User login
Opioid prescriptions got shorter in 2017
Use of opioids was down among enrollees of all types of payers in 2017, while the use of drugs to treat opioid dependence went up for three of the four payer categories, according to pharmacy benefits manager Express Scripts.
The days’ worth of opioids dispensed per person per year was down 16.6% from 2016 to 2017 for enrollees with plans on the health exchanges received managed by Express Scripts. Medicaid patients received 16% fewer days’ worth, patients with commercial plans received 10.3% fewer days’ worth, and Medicare patients received 4.9% fewer days’ worth, Express Scripts said in its 2017 Drug Trend Report, which was based on data for 34.3 million members of pharmacy benefits plans the company administers.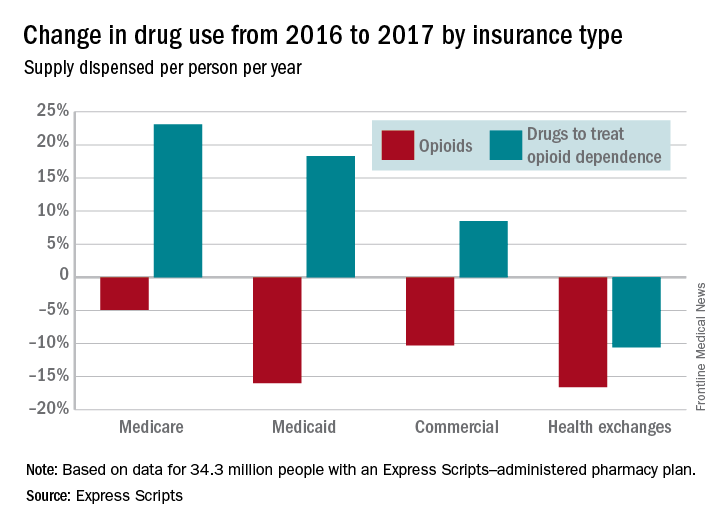
Plans that participated in Express Scripts’ Advanced Opioid Management solution, which was launched in September, experienced “a 60% reduction in the average days’ supply per initial fill, from 18.6 days to just 7.5 days,” according to the report.
Use of opioids was down among enrollees of all types of payers in 2017, while the use of drugs to treat opioid dependence went up for three of the four payer categories, according to pharmacy benefits manager Express Scripts.
The days’ worth of opioids dispensed per person per year was down 16.6% from 2016 to 2017 for enrollees with plans on the health exchanges received managed by Express Scripts. Medicaid patients received 16% fewer days’ worth, patients with commercial plans received 10.3% fewer days’ worth, and Medicare patients received 4.9% fewer days’ worth, Express Scripts said in its 2017 Drug Trend Report, which was based on data for 34.3 million members of pharmacy benefits plans the company administers.
Plans that participated in Express Scripts’ Advanced Opioid Management solution, which was launched in September, experienced “a 60% reduction in the average days’ supply per initial fill, from 18.6 days to just 7.5 days,” according to the report.
Use of opioids was down among enrollees of all types of payers in 2017, while the use of drugs to treat opioid dependence went up for three of the four payer categories, according to pharmacy benefits manager Express Scripts.
The days’ worth of opioids dispensed per person per year was down 16.6% from 2016 to 2017 for enrollees with plans on the health exchanges received managed by Express Scripts. Medicaid patients received 16% fewer days’ worth, patients with commercial plans received 10.3% fewer days’ worth, and Medicare patients received 4.9% fewer days’ worth, Express Scripts said in its 2017 Drug Trend Report, which was based on data for 34.3 million members of pharmacy benefits plans the company administers.
Plans that participated in Express Scripts’ Advanced Opioid Management solution, which was launched in September, experienced “a 60% reduction in the average days’ supply per initial fill, from 18.6 days to just 7.5 days,” according to the report.
Breast cancer care delayed when patients have high deductibles
High-deductible health insurance plans may be bad for women’s health, suggest results of a new study.
An analysis of data on women without evidence of breast cancer who were covered for at least 1 year in a low annual deductible plan and then switched by their employers to high annual deductible plans showed that when women were forced to shell out substantially more money before their insurance kicked in, they were significantly more likely to have delays in diagnostic breast imaging, breast biopsy, and initiation of chemotherapy.
“Such delays might lead to adverse long-term breast cancer outcomes. Policymakers, health insurers, and employers should consider designing or incentivizing health insurance benefits that facilitate transitions through key steps along the cancer care pathway,” wrote J. Frank Wharam, MB, and colleagues at Harvard Medical School and Harvard Pilgrim Health Care Institute in Boston. The report was published in Journal of Clinical Oncology.
The investigators conducted a controlled pre-post study to measure the occurrence of outcomes both before and after women were switched from a low-deductible health plan, defined as a maximum annual deductible of $500 or less, to a high-deductible plan, defined as an annual deductible of $1,000 or more.
The study population comprised 273,499 women aged 25-64 years who had no evidence of breast cancer before they were included in the study. The women had all been enrolled in a low-deductible plan for at least 1 year, and were then switched by employer mandate to a high-deductible plan and followed for up to 4 additional years.
Controls included 2.4 million women matched by time of inclusion whose employers continued to offer only low-deductible health plans.
Although at baseline there were no differences between the study sample and the controls in time to first diagnostic breast imaging, breast biopsy, diagnosis of early stage breast cancer, or initiation of breast cancer chemotherapy, at follow-up the women who had been switched to the high-deductible plans had significant delays in all categories.
Compared with controls, the hazard ratios (HR) for each parameter were as follows:
Time to first diagnostic breast imaging: HR = 0.96 (95% confidence interval 0.94-0.96)
Time to first breast biopsy: HR = 0.92 (0.89-0.95)
Time to early stage breast cancer diagnosis: HR = 0.83 (0.78-0.90)
Time to breast cancer chemotherapy: HR = 0.79 (0.72-0.86)
“The findings imply that the high out-of-pocket obligations under HDHPs [high-deductible health plans] might be a barrier to timely receipt of essential breast cancer services. Women in HDHPs might either delay presenting for concerning symptoms or, if proceeding along the pathway from breast cancer screening to diagnostic testing to treatment, be hesitant to undergo subsequent (and generally more expensive) care,” the authors wrote.
They noted that initially modest delays in diagnostic imaging appeared to snowball into longer delays as women proceeded through stages of care.
They recommend a strategy whereby insurers carve out exemptions to high deductibles for services such as diagnostic imaging and breast biopsy.
SOURCE: Wharam et al. J Clin Oncol. 2018 Feb 28. doi: 10.1200/JCO.2017.75.2501.
High-deductible health insurance plans may be bad for women’s health, suggest results of a new study.
An analysis of data on women without evidence of breast cancer who were covered for at least 1 year in a low annual deductible plan and then switched by their employers to high annual deductible plans showed that when women were forced to shell out substantially more money before their insurance kicked in, they were significantly more likely to have delays in diagnostic breast imaging, breast biopsy, and initiation of chemotherapy.
“Such delays might lead to adverse long-term breast cancer outcomes. Policymakers, health insurers, and employers should consider designing or incentivizing health insurance benefits that facilitate transitions through key steps along the cancer care pathway,” wrote J. Frank Wharam, MB, and colleagues at Harvard Medical School and Harvard Pilgrim Health Care Institute in Boston. The report was published in Journal of Clinical Oncology.
The investigators conducted a controlled pre-post study to measure the occurrence of outcomes both before and after women were switched from a low-deductible health plan, defined as a maximum annual deductible of $500 or less, to a high-deductible plan, defined as an annual deductible of $1,000 or more.
The study population comprised 273,499 women aged 25-64 years who had no evidence of breast cancer before they were included in the study. The women had all been enrolled in a low-deductible plan for at least 1 year, and were then switched by employer mandate to a high-deductible plan and followed for up to 4 additional years.
Controls included 2.4 million women matched by time of inclusion whose employers continued to offer only low-deductible health plans.
Although at baseline there were no differences between the study sample and the controls in time to first diagnostic breast imaging, breast biopsy, diagnosis of early stage breast cancer, or initiation of breast cancer chemotherapy, at follow-up the women who had been switched to the high-deductible plans had significant delays in all categories.
Compared with controls, the hazard ratios (HR) for each parameter were as follows:
Time to first diagnostic breast imaging: HR = 0.96 (95% confidence interval 0.94-0.96)
Time to first breast biopsy: HR = 0.92 (0.89-0.95)
Time to early stage breast cancer diagnosis: HR = 0.83 (0.78-0.90)
Time to breast cancer chemotherapy: HR = 0.79 (0.72-0.86)
“The findings imply that the high out-of-pocket obligations under HDHPs [high-deductible health plans] might be a barrier to timely receipt of essential breast cancer services. Women in HDHPs might either delay presenting for concerning symptoms or, if proceeding along the pathway from breast cancer screening to diagnostic testing to treatment, be hesitant to undergo subsequent (and generally more expensive) care,” the authors wrote.
They noted that initially modest delays in diagnostic imaging appeared to snowball into longer delays as women proceeded through stages of care.
They recommend a strategy whereby insurers carve out exemptions to high deductibles for services such as diagnostic imaging and breast biopsy.
SOURCE: Wharam et al. J Clin Oncol. 2018 Feb 28. doi: 10.1200/JCO.2017.75.2501.
High-deductible health insurance plans may be bad for women’s health, suggest results of a new study.
An analysis of data on women without evidence of breast cancer who were covered for at least 1 year in a low annual deductible plan and then switched by their employers to high annual deductible plans showed that when women were forced to shell out substantially more money before their insurance kicked in, they were significantly more likely to have delays in diagnostic breast imaging, breast biopsy, and initiation of chemotherapy.
“Such delays might lead to adverse long-term breast cancer outcomes. Policymakers, health insurers, and employers should consider designing or incentivizing health insurance benefits that facilitate transitions through key steps along the cancer care pathway,” wrote J. Frank Wharam, MB, and colleagues at Harvard Medical School and Harvard Pilgrim Health Care Institute in Boston. The report was published in Journal of Clinical Oncology.
The investigators conducted a controlled pre-post study to measure the occurrence of outcomes both before and after women were switched from a low-deductible health plan, defined as a maximum annual deductible of $500 or less, to a high-deductible plan, defined as an annual deductible of $1,000 or more.
The study population comprised 273,499 women aged 25-64 years who had no evidence of breast cancer before they were included in the study. The women had all been enrolled in a low-deductible plan for at least 1 year, and were then switched by employer mandate to a high-deductible plan and followed for up to 4 additional years.
Controls included 2.4 million women matched by time of inclusion whose employers continued to offer only low-deductible health plans.
Although at baseline there were no differences between the study sample and the controls in time to first diagnostic breast imaging, breast biopsy, diagnosis of early stage breast cancer, or initiation of breast cancer chemotherapy, at follow-up the women who had been switched to the high-deductible plans had significant delays in all categories.
Compared with controls, the hazard ratios (HR) for each parameter were as follows:
Time to first diagnostic breast imaging: HR = 0.96 (95% confidence interval 0.94-0.96)
Time to first breast biopsy: HR = 0.92 (0.89-0.95)
Time to early stage breast cancer diagnosis: HR = 0.83 (0.78-0.90)
Time to breast cancer chemotherapy: HR = 0.79 (0.72-0.86)
“The findings imply that the high out-of-pocket obligations under HDHPs [high-deductible health plans] might be a barrier to timely receipt of essential breast cancer services. Women in HDHPs might either delay presenting for concerning symptoms or, if proceeding along the pathway from breast cancer screening to diagnostic testing to treatment, be hesitant to undergo subsequent (and generally more expensive) care,” the authors wrote.
They noted that initially modest delays in diagnostic imaging appeared to snowball into longer delays as women proceeded through stages of care.
They recommend a strategy whereby insurers carve out exemptions to high deductibles for services such as diagnostic imaging and breast biopsy.
SOURCE: Wharam et al. J Clin Oncol. 2018 Feb 28. doi: 10.1200/JCO.2017.75.2501.
FROM JOURNAL OF CLINICAL ONCOLOGY
Key clinical point: Many women have high-deductible health plans that may discourage them from seeking essential care when needed.
Major finding: Women with an employer-mandated switch from a low- to high-deductible health plan had significant delays in diagnostic imaging, biopsy, diagnosis, and cancer care.
Study details: Controlled pre-post study of data on 273,499 women and 2.4 million controls.
Disclosures: The study was supported by National Cancer Institute and National Institute of Health grants. Dr. Wharam and three coauthors reported no conflicts of interest. Three coauthors reported honoraria and/or consulting/advisory roles with various companies.
Source: Wharam et al. J Clin Oncol. 2018 Feb 28. doi: 10.1200/JCO.2017.75.2501.
Ibrutinib linked to invasive fungal infections
The tyrosine kinase inhibitor ibrutinib (Imbruvica) may be associated with early-onset invasive fungal infections (IFI) in patients with hematologic malignancies, investigators caution.
French investigators identified 33 cases of invasive fungal infections occurring among patients who had been treated with ibrutinib as monotherapy or in combination with other agents. Of the 33 cases, 27 were invasive aspergillosis, and 40% of these were localized in the central nervous system. The findings were published in the journal Blood.
“IFI tend to occur within the first months of treatment and are infrequent thereafter. Whilst it seems difficult at this point to advocate for systematic antifungal prophylaxis in all patients, an increased awareness about the potential risk of IFI after initiating ibrutinib is warranted, especially when other predisposing factors are associated,” wrote David Ghez, MD, PhD, and colleagues at the Gustave Roussy Institute in Villejuif and other centers in France.
Although ibrutinib, an inhibitor of Bruton’s tyrosine kinase, is generally considered to be less immunosuppressive than other therapies, it was associated with five cases of Pneumocystis jirovecii pneumonia in patients with chronic lymphocytic leukemia (CLL) treated with ibrutinib monotherapy in a 2016 report (Blood. 2016;128:1940-3). Of these five patients, four were treatment naive, suggesting that ibrutinib itself could increase risk for invasive opportunistic infections, Dr. Ghez and his colleagues noted.
Based on this finding and on case reports of invasive infections in other patients being treated with ibrutinib, the authors conducted a retrospective survey of centers in the French Innovative Leukemia Organization CLL group.
They identified 33 cases, including 30 patients with CLL (15 of whom had deleterious 17p deletions), 1 with mantle cell lymphoma, and 2 with Waldenstrom macroglobulinemia.
Invasive aspergillosis accounted for 27 of the 33 cases, and 11 cases had CNS localization. There were four cases of disseminated cryptococcosis, and one each of mucormycosis and pneumocystis pneumonia.
The median time between the start of ibrutinib therapy and a diagnosis of invasive fungal infection was 3 months, with some cases occurring as early as 1 month, and others occurring 30 months out. However, the majority of cases – 28 – were diagnosed within 6 months of the start of therapy, including 20 that occurred within 3 months of ibrutinib initiation.
In 21 patients, the diagnosis of an invasive fungal infection led to drug discontinuation. In the remaining patients, the drug was either resumed after resolution of the IFI, or continued at a lower dose because of potential for interaction between ibrutinib and the antifungal agent voriconazole.
Dr. Ghez reported receiving a research grant from Janssen, and coauthor Loic Ysebaert, MD, PhD, reported consultancy fees from the company. All other authors declared no competing financial interests.
SOURCE: Ghez D et al., Blood. 2018 Feb 1. doi: 10.1182/blood-2017-11-818286.
The tyrosine kinase inhibitor ibrutinib (Imbruvica) may be associated with early-onset invasive fungal infections (IFI) in patients with hematologic malignancies, investigators caution.
French investigators identified 33 cases of invasive fungal infections occurring among patients who had been treated with ibrutinib as monotherapy or in combination with other agents. Of the 33 cases, 27 were invasive aspergillosis, and 40% of these were localized in the central nervous system. The findings were published in the journal Blood.
“IFI tend to occur within the first months of treatment and are infrequent thereafter. Whilst it seems difficult at this point to advocate for systematic antifungal prophylaxis in all patients, an increased awareness about the potential risk of IFI after initiating ibrutinib is warranted, especially when other predisposing factors are associated,” wrote David Ghez, MD, PhD, and colleagues at the Gustave Roussy Institute in Villejuif and other centers in France.
Although ibrutinib, an inhibitor of Bruton’s tyrosine kinase, is generally considered to be less immunosuppressive than other therapies, it was associated with five cases of Pneumocystis jirovecii pneumonia in patients with chronic lymphocytic leukemia (CLL) treated with ibrutinib monotherapy in a 2016 report (Blood. 2016;128:1940-3). Of these five patients, four were treatment naive, suggesting that ibrutinib itself could increase risk for invasive opportunistic infections, Dr. Ghez and his colleagues noted.
Based on this finding and on case reports of invasive infections in other patients being treated with ibrutinib, the authors conducted a retrospective survey of centers in the French Innovative Leukemia Organization CLL group.
They identified 33 cases, including 30 patients with CLL (15 of whom had deleterious 17p deletions), 1 with mantle cell lymphoma, and 2 with Waldenstrom macroglobulinemia.
Invasive aspergillosis accounted for 27 of the 33 cases, and 11 cases had CNS localization. There were four cases of disseminated cryptococcosis, and one each of mucormycosis and pneumocystis pneumonia.
The median time between the start of ibrutinib therapy and a diagnosis of invasive fungal infection was 3 months, with some cases occurring as early as 1 month, and others occurring 30 months out. However, the majority of cases – 28 – were diagnosed within 6 months of the start of therapy, including 20 that occurred within 3 months of ibrutinib initiation.
In 21 patients, the diagnosis of an invasive fungal infection led to drug discontinuation. In the remaining patients, the drug was either resumed after resolution of the IFI, or continued at a lower dose because of potential for interaction between ibrutinib and the antifungal agent voriconazole.
Dr. Ghez reported receiving a research grant from Janssen, and coauthor Loic Ysebaert, MD, PhD, reported consultancy fees from the company. All other authors declared no competing financial interests.
SOURCE: Ghez D et al., Blood. 2018 Feb 1. doi: 10.1182/blood-2017-11-818286.
The tyrosine kinase inhibitor ibrutinib (Imbruvica) may be associated with early-onset invasive fungal infections (IFI) in patients with hematologic malignancies, investigators caution.
French investigators identified 33 cases of invasive fungal infections occurring among patients who had been treated with ibrutinib as monotherapy or in combination with other agents. Of the 33 cases, 27 were invasive aspergillosis, and 40% of these were localized in the central nervous system. The findings were published in the journal Blood.
“IFI tend to occur within the first months of treatment and are infrequent thereafter. Whilst it seems difficult at this point to advocate for systematic antifungal prophylaxis in all patients, an increased awareness about the potential risk of IFI after initiating ibrutinib is warranted, especially when other predisposing factors are associated,” wrote David Ghez, MD, PhD, and colleagues at the Gustave Roussy Institute in Villejuif and other centers in France.
Although ibrutinib, an inhibitor of Bruton’s tyrosine kinase, is generally considered to be less immunosuppressive than other therapies, it was associated with five cases of Pneumocystis jirovecii pneumonia in patients with chronic lymphocytic leukemia (CLL) treated with ibrutinib monotherapy in a 2016 report (Blood. 2016;128:1940-3). Of these five patients, four were treatment naive, suggesting that ibrutinib itself could increase risk for invasive opportunistic infections, Dr. Ghez and his colleagues noted.
Based on this finding and on case reports of invasive infections in other patients being treated with ibrutinib, the authors conducted a retrospective survey of centers in the French Innovative Leukemia Organization CLL group.
They identified 33 cases, including 30 patients with CLL (15 of whom had deleterious 17p deletions), 1 with mantle cell lymphoma, and 2 with Waldenstrom macroglobulinemia.
Invasive aspergillosis accounted for 27 of the 33 cases, and 11 cases had CNS localization. There were four cases of disseminated cryptococcosis, and one each of mucormycosis and pneumocystis pneumonia.
The median time between the start of ibrutinib therapy and a diagnosis of invasive fungal infection was 3 months, with some cases occurring as early as 1 month, and others occurring 30 months out. However, the majority of cases – 28 – were diagnosed within 6 months of the start of therapy, including 20 that occurred within 3 months of ibrutinib initiation.
In 21 patients, the diagnosis of an invasive fungal infection led to drug discontinuation. In the remaining patients, the drug was either resumed after resolution of the IFI, or continued at a lower dose because of potential for interaction between ibrutinib and the antifungal agent voriconazole.
Dr. Ghez reported receiving a research grant from Janssen, and coauthor Loic Ysebaert, MD, PhD, reported consultancy fees from the company. All other authors declared no competing financial interests.
SOURCE: Ghez D et al., Blood. 2018 Feb 1. doi: 10.1182/blood-2017-11-818286.
FROM BLOOD
Key clinical point:
Major finding: Of 33 identified cases, 27 were invasive aspergillosis.
Study details: Retrospective review of case reports from 16 French centers.
Disclosures: Dr. Ghez reported receiving a research grant from Janssen, and coauthor Loic Ysebaert, MD, PhD, reported consultancy fees with the company. All other authors declared no competing financial interests.
Source: Ghez D et al. Blood. 2018 Feb 1. doi: 10.1182/blood-2017-11-818286.
FDA authorizes first direct-to-consumer BRCA1/2 test
The Food and Drug Administration has authorized the first direct-to-consumer (DTC) test to report on three specific BRCA1/BRCA2 breast cancer gene mutations.
Personal Genome Service Genetic Health Risk (GHR) Report for BRCA1/BRCA2 (Selected Variants) does not identify the most common BRCA1/2 mutations but rather the three most common in people of Ashkenazi (Eastern European) Jewish descent, the FDA said in a press statement.
The test, marketed by 23andMe, analyzes DNA from a self-collected saliva sample.
The three mutations identified by the test are present in about 2% of Ashkenazi Jewish women, but rarely in other ethnic populations. Any individual who takes the test may have other mutations in BRCA1 or BRCA2 genes, or other cancer-related gene mutations that are not detected by this test.
“This test provides information to certain individuals who may be at increased breast, ovarian, or prostate cancer risk and who might not otherwise get genetic screening and is a step forward in the availability of DTC genetic tests. But it has a lot of caveats,” Donald St. Pierre, acting director of the Office of In Vitro Diagnostics and Radiological Health in the FDA’s Center for Devices and Radiological Health, said in the press statement. “While the detection of a BRCA mutation on this test does indicate an increased risk, only a small percentage of Americans carry one of these three mutations and most BRCA mutations that increase an individual’s risk are not detected by this test. The test should not be used as a substitute for seeing your doctor for cancer screenings or counseling on genetic and lifestyle factors that can increase or decrease cancer risk.”
The authorization was based on data provided by the company to indicate the test correctly identifies the three genetic variants in saliva samples and is reproducible. In addition, the company submitted data to demonstrate that the instructions are comprehensible and easy to follow.
The FDA cautions that consumers and health care professionals “should not use the test results to determine any treatments, including antihormone therapies and prophylactic removal of the breasts or ovaries.” Decisions should be made only after confirmatory testing and genetic counseling, they said.
The Food and Drug Administration has authorized the first direct-to-consumer (DTC) test to report on three specific BRCA1/BRCA2 breast cancer gene mutations.
Personal Genome Service Genetic Health Risk (GHR) Report for BRCA1/BRCA2 (Selected Variants) does not identify the most common BRCA1/2 mutations but rather the three most common in people of Ashkenazi (Eastern European) Jewish descent, the FDA said in a press statement.
The test, marketed by 23andMe, analyzes DNA from a self-collected saliva sample.
The three mutations identified by the test are present in about 2% of Ashkenazi Jewish women, but rarely in other ethnic populations. Any individual who takes the test may have other mutations in BRCA1 or BRCA2 genes, or other cancer-related gene mutations that are not detected by this test.
“This test provides information to certain individuals who may be at increased breast, ovarian, or prostate cancer risk and who might not otherwise get genetic screening and is a step forward in the availability of DTC genetic tests. But it has a lot of caveats,” Donald St. Pierre, acting director of the Office of In Vitro Diagnostics and Radiological Health in the FDA’s Center for Devices and Radiological Health, said in the press statement. “While the detection of a BRCA mutation on this test does indicate an increased risk, only a small percentage of Americans carry one of these three mutations and most BRCA mutations that increase an individual’s risk are not detected by this test. The test should not be used as a substitute for seeing your doctor for cancer screenings or counseling on genetic and lifestyle factors that can increase or decrease cancer risk.”
The authorization was based on data provided by the company to indicate the test correctly identifies the three genetic variants in saliva samples and is reproducible. In addition, the company submitted data to demonstrate that the instructions are comprehensible and easy to follow.
The FDA cautions that consumers and health care professionals “should not use the test results to determine any treatments, including antihormone therapies and prophylactic removal of the breasts or ovaries.” Decisions should be made only after confirmatory testing and genetic counseling, they said.
The Food and Drug Administration has authorized the first direct-to-consumer (DTC) test to report on three specific BRCA1/BRCA2 breast cancer gene mutations.
Personal Genome Service Genetic Health Risk (GHR) Report for BRCA1/BRCA2 (Selected Variants) does not identify the most common BRCA1/2 mutations but rather the three most common in people of Ashkenazi (Eastern European) Jewish descent, the FDA said in a press statement.
The test, marketed by 23andMe, analyzes DNA from a self-collected saliva sample.
The three mutations identified by the test are present in about 2% of Ashkenazi Jewish women, but rarely in other ethnic populations. Any individual who takes the test may have other mutations in BRCA1 or BRCA2 genes, or other cancer-related gene mutations that are not detected by this test.
“This test provides information to certain individuals who may be at increased breast, ovarian, or prostate cancer risk and who might not otherwise get genetic screening and is a step forward in the availability of DTC genetic tests. But it has a lot of caveats,” Donald St. Pierre, acting director of the Office of In Vitro Diagnostics and Radiological Health in the FDA’s Center for Devices and Radiological Health, said in the press statement. “While the detection of a BRCA mutation on this test does indicate an increased risk, only a small percentage of Americans carry one of these three mutations and most BRCA mutations that increase an individual’s risk are not detected by this test. The test should not be used as a substitute for seeing your doctor for cancer screenings or counseling on genetic and lifestyle factors that can increase or decrease cancer risk.”
The authorization was based on data provided by the company to indicate the test correctly identifies the three genetic variants in saliva samples and is reproducible. In addition, the company submitted data to demonstrate that the instructions are comprehensible and easy to follow.
The FDA cautions that consumers and health care professionals “should not use the test results to determine any treatments, including antihormone therapies and prophylactic removal of the breasts or ovaries.” Decisions should be made only after confirmatory testing and genetic counseling, they said.
Immunotherapy regimen influences inflammatory arthritis presentation
Variations in the clinical presentation of immunotherapy-induced inflammatory arthritis is partly explained by which treatment regimen was used to treat the cancer, a single-center study suggests.
While immune checkpoint inhibitors (ICI) have revolutionized the field of oncology, their use for an ever-widening range of indications had created an increasing population of patients referred to rheumatologists for the management of immune-related adverse events (IrAEs), according to Laura C. Cappelli, MD, and her colleagues at John Hopkins University, Baltimore.
Well-established guidelines exist for managing adverse events such as colitis and pneumonitis, but there are only preliminary guidelines for evaluating and treating immunotherapy-induced inflammatory arthritis (IA). “This may stem from a lack of consistent reporting of rheumatologic IrAEs in clinical trials, the non–life threatening nature of [inflammatory arthritis], or lack of recognition of musculoskeletal symptoms by treating providers,” they wrote in Seminars in Arthritis and Rheumatism.
Clinical trials have reported ranges of arthralgia in 1%-43% of patients treated with ICIs, but no accurate estimate of the incidence of IA exists.
The researchers noted that treating patients with ICI-induced IA is complicated by a history of active or recently treated cancer and concerns over using immunosuppression in the context of ICI therapy.
They set out to evaluate the clinical presentations of 30 patients seen in their clinic with ICI-induced IA. Patients were a median of 59 years old and 12 (40%) were female. Tumor types included metastatic melanoma, non–small cell lung cancer, small cell lung cancer, colorectal cancer, Hodgkin lymphoma, cutaneous lymphoma, renal cell carcinoma, duodenal carcinoma, Merkel cell carcinoma, cutaneous basal cell carcinoma, and cutaneous squamous cell carcinoma.
Sixteen patients were treated with anti–programmed cell death protein 1 (PD-1)/programmed death ligand 1 monotherapy, and 14 were treated with combination anti–CTLA-4/PD-1 therapy.
Patients on combination therapy were significantly younger (7.5 years, P = 0.01) and were more likely to have metastatic melanoma as their underlying cancer.
Patients who received combination therapy were more likely to present first with knee IA (n = 10) and none had small joint involvement. In contrast, initial small joint involvement was more common in the monotherapy group (n = 6).
C-reactive protein levels were significantly higher in the combination therapy group (4mg/dL vs. 0.5mg/dL, P = 0.03). Only monotherapy patients were positive for anti–citrullinated peptide antibodies, rheumatoid factor, or antinuclear antibodies.
Most of the patients in the study had an additional IrAE, with colitis being the most common (n=10), followed by thyroid disease, pneumonitis, and rash. Patients on PD-1 or programmed death ligand 1 monotherapy were more likely to have IA as their first IrAE.
The research team noted that the median time to symptom onset was 5 months after ICI initiation.
Diagnosis of IA following patient-reported symptoms was an average of 5.2 months, with a significant difference in lag time to diagnosis depending on initial joint presentation. For example, patients with initial small joint involvement had a 10 month longer lag time to IA diagnosis than those with knees as the initial joint involved.
In terms of treatment, 24 patients were treated with systemic corticosteroids and 10 required additional immunosuppression. The need for corticosteroids did not differ by ICI treatment regimen, but those treated with combination therapy were more likely to require additional immunosuppression (P = 0.02).
Tumor necrosis factor inhibitors with or without methotrexate were prescribed for seven patients. All of the patients had a clinical improvement in their arthritis symptoms. Four had a complete tumor response at the time of tumor necrosis factor inhibitor initiation with none having tumor progression.
The three patients treated with methotrexate monotherapy had a complete or sustained partial tumor response to ICI therapy and their cancer did not develop during IA management follow-up.
The authors went on to look at the persistence of IA after cessation of therapy in a subset of 21 patients. They found that 18 of these patients still had IA symptoms months after stopping treatment. They suggested that the delay in diagnosis and treatment seen in their study might explain the finding.
The study provides “critical information, not just for rheumatologists as they try to recognize subgroups in ICI-induced IA and diagnose patients with this new entity, but also for oncology providers who are usually first to encounter patients with ICI-induced IA and subsequently refer patients to rheumatology,” Dr. Cappelli and colleagues wrote.
The experience so far with using immunosuppression in ICI-induced IA “has been reassuring in terms of cancer outcomes, but more studies are needed to confirm this finding,” they concluded.
SOURCE: Cappelli LC et al. Semin Arthritis Rheum. doi: 10.1016/j.semarthrit. 2018.02.011.
Variations in the clinical presentation of immunotherapy-induced inflammatory arthritis is partly explained by which treatment regimen was used to treat the cancer, a single-center study suggests.
While immune checkpoint inhibitors (ICI) have revolutionized the field of oncology, their use for an ever-widening range of indications had created an increasing population of patients referred to rheumatologists for the management of immune-related adverse events (IrAEs), according to Laura C. Cappelli, MD, and her colleagues at John Hopkins University, Baltimore.
Well-established guidelines exist for managing adverse events such as colitis and pneumonitis, but there are only preliminary guidelines for evaluating and treating immunotherapy-induced inflammatory arthritis (IA). “This may stem from a lack of consistent reporting of rheumatologic IrAEs in clinical trials, the non–life threatening nature of [inflammatory arthritis], or lack of recognition of musculoskeletal symptoms by treating providers,” they wrote in Seminars in Arthritis and Rheumatism.
Clinical trials have reported ranges of arthralgia in 1%-43% of patients treated with ICIs, but no accurate estimate of the incidence of IA exists.
The researchers noted that treating patients with ICI-induced IA is complicated by a history of active or recently treated cancer and concerns over using immunosuppression in the context of ICI therapy.
They set out to evaluate the clinical presentations of 30 patients seen in their clinic with ICI-induced IA. Patients were a median of 59 years old and 12 (40%) were female. Tumor types included metastatic melanoma, non–small cell lung cancer, small cell lung cancer, colorectal cancer, Hodgkin lymphoma, cutaneous lymphoma, renal cell carcinoma, duodenal carcinoma, Merkel cell carcinoma, cutaneous basal cell carcinoma, and cutaneous squamous cell carcinoma.
Sixteen patients were treated with anti–programmed cell death protein 1 (PD-1)/programmed death ligand 1 monotherapy, and 14 were treated with combination anti–CTLA-4/PD-1 therapy.
Patients on combination therapy were significantly younger (7.5 years, P = 0.01) and were more likely to have metastatic melanoma as their underlying cancer.
Patients who received combination therapy were more likely to present first with knee IA (n = 10) and none had small joint involvement. In contrast, initial small joint involvement was more common in the monotherapy group (n = 6).
C-reactive protein levels were significantly higher in the combination therapy group (4mg/dL vs. 0.5mg/dL, P = 0.03). Only monotherapy patients were positive for anti–citrullinated peptide antibodies, rheumatoid factor, or antinuclear antibodies.
Most of the patients in the study had an additional IrAE, with colitis being the most common (n=10), followed by thyroid disease, pneumonitis, and rash. Patients on PD-1 or programmed death ligand 1 monotherapy were more likely to have IA as their first IrAE.
The research team noted that the median time to symptom onset was 5 months after ICI initiation.
Diagnosis of IA following patient-reported symptoms was an average of 5.2 months, with a significant difference in lag time to diagnosis depending on initial joint presentation. For example, patients with initial small joint involvement had a 10 month longer lag time to IA diagnosis than those with knees as the initial joint involved.
In terms of treatment, 24 patients were treated with systemic corticosteroids and 10 required additional immunosuppression. The need for corticosteroids did not differ by ICI treatment regimen, but those treated with combination therapy were more likely to require additional immunosuppression (P = 0.02).
Tumor necrosis factor inhibitors with or without methotrexate were prescribed for seven patients. All of the patients had a clinical improvement in their arthritis symptoms. Four had a complete tumor response at the time of tumor necrosis factor inhibitor initiation with none having tumor progression.
