, 1.88; CI, 1.30-2.73).
However, they also found that BMI was not an independent predictor of overall mortality, and did identify those patients who were at risk because of their body composition.
“We demonstrate that sarcopenia is not a condition restricted to patients with later-stage disease but rather is highly prevalent among patients with nonmetastatic disease across all levels of BMI. Our findings are likely generalizable across many other nonmetastatic cancers because the associations with muscle and improved survival for those with metastatic cancer has been observed across a variety of solid tumors,” Dr. Caan and her associates wrote in their conclusion.
The article did not report a funding source for the study. The investigators reported having no conflicts of interest to disclose.
SOURCE: Cann BJ et al. JAMA Oncol. 2018 Apr 5. doi: 10.1001/jamaoncol.2018.0137.
Obesity is highly prevalent among breast cancer survivors, and in addition to its effects on cancer development and outcomes, it also can affect treatment efficacy and adverse effects and complicate clinical management of breast cancer from obesity-related comorbidities such as hypertension and diabetes. As such, the American Society of Clinical Oncology made obesity and cancer one of their core priorities in 2013 and launched the Obesity & Cancer Initiative with activities ranging from education and awareness to clinical guidance, promotion of research, and policy and advocacy.
Despite its limitations, body mass index remains an easy tool to help health care clinicians identify patients at greater risk for poor outcomes and adverse effects and guide their recommendations, as well as to educate patients in self-assessing their weight status. Weight management and control are likely to have many benefits for breast cancer survivors but should always be tailored to individual patients’ needs. When CT imaging is available, the study by Caan et al. suggests that body composition measures can be useful in identifying women at higher risk of mortality. Their findings are an important reminder that weight loss and/or weight control programs must always incorporate physical activity with the goal of not just reducing adiposity, but also maintaining and increasing muscle mass, which would not only reduce the risk of death, but might also help improve quality of life after a cancer diagnosis.
Elisa V. Bandera, MD, PhD, is with the Rutgers Cancer Institute of New Jersey, Rutgers University, New Brunswick. Esther M. John, PhD, is with Stanford (Calif.) University. Both editorialists reported having no conflicts of interest to disclose. Their remarks are adapted from an accompanying invited commentary (JAMA Oncol. doi: 10.1001/jamaoncol.2018.0137).
Obesity is highly prevalent among breast cancer survivors, and in addition to its effects on cancer development and outcomes, it also can affect treatment efficacy and adverse effects and complicate clinical management of breast cancer from obesity-related comorbidities such as hypertension and diabetes. As such, the American Society of Clinical Oncology made obesity and cancer one of their core priorities in 2013 and launched the Obesity & Cancer Initiative with activities ranging from education and awareness to clinical guidance, promotion of research, and policy and advocacy.
Despite its limitations, body mass index remains an easy tool to help health care clinicians identify patients at greater risk for poor outcomes and adverse effects and guide their recommendations, as well as to educate patients in self-assessing their weight status. Weight management and control are likely to have many benefits for breast cancer survivors but should always be tailored to individual patients’ needs. When CT imaging is available, the study by Caan et al. suggests that body composition measures can be useful in identifying women at higher risk of mortality. Their findings are an important reminder that weight loss and/or weight control programs must always incorporate physical activity with the goal of not just reducing adiposity, but also maintaining and increasing muscle mass, which would not only reduce the risk of death, but might also help improve quality of life after a cancer diagnosis.
Elisa V. Bandera, MD, PhD, is with the Rutgers Cancer Institute of New Jersey, Rutgers University, New Brunswick. Esther M. John, PhD, is with Stanford (Calif.) University. Both editorialists reported having no conflicts of interest to disclose. Their remarks are adapted from an accompanying invited commentary (JAMA Oncol. doi: 10.1001/jamaoncol.2018.0137).
