User login
Early estrogen likely prevents bone fractures in Turner syndrome
BOSTON – The longer that estrogen therapy is delayed in girls with Turner syndrome, the lower their bone density will be in subsequent years, based on results of a retrospective, cross-sectional study from Monash University, in Melbourne, Australia.
For every year after age 11 that Turner patients went without estrogen – generally due to delayed initiation, but sometimes noncompliance – there was a significant reduction in bone mineral density in both the lumbar spine (Beta -0.582, P less than 0.001) and femoral neck (Beta -0.383, P = 0.008).
Estrogen deficiency and subsequent suboptimal bone mass accrual are known to contribute to the increased risk of osteoporosis in women with Turner syndrome, and about a doubling of the risk of fragility fractures, mostly of the forearm. About a third of the 76 women in the study had at least one fracture, explained investigator Dr. Amanda Vincent, head of the Midlife Health and Menopause Program at Monash.
“Avoiding estrogen deficiency is important to optimize bone health in Turner syndrome.” It “depends on early diagnosis, age-appropriate pubertal induction, and optimization of compliance,” Dr. Vincent said at the Endocrine Society annual meeting.
The median age of Turner syndrome diagnosis was 11 years, but estrogen treatments didn’t begin until a median age of 15. The women in the study were a median of about 30 years old, which means that they were adolescents at the time when estrogen treatment was often delayed in the mistaken belief that growth hormone therapy would be more effective before puberty was induced.
It’s now known that estrogen replacement works synergistically with, and even potentiates, the effects of growth hormone. Current guidelines recommend pubertal induction by age 13 (J Clin Endocrinol Metab. 2007 Jan;92(1):10-25).
The women had at least one dual-energy x-ray absorptiometry scan at Monash since 1998. Z-scores below - 2, indicating low bone density, were found in the lumbar spines of about a quarter the subjects, and in the femoral necks of about 8%. Primary amenorrhea and premature menopause, followed by vitamin D deficiency, were the most common risk factors for low bone mass. Almost 40% of the women reported non-continuous use of estrogen. About half had undergone growth hormone therapy.
At a median height of 149 cm, the subjects were about 15 cm shorter than age-matched, healthy controls, and also had a slightly higher median body mass index of 25.6 kg/m2. Lumbar spine bone area, bone mineral content, areal bone mineral density, and bone mineral apparent density were significantly lower in Turner syndrome patients. In the femoral neck, areal bone mineral density was significantly lower.
There was no relationship between bone markers and growth hormone use or Turner syndrome karyotype; the predominant karyotype was 45XO, but the study also included mosaic karyotypes.
The investigators had no disclosures.
These are important observations. The bottom line is early recognition and early referral. It’s clear from this study and others that earlier institution of estrogen is beneficial for height, bone density, and fracture risk throughout life. It’s not just an issue of a 20 year old with low bone density; that 20 year old later becomes a 60 year old with low bone density.

|
Dr. Michael Levine |
[However,] we still have a problem with delayed recognition and referral of young girls with Turner syndrome. Most girls with Turner syndrome have some typical phenotypic features, but some do not, so the diagnosis is often made too late. [To get around that problem,] we recommend that all children below the 5th percentile for height – or who flatten out too early on growth curves – be referred to rule out Turner syndrome and other problems.
Dr. Michael Levine is chief of the Division of Endocrinology at The Children’s Hospital of Philadelphia. He made his comments after the study presentation, and was not involved in the work.
These are important observations. The bottom line is early recognition and early referral. It’s clear from this study and others that earlier institution of estrogen is beneficial for height, bone density, and fracture risk throughout life. It’s not just an issue of a 20 year old with low bone density; that 20 year old later becomes a 60 year old with low bone density.

|
Dr. Michael Levine |
[However,] we still have a problem with delayed recognition and referral of young girls with Turner syndrome. Most girls with Turner syndrome have some typical phenotypic features, but some do not, so the diagnosis is often made too late. [To get around that problem,] we recommend that all children below the 5th percentile for height – or who flatten out too early on growth curves – be referred to rule out Turner syndrome and other problems.
Dr. Michael Levine is chief of the Division of Endocrinology at The Children’s Hospital of Philadelphia. He made his comments after the study presentation, and was not involved in the work.
These are important observations. The bottom line is early recognition and early referral. It’s clear from this study and others that earlier institution of estrogen is beneficial for height, bone density, and fracture risk throughout life. It’s not just an issue of a 20 year old with low bone density; that 20 year old later becomes a 60 year old with low bone density.

|
Dr. Michael Levine |
[However,] we still have a problem with delayed recognition and referral of young girls with Turner syndrome. Most girls with Turner syndrome have some typical phenotypic features, but some do not, so the diagnosis is often made too late. [To get around that problem,] we recommend that all children below the 5th percentile for height – or who flatten out too early on growth curves – be referred to rule out Turner syndrome and other problems.
Dr. Michael Levine is chief of the Division of Endocrinology at The Children’s Hospital of Philadelphia. He made his comments after the study presentation, and was not involved in the work.
BOSTON – The longer that estrogen therapy is delayed in girls with Turner syndrome, the lower their bone density will be in subsequent years, based on results of a retrospective, cross-sectional study from Monash University, in Melbourne, Australia.
For every year after age 11 that Turner patients went without estrogen – generally due to delayed initiation, but sometimes noncompliance – there was a significant reduction in bone mineral density in both the lumbar spine (Beta -0.582, P less than 0.001) and femoral neck (Beta -0.383, P = 0.008).
Estrogen deficiency and subsequent suboptimal bone mass accrual are known to contribute to the increased risk of osteoporosis in women with Turner syndrome, and about a doubling of the risk of fragility fractures, mostly of the forearm. About a third of the 76 women in the study had at least one fracture, explained investigator Dr. Amanda Vincent, head of the Midlife Health and Menopause Program at Monash.
“Avoiding estrogen deficiency is important to optimize bone health in Turner syndrome.” It “depends on early diagnosis, age-appropriate pubertal induction, and optimization of compliance,” Dr. Vincent said at the Endocrine Society annual meeting.
The median age of Turner syndrome diagnosis was 11 years, but estrogen treatments didn’t begin until a median age of 15. The women in the study were a median of about 30 years old, which means that they were adolescents at the time when estrogen treatment was often delayed in the mistaken belief that growth hormone therapy would be more effective before puberty was induced.
It’s now known that estrogen replacement works synergistically with, and even potentiates, the effects of growth hormone. Current guidelines recommend pubertal induction by age 13 (J Clin Endocrinol Metab. 2007 Jan;92(1):10-25).
The women had at least one dual-energy x-ray absorptiometry scan at Monash since 1998. Z-scores below - 2, indicating low bone density, were found in the lumbar spines of about a quarter the subjects, and in the femoral necks of about 8%. Primary amenorrhea and premature menopause, followed by vitamin D deficiency, were the most common risk factors for low bone mass. Almost 40% of the women reported non-continuous use of estrogen. About half had undergone growth hormone therapy.
At a median height of 149 cm, the subjects were about 15 cm shorter than age-matched, healthy controls, and also had a slightly higher median body mass index of 25.6 kg/m2. Lumbar spine bone area, bone mineral content, areal bone mineral density, and bone mineral apparent density were significantly lower in Turner syndrome patients. In the femoral neck, areal bone mineral density was significantly lower.
There was no relationship between bone markers and growth hormone use or Turner syndrome karyotype; the predominant karyotype was 45XO, but the study also included mosaic karyotypes.
The investigators had no disclosures.
BOSTON – The longer that estrogen therapy is delayed in girls with Turner syndrome, the lower their bone density will be in subsequent years, based on results of a retrospective, cross-sectional study from Monash University, in Melbourne, Australia.
For every year after age 11 that Turner patients went without estrogen – generally due to delayed initiation, but sometimes noncompliance – there was a significant reduction in bone mineral density in both the lumbar spine (Beta -0.582, P less than 0.001) and femoral neck (Beta -0.383, P = 0.008).
Estrogen deficiency and subsequent suboptimal bone mass accrual are known to contribute to the increased risk of osteoporosis in women with Turner syndrome, and about a doubling of the risk of fragility fractures, mostly of the forearm. About a third of the 76 women in the study had at least one fracture, explained investigator Dr. Amanda Vincent, head of the Midlife Health and Menopause Program at Monash.
“Avoiding estrogen deficiency is important to optimize bone health in Turner syndrome.” It “depends on early diagnosis, age-appropriate pubertal induction, and optimization of compliance,” Dr. Vincent said at the Endocrine Society annual meeting.
The median age of Turner syndrome diagnosis was 11 years, but estrogen treatments didn’t begin until a median age of 15. The women in the study were a median of about 30 years old, which means that they were adolescents at the time when estrogen treatment was often delayed in the mistaken belief that growth hormone therapy would be more effective before puberty was induced.
It’s now known that estrogen replacement works synergistically with, and even potentiates, the effects of growth hormone. Current guidelines recommend pubertal induction by age 13 (J Clin Endocrinol Metab. 2007 Jan;92(1):10-25).
The women had at least one dual-energy x-ray absorptiometry scan at Monash since 1998. Z-scores below - 2, indicating low bone density, were found in the lumbar spines of about a quarter the subjects, and in the femoral necks of about 8%. Primary amenorrhea and premature menopause, followed by vitamin D deficiency, were the most common risk factors for low bone mass. Almost 40% of the women reported non-continuous use of estrogen. About half had undergone growth hormone therapy.
At a median height of 149 cm, the subjects were about 15 cm shorter than age-matched, healthy controls, and also had a slightly higher median body mass index of 25.6 kg/m2. Lumbar spine bone area, bone mineral content, areal bone mineral density, and bone mineral apparent density were significantly lower in Turner syndrome patients. In the femoral neck, areal bone mineral density was significantly lower.
There was no relationship between bone markers and growth hormone use or Turner syndrome karyotype; the predominant karyotype was 45XO, but the study also included mosaic karyotypes.
The investigators had no disclosures.
AT ENDO 2016
Key clinical point: Induce puberty by age 13 in Turner syndrome.
Major finding: For every year after age 11 that Turner patients went without estrogen – generally due to delayed initiation, but sometimes noncompliance – there was a significant reduction in bone mineral density in both the lubar spine (Beta -0.582, P less than 0.001) and femoral neck (Beta -0.383, P = 0.008).
Data source: Retrospective, cross-section study of 76 Turner syndrome patients
Disclosures: The investigators had no disclosures.
Higher levels of function before hip fracture tied to greater fears of falling at 1 year
WASHINGTON – Fear of falling at 12 weeks was associated with poorer functional recovery up to 1 year after hip fracture, particularly if the person had a high level of function before the fracture, a study has shown.
Inherent in this fear is a tendency of the patient to limit his activities, which in turn affects his sense of balance, visual attention, and gait. This leads to an increased risk of further falls, according to Emily Bower, a doctoral candidate who presented the findings at the annual meeting of the American Association for Geriatric Psychiatry.
The study included 241 hip fracture patients, three-quarters of whom were female, with an average age of 77 years. All of the patients lived in the community, nearly all were able to participate in basic activities of daily living, and three-quarters could walk without assistance.
Patients were assessed for their level of functionality at week 4, week 12, week 26, and week 52, as well as their fear of falling using the Falls Efficacy Scale International (FES-I).
The investigators found that by week 52, 48% of all patients had reached full recovery of functional status. At week 12, 53% of all patients had FES-I scores indicating a lower fear of falling.
“That means that almost half of our participants were reporting high fear of falling 3 months after hip fracture, which is a substantial number when you consider that in the general population, fear of falling is reported by about one in five older adults,” Ms. Bower, who is enrolled at the San Diego State University/University of California, San Diego, joint doctoral program in clinical psychology, said in an interview.
At 1-year after fracture, for each 1 point increase in FES-I scores, the odds of reaching full recovery were lowered by 12% (P = .003). Meanwhile, in patients with low baseline levels of functioning, there was “no effect of fear of falling,” according to Ms. Bower.
The upshot is that for patients with high levels of functional independence prior to injury, more intervention may be required to bring them back to their levels of activity before the fracture, according to Ms. Bower, who suggested multicomponent activities that combine psychological, physical, and environmental factors, such as tai chi.
On Twitter @whitneymcknight
WASHINGTON – Fear of falling at 12 weeks was associated with poorer functional recovery up to 1 year after hip fracture, particularly if the person had a high level of function before the fracture, a study has shown.
Inherent in this fear is a tendency of the patient to limit his activities, which in turn affects his sense of balance, visual attention, and gait. This leads to an increased risk of further falls, according to Emily Bower, a doctoral candidate who presented the findings at the annual meeting of the American Association for Geriatric Psychiatry.
The study included 241 hip fracture patients, three-quarters of whom were female, with an average age of 77 years. All of the patients lived in the community, nearly all were able to participate in basic activities of daily living, and three-quarters could walk without assistance.
Patients were assessed for their level of functionality at week 4, week 12, week 26, and week 52, as well as their fear of falling using the Falls Efficacy Scale International (FES-I).
The investigators found that by week 52, 48% of all patients had reached full recovery of functional status. At week 12, 53% of all patients had FES-I scores indicating a lower fear of falling.
“That means that almost half of our participants were reporting high fear of falling 3 months after hip fracture, which is a substantial number when you consider that in the general population, fear of falling is reported by about one in five older adults,” Ms. Bower, who is enrolled at the San Diego State University/University of California, San Diego, joint doctoral program in clinical psychology, said in an interview.
