User login
Oncologist-led BRCA mutation testing and counseling may reduce wait times for women with ovarian cancer
For women with ovarian cancer, an oncologist-led BRCA1/2 (BRCAm) counseling process is associated with favorable waiting times for test results and high levels of satisfaction, according to results of a prospective observational study.
The median turnaround time from initial counseling to receiving a test result was 9.1 weeks, investigators reported in the Journal of Clinical Oncology.
“Following a pathway similar to the one used in this study could allow faster treatment decisions and better use of resources in the management of patients with ovarian cancer,” said lead author Nicoletta Colombo, MD, of European Institute of Oncology, University of Milan-Bicocca, Italy, and her associates.
Establishing an ovarian cancer patient’s BRCAm status provides useful prognostic information and helps identify patients most likely to benefit from therapy with poly(ADP-ribose) polymerase (PARP) inhibitors, Dr. Colombo and her colleagues wrote.
However, despite guideline recommendations, many patients with an ovarian cancer diagnosis are currently not receiving BRCAm testing, they added.
“Given the high volume of BRCAm tests now being ordered, a new, more streamlined testing approach is needed to shorten testing turnaround times and to ease the pressure on genetic counselors,” the authors said.
In a pilot study from the United Kingdom, a streamlined, oncologist-led BRCAm testing model reduced a 20-week average turnaround time by fourfold, Dr. Colombo and her colleagues said.
Accordingly, the prospective, observational ENGAGE study sought to evaluate a streamlined oncologist-led BRCAm testing pathway in 700 patients with ovarian cancer at 26 sites in the United States, Spain, and Italy.
Oncologists and oncology nurses involved in the study received training on pretest genetic counseling techniques and on how to discuss the role of BRCAm testing with patients, according to the study description. Patients with a positive test were recommended for an appointment with a geneticist or genetic counselor.
The median time from initial counseling to receiving a test result was 9.1 weeks, the investigators reported. For patients in the United States, that median turnaround time was 4.1 weeks, while turnaround times in Spain and Italy were 12.0 and 20.4 weeks, respectively.
“BRCAm testing usually occurred shortly after the initial oncology team counseling, whereas the average time from patient consent to BRCAm testing was expected to be more than 1 month in approximately 25% of patients using standard procedures,” the investigators said in their report.
More than 99% of patients expressed satisfaction with the oncologist-led testing pathway, Dr. Colombo and her associates said. In addition, more than 80% of oncologists said the testing worked well and that counseling was an efficient use of their time.
Geneticists and genetic counselors showed less enthusiasm for the oncologist-led approach, according to investigators.
Less than half of surveyed geneticists or genetic counselors felt that patients received accurate information about the BRCAm test in the pretest counseling session, according to the report.
“It should be noted that the purpose of the oncologist-led pretest counseling was to provide enough information on why the patient should have the test, rather than full genetic counseling, which is appropriate once the test result is known,” investigators said in the report.
The study was supported by AstraZeneca. Dr. Colombo and her associates reported potential conflicts of interest related to AstraZeneca, Genentech, PharmaMar, Amgen, Clovis Oncology, Pfizer, MSD, Tesaro, and others.
SOURCE: Colombo N et al. J Clin Oncol. 2018 Mar 20. doi: 10.1200/JCO.2017.76.278.
For women with ovarian cancer, an oncologist-led BRCA1/2 (BRCAm) counseling process is associated with favorable waiting times for test results and high levels of satisfaction, according to results of a prospective observational study.
The median turnaround time from initial counseling to receiving a test result was 9.1 weeks, investigators reported in the Journal of Clinical Oncology.
“Following a pathway similar to the one used in this study could allow faster treatment decisions and better use of resources in the management of patients with ovarian cancer,” said lead author Nicoletta Colombo, MD, of European Institute of Oncology, University of Milan-Bicocca, Italy, and her associates.
Establishing an ovarian cancer patient’s BRCAm status provides useful prognostic information and helps identify patients most likely to benefit from therapy with poly(ADP-ribose) polymerase (PARP) inhibitors, Dr. Colombo and her colleagues wrote.
However, despite guideline recommendations, many patients with an ovarian cancer diagnosis are currently not receiving BRCAm testing, they added.
“Given the high volume of BRCAm tests now being ordered, a new, more streamlined testing approach is needed to shorten testing turnaround times and to ease the pressure on genetic counselors,” the authors said.
In a pilot study from the United Kingdom, a streamlined, oncologist-led BRCAm testing model reduced a 20-week average turnaround time by fourfold, Dr. Colombo and her colleagues said.
Accordingly, the prospective, observational ENGAGE study sought to evaluate a streamlined oncologist-led BRCAm testing pathway in 700 patients with ovarian cancer at 26 sites in the United States, Spain, and Italy.
Oncologists and oncology nurses involved in the study received training on pretest genetic counseling techniques and on how to discuss the role of BRCAm testing with patients, according to the study description. Patients with a positive test were recommended for an appointment with a geneticist or genetic counselor.
The median time from initial counseling to receiving a test result was 9.1 weeks, the investigators reported. For patients in the United States, that median turnaround time was 4.1 weeks, while turnaround times in Spain and Italy were 12.0 and 20.4 weeks, respectively.
“BRCAm testing usually occurred shortly after the initial oncology team counseling, whereas the average time from patient consent to BRCAm testing was expected to be more than 1 month in approximately 25% of patients using standard procedures,” the investigators said in their report.
More than 99% of patients expressed satisfaction with the oncologist-led testing pathway, Dr. Colombo and her associates said. In addition, more than 80% of oncologists said the testing worked well and that counseling was an efficient use of their time.
Geneticists and genetic counselors showed less enthusiasm for the oncologist-led approach, according to investigators.
Less than half of surveyed geneticists or genetic counselors felt that patients received accurate information about the BRCAm test in the pretest counseling session, according to the report.
“It should be noted that the purpose of the oncologist-led pretest counseling was to provide enough information on why the patient should have the test, rather than full genetic counseling, which is appropriate once the test result is known,” investigators said in the report.
The study was supported by AstraZeneca. Dr. Colombo and her associates reported potential conflicts of interest related to AstraZeneca, Genentech, PharmaMar, Amgen, Clovis Oncology, Pfizer, MSD, Tesaro, and others.
SOURCE: Colombo N et al. J Clin Oncol. 2018 Mar 20. doi: 10.1200/JCO.2017.76.278.
For women with ovarian cancer, an oncologist-led BRCA1/2 (BRCAm) counseling process is associated with favorable waiting times for test results and high levels of satisfaction, according to results of a prospective observational study.
The median turnaround time from initial counseling to receiving a test result was 9.1 weeks, investigators reported in the Journal of Clinical Oncology.
“Following a pathway similar to the one used in this study could allow faster treatment decisions and better use of resources in the management of patients with ovarian cancer,” said lead author Nicoletta Colombo, MD, of European Institute of Oncology, University of Milan-Bicocca, Italy, and her associates.
Establishing an ovarian cancer patient’s BRCAm status provides useful prognostic information and helps identify patients most likely to benefit from therapy with poly(ADP-ribose) polymerase (PARP) inhibitors, Dr. Colombo and her colleagues wrote.
However, despite guideline recommendations, many patients with an ovarian cancer diagnosis are currently not receiving BRCAm testing, they added.
“Given the high volume of BRCAm tests now being ordered, a new, more streamlined testing approach is needed to shorten testing turnaround times and to ease the pressure on genetic counselors,” the authors said.
In a pilot study from the United Kingdom, a streamlined, oncologist-led BRCAm testing model reduced a 20-week average turnaround time by fourfold, Dr. Colombo and her colleagues said.
Accordingly, the prospective, observational ENGAGE study sought to evaluate a streamlined oncologist-led BRCAm testing pathway in 700 patients with ovarian cancer at 26 sites in the United States, Spain, and Italy.
Oncologists and oncology nurses involved in the study received training on pretest genetic counseling techniques and on how to discuss the role of BRCAm testing with patients, according to the study description. Patients with a positive test were recommended for an appointment with a geneticist or genetic counselor.
The median time from initial counseling to receiving a test result was 9.1 weeks, the investigators reported. For patients in the United States, that median turnaround time was 4.1 weeks, while turnaround times in Spain and Italy were 12.0 and 20.4 weeks, respectively.
“BRCAm testing usually occurred shortly after the initial oncology team counseling, whereas the average time from patient consent to BRCAm testing was expected to be more than 1 month in approximately 25% of patients using standard procedures,” the investigators said in their report.
More than 99% of patients expressed satisfaction with the oncologist-led testing pathway, Dr. Colombo and her associates said. In addition, more than 80% of oncologists said the testing worked well and that counseling was an efficient use of their time.
Geneticists and genetic counselors showed less enthusiasm for the oncologist-led approach, according to investigators.
Less than half of surveyed geneticists or genetic counselors felt that patients received accurate information about the BRCAm test in the pretest counseling session, according to the report.
“It should be noted that the purpose of the oncologist-led pretest counseling was to provide enough information on why the patient should have the test, rather than full genetic counseling, which is appropriate once the test result is known,” investigators said in the report.
The study was supported by AstraZeneca. Dr. Colombo and her associates reported potential conflicts of interest related to AstraZeneca, Genentech, PharmaMar, Amgen, Clovis Oncology, Pfizer, MSD, Tesaro, and others.
SOURCE: Colombo N et al. J Clin Oncol. 2018 Mar 20. doi: 10.1200/JCO.2017.76.278.
FROM THE JOURNAL OF CLINICAL ONCOLOGY
Key clinical point: An oncologist-led BRCA1/2 (BRCAm) counseling process is associated with favorable waiting times for test results and high levels of satisfaction among women with ovarian cancer.
Major finding: The median turnaround time from initial counseling to receiving a test result was 9.1 weeks.
Study details: The prospective, observational ENGAGE study evaluating a streamlined oncologist-led BRCAm testing pathway in 700 patients with ovarian cancer at 26 sites in the United States, Spain, and Italy.
Disclosures: The study was supported by AstraZeneca. Study authors reported potential conflicts of interest related to AstraZeneca, Genentech, PharmaMar, Amgen, Clovis Oncology, Pfizer, MSD, Tesaro, and others.
Source: Colombo N et al. J Clin Oncol. 2018 Mar 20. doi: 10.1200/JCO.2017.76.278.
Over one-third report financial burden from breast cancer treatment
CHICAGO – Women who have treatment for breast cancer seldom talk about the costs of care with their medical team, but a study out of Duke University has found that more than one-third reported having a financial burden from their breast cancer treatment, even among women with health insurance, according to a report presented at the Society of Surgical Oncology Annual Cancer Symposium.
“The financial harm associated with cancer treatment is now known as ‘financial toxicity,’ ” Rachel A. Greenup, MD, MPH, said in reporting the results of an 88-item survey completed by 654 adult women who had treatment for breast cancer. The women were recruited through the Army of Women of the Dr. Susan Love Research Foundation and The Sister’s Network of North Carolina, an African-American breast cancer survivors’ organization.
Overall, 69% of survey respondents had private insurance and 26% had Medicare. Of the patients surveyed, 94% had breast cancer surgery: 40.6% lumpectomy, 23.7% mastectomy, and 29.7% bilateral mastectomy; 34% also had breast reconstruction. Among those surveyed, 43% reported considering costs in their treatment decision. Of these, 29% considered costs when making surgical treatment decisions, including 14% who reported that costs were “extremely” important.
Despite the high levels of insurance coverage, 35% of the study participants reported a financial burden resulting from cancer treatment, ranging from “somewhat” burdensome to “catastrophic.” The median out-of-pocket cost for the study participants was $4,000, and 5% exceeded $40,000 in such costs, Dr. Greenup said. “The risk of financial harm and increased out-of-pocket costs to patients differed by surgery type,” with higher financial burdens seen in women who underwent bilateral mastectomy.
Cost was one of many factors survey participants reported considering when making surgical treatment decisions, but the most important factors were the opinions and advice of the medical team and the individual patient’s fear of recurrence. However, in lower-income women, cost factored more significantly in decision making. “In a subset of women who reported an annual income of $45,000 a year or less, cost of treatment gained importance and, interestingly, became more important than many variables we routinely discuss – for example, appearance of the breast, sexuality, avoiding radiation, and breast preservation,” Dr. Greenup said. “An income of $74,000 a year was the tipping point at which women reported incorporating costs into their cancer treatment decisions.”
She added that younger, minority women who did not have Medicare coverage were more likely to consider costs in breast cancer treatment decisions.
Most women surveyed (79%) said they preferred to know their out-of-pocket costs before they begin treatment, Dr. Greenup said, “and 40% believed that we as physicians should be considering out-of-pocket costs while making medical decisions.” However, 78% of those surveyed said they never discussed costs with their cancer team – despite American Society of Clinical Oncologists guidelines, she pointed out – and 35% said their treatment costs were higher than expected.
Dr. Greenup described the study population as “well engaged … with good insurance and strong educational background that likely does not reflect the general population.” The results may not be generalizable. “We expect that in a general cohort of women, our findings would be even more exaggerated,” she said.
The study points out the need to better understand how cost transparency may affect breast cancer treatment decisions, Dr. Greenup said. “As eligible women with breast cancer choose between surgical options, it’s important that we consider the potential risk of financial harm as we guide them through these difficult treatment decisions,” she said.
Dr. Greenup and her study coauthors reported having no financial disclosures.
SOURCE: Greenup RA. SSO 2018, Abstract No. 24.
CHICAGO – Women who have treatment for breast cancer seldom talk about the costs of care with their medical team, but a study out of Duke University has found that more than one-third reported having a financial burden from their breast cancer treatment, even among women with health insurance, according to a report presented at the Society of Surgical Oncology Annual Cancer Symposium.
“The financial harm associated with cancer treatment is now known as ‘financial toxicity,’ ” Rachel A. Greenup, MD, MPH, said in reporting the results of an 88-item survey completed by 654 adult women who had treatment for breast cancer. The women were recruited through the Army of Women of the Dr. Susan Love Research Foundation and The Sister’s Network of North Carolina, an African-American breast cancer survivors’ organization.
Overall, 69% of survey respondents had private insurance and 26% had Medicare. Of the patients surveyed, 94% had breast cancer surgery: 40.6% lumpectomy, 23.7% mastectomy, and 29.7% bilateral mastectomy; 34% also had breast reconstruction. Among those surveyed, 43% reported considering costs in their treatment decision. Of these, 29% considered costs when making surgical treatment decisions, including 14% who reported that costs were “extremely” important.
Despite the high levels of insurance coverage, 35% of the study participants reported a financial burden resulting from cancer treatment, ranging from “somewhat” burdensome to “catastrophic.” The median out-of-pocket cost for the study participants was $4,000, and 5% exceeded $40,000 in such costs, Dr. Greenup said. “The risk of financial harm and increased out-of-pocket costs to patients differed by surgery type,” with higher financial burdens seen in women who underwent bilateral mastectomy.