Obesity is highly prevalent among breast cancer survivors, and in addition to its effects on cancer development and outcomes, it also can affect treatment efficacy and adverse effects and complicate clinical management of breast cancer from obesity-related comorbidities such as hypertension and diabetes. As such, the American Society of Clinical Oncology made obesity and cancer one of their core priorities in 2013 and launched the Obesity & Cancer Initiative with activities ranging from education and awareness to clinical guidance, promotion of research, and policy and advocacy.
Despite its limitations, body mass index remains an easy tool to help health care clinicians identify patients at greater risk for poor outcomes and adverse effects and guide their recommendations, as well as to educate patients in self-assessing their weight status. Weight management and control are likely to have many benefits for breast cancer survivors but should always be tailored to individual patients’ needs. When CT imaging is available, the study by Caan et al. suggests that body composition measures can be useful in identifying women at higher risk of mortality. Their findings are an important reminder that weight loss and/or weight control programs must always incorporate physical activity with the goal of not just reducing adiposity, but also maintaining and increasing muscle mass, which would not only reduce the risk of death, but might also help improve quality of life after a cancer diagnosis.
Elisa V. Bandera, MD, PhD, is with the Rutgers Cancer Institute of New Jersey, Rutgers University, New Brunswick. Esther M. John, PhD, is with Stanford (Calif.) University. Both editorialists reported having no conflicts of interest to disclose. Their remarks are adapted from an accompanying invited commentary (JAMA Oncol. doi: 10.1001/jamaoncol.2018.0137).
Among women with nonmetastatic breast cancer, low muscle mass and excess body fat are significantly associated with worse survival, investigators report.
An observational study of 3,241 women diagnosed with stage II or III breast cancer showed that low muscle mass (sarcopenia) was independently associated with a 41% increase in risk for overall mortality, and that total adipose tissue (TAT) measures were associated with a 35% increase in overall mortality.
Women with sarcopenia and high total TAT scores had a nearly twofold higher risk for death, reported Bette J. Caan, DrPH, of Kaiser Permanente in Oakland, Calif., and her colleagues.
Although low muscle mass was found to be a significant risk factor for death, neither poor muscle quality, measured by radiodensity, nor body mass index (BMI) were significantly associated with overall mortality, the investigators reported in a study published online in JAMA Oncology.
“Both muscle and adiposity represent modifiable risk factors in patients with breast cancer. In addition to weight loss, we should also consider interventions to improve muscle mass, such as resistance training or protein supplementation. In the era of precision medicine, the direct measurement of muscle and adiposity will help to guide treatment plans and interventions to optimize survival outcomes,” they wrote.
Although moderate to severe obesity measured by high BMI has been associated with worse outcomes for patients with breast cancer and other malignancies, the evidence is mixed for those who are merely overweight or have borderline obesity, the authors noted.
BMI is a simple ratio of height to weight, and does not measure body composition, and “low BMI can mask excess adiposity while high BMI can mask low muscularity,” they wrote.
To determine whether associations between measures of body composition could be prognostic for overall mortality, the investigators conducted a retrospective cohort study with patients from Kaiser Permanente Northern California and the Dana-Farber Cancer Institute in Boston.
The cohort included 3,241 women diagnosed with stage II or III invasive breast cancer during 2005-2013 in California and during 2000-2012 in Boston. All of the patients included had either abdominal or pelvic CT scans or PET-CT scans at the time of diagnosis.
The investigators looked at the associations between sarcopenia, TAT, and low muscle radiodensity, and created hazard ratio (HR) estimates of the effects of the various interactions on overall mortality, adjusted for sociodemographics, tumor characteristics, treatment, BMI, and other body composition measures.
They found that after a median follow-up of 6 years, patients with sarcopenia had a significantly greater risk for overall mortality than did patients without sarcopenia (HR, 1.41; 95% confidence interval, 1.18-1.69).
Additionally, patients in the highest tertile of TAT also had significantly higher overall mortality, compared with patients in the lowest tertile (HR, 1.35; CI, 1.08-1.69).
As noted before, poor muscle quality was not significantly associated with overall mortality.
Looking at both sarcopenia and TAT, the authors found that the highest risk for death was in those patients with both sarcopenia and high TAT (HR, 1.88; CI, 1.30-2.73).