The three patients treated with methotrexate monotherapy had a complete or sustained partial tumor response to ICI therapy and their cancer did not develop during IA management follow-up.
The authors went on to look at the persistence of IA after cessation of therapy in a subset of 21 patients. They found that 18 of these patients still had IA symptoms months after stopping treatment. They suggested that the delay in diagnosis and treatment seen in their study might explain the finding.
The study provides “critical information, not just for rheumatologists as they try to recognize subgroups in ICI-induced IA and diagnose patients with this new entity, but also for oncology providers who are usually first to encounter patients with ICI-induced IA and subsequently refer patients to rheumatology,” Dr. Cappelli and colleagues wrote.
The experience so far with using immunosuppression in ICI-induced IA “has been reassuring in terms of cancer outcomes, but more studies are needed to confirm this finding,” they concluded.
SOURCE: Cappelli LC et al. Semin Arthritis Rheum. doi: 10.1016/j.semarthrit. 2018.02.011.
Variations in the clinical presentation of immunotherapy-induced inflammatory arthritis is partly explained by which treatment regimen was used to treat the cancer, a single-center study suggests.
While immune checkpoint inhibitors (ICI) have revolutionized the field of oncology, their use for an ever-widening range of indications had created an increasing population of patients referred to rheumatologists for the management of immune-related adverse events (IrAEs), according to Laura C. Cappelli, MD, and her colleagues at John Hopkins University, Baltimore.
Well-established guidelines exist for managing adverse events such as colitis and pneumonitis, but there are only preliminary guidelines for evaluating and treating immunotherapy-induced inflammatory arthritis (IA). “This may stem from a lack of consistent reporting of rheumatologic IrAEs in clinical trials, the non–life threatening nature of [inflammatory arthritis], or lack of recognition of musculoskeletal symptoms by treating providers,” they wrote in Seminars in Arthritis and Rheumatism.
Clinical trials have reported ranges of arthralgia in 1%-43% of patients treated with ICIs, but no accurate estimate of the incidence of IA exists.
The researchers noted that treating patients with ICI-induced IA is complicated by a history of active or recently treated cancer and concerns over using immunosuppression in the context of ICI therapy.
They set out to evaluate the clinical presentations of 30 patients seen in their clinic with ICI-induced IA. Patients were a median of 59 years old and 12 (40%) were female. Tumor types included metastatic melanoma, non–small cell lung cancer, small cell lung cancer, colorectal cancer, Hodgkin lymphoma, cutaneous lymphoma, renal cell carcinoma, duodenal carcinoma, Merkel cell carcinoma, cutaneous basal cell carcinoma, and cutaneous squamous cell carcinoma.
Sixteen patients were treated with anti–programmed cell death protein 1 (PD-1)/programmed death ligand 1 monotherapy, and 14 were treated with combination anti–CTLA-4/PD-1 therapy.
Patients on combination therapy were significantly younger (7.5 years, P = 0.01) and were more likely to have metastatic melanoma as their underlying cancer.
Patients who received combination therapy were more likely to present first with knee IA (n = 10) and none had small joint involvement. In contrast, initial small joint involvement was more common in the monotherapy group (n = 6).
C-reactive protein levels were significantly higher in the combination therapy group (4mg/dL vs. 0.5mg/dL, P = 0.03). Only monotherapy patients were positive for anti–citrullinated peptide antibodies, rheumatoid factor, or antinuclear antibodies.
Most of the patients in the study had an additional IrAE, with colitis being the most common (n=10), followed by thyroid disease, pneumonitis, and rash. Patients on PD-1 or programmed death ligand 1 monotherapy were more likely to have IA as their first IrAE.
The research team noted that the median time to symptom onset was 5 months after ICI initiation.
Diagnosis of IA following patient-reported symptoms was an average of 5.2 months, with a significant difference in lag time to diagnosis depending on initial joint presentation. For example, patients with initial small joint involvement had a 10 month longer lag time to IA diagnosis than those with knees as the initial joint involved.
In terms of treatment, 24 patients were treated with systemic corticosteroids and 10 required additional immunosuppression. The need for corticosteroids did not differ by ICI treatment regimen, but those treated with combination therapy were more likely to require additional immunosuppression (P = 0.02).
Tumor necrosis factor inhibitors with or without methotrexate were prescribed for seven patients. All of the patients had a clinical improvement in their arthritis symptoms. Four had a complete tumor response at the time of tumor necrosis factor inhibitor initiation with none having tumor progression.
The three patients treated with methotrexate monotherapy had a complete or sustained partial tumor response to ICI therapy and their cancer did not develop during IA management follow-up.
The authors went on to look at the persistence of IA after cessation of therapy in a subset of 21 patients. They found that 18 of these patients still had IA symptoms months after stopping treatment. They suggested that the delay in diagnosis and treatment seen in their study might explain the finding.
The study provides “critical information, not just for rheumatologists as they try to recognize subgroups in ICI-induced IA and diagnose patients with this new entity, but also for oncology providers who are usually first to encounter patients with ICI-induced IA and subsequently refer patients to rheumatology,” Dr. Cappelli and colleagues wrote.
The experience so far with using immunosuppression in ICI-induced IA “has been reassuring in terms of cancer outcomes, but more studies are needed to confirm this finding,” they concluded.
SOURCE: Cappelli LC et al. Semin Arthritis Rheum. doi: 10.1016/j.semarthrit. 2018.02.011.
FROM SEMINARS IN ARTHRITIS AND RHEUMATISM
Key clinical point: The clinical features of patients with immunotherapy-induced inflammatory arthritis differ according to the treatment regimen used.
Major findings: Combination immune checkpoint inhibitor therapy was associated with higher C-reactive protein levels and a higher likelihood of having a large joint affected first.
Study details: A single-center, retrospective cohort study of 30 patients with rheumatologist-confirmed inflammatory arthritis after receiving immune checkpoint inhibitor therapy.
Disclosures: The study was funded by the National Institute of Arthritis and Musculoskeletal and Skin Disease and the Jerome L. Greene Foundation.
Source: Cappelli LC et al. Semin Arthritis Rheum. doi: 10.1016/j.semarthrit. 2018.02.011.
Hospitalizations for fracture in patients with metastatic disease: primary source lesions in the United States
It has been well established that metastatic disease to bone has major significance in the morbidity associated with the diagnosis of cancer.1 More than 75% of patients with metastatic cancer will have bone involvement at the time of death.2-4 Moreover, there is a reported 8% incidence of a pathologic fracture in patients who carry the diagnosis of cancer.5 Common sites of involvement include the spine, ribs, pelvis, and long bones such as humerus and femur.6 Pathologic fracture is fracture caused by disease rather than injury or trauma (referred to here as nonpathologic). In any bone, pathologic fracture will be associated with increased morbidity for the patient, but it is the spine and long bones that frequently require surgical intervention and are associated with high mortality and morbidity. Advanced cancer can also increase fracture risk through increasing falls; in one prospective study of patients with advanced cancer, more than half the patients experienced a fall.7
Based on historical studies of patients who have died from common cancers,4,6 it is commonly believed that breast, lung, thyroid, kidney, and prostate cancers are the most common sources of metastasis to bone, and that other common cancers, such as colorectal carcinoma (CRC), have lower rates of metastasis to bone.6,8,9 It has been inferred from this data that cancers such as CRC thereby have lower rates of pathologic fracture.
Presence of bone metastasis at time of death may be less clinically relevant than occurrence of pathologic fracture and, especially, pathologic fracture requiring hospitalization. The authors are aware of no studies that have determined the number of patients hospitalized as a result of pathologic fracture from common tumors. Despite cadaveric findings, clinical experience dictates that colorectal carcinoma is not an uncommon primary tumor in patients presenting with metastatic disease and pathologic fracture, whereas thyroid carcinoma is more rare.
Despite lower rates of metastasis to bone from CRC, progression to advanced disease is common, with projected 50,000 deaths in the United States in 2014, and tumor progression is associated with metastasis to bone.10 Patterns of health care use and costs associated with skeletal-related events in more common metastatic prostate and breast cancer are well documented.11-13 The authors are aware of no population-based studies examining the burden from metastatic fractures or hospitalization incidence attributed to CRC.
Methods
This is a retrospective study of patients hospitalized in the United States with metastatic disease. Data for this study were obtained from the 2003-2010 National (Nationwide) Inpatient Sample (NIS), the Healthcare Cost and Utilization Project (HCUP), and the Agency for Healthcare Research and Quality.14 The NIS is a stratified sample of approximately 20% of inpatient hospitalization discharges in the United States with more than 7 million hospital stays each year. The dataset contains basic patient demographics, dates of admission, discharge, and procedures, as well as diagnosis and procedure codes for unique hospitalizations. The numbers of new cases of each type of cancer diagnosed in the United States during 2003-2010 were determined from fact sheets published by the American Cancer Society.15
In all, 1,008,641 patients with metastatic disease in the NIS database, were identified by the presence of International Classification of Diseases, 9th revision, Clinical Modification (ICD-9-CM) diagnosis codes 196.0-199.1. Patients were then classified by primary cancer type based on the presence of additional ICD-9-CM codes for a specific cancer type (140.x-189.x) or for a history of a specific cancer type (V10.00 – V10.91). The analysis was limited to the 10 most common types of cancer. Multiple myeloma, leukemia, lymphoma, and primary cancers of bone also cause pathologic fractures, but they were purposefully excluded from the analysis because they do not represent truly metastatic disease. Patients were excluded if they were younger than 18 years (n = 9,425), had been admitted with major significant trauma (Major Diagnostic Category 24; n = 287), or if the cancer type was either not listed in discharge billing data or not one of the 10 most common types (n = 324,249). Therefore, the final study sample consisted of 674,680 hospitalizations.
The primary outcome assessed was pathologic fracture, identified with ICD-9-CM codes 733.10-733,19. Fractures not due to bone metastasis can occur in patients with metastatic disease owing to falls and general debility; therefore, the secondary outcome was nonpathologic fracture, identified with ICD-9-CM codes for fracture (805.0-829.0) in the absence of a code for pathologic fracture. Fractures classified as a “stress fracture” (ICD-9-CM code 733.9x) or where there was a concomitant diagnosis of osteoporosis (ICD-9-CM cod 733.0x) were also considered nonpathologic for the purpose of this study. Thus there were 3 groups of hospitalized patients identified: metastatic disease without fracture (No Fracture); Pathologic Fracture; and Nonpathologic Fracture. The study was limited to the 10 types of cancer with the highest numbers of pathologic fracture, leaving 647,680 hospitalizations for analysis.
Univariate analyses comparing the Pathologic, Nonpathologic, and No Fracture groups were performed with the Student t test for continuous characteristics and chi-square test for categorical characteristics. All analyses were performed with use of Stata 13.1 (StataCorp, College Station, TX).
This study protocol (RSRB00055625) was reviewed by the Office for Human Subject Protection Research Subjects Review Board at the University of Rochester and was determined to meet exemption criteria.
Results
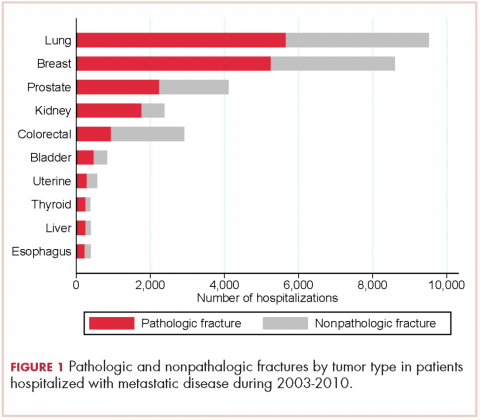
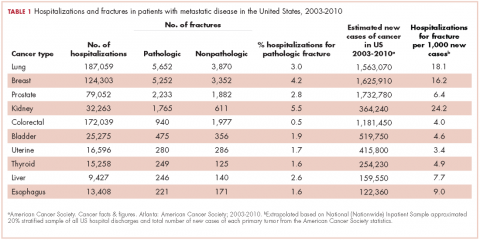
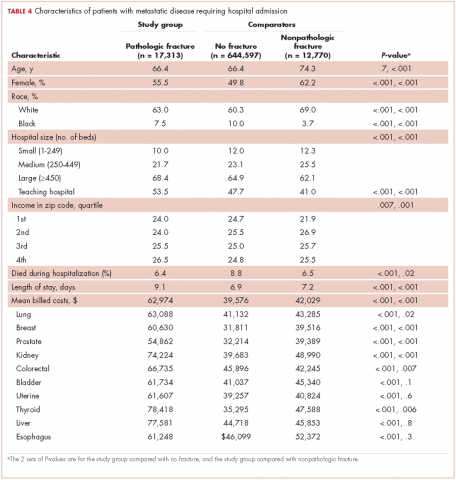
From 2003-2010 there were 674,680 hospitalizations in patients with metastatic cancer that met the inclusion criteria. Hospitalization was most frequent for lung cancer (187,059 admissions), colorectal cancer (172,039), and breast cancer (124,303; Table 1).
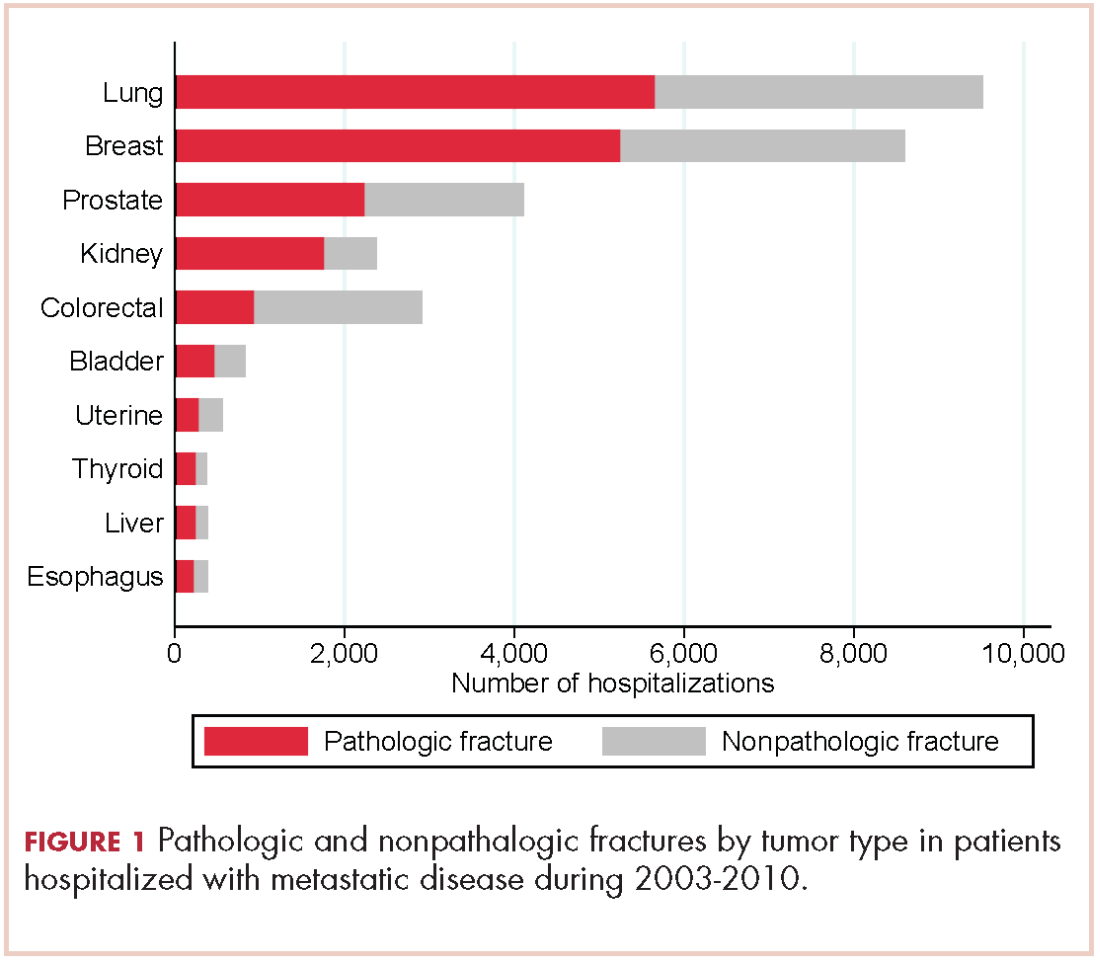
There were 17,303 hospitalizations with pathologic fracture and 12,770 hospitalizations with nonpathologic fracture (Figure 1).
Among the most commonly occurring primary cancers in hospitalizations with pathologic fracture were lung, breast, prostate, kidney, and colorectal cancers (Table 1).
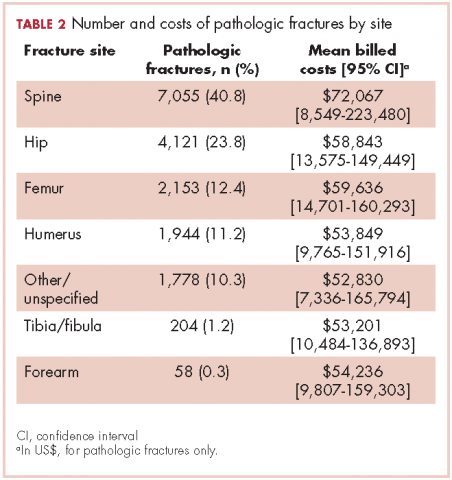
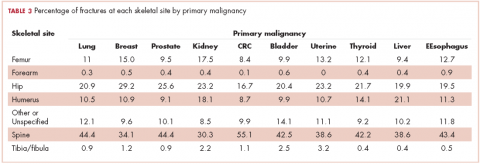
Relative to the annual incidence,15 kidney, lung, and breast cancer had the highest rates of hospital admission for pathologic fracture during the study period. Hospital admission with pathologic fracture was more common than nonpathologic fracture for every type of metastatic disease except colorectal and uterine cancer. Pathologic fracture in patients with metastatic disease was most likely to occur in the spine, hip, and femur (Table 2), and ratio of anatomic sites fractured was relatively consistent across each of the 10 primary malignancies (Table 3).
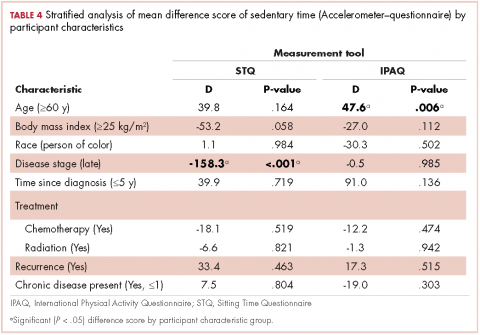
Demographic characteristics of patients in the 3 study groups are shown in Table 4. Patients with pathologic fracture were more likely than those in the no-fracture group to be white (63.0% vs 60.3%, respectively; P < .001) and female (55.5% vs 49.8%; P < .001), but were similar in age (66.4 years; P = 0.7). In-hospital mortality was lower in the pathologic fracture group compared with the no-fracture group (6.4% vs 8.8%; P < .001). People in the pathologic fracture group were more likely than others to be treated at a teaching hospital (P < .001) with ≥450 beds (P < .001), and reside in a zip code with higher income (P < .01).
Pathologic fracture hospitalizations, on average, had higher billed costs and longer length of stay ($62,974, 9.1 days; Table 4), compared with the no-fracture group ($39,576, 6.9 days; both P < .001) and the nonpathologic fracture group ($42,029, 7.2 days; both P < .001). Pathologic fracture in patients with thyroid, liver, and kidney cancer was associated with the highest costs of hospitalization.
In patients with metastatic disease, differences were found between those with pathologic and nonpathologic fractures: those with pathologic fracture were younger (66.4 vs 74.3 years; P < .001), less likely to be white (63.0% vs 69.0%; P < .001), and more commonly treated at a large hospital (68.4% vs 62.1%; P < .001) or a teaching hospital (53.5% vs 41.0%; P < .001).
Discussion
Other investigators have looked at risk factors for pathologic fracture, such as degree of bone involvement, location, and the presence of lytic versus blastic disease, as well as the optimal management of such patients.16-20 In those analyses, there is an emphasis on large, lytic lesions with cortical destruction in weight-bearing long bones, and on functional pain as a key determinant of fracture risk. Although the guidelines outlined by Mirel and others are helpful in predicting fractures, they are not widely applied by practicing oncologists.18 Oncologists and surgeons lack foolproof criteria to predict impending pathologic fracture despite evidence that the pathologic fracture event greatly increases mortality and morbidity.1,4,21,22 As far as we know, this is the first study to determine which types of primary carcinomas were most associated with pathologic fracture requiring hospitalization. This finding will hopefully raise awareness among doctors who care for these patients to be particularly conscientious with patients who present with symptoms of bone pain with activity (functional bone pain) or with lytic disease in the long bones. The results of the present study are similar to those from cadaveric studies, which emphasize the importance of lung, breast, prostate, and kidney cancers as primary tumors that metastasize to bone and lead to pathologic fracture. A novel finding is the nearly 4-fold greater number of pathologic fractures from colorectal carcinoma than thyroid carcinoma.
The importance of detecting patients at risk for pathologic fracture is now more relevant than ever because there are treatment modalities that are readily available to patients with metastatic bone involvement. Two classes of medications, the RANK-ligand inhibitors and bisphosphonates, reduce the number of skeletal events, such as pathologic fracture, in patients with metastatic disease to bone.23-26 However, most of those studies focused on the 3 most common carcinomas (breast, lung, and prostate) to metastasize to bone and cause pathologic fracture. There is greater variability in the prophylactic treatment of other forms of cancer that have metastasized to bone amongst oncologists.
Despite a lower proportion of hospitalizations for fracture in patients with CRC than for thyroid carcinoma (0.5% vs 1.6%, respectively), there were more pathologic fractures from CRC than from thyroid carcinoma because there are far more cases of CRC. SEER data estimate that in 2014 there were 62,000 cases of thyroid cancer and 1,890 deaths, compared with 136,000 cases of CRC and 50,000 deaths.10 Previous findings have shown that bone metastasis from CRC is more common than originally thought, based on autopsies of CRC patients.3 However, the lower rate of bone metastasis in CRC compared with other malignancies has led to a decreased focus on skeletal-related events in CRC. Our results suggest vigilance to bone health is warranted in patients with metastatic CRC. A novel finding is that patients with metastatic CRC also have a high number of hospital admissions for nonpathologic fracture. In establishing that patients with metastatic CRC with bone involvement have a real and significant risk of developing both pathologic and nonpathologic fractures, it may alter the treatment practice for these patients going forward, with greater consideration for an antiresorptive therapy, fall prevention education, or other preventive modalities, such as external-beam radiation therapy after it has been established that patients have metastatic bone disease.
There were some demographic differences between patients with metastatic disease who sustain pathologic fractures and those who do not fracture or sustain nonpathologic fractures. Patients with pathologic fracture were younger than those with nonpathologic fractures, and patients who sustained any fracture were more likely to be white than were patients in the no-fracture group. Known osteoporosis risk factors including older, female, and white with Northern European descent.27 Those findings emphasize the importance of osteoporosis screening and fracture prevention in patients with metastatic disease in general, regardless of the presence of bony metastasis. The present study found that patients who reside in zip codes areas with higher incomes were at slightly increased risk of hospitalization for pathologic fracture. Economic disparities in access to health care and cancer care are well documented,28 and the basis for this finding is a direction for future research.
Both mean billed costs and length of stay were greatest in the pathologic fracture group. The large number of admissions for no-fractured patients may be a final opportunity for intervention and preventative measures in this fragile population. Improved surveillance for bony lesions and attention to pain, especially at night, or unexplained hypercalcemia may help with early diagnosis and prevent some pathologic fractures. Patients with pathologic fracture often undergo additional treatments such as radiation therapy or chemotherapy. These additional treatments may partially explain the higher billed costs associated with inpatient hospitalization; future studies may be able to elucidate treatment differences or other reasons for the increased costs associated with pathologic fractures and identify targets to reduce expenditures.
Limitations
This study is subject to the limitations of a retrospective analysis based on hospital administrative discharge data. It evaluates only billed charges and does not account for costs associated with rehabilitation stays. However, it represents a stratified cross-sample of hospitalizations in the United States, in both teaching and nonteaching hospitals, and is the largest study to date that the authors are aware of looking at the burden of pathologic fractures in patients with metastatic disease.
This study specifically included only patients with metastatic disease, which therefore limits comparisons with the rate of hospitalization for nonpathologic fracture in patients without metastatic disease. Patients with metastatic disease who were not hospitalized during the study period are nevertheless at risk for fracture but would not have been captured in this study. It is also likely that some patients with metastatic disease had multiple hospitalizations, including some that were not for fracture; therefore, this study likely underestimates the percentage of patients with metastatic disease who sustain pathologic and nonpathologic fracture.
Some patients were excluded because we were not able to identify a primary cancer from hospital discharge records. The lack of an included diagnosis may be a result of indeterminate primary during the fracture admission or may represent a failure to accurately code a primary, known cancer. Although the NIS does not permit identification of these patients to determine if a primary cancer was subsequently identified, future studies using other databases may target patients presenting with pathologic fracture and an unknown primary tumor to evaluate subsequent cancer diagnosis.
Summary
The significance of bone metastasis in causing pathologic fractures in lung, breast, prostate, and kidney cancers was confirmed. Colorectal carcinoma has been established as the fifth most common primary cancer in patients with metastatic disease who are hospitalized with pathologic fracture, and a large number of patients with metastatic CRC sustain nonpathologic fractures requiring hospitalization. In patients with metastatic CRC or new skeletal pain, education on fall prevention and increased vigilance should be considered. Further studies are needed to determine the best method for prevention of pathologic fractures in all highly prevalent cancers, with previous hospitalizations without fracture as an appropriate target. Previous paradigms about which cancers metastasize to bone should be reconsidered in the context of which lead to clinically important fractures and hospitalization.
1. Carter JA, Ji X, Botteman MF. Clinical, economic and humanistic burdens of skeletal-related events associated with bone metastases. Expert Rev Pharmacoecon Outcomes Res. 2013;13(4):483-496.
2. Clain A. Secondary malignant disease of bone. Br J Cancer. 1965;19:15-29.
3. Coleman RE. Clinical features of metastatic bone disease and risk of skeletal morbidity. Clin Cancer Res. 2006;12(20 Pt 2):6243s-6249s.
4. Coleman RE. Skeletal complications of malignancy. Cancer. 1997;80(8 Suppl):1588-1594.
5. Higinbotham NL, Marcove RC. The management of pathological fractures. J Trauma. 1965;5(6):792-798.
6. Hess KR, Varadhachary GR, Taylor SH, et al. Metastatic patterns in adenocarcinoma. Cancer. 2006;106(7):1624-1633.
7. Stone CA, Lawlor PG, Savva GM, Bennett K, Kenny RA. Prospective study of falls and risk factors for falls in adults with advanced cancer. J Clin Oncol. 2012;30(17):2128-2133.
8. Coleman RE. Metastatic bone disease: clinical features, pathophysiology and treatment strategies. Cancer Treat Rev. 2001;27(3):165-176.
9. Katoh M, Unakami M, Hara M, Fukuchi S. Bone metastasis from colorectal cancer in autopsy cases. J Gastroenterol. 1995;30(5):615-618.
10. Howlader N NA, Krapcho M, Garshell J, Miller D, Altekruse SF, Kosary CL, Yu M, Ruhl J, Tatalovich Z,Mariotto A, Lewis DR, Chen HS, Feuer EJ, Cronin KA (eds). SEER Cancer Statistics Review, 1975-2011, National Cancer Institute. Bethesda, MD, http://seer.cancer.gov/csr/1975_2011/, based on November 2013 SEER data submission. Posted April 2014. Accessed January 19, 2018.
11. Hagiwara M, Delea TE, Saville MW, Chung K. Healthcare utilization and costs associated with skeletal-related events in prostate cancer patients with bone metastases. Prostate Cancer Prostatic Dis. 2013;16(1):23-27.
12. Hagiwara M, Delea TE, Chung K. Healthcare costs associated with skeletal-related events in breast cancer patients with bone metastases. J Med Econ. 2014;17(3):223-230.
13. Yong C, Onukwugha E, Mullins CD. Clinical and economic burden of bone metastasis and skeletal-related events in prostate cancer. Curr Opin Oncol. 2014;26(3):274-283.
14. HCUP Nationwide Inpatient Sample (NIS). Healthcare Cost and Utilization Project (HCUP). 2011. Agency for Healthcare Research and Quality R, MD. http://www.hcup-us.ahrq.gov/nisoverview.jsp. Last modified January 17, 2018. Accessed January 18, 2018.
15. American Cancer Society. Cancer Facts & Figures. Atlanta: American Cancer Society; 2003-2010. https://www.cancer.org/research/cancer-facts-statistics/all-cancer-facts-figures/cancer-facts-figures-2010.html Published January 2010. Accessed January 17, 2018.
16. Harrington KD. Orthopedic surgical management of skeletal complications of malignancy. Cancer. 1997;80(8 Suppl):1614-1627.
17. Harrington KD. Impending pathologic fractures from metastatic malignancy: evaluation and management. Instr Course Lect. 1986;35:357-381.
18. Mirels H. Metastatic disease in long bones. A proposed scoring system for diagnosing impending pathologic fractures. Clin Orthop Relat Res. 1989;249:256-264.
19. Weber KL. Evaluation of the adult patient (aged >40 years) with a destructive bone lesion. J Am Acad Orthop Surg. 2010;18(3):169-179.
20. Rougraff BT. Evaluation of the patient with carcinoma of unknown origin metastatic to bone. Clin Orthop Relat Res. 2003(415 Suppl):S105-109.
21. Coleman RE, Rubens RD. The clinical course of bone metastases from breast cancer. Br J Cancer. 1987;55(1):61-66.
22. Dijstra S, Wiggers T, van Geel BN, Boxma H. Impending and actual pathological fractures in patients with bone metastases of the long bones. A retrospective study of 233 surgically treated fractures. Eur J Surg. 1994;160(10):535-542.
23. Henry D, Vadhan-Raj S, Hirsh V, et al. Delaying skeletal-related events in a randomized phase 3 study of denosumab versus zoledronic acid in patients with advanced cancer: an analysis of data from patients with solid tumors. Support Care Cancer. 2014;22(3):679-687.
24. Lorusso V, Duran I, Garzon-Rodriguez C, et al. Health resource utilisation associated with skeletal-related events in European patients with lung cancer: Alpha subgroup analysis from a prospective multinational study. Mol Clin Oncol. 2014;2(5):701-708.
25. Lothgren M, Ribnicsek E, Schmidt L, et al. Cost per patient and potential budget implications of denosumab compared with zoledronic acid in adults with bone metastases from solid tumours who are at risk of skeletal-related events: an analysis for Austria, Sweden and Switzerland. Eu J Hosp Pharm Sci Pract. 2013;20(4):227-231.
26. Luftner D, Lorusso V, Duran I, et al. Health resource utilization associated with skeletal-related events in patients with advanced breast cancer: results from a prospective, multinational observational study. SpringerPlus. 2014;3:328.
27. Cauley JA. Defining ethnic and racial differences in osteoporosis and fragility fractures. Clin Orthop Relat Res. 2011;469(7):1891-1899.
28. VanEenwyk J, Campo JS, Ossiander EM. Socioeconomic and demographic disparities in treatment for carcinomas of the colon and rectum. Cancer. 2002;95(1):39-46.
It has been well established that metastatic disease to bone has major significance in the morbidity associated with the diagnosis of cancer.1 More than 75% of patients with metastatic cancer will have bone involvement at the time of death.2-4 Moreover, there is a reported 8% incidence of a pathologic fracture in patients who carry the diagnosis of cancer.5 Common sites of involvement include the spine, ribs, pelvis, and long bones such as humerus and femur.6 Pathologic fracture is fracture caused by disease rather than injury or trauma (referred to here as nonpathologic). In any bone, pathologic fracture will be associated with increased morbidity for the patient, but it is the spine and long bones that frequently require surgical intervention and are associated with high mortality and morbidity. Advanced cancer can also increase fracture risk through increasing falls; in one prospective study of patients with advanced cancer, more than half the patients experienced a fall.7
Based on historical studies of patients who have died from common cancers,4,6 it is commonly believed that breast, lung, thyroid, kidney, and prostate cancers are the most common sources of metastasis to bone, and that other common cancers, such as colorectal carcinoma (CRC), have lower rates of metastasis to bone.6,8,9 It has been inferred from this data that cancers such as CRC thereby have lower rates of pathologic fracture.
Presence of bone metastasis at time of death may be less clinically relevant than occurrence of pathologic fracture and, especially, pathologic fracture requiring hospitalization. The authors are aware of no studies that have determined the number of patients hospitalized as a result of pathologic fracture from common tumors. Despite cadaveric findings, clinical experience dictates that colorectal carcinoma is not an uncommon primary tumor in patients presenting with metastatic disease and pathologic fracture, whereas thyroid carcinoma is more rare.
Despite lower rates of metastasis to bone from CRC, progression to advanced disease is common, with projected 50,000 deaths in the United States in 2014, and tumor progression is associated with metastasis to bone.10 Patterns of health care use and costs associated with skeletal-related events in more common metastatic prostate and breast cancer are well documented.11-13 The authors are aware of no population-based studies examining the burden from metastatic fractures or hospitalization incidence attributed to CRC.
Methods
This is a retrospective study of patients hospitalized in the United States with metastatic disease. Data for this study were obtained from the 2003-2010 National (Nationwide) Inpatient Sample (NIS), the Healthcare Cost and Utilization Project (HCUP), and the Agency for Healthcare Research and Quality.14 The NIS is a stratified sample of approximately 20% of inpatient hospitalization discharges in the United States with more than 7 million hospital stays each year. The dataset contains basic patient demographics, dates of admission, discharge, and procedures, as well as diagnosis and procedure codes for unique hospitalizations. The numbers of new cases of each type of cancer diagnosed in the United States during 2003-2010 were determined from fact sheets published by the American Cancer Society.15
In all, 1,008,641 patients with metastatic disease in the NIS database, were identified by the presence of International Classification of Diseases, 9th revision, Clinical Modification (ICD-9-CM) diagnosis codes 196.0-199.1. Patients were then classified by primary cancer type based on the presence of additional ICD-9-CM codes for a specific cancer type (140.x-189.x) or for a history of a specific cancer type (V10.00 – V10.91). The analysis was limited to the 10 most common types of cancer. Multiple myeloma, leukemia, lymphoma, and primary cancers of bone also cause pathologic fractures, but they were purposefully excluded from the analysis because they do not represent truly metastatic disease. Patients were excluded if they were younger than 18 years (n = 9,425), had been admitted with major significant trauma (Major Diagnostic Category 24; n = 287), or if the cancer type was either not listed in discharge billing data or not one of the 10 most common types (n = 324,249). Therefore, the final study sample consisted of 674,680 hospitalizations.
