User login
Marijuana: Most oncologists are having the conversation
The vast majority of oncologists discuss medical marijuana use with their patients, and around one-half recommend it to patients, yet many also say they do not feel equipped to make clinical recommendations on its use, new research has found.
A survey on medical marijuana was mailed to a nationally-representative, random sample of 400 medical oncologists and had a response rate of 63%; results from the 237 responders revealed that 79.8% had discussed medical marijuana use with patients or their families and 45.9% had recommended medical marijuana for cancer-related issues to at least one patient in the previous year.
Oncologists in the western United States were significantly more likely to recommend medical marijuana use, compared with those in the south of the country (84.2% vs. 34.7%, respectively; P less than .001), Ilana M. Braun, MD, and her associates reported in Journal of Clinical Oncology.
Doctors who practiced outside a hospital setting were also significantly more likely to recommend medical marijuana to their patients, as were medical oncologists with higher practice volumes.
Among the oncologists who reported discussing medical marijuana use with patients, 78% said these conversations were more likely to be initiated by the patient and their family than by the oncologist themselves.
However only 29.4% of oncologists surveyed said they felt “sufficiently knowledgeable” to make recommendations to patients about medical marijuana. Even among those who said they had recommended medical marijuana to a patient in the past year, 56.2% said they didn’t feel they had enough knowledge to make a recommendation.
Overall, oncologists had mixed views about medical marijuana. About one-third viewed it as equal to or more effective than standard pain treatments, one-third viewed it as less effective, and one-third said they did not know. However two-thirds viewed medical marijuana as a useful adjunct to standard pain therapies.
Two-thirds of oncologists surveyed believed medical marijuana was as good as or better than standard treatments for poor appetite or cachexia, but less than half felt it was equal to or better than standard antinausea therapies.
The data revealed a “clinically problematic discrepancy” between medical oncologists’ their perceived knowledge about medical marijuana use and their actual beliefs and practices, said Dr. Braun of the Dana-Farber Cancer Institute and her associates.
“Although our survey could not determine why oncologists recommend medical marijuana, it may be because they regard medical marijuana as an alternative therapy that is difficult to evaluate given sparse randomized, controlled trial data,” they wrote.
“[The results] highlight a crucial need for expedited clinical trials exploring marijuana’s potential medicinal effects in oncology (e.g. as an adjunctive pain management strategy or as a treatment of anorexia/cachexia) and the need for educational programs about medical marijuana to inform oncologists who frequently confront questions regarding medical marijuana in daily practice.”
No funding was declared. Two authors declared royalties and honoraria from medical publishing and a research institute, and one declared fees for expert testimony.
SOURCE: Braun I et al. J Clin Oncol. 2018 May 10. doi: 10.1200/JCO.2017.76.1221.
The vast majority of oncologists discuss medical marijuana use with their patients, and around one-half recommend it to patients, yet many also say they do not feel equipped to make clinical recommendations on its use, new research has found.
A survey on medical marijuana was mailed to a nationally-representative, random sample of 400 medical oncologists and had a response rate of 63%; results from the 237 responders revealed that 79.8% had discussed medical marijuana use with patients or their families and 45.9% had recommended medical marijuana for cancer-related issues to at least one patient in the previous year.
Oncologists in the western United States were significantly more likely to recommend medical marijuana use, compared with those in the south of the country (84.2% vs. 34.7%, respectively; P less than .001), Ilana M. Braun, MD, and her associates reported in Journal of Clinical Oncology.
Doctors who practiced outside a hospital setting were also significantly more likely to recommend medical marijuana to their patients, as were medical oncologists with higher practice volumes.
Among the oncologists who reported discussing medical marijuana use with patients, 78% said these conversations were more likely to be initiated by the patient and their family than by the oncologist themselves.
However only 29.4% of oncologists surveyed said they felt “sufficiently knowledgeable” to make recommendations to patients about medical marijuana. Even among those who said they had recommended medical marijuana to a patient in the past year, 56.2% said they didn’t feel they had enough knowledge to make a recommendation.
Overall, oncologists had mixed views about medical marijuana. About one-third viewed it as equal to or more effective than standard pain treatments, one-third viewed it as less effective, and one-third said they did not know. However two-thirds viewed medical marijuana as a useful adjunct to standard pain therapies.
Two-thirds of oncologists surveyed believed medical marijuana was as good as or better than standard treatments for poor appetite or cachexia, but less than half felt it was equal to or better than standard antinausea therapies.
The data revealed a “clinically problematic discrepancy” between medical oncologists’ their perceived knowledge about medical marijuana use and their actual beliefs and practices, said Dr. Braun of the Dana-Farber Cancer Institute and her associates.
“Although our survey could not determine why oncologists recommend medical marijuana, it may be because they regard medical marijuana as an alternative therapy that is difficult to evaluate given sparse randomized, controlled trial data,” they wrote.
“[The results] highlight a crucial need for expedited clinical trials exploring marijuana’s potential medicinal effects in oncology (e.g. as an adjunctive pain management strategy or as a treatment of anorexia/cachexia) and the need for educational programs about medical marijuana to inform oncologists who frequently confront questions regarding medical marijuana in daily practice.”
No funding was declared. Two authors declared royalties and honoraria from medical publishing and a research institute, and one declared fees for expert testimony.
SOURCE: Braun I et al. J Clin Oncol. 2018 May 10. doi: 10.1200/JCO.2017.76.1221.
The vast majority of oncologists discuss medical marijuana use with their patients, and around one-half recommend it to patients, yet many also say they do not feel equipped to make clinical recommendations on its use, new research has found.
A survey on medical marijuana was mailed to a nationally-representative, random sample of 400 medical oncologists and had a response rate of 63%; results from the 237 responders revealed that 79.8% had discussed medical marijuana use with patients or their families and 45.9% had recommended medical marijuana for cancer-related issues to at least one patient in the previous year.
Oncologists in the western United States were significantly more likely to recommend medical marijuana use, compared with those in the south of the country (84.2% vs. 34.7%, respectively; P less than .001), Ilana M. Braun, MD, and her associates reported in Journal of Clinical Oncology.
Doctors who practiced outside a hospital setting were also significantly more likely to recommend medical marijuana to their patients, as were medical oncologists with higher practice volumes.
Among the oncologists who reported discussing medical marijuana use with patients, 78% said these conversations were more likely to be initiated by the patient and their family than by the oncologist themselves.
However only 29.4% of oncologists surveyed said they felt “sufficiently knowledgeable” to make recommendations to patients about medical marijuana. Even among those who said they had recommended medical marijuana to a patient in the past year, 56.2% said they didn’t feel they had enough knowledge to make a recommendation.
Overall, oncologists had mixed views about medical marijuana. About one-third viewed it as equal to or more effective than standard pain treatments, one-third viewed it as less effective, and one-third said they did not know. However two-thirds viewed medical marijuana as a useful adjunct to standard pain therapies.
Two-thirds of oncologists surveyed believed medical marijuana was as good as or better than standard treatments for poor appetite or cachexia, but less than half felt it was equal to or better than standard antinausea therapies.
The data revealed a “clinically problematic discrepancy” between medical oncologists’ their perceived knowledge about medical marijuana use and their actual beliefs and practices, said Dr. Braun of the Dana-Farber Cancer Institute and her associates.
“Although our survey could not determine why oncologists recommend medical marijuana, it may be because they regard medical marijuana as an alternative therapy that is difficult to evaluate given sparse randomized, controlled trial data,” they wrote.
“[The results] highlight a crucial need for expedited clinical trials exploring marijuana’s potential medicinal effects in oncology (e.g. as an adjunctive pain management strategy or as a treatment of anorexia/cachexia) and the need for educational programs about medical marijuana to inform oncologists who frequently confront questions regarding medical marijuana in daily practice.”
No funding was declared. Two authors declared royalties and honoraria from medical publishing and a research institute, and one declared fees for expert testimony.
SOURCE: Braun I et al. J Clin Oncol. 2018 May 10. doi: 10.1200/JCO.2017.76.1221.
FROM THE JOURNAL OF CLINICAL ONCOLOGY
Key clinical point: The majority of medical oncologists discuss medical marijuana use with their patients, though few feel knowledgeable on the subject.
Major finding: Nearly 80% of medical oncologists have discussed medical marijuana use with their patients, but only 29.4% of those surveyed felt sufficiently knowledgeable.
Study details: Survey of 237 medical oncologists.
Disclosures: No funding was declared. Two authors declared royalties and honoraria from medical publishing and a research institute, and one declared fees for expert testimony.
Source: Braun I et al. J Clin Oncol. 2018 May 10. doi: 10.1200/JCO.2017.76.1221.
Patients with CF at increased risk for GI cancers
Patients with cystic fibrosis have a significantly greater risk for developing cancers of the gastrointestinal tract compared with the general population, results of a systematic review and meta-analysis indicate.
Among persons with cystic fibrosis (CF) the standardized incidence ratio (SIR) for any gastrointestinal cancer compared with the general population was 8.13 (P less than .0001). Patients with CF were at significantly elevated risk for cancers of the small bowel, colon, biliary tract, and pancreas, reported Atsushi Sakuraba, MD, PhD, of the University of Chicago, and his colleagues.
“Additionally, our findings suggest that patients who had an organ transplant have a higher risk of developing gastrointestinal cancer than those who did not. Although further studies are needed to monitor gastrointestinal cancer incidence over time in patients with cystic fibrosis, the development of a screening strategy for gastrointestinal cancer in these patients is warranted,” they wrote in Lancet Oncology.
As patients with CF live longer because of improvements in therapies and management of comorbidities, their risks for cancer and other diseases will increase, the authors noted.
To get a more accurate estimate of the degree of risk than what smaller cohort studies could provide, the investigators conducted a systematic review and meta-analysis. They narrowed their search to six cohort studies including 99,925 patients with CF followed for a total of 5,444,695 person-years.
As noted, patients with CF overall had an eightfold higher risk for gastrointestinal cancers compared with the general population, and for the subgroup of patients who had undergone a lung transplant, the SIR was 21.13 compared with 4.18 for those with CF but no lung transplant (P less than .0001).
- The SIRs for site-specific gastrointestinal cancers were as follows:
- Small bowel: SIR= 18.94 (P less than .0001).
- Colon: SIR = 10.91 (P less than .0001).
- Biliary tract: SIR = 17.87 (P less than .0001).
- Pancreas: SIR = 6.18 (P = .022).
The investigators recommend screening for gastrointestinal cancers in patients with CF in general, and especially for patients who have undergone lung transplantation and are receiving immunosuppressive therapies.
Because of the elevated risk of pancreatic and biliary tract cancers, the authors propose a screening program including magnetic resonance cholangiopancreatography, endoscopic ultrasound, or abdominal ultrasound and measurement of cancer antigen 19-9.
The authors reported that the study had no funding source, and they declared no competing interests.
SOURCE: Yamada A et al. Lancet Oncol. 2018 Apr 26. doi: 10.1016/S1470-2045(18)30188-8.
The emergence of gastrointestinal cancer as a clinical complication in adults with cystic fibrosis is a consequence of the substantial improvement in life expectancy. Novel CFTR modulator therapies, which correct the malfunctioning protein and increase its expression at the apical surface, might decrease the incidence of gastrointestinal cancers in patients with cystic fibrosis.
In conclusion, the meta-analysis by Yamada and colleagues shows that cystic fibrosis can be considered a gastrointestinal cancer syndrome, for which screening and surveillance protocols should be implemented. Oncologists and gastroenterologists managing patients with cystic fibrosis should consider the best methods for screening of the small bowel, biliary tract, pancreas, and colon to prevent gastrointestinal malignancies in these patients.
Mordechai Slae, MD, and Michael Wilschanski, MD, are with the Hadassah Medical Center in Jerusalem. These comments are condensed from their editorial in The Lancet Oncology.
The emergence of gastrointestinal cancer as a clinical complication in adults with cystic fibrosis is a consequence of the substantial improvement in life expectancy. Novel CFTR modulator therapies, which correct the malfunctioning protein and increase its expression at the apical surface, might decrease the incidence of gastrointestinal cancers in patients with cystic fibrosis.
In conclusion, the meta-analysis by Yamada and colleagues shows that cystic fibrosis can be considered a gastrointestinal cancer syndrome, for which screening and surveillance protocols should be implemented. Oncologists and gastroenterologists managing patients with cystic fibrosis should consider the best methods for screening of the small bowel, biliary tract, pancreas, and colon to prevent gastrointestinal malignancies in these patients.
Mordechai Slae, MD, and Michael Wilschanski, MD, are with the Hadassah Medical Center in Jerusalem. These comments are condensed from their editorial in The Lancet Oncology.
The emergence of gastrointestinal cancer as a clinical complication in adults with cystic fibrosis is a consequence of the substantial improvement in life expectancy. Novel CFTR modulator therapies, which correct the malfunctioning protein and increase its expression at the apical surface, might decrease the incidence of gastrointestinal cancers in patients with cystic fibrosis.
In conclusion, the meta-analysis by Yamada and colleagues shows that cystic fibrosis can be considered a gastrointestinal cancer syndrome, for which screening and surveillance protocols should be implemented. Oncologists and gastroenterologists managing patients with cystic fibrosis should consider the best methods for screening of the small bowel, biliary tract, pancreas, and colon to prevent gastrointestinal malignancies in these patients.
Mordechai Slae, MD, and Michael Wilschanski, MD, are with the Hadassah Medical Center in Jerusalem. These comments are condensed from their editorial in The Lancet Oncology.
Patients with cystic fibrosis have a significantly greater risk for developing cancers of the gastrointestinal tract compared with the general population, results of a systematic review and meta-analysis indicate.
Among persons with cystic fibrosis (CF) the standardized incidence ratio (SIR) for any gastrointestinal cancer compared with the general population was 8.13 (P less than .0001). Patients with CF were at significantly elevated risk for cancers of the small bowel, colon, biliary tract, and pancreas, reported Atsushi Sakuraba, MD, PhD, of the University of Chicago, and his colleagues.
“Additionally, our findings suggest that patients who had an organ transplant have a higher risk of developing gastrointestinal cancer than those who did not. Although further studies are needed to monitor gastrointestinal cancer incidence over time in patients with cystic fibrosis, the development of a screening strategy for gastrointestinal cancer in these patients is warranted,” they wrote in Lancet Oncology.
As patients with CF live longer because of improvements in therapies and management of comorbidities, their risks for cancer and other diseases will increase, the authors noted.
To get a more accurate estimate of the degree of risk than what smaller cohort studies could provide, the investigators conducted a systematic review and meta-analysis. They narrowed their search to six cohort studies including 99,925 patients with CF followed for a total of 5,444,695 person-years.
As noted, patients with CF overall had an eightfold higher risk for gastrointestinal cancers compared with the general population, and for the subgroup of patients who had undergone a lung transplant, the SIR was 21.13 compared with 4.18 for those with CF but no lung transplant (P less than .0001).
- The SIRs for site-specific gastrointestinal cancers were as follows:
- Small bowel: SIR= 18.94 (P less than .0001).
- Colon: SIR = 10.91 (P less than .0001).
- Biliary tract: SIR = 17.87 (P less than .0001).
- Pancreas: SIR = 6.18 (P = .022).
The investigators recommend screening for gastrointestinal cancers in patients with CF in general, and especially for patients who have undergone lung transplantation and are receiving immunosuppressive therapies.
Because of the elevated risk of pancreatic and biliary tract cancers, the authors propose a screening program including magnetic resonance cholangiopancreatography, endoscopic ultrasound, or abdominal ultrasound and measurement of cancer antigen 19-9.
The authors reported that the study had no funding source, and they declared no competing interests.
SOURCE: Yamada A et al. Lancet Oncol. 2018 Apr 26. doi: 10.1016/S1470-2045(18)30188-8.
Patients with cystic fibrosis have a significantly greater risk for developing cancers of the gastrointestinal tract compared with the general population, results of a systematic review and meta-analysis indicate.
Among persons with cystic fibrosis (CF) the standardized incidence ratio (SIR) for any gastrointestinal cancer compared with the general population was 8.13 (P less than .0001). Patients with CF were at significantly elevated risk for cancers of the small bowel, colon, biliary tract, and pancreas, reported Atsushi Sakuraba, MD, PhD, of the University of Chicago, and his colleagues.
“Additionally, our findings suggest that patients who had an organ transplant have a higher risk of developing gastrointestinal cancer than those who did not. Although further studies are needed to monitor gastrointestinal cancer incidence over time in patients with cystic fibrosis, the development of a screening strategy for gastrointestinal cancer in these patients is warranted,” they wrote in Lancet Oncology.
As patients with CF live longer because of improvements in therapies and management of comorbidities, their risks for cancer and other diseases will increase, the authors noted.
To get a more accurate estimate of the degree of risk than what smaller cohort studies could provide, the investigators conducted a systematic review and meta-analysis. They narrowed their search to six cohort studies including 99,925 patients with CF followed for a total of 5,444,695 person-years.
As noted, patients with CF overall had an eightfold higher risk for gastrointestinal cancers compared with the general population, and for the subgroup of patients who had undergone a lung transplant, the SIR was 21.13 compared with 4.18 for those with CF but no lung transplant (P less than .0001).
- The SIRs for site-specific gastrointestinal cancers were as follows:
- Small bowel: SIR= 18.94 (P less than .0001).
- Colon: SIR = 10.91 (P less than .0001).
- Biliary tract: SIR = 17.87 (P less than .0001).
- Pancreas: SIR = 6.18 (P = .022).
The investigators recommend screening for gastrointestinal cancers in patients with CF in general, and especially for patients who have undergone lung transplantation and are receiving immunosuppressive therapies.
Because of the elevated risk of pancreatic and biliary tract cancers, the authors propose a screening program including magnetic resonance cholangiopancreatography, endoscopic ultrasound, or abdominal ultrasound and measurement of cancer antigen 19-9.
The authors reported that the study had no funding source, and they declared no competing interests.
SOURCE: Yamada A et al. Lancet Oncol. 2018 Apr 26. doi: 10.1016/S1470-2045(18)30188-8.
FROM THE LANCET ONCOLOGY
Key clinical point: Patients with cystic fibrosis, especially those who have received a lung transplant, are at increased risk for gastrointestinal tract cancers.
Major finding: The standard incidence ratio for GI cancers among patients with CF was 8.13 compared with the general population.
Study details: Systematic review and meta-analysis of published studies including 99,925 patients with CF followed for 5,444,695 person-years.
Disclosures: The authors reported that the study had no funding source, and they declared no competing interests.
Source: Yamada A et al. Lancet Oncol 2018 Apr 26. doi: 10.1016/S1470-2045(18)30188-8.
Novel targeted cancer drugs cause fewer arrhythmias
ORLANDO – Not all oncology drugs are equal when it comes to their risk of treatment-induced cardiac arrhythmias.
Indeed, compared with anthracycline-based regimens, long the workhorse in treating many forms of cancer, the novel targeted agents – tyrosine kinase inhibitors, immune checkpoint inhibitors, and monoclonal antibodies – were 40% less likely to result in a new arrhythmia diagnosis within 6 months of treatment initiation, in a large, single-center retrospective study reported by Andrew Nickel at the annual meeting of the American College of Cardiology.
Overall, 14% of cancer patients developed a first-ever cardiac arrhythmia within the first 6 months after treatment began. In a Cox multivariate analysis, treatment with a targeted cancer agent was independently associated with a 40% lower risk of arrhythmia, compared with anthracycline-containing therapy. Of note, the incidence of new-onset atrial fibrillation was closely similar in the two groups.
Several patient factors emerged as independent predictors of increased risk of cancer treatment–induced arrhythmia in the multivariate analysis: male sex, with a 1.2-fold increased risk; baseline heart failure, with a 2.2-fold risk; and hypertension, which conferred a 1.6-fold increased risk. These are patient groups in which the novel targeted cancer treatments are a particularly attractive option from the standpoint of mitigating arrhythmia risk, provided their use would be appropriate, he observed.
Mr. Nickel reported having no financial conflicts regarding his study, which was conducted free of commercial support.
SOURCE: Nickel A et al. ACC 18. Abstract 900-06.
ORLANDO – Not all oncology drugs are equal when it comes to their risk of treatment-induced cardiac arrhythmias.
Indeed, compared with anthracycline-based regimens, long the workhorse in treating many forms of cancer, the novel targeted agents – tyrosine kinase inhibitors, immune checkpoint inhibitors, and monoclonal antibodies – were 40% less likely to result in a new arrhythmia diagnosis within 6 months of treatment initiation, in a large, single-center retrospective study reported by Andrew Nickel at the annual meeting of the American College of Cardiology.
Overall, 14% of cancer patients developed a first-ever cardiac arrhythmia within the first 6 months after treatment began. In a Cox multivariate analysis, treatment with a targeted cancer agent was independently associated with a 40% lower risk of arrhythmia, compared with anthracycline-containing therapy. Of note, the incidence of new-onset atrial fibrillation was closely similar in the two groups.
Several patient factors emerged as independent predictors of increased risk of cancer treatment–induced arrhythmia in the multivariate analysis: male sex, with a 1.2-fold increased risk; baseline heart failure, with a 2.2-fold risk; and hypertension, which conferred a 1.6-fold increased risk. These are patient groups in which the novel targeted cancer treatments are a particularly attractive option from the standpoint of mitigating arrhythmia risk, provided their use would be appropriate, he observed.
Mr. Nickel reported having no financial conflicts regarding his study, which was conducted free of commercial support.
SOURCE: Nickel A et al. ACC 18. Abstract 900-06.
ORLANDO – Not all oncology drugs are equal when it comes to their risk of treatment-induced cardiac arrhythmias.
Indeed, compared with anthracycline-based regimens, long the workhorse in treating many forms of cancer, the novel targeted agents – tyrosine kinase inhibitors, immune checkpoint inhibitors, and monoclonal antibodies – were 40% less likely to result in a new arrhythmia diagnosis within 6 months of treatment initiation, in a large, single-center retrospective study reported by Andrew Nickel at the annual meeting of the American College of Cardiology.
Overall, 14% of cancer patients developed a first-ever cardiac arrhythmia within the first 6 months after treatment began. In a Cox multivariate analysis, treatment with a targeted cancer agent was independently associated with a 40% lower risk of arrhythmia, compared with anthracycline-containing therapy. Of note, the incidence of new-onset atrial fibrillation was closely similar in the two groups.
Several patient factors emerged as independent predictors of increased risk of cancer treatment–induced arrhythmia in the multivariate analysis: male sex, with a 1.2-fold increased risk; baseline heart failure, with a 2.2-fold risk; and hypertension, which conferred a 1.6-fold increased risk. These are patient groups in which the novel targeted cancer treatments are a particularly attractive option from the standpoint of mitigating arrhythmia risk, provided their use would be appropriate, he observed.
Mr. Nickel reported having no financial conflicts regarding his study, which was conducted free of commercial support.
SOURCE: Nickel A et al. ACC 18. Abstract 900-06.
REPORTING FROM ACC 2018
Key clinical point: The novel targeted cancer therapies cause markedly fewer cardiac arrhythmias.
Major finding: Cancer patients treated with a tyrosine kinase inhibitor, immune checkpoint inhibitor, or another of the novel targeted therapies were 40% less likely than were those on anthracycline-based therapy to develop a treatment-induced cardiac arrhythmia up to 6 months after treatment initiation.
Study details: This was a retrospective single-center study including more than 5,000 cancer patients.
Disclosures: The presenter reported having no financial conflicts regarding his study, which was conducted free of commercial support.
Source: Nickel A et al. ACC 18, Abstract #900-06.
Reduced intensity conditioning doesn’t protect fertility
SALT LAKE CITY – Both male and female recipients of childhood hematopoietic stem cell transplantation (HSCT) were very likely to have severely decreased fertility potential, even in the setting of preserved puberty, according to a recent study of adolescent and young adult HSCT recipients.
A reduced intensity conditioning regimen did not protect this cohort from decreased fertility, a finding that surprised the study’s lead author.
“We had hypothesized that, as compared to myeloablative conditioning, reduced intensity conditioning in children who received HSCT would lower the risk of infertility and lessen gonadal failure,” said Helen Oquendo del Toro, MD. In fact, Dr. Oquendo del Toro and her collaborators found that more than 90% of semen samples available for analysis had results that indicated infertility or severely impaired fertility, regardless of the type of pretransplant conditioning the patient had received.
The study highlights the need for fertility preservation when possible before HSCT, and makes clear that “normal puberty does not equate to normal fertility,” said Dr. Oquendo del Toro, of Cincinnati Children’s Hospital Medical Center.
Dr. Oquendo del Toro presented results of an observational cohort study of late effects of HSCT that included individuals aged 1-40 years old who received a single HSCT at, or after, 1 year of age.
Twenty-one males in the study had semen available for analysis. Of the 10 males who received myeloablative conditioning (MAC), 8 had azoospermia, and 2 more had oligoteratospermia (low sperm count with abnormal morphology). For the 11 males who received reduced intensity conditioning (RIC), eight had azoospermia, two had semen samples that showed oligoteratospermia, and one had a normal semen analysis.
The median age at transplant for these males was 14.5 years, and patients were a median of 19 years old at follow-up, Dr. Oquendo del Toro said at the combined annual meetings of the Center for International Blood & Marrow Transplant Research and the American Society for Blood and Marrow Transplantation.
For females in the study, low levels of anti-Müllerian hormone (AMH) – generally considered the best surrogate lab value for ovarian reserve – were nearly as common. Of 14 females receiving MAC, 13 (93%) had low AMH, as did 6 of 8 (75%) female patients who received RIC.
Individuals with more than one HSCT were excluded, as were those with Fanconi anemia, which itself carries a risk of gonadal failure. The study’s two aims were to investigate gonadal function as well as fertility potential after receipt of either RIC or MAC for HSCT.
Patients were seen by an endocrinologist who assessed testicular volume and assigned a Tanner stage. At age 11 and older, patients’ gonadal function was assessed on an annual basis by obtaining levels of luteinizing hormone and follicle stimulating hormone for all patients; female estradiol levels were tracked, as were male testosterone levels.
Assessment of fertility potential required additional laboratory testing: For females, the investigators obtained AMH levels, while for males, semen analysis was coupled with serum levels of inhibin B, an indicator of Sertoli cell function.
A total of 72 males were more than 1 year post-HSCT in the cohort, and of these, 41 were at least 11 years old and had achieved pubertal status according to laboratory evaluation. In all, 22 of the male patients received RIC, and 19 received MAC.
Males receiving MAC were a median 20 years old at their follow-up evaluation, and a median 6 years post-HSCT, while the RIC group were a median of 18.5 years old and 5.5 years out from their transplant.
Of the 50 females who were more than 1 year post-HSCT, 25 were pubertal and 11 years old or older. Nine of the female patients received RIC, and 16 received MAC.
Females who received MAC were a median 12.1 years old and 4.1 years post-HSCT at their follow-up evaluation. Females receiving RIC were a median 16 years old, and 6.5 years post-HSCT at the time of evaluation.
Patients received their transplants for a variety of malignant and nonmalignant conditions.
“We saw relatively normal gonadotropins after both reduced intensity and myeloablative conditioning in males,” Dr. Oquendo del Toro said. Of the MAC group, 4 of 15 (27%) had elevated follicle stimulating hormone levels, as did 2 of 17 (12%) of the RIC group. Elevated luteinizing hormone levels were seen in 2 of 15 (13%) of the MAC group and 1 of 17 (6%) of the RIC group. Four patients in each group had abnormally low testosterone levels.
However, when the investigators looked at inhibin B levels in males, they found abnormally low levels in 9 of 15 (60%) of those who received MAC, and in 6 of 15 (40%) of those who received RIC. These results meshed with the severely abnormal semen analyses investigators found from those participants for whom a sample was available, Dr. Oquendo del Toro said.
For females, estradiol levels were significantly lower for those who had received MAC, with 7 of 11 (64%) of that group having abnormally low estradiol levels. The levels approached 0 pg/mL for many, said Dr. Oquendo del Toro. None of the eight patients who had received RIC had abnormally low estradiol levels (P = .0008).
“Male puberty is relatively well preserved after both myeloablative and reduced intensity conditioning, but there is a greater than 90% risk of male infertility associated with both reduced intensity and myeloablative conditioning for HSCT,” Dr. Oquendo del Toro said.
For females, the study paints a different picture. “We saw decreased premature ovarian failure after reduced intensity conditioning … but the fertility potential as assessed by anti-Müllerian hormone was decreased” after both conditioning regimens, she said.
Dr. Oquendo del Toro reported having no conflicts of interest.
SOURCE: Oquendo del Toro H et al. The 2018 BMT Tandem Meetings, Abstract 88.
SALT LAKE CITY – Both male and female recipients of childhood hematopoietic stem cell transplantation (HSCT) were very likely to have severely decreased fertility potential, even in the setting of preserved puberty, according to a recent study of adolescent and young adult HSCT recipients.
A reduced intensity conditioning regimen did not protect this cohort from decreased fertility, a finding that surprised the study’s lead author.
“We had hypothesized that, as compared to myeloablative conditioning, reduced intensity conditioning in children who received HSCT would lower the risk of infertility and lessen gonadal failure,” said Helen Oquendo del Toro, MD. In fact, Dr. Oquendo del Toro and her collaborators found that more than 90% of semen samples available for analysis had results that indicated infertility or severely impaired fertility, regardless of the type of pretransplant conditioning the patient had received.
The study highlights the need for fertility preservation when possible before HSCT, and makes clear that “normal puberty does not equate to normal fertility,” said Dr. Oquendo del Toro, of Cincinnati Children’s Hospital Medical Center.
Dr. Oquendo del Toro presented results of an observational cohort study of late effects of HSCT that included individuals aged 1-40 years old who received a single HSCT at, or after, 1 year of age.
Twenty-one males in the study had semen available for analysis. Of the 10 males who received myeloablative conditioning (MAC), 8 had azoospermia, and 2 more had oligoteratospermia (low sperm count with abnormal morphology). For the 11 males who received reduced intensity conditioning (RIC), eight had azoospermia, two had semen samples that showed oligoteratospermia, and one had a normal semen analysis.
The median age at transplant for these males was 14.5 years, and patients were a median of 19 years old at follow-up, Dr. Oquendo del Toro said at the combined annual meetings of the Center for International Blood & Marrow Transplant Research and the American Society for Blood and Marrow Transplantation.
For females in the study, low levels of anti-Müllerian hormone (AMH) – generally considered the best surrogate lab value for ovarian reserve – were nearly as common. Of 14 females receiving MAC, 13 (93%) had low AMH, as did 6 of 8 (75%) female patients who received RIC.
Individuals with more than one HSCT were excluded, as were those with Fanconi anemia, which itself carries a risk of gonadal failure. The study’s two aims were to investigate gonadal function as well as fertility potential after receipt of either RIC or MAC for HSCT.
Patients were seen by an endocrinologist who assessed testicular volume and assigned a Tanner stage. At age 11 and older, patients’ gonadal function was assessed on an annual basis by obtaining levels of luteinizing hormone and follicle stimulating hormone for all patients; female estradiol levels were tracked, as were male testosterone levels.
Assessment of fertility potential required additional laboratory testing: For females, the investigators obtained AMH levels, while for males, semen analysis was coupled with serum levels of inhibin B, an indicator of Sertoli cell function.
A total of 72 males were more than 1 year post-HSCT in the cohort, and of these, 41 were at least 11 years old and had achieved pubertal status according to laboratory evaluation. In all, 22 of the male patients received RIC, and 19 received MAC.
Males receiving MAC were a median 20 years old at their follow-up evaluation, and a median 6 years post-HSCT, while the RIC group were a median of 18.5 years old and 5.5 years out from their transplant.
Of the 50 females who were more than 1 year post-HSCT, 25 were pubertal and 11 years old or older. Nine of the female patients received RIC, and 16 received MAC.
Females who received MAC were a median 12.1 years old and 4.1 years post-HSCT at their follow-up evaluation. Females receiving RIC were a median 16 years old, and 6.5 years post-HSCT at the time of evaluation.
Patients received their transplants for a variety of malignant and nonmalignant conditions.
“We saw relatively normal gonadotropins after both reduced intensity and myeloablative conditioning in males,” Dr. Oquendo del Toro said. Of the MAC group, 4 of 15 (27%) had elevated follicle stimulating hormone levels, as did 2 of 17 (12%) of the RIC group. Elevated luteinizing hormone levels were seen in 2 of 15 (13%) of the MAC group and 1 of 17 (6%) of the RIC group. Four patients in each group had abnormally low testosterone levels.
However, when the investigators looked at inhibin B levels in males, they found abnormally low levels in 9 of 15 (60%) of those who received MAC, and in 6 of 15 (40%) of those who received RIC. These results meshed with the severely abnormal semen analyses investigators found from those participants for whom a sample was available, Dr. Oquendo del Toro said.
For females, estradiol levels were significantly lower for those who had received MAC, with 7 of 11 (64%) of that group having abnormally low estradiol levels. The levels approached 0 pg/mL for many, said Dr. Oquendo del Toro. None of the eight patients who had received RIC had abnormally low estradiol levels (P = .0008).
“Male puberty is relatively well preserved after both myeloablative and reduced intensity conditioning, but there is a greater than 90% risk of male infertility associated with both reduced intensity and myeloablative conditioning for HSCT,” Dr. Oquendo del Toro said.
For females, the study paints a different picture. “We saw decreased premature ovarian failure after reduced intensity conditioning … but the fertility potential as assessed by anti-Müllerian hormone was decreased” after both conditioning regimens, she said.
Dr. Oquendo del Toro reported having no conflicts of interest.
SOURCE: Oquendo del Toro H et al. The 2018 BMT Tandem Meetings, Abstract 88.
SALT LAKE CITY – Both male and female recipients of childhood hematopoietic stem cell transplantation (HSCT) were very likely to have severely decreased fertility potential, even in the setting of preserved puberty, according to a recent study of adolescent and young adult HSCT recipients.
A reduced intensity conditioning regimen did not protect this cohort from decreased fertility, a finding that surprised the study’s lead author.
“We had hypothesized that, as compared to myeloablative conditioning, reduced intensity conditioning in children who received HSCT would lower the risk of infertility and lessen gonadal failure,” said Helen Oquendo del Toro, MD. In fact, Dr. Oquendo del Toro and her collaborators found that more than 90% of semen samples available for analysis had results that indicated infertility or severely impaired fertility, regardless of the type of pretransplant conditioning the patient had received.
The study highlights the need for fertility preservation when possible before HSCT, and makes clear that “normal puberty does not equate to normal fertility,” said Dr. Oquendo del Toro, of Cincinnati Children’s Hospital Medical Center.
Dr. Oquendo del Toro presented results of an observational cohort study of late effects of HSCT that included individuals aged 1-40 years old who received a single HSCT at, or after, 1 year of age.
Twenty-one males in the study had semen available for analysis. Of the 10 males who received myeloablative conditioning (MAC), 8 had azoospermia, and 2 more had oligoteratospermia (low sperm count with abnormal morphology). For the 11 males who received reduced intensity conditioning (RIC), eight had azoospermia, two had semen samples that showed oligoteratospermia, and one had a normal semen analysis.
The median age at transplant for these males was 14.5 years, and patients were a median of 19 years old at follow-up, Dr. Oquendo del Toro said at the combined annual meetings of the Center for International Blood & Marrow Transplant Research and the American Society for Blood and Marrow Transplantation.
For females in the study, low levels of anti-Müllerian hormone (AMH) – generally considered the best surrogate lab value for ovarian reserve – were nearly as common. Of 14 females receiving MAC, 13 (93%) had low AMH, as did 6 of 8 (75%) female patients who received RIC.
Individuals with more than one HSCT were excluded, as were those with Fanconi anemia, which itself carries a risk of gonadal failure. The study’s two aims were to investigate gonadal function as well as fertility potential after receipt of either RIC or MAC for HSCT.
Patients were seen by an endocrinologist who assessed testicular volume and assigned a Tanner stage. At age 11 and older, patients’ gonadal function was assessed on an annual basis by obtaining levels of luteinizing hormone and follicle stimulating hormone for all patients; female estradiol levels were tracked, as were male testosterone levels.
Assessment of fertility potential required additional laboratory testing: For females, the investigators obtained AMH levels, while for males, semen analysis was coupled with serum levels of inhibin B, an indicator of Sertoli cell function.
A total of 72 males were more than 1 year post-HSCT in the cohort, and of these, 41 were at least 11 years old and had achieved pubertal status according to laboratory evaluation. In all, 22 of the male patients received RIC, and 19 received MAC.
Males receiving MAC were a median 20 years old at their follow-up evaluation, and a median 6 years post-HSCT, while the RIC group were a median of 18.5 years old and 5.5 years out from their transplant.
Of the 50 females who were more than 1 year post-HSCT, 25 were pubertal and 11 years old or older. Nine of the female patients received RIC, and 16 received MAC.
Females who received MAC were a median 12.1 years old and 4.1 years post-HSCT at their follow-up evaluation. Females receiving RIC were a median 16 years old, and 6.5 years post-HSCT at the time of evaluation.
Patients received their transplants for a variety of malignant and nonmalignant conditions.
“We saw relatively normal gonadotropins after both reduced intensity and myeloablative conditioning in males,” Dr. Oquendo del Toro said. Of the MAC group, 4 of 15 (27%) had elevated follicle stimulating hormone levels, as did 2 of 17 (12%) of the RIC group. Elevated luteinizing hormone levels were seen in 2 of 15 (13%) of the MAC group and 1 of 17 (6%) of the RIC group. Four patients in each group had abnormally low testosterone levels.
However, when the investigators looked at inhibin B levels in males, they found abnormally low levels in 9 of 15 (60%) of those who received MAC, and in 6 of 15 (40%) of those who received RIC. These results meshed with the severely abnormal semen analyses investigators found from those participants for whom a sample was available, Dr. Oquendo del Toro said.
For females, estradiol levels were significantly lower for those who had received MAC, with 7 of 11 (64%) of that group having abnormally low estradiol levels. The levels approached 0 pg/mL for many, said Dr. Oquendo del Toro. None of the eight patients who had received RIC had abnormally low estradiol levels (P = .0008).
“Male puberty is relatively well preserved after both myeloablative and reduced intensity conditioning, but there is a greater than 90% risk of male infertility associated with both reduced intensity and myeloablative conditioning for HSCT,” Dr. Oquendo del Toro said.
For females, the study paints a different picture. “We saw decreased premature ovarian failure after reduced intensity conditioning … but the fertility potential as assessed by anti-Müllerian hormone was decreased” after both conditioning regimens, she said.
Dr. Oquendo del Toro reported having no conflicts of interest.
SOURCE: Oquendo del Toro H et al. The 2018 BMT Tandem Meetings, Abstract 88.
