User login
Stewart Tepper: Emgality approval ‘very exciting’
The drug, a humanized monoclonal antibody that binds to calcitonin gene-related peptide (CGRP), is administered by self-injection in 300-mg doses.
Galcanezumab is the first medication for episodic cluster headache that reduces the frequency of attacks, the agency said in an announcement.
Cluster headache can be more intense than migraine. The pain is unilateral and occurs in the orbital, supraorbital, or temporal regions. It reaches its peak intensity within 5-10 minutes and generally lasts for 30-90 minutes. Symptoms include a burning sensation, conjunctival injection, rhinorrhea, and photosensitivity. Patients often have one to three of these headaches per day, and the headaches appear to be linked to the circadian rhythm. An episodic cluster cycle can last for weeks to months of daily or near daily attacks.
A study presented at the recent meeting of the American Academy of Neurology provided evidence of the drug’s efficacy in cluster headache. In this trial, researchers randomized 106 patients with episodic cluster headache to galcanezumab or placebo. The baseline cluster headache frequency was 17.3 attacks per week, and galcanezumab reduced this frequency to 9.1 attacks per week, compared with 12.1 attacks per week with placebo. The most common side effect reported in this and other clinical trials was injection-site reactions.
Galcanezumab entails a risk of hypersensitivity reactions, according to the FDA. These reactions may occur several days after administration and may be prolonged. “If a serious hypersensitivity reaction occurs, treatment should be discontinued,” the agency said.
“It’s a very exciting day. There had never been a drug approved for prevention of cluster headache,” said Stewart J. Tepper, MD, professor of neurology at the Geisel School of Medicine at Dartmouth and director of the Dartmouth Headache Center, Dartmouth-Hitchcock Medical Center, Lebanon, N.H.
It is difficult to achieve therapeutic concentrations of current preventive medications that do not have FDA approval for this indication, such as verapamil, lithium, or antiepileptic drugs. Galcanezumab, in contrast, works quickly. It is important to note that the approval was for preventive treatment of episodic cluster headache, not for prevention of chronic cluster headache, and not for acute treatment, Dr. Tepper said.
“It’s important to get optimal therapy for cluster headache. It is one of the most disabling, terrible disorders on Earth,” Dr. Tepper said. “The importance [of this approval] cannot be overestimated.”
When asked for comment, Alan M. Rapoport, MD, clinical professor of neurology at the University of California, Los Angeles, said “If this monoclonal antibody to the CGRP ligand works as well in real life as in the trial, it will be an important advance in the treatment of cluster headache.”
Prior to the approval of galcanezumab, noninvasive vagal nerve stimulation was approved in November 2018 for adjunctive use in the preventive treatment of cluster headache in adults.
The FDA granted the application for galcanezumab using a Priority Review and Breakthrough Therapy designation. The agency approved galcanezumab for the preventive treatment of migraine in adults in September 2018. The drug appears to have a similar safety profile in both patient populations. Eli Lilly, which is based in Indianapolis, Indiana, manufactures the drug.
This article was updated June 5, 2019.
The drug, a humanized monoclonal antibody that binds to calcitonin gene-related peptide (CGRP), is administered by self-injection in 300-mg doses.
Galcanezumab is the first medication for episodic cluster headache that reduces the frequency of attacks, the agency said in an announcement.
Cluster headache can be more intense than migraine. The pain is unilateral and occurs in the orbital, supraorbital, or temporal regions. It reaches its peak intensity within 5-10 minutes and generally lasts for 30-90 minutes. Symptoms include a burning sensation, conjunctival injection, rhinorrhea, and photosensitivity. Patients often have one to three of these headaches per day, and the headaches appear to be linked to the circadian rhythm. An episodic cluster cycle can last for weeks to months of daily or near daily attacks.
A study presented at the recent meeting of the American Academy of Neurology provided evidence of the drug’s efficacy in cluster headache. In this trial, researchers randomized 106 patients with episodic cluster headache to galcanezumab or placebo. The baseline cluster headache frequency was 17.3 attacks per week, and galcanezumab reduced this frequency to 9.1 attacks per week, compared with 12.1 attacks per week with placebo. The most common side effect reported in this and other clinical trials was injection-site reactions.
Galcanezumab entails a risk of hypersensitivity reactions, according to the FDA. These reactions may occur several days after administration and may be prolonged. “If a serious hypersensitivity reaction occurs, treatment should be discontinued,” the agency said.
“It’s a very exciting day. There had never been a drug approved for prevention of cluster headache,” said Stewart J. Tepper, MD, professor of neurology at the Geisel School of Medicine at Dartmouth and director of the Dartmouth Headache Center, Dartmouth-Hitchcock Medical Center, Lebanon, N.H.
It is difficult to achieve therapeutic concentrations of current preventive medications that do not have FDA approval for this indication, such as verapamil, lithium, or antiepileptic drugs. Galcanezumab, in contrast, works quickly. It is important to note that the approval was for preventive treatment of episodic cluster headache, not for prevention of chronic cluster headache, and not for acute treatment, Dr. Tepper said.
“It’s important to get optimal therapy for cluster headache. It is one of the most disabling, terrible disorders on Earth,” Dr. Tepper said. “The importance [of this approval] cannot be overestimated.”
When asked for comment, Alan M. Rapoport, MD, clinical professor of neurology at the University of California, Los Angeles, said “If this monoclonal antibody to the CGRP ligand works as well in real life as in the trial, it will be an important advance in the treatment of cluster headache.”
Prior to the approval of galcanezumab, noninvasive vagal nerve stimulation was approved in November 2018 for adjunctive use in the preventive treatment of cluster headache in adults.
The FDA granted the application for galcanezumab using a Priority Review and Breakthrough Therapy designation. The agency approved galcanezumab for the preventive treatment of migraine in adults in September 2018. The drug appears to have a similar safety profile in both patient populations. Eli Lilly, which is based in Indianapolis, Indiana, manufactures the drug.
This article was updated June 5, 2019.
The drug, a humanized monoclonal antibody that binds to calcitonin gene-related peptide (CGRP), is administered by self-injection in 300-mg doses.
Galcanezumab is the first medication for episodic cluster headache that reduces the frequency of attacks, the agency said in an announcement.
Cluster headache can be more intense than migraine. The pain is unilateral and occurs in the orbital, supraorbital, or temporal regions. It reaches its peak intensity within 5-10 minutes and generally lasts for 30-90 minutes. Symptoms include a burning sensation, conjunctival injection, rhinorrhea, and photosensitivity. Patients often have one to three of these headaches per day, and the headaches appear to be linked to the circadian rhythm. An episodic cluster cycle can last for weeks to months of daily or near daily attacks.
A study presented at the recent meeting of the American Academy of Neurology provided evidence of the drug’s efficacy in cluster headache. In this trial, researchers randomized 106 patients with episodic cluster headache to galcanezumab or placebo. The baseline cluster headache frequency was 17.3 attacks per week, and galcanezumab reduced this frequency to 9.1 attacks per week, compared with 12.1 attacks per week with placebo. The most common side effect reported in this and other clinical trials was injection-site reactions.
Galcanezumab entails a risk of hypersensitivity reactions, according to the FDA. These reactions may occur several days after administration and may be prolonged. “If a serious hypersensitivity reaction occurs, treatment should be discontinued,” the agency said.
“It’s a very exciting day. There had never been a drug approved for prevention of cluster headache,” said Stewart J. Tepper, MD, professor of neurology at the Geisel School of Medicine at Dartmouth and director of the Dartmouth Headache Center, Dartmouth-Hitchcock Medical Center, Lebanon, N.H.
It is difficult to achieve therapeutic concentrations of current preventive medications that do not have FDA approval for this indication, such as verapamil, lithium, or antiepileptic drugs. Galcanezumab, in contrast, works quickly. It is important to note that the approval was for preventive treatment of episodic cluster headache, not for prevention of chronic cluster headache, and not for acute treatment, Dr. Tepper said.
“It’s important to get optimal therapy for cluster headache. It is one of the most disabling, terrible disorders on Earth,” Dr. Tepper said. “The importance [of this approval] cannot be overestimated.”
When asked for comment, Alan M. Rapoport, MD, clinical professor of neurology at the University of California, Los Angeles, said “If this monoclonal antibody to the CGRP ligand works as well in real life as in the trial, it will be an important advance in the treatment of cluster headache.”
Prior to the approval of galcanezumab, noninvasive vagal nerve stimulation was approved in November 2018 for adjunctive use in the preventive treatment of cluster headache in adults.
The FDA granted the application for galcanezumab using a Priority Review and Breakthrough Therapy designation. The agency approved galcanezumab for the preventive treatment of migraine in adults in September 2018. The drug appears to have a similar safety profile in both patient populations. Eli Lilly, which is based in Indianapolis, Indiana, manufactures the drug.
This article was updated June 5, 2019.
Slow breathing: An effective, pragmatic analgesic technique?
MILWAUKEE – Mindfulness-based practices are effective in reducing pain perceptions, but a more easily taught breath control technique also showed efficacy in a recent study. Slow, rhythmic breathing alone, even without the additional attentional components of mindfulness, had significant analgesic effects in a human experimental model of pain.
“Slow breathing is much easier to perform” than mindfulness-based meditation, Fadel Zeidan, PhD, said at the scientific meeting of the American Pain Society. More research into the technique may offer a “clinically pragmatic” nonpharmacologic option for pain control, he said. And there may be some similarities between how the two techniques work: like mindfulness meditation, slow, rhythmic breathing’s analgesic properties are not dependent on the endogenous opioid system, said Dr. Zeidan, assistant professor of anesthesiology at the University of California, San Diego. His interests include mindfulness meditation–based pain relief.
In previous work, Dr. Zeidan and his collaborators had shown that the analgesic effect of mindfulness practices is not mediated by endogenous opioids. Participants in a study were trained in mindfulness meditation, and then exposed to a pain stimulus. Compared with a control group who listened to an audiobook rather than using mindfulness practices when exposed to pain, the meditators experienced a significant reduction in pain unpleasantness (J Neurosci. 16 March 2016;36[11]:3391-7).
In the experiment, both the meditation and the control group received first an intravenous saline solution, and then the opioid antagonist naloxone, which blocks endogenous opioids. When receiving naloxone, the meditators experienced reductions in the perceived unpleasantness of pain that were similar to what they experienced when they had received saline, showing that endogenous opioids weren’t responsible for meditation’s analgesic effects.
After verifying those findings, said Dr. Zeidan, he became interested in conducting a “graded analytical dissection of mindfulness,” to see exactly which components of the practice are nonopioidergic.
With mindfulness meditation, participants engage in slow, rhythmic breathing, and they learn about observation and appraisal practices, which can briefly be described as “the awareness of arising sensory events without reaction,” Dr. Zeidan said.
Mere belief in meditation in combination with the slow rhythmic breathing might have an analgesic effect, he said. In effect, this is sham mindfulness.
To try to tease out the contributions of each component of mindfulness meditation, Dr. Zeidan and his colleagues devised an experiment that trained participants in one of three ways. Over the course of four 20-minute sessions, randomized participants were trained in slow breathing techniques, with a goal respiratory rate of 6 breaths per minute; in mindfulness meditation techniques; or in a sham mindfulness technique that did not teach specific mindfulness principles.
The randomized participants were subject to a painful heat stimulus before the training to establish a baseline.
After training, they returned for two further sessions. At each visit, they experienced the noxious stimulus with no medication. After a rest period, they then received either high-dose intravenous naloxone or saline. The allocation was randomized and administration of the study drug was double-blinded.