At 1-year after fracture, for each 1 point increase in FES-I scores, the odds of reaching full recovery were lowered by 12% (P = .003). Meanwhile, in patients with low baseline levels of functioning, there was “no effect of fear of falling,” according to Ms. Bower.
The upshot is that for patients with high levels of functional independence prior to injury, more intervention may be required to bring them back to their levels of activity before the fracture, according to Ms. Bower, who suggested multicomponent activities that combine psychological, physical, and environmental factors, such as tai chi.
On Twitter @whitneymcknight
WASHINGTON – Fear of falling at 12 weeks was associated with poorer functional recovery up to 1 year after hip fracture, particularly if the person had a high level of function before the fracture, a study has shown.
Inherent in this fear is a tendency of the patient to limit his activities, which in turn affects his sense of balance, visual attention, and gait. This leads to an increased risk of further falls, according to Emily Bower, a doctoral candidate who presented the findings at the annual meeting of the American Association for Geriatric Psychiatry.
The study included 241 hip fracture patients, three-quarters of whom were female, with an average age of 77 years. All of the patients lived in the community, nearly all were able to participate in basic activities of daily living, and three-quarters could walk without assistance.
Patients were assessed for their level of functionality at week 4, week 12, week 26, and week 52, as well as their fear of falling using the Falls Efficacy Scale International (FES-I).
The investigators found that by week 52, 48% of all patients had reached full recovery of functional status. At week 12, 53% of all patients had FES-I scores indicating a lower fear of falling.
“That means that almost half of our participants were reporting high fear of falling 3 months after hip fracture, which is a substantial number when you consider that in the general population, fear of falling is reported by about one in five older adults,” Ms. Bower, who is enrolled at the San Diego State University/University of California, San Diego, joint doctoral program in clinical psychology, said in an interview.
At 1-year after fracture, for each 1 point increase in FES-I scores, the odds of reaching full recovery were lowered by 12% (P = .003). Meanwhile, in patients with low baseline levels of functioning, there was “no effect of fear of falling,” according to Ms. Bower.
The upshot is that for patients with high levels of functional independence prior to injury, more intervention may be required to bring them back to their levels of activity before the fracture, according to Ms. Bower, who suggested multicomponent activities that combine psychological, physical, and environmental factors, such as tai chi.
On Twitter @whitneymcknight
EXPERT ANALYSIS FROM AAGP 2016
Antisclerostin osteoporosis drugs might worsen or unmask rheumatoid arthritis
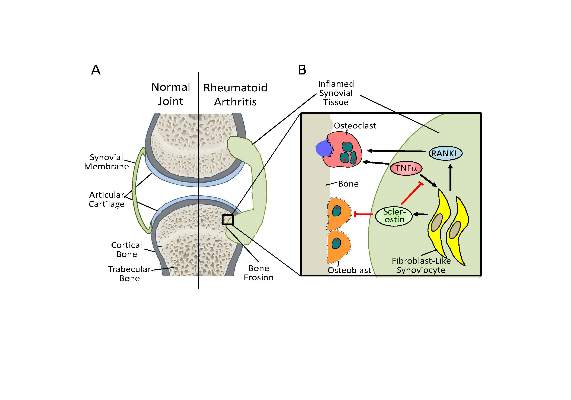
Antisclerostin monoclonal antibodies have shown their ability to increase bone density in phase II and III trials of men and women with osteoporosis but could potentially have the opposite effect in patients with rheumatoid arthritis or other chronic inflammatory diseases in which tumor necrosis factor–alpha (TNF-alpha) plays an important role, according to new research.
The new work, conducted by Corinna Wehmeyer, Ph.D., of the Institute of Experimental Musculoskeletal Medicine at University Hospital Muenster (Germany) and her colleagues, shows that the bone formation–inhibiting protein sclerostin is not expressed in bone only, as was previously thought, but is also expressed on the synovial cells of patients with rheumatoid arthritis (RA).
Dr. Wehmeyer and her associates were surprised to find that inhibiting sclerostin in a human TNF-alpha transgenic mouse model of RA actually accelerated joint damage rather than prevented it, suggesting that sclerostin actually had a protective role in the presence of chronic TNF-alpha–mediated inflammation. They confirmed this by demonstrating that sclerostin inhibited TNF-alpha signaling in fibroblast-like synoviocytes and showing that blocking sclerostin caused less or little worsening of bone erosions in mouse models of RA that are more dependent on a robust T and B cell response accompanied by high cytokine expression within the joint, rather than damage driven by TNF-alpha.
“These findings strongly suggest that in chronic TNF-alpha–mediated inflammation, sclerostin expression is upregulated as part of an attempt to reestablish bone homeostasis, where it exerts protective functions,” the authors wrote (Sci Transl Med. 2016 Mar 16;8:330ra34. doi: 10.1126/scitranslmed.aac4351).
The research needs confirmation in humans with RA and potentially in other chronic inflammatory diseases in which TNF-alpha plays an important role. “Nevertheless, the preliminary data in three different models indicate that sclerostin antibody therapy could be contraindicated in patients with chronic TNF-alpha–dependent inflammatory conditions. The possibility of adverse pathological effects means that caution should be taken both when considering such treatment in RA or in patients with chronic TNF-alpha–dependent comorbidities. Thus, to translate these findings to patients, first strategies to use sclerostin inhibition should exclude inflammatory comorbidities and very thoroughly monitor inflammatory events in patients to which such therapies are applied,” the researchers advised.
In an editorial, Dr. Frank Rauch of McGill University, Montreal, and Dr. Rick Adachi of the department of rheumatology at McMaster University, Hamilton, Ont., wrote that antisclerostin “treatment might accelerate joint destruction, at least when the inflammatory process is not quelled first. Patients with established RA usually undergo anti-inflammatory treatment, and it is unclear whether sclerostin inactivation would be detrimental in this context. Mouse data suggest that antisclerostin treatment might bring about regression of bone erosions when combined with TNF-alpha inhibition. The new work mirrors the situation of patients who have unrecognized RA while on antisclerostin therapy or who develop RA while receiving this treatment” (Sci Transl Med. 2016 Mar 16;8:330fs7. doi: 10.1126/scitranslmed.aaf4628).
Antisclerostin antibodies in trials
Trials of the antisclerostin monoclonal antibodies romosozumab and blosozumab have been successful in treating postmenopausal women and men with osteoporosis.
Romosozumab codevelopers UCB and Amgen reported that the biologic agent significantly reduced the rate of new vertebral fractures by 73% versus placebo at 12 months in the randomized, double-blind phase III FRAME (Fracture Study in Postmenopausal Women With Osteoporosis) study. In the 7,180-patient trial, the reduction was 75% versus placebo at 24 months after both treatment groups had been transitioned to denosumab given every 6 months in the second year of treatment. Romosozumab also significantly lowered the relative risk of clinical fractures (composite of vertebral and nonvertebral fractures) by 36% at 12 months, but the difference was not statistically significant at 24 months.
In the initial 12-month treatment period, the most commonly reported adverse events in both arms (greater than 10%) were arthralgia, nasopharyngitis, and back pain. There were no differences in the proportions of patients who reported hearing loss or worsening of knee osteoarthritis. There were two positively adjudicated events of osteonecrosis of the jaw in the romosozumab treatment group, one after completing romosozumab dosing and the other after completing romosozumab treatment and receiving the initial dose of denosumab. There was one positively adjudicated event of atypical femoral fracture after 3 months of romosozumab treatment.
Phase III results from the 244-patient BRIDGE (Placebo-Controlled Study Evaluating the Efficacy and Safety of Romosozumab in Treating Men With Osteoporosis) trial found a significant increase in bone mineral density (BMD) at the lumbar spine at 12 months, which was the study’s primary endpoint. Other significant increases in femoral neck and total hip BMD were detected at 12 months. Cardiovascular severe adverse events occurred in 4.9% of men on romosozumab and 2.5% on placebo, including death in 0.6% and 1.2%, respectively. At least 5% or more of patients who received romosozumab reported nasopharyngitis, back pain, hypertension, headache, and constipation. About 5% of patients who received romosozumab in each trial had injection-site reactions, most of which were mild.
A phase II trial of blosozumab in 120 postmenopausal women with low bone mineral density (mean lumbar spine T-score –2.8) showed that the drug increased BMD in the lumbar spine by 17.7% above baseline at 52 weeks, femoral neck by 8.4%, and total hip by 6.2%, compared with decreases of 1.6%, 0.6%, and 0.7%, respectively, with placebo (J Bone Miner Res. 2015 Feb;30[2]:216-24). However, mild injection-site reactions were reported by up to 40% of women taking blosozumab, and 35% developed antidrug antibodies after exposure to blosozumab. Eli Lilly, its developer, is looking at possible ways to reformulate the drug before it moves to phase III.
The study in Science Translational Medicine was supported by the German Research Foundation. The authors had no competing interests to disclose.
Antisclerostin monoclonal antibodies have shown their ability to increase bone density in phase II and III trials of men and women with osteoporosis but could potentially have the opposite effect in patients with rheumatoid arthritis or other chronic inflammatory diseases in which tumor necrosis factor–alpha (TNF-alpha) plays an important role, according to new research.
The new work, conducted by Corinna Wehmeyer, Ph.D., of the Institute of Experimental Musculoskeletal Medicine at University Hospital Muenster (Germany) and her colleagues, shows that the bone formation–inhibiting protein sclerostin is not expressed in bone only, as was previously thought, but is also expressed on the synovial cells of patients with rheumatoid arthritis (RA).
Dr. Wehmeyer and her associates were surprised to find that inhibiting sclerostin in a human TNF-alpha transgenic mouse model of RA actually accelerated joint damage rather than prevented it, suggesting that sclerostin actually had a protective role in the presence of chronic TNF-alpha–mediated inflammation. They confirmed this by demonstrating that sclerostin inhibited TNF-alpha signaling in fibroblast-like synoviocytes and showing that blocking sclerostin caused less or little worsening of bone erosions in mouse models of RA that are more dependent on a robust T and B cell response accompanied by high cytokine expression within the joint, rather than damage driven by TNF-alpha.
“These findings strongly suggest that in chronic TNF-alpha–mediated inflammation, sclerostin expression is upregulated as part of an attempt to reestablish bone homeostasis, where it exerts protective functions,” the authors wrote (Sci Transl Med. 2016 Mar 16;8:330ra34. doi: 10.1126/scitranslmed.aac4351).
The research needs confirmation in humans with RA and potentially in other chronic inflammatory diseases in which TNF-alpha plays an important role. “Nevertheless, the preliminary data in three different models indicate that sclerostin antibody therapy could be contraindicated in patients with chronic TNF-alpha–dependent inflammatory conditions. The possibility of adverse pathological effects means that caution should be taken both when considering such treatment in RA or in patients with chronic TNF-alpha–dependent comorbidities. Thus, to translate these findings to patients, first strategies to use sclerostin inhibition should exclude inflammatory comorbidities and very thoroughly monitor inflammatory events in patients to which such therapies are applied,” the researchers advised.
In an editorial, Dr. Frank Rauch of McGill University, Montreal, and Dr. Rick Adachi of the department of rheumatology at McMaster University, Hamilton, Ont., wrote that antisclerostin “treatment might accelerate joint destruction, at least when the inflammatory process is not quelled first. Patients with established RA usually undergo anti-inflammatory treatment, and it is unclear whether sclerostin inactivation would be detrimental in this context. Mouse data suggest that antisclerostin treatment might bring about regression of bone erosions when combined with TNF-alpha inhibition. The new work mirrors the situation of patients who have unrecognized RA while on antisclerostin therapy or who develop RA while receiving this treatment” (Sci Transl Med. 2016 Mar 16;8:330fs7. doi: 10.1126/scitranslmed.aaf4628).
Antisclerostin antibodies in trials
Trials of the antisclerostin monoclonal antibodies romosozumab and blosozumab have been successful in treating postmenopausal women and men with osteoporosis.
Romosozumab codevelopers UCB and Amgen reported that the biologic agent significantly reduced the rate of new vertebral fractures by 73% versus placebo at 12 months in the randomized, double-blind phase III FRAME (Fracture Study in Postmenopausal Women With Osteoporosis) study. In the 7,180-patient trial, the reduction was 75% versus placebo at 24 months after both treatment groups had been transitioned to denosumab given every 6 months in the second year of treatment. Romosozumab also significantly lowered the relative risk of clinical fractures (composite of vertebral and nonvertebral fractures) by 36% at 12 months, but the difference was not statistically significant at 24 months.
In the initial 12-month treatment period, the most commonly reported adverse events in both arms (greater than 10%) were arthralgia, nasopharyngitis, and back pain. There were no differences in the proportions of patients who reported hearing loss or worsening of knee osteoarthritis. There were two positively adjudicated events of osteonecrosis of the jaw in the romosozumab treatment group, one after completing romosozumab dosing and the other after completing romosozumab treatment and receiving the initial dose of denosumab. There was one positively adjudicated event of atypical femoral fracture after 3 months of romosozumab treatment.
Phase III results from the 244-patient BRIDGE (Placebo-Controlled Study Evaluating the Efficacy and Safety of Romosozumab in Treating Men With Osteoporosis) trial found a significant increase in bone mineral density (BMD) at the lumbar spine at 12 months, which was the study’s primary endpoint. Other significant increases in femoral neck and total hip BMD were detected at 12 months. Cardiovascular severe adverse events occurred in 4.9% of men on romosozumab and 2.5% on placebo, including death in 0.6% and 1.2%, respectively. At least 5% or more of patients who received romosozumab reported nasopharyngitis, back pain, hypertension, headache, and constipation. About 5% of patients who received romosozumab in each trial had injection-site reactions, most of which were mild.