The primary outcome assessed was pathologic fracture, identified with ICD-9-CM codes 733.10-733,19. Fractures not due to bone metastasis can occur in patients with metastatic disease owing to falls and general debility; therefore, the secondary outcome was nonpathologic fracture, identified with ICD-9-CM codes for fracture (805.0-829.0) in the absence of a code for pathologic fracture. Fractures classified as a “stress fracture” (ICD-9-CM code 733.9x) or where there was a concomitant diagnosis of osteoporosis (ICD-9-CM cod 733.0x) were also considered nonpathologic for the purpose of this study. Thus there were 3 groups of hospitalized patients identified: metastatic disease without fracture (No Fracture); Pathologic Fracture; and Nonpathologic Fracture. The study was limited to the 10 types of cancer with the highest numbers of pathologic fracture, leaving 647,680 hospitalizations for analysis.
Univariate analyses comparing the Pathologic, Nonpathologic, and No Fracture groups were performed with the Student t test for continuous characteristics and chi-square test for categorical characteristics. All analyses were performed with use of Stata 13.1 (StataCorp, College Station, TX).
This study protocol (RSRB00055625) was reviewed by the Office for Human Subject Protection Research Subjects Review Board at the University of Rochester and was determined to meet exemption criteria.
Results
From 2003-2010 there were 674,680 hospitalizations in patients with metastatic cancer that met the inclusion criteria. Hospitalization was most frequent for lung cancer (187,059 admissions), colorectal cancer (172,039), and breast cancer (124,303; Table 1).
There were 17,303 hospitalizations with pathologic fracture and 12,770 hospitalizations with nonpathologic fracture (Figure 1).
Among the most commonly occurring primary cancers in hospitalizations with pathologic fracture were lung, breast, prostate, kidney, and colorectal cancers (Table 1).
Relative to the annual incidence,15 kidney, lung, and breast cancer had the highest rates of hospital admission for pathologic fracture during the study period. Hospital admission with pathologic fracture was more common than nonpathologic fracture for every type of metastatic disease except colorectal and uterine cancer. Pathologic fracture in patients with metastatic disease was most likely to occur in the spine, hip, and femur (Table 2), and ratio of anatomic sites fractured was relatively consistent across each of the 10 primary malignancies (Table 3).
Demographic characteristics of patients in the 3 study groups are shown in Table 4. Patients with pathologic fracture were more likely than those in the no-fracture group to be white (63.0% vs 60.3%, respectively; P < .001) and female (55.5% vs 49.8%; P < .001), but were similar in age (66.4 years; P = 0.7). In-hospital mortality was lower in the pathologic fracture group compared with the no-fracture group (6.4% vs 8.8%; P < .001). People in the pathologic fracture group were more likely than others to be treated at a teaching hospital (P < .001) with ≥450 beds (P < .001), and reside in a zip code with higher income (P < .01).
Pathologic fracture hospitalizations, on average, had higher billed costs and longer length of stay ($62,974, 9.1 days; Table 4), compared with the no-fracture group ($39,576, 6.9 days; both P < .001) and the nonpathologic fracture group ($42,029, 7.2 days; both P < .001). Pathologic fracture in patients with thyroid, liver, and kidney cancer was associated with the highest costs of hospitalization.
In patients with metastatic disease, differences were found between those with pathologic and nonpathologic fractures: those with pathologic fracture were younger (66.4 vs 74.3 years; P < .001), less likely to be white (63.0% vs 69.0%; P < .001), and more commonly treated at a large hospital (68.4% vs 62.1%; P < .001) or a teaching hospital (53.5% vs 41.0%; P < .001).
Discussion
Other investigators have looked at risk factors for pathologic fracture, such as degree of bone involvement, location, and the presence of lytic versus blastic disease, as well as the optimal management of such patients.16-20 In those analyses, there is an emphasis on large, lytic lesions with cortical destruction in weight-bearing long bones, and on functional pain as a key determinant of fracture risk. Although the guidelines outlined by Mirel and others are helpful in predicting fractures, they are not widely applied by practicing oncologists.18 Oncologists and surgeons lack foolproof criteria to predict impending pathologic fracture despite evidence that the pathologic fracture event greatly increases mortality and morbidity.1,4,21,22 As far as we know, this is the first study to determine which types of primary carcinomas were most associated with pathologic fracture requiring hospitalization. This finding will hopefully raise awareness among doctors who care for these patients to be particularly conscientious with patients who present with symptoms of bone pain with activity (functional bone pain) or with lytic disease in the long bones. The results of the present study are similar to those from cadaveric studies, which emphasize the importance of lung, breast, prostate, and kidney cancers as primary tumors that metastasize to bone and lead to pathologic fracture. A novel finding is the nearly 4-fold greater number of pathologic fractures from colorectal carcinoma than thyroid carcinoma.
The importance of detecting patients at risk for pathologic fracture is now more relevant than ever because there are treatment modalities that are readily available to patients with metastatic bone involvement. Two classes of medications, the RANK-ligand inhibitors and bisphosphonates, reduce the number of skeletal events, such as pathologic fracture, in patients with metastatic disease to bone.23-26 However, most of those studies focused on the 3 most common carcinomas (breast, lung, and prostate) to metastasize to bone and cause pathologic fracture. There is greater variability in the prophylactic treatment of other forms of cancer that have metastasized to bone amongst oncologists.
Despite a lower proportion of hospitalizations for fracture in patients with CRC than for thyroid carcinoma (0.5% vs 1.6%, respectively), there were more pathologic fractures from CRC than from thyroid carcinoma because there are far more cases of CRC. SEER data estimate that in 2014 there were 62,000 cases of thyroid cancer and 1,890 deaths, compared with 136,000 cases of CRC and 50,000 deaths.10 Previous findings have shown that bone metastasis from CRC is more common than originally thought, based on autopsies of CRC patients.3 However, the lower rate of bone metastasis in CRC compared with other malignancies has led to a decreased focus on skeletal-related events in CRC. Our results suggest vigilance to bone health is warranted in patients with metastatic CRC. A novel finding is that patients with metastatic CRC also have a high number of hospital admissions for nonpathologic fracture. In establishing that patients with metastatic CRC with bone involvement have a real and significant risk of developing both pathologic and nonpathologic fractures, it may alter the treatment practice for these patients going forward, with greater consideration for an antiresorptive therapy, fall prevention education, or other preventive modalities, such as external-beam radiation therapy after it has been established that patients have metastatic bone disease.
There were some demographic differences between patients with metastatic disease who sustain pathologic fractures and those who do not fracture or sustain nonpathologic fractures. Patients with pathologic fracture were younger than those with nonpathologic fractures, and patients who sustained any fracture were more likely to be white than were patients in the no-fracture group. Known osteoporosis risk factors including older, female, and white with Northern European descent.27 Those findings emphasize the importance of osteoporosis screening and fracture prevention in patients with metastatic disease in general, regardless of the presence of bony metastasis. The present study found that patients who reside in zip codes areas with higher incomes were at slightly increased risk of hospitalization for pathologic fracture. Economic disparities in access to health care and cancer care are well documented,28 and the basis for this finding is a direction for future research.
Both mean billed costs and length of stay were greatest in the pathologic fracture group. The large number of admissions for no-fractured patients may be a final opportunity for intervention and preventative measures in this fragile population. Improved surveillance for bony lesions and attention to pain, especially at night, or unexplained hypercalcemia may help with early diagnosis and prevent some pathologic fractures. Patients with pathologic fracture often undergo additional treatments such as radiation therapy or chemotherapy. These additional treatments may partially explain the higher billed costs associated with inpatient hospitalization; future studies may be able to elucidate treatment differences or other reasons for the increased costs associated with pathologic fractures and identify targets to reduce expenditures.
Limitations
This study is subject to the limitations of a retrospective analysis based on hospital administrative discharge data. It evaluates only billed charges and does not account for costs associated with rehabilitation stays. However, it represents a stratified cross-sample of hospitalizations in the United States, in both teaching and nonteaching hospitals, and is the largest study to date that the authors are aware of looking at the burden of pathologic fractures in patients with metastatic disease.
This study specifically included only patients with metastatic disease, which therefore limits comparisons with the rate of hospitalization for nonpathologic fracture in patients without metastatic disease. Patients with metastatic disease who were not hospitalized during the study period are nevertheless at risk for fracture but would not have been captured in this study. It is also likely that some patients with metastatic disease had multiple hospitalizations, including some that were not for fracture; therefore, this study likely underestimates the percentage of patients with metastatic disease who sustain pathologic and nonpathologic fracture.
Some patients were excluded because we were not able to identify a primary cancer from hospital discharge records. The lack of an included diagnosis may be a result of indeterminate primary during the fracture admission or may represent a failure to accurately code a primary, known cancer. Although the NIS does not permit identification of these patients to determine if a primary cancer was subsequently identified, future studies using other databases may target patients presenting with pathologic fracture and an unknown primary tumor to evaluate subsequent cancer diagnosis.
Summary
The significance of bone metastasis in causing pathologic fractures in lung, breast, prostate, and kidney cancers was confirmed. Colorectal carcinoma has been established as the fifth most common primary cancer in patients with metastatic disease who are hospitalized with pathologic fracture, and a large number of patients with metastatic CRC sustain nonpathologic fractures requiring hospitalization. In patients with metastatic CRC or new skeletal pain, education on fall prevention and increased vigilance should be considered. Further studies are needed to determine the best method for prevention of pathologic fractures in all highly prevalent cancers, with previous hospitalizations without fracture as an appropriate target. Previous paradigms about which cancers metastasize to bone should be reconsidered in the context of which lead to clinically important fractures and hospitalization.
It has been well established that metastatic disease to bone has major significance in the morbidity associated with the diagnosis of cancer.1 More than 75% of patients with metastatic cancer will have bone involvement at the time of death.2-4 Moreover, there is a reported 8% incidence of a pathologic fracture in patients who carry the diagnosis of cancer.5 Common sites of involvement include the spine, ribs, pelvis, and long bones such as humerus and femur.6 Pathologic fracture is fracture caused by disease rather than injury or trauma (referred to here as nonpathologic). In any bone, pathologic fracture will be associated with increased morbidity for the patient, but it is the spine and long bones that frequently require surgical intervention and are associated with high mortality and morbidity. Advanced cancer can also increase fracture risk through increasing falls; in one prospective study of patients with advanced cancer, more than half the patients experienced a fall.7
Based on historical studies of patients who have died from common cancers,4,6 it is commonly believed that breast, lung, thyroid, kidney, and prostate cancers are the most common sources of metastasis to bone, and that other common cancers, such as colorectal carcinoma (CRC), have lower rates of metastasis to bone.6,8,9 It has been inferred from this data that cancers such as CRC thereby have lower rates of pathologic fracture.
Presence of bone metastasis at time of death may be less clinically relevant than occurrence of pathologic fracture and, especially, pathologic fracture requiring hospitalization. The authors are aware of no studies that have determined the number of patients hospitalized as a result of pathologic fracture from common tumors. Despite cadaveric findings, clinical experience dictates that colorectal carcinoma is not an uncommon primary tumor in patients presenting with metastatic disease and pathologic fracture, whereas thyroid carcinoma is more rare.
Despite lower rates of metastasis to bone from CRC, progression to advanced disease is common, with projected 50,000 deaths in the United States in 2014, and tumor progression is associated with metastasis to bone.10 Patterns of health care use and costs associated with skeletal-related events in more common metastatic prostate and breast cancer are well documented.11-13 The authors are aware of no population-based studies examining the burden from metastatic fractures or hospitalization incidence attributed to CRC.
Methods
This is a retrospective study of patients hospitalized in the United States with metastatic disease. Data for this study were obtained from the 2003-2010 National (Nationwide) Inpatient Sample (NIS), the Healthcare Cost and Utilization Project (HCUP), and the Agency for Healthcare Research and Quality.14 The NIS is a stratified sample of approximately 20% of inpatient hospitalization discharges in the United States with more than 7 million hospital stays each year. The dataset contains basic patient demographics, dates of admission, discharge, and procedures, as well as diagnosis and procedure codes for unique hospitalizations. The numbers of new cases of each type of cancer diagnosed in the United States during 2003-2010 were determined from fact sheets published by the American Cancer Society.15
In all, 1,008,641 patients with metastatic disease in the NIS database, were identified by the presence of International Classification of Diseases, 9th revision, Clinical Modification (ICD-9-CM) diagnosis codes 196.0-199.1. Patients were then classified by primary cancer type based on the presence of additional ICD-9-CM codes for a specific cancer type (140.x-189.x) or for a history of a specific cancer type (V10.00 – V10.91). The analysis was limited to the 10 most common types of cancer. Multiple myeloma, leukemia, lymphoma, and primary cancers of bone also cause pathologic fractures, but they were purposefully excluded from the analysis because they do not represent truly metastatic disease. Patients were excluded if they were younger than 18 years (n = 9,425), had been admitted with major significant trauma (Major Diagnostic Category 24; n = 287), or if the cancer type was either not listed in discharge billing data or not one of the 10 most common types (n = 324,249). Therefore, the final study sample consisted of 674,680 hospitalizations.
The primary outcome assessed was pathologic fracture, identified with ICD-9-CM codes 733.10-733,19. Fractures not due to bone metastasis can occur in patients with metastatic disease owing to falls and general debility; therefore, the secondary outcome was nonpathologic fracture, identified with ICD-9-CM codes for fracture (805.0-829.0) in the absence of a code for pathologic fracture. Fractures classified as a “stress fracture” (ICD-9-CM code 733.9x) or where there was a concomitant diagnosis of osteoporosis (ICD-9-CM cod 733.0x) were also considered nonpathologic for the purpose of this study. Thus there were 3 groups of hospitalized patients identified: metastatic disease without fracture (No Fracture); Pathologic Fracture; and Nonpathologic Fracture. The study was limited to the 10 types of cancer with the highest numbers of pathologic fracture, leaving 647,680 hospitalizations for analysis.
Univariate analyses comparing the Pathologic, Nonpathologic, and No Fracture groups were performed with the Student t test for continuous characteristics and chi-square test for categorical characteristics. All analyses were performed with use of Stata 13.1 (StataCorp, College Station, TX).
This study protocol (RSRB00055625) was reviewed by the Office for Human Subject Protection Research Subjects Review Board at the University of Rochester and was determined to meet exemption criteria.
Results
From 2003-2010 there were 674,680 hospitalizations in patients with metastatic cancer that met the inclusion criteria. Hospitalization was most frequent for lung cancer (187,059 admissions), colorectal cancer (172,039), and breast cancer (124,303; Table 1).
There were 17,303 hospitalizations with pathologic fracture and 12,770 hospitalizations with nonpathologic fracture (Figure 1).
Among the most commonly occurring primary cancers in hospitalizations with pathologic fracture were lung, breast, prostate, kidney, and colorectal cancers (Table 1).
Relative to the annual incidence,15 kidney, lung, and breast cancer had the highest rates of hospital admission for pathologic fracture during the study period. Hospital admission with pathologic fracture was more common than nonpathologic fracture for every type of metastatic disease except colorectal and uterine cancer. Pathologic fracture in patients with metastatic disease was most likely to occur in the spine, hip, and femur (Table 2), and ratio of anatomic sites fractured was relatively consistent across each of the 10 primary malignancies (Table 3).
Demographic characteristics of patients in the 3 study groups are shown in Table 4. Patients with pathologic fracture were more likely than those in the no-fracture group to be white (63.0% vs 60.3%, respectively; P < .001) and female (55.5% vs 49.8%; P < .001), but were similar in age (66.4 years; P = 0.7). In-hospital mortality was lower in the pathologic fracture group compared with the no-fracture group (6.4% vs 8.8%; P < .001). People in the pathologic fracture group were more likely than others to be treated at a teaching hospital (P < .001) with ≥450 beds (P < .001), and reside in a zip code with higher income (P < .01).
Pathologic fracture hospitalizations, on average, had higher billed costs and longer length of stay ($62,974, 9.1 days; Table 4), compared with the no-fracture group ($39,576, 6.9 days; both P < .001) and the nonpathologic fracture group ($42,029, 7.2 days; both P < .001). Pathologic fracture in patients with thyroid, liver, and kidney cancer was associated with the highest costs of hospitalization.
In patients with metastatic disease, differences were found between those with pathologic and nonpathologic fractures: those with pathologic fracture were younger (66.4 vs 74.3 years; P < .001), less likely to be white (63.0% vs 69.0%; P < .001), and more commonly treated at a large hospital (68.4% vs 62.1%; P < .001) or a teaching hospital (53.5% vs 41.0%; P < .001).
Discussion
Other investigators have looked at risk factors for pathologic fracture, such as degree of bone involvement, location, and the presence of lytic versus blastic disease, as well as the optimal management of such patients.16-20 In those analyses, there is an emphasis on large, lytic lesions with cortical destruction in weight-bearing long bones, and on functional pain as a key determinant of fracture risk. Although the guidelines outlined by Mirel and others are helpful in predicting fractures, they are not widely applied by practicing oncologists.18 Oncologists and surgeons lack foolproof criteria to predict impending pathologic fracture despite evidence that the pathologic fracture event greatly increases mortality and morbidity.1,4,21,22 As far as we know, this is the first study to determine which types of primary carcinomas were most associated with pathologic fracture requiring hospitalization. This finding will hopefully raise awareness among doctors who care for these patients to be particularly conscientious with patients who present with symptoms of bone pain with activity (functional bone pain) or with lytic disease in the long bones. The results of the present study are similar to those from cadaveric studies, which emphasize the importance of lung, breast, prostate, and kidney cancers as primary tumors that metastasize to bone and lead to pathologic fracture. A novel finding is the nearly 4-fold greater number of pathologic fractures from colorectal carcinoma than thyroid carcinoma.
The importance of detecting patients at risk for pathologic fracture is now more relevant than ever because there are treatment modalities that are readily available to patients with metastatic bone involvement. Two classes of medications, the RANK-ligand inhibitors and bisphosphonates, reduce the number of skeletal events, such as pathologic fracture, in patients with metastatic disease to bone.23-26 However, most of those studies focused on the 3 most common carcinomas (breast, lung, and prostate) to metastasize to bone and cause pathologic fracture. There is greater variability in the prophylactic treatment of other forms of cancer that have metastasized to bone amongst oncologists.
Despite a lower proportion of hospitalizations for fracture in patients with CRC than for thyroid carcinoma (0.5% vs 1.6%, respectively), there were more pathologic fractures from CRC than from thyroid carcinoma because there are far more cases of CRC. SEER data estimate that in 2014 there were 62,000 cases of thyroid cancer and 1,890 deaths, compared with 136,000 cases of CRC and 50,000 deaths.10 Previous findings have shown that bone metastasis from CRC is more common than originally thought, based on autopsies of CRC patients.3 However, the lower rate of bone metastasis in CRC compared with other malignancies has led to a decreased focus on skeletal-related events in CRC. Our results suggest vigilance to bone health is warranted in patients with metastatic CRC. A novel finding is that patients with metastatic CRC also have a high number of hospital admissions for nonpathologic fracture. In establishing that patients with metastatic CRC with bone involvement have a real and significant risk of developing both pathologic and nonpathologic fractures, it may alter the treatment practice for these patients going forward, with greater consideration for an antiresorptive therapy, fall prevention education, or other preventive modalities, such as external-beam radiation therapy after it has been established that patients have metastatic bone disease.
There were some demographic differences between patients with metastatic disease who sustain pathologic fractures and those who do not fracture or sustain nonpathologic fractures. Patients with pathologic fracture were younger than those with nonpathologic fractures, and patients who sustained any fracture were more likely to be white than were patients in the no-fracture group. Known osteoporosis risk factors including older, female, and white with Northern European descent.27 Those findings emphasize the importance of osteoporosis screening and fracture prevention in patients with metastatic disease in general, regardless of the presence of bony metastasis. The present study found that patients who reside in zip codes areas with higher incomes were at slightly increased risk of hospitalization for pathologic fracture. Economic disparities in access to health care and cancer care are well documented,28 and the basis for this finding is a direction for future research.
Both mean billed costs and length of stay were greatest in the pathologic fracture group. The large number of admissions for no-fractured patients may be a final opportunity for intervention and preventative measures in this fragile population. Improved surveillance for bony lesions and attention to pain, especially at night, or unexplained hypercalcemia may help with early diagnosis and prevent some pathologic fractures. Patients with pathologic fracture often undergo additional treatments such as radiation therapy or chemotherapy. These additional treatments may partially explain the higher billed costs associated with inpatient hospitalization; future studies may be able to elucidate treatment differences or other reasons for the increased costs associated with pathologic fractures and identify targets to reduce expenditures.
Limitations
This study is subject to the limitations of a retrospective analysis based on hospital administrative discharge data. It evaluates only billed charges and does not account for costs associated with rehabilitation stays. However, it represents a stratified cross-sample of hospitalizations in the United States, in both teaching and nonteaching hospitals, and is the largest study to date that the authors are aware of looking at the burden of pathologic fractures in patients with metastatic disease.
This study specifically included only patients with metastatic disease, which therefore limits comparisons with the rate of hospitalization for nonpathologic fracture in patients without metastatic disease. Patients with metastatic disease who were not hospitalized during the study period are nevertheless at risk for fracture but would not have been captured in this study. It is also likely that some patients with metastatic disease had multiple hospitalizations, including some that were not for fracture; therefore, this study likely underestimates the percentage of patients with metastatic disease who sustain pathologic and nonpathologic fracture.
Some patients were excluded because we were not able to identify a primary cancer from hospital discharge records. The lack of an included diagnosis may be a result of indeterminate primary during the fracture admission or may represent a failure to accurately code a primary, known cancer. Although the NIS does not permit identification of these patients to determine if a primary cancer was subsequently identified, future studies using other databases may target patients presenting with pathologic fracture and an unknown primary tumor to evaluate subsequent cancer diagnosis.
Summary
The significance of bone metastasis in causing pathologic fractures in lung, breast, prostate, and kidney cancers was confirmed. Colorectal carcinoma has been established as the fifth most common primary cancer in patients with metastatic disease who are hospitalized with pathologic fracture, and a large number of patients with metastatic CRC sustain nonpathologic fractures requiring hospitalization. In patients with metastatic CRC or new skeletal pain, education on fall prevention and increased vigilance should be considered. Further studies are needed to determine the best method for prevention of pathologic fractures in all highly prevalent cancers, with previous hospitalizations without fracture as an appropriate target. Previous paradigms about which cancers metastasize to bone should be reconsidered in the context of which lead to clinically important fractures and hospitalization.
1. Carter JA, Ji X, Botteman MF. Clinical, economic and humanistic burdens of skeletal-related events associated with bone metastases. Expert Rev Pharmacoecon Outcomes Res. 2013;13(4):483-496.
2. Clain A. Secondary malignant disease of bone. Br J Cancer. 1965;19:15-29.
3. Coleman RE. Clinical features of metastatic bone disease and risk of skeletal morbidity. Clin Cancer Res. 2006;12(20 Pt 2):6243s-6249s.
4. Coleman RE. Skeletal complications of malignancy. Cancer. 1997;80(8 Suppl):1588-1594.
5. Higinbotham NL, Marcove RC. The management of pathological fractures. J Trauma. 1965;5(6):792-798.
6. Hess KR, Varadhachary GR, Taylor SH, et al. Metastatic patterns in adenocarcinoma. Cancer. 2006;106(7):1624-1633.
7. Stone CA, Lawlor PG, Savva GM, Bennett K, Kenny RA. Prospective study of falls and risk factors for falls in adults with advanced cancer. J Clin Oncol. 2012;30(17):2128-2133.
8. Coleman RE. Metastatic bone disease: clinical features, pathophysiology and treatment strategies. Cancer Treat Rev. 2001;27(3):165-176.
9. Katoh M, Unakami M, Hara M, Fukuchi S. Bone metastasis from colorectal cancer in autopsy cases. J Gastroenterol. 1995;30(5):615-618.
10. Howlader N NA, Krapcho M, Garshell J, Miller D, Altekruse SF, Kosary CL, Yu M, Ruhl J, Tatalovich Z,Mariotto A, Lewis DR, Chen HS, Feuer EJ, Cronin KA (eds). SEER Cancer Statistics Review, 1975-2011, National Cancer Institute. Bethesda, MD, http://seer.cancer.gov/csr/1975_2011/, based on November 2013 SEER data submission. Posted April 2014. Accessed January 19, 2018.
11. Hagiwara M, Delea TE, Saville MW, Chung K. Healthcare utilization and costs associated with skeletal-related events in prostate cancer patients with bone metastases. Prostate Cancer Prostatic Dis. 2013;16(1):23-27.
12. Hagiwara M, Delea TE, Chung K. Healthcare costs associated with skeletal-related events in breast cancer patients with bone metastases. J Med Econ. 2014;17(3):223-230.
13. Yong C, Onukwugha E, Mullins CD. Clinical and economic burden of bone metastasis and skeletal-related events in prostate cancer. Curr Opin Oncol. 2014;26(3):274-283.
14. HCUP Nationwide Inpatient Sample (NIS). Healthcare Cost and Utilization Project (HCUP). 2011. Agency for Healthcare Research and Quality R, MD. http://www.hcup-us.ahrq.gov/nisoverview.jsp. Last modified January 17, 2018. Accessed January 18, 2018.
15. American Cancer Society. Cancer Facts & Figures. Atlanta: American Cancer Society; 2003-2010. https://www.cancer.org/research/cancer-facts-statistics/all-cancer-facts-figures/cancer-facts-figures-2010.html Published January 2010. Accessed January 17, 2018.
16. Harrington KD. Orthopedic surgical management of skeletal complications of malignancy. Cancer. 1997;80(8 Suppl):1614-1627.
17. Harrington KD. Impending pathologic fractures from metastatic malignancy: evaluation and management. Instr Course Lect. 1986;35:357-381.
18. Mirels H. Metastatic disease in long bones. A proposed scoring system for diagnosing impending pathologic fractures. Clin Orthop Relat Res. 1989;249:256-264.
19. Weber KL. Evaluation of the adult patient (aged >40 years) with a destructive bone lesion. J Am Acad Orthop Surg. 2010;18(3):169-179.
20. Rougraff BT. Evaluation of the patient with carcinoma of unknown origin metastatic to bone. Clin Orthop Relat Res. 2003(415 Suppl):S105-109.
21. Coleman RE, Rubens RD. The clinical course of bone metastases from breast cancer. Br J Cancer. 1987;55(1):61-66.
22. Dijstra S, Wiggers T, van Geel BN, Boxma H. Impending and actual pathological fractures in patients with bone metastases of the long bones. A retrospective study of 233 surgically treated fractures. Eur J Surg. 1994;160(10):535-542.
23. Henry D, Vadhan-Raj S, Hirsh V, et al. Delaying skeletal-related events in a randomized phase 3 study of denosumab versus zoledronic acid in patients with advanced cancer: an analysis of data from patients with solid tumors. Support Care Cancer. 2014;22(3):679-687.
24. Lorusso V, Duran I, Garzon-Rodriguez C, et al. Health resource utilisation associated with skeletal-related events in European patients with lung cancer: Alpha subgroup analysis from a prospective multinational study. Mol Clin Oncol. 2014;2(5):701-708.
25. Lothgren M, Ribnicsek E, Schmidt L, et al. Cost per patient and potential budget implications of denosumab compared with zoledronic acid in adults with bone metastases from solid tumours who are at risk of skeletal-related events: an analysis for Austria, Sweden and Switzerland. Eu J Hosp Pharm Sci Pract. 2013;20(4):227-231.
26. Luftner D, Lorusso V, Duran I, et al. Health resource utilization associated with skeletal-related events in patients with advanced breast cancer: results from a prospective, multinational observational study. SpringerPlus. 2014;3:328.
27. Cauley JA. Defining ethnic and racial differences in osteoporosis and fragility fractures. Clin Orthop Relat Res. 2011;469(7):1891-1899.
28. VanEenwyk J, Campo JS, Ossiander EM. Socioeconomic and demographic disparities in treatment for carcinomas of the colon and rectum. Cancer. 2002;95(1):39-46.
1. Carter JA, Ji X, Botteman MF. Clinical, economic and humanistic burdens of skeletal-related events associated with bone metastases. Expert Rev Pharmacoecon Outcomes Res. 2013;13(4):483-496.
2. Clain A. Secondary malignant disease of bone. Br J Cancer. 1965;19:15-29.
3. Coleman RE. Clinical features of metastatic bone disease and risk of skeletal morbidity. Clin Cancer Res. 2006;12(20 Pt 2):6243s-6249s.
4. Coleman RE. Skeletal complications of malignancy. Cancer. 1997;80(8 Suppl):1588-1594.
5. Higinbotham NL, Marcove RC. The management of pathological fractures. J Trauma. 1965;5(6):792-798.
6. Hess KR, Varadhachary GR, Taylor SH, et al. Metastatic patterns in adenocarcinoma. Cancer. 2006;106(7):1624-1633.
7. Stone CA, Lawlor PG, Savva GM, Bennett K, Kenny RA. Prospective study of falls and risk factors for falls in adults with advanced cancer. J Clin Oncol. 2012;30(17):2128-2133.
8. Coleman RE. Metastatic bone disease: clinical features, pathophysiology and treatment strategies. Cancer Treat Rev. 2001;27(3):165-176.
9. Katoh M, Unakami M, Hara M, Fukuchi S. Bone metastasis from colorectal cancer in autopsy cases. J Gastroenterol. 1995;30(5):615-618.
10. Howlader N NA, Krapcho M, Garshell J, Miller D, Altekruse SF, Kosary CL, Yu M, Ruhl J, Tatalovich Z,Mariotto A, Lewis DR, Chen HS, Feuer EJ, Cronin KA (eds). SEER Cancer Statistics Review, 1975-2011, National Cancer Institute. Bethesda, MD, http://seer.cancer.gov/csr/1975_2011/, based on November 2013 SEER data submission. Posted April 2014. Accessed January 19, 2018.
11. Hagiwara M, Delea TE, Saville MW, Chung K. Healthcare utilization and costs associated with skeletal-related events in prostate cancer patients with bone metastases. Prostate Cancer Prostatic Dis. 2013;16(1):23-27.
12. Hagiwara M, Delea TE, Chung K. Healthcare costs associated with skeletal-related events in breast cancer patients with bone metastases. J Med Econ. 2014;17(3):223-230.
13. Yong C, Onukwugha E, Mullins CD. Clinical and economic burden of bone metastasis and skeletal-related events in prostate cancer. Curr Opin Oncol. 2014;26(3):274-283.
14. HCUP Nationwide Inpatient Sample (NIS). Healthcare Cost and Utilization Project (HCUP). 2011. Agency for Healthcare Research and Quality R, MD. http://www.hcup-us.ahrq.gov/nisoverview.jsp. Last modified January 17, 2018. Accessed January 18, 2018.
15. American Cancer Society. Cancer Facts & Figures. Atlanta: American Cancer Society; 2003-2010. https://www.cancer.org/research/cancer-facts-statistics/all-cancer-facts-figures/cancer-facts-figures-2010.html Published January 2010. Accessed January 17, 2018.
16. Harrington KD. Orthopedic surgical management of skeletal complications of malignancy. Cancer. 1997;80(8 Suppl):1614-1627.
17. Harrington KD. Impending pathologic fractures from metastatic malignancy: evaluation and management. Instr Course Lect. 1986;35:357-381.
18. Mirels H. Metastatic disease in long bones. A proposed scoring system for diagnosing impending pathologic fractures. Clin Orthop Relat Res. 1989;249:256-264.
19. Weber KL. Evaluation of the adult patient (aged >40 years) with a destructive bone lesion. J Am Acad Orthop Surg. 2010;18(3):169-179.
20. Rougraff BT. Evaluation of the patient with carcinoma of unknown origin metastatic to bone. Clin Orthop Relat Res. 2003(415 Suppl):S105-109.
21. Coleman RE, Rubens RD. The clinical course of bone metastases from breast cancer. Br J Cancer. 1987;55(1):61-66.
22. Dijstra S, Wiggers T, van Geel BN, Boxma H. Impending and actual pathological fractures in patients with bone metastases of the long bones. A retrospective study of 233 surgically treated fractures. Eur J Surg. 1994;160(10):535-542.
23. Henry D, Vadhan-Raj S, Hirsh V, et al. Delaying skeletal-related events in a randomized phase 3 study of denosumab versus zoledronic acid in patients with advanced cancer: an analysis of data from patients with solid tumors. Support Care Cancer. 2014;22(3):679-687.
24. Lorusso V, Duran I, Garzon-Rodriguez C, et al. Health resource utilisation associated with skeletal-related events in European patients with lung cancer: Alpha subgroup analysis from a prospective multinational study. Mol Clin Oncol. 2014;2(5):701-708.
25. Lothgren M, Ribnicsek E, Schmidt L, et al. Cost per patient and potential budget implications of denosumab compared with zoledronic acid in adults with bone metastases from solid tumours who are at risk of skeletal-related events: an analysis for Austria, Sweden and Switzerland. Eu J Hosp Pharm Sci Pract. 2013;20(4):227-231.
26. Luftner D, Lorusso V, Duran I, et al. Health resource utilization associated with skeletal-related events in patients with advanced breast cancer: results from a prospective, multinational observational study. SpringerPlus. 2014;3:328.
27. Cauley JA. Defining ethnic and racial differences in osteoporosis and fragility fractures. Clin Orthop Relat Res. 2011;469(7):1891-1899.
28. VanEenwyk J, Campo JS, Ossiander EM. Socioeconomic and demographic disparities in treatment for carcinomas of the colon and rectum. Cancer. 2002;95(1):39-46.