However, they also found that BMI was not an independent predictor of overall mortality, and did identify those patients who were at risk because of their body composition.
“We demonstrate that sarcopenia is not a condition restricted to patients with later-stage disease but rather is highly prevalent among patients with nonmetastatic disease across all levels of BMI. Our findings are likely generalizable across many other nonmetastatic cancers because the associations with muscle and improved survival for those with metastatic cancer has been observed across a variety of solid tumors,” Dr. Caan and her associates wrote in their conclusion.
The article did not report a funding source for the study. The investigators reported having no conflicts of interest to disclose.
SOURCE: Cann BJ et al. JAMA Oncol. 2018 Apr 5. doi: 10.1001/jamaoncol.2018.0137.
Among women with nonmetastatic breast cancer, low muscle mass and excess body fat are significantly associated with worse survival, investigators report.
An observational study of 3,241 women diagnosed with stage II or III breast cancer showed that low muscle mass (sarcopenia) was independently associated with a 41% increase in risk for overall mortality, and that total adipose tissue (TAT) measures were associated with a 35% increase in overall mortality.
Women with sarcopenia and high total TAT scores had a nearly twofold higher risk for death, reported Bette J. Caan, DrPH, of Kaiser Permanente in Oakland, Calif., and her colleagues.
Although low muscle mass was found to be a significant risk factor for death, neither poor muscle quality, measured by radiodensity, nor body mass index (BMI) were significantly associated with overall mortality, the investigators reported in a study published online in JAMA Oncology.
“Both muscle and adiposity represent modifiable risk factors in patients with breast cancer. In addition to weight loss, we should also consider interventions to improve muscle mass, such as resistance training or protein supplementation. In the era of precision medicine, the direct measurement of muscle and adiposity will help to guide treatment plans and interventions to optimize survival outcomes,” they wrote.
Although moderate to severe obesity measured by high BMI has been associated with worse outcomes for patients with breast cancer and other malignancies, the evidence is mixed for those who are merely overweight or have borderline obesity, the authors noted.
BMI is a simple ratio of height to weight, and does not measure body composition, and “low BMI can mask excess adiposity while high BMI can mask low muscularity,” they wrote.
To determine whether associations between measures of body composition could be prognostic for overall mortality, the investigators conducted a retrospective cohort study with patients from Kaiser Permanente Northern California and the Dana-Farber Cancer Institute in Boston.
The cohort included 3,241 women diagnosed with stage II or III invasive breast cancer during 2005-2013 in California and during 2000-2012 in Boston. All of the patients included had either abdominal or pelvic CT scans or PET-CT scans at the time of diagnosis.
The investigators looked at the associations between sarcopenia, TAT, and low muscle radiodensity, and created hazard ratio (HR) estimates of the effects of the various interactions on overall mortality, adjusted for sociodemographics, tumor characteristics, treatment, BMI, and other body composition measures.
They found that after a median follow-up of 6 years, patients with sarcopenia had a significantly greater risk for overall mortality than did patients without sarcopenia (HR, 1.41; 95% confidence interval, 1.18-1.69).
Additionally, patients in the highest tertile of TAT also had significantly higher overall mortality, compared with patients in the lowest tertile (HR, 1.35; CI, 1.08-1.69).
As noted before, poor muscle quality was not significantly associated with overall mortality.
Looking at both sarcopenia and TAT, the authors found that the highest risk for death was in those patients with both sarcopenia and high TAT (HR, 1.88; CI, 1.30-2.73).
However, they also found that BMI was not an independent predictor of overall mortality, and did identify those patients who were at risk because of their body composition.
“We demonstrate that sarcopenia is not a condition restricted to patients with later-stage disease but rather is highly prevalent among patients with nonmetastatic disease across all levels of BMI. Our findings are likely generalizable across many other nonmetastatic cancers because the associations with muscle and improved survival for those with metastatic cancer has been observed across a variety of solid tumors,” Dr. Caan and her associates wrote in their conclusion.
