User login
Progress in treating diabetic foot osteomyelitis
SAN DIEGO – The evidence-based treatment of diabetic foot osteomyelitis has jumped up to the next level as a result of two recent randomized clinical trials, the first-ever to address a couple of key contentious issues, experts agreed recently at the annual Interscience Conference on Antimicrobial Agents and Chemotherapy.
Dr. Eric Senneville presented highlights of the two randomized trials, one of which examined the optimal duration of antibiotic therapy in patients with nonsurgically treated diabetic foot osteomyelitis. The other study was the first-ever head-to-head randomized comparison of antibiotics versus conservative surgery.
He also touched on another new development in the treatment of diabetic foot osteomyelitis: surgically implanted topical antibiotics, which show promise in specific situations.
Dr. Senneville of Gustave Dron Hospital in Tourcoing, France, was senior investigator in the prospective, randomized, multicenter comparison of outcomes with 6 versus 12 weeks of open-label antibiotic therapy in 40 patients. All participants had bone biopsy–confirmed osteomyelitis with no ischemia, and none underwent any bone resection during the treatment period.
The 6-week regimen proved to be the winning strategy. It resulted in remission in 12 of 20 patients, a result not significantly different from the 14 of 20 remission rate with 12 weeks of treatment. Moreover, significant drug-related gastrointestinal side effects occurred in only three patients in the 6-week-treatment arm, compared with nine patients treated for 12 weeks. There was no difference between the two groups in rates of relapse, need for later bone resection, or spread of osteomyelitis (Diabetes Care. 2015 Feb;38[2]:302-7).
In the surgery-versus-antibiotics trial, investigators at the University of Madrid prospectively randomized 52 patients with diabetic foot osteomyelitis to 90 days of antibiotics with no surgery or to conservative surgery with 10 days of postoperative antibiotics. Dr. Senneville emphasized that this was a select patient population and at this point the results apply only to similar groups; that is, all participants had forefoot osteomyelitis without ischemia or necrosis. There were six dropouts: one in the medically treated arm and five in the surgical group.
The key finding: At 12 weeks of follow-up, main outcomes were similar in the two groups. Eighteen of 24 patients in the medically managed group achieved primary healing, for a 75% cure rate, not significantly different from the 86% rate – 19 of 22 patients cured – in the surgical group. Median time to healing was 7 weeks with antibiotics only and similar at 6 weeks with surgery. Four patients in the antibiotic group worsened and required surgery, while three in the surgery group required reoperation (Diabetes Care. 2014 Mar;37[3]:789-95).
Dr. Senneville noted that the reulceration rate was 10% in the medically treated group and twice that in the surgical group. A higher reulceration rate has also been seen in retrospective studies. It’s thought to result from what has been termed pressure transfer syndrome, which is particularly common among patients who undergo surgery on the first metatarsal head.
Dr. Edgar J. G. Peters of VU Academic Medical Center, Amsterdam, commented that the Spanish randomized trial of antibiotics versus surgery in diabetic osteomyelitis was sorely needed. All too often, he observed, earlier retrospective studies conducted by surgeons concluded that surgery was best, while those carried out by infectious disease specialists found the antiobiotics-only strategy to be superior. Skeptical unbiased physicians were left in the dark.
He noted that this important clinical trial as well as the major randomized trial of 6 versus 12 weeks of antibiotic therapy were published too late for inclusion in the recently released systematic review of treatments for diabetic foot infections conducted by the International Working Group on the Diabetic Foot (Diabetes Metab Res Rev. 2015 Sep 7. doi: 10.1002/dmrr.2706), for which both Dr. Peters and Dr. Senneville were coauthors.
Turning to the novel use of topical antibiotics in patients with diabetic foot osteomyelitis, Dr. Senneville described several potential advantages, including the attainment of optimal drug levels in the presence of peripheral vascular disease and in avascular spaces.
The idea is to place antibiotic-impregnated beads or bone cement in the space created by debridement and removal of infected bone. By filling the dead space, the antibiotic-impregnated cement may control the infection simmering in any areas of infected bone unintentionally left behind, while also reducing the risk of pressure transfer syndrome. The use of antibiotic-eluting bone cement has recently been shown to reduce the need for reoperation (J Foot Ankle Surg. 2015 Jul-Aug;54[4]:536-40).
Dr. Senneville reported serving on speakers’ bureaus for Novartis and Merck and as an advisor to Pfizer.
SAN DIEGO – The evidence-based treatment of diabetic foot osteomyelitis has jumped up to the next level as a result of two recent randomized clinical trials, the first-ever to address a couple of key contentious issues, experts agreed recently at the annual Interscience Conference on Antimicrobial Agents and Chemotherapy.
Dr. Eric Senneville presented highlights of the two randomized trials, one of which examined the optimal duration of antibiotic therapy in patients with nonsurgically treated diabetic foot osteomyelitis. The other study was the first-ever head-to-head randomized comparison of antibiotics versus conservative surgery.
He also touched on another new development in the treatment of diabetic foot osteomyelitis: surgically implanted topical antibiotics, which show promise in specific situations.
Dr. Senneville of Gustave Dron Hospital in Tourcoing, France, was senior investigator in the prospective, randomized, multicenter comparison of outcomes with 6 versus 12 weeks of open-label antibiotic therapy in 40 patients. All participants had bone biopsy–confirmed osteomyelitis with no ischemia, and none underwent any bone resection during the treatment period.
The 6-week regimen proved to be the winning strategy. It resulted in remission in 12 of 20 patients, a result not significantly different from the 14 of 20 remission rate with 12 weeks of treatment. Moreover, significant drug-related gastrointestinal side effects occurred in only three patients in the 6-week-treatment arm, compared with nine patients treated for 12 weeks. There was no difference between the two groups in rates of relapse, need for later bone resection, or spread of osteomyelitis (Diabetes Care. 2015 Feb;38[2]:302-7).
In the surgery-versus-antibiotics trial, investigators at the University of Madrid prospectively randomized 52 patients with diabetic foot osteomyelitis to 90 days of antibiotics with no surgery or to conservative surgery with 10 days of postoperative antibiotics. Dr. Senneville emphasized that this was a select patient population and at this point the results apply only to similar groups; that is, all participants had forefoot osteomyelitis without ischemia or necrosis. There were six dropouts: one in the medically treated arm and five in the surgical group.
The key finding: At 12 weeks of follow-up, main outcomes were similar in the two groups. Eighteen of 24 patients in the medically managed group achieved primary healing, for a 75% cure rate, not significantly different from the 86% rate – 19 of 22 patients cured – in the surgical group. Median time to healing was 7 weeks with antibiotics only and similar at 6 weeks with surgery. Four patients in the antibiotic group worsened and required surgery, while three in the surgery group required reoperation (Diabetes Care. 2014 Mar;37[3]:789-95).
Dr. Senneville noted that the reulceration rate was 10% in the medically treated group and twice that in the surgical group. A higher reulceration rate has also been seen in retrospective studies. It’s thought to result from what has been termed pressure transfer syndrome, which is particularly common among patients who undergo surgery on the first metatarsal head.
Dr. Edgar J. G. Peters of VU Academic Medical Center, Amsterdam, commented that the Spanish randomized trial of antibiotics versus surgery in diabetic osteomyelitis was sorely needed. All too often, he observed, earlier retrospective studies conducted by surgeons concluded that surgery was best, while those carried out by infectious disease specialists found the antiobiotics-only strategy to be superior. Skeptical unbiased physicians were left in the dark.
He noted that this important clinical trial as well as the major randomized trial of 6 versus 12 weeks of antibiotic therapy were published too late for inclusion in the recently released systematic review of treatments for diabetic foot infections conducted by the International Working Group on the Diabetic Foot (Diabetes Metab Res Rev. 2015 Sep 7. doi: 10.1002/dmrr.2706), for which both Dr. Peters and Dr. Senneville were coauthors.
Turning to the novel use of topical antibiotics in patients with diabetic foot osteomyelitis, Dr. Senneville described several potential advantages, including the attainment of optimal drug levels in the presence of peripheral vascular disease and in avascular spaces.
The idea is to place antibiotic-impregnated beads or bone cement in the space created by debridement and removal of infected bone. By filling the dead space, the antibiotic-impregnated cement may control the infection simmering in any areas of infected bone unintentionally left behind, while also reducing the risk of pressure transfer syndrome. The use of antibiotic-eluting bone cement has recently been shown to reduce the need for reoperation (J Foot Ankle Surg. 2015 Jul-Aug;54[4]:536-40).
Dr. Senneville reported serving on speakers’ bureaus for Novartis and Merck and as an advisor to Pfizer.
SAN DIEGO – The evidence-based treatment of diabetic foot osteomyelitis has jumped up to the next level as a result of two recent randomized clinical trials, the first-ever to address a couple of key contentious issues, experts agreed recently at the annual Interscience Conference on Antimicrobial Agents and Chemotherapy.
Dr. Eric Senneville presented highlights of the two randomized trials, one of which examined the optimal duration of antibiotic therapy in patients with nonsurgically treated diabetic foot osteomyelitis. The other study was the first-ever head-to-head randomized comparison of antibiotics versus conservative surgery.
He also touched on another new development in the treatment of diabetic foot osteomyelitis: surgically implanted topical antibiotics, which show promise in specific situations.
Dr. Senneville of Gustave Dron Hospital in Tourcoing, France, was senior investigator in the prospective, randomized, multicenter comparison of outcomes with 6 versus 12 weeks of open-label antibiotic therapy in 40 patients. All participants had bone biopsy–confirmed osteomyelitis with no ischemia, and none underwent any bone resection during the treatment period.
The 6-week regimen proved to be the winning strategy. It resulted in remission in 12 of 20 patients, a result not significantly different from the 14 of 20 remission rate with 12 weeks of treatment. Moreover, significant drug-related gastrointestinal side effects occurred in only three patients in the 6-week-treatment arm, compared with nine patients treated for 12 weeks. There was no difference between the two groups in rates of relapse, need for later bone resection, or spread of osteomyelitis (Diabetes Care. 2015 Feb;38[2]:302-7).
In the surgery-versus-antibiotics trial, investigators at the University of Madrid prospectively randomized 52 patients with diabetic foot osteomyelitis to 90 days of antibiotics with no surgery or to conservative surgery with 10 days of postoperative antibiotics. Dr. Senneville emphasized that this was a select patient population and at this point the results apply only to similar groups; that is, all participants had forefoot osteomyelitis without ischemia or necrosis. There were six dropouts: one in the medically treated arm and five in the surgical group.
