User login
Study finds discrepancies in biopsy decisions, diagnoses based on skin type
BOSTON – compared with White patients, new research shows.
“Our findings suggest diagnostic biases based on skin color exist in dermatology practice,” lead author Loren Krueger, MD, assistant professor in the department of dermatology, Emory University School of Medicine, Atlanta, said at the Annual Skin of Color Society Scientific Symposium. “A lower likelihood of biopsy of malignancy in darker skin types could contribute to disparities in cutaneous malignancies,” she added.
Disparities in dermatologic care among Black patients, compared with White patients, have been well documented. Recent evidence includes a 2020 study that showed significant shortcomings among medical students in correctly diagnosing squamous cell carcinoma, urticaria, and atopic dermatitis for patients with skin of color.
“It’s no secret that our images do not accurately or in the right quantity include skin of color,” Dr. Krueger said. “Yet few papers talk about how these biases actually impact our care. Importantly, this study demonstrates that diagnostic bias develops as early as the medical student level.”
To further investigate the role of skin color in the assessment of neoplastic and inflammatory skin conditions and decisions to perform biopsy, Dr. Krueger and her colleagues surveyed 144 dermatology residents and attending dermatologists to evaluate their clinical decisionmaking skills in assessing skin conditions for patients with lighter skin and those with darker skin. Almost 80% (113) provided complete responses and were included in the study.
For the survey, participants were shown photos of 10 neoplastic and 10 inflammatory skin conditions. Each image was matched in lighter (skin types I-II) and darker (skin types IV-VI) skinned patients in random order. Participants were asked to identify the suspected underlying etiology (neoplastic–benign, neoplastic–malignant, papulosquamous, lichenoid, infectious, bullous, or no suspected etiology) and whether they would choose to perform biopsy for the pictured condition.
Overall, their responses showed a slightly higher probability of recommending a biopsy for patients with skin types IV-V (odds ratio, 1.18; P = .054).
However, respondents were more than twice as likely to recommend a biopsy for benign neoplasms for patients with skin of color, compared with those with lighter skin types (OR, 2.57; P < .0001). They were significantly less likely to recommend a biopsy for a malignant neoplasm for patients with skin of color (OR, 0.42; P < .0001).
In addition, the correct etiology was much more commonly missed in diagnosing patients with skin of color, even after adjusting for years in dermatology practice (OR, 0.569; P < .0001).
Conversely, respondents were significantly less likely to recommend a biopsy for benign neoplasms and were more likely to recommend a biopsy for malignant neoplasms among White patients. Etiology was more commonly correct.
The findings underscore that “for skin of color patients, you’re more likely to have a benign neoplasm biopsied, you’re less likely to have a malignant neoplasm biopsied, and more often, your etiology may be missed,” Dr. Krueger said at the meeting.
Of note, while 45% of respondents were dermatology residents or fellows, 20.4% had 1-5 years of experience, and about 28% had 10 to more than 25 years of experience.
And while 75% of the dermatology residents, fellows, and attendings were White, there was no difference in the probability of correctly identifying the underlying etiology in dark or light skin types based on the provider’s self-identified race.
Importantly, the patterns in the study of diagnostic discrepancies are reflected in broader dermatologic outcomes. The 5-year melanoma survival rate is 74.1% among Black patients and 92.9% among White patients. Dr. Krueger referred to data showing that only 52.6% of Black patients have stage I melanoma at diagnosis, whereas among White patients, the rate is much higher, at 75.9%.
“We know skin malignancy can be more aggressive and late-stage in skin of color populations, leading to increased morbidity and later stage at initial diagnosis,” Dr. Krueger told this news organization. “We routinely attribute this to limited access to care and lack of awareness on skin malignancy. However, we have no evidence on how we, as dermatologists, may be playing a role.”
Furthermore, the decision to perform biopsy or not can affect the size and stage at diagnosis of a cutaneous malignancy, she noted.
Key changes needed to prevent the disparities – and their implications – should start at the training level, she emphasized. “I would love to see increased photo representation in training materials – this is a great place to start,” Dr. Krueger said.
In addition, “encouraging medical students, residents, and dermatologists to learn from skin of color experts is vital,” she said. “We should also provide hands-on experience and training with diverse patient populations.”
The first step to addressing biases “is to acknowledge they exist,” Dr. Krueger added. “I am hopeful this inspires others to continue to investigate these biases, as well as how we can eliminate them.”
The study was funded by the Rudin Resident Research Award. The authors have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
BOSTON – compared with White patients, new research shows.
“Our findings suggest diagnostic biases based on skin color exist in dermatology practice,” lead author Loren Krueger, MD, assistant professor in the department of dermatology, Emory University School of Medicine, Atlanta, said at the Annual Skin of Color Society Scientific Symposium. “A lower likelihood of biopsy of malignancy in darker skin types could contribute to disparities in cutaneous malignancies,” she added.
Disparities in dermatologic care among Black patients, compared with White patients, have been well documented. Recent evidence includes a 2020 study that showed significant shortcomings among medical students in correctly diagnosing squamous cell carcinoma, urticaria, and atopic dermatitis for patients with skin of color.
“It’s no secret that our images do not accurately or in the right quantity include skin of color,” Dr. Krueger said. “Yet few papers talk about how these biases actually impact our care. Importantly, this study demonstrates that diagnostic bias develops as early as the medical student level.”
To further investigate the role of skin color in the assessment of neoplastic and inflammatory skin conditions and decisions to perform biopsy, Dr. Krueger and her colleagues surveyed 144 dermatology residents and attending dermatologists to evaluate their clinical decisionmaking skills in assessing skin conditions for patients with lighter skin and those with darker skin. Almost 80% (113) provided complete responses and were included in the study.
For the survey, participants were shown photos of 10 neoplastic and 10 inflammatory skin conditions. Each image was matched in lighter (skin types I-II) and darker (skin types IV-VI) skinned patients in random order. Participants were asked to identify the suspected underlying etiology (neoplastic–benign, neoplastic–malignant, papulosquamous, lichenoid, infectious, bullous, or no suspected etiology) and whether they would choose to perform biopsy for the pictured condition.
Overall, their responses showed a slightly higher probability of recommending a biopsy for patients with skin types IV-V (odds ratio, 1.18; P = .054).
However, respondents were more than twice as likely to recommend a biopsy for benign neoplasms for patients with skin of color, compared with those with lighter skin types (OR, 2.57; P < .0001). They were significantly less likely to recommend a biopsy for a malignant neoplasm for patients with skin of color (OR, 0.42; P < .0001).
In addition, the correct etiology was much more commonly missed in diagnosing patients with skin of color, even after adjusting for years in dermatology practice (OR, 0.569; P < .0001).
Conversely, respondents were significantly less likely to recommend a biopsy for benign neoplasms and were more likely to recommend a biopsy for malignant neoplasms among White patients. Etiology was more commonly correct.
The findings underscore that “for skin of color patients, you’re more likely to have a benign neoplasm biopsied, you’re less likely to have a malignant neoplasm biopsied, and more often, your etiology may be missed,” Dr. Krueger said at the meeting.
Of note, while 45% of respondents were dermatology residents or fellows, 20.4% had 1-5 years of experience, and about 28% had 10 to more than 25 years of experience.
And while 75% of the dermatology residents, fellows, and attendings were White, there was no difference in the probability of correctly identifying the underlying etiology in dark or light skin types based on the provider’s self-identified race.
Importantly, the patterns in the study of diagnostic discrepancies are reflected in broader dermatologic outcomes. The 5-year melanoma survival rate is 74.1% among Black patients and 92.9% among White patients. Dr. Krueger referred to data showing that only 52.6% of Black patients have stage I melanoma at diagnosis, whereas among White patients, the rate is much higher, at 75.9%.
“We know skin malignancy can be more aggressive and late-stage in skin of color populations, leading to increased morbidity and later stage at initial diagnosis,” Dr. Krueger told this news organization. “We routinely attribute this to limited access to care and lack of awareness on skin malignancy. However, we have no evidence on how we, as dermatologists, may be playing a role.”
Furthermore, the decision to perform biopsy or not can affect the size and stage at diagnosis of a cutaneous malignancy, she noted.
Key changes needed to prevent the disparities – and their implications – should start at the training level, she emphasized. “I would love to see increased photo representation in training materials – this is a great place to start,” Dr. Krueger said.
In addition, “encouraging medical students, residents, and dermatologists to learn from skin of color experts is vital,” she said. “We should also provide hands-on experience and training with diverse patient populations.”
The first step to addressing biases “is to acknowledge they exist,” Dr. Krueger added. “I am hopeful this inspires others to continue to investigate these biases, as well as how we can eliminate them.”
The study was funded by the Rudin Resident Research Award. The authors have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
BOSTON – compared with White patients, new research shows.
“Our findings suggest diagnostic biases based on skin color exist in dermatology practice,” lead author Loren Krueger, MD, assistant professor in the department of dermatology, Emory University School of Medicine, Atlanta, said at the Annual Skin of Color Society Scientific Symposium. “A lower likelihood of biopsy of malignancy in darker skin types could contribute to disparities in cutaneous malignancies,” she added.
Disparities in dermatologic care among Black patients, compared with White patients, have been well documented. Recent evidence includes a 2020 study that showed significant shortcomings among medical students in correctly diagnosing squamous cell carcinoma, urticaria, and atopic dermatitis for patients with skin of color.
“It’s no secret that our images do not accurately or in the right quantity include skin of color,” Dr. Krueger said. “Yet few papers talk about how these biases actually impact our care. Importantly, this study demonstrates that diagnostic bias develops as early as the medical student level.”
To further investigate the role of skin color in the assessment of neoplastic and inflammatory skin conditions and decisions to perform biopsy, Dr. Krueger and her colleagues surveyed 144 dermatology residents and attending dermatologists to evaluate their clinical decisionmaking skills in assessing skin conditions for patients with lighter skin and those with darker skin. Almost 80% (113) provided complete responses and were included in the study.
For the survey, participants were shown photos of 10 neoplastic and 10 inflammatory skin conditions. Each image was matched in lighter (skin types I-II) and darker (skin types IV-VI) skinned patients in random order. Participants were asked to identify the suspected underlying etiology (neoplastic–benign, neoplastic–malignant, papulosquamous, lichenoid, infectious, bullous, or no suspected etiology) and whether they would choose to perform biopsy for the pictured condition.
Overall, their responses showed a slightly higher probability of recommending a biopsy for patients with skin types IV-V (odds ratio, 1.18; P = .054).
However, respondents were more than twice as likely to recommend a biopsy for benign neoplasms for patients with skin of color, compared with those with lighter skin types (OR, 2.57; P < .0001). They were significantly less likely to recommend a biopsy for a malignant neoplasm for patients with skin of color (OR, 0.42; P < .0001).
In addition, the correct etiology was much more commonly missed in diagnosing patients with skin of color, even after adjusting for years in dermatology practice (OR, 0.569; P < .0001).
Conversely, respondents were significantly less likely to recommend a biopsy for benign neoplasms and were more likely to recommend a biopsy for malignant neoplasms among White patients. Etiology was more commonly correct.
The findings underscore that “for skin of color patients, you’re more likely to have a benign neoplasm biopsied, you’re less likely to have a malignant neoplasm biopsied, and more often, your etiology may be missed,” Dr. Krueger said at the meeting.
Of note, while 45% of respondents were dermatology residents or fellows, 20.4% had 1-5 years of experience, and about 28% had 10 to more than 25 years of experience.
And while 75% of the dermatology residents, fellows, and attendings were White, there was no difference in the probability of correctly identifying the underlying etiology in dark or light skin types based on the provider’s self-identified race.
Importantly, the patterns in the study of diagnostic discrepancies are reflected in broader dermatologic outcomes. The 5-year melanoma survival rate is 74.1% among Black patients and 92.9% among White patients. Dr. Krueger referred to data showing that only 52.6% of Black patients have stage I melanoma at diagnosis, whereas among White patients, the rate is much higher, at 75.9%.
“We know skin malignancy can be more aggressive and late-stage in skin of color populations, leading to increased morbidity and later stage at initial diagnosis,” Dr. Krueger told this news organization. “We routinely attribute this to limited access to care and lack of awareness on skin malignancy. However, we have no evidence on how we, as dermatologists, may be playing a role.”
Furthermore, the decision to perform biopsy or not can affect the size and stage at diagnosis of a cutaneous malignancy, she noted.
Key changes needed to prevent the disparities – and their implications – should start at the training level, she emphasized. “I would love to see increased photo representation in training materials – this is a great place to start,” Dr. Krueger said.
In addition, “encouraging medical students, residents, and dermatologists to learn from skin of color experts is vital,” she said. “We should also provide hands-on experience and training with diverse patient populations.”
The first step to addressing biases “is to acknowledge they exist,” Dr. Krueger added. “I am hopeful this inspires others to continue to investigate these biases, as well as how we can eliminate them.”
The study was funded by the Rudin Resident Research Award. The authors have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Verrucous Carcinoma of the Foot: A Retrospective Study of 19 Cases and Analysis of Prognostic Factors Influencing Recurrence
Verrucous carcinoma is a rare cancer with the greatest predilection for the foot. Multiple case reports with only a few large case series have been published. 1-3 Plantar verrucous carcinoma is characterized as a slowly but relentlessly enlarging warty tumor with low metastatic potential and high risk for local invasion. The tumor occurs most frequently in patients aged 60 to 70 years, predominantly in White males. 1 It often is misdiagnosed for years as an ulcer or wart that is highly resistant to therapy. Size typically ranges from 1 to 12 cm in greatest dimension. 1
The pathogenesis of plantar verrucous carcinoma remains unclear, but some contributing factors have been proposed, including trauma, chronic irritation, infection, and poor local hygiene.2 This tumor has been reported to occur in chronic foot ulcerations, particularly in the diabetic population.4 It has been proposed that abnormal expression of the p53 tumor suppressor protein and several types of human papillomavirus (HPV) may have a role in the pathogenesis of verrucous carcinoma.5
The pathologic hallmarks of this tumor include a verrucous/hyperkeratotic surface with a deeply endophytic, broad, pushing base. Tumor cells are well differentiated, and atypia is either absent or confined to 1 or 2 layers at the base of the tumor. Overt invasion at the base is lacking, except in cases with a component of conventional invasive squamous cell carcinoma. Human papillomavirus viropathic changes are classically absent.1,3 Studies of the histopathology of verrucous carcinoma have been complicated by similar entities, nomenclatural uncertainty, and variable diagnostic criteria. For example, epithelioma cuniculatum variously has been defined as being synonymous with verrucous carcinoma, a distinct clinical verrucous carcinoma subtype occurring on the soles, a histologic subtype (characterized by prominent burrowing sinuses), or a separate entity entirely.1,2,6,7 Furthermore, in the genital area, several different types of carcinomas have verruciform features but display distinct microscopic findings and outcomes from verrucous carcinoma.8
Verrucous carcinoma represents an unusual variant of squamous cell carcinoma and is treated as such. Treatments have included laser surgery; immunotherapy; retinoid therapy; and chemotherapy by oral, intralesional, or iontophoretic routes in select patients.9 Radiotherapy presents another option, though reports have described progression to aggressive squamous cell carcinoma in some cases.9 Surgery is the best course of treatment, and as more case reports have been published, a transition from radical resection to wide excision with tumor-free margins is the treatment of choice.2,3,10,11 To minimize soft-tissue deficits, Mohs micrographic surgery has been discussed as a treatment option for verrucous carcinoma.11-13
Few studies have described verrucous carcinoma recurrence, and none have systematically examined recurrence rate, risk factors, or prognosis
Methods
Patient cases were
Of the 19 cases, 16 were treated at the University of Michigan and are included in the treatment analyses. Specific attention was then paid to the cases with a clinical recurrence despite negative surgical margins. We compared the clinical and surgical differences between recurrent cases and nonrecurrent cases.
Pathology was rereviewed for selected cases, including 2 cases with recurrence and matched primary, 2 cases with recurrence (for which the matched primary was unavailable for review), and 5 representative primary cases that were not complicated by recurrence. Pathology review was conducted in a blinded manner by one of the authors (P.W.H) who is a board-certified dermatopathologist for approximate depth of invasion from the granular layer, perineural invasion, bone invasion, infiltrative growth, presence of conventional squamous cell carcinoma, and margin status.
Statistical analysis was performed when appropriate using an N1 χ2 test or Student t test.
Results
Demographics and Comorbidities—The median age of the patients at the time of diagnosis was 55 years (range, 34–77 years). There were 12 males and 7 females (Table 1). Two patients were Black and 17 were White. Almost all patients had additional comorbidities including tobacco use (68%), alcohol use (47%), and diabetes (47%). Only 1 patient had an autoimmune disease and was on chronic steroids. No significant difference was found between the demographics of patients with recurrent lesions and those without recurrence.