Cost was one of many factors survey participants reported considering when making surgical treatment decisions, but the most important factors were the opinions and advice of the medical team and the individual patient’s fear of recurrence. However, in lower-income women, cost factored more significantly in decision making. “In a subset of women who reported an annual income of $45,000 a year or less, cost of treatment gained importance and, interestingly, became more important than many variables we routinely discuss – for example, appearance of the breast, sexuality, avoiding radiation, and breast preservation,” Dr. Greenup said. “An income of $74,000 a year was the tipping point at which women reported incorporating costs into their cancer treatment decisions.”
She added that younger, minority women who did not have Medicare coverage were more likely to consider costs in breast cancer treatment decisions.
Most women surveyed (79%) said they preferred to know their out-of-pocket costs before they begin treatment, Dr. Greenup said, “and 40% believed that we as physicians should be considering out-of-pocket costs while making medical decisions.” However, 78% of those surveyed said they never discussed costs with their cancer team – despite American Society of Clinical Oncologists guidelines, she pointed out – and 35% said their treatment costs were higher than expected.
Dr. Greenup described the study population as “well engaged … with good insurance and strong educational background that likely does not reflect the general population.” The results may not be generalizable. “We expect that in a general cohort of women, our findings would be even more exaggerated,” she said.
The study points out the need to better understand how cost transparency may affect breast cancer treatment decisions, Dr. Greenup said. “As eligible women with breast cancer choose between surgical options, it’s important that we consider the potential risk of financial harm as we guide them through these difficult treatment decisions,” she said.
Dr. Greenup and her study coauthors reported having no financial disclosures.
SOURCE: Greenup RA. SSO 2018, Abstract No. 24.
CHICAGO – Women who have treatment for breast cancer seldom talk about the costs of care with their medical team, but a study out of Duke University has found that more than one-third reported having a financial burden from their breast cancer treatment, even among women with health insurance, according to a report presented at the Society of Surgical Oncology Annual Cancer Symposium.
“The financial harm associated with cancer treatment is now known as ‘financial toxicity,’ ” Rachel A. Greenup, MD, MPH, said in reporting the results of an 88-item survey completed by 654 adult women who had treatment for breast cancer. The women were recruited through the Army of Women of the Dr. Susan Love Research Foundation and The Sister’s Network of North Carolina, an African-American breast cancer survivors’ organization.
Overall, 69% of survey respondents had private insurance and 26% had Medicare. Of the patients surveyed, 94% had breast cancer surgery: 40.6% lumpectomy, 23.7% mastectomy, and 29.7% bilateral mastectomy; 34% also had breast reconstruction. Among those surveyed, 43% reported considering costs in their treatment decision. Of these, 29% considered costs when making surgical treatment decisions, including 14% who reported that costs were “extremely” important.
Despite the high levels of insurance coverage, 35% of the study participants reported a financial burden resulting from cancer treatment, ranging from “somewhat” burdensome to “catastrophic.” The median out-of-pocket cost for the study participants was $4,000, and 5% exceeded $40,000 in such costs, Dr. Greenup said. “The risk of financial harm and increased out-of-pocket costs to patients differed by surgery type,” with higher financial burdens seen in women who underwent bilateral mastectomy.
Cost was one of many factors survey participants reported considering when making surgical treatment decisions, but the most important factors were the opinions and advice of the medical team and the individual patient’s fear of recurrence. However, in lower-income women, cost factored more significantly in decision making. “In a subset of women who reported an annual income of $45,000 a year or less, cost of treatment gained importance and, interestingly, became more important than many variables we routinely discuss – for example, appearance of the breast, sexuality, avoiding radiation, and breast preservation,” Dr. Greenup said. “An income of $74,000 a year was the tipping point at which women reported incorporating costs into their cancer treatment decisions.”
She added that younger, minority women who did not have Medicare coverage were more likely to consider costs in breast cancer treatment decisions.
Most women surveyed (79%) said they preferred to know their out-of-pocket costs before they begin treatment, Dr. Greenup said, “and 40% believed that we as physicians should be considering out-of-pocket costs while making medical decisions.” However, 78% of those surveyed said they never discussed costs with their cancer team – despite American Society of Clinical Oncologists guidelines, she pointed out – and 35% said their treatment costs were higher than expected.
Dr. Greenup described the study population as “well engaged … with good insurance and strong educational background that likely does not reflect the general population.” The results may not be generalizable. “We expect that in a general cohort of women, our findings would be even more exaggerated,” she said.
The study points out the need to better understand how cost transparency may affect breast cancer treatment decisions, Dr. Greenup said. “As eligible women with breast cancer choose between surgical options, it’s important that we consider the potential risk of financial harm as we guide them through these difficult treatment decisions,” she said.
Dr. Greenup and her study coauthors reported having no financial disclosures.
SOURCE: Greenup RA. SSO 2018, Abstract No. 24.
REPORTING FROM SSO 2018
Key clinical point: Treatment costs are important to many women with breast cancer, although most report not having cost discussions with their physicians.
Major finding: Despite the high levels of insurance coverage, 35% of study participants reported a financial burden resulting from cancer treatment, ranging from “somewhat” burdensome to “catastrophic.”
Study details: An 88-item survey completed by 654 adult women who had treatment for breast cancer.
Disclosures: Dr. Greenup and her coauthors reported having no financial disclosures.
Source: Greenup RA. SSO 2018, Abstract No. 24.
Lower socioeconomic status linked with poor NSCLC prognosis in those with pretreatment weight loss
, in a retrospective review of medical records.
Investigators identified 1,366 patients with NSCLC who had been consecutively treated at a tertiary care health system between Jan. 1, 2006, and Dec. 31, 2013, and obtained their insurance status from an institutional tumor registry.
Cancer-associated weight loss was present at the time of diagnosis in 30% of the patients. Among those patients with pretreatment weight loss, uninsured patients had worse survival compared with those who had private insurance (hazard ratio, 1.63; 95% confidence interval, 1.14-2.35). However, no association was found for patients without pretreatment weight loss, wrote Steven Lau, MD, of the University of Texas Southwestern Medical Center, Dallas, and his associates. The report was published in the Journal of Oncology Practice.
After the researchers controlled for other prognostic factors, Medicaid insurance (odds ratio, 2.17; 95% CI, 1.42-3.30) and lack of insurance (OR, 2.32; 95% CI, 1.50-3.58) were independently associated with pretreatment weight loss.
“Patients with NSCLC of lower SES as measured by primary payer are disproportionately affected by cancer-associated weight loss, which in turn is prognostic of diminished survival ... These findings suggest early recognition and management of cachexia, even at the time of cancer diagnosis, could result in improved survival,” Dr. Lau and his associates concluded.
The study was supported in part by National Center for Advancing Translational Sciences grants TL1TR001104 and UL1TR001105. Dr. Lau reported employment with LabCorp by an immediate family member. Coauthors reported financial ties to Advenchen Laboratories, Macrogen, Peregrine Pharmaceuticals, and DFINE.
SOURCE: Lau S et al. J Oncol Pract. 2018 Mar 20. doi: 10.1200/JOP.2017.025239
, in a retrospective review of medical records.
Investigators identified 1,366 patients with NSCLC who had been consecutively treated at a tertiary care health system between Jan. 1, 2006, and Dec. 31, 2013, and obtained their insurance status from an institutional tumor registry.
Cancer-associated weight loss was present at the time of diagnosis in 30% of the patients. Among those patients with pretreatment weight loss, uninsured patients had worse survival compared with those who had private insurance (hazard ratio, 1.63; 95% confidence interval, 1.14-2.35). However, no association was found for patients without pretreatment weight loss, wrote Steven Lau, MD, of the University of Texas Southwestern Medical Center, Dallas, and his associates. The report was published in the Journal of Oncology Practice.
After the researchers controlled for other prognostic factors, Medicaid insurance (odds ratio, 2.17; 95% CI, 1.42-3.30) and lack of insurance (OR, 2.32; 95% CI, 1.50-3.58) were independently associated with pretreatment weight loss.
“Patients with NSCLC of lower SES as measured by primary payer are disproportionately affected by cancer-associated weight loss, which in turn is prognostic of diminished survival ... These findings suggest early recognition and management of cachexia, even at the time of cancer diagnosis, could result in improved survival,” Dr. Lau and his associates concluded.
The study was supported in part by National Center for Advancing Translational Sciences grants TL1TR001104 and UL1TR001105. Dr. Lau reported employment with LabCorp by an immediate family member. Coauthors reported financial ties to Advenchen Laboratories, Macrogen, Peregrine Pharmaceuticals, and DFINE.
SOURCE: Lau S et al. J Oncol Pract. 2018 Mar 20. doi: 10.1200/JOP.2017.025239
, in a retrospective review of medical records.
Investigators identified 1,366 patients with NSCLC who had been consecutively treated at a tertiary care health system between Jan. 1, 2006, and Dec. 31, 2013, and obtained their insurance status from an institutional tumor registry.
Cancer-associated weight loss was present at the time of diagnosis in 30% of the patients. Among those patients with pretreatment weight loss, uninsured patients had worse survival compared with those who had private insurance (hazard ratio, 1.63; 95% confidence interval, 1.14-2.35). However, no association was found for patients without pretreatment weight loss, wrote Steven Lau, MD, of the University of Texas Southwestern Medical Center, Dallas, and his associates. The report was published in the Journal of Oncology Practice.
After the researchers controlled for other prognostic factors, Medicaid insurance (odds ratio, 2.17; 95% CI, 1.42-3.30) and lack of insurance (OR, 2.32; 95% CI, 1.50-3.58) were independently associated with pretreatment weight loss.
“Patients with NSCLC of lower SES as measured by primary payer are disproportionately affected by cancer-associated weight loss, which in turn is prognostic of diminished survival ... These findings suggest early recognition and management of cachexia, even at the time of cancer diagnosis, could result in improved survival,” Dr. Lau and his associates concluded.
The study was supported in part by National Center for Advancing Translational Sciences grants TL1TR001104 and UL1TR001105. Dr. Lau reported employment with LabCorp by an immediate family member. Coauthors reported financial ties to Advenchen Laboratories, Macrogen, Peregrine Pharmaceuticals, and DFINE.
SOURCE: Lau S et al. J Oncol Pract. 2018 Mar 20. doi: 10.1200/JOP.2017.025239
FROM JOURNAL OF ONCOLOGY PRACTICE
Key clinical point: Early recognition and management of cancer-associated weight loss in patients with NSCLC and low socioeconomic status may improve outcomes.
Major finding: Lack of insurance was significantly prognostic among patients with NSCLC and pretreatment weight loss (hazard ratio, 1.63 95% CI, 1.14-2.35).
Study details: 1,366 adult patients with NSCLC consecutively treated at a tertiary care health system between Jan. 1, 2006, and Dec. 31, 2013.
Disclosures: The study was supported in part by National Center for Advancing Translational Sciences grants TL1TR001104 and UL1TR001105. Dr. Lau reported employment with LabCorp by an immediate family member. Coauthors reported financial ties to Advenchen Laboratories, Macrogen, Peregrine Pharmaceuticals, and DFINE.
Source: Lau S et al. J Oncol Pract. 2018 Mar 20. doi: 10.1200/JOP.2017.025239.
Late toxicities with PARP inhibitor plus RT in inflammatory breast cancer
Using the PARP inhibitor veliparib as a radiosensitizer for chest wall radiation in women with inflammatory or locally recurrent breast cancer was associated with a high rate of late grade 3 adverse events, results of a phase 1 study show.
Although the trial’s upper limit of dose-limiting toxicities during 6 weeks of treatment and 4 weeks of follow-up was not met, 46.7% of 30 patients treated with veliparib and radiation after complete surgical resection had at least one grade 3 adverse event by 3 years of follow-up, reported Reshma Jagsi, MD, of the University of Michigan, Ann Arbor.
“In this multicenter phase 1 trial, severe acute toxicity did not exceed the prespecified target of 30%, even at the highest tested dose of veliparib (200 mg twice a day), and we observed no grade 4 or 5 events. However, given observations of grade 3 late toxicity in nearly one-half of all patients evaluated at 3 years, we recommend a phase 2 dose of 50 mg twice a day if veliparib is investigated further for radiosensitization in patients with breast cancer at high risk of locoregional recurrence and in need of treatment intensification,” they wrote in the Journal of Clinical Oncology.
In preclinical studies, PARP (poly [ADP-ribose] polymerase) inhibitors have been shown to enhance radiosensitivty of breast malignancies when given concurrently with radiation.
In a phase 1 dosing and safety study, 30 women with inflammatory or locally recurrent breast cancer of the chest wall underwent complete surgical resection and were then assigned to radiation consisting of 50 Gy to the chest wall and regional lymph nodes, plus a 10 Gy boost. The patients also received oral veliparib at a dose of either 50, 100, 150, or 200 mg taken twice daily during the 6-week course of radiotherapy.
During the 6 weeks of therapy and 4 weeks of follow-up, there were five dose-limiting toxicities, including two cases each of confluent moist desquamation greater than 100 cm2 in the 100- and 150-mg dose groups, and one case of neutropenia in a patient at the 200-mg dose level.
The respective rates of any grade 3 toxicity, treatment related or otherwise, at 1, 2, and 3 years of follow-up were 10%, 16.7%, and 46.7%.
The investigators noted that, at year 3, severe fibrosis in the treatment field was seen in 6 of the 15 surviving patients. Of the six patients, two also had grade 3 skin induration, and two had grade 3 lymphedema.
“Although some of the late adverse events we observed might have occurred even in the absence of the investigational agent and with standard therapy, severe late toxicity is relatively uncommon with standard therapy alone, so we believe that a cautious approach is prudent,” Dr. Jagsi and associates wrote.
The study was supported by the Translational Breast Cancer Research Consortium, Breast Cancer Research Foundation, University of Michigan Comprehensive Cancer Center, and Michigan Institute for Clinical and Health Research. Dr. Jagsi reported institutional research support from AbbVie, which donated the veliparib used in the study.
SOURCE: Jagsi R et al. J Clin Oncol. 2018 Mar 20. doi: 10.1200/JCO.2017.77.2665
Using the PARP inhibitor veliparib as a radiosensitizer for chest wall radiation in women with inflammatory or locally recurrent breast cancer was associated with a high rate of late grade 3 adverse events, results of a phase 1 study show.
Although the trial’s upper limit of dose-limiting toxicities during 6 weeks of treatment and 4 weeks of follow-up was not met, 46.7% of 30 patients treated with veliparib and radiation after complete surgical resection had at least one grade 3 adverse event by 3 years of follow-up, reported Reshma Jagsi, MD, of the University of Michigan, Ann Arbor.
“In this multicenter phase 1 trial, severe acute toxicity did not exceed the prespecified target of 30%, even at the highest tested dose of veliparib (200 mg twice a day), and we observed no grade 4 or 5 events. However, given observations of grade 3 late toxicity in nearly one-half of all patients evaluated at 3 years, we recommend a phase 2 dose of 50 mg twice a day if veliparib is investigated further for radiosensitization in patients with breast cancer at high risk of locoregional recurrence and in need of treatment intensification,” they wrote in the Journal of Clinical Oncology.