The key finding: At 12 weeks of follow-up, main outcomes were similar in the two groups. Eighteen of 24 patients in the medically managed group achieved primary healing, for a 75% cure rate, not significantly different from the 86% rate – 19 of 22 patients cured – in the surgical group. Median time to healing was 7 weeks with antibiotics only and similar at 6 weeks with surgery. Four patients in the antibiotic group worsened and required surgery, while three in the surgery group required reoperation (Diabetes Care. 2014 Mar;37[3]:789-95).
Dr. Senneville noted that the reulceration rate was 10% in the medically treated group and twice that in the surgical group. A higher reulceration rate has also been seen in retrospective studies. It’s thought to result from what has been termed pressure transfer syndrome, which is particularly common among patients who undergo surgery on the first metatarsal head.
Dr. Edgar J. G. Peters of VU Academic Medical Center, Amsterdam, commented that the Spanish randomized trial of antibiotics versus surgery in diabetic osteomyelitis was sorely needed. All too often, he observed, earlier retrospective studies conducted by surgeons concluded that surgery was best, while those carried out by infectious disease specialists found the antiobiotics-only strategy to be superior. Skeptical unbiased physicians were left in the dark.
He noted that this important clinical trial as well as the major randomized trial of 6 versus 12 weeks of antibiotic therapy were published too late for inclusion in the recently released systematic review of treatments for diabetic foot infections conducted by the International Working Group on the Diabetic Foot (Diabetes Metab Res Rev. 2015 Sep 7. doi: 10.1002/dmrr.2706), for which both Dr. Peters and Dr. Senneville were coauthors.
Turning to the novel use of topical antibiotics in patients with diabetic foot osteomyelitis, Dr. Senneville described several potential advantages, including the attainment of optimal drug levels in the presence of peripheral vascular disease and in avascular spaces.
The idea is to place antibiotic-impregnated beads or bone cement in the space created by debridement and removal of infected bone. By filling the dead space, the antibiotic-impregnated cement may control the infection simmering in any areas of infected bone unintentionally left behind, while also reducing the risk of pressure transfer syndrome. The use of antibiotic-eluting bone cement has recently been shown to reduce the need for reoperation (J Foot Ankle Surg. 2015 Jul-Aug;54[4]:536-40).
Dr. Senneville reported serving on speakers’ bureaus for Novartis and Merck and as an advisor to Pfizer.
EXPERT ANALYSIS FROM ICAAC 2015
S. lugdunensis osteoarticular infection often linked to orthopedic devices
SAN DIEGO – Bone and joint infections caused by Staphylococcus lugdunensis are an underestimated hospital-acquired infection often associated with orthopedic devices, according to a multicenter study.
“Consider potential relapse even after 1 year of the end of antibiotic treatment and follow patients with bone and joint infections caused by S. lugdunensis for a minimum 2 years after the end of treatment,” lead study author Dr. Piseth Seng said in an interview at the annual Interscience Conference on Antimicrobial Agents and Chemotherapy.
S. lugdunensis is a virulent coagulase-negative staphylococcus which behaves like S. aureus. Prior to the current study, only 47 cases are believed to be published in the medical literature, according to Dr. Seng of the department of internal medicine at Assistance Publique des Hôpitaux de Marseille (France). The purpose of the current study was to report a series of 138 cases of S. lugdunensis osteoarticular infection managed in nine hospital centers and three private clinics in France from January 1995 to December 2014.
The mean age of patients was 61 years, and 68% were male. Of the 138 cases, 113 (82%) were associated with an orthopedic device, including 2 cases of infection after anterior cruciate ligament reconstruction, 66 cases of prosthetic joint infection, and 3 cases of vertebral orthopedic device infection. The majority of orthopedic device infections (88%) occurred more than 1 month after implantation, while the remaining 12% occurred within the first month of implantation.
The researchers identified 30 cases (22%) of bone and joint infection that occurred in the absence of an orthopedic device, including 7 cases of arthritis, 21 cases of osteitis, and 2 cases of vertebral osteomyelitis.
The majority of patients (91%) received a combination of antibiotic and surgical treatment, including amputation (6%), orthopedic prosthesis removal (14%), internal orthopedic device removal (23%), and surgical debridement and retention of the orthopedic device (41%). The proportion of S. lugdunensis strains with reduced susceptibility to antistaphylococcal agents was low. Resistant strains included five to oxacillin, four to fosfomycin, two to fusidic acid, two to co-trimoxazole, one to rifampicin, and one to clindamycin.
To date, relapses have occurred in 19% of the 123 patients in whom researchers have complete follow-up data. The readmission rate among these patients was 76%, and four (3%) died of their infection. “These relapses were not associated with risk factor or comorbidity or polymicrobial infection,” noted Dr. Seng, who characterized the incidence of bone and joint infections caused by S. lugdunensis as being under reported. “S. lugdunensis is known as an organism forming biofilms, but treatment options (surgical debridement or prosthesis removal) did not influence clinical outcomes.”
The mean time to relapse was 305 days and no risk factor or comorbidity was associated with relapse.
Dr. Seng acknowledged that the study was limited by its retrospective design. He and his associates reported having no financial disclosures.
SAN DIEGO – Bone and joint infections caused by Staphylococcus lugdunensis are an underestimated hospital-acquired infection often associated with orthopedic devices, according to a multicenter study.
“Consider potential relapse even after 1 year of the end of antibiotic treatment and follow patients with bone and joint infections caused by S. lugdunensis for a minimum 2 years after the end of treatment,” lead study author Dr. Piseth Seng said in an interview at the annual Interscience Conference on Antimicrobial Agents and Chemotherapy.
S. lugdunensis is a virulent coagulase-negative staphylococcus which behaves like S. aureus. Prior to the current study, only 47 cases are believed to be published in the medical literature, according to Dr. Seng of the department of internal medicine at Assistance Publique des Hôpitaux de Marseille (France). The purpose of the current study was to report a series of 138 cases of S. lugdunensis osteoarticular infection managed in nine hospital centers and three private clinics in France from January 1995 to December 2014.
The mean age of patients was 61 years, and 68% were male. Of the 138 cases, 113 (82%) were associated with an orthopedic device, including 2 cases of infection after anterior cruciate ligament reconstruction, 66 cases of prosthetic joint infection, and 3 cases of vertebral orthopedic device infection. The majority of orthopedic device infections (88%) occurred more than 1 month after implantation, while the remaining 12% occurred within the first month of implantation.
The researchers identified 30 cases (22%) of bone and joint infection that occurred in the absence of an orthopedic device, including 7 cases of arthritis, 21 cases of osteitis, and 2 cases of vertebral osteomyelitis.
The majority of patients (91%) received a combination of antibiotic and surgical treatment, including amputation (6%), orthopedic prosthesis removal (14%), internal orthopedic device removal (23%), and surgical debridement and retention of the orthopedic device (41%). The proportion of S. lugdunensis strains with reduced susceptibility to antistaphylococcal agents was low. Resistant strains included five to oxacillin, four to fosfomycin, two to fusidic acid, two to co-trimoxazole, one to rifampicin, and one to clindamycin.
To date, relapses have occurred in 19% of the 123 patients in whom researchers have complete follow-up data. The readmission rate among these patients was 76%, and four (3%) died of their infection. “These relapses were not associated with risk factor or comorbidity or polymicrobial infection,” noted Dr. Seng, who characterized the incidence of bone and joint infections caused by S. lugdunensis as being under reported. “S. lugdunensis is known as an organism forming biofilms, but treatment options (surgical debridement or prosthesis removal) did not influence clinical outcomes.”
The mean time to relapse was 305 days and no risk factor or comorbidity was associated with relapse.
Dr. Seng acknowledged that the study was limited by its retrospective design. He and his associates reported having no financial disclosures.
SAN DIEGO – Bone and joint infections caused by Staphylococcus lugdunensis are an underestimated hospital-acquired infection often associated with orthopedic devices, according to a multicenter study.
“Consider potential relapse even after 1 year of the end of antibiotic treatment and follow patients with bone and joint infections caused by S. lugdunensis for a minimum 2 years after the end of treatment,” lead study author Dr. Piseth Seng said in an interview at the annual Interscience Conference on Antimicrobial Agents and Chemotherapy.
S. lugdunensis is a virulent coagulase-negative staphylococcus which behaves like S. aureus. Prior to the current study, only 47 cases are believed to be published in the medical literature, according to Dr. Seng of the department of internal medicine at Assistance Publique des Hôpitaux de Marseille (France). The purpose of the current study was to report a series of 138 cases of S. lugdunensis osteoarticular infection managed in nine hospital centers and three private clinics in France from January 1995 to December 2014.
The mean age of patients was 61 years, and 68% were male. Of the 138 cases, 113 (82%) were associated with an orthopedic device, including 2 cases of infection after anterior cruciate ligament reconstruction, 66 cases of prosthetic joint infection, and 3 cases of vertebral orthopedic device infection. The majority of orthopedic device infections (88%) occurred more than 1 month after implantation, while the remaining 12% occurred within the first month of implantation.
The researchers identified 30 cases (22%) of bone and joint infection that occurred in the absence of an orthopedic device, including 7 cases of arthritis, 21 cases of osteitis, and 2 cases of vertebral osteomyelitis.
The majority of patients (91%) received a combination of antibiotic and surgical treatment, including amputation (6%), orthopedic prosthesis removal (14%), internal orthopedic device removal (23%), and surgical debridement and retention of the orthopedic device (41%). The proportion of S. lugdunensis strains with reduced susceptibility to antistaphylococcal agents was low. Resistant strains included five to oxacillin, four to fosfomycin, two to fusidic acid, two to co-trimoxazole, one to rifampicin, and one to clindamycin.
To date, relapses have occurred in 19% of the 123 patients in whom researchers have complete follow-up data. The readmission rate among these patients was 76%, and four (3%) died of their infection. “These relapses were not associated with risk factor or comorbidity or polymicrobial infection,” noted Dr. Seng, who characterized the incidence of bone and joint infections caused by S. lugdunensis as being under reported. “S. lugdunensis is known as an organism forming biofilms, but treatment options (surgical debridement or prosthesis removal) did not influence clinical outcomes.”