REPORTING FROM THE 2018 BMT TANDEM MEETINGS
Key clinical point:
Major finding: Of 21 males receiving reduced intensity conditioning or myeloablative conditioning, all but one had azoospermia or oligoteratospermia.
Study details: Observational cohort study of 41 males and 25 females receiving pediatric HSCT.
Disclosures: Dr. Oquendo del Toro reported having no conflicts of interest.
Source: Oquendo del Toro H et al. The 2018 BMT Tandem Meetings, Abstract 88.
Prompt palliative care cut hospital costs in pooled study
For adults with serious illness, consulting with a palliative care team within 3 days of hospital admission significantly reduced hospital costs, according to findings from a systematic review and meta-analysis.
In a pooled analysis of six cohort studies, average cost savings per admission were $3,237 (95% confidence interval, –$3,581 to −$2,893) overall, $4,251 for patients with cancer, and $2,105 for patients with other serious illnesses (all P values less than .001), reported Peter May, PhD, of Trinity College Dublin, and his associates.
In this latter group, prompt palliative care consultations saved more when patients had at least four comorbidities rather than two or fewer comorbidities, the reviewers wrote. The report was published in JAMA Internal Medicine.
About one in four Medicare beneficiaries dies in acute care hospitals, often after weeks of intensive, costly care that may not reflect personal wishes, according to an earlier study (JAMA. 2013;309:470-7). Economic studies have tried to pinpoint the cost savings of palliative care. These studies have found it important to consider both the clinical characteristics of patients and the amount of time between admission and palliative consultations, the reviewers noted. However, heterogeneity among older studies had precluded pooled analyses.
The six studies in this meta-analysis were identified by a search of Embase, PsycINFO, CENTRAL, PubMed, CINAHL, and EconLit databases for economic studies of hospital-based palliative care consultations. The studies were published between 2008 and 2017 and included 133,118 adults with cancer, chronic obstructive pulmonary disease, major organ failure, AIDS/HIV, or serious neurodegenerative disease. Patients tended to be in their 60s and were usually Medicare beneficiaries, although one study focused only on Medicaid enrollees. Forty-one percent of patients had a primary diagnosis of cancer, and 93% were discharged alive. Most also had at least two comorbidities. Only 3.6% received a palliative care consultation (range, 2.2% to 22.3%).
The link that they found between more comorbidities and greater cost savings “is the reverse of prior research that assumed that long-stay, high-cost hospitalized patients could not have their care trajectories affected by palliative care,” the researchers wrote. “Current palliative care provision in the United States is characterized by widespread understaffing. Our results suggest that acute care hospitals may be able to reduce costs for this population by increasing palliative care capacity to meet national guidelines.”
Dr. May received grant support from The Atlantic Philanthropies. The reviewers reported having no conflicts of interest.
SOURCE: May P et al. JAMA Intern Med. 2018 Apr 30. doi: 10.1001/jamainternmed.2018.0750.
For adults with serious illness, consulting with a palliative care team within 3 days of hospital admission significantly reduced hospital costs, according to findings from a systematic review and meta-analysis.
In a pooled analysis of six cohort studies, average cost savings per admission were $3,237 (95% confidence interval, –$3,581 to −$2,893) overall, $4,251 for patients with cancer, and $2,105 for patients with other serious illnesses (all P values less than .001), reported Peter May, PhD, of Trinity College Dublin, and his associates.
In this latter group, prompt palliative care consultations saved more when patients had at least four comorbidities rather than two or fewer comorbidities, the reviewers wrote. The report was published in JAMA Internal Medicine.
About one in four Medicare beneficiaries dies in acute care hospitals, often after weeks of intensive, costly care that may not reflect personal wishes, according to an earlier study (JAMA. 2013;309:470-7). Economic studies have tried to pinpoint the cost savings of palliative care. These studies have found it important to consider both the clinical characteristics of patients and the amount of time between admission and palliative consultations, the reviewers noted. However, heterogeneity among older studies had precluded pooled analyses.
The six studies in this meta-analysis were identified by a search of Embase, PsycINFO, CENTRAL, PubMed, CINAHL, and EconLit databases for economic studies of hospital-based palliative care consultations. The studies were published between 2008 and 2017 and included 133,118 adults with cancer, chronic obstructive pulmonary disease, major organ failure, AIDS/HIV, or serious neurodegenerative disease. Patients tended to be in their 60s and were usually Medicare beneficiaries, although one study focused only on Medicaid enrollees. Forty-one percent of patients had a primary diagnosis of cancer, and 93% were discharged alive. Most also had at least two comorbidities. Only 3.6% received a palliative care consultation (range, 2.2% to 22.3%).
The link that they found between more comorbidities and greater cost savings “is the reverse of prior research that assumed that long-stay, high-cost hospitalized patients could not have their care trajectories affected by palliative care,” the researchers wrote. “Current palliative care provision in the United States is characterized by widespread understaffing. Our results suggest that acute care hospitals may be able to reduce costs for this population by increasing palliative care capacity to meet national guidelines.”
Dr. May received grant support from The Atlantic Philanthropies. The reviewers reported having no conflicts of interest.
SOURCE: May P et al. JAMA Intern Med. 2018 Apr 30. doi: 10.1001/jamainternmed.2018.0750.
For adults with serious illness, consulting with a palliative care team within 3 days of hospital admission significantly reduced hospital costs, according to findings from a systematic review and meta-analysis.
In a pooled analysis of six cohort studies, average cost savings per admission were $3,237 (95% confidence interval, –$3,581 to −$2,893) overall, $4,251 for patients with cancer, and $2,105 for patients with other serious illnesses (all P values less than .001), reported Peter May, PhD, of Trinity College Dublin, and his associates.
In this latter group, prompt palliative care consultations saved more when patients had at least four comorbidities rather than two or fewer comorbidities, the reviewers wrote. The report was published in JAMA Internal Medicine.
About one in four Medicare beneficiaries dies in acute care hospitals, often after weeks of intensive, costly care that may not reflect personal wishes, according to an earlier study (JAMA. 2013;309:470-7). Economic studies have tried to pinpoint the cost savings of palliative care. These studies have found it important to consider both the clinical characteristics of patients and the amount of time between admission and palliative consultations, the reviewers noted. However, heterogeneity among older studies had precluded pooled analyses.
The six studies in this meta-analysis were identified by a search of Embase, PsycINFO, CENTRAL, PubMed, CINAHL, and EconLit databases for economic studies of hospital-based palliative care consultations. The studies were published between 2008 and 2017 and included 133,118 adults with cancer, chronic obstructive pulmonary disease, major organ failure, AIDS/HIV, or serious neurodegenerative disease. Patients tended to be in their 60s and were usually Medicare beneficiaries, although one study focused only on Medicaid enrollees. Forty-one percent of patients had a primary diagnosis of cancer, and 93% were discharged alive. Most also had at least two comorbidities. Only 3.6% received a palliative care consultation (range, 2.2% to 22.3%).
The link that they found between more comorbidities and greater cost savings “is the reverse of prior research that assumed that long-stay, high-cost hospitalized patients could not have their care trajectories affected by palliative care,” the researchers wrote. “Current palliative care provision in the United States is characterized by widespread understaffing. Our results suggest that acute care hospitals may be able to reduce costs for this population by increasing palliative care capacity to meet national guidelines.”
Dr. May received grant support from The Atlantic Philanthropies. The reviewers reported having no conflicts of interest.
SOURCE: May P et al. JAMA Intern Med. 2018 Apr 30. doi: 10.1001/jamainternmed.2018.0750.
FROM JAMA INTERNAL MEDICINE
Key clinical point:
Major finding: Average cost savings per admission were $3,237 overall, $4,251 for patients with cancer, and $2,105 for patients with other serious illnesses (all P-values less than .001).
Study details: Systematic review and meta-analysis of six cohort studies of 133,118 adults with cancer, chronic obstructive pulmonary disease, major organ failure, AIDS/HIV, or serious neurodegenerative disease.
Disclosures: Dr. May received grant support from The Atlantic Philanthropies. The reviewers reported having no conflicts of interest.
Source: May P et al. JAMA Intern Med. 2018 Apr 30. doi: 10.1001/jamainternmed.2018.0750.
Qualitative assessment of organizational barriers to optimal lung cancer care in a community hospital setting in the United States
Lung cancer is a major public health challenge in the United States. It is the leading cause of cancer death in the United States, accounting for 27% of all cancer deaths, and it has an aggregate 5-year survival rate of 18%.1 Advances in diagnostic and treatment options are rapidly increasing the complexity of lung cancer care delivery, which involves multiple specialty providers and often cuts across health care institutions.2-4 Navigating the process of care while coping with the complexities of the illness can be overwhelming for both the patient and the caregiver.5 With increasing regulations and cost-cutting measures, the health care system in the United States can pose many challenges, especially for those dealing with catastrophic and life-threatening illnesses. Any barrier to accessing care often increases anxiety in patients, who are already trying to cope with the management of their disease.6-8
The concept of barriers to quality care (such as the receipt of timely and appropriate diagnostic and staging work-up and treatment selection according to evidence-based guidelines) is generally used in the context of improving health care management or prevention programs.9-13 Barriers might include high costs, transportation, distance, underinsurance, limited hours for access to care, patient sharing by physicians, and a lack of access to information about physicians’ recommendations.10,14-16 Such barriers have been categorized as organizational (leadership and workforce), structural (process of care), clinical (provider-patient encounter), and macro (policy and population).17,18 Organizational barriers are defined as impediments encountered within the medical system and health care organizations when accessing, receiving, and delivering care.12 Several organizational barriers have been identified in the literature based on characteristics of the targeted population (eg, race, ethnicity, type of illness), key stakeholder views, and aspects of care (eg, screening, preventive practice, care, and treatment).
In a systematic review, Betancourt and colleagues reported provider-patient interactions, processes of care, and language as some of the barriers to receiving quality care.17 Although cancer screening has been shown to reduce mortality in the adult population for several types of cancer,19-21 barriers that impede access to services have been identified as emanating not only from the macro level (eg, age of screening, reimbursement problems, screening guidelines) or inter- and intra-individual levels (eg, awareness of screening, various perspectives on life and cancer, comorbidities, social support), but also from the organization (organizational infrastructure that inhibits screening because of limited participation in research trials) and provider levels (impaired communication regarding screening between patient and physician, low commitment to shared decision-making, provider’s awareness of screening and screening guidelines).18 Other organizational barriers, such as difficulty navigating the health care system, poor interaction between patients and medical staff, and language barriers, have been identified in a systematic review of breast cancer screening in immigrant and minority women.22
Other barriers to quality cancer care reported by patients include knowledge about the disease and treatment, poor communication with providers, lack of coordination and timeliness of care, and lack of attention to care. Providers have identified other barriers to quality care, which include a lack of access to care, reimbursement problems, poor psychosocial support services, accountability of care, provider workload, and inadequate patient education.23 Few qualitative studies have been conducted to understand the organizational barriers that lung cancer patients and their caregivers face within the health care system.
Through the use of focus groups, we sought the perspectives of lung cancer patients and their caregivers on the organizational barriers that they experience while navigating the health care system. Identifying and understanding these barriers can help health care professionals work with patients and their caregivers to alleviate these stressors in an already difficult time.3,24 In addition, a more thorough understanding of patients’ and caregivers’ perspectives on organizational barriers may help improve health care delivery and, thus, patient satisfaction.
Methods
With the approval of the Institutional Review Boards of the University of Memphis and the Baptist Memorial Health Care Corporation, we conducted focus groups with lung cancer patients and their informal caregivers to understand the challenges they encounter while navigating the health care system during their illness. The Baptist Memorial Health Care system is centered in the Mid-South region of the United States, which has some of the highest US lung cancer incidence rates.25
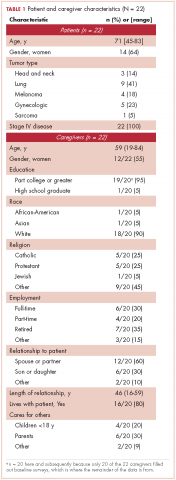
Research staff identified potential participants from a roster of patients provided by treatment clinics within the system. Patients eligible for this study had received care for suspected lung cancer within a community-based health care system within 6 months preceding the date of the focus group. Eligible patients were approached by the research staff by cold calling or in-person contact during clinic visits for their consent to participate in the study. From a compiled list of 219 patients, 89 received initial contact to gauge interest. Of those, 42 patients were formally approached and asked to participate; 22 agreed to participate, and 20 did not participate for reasons including illness, previous participation in other forms of patient feedback, lack of interest, failure to show up to focus group sessions, change of mind, lack of transportation, or other commitments. Patients identified their informal caregivers to form patient-caregiver dyads. All patients and caregivers provided written informed consent before participating in the focus groups.
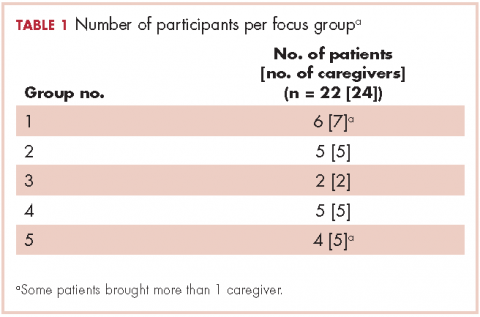
We conducted 10 focus groups during March 2013 through January 2014 – 5 with 22 patients and 5 with 24 caregivers (Table 1). Eight of the focus groups were conducted in Memphis, Tennessee, and to obtain the perspectives of patients from a rural setting, we conducted 2 focus groups in Grenada, Mississippi. All of the focus groups were facilitated by a medical anthropologist (SK) and a clinical psychologist (KDW), neither of whom was affiliated with the health care system. Each facilitator was accompanied by a note-taker. Patient-caregiver dyads came to the designated location together. Two focus groups (one for patients, the other for caregivers) were then conducted simultaneously in 2 separate rooms. The facilitators used a pilot-tested focus group interview guide during each session. The items in the focus group guide revolved around experience with the health care system in diagnosis and treatment; timeliness with appointments and procedures for diagnosis and subsequent care; physician communication in being informed about the disease, treatment, and getting questions answered; coordination of care; other challenges in receiving quality care; and suggestions for improving the patient and caregiver experience with the health care system.
The focus group sessions lasted 1 to 2 hours and were audiorecorded and transcribed verbatim. The data were analyzed by using Dedoose software version 5.0.11 (Sociocultural Research Consultants, Los Angeles, California). Data collection and analysis were conducted concurrently to achieve theoretical saturation. Creswell’s 7-step analysis framework was used as a guide to code and interpret the data.26 The process involved collecting raw data, preparing and organizing transcripts, reading the transcripts, coding the data with the help of qualitative software, analyzing the data for themes and subthemes, interpreting the themes, and devising the meaning of the themes.26 Initial codes were categorized and compared to determine recurrent themes. Three members of the research team independently reviewed the transcripts, extensively discussed the content, and developed consensus around the identified themes. Critical and rigorous steps were taken throughout data collection and analysis to ensure the credibility, transferability, dependability, and confirmability of the qualitative data.27-29 In addition, elements of the Consolidated Criteria for Reporting Qualitative Research checklist were used to strengthen the data collection, analysis, and reporting process.30
Results
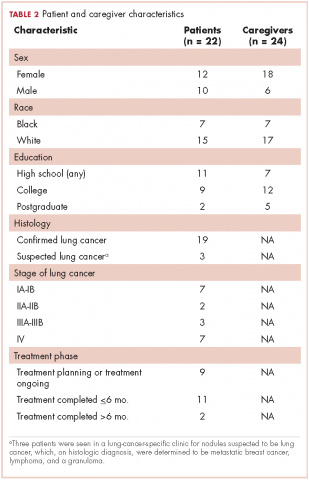
The 10 focus groups included 46 participants: 22 patients and 24 caregivers (some patients brought multiple caregivers). Of the 22 patients, 12 were women and 7 were black. An equal proportion of patients had at least a high school education as had a college or postgraduate degree. Although all of the patients had had a lung lesion suspicious for lung cancer, 19 eventually had a histologic diagnosis of lung cancer. The remaining 3 patients were all evaluated in a thoracic oncology clinic but were eventually found to have metastatic breast cancer, lymphoma, and a granuloma. Nine patients were either currently in the treatment decision-making process or actively receiving treatment, 11 had completed treatment within the preceding 6 months, and 2 had completed treatment more than 6 months previously. Treatment covered the spectrum from curative intent to palliative care. Of the 24 caregivers, 18 were women and 7 were black; 12 caregivers had at least a college education, of whom 2 had postgraduate degrees (Table 2).
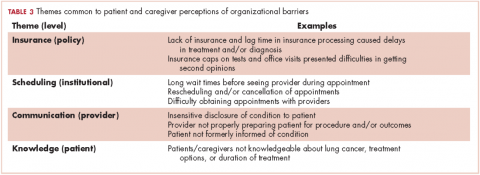
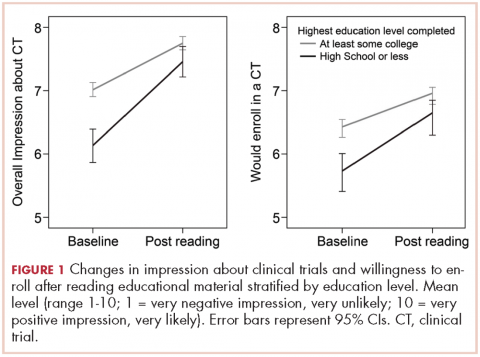
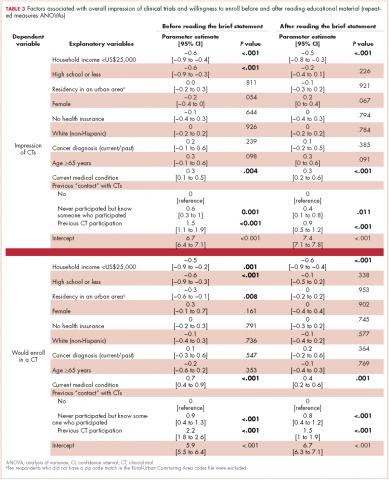
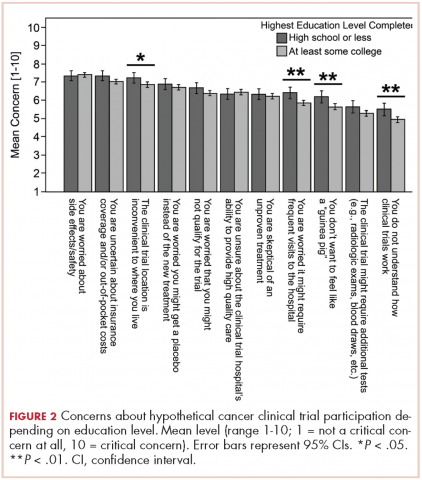
Based on participants’ feedback, we identified 4 main levels within the system where barriers to optimal care occurred: policy, institutional, provider, and patient. From our qualitative analyses, we identified a central theme associated with each level, around which the barriers coalesced. The themes were insurance, scheduling, provider communication, and patient knowledge. At the policy level, medical insurance was perceived to affect the timeliness of care and to be a deterrent to timely diagnosis and quality treatment. Lack of insurance was a daunting obstacle for indigent patients. However, even those who were insured felt that dealing with insurance companies was a significant barrier to care. At the institutional level, appointment scheduling caused problems for both patients and their caregivers. At the health care provider level, communication was perceived as a major problem. And finally, at the patient level, both patient and caregiver lack of knowledge of lung cancer and the processes inherent in lung cancer treatment were barriers for optimal diagnosis and treatment (Table 3).
Insurance barriers
At the policy level, health insurance was reported as a significant barrier to accessing health care. Patients and caregivers reported delays in diagnosis and/or treatment because of either lack of insurance or lag time in insurance processing of clinician requests. Insurance restrictions on tests, procedures, and office visits presented difficulties in getting additional opinions from providers regarding diagnosis, prognosis, or treatment plans. Some patients were no longer able to see providers after they had met the test or office visit limit allotted by insurance providers. One patient shared the following experience with office visits leading up to their lung cancer diagnosis:
Patient … with my insurance I just had only 12 office visits … and I had already maxed those out.
In other instances, insurers would not cover hospital or clinic visits if certain logistic protocols were not met. This would sometimes leave patients stranded for a period of time without receiving any care.
Caretaker … went home and went to one of those minor emergency clinics and they sent her to [xx hospital in another city]. There they did nothing. That was then over the weekend, and my son wanted to get her out of there to get her into practice because they weren’t doing anything. They said, ‘No. Your insurance won’t pay for it unless you stay here till we sign the papers to be transferred.’ … It was a bandage. Period.
Some insurers would not cover certain health services outside of routine testing protocols for the patients’ conditions. This lack of coverage caused patients to pay out of pocket for needed care.
Patient Nothing in the lymph nodes, but if it hadn’t been for me going ahead with this [coronary] calcium score, the insurance wasn’t gonna pay anything. If it wouldn’t been for the 79 bucks or the family situation, I wouldn’t be sitting [here] today.
Individuals who had not yet met the age requirement for Medicare reported being without insurance for a period of time, which contributed to delays in accessing care.
Caregiver [xx patient] probably … could’ve been diagnosed maybe even months ago, but she is in that in-between where she gets Social Security but she’s not 65 until November, so she has no insurance.
Scheduling barriers
At the institutional level, patients and caregivers reported problems with appointment scheduling. Logistic problems with adjusting work schedules and arranging for transportation as well as long wait times before evaluation by a provider were recurrent themes expressed by both patients and caregivers. Many had become resigned to the expectation of long wait times during appointments.
Patient I have to call the month before to make the appointment because they don’t take appointments so far—‘Oh, we’re not working on that yet.’ I find that very annoying ....
Caregiver The last time I was there I waited four hours.
Caregiver … your appointment at 9:00 and you get called back at 9:30 or 10:00 and you get to see the doctor by 11:00, but that’s not any different than anywhere, unfortunately ….
Rescheduled appointments also posed a problem for participants. Constant rescheduling was an inconvenience for both patients and caregivers. Many were unhappy with rescheduling because both patients and caregivers had prepared mentally and physically for an appointment, only to be told that they would have to reschedule, which caused delays in the care process.
Patient I think every single visit I had with him gets rescheduled at least twice ....
Caregiver Three times this week we’ve been geared up, ready to have chemo and they keep changing it.
Patient Everything was fine with me, but they keep cancelling my appointments ....
Some participants perceived that the popularity of physicians might explain the difficulty with scheduling. Patients suggested that it is challenging to get appointments with better-known physicians, so they are more accepting of appointments at any time, even if the time is inconvenient for them.
Caregiver Of course, … if you have a popular doctor, sometimes you don’t always get the appointment you want …
Participants also expressed frustration with the way appointments were rescheduled. They felt as though the physicians were not concerned about their lives outside of office visits.
Patient … patients actually have lives. Many of them have jobs or families or responsibilities.
Communication barriers
At the provider level, poor communication between health care providers and patients was perceived as a major impediment to the quality of care patients received. Both patients and caregivers emphasized the importance of open patient-provider interactions and that there was a lack of such open communications in many instances. There was concern regarding the way diagnoses or prognoses were relayed to patients. Many times, physicians were insensitive and disregarded the sentiments of the patients and caregivers when delivering news about the patients’ condition, as one caregiver shared,
Caregiver … the pulmonary man came … in the room and said, ‘Oh, don’t worry about your lungs. Something else will get you first,’ which was a very, very bad thing to say.
Participants also expressed concerns that they were not properly prepared for treatments by their physicians because vital information was not discussed. They felt as if physicians were not realistic about potential outcomes. This resulted in patients and caregivers being too optimistic and later disappointed when the outcome was not what they had originally expected.
Patient Until I got to this office, I was totally oversold on everything. I was told surgery … robotic, not invasive. Day one, surgery. Day two, tubes out. Day three, go home. I expected to be home on Sunday night, stir-frying vegetables, and making dinner, feeding my cat. I was in ICU four days … I went home with oxygen. I mean I thought I was just gonna walk outta there…. You take a little thing out and you put a Band-Aid on, and you go home.
Data also revealed that patients were unsure of their condition, even following treatment. Information was not communicated to patients about the specifics of their disease, either because of miscommunication or minimal patient-provider time spent during office visits. This lack of communication between patients and providers often left patients and caregivers uncertain about exactly what condition they had or what they were being treated for.
Patient I just can’t have the time with Dr. xx, cuz he’s so busy...
Patient … I didn’t understand. Which exactly what type of cancer did I have, cuz I’m—really to tell you the truth—I’m still wondering.
Knowledge barriers
Patients and caregivers also identified a lack of education and knowledge about lung cancer diagnosis and treatment as a barrier to their care. Patients and caregivers were not always fully knowledgeable about lung cancer, treatment options, or the duration of treatments. They relied on the provider to disclose such information or direct them to credible sources. In many instances, patients were misinformed about the causes of lung cancer. There were misconceptions that lung cancer was only caused by a history of smoking or genetic predisposition. Patients who did not smoke or did not have a family history of lung cancer were often confused and dismayed by the diagnosis.
Patient I was trying to figure out, why do I have lung cancer. Never smoked a day in my life.
Patients were often unaware of treatment options or side effects of various treatments. They relied on physicians to relay information and make decisions for them about treatment plans.
Patient I was told chemo would probably be the best thing for me, and I just had faith that Dr. xx knows more about it than me.
Patient I’m doing chemo but it’s — what I’m doing is different. Of course, I don’t know anything about it actually either. It’s what I hear from other people.
Other patients relied on their own sources for information about their condition, either through the Internet, from family members and/or friends, or from preconceived notions.
Patient Well — of course in the meantime, I read — because they said it was a small cell, very aggressive, so I felt like everything I read — that’s the deal. I think we’ve become where we can get on the internet and look up so much, that to me, I was gonna be gone.
Patient When he said, ‘Cancer,’ I said, ‘Well, I thought cancer was a heredity thing? That you have to have somebody in your family that has it…’
Discussion
Organizational barriers are an important consideration in the delivery and receipt of high-quality, patient-centered lung cancer care. This qualitative study of patients being treated for lung cancer and their informal caregivers revealed several common perceived organizational barriers to receiving care, including health insurance coverage restrictions, appointment scheduling difficulties, quality of communication with physicians, and failure to properly educate the patient and family about the disease and what to expect of the treatment process.
The provider communication and patient knowledge barriers seem to reinforce each other and could be improved through focused efforts on the quality of communication between patients and their caregivers and clinical care providers. Patients expect, but are often deprived of, open and active dialogue with their providers. Improved communication can be helpful in educating patients and their caregivers about their disease, prognosis, and treatment goals. Although communication ranks highly as a patient and caregiver priority, there is often a disconnect between patients and caregivers and their physicians.31 Patients and caregivers often want to be more involved in the decision-making process, and effective communication between physicians and patients has been linked to the patient’s ability to understand, and also receive high-quality care.32,33 Failure to communicate effectively and educate patients on key aspects of their condition strips them of their autonomy in decision-making.
The involvement of a navigator for patients being treated for lung cancer could be pivotal in relieving the communication and scheduling barriers. The nurse navigator assists with coordinating effective communication and providing needed information between providers and patients and their caregivers. A navigator also serves as a single point of contact for patients and caregivers to communicate questions outside of physician visits or concerns that may not be urgent enough to warrant immediate physician response.34 The navigator coordinates patients’ appointment schedules and physician referrals and communicates the details of the next steps in the care-delivery process. This helps remove the barriers to care and improve patient outcomes and the quality of health care delivery, especially for patients and caregivers dealing with a life-threatening illness within a complex referral process.35
Multidisciplinary care, a much-recommended alternative care-delivery model, should, in theory, promote connectivity of providers and collaboration between providers, patients, and family members. This model could help reduce barriers for patients and caregivers.3,24,36 A network of connected providers can better coordinate treatment plans, easily share test results, and provide built-in second opinions. Given the increasingly multimodal approach to the diagnosis, staging, and treatment of lung cancer, the multidisciplinary model could allow physicians to consider multiple perspectives and care-delivery options and, ideally, develop consensus around the optimal approach for each individual patient in one setting. This can shorten the length of time before treatment and establish a plan that is tailored to the patient’s needs.24
The National Academy of Medicine (formerly, Institute of Medicine) proposes that modern health care systems have 6 aims for quality improvement: safety, effectiveness, patient-centeredness, timeliness, efficiency, and equity.37 It would take changes in the design and implementation of organizational support systems at the policy, institutional, and provider level for those aims to be achieved. Further investigation of the problems identified by patients and caregivers could lead to innovative solutions to improve lung cancer care. Future work should evaluate the most effective communication styles in patient-provider interactions, particularly in regard to to lung cancer diagnosis and treatment, and investigate how multidisciplinary models influence patient-provider communication and patient care.
Limitations
This study has several limitations. Less than half of those approached for the study participated in the study for various reasons, which may have introduced selection bias in terms of not having the perspectives of patients not willing or able to participate in the study. Though focus groups are known to generate rich in-depth views of certain issues, they have been criticized as potentially lacking rigor and generalizability. To address this concern, we used a standardized script for each focus group and involved multiple members of the research team in data analysis and interpretation. Also, this study enrolled participants from a single health care institution and did not use a comparison group. There might be institutional and geographic differences in the experience of lung cancer care, which might further limit the generalizability of the results of this study.
Conclusions
Despite those limitations, this study offers valuable insight into the barriers that lung cancer patients and caregivers encounter while navigating a community-level health care system. Eliminating or minimizing these barriers will require strategic plans that help mitigate insurance-related, scheduling, provider-patient communication, and patient/caregiver knowledge acquisition problems and translate them into tactical actions for quality improvement. This is one of the first qualitative studies conducted to understand the organizational barriers that lung cancer patients and their caregivers face within a health care system. Additional research is needed to explore these barriers and develop viable solutions.
Disclaimer
All statements in this report, including its findings and conclusions, are solely those of the authors and do not necessarily represent the views of the Patient-Centered Outcomes Research Institute or its Board of Governors or Methodology Committee.
1. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin. 2016;66(1):7-30.
2. Riedel RF, Wang X, McCormack M, et al. Impact of a multidisciplinary thoracic oncology clinic on the timeliness of care. J Thorac Oncol. 2006;1(7):692-696.
3. Seek A, Hogle WP. Modeling a better way: navigating the health care system for patients with lung cancer. Clin J Oncol Nurs. 2007;11(1):81-85.
4. Sorensen R, Iedema R. Redefining accountability in health care: managing the plurality of medical interests. Health. 2008;12(1):87-106.
5. Mosher CE, Jaynes HA, Hanna N, Ostroff JS. Distressed family caregivers of lung cancer patients: an examination of psychosocial and practical challenges. Support Care Cancer. 2013;21(2):431-437.
6. Cantril C, Haylock PJ. Patient navigation in the oncology care setting. Semin Oncol Nurs. 2013;29(2):76-90.
7. Freeman HP. Patient navigation: a community based strategy to reduce cancer disparities. J Urban Health. 2006;83(2):139-141.
8. Kwon DH, Tisnado DM, Keating NL, et al. Physician reported barriers to referring cancer patients to specialists: prevalence, factors, and association with career satisfaction. Cancer. 2015;121(1):113-122.
9. Andersen SE. Implementation of a new prescription system. A qualitative survey of organizational barriers. Ugeskr Laeger. 2002;164(38):4449-4453.
10. Rousseau L, Guay M, Archambault D, El m’ala Z, Abdelaziz N. Do organizational barriers to pneumococcal and influenza vaccine access exist? Can J Public Health. 2007;98(2):105-110.
11. Storey J, Buchanan D. Health care governance and organizational barriers to learning from mistakes. J Health Organ Manag. 2008;22(6):642-651.
12. Ziegenfuss JT Jr. Organizational barriers to quality improvement in medical and health care organizations. Qual Assur Util Rev. 1991;6(4):115-122.
13. Rihari-Thomas J, DiGiacomo M, Phillips J, Newton P, Davidson PM. Clinician perspectives of barriers to effective implementation of a rapid response system in an academic health centre: a focus group study. Int J Health Policy Manag. 2017;6(8):447-456.
14. Dutton D. Financial, organizational and professional factors affecting health care utilization. Soc Sci Med. 1986;23(7):721-735.
15. King CJ, Chen J, Dagher RK, Holt CL, Thomas SB. Decomposing differences in medical care access among cancer survivors by race and ethnicity. Am J Med Qual. 2015;30(5):459-469.
16. Renzaho AM, Romios P, Crock C, Sønderlund AL. The effectiveness of cultural competence programs in ethnic minority patient-centered health care—a systematic review of the literature. Int J Qual Health Care. 2013;25(3):261-269.
17. Betancourt JR, Green AR, Carrillo JE, Ananeh-Firempong O. Defining cultural competence: a practical framework for addressing racial/ethnic disparities in health and health care. Public Health Rep. 2003;118(4):293-302.
18. Scheinfeld Gorin S, Gauthier J, Hay J, Miles A, Wardle J. Cancer screening and aging: research barriers and opportunities. Cancer. 2008;113(suppl 12):3493-3504.
19. Humphrey L, Deffebach M, Pappas M, et al. Screening for lung cancer: systematic review to update the US preventive services task force recommendation. Evidence syntheses No. 105. Rockville, MD: Agency for Health Care Research and Quality; 2013.
20. Nelson HD, Fu R, Cantor A, Pappas M, Daeges M, Humphrey L. Effectiveness of breast cancer screening: systematic review and meta-analysis to update the 2009 U.S. Preventive Services Task Force Recommendation. Ann Intern Med. 2016;164(4):244-255.
21. Zauber AG. The impact of screening on colorectal cancer mortality and incidence: has it really made a difference? Dig Dis Sci. 2015;60(3):681-691.
22. Remennick L. The challenge of early breast cancer detection among immigrant and minority women in multicultural societies. Breast J. 2006;12(suppl 1):103-110.
23. Hess LM, Pohl G. Perspectives of quality care in cancer treatment: a review of the literature. Am Health Drug Benefits. 2013;6(6):321-329.
24. Kedia SK, Ward KD, Digney SA, et al. ‘One-stop shop’: lung cancer patients’ and caregivers’ perceptions of multidisciplinary care in a community health care setting. Transl Lung Cancer Res. 2015;4(4):456-464.
25. US Cancer Statistics Working Group. United States cancer statistics: 1999-2014 incidence and mortality web-based report. Atlanta, GA: Department of Health and Human Services, Centers for Disease Control and Prevention, and National Cancer Institute. http://www.cdc.gov/uscs. Published 2017. Accessed November 21, 2017.
26. Creswell JW. Research design: qualitative, quantitative, and mixed methods approaches. 4th ed. Thousand Oaks, CA: SAGE Publications; 2014.
27. Ballinger C. Demonstrating rigour and quality? In Finlay L, Ballinger C, eds. Qualitative research for allied health professionals: challenging choices. Chichester, England: John Wiley & Sons Ltd; 2006:235-246.
28. Finlay L. ‘Rigour’, ‘ethical integrity’ or ‘artistry’? Reflexively reviewing criteria for evaluating qualitative research. Br J Occup Ther. 2006;69(7):319-326.
29. Lincoln YS, Guba EG. Naturalistic inquiry. Newbury Park, CA: SAGE Publications; 1985.
30. Tong A, Sainsbury P, Craig J. Consolidated criteria for reporting qualitative research (COREQ): a 32-item checklist for interviews and focus group. Int J Qual Health Care. 2007;19(6):349-357.
31. Neeman N, Quinn K, Shoeb M, Mourad M, Sehgal NL, Sliwka D. Postdischarge focus groups to improve the hospital experience. Am J Med Qual. 2013;28(6):536-538.
32. O’Day BL, Killeen M, Iezzoni LI. Improving health care experiences of persons who are blind or have low vision: suggestions from focus groups. Am J Med Qual. 2004;19(5):193-200.
33. Smith B, Lynch WD, Markow C, Lifsey S, Slover M. Consumers’ understanding of and interest in provider- versus practice-level quality characteristics: findings from a focus group study. Am J Med Qual. 2015;30(4):367-373.
34. Islam KM, Opoku ST, Apenteng BA, et al. Coping with an advanced stage lung cancer diagnosis: patient, caregiver, and provider perspectives on the role of the health care system. J Cancer Educ. 2016;31(3):554-558.
35. Pedersen A, Hack TF. Pilots of oncology health care: a concept analysis of the patient navigator role. Oncol Nurs Forum. 2010;37(1):55-60.
36. Gopal R. How to maintain multidisciplinary treatment schedules. J Support Oncol. 2005;3(3):248-256.
37. Committee on Quality of Health Care in America. Improving the 21st century health care system. In: Institute of Medicine, ed. Crossing the quality chasm: a new health system for the 21st century. Washington, DC: National Academies Press; 2001:39-60.
Lung cancer is a major public health challenge in the United States. It is the leading cause of cancer death in the United States, accounting for 27% of all cancer deaths, and it has an aggregate 5-year survival rate of 18%.1 Advances in diagnostic and treatment options are rapidly increasing the complexity of lung cancer care delivery, which involves multiple specialty providers and often cuts across health care institutions.2-4 Navigating the process of care while coping with the complexities of the illness can be overwhelming for both the patient and the caregiver.5 With increasing regulations and cost-cutting measures, the health care system in the United States can pose many challenges, especially for those dealing with catastrophic and life-threatening illnesses. Any barrier to accessing care often increases anxiety in patients, who are already trying to cope with the management of their disease.6-8
The concept of barriers to quality care (such as the receipt of timely and appropriate diagnostic and staging work-up and treatment selection according to evidence-based guidelines) is generally used in the context of improving health care management or prevention programs.9-13 Barriers might include high costs, transportation, distance, underinsurance, limited hours for access to care, patient sharing by physicians, and a lack of access to information about physicians’ recommendations.10,14-16 Such barriers have been categorized as organizational (leadership and workforce), structural (process of care), clinical (provider-patient encounter), and macro (policy and population).17,18 Organizational barriers are defined as impediments encountered within the medical system and health care organizations when accessing, receiving, and delivering care.12 Several organizational barriers have been identified in the literature based on characteristics of the targeted population (eg, race, ethnicity, type of illness), key stakeholder views, and aspects of care (eg, screening, preventive practice, care, and treatment).