A phase II trial of blosozumab in 120 postmenopausal women with low bone mineral density (mean lumbar spine T-score –2.8) showed that the drug increased BMD in the lumbar spine by 17.7% above baseline at 52 weeks, femoral neck by 8.4%, and total hip by 6.2%, compared with decreases of 1.6%, 0.6%, and 0.7%, respectively, with placebo (J Bone Miner Res. 2015 Feb;30[2]:216-24). However, mild injection-site reactions were reported by up to 40% of women taking blosozumab, and 35% developed antidrug antibodies after exposure to blosozumab. Eli Lilly, its developer, is looking at possible ways to reformulate the drug before it moves to phase III.
The study in Science Translational Medicine was supported by the German Research Foundation. The authors had no competing interests to disclose.
Antisclerostin monoclonal antibodies have shown their ability to increase bone density in phase II and III trials of men and women with osteoporosis but could potentially have the opposite effect in patients with rheumatoid arthritis or other chronic inflammatory diseases in which tumor necrosis factor–alpha (TNF-alpha) plays an important role, according to new research.
The new work, conducted by Corinna Wehmeyer, Ph.D., of the Institute of Experimental Musculoskeletal Medicine at University Hospital Muenster (Germany) and her colleagues, shows that the bone formation–inhibiting protein sclerostin is not expressed in bone only, as was previously thought, but is also expressed on the synovial cells of patients with rheumatoid arthritis (RA).
Dr. Wehmeyer and her associates were surprised to find that inhibiting sclerostin in a human TNF-alpha transgenic mouse model of RA actually accelerated joint damage rather than prevented it, suggesting that sclerostin actually had a protective role in the presence of chronic TNF-alpha–mediated inflammation. They confirmed this by demonstrating that sclerostin inhibited TNF-alpha signaling in fibroblast-like synoviocytes and showing that blocking sclerostin caused less or little worsening of bone erosions in mouse models of RA that are more dependent on a robust T and B cell response accompanied by high cytokine expression within the joint, rather than damage driven by TNF-alpha.
“These findings strongly suggest that in chronic TNF-alpha–mediated inflammation, sclerostin expression is upregulated as part of an attempt to reestablish bone homeostasis, where it exerts protective functions,” the authors wrote (Sci Transl Med. 2016 Mar 16;8:330ra34. doi: 10.1126/scitranslmed.aac4351).
The research needs confirmation in humans with RA and potentially in other chronic inflammatory diseases in which TNF-alpha plays an important role. “Nevertheless, the preliminary data in three different models indicate that sclerostin antibody therapy could be contraindicated in patients with chronic TNF-alpha–dependent inflammatory conditions. The possibility of adverse pathological effects means that caution should be taken both when considering such treatment in RA or in patients with chronic TNF-alpha–dependent comorbidities. Thus, to translate these findings to patients, first strategies to use sclerostin inhibition should exclude inflammatory comorbidities and very thoroughly monitor inflammatory events in patients to which such therapies are applied,” the researchers advised.
In an editorial, Dr. Frank Rauch of McGill University, Montreal, and Dr. Rick Adachi of the department of rheumatology at McMaster University, Hamilton, Ont., wrote that antisclerostin “treatment might accelerate joint destruction, at least when the inflammatory process is not quelled first. Patients with established RA usually undergo anti-inflammatory treatment, and it is unclear whether sclerostin inactivation would be detrimental in this context. Mouse data suggest that antisclerostin treatment might bring about regression of bone erosions when combined with TNF-alpha inhibition. The new work mirrors the situation of patients who have unrecognized RA while on antisclerostin therapy or who develop RA while receiving this treatment” (Sci Transl Med. 2016 Mar 16;8:330fs7. doi: 10.1126/scitranslmed.aaf4628).
Antisclerostin antibodies in trials
Trials of the antisclerostin monoclonal antibodies romosozumab and blosozumab have been successful in treating postmenopausal women and men with osteoporosis.
Romosozumab codevelopers UCB and Amgen reported that the biologic agent significantly reduced the rate of new vertebral fractures by 73% versus placebo at 12 months in the randomized, double-blind phase III FRAME (Fracture Study in Postmenopausal Women With Osteoporosis) study. In the 7,180-patient trial, the reduction was 75% versus placebo at 24 months after both treatment groups had been transitioned to denosumab given every 6 months in the second year of treatment. Romosozumab also significantly lowered the relative risk of clinical fractures (composite of vertebral and nonvertebral fractures) by 36% at 12 months, but the difference was not statistically significant at 24 months.
In the initial 12-month treatment period, the most commonly reported adverse events in both arms (greater than 10%) were arthralgia, nasopharyngitis, and back pain. There were no differences in the proportions of patients who reported hearing loss or worsening of knee osteoarthritis. There were two positively adjudicated events of osteonecrosis of the jaw in the romosozumab treatment group, one after completing romosozumab dosing and the other after completing romosozumab treatment and receiving the initial dose of denosumab. There was one positively adjudicated event of atypical femoral fracture after 3 months of romosozumab treatment.
Phase III results from the 244-patient BRIDGE (Placebo-Controlled Study Evaluating the Efficacy and Safety of Romosozumab in Treating Men With Osteoporosis) trial found a significant increase in bone mineral density (BMD) at the lumbar spine at 12 months, which was the study’s primary endpoint. Other significant increases in femoral neck and total hip BMD were detected at 12 months. Cardiovascular severe adverse events occurred in 4.9% of men on romosozumab and 2.5% on placebo, including death in 0.6% and 1.2%, respectively. At least 5% or more of patients who received romosozumab reported nasopharyngitis, back pain, hypertension, headache, and constipation. About 5% of patients who received romosozumab in each trial had injection-site reactions, most of which were mild.
A phase II trial of blosozumab in 120 postmenopausal women with low bone mineral density (mean lumbar spine T-score –2.8) showed that the drug increased BMD in the lumbar spine by 17.7% above baseline at 52 weeks, femoral neck by 8.4%, and total hip by 6.2%, compared with decreases of 1.6%, 0.6%, and 0.7%, respectively, with placebo (J Bone Miner Res. 2015 Feb;30[2]:216-24). However, mild injection-site reactions were reported by up to 40% of women taking blosozumab, and 35% developed antidrug antibodies after exposure to blosozumab. Eli Lilly, its developer, is looking at possible ways to reformulate the drug before it moves to phase III.
The study in Science Translational Medicine was supported by the German Research Foundation. The authors had no competing interests to disclose.
FROM SCIENCE TRANSLATIONAL MEDICINE
Getting a leg up on bone comorbidities in lupus
MAUI, HAWAII – Patients with systemic lupus erythematosus (SLE) really need to be placed on bone protection therapy as soon as they start on corticosteroids because their risks of steroid-related osteoporosis and osteonecrosis are so high, Dr. Dafna D. Gladman advised at the 2016 Rheumatology Winter Clinical Symposium.
Investigators for new studies of large cohorts of SLE patients seen at the University of Toronto Lupus Clinic have examined numerous potential predictors of bone comorbidities, but only one independent risk factor emerged: a high cumulative dose of corticosteroids, said Dr. Gladman, professor of medicine and codirector of the clinic.
“The dose of steroids is certainly important. It’s relevant to patient management because we obviously want to try to minimize the amount of steroids that patients with lupus get,” the rheumatologist observed.
The Toronto experience underscores just how common and serious these bone comorbidities are.
Among 1,729 SLE patients in the clinic database, 13.6% developed symptomatic osteonecrosis as defined by clinical symptoms plus positive imaging findings. Overall, 86% were female. The mean age at diagnosis of SLE was 26.6 years, with a mean 8.2-year interval from SLE diagnosis to the first episode of osteonecrosis. The 235 patients with osteonecrosis had a collective 382 affected joints at the time of their first osteonecrosis diagnosis, with an additional 160 joints becoming osteonecrotic later.
Particularly noteworthy was the finding that fully 47% of patients had more than one site involved at the time of their first osteonecrotic event, according to Dr. Gladman.
By far the most frequently affected joints were the hips, followed by knees. Surgery was often required in order to manage the injuries. Indeed, surgery was performed on more than half of osteonecrotic hips, 30% of affected wrists, and 20% of knees.
In a multivariate regression analysis involving 162 SLE patients with osteonecrosis and an equal number of matched controls who had SLE but not osteonecrosis, the only independent predictor of osteonecrosis was a high cumulative dose of corticosteroids; in the osteonecrosis group, it averaged 31 g. Factors that didn’t pan out as predictors included patient age, gender, race, smoking status, current or past use of antimalarial drugs, duration of immunosuppressive therapy, disease severity as reflected by the adjusted mean SLE Disease Activity Index score 3 years prior to osteonecrosis or the last clinic visit, total serum cholesterol, and a history of Raynaud’s, vasculitis, or renal or CNS involvement.
Turning to osteoporosis in SLE patients, Dr. Gladman said that among 286 patients who underwent bone mineral density (BMD) measurement at the time they were first seen in the clinic, 31.5% had an abnormal result. Among the 173 premenopausal females, 17% had a BMD below the lower limit of normal for their age. So did 27% of men below age 50. In postmenopausal women, the prevalences of osteoporosis and osteopenia were 12% and 43%, respectively. One of 10 men over age 50 had osteoporosis, while 8 had low BMD.
Twenty patients had a symptomatic fragility fracture at the time of their BMD test, and, of note, only half of them had an abnormal BMD.
“So the BMD does not actually identify all those patients who are at risk for the adverse outcome of osteoporosis, which will be a fragility fracture,” Dr. Gladman said.
She reported having no financial conflicts of interest regarding her presentation.
MAUI, HAWAII – Patients with systemic lupus erythematosus (SLE) really need to be placed on bone protection therapy as soon as they start on corticosteroids because their risks of steroid-related osteoporosis and osteonecrosis are so high, Dr. Dafna D. Gladman advised at the 2016 Rheumatology Winter Clinical Symposium.
Investigators for new studies of large cohorts of SLE patients seen at the University of Toronto Lupus Clinic have examined numerous potential predictors of bone comorbidities, but only one independent risk factor emerged: a high cumulative dose of corticosteroids, said Dr. Gladman, professor of medicine and codirector of the clinic.
“The dose of steroids is certainly important. It’s relevant to patient management because we obviously want to try to minimize the amount of steroids that patients with lupus get,” the rheumatologist observed.
The Toronto experience underscores just how common and serious these bone comorbidities are.
Among 1,729 SLE patients in the clinic database, 13.6% developed symptomatic osteonecrosis as defined by clinical symptoms plus positive imaging findings. Overall, 86% were female. The mean age at diagnosis of SLE was 26.6 years, with a mean 8.2-year interval from SLE diagnosis to the first episode of osteonecrosis. The 235 patients with osteonecrosis had a collective 382 affected joints at the time of their first osteonecrosis diagnosis, with an additional 160 joints becoming osteonecrotic later.
Particularly noteworthy was the finding that fully 47% of patients had more than one site involved at the time of their first osteonecrotic event, according to Dr. Gladman.
By far the most frequently affected joints were the hips, followed by knees. Surgery was often required in order to manage the injuries. Indeed, surgery was performed on more than half of osteonecrotic hips, 30% of affected wrists, and 20% of knees.
In a multivariate regression analysis involving 162 SLE patients with osteonecrosis and an equal number of matched controls who had SLE but not osteonecrosis, the only independent predictor of osteonecrosis was a high cumulative dose of corticosteroids; in the osteonecrosis group, it averaged 31 g. Factors that didn’t pan out as predictors included patient age, gender, race, smoking status, current or past use of antimalarial drugs, duration of immunosuppressive therapy, disease severity as reflected by the adjusted mean SLE Disease Activity Index score 3 years prior to osteonecrosis or the last clinic visit, total serum cholesterol, and a history of Raynaud’s, vasculitis, or renal or CNS involvement.
Turning to osteoporosis in SLE patients, Dr. Gladman said that among 286 patients who underwent bone mineral density (BMD) measurement at the time they were first seen in the clinic, 31.5% had an abnormal result. Among the 173 premenopausal females, 17% had a BMD below the lower limit of normal for their age. So did 27% of men below age 50. In postmenopausal women, the prevalences of osteoporosis and osteopenia were 12% and 43%, respectively. One of 10 men over age 50 had osteoporosis, while 8 had low BMD.
Twenty patients had a symptomatic fragility fracture at the time of their BMD test, and, of note, only half of them had an abnormal BMD.
“So the BMD does not actually identify all those patients who are at risk for the adverse outcome of osteoporosis, which will be a fragility fracture,” Dr. Gladman said.
She reported having no financial conflicts of interest regarding her presentation.
MAUI, HAWAII – Patients with systemic lupus erythematosus (SLE) really need to be placed on bone protection therapy as soon as they start on corticosteroids because their risks of steroid-related osteoporosis and osteonecrosis are so high, Dr. Dafna D. Gladman advised at the 2016 Rheumatology Winter Clinical Symposium.
Investigators for new studies of large cohorts of SLE patients seen at the University of Toronto Lupus Clinic have examined numerous potential predictors of bone comorbidities, but only one independent risk factor emerged: a high cumulative dose of corticosteroids, said Dr. Gladman, professor of medicine and codirector of the clinic.
“The dose of steroids is certainly important. It’s relevant to patient management because we obviously want to try to minimize the amount of steroids that patients with lupus get,” the rheumatologist observed.
