User login
Pro-inflammatory diets tied to osteoporosis risk
Key clinical point: Diets rich in pro-inflammatory components may increase the risk of osteoporosis, fractures, and lower bone mineral density (BMD) of lumbar spine and total hip.
Major finding: Dietary Inflammatory Index (DII) was negatively linked with BMD of lumbar spine (odds ratios [OR], 0.990; P = .144) and total hip (OR, 0.995; P = .392). The highest category of DII was associated with an elevated risk of osteoporosis (pooled effect size [ES], 1.31; 95% confidence interval [CI], 1.16-1.48) and fractures (pooled ES, 1.26; 95% CI, 1.03-1.59) compared with the lowest category of DII.
Study details: A meta-analysis of 11 studies (4 cohort, 1 case-control, and 6 cross-sectional) involving 127,769 participants.
Disclosures: The study was supported by the National Natural Science Foundation of China. The authors declared no conflicts of interest.
Source: Fang Y et al. Osteoporos Int. 2020 Aug 1. doi: 10.1007/s00198-020-05578-8.
Key clinical point: Diets rich in pro-inflammatory components may increase the risk of osteoporosis, fractures, and lower bone mineral density (BMD) of lumbar spine and total hip.
Major finding: Dietary Inflammatory Index (DII) was negatively linked with BMD of lumbar spine (odds ratios [OR], 0.990; P = .144) and total hip (OR, 0.995; P = .392). The highest category of DII was associated with an elevated risk of osteoporosis (pooled effect size [ES], 1.31; 95% confidence interval [CI], 1.16-1.48) and fractures (pooled ES, 1.26; 95% CI, 1.03-1.59) compared with the lowest category of DII.
Study details: A meta-analysis of 11 studies (4 cohort, 1 case-control, and 6 cross-sectional) involving 127,769 participants.
Disclosures: The study was supported by the National Natural Science Foundation of China. The authors declared no conflicts of interest.
Source: Fang Y et al. Osteoporos Int. 2020 Aug 1. doi: 10.1007/s00198-020-05578-8.
Key clinical point: Diets rich in pro-inflammatory components may increase the risk of osteoporosis, fractures, and lower bone mineral density (BMD) of lumbar spine and total hip.
Major finding: Dietary Inflammatory Index (DII) was negatively linked with BMD of lumbar spine (odds ratios [OR], 0.990; P = .144) and total hip (OR, 0.995; P = .392). The highest category of DII was associated with an elevated risk of osteoporosis (pooled effect size [ES], 1.31; 95% confidence interval [CI], 1.16-1.48) and fractures (pooled ES, 1.26; 95% CI, 1.03-1.59) compared with the lowest category of DII.
Study details: A meta-analysis of 11 studies (4 cohort, 1 case-control, and 6 cross-sectional) involving 127,769 participants.
Disclosures: The study was supported by the National Natural Science Foundation of China. The authors declared no conflicts of interest.
Source: Fang Y et al. Osteoporos Int. 2020 Aug 1. doi: 10.1007/s00198-020-05578-8.
Risk factors for osteosarcopenia in postmenopausal women with osteoporosis
Key clinical point: Postmenopausal osteoporotic women with low body mass index (BMI) have a higher risk of developing osteosarcopenia. Moreover, appropriate assessments, including the presence of comorbidities, will help in identifying patients at a greater risk of developing osteosarcopenia, especially for patients aged 65-74 years.
Major finding: Patients with osteosarcopenia had a higher risk of frailty vs. those with osteoporosis alone (odds ratio [OR], 2.33; P = .028). BMI (per 1 kg/m2 decrease) seemed to be the strongest factor associated with osteosarcopenia risk (adjusted OR [aOR], 1.71; P less than .01). In patients aged 65-74 years, estimated glomerular filtration rate (per 10 mL/min/1.73m2 decrease) and glycated hemoglobin (per 1% decrease) were identified as independent predictors of osteosarcopenia (aOR, 1.75; P = .01 and aOR, 5.01; P = .01, respectively).
Study details: The data come from a retrospective study of 276 patients with postmenopausal osteoporosis (osteoporosis alone: n=222 and osteosarcopenia: n=54).
Disclosures: The study was supported by grants awarded to Dr. Koji Ishikawa by KAKENHI and Grant of Japan Orthopaedics and Traumatology Research Foundation. The authors declared no conflicts of interest.
Source: Okamura H et al. PLoS One. 2020 Aug 7. doi: 10.1371/journal.pone.0237454.
Key clinical point: Postmenopausal osteoporotic women with low body mass index (BMI) have a higher risk of developing osteosarcopenia. Moreover, appropriate assessments, including the presence of comorbidities, will help in identifying patients at a greater risk of developing osteosarcopenia, especially for patients aged 65-74 years.
Major finding: Patients with osteosarcopenia had a higher risk of frailty vs. those with osteoporosis alone (odds ratio [OR], 2.33; P = .028). BMI (per 1 kg/m2 decrease) seemed to be the strongest factor associated with osteosarcopenia risk (adjusted OR [aOR], 1.71; P less than .01). In patients aged 65-74 years, estimated glomerular filtration rate (per 10 mL/min/1.73m2 decrease) and glycated hemoglobin (per 1% decrease) were identified as independent predictors of osteosarcopenia (aOR, 1.75; P = .01 and aOR, 5.01; P = .01, respectively).
Study details: The data come from a retrospective study of 276 patients with postmenopausal osteoporosis (osteoporosis alone: n=222 and osteosarcopenia: n=54).
Disclosures: The study was supported by grants awarded to Dr. Koji Ishikawa by KAKENHI and Grant of Japan Orthopaedics and Traumatology Research Foundation. The authors declared no conflicts of interest.
Source: Okamura H et al. PLoS One. 2020 Aug 7. doi: 10.1371/journal.pone.0237454.
Key clinical point: Postmenopausal osteoporotic women with low body mass index (BMI) have a higher risk of developing osteosarcopenia. Moreover, appropriate assessments, including the presence of comorbidities, will help in identifying patients at a greater risk of developing osteosarcopenia, especially for patients aged 65-74 years.
Major finding: Patients with osteosarcopenia had a higher risk of frailty vs. those with osteoporosis alone (odds ratio [OR], 2.33; P = .028). BMI (per 1 kg/m2 decrease) seemed to be the strongest factor associated with osteosarcopenia risk (adjusted OR [aOR], 1.71; P less than .01). In patients aged 65-74 years, estimated glomerular filtration rate (per 10 mL/min/1.73m2 decrease) and glycated hemoglobin (per 1% decrease) were identified as independent predictors of osteosarcopenia (aOR, 1.75; P = .01 and aOR, 5.01; P = .01, respectively).
Study details: The data come from a retrospective study of 276 patients with postmenopausal osteoporosis (osteoporosis alone: n=222 and osteosarcopenia: n=54).
Disclosures: The study was supported by grants awarded to Dr. Koji Ishikawa by KAKENHI and Grant of Japan Orthopaedics and Traumatology Research Foundation. The authors declared no conflicts of interest.
Source: Okamura H et al. PLoS One. 2020 Aug 7. doi: 10.1371/journal.pone.0237454.
Asthma tied to increased risk of osteoporosis and fragility fractures
Key clinical point: Asthma is associated with an increased risk of osteoporosis and fragility fractures (FF), particularly vertebral and humerus fractures.
Major finding: The risk of osteoporosis and FF was higher in asthma vs. non-asthma group (adjusted hazard ratio [aHR]: 1.18 and 1.12, respectively). The effect was stronger in younger age groups (Pinteraction less than .0001). Forearm-wrist (aHR, 1.21) and vertebra (aHR, 1.19) were the sites associated with a higher risk. Among patients with asthma, a single oral corticosteroid course increased the risk of osteoporosis, and greater use of inhaled corticosteroids increased the risk of both bone diseases.
Study details: This population-based matched cohort study included 138,123 patients with asthma and 520,626 age-, sex-, and practice-matched people without asthma using data from the U.K. Clinical Practice Research Datalink.
Disclosures: The study was funded by a research grant from the British Medical Association. The authors declared no conflicts of interest.
Source: Chalitsios CV et al. Eur Respir J. 2020 Aug 6. doi: 10.1183/13993003.01251-2020.
Key clinical point: Asthma is associated with an increased risk of osteoporosis and fragility fractures (FF), particularly vertebral and humerus fractures.
Major finding: The risk of osteoporosis and FF was higher in asthma vs. non-asthma group (adjusted hazard ratio [aHR]: 1.18 and 1.12, respectively). The effect was stronger in younger age groups (Pinteraction less than .0001). Forearm-wrist (aHR, 1.21) and vertebra (aHR, 1.19) were the sites associated with a higher risk. Among patients with asthma, a single oral corticosteroid course increased the risk of osteoporosis, and greater use of inhaled corticosteroids increased the risk of both bone diseases.
Study details: This population-based matched cohort study included 138,123 patients with asthma and 520,626 age-, sex-, and practice-matched people without asthma using data from the U.K. Clinical Practice Research Datalink.
Disclosures: The study was funded by a research grant from the British Medical Association. The authors declared no conflicts of interest.
Source: Chalitsios CV et al. Eur Respir J. 2020 Aug 6. doi: 10.1183/13993003.01251-2020.
Key clinical point: Asthma is associated with an increased risk of osteoporosis and fragility fractures (FF), particularly vertebral and humerus fractures.
Major finding: The risk of osteoporosis and FF was higher in asthma vs. non-asthma group (adjusted hazard ratio [aHR]: 1.18 and 1.12, respectively). The effect was stronger in younger age groups (Pinteraction less than .0001). Forearm-wrist (aHR, 1.21) and vertebra (aHR, 1.19) were the sites associated with a higher risk. Among patients with asthma, a single oral corticosteroid course increased the risk of osteoporosis, and greater use of inhaled corticosteroids increased the risk of both bone diseases.
Study details: This population-based matched cohort study included 138,123 patients with asthma and 520,626 age-, sex-, and practice-matched people without asthma using data from the U.K. Clinical Practice Research Datalink.
Disclosures: The study was funded by a research grant from the British Medical Association. The authors declared no conflicts of interest.
Source: Chalitsios CV et al. Eur Respir J. 2020 Aug 6. doi: 10.1183/13993003.01251-2020.
Reassuring rheumatic disease patients on value of bisphosphonates
“When we think about bisphosphonates, we have to think about whether they are good players or bad players,” Marcy B. Bolster, MD, of Harvard Medical School, Boston, said in a virtual presentation at the annual Perspectives in Rheumatic Diseases held by Global Academy for Medical Education.
Although bisphosphonates are a first-line treatment for many patients to reduce fracture risk, rheumatology patients have distinct concerns about these medications, said Dr. Bolster, who is director of the Rheumatology Fellowship Training Program at Massachusetts General Hospital, Boston, and medical lead for the Fracture Liaison Service there.
She shared her insights on four questions most often asked by patients in her practice:
How long do I need to take this medication?
When discussing bisphosphonates with a patient for the first time, “I typically start with the benefits, and include the risks,” she said. “Then I outline my plans for treatment, which would include treatment duration.”
Setting expectations with the patient about planned duration of therapy, reviewing risks and benefits, and preparing to be flexible if changes are needed can help relieve patients’ concerns, she said.
For example, in a hypothetical case of a 69-year-old woman with a 17% chance of a major osteoporotic fracture and 3.8% chance of hip fracture in the next 10 years based on FRAX scores, Dr. Bolster said she would treat with alendronate or zoledronic acid.
Duration must be a clinical decision individualized to the patient, she noted. Research studies support that some patients benefit from a longer duration of therapy. In the Fracture Intervention Trial (FIT) Long-Term Extension, which included 1,099 women, the risk of clinical vertebral fractures significantly declined with 10 years of alendronate treatment, compared with 5 years of treatment, she said.
In the HORIZON trial of 1,233 postmenopausal women, the risk of new morphometric vertebral fractures was significantly lower in those treated with IV zoledronic acid for 6 years versus those treated for 3 years. These studies support that patients at particularly high risk for vertebral fractures may benefit from a longer duration of bisphosphonate therapy, she said.
What should I know about infusion side effects?
Infusion side effects remain a concern, and the acute phase reaction of zoledronic acid occurs in about 30% of patients, but most of these are mild and not recurring, Dr. Bolster said. “I tell patients that 90% report mild to moderate infusion side effects, and that it usually occurs only with the first infusion,” she noted.
To potentially prevent an acute-phase reaction, Dr. Bolster has advised patients to take acetaminophen prior to infusion. “I would tend to recommend acetaminophen over NSAIDs to avoid gastric and renal toxicities,” she said.
“Determining the risks of atypical femoral fractures are challenging” but are another potential side effect that worries patients, she said. An atypical femoral fracture (AFF) is a femur fracture in the proximal third of the shaft, she said.
AFF may occur in patients with osteoporosis even in the absence of bisphosphonate use, Dr. Bolster noted. However, AFF “may occur at increased frequency in those patients with osteoporosis and prolonged bisphosphonate use,” she said. AFF is rare overall, and known risk factors include Asian race (in North America), as well as femoral bowing and glucocorticoid use, she said.
A 2019 meta-analysis favored fracture prevention benefits over potential risk associated with bisphosphonate use. Predicting the risk of AFF remains difficult given several factors, including the low incidence of AFF, the unavailability of radiographs in all studies, not accounting for potential confounding by indication in some studies, and lack of adjustment for low bone mineral density or fracture risk, she added.
Osteonecrosis of the jaw has been linked to bisphosphonate use, and some patients ask about it, Dr. Bolster said. Current data show an incidence of 1 in 10,000 to 1 in 100,000 in patients with osteoporosis, while the incidence in the general population is 1 in 100,000, she noted. The highest risk is associated with use of IV bisphosphonates, although it does occur in patients on oral bisphosphonates and denosumab (Prolia), she added. Given the relatively low risk, the American Dental Association states that there is “no need to discontinue bisphosphonates prior to procedures.” Based on current evidence, bisphosphonate treatment outweighs the low risk of medication-related osteonecrosis of the jaw in patients in need of osteoporosis treatment because of the high risk of fragility fractures in the osteoporosis population, she emphasized.
When will I need another dual x-ray absorptiometry scan?
Osteoporosis develops in fewer than 10% of older postmenopausal women using a 15-year screening interval for those with normal bone mineral density or mild osteopenia at an initial scan, with T-scores of –1.49 or higher, she noted. Therefore, the need for repeat dual x-ray absorptiometry (DXA) scans should be individualized, so some patients with normal bone density or osteopenia and few comorbidities and risk factors for osteoporosis may not need frequent DXA scans, she added.
Although little evidence exists to specifically demonstrate the value of monitoring bone mineral density during a 5-year drug treatment period, as is noted by the American College of Physicians 2017 clinical practice guideline published in Annals of Internal Medicine, Dr. Bolster said that a DXA scan showing loss of bone mineral density during treatment could indicate incorrect drug use or noncompliance, or a secondary cause for bone loss that may otherwise go unnoticed. For IV zoledronic acid in particular, a DXA scan at 3-4 years can determine whether a drug holiday is warranted, or for patients with severe osteoporosis, whether another 3 years of treatment is necessary. She suggested considering a DXA scan at 2-3 years with alendronate and at 3-4 years with IV zoledronic acid.
Will I need a new medication if I fracture while on treatment?
For patients who ask whether to change medications following a new fracture, Dr. Bolster said it is important to evaluate the patient’s compliance with the treatment regimen and also consider the presence of secondary causes of bone loss. Consideration can be given to keeping the patient on the same regimen because osteoporosis treatment regimens have demonstrated a 50%-70% fracture-risk reduction so they do not prevent all fractures, she said. “It is therefore reasonable, after confirming compliance and ruling out secondary causes of bone loss, to keep a patient on the same regimen following a fracture. For patients using denosumab, there is an increased risk of rapid bone loss and sustaining multiple vertebral fracture with missed doses or discontinuation,” she said.
It is important to evaluate patients who fracture while on therapy for secondary causes of bone loss, assess compliance, and consider strategies such as modifying the route of administration, seeking a different mechanism of action, or continuing on the same regimen, Dr. Bolster noted.
Dr. Bolster disclosed participation in clinical trials for Corbus, Cumberland, and Genentech, as well as research grants from the Rheumatology Research Foundation. She also disclosed serving on advisory boards for Gilead Sciences and Clinical Learning Designs, serving on the American College of Rheumatology’s Committee on Marketing and Communications, and holding investments in Johnson & Johnson.
Global Academy for Medical Education and this news organization are owned by the same parent company.