With naloxone or saline infusion ongoing, participants were then again subjected to the painful heat stimulus.
“All manipulations effectively reduced the respiration rate,” by 18%-21%, Dr. Zeidan said.
However, with the introduction of naloxone, both the slow-breathing group and the mindfulness group maintained reductions in pain unpleasantness, while those in the sham group had significant increases in pain unpleasantness. Reductions in pain unpleasantness ranged from 11% to 18% for these two groups, while the initial 8% reduction for the sham group climbed to a 13% increase in pain unpleasantness when this group received naloxone.
An unexpected finding was how effective slow breathing alone was as an analgesic. “There’s really something here,” said Dr. Zeidan, in reference to the analgesic effect of breath control. He explained that the slow breathing technique training was done with the aid of a device that emitted a blue glow that dimmed and brightened at the target respiratory rate.
Dr. Zeidan added that few participants were able to slow their breathing to 6 respirations per minute, but that the average rate did slow to about 12 from the normal 16 or so breaths per minute.
Dr. Zeidan reported no conflicts of interest. The National Institutes of Health funded the research.
MILWAUKEE – Mindfulness-based practices are effective in reducing pain perceptions, but a more easily taught breath control technique also showed efficacy in a recent study. Slow, rhythmic breathing alone, even without the additional attentional components of mindfulness, had significant analgesic effects in a human experimental model of pain.
“Slow breathing is much easier to perform” than mindfulness-based meditation, Fadel Zeidan, PhD, said at the scientific meeting of the American Pain Society. More research into the technique may offer a “clinically pragmatic” nonpharmacologic option for pain control, he said. And there may be some similarities between how the two techniques work: like mindfulness meditation, slow, rhythmic breathing’s analgesic properties are not dependent on the endogenous opioid system, said Dr. Zeidan, assistant professor of anesthesiology at the University of California, San Diego. His interests include mindfulness meditation–based pain relief.
In previous work, Dr. Zeidan and his collaborators had shown that the analgesic effect of mindfulness practices is not mediated by endogenous opioids. Participants in a study were trained in mindfulness meditation, and then exposed to a pain stimulus. Compared with a control group who listened to an audiobook rather than using mindfulness practices when exposed to pain, the meditators experienced a significant reduction in pain unpleasantness (J Neurosci. 16 March 2016;36[11]:3391-7).
In the experiment, both the meditation and the control group received first an intravenous saline solution, and then the opioid antagonist naloxone, which blocks endogenous opioids. When receiving naloxone, the meditators experienced reductions in the perceived unpleasantness of pain that were similar to what they experienced when they had received saline, showing that endogenous opioids weren’t responsible for meditation’s analgesic effects.
After verifying those findings, said Dr. Zeidan, he became interested in conducting a “graded analytical dissection of mindfulness,” to see exactly which components of the practice are nonopioidergic.
With mindfulness meditation, participants engage in slow, rhythmic breathing, and they learn about observation and appraisal practices, which can briefly be described as “the awareness of arising sensory events without reaction,” Dr. Zeidan said.
Mere belief in meditation in combination with the slow rhythmic breathing might have an analgesic effect, he said. In effect, this is sham mindfulness.
To try to tease out the contributions of each component of mindfulness meditation, Dr. Zeidan and his colleagues devised an experiment that trained participants in one of three ways. Over the course of four 20-minute sessions, randomized participants were trained in slow breathing techniques, with a goal respiratory rate of 6 breaths per minute; in mindfulness meditation techniques; or in a sham mindfulness technique that did not teach specific mindfulness principles.
The randomized participants were subject to a painful heat stimulus before the training to establish a baseline.
After training, they returned for two further sessions. At each visit, they experienced the noxious stimulus with no medication. After a rest period, they then received either high-dose intravenous naloxone or saline. The allocation was randomized and administration of the study drug was double-blinded.
With naloxone or saline infusion ongoing, participants were then again subjected to the painful heat stimulus.
“All manipulations effectively reduced the respiration rate,” by 18%-21%, Dr. Zeidan said.
However, with the introduction of naloxone, both the slow-breathing group and the mindfulness group maintained reductions in pain unpleasantness, while those in the sham group had significant increases in pain unpleasantness. Reductions in pain unpleasantness ranged from 11% to 18% for these two groups, while the initial 8% reduction for the sham group climbed to a 13% increase in pain unpleasantness when this group received naloxone.
An unexpected finding was how effective slow breathing alone was as an analgesic. “There’s really something here,” said Dr. Zeidan, in reference to the analgesic effect of breath control. He explained that the slow breathing technique training was done with the aid of a device that emitted a blue glow that dimmed and brightened at the target respiratory rate.
Dr. Zeidan added that few participants were able to slow their breathing to 6 respirations per minute, but that the average rate did slow to about 12 from the normal 16 or so breaths per minute.
Dr. Zeidan reported no conflicts of interest. The National Institutes of Health funded the research.
MILWAUKEE – Mindfulness-based practices are effective in reducing pain perceptions, but a more easily taught breath control technique also showed efficacy in a recent study. Slow, rhythmic breathing alone, even without the additional attentional components of mindfulness, had significant analgesic effects in a human experimental model of pain.
“Slow breathing is much easier to perform” than mindfulness-based meditation, Fadel Zeidan, PhD, said at the scientific meeting of the American Pain Society. More research into the technique may offer a “clinically pragmatic” nonpharmacologic option for pain control, he said. And there may be some similarities between how the two techniques work: like mindfulness meditation, slow, rhythmic breathing’s analgesic properties are not dependent on the endogenous opioid system, said Dr. Zeidan, assistant professor of anesthesiology at the University of California, San Diego. His interests include mindfulness meditation–based pain relief.
In previous work, Dr. Zeidan and his collaborators had shown that the analgesic effect of mindfulness practices is not mediated by endogenous opioids. Participants in a study were trained in mindfulness meditation, and then exposed to a pain stimulus. Compared with a control group who listened to an audiobook rather than using mindfulness practices when exposed to pain, the meditators experienced a significant reduction in pain unpleasantness (J Neurosci. 16 March 2016;36[11]:3391-7).
In the experiment, both the meditation and the control group received first an intravenous saline solution, and then the opioid antagonist naloxone, which blocks endogenous opioids. When receiving naloxone, the meditators experienced reductions in the perceived unpleasantness of pain that were similar to what they experienced when they had received saline, showing that endogenous opioids weren’t responsible for meditation’s analgesic effects.
After verifying those findings, said Dr. Zeidan, he became interested in conducting a “graded analytical dissection of mindfulness,” to see exactly which components of the practice are nonopioidergic.
With mindfulness meditation, participants engage in slow, rhythmic breathing, and they learn about observation and appraisal practices, which can briefly be described as “the awareness of arising sensory events without reaction,” Dr. Zeidan said.
Mere belief in meditation in combination with the slow rhythmic breathing might have an analgesic effect, he said. In effect, this is sham mindfulness.
To try to tease out the contributions of each component of mindfulness meditation, Dr. Zeidan and his colleagues devised an experiment that trained participants in one of three ways. Over the course of four 20-minute sessions, randomized participants were trained in slow breathing techniques, with a goal respiratory rate of 6 breaths per minute; in mindfulness meditation techniques; or in a sham mindfulness technique that did not teach specific mindfulness principles.
The randomized participants were subject to a painful heat stimulus before the training to establish a baseline.
After training, they returned for two further sessions. At each visit, they experienced the noxious stimulus with no medication. After a rest period, they then received either high-dose intravenous naloxone or saline. The allocation was randomized and administration of the study drug was double-blinded.
With naloxone or saline infusion ongoing, participants were then again subjected to the painful heat stimulus.
“All manipulations effectively reduced the respiration rate,” by 18%-21%, Dr. Zeidan said.
However, with the introduction of naloxone, both the slow-breathing group and the mindfulness group maintained reductions in pain unpleasantness, while those in the sham group had significant increases in pain unpleasantness. Reductions in pain unpleasantness ranged from 11% to 18% for these two groups, while the initial 8% reduction for the sham group climbed to a 13% increase in pain unpleasantness when this group received naloxone.
An unexpected finding was how effective slow breathing alone was as an analgesic. “There’s really something here,” said Dr. Zeidan, in reference to the analgesic effect of breath control. He explained that the slow breathing technique training was done with the aid of a device that emitted a blue glow that dimmed and brightened at the target respiratory rate.
Dr. Zeidan added that few participants were able to slow their breathing to 6 respirations per minute, but that the average rate did slow to about 12 from the normal 16 or so breaths per minute.
Dr. Zeidan reported no conflicts of interest. The National Institutes of Health funded the research.
REPORTING FROM APS 2019
Methotrexate significantly reduced knee OA pain
TORONTO – Philip G. Conaghan, MD, PhD, reported at the OARSI 2019 World Congress.
There is, however, an asterisk attached to these findings. “Despite a moderate standard effect size, the treatment effect was smaller than some of the thresholds for what is considered clinically meaningful,” he noted at the meeting sponsored by the Osteoarthritis Research Society International.
That being said, the rheumatologist is convinced further investigation of methotrexate in osteoarthritis is warranted.
“I have to say that, unlike our earlier hydroxychloroquine trial, which was robustly negative with nothing more to say, I think there is a signal in this study. I need to understand the results of this trial better to understand if there is a subgroup we could treat with methotrexate. It’s a cheap drug, it’s readily available, and we’ve got a lot of experience with it,” noted Dr. Conaghan, professor of musculoskeletal medicine at the University of Leeds (England) and director of the Leeds Institute of Rheumatic and Musculoskeletal Medicine.
The rationale for the 15-center PROMOTE trial is that synovitis is common in OA. Synovitis is associated with pain, methotrexate is the gold-standard treatment for synovitis in inflammatory forms of arthritis, and current treatments for OA are, to say the least, severely limited. Also, an earlier 30-patient, open-label pilot study of methotrexate in patients with painful knee OA conducted by Dr. Conaghan and coworkers suggested the drug was promising (Rheumatology [Oxford]. 2013 May;52[5]:888-92).
PROMOTE included 134 patients with symptomatic and radiographic knee OA who were randomized in double-blind fashion to 6 months of oral methotrexate at 10 mg titrated to a target dose of 25 mg/week or to placebo. All patients also received usual care with oral NSAIDs and/or acetaminophen. Their mean baseline knee pain on a 0-10 numeric rating scale was 6.6.
The primary endpoint, assessed at 6 months, was the difference between the two study arms in average knee pain during the previous week on a 0-10 scale. The score was 5.1 in the methotrexate group and 6.2 in the placebo arm, for a baseline-adjusted treatment difference of 0.83 points, which works out to a standard effect size of 0.36. When the data were reanalyzed after excluding the 15 patients who missed more than four doses of medication within any 3-month period, the between-group difference in pain scores increased to 0.95 points in favor of the methotrexate group.
A significant difference in favor of the methotrexate group was documented in the OARSI-OMERACT response rate at 6 months: 45% in the methotrexate group and 26% in the controls. Some secondary endpoints were positive as well, with statistically significant differences seen at 6 months in Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC) stiffness, WOMAC physical function, and several other endpoints. But there were no significant differences in WOMAC pain, SF-12 physical component or SF-12 mental component scores, or in an OA quality of life measure.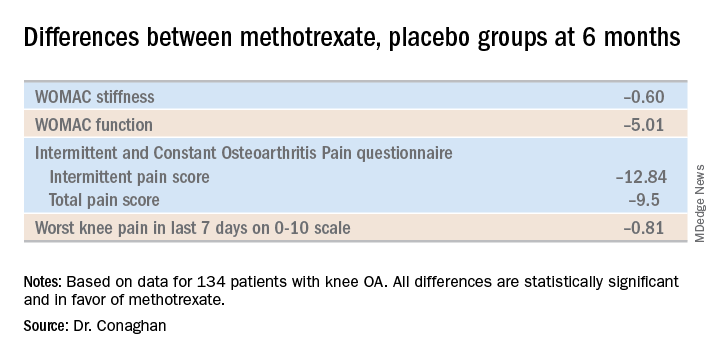
The mean dose of methotrexate used in the study was about 17 mg/week. Dr. Conaghan said that if he could do the trial over again, he would have used subcutaneous methotrexate.