The Toronto experience underscores just how common and serious these bone comorbidities are.
Among 1,729 SLE patients in the clinic database, 13.6% developed symptomatic osteonecrosis as defined by clinical symptoms plus positive imaging findings. Overall, 86% were female. The mean age at diagnosis of SLE was 26.6 years, with a mean 8.2-year interval from SLE diagnosis to the first episode of osteonecrosis. The 235 patients with osteonecrosis had a collective 382 affected joints at the time of their first osteonecrosis diagnosis, with an additional 160 joints becoming osteonecrotic later.
Particularly noteworthy was the finding that fully 47% of patients had more than one site involved at the time of their first osteonecrotic event, according to Dr. Gladman.
By far the most frequently affected joints were the hips, followed by knees. Surgery was often required in order to manage the injuries. Indeed, surgery was performed on more than half of osteonecrotic hips, 30% of affected wrists, and 20% of knees.
In a multivariate regression analysis involving 162 SLE patients with osteonecrosis and an equal number of matched controls who had SLE but not osteonecrosis, the only independent predictor of osteonecrosis was a high cumulative dose of corticosteroids; in the osteonecrosis group, it averaged 31 g. Factors that didn’t pan out as predictors included patient age, gender, race, smoking status, current or past use of antimalarial drugs, duration of immunosuppressive therapy, disease severity as reflected by the adjusted mean SLE Disease Activity Index score 3 years prior to osteonecrosis or the last clinic visit, total serum cholesterol, and a history of Raynaud’s, vasculitis, or renal or CNS involvement.
Turning to osteoporosis in SLE patients, Dr. Gladman said that among 286 patients who underwent bone mineral density (BMD) measurement at the time they were first seen in the clinic, 31.5% had an abnormal result. Among the 173 premenopausal females, 17% had a BMD below the lower limit of normal for their age. So did 27% of men below age 50. In postmenopausal women, the prevalences of osteoporosis and osteopenia were 12% and 43%, respectively. One of 10 men over age 50 had osteoporosis, while 8 had low BMD.
Twenty patients had a symptomatic fragility fracture at the time of their BMD test, and, of note, only half of them had an abnormal BMD.
“So the BMD does not actually identify all those patients who are at risk for the adverse outcome of osteoporosis, which will be a fragility fracture,” Dr. Gladman said.
She reported having no financial conflicts of interest regarding her presentation.
EXPERT ANALYSIS FROM RWCS 2016
ACR’s 2016-2020 research agenda built through consensus
Therapeutic goals set the tone for the American College of Rheumatology National Research Agenda 2016-2020 by calling for the discovery and development of new therapies for rheumatic disease; finding predictors of response and nonresponse to, and adverse events from therapy; and improving the understanding of how therapies should be used.
Those are the top 3 out of 15 goals facilitated by the ACR’s Committee on Research, which finalized the agenda after seeking input from members of the ACR and Association of Rheumatology Health Professionals (ARHP) living in the United States, and going through several rounds of refining and prioritizing the importance of goals through the input of clinicians, researchers, patients, and stakeholders. The Committee on Research uses the agenda to “set the compass for the organization in terms of research initiatives and facilitate the ACR’s advocacy for the research goals identified.”
Dr. Alexis R. Ogdie-Beatty, who jointly led the development of the agenda for the Committee on Research along with Dr. S. Louis Bridges, said that while the goals for 2016-2020 had a great deal of overlap with those of 2011-2015, “some of the topics that came up were different. Some of the topics were more specific than in the previous agenda. We have some idea how important these issues were to rheumatologists, given that rheumatologists (and patients) rated the importance of the items. Defining new therapeutic targets and developing new therapies for rheumatic diseases was by far the most highly rated goal by rheumatologists. Next most highly rated was to advocate for increased support for rheumatology research and rheumatology investigators – this was included as a supplementary goal that supports the rest of the agenda. Other newer items were those around determining how the changing health care landscape affects rheumatology patients and clinicians. In addition, nonpharmacologic therapy, adult outcomes of pediatric disease, and optimizing patient engagement were topics that were felt to be important. I think these highlight the input of clinicians in identifying research objectives.”
The 2016-2020 agenda is the third set of goals developed by the committee since 2005, and the first to “crowdsource” the important questions to ACR and ARHP members rather than be assembled solely by the committee.
The agenda arose from a multistage process that began with a web-based survey to the ACR/ARHP membership that asked respondents to “list the five most important research questions that need to be addressed over the next 5 years in order to improve the care for patients with rheumatic disease.” A selected group of 100 individuals representing patients, clinicians (academic and community), research (all types with diverse areas/diseases of interest), allied health professionals, pediatric and adult rheumatology, men and women, all career stages, and all regions of the country, used a Delphi exercise to rate 30 statements generated from the survey on a scale from 1 (not important) to 10 (very important). They had the option to provide comments. At a Leadership Summit, stakeholders from various nonprofit foundations associated with rheumatic diseases, the National Institutes of Health, and the president of the Rheumatology Research Foundation gave comments on a draft agenda to the Committee on Research, after which the committee discussed the results and input and then solicited further 1-10 ratings and comments on preliminary agenda goals from the same group of 100 individuals as in the second phase, plus an additional 17 clinicians.
Up next in the rank-ordering after therapeutic goals were three goals about understanding:
• The etiology, pathogenesis, and genetic basis of rheumatic diseases.
• Early disease states to improve early diagnosis, develop biomarkers for early detection, and determine how earlier treatment changes outcomes.
• The immune system and autoimmunity by defining autoimmunity triggers and determining how epigenetics affect disease susceptibility and inflammation.
The 5-year plan proposed developing improved outcome measures that incorporate patient self-reports, imaging, and measures of clinical response and disease activity. The agenda also seeks to gain better understanding of how patients with rheumatic disease, rheumatologists, and rheumatology health professionals are being affected by the changing U.S. health care landscape.
The plan calls for determining the role of nonpharmacologic therapy in the management of rheumatic disease (promoting and improving adherence to physical activity, finding optimal exercise prescriptions, and determining the role of diet on disease activity), as well as evaluating the role of regenerative medicine.
The agenda spells out the need for better engagement of patients in their care as well as for understanding how comorbidities are influenced by rheumatic disease and how pain and fatigue arise in rheumatic disease.
In two separate goals, committee members listed the importance of determining adult outcomes of pediatric rheumatic diseases and the effect of aging on the development, progression, and management of rheumatic diseases.
The Committee on Research identified three supplemental goals that support the others:
• Advocating for increased support for rheumatology research and rheumatology investigators.
• Harmonizing data from existing cohorts and registries to optimize research capabilities.
• Improving patient research partner involvement in research protocols.
Therapeutic goals set the tone for the American College of Rheumatology National Research Agenda 2016-2020 by calling for the discovery and development of new therapies for rheumatic disease; finding predictors of response and nonresponse to, and adverse events from therapy; and improving the understanding of how therapies should be used.
Those are the top 3 out of 15 goals facilitated by the ACR’s Committee on Research, which finalized the agenda after seeking input from members of the ACR and Association of Rheumatology Health Professionals (ARHP) living in the United States, and going through several rounds of refining and prioritizing the importance of goals through the input of clinicians, researchers, patients, and stakeholders. The Committee on Research uses the agenda to “set the compass for the organization in terms of research initiatives and facilitate the ACR’s advocacy for the research goals identified.”
Dr. Alexis R. Ogdie-Beatty, who jointly led the development of the agenda for the Committee on Research along with Dr. S. Louis Bridges, said that while the goals for 2016-2020 had a great deal of overlap with those of 2011-2015, “some of the topics that came up were different. Some of the topics were more specific than in the previous agenda. We have some idea how important these issues were to rheumatologists, given that rheumatologists (and patients) rated the importance of the items. Defining new therapeutic targets and developing new therapies for rheumatic diseases was by far the most highly rated goal by rheumatologists. Next most highly rated was to advocate for increased support for rheumatology research and rheumatology investigators – this was included as a supplementary goal that supports the rest of the agenda. Other newer items were those around determining how the changing health care landscape affects rheumatology patients and clinicians. In addition, nonpharmacologic therapy, adult outcomes of pediatric disease, and optimizing patient engagement were topics that were felt to be important. I think these highlight the input of clinicians in identifying research objectives.”
The 2016-2020 agenda is the third set of goals developed by the committee since 2005, and the first to “crowdsource” the important questions to ACR and ARHP members rather than be assembled solely by the committee.
The agenda arose from a multistage process that began with a web-based survey to the ACR/ARHP membership that asked respondents to “list the five most important research questions that need to be addressed over the next 5 years in order to improve the care for patients with rheumatic disease.” A selected group of 100 individuals representing patients, clinicians (academic and community), research (all types with diverse areas/diseases of interest), allied health professionals, pediatric and adult rheumatology, men and women, all career stages, and all regions of the country, used a Delphi exercise to rate 30 statements generated from the survey on a scale from 1 (not important) to 10 (very important). They had the option to provide comments. At a Leadership Summit, stakeholders from various nonprofit foundations associated with rheumatic diseases, the National Institutes of Health, and the president of the Rheumatology Research Foundation gave comments on a draft agenda to the Committee on Research, after which the committee discussed the results and input and then solicited further 1-10 ratings and comments on preliminary agenda goals from the same group of 100 individuals as in the second phase, plus an additional 17 clinicians.
Up next in the rank-ordering after therapeutic goals were three goals about understanding:
• The etiology, pathogenesis, and genetic basis of rheumatic diseases.
• Early disease states to improve early diagnosis, develop biomarkers for early detection, and determine how earlier treatment changes outcomes.
• The immune system and autoimmunity by defining autoimmunity triggers and determining how epigenetics affect disease susceptibility and inflammation.
The 5-year plan proposed developing improved outcome measures that incorporate patient self-reports, imaging, and measures of clinical response and disease activity. The agenda also seeks to gain better understanding of how patients with rheumatic disease, rheumatologists, and rheumatology health professionals are being affected by the changing U.S. health care landscape.
The plan calls for determining the role of nonpharmacologic therapy in the management of rheumatic disease (promoting and improving adherence to physical activity, finding optimal exercise prescriptions, and determining the role of diet on disease activity), as well as evaluating the role of regenerative medicine.
The agenda spells out the need for better engagement of patients in their care as well as for understanding how comorbidities are influenced by rheumatic disease and how pain and fatigue arise in rheumatic disease.
In two separate goals, committee members listed the importance of determining adult outcomes of pediatric rheumatic diseases and the effect of aging on the development, progression, and management of rheumatic diseases.
The Committee on Research identified three supplemental goals that support the others:
• Advocating for increased support for rheumatology research and rheumatology investigators.
• Harmonizing data from existing cohorts and registries to optimize research capabilities.
• Improving patient research partner involvement in research protocols.
Therapeutic goals set the tone for the American College of Rheumatology National Research Agenda 2016-2020 by calling for the discovery and development of new therapies for rheumatic disease; finding predictors of response and nonresponse to, and adverse events from therapy; and improving the understanding of how therapies should be used.
Those are the top 3 out of 15 goals facilitated by the ACR’s Committee on Research, which finalized the agenda after seeking input from members of the ACR and Association of Rheumatology Health Professionals (ARHP) living in the United States, and going through several rounds of refining and prioritizing the importance of goals through the input of clinicians, researchers, patients, and stakeholders. The Committee on Research uses the agenda to “set the compass for the organization in terms of research initiatives and facilitate the ACR’s advocacy for the research goals identified.”
Dr. Alexis R. Ogdie-Beatty, who jointly led the development of the agenda for the Committee on Research along with Dr. S. Louis Bridges, said that while the goals for 2016-2020 had a great deal of overlap with those of 2011-2015, “some of the topics that came up were different. Some of the topics were more specific than in the previous agenda. We have some idea how important these issues were to rheumatologists, given that rheumatologists (and patients) rated the importance of the items. Defining new therapeutic targets and developing new therapies for rheumatic diseases was by far the most highly rated goal by rheumatologists. Next most highly rated was to advocate for increased support for rheumatology research and rheumatology investigators – this was included as a supplementary goal that supports the rest of the agenda. Other newer items were those around determining how the changing health care landscape affects rheumatology patients and clinicians. In addition, nonpharmacologic therapy, adult outcomes of pediatric disease, and optimizing patient engagement were topics that were felt to be important. I think these highlight the input of clinicians in identifying research objectives.”
The 2016-2020 agenda is the third set of goals developed by the committee since 2005, and the first to “crowdsource” the important questions to ACR and ARHP members rather than be assembled solely by the committee.
The agenda arose from a multistage process that began with a web-based survey to the ACR/ARHP membership that asked respondents to “list the five most important research questions that need to be addressed over the next 5 years in order to improve the care for patients with rheumatic disease.” A selected group of 100 individuals representing patients, clinicians (academic and community), research (all types with diverse areas/diseases of interest), allied health professionals, pediatric and adult rheumatology, men and women, all career stages, and all regions of the country, used a Delphi exercise to rate 30 statements generated from the survey on a scale from 1 (not important) to 10 (very important). They had the option to provide comments. At a Leadership Summit, stakeholders from various nonprofit foundations associated with rheumatic diseases, the National Institutes of Health, and the president of the Rheumatology Research Foundation gave comments on a draft agenda to the Committee on Research, after which the committee discussed the results and input and then solicited further 1-10 ratings and comments on preliminary agenda goals from the same group of 100 individuals as in the second phase, plus an additional 17 clinicians.