The mean time to relapse was 305 days and no risk factor or comorbidity was associated with relapse.
Dr. Seng acknowledged that the study was limited by its retrospective design. He and his associates reported having no financial disclosures.
AT ICAAC 2015
Key clinical point: S. lugdunensis infections are often associated with orthopedic devices.
Major finding: Of 138 cases of S. lugdunensis osteoarticular infection, 113 (82%) were associated with an orthopedic device.
Data source: A retrospective study of 138 cases of S. lugdunensis osteoarticular infection managed in nine hospitals and three private clinics in France.
Disclosures: The researchers reported having no financial disclosures.
FDA issues revised warning for adverse effects associated with canagliflozin
The Food and Drug Administration has issued a new warning and precaution for the type 2 diabetes drug canagliflozin, saying that risks of decreased bone density associated with the drug are more serious than previously stated.
Canagliflozin, a sodium-glucose cotransporter-2 (SGLT2) inhibitor, will now have a revised adverse reactions section on its drug label. Taking canagliflozin can significantly increase an individual’s chances of incurring bone fractures, due to the decreased bone mineral density caused by the drug. According to the FDA, these fractures can start to appear 12 weeks after starting a canagliflozin regimen, and can lead to bone mineral density loss in the hip and lower spine.
“FDA is continuing to evaluate the risk of bone fractures with other drugs in the SGLT2 inhibitor class, including dapagliflozin (Farxiga, Xigduo XR) and empagliflozin (Jardiance, Glyxambi, Synjardy), to determine if additional label changes or studies are needed,” the FDA stated, adding that all health care providers and patients are urged to contact the FDA if they experience adverse effects while taking any of these drugs.
Canagliflozin is marketed as Invokana and Invokamet by Janssen Pharmaceuticals, and was approved by the FDA in March 2013. The FDA is advising all health care professionals to carefully assess patients’ risk for developing bone fractures before prescribing the drug. Individuals who experience side effects while taking canagliflozin should submit a report through the FDA’s MedWatch program, or contact 1-800-332-1088 for more information.
The Food and Drug Administration has issued a new warning and precaution for the type 2 diabetes drug canagliflozin, saying that risks of decreased bone density associated with the drug are more serious than previously stated.
Canagliflozin, a sodium-glucose cotransporter-2 (SGLT2) inhibitor, will now have a revised adverse reactions section on its drug label. Taking canagliflozin can significantly increase an individual’s chances of incurring bone fractures, due to the decreased bone mineral density caused by the drug. According to the FDA, these fractures can start to appear 12 weeks after starting a canagliflozin regimen, and can lead to bone mineral density loss in the hip and lower spine.
“FDA is continuing to evaluate the risk of bone fractures with other drugs in the SGLT2 inhibitor class, including dapagliflozin (Farxiga, Xigduo XR) and empagliflozin (Jardiance, Glyxambi, Synjardy), to determine if additional label changes or studies are needed,” the FDA stated, adding that all health care providers and patients are urged to contact the FDA if they experience adverse effects while taking any of these drugs.
Canagliflozin is marketed as Invokana and Invokamet by Janssen Pharmaceuticals, and was approved by the FDA in March 2013. The FDA is advising all health care professionals to carefully assess patients’ risk for developing bone fractures before prescribing the drug. Individuals who experience side effects while taking canagliflozin should submit a report through the FDA’s MedWatch program, or contact 1-800-332-1088 for more information.
The Food and Drug Administration has issued a new warning and precaution for the type 2 diabetes drug canagliflozin, saying that risks of decreased bone density associated with the drug are more serious than previously stated.
Canagliflozin, a sodium-glucose cotransporter-2 (SGLT2) inhibitor, will now have a revised adverse reactions section on its drug label. Taking canagliflozin can significantly increase an individual’s chances of incurring bone fractures, due to the decreased bone mineral density caused by the drug. According to the FDA, these fractures can start to appear 12 weeks after starting a canagliflozin regimen, and can lead to bone mineral density loss in the hip and lower spine.
“FDA is continuing to evaluate the risk of bone fractures with other drugs in the SGLT2 inhibitor class, including dapagliflozin (Farxiga, Xigduo XR) and empagliflozin (Jardiance, Glyxambi, Synjardy), to determine if additional label changes or studies are needed,” the FDA stated, adding that all health care providers and patients are urged to contact the FDA if they experience adverse effects while taking any of these drugs.
Canagliflozin is marketed as Invokana and Invokamet by Janssen Pharmaceuticals, and was approved by the FDA in March 2013. The FDA is advising all health care professionals to carefully assess patients’ risk for developing bone fractures before prescribing the drug. Individuals who experience side effects while taking canagliflozin should submit a report through the FDA’s MedWatch program, or contact 1-800-332-1088 for more information.
Osteoporosis trends collide for Mexican American women
Among adults aged 65 years and over, women were 4.4 times as likely as men to have osteoporosis, and Mexican Americans were 2.4 times more likely than were blacks to have osteoporosis from 2005 to 2010, the National Center for Health Statistics reported.
So where does leave those who are both women and Mexican American?
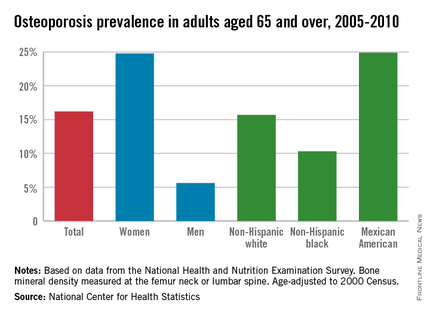
First, a little background: The age-adjusted prevalence of osteoporosis measured at either the lumbar spine or femur neck among adults aged 65 years and over was 24.8% for women and 5.6% for men, for an overall prevalence of 16.2%. Adults aged 65-79 years had an unadjusted prevalence of 12.8%, compared with 25.7% for those aged 80 years and over, according to data from the 2005-2010 National Health and Nutrition Examination Survey.
Age-adjusted prevalence over that time period for Mexican Americans aged 65 years and older was 24.9%, compared with 15.7% for non-Hispanic whites and 10.3% for non-Hispanic blacks.
Mexican American women, who find themselves at the intersection of these two trends, had an adjusted osteoporosis rate of 36.8%, the NCHS reported.
Osteoporosis was defined as a bone mineral density value that was more than 2.5 standard deviations below the mean value for young, non-Hispanic white females.
Among adults aged 65 years and over, women were 4.4 times as likely as men to have osteoporosis, and Mexican Americans were 2.4 times more likely than were blacks to have osteoporosis from 2005 to 2010, the National Center for Health Statistics reported.
So where does leave those who are both women and Mexican American?

First, a little background: The age-adjusted prevalence of osteoporosis measured at either the lumbar spine or femur neck among adults aged 65 years and over was 24.8% for women and 5.6% for men, for an overall prevalence of 16.2%. Adults aged 65-79 years had an unadjusted prevalence of 12.8%, compared with 25.7% for those aged 80 years and over, according to data from the 2005-2010 National Health and Nutrition Examination Survey.
Age-adjusted prevalence over that time period for Mexican Americans aged 65 years and older was 24.9%, compared with 15.7% for non-Hispanic whites and 10.3% for non-Hispanic blacks.
Mexican American women, who find themselves at the intersection of these two trends, had an adjusted osteoporosis rate of 36.8%, the NCHS reported.
Osteoporosis was defined as a bone mineral density value that was more than 2.5 standard deviations below the mean value for young, non-Hispanic white females.
Among adults aged 65 years and over, women were 4.4 times as likely as men to have osteoporosis, and Mexican Americans were 2.4 times more likely than were blacks to have osteoporosis from 2005 to 2010, the National Center for Health Statistics reported.
So where does leave those who are both women and Mexican American?

First, a little background: The age-adjusted prevalence of osteoporosis measured at either the lumbar spine or femur neck among adults aged 65 years and over was 24.8% for women and 5.6% for men, for an overall prevalence of 16.2%. Adults aged 65-79 years had an unadjusted prevalence of 12.8%, compared with 25.7% for those aged 80 years and over, according to data from the 2005-2010 National Health and Nutrition Examination Survey.
Age-adjusted prevalence over that time period for Mexican Americans aged 65 years and older was 24.9%, compared with 15.7% for non-Hispanic whites and 10.3% for non-Hispanic blacks.
Mexican American women, who find themselves at the intersection of these two trends, had an adjusted osteoporosis rate of 36.8%, the NCHS reported.
Osteoporosis was defined as a bone mineral density value that was more than 2.5 standard deviations below the mean value for young, non-Hispanic white females.
HLA-matched sibling transplants provide best outcomes in infantile osteopetrosis
Long-term survival after hematopoietic stem cell transplantation for infantile osteopetrosis was highest when grafts were taken from human leukocyte antigen (HLA)-matched siblings, according to the largest cohort of patients with the disease that has been compiled to date.
For HLA-matched sibling transplants, 5- and 10-year survival probabilities were both 62%, whereas the combined average survival probability for HLA-mismatched relative donors, HLA-matched unrelated donors, and HLA-unmatched unrelated donors was 42% after 5 years and 39% after 10 years, Dr. Paul J. Orchard of the University of Minnesota, Minneapolis, and his colleagues reported.
Of surviving patients, 70% have visual impairment and 10% have auditory impairment and motor delay. Despite this, most survivors are attending a public or specialized school, and 65% of survivors reported performance scores of 90 or 100 at last contact, the investigators said.
Graft failure was the most common cause of death, occurring in 50% of HLA-matched sibling transplant patients and in 43% of alternative HLA transplant patients. Veno-occlusive disease and interstitial pneumonitis rates were also high, both at about 20%.
“There is an urgent need to improve engraftment by developing novel strategies that target the microenvironment and study the association between genetic variants of osteopetrosis and transplantation outcomes,” the researchers said.
Find the full article in the July 9 issue of Blood (Blood 2015;126:270-6).
Long-term survival after hematopoietic stem cell transplantation for infantile osteopetrosis was highest when grafts were taken from human leukocyte antigen (HLA)-matched siblings, according to the largest cohort of patients with the disease that has been compiled to date.
For HLA-matched sibling transplants, 5- and 10-year survival probabilities were both 62%, whereas the combined average survival probability for HLA-mismatched relative donors, HLA-matched unrelated donors, and HLA-unmatched unrelated donors was 42% after 5 years and 39% after 10 years, Dr. Paul J. Orchard of the University of Minnesota, Minneapolis, and his colleagues reported.