“It’s a more reliable way of getting a dose into people and probably of getting a slightly higher dose into people. In the rheumatoid arthritis world, we use a lot more subcutaneous methotrexate now than we did 10 years ago because it gets around a lot of the minor side effects and helps compliance,” he said.
One audience member suggested that one potentially useful way to zero in on a subgroup of knee OA patients likely to derive the most benefit from methotrexate would be to have screened potential study participants for comorbid fibromyalgia and exclude those with the disorder. Dr. Conaghan replied that the PROMOTE investigators did gather data on participants’ pain at sites other than the knee. That data can be used to identify those at increased likelihood of fibromyalgia, and he agreed that’s worth looking into.
Dr. Conaghan reported having no financial conflicts regarding PROMOTE, which was funded by the U.K. National Institute for Health Research and Versus Arthritis.
SOURCE: Conaghan PG et al. OARSI 2019, Abstract 86.
TORONTO – Philip G. Conaghan, MD, PhD, reported at the OARSI 2019 World Congress.
There is, however, an asterisk attached to these findings. “Despite a moderate standard effect size, the treatment effect was smaller than some of the thresholds for what is considered clinically meaningful,” he noted at the meeting sponsored by the Osteoarthritis Research Society International.
That being said, the rheumatologist is convinced further investigation of methotrexate in osteoarthritis is warranted.
“I have to say that, unlike our earlier hydroxychloroquine trial, which was robustly negative with nothing more to say, I think there is a signal in this study. I need to understand the results of this trial better to understand if there is a subgroup we could treat with methotrexate. It’s a cheap drug, it’s readily available, and we’ve got a lot of experience with it,” noted Dr. Conaghan, professor of musculoskeletal medicine at the University of Leeds (England) and director of the Leeds Institute of Rheumatic and Musculoskeletal Medicine.
The rationale for the 15-center PROMOTE trial is that synovitis is common in OA. Synovitis is associated with pain, methotrexate is the gold-standard treatment for synovitis in inflammatory forms of arthritis, and current treatments for OA are, to say the least, severely limited. Also, an earlier 30-patient, open-label pilot study of methotrexate in patients with painful knee OA conducted by Dr. Conaghan and coworkers suggested the drug was promising (Rheumatology [Oxford]. 2013 May;52[5]:888-92).
PROMOTE included 134 patients with symptomatic and radiographic knee OA who were randomized in double-blind fashion to 6 months of oral methotrexate at 10 mg titrated to a target dose of 25 mg/week or to placebo. All patients also received usual care with oral NSAIDs and/or acetaminophen. Their mean baseline knee pain on a 0-10 numeric rating scale was 6.6.
The primary endpoint, assessed at 6 months, was the difference between the two study arms in average knee pain during the previous week on a 0-10 scale. The score was 5.1 in the methotrexate group and 6.2 in the placebo arm, for a baseline-adjusted treatment difference of 0.83 points, which works out to a standard effect size of 0.36. When the data were reanalyzed after excluding the 15 patients who missed more than four doses of medication within any 3-month period, the between-group difference in pain scores increased to 0.95 points in favor of the methotrexate group.
A significant difference in favor of the methotrexate group was documented in the OARSI-OMERACT response rate at 6 months: 45% in the methotrexate group and 26% in the controls. Some secondary endpoints were positive as well, with statistically significant differences seen at 6 months in Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC) stiffness, WOMAC physical function, and several other endpoints. But there were no significant differences in WOMAC pain, SF-12 physical component or SF-12 mental component scores, or in an OA quality of life measure.
The mean dose of methotrexate used in the study was about 17 mg/week. Dr. Conaghan said that if he could do the trial over again, he would have used subcutaneous methotrexate.
“It’s a more reliable way of getting a dose into people and probably of getting a slightly higher dose into people. In the rheumatoid arthritis world, we use a lot more subcutaneous methotrexate now than we did 10 years ago because it gets around a lot of the minor side effects and helps compliance,” he said.
One audience member suggested that one potentially useful way to zero in on a subgroup of knee OA patients likely to derive the most benefit from methotrexate would be to have screened potential study participants for comorbid fibromyalgia and exclude those with the disorder. Dr. Conaghan replied that the PROMOTE investigators did gather data on participants’ pain at sites other than the knee. That data can be used to identify those at increased likelihood of fibromyalgia, and he agreed that’s worth looking into.
Dr. Conaghan reported having no financial conflicts regarding PROMOTE, which was funded by the U.K. National Institute for Health Research and Versus Arthritis.
SOURCE: Conaghan PG et al. OARSI 2019, Abstract 86.
TORONTO – Philip G. Conaghan, MD, PhD, reported at the OARSI 2019 World Congress.
There is, however, an asterisk attached to these findings. “Despite a moderate standard effect size, the treatment effect was smaller than some of the thresholds for what is considered clinically meaningful,” he noted at the meeting sponsored by the Osteoarthritis Research Society International.
That being said, the rheumatologist is convinced further investigation of methotrexate in osteoarthritis is warranted.
“I have to say that, unlike our earlier hydroxychloroquine trial, which was robustly negative with nothing more to say, I think there is a signal in this study. I need to understand the results of this trial better to understand if there is a subgroup we could treat with methotrexate. It’s a cheap drug, it’s readily available, and we’ve got a lot of experience with it,” noted Dr. Conaghan, professor of musculoskeletal medicine at the University of Leeds (England) and director of the Leeds Institute of Rheumatic and Musculoskeletal Medicine.
The rationale for the 15-center PROMOTE trial is that synovitis is common in OA. Synovitis is associated with pain, methotrexate is the gold-standard treatment for synovitis in inflammatory forms of arthritis, and current treatments for OA are, to say the least, severely limited. Also, an earlier 30-patient, open-label pilot study of methotrexate in patients with painful knee OA conducted by Dr. Conaghan and coworkers suggested the drug was promising (Rheumatology [Oxford]. 2013 May;52[5]:888-92).
PROMOTE included 134 patients with symptomatic and radiographic knee OA who were randomized in double-blind fashion to 6 months of oral methotrexate at 10 mg titrated to a target dose of 25 mg/week or to placebo. All patients also received usual care with oral NSAIDs and/or acetaminophen. Their mean baseline knee pain on a 0-10 numeric rating scale was 6.6.
The primary endpoint, assessed at 6 months, was the difference between the two study arms in average knee pain during the previous week on a 0-10 scale. The score was 5.1 in the methotrexate group and 6.2 in the placebo arm, for a baseline-adjusted treatment difference of 0.83 points, which works out to a standard effect size of 0.36. When the data were reanalyzed after excluding the 15 patients who missed more than four doses of medication within any 3-month period, the between-group difference in pain scores increased to 0.95 points in favor of the methotrexate group.
A significant difference in favor of the methotrexate group was documented in the OARSI-OMERACT response rate at 6 months: 45% in the methotrexate group and 26% in the controls. Some secondary endpoints were positive as well, with statistically significant differences seen at 6 months in Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC) stiffness, WOMAC physical function, and several other endpoints. But there were no significant differences in WOMAC pain, SF-12 physical component or SF-12 mental component scores, or in an OA quality of life measure.
The mean dose of methotrexate used in the study was about 17 mg/week. Dr. Conaghan said that if he could do the trial over again, he would have used subcutaneous methotrexate.
“It’s a more reliable way of getting a dose into people and probably of getting a slightly higher dose into people. In the rheumatoid arthritis world, we use a lot more subcutaneous methotrexate now than we did 10 years ago because it gets around a lot of the minor side effects and helps compliance,” he said.
One audience member suggested that one potentially useful way to zero in on a subgroup of knee OA patients likely to derive the most benefit from methotrexate would be to have screened potential study participants for comorbid fibromyalgia and exclude those with the disorder. Dr. Conaghan replied that the PROMOTE investigators did gather data on participants’ pain at sites other than the knee. That data can be used to identify those at increased likelihood of fibromyalgia, and he agreed that’s worth looking into.
Dr. Conaghan reported having no financial conflicts regarding PROMOTE, which was funded by the U.K. National Institute for Health Research and Versus Arthritis.
SOURCE: Conaghan PG et al. OARSI 2019, Abstract 86.
REPORTING FROM OARSI 2019
Click for Credit: Biomarkers for VTE risk; Exercise & concussion recovery; more
Here are 5 articles from the June issue of Clinician Reviews (individual articles are valid for one year from date of publication—expiration dates below):
1. Expert: There’s no single treatment for fibromyalgia
To take the posttest, go to: https://bit.ly/2EAI5v1
Expires February 3, 2020
2. Mood and behavior are different targets for irritability in children
To take the posttest, go to: https://bit.ly/2wpLS9X
Expires February 6, 2020
3. Biomarkers predict VTE risk with menopausal oral hormone therapy
To take the posttest, go to: https://bit.ly/2JKEQFC
Expires February 6, 2020
4. Mild aerobic exercise speeds sports concussion recovery
To take the posttest, go to: https://bit.ly/30RuYiE
Expires February 4, 2020
5. For CABG, multiple and single arterial grafts show no survival difference
To take the posttest, go to: https://bit.ly/2wtiCiF
Expires January 31, 2020
Here are 5 articles from the June issue of Clinician Reviews (individual articles are valid for one year from date of publication—expiration dates below):
1. Expert: There’s no single treatment for fibromyalgia
To take the posttest, go to: https://bit.ly/2EAI5v1
Expires February 3, 2020
2. Mood and behavior are different targets for irritability in children
To take the posttest, go to: https://bit.ly/2wpLS9X
Expires February 6, 2020
3. Biomarkers predict VTE risk with menopausal oral hormone therapy
To take the posttest, go to: https://bit.ly/2JKEQFC
Expires February 6, 2020
4. Mild aerobic exercise speeds sports concussion recovery
To take the posttest, go to: https://bit.ly/30RuYiE
Expires February 4, 2020
5. For CABG, multiple and single arterial grafts show no survival difference
To take the posttest, go to: https://bit.ly/2wtiCiF
Expires January 31, 2020
Here are 5 articles from the June issue of Clinician Reviews (individual articles are valid for one year from date of publication—expiration dates below):
1. Expert: There’s no single treatment for fibromyalgia
To take the posttest, go to: https://bit.ly/2EAI5v1
Expires February 3, 2020
2. Mood and behavior are different targets for irritability in children
To take the posttest, go to: https://bit.ly/2wpLS9X
Expires February 6, 2020
3. Biomarkers predict VTE risk with menopausal oral hormone therapy
To take the posttest, go to: https://bit.ly/2JKEQFC
Expires February 6, 2020
4. Mild aerobic exercise speeds sports concussion recovery
To take the posttest, go to: https://bit.ly/30RuYiE
Expires February 4, 2020
5. For CABG, multiple and single arterial grafts show no survival difference
To take the posttest, go to: https://bit.ly/2wtiCiF
Expires January 31, 2020
Questions remain as marijuana enters clinic use
SAN FRANCISCO – Medical marijuana skipped the usual phased testing of pharmaceuticals, so questions abound about how to counsel patients as legalization rolls out across the country, speakers said at the American Psychiatric Association annual meeting.