Tumor Location and Clinical Presentation—The most common clinical presentation included a nonhealing ulceration with warty edges, pain, bleeding, and lowered mobility. In most cases, there was history of prior treatment over a duration ranging from 1 to 8 years, with a median of 5 years prior to biopsy-based diagnosis (Table 1). Six patients had a history of osteomyelitis, diagnosed by imaging or biopsy, within a year before tumor diagnosis. The size of the primary tumor ranged from 2.4 to 6 cm, with a mean of 4 cm (P=.20). The clinical presentation, time before diagnosis, and size of the tumors did not differ significantly between recurrent and nonrecurrent cases.
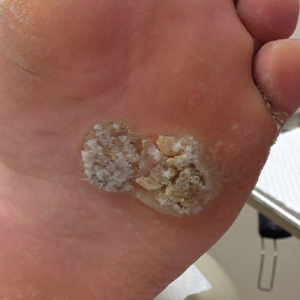
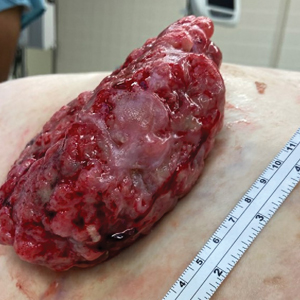
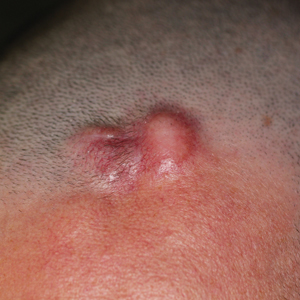
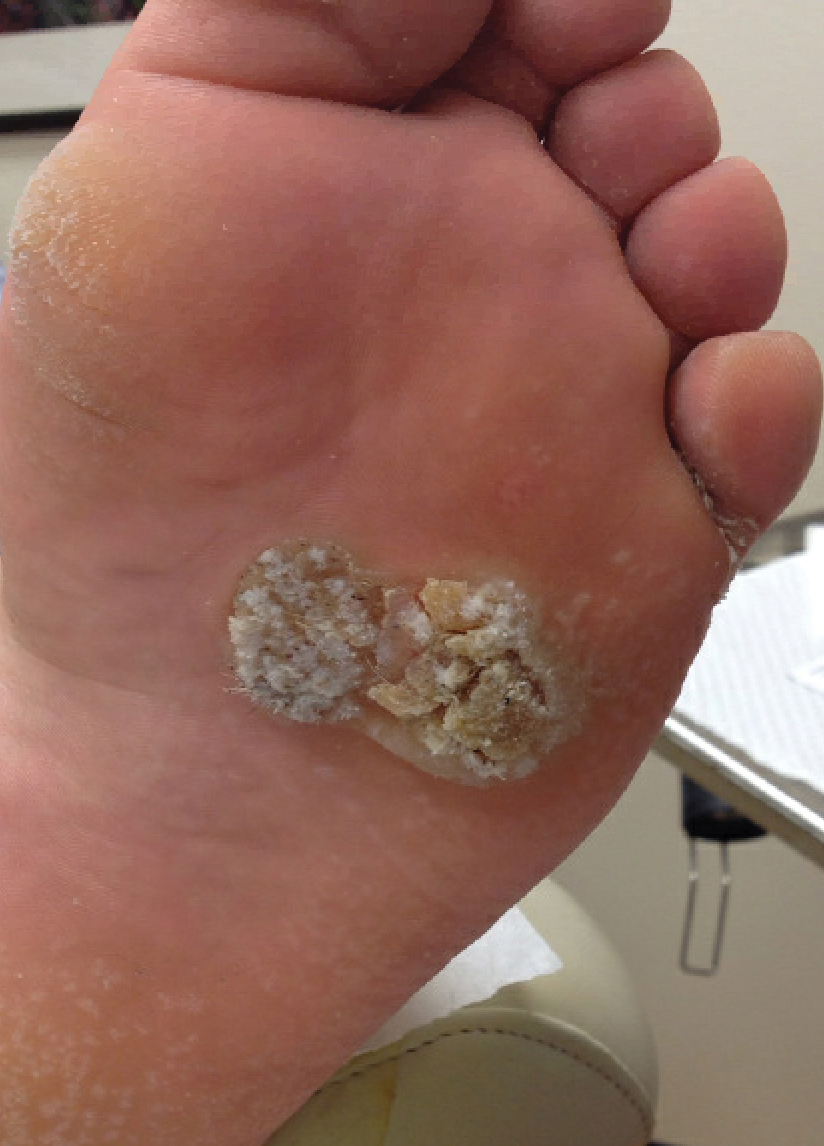
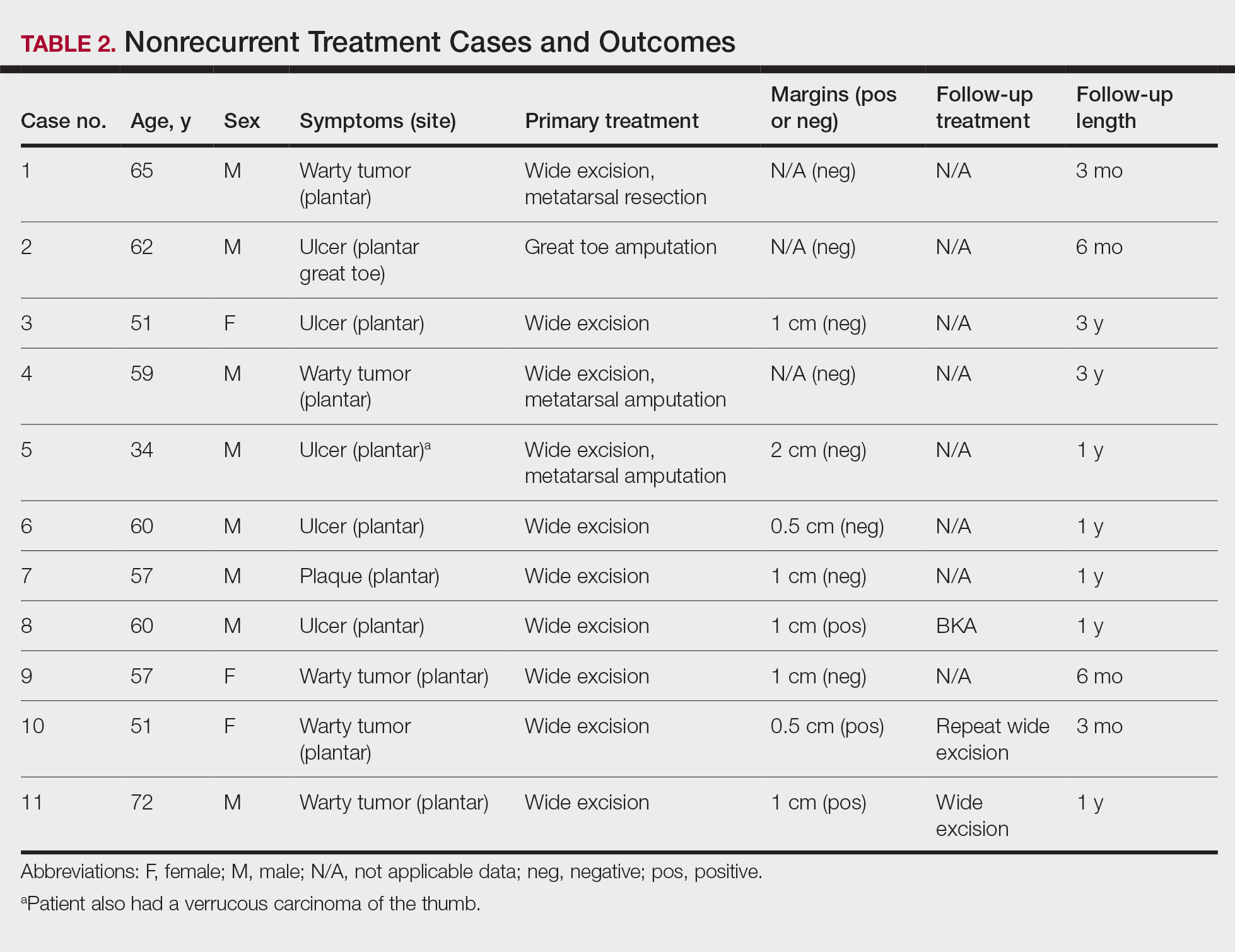
The tumor location for the recurrent cases differed significantly compared to nonrecurrent cases. All 5 of the patients with a recurrence presented with a tumor on the nonglabrous part of the foot. Four patients (80%) had lesions on the dorsal or lateral aspect of the great toe (P=.002), and 1 patient (20%) had a lesion on the low ankle (P=.09)(Table 1). Of the nonrecurrent cases, 1 patient (7%) presented with a tumor on the plantar surface of the great toe (P=.002), 13 patients (93%) presented with tumors on the distal plantar surface of the foot (P=.0002), and 1 patient with a plantar foot tumor (Figure 1) also had verrucous carcinoma on the thumb (Table 1 and Figure 2).
Histopathology—Available pathology slides for recurrent cases of verrucous carcinoma were reviewed alongside representative cases of verrucous carcinomas that did not progress to recurrence. The diagnosis of verrucous carcinoma was confirmed in all cases, with no evidence of conventional squamous cell carcinoma, perineural invasion, extension beyond the dermis, or bone invasion in any case. The median size of the tumors was 4.2 cm and 4 cm for nonrecurrent and recurrent specimens, respectively. Recurrences displayed a trend toward increased depth compared to primary tumors without recurrence (average depth, 5.5 mm vs 3.7 mm); however, this did not reach statistical significance (P=.24). Primary tumors that progressed to recurrence (n=2) displayed similar findings to the other cases, with invasive depths of 3.5 and 5.5 mm, and there was no evidence of conventional squamous cell carcinoma, perineural invasion, or extension beyond the dermis.
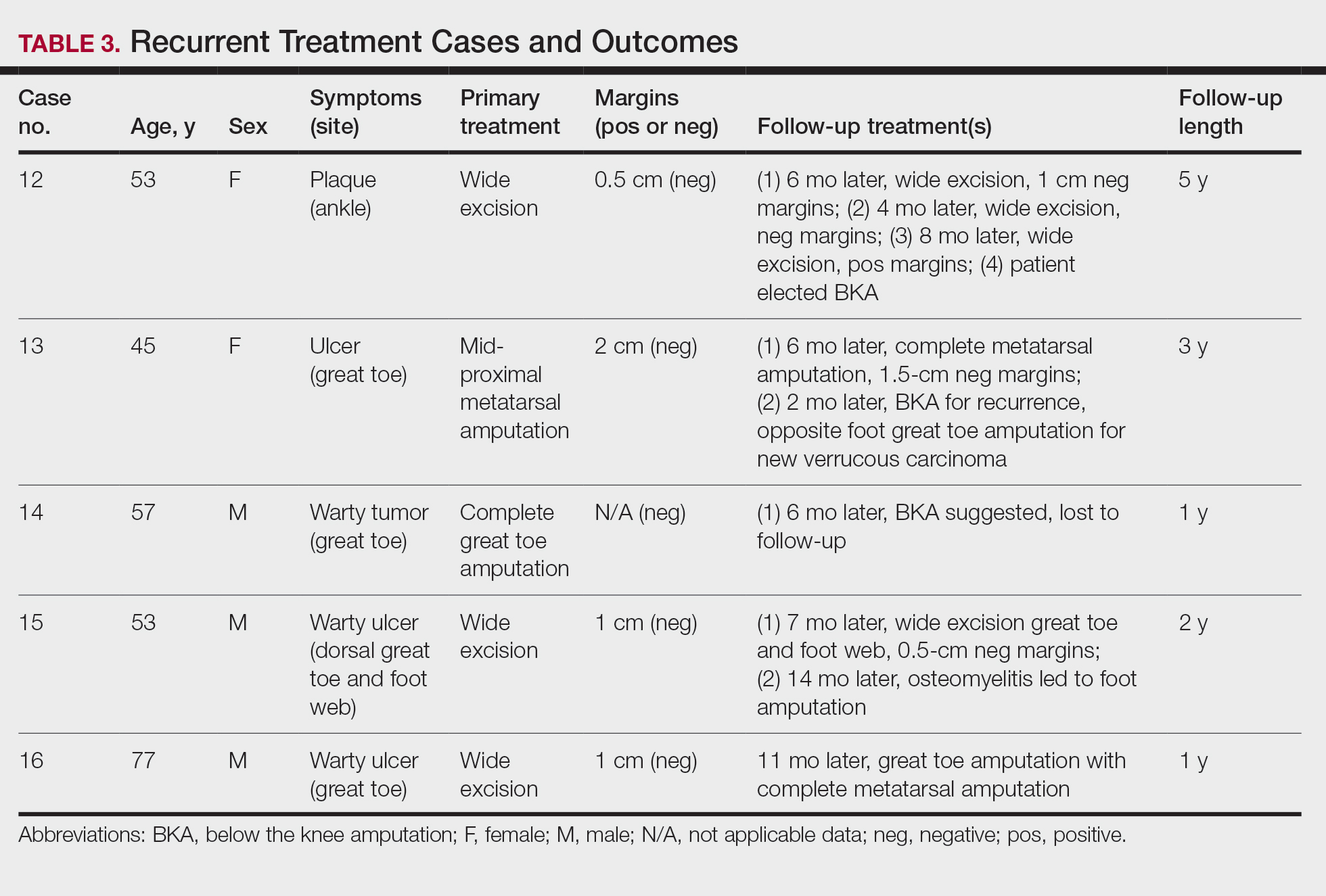
Treatment of Nonrecurrent Cases—Of the 16 total cases treated at the University of Michigan, surgery was the primary mode of therapy in every case (Tables 2 and 3). Of the 11 nonrecurrent cases, 7 patients had wide local excision with a dermal regeneration template, and delayed split-thickness graft reconstruction. Three cases had wide local excision with metatarsal resection, dermal regeneration template, and delayed skin grafting. One case had a great toe amputation
Treatment of Recurrent Cases—For the 5 patients with recurrence, surgical margins were not reported in all the cases but ranged from 0.5 to 2 cm (4/5 [80%] reported). On average, follow-up for this group of patients was 29 months, with a range of 12 to 60 months (Table 3).
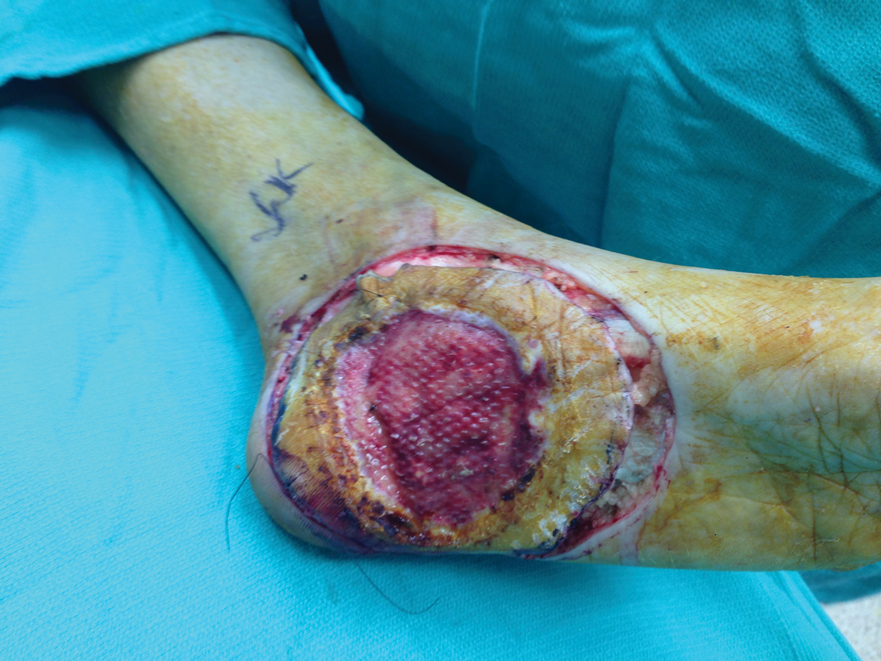
The first case with a recurrence (patient 12) initially presented with a chronic calluslike growth of the medial ankle. The lesion initially was treated with wide local excision with negative margins. Reconstruction was performed in a staged fashion with use of a dermal regenerative template followed later by split-thickness skin grafting. Tumor recurrence with negative margins occurred 3 times over the next 2 years despite re-resections with negative pathologic margins. Each recurrence presented as graft breakdown and surrounding hyperkeratosis (Figure 3). After the third graft placement failed, the patient elected for a BKA. There has not been recurrence since the BKA after 5 years total follow-up from the time of primary tumor resection. Of note, this was the only patient in our cohort who was immunosuppressed and evaluated for regional nodal involvement by positron emission tomography.
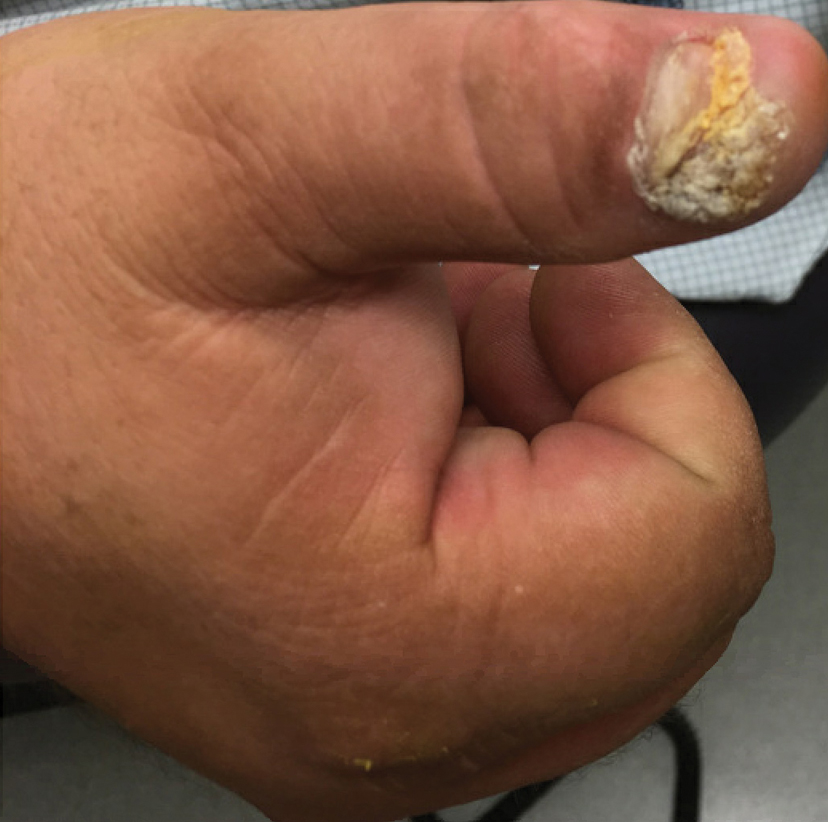
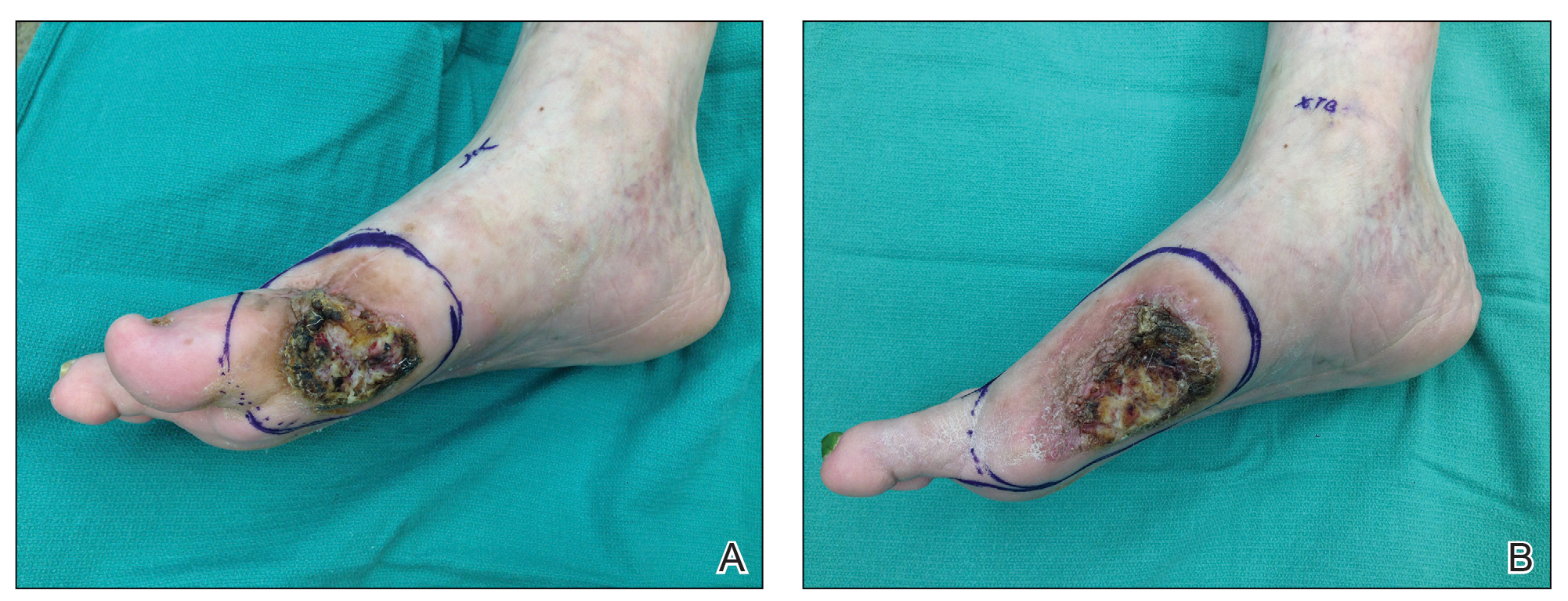
Another patient with recurrence (patient 13) presented with a chronic great toe ulcer of 5 years’ duration that formed on the dorsal aspect of the great toe after a previously excised wart (Figure 4A). This patient underwent mid-proximal metatarsal amputation with 2-cm margins and subsequent skin graft. Pathologic margins were negative. Within 6 months, there was hyperkeratosis and a draining wound (Figure 4B). Biopsy results confirmed recurrent disease that was treated with re-resection, including complete metatarsal amputation with negative margins and skin graft placement. Verrucous carcinoma recurred at the edges of the graft within 8 months, and the patient elected for a BKA. In addition, this patient also presented with a verrucous carcinoma of the contralateral great toe. The tumor presented as a warty ulcer of 4 months’ duration in the setting of osteomyelitis and was resected by great toe amputation that was performed concurrently with the opposite leg BKA; there has been no recurrence. Of note, this was the only patient to have right inguinal sentinel lymph node tissue sampled and HPV testing conducted, which were negative for verrucous carcinoma and high or low strains of HPV.
Another recurrent case (patient 14) presented with a large warty lesion on the dorsal great toe positive for verrucous carcinoma. He underwent a complete great toe amputation with skin graft placement. Verrucous carcinoma recurred on the edges of the graft within 6 months, and the patient was lost to follow-up when a BKA was suggested.
The fourth recurrent case (patient 15) initially had been treated for 1 year as a viral verruca of the dorsal aspect of the great toe. He had an exophytic mass positive for verrucous carcinoma growing on the dorsal aspect of the great toe around the prior excision site. After primary wide excision with negative 1-cm margins and graft placement, the tumor was re-excised twice within the next 2 years with pathologic negative margins. The patient underwent a foot amputation due to a severe osteomyelitis infection at the reconstruction site.
The final recurrent case (patient 16) presented with a mass on the lateral great toe that initially was treated as a viral verruca (for unknown duration) that had begun to ulcerate. The patient underwent wide excision with 1-cm margins and graft placement. Final pathology was consistent with verrucous carcinoma with negative margins. Recurrence occurred within 11 months on the edge of the graft, and a great toe amputation through the metatarsal phalangeal joint was performed.
Comment
Our series of 19 cases of verrucous carcinoma adds to the limited number of reported cases in the literature. We sought to evaluate the potential risk factors for early recurrence. Consistent with prior studies, our series found verrucous carcinoma of the foot to occur most frequently in patients aged 50 to 70 years, predominantly in White men.1 These tumors grew in the setting of chronic inflammation, tissue regeneration, multiple comorbidities, and poor wound hygiene. Misdiagnosis of verrucous carcinoma often leads to ineffective treatments and local invasion of nerves, muscle, and bone tissue.9,15,16 Our case series also clearly demonstrated the diagnostic challenge verrucous carcinoma presents, with an average delay in diagnosis of 5 years; correct diagnosis often did not occur until the tumor was 4 cm in size (average) and more than 50% had chronic ulceration.
The histologic features of the tumors showed striking uniformity. Within the literature, there is confusion regarding the use of the terms verrucous carcinoma and carcinoma (epithelioma) cuniculatum and the possible pathologic differences between the two. The World Health Organization’s classification of skin tumors describes epithelioma cuniculatum as verrucous carcinoma located on the sole of the foot.7 Kubik and Rhatigan6 pointed out that carcinoma cuniculatum does not have a warty or verrucous surface, which is a defining feature of verrucous carcinoma. Multiple authors have further surmised that the deep burrowing sinus tracts of epithelioma cuniculatum are different than those seen in verrucous carcinoma formed by the undulations extending from the papillomatous and verrucous surface.1,6 We did not observe these notable pathologic differences in recurrent or nonrecurrent primary tumors or differences between primary and recurrent cases. Although our cohort was small, the findings suggest that standard histologic features do not predict aggressive behavior in verrucous carcinomas. Furthermore, our observations support a model wherein recurrence is an inherent property of certain verrucous carcinomas rather than a consequence of histologic progression to conventional squamous cell carcinoma. The lack of overt malignant features in such cases underscores the need for distinction of verrucous carcinoma from benign mimics such as viral verruca or reactive epidermal hyperplasia.
Our recurrent cases showed a greater predilection for nonplantar surfaces and the great toe (P=.002). Five of 6 cases on the nonplantar surface—1 on the ankle and 5 on the great toe—recurred despite negative pathologic margins. There was no significant difference in demographics, pathogenesis, tumor size, chronicity, phenotype, or metastatic spread in recurrent and nonrecurrent cases in our cohort.
The tumor has only been described in rare instances at extrapedal cutaneous sites including the hand, scalp, and abdomen.14,17,18 Our series did include a case of synchronous presentation with a verrucous carcinoma on the thumb. Given the rarity of this presentation, thus far there are no data supporting any atypical locations of verrucous carcinoma having greater instances of recurrence. Our recurrent cases displaying atypical location on nonglabrous skin could suggest an underlying pathologic mechanism distinct from tumors on glabrous skin and relevant to increased recurrence risk. Such a mechanism might relate to distinct genetic insults, tumor-microenvironment interactions, or field effects. There are few studies regarding physiologic differences between the plantar surface and the nonglabrous surface and how that influences cancer genesis. Within acral melanoma studies, nonglabrous skin of more sun-exposed surfaces has a higher burden of genetic insults including BRAF mutations.19 Genetic testing of verrucous carcinoma is highly limited, with abnormal expression of the p53 tumor suppressor protein and possible association with several types of HPV. Verrucous carcinoma in general has been found to contain HPV types 6 and 11, nononcogenic forms, and higher risk from HPV types 16 and 18.9,20 However, only a few cases of HPV type 16 as well as 1 case each of HPV type 2 and type 11 have been found within verrucous carcinoma of the foot.21,22 In squamous cell carcinoma of the head and neck, HPV-positive tumors have shown better response to treatment. Further investigation of HPV and genetic contributors in verrucous carcinoma is warranted.
There is notable evidence that surgical resection is the best mode of treatment of verrucous carcinoma.2,3,10,11 Our case series was treated with wide local excision, with partial metatarsal amputation or great toe amputation, in cases with bone invasion or osteomyelitis. Surgical margins were not reported in all the cases but ranged from 0.5 to 2 cm with no significant differences between the recurrent and nonrecurrent groups. After excision, closure was conducted by incorporating primary, secondary, and delayed closure techniques, along with skin grafts for larger defects. Lymph node biopsy traditionally has not been recommended due to reported low metastatic potential. In all 5 recurrent cases, the tumors recurred after multiple attempts at wide excision and greater resection of bone and tissue, with negative margins. The tumors regrew quickly, within months, on the edges of the new graft or in the middle of the graft. The sites of recurrent tumor growth would suggest regrowth in the areas of greatest tissue stress and proliferation. We recommend a low threshold for biopsy and aggressive retreatment in the setting of exophytic growth at reconstruction sites.
Recurrence is uncommon in the setting of verrucous carcinoma, with our series being the first to analyze prognostic factors.3,9,14 Our findings indicate that
- Kao GF, Graham JH, Helwig EB. Carcinoma cuniculatum (verrucous carcinoma of the skin): a clinicopathologic study of 46 cases with ultrastructural observations. Cancer. 1982;49:2395-2403.
- McKee PH, Wilkinson JD, Black M, et al. Carcinoma (epithelioma) cuniculatum: a clinic-pathologic study of nineteen cases and review of the literature. Histopathology. 1981;5:425-436.
- Penera KE, Manji KA, Craig AB, et al. Atypical presentation of verrucous carcinoma: a case study and review of the literature. Foot Ankle Spec. 2013;6:318-322.
- Rosales MA, Martin BR, Armstrong DG, et al. Verrucous hyperplasia: a common and problematic finding in the high-risk diabetic foot. J Am Podiatr Assoc. 2006:4:348-350.
- Noel JC, Peny MO, De Dobbeleer G, et al. p53 Protein overexpression in verrucous carcinoma of the skin. Dermatology. 1996;192:12-15.
- Kubik MJ, Rhatigan RM. Carcinoma cuniculatum: not a verrucous carcinoma. J Cutan Pathol. 2012;39:1083-1087
- Elder D, Massi D, Scolver R, et al. Verrucous squamous cell carcinoma. WHO Classification of Tumours (Medicine). Vol 11. 4th ed. International Agency for Research on Cancer: 2018;35-57.
- Chan MP. Verruciform and condyloma-like squamous proliferations in the anogenital region. Arch Pathol Lab Med. 2019;143:821-831
- Schwartz RA. Verrucous carcinoma of the skin and mucosa. J Am Acad Dermatol. 1995;32:1-21.
- Flynn K, Wiemer D. Treatment of an epithelioma cuniculatum plantare by local excision and a plantar skin flap. J Dermatol Surg Oncol. 1978;4:773-775.
- Spyriounis P, Tentis D, Sparveri I, et al. Plantar epithelioma cuniculatum: a case report with review of the literature. Eur J Plast Surg. 2004;27:253-256.
- Swanson NA, Taylor WB. Plantar verrucous carcinoma: literature review and treatment by the Moh’s chemosurgery technique. Arch Dermatol. 1980;116:794-797.
- Alkalay R, Alcalay J, Shiri J. Plantar verrucous carcinoma treated with Mohs micrographic surgery: a case report and literature review. J Drugs Dermatol. 2006:5:68-73.
- Kotwal M, Poflee S, Bobhate, S. Carcinoma cuniculatum at various anatomical sites. Indian J Dermatol. 2005;50:216-220.
- Nagarajan D, Chandrasekhar M, Jebakumar J, et al. Verrucous carcinoma of foot at an unusual site: lessons to be learnt. South Asian J Cancer. 2017;6:63.
- Pempinello C, Bova A, Pempinello R, et al Verrucous carcinoma of the foot with bone invasion: a case report. Case Rep Oncol Med. 2013;2013:135307.
- Vandeweyer E, Sales F, Deramaecker R. Cutaneous verrucous carcinoma. Br J Plastic Surg. 2001;54:168-170.
- Joybari A, Azadeh P, Honar B. Cutaneous verrucous carcinoma superimposed on chronically inflamed ileostomy site skin. Iran J Pathol. 2018;13:285-288.
- Davis EJ, Johnson DB, Sosman JA, et al. Melanoma: what do all the mutations mean? Cancer. 2018;124:3490-3499.
- Gissmann L, Wolnik L, Ikenberg H, et al. Human papillomavirus types 6 and 11 DNA sequences in genital and laryngeal papillomas and in some cervical cancers. Proc Natl Acad Sci U S A. 1983;80:560-563.
- Knobler RM, Schneider S, Neumann RA, et al. DNA dot-blot hybridization implicates human papillomavirus type 11-DNA in epithelioma cuniculatum. J Med Virol. 1989;29:33-37.
- Noel JC, Peny MO, Detremmerie O, et al. Demonstration of human papillomavirus type 2 in a verrucous carcinoma of the foot. Dermatology. 1993;187:58-61.
Verrucous carcinoma is a rare cancer with the greatest predilection for the foot. Multiple case reports with only a few large case series have been published. 1-3 Plantar verrucous carcinoma is characterized as a slowly but relentlessly enlarging warty tumor with low metastatic potential and high risk for local invasion. The tumor occurs most frequently in patients aged 60 to 70 years, predominantly in White males. 1 It often is misdiagnosed for years as an ulcer or wart that is highly resistant to therapy. Size typically ranges from 1 to 12 cm in greatest dimension. 1
The pathogenesis of plantar verrucous carcinoma remains unclear, but some contributing factors have been proposed, including trauma, chronic irritation, infection, and poor local hygiene.2 This tumor has been reported to occur in chronic foot ulcerations, particularly in the diabetic population.4 It has been proposed that abnormal expression of the p53 tumor suppressor protein and several types of human papillomavirus (HPV) may have a role in the pathogenesis of verrucous carcinoma.5
The pathologic hallmarks of this tumor include a verrucous/hyperkeratotic surface with a deeply endophytic, broad, pushing base. Tumor cells are well differentiated, and atypia is either absent or confined to 1 or 2 layers at the base of the tumor. Overt invasion at the base is lacking, except in cases with a component of conventional invasive squamous cell carcinoma. Human papillomavirus viropathic changes are classically absent.1,3 Studies of the histopathology of verrucous carcinoma have been complicated by similar entities, nomenclatural uncertainty, and variable diagnostic criteria. For example, epithelioma cuniculatum variously has been defined as being synonymous with verrucous carcinoma, a distinct clinical verrucous carcinoma subtype occurring on the soles, a histologic subtype (characterized by prominent burrowing sinuses), or a separate entity entirely.1,2,6,7 Furthermore, in the genital area, several different types of carcinomas have verruciform features but display distinct microscopic findings and outcomes from verrucous carcinoma.8
Verrucous carcinoma represents an unusual variant of squamous cell carcinoma and is treated as such. Treatments have included laser surgery; immunotherapy; retinoid therapy; and chemotherapy by oral, intralesional, or iontophoretic routes in select patients.9 Radiotherapy presents another option, though reports have described progression to aggressive squamous cell carcinoma in some cases.9 Surgery is the best course of treatment, and as more case reports have been published, a transition from radical resection to wide excision with tumor-free margins is the treatment of choice.2,3,10,11 To minimize soft-tissue deficits, Mohs micrographic surgery has been discussed as a treatment option for verrucous carcinoma.11-13
Few studies have described verrucous carcinoma recurrence, and none have systematically examined recurrence rate, risk factors, or prognosis
Methods
Patient cases were
Of the 19 cases, 16 were treated at the University of Michigan and are included in the treatment analyses. Specific attention was then paid to the cases with a clinical recurrence despite negative surgical margins. We compared the clinical and surgical differences between recurrent cases and nonrecurrent cases.
Pathology was rereviewed for selected cases, including 2 cases with recurrence and matched primary, 2 cases with recurrence (for which the matched primary was unavailable for review), and 5 representative primary cases that were not complicated by recurrence. Pathology review was conducted in a blinded manner by one of the authors (P.W.H) who is a board-certified dermatopathologist for approximate depth of invasion from the granular layer, perineural invasion, bone invasion, infiltrative growth, presence of conventional squamous cell carcinoma, and margin status.
Statistical analysis was performed when appropriate using an N1 χ2 test or Student t test.
Results
Demographics and Comorbidities—The median age of the patients at the time of diagnosis was 55 years (range, 34–77 years). There were 12 males and 7 females (Table 1). Two patients were Black and 17 were White. Almost all patients had additional comorbidities including tobacco use (68%), alcohol use (47%), and diabetes (47%). Only 1 patient had an autoimmune disease and was on chronic steroids. No significant difference was found between the demographics of patients with recurrent lesions and those without recurrence.