Of surviving patients, 70% have visual impairment and 10% have auditory impairment and motor delay. Despite this, most survivors are attending a public or specialized school, and 65% of survivors reported performance scores of 90 or 100 at last contact, the investigators said.
Graft failure was the most common cause of death, occurring in 50% of HLA-matched sibling transplant patients and in 43% of alternative HLA transplant patients. Veno-occlusive disease and interstitial pneumonitis rates were also high, both at about 20%.
“There is an urgent need to improve engraftment by developing novel strategies that target the microenvironment and study the association between genetic variants of osteopetrosis and transplantation outcomes,” the researchers said.
Find the full article in the July 9 issue of Blood (Blood 2015;126:270-6).
Long-term survival after hematopoietic stem cell transplantation for infantile osteopetrosis was highest when grafts were taken from human leukocyte antigen (HLA)-matched siblings, according to the largest cohort of patients with the disease that has been compiled to date.
For HLA-matched sibling transplants, 5- and 10-year survival probabilities were both 62%, whereas the combined average survival probability for HLA-mismatched relative donors, HLA-matched unrelated donors, and HLA-unmatched unrelated donors was 42% after 5 years and 39% after 10 years, Dr. Paul J. Orchard of the University of Minnesota, Minneapolis, and his colleagues reported.
Of surviving patients, 70% have visual impairment and 10% have auditory impairment and motor delay. Despite this, most survivors are attending a public or specialized school, and 65% of survivors reported performance scores of 90 or 100 at last contact, the investigators said.
Graft failure was the most common cause of death, occurring in 50% of HLA-matched sibling transplant patients and in 43% of alternative HLA transplant patients. Veno-occlusive disease and interstitial pneumonitis rates were also high, both at about 20%.
“There is an urgent need to improve engraftment by developing novel strategies that target the microenvironment and study the association between genetic variants of osteopetrosis and transplantation outcomes,” the researchers said.
Find the full article in the July 9 issue of Blood (Blood 2015;126:270-6).
MDD tied to lower bone mineral density in men
In men, major depressive disorder has a negative impact on bone mineral density, a cross-sectional study shows.
The study included 928 men, aged 24-98. Each study participant’s ultradistal forearm, lumbar spine, total hip, and total body bone mineral density (BMD) (g/cm2) were measured using dual-energy x-ray absorptiometry. Clinicians queried patients on their history of major depressive disorder (MDD) and whether they were currently using antidepressants. Of the study population, 84 (9.1%) had a single manic episode, 50 (5.4%) had recurrent (at least two ) manic episodes, and 65 (7.0%) were using antidepressants.
Study participants with recurrent MDD had lower forearm, total hip, lumbar spine, and total body BMDs than study participants who had one manic episode or had no history of MDD. After age and weight adjustments, recurrent MDD was significantly associated with lower forearm and total body BMDs, with forearm BMDs having been 6.5% lower and total body BMDs having been 2.5% lower in study participants with recurrent MDD than in those with no history of MDD.
Those men who had experienced a single manic episode actually had higher forearm, total hip, and total body BMDs than men with no history of MDD. Also, single-episode MDD was positively associated with total hip BMD – a finding that Paivi H. Rauma, a PhD student and researcher at University of Eastern Finland, Kuopio, and her colleagues said they could not explain.
Among the study’s other results was that antidepressant use was associated with lower BMD for the men studied with the lowest body weights (between 75 kg and 110 kg).
“We found that MDD and antidepressant use were independently associated with BMD; however, separation of these two issues is difficult,” the researchers wrote. “In all, prevention of depression, its early detection, and appropriate medical care are important issues in the prevention and care of osteoporosis in men. Lastly, these data raise the issue of screening for BMD in risk populations.”
Read the full study in Journal of Musculoskeletal and Neuronal Interactions.
In men, major depressive disorder has a negative impact on bone mineral density, a cross-sectional study shows.
The study included 928 men, aged 24-98. Each study participant’s ultradistal forearm, lumbar spine, total hip, and total body bone mineral density (BMD) (g/cm2) were measured using dual-energy x-ray absorptiometry. Clinicians queried patients on their history of major depressive disorder (MDD) and whether they were currently using antidepressants. Of the study population, 84 (9.1%) had a single manic episode, 50 (5.4%) had recurrent (at least two ) manic episodes, and 65 (7.0%) were using antidepressants.
Study participants with recurrent MDD had lower forearm, total hip, lumbar spine, and total body BMDs than study participants who had one manic episode or had no history of MDD. After age and weight adjustments, recurrent MDD was significantly associated with lower forearm and total body BMDs, with forearm BMDs having been 6.5% lower and total body BMDs having been 2.5% lower in study participants with recurrent MDD than in those with no history of MDD.
Those men who had experienced a single manic episode actually had higher forearm, total hip, and total body BMDs than men with no history of MDD. Also, single-episode MDD was positively associated with total hip BMD – a finding that Paivi H. Rauma, a PhD student and researcher at University of Eastern Finland, Kuopio, and her colleagues said they could not explain.
Among the study’s other results was that antidepressant use was associated with lower BMD for the men studied with the lowest body weights (between 75 kg and 110 kg).
“We found that MDD and antidepressant use were independently associated with BMD; however, separation of these two issues is difficult,” the researchers wrote. “In all, prevention of depression, its early detection, and appropriate medical care are important issues in the prevention and care of osteoporosis in men. Lastly, these data raise the issue of screening for BMD in risk populations.”
Read the full study in Journal of Musculoskeletal and Neuronal Interactions.
In men, major depressive disorder has a negative impact on bone mineral density, a cross-sectional study shows.
The study included 928 men, aged 24-98. Each study participant’s ultradistal forearm, lumbar spine, total hip, and total body bone mineral density (BMD) (g/cm2) were measured using dual-energy x-ray absorptiometry. Clinicians queried patients on their history of major depressive disorder (MDD) and whether they were currently using antidepressants. Of the study population, 84 (9.1%) had a single manic episode, 50 (5.4%) had recurrent (at least two ) manic episodes, and 65 (7.0%) were using antidepressants.
Study participants with recurrent MDD had lower forearm, total hip, lumbar spine, and total body BMDs than study participants who had one manic episode or had no history of MDD. After age and weight adjustments, recurrent MDD was significantly associated with lower forearm and total body BMDs, with forearm BMDs having been 6.5% lower and total body BMDs having been 2.5% lower in study participants with recurrent MDD than in those with no history of MDD.
Those men who had experienced a single manic episode actually had higher forearm, total hip, and total body BMDs than men with no history of MDD. Also, single-episode MDD was positively associated with total hip BMD – a finding that Paivi H. Rauma, a PhD student and researcher at University of Eastern Finland, Kuopio, and her colleagues said they could not explain.
Among the study’s other results was that antidepressant use was associated with lower BMD for the men studied with the lowest body weights (between 75 kg and 110 kg).
“We found that MDD and antidepressant use were independently associated with BMD; however, separation of these two issues is difficult,” the researchers wrote. “In all, prevention of depression, its early detection, and appropriate medical care are important issues in the prevention and care of osteoporosis in men. Lastly, these data raise the issue of screening for BMD in risk populations.”
Read the full study in Journal of Musculoskeletal and Neuronal Interactions.
ASCO: Adjuvant denosumab halves fracture risk for breast cancer patients on AIs
CHICAGO – Adjuvant denosumab is efficacious and safe for reducing fracture risk among women taking aromatase inhibitors (AIs) as part of their treatment for early breast cancer, finds the Austrian Breast & Colorectal Cancer Study Group’s study 18 (ABCSG-18).
Compared with peers randomized to placebo in the phase III trial, women randomized to the antiresorptive monoclonal antibody at the dose typically used to treat osteoporosis were half as likely to experience a first clinical fracture, first author Dr. Michael Gnant reported at the annual meeting of the American Society of Clinical Oncology. The benefit was similar whether women had normal bone mineral density at baseline or already had osteopenia.
Patients in the denosumab group did not have a significantly higher rate of adverse events, including the much-feared complication of osteonecrosis of the jaw.
“The actual fracture risk of postmenopausal breast cancer patients on AIs is substantial and may have been underestimated until today,” commented Dr. Gnant, professor of surgery at the Medical University of Vienna. “In these patients with only a modest risk of disease recurrence, adjuvant denosumab significantly reduced the bone side effects of AI treatment. We therefore believe that denosumab 60 mg every 6 months should be considered for clinical practice.”
“Today, several clinical practice guidelines advocate the use of bisphosphonates for breast cancer patients receiving AIs, however, only if they are at high risk for fractures,” he further noted. However, “patients with normal baseline bone mineral density showed a similar fracture risk but also similar benefit from denosumab as compared to patients with baseline T scores below –1, indicating that DEXA scans may be an insufficient way to assess the individual patient’s fracture risk. In view of the benefits in this particular patient subgroup, we may have to rediscuss our current clinical practice guidelines.”
Invited discussant Dr. Robert E. Coleman of the University of Sheffield and Weston Park Hospital in England, said, “It’s very important to dissect out fractures related to subsequent recurrence from fractures due to poor bone health.” Most of the reduction in fracture risk in ABCSG-18 appeared to be because of prevention of fractures before any recurrence, whereas most of that in the AZURE trial (Adjuvant Zoledronic Acid to Reduce Recurrence) of an adjuvant bisphosphonate, another type of antiresorptive agent, appeared to be because of prevention of fractures from bone metastases. “So I think we are seeing something very different with denosumab to what we’ve seen to date with a bisphosphonate,” he said.
“As oncologists, we are somewhat wedded to measuring bone mineral density as the reason for giving bone-targeted therapy to protect [against] bone loss, but there are much better ways of predicting fracture with online algorithms such as FRAX [Fracture Risk Assessment Tool] and others,” Dr. Coleman further commented. “And bone mineral density is a pretty poor predictor of fracture, so it’s perhaps not surprising that the risk reductions were fairly similar” across bone mineral density subgroups.
During a question and answer period, session attendee Dr. Toru Watanabe, Hamamatsu (Japan) Oncology Center, said, “It is really clear that the osteoporosis-related fracture is prevented by denosumab at the dose usually used for the treatment of osteoporosis. That part is very clear. My question is, the same dose is being tested for modifying overall survival or progression-free survival. Don’t you think it’s necessary to conduct some kind of dose-finding trial?”