The article did not report a funding source for the study. The investigators reported having no conflicts of interest to disclose.
SOURCE: Cann BJ et al. JAMA Oncol. 2018 Apr 5. doi: 10.1001/jamaoncol.2018.0137.
FROM JAMA ONCOLOGY
Key clinical point: Helping women with nonmetastatic breast cancer control weight and improve muscle strength could lower their risk of death.
Major finding: Women with sarcopenia and high total adipose tissue had a hazard ratio of 1.89 for overall mortality.
Study details: Retrospective cohort study of 3,241 women diagnosed with stage II or III invasive breast cancer in California and Massachusetts.
Disclosures: The article did not report a funding source for the study. The investigators reported having no conflicts of interest to disclose.
Source: Cann BJ et al. JAMA Oncol. 2018 Apr 5. doi:10.1001/jamaoncol.2018.0137.
21-gene assay predicts survival in male and female breast cancer
A study of the molecular and genomic features of breast cancer in men, compared with those in women, highlights the prognostic value of a 21-gene breast recurrence score in both sexes, investigators say.
Men and women with estrogen receptor (ER)–positive breast cancer who had recurrence scores (RS) of 0 to 30 on the 21-gene assay (Oncotype DX) had excellent breast cancer–specific survival rates, which suggests that such patients could be spared from more aggressive treatments, such as chemotherapy, according to Suleiman Alfred Massarweh, MD, of Stanford (Calif.) University and his colleagues.
“Future adjuvant trials in ER-positive breast cancer may need to focus on targeting endocrine resistance in those patients with RS greater than 31 and may need to consider the weight of competing mortality risk when investigating the value of any additional treatment beyond endocrine therapy,” they wrote in the Journal of Clinical Oncology.
In 2016, an estimated 2,600 men were diagnosed with breast cancer in the United States.
“Approximately 95% of breast cancers diagnosed in men express the estrogen receptor and progesterone receptor (PR), which is a higher percentage than in women and suggests a key role for ER in the biology of breast cancer in men,” the investigators noted.
Although treatment of men with breast cancer has traditionally been extrapolated from treatment of women with breast cancer, genomic studies have suggested some key differences, the investigators noted, citing a study of the genomic landscape of male breast cancers presented at the 2014 San Antonio Breast Cancer Symposium.
In that study, investigators from the Memorial Sloan Kettering Cancer Center in New York and other institutions found that all male breast cancers in their sample of 64 patients were ER+ and human epidermal growth factor receptor 2 (HER2)–negative, predominantly of the luminal B subtype, and that the genetic alterations seen in male breast cancers frequently target DNA-repair fibroblast growth factor pathways. However, the pathways that are known to drive luminal cancers when mutated in women are seen less often among men, said Salvatore Piscuoglio, PhD, then a research fellow at MSKCC.
The current study helps to confirm and expand on the findings from that study, commented Steven J. Isakoff, MD, PhD, of the Massachusetts General Hospital Cancer Center in Boston, who was not involved in either study.
“I think it’s helpful to see in a larger dataset what the spectrum of oncotypes [Oncotype DX] looks like in men. In general, as the study described, we have a real lack of large-scale data in men and certainly no prospective data with oncotypes,” he said in an interview.
To get a better idea of the molecular characteristics of breast cancer in men and how they relate to breast cancer–specific mortality, Dr. Massarweh and his associates looked at deidentified 21-gene assay data from the Genomic Health Laboratory database on 3,806 men and 571,115 women with breast cancer with either no nodal involvement, micrometastases only, or one to three involved lymph nodes.
They also looked at survival data from the National Cancer Institute’s Surveillance, Epidemiology, and End Results (SEER) population of patients with breast cancer diagnosed during 2004-2012, which included data on 332 men and 55,842 women with ER-positive and/or PR-positive invasive breast cancer.
Among the entire 21-gene assay sample, they found that men were significantly older than women at the time of diagnosis, at a mean age of 64.2 vs. 59.1 years (P less than .001).