“When we think about bisphosphonates, we have to think about whether they are good players or bad players,” Marcy B. Bolster, MD, of Harvard Medical School, Boston, said in a virtual presentation at the annual Perspectives in Rheumatic Diseases held by Global Academy for Medical Education.
Although bisphosphonates are a first-line treatment for many patients to reduce fracture risk, rheumatology patients have distinct concerns about these medications, said Dr. Bolster, who is director of the Rheumatology Fellowship Training Program at Massachusetts General Hospital, Boston, and medical lead for the Fracture Liaison Service there.
She shared her insights on four questions most often asked by patients in her practice:
How long do I need to take this medication?
When discussing bisphosphonates with a patient for the first time, “I typically start with the benefits, and include the risks,” she said. “Then I outline my plans for treatment, which would include treatment duration.”
Setting expectations with the patient about planned duration of therapy, reviewing risks and benefits, and preparing to be flexible if changes are needed can help relieve patients’ concerns, she said.
For example, in a hypothetical case of a 69-year-old woman with a 17% chance of a major osteoporotic fracture and 3.8% chance of hip fracture in the next 10 years based on FRAX scores, Dr. Bolster said she would treat with alendronate or zoledronic acid.
Duration must be a clinical decision individualized to the patient, she noted. Research studies support that some patients benefit from a longer duration of therapy. In the Fracture Intervention Trial (FIT) Long-Term Extension, which included 1,099 women, the risk of clinical vertebral fractures significantly declined with 10 years of alendronate treatment, compared with 5 years of treatment, she said.
In the HORIZON trial of 1,233 postmenopausal women, the risk of new morphometric vertebral fractures was significantly lower in those treated with IV zoledronic acid for 6 years versus those treated for 3 years. These studies support that patients at particularly high risk for vertebral fractures may benefit from a longer duration of bisphosphonate therapy, she said.
What should I know about infusion side effects?
Infusion side effects remain a concern, and the acute phase reaction of zoledronic acid occurs in about 30% of patients, but most of these are mild and not recurring, Dr. Bolster said. “I tell patients that 90% report mild to moderate infusion side effects, and that it usually occurs only with the first infusion,” she noted.
To potentially prevent an acute-phase reaction, Dr. Bolster has advised patients to take acetaminophen prior to infusion. “I would tend to recommend acetaminophen over NSAIDs to avoid gastric and renal toxicities,” she said.
“Determining the risks of atypical femoral fractures are challenging” but are another potential side effect that worries patients, she said. An atypical femoral fracture (AFF) is a femur fracture in the proximal third of the shaft, she said.
AFF may occur in patients with osteoporosis even in the absence of bisphosphonate use, Dr. Bolster noted. However, AFF “may occur at increased frequency in those patients with osteoporosis and prolonged bisphosphonate use,” she said. AFF is rare overall, and known risk factors include Asian race (in North America), as well as femoral bowing and glucocorticoid use, she said.
A 2019 meta-analysis favored fracture prevention benefits over potential risk associated with bisphosphonate use. Predicting the risk of AFF remains difficult given several factors, including the low incidence of AFF, the unavailability of radiographs in all studies, not accounting for potential confounding by indication in some studies, and lack of adjustment for low bone mineral density or fracture risk, she added.
Osteonecrosis of the jaw has been linked to bisphosphonate use, and some patients ask about it, Dr. Bolster said. Current data show an incidence of 1 in 10,000 to 1 in 100,000 in patients with osteoporosis, while the incidence in the general population is 1 in 100,000, she noted. The highest risk is associated with use of IV bisphosphonates, although it does occur in patients on oral bisphosphonates and denosumab (Prolia), she added. Given the relatively low risk, the American Dental Association states that there is “no need to discontinue bisphosphonates prior to procedures.” Based on current evidence, bisphosphonate treatment outweighs the low risk of medication-related osteonecrosis of the jaw in patients in need of osteoporosis treatment because of the high risk of fragility fractures in the osteoporosis population, she emphasized.
When will I need another dual x-ray absorptiometry scan?
Osteoporosis develops in fewer than 10% of older postmenopausal women using a 15-year screening interval for those with normal bone mineral density or mild osteopenia at an initial scan, with T-scores of –1.49 or higher, she noted. Therefore, the need for repeat dual x-ray absorptiometry (DXA) scans should be individualized, so some patients with normal bone density or osteopenia and few comorbidities and risk factors for osteoporosis may not need frequent DXA scans, she added.
Although little evidence exists to specifically demonstrate the value of monitoring bone mineral density during a 5-year drug treatment period, as is noted by the American College of Physicians 2017 clinical practice guideline published in Annals of Internal Medicine, Dr. Bolster said that a DXA scan showing loss of bone mineral density during treatment could indicate incorrect drug use or noncompliance, or a secondary cause for bone loss that may otherwise go unnoticed. For IV zoledronic acid in particular, a DXA scan at 3-4 years can determine whether a drug holiday is warranted, or for patients with severe osteoporosis, whether another 3 years of treatment is necessary. She suggested considering a DXA scan at 2-3 years with alendronate and at 3-4 years with IV zoledronic acid.
Will I need a new medication if I fracture while on treatment?
For patients who ask whether to change medications following a new fracture, Dr. Bolster said it is important to evaluate the patient’s compliance with the treatment regimen and also consider the presence of secondary causes of bone loss. Consideration can be given to keeping the patient on the same regimen because osteoporosis treatment regimens have demonstrated a 50%-70% fracture-risk reduction so they do not prevent all fractures, she said. “It is therefore reasonable, after confirming compliance and ruling out secondary causes of bone loss, to keep a patient on the same regimen following a fracture. For patients using denosumab, there is an increased risk of rapid bone loss and sustaining multiple vertebral fracture with missed doses or discontinuation,” she said.
It is important to evaluate patients who fracture while on therapy for secondary causes of bone loss, assess compliance, and consider strategies such as modifying the route of administration, seeking a different mechanism of action, or continuing on the same regimen, Dr. Bolster noted.
Dr. Bolster disclosed participation in clinical trials for Corbus, Cumberland, and Genentech, as well as research grants from the Rheumatology Research Foundation. She also disclosed serving on advisory boards for Gilead Sciences and Clinical Learning Designs, serving on the American College of Rheumatology’s Committee on Marketing and Communications, and holding investments in Johnson & Johnson.
Global Academy for Medical Education and this news organization are owned by the same parent company.
“When we think about bisphosphonates, we have to think about whether they are good players or bad players,” Marcy B. Bolster, MD, of Harvard Medical School, Boston, said in a virtual presentation at the annual Perspectives in Rheumatic Diseases held by Global Academy for Medical Education.
Although bisphosphonates are a first-line treatment for many patients to reduce fracture risk, rheumatology patients have distinct concerns about these medications, said Dr. Bolster, who is director of the Rheumatology Fellowship Training Program at Massachusetts General Hospital, Boston, and medical lead for the Fracture Liaison Service there.
She shared her insights on four questions most often asked by patients in her practice:
How long do I need to take this medication?
When discussing bisphosphonates with a patient for the first time, “I typically start with the benefits, and include the risks,” she said. “Then I outline my plans for treatment, which would include treatment duration.”
Setting expectations with the patient about planned duration of therapy, reviewing risks and benefits, and preparing to be flexible if changes are needed can help relieve patients’ concerns, she said.
For example, in a hypothetical case of a 69-year-old woman with a 17% chance of a major osteoporotic fracture and 3.8% chance of hip fracture in the next 10 years based on FRAX scores, Dr. Bolster said she would treat with alendronate or zoledronic acid.
Duration must be a clinical decision individualized to the patient, she noted. Research studies support that some patients benefit from a longer duration of therapy. In the Fracture Intervention Trial (FIT) Long-Term Extension, which included 1,099 women, the risk of clinical vertebral fractures significantly declined with 10 years of alendronate treatment, compared with 5 years of treatment, she said.
In the HORIZON trial of 1,233 postmenopausal women, the risk of new morphometric vertebral fractures was significantly lower in those treated with IV zoledronic acid for 6 years versus those treated for 3 years. These studies support that patients at particularly high risk for vertebral fractures may benefit from a longer duration of bisphosphonate therapy, she said.
What should I know about infusion side effects?
Infusion side effects remain a concern, and the acute phase reaction of zoledronic acid occurs in about 30% of patients, but most of these are mild and not recurring, Dr. Bolster said. “I tell patients that 90% report mild to moderate infusion side effects, and that it usually occurs only with the first infusion,” she noted.
To potentially prevent an acute-phase reaction, Dr. Bolster has advised patients to take acetaminophen prior to infusion. “I would tend to recommend acetaminophen over NSAIDs to avoid gastric and renal toxicities,” she said.
“Determining the risks of atypical femoral fractures are challenging” but are another potential side effect that worries patients, she said. An atypical femoral fracture (AFF) is a femur fracture in the proximal third of the shaft, she said.
AFF may occur in patients with osteoporosis even in the absence of bisphosphonate use, Dr. Bolster noted. However, AFF “may occur at increased frequency in those patients with osteoporosis and prolonged bisphosphonate use,” she said. AFF is rare overall, and known risk factors include Asian race (in North America), as well as femoral bowing and glucocorticoid use, she said.
A 2019 meta-analysis favored fracture prevention benefits over potential risk associated with bisphosphonate use. Predicting the risk of AFF remains difficult given several factors, including the low incidence of AFF, the unavailability of radiographs in all studies, not accounting for potential confounding by indication in some studies, and lack of adjustment for low bone mineral density or fracture risk, she added.
Osteonecrosis of the jaw has been linked to bisphosphonate use, and some patients ask about it, Dr. Bolster said. Current data show an incidence of 1 in 10,000 to 1 in 100,000 in patients with osteoporosis, while the incidence in the general population is 1 in 100,000, she noted. The highest risk is associated with use of IV bisphosphonates, although it does occur in patients on oral bisphosphonates and denosumab (Prolia), she added. Given the relatively low risk, the American Dental Association states that there is “no need to discontinue bisphosphonates prior to procedures.” Based on current evidence, bisphosphonate treatment outweighs the low risk of medication-related osteonecrosis of the jaw in patients in need of osteoporosis treatment because of the high risk of fragility fractures in the osteoporosis population, she emphasized.
When will I need another dual x-ray absorptiometry scan?
Osteoporosis develops in fewer than 10% of older postmenopausal women using a 15-year screening interval for those with normal bone mineral density or mild osteopenia at an initial scan, with T-scores of –1.49 or higher, she noted. Therefore, the need for repeat dual x-ray absorptiometry (DXA) scans should be individualized, so some patients with normal bone density or osteopenia and few comorbidities and risk factors for osteoporosis may not need frequent DXA scans, she added.
Although little evidence exists to specifically demonstrate the value of monitoring bone mineral density during a 5-year drug treatment period, as is noted by the American College of Physicians 2017 clinical practice guideline published in Annals of Internal Medicine, Dr. Bolster said that a DXA scan showing loss of bone mineral density during treatment could indicate incorrect drug use or noncompliance, or a secondary cause for bone loss that may otherwise go unnoticed. For IV zoledronic acid in particular, a DXA scan at 3-4 years can determine whether a drug holiday is warranted, or for patients with severe osteoporosis, whether another 3 years of treatment is necessary. She suggested considering a DXA scan at 2-3 years with alendronate and at 3-4 years with IV zoledronic acid.
Will I need a new medication if I fracture while on treatment?
For patients who ask whether to change medications following a new fracture, Dr. Bolster said it is important to evaluate the patient’s compliance with the treatment regimen and also consider the presence of secondary causes of bone loss. Consideration can be given to keeping the patient on the same regimen because osteoporosis treatment regimens have demonstrated a 50%-70% fracture-risk reduction so they do not prevent all fractures, she said. “It is therefore reasonable, after confirming compliance and ruling out secondary causes of bone loss, to keep a patient on the same regimen following a fracture. For patients using denosumab, there is an increased risk of rapid bone loss and sustaining multiple vertebral fracture with missed doses or discontinuation,” she said.
It is important to evaluate patients who fracture while on therapy for secondary causes of bone loss, assess compliance, and consider strategies such as modifying the route of administration, seeking a different mechanism of action, or continuing on the same regimen, Dr. Bolster noted.
Dr. Bolster disclosed participation in clinical trials for Corbus, Cumberland, and Genentech, as well as research grants from the Rheumatology Research Foundation. She also disclosed serving on advisory boards for Gilead Sciences and Clinical Learning Designs, serving on the American College of Rheumatology’s Committee on Marketing and Communications, and holding investments in Johnson & Johnson.
Global Academy for Medical Education and this news organization are owned by the same parent company.
FROM PRD 2020
Longer bisphosphonate use ups AFF risk, but not all is tied to drug
In a national study of older Danes who had previously had a fracture and were taking bisphosphonates, the risk of having a serious though rare atypical femoral fracture (AFF) was greater after 3-5 years of bisphosphonate use.
The risk quickly dropped after patients stopped taking a bisphosphonate, which suggests that bisphosphonate “holidays” may be useful for some patients, the researchers said. These findings support previous work.
But the study also found that 34% of the AFFs occurred in patients who had not been taking a bisphosphonate. That rate is higher than the 6%-22% that has been reported by others.
Doug Bauer, MD, from the University of California, San Francisco, presented the new study findings during the virtual American Society of Bone and Mineral Research 2020 annual meeting.
“We found no clear risk factor that accounts for this increased risk [for AFFs] among those not exposed to bisphosphonates,” he said, “but we believe this was a real finding, as our study protocol ensured that the study radiologists were completely blinded to treatments received.”
Suzanne N. Morin, MD, who was not involved in this research, pointed out that the reported AFF risks related to bisphosphonate dose and cessation are in keeping with findings of other studies, including a recent large study by Dennis M. Black, MD, and colleagues that was published in the New England Journal of Medicine.
That study found that Asians are at higher risk for AFFs than White persons. Others have reported that specific femur geometry or physique and use of glucocorticoids increase AFF risk, Dr. Morin, from the Research Institute of the McGill University Health Center, Montreal, said in an interview.
The current study suggests that rheumatoid arthritis may be a risk factor, she added.
The fact that the rate of AFFs among patients who had not been exposed to bisphosphonates was higher than previously reported “may be due to differences in the method they used to ascertain the fractures or in medication use,” she speculated.
The clinical implications of research to date are that “the risk of AFF should not dissuade patients and providers from short-term use of bisphosphonates [3-5 years],” Dr. Bauer said. He noted that most patients should not take a bisphosphonate for longer than this unless they have a very high fracture risk.
Similarly, Dr. Morin said that clinicians “should consider initiating bisphosphonate in those at high risk for fractures and reevaluate their use after 3-6 years, depending on individual’s risk profile.”
AFF is serious but rare complication of bisphosphonate use
“Since first reported over 10 years ago, it has become clear that AFFs are a rare but serious complication of bisphosphonate therapy,” Dr. Bauer explained. However, there is still uncertainty about the magnitude of this risk, including the absolute risk for AFFs among adults who take bisphosphonates and those who do not.
To study this, the researchers analyzed data from national health care and pharmacy records and a radiology image database in Denmark. They identified almost 5,000 adults who were aged 50 years or older and who experienced a subtrochanteric and femoral shaft fracture during the period from 2010 to 2015. Two expert radiologists who were blinded to the patients’ clinical history or treatment identified AFF on the basis of ASBMR 2014 criteria.
The researchers compared three patient groups: 189 patients with AFF, 2,397 patients with typical subtrochanteric and femoral shaft fractures (no AFF), and35,946 adults aged older than 50 years (control persons).
Compared with patients with typical fractures, patients with AFF were younger (aged 71 vs. 77), more likely to be women (79% vs. 69%), and more likely to have RA (12% vs. 2.5%).
Compared with patients in the other two groups, those with AFF were more likely to use corticosteroids, proton pump inhibitors, statins, and hormone replacement therapy.
They were also more likely to use bisphosphonates (58%) than patients with typical subtrochanteric and femoral shaft fractures (19%) or control patients (10%).
The bisphosphonates used in Denmark at the time were mostly alendronate (85%) and rarely ibandronate (6%), intravenous zoledronic acid (5%), etidronate (3%), or risedronate (1%).
One-third of patients with AFFs had no bisphosphonate exposure
In this national cohort of adults aged older than 50 years, the absolute rates of AFF per 10,000 person-years were as follows: 0.07 in nonusers of bisphosphonates, 1.84 in those with 3-5 years of bisphosphonate use, and 4.63 in those with >7 years of bisphosphonate use. As a comparison, the rate of classic hip fracture was 43.8 per 10,000 person-years.