In preclinical studies, PARP (poly [ADP-ribose] polymerase) inhibitors have been shown to enhance radiosensitivty of breast malignancies when given concurrently with radiation.
In a phase 1 dosing and safety study, 30 women with inflammatory or locally recurrent breast cancer of the chest wall underwent complete surgical resection and were then assigned to radiation consisting of 50 Gy to the chest wall and regional lymph nodes, plus a 10 Gy boost. The patients also received oral veliparib at a dose of either 50, 100, 150, or 200 mg taken twice daily during the 6-week course of radiotherapy.
During the 6 weeks of therapy and 4 weeks of follow-up, there were five dose-limiting toxicities, including two cases each of confluent moist desquamation greater than 100 cm2 in the 100- and 150-mg dose groups, and one case of neutropenia in a patient at the 200-mg dose level.
The respective rates of any grade 3 toxicity, treatment related or otherwise, at 1, 2, and 3 years of follow-up were 10%, 16.7%, and 46.7%.
The investigators noted that, at year 3, severe fibrosis in the treatment field was seen in 6 of the 15 surviving patients. Of the six patients, two also had grade 3 skin induration, and two had grade 3 lymphedema.
“Although some of the late adverse events we observed might have occurred even in the absence of the investigational agent and with standard therapy, severe late toxicity is relatively uncommon with standard therapy alone, so we believe that a cautious approach is prudent,” Dr. Jagsi and associates wrote.
The study was supported by the Translational Breast Cancer Research Consortium, Breast Cancer Research Foundation, University of Michigan Comprehensive Cancer Center, and Michigan Institute for Clinical and Health Research. Dr. Jagsi reported institutional research support from AbbVie, which donated the veliparib used in the study.
SOURCE: Jagsi R et al. J Clin Oncol. 2018 Mar 20. doi: 10.1200/JCO.2017.77.2665
Using the PARP inhibitor veliparib as a radiosensitizer for chest wall radiation in women with inflammatory or locally recurrent breast cancer was associated with a high rate of late grade 3 adverse events, results of a phase 1 study show.
Although the trial’s upper limit of dose-limiting toxicities during 6 weeks of treatment and 4 weeks of follow-up was not met, 46.7% of 30 patients treated with veliparib and radiation after complete surgical resection had at least one grade 3 adverse event by 3 years of follow-up, reported Reshma Jagsi, MD, of the University of Michigan, Ann Arbor.
“In this multicenter phase 1 trial, severe acute toxicity did not exceed the prespecified target of 30%, even at the highest tested dose of veliparib (200 mg twice a day), and we observed no grade 4 or 5 events. However, given observations of grade 3 late toxicity in nearly one-half of all patients evaluated at 3 years, we recommend a phase 2 dose of 50 mg twice a day if veliparib is investigated further for radiosensitization in patients with breast cancer at high risk of locoregional recurrence and in need of treatment intensification,” they wrote in the Journal of Clinical Oncology.
In preclinical studies, PARP (poly [ADP-ribose] polymerase) inhibitors have been shown to enhance radiosensitivty of breast malignancies when given concurrently with radiation.
In a phase 1 dosing and safety study, 30 women with inflammatory or locally recurrent breast cancer of the chest wall underwent complete surgical resection and were then assigned to radiation consisting of 50 Gy to the chest wall and regional lymph nodes, plus a 10 Gy boost. The patients also received oral veliparib at a dose of either 50, 100, 150, or 200 mg taken twice daily during the 6-week course of radiotherapy.
During the 6 weeks of therapy and 4 weeks of follow-up, there were five dose-limiting toxicities, including two cases each of confluent moist desquamation greater than 100 cm2 in the 100- and 150-mg dose groups, and one case of neutropenia in a patient at the 200-mg dose level.
The respective rates of any grade 3 toxicity, treatment related or otherwise, at 1, 2, and 3 years of follow-up were 10%, 16.7%, and 46.7%.
The investigators noted that, at year 3, severe fibrosis in the treatment field was seen in 6 of the 15 surviving patients. Of the six patients, two also had grade 3 skin induration, and two had grade 3 lymphedema.
“Although some of the late adverse events we observed might have occurred even in the absence of the investigational agent and with standard therapy, severe late toxicity is relatively uncommon with standard therapy alone, so we believe that a cautious approach is prudent,” Dr. Jagsi and associates wrote.
The study was supported by the Translational Breast Cancer Research Consortium, Breast Cancer Research Foundation, University of Michigan Comprehensive Cancer Center, and Michigan Institute for Clinical and Health Research. Dr. Jagsi reported institutional research support from AbbVie, which donated the veliparib used in the study.
SOURCE: Jagsi R et al. J Clin Oncol. 2018 Mar 20. doi: 10.1200/JCO.2017.77.2665
FROM JOURNAL OF CLINICAL ONCOLOGY
Key clinical point: PARP inhibitors have a radiosensitizing effect when used in treatment of inflammatory breast cancer but are associated with late grade 3 adverse events.
Major finding: At 3 years, 46.7% of patients had a grade 3 adverse event of any kind.
Study details: Phase 1 dose-finding and safety study in 30 women treated with radiation and veliparib after complete surgical resection of inflammatory or recurrent breast cancer of the chest wall and regional lymph nodes.
Disclosures: The study was supported by the Translational Breast Cancer Research Consortium, Breast Cancer Research Foundation, University of Michigan Comprehensive Cancer Center, and Michigan Institute for Clinical and Health Research. Dr. Jagsi reported institutional research support from AbbVie, which donated the veliparib used in the study.
Source: Jagsi R et al. J Clin Oncol. 2018 Mar 20. doi: 10.1200/JCO.2017.77.2665.
‘Right to try’ bill passes House
Terminal patients who have exhausted all approved drug options would be able to seek out investigational treatments – even if they do not qualify for clinical trials – under a bill passed in the U.S. House, despite opposition from more than 100 patient and physician groups.
The Trickett Wendler, Frank Mongiello, Jordan McLinn, and Matthew Bellina
For an unapproved drug to be made available to patients, it must have an active application that is not subject to any kind of clinical hold. Sponsors and manufacturers must notify the Food and Drug Administration when an unapproved drug is made available to the patient.
The bill also includes safeguards to prevent manufacturers from purposefully misbranding or mislabeling drugs.
H.R. 5247 provides liability protections to manufacturers, sponsors, physicians, clinical investigators, and hospitals that participate in providing experimental drugs to terminal patients through this new alternative pathway, although it does not shield them from liability stemming from reckless misconduct, gross negligence, or any other intentional violations. It requires sponsors and manufacturers to report all adverse events to the FDA.
It also provides certainty to manufacturers as to how the FDA will use patient outcomes from the use of treatments outside of clinical trials when it is evaluating the applications on these new drugs.
Rep. Michael Burgess (R-Tex.), the House Energy & Commerce Health Subcommittee chairman and a physician, spoke in support of the bill during a debate on the House floor.
“Mr. Speaker, as a physician, I understand that access to investigational drugs and therapies is a deeply personal priority for those seeking treatment for their loved ones with serious, life-threatening conditions,” he said. “To my friends on the other side of the aisle, I have a simple question: Why do you not want to allow these patients to exercise their right to fight for their future?”
Rep. Frank Pallone (D-N.J.), the top-ranking Democrat on the House Energy & Commerce Committee, responded by asking, “if this is such a patient-centered bill, then why does every major patient organization overwhelmingly oppose it?”
In a March 19 letter to congressional leaders, a coalition of more than 100 physician and patient advocacy groups called the alternative pathway laid out in legislation “less safe” for patients than the FDA’s current expanded access process.
“This alternative pathway would allow for a 7-day lag between access to investigational therapies (as well as potential ensuing adverse effects) and FDA notification. FDA also is prohibited from halting access to these experimental therapies short of placing a clinical hold on all clinical research on the therapy in question, which is a blunt and disproportionate measure. The legislation would also remove FDA’s consultation on dosing, route of administration, dosing schedule, and other important safety measures available under FDA’s current expanded access program,” the groups wrote.
The groups that signed the letter included the American Society of Clinical Oncology, the Cystic Fibrosis Foundation, Friends of Cancer Research, the Leukemia & Lymphoma Society, the National Comprehensive Cancer Network, the National Organization for Rare Disorders, the Platelet Disorder Support Association, and Vietnam Veterans of America.
“The current compassionate use program at the Food and Drug Administration does make a good faith effort to help patients who do not qualify for clinical trials,” Rep. Burgess said. “But ‘right to try’ would actually offer patients an alternative pathway to access eligible investigational drugs, so long as they are certified by a physician who is in good standing and abides by the rules laid out in the bill.”
But Rep. Pallone noted that a review by the Government Accountability Office found that the FDA approves 99% of the requests submitted to the agency. Of the nearly 1,700 requests the FDA received in 2017, just 9 were not approved. However, the agency also adjusted applications for 11% of the patients in order to improve patient safety protections and that type of review should be allowed to continue, he said.
Patient groups expressed disappointment following the House vote. “The House has voted for a proposal that would create a less-safe, redundant pathway for accessing investigational therapies outside of clinical trials,” the National Organization for Rare Disorders said in a statement. “We hope the Senate will recognize that patients deserve legislation that will genuinely increase access. For example, senators should focus on legislation that reduces the financial disincentives companies encounter in offering their therapy through expanded access.”
The Senate passed a version of “right to try” in 2017 through the unanimous consent process (S. 204). No schedule has been set yet to either combine the two bills in committee or for the Senate to take up the House bill. President Trump voiced support for “right to try” legislation during his 2018 State of the Union address.
Terminal patients who have exhausted all approved drug options would be able to seek out investigational treatments – even if they do not qualify for clinical trials – under a bill passed in the U.S. House, despite opposition from more than 100 patient and physician groups.
The Trickett Wendler, Frank Mongiello, Jordan McLinn, and Matthew Bellina
For an unapproved drug to be made available to patients, it must have an active application that is not subject to any kind of clinical hold. Sponsors and manufacturers must notify the Food and Drug Administration when an unapproved drug is made available to the patient.
The bill also includes safeguards to prevent manufacturers from purposefully misbranding or mislabeling drugs.
H.R. 5247 provides liability protections to manufacturers, sponsors, physicians, clinical investigators, and hospitals that participate in providing experimental drugs to terminal patients through this new alternative pathway, although it does not shield them from liability stemming from reckless misconduct, gross negligence, or any other intentional violations. It requires sponsors and manufacturers to report all adverse events to the FDA.
It also provides certainty to manufacturers as to how the FDA will use patient outcomes from the use of treatments outside of clinical trials when it is evaluating the applications on these new drugs.
Rep. Michael Burgess (R-Tex.), the House Energy & Commerce Health Subcommittee chairman and a physician, spoke in support of the bill during a debate on the House floor.
“Mr. Speaker, as a physician, I understand that access to investigational drugs and therapies is a deeply personal priority for those seeking treatment for their loved ones with serious, life-threatening conditions,” he said. “To my friends on the other side of the aisle, I have a simple question: Why do you not want to allow these patients to exercise their right to fight for their future?”
Rep. Frank Pallone (D-N.J.), the top-ranking Democrat on the House Energy & Commerce Committee, responded by asking, “if this is such a patient-centered bill, then why does every major patient organization overwhelmingly oppose it?”
In a March 19 letter to congressional leaders, a coalition of more than 100 physician and patient advocacy groups called the alternative pathway laid out in legislation “less safe” for patients than the FDA’s current expanded access process.
“This alternative pathway would allow for a 7-day lag between access to investigational therapies (as well as potential ensuing adverse effects) and FDA notification. FDA also is prohibited from halting access to these experimental therapies short of placing a clinical hold on all clinical research on the therapy in question, which is a blunt and disproportionate measure. The legislation would also remove FDA’s consultation on dosing, route of administration, dosing schedule, and other important safety measures available under FDA’s current expanded access program,” the groups wrote.
The groups that signed the letter included the American Society of Clinical Oncology, the Cystic Fibrosis Foundation, Friends of Cancer Research, the Leukemia & Lymphoma Society, the National Comprehensive Cancer Network, the National Organization for Rare Disorders, the Platelet Disorder Support Association, and Vietnam Veterans of America.
“The current compassionate use program at the Food and Drug Administration does make a good faith effort to help patients who do not qualify for clinical trials,” Rep. Burgess said. “But ‘right to try’ would actually offer patients an alternative pathway to access eligible investigational drugs, so long as they are certified by a physician who is in good standing and abides by the rules laid out in the bill.”
But Rep. Pallone noted that a review by the Government Accountability Office found that the FDA approves 99% of the requests submitted to the agency. Of the nearly 1,700 requests the FDA received in 2017, just 9 were not approved. However, the agency also adjusted applications for 11% of the patients in order to improve patient safety protections and that type of review should be allowed to continue, he said.
Patient groups expressed disappointment following the House vote. “The House has voted for a proposal that would create a less-safe, redundant pathway for accessing investigational therapies outside of clinical trials,” the National Organization for Rare Disorders said in a statement. “We hope the Senate will recognize that patients deserve legislation that will genuinely increase access. For example, senators should focus on legislation that reduces the financial disincentives companies encounter in offering their therapy through expanded access.”
The Senate passed a version of “right to try” in 2017 through the unanimous consent process (S. 204). No schedule has been set yet to either combine the two bills in committee or for the Senate to take up the House bill. President Trump voiced support for “right to try” legislation during his 2018 State of the Union address.
Terminal patients who have exhausted all approved drug options would be able to seek out investigational treatments – even if they do not qualify for clinical trials – under a bill passed in the U.S. House, despite opposition from more than 100 patient and physician groups.
The Trickett Wendler, Frank Mongiello, Jordan McLinn, and Matthew Bellina
For an unapproved drug to be made available to patients, it must have an active application that is not subject to any kind of clinical hold. Sponsors and manufacturers must notify the Food and Drug Administration when an unapproved drug is made available to the patient.
The bill also includes safeguards to prevent manufacturers from purposefully misbranding or mislabeling drugs.
H.R. 5247 provides liability protections to manufacturers, sponsors, physicians, clinical investigators, and hospitals that participate in providing experimental drugs to terminal patients through this new alternative pathway, although it does not shield them from liability stemming from reckless misconduct, gross negligence, or any other intentional violations. It requires sponsors and manufacturers to report all adverse events to the FDA.
It also provides certainty to manufacturers as to how the FDA will use patient outcomes from the use of treatments outside of clinical trials when it is evaluating the applications on these new drugs.
Rep. Michael Burgess (R-Tex.), the House Energy & Commerce Health Subcommittee chairman and a physician, spoke in support of the bill during a debate on the House floor.
“Mr. Speaker, as a physician, I understand that access to investigational drugs and therapies is a deeply personal priority for those seeking treatment for their loved ones with serious, life-threatening conditions,” he said. “To my friends on the other side of the aisle, I have a simple question: Why do you not want to allow these patients to exercise their right to fight for their future?”
Rep. Frank Pallone (D-N.J.), the top-ranking Democrat on the House Energy & Commerce Committee, responded by asking, “if this is such a patient-centered bill, then why does every major patient organization overwhelmingly oppose it?”