Up next in the rank-ordering after therapeutic goals were three goals about understanding:
• The etiology, pathogenesis, and genetic basis of rheumatic diseases.
• Early disease states to improve early diagnosis, develop biomarkers for early detection, and determine how earlier treatment changes outcomes.
• The immune system and autoimmunity by defining autoimmunity triggers and determining how epigenetics affect disease susceptibility and inflammation.
The 5-year plan proposed developing improved outcome measures that incorporate patient self-reports, imaging, and measures of clinical response and disease activity. The agenda also seeks to gain better understanding of how patients with rheumatic disease, rheumatologists, and rheumatology health professionals are being affected by the changing U.S. health care landscape.
The plan calls for determining the role of nonpharmacologic therapy in the management of rheumatic disease (promoting and improving adherence to physical activity, finding optimal exercise prescriptions, and determining the role of diet on disease activity), as well as evaluating the role of regenerative medicine.
The agenda spells out the need for better engagement of patients in their care as well as for understanding how comorbidities are influenced by rheumatic disease and how pain and fatigue arise in rheumatic disease.
In two separate goals, committee members listed the importance of determining adult outcomes of pediatric rheumatic diseases and the effect of aging on the development, progression, and management of rheumatic diseases.
The Committee on Research identified three supplemental goals that support the others:
• Advocating for increased support for rheumatology research and rheumatology investigators.
• Harmonizing data from existing cohorts and registries to optimize research capabilities.
• Improving patient research partner involvement in research protocols.
Romosozumab, coming ACR guidelines mark recent high points in osteoporosis
MAUI, HAWAII – The investigational bone-building agent romosozumab provided the therapeutic highlight in the field of osteoporosis during the past year, Dr. Martin J. Bergman said at the 2016 Rheumatology Winter Clinical Symposium.
Romosozumab is a monoclonal antibody directed against sclerostin, a glycoprotein that prevents mesenchymal cells from becoming osteoblasts. By inhibiting sclerostin, romosozumab promotes osteoblast production. The result is increased bone mineral density and bone formation coupled with decreased bone resorption, providing physicians with a promising new avenue for rapidly building strong bone, explained Dr. Bergman of Drexel University in Philadelphia and chief of the section of rheumatology at Taylor Hospital in Ridley Park, Pa.
Romosozumab caught his eye in a 12-month randomized trial presented last fall at the annual meeting of the American College of Rheumatology. The 430 postmenopausal participants were assigned to blinded romosozumab at 210 mg delivered by subcutaneous injection once per month, blinded placebo, or open-label teriparatide (Forteo). The primary endpoint in this secondary analysis was change in bone strength as measured using the Food and Drug Administration–approved method of finite element analysis based upon quantitative CT imaging.
Romosozumab boosted bone strength at the spine by 27.3% at 12 months, compared with a 3.9% reduction from baseline with placebo and an 18.5% increase with teriparatide. At the hip, romosozumab delivered a 3.6% increase in bone strength versus no significant change from baseline in the other two study arms. Thus, romosozumab increased bone strength both in the cortical and trabecular compartments even more than did teriparatide, the most potent drug currently available for building bone mass.
“The numbers are very impressive,” Dr. Bergman observed. “Trabecular bone, cortical bone, whole bone – across the board, we haven’t seen similar numbers before. I think this is going to be a very exciting new approach to the treatment of osteoporosis. We need to keep an eye on this.”
Romosozumab, which is being codeveloped by Amgen and UCB, is now in phase III testing.
The other big news in osteoporosis is that later this year the ACR will undertake a revision of its 2010 guidelines for the prevention and treatment of glucocorticoid-induced osteoporosis (Arthritis Care Res [Hoboken]. 2010 Nov;62[11]:1515-26).
Among the actions that need to be taken are the incorporation of denosumab (Prolia) and ibandronate (Boniva) into the treatment recommendations, as well as clarification of the recommendation for supplemental calcium in light of recent evidence of an association between high serum calcium and increased cardiovascular risk. Most of the lifestyle modification recommendations in the current guidelines are supported by a weak level of evidence C, meaning “expert opinion,” and the hope is that the evidence has become stronger since 2010, he said.
Dr. Bergman reported having no financial conflicts regarding his presentation.
MAUI, HAWAII – The investigational bone-building agent romosozumab provided the therapeutic highlight in the field of osteoporosis during the past year, Dr. Martin J. Bergman said at the 2016 Rheumatology Winter Clinical Symposium.
Romosozumab is a monoclonal antibody directed against sclerostin, a glycoprotein that prevents mesenchymal cells from becoming osteoblasts. By inhibiting sclerostin, romosozumab promotes osteoblast production. The result is increased bone mineral density and bone formation coupled with decreased bone resorption, providing physicians with a promising new avenue for rapidly building strong bone, explained Dr. Bergman of Drexel University in Philadelphia and chief of the section of rheumatology at Taylor Hospital in Ridley Park, Pa.
Romosozumab caught his eye in a 12-month randomized trial presented last fall at the annual meeting of the American College of Rheumatology. The 430 postmenopausal participants were assigned to blinded romosozumab at 210 mg delivered by subcutaneous injection once per month, blinded placebo, or open-label teriparatide (Forteo). The primary endpoint in this secondary analysis was change in bone strength as measured using the Food and Drug Administration–approved method of finite element analysis based upon quantitative CT imaging.
Romosozumab boosted bone strength at the spine by 27.3% at 12 months, compared with a 3.9% reduction from baseline with placebo and an 18.5% increase with teriparatide. At the hip, romosozumab delivered a 3.6% increase in bone strength versus no significant change from baseline in the other two study arms. Thus, romosozumab increased bone strength both in the cortical and trabecular compartments even more than did teriparatide, the most potent drug currently available for building bone mass.
“The numbers are very impressive,” Dr. Bergman observed. “Trabecular bone, cortical bone, whole bone – across the board, we haven’t seen similar numbers before. I think this is going to be a very exciting new approach to the treatment of osteoporosis. We need to keep an eye on this.”
Romosozumab, which is being codeveloped by Amgen and UCB, is now in phase III testing.
The other big news in osteoporosis is that later this year the ACR will undertake a revision of its 2010 guidelines for the prevention and treatment of glucocorticoid-induced osteoporosis (Arthritis Care Res [Hoboken]. 2010 Nov;62[11]:1515-26).
Among the actions that need to be taken are the incorporation of denosumab (Prolia) and ibandronate (Boniva) into the treatment recommendations, as well as clarification of the recommendation for supplemental calcium in light of recent evidence of an association between high serum calcium and increased cardiovascular risk. Most of the lifestyle modification recommendations in the current guidelines are supported by a weak level of evidence C, meaning “expert opinion,” and the hope is that the evidence has become stronger since 2010, he said.
Dr. Bergman reported having no financial conflicts regarding his presentation.
MAUI, HAWAII – The investigational bone-building agent romosozumab provided the therapeutic highlight in the field of osteoporosis during the past year, Dr. Martin J. Bergman said at the 2016 Rheumatology Winter Clinical Symposium.
Romosozumab is a monoclonal antibody directed against sclerostin, a glycoprotein that prevents mesenchymal cells from becoming osteoblasts. By inhibiting sclerostin, romosozumab promotes osteoblast production. The result is increased bone mineral density and bone formation coupled with decreased bone resorption, providing physicians with a promising new avenue for rapidly building strong bone, explained Dr. Bergman of Drexel University in Philadelphia and chief of the section of rheumatology at Taylor Hospital in Ridley Park, Pa.
Romosozumab caught his eye in a 12-month randomized trial presented last fall at the annual meeting of the American College of Rheumatology. The 430 postmenopausal participants were assigned to blinded romosozumab at 210 mg delivered by subcutaneous injection once per month, blinded placebo, or open-label teriparatide (Forteo). The primary endpoint in this secondary analysis was change in bone strength as measured using the Food and Drug Administration–approved method of finite element analysis based upon quantitative CT imaging.
Romosozumab boosted bone strength at the spine by 27.3% at 12 months, compared with a 3.9% reduction from baseline with placebo and an 18.5% increase with teriparatide. At the hip, romosozumab delivered a 3.6% increase in bone strength versus no significant change from baseline in the other two study arms. Thus, romosozumab increased bone strength both in the cortical and trabecular compartments even more than did teriparatide, the most potent drug currently available for building bone mass.
“The numbers are very impressive,” Dr. Bergman observed. “Trabecular bone, cortical bone, whole bone – across the board, we haven’t seen similar numbers before. I think this is going to be a very exciting new approach to the treatment of osteoporosis. We need to keep an eye on this.”
Romosozumab, which is being codeveloped by Amgen and UCB, is now in phase III testing.
The other big news in osteoporosis is that later this year the ACR will undertake a revision of its 2010 guidelines for the prevention and treatment of glucocorticoid-induced osteoporosis (Arthritis Care Res [Hoboken]. 2010 Nov;62[11]:1515-26).
Among the actions that need to be taken are the incorporation of denosumab (Prolia) and ibandronate (Boniva) into the treatment recommendations, as well as clarification of the recommendation for supplemental calcium in light of recent evidence of an association between high serum calcium and increased cardiovascular risk. Most of the lifestyle modification recommendations in the current guidelines are supported by a weak level of evidence C, meaning “expert opinion,” and the hope is that the evidence has become stronger since 2010, he said.
Dr. Bergman reported having no financial conflicts regarding his presentation.
EXPERT ANALYSIS FROM RWCS 2016
Sleep duration linked with gestational weight gain
ATLANTA – Both short and long sleep duration during pregnancy are associated with extremes of gestational weight gain, according to findings from a multicenter prospective cohort study.
Among 760 nulliparous women with a singleton gestation who were part of the nuMoM2b (Nulliparous Pregnancy Outcomes Study: Monitoring mothers-to-be) network – a National Institute of Child Health and Human Development cohort of more than 10,000 women – the 2.1% with average sleep duration of fewer than 6 hours and the 5.2% with sleep duration greater than 9 hours had the highest rates of low gestational weight gain (z less than –1). The differences were statistically significant, compared with those with average sleep duration of 7 to fewer than 9 hours, at visits between 16 and 21 weeks and between 22 and 29 weeks (P less than .0001, P = .04, respectively), Dr. Francesca Facco reported at the annual Pregnancy Meeting sponsored by the Society for Maternal-Fetal Medicine.
For example, at visit 2, the rate of low gestational weight gain was 18.8% and 35.5% for those with sleep duration fewer than 6 hours and more than 9 hours, respectively, vs. 8.2% for those with sleep duration of 6 to fewer than 7 hours, and 12% for those with 7 to fewer than 8 hours.
The differences were similar in magnitude at the last weight measure prior to delivery but did not reach statistical significance, said Dr. Facco of Magee-Women’s Research Institute, University of Pittsburgh.
“Nonlinear relationships were observed between sleep duration and gestational weight gain,” she said, adding that at all gestational weight gain assessments, high gestational weight gain occurred more frequently as sleep duration shortened.
“We found a U-shaped relationship between sleep and low gestational weight gain; women with the shortest and the longest sleep duration had the highest rates of low gestational weight gain,” she said.
The findings suggest that both long and short sleep duration are associated with extremes of gestational weight gain.
Study subjects were enrolled in the nuMoM2b study and were recruited at the second study visit (16-21 weeks) to wear an actigraph to measure sleep activity for 7 consecutive days. The women, who had a mean age of 27 years, also kept a sleep diary. A little over half (51.5%) were normal weight, 3% were underweight, and 45.5% were overweight or obese. Gestational weight gain was examined using age-standardized z scores, which are a measure of gestational weight gain uncorrelated with gestational age and body mass index.
Sleep is getting more and more attention as an important health behavior, especially in relation to weight and metabolism, Dr. Facco said, noting that short sleep duration has consistently been associated with higher body mass index, and studies show that short sleep duration hinders weight loss efforts.
Data on long sleep duration are less clear but suggest an age-dependent relationship, she said.
The current study was undertaken to evaluate whether the findings in nonpregnant women also apply during pregnancy.
Data from the same cohort, which were presented at the 2015 Pregnancy Meeting, showed that women with sleep duration of fewer than 7 hours had twice the rate of gestational diabetes, compared with those who slept 7 or more hours. The finding remained significant even after adjusting for age and body mass index. Those findings are congruous with the current findings, Dr. Facco said, explaining that the short sleepers were those most likely to have the greatest weight gain, thus putting them at higher risk of gestational diabetes.
“Poor sleep in pregnancy has been linked to adverse pregnancy outcomes, and this association between sleep and gestational weight gain suggests one possible mechanism for this association,” she concluded.
The nuMoM2b study is funded by the National Institutes of Health. Dr. Facco reported having no conflicts of interest.
ATLANTA – Both short and long sleep duration during pregnancy are associated with extremes of gestational weight gain, according to findings from a multicenter prospective cohort study.
Among 760 nulliparous women with a singleton gestation who were part of the nuMoM2b (Nulliparous Pregnancy Outcomes Study: Monitoring mothers-to-be) network – a National Institute of Child Health and Human Development cohort of more than 10,000 women – the 2.1% with average sleep duration of fewer than 6 hours and the 5.2% with sleep duration greater than 9 hours had the highest rates of low gestational weight gain (z less than –1). The differences were statistically significant, compared with those with average sleep duration of 7 to fewer than 9 hours, at visits between 16 and 21 weeks and between 22 and 29 weeks (P less than .0001, P = .04, respectively), Dr. Francesca Facco reported at the annual Pregnancy Meeting sponsored by the Society for Maternal-Fetal Medicine.