In a systematic review, Betancourt and colleagues reported provider-patient interactions, processes of care, and language as some of the barriers to receiving quality care.17 Although cancer screening has been shown to reduce mortality in the adult population for several types of cancer,19-21 barriers that impede access to services have been identified as emanating not only from the macro level (eg, age of screening, reimbursement problems, screening guidelines) or inter- and intra-individual levels (eg, awareness of screening, various perspectives on life and cancer, comorbidities, social support), but also from the organization (organizational infrastructure that inhibits screening because of limited participation in research trials) and provider levels (impaired communication regarding screening between patient and physician, low commitment to shared decision-making, provider’s awareness of screening and screening guidelines).18 Other organizational barriers, such as difficulty navigating the health care system, poor interaction between patients and medical staff, and language barriers, have been identified in a systematic review of breast cancer screening in immigrant and minority women.22
Other barriers to quality cancer care reported by patients include knowledge about the disease and treatment, poor communication with providers, lack of coordination and timeliness of care, and lack of attention to care. Providers have identified other barriers to quality care, which include a lack of access to care, reimbursement problems, poor psychosocial support services, accountability of care, provider workload, and inadequate patient education.23 Few qualitative studies have been conducted to understand the organizational barriers that lung cancer patients and their caregivers face within the health care system.
Through the use of focus groups, we sought the perspectives of lung cancer patients and their caregivers on the organizational barriers that they experience while navigating the health care system. Identifying and understanding these barriers can help health care professionals work with patients and their caregivers to alleviate these stressors in an already difficult time.3,24 In addition, a more thorough understanding of patients’ and caregivers’ perspectives on organizational barriers may help improve health care delivery and, thus, patient satisfaction.
Methods
With the approval of the Institutional Review Boards of the University of Memphis and the Baptist Memorial Health Care Corporation, we conducted focus groups with lung cancer patients and their informal caregivers to understand the challenges they encounter while navigating the health care system during their illness. The Baptist Memorial Health Care system is centered in the Mid-South region of the United States, which has some of the highest US lung cancer incidence rates.25
Research staff identified potential participants from a roster of patients provided by treatment clinics within the system. Patients eligible for this study had received care for suspected lung cancer within a community-based health care system within 6 months preceding the date of the focus group. Eligible patients were approached by the research staff by cold calling or in-person contact during clinic visits for their consent to participate in the study. From a compiled list of 219 patients, 89 received initial contact to gauge interest. Of those, 42 patients were formally approached and asked to participate; 22 agreed to participate, and 20 did not participate for reasons including illness, previous participation in other forms of patient feedback, lack of interest, failure to show up to focus group sessions, change of mind, lack of transportation, or other commitments. Patients identified their informal caregivers to form patient-caregiver dyads. All patients and caregivers provided written informed consent before participating in the focus groups.
We conducted 10 focus groups during March 2013 through January 2014 – 5 with 22 patients and 5 with 24 caregivers (Table 1). Eight of the focus groups were conducted in Memphis, Tennessee, and to obtain the perspectives of patients from a rural setting, we conducted 2 focus groups in Grenada, Mississippi. All of the focus groups were facilitated by a medical anthropologist (SK) and a clinical psychologist (KDW), neither of whom was affiliated with the health care system. Each facilitator was accompanied by a note-taker. Patient-caregiver dyads came to the designated location together. Two focus groups (one for patients, the other for caregivers) were then conducted simultaneously in 2 separate rooms. The facilitators used a pilot-tested focus group interview guide during each session. The items in the focus group guide revolved around experience with the health care system in diagnosis and treatment; timeliness with appointments and procedures for diagnosis and subsequent care; physician communication in being informed about the disease, treatment, and getting questions answered; coordination of care; other challenges in receiving quality care; and suggestions for improving the patient and caregiver experience with the health care system.
The focus group sessions lasted 1 to 2 hours and were audiorecorded and transcribed verbatim. The data were analyzed by using Dedoose software version 5.0.11 (Sociocultural Research Consultants, Los Angeles, California). Data collection and analysis were conducted concurrently to achieve theoretical saturation. Creswell’s 7-step analysis framework was used as a guide to code and interpret the data.26 The process involved collecting raw data, preparing and organizing transcripts, reading the transcripts, coding the data with the help of qualitative software, analyzing the data for themes and subthemes, interpreting the themes, and devising the meaning of the themes.26 Initial codes were categorized and compared to determine recurrent themes. Three members of the research team independently reviewed the transcripts, extensively discussed the content, and developed consensus around the identified themes. Critical and rigorous steps were taken throughout data collection and analysis to ensure the credibility, transferability, dependability, and confirmability of the qualitative data.27-29 In addition, elements of the Consolidated Criteria for Reporting Qualitative Research checklist were used to strengthen the data collection, analysis, and reporting process.30
Results
The 10 focus groups included 46 participants: 22 patients and 24 caregivers (some patients brought multiple caregivers). Of the 22 patients, 12 were women and 7 were black. An equal proportion of patients had at least a high school education as had a college or postgraduate degree. Although all of the patients had had a lung lesion suspicious for lung cancer, 19 eventually had a histologic diagnosis of lung cancer. The remaining 3 patients were all evaluated in a thoracic oncology clinic but were eventually found to have metastatic breast cancer, lymphoma, and a granuloma. Nine patients were either currently in the treatment decision-making process or actively receiving treatment, 11 had completed treatment within the preceding 6 months, and 2 had completed treatment more than 6 months previously. Treatment covered the spectrum from curative intent to palliative care. Of the 24 caregivers, 18 were women and 7 were black; 12 caregivers had at least a college education, of whom 2 had postgraduate degrees (Table 2).
Based on participants’ feedback, we identified 4 main levels within the system where barriers to optimal care occurred: policy, institutional, provider, and patient. From our qualitative analyses, we identified a central theme associated with each level, around which the barriers coalesced. The themes were insurance, scheduling, provider communication, and patient knowledge. At the policy level, medical insurance was perceived to affect the timeliness of care and to be a deterrent to timely diagnosis and quality treatment. Lack of insurance was a daunting obstacle for indigent patients. However, even those who were insured felt that dealing with insurance companies was a significant barrier to care. At the institutional level, appointment scheduling caused problems for both patients and their caregivers. At the health care provider level, communication was perceived as a major problem. And finally, at the patient level, both patient and caregiver lack of knowledge of lung cancer and the processes inherent in lung cancer treatment were barriers for optimal diagnosis and treatment (Table 3).
Insurance barriers
At the policy level, health insurance was reported as a significant barrier to accessing health care. Patients and caregivers reported delays in diagnosis and/or treatment because of either lack of insurance or lag time in insurance processing of clinician requests. Insurance restrictions on tests, procedures, and office visits presented difficulties in getting additional opinions from providers regarding diagnosis, prognosis, or treatment plans. Some patients were no longer able to see providers after they had met the test or office visit limit allotted by insurance providers. One patient shared the following experience with office visits leading up to their lung cancer diagnosis:
Patient … with my insurance I just had only 12 office visits … and I had already maxed those out.
In other instances, insurers would not cover hospital or clinic visits if certain logistic protocols were not met. This would sometimes leave patients stranded for a period of time without receiving any care.
Caretaker … went home and went to one of those minor emergency clinics and they sent her to [xx hospital in another city]. There they did nothing. That was then over the weekend, and my son wanted to get her out of there to get her into practice because they weren’t doing anything. They said, ‘No. Your insurance won’t pay for it unless you stay here till we sign the papers to be transferred.’ … It was a bandage. Period.
Some insurers would not cover certain health services outside of routine testing protocols for the patients’ conditions. This lack of coverage caused patients to pay out of pocket for needed care.
Patient Nothing in the lymph nodes, but if it hadn’t been for me going ahead with this [coronary] calcium score, the insurance wasn’t gonna pay anything. If it wouldn’t been for the 79 bucks or the family situation, I wouldn’t be sitting [here] today.
Individuals who had not yet met the age requirement for Medicare reported being without insurance for a period of time, which contributed to delays in accessing care.
Caregiver [xx patient] probably … could’ve been diagnosed maybe even months ago, but she is in that in-between where she gets Social Security but she’s not 65 until November, so she has no insurance.
Scheduling barriers
At the institutional level, patients and caregivers reported problems with appointment scheduling. Logistic problems with adjusting work schedules and arranging for transportation as well as long wait times before evaluation by a provider were recurrent themes expressed by both patients and caregivers. Many had become resigned to the expectation of long wait times during appointments.
Patient I have to call the month before to make the appointment because they don’t take appointments so far—‘Oh, we’re not working on that yet.’ I find that very annoying ....
Caregiver The last time I was there I waited four hours.
Caregiver … your appointment at 9:00 and you get called back at 9:30 or 10:00 and you get to see the doctor by 11:00, but that’s not any different than anywhere, unfortunately ….
Rescheduled appointments also posed a problem for participants. Constant rescheduling was an inconvenience for both patients and caregivers. Many were unhappy with rescheduling because both patients and caregivers had prepared mentally and physically for an appointment, only to be told that they would have to reschedule, which caused delays in the care process.
Patient I think every single visit I had with him gets rescheduled at least twice ....
Caregiver Three times this week we’ve been geared up, ready to have chemo and they keep changing it.
Patient Everything was fine with me, but they keep cancelling my appointments ....
Some participants perceived that the popularity of physicians might explain the difficulty with scheduling. Patients suggested that it is challenging to get appointments with better-known physicians, so they are more accepting of appointments at any time, even if the time is inconvenient for them.
Caregiver Of course, … if you have a popular doctor, sometimes you don’t always get the appointment you want …
Participants also expressed frustration with the way appointments were rescheduled. They felt as though the physicians were not concerned about their lives outside of office visits.
Patient … patients actually have lives. Many of them have jobs or families or responsibilities.
Communication barriers
At the provider level, poor communication between health care providers and patients was perceived as a major impediment to the quality of care patients received. Both patients and caregivers emphasized the importance of open patient-provider interactions and that there was a lack of such open communications in many instances. There was concern regarding the way diagnoses or prognoses were relayed to patients. Many times, physicians were insensitive and disregarded the sentiments of the patients and caregivers when delivering news about the patients’ condition, as one caregiver shared,
Caregiver … the pulmonary man came … in the room and said, ‘Oh, don’t worry about your lungs. Something else will get you first,’ which was a very, very bad thing to say.
Participants also expressed concerns that they were not properly prepared for treatments by their physicians because vital information was not discussed. They felt as if physicians were not realistic about potential outcomes. This resulted in patients and caregivers being too optimistic and later disappointed when the outcome was not what they had originally expected.
Patient Until I got to this office, I was totally oversold on everything. I was told surgery … robotic, not invasive. Day one, surgery. Day two, tubes out. Day three, go home. I expected to be home on Sunday night, stir-frying vegetables, and making dinner, feeding my cat. I was in ICU four days … I went home with oxygen. I mean I thought I was just gonna walk outta there…. You take a little thing out and you put a Band-Aid on, and you go home.
Data also revealed that patients were unsure of their condition, even following treatment. Information was not communicated to patients about the specifics of their disease, either because of miscommunication or minimal patient-provider time spent during office visits. This lack of communication between patients and providers often left patients and caregivers uncertain about exactly what condition they had or what they were being treated for.
Patient I just can’t have the time with Dr. xx, cuz he’s so busy...
Patient … I didn’t understand. Which exactly what type of cancer did I have, cuz I’m—really to tell you the truth—I’m still wondering.
Knowledge barriers
Patients and caregivers also identified a lack of education and knowledge about lung cancer diagnosis and treatment as a barrier to their care. Patients and caregivers were not always fully knowledgeable about lung cancer, treatment options, or the duration of treatments. They relied on the provider to disclose such information or direct them to credible sources. In many instances, patients were misinformed about the causes of lung cancer. There were misconceptions that lung cancer was only caused by a history of smoking or genetic predisposition. Patients who did not smoke or did not have a family history of lung cancer were often confused and dismayed by the diagnosis.
Patient I was trying to figure out, why do I have lung cancer. Never smoked a day in my life.
Patients were often unaware of treatment options or side effects of various treatments. They relied on physicians to relay information and make decisions for them about treatment plans.
Patient I was told chemo would probably be the best thing for me, and I just had faith that Dr. xx knows more about it than me.
Patient I’m doing chemo but it’s — what I’m doing is different. Of course, I don’t know anything about it actually either. It’s what I hear from other people.
Other patients relied on their own sources for information about their condition, either through the Internet, from family members and/or friends, or from preconceived notions.
Patient Well — of course in the meantime, I read — because they said it was a small cell, very aggressive, so I felt like everything I read — that’s the deal. I think we’ve become where we can get on the internet and look up so much, that to me, I was gonna be gone.
Patient When he said, ‘Cancer,’ I said, ‘Well, I thought cancer was a heredity thing? That you have to have somebody in your family that has it…’
Discussion
Organizational barriers are an important consideration in the delivery and receipt of high-quality, patient-centered lung cancer care. This qualitative study of patients being treated for lung cancer and their informal caregivers revealed several common perceived organizational barriers to receiving care, including health insurance coverage restrictions, appointment scheduling difficulties, quality of communication with physicians, and failure to properly educate the patient and family about the disease and what to expect of the treatment process.
The provider communication and patient knowledge barriers seem to reinforce each other and could be improved through focused efforts on the quality of communication between patients and their caregivers and clinical care providers. Patients expect, but are often deprived of, open and active dialogue with their providers. Improved communication can be helpful in educating patients and their caregivers about their disease, prognosis, and treatment goals. Although communication ranks highly as a patient and caregiver priority, there is often a disconnect between patients and caregivers and their physicians.31 Patients and caregivers often want to be more involved in the decision-making process, and effective communication between physicians and patients has been linked to the patient’s ability to understand, and also receive high-quality care.32,33 Failure to communicate effectively and educate patients on key aspects of their condition strips them of their autonomy in decision-making.
The involvement of a navigator for patients being treated for lung cancer could be pivotal in relieving the communication and scheduling barriers. The nurse navigator assists with coordinating effective communication and providing needed information between providers and patients and their caregivers. A navigator also serves as a single point of contact for patients and caregivers to communicate questions outside of physician visits or concerns that may not be urgent enough to warrant immediate physician response.34 The navigator coordinates patients’ appointment schedules and physician referrals and communicates the details of the next steps in the care-delivery process. This helps remove the barriers to care and improve patient outcomes and the quality of health care delivery, especially for patients and caregivers dealing with a life-threatening illness within a complex referral process.35
Multidisciplinary care, a much-recommended alternative care-delivery model, should, in theory, promote connectivity of providers and collaboration between providers, patients, and family members. This model could help reduce barriers for patients and caregivers.3,24,36 A network of connected providers can better coordinate treatment plans, easily share test results, and provide built-in second opinions. Given the increasingly multimodal approach to the diagnosis, staging, and treatment of lung cancer, the multidisciplinary model could allow physicians to consider multiple perspectives and care-delivery options and, ideally, develop consensus around the optimal approach for each individual patient in one setting. This can shorten the length of time before treatment and establish a plan that is tailored to the patient’s needs.24
The National Academy of Medicine (formerly, Institute of Medicine) proposes that modern health care systems have 6 aims for quality improvement: safety, effectiveness, patient-centeredness, timeliness, efficiency, and equity.37 It would take changes in the design and implementation of organizational support systems at the policy, institutional, and provider level for those aims to be achieved. Further investigation of the problems identified by patients and caregivers could lead to innovative solutions to improve lung cancer care. Future work should evaluate the most effective communication styles in patient-provider interactions, particularly in regard to to lung cancer diagnosis and treatment, and investigate how multidisciplinary models influence patient-provider communication and patient care.
Limitations
This study has several limitations. Less than half of those approached for the study participated in the study for various reasons, which may have introduced selection bias in terms of not having the perspectives of patients not willing or able to participate in the study. Though focus groups are known to generate rich in-depth views of certain issues, they have been criticized as potentially lacking rigor and generalizability. To address this concern, we used a standardized script for each focus group and involved multiple members of the research team in data analysis and interpretation. Also, this study enrolled participants from a single health care institution and did not use a comparison group. There might be institutional and geographic differences in the experience of lung cancer care, which might further limit the generalizability of the results of this study.
Conclusions
Despite those limitations, this study offers valuable insight into the barriers that lung cancer patients and caregivers encounter while navigating a community-level health care system. Eliminating or minimizing these barriers will require strategic plans that help mitigate insurance-related, scheduling, provider-patient communication, and patient/caregiver knowledge acquisition problems and translate them into tactical actions for quality improvement. This is one of the first qualitative studies conducted to understand the organizational barriers that lung cancer patients and their caregivers face within a health care system. Additional research is needed to explore these barriers and develop viable solutions.
Disclaimer
All statements in this report, including its findings and conclusions, are solely those of the authors and do not necessarily represent the views of the Patient-Centered Outcomes Research Institute or its Board of Governors or Methodology Committee.
Lung cancer is a major public health challenge in the United States. It is the leading cause of cancer death in the United States, accounting for 27% of all cancer deaths, and it has an aggregate 5-year survival rate of 18%.1 Advances in diagnostic and treatment options are rapidly increasing the complexity of lung cancer care delivery, which involves multiple specialty providers and often cuts across health care institutions.2-4 Navigating the process of care while coping with the complexities of the illness can be overwhelming for both the patient and the caregiver.5 With increasing regulations and cost-cutting measures, the health care system in the United States can pose many challenges, especially for those dealing with catastrophic and life-threatening illnesses. Any barrier to accessing care often increases anxiety in patients, who are already trying to cope with the management of their disease.6-8
The concept of barriers to quality care (such as the receipt of timely and appropriate diagnostic and staging work-up and treatment selection according to evidence-based guidelines) is generally used in the context of improving health care management or prevention programs.9-13 Barriers might include high costs, transportation, distance, underinsurance, limited hours for access to care, patient sharing by physicians, and a lack of access to information about physicians’ recommendations.10,14-16 Such barriers have been categorized as organizational (leadership and workforce), structural (process of care), clinical (provider-patient encounter), and macro (policy and population).17,18 Organizational barriers are defined as impediments encountered within the medical system and health care organizations when accessing, receiving, and delivering care.12 Several organizational barriers have been identified in the literature based on characteristics of the targeted population (eg, race, ethnicity, type of illness), key stakeholder views, and aspects of care (eg, screening, preventive practice, care, and treatment).
In a systematic review, Betancourt and colleagues reported provider-patient interactions, processes of care, and language as some of the barriers to receiving quality care.17 Although cancer screening has been shown to reduce mortality in the adult population for several types of cancer,19-21 barriers that impede access to services have been identified as emanating not only from the macro level (eg, age of screening, reimbursement problems, screening guidelines) or inter- and intra-individual levels (eg, awareness of screening, various perspectives on life and cancer, comorbidities, social support), but also from the organization (organizational infrastructure that inhibits screening because of limited participation in research trials) and provider levels (impaired communication regarding screening between patient and physician, low commitment to shared decision-making, provider’s awareness of screening and screening guidelines).18 Other organizational barriers, such as difficulty navigating the health care system, poor interaction between patients and medical staff, and language barriers, have been identified in a systematic review of breast cancer screening in immigrant and minority women.22
Other barriers to quality cancer care reported by patients include knowledge about the disease and treatment, poor communication with providers, lack of coordination and timeliness of care, and lack of attention to care. Providers have identified other barriers to quality care, which include a lack of access to care, reimbursement problems, poor psychosocial support services, accountability of care, provider workload, and inadequate patient education.23 Few qualitative studies have been conducted to understand the organizational barriers that lung cancer patients and their caregivers face within the health care system.
Through the use of focus groups, we sought the perspectives of lung cancer patients and their caregivers on the organizational barriers that they experience while navigating the health care system. Identifying and understanding these barriers can help health care professionals work with patients and their caregivers to alleviate these stressors in an already difficult time.3,24 In addition, a more thorough understanding of patients’ and caregivers’ perspectives on organizational barriers may help improve health care delivery and, thus, patient satisfaction.
Methods
With the approval of the Institutional Review Boards of the University of Memphis and the Baptist Memorial Health Care Corporation, we conducted focus groups with lung cancer patients and their informal caregivers to understand the challenges they encounter while navigating the health care system during their illness. The Baptist Memorial Health Care system is centered in the Mid-South region of the United States, which has some of the highest US lung cancer incidence rates.25
Research staff identified potential participants from a roster of patients provided by treatment clinics within the system. Patients eligible for this study had received care for suspected lung cancer within a community-based health care system within 6 months preceding the date of the focus group. Eligible patients were approached by the research staff by cold calling or in-person contact during clinic visits for their consent to participate in the study. From a compiled list of 219 patients, 89 received initial contact to gauge interest. Of those, 42 patients were formally approached and asked to participate; 22 agreed to participate, and 20 did not participate for reasons including illness, previous participation in other forms of patient feedback, lack of interest, failure to show up to focus group sessions, change of mind, lack of transportation, or other commitments. Patients identified their informal caregivers to form patient-caregiver dyads. All patients and caregivers provided written informed consent before participating in the focus groups.
We conducted 10 focus groups during March 2013 through January 2014 – 5 with 22 patients and 5 with 24 caregivers (Table 1). Eight of the focus groups were conducted in Memphis, Tennessee, and to obtain the perspectives of patients from a rural setting, we conducted 2 focus groups in Grenada, Mississippi. All of the focus groups were facilitated by a medical anthropologist (SK) and a clinical psychologist (KDW), neither of whom was affiliated with the health care system. Each facilitator was accompanied by a note-taker. Patient-caregiver dyads came to the designated location together. Two focus groups (one for patients, the other for caregivers) were then conducted simultaneously in 2 separate rooms. The facilitators used a pilot-tested focus group interview guide during each session. The items in the focus group guide revolved around experience with the health care system in diagnosis and treatment; timeliness with appointments and procedures for diagnosis and subsequent care; physician communication in being informed about the disease, treatment, and getting questions answered; coordination of care; other challenges in receiving quality care; and suggestions for improving the patient and caregiver experience with the health care system.
The focus group sessions lasted 1 to 2 hours and were audiorecorded and transcribed verbatim. The data were analyzed by using Dedoose software version 5.0.11 (Sociocultural Research Consultants, Los Angeles, California). Data collection and analysis were conducted concurrently to achieve theoretical saturation. Creswell’s 7-step analysis framework was used as a guide to code and interpret the data.26 The process involved collecting raw data, preparing and organizing transcripts, reading the transcripts, coding the data with the help of qualitative software, analyzing the data for themes and subthemes, interpreting the themes, and devising the meaning of the themes.26 Initial codes were categorized and compared to determine recurrent themes. Three members of the research team independently reviewed the transcripts, extensively discussed the content, and developed consensus around the identified themes. Critical and rigorous steps were taken throughout data collection and analysis to ensure the credibility, transferability, dependability, and confirmability of the qualitative data.27-29 In addition, elements of the Consolidated Criteria for Reporting Qualitative Research checklist were used to strengthen the data collection, analysis, and reporting process.30
Results
The 10 focus groups included 46 participants: 22 patients and 24 caregivers (some patients brought multiple caregivers). Of the 22 patients, 12 were women and 7 were black. An equal proportion of patients had at least a high school education as had a college or postgraduate degree. Although all of the patients had had a lung lesion suspicious for lung cancer, 19 eventually had a histologic diagnosis of lung cancer. The remaining 3 patients were all evaluated in a thoracic oncology clinic but were eventually found to have metastatic breast cancer, lymphoma, and a granuloma. Nine patients were either currently in the treatment decision-making process or actively receiving treatment, 11 had completed treatment within the preceding 6 months, and 2 had completed treatment more than 6 months previously. Treatment covered the spectrum from curative intent to palliative care. Of the 24 caregivers, 18 were women and 7 were black; 12 caregivers had at least a college education, of whom 2 had postgraduate degrees (Table 2).
Based on participants’ feedback, we identified 4 main levels within the system where barriers to optimal care occurred: policy, institutional, provider, and patient. From our qualitative analyses, we identified a central theme associated with each level, around which the barriers coalesced. The themes were insurance, scheduling, provider communication, and patient knowledge. At the policy level, medical insurance was perceived to affect the timeliness of care and to be a deterrent to timely diagnosis and quality treatment. Lack of insurance was a daunting obstacle for indigent patients. However, even those who were insured felt that dealing with insurance companies was a significant barrier to care. At the institutional level, appointment scheduling caused problems for both patients and their caregivers. At the health care provider level, communication was perceived as a major problem. And finally, at the patient level, both patient and caregiver lack of knowledge of lung cancer and the processes inherent in lung cancer treatment were barriers for optimal diagnosis and treatment (Table 3).
Insurance barriers
At the policy level, health insurance was reported as a significant barrier to accessing health care. Patients and caregivers reported delays in diagnosis and/or treatment because of either lack of insurance or lag time in insurance processing of clinician requests. Insurance restrictions on tests, procedures, and office visits presented difficulties in getting additional opinions from providers regarding diagnosis, prognosis, or treatment plans. Some patients were no longer able to see providers after they had met the test or office visit limit allotted by insurance providers. One patient shared the following experience with office visits leading up to their lung cancer diagnosis:
Patient … with my insurance I just had only 12 office visits … and I had already maxed those out.
In other instances, insurers would not cover hospital or clinic visits if certain logistic protocols were not met. This would sometimes leave patients stranded for a period of time without receiving any care.
Caretaker … went home and went to one of those minor emergency clinics and they sent her to [xx hospital in another city]. There they did nothing. That was then over the weekend, and my son wanted to get her out of there to get her into practice because they weren’t doing anything. They said, ‘No. Your insurance won’t pay for it unless you stay here till we sign the papers to be transferred.’ … It was a bandage. Period.
Some insurers would not cover certain health services outside of routine testing protocols for the patients’ conditions. This lack of coverage caused patients to pay out of pocket for needed care.
Patient Nothing in the lymph nodes, but if it hadn’t been for me going ahead with this [coronary] calcium score, the insurance wasn’t gonna pay anything. If it wouldn’t been for the 79 bucks or the family situation, I wouldn’t be sitting [here] today.
Individuals who had not yet met the age requirement for Medicare reported being without insurance for a period of time, which contributed to delays in accessing care.
Caregiver [xx patient] probably … could’ve been diagnosed maybe even months ago, but she is in that in-between where she gets Social Security but she’s not 65 until November, so she has no insurance.
Scheduling barriers
At the institutional level, patients and caregivers reported problems with appointment scheduling. Logistic problems with adjusting work schedules and arranging for transportation as well as long wait times before evaluation by a provider were recurrent themes expressed by both patients and caregivers. Many had become resigned to the expectation of long wait times during appointments.
Patient I have to call the month before to make the appointment because they don’t take appointments so far—‘Oh, we’re not working on that yet.’ I find that very annoying ....
Caregiver The last time I was there I waited four hours.
Caregiver … your appointment at 9:00 and you get called back at 9:30 or 10:00 and you get to see the doctor by 11:00, but that’s not any different than anywhere, unfortunately ….
Rescheduled appointments also posed a problem for participants. Constant rescheduling was an inconvenience for both patients and caregivers. Many were unhappy with rescheduling because both patients and caregivers had prepared mentally and physically for an appointment, only to be told that they would have to reschedule, which caused delays in the care process.
Patient I think every single visit I had with him gets rescheduled at least twice ....
Caregiver Three times this week we’ve been geared up, ready to have chemo and they keep changing it.
Patient Everything was fine with me, but they keep cancelling my appointments ....
Some participants perceived that the popularity of physicians might explain the difficulty with scheduling. Patients suggested that it is challenging to get appointments with better-known physicians, so they are more accepting of appointments at any time, even if the time is inconvenient for them.
Caregiver Of course, … if you have a popular doctor, sometimes you don’t always get the appointment you want …
Participants also expressed frustration with the way appointments were rescheduled. They felt as though the physicians were not concerned about their lives outside of office visits.
Patient … patients actually have lives. Many of them have jobs or families or responsibilities.
Communication barriers
At the provider level, poor communication between health care providers and patients was perceived as a major impediment to the quality of care patients received. Both patients and caregivers emphasized the importance of open patient-provider interactions and that there was a lack of such open communications in many instances. There was concern regarding the way diagnoses or prognoses were relayed to patients. Many times, physicians were insensitive and disregarded the sentiments of the patients and caregivers when delivering news about the patients’ condition, as one caregiver shared,
Caregiver … the pulmonary man came … in the room and said, ‘Oh, don’t worry about your lungs. Something else will get you first,’ which was a very, very bad thing to say.
Participants also expressed concerns that they were not properly prepared for treatments by their physicians because vital information was not discussed. They felt as if physicians were not realistic about potential outcomes. This resulted in patients and caregivers being too optimistic and later disappointed when the outcome was not what they had originally expected.
Patient Until I got to this office, I was totally oversold on everything. I was told surgery … robotic, not invasive. Day one, surgery. Day two, tubes out. Day three, go home. I expected to be home on Sunday night, stir-frying vegetables, and making dinner, feeding my cat. I was in ICU four days … I went home with oxygen. I mean I thought I was just gonna walk outta there…. You take a little thing out and you put a Band-Aid on, and you go home.
Data also revealed that patients were unsure of their condition, even following treatment. Information was not communicated to patients about the specifics of their disease, either because of miscommunication or minimal patient-provider time spent during office visits. This lack of communication between patients and providers often left patients and caregivers uncertain about exactly what condition they had or what they were being treated for.
Patient I just can’t have the time with Dr. xx, cuz he’s so busy...
Patient … I didn’t understand. Which exactly what type of cancer did I have, cuz I’m—really to tell you the truth—I’m still wondering.
Knowledge barriers
Patients and caregivers also identified a lack of education and knowledge about lung cancer diagnosis and treatment as a barrier to their care. Patients and caregivers were not always fully knowledgeable about lung cancer, treatment options, or the duration of treatments. They relied on the provider to disclose such information or direct them to credible sources. In many instances, patients were misinformed about the causes of lung cancer. There were misconceptions that lung cancer was only caused by a history of smoking or genetic predisposition. Patients who did not smoke or did not have a family history of lung cancer were often confused and dismayed by the diagnosis.
Patient I was trying to figure out, why do I have lung cancer. Never smoked a day in my life.
Patients were often unaware of treatment options or side effects of various treatments. They relied on physicians to relay information and make decisions for them about treatment plans.
Patient I was told chemo would probably be the best thing for me, and I just had faith that Dr. xx knows more about it than me.
Patient I’m doing chemo but it’s — what I’m doing is different. Of course, I don’t know anything about it actually either. It’s what I hear from other people.
Other patients relied on their own sources for information about their condition, either through the Internet, from family members and/or friends, or from preconceived notions.
Patient Well — of course in the meantime, I read — because they said it was a small cell, very aggressive, so I felt like everything I read — that’s the deal. I think we’ve become where we can get on the internet and look up so much, that to me, I was gonna be gone.
Patient When he said, ‘Cancer,’ I said, ‘Well, I thought cancer was a heredity thing? That you have to have somebody in your family that has it…’
Discussion
Organizational barriers are an important consideration in the delivery and receipt of high-quality, patient-centered lung cancer care. This qualitative study of patients being treated for lung cancer and their informal caregivers revealed several common perceived organizational barriers to receiving care, including health insurance coverage restrictions, appointment scheduling difficulties, quality of communication with physicians, and failure to properly educate the patient and family about the disease and what to expect of the treatment process.
The provider communication and patient knowledge barriers seem to reinforce each other and could be improved through focused efforts on the quality of communication between patients and their caregivers and clinical care providers. Patients expect, but are often deprived of, open and active dialogue with their providers. Improved communication can be helpful in educating patients and their caregivers about their disease, prognosis, and treatment goals. Although communication ranks highly as a patient and caregiver priority, there is often a disconnect between patients and caregivers and their physicians.31 Patients and caregivers often want to be more involved in the decision-making process, and effective communication between physicians and patients has been linked to the patient’s ability to understand, and also receive high-quality care.32,33 Failure to communicate effectively and educate patients on key aspects of their condition strips them of their autonomy in decision-making.
The involvement of a navigator for patients being treated for lung cancer could be pivotal in relieving the communication and scheduling barriers. The nurse navigator assists with coordinating effective communication and providing needed information between providers and patients and their caregivers. A navigator also serves as a single point of contact for patients and caregivers to communicate questions outside of physician visits or concerns that may not be urgent enough to warrant immediate physician response.34 The navigator coordinates patients’ appointment schedules and physician referrals and communicates the details of the next steps in the care-delivery process. This helps remove the barriers to care and improve patient outcomes and the quality of health care delivery, especially for patients and caregivers dealing with a life-threatening illness within a complex referral process.35
Multidisciplinary care, a much-recommended alternative care-delivery model, should, in theory, promote connectivity of providers and collaboration between providers, patients, and family members. This model could help reduce barriers for patients and caregivers.3,24,36 A network of connected providers can better coordinate treatment plans, easily share test results, and provide built-in second opinions. Given the increasingly multimodal approach to the diagnosis, staging, and treatment of lung cancer, the multidisciplinary model could allow physicians to consider multiple perspectives and care-delivery options and, ideally, develop consensus around the optimal approach for each individual patient in one setting. This can shorten the length of time before treatment and establish a plan that is tailored to the patient’s needs.24
The National Academy of Medicine (formerly, Institute of Medicine) proposes that modern health care systems have 6 aims for quality improvement: safety, effectiveness, patient-centeredness, timeliness, efficiency, and equity.37 It would take changes in the design and implementation of organizational support systems at the policy, institutional, and provider level for those aims to be achieved. Further investigation of the problems identified by patients and caregivers could lead to innovative solutions to improve lung cancer care. Future work should evaluate the most effective communication styles in patient-provider interactions, particularly in regard to to lung cancer diagnosis and treatment, and investigate how multidisciplinary models influence patient-provider communication and patient care.
Limitations
This study has several limitations. Less than half of those approached for the study participated in the study for various reasons, which may have introduced selection bias in terms of not having the perspectives of patients not willing or able to participate in the study. Though focus groups are known to generate rich in-depth views of certain issues, they have been criticized as potentially lacking rigor and generalizability. To address this concern, we used a standardized script for each focus group and involved multiple members of the research team in data analysis and interpretation. Also, this study enrolled participants from a single health care institution and did not use a comparison group. There might be institutional and geographic differences in the experience of lung cancer care, which might further limit the generalizability of the results of this study.
Conclusions
Despite those limitations, this study offers valuable insight into the barriers that lung cancer patients and caregivers encounter while navigating a community-level health care system. Eliminating or minimizing these barriers will require strategic plans that help mitigate insurance-related, scheduling, provider-patient communication, and patient/caregiver knowledge acquisition problems and translate them into tactical actions for quality improvement. This is one of the first qualitative studies conducted to understand the organizational barriers that lung cancer patients and their caregivers face within a health care system. Additional research is needed to explore these barriers and develop viable solutions.
Disclaimer
All statements in this report, including its findings and conclusions, are solely those of the authors and do not necessarily represent the views of the Patient-Centered Outcomes Research Institute or its Board of Governors or Methodology Committee.
1. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin. 2016;66(1):7-30.
2. Riedel RF, Wang X, McCormack M, et al. Impact of a multidisciplinary thoracic oncology clinic on the timeliness of care. J Thorac Oncol. 2006;1(7):692-696.
3. Seek A, Hogle WP. Modeling a better way: navigating the health care system for patients with lung cancer. Clin J Oncol Nurs. 2007;11(1):81-85.
4. Sorensen R, Iedema R. Redefining accountability in health care: managing the plurality of medical interests. Health. 2008;12(1):87-106.
5. Mosher CE, Jaynes HA, Hanna N, Ostroff JS. Distressed family caregivers of lung cancer patients: an examination of psychosocial and practical challenges. Support Care Cancer. 2013;21(2):431-437.
6. Cantril C, Haylock PJ. Patient navigation in the oncology care setting. Semin Oncol Nurs. 2013;29(2):76-90.
7. Freeman HP. Patient navigation: a community based strategy to reduce cancer disparities. J Urban Health. 2006;83(2):139-141.
8. Kwon DH, Tisnado DM, Keating NL, et al. Physician reported barriers to referring cancer patients to specialists: prevalence, factors, and association with career satisfaction. Cancer. 2015;121(1):113-122.
9. Andersen SE. Implementation of a new prescription system. A qualitative survey of organizational barriers. Ugeskr Laeger. 2002;164(38):4449-4453.
10. Rousseau L, Guay M, Archambault D, El m’ala Z, Abdelaziz N. Do organizational barriers to pneumococcal and influenza vaccine access exist? Can J Public Health. 2007;98(2):105-110.
11. Storey J, Buchanan D. Health care governance and organizational barriers to learning from mistakes. J Health Organ Manag. 2008;22(6):642-651.
12. Ziegenfuss JT Jr. Organizational barriers to quality improvement in medical and health care organizations. Qual Assur Util Rev. 1991;6(4):115-122.
13. Rihari-Thomas J, DiGiacomo M, Phillips J, Newton P, Davidson PM. Clinician perspectives of barriers to effective implementation of a rapid response system in an academic health centre: a focus group study. Int J Health Policy Manag. 2017;6(8):447-456.
14. Dutton D. Financial, organizational and professional factors affecting health care utilization. Soc Sci Med. 1986;23(7):721-735.
15. King CJ, Chen J, Dagher RK, Holt CL, Thomas SB. Decomposing differences in medical care access among cancer survivors by race and ethnicity. Am J Med Qual. 2015;30(5):459-469.
16. Renzaho AM, Romios P, Crock C, Sønderlund AL. The effectiveness of cultural competence programs in ethnic minority patient-centered health care—a systematic review of the literature. Int J Qual Health Care. 2013;25(3):261-269.
17. Betancourt JR, Green AR, Carrillo JE, Ananeh-Firempong O. Defining cultural competence: a practical framework for addressing racial/ethnic disparities in health and health care. Public Health Rep. 2003;118(4):293-302.
18. Scheinfeld Gorin S, Gauthier J, Hay J, Miles A, Wardle J. Cancer screening and aging: research barriers and opportunities. Cancer. 2008;113(suppl 12):3493-3504.
19. Humphrey L, Deffebach M, Pappas M, et al. Screening for lung cancer: systematic review to update the US preventive services task force recommendation. Evidence syntheses No. 105. Rockville, MD: Agency for Health Care Research and Quality; 2013.
20. Nelson HD, Fu R, Cantor A, Pappas M, Daeges M, Humphrey L. Effectiveness of breast cancer screening: systematic review and meta-analysis to update the 2009 U.S. Preventive Services Task Force Recommendation. Ann Intern Med. 2016;164(4):244-255.
21. Zauber AG. The impact of screening on colorectal cancer mortality and incidence: has it really made a difference? Dig Dis Sci. 2015;60(3):681-691.
22. Remennick L. The challenge of early breast cancer detection among immigrant and minority women in multicultural societies. Breast J. 2006;12(suppl 1):103-110.
23. Hess LM, Pohl G. Perspectives of quality care in cancer treatment: a review of the literature. Am Health Drug Benefits. 2013;6(6):321-329.
24. Kedia SK, Ward KD, Digney SA, et al. ‘One-stop shop’: lung cancer patients’ and caregivers’ perceptions of multidisciplinary care in a community health care setting. Transl Lung Cancer Res. 2015;4(4):456-464.