Tumor Location and Clinical Presentation—The most common clinical presentation included a nonhealing ulceration with warty edges, pain, bleeding, and lowered mobility. In most cases, there was history of prior treatment over a duration ranging from 1 to 8 years, with a median of 5 years prior to biopsy-based diagnosis (Table 1). Six patients had a history of osteomyelitis, diagnosed by imaging or biopsy, within a year before tumor diagnosis. The size of the primary tumor ranged from 2.4 to 6 cm, with a mean of 4 cm (P=.20). The clinical presentation, time before diagnosis, and size of the tumors did not differ significantly between recurrent and nonrecurrent cases.
The tumor location for the recurrent cases differed significantly compared to nonrecurrent cases. All 5 of the patients with a recurrence presented with a tumor on the nonglabrous part of the foot. Four patients (80%) had lesions on the dorsal or lateral aspect of the great toe (P=.002), and 1 patient (20%) had a lesion on the low ankle (P=.09)(Table 1). Of the nonrecurrent cases, 1 patient (7%) presented with a tumor on the plantar surface of the great toe (P=.002), 13 patients (93%) presented with tumors on the distal plantar surface of the foot (P=.0002), and 1 patient with a plantar foot tumor (Figure 1) also had verrucous carcinoma on the thumb (Table 1 and Figure 2).