Drug interactions are an issue but remain under the radar. Tetrahydrocannabinol (THC) and cannabidiol (CBD) are inhibitors of cytochrome P450, specifically the CYP2C enzyme and CYP3A liver enzymes, which means possible interactions with drug classes such as antidepressants and antipsychotics might come into play.
Concomitant use could affect, or be affected by, fluoxetine, clozapine, duloxetine, and olanzapine, among other medications. One case study suggested that warfarin doses should be reduced by 30% in a patient who had started with a liquid formulation of CBD for managing epilepsy (Basic Clin Pharmacol Toxicol. 2019 Jan;124[1]:28-31).
At this point, it’s “not clear what the clinic implications are,” but “it’s not unreasonable to consider that your patients’ response to their psychiatric medications might change based on the introduction of cannabinoids,” said Arthur Williams, MD, assistant professor of clinical psychiatry at Columbia University, New York, and one of many researchers playing catch-up as marijuana and its derivatives enter the clinic.
Another question is what, exactly, is a standard dose?
Dosing mostly has been a question of THC, the psychoactive component of marijuana. Washington state and Colorado opted for 10-mg THC when those jurisdictions legalized recreational use; Oregon chose 5 mg. Both are in line with Food and Drug Administration formulations already on the market, including dronabinol (Marinol), a synthetic THC approved in 2.5-mg, 5-mg, and 10-mg doses for AIDS wasting, and chemotherapy nausea and vomiting.
A typical .7-g joint of 8% THC delivers about 5 mg or so, but newer strains range up to 20% THC, and could deliver over 13 mg per joint; occasional users, meanwhile, feel high from just 2-3 mg.
The ratio of THC to CBD matters, as well. Generally, “whole plant marijuana on the black market is much higher in THC and much lower in CBD,” Dr. Williams said. CBD is thought to deliver most of the medical benefits of marijuana.
It’s best to ask people what they’re using, and to counsel new users – especially the elderly – to start low and go slow. But keep in mind that many medical users have years of recreational use and have built up tolerance, he said.
Vaping is not a bad idea for those interested. It heats the plant material to high enough temperatures to release cannabinoids but without combusting. It’s a much more efficient THC delivery system than smoking, and there’s no smoke in the lungs. Vape patients often feel they can titrate their dose exactly.
Edibles are another matter. It can take hours for them to hit. Although THC levels do not spike with edibles as they do when the substance is inhaled, the effects last longer. A lot depends on how much food is in the gut.
The risk with edibles is that people may keep popping gummy bears and brownies because they don’t feel anything but end up overdosing. Children might be tempted by the treats, too, and for those under 4 years old, overdose can lead to fatal encephalopathic comas, “something we never really saw until edibles came around,” Dr. Williams said.
With edibles, “you have no idea what’s actually in the product.” Labels can be “inaccurate by an order of magnitude. Patients should be cautioned about that,” he said.
Pregnant and breastfeeding women, especially, should be warned away from marijuana. Some of the literature suggests a link between exposure to marijuana and preterm birth – in addition to early psychosis in vulnerable children.
Dr. Williams had no relevant disclosures.
SAN FRANCISCO – Medical marijuana skipped the usual phased testing of pharmaceuticals, so questions abound about how to counsel patients as legalization rolls out across the country, speakers said at the American Psychiatric Association annual meeting.
Drug interactions are an issue but remain under the radar. Tetrahydrocannabinol (THC) and cannabidiol (CBD) are inhibitors of cytochrome P450, specifically the CYP2C enzyme and CYP3A liver enzymes, which means possible interactions with drug classes such as antidepressants and antipsychotics might come into play.
Concomitant use could affect, or be affected by, fluoxetine, clozapine, duloxetine, and olanzapine, among other medications. One case study suggested that warfarin doses should be reduced by 30% in a patient who had started with a liquid formulation of CBD for managing epilepsy (Basic Clin Pharmacol Toxicol. 2019 Jan;124[1]:28-31).
At this point, it’s “not clear what the clinic implications are,” but “it’s not unreasonable to consider that your patients’ response to their psychiatric medications might change based on the introduction of cannabinoids,” said Arthur Williams, MD, assistant professor of clinical psychiatry at Columbia University, New York, and one of many researchers playing catch-up as marijuana and its derivatives enter the clinic.
Another question is what, exactly, is a standard dose?
Dosing mostly has been a question of THC, the psychoactive component of marijuana. Washington state and Colorado opted for 10-mg THC when those jurisdictions legalized recreational use; Oregon chose 5 mg. Both are in line with Food and Drug Administration formulations already on the market, including dronabinol (Marinol), a synthetic THC approved in 2.5-mg, 5-mg, and 10-mg doses for AIDS wasting, and chemotherapy nausea and vomiting.
A typical .7-g joint of 8% THC delivers about 5 mg or so, but newer strains range up to 20% THC, and could deliver over 13 mg per joint; occasional users, meanwhile, feel high from just 2-3 mg.
The ratio of THC to CBD matters, as well. Generally, “whole plant marijuana on the black market is much higher in THC and much lower in CBD,” Dr. Williams said. CBD is thought to deliver most of the medical benefits of marijuana.
It’s best to ask people what they’re using, and to counsel new users – especially the elderly – to start low and go slow. But keep in mind that many medical users have years of recreational use and have built up tolerance, he said.
Vaping is not a bad idea for those interested. It heats the plant material to high enough temperatures to release cannabinoids but without combusting. It’s a much more efficient THC delivery system than smoking, and there’s no smoke in the lungs. Vape patients often feel they can titrate their dose exactly.
Edibles are another matter. It can take hours for them to hit. Although THC levels do not spike with edibles as they do when the substance is inhaled, the effects last longer. A lot depends on how much food is in the gut.
The risk with edibles is that people may keep popping gummy bears and brownies because they don’t feel anything but end up overdosing. Children might be tempted by the treats, too, and for those under 4 years old, overdose can lead to fatal encephalopathic comas, “something we never really saw until edibles came around,” Dr. Williams said.
With edibles, “you have no idea what’s actually in the product.” Labels can be “inaccurate by an order of magnitude. Patients should be cautioned about that,” he said.
Pregnant and breastfeeding women, especially, should be warned away from marijuana. Some of the literature suggests a link between exposure to marijuana and preterm birth – in addition to early psychosis in vulnerable children.
Dr. Williams had no relevant disclosures.
SAN FRANCISCO – Medical marijuana skipped the usual phased testing of pharmaceuticals, so questions abound about how to counsel patients as legalization rolls out across the country, speakers said at the American Psychiatric Association annual meeting.
Drug interactions are an issue but remain under the radar. Tetrahydrocannabinol (THC) and cannabidiol (CBD) are inhibitors of cytochrome P450, specifically the CYP2C enzyme and CYP3A liver enzymes, which means possible interactions with drug classes such as antidepressants and antipsychotics might come into play.
Concomitant use could affect, or be affected by, fluoxetine, clozapine, duloxetine, and olanzapine, among other medications. One case study suggested that warfarin doses should be reduced by 30% in a patient who had started with a liquid formulation of CBD for managing epilepsy (Basic Clin Pharmacol Toxicol. 2019 Jan;124[1]:28-31).
At this point, it’s “not clear what the clinic implications are,” but “it’s not unreasonable to consider that your patients’ response to their psychiatric medications might change based on the introduction of cannabinoids,” said Arthur Williams, MD, assistant professor of clinical psychiatry at Columbia University, New York, and one of many researchers playing catch-up as marijuana and its derivatives enter the clinic.
Another question is what, exactly, is a standard dose?
Dosing mostly has been a question of THC, the psychoactive component of marijuana. Washington state and Colorado opted for 10-mg THC when those jurisdictions legalized recreational use; Oregon chose 5 mg. Both are in line with Food and Drug Administration formulations already on the market, including dronabinol (Marinol), a synthetic THC approved in 2.5-mg, 5-mg, and 10-mg doses for AIDS wasting, and chemotherapy nausea and vomiting.
A typical .7-g joint of 8% THC delivers about 5 mg or so, but newer strains range up to 20% THC, and could deliver over 13 mg per joint; occasional users, meanwhile, feel high from just 2-3 mg.
The ratio of THC to CBD matters, as well. Generally, “whole plant marijuana on the black market is much higher in THC and much lower in CBD,” Dr. Williams said. CBD is thought to deliver most of the medical benefits of marijuana.
It’s best to ask people what they’re using, and to counsel new users – especially the elderly – to start low and go slow. But keep in mind that many medical users have years of recreational use and have built up tolerance, he said.
Vaping is not a bad idea for those interested. It heats the plant material to high enough temperatures to release cannabinoids but without combusting. It’s a much more efficient THC delivery system than smoking, and there’s no smoke in the lungs. Vape patients often feel they can titrate their dose exactly.
Edibles are another matter. It can take hours for them to hit. Although THC levels do not spike with edibles as they do when the substance is inhaled, the effects last longer. A lot depends on how much food is in the gut.
The risk with edibles is that people may keep popping gummy bears and brownies because they don’t feel anything but end up overdosing. Children might be tempted by the treats, too, and for those under 4 years old, overdose can lead to fatal encephalopathic comas, “something we never really saw until edibles came around,” Dr. Williams said.
With edibles, “you have no idea what’s actually in the product.” Labels can be “inaccurate by an order of magnitude. Patients should be cautioned about that,” he said.
Pregnant and breastfeeding women, especially, should be warned away from marijuana. Some of the literature suggests a link between exposure to marijuana and preterm birth – in addition to early psychosis in vulnerable children.
Dr. Williams had no relevant disclosures.
REPORTING FROM APA 2019
Pain, fatigue, depression, and anxiety are common in the year after MS diagnosis
SEATTLE – researchers reported at the annual meeting of the Consortium of Multiple Sclerosis Centers. In a novel study, about half of patients with MS reported clinically significant symptoms of depression or pain, and approximately 60% reported fatigue during that time.
Pain, fatigue, depression, and anxiety are common in MS, but their prevalence in the first year after diagnosis is not well understood. To examine the rates of these conditions and how often they co-occur during that period, Anna L. Kratz, PhD, associate professor of physical medicine and rehabilitation at the University of Michigan in Ann Arbor, and her research colleagues had 231 adults with MS complete validated surveys at 1, 2, 3, 6, 9, and 12 months after diagnosis to assess symptoms of these conditions.
Overall, 47.2% of patients reported clinically significant levels of depression, 38.5% reported clinically significant levels of anxiety, 50.4% reported clinically significant pain, and 62.2% reported clinically significant fatigue at any point during the year after diagnosis. “Of those who did not have clinically significant symptoms at time of diagnosis, 21.3% went on to develop clinically significant depression, 17.0% anxiety, 30.9% pain, and 34.1% fatigue,” the authors reported.
About 23% of patients did not have clinically significant symptoms for any condition, while 20% had clinically significant symptoms for one condition, 21% for two, 19% for three, and 17% for all four.
Depression and fatigue had the highest rate of comorbidity, whereas pain and anxiety had the lowest rate of comorbidity.
“Important clinical symptoms associated with MS are present at high levels in the first year post diagnosis,” Dr. Kratz and colleagues concluded. “While the rates and severity are marginally lower than have been identified in studies of individuals farther into the MS disease course, this study is a reminder that early MS intervention should incorporate interventions for these symptoms that are known to have strong associations with quality of life.”
The researchers had no disclosures.