25. US Cancer Statistics Working Group. United States cancer statistics: 1999-2014 incidence and mortality web-based report. Atlanta, GA: Department of Health and Human Services, Centers for Disease Control and Prevention, and National Cancer Institute. http://www.cdc.gov/uscs. Published 2017. Accessed November 21, 2017.
26. Creswell JW. Research design: qualitative, quantitative, and mixed methods approaches. 4th ed. Thousand Oaks, CA: SAGE Publications; 2014.
27. Ballinger C. Demonstrating rigour and quality? In Finlay L, Ballinger C, eds. Qualitative research for allied health professionals: challenging choices. Chichester, England: John Wiley & Sons Ltd; 2006:235-246.
28. Finlay L. ‘Rigour’, ‘ethical integrity’ or ‘artistry’? Reflexively reviewing criteria for evaluating qualitative research. Br J Occup Ther. 2006;69(7):319-326.
29. Lincoln YS, Guba EG. Naturalistic inquiry. Newbury Park, CA: SAGE Publications; 1985.
30. Tong A, Sainsbury P, Craig J. Consolidated criteria for reporting qualitative research (COREQ): a 32-item checklist for interviews and focus group. Int J Qual Health Care. 2007;19(6):349-357.
31. Neeman N, Quinn K, Shoeb M, Mourad M, Sehgal NL, Sliwka D. Postdischarge focus groups to improve the hospital experience. Am J Med Qual. 2013;28(6):536-538.
32. O’Day BL, Killeen M, Iezzoni LI. Improving health care experiences of persons who are blind or have low vision: suggestions from focus groups. Am J Med Qual. 2004;19(5):193-200.
33. Smith B, Lynch WD, Markow C, Lifsey S, Slover M. Consumers’ understanding of and interest in provider- versus practice-level quality characteristics: findings from a focus group study. Am J Med Qual. 2015;30(4):367-373.
34. Islam KM, Opoku ST, Apenteng BA, et al. Coping with an advanced stage lung cancer diagnosis: patient, caregiver, and provider perspectives on the role of the health care system. J Cancer Educ. 2016;31(3):554-558.
35. Pedersen A, Hack TF. Pilots of oncology health care: a concept analysis of the patient navigator role. Oncol Nurs Forum. 2010;37(1):55-60.
36. Gopal R. How to maintain multidisciplinary treatment schedules. J Support Oncol. 2005;3(3):248-256.
37. Committee on Quality of Health Care in America. Improving the 21st century health care system. In: Institute of Medicine, ed. Crossing the quality chasm: a new health system for the 21st century. Washington, DC: National Academies Press; 2001:39-60.
1. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin. 2016;66(1):7-30.
2. Riedel RF, Wang X, McCormack M, et al. Impact of a multidisciplinary thoracic oncology clinic on the timeliness of care. J Thorac Oncol. 2006;1(7):692-696.
3. Seek A, Hogle WP. Modeling a better way: navigating the health care system for patients with lung cancer. Clin J Oncol Nurs. 2007;11(1):81-85.
4. Sorensen R, Iedema R. Redefining accountability in health care: managing the plurality of medical interests. Health. 2008;12(1):87-106.
5. Mosher CE, Jaynes HA, Hanna N, Ostroff JS. Distressed family caregivers of lung cancer patients: an examination of psychosocial and practical challenges. Support Care Cancer. 2013;21(2):431-437.
6. Cantril C, Haylock PJ. Patient navigation in the oncology care setting. Semin Oncol Nurs. 2013;29(2):76-90.
7. Freeman HP. Patient navigation: a community based strategy to reduce cancer disparities. J Urban Health. 2006;83(2):139-141.
8. Kwon DH, Tisnado DM, Keating NL, et al. Physician reported barriers to referring cancer patients to specialists: prevalence, factors, and association with career satisfaction. Cancer. 2015;121(1):113-122.
9. Andersen SE. Implementation of a new prescription system. A qualitative survey of organizational barriers. Ugeskr Laeger. 2002;164(38):4449-4453.
10. Rousseau L, Guay M, Archambault D, El m’ala Z, Abdelaziz N. Do organizational barriers to pneumococcal and influenza vaccine access exist? Can J Public Health. 2007;98(2):105-110.
11. Storey J, Buchanan D. Health care governance and organizational barriers to learning from mistakes. J Health Organ Manag. 2008;22(6):642-651.
12. Ziegenfuss JT Jr. Organizational barriers to quality improvement in medical and health care organizations. Qual Assur Util Rev. 1991;6(4):115-122.
13. Rihari-Thomas J, DiGiacomo M, Phillips J, Newton P, Davidson PM. Clinician perspectives of barriers to effective implementation of a rapid response system in an academic health centre: a focus group study. Int J Health Policy Manag. 2017;6(8):447-456.
14. Dutton D. Financial, organizational and professional factors affecting health care utilization. Soc Sci Med. 1986;23(7):721-735.
15. King CJ, Chen J, Dagher RK, Holt CL, Thomas SB. Decomposing differences in medical care access among cancer survivors by race and ethnicity. Am J Med Qual. 2015;30(5):459-469.
16. Renzaho AM, Romios P, Crock C, Sønderlund AL. The effectiveness of cultural competence programs in ethnic minority patient-centered health care—a systematic review of the literature. Int J Qual Health Care. 2013;25(3):261-269.
17. Betancourt JR, Green AR, Carrillo JE, Ananeh-Firempong O. Defining cultural competence: a practical framework for addressing racial/ethnic disparities in health and health care. Public Health Rep. 2003;118(4):293-302.
18. Scheinfeld Gorin S, Gauthier J, Hay J, Miles A, Wardle J. Cancer screening and aging: research barriers and opportunities. Cancer. 2008;113(suppl 12):3493-3504.
19. Humphrey L, Deffebach M, Pappas M, et al. Screening for lung cancer: systematic review to update the US preventive services task force recommendation. Evidence syntheses No. 105. Rockville, MD: Agency for Health Care Research and Quality; 2013.
20. Nelson HD, Fu R, Cantor A, Pappas M, Daeges M, Humphrey L. Effectiveness of breast cancer screening: systematic review and meta-analysis to update the 2009 U.S. Preventive Services Task Force Recommendation. Ann Intern Med. 2016;164(4):244-255.
21. Zauber AG. The impact of screening on colorectal cancer mortality and incidence: has it really made a difference? Dig Dis Sci. 2015;60(3):681-691.
22. Remennick L. The challenge of early breast cancer detection among immigrant and minority women in multicultural societies. Breast J. 2006;12(suppl 1):103-110.
23. Hess LM, Pohl G. Perspectives of quality care in cancer treatment: a review of the literature. Am Health Drug Benefits. 2013;6(6):321-329.
24. Kedia SK, Ward KD, Digney SA, et al. ‘One-stop shop’: lung cancer patients’ and caregivers’ perceptions of multidisciplinary care in a community health care setting. Transl Lung Cancer Res. 2015;4(4):456-464.
25. US Cancer Statistics Working Group. United States cancer statistics: 1999-2014 incidence and mortality web-based report. Atlanta, GA: Department of Health and Human Services, Centers for Disease Control and Prevention, and National Cancer Institute. http://www.cdc.gov/uscs. Published 2017. Accessed November 21, 2017.
26. Creswell JW. Research design: qualitative, quantitative, and mixed methods approaches. 4th ed. Thousand Oaks, CA: SAGE Publications; 2014.
27. Ballinger C. Demonstrating rigour and quality? In Finlay L, Ballinger C, eds. Qualitative research for allied health professionals: challenging choices. Chichester, England: John Wiley & Sons Ltd; 2006:235-246.
28. Finlay L. ‘Rigour’, ‘ethical integrity’ or ‘artistry’? Reflexively reviewing criteria for evaluating qualitative research. Br J Occup Ther. 2006;69(7):319-326.
29. Lincoln YS, Guba EG. Naturalistic inquiry. Newbury Park, CA: SAGE Publications; 1985.
30. Tong A, Sainsbury P, Craig J. Consolidated criteria for reporting qualitative research (COREQ): a 32-item checklist for interviews and focus group. Int J Qual Health Care. 2007;19(6):349-357.
31. Neeman N, Quinn K, Shoeb M, Mourad M, Sehgal NL, Sliwka D. Postdischarge focus groups to improve the hospital experience. Am J Med Qual. 2013;28(6):536-538.
32. O’Day BL, Killeen M, Iezzoni LI. Improving health care experiences of persons who are blind or have low vision: suggestions from focus groups. Am J Med Qual. 2004;19(5):193-200.
33. Smith B, Lynch WD, Markow C, Lifsey S, Slover M. Consumers’ understanding of and interest in provider- versus practice-level quality characteristics: findings from a focus group study. Am J Med Qual. 2015;30(4):367-373.
34. Islam KM, Opoku ST, Apenteng BA, et al. Coping with an advanced stage lung cancer diagnosis: patient, caregiver, and provider perspectives on the role of the health care system. J Cancer Educ. 2016;31(3):554-558.
35. Pedersen A, Hack TF. Pilots of oncology health care: a concept analysis of the patient navigator role. Oncol Nurs Forum. 2010;37(1):55-60.
36. Gopal R. How to maintain multidisciplinary treatment schedules. J Support Oncol. 2005;3(3):248-256.
37. Committee on Quality of Health Care in America. Improving the 21st century health care system. In: Institute of Medicine, ed. Crossing the quality chasm: a new health system for the 21st century. Washington, DC: National Academies Press; 2001:39-60.
The impact of patient education on consideration of enrollment in clinical trials
The low rate of participation in clinical trials is partly owing to the lack of awareness of these trials not only among potential participants but the US population as a whole.1 This lack of awareness, however, can be reversed. For example, findings from a single-institution observational study showed that systematically sending letters about clinical trial participation to all new lung cancer patients was associated with increased trial participation.2 More recently, a large, multicenter, randomized experiment showed that attitudes toward clinical trials were improved through preparatory education about clinical trials before a patient’s first oncologic visit.3
Such clinical trial education can be used before any medical diagnosis to increase clinical trial awareness in the general population. It may be advantageous to do so because people tend to process information more effectively during less stressful times.4 Clinical trial awareness in the US population has increased slightly over time, but in 2012, one study reported that 26% of its participants lacked general awareness about clinical trials.5
Comprehensive educational material, such as a multimedia psychoeducational intervention,6 a 28-video library,3 or a 160-page book,7 which have been proposed for oncology patients, may be too intensive for someone who is not immediately deciding whether to participate in a clinical trial. However, a simple, concise form of education might be preferable and appropriate to increase basic knowledge and awareness among the general population, especially among those who are less educated.8
Our aim in the present study was to evaluate whether providing brief educational material about clinical trials would increase patient willingness to participate in these trials.
Methods
This is a single-group, cross-sectional design study in which all participants were administered the questions and the 240-word educational statement in the same order.
Sample
An electronic survey was conducted by Marketing and Planning Systems (the analytics practice of Kantar Millwardbrown) on behalf of the Memorial Sloan Kettering Cancer Center (MSK). The survey included a national sample of 1011 participants and a local sample of 500 participants from the MSK catchment area (22 counties across the 5 boroughs of New York City, Long Island, southern New York State, northern New Jersey, and southwestern Connecticut).
Survey participants were aged 18 to 69 years in the national sample and 25 to 69 years in the local sample, representing 87% and 75% of the adult populations of those areas, respectively. Respondents who were or who had a family member currently working in the fields of news, advertising/marketing, or medical care were not surveyed. Participants were sourced from an online incentivized panel with millions of potential respondents representative of the US adult population.
Questionnaire
The questionnaire collected data on participant demographics and main medical history (including previous participation in a clinical trial), and asked questions about clinical trials, focusing on:
Analyses
Descriptive and bivariate statistical analyses of participants’ characteristics were weighted to ensure national representativeness for gender, age, ethnicity, and income. Mean standard deviation (SD) was computed for every quantitative variable. Categorical variables were expressed as proportions.
Student t tests and analyses of variance (ANOVAs) were used to compare continuous variables, while chi-square tests were used to compare categorical data. Repeated measures ANOVAs were then used to determine the sociodemographic and medical characteristics associated with the impression of and willingness to enroll in a clinical trial before and after reading the educational material. The interaction between education level and time (pre- or postreading assessment) was tested to determine if the changes after reading the brief statement were different depending on education level.
All statistical analyses were 2-tailed and considered statistically significant at P < .05. Analyses were performed using SPSS PAWS Statistics 24 (IBM Inc, Armonk, New York). Effect sizes (standardized mean differences) and their 95% confidence intervals (CIs) were computed using the compute.es package for R 3.3 (R Foundation for Statistical Computing, Vienna, Austria).
Results
Participants
From October 23, 2015, through November 12, 2015, 1511 US participants responded to the survey request, including 1507 respondents (99.7%) who reported their education level and are included in the analyses of this report. The mean age of the respondents was 43.5 years (SD, 4.6). More than half of the respondents (57.8%) reported a current medical condition, mainly cardiovascular (20.0%), arthritis (20.0%), or other type or chronic pain (20.0%), and 9.0% reported a cancer diagnosis (current, 2.9%; previous, 6.1%).
Participants who at most had completed high school (18.9%, including 1.4% who had never even attended high school) were more often white women, lived outside urban areas, had lower household income, and were less likely to have health care insurance (Table 2). They also reported a current or previous cancer diagnosis less often than those of similar age who had attended college.
Previous participation in a clinical trial was reported by 9.6% of participants. Most of the clinical trials (75.0%) were testing a new drug. Previous trial participants were more likely to be older than those who had not participated in trials (46.1 years [SD, 14.8] vs 43.3 [SD, 4.6], repectively; P = .033), have a current health condition (86.2% vs 54.8%; P < .001), and know another trial participant (39.9% vs 9.5%; P < .001).
Education level and baseline impression of and
willingness to enroll in a clinical trial
A lower level of education was associated with a decreased likelihood of previous trial participation or of knowing a trial participant, as well as with less awareness and inaccurate perceptions of clinical trials (Table 2).
Participants with a high school degree or less were more likely to have a worse impression of and were less likely to enroll in a future hypothetical clinical trial before reading the educational material (Figure 1). Multivariable analyses confirmed that lower education level was associated with lower baseline overall impression, regardless of other personal characteristics (Table 3). Lowest household income was also associated with a more negative impression of trials, whereas participants with a current medical condition and with previous contact with clinical trials had a more positive impression of them. The same effects were observed with likeliness to enroll in a future hypothetical clinical trial (correlated with the overall impression: r, 0.63; P < .001), except that the negative effect of female gender was statistically significant (Table 3).
Posteducation impression of and willingnes to enroll
willingness to enroll in a clinical trial
The brief educational material was mostly considered believable (86.2%), easy to understand (84.8%), and included information that was new to participants (81.5%; Table 2). Participants with a high school diploma or less more often noted that the material provided them with new information, but they also reported more difficulties in fully understanding and believing the information. Overall, however, few participants found the information difficult to understand (4.6%) or hard to believe (1.8%; Table 2).
Most participants had an improved overall impression of clinical trials (standardized mean difference, 0.42; 95% CI, 0.35-0.50; P < .001) after reading the educational material. This increase was higher among participants with a lower completed level of education (Figure 1; standardized mean difference, 0.62; 95% CI, 0.45-0.79; Pinteraction < .001). The same effects were observed for likeliness to enroll in a future hypothetical clinical trial (P < .001; Figure 1).
After reading the informational statement, education level effect was no longer significantly associated with the overall impression of clinical trials (P = .23) and willingness to enroll in a clinical trial (P = .34), whereas the effects of income, current medical condition, and previous engangement with clinical trials remained statistically significant (Table 3).
Remaining challenges
Regarding hypothetical participation in a future clinical trial after a cancer diagnosis, the most critical concerns were related to side effects and the uncertainty of insurance coverage (Figure 2).10 The lack of understanding of clinical trials was the least critical concern; however, it was significantly higher among participants with a lower completed level of education. These participants also expressed more critical concerns about feeling like a “guinea pig,” inconvenient trial location, and the frequent visits needed.
Discussion
Findings from this survey demonstrated that providing brief educational material about cancer clinical trials was associated with a more favorable impression of clinical trials and higher interest in trial participation. Furthermore, as far as we can ascertain, this is the first report showing how a simple intervention such as this may help close the knowledge gap on clinical trials among people of different educational backgrounds. Although most respondents in this interventional survey noted an increased willingness to consider participation in a clinical trial after reading the educational material, those with a lower level of education and knowledge about clinical trials received the most benefit. Previous participation in a clinical trial was also strongly associated with the impression of and willingness to enroll in a trial, both before and after reading the statement.11
Most of the interventions evaluated to date12 have focused on patients who are faced with having to decide whether to participate in a clinical trial2,3 or on very specific populations, such as select ethnic communities.13,14 However, it may be beneficial to provide simple, concise educational information about clinical trials to the general population, especially to those with minimal education. Although level of education concerns the minority of our sample (19%) sourced from an online panel, in the 2015 Current Population Survey, 41% of US participants aged 25 and older had not reached college education.15 Those with a lower level of education have reported a general lack of familiarity with clinical trials and were more likely to have inaccurate perceptions about trials. This is consistent with previous studies that have shown the lack of awareness and knowledge of clinical trials in this population.5,16,17 Our findings suggest that this knowledge gap can be reversed through a simple educational intervention and result in an increased willingness to participate.
The provision of information on clinical trials was positively associated with the 2 outcomes analyzed – improved impression of clinical trials and increased likelihood to enroll in a hypothetical trial. Such improvement might not translate to improved accrual,18 but it is a step toward closing the overall knowledge gap related to clinical trials and increasing the number of people who would consider trial participation. The lack of awareness of clinical trials has been reported as a legitimate explanation for why participation rates are lower in less-educated patient populations.19,20 This brief educational intervention is a simple, technology-sparing way to increase clinical trial awareness in the general population. In a similar survey, most physicians who reviewed an educational statement noted they were likely to use it with patients.21Less-educated patients, those who lived outside of urban areas, and those with lower household incomes were most concerned about trial location and the frequent visits needed when participating in a trial (Figure 2). Living in a nonurban area was not associated with participant impression of clinical trials or willingness to enroll in a trial. However, rural residency may be a barrier to enrollment depending on distance to the hospital22 and out-of-pocket expenses related to travel.23 Some comprehensive cancer centers, such as MSK, have developed alliances with community centers24 as a means of overcoming geographical barriers and increasing clinical trial participation rates.
Another concern shared by most respondents was the uncertainty in insurance coverage and potential out-of-pocket costs related to care. Lower household income, unlike location of residence and lack of insurance, was significantly associated with negative impressions of clinical trials and lower willingness to enroll in a trial, even after adjusting for education level. Cancer patients with higher financial burden have reported more attitudinal barriers, even after accounting for the negative effect of lower education level.25 Recent studies have also discussed the negative impact of lower income on cancer clinical trial participation,19,20,26,27 and new attention has been paid to the negative financial implications or “financial toxicity” of participating in a trial.23,28
White and older survey participants showed similar interest in clinical trial participation after accounting for other characteristics. There is growing evidence that outcome differences attributed to race may in fact be more dependent on socioeconomic status.8 A recent study among breast cancer patients showed that low socioeconomic status, but not race, was associated with decreased participation in clinical trials.29,30 Previous findings have also indicated that interest in clinical trials and barriers to enrollment among older, less-educated patients31 are often related to ineligibility, comorbidity, or communication difficulties.
Among our participants, the fear of side effects also was a common attitudinal barrier to clinical trial participation, as has been reported in previous studies.3,20 However, contrary to one previous study,20 this fear was not significantly increased among our less-educated participants.
Less-educated participants also reported more difficulties in understanding the information they were provided with, and they remained more concerned about being treated like “guinea pigs.” These concerns are consistent with other results showing that decisional conflict about clinical trial participation among patients with a high school diploma or less remained high even after they had received a National Cancer Institute text as pre-education material.3
Limitations
The lack of randomization makes it difficult to attribute with certainty that the change in acceptability of clinical trial participation is owing to the reading of the educational statement. The survey also sampled only English-speaking and well-educated participants from an online panel (81.1% had at least attended college) despite the use of a weighting procedure to ensure representativeness regarding gender, age, ethnicity, and income. Health literacy level or more specific trial literacy level was not evaluated; however, we were able to show less accurate perceptions of clinical trials among participants with a lower level of education by using agreement toward 8 statements about trials. The responses to hypothetical questions from these participants in the general population may also not be generalizable to a restricted population of patients with cancer. In addition, we measured impression of and willingness to enroll in a clinical trial immediately after providing participants with the educational material. We would have to confirm whether the positive effects of the education persist over time and translate to higher clinical trial participation rates.
Conclusions
Participants were receptive of educational material and expressed greater interest in and likelihood of enrolling in a clinical trial after reading it. The information had a greater effect on those with less education, but increased the willingness of all participants to enroll.
1. Ford JG, Howerton MW, Lai GY, et al. Barriers to recruiting underrepresented populations to cancer clinical trials: a systematic review. Cancer. 2008;112(2):228-242.
2. Quinn GP, Bell BA, Bell MY, et al. The guinea pig syndrome: improving clinical trial participation among thoracic patients. J Thorac Oncol. 2007;2(3):191-196.
3. Meropol NJ, Wong Y-N, Albrecht T, et al. Randomized trial of a web-based intervention to address barriers to clinical trials. J Clin Oncol. 2016;34(5):469-478.
4. Goldberg RJ. Disclosure of information to adult cancer patients: issues and update. J Clin Oncol. 1984;2(8):948-955.
5. Leiter A, Diefenbach MA, Doucette J, Oh WK, Galsky MD. Clinical trial awareness: changes over time and sociodemographic disparities. Clin Trials. 2015;12(3):215-223.
6. Jacobsen PB, Wells KJ, Meade CD, et al. Effects of a brief multimedia psychoeducational intervention on the attitudes and interest of patients with cancer regarding clinical trial participation: a multicenter randomized controlled trial. J Clin Oncol. 2012;30(20):2516-2521.
7. Carney PA, Tucker EK, Newby TA, Beer TM. Feasibility, acceptability and findings from a pilot randomized controlled intervention study on the impact of a book designed to inform patients about cancer clinical trials. J Cancer Educ. 2014;29(1):181-187.
8. Sharrocks K, Spicer J, Camidge DR, Papa S. The impact of socioeconomic status on access to cancer clinical trials. Br J Cancer. 2014;111(9):1684-1687.
9. Regan J, Hickey C, Targett C, Masuda S, Sabbatini P. Framing clinical research and the importance of trial participation: patient and physician perspective. Poster presented at: 8th Annual AACI Clinical Research Initiative Meeting; July 20-21 2016; Chicago, IL.
10. Mancini J, Genre D, Dalenc F, et al. Patients’ regrets after participating in a randomized controlled trials depended on their involvement in the decision making. J Clin Epidemiol. 2012;65(6):635-642
11. Murphy ST, Frank LB, Chatterjee JS, et al. Comparing the relative efficacy of narrative vs nonnarrative health messages in reducing health disparities using a randomized trial. Am J Public Health. 2015;105(10):2117-2123.
12. Treweek S, Lockhart P, Pitkethly M, et al. Methods to improve recruitment to randomised controlled trials: Cochrane systematic review and meta-analysis. BMJ Open. 2013;3(2):e002360. doi:10.1136/bmjopen-2012-002360.
13. Ma GX, Tan Y, Blakeney NC, et al. The impact of a community-based clinical trial educational intervention among underrepresented Chinese Americans. Cancer Epidemiol Biomarkers Prev. 2014;23(3):424-432.
14. Cupertino AP, Molina CSP, de los Rios JB, et al. Knowledge, awareness, and interest in cancer clinical trials among rural latinos following brief education by promotores de salud. J Community Med Health Educ. 2015;5:2161. doi:10.4172/2161-0711.1000358
15. Ryan CL, Bauman K. Educational attainment in the United States: 2015. https://www.census.gov/content/dam/Census/library/publications/2016/demo/p20-578.pdf. Published March 2016. Accessed October 18, 2016.
16. Lara PN Jr, Paterniti DA, Chiechi C, et al. Evaluation of factors affecting awareness of and willingness to participate in cancer clinical trials. J Clin Oncol. 2005;23(36):9282-9289.
17. Dhai A, Etheredge H, Cleaton-Jones P. A pilot study evaluating an intervention designed to raise awareness of clinical trials among potential participants in the developing world. J Med Ethics. 2010;36(4):238-242.
18. Denicoff AM, McCaskill-Stevens W, Grubbs SS, et al. The National Cancer Institute–American Society of Clinical Oncology Cancer Trial Accrual Symposium: summary and recommendations. J Oncol Pract. 2013;9(6):267-276.
19. Davison BJ, So A, Goldenberg SL, Berkowitz J, Gleave ME. Measurement of factors influencing the participation of patients with prostate cancer in clinical trials: a Canadian perspective. BJU Int. 2008;101(8):982-987.
20. Unger JM, Hershman DL, Albain KS, et al. Patient income level and cancer clinical trial participation. J Clin Oncol. 2013;31(5):536-542.
21. IRB Advisor. Education may overcome reticence to join trials. Relias website. http://www.ahcmedia.com/articles/138088-education-may-overcome-reticence-to-join-trials. Published July 1, 2016. Accessed July 29, 2016.
22. Vanderpool RC, Kornfeld J, Mills L, Byrne MM. Rural-urban differences in discussions of cancer treatment clinical trials. Patient Educ Couns. 2011;85(2):e69-e74.
23. Meropol NJ. Health policy: overcoming cost barriers to clinical trial participation. Nat Rev Clin Oncol. 2016;13(6):333-334.
24. MSK Cancer Alliance. Memorial Sloan Kettering Cancer Center website. https://www.mskcc.org/about/innovative-collaborations/msk-alliance. Updated March 7, 2018. Accessed August 18, 2016.
25. Manne S, Kashy D, Albrecht T, et al. Attitudinal barriers to participation in oncology clinical trials: factor analysis and correlates of barriers. Eur J Cancer Care (Engl). 2015;24(1):28-38.
26. Unger JM, Gralow JR, Albain KS, Ramsey SD, Hershman DL. Patient income level and cancer clinical trial participation: a prospective survey study. JAMA Oncol. 2016;2(1):137-139.
27. Moy B. Clinical trials, disparities, and financial burden: it’s time to intervene. Oncologist. 2015;20(6):571.
28. Bonevski B, Randell M, Paul C, et al. Reaching the hard-to-reach: a systematic review of strategies for improving health and medical research with socially disadvantaged groups. BMC Med Res Methodol. 2014;14:42.
29. Gross CP, Filardo G, Mayne ST, Krumholz HM. The impact of socioeconomic status and race on trial participation for older women with breast cancer. Cancer. 2005;103(3):483-491.
30. Nickell A, Burke NJ, Cohen E, Caprio M, Joseph G. Educating low-SES and LEP survivors about breast cancer research: pilot test of the Health Research Engagement Intervention. J Cancer Educ. 2014;29(4):746-752.
31. Mancini J, Jansen J, Julian-Reynier C, Bechlian D, Vey N, Chabannon C. Preferences of older adults with cancer for involvement in decision-making about research participation. J Am Geriatr Soc. 2014;62(6):1191-1193.
The low rate of participation in clinical trials is partly owing to the lack of awareness of these trials not only among potential participants but the US population as a whole.1 This lack of awareness, however, can be reversed. For example, findings from a single-institution observational study showed that systematically sending letters about clinical trial participation to all new lung cancer patients was associated with increased trial participation.2 More recently, a large, multicenter, randomized experiment showed that attitudes toward clinical trials were improved through preparatory education about clinical trials before a patient’s first oncologic visit.3
Such clinical trial education can be used before any medical diagnosis to increase clinical trial awareness in the general population. It may be advantageous to do so because people tend to process information more effectively during less stressful times.4 Clinical trial awareness in the US population has increased slightly over time, but in 2012, one study reported that 26% of its participants lacked general awareness about clinical trials.5
Comprehensive educational material, such as a multimedia psychoeducational intervention,6 a 28-video library,3 or a 160-page book,7 which have been proposed for oncology patients, may be too intensive for someone who is not immediately deciding whether to participate in a clinical trial. However, a simple, concise form of education might be preferable and appropriate to increase basic knowledge and awareness among the general population, especially among those who are less educated.8
Our aim in the present study was to evaluate whether providing brief educational material about clinical trials would increase patient willingness to participate in these trials.
Methods
This is a single-group, cross-sectional design study in which all participants were administered the questions and the 240-word educational statement in the same order.
Sample
An electronic survey was conducted by Marketing and Planning Systems (the analytics practice of Kantar Millwardbrown) on behalf of the Memorial Sloan Kettering Cancer Center (MSK). The survey included a national sample of 1011 participants and a local sample of 500 participants from the MSK catchment area (22 counties across the 5 boroughs of New York City, Long Island, southern New York State, northern New Jersey, and southwestern Connecticut).
Survey participants were aged 18 to 69 years in the national sample and 25 to 69 years in the local sample, representing 87% and 75% of the adult populations of those areas, respectively. Respondents who were or who had a family member currently working in the fields of news, advertising/marketing, or medical care were not surveyed. Participants were sourced from an online incentivized panel with millions of potential respondents representative of the US adult population.
Questionnaire
The questionnaire collected data on participant demographics and main medical history (including previous participation in a clinical trial), and asked questions about clinical trials, focusing on:
Analyses
Descriptive and bivariate statistical analyses of participants’ characteristics were weighted to ensure national representativeness for gender, age, ethnicity, and income. Mean standard deviation (SD) was computed for every quantitative variable. Categorical variables were expressed as proportions.
Student t tests and analyses of variance (ANOVAs) were used to compare continuous variables, while chi-square tests were used to compare categorical data. Repeated measures ANOVAs were then used to determine the sociodemographic and medical characteristics associated with the impression of and willingness to enroll in a clinical trial before and after reading the educational material. The interaction between education level and time (pre- or postreading assessment) was tested to determine if the changes after reading the brief statement were different depending on education level.
All statistical analyses were 2-tailed and considered statistically significant at P < .05. Analyses were performed using SPSS PAWS Statistics 24 (IBM Inc, Armonk, New York). Effect sizes (standardized mean differences) and their 95% confidence intervals (CIs) were computed using the compute.es package for R 3.3 (R Foundation for Statistical Computing, Vienna, Austria).
Results
Participants
From October 23, 2015, through November 12, 2015, 1511 US participants responded to the survey request, including 1507 respondents (99.7%) who reported their education level and are included in the analyses of this report. The mean age of the respondents was 43.5 years (SD, 4.6). More than half of the respondents (57.8%) reported a current medical condition, mainly cardiovascular (20.0%), arthritis (20.0%), or other type or chronic pain (20.0%), and 9.0% reported a cancer diagnosis (current, 2.9%; previous, 6.1%).
Participants who at most had completed high school (18.9%, including 1.4% who had never even attended high school) were more often white women, lived outside urban areas, had lower household income, and were less likely to have health care insurance (Table 2). They also reported a current or previous cancer diagnosis less often than those of similar age who had attended college.
Previous participation in a clinical trial was reported by 9.6% of participants. Most of the clinical trials (75.0%) were testing a new drug. Previous trial participants were more likely to be older than those who had not participated in trials (46.1 years [SD, 14.8] vs 43.3 [SD, 4.6], repectively; P = .033), have a current health condition (86.2% vs 54.8%; P < .001), and know another trial participant (39.9% vs 9.5%; P < .001).
Education level and baseline impression of and
willingness to enroll in a clinical trial
A lower level of education was associated with a decreased likelihood of previous trial participation or of knowing a trial participant, as well as with less awareness and inaccurate perceptions of clinical trials (Table 2).
Participants with a high school degree or less were more likely to have a worse impression of and were less likely to enroll in a future hypothetical clinical trial before reading the educational material (Figure 1). Multivariable analyses confirmed that lower education level was associated with lower baseline overall impression, regardless of other personal characteristics (Table 3). Lowest household income was also associated with a more negative impression of trials, whereas participants with a current medical condition and with previous contact with clinical trials had a more positive impression of them. The same effects were observed with likeliness to enroll in a future hypothetical clinical trial (correlated with the overall impression: r, 0.63; P < .001), except that the negative effect of female gender was statistically significant (Table 3).
Posteducation impression of and willingnes to enroll
willingness to enroll in a clinical trial
The brief educational material was mostly considered believable (86.2%), easy to understand (84.8%), and included information that was new to participants (81.5%; Table 2). Participants with a high school diploma or less more often noted that the material provided them with new information, but they also reported more difficulties in fully understanding and believing the information. Overall, however, few participants found the information difficult to understand (4.6%) or hard to believe (1.8%; Table 2).
Most participants had an improved overall impression of clinical trials (standardized mean difference, 0.42; 95% CI, 0.35-0.50; P < .001) after reading the educational material. This increase was higher among participants with a lower completed level of education (Figure 1; standardized mean difference, 0.62; 95% CI, 0.45-0.79; Pinteraction < .001). The same effects were observed for likeliness to enroll in a future hypothetical clinical trial (P < .001; Figure 1).
After reading the informational statement, education level effect was no longer significantly associated with the overall impression of clinical trials (P = .23) and willingness to enroll in a clinical trial (P = .34), whereas the effects of income, current medical condition, and previous engangement with clinical trials remained statistically significant (Table 3).
Remaining challenges
Regarding hypothetical participation in a future clinical trial after a cancer diagnosis, the most critical concerns were related to side effects and the uncertainty of insurance coverage (Figure 2).10 The lack of understanding of clinical trials was the least critical concern; however, it was significantly higher among participants with a lower completed level of education. These participants also expressed more critical concerns about feeling like a “guinea pig,” inconvenient trial location, and the frequent visits needed.
Discussion
Findings from this survey demonstrated that providing brief educational material about cancer clinical trials was associated with a more favorable impression of clinical trials and higher interest in trial participation. Furthermore, as far as we can ascertain, this is the first report showing how a simple intervention such as this may help close the knowledge gap on clinical trials among people of different educational backgrounds. Although most respondents in this interventional survey noted an increased willingness to consider participation in a clinical trial after reading the educational material, those with a lower level of education and knowledge about clinical trials received the most benefit. Previous participation in a clinical trial was also strongly associated with the impression of and willingness to enroll in a trial, both before and after reading the statement.11
Most of the interventions evaluated to date12 have focused on patients who are faced with having to decide whether to participate in a clinical trial2,3 or on very specific populations, such as select ethnic communities.13,14 However, it may be beneficial to provide simple, concise educational information about clinical trials to the general population, especially to those with minimal education. Although level of education concerns the minority of our sample (19%) sourced from an online panel, in the 2015 Current Population Survey, 41% of US participants aged 25 and older had not reached college education.15 Those with a lower level of education have reported a general lack of familiarity with clinical trials and were more likely to have inaccurate perceptions about trials. This is consistent with previous studies that have shown the lack of awareness and knowledge of clinical trials in this population.5,16,17 Our findings suggest that this knowledge gap can be reversed through a simple educational intervention and result in an increased willingness to participate.
The provision of information on clinical trials was positively associated with the 2 outcomes analyzed – improved impression of clinical trials and increased likelihood to enroll in a hypothetical trial. Such improvement might not translate to improved accrual,18 but it is a step toward closing the overall knowledge gap related to clinical trials and increasing the number of people who would consider trial participation. The lack of awareness of clinical trials has been reported as a legitimate explanation for why participation rates are lower in less-educated patient populations.19,20 This brief educational intervention is a simple, technology-sparing way to increase clinical trial awareness in the general population. In a similar survey, most physicians who reviewed an educational statement noted they were likely to use it with patients.21Less-educated patients, those who lived outside of urban areas, and those with lower household incomes were most concerned about trial location and the frequent visits needed when participating in a trial (Figure 2). Living in a nonurban area was not associated with participant impression of clinical trials or willingness to enroll in a trial. However, rural residency may be a barrier to enrollment depending on distance to the hospital22 and out-of-pocket expenses related to travel.23 Some comprehensive cancer centers, such as MSK, have developed alliances with community centers24 as a means of overcoming geographical barriers and increasing clinical trial participation rates.
Another concern shared by most respondents was the uncertainty in insurance coverage and potential out-of-pocket costs related to care. Lower household income, unlike location of residence and lack of insurance, was significantly associated with negative impressions of clinical trials and lower willingness to enroll in a trial, even after adjusting for education level. Cancer patients with higher financial burden have reported more attitudinal barriers, even after accounting for the negative effect of lower education level.25 Recent studies have also discussed the negative impact of lower income on cancer clinical trial participation,19,20,26,27 and new attention has been paid to the negative financial implications or “financial toxicity” of participating in a trial.23,28
White and older survey participants showed similar interest in clinical trial participation after accounting for other characteristics. There is growing evidence that outcome differences attributed to race may in fact be more dependent on socioeconomic status.8 A recent study among breast cancer patients showed that low socioeconomic status, but not race, was associated with decreased participation in clinical trials.29,30 Previous findings have also indicated that interest in clinical trials and barriers to enrollment among older, less-educated patients31 are often related to ineligibility, comorbidity, or communication difficulties.
Among our participants, the fear of side effects also was a common attitudinal barrier to clinical trial participation, as has been reported in previous studies.3,20 However, contrary to one previous study,20 this fear was not significantly increased among our less-educated participants.
Less-educated participants also reported more difficulties in understanding the information they were provided with, and they remained more concerned about being treated like “guinea pigs.” These concerns are consistent with other results showing that decisional conflict about clinical trial participation among patients with a high school diploma or less remained high even after they had received a National Cancer Institute text as pre-education material.3
Limitations
The lack of randomization makes it difficult to attribute with certainty that the change in acceptability of clinical trial participation is owing to the reading of the educational statement. The survey also sampled only English-speaking and well-educated participants from an online panel (81.1% had at least attended college) despite the use of a weighting procedure to ensure representativeness regarding gender, age, ethnicity, and income. Health literacy level or more specific trial literacy level was not evaluated; however, we were able to show less accurate perceptions of clinical trials among participants with a lower level of education by using agreement toward 8 statements about trials. The responses to hypothetical questions from these participants in the general population may also not be generalizable to a restricted population of patients with cancer. In addition, we measured impression of and willingness to enroll in a clinical trial immediately after providing participants with the educational material. We would have to confirm whether the positive effects of the education persist over time and translate to higher clinical trial participation rates.
Conclusions
Participants were receptive of educational material and expressed greater interest in and likelihood of enrolling in a clinical trial after reading it. The information had a greater effect on those with less education, but increased the willingness of all participants to enroll.