Compared with no bisphosphonate use, the relative risk for AFF was close to 40 times higher with more than 7 years of use, after adjusting for multiple confounders. The risk for AFF was also significantly higher among patients with RA or hypertension and for those who used proton pump inhibitors.
“Note that age, gender, and previous fracture were not associated with the risk of AFF” after controlling for multiple confounders, Dr. Bauer stressed.
The relative risk for AFF fell significantly after it had been withheld from use for more than 1 year.
Among the 189 patients with confirmed AFF, 64 patients (34%) had never taken a bisphosphonate.
Preliminary analysis showed that, among patients with AFF, those who had not been exposed to bisphosphonates were younger, more likely to be male, and less likely to have had a previous fracture, RA, or to have used corticosteroids, proton pump inhibitors, statins, or hormone-replacement therapy.
The study was funded by the National Institute of Arthritis and Musculoskeletal and Skin Diseases. Dr. Bauer and Dr. Morin disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
In a national study of older Danes who had previously had a fracture and were taking bisphosphonates, the risk of having a serious though rare atypical femoral fracture (AFF) was greater after 3-5 years of bisphosphonate use.
The risk quickly dropped after patients stopped taking a bisphosphonate, which suggests that bisphosphonate “holidays” may be useful for some patients, the researchers said. These findings support previous work.
But the study also found that 34% of the AFFs occurred in patients who had not been taking a bisphosphonate. That rate is higher than the 6%-22% that has been reported by others.
Doug Bauer, MD, from the University of California, San Francisco, presented the new study findings during the virtual American Society of Bone and Mineral Research 2020 annual meeting.
“We found no clear risk factor that accounts for this increased risk [for AFFs] among those not exposed to bisphosphonates,” he said, “but we believe this was a real finding, as our study protocol ensured that the study radiologists were completely blinded to treatments received.”
Suzanne N. Morin, MD, who was not involved in this research, pointed out that the reported AFF risks related to bisphosphonate dose and cessation are in keeping with findings of other studies, including a recent large study by Dennis M. Black, MD, and colleagues that was published in the New England Journal of Medicine.
That study found that Asians are at higher risk for AFFs than White persons. Others have reported that specific femur geometry or physique and use of glucocorticoids increase AFF risk, Dr. Morin, from the Research Institute of the McGill University Health Center, Montreal, said in an interview.
The current study suggests that rheumatoid arthritis may be a risk factor, she added.
The fact that the rate of AFFs among patients who had not been exposed to bisphosphonates was higher than previously reported “may be due to differences in the method they used to ascertain the fractures or in medication use,” she speculated.
The clinical implications of research to date are that “the risk of AFF should not dissuade patients and providers from short-term use of bisphosphonates [3-5 years],” Dr. Bauer said. He noted that most patients should not take a bisphosphonate for longer than this unless they have a very high fracture risk.
Similarly, Dr. Morin said that clinicians “should consider initiating bisphosphonate in those at high risk for fractures and reevaluate their use after 3-6 years, depending on individual’s risk profile.”
AFF is serious but rare complication of bisphosphonate use
“Since first reported over 10 years ago, it has become clear that AFFs are a rare but serious complication of bisphosphonate therapy,” Dr. Bauer explained. However, there is still uncertainty about the magnitude of this risk, including the absolute risk for AFFs among adults who take bisphosphonates and those who do not.
To study this, the researchers analyzed data from national health care and pharmacy records and a radiology image database in Denmark. They identified almost 5,000 adults who were aged 50 years or older and who experienced a subtrochanteric and femoral shaft fracture during the period from 2010 to 2015. Two expert radiologists who were blinded to the patients’ clinical history or treatment identified AFF on the basis of ASBMR 2014 criteria.
The researchers compared three patient groups: 189 patients with AFF, 2,397 patients with typical subtrochanteric and femoral shaft fractures (no AFF), and35,946 adults aged older than 50 years (control persons).
Compared with patients with typical fractures, patients with AFF were younger (aged 71 vs. 77), more likely to be women (79% vs. 69%), and more likely to have RA (12% vs. 2.5%).
Compared with patients in the other two groups, those with AFF were more likely to use corticosteroids, proton pump inhibitors, statins, and hormone replacement therapy.
They were also more likely to use bisphosphonates (58%) than patients with typical subtrochanteric and femoral shaft fractures (19%) or control patients (10%).
The bisphosphonates used in Denmark at the time were mostly alendronate (85%) and rarely ibandronate (6%), intravenous zoledronic acid (5%), etidronate (3%), or risedronate (1%).
One-third of patients with AFFs had no bisphosphonate exposure
In this national cohort of adults aged older than 50 years, the absolute rates of AFF per 10,000 person-years were as follows: 0.07 in nonusers of bisphosphonates, 1.84 in those with 3-5 years of bisphosphonate use, and 4.63 in those with >7 years of bisphosphonate use. As a comparison, the rate of classic hip fracture was 43.8 per 10,000 person-years.
Compared with no bisphosphonate use, the relative risk for AFF was close to 40 times higher with more than 7 years of use, after adjusting for multiple confounders. The risk for AFF was also significantly higher among patients with RA or hypertension and for those who used proton pump inhibitors.
“Note that age, gender, and previous fracture were not associated with the risk of AFF” after controlling for multiple confounders, Dr. Bauer stressed.
The relative risk for AFF fell significantly after it had been withheld from use for more than 1 year.
Among the 189 patients with confirmed AFF, 64 patients (34%) had never taken a bisphosphonate.
Preliminary analysis showed that, among patients with AFF, those who had not been exposed to bisphosphonates were younger, more likely to be male, and less likely to have had a previous fracture, RA, or to have used corticosteroids, proton pump inhibitors, statins, or hormone-replacement therapy.
The study was funded by the National Institute of Arthritis and Musculoskeletal and Skin Diseases. Dr. Bauer and Dr. Morin disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
In a national study of older Danes who had previously had a fracture and were taking bisphosphonates, the risk of having a serious though rare atypical femoral fracture (AFF) was greater after 3-5 years of bisphosphonate use.
The risk quickly dropped after patients stopped taking a bisphosphonate, which suggests that bisphosphonate “holidays” may be useful for some patients, the researchers said. These findings support previous work.
But the study also found that 34% of the AFFs occurred in patients who had not been taking a bisphosphonate. That rate is higher than the 6%-22% that has been reported by others.
Doug Bauer, MD, from the University of California, San Francisco, presented the new study findings during the virtual American Society of Bone and Mineral Research 2020 annual meeting.
“We found no clear risk factor that accounts for this increased risk [for AFFs] among those not exposed to bisphosphonates,” he said, “but we believe this was a real finding, as our study protocol ensured that the study radiologists were completely blinded to treatments received.”
Suzanne N. Morin, MD, who was not involved in this research, pointed out that the reported AFF risks related to bisphosphonate dose and cessation are in keeping with findings of other studies, including a recent large study by Dennis M. Black, MD, and colleagues that was published in the New England Journal of Medicine.
That study found that Asians are at higher risk for AFFs than White persons. Others have reported that specific femur geometry or physique and use of glucocorticoids increase AFF risk, Dr. Morin, from the Research Institute of the McGill University Health Center, Montreal, said in an interview.
The current study suggests that rheumatoid arthritis may be a risk factor, she added.
The fact that the rate of AFFs among patients who had not been exposed to bisphosphonates was higher than previously reported “may be due to differences in the method they used to ascertain the fractures or in medication use,” she speculated.
The clinical implications of research to date are that “the risk of AFF should not dissuade patients and providers from short-term use of bisphosphonates [3-5 years],” Dr. Bauer said. He noted that most patients should not take a bisphosphonate for longer than this unless they have a very high fracture risk.
Similarly, Dr. Morin said that clinicians “should consider initiating bisphosphonate in those at high risk for fractures and reevaluate their use after 3-6 years, depending on individual’s risk profile.”
AFF is serious but rare complication of bisphosphonate use
“Since first reported over 10 years ago, it has become clear that AFFs are a rare but serious complication of bisphosphonate therapy,” Dr. Bauer explained. However, there is still uncertainty about the magnitude of this risk, including the absolute risk for AFFs among adults who take bisphosphonates and those who do not.
To study this, the researchers analyzed data from national health care and pharmacy records and a radiology image database in Denmark. They identified almost 5,000 adults who were aged 50 years or older and who experienced a subtrochanteric and femoral shaft fracture during the period from 2010 to 2015. Two expert radiologists who were blinded to the patients’ clinical history or treatment identified AFF on the basis of ASBMR 2014 criteria.
The researchers compared three patient groups: 189 patients with AFF, 2,397 patients with typical subtrochanteric and femoral shaft fractures (no AFF), and35,946 adults aged older than 50 years (control persons).
Compared with patients with typical fractures, patients with AFF were younger (aged 71 vs. 77), more likely to be women (79% vs. 69%), and more likely to have RA (12% vs. 2.5%).
Compared with patients in the other two groups, those with AFF were more likely to use corticosteroids, proton pump inhibitors, statins, and hormone replacement therapy.
They were also more likely to use bisphosphonates (58%) than patients with typical subtrochanteric and femoral shaft fractures (19%) or control patients (10%).
The bisphosphonates used in Denmark at the time were mostly alendronate (85%) and rarely ibandronate (6%), intravenous zoledronic acid (5%), etidronate (3%), or risedronate (1%).
One-third of patients with AFFs had no bisphosphonate exposure
In this national cohort of adults aged older than 50 years, the absolute rates of AFF per 10,000 person-years were as follows: 0.07 in nonusers of bisphosphonates, 1.84 in those with 3-5 years of bisphosphonate use, and 4.63 in those with >7 years of bisphosphonate use. As a comparison, the rate of classic hip fracture was 43.8 per 10,000 person-years.
Compared with no bisphosphonate use, the relative risk for AFF was close to 40 times higher with more than 7 years of use, after adjusting for multiple confounders. The risk for AFF was also significantly higher among patients with RA or hypertension and for those who used proton pump inhibitors.
“Note that age, gender, and previous fracture were not associated with the risk of AFF” after controlling for multiple confounders, Dr. Bauer stressed.
The relative risk for AFF fell significantly after it had been withheld from use for more than 1 year.
Among the 189 patients with confirmed AFF, 64 patients (34%) had never taken a bisphosphonate.
Preliminary analysis showed that, among patients with AFF, those who had not been exposed to bisphosphonates were younger, more likely to be male, and less likely to have had a previous fracture, RA, or to have used corticosteroids, proton pump inhibitors, statins, or hormone-replacement therapy.
The study was funded by the National Institute of Arthritis and Musculoskeletal and Skin Diseases. Dr. Bauer and Dr. Morin disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
FROM ASBMR 2020
Treat-to-target strategy ‘not ready for primetime’ in osteoporosis
“A treat-to-target approach is useful in the management of osteoporosis” was the motion proposed in a debate during the recent virtual American Society of Bone and Mineral Research (ASBMR) 2020 annual meeting, and when the votes came in, Michael McClung, MD, who argued against the motion, carried the day.
Agreement with the motion dropped from 63%-46% after McClung, of the Oregon Osteoporosis Center, Portland, put his views forward in opposition to those of Celia L. Gregson, PhD, University of Bristol (England), who argued for the motion on behalf of the European Calcified Tissue Society (ECTS).
Disagreement with the statement rose from 37% predebate to 54% in the postdebate audience polls.
“The debate is part education and part entertainment,” said Dr. McClung, who represented the ASBMR. “I could just as easily have made a strong argument for the motion,” he emphasized in an interview.
On the other hand, “had I been in the audience, as a member of ASBMR relying on data and evidence to make clinical decisions, I would have voted against the motion. As appealing as the strategy sounds, we don’t yet have the hard evidence to support its use nor is there a consensus about what an appropriate target should be, he noted.
Similarly, the debate comoderator and incoming ASBMR President, Suzanne M. Jan de Beur, MD, from Johns Hopkins University, Baltimore, said that a treat-to-target strategy for osteoporosis is an attractive idea, but there is no consensus on how to apply it nor evidence that it improves clinical outcomes.
Treat to target to guide osteoporosis therapy is like going “backwards”
In treat to target, the target – such as bone mineral density (BMD) (the most common one) – is identified before treatment is started, Dr. McClung explained (and as stated in a review article in the New England Journal of Medicine he coauthored on the topic).
“While treat to target has appealing concepts, using risk factors to guide therapy is almost backwards,” he said. “We can’t change bone density very much.”
Treat to target is “not quite ready for prime time,” he concluded in his rebuttal.
Invited to speculate on which of Dr. McClung’s arguments swayed the audience, Dr. Gregson conceded that with a treat-to-target strategy “there is too much focus on getting one target for the whole global population with osteoporosis.”
“This is an oversimplification of a complex disease, and it misses the main message that the target should be decided with the patient not for the patient, which means one can’t just have one rule for everyone. There has to be scope to have different targets for different people so that we can deliver individualized care.”
Also, she noted, “generally people don’t vote to change familiar systems.”
Arguments for treat to target
Dr. Gregson began her argument, however, by stating that treat to target “is now a feasible and useful approach in osteoporosis care.”
The main reasons for adopting this treatment strategy are as follows:
- It provides a proactive approach with a clear goal.
- It includes periodic treatment reassessment, which allows for prompt revisions to treatment.
- It can use targets to guide treatment timing and patient monitoring.
- It includes shared decision-making, the preferred method of patient care.
- It could improve treatment adherence through patient “buy-in” of the target.
- It can use targets to address the risk of rare side effects.
- It allows for sequential treatments, especially for patients at highest risk of fracture.
- It can include more patient-centered outcomes such as reduced , restored range of movement, and ability to live independently.
“Patients are not interested in their T-score. They are interested in pain,” said Dr. Gregson.
“Reduced fracture risk is a very important goal,” she emphasized. Patients “with osteoporosis and a high fracture risk have the most to gain from a treat-to-target approach.”
“Improved access to anabolic osteoporosis treatments mean achieving those goals or targets are now more achievable than ever,” she concluded.
Arguments against treat to target
“Do we truly have an appropriate, meaningful target for osteoporosis?” Dr. McClung began in his counterargument, which cast a seed of doubt in the minds of the audience.
Targets such as no fractures, fracture risk (FRAX score), bone turnover markers, and bone strength have limitations.
Moreover, “do we have treatment strategies to move patients to the chosen target?” he continued. “What is the evidence that a treat-to-target strategy provides better outcomes than our current treatment paradigm?”
After pointing out a lack of evidence that treat to target leads to better outcomes in osteoporosis, he did allow that “recent data about the relationship between treatment-related BMD values and current fracture risk are appreciated and welcomed.”
“However, a treat-to-target strategy will only be successful if the targets are individualized for each patient, those targets are attainable for most patients, and we have evidence that adopting this strategy improves clinical outcomes,” he summarized.
He then quoted his late wife Betsy Love McClung, RN, MN, who had said, “We don’t treat osteoporosis; we treat patients with osteoporosis.”
Dr. McClung wrapped up by stressing: “We should not treat T-scores or any other specific target. We should individualize our therapy based upon the patient’s risk of fracture and other clinical factors.”
As members of the ECTS and ASBMR, and “proud of our reputation of our societies as being scientifically based and driven,” Dr. McClung concluded, “recognizing that a treat-to-target strategy has appeal, we should certainly encourage more research and be attentive to those results.
“But we must hold off on the adoption of the strategy until we have evidence convincing us of its clinical value.”
When to use a treat-to-target strategy
However, “there are some specific situations where I use something like a treat-to-target strategy,” Dr. McClung conceded. “That is, I make decisions and recommendations to the patients about one drug rather than another because I want to maximize the improvement in their bone density.”
For example, “We have known for 15 years that denosumab results in greater increases in bone density than do bisphosphonates,” he continued.
“So I have used that information to make treatment decisions long before the term ‘treat to target’ entered the vocabulary of osteoporosis experts. I simply wanted to induce the largest possible gains in bone density – but I didn’t have a ‘target’ in mind.”
But for most patients, treatment decisions are made based on other factors, such as their fracture risk, he added. BMD is an important risk factor for fracture, but not as important as having had a recent fracture or being old and frail.
“Unfortunately, in most of today’s health systems, decisions about treatment are made on the basis of cost,” he continued. “More often than not, the health plan rules rather than optimal medical practice are the main guides to treatment decisions.”
According to Dr. Gregson, “in some instances, treat to target would be very helpful. I don’t think it will suit everyone, but I think we should have it in our portfolio of management approaches, and we should as an osteoporosis community be trained in its use.”
“Attractive idea, but ...”