Two studies are addressing the impact of denosumab on breast cancer outcomes, according to Dr. Gnant: the investigators’ ABCSG-18 study and the Study of Denosumab as Adjuvant Treatment for Women With High-Risk Early Breast Cancer Receiving Neoadjuvant or Adjuvant Therapy (D-CARE), which is using a higher initial dose and tapering after 1 year. “So we will have that indirect comparison at least. My personal expectation would be that there is a trade-off potentially between efficacy and tolerability,” he commented.
The 3,425 postmenopausal breast cancer patients in ABCSG-18 were randomized evenly to receive 60 mg of denosumab or placebo every 6 months. Denosumab is approved by the Food and Drug Administration for the prevention and treatment of fractures due to bone metastases (brand name Xgeva) and osteoporosis after menopause (brand name Prolia), as well as other indications. The study used the dose for postmenopausal osteoporosis, which is much lower than that typically used for bone metastases (120 mg every 4 weeks), Dr. Gnant noted.
Main results showed that denosumab was highly efficacious in reducing the risk of first clinical fractures, meaning those that were clinically evident and causing symptoms (hazard ratio, 0.50; P less than .0001), according to data presented at the meeting and simultaneously published (Lancet 2015 May 31).
The estimated 6-year fracture rate was about 10% in the denosumab group and 20% in the placebo group. “Please note that the frequency of clinical fractures reported in this trial that is focusing on bone health is markedly higher than fracture rates reported in previous large AI trials. Obviously, we had a tendency to underreport them in those trials,” Dr. Gnant commented. “The true magnitude of the problem in clinical practice is likely reflected in the placebo group … with approximately one out of five patients experiencing a new clinical fracture within 5-6 years of adjuvant AI treatment.”
Benefit was similar across numerous patient subgroups studied, including the subgroups of women who had a baseline bone mineral density T-score of less than –1 and women who had a baseline bone mineral density T-score of –1 or greater.
Additionally, the denosumab group had improvements from baseline in bone mineral density of the lumbar spine, total hip, and femoral neck, whereas the placebo group had worsening at all sites (P less than .0001 between groups for each site). And at 36 months, the denosumab group had significantly lower risks of both new vertebral fractures and new or worsening vertebral fractures.
“Adjuvant denosumab at this dose and schedule is safe,” Dr. Gnant maintained. The two groups had similar rates of various adverse events, with musculoskeletal disorders and vascular disorders (including hot flashes) predominating. “This means that we are in essence reporting the side effects of the underlying adjuvant AI treatment,” he noted.
There were 31 cases of dental issues, but none met diagnostic criteria for osteonecrosis of the jaw. “We can safely say that at this dose of denosumab, 60 mg twice yearly, ONJ is not an issue,” Dr. Gnant commented. Additionally, none of the women experienced atypical fractures.
Dr. Gnant disclosed employment of an immediate family member with Sandoz; receipt of honoraria from Amgen, AstraZeneca, GlaxoSmithKline, NanoString Technologies, Novartis, and Roche Pharma AG; a consulting or advisory role with Accelsiors, AstraZeneca, and Novartis; and receipt of research funding from GlaxoSmithKline, Novartis, Pfizer, Roche Pharma AG, Sanofi, and Smiths Medical. The trial was sponsored by Amgen.
CHICAGO – Adjuvant denosumab is efficacious and safe for reducing fracture risk among women taking aromatase inhibitors (AIs) as part of their treatment for early breast cancer, finds the Austrian Breast & Colorectal Cancer Study Group’s study 18 (ABCSG-18).
Compared with peers randomized to placebo in the phase III trial, women randomized to the antiresorptive monoclonal antibody at the dose typically used to treat osteoporosis were half as likely to experience a first clinical fracture, first author Dr. Michael Gnant reported at the annual meeting of the American Society of Clinical Oncology. The benefit was similar whether women had normal bone mineral density at baseline or already had osteopenia.
Patients in the denosumab group did not have a significantly higher rate of adverse events, including the much-feared complication of osteonecrosis of the jaw.
“The actual fracture risk of postmenopausal breast cancer patients on AIs is substantial and may have been underestimated until today,” commented Dr. Gnant, professor of surgery at the Medical University of Vienna. “In these patients with only a modest risk of disease recurrence, adjuvant denosumab significantly reduced the bone side effects of AI treatment. We therefore believe that denosumab 60 mg every 6 months should be considered for clinical practice.”
“Today, several clinical practice guidelines advocate the use of bisphosphonates for breast cancer patients receiving AIs, however, only if they are at high risk for fractures,” he further noted. However, “patients with normal baseline bone mineral density showed a similar fracture risk but also similar benefit from denosumab as compared to patients with baseline T scores below –1, indicating that DEXA scans may be an insufficient way to assess the individual patient’s fracture risk. In view of the benefits in this particular patient subgroup, we may have to rediscuss our current clinical practice guidelines.”
Invited discussant Dr. Robert E. Coleman of the University of Sheffield and Weston Park Hospital in England, said, “It’s very important to dissect out fractures related to subsequent recurrence from fractures due to poor bone health.” Most of the reduction in fracture risk in ABCSG-18 appeared to be because of prevention of fractures before any recurrence, whereas most of that in the AZURE trial (Adjuvant Zoledronic Acid to Reduce Recurrence) of an adjuvant bisphosphonate, another type of antiresorptive agent, appeared to be because of prevention of fractures from bone metastases. “So I think we are seeing something very different with denosumab to what we’ve seen to date with a bisphosphonate,” he said.
“As oncologists, we are somewhat wedded to measuring bone mineral density as the reason for giving bone-targeted therapy to protect [against] bone loss, but there are much better ways of predicting fracture with online algorithms such as FRAX [Fracture Risk Assessment Tool] and others,” Dr. Coleman further commented. “And bone mineral density is a pretty poor predictor of fracture, so it’s perhaps not surprising that the risk reductions were fairly similar” across bone mineral density subgroups.
During a question and answer period, session attendee Dr. Toru Watanabe, Hamamatsu (Japan) Oncology Center, said, “It is really clear that the osteoporosis-related fracture is prevented by denosumab at the dose usually used for the treatment of osteoporosis. That part is very clear. My question is, the same dose is being tested for modifying overall survival or progression-free survival. Don’t you think it’s necessary to conduct some kind of dose-finding trial?”
Two studies are addressing the impact of denosumab on breast cancer outcomes, according to Dr. Gnant: the investigators’ ABCSG-18 study and the Study of Denosumab as Adjuvant Treatment for Women With High-Risk Early Breast Cancer Receiving Neoadjuvant or Adjuvant Therapy (D-CARE), which is using a higher initial dose and tapering after 1 year. “So we will have that indirect comparison at least. My personal expectation would be that there is a trade-off potentially between efficacy and tolerability,” he commented.
The 3,425 postmenopausal breast cancer patients in ABCSG-18 were randomized evenly to receive 60 mg of denosumab or placebo every 6 months. Denosumab is approved by the Food and Drug Administration for the prevention and treatment of fractures due to bone metastases (brand name Xgeva) and osteoporosis after menopause (brand name Prolia), as well as other indications. The study used the dose for postmenopausal osteoporosis, which is much lower than that typically used for bone metastases (120 mg every 4 weeks), Dr. Gnant noted.
Main results showed that denosumab was highly efficacious in reducing the risk of first clinical fractures, meaning those that were clinically evident and causing symptoms (hazard ratio, 0.50; P less than .0001), according to data presented at the meeting and simultaneously published (Lancet 2015 May 31).
The estimated 6-year fracture rate was about 10% in the denosumab group and 20% in the placebo group. “Please note that the frequency of clinical fractures reported in this trial that is focusing on bone health is markedly higher than fracture rates reported in previous large AI trials. Obviously, we had a tendency to underreport them in those trials,” Dr. Gnant commented. “The true magnitude of the problem in clinical practice is likely reflected in the placebo group … with approximately one out of five patients experiencing a new clinical fracture within 5-6 years of adjuvant AI treatment.”
Benefit was similar across numerous patient subgroups studied, including the subgroups of women who had a baseline bone mineral density T-score of less than –1 and women who had a baseline bone mineral density T-score of –1 or greater.
Additionally, the denosumab group had improvements from baseline in bone mineral density of the lumbar spine, total hip, and femoral neck, whereas the placebo group had worsening at all sites (P less than .0001 between groups for each site). And at 36 months, the denosumab group had significantly lower risks of both new vertebral fractures and new or worsening vertebral fractures.
“Adjuvant denosumab at this dose and schedule is safe,” Dr. Gnant maintained. The two groups had similar rates of various adverse events, with musculoskeletal disorders and vascular disorders (including hot flashes) predominating. “This means that we are in essence reporting the side effects of the underlying adjuvant AI treatment,” he noted.
There were 31 cases of dental issues, but none met diagnostic criteria for osteonecrosis of the jaw. “We can safely say that at this dose of denosumab, 60 mg twice yearly, ONJ is not an issue,” Dr. Gnant commented. Additionally, none of the women experienced atypical fractures.
Dr. Gnant disclosed employment of an immediate family member with Sandoz; receipt of honoraria from Amgen, AstraZeneca, GlaxoSmithKline, NanoString Technologies, Novartis, and Roche Pharma AG; a consulting or advisory role with Accelsiors, AstraZeneca, and Novartis; and receipt of research funding from GlaxoSmithKline, Novartis, Pfizer, Roche Pharma AG, Sanofi, and Smiths Medical. The trial was sponsored by Amgen.
CHICAGO – Adjuvant denosumab is efficacious and safe for reducing fracture risk among women taking aromatase inhibitors (AIs) as part of their treatment for early breast cancer, finds the Austrian Breast & Colorectal Cancer Study Group’s study 18 (ABCSG-18).
Compared with peers randomized to placebo in the phase III trial, women randomized to the antiresorptive monoclonal antibody at the dose typically used to treat osteoporosis were half as likely to experience a first clinical fracture, first author Dr. Michael Gnant reported at the annual meeting of the American Society of Clinical Oncology. The benefit was similar whether women had normal bone mineral density at baseline or already had osteopenia.
Patients in the denosumab group did not have a significantly higher rate of adverse events, including the much-feared complication of osteonecrosis of the jaw.