The low rate of participation in clinical trials is partly owing to the lack of awareness of these trials not only among potential participants but the US population as a whole.1 This lack of awareness, however, can be reversed. For example, findings from a single-institution observational study showed that systematically sending letters about clinical trial participation to all new lung cancer patients was associated with increased trial participation.2 More recently, a large, multicenter, randomized experiment showed that attitudes toward clinical trials were improved through preparatory education about clinical trials before a patient’s first oncologic visit.3
Such clinical trial education can be used before any medical diagnosis to increase clinical trial awareness in the general population. It may be advantageous to do so because people tend to process information more effectively during less stressful times.4 Clinical trial awareness in the US population has increased slightly over time, but in 2012, one study reported that 26% of its participants lacked general awareness about clinical trials.5
Comprehensive educational material, such as a multimedia psychoeducational intervention,6 a 28-video library,3 or a 160-page book,7 which have been proposed for oncology patients, may be too intensive for someone who is not immediately deciding whether to participate in a clinical trial. However, a simple, concise form of education might be preferable and appropriate to increase basic knowledge and awareness among the general population, especially among those who are less educated.8
Our aim in the present study was to evaluate whether providing brief educational material about clinical trials would increase patient willingness to participate in these trials.
Methods
This is a single-group, cross-sectional design study in which all participants were administered the questions and the 240-word educational statement in the same order.
Sample
An electronic survey was conducted by Marketing and Planning Systems (the analytics practice of Kantar Millwardbrown) on behalf of the Memorial Sloan Kettering Cancer Center (MSK). The survey included a national sample of 1011 participants and a local sample of 500 participants from the MSK catchment area (22 counties across the 5 boroughs of New York City, Long Island, southern New York State, northern New Jersey, and southwestern Connecticut).
Survey participants were aged 18 to 69 years in the national sample and 25 to 69 years in the local sample, representing 87% and 75% of the adult populations of those areas, respectively. Respondents who were or who had a family member currently working in the fields of news, advertising/marketing, or medical care were not surveyed. Participants were sourced from an online incentivized panel with millions of potential respondents representative of the US adult population.
Questionnaire
The questionnaire collected data on participant demographics and main medical history (including previous participation in a clinical trial), and asked questions about clinical trials, focusing on:
Analyses
Descriptive and bivariate statistical analyses of participants’ characteristics were weighted to ensure national representativeness for gender, age, ethnicity, and income. Mean standard deviation (SD) was computed for every quantitative variable. Categorical variables were expressed as proportions.
Student t tests and analyses of variance (ANOVAs) were used to compare continuous variables, while chi-square tests were used to compare categorical data. Repeated measures ANOVAs were then used to determine the sociodemographic and medical characteristics associated with the impression of and willingness to enroll in a clinical trial before and after reading the educational material. The interaction between education level and time (pre- or postreading assessment) was tested to determine if the changes after reading the brief statement were different depending on education level.
All statistical analyses were 2-tailed and considered statistically significant at P < .05. Analyses were performed using SPSS PAWS Statistics 24 (IBM Inc, Armonk, New York). Effect sizes (standardized mean differences) and their 95% confidence intervals (CIs) were computed using the compute.es package for R 3.3 (R Foundation for Statistical Computing, Vienna, Austria).
Results
Participants
From October 23, 2015, through November 12, 2015, 1511 US participants responded to the survey request, including 1507 respondents (99.7%) who reported their education level and are included in the analyses of this report. The mean age of the respondents was 43.5 years (SD, 4.6). More than half of the respondents (57.8%) reported a current medical condition, mainly cardiovascular (20.0%), arthritis (20.0%), or other type or chronic pain (20.0%), and 9.0% reported a cancer diagnosis (current, 2.9%; previous, 6.1%).
Participants who at most had completed high school (18.9%, including 1.4% who had never even attended high school) were more often white women, lived outside urban areas, had lower household income, and were less likely to have health care insurance (Table 2). They also reported a current or previous cancer diagnosis less often than those of similar age who had attended college.
Previous participation in a clinical trial was reported by 9.6% of participants. Most of the clinical trials (75.0%) were testing a new drug. Previous trial participants were more likely to be older than those who had not participated in trials (46.1 years [SD, 14.8] vs 43.3 [SD, 4.6], repectively; P = .033), have a current health condition (86.2% vs 54.8%; P < .001), and know another trial participant (39.9% vs 9.5%; P < .001).
Education level and baseline impression of and
willingness to enroll in a clinical trial
A lower level of education was associated with a decreased likelihood of previous trial participation or of knowing a trial participant, as well as with less awareness and inaccurate perceptions of clinical trials (Table 2).
Participants with a high school degree or less were more likely to have a worse impression of and were less likely to enroll in a future hypothetical clinical trial before reading the educational material (Figure 1). Multivariable analyses confirmed that lower education level was associated with lower baseline overall impression, regardless of other personal characteristics (Table 3). Lowest household income was also associated with a more negative impression of trials, whereas participants with a current medical condition and with previous contact with clinical trials had a more positive impression of them. The same effects were observed with likeliness to enroll in a future hypothetical clinical trial (correlated with the overall impression: r, 0.63; P < .001), except that the negative effect of female gender was statistically significant (Table 3).
Posteducation impression of and willingnes to enroll
willingness to enroll in a clinical trial
The brief educational material was mostly considered believable (86.2%), easy to understand (84.8%), and included information that was new to participants (81.5%; Table 2). Participants with a high school diploma or less more often noted that the material provided them with new information, but they also reported more difficulties in fully understanding and believing the information. Overall, however, few participants found the information difficult to understand (4.6%) or hard to believe (1.8%; Table 2).
Most participants had an improved overall impression of clinical trials (standardized mean difference, 0.42; 95% CI, 0.35-0.50; P < .001) after reading the educational material. This increase was higher among participants with a lower completed level of education (Figure 1; standardized mean difference, 0.62; 95% CI, 0.45-0.79; Pinteraction < .001). The same effects were observed for likeliness to enroll in a future hypothetical clinical trial (P < .001; Figure 1).
After reading the informational statement, education level effect was no longer significantly associated with the overall impression of clinical trials (P = .23) and willingness to enroll in a clinical trial (P = .34), whereas the effects of income, current medical condition, and previous engangement with clinical trials remained statistically significant (Table 3).
Remaining challenges
Regarding hypothetical participation in a future clinical trial after a cancer diagnosis, the most critical concerns were related to side effects and the uncertainty of insurance coverage (Figure 2).10 The lack of understanding of clinical trials was the least critical concern; however, it was significantly higher among participants with a lower completed level of education. These participants also expressed more critical concerns about feeling like a “guinea pig,” inconvenient trial location, and the frequent visits needed.
Discussion
Findings from this survey demonstrated that providing brief educational material about cancer clinical trials was associated with a more favorable impression of clinical trials and higher interest in trial participation. Furthermore, as far as we can ascertain, this is the first report showing how a simple intervention such as this may help close the knowledge gap on clinical trials among people of different educational backgrounds. Although most respondents in this interventional survey noted an increased willingness to consider participation in a clinical trial after reading the educational material, those with a lower level of education and knowledge about clinical trials received the most benefit. Previous participation in a clinical trial was also strongly associated with the impression of and willingness to enroll in a trial, both before and after reading the statement.11
Most of the interventions evaluated to date12 have focused on patients who are faced with having to decide whether to participate in a clinical trial2,3 or on very specific populations, such as select ethnic communities.13,14 However, it may be beneficial to provide simple, concise educational information about clinical trials to the general population, especially to those with minimal education. Although level of education concerns the minority of our sample (19%) sourced from an online panel, in the 2015 Current Population Survey, 41% of US participants aged 25 and older had not reached college education.15 Those with a lower level of education have reported a general lack of familiarity with clinical trials and were more likely to have inaccurate perceptions about trials. This is consistent with previous studies that have shown the lack of awareness and knowledge of clinical trials in this population.5,16,17 Our findings suggest that this knowledge gap can be reversed through a simple educational intervention and result in an increased willingness to participate.
The provision of information on clinical trials was positively associated with the 2 outcomes analyzed – improved impression of clinical trials and increased likelihood to enroll in a hypothetical trial. Such improvement might not translate to improved accrual,18 but it is a step toward closing the overall knowledge gap related to clinical trials and increasing the number of people who would consider trial participation. The lack of awareness of clinical trials has been reported as a legitimate explanation for why participation rates are lower in less-educated patient populations.19,20 This brief educational intervention is a simple, technology-sparing way to increase clinical trial awareness in the general population. In a similar survey, most physicians who reviewed an educational statement noted they were likely to use it with patients.21Less-educated patients, those who lived outside of urban areas, and those with lower household incomes were most concerned about trial location and the frequent visits needed when participating in a trial (Figure 2). Living in a nonurban area was not associated with participant impression of clinical trials or willingness to enroll in a trial. However, rural residency may be a barrier to enrollment depending on distance to the hospital22 and out-of-pocket expenses related to travel.23 Some comprehensive cancer centers, such as MSK, have developed alliances with community centers24 as a means of overcoming geographical barriers and increasing clinical trial participation rates.
Another concern shared by most respondents was the uncertainty in insurance coverage and potential out-of-pocket costs related to care. Lower household income, unlike location of residence and lack of insurance, was significantly associated with negative impressions of clinical trials and lower willingness to enroll in a trial, even after adjusting for education level. Cancer patients with higher financial burden have reported more attitudinal barriers, even after accounting for the negative effect of lower education level.25 Recent studies have also discussed the negative impact of lower income on cancer clinical trial participation,19,20,26,27 and new attention has been paid to the negative financial implications or “financial toxicity” of participating in a trial.23,28
White and older survey participants showed similar interest in clinical trial participation after accounting for other characteristics. There is growing evidence that outcome differences attributed to race may in fact be more dependent on socioeconomic status.8 A recent study among breast cancer patients showed that low socioeconomic status, but not race, was associated with decreased participation in clinical trials.29,30 Previous findings have also indicated that interest in clinical trials and barriers to enrollment among older, less-educated patients31 are often related to ineligibility, comorbidity, or communication difficulties.
Among our participants, the fear of side effects also was a common attitudinal barrier to clinical trial participation, as has been reported in previous studies.3,20 However, contrary to one previous study,20 this fear was not significantly increased among our less-educated participants.
Less-educated participants also reported more difficulties in understanding the information they were provided with, and they remained more concerned about being treated like “guinea pigs.” These concerns are consistent with other results showing that decisional conflict about clinical trial participation among patients with a high school diploma or less remained high even after they had received a National Cancer Institute text as pre-education material.3
Limitations
The lack of randomization makes it difficult to attribute with certainty that the change in acceptability of clinical trial participation is owing to the reading of the educational statement. The survey also sampled only English-speaking and well-educated participants from an online panel (81.1% had at least attended college) despite the use of a weighting procedure to ensure representativeness regarding gender, age, ethnicity, and income. Health literacy level or more specific trial literacy level was not evaluated; however, we were able to show less accurate perceptions of clinical trials among participants with a lower level of education by using agreement toward 8 statements about trials. The responses to hypothetical questions from these participants in the general population may also not be generalizable to a restricted population of patients with cancer. In addition, we measured impression of and willingness to enroll in a clinical trial immediately after providing participants with the educational material. We would have to confirm whether the positive effects of the education persist over time and translate to higher clinical trial participation rates.
Conclusions
Participants were receptive of educational material and expressed greater interest in and likelihood of enrolling in a clinical trial after reading it. The information had a greater effect on those with less education, but increased the willingness of all participants to enroll.
1. Ford JG, Howerton MW, Lai GY, et al. Barriers to recruiting underrepresented populations to cancer clinical trials: a systematic review. Cancer. 2008;112(2):228-242.
2. Quinn GP, Bell BA, Bell MY, et al. The guinea pig syndrome: improving clinical trial participation among thoracic patients. J Thorac Oncol. 2007;2(3):191-196.
3. Meropol NJ, Wong Y-N, Albrecht T, et al. Randomized trial of a web-based intervention to address barriers to clinical trials. J Clin Oncol. 2016;34(5):469-478.
4. Goldberg RJ. Disclosure of information to adult cancer patients: issues and update. J Clin Oncol. 1984;2(8):948-955.
5. Leiter A, Diefenbach MA, Doucette J, Oh WK, Galsky MD. Clinical trial awareness: changes over time and sociodemographic disparities. Clin Trials. 2015;12(3):215-223.
6. Jacobsen PB, Wells KJ, Meade CD, et al. Effects of a brief multimedia psychoeducational intervention on the attitudes and interest of patients with cancer regarding clinical trial participation: a multicenter randomized controlled trial. J Clin Oncol. 2012;30(20):2516-2521.
7. Carney PA, Tucker EK, Newby TA, Beer TM. Feasibility, acceptability and findings from a pilot randomized controlled intervention study on the impact of a book designed to inform patients about cancer clinical trials. J Cancer Educ. 2014;29(1):181-187.
8. Sharrocks K, Spicer J, Camidge DR, Papa S. The impact of socioeconomic status on access to cancer clinical trials. Br J Cancer. 2014;111(9):1684-1687.
9. Regan J, Hickey C, Targett C, Masuda S, Sabbatini P. Framing clinical research and the importance of trial participation: patient and physician perspective. Poster presented at: 8th Annual AACI Clinical Research Initiative Meeting; July 20-21 2016; Chicago, IL.
10. Mancini J, Genre D, Dalenc F, et al. Patients’ regrets after participating in a randomized controlled trials depended on their involvement in the decision making. J Clin Epidemiol. 2012;65(6):635-642
11. Murphy ST, Frank LB, Chatterjee JS, et al. Comparing the relative efficacy of narrative vs nonnarrative health messages in reducing health disparities using a randomized trial. Am J Public Health. 2015;105(10):2117-2123.
12. Treweek S, Lockhart P, Pitkethly M, et al. Methods to improve recruitment to randomised controlled trials: Cochrane systematic review and meta-analysis. BMJ Open. 2013;3(2):e002360. doi:10.1136/bmjopen-2012-002360.
13. Ma GX, Tan Y, Blakeney NC, et al. The impact of a community-based clinical trial educational intervention among underrepresented Chinese Americans. Cancer Epidemiol Biomarkers Prev. 2014;23(3):424-432.
14. Cupertino AP, Molina CSP, de los Rios JB, et al. Knowledge, awareness, and interest in cancer clinical trials among rural latinos following brief education by promotores de salud. J Community Med Health Educ. 2015;5:2161. doi:10.4172/2161-0711.1000358
15. Ryan CL, Bauman K. Educational attainment in the United States: 2015. https://www.census.gov/content/dam/Census/library/publications/2016/demo/p20-578.pdf. Published March 2016. Accessed October 18, 2016.
16. Lara PN Jr, Paterniti DA, Chiechi C, et al. Evaluation of factors affecting awareness of and willingness to participate in cancer clinical trials. J Clin Oncol. 2005;23(36):9282-9289.
17. Dhai A, Etheredge H, Cleaton-Jones P. A pilot study evaluating an intervention designed to raise awareness of clinical trials among potential participants in the developing world. J Med Ethics. 2010;36(4):238-242.
18. Denicoff AM, McCaskill-Stevens W, Grubbs SS, et al. The National Cancer Institute–American Society of Clinical Oncology Cancer Trial Accrual Symposium: summary and recommendations. J Oncol Pract. 2013;9(6):267-276.
19. Davison BJ, So A, Goldenberg SL, Berkowitz J, Gleave ME. Measurement of factors influencing the participation of patients with prostate cancer in clinical trials: a Canadian perspective. BJU Int. 2008;101(8):982-987.
20. Unger JM, Hershman DL, Albain KS, et al. Patient income level and cancer clinical trial participation. J Clin Oncol. 2013;31(5):536-542.
21. IRB Advisor. Education may overcome reticence to join trials. Relias website. http://www.ahcmedia.com/articles/138088-education-may-overcome-reticence-to-join-trials. Published July 1, 2016. Accessed July 29, 2016.
22. Vanderpool RC, Kornfeld J, Mills L, Byrne MM. Rural-urban differences in discussions of cancer treatment clinical trials. Patient Educ Couns. 2011;85(2):e69-e74.
23. Meropol NJ. Health policy: overcoming cost barriers to clinical trial participation. Nat Rev Clin Oncol. 2016;13(6):333-334.
24. MSK Cancer Alliance. Memorial Sloan Kettering Cancer Center website. https://www.mskcc.org/about/innovative-collaborations/msk-alliance. Updated March 7, 2018. Accessed August 18, 2016.
25. Manne S, Kashy D, Albrecht T, et al. Attitudinal barriers to participation in oncology clinical trials: factor analysis and correlates of barriers. Eur J Cancer Care (Engl). 2015;24(1):28-38.
26. Unger JM, Gralow JR, Albain KS, Ramsey SD, Hershman DL. Patient income level and cancer clinical trial participation: a prospective survey study. JAMA Oncol. 2016;2(1):137-139.
27. Moy B. Clinical trials, disparities, and financial burden: it’s time to intervene. Oncologist. 2015;20(6):571.
28. Bonevski B, Randell M, Paul C, et al. Reaching the hard-to-reach: a systematic review of strategies for improving health and medical research with socially disadvantaged groups. BMC Med Res Methodol. 2014;14:42.
29. Gross CP, Filardo G, Mayne ST, Krumholz HM. The impact of socioeconomic status and race on trial participation for older women with breast cancer. Cancer. 2005;103(3):483-491.
30. Nickell A, Burke NJ, Cohen E, Caprio M, Joseph G. Educating low-SES and LEP survivors about breast cancer research: pilot test of the Health Research Engagement Intervention. J Cancer Educ. 2014;29(4):746-752.
31. Mancini J, Jansen J, Julian-Reynier C, Bechlian D, Vey N, Chabannon C. Preferences of older adults with cancer for involvement in decision-making about research participation. J Am Geriatr Soc. 2014;62(6):1191-1193.
1. Ford JG, Howerton MW, Lai GY, et al. Barriers to recruiting underrepresented populations to cancer clinical trials: a systematic review. Cancer. 2008;112(2):228-242.
2. Quinn GP, Bell BA, Bell MY, et al. The guinea pig syndrome: improving clinical trial participation among thoracic patients. J Thorac Oncol. 2007;2(3):191-196.
3. Meropol NJ, Wong Y-N, Albrecht T, et al. Randomized trial of a web-based intervention to address barriers to clinical trials. J Clin Oncol. 2016;34(5):469-478.
4. Goldberg RJ. Disclosure of information to adult cancer patients: issues and update. J Clin Oncol. 1984;2(8):948-955.
5. Leiter A, Diefenbach MA, Doucette J, Oh WK, Galsky MD. Clinical trial awareness: changes over time and sociodemographic disparities. Clin Trials. 2015;12(3):215-223.
6. Jacobsen PB, Wells KJ, Meade CD, et al. Effects of a brief multimedia psychoeducational intervention on the attitudes and interest of patients with cancer regarding clinical trial participation: a multicenter randomized controlled trial. J Clin Oncol. 2012;30(20):2516-2521.
7. Carney PA, Tucker EK, Newby TA, Beer TM. Feasibility, acceptability and findings from a pilot randomized controlled intervention study on the impact of a book designed to inform patients about cancer clinical trials. J Cancer Educ. 2014;29(1):181-187.
8. Sharrocks K, Spicer J, Camidge DR, Papa S. The impact of socioeconomic status on access to cancer clinical trials. Br J Cancer. 2014;111(9):1684-1687.
9. Regan J, Hickey C, Targett C, Masuda S, Sabbatini P. Framing clinical research and the importance of trial participation: patient and physician perspective. Poster presented at: 8th Annual AACI Clinical Research Initiative Meeting; July 20-21 2016; Chicago, IL.
10. Mancini J, Genre D, Dalenc F, et al. Patients’ regrets after participating in a randomized controlled trials depended on their involvement in the decision making. J Clin Epidemiol. 2012;65(6):635-642
11. Murphy ST, Frank LB, Chatterjee JS, et al. Comparing the relative efficacy of narrative vs nonnarrative health messages in reducing health disparities using a randomized trial. Am J Public Health. 2015;105(10):2117-2123.
12. Treweek S, Lockhart P, Pitkethly M, et al. Methods to improve recruitment to randomised controlled trials: Cochrane systematic review and meta-analysis. BMJ Open. 2013;3(2):e002360. doi:10.1136/bmjopen-2012-002360.
13. Ma GX, Tan Y, Blakeney NC, et al. The impact of a community-based clinical trial educational intervention among underrepresented Chinese Americans. Cancer Epidemiol Biomarkers Prev. 2014;23(3):424-432.
14. Cupertino AP, Molina CSP, de los Rios JB, et al. Knowledge, awareness, and interest in cancer clinical trials among rural latinos following brief education by promotores de salud. J Community Med Health Educ. 2015;5:2161. doi:10.4172/2161-0711.1000358
15. Ryan CL, Bauman K. Educational attainment in the United States: 2015. https://www.census.gov/content/dam/Census/library/publications/2016/demo/p20-578.pdf. Published March 2016. Accessed October 18, 2016.
16. Lara PN Jr, Paterniti DA, Chiechi C, et al. Evaluation of factors affecting awareness of and willingness to participate in cancer clinical trials. J Clin Oncol. 2005;23(36):9282-9289.
17. Dhai A, Etheredge H, Cleaton-Jones P. A pilot study evaluating an intervention designed to raise awareness of clinical trials among potential participants in the developing world. J Med Ethics. 2010;36(4):238-242.
18. Denicoff AM, McCaskill-Stevens W, Grubbs SS, et al. The National Cancer Institute–American Society of Clinical Oncology Cancer Trial Accrual Symposium: summary and recommendations. J Oncol Pract. 2013;9(6):267-276.
19. Davison BJ, So A, Goldenberg SL, Berkowitz J, Gleave ME. Measurement of factors influencing the participation of patients with prostate cancer in clinical trials: a Canadian perspective. BJU Int. 2008;101(8):982-987.
20. Unger JM, Hershman DL, Albain KS, et al. Patient income level and cancer clinical trial participation. J Clin Oncol. 2013;31(5):536-542.
21. IRB Advisor. Education may overcome reticence to join trials. Relias website. http://www.ahcmedia.com/articles/138088-education-may-overcome-reticence-to-join-trials. Published July 1, 2016. Accessed July 29, 2016.
22. Vanderpool RC, Kornfeld J, Mills L, Byrne MM. Rural-urban differences in discussions of cancer treatment clinical trials. Patient Educ Couns. 2011;85(2):e69-e74.
23. Meropol NJ. Health policy: overcoming cost barriers to clinical trial participation. Nat Rev Clin Oncol. 2016;13(6):333-334.
24. MSK Cancer Alliance. Memorial Sloan Kettering Cancer Center website. https://www.mskcc.org/about/innovative-collaborations/msk-alliance. Updated March 7, 2018. Accessed August 18, 2016.
25. Manne S, Kashy D, Albrecht T, et al. Attitudinal barriers to participation in oncology clinical trials: factor analysis and correlates of barriers. Eur J Cancer Care (Engl). 2015;24(1):28-38.
26. Unger JM, Gralow JR, Albain KS, Ramsey SD, Hershman DL. Patient income level and cancer clinical trial participation: a prospective survey study. JAMA Oncol. 2016;2(1):137-139.
27. Moy B. Clinical trials, disparities, and financial burden: it’s time to intervene. Oncologist. 2015;20(6):571.
28. Bonevski B, Randell M, Paul C, et al. Reaching the hard-to-reach: a systematic review of strategies for improving health and medical research with socially disadvantaged groups. BMC Med Res Methodol. 2014;14:42.
29. Gross CP, Filardo G, Mayne ST, Krumholz HM. The impact of socioeconomic status and race on trial participation for older women with breast cancer. Cancer. 2005;103(3):483-491.
30. Nickell A, Burke NJ, Cohen E, Caprio M, Joseph G. Educating low-SES and LEP survivors about breast cancer research: pilot test of the Health Research Engagement Intervention. J Cancer Educ. 2014;29(4):746-752.
31. Mancini J, Jansen J, Julian-Reynier C, Bechlian D, Vey N, Chabannon C. Preferences of older adults with cancer for involvement in decision-making about research participation. J Am Geriatr Soc. 2014;62(6):1191-1193.
Enhancing communication between oncology care providers and patient caregivers during hospice
Improving the delivery of end-of-life care for patients with advanced cancer has become a priority in the United States.1,2 Quality metrics identifying the components of high-quality end-of-life care have focused on improved symptom management, decreased use of chemotherapy at the end of life, fewer hospitalizations, and increased use of hospice care. Patients and caregivers also consider good communication with the medical team to be a critical component of end-of-life care.3-5 Interventions to improve the quality of end-of-life care are needed.
Caregivers of patients with advanced cancer who receive hospice services report better quality of care and death than those receiving end-of-life care in other settings.6-9 However, the transition for patients from active cancer therapy delivered by their oncologists to end-of-life care delivered by a hospice care team can be abrupt. Patients and their caregivers often feel abandoned by oncology clinicians because of the lack of continuity of care and poor communication.10-13 Caregivers who note continued involvement and communication with their oncology clinicians experience a lower caregiving burden, report higher satisfaction with care, and recount a higher quality of death for their loved one.14-16 Therefore, interventions that prevent abrupt transitions in care from oncology to hospice by ensuring continued communication with oncology clinicians are needed to improve the quality of end-of-life care.17 Recent findings have shown that providing concurrent oncology and palliative care is not only feasible but beneficial for patients with advanced cancer and their caregivers.18-24 However, there is no standard of care for the involvement of oncology clinicians in the care of patients receiving hospice services and their families.
Although interventions may be needed, it could be challenging to deliver them given the multiple demands of caregiving during hospice and the lack of regular contact in clinic. We sought to assess the feasibility of an intervention, Ensuring Communication in Hospice by Oncology (ECHO), to facilitate communication between oncology clinicians and caregivers of patients who enroll in hospice. We also explored caregiver-reported outcomes during hospice care, including satisfaction with care, attitudes toward caregiving, stress, decision regret, and perception of the quality of patients’ end-of-life care.
Methods
Study design
During March 2014-June 2015, caregivers of patients with advanced cancer who enrolled in home hospice services were eligible to participate in the study at Massachusetts General Hospital (MGH) in Boston. The Dana Farber/Harvard Cancer Center Institutional Review Board approved all methods and materials. The study opened with an enrollment goal of 30 participating caregivers. However, due to staff transitions, we closed the study early in June 2015 after 25 caregivers enrolled.
Participants
Caregivers of patients receiving care at the cancer center's thoracic, head and neck, sarcoma, melanoma, and gynecological disease centers were eligible within 10 days after a patient’s enrollment in hospice. Five disease sites were selected to participate in the intervention. We defined caregivers as relatives or friends serving as the primary caregiver of the patient at home during hospice care. Other caregiver eligibility criteria included the ability to read and respond to questions in English or with a translator, access to a telephone and/or computer to communicate with oncology clinicians, and willingness to complete questionnaires. Caregivers were ineligible if the patient was participating in an ongoing palliative care trial.
To identify eligible caregivers, case managers from both the inpatient and outpatient settings, as well as the nurses based in participating disease centers, notified the research team of all patients referred to hospice. If the patient had received oncology care in one of our participating disease centers, the research team contacted their oncology clinician/s (physicians, nurse practitioners [NP], registered nurses [RN], and/or physician assistants [PA]) to inquire if the patient had an involved caregiver and to obtain permission to offer study participation. If the oncology clinician/s did not grant permission, we documented the reason. Otherwise, with permission, research staff contacted the caregiver by telephone to offer study participation and obtain verbal consent. We then sent participating caregivers a copy of the informed consent by mail or e-mail.
Intervention
The ECHO intervention consisted of: supportive phone calls from an oncology clinician to the caregiver; an optional clinic visit with the oncology clinician for the patient to address clinical questions or concerns that was offered during the initial telephone consent; a bereavement call to the participating caregiver (Figure 1). Initially, we designed the intervention to have phone calls occurring twice weekly until the patient died. However, 3 months after starting the study, we received feedback from oncology clinicians and caregivers that calls were too frequent, so we amended the protocol to include phone calls twice weekly for the first 2 weeks of the study and then weekly thereafter. Seven months into the study, we again decreased the number of phone calls to weekly for the first 4 weeks, every other week for 4 weeks, and then monthly until patient death. We informed caregivers of changes by e-mail.
Before we started the study, we conducted training sessions with oncology clinicians from the participating disease centers to review study procedures and expectations of the phone calls. Supportive phone calls during hospice were not a part of standard practice prior to the study. The RN, NP or PA, and/or physician who had an established relationship with the patient and caregiver completed the phone calls. They decided based on their respective relationships with the patients and their workloads who would call each week, though the majority of calls were conducted by the RN or NP. All the clinicians had experience comanaging patients with hospice agencies, and our general practice is for the oncology physician to serve as the hospice attending of record. The calls were intended to offer support and reassurance to caregivers. We did not script the calls so that clinicians could tailor their content to the individual needs of the caregiver, as informed by their established relationship. The calls could include the patient if he/she was able to and interested in speaking to the clinician. There was no standardized communication with hospice as part of the intervention. If a caregiver raised concerns about symptom management during a call, the clinician would advise the caregiver to contact the hospice team directly or the clinician would call the hospice to discuss, depending on the clinical scenario and the clinician’s judgment. Research staff reminded oncology clinicians to call caregivers on the scheduled date and to document the discussion in the electronic medical record. The hospice phone number was included in the e-mail. If the call was not documented, research staff sent a reminder e-mail to the oncology clinicians 24 hours after the call was due.
Caregiver-reported measures
Caregivers completed a demographic questionnaire at baseline in which they reported their age, gender, race, ethnicity, religion, employment status, and relationship to the patient. We collected information about patient characteristics from the electronic medical record, including age, gender, and cancer type. In addition, we administered validated, self-report measures (see below). We limited the number of measures to decrease caregiver burden:
The baseline questionnaire included the FACQ-PC, the FAMCARE scale, the PSS, and the Decision Regret Scale. Initially, the study involved weekly questionnaires after baseline that included the FACQ-PC, the FAMCARE scale, and the PSS. However, after 3 months of study enrollment, we received feedback that the questionnaires were too frequent, so we amended the protocol and changed the frequency to weekly for 2 weeks, then monthly thereafter until the patient died.
Caregiver exit interview
Exit interviews included the toolkit interview, the Quality of End-of-Life Care scale, and the Decision Regret Scale. Caregivers also reported patients’ place and date of death. After the first 6 caregivers enrolled, we amended the exit interview to include open-ended feedback from caregivers. Specifically, we evaluated caregivers’ perceptions of the ECHO intervention by asking them about their perception of and satisfaction with the content and frequency of the oncology clinicians’ phone calls, whether they had an in-person visit with their oncology clinicians after the start of hospice care, whether the clinician/s contacted them after the patient died, and whether there were ways in which the clinician/s could help in the future.
Data collection and storage
Caregivers were given the option of completing study measures by telephone or e-mail so that they could complete them on a computer when it was convenient for them. Caregivers received a link to Research Electronic Data Capture (REDCap), a web-based, HIPAA-compliant application that allows participants to answer questionnaires online. The exit interviews were completed by phone, and research staff entered the data into the REDCap database. In addition, with we obtained caregiver permission to audiorecord the exit interviews, which were then transcribed and de-identified.
Statistical analysis
The primary outcome for the study was feasibility, which we defined as >70% of the caregivers receiving >50% of the phone calls from an oncology clinician, and >70% of the caregivers completing >50% of the questionnaires. All time points for the questionnaires and the exit interview counted toward feasibility. Exploratory endpoints included caregiver-reported satisfaction, stress, quality of end-of-life care, and decision-making regret.
Using STATA (v9.3; StataCorp, College Station, Texas) for all statistical analyses, we summarized participants’ characteristics and outcomes as frequencies and percentages for categorical variables and mean standard deviation for continuous variables. We used the repeated-measures t test to assess changes in caregiver outcomes over time. We used the Fisher exact test to compare clinically meaningful threshold scores of perceived stress between men and women.
We examined caregivers’ open-ended feedback using descriptive analyses to summarize comments about the intervention and to inform possible refinements for a future study.
Results
Baseline characteristics
During March 2014-June 2015, we enrolled caregivers of patients with advanced cancer from 5 participating disease centers: thoracic, head and neck, sarcoma, melanoma, and gynecological malignancy. We screened 123 patients to determine the eligibility of their caregivers (Figure 2). Of 38 eligible caregivers, 7 could not be reached, 6 declined participation, and 25 enrolled in the study (81% enrollment rate). Of the 25 caregivers who enrolled, 3 withdrew – 2 because the patients they were caring for died before the intervention began, and 1 who withdrew from the study because the family wanted less contact with the oncology clinician/s. Thus, we had data for 22 caregivers for our feasibility evaluation. One caregiver stopped study assessments after 3 months because the patient dis-enrolled from hospice. Median time from the patients’ hospice enrollment to caregiver study enrollment was 3 days (range, 1-9). Median time from study enrollment to patient death was 36 days (range, 2-135). Patients were receiving care from 10 different hospice agencies.
All of the patients had metastatic cancer, and 64% were women (Table 1).
Most of the caregivers were white (n = 18, 90%) and women (n = 12, 55%). The majority were the patient’s spouse (n = 12, 60%) or child (n = 6, 30%), and they lived with the patient (n = 16, 80%). Many of the caregivers had other responsibilities in addition to caring for the patients, including part- or full-time work (n = 10, 50%) or caring for others in addition to the patient (n = 10, 50%).
Feasibility
Over the study period, oncology clinicians completed 164 of 180 possible phone calls (91%). All 22 caregivers received >50% of the phone calls. Caregivers completed 78 of 99 possible questionnaires (79%), and 16 of 22 completed >50% of the questionnaires (73%). None of the caregivers/patients wanted to schedule the optional visit with an oncology clinician that was offered as part of the intervention; however, 5 patients had a clinic visit after hospice enrollment. In addition, 2 oncologists visited a patient and caregiver at home. All caregivers received bereavement contact from the oncology team.
Caregiver-reported outcomes
In all, 20 of the 22 enrolled caregivers completed baseline measures (Table 2), and they all chose to complete questionnaires by e-mail. Caregivers’ attitudes toward caregiving and satisfaction with hospice services were overall positive. They reported high mean scores on the 2 domains on the FACQ-PC of positive caregiver appraisal (mean, 4.25; SD, 0.52) and family well-being (mean, 4.09; SD, 0.45). The majority of caregivers (75%-95%) reported they were satisfied or very satisfied with various dimensions of hospice care based on the FAMCARE questionnaire.
Overall, caregivers reported moderate levels of stress (mean, 13.55; SD, 6.08) based on the PSS scores. Of the 20 caregivers who completed the baseline measures, 8 had clinically meaningful stress, and stress was numerically higher in female caregivers than in their male counterparts, but it did not reach statistical significance (55% vs 22%, P = .197). Finally, at baseline, caregivers indicated relatively low levels of decisional regret about enrolling in hospice, although there was considerable variation (mean, 10.25; SD, 14.37).
We conducted exit interviews with 15 caregivers because we were not able to reach 6 of the original 21 (Table 2). Caregivers rated hospice services highly for communication, symptom control, emotional support, and overall care. They also rated quality of death highly (mean, 8.46; SD 1.13). Regret was lower at the end of the study, but this did not reach statistical significance (baseline mean 9.29; end of study mean 3.57; P = .161).
In the recorded exit interviews, all of the caregivers responded they were satisfied with the phone calls. Two caregivers commented that they would have preferred the calls were more scheduled or at more suitable times. Overall, they described the phone calls as excellent, supportive, responsive, comfortable, and appreciated. All caregivers reported contact with the oncology team after the patient died, but one caregiver was disappointed she was only contacted by the nurse practitioner and not the oncologist. Participants did not feel as if there were other ways that the oncology team could have been helpful for them while their loved one was in hospice.
Table 3 highlights other representative comments from the exit interviews.
Discussion
As far as we know, this is the first study to assess the feasibility of an intervention to facilitate communication between oncology clinicians and caregivers of patients with advanced cancer who are receiving hospice care. Despite the challenges of oncology clinicians delivering an intervention to caregivers during hospice, we found this intervention was feasible and acceptable. Although the transition to hospice can be stressful, caregivers reported high satisfaction with hospice care and the quality of the patient’s death. In exit interviews, they also reported high satisfaction with the intervention and appreciation for maintaining their relationship with the oncology team. It is worth noting that no caregiver requested the optional clinic visit after hospice enrollment, and most caregivers were not seen again in clinic after hospice enrollment.
These results suggest that a simple, telephone-based intervention of scheduled calls from the oncology team at prompted intervals is not only feasible, but may also help foster continuity between the patient and caregiver and the oncology team. We received feedback from both oncology clinicians and caregivers that the initial call frequency was too often, suggesting that communication may not need to be very frequent to maintain continuity and provide support. This also suggests that if the calls are too frequent, they may be more intrusive than helpful for both oncologists and caregivers. Alternatively, caregiver suggestion for fewer phone calls may indicate that concerns about abandonment are less prevalent than existing literature has suggested.
Limitations
Our study has several important limitations. The sample size was small as this was a feasibility study conducted at a single tertiary care hospital, and the population was 90% white and 95% college educated, which may limit the generalizability of our results. In addition, the median length of stay in hospice for patients on this study was 36 days, which is long compared with national averages,32,33 and thus the outcomes may not represent the experience of a more heterogeneous population. The longer length of stay in hospice may have contributed to caregivers’ high satisfaction with the quality of end-of-life care.
Oncologists did not grant permission for the study team to approach all eligible caregivers, which may have introduced selection bias. We were also not able to reach 6 participants for exit interviews. People less satisfied with the intervention or with hospice may be more likely to have missing data, which could introduce bias into the satisfaction ratings. Furthermore, we did not explore the oncology clinicians’ perspective of the intervention or assess the time commitment of the calls. Oncology clinicians have many competing responsibilities and have variable experience and comfort with hospice care. Therefore, future studies should explore the perspective of oncology clinicians in regard to the intervention.
Finally, we did not require communication with the hospice agency as part of the intervention as there were ten different hospices involved. Thus, we do not know how the intervention impacted the hospice team’s care of the patient. However, based upon the success of this pilot study, future larger studies should explore the impact of the intervention from the perspective of the hospice care team and include oncology clinician communication with the hospice agency.
Conclusion
These findings demonstrate the feasibility and acceptability of an intervention to enhance communication between oncology clinicians and caregivers of patients with advanced cancer receiving hospice care. Importantly, the high caregiver satisfaction with the intervention in this study suggests that maintaining communication with the primary oncology team during hospice care may be an important component of high quality end-of-life care, though the desire for decreased calls suggests that this communication need not be frequent to maintain the continuity. A randomized study with a larger and more diverse patient/caregiver sample would allow us to explore the impact of the intervention on caregiver feelings of abandonment by the oncology team and short- and long-term caregiver outcomes, as well as to understand the perspective of the oncology and hospice clinicians involved.
1. Institute of Medicine. 2015. Dying in america: improving quality and honoring individual preferences near the end of life. Washington, DC: The National Academies Press. https://doi.org/10.17226/18748.