Invited to weigh in, Dr. Jan de Beur noted that A1c, blood pressure, and LDL cholesterol targets are used to improve clinical outcomes in patients with diabetes, hypertension, and hyperlipidemia, respectively.
However, “treat to target for the treatment of low BMD is controversial because it is an attractive idea but without consensus on what the target should be and without evidence that treat to target improves clinical outcomes,” she reiterated.
“The potential benefits of treat to target are proactive, clear goals to achieve, shared decision-making with the patient, the possibility for improved adherence, justification for sequence treatments, and balancing risk of rare side effects.”
On the other hand, “barriers to operationalizing the treat-to-target concept is that there is lack of consensus on the target to be achieved [as any specific target may minimize other important risk factors],” she noted.
There is also a “lack of evidence that demonstrates improved clinical outcomes over choosing therapy based on fracture risk, and lack of ability to achieve the target with available therapies in those with very-low bone density,” she concluded.
Dr. McClung has reported receiving consulting fees from Amgen and Myovant and speaker honoraria from Amgen. Dr. Gregson and Dr. Jan de Beur have reported no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
“A treat-to-target approach is useful in the management of osteoporosis” was the motion proposed in a debate during the recent virtual American Society of Bone and Mineral Research (ASBMR) 2020 annual meeting, and when the votes came in, Michael McClung, MD, who argued against the motion, carried the day.
Agreement with the motion dropped from 63%-46% after McClung, of the Oregon Osteoporosis Center, Portland, put his views forward in opposition to those of Celia L. Gregson, PhD, University of Bristol (England), who argued for the motion on behalf of the European Calcified Tissue Society (ECTS).
Disagreement with the statement rose from 37% predebate to 54% in the postdebate audience polls.
“The debate is part education and part entertainment,” said Dr. McClung, who represented the ASBMR. “I could just as easily have made a strong argument for the motion,” he emphasized in an interview.
On the other hand, “had I been in the audience, as a member of ASBMR relying on data and evidence to make clinical decisions, I would have voted against the motion. As appealing as the strategy sounds, we don’t yet have the hard evidence to support its use nor is there a consensus about what an appropriate target should be, he noted.
Similarly, the debate comoderator and incoming ASBMR President, Suzanne M. Jan de Beur, MD, from Johns Hopkins University, Baltimore, said that a treat-to-target strategy for osteoporosis is an attractive idea, but there is no consensus on how to apply it nor evidence that it improves clinical outcomes.
Treat to target to guide osteoporosis therapy is like going “backwards”
In treat to target, the target – such as bone mineral density (BMD) (the most common one) – is identified before treatment is started, Dr. McClung explained (and as stated in a review article in the New England Journal of Medicine he coauthored on the topic).
“While treat to target has appealing concepts, using risk factors to guide therapy is almost backwards,” he said. “We can’t change bone density very much.”
Treat to target is “not quite ready for prime time,” he concluded in his rebuttal.
Invited to speculate on which of Dr. McClung’s arguments swayed the audience, Dr. Gregson conceded that with a treat-to-target strategy “there is too much focus on getting one target for the whole global population with osteoporosis.”
“This is an oversimplification of a complex disease, and it misses the main message that the target should be decided with the patient not for the patient, which means one can’t just have one rule for everyone. There has to be scope to have different targets for different people so that we can deliver individualized care.”
Also, she noted, “generally people don’t vote to change familiar systems.”
Arguments for treat to target
Dr. Gregson began her argument, however, by stating that treat to target “is now a feasible and useful approach in osteoporosis care.”
The main reasons for adopting this treatment strategy are as follows:
- It provides a proactive approach with a clear goal.
- It includes periodic treatment reassessment, which allows for prompt revisions to treatment.
- It can use targets to guide treatment timing and patient monitoring.
- It includes shared decision-making, the preferred method of patient care.
- It could improve treatment adherence through patient “buy-in” of the target.
- It can use targets to address the risk of rare side effects.
- It allows for sequential treatments, especially for patients at highest risk of fracture.
- It can include more patient-centered outcomes such as reduced , restored range of movement, and ability to live independently.
“Patients are not interested in their T-score. They are interested in pain,” said Dr. Gregson.
“Reduced fracture risk is a very important goal,” she emphasized. Patients “with osteoporosis and a high fracture risk have the most to gain from a treat-to-target approach.”
“Improved access to anabolic osteoporosis treatments mean achieving those goals or targets are now more achievable than ever,” she concluded.
Arguments against treat to target
“Do we truly have an appropriate, meaningful target for osteoporosis?” Dr. McClung began in his counterargument, which cast a seed of doubt in the minds of the audience.
Targets such as no fractures, fracture risk (FRAX score), bone turnover markers, and bone strength have limitations.
Moreover, “do we have treatment strategies to move patients to the chosen target?” he continued. “What is the evidence that a treat-to-target strategy provides better outcomes than our current treatment paradigm?”
After pointing out a lack of evidence that treat to target leads to better outcomes in osteoporosis, he did allow that “recent data about the relationship between treatment-related BMD values and current fracture risk are appreciated and welcomed.”
“However, a treat-to-target strategy will only be successful if the targets are individualized for each patient, those targets are attainable for most patients, and we have evidence that adopting this strategy improves clinical outcomes,” he summarized.
He then quoted his late wife Betsy Love McClung, RN, MN, who had said, “We don’t treat osteoporosis; we treat patients with osteoporosis.”
Dr. McClung wrapped up by stressing: “We should not treat T-scores or any other specific target. We should individualize our therapy based upon the patient’s risk of fracture and other clinical factors.”
As members of the ECTS and ASBMR, and “proud of our reputation of our societies as being scientifically based and driven,” Dr. McClung concluded, “recognizing that a treat-to-target strategy has appeal, we should certainly encourage more research and be attentive to those results.
“But we must hold off on the adoption of the strategy until we have evidence convincing us of its clinical value.”
When to use a treat-to-target strategy
However, “there are some specific situations where I use something like a treat-to-target strategy,” Dr. McClung conceded. “That is, I make decisions and recommendations to the patients about one drug rather than another because I want to maximize the improvement in their bone density.”
For example, “We have known for 15 years that denosumab results in greater increases in bone density than do bisphosphonates,” he continued.
“So I have used that information to make treatment decisions long before the term ‘treat to target’ entered the vocabulary of osteoporosis experts. I simply wanted to induce the largest possible gains in bone density – but I didn’t have a ‘target’ in mind.”
But for most patients, treatment decisions are made based on other factors, such as their fracture risk, he added. BMD is an important risk factor for fracture, but not as important as having had a recent fracture or being old and frail.
“Unfortunately, in most of today’s health systems, decisions about treatment are made on the basis of cost,” he continued. “More often than not, the health plan rules rather than optimal medical practice are the main guides to treatment decisions.”
According to Dr. Gregson, “in some instances, treat to target would be very helpful. I don’t think it will suit everyone, but I think we should have it in our portfolio of management approaches, and we should as an osteoporosis community be trained in its use.”
“Attractive idea, but ...”
Invited to weigh in, Dr. Jan de Beur noted that A1c, blood pressure, and LDL cholesterol targets are used to improve clinical outcomes in patients with diabetes, hypertension, and hyperlipidemia, respectively.
However, “treat to target for the treatment of low BMD is controversial because it is an attractive idea but without consensus on what the target should be and without evidence that treat to target improves clinical outcomes,” she reiterated.
“The potential benefits of treat to target are proactive, clear goals to achieve, shared decision-making with the patient, the possibility for improved adherence, justification for sequence treatments, and balancing risk of rare side effects.”
On the other hand, “barriers to operationalizing the treat-to-target concept is that there is lack of consensus on the target to be achieved [as any specific target may minimize other important risk factors],” she noted.
There is also a “lack of evidence that demonstrates improved clinical outcomes over choosing therapy based on fracture risk, and lack of ability to achieve the target with available therapies in those with very-low bone density,” she concluded.
Dr. McClung has reported receiving consulting fees from Amgen and Myovant and speaker honoraria from Amgen. Dr. Gregson and Dr. Jan de Beur have reported no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
“A treat-to-target approach is useful in the management of osteoporosis” was the motion proposed in a debate during the recent virtual American Society of Bone and Mineral Research (ASBMR) 2020 annual meeting, and when the votes came in, Michael McClung, MD, who argued against the motion, carried the day.
Agreement with the motion dropped from 63%-46% after McClung, of the Oregon Osteoporosis Center, Portland, put his views forward in opposition to those of Celia L. Gregson, PhD, University of Bristol (England), who argued for the motion on behalf of the European Calcified Tissue Society (ECTS).
Disagreement with the statement rose from 37% predebate to 54% in the postdebate audience polls.
“The debate is part education and part entertainment,” said Dr. McClung, who represented the ASBMR. “I could just as easily have made a strong argument for the motion,” he emphasized in an interview.
On the other hand, “had I been in the audience, as a member of ASBMR relying on data and evidence to make clinical decisions, I would have voted against the motion. As appealing as the strategy sounds, we don’t yet have the hard evidence to support its use nor is there a consensus about what an appropriate target should be, he noted.
Similarly, the debate comoderator and incoming ASBMR President, Suzanne M. Jan de Beur, MD, from Johns Hopkins University, Baltimore, said that a treat-to-target strategy for osteoporosis is an attractive idea, but there is no consensus on how to apply it nor evidence that it improves clinical outcomes.
Treat to target to guide osteoporosis therapy is like going “backwards”
In treat to target, the target – such as bone mineral density (BMD) (the most common one) – is identified before treatment is started, Dr. McClung explained (and as stated in a review article in the New England Journal of Medicine he coauthored on the topic).
“While treat to target has appealing concepts, using risk factors to guide therapy is almost backwards,” he said. “We can’t change bone density very much.”
Treat to target is “not quite ready for prime time,” he concluded in his rebuttal.
Invited to speculate on which of Dr. McClung’s arguments swayed the audience, Dr. Gregson conceded that with a treat-to-target strategy “there is too much focus on getting one target for the whole global population with osteoporosis.”
“This is an oversimplification of a complex disease, and it misses the main message that the target should be decided with the patient not for the patient, which means one can’t just have one rule for everyone. There has to be scope to have different targets for different people so that we can deliver individualized care.”
Also, she noted, “generally people don’t vote to change familiar systems.”
Arguments for treat to target
Dr. Gregson began her argument, however, by stating that treat to target “is now a feasible and useful approach in osteoporosis care.”
The main reasons for adopting this treatment strategy are as follows:
- It provides a proactive approach with a clear goal.
- It includes periodic treatment reassessment, which allows for prompt revisions to treatment.
- It can use targets to guide treatment timing and patient monitoring.
- It includes shared decision-making, the preferred method of patient care.
- It could improve treatment adherence through patient “buy-in” of the target.
- It can use targets to address the risk of rare side effects.
- It allows for sequential treatments, especially for patients at highest risk of fracture.
- It can include more patient-centered outcomes such as reduced , restored range of movement, and ability to live independently.
“Patients are not interested in their T-score. They are interested in pain,” said Dr. Gregson.
“Reduced fracture risk is a very important goal,” she emphasized. Patients “with osteoporosis and a high fracture risk have the most to gain from a treat-to-target approach.”
“Improved access to anabolic osteoporosis treatments mean achieving those goals or targets are now more achievable than ever,” she concluded.
Arguments against treat to target
“Do we truly have an appropriate, meaningful target for osteoporosis?” Dr. McClung began in his counterargument, which cast a seed of doubt in the minds of the audience.
Targets such as no fractures, fracture risk (FRAX score), bone turnover markers, and bone strength have limitations.
Moreover, “do we have treatment strategies to move patients to the chosen target?” he continued. “What is the evidence that a treat-to-target strategy provides better outcomes than our current treatment paradigm?”
After pointing out a lack of evidence that treat to target leads to better outcomes in osteoporosis, he did allow that “recent data about the relationship between treatment-related BMD values and current fracture risk are appreciated and welcomed.”
“However, a treat-to-target strategy will only be successful if the targets are individualized for each patient, those targets are attainable for most patients, and we have evidence that adopting this strategy improves clinical outcomes,” he summarized.
He then quoted his late wife Betsy Love McClung, RN, MN, who had said, “We don’t treat osteoporosis; we treat patients with osteoporosis.”
Dr. McClung wrapped up by stressing: “We should not treat T-scores or any other specific target. We should individualize our therapy based upon the patient’s risk of fracture and other clinical factors.”
As members of the ECTS and ASBMR, and “proud of our reputation of our societies as being scientifically based and driven,” Dr. McClung concluded, “recognizing that a treat-to-target strategy has appeal, we should certainly encourage more research and be attentive to those results.
“But we must hold off on the adoption of the strategy until we have evidence convincing us of its clinical value.”
When to use a treat-to-target strategy
However, “there are some specific situations where I use something like a treat-to-target strategy,” Dr. McClung conceded. “That is, I make decisions and recommendations to the patients about one drug rather than another because I want to maximize the improvement in their bone density.”
For example, “We have known for 15 years that denosumab results in greater increases in bone density than do bisphosphonates,” he continued.
“So I have used that information to make treatment decisions long before the term ‘treat to target’ entered the vocabulary of osteoporosis experts. I simply wanted to induce the largest possible gains in bone density – but I didn’t have a ‘target’ in mind.”
But for most patients, treatment decisions are made based on other factors, such as their fracture risk, he added. BMD is an important risk factor for fracture, but not as important as having had a recent fracture or being old and frail.
“Unfortunately, in most of today’s health systems, decisions about treatment are made on the basis of cost,” he continued. “More often than not, the health plan rules rather than optimal medical practice are the main guides to treatment decisions.”
According to Dr. Gregson, “in some instances, treat to target would be very helpful. I don’t think it will suit everyone, but I think we should have it in our portfolio of management approaches, and we should as an osteoporosis community be trained in its use.”
“Attractive idea, but ...”
Invited to weigh in, Dr. Jan de Beur noted that A1c, blood pressure, and LDL cholesterol targets are used to improve clinical outcomes in patients with diabetes, hypertension, and hyperlipidemia, respectively.
However, “treat to target for the treatment of low BMD is controversial because it is an attractive idea but without consensus on what the target should be and without evidence that treat to target improves clinical outcomes,” she reiterated.
“The potential benefits of treat to target are proactive, clear goals to achieve, shared decision-making with the patient, the possibility for improved adherence, justification for sequence treatments, and balancing risk of rare side effects.”
On the other hand, “barriers to operationalizing the treat-to-target concept is that there is lack of consensus on the target to be achieved [as any specific target may minimize other important risk factors],” she noted.
There is also a “lack of evidence that demonstrates improved clinical outcomes over choosing therapy based on fracture risk, and lack of ability to achieve the target with available therapies in those with very-low bone density,” she concluded.
Dr. McClung has reported receiving consulting fees from Amgen and Myovant and speaker honoraria from Amgen. Dr. Gregson and Dr. Jan de Beur have reported no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
FROM ASBMR 2020
Trabecular bone loss may contribute to axial spondyloarthritis progression
A paper published in Seminars in Arthritis and Rheumatism reports the outcomes of a prospective observational cohort study in 245 patients with axial spondyloarthritis that sought to identify possible predictors of spinal radiographic progression of the disease.
Joon-Yong Jung, MD, PhD, and colleagues from the Catholic University of Korea, Seoul, South Korea, wrote that inflammation of the vertebrae is the first stage of progression of axial spondyloarthritis. One hypothesis is that this inflammation is associated with trabecular bone loss, which then leads to spinal instability, which in turns causes biomechanical stress on the area and the formation of new bone as syndesmophytes.
To evaluate the possible relationship between trabecular bone loss and syndesmophytes, researchers used dual-energy x-ray absorptiometry imaging of the lumbar spine, which can assess the microarchitecture of trabecular bone, as well as radiographs of the cervical and lumbar spine at 2 and 4 years of follow-up to assess the presence of syndesmophytes.
At baseline, 40% of patients had syndesmophytes, and the mean number of syndesmophytes was 3.3. A total of 11% of patients at baseline had mild trabecular bone loss, defined as trabecular bone score values between 1.230 and 1.310, and 10% had severe trabecular bone loss, with a score of 1.230 or less.
While on average patients had an increase of 1.41 syndesmophytes every 2 years during the study, patients with severe trabecular bone loss at baseline formed 1.26 more syndesmophytes every 2 years than did patients with normal trabecular bone loss score. After adjusting for variables such as disease activity and clinical factors, the authors found that both mild and severe trabecular bone loss were independently associated with progression of structural damage in the cervical and lumbar spine.