For example, at visit 2, the rate of low gestational weight gain was 18.8% and 35.5% for those with sleep duration fewer than 6 hours and more than 9 hours, respectively, vs. 8.2% for those with sleep duration of 6 to fewer than 7 hours, and 12% for those with 7 to fewer than 8 hours.
The differences were similar in magnitude at the last weight measure prior to delivery but did not reach statistical significance, said Dr. Facco of Magee-Women’s Research Institute, University of Pittsburgh.
“Nonlinear relationships were observed between sleep duration and gestational weight gain,” she said, adding that at all gestational weight gain assessments, high gestational weight gain occurred more frequently as sleep duration shortened.
“We found a U-shaped relationship between sleep and low gestational weight gain; women with the shortest and the longest sleep duration had the highest rates of low gestational weight gain,” she said.
The findings suggest that both long and short sleep duration are associated with extremes of gestational weight gain.
Study subjects were enrolled in the nuMoM2b study and were recruited at the second study visit (16-21 weeks) to wear an actigraph to measure sleep activity for 7 consecutive days. The women, who had a mean age of 27 years, also kept a sleep diary. A little over half (51.5%) were normal weight, 3% were underweight, and 45.5% were overweight or obese. Gestational weight gain was examined using age-standardized z scores, which are a measure of gestational weight gain uncorrelated with gestational age and body mass index.
Sleep is getting more and more attention as an important health behavior, especially in relation to weight and metabolism, Dr. Facco said, noting that short sleep duration has consistently been associated with higher body mass index, and studies show that short sleep duration hinders weight loss efforts.
Data on long sleep duration are less clear but suggest an age-dependent relationship, she said.
The current study was undertaken to evaluate whether the findings in nonpregnant women also apply during pregnancy.
Data from the same cohort, which were presented at the 2015 Pregnancy Meeting, showed that women with sleep duration of fewer than 7 hours had twice the rate of gestational diabetes, compared with those who slept 7 or more hours. The finding remained significant even after adjusting for age and body mass index. Those findings are congruous with the current findings, Dr. Facco said, explaining that the short sleepers were those most likely to have the greatest weight gain, thus putting them at higher risk of gestational diabetes.
“Poor sleep in pregnancy has been linked to adverse pregnancy outcomes, and this association between sleep and gestational weight gain suggests one possible mechanism for this association,” she concluded.
The nuMoM2b study is funded by the National Institutes of Health. Dr. Facco reported having no conflicts of interest.
ATLANTA – Both short and long sleep duration during pregnancy are associated with extremes of gestational weight gain, according to findings from a multicenter prospective cohort study.
Among 760 nulliparous women with a singleton gestation who were part of the nuMoM2b (Nulliparous Pregnancy Outcomes Study: Monitoring mothers-to-be) network – a National Institute of Child Health and Human Development cohort of more than 10,000 women – the 2.1% with average sleep duration of fewer than 6 hours and the 5.2% with sleep duration greater than 9 hours had the highest rates of low gestational weight gain (z less than –1). The differences were statistically significant, compared with those with average sleep duration of 7 to fewer than 9 hours, at visits between 16 and 21 weeks and between 22 and 29 weeks (P less than .0001, P = .04, respectively), Dr. Francesca Facco reported at the annual Pregnancy Meeting sponsored by the Society for Maternal-Fetal Medicine.
For example, at visit 2, the rate of low gestational weight gain was 18.8% and 35.5% for those with sleep duration fewer than 6 hours and more than 9 hours, respectively, vs. 8.2% for those with sleep duration of 6 to fewer than 7 hours, and 12% for those with 7 to fewer than 8 hours.
The differences were similar in magnitude at the last weight measure prior to delivery but did not reach statistical significance, said Dr. Facco of Magee-Women’s Research Institute, University of Pittsburgh.
“Nonlinear relationships were observed between sleep duration and gestational weight gain,” she said, adding that at all gestational weight gain assessments, high gestational weight gain occurred more frequently as sleep duration shortened.
“We found a U-shaped relationship between sleep and low gestational weight gain; women with the shortest and the longest sleep duration had the highest rates of low gestational weight gain,” she said.
The findings suggest that both long and short sleep duration are associated with extremes of gestational weight gain.
Study subjects were enrolled in the nuMoM2b study and were recruited at the second study visit (16-21 weeks) to wear an actigraph to measure sleep activity for 7 consecutive days. The women, who had a mean age of 27 years, also kept a sleep diary. A little over half (51.5%) were normal weight, 3% were underweight, and 45.5% were overweight or obese. Gestational weight gain was examined using age-standardized z scores, which are a measure of gestational weight gain uncorrelated with gestational age and body mass index.
Sleep is getting more and more attention as an important health behavior, especially in relation to weight and metabolism, Dr. Facco said, noting that short sleep duration has consistently been associated with higher body mass index, and studies show that short sleep duration hinders weight loss efforts.
Data on long sleep duration are less clear but suggest an age-dependent relationship, she said.
The current study was undertaken to evaluate whether the findings in nonpregnant women also apply during pregnancy.
Data from the same cohort, which were presented at the 2015 Pregnancy Meeting, showed that women with sleep duration of fewer than 7 hours had twice the rate of gestational diabetes, compared with those who slept 7 or more hours. The finding remained significant even after adjusting for age and body mass index. Those findings are congruous with the current findings, Dr. Facco said, explaining that the short sleepers were those most likely to have the greatest weight gain, thus putting them at higher risk of gestational diabetes.
“Poor sleep in pregnancy has been linked to adverse pregnancy outcomes, and this association between sleep and gestational weight gain suggests one possible mechanism for this association,” she concluded.
The nuMoM2b study is funded by the National Institutes of Health. Dr. Facco reported having no conflicts of interest.
AT THE PREGNANCY MEETING
Key clinical point: Both short and long sleep duration during pregnancy are associated with extremes of gestational weight gain, according to findings from a multicenter prospective cohort study.
Major finding: Women with average sleep duration less than 6 hours and greater than 9 hours had the highest rates of low gestational weight gain (18.8% and 35.5%, respectively, vs. 8.2% for those with 6 to under 7 hours, and 12% for those with 7 to under 8 hours).
Data source: A study of 760 women from a large prospective cohort.
Disclosures: The nuMoM2b study is funded by the National Institutes of Health. Dr. Facco reported having no conflicts of interest.
Denosumab boosts BMD in kidney transplant recipients
SAN DIEGO – Twice-yearly denosumab effectively increased bone mineral density in kidney transplant recipients, but was associated with more frequent episodes of urinary tract infections and hypocalcemia, results from a randomized trial showed.
“Kidney transplant recipients lose bone mass and are at increased risk for fractures, more so in females than in males,” Dr. Rudolf P. Wuthrich said at Kidney Week 2015, sponsored by the American Society of Nephrology. Results from previous studies suggest that one in five patients may develop a fracture within 5 years after kidney transplantation.
Considering that current therapeutic options to prevent bone loss are limited, Dr. Wuthrich, director of the Clinic for Nephrology at University Hospital Zurich, and his associates assessed the efficacy and safety of receptor activator of nuclear factor–kappaB ligand (RANKL) inhibition with denosumab to improve bone mineralization in the first year after kidney transplantation. They recruited 108 patients from June 2011 to May 2014. Of these, 90 were randomized within 4 weeks after kidney transplant surgery in a 1:1 ratio to receive subcutaneous injections of 60 mg denosumab at baseline and after 6 months, or no treatment. The study’s primary endpoint was the percentage change in bone mineral density measured by DXA at the lumbar spine at 12 months. The study, known as Denosumab for Prevention of Osteoporosis in Renal Transplant Recipients (POSTOP), was limited to adults who had undergone kidney transplantation within 28 days and who were on standard triple immunosuppression, including a calcineurin antagonist, mycophenolate, and steroids.
Dr. Wuthrich reported results from 46 patients in the denosumab group and 44 patients in the control group. At baseline, their mean age was 50 years, 63% were male, and 96% were white. After 12 months, the total lumbar spine BMD increased by 4.6% in the denosumab group and decreased by 0.5% in the control group, for a between-group difference of 5.1% (P less than .0001). Denosumab also significantly increased BMD at the total hip by 1.9% (P = .035) over that in the control group at 12 months.
High-resolution peripheral quantitative computed tomography in a subgroup of 24 patients showed that denosumab also significantly increased BMD and cortical thickness at the distal tibia and radius (P less than .05). Two biomarkers of bone resorption in beta C-terminal telopeptide and urine deoxypyridinoline markedly decreased in the denosumab group, as did two biomarkers of bone formation in procollagen type 1 N-terminal propeptide and bone-specific alkaline phosphatase (P less than .0001).
In terms of adverse events, there were significantly more urinary tract infections in the denosumab group, compared with the control group (15% vs. 9%, respectively), as well as more episodes of diarrhea (9% vs. 5%), and transient hypocalcemia (3% vs. 0.3%). The number of serious adverse events was similar between groups, at 17% and 19%, respectively.
“We had significantly increased bone mineral density at all measured skeletal sites in response to denosumab,” Dr. Wuthrich concluded. “We had a significant increase in bone biomarkers and we can say that denosumab was generally safe in a complex population of immunosuppressed kidney transplant recipients. But it was associated with a higher incidence of urinary tract infections. At this point we have no good explanation as to why this is. We also had a few episodes of transient and asymptomatic hypocalcemia.”
The researchers reported having no financial disclosures.
SAN DIEGO – Twice-yearly denosumab effectively increased bone mineral density in kidney transplant recipients, but was associated with more frequent episodes of urinary tract infections and hypocalcemia, results from a randomized trial showed.
“Kidney transplant recipients lose bone mass and are at increased risk for fractures, more so in females than in males,” Dr. Rudolf P. Wuthrich said at Kidney Week 2015, sponsored by the American Society of Nephrology. Results from previous studies suggest that one in five patients may develop a fracture within 5 years after kidney transplantation.
Considering that current therapeutic options to prevent bone loss are limited, Dr. Wuthrich, director of the Clinic for Nephrology at University Hospital Zurich, and his associates assessed the efficacy and safety of receptor activator of nuclear factor–kappaB ligand (RANKL) inhibition with denosumab to improve bone mineralization in the first year after kidney transplantation. They recruited 108 patients from June 2011 to May 2014. Of these, 90 were randomized within 4 weeks after kidney transplant surgery in a 1:1 ratio to receive subcutaneous injections of 60 mg denosumab at baseline and after 6 months, or no treatment. The study’s primary endpoint was the percentage change in bone mineral density measured by DXA at the lumbar spine at 12 months. The study, known as Denosumab for Prevention of Osteoporosis in Renal Transplant Recipients (POSTOP), was limited to adults who had undergone kidney transplantation within 28 days and who were on standard triple immunosuppression, including a calcineurin antagonist, mycophenolate, and steroids.
Dr. Wuthrich reported results from 46 patients in the denosumab group and 44 patients in the control group. At baseline, their mean age was 50 years, 63% were male, and 96% were white. After 12 months, the total lumbar spine BMD increased by 4.6% in the denosumab group and decreased by 0.5% in the control group, for a between-group difference of 5.1% (P less than .0001). Denosumab also significantly increased BMD at the total hip by 1.9% (P = .035) over that in the control group at 12 months.
High-resolution peripheral quantitative computed tomography in a subgroup of 24 patients showed that denosumab also significantly increased BMD and cortical thickness at the distal tibia and radius (P less than .05). Two biomarkers of bone resorption in beta C-terminal telopeptide and urine deoxypyridinoline markedly decreased in the denosumab group, as did two biomarkers of bone formation in procollagen type 1 N-terminal propeptide and bone-specific alkaline phosphatase (P less than .0001).
In terms of adverse events, there were significantly more urinary tract infections in the denosumab group, compared with the control group (15% vs. 9%, respectively), as well as more episodes of diarrhea (9% vs. 5%), and transient hypocalcemia (3% vs. 0.3%). The number of serious adverse events was similar between groups, at 17% and 19%, respectively.
“We had significantly increased bone mineral density at all measured skeletal sites in response to denosumab,” Dr. Wuthrich concluded. “We had a significant increase in bone biomarkers and we can say that denosumab was generally safe in a complex population of immunosuppressed kidney transplant recipients. But it was associated with a higher incidence of urinary tract infections. At this point we have no good explanation as to why this is. We also had a few episodes of transient and asymptomatic hypocalcemia.”
The researchers reported having no financial disclosures.
SAN DIEGO – Twice-yearly denosumab effectively increased bone mineral density in kidney transplant recipients, but was associated with more frequent episodes of urinary tract infections and hypocalcemia, results from a randomized trial showed.
“Kidney transplant recipients lose bone mass and are at increased risk for fractures, more so in females than in males,” Dr. Rudolf P. Wuthrich said at Kidney Week 2015, sponsored by the American Society of Nephrology. Results from previous studies suggest that one in five patients may develop a fracture within 5 years after kidney transplantation.
Considering that current therapeutic options to prevent bone loss are limited, Dr. Wuthrich, director of the Clinic for Nephrology at University Hospital Zurich, and his associates assessed the efficacy and safety of receptor activator of nuclear factor–kappaB ligand (RANKL) inhibition with denosumab to improve bone mineralization in the first year after kidney transplantation. They recruited 108 patients from June 2011 to May 2014. Of these, 90 were randomized within 4 weeks after kidney transplant surgery in a 1:1 ratio to receive subcutaneous injections of 60 mg denosumab at baseline and after 6 months, or no treatment. The study’s primary endpoint was the percentage change in bone mineral density measured by DXA at the lumbar spine at 12 months. The study, known as Denosumab for Prevention of Osteoporosis in Renal Transplant Recipients (POSTOP), was limited to adults who had undergone kidney transplantation within 28 days and who were on standard triple immunosuppression, including a calcineurin antagonist, mycophenolate, and steroids.