SEATTLE – researchers reported at the annual meeting of the Consortium of Multiple Sclerosis Centers. In a novel study, about half of patients with MS reported clinically significant symptoms of depression or pain, and approximately 60% reported fatigue during that time.
Pain, fatigue, depression, and anxiety are common in MS, but their prevalence in the first year after diagnosis is not well understood. To examine the rates of these conditions and how often they co-occur during that period, Anna L. Kratz, PhD, associate professor of physical medicine and rehabilitation at the University of Michigan in Ann Arbor, and her research colleagues had 231 adults with MS complete validated surveys at 1, 2, 3, 6, 9, and 12 months after diagnosis to assess symptoms of these conditions.
Overall, 47.2% of patients reported clinically significant levels of depression, 38.5% reported clinically significant levels of anxiety, 50.4% reported clinically significant pain, and 62.2% reported clinically significant fatigue at any point during the year after diagnosis. “Of those who did not have clinically significant symptoms at time of diagnosis, 21.3% went on to develop clinically significant depression, 17.0% anxiety, 30.9% pain, and 34.1% fatigue,” the authors reported.
About 23% of patients did not have clinically significant symptoms for any condition, while 20% had clinically significant symptoms for one condition, 21% for two, 19% for three, and 17% for all four.
Depression and fatigue had the highest rate of comorbidity, whereas pain and anxiety had the lowest rate of comorbidity.
“Important clinical symptoms associated with MS are present at high levels in the first year post diagnosis,” Dr. Kratz and colleagues concluded. “While the rates and severity are marginally lower than have been identified in studies of individuals farther into the MS disease course, this study is a reminder that early MS intervention should incorporate interventions for these symptoms that are known to have strong associations with quality of life.”
The researchers had no disclosures.
SEATTLE – researchers reported at the annual meeting of the Consortium of Multiple Sclerosis Centers. In a novel study, about half of patients with MS reported clinically significant symptoms of depression or pain, and approximately 60% reported fatigue during that time.
Pain, fatigue, depression, and anxiety are common in MS, but their prevalence in the first year after diagnosis is not well understood. To examine the rates of these conditions and how often they co-occur during that period, Anna L. Kratz, PhD, associate professor of physical medicine and rehabilitation at the University of Michigan in Ann Arbor, and her research colleagues had 231 adults with MS complete validated surveys at 1, 2, 3, 6, 9, and 12 months after diagnosis to assess symptoms of these conditions.
Overall, 47.2% of patients reported clinically significant levels of depression, 38.5% reported clinically significant levels of anxiety, 50.4% reported clinically significant pain, and 62.2% reported clinically significant fatigue at any point during the year after diagnosis. “Of those who did not have clinically significant symptoms at time of diagnosis, 21.3% went on to develop clinically significant depression, 17.0% anxiety, 30.9% pain, and 34.1% fatigue,” the authors reported.
About 23% of patients did not have clinically significant symptoms for any condition, while 20% had clinically significant symptoms for one condition, 21% for two, 19% for three, and 17% for all four.
Depression and fatigue had the highest rate of comorbidity, whereas pain and anxiety had the lowest rate of comorbidity.
“Important clinical symptoms associated with MS are present at high levels in the first year post diagnosis,” Dr. Kratz and colleagues concluded. “While the rates and severity are marginally lower than have been identified in studies of individuals farther into the MS disease course, this study is a reminder that early MS intervention should incorporate interventions for these symptoms that are known to have strong associations with quality of life.”
The researchers had no disclosures.
REPORTING FROM CMSC 2019
Key clinical point: Pain, fatigue, depression, and anxiety are common among patients with multiple sclerosis in the 12 months after diagnosis.
Major finding: About half of patients with multiple sclerosis reported clinically significant symptoms of depression or pain, and approximately 60% reported fatigue.
Study details: An analysis of data from 231 adults with multiple sclerosis who completed validated surveys at 1, 2, 3, 6, 9, and 12 months after diagnosis to assess symptoms of pain, fatigue, depression, and anxiety.
Disclosures: The researchers had no disclosures.
When adolescents visit the ED, 10% leave with an opioid
although there was a small but significant decrease in prescriptions over that time, according to an analysis of two nationwide ambulatory care surveys.
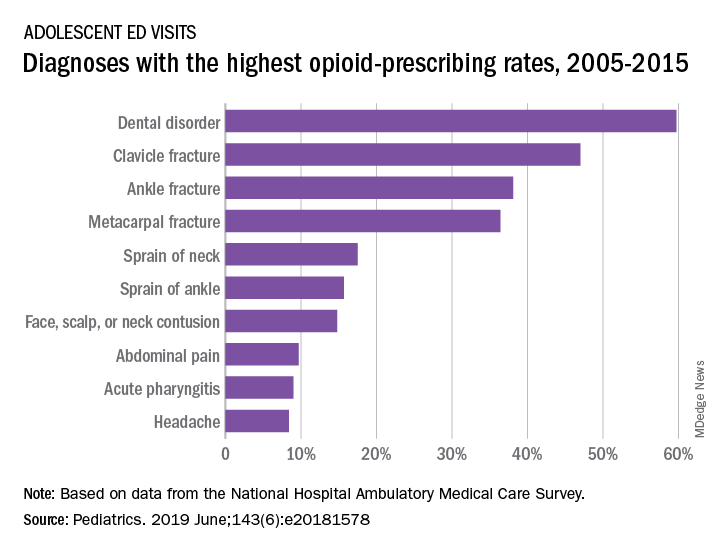
For adolescents aged 13-17 years, 10.4% of ED visits were associated with a prescription for an opioid versus 1.6% among outpatient visits. There was a slight but significant decrease in the rate of opioid prescriptions in the ED setting over the study period, with an odds ratio of 0.95 (95% confidence interval, 0.92-0.97), but there was no significant change in the trend over time in the outpatient setting (OR, 1.02; 95% CI, 0.99-1.09), Joel D. Hudgins, MD, and associates reported in Pediatrics.
“Opioid prescribing in ambulatory care visits is particularly high in the ED setting and … certain diagnoses appear to be routinely treated with an opioid,” said Dr. Hudgins and associates from Boston Children’s Hospital.
The highest rates of opioid prescribing among adolescents visiting the ED involved dental disorders (60%) and acute injuries such as fractures of the clavicle (47%), ankle (38%), and metacarpals (36%). “However, when considering the total volume of opioid prescriptions dispensed [over 7.8 million during 2005-2015], certain common conditions, including abdominal pain, acute pharyngitis, urinary tract infection, and headache, contributed large numbers of prescriptions as well,” they added.
The study involved data from the National Hospital Ambulatory Medical Care Survey (hospital-based EDs) and the National Ambulatory Medical Care Survey (office-based practices), which both are conducted annually by the National Center for Health Statistics.
The senior investigator is supported by an award from the Burroughs Wellcome Fund by the Harvard-MIT Center for Regulatory Science. The authors said that they have no relevant financial relationships.
SOURCE: Hudgins JD et al. Pediatrics. 2019 June. doi: 10.1542/peds.2018-1578.
although there was a small but significant decrease in prescriptions over that time, according to an analysis of two nationwide ambulatory care surveys.

For adolescents aged 13-17 years, 10.4% of ED visits were associated with a prescription for an opioid versus 1.6% among outpatient visits. There was a slight but significant decrease in the rate of opioid prescriptions in the ED setting over the study period, with an odds ratio of 0.95 (95% confidence interval, 0.92-0.97), but there was no significant change in the trend over time in the outpatient setting (OR, 1.02; 95% CI, 0.99-1.09), Joel D. Hudgins, MD, and associates reported in Pediatrics.
“Opioid prescribing in ambulatory care visits is particularly high in the ED setting and … certain diagnoses appear to be routinely treated with an opioid,” said Dr. Hudgins and associates from Boston Children’s Hospital.
The highest rates of opioid prescribing among adolescents visiting the ED involved dental disorders (60%) and acute injuries such as fractures of the clavicle (47%), ankle (38%), and metacarpals (36%). “However, when considering the total volume of opioid prescriptions dispensed [over 7.8 million during 2005-2015], certain common conditions, including abdominal pain, acute pharyngitis, urinary tract infection, and headache, contributed large numbers of prescriptions as well,” they added.
The study involved data from the National Hospital Ambulatory Medical Care Survey (hospital-based EDs) and the National Ambulatory Medical Care Survey (office-based practices), which both are conducted annually by the National Center for Health Statistics.
The senior investigator is supported by an award from the Burroughs Wellcome Fund by the Harvard-MIT Center for Regulatory Science. The authors said that they have no relevant financial relationships.
SOURCE: Hudgins JD et al. Pediatrics. 2019 June. doi: 10.1542/peds.2018-1578.
although there was a small but significant decrease in prescriptions over that time, according to an analysis of two nationwide ambulatory care surveys.

For adolescents aged 13-17 years, 10.4% of ED visits were associated with a prescription for an opioid versus 1.6% among outpatient visits. There was a slight but significant decrease in the rate of opioid prescriptions in the ED setting over the study period, with an odds ratio of 0.95 (95% confidence interval, 0.92-0.97), but there was no significant change in the trend over time in the outpatient setting (OR, 1.02; 95% CI, 0.99-1.09), Joel D. Hudgins, MD, and associates reported in Pediatrics.
“Opioid prescribing in ambulatory care visits is particularly high in the ED setting and … certain diagnoses appear to be routinely treated with an opioid,” said Dr. Hudgins and associates from Boston Children’s Hospital.
The highest rates of opioid prescribing among adolescents visiting the ED involved dental disorders (60%) and acute injuries such as fractures of the clavicle (47%), ankle (38%), and metacarpals (36%). “However, when considering the total volume of opioid prescriptions dispensed [over 7.8 million during 2005-2015], certain common conditions, including abdominal pain, acute pharyngitis, urinary tract infection, and headache, contributed large numbers of prescriptions as well,” they added.
The study involved data from the National Hospital Ambulatory Medical Care Survey (hospital-based EDs) and the National Ambulatory Medical Care Survey (office-based practices), which both are conducted annually by the National Center for Health Statistics.
The senior investigator is supported by an award from the Burroughs Wellcome Fund by the Harvard-MIT Center for Regulatory Science. The authors said that they have no relevant financial relationships.
SOURCE: Hudgins JD et al. Pediatrics. 2019 June. doi: 10.1542/peds.2018-1578.
FROM PEDIATRICS
Tanezumab acts fast for OA pain relief
TORONTO – , according to a secondary analysis of a phase 3 randomized trial.
“The onset is relatively quick. It’s a monoclonal antibody, so it doesn’t work overnight, but by 3-5 days you see a significant difference,” Thomas J. Schnitzer, MD, PhD, reported at the OARSI 2019 World Congress.
He had previously presented the primary outcomes of this 696-patient, phase 3, randomized trial at the 2018 annual meeting of the American College of Rheumatology. At OARSI 2019, the rheumatologist presented new data focusing on the speed and durability of the pain relief provided by tanezumab, a humanized monoclonal antibody designed to help keep pain signals produced in the periphery from reaching the CNS.
The double-blind trial included U.S. patients with an average 9.3-year disease duration who were randomized to either two 2.5-mg subcutaneous injections of the nerve growth factor inhibitor 8 weeks apart, a 2.5-mg dose followed 8 weeks later by a 5-mg dose, or two placebo injections. Eighty-five percent of subjects had knee OA, and the rest had hip OA. The patients had fairly severe pain, with average baseline Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC) pain scores of 7.1-7.4. Notably, all study participants had to have a documented history of previous failure to respond to at least three pain relievers: acetaminophen, oral NSAIDs, and tramadol or opioids, according to Dr. Schnitzer, a rheumatologist who is professor of physical medicine and rehabilitation, anesthesiology, and medicine at Northwestern University in Chicago.