In a March 19 letter to congressional leaders, a coalition of more than 100 physician and patient advocacy groups called the alternative pathway laid out in legislation “less safe” for patients than the FDA’s current expanded access process.
“This alternative pathway would allow for a 7-day lag between access to investigational therapies (as well as potential ensuing adverse effects) and FDA notification. FDA also is prohibited from halting access to these experimental therapies short of placing a clinical hold on all clinical research on the therapy in question, which is a blunt and disproportionate measure. The legislation would also remove FDA’s consultation on dosing, route of administration, dosing schedule, and other important safety measures available under FDA’s current expanded access program,” the groups wrote.
The groups that signed the letter included the American Society of Clinical Oncology, the Cystic Fibrosis Foundation, Friends of Cancer Research, the Leukemia & Lymphoma Society, the National Comprehensive Cancer Network, the National Organization for Rare Disorders, the Platelet Disorder Support Association, and Vietnam Veterans of America.
“The current compassionate use program at the Food and Drug Administration does make a good faith effort to help patients who do not qualify for clinical trials,” Rep. Burgess said. “But ‘right to try’ would actually offer patients an alternative pathway to access eligible investigational drugs, so long as they are certified by a physician who is in good standing and abides by the rules laid out in the bill.”
But Rep. Pallone noted that a review by the Government Accountability Office found that the FDA approves 99% of the requests submitted to the agency. Of the nearly 1,700 requests the FDA received in 2017, just 9 were not approved. However, the agency also adjusted applications for 11% of the patients in order to improve patient safety protections and that type of review should be allowed to continue, he said.
Patient groups expressed disappointment following the House vote. “The House has voted for a proposal that would create a less-safe, redundant pathway for accessing investigational therapies outside of clinical trials,” the National Organization for Rare Disorders said in a statement. “We hope the Senate will recognize that patients deserve legislation that will genuinely increase access. For example, senators should focus on legislation that reduces the financial disincentives companies encounter in offering their therapy through expanded access.”
The Senate passed a version of “right to try” in 2017 through the unanimous consent process (S. 204). No schedule has been set yet to either combine the two bills in committee or for the Senate to take up the House bill. President Trump voiced support for “right to try” legislation during his 2018 State of the Union address.
Omalizumab may help with chemotherapy hypersensitivity
ORLANDO – Omalizumab increased reaction-free rapid drug desensitization for patients with hypersensitivity to chemotherapy, according to research presented at the joint congress of the American Academy of Allergy, Asthma, and Immunology and the World Asthma Organization.
“In about 99% of patients, a desensitization protocol will be effective enough to allow patients to receive the relevant chemotherapy treatment,” Dr. Hong said in an interview. “However, in a small minority of patents, no matter what we try, we simply cannot desensitize them – those are the patients we looked at in this study.”
Patients received 300 mg of omalizumab every 4 weeks for three treatment sessions. During the 12-week treatment period, patients continued their normal chemotherapy regimens via rapid drug desensitization following protocols previously published by Castelles et al.
The primary outcome was the number of rapid drug desensitizations that were free from hypersensitivity reactions. In a secondary analysis, the researchers compared results of chemotherapy skin tests taken before and after the trial.
Of the five patients included in the study, four were female, received carboplatin, and had a skin tests, while the fifth patient was male, received rituximab, and did not receive a skin test.
In an intention-to-treat approach, Dr. Hong and his colleagues reported that 33% of all rapid drug desensitizations for omalizumab had no reaction.
In the poster, the researchers noted that these data could be significant because the 95% confidence interval for nonreactivity on omalizumab ranged from 11% to 98%.
“This is a unique situation,” Dr. Hong said in an interview. Because ovarian cancer diagnoses tend to come later in the disease course, patients often can receive extended chemotherapy regimens to treat disease metastases.
He speculated that there could be more uses for omalizumab. “In this study, we looked at the most sensitive patients, but perhaps this drug could have a significant impact on patients with a more mild form of sensitivity,” Dr. Hong said. “Maybe [omalizumab] could take the place of desensitization for patients with a milder sensitivity – we don’t know.”
Dr. Hong reports no relevant financial disclosures. Novartis Pharmaceuticals funded this research and provided omalizumab.
SOURCE: Hong DI et al. AAAAI/WAO Joint Congress, Poster L33.
ORLANDO – Omalizumab increased reaction-free rapid drug desensitization for patients with hypersensitivity to chemotherapy, according to research presented at the joint congress of the American Academy of Allergy, Asthma, and Immunology and the World Asthma Organization.
“In about 99% of patients, a desensitization protocol will be effective enough to allow patients to receive the relevant chemotherapy treatment,” Dr. Hong said in an interview. “However, in a small minority of patents, no matter what we try, we simply cannot desensitize them – those are the patients we looked at in this study.”
Patients received 300 mg of omalizumab every 4 weeks for three treatment sessions. During the 12-week treatment period, patients continued their normal chemotherapy regimens via rapid drug desensitization following protocols previously published by Castelles et al.
The primary outcome was the number of rapid drug desensitizations that were free from hypersensitivity reactions. In a secondary analysis, the researchers compared results of chemotherapy skin tests taken before and after the trial.
Of the five patients included in the study, four were female, received carboplatin, and had a skin tests, while the fifth patient was male, received rituximab, and did not receive a skin test.
In an intention-to-treat approach, Dr. Hong and his colleagues reported that 33% of all rapid drug desensitizations for omalizumab had no reaction.
In the poster, the researchers noted that these data could be significant because the 95% confidence interval for nonreactivity on omalizumab ranged from 11% to 98%.
“This is a unique situation,” Dr. Hong said in an interview. Because ovarian cancer diagnoses tend to come later in the disease course, patients often can receive extended chemotherapy regimens to treat disease metastases.
He speculated that there could be more uses for omalizumab. “In this study, we looked at the most sensitive patients, but perhaps this drug could have a significant impact on patients with a more mild form of sensitivity,” Dr. Hong said. “Maybe [omalizumab] could take the place of desensitization for patients with a milder sensitivity – we don’t know.”
Dr. Hong reports no relevant financial disclosures. Novartis Pharmaceuticals funded this research and provided omalizumab.
SOURCE: Hong DI et al. AAAAI/WAO Joint Congress, Poster L33.
ORLANDO – Omalizumab increased reaction-free rapid drug desensitization for patients with hypersensitivity to chemotherapy, according to research presented at the joint congress of the American Academy of Allergy, Asthma, and Immunology and the World Asthma Organization.
“In about 99% of patients, a desensitization protocol will be effective enough to allow patients to receive the relevant chemotherapy treatment,” Dr. Hong said in an interview. “However, in a small minority of patents, no matter what we try, we simply cannot desensitize them – those are the patients we looked at in this study.”
Patients received 300 mg of omalizumab every 4 weeks for three treatment sessions. During the 12-week treatment period, patients continued their normal chemotherapy regimens via rapid drug desensitization following protocols previously published by Castelles et al.
The primary outcome was the number of rapid drug desensitizations that were free from hypersensitivity reactions. In a secondary analysis, the researchers compared results of chemotherapy skin tests taken before and after the trial.
Of the five patients included in the study, four were female, received carboplatin, and had a skin tests, while the fifth patient was male, received rituximab, and did not receive a skin test.
In an intention-to-treat approach, Dr. Hong and his colleagues reported that 33% of all rapid drug desensitizations for omalizumab had no reaction.
In the poster, the researchers noted that these data could be significant because the 95% confidence interval for nonreactivity on omalizumab ranged from 11% to 98%.
“This is a unique situation,” Dr. Hong said in an interview. Because ovarian cancer diagnoses tend to come later in the disease course, patients often can receive extended chemotherapy regimens to treat disease metastases.
He speculated that there could be more uses for omalizumab. “In this study, we looked at the most sensitive patients, but perhaps this drug could have a significant impact on patients with a more mild form of sensitivity,” Dr. Hong said. “Maybe [omalizumab] could take the place of desensitization for patients with a milder sensitivity – we don’t know.”
Dr. Hong reports no relevant financial disclosures. Novartis Pharmaceuticals funded this research and provided omalizumab.
SOURCE: Hong DI et al. AAAAI/WAO Joint Congress, Poster L33.
REPORTING FROM AAAAI/WAO JOINT CONGRESS
Key clinical point: Omalizumab increased reaction-free rapid drug desensitization for patients with hypersensitivity to chemotherapy.
Major finding: Despite low enrollment, omalizumab appeared to significantly increase the number of reaction-free rapid drug desensitizations.
Data source: A 12-week, open-label, non-randomized trial of omalizumab.
Disclosures: Dr. Hong reports no relevant financial disclosures. Novartis Pharmaceuticals funded the research and provided omalizumab.
Source: Hong DI et al. AAAAI/WAO Joint Congress, Poster L33.
Atraumatic splenic rupture as an initial presentation of chronic myelogenous leukemia
Chronic myelogenous leukemia (CML) is a myeloproliferative neoplasm associated with the fusion of the BCR gene located on chromosome 22 and the ABL1 gene on chromosome 9. The fusion results in a reciprocal translocation between chromosomes 9 and 22, leading to the formation of the Philadelphia (Ph) chromosome found in 90%-95% of patients with CML. The incidence of CML is 1.5 per 100,000 people per year, with a male predominance and an average age at diagnosis of 64.1
About 85%-90% of newly diagnosed patients present in the chronic phase and therefore many of them are asymptomatic at the time of diagnosis. If symptoms are present, they often include fatigue, malaise, unintentional weight loss, early satiety, or left upper quadrant pain. Progression of the disease is associated with worsening symptoms such as unexplained fever, significant weight loss, bone or joint pain, bleeding, thrombosis, and infections suggestive of transformation to the accelerated phase or blast crisis. Physical exam findings most commonly include splenomegaly and occasionally mild hepatomegaly.
Atraumatic splenic rupture is a rare complication of this hematologic malignancy, and there are almost no reported cases of CML as the underlying cause.2-4 Here we present the case of a man with sudden-onset generalized abdominal pain and leukocytosis. A computed-tomography scan showed splenic rupture, and the patient was taken for emergency splenectomy. The patient was subsequently positive for t(9,22)(q34;q11.2).
Case presentation and summary
A 59-year-old white man with a history of hypertension and kidney stones presented to a community emergency department with a chief complaint of abdominal pain. About 30 minutes before his arrival, the patient had woken up from sleep with generalized, nonradiating, abdominal pain, which he described as “like my previous kidney stones.” He also reported worsening dyspnea, nausea without vomiting, and lightheadedness without loss of consciousness. The remainder of the review of systems was negative. A physical exam revealed that he was in moderate distress with clear lung fields and had tachycardia without murmur, no CVA tenderness, and a diffusely tender abdomen.
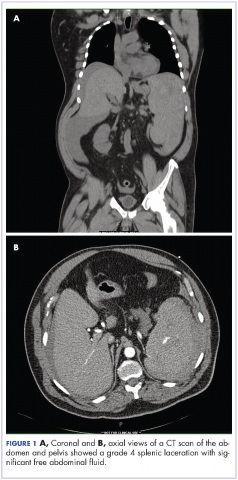
Complete blood count with differential showed leukocytosis (109.1 x 103/uL), normocytic anemia (8.1 g/dL), thrombocytopenia (100,000 cells/uL), neutrophils (71.06 cells/uL), bands (27.13 cells/uL), and monocytes (11.63 cells/uL). A CT scan of the abdomen and pelvis showed a grade 4 splenic laceration with significant free abdominal fluid (Figure 1).
The patient was taken to the operating room where he underwent a splenectomy which was complicated by partial gastrectomy and partial omentectomy. He remained intubated on mechanical ventilation in the intensive care for 7 days. His progress was complicated by profound hypotension that required significant fluid administration and ultimately multiple pressors for blood pressure support. Hypotensive shock was beginning to improve on day 3 and was completely resolved by day 5. The patient underwent continuous positive airway pressure (CPAP) trials on day 6 and was successfully extubated on day 7.
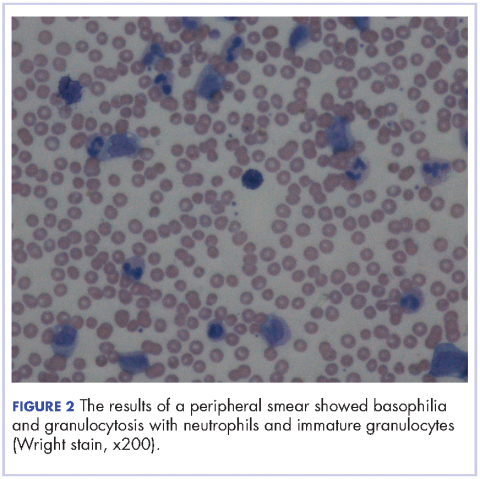
After extubation a more thorough history could be obtained from the patient. He denied any history of weight loss, night sweats, or fatigue. Patient denied any known family history of hematologic malignancies. His peripheral smear showed basophilia and granulocytosis with neutrophils and immature granulocytes (Figure 2). The patient was evaluated by the hematology service and was started on allopurinol and hydroxyurea for presumed hematologic malignancy. He was given the meningococcus and streptococcus pneumoniae vaccine and was discharged home in stable condition on day eleven. Patient was subsequently positive for t(9,22)(q34;q11.2) and was started on imatinib. He has continued to follow in the clinic and is currently in remission.
Discussion
CML has a triphasic clinical course and treatment is based on the specific disease phase. The 3 phases of the disease include the chronic (more indolent) phase, accelerated (more aggressive) phase, and blast crisis. If the disease is left untreated, it will inevitably transition from a chronic to an accelerated phase and finally to blast crisis within a median time of 4 years.