Both men and women had infiltrating ductal carcinoma as the most common histology; the prevalence was slightly higher among men at 87.6% versus 81.3% for women.
The average recurrence score in men was 16.8 versus 17.0 in women, a difference that was not statistically significant. A majority of both men and women had RS scores below 18 (65.8% and 58.2%, respectively), although significantly more men than women had RS scores of 31 or higher (12.4% vs. 7.4%; P less than .001).
“This relative predominance of high RS results in men was encountered across age groups but was most prominent in men younger than 40 years of age,” the investigators wrote.
At the other end of the scale, RS lower than 11, especially RS 0, were seen more frequently in men than in women, except among those younger than 40 years.
Looking at individual gene expression profiles, the authors found that mean gene expression was higher in men for genes associated with ER, proliferation, and invasion. ER expression was lowest and PR expression was highest in women younger than 50 years, but ER expression increased progressively with age.
Among men, those younger than 50 years had slightly lower ER and PR expression than did older men.
In the analysis of SEER survival data, they found that 5-year breast cancer severity score (BCSS) was 99% for men with RS below 18, 95.7% for those with RS between18 and 30, and 81% for those with RS of 31 or higher. Among women, 5-year BCSS was 99.5% for those with RS under 18, 98.6% for those with RS between 18 and 30, and 94.9% for those with RS of 31 or higher.
Five-year overall survival estimates were 92.6% for men with RS below 18, 86% for those with RS between 18 and 30, and 69% for those with RS of 31 or higher. Respective 5-year OS rates for women were 95%, 94.2%, and 89.9%.
“The 21-gene RS provided clear prognostic information in our cohort, with a significantly different 5-year BCSS determined by RS in both men and women,” the investigators wrote.
They noted that patients with low and intermediate RS have excellent prognoses regardless of nodal status, which suggests that these patients have more indolent disease and better outcomes than do patients with higher RS.
The more frequent use of adjuvant chemotherapy in the RS 31 and higher group indicates that “the prognostic utility of RS results is evident despite adjuvant chemotherapy use,” they wrote.
Dr. Isakoff pointed out, however, that the population in the study is from a registry of patients eligible for the 21-gene assay, which can only be used for patients with ER-positive and HER2-negative tumors.
“In other words, this is not a random sample. This is a sample of patients for whom the treating physician was on the fence about chemotherapy and in some way thought that getting an oncotype might be helpful,” he said.
He added that although the study findings “don’t change anything we have been doing, they provide reassurance that oncotype is a reasonable test to consider in patients with male breast cancer for whom we’re considering including or avoiding chemotherapy,” he said.
A funding source for the study was not reported. Dr. Massarweh disclosed stock or ownership in Radius Health, consulting for Novartis, and institutional research funding from multiple companies. Three coauthors are employees and stockholders of Genomic Health, maker of the Oncotype DX assay used in the study. Dr. Isakoff reported no conflicts of interest related to the study
SOURCE: Massarweh SA et al. 2018 Mar 27. doi: 10.1200/JCO.2017.76.8861.
A study of the molecular and genomic features of breast cancer in men, compared with those in women, highlights the prognostic value of a 21-gene breast recurrence score in both sexes, investigators say.
Men and women with estrogen receptor (ER)–positive breast cancer who had recurrence scores (RS) of 0 to 30 on the 21-gene assay (Oncotype DX) had excellent breast cancer–specific survival rates, which suggests that such patients could be spared from more aggressive treatments, such as chemotherapy, according to Suleiman Alfred Massarweh, MD, of Stanford (Calif.) University and his colleagues.
“Future adjuvant trials in ER-positive breast cancer may need to focus on targeting endocrine resistance in those patients with RS greater than 31 and may need to consider the weight of competing mortality risk when investigating the value of any additional treatment beyond endocrine therapy,” they wrote in the Journal of Clinical Oncology.
In 2016, an estimated 2,600 men were diagnosed with breast cancer in the United States.