As previously reported, the co–primary endpoint of change from baseline to week 16 in WOMAC pain was –3.22 points with the 2.5-mg tanezumab regimen and –3.45 with the 2.5/5–mg strategy, both significantly better than the 2.56-point improvement with placebo. Improvement in WOMAC physical function followed suit, he said at the meeting sponsored by the Osteoarthritis Research Society International.
Assessments were made at office visits every 2 weeks during the study. By the first visit at week 2, tanezumab was significantly better than placebo on both WOMAC measures, an advantage maintained for the rest of the 16 weeks. Pain relief in the tanezumab-treated groups was maximum at weeks 4 and 12; that is, 4 weeks following the first and second injections.
“This suggests that there’s an immediate effect of the antibody, which then tends to wane as the antibody begins to get cleared,” Dr. Schnitzer observed.
Study participants kept a structured daily pain diary, which enabled investigators to zero in on the timing of pain relief. Statistically significant separation from placebo was documented by day 3 in one group on tanezumab and by day 5 in the other.
An increased rate of rapidly progressive OA was a concern years ago in earlier studies of a now-abandoned intravenous formulation of tanezumab. However, in the phase 3 trial of the subcutaneous humanized monoclonal antibody, rapidly progressive OA occurred in only six patients, or 1.3%, during the 24-week safety follow-up period. Interestingly, the phenomenon was not dose related, as five of the six cases occurred in patients on the twin 2.5-mg regimen, and only one in the 2.5/5-mg group. No cases of osteonecrosis occurred in the trial.
One audience member rose to say she and her fellow rheumatologists are very excited about the prospect of possible access to a novel and more effective OA therapy. But she took issue with the trial’s reliance on WOMAC pain and physical function scores as primary endpoints, noting that OARSI experts have developed and validated several more comprehensive and globally informative assessment tools. Dr. Schnitzer readily agreed. The investigators utilized WOMAC pain and physical function because that’s what the U.S. and European regulatory agencies insist upon, he explained.
Clinicians should stay tuned because the results of much larger, longer-term phase 3 trials of tanezumab are due to be presented soon, he added.
Dr. Schnitzer reported serving as a consultant to Pfizer and Eli Lilly, which are jointly developing tanezumab and sponsored the trial, as well as to a handful of other pharmaceutical companies.
SOURCE: Bessette L et al. Osteoarthritis Cartilage. 2019 Apr;27[suppl 1]:S85-6, Abstract 88.
TORONTO – , according to a secondary analysis of a phase 3 randomized trial.
“The onset is relatively quick. It’s a monoclonal antibody, so it doesn’t work overnight, but by 3-5 days you see a significant difference,” Thomas J. Schnitzer, MD, PhD, reported at the OARSI 2019 World Congress.
He had previously presented the primary outcomes of this 696-patient, phase 3, randomized trial at the 2018 annual meeting of the American College of Rheumatology. At OARSI 2019, the rheumatologist presented new data focusing on the speed and durability of the pain relief provided by tanezumab, a humanized monoclonal antibody designed to help keep pain signals produced in the periphery from reaching the CNS.
The double-blind trial included U.S. patients with an average 9.3-year disease duration who were randomized to either two 2.5-mg subcutaneous injections of the nerve growth factor inhibitor 8 weeks apart, a 2.5-mg dose followed 8 weeks later by a 5-mg dose, or two placebo injections. Eighty-five percent of subjects had knee OA, and the rest had hip OA. The patients had fairly severe pain, with average baseline Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC) pain scores of 7.1-7.4. Notably, all study participants had to have a documented history of previous failure to respond to at least three pain relievers: acetaminophen, oral NSAIDs, and tramadol or opioids, according to Dr. Schnitzer, a rheumatologist who is professor of physical medicine and rehabilitation, anesthesiology, and medicine at Northwestern University in Chicago.
As previously reported, the co–primary endpoint of change from baseline to week 16 in WOMAC pain was –3.22 points with the 2.5-mg tanezumab regimen and –3.45 with the 2.5/5–mg strategy, both significantly better than the 2.56-point improvement with placebo. Improvement in WOMAC physical function followed suit, he said at the meeting sponsored by the Osteoarthritis Research Society International.
Assessments were made at office visits every 2 weeks during the study. By the first visit at week 2, tanezumab was significantly better than placebo on both WOMAC measures, an advantage maintained for the rest of the 16 weeks. Pain relief in the tanezumab-treated groups was maximum at weeks 4 and 12; that is, 4 weeks following the first and second injections.
“This suggests that there’s an immediate effect of the antibody, which then tends to wane as the antibody begins to get cleared,” Dr. Schnitzer observed.
Study participants kept a structured daily pain diary, which enabled investigators to zero in on the timing of pain relief. Statistically significant separation from placebo was documented by day 3 in one group on tanezumab and by day 5 in the other.
An increased rate of rapidly progressive OA was a concern years ago in earlier studies of a now-abandoned intravenous formulation of tanezumab. However, in the phase 3 trial of the subcutaneous humanized monoclonal antibody, rapidly progressive OA occurred in only six patients, or 1.3%, during the 24-week safety follow-up period. Interestingly, the phenomenon was not dose related, as five of the six cases occurred in patients on the twin 2.5-mg regimen, and only one in the 2.5/5-mg group. No cases of osteonecrosis occurred in the trial.
One audience member rose to say she and her fellow rheumatologists are very excited about the prospect of possible access to a novel and more effective OA therapy. But she took issue with the trial’s reliance on WOMAC pain and physical function scores as primary endpoints, noting that OARSI experts have developed and validated several more comprehensive and globally informative assessment tools. Dr. Schnitzer readily agreed. The investigators utilized WOMAC pain and physical function because that’s what the U.S. and European regulatory agencies insist upon, he explained.
Clinicians should stay tuned because the results of much larger, longer-term phase 3 trials of tanezumab are due to be presented soon, he added.
Dr. Schnitzer reported serving as a consultant to Pfizer and Eli Lilly, which are jointly developing tanezumab and sponsored the trial, as well as to a handful of other pharmaceutical companies.
SOURCE: Bessette L et al. Osteoarthritis Cartilage. 2019 Apr;27[suppl 1]:S85-6, Abstract 88.
TORONTO – , according to a secondary analysis of a phase 3 randomized trial.
“The onset is relatively quick. It’s a monoclonal antibody, so it doesn’t work overnight, but by 3-5 days you see a significant difference,” Thomas J. Schnitzer, MD, PhD, reported at the OARSI 2019 World Congress.
He had previously presented the primary outcomes of this 696-patient, phase 3, randomized trial at the 2018 annual meeting of the American College of Rheumatology. At OARSI 2019, the rheumatologist presented new data focusing on the speed and durability of the pain relief provided by tanezumab, a humanized monoclonal antibody designed to help keep pain signals produced in the periphery from reaching the CNS.
The double-blind trial included U.S. patients with an average 9.3-year disease duration who were randomized to either two 2.5-mg subcutaneous injections of the nerve growth factor inhibitor 8 weeks apart, a 2.5-mg dose followed 8 weeks later by a 5-mg dose, or two placebo injections. Eighty-five percent of subjects had knee OA, and the rest had hip OA. The patients had fairly severe pain, with average baseline Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC) pain scores of 7.1-7.4. Notably, all study participants had to have a documented history of previous failure to respond to at least three pain relievers: acetaminophen, oral NSAIDs, and tramadol or opioids, according to Dr. Schnitzer, a rheumatologist who is professor of physical medicine and rehabilitation, anesthesiology, and medicine at Northwestern University in Chicago.
As previously reported, the co–primary endpoint of change from baseline to week 16 in WOMAC pain was –3.22 points with the 2.5-mg tanezumab regimen and –3.45 with the 2.5/5–mg strategy, both significantly better than the 2.56-point improvement with placebo. Improvement in WOMAC physical function followed suit, he said at the meeting sponsored by the Osteoarthritis Research Society International.
Assessments were made at office visits every 2 weeks during the study. By the first visit at week 2, tanezumab was significantly better than placebo on both WOMAC measures, an advantage maintained for the rest of the 16 weeks. Pain relief in the tanezumab-treated groups was maximum at weeks 4 and 12; that is, 4 weeks following the first and second injections.
“This suggests that there’s an immediate effect of the antibody, which then tends to wane as the antibody begins to get cleared,” Dr. Schnitzer observed.
Study participants kept a structured daily pain diary, which enabled investigators to zero in on the timing of pain relief. Statistically significant separation from placebo was documented by day 3 in one group on tanezumab and by day 5 in the other.
An increased rate of rapidly progressive OA was a concern years ago in earlier studies of a now-abandoned intravenous formulation of tanezumab. However, in the phase 3 trial of the subcutaneous humanized monoclonal antibody, rapidly progressive OA occurred in only six patients, or 1.3%, during the 24-week safety follow-up period. Interestingly, the phenomenon was not dose related, as five of the six cases occurred in patients on the twin 2.5-mg regimen, and only one in the 2.5/5-mg group. No cases of osteonecrosis occurred in the trial.
One audience member rose to say she and her fellow rheumatologists are very excited about the prospect of possible access to a novel and more effective OA therapy. But she took issue with the trial’s reliance on WOMAC pain and physical function scores as primary endpoints, noting that OARSI experts have developed and validated several more comprehensive and globally informative assessment tools. Dr. Schnitzer readily agreed. The investigators utilized WOMAC pain and physical function because that’s what the U.S. and European regulatory agencies insist upon, he explained.
Clinicians should stay tuned because the results of much larger, longer-term phase 3 trials of tanezumab are due to be presented soon, he added.
Dr. Schnitzer reported serving as a consultant to Pfizer and Eli Lilly, which are jointly developing tanezumab and sponsored the trial, as well as to a handful of other pharmaceutical companies.
SOURCE: Bessette L et al. Osteoarthritis Cartilage. 2019 Apr;27[suppl 1]:S85-6, Abstract 88.
REPORTING FROM OARSI 2019
Key clinical point: The nerve growth factor inhibitor tanezumab brings rapid improvement in pain.
Major finding: Tanezumab-treated patients experienced significant pain reduction within 3-5 days after their first dose.
Study details: This was a phase 3, prospective, multicenter, double-blind, placebo-controlled trial in 696 patients with refractory pain attributable to knee or hip OA.
Disclosures: The presenter reported serving as a consultant to Pfizer and Eli Lilly, which cosponsored the phase 3 trial.
Source: Bessette L et al. Osteoarthritis Cartilage. 2019 Apr;27[suppl 1]:S85-6, Abstract 88.
N.J. law, EMR alerts appear effective at reducing opioid prescriptions
WASHINGTON –
Researchers looked at prescribing patterns of doctors in the Penn Medicine health system, which straddles both the Philadelphia area and southern New Jersey, following the implementation of prescribing limits in New Jersey.
The law in question is a 5-day limit on new opioid prescriptions, which was passed in February 2017 and implemented in May 2017. Penn Medicine implemented an EMR alert in their New Jersey locations to alert physicians within the Penn Medicine system of the change in their state law 2 months after the law went into effect. Researchers looked at prescribing patterns before passage, during the transition between passage and the implementation of the EMR alert and following implementation of the EMR alert, as well as secondary outcomes such as rate of refills, telephone calls, and utilization.
“The implementation of the prescribing limit and EMR alert was associated with a decrease in the volume of opioids prescribed in acute prescriptions without changes in the rates of refills, telephone calls or utilization,” Margaret Lowenstein, MD, of the University of Pennsylvania, Philadelphia, said at the annual meeting of the Society of General Internal Medicine.