The chronic phase is the most common, representing 85% of diagnoses. Patients can be asymptomatic and many in this phase will be diagnosed by routine lab testing.5 According to the World Health Organization, the accelerated phase is defined as CML patients with one of the following: 10%-19% blasts, basophils ≥20%, platelets <100,000/microL or >1,000,000/microL, unresponsive to therapy, splenomegaly unresponsive to therapy, an increasing white cell count unresponsive to therapy, or cytogenetic evolution.6 Blast crisis is the most aggressive phase and is usually defined by ≥20% blasts, large foci or clusters of blasts on the bone marrow biopsy, or the presence of extramedullary blastic infiltrates.7,8
The diagnosis of CML should be suspected in the presence of distinct lab abnormalities in the peripheral blood. These include elevated white blood cell counts with a median count of 100,000 cells/microL, elevated platelet counts, and a mild normocytic normochromic anemia. Platelet counts of 600,000 or greater have been seen in 15%-30% of patients at the time of diagnosis. The white count differential can show a variety of cells but there will be a notably greater percentage of myelocytes than metamyelocytes. Bone marrow biopsy will reveal increased cellularity, normal to slightly elevated percentage of blasts, and reticulin fibrosis. The diagnosis should be confirmed by the presence of the Philadelphia chromosome either by cytogenetics, fluorescence in situ hybridization, or reverse-transcription polymerase chain reaction (RT-PCR). The Philadelphia chromosome is found in 90%-95% of patients with CML. Most of the remaining patients will have other translocations, but a small minority will have no detectable genetic abnormalities and those patients are known as Ph-negative.9
Treatment options for CML include potential cure with allogeneic hematopoietic stem-cell transplant (HSCT) or disease control using tyrosine kinase inhibitors (TKIs). TKIs are the initial treatment of choice for newly diagnosed patients and are able to produce long-term remission in most patients. The drugs in this category include imatinib, dasatinib, and nilotinib. They work by inhibiting the Bcr-Abl tyrosine kinase, thereby blocking proliferation and inducing apoptosis in Bcr-Abl-positive cells. The majority of patients with chronic-phase CML will have an excellent response to initial treatment with a TKI. It is critical to follow these patients on a regular basis and monitor their disease status. Although the gold standard for assessing cytogenetic response is cytogenetic analysis of a bone marrow biopsy, more sensitive methods such as quantitative PCR using peripheral blood are now available, thereby minimizing the need for bone marrow biopsy. Patients in the accelerated phase are more difficult to manage because they are resistant to most forms of treatment and have short-lived responses to TKI therapy. These patients should strongly be considered for transplantation. Patients in blast crisis have aggressive disease that is more complex and requires more extensive testing. These patients should ideally be treated at tertiary care centers and treatment often involves chemotherapy in addition to TKI therapy usually followed by HSCT.
Atraumatic splenic rupture (ASR) presents similarly to traumatic splenic rupture with typical symptoms being acute onset of upper abdominal, left chest wall, or left shoulder pain (Kehr’s sign) but without a known history of trauma. Quick recognition and surgical intervention represent the best means of definitive care.10 Renzulli and colleagues conducted a literature review for all ASR cases from 1980-2008, examining 632 publications representing 845 cases. They examined the cases using logistic regression analysis to better define the clinicopathology behind ASR. The reported causes of ASR are neoplastic processes (30.3%), infectious (27.3%), inflammatory noninfectious (20.0%), drug- and treatment-related (9.2%), mechanical (6.8%), and normal spleen (6.4%). Treatment included total splenectomy in 84.1% of cases, organ-preserving surgery in 1.2%, and conservative measures in 14.7%. They reported an ASR-related mortality of 12.2%, with being older than 40 and neoplastic disorders associated with increased mortality – although male sex and splenomegaly have also been reported.11-13 Thomas and colleagues have reported on 48 cases of ASR related to hematologic malignancy showing acute myeloid leukemia being the most common cause (21%), followed by acute lymphoblastic leukemia (19%).2
Hematologic malignancies commonly cause splenic engorgement and pain although splenic rupture is an extremely rare event. Recent literature review has shown fewer than a thousand reported cases since 1980.4 There far fewer reported cases of ASR being related to CML, with most being reported as a complication.3,14 Based on our review, we could identify only a handful cases of CML with ASR being the initial symptom. These include a patient with Ph-negative CML and ASR following blast crisis, a patient with Phil-negative BCR-ABL-positive essential thrombocythemia, several cases in which the patient ultimately died, and 1 in which the patient survived into remission.4,14-16 Our case is different because the patient was ultimately positive for t(9,22)(q34;q11.2) and although he experienced multiple complications, he is currently functioning at his baseline and in remission. We hope this case will remind others that CML should be considered in the differential diagnosis of patients ASR.
1. American Cancer Society. Cancer Facts & Figures 2015. Atlanta, Ga: American Cancer Society; 2015.
2. Bauer TW, Haskins GE, Armitage JO. Splenic rupture in patients with hematologic malignancies. Cancer. 1981;48:2729-2733.
3. Giagounidis AA, Burk M, Meckenstock G, Koch AJ, Schneider W. Pathologic rupture of the spleen in hematologic malignancies: two additional cases. Ann Hematol. 1996;73(6):297-302.
4. Goodard SL, Chesney AE, Reis MD, et al. Pathologic splenic rupture: a rare complication of chronic myelomonocytic leukemia. Am J Hematology. 2007;82:405-408.
5. Faderl S, Talpaz M, Estrov Z, et al. The biology of chronic myeloid leukemia. N Engl J Med. 1999;341:164-172.
6. Cortes JE, Talpaz M, O’Brien S, et al. Staging of chronic myeloid leukemia in the imatinib era: an evaluation of the World Health Organization proposal. Cancer. 2006;106:1306-1315.
7. Vardiman JW, Harris NL, Brunning RD. The World Health Organization (WHO) classification of the myeloid neoplasms. Blood. 2002;100:2292-2302.
8. Kantarjian HM, O’Brien S, Cortes J, et al. Results of decitabine (5-aza-2’deoxycytidine) therapy in 130 patients with chronic myelogenous leukemia. Cancer.2003; 98:522-528.
9. Swerdlow SH, Campo E, Harris NL, et al, editors. WHO classification of tumours of haematopoietic and lymphoid tissues. Lyon, France: IARC Press; 2008.
10. Maung A, KaplanL. Management of splenic injury in the adult trauma patient. In: UpToDate, Basow DS (ed), Waltham, MA, 2013.
11. Renzulli P, Hostettler A, Schoepfer AM, Gloor B, Candinas D. Systematic review of atraumatic splenic rupture. Br J Surg. 2009;8(10):1114-1121.
12. Hehlmann R, Heimpel H, Hasford J, et al. Randomized comparison of interferon-alpha with busulfan and hydroxyurea in chronic myelogenous leukemia. The German CML Study Group. Blood. 1994;84:4064-4077.
13. Cortes J, Kantarjian H. How I treat newly diagnosed chronic phase CML. Blood. 2012;120:1390-1397.
14. Nestok BR, Goldstein JD, Lipkovic P. Splenic rupture as a cause of sudden death in undiagnosed chronic myelogenous leukemia. Am J Forensic Med Pathol. 1988;9:241-245.
15. Sachithanandan A, Gleadhil I, Alexander HD, Morris TC. Spontaneous splenic rupture in atypical (Philadelphia chromosome negative) chronic myeloid leukemia following blastic crisis. Ir Med J. 2003;96(6):181-182.
16. Chim CS, Kwong YL, Shek TW, Ma SK, Ooi GC. Splenic rupture as the presenting symptom of blastic crisis in a patient with Philadelphia-negative, BCR-ABL-positive ET. Am J Hematology. 2001;66:70-71.
Chronic myelogenous leukemia (CML) is a myeloproliferative neoplasm associated with the fusion of the BCR gene located on chromosome 22 and the ABL1 gene on chromosome 9. The fusion results in a reciprocal translocation between chromosomes 9 and 22, leading to the formation of the Philadelphia (Ph) chromosome found in 90%-95% of patients with CML. The incidence of CML is 1.5 per 100,000 people per year, with a male predominance and an average age at diagnosis of 64.1
About 85%-90% of newly diagnosed patients present in the chronic phase and therefore many of them are asymptomatic at the time of diagnosis. If symptoms are present, they often include fatigue, malaise, unintentional weight loss, early satiety, or left upper quadrant pain. Progression of the disease is associated with worsening symptoms such as unexplained fever, significant weight loss, bone or joint pain, bleeding, thrombosis, and infections suggestive of transformation to the accelerated phase or blast crisis. Physical exam findings most commonly include splenomegaly and occasionally mild hepatomegaly.
Atraumatic splenic rupture is a rare complication of this hematologic malignancy, and there are almost no reported cases of CML as the underlying cause.2-4 Here we present the case of a man with sudden-onset generalized abdominal pain and leukocytosis. A computed-tomography scan showed splenic rupture, and the patient was taken for emergency splenectomy. The patient was subsequently positive for t(9,22)(q34;q11.2).
Case presentation and summary
A 59-year-old white man with a history of hypertension and kidney stones presented to a community emergency department with a chief complaint of abdominal pain. About 30 minutes before his arrival, the patient had woken up from sleep with generalized, nonradiating, abdominal pain, which he described as “like my previous kidney stones.” He also reported worsening dyspnea, nausea without vomiting, and lightheadedness without loss of consciousness. The remainder of the review of systems was negative. A physical exam revealed that he was in moderate distress with clear lung fields and had tachycardia without murmur, no CVA tenderness, and a diffusely tender abdomen.
Complete blood count with differential showed leukocytosis (109.1 x 103/uL), normocytic anemia (8.1 g/dL), thrombocytopenia (100,000 cells/uL), neutrophils (71.06 cells/uL), bands (27.13 cells/uL), and monocytes (11.63 cells/uL). A CT scan of the abdomen and pelvis showed a grade 4 splenic laceration with significant free abdominal fluid (Figure 1).
The patient was taken to the operating room where he underwent a splenectomy which was complicated by partial gastrectomy and partial omentectomy. He remained intubated on mechanical ventilation in the intensive care for 7 days. His progress was complicated by profound hypotension that required significant fluid administration and ultimately multiple pressors for blood pressure support. Hypotensive shock was beginning to improve on day 3 and was completely resolved by day 5. The patient underwent continuous positive airway pressure (CPAP) trials on day 6 and was successfully extubated on day 7.
After extubation a more thorough history could be obtained from the patient. He denied any history of weight loss, night sweats, or fatigue. Patient denied any known family history of hematologic malignancies. His peripheral smear showed basophilia and granulocytosis with neutrophils and immature granulocytes (Figure 2). The patient was evaluated by the hematology service and was started on allopurinol and hydroxyurea for presumed hematologic malignancy. He was given the meningococcus and streptococcus pneumoniae vaccine and was discharged home in stable condition on day eleven. Patient was subsequently positive for t(9,22)(q34;q11.2) and was started on imatinib. He has continued to follow in the clinic and is currently in remission.
Discussion
CML has a triphasic clinical course and treatment is based on the specific disease phase. The 3 phases of the disease include the chronic (more indolent) phase, accelerated (more aggressive) phase, and blast crisis. If the disease is left untreated, it will inevitably transition from a chronic to an accelerated phase and finally to blast crisis within a median time of 4 years.
The chronic phase is the most common, representing 85% of diagnoses. Patients can be asymptomatic and many in this phase will be diagnosed by routine lab testing.5 According to the World Health Organization, the accelerated phase is defined as CML patients with one of the following: 10%-19% blasts, basophils ≥20%, platelets <100,000/microL or >1,000,000/microL, unresponsive to therapy, splenomegaly unresponsive to therapy, an increasing white cell count unresponsive to therapy, or cytogenetic evolution.6 Blast crisis is the most aggressive phase and is usually defined by ≥20% blasts, large foci or clusters of blasts on the bone marrow biopsy, or the presence of extramedullary blastic infiltrates.7,8
The diagnosis of CML should be suspected in the presence of distinct lab abnormalities in the peripheral blood. These include elevated white blood cell counts with a median count of 100,000 cells/microL, elevated platelet counts, and a mild normocytic normochromic anemia. Platelet counts of 600,000 or greater have been seen in 15%-30% of patients at the time of diagnosis. The white count differential can show a variety of cells but there will be a notably greater percentage of myelocytes than metamyelocytes. Bone marrow biopsy will reveal increased cellularity, normal to slightly elevated percentage of blasts, and reticulin fibrosis. The diagnosis should be confirmed by the presence of the Philadelphia chromosome either by cytogenetics, fluorescence in situ hybridization, or reverse-transcription polymerase chain reaction (RT-PCR). The Philadelphia chromosome is found in 90%-95% of patients with CML. Most of the remaining patients will have other translocations, but a small minority will have no detectable genetic abnormalities and those patients are known as Ph-negative.9
Treatment options for CML include potential cure with allogeneic hematopoietic stem-cell transplant (HSCT) or disease control using tyrosine kinase inhibitors (TKIs). TKIs are the initial treatment of choice for newly diagnosed patients and are able to produce long-term remission in most patients. The drugs in this category include imatinib, dasatinib, and nilotinib. They work by inhibiting the Bcr-Abl tyrosine kinase, thereby blocking proliferation and inducing apoptosis in Bcr-Abl-positive cells. The majority of patients with chronic-phase CML will have an excellent response to initial treatment with a TKI. It is critical to follow these patients on a regular basis and monitor their disease status. Although the gold standard for assessing cytogenetic response is cytogenetic analysis of a bone marrow biopsy, more sensitive methods such as quantitative PCR using peripheral blood are now available, thereby minimizing the need for bone marrow biopsy. Patients in the accelerated phase are more difficult to manage because they are resistant to most forms of treatment and have short-lived responses to TKI therapy. These patients should strongly be considered for transplantation. Patients in blast crisis have aggressive disease that is more complex and requires more extensive testing. These patients should ideally be treated at tertiary care centers and treatment often involves chemotherapy in addition to TKI therapy usually followed by HSCT.
Atraumatic splenic rupture (ASR) presents similarly to traumatic splenic rupture with typical symptoms being acute onset of upper abdominal, left chest wall, or left shoulder pain (Kehr’s sign) but without a known history of trauma. Quick recognition and surgical intervention represent the best means of definitive care.10 Renzulli and colleagues conducted a literature review for all ASR cases from 1980-2008, examining 632 publications representing 845 cases. They examined the cases using logistic regression analysis to better define the clinicopathology behind ASR. The reported causes of ASR are neoplastic processes (30.3%), infectious (27.3%), inflammatory noninfectious (20.0%), drug- and treatment-related (9.2%), mechanical (6.8%), and normal spleen (6.4%). Treatment included total splenectomy in 84.1% of cases, organ-preserving surgery in 1.2%, and conservative measures in 14.7%. They reported an ASR-related mortality of 12.2%, with being older than 40 and neoplastic disorders associated with increased mortality – although male sex and splenomegaly have also been reported.11-13 Thomas and colleagues have reported on 48 cases of ASR related to hematologic malignancy showing acute myeloid leukemia being the most common cause (21%), followed by acute lymphoblastic leukemia (19%).2
Hematologic malignancies commonly cause splenic engorgement and pain although splenic rupture is an extremely rare event. Recent literature review has shown fewer than a thousand reported cases since 1980.4 There far fewer reported cases of ASR being related to CML, with most being reported as a complication.3,14 Based on our review, we could identify only a handful cases of CML with ASR being the initial symptom. These include a patient with Ph-negative CML and ASR following blast crisis, a patient with Phil-negative BCR-ABL-positive essential thrombocythemia, several cases in which the patient ultimately died, and 1 in which the patient survived into remission.4,14-16 Our case is different because the patient was ultimately positive for t(9,22)(q34;q11.2) and although he experienced multiple complications, he is currently functioning at his baseline and in remission. We hope this case will remind others that CML should be considered in the differential diagnosis of patients ASR.