“Approximately 95% of breast cancers diagnosed in men express the estrogen receptor and progesterone receptor (PR), which is a higher percentage than in women and suggests a key role for ER in the biology of breast cancer in men,” the investigators noted.
Although treatment of men with breast cancer has traditionally been extrapolated from treatment of women with breast cancer, genomic studies have suggested some key differences, the investigators noted, citing a study of the genomic landscape of male breast cancers presented at the 2014 San Antonio Breast Cancer Symposium.
In that study, investigators from the Memorial Sloan Kettering Cancer Center in New York and other institutions found that all male breast cancers in their sample of 64 patients were ER+ and human epidermal growth factor receptor 2 (HER2)–negative, predominantly of the luminal B subtype, and that the genetic alterations seen in male breast cancers frequently target DNA-repair fibroblast growth factor pathways. However, the pathways that are known to drive luminal cancers when mutated in women are seen less often among men, said Salvatore Piscuoglio, PhD, then a research fellow at MSKCC.
The current study helps to confirm and expand on the findings from that study, commented Steven J. Isakoff, MD, PhD, of the Massachusetts General Hospital Cancer Center in Boston, who was not involved in either study.
“I think it’s helpful to see in a larger dataset what the spectrum of oncotypes [Oncotype DX] looks like in men. In general, as the study described, we have a real lack of large-scale data in men and certainly no prospective data with oncotypes,” he said in an interview.
To get a better idea of the molecular characteristics of breast cancer in men and how they relate to breast cancer–specific mortality, Dr. Massarweh and his associates looked at deidentified 21-gene assay data from the Genomic Health Laboratory database on 3,806 men and 571,115 women with breast cancer with either no nodal involvement, micrometastases only, or one to three involved lymph nodes.
They also looked at survival data from the National Cancer Institute’s Surveillance, Epidemiology, and End Results (SEER) population of patients with breast cancer diagnosed during 2004-2012, which included data on 332 men and 55,842 women with ER-positive and/or PR-positive invasive breast cancer.
Among the entire 21-gene assay sample, they found that men were significantly older than women at the time of diagnosis, at a mean age of 64.2 vs. 59.1 years (P less than .001).
Both men and women had infiltrating ductal carcinoma as the most common histology; the prevalence was slightly higher among men at 87.6% versus 81.3% for women.
The average recurrence score in men was 16.8 versus 17.0 in women, a difference that was not statistically significant. A majority of both men and women had RS scores below 18 (65.8% and 58.2%, respectively), although significantly more men than women had RS scores of 31 or higher (12.4% vs. 7.4%; P less than .001).
“This relative predominance of high RS results in men was encountered across age groups but was most prominent in men younger than 40 years of age,” the investigators wrote.
At the other end of the scale, RS lower than 11, especially RS 0, were seen more frequently in men than in women, except among those younger than 40 years.
Looking at individual gene expression profiles, the authors found that mean gene expression was higher in men for genes associated with ER, proliferation, and invasion. ER expression was lowest and PR expression was highest in women younger than 50 years, but ER expression increased progressively with age.
Among men, those younger than 50 years had slightly lower ER and PR expression than did older men.
In the analysis of SEER survival data, they found that 5-year breast cancer severity score (BCSS) was 99% for men with RS below 18, 95.7% for those with RS between18 and 30, and 81% for those with RS of 31 or higher. Among women, 5-year BCSS was 99.5% for those with RS under 18, 98.6% for those with RS between 18 and 30, and 94.9% for those with RS of 31 or higher.
Five-year overall survival estimates were 92.6% for men with RS below 18, 86% for those with RS between 18 and 30, and 69% for those with RS of 31 or higher. Respective 5-year OS rates for women were 95%, 94.2%, and 89.9%.
“The 21-gene RS provided clear prognostic information in our cohort, with a significantly different 5-year BCSS determined by RS in both men and women,” the investigators wrote.
They noted that patients with low and intermediate RS have excellent prognoses regardless of nodal status, which suggests that these patients have more indolent disease and better outcomes than do patients with higher RS.