Histopathology—Available pathology slides for recurrent cases of verrucous carcinoma were reviewed alongside representative cases of verrucous carcinomas that did not progress to recurrence. The diagnosis of verrucous carcinoma was confirmed in all cases, with no evidence of conventional squamous cell carcinoma, perineural invasion, extension beyond the dermis, or bone invasion in any case. The median size of the tumors was 4.2 cm and 4 cm for nonrecurrent and recurrent specimens, respectively. Recurrences displayed a trend toward increased depth compared to primary tumors without recurrence (average depth, 5.5 mm vs 3.7 mm); however, this did not reach statistical significance (P=.24). Primary tumors that progressed to recurrence (n=2) displayed similar findings to the other cases, with invasive depths of 3.5 and 5.5 mm, and there was no evidence of conventional squamous cell carcinoma, perineural invasion, or extension beyond the dermis.

Treatment of Nonrecurrent Cases—Of the 16 total cases treated at the University of Michigan, surgery was the primary mode of therapy in every case (Tables 2 and 3). Of the 11 nonrecurrent cases, 7 patients had wide local excision with a dermal regeneration template, and delayed split-thickness graft reconstruction. Three cases had wide local excision with metatarsal resection, dermal regeneration template, and delayed skin grafting. One case had a great toe amputation

Treatment of Recurrent Cases—For the 5 patients with recurrence, surgical margins were not reported in all the cases but ranged from 0.5 to 2 cm (4/5 [80%] reported). On average, follow-up for this group of patients was 29 months, with a range of 12 to 60 months (Table 3).

The first case with a recurrence (patient 12) initially presented with a chronic calluslike growth of the medial ankle. The lesion initially was treated with wide local excision with negative margins. Reconstruction was performed in a staged fashion with use of a dermal regenerative template followed later by split-thickness skin grafting. Tumor recurrence with negative margins occurred 3 times over the next 2 years despite re-resections with negative pathologic margins. Each recurrence presented as graft breakdown and surrounding hyperkeratosis (Figure 3). After the third graft placement failed, the patient elected for a BKA. There has not been recurrence since the BKA after 5 years total follow-up from the time of primary tumor resection. Of note, this was the only patient in our cohort who was immunosuppressed and evaluated for regional nodal involvement by positron emission tomography.

Another patient with recurrence (patient 13) presented with a chronic great toe ulcer of 5 years’ duration that formed on the dorsal aspect of the great toe after a previously excised wart (Figure 4A). This patient underwent mid-proximal metatarsal amputation with 2-cm margins and subsequent skin graft. Pathologic margins were negative. Within 6 months, there was hyperkeratosis and a draining wound (Figure 4B). Biopsy results confirmed recurrent disease that was treated with re-resection, including complete metatarsal amputation with negative margins and skin graft placement. Verrucous carcinoma recurred at the edges of the graft within 8 months, and the patient elected for a BKA. In addition, this patient also presented with a verrucous carcinoma of the contralateral great toe. The tumor presented as a warty ulcer of 4 months’ duration in the setting of osteomyelitis and was resected by great toe amputation that was performed concurrently with the opposite leg BKA; there has been no recurrence. Of note, this was the only patient to have right inguinal sentinel lymph node tissue sampled and HPV testing conducted, which were negative for verrucous carcinoma and high or low strains of HPV.