Measurement of physical activity and sedentary behavior in breast cancer survivors
Physical activity has numerous physical, mental, and psychosocial benefits for cancer survivors, such as a reduction in the risk of mobility disability, depression, and anxiety, and improved patient quality of life.1,2 In addition, higher levels of physical activity are associated with reduced cancer-specific and all-causes mortality as well as cancer-specific outcomes including reduced risk of cancer progression and recurrence and new primary cancers.3-5 However, fewer than one-third of cancer survivors are meeting government and cancer-specific recommendations of 150 minutes a week of moderate to vigorous physical activity (MPVA; ≥3 metabolic equivalents [METs]).6,7 Growing evidence also demonstrates a significant association between higher levels of sedentary behavior and many deleterious health effects after cancer, including an increased risk for decreased physical functioning and development of other chronic diseases such as cardiovascular disease or diabetes.8 Distinct from physical activity, sedentary behavior is defined as any waking activity resulting in low levels of energy expenditure (≤1.5 METs) while in a seated or reclined position.9 Increased sedentary behavior, even when controlling for moderate and vigorous physical activity (MVPA), is associated with poor quality of life and increased all-cause mortality in cancer survivors.10,11 Given the associations observed between higher levels of physical activity, lower levels of sedentary behavior, and improved health and disease outcomes among the large and increasing number of cancer survivors in the United States, it is important to identify low-cost methods that can be used in a in a variety of settings (ie, research, clinical, community) to accurately and efficiently measure survivors’ lifestyle behaviors to identify high-risk survivors for early intervention, better understand the effects of these behaviors on survivors’ health outcomes and disease trajectories, and ultimately, improve survivors’ health and quality of life.12,13
Two methods commonly used to capture physical activity and sedentary behavior across the lifespan are accelerometry (Actigraph, Pensacola, FL) and self-report questionnaires such as the Godin Leisure-Time Questionnaire (GLTEQ), International Physical Activity Questionnaire (IPAQ), and Sitting Time Questionnaire (STQ).14-17 Each method has unique strengths and weaknesses. Sending accelerometers to multiple individuals at a single time point can be costly, particularly in large-scale epidemiological studies, and the accelerometer’s waist-worn, nonwaterproof design may prevent researchers from capturing certain activities such as swimming and resistance training. However, the accelerometer provides objective, precise assessments of most physical activities and may help remove response bias.18 Conversely, self-report questionnaires rely solely on individuals’ memories and often result in recall bias, inaccurate reporting, and under- or overestimation of physical activity engagement.19,20 Nevertheless, these questionnaires can be widely disseminated at low cost in a variety of settings (eg, clinical, research, community) and are less of a burden to participants.
Recent studies comparing objective (eg, accelerometer) with subjective (eg, self-report) methods of measuring physical activity and sedentary behavior in healthy middle-aged adults and older adults have demonstrated mixed findings with no distinct trends in the degree to which these methods differ.19,21,22 To date, little consideration has been given to the measurement of these lifestyle behaviors in cancer survivors. Boyle and colleagues recently investigated the concurrent validity of an accelerometer to the GLTEQ in colon cancer survivors, finding significant differences in estimated MVPA (~11 minutes). However, no studies, to our knowledge, have compared accelerometer and self-report measures in breast cancer survivors, so it remains unclear how these different measurement tools relate to each another in this population.
It is particularly important to compare these measurement tools among breast cancer survivors because evidence indicates this population’s behavioral habits, self-perceived activity, and sitting time and movement patterns may differ significantly from the general population and other survivor groups across the lifespan.23,24 Further, previous studies examining these behaviors in cancer survivors focused primarily on sitting time and MVPA.15,25,26 Examining other lower-intensity intensities (eg, light activity or lifestyle) in cancer survivors may also be important given that increased levels of activity are associated with health benefits, ranging from reduced disability and fatigue to improved cardiovascular health and quality of life, and that breast cancer survivors engage in fewer of these activities compared with noncancer controls.23 These lower levels of physical activity may be more prevalent among cancer survivors of their high levels of fatigue and propensity toward increased sitting time during the first year of treatment,11 so it is important to be able to accurately assess these activities in this population. The purpose of the present study was to compare estimates of time spent in light physical activity (LPA), MVPA, and sitting time (ST) obtained from an accelerometer and 3 self-report measurement tools (GLTEQ, IPAQ, STQ) in a large, US-based sample of breast cancer survivors. A secondary purpose was to determine whether estimate comparisons among measurements changed by participant characteristics.
Methods
Participants and procedures
This study consisted of a subsample of women who participated in a larger study whose findings have been reported elsewhere by Phillips and McAuley.27 In that study, breast cancer survivors (n = 1,631) were recruited nationally to participate in a 6-month prospective study on quality of life. Eligibility criteria included being aged 18 years or older, having had a diagnosis of breast cancer, being English speaking, and having access to the internet. Once consented to participate in the study, 500 women were randomly selected to wear the accelerometer.
Participants in this group were mailed an accelerometer, an activity log, instructions for use, and a self-addressed stamped envelope to return the monitor. They were asked to wear the accelerometer during all waking hours for 7 consecutive days of usual activity. They were also sent a secure link to complete 3 activity questionnaires online. The questionnaires were to be completed by the end of the 7-day monitoring period. Only women with 3 or more valid days of accelerometer data and complete data on variables of interest (n = 414) were included in the present analyses. All of the participants consented to the study procedures approved by the University of Illinois Institutional Review Board.
Measures
Demographics. The participants self-reported their age, level of education, height, and weight. Their body mass index (BMI; kg/m2) was estimated using the standard equation. They also self-reported their health and cancer history, detailing breast cancer disease stage, time since diagnosis, treatment type, and whether they had had a cancer recurrence. They were also asked to report whether they had ever been diagnosed (Yes/No) with 18 chronic conditions (eg, diabetes, arthritis).
Godin Leisure-Time Exercise Questionnaire.16 The GLTEQ assessed participants’ weekly frequency and mean amount of time performing MVPA (moderate exercise, such as fast walking, combined with vigorous exercise, such as jogging), and LPA (light/mild exercise, eg, easy walking) during the previous 7 days. The mean daily duration (in minutes) for each intensity category (MVPA, LPA) was calculated using activity frequencies and the amount of time spent in each activity presented as minutes/day.
The International Physical Activity Questionnaire.14 The IPAQ evaluated participants’ physical activity of at least moderate intensity in 4 domains of everyday life: job-related physical activity, transportation, housework/caring for family, and leisure-time activity. Within each domain, participants were asked the number of days per week and time per day (hours and minutes) spent performing MVPA. To estimate sitting time, the questionnaire asks participants to report the total amount of time spent sitting per day in 2 conditions, during weekdays and during weekends. The present analysis averaged sitting time for a typical 7-day (5 week days, 2 weekend days) period. We multiplied reported minutes per day and frequency per week of each activity category (MVPA and ST) to calculate the mean number of minutes per day.29,30
Sitting Time Questionnaire.17,28 The STQ estimated the mean time (hours and minutes) participants spent sitting each day on weekdays and at weekends within 5 domains: while traveling to and from places, at work, watching television, using a computer at home, and at leisure, not including watching television (eg, visiting friends, movies, dining out). Mean minutes per day of ST were calculated using all sitting domains.
Actigraph accelerometer (model GT1M, Health One Technology, Fort Walton Beach, FL). The Actigraph GT1M is a reliable and objective measure of physical activity.31-33 Participants wore the monitor on the right hip for 7 consecutive days during all waking hours, except when bathing or swimming. Activity data was analyzed in 1-minute intervals. A valid day of accelerometer wear time was defined as ≥600 minutes with no more than 60 minutes of consecutive zero-values, with allowance of 2 minutes or fewer of observations <100 counts/minute within the nonwear interval.34 Each minute of wear time was classified according to intensity (counts/min) using the following cut-points:34 sedentary, <100 counts/min; LPA, 100-2,019 counts/min; and MVPA, ≥2,020 count/min. Mean daily durations (min/day) spent in each behavior were estimated by dividing the number of minutes in each category by the number of valid days.
Statistical analysis
All statistical analyses were completed in SPSS Statistics 23 (IBM, Chicago, IL). Descriptive statistics were used to define participant characteristics. Rank-order correlation between the methods was assessed using Spearman’s rho (rs) and results were interpreted as follows: rs = 0.10, small; 0.30, moderate; and 0.50, strong.35 Within each activity intensity group, we jointly modeled daily minutes of self-report and accelerometer data using a random-intercept mixed-effects regression model. Differences between measurement tools were assessed based on regression coefficients with accelerometer as the reference category. Finally, we did a post hoc analysis of leisure-time–only MVPA from the IPAQ to compare with other estimates of MVPA.
We calculated the measurement tool difference scores for each estimated intensity category (ST, LPA, MVPA), that is, accelerometer estimated ST minus STQ estimated ST, and GLTEQ estimated MVPA minus IPAQ estimated MVPA. We used these data in an exploratory analysis to examine whether there were statistically significant differences between measurement difference scores by demographic or disease characteristics using linear regression stratified analyses. For example, we were interested in whether there was a significant difference in measurement tool estimates for sitting time in older compared with younger survivors. Analyses were stratified by age (<60/≥60 years), body mass index (<25 kg/m2/≥25 kg/m2), race (white/people of color), disease stage (I and II/III and IV), years since diagnosis (≤5 years/>5 years), recurrence (Yes/No), received chemotherapy (Yes/No ), received radiation (Yes/No ), and the presence of 1 or more chronic diseases (Yes/No ).
Results
Participants
The mean age of the participants was 56.8 years [9.2], they were overweight (BMI, 26.2 kg/m2 [5.4]), and predominantly white (96.7%; Table 1). Table 2 provides a summary of mean daily duration of activity estimates for ST, LPA, and MVPA and the estimate mean difference scores between measurements.
Moderate and vigorous physical activity
Accelerometer−GLTEQ. The mean difference in MVPA estimates between the accelerometer and GLTEQ was less than 5 minutes (Maccelerometer = 20.2 minutes; MGLTEQ = 23.6 minutes), even though the difference was statistically significant (P = .02). Estimates of MVPA from the accelerometer and GLTEQ (rs = 0.564, P < .001) showed a strong relationship. Stratified analyses showed that the difference scores between the GLTEQ and accelerometer were lower for older survivors (≥60 years) compared with younger survivors such that older survivors reported significantly less time in MVPA on the GLTEQ compared with accelerometer estimates (difference score [D] = 6.8 minutes less, P = .001).
Accelerometer−IPAQ. The accelerometer estimated significantly fewer minutes of MVPA per day when compared with the IPAQ (Mdiff = -67.4; 95% confidence interval [CI], -78.6, -55.8; P < .001). Estimates of MVPA from the accelerometer and IPAQ (rs = 0.011, P = .680) were poorly related. Differences between the IPAQ and accelerometer were greater for later-stage breast cancer, compared with early-stage diagnoses such that participants with late-stage disease reported significantly less MVPA on the IPAQ compared with accelerometer estimates (D = 41.8 minutes less than early-stage disease, P = .018). Finally, participants of color reported a greater difference in MVPA between the accelerometer and the IPAQ than did their white counterparts (D = 47.5 minutes, P = .033).
GLTEQ−IPAQ. GLTEQ estimated significantly fewer minutes of MVPA per day compared with the IPAQ (Mdiff = -64.6; 95% CI, -76.6, -52.5; P < .001). The estimates of MVPA from the GLTEQ had a small correlation with IPAQ estimates (rs = 0.128, P = .011).
IPAQ estimates showed almost triple the MVPA minutes per day as were estimated by the accelerometer and GLTEQ. As the MVPA estimate for the IPAQ include nonleisure activities, we conducted a post hoc analyses that only included the leisure-time items from the IPAQ. Leisure-time only IPAQ items, estimates indicated survivors spent a mean 18.5 [SD, 14.2] min/day in MVPA. Although the magnitude of the difference between the accelerometer and GLTEQ estimates (~10 minutes) was much smaller using the leisure-time only IPAQ items, a repeated measures analysis of variance revealed there was still a significant difference between these estimates (P < .05 for both) and negligible correlation.
Light intensity physical activity
Accelerometer−GLTEQ. There was a large and significant difference between LPA estimates from the GLTEQ and accelerometer (Mdiff = 224.5; 95% CI, 218.2, 230.7; P < .001) with estimates from the accelerometer being higher than those for the GLTEQ. Additionally, the measurements showed a negligible correlation (rs = 0.004, P = .94). Difference scores for GLTEQ and accelerometer estimated LPA were significantly different by age, with survivors aged 60 years or older demonstrating a difference that was 18.3 minutes shorter (P = .005) than the difference in younger survivors (<60 years).
Sitting time
Accelerometer−IPAQ. Mean IPAQ estimates were significantly lower (M = 303.8 [63.4]) than accelerometer estimates (M = 603.9 [78.0]). Rank-order correlations between IPAQ and accelerometer estimated ST was small (rs =0.26, P < .001). Difference scores between IPAQ and accelerometer estimates were significantly greater for survivors who were 60 years or older, compared with those younger than 60 years (D = 47.6 minutes, P = .006), indicating that older survivors tended to self-report significantly more ST than estimated by the accelerometer.
Accelerometer−STQ. There was no significant difference in estimated mean ST minutes per day between the STQ and the accelerometer, but the correlation between estimates was low (rs = 0.30, P < .001). Stratified analyses revealed estimates for the difference scores for mean daily ST between the STQ and accelerometer were greater for participants who were diagnosed with later-stage breast cancer (D= -158.3 minutes, P < .001) and those who had received chemotherapy (D= -61.7 minutes, P = .028; Table 2) than for those who were diagnosed with early-stage breast cancer or had not received chemotherapy. Women who had later-stage disease reported significantly less ST than did women diagnosed with early-stage disease, when compared with estimates by the accelerometer.
IPAQ−STQ. The estimated mean ST was significantly lower for IPAQ (M = 303.8 minutes [163.4]) than for the STQ (M = 605.2 minutes [296.2]). There were no significant estimate differences among the stratified groups.
Discussion
The purpose of the present study was to compare 4 measurement tools, an accelerometer-based activity monitor and 3 self-report questionnaires, to estimate ST, LPA, and MVPA in breast cancer survivors. Developing and evaluating accurate and precise measurement tools to assess physical activity and ST in breast cancer survivors remains a critical step toward better understanding the role of physical activity in cancer survivorship. Our results indicate that the congruency of the measurement tools examined was highly dependent on the activity intensity of interest and participants’ demographic or disease characteristics. Overall, the accelerometer estimated a greater amount of time spent sitting and engaging in LPA and less time in MVPA than was estimated on the STQ, GLTEQ, and IPAQ. In addition, our findings suggest significant subgroup differences that will be important in future development and implementation of physical activity measurement for breast cancer survivors.
MVPA has been the most commonly measured activity intensity among cancer survivors to date.15,25,26 The present results indicate mean daily MVPA estimates were significantly higher for the GLTEQ compared with the accelerometer (Mdiff = 2.8 min/d, P = .019), although the magnitude of these differences was relatively small. This difference is lower than in another study that compared these measures in colon cancer survivors and found the GLTEQ over-estimated MVPA by 10.6 min/day compared with the accelerometer (P < .01).15 However, the correlation between the 2 tools in our study was similar to that of Boyle and colleagues (rs = 0.56 and rs = 0.51, respectively). A possible explanation for the equivocal findings across these studies may lie in the difference in study sample demographics; a previous study results finding breast cancer survivors may be better at recalling their physical activities because they may be more attentive to activities they perform daily.26
The IPAQ significantly estimated more than an hour more of MVPA minutes per day compared with the accelerometer and GLTEQ. There are a number of limitations to the reporting of MVPA on the IPAQ. These limitations have been previously reported in the literature and include cross-cultural differences as well as overreporting of nonleisure-time MVPA (eg, occupational or household activities). However, the IPAQ has consistently been shown to be a valid and reliable tool for physical activity surveillance in different populations across the world.29,36,37 This shows that although MVPA was overestimated in our population, we do not mean to undermine the IPAQ value in other populations in which it has shown great utility for overall physical activity surveillance. When we excluded nonleisure-time MVPA, MVPA equated to about 18 min/day, which was closer in magnitude to the GLTEQ and accelerometer. These data highlight the importance of identifying the specific activity parameters of interest when selecting a measurement tool to ensure congruency between the tool and construct of interest.
The differences in MVPA estimation from the 3 tools have significant translational consequences, notably the potential for misclassification of meeting physical activity guidelines. For example, the percentage of women in the present sample that met physical activity guidelines ranged from 0% (using the accelerometer) to 19.5% (using the IPAQ), depending on the measurement tool used. These findings have meaningful implications for future physical activity assessment because multiple measurement tools are cur
For example, scores from the IPAQ may result in a survivor being classified as meeting physical activity guidelines when in fact they are not, and thereby missing the opportunity for intervention; or the accelerometer may classify an active survivor as inactive, which could result in using time and resources for a behavior change intervention that is not necessary. The clinical significance of these findings is to provide providers with data-based information on the strengths and limitations of the measurement tools so that they can accurately estimate physical activity and ST and appropriately optimize resources and treatments.
The degree of measurement tool congruence is likely influenced by a number of factors. First, survivors’ perceptions of the intensity of their activity are relative and subjective to their state of feeling during the activity. For example, breast cancer survivors with lower functional capacity may perceive activities with lower absolute intensity as having a higher relative intensity (ie, they think they are working at a moderate intensity so record an activity as such, but the activity is classified as light by the accelerometer). Second, although our self-report measures asked survivors to record the time they had spent active over the previous 7 days, survivors might report on what they consider a “usual” week, which may reflect the ideal rather than the reality. Third, the accelerometer cut-points used were derived from young, healthy adults on a treadmill. Thus, generalization to an older, sick, less active population that could be experiencing treatment-related side effects could lead to underestimation of time spent in MVPA. To better understand measurement congruency in breast cancer survivors, future research should investigate how functional capacity and activity intensity perceptions are influenced by a breast cancer diagnosis and how those factors may influence subjective and objective physical activity measurement. If those factors were found to have significant influence on activity in breast cancer survivors, it would warrant future development of breast-cancer–specific accelerometer reduction techniques.
The comparison of LPA presented another interesting significant contrast between self-report (GLTEQ) and accelerometry. Results indicated the GLTEQ underestimated LPA by 224.5 [3.2] min/day compared with the accelerometer. This equates to over 3.5 h/day of active time (or about 280 kcal/day) that was potentially unaccounted for by the GLTEQ. The difference between these estimates could be due to the fact that the GLTEQ was designed to measure exercise time and therefore may not be as sensitive as the accelerometer to nonexercise-related LPA. Light intensity activities typically span a large range of domains (ie, occupational, leisure time, household) and tend to occur in higher volumes than MVPA, which may lead to some challenges with recall. Expanding existing LPA questionnaires to encompass these domains would likely provide increased congruency between self-reported and accelerometer-derived estimates for LPA, as it may provide a better trigger for recalling these high volume activities. With increasing literature advocating the important role of LPA in adults’ health in concert with data suggesting survivors may engage in lower levels of LPA than healthy controls,23, accurately accounting for these lower intensity activities to provide a “whole picture” of a survivor’s active day remains an important future research direction. Combining accelerometer and self-report data using ecological momentary assessment to capture these behaviors in real-time in the real world could provide a better understanding of the context in which LPA occurs as well as survivors’ perceptions of intensity to build more accurate and scalable measurement tools for LPA.
Our ST results indicate nonsignificant difference estimates from the accelerometer and the STQ (Mdiff = 1.3 [15.3] min/day) with slightly higher estimates for the STQ versus accelerometer. This finding is consistent with the one other study that has examined these relationships in cancer survivors.15 However, our findings also indicate the IPAQ significantly underestimated ST compared with the accelerometer and the STQ by about half (Table 1). These differences may be because both the STQ and Marshall questionnaire used in the previous study measure multiple domains of sitting (ie, computer, television, travel) on both weekdays and weekends whereas the IPAQ uses only two recall items of overall sitting time (for weekday and weekend separately). The domain-specific, structured approach has been shown to improve recall and may help to prevent underestimation and general underreporting of the high volume, ubiquitous behavior of sitting.17,38 Finally, we would be remiss to not acknowledge the known limitations to estimating ST using the count-based approach on the waist-worn accelerometer. Due to the monitor’s orientation at the hip, the accelerometer may misrepresent total ST by misclassifying standing still as sitting. However, Kozey-Keadle and colleagues have previously examined estimation of ST using waist-worn accelerometers and have shown the 100 count per minute cut off yields ST estimates within 5% range of accuracy for a seated position compared with direct observation.39
Of further interest are our exploratory results indicating that age and disease stage may modify the congruency between activity and ST measures. Specifically, older survivors and those with more advanced disease stage generally reported more PA and less ST than were measured by the accelerometer. These differences raise the question of whether these subgroups are systematically reporting more time physically active, overestimating their intensity, or the accelerometer is misclassifying their activity intensity. These misclassifications could be due to their age, disease stage, fatigue status, functional status, cognitive function, occupational status, etc. and would be important next steps for exploration of measurement of physical activity in breast cancer survivors. Finally, the difference score for MVPA was greater for survivors of color than for white survivors, with survivors of color overreporting MVPA compared with accelerometer-derived estimates. This may be due in part to cultural differences between white survivors and survivors of color. Previous research has suggested that people of color may accumulate a majority of their activity in occupational or household-related domains, thus explaining lower levels of leisure-time MVPA but high levels of reported total MVPA from other nonleisure domains.20 However, given the small number of survivors of color in the present study, these results should be interpreted with caution.
With the multitude of physical activity and ST measurement tools available, many factors including cost, sample size, primary outcome of interest, and activity characteristics of interest (eg, duration, intensity, energy expenditure) need to be considered40 when choosing a tool. Our findings may help inform these decisions for breast cancer survivors. For example, if LPA is of interest, an accelerometer may provide a more comprehensive assessment of these activities than the GLTEQ. In contrast, if MVPA is the activity of interest, our results suggest the GLTEQ and accelerometer were more congruent than the IPAQ was with either measure, therefore, if budgetary constraints are a concern, the more cost-efficient GLTEQ could provide similar results to an accelerometer. In addition to considering measurement congruency, it is also critically important to carefully consider the population (breast cancer survivors) and subsequent burden that accompanies the measurement tool of choice. Overall, our results indicate, when choosing a questionnaire for ST or LPA for breast cancer survivors, the more comprehensive the questions, to encompass multiple domains or time of day, the greater amount of time that will be captured within that activity category. Conversely, since the majority of MVPA is completed in leisure-time, dependent on the age and race of the population, a shorter questionnaire may be sufficient. Additionally, dependent on time since diagnosis and treatment received, activity recall or body movement patterns may be affected which could influence measurement tool selection.23,24 Finally, it is also important to consider the setting in which measurement is taking place. In busy clinical settings, shorter, self-report measures may have a greater chance of being implemented than accelerometers or longer self-report measures and would still provide useful information regarding an overall snapshot of survivors’ MVPA or ST that could be used to initiate a conversation or referral for a program to help survivors positively change one or both of these behaviors.
Limitations
There were a few limitations within the current study that should be taken into account. First, the accelerometer cut-points used were developed with healthy, young adults; therefore using different cut-points may have yielded different results.34 Given the large age range in our participants (23-84 years), we believe the use of these cut-points was justified, in lieu of population-specific (ie, older adults) cut-points. In addition, limitations to estimating activity from an accelerometer include the inability to capture certain activities such as swimming and cycling and the aforementioned inability to distinguish between body postures (ie, sitting vs standing).41 The participants were predominantly white, highly educated, and high earners (85.2% earned ≥$40,000 per year), therefore, the present results may not be generalizable to survivors from more diverse backgrounds. However, as far as we know, this is the first study to report the congruency of estimated ST, LPA, and MVPA across multiple measurement tools in a nationwide sample of breast cancer survivors who were heterogeneous in terms of disease characteristics (ie, stage, treatment, time since diagnosis).
Conclusions
Our findings suggest that physical activity and ST estimates in breast cancer survivors may be dependent on the measurement tool used. In addition, congruency of measurement tools was dependent on activity intensity of interest, and participant age, race, and disease history may also influence these factors. Therefore, researchers should consider the intended outcomes of interest, the context in which the tool is being used (ie, clinical versus research), the available resources, and the participant population before they select a measurement tool for estimating physical activity and sitting time in breast cancer survivors.
Acknowledgment
This work was supported by grant #F31AG034025 from the National Institute on Aging (Dr Phillips); Shahid and Ann Carlson Khan endowed professorship and grant #AG020118 from the National Institute on Aging (Dr McAuley). Dr Phillips is supported by the National Cancer Institute #K07CA196840, and Dr Welch is supported by National Institute of Health/National Cancer Institute training grant CA193193. All data for this study were collected at the University of Illinois Urbana Champaign.
1. Speck RM, Courneya KS, Masse LC, Duval S, Schmitz KH. An update of controlled physical activity trials in cancer survivors: a systematic review and meta-analysis. J Cancer Surviv. 2010;4(2):87-100.
2. Brenner DR. Cancer incidence due to excess body weight and leisure-time physical inactivity in Canada: implications for prevention. Prev Med. 2014;66:131-139.
3. Courneya KS, Friedenreich CM. Relationship between exercise pattern across the cancer experience and current quality of life in colorectal cancer survivors. J Altern Complement Med. 1997;3(3):215-226.
4. Ibrahim EM, Al-Homaidh A. Physical activity and survival after breast cancer diagnosis: meta-analysis of published studies. Med Oncol. 2011;28(3):753-765.
5. Lahart IM, Metsios GS, Nevill AM, Carmichael AR. Physical activity, risk of death and recurrence in breast cancer survivors: a systematic review and meta-analysis of epidemiological studies. Acta Oncol. 2015;54(5):635-654.
6. Irwin ML, McTiernan A, Bernstein L, et al. Physical activity levels among breast cancer survivors. Med Sci Sports Exerc. 2004;36(9):1484-1491.
7. Schmitz KH, Courneya KS, Matthews C, et al. American College of Sports Medicine roundtable on exercise guidelines for cancer survivors. Med Sci Sports Exerc. 2010;42(7):1409-1426.
8. Lynch BM. Sedentary behavior and cancer: a systematic review of the literature and proposed biological mechanisms. Cancer Epidemiol Biomarkers Prev. 2010;19(11):2691-2709.
9. Owen N, Healy GN, Matthews CE, Dunstan DW. Too much sitting: the population health science of sedentary behavior. Exerc Sport Sci Rev. 2010;38(3):105-113.
10. Campbell PT, Patel AV, Newton CC, Jacobs EJ, Gapstur SM. Associations of recreational physical activity and leisure time spent sitting with colorectal cancer survival. J Clin Oncol. 2013;31(7):876-885.
11. Lynch BM, Dunstan DW, Vallance JK, Owen N. Don’t take cancer sitting down: a new survivorship research agenda. Cancer. 2013;119(11):1928-1935.
12. Bluethmann SM, Mariotto AB, Rowland JH. Anticipating the ‘silver tsunami:’ prevalence trajectories and comorbidity burden among older cancer survivors in the United States. Cancer Epidemiol Biomarkers Prev. 2016;25(7):1029-1036.
13. Miller KD, Siegel RL, Lin CC, et al. Cancer treatment and survivorship statistics, 2016. CA Cancer J Clin. 2016;66(4):271-289.
14. Booth M. Assessment of physical activity: an international perspective. Res Q Exerc Sport. 2000;71(2 suppl):S114-120.
15. Boyle T, Lynch BM, Courneya KS, Vallance JK. Agreement between accelerometer-assessed and self-reported physical activity and sedentary time in colon cancer survivors. Support Care Cancer. 2015;23(4):1121-1126.
16. Godin G, Shephard RJ. A simple method to assess exercise behavior in the community. Canadian journal of applied sport sciences. Can J Appl Sport Sci. 1985;10(3):141-146.
17. Marshall AL, Miller YD, Burton NW, Brown WJ. Measuring total and domain-specific sitting: a study of reliability and validity. Med Sci Sports Exerc. 2010;42(6):1094-1102.
18. Matthews CE, Hagstromer M, Pober DM, Bowles HR. Best practices for using physical activity monitors in population-based research. Med Sci Sports Exerc. 2012;44(1 Suppl 1):S68-76.
19. Prince SA, Adamo KB, Hamel ME, Hardt J, Connor Gorber S, Tremblay M. A comparison of direct versus self-report measures for assessing physical activity in adults: a systematic review. Int J Behav Nutr Phys Act. 2008;5:56.
20. Sallis JF, Saelens BE. Assessment of physical activity by self-report: status, limitations, and future directions. Res Q Exerc Sport. 2000;71(2 Suppl):S1-14.
21. Hart TL, Swartz AM, Cashin SE, Strath SJ. How many days of monitoring predict physical activity and sedentary behaviour in older adults? Int J Behav Nutr Phys Act. 2011;8:62.
22. Hart TL, Ainsworth BE, Tudor-Locke C. Objective and subjective measures of sedentary behavior and physical activity. Med Sci Sports Exerc. 2011;43(3):449-456.
23. Phillips SM, Dodd KW, Steeves J, McClain J, Alfano CM, McAuley E. Physical activity and sedentary behavior in breast cancer survivors: new insight into activity patterns and potential intervention targets. Gynecol Oncol. 2015;138(2):398-404.
24. Boyle T, Vallance JK, Ransom EK, Lynch BM. How sedentary and physically active are breast cancer survivors, and which population subgroups have higher or lower levels of these behaviors? Support Care Cancer. 2016;24(5):2181-2190.
25. Broderick JM, Guinan E, Kennedy MJ, et al. Feasibility and efficacy of a supervised exercise intervention in de-conditioned cancer survivors during the early survivorship phase: the PEACH trial. J Cancer Surviv. 2013;7(4):551-562.
26. Su CC, Lee KD, Yeh CH, Kao CC, Lin CC. Measurement of physical activity in cancer survivors: a validity study. J Cancer Surviv. 2014;8(2):205-212.
27. Phillips SM, McAuley E. Social cognitive influences on physical activity participation in long-term breast cancer survivors. Psychooncology. 2013;22(4):783-791.
28. Wojcicki TR, White SM, McAuley E. Assessing outcome expectations in older adults: the multidimensional outcome expectations for exercise scale. J Gerontol B Psychol Sci Soc Sci. 2009;64(1):33-40.
29. Craig CL, Marshall AL, Sjostrom M, et al. International physical activity questionnaire: 12-country reliability and validity. Med Sci Sports Exerc. 2003;35(8):1381-1395.
30. Ainsworth BE, Haskell WL, Whitt MC, et al. Compendium of physical activities: an update of activity codes and MET intensities. Med Sci Sports Exerc. 2000;32(9 Suppl):S498-504.
31. Hacker ED, Ferrans CE. Ecological momentary assessment of fatigue in patients receiving intensive cancer therapy. J Pain Symptom Manage. 2007;33(3):267-275.
32. Swartz AM, Strath SJ, Bassett DR, Jr, O’Brien WL, King GA, Ainsworth BE. Estimation of energy expenditure using CSA accelerometers at hip and wrist sites. Med Sci Sports Exerc. 2000;32(9 Suppl):S450-456.
33. Jim HS, Small B, Faul LA, Franzen J, Apte S, Jacobsen PB. Fatigue, depression, sleep, and activity during chemotherapy: daily and intraday variation and relationships among symptom changes. Ann Behav Med. 2011;42(3):321-333.
34. Troiano RP, Berrigan D, Dodd KW, Masse LC, Tilert T, McDowell M. Physical activity in the United States measured by accelerometer. Med Sci Sports Exerc. 2008;40(1):181-188.
35. Cohen J. Statistical power analysis for the behavioral sciences. 2nd ed. Hillsdale, NJ: L Erlbaum Associates; 1988.
36. Bauman A, Ainsworth BE, Bull F, et al. Progress and pitfalls in the use of the International Physical Activity Questionnaire (IPAQ) for adult physical activity surveillance. J Phys Act Health. 2009;6 Suppl 1:S5-8.
37. Hagströmer M1, Oja P, Sjöström M. The International Physical Activity Questionnaire (IPAQ): a study of concurrent and construct validity. Public Health Nutr. 2006;9(6):755-762.
38. Johnson-Kozlow M, Sallis JF, Gilpin EA, Rock CL, Pierce JP. Comparative validation of the IPAQ and the 7-Day PAR among women diagnosed with breast cancer. Int J Behav Nutr Phys Act. 2006;3:7.
39. Kozey-Keadle S, Libertine A, Lyden K, Staudenmayer J, Freedson PS. Validation of wearable monitors for assessing sedentary behavior. Med Sci Sports Exerc. 2011;43(8):1561-1567.
40. Strath SJ, Kaminsky LA, Ainsworth BE, et al. Guide to the assessment of physical activity: clinical and research applications. Circulation. 2013;128(20):2259-2279.
41. Bassett DR. Device-based monitoring in physical activity and public health research. Physiol Meas. 2012;33(11):1769-1783.
Physical activity has numerous physical, mental, and psychosocial benefits for cancer survivors, such as a reduction in the risk of mobility disability, depression, and anxiety, and improved patient quality of life.1,2 In addition, higher levels of physical activity are associated with reduced cancer-specific and all-causes mortality as well as cancer-specific outcomes including reduced risk of cancer progression and recurrence and new primary cancers.3-5 However, fewer than one-third of cancer survivors are meeting government and cancer-specific recommendations of 150 minutes a week of moderate to vigorous physical activity (MPVA; ≥3 metabolic equivalents [METs]).6,7 Growing evidence also demonstrates a significant association between higher levels of sedentary behavior and many deleterious health effects after cancer, including an increased risk for decreased physical functioning and development of other chronic diseases such as cardiovascular disease or diabetes.8 Distinct from physical activity, sedentary behavior is defined as any waking activity resulting in low levels of energy expenditure (≤1.5 METs) while in a seated or reclined position.9 Increased sedentary behavior, even when controlling for moderate and vigorous physical activity (MVPA), is associated with poor quality of life and increased all-cause mortality in cancer survivors.10,11 Given the associations observed between higher levels of physical activity, lower levels of sedentary behavior, and improved health and disease outcomes among the large and increasing number of cancer survivors in the United States, it is important to identify low-cost methods that can be used in a in a variety of settings (ie, research, clinical, community) to accurately and efficiently measure survivors’ lifestyle behaviors to identify high-risk survivors for early intervention, better understand the effects of these behaviors on survivors’ health outcomes and disease trajectories, and ultimately, improve survivors’ health and quality of life.12,13
Two methods commonly used to capture physical activity and sedentary behavior across the lifespan are accelerometry (Actigraph, Pensacola, FL) and self-report questionnaires such as the Godin Leisure-Time Questionnaire (GLTEQ), International Physical Activity Questionnaire (IPAQ), and Sitting Time Questionnaire (STQ).14-17 Each method has unique strengths and weaknesses. Sending accelerometers to multiple individuals at a single time point can be costly, particularly in large-scale epidemiological studies, and the accelerometer’s waist-worn, nonwaterproof design may prevent researchers from capturing certain activities such as swimming and resistance training. However, the accelerometer provides objective, precise assessments of most physical activities and may help remove response bias.18 Conversely, self-report questionnaires rely solely on individuals’ memories and often result in recall bias, inaccurate reporting, and under- or overestimation of physical activity engagement.19,20 Nevertheless, these questionnaires can be widely disseminated at low cost in a variety of settings (eg, clinical, research, community) and are less of a burden to participants.