Chronic myelogenous leukemia (CML) is a myeloproliferative neoplasm associated with the fusion of the BCR gene located on chromosome 22 and the ABL1 gene on chromosome 9. The fusion results in a reciprocal translocation between chromosomes 9 and 22, leading to the formation of the Philadelphia (Ph) chromosome found in 90%-95% of patients with CML. The incidence of CML is 1.5 per 100,000 people per year, with a male predominance and an average age at diagnosis of 64.1
About 85%-90% of newly diagnosed patients present in the chronic phase and therefore many of them are asymptomatic at the time of diagnosis. If symptoms are present, they often include fatigue, malaise, unintentional weight loss, early satiety, or left upper quadrant pain. Progression of the disease is associated with worsening symptoms such as unexplained fever, significant weight loss, bone or joint pain, bleeding, thrombosis, and infections suggestive of transformation to the accelerated phase or blast crisis. Physical exam findings most commonly include splenomegaly and occasionally mild hepatomegaly.
Atraumatic splenic rupture is a rare complication of this hematologic malignancy, and there are almost no reported cases of CML as the underlying cause.2-4 Here we present the case of a man with sudden-onset generalized abdominal pain and leukocytosis. A computed-tomography scan showed splenic rupture, and the patient was taken for emergency splenectomy. The patient was subsequently positive for t(9,22)(q34;q11.2).
Case presentation and summary
A 59-year-old white man with a history of hypertension and kidney stones presented to a community emergency department with a chief complaint of abdominal pain. About 30 minutes before his arrival, the patient had woken up from sleep with generalized, nonradiating, abdominal pain, which he described as “like my previous kidney stones.” He also reported worsening dyspnea, nausea without vomiting, and lightheadedness without loss of consciousness. The remainder of the review of systems was negative. A physical exam revealed that he was in moderate distress with clear lung fields and had tachycardia without murmur, no CVA tenderness, and a diffusely tender abdomen.
Complete blood count with differential showed leukocytosis (109.1 x 103/uL), normocytic anemia (8.1 g/dL), thrombocytopenia (100,000 cells/uL), neutrophils (71.06 cells/uL), bands (27.13 cells/uL), and monocytes (11.63 cells/uL). A CT scan of the abdomen and pelvis showed a grade 4 splenic laceration with significant free abdominal fluid (Figure 1).
The patient was taken to the operating room where he underwent a splenectomy which was complicated by partial gastrectomy and partial omentectomy. He remained intubated on mechanical ventilation in the intensive care for 7 days. His progress was complicated by profound hypotension that required significant fluid administration and ultimately multiple pressors for blood pressure support. Hypotensive shock was beginning to improve on day 3 and was completely resolved by day 5. The patient underwent continuous positive airway pressure (CPAP) trials on day 6 and was successfully extubated on day 7.
After extubation a more thorough history could be obtained from the patient. He denied any history of weight loss, night sweats, or fatigue. Patient denied any known family history of hematologic malignancies. His peripheral smear showed basophilia and granulocytosis with neutrophils and immature granulocytes (Figure 2). The patient was evaluated by the hematology service and was started on allopurinol and hydroxyurea for presumed hematologic malignancy. He was given the meningococcus and streptococcus pneumoniae vaccine and was discharged home in stable condition on day eleven. Patient was subsequently positive for t(9,22)(q34;q11.2) and was started on imatinib. He has continued to follow in the clinic and is currently in remission.
Discussion
CML has a triphasic clinical course and treatment is based on the specific disease phase. The 3 phases of the disease include the chronic (more indolent) phase, accelerated (more aggressive) phase, and blast crisis. If the disease is left untreated, it will inevitably transition from a chronic to an accelerated phase and finally to blast crisis within a median time of 4 years.
The chronic phase is the most common, representing 85% of diagnoses. Patients can be asymptomatic and many in this phase will be diagnosed by routine lab testing.5 According to the World Health Organization, the accelerated phase is defined as CML patients with one of the following: 10%-19% blasts, basophils ≥20%, platelets <100,000/microL or >1,000,000/microL, unresponsive to therapy, splenomegaly unresponsive to therapy, an increasing white cell count unresponsive to therapy, or cytogenetic evolution.6 Blast crisis is the most aggressive phase and is usually defined by ≥20% blasts, large foci or clusters of blasts on the bone marrow biopsy, or the presence of extramedullary blastic infiltrates.7,8
The diagnosis of CML should be suspected in the presence of distinct lab abnormalities in the peripheral blood. These include elevated white blood cell counts with a median count of 100,000 cells/microL, elevated platelet counts, and a mild normocytic normochromic anemia. Platelet counts of 600,000 or greater have been seen in 15%-30% of patients at the time of diagnosis. The white count differential can show a variety of cells but there will be a notably greater percentage of myelocytes than metamyelocytes. Bone marrow biopsy will reveal increased cellularity, normal to slightly elevated percentage of blasts, and reticulin fibrosis. The diagnosis should be confirmed by the presence of the Philadelphia chromosome either by cytogenetics, fluorescence in situ hybridization, or reverse-transcription polymerase chain reaction (RT-PCR). The Philadelphia chromosome is found in 90%-95% of patients with CML. Most of the remaining patients will have other translocations, but a small minority will have no detectable genetic abnormalities and those patients are known as Ph-negative.9
Treatment options for CML include potential cure with allogeneic hematopoietic stem-cell transplant (HSCT) or disease control using tyrosine kinase inhibitors (TKIs). TKIs are the initial treatment of choice for newly diagnosed patients and are able to produce long-term remission in most patients. The drugs in this category include imatinib, dasatinib, and nilotinib. They work by inhibiting the Bcr-Abl tyrosine kinase, thereby blocking proliferation and inducing apoptosis in Bcr-Abl-positive cells. The majority of patients with chronic-phase CML will have an excellent response to initial treatment with a TKI. It is critical to follow these patients on a regular basis and monitor their disease status. Although the gold standard for assessing cytogenetic response is cytogenetic analysis of a bone marrow biopsy, more sensitive methods such as quantitative PCR using peripheral blood are now available, thereby minimizing the need for bone marrow biopsy. Patients in the accelerated phase are more difficult to manage because they are resistant to most forms of treatment and have short-lived responses to TKI therapy. These patients should strongly be considered for transplantation. Patients in blast crisis have aggressive disease that is more complex and requires more extensive testing. These patients should ideally be treated at tertiary care centers and treatment often involves chemotherapy in addition to TKI therapy usually followed by HSCT.
Atraumatic splenic rupture (ASR) presents similarly to traumatic splenic rupture with typical symptoms being acute onset of upper abdominal, left chest wall, or left shoulder pain (Kehr’s sign) but without a known history of trauma. Quick recognition and surgical intervention represent the best means of definitive care.10 Renzulli and colleagues conducted a literature review for all ASR cases from 1980-2008, examining 632 publications representing 845 cases. They examined the cases using logistic regression analysis to better define the clinicopathology behind ASR. The reported causes of ASR are neoplastic processes (30.3%), infectious (27.3%), inflammatory noninfectious (20.0%), drug- and treatment-related (9.2%), mechanical (6.8%), and normal spleen (6.4%). Treatment included total splenectomy in 84.1% of cases, organ-preserving surgery in 1.2%, and conservative measures in 14.7%. They reported an ASR-related mortality of 12.2%, with being older than 40 and neoplastic disorders associated with increased mortality – although male sex and splenomegaly have also been reported.11-13 Thomas and colleagues have reported on 48 cases of ASR related to hematologic malignancy showing acute myeloid leukemia being the most common cause (21%), followed by acute lymphoblastic leukemia (19%).2
Hematologic malignancies commonly cause splenic engorgement and pain although splenic rupture is an extremely rare event. Recent literature review has shown fewer than a thousand reported cases since 1980.4 There far fewer reported cases of ASR being related to CML, with most being reported as a complication.3,14 Based on our review, we could identify only a handful cases of CML with ASR being the initial symptom. These include a patient with Ph-negative CML and ASR following blast crisis, a patient with Phil-negative BCR-ABL-positive essential thrombocythemia, several cases in which the patient ultimately died, and 1 in which the patient survived into remission.4,14-16 Our case is different because the patient was ultimately positive for t(9,22)(q34;q11.2) and although he experienced multiple complications, he is currently functioning at his baseline and in remission. We hope this case will remind others that CML should be considered in the differential diagnosis of patients ASR.
1. American Cancer Society. Cancer Facts & Figures 2015. Atlanta, Ga: American Cancer Society; 2015.
2. Bauer TW, Haskins GE, Armitage JO. Splenic rupture in patients with hematologic malignancies. Cancer. 1981;48:2729-2733.
3. Giagounidis AA, Burk M, Meckenstock G, Koch AJ, Schneider W. Pathologic rupture of the spleen in hematologic malignancies: two additional cases. Ann Hematol. 1996;73(6):297-302.
4. Goodard SL, Chesney AE, Reis MD, et al. Pathologic splenic rupture: a rare complication of chronic myelomonocytic leukemia. Am J Hematology. 2007;82:405-408.
5. Faderl S, Talpaz M, Estrov Z, et al. The biology of chronic myeloid leukemia. N Engl J Med. 1999;341:164-172.
6. Cortes JE, Talpaz M, O’Brien S, et al. Staging of chronic myeloid leukemia in the imatinib era: an evaluation of the World Health Organization proposal. Cancer. 2006;106:1306-1315.
7. Vardiman JW, Harris NL, Brunning RD. The World Health Organization (WHO) classification of the myeloid neoplasms. Blood. 2002;100:2292-2302.
8. Kantarjian HM, O’Brien S, Cortes J, et al. Results of decitabine (5-aza-2’deoxycytidine) therapy in 130 patients with chronic myelogenous leukemia. Cancer.2003; 98:522-528.
9. Swerdlow SH, Campo E, Harris NL, et al, editors. WHO classification of tumours of haematopoietic and lymphoid tissues. Lyon, France: IARC Press; 2008.
10. Maung A, KaplanL. Management of splenic injury in the adult trauma patient. In: UpToDate, Basow DS (ed), Waltham, MA, 2013.
11. Renzulli P, Hostettler A, Schoepfer AM, Gloor B, Candinas D. Systematic review of atraumatic splenic rupture. Br J Surg. 2009;8(10):1114-1121.
12. Hehlmann R, Heimpel H, Hasford J, et al. Randomized comparison of interferon-alpha with busulfan and hydroxyurea in chronic myelogenous leukemia. The German CML Study Group. Blood. 1994;84:4064-4077.
13. Cortes J, Kantarjian H. How I treat newly diagnosed chronic phase CML. Blood. 2012;120:1390-1397.
14. Nestok BR, Goldstein JD, Lipkovic P. Splenic rupture as a cause of sudden death in undiagnosed chronic myelogenous leukemia. Am J Forensic Med Pathol. 1988;9:241-245.
15. Sachithanandan A, Gleadhil I, Alexander HD, Morris TC. Spontaneous splenic rupture in atypical (Philadelphia chromosome negative) chronic myeloid leukemia following blastic crisis. Ir Med J. 2003;96(6):181-182.
16. Chim CS, Kwong YL, Shek TW, Ma SK, Ooi GC. Splenic rupture as the presenting symptom of blastic crisis in a patient with Philadelphia-negative, BCR-ABL-positive ET. Am J Hematology. 2001;66:70-71.
1. American Cancer Society. Cancer Facts & Figures 2015. Atlanta, Ga: American Cancer Society; 2015.
2. Bauer TW, Haskins GE, Armitage JO. Splenic rupture in patients with hematologic malignancies. Cancer. 1981;48:2729-2733.
3. Giagounidis AA, Burk M, Meckenstock G, Koch AJ, Schneider W. Pathologic rupture of the spleen in hematologic malignancies: two additional cases. Ann Hematol. 1996;73(6):297-302.
4. Goodard SL, Chesney AE, Reis MD, et al. Pathologic splenic rupture: a rare complication of chronic myelomonocytic leukemia. Am J Hematology. 2007;82:405-408.
5. Faderl S, Talpaz M, Estrov Z, et al. The biology of chronic myeloid leukemia. N Engl J Med. 1999;341:164-172.
6. Cortes JE, Talpaz M, O’Brien S, et al. Staging of chronic myeloid leukemia in the imatinib era: an evaluation of the World Health Organization proposal. Cancer. 2006;106:1306-1315.
7. Vardiman JW, Harris NL, Brunning RD. The World Health Organization (WHO) classification of the myeloid neoplasms. Blood. 2002;100:2292-2302.
8. Kantarjian HM, O’Brien S, Cortes J, et al. Results of decitabine (5-aza-2’deoxycytidine) therapy in 130 patients with chronic myelogenous leukemia. Cancer.2003; 98:522-528.
9. Swerdlow SH, Campo E, Harris NL, et al, editors. WHO classification of tumours of haematopoietic and lymphoid tissues. Lyon, France: IARC Press; 2008.
10. Maung A, KaplanL. Management of splenic injury in the adult trauma patient. In: UpToDate, Basow DS (ed), Waltham, MA, 2013.
11. Renzulli P, Hostettler A, Schoepfer AM, Gloor B, Candinas D. Systematic review of atraumatic splenic rupture. Br J Surg. 2009;8(10):1114-1121.
12. Hehlmann R, Heimpel H, Hasford J, et al. Randomized comparison of interferon-alpha with busulfan and hydroxyurea in chronic myelogenous leukemia. The German CML Study Group. Blood. 1994;84:4064-4077.
13. Cortes J, Kantarjian H. How I treat newly diagnosed chronic phase CML. Blood. 2012;120:1390-1397.
14. Nestok BR, Goldstein JD, Lipkovic P. Splenic rupture as a cause of sudden death in undiagnosed chronic myelogenous leukemia. Am J Forensic Med Pathol. 1988;9:241-245.
15. Sachithanandan A, Gleadhil I, Alexander HD, Morris TC. Spontaneous splenic rupture in atypical (Philadelphia chromosome negative) chronic myeloid leukemia following blastic crisis. Ir Med J. 2003;96(6):181-182.
16. Chim CS, Kwong YL, Shek TW, Ma SK, Ooi GC. Splenic rupture as the presenting symptom of blastic crisis in a patient with Philadelphia-negative, BCR-ABL-positive ET. Am J Hematology. 2001;66:70-71.
Gaps exist in receipt of clinically indicated genetic counseling after breast cancer diagnosis
, according to an analysis of NCI Surveillance, Epidemiology, and End Results (SEER) data published in Journal of Clinical Oncology.
More expertise is required in genetic counseling, either formal counseling given by an expert, or by a cancer physician (physician-directed), wrote Steven J. Katz and his colleagues at the University of Michigan, Ann Arbor. With BRCA1/2-only testing, being replaced by multi-gene panel testing, further consideration and/or discussion of results and formulation of a management plan is required, they said.
Of those, 47.4% did not get tested, 40.7% tested negative, 7.4% had a variant of uncertain significance only, and 4.5% had a pathogenic mutation. Three quarters (74.6%) received some form of genetic counseling (43.5%, formal counseling and 31.1%, physician-directed discussion). Almost all tested patients (96.1%) reported some form of genetic discussion. One half (50.6%) of those not tested received any discussion about genetics, reported the authors.
In addition, younger women more often reported some type of counseling (odds ratio, 4.5;95% confidence interval, 2.6-8.0; 1.9;95% CI, 1.1-3.3; and 1.5;95% CI, 1.0-2.3 for women younger than 50 years of age, 50-59 years of age, and 60-69 years of age, respectively, versus those 70 years of age and older).