2. Ferrell BR, Temel JS, Temin S, et al. Integration of palliative care into standard oncology care: American Society of Clinical Oncology clinical practice guideline update. J Clin Oncol. 2017;35(1):96-112.
3. Wenrich MD, Curtis JR, Shannon SE, Carline JD, Ambrozy DM, Ramsey PG. Communicating with dying patients within the spectrum of medical care from terminal diagnosis to death. Arch Intern Med. 2001;161(6):868-874.
4. Parker SM, Clayton JM, Hancock K, et al. A systematic review of prognostic/end-of-life communication with adults in the advanced stages of a life-limiting illness: patient/caregiver preferences for the content, style, and timing of information. J Pain Symptom Manage. 2007;34(1):81-93.
5. Steinhauser KE, Clipp EC, McNeilly M, Christakis NA, McIntyre LM, Tulsky JA. In search of a good death: observations of patients, families, and providers. Ann Intern Med. 2000;132(10):825-832.
6. Teno JM, Clarridge BR, Casey V, et al. Family perspectives on end-of-life care at the last place of care. JAMA. 2004;291(1):88-93.
7. Kumar P, Wright AA, Hatfield LA, Temel JS, Keating NL. Family perspectives on hospice care experiences of patients with cancer. J Clin Oncol. 2017;35(4):432-439.
8. Wright AA, Keating NL, Ayanian JZ, et al. Family perspectives on aggressive cancer care near the end of life. JAMA. 2016;315(3):284-292.
9. Duggan KT, Hildebrand Duffus S, D’Agostino RB Jr, Petty WJ, Streer NP, Stephenson RC. The impact of hospice services in the care of patients with advanced stage nonsmall cell lung cancer. J Palliat Med. 2017;20(1):29-34.
10. Curtis JR, Wenrich MD, Carline JD, Shannon SE, Ambrozy DM, Ramsey PG. Understanding physicians’ skills at providing end-of-life care perspectives of patients, families, and health care workers. J Gen Intern Med. 2001;16(1):41-49.
11. Back AL, Young JP, McCown E, et al. Abandonment at the end of life from patient, caregiver, nurse, and physician perspectives: loss of continuity and lack of closure. Arch Intern Med. 2009;169(5):474-479.
12. Vig EK, Starks H, Taylor JS, Hopley EK, Fryer-Edwards K. Why don’t patients enroll in hospice? Can we do anything about it? J Gen Intern Med. 2010;25(10):1009-1019.
13. Waldrop DP, Meeker MA, Kerr C, Skretny J, Tangeman J, Milch R. The nature and timing of family-provider communication in late-stage cancer: a qualitative study of caregivers’ experiences. J Pain Symptom Manage. 2012;43(2):182-194.
14. Emanuel EJ, Fairclough DL, Slutsman J, Emanuel LL. Understanding economic and other burdens of terminal illness: the experience of patients and their caregivers. Ann Intern Med. 2000;132(6):451-459.
15. Curtis JR, Patrick DL, Engelberg RA, Norris K, Asp C, Byock I. A measure of the quality of dying and death. Initial validation using after-death interviews with family members. J Pain Symptom Manage. 2002;24(1):17-31.
16. Fleming DA, Sheppard VB, Mangan PA, et al. Caregiving at the end of life: Perceptions of health care quality and quality of life among patients and caregivers. J Pain Symptom Manage. 2006;31(5):407-420.
17. McMillan SC. Interventions to facilitate family caregiving at the end of life. J Palliat Med. 2005;8(Suppl 1):S132-S139.
18. Bakitas M, Lyons KD, Hegel MT, et al. Effects of a palliative care intervention on clinical outcomes in patients with advanced cancer: the Project ENABLE II randomized controlled trial. JAMA. 2009;302(7):741-749.
19. Temel JS, Greer JA, Muzikansky A, et al. Early palliative care for patients with metastatic non-small-cell lung cancer. N Engl J Med. 2010;363(8):733-742.
20. Zimmermann C, Swami N, Krzyzanowska M, et al. Early palliative care for patients with advanced cancer: a cluster-randomised controlled trial. Lancet. 2014;383(9930):1721-1730.
21. Maltoni M, Scarpi E, Dall’Agata M, et al. Systematic versus on-demand early palliative care: a randomised clinical trial assessing quality of care and treatment aggressiveness near the end of life. Eur J Cancer. 2016;69:110-118.
22. El-Jawahri A ea. Early integrated palliative care to improve family caregivers (FC) outcomes for patients with gastrointestinal and lung cancer. J Clin Oncol. 2016;34(suppl; abstr 10131).
23. Temel JS, Greer JA, El-Jawahri A, et al. Effects of early integrated palliative care in patients with lung and gi cancer: a randomized clinical trial. J Clin Oncol. 2017;35(8):834-841.
24. El-Jawahri A, LeBlanc T, VanDusen H, et al. Effect of inpatient palliative care on quality of life 2 weeks after hematopoietic stem cell transplantation: a randomized clinical trial. JAMA. 2016;316(20):2094-2103.
25. Cooper B, Kinsella GJ, Picton C. Development and initial validation of a family appraisal of caregiving questionnaire for palliative care. Psychooncology. 2006;15(7):613-622.
26. Kristjanson LJ. Validity and reliability testing of the FAMCARE Scale: measuring family satisfaction with advanced cancer care. Soc Sci Med. 1993;36(5):693-701.
27. Cohen S, Kamarck T, Mermelstein R. A global measure of perceived stress. J Health Soc Behav. 1983;24(4):385-396.
28. Keir ST, Guill AB, Carter KE, Boole LC, Gonzales L, Friedman HS. Differential levels of stress in caregivers of brain tumor patients--observations from a pilot study. Support Care Cancer. 2006;14(12):1258-1261.
29. Brehaut JC, O’Connor AM, Wood TJ, et al. Validation of a decision regret scale. Med Decis Making. 2003;23(4):281-292.
30. Teno JM, Clarridge B, Casey V, Edgman-Levitan S, Fowler J. Validation of toolkit after-death bereaved family member interview. J Pain Symptom Manage. 2001;22(3):752-758.
31. Wright AA, Zhang B, Ray A, Mack JW, Trice E, Balboni T, et al. Associations between end-of-life discussions, patient mental health, medical care near death, and caregiver bereavement adjustment. JAMA. 2008;300(14):1665-1673.
32. Earle CC, Neville BA, Landrum MB, Ayanian JZ, Block SD, Weeks JC. Trends in the aggressiveness of cancer care near the end of life. J Clin Oncol. 2004;22(2):315-321.
33. Obermeyer Z, Makar M, Abujaber S, Dominici F, Block S, Cutler DM. Association between the Medicare hospice benefit and health care utilization and costs for patients with poor-prognosis cancer. JAMA. 2014;312(18):1888-1896.
Improving the delivery of end-of-life care for patients with advanced cancer has become a priority in the United States.1,2 Quality metrics identifying the components of high-quality end-of-life care have focused on improved symptom management, decreased use of chemotherapy at the end of life, fewer hospitalizations, and increased use of hospice care. Patients and caregivers also consider good communication with the medical team to be a critical component of end-of-life care.3-5 Interventions to improve the quality of end-of-life care are needed.
Caregivers of patients with advanced cancer who receive hospice services report better quality of care and death than those receiving end-of-life care in other settings.6-9 However, the transition for patients from active cancer therapy delivered by their oncologists to end-of-life care delivered by a hospice care team can be abrupt. Patients and their caregivers often feel abandoned by oncology clinicians because of the lack of continuity of care and poor communication.10-13 Caregivers who note continued involvement and communication with their oncology clinicians experience a lower caregiving burden, report higher satisfaction with care, and recount a higher quality of death for their loved one.14-16 Therefore, interventions that prevent abrupt transitions in care from oncology to hospice by ensuring continued communication with oncology clinicians are needed to improve the quality of end-of-life care.17 Recent findings have shown that providing concurrent oncology and palliative care is not only feasible but beneficial for patients with advanced cancer and their caregivers.18-24 However, there is no standard of care for the involvement of oncology clinicians in the care of patients receiving hospice services and their families.
Although interventions may be needed, it could be challenging to deliver them given the multiple demands of caregiving during hospice and the lack of regular contact in clinic. We sought to assess the feasibility of an intervention, Ensuring Communication in Hospice by Oncology (ECHO), to facilitate communication between oncology clinicians and caregivers of patients who enroll in hospice. We also explored caregiver-reported outcomes during hospice care, including satisfaction with care, attitudes toward caregiving, stress, decision regret, and perception of the quality of patients’ end-of-life care.
Methods
Study design
During March 2014-June 2015, caregivers of patients with advanced cancer who enrolled in home hospice services were eligible to participate in the study at Massachusetts General Hospital (MGH) in Boston. The Dana Farber/Harvard Cancer Center Institutional Review Board approved all methods and materials. The study opened with an enrollment goal of 30 participating caregivers. However, due to staff transitions, we closed the study early in June 2015 after 25 caregivers enrolled.
Participants
Caregivers of patients receiving care at the cancer center's thoracic, head and neck, sarcoma, melanoma, and gynecological disease centers were eligible within 10 days after a patient’s enrollment in hospice. Five disease sites were selected to participate in the intervention. We defined caregivers as relatives or friends serving as the primary caregiver of the patient at home during hospice care. Other caregiver eligibility criteria included the ability to read and respond to questions in English or with a translator, access to a telephone and/or computer to communicate with oncology clinicians, and willingness to complete questionnaires. Caregivers were ineligible if the patient was participating in an ongoing palliative care trial.
To identify eligible caregivers, case managers from both the inpatient and outpatient settings, as well as the nurses based in participating disease centers, notified the research team of all patients referred to hospice. If the patient had received oncology care in one of our participating disease centers, the research team contacted their oncology clinician/s (physicians, nurse practitioners [NP], registered nurses [RN], and/or physician assistants [PA]) to inquire if the patient had an involved caregiver and to obtain permission to offer study participation. If the oncology clinician/s did not grant permission, we documented the reason. Otherwise, with permission, research staff contacted the caregiver by telephone to offer study participation and obtain verbal consent. We then sent participating caregivers a copy of the informed consent by mail or e-mail.
Intervention
The ECHO intervention consisted of: supportive phone calls from an oncology clinician to the caregiver; an optional clinic visit with the oncology clinician for the patient to address clinical questions or concerns that was offered during the initial telephone consent; a bereavement call to the participating caregiver (Figure 1). Initially, we designed the intervention to have phone calls occurring twice weekly until the patient died. However, 3 months after starting the study, we received feedback from oncology clinicians and caregivers that calls were too frequent, so we amended the protocol to include phone calls twice weekly for the first 2 weeks of the study and then weekly thereafter. Seven months into the study, we again decreased the number of phone calls to weekly for the first 4 weeks, every other week for 4 weeks, and then monthly until patient death. We informed caregivers of changes by e-mail.
Before we started the study, we conducted training sessions with oncology clinicians from the participating disease centers to review study procedures and expectations of the phone calls. Supportive phone calls during hospice were not a part of standard practice prior to the study. The RN, NP or PA, and/or physician who had an established relationship with the patient and caregiver completed the phone calls. They decided based on their respective relationships with the patients and their workloads who would call each week, though the majority of calls were conducted by the RN or NP. All the clinicians had experience comanaging patients with hospice agencies, and our general practice is for the oncology physician to serve as the hospice attending of record. The calls were intended to offer support and reassurance to caregivers. We did not script the calls so that clinicians could tailor their content to the individual needs of the caregiver, as informed by their established relationship. The calls could include the patient if he/she was able to and interested in speaking to the clinician. There was no standardized communication with hospice as part of the intervention. If a caregiver raised concerns about symptom management during a call, the clinician would advise the caregiver to contact the hospice team directly or the clinician would call the hospice to discuss, depending on the clinical scenario and the clinician’s judgment. Research staff reminded oncology clinicians to call caregivers on the scheduled date and to document the discussion in the electronic medical record. The hospice phone number was included in the e-mail. If the call was not documented, research staff sent a reminder e-mail to the oncology clinicians 24 hours after the call was due.
Caregiver-reported measures
Caregivers completed a demographic questionnaire at baseline in which they reported their age, gender, race, ethnicity, religion, employment status, and relationship to the patient. We collected information about patient characteristics from the electronic medical record, including age, gender, and cancer type. In addition, we administered validated, self-report measures (see below). We limited the number of measures to decrease caregiver burden:
The baseline questionnaire included the FACQ-PC, the FAMCARE scale, the PSS, and the Decision Regret Scale. Initially, the study involved weekly questionnaires after baseline that included the FACQ-PC, the FAMCARE scale, and the PSS. However, after 3 months of study enrollment, we received feedback that the questionnaires were too frequent, so we amended the protocol and changed the frequency to weekly for 2 weeks, then monthly thereafter until the patient died.
Caregiver exit interview
Exit interviews included the toolkit interview, the Quality of End-of-Life Care scale, and the Decision Regret Scale. Caregivers also reported patients’ place and date of death. After the first 6 caregivers enrolled, we amended the exit interview to include open-ended feedback from caregivers. Specifically, we evaluated caregivers’ perceptions of the ECHO intervention by asking them about their perception of and satisfaction with the content and frequency of the oncology clinicians’ phone calls, whether they had an in-person visit with their oncology clinicians after the start of hospice care, whether the clinician/s contacted them after the patient died, and whether there were ways in which the clinician/s could help in the future.
Data collection and storage
Caregivers were given the option of completing study measures by telephone or e-mail so that they could complete them on a computer when it was convenient for them. Caregivers received a link to Research Electronic Data Capture (REDCap), a web-based, HIPAA-compliant application that allows participants to answer questionnaires online. The exit interviews were completed by phone, and research staff entered the data into the REDCap database. In addition, with we obtained caregiver permission to audiorecord the exit interviews, which were then transcribed and de-identified.
Statistical analysis
The primary outcome for the study was feasibility, which we defined as >70% of the caregivers receiving >50% of the phone calls from an oncology clinician, and >70% of the caregivers completing >50% of the questionnaires. All time points for the questionnaires and the exit interview counted toward feasibility. Exploratory endpoints included caregiver-reported satisfaction, stress, quality of end-of-life care, and decision-making regret.
Using STATA (v9.3; StataCorp, College Station, Texas) for all statistical analyses, we summarized participants’ characteristics and outcomes as frequencies and percentages for categorical variables and mean standard deviation for continuous variables. We used the repeated-measures t test to assess changes in caregiver outcomes over time. We used the Fisher exact test to compare clinically meaningful threshold scores of perceived stress between men and women.
We examined caregivers’ open-ended feedback using descriptive analyses to summarize comments about the intervention and to inform possible refinements for a future study.
Results
Baseline characteristics
During March 2014-June 2015, we enrolled caregivers of patients with advanced cancer from 5 participating disease centers: thoracic, head and neck, sarcoma, melanoma, and gynecological malignancy. We screened 123 patients to determine the eligibility of their caregivers (Figure 2). Of 38 eligible caregivers, 7 could not be reached, 6 declined participation, and 25 enrolled in the study (81% enrollment rate). Of the 25 caregivers who enrolled, 3 withdrew – 2 because the patients they were caring for died before the intervention began, and 1 who withdrew from the study because the family wanted less contact with the oncology clinician/s. Thus, we had data for 22 caregivers for our feasibility evaluation. One caregiver stopped study assessments after 3 months because the patient dis-enrolled from hospice. Median time from the patients’ hospice enrollment to caregiver study enrollment was 3 days (range, 1-9). Median time from study enrollment to patient death was 36 days (range, 2-135). Patients were receiving care from 10 different hospice agencies.
All of the patients had metastatic cancer, and 64% were women (Table 1).
Most of the caregivers were white (n = 18, 90%) and women (n = 12, 55%). The majority were the patient’s spouse (n = 12, 60%) or child (n = 6, 30%), and they lived with the patient (n = 16, 80%). Many of the caregivers had other responsibilities in addition to caring for the patients, including part- or full-time work (n = 10, 50%) or caring for others in addition to the patient (n = 10, 50%).
Feasibility
Over the study period, oncology clinicians completed 164 of 180 possible phone calls (91%). All 22 caregivers received >50% of the phone calls. Caregivers completed 78 of 99 possible questionnaires (79%), and 16 of 22 completed >50% of the questionnaires (73%). None of the caregivers/patients wanted to schedule the optional visit with an oncology clinician that was offered as part of the intervention; however, 5 patients had a clinic visit after hospice enrollment. In addition, 2 oncologists visited a patient and caregiver at home. All caregivers received bereavement contact from the oncology team.
Caregiver-reported outcomes
In all, 20 of the 22 enrolled caregivers completed baseline measures (Table 2), and they all chose to complete questionnaires by e-mail. Caregivers’ attitudes toward caregiving and satisfaction with hospice services were overall positive. They reported high mean scores on the 2 domains on the FACQ-PC of positive caregiver appraisal (mean, 4.25; SD, 0.52) and family well-being (mean, 4.09; SD, 0.45). The majority of caregivers (75%-95%) reported they were satisfied or very satisfied with various dimensions of hospice care based on the FAMCARE questionnaire.
Overall, caregivers reported moderate levels of stress (mean, 13.55; SD, 6.08) based on the PSS scores. Of the 20 caregivers who completed the baseline measures, 8 had clinically meaningful stress, and stress was numerically higher in female caregivers than in their male counterparts, but it did not reach statistical significance (55% vs 22%, P = .197). Finally, at baseline, caregivers indicated relatively low levels of decisional regret about enrolling in hospice, although there was considerable variation (mean, 10.25; SD, 14.37).
We conducted exit interviews with 15 caregivers because we were not able to reach 6 of the original 21 (Table 2). Caregivers rated hospice services highly for communication, symptom control, emotional support, and overall care. They also rated quality of death highly (mean, 8.46; SD 1.13). Regret was lower at the end of the study, but this did not reach statistical significance (baseline mean 9.29; end of study mean 3.57; P = .161).
In the recorded exit interviews, all of the caregivers responded they were satisfied with the phone calls. Two caregivers commented that they would have preferred the calls were more scheduled or at more suitable times. Overall, they described the phone calls as excellent, supportive, responsive, comfortable, and appreciated. All caregivers reported contact with the oncology team after the patient died, but one caregiver was disappointed she was only contacted by the nurse practitioner and not the oncologist. Participants did not feel as if there were other ways that the oncology team could have been helpful for them while their loved one was in hospice.
Table 3 highlights other representative comments from the exit interviews.
Discussion
As far as we know, this is the first study to assess the feasibility of an intervention to facilitate communication between oncology clinicians and caregivers of patients with advanced cancer who are receiving hospice care. Despite the challenges of oncology clinicians delivering an intervention to caregivers during hospice, we found this intervention was feasible and acceptable. Although the transition to hospice can be stressful, caregivers reported high satisfaction with hospice care and the quality of the patient’s death. In exit interviews, they also reported high satisfaction with the intervention and appreciation for maintaining their relationship with the oncology team. It is worth noting that no caregiver requested the optional clinic visit after hospice enrollment, and most caregivers were not seen again in clinic after hospice enrollment.
These results suggest that a simple, telephone-based intervention of scheduled calls from the oncology team at prompted intervals is not only feasible, but may also help foster continuity between the patient and caregiver and the oncology team. We received feedback from both oncology clinicians and caregivers that the initial call frequency was too often, suggesting that communication may not need to be very frequent to maintain continuity and provide support. This also suggests that if the calls are too frequent, they may be more intrusive than helpful for both oncologists and caregivers. Alternatively, caregiver suggestion for fewer phone calls may indicate that concerns about abandonment are less prevalent than existing literature has suggested.
Limitations
Our study has several important limitations. The sample size was small as this was a feasibility study conducted at a single tertiary care hospital, and the population was 90% white and 95% college educated, which may limit the generalizability of our results. In addition, the median length of stay in hospice for patients on this study was 36 days, which is long compared with national averages,32,33 and thus the outcomes may not represent the experience of a more heterogeneous population. The longer length of stay in hospice may have contributed to caregivers’ high satisfaction with the quality of end-of-life care.
Oncologists did not grant permission for the study team to approach all eligible caregivers, which may have introduced selection bias. We were also not able to reach 6 participants for exit interviews. People less satisfied with the intervention or with hospice may be more likely to have missing data, which could introduce bias into the satisfaction ratings. Furthermore, we did not explore the oncology clinicians’ perspective of the intervention or assess the time commitment of the calls. Oncology clinicians have many competing responsibilities and have variable experience and comfort with hospice care. Therefore, future studies should explore the perspective of oncology clinicians in regard to the intervention.
Finally, we did not require communication with the hospice agency as part of the intervention as there were ten different hospices involved. Thus, we do not know how the intervention impacted the hospice team’s care of the patient. However, based upon the success of this pilot study, future larger studies should explore the impact of the intervention from the perspective of the hospice care team and include oncology clinician communication with the hospice agency.
Conclusion
These findings demonstrate the feasibility and acceptability of an intervention to enhance communication between oncology clinicians and caregivers of patients with advanced cancer receiving hospice care. Importantly, the high caregiver satisfaction with the intervention in this study suggests that maintaining communication with the primary oncology team during hospice care may be an important component of high quality end-of-life care, though the desire for decreased calls suggests that this communication need not be frequent to maintain the continuity. A randomized study with a larger and more diverse patient/caregiver sample would allow us to explore the impact of the intervention on caregiver feelings of abandonment by the oncology team and short- and long-term caregiver outcomes, as well as to understand the perspective of the oncology and hospice clinicians involved.
Improving the delivery of end-of-life care for patients with advanced cancer has become a priority in the United States.1,2 Quality metrics identifying the components of high-quality end-of-life care have focused on improved symptom management, decreased use of chemotherapy at the end of life, fewer hospitalizations, and increased use of hospice care. Patients and caregivers also consider good communication with the medical team to be a critical component of end-of-life care.3-5 Interventions to improve the quality of end-of-life care are needed.
Caregivers of patients with advanced cancer who receive hospice services report better quality of care and death than those receiving end-of-life care in other settings.6-9 However, the transition for patients from active cancer therapy delivered by their oncologists to end-of-life care delivered by a hospice care team can be abrupt. Patients and their caregivers often feel abandoned by oncology clinicians because of the lack of continuity of care and poor communication.10-13 Caregivers who note continued involvement and communication with their oncology clinicians experience a lower caregiving burden, report higher satisfaction with care, and recount a higher quality of death for their loved one.14-16 Therefore, interventions that prevent abrupt transitions in care from oncology to hospice by ensuring continued communication with oncology clinicians are needed to improve the quality of end-of-life care.17 Recent findings have shown that providing concurrent oncology and palliative care is not only feasible but beneficial for patients with advanced cancer and their caregivers.18-24 However, there is no standard of care for the involvement of oncology clinicians in the care of patients receiving hospice services and their families.
Although interventions may be needed, it could be challenging to deliver them given the multiple demands of caregiving during hospice and the lack of regular contact in clinic. We sought to assess the feasibility of an intervention, Ensuring Communication in Hospice by Oncology (ECHO), to facilitate communication between oncology clinicians and caregivers of patients who enroll in hospice. We also explored caregiver-reported outcomes during hospice care, including satisfaction with care, attitudes toward caregiving, stress, decision regret, and perception of the quality of patients’ end-of-life care.
Methods
Study design
During March 2014-June 2015, caregivers of patients with advanced cancer who enrolled in home hospice services were eligible to participate in the study at Massachusetts General Hospital (MGH) in Boston. The Dana Farber/Harvard Cancer Center Institutional Review Board approved all methods and materials. The study opened with an enrollment goal of 30 participating caregivers. However, due to staff transitions, we closed the study early in June 2015 after 25 caregivers enrolled.
Participants
Caregivers of patients receiving care at the cancer center's thoracic, head and neck, sarcoma, melanoma, and gynecological disease centers were eligible within 10 days after a patient’s enrollment in hospice. Five disease sites were selected to participate in the intervention. We defined caregivers as relatives or friends serving as the primary caregiver of the patient at home during hospice care. Other caregiver eligibility criteria included the ability to read and respond to questions in English or with a translator, access to a telephone and/or computer to communicate with oncology clinicians, and willingness to complete questionnaires. Caregivers were ineligible if the patient was participating in an ongoing palliative care trial.
To identify eligible caregivers, case managers from both the inpatient and outpatient settings, as well as the nurses based in participating disease centers, notified the research team of all patients referred to hospice. If the patient had received oncology care in one of our participating disease centers, the research team contacted their oncology clinician/s (physicians, nurse practitioners [NP], registered nurses [RN], and/or physician assistants [PA]) to inquire if the patient had an involved caregiver and to obtain permission to offer study participation. If the oncology clinician/s did not grant permission, we documented the reason. Otherwise, with permission, research staff contacted the caregiver by telephone to offer study participation and obtain verbal consent. We then sent participating caregivers a copy of the informed consent by mail or e-mail.
Intervention
The ECHO intervention consisted of: supportive phone calls from an oncology clinician to the caregiver; an optional clinic visit with the oncology clinician for the patient to address clinical questions or concerns that was offered during the initial telephone consent; a bereavement call to the participating caregiver (Figure 1). Initially, we designed the intervention to have phone calls occurring twice weekly until the patient died. However, 3 months after starting the study, we received feedback from oncology clinicians and caregivers that calls were too frequent, so we amended the protocol to include phone calls twice weekly for the first 2 weeks of the study and then weekly thereafter. Seven months into the study, we again decreased the number of phone calls to weekly for the first 4 weeks, every other week for 4 weeks, and then monthly until patient death. We informed caregivers of changes by e-mail.
Before we started the study, we conducted training sessions with oncology clinicians from the participating disease centers to review study procedures and expectations of the phone calls. Supportive phone calls during hospice were not a part of standard practice prior to the study. The RN, NP or PA, and/or physician who had an established relationship with the patient and caregiver completed the phone calls. They decided based on their respective relationships with the patients and their workloads who would call each week, though the majority of calls were conducted by the RN or NP. All the clinicians had experience comanaging patients with hospice agencies, and our general practice is for the oncology physician to serve as the hospice attending of record. The calls were intended to offer support and reassurance to caregivers. We did not script the calls so that clinicians could tailor their content to the individual needs of the caregiver, as informed by their established relationship. The calls could include the patient if he/she was able to and interested in speaking to the clinician. There was no standardized communication with hospice as part of the intervention. If a caregiver raised concerns about symptom management during a call, the clinician would advise the caregiver to contact the hospice team directly or the clinician would call the hospice to discuss, depending on the clinical scenario and the clinician’s judgment. Research staff reminded oncology clinicians to call caregivers on the scheduled date and to document the discussion in the electronic medical record. The hospice phone number was included in the e-mail. If the call was not documented, research staff sent a reminder e-mail to the oncology clinicians 24 hours after the call was due.
Caregiver-reported measures
Caregivers completed a demographic questionnaire at baseline in which they reported their age, gender, race, ethnicity, religion, employment status, and relationship to the patient. We collected information about patient characteristics from the electronic medical record, including age, gender, and cancer type. In addition, we administered validated, self-report measures (see below). We limited the number of measures to decrease caregiver burden:
The baseline questionnaire included the FACQ-PC, the FAMCARE scale, the PSS, and the Decision Regret Scale. Initially, the study involved weekly questionnaires after baseline that included the FACQ-PC, the FAMCARE scale, and the PSS. However, after 3 months of study enrollment, we received feedback that the questionnaires were too frequent, so we amended the protocol and changed the frequency to weekly for 2 weeks, then monthly thereafter until the patient died.
Caregiver exit interview
Exit interviews included the toolkit interview, the Quality of End-of-Life Care scale, and the Decision Regret Scale. Caregivers also reported patients’ place and date of death. After the first 6 caregivers enrolled, we amended the exit interview to include open-ended feedback from caregivers. Specifically, we evaluated caregivers’ perceptions of the ECHO intervention by asking them about their perception of and satisfaction with the content and frequency of the oncology clinicians’ phone calls, whether they had an in-person visit with their oncology clinicians after the start of hospice care, whether the clinician/s contacted them after the patient died, and whether there were ways in which the clinician/s could help in the future.
Data collection and storage
Caregivers were given the option of completing study measures by telephone or e-mail so that they could complete them on a computer when it was convenient for them. Caregivers received a link to Research Electronic Data Capture (REDCap), a web-based, HIPAA-compliant application that allows participants to answer questionnaires online. The exit interviews were completed by phone, and research staff entered the data into the REDCap database. In addition, with we obtained caregiver permission to audiorecord the exit interviews, which were then transcribed and de-identified.
Statistical analysis
The primary outcome for the study was feasibility, which we defined as >70% of the caregivers receiving >50% of the phone calls from an oncology clinician, and >70% of the caregivers completing >50% of the questionnaires. All time points for the questionnaires and the exit interview counted toward feasibility. Exploratory endpoints included caregiver-reported satisfaction, stress, quality of end-of-life care, and decision-making regret.
Using STATA (v9.3; StataCorp, College Station, Texas) for all statistical analyses, we summarized participants’ characteristics and outcomes as frequencies and percentages for categorical variables and mean standard deviation for continuous variables. We used the repeated-measures t test to assess changes in caregiver outcomes over time. We used the Fisher exact test to compare clinically meaningful threshold scores of perceived stress between men and women.
We examined caregivers’ open-ended feedback using descriptive analyses to summarize comments about the intervention and to inform possible refinements for a future study.
Results
Baseline characteristics
During March 2014-June 2015, we enrolled caregivers of patients with advanced cancer from 5 participating disease centers: thoracic, head and neck, sarcoma, melanoma, and gynecological malignancy. We screened 123 patients to determine the eligibility of their caregivers (Figure 2). Of 38 eligible caregivers, 7 could not be reached, 6 declined participation, and 25 enrolled in the study (81% enrollment rate). Of the 25 caregivers who enrolled, 3 withdrew – 2 because the patients they were caring for died before the intervention began, and 1 who withdrew from the study because the family wanted less contact with the oncology clinician/s. Thus, we had data for 22 caregivers for our feasibility evaluation. One caregiver stopped study assessments after 3 months because the patient dis-enrolled from hospice. Median time from the patients’ hospice enrollment to caregiver study enrollment was 3 days (range, 1-9). Median time from study enrollment to patient death was 36 days (range, 2-135). Patients were receiving care from 10 different hospice agencies.
All of the patients had metastatic cancer, and 64% were women (Table 1).
Most of the caregivers were white (n = 18, 90%) and women (n = 12, 55%). The majority were the patient’s spouse (n = 12, 60%) or child (n = 6, 30%), and they lived with the patient (n = 16, 80%). Many of the caregivers had other responsibilities in addition to caring for the patients, including part- or full-time work (n = 10, 50%) or caring for others in addition to the patient (n = 10, 50%).
Feasibility
Over the study period, oncology clinicians completed 164 of 180 possible phone calls (91%). All 22 caregivers received >50% of the phone calls. Caregivers completed 78 of 99 possible questionnaires (79%), and 16 of 22 completed >50% of the questionnaires (73%). None of the caregivers/patients wanted to schedule the optional visit with an oncology clinician that was offered as part of the intervention; however, 5 patients had a clinic visit after hospice enrollment. In addition, 2 oncologists visited a patient and caregiver at home. All caregivers received bereavement contact from the oncology team.
Caregiver-reported outcomes
In all, 20 of the 22 enrolled caregivers completed baseline measures (Table 2), and they all chose to complete questionnaires by e-mail. Caregivers’ attitudes toward caregiving and satisfaction with hospice services were overall positive. They reported high mean scores on the 2 domains on the FACQ-PC of positive caregiver appraisal (mean, 4.25; SD, 0.52) and family well-being (mean, 4.09; SD, 0.45). The majority of caregivers (75%-95%) reported they were satisfied or very satisfied with various dimensions of hospice care based on the FAMCARE questionnaire.
Overall, caregivers reported moderate levels of stress (mean, 13.55; SD, 6.08) based on the PSS scores. Of the 20 caregivers who completed the baseline measures, 8 had clinically meaningful stress, and stress was numerically higher in female caregivers than in their male counterparts, but it did not reach statistical significance (55% vs 22%, P = .197). Finally, at baseline, caregivers indicated relatively low levels of decisional regret about enrolling in hospice, although there was considerable variation (mean, 10.25; SD, 14.37).
We conducted exit interviews with 15 caregivers because we were not able to reach 6 of the original 21 (Table 2). Caregivers rated hospice services highly for communication, symptom control, emotional support, and overall care. They also rated quality of death highly (mean, 8.46; SD 1.13). Regret was lower at the end of the study, but this did not reach statistical significance (baseline mean 9.29; end of study mean 3.57; P = .161).
In the recorded exit interviews, all of the caregivers responded they were satisfied with the phone calls. Two caregivers commented that they would have preferred the calls were more scheduled or at more suitable times. Overall, they described the phone calls as excellent, supportive, responsive, comfortable, and appreciated. All caregivers reported contact with the oncology team after the patient died, but one caregiver was disappointed she was only contacted by the nurse practitioner and not the oncologist. Participants did not feel as if there were other ways that the oncology team could have been helpful for them while their loved one was in hospice.
Table 3 highlights other representative comments from the exit interviews.
Discussion
As far as we know, this is the first study to assess the feasibility of an intervention to facilitate communication between oncology clinicians and caregivers of patients with advanced cancer who are receiving hospice care. Despite the challenges of oncology clinicians delivering an intervention to caregivers during hospice, we found this intervention was feasible and acceptable. Although the transition to hospice can be stressful, caregivers reported high satisfaction with hospice care and the quality of the patient’s death. In exit interviews, they also reported high satisfaction with the intervention and appreciation for maintaining their relationship with the oncology team. It is worth noting that no caregiver requested the optional clinic visit after hospice enrollment, and most caregivers were not seen again in clinic after hospice enrollment.
These results suggest that a simple, telephone-based intervention of scheduled calls from the oncology team at prompted intervals is not only feasible, but may also help foster continuity between the patient and caregiver and the oncology team. We received feedback from both oncology clinicians and caregivers that the initial call frequency was too often, suggesting that communication may not need to be very frequent to maintain continuity and provide support. This also suggests that if the calls are too frequent, they may be more intrusive than helpful for both oncologists and caregivers. Alternatively, caregiver suggestion for fewer phone calls may indicate that concerns about abandonment are less prevalent than existing literature has suggested.
Limitations
Our study has several important limitations. The sample size was small as this was a feasibility study conducted at a single tertiary care hospital, and the population was 90% white and 95% college educated, which may limit the generalizability of our results. In addition, the median length of stay in hospice for patients on this study was 36 days, which is long compared with national averages,32,33 and thus the outcomes may not represent the experience of a more heterogeneous population. The longer length of stay in hospice may have contributed to caregivers’ high satisfaction with the quality of end-of-life care.
Oncologists did not grant permission for the study team to approach all eligible caregivers, which may have introduced selection bias. We were also not able to reach 6 participants for exit interviews. People less satisfied with the intervention or with hospice may be more likely to have missing data, which could introduce bias into the satisfaction ratings. Furthermore, we did not explore the oncology clinicians’ perspective of the intervention or assess the time commitment of the calls. Oncology clinicians have many competing responsibilities and have variable experience and comfort with hospice care. Therefore, future studies should explore the perspective of oncology clinicians in regard to the intervention.
Finally, we did not require communication with the hospice agency as part of the intervention as there were ten different hospices involved. Thus, we do not know how the intervention impacted the hospice team’s care of the patient. However, based upon the success of this pilot study, future larger studies should explore the impact of the intervention from the perspective of the hospice care team and include oncology clinician communication with the hospice agency.
Conclusion
These findings demonstrate the feasibility and acceptability of an intervention to enhance communication between oncology clinicians and caregivers of patients with advanced cancer receiving hospice care. Importantly, the high caregiver satisfaction with the intervention in this study suggests that maintaining communication with the primary oncology team during hospice care may be an important component of high quality end-of-life care, though the desire for decreased calls suggests that this communication need not be frequent to maintain the continuity. A randomized study with a larger and more diverse patient/caregiver sample would allow us to explore the impact of the intervention on caregiver feelings of abandonment by the oncology team and short- and long-term caregiver outcomes, as well as to understand the perspective of the oncology and hospice clinicians involved.
1. Institute of Medicine. 2015. Dying in america: improving quality and honoring individual preferences near the end of life. Washington, DC: The National Academies Press. https://doi.org/10.17226/18748.
2. Ferrell BR, Temel JS, Temin S, et al. Integration of palliative care into standard oncology care: American Society of Clinical Oncology clinical practice guideline update. J Clin Oncol. 2017;35(1):96-112.
3. Wenrich MD, Curtis JR, Shannon SE, Carline JD, Ambrozy DM, Ramsey PG. Communicating with dying patients within the spectrum of medical care from terminal diagnosis to death. Arch Intern Med. 2001;161(6):868-874.
4. Parker SM, Clayton JM, Hancock K, et al. A systematic review of prognostic/end-of-life communication with adults in the advanced stages of a life-limiting illness: patient/caregiver preferences for the content, style, and timing of information. J Pain Symptom Manage. 2007;34(1):81-93.
5. Steinhauser KE, Clipp EC, McNeilly M, Christakis NA, McIntyre LM, Tulsky JA. In search of a good death: observations of patients, families, and providers. Ann Intern Med. 2000;132(10):825-832.
6. Teno JM, Clarridge BR, Casey V, et al. Family perspectives on end-of-life care at the last place of care. JAMA. 2004;291(1):88-93.
7. Kumar P, Wright AA, Hatfield LA, Temel JS, Keating NL. Family perspectives on hospice care experiences of patients with cancer. J Clin Oncol. 2017;35(4):432-439.
8. Wright AA, Keating NL, Ayanian JZ, et al. Family perspectives on aggressive cancer care near the end of life. JAMA. 2016;315(3):284-292.
9. Duggan KT, Hildebrand Duffus S, D’Agostino RB Jr, Petty WJ, Streer NP, Stephenson RC. The impact of hospice services in the care of patients with advanced stage nonsmall cell lung cancer. J Palliat Med. 2017;20(1):29-34.
10. Curtis JR, Wenrich MD, Carline JD, Shannon SE, Ambrozy DM, Ramsey PG. Understanding physicians’ skills at providing end-of-life care perspectives of patients, families, and health care workers. J Gen Intern Med. 2001;16(1):41-49.
11. Back AL, Young JP, McCown E, et al. Abandonment at the end of life from patient, caregiver, nurse, and physician perspectives: loss of continuity and lack of closure. Arch Intern Med. 2009;169(5):474-479.
12. Vig EK, Starks H, Taylor JS, Hopley EK, Fryer-Edwards K. Why don’t patients enroll in hospice? Can we do anything about it? J Gen Intern Med. 2010;25(10):1009-1019.