Recent studies comparing objective (eg, accelerometer) with subjective (eg, self-report) methods of measuring physical activity and sedentary behavior in healthy middle-aged adults and older adults have demonstrated mixed findings with no distinct trends in the degree to which these methods differ.19,21,22 To date, little consideration has been given to the measurement of these lifestyle behaviors in cancer survivors. Boyle and colleagues recently investigated the concurrent validity of an accelerometer to the GLTEQ in colon cancer survivors, finding significant differences in estimated MVPA (~11 minutes). However, no studies, to our knowledge, have compared accelerometer and self-report measures in breast cancer survivors, so it remains unclear how these different measurement tools relate to each another in this population.
It is particularly important to compare these measurement tools among breast cancer survivors because evidence indicates this population’s behavioral habits, self-perceived activity, and sitting time and movement patterns may differ significantly from the general population and other survivor groups across the lifespan.23,24 Further, previous studies examining these behaviors in cancer survivors focused primarily on sitting time and MVPA.15,25,26 Examining other lower-intensity intensities (eg, light activity or lifestyle) in cancer survivors may also be important given that increased levels of activity are associated with health benefits, ranging from reduced disability and fatigue to improved cardiovascular health and quality of life, and that breast cancer survivors engage in fewer of these activities compared with noncancer controls.23 These lower levels of physical activity may be more prevalent among cancer survivors of their high levels of fatigue and propensity toward increased sitting time during the first year of treatment,11 so it is important to be able to accurately assess these activities in this population. The purpose of the present study was to compare estimates of time spent in light physical activity (LPA), MVPA, and sitting time (ST) obtained from an accelerometer and 3 self-report measurement tools (GLTEQ, IPAQ, STQ) in a large, US-based sample of breast cancer survivors. A secondary purpose was to determine whether estimate comparisons among measurements changed by participant characteristics.
Methods
Participants and procedures
This study consisted of a subsample of women who participated in a larger study whose findings have been reported elsewhere by Phillips and McAuley.27 In that study, breast cancer survivors (n = 1,631) were recruited nationally to participate in a 6-month prospective study on quality of life. Eligibility criteria included being aged 18 years or older, having had a diagnosis of breast cancer, being English speaking, and having access to the internet. Once consented to participate in the study, 500 women were randomly selected to wear the accelerometer.
Participants in this group were mailed an accelerometer, an activity log, instructions for use, and a self-addressed stamped envelope to return the monitor. They were asked to wear the accelerometer during all waking hours for 7 consecutive days of usual activity. They were also sent a secure link to complete 3 activity questionnaires online. The questionnaires were to be completed by the end of the 7-day monitoring period. Only women with 3 or more valid days of accelerometer data and complete data on variables of interest (n = 414) were included in the present analyses. All of the participants consented to the study procedures approved by the University of Illinois Institutional Review Board.
Measures
Demographics. The participants self-reported their age, level of education, height, and weight. Their body mass index (BMI; kg/m2) was estimated using the standard equation. They also self-reported their health and cancer history, detailing breast cancer disease stage, time since diagnosis, treatment type, and whether they had had a cancer recurrence. They were also asked to report whether they had ever been diagnosed (Yes/No) with 18 chronic conditions (eg, diabetes, arthritis).
Godin Leisure-Time Exercise Questionnaire.16 The GLTEQ assessed participants’ weekly frequency and mean amount of time performing MVPA (moderate exercise, such as fast walking, combined with vigorous exercise, such as jogging), and LPA (light/mild exercise, eg, easy walking) during the previous 7 days. The mean daily duration (in minutes) for each intensity category (MVPA, LPA) was calculated using activity frequencies and the amount of time spent in each activity presented as minutes/day.
The International Physical Activity Questionnaire.14 The IPAQ evaluated participants’ physical activity of at least moderate intensity in 4 domains of everyday life: job-related physical activity, transportation, housework/caring for family, and leisure-time activity. Within each domain, participants were asked the number of days per week and time per day (hours and minutes) spent performing MVPA. To estimate sitting time, the questionnaire asks participants to report the total amount of time spent sitting per day in 2 conditions, during weekdays and during weekends. The present analysis averaged sitting time for a typical 7-day (5 week days, 2 weekend days) period. We multiplied reported minutes per day and frequency per week of each activity category (MVPA and ST) to calculate the mean number of minutes per day.29,30
Sitting Time Questionnaire.17,28 The STQ estimated the mean time (hours and minutes) participants spent sitting each day on weekdays and at weekends within 5 domains: while traveling to and from places, at work, watching television, using a computer at home, and at leisure, not including watching television (eg, visiting friends, movies, dining out). Mean minutes per day of ST were calculated using all sitting domains.
Actigraph accelerometer (model GT1M, Health One Technology, Fort Walton Beach, FL). The Actigraph GT1M is a reliable and objective measure of physical activity.31-33 Participants wore the monitor on the right hip for 7 consecutive days during all waking hours, except when bathing or swimming. Activity data was analyzed in 1-minute intervals. A valid day of accelerometer wear time was defined as ≥600 minutes with no more than 60 minutes of consecutive zero-values, with allowance of 2 minutes or fewer of observations <100 counts/minute within the nonwear interval.34 Each minute of wear time was classified according to intensity (counts/min) using the following cut-points:34 sedentary, <100 counts/min; LPA, 100-2,019 counts/min; and MVPA, ≥2,020 count/min. Mean daily durations (min/day) spent in each behavior were estimated by dividing the number of minutes in each category by the number of valid days.
Statistical analysis
All statistical analyses were completed in SPSS Statistics 23 (IBM, Chicago, IL). Descriptive statistics were used to define participant characteristics. Rank-order correlation between the methods was assessed using Spearman’s rho (rs) and results were interpreted as follows: rs = 0.10, small; 0.30, moderate; and 0.50, strong.35 Within each activity intensity group, we jointly modeled daily minutes of self-report and accelerometer data using a random-intercept mixed-effects regression model. Differences between measurement tools were assessed based on regression coefficients with accelerometer as the reference category. Finally, we did a post hoc analysis of leisure-time–only MVPA from the IPAQ to compare with other estimates of MVPA.
We calculated the measurement tool difference scores for each estimated intensity category (ST, LPA, MVPA), that is, accelerometer estimated ST minus STQ estimated ST, and GLTEQ estimated MVPA minus IPAQ estimated MVPA. We used these data in an exploratory analysis to examine whether there were statistically significant differences between measurement difference scores by demographic or disease characteristics using linear regression stratified analyses. For example, we were interested in whether there was a significant difference in measurement tool estimates for sitting time in older compared with younger survivors. Analyses were stratified by age (<60/≥60 years), body mass index (<25 kg/m2/≥25 kg/m2), race (white/people of color), disease stage (I and II/III and IV), years since diagnosis (≤5 years/>5 years), recurrence (Yes/No), received chemotherapy (Yes/No ), received radiation (Yes/No ), and the presence of 1 or more chronic diseases (Yes/No ).
Results
Participants
The mean age of the participants was 56.8 years [9.2], they were overweight (BMI, 26.2 kg/m2 [5.4]), and predominantly white (96.7%; Table 1). Table 2 provides a summary of mean daily duration of activity estimates for ST, LPA, and MVPA and the estimate mean difference scores between measurements.
Moderate and vigorous physical activity
Accelerometer−GLTEQ. The mean difference in MVPA estimates between the accelerometer and GLTEQ was less than 5 minutes (Maccelerometer = 20.2 minutes; MGLTEQ = 23.6 minutes), even though the difference was statistically significant (P = .02). Estimates of MVPA from the accelerometer and GLTEQ (rs = 0.564, P < .001) showed a strong relationship. Stratified analyses showed that the difference scores between the GLTEQ and accelerometer were lower for older survivors (≥60 years) compared with younger survivors such that older survivors reported significantly less time in MVPA on the GLTEQ compared with accelerometer estimates (difference score [D] = 6.8 minutes less, P = .001).
Accelerometer−IPAQ. The accelerometer estimated significantly fewer minutes of MVPA per day when compared with the IPAQ (Mdiff = -67.4; 95% confidence interval [CI], -78.6, -55.8; P < .001). Estimates of MVPA from the accelerometer and IPAQ (rs = 0.011, P = .680) were poorly related. Differences between the IPAQ and accelerometer were greater for later-stage breast cancer, compared with early-stage diagnoses such that participants with late-stage disease reported significantly less MVPA on the IPAQ compared with accelerometer estimates (D = 41.8 minutes less than early-stage disease, P = .018). Finally, participants of color reported a greater difference in MVPA between the accelerometer and the IPAQ than did their white counterparts (D = 47.5 minutes, P = .033).
GLTEQ−IPAQ. GLTEQ estimated significantly fewer minutes of MVPA per day compared with the IPAQ (Mdiff = -64.6; 95% CI, -76.6, -52.5; P < .001). The estimates of MVPA from the GLTEQ had a small correlation with IPAQ estimates (rs = 0.128, P = .011).
IPAQ estimates showed almost triple the MVPA minutes per day as were estimated by the accelerometer and GLTEQ. As the MVPA estimate for the IPAQ include nonleisure activities, we conducted a post hoc analyses that only included the leisure-time items from the IPAQ. Leisure-time only IPAQ items, estimates indicated survivors spent a mean 18.5 [SD, 14.2] min/day in MVPA. Although the magnitude of the difference between the accelerometer and GLTEQ estimates (~10 minutes) was much smaller using the leisure-time only IPAQ items, a repeated measures analysis of variance revealed there was still a significant difference between these estimates (P < .05 for both) and negligible correlation.
Light intensity physical activity
Accelerometer−GLTEQ. There was a large and significant difference between LPA estimates from the GLTEQ and accelerometer (Mdiff = 224.5; 95% CI, 218.2, 230.7; P < .001) with estimates from the accelerometer being higher than those for the GLTEQ. Additionally, the measurements showed a negligible correlation (rs = 0.004, P = .94). Difference scores for GLTEQ and accelerometer estimated LPA were significantly different by age, with survivors aged 60 years or older demonstrating a difference that was 18.3 minutes shorter (P = .005) than the difference in younger survivors (<60 years).
Sitting time
Accelerometer−IPAQ. Mean IPAQ estimates were significantly lower (M = 303.8 [63.4]) than accelerometer estimates (M = 603.9 [78.0]). Rank-order correlations between IPAQ and accelerometer estimated ST was small (rs =0.26, P < .001). Difference scores between IPAQ and accelerometer estimates were significantly greater for survivors who were 60 years or older, compared with those younger than 60 years (D = 47.6 minutes, P = .006), indicating that older survivors tended to self-report significantly more ST than estimated by the accelerometer.
Accelerometer−STQ. There was no significant difference in estimated mean ST minutes per day between the STQ and the accelerometer, but the correlation between estimates was low (rs = 0.30, P < .001). Stratified analyses revealed estimates for the difference scores for mean daily ST between the STQ and accelerometer were greater for participants who were diagnosed with later-stage breast cancer (D= -158.3 minutes, P < .001) and those who had received chemotherapy (D= -61.7 minutes, P = .028; Table 2) than for those who were diagnosed with early-stage breast cancer or had not received chemotherapy. Women who had later-stage disease reported significantly less ST than did women diagnosed with early-stage disease, when compared with estimates by the accelerometer.
IPAQ−STQ. The estimated mean ST was significantly lower for IPAQ (M = 303.8 minutes [163.4]) than for the STQ (M = 605.2 minutes [296.2]). There were no significant estimate differences among the stratified groups.
Discussion
The purpose of the present study was to compare 4 measurement tools, an accelerometer-based activity monitor and 3 self-report questionnaires, to estimate ST, LPA, and MVPA in breast cancer survivors. Developing and evaluating accurate and precise measurement tools to assess physical activity and ST in breast cancer survivors remains a critical step toward better understanding the role of physical activity in cancer survivorship. Our results indicate that the congruency of the measurement tools examined was highly dependent on the activity intensity of interest and participants’ demographic or disease characteristics. Overall, the accelerometer estimated a greater amount of time spent sitting and engaging in LPA and less time in MVPA than was estimated on the STQ, GLTEQ, and IPAQ. In addition, our findings suggest significant subgroup differences that will be important in future development and implementation of physical activity measurement for breast cancer survivors.
MVPA has been the most commonly measured activity intensity among cancer survivors to date.15,25,26 The present results indicate mean daily MVPA estimates were significantly higher for the GLTEQ compared with the accelerometer (Mdiff = 2.8 min/d, P = .019), although the magnitude of these differences was relatively small. This difference is lower than in another study that compared these measures in colon cancer survivors and found the GLTEQ over-estimated MVPA by 10.6 min/day compared with the accelerometer (P < .01).15 However, the correlation between the 2 tools in our study was similar to that of Boyle and colleagues (rs = 0.56 and rs = 0.51, respectively). A possible explanation for the equivocal findings across these studies may lie in the difference in study sample demographics; a previous study results finding breast cancer survivors may be better at recalling their physical activities because they may be more attentive to activities they perform daily.26
The IPAQ significantly estimated more than an hour more of MVPA minutes per day compared with the accelerometer and GLTEQ. There are a number of limitations to the reporting of MVPA on the IPAQ. These limitations have been previously reported in the literature and include cross-cultural differences as well as overreporting of nonleisure-time MVPA (eg, occupational or household activities). However, the IPAQ has consistently been shown to be a valid and reliable tool for physical activity surveillance in different populations across the world.29,36,37 This shows that although MVPA was overestimated in our population, we do not mean to undermine the IPAQ value in other populations in which it has shown great utility for overall physical activity surveillance. When we excluded nonleisure-time MVPA, MVPA equated to about 18 min/day, which was closer in magnitude to the GLTEQ and accelerometer. These data highlight the importance of identifying the specific activity parameters of interest when selecting a measurement tool to ensure congruency between the tool and construct of interest.
The differences in MVPA estimation from the 3 tools have significant translational consequences, notably the potential for misclassification of meeting physical activity guidelines. For example, the percentage of women in the present sample that met physical activity guidelines ranged from 0% (using the accelerometer) to 19.5% (using the IPAQ), depending on the measurement tool used. These findings have meaningful implications for future physical activity assessment because multiple measurement tools are cur
For example, scores from the IPAQ may result in a survivor being classified as meeting physical activity guidelines when in fact they are not, and thereby missing the opportunity for intervention; or the accelerometer may classify an active survivor as inactive, which could result in using time and resources for a behavior change intervention that is not necessary. The clinical significance of these findings is to provide providers with data-based information on the strengths and limitations of the measurement tools so that they can accurately estimate physical activity and ST and appropriately optimize resources and treatments.
The degree of measurement tool congruence is likely influenced by a number of factors. First, survivors’ perceptions of the intensity of their activity are relative and subjective to their state of feeling during the activity. For example, breast cancer survivors with lower functional capacity may perceive activities with lower absolute intensity as having a higher relative intensity (ie, they think they are working at a moderate intensity so record an activity as such, but the activity is classified as light by the accelerometer). Second, although our self-report measures asked survivors to record the time they had spent active over the previous 7 days, survivors might report on what they consider a “usual” week, which may reflect the ideal rather than the reality. Third, the accelerometer cut-points used were derived from young, healthy adults on a treadmill. Thus, generalization to an older, sick, less active population that could be experiencing treatment-related side effects could lead to underestimation of time spent in MVPA. To better understand measurement congruency in breast cancer survivors, future research should investigate how functional capacity and activity intensity perceptions are influenced by a breast cancer diagnosis and how those factors may influence subjective and objective physical activity measurement. If those factors were found to have significant influence on activity in breast cancer survivors, it would warrant future development of breast-cancer–specific accelerometer reduction techniques.
The comparison of LPA presented another interesting significant contrast between self-report (GLTEQ) and accelerometry. Results indicated the GLTEQ underestimated LPA by 224.5 [3.2] min/day compared with the accelerometer. This equates to over 3.5 h/day of active time (or about 280 kcal/day) that was potentially unaccounted for by the GLTEQ. The difference between these estimates could be due to the fact that the GLTEQ was designed to measure exercise time and therefore may not be as sensitive as the accelerometer to nonexercise-related LPA. Light intensity activities typically span a large range of domains (ie, occupational, leisure time, household) and tend to occur in higher volumes than MVPA, which may lead to some challenges with recall. Expanding existing LPA questionnaires to encompass these domains would likely provide increased congruency between self-reported and accelerometer-derived estimates for LPA, as it may provide a better trigger for recalling these high volume activities. With increasing literature advocating the important role of LPA in adults’ health in concert with data suggesting survivors may engage in lower levels of LPA than healthy controls,23, accurately accounting for these lower intensity activities to provide a “whole picture” of a survivor’s active day remains an important future research direction. Combining accelerometer and self-report data using ecological momentary assessment to capture these behaviors in real-time in the real world could provide a better understanding of the context in which LPA occurs as well as survivors’ perceptions of intensity to build more accurate and scalable measurement tools for LPA.
Our ST results indicate nonsignificant difference estimates from the accelerometer and the STQ (Mdiff = 1.3 [15.3] min/day) with slightly higher estimates for the STQ versus accelerometer. This finding is consistent with the one other study that has examined these relationships in cancer survivors.15 However, our findings also indicate the IPAQ significantly underestimated ST compared with the accelerometer and the STQ by about half (Table 1). These differences may be because both the STQ and Marshall questionnaire used in the previous study measure multiple domains of sitting (ie, computer, television, travel) on both weekdays and weekends whereas the IPAQ uses only two recall items of overall sitting time (for weekday and weekend separately). The domain-specific, structured approach has been shown to improve recall and may help to prevent underestimation and general underreporting of the high volume, ubiquitous behavior of sitting.17,38 Finally, we would be remiss to not acknowledge the known limitations to estimating ST using the count-based approach on the waist-worn accelerometer. Due to the monitor’s orientation at the hip, the accelerometer may misrepresent total ST by misclassifying standing still as sitting. However, Kozey-Keadle and colleagues have previously examined estimation of ST using waist-worn accelerometers and have shown the 100 count per minute cut off yields ST estimates within 5% range of accuracy for a seated position compared with direct observation.39
Of further interest are our exploratory results indicating that age and disease stage may modify the congruency between activity and ST measures. Specifically, older survivors and those with more advanced disease stage generally reported more PA and less ST than were measured by the accelerometer. These differences raise the question of whether these subgroups are systematically reporting more time physically active, overestimating their intensity, or the accelerometer is misclassifying their activity intensity. These misclassifications could be due to their age, disease stage, fatigue status, functional status, cognitive function, occupational status, etc. and would be important next steps for exploration of measurement of physical activity in breast cancer survivors. Finally, the difference score for MVPA was greater for survivors of color than for white survivors, with survivors of color overreporting MVPA compared with accelerometer-derived estimates. This may be due in part to cultural differences between white survivors and survivors of color. Previous research has suggested that people of color may accumulate a majority of their activity in occupational or household-related domains, thus explaining lower levels of leisure-time MVPA but high levels of reported total MVPA from other nonleisure domains.20 However, given the small number of survivors of color in the present study, these results should be interpreted with caution.
With the multitude of physical activity and ST measurement tools available, many factors including cost, sample size, primary outcome of interest, and activity characteristics of interest (eg, duration, intensity, energy expenditure) need to be considered40 when choosing a tool. Our findings may help inform these decisions for breast cancer survivors. For example, if LPA is of interest, an accelerometer may provide a more comprehensive assessment of these activities than the GLTEQ. In contrast, if MVPA is the activity of interest, our results suggest the GLTEQ and accelerometer were more congruent than the IPAQ was with either measure, therefore, if budgetary constraints are a concern, the more cost-efficient GLTEQ could provide similar results to an accelerometer. In addition to considering measurement congruency, it is also critically important to carefully consider the population (breast cancer survivors) and subsequent burden that accompanies the measurement tool of choice. Overall, our results indicate, when choosing a questionnaire for ST or LPA for breast cancer survivors, the more comprehensive the questions, to encompass multiple domains or time of day, the greater amount of time that will be captured within that activity category. Conversely, since the majority of MVPA is completed in leisure-time, dependent on the age and race of the population, a shorter questionnaire may be sufficient. Additionally, dependent on time since diagnosis and treatment received, activity recall or body movement patterns may be affected which could influence measurement tool selection.23,24 Finally, it is also important to consider the setting in which measurement is taking place. In busy clinical settings, shorter, self-report measures may have a greater chance of being implemented than accelerometers or longer self-report measures and would still provide useful information regarding an overall snapshot of survivors’ MVPA or ST that could be used to initiate a conversation or referral for a program to help survivors positively change one or both of these behaviors.
Limitations
There were a few limitations within the current study that should be taken into account. First, the accelerometer cut-points used were developed with healthy, young adults; therefore using different cut-points may have yielded different results.34 Given the large age range in our participants (23-84 years), we believe the use of these cut-points was justified, in lieu of population-specific (ie, older adults) cut-points. In addition, limitations to estimating activity from an accelerometer include the inability to capture certain activities such as swimming and cycling and the aforementioned inability to distinguish between body postures (ie, sitting vs standing).41 The participants were predominantly white, highly educated, and high earners (85.2% earned ≥$40,000 per year), therefore, the present results may not be generalizable to survivors from more diverse backgrounds. However, as far as we know, this is the first study to report the congruency of estimated ST, LPA, and MVPA across multiple measurement tools in a nationwide sample of breast cancer survivors who were heterogeneous in terms of disease characteristics (ie, stage, treatment, time since diagnosis).
Conclusions
Our findings suggest that physical activity and ST estimates in breast cancer survivors may be dependent on the measurement tool used. In addition, congruency of measurement tools was dependent on activity intensity of interest, and participant age, race, and disease history may also influence these factors. Therefore, researchers should consider the intended outcomes of interest, the context in which the tool is being used (ie, clinical versus research), the available resources, and the participant population before they select a measurement tool for estimating physical activity and sitting time in breast cancer survivors.
Acknowledgment
This work was supported by grant #F31AG034025 from the National Institute on Aging (Dr Phillips); Shahid and Ann Carlson Khan endowed professorship and grant #AG020118 from the National Institute on Aging (Dr McAuley). Dr Phillips is supported by the National Cancer Institute #K07CA196840, and Dr Welch is supported by National Institute of Health/National Cancer Institute training grant CA193193. All data for this study were collected at the University of Illinois Urbana Champaign.
Physical activity has numerous physical, mental, and psychosocial benefits for cancer survivors, such as a reduction in the risk of mobility disability, depression, and anxiety, and improved patient quality of life.1,2 In addition, higher levels of physical activity are associated with reduced cancer-specific and all-causes mortality as well as cancer-specific outcomes including reduced risk of cancer progression and recurrence and new primary cancers.3-5 However, fewer than one-third of cancer survivors are meeting government and cancer-specific recommendations of 150 minutes a week of moderate to vigorous physical activity (MPVA; ≥3 metabolic equivalents [METs]).6,7 Growing evidence also demonstrates a significant association between higher levels of sedentary behavior and many deleterious health effects after cancer, including an increased risk for decreased physical functioning and development of other chronic diseases such as cardiovascular disease or diabetes.8 Distinct from physical activity, sedentary behavior is defined as any waking activity resulting in low levels of energy expenditure (≤1.5 METs) while in a seated or reclined position.9 Increased sedentary behavior, even when controlling for moderate and vigorous physical activity (MVPA), is associated with poor quality of life and increased all-cause mortality in cancer survivors.10,11 Given the associations observed between higher levels of physical activity, lower levels of sedentary behavior, and improved health and disease outcomes among the large and increasing number of cancer survivors in the United States, it is important to identify low-cost methods that can be used in a in a variety of settings (ie, research, clinical, community) to accurately and efficiently measure survivors’ lifestyle behaviors to identify high-risk survivors for early intervention, better understand the effects of these behaviors on survivors’ health outcomes and disease trajectories, and ultimately, improve survivors’ health and quality of life.12,13
Two methods commonly used to capture physical activity and sedentary behavior across the lifespan are accelerometry (Actigraph, Pensacola, FL) and self-report questionnaires such as the Godin Leisure-Time Questionnaire (GLTEQ), International Physical Activity Questionnaire (IPAQ), and Sitting Time Questionnaire (STQ).14-17 Each method has unique strengths and weaknesses. Sending accelerometers to multiple individuals at a single time point can be costly, particularly in large-scale epidemiological studies, and the accelerometer’s waist-worn, nonwaterproof design may prevent researchers from capturing certain activities such as swimming and resistance training. However, the accelerometer provides objective, precise assessments of most physical activities and may help remove response bias.18 Conversely, self-report questionnaires rely solely on individuals’ memories and often result in recall bias, inaccurate reporting, and under- or overestimation of physical activity engagement.19,20 Nevertheless, these questionnaires can be widely disseminated at low cost in a variety of settings (eg, clinical, research, community) and are less of a burden to participants.
Recent studies comparing objective (eg, accelerometer) with subjective (eg, self-report) methods of measuring physical activity and sedentary behavior in healthy middle-aged adults and older adults have demonstrated mixed findings with no distinct trends in the degree to which these methods differ.19,21,22 To date, little consideration has been given to the measurement of these lifestyle behaviors in cancer survivors. Boyle and colleagues recently investigated the concurrent validity of an accelerometer to the GLTEQ in colon cancer survivors, finding significant differences in estimated MVPA (~11 minutes). However, no studies, to our knowledge, have compared accelerometer and self-report measures in breast cancer survivors, so it remains unclear how these different measurement tools relate to each another in this population.
It is particularly important to compare these measurement tools among breast cancer survivors because evidence indicates this population’s behavioral habits, self-perceived activity, and sitting time and movement patterns may differ significantly from the general population and other survivor groups across the lifespan.23,24 Further, previous studies examining these behaviors in cancer survivors focused primarily on sitting time and MVPA.15,25,26 Examining other lower-intensity intensities (eg, light activity or lifestyle) in cancer survivors may also be important given that increased levels of activity are associated with health benefits, ranging from reduced disability and fatigue to improved cardiovascular health and quality of life, and that breast cancer survivors engage in fewer of these activities compared with noncancer controls.23 These lower levels of physical activity may be more prevalent among cancer survivors of their high levels of fatigue and propensity toward increased sitting time during the first year of treatment,11 so it is important to be able to accurately assess these activities in this population. The purpose of the present study was to compare estimates of time spent in light physical activity (LPA), MVPA, and sitting time (ST) obtained from an accelerometer and 3 self-report measurement tools (GLTEQ, IPAQ, STQ) in a large, US-based sample of breast cancer survivors. A secondary purpose was to determine whether estimate comparisons among measurements changed by participant characteristics.
Methods
Participants and procedures
This study consisted of a subsample of women who participated in a larger study whose findings have been reported elsewhere by Phillips and McAuley.27 In that study, breast cancer survivors (n = 1,631) were recruited nationally to participate in a 6-month prospective study on quality of life. Eligibility criteria included being aged 18 years or older, having had a diagnosis of breast cancer, being English speaking, and having access to the internet. Once consented to participate in the study, 500 women were randomly selected to wear the accelerometer.
Participants in this group were mailed an accelerometer, an activity log, instructions for use, and a self-addressed stamped envelope to return the monitor. They were asked to wear the accelerometer during all waking hours for 7 consecutive days of usual activity. They were also sent a secure link to complete 3 activity questionnaires online. The questionnaires were to be completed by the end of the 7-day monitoring period. Only women with 3 or more valid days of accelerometer data and complete data on variables of interest (n = 414) were included in the present analyses. All of the participants consented to the study procedures approved by the University of Illinois Institutional Review Board.
Measures
Demographics. The participants self-reported their age, level of education, height, and weight. Their body mass index (BMI; kg/m2) was estimated using the standard equation. They also self-reported their health and cancer history, detailing breast cancer disease stage, time since diagnosis, treatment type, and whether they had had a cancer recurrence. They were also asked to report whether they had ever been diagnosed (Yes/No) with 18 chronic conditions (eg, diabetes, arthritis).
Godin Leisure-Time Exercise Questionnaire.16 The GLTEQ assessed participants’ weekly frequency and mean amount of time performing MVPA (moderate exercise, such as fast walking, combined with vigorous exercise, such as jogging), and LPA (light/mild exercise, eg, easy walking) during the previous 7 days. The mean daily duration (in minutes) for each intensity category (MVPA, LPA) was calculated using activity frequencies and the amount of time spent in each activity presented as minutes/day.
The International Physical Activity Questionnaire.14 The IPAQ evaluated participants’ physical activity of at least moderate intensity in 4 domains of everyday life: job-related physical activity, transportation, housework/caring for family, and leisure-time activity. Within each domain, participants were asked the number of days per week and time per day (hours and minutes) spent performing MVPA. To estimate sitting time, the questionnaire asks participants to report the total amount of time spent sitting per day in 2 conditions, during weekdays and during weekends. The present analysis averaged sitting time for a typical 7-day (5 week days, 2 weekend days) period. We multiplied reported minutes per day and frequency per week of each activity category (MVPA and ST) to calculate the mean number of minutes per day.29,30
Sitting Time Questionnaire.17,28 The STQ estimated the mean time (hours and minutes) participants spent sitting each day on weekdays and at weekends within 5 domains: while traveling to and from places, at work, watching television, using a computer at home, and at leisure, not including watching television (eg, visiting friends, movies, dining out). Mean minutes per day of ST were calculated using all sitting domains.
Actigraph accelerometer (model GT1M, Health One Technology, Fort Walton Beach, FL). The Actigraph GT1M is a reliable and objective measure of physical activity.31-33 Participants wore the monitor on the right hip for 7 consecutive days during all waking hours, except when bathing or swimming. Activity data was analyzed in 1-minute intervals. A valid day of accelerometer wear time was defined as ≥600 minutes with no more than 60 minutes of consecutive zero-values, with allowance of 2 minutes or fewer of observations <100 counts/minute within the nonwear interval.34 Each minute of wear time was classified according to intensity (counts/min) using the following cut-points:34 sedentary, <100 counts/min; LPA, 100-2,019 counts/min; and MVPA, ≥2,020 count/min. Mean daily durations (min/day) spent in each behavior were estimated by dividing the number of minutes in each category by the number of valid days.
Statistical analysis
All statistical analyses were completed in SPSS Statistics 23 (IBM, Chicago, IL). Descriptive statistics were used to define participant characteristics. Rank-order correlation between the methods was assessed using Spearman’s rho (rs) and results were interpreted as follows: rs = 0.10, small; 0.30, moderate; and 0.50, strong.35 Within each activity intensity group, we jointly modeled daily minutes of self-report and accelerometer data using a random-intercept mixed-effects regression model. Differences between measurement tools were assessed based on regression coefficients with accelerometer as the reference category. Finally, we did a post hoc analysis of leisure-time–only MVPA from the IPAQ to compare with other estimates of MVPA.
We calculated the measurement tool difference scores for each estimated intensity category (ST, LPA, MVPA), that is, accelerometer estimated ST minus STQ estimated ST, and GLTEQ estimated MVPA minus IPAQ estimated MVPA. We used these data in an exploratory analysis to examine whether there were statistically significant differences between measurement difference scores by demographic or disease characteristics using linear regression stratified analyses. For example, we were interested in whether there was a significant difference in measurement tool estimates for sitting time in older compared with younger survivors. Analyses were stratified by age (<60/≥60 years), body mass index (<25 kg/m2/≥25 kg/m2), race (white/people of color), disease stage (I and II/III and IV), years since diagnosis (≤5 years/>5 years), recurrence (Yes/No), received chemotherapy (Yes/No ), received radiation (Yes/No ), and the presence of 1 or more chronic diseases (Yes/No ).
Results
Participants
The mean age of the participants was 56.8 years [9.2], they were overweight (BMI, 26.2 kg/m2 [5.4]), and predominantly white (96.7%; Table 1). Table 2 provides a summary of mean daily duration of activity estimates for ST, LPA, and MVPA and the estimate mean difference scores between measurements.
Moderate and vigorous physical activity
Accelerometer−GLTEQ. The mean difference in MVPA estimates between the accelerometer and GLTEQ was less than 5 minutes (Maccelerometer = 20.2 minutes; MGLTEQ = 23.6 minutes), even though the difference was statistically significant (P = .02). Estimates of MVPA from the accelerometer and GLTEQ (rs = 0.564, P < .001) showed a strong relationship. Stratified analyses showed that the difference scores between the GLTEQ and accelerometer were lower for older survivors (≥60 years) compared with younger survivors such that older survivors reported significantly less time in MVPA on the GLTEQ compared with accelerometer estimates (difference score [D] = 6.8 minutes less, P = .001).
Accelerometer−IPAQ. The accelerometer estimated significantly fewer minutes of MVPA per day when compared with the IPAQ (Mdiff = -67.4; 95% confidence interval [CI], -78.6, -55.8; P < .001). Estimates of MVPA from the accelerometer and IPAQ (rs = 0.011, P = .680) were poorly related. Differences between the IPAQ and accelerometer were greater for later-stage breast cancer, compared with early-stage diagnoses such that participants with late-stage disease reported significantly less MVPA on the IPAQ compared with accelerometer estimates (D = 41.8 minutes less than early-stage disease, P = .018). Finally, participants of color reported a greater difference in MVPA between the accelerometer and the IPAQ than did their white counterparts (D = 47.5 minutes, P = .033).
GLTEQ−IPAQ. GLTEQ estimated significantly fewer minutes of MVPA per day compared with the IPAQ (Mdiff = -64.6; 95% CI, -76.6, -52.5; P < .001). The estimates of MVPA from the GLTEQ had a small correlation with IPAQ estimates (rs = 0.128, P = .011).