Patients with mild trabecular bone loss had a 120% greater odds of new syndesmophyte formation over the next 2 years, compared with those with normal trabecular bone loss scores, while those with severe loss had a 280% greater odds.
“The more severe the trabecular bone loss, the stronger the effect on the progression of the spine,” the authors wrote. Other factors associated with new syndesmophyte formation included higher C-reactive protein levels, longer symptom duration, smoking, and high NSAID index.
The study also pointed to an association between trabecular bone loss and modified Stoke Ankylosing Spondylitis Spinal Score. Patients with severe trabecular bone loss showed an average increase of 0.37 in their modified Stoke Ankylosing Spondylitis Spinal Score over 2 years, compared with patients with normal trabecular bone loss score at baseline, even after adjusting for confounders.
The authors commented that inflammation is hypothesized to lead to structural damage in two ways. “Inflammation-induced bone loss in the spine results in instability, another type of biomechanical stress, which then triggers a biomechanical response in an attempt to increase stability,” they wrote. Or inflammation leads to the formation of granulated repair tissue which then triggers new bone formation.
Whatever the mechanism, the authors said finding that trabecular bone loss is associated with disease progression suggests a possible use for the trabecular bone score as a practical and noninvasive means to predict spinal progression in patients with axial spondyloarthritis.
The study received no funding, and the authors said they had no conflicts of interest to declare.
SOURCE: Jung J-Y et al. Semin Arthritis Rheum. 2020;50(5):827-33.
A paper published in Seminars in Arthritis and Rheumatism reports the outcomes of a prospective observational cohort study in 245 patients with axial spondyloarthritis that sought to identify possible predictors of spinal radiographic progression of the disease.
Joon-Yong Jung, MD, PhD, and colleagues from the Catholic University of Korea, Seoul, South Korea, wrote that inflammation of the vertebrae is the first stage of progression of axial spondyloarthritis. One hypothesis is that this inflammation is associated with trabecular bone loss, which then leads to spinal instability, which in turns causes biomechanical stress on the area and the formation of new bone as syndesmophytes.
To evaluate the possible relationship between trabecular bone loss and syndesmophytes, researchers used dual-energy x-ray absorptiometry imaging of the lumbar spine, which can assess the microarchitecture of trabecular bone, as well as radiographs of the cervical and lumbar spine at 2 and 4 years of follow-up to assess the presence of syndesmophytes.
At baseline, 40% of patients had syndesmophytes, and the mean number of syndesmophytes was 3.3. A total of 11% of patients at baseline had mild trabecular bone loss, defined as trabecular bone score values between 1.230 and 1.310, and 10% had severe trabecular bone loss, with a score of 1.230 or less.
While on average patients had an increase of 1.41 syndesmophytes every 2 years during the study, patients with severe trabecular bone loss at baseline formed 1.26 more syndesmophytes every 2 years than did patients with normal trabecular bone loss score. After adjusting for variables such as disease activity and clinical factors, the authors found that both mild and severe trabecular bone loss were independently associated with progression of structural damage in the cervical and lumbar spine.
Patients with mild trabecular bone loss had a 120% greater odds of new syndesmophyte formation over the next 2 years, compared with those with normal trabecular bone loss scores, while those with severe loss had a 280% greater odds.
“The more severe the trabecular bone loss, the stronger the effect on the progression of the spine,” the authors wrote. Other factors associated with new syndesmophyte formation included higher C-reactive protein levels, longer symptom duration, smoking, and high NSAID index.
The study also pointed to an association between trabecular bone loss and modified Stoke Ankylosing Spondylitis Spinal Score. Patients with severe trabecular bone loss showed an average increase of 0.37 in their modified Stoke Ankylosing Spondylitis Spinal Score over 2 years, compared with patients with normal trabecular bone loss score at baseline, even after adjusting for confounders.
The authors commented that inflammation is hypothesized to lead to structural damage in two ways. “Inflammation-induced bone loss in the spine results in instability, another type of biomechanical stress, which then triggers a biomechanical response in an attempt to increase stability,” they wrote. Or inflammation leads to the formation of granulated repair tissue which then triggers new bone formation.
Whatever the mechanism, the authors said finding that trabecular bone loss is associated with disease progression suggests a possible use for the trabecular bone score as a practical and noninvasive means to predict spinal progression in patients with axial spondyloarthritis.
The study received no funding, and the authors said they had no conflicts of interest to declare.
SOURCE: Jung J-Y et al. Semin Arthritis Rheum. 2020;50(5):827-33.
A paper published in Seminars in Arthritis and Rheumatism reports the outcomes of a prospective observational cohort study in 245 patients with axial spondyloarthritis that sought to identify possible predictors of spinal radiographic progression of the disease.
Joon-Yong Jung, MD, PhD, and colleagues from the Catholic University of Korea, Seoul, South Korea, wrote that inflammation of the vertebrae is the first stage of progression of axial spondyloarthritis. One hypothesis is that this inflammation is associated with trabecular bone loss, which then leads to spinal instability, which in turns causes biomechanical stress on the area and the formation of new bone as syndesmophytes.
To evaluate the possible relationship between trabecular bone loss and syndesmophytes, researchers used dual-energy x-ray absorptiometry imaging of the lumbar spine, which can assess the microarchitecture of trabecular bone, as well as radiographs of the cervical and lumbar spine at 2 and 4 years of follow-up to assess the presence of syndesmophytes.
At baseline, 40% of patients had syndesmophytes, and the mean number of syndesmophytes was 3.3. A total of 11% of patients at baseline had mild trabecular bone loss, defined as trabecular bone score values between 1.230 and 1.310, and 10% had severe trabecular bone loss, with a score of 1.230 or less.
While on average patients had an increase of 1.41 syndesmophytes every 2 years during the study, patients with severe trabecular bone loss at baseline formed 1.26 more syndesmophytes every 2 years than did patients with normal trabecular bone loss score. After adjusting for variables such as disease activity and clinical factors, the authors found that both mild and severe trabecular bone loss were independently associated with progression of structural damage in the cervical and lumbar spine.
Patients with mild trabecular bone loss had a 120% greater odds of new syndesmophyte formation over the next 2 years, compared with those with normal trabecular bone loss scores, while those with severe loss had a 280% greater odds.
“The more severe the trabecular bone loss, the stronger the effect on the progression of the spine,” the authors wrote. Other factors associated with new syndesmophyte formation included higher C-reactive protein levels, longer symptom duration, smoking, and high NSAID index.
The study also pointed to an association between trabecular bone loss and modified Stoke Ankylosing Spondylitis Spinal Score. Patients with severe trabecular bone loss showed an average increase of 0.37 in their modified Stoke Ankylosing Spondylitis Spinal Score over 2 years, compared with patients with normal trabecular bone loss score at baseline, even after adjusting for confounders.
The authors commented that inflammation is hypothesized to lead to structural damage in two ways. “Inflammation-induced bone loss in the spine results in instability, another type of biomechanical stress, which then triggers a biomechanical response in an attempt to increase stability,” they wrote. Or inflammation leads to the formation of granulated repair tissue which then triggers new bone formation.
Whatever the mechanism, the authors said finding that trabecular bone loss is associated with disease progression suggests a possible use for the trabecular bone score as a practical and noninvasive means to predict spinal progression in patients with axial spondyloarthritis.
The study received no funding, and the authors said they had no conflicts of interest to declare.
SOURCE: Jung J-Y et al. Semin Arthritis Rheum. 2020;50(5):827-33.
FROM SEMINARS IN ARTHRITIS AND RHEUMATISM
More dairy lowers risk of falls, fractures in frail elderly
Consuming more milk, cheese, or yogurt might be a simple, low-cost way to boost bone health and prevent some falls and fractures in older people living in long-term care facilities, according to a new randomized study from Australia.
“Supplementation using dairy foods is likely to be an effective, safe, widely available, and low cost means of curtailing the public health burden of fractures,” said Sandra Iuliano, PhD, from the University of Melbourne, who presented the findings during the virtual American Society of Bone and Mineral Research 2020 annual meeting.
The researchers randomized 60 old-age institutions to provide residents with their usual menus or a diet with more milk, cheese, or yogurt for 2 years.
The residents with the altered menus increased their dairy consumption from 2 servings/day to 3.5 servings/day, which was reflected in a greater intake of calcium and protein, along with fewer falls, total fractures, and hip fractures than in the control group.
“This is the first randomized trial to show a benefit of dairy food intake on risk of fractures,” Walter Willett, MD, DrPH, professor of nutrition and epidemiology at the Harvard School of Public Health, Boston, said in an interview.
The results are “not surprising” because supplements of calcium plus vitamin D have reduced the risk of fractures in a similar population of older residents living in special living facilities, said Dr. Willett, coauthor of a recent review article, “Milk and Health,” published in the New England Journal of Medicine.
“It is important for everyone to have adequate intake of calcium and vitamin D,” he said. However, “it isn’t clear whether it is better to ensure this clinically by supplements, overall healthy diet, or extra dairy intake,” he added, noting that consuming the amount of dairy given in this Australian study is not environmentally sustainable.
Clifford Rosen, MD, professor of medicine, Tufts University, Boston, said in an interview that the Australian researchers studied the impact of increased dietary calcium and protein, not the impact of vitamin D via supplements.
“This is progress toward getting interventions to our most needy residents to prevent fractures – probably the most compelling data that we have had in a number of years,” he noted.
The current study shows “it’s not [the] vitamin D,” because the residents had initial low calcium levels but normal vitamin D levels. “For too long we’ve been stuck on the idea that it is [increasing] vitamin D in the elderly that causes a reduction in fractures,” said Dr. Rosen. “The data are not very supportive of it, but people continue to think that’s the most important element.”
On the other hand, the current study raises certain questions. “What we don’t know is, is it the calcium, or is it the protein, or the combination, that had an impact?”
Would upping dairy decrease falls?
Older adults living in institutions have a high risk of falls and fractures, including hip fractures, and “malnutrition is common,” said Dr. Iuliano during her presentation.
Prior studies have reported that such residents have a daily dietary calcium intake of 635 mg (half the recommended 1,300 mg), a protein intake of 0.8 g/kg body weight (less than the recommended 1 g/kg body weight), and a dairy intake of 1.5 servings (about a third of the recommended amount), she said.
The group hypothesized that upping dairy intake of elderly residents living in long-term care institutions would reduce the risk of fractures. They performed a 2-year cluster-randomized trial in 60 facilities in Melbourne and surrounding areas.
Half gave their 3,301 residents menus with a higher dairy content, and the other half gave their 3,894 residents (controls) the usual menus.
The residents in both groups had similar characteristics: they were a mean age of 87 years and 68% were women. A subgroup had blood tests and bone morphology studies at baseline and 1 year.
Researchers verified nutrient intake by analyzing the menus and doing plate waste analysis for a subgroup, and they determined the number of falls and fractures from incident and hospital x-ray reports, respectively.
One-third fewer fractures in the higher-dairy group
At the study start, residents in both groups had similar vitamin D levels (72 nmol/L) and bone morphology. They were consuming two servings of dairy food and drink a day, where a serving was 250 mL of milk (including lactose-free milk) or 200 g of yogurt or 40 g of cheese.
Their initial daily calcium intake was 650 mg, which stayed the same in the control group, but increased to >1100 mg in the intervention group.
Their initial daily protein intake was around 59 g, which remained the same in the control group, but increased to about 72 grams (1.1 g/kg body weight) in the intervention group.
At 2 years, the 1.5 servings/day increase in dairy intake in the control versus intervention group was associated with an 11% reduction in falls (62% vs. 57%), a 33% reduction in fractures (5.2% vs. 3.7%), a 46% reduction in hip fractures (2.4% vs. 1.3%), and no difference in mortality (28% in both groups).
The intervention was also associated with a slowing in bone loss and an increase in insulinlike growth factor–1.
Four dairy servings a day “is high”
Dr. Willett said that “it is reasonable for seniors to take one or two servings of dairy per day, but four servings per day, as in this study, is probably not necessary.”
Moreover, “dairy production has a major impact on greenhouse gas emissions, and even two servings per day would not be environmentally sustainable if everyone were to consume this amount,” he observed.
“Because the world is facing an existential threat from climate change, general advice to consume high amounts of dairy products would be irresponsible as we can get all essential nutrients from other sources,” he added. “That said, modest amounts of dairy foods, such as one to two servings per day could be reasonable. There is some suggestive evidence that dairy in the form of yogurt may have particular benefits.”
The study was funded by Melbourne University and various dietary councils. Dr. Iuliano reported receiving lecture fees from Abbott. Dr. Rosen and Dr. Willett reported no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
Consuming more milk, cheese, or yogurt might be a simple, low-cost way to boost bone health and prevent some falls and fractures in older people living in long-term care facilities, according to a new randomized study from Australia.
“Supplementation using dairy foods is likely to be an effective, safe, widely available, and low cost means of curtailing the public health burden of fractures,” said Sandra Iuliano, PhD, from the University of Melbourne, who presented the findings during the virtual American Society of Bone and Mineral Research 2020 annual meeting.
The researchers randomized 60 old-age institutions to provide residents with their usual menus or a diet with more milk, cheese, or yogurt for 2 years.
The residents with the altered menus increased their dairy consumption from 2 servings/day to 3.5 servings/day, which was reflected in a greater intake of calcium and protein, along with fewer falls, total fractures, and hip fractures than in the control group.
“This is the first randomized trial to show a benefit of dairy food intake on risk of fractures,” Walter Willett, MD, DrPH, professor of nutrition and epidemiology at the Harvard School of Public Health, Boston, said in an interview.
The results are “not surprising” because supplements of calcium plus vitamin D have reduced the risk of fractures in a similar population of older residents living in special living facilities, said Dr. Willett, coauthor of a recent review article, “Milk and Health,” published in the New England Journal of Medicine.
“It is important for everyone to have adequate intake of calcium and vitamin D,” he said. However, “it isn’t clear whether it is better to ensure this clinically by supplements, overall healthy diet, or extra dairy intake,” he added, noting that consuming the amount of dairy given in this Australian study is not environmentally sustainable.
Clifford Rosen, MD, professor of medicine, Tufts University, Boston, said in an interview that the Australian researchers studied the impact of increased dietary calcium and protein, not the impact of vitamin D via supplements.
“This is progress toward getting interventions to our most needy residents to prevent fractures – probably the most compelling data that we have had in a number of years,” he noted.
The current study shows “it’s not [the] vitamin D,” because the residents had initial low calcium levels but normal vitamin D levels. “For too long we’ve been stuck on the idea that it is [increasing] vitamin D in the elderly that causes a reduction in fractures,” said Dr. Rosen. “The data are not very supportive of it, but people continue to think that’s the most important element.”
On the other hand, the current study raises certain questions. “What we don’t know is, is it the calcium, or is it the protein, or the combination, that had an impact?”
Would upping dairy decrease falls?
Older adults living in institutions have a high risk of falls and fractures, including hip fractures, and “malnutrition is common,” said Dr. Iuliano during her presentation.
Prior studies have reported that such residents have a daily dietary calcium intake of 635 mg (half the recommended 1,300 mg), a protein intake of 0.8 g/kg body weight (less than the recommended 1 g/kg body weight), and a dairy intake of 1.5 servings (about a third of the recommended amount), she said.
The group hypothesized that upping dairy intake of elderly residents living in long-term care institutions would reduce the risk of fractures. They performed a 2-year cluster-randomized trial in 60 facilities in Melbourne and surrounding areas.
Half gave their 3,301 residents menus with a higher dairy content, and the other half gave their 3,894 residents (controls) the usual menus.
The residents in both groups had similar characteristics: they were a mean age of 87 years and 68% were women. A subgroup had blood tests and bone morphology studies at baseline and 1 year.
Researchers verified nutrient intake by analyzing the menus and doing plate waste analysis for a subgroup, and they determined the number of falls and fractures from incident and hospital x-ray reports, respectively.
One-third fewer fractures in the higher-dairy group
At the study start, residents in both groups had similar vitamin D levels (72 nmol/L) and bone morphology. They were consuming two servings of dairy food and drink a day, where a serving was 250 mL of milk (including lactose-free milk) or 200 g of yogurt or 40 g of cheese.
Their initial daily calcium intake was 650 mg, which stayed the same in the control group, but increased to >1100 mg in the intervention group.
Their initial daily protein intake was around 59 g, which remained the same in the control group, but increased to about 72 grams (1.1 g/kg body weight) in the intervention group.
At 2 years, the 1.5 servings/day increase in dairy intake in the control versus intervention group was associated with an 11% reduction in falls (62% vs. 57%), a 33% reduction in fractures (5.2% vs. 3.7%), a 46% reduction in hip fractures (2.4% vs. 1.3%), and no difference in mortality (28% in both groups).