Patients’ assessment of the amount of information they received about whether to have testing was high, “whether they received formal genetic counseling or a physician-directed discussion only (80.8% v 79.4% stated information was ‘just right’; P = .58),” the researchers noted.
As high-throughput molecular testing becomes increasingly complex, personalizing and tailoring the information to a individual patients’ need is crucial, the authors said. They further suggest a multipronged strategy that will train oncologists to integrate genetic testing into clinical decision making; including timely testing of patients at an elevated risk.
The study was supported by Grant P01 CA163233 to the University of Michigan from the National Cancer Institute. Potential conflict of interests were reported by Lauren P. Wallner, PhD (GlaxoSmithKline); Monica Morrow, MD (Genomic Health); Reshma Jagsi, MD (Amgen and AbbVie); and Allison W. Kurian, MD (Myriad Genetics, Invitae, Ambry Genetics, Genomic Health, GeneDx/BioReference, Genentech (a member of the Roche Group).
SOURCE: Katz SJ et al. J Clin Oncol. 2018 Mar 12. doi: 10.1200/JCO.2017.76.2369.
, according to an analysis of NCI Surveillance, Epidemiology, and End Results (SEER) data published in Journal of Clinical Oncology.
More expertise is required in genetic counseling, either formal counseling given by an expert, or by a cancer physician (physician-directed), wrote Steven J. Katz and his colleagues at the University of Michigan, Ann Arbor. With BRCA1/2-only testing, being replaced by multi-gene panel testing, further consideration and/or discussion of results and formulation of a management plan is required, they said.
Of those, 47.4% did not get tested, 40.7% tested negative, 7.4% had a variant of uncertain significance only, and 4.5% had a pathogenic mutation. Three quarters (74.6%) received some form of genetic counseling (43.5%, formal counseling and 31.1%, physician-directed discussion). Almost all tested patients (96.1%) reported some form of genetic discussion. One half (50.6%) of those not tested received any discussion about genetics, reported the authors.
In addition, younger women more often reported some type of counseling (odds ratio, 4.5;95% confidence interval, 2.6-8.0; 1.9;95% CI, 1.1-3.3; and 1.5;95% CI, 1.0-2.3 for women younger than 50 years of age, 50-59 years of age, and 60-69 years of age, respectively, versus those 70 years of age and older).
Patients’ assessment of the amount of information they received about whether to have testing was high, “whether they received formal genetic counseling or a physician-directed discussion only (80.8% v 79.4% stated information was ‘just right’; P = .58),” the researchers noted.
As high-throughput molecular testing becomes increasingly complex, personalizing and tailoring the information to a individual patients’ need is crucial, the authors said. They further suggest a multipronged strategy that will train oncologists to integrate genetic testing into clinical decision making; including timely testing of patients at an elevated risk.
The study was supported by Grant P01 CA163233 to the University of Michigan from the National Cancer Institute. Potential conflict of interests were reported by Lauren P. Wallner, PhD (GlaxoSmithKline); Monica Morrow, MD (Genomic Health); Reshma Jagsi, MD (Amgen and AbbVie); and Allison W. Kurian, MD (Myriad Genetics, Invitae, Ambry Genetics, Genomic Health, GeneDx/BioReference, Genentech (a member of the Roche Group).
SOURCE: Katz SJ et al. J Clin Oncol. 2018 Mar 12. doi: 10.1200/JCO.2017.76.2369.
, according to an analysis of NCI Surveillance, Epidemiology, and End Results (SEER) data published in Journal of Clinical Oncology.
More expertise is required in genetic counseling, either formal counseling given by an expert, or by a cancer physician (physician-directed), wrote Steven J. Katz and his colleagues at the University of Michigan, Ann Arbor. With BRCA1/2-only testing, being replaced by multi-gene panel testing, further consideration and/or discussion of results and formulation of a management plan is required, they said.
Of those, 47.4% did not get tested, 40.7% tested negative, 7.4% had a variant of uncertain significance only, and 4.5% had a pathogenic mutation. Three quarters (74.6%) received some form of genetic counseling (43.5%, formal counseling and 31.1%, physician-directed discussion). Almost all tested patients (96.1%) reported some form of genetic discussion. One half (50.6%) of those not tested received any discussion about genetics, reported the authors.
In addition, younger women more often reported some type of counseling (odds ratio, 4.5;95% confidence interval, 2.6-8.0; 1.9;95% CI, 1.1-3.3; and 1.5;95% CI, 1.0-2.3 for women younger than 50 years of age, 50-59 years of age, and 60-69 years of age, respectively, versus those 70 years of age and older).
Patients’ assessment of the amount of information they received about whether to have testing was high, “whether they received formal genetic counseling or a physician-directed discussion only (80.8% v 79.4% stated information was ‘just right’; P = .58),” the researchers noted.
As high-throughput molecular testing becomes increasingly complex, personalizing and tailoring the information to a individual patients’ need is crucial, the authors said. They further suggest a multipronged strategy that will train oncologists to integrate genetic testing into clinical decision making; including timely testing of patients at an elevated risk.
The study was supported by Grant P01 CA163233 to the University of Michigan from the National Cancer Institute. Potential conflict of interests were reported by Lauren P. Wallner, PhD (GlaxoSmithKline); Monica Morrow, MD (Genomic Health); Reshma Jagsi, MD (Amgen and AbbVie); and Allison W. Kurian, MD (Myriad Genetics, Invitae, Ambry Genetics, Genomic Health, GeneDx/BioReference, Genentech (a member of the Roche Group).
SOURCE: Katz SJ et al. J Clin Oncol. 2018 Mar 12. doi: 10.1200/JCO.2017.76.2369.
FROM JOURNAL OF CLINICAL ONCOLOGY
Key clinical point: There exists a large gap between mandates for timely pretest formal genetic counseling of higher-risk, breast cancer patients and its implementation in clinical practice.
Major finding: Almost half (47.4%) of patients diagnosed with early breast cancer with an indication for genetic risk evaluation did not get genetic tests. Of those who got genetic testing, 43.5% received formal counseling and 31.1% received physician-directed discussion.
Study details: Data on 5,080 women aged 20-79 years diagnosed with early stage breast during 2013-2015, reported to National Cancer Institute Surveillance, Epidemiology, and End Results (SEER) registries of Georgia and Los Angeles County.
Disclosures: Potential conflict of interests were reported by Lauren P. Wallner, PhD (GlaxoSmithKline); Monica Morrow, MD (Genomic Health); Reshma Jagsi, MD (Amgen and AbbVie); and Allison W. Kurian, MD (Myriad Genetics, Invitae, Ambry Genetics, Genomic Health, GeneDx/BioReference, Genentech (a member of the Roche Group).
Source: Katz SJ et al. J Clin Oncol. 2018 Mar 12. doi: 10.1200/JCO.2017.76.2369.
Colorectal cancer risk stratification enhanced by combining family history and genetic risk scores
Stratification of colorectal cancer (CRC) risk was enhanced by joint consideration of the independent family history and genetic risk score predictors, according to an ongoing population-based, case-control study of patients recruited during 2003-2010.
The research was conducted using data from DACHS (Colorectal Cancer: Chances for Prevention Through Screening), an ongoing population-based, case-control study in Germany, reported Korbinian Weigl, PhD, and his colleagues in the journal Clinical Epidemiology (doi: 10.2147/CLEP.S145636). They included 2,363 eligible CRC patients who were identified by 22 participating hospitals and frequency matched with respect to sex, age, and residential location to 2,198 randomly selected controls that had genome-wide association studies data. The population consisted of 40% women, and the median ages for cases and controls were 69 and 70 years, respectively.
Genetic risk score was calculated by genotyping 53 single-nucleotide polymorphisms reported in published literature to be associated with higher CRC risk for individuals of European descent. Seven genetic risk score groups – very low, low, low-medium, medium, medium-high, high, and very high – were established according to categories generated on the basis of weighted risk allele distribution among controls. Family history referred to CRC in first-degree and second-degree relatives. Selected potential confounders included age, sex, body mass index, education, hormone replacement therapy in women, smoking, and colonoscopy history. Odds ratios with 95% confidence intervals were estimated by multiple logistic regression models that included adjustment for potential confounders. Statistical calculations examined individual and joint family history and genetic risk score associations with risk for CRC and the effect of potential confounding factors.
At least one colonoscopy was performed on over half the individuals in the control group, while a significantly lower number (P less than .0001) were performed on case individuals (22.1%). Family history of CRC in first-degree relatives was reported by 316 case participants (13.4%) and 214 controls (9.7%; P less than .0001). The calculated genetic risk score ranged from 20 to 48, with a substantially higher proportion of cases in the higher deciles.
Investigators compared the risk for CRC in the top decile with that in the lowest and found an increased risk of 2.9-fold (OR, 2.94) based on genetic risk analysis adjusted for sex and age and an increased risk of 3.0-fold (OR, 3.0) when all other covariates except family history were included. Comparing results against analysis with the 27 single-nucleotide polymorphisms that had been used in previous studies indicated a sizable improvement in genetic risk stratification as a result of increasing the number of single-nucleotide polymorphisms (P value for increase in c statistic = .003) included in the analysis.
Risk associated with having a family history of CRC in a first-degree relative was 1.5-fold (OR, 1.47) higher in an age- and sex-adjusted analysis. Risk prediction increased to an OR of 1.86 when calculations were adjusted with covariates, especially with previous colonoscopies. Using genetic risk scoring as a calculation adjustment only slightly changed the result (OR, 1.83). A similar trend, but with lower-magnitude associations, was observed with family history of CRC in second-degree relatives.
A dose-response association between the number of risk alleles and CRC risk determined by a logistic regression model revealed a curvilinear relationship between genetic risk score and CRC risk. At higher genetic risk score levels, the increase in CRC risk was particularly strong. The dose-response association indicated an independent relationship between family history and CRC such that individuals with first-degree relatives with CRC will reach the same risk level with a lower genetic risk score as those with a higher genetic risk score but no first-degree relatives with CRC.
Joint risk stratification that combined family history and genetic risk scores was compared with risks determined by each predictor. As the genetic risk score increased there was an observed increased risk for individuals with first-degree relatives, second-degree relatives, or without family history. Considering only genetic risk score, the increase in risk from the lowest to highest decile was 2.8-fold. In contrast, the increased risk from the lowest to highest decile was 6.14-fold when stratification included both genetic risk score and considering family history in first-degree relatives, thus demonstrating the enhancing effect of combining the independent relationship of these two predictors.
The investigators concluded from their results that, by combining the genetic risk scores with family history and other easy-to-collect risk factor information,
The authors reported that they had no conflicts of interest.
SOURCE: Weigl K et al. Clin Epidemiol. 2018;10:143-52.
Stratification of colorectal cancer (CRC) risk was enhanced by joint consideration of the independent family history and genetic risk score predictors, according to an ongoing population-based, case-control study of patients recruited during 2003-2010.
The research was conducted using data from DACHS (Colorectal Cancer: Chances for Prevention Through Screening), an ongoing population-based, case-control study in Germany, reported Korbinian Weigl, PhD, and his colleagues in the journal Clinical Epidemiology (doi: 10.2147/CLEP.S145636). They included 2,363 eligible CRC patients who were identified by 22 participating hospitals and frequency matched with respect to sex, age, and residential location to 2,198 randomly selected controls that had genome-wide association studies data. The population consisted of 40% women, and the median ages for cases and controls were 69 and 70 years, respectively.
Genetic risk score was calculated by genotyping 53 single-nucleotide polymorphisms reported in published literature to be associated with higher CRC risk for individuals of European descent. Seven genetic risk score groups – very low, low, low-medium, medium, medium-high, high, and very high – were established according to categories generated on the basis of weighted risk allele distribution among controls. Family history referred to CRC in first-degree and second-degree relatives. Selected potential confounders included age, sex, body mass index, education, hormone replacement therapy in women, smoking, and colonoscopy history. Odds ratios with 95% confidence intervals were estimated by multiple logistic regression models that included adjustment for potential confounders. Statistical calculations examined individual and joint family history and genetic risk score associations with risk for CRC and the effect of potential confounding factors.
At least one colonoscopy was performed on over half the individuals in the control group, while a significantly lower number (P less than .0001) were performed on case individuals (22.1%). Family history of CRC in first-degree relatives was reported by 316 case participants (13.4%) and 214 controls (9.7%; P less than .0001). The calculated genetic risk score ranged from 20 to 48, with a substantially higher proportion of cases in the higher deciles.
Investigators compared the risk for CRC in the top decile with that in the lowest and found an increased risk of 2.9-fold (OR, 2.94) based on genetic risk analysis adjusted for sex and age and an increased risk of 3.0-fold (OR, 3.0) when all other covariates except family history were included. Comparing results against analysis with the 27 single-nucleotide polymorphisms that had been used in previous studies indicated a sizable improvement in genetic risk stratification as a result of increasing the number of single-nucleotide polymorphisms (P value for increase in c statistic = .003) included in the analysis.
Risk associated with having a family history of CRC in a first-degree relative was 1.5-fold (OR, 1.47) higher in an age- and sex-adjusted analysis. Risk prediction increased to an OR of 1.86 when calculations were adjusted with covariates, especially with previous colonoscopies. Using genetic risk scoring as a calculation adjustment only slightly changed the result (OR, 1.83). A similar trend, but with lower-magnitude associations, was observed with family history of CRC in second-degree relatives.
A dose-response association between the number of risk alleles and CRC risk determined by a logistic regression model revealed a curvilinear relationship between genetic risk score and CRC risk. At higher genetic risk score levels, the increase in CRC risk was particularly strong. The dose-response association indicated an independent relationship between family history and CRC such that individuals with first-degree relatives with CRC will reach the same risk level with a lower genetic risk score as those with a higher genetic risk score but no first-degree relatives with CRC.
Joint risk stratification that combined family history and genetic risk scores was compared with risks determined by each predictor. As the genetic risk score increased there was an observed increased risk for individuals with first-degree relatives, second-degree relatives, or without family history. Considering only genetic risk score, the increase in risk from the lowest to highest decile was 2.8-fold. In contrast, the increased risk from the lowest to highest decile was 6.14-fold when stratification included both genetic risk score and considering family history in first-degree relatives, thus demonstrating the enhancing effect of combining the independent relationship of these two predictors.
The investigators concluded from their results that, by combining the genetic risk scores with family history and other easy-to-collect risk factor information,
The authors reported that they had no conflicts of interest.
SOURCE: Weigl K et al. Clin Epidemiol. 2018;10:143-52.
Stratification of colorectal cancer (CRC) risk was enhanced by joint consideration of the independent family history and genetic risk score predictors, according to an ongoing population-based, case-control study of patients recruited during 2003-2010.
The research was conducted using data from DACHS (Colorectal Cancer: Chances for Prevention Through Screening), an ongoing population-based, case-control study in Germany, reported Korbinian Weigl, PhD, and his colleagues in the journal Clinical Epidemiology (doi: 10.2147/CLEP.S145636). They included 2,363 eligible CRC patients who were identified by 22 participating hospitals and frequency matched with respect to sex, age, and residential location to 2,198 randomly selected controls that had genome-wide association studies data. The population consisted of 40% women, and the median ages for cases and controls were 69 and 70 years, respectively.