IPAQ estimates showed almost triple the MVPA minutes per day as were estimated by the accelerometer and GLTEQ. As the MVPA estimate for the IPAQ include nonleisure activities, we conducted a post hoc analyses that only included the leisure-time items from the IPAQ. Leisure-time only IPAQ items, estimates indicated survivors spent a mean 18.5 [SD, 14.2] min/day in MVPA. Although the magnitude of the difference between the accelerometer and GLTEQ estimates (~10 minutes) was much smaller using the leisure-time only IPAQ items, a repeated measures analysis of variance revealed there was still a significant difference between these estimates (P < .05 for both) and negligible correlation.
Light intensity physical activity
Accelerometer−GLTEQ. There was a large and significant difference between LPA estimates from the GLTEQ and accelerometer (Mdiff = 224.5; 95% CI, 218.2, 230.7; P < .001) with estimates from the accelerometer being higher than those for the GLTEQ. Additionally, the measurements showed a negligible correlation (rs = 0.004, P = .94). Difference scores for GLTEQ and accelerometer estimated LPA were significantly different by age, with survivors aged 60 years or older demonstrating a difference that was 18.3 minutes shorter (P = .005) than the difference in younger survivors (<60 years).
Sitting time
Accelerometer−IPAQ. Mean IPAQ estimates were significantly lower (M = 303.8 [63.4]) than accelerometer estimates (M = 603.9 [78.0]). Rank-order correlations between IPAQ and accelerometer estimated ST was small (rs =0.26, P < .001). Difference scores between IPAQ and accelerometer estimates were significantly greater for survivors who were 60 years or older, compared with those younger than 60 years (D = 47.6 minutes, P = .006), indicating that older survivors tended to self-report significantly more ST than estimated by the accelerometer.
Accelerometer−STQ. There was no significant difference in estimated mean ST minutes per day between the STQ and the accelerometer, but the correlation between estimates was low (rs = 0.30, P < .001). Stratified analyses revealed estimates for the difference scores for mean daily ST between the STQ and accelerometer were greater for participants who were diagnosed with later-stage breast cancer (D= -158.3 minutes, P < .001) and those who had received chemotherapy (D= -61.7 minutes, P = .028; Table 2) than for those who were diagnosed with early-stage breast cancer or had not received chemotherapy. Women who had later-stage disease reported significantly less ST than did women diagnosed with early-stage disease, when compared with estimates by the accelerometer.
IPAQ−STQ. The estimated mean ST was significantly lower for IPAQ (M = 303.8 minutes [163.4]) than for the STQ (M = 605.2 minutes [296.2]). There were no significant estimate differences among the stratified groups.
Discussion
The purpose of the present study was to compare 4 measurement tools, an accelerometer-based activity monitor and 3 self-report questionnaires, to estimate ST, LPA, and MVPA in breast cancer survivors. Developing and evaluating accurate and precise measurement tools to assess physical activity and ST in breast cancer survivors remains a critical step toward better understanding the role of physical activity in cancer survivorship. Our results indicate that the congruency of the measurement tools examined was highly dependent on the activity intensity of interest and participants’ demographic or disease characteristics. Overall, the accelerometer estimated a greater amount of time spent sitting and engaging in LPA and less time in MVPA than was estimated on the STQ, GLTEQ, and IPAQ. In addition, our findings suggest significant subgroup differences that will be important in future development and implementation of physical activity measurement for breast cancer survivors.
MVPA has been the most commonly measured activity intensity among cancer survivors to date.15,25,26 The present results indicate mean daily MVPA estimates were significantly higher for the GLTEQ compared with the accelerometer (Mdiff = 2.8 min/d, P = .019), although the magnitude of these differences was relatively small. This difference is lower than in another study that compared these measures in colon cancer survivors and found the GLTEQ over-estimated MVPA by 10.6 min/day compared with the accelerometer (P < .01).15 However, the correlation between the 2 tools in our study was similar to that of Boyle and colleagues (rs = 0.56 and rs = 0.51, respectively). A possible explanation for the equivocal findings across these studies may lie in the difference in study sample demographics; a previous study results finding breast cancer survivors may be better at recalling their physical activities because they may be more attentive to activities they perform daily.26
The IPAQ significantly estimated more than an hour more of MVPA minutes per day compared with the accelerometer and GLTEQ. There are a number of limitations to the reporting of MVPA on the IPAQ. These limitations have been previously reported in the literature and include cross-cultural differences as well as overreporting of nonleisure-time MVPA (eg, occupational or household activities). However, the IPAQ has consistently been shown to be a valid and reliable tool for physical activity surveillance in different populations across the world.29,36,37 This shows that although MVPA was overestimated in our population, we do not mean to undermine the IPAQ value in other populations in which it has shown great utility for overall physical activity surveillance. When we excluded nonleisure-time MVPA, MVPA equated to about 18 min/day, which was closer in magnitude to the GLTEQ and accelerometer. These data highlight the importance of identifying the specific activity parameters of interest when selecting a measurement tool to ensure congruency between the tool and construct of interest.
The differences in MVPA estimation from the 3 tools have significant translational consequences, notably the potential for misclassification of meeting physical activity guidelines. For example, the percentage of women in the present sample that met physical activity guidelines ranged from 0% (using the accelerometer) to 19.5% (using the IPAQ), depending on the measurement tool used. These findings have meaningful implications for future physical activity assessment because multiple measurement tools are cur
For example, scores from the IPAQ may result in a survivor being classified as meeting physical activity guidelines when in fact they are not, and thereby missing the opportunity for intervention; or the accelerometer may classify an active survivor as inactive, which could result in using time and resources for a behavior change intervention that is not necessary. The clinical significance of these findings is to provide providers with data-based information on the strengths and limitations of the measurement tools so that they can accurately estimate physical activity and ST and appropriately optimize resources and treatments.
The degree of measurement tool congruence is likely influenced by a number of factors. First, survivors’ perceptions of the intensity of their activity are relative and subjective to their state of feeling during the activity. For example, breast cancer survivors with lower functional capacity may perceive activities with lower absolute intensity as having a higher relative intensity (ie, they think they are working at a moderate intensity so record an activity as such, but the activity is classified as light by the accelerometer). Second, although our self-report measures asked survivors to record the time they had spent active over the previous 7 days, survivors might report on what they consider a “usual” week, which may reflect the ideal rather than the reality. Third, the accelerometer cut-points used were derived from young, healthy adults on a treadmill. Thus, generalization to an older, sick, less active population that could be experiencing treatment-related side effects could lead to underestimation of time spent in MVPA. To better understand measurement congruency in breast cancer survivors, future research should investigate how functional capacity and activity intensity perceptions are influenced by a breast cancer diagnosis and how those factors may influence subjective and objective physical activity measurement. If those factors were found to have significant influence on activity in breast cancer survivors, it would warrant future development of breast-cancer–specific accelerometer reduction techniques.
The comparison of LPA presented another interesting significant contrast between self-report (GLTEQ) and accelerometry. Results indicated the GLTEQ underestimated LPA by 224.5 [3.2] min/day compared with the accelerometer. This equates to over 3.5 h/day of active time (or about 280 kcal/day) that was potentially unaccounted for by the GLTEQ. The difference between these estimates could be due to the fact that the GLTEQ was designed to measure exercise time and therefore may not be as sensitive as the accelerometer to nonexercise-related LPA. Light intensity activities typically span a large range of domains (ie, occupational, leisure time, household) and tend to occur in higher volumes than MVPA, which may lead to some challenges with recall. Expanding existing LPA questionnaires to encompass these domains would likely provide increased congruency between self-reported and accelerometer-derived estimates for LPA, as it may provide a better trigger for recalling these high volume activities. With increasing literature advocating the important role of LPA in adults’ health in concert with data suggesting survivors may engage in lower levels of LPA than healthy controls,23, accurately accounting for these lower intensity activities to provide a “whole picture” of a survivor’s active day remains an important future research direction. Combining accelerometer and self-report data using ecological momentary assessment to capture these behaviors in real-time in the real world could provide a better understanding of the context in which LPA occurs as well as survivors’ perceptions of intensity to build more accurate and scalable measurement tools for LPA.
Our ST results indicate nonsignificant difference estimates from the accelerometer and the STQ (Mdiff = 1.3 [15.3] min/day) with slightly higher estimates for the STQ versus accelerometer. This finding is consistent with the one other study that has examined these relationships in cancer survivors.15 However, our findings also indicate the IPAQ significantly underestimated ST compared with the accelerometer and the STQ by about half (Table 1). These differences may be because both the STQ and Marshall questionnaire used in the previous study measure multiple domains of sitting (ie, computer, television, travel) on both weekdays and weekends whereas the IPAQ uses only two recall items of overall sitting time (for weekday and weekend separately). The domain-specific, structured approach has been shown to improve recall and may help to prevent underestimation and general underreporting of the high volume, ubiquitous behavior of sitting.17,38 Finally, we would be remiss to not acknowledge the known limitations to estimating ST using the count-based approach on the waist-worn accelerometer. Due to the monitor’s orientation at the hip, the accelerometer may misrepresent total ST by misclassifying standing still as sitting. However, Kozey-Keadle and colleagues have previously examined estimation of ST using waist-worn accelerometers and have shown the 100 count per minute cut off yields ST estimates within 5% range of accuracy for a seated position compared with direct observation.39
Of further interest are our exploratory results indicating that age and disease stage may modify the congruency between activity and ST measures. Specifically, older survivors and those with more advanced disease stage generally reported more PA and less ST than were measured by the accelerometer. These differences raise the question of whether these subgroups are systematically reporting more time physically active, overestimating their intensity, or the accelerometer is misclassifying their activity intensity. These misclassifications could be due to their age, disease stage, fatigue status, functional status, cognitive function, occupational status, etc. and would be important next steps for exploration of measurement of physical activity in breast cancer survivors. Finally, the difference score for MVPA was greater for survivors of color than for white survivors, with survivors of color overreporting MVPA compared with accelerometer-derived estimates. This may be due in part to cultural differences between white survivors and survivors of color. Previous research has suggested that people of color may accumulate a majority of their activity in occupational or household-related domains, thus explaining lower levels of leisure-time MVPA but high levels of reported total MVPA from other nonleisure domains.20 However, given the small number of survivors of color in the present study, these results should be interpreted with caution.
With the multitude of physical activity and ST measurement tools available, many factors including cost, sample size, primary outcome of interest, and activity characteristics of interest (eg, duration, intensity, energy expenditure) need to be considered40 when choosing a tool. Our findings may help inform these decisions for breast cancer survivors. For example, if LPA is of interest, an accelerometer may provide a more comprehensive assessment of these activities than the GLTEQ. In contrast, if MVPA is the activity of interest, our results suggest the GLTEQ and accelerometer were more congruent than the IPAQ was with either measure, therefore, if budgetary constraints are a concern, the more cost-efficient GLTEQ could provide similar results to an accelerometer. In addition to considering measurement congruency, it is also critically important to carefully consider the population (breast cancer survivors) and subsequent burden that accompanies the measurement tool of choice. Overall, our results indicate, when choosing a questionnaire for ST or LPA for breast cancer survivors, the more comprehensive the questions, to encompass multiple domains or time of day, the greater amount of time that will be captured within that activity category. Conversely, since the majority of MVPA is completed in leisure-time, dependent on the age and race of the population, a shorter questionnaire may be sufficient. Additionally, dependent on time since diagnosis and treatment received, activity recall or body movement patterns may be affected which could influence measurement tool selection.23,24 Finally, it is also important to consider the setting in which measurement is taking place. In busy clinical settings, shorter, self-report measures may have a greater chance of being implemented than accelerometers or longer self-report measures and would still provide useful information regarding an overall snapshot of survivors’ MVPA or ST that could be used to initiate a conversation or referral for a program to help survivors positively change one or both of these behaviors.
Limitations
There were a few limitations within the current study that should be taken into account. First, the accelerometer cut-points used were developed with healthy, young adults; therefore using different cut-points may have yielded different results.34 Given the large age range in our participants (23-84 years), we believe the use of these cut-points was justified, in lieu of population-specific (ie, older adults) cut-points. In addition, limitations to estimating activity from an accelerometer include the inability to capture certain activities such as swimming and cycling and the aforementioned inability to distinguish between body postures (ie, sitting vs standing).41 The participants were predominantly white, highly educated, and high earners (85.2% earned ≥$40,000 per year), therefore, the present results may not be generalizable to survivors from more diverse backgrounds. However, as far as we know, this is the first study to report the congruency of estimated ST, LPA, and MVPA across multiple measurement tools in a nationwide sample of breast cancer survivors who were heterogeneous in terms of disease characteristics (ie, stage, treatment, time since diagnosis).
Conclusions
Our findings suggest that physical activity and ST estimates in breast cancer survivors may be dependent on the measurement tool used. In addition, congruency of measurement tools was dependent on activity intensity of interest, and participant age, race, and disease history may also influence these factors. Therefore, researchers should consider the intended outcomes of interest, the context in which the tool is being used (ie, clinical versus research), the available resources, and the participant population before they select a measurement tool for estimating physical activity and sitting time in breast cancer survivors.
Acknowledgment
This work was supported by grant #F31AG034025 from the National Institute on Aging (Dr Phillips); Shahid and Ann Carlson Khan endowed professorship and grant #AG020118 from the National Institute on Aging (Dr McAuley). Dr Phillips is supported by the National Cancer Institute #K07CA196840, and Dr Welch is supported by National Institute of Health/National Cancer Institute training grant CA193193. All data for this study were collected at the University of Illinois Urbana Champaign.
1. Speck RM, Courneya KS, Masse LC, Duval S, Schmitz KH. An update of controlled physical activity trials in cancer survivors: a systematic review and meta-analysis. J Cancer Surviv. 2010;4(2):87-100.
2. Brenner DR. Cancer incidence due to excess body weight and leisure-time physical inactivity in Canada: implications for prevention. Prev Med. 2014;66:131-139.
3. Courneya KS, Friedenreich CM. Relationship between exercise pattern across the cancer experience and current quality of life in colorectal cancer survivors. J Altern Complement Med. 1997;3(3):215-226.
4. Ibrahim EM, Al-Homaidh A. Physical activity and survival after breast cancer diagnosis: meta-analysis of published studies. Med Oncol. 2011;28(3):753-765.
5. Lahart IM, Metsios GS, Nevill AM, Carmichael AR. Physical activity, risk of death and recurrence in breast cancer survivors: a systematic review and meta-analysis of epidemiological studies. Acta Oncol. 2015;54(5):635-654.
6. Irwin ML, McTiernan A, Bernstein L, et al. Physical activity levels among breast cancer survivors. Med Sci Sports Exerc. 2004;36(9):1484-1491.
7. Schmitz KH, Courneya KS, Matthews C, et al. American College of Sports Medicine roundtable on exercise guidelines for cancer survivors. Med Sci Sports Exerc. 2010;42(7):1409-1426.
8. Lynch BM. Sedentary behavior and cancer: a systematic review of the literature and proposed biological mechanisms. Cancer Epidemiol Biomarkers Prev. 2010;19(11):2691-2709.
9. Owen N, Healy GN, Matthews CE, Dunstan DW. Too much sitting: the population health science of sedentary behavior. Exerc Sport Sci Rev. 2010;38(3):105-113.
10. Campbell PT, Patel AV, Newton CC, Jacobs EJ, Gapstur SM. Associations of recreational physical activity and leisure time spent sitting with colorectal cancer survival. J Clin Oncol. 2013;31(7):876-885.
11. Lynch BM, Dunstan DW, Vallance JK, Owen N. Don’t take cancer sitting down: a new survivorship research agenda. Cancer. 2013;119(11):1928-1935.
12. Bluethmann SM, Mariotto AB, Rowland JH. Anticipating the ‘silver tsunami:’ prevalence trajectories and comorbidity burden among older cancer survivors in the United States. Cancer Epidemiol Biomarkers Prev. 2016;25(7):1029-1036.
13. Miller KD, Siegel RL, Lin CC, et al. Cancer treatment and survivorship statistics, 2016. CA Cancer J Clin. 2016;66(4):271-289.
14. Booth M. Assessment of physical activity: an international perspective. Res Q Exerc Sport. 2000;71(2 suppl):S114-120.
15. Boyle T, Lynch BM, Courneya KS, Vallance JK. Agreement between accelerometer-assessed and self-reported physical activity and sedentary time in colon cancer survivors. Support Care Cancer. 2015;23(4):1121-1126.
16. Godin G, Shephard RJ. A simple method to assess exercise behavior in the community. Canadian journal of applied sport sciences. Can J Appl Sport Sci. 1985;10(3):141-146.
17. Marshall AL, Miller YD, Burton NW, Brown WJ. Measuring total and domain-specific sitting: a study of reliability and validity. Med Sci Sports Exerc. 2010;42(6):1094-1102.
18. Matthews CE, Hagstromer M, Pober DM, Bowles HR. Best practices for using physical activity monitors in population-based research. Med Sci Sports Exerc. 2012;44(1 Suppl 1):S68-76.
19. Prince SA, Adamo KB, Hamel ME, Hardt J, Connor Gorber S, Tremblay M. A comparison of direct versus self-report measures for assessing physical activity in adults: a systematic review. Int J Behav Nutr Phys Act. 2008;5:56.
20. Sallis JF, Saelens BE. Assessment of physical activity by self-report: status, limitations, and future directions. Res Q Exerc Sport. 2000;71(2 Suppl):S1-14.
21. Hart TL, Swartz AM, Cashin SE, Strath SJ. How many days of monitoring predict physical activity and sedentary behaviour in older adults? Int J Behav Nutr Phys Act. 2011;8:62.
22. Hart TL, Ainsworth BE, Tudor-Locke C. Objective and subjective measures of sedentary behavior and physical activity. Med Sci Sports Exerc. 2011;43(3):449-456.
23. Phillips SM, Dodd KW, Steeves J, McClain J, Alfano CM, McAuley E. Physical activity and sedentary behavior in breast cancer survivors: new insight into activity patterns and potential intervention targets. Gynecol Oncol. 2015;138(2):398-404.
24. Boyle T, Vallance JK, Ransom EK, Lynch BM. How sedentary and physically active are breast cancer survivors, and which population subgroups have higher or lower levels of these behaviors? Support Care Cancer. 2016;24(5):2181-2190.
25. Broderick JM, Guinan E, Kennedy MJ, et al. Feasibility and efficacy of a supervised exercise intervention in de-conditioned cancer survivors during the early survivorship phase: the PEACH trial. J Cancer Surviv. 2013;7(4):551-562.
26. Su CC, Lee KD, Yeh CH, Kao CC, Lin CC. Measurement of physical activity in cancer survivors: a validity study. J Cancer Surviv. 2014;8(2):205-212.
27. Phillips SM, McAuley E. Social cognitive influences on physical activity participation in long-term breast cancer survivors. Psychooncology. 2013;22(4):783-791.
28. Wojcicki TR, White SM, McAuley E. Assessing outcome expectations in older adults: the multidimensional outcome expectations for exercise scale. J Gerontol B Psychol Sci Soc Sci. 2009;64(1):33-40.
29. Craig CL, Marshall AL, Sjostrom M, et al. International physical activity questionnaire: 12-country reliability and validity. Med Sci Sports Exerc. 2003;35(8):1381-1395.
30. Ainsworth BE, Haskell WL, Whitt MC, et al. Compendium of physical activities: an update of activity codes and MET intensities. Med Sci Sports Exerc. 2000;32(9 Suppl):S498-504.
31. Hacker ED, Ferrans CE. Ecological momentary assessment of fatigue in patients receiving intensive cancer therapy. J Pain Symptom Manage. 2007;33(3):267-275.
32. Swartz AM, Strath SJ, Bassett DR, Jr, O’Brien WL, King GA, Ainsworth BE. Estimation of energy expenditure using CSA accelerometers at hip and wrist sites. Med Sci Sports Exerc. 2000;32(9 Suppl):S450-456.
33. Jim HS, Small B, Faul LA, Franzen J, Apte S, Jacobsen PB. Fatigue, depression, sleep, and activity during chemotherapy: daily and intraday variation and relationships among symptom changes. Ann Behav Med. 2011;42(3):321-333.
34. Troiano RP, Berrigan D, Dodd KW, Masse LC, Tilert T, McDowell M. Physical activity in the United States measured by accelerometer. Med Sci Sports Exerc. 2008;40(1):181-188.
35. Cohen J. Statistical power analysis for the behavioral sciences. 2nd ed. Hillsdale, NJ: L Erlbaum Associates; 1988.
36. Bauman A, Ainsworth BE, Bull F, et al. Progress and pitfalls in the use of the International Physical Activity Questionnaire (IPAQ) for adult physical activity surveillance. J Phys Act Health. 2009;6 Suppl 1:S5-8.
37. Hagströmer M1, Oja P, Sjöström M. The International Physical Activity Questionnaire (IPAQ): a study of concurrent and construct validity. Public Health Nutr. 2006;9(6):755-762.
38. Johnson-Kozlow M, Sallis JF, Gilpin EA, Rock CL, Pierce JP. Comparative validation of the IPAQ and the 7-Day PAR among women diagnosed with breast cancer. Int J Behav Nutr Phys Act. 2006;3:7.
39. Kozey-Keadle S, Libertine A, Lyden K, Staudenmayer J, Freedson PS. Validation of wearable monitors for assessing sedentary behavior. Med Sci Sports Exerc. 2011;43(8):1561-1567.
40. Strath SJ, Kaminsky LA, Ainsworth BE, et al. Guide to the assessment of physical activity: clinical and research applications. Circulation. 2013;128(20):2259-2279.
41. Bassett DR. Device-based monitoring in physical activity and public health research. Physiol Meas. 2012;33(11):1769-1783.
1. Speck RM, Courneya KS, Masse LC, Duval S, Schmitz KH. An update of controlled physical activity trials in cancer survivors: a systematic review and meta-analysis. J Cancer Surviv. 2010;4(2):87-100.
2. Brenner DR. Cancer incidence due to excess body weight and leisure-time physical inactivity in Canada: implications for prevention. Prev Med. 2014;66:131-139.
3. Courneya KS, Friedenreich CM. Relationship between exercise pattern across the cancer experience and current quality of life in colorectal cancer survivors. J Altern Complement Med. 1997;3(3):215-226.
4. Ibrahim EM, Al-Homaidh A. Physical activity and survival after breast cancer diagnosis: meta-analysis of published studies. Med Oncol. 2011;28(3):753-765.
5. Lahart IM, Metsios GS, Nevill AM, Carmichael AR. Physical activity, risk of death and recurrence in breast cancer survivors: a systematic review and meta-analysis of epidemiological studies. Acta Oncol. 2015;54(5):635-654.
6. Irwin ML, McTiernan A, Bernstein L, et al. Physical activity levels among breast cancer survivors. Med Sci Sports Exerc. 2004;36(9):1484-1491.
7. Schmitz KH, Courneya KS, Matthews C, et al. American College of Sports Medicine roundtable on exercise guidelines for cancer survivors. Med Sci Sports Exerc. 2010;42(7):1409-1426.
8. Lynch BM. Sedentary behavior and cancer: a systematic review of the literature and proposed biological mechanisms. Cancer Epidemiol Biomarkers Prev. 2010;19(11):2691-2709.
9. Owen N, Healy GN, Matthews CE, Dunstan DW. Too much sitting: the population health science of sedentary behavior. Exerc Sport Sci Rev. 2010;38(3):105-113.
10. Campbell PT, Patel AV, Newton CC, Jacobs EJ, Gapstur SM. Associations of recreational physical activity and leisure time spent sitting with colorectal cancer survival. J Clin Oncol. 2013;31(7):876-885.
11. Lynch BM, Dunstan DW, Vallance JK, Owen N. Don’t take cancer sitting down: a new survivorship research agenda. Cancer. 2013;119(11):1928-1935.
12. Bluethmann SM, Mariotto AB, Rowland JH. Anticipating the ‘silver tsunami:’ prevalence trajectories and comorbidity burden among older cancer survivors in the United States. Cancer Epidemiol Biomarkers Prev. 2016;25(7):1029-1036.
13. Miller KD, Siegel RL, Lin CC, et al. Cancer treatment and survivorship statistics, 2016. CA Cancer J Clin. 2016;66(4):271-289.
14. Booth M. Assessment of physical activity: an international perspective. Res Q Exerc Sport. 2000;71(2 suppl):S114-120.
15. Boyle T, Lynch BM, Courneya KS, Vallance JK. Agreement between accelerometer-assessed and self-reported physical activity and sedentary time in colon cancer survivors. Support Care Cancer. 2015;23(4):1121-1126.
16. Godin G, Shephard RJ. A simple method to assess exercise behavior in the community. Canadian journal of applied sport sciences. Can J Appl Sport Sci. 1985;10(3):141-146.
17. Marshall AL, Miller YD, Burton NW, Brown WJ. Measuring total and domain-specific sitting: a study of reliability and validity. Med Sci Sports Exerc. 2010;42(6):1094-1102.
18. Matthews CE, Hagstromer M, Pober DM, Bowles HR. Best practices for using physical activity monitors in population-based research. Med Sci Sports Exerc. 2012;44(1 Suppl 1):S68-76.
19. Prince SA, Adamo KB, Hamel ME, Hardt J, Connor Gorber S, Tremblay M. A comparison of direct versus self-report measures for assessing physical activity in adults: a systematic review. Int J Behav Nutr Phys Act. 2008;5:56.
20. Sallis JF, Saelens BE. Assessment of physical activity by self-report: status, limitations, and future directions. Res Q Exerc Sport. 2000;71(2 Suppl):S1-14.
21. Hart TL, Swartz AM, Cashin SE, Strath SJ. How many days of monitoring predict physical activity and sedentary behaviour in older adults? Int J Behav Nutr Phys Act. 2011;8:62.
22. Hart TL, Ainsworth BE, Tudor-Locke C. Objective and subjective measures of sedentary behavior and physical activity. Med Sci Sports Exerc. 2011;43(3):449-456.
23. Phillips SM, Dodd KW, Steeves J, McClain J, Alfano CM, McAuley E. Physical activity and sedentary behavior in breast cancer survivors: new insight into activity patterns and potential intervention targets. Gynecol Oncol. 2015;138(2):398-404.
24. Boyle T, Vallance JK, Ransom EK, Lynch BM. How sedentary and physically active are breast cancer survivors, and which population subgroups have higher or lower levels of these behaviors? Support Care Cancer. 2016;24(5):2181-2190.
25. Broderick JM, Guinan E, Kennedy MJ, et al. Feasibility and efficacy of a supervised exercise intervention in de-conditioned cancer survivors during the early survivorship phase: the PEACH trial. J Cancer Surviv. 2013;7(4):551-562.
26. Su CC, Lee KD, Yeh CH, Kao CC, Lin CC. Measurement of physical activity in cancer survivors: a validity study. J Cancer Surviv. 2014;8(2):205-212.
27. Phillips SM, McAuley E. Social cognitive influences on physical activity participation in long-term breast cancer survivors. Psychooncology. 2013;22(4):783-791.
28. Wojcicki TR, White SM, McAuley E. Assessing outcome expectations in older adults: the multidimensional outcome expectations for exercise scale. J Gerontol B Psychol Sci Soc Sci. 2009;64(1):33-40.
29. Craig CL, Marshall AL, Sjostrom M, et al. International physical activity questionnaire: 12-country reliability and validity. Med Sci Sports Exerc. 2003;35(8):1381-1395.
30. Ainsworth BE, Haskell WL, Whitt MC, et al. Compendium of physical activities: an update of activity codes and MET intensities. Med Sci Sports Exerc. 2000;32(9 Suppl):S498-504.
31. Hacker ED, Ferrans CE. Ecological momentary assessment of fatigue in patients receiving intensive cancer therapy. J Pain Symptom Manage. 2007;33(3):267-275.
32. Swartz AM, Strath SJ, Bassett DR, Jr, O’Brien WL, King GA, Ainsworth BE. Estimation of energy expenditure using CSA accelerometers at hip and wrist sites. Med Sci Sports Exerc. 2000;32(9 Suppl):S450-456.
33. Jim HS, Small B, Faul LA, Franzen J, Apte S, Jacobsen PB. Fatigue, depression, sleep, and activity during chemotherapy: daily and intraday variation and relationships among symptom changes. Ann Behav Med. 2011;42(3):321-333.
34. Troiano RP, Berrigan D, Dodd KW, Masse LC, Tilert T, McDowell M. Physical activity in the United States measured by accelerometer. Med Sci Sports Exerc. 2008;40(1):181-188.
35. Cohen J. Statistical power analysis for the behavioral sciences. 2nd ed. Hillsdale, NJ: L Erlbaum Associates; 1988.
36. Bauman A, Ainsworth BE, Bull F, et al. Progress and pitfalls in the use of the International Physical Activity Questionnaire (IPAQ) for adult physical activity surveillance. J Phys Act Health. 2009;6 Suppl 1:S5-8.
37. Hagströmer M1, Oja P, Sjöström M. The International Physical Activity Questionnaire (IPAQ): a study of concurrent and construct validity. Public Health Nutr. 2006;9(6):755-762.
38. Johnson-Kozlow M, Sallis JF, Gilpin EA, Rock CL, Pierce JP. Comparative validation of the IPAQ and the 7-Day PAR among women diagnosed with breast cancer. Int J Behav Nutr Phys Act. 2006;3:7.
39. Kozey-Keadle S, Libertine A, Lyden K, Staudenmayer J, Freedson PS. Validation of wearable monitors for assessing sedentary behavior. Med Sci Sports Exerc. 2011;43(8):1561-1567.
40. Strath SJ, Kaminsky LA, Ainsworth BE, et al. Guide to the assessment of physical activity: clinical and research applications. Circulation. 2013;128(20):2259-2279.
41. Bassett DR. Device-based monitoring in physical activity and public health research. Physiol Meas. 2012;33(11):1769-1783.
Concurrent ipilimumab and CMV colitis refractory to oral steroids
Immune checkpoint inhibitors, including anti-cytotoxic T-lymphocyte antigen 4 (anti-CTLA4) and anti-programmed cell death protein-1 (anti-PD-1) antibodies, have demonstrated clinical and survival benefits in a variety of malignancies, which has led to an expansion in their role in oncology. In melanoma, the anti-CTLA-4 antibody, ipilimumab, has demonstrated a survival benefit in patients with advanced metastatic melanoma and in patients with resectable disease with lymph node involvement.1,2
Ipilimumab exerts its effect by binding CTLA-4 on conventional and regulatory T cells, thus blocking inhibitory signals on T cells, which leads to an antitumor response.3 The increased immune response counteracts the immune-evading mechanisms of the tumor. With increased use of these agents, immune-related adverse events (irAEs) have become more prevalent. The most common irAEs secondary to ipilimumab are skin rash, colitis/diarrhea, hepatitis, pneumonitis, and various endocrinopathies.4 In a phase 3 trial of adjuvant ipilimumab in patients with resected stage III melanoma, grade 3 or 4 adverse events occurred in 54.1% of participants in the ipilimumab arm, the most common being diarrhea and colitis (9.8% and 6.8%, respectively).2Recognition and management of irAEs has led to the implementation of treatment guidelines.4,5 Management of irAEs includes checkpoint inhibitor discontinuation and reversal of the immune response by institution of immunosuppression with corticosteroids.
Case presentation and summary
A 40-year-old white woman with stage IIIB BRAF V600E-positive melanoma presented with diarrhea refractory to high-dose prednisone (1 mg/kg BID). She had recently undergone wide local excision and sentinel node biopsy and received her inaugural dose of ipilimumab (10 mg/kg).
The patient first presented with loose, watery stools that had begun 8 days after she had received her first dose of adjuvant ipilimumab. She was admitted to the hospital, and intravenous methylprednisolone was initiated along with empiric ciprofloxacin (400 mg, IVPB Q12h) and metronidazole (500 mg, IVPB Q8h) as infectious causes were concurrently ruled out. During this initial admission, the patient’s stool was negative for Clostridium difficile toxin, ova, and parasites, as well as enteric pathogens by culture. After infectious causes were excluded, she was diagnosed with ipilimumab-induced colitis. Antibiotics were discontinued, and the patient ultimately noted improvement in her symptoms. On hospital day 7, she was experiencing only 2 bowel movements a day and was discharged on 80 mg of prednisone twice daily.
After discharge the patient noted persistence of her symptoms. At her follow-up, 9 days after discharge, the patient noted continued symptoms of low-grade diarrhea. She failed a trial of steroid tapering due to exacerbation of her abdominal pain and frequency of diarrhea. Further investigation was negative for C. diff toxin and a computed-tomography scan was consistent with continuing colitis. The patient’s symptoms continued to worsen, with recurrence of grade 3 diarrhea, and she was ultimately readmitted 17 days after her earlier discharge (36 days after her first ipilimumab dosing).
On re-admission, the patient was again given intravenous methylprednisolone and experienced interval improvement in the frequency of diarrhea. A gastroenterology expert was consulted, and the patient underwent a flexible sigmoidoscopy that demonstrated findings of diffuse and severe inflammation and biopsies were obtained (Figure 1). After several days of continued symptoms, the patient received infliximab 5 mg/kg for treatment of her adverse autoimmune reaction. After administration, the patient noted improvement in the frequency and volume of diarrhea, however, her symptoms still persisted.
Biopsy results subsequently revealed findings compatible with ipilimumab-induced colitis, and immunohistochemical staining demonstrated positivity for cytomegalovirus (CMV). Specifically, histologic examination showed lymphoplasmacytic expansion of the lamina propria, some architectural distortion, and increased crypt apoptosis. Scattered cryptitis and crypt abscesses were also noted, as were rare stromal and endothelial cells with characteristic CMV inclusions (Figure 2 and Figure 3).
Serum CMV polymerase chain reaction (PCR) was also positive at 652,000 IU/mL (lower limit of detection 100 IU/mL). Induction dosing of ganciclovir (5 mg/kg IV Q12h) was initiated. The combined treatment with intravenous methylprednisone and ganciclovir led to an improvement in diarrhea frequency and resolution of blood in the stool. She was transitioned to oral prednisone, but it resulted in redevelopment of grade 3 diarrhea. The patient was therefore resumed on and discharged on daily intravenous methylprednisolone.
After discharge, the patient was started on budesonide 9 mg daily. Her serum CMV PCR level reduced and she was transitioned to oral valgancyclovir (900 mg daily) for maintenance. Another unsuccessful attempt was made to switch her to oral prednisone.