13. Waldrop DP, Meeker MA, Kerr C, Skretny J, Tangeman J, Milch R. The nature and timing of family-provider communication in late-stage cancer: a qualitative study of caregivers’ experiences. J Pain Symptom Manage. 2012;43(2):182-194.
14. Emanuel EJ, Fairclough DL, Slutsman J, Emanuel LL. Understanding economic and other burdens of terminal illness: the experience of patients and their caregivers. Ann Intern Med. 2000;132(6):451-459.
15. Curtis JR, Patrick DL, Engelberg RA, Norris K, Asp C, Byock I. A measure of the quality of dying and death. Initial validation using after-death interviews with family members. J Pain Symptom Manage. 2002;24(1):17-31.
16. Fleming DA, Sheppard VB, Mangan PA, et al. Caregiving at the end of life: Perceptions of health care quality and quality of life among patients and caregivers. J Pain Symptom Manage. 2006;31(5):407-420.
17. McMillan SC. Interventions to facilitate family caregiving at the end of life. J Palliat Med. 2005;8(Suppl 1):S132-S139.
18. Bakitas M, Lyons KD, Hegel MT, et al. Effects of a palliative care intervention on clinical outcomes in patients with advanced cancer: the Project ENABLE II randomized controlled trial. JAMA. 2009;302(7):741-749.
19. Temel JS, Greer JA, Muzikansky A, et al. Early palliative care for patients with metastatic non-small-cell lung cancer. N Engl J Med. 2010;363(8):733-742.
20. Zimmermann C, Swami N, Krzyzanowska M, et al. Early palliative care for patients with advanced cancer: a cluster-randomised controlled trial. Lancet. 2014;383(9930):1721-1730.
21. Maltoni M, Scarpi E, Dall’Agata M, et al. Systematic versus on-demand early palliative care: a randomised clinical trial assessing quality of care and treatment aggressiveness near the end of life. Eur J Cancer. 2016;69:110-118.
22. El-Jawahri A ea. Early integrated palliative care to improve family caregivers (FC) outcomes for patients with gastrointestinal and lung cancer. J Clin Oncol. 2016;34(suppl; abstr 10131).
23. Temel JS, Greer JA, El-Jawahri A, et al. Effects of early integrated palliative care in patients with lung and gi cancer: a randomized clinical trial. J Clin Oncol. 2017;35(8):834-841.
24. El-Jawahri A, LeBlanc T, VanDusen H, et al. Effect of inpatient palliative care on quality of life 2 weeks after hematopoietic stem cell transplantation: a randomized clinical trial. JAMA. 2016;316(20):2094-2103.
25. Cooper B, Kinsella GJ, Picton C. Development and initial validation of a family appraisal of caregiving questionnaire for palliative care. Psychooncology. 2006;15(7):613-622.
26. Kristjanson LJ. Validity and reliability testing of the FAMCARE Scale: measuring family satisfaction with advanced cancer care. Soc Sci Med. 1993;36(5):693-701.
27. Cohen S, Kamarck T, Mermelstein R. A global measure of perceived stress. J Health Soc Behav. 1983;24(4):385-396.
28. Keir ST, Guill AB, Carter KE, Boole LC, Gonzales L, Friedman HS. Differential levels of stress in caregivers of brain tumor patients--observations from a pilot study. Support Care Cancer. 2006;14(12):1258-1261.
29. Brehaut JC, O’Connor AM, Wood TJ, et al. Validation of a decision regret scale. Med Decis Making. 2003;23(4):281-292.
30. Teno JM, Clarridge B, Casey V, Edgman-Levitan S, Fowler J. Validation of toolkit after-death bereaved family member interview. J Pain Symptom Manage. 2001;22(3):752-758.
31. Wright AA, Zhang B, Ray A, Mack JW, Trice E, Balboni T, et al. Associations between end-of-life discussions, patient mental health, medical care near death, and caregiver bereavement adjustment. JAMA. 2008;300(14):1665-1673.
32. Earle CC, Neville BA, Landrum MB, Ayanian JZ, Block SD, Weeks JC. Trends in the aggressiveness of cancer care near the end of life. J Clin Oncol. 2004;22(2):315-321.
33. Obermeyer Z, Makar M, Abujaber S, Dominici F, Block S, Cutler DM. Association between the Medicare hospice benefit and health care utilization and costs for patients with poor-prognosis cancer. JAMA. 2014;312(18):1888-1896.
1. Institute of Medicine. 2015. Dying in america: improving quality and honoring individual preferences near the end of life. Washington, DC: The National Academies Press. https://doi.org/10.17226/18748.
2. Ferrell BR, Temel JS, Temin S, et al. Integration of palliative care into standard oncology care: American Society of Clinical Oncology clinical practice guideline update. J Clin Oncol. 2017;35(1):96-112.
3. Wenrich MD, Curtis JR, Shannon SE, Carline JD, Ambrozy DM, Ramsey PG. Communicating with dying patients within the spectrum of medical care from terminal diagnosis to death. Arch Intern Med. 2001;161(6):868-874.
4. Parker SM, Clayton JM, Hancock K, et al. A systematic review of prognostic/end-of-life communication with adults in the advanced stages of a life-limiting illness: patient/caregiver preferences for the content, style, and timing of information. J Pain Symptom Manage. 2007;34(1):81-93.
5. Steinhauser KE, Clipp EC, McNeilly M, Christakis NA, McIntyre LM, Tulsky JA. In search of a good death: observations of patients, families, and providers. Ann Intern Med. 2000;132(10):825-832.
6. Teno JM, Clarridge BR, Casey V, et al. Family perspectives on end-of-life care at the last place of care. JAMA. 2004;291(1):88-93.
7. Kumar P, Wright AA, Hatfield LA, Temel JS, Keating NL. Family perspectives on hospice care experiences of patients with cancer. J Clin Oncol. 2017;35(4):432-439.
8. Wright AA, Keating NL, Ayanian JZ, et al. Family perspectives on aggressive cancer care near the end of life. JAMA. 2016;315(3):284-292.
9. Duggan KT, Hildebrand Duffus S, D’Agostino RB Jr, Petty WJ, Streer NP, Stephenson RC. The impact of hospice services in the care of patients with advanced stage nonsmall cell lung cancer. J Palliat Med. 2017;20(1):29-34.
10. Curtis JR, Wenrich MD, Carline JD, Shannon SE, Ambrozy DM, Ramsey PG. Understanding physicians’ skills at providing end-of-life care perspectives of patients, families, and health care workers. J Gen Intern Med. 2001;16(1):41-49.
11. Back AL, Young JP, McCown E, et al. Abandonment at the end of life from patient, caregiver, nurse, and physician perspectives: loss of continuity and lack of closure. Arch Intern Med. 2009;169(5):474-479.
12. Vig EK, Starks H, Taylor JS, Hopley EK, Fryer-Edwards K. Why don’t patients enroll in hospice? Can we do anything about it? J Gen Intern Med. 2010;25(10):1009-1019.
13. Waldrop DP, Meeker MA, Kerr C, Skretny J, Tangeman J, Milch R. The nature and timing of family-provider communication in late-stage cancer: a qualitative study of caregivers’ experiences. J Pain Symptom Manage. 2012;43(2):182-194.
14. Emanuel EJ, Fairclough DL, Slutsman J, Emanuel LL. Understanding economic and other burdens of terminal illness: the experience of patients and their caregivers. Ann Intern Med. 2000;132(6):451-459.
15. Curtis JR, Patrick DL, Engelberg RA, Norris K, Asp C, Byock I. A measure of the quality of dying and death. Initial validation using after-death interviews with family members. J Pain Symptom Manage. 2002;24(1):17-31.
16. Fleming DA, Sheppard VB, Mangan PA, et al. Caregiving at the end of life: Perceptions of health care quality and quality of life among patients and caregivers. J Pain Symptom Manage. 2006;31(5):407-420.
17. McMillan SC. Interventions to facilitate family caregiving at the end of life. J Palliat Med. 2005;8(Suppl 1):S132-S139.
18. Bakitas M, Lyons KD, Hegel MT, et al. Effects of a palliative care intervention on clinical outcomes in patients with advanced cancer: the Project ENABLE II randomized controlled trial. JAMA. 2009;302(7):741-749.
19. Temel JS, Greer JA, Muzikansky A, et al. Early palliative care for patients with metastatic non-small-cell lung cancer. N Engl J Med. 2010;363(8):733-742.
20. Zimmermann C, Swami N, Krzyzanowska M, et al. Early palliative care for patients with advanced cancer: a cluster-randomised controlled trial. Lancet. 2014;383(9930):1721-1730.
21. Maltoni M, Scarpi E, Dall’Agata M, et al. Systematic versus on-demand early palliative care: a randomised clinical trial assessing quality of care and treatment aggressiveness near the end of life. Eur J Cancer. 2016;69:110-118.
22. El-Jawahri A ea. Early integrated palliative care to improve family caregivers (FC) outcomes for patients with gastrointestinal and lung cancer. J Clin Oncol. 2016;34(suppl; abstr 10131).
23. Temel JS, Greer JA, El-Jawahri A, et al. Effects of early integrated palliative care in patients with lung and gi cancer: a randomized clinical trial. J Clin Oncol. 2017;35(8):834-841.
24. El-Jawahri A, LeBlanc T, VanDusen H, et al. Effect of inpatient palliative care on quality of life 2 weeks after hematopoietic stem cell transplantation: a randomized clinical trial. JAMA. 2016;316(20):2094-2103.
25. Cooper B, Kinsella GJ, Picton C. Development and initial validation of a family appraisal of caregiving questionnaire for palliative care. Psychooncology. 2006;15(7):613-622.
26. Kristjanson LJ. Validity and reliability testing of the FAMCARE Scale: measuring family satisfaction with advanced cancer care. Soc Sci Med. 1993;36(5):693-701.
27. Cohen S, Kamarck T, Mermelstein R. A global measure of perceived stress. J Health Soc Behav. 1983;24(4):385-396.
28. Keir ST, Guill AB, Carter KE, Boole LC, Gonzales L, Friedman HS. Differential levels of stress in caregivers of brain tumor patients--observations from a pilot study. Support Care Cancer. 2006;14(12):1258-1261.
29. Brehaut JC, O’Connor AM, Wood TJ, et al. Validation of a decision regret scale. Med Decis Making. 2003;23(4):281-292.
30. Teno JM, Clarridge B, Casey V, Edgman-Levitan S, Fowler J. Validation of toolkit after-death bereaved family member interview. J Pain Symptom Manage. 2001;22(3):752-758.
31. Wright AA, Zhang B, Ray A, Mack JW, Trice E, Balboni T, et al. Associations between end-of-life discussions, patient mental health, medical care near death, and caregiver bereavement adjustment. JAMA. 2008;300(14):1665-1673.
32. Earle CC, Neville BA, Landrum MB, Ayanian JZ, Block SD, Weeks JC. Trends in the aggressiveness of cancer care near the end of life. J Clin Oncol. 2004;22(2):315-321.
33. Obermeyer Z, Makar M, Abujaber S, Dominici F, Block S, Cutler DM. Association between the Medicare hospice benefit and health care utilization and costs for patients with poor-prognosis cancer. JAMA. 2014;312(18):1888-1896.
Integrating survivorship care planning in radiation oncology workflow
In January 2016 there were an estimated 15.5 million people in the United States who were living with a cancer diagnosis, representing 4.8% of the population. That number is expected to increase to 20.3 million by 2026.1 The 5-year relative survival rate for all cancers diagnosed during 2005 to 2011 was 69%.2 As more individuals with a cancer diagnosis now live longer, cancer survivorship is receiving increased attention. A report from the Institute of Medicine3 identified the essential components of survivorship care, including the provision of a survivorship care plan (SCP) containing specific diagnosis, treatment, and follow-up information (Table 1). To maintain accreditation in their respective organizations, the American College of Surgeons’ Commission on Cancer and the National Accreditation Program for Breast Centers (NAPBC) have included standards on providing treatment summaries and SCPs in person to those patients who have completed cancer treatments given with curative intent.4,5
SCPs are personalized documents presented to cancer patients at the end of treatment that summarize key aspects of cancer treatment and recommend appropriate ongoing medical care and self-management. The purpose of the SCP is both to educate cancer survivors and to create a portable document that can be shared with primary care providers to facilitate coordinated care.6 There are multiple barriers to SCP implementation, which may include the time required to create an SCP, inadequate reimbursement for the time spent creating and delivering the plan, a lack of risk-stratified guidelines for coordinated care, and the incomplete automation of diagnosis and treatment summarization by the electronic health record (EHR).7
Survivorship care in radiation oncology
The American College of Radiology includes the recommendation for regular, ongoing follow-up in the standards for accreditation for radiation oncology practice.8 Radiation oncology practices often provide the initial follow-up appointment about a month after the prescribed radiation treatment has been completed. The twofold purpose of this appointment is to assess the response to treatment and to evaluate acute treatment-related effects.9 The appointment may include a skin evaluation, assessment for any acute treatment effects, informal counseling on maintaining a healthy lifestyle, and recommendations for posttreatment care and follow-up. The appointment may also be an opportune time for delivering the SCP because radiation therapy is often the final treatment modality in active therapy for breast cancer patients.
A review of the literature yields scant data on the incorporation of SCPs into a radiation oncology practice. A 2014 survey of members of the American Society of Radiation Oncology10for a response percentage of 14.7%. Almost all providers follow their patients after treatment (97% (n = 574 respondents/3987 total membership, 14.4% response rate) showed that although most radiation oncologists provide long-term follow-up care to their patients after treatment completion (97%), fewer than half of those surveyed indicated that they delivered SCPs for curative-intent patients (40%), and even fewer delivered for palliative-intent patients (19%). Standards for the American Society for Radiation Oncology’s Accreditation Program for Excellence11 outline content for end-of-treatment documentation. Typically, the documentation includes a detailed treatment summary prepared by the treating radiation oncologist. This treatment summary includes the patient’s diagnosis, the area treated, radiation doses received, number of fractions delivered, therapy start date, therapy completion date, and overall tolerance of treatment in a clinical summary. The treatment summary is communicated to other providers involved in the patient’s care to promote care coordination, but it is not typically provided to patients.
Development of University of Wisconsin survivorship care planning
As an important component of maintaining NAPBC accreditation, the University of Wisconsin (UW) Health Breast Center began the process of formalizing and optimizing SCPs for breast cancer survivors who are followed at the center. Multidisciplinary input from surgical, medical, and radiation oncology was obtained. Representatives from those disciplines met regularly to reach consensus on the treatment summary and SCP content. The following 3 documents were created for use during a transition visit at the end of treatment: the written individualized SCP to be provided to the survivor and his/her primary care providers, a general survivorship patient education booklet, and a patient questionnaire to identify survivors’ concerns and additional resources that may be beneficial.
Treatment summary
Working in collaboration with IT specialists, we enabled out-of-the-box functionality within our EHR. This cancer-specific functionality provides a central and standard location within each survivor’s problem list to systematically document information regarding cancer diagnosis, stage, and treatment associated with a specific cancer diagnosis. Each treating provider (surgeon, medical oncologist, radiation oncologist, genetic counselor, etc) is responsible for entering and updating the relevant components within the treatment summary (ie, the surgeon enters and maintains the surgical details, the medical oncologist does likewise for chemotherapy and other medical therapies, etc). Information is updated and current, creating a dynamic documentation of diagnosis and treatment that can be used in clinic notes, patient after-visit summaries, and SCPs.
Survivorship care plan
This same EHR functionality is leveraged to generate, populate, and maintain the individualized SCP for each breast cancer survivor. The Treatment Summary section of the SCP can be quickly prepared within the EHR by autopopulating data previously entered by treating providers. Content and language for SCP templates in breast, colorectal, prostate, and gynecologic cancers are in use at the time of publication. The templates are developed as a collaborative effort between oncology subspecialists, with input from the UW Health survivor and family advocacy councils.
Each template contains a Treatment Summary section and an SCP section. The Treatment Summary section includes survivor general information, diagnosis and treatment information, and the clinical and supportive/survivor care team names and contact information. The SCP section includes follow-up recommendations, signs of recurrence and/or symptoms to report, healthy lifestyle and maintenance, chronic or late effects of specific treatment if applicable (eg, surgery, chemotherapy by drug, radiation therapy, and endocrine therapy), and general resources for common psychosocial concerns (Table 1).12,13
Each SCP is visible to the entire health care team, including other specialists and primary care, as long as they have access to UW Health’s EHR.14 The result is a readily accessible, comprehensive document that is individualized for each survivor, residing in a standard location with standardized format and content to facilitate review and use.15
General survivorship patient education booklet
Many cancer survivors request additional information about their posttreatment concerns. The “UW Health Facts for You: Cancer Survivorship, Carbone Cancer Center” booklet was developed by a multidisciplinary team including oncologists, advanced practice providers (APPs), navigators, social workers, program leadership, cancer survivors, and caregivers. The guide includes detailed information for the cancer survivor on topics including nutrition, exercise, sleep, tobacco cessation, sexual health, and spirituality. Common concerns and symptom management are addressed as well as a comprehensive list of community resources. The booklet can be found at http://www.uwhealth.org/healthfacts/cancer/7834.
Survivorship questionnaire
Breast cancer survivors often have multiple concerns as they transition from active treatment to the survivorship phase of their cancer journey. Specific concerns may vary slightly form one survivor to another. Guided by recommendations for the American Society of Clinical Oncology and the National Comprehensive Cancer Network, we developed a 10-question, 2-page questionnaire to identify those concerns with input from members of the Breast Cancer Steering Committee. Members of the committee include surgical, medical, and radiation oncologists, AAPs, radiologists, pathologists, program leadership, and nurses, along with breast cancer survivors. Elements in the questionnaire include nutrition, activity, mood, sleep, sexual health, employment/insurance, pain/swelling, desires regarding pregnancy or prevention, memory/concentration, smoking, alcohol, genetic testing/counseling, and assistance with establishing care with a primary care provider. By completing the questionnaire, breast cancer survivors identify specific concerns within each category and are able to request additional information about those concerns and/or a referral to appropriate resources. They may also select the I need nothing further option if the concern is present but already being addressed.
SCP delivery and the transition visit
The next task in implementation of the care process for survivors encompassed the development of clinical workflows and processes to provide the document to the breast cancer survivor at the completion of treatment. In a study of breast cancer survivors, it was found that the preferred format for survivorship care planning is generally an in-person consultation at completion of treatment with an oncology professional.16 The best time for distribution of the written SCP is, however, unclear. Intuitively, it seems optimal to distribute SCPs around the time of completion of active treatment. However, for SCP delivery to be feasible and sustainable, delivery must be integrated into existing clinical care-delivery processes, and content must be streamlined and focused to meet the needs of their intended recipients without becoming overly burdensome to prepare and deliver.17
Ultimately, and after significant multidisciplinary discussion, it was determined that Stage 0-III breast cancer patients would have a visit focusing on symptoms and transitioning to surveillance follow-up (Transition Visit) as they completed active curative-intent cancer treatment. During this Transition Visit, the SCP document would be provided and reviewed with survivors. The Transition Visit for breast cancer survivors would be conducted by an APP following the completion of their final stage of active, primary treatment (surgery, chemotherapy, and/or radiation therapy). Additional long-term adjuvant therapy for breast cancer survivors (ie, trastuzumab, endocrine therapy) would continue as indicated during and after delivery of the SCP.
The radiation oncology clinic was chosen as a venue for these Transition Visits for breast cancer survivors whose treatment included radiotherapy. Despite little historical experience with delivery of SCPs in radiation oncology clinics, this was a logical choice given that radiotherapy is usually the final phase of active treatment for these breast cancer survivors, and a follow-up visit about a month after completing radiotherapy is already part of standard practice. Collaborating with the multidisciplinary UW Health Breast Center, we therefore integrated the formal breast survivorship care planning process and provision of the SCP into the current radiation oncology workflow. About 40% of the roughly 600 breast cancer patients treated by surgical and/or medical oncology at our institution annually also receive radiation therapy at our site. For the remaining 60% of breast cancer survivors who do not receive radiation therapy or who completed radiotherapy at an outside facility, the SCP is provided by an APP within the UW Health Breast Center.
UW radiation oncology survivorship transition visit
The overall workflow of our Transition Visit is depicted in the Figure. Toward the end of the breast cancer survivor’s radiation treatments, the radiation oncologist instructs the schedulers to arrange the 1-month, post-radiation Transition Visit with the APP and informs the survivor about the nature of the appointment. The Transition Visit is scheduled as a 60-minute appointment. Before the survivor’s arrival, an APP generates the written SCP. The activity includes completing the Treatment Summary, or verifying the accuracy of a prepopulated Treatment Summary, and individualizing the SCP section for the patient based on treatment received and follow-up recommendations using drop-down functionality. As the SCP is printed for review with the survivor, it is simultaneously sent to the survivor’s primary care provider. This is accomplished by using EHR functionality to route the document internally to UW primary care providers or automatically faxing the document to external primary care providers. Each SCP is also marked as complete within the EHR for the purposes of documenting compliance with this activity for later data analysis.
On arrival for the appointment, each breast cancer survivor completes the survivorship questionnaire. During the Transition Visit, the questionnaire is reviewed with the survivor and additional information is provided. Referral options are discussed if indicated with desired referrals made by the APP. The survivor is interviewed and examined for any persistent side effects of treatment. Next, the Treatment Summary and SCP are reviewed with the survivor, emphasizing the follow-up plan, signs or symptoms of breast cancer recurrence, and chronic or late treatment-related toxicities. Ample opportunity is provided for the survivor to ask questions and voice concerns.
Follow-up appointments with members of the patient’s care team (ie, medical, surgical, or radiation oncology) as well as necessary breast imaging (ie, mammogram, MRI) are coordinated and scheduled before the survivor leaves the department. A survey of oncologists (medical, surgical, radiation) identified specific cancer-related components of survivorship care that oncologists felt most responsible for as well as opportunities to improve the quality and efficiency of care provided by oncologists.18 At our institution, the breast surgical, medical, and radiation oncologists all generally participate in follow-up care through at least 1 year following completion of active, primary treatment.
Outcomes, quality improvement opportunities, and continued challenges with the process
There is presently a lack of long-term outcome data about the impact of SCPs. As mandates for the provision of SCPs are made, research focusing on whether SCPs result in improved health behaviors and outcomes, reduced burden in care transitions from the oncology setting, and increased cost-effectiveness will be needed.19 The long-term effects of SCPs on psychological, oncologic, and resource outcomes should be evaluated,20 as well as the impact on health behaviors, such as smoking cessation or participation in rehabilitation programs.21
Following the implementation of our Transition Visits in 2015, we conducted a quality improvement review. This review included summation of 69 recent breast cancer questionnaires from Transition Visits with our APPs (Table 2 and Table 3). The most common concerns raised by our breast cancer survivors include desire for weight loss, improving diet, and increasing physical activity. Of note, concerns did not often translate into a desire for more information or referrals.22 Survivors were generally satisfied with the timing of the Transition Visits and generally indicated that the visits were helpful, with self-reported improvements in their understanding of planned follow-up. A Canadian group evaluating breast and head and neck cancer survivors has suggested that SCPs could produce long-term improvements in healthy lifestyle behaviors; however, further research is needed to determine the extent to which SCPs might improve follow-up care over the long term.23
Finally, although efforts to date have been focused on the breast cancer survivor at the completion of treatment, long-term survivors may also benefit from receiving the SCP. A study by the American Cancer Society found that long-term cancer survivors had unmet informational needs, particularly with regard to screening, long-term cancer and treatment effects, and healthy lifestyle behaviors.24 Identifying and subsequently delivering an SCP to eligible long-term survivors is a challenging prospect, which depends on further refinement of EHR-based tracking of the date of diagnosis, cancer stage, and end-of-treatment date.
Summary and recommendations
Survivorship care has been efficiently integrated into our 1-month post-radiation follow-up appointment for breast cancer survivors. By using current resources in the radiation oncology department, the process has provided an effective way to deliver the SCP to breast cancer survivors. Future plans include implementing the process for all patients receiving curative-intent radiation for additional solid tumor survivors. Quality improvement projects will be developed to assess survivor satisfaction and the impact on health behaviors.
Acknowledgments
The authors thank Amy Heath, MS, RTT, for editorial and manuscript preparation assistance.
1. Statistics. National Cancer Institute, Division of Cancer Control & Population Sciences website. http:///cancercontrol.cancer.gov/ocs/statistics/statistics.html. Updated October 17, 2016. Accessed March 6, 2018.
2. Cancer facts & figures 2016. American Cancer Society website. https://www.cancer.org/research/cancer-facts-statistics/all-cancer-facts-figures/cancer-facts-figures-2016.html. Published 2016. Accessed February 27, 2018.
3. Hewitt M, Greenfield S, Stovall E, eds. From cancer patient to cancer survivor: lost in transition. Washington, DC: National Academies Press; 2006.
4. Knutson A, McNamara E. Cancer program standards: ensuring patient-centered care. American College of Surgeons website. https://www.facs.org/quality-programs/cancer/coc/standards. Published August 2016. Accessed March 6, 2018.
5. National Accreditation Program for Breast Centers. NAPBC standards manual. American College of Surgeons website. https://www.facs.org/~/media/files/quality%20programs/napbc/2014%20napbc%20standards%20manual.ashx. Published 2014. Accessed March 6, 2018.
6. Salz T, McCabe MS, Onstad EE, et al. Survivorship care plans: is there buy-in from community oncology providers? Cancer. 2014;120(5):722-730.
7. Mayer DK, Nekhlyudov L, Snyder CF, Merrill JK, Wollins DS, Shulman LN. American Society of Clinical Oncology clinical expert statement on cancer survivorship care planning. J Oncol Pract. 2014;10(6):345-351.
8. Dobelbower RR, Cotter G, Schilling PJ, Parsai EI, Carroll JM. Radiation oncology practice accreditation. Rays. 2001;26(3):191-198.
9. Hartford AC, Conway PD, Desai NB, et al. ACR-ASTRO practice parameter for communication: radiation oncology. The American College of Radiology website. http://www.acr.org/-/media/ACR/Files/Practice-Parameters/RadOnc.pdf. Updated 2014. Accessed March 6, 2018.
10. Koontz BF, Benda R, De Los Santos J, et al. US radiation oncology practice patterns for posttreatment survivor care. Pract Radiat Oncol. 2016;6(1):50-56.
11. American Society of Therapeutic Radiation Oncologists. APEx program standards. ASTRO website. http://www.astro.org/uploadedFiles/_MAIN_SITE/Daily_Practice/Accreditation/Content_Pieces/ProgramStandards.pdf. Published February 1, 2016. Accessed March 6, 2018.
12. Clinical practice survivorship guidelines and adaptations. American Society of Clinical Oncology website. http://www.asco.org/practice-guidelines/cancer-care-initiatives/prevention-survivorship. Published 2013. Accessed March 6, 2018.
13. National Comprehensive Cancer Network. Supportive care guidelines. NCNN website. http://www.nccn.org/professionals/physician_gls/f_guidelines.asp#supportive. Updated February 16, 2018. Accessed March 6, 2018.
14. Donohue S, Sesto ME, Hahn DL, et al. Evaluating primary care providers’ views on survivorship care plans generated by an electronic health record system. J Oncol Pract. 2015;11(3):e329-e335.
15. Tevaarwerk AJ, Wisinski KB, Buhr KA, et al. Leveraging electronic health record systems to create and provide electronic cancer survivorship care plans: a pilot study. J Oncol Pract. 2014;10(3):e150-e159.
16. Smith SL, Singh-Carlson S, Downie L, Payeur N, Wai ES. Survivors of breast cancer: patient perspectives on survivorship care planning. J Cancer Surviv. 2011;5(4):337-344.
17. Stricker CT, O’Brien M. Implementing the commission on cancer standards for survivorship care plans. Clin J Oncol Nurs. 2014;18(suppl 1):15-22.
18. Neuman HB, Steffens NM, Jacobson N, et al. Oncologists’ perspectives of their roles and responsibilities during multi-disciplinary breast cancer follow-up. Ann Surg Oncol. 2016;23(3):708-714.
19. Palmer SC, Stricker CT, Panzer SL, et al. Outcomes and satisfaction after delivery of a breast cancer survivorship care plan: Results of a multicenter trial. J Oncol Pract. 2015;11(2):e222-e229.
20. Brennan ME, Gormally JF, Butow P, Boyle FM, Spillane AJ. Survivorship care plans in cancer: a systematic review of care plan outcomes. Br J Cancer. 2014;111(10):1899-1908.
21. Chen RC, Hoffman KE, Sher DJ, et al. Development of a standard survivorship care plan template for radiation oncologists. Pract Radiat Oncol. 2016;6(1):57-65.
22. Seaborne LA, Huenerberg KJ, Bohler A, et al. Developing electronic health record based program to deliver survivorship care plans and visits at the UW breast center. Poster presented at American Society of Clinical Oncology Survivorship Symposium; January 15-16, 2016; San Francisco CA.
23. Collie K, McCormick J, Waller A, et al. Qualitative evaluation of care plans for Canadian breast and head-and-neck cancer survivors. Curr Oncol. 2014;21(1):18-28.
24. Playdon M, Ferrucci LM, McCorkle R, et al. Health information needs and preferences in relation to survivorship care plans of long-term cancer survivors in the American Cancer Society’s study of cancer survivors-I. J Cancer Surviv. 2016;10(4):674-685.
In January 2016 there were an estimated 15.5 million people in the United States who were living with a cancer diagnosis, representing 4.8% of the population. That number is expected to increase to 20.3 million by 2026.1 The 5-year relative survival rate for all cancers diagnosed during 2005 to 2011 was 69%.2 As more individuals with a cancer diagnosis now live longer, cancer survivorship is receiving increased attention. A report from the Institute of Medicine3 identified the essential components of survivorship care, including the provision of a survivorship care plan (SCP) containing specific diagnosis, treatment, and follow-up information (Table 1). To maintain accreditation in their respective organizations, the American College of Surgeons’ Commission on Cancer and the National Accreditation Program for Breast Centers (NAPBC) have included standards on providing treatment summaries and SCPs in person to those patients who have completed cancer treatments given with curative intent.4,5
SCPs are personalized documents presented to cancer patients at the end of treatment that summarize key aspects of cancer treatment and recommend appropriate ongoing medical care and self-management. The purpose of the SCP is both to educate cancer survivors and to create a portable document that can be shared with primary care providers to facilitate coordinated care.6 There are multiple barriers to SCP implementation, which may include the time required to create an SCP, inadequate reimbursement for the time spent creating and delivering the plan, a lack of risk-stratified guidelines for coordinated care, and the incomplete automation of diagnosis and treatment summarization by the electronic health record (EHR).7
Survivorship care in radiation oncology
The American College of Radiology includes the recommendation for regular, ongoing follow-up in the standards for accreditation for radiation oncology practice.8 Radiation oncology practices often provide the initial follow-up appointment about a month after the prescribed radiation treatment has been completed. The twofold purpose of this appointment is to assess the response to treatment and to evaluate acute treatment-related effects.9 The appointment may include a skin evaluation, assessment for any acute treatment effects, informal counseling on maintaining a healthy lifestyle, and recommendations for posttreatment care and follow-up. The appointment may also be an opportune time for delivering the SCP because radiation therapy is often the final treatment modality in active therapy for breast cancer patients.
A review of the literature yields scant data on the incorporation of SCPs into a radiation oncology practice. A 2014 survey of members of the American Society of Radiation Oncology10for a response percentage of 14.7%. Almost all providers follow their patients after treatment (97% (n = 574 respondents/3987 total membership, 14.4% response rate) showed that although most radiation oncologists provide long-term follow-up care to their patients after treatment completion (97%), fewer than half of those surveyed indicated that they delivered SCPs for curative-intent patients (40%), and even fewer delivered for palliative-intent patients (19%). Standards for the American Society for Radiation Oncology’s Accreditation Program for Excellence11 outline content for end-of-treatment documentation. Typically, the documentation includes a detailed treatment summary prepared by the treating radiation oncologist. This treatment summary includes the patient’s diagnosis, the area treated, radiation doses received, number of fractions delivered, therapy start date, therapy completion date, and overall tolerance of treatment in a clinical summary. The treatment summary is communicated to other providers involved in the patient’s care to promote care coordination, but it is not typically provided to patients.
Development of University of Wisconsin survivorship care planning
As an important component of maintaining NAPBC accreditation, the University of Wisconsin (UW) Health Breast Center began the process of formalizing and optimizing SCPs for breast cancer survivors who are followed at the center. Multidisciplinary input from surgical, medical, and radiation oncology was obtained. Representatives from those disciplines met regularly to reach consensus on the treatment summary and SCP content. The following 3 documents were created for use during a transition visit at the end of treatment: the written individualized SCP to be provided to the survivor and his/her primary care providers, a general survivorship patient education booklet, and a patient questionnaire to identify survivors’ concerns and additional resources that may be beneficial.
Treatment summary
Working in collaboration with IT specialists, we enabled out-of-the-box functionality within our EHR. This cancer-specific functionality provides a central and standard location within each survivor’s problem list to systematically document information regarding cancer diagnosis, stage, and treatment associated with a specific cancer diagnosis. Each treating provider (surgeon, medical oncologist, radiation oncologist, genetic counselor, etc) is responsible for entering and updating the relevant components within the treatment summary (ie, the surgeon enters and maintains the surgical details, the medical oncologist does likewise for chemotherapy and other medical therapies, etc). Information is updated and current, creating a dynamic documentation of diagnosis and treatment that can be used in clinic notes, patient after-visit summaries, and SCPs.
Survivorship care plan
This same EHR functionality is leveraged to generate, populate, and maintain the individualized SCP for each breast cancer survivor. The Treatment Summary section of the SCP can be quickly prepared within the EHR by autopopulating data previously entered by treating providers. Content and language for SCP templates in breast, colorectal, prostate, and gynecologic cancers are in use at the time of publication. The templates are developed as a collaborative effort between oncology subspecialists, with input from the UW Health survivor and family advocacy councils.
Each template contains a Treatment Summary section and an SCP section. The Treatment Summary section includes survivor general information, diagnosis and treatment information, and the clinical and supportive/survivor care team names and contact information. The SCP section includes follow-up recommendations, signs of recurrence and/or symptoms to report, healthy lifestyle and maintenance, chronic or late effects of specific treatment if applicable (eg, surgery, chemotherapy by drug, radiation therapy, and endocrine therapy), and general resources for common psychosocial concerns (Table 1).12,13
Each SCP is visible to the entire health care team, including other specialists and primary care, as long as they have access to UW Health’s EHR.14 The result is a readily accessible, comprehensive document that is individualized for each survivor, residing in a standard location with standardized format and content to facilitate review and use.15
General survivorship patient education booklet
Many cancer survivors request additional information about their posttreatment concerns. The “UW Health Facts for You: Cancer Survivorship, Carbone Cancer Center” booklet was developed by a multidisciplinary team including oncologists, advanced practice providers (APPs), navigators, social workers, program leadership, cancer survivors, and caregivers. The guide includes detailed information for the cancer survivor on topics including nutrition, exercise, sleep, tobacco cessation, sexual health, and spirituality. Common concerns and symptom management are addressed as well as a comprehensive list of community resources. The booklet can be found at http://www.uwhealth.org/healthfacts/cancer/7834.
Survivorship questionnaire
Breast cancer survivors often have multiple concerns as they transition from active treatment to the survivorship phase of their cancer journey. Specific concerns may vary slightly form one survivor to another. Guided by recommendations for the American Society of Clinical Oncology and the National Comprehensive Cancer Network, we developed a 10-question, 2-page questionnaire to identify those concerns with input from members of the Breast Cancer Steering Committee. Members of the committee include surgical, medical, and radiation oncologists, AAPs, radiologists, pathologists, program leadership, and nurses, along with breast cancer survivors. Elements in the questionnaire include nutrition, activity, mood, sleep, sexual health, employment/insurance, pain/swelling, desires regarding pregnancy or prevention, memory/concentration, smoking, alcohol, genetic testing/counseling, and assistance with establishing care with a primary care provider. By completing the questionnaire, breast cancer survivors identify specific concerns within each category and are able to request additional information about those concerns and/or a referral to appropriate resources. They may also select the I need nothing further option if the concern is present but already being addressed.
SCP delivery and the transition visit
The next task in implementation of the care process for survivors encompassed the development of clinical workflows and processes to provide the document to the breast cancer survivor at the completion of treatment. In a study of breast cancer survivors, it was found that the preferred format for survivorship care planning is generally an in-person consultation at completion of treatment with an oncology professional.16 The best time for distribution of the written SCP is, however, unclear. Intuitively, it seems optimal to distribute SCPs around the time of completion of active treatment. However, for SCP delivery to be feasible and sustainable, delivery must be integrated into existing clinical care-delivery processes, and content must be streamlined and focused to meet the needs of their intended recipients without becoming overly burdensome to prepare and deliver.17
Ultimately, and after significant multidisciplinary discussion, it was determined that Stage 0-III breast cancer patients would have a visit focusing on symptoms and transitioning to surveillance follow-up (Transition Visit) as they completed active curative-intent cancer treatment. During this Transition Visit, the SCP document would be provided and reviewed with survivors. The Transition Visit for breast cancer survivors would be conducted by an APP following the completion of their final stage of active, primary treatment (surgery, chemotherapy, and/or radiation therapy). Additional long-term adjuvant therapy for breast cancer survivors (ie, trastuzumab, endocrine therapy) would continue as indicated during and after delivery of the SCP.
The radiation oncology clinic was chosen as a venue for these Transition Visits for breast cancer survivors whose treatment included radiotherapy. Despite little historical experience with delivery of SCPs in radiation oncology clinics, this was a logical choice given that radiotherapy is usually the final phase of active treatment for these breast cancer survivors, and a follow-up visit about a month after completing radiotherapy is already part of standard practice. Collaborating with the multidisciplinary UW Health Breast Center, we therefore integrated the formal breast survivorship care planning process and provision of the SCP into the current radiation oncology workflow. About 40% of the roughly 600 breast cancer patients treated by surgical and/or medical oncology at our institution annually also receive radiation therapy at our site. For the remaining 60% of breast cancer survivors who do not receive radiation therapy or who completed radiotherapy at an outside facility, the SCP is provided by an APP within the UW Health Breast Center.
UW radiation oncology survivorship transition visit
The overall workflow of our Transition Visit is depicted in the Figure. Toward the end of the breast cancer survivor’s radiation treatments, the radiation oncologist instructs the schedulers to arrange the 1-month, post-radiation Transition Visit with the APP and informs the survivor about the nature of the appointment. The Transition Visit is scheduled as a 60-minute appointment. Before the survivor’s arrival, an APP generates the written SCP. The activity includes completing the Treatment Summary, or verifying the accuracy of a prepopulated Treatment Summary, and individualizing the SCP section for the patient based on treatment received and follow-up recommendations using drop-down functionality. As the SCP is printed for review with the survivor, it is simultaneously sent to the survivor’s primary care provider. This is accomplished by using EHR functionality to route the document internally to UW primary care providers or automatically faxing the document to external primary care providers. Each SCP is also marked as complete within the EHR for the purposes of documenting compliance with this activity for later data analysis.