Another recurrent case (patient 14) presented with a large warty lesion on the dorsal great toe positive for verrucous carcinoma. He underwent a complete great toe amputation with skin graft placement. Verrucous carcinoma recurred on the edges of the graft within 6 months, and the patient was lost to follow-up when a BKA was suggested.
The fourth recurrent case (patient 15) initially had been treated for 1 year as a viral verruca of the dorsal aspect of the great toe. He had an exophytic mass positive for verrucous carcinoma growing on the dorsal aspect of the great toe around the prior excision site. After primary wide excision with negative 1-cm margins and graft placement, the tumor was re-excised twice within the next 2 years with pathologic negative margins. The patient underwent a foot amputation due to a severe osteomyelitis infection at the reconstruction site.
The final recurrent case (patient 16) presented with a mass on the lateral great toe that initially was treated as a viral verruca (for unknown duration) that had begun to ulcerate. The patient underwent wide excision with 1-cm margins and graft placement. Final pathology was consistent with verrucous carcinoma with negative margins. Recurrence occurred within 11 months on the edge of the graft, and a great toe amputation through the metatarsal phalangeal joint was performed.
Comment
Our series of 19 cases of verrucous carcinoma adds to the limited number of reported cases in the literature. We sought to evaluate the potential risk factors for early recurrence. Consistent with prior studies, our series found verrucous carcinoma of the foot to occur most frequently in patients aged 50 to 70 years, predominantly in White men.1 These tumors grew in the setting of chronic inflammation, tissue regeneration, multiple comorbidities, and poor wound hygiene. Misdiagnosis of verrucous carcinoma often leads to ineffective treatments and local invasion of nerves, muscle, and bone tissue.9,15,16 Our case series also clearly demonstrated the diagnostic challenge verrucous carcinoma presents, with an average delay in diagnosis of 5 years; correct diagnosis often did not occur until the tumor was 4 cm in size (average) and more than 50% had chronic ulceration.
The histologic features of the tumors showed striking uniformity. Within the literature, there is confusion regarding the use of the terms verrucous carcinoma and carcinoma (epithelioma) cuniculatum and the possible pathologic differences between the two. The World Health Organization’s classification of skin tumors describes epithelioma cuniculatum as verrucous carcinoma located on the sole of the foot.7 Kubik and Rhatigan6 pointed out that carcinoma cuniculatum does not have a warty or verrucous surface, which is a defining feature of verrucous carcinoma. Multiple authors have further surmised that the deep burrowing sinus tracts of epithelioma cuniculatum are different than those seen in verrucous carcinoma formed by the undulations extending from the papillomatous and verrucous surface.1,6 We did not observe these notable pathologic differences in recurrent or nonrecurrent primary tumors or differences between primary and recurrent cases. Although our cohort was small, the findings suggest that standard histologic features do not predict aggressive behavior in verrucous carcinomas. Furthermore, our observations support a model wherein recurrence is an inherent property of certain verrucous carcinomas rather than a consequence of histologic progression to conventional squamous cell carcinoma. The lack of overt malignant features in such cases underscores the need for distinction of verrucous carcinoma from benign mimics such as viral verruca or reactive epidermal hyperplasia.
Our recurrent cases showed a greater predilection for nonplantar surfaces and the great toe (P=.002). Five of 6 cases on the nonplantar surface—1 on the ankle and 5 on the great toe—recurred despite negative pathologic margins. There was no significant difference in demographics, pathogenesis, tumor size, chronicity, phenotype, or metastatic spread in recurrent and nonrecurrent cases in our cohort.
The tumor has only been described in rare instances at extrapedal cutaneous sites including the hand, scalp, and abdomen.14,17,18 Our series did include a case of synchronous presentation with a verrucous carcinoma on the thumb. Given the rarity of this presentation, thus far there are no data supporting any atypical locations of verrucous carcinoma having greater instances of recurrence. Our recurrent cases displaying atypical location on nonglabrous skin could suggest an underlying pathologic mechanism distinct from tumors on glabrous skin and relevant to increased recurrence risk. Such a mechanism might relate to distinct genetic insults, tumor-microenvironment interactions, or field effects. There are few studies regarding physiologic differences between the plantar surface and the nonglabrous surface and how that influences cancer genesis. Within acral melanoma studies, nonglabrous skin of more sun-exposed surfaces has a higher burden of genetic insults including BRAF mutations.19 Genetic testing of verrucous carcinoma is highly limited, with abnormal expression of the p53 tumor suppressor protein and possible association with several types of HPV. Verrucous carcinoma in general has been found to contain HPV types 6 and 11, nononcogenic forms, and higher risk from HPV types 16 and 18.9,20 However, only a few cases of HPV type 16 as well as 1 case each of HPV type 2 and type 11 have been found within verrucous carcinoma of the foot.21,22 In squamous cell carcinoma of the head and neck, HPV-positive tumors have shown better response to treatment. Further investigation of HPV and genetic contributors in verrucous carcinoma is warranted.
There is notable evidence that surgical resection is the best mode of treatment of verrucous carcinoma.2,3,10,11 Our case series was treated with wide local excision, with partial metatarsal amputation or great toe amputation, in cases with bone invasion or osteomyelitis. Surgical margins were not reported in all the cases but ranged from 0.5 to 2 cm with no significant differences between the recurrent and nonrecurrent groups. After excision, closure was conducted by incorporating primary, secondary, and delayed closure techniques, along with skin grafts for larger defects. Lymph node biopsy traditionally has not been recommended due to reported low metastatic potential. In all 5 recurrent cases, the tumors recurred after multiple attempts at wide excision and greater resection of bone and tissue, with negative margins. The tumors regrew quickly, within months, on the edges of the new graft or in the middle of the graft. The sites of recurrent tumor growth would suggest regrowth in the areas of greatest tissue stress and proliferation. We recommend a low threshold for biopsy and aggressive retreatment in the setting of exophytic growth at reconstruction sites.
Recurrence is uncommon in the setting of verrucous carcinoma, with our series being the first to analyze prognostic factors.3,9,14 Our findings indicate that
Verrucous carcinoma is a rare cancer with the greatest predilection for the foot. Multiple case reports with only a few large case series have been published. 1-3 Plantar verrucous carcinoma is characterized as a slowly but relentlessly enlarging warty tumor with low metastatic potential and high risk for local invasion. The tumor occurs most frequently in patients aged 60 to 70 years, predominantly in White males. 1 It often is misdiagnosed for years as an ulcer or wart that is highly resistant to therapy. Size typically ranges from 1 to 12 cm in greatest dimension. 1
The pathogenesis of plantar verrucous carcinoma remains unclear, but some contributing factors have been proposed, including trauma, chronic irritation, infection, and poor local hygiene.2 This tumor has been reported to occur in chronic foot ulcerations, particularly in the diabetic population.4 It has been proposed that abnormal expression of the p53 tumor suppressor protein and several types of human papillomavirus (HPV) may have a role in the pathogenesis of verrucous carcinoma.5
The pathologic hallmarks of this tumor include a verrucous/hyperkeratotic surface with a deeply endophytic, broad, pushing base. Tumor cells are well differentiated, and atypia is either absent or confined to 1 or 2 layers at the base of the tumor. Overt invasion at the base is lacking, except in cases with a component of conventional invasive squamous cell carcinoma. Human papillomavirus viropathic changes are classically absent.1,3 Studies of the histopathology of verrucous carcinoma have been complicated by similar entities, nomenclatural uncertainty, and variable diagnostic criteria. For example, epithelioma cuniculatum variously has been defined as being synonymous with verrucous carcinoma, a distinct clinical verrucous carcinoma subtype occurring on the soles, a histologic subtype (characterized by prominent burrowing sinuses), or a separate entity entirely.1,2,6,7 Furthermore, in the genital area, several different types of carcinomas have verruciform features but display distinct microscopic findings and outcomes from verrucous carcinoma.8
Verrucous carcinoma represents an unusual variant of squamous cell carcinoma and is treated as such. Treatments have included laser surgery; immunotherapy; retinoid therapy; and chemotherapy by oral, intralesional, or iontophoretic routes in select patients.9 Radiotherapy presents another option, though reports have described progression to aggressive squamous cell carcinoma in some cases.9 Surgery is the best course of treatment, and as more case reports have been published, a transition from radical resection to wide excision with tumor-free margins is the treatment of choice.2,3,10,11 To minimize soft-tissue deficits, Mohs micrographic surgery has been discussed as a treatment option for verrucous carcinoma.11-13
Few studies have described verrucous carcinoma recurrence, and none have systematically examined recurrence rate, risk factors, or prognosis
Methods
Patient cases were
Of the 19 cases, 16 were treated at the University of Michigan and are included in the treatment analyses. Specific attention was then paid to the cases with a clinical recurrence despite negative surgical margins. We compared the clinical and surgical differences between recurrent cases and nonrecurrent cases.
Pathology was rereviewed for selected cases, including 2 cases with recurrence and matched primary, 2 cases with recurrence (for which the matched primary was unavailable for review), and 5 representative primary cases that were not complicated by recurrence. Pathology review was conducted in a blinded manner by one of the authors (P.W.H) who is a board-certified dermatopathologist for approximate depth of invasion from the granular layer, perineural invasion, bone invasion, infiltrative growth, presence of conventional squamous cell carcinoma, and margin status.
Statistical analysis was performed when appropriate using an N1 χ2 test or Student t test.
Results
Demographics and Comorbidities—The median age of the patients at the time of diagnosis was 55 years (range, 34–77 years). There were 12 males and 7 females (Table 1). Two patients were Black and 17 were White. Almost all patients had additional comorbidities including tobacco use (68%), alcohol use (47%), and diabetes (47%). Only 1 patient had an autoimmune disease and was on chronic steroids. No significant difference was found between the demographics of patients with recurrent lesions and those without recurrence.

Tumor Location and Clinical Presentation—The most common clinical presentation included a nonhealing ulceration with warty edges, pain, bleeding, and lowered mobility. In most cases, there was history of prior treatment over a duration ranging from 1 to 8 years, with a median of 5 years prior to biopsy-based diagnosis (Table 1). Six patients had a history of osteomyelitis, diagnosed by imaging or biopsy, within a year before tumor diagnosis. The size of the primary tumor ranged from 2.4 to 6 cm, with a mean of 4 cm (P=.20). The clinical presentation, time before diagnosis, and size of the tumors did not differ significantly between recurrent and nonrecurrent cases.
The tumor location for the recurrent cases differed significantly compared to nonrecurrent cases. All 5 of the patients with a recurrence presented with a tumor on the nonglabrous part of the foot. Four patients (80%) had lesions on the dorsal or lateral aspect of the great toe (P=.002), and 1 patient (20%) had a lesion on the low ankle (P=.09)(Table 1). Of the nonrecurrent cases, 1 patient (7%) presented with a tumor on the plantar surface of the great toe (P=.002), 13 patients (93%) presented with tumors on the distal plantar surface of the foot (P=.0002), and 1 patient with a plantar foot tumor (Figure 1) also had verrucous carcinoma on the thumb (Table 1 and Figure 2).

Histopathology—Available pathology slides for recurrent cases of verrucous carcinoma were reviewed alongside representative cases of verrucous carcinomas that did not progress to recurrence. The diagnosis of verrucous carcinoma was confirmed in all cases, with no evidence of conventional squamous cell carcinoma, perineural invasion, extension beyond the dermis, or bone invasion in any case. The median size of the tumors was 4.2 cm and 4 cm for nonrecurrent and recurrent specimens, respectively. Recurrences displayed a trend toward increased depth compared to primary tumors without recurrence (average depth, 5.5 mm vs 3.7 mm); however, this did not reach statistical significance (P=.24). Primary tumors that progressed to recurrence (n=2) displayed similar findings to the other cases, with invasive depths of 3.5 and 5.5 mm, and there was no evidence of conventional squamous cell carcinoma, perineural invasion, or extension beyond the dermis.

Treatment of Nonrecurrent Cases—Of the 16 total cases treated at the University of Michigan, surgery was the primary mode of therapy in every case (Tables 2 and 3). Of the 11 nonrecurrent cases, 7 patients had wide local excision with a dermal regeneration template, and delayed split-thickness graft reconstruction. Three cases had wide local excision with metatarsal resection, dermal regeneration template, and delayed skin grafting. One case had a great toe amputation

Treatment of Recurrent Cases—For the 5 patients with recurrence, surgical margins were not reported in all the cases but ranged from 0.5 to 2 cm (4/5 [80%] reported). On average, follow-up for this group of patients was 29 months, with a range of 12 to 60 months (Table 3).

The first case with a recurrence (patient 12) initially presented with a chronic calluslike growth of the medial ankle. The lesion initially was treated with wide local excision with negative margins. Reconstruction was performed in a staged fashion with use of a dermal regenerative template followed later by split-thickness skin grafting. Tumor recurrence with negative margins occurred 3 times over the next 2 years despite re-resections with negative pathologic margins. Each recurrence presented as graft breakdown and surrounding hyperkeratosis (Figure 3). After the third graft placement failed, the patient elected for a BKA. There has not been recurrence since the BKA after 5 years total follow-up from the time of primary tumor resection. Of note, this was the only patient in our cohort who was immunosuppressed and evaluated for regional nodal involvement by positron emission tomography.

Another patient with recurrence (patient 13) presented with a chronic great toe ulcer of 5 years’ duration that formed on the dorsal aspect of the great toe after a previously excised wart (Figure 4A). This patient underwent mid-proximal metatarsal amputation with 2-cm margins and subsequent skin graft. Pathologic margins were negative. Within 6 months, there was hyperkeratosis and a draining wound (Figure 4B). Biopsy results confirmed recurrent disease that was treated with re-resection, including complete metatarsal amputation with negative margins and skin graft placement. Verrucous carcinoma recurred at the edges of the graft within 8 months, and the patient elected for a BKA. In addition, this patient also presented with a verrucous carcinoma of the contralateral great toe. The tumor presented as a warty ulcer of 4 months’ duration in the setting of osteomyelitis and was resected by great toe amputation that was performed concurrently with the opposite leg BKA; there has been no recurrence. Of note, this was the only patient to have right inguinal sentinel lymph node tissue sampled and HPV testing conducted, which were negative for verrucous carcinoma and high or low strains of HPV.