The intervention was also associated with a slowing in bone loss and an increase in insulinlike growth factor–1.
Four dairy servings a day “is high”
Dr. Willett said that “it is reasonable for seniors to take one or two servings of dairy per day, but four servings per day, as in this study, is probably not necessary.”
Moreover, “dairy production has a major impact on greenhouse gas emissions, and even two servings per day would not be environmentally sustainable if everyone were to consume this amount,” he observed.
“Because the world is facing an existential threat from climate change, general advice to consume high amounts of dairy products would be irresponsible as we can get all essential nutrients from other sources,” he added. “That said, modest amounts of dairy foods, such as one to two servings per day could be reasonable. There is some suggestive evidence that dairy in the form of yogurt may have particular benefits.”
The study was funded by Melbourne University and various dietary councils. Dr. Iuliano reported receiving lecture fees from Abbott. Dr. Rosen and Dr. Willett reported no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
Consuming more milk, cheese, or yogurt might be a simple, low-cost way to boost bone health and prevent some falls and fractures in older people living in long-term care facilities, according to a new randomized study from Australia.
“Supplementation using dairy foods is likely to be an effective, safe, widely available, and low cost means of curtailing the public health burden of fractures,” said Sandra Iuliano, PhD, from the University of Melbourne, who presented the findings during the virtual American Society of Bone and Mineral Research 2020 annual meeting.
The researchers randomized 60 old-age institutions to provide residents with their usual menus or a diet with more milk, cheese, or yogurt for 2 years.
The residents with the altered menus increased their dairy consumption from 2 servings/day to 3.5 servings/day, which was reflected in a greater intake of calcium and protein, along with fewer falls, total fractures, and hip fractures than in the control group.
“This is the first randomized trial to show a benefit of dairy food intake on risk of fractures,” Walter Willett, MD, DrPH, professor of nutrition and epidemiology at the Harvard School of Public Health, Boston, said in an interview.
The results are “not surprising” because supplements of calcium plus vitamin D have reduced the risk of fractures in a similar population of older residents living in special living facilities, said Dr. Willett, coauthor of a recent review article, “Milk and Health,” published in the New England Journal of Medicine.
“It is important for everyone to have adequate intake of calcium and vitamin D,” he said. However, “it isn’t clear whether it is better to ensure this clinically by supplements, overall healthy diet, or extra dairy intake,” he added, noting that consuming the amount of dairy given in this Australian study is not environmentally sustainable.
Clifford Rosen, MD, professor of medicine, Tufts University, Boston, said in an interview that the Australian researchers studied the impact of increased dietary calcium and protein, not the impact of vitamin D via supplements.
“This is progress toward getting interventions to our most needy residents to prevent fractures – probably the most compelling data that we have had in a number of years,” he noted.
The current study shows “it’s not [the] vitamin D,” because the residents had initial low calcium levels but normal vitamin D levels. “For too long we’ve been stuck on the idea that it is [increasing] vitamin D in the elderly that causes a reduction in fractures,” said Dr. Rosen. “The data are not very supportive of it, but people continue to think that’s the most important element.”
On the other hand, the current study raises certain questions. “What we don’t know is, is it the calcium, or is it the protein, or the combination, that had an impact?”
Would upping dairy decrease falls?
Older adults living in institutions have a high risk of falls and fractures, including hip fractures, and “malnutrition is common,” said Dr. Iuliano during her presentation.
Prior studies have reported that such residents have a daily dietary calcium intake of 635 mg (half the recommended 1,300 mg), a protein intake of 0.8 g/kg body weight (less than the recommended 1 g/kg body weight), and a dairy intake of 1.5 servings (about a third of the recommended amount), she said.
The group hypothesized that upping dairy intake of elderly residents living in long-term care institutions would reduce the risk of fractures. They performed a 2-year cluster-randomized trial in 60 facilities in Melbourne and surrounding areas.
Half gave their 3,301 residents menus with a higher dairy content, and the other half gave their 3,894 residents (controls) the usual menus.
The residents in both groups had similar characteristics: they were a mean age of 87 years and 68% were women. A subgroup had blood tests and bone morphology studies at baseline and 1 year.
Researchers verified nutrient intake by analyzing the menus and doing plate waste analysis for a subgroup, and they determined the number of falls and fractures from incident and hospital x-ray reports, respectively.
One-third fewer fractures in the higher-dairy group
At the study start, residents in both groups had similar vitamin D levels (72 nmol/L) and bone morphology. They were consuming two servings of dairy food and drink a day, where a serving was 250 mL of milk (including lactose-free milk) or 200 g of yogurt or 40 g of cheese.
Their initial daily calcium intake was 650 mg, which stayed the same in the control group, but increased to >1100 mg in the intervention group.
Their initial daily protein intake was around 59 g, which remained the same in the control group, but increased to about 72 grams (1.1 g/kg body weight) in the intervention group.
At 2 years, the 1.5 servings/day increase in dairy intake in the control versus intervention group was associated with an 11% reduction in falls (62% vs. 57%), a 33% reduction in fractures (5.2% vs. 3.7%), a 46% reduction in hip fractures (2.4% vs. 1.3%), and no difference in mortality (28% in both groups).
The intervention was also associated with a slowing in bone loss and an increase in insulinlike growth factor–1.
Four dairy servings a day “is high”
Dr. Willett said that “it is reasonable for seniors to take one or two servings of dairy per day, but four servings per day, as in this study, is probably not necessary.”
Moreover, “dairy production has a major impact on greenhouse gas emissions, and even two servings per day would not be environmentally sustainable if everyone were to consume this amount,” he observed.
“Because the world is facing an existential threat from climate change, general advice to consume high amounts of dairy products would be irresponsible as we can get all essential nutrients from other sources,” he added. “That said, modest amounts of dairy foods, such as one to two servings per day could be reasonable. There is some suggestive evidence that dairy in the form of yogurt may have particular benefits.”
The study was funded by Melbourne University and various dietary councils. Dr. Iuliano reported receiving lecture fees from Abbott. Dr. Rosen and Dr. Willett reported no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
FROM ASBMR 2020
Mega vitamin D harms bone in women, not men, without osteoporosis
“More is not necessarily better” when it comes to vitamin D supplements for women with adequate serum levels, new research suggests.
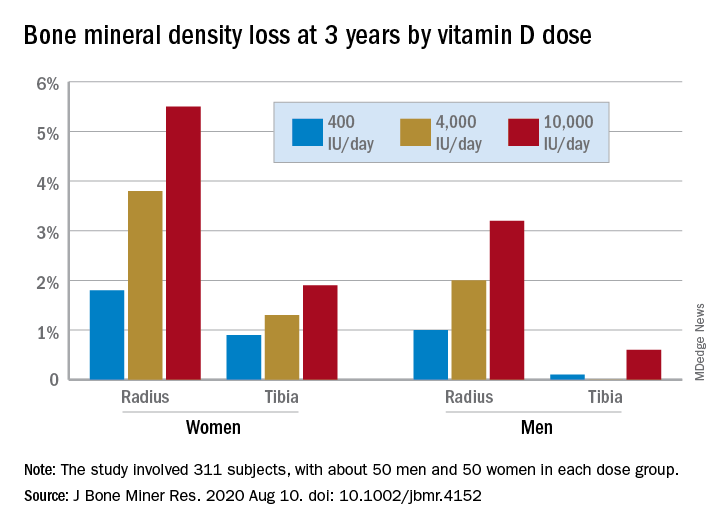
In a study of healthy 55- to 70-year-old women who took very-high-dose vitamin D supplements – either 4,000 IU/day or the previously identified “upper safe limit” of 10,000 IU/day – for 3 years had a significantly greater loss of total bone mineral density (BMD) at the radius and tibia than did women who took 400 IU/day. However, this effect was not seen in men. And the higher-dose vitamin D supplements did not improve bone strength in men or women.
But this was an exploratory post hoc analysis, and these were healthy community-dwelling adults with sufficient serum vitamin D levels (and no osteoporosis) at study entry, stressed lead researcher Lauren A. Burt, PhD, from the University of Calgary, in Alberta, Canada.
Dr. Burt presented these findings Sept. 11 at the virtual American Society of Bone and Mineral Research (ASBMR) 2020 annual meeting, and the study was also recently published online in the Journal of Bone and Mineral Research.
The results suggest that, “if you have normal bone density and adequate levels of vitamin D, there is no bone benefit in taking doses of vitamin D above the standard recommendations designed to prevent vitamin D deficiency, and doses at or above 4,000 IU/day might even be detrimental to bone, especially in females,” Dr. Burt said in an interview.
“These results are clinically relevant,” Dr. Burt and her coauthors wrote, “as vitamin D supplementation is widely administered to postmenopausal females for osteoporosis prevention.”
“Our findings do not support a benefit of high-dose vitamin D supplementation for bone health and raise the possibility of harm for females.”
Invited to comment, Meryl S. LeBoff, MD, of Harvard Medical School, Boston, said in an interview that this finding “warrants further research” because it is “important” to discover sex differences in bone responses to vitamin D.
“This doesn’t apply to osteoporosis”
Dr. LeBoff was lead author of a subanalysis of the Vitamin D and Omega-3 Trial (VITAL).
As she reported at last year’s ASBMR meeting, that analysis showed that, in healthy adults who did not have vitamin D insufficiency, taking vitamin D3 supplements for 2 years did not improve BMD, compared with placebo (recently published), nor was this linked with fewer fractures.
Dr. LeBoff pointed out that the current study investigated “very high doses of vitamin D” – at least double the 2,000 IU/day doses examined in VITAL.
Also, the serum vitamin D levels in this study were “above what we considered the upper normal limit for our assay in our hospital,” she noted, and there was no placebo control.
“We did not see any adverse effects of 2,000 IU/day vitamin D,” Dr. LeBoff stressed.
“At the same time, we didn’t see any significant benefits in terms of bone density because they already had achieved a normal level of vitamin D sufficient for bone.”
But “this doesn’t apply to patients with vitamin D deficiency, patients with osteoporosis, or low bone mass, in which case we would recommend vitamin D.”
Some patients take more vitamin D than they need because they think more is better, said LeBoff, but this study suggests “more is not necessarily better.”
“There’s been a concern for several years that too much vitamin D may be associated with increased fractures,” she emphasized.
Post hoc analysis
The current study analyzed new data from the Calgary Vitamin D study.
That study found no benefit in BMD or bone strength (JAMA. 2019;322[8]:736-45), contrary to the researchers’ hypothesis that high-dose vitamin D supplements would be associated with greater calcium absorption and parathyroid hormone suppression and, thus, reduced age-related bone loss (improved bone density and strength).
Instead, they found a negative dose-response relationship, which “should be regarded as hypothesis generating, requiring confirmation with further research,” they wrote.
The current study sought to determine if there were sex differences in the effect of vitamin D supplements on bone health in this population.
From October 2013 to December 2017, the Canada Vitamin D study enrolled 311 participants (53% male). To be eligible for the study, participants had to have serum 25-hydroxyvitamin D levels greater than 30 nmol/L and less than 125 nmol/L. They also needed to have adequate calcium intake (1,200 mg/day, as defined by the U.S. Institute of Medicine), or if not, they were instructed to take an appropriate calcium supplement dose.
Patients were randomized to receive 400, 4,000, or 10,000 IU/day of vitamin D3 cholecalciferol, given as 5 drops/day of liquid (Ddrops), with roughly 50 men and 50 women in each dose group.
Researchers selected the 400 IU/day dose as the comparator because the Institute of Medicine recommends a vitamin D intake of 600 IU/day for adults under age 70 years to provide the vitamin D needed for bone health. The typical Canadian diet includes 200-300 IU/day of vitamin D, so individuals would need a supplement of 400 IU/day to reach the recommended intake. The 4,000 IU/day dose is the recommended tolerable upper intake level, according to the Institute of Medicine. And the 10,000 IU/day dose is the tolerable upper intake level of vitamin D as identified in a review by Hathcock and colleagues (Am J Clin Nutr. 2007;85:6-18).
Participants underwent scans with high-resolution peripheral quantitative computed tomography (HR-pQCT) to measure total volumetric BMD at the radius and tibia at baseline, 6, 12, 24, and 36 months. Finite element analysis was used to estimate bone strength.
After 3 years, women had lost significantly more BMD at the radius after taking high-dose versus 400 IU/day of vitamin D. Losses in BMD at the tibia followed a similar trend but were smaller (Figure 1). There were no significant changes in this measure among men (Figure 2).
There were also no significant changes in bone strength among men or women.
Biological mechanism remains to be determined
Dr. LeBoff said a “possible biological explanation” for the findings is that “women, particularly when they are younger, lose more bone than men.”
“Postmenopausal females do lose bone at an accelerated rate compared with males,” Dr. Burt agreed, “but at the time the study was designed, there was no reason to believe that high-dose vitamin D supplementation would accelerate the problem.”
“The biological mechanism of the vitamin D–related bone loss needs further investigation,” Dr. Burt added, “but there are laboratory data suggesting that supraphysiologic doses of active metabolites of vitamin D may stimulate bone resorption.”
The study was funded by the Pure North S’Energy Foundation. Dr. Burt has reported no relevant financial relationships. Disclosures for the other authors are listed with the article. Dr. LeBoff has reported receiving grants from the National Institutes of Health for the VITAL analysis.
A version of this article originally appeared on Medscape.com.
“More is not necessarily better” when it comes to vitamin D supplements for women with adequate serum levels, new research suggests.

In a study of healthy 55- to 70-year-old women who took very-high-dose vitamin D supplements – either 4,000 IU/day or the previously identified “upper safe limit” of 10,000 IU/day – for 3 years had a significantly greater loss of total bone mineral density (BMD) at the radius and tibia than did women who took 400 IU/day. However, this effect was not seen in men. And the higher-dose vitamin D supplements did not improve bone strength in men or women.
But this was an exploratory post hoc analysis, and these were healthy community-dwelling adults with sufficient serum vitamin D levels (and no osteoporosis) at study entry, stressed lead researcher Lauren A. Burt, PhD, from the University of Calgary, in Alberta, Canada.
Dr. Burt presented these findings Sept. 11 at the virtual American Society of Bone and Mineral Research (ASBMR) 2020 annual meeting, and the study was also recently published online in the Journal of Bone and Mineral Research.
The results suggest that, “if you have normal bone density and adequate levels of vitamin D, there is no bone benefit in taking doses of vitamin D above the standard recommendations designed to prevent vitamin D deficiency, and doses at or above 4,000 IU/day might even be detrimental to bone, especially in females,” Dr. Burt said in an interview.
“These results are clinically relevant,” Dr. Burt and her coauthors wrote, “as vitamin D supplementation is widely administered to postmenopausal females for osteoporosis prevention.”
“Our findings do not support a benefit of high-dose vitamin D supplementation for bone health and raise the possibility of harm for females.”
Invited to comment, Meryl S. LeBoff, MD, of Harvard Medical School, Boston, said in an interview that this finding “warrants further research” because it is “important” to discover sex differences in bone responses to vitamin D.
“This doesn’t apply to osteoporosis”
Dr. LeBoff was lead author of a subanalysis of the Vitamin D and Omega-3 Trial (VITAL).
As she reported at last year’s ASBMR meeting, that analysis showed that, in healthy adults who did not have vitamin D insufficiency, taking vitamin D3 supplements for 2 years did not improve BMD, compared with placebo (recently published), nor was this linked with fewer fractures.
Dr. LeBoff pointed out that the current study investigated “very high doses of vitamin D” – at least double the 2,000 IU/day doses examined in VITAL.
Also, the serum vitamin D levels in this study were “above what we considered the upper normal limit for our assay in our hospital,” she noted, and there was no placebo control.
“We did not see any adverse effects of 2,000 IU/day vitamin D,” Dr. LeBoff stressed.
“At the same time, we didn’t see any significant benefits in terms of bone density because they already had achieved a normal level of vitamin D sufficient for bone.”
But “this doesn’t apply to patients with vitamin D deficiency, patients with osteoporosis, or low bone mass, in which case we would recommend vitamin D.”
Some patients take more vitamin D than they need because they think more is better, said LeBoff, but this study suggests “more is not necessarily better.”
“There’s been a concern for several years that too much vitamin D may be associated with increased fractures,” she emphasized.
Post hoc analysis
The current study analyzed new data from the Calgary Vitamin D study.