“This combination of the policy and the EMR alert may be an effective strategy to influence prescriber behavior,” she added.
Researchers compared outcomes before and after the implementation of the law in New Jersey, using prescribing patterns in Pennsylvania as the control. The cohort of patients was those with a new opioid prescription within Penn Medicine ambulatory nonteaching practices. It excluded specialties not represented in both states as well as patients with cancer, those in hospice and palliative care and those in treatment for opioid use disorder, since the law does not apply to those groups.
In New Jersey, there were 434 patients receiving new prescriptions in the 12 months prior to the implementation of the law, with 234 patients receiving new prescriptions in the 9 months after the EMR alert was implemented in New Jersey. In Pennsylvania, the cohort included 2,961 patients prior to the law going into effect and 1,677 after the EMR intervention went live in New Jersey.
For New Jersey, the morphine milligram equivalent (MME) per prescription was steady at about 350 during the period prior to the law’s implementation, but dropped to nearly 250 by the end of the postintervention period examined. In Pennsylvania, the prelaw implementation period had an MME per prescription a little higher than 200, which leveled off at around 200 during the postintervention period.
“In New Jersey, there is a significantly higher MME than in Pennsylvania and this difference persists in the transition period but what you see in the post period is a significantly greater decline in the MME per prescription in New Jersey as compared to the rate of change in Pa.,” Dr. Lowenstein said. “That difference was statistically significant.”
She said similar results were seen regarding the quantity of tablets prescribed. In New Jersey before the law’s passage, the number of tablets per prescription was close to 50, dropping down to about 35 post period. Pennsylvania saw a slight decrease from about 35 pills per prescription to about 33 during the same period.
No significant changes occurred in the other outcomes measured following implementation of the EMR alert.
Dr. Lowenstein noted that, because the transition period between the law going into effect and the implementation of the EMR alert was so short, whether the greater decreases in opioid prescriptions in New Jersey relative to Pennsylvania was because of the law alone, the EMR alert alone, or both changes is unclear.
Based on the limited amount of change in prescribing patterns during the transition period, it appears that the EMR intervention may be driving the change, “but we weren’t powered to make that determination,” she added.
Dr. Lowenstein and her colleagues reported no disclosures.
WASHINGTON –
Researchers looked at prescribing patterns of doctors in the Penn Medicine health system, which straddles both the Philadelphia area and southern New Jersey, following the implementation of prescribing limits in New Jersey.
The law in question is a 5-day limit on new opioid prescriptions, which was passed in February 2017 and implemented in May 2017. Penn Medicine implemented an EMR alert in their New Jersey locations to alert physicians within the Penn Medicine system of the change in their state law 2 months after the law went into effect. Researchers looked at prescribing patterns before passage, during the transition between passage and the implementation of the EMR alert and following implementation of the EMR alert, as well as secondary outcomes such as rate of refills, telephone calls, and utilization.
“The implementation of the prescribing limit and EMR alert was associated with a decrease in the volume of opioids prescribed in acute prescriptions without changes in the rates of refills, telephone calls or utilization,” Margaret Lowenstein, MD, of the University of Pennsylvania, Philadelphia, said at the annual meeting of the Society of General Internal Medicine.
“This combination of the policy and the EMR alert may be an effective strategy to influence prescriber behavior,” she added.
Researchers compared outcomes before and after the implementation of the law in New Jersey, using prescribing patterns in Pennsylvania as the control. The cohort of patients was those with a new opioid prescription within Penn Medicine ambulatory nonteaching practices. It excluded specialties not represented in both states as well as patients with cancer, those in hospice and palliative care and those in treatment for opioid use disorder, since the law does not apply to those groups.
In New Jersey, there were 434 patients receiving new prescriptions in the 12 months prior to the implementation of the law, with 234 patients receiving new prescriptions in the 9 months after the EMR alert was implemented in New Jersey. In Pennsylvania, the cohort included 2,961 patients prior to the law going into effect and 1,677 after the EMR intervention went live in New Jersey.
For New Jersey, the morphine milligram equivalent (MME) per prescription was steady at about 350 during the period prior to the law’s implementation, but dropped to nearly 250 by the end of the postintervention period examined. In Pennsylvania, the prelaw implementation period had an MME per prescription a little higher than 200, which leveled off at around 200 during the postintervention period.
“In New Jersey, there is a significantly higher MME than in Pennsylvania and this difference persists in the transition period but what you see in the post period is a significantly greater decline in the MME per prescription in New Jersey as compared to the rate of change in Pa.,” Dr. Lowenstein said. “That difference was statistically significant.”
She said similar results were seen regarding the quantity of tablets prescribed. In New Jersey before the law’s passage, the number of tablets per prescription was close to 50, dropping down to about 35 post period. Pennsylvania saw a slight decrease from about 35 pills per prescription to about 33 during the same period.
No significant changes occurred in the other outcomes measured following implementation of the EMR alert.
Dr. Lowenstein noted that, because the transition period between the law going into effect and the implementation of the EMR alert was so short, whether the greater decreases in opioid prescriptions in New Jersey relative to Pennsylvania was because of the law alone, the EMR alert alone, or both changes is unclear.
Based on the limited amount of change in prescribing patterns during the transition period, it appears that the EMR intervention may be driving the change, “but we weren’t powered to make that determination,” she added.
Dr. Lowenstein and her colleagues reported no disclosures.
WASHINGTON –
Researchers looked at prescribing patterns of doctors in the Penn Medicine health system, which straddles both the Philadelphia area and southern New Jersey, following the implementation of prescribing limits in New Jersey.
The law in question is a 5-day limit on new opioid prescriptions, which was passed in February 2017 and implemented in May 2017. Penn Medicine implemented an EMR alert in their New Jersey locations to alert physicians within the Penn Medicine system of the change in their state law 2 months after the law went into effect. Researchers looked at prescribing patterns before passage, during the transition between passage and the implementation of the EMR alert and following implementation of the EMR alert, as well as secondary outcomes such as rate of refills, telephone calls, and utilization.
“The implementation of the prescribing limit and EMR alert was associated with a decrease in the volume of opioids prescribed in acute prescriptions without changes in the rates of refills, telephone calls or utilization,” Margaret Lowenstein, MD, of the University of Pennsylvania, Philadelphia, said at the annual meeting of the Society of General Internal Medicine.
“This combination of the policy and the EMR alert may be an effective strategy to influence prescriber behavior,” she added.
Researchers compared outcomes before and after the implementation of the law in New Jersey, using prescribing patterns in Pennsylvania as the control. The cohort of patients was those with a new opioid prescription within Penn Medicine ambulatory nonteaching practices. It excluded specialties not represented in both states as well as patients with cancer, those in hospice and palliative care and those in treatment for opioid use disorder, since the law does not apply to those groups.
In New Jersey, there were 434 patients receiving new prescriptions in the 12 months prior to the implementation of the law, with 234 patients receiving new prescriptions in the 9 months after the EMR alert was implemented in New Jersey. In Pennsylvania, the cohort included 2,961 patients prior to the law going into effect and 1,677 after the EMR intervention went live in New Jersey.
For New Jersey, the morphine milligram equivalent (MME) per prescription was steady at about 350 during the period prior to the law’s implementation, but dropped to nearly 250 by the end of the postintervention period examined. In Pennsylvania, the prelaw implementation period had an MME per prescription a little higher than 200, which leveled off at around 200 during the postintervention period.
“In New Jersey, there is a significantly higher MME than in Pennsylvania and this difference persists in the transition period but what you see in the post period is a significantly greater decline in the MME per prescription in New Jersey as compared to the rate of change in Pa.,” Dr. Lowenstein said. “That difference was statistically significant.”
She said similar results were seen regarding the quantity of tablets prescribed. In New Jersey before the law’s passage, the number of tablets per prescription was close to 50, dropping down to about 35 post period. Pennsylvania saw a slight decrease from about 35 pills per prescription to about 33 during the same period.
No significant changes occurred in the other outcomes measured following implementation of the EMR alert.
Dr. Lowenstein noted that, because the transition period between the law going into effect and the implementation of the EMR alert was so short, whether the greater decreases in opioid prescriptions in New Jersey relative to Pennsylvania was because of the law alone, the EMR alert alone, or both changes is unclear.
Based on the limited amount of change in prescribing patterns during the transition period, it appears that the EMR intervention may be driving the change, “but we weren’t powered to make that determination,” she added.
Dr. Lowenstein and her colleagues reported no disclosures.
REPORTING FROM SGIM 2019
Zoster vaccination is underused but looks effective in IBD
For men with inflammatory bowel disease, herpes zoster vaccination was associated with about a 46% decrease in risk of associated infection, according to the results of a retrospective study from the national Veterans Affairs Healthcare System.
Crude rates of herpes zoster infection were 4.09 cases per 1,000 person-years among vaccinated patients versus 6.97 cases per 1,000 person-years among unvaccinated patients, for an adjusted hazard ratio of 0.54 (95% confidence interval, 0.44-0.68), reported Nabeel Khan, MD, of the University of Pennsylvania, Philadelphia, and associates. “This vaccine is therefore effective in patients with IBD, but underused,” they wrote in Clinical Gastroenterology and Hepatology.
Studies have linked IBD with a 1.2- to 1.8-fold increased risk of herpes zoster infection, the researchers noted. Relevant risk factors include older age, disease flare, recent use or high cumulative use of prednisone, and use of thiopurines, either alone or in combination with a tumor necrosis factor (TNF) inhibitor. Although the American College of Gastroenterology recommends that all patients with IBD receive the herpes zoster vaccine by age 50 years, the efficacy of the vaccine in these patients remains unclear.
For their study, Dr. Khan and associates analyzed International Classification of Diseases (ICD) codes and other medical record data from 39,983 veterans with IBD who had not received the herpes zoster vaccine by age 60 years. In all, 97% of patients were male, and 94% were white. Most patients had high rates of health care utilization: Approximately half visited VA clinics or hospitals at least 13 times per year, and another third made 6-12 annual visits.
Despite their many contacts with VA health care systems, only 7,170 (17.9%) patients received the herpes zoster vaccine during 2000-2016, the researchers found. Vaccination rates varied substantially by region – they were highest in the Midwest (35%) and North Atlantic states (29%) but reached only 9% in Montana, Utah, Wyoming, Colorado, Oklahoma, Texas, Arkansas, and Louisiana, collectively.
The crude rate of herpes zoster infection among unvaccinated patients with IBD resembled the incidence reported in prior studies, the researchers said. After researchers accounted for differences in geography, demographics, and health care utilization between vaccinated and unvaccinated veterans with IBD, they found that vaccination was associated with an approximately 46% decrease in the risk of herpes zoster infection.
Very few patients were vaccinated for herpes zoster while on a TNF inhibitor, precluding the ability to study this subgroup. However, the vaccine showed a protective effect (adjusted HR, 0.63) among patients who received thiopurines without a TNF inhibitor. This effect did not reach statistical significance, perhaps because of lack of power, the researchers noted. “Among the 315 patients who were [vaccinated while] on thiopurines, none developed a documented painful or painless vesicular rash within 42 days of herpes zoster vaccination,” they added. One patient developed a painful blister 20 days post vaccination without vesicles or long-term sequelae.
Pfizer provided funding. Dr. Khan disclosed research funding from Pfizer, Luitpold, and Takeda Pharmaceuticals. One coinvestigator disclosed ties to Pfizer, Gilead, Merck, AbbVie, Lilly, Janssen, Johnson & Johnson, UCB, and Nestle Health Science. The remaining researchers disclosed no conflicts.