About 14 weeks after the initial ipilimumab dosing, the patient underwent another flexible sigmoidoscopy that again demonstrated severe colitis from the rectum to sigmoid colon. Biopsies were negative for CMV. Patient was readmitted for recurrence of diarrhea the following week. Treatment with IV methylprednisone (1mg/kg BID) and infliximab (5 mg/kg) again led to an improvement of symptoms. She was again discharged on IV methylprednisone (1 mg/kg BID) with a taper.
In the 15th week after her initial ipilimumab dose, the patient presented with a perforated bowel, requiring a subtotal colectomy and end ileostomy. She continued on a slow taper of oral prednisone (50 mg daily and decrease by 10 mg every 5 days).
At her last documented follow-up, 8 months after her first ipilimumab dose, she was having normal output from her ileostomy. She developed secondary adrenal insufficiency because of the long-term steroids and continued to take prednisone 5 mg daily.
Discussion
Diarrhea and colitis are common irAEs attributable to checkpoint-inhibitor therapy used for the treatment of melanoma. This case of ipilimumab-induced colitis refractory to high-dose oral steroids demonstrates the risks associated with management of anti-CTLA-4 induced colitis. In particular, the high-dose corticosteroids required to treat the autoimmune component of this patient’s colitis increased her susceptibility to CMV reactivation.
The diagnosis of colitis secondary to ipilimumab is made primarily in the appropriate clinical setting, and typically onsets during the induction period (within 12 weeks of initial dosing) and most resolve within 6-8 weeks.6 Histopathologically, there is lymphoplasmacytic expansion of lamina propria, increased intraepithelial lymphocytes, and increased epithelial apoptosis of crypts. One can also see acute cryptitis and crypt abscesses. Reactive epithelial changes with mucin depletion are also often seen in epithelial cells.
Findings from immunohistochemical studies have shown the increased intraepithelial lymphocytes to be predominantly CD8-positive T cells, while the lamina propria contains an increase in the mixture of CD4- and CD8-positive T cells. In addition, small intestinal samples show villous blunting. There is an absence of significant architectural distortion and well-developed basal lymphoplasmacytic infiltrates characteristic of chronic mucosal injury, such as idiopathic inflammatory bowel disease.7 Granulomas are also absent in most series, though they have been reported in some cases.8 The features are similar to those seen in autoimmune enteropathy, but goblet and endocrine cells remain preserved. Graft-versus-host disease has similar histologic features, however, the clinical setting usually makes the distinction between these obvious.
Current treatment algorithms for ipilimumab-related diarrhea, begin with immediate treatment with intravenous methylprednisolone (125 mg once). This is followed with oral prednisone at a dose of 1-2 mg/kg tapered over 4 to 8 weeks.4 In patients with persistent symptoms despite adequate doses of corticosteroids, infliximab (5 mg/kg every 2 weeks) is recommended until the resolution of symptoms, and a longer taper of prednisone is often necessary.
Institution of high-dose corticosteroids to treat grade 3 or 4 irAEs can increase the risk for infection, including opportunistic infections. One retrospective review of patients administered checkpoint inhibitors at a single institution revealed that 7.3% of 740 patients developed a severe infection that lead to hospitalization or treatment with intravenous antibiotics.9 In that patient cohort, only 0.6% had a serious infection secondary to a viral etiology, and 1 patient developed CMV enterocolitis. Most patients who developed an infection in this cohort had received corticosteroids (46/54 patients, 85%) and/or infliximab (13/54 patients, 24%).9
CMV is a member of the Herpesviridae family. After a primary infection, which can often go unrecognized in an immunocompetent host, CMV can persist in a latent state.10 In a study by Bate and colleagues, the age-adjusted seropositivity of CMV was found to be 50.4%.11 Based on those results, immunosuppression in a patient who has previously been infected with CMV can lead to a risk of reactivation or even reinfection. In the era of checkpoint-inhibitor therapy, reactivation of CMV has been described previously in a case of CMV hepatitis and a report of CMV colitis.12,13 Immunosuppression, such as that caused by corticosteroids, is a risk factor for CMV infection.14 Colitis caused by CMV usually presents with abdominal pain, diarrhea, and bloody diarrhea.15 In suspected cases of CMV colitis, endoscopy should be pursued with biopsy for tissue examination. A tissue diagnosis is required for CMV colitis because serum PCR can be negative in isolated cases of gastrointestinal CMV infection.15
Conclusion
Despite appropriate treatment with ganciclovir and the noted response in the patient’s serum CMV PCR, symptom exacerbation was observed with the transition to oral prednisone. The requirement for intravenous corticosteroids in the present case demonstrates the prolonged effects exerted by irAEs secondary to checkpoint-inhibitor therapy. Those effects are attributable to the design of the antibody – ipilimumab is a fully humanized monoclonal antibody and has a plasma half-life of about 15 days.1,4
By the identification of CMV histopathologically, this case, along with the case presented by Lankes and colleagues,13 illustrates the importance of considering CMV colitis in patients who are being treated with ipilimumab and who develop persistent or worsening diarrhea after initial treatment with high-dose steroids.
Early recognition of possible coexistent CMV colitis in patients with a history of treatment with ipilimumab can have important clinical consequences. It can lead to quicker implementation of proper antiviral therapy and minimization of immune suppression to levels required to maintain control of the patient’s symptoms.
1. Hodi FS, O’Day SJ, McDermott DF, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med. 2010;363(8):711-723.
2. Eggermont AM, Chiarion-Sileni V, Grob JJ, et al. Prolonged survival in stage III melanoma with ipilimumab adjuvant therapy. N Engl J Med. 2016;375(19):1845-1855.
3. Glassman PM, Balthasar JP. Mechanistic considerations for the use of monoclonal antibodies for cancer therapy. Cancer Biol Med. 2014;11(1):20-33.
4. Weber JS, Kahler KC, Hauschild A. Management of immune-related adverse events and kinetics of response with ipilimumab. J Clin Oncol. 2012;30(21):2691-2697.
5. Fecher LA, Agarwala SS, Hodi FS, Weber JS. Ipilimumab and its toxicities: a multidisciplinary approach. Oncologist. 2013;18(6):733-743.
6. Weber JS, Dummer R, de Pril V, Lebbe C, Hodi FS, Investigators MDX. Patterns of onset and resolution of immune-related adverse events of special interest with ipilimumab: detailed safety analysis from a phase 3 trial in patients with advanced melanoma. Cancer. 2013;119(9):1675-1682.
7. Oble DA, Mino-Kenudson M, Goldsmith J, et al. Alpha-CTLA-4 mAb-associated panenteritis: a histologic and immunohistochemical analysis. Am J Surg Pathol. 2008;32(8):1130-1137.
8. Beck KE, Blansfield JA, Tran KQ, et al. Enterocolitis in patients with cancer after antibody blockade of cytotoxic T-lymphocyte-associated antigen 4. J Clin Oncol. 2006;24(15):2283-2289.
9. Del Castillo M, Romero FA, Arguello E, Kyi C, Postow MA, Redelman-Sidi G. The spectrum of serious infections among patients receiving immune checkpoint blockade for the treatment of melanoma. Clin Infect Dis. 2016;63(11):1490-1493.
10. Pillet S, Pozzetto B, Roblin X. Cytomegalovirus and ulcerative colitis: place of antiviral therapy. World J Gastroenterol. 2016;22(6):2030-2045.
11. Bate SL, Dollard SC, Cannon MJ. Cytomegalovirus seroprevalence in the United States: the national health and nutrition examination surveys, 1988-2004. Clin Infect Dis. 2010;50(11):1439-1447.
12. Uslu U, Agaimy A, Hundorfean G, Harrer T, Schuler G, Heinzerling L. autoimmune colitis and subsequent CMV-induced hepatitis after treatment with ipilimumab. J Immunother. 2015;38(5):212-215.
13. Lankes K, Hundorfean G, Harrer T, et al. Anti-TNF-refractory colitis after checkpoint inhibitor therapy: possible role of CMV-mediated immunopathogenesis. Oncoimmunology. 2016;5(6):e1128611.
14. Ko JH, Peck KR, Lee WJ, et al. Clinical presentation and risk factors for cytomegalovirus colitis in immunocompetent adult patients. Clin Infect Dis. 2015;60(6):e20-26.
15. You DM, Johnson MD. Cytomegalovirus infection and the gastrointestinal tract. Curr Gastroenterol Rep. 2012;14(4):334-342.
Immune checkpoint inhibitors, including anti-cytotoxic T-lymphocyte antigen 4 (anti-CTLA4) and anti-programmed cell death protein-1 (anti-PD-1) antibodies, have demonstrated clinical and survival benefits in a variety of malignancies, which has led to an expansion in their role in oncology. In melanoma, the anti-CTLA-4 antibody, ipilimumab, has demonstrated a survival benefit in patients with advanced metastatic melanoma and in patients with resectable disease with lymph node involvement.1,2
Ipilimumab exerts its effect by binding CTLA-4 on conventional and regulatory T cells, thus blocking inhibitory signals on T cells, which leads to an antitumor response.3 The increased immune response counteracts the immune-evading mechanisms of the tumor. With increased use of these agents, immune-related adverse events (irAEs) have become more prevalent. The most common irAEs secondary to ipilimumab are skin rash, colitis/diarrhea, hepatitis, pneumonitis, and various endocrinopathies.4 In a phase 3 trial of adjuvant ipilimumab in patients with resected stage III melanoma, grade 3 or 4 adverse events occurred in 54.1% of participants in the ipilimumab arm, the most common being diarrhea and colitis (9.8% and 6.8%, respectively).2Recognition and management of irAEs has led to the implementation of treatment guidelines.4,5 Management of irAEs includes checkpoint inhibitor discontinuation and reversal of the immune response by institution of immunosuppression with corticosteroids.
Case presentation and summary
A 40-year-old white woman with stage IIIB BRAF V600E-positive melanoma presented with diarrhea refractory to high-dose prednisone (1 mg/kg BID). She had recently undergone wide local excision and sentinel node biopsy and received her inaugural dose of ipilimumab (10 mg/kg).
The patient first presented with loose, watery stools that had begun 8 days after she had received her first dose of adjuvant ipilimumab. She was admitted to the hospital, and intravenous methylprednisolone was initiated along with empiric ciprofloxacin (400 mg, IVPB Q12h) and metronidazole (500 mg, IVPB Q8h) as infectious causes were concurrently ruled out. During this initial admission, the patient’s stool was negative for Clostridium difficile toxin, ova, and parasites, as well as enteric pathogens by culture. After infectious causes were excluded, she was diagnosed with ipilimumab-induced colitis. Antibiotics were discontinued, and the patient ultimately noted improvement in her symptoms. On hospital day 7, she was experiencing only 2 bowel movements a day and was discharged on 80 mg of prednisone twice daily.
After discharge the patient noted persistence of her symptoms. At her follow-up, 9 days after discharge, the patient noted continued symptoms of low-grade diarrhea. She failed a trial of steroid tapering due to exacerbation of her abdominal pain and frequency of diarrhea. Further investigation was negative for C. diff toxin and a computed-tomography scan was consistent with continuing colitis. The patient’s symptoms continued to worsen, with recurrence of grade 3 diarrhea, and she was ultimately readmitted 17 days after her earlier discharge (36 days after her first ipilimumab dosing).
On re-admission, the patient was again given intravenous methylprednisolone and experienced interval improvement in the frequency of diarrhea. A gastroenterology expert was consulted, and the patient underwent a flexible sigmoidoscopy that demonstrated findings of diffuse and severe inflammation and biopsies were obtained (Figure 1). After several days of continued symptoms, the patient received infliximab 5 mg/kg for treatment of her adverse autoimmune reaction. After administration, the patient noted improvement in the frequency and volume of diarrhea, however, her symptoms still persisted.
Biopsy results subsequently revealed findings compatible with ipilimumab-induced colitis, and immunohistochemical staining demonstrated positivity for cytomegalovirus (CMV). Specifically, histologic examination showed lymphoplasmacytic expansion of the lamina propria, some architectural distortion, and increased crypt apoptosis. Scattered cryptitis and crypt abscesses were also noted, as were rare stromal and endothelial cells with characteristic CMV inclusions (Figure 2 and Figure 3).
Serum CMV polymerase chain reaction (PCR) was also positive at 652,000 IU/mL (lower limit of detection 100 IU/mL). Induction dosing of ganciclovir (5 mg/kg IV Q12h) was initiated. The combined treatment with intravenous methylprednisone and ganciclovir led to an improvement in diarrhea frequency and resolution of blood in the stool. She was transitioned to oral prednisone, but it resulted in redevelopment of grade 3 diarrhea. The patient was therefore resumed on and discharged on daily intravenous methylprednisolone.
After discharge, the patient was started on budesonide 9 mg daily. Her serum CMV PCR level reduced and she was transitioned to oral valgancyclovir (900 mg daily) for maintenance. Another unsuccessful attempt was made to switch her to oral prednisone.
About 14 weeks after the initial ipilimumab dosing, the patient underwent another flexible sigmoidoscopy that again demonstrated severe colitis from the rectum to sigmoid colon. Biopsies were negative for CMV. Patient was readmitted for recurrence of diarrhea the following week. Treatment with IV methylprednisone (1mg/kg BID) and infliximab (5 mg/kg) again led to an improvement of symptoms. She was again discharged on IV methylprednisone (1 mg/kg BID) with a taper.
In the 15th week after her initial ipilimumab dose, the patient presented with a perforated bowel, requiring a subtotal colectomy and end ileostomy. She continued on a slow taper of oral prednisone (50 mg daily and decrease by 10 mg every 5 days).
At her last documented follow-up, 8 months after her first ipilimumab dose, she was having normal output from her ileostomy. She developed secondary adrenal insufficiency because of the long-term steroids and continued to take prednisone 5 mg daily.
Discussion
Diarrhea and colitis are common irAEs attributable to checkpoint-inhibitor therapy used for the treatment of melanoma. This case of ipilimumab-induced colitis refractory to high-dose oral steroids demonstrates the risks associated with management of anti-CTLA-4 induced colitis. In particular, the high-dose corticosteroids required to treat the autoimmune component of this patient’s colitis increased her susceptibility to CMV reactivation.
The diagnosis of colitis secondary to ipilimumab is made primarily in the appropriate clinical setting, and typically onsets during the induction period (within 12 weeks of initial dosing) and most resolve within 6-8 weeks.6 Histopathologically, there is lymphoplasmacytic expansion of lamina propria, increased intraepithelial lymphocytes, and increased epithelial apoptosis of crypts. One can also see acute cryptitis and crypt abscesses. Reactive epithelial changes with mucin depletion are also often seen in epithelial cells.
Findings from immunohistochemical studies have shown the increased intraepithelial lymphocytes to be predominantly CD8-positive T cells, while the lamina propria contains an increase in the mixture of CD4- and CD8-positive T cells. In addition, small intestinal samples show villous blunting. There is an absence of significant architectural distortion and well-developed basal lymphoplasmacytic infiltrates characteristic of chronic mucosal injury, such as idiopathic inflammatory bowel disease.7 Granulomas are also absent in most series, though they have been reported in some cases.8 The features are similar to those seen in autoimmune enteropathy, but goblet and endocrine cells remain preserved. Graft-versus-host disease has similar histologic features, however, the clinical setting usually makes the distinction between these obvious.
Current treatment algorithms for ipilimumab-related diarrhea, begin with immediate treatment with intravenous methylprednisolone (125 mg once). This is followed with oral prednisone at a dose of 1-2 mg/kg tapered over 4 to 8 weeks.4 In patients with persistent symptoms despite adequate doses of corticosteroids, infliximab (5 mg/kg every 2 weeks) is recommended until the resolution of symptoms, and a longer taper of prednisone is often necessary.
Institution of high-dose corticosteroids to treat grade 3 or 4 irAEs can increase the risk for infection, including opportunistic infections. One retrospective review of patients administered checkpoint inhibitors at a single institution revealed that 7.3% of 740 patients developed a severe infection that lead to hospitalization or treatment with intravenous antibiotics.9 In that patient cohort, only 0.6% had a serious infection secondary to a viral etiology, and 1 patient developed CMV enterocolitis. Most patients who developed an infection in this cohort had received corticosteroids (46/54 patients, 85%) and/or infliximab (13/54 patients, 24%).9
CMV is a member of the Herpesviridae family. After a primary infection, which can often go unrecognized in an immunocompetent host, CMV can persist in a latent state.10 In a study by Bate and colleagues, the age-adjusted seropositivity of CMV was found to be 50.4%.11 Based on those results, immunosuppression in a patient who has previously been infected with CMV can lead to a risk of reactivation or even reinfection. In the era of checkpoint-inhibitor therapy, reactivation of CMV has been described previously in a case of CMV hepatitis and a report of CMV colitis.12,13 Immunosuppression, such as that caused by corticosteroids, is a risk factor for CMV infection.14 Colitis caused by CMV usually presents with abdominal pain, diarrhea, and bloody diarrhea.15 In suspected cases of CMV colitis, endoscopy should be pursued with biopsy for tissue examination. A tissue diagnosis is required for CMV colitis because serum PCR can be negative in isolated cases of gastrointestinal CMV infection.15
Conclusion
Despite appropriate treatment with ganciclovir and the noted response in the patient’s serum CMV PCR, symptom exacerbation was observed with the transition to oral prednisone. The requirement for intravenous corticosteroids in the present case demonstrates the prolonged effects exerted by irAEs secondary to checkpoint-inhibitor therapy. Those effects are attributable to the design of the antibody – ipilimumab is a fully humanized monoclonal antibody and has a plasma half-life of about 15 days.1,4
By the identification of CMV histopathologically, this case, along with the case presented by Lankes and colleagues,13 illustrates the importance of considering CMV colitis in patients who are being treated with ipilimumab and who develop persistent or worsening diarrhea after initial treatment with high-dose steroids.
Early recognition of possible coexistent CMV colitis in patients with a history of treatment with ipilimumab can have important clinical consequences. It can lead to quicker implementation of proper antiviral therapy and minimization of immune suppression to levels required to maintain control of the patient’s symptoms.
Immune checkpoint inhibitors, including anti-cytotoxic T-lymphocyte antigen 4 (anti-CTLA4) and anti-programmed cell death protein-1 (anti-PD-1) antibodies, have demonstrated clinical and survival benefits in a variety of malignancies, which has led to an expansion in their role in oncology. In melanoma, the anti-CTLA-4 antibody, ipilimumab, has demonstrated a survival benefit in patients with advanced metastatic melanoma and in patients with resectable disease with lymph node involvement.1,2
Ipilimumab exerts its effect by binding CTLA-4 on conventional and regulatory T cells, thus blocking inhibitory signals on T cells, which leads to an antitumor response.3 The increased immune response counteracts the immune-evading mechanisms of the tumor. With increased use of these agents, immune-related adverse events (irAEs) have become more prevalent. The most common irAEs secondary to ipilimumab are skin rash, colitis/diarrhea, hepatitis, pneumonitis, and various endocrinopathies.4 In a phase 3 trial of adjuvant ipilimumab in patients with resected stage III melanoma, grade 3 or 4 adverse events occurred in 54.1% of participants in the ipilimumab arm, the most common being diarrhea and colitis (9.8% and 6.8%, respectively).2Recognition and management of irAEs has led to the implementation of treatment guidelines.4,5 Management of irAEs includes checkpoint inhibitor discontinuation and reversal of the immune response by institution of immunosuppression with corticosteroids.
Case presentation and summary
A 40-year-old white woman with stage IIIB BRAF V600E-positive melanoma presented with diarrhea refractory to high-dose prednisone (1 mg/kg BID). She had recently undergone wide local excision and sentinel node biopsy and received her inaugural dose of ipilimumab (10 mg/kg).
The patient first presented with loose, watery stools that had begun 8 days after she had received her first dose of adjuvant ipilimumab. She was admitted to the hospital, and intravenous methylprednisolone was initiated along with empiric ciprofloxacin (400 mg, IVPB Q12h) and metronidazole (500 mg, IVPB Q8h) as infectious causes were concurrently ruled out. During this initial admission, the patient’s stool was negative for Clostridium difficile toxin, ova, and parasites, as well as enteric pathogens by culture. After infectious causes were excluded, she was diagnosed with ipilimumab-induced colitis. Antibiotics were discontinued, and the patient ultimately noted improvement in her symptoms. On hospital day 7, she was experiencing only 2 bowel movements a day and was discharged on 80 mg of prednisone twice daily.
After discharge the patient noted persistence of her symptoms. At her follow-up, 9 days after discharge, the patient noted continued symptoms of low-grade diarrhea. She failed a trial of steroid tapering due to exacerbation of her abdominal pain and frequency of diarrhea. Further investigation was negative for C. diff toxin and a computed-tomography scan was consistent with continuing colitis. The patient’s symptoms continued to worsen, with recurrence of grade 3 diarrhea, and she was ultimately readmitted 17 days after her earlier discharge (36 days after her first ipilimumab dosing).
On re-admission, the patient was again given intravenous methylprednisolone and experienced interval improvement in the frequency of diarrhea. A gastroenterology expert was consulted, and the patient underwent a flexible sigmoidoscopy that demonstrated findings of diffuse and severe inflammation and biopsies were obtained (Figure 1). After several days of continued symptoms, the patient received infliximab 5 mg/kg for treatment of her adverse autoimmune reaction. After administration, the patient noted improvement in the frequency and volume of diarrhea, however, her symptoms still persisted.
Biopsy results subsequently revealed findings compatible with ipilimumab-induced colitis, and immunohistochemical staining demonstrated positivity for cytomegalovirus (CMV). Specifically, histologic examination showed lymphoplasmacytic expansion of the lamina propria, some architectural distortion, and increased crypt apoptosis. Scattered cryptitis and crypt abscesses were also noted, as were rare stromal and endothelial cells with characteristic CMV inclusions (Figure 2 and Figure 3).
Serum CMV polymerase chain reaction (PCR) was also positive at 652,000 IU/mL (lower limit of detection 100 IU/mL). Induction dosing of ganciclovir (5 mg/kg IV Q12h) was initiated. The combined treatment with intravenous methylprednisone and ganciclovir led to an improvement in diarrhea frequency and resolution of blood in the stool. She was transitioned to oral prednisone, but it resulted in redevelopment of grade 3 diarrhea. The patient was therefore resumed on and discharged on daily intravenous methylprednisolone.
After discharge, the patient was started on budesonide 9 mg daily. Her serum CMV PCR level reduced and she was transitioned to oral valgancyclovir (900 mg daily) for maintenance. Another unsuccessful attempt was made to switch her to oral prednisone.
About 14 weeks after the initial ipilimumab dosing, the patient underwent another flexible sigmoidoscopy that again demonstrated severe colitis from the rectum to sigmoid colon. Biopsies were negative for CMV. Patient was readmitted for recurrence of diarrhea the following week. Treatment with IV methylprednisone (1mg/kg BID) and infliximab (5 mg/kg) again led to an improvement of symptoms. She was again discharged on IV methylprednisone (1 mg/kg BID) with a taper.
In the 15th week after her initial ipilimumab dose, the patient presented with a perforated bowel, requiring a subtotal colectomy and end ileostomy. She continued on a slow taper of oral prednisone (50 mg daily and decrease by 10 mg every 5 days).
At her last documented follow-up, 8 months after her first ipilimumab dose, she was having normal output from her ileostomy. She developed secondary adrenal insufficiency because of the long-term steroids and continued to take prednisone 5 mg daily.
Discussion
Diarrhea and colitis are common irAEs attributable to checkpoint-inhibitor therapy used for the treatment of melanoma. This case of ipilimumab-induced colitis refractory to high-dose oral steroids demonstrates the risks associated with management of anti-CTLA-4 induced colitis. In particular, the high-dose corticosteroids required to treat the autoimmune component of this patient’s colitis increased her susceptibility to CMV reactivation.
The diagnosis of colitis secondary to ipilimumab is made primarily in the appropriate clinical setting, and typically onsets during the induction period (within 12 weeks of initial dosing) and most resolve within 6-8 weeks.6 Histopathologically, there is lymphoplasmacytic expansion of lamina propria, increased intraepithelial lymphocytes, and increased epithelial apoptosis of crypts. One can also see acute cryptitis and crypt abscesses. Reactive epithelial changes with mucin depletion are also often seen in epithelial cells.
Findings from immunohistochemical studies have shown the increased intraepithelial lymphocytes to be predominantly CD8-positive T cells, while the lamina propria contains an increase in the mixture of CD4- and CD8-positive T cells. In addition, small intestinal samples show villous blunting. There is an absence of significant architectural distortion and well-developed basal lymphoplasmacytic infiltrates characteristic of chronic mucosal injury, such as idiopathic inflammatory bowel disease.7 Granulomas are also absent in most series, though they have been reported in some cases.8 The features are similar to those seen in autoimmune enteropathy, but goblet and endocrine cells remain preserved. Graft-versus-host disease has similar histologic features, however, the clinical setting usually makes the distinction between these obvious.
Current treatment algorithms for ipilimumab-related diarrhea, begin with immediate treatment with intravenous methylprednisolone (125 mg once). This is followed with oral prednisone at a dose of 1-2 mg/kg tapered over 4 to 8 weeks.4 In patients with persistent symptoms despite adequate doses of corticosteroids, infliximab (5 mg/kg every 2 weeks) is recommended until the resolution of symptoms, and a longer taper of prednisone is often necessary.
Institution of high-dose corticosteroids to treat grade 3 or 4 irAEs can increase the risk for infection, including opportunistic infections. One retrospective review of patients administered checkpoint inhibitors at a single institution revealed that 7.3% of 740 patients developed a severe infection that lead to hospitalization or treatment with intravenous antibiotics.9 In that patient cohort, only 0.6% had a serious infection secondary to a viral etiology, and 1 patient developed CMV enterocolitis. Most patients who developed an infection in this cohort had received corticosteroids (46/54 patients, 85%) and/or infliximab (13/54 patients, 24%).9
CMV is a member of the Herpesviridae family. After a primary infection, which can often go unrecognized in an immunocompetent host, CMV can persist in a latent state.10 In a study by Bate and colleagues, the age-adjusted seropositivity of CMV was found to be 50.4%.11 Based on those results, immunosuppression in a patient who has previously been infected with CMV can lead to a risk of reactivation or even reinfection. In the era of checkpoint-inhibitor therapy, reactivation of CMV has been described previously in a case of CMV hepatitis and a report of CMV colitis.12,13 Immunosuppression, such as that caused by corticosteroids, is a risk factor for CMV infection.14 Colitis caused by CMV usually presents with abdominal pain, diarrhea, and bloody diarrhea.15 In suspected cases of CMV colitis, endoscopy should be pursued with biopsy for tissue examination. A tissue diagnosis is required for CMV colitis because serum PCR can be negative in isolated cases of gastrointestinal CMV infection.15
Conclusion
Despite appropriate treatment with ganciclovir and the noted response in the patient’s serum CMV PCR, symptom exacerbation was observed with the transition to oral prednisone. The requirement for intravenous corticosteroids in the present case demonstrates the prolonged effects exerted by irAEs secondary to checkpoint-inhibitor therapy. Those effects are attributable to the design of the antibody – ipilimumab is a fully humanized monoclonal antibody and has a plasma half-life of about 15 days.1,4
By the identification of CMV histopathologically, this case, along with the case presented by Lankes and colleagues,13 illustrates the importance of considering CMV colitis in patients who are being treated with ipilimumab and who develop persistent or worsening diarrhea after initial treatment with high-dose steroids.
Early recognition of possible coexistent CMV colitis in patients with a history of treatment with ipilimumab can have important clinical consequences. It can lead to quicker implementation of proper antiviral therapy and minimization of immune suppression to levels required to maintain control of the patient’s symptoms.
1. Hodi FS, O’Day SJ, McDermott DF, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med. 2010;363(8):711-723.
2. Eggermont AM, Chiarion-Sileni V, Grob JJ, et al. Prolonged survival in stage III melanoma with ipilimumab adjuvant therapy. N Engl J Med. 2016;375(19):1845-1855.
3. Glassman PM, Balthasar JP. Mechanistic considerations for the use of monoclonal antibodies for cancer therapy. Cancer Biol Med. 2014;11(1):20-33.
4. Weber JS, Kahler KC, Hauschild A. Management of immune-related adverse events and kinetics of response with ipilimumab. J Clin Oncol. 2012;30(21):2691-2697.
5. Fecher LA, Agarwala SS, Hodi FS, Weber JS. Ipilimumab and its toxicities: a multidisciplinary approach. Oncologist. 2013;18(6):733-743.
6. Weber JS, Dummer R, de Pril V, Lebbe C, Hodi FS, Investigators MDX. Patterns of onset and resolution of immune-related adverse events of special interest with ipilimumab: detailed safety analysis from a phase 3 trial in patients with advanced melanoma. Cancer. 2013;119(9):1675-1682.
7. Oble DA, Mino-Kenudson M, Goldsmith J, et al. Alpha-CTLA-4 mAb-associated panenteritis: a histologic and immunohistochemical analysis. Am J Surg Pathol. 2008;32(8):1130-1137.
8. Beck KE, Blansfield JA, Tran KQ, et al. Enterocolitis in patients with cancer after antibody blockade of cytotoxic T-lymphocyte-associated antigen 4. J Clin Oncol. 2006;24(15):2283-2289.
9. Del Castillo M, Romero FA, Arguello E, Kyi C, Postow MA, Redelman-Sidi G. The spectrum of serious infections among patients receiving immune checkpoint blockade for the treatment of melanoma. Clin Infect Dis. 2016;63(11):1490-1493.
10. Pillet S, Pozzetto B, Roblin X. Cytomegalovirus and ulcerative colitis: place of antiviral therapy. World J Gastroenterol. 2016;22(6):2030-2045.
11. Bate SL, Dollard SC, Cannon MJ. Cytomegalovirus seroprevalence in the United States: the national health and nutrition examination surveys, 1988-2004. Clin Infect Dis. 2010;50(11):1439-1447.
12. Uslu U, Agaimy A, Hundorfean G, Harrer T, Schuler G, Heinzerling L. autoimmune colitis and subsequent CMV-induced hepatitis after treatment with ipilimumab. J Immunother. 2015;38(5):212-215.
13. Lankes K, Hundorfean G, Harrer T, et al. Anti-TNF-refractory colitis after checkpoint inhibitor therapy: possible role of CMV-mediated immunopathogenesis. Oncoimmunology. 2016;5(6):e1128611.
14. Ko JH, Peck KR, Lee WJ, et al. Clinical presentation and risk factors for cytomegalovirus colitis in immunocompetent adult patients. Clin Infect Dis. 2015;60(6):e20-26.
15. You DM, Johnson MD. Cytomegalovirus infection and the gastrointestinal tract. Curr Gastroenterol Rep. 2012;14(4):334-342.
1. Hodi FS, O’Day SJ, McDermott DF, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med. 2010;363(8):711-723.
2. Eggermont AM, Chiarion-Sileni V, Grob JJ, et al. Prolonged survival in stage III melanoma with ipilimumab adjuvant therapy. N Engl J Med. 2016;375(19):1845-1855.
3. Glassman PM, Balthasar JP. Mechanistic considerations for the use of monoclonal antibodies for cancer therapy. Cancer Biol Med. 2014;11(1):20-33.
4. Weber JS, Kahler KC, Hauschild A. Management of immune-related adverse events and kinetics of response with ipilimumab. J Clin Oncol. 2012;30(21):2691-2697.
5. Fecher LA, Agarwala SS, Hodi FS, Weber JS. Ipilimumab and its toxicities: a multidisciplinary approach. Oncologist. 2013;18(6):733-743.
6. Weber JS, Dummer R, de Pril V, Lebbe C, Hodi FS, Investigators MDX. Patterns of onset and resolution of immune-related adverse events of special interest with ipilimumab: detailed safety analysis from a phase 3 trial in patients with advanced melanoma. Cancer. 2013;119(9):1675-1682.
7. Oble DA, Mino-Kenudson M, Goldsmith J, et al. Alpha-CTLA-4 mAb-associated panenteritis: a histologic and immunohistochemical analysis. Am J Surg Pathol. 2008;32(8):1130-1137.
8. Beck KE, Blansfield JA, Tran KQ, et al. Enterocolitis in patients with cancer after antibody blockade of cytotoxic T-lymphocyte-associated antigen 4. J Clin Oncol. 2006;24(15):2283-2289.
9. Del Castillo M, Romero FA, Arguello E, Kyi C, Postow MA, Redelman-Sidi G. The spectrum of serious infections among patients receiving immune checkpoint blockade for the treatment of melanoma. Clin Infect Dis. 2016;63(11):1490-1493.
10. Pillet S, Pozzetto B, Roblin X. Cytomegalovirus and ulcerative colitis: place of antiviral therapy. World J Gastroenterol. 2016;22(6):2030-2045.
11. Bate SL, Dollard SC, Cannon MJ. Cytomegalovirus seroprevalence in the United States: the national health and nutrition examination surveys, 1988-2004. Clin Infect Dis. 2010;50(11):1439-1447.
12. Uslu U, Agaimy A, Hundorfean G, Harrer T, Schuler G, Heinzerling L. autoimmune colitis and subsequent CMV-induced hepatitis after treatment with ipilimumab. J Immunother. 2015;38(5):212-215.
13. Lankes K, Hundorfean G, Harrer T, et al. Anti-TNF-refractory colitis after checkpoint inhibitor therapy: possible role of CMV-mediated immunopathogenesis. Oncoimmunology. 2016;5(6):e1128611.
14. Ko JH, Peck KR, Lee WJ, et al. Clinical presentation and risk factors for cytomegalovirus colitis in immunocompetent adult patients. Clin Infect Dis. 2015;60(6):e20-26.
15. You DM, Johnson MD. Cytomegalovirus infection and the gastrointestinal tract. Curr Gastroenterol Rep. 2012;14(4):334-342.
Recurrent head and neck cancer presenting as a large retroperitoneal mass
Worldwide, head and neck cancers account for more than half a million cases annually and nearly 400,000 deaths.1 Although the exact incidence of metastatic disease of these primarily squamous cell tumors is difficult to determine, the incidence is thought to be much lower than that of other solid tumors.2 When the different sites of metastatic disease of these tumors have been studied previously, the most common have been (in descending order of frequency) the lungs, bones, liver, skin, mediastinum, and bone marrow.2,3 It is extremely rare area for head and neck squamous cell cancers to metastasize to the retroperitoneum. To our knowledge, only 2 other such cases have been reported in the literature.4,5 In those two cases, the metastatic recurrence occurred at 6 and 13 months after definitive treatment of the primary cancer.