That study found no benefit in BMD or bone strength (JAMA. 2019;322[8]:736-45), contrary to the researchers’ hypothesis that high-dose vitamin D supplements would be associated with greater calcium absorption and parathyroid hormone suppression and, thus, reduced age-related bone loss (improved bone density and strength).
Instead, they found a negative dose-response relationship, which “should be regarded as hypothesis generating, requiring confirmation with further research,” they wrote.
The current study sought to determine if there were sex differences in the effect of vitamin D supplements on bone health in this population.
From October 2013 to December 2017, the Canada Vitamin D study enrolled 311 participants (53% male). To be eligible for the study, participants had to have serum 25-hydroxyvitamin D levels greater than 30 nmol/L and less than 125 nmol/L. They also needed to have adequate calcium intake (1,200 mg/day, as defined by the U.S. Institute of Medicine), or if not, they were instructed to take an appropriate calcium supplement dose.
Patients were randomized to receive 400, 4,000, or 10,000 IU/day of vitamin D3 cholecalciferol, given as 5 drops/day of liquid (Ddrops), with roughly 50 men and 50 women in each dose group.
Researchers selected the 400 IU/day dose as the comparator because the Institute of Medicine recommends a vitamin D intake of 600 IU/day for adults under age 70 years to provide the vitamin D needed for bone health. The typical Canadian diet includes 200-300 IU/day of vitamin D, so individuals would need a supplement of 400 IU/day to reach the recommended intake. The 4,000 IU/day dose is the recommended tolerable upper intake level, according to the Institute of Medicine. And the 10,000 IU/day dose is the tolerable upper intake level of vitamin D as identified in a review by Hathcock and colleagues (Am J Clin Nutr. 2007;85:6-18).
Participants underwent scans with high-resolution peripheral quantitative computed tomography (HR-pQCT) to measure total volumetric BMD at the radius and tibia at baseline, 6, 12, 24, and 36 months. Finite element analysis was used to estimate bone strength.
After 3 years, women had lost significantly more BMD at the radius after taking high-dose versus 400 IU/day of vitamin D. Losses in BMD at the tibia followed a similar trend but were smaller (Figure 1). There were no significant changes in this measure among men (Figure 2).
There were also no significant changes in bone strength among men or women.
Biological mechanism remains to be determined
Dr. LeBoff said a “possible biological explanation” for the findings is that “women, particularly when they are younger, lose more bone than men.”
“Postmenopausal females do lose bone at an accelerated rate compared with males,” Dr. Burt agreed, “but at the time the study was designed, there was no reason to believe that high-dose vitamin D supplementation would accelerate the problem.”
“The biological mechanism of the vitamin D–related bone loss needs further investigation,” Dr. Burt added, “but there are laboratory data suggesting that supraphysiologic doses of active metabolites of vitamin D may stimulate bone resorption.”
The study was funded by the Pure North S’Energy Foundation. Dr. Burt has reported no relevant financial relationships. Disclosures for the other authors are listed with the article. Dr. LeBoff has reported receiving grants from the National Institutes of Health for the VITAL analysis.
A version of this article originally appeared on Medscape.com.
“More is not necessarily better” when it comes to vitamin D supplements for women with adequate serum levels, new research suggests.

In a study of healthy 55- to 70-year-old women who took very-high-dose vitamin D supplements – either 4,000 IU/day or the previously identified “upper safe limit” of 10,000 IU/day – for 3 years had a significantly greater loss of total bone mineral density (BMD) at the radius and tibia than did women who took 400 IU/day. However, this effect was not seen in men. And the higher-dose vitamin D supplements did not improve bone strength in men or women.
But this was an exploratory post hoc analysis, and these were healthy community-dwelling adults with sufficient serum vitamin D levels (and no osteoporosis) at study entry, stressed lead researcher Lauren A. Burt, PhD, from the University of Calgary, in Alberta, Canada.
Dr. Burt presented these findings Sept. 11 at the virtual American Society of Bone and Mineral Research (ASBMR) 2020 annual meeting, and the study was also recently published online in the Journal of Bone and Mineral Research.
The results suggest that, “if you have normal bone density and adequate levels of vitamin D, there is no bone benefit in taking doses of vitamin D above the standard recommendations designed to prevent vitamin D deficiency, and doses at or above 4,000 IU/day might even be detrimental to bone, especially in females,” Dr. Burt said in an interview.
“These results are clinically relevant,” Dr. Burt and her coauthors wrote, “as vitamin D supplementation is widely administered to postmenopausal females for osteoporosis prevention.”
“Our findings do not support a benefit of high-dose vitamin D supplementation for bone health and raise the possibility of harm for females.”
Invited to comment, Meryl S. LeBoff, MD, of Harvard Medical School, Boston, said in an interview that this finding “warrants further research” because it is “important” to discover sex differences in bone responses to vitamin D.
“This doesn’t apply to osteoporosis”
Dr. LeBoff was lead author of a subanalysis of the Vitamin D and Omega-3 Trial (VITAL).
As she reported at last year’s ASBMR meeting, that analysis showed that, in healthy adults who did not have vitamin D insufficiency, taking vitamin D3 supplements for 2 years did not improve BMD, compared with placebo (recently published), nor was this linked with fewer fractures.
Dr. LeBoff pointed out that the current study investigated “very high doses of vitamin D” – at least double the 2,000 IU/day doses examined in VITAL.
Also, the serum vitamin D levels in this study were “above what we considered the upper normal limit for our assay in our hospital,” she noted, and there was no placebo control.
“We did not see any adverse effects of 2,000 IU/day vitamin D,” Dr. LeBoff stressed.
“At the same time, we didn’t see any significant benefits in terms of bone density because they already had achieved a normal level of vitamin D sufficient for bone.”
But “this doesn’t apply to patients with vitamin D deficiency, patients with osteoporosis, or low bone mass, in which case we would recommend vitamin D.”
Some patients take more vitamin D than they need because they think more is better, said LeBoff, but this study suggests “more is not necessarily better.”
“There’s been a concern for several years that too much vitamin D may be associated with increased fractures,” she emphasized.
Post hoc analysis
The current study analyzed new data from the Calgary Vitamin D study.
That study found no benefit in BMD or bone strength (JAMA. 2019;322[8]:736-45), contrary to the researchers’ hypothesis that high-dose vitamin D supplements would be associated with greater calcium absorption and parathyroid hormone suppression and, thus, reduced age-related bone loss (improved bone density and strength).
Instead, they found a negative dose-response relationship, which “should be regarded as hypothesis generating, requiring confirmation with further research,” they wrote.
The current study sought to determine if there were sex differences in the effect of vitamin D supplements on bone health in this population.
From October 2013 to December 2017, the Canada Vitamin D study enrolled 311 participants (53% male). To be eligible for the study, participants had to have serum 25-hydroxyvitamin D levels greater than 30 nmol/L and less than 125 nmol/L. They also needed to have adequate calcium intake (1,200 mg/day, as defined by the U.S. Institute of Medicine), or if not, they were instructed to take an appropriate calcium supplement dose.
Patients were randomized to receive 400, 4,000, or 10,000 IU/day of vitamin D3 cholecalciferol, given as 5 drops/day of liquid (Ddrops), with roughly 50 men and 50 women in each dose group.
Researchers selected the 400 IU/day dose as the comparator because the Institute of Medicine recommends a vitamin D intake of 600 IU/day for adults under age 70 years to provide the vitamin D needed for bone health. The typical Canadian diet includes 200-300 IU/day of vitamin D, so individuals would need a supplement of 400 IU/day to reach the recommended intake. The 4,000 IU/day dose is the recommended tolerable upper intake level, according to the Institute of Medicine. And the 10,000 IU/day dose is the tolerable upper intake level of vitamin D as identified in a review by Hathcock and colleagues (Am J Clin Nutr. 2007;85:6-18).
Participants underwent scans with high-resolution peripheral quantitative computed tomography (HR-pQCT) to measure total volumetric BMD at the radius and tibia at baseline, 6, 12, 24, and 36 months. Finite element analysis was used to estimate bone strength.
After 3 years, women had lost significantly more BMD at the radius after taking high-dose versus 400 IU/day of vitamin D. Losses in BMD at the tibia followed a similar trend but were smaller (Figure 1). There were no significant changes in this measure among men (Figure 2).
There were also no significant changes in bone strength among men or women.
Biological mechanism remains to be determined
Dr. LeBoff said a “possible biological explanation” for the findings is that “women, particularly when they are younger, lose more bone than men.”
“Postmenopausal females do lose bone at an accelerated rate compared with males,” Dr. Burt agreed, “but at the time the study was designed, there was no reason to believe that high-dose vitamin D supplementation would accelerate the problem.”
“The biological mechanism of the vitamin D–related bone loss needs further investigation,” Dr. Burt added, “but there are laboratory data suggesting that supraphysiologic doses of active metabolites of vitamin D may stimulate bone resorption.”
The study was funded by the Pure North S’Energy Foundation. Dr. Burt has reported no relevant financial relationships. Disclosures for the other authors are listed with the article. Dr. LeBoff has reported receiving grants from the National Institutes of Health for the VITAL analysis.
A version of this article originally appeared on Medscape.com.
ASBMR 2020: Sequential osteoporosis meds, AI, bone cancer, and more
The virtual American Society for Bone and Mineral Research 2020 annual meeting “is full of highlights,” says Lorenz Hofbauer, MD, scientific chair, but “this year you won’t lose time in the hallways to switch between the talks,” he quipped.
Nevertheless, “although we won’t be coming together face to face this year, you will have the flexibility to virtually connect with peers and colleagues from around the world,” Teresita Bellido, PhD, ASBMR president emphasized in a message to members.
Like other medical organizations, with the advent of the COVID-19 pandemic, the ASBMR had to quickly pivot to provide a virtual meeting.
The meeting will take place September 11-15 and is free for ASBMR members.
Speaking to Medscape Medical News, Bellido and Hofbauer drew attention to some of the meeting’s major themes, key sessions, and top clinical oral abstracts.
Attendees at this year’s virtual meeting will hear the latest information on optimal sequential treatment for osteoporosis, the latest research using artificial intelligence (AI), and bone and cancer, among other topics.
Sequential osteoporosis treatment a recurring theme
According to Hofbauer, from Dresden Technical University, Germany, the September 13 Cutting Edge symposium entitled, “Optimizing Sequential Osteoporosis Treatment,” is not to be missed, and the topic “will be a leitmotiv [recurrent theme] for the entire meeting.”
During this session speakers will present findings from two perspectives – basic science and clinical applications – with the latter being another recurring theme at the meeting.
Bellido, from the University of Arkansas for Medical Sciences, in Little Rock, pointed out that romosozumab (Evenity, Amgen), recently approved by the US Food and Drug Administration, is an example of how basic laboratory research can lead to important new therapies.
Anabolic therapies for osteoporosis that “build up bone” include teriparatide, abaloparatide, and now romosozumab, whereas antiresorptive therapies that stop bone resorption include the bisphosphonates (alendronate, risedronate, ibandronate, and zoledronic acid) and the monoclonal antibody denosumab, Bellido explained.
As osteoporosis treatment options have expanded, the timing and sequencing of optimal therapies have become much more complex, and so this session on sequencing, as well as the September 13 Concurrent Orals session, “Issues of Long-term Treatment and Discontinuation,” is sure to spark interest.
The ASBMR/European Calcified Tissue Society debate, entitled, “A Treat to Target Approach is Helpful for Osteoporosis Management,” is also expected to be lively and generate wide interest, according to Bellido and Hofbauer.
Michael R. McClung, MD, Oregon Osteoporosis Center, Portland, will argue against the motion and Celia Gregson, PhD, University of Bristol, UK, will argue for it. Attendees will be able to vote for/against the motion before and after the debate, and the result will indicate which speaker was more persuasive.
Bone cancer ultimately damages other tissues
The meeting will also offer attendees a close look at bone and cancer, which is an example of how “all the homeostatic processes that occur with bone not only affect bone but also impact other tissues and organs,” said Bellido.
In other words, “what happens in bone impacts other tissues – for example, skeletal muscle, the pancreas, and even frailty and fractures.”
Theresa A. Guise, MD, from the University of Texas MD Anderson Cancer Center, in Austin, will present the Louis V. Avioli Lecture on September 11, entitled, “Cancer, Bone and Beyond: An Integrated View of the Bone Microenvironment.”
“Local events in the bone microenvironment due to cancer and cancer treatment which result in pathologic bone destruction may have widespread systemic consequences that further increase morbidity and mortality,” Hofbauer noted.
Guise “will highlight cutting-edge concepts, potential mechanisms, and therapy for bone metastases,” he said.
These concepts will also be discussed in more detail during a 2-day virtual premeeting symposium, presented on September 9 and 10 by the ASBMR along with the Cancer and Bone Society, entitled, “The Seed and Soil: Therapeutic Targets for Cancer in Bone.”
The symposium will cover tumor dormancy, imaging, adiposity in the bone tumor microenvironment, a history of bone-targeted therapies in cancer, advances in breast cancer bone metastasis, and new approaches in myeloma bone disease.
“We have evidence from breast cancer, multiple myeloma, as well as from prostate cancer,” Bellido noted, that “all those cancer cells make their home in bone and transform the bone in such a way that not only the bone is damaged but also other tissues.”
“We have skeletal muscle weakness (that is directed by the effects that occur in bone), as well as changes in the pancreas – all directed by proteins and genes in bone cells.”
AI, bench to bedside research
“Every field is moving towards the use of AI,” Bellido noted, and the September 11 plenary symposium entitled, “Artificial Intelligence and Precision Medicine in Musculoskeletal Health,” will shed light on how AI is being used to study bone health.
The session “will give us a glimpse of the future,” said Hofbauer.
Session topics include principles of applications to research and clinical care in bone and mineral research; how AI can help detect rare diseases; and combining genomics with medical data using AI in precision medicine for drug discovery.
“The Bench to Bedside presentation on ‘Beta Blockers and Bone’ is a great example of translational research, while the Basic Symposium on ‘Bones, Guts and Brains’ provides inspiring and thought-provoking insights into novel physiology and tempting teleology,” Hofbauer explained.
“Another fascinating Cutting Edge symposium,” he added, “is on ‘Inspiring Mechanistic Bone Stories from Around the Animal Kingdom,’ a must-see for those employing preclinical animal models.”
For more insight into early research and a research pioneer, attendees can listen to Selma Masri, PhD, from the University of California, Irvine, who will deliver the Gerald D. Aurbach Lecture entitled, “The Scientific Legacy of Paolo Sassone-Corsi: A Tour Through the Fields of Transcriptional Regulation, Epigenetics, Metabolism and Circadian Rhythms.”
Masri’s lab is dissecting how genetic disruption of the circadian clock in mouse models affects cancer, and she will discuss the work and legacy of the late Sassone-Corsi, as well as the future of the field.
Rare disease, fragility fractures
The ASBMR meeting will also feature the latest research into rare diseases and fragility fractures.
Rare diseases are often about “more bone or less bone,” said Bellido. “Understanding the mechanisms of these rare diseases can give us very important clues of treating the more common diseases.”
A fragility fracture is a diagnosis of osteoporosis, but most are not treated, she continued. “This is equivalent to having, for example, a heart attack and leaving the hospital after the incident was resolved and not treating it.”
“We’re trying to address this gap,” she said, and a symposium on September 14 will present some of the latest knowledge.
During the “Long-term Management of Fragility Fracture” symposium, speakers will discuss reducing mortality with antiosteoporotic treatment, new scenarios to prevent postfracture frailty, as well as fracture and postfracture management – surgeon and nursing perspectives.
COVID-19, nutrition, microbiome, and top 5 clinical abstracts
In addition to plenary sessions and symposiums, there are many oral abstracts and posters on important studies in the field of bone health, including, for example, a topical study of vitamin D and COVID-19.
There are also many abstracts on nutrition, the microbiome, and treating bone loss, said Bellido.
“We have a huge increase in the number of abstracts submitted from South America and Australia compared to previous years,” she noted, “and a 10% increase (from 50% to 61%) in the number of abstracts submitted by young investigators, which is crucial.”
Close to 1000 abstracts (988) were submitted, two thirds of which were clinical.
The top 5 clinical abstracts reflect important current issues in the field, said Hofbauer.
“One major theme is on long-term and sequential therapy efficacy and safety,” he said. And “burosumab is a game-changing new drug, and nutritional aspects are evergreens [perennial favorites], especially in the elderly population.”
The top 5 clinical oral abstracts at the ASBMR 2020 meeting are:
- Dairy supplementation reduces fractures and falls in institutionalized older adults: A cluster-randomized placebo-controlled trial (abstract 1022).