“The actual fracture risk of postmenopausal breast cancer patients on AIs is substantial and may have been underestimated until today,” commented Dr. Gnant, professor of surgery at the Medical University of Vienna. “In these patients with only a modest risk of disease recurrence, adjuvant denosumab significantly reduced the bone side effects of AI treatment. We therefore believe that denosumab 60 mg every 6 months should be considered for clinical practice.”
“Today, several clinical practice guidelines advocate the use of bisphosphonates for breast cancer patients receiving AIs, however, only if they are at high risk for fractures,” he further noted. However, “patients with normal baseline bone mineral density showed a similar fracture risk but also similar benefit from denosumab as compared to patients with baseline T scores below –1, indicating that DEXA scans may be an insufficient way to assess the individual patient’s fracture risk. In view of the benefits in this particular patient subgroup, we may have to rediscuss our current clinical practice guidelines.”
Invited discussant Dr. Robert E. Coleman of the University of Sheffield and Weston Park Hospital in England, said, “It’s very important to dissect out fractures related to subsequent recurrence from fractures due to poor bone health.” Most of the reduction in fracture risk in ABCSG-18 appeared to be because of prevention of fractures before any recurrence, whereas most of that in the AZURE trial (Adjuvant Zoledronic Acid to Reduce Recurrence) of an adjuvant bisphosphonate, another type of antiresorptive agent, appeared to be because of prevention of fractures from bone metastases. “So I think we are seeing something very different with denosumab to what we’ve seen to date with a bisphosphonate,” he said.
“As oncologists, we are somewhat wedded to measuring bone mineral density as the reason for giving bone-targeted therapy to protect [against] bone loss, but there are much better ways of predicting fracture with online algorithms such as FRAX [Fracture Risk Assessment Tool] and others,” Dr. Coleman further commented. “And bone mineral density is a pretty poor predictor of fracture, so it’s perhaps not surprising that the risk reductions were fairly similar” across bone mineral density subgroups.
During a question and answer period, session attendee Dr. Toru Watanabe, Hamamatsu (Japan) Oncology Center, said, “It is really clear that the osteoporosis-related fracture is prevented by denosumab at the dose usually used for the treatment of osteoporosis. That part is very clear. My question is, the same dose is being tested for modifying overall survival or progression-free survival. Don’t you think it’s necessary to conduct some kind of dose-finding trial?”
Two studies are addressing the impact of denosumab on breast cancer outcomes, according to Dr. Gnant: the investigators’ ABCSG-18 study and the Study of Denosumab as Adjuvant Treatment for Women With High-Risk Early Breast Cancer Receiving Neoadjuvant or Adjuvant Therapy (D-CARE), which is using a higher initial dose and tapering after 1 year. “So we will have that indirect comparison at least. My personal expectation would be that there is a trade-off potentially between efficacy and tolerability,” he commented.
The 3,425 postmenopausal breast cancer patients in ABCSG-18 were randomized evenly to receive 60 mg of denosumab or placebo every 6 months. Denosumab is approved by the Food and Drug Administration for the prevention and treatment of fractures due to bone metastases (brand name Xgeva) and osteoporosis after menopause (brand name Prolia), as well as other indications. The study used the dose for postmenopausal osteoporosis, which is much lower than that typically used for bone metastases (120 mg every 4 weeks), Dr. Gnant noted.
Main results showed that denosumab was highly efficacious in reducing the risk of first clinical fractures, meaning those that were clinically evident and causing symptoms (hazard ratio, 0.50; P less than .0001), according to data presented at the meeting and simultaneously published (Lancet 2015 May 31).
The estimated 6-year fracture rate was about 10% in the denosumab group and 20% in the placebo group. “Please note that the frequency of clinical fractures reported in this trial that is focusing on bone health is markedly higher than fracture rates reported in previous large AI trials. Obviously, we had a tendency to underreport them in those trials,” Dr. Gnant commented. “The true magnitude of the problem in clinical practice is likely reflected in the placebo group … with approximately one out of five patients experiencing a new clinical fracture within 5-6 years of adjuvant AI treatment.”
Benefit was similar across numerous patient subgroups studied, including the subgroups of women who had a baseline bone mineral density T-score of less than –1 and women who had a baseline bone mineral density T-score of –1 or greater.
Additionally, the denosumab group had improvements from baseline in bone mineral density of the lumbar spine, total hip, and femoral neck, whereas the placebo group had worsening at all sites (P less than .0001 between groups for each site). And at 36 months, the denosumab group had significantly lower risks of both new vertebral fractures and new or worsening vertebral fractures.
“Adjuvant denosumab at this dose and schedule is safe,” Dr. Gnant maintained. The two groups had similar rates of various adverse events, with musculoskeletal disorders and vascular disorders (including hot flashes) predominating. “This means that we are in essence reporting the side effects of the underlying adjuvant AI treatment,” he noted.
There were 31 cases of dental issues, but none met diagnostic criteria for osteonecrosis of the jaw. “We can safely say that at this dose of denosumab, 60 mg twice yearly, ONJ is not an issue,” Dr. Gnant commented. Additionally, none of the women experienced atypical fractures.
Dr. Gnant disclosed employment of an immediate family member with Sandoz; receipt of honoraria from Amgen, AstraZeneca, GlaxoSmithKline, NanoString Technologies, Novartis, and Roche Pharma AG; a consulting or advisory role with Accelsiors, AstraZeneca, and Novartis; and receipt of research funding from GlaxoSmithKline, Novartis, Pfizer, Roche Pharma AG, Sanofi, and Smiths Medical. The trial was sponsored by Amgen.
AT THE 2015 ASCO ANNUAL MEETING
Key clinical point: Denosumab reduces the risk of clinical fractures in postmenopausal women taking AIs for early breast cancer.
Major finding: The denosumab group was half as likely to have a first clinical fracture as the placebo group (HR, 0.50).
Data source: A randomized phase III trial among 3,425 postmenopausal women with early breast cancer taking AIs.
Disclosures: Dr. Gnant disclosed employment of an immediate family member with Sandoz; receipt of honoraria from Amgen, AstraZeneca, GlaxoSmithKline, NanoString Technologies, Novartis, and Roche Pharma AG; a consulting or advisory role with Accelsiors, AstraZeneca, and Novartis; and receipt of research funding from GlaxoSmithKline, Novartis, Pfizer, Roche Pharma AG, Sanofi, and Smiths Medical. The trial was sponsored by Amgen.
AACE: Bisphosphonates do not prevent fractures in adults with osteogenesis imperfecta
NASHVILLE, TENN. – Bisphosphonates help prevent fractures in some children with osteogenesis imperfecta, but they don't do the same for adults with the condition, according to a review from Johns Hopkins University and the Kennedy Krieger Institute.
Even so, bisphosphonates are used widely for adult osteogenesis imperfecta (OI) “because people have nothing else to hang their hat on, and the average physician doesn’t understand that osteogenesis imperfecta is not the same as age-related osteoporosis,” said senior investigator Dr. Jay R. Shapiro, director of Kennedy Krieger’s osteogenesis imperfecta program in Baltimore.
“We see adults with OI all the time who have been on bisphosphonates for 10 years, 12 years. It’s not doing anything for them, and sooner or later they come to realize that.” Meanwhile, “I think people are getting a little bit of a queasy feeling about not fully understanding the effectiveness and side effects of long-term treatment with bisphosphonates,” said Dr. Shapiro, also professor in the department of physical medicine and rehabilitation at Johns Hopkins University, Baltimore.
Adults with OI “also don’t respond to Forteo [teriparatide]. I do not recommend treatment with bisphosphonates or Forteo in” adults, he said at the annual meeting of the American Association of Clinical Endocrinologists.
Dr. Shapiro shared his thoughts during an interview regarding his latest study, an analysis of five children with OI under 18 years of age who responded to pamidronate (Aredia) and 11 who did not, meaning that they had two or more fractures per year while on the drug.
His team also compared fracture outcomes in 34 adults with OI treated with oral or intravenous bisphosphonates with 12 untreated adults. The adults were, on average, 52 years old.
The goal was to see if common bone markers predicted who would respond to bisphosphonates, but they did not. Vitamin D, phosphorus, alkaline phosphatase, C-telopeptide, and other measures were the same in children regardless of their response to pamidronate, and the same in adults with OI whether or not they were on bisphosphonates.
“We have not yet defined what the difference is between responders and nonresponders. If you take a crack at the simple things, they don’t help,” Dr. Shapiro said.
Children who responded had a mean of 4.8 fractures over an average of 42.6 months of treatment. Nonresponders had a mean of 15.6 fractures over an average of 72.7 months of treatment.
“For a period of time, you can expect about two-thirds of kids to respond. I would look to see a decrease in fracture rates within 2 years of treatment. If they haven’t decreased their fracture rate [by then], I would be very cautious about continuing,” he said, adding that the optimal duration of treatment in children is unknown.
In adults, the team found no difference in fracture rates at 5 and 10 years. Treated adults had an average of 1.71 fractures over 10 years, versus 1.23 in untreated adults (P = 0.109).
The numbers in the study were too small for meaningful subgroup analysis by OI type.
The findings parallel recent meta-analyses; some have found that bisphosphonates help OI children, but none has found benefits for adults (J. Bone. Miner. Res. 2015;30:929-33). “To date, there is no evidence indicating that bisphosphonates have a positive effect on fracture rates in adults,” Dr. Shapiro said.
Bone turnover declines after puberty, which may explain why the drugs lose their effectiveness after age 18 or so. “What happens in OI anyway is that, after puberty, the fracture rates normally go way down,” Dr. Shapiro said.
Dr. Shapiro said that he had no relevant disclosures, and that there was no outside funding for the work.
NASHVILLE, TENN. – Bisphosphonates help prevent fractures in some children with osteogenesis imperfecta, but they don't do the same for adults with the condition, according to a review from Johns Hopkins University and the Kennedy Krieger Institute.
Even so, bisphosphonates are used widely for adult osteogenesis imperfecta (OI) “because people have nothing else to hang their hat on, and the average physician doesn’t understand that osteogenesis imperfecta is not the same as age-related osteoporosis,” said senior investigator Dr. Jay R. Shapiro, director of Kennedy Krieger’s osteogenesis imperfecta program in Baltimore.