Another recurrent case (patient 14) presented with a large warty lesion on the dorsal great toe positive for verrucous carcinoma. He underwent a complete great toe amputation with skin graft placement. Verrucous carcinoma recurred on the edges of the graft within 6 months, and the patient was lost to follow-up when a BKA was suggested.
The fourth recurrent case (patient 15) initially had been treated for 1 year as a viral verruca of the dorsal aspect of the great toe. He had an exophytic mass positive for verrucous carcinoma growing on the dorsal aspect of the great toe around the prior excision site. After primary wide excision with negative 1-cm margins and graft placement, the tumor was re-excised twice within the next 2 years with pathologic negative margins. The patient underwent a foot amputation due to a severe osteomyelitis infection at the reconstruction site.
The final recurrent case (patient 16) presented with a mass on the lateral great toe that initially was treated as a viral verruca (for unknown duration) that had begun to ulcerate. The patient underwent wide excision with 1-cm margins and graft placement. Final pathology was consistent with verrucous carcinoma with negative margins. Recurrence occurred within 11 months on the edge of the graft, and a great toe amputation through the metatarsal phalangeal joint was performed.
Comment
Our series of 19 cases of verrucous carcinoma adds to the limited number of reported cases in the literature. We sought to evaluate the potential risk factors for early recurrence. Consistent with prior studies, our series found verrucous carcinoma of the foot to occur most frequently in patients aged 50 to 70 years, predominantly in White men.1 These tumors grew in the setting of chronic inflammation, tissue regeneration, multiple comorbidities, and poor wound hygiene. Misdiagnosis of verrucous carcinoma often leads to ineffective treatments and local invasion of nerves, muscle, and bone tissue.9,15,16 Our case series also clearly demonstrated the diagnostic challenge verrucous carcinoma presents, with an average delay in diagnosis of 5 years; correct diagnosis often did not occur until the tumor was 4 cm in size (average) and more than 50% had chronic ulceration.
The histologic features of the tumors showed striking uniformity. Within the literature, there is confusion regarding the use of the terms verrucous carcinoma and carcinoma (epithelioma) cuniculatum and the possible pathologic differences between the two. The World Health Organization’s classification of skin tumors describes epithelioma cuniculatum as verrucous carcinoma located on the sole of the foot.7 Kubik and Rhatigan6 pointed out that carcinoma cuniculatum does not have a warty or verrucous surface, which is a defining feature of verrucous carcinoma. Multiple authors have further surmised that the deep burrowing sinus tracts of epithelioma cuniculatum are different than those seen in verrucous carcinoma formed by the undulations extending from the papillomatous and verrucous surface.1,6 We did not observe these notable pathologic differences in recurrent or nonrecurrent primary tumors or differences between primary and recurrent cases. Although our cohort was small, the findings suggest that standard histologic features do not predict aggressive behavior in verrucous carcinomas. Furthermore, our observations support a model wherein recurrence is an inherent property of certain verrucous carcinomas rather than a consequence of histologic progression to conventional squamous cell carcinoma. The lack of overt malignant features in such cases underscores the need for distinction of verrucous carcinoma from benign mimics such as viral verruca or reactive epidermal hyperplasia.
Our recurrent cases showed a greater predilection for nonplantar surfaces and the great toe (P=.002). Five of 6 cases on the nonplantar surface—1 on the ankle and 5 on the great toe—recurred despite negative pathologic margins. There was no significant difference in demographics, pathogenesis, tumor size, chronicity, phenotype, or metastatic spread in recurrent and nonrecurrent cases in our cohort.
The tumor has only been described in rare instances at extrapedal cutaneous sites including the hand, scalp, and abdomen.14,17,18 Our series did include a case of synchronous presentation with a verrucous carcinoma on the thumb. Given the rarity of this presentation, thus far there are no data supporting any atypical locations of verrucous carcinoma having greater instances of recurrence. Our recurrent cases displaying atypical location on nonglabrous skin could suggest an underlying pathologic mechanism distinct from tumors on glabrous skin and relevant to increased recurrence risk. Such a mechanism might relate to distinct genetic insults, tumor-microenvironment interactions, or field effects. There are few studies regarding physiologic differences between the plantar surface and the nonglabrous surface and how that influences cancer genesis. Within acral melanoma studies, nonglabrous skin of more sun-exposed surfaces has a higher burden of genetic insults including BRAF mutations.19 Genetic testing of verrucous carcinoma is highly limited, with abnormal expression of the p53 tumor suppressor protein and possible association with several types of HPV. Verrucous carcinoma in general has been found to contain HPV types 6 and 11, nononcogenic forms, and higher risk from HPV types 16 and 18.9,20 However, only a few cases of HPV type 16 as well as 1 case each of HPV type 2 and type 11 have been found within verrucous carcinoma of the foot.21,22 In squamous cell carcinoma of the head and neck, HPV-positive tumors have shown better response to treatment. Further investigation of HPV and genetic contributors in verrucous carcinoma is warranted.
There is notable evidence that surgical resection is the best mode of treatment of verrucous carcinoma.2,3,10,11 Our case series was treated with wide local excision, with partial metatarsal amputation or great toe amputation, in cases with bone invasion or osteomyelitis. Surgical margins were not reported in all the cases but ranged from 0.5 to 2 cm with no significant differences between the recurrent and nonrecurrent groups. After excision, closure was conducted by incorporating primary, secondary, and delayed closure techniques, along with skin grafts for larger defects. Lymph node biopsy traditionally has not been recommended due to reported low metastatic potential. In all 5 recurrent cases, the tumors recurred after multiple attempts at wide excision and greater resection of bone and tissue, with negative margins. The tumors regrew quickly, within months, on the edges of the new graft or in the middle of the graft. The sites of recurrent tumor growth would suggest regrowth in the areas of greatest tissue stress and proliferation. We recommend a low threshold for biopsy and aggressive retreatment in the setting of exophytic growth at reconstruction sites.
Recurrence is uncommon in the setting of verrucous carcinoma, with our series being the first to analyze prognostic factors.3,9,14 Our findings indicate that
- Kao GF, Graham JH, Helwig EB. Carcinoma cuniculatum (verrucous carcinoma of the skin): a clinicopathologic study of 46 cases with ultrastructural observations. Cancer. 1982;49:2395-2403.
- McKee PH, Wilkinson JD, Black M, et al. Carcinoma (epithelioma) cuniculatum: a clinic-pathologic study of nineteen cases and review of the literature. Histopathology. 1981;5:425-436.
- Penera KE, Manji KA, Craig AB, et al. Atypical presentation of verrucous carcinoma: a case study and review of the literature. Foot Ankle Spec. 2013;6:318-322.
- Rosales MA, Martin BR, Armstrong DG, et al. Verrucous hyperplasia: a common and problematic finding in the high-risk diabetic foot. J Am Podiatr Assoc. 2006:4:348-350.
- Noel JC, Peny MO, De Dobbeleer G, et al. p53 Protein overexpression in verrucous carcinoma of the skin. Dermatology. 1996;192:12-15.
- Kubik MJ, Rhatigan RM. Carcinoma cuniculatum: not a verrucous carcinoma. J Cutan Pathol. 2012;39:1083-1087
- Elder D, Massi D, Scolver R, et al. Verrucous squamous cell carcinoma. WHO Classification of Tumours (Medicine). Vol 11. 4th ed. International Agency for Research on Cancer: 2018;35-57.
- Chan MP. Verruciform and condyloma-like squamous proliferations in the anogenital region. Arch Pathol Lab Med. 2019;143:821-831
- Schwartz RA. Verrucous carcinoma of the skin and mucosa. J Am Acad Dermatol. 1995;32:1-21.
- Flynn K, Wiemer D. Treatment of an epithelioma cuniculatum plantare by local excision and a plantar skin flap. J Dermatol Surg Oncol. 1978;4:773-775.
- Spyriounis P, Tentis D, Sparveri I, et al. Plantar epithelioma cuniculatum: a case report with review of the literature. Eur J Plast Surg. 2004;27:253-256.
- Swanson NA, Taylor WB. Plantar verrucous carcinoma: literature review and treatment by the Moh’s chemosurgery technique. Arch Dermatol. 1980;116:794-797.
- Alkalay R, Alcalay J, Shiri J. Plantar verrucous carcinoma treated with Mohs micrographic surgery: a case report and literature review. J Drugs Dermatol. 2006:5:68-73.
- Kotwal M, Poflee S, Bobhate, S. Carcinoma cuniculatum at various anatomical sites. Indian J Dermatol. 2005;50:216-220.
- Nagarajan D, Chandrasekhar M, Jebakumar J, et al. Verrucous carcinoma of foot at an unusual site: lessons to be learnt. South Asian J Cancer. 2017;6:63.
- Pempinello C, Bova A, Pempinello R, et al Verrucous carcinoma of the foot with bone invasion: a case report. Case Rep Oncol Med. 2013;2013:135307.
- Vandeweyer E, Sales F, Deramaecker R. Cutaneous verrucous carcinoma. Br J Plastic Surg. 2001;54:168-170.
- Joybari A, Azadeh P, Honar B. Cutaneous verrucous carcinoma superimposed on chronically inflamed ileostomy site skin. Iran J Pathol. 2018;13:285-288.
- Davis EJ, Johnson DB, Sosman JA, et al. Melanoma: what do all the mutations mean? Cancer. 2018;124:3490-3499.
- Gissmann L, Wolnik L, Ikenberg H, et al. Human papillomavirus types 6 and 11 DNA sequences in genital and laryngeal papillomas and in some cervical cancers. Proc Natl Acad Sci U S A. 1983;80:560-563.
- Knobler RM, Schneider S, Neumann RA, et al. DNA dot-blot hybridization implicates human papillomavirus type 11-DNA in epithelioma cuniculatum. J Med Virol. 1989;29:33-37.
- Noel JC, Peny MO, Detremmerie O, et al. Demonstration of human papillomavirus type 2 in a verrucous carcinoma of the foot. Dermatology. 1993;187:58-61.
- Kao GF, Graham JH, Helwig EB. Carcinoma cuniculatum (verrucous carcinoma of the skin): a clinicopathologic study of 46 cases with ultrastructural observations. Cancer. 1982;49:2395-2403.
- McKee PH, Wilkinson JD, Black M, et al. Carcinoma (epithelioma) cuniculatum: a clinic-pathologic study of nineteen cases and review of the literature. Histopathology. 1981;5:425-436.
- Penera KE, Manji KA, Craig AB, et al. Atypical presentation of verrucous carcinoma: a case study and review of the literature. Foot Ankle Spec. 2013;6:318-322.
- Rosales MA, Martin BR, Armstrong DG, et al. Verrucous hyperplasia: a common and problematic finding in the high-risk diabetic foot. J Am Podiatr Assoc. 2006:4:348-350.
- Noel JC, Peny MO, De Dobbeleer G, et al. p53 Protein overexpression in verrucous carcinoma of the skin. Dermatology. 1996;192:12-15.
- Kubik MJ, Rhatigan RM. Carcinoma cuniculatum: not a verrucous carcinoma. J Cutan Pathol. 2012;39:1083-1087
- Elder D, Massi D, Scolver R, et al. Verrucous squamous cell carcinoma. WHO Classification of Tumours (Medicine). Vol 11. 4th ed. International Agency for Research on Cancer: 2018;35-57.
- Chan MP. Verruciform and condyloma-like squamous proliferations in the anogenital region. Arch Pathol Lab Med. 2019;143:821-831
- Schwartz RA. Verrucous carcinoma of the skin and mucosa. J Am Acad Dermatol. 1995;32:1-21.
- Flynn K, Wiemer D. Treatment of an epithelioma cuniculatum plantare by local excision and a plantar skin flap. J Dermatol Surg Oncol. 1978;4:773-775.
- Spyriounis P, Tentis D, Sparveri I, et al. Plantar epithelioma cuniculatum: a case report with review of the literature. Eur J Plast Surg. 2004;27:253-256.
- Swanson NA, Taylor WB. Plantar verrucous carcinoma: literature review and treatment by the Moh’s chemosurgery technique. Arch Dermatol. 1980;116:794-797.
- Alkalay R, Alcalay J, Shiri J. Plantar verrucous carcinoma treated with Mohs micrographic surgery: a case report and literature review. J Drugs Dermatol. 2006:5:68-73.
- Kotwal M, Poflee S, Bobhate, S. Carcinoma cuniculatum at various anatomical sites. Indian J Dermatol. 2005;50:216-220.
- Nagarajan D, Chandrasekhar M, Jebakumar J, et al. Verrucous carcinoma of foot at an unusual site: lessons to be learnt. South Asian J Cancer. 2017;6:63.
- Pempinello C, Bova A, Pempinello R, et al Verrucous carcinoma of the foot with bone invasion: a case report. Case Rep Oncol Med. 2013;2013:135307.
- Vandeweyer E, Sales F, Deramaecker R. Cutaneous verrucous carcinoma. Br J Plastic Surg. 2001;54:168-170.
- Joybari A, Azadeh P, Honar B. Cutaneous verrucous carcinoma superimposed on chronically inflamed ileostomy site skin. Iran J Pathol. 2018;13:285-288.
- Davis EJ, Johnson DB, Sosman JA, et al. Melanoma: what do all the mutations mean? Cancer. 2018;124:3490-3499.
- Gissmann L, Wolnik L, Ikenberg H, et al. Human papillomavirus types 6 and 11 DNA sequences in genital and laryngeal papillomas and in some cervical cancers. Proc Natl Acad Sci U S A. 1983;80:560-563.
- Knobler RM, Schneider S, Neumann RA, et al. DNA dot-blot hybridization implicates human papillomavirus type 11-DNA in epithelioma cuniculatum. J Med Virol. 1989;29:33-37.
- Noel JC, Peny MO, Detremmerie O, et al. Demonstration of human papillomavirus type 2 in a verrucous carcinoma of the foot. Dermatology. 1993;187:58-61.
Practice Points
- Clinicians should have a high suspicion for verrucous carcinoma in the setting of a chronic ulceration or warty lesion that is resistant to traditional treatment. Early biopsy with tissue collection of the raised ulcer borders and the deep dermis layer of warty lesions is imperative for diagnosis.
- Verrucous carcinoma originating on the nonglabrous surface of the foot may have a higher rate of recurrence often occurring within months of previous treatment. Patients presenting with nonhealing surgical sites in this area should be treated with a high level of suspicion for recurrence.
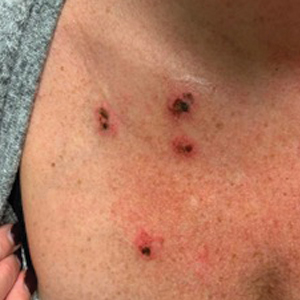
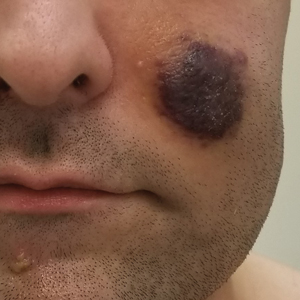
Inpatient Dermatology Consultations for Suspected Skin Cancer: A Retrospective Review
To the Editor:
Dermatologists sometimes are consulted in the inpatient setting to rule out possible skin cancer. This scenario provides an opportunity to facilitate the diagnosis and treatment of cutaneous malignancy, often in patients who might not have sought regular outpatient dermatology care. Few studies have described the outcomes of inpatient biopsies to identify skin cancer.1,2
Seeking to better understand the nature of these patient encounters, we reviewed all consultations at a medical center for which the referring physician suspected skin cancer rather than only those lesions that were biopsied by the dermatologist. We also collected data about subsequent treatment to better understand the outcomes of these patient encounters.
We conducted a retrospective review of inpatient dermatology referrals at an academic-affiliated tertiary medical center. We identified all patients who were provided with an inpatient dermatology consultation for suspected skin cancer or what was identified as a “skin lesion” between July 1, 2013, and July 1, 2019. We collected information on each patient’s sex, age at time of consultation, and race, as well as the specialty of the referring provider, lesion location, maximum diameter of the lesion, whether a biopsy was performed, where the biopsy was performed (inpatient or outpatient setting), clinical diagnosis, histopathologic diagnosis, and subsequent treatment.
The institutional review board at Eastern Virginia Medical School (Norfolk, Virginia) approved this study, and all protocol conformed to the ethical guidelines of the Declaration of Helsinki.
Thirty-eight patients met the inclusion criteria. Their characteristics are listed in the Table. Consultations for possible skin cancer accounted for 4% (38/950) of all inpatient dermatology consultations over the study period. Outcomes of the referrals are shown in the Figure. Consultations were received from 12 different physician specialties.

In the 38 patients, 47 lesions were identified; most (66% [31/47]) were on the head and neck. Twenty of 38 patients were found to have at least 1 biopsy-confirmed cutaneous malignancy (23 total tumors). Of those 23 identified malignancies, 10 were basal cell carcinoma, 11 squamous cell carcinoma, 1 malignant melanoma, and 1 anaplastic T-cell lymphoma. Of note, 17 of 23 (74%) identified cutaneous malignancies were 2.0 cm in diameter at biopsy or larger. Subsequently performed treatments for these patients included wide local excision (n=3), Mohs micrographic surgery (n=5), radiation therapy (n=3), topical fluorouracil (n=1), electrodesiccation and curettage (n=4), and chemotherapy or immunotherapy (n=2). Two patients who were diagnosed with skin cancer died of unrelated causes before treatment was completed.
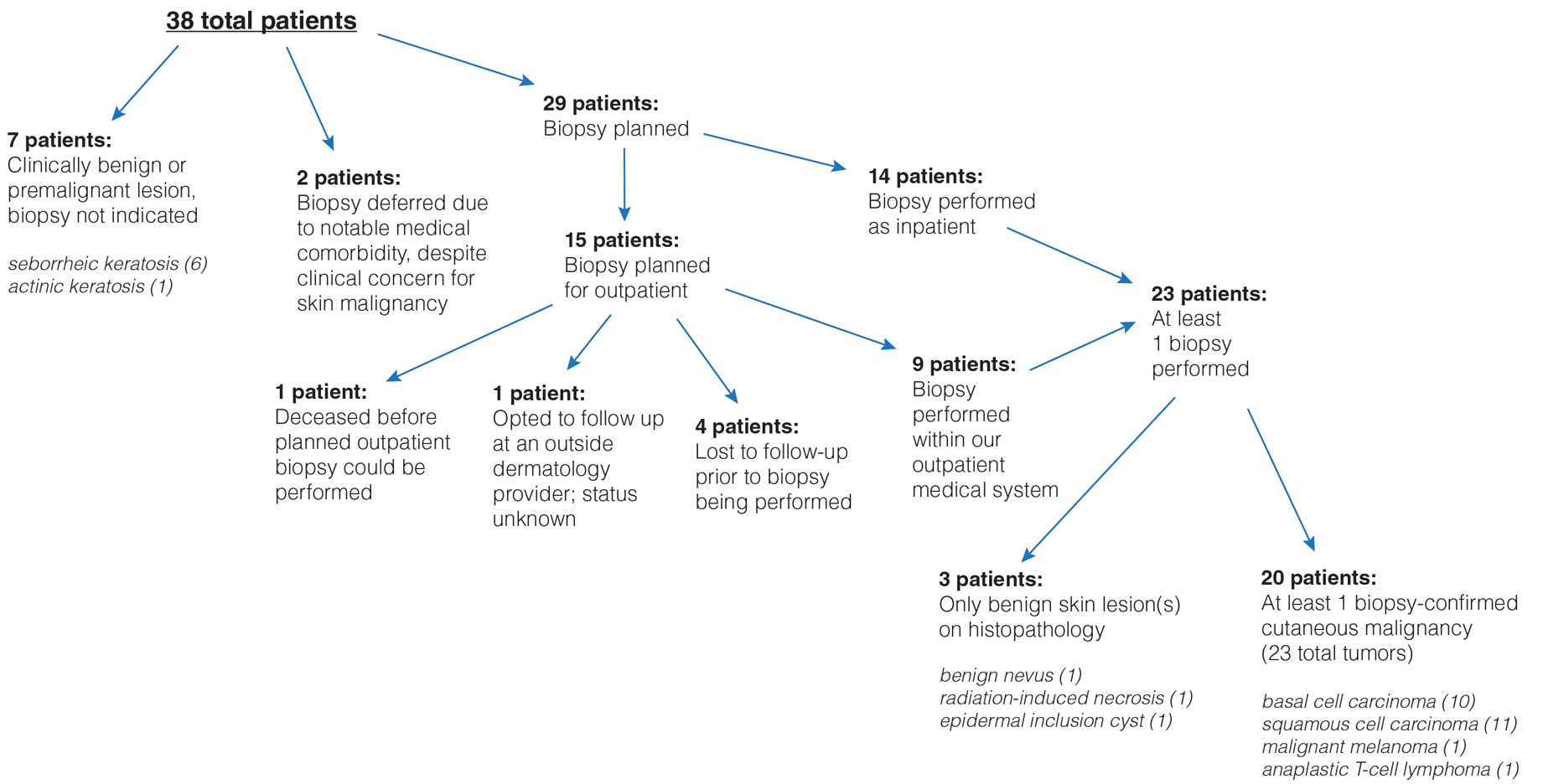
In 10 of 38 patients, only nonmalignant entities were diagnosed, including seborrheic keratosis (n=6), benign melanocytic nevus (n=1), epidermal inclusion cyst (n=1), actinic keratosis (n=1), and radiation-induced necrosis (n=1). Of the 8 remaining patients, 4 were ultimately lost to follow-up before planned outpatient biopsy could be completed; 1 opted to follow up for biopsy at an unaffiliated outpatient dermatology provider. For 2 patients, the decision was made to forgo biopsy despite clinical suspicion of skin cancer because of overall poor health status, and 1 additional patient died before a planned outpatient biopsy could be performed.
In summary, approximately half of the inpatient dermatology consultations for suspected cutaneous malignancy resulted in a diagnosis of skin cancer. The patients in this population were admitted for a range of diagnoses, most unrelated to their cutaneous malignancy, suggesting that the inpatient setting offers the opportunity for physicians in a variety of specialties to help identify skin cancer that might otherwise be unaddressed and then facilitate management, whether ultimately in an inpatient or outpatient setting.
In many of these cases, it might be most appropriate to arrange subsequent outpatient dermatology follow-up after hospitalization, rather than making an inpatient consultation, as these situations usually are nonurgent and not directly related to hospitalization. However, in cases in which the lesion is directly related to admission, the lesion is advanced, there is concern for metastatic disease, or extenuating circumstances make outpatient follow-up difficult, inpatient dermatology consultation may be reasonable. There sometimes can be compelling reasons to expedite diagnosis and treatment as an inpatient.
In hospitalized, medically complex patients, in whom a new cutaneous malignancy is identified, dermatologists should discuss the situation thoughtfully with the patient, the patient’s family (when appropriate), and other physicians on the treatment team to determine the most appropriate course of action. In some cases, the most appropriate course might be to delay biopsy or treatment until the outpatient setting or to even defer further action completely when the prognosis is very limited. Consulting dermatologists must be mindful of patients’ overall medical situation in planning care for a cutaneous malignancy in these inpatient situations.
This study also highlights the surprising number of large-diameter, high-risk tumors identified in these scenarios. Limitations of this study include a relatively small sample size from a single facility that might not be representative of other practice settings and locations. Future multicenter studies could further explore the impact of inpatient dermatologic consultation on the diagnosis and management of skin cancer.
- Bauer J, Maroon M. Dermatology inpatient consultations: a retrospective study. J Am Acad Dermatol. 2010;62:518-519. doi:10.1016/j.jaad.2009.06.030
- Tsai S, Scott JF, Keller JJ, et al. Cutaneous malignancies identified in an inpatient dermatology consultation service. Br J Dermatol. 2017;177:E116-E118. doi:10.1111/bjd.15401
To the Editor:
Dermatologists sometimes are consulted in the inpatient setting to rule out possible skin cancer. This scenario provides an opportunity to facilitate the diagnosis and treatment of cutaneous malignancy, often in patients who might not have sought regular outpatient dermatology care. Few studies have described the outcomes of inpatient biopsies to identify skin cancer.1,2
Seeking to better understand the nature of these patient encounters, we reviewed all consultations at a medical center for which the referring physician suspected skin cancer rather than only those lesions that were biopsied by the dermatologist. We also collected data about subsequent treatment to better understand the outcomes of these patient encounters.
We conducted a retrospective review of inpatient dermatology referrals at an academic-affiliated tertiary medical center. We identified all patients who were provided with an inpatient dermatology consultation for suspected skin cancer or what was identified as a “skin lesion” between July 1, 2013, and July 1, 2019. We collected information on each patient’s sex, age at time of consultation, and race, as well as the specialty of the referring provider, lesion location, maximum diameter of the lesion, whether a biopsy was performed, where the biopsy was performed (inpatient or outpatient setting), clinical diagnosis, histopathologic diagnosis, and subsequent treatment.
The institutional review board at Eastern Virginia Medical School (Norfolk, Virginia) approved this study, and all protocol conformed to the ethical guidelines of the Declaration of Helsinki.
Thirty-eight patients met the inclusion criteria. Their characteristics are listed in the Table. Consultations for possible skin cancer accounted for 4% (38/950) of all inpatient dermatology consultations over the study period. Outcomes of the referrals are shown in the Figure. Consultations were received from 12 different physician specialties.