- Treatment with zoledronate subsequent to denosumab in osteoporosis: A randomized trial (abstract 1065).
- Efficacy of burosumab in adults with X-linked hypophosphatemia (XLH): A subgroup analysis of a randomized, double-blind, placebo-controlled, phase 3 study (abstract 1044).
- High-dose vitamin D supplementation affects bone density differently in females than males (abstract 1019).
- Bisphosphonate use and risk of atypical femoral fractures: A nationwide Danish analysis with blinded radiographic review (abstract 1061).
This article first appeared on Medscape.com.
The virtual American Society for Bone and Mineral Research 2020 annual meeting “is full of highlights,” says Lorenz Hofbauer, MD, scientific chair, but “this year you won’t lose time in the hallways to switch between the talks,” he quipped.
Nevertheless, “although we won’t be coming together face to face this year, you will have the flexibility to virtually connect with peers and colleagues from around the world,” Teresita Bellido, PhD, ASBMR president emphasized in a message to members.
Like other medical organizations, with the advent of the COVID-19 pandemic, the ASBMR had to quickly pivot to provide a virtual meeting.
The meeting will take place September 11-15 and is free for ASBMR members.
Speaking to Medscape Medical News, Bellido and Hofbauer drew attention to some of the meeting’s major themes, key sessions, and top clinical oral abstracts.
Attendees at this year’s virtual meeting will hear the latest information on optimal sequential treatment for osteoporosis, the latest research using artificial intelligence (AI), and bone and cancer, among other topics.
Sequential osteoporosis treatment a recurring theme
According to Hofbauer, from Dresden Technical University, Germany, the September 13 Cutting Edge symposium entitled, “Optimizing Sequential Osteoporosis Treatment,” is not to be missed, and the topic “will be a leitmotiv [recurrent theme] for the entire meeting.”
During this session speakers will present findings from two perspectives – basic science and clinical applications – with the latter being another recurring theme at the meeting.
Bellido, from the University of Arkansas for Medical Sciences, in Little Rock, pointed out that romosozumab (Evenity, Amgen), recently approved by the US Food and Drug Administration, is an example of how basic laboratory research can lead to important new therapies.
Anabolic therapies for osteoporosis that “build up bone” include teriparatide, abaloparatide, and now romosozumab, whereas antiresorptive therapies that stop bone resorption include the bisphosphonates (alendronate, risedronate, ibandronate, and zoledronic acid) and the monoclonal antibody denosumab, Bellido explained.
As osteoporosis treatment options have expanded, the timing and sequencing of optimal therapies have become much more complex, and so this session on sequencing, as well as the September 13 Concurrent Orals session, “Issues of Long-term Treatment and Discontinuation,” is sure to spark interest.
The ASBMR/European Calcified Tissue Society debate, entitled, “A Treat to Target Approach is Helpful for Osteoporosis Management,” is also expected to be lively and generate wide interest, according to Bellido and Hofbauer.
Michael R. McClung, MD, Oregon Osteoporosis Center, Portland, will argue against the motion and Celia Gregson, PhD, University of Bristol, UK, will argue for it. Attendees will be able to vote for/against the motion before and after the debate, and the result will indicate which speaker was more persuasive.
Bone cancer ultimately damages other tissues
The meeting will also offer attendees a close look at bone and cancer, which is an example of how “all the homeostatic processes that occur with bone not only affect bone but also impact other tissues and organs,” said Bellido.
In other words, “what happens in bone impacts other tissues – for example, skeletal muscle, the pancreas, and even frailty and fractures.”
Theresa A. Guise, MD, from the University of Texas MD Anderson Cancer Center, in Austin, will present the Louis V. Avioli Lecture on September 11, entitled, “Cancer, Bone and Beyond: An Integrated View of the Bone Microenvironment.”
“Local events in the bone microenvironment due to cancer and cancer treatment which result in pathologic bone destruction may have widespread systemic consequences that further increase morbidity and mortality,” Hofbauer noted.
Guise “will highlight cutting-edge concepts, potential mechanisms, and therapy for bone metastases,” he said.
These concepts will also be discussed in more detail during a 2-day virtual premeeting symposium, presented on September 9 and 10 by the ASBMR along with the Cancer and Bone Society, entitled, “The Seed and Soil: Therapeutic Targets for Cancer in Bone.”
The symposium will cover tumor dormancy, imaging, adiposity in the bone tumor microenvironment, a history of bone-targeted therapies in cancer, advances in breast cancer bone metastasis, and new approaches in myeloma bone disease.
“We have evidence from breast cancer, multiple myeloma, as well as from prostate cancer,” Bellido noted, that “all those cancer cells make their home in bone and transform the bone in such a way that not only the bone is damaged but also other tissues.”
“We have skeletal muscle weakness (that is directed by the effects that occur in bone), as well as changes in the pancreas – all directed by proteins and genes in bone cells.”
AI, bench to bedside research
“Every field is moving towards the use of AI,” Bellido noted, and the September 11 plenary symposium entitled, “Artificial Intelligence and Precision Medicine in Musculoskeletal Health,” will shed light on how AI is being used to study bone health.
The session “will give us a glimpse of the future,” said Hofbauer.
Session topics include principles of applications to research and clinical care in bone and mineral research; how AI can help detect rare diseases; and combining genomics with medical data using AI in precision medicine for drug discovery.
“The Bench to Bedside presentation on ‘Beta Blockers and Bone’ is a great example of translational research, while the Basic Symposium on ‘Bones, Guts and Brains’ provides inspiring and thought-provoking insights into novel physiology and tempting teleology,” Hofbauer explained.
“Another fascinating Cutting Edge symposium,” he added, “is on ‘Inspiring Mechanistic Bone Stories from Around the Animal Kingdom,’ a must-see for those employing preclinical animal models.”
For more insight into early research and a research pioneer, attendees can listen to Selma Masri, PhD, from the University of California, Irvine, who will deliver the Gerald D. Aurbach Lecture entitled, “The Scientific Legacy of Paolo Sassone-Corsi: A Tour Through the Fields of Transcriptional Regulation, Epigenetics, Metabolism and Circadian Rhythms.”
Masri’s lab is dissecting how genetic disruption of the circadian clock in mouse models affects cancer, and she will discuss the work and legacy of the late Sassone-Corsi, as well as the future of the field.
Rare disease, fragility fractures
The ASBMR meeting will also feature the latest research into rare diseases and fragility fractures.
Rare diseases are often about “more bone or less bone,” said Bellido. “Understanding the mechanisms of these rare diseases can give us very important clues of treating the more common diseases.”
A fragility fracture is a diagnosis of osteoporosis, but most are not treated, she continued. “This is equivalent to having, for example, a heart attack and leaving the hospital after the incident was resolved and not treating it.”
“We’re trying to address this gap,” she said, and a symposium on September 14 will present some of the latest knowledge.
During the “Long-term Management of Fragility Fracture” symposium, speakers will discuss reducing mortality with antiosteoporotic treatment, new scenarios to prevent postfracture frailty, as well as fracture and postfracture management – surgeon and nursing perspectives.
COVID-19, nutrition, microbiome, and top 5 clinical abstracts
In addition to plenary sessions and symposiums, there are many oral abstracts and posters on important studies in the field of bone health, including, for example, a topical study of vitamin D and COVID-19.
There are also many abstracts on nutrition, the microbiome, and treating bone loss, said Bellido.
“We have a huge increase in the number of abstracts submitted from South America and Australia compared to previous years,” she noted, “and a 10% increase (from 50% to 61%) in the number of abstracts submitted by young investigators, which is crucial.”
Close to 1000 abstracts (988) were submitted, two thirds of which were clinical.
The top 5 clinical abstracts reflect important current issues in the field, said Hofbauer.
“One major theme is on long-term and sequential therapy efficacy and safety,” he said. And “burosumab is a game-changing new drug, and nutritional aspects are evergreens [perennial favorites], especially in the elderly population.”
The top 5 clinical oral abstracts at the ASBMR 2020 meeting are:
- Dairy supplementation reduces fractures and falls in institutionalized older adults: A cluster-randomized placebo-controlled trial (abstract 1022).
- Treatment with zoledronate subsequent to denosumab in osteoporosis: A randomized trial (abstract 1065).
- Efficacy of burosumab in adults with X-linked hypophosphatemia (XLH): A subgroup analysis of a randomized, double-blind, placebo-controlled, phase 3 study (abstract 1044).
- High-dose vitamin D supplementation affects bone density differently in females than males (abstract 1019).
- Bisphosphonate use and risk of atypical femoral fractures: A nationwide Danish analysis with blinded radiographic review (abstract 1061).
This article first appeared on Medscape.com.
The virtual American Society for Bone and Mineral Research 2020 annual meeting “is full of highlights,” says Lorenz Hofbauer, MD, scientific chair, but “this year you won’t lose time in the hallways to switch between the talks,” he quipped.
Nevertheless, “although we won’t be coming together face to face this year, you will have the flexibility to virtually connect with peers and colleagues from around the world,” Teresita Bellido, PhD, ASBMR president emphasized in a message to members.
Like other medical organizations, with the advent of the COVID-19 pandemic, the ASBMR had to quickly pivot to provide a virtual meeting.
The meeting will take place September 11-15 and is free for ASBMR members.
Speaking to Medscape Medical News, Bellido and Hofbauer drew attention to some of the meeting’s major themes, key sessions, and top clinical oral abstracts.
Attendees at this year’s virtual meeting will hear the latest information on optimal sequential treatment for osteoporosis, the latest research using artificial intelligence (AI), and bone and cancer, among other topics.
Sequential osteoporosis treatment a recurring theme
According to Hofbauer, from Dresden Technical University, Germany, the September 13 Cutting Edge symposium entitled, “Optimizing Sequential Osteoporosis Treatment,” is not to be missed, and the topic “will be a leitmotiv [recurrent theme] for the entire meeting.”
During this session speakers will present findings from two perspectives – basic science and clinical applications – with the latter being another recurring theme at the meeting.
Bellido, from the University of Arkansas for Medical Sciences, in Little Rock, pointed out that romosozumab (Evenity, Amgen), recently approved by the US Food and Drug Administration, is an example of how basic laboratory research can lead to important new therapies.
Anabolic therapies for osteoporosis that “build up bone” include teriparatide, abaloparatide, and now romosozumab, whereas antiresorptive therapies that stop bone resorption include the bisphosphonates (alendronate, risedronate, ibandronate, and zoledronic acid) and the monoclonal antibody denosumab, Bellido explained.
As osteoporosis treatment options have expanded, the timing and sequencing of optimal therapies have become much more complex, and so this session on sequencing, as well as the September 13 Concurrent Orals session, “Issues of Long-term Treatment and Discontinuation,” is sure to spark interest.
The ASBMR/European Calcified Tissue Society debate, entitled, “A Treat to Target Approach is Helpful for Osteoporosis Management,” is also expected to be lively and generate wide interest, according to Bellido and Hofbauer.
Michael R. McClung, MD, Oregon Osteoporosis Center, Portland, will argue against the motion and Celia Gregson, PhD, University of Bristol, UK, will argue for it. Attendees will be able to vote for/against the motion before and after the debate, and the result will indicate which speaker was more persuasive.
Bone cancer ultimately damages other tissues
The meeting will also offer attendees a close look at bone and cancer, which is an example of how “all the homeostatic processes that occur with bone not only affect bone but also impact other tissues and organs,” said Bellido.
In other words, “what happens in bone impacts other tissues – for example, skeletal muscle, the pancreas, and even frailty and fractures.”
Theresa A. Guise, MD, from the University of Texas MD Anderson Cancer Center, in Austin, will present the Louis V. Avioli Lecture on September 11, entitled, “Cancer, Bone and Beyond: An Integrated View of the Bone Microenvironment.”
“Local events in the bone microenvironment due to cancer and cancer treatment which result in pathologic bone destruction may have widespread systemic consequences that further increase morbidity and mortality,” Hofbauer noted.
Guise “will highlight cutting-edge concepts, potential mechanisms, and therapy for bone metastases,” he said.
These concepts will also be discussed in more detail during a 2-day virtual premeeting symposium, presented on September 9 and 10 by the ASBMR along with the Cancer and Bone Society, entitled, “The Seed and Soil: Therapeutic Targets for Cancer in Bone.”
The symposium will cover tumor dormancy, imaging, adiposity in the bone tumor microenvironment, a history of bone-targeted therapies in cancer, advances in breast cancer bone metastasis, and new approaches in myeloma bone disease.
“We have evidence from breast cancer, multiple myeloma, as well as from prostate cancer,” Bellido noted, that “all those cancer cells make their home in bone and transform the bone in such a way that not only the bone is damaged but also other tissues.”
“We have skeletal muscle weakness (that is directed by the effects that occur in bone), as well as changes in the pancreas – all directed by proteins and genes in bone cells.”
AI, bench to bedside research
“Every field is moving towards the use of AI,” Bellido noted, and the September 11 plenary symposium entitled, “Artificial Intelligence and Precision Medicine in Musculoskeletal Health,” will shed light on how AI is being used to study bone health.
The session “will give us a glimpse of the future,” said Hofbauer.
Session topics include principles of applications to research and clinical care in bone and mineral research; how AI can help detect rare diseases; and combining genomics with medical data using AI in precision medicine for drug discovery.
“The Bench to Bedside presentation on ‘Beta Blockers and Bone’ is a great example of translational research, while the Basic Symposium on ‘Bones, Guts and Brains’ provides inspiring and thought-provoking insights into novel physiology and tempting teleology,” Hofbauer explained.
“Another fascinating Cutting Edge symposium,” he added, “is on ‘Inspiring Mechanistic Bone Stories from Around the Animal Kingdom,’ a must-see for those employing preclinical animal models.”
For more insight into early research and a research pioneer, attendees can listen to Selma Masri, PhD, from the University of California, Irvine, who will deliver the Gerald D. Aurbach Lecture entitled, “The Scientific Legacy of Paolo Sassone-Corsi: A Tour Through the Fields of Transcriptional Regulation, Epigenetics, Metabolism and Circadian Rhythms.”
Masri’s lab is dissecting how genetic disruption of the circadian clock in mouse models affects cancer, and she will discuss the work and legacy of the late Sassone-Corsi, as well as the future of the field.
Rare disease, fragility fractures
The ASBMR meeting will also feature the latest research into rare diseases and fragility fractures.
Rare diseases are often about “more bone or less bone,” said Bellido. “Understanding the mechanisms of these rare diseases can give us very important clues of treating the more common diseases.”
A fragility fracture is a diagnosis of osteoporosis, but most are not treated, she continued. “This is equivalent to having, for example, a heart attack and leaving the hospital after the incident was resolved and not treating it.”
“We’re trying to address this gap,” she said, and a symposium on September 14 will present some of the latest knowledge.
During the “Long-term Management of Fragility Fracture” symposium, speakers will discuss reducing mortality with antiosteoporotic treatment, new scenarios to prevent postfracture frailty, as well as fracture and postfracture management – surgeon and nursing perspectives.
COVID-19, nutrition, microbiome, and top 5 clinical abstracts
In addition to plenary sessions and symposiums, there are many oral abstracts and posters on important studies in the field of bone health, including, for example, a topical study of vitamin D and COVID-19.
There are also many abstracts on nutrition, the microbiome, and treating bone loss, said Bellido.
“We have a huge increase in the number of abstracts submitted from South America and Australia compared to previous years,” she noted, “and a 10% increase (from 50% to 61%) in the number of abstracts submitted by young investigators, which is crucial.”
Close to 1000 abstracts (988) were submitted, two thirds of which were clinical.
The top 5 clinical abstracts reflect important current issues in the field, said Hofbauer.
“One major theme is on long-term and sequential therapy efficacy and safety,” he said. And “burosumab is a game-changing new drug, and nutritional aspects are evergreens [perennial favorites], especially in the elderly population.”
The top 5 clinical oral abstracts at the ASBMR 2020 meeting are:
- Dairy supplementation reduces fractures and falls in institutionalized older adults: A cluster-randomized placebo-controlled trial (abstract 1022).
- Treatment with zoledronate subsequent to denosumab in osteoporosis: A randomized trial (abstract 1065).
- Efficacy of burosumab in adults with X-linked hypophosphatemia (XLH): A subgroup analysis of a randomized, double-blind, placebo-controlled, phase 3 study (abstract 1044).
- High-dose vitamin D supplementation affects bone density differently in females than males (abstract 1019).
- Bisphosphonate use and risk of atypical femoral fractures: A nationwide Danish analysis with blinded radiographic review (abstract 1061).
This article first appeared on Medscape.com.