Genetic risk score was calculated by genotyping 53 single-nucleotide polymorphisms reported in published literature to be associated with higher CRC risk for individuals of European descent. Seven genetic risk score groups – very low, low, low-medium, medium, medium-high, high, and very high – were established according to categories generated on the basis of weighted risk allele distribution among controls. Family history referred to CRC in first-degree and second-degree relatives. Selected potential confounders included age, sex, body mass index, education, hormone replacement therapy in women, smoking, and colonoscopy history. Odds ratios with 95% confidence intervals were estimated by multiple logistic regression models that included adjustment for potential confounders. Statistical calculations examined individual and joint family history and genetic risk score associations with risk for CRC and the effect of potential confounding factors.
At least one colonoscopy was performed on over half the individuals in the control group, while a significantly lower number (P less than .0001) were performed on case individuals (22.1%). Family history of CRC in first-degree relatives was reported by 316 case participants (13.4%) and 214 controls (9.7%; P less than .0001). The calculated genetic risk score ranged from 20 to 48, with a substantially higher proportion of cases in the higher deciles.
Investigators compared the risk for CRC in the top decile with that in the lowest and found an increased risk of 2.9-fold (OR, 2.94) based on genetic risk analysis adjusted for sex and age and an increased risk of 3.0-fold (OR, 3.0) when all other covariates except family history were included. Comparing results against analysis with the 27 single-nucleotide polymorphisms that had been used in previous studies indicated a sizable improvement in genetic risk stratification as a result of increasing the number of single-nucleotide polymorphisms (P value for increase in c statistic = .003) included in the analysis.
Risk associated with having a family history of CRC in a first-degree relative was 1.5-fold (OR, 1.47) higher in an age- and sex-adjusted analysis. Risk prediction increased to an OR of 1.86 when calculations were adjusted with covariates, especially with previous colonoscopies. Using genetic risk scoring as a calculation adjustment only slightly changed the result (OR, 1.83). A similar trend, but with lower-magnitude associations, was observed with family history of CRC in second-degree relatives.
A dose-response association between the number of risk alleles and CRC risk determined by a logistic regression model revealed a curvilinear relationship between genetic risk score and CRC risk. At higher genetic risk score levels, the increase in CRC risk was particularly strong. The dose-response association indicated an independent relationship between family history and CRC such that individuals with first-degree relatives with CRC will reach the same risk level with a lower genetic risk score as those with a higher genetic risk score but no first-degree relatives with CRC.
Joint risk stratification that combined family history and genetic risk scores was compared with risks determined by each predictor. As the genetic risk score increased there was an observed increased risk for individuals with first-degree relatives, second-degree relatives, or without family history. Considering only genetic risk score, the increase in risk from the lowest to highest decile was 2.8-fold. In contrast, the increased risk from the lowest to highest decile was 6.14-fold when stratification included both genetic risk score and considering family history in first-degree relatives, thus demonstrating the enhancing effect of combining the independent relationship of these two predictors.
The investigators concluded from their results that, by combining the genetic risk scores with family history and other easy-to-collect risk factor information,
The authors reported that they had no conflicts of interest.
SOURCE: Weigl K et al. Clin Epidemiol. 2018;10:143-52.
FROM CLINICAL EPIDEMIOLOGY
Key clinical point: Jointly using family history and genetic risk scores enhances their independent considerations for CRC risk stratifications.
Major finding: CRC in the highest decile compared with lowest decile was associated with 3.0-fold and 6.1-fold increased risks when considering genetic risk scores independently and jointly with family history, respectively.
Study details: Ongoing population-based study involving 22 hospitals in Germany with 2,363 CRC cases compared to 2,198 controls.
Disclosures: The authors reported that they had no conflicts of interest.
Source: Weigl K et al. Clin Epidemiol. 2018;10:143-52.
Cancer groups offer guidance on immune-related adverse events
The American Society of Clinical Oncology (ASCO) and the National Comprehensive Cancer Network (NCCN) have released new guidelines designed to help clinicians manage the unique and sometimes severe side effects associated with cancer immunotherapy agents.
These guidelines meet a growing need to help practicing clinicians identify and best manage immune-related adverse events, according to Bryan J. Schneider, MD, of the University of Michigan Comprehensive Cancer Center, and vice chair of the NCCN Panel on Management of Immunotherapy-Related Toxicities.
“The mechanism of action of these anticancer therapies is so much different from anything that we’re used to,” Dr. Schneider said in an interview.
Critical need for guidance
The ASCO and NCCN guidelines are “critically important” to ensure uniform management of common immune-related adverse events, according to Stephen M. Ansell, MD, PhD, professor of medicine and chair of the Lymphoma Group at Mayo Clinic, Rochester, Minn.
“I think it also specifically highlights a few side effects that many people may not necessarily think about, from eye toxicities to thyroid effects, or the type of things that the average oncologist who is now using this in their practice quite regularly may not necessarily think about,” Dr. Ansell said. “Those kind of effects are now clearly outlined with clear guidance about what should be done, and I think that allows oncologists a resource to go and look at this carefully so that they do the right thing.”
The spectrum of adverse effects associated with checkpoint inhibitors is markedly different from what is seen with cytotoxic chemotherapy, the guidelines note. Most often, the side effects are seen in the skin, GI tract, and lungs, as well as the endocrine, adrenal, nervous, thyroid, pituitary, musculoskeletal, cardiovascular, ocular, and hematologic systems.
Stepwise approach
Side effects of checkpoint inhibitors are typically mild, but they can be severe and sometimes life-threatening, according to ASCO and NCCN.
If immune-related adverse events are mild (i.e., grade 1), treatment can continue with close monitoring, according to the guidelines. By contrast, moderate to severe immune-related adverse events can lead to severe declines in organ function and quality of life, or even fatal outcomes, so early detection and proper management are needed.
Grade 2 toxicities warrant suspending immune checkpoint inhibitor treatment, and resuming it once symptoms subside to grade 1 or less, according to the guidelines. Grade 3 toxicities should also prompt suspension of treatment, plus initiation of high-dose corticosteroids tapered over at least 4-6 weeks.
For most toxicities that reach grade 4, permanent discontinuation of checkpoint inhibitors is recommended.
A thoughtful discussion of potential risks and benefits is needed before using immune checkpoint inhibitors in patients who have autoimmune disease or prior organ transplant, according to the guidelines.
Vigilance required
Checkpoint inhibitors have been approved by the Food and Drug Administration to treat a variety of cancers, including melanoma, lung cancer, and Hodgkin lymphoma, as well as lung, liver, kidney, and bladder cancers.
Clinicians managing patients on checkpoint inhibitors should always be vigilant because immune-related adverse event symptoms can be subtle, according to Julie Brahmer, MD, of The Sidney Kimmel Comprehensive Cancer Center at Johns Hopkins in Baltimore.
“Everyone has to work as a team, which includes being educated on possible side effects to immunotherapy prior to prescribing it,” said Dr. Brahmer, chair of the ASCO panel and vice chair of the NCCN panel that developed the guidelines.
The guidelines were published Feb. 14 in two documents that are similar in content, but different in format. The ASCO guideline was published in the Journal of Clinical Oncology (doi: 10.1200/JCO.2017.77.6385) and the NCCN Clinical Practice Guidelines in Oncology were posted on the NCCN website.
While the first edition of the guidelines focuses specifically on immune checkpoint inhibitors, an update anticipated for 2019 will include guidance on chimeric antigen receptor (CAR) T-cell therapy, which is associated with several important side effects, notably cytokine release syndrome.
The American Society of Clinical Oncology (ASCO) and the National Comprehensive Cancer Network (NCCN) have released new guidelines designed to help clinicians manage the unique and sometimes severe side effects associated with cancer immunotherapy agents.
These guidelines meet a growing need to help practicing clinicians identify and best manage immune-related adverse events, according to Bryan J. Schneider, MD, of the University of Michigan Comprehensive Cancer Center, and vice chair of the NCCN Panel on Management of Immunotherapy-Related Toxicities.
“The mechanism of action of these anticancer therapies is so much different from anything that we’re used to,” Dr. Schneider said in an interview.
Critical need for guidance
The ASCO and NCCN guidelines are “critically important” to ensure uniform management of common immune-related adverse events, according to Stephen M. Ansell, MD, PhD, professor of medicine and chair of the Lymphoma Group at Mayo Clinic, Rochester, Minn.
“I think it also specifically highlights a few side effects that many people may not necessarily think about, from eye toxicities to thyroid effects, or the type of things that the average oncologist who is now using this in their practice quite regularly may not necessarily think about,” Dr. Ansell said. “Those kind of effects are now clearly outlined with clear guidance about what should be done, and I think that allows oncologists a resource to go and look at this carefully so that they do the right thing.”
The spectrum of adverse effects associated with checkpoint inhibitors is markedly different from what is seen with cytotoxic chemotherapy, the guidelines note. Most often, the side effects are seen in the skin, GI tract, and lungs, as well as the endocrine, adrenal, nervous, thyroid, pituitary, musculoskeletal, cardiovascular, ocular, and hematologic systems.
Stepwise approach
Side effects of checkpoint inhibitors are typically mild, but they can be severe and sometimes life-threatening, according to ASCO and NCCN.
If immune-related adverse events are mild (i.e., grade 1), treatment can continue with close monitoring, according to the guidelines. By contrast, moderate to severe immune-related adverse events can lead to severe declines in organ function and quality of life, or even fatal outcomes, so early detection and proper management are needed.
Grade 2 toxicities warrant suspending immune checkpoint inhibitor treatment, and resuming it once symptoms subside to grade 1 or less, according to the guidelines. Grade 3 toxicities should also prompt suspension of treatment, plus initiation of high-dose corticosteroids tapered over at least 4-6 weeks.
For most toxicities that reach grade 4, permanent discontinuation of checkpoint inhibitors is recommended.
A thoughtful discussion of potential risks and benefits is needed before using immune checkpoint inhibitors in patients who have autoimmune disease or prior organ transplant, according to the guidelines.
Vigilance required
Checkpoint inhibitors have been approved by the Food and Drug Administration to treat a variety of cancers, including melanoma, lung cancer, and Hodgkin lymphoma, as well as lung, liver, kidney, and bladder cancers.
Clinicians managing patients on checkpoint inhibitors should always be vigilant because immune-related adverse event symptoms can be subtle, according to Julie Brahmer, MD, of The Sidney Kimmel Comprehensive Cancer Center at Johns Hopkins in Baltimore.
“Everyone has to work as a team, which includes being educated on possible side effects to immunotherapy prior to prescribing it,” said Dr. Brahmer, chair of the ASCO panel and vice chair of the NCCN panel that developed the guidelines.
The guidelines were published Feb. 14 in two documents that are similar in content, but different in format. The ASCO guideline was published in the Journal of Clinical Oncology (doi: 10.1200/JCO.2017.77.6385) and the NCCN Clinical Practice Guidelines in Oncology were posted on the NCCN website.
While the first edition of the guidelines focuses specifically on immune checkpoint inhibitors, an update anticipated for 2019 will include guidance on chimeric antigen receptor (CAR) T-cell therapy, which is associated with several important side effects, notably cytokine release syndrome.
The American Society of Clinical Oncology (ASCO) and the National Comprehensive Cancer Network (NCCN) have released new guidelines designed to help clinicians manage the unique and sometimes severe side effects associated with cancer immunotherapy agents.
These guidelines meet a growing need to help practicing clinicians identify and best manage immune-related adverse events, according to Bryan J. Schneider, MD, of the University of Michigan Comprehensive Cancer Center, and vice chair of the NCCN Panel on Management of Immunotherapy-Related Toxicities.
“The mechanism of action of these anticancer therapies is so much different from anything that we’re used to,” Dr. Schneider said in an interview.
Critical need for guidance
The ASCO and NCCN guidelines are “critically important” to ensure uniform management of common immune-related adverse events, according to Stephen M. Ansell, MD, PhD, professor of medicine and chair of the Lymphoma Group at Mayo Clinic, Rochester, Minn.
“I think it also specifically highlights a few side effects that many people may not necessarily think about, from eye toxicities to thyroid effects, or the type of things that the average oncologist who is now using this in their practice quite regularly may not necessarily think about,” Dr. Ansell said. “Those kind of effects are now clearly outlined with clear guidance about what should be done, and I think that allows oncologists a resource to go and look at this carefully so that they do the right thing.”
The spectrum of adverse effects associated with checkpoint inhibitors is markedly different from what is seen with cytotoxic chemotherapy, the guidelines note. Most often, the side effects are seen in the skin, GI tract, and lungs, as well as the endocrine, adrenal, nervous, thyroid, pituitary, musculoskeletal, cardiovascular, ocular, and hematologic systems.
Stepwise approach
Side effects of checkpoint inhibitors are typically mild, but they can be severe and sometimes life-threatening, according to ASCO and NCCN.
If immune-related adverse events are mild (i.e., grade 1), treatment can continue with close monitoring, according to the guidelines. By contrast, moderate to severe immune-related adverse events can lead to severe declines in organ function and quality of life, or even fatal outcomes, so early detection and proper management are needed.
Grade 2 toxicities warrant suspending immune checkpoint inhibitor treatment, and resuming it once symptoms subside to grade 1 or less, according to the guidelines. Grade 3 toxicities should also prompt suspension of treatment, plus initiation of high-dose corticosteroids tapered over at least 4-6 weeks.
For most toxicities that reach grade 4, permanent discontinuation of checkpoint inhibitors is recommended.
A thoughtful discussion of potential risks and benefits is needed before using immune checkpoint inhibitors in patients who have autoimmune disease or prior organ transplant, according to the guidelines.
Vigilance required
Checkpoint inhibitors have been approved by the Food and Drug Administration to treat a variety of cancers, including melanoma, lung cancer, and Hodgkin lymphoma, as well as lung, liver, kidney, and bladder cancers.
Clinicians managing patients on checkpoint inhibitors should always be vigilant because immune-related adverse event symptoms can be subtle, according to Julie Brahmer, MD, of The Sidney Kimmel Comprehensive Cancer Center at Johns Hopkins in Baltimore.
“Everyone has to work as a team, which includes being educated on possible side effects to immunotherapy prior to prescribing it,” said Dr. Brahmer, chair of the ASCO panel and vice chair of the NCCN panel that developed the guidelines.
The guidelines were published Feb. 14 in two documents that are similar in content, but different in format. The ASCO guideline was published in the Journal of Clinical Oncology (doi: 10.1200/JCO.2017.77.6385) and the NCCN Clinical Practice Guidelines in Oncology were posted on the NCCN website.
While the first edition of the guidelines focuses specifically on immune checkpoint inhibitors, an update anticipated for 2019 will include guidance on chimeric antigen receptor (CAR) T-cell therapy, which is associated with several important side effects, notably cytokine release syndrome.