In the 38 patients, 47 lesions were identified; most (66% [31/47]) were on the head and neck. Twenty of 38 patients were found to have at least 1 biopsy-confirmed cutaneous malignancy (23 total tumors). Of those 23 identified malignancies, 10 were basal cell carcinoma, 11 squamous cell carcinoma, 1 malignant melanoma, and 1 anaplastic T-cell lymphoma. Of note, 17 of 23 (74%) identified cutaneous malignancies were 2.0 cm in diameter at biopsy or larger. Subsequently performed treatments for these patients included wide local excision (n=3), Mohs micrographic surgery (n=5), radiation therapy (n=3), topical fluorouracil (n=1), electrodesiccation and curettage (n=4), and chemotherapy or immunotherapy (n=2). Two patients who were diagnosed with skin cancer died of unrelated causes before treatment was completed.

In 10 of 38 patients, only nonmalignant entities were diagnosed, including seborrheic keratosis (n=6), benign melanocytic nevus (n=1), epidermal inclusion cyst (n=1), actinic keratosis (n=1), and radiation-induced necrosis (n=1). Of the 8 remaining patients, 4 were ultimately lost to follow-up before planned outpatient biopsy could be completed; 1 opted to follow up for biopsy at an unaffiliated outpatient dermatology provider. For 2 patients, the decision was made to forgo biopsy despite clinical suspicion of skin cancer because of overall poor health status, and 1 additional patient died before a planned outpatient biopsy could be performed.
In summary, approximately half of the inpatient dermatology consultations for suspected cutaneous malignancy resulted in a diagnosis of skin cancer. The patients in this population were admitted for a range of diagnoses, most unrelated to their cutaneous malignancy, suggesting that the inpatient setting offers the opportunity for physicians in a variety of specialties to help identify skin cancer that might otherwise be unaddressed and then facilitate management, whether ultimately in an inpatient or outpatient setting.
In many of these cases, it might be most appropriate to arrange subsequent outpatient dermatology follow-up after hospitalization, rather than making an inpatient consultation, as these situations usually are nonurgent and not directly related to hospitalization. However, in cases in which the lesion is directly related to admission, the lesion is advanced, there is concern for metastatic disease, or extenuating circumstances make outpatient follow-up difficult, inpatient dermatology consultation may be reasonable. There sometimes can be compelling reasons to expedite diagnosis and treatment as an inpatient.
In hospitalized, medically complex patients, in whom a new cutaneous malignancy is identified, dermatologists should discuss the situation thoughtfully with the patient, the patient’s family (when appropriate), and other physicians on the treatment team to determine the most appropriate course of action. In some cases, the most appropriate course might be to delay biopsy or treatment until the outpatient setting or to even defer further action completely when the prognosis is very limited. Consulting dermatologists must be mindful of patients’ overall medical situation in planning care for a cutaneous malignancy in these inpatient situations.
This study also highlights the surprising number of large-diameter, high-risk tumors identified in these scenarios. Limitations of this study include a relatively small sample size from a single facility that might not be representative of other practice settings and locations. Future multicenter studies could further explore the impact of inpatient dermatologic consultation on the diagnosis and management of skin cancer.
To the Editor:
Dermatologists sometimes are consulted in the inpatient setting to rule out possible skin cancer. This scenario provides an opportunity to facilitate the diagnosis and treatment of cutaneous malignancy, often in patients who might not have sought regular outpatient dermatology care. Few studies have described the outcomes of inpatient biopsies to identify skin cancer.1,2
Seeking to better understand the nature of these patient encounters, we reviewed all consultations at a medical center for which the referring physician suspected skin cancer rather than only those lesions that were biopsied by the dermatologist. We also collected data about subsequent treatment to better understand the outcomes of these patient encounters.
We conducted a retrospective review of inpatient dermatology referrals at an academic-affiliated tertiary medical center. We identified all patients who were provided with an inpatient dermatology consultation for suspected skin cancer or what was identified as a “skin lesion” between July 1, 2013, and July 1, 2019. We collected information on each patient’s sex, age at time of consultation, and race, as well as the specialty of the referring provider, lesion location, maximum diameter of the lesion, whether a biopsy was performed, where the biopsy was performed (inpatient or outpatient setting), clinical diagnosis, histopathologic diagnosis, and subsequent treatment.
The institutional review board at Eastern Virginia Medical School (Norfolk, Virginia) approved this study, and all protocol conformed to the ethical guidelines of the Declaration of Helsinki.
Thirty-eight patients met the inclusion criteria. Their characteristics are listed in the Table. Consultations for possible skin cancer accounted for 4% (38/950) of all inpatient dermatology consultations over the study period. Outcomes of the referrals are shown in the Figure. Consultations were received from 12 different physician specialties.

In the 38 patients, 47 lesions were identified; most (66% [31/47]) were on the head and neck. Twenty of 38 patients were found to have at least 1 biopsy-confirmed cutaneous malignancy (23 total tumors). Of those 23 identified malignancies, 10 were basal cell carcinoma, 11 squamous cell carcinoma, 1 malignant melanoma, and 1 anaplastic T-cell lymphoma. Of note, 17 of 23 (74%) identified cutaneous malignancies were 2.0 cm in diameter at biopsy or larger. Subsequently performed treatments for these patients included wide local excision (n=3), Mohs micrographic surgery (n=5), radiation therapy (n=3), topical fluorouracil (n=1), electrodesiccation and curettage (n=4), and chemotherapy or immunotherapy (n=2). Two patients who were diagnosed with skin cancer died of unrelated causes before treatment was completed.

In 10 of 38 patients, only nonmalignant entities were diagnosed, including seborrheic keratosis (n=6), benign melanocytic nevus (n=1), epidermal inclusion cyst (n=1), actinic keratosis (n=1), and radiation-induced necrosis (n=1). Of the 8 remaining patients, 4 were ultimately lost to follow-up before planned outpatient biopsy could be completed; 1 opted to follow up for biopsy at an unaffiliated outpatient dermatology provider. For 2 patients, the decision was made to forgo biopsy despite clinical suspicion of skin cancer because of overall poor health status, and 1 additional patient died before a planned outpatient biopsy could be performed.
In summary, approximately half of the inpatient dermatology consultations for suspected cutaneous malignancy resulted in a diagnosis of skin cancer. The patients in this population were admitted for a range of diagnoses, most unrelated to their cutaneous malignancy, suggesting that the inpatient setting offers the opportunity for physicians in a variety of specialties to help identify skin cancer that might otherwise be unaddressed and then facilitate management, whether ultimately in an inpatient or outpatient setting.
In many of these cases, it might be most appropriate to arrange subsequent outpatient dermatology follow-up after hospitalization, rather than making an inpatient consultation, as these situations usually are nonurgent and not directly related to hospitalization. However, in cases in which the lesion is directly related to admission, the lesion is advanced, there is concern for metastatic disease, or extenuating circumstances make outpatient follow-up difficult, inpatient dermatology consultation may be reasonable. There sometimes can be compelling reasons to expedite diagnosis and treatment as an inpatient.
In hospitalized, medically complex patients, in whom a new cutaneous malignancy is identified, dermatologists should discuss the situation thoughtfully with the patient, the patient’s family (when appropriate), and other physicians on the treatment team to determine the most appropriate course of action. In some cases, the most appropriate course might be to delay biopsy or treatment until the outpatient setting or to even defer further action completely when the prognosis is very limited. Consulting dermatologists must be mindful of patients’ overall medical situation in planning care for a cutaneous malignancy in these inpatient situations.
This study also highlights the surprising number of large-diameter, high-risk tumors identified in these scenarios. Limitations of this study include a relatively small sample size from a single facility that might not be representative of other practice settings and locations. Future multicenter studies could further explore the impact of inpatient dermatologic consultation on the diagnosis and management of skin cancer.
- Bauer J, Maroon M. Dermatology inpatient consultations: a retrospective study. J Am Acad Dermatol. 2010;62:518-519. doi:10.1016/j.jaad.2009.06.030
- Tsai S, Scott JF, Keller JJ, et al. Cutaneous malignancies identified in an inpatient dermatology consultation service. Br J Dermatol. 2017;177:E116-E118. doi:10.1111/bjd.15401
- Bauer J, Maroon M. Dermatology inpatient consultations: a retrospective study. J Am Acad Dermatol. 2010;62:518-519. doi:10.1016/j.jaad.2009.06.030
- Tsai S, Scott JF, Keller JJ, et al. Cutaneous malignancies identified in an inpatient dermatology consultation service. Br J Dermatol. 2017;177:E116-E118. doi:10.1111/bjd.15401
Practice Points
- Dermatologists who perform inpatient consultations should be prepared to be consulted for cutaneous malignancies.
- Relatively large skin tumors may be identified, often incidentally, in the inpatient population.
- Careful consideration should be involved when deciding how to diagnose and manage cutaneous malignancies identified in the inpatient setting, taking the overall medical and social context into account.
Necrotic Ulcerations After the Use of an Over-the-counter Mole and Skin Tag Removal Product
To the Editor:
Several mole and skin tag removal products are available online and over the counter (OTC).1 Patients concerned with the cosmetic appearance of nevi may use these products as a do-it-yourself alternative to surgical removal. However, these products have the potential to cause harm.2 Beyond the cosmetic adverse effects of skin necrosis and scar formation, these products can mask premalignant and malignant skin lesions.2 Herein, we describe a patient with a family history of melanoma who developed facial and chest ulcerations with necrosis after applying an OTC mole and skin tag removal product.
A 45-year-old woman with fair skin presented to a clinic with multiple superficial ulcerations measuring approximately 1 cm in diameter with necrotic black bases and erythematous rims on the face, right side of the upper chest, and left earlobe after using the Ariella Mole Corrector and Skin Tag Remover and Repair Lotion Set, an OTC mole and skin tag removal product. The patient reported using the product 24 hours prior for the cosmetic removal of multiple nevi. After applying the product, she observed that it “immediately melted [her] skin” and the areas where the product was applied “turned black.” She reported that the product was applied to the skin for no longer than 30 seconds, after which she developed the necrotic lesions (Figure). After removing the product, she applied an OTC ointment containing bacitracin, neomycin, and polymyxin B to the lesions.
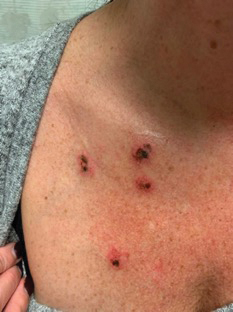
The patient had no history of nonmelanoma skin cancers or atypical nevi. She had a family history of melanoma in her mother and maternal uncle. The treatment plan was aimed primarily at reducing scar formation. We advised frequent application of petroleum-based ointments for moisture and overlying silicone scar tape to protect the area from photodamage and promote wound healing. We further advocated for sun protection and the use of a physical sunscreen on the lesions as they healed. We discussed potential laser-based scar revision options in the future.
With more than 180 reviews on Amazon and almost 70% of these reviews made within the month prior to compiling this manuscript, the Ariella Mole Corrector and Skin Tag Remover and Repair Lotion Set appeared to be popular; however, the product currently is unavailable on Amazon. Testimonials and before-and-after pictures advertising the product show an all-natural, safe, and effective method as an alternative to surgical removal of skin tags and nevi. The product website claims that skin tags and moles will “fall off naturally within 7 to 10 days” and the product can be used for “almost all skin types.” Users are instructed to apply the removal product and wipe it off when the skin surrounding the mole becomes swollen. The product kit also includes a repair lotion, which claims to help heal the skin after scab formation and scar development.
The ingredients listed on the product packaging are salicylic acid 25%, Melaleuca alternifolia (tea tree) leaf oil, propylene glycol, hydroxyethylcellulose, and alcohol. Salicylic acid 25% is a superficial peeling agent that penetrates the epidermis to the dermoepidermal junction. The potential side effects are mild and include superficial desquamation and epidermolysis.3 The Ariella Mole Corrector and Skin Tag Remover and Repair Lotion Set is not regulated by the US Food and Drug Administration and may contain variable concentrations of salicylic acid and other unknown compounds. Higher concentrations of salicylic acid can penetrate the full thickness of the epidermis into the papillary dermis, which can result in postinflammatory pigmentation, superficial infection, scarring, and deeper desquamation and epidermolysis.3 The product website advertises the use of only natural ingredients and an “advanced blend of concentrated natural ingredients contributing a broad spectrum of healing properties” in the formula. Although these claims are attractive to patients seeking alternatives to surgical approaches to nevi removal, the unfounded claims and unregulated ingredients may pose a threat to unsuspecting consumers.
Other OTC and “all-natural” mole removal products previously have been reported to cause harm.2Sanguinaria canadensis, also known as bloodroot, contains an alkaloid compound (sanguinarine) that has been shown to induce mitochondrial apoptosis and activation of Bcl-2 proteins in keratinocytes.4 Some products, such as Wart & Mole Vanish cream, may claim not to contain bloodroot specifically. However, sanguinarine can be extracted from other plants and may be listed as Argemone mexicana, Chelidonium majus, or Macleaya cordata in the ingredients list.5 The use of alternative medicine products such as black or yellow salve for the removal of suspected skin cancers also is not recommended because these escharotic treatments have not been proven safe or effective, and the manufacturing process for these compounds is unregulated.6,7 Self-treatment with alternative remedies for nevi or suspected skin cancers has been associated with progression of disease and even death due to metastatic spread.2
Self-removal of moles is concerning because the nevi are masked by necrotic lesions and can no longer be assessed by dermoscopy or histopathology. Furthermore, the compounds in the Ariella Mole Corrector and Skin Tag Remover and Repair Lotion Set may have unknown effects on the transformation of premalignant cells. They also may mask an underlying process for which clinically proven and effective treatments such as cryotherapy, prescription topical agents, and surgical excision are warranted. Awareness of this product and similar products is important to educate patients on the harmful effects they may cause.
- Clayton R, Turner R. Cosmetic surgery: who needs surgeons when you’ve got creams? Br J Dermatol. 2007;156:1383-1384.
- McAllister JC, Petzold CR, Lio PA. Adverse effects of a mole removal cream. Pediatr Dermatol. 2009;26:628-629.
- Soleymani T, Lanoue J, Rahman Z. A practical approach to chemical peels: a review of fundamentals and step-by-step algorithmic protocol for treatment. J Clin Aesthet Dermatol. 2018;11:21-28.
- Adhami VM, Aziz MH, Mukhatar M, et al. Activation of prodeath Bcl-2 family proteins and mitochondrial apoptosis pathway by sanguinarine in immortalized human HaCaT keratinocytes. Clin Cancer Res. 2003;9:3176-3182.
- Santos AC, Adkilen P. The alkaloids of Argemone mexicana. J Am Chem Soc. 1932;54:2923-2924.
- Osswald SS, Elston DM, Farley MF, et al. Self-treatment of a basal cell carcinoma with “black and yellow salve.” J Am Acad Dermatol. 2005;53:509-511.
- McDaniel S, Goldman GD. Consequences of using escharotic agents as primary treatment for nonmelanoma skin cancer. Arch Dermatol. 2002;138:1593-1596.
To the Editor:
Several mole and skin tag removal products are available online and over the counter (OTC).1 Patients concerned with the cosmetic appearance of nevi may use these products as a do-it-yourself alternative to surgical removal. However, these products have the potential to cause harm.2 Beyond the cosmetic adverse effects of skin necrosis and scar formation, these products can mask premalignant and malignant skin lesions.2 Herein, we describe a patient with a family history of melanoma who developed facial and chest ulcerations with necrosis after applying an OTC mole and skin tag removal product.
A 45-year-old woman with fair skin presented to a clinic with multiple superficial ulcerations measuring approximately 1 cm in diameter with necrotic black bases and erythematous rims on the face, right side of the upper chest, and left earlobe after using the Ariella Mole Corrector and Skin Tag Remover and Repair Lotion Set, an OTC mole and skin tag removal product. The patient reported using the product 24 hours prior for the cosmetic removal of multiple nevi. After applying the product, she observed that it “immediately melted [her] skin” and the areas where the product was applied “turned black.” She reported that the product was applied to the skin for no longer than 30 seconds, after which she developed the necrotic lesions (Figure). After removing the product, she applied an OTC ointment containing bacitracin, neomycin, and polymyxin B to the lesions.

The patient had no history of nonmelanoma skin cancers or atypical nevi. She had a family history of melanoma in her mother and maternal uncle. The treatment plan was aimed primarily at reducing scar formation. We advised frequent application of petroleum-based ointments for moisture and overlying silicone scar tape to protect the area from photodamage and promote wound healing. We further advocated for sun protection and the use of a physical sunscreen on the lesions as they healed. We discussed potential laser-based scar revision options in the future.
With more than 180 reviews on Amazon and almost 70% of these reviews made within the month prior to compiling this manuscript, the Ariella Mole Corrector and Skin Tag Remover and Repair Lotion Set appeared to be popular; however, the product currently is unavailable on Amazon. Testimonials and before-and-after pictures advertising the product show an all-natural, safe, and effective method as an alternative to surgical removal of skin tags and nevi. The product website claims that skin tags and moles will “fall off naturally within 7 to 10 days” and the product can be used for “almost all skin types.” Users are instructed to apply the removal product and wipe it off when the skin surrounding the mole becomes swollen. The product kit also includes a repair lotion, which claims to help heal the skin after scab formation and scar development.
The ingredients listed on the product packaging are salicylic acid 25%, Melaleuca alternifolia (tea tree) leaf oil, propylene glycol, hydroxyethylcellulose, and alcohol. Salicylic acid 25% is a superficial peeling agent that penetrates the epidermis to the dermoepidermal junction. The potential side effects are mild and include superficial desquamation and epidermolysis.3 The Ariella Mole Corrector and Skin Tag Remover and Repair Lotion Set is not regulated by the US Food and Drug Administration and may contain variable concentrations of salicylic acid and other unknown compounds. Higher concentrations of salicylic acid can penetrate the full thickness of the epidermis into the papillary dermis, which can result in postinflammatory pigmentation, superficial infection, scarring, and deeper desquamation and epidermolysis.3 The product website advertises the use of only natural ingredients and an “advanced blend of concentrated natural ingredients contributing a broad spectrum of healing properties” in the formula. Although these claims are attractive to patients seeking alternatives to surgical approaches to nevi removal, the unfounded claims and unregulated ingredients may pose a threat to unsuspecting consumers.
Other OTC and “all-natural” mole removal products previously have been reported to cause harm.2Sanguinaria canadensis, also known as bloodroot, contains an alkaloid compound (sanguinarine) that has been shown to induce mitochondrial apoptosis and activation of Bcl-2 proteins in keratinocytes.4 Some products, such as Wart & Mole Vanish cream, may claim not to contain bloodroot specifically. However, sanguinarine can be extracted from other plants and may be listed as Argemone mexicana, Chelidonium majus, or Macleaya cordata in the ingredients list.5 The use of alternative medicine products such as black or yellow salve for the removal of suspected skin cancers also is not recommended because these escharotic treatments have not been proven safe or effective, and the manufacturing process for these compounds is unregulated.6,7 Self-treatment with alternative remedies for nevi or suspected skin cancers has been associated with progression of disease and even death due to metastatic spread.2
Self-removal of moles is concerning because the nevi are masked by necrotic lesions and can no longer be assessed by dermoscopy or histopathology. Furthermore, the compounds in the Ariella Mole Corrector and Skin Tag Remover and Repair Lotion Set may have unknown effects on the transformation of premalignant cells. They also may mask an underlying process for which clinically proven and effective treatments such as cryotherapy, prescription topical agents, and surgical excision are warranted. Awareness of this product and similar products is important to educate patients on the harmful effects they may cause.
To the Editor:
Several mole and skin tag removal products are available online and over the counter (OTC).1 Patients concerned with the cosmetic appearance of nevi may use these products as a do-it-yourself alternative to surgical removal. However, these products have the potential to cause harm.2 Beyond the cosmetic adverse effects of skin necrosis and scar formation, these products can mask premalignant and malignant skin lesions.2 Herein, we describe a patient with a family history of melanoma who developed facial and chest ulcerations with necrosis after applying an OTC mole and skin tag removal product.
A 45-year-old woman with fair skin presented to a clinic with multiple superficial ulcerations measuring approximately 1 cm in diameter with necrotic black bases and erythematous rims on the face, right side of the upper chest, and left earlobe after using the Ariella Mole Corrector and Skin Tag Remover and Repair Lotion Set, an OTC mole and skin tag removal product. The patient reported using the product 24 hours prior for the cosmetic removal of multiple nevi. After applying the product, she observed that it “immediately melted [her] skin” and the areas where the product was applied “turned black.” She reported that the product was applied to the skin for no longer than 30 seconds, after which she developed the necrotic lesions (Figure). After removing the product, she applied an OTC ointment containing bacitracin, neomycin, and polymyxin B to the lesions.