Dr. Wuthrich reported results from 46 patients in the denosumab group and 44 patients in the control group. At baseline, their mean age was 50 years, 63% were male, and 96% were white. After 12 months, the total lumbar spine BMD increased by 4.6% in the denosumab group and decreased by 0.5% in the control group, for a between-group difference of 5.1% (P less than .0001). Denosumab also significantly increased BMD at the total hip by 1.9% (P = .035) over that in the control group at 12 months.
High-resolution peripheral quantitative computed tomography in a subgroup of 24 patients showed that denosumab also significantly increased BMD and cortical thickness at the distal tibia and radius (P less than .05). Two biomarkers of bone resorption in beta C-terminal telopeptide and urine deoxypyridinoline markedly decreased in the denosumab group, as did two biomarkers of bone formation in procollagen type 1 N-terminal propeptide and bone-specific alkaline phosphatase (P less than .0001).
In terms of adverse events, there were significantly more urinary tract infections in the denosumab group, compared with the control group (15% vs. 9%, respectively), as well as more episodes of diarrhea (9% vs. 5%), and transient hypocalcemia (3% vs. 0.3%). The number of serious adverse events was similar between groups, at 17% and 19%, respectively.
“We had significantly increased bone mineral density at all measured skeletal sites in response to denosumab,” Dr. Wuthrich concluded. “We had a significant increase in bone biomarkers and we can say that denosumab was generally safe in a complex population of immunosuppressed kidney transplant recipients. But it was associated with a higher incidence of urinary tract infections. At this point we have no good explanation as to why this is. We also had a few episodes of transient and asymptomatic hypocalcemia.”
The researchers reported having no financial disclosures.
AT KIDNEY WEEK 2015
Key clinical point: Denosumab effectively increased bone mineral density in kidney transplant recipients in the POSTOP trial.
Major finding: After 12 months, total lumbar spine BMD increased by 4.6% in the denosumab group and decreased by 0.5% in the control group, for a between-group difference of 5.1% (P less than .0001).
Data source: POSTOP, a study of 90 patients who were randomized within 4 weeks after kidney transplant surgery in a 1:1 ratio to receive subcutaneous injections of 60 mg denosumab at baseline and after 6 months, or no treatment.
Disclosures: The researchers reported having no financial disclosures.
High-dose vitamin D increases fall risk in study of 200 patients
Older adults treated with high-dose vitamin D as part of a double-blind, randomized clinical trial achieved target 25-hydroxyvitamin D levels at 6 and 12 months, but did not achieve the primary endpoint of improved lower extremity function.
In fact, the highest dose used in the study – 60,000 IU of vitamin D3 monthly – was associated with an increased risk of falls, according to Dr. Heike A. Bischoff-Ferrari of University Hospital Zurich, and her colleagues.
In the 200 home-dwelling adults aged older than 70 years who participated in the 1-year study, monthly doses of 60,000 IU of vitamin D3 and 24,000 IU of vitamin D3 plus 300 mcg of calcifediol were significantly more likely than a 24,000 IU dose of vitamin D3 alone to result in 25-hydroxyvitamin D levels of at least 30 ng/mL at 6 and 12 months, but lower extremity function did not differ among the groups, the investigators reported in a study published online Jan. 4 in JAMA Internal Medicine.
For example, the adjusted changes in Short Physical Performance Battery scores – a measure of walking speed, successive chair stands, and balance – were 0.17, 0.16, and 0.16 at 6 months in the 24,000 IU, 60,000 IU, and 24,000 IU plus calcifediol groups, respectively, and 0.38, 0.10, and 0.11 at 12 months (JAMA Intern Med. 2016 Jan 4. doi: 10.1001/jamainternmed.2015.7148).
However, the incidence of falls was 66.9%, 66.1%, and 47.9% in the groups, respectively, and the mean number of falls was higher in the 60,000 IU group (1.47) and 24,000 IU plus calcifediol group (1.24) than in the 24,000 IU group (0.94), they said, noting that a similar pattern was observed during study months 0-6 and months 7-12.
Study subjects had a mean age of 78 years, 58% were vitamin D deficient with levels of less than 20 ng/mL at baseline, and 13% were severely deficient (levels less than 10 ng/mL). All had experienced a low-trauma fall in the previous 12 months and were thus considered a high-risk group for vitamin D deficiency and functional decline. Vitamin D supplementation was given via one 5-mL drink solution each month that provided 24,000 IU of vitamin D3 – equivalent to the current recommendation of 800 IU/day – plus three placebo capsules; a 5-mL drink solution that provided 60,000 IU of vitamin D3 – the equivalent of 2,000 IU/day – plus three placebo capsules; or a 5-mL placebo drink, two capsules containing 12,000 IU of vitamin D3 each, and one capsule containing 300 mcg of calcifediol, which is a potent liver metabolite of vitamin D.
Although vitamin D supplementation has been proposed as a possible strategy for delaying functional decline because of its effects on muscle strength, the current findings, which are consistent with some prior studies, suggest that high-doses of vitamin D confer no benefit with respect to function decline, compared with a standard-of-care dose of 24,000 IU of D3, and that high doses may increase falls in those with a prior fall event. Further research is needed to confirm the findings for daily dosing regimens, as well as to explore the physiology behind a possible detrimental effect of a high monthly bolus dose of vitamin D on muscle function and falls, they concluded.
This study was funded by the Swiss National Science Foundation and the VELUX Foundations, as well as by investigator-initiated funds from Merck Sharp & Dohme AG, WILD, and DSM Nutritional Products. Dr. Bischoff-Ferrari reported receiving speaker fees from and serving on advisory boards for Merck Sharp & Dohme AG, Amgen, WILD, DSM Nutritional Products, Roche Diagnostics, Nestle, Pfizer, and Sanofi.
The vitamin D storyline may be similar to that of antioxidant vitamins, Dr. Steven R. Cummings and his colleagues wrote in an editorial.
“Enthusiasm for the health benefits of vitamin supplements is coupled with the belief that ‘vitamins’ are inherently safe and reinforced by observational studies showing, essentially, that healthy people have higher vitamin levels. Then [randomized, controlled trials] and meta-analyses proved that the supplements in fact increase mortality (beta carotene, vitamin E), or have no health benefits (vitamin A, vitamin C),” they wrote (JAMA Intern Med. 2016 Jan 4. doi: 10.1001/jamainternmed.2015.7568).
The strategy of supplementation with vitamin D to achieve serum levels of at least 30 ng/mL to reduce the risk of falls and fractures has not been established, and according to the findings by Bischoff-Ferrari et al., it may increase the risk of falling.
“Until that approach is supported by randomized trials with updated meta-analyses, it would be prudent to follow recommendation from the Institute of Medicine (IOM) that people 70 years or older have a total daily intake of 800 IU of vitamin D without routine measurement of serum 25(OH)D levels,” they wrote, adding that recommended intakes are best achieved through a balanced diet.
Dr. Cummings is with the California Pacific Medical Center Research Institute, San Francisco, and the University of California, San Francisco. He and his coauthors reported having no disclosures.
The vitamin D storyline may be similar to that of antioxidant vitamins, Dr. Steven R. Cummings and his colleagues wrote in an editorial.
“Enthusiasm for the health benefits of vitamin supplements is coupled with the belief that ‘vitamins’ are inherently safe and reinforced by observational studies showing, essentially, that healthy people have higher vitamin levels. Then [randomized, controlled trials] and meta-analyses proved that the supplements in fact increase mortality (beta carotene, vitamin E), or have no health benefits (vitamin A, vitamin C),” they wrote (JAMA Intern Med. 2016 Jan 4. doi: 10.1001/jamainternmed.2015.7568).
The strategy of supplementation with vitamin D to achieve serum levels of at least 30 ng/mL to reduce the risk of falls and fractures has not been established, and according to the findings by Bischoff-Ferrari et al., it may increase the risk of falling.
“Until that approach is supported by randomized trials with updated meta-analyses, it would be prudent to follow recommendation from the Institute of Medicine (IOM) that people 70 years or older have a total daily intake of 800 IU of vitamin D without routine measurement of serum 25(OH)D levels,” they wrote, adding that recommended intakes are best achieved through a balanced diet.
Dr. Cummings is with the California Pacific Medical Center Research Institute, San Francisco, and the University of California, San Francisco. He and his coauthors reported having no disclosures.
The vitamin D storyline may be similar to that of antioxidant vitamins, Dr. Steven R. Cummings and his colleagues wrote in an editorial.
“Enthusiasm for the health benefits of vitamin supplements is coupled with the belief that ‘vitamins’ are inherently safe and reinforced by observational studies showing, essentially, that healthy people have higher vitamin levels. Then [randomized, controlled trials] and meta-analyses proved that the supplements in fact increase mortality (beta carotene, vitamin E), or have no health benefits (vitamin A, vitamin C),” they wrote (JAMA Intern Med. 2016 Jan 4. doi: 10.1001/jamainternmed.2015.7568).
The strategy of supplementation with vitamin D to achieve serum levels of at least 30 ng/mL to reduce the risk of falls and fractures has not been established, and according to the findings by Bischoff-Ferrari et al., it may increase the risk of falling.
“Until that approach is supported by randomized trials with updated meta-analyses, it would be prudent to follow recommendation from the Institute of Medicine (IOM) that people 70 years or older have a total daily intake of 800 IU of vitamin D without routine measurement of serum 25(OH)D levels,” they wrote, adding that recommended intakes are best achieved through a balanced diet.
Dr. Cummings is with the California Pacific Medical Center Research Institute, San Francisco, and the University of California, San Francisco. He and his coauthors reported having no disclosures.
Older adults treated with high-dose vitamin D as part of a double-blind, randomized clinical trial achieved target 25-hydroxyvitamin D levels at 6 and 12 months, but did not achieve the primary endpoint of improved lower extremity function.
In fact, the highest dose used in the study – 60,000 IU of vitamin D3 monthly – was associated with an increased risk of falls, according to Dr. Heike A. Bischoff-Ferrari of University Hospital Zurich, and her colleagues.
In the 200 home-dwelling adults aged older than 70 years who participated in the 1-year study, monthly doses of 60,000 IU of vitamin D3 and 24,000 IU of vitamin D3 plus 300 mcg of calcifediol were significantly more likely than a 24,000 IU dose of vitamin D3 alone to result in 25-hydroxyvitamin D levels of at least 30 ng/mL at 6 and 12 months, but lower extremity function did not differ among the groups, the investigators reported in a study published online Jan. 4 in JAMA Internal Medicine.
For example, the adjusted changes in Short Physical Performance Battery scores – a measure of walking speed, successive chair stands, and balance – were 0.17, 0.16, and 0.16 at 6 months in the 24,000 IU, 60,000 IU, and 24,000 IU plus calcifediol groups, respectively, and 0.38, 0.10, and 0.11 at 12 months (JAMA Intern Med. 2016 Jan 4. doi: 10.1001/jamainternmed.2015.7148).
However, the incidence of falls was 66.9%, 66.1%, and 47.9% in the groups, respectively, and the mean number of falls was higher in the 60,000 IU group (1.47) and 24,000 IU plus calcifediol group (1.24) than in the 24,000 IU group (0.94), they said, noting that a similar pattern was observed during study months 0-6 and months 7-12.
Study subjects had a mean age of 78 years, 58% were vitamin D deficient with levels of less than 20 ng/mL at baseline, and 13% were severely deficient (levels less than 10 ng/mL). All had experienced a low-trauma fall in the previous 12 months and were thus considered a high-risk group for vitamin D deficiency and functional decline. Vitamin D supplementation was given via one 5-mL drink solution each month that provided 24,000 IU of vitamin D3 – equivalent to the current recommendation of 800 IU/day – plus three placebo capsules; a 5-mL drink solution that provided 60,000 IU of vitamin D3 – the equivalent of 2,000 IU/day – plus three placebo capsules; or a 5-mL placebo drink, two capsules containing 12,000 IU of vitamin D3 each, and one capsule containing 300 mcg of calcifediol, which is a potent liver metabolite of vitamin D.
Although vitamin D supplementation has been proposed as a possible strategy for delaying functional decline because of its effects on muscle strength, the current findings, which are consistent with some prior studies, suggest that high-doses of vitamin D confer no benefit with respect to function decline, compared with a standard-of-care dose of 24,000 IU of D3, and that high doses may increase falls in those with a prior fall event. Further research is needed to confirm the findings for daily dosing regimens, as well as to explore the physiology behind a possible detrimental effect of a high monthly bolus dose of vitamin D on muscle function and falls, they concluded.
This study was funded by the Swiss National Science Foundation and the VELUX Foundations, as well as by investigator-initiated funds from Merck Sharp & Dohme AG, WILD, and DSM Nutritional Products. Dr. Bischoff-Ferrari reported receiving speaker fees from and serving on advisory boards for Merck Sharp & Dohme AG, Amgen, WILD, DSM Nutritional Products, Roche Diagnostics, Nestle, Pfizer, and Sanofi.
Older adults treated with high-dose vitamin D as part of a double-blind, randomized clinical trial achieved target 25-hydroxyvitamin D levels at 6 and 12 months, but did not achieve the primary endpoint of improved lower extremity function.