“We see adults with OI all the time who have been on bisphosphonates for 10 years, 12 years. It’s not doing anything for them, and sooner or later they come to realize that.” Meanwhile, “I think people are getting a little bit of a queasy feeling about not fully understanding the effectiveness and side effects of long-term treatment with bisphosphonates,” said Dr. Shapiro, also professor in the department of physical medicine and rehabilitation at Johns Hopkins University, Baltimore.
Adults with OI “also don’t respond to Forteo [teriparatide]. I do not recommend treatment with bisphosphonates or Forteo in” adults, he said at the annual meeting of the American Association of Clinical Endocrinologists.
Dr. Shapiro shared his thoughts during an interview regarding his latest study, an analysis of five children with OI under 18 years of age who responded to pamidronate (Aredia) and 11 who did not, meaning that they had two or more fractures per year while on the drug.
His team also compared fracture outcomes in 34 adults with OI treated with oral or intravenous bisphosphonates with 12 untreated adults. The adults were, on average, 52 years old.
The goal was to see if common bone markers predicted who would respond to bisphosphonates, but they did not. Vitamin D, phosphorus, alkaline phosphatase, C-telopeptide, and other measures were the same in children regardless of their response to pamidronate, and the same in adults with OI whether or not they were on bisphosphonates.
“We have not yet defined what the difference is between responders and nonresponders. If you take a crack at the simple things, they don’t help,” Dr. Shapiro said.
Children who responded had a mean of 4.8 fractures over an average of 42.6 months of treatment. Nonresponders had a mean of 15.6 fractures over an average of 72.7 months of treatment.
“For a period of time, you can expect about two-thirds of kids to respond. I would look to see a decrease in fracture rates within 2 years of treatment. If they haven’t decreased their fracture rate [by then], I would be very cautious about continuing,” he said, adding that the optimal duration of treatment in children is unknown.
In adults, the team found no difference in fracture rates at 5 and 10 years. Treated adults had an average of 1.71 fractures over 10 years, versus 1.23 in untreated adults (P = 0.109).
The numbers in the study were too small for meaningful subgroup analysis by OI type.
The findings parallel recent meta-analyses; some have found that bisphosphonates help OI children, but none has found benefits for adults (J. Bone. Miner. Res. 2015;30:929-33). “To date, there is no evidence indicating that bisphosphonates have a positive effect on fracture rates in adults,” Dr. Shapiro said.
Bone turnover declines after puberty, which may explain why the drugs lose their effectiveness after age 18 or so. “What happens in OI anyway is that, after puberty, the fracture rates normally go way down,” Dr. Shapiro said.
Dr. Shapiro said that he had no relevant disclosures, and that there was no outside funding for the work.
NASHVILLE, TENN. – Bisphosphonates help prevent fractures in some children with osteogenesis imperfecta, but they don't do the same for adults with the condition, according to a review from Johns Hopkins University and the Kennedy Krieger Institute.
Even so, bisphosphonates are used widely for adult osteogenesis imperfecta (OI) “because people have nothing else to hang their hat on, and the average physician doesn’t understand that osteogenesis imperfecta is not the same as age-related osteoporosis,” said senior investigator Dr. Jay R. Shapiro, director of Kennedy Krieger’s osteogenesis imperfecta program in Baltimore.
“We see adults with OI all the time who have been on bisphosphonates for 10 years, 12 years. It’s not doing anything for them, and sooner or later they come to realize that.” Meanwhile, “I think people are getting a little bit of a queasy feeling about not fully understanding the effectiveness and side effects of long-term treatment with bisphosphonates,” said Dr. Shapiro, also professor in the department of physical medicine and rehabilitation at Johns Hopkins University, Baltimore.
Adults with OI “also don’t respond to Forteo [teriparatide]. I do not recommend treatment with bisphosphonates or Forteo in” adults, he said at the annual meeting of the American Association of Clinical Endocrinologists.
Dr. Shapiro shared his thoughts during an interview regarding his latest study, an analysis of five children with OI under 18 years of age who responded to pamidronate (Aredia) and 11 who did not, meaning that they had two or more fractures per year while on the drug.
His team also compared fracture outcomes in 34 adults with OI treated with oral or intravenous bisphosphonates with 12 untreated adults. The adults were, on average, 52 years old.
The goal was to see if common bone markers predicted who would respond to bisphosphonates, but they did not. Vitamin D, phosphorus, alkaline phosphatase, C-telopeptide, and other measures were the same in children regardless of their response to pamidronate, and the same in adults with OI whether or not they were on bisphosphonates.
“We have not yet defined what the difference is between responders and nonresponders. If you take a crack at the simple things, they don’t help,” Dr. Shapiro said.
Children who responded had a mean of 4.8 fractures over an average of 42.6 months of treatment. Nonresponders had a mean of 15.6 fractures over an average of 72.7 months of treatment.
“For a period of time, you can expect about two-thirds of kids to respond. I would look to see a decrease in fracture rates within 2 years of treatment. If they haven’t decreased their fracture rate [by then], I would be very cautious about continuing,” he said, adding that the optimal duration of treatment in children is unknown.
In adults, the team found no difference in fracture rates at 5 and 10 years. Treated adults had an average of 1.71 fractures over 10 years, versus 1.23 in untreated adults (P = 0.109).
The numbers in the study were too small for meaningful subgroup analysis by OI type.
The findings parallel recent meta-analyses; some have found that bisphosphonates help OI children, but none has found benefits for adults (J. Bone. Miner. Res. 2015;30:929-33). “To date, there is no evidence indicating that bisphosphonates have a positive effect on fracture rates in adults,” Dr. Shapiro said.
Bone turnover declines after puberty, which may explain why the drugs lose their effectiveness after age 18 or so. “What happens in OI anyway is that, after puberty, the fracture rates normally go way down,” Dr. Shapiro said.
Dr. Shapiro said that he had no relevant disclosures, and that there was no outside funding for the work.
AT AACE 2015
Key clinical point: Adult osteogenesis imperfecta patients don’t need bisphosphonates.
Major finding: Treated adults had an average of 1.71 fractures over 10 years; untreated adults had an average of 1.23 (P = 0.109).
Data source: Retrospective study of 16 pediatric and 46 adult OI patients.
Disclosures: There was no outside funding for the work, and the senior investigator had no relevant disclosures.
Subclinical hyperthyroidism linked to higher fracture risk
Individuals with subclinical hyperthyroidism are at increased risk of hip and other fractures, according to the authors of a meta-analysis.
Researchers examined data from 70,298 individuals – 4,092 with subclinical hypothyroidism and 2,219 with subclinical hyperthyroidism – enrolled in 13 prospective cohort studies.
After adjusting for age, sex, and other fracture risk factors, the researchers found that individuals with subclinical hyperthyroidism had a 28% increase in the risk of any fracture and a 36% increased risk of hip fracture compared to individuals with normal thyroid function.
Subclinical hyperthyroidism – defined as a thyroid-stimulating hormone (TSH) level of less than 0.45 mIU/L with normal FT4 levels – was also associated with a 16% increase in the risk of nonspine fracture, according to a paper published online in the May 26 edition of JAMA.
Men with subclinical hyperthyroidism had a more than 3.5-fold increased in the risk of spine fracture, but the increase was not significant in women.
Lower TSH was associated with higher fracture rates, and the analysis showed a 61% increase in the risk of hip fracture and more than a 3.5-fold increase in spine fracture risk among individuals with a TSH less than 0.10 mIU/L.
The analysis yielded no link between subclinical hypothyroidism and fracture risk, and a comparison of fracture risk between individuals treated with thyroxine at baseline and untreated participants also showed no impact of thyroxine therapy on fracture outcomes (JAMA 2015, May 26 [doi:10.1001/jama.2015.5161].
“In prospective cohort studies, data about the association between subclinical thyroid dysfunction and fracture risk are in conflict because of inclusion of participants with overt thyroid disease and small sample sizes of participants with thyroid dysfunction or fracture events,” wrote Dr. Manuel R. Blum of Bern University Hospital, Switzerland, and an international team of coauthors.
They proposed three mechanisms by which thyroid dysfunction may affect fracture risk.
“First, thyroid hormones have been shown to have effects on osteoclasts and osteoblasts, with thyroid status in the upper normal range or excess thyroid hormones leading to accelerated bone turnover with bone loss and increased fracture risk,” they wrote.
Subclinical hyperthyroidism may also increase the risk of falls by affecting muscle strength and coordination, and thyroxine supplementation was also suggested as impacting fracture risk.
“Endogenous subclinical hyperthyroidism may be undetected for years because symptoms of subclinical hyperthyroidism are often nonspecific or absent,” the authors wrote. “This phenomenon has the potential to lead to a greater length of time for adverse associations with bone metabolism.”
The authors stressed the limitations of the observational data; for example, that thyroid function was assessed only at baseline and that some individuals may have progressed to overt thyroid dysfunction over the course of the study, and a lack of uniform definition of fracture type across the cohorts.
They said their findings supported current guideline recommendations that anyone aged 65 years or older, with subclinical hyperthyroidism and a TSH persistently lower than 0.1 mIU/L, should be treated, and treatment should be considered in those individuals with low TSH but still above 0.1 mIU/L.
The Swiss National Science Foundation and Swiss Heart Foundation supported the study. Some authors disclosed personal fees, grants and funding from a range of pharmaceutical companies.
Individuals with subclinical hyperthyroidism are at increased risk of hip and other fractures, according to the authors of a meta-analysis.
Researchers examined data from 70,298 individuals – 4,092 with subclinical hypothyroidism and 2,219 with subclinical hyperthyroidism – enrolled in 13 prospective cohort studies.
After adjusting for age, sex, and other fracture risk factors, the researchers found that individuals with subclinical hyperthyroidism had a 28% increase in the risk of any fracture and a 36% increased risk of hip fracture compared to individuals with normal thyroid function.
Subclinical hyperthyroidism – defined as a thyroid-stimulating hormone (TSH) level of less than 0.45 mIU/L with normal FT4 levels – was also associated with a 16% increase in the risk of nonspine fracture, according to a paper published online in the May 26 edition of JAMA.
Men with subclinical hyperthyroidism had a more than 3.5-fold increased in the risk of spine fracture, but the increase was not significant in women.
Lower TSH was associated with higher fracture rates, and the analysis showed a 61% increase in the risk of hip fracture and more than a 3.5-fold increase in spine fracture risk among individuals with a TSH less than 0.10 mIU/L.