The more frequent use of adjuvant chemotherapy in the RS 31 and higher group indicates that “the prognostic utility of RS results is evident despite adjuvant chemotherapy use,” they wrote.
Dr. Isakoff pointed out, however, that the population in the study is from a registry of patients eligible for the 21-gene assay, which can only be used for patients with ER-positive and HER2-negative tumors.
“In other words, this is not a random sample. This is a sample of patients for whom the treating physician was on the fence about chemotherapy and in some way thought that getting an oncotype might be helpful,” he said.
He added that although the study findings “don’t change anything we have been doing, they provide reassurance that oncotype is a reasonable test to consider in patients with male breast cancer for whom we’re considering including or avoiding chemotherapy,” he said.
A funding source for the study was not reported. Dr. Massarweh disclosed stock or ownership in Radius Health, consulting for Novartis, and institutional research funding from multiple companies. Three coauthors are employees and stockholders of Genomic Health, maker of the Oncotype DX assay used in the study. Dr. Isakoff reported no conflicts of interest related to the study
SOURCE: Massarweh SA et al. 2018 Mar 27. doi: 10.1200/JCO.2017.76.8861.
A study of the molecular and genomic features of breast cancer in men, compared with those in women, highlights the prognostic value of a 21-gene breast recurrence score in both sexes, investigators say.
Men and women with estrogen receptor (ER)–positive breast cancer who had recurrence scores (RS) of 0 to 30 on the 21-gene assay (Oncotype DX) had excellent breast cancer–specific survival rates, which suggests that such patients could be spared from more aggressive treatments, such as chemotherapy, according to Suleiman Alfred Massarweh, MD, of Stanford (Calif.) University and his colleagues.
“Future adjuvant trials in ER-positive breast cancer may need to focus on targeting endocrine resistance in those patients with RS greater than 31 and may need to consider the weight of competing mortality risk when investigating the value of any additional treatment beyond endocrine therapy,” they wrote in the Journal of Clinical Oncology.
In 2016, an estimated 2,600 men were diagnosed with breast cancer in the United States.
“Approximately 95% of breast cancers diagnosed in men express the estrogen receptor and progesterone receptor (PR), which is a higher percentage than in women and suggests a key role for ER in the biology of breast cancer in men,” the investigators noted.
Although treatment of men with breast cancer has traditionally been extrapolated from treatment of women with breast cancer, genomic studies have suggested some key differences, the investigators noted, citing a study of the genomic landscape of male breast cancers presented at the 2014 San Antonio Breast Cancer Symposium.
In that study, investigators from the Memorial Sloan Kettering Cancer Center in New York and other institutions found that all male breast cancers in their sample of 64 patients were ER+ and human epidermal growth factor receptor 2 (HER2)–negative, predominantly of the luminal B subtype, and that the genetic alterations seen in male breast cancers frequently target DNA-repair fibroblast growth factor pathways. However, the pathways that are known to drive luminal cancers when mutated in women are seen less often among men, said Salvatore Piscuoglio, PhD, then a research fellow at MSKCC.
The current study helps to confirm and expand on the findings from that study, commented Steven J. Isakoff, MD, PhD, of the Massachusetts General Hospital Cancer Center in Boston, who was not involved in either study.
“I think it’s helpful to see in a larger dataset what the spectrum of oncotypes [Oncotype DX] looks like in men. In general, as the study described, we have a real lack of large-scale data in men and certainly no prospective data with oncotypes,” he said in an interview.
To get a better idea of the molecular characteristics of breast cancer in men and how they relate to breast cancer–specific mortality, Dr. Massarweh and his associates looked at deidentified 21-gene assay data from the Genomic Health Laboratory database on 3,806 men and 571,115 women with breast cancer with either no nodal involvement, micrometastases only, or one to three involved lymph nodes.
They also looked at survival data from the National Cancer Institute’s Surveillance, Epidemiology, and End Results (SEER) population of patients with breast cancer diagnosed during 2004-2012, which included data on 332 men and 55,842 women with ER-positive and/or PR-positive invasive breast cancer.