On arrival for the appointment, each breast cancer survivor completes the survivorship questionnaire. During the Transition Visit, the questionnaire is reviewed with the survivor and additional information is provided. Referral options are discussed if indicated with desired referrals made by the APP. The survivor is interviewed and examined for any persistent side effects of treatment. Next, the Treatment Summary and SCP are reviewed with the survivor, emphasizing the follow-up plan, signs or symptoms of breast cancer recurrence, and chronic or late treatment-related toxicities. Ample opportunity is provided for the survivor to ask questions and voice concerns.
Follow-up appointments with members of the patient’s care team (ie, medical, surgical, or radiation oncology) as well as necessary breast imaging (ie, mammogram, MRI) are coordinated and scheduled before the survivor leaves the department. A survey of oncologists (medical, surgical, radiation) identified specific cancer-related components of survivorship care that oncologists felt most responsible for as well as opportunities to improve the quality and efficiency of care provided by oncologists.18 At our institution, the breast surgical, medical, and radiation oncologists all generally participate in follow-up care through at least 1 year following completion of active, primary treatment.
Outcomes, quality improvement opportunities, and continued challenges with the process
There is presently a lack of long-term outcome data about the impact of SCPs. As mandates for the provision of SCPs are made, research focusing on whether SCPs result in improved health behaviors and outcomes, reduced burden in care transitions from the oncology setting, and increased cost-effectiveness will be needed.19 The long-term effects of SCPs on psychological, oncologic, and resource outcomes should be evaluated,20 as well as the impact on health behaviors, such as smoking cessation or participation in rehabilitation programs.21
Following the implementation of our Transition Visits in 2015, we conducted a quality improvement review. This review included summation of 69 recent breast cancer questionnaires from Transition Visits with our APPs (Table 2 and Table 3). The most common concerns raised by our breast cancer survivors include desire for weight loss, improving diet, and increasing physical activity. Of note, concerns did not often translate into a desire for more information or referrals.22 Survivors were generally satisfied with the timing of the Transition Visits and generally indicated that the visits were helpful, with self-reported improvements in their understanding of planned follow-up. A Canadian group evaluating breast and head and neck cancer survivors has suggested that SCPs could produce long-term improvements in healthy lifestyle behaviors; however, further research is needed to determine the extent to which SCPs might improve follow-up care over the long term.23
Finally, although efforts to date have been focused on the breast cancer survivor at the completion of treatment, long-term survivors may also benefit from receiving the SCP. A study by the American Cancer Society found that long-term cancer survivors had unmet informational needs, particularly with regard to screening, long-term cancer and treatment effects, and healthy lifestyle behaviors.24 Identifying and subsequently delivering an SCP to eligible long-term survivors is a challenging prospect, which depends on further refinement of EHR-based tracking of the date of diagnosis, cancer stage, and end-of-treatment date.
Summary and recommendations
Survivorship care has been efficiently integrated into our 1-month post-radiation follow-up appointment for breast cancer survivors. By using current resources in the radiation oncology department, the process has provided an effective way to deliver the SCP to breast cancer survivors. Future plans include implementing the process for all patients receiving curative-intent radiation for additional solid tumor survivors. Quality improvement projects will be developed to assess survivor satisfaction and the impact on health behaviors.
Acknowledgments
The authors thank Amy Heath, MS, RTT, for editorial and manuscript preparation assistance.
In January 2016 there were an estimated 15.5 million people in the United States who were living with a cancer diagnosis, representing 4.8% of the population. That number is expected to increase to 20.3 million by 2026.1 The 5-year relative survival rate for all cancers diagnosed during 2005 to 2011 was 69%.2 As more individuals with a cancer diagnosis now live longer, cancer survivorship is receiving increased attention. A report from the Institute of Medicine3 identified the essential components of survivorship care, including the provision of a survivorship care plan (SCP) containing specific diagnosis, treatment, and follow-up information (Table 1). To maintain accreditation in their respective organizations, the American College of Surgeons’ Commission on Cancer and the National Accreditation Program for Breast Centers (NAPBC) have included standards on providing treatment summaries and SCPs in person to those patients who have completed cancer treatments given with curative intent.4,5
SCPs are personalized documents presented to cancer patients at the end of treatment that summarize key aspects of cancer treatment and recommend appropriate ongoing medical care and self-management. The purpose of the SCP is both to educate cancer survivors and to create a portable document that can be shared with primary care providers to facilitate coordinated care.6 There are multiple barriers to SCP implementation, which may include the time required to create an SCP, inadequate reimbursement for the time spent creating and delivering the plan, a lack of risk-stratified guidelines for coordinated care, and the incomplete automation of diagnosis and treatment summarization by the electronic health record (EHR).7
Survivorship care in radiation oncology
The American College of Radiology includes the recommendation for regular, ongoing follow-up in the standards for accreditation for radiation oncology practice.8 Radiation oncology practices often provide the initial follow-up appointment about a month after the prescribed radiation treatment has been completed. The twofold purpose of this appointment is to assess the response to treatment and to evaluate acute treatment-related effects.9 The appointment may include a skin evaluation, assessment for any acute treatment effects, informal counseling on maintaining a healthy lifestyle, and recommendations for posttreatment care and follow-up. The appointment may also be an opportune time for delivering the SCP because radiation therapy is often the final treatment modality in active therapy for breast cancer patients.
A review of the literature yields scant data on the incorporation of SCPs into a radiation oncology practice. A 2014 survey of members of the American Society of Radiation Oncology10for a response percentage of 14.7%. Almost all providers follow their patients after treatment (97% (n = 574 respondents/3987 total membership, 14.4% response rate) showed that although most radiation oncologists provide long-term follow-up care to their patients after treatment completion (97%), fewer than half of those surveyed indicated that they delivered SCPs for curative-intent patients (40%), and even fewer delivered for palliative-intent patients (19%). Standards for the American Society for Radiation Oncology’s Accreditation Program for Excellence11 outline content for end-of-treatment documentation. Typically, the documentation includes a detailed treatment summary prepared by the treating radiation oncologist. This treatment summary includes the patient’s diagnosis, the area treated, radiation doses received, number of fractions delivered, therapy start date, therapy completion date, and overall tolerance of treatment in a clinical summary. The treatment summary is communicated to other providers involved in the patient’s care to promote care coordination, but it is not typically provided to patients.
Development of University of Wisconsin survivorship care planning
As an important component of maintaining NAPBC accreditation, the University of Wisconsin (UW) Health Breast Center began the process of formalizing and optimizing SCPs for breast cancer survivors who are followed at the center. Multidisciplinary input from surgical, medical, and radiation oncology was obtained. Representatives from those disciplines met regularly to reach consensus on the treatment summary and SCP content. The following 3 documents were created for use during a transition visit at the end of treatment: the written individualized SCP to be provided to the survivor and his/her primary care providers, a general survivorship patient education booklet, and a patient questionnaire to identify survivors’ concerns and additional resources that may be beneficial.
Treatment summary
Working in collaboration with IT specialists, we enabled out-of-the-box functionality within our EHR. This cancer-specific functionality provides a central and standard location within each survivor’s problem list to systematically document information regarding cancer diagnosis, stage, and treatment associated with a specific cancer diagnosis. Each treating provider (surgeon, medical oncologist, radiation oncologist, genetic counselor, etc) is responsible for entering and updating the relevant components within the treatment summary (ie, the surgeon enters and maintains the surgical details, the medical oncologist does likewise for chemotherapy and other medical therapies, etc). Information is updated and current, creating a dynamic documentation of diagnosis and treatment that can be used in clinic notes, patient after-visit summaries, and SCPs.
Survivorship care plan
This same EHR functionality is leveraged to generate, populate, and maintain the individualized SCP for each breast cancer survivor. The Treatment Summary section of the SCP can be quickly prepared within the EHR by autopopulating data previously entered by treating providers. Content and language for SCP templates in breast, colorectal, prostate, and gynecologic cancers are in use at the time of publication. The templates are developed as a collaborative effort between oncology subspecialists, with input from the UW Health survivor and family advocacy councils.
Each template contains a Treatment Summary section and an SCP section. The Treatment Summary section includes survivor general information, diagnosis and treatment information, and the clinical and supportive/survivor care team names and contact information. The SCP section includes follow-up recommendations, signs of recurrence and/or symptoms to report, healthy lifestyle and maintenance, chronic or late effects of specific treatment if applicable (eg, surgery, chemotherapy by drug, radiation therapy, and endocrine therapy), and general resources for common psychosocial concerns (Table 1).12,13
Each SCP is visible to the entire health care team, including other specialists and primary care, as long as they have access to UW Health’s EHR.14 The result is a readily accessible, comprehensive document that is individualized for each survivor, residing in a standard location with standardized format and content to facilitate review and use.15
General survivorship patient education booklet
Many cancer survivors request additional information about their posttreatment concerns. The “UW Health Facts for You: Cancer Survivorship, Carbone Cancer Center” booklet was developed by a multidisciplinary team including oncologists, advanced practice providers (APPs), navigators, social workers, program leadership, cancer survivors, and caregivers. The guide includes detailed information for the cancer survivor on topics including nutrition, exercise, sleep, tobacco cessation, sexual health, and spirituality. Common concerns and symptom management are addressed as well as a comprehensive list of community resources. The booklet can be found at http://www.uwhealth.org/healthfacts/cancer/7834.
Survivorship questionnaire
Breast cancer survivors often have multiple concerns as they transition from active treatment to the survivorship phase of their cancer journey. Specific concerns may vary slightly form one survivor to another. Guided by recommendations for the American Society of Clinical Oncology and the National Comprehensive Cancer Network, we developed a 10-question, 2-page questionnaire to identify those concerns with input from members of the Breast Cancer Steering Committee. Members of the committee include surgical, medical, and radiation oncologists, AAPs, radiologists, pathologists, program leadership, and nurses, along with breast cancer survivors. Elements in the questionnaire include nutrition, activity, mood, sleep, sexual health, employment/insurance, pain/swelling, desires regarding pregnancy or prevention, memory/concentration, smoking, alcohol, genetic testing/counseling, and assistance with establishing care with a primary care provider. By completing the questionnaire, breast cancer survivors identify specific concerns within each category and are able to request additional information about those concerns and/or a referral to appropriate resources. They may also select the I need nothing further option if the concern is present but already being addressed.
SCP delivery and the transition visit
The next task in implementation of the care process for survivors encompassed the development of clinical workflows and processes to provide the document to the breast cancer survivor at the completion of treatment. In a study of breast cancer survivors, it was found that the preferred format for survivorship care planning is generally an in-person consultation at completion of treatment with an oncology professional.16 The best time for distribution of the written SCP is, however, unclear. Intuitively, it seems optimal to distribute SCPs around the time of completion of active treatment. However, for SCP delivery to be feasible and sustainable, delivery must be integrated into existing clinical care-delivery processes, and content must be streamlined and focused to meet the needs of their intended recipients without becoming overly burdensome to prepare and deliver.17
Ultimately, and after significant multidisciplinary discussion, it was determined that Stage 0-III breast cancer patients would have a visit focusing on symptoms and transitioning to surveillance follow-up (Transition Visit) as they completed active curative-intent cancer treatment. During this Transition Visit, the SCP document would be provided and reviewed with survivors. The Transition Visit for breast cancer survivors would be conducted by an APP following the completion of their final stage of active, primary treatment (surgery, chemotherapy, and/or radiation therapy). Additional long-term adjuvant therapy for breast cancer survivors (ie, trastuzumab, endocrine therapy) would continue as indicated during and after delivery of the SCP.
The radiation oncology clinic was chosen as a venue for these Transition Visits for breast cancer survivors whose treatment included radiotherapy. Despite little historical experience with delivery of SCPs in radiation oncology clinics, this was a logical choice given that radiotherapy is usually the final phase of active treatment for these breast cancer survivors, and a follow-up visit about a month after completing radiotherapy is already part of standard practice. Collaborating with the multidisciplinary UW Health Breast Center, we therefore integrated the formal breast survivorship care planning process and provision of the SCP into the current radiation oncology workflow. About 40% of the roughly 600 breast cancer patients treated by surgical and/or medical oncology at our institution annually also receive radiation therapy at our site. For the remaining 60% of breast cancer survivors who do not receive radiation therapy or who completed radiotherapy at an outside facility, the SCP is provided by an APP within the UW Health Breast Center.
UW radiation oncology survivorship transition visit
The overall workflow of our Transition Visit is depicted in the Figure. Toward the end of the breast cancer survivor’s radiation treatments, the radiation oncologist instructs the schedulers to arrange the 1-month, post-radiation Transition Visit with the APP and informs the survivor about the nature of the appointment. The Transition Visit is scheduled as a 60-minute appointment. Before the survivor’s arrival, an APP generates the written SCP. The activity includes completing the Treatment Summary, or verifying the accuracy of a prepopulated Treatment Summary, and individualizing the SCP section for the patient based on treatment received and follow-up recommendations using drop-down functionality. As the SCP is printed for review with the survivor, it is simultaneously sent to the survivor’s primary care provider. This is accomplished by using EHR functionality to route the document internally to UW primary care providers or automatically faxing the document to external primary care providers. Each SCP is also marked as complete within the EHR for the purposes of documenting compliance with this activity for later data analysis.
On arrival for the appointment, each breast cancer survivor completes the survivorship questionnaire. During the Transition Visit, the questionnaire is reviewed with the survivor and additional information is provided. Referral options are discussed if indicated with desired referrals made by the APP. The survivor is interviewed and examined for any persistent side effects of treatment. Next, the Treatment Summary and SCP are reviewed with the survivor, emphasizing the follow-up plan, signs or symptoms of breast cancer recurrence, and chronic or late treatment-related toxicities. Ample opportunity is provided for the survivor to ask questions and voice concerns.
Follow-up appointments with members of the patient’s care team (ie, medical, surgical, or radiation oncology) as well as necessary breast imaging (ie, mammogram, MRI) are coordinated and scheduled before the survivor leaves the department. A survey of oncologists (medical, surgical, radiation) identified specific cancer-related components of survivorship care that oncologists felt most responsible for as well as opportunities to improve the quality and efficiency of care provided by oncologists.18 At our institution, the breast surgical, medical, and radiation oncologists all generally participate in follow-up care through at least 1 year following completion of active, primary treatment.
Outcomes, quality improvement opportunities, and continued challenges with the process
There is presently a lack of long-term outcome data about the impact of SCPs. As mandates for the provision of SCPs are made, research focusing on whether SCPs result in improved health behaviors and outcomes, reduced burden in care transitions from the oncology setting, and increased cost-effectiveness will be needed.19 The long-term effects of SCPs on psychological, oncologic, and resource outcomes should be evaluated,20 as well as the impact on health behaviors, such as smoking cessation or participation in rehabilitation programs.21
Following the implementation of our Transition Visits in 2015, we conducted a quality improvement review. This review included summation of 69 recent breast cancer questionnaires from Transition Visits with our APPs (Table 2 and Table 3). The most common concerns raised by our breast cancer survivors include desire for weight loss, improving diet, and increasing physical activity. Of note, concerns did not often translate into a desire for more information or referrals.22 Survivors were generally satisfied with the timing of the Transition Visits and generally indicated that the visits were helpful, with self-reported improvements in their understanding of planned follow-up. A Canadian group evaluating breast and head and neck cancer survivors has suggested that SCPs could produce long-term improvements in healthy lifestyle behaviors; however, further research is needed to determine the extent to which SCPs might improve follow-up care over the long term.23
Finally, although efforts to date have been focused on the breast cancer survivor at the completion of treatment, long-term survivors may also benefit from receiving the SCP. A study by the American Cancer Society found that long-term cancer survivors had unmet informational needs, particularly with regard to screening, long-term cancer and treatment effects, and healthy lifestyle behaviors.24 Identifying and subsequently delivering an SCP to eligible long-term survivors is a challenging prospect, which depends on further refinement of EHR-based tracking of the date of diagnosis, cancer stage, and end-of-treatment date.
Summary and recommendations
Survivorship care has been efficiently integrated into our 1-month post-radiation follow-up appointment for breast cancer survivors. By using current resources in the radiation oncology department, the process has provided an effective way to deliver the SCP to breast cancer survivors. Future plans include implementing the process for all patients receiving curative-intent radiation for additional solid tumor survivors. Quality improvement projects will be developed to assess survivor satisfaction and the impact on health behaviors.
Acknowledgments
The authors thank Amy Heath, MS, RTT, for editorial and manuscript preparation assistance.
1. Statistics. National Cancer Institute, Division of Cancer Control & Population Sciences website. http:///cancercontrol.cancer.gov/ocs/statistics/statistics.html. Updated October 17, 2016. Accessed March 6, 2018.
2. Cancer facts & figures 2016. American Cancer Society website. https://www.cancer.org/research/cancer-facts-statistics/all-cancer-facts-figures/cancer-facts-figures-2016.html. Published 2016. Accessed February 27, 2018.
3. Hewitt M, Greenfield S, Stovall E, eds. From cancer patient to cancer survivor: lost in transition. Washington, DC: National Academies Press; 2006.
4. Knutson A, McNamara E. Cancer program standards: ensuring patient-centered care. American College of Surgeons website. https://www.facs.org/quality-programs/cancer/coc/standards. Published August 2016. Accessed March 6, 2018.
5. National Accreditation Program for Breast Centers. NAPBC standards manual. American College of Surgeons website. https://www.facs.org/~/media/files/quality%20programs/napbc/2014%20napbc%20standards%20manual.ashx. Published 2014. Accessed March 6, 2018.
6. Salz T, McCabe MS, Onstad EE, et al. Survivorship care plans: is there buy-in from community oncology providers? Cancer. 2014;120(5):722-730.
7. Mayer DK, Nekhlyudov L, Snyder CF, Merrill JK, Wollins DS, Shulman LN. American Society of Clinical Oncology clinical expert statement on cancer survivorship care planning. J Oncol Pract. 2014;10(6):345-351.
8. Dobelbower RR, Cotter G, Schilling PJ, Parsai EI, Carroll JM. Radiation oncology practice accreditation. Rays. 2001;26(3):191-198.
9. Hartford AC, Conway PD, Desai NB, et al. ACR-ASTRO practice parameter for communication: radiation oncology. The American College of Radiology website. http://www.acr.org/-/media/ACR/Files/Practice-Parameters/RadOnc.pdf. Updated 2014. Accessed March 6, 2018.
10. Koontz BF, Benda R, De Los Santos J, et al. US radiation oncology practice patterns for posttreatment survivor care. Pract Radiat Oncol. 2016;6(1):50-56.
11. American Society of Therapeutic Radiation Oncologists. APEx program standards. ASTRO website. http://www.astro.org/uploadedFiles/_MAIN_SITE/Daily_Practice/Accreditation/Content_Pieces/ProgramStandards.pdf. Published February 1, 2016. Accessed March 6, 2018.
12. Clinical practice survivorship guidelines and adaptations. American Society of Clinical Oncology website. http://www.asco.org/practice-guidelines/cancer-care-initiatives/prevention-survivorship. Published 2013. Accessed March 6, 2018.
13. National Comprehensive Cancer Network. Supportive care guidelines. NCNN website. http://www.nccn.org/professionals/physician_gls/f_guidelines.asp#supportive. Updated February 16, 2018. Accessed March 6, 2018.
14. Donohue S, Sesto ME, Hahn DL, et al. Evaluating primary care providers’ views on survivorship care plans generated by an electronic health record system. J Oncol Pract. 2015;11(3):e329-e335.
15. Tevaarwerk AJ, Wisinski KB, Buhr KA, et al. Leveraging electronic health record systems to create and provide electronic cancer survivorship care plans: a pilot study. J Oncol Pract. 2014;10(3):e150-e159.
16. Smith SL, Singh-Carlson S, Downie L, Payeur N, Wai ES. Survivors of breast cancer: patient perspectives on survivorship care planning. J Cancer Surviv. 2011;5(4):337-344.
17. Stricker CT, O’Brien M. Implementing the commission on cancer standards for survivorship care plans. Clin J Oncol Nurs. 2014;18(suppl 1):15-22.
18. Neuman HB, Steffens NM, Jacobson N, et al. Oncologists’ perspectives of their roles and responsibilities during multi-disciplinary breast cancer follow-up. Ann Surg Oncol. 2016;23(3):708-714.
19. Palmer SC, Stricker CT, Panzer SL, et al. Outcomes and satisfaction after delivery of a breast cancer survivorship care plan: Results of a multicenter trial. J Oncol Pract. 2015;11(2):e222-e229.
20. Brennan ME, Gormally JF, Butow P, Boyle FM, Spillane AJ. Survivorship care plans in cancer: a systematic review of care plan outcomes. Br J Cancer. 2014;111(10):1899-1908.
21. Chen RC, Hoffman KE, Sher DJ, et al. Development of a standard survivorship care plan template for radiation oncologists. Pract Radiat Oncol. 2016;6(1):57-65.
22. Seaborne LA, Huenerberg KJ, Bohler A, et al. Developing electronic health record based program to deliver survivorship care plans and visits at the UW breast center. Poster presented at American Society of Clinical Oncology Survivorship Symposium; January 15-16, 2016; San Francisco CA.
23. Collie K, McCormick J, Waller A, et al. Qualitative evaluation of care plans for Canadian breast and head-and-neck cancer survivors. Curr Oncol. 2014;21(1):18-28.
24. Playdon M, Ferrucci LM, McCorkle R, et al. Health information needs and preferences in relation to survivorship care plans of long-term cancer survivors in the American Cancer Society’s study of cancer survivors-I. J Cancer Surviv. 2016;10(4):674-685.
1. Statistics. National Cancer Institute, Division of Cancer Control & Population Sciences website. http:///cancercontrol.cancer.gov/ocs/statistics/statistics.html. Updated October 17, 2016. Accessed March 6, 2018.
2. Cancer facts & figures 2016. American Cancer Society website. https://www.cancer.org/research/cancer-facts-statistics/all-cancer-facts-figures/cancer-facts-figures-2016.html. Published 2016. Accessed February 27, 2018.
3. Hewitt M, Greenfield S, Stovall E, eds. From cancer patient to cancer survivor: lost in transition. Washington, DC: National Academies Press; 2006.
4. Knutson A, McNamara E. Cancer program standards: ensuring patient-centered care. American College of Surgeons website. https://www.facs.org/quality-programs/cancer/coc/standards. Published August 2016. Accessed March 6, 2018.
5. National Accreditation Program for Breast Centers. NAPBC standards manual. American College of Surgeons website. https://www.facs.org/~/media/files/quality%20programs/napbc/2014%20napbc%20standards%20manual.ashx. Published 2014. Accessed March 6, 2018.
6. Salz T, McCabe MS, Onstad EE, et al. Survivorship care plans: is there buy-in from community oncology providers? Cancer. 2014;120(5):722-730.
7. Mayer DK, Nekhlyudov L, Snyder CF, Merrill JK, Wollins DS, Shulman LN. American Society of Clinical Oncology clinical expert statement on cancer survivorship care planning. J Oncol Pract. 2014;10(6):345-351.
8. Dobelbower RR, Cotter G, Schilling PJ, Parsai EI, Carroll JM. Radiation oncology practice accreditation. Rays. 2001;26(3):191-198.
9. Hartford AC, Conway PD, Desai NB, et al. ACR-ASTRO practice parameter for communication: radiation oncology. The American College of Radiology website. http://www.acr.org/-/media/ACR/Files/Practice-Parameters/RadOnc.pdf. Updated 2014. Accessed March 6, 2018.
10. Koontz BF, Benda R, De Los Santos J, et al. US radiation oncology practice patterns for posttreatment survivor care. Pract Radiat Oncol. 2016;6(1):50-56.
11. American Society of Therapeutic Radiation Oncologists. APEx program standards. ASTRO website. http://www.astro.org/uploadedFiles/_MAIN_SITE/Daily_Practice/Accreditation/Content_Pieces/ProgramStandards.pdf. Published February 1, 2016. Accessed March 6, 2018.
12. Clinical practice survivorship guidelines and adaptations. American Society of Clinical Oncology website. http://www.asco.org/practice-guidelines/cancer-care-initiatives/prevention-survivorship. Published 2013. Accessed March 6, 2018.
13. National Comprehensive Cancer Network. Supportive care guidelines. NCNN website. http://www.nccn.org/professionals/physician_gls/f_guidelines.asp#supportive. Updated February 16, 2018. Accessed March 6, 2018.
14. Donohue S, Sesto ME, Hahn DL, et al. Evaluating primary care providers’ views on survivorship care plans generated by an electronic health record system. J Oncol Pract. 2015;11(3):e329-e335.
15. Tevaarwerk AJ, Wisinski KB, Buhr KA, et al. Leveraging electronic health record systems to create and provide electronic cancer survivorship care plans: a pilot study. J Oncol Pract. 2014;10(3):e150-e159.
16. Smith SL, Singh-Carlson S, Downie L, Payeur N, Wai ES. Survivors of breast cancer: patient perspectives on survivorship care planning. J Cancer Surviv. 2011;5(4):337-344.
17. Stricker CT, O’Brien M. Implementing the commission on cancer standards for survivorship care plans. Clin J Oncol Nurs. 2014;18(suppl 1):15-22.
18. Neuman HB, Steffens NM, Jacobson N, et al. Oncologists’ perspectives of their roles and responsibilities during multi-disciplinary breast cancer follow-up. Ann Surg Oncol. 2016;23(3):708-714.
19. Palmer SC, Stricker CT, Panzer SL, et al. Outcomes and satisfaction after delivery of a breast cancer survivorship care plan: Results of a multicenter trial. J Oncol Pract. 2015;11(2):e222-e229.
20. Brennan ME, Gormally JF, Butow P, Boyle FM, Spillane AJ. Survivorship care plans in cancer: a systematic review of care plan outcomes. Br J Cancer. 2014;111(10):1899-1908.
21. Chen RC, Hoffman KE, Sher DJ, et al. Development of a standard survivorship care plan template for radiation oncologists. Pract Radiat Oncol. 2016;6(1):57-65.
22. Seaborne LA, Huenerberg KJ, Bohler A, et al. Developing electronic health record based program to deliver survivorship care plans and visits at the UW breast center. Poster presented at American Society of Clinical Oncology Survivorship Symposium; January 15-16, 2016; San Francisco CA.
23. Collie K, McCormick J, Waller A, et al. Qualitative evaluation of care plans for Canadian breast and head-and-neck cancer survivors. Curr Oncol. 2014;21(1):18-28.
24. Playdon M, Ferrucci LM, McCorkle R, et al. Health information needs and preferences in relation to survivorship care plans of long-term cancer survivors in the American Cancer Society’s study of cancer survivors-I. J Cancer Surviv. 2016;10(4):674-685.
Biosimilars: same ol’ – but with a suffix, and cheaper
Biosimilars have arrived, and chances are that you’re already prescribing them. Last September, the US Food and Drug Administration (FDA) approved the first cancer-specific biosimilar, bevacizumab-awwb, for multiple cancer types (p. e60);1 and in November, it approved trastuzumab-dkst for HER2-positive breast and gastrointestinal cancers (p. e63).1 Briefly, biosimilars are biologic products that show comparable quality, efficacy, and safety to an existing, approved biologic known as the reference product.
Small-molecule drugs such as aspirin are easy to replicate identically, whereas biosimilars are large, complex proteins that are manufactured in nature’s factory, a micro-organism or biologic cell.2 The manufacturing process must be nearly identical to that for the reference product, so that only insignificant/nonclinically significant impurities occur in the final product. The protein-amino acid sequence is key and must therefore be identical. The 2010 Biologics Price Competition and Innovation Act established an abbreviated pathway for the FDA to consider and approve biosimilars, and 5 years later, the bone marrow stimulant filgrastim-sndz became the first biosimilar approved for use in the United States.3 The development of biosimilars is not inexpensive. The law and the FDA approval system require preclinical and phase 1 testing, and a robust phase 3 trial against the reference product to demonstrate that safety and efficacy are statistically not different and that any chemical differences between the biosimilar and reference product are clinically and safety or immunogenically insignificant. When those criteria have been met, and the biosimilar approved, the clinical and cost benefits to patients could be significant. In general, the cost of a biosimilar is about 20% to 30% lower than that of the reference product.
Biosimilarity does not yet allow interchangeability. Small-molecule generics under FDA regulations are interchangeable in the drug store and the hospital without the prescriber or patient being aware. That is not yet the case with biosimilars, but their lower prices could have a notable impact on overall cost of care. In 2013, 7 of the top 8 best-selling drugs in the global market were biologics.4 Three of the top 8 – rituximab, trastuzumab, and bevacizumab – were used to treat cancer, and 1 (pegfilgrastim) was for therapy-related neutropenia. Their total cost was US$27 billion. Biosimilars of those therapies could significantly lower that amount.
Nabhan and colleagues interviewed 510 US-based community oncologists about their understanding of biosimilars. They found that only 29% of respondents said they prescribed filgrastim-sndz for supportive care by personal choice, but upward of 73% said they would prescribe biosimilars for the active anticancer therapies, trastuzumab and bevacizumab. There’s no question that biosimilars are here to stay. The requirements to make them have been well worked out. Their safety and efficacy therefore can be assured, and their lower prices promise cost savings for patients and society as a whole.
1. Bosserman L. Cancer care in 2017: the promise of more cures with the challenges of an unstable health care system. https://www. mdedge.com/jcso/article/154559/cancer-care-2017-promise-morecures- challenges-unstable-health-care-system. December 15, 2017. Accessed April 23, 2018.
2. Biosimilar and interchangeable products. FDA website. https://www. fda.gov/Drugs/DevelopmentApprovalProcess/HowDrugsare DevelopedandApproved/ApprovalApplications/Therapeutic BiologicApplications/Biosimilars/ucm580419.htm#biological. Last updated October 23, 2017. Accessed April 25, 2018.
3. de Lartigue J. Filgrastim-sndz debuts as the first biosimilar approved in United States. https://www.mdedge.com/jcso/article/105177/patientsurvivor- care/filgrastim-sndz-debuts-first-biosimilar-approved-united. Published December 2015. Accessed April 23, 2018.
4 . The Dish. Biologics still on top in best selling drugs of 2013. http:// cellculturedish.com/2014/03/top-ten-biologics-2013-us-pharmaceutical- sales-2/. March 13, 2014. Accessed April 26, 2018.
5. Nabhan C, Jeune-Smith Y, Valley A, Feinberg BA. Community Oncologists’ Perception and Acceptance of Biosimilars in Oncology. https://www.journalofclinicalpathways.com/article/communityoncologists- perception-and-acceptance-biosimilars-oncology. Published March 2018. Accessed April 24, 2018.
Biosimilars have arrived, and chances are that you’re already prescribing them. Last September, the US Food and Drug Administration (FDA) approved the first cancer-specific biosimilar, bevacizumab-awwb, for multiple cancer types (p. e60);1 and in November, it approved trastuzumab-dkst for HER2-positive breast and gastrointestinal cancers (p. e63).1 Briefly, biosimilars are biologic products that show comparable quality, efficacy, and safety to an existing, approved biologic known as the reference product.
Small-molecule drugs such as aspirin are easy to replicate identically, whereas biosimilars are large, complex proteins that are manufactured in nature’s factory, a micro-organism or biologic cell.2 The manufacturing process must be nearly identical to that for the reference product, so that only insignificant/nonclinically significant impurities occur in the final product. The protein-amino acid sequence is key and must therefore be identical. The 2010 Biologics Price Competition and Innovation Act established an abbreviated pathway for the FDA to consider and approve biosimilars, and 5 years later, the bone marrow stimulant filgrastim-sndz became the first biosimilar approved for use in the United States.3 The development of biosimilars is not inexpensive. The law and the FDA approval system require preclinical and phase 1 testing, and a robust phase 3 trial against the reference product to demonstrate that safety and efficacy are statistically not different and that any chemical differences between the biosimilar and reference product are clinically and safety or immunogenically insignificant. When those criteria have been met, and the biosimilar approved, the clinical and cost benefits to patients could be significant. In general, the cost of a biosimilar is about 20% to 30% lower than that of the reference product.
Biosimilarity does not yet allow interchangeability. Small-molecule generics under FDA regulations are interchangeable in the drug store and the hospital without the prescriber or patient being aware. That is not yet the case with biosimilars, but their lower prices could have a notable impact on overall cost of care. In 2013, 7 of the top 8 best-selling drugs in the global market were biologics.4 Three of the top 8 – rituximab, trastuzumab, and bevacizumab – were used to treat cancer, and 1 (pegfilgrastim) was for therapy-related neutropenia. Their total cost was US$27 billion. Biosimilars of those therapies could significantly lower that amount.
Nabhan and colleagues interviewed 510 US-based community oncologists about their understanding of biosimilars. They found that only 29% of respondents said they prescribed filgrastim-sndz for supportive care by personal choice, but upward of 73% said they would prescribe biosimilars for the active anticancer therapies, trastuzumab and bevacizumab. There’s no question that biosimilars are here to stay. The requirements to make them have been well worked out. Their safety and efficacy therefore can be assured, and their lower prices promise cost savings for patients and society as a whole.
Biosimilars have arrived, and chances are that you’re already prescribing them. Last September, the US Food and Drug Administration (FDA) approved the first cancer-specific biosimilar, bevacizumab-awwb, for multiple cancer types (p. e60);1 and in November, it approved trastuzumab-dkst for HER2-positive breast and gastrointestinal cancers (p. e63).1 Briefly, biosimilars are biologic products that show comparable quality, efficacy, and safety to an existing, approved biologic known as the reference product.
Small-molecule drugs such as aspirin are easy to replicate identically, whereas biosimilars are large, complex proteins that are manufactured in nature’s factory, a micro-organism or biologic cell.2 The manufacturing process must be nearly identical to that for the reference product, so that only insignificant/nonclinically significant impurities occur in the final product. The protein-amino acid sequence is key and must therefore be identical. The 2010 Biologics Price Competition and Innovation Act established an abbreviated pathway for the FDA to consider and approve biosimilars, and 5 years later, the bone marrow stimulant filgrastim-sndz became the first biosimilar approved for use in the United States.3 The development of biosimilars is not inexpensive. The law and the FDA approval system require preclinical and phase 1 testing, and a robust phase 3 trial against the reference product to demonstrate that safety and efficacy are statistically not different and that any chemical differences between the biosimilar and reference product are clinically and safety or immunogenically insignificant. When those criteria have been met, and the biosimilar approved, the clinical and cost benefits to patients could be significant. In general, the cost of a biosimilar is about 20% to 30% lower than that of the reference product.
Biosimilarity does not yet allow interchangeability. Small-molecule generics under FDA regulations are interchangeable in the drug store and the hospital without the prescriber or patient being aware. That is not yet the case with biosimilars, but their lower prices could have a notable impact on overall cost of care. In 2013, 7 of the top 8 best-selling drugs in the global market were biologics.4 Three of the top 8 – rituximab, trastuzumab, and bevacizumab – were used to treat cancer, and 1 (pegfilgrastim) was for therapy-related neutropenia. Their total cost was US$27 billion. Biosimilars of those therapies could significantly lower that amount.
Nabhan and colleagues interviewed 510 US-based community oncologists about their understanding of biosimilars. They found that only 29% of respondents said they prescribed filgrastim-sndz for supportive care by personal choice, but upward of 73% said they would prescribe biosimilars for the active anticancer therapies, trastuzumab and bevacizumab. There’s no question that biosimilars are here to stay. The requirements to make them have been well worked out. Their safety and efficacy therefore can be assured, and their lower prices promise cost savings for patients and society as a whole.
1. Bosserman L. Cancer care in 2017: the promise of more cures with the challenges of an unstable health care system. https://www. mdedge.com/jcso/article/154559/cancer-care-2017-promise-morecures- challenges-unstable-health-care-system. December 15, 2017. Accessed April 23, 2018.
2. Biosimilar and interchangeable products. FDA website. https://www. fda.gov/Drugs/DevelopmentApprovalProcess/HowDrugsare DevelopedandApproved/ApprovalApplications/Therapeutic BiologicApplications/Biosimilars/ucm580419.htm#biological. Last updated October 23, 2017. Accessed April 25, 2018.
3. de Lartigue J. Filgrastim-sndz debuts as the first biosimilar approved in United States. https://www.mdedge.com/jcso/article/105177/patientsurvivor- care/filgrastim-sndz-debuts-first-biosimilar-approved-united. Published December 2015. Accessed April 23, 2018.
4 . The Dish. Biologics still on top in best selling drugs of 2013. http:// cellculturedish.com/2014/03/top-ten-biologics-2013-us-pharmaceutical- sales-2/. March 13, 2014. Accessed April 26, 2018.
5. Nabhan C, Jeune-Smith Y, Valley A, Feinberg BA. Community Oncologists’ Perception and Acceptance of Biosimilars in Oncology. https://www.journalofclinicalpathways.com/article/communityoncologists- perception-and-acceptance-biosimilars-oncology. Published March 2018. Accessed April 24, 2018.
1. Bosserman L. Cancer care in 2017: the promise of more cures with the challenges of an unstable health care system. https://www. mdedge.com/jcso/article/154559/cancer-care-2017-promise-morecures- challenges-unstable-health-care-system. December 15, 2017. Accessed April 23, 2018.
2. Biosimilar and interchangeable products. FDA website. https://www. fda.gov/Drugs/DevelopmentApprovalProcess/HowDrugsare DevelopedandApproved/ApprovalApplications/Therapeutic BiologicApplications/Biosimilars/ucm580419.htm#biological. Last updated October 23, 2017. Accessed April 25, 2018.
3. de Lartigue J. Filgrastim-sndz debuts as the first biosimilar approved in United States. https://www.mdedge.com/jcso/article/105177/patientsurvivor- care/filgrastim-sndz-debuts-first-biosimilar-approved-united. Published December 2015. Accessed April 23, 2018.
4 . The Dish. Biologics still on top in best selling drugs of 2013. http:// cellculturedish.com/2014/03/top-ten-biologics-2013-us-pharmaceutical- sales-2/. March 13, 2014. Accessed April 26, 2018.
5. Nabhan C, Jeune-Smith Y, Valley A, Feinberg BA. Community Oncologists’ Perception and Acceptance of Biosimilars in Oncology. https://www.journalofclinicalpathways.com/article/communityoncologists- perception-and-acceptance-biosimilars-oncology. Published March 2018. Accessed April 24, 2018.