The analysis yielded no link between subclinical hypothyroidism and fracture risk, and a comparison of fracture risk between individuals treated with thyroxine at baseline and untreated participants also showed no impact of thyroxine therapy on fracture outcomes (JAMA 2015, May 26 [doi:10.1001/jama.2015.5161].
“In prospective cohort studies, data about the association between subclinical thyroid dysfunction and fracture risk are in conflict because of inclusion of participants with overt thyroid disease and small sample sizes of participants with thyroid dysfunction or fracture events,” wrote Dr. Manuel R. Blum of Bern University Hospital, Switzerland, and an international team of coauthors.
They proposed three mechanisms by which thyroid dysfunction may affect fracture risk.
“First, thyroid hormones have been shown to have effects on osteoclasts and osteoblasts, with thyroid status in the upper normal range or excess thyroid hormones leading to accelerated bone turnover with bone loss and increased fracture risk,” they wrote.
Subclinical hyperthyroidism may also increase the risk of falls by affecting muscle strength and coordination, and thyroxine supplementation was also suggested as impacting fracture risk.
“Endogenous subclinical hyperthyroidism may be undetected for years because symptoms of subclinical hyperthyroidism are often nonspecific or absent,” the authors wrote. “This phenomenon has the potential to lead to a greater length of time for adverse associations with bone metabolism.”
The authors stressed the limitations of the observational data; for example, that thyroid function was assessed only at baseline and that some individuals may have progressed to overt thyroid dysfunction over the course of the study, and a lack of uniform definition of fracture type across the cohorts.
They said their findings supported current guideline recommendations that anyone aged 65 years or older, with subclinical hyperthyroidism and a TSH persistently lower than 0.1 mIU/L, should be treated, and treatment should be considered in those individuals with low TSH but still above 0.1 mIU/L.
The Swiss National Science Foundation and Swiss Heart Foundation supported the study. Some authors disclosed personal fees, grants and funding from a range of pharmaceutical companies.
Individuals with subclinical hyperthyroidism are at increased risk of hip and other fractures, according to the authors of a meta-analysis.
Researchers examined data from 70,298 individuals – 4,092 with subclinical hypothyroidism and 2,219 with subclinical hyperthyroidism – enrolled in 13 prospective cohort studies.
After adjusting for age, sex, and other fracture risk factors, the researchers found that individuals with subclinical hyperthyroidism had a 28% increase in the risk of any fracture and a 36% increased risk of hip fracture compared to individuals with normal thyroid function.
Subclinical hyperthyroidism – defined as a thyroid-stimulating hormone (TSH) level of less than 0.45 mIU/L with normal FT4 levels – was also associated with a 16% increase in the risk of nonspine fracture, according to a paper published online in the May 26 edition of JAMA.
Men with subclinical hyperthyroidism had a more than 3.5-fold increased in the risk of spine fracture, but the increase was not significant in women.
Lower TSH was associated with higher fracture rates, and the analysis showed a 61% increase in the risk of hip fracture and more than a 3.5-fold increase in spine fracture risk among individuals with a TSH less than 0.10 mIU/L.
The analysis yielded no link between subclinical hypothyroidism and fracture risk, and a comparison of fracture risk between individuals treated with thyroxine at baseline and untreated participants also showed no impact of thyroxine therapy on fracture outcomes (JAMA 2015, May 26 [doi:10.1001/jama.2015.5161].
“In prospective cohort studies, data about the association between subclinical thyroid dysfunction and fracture risk are in conflict because of inclusion of participants with overt thyroid disease and small sample sizes of participants with thyroid dysfunction or fracture events,” wrote Dr. Manuel R. Blum of Bern University Hospital, Switzerland, and an international team of coauthors.
They proposed three mechanisms by which thyroid dysfunction may affect fracture risk.
“First, thyroid hormones have been shown to have effects on osteoclasts and osteoblasts, with thyroid status in the upper normal range or excess thyroid hormones leading to accelerated bone turnover with bone loss and increased fracture risk,” they wrote.
Subclinical hyperthyroidism may also increase the risk of falls by affecting muscle strength and coordination, and thyroxine supplementation was also suggested as impacting fracture risk.
“Endogenous subclinical hyperthyroidism may be undetected for years because symptoms of subclinical hyperthyroidism are often nonspecific or absent,” the authors wrote. “This phenomenon has the potential to lead to a greater length of time for adverse associations with bone metabolism.”
The authors stressed the limitations of the observational data; for example, that thyroid function was assessed only at baseline and that some individuals may have progressed to overt thyroid dysfunction over the course of the study, and a lack of uniform definition of fracture type across the cohorts.
They said their findings supported current guideline recommendations that anyone aged 65 years or older, with subclinical hyperthyroidism and a TSH persistently lower than 0.1 mIU/L, should be treated, and treatment should be considered in those individuals with low TSH but still above 0.1 mIU/L.
The Swiss National Science Foundation and Swiss Heart Foundation supported the study. Some authors disclosed personal fees, grants and funding from a range of pharmaceutical companies.
FROM JAMA
Key clinical point: Subclinical hyperthyroidism is associated with an increased risk of hip and other fractures.
Major finding: Individuals with subclinical hyperthyroidism had a 28% increase in their risk of any fracture compared to individuals with normal thyroid function.
Data source: A meta-analysis of 13 prospective cohort studies comprising 70,298 individuals.
Disclosures: The Swiss National Science Foundation and Swiss Heart Foundation supported the study. Some authors disclosed personal fees, grants, and funding from a range of pharmaceutical companies.
DXA screening: You’re doing it wrong
Dual-energy x-ray absorptiometry to screen for osteoporosis is both underused in women who meet the U.S. Preventive Services Task Force criteria for screening, and overused in low-risk younger women, new data suggest.
A retrospective, longitudinal cohort study of 50,995 women attending 13 primary care clinics found that among previously unscreened women who met the criteria for screening, the 7-year cumulative incidence of DXA screening ranged from 58.8% in women aged 60-64 years with at least one risk factor, to 42.7% in women aged 75 years and older.
But among women who didn’t meet screening criteria, the 7-year cumulative incidence was 45.5% in women aged 50-59 years and 58.6% in women aged 60-64 years without risk factors, according to a paper published May 19 in the Journal of General Internal Medicine (doi:10.1007/s11606-015-3349-8).
The U.S. Preventive Services Task Force currently recommends screening for osteoporosis with DXA for women aged 65 and older, as well as young women at increased risk for fracture. The American Academy of Family Physicians also advised against DXA screening in women younger than age 65 without osteoporosis risk factors as part of the “Choosing Wisely” campaign.
“Additional research is needed to elucidate patient, physician, and health system barriers to evidence-based screening so that interventions can maximize the value of population screening for osteoporosis,” wrote Dr. Anna Lee D. Amarnath of the University of California, Davis Health System, and her coauthors.
The study was supported by the National Institutes of Health and the Agency for Healthcare Research and Quality. The researchers reported having no financial disclosures.
Dual-energy x-ray absorptiometry to screen for osteoporosis is both underused in women who meet the U.S. Preventive Services Task Force criteria for screening, and overused in low-risk younger women, new data suggest.
A retrospective, longitudinal cohort study of 50,995 women attending 13 primary care clinics found that among previously unscreened women who met the criteria for screening, the 7-year cumulative incidence of DXA screening ranged from 58.8% in women aged 60-64 years with at least one risk factor, to 42.7% in women aged 75 years and older.
But among women who didn’t meet screening criteria, the 7-year cumulative incidence was 45.5% in women aged 50-59 years and 58.6% in women aged 60-64 years without risk factors, according to a paper published May 19 in the Journal of General Internal Medicine (doi:10.1007/s11606-015-3349-8).
The U.S. Preventive Services Task Force currently recommends screening for osteoporosis with DXA for women aged 65 and older, as well as young women at increased risk for fracture. The American Academy of Family Physicians also advised against DXA screening in women younger than age 65 without osteoporosis risk factors as part of the “Choosing Wisely” campaign.
“Additional research is needed to elucidate patient, physician, and health system barriers to evidence-based screening so that interventions can maximize the value of population screening for osteoporosis,” wrote Dr. Anna Lee D. Amarnath of the University of California, Davis Health System, and her coauthors.
The study was supported by the National Institutes of Health and the Agency for Healthcare Research and Quality. The researchers reported having no financial disclosures.
Dual-energy x-ray absorptiometry to screen for osteoporosis is both underused in women who meet the U.S. Preventive Services Task Force criteria for screening, and overused in low-risk younger women, new data suggest.
A retrospective, longitudinal cohort study of 50,995 women attending 13 primary care clinics found that among previously unscreened women who met the criteria for screening, the 7-year cumulative incidence of DXA screening ranged from 58.8% in women aged 60-64 years with at least one risk factor, to 42.7% in women aged 75 years and older.
But among women who didn’t meet screening criteria, the 7-year cumulative incidence was 45.5% in women aged 50-59 years and 58.6% in women aged 60-64 years without risk factors, according to a paper published May 19 in the Journal of General Internal Medicine (doi:10.1007/s11606-015-3349-8).
The U.S. Preventive Services Task Force currently recommends screening for osteoporosis with DXA for women aged 65 and older, as well as young women at increased risk for fracture. The American Academy of Family Physicians also advised against DXA screening in women younger than age 65 without osteoporosis risk factors as part of the “Choosing Wisely” campaign.
“Additional research is needed to elucidate patient, physician, and health system barriers to evidence-based screening so that interventions can maximize the value of population screening for osteoporosis,” wrote Dr. Anna Lee D. Amarnath of the University of California, Davis Health System, and her coauthors.
The study was supported by the National Institutes of Health and the Agency for Healthcare Research and Quality. The researchers reported having no financial disclosures.
FROM THE JOURNAL OF GENERAL INTERNAL MEDICINE
Key clinical point: DXA is underused in women who meet the U.S. Preventive Services Task Force criteria for osteoporosis screening and overused in women who do not.
Major finding: The 7-year cumulative incidence of DXA screening was virtually the same for women aged 60-64 years whether they met screening criteria (58.8%) or did not (58.6%).
Data source: A retrospective longitudinal cohort study of 50,995 women attending 13 primary care clinics.
Disclosures: The study was supported by the National Institutes of Health and the Agency for Healthcare Research and Quality. The researchers reported having no financial disclosures.