Case presentation and summary
The patient in this case is a 60-year-old man with a history of stage IV moderately differentiated invasive squamous cell carcinoma (p16 negative, Bcl-2 negative, EGFR positive) of the hypopharynx that had been initially diagnosed in 2012. At that time, he underwent a total laryngectomy, partial pharyngectomy, and total thyroidectomy. A 2-centimeter mediastinal mass was also identified on a computed-tomography scan of the thorax and resected during the initial curative surgery. Final surgical pathology on the primary hypopharygeal tumor revealed a 4.1-cm moderately differentiated squamous cell carcinoma with negative margins, but positive lymphovascular invasion (Figure 1). The 2-cm mediastinal mass also revealed the same squamous cell carcinoma as the hypopharyngeal primary. Final surgical margins were negative.
The patient went on to receive adjuvant treatment in the form of concurrent chemoradiation with cisplatin (100 mg/m2 every 21 days for 3 doses, with 70 Gy of radiation]. After his initial treatment, he was followed closely by a multidisciplinary team, including otolaryngology, radiation oncology, and medical oncology specialists. He underwent a positron-emission tomography–CT scan 1 year after the conclusion of adjuvant therapy that showed no evidence of local or distant disease. The patient underwent 12 fiberoptic pharyngoscopy procedures over the course of 4 years without any evidence of local disease recurrence. He underwent a CT scan of the neck in October of 2016 without any evidence of local disease recurrence.
In early 2017, the patient presented with fatigue, abdominal pain, and back pain during the previous month. CT imaging revealed a left retroperitoneal mass of 8.8 x 4.0 x 6.6 cm, with bony destruction of L3-L4 causing left hydronephrosis (Figure 2 and Figure 3). Other staging work-up and imaging did not reveal any other distant disease or locoregional disease recurrence in the head and neck. Lab work was significant for an acute kidney injury that was likely secondary to mass effect from the tumor.
The mass was biopsied, with pathology revealing squamous cell carcinoma consistent with metastatic, recurrent disease from the previously known head and neck primary, and it was also p16 negative, Bcl-2 negative, and EGFR positive (Figure 4).
After a multidisciplinary discussion it was determined that the best front-line treatment option would be to treat with definitive concurrent chemoradiation. However, due to the size and location of the mass, it was not possible to deliver an effective therapeutic dose of radiation without unacceptable toxicity to the adjacent structures. Therefore, palliative systemic therapy was the only option. These treatment options, including systemic chemotherapy and immunotherapy, were discussed with the patient. However, he did not want to pursue any further cancer treatment and wanted instead to focus on palliation (pain control, antiemetics and nephrostomy to relieve obstruction) and hospice. He passed away 3 months later.
Discussion
Masses of the retroperitoneum have a wide differential diagnosis.6 Primary malignancies including lymphomas, sarcomas, neurogenic tumors, and germ cell tumors may all present primarily as retroperitoneal masses.6,7 Nonmalignant processes such as retroperitoneal fibrosis may also present in this manner.7 Certain tumors are known to metastasize to the retroperitoneum, namely carcinomas of the gastrointestinal tract and ovary as well as lung cancer or melanoma.5,8 Some primary retroperitoneal masses in women have been described in the literature as being HPV-associated squamous cell cancers of unknown primaries.9
When head and neck cancers metastasize they typically metastasize to the lungs, bone, liver, mediastinum, skin, and bone marrow. Most metastasis is pulmonary in origin, with the literature indicating it accounts for 52%-66% of head and neck cancer metastases, with bone metastases next in frequency at 12%-22%.2,3,10 In general, the incidence of distant metastatic disease in head and neck cancers is not as common as its other solid tumor counterparts, and even metastasis to other lymph node groups other than locoregional cervical nodes is rare.11 Furthermore, late metastasis occurring more than 2 years after definitive treatment is also an infrequent occurrence.12
When discussing distant metastatic disease in head and neck cancer, previous literature has described an increasing likelihood of distant metastases when there is locoregional disease recurrence.13 Moreover, the retroperitoneum is an exceedingly rare site of distant metastatic disease for head and neck cancer. There have been only 2 previous cases that have described this phenomenon, and in both cases the metastases occurred within or close to 1 year of definitive locoregional treatment.4,5
Conclusion
We present our case to present an exceedingly rare case of distant metastatic, recurrent disease from head and neck cancer to the retroperitoneum (without locoregional recurrence) that occurred 4 years after definitive treatment. We believe this to be the first case of its kind to be described when taking into consideration the site of metastases, when the metastatic recurrence occurred and that it happened without loco-regional disease recurrence. This case highlights the importance of keeping a wide differential diagnosis when encountering a retroperitoneal mass in a patient with even a remote history of head and neck cancer.
Acknowledgments
The authors thank the following members of the Department of Pathology at the University of Texas Medical Branch: Asad Ahmad, MD; Eduardo Eyzaguirre, MD; Timothy C Allen, MD, JD, FACP; and Suimmin Qiu, MD, PHD.
1. Fitzmaurice C, Allen C, Barber RM, et al. Global, regional, and national cancer incidence, mortality, years of life lost, years lived with disability, and disability-adjusted life-years for 32 cancer groups, 1990 to 2015: a systematic analysis for the Global Burden of Disease Study. JAMA Oncol. 2017;3:524-548.
2. Ferlito A, Shaha AR, Silver CE, Rinaldo A, Mondin V. Incidence and sites of distant metastases from head and neck cancer. ORL J Otorhinolaryngol Relat Spec. 2001;63:202-207.
3. Wiegand S, Zimmermann A, Wilhelm T, Werner JA. Survival after distant metastasis in head and neck cancer. Anticancer Res. 2015;35:5499-5502.
4. Hofmann U, O’Connor JP, Biyani CS, Harnden P, Selby P, Weston PM. Retroperitoneal metastatic squamous cell carcinoma of the tonsil (with elevated beta human chorionic gonadotrophin): a misdiagnosis as extra-gonadal germ cell tumour. J Laryngol Otol. 2006;120:885-887.
5. Purkayastha A, Sharma N, Suhag V. Extremely rare and unusual case of retroperitoneal and pelvic metastasis from squamous cell carcinoma of vallecula. Int J Cancer Ther Oncol. 2016;4(2):1-4.
6. Rajiah P, Sinha R, Cuevas C, Dubinsky TJ, Bush WH, Kolokythas O. Imaging of uncommon retroperitoneal masses. Radiographics 2011;31:949-976.
7. Scali EP, Chandler TM, Heffernan EJ, Coyle J, Harris AC, Chang SD. Primary retroperitoneal masses: what is the differential diagnosis? Abdom Imaging. 2015;40:1887-1903.
8. Levy AD, Shaw JC, Sobin LH. Secondary tumors and tumorlike lesions of the peritoneal cavity: imaging features with pathologic correlation. Radiographics. 2009;29:347-373.
9. Isbell A, Fields EC. Three cases of women with HPV-related squamous cell carcinoma of unknown primary in the pelvis and retroperitoneum: a case series. Gynecol Oncol Rep. 2016;16:5-8.
10. León X, Quer M, Orús C, del Prado Venegas M, López M. Distant metastases in head and neck cancer patients who achieved loco-regional control. Head Neck. 2000;22:680-686.
11. Alavi S, Namazie A, Sercarz JA, Wang MB, Blackwell KE. Distant lymphatic metastasis from head and neck cancer. Ann Otol Rhinol Laryngol. 1999;108:860-863.
12. Krishnatry R, Gupta T, Murthy V, et al. Factors predicting ‘time to distant metastasis’ in radically treated head and neck cancer. Indian J Cancer. 2014;51:231-235.
13. Goodwin WJ. Distant metastases from oropharyngeal cancer. ORL J Otorhinolaryngol Relat Spec. 2001;63:222-223.
Worldwide, head and neck cancers account for more than half a million cases annually and nearly 400,000 deaths.1 Although the exact incidence of metastatic disease of these primarily squamous cell tumors is difficult to determine, the incidence is thought to be much lower than that of other solid tumors.2 When the different sites of metastatic disease of these tumors have been studied previously, the most common have been (in descending order of frequency) the lungs, bones, liver, skin, mediastinum, and bone marrow.2,3 It is extremely rare area for head and neck squamous cell cancers to metastasize to the retroperitoneum. To our knowledge, only 2 other such cases have been reported in the literature.4,5 In those two cases, the metastatic recurrence occurred at 6 and 13 months after definitive treatment of the primary cancer.
Case presentation and summary
The patient in this case is a 60-year-old man with a history of stage IV moderately differentiated invasive squamous cell carcinoma (p16 negative, Bcl-2 negative, EGFR positive) of the hypopharynx that had been initially diagnosed in 2012. At that time, he underwent a total laryngectomy, partial pharyngectomy, and total thyroidectomy. A 2-centimeter mediastinal mass was also identified on a computed-tomography scan of the thorax and resected during the initial curative surgery. Final surgical pathology on the primary hypopharygeal tumor revealed a 4.1-cm moderately differentiated squamous cell carcinoma with negative margins, but positive lymphovascular invasion (Figure 1). The 2-cm mediastinal mass also revealed the same squamous cell carcinoma as the hypopharyngeal primary. Final surgical margins were negative.
The patient went on to receive adjuvant treatment in the form of concurrent chemoradiation with cisplatin (100 mg/m2 every 21 days for 3 doses, with 70 Gy of radiation]. After his initial treatment, he was followed closely by a multidisciplinary team, including otolaryngology, radiation oncology, and medical oncology specialists. He underwent a positron-emission tomography–CT scan 1 year after the conclusion of adjuvant therapy that showed no evidence of local or distant disease. The patient underwent 12 fiberoptic pharyngoscopy procedures over the course of 4 years without any evidence of local disease recurrence. He underwent a CT scan of the neck in October of 2016 without any evidence of local disease recurrence.
In early 2017, the patient presented with fatigue, abdominal pain, and back pain during the previous month. CT imaging revealed a left retroperitoneal mass of 8.8 x 4.0 x 6.6 cm, with bony destruction of L3-L4 causing left hydronephrosis (Figure 2 and Figure 3). Other staging work-up and imaging did not reveal any other distant disease or locoregional disease recurrence in the head and neck. Lab work was significant for an acute kidney injury that was likely secondary to mass effect from the tumor.
The mass was biopsied, with pathology revealing squamous cell carcinoma consistent with metastatic, recurrent disease from the previously known head and neck primary, and it was also p16 negative, Bcl-2 negative, and EGFR positive (Figure 4).
After a multidisciplinary discussion it was determined that the best front-line treatment option would be to treat with definitive concurrent chemoradiation. However, due to the size and location of the mass, it was not possible to deliver an effective therapeutic dose of radiation without unacceptable toxicity to the adjacent structures. Therefore, palliative systemic therapy was the only option. These treatment options, including systemic chemotherapy and immunotherapy, were discussed with the patient. However, he did not want to pursue any further cancer treatment and wanted instead to focus on palliation (pain control, antiemetics and nephrostomy to relieve obstruction) and hospice. He passed away 3 months later.
Discussion
Masses of the retroperitoneum have a wide differential diagnosis.6 Primary malignancies including lymphomas, sarcomas, neurogenic tumors, and germ cell tumors may all present primarily as retroperitoneal masses.6,7 Nonmalignant processes such as retroperitoneal fibrosis may also present in this manner.7 Certain tumors are known to metastasize to the retroperitoneum, namely carcinomas of the gastrointestinal tract and ovary as well as lung cancer or melanoma.5,8 Some primary retroperitoneal masses in women have been described in the literature as being HPV-associated squamous cell cancers of unknown primaries.9
When head and neck cancers metastasize they typically metastasize to the lungs, bone, liver, mediastinum, skin, and bone marrow. Most metastasis is pulmonary in origin, with the literature indicating it accounts for 52%-66% of head and neck cancer metastases, with bone metastases next in frequency at 12%-22%.2,3,10 In general, the incidence of distant metastatic disease in head and neck cancers is not as common as its other solid tumor counterparts, and even metastasis to other lymph node groups other than locoregional cervical nodes is rare.11 Furthermore, late metastasis occurring more than 2 years after definitive treatment is also an infrequent occurrence.12
When discussing distant metastatic disease in head and neck cancer, previous literature has described an increasing likelihood of distant metastases when there is locoregional disease recurrence.13 Moreover, the retroperitoneum is an exceedingly rare site of distant metastatic disease for head and neck cancer. There have been only 2 previous cases that have described this phenomenon, and in both cases the metastases occurred within or close to 1 year of definitive locoregional treatment.4,5
Conclusion
We present our case to present an exceedingly rare case of distant metastatic, recurrent disease from head and neck cancer to the retroperitoneum (without locoregional recurrence) that occurred 4 years after definitive treatment. We believe this to be the first case of its kind to be described when taking into consideration the site of metastases, when the metastatic recurrence occurred and that it happened without loco-regional disease recurrence. This case highlights the importance of keeping a wide differential diagnosis when encountering a retroperitoneal mass in a patient with even a remote history of head and neck cancer.
Acknowledgments
The authors thank the following members of the Department of Pathology at the University of Texas Medical Branch: Asad Ahmad, MD; Eduardo Eyzaguirre, MD; Timothy C Allen, MD, JD, FACP; and Suimmin Qiu, MD, PHD.
Worldwide, head and neck cancers account for more than half a million cases annually and nearly 400,000 deaths.1 Although the exact incidence of metastatic disease of these primarily squamous cell tumors is difficult to determine, the incidence is thought to be much lower than that of other solid tumors.2 When the different sites of metastatic disease of these tumors have been studied previously, the most common have been (in descending order of frequency) the lungs, bones, liver, skin, mediastinum, and bone marrow.2,3 It is extremely rare area for head and neck squamous cell cancers to metastasize to the retroperitoneum. To our knowledge, only 2 other such cases have been reported in the literature.4,5 In those two cases, the metastatic recurrence occurred at 6 and 13 months after definitive treatment of the primary cancer.
Case presentation and summary
The patient in this case is a 60-year-old man with a history of stage IV moderately differentiated invasive squamous cell carcinoma (p16 negative, Bcl-2 negative, EGFR positive) of the hypopharynx that had been initially diagnosed in 2012. At that time, he underwent a total laryngectomy, partial pharyngectomy, and total thyroidectomy. A 2-centimeter mediastinal mass was also identified on a computed-tomography scan of the thorax and resected during the initial curative surgery. Final surgical pathology on the primary hypopharygeal tumor revealed a 4.1-cm moderately differentiated squamous cell carcinoma with negative margins, but positive lymphovascular invasion (Figure 1). The 2-cm mediastinal mass also revealed the same squamous cell carcinoma as the hypopharyngeal primary. Final surgical margins were negative.
The patient went on to receive adjuvant treatment in the form of concurrent chemoradiation with cisplatin (100 mg/m2 every 21 days for 3 doses, with 70 Gy of radiation]. After his initial treatment, he was followed closely by a multidisciplinary team, including otolaryngology, radiation oncology, and medical oncology specialists. He underwent a positron-emission tomography–CT scan 1 year after the conclusion of adjuvant therapy that showed no evidence of local or distant disease. The patient underwent 12 fiberoptic pharyngoscopy procedures over the course of 4 years without any evidence of local disease recurrence. He underwent a CT scan of the neck in October of 2016 without any evidence of local disease recurrence.
In early 2017, the patient presented with fatigue, abdominal pain, and back pain during the previous month. CT imaging revealed a left retroperitoneal mass of 8.8 x 4.0 x 6.6 cm, with bony destruction of L3-L4 causing left hydronephrosis (Figure 2 and Figure 3). Other staging work-up and imaging did not reveal any other distant disease or locoregional disease recurrence in the head and neck. Lab work was significant for an acute kidney injury that was likely secondary to mass effect from the tumor.
The mass was biopsied, with pathology revealing squamous cell carcinoma consistent with metastatic, recurrent disease from the previously known head and neck primary, and it was also p16 negative, Bcl-2 negative, and EGFR positive (Figure 4).
After a multidisciplinary discussion it was determined that the best front-line treatment option would be to treat with definitive concurrent chemoradiation. However, due to the size and location of the mass, it was not possible to deliver an effective therapeutic dose of radiation without unacceptable toxicity to the adjacent structures. Therefore, palliative systemic therapy was the only option. These treatment options, including systemic chemotherapy and immunotherapy, were discussed with the patient. However, he did not want to pursue any further cancer treatment and wanted instead to focus on palliation (pain control, antiemetics and nephrostomy to relieve obstruction) and hospice. He passed away 3 months later.
Discussion
Masses of the retroperitoneum have a wide differential diagnosis.6 Primary malignancies including lymphomas, sarcomas, neurogenic tumors, and germ cell tumors may all present primarily as retroperitoneal masses.6,7 Nonmalignant processes such as retroperitoneal fibrosis may also present in this manner.7 Certain tumors are known to metastasize to the retroperitoneum, namely carcinomas of the gastrointestinal tract and ovary as well as lung cancer or melanoma.5,8 Some primary retroperitoneal masses in women have been described in the literature as being HPV-associated squamous cell cancers of unknown primaries.9
When head and neck cancers metastasize they typically metastasize to the lungs, bone, liver, mediastinum, skin, and bone marrow. Most metastasis is pulmonary in origin, with the literature indicating it accounts for 52%-66% of head and neck cancer metastases, with bone metastases next in frequency at 12%-22%.2,3,10 In general, the incidence of distant metastatic disease in head and neck cancers is not as common as its other solid tumor counterparts, and even metastasis to other lymph node groups other than locoregional cervical nodes is rare.11 Furthermore, late metastasis occurring more than 2 years after definitive treatment is also an infrequent occurrence.12
When discussing distant metastatic disease in head and neck cancer, previous literature has described an increasing likelihood of distant metastases when there is locoregional disease recurrence.13 Moreover, the retroperitoneum is an exceedingly rare site of distant metastatic disease for head and neck cancer. There have been only 2 previous cases that have described this phenomenon, and in both cases the metastases occurred within or close to 1 year of definitive locoregional treatment.4,5
Conclusion
We present our case to present an exceedingly rare case of distant metastatic, recurrent disease from head and neck cancer to the retroperitoneum (without locoregional recurrence) that occurred 4 years after definitive treatment. We believe this to be the first case of its kind to be described when taking into consideration the site of metastases, when the metastatic recurrence occurred and that it happened without loco-regional disease recurrence. This case highlights the importance of keeping a wide differential diagnosis when encountering a retroperitoneal mass in a patient with even a remote history of head and neck cancer.
Acknowledgments
The authors thank the following members of the Department of Pathology at the University of Texas Medical Branch: Asad Ahmad, MD; Eduardo Eyzaguirre, MD; Timothy C Allen, MD, JD, FACP; and Suimmin Qiu, MD, PHD.
1. Fitzmaurice C, Allen C, Barber RM, et al. Global, regional, and national cancer incidence, mortality, years of life lost, years lived with disability, and disability-adjusted life-years for 32 cancer groups, 1990 to 2015: a systematic analysis for the Global Burden of Disease Study. JAMA Oncol. 2017;3:524-548.
2. Ferlito A, Shaha AR, Silver CE, Rinaldo A, Mondin V. Incidence and sites of distant metastases from head and neck cancer. ORL J Otorhinolaryngol Relat Spec. 2001;63:202-207.
3. Wiegand S, Zimmermann A, Wilhelm T, Werner JA. Survival after distant metastasis in head and neck cancer. Anticancer Res. 2015;35:5499-5502.
4. Hofmann U, O’Connor JP, Biyani CS, Harnden P, Selby P, Weston PM. Retroperitoneal metastatic squamous cell carcinoma of the tonsil (with elevated beta human chorionic gonadotrophin): a misdiagnosis as extra-gonadal germ cell tumour. J Laryngol Otol. 2006;120:885-887.
5. Purkayastha A, Sharma N, Suhag V. Extremely rare and unusual case of retroperitoneal and pelvic metastasis from squamous cell carcinoma of vallecula. Int J Cancer Ther Oncol. 2016;4(2):1-4.
6. Rajiah P, Sinha R, Cuevas C, Dubinsky TJ, Bush WH, Kolokythas O. Imaging of uncommon retroperitoneal masses. Radiographics 2011;31:949-976.
7. Scali EP, Chandler TM, Heffernan EJ, Coyle J, Harris AC, Chang SD. Primary retroperitoneal masses: what is the differential diagnosis? Abdom Imaging. 2015;40:1887-1903.
8. Levy AD, Shaw JC, Sobin LH. Secondary tumors and tumorlike lesions of the peritoneal cavity: imaging features with pathologic correlation. Radiographics. 2009;29:347-373.
9. Isbell A, Fields EC. Three cases of women with HPV-related squamous cell carcinoma of unknown primary in the pelvis and retroperitoneum: a case series. Gynecol Oncol Rep. 2016;16:5-8.
10. León X, Quer M, Orús C, del Prado Venegas M, López M. Distant metastases in head and neck cancer patients who achieved loco-regional control. Head Neck. 2000;22:680-686.
11. Alavi S, Namazie A, Sercarz JA, Wang MB, Blackwell KE. Distant lymphatic metastasis from head and neck cancer. Ann Otol Rhinol Laryngol. 1999;108:860-863.
12. Krishnatry R, Gupta T, Murthy V, et al. Factors predicting ‘time to distant metastasis’ in radically treated head and neck cancer. Indian J Cancer. 2014;51:231-235.
13. Goodwin WJ. Distant metastases from oropharyngeal cancer. ORL J Otorhinolaryngol Relat Spec. 2001;63:222-223.
1. Fitzmaurice C, Allen C, Barber RM, et al. Global, regional, and national cancer incidence, mortality, years of life lost, years lived with disability, and disability-adjusted life-years for 32 cancer groups, 1990 to 2015: a systematic analysis for the Global Burden of Disease Study. JAMA Oncol. 2017;3:524-548.
2. Ferlito A, Shaha AR, Silver CE, Rinaldo A, Mondin V. Incidence and sites of distant metastases from head and neck cancer. ORL J Otorhinolaryngol Relat Spec. 2001;63:202-207.
3. Wiegand S, Zimmermann A, Wilhelm T, Werner JA. Survival after distant metastasis in head and neck cancer. Anticancer Res. 2015;35:5499-5502.
4. Hofmann U, O’Connor JP, Biyani CS, Harnden P, Selby P, Weston PM. Retroperitoneal metastatic squamous cell carcinoma of the tonsil (with elevated beta human chorionic gonadotrophin): a misdiagnosis as extra-gonadal germ cell tumour. J Laryngol Otol. 2006;120:885-887.
5. Purkayastha A, Sharma N, Suhag V. Extremely rare and unusual case of retroperitoneal and pelvic metastasis from squamous cell carcinoma of vallecula. Int J Cancer Ther Oncol. 2016;4(2):1-4.
6. Rajiah P, Sinha R, Cuevas C, Dubinsky TJ, Bush WH, Kolokythas O. Imaging of uncommon retroperitoneal masses. Radiographics 2011;31:949-976.
7. Scali EP, Chandler TM, Heffernan EJ, Coyle J, Harris AC, Chang SD. Primary retroperitoneal masses: what is the differential diagnosis? Abdom Imaging. 2015;40:1887-1903.
8. Levy AD, Shaw JC, Sobin LH. Secondary tumors and tumorlike lesions of the peritoneal cavity: imaging features with pathologic correlation. Radiographics. 2009;29:347-373.
9. Isbell A, Fields EC. Three cases of women with HPV-related squamous cell carcinoma of unknown primary in the pelvis and retroperitoneum: a case series. Gynecol Oncol Rep. 2016;16:5-8.
10. León X, Quer M, Orús C, del Prado Venegas M, López M. Distant metastases in head and neck cancer patients who achieved loco-regional control. Head Neck. 2000;22:680-686.
11. Alavi S, Namazie A, Sercarz JA, Wang MB, Blackwell KE. Distant lymphatic metastasis from head and neck cancer. Ann Otol Rhinol Laryngol. 1999;108:860-863.
12. Krishnatry R, Gupta T, Murthy V, et al. Factors predicting ‘time to distant metastasis’ in radically treated head and neck cancer. Indian J Cancer. 2014;51:231-235.
13. Goodwin WJ. Distant metastases from oropharyngeal cancer. ORL J Otorhinolaryngol Relat Spec. 2001;63:222-223.
Balancing quality and cost of care with patient well-being
Welcome to the first issue of The Journal of Community and Supportive Oncology for this year. 2017 was a rollercoaster year for the oncology community, literally from day 1. January 1 saw the kick-off for participation in the MACRA [Medicare Access and CHIP Reauthorization Act] Quality Payment Program, and soon after came the growing concern and uncertainty around the future of President Barack Obama’s Affordable Health Care Act. Attempts during the year to repeal the ACA failed, but with the December passage of the tax bill came Medicare cuts and the repeal of the individual mandate, which will effectively sever crucial revenue sources for the ACA. Nevertheless, against that backdrop, there was a slew of exciting therapeutic approvals – some of them landmark, as my fellow Editor, Linda Bosserman, noted in her year-end editorial (JCSO 2017;15[6]:e283-e290). As often happens, and as noted in the editorial, such advances come with concerns about the high cost of the therapies and their related toxicities, and the combined negative impact of those on quality and cost of care and patient quality of life. (QoL).
In this issue, 2 research articles examine bone metastasis in late-stage disease and their findings underscore the aforementioned importance of care cost and quality and patient QoL. Bone metastases are a common cause of pain in patients with advanced cancer. That pain is often associated with higher rates of depression, anxiety, and fatigue, and patient QoL will diminish if the pain is not adequately treated. Although radiotherapy is effective in palliating painful bone metastases, relief may be delayed and interim analgesic management needed. Garcia and colleagues (p. e8) examined the frequency of analgesic regimen assessment and intervention during radiation oncology consultations for bone metastases and evaluated the impact on analgesic management before and after implementation of a dedicated palliative radiation oncology service. They found that pain assessment and intervention were not common in the radiation oncology setting before establishment of the service and suggest that integrating palliative care within radiation oncology could improve the quality of pain management and by extension, patient well-being.
Patients with bone metastases are also at greater risk of bone fracture, for which they often are hospitalized at great cost. Nikkel and colleagues sought to determine the primary tumors in patients hospitalized with metastatic disease and who sustained pathologic and nonpathologic fractures, and to estimate the costs and lengths of stay for those hospitalizations (p. e14). The most common primary cancers in these patients were lung, breast, prostate, kidney, and colorectal – a novel finding in this study was that there were almost 4 times as many pathologic fractures from colorectal than from thyroid carcinoma. Patients hospitalized for pathologic fracture had higher billed costs and longer length of stay. The authors emphasize the importance of identifying patients at risk for pathological fracture based on primary tumor type, age, and socio-economic group; improving surveillance; and doing timely osteoporosis screening.
Therapeutic advances and the ensuing new options and combination possibilities are the substrate for our daily engagement with our patients. On page e53, Dr David Henry, the JCSO Editor-in-Chief, talks with Dr Kenneth Anderson of Harvard Medical School about advances in multiple myeloma therapies and how numerous therapy approvals have pushed the disease closer to becoming a manageable, chronic disease. On page e47, Jane de Lartigue describes the latest developments in the therapeutic targeting of altered metabolic pathways in cancer cells.
Also in this issue are new approval updates for abemaciclib as the first CDK inhibitor for breast cancer (p. e2) and the checkpoint inhibitors avelumab and durvalumab for metastatic bladder cancer (p. e5), a brief report on whether patient navigators’ personal experience with cancer has any effect on patient experience (p. e43), a research article on physical activity and sedentary behavior in survivors of breast cancer, and Case Reports (pp. e30-e42).
Welcome to the first issue of The Journal of Community and Supportive Oncology for this year. 2017 was a rollercoaster year for the oncology community, literally from day 1. January 1 saw the kick-off for participation in the MACRA [Medicare Access and CHIP Reauthorization Act] Quality Payment Program, and soon after came the growing concern and uncertainty around the future of President Barack Obama’s Affordable Health Care Act. Attempts during the year to repeal the ACA failed, but with the December passage of the tax bill came Medicare cuts and the repeal of the individual mandate, which will effectively sever crucial revenue sources for the ACA. Nevertheless, against that backdrop, there was a slew of exciting therapeutic approvals – some of them landmark, as my fellow Editor, Linda Bosserman, noted in her year-end editorial (JCSO 2017;15[6]:e283-e290). As often happens, and as noted in the editorial, such advances come with concerns about the high cost of the therapies and their related toxicities, and the combined negative impact of those on quality and cost of care and patient quality of life. (QoL).
In this issue, 2 research articles examine bone metastasis in late-stage disease and their findings underscore the aforementioned importance of care cost and quality and patient QoL. Bone metastases are a common cause of pain in patients with advanced cancer. That pain is often associated with higher rates of depression, anxiety, and fatigue, and patient QoL will diminish if the pain is not adequately treated. Although radiotherapy is effective in palliating painful bone metastases, relief may be delayed and interim analgesic management needed. Garcia and colleagues (p. e8) examined the frequency of analgesic regimen assessment and intervention during radiation oncology consultations for bone metastases and evaluated the impact on analgesic management before and after implementation of a dedicated palliative radiation oncology service. They found that pain assessment and intervention were not common in the radiation oncology setting before establishment of the service and suggest that integrating palliative care within radiation oncology could improve the quality of pain management and by extension, patient well-being.
Patients with bone metastases are also at greater risk of bone fracture, for which they often are hospitalized at great cost. Nikkel and colleagues sought to determine the primary tumors in patients hospitalized with metastatic disease and who sustained pathologic and nonpathologic fractures, and to estimate the costs and lengths of stay for those hospitalizations (p. e14). The most common primary cancers in these patients were lung, breast, prostate, kidney, and colorectal – a novel finding in this study was that there were almost 4 times as many pathologic fractures from colorectal than from thyroid carcinoma. Patients hospitalized for pathologic fracture had higher billed costs and longer length of stay. The authors emphasize the importance of identifying patients at risk for pathological fracture based on primary tumor type, age, and socio-economic group; improving surveillance; and doing timely osteoporosis screening.
Therapeutic advances and the ensuing new options and combination possibilities are the substrate for our daily engagement with our patients. On page e53, Dr David Henry, the JCSO Editor-in-Chief, talks with Dr Kenneth Anderson of Harvard Medical School about advances in multiple myeloma therapies and how numerous therapy approvals have pushed the disease closer to becoming a manageable, chronic disease. On page e47, Jane de Lartigue describes the latest developments in the therapeutic targeting of altered metabolic pathways in cancer cells.
Also in this issue are new approval updates for abemaciclib as the first CDK inhibitor for breast cancer (p. e2) and the checkpoint inhibitors avelumab and durvalumab for metastatic bladder cancer (p. e5), a brief report on whether patient navigators’ personal experience with cancer has any effect on patient experience (p. e43), a research article on physical activity and sedentary behavior in survivors of breast cancer, and Case Reports (pp. e30-e42).
Welcome to the first issue of The Journal of Community and Supportive Oncology for this year. 2017 was a rollercoaster year for the oncology community, literally from day 1. January 1 saw the kick-off for participation in the MACRA [Medicare Access and CHIP Reauthorization Act] Quality Payment Program, and soon after came the growing concern and uncertainty around the future of President Barack Obama’s Affordable Health Care Act. Attempts during the year to repeal the ACA failed, but with the December passage of the tax bill came Medicare cuts and the repeal of the individual mandate, which will effectively sever crucial revenue sources for the ACA. Nevertheless, against that backdrop, there was a slew of exciting therapeutic approvals – some of them landmark, as my fellow Editor, Linda Bosserman, noted in her year-end editorial (JCSO 2017;15[6]:e283-e290). As often happens, and as noted in the editorial, such advances come with concerns about the high cost of the therapies and their related toxicities, and the combined negative impact of those on quality and cost of care and patient quality of life. (QoL).
In this issue, 2 research articles examine bone metastasis in late-stage disease and their findings underscore the aforementioned importance of care cost and quality and patient QoL. Bone metastases are a common cause of pain in patients with advanced cancer. That pain is often associated with higher rates of depression, anxiety, and fatigue, and patient QoL will diminish if the pain is not adequately treated. Although radiotherapy is effective in palliating painful bone metastases, relief may be delayed and interim analgesic management needed. Garcia and colleagues (p. e8) examined the frequency of analgesic regimen assessment and intervention during radiation oncology consultations for bone metastases and evaluated the impact on analgesic management before and after implementation of a dedicated palliative radiation oncology service. They found that pain assessment and intervention were not common in the radiation oncology setting before establishment of the service and suggest that integrating palliative care within radiation oncology could improve the quality of pain management and by extension, patient well-being.
Patients with bone metastases are also at greater risk of bone fracture, for which they often are hospitalized at great cost. Nikkel and colleagues sought to determine the primary tumors in patients hospitalized with metastatic disease and who sustained pathologic and nonpathologic fractures, and to estimate the costs and lengths of stay for those hospitalizations (p. e14). The most common primary cancers in these patients were lung, breast, prostate, kidney, and colorectal – a novel finding in this study was that there were almost 4 times as many pathologic fractures from colorectal than from thyroid carcinoma. Patients hospitalized for pathologic fracture had higher billed costs and longer length of stay. The authors emphasize the importance of identifying patients at risk for pathological fracture based on primary tumor type, age, and socio-economic group; improving surveillance; and doing timely osteoporosis screening.
Therapeutic advances and the ensuing new options and combination possibilities are the substrate for our daily engagement with our patients. On page e53, Dr David Henry, the JCSO Editor-in-Chief, talks with Dr Kenneth Anderson of Harvard Medical School about advances in multiple myeloma therapies and how numerous therapy approvals have pushed the disease closer to becoming a manageable, chronic disease. On page e47, Jane de Lartigue describes the latest developments in the therapeutic targeting of altered metabolic pathways in cancer cells.
Also in this issue are new approval updates for abemaciclib as the first CDK inhibitor for breast cancer (p. e2) and the checkpoint inhibitors avelumab and durvalumab for metastatic bladder cancer (p. e5), a brief report on whether patient navigators’ personal experience with cancer has any effect on patient experience (p. e43), a research article on physical activity and sedentary behavior in survivors of breast cancer, and Case Reports (pp. e30-e42).