SOURCE: Khan N et al. Clin Gastroenterol Hepatol. 2018 Oct 13. doi: 10.1016/j.cgh.2018.10.016.
Preventive care is an underemphasized component of IBD management because the primary focus tends to be control of active symptoms. However, as patients are treated with immunosuppression, particularly combinations of therapies and newer mechanisms of action such as the Janus kinase inhibitors, the risk of infections increases, including those that are vaccine preventable including shingles and its related complications.
This study by Khan et al. highlights several important messages for patients and providers. First, in this large older IBD cohort, the vaccination rates were very low at 18% even though more than 80% of patients had more than six annual visits to the VA Health Systems during the study period. These represent multiple missed opportunities to discuss and administer vaccinations. Second, the authors highlighted the vaccine’s efficacy: Persons receiving herpes zoster vaccination had a clearly decreased risk of subsequent infection. While the number of vaccinated patients on immunosuppression was too small to draw conclusions about efficacy, the live attenuated vaccination is contraindicated for immunosuppressed patients. However, the newer recombinant shingles vaccine offers the opportunity to extend the reach of shingles vaccination to include those on immunosuppression. As utilization of the newer vaccine series increases, we will be able to evaluate the efficacy for immunosuppressed IBD patients, although studies from other disease states suggest efficacy. However, vaccinations will never work if they aren’t administered. Counseling patients and providers regarding the importance of vaccinations is a low-risk, efficacious means to decrease infection and associated morbidity.
Christina Ha, MD, AGAF, associate professor of medicine, Inflammatory Bowel Disease Center, division of digestive diseases, Cedars-Sinai Medical Center, Los Angeles. She is a speaker, consultant, or on the advisory board for AbbVie, Janssen, Genentech, Samsung Bioepis, and Takeda. She received grant funding from Pfizer.
Preventive care is an underemphasized component of IBD management because the primary focus tends to be control of active symptoms. However, as patients are treated with immunosuppression, particularly combinations of therapies and newer mechanisms of action such as the Janus kinase inhibitors, the risk of infections increases, including those that are vaccine preventable including shingles and its related complications.
This study by Khan et al. highlights several important messages for patients and providers. First, in this large older IBD cohort, the vaccination rates were very low at 18% even though more than 80% of patients had more than six annual visits to the VA Health Systems during the study period. These represent multiple missed opportunities to discuss and administer vaccinations. Second, the authors highlighted the vaccine’s efficacy: Persons receiving herpes zoster vaccination had a clearly decreased risk of subsequent infection. While the number of vaccinated patients on immunosuppression was too small to draw conclusions about efficacy, the live attenuated vaccination is contraindicated for immunosuppressed patients. However, the newer recombinant shingles vaccine offers the opportunity to extend the reach of shingles vaccination to include those on immunosuppression. As utilization of the newer vaccine series increases, we will be able to evaluate the efficacy for immunosuppressed IBD patients, although studies from other disease states suggest efficacy. However, vaccinations will never work if they aren’t administered. Counseling patients and providers regarding the importance of vaccinations is a low-risk, efficacious means to decrease infection and associated morbidity.
Christina Ha, MD, AGAF, associate professor of medicine, Inflammatory Bowel Disease Center, division of digestive diseases, Cedars-Sinai Medical Center, Los Angeles. She is a speaker, consultant, or on the advisory board for AbbVie, Janssen, Genentech, Samsung Bioepis, and Takeda. She received grant funding from Pfizer.
Preventive care is an underemphasized component of IBD management because the primary focus tends to be control of active symptoms. However, as patients are treated with immunosuppression, particularly combinations of therapies and newer mechanisms of action such as the Janus kinase inhibitors, the risk of infections increases, including those that are vaccine preventable including shingles and its related complications.
This study by Khan et al. highlights several important messages for patients and providers. First, in this large older IBD cohort, the vaccination rates were very low at 18% even though more than 80% of patients had more than six annual visits to the VA Health Systems during the study period. These represent multiple missed opportunities to discuss and administer vaccinations. Second, the authors highlighted the vaccine’s efficacy: Persons receiving herpes zoster vaccination had a clearly decreased risk of subsequent infection. While the number of vaccinated patients on immunosuppression was too small to draw conclusions about efficacy, the live attenuated vaccination is contraindicated for immunosuppressed patients. However, the newer recombinant shingles vaccine offers the opportunity to extend the reach of shingles vaccination to include those on immunosuppression. As utilization of the newer vaccine series increases, we will be able to evaluate the efficacy for immunosuppressed IBD patients, although studies from other disease states suggest efficacy. However, vaccinations will never work if they aren’t administered. Counseling patients and providers regarding the importance of vaccinations is a low-risk, efficacious means to decrease infection and associated morbidity.
Christina Ha, MD, AGAF, associate professor of medicine, Inflammatory Bowel Disease Center, division of digestive diseases, Cedars-Sinai Medical Center, Los Angeles. She is a speaker, consultant, or on the advisory board for AbbVie, Janssen, Genentech, Samsung Bioepis, and Takeda. She received grant funding from Pfizer.
For men with inflammatory bowel disease, herpes zoster vaccination was associated with about a 46% decrease in risk of associated infection, according to the results of a retrospective study from the national Veterans Affairs Healthcare System.
Crude rates of herpes zoster infection were 4.09 cases per 1,000 person-years among vaccinated patients versus 6.97 cases per 1,000 person-years among unvaccinated patients, for an adjusted hazard ratio of 0.54 (95% confidence interval, 0.44-0.68), reported Nabeel Khan, MD, of the University of Pennsylvania, Philadelphia, and associates. “This vaccine is therefore effective in patients with IBD, but underused,” they wrote in Clinical Gastroenterology and Hepatology.
Studies have linked IBD with a 1.2- to 1.8-fold increased risk of herpes zoster infection, the researchers noted. Relevant risk factors include older age, disease flare, recent use or high cumulative use of prednisone, and use of thiopurines, either alone or in combination with a tumor necrosis factor (TNF) inhibitor. Although the American College of Gastroenterology recommends that all patients with IBD receive the herpes zoster vaccine by age 50 years, the efficacy of the vaccine in these patients remains unclear.
For their study, Dr. Khan and associates analyzed International Classification of Diseases (ICD) codes and other medical record data from 39,983 veterans with IBD who had not received the herpes zoster vaccine by age 60 years. In all, 97% of patients were male, and 94% were white. Most patients had high rates of health care utilization: Approximately half visited VA clinics or hospitals at least 13 times per year, and another third made 6-12 annual visits.
Despite their many contacts with VA health care systems, only 7,170 (17.9%) patients received the herpes zoster vaccine during 2000-2016, the researchers found. Vaccination rates varied substantially by region – they were highest in the Midwest (35%) and North Atlantic states (29%) but reached only 9% in Montana, Utah, Wyoming, Colorado, Oklahoma, Texas, Arkansas, and Louisiana, collectively.
The crude rate of herpes zoster infection among unvaccinated patients with IBD resembled the incidence reported in prior studies, the researchers said. After researchers accounted for differences in geography, demographics, and health care utilization between vaccinated and unvaccinated veterans with IBD, they found that vaccination was associated with an approximately 46% decrease in the risk of herpes zoster infection.
Very few patients were vaccinated for herpes zoster while on a TNF inhibitor, precluding the ability to study this subgroup. However, the vaccine showed a protective effect (adjusted HR, 0.63) among patients who received thiopurines without a TNF inhibitor. This effect did not reach statistical significance, perhaps because of lack of power, the researchers noted. “Among the 315 patients who were [vaccinated while] on thiopurines, none developed a documented painful or painless vesicular rash within 42 days of herpes zoster vaccination,” they added. One patient developed a painful blister 20 days post vaccination without vesicles or long-term sequelae.
Pfizer provided funding. Dr. Khan disclosed research funding from Pfizer, Luitpold, and Takeda Pharmaceuticals. One coinvestigator disclosed ties to Pfizer, Gilead, Merck, AbbVie, Lilly, Janssen, Johnson & Johnson, UCB, and Nestle Health Science. The remaining researchers disclosed no conflicts.
SOURCE: Khan N et al. Clin Gastroenterol Hepatol. 2018 Oct 13. doi: 10.1016/j.cgh.2018.10.016.
For men with inflammatory bowel disease, herpes zoster vaccination was associated with about a 46% decrease in risk of associated infection, according to the results of a retrospective study from the national Veterans Affairs Healthcare System.
Crude rates of herpes zoster infection were 4.09 cases per 1,000 person-years among vaccinated patients versus 6.97 cases per 1,000 person-years among unvaccinated patients, for an adjusted hazard ratio of 0.54 (95% confidence interval, 0.44-0.68), reported Nabeel Khan, MD, of the University of Pennsylvania, Philadelphia, and associates. “This vaccine is therefore effective in patients with IBD, but underused,” they wrote in Clinical Gastroenterology and Hepatology.
Studies have linked IBD with a 1.2- to 1.8-fold increased risk of herpes zoster infection, the researchers noted. Relevant risk factors include older age, disease flare, recent use or high cumulative use of prednisone, and use of thiopurines, either alone or in combination with a tumor necrosis factor (TNF) inhibitor. Although the American College of Gastroenterology recommends that all patients with IBD receive the herpes zoster vaccine by age 50 years, the efficacy of the vaccine in these patients remains unclear.
For their study, Dr. Khan and associates analyzed International Classification of Diseases (ICD) codes and other medical record data from 39,983 veterans with IBD who had not received the herpes zoster vaccine by age 60 years. In all, 97% of patients were male, and 94% were white. Most patients had high rates of health care utilization: Approximately half visited VA clinics or hospitals at least 13 times per year, and another third made 6-12 annual visits.
Despite their many contacts with VA health care systems, only 7,170 (17.9%) patients received the herpes zoster vaccine during 2000-2016, the researchers found. Vaccination rates varied substantially by region – they were highest in the Midwest (35%) and North Atlantic states (29%) but reached only 9% in Montana, Utah, Wyoming, Colorado, Oklahoma, Texas, Arkansas, and Louisiana, collectively.
The crude rate of herpes zoster infection among unvaccinated patients with IBD resembled the incidence reported in prior studies, the researchers said. After researchers accounted for differences in geography, demographics, and health care utilization between vaccinated and unvaccinated veterans with IBD, they found that vaccination was associated with an approximately 46% decrease in the risk of herpes zoster infection.
Very few patients were vaccinated for herpes zoster while on a TNF inhibitor, precluding the ability to study this subgroup. However, the vaccine showed a protective effect (adjusted HR, 0.63) among patients who received thiopurines without a TNF inhibitor. This effect did not reach statistical significance, perhaps because of lack of power, the researchers noted. “Among the 315 patients who were [vaccinated while] on thiopurines, none developed a documented painful or painless vesicular rash within 42 days of herpes zoster vaccination,” they added. One patient developed a painful blister 20 days post vaccination without vesicles or long-term sequelae.
Pfizer provided funding. Dr. Khan disclosed research funding from Pfizer, Luitpold, and Takeda Pharmaceuticals. One coinvestigator disclosed ties to Pfizer, Gilead, Merck, AbbVie, Lilly, Janssen, Johnson & Johnson, UCB, and Nestle Health Science. The remaining researchers disclosed no conflicts.
SOURCE: Khan N et al. Clin Gastroenterol Hepatol. 2018 Oct 13. doi: 10.1016/j.cgh.2018.10.016.
FROM CLINICAL GASTROENTEROLOGY AND HEPATOLOGY