Among the entire 21-gene assay sample, they found that men were significantly older than women at the time of diagnosis, at a mean age of 64.2 vs. 59.1 years (P less than .001).
Both men and women had infiltrating ductal carcinoma as the most common histology; the prevalence was slightly higher among men at 87.6% versus 81.3% for women.
The average recurrence score in men was 16.8 versus 17.0 in women, a difference that was not statistically significant. A majority of both men and women had RS scores below 18 (65.8% and 58.2%, respectively), although significantly more men than women had RS scores of 31 or higher (12.4% vs. 7.4%; P less than .001).
“This relative predominance of high RS results in men was encountered across age groups but was most prominent in men younger than 40 years of age,” the investigators wrote.
At the other end of the scale, RS lower than 11, especially RS 0, were seen more frequently in men than in women, except among those younger than 40 years.
Looking at individual gene expression profiles, the authors found that mean gene expression was higher in men for genes associated with ER, proliferation, and invasion. ER expression was lowest and PR expression was highest in women younger than 50 years, but ER expression increased progressively with age.
Among men, those younger than 50 years had slightly lower ER and PR expression than did older men.
In the analysis of SEER survival data, they found that 5-year breast cancer severity score (BCSS) was 99% for men with RS below 18, 95.7% for those with RS between18 and 30, and 81% for those with RS of 31 or higher. Among women, 5-year BCSS was 99.5% for those with RS under 18, 98.6% for those with RS between 18 and 30, and 94.9% for those with RS of 31 or higher.
Five-year overall survival estimates were 92.6% for men with RS below 18, 86% for those with RS between 18 and 30, and 69% for those with RS of 31 or higher. Respective 5-year OS rates for women were 95%, 94.2%, and 89.9%.
“The 21-gene RS provided clear prognostic information in our cohort, with a significantly different 5-year BCSS determined by RS in both men and women,” the investigators wrote.
They noted that patients with low and intermediate RS have excellent prognoses regardless of nodal status, which suggests that these patients have more indolent disease and better outcomes than do patients with higher RS.
The more frequent use of adjuvant chemotherapy in the RS 31 and higher group indicates that “the prognostic utility of RS results is evident despite adjuvant chemotherapy use,” they wrote.
Dr. Isakoff pointed out, however, that the population in the study is from a registry of patients eligible for the 21-gene assay, which can only be used for patients with ER-positive and HER2-negative tumors.
“In other words, this is not a random sample. This is a sample of patients for whom the treating physician was on the fence about chemotherapy and in some way thought that getting an oncotype might be helpful,” he said.
He added that although the study findings “don’t change anything we have been doing, they provide reassurance that oncotype is a reasonable test to consider in patients with male breast cancer for whom we’re considering including or avoiding chemotherapy,” he said.
A funding source for the study was not reported. Dr. Massarweh disclosed stock or ownership in Radius Health, consulting for Novartis, and institutional research funding from multiple companies. Three coauthors are employees and stockholders of Genomic Health, maker of the Oncotype DX assay used in the study. Dr. Isakoff reported no conflicts of interest related to the study
SOURCE: Massarweh SA et al. 2018 Mar 27. doi: 10.1200/JCO.2017.76.8861.
FROM THE JOURNAL OF CLINICAL ONCOLOGY
Key clinical point: A 21-gene assay provides useful information about survival odds for men and women with breast cancer.
Major finding: A recurrence score of 31 or greater was associated with worse survival, particularly in men.
Study details: Retrospective review of genomic and surveillance data on 3,806 men and 571,115 women with breast cancer.
Disclosures: A funding source for the study was not reported. Dr. Massarweh disclosed stock or ownership in Radius Health, consulting for Novartis, and institutional research funding from multiple companies. Three coauthors are employees and stockholders of Genomic Health, maker of the Oncotype DX assay used in the study. Dr. Isakoff reported no conflicts of interest related to the study.
Source: Massarweh SA et al. 2018 Mar 27. doi: 10.1200/JCO.2017.76.8861.