In fact, the highest dose used in the study – 60,000 IU of vitamin D3 monthly – was associated with an increased risk of falls, according to Dr. Heike A. Bischoff-Ferrari of University Hospital Zurich, and her colleagues.
In the 200 home-dwelling adults aged older than 70 years who participated in the 1-year study, monthly doses of 60,000 IU of vitamin D3 and 24,000 IU of vitamin D3 plus 300 mcg of calcifediol were significantly more likely than a 24,000 IU dose of vitamin D3 alone to result in 25-hydroxyvitamin D levels of at least 30 ng/mL at 6 and 12 months, but lower extremity function did not differ among the groups, the investigators reported in a study published online Jan. 4 in JAMA Internal Medicine.
For example, the adjusted changes in Short Physical Performance Battery scores – a measure of walking speed, successive chair stands, and balance – were 0.17, 0.16, and 0.16 at 6 months in the 24,000 IU, 60,000 IU, and 24,000 IU plus calcifediol groups, respectively, and 0.38, 0.10, and 0.11 at 12 months (JAMA Intern Med. 2016 Jan 4. doi: 10.1001/jamainternmed.2015.7148).
However, the incidence of falls was 66.9%, 66.1%, and 47.9% in the groups, respectively, and the mean number of falls was higher in the 60,000 IU group (1.47) and 24,000 IU plus calcifediol group (1.24) than in the 24,000 IU group (0.94), they said, noting that a similar pattern was observed during study months 0-6 and months 7-12.
Study subjects had a mean age of 78 years, 58% were vitamin D deficient with levels of less than 20 ng/mL at baseline, and 13% were severely deficient (levels less than 10 ng/mL). All had experienced a low-trauma fall in the previous 12 months and were thus considered a high-risk group for vitamin D deficiency and functional decline. Vitamin D supplementation was given via one 5-mL drink solution each month that provided 24,000 IU of vitamin D3 – equivalent to the current recommendation of 800 IU/day – plus three placebo capsules; a 5-mL drink solution that provided 60,000 IU of vitamin D3 – the equivalent of 2,000 IU/day – plus three placebo capsules; or a 5-mL placebo drink, two capsules containing 12,000 IU of vitamin D3 each, and one capsule containing 300 mcg of calcifediol, which is a potent liver metabolite of vitamin D.
Although vitamin D supplementation has been proposed as a possible strategy for delaying functional decline because of its effects on muscle strength, the current findings, which are consistent with some prior studies, suggest that high-doses of vitamin D confer no benefit with respect to function decline, compared with a standard-of-care dose of 24,000 IU of D3, and that high doses may increase falls in those with a prior fall event. Further research is needed to confirm the findings for daily dosing regimens, as well as to explore the physiology behind a possible detrimental effect of a high monthly bolus dose of vitamin D on muscle function and falls, they concluded.
This study was funded by the Swiss National Science Foundation and the VELUX Foundations, as well as by investigator-initiated funds from Merck Sharp & Dohme AG, WILD, and DSM Nutritional Products. Dr. Bischoff-Ferrari reported receiving speaker fees from and serving on advisory boards for Merck Sharp & Dohme AG, Amgen, WILD, DSM Nutritional Products, Roche Diagnostics, Nestle, Pfizer, and Sanofi.
FROM JAMA INTERNAL MEDICINE
Key clinical point: Older adults treated with high-dose vitamin D as part of a double-blind, randomized clinical trial achieved target 25-hydroxyvitamin D levels at 6 and 12 months, but did not achieve the primary endpoint of improved lower extremity function.
Major finding: Fall incidence was 66.9%, 66.1%, and 47.9% with vitamin D3 at doses of 24,000 IU, 60,000 IU, and 24,000 IU, respectively, plus 300 mcg calcifediol.
Data source: A double-blind, randomized trial involving 200 patients.
Disclosures: This study was funded by the Swiss National Science Foundation and the VELUX Foundations, as well as by investigator-initiated funds from Merck Sharp & Dohme AG, WILD, and DSM Nutritional Products. Dr. Bischoff-Ferrari disclosed ties with Merck Sharp & Dohme AG, Amgen, WILD, DSM Nutritional Products, Roche Diagnostics, Nestle, Pfizer, and Sanofi.
Denosumab better at building bone than zoledronic acid
SAN FRANCISCO – Denosumab was superior to zoledronic acid (ZA) in building bone at the lumber spine, total hip, and femoral neck in postmenopausal women with osteoporosis previously treated with oral bisphosphonate therapy in a randomized phase III trial. This study is one more piece of level I evidence to support the effectiveness of transitioning to denosumab in such patients.
“In postmenopausal women with osteoporosis on prior oral bisphosphonates for 2 or more years, transitioning to denosumab resulted in significantly greater increases in bone mineral density [BMD], compared with zoledronic acid at all measured skeletal sites,” said lead author Dr. Paul D. Miller of the Colorado Center for Bone Research, Lakewood, Colo.
Speaking at the annual meeting of the American College of Rheumatology, Dr. Miller said: “This study completes a suite of trials that show transitioning from oral bisphosphonates to denosumab provides greater increases in bone mineral density and reduction in bone turnover markers, compared to maintaining therapy with another bisphosphonate.”
“Adherence rates to oral bisphosphonates are low,” he continued. “Patients who are intolerant to or fail other bisphosphonates may cycle from one bisphosphonate to another, but clinical benefits are not shown. Previous trials have shown that denosumab increased bone mineral density in women previously treated with oral bisphosphonates, whereas zoledronic acid did not, in women previously treated with alendronate.”
A prospective, double-blind, double-dummy, randomized trial was mounted to compare denosumab given subcutaneously at 60 mg every 6 months (n = 321) vs. ZA 5 mg once a year (n = 322) in postmenopausal women with osteoporosis who were intolerant to or who failed oral bisphosphonate therapy after at least 2 years of treatment.
Baseline characteristics were similar between both treatment arms. Median age was 69 years. About 38% had previous osteoporotic fracture. Bone mineral density T scores were similar between groups. Prior oral bisphosphonate exposure was a median of 6.4 years. Bone turnover marker levels were similar between the two arms.
For the primary endpoint, at 12 months, denosumab achieved a greater increase in BMD at all sites, compared with ZA: a 2.1% difference was observed at the lumbar spine and a 1.4% difference at the total hip.
Changes in bone turnover markers over time were more favorable with denosumab, he continued. Serum C-terminal cross-linking telopeptide (CTX) level was maintained in the denosumab arm and increased with ZA over 12 months. A similar pattern was observed with procollagen type 1 N-terminal propeptide (P1NP).
Adverse events were comparable between both groups. Serious adverse events were reported in 7.8% in the denosumab arm versus 9.1% in the ZA arm, but this difference was not statistically significant. Slightly more hypersensitivity adverse events were reported in the denosumab group: 3.8% versus 1.9% for ZA. The number of osteoporotic-related fractures was doubled in the ZA arm: 7 for denosumab vs. 15 for ZA.
Dr. Miller told listeners that this is the fourth study to show that denosumab is effective in osteoporotic patients previously on oral bisphosphonates, including prior risedronate, ibandronate, and alendronate.
SAN FRANCISCO – Denosumab was superior to zoledronic acid (ZA) in building bone at the lumber spine, total hip, and femoral neck in postmenopausal women with osteoporosis previously treated with oral bisphosphonate therapy in a randomized phase III trial. This study is one more piece of level I evidence to support the effectiveness of transitioning to denosumab in such patients.
“In postmenopausal women with osteoporosis on prior oral bisphosphonates for 2 or more years, transitioning to denosumab resulted in significantly greater increases in bone mineral density [BMD], compared with zoledronic acid at all measured skeletal sites,” said lead author Dr. Paul D. Miller of the Colorado Center for Bone Research, Lakewood, Colo.
Speaking at the annual meeting of the American College of Rheumatology, Dr. Miller said: “This study completes a suite of trials that show transitioning from oral bisphosphonates to denosumab provides greater increases in bone mineral density and reduction in bone turnover markers, compared to maintaining therapy with another bisphosphonate.”
“Adherence rates to oral bisphosphonates are low,” he continued. “Patients who are intolerant to or fail other bisphosphonates may cycle from one bisphosphonate to another, but clinical benefits are not shown. Previous trials have shown that denosumab increased bone mineral density in women previously treated with oral bisphosphonates, whereas zoledronic acid did not, in women previously treated with alendronate.”
A prospective, double-blind, double-dummy, randomized trial was mounted to compare denosumab given subcutaneously at 60 mg every 6 months (n = 321) vs. ZA 5 mg once a year (n = 322) in postmenopausal women with osteoporosis who were intolerant to or who failed oral bisphosphonate therapy after at least 2 years of treatment.
Baseline characteristics were similar between both treatment arms. Median age was 69 years. About 38% had previous osteoporotic fracture. Bone mineral density T scores were similar between groups. Prior oral bisphosphonate exposure was a median of 6.4 years. Bone turnover marker levels were similar between the two arms.
For the primary endpoint, at 12 months, denosumab achieved a greater increase in BMD at all sites, compared with ZA: a 2.1% difference was observed at the lumbar spine and a 1.4% difference at the total hip.
Changes in bone turnover markers over time were more favorable with denosumab, he continued. Serum C-terminal cross-linking telopeptide (CTX) level was maintained in the denosumab arm and increased with ZA over 12 months. A similar pattern was observed with procollagen type 1 N-terminal propeptide (P1NP).
Adverse events were comparable between both groups. Serious adverse events were reported in 7.8% in the denosumab arm versus 9.1% in the ZA arm, but this difference was not statistically significant. Slightly more hypersensitivity adverse events were reported in the denosumab group: 3.8% versus 1.9% for ZA. The number of osteoporotic-related fractures was doubled in the ZA arm: 7 for denosumab vs. 15 for ZA.
Dr. Miller told listeners that this is the fourth study to show that denosumab is effective in osteoporotic patients previously on oral bisphosphonates, including prior risedronate, ibandronate, and alendronate.
SAN FRANCISCO – Denosumab was superior to zoledronic acid (ZA) in building bone at the lumber spine, total hip, and femoral neck in postmenopausal women with osteoporosis previously treated with oral bisphosphonate therapy in a randomized phase III trial. This study is one more piece of level I evidence to support the effectiveness of transitioning to denosumab in such patients.
“In postmenopausal women with osteoporosis on prior oral bisphosphonates for 2 or more years, transitioning to denosumab resulted in significantly greater increases in bone mineral density [BMD], compared with zoledronic acid at all measured skeletal sites,” said lead author Dr. Paul D. Miller of the Colorado Center for Bone Research, Lakewood, Colo.
Speaking at the annual meeting of the American College of Rheumatology, Dr. Miller said: “This study completes a suite of trials that show transitioning from oral bisphosphonates to denosumab provides greater increases in bone mineral density and reduction in bone turnover markers, compared to maintaining therapy with another bisphosphonate.”
“Adherence rates to oral bisphosphonates are low,” he continued. “Patients who are intolerant to or fail other bisphosphonates may cycle from one bisphosphonate to another, but clinical benefits are not shown. Previous trials have shown that denosumab increased bone mineral density in women previously treated with oral bisphosphonates, whereas zoledronic acid did not, in women previously treated with alendronate.”
A prospective, double-blind, double-dummy, randomized trial was mounted to compare denosumab given subcutaneously at 60 mg every 6 months (n = 321) vs. ZA 5 mg once a year (n = 322) in postmenopausal women with osteoporosis who were intolerant to or who failed oral bisphosphonate therapy after at least 2 years of treatment.
Baseline characteristics were similar between both treatment arms. Median age was 69 years. About 38% had previous osteoporotic fracture. Bone mineral density T scores were similar between groups. Prior oral bisphosphonate exposure was a median of 6.4 years. Bone turnover marker levels were similar between the two arms.
For the primary endpoint, at 12 months, denosumab achieved a greater increase in BMD at all sites, compared with ZA: a 2.1% difference was observed at the lumbar spine and a 1.4% difference at the total hip.
Changes in bone turnover markers over time were more favorable with denosumab, he continued. Serum C-terminal cross-linking telopeptide (CTX) level was maintained in the denosumab arm and increased with ZA over 12 months. A similar pattern was observed with procollagen type 1 N-terminal propeptide (P1NP).
Adverse events were comparable between both groups. Serious adverse events were reported in 7.8% in the denosumab arm versus 9.1% in the ZA arm, but this difference was not statistically significant. Slightly more hypersensitivity adverse events were reported in the denosumab group: 3.8% versus 1.9% for ZA. The number of osteoporotic-related fractures was doubled in the ZA arm: 7 for denosumab vs. 15 for ZA.
Dr. Miller told listeners that this is the fourth study to show that denosumab is effective in osteoporotic patients previously on oral bisphosphonates, including prior risedronate, ibandronate, and alendronate.
AT THE ACR ANNUAL MEETING
Key clinical point: Denosumab was superior to zoledronic acid in building bone mineral density in postmenopausal women with osteoporosis who had previously been treated with oral bisphosphonates.
Major finding: Denosumab achieved a 2.1% difference in BMD at the lumbar spine and a 1.4% difference at the hip, compared with zoledronic acid.
Data source: Prospective, randomized, double-blind, double-dummy trial that included 643 patients.
Disclosures: Dr. Miller’s financial disclosures include Alexion, Amgen, Merck, Merck Serono, Boehringer Ingelheim, Regeneron, National Bone Health Alliance, Eli Lilly, Pfizer, Radius, and he is owner and director of the Colorado Center for Bone Research. The study was supported by Amgen.