The patient had no history of nonmelanoma skin cancers or atypical nevi. She had a family history of melanoma in her mother and maternal uncle. The treatment plan was aimed primarily at reducing scar formation. We advised frequent application of petroleum-based ointments for moisture and overlying silicone scar tape to protect the area from photodamage and promote wound healing. We further advocated for sun protection and the use of a physical sunscreen on the lesions as they healed. We discussed potential laser-based scar revision options in the future.
With more than 180 reviews on Amazon and almost 70% of these reviews made within the month prior to compiling this manuscript, the Ariella Mole Corrector and Skin Tag Remover and Repair Lotion Set appeared to be popular; however, the product currently is unavailable on Amazon. Testimonials and before-and-after pictures advertising the product show an all-natural, safe, and effective method as an alternative to surgical removal of skin tags and nevi. The product website claims that skin tags and moles will “fall off naturally within 7 to 10 days” and the product can be used for “almost all skin types.” Users are instructed to apply the removal product and wipe it off when the skin surrounding the mole becomes swollen. The product kit also includes a repair lotion, which claims to help heal the skin after scab formation and scar development.
The ingredients listed on the product packaging are salicylic acid 25%, Melaleuca alternifolia (tea tree) leaf oil, propylene glycol, hydroxyethylcellulose, and alcohol. Salicylic acid 25% is a superficial peeling agent that penetrates the epidermis to the dermoepidermal junction. The potential side effects are mild and include superficial desquamation and epidermolysis.3 The Ariella Mole Corrector and Skin Tag Remover and Repair Lotion Set is not regulated by the US Food and Drug Administration and may contain variable concentrations of salicylic acid and other unknown compounds. Higher concentrations of salicylic acid can penetrate the full thickness of the epidermis into the papillary dermis, which can result in postinflammatory pigmentation, superficial infection, scarring, and deeper desquamation and epidermolysis.3 The product website advertises the use of only natural ingredients and an “advanced blend of concentrated natural ingredients contributing a broad spectrum of healing properties” in the formula. Although these claims are attractive to patients seeking alternatives to surgical approaches to nevi removal, the unfounded claims and unregulated ingredients may pose a threat to unsuspecting consumers.
Other OTC and “all-natural” mole removal products previously have been reported to cause harm.2Sanguinaria canadensis, also known as bloodroot, contains an alkaloid compound (sanguinarine) that has been shown to induce mitochondrial apoptosis and activation of Bcl-2 proteins in keratinocytes.4 Some products, such as Wart & Mole Vanish cream, may claim not to contain bloodroot specifically. However, sanguinarine can be extracted from other plants and may be listed as Argemone mexicana, Chelidonium majus, or Macleaya cordata in the ingredients list.5 The use of alternative medicine products such as black or yellow salve for the removal of suspected skin cancers also is not recommended because these escharotic treatments have not been proven safe or effective, and the manufacturing process for these compounds is unregulated.6,7 Self-treatment with alternative remedies for nevi or suspected skin cancers has been associated with progression of disease and even death due to metastatic spread.2
Self-removal of moles is concerning because the nevi are masked by necrotic lesions and can no longer be assessed by dermoscopy or histopathology. Furthermore, the compounds in the Ariella Mole Corrector and Skin Tag Remover and Repair Lotion Set may have unknown effects on the transformation of premalignant cells. They also may mask an underlying process for which clinically proven and effective treatments such as cryotherapy, prescription topical agents, and surgical excision are warranted. Awareness of this product and similar products is important to educate patients on the harmful effects they may cause.
- Clayton R, Turner R. Cosmetic surgery: who needs surgeons when you’ve got creams? Br J Dermatol. 2007;156:1383-1384.
- McAllister JC, Petzold CR, Lio PA. Adverse effects of a mole removal cream. Pediatr Dermatol. 2009;26:628-629.
- Soleymani T, Lanoue J, Rahman Z. A practical approach to chemical peels: a review of fundamentals and step-by-step algorithmic protocol for treatment. J Clin Aesthet Dermatol. 2018;11:21-28.
- Adhami VM, Aziz MH, Mukhatar M, et al. Activation of prodeath Bcl-2 family proteins and mitochondrial apoptosis pathway by sanguinarine in immortalized human HaCaT keratinocytes. Clin Cancer Res. 2003;9:3176-3182.
- Santos AC, Adkilen P. The alkaloids of Argemone mexicana. J Am Chem Soc. 1932;54:2923-2924.
- Osswald SS, Elston DM, Farley MF, et al. Self-treatment of a basal cell carcinoma with “black and yellow salve.” J Am Acad Dermatol. 2005;53:509-511.
- McDaniel S, Goldman GD. Consequences of using escharotic agents as primary treatment for nonmelanoma skin cancer. Arch Dermatol. 2002;138:1593-1596.
- Clayton R, Turner R. Cosmetic surgery: who needs surgeons when you’ve got creams? Br J Dermatol. 2007;156:1383-1384.
- McAllister JC, Petzold CR, Lio PA. Adverse effects of a mole removal cream. Pediatr Dermatol. 2009;26:628-629.
- Soleymani T, Lanoue J, Rahman Z. A practical approach to chemical peels: a review of fundamentals and step-by-step algorithmic protocol for treatment. J Clin Aesthet Dermatol. 2018;11:21-28.
- Adhami VM, Aziz MH, Mukhatar M, et al. Activation of prodeath Bcl-2 family proteins and mitochondrial apoptosis pathway by sanguinarine in immortalized human HaCaT keratinocytes. Clin Cancer Res. 2003;9:3176-3182.
- Santos AC, Adkilen P. The alkaloids of Argemone mexicana. J Am Chem Soc. 1932;54:2923-2924.
- Osswald SS, Elston DM, Farley MF, et al. Self-treatment of a basal cell carcinoma with “black and yellow salve.” J Am Acad Dermatol. 2005;53:509-511.
- McDaniel S, Goldman GD. Consequences of using escharotic agents as primary treatment for nonmelanoma skin cancer. Arch Dermatol. 2002;138:1593-1596.
Practice Point
- Self-administered mole and skin tag removal products are rising in popularity, but unregulated ingredients in over-the-counter products that are not approved by the US Food and Drug Administration may mask underlying transformation of atypical nevi.
At-Home Treatment of Pigmented Lesions With a Zinc Chloride Preparation
To the Editor:
Zinc chloride originally was used by Dr. Frederic Mohs as an in vivo tissue fixative during the early phases of Mohs micrographic surgery.1 Although this technique has since been replaced with fresh frozen tissue fixation, zinc chloride still is found in topical preparations that are readily available to patients. Specifically, black salve describes variably composed topical preparations that share the common ingredients zinc chloride and Sanguinaria canadensis (bloodroot).2 Patients self-treat with these unregulated compounds, but the majority do not have their lesions evaluated by a clinician prior to use and are unaware of the potential risks.3-5 Products containing zinc chloride and S canadensis that are not marketed as black salve present a new problem for the dermatology community.
A 73-year-old man presented to our dermatology clinic for the focused evaluation of scaly lesions on the face and nose. At this visit, it was recommended he undergo a total-body skin examination for skin cancer screening given his age and substantial photodamage.
Physical examination revealed more than 20 superficial, 3- to 10-mm scars predominantly over the trunk. One scar over the left mid-back had a large, 1.2-cm peripheral rim of dark brown pigment that was clinically concerning for a melanocytic neoplasm. Shave removal of this lesion was performed. Histologic examination showed melanoma in situ with a central scar. The central scar spanned the depth of the dermis, and the melanocytic component was absent in this area, raising the question if prior biopsy or treatment had been performed on this lesion. During a discussion of the results with the patient, he was questioned about prior biopsy or treatment of this lesion. He reported prior use of a topical all-natural cream containing zinc chloride and S canadensis that he purchased online, which he had used to treat this lesion as well as numerous presumed moles.
The trend of at-home mole removal products containing the traditional ingredients in black salve seems to be one of rapidly shifting product availability as well as a departure from marketing items as black salve. Many prior black salve products are no longer available.4 The product that our patient used is a topical cream marketed as a treatment for moles and skin tags.6 Despite not being marketed as black salve, it does contain zinc chloride and S canadensis. The product’s website highlights these ingredients as being a safe and effective treatment for mole removal, with claims that the product will remove the mole or skin tag without irritating the surrounding skin and can be safely used anywhere on the body without scarring.6 A Google search at the time this article was written using the term skin tag remover revealed similar products marketed as all-natural “skin tag remover and mole corrector creams.” These similar products containing zinc chloride and S canadensis were available in the United States at the time of our initial research but have since been removed and only are available outside of the United States.7
Prior reports of melanoma masked by zinc chloride and S canadensis described the use of topical agents marketed as black salve. This new wave of products marketed as all-natural creams makes continued education on the available products and their associated risks necessary for clinicians. The lack of US Food and Drug Administration oversight for these products and their frequent introduction and discontinuation in the market makes keeping updated even more challenging. Because many patients self-treat without prior evaluation by a health care provider, treatment with these products can lead to a delay in diagnosis or inaccurate staging due to scars from the chemical destruction, both of which may have occurred in our patient.5 Until these products become regulated by the US Food and Drug Administration, it is imperative that clinicians continue to educate their patients on the lack of documented benefit and clear risks of their use as well as remain up-to-date on product trends.
- Cohen DK. Mohs micrographic surgery: past, present, and future. Dermatol Surg. 2019;45:329-339. doi:10.1097/DSS.0000000000001701
- Eastman KL. A review of topical corrosive black salve. J Altern Complement Med. 2014;20:284-289. doi:10.1089/acm.2012.0377
- Sivyer GW, Rosendahl C. Application of black salve to a thin melanoma that subsequently progressed to metastatic melanoma: a case study. Dermatol Pract Concept. 2014;4:77-80. doi:10.5826/dpc.0403a16
- McDaniel S. Consequences of using escharotic agents as primary treatment for nonmelanoma skin cancer. Arch Dermatol. 2002;138:1593-1596.
- Clark JJ. Community perceptions about the use of black salve. J Am Acad Dermatol. 2016;74:1021-1023. doi:10.1016/j.jaad.2015.10.016
- Skinprov Cream. Skinprov. Accessed February 22, 2022. https://skinprov.net
- HaloDerm. HaloDerm Inc. Accessed February 22, 2022. https://haloderm.com/
To the Editor:
Zinc chloride originally was used by Dr. Frederic Mohs as an in vivo tissue fixative during the early phases of Mohs micrographic surgery.1 Although this technique has since been replaced with fresh frozen tissue fixation, zinc chloride still is found in topical preparations that are readily available to patients. Specifically, black salve describes variably composed topical preparations that share the common ingredients zinc chloride and Sanguinaria canadensis (bloodroot).2 Patients self-treat with these unregulated compounds, but the majority do not have their lesions evaluated by a clinician prior to use and are unaware of the potential risks.3-5 Products containing zinc chloride and S canadensis that are not marketed as black salve present a new problem for the dermatology community.
A 73-year-old man presented to our dermatology clinic for the focused evaluation of scaly lesions on the face and nose. At this visit, it was recommended he undergo a total-body skin examination for skin cancer screening given his age and substantial photodamage.
Physical examination revealed more than 20 superficial, 3- to 10-mm scars predominantly over the trunk. One scar over the left mid-back had a large, 1.2-cm peripheral rim of dark brown pigment that was clinically concerning for a melanocytic neoplasm. Shave removal of this lesion was performed. Histologic examination showed melanoma in situ with a central scar. The central scar spanned the depth of the dermis, and the melanocytic component was absent in this area, raising the question if prior biopsy or treatment had been performed on this lesion. During a discussion of the results with the patient, he was questioned about prior biopsy or treatment of this lesion. He reported prior use of a topical all-natural cream containing zinc chloride and S canadensis that he purchased online, which he had used to treat this lesion as well as numerous presumed moles.
The trend of at-home mole removal products containing the traditional ingredients in black salve seems to be one of rapidly shifting product availability as well as a departure from marketing items as black salve. Many prior black salve products are no longer available.4 The product that our patient used is a topical cream marketed as a treatment for moles and skin tags.6 Despite not being marketed as black salve, it does contain zinc chloride and S canadensis. The product’s website highlights these ingredients as being a safe and effective treatment for mole removal, with claims that the product will remove the mole or skin tag without irritating the surrounding skin and can be safely used anywhere on the body without scarring.6 A Google search at the time this article was written using the term skin tag remover revealed similar products marketed as all-natural “skin tag remover and mole corrector creams.” These similar products containing zinc chloride and S canadensis were available in the United States at the time of our initial research but have since been removed and only are available outside of the United States.7
Prior reports of melanoma masked by zinc chloride and S canadensis described the use of topical agents marketed as black salve. This new wave of products marketed as all-natural creams makes continued education on the available products and their associated risks necessary for clinicians. The lack of US Food and Drug Administration oversight for these products and their frequent introduction and discontinuation in the market makes keeping updated even more challenging. Because many patients self-treat without prior evaluation by a health care provider, treatment with these products can lead to a delay in diagnosis or inaccurate staging due to scars from the chemical destruction, both of which may have occurred in our patient.5 Until these products become regulated by the US Food and Drug Administration, it is imperative that clinicians continue to educate their patients on the lack of documented benefit and clear risks of their use as well as remain up-to-date on product trends.
To the Editor:
Zinc chloride originally was used by Dr. Frederic Mohs as an in vivo tissue fixative during the early phases of Mohs micrographic surgery.1 Although this technique has since been replaced with fresh frozen tissue fixation, zinc chloride still is found in topical preparations that are readily available to patients. Specifically, black salve describes variably composed topical preparations that share the common ingredients zinc chloride and Sanguinaria canadensis (bloodroot).2 Patients self-treat with these unregulated compounds, but the majority do not have their lesions evaluated by a clinician prior to use and are unaware of the potential risks.3-5 Products containing zinc chloride and S canadensis that are not marketed as black salve present a new problem for the dermatology community.
A 73-year-old man presented to our dermatology clinic for the focused evaluation of scaly lesions on the face and nose. At this visit, it was recommended he undergo a total-body skin examination for skin cancer screening given his age and substantial photodamage.
Physical examination revealed more than 20 superficial, 3- to 10-mm scars predominantly over the trunk. One scar over the left mid-back had a large, 1.2-cm peripheral rim of dark brown pigment that was clinically concerning for a melanocytic neoplasm. Shave removal of this lesion was performed. Histologic examination showed melanoma in situ with a central scar. The central scar spanned the depth of the dermis, and the melanocytic component was absent in this area, raising the question if prior biopsy or treatment had been performed on this lesion. During a discussion of the results with the patient, he was questioned about prior biopsy or treatment of this lesion. He reported prior use of a topical all-natural cream containing zinc chloride and S canadensis that he purchased online, which he had used to treat this lesion as well as numerous presumed moles.
The trend of at-home mole removal products containing the traditional ingredients in black salve seems to be one of rapidly shifting product availability as well as a departure from marketing items as black salve. Many prior black salve products are no longer available.4 The product that our patient used is a topical cream marketed as a treatment for moles and skin tags.6 Despite not being marketed as black salve, it does contain zinc chloride and S canadensis. The product’s website highlights these ingredients as being a safe and effective treatment for mole removal, with claims that the product will remove the mole or skin tag without irritating the surrounding skin and can be safely used anywhere on the body without scarring.6 A Google search at the time this article was written using the term skin tag remover revealed similar products marketed as all-natural “skin tag remover and mole corrector creams.” These similar products containing zinc chloride and S canadensis were available in the United States at the time of our initial research but have since been removed and only are available outside of the United States.7
Prior reports of melanoma masked by zinc chloride and S canadensis described the use of topical agents marketed as black salve. This new wave of products marketed as all-natural creams makes continued education on the available products and their associated risks necessary for clinicians. The lack of US Food and Drug Administration oversight for these products and their frequent introduction and discontinuation in the market makes keeping updated even more challenging. Because many patients self-treat without prior evaluation by a health care provider, treatment with these products can lead to a delay in diagnosis or inaccurate staging due to scars from the chemical destruction, both of which may have occurred in our patient.5 Until these products become regulated by the US Food and Drug Administration, it is imperative that clinicians continue to educate their patients on the lack of documented benefit and clear risks of their use as well as remain up-to-date on product trends.
- Cohen DK. Mohs micrographic surgery: past, present, and future. Dermatol Surg. 2019;45:329-339. doi:10.1097/DSS.0000000000001701
- Eastman KL. A review of topical corrosive black salve. J Altern Complement Med. 2014;20:284-289. doi:10.1089/acm.2012.0377
- Sivyer GW, Rosendahl C. Application of black salve to a thin melanoma that subsequently progressed to metastatic melanoma: a case study. Dermatol Pract Concept. 2014;4:77-80. doi:10.5826/dpc.0403a16
- McDaniel S. Consequences of using escharotic agents as primary treatment for nonmelanoma skin cancer. Arch Dermatol. 2002;138:1593-1596.
- Clark JJ. Community perceptions about the use of black salve. J Am Acad Dermatol. 2016;74:1021-1023. doi:10.1016/j.jaad.2015.10.016
- Skinprov Cream. Skinprov. Accessed February 22, 2022. https://skinprov.net
- HaloDerm. HaloDerm Inc. Accessed February 22, 2022. https://haloderm.com/
- Cohen DK. Mohs micrographic surgery: past, present, and future. Dermatol Surg. 2019;45:329-339. doi:10.1097/DSS.0000000000001701
- Eastman KL. A review of topical corrosive black salve. J Altern Complement Med. 2014;20:284-289. doi:10.1089/acm.2012.0377
- Sivyer GW, Rosendahl C. Application of black salve to a thin melanoma that subsequently progressed to metastatic melanoma: a case study. Dermatol Pract Concept. 2014;4:77-80. doi:10.5826/dpc.0403a16
- McDaniel S. Consequences of using escharotic agents as primary treatment for nonmelanoma skin cancer. Arch Dermatol. 2002;138:1593-1596.
- Clark JJ. Community perceptions about the use of black salve. J Am Acad Dermatol. 2016;74:1021-1023. doi:10.1016/j.jaad.2015.10.016
- Skinprov Cream. Skinprov. Accessed February 22, 2022. https://skinprov.net
- HaloDerm. HaloDerm Inc. Accessed February 22, 2022. https://haloderm.com/
Practice Points
- Zinc chloride preparations are readily available over the counter and unregulated.
- Patients may attempt to self-treat pigmented lesions based on claims they see online.
- When asking patients about prior treatments, it may be prudent to specifically ask about over-the-counter products and their ingredients.
Survey: Artificial intelligence finds support among dermatologists
according to the results of a small survey.
Just 9% of the 90 respondents acknowledged that they have used AI in their practices, while 81% said they had not, and 10% weren’t sure or didn’t know. Despite that lack of familiarity, however, “many embrace the potential positive benefits, such as reducing misdiagnoses” and a majority (94.5%) “would use it at least in certain scenarios,” Vishal A. Patel, MD, and associates said in the Journal of Drugs in Dermatology.
Dermatologists aged 40 years and under were more likely to have used AI previously: 15% reported previous experience, compared with 4% of those over age 40 – but the difference in “age did not have a significant effect on perception of AI,” the investigators noted, adding that most of the dermatologists over 40 believe “that AI would be most beneficial and used for detection of malignant skin lesions.”
The survey also asked about ways the respondents would use AI to help their patients. Almost two-thirds of respondents (66%) chose analysis and management of electronic health records “for research purposes to improve patient outcomes,” compared with 56% who chose identifying unknown/screening skin lesions “with a list of differential diagnoses,” 32% who chose telemedicine, and 26% who chose primary surveys of skin, said Dr. Patel, director of cutaneous oncology at the George Washington University Cancer Center in Washington, and coauthors.
The respondents were fairly evenly split when asked about the possible impact of nondermatologists using AI in the near future to detect skin lesions, such as melanomas, on the need for dermatologists. Just over a quarter said that the need for dermatologists will be decreased all (about 4.4%) or some (about 21.1%) of the time, and 24.4% said that the need will be increased, with the largest share (39.9%) of respondents choosing the middle ground: neither increased or decreased, the investigators reported.
The survey form was emailed to 850 members of the Orlando Dermatology, Aesthetic & Surgical Conference listserv, with responses accepted from April 13 to May 14, 2021. The investigators noted that the response rate was low enough to be a limiting factor, making selection bias “by those with a particular interest in the topic” a possibility.
No funding sources for the study were disclosed. Dr. Patel disclosed that he is chief medical officer for Lazarus AI, the other authors had no disclosures listed.
according to the results of a small survey.
Just 9% of the 90 respondents acknowledged that they have used AI in their practices, while 81% said they had not, and 10% weren’t sure or didn’t know. Despite that lack of familiarity, however, “many embrace the potential positive benefits, such as reducing misdiagnoses” and a majority (94.5%) “would use it at least in certain scenarios,” Vishal A. Patel, MD, and associates said in the Journal of Drugs in Dermatology.
Dermatologists aged 40 years and under were more likely to have used AI previously: 15% reported previous experience, compared with 4% of those over age 40 – but the difference in “age did not have a significant effect on perception of AI,” the investigators noted, adding that most of the dermatologists over 40 believe “that AI would be most beneficial and used for detection of malignant skin lesions.”
The survey also asked about ways the respondents would use AI to help their patients. Almost two-thirds of respondents (66%) chose analysis and management of electronic health records “for research purposes to improve patient outcomes,” compared with 56% who chose identifying unknown/screening skin lesions “with a list of differential diagnoses,” 32% who chose telemedicine, and 26% who chose primary surveys of skin, said Dr. Patel, director of cutaneous oncology at the George Washington University Cancer Center in Washington, and coauthors.
The respondents were fairly evenly split when asked about the possible impact of nondermatologists using AI in the near future to detect skin lesions, such as melanomas, on the need for dermatologists. Just over a quarter said that the need for dermatologists will be decreased all (about 4.4%) or some (about 21.1%) of the time, and 24.4% said that the need will be increased, with the largest share (39.9%) of respondents choosing the middle ground: neither increased or decreased, the investigators reported.
The survey form was emailed to 850 members of the Orlando Dermatology, Aesthetic & Surgical Conference listserv, with responses accepted from April 13 to May 14, 2021. The investigators noted that the response rate was low enough to be a limiting factor, making selection bias “by those with a particular interest in the topic” a possibility.
No funding sources for the study were disclosed. Dr. Patel disclosed that he is chief medical officer for Lazarus AI, the other authors had no disclosures listed.
according to the results of a small survey.
Just 9% of the 90 respondents acknowledged that they have used AI in their practices, while 81% said they had not, and 10% weren’t sure or didn’t know. Despite that lack of familiarity, however, “many embrace the potential positive benefits, such as reducing misdiagnoses” and a majority (94.5%) “would use it at least in certain scenarios,” Vishal A. Patel, MD, and associates said in the Journal of Drugs in Dermatology.
Dermatologists aged 40 years and under were more likely to have used AI previously: 15% reported previous experience, compared with 4% of those over age 40 – but the difference in “age did not have a significant effect on perception of AI,” the investigators noted, adding that most of the dermatologists over 40 believe “that AI would be most beneficial and used for detection of malignant skin lesions.”
The survey also asked about ways the respondents would use AI to help their patients. Almost two-thirds of respondents (66%) chose analysis and management of electronic health records “for research purposes to improve patient outcomes,” compared with 56% who chose identifying unknown/screening skin lesions “with a list of differential diagnoses,” 32% who chose telemedicine, and 26% who chose primary surveys of skin, said Dr. Patel, director of cutaneous oncology at the George Washington University Cancer Center in Washington, and coauthors.
The respondents were fairly evenly split when asked about the possible impact of nondermatologists using AI in the near future to detect skin lesions, such as melanomas, on the need for dermatologists. Just over a quarter said that the need for dermatologists will be decreased all (about 4.4%) or some (about 21.1%) of the time, and 24.4% said that the need will be increased, with the largest share (39.9%) of respondents choosing the middle ground: neither increased or decreased, the investigators reported.
The survey form was emailed to 850 members of the Orlando Dermatology, Aesthetic & Surgical Conference listserv, with responses accepted from April 13 to May 14, 2021. The investigators noted that the response rate was low enough to be a limiting factor, making selection bias “by those with a particular interest in the topic” a possibility.
No funding sources for the study were disclosed. Dr. Patel disclosed that he is chief medical officer for Lazarus AI, the other authors had no disclosures listed.
FROM JOURNAL OF DRUGS IN DERMATOLOGY
Why dermatologists should support artificial intelligence efforts
“AI is meant to be an enhancement strategy, a support tool to improve our diagnostic abilities,” Dr. Patel, a Mohs surgeon who is director of cutaneous oncology at the George Washington University Cancer Center, Washington, said during the ODAC Dermatology, Aesthetic & Surgical Conference. “Dermatologists should embrace AI and drive how it is utilized – be the captain of the plane (technology) and the passenger (patient). If we’re not in the forefront of the plane, we’re not to be able to dictate which way we are going with this.”
In 2019, a group of German researchers found that AI can improve accuracy and efficiency of specialists in classifying skin cancer based on dermoscopic images. “I really do believe this is going to be the future,” said Dr. Patel, who was not involved with the study. “Current research involves using supervised learning on known outcomes to determine inputs to predict them. In dermatology, think of identifying melanoma from clinical or dermoscopic images or predicting metastasis risk from digitized pathology slides.”
However, there are currently no universal guidelines on how large an AI dataset needs to be to yield accurate results. In the dermatology literature, most AI datasets range between 600 and 14,000 examples, Dr. Patel said, with a large study-specific variation in performance. “Misleading results can result from unanticipated training errors,” he said.
“The AI network may learn its intended task or an unrelated situational cue. For example, you can use great images to predict melanoma, but you may have an unintended poor outcome related to images that have, say, a ruler inside of them clustered within the melanoma diagnoses.” And unbeknown to the system’s developer, “the algorithm picks up that the ruler is predictive of an image being a melanoma and not the pigmented lesion itself.” In other words, the algorithm is only as good as the dataset being used, he said. “This is the key element, to ask what the dataset is that’s training the tool that you may one day use.”
Convolutional neural network
In 2017, a seminal study published in Nature showed that for classification of melanoma and epidermal lesions, a type of AI used in image processing known as a convolutional neural network (CNN) was on par with dermatologists and outperformed the average. For epidermal lesions, the network was one standard deviation higher above the average for dermatologists, while for melanocytic lesions, the network was just below one standard deviation above the average of the dermatologists. A CNN “clearly can perform well because it works on a different level than how our brains work,” Dr. Patel said.
In a separate study, a CNN trained to recognize melanoma in dermoscopic images was compared to 58 international dermatologists with varying levels of dermoscopy experience; 29% were “beginners,” with less than 2 years of experience; 19% were “skilled,” with 2-5 years of experience; and 52% were “experts,” with at least 5 years of experience. The analysis consisted of two experiments: In level I, dermatologists classified lesions based on dermoscopy only. In level II, dermatologists were provided dermoscopy, clinical images, and additional clinical information, while the CNN was trained on images only. The researchers found that most dermatologists were outperformed by the CNN. “Physicians of all different levels of training and experience may benefit from assistance by a CNN’s image classification,” they concluded.
Gene expression profiling
Another aspect of AI is gene expression profiling (GEP), which Dr. Patel defined as the evaluation of frequency and intensity of genetic activity at once to create a global picture of cellular function. “It’s AI that uses machine learning to evaluate genetic expression to assess lesion behavior,” he explained.
One GEP test on the market is the Pigmented Lesion Assay (PLA) from DermTech, a noninvasive test that looks at the expression of two genes to predict if a lesion is malignant or not. “Based on their validation set, they have shown some impressive numbers,” with sensitivities above 90%, and published registry data that have shown higher sensitivities “and even specificities above 90%,” he said.
“On the surface, it looks like this would be a useful test,” Dr. Patel said. A study published in 2021 looked at the evidence of applying real-world evidence with this test to see if results held up. Based on the authors’ analysis, he noted, “you would need a sensitivity and specificity of 95% to yield a positivity rate of 9.5% for the PLA test, which is what has been reported in real-world use. So, there’s a disconnect somewhere and we are not quite there yet.” That may be a result of the dataset itself not being as uniform between the validation and the training datasets, he continued. Also, the expression of certain genes is different “if you don’t have a clean input variable” of what the test is being used for, he added.
“If you’re not mirroring the dataset, you’re not going to get clean data,” he said. “So, if you’re using this on younger patients or for sun-damaged lesional skin or nonmelanocytic lesions around sun-damaged areas, there are variable expressions that may not be accurately captured by that algorithm. This might help explain the real-world variation that we’re seeing.”
Another GEP test in use is the 31-Gene Expression Profile Test for Melanoma, which evaluates gene expressions in melanoma tumors and what the behavior of that tumor may be. The test has been available for more than a decade “and there is a lot of speculation about its use,” Dr. Patel said. “A recent paper attempted to come up with an algorithm of how to use this, but there’s a lot of concern about the endpoints of what changes in management might result from this test. That is what we need to be thinking about. There’s a lot of back and forth about this.”
In 2020, authors of a consensus statement on prognostic GEP in cutaneous melanoma concluded that before GEP testing is routinely used, the clinical benefit in the management of patients with melanoma should be established through further clinical investigation. Dr. Patel recommended the accompanying editorial on GEP in melanoma, written by Hensin Tsao, MD, PhD, and Warren H. Chan, MS, in JAMA Dermatology.
In Dr. Patel’s opinion, T1a melanomas (0.8 mm, nonulcerated) do not need routine GEP, but the GEP test may be useful in cases that are in the “gray zone,” such as those with T1b or some borderline T2a melanomas (> 0.8 mm, < 1.2mm, nonulcerated, but with high mitosis, etc.); patients with unique coexisting conditions such as pregnancy, and patients who may not tolerate sentinel lymph node biopsy (SLNB) or adjuvant therapy.
Echoing sentiments expressed in the JAMA Dermatology editorial, he advised dermatologists to “remember your training and know the data. GEP predicting survival is not the same as SLNB positive rate. GEP should not replace standard guidelines in T2a and higher melanomas. Nodal sampling remains part of all major guidelines and determines adjuvant therapy.”
He cited the characterization of GEP in the editorial as “a powerful technology” that heralds the age of personalized medicine, but it is not ready for ubiquitous use. Prospective studies and time will lead to highly accurate tools.”
Dr. Patel disclosed that he is chief medical officer for Lazarus AI.
“AI is meant to be an enhancement strategy, a support tool to improve our diagnostic abilities,” Dr. Patel, a Mohs surgeon who is director of cutaneous oncology at the George Washington University Cancer Center, Washington, said during the ODAC Dermatology, Aesthetic & Surgical Conference. “Dermatologists should embrace AI and drive how it is utilized – be the captain of the plane (technology) and the passenger (patient). If we’re not in the forefront of the plane, we’re not to be able to dictate which way we are going with this.”
In 2019, a group of German researchers found that AI can improve accuracy and efficiency of specialists in classifying skin cancer based on dermoscopic images. “I really do believe this is going to be the future,” said Dr. Patel, who was not involved with the study. “Current research involves using supervised learning on known outcomes to determine inputs to predict them. In dermatology, think of identifying melanoma from clinical or dermoscopic images or predicting metastasis risk from digitized pathology slides.”
However, there are currently no universal guidelines on how large an AI dataset needs to be to yield accurate results. In the dermatology literature, most AI datasets range between 600 and 14,000 examples, Dr. Patel said, with a large study-specific variation in performance. “Misleading results can result from unanticipated training errors,” he said.
“The AI network may learn its intended task or an unrelated situational cue. For example, you can use great images to predict melanoma, but you may have an unintended poor outcome related to images that have, say, a ruler inside of them clustered within the melanoma diagnoses.” And unbeknown to the system’s developer, “the algorithm picks up that the ruler is predictive of an image being a melanoma and not the pigmented lesion itself.” In other words, the algorithm is only as good as the dataset being used, he said. “This is the key element, to ask what the dataset is that’s training the tool that you may one day use.”
Convolutional neural network
In 2017, a seminal study published in Nature showed that for classification of melanoma and epidermal lesions, a type of AI used in image processing known as a convolutional neural network (CNN) was on par with dermatologists and outperformed the average. For epidermal lesions, the network was one standard deviation higher above the average for dermatologists, while for melanocytic lesions, the network was just below one standard deviation above the average of the dermatologists. A CNN “clearly can perform well because it works on a different level than how our brains work,” Dr. Patel said.
In a separate study, a CNN trained to recognize melanoma in dermoscopic images was compared to 58 international dermatologists with varying levels of dermoscopy experience; 29% were “beginners,” with less than 2 years of experience; 19% were “skilled,” with 2-5 years of experience; and 52% were “experts,” with at least 5 years of experience. The analysis consisted of two experiments: In level I, dermatologists classified lesions based on dermoscopy only. In level II, dermatologists were provided dermoscopy, clinical images, and additional clinical information, while the CNN was trained on images only. The researchers found that most dermatologists were outperformed by the CNN. “Physicians of all different levels of training and experience may benefit from assistance by a CNN’s image classification,” they concluded.
Gene expression profiling
Another aspect of AI is gene expression profiling (GEP), which Dr. Patel defined as the evaluation of frequency and intensity of genetic activity at once to create a global picture of cellular function. “It’s AI that uses machine learning to evaluate genetic expression to assess lesion behavior,” he explained.
One GEP test on the market is the Pigmented Lesion Assay (PLA) from DermTech, a noninvasive test that looks at the expression of two genes to predict if a lesion is malignant or not. “Based on their validation set, they have shown some impressive numbers,” with sensitivities above 90%, and published registry data that have shown higher sensitivities “and even specificities above 90%,” he said.
“On the surface, it looks like this would be a useful test,” Dr. Patel said. A study published in 2021 looked at the evidence of applying real-world evidence with this test to see if results held up. Based on the authors’ analysis, he noted, “you would need a sensitivity and specificity of 95% to yield a positivity rate of 9.5% for the PLA test, which is what has been reported in real-world use. So, there’s a disconnect somewhere and we are not quite there yet.” That may be a result of the dataset itself not being as uniform between the validation and the training datasets, he continued. Also, the expression of certain genes is different “if you don’t have a clean input variable” of what the test is being used for, he added.
“If you’re not mirroring the dataset, you’re not going to get clean data,” he said. “So, if you’re using this on younger patients or for sun-damaged lesional skin or nonmelanocytic lesions around sun-damaged areas, there are variable expressions that may not be accurately captured by that algorithm. This might help explain the real-world variation that we’re seeing.”
Another GEP test in use is the 31-Gene Expression Profile Test for Melanoma, which evaluates gene expressions in melanoma tumors and what the behavior of that tumor may be. The test has been available for more than a decade “and there is a lot of speculation about its use,” Dr. Patel said. “A recent paper attempted to come up with an algorithm of how to use this, but there’s a lot of concern about the endpoints of what changes in management might result from this test. That is what we need to be thinking about. There’s a lot of back and forth about this.”
In 2020, authors of a consensus statement on prognostic GEP in cutaneous melanoma concluded that before GEP testing is routinely used, the clinical benefit in the management of patients with melanoma should be established through further clinical investigation. Dr. Patel recommended the accompanying editorial on GEP in melanoma, written by Hensin Tsao, MD, PhD, and Warren H. Chan, MS, in JAMA Dermatology.
In Dr. Patel’s opinion, T1a melanomas (0.8 mm, nonulcerated) do not need routine GEP, but the GEP test may be useful in cases that are in the “gray zone,” such as those with T1b or some borderline T2a melanomas (> 0.8 mm, < 1.2mm, nonulcerated, but with high mitosis, etc.); patients with unique coexisting conditions such as pregnancy, and patients who may not tolerate sentinel lymph node biopsy (SLNB) or adjuvant therapy.
Echoing sentiments expressed in the JAMA Dermatology editorial, he advised dermatologists to “remember your training and know the data. GEP predicting survival is not the same as SLNB positive rate. GEP should not replace standard guidelines in T2a and higher melanomas. Nodal sampling remains part of all major guidelines and determines adjuvant therapy.”
He cited the characterization of GEP in the editorial as “a powerful technology” that heralds the age of personalized medicine, but it is not ready for ubiquitous use. Prospective studies and time will lead to highly accurate tools.”
Dr. Patel disclosed that he is chief medical officer for Lazarus AI.
“AI is meant to be an enhancement strategy, a support tool to improve our diagnostic abilities,” Dr. Patel, a Mohs surgeon who is director of cutaneous oncology at the George Washington University Cancer Center, Washington, said during the ODAC Dermatology, Aesthetic & Surgical Conference. “Dermatologists should embrace AI and drive how it is utilized – be the captain of the plane (technology) and the passenger (patient). If we’re not in the forefront of the plane, we’re not to be able to dictate which way we are going with this.”
In 2019, a group of German researchers found that AI can improve accuracy and efficiency of specialists in classifying skin cancer based on dermoscopic images. “I really do believe this is going to be the future,” said Dr. Patel, who was not involved with the study. “Current research involves using supervised learning on known outcomes to determine inputs to predict them. In dermatology, think of identifying melanoma from clinical or dermoscopic images or predicting metastasis risk from digitized pathology slides.”
However, there are currently no universal guidelines on how large an AI dataset needs to be to yield accurate results. In the dermatology literature, most AI datasets range between 600 and 14,000 examples, Dr. Patel said, with a large study-specific variation in performance. “Misleading results can result from unanticipated training errors,” he said.
“The AI network may learn its intended task or an unrelated situational cue. For example, you can use great images to predict melanoma, but you may have an unintended poor outcome related to images that have, say, a ruler inside of them clustered within the melanoma diagnoses.” And unbeknown to the system’s developer, “the algorithm picks up that the ruler is predictive of an image being a melanoma and not the pigmented lesion itself.” In other words, the algorithm is only as good as the dataset being used, he said. “This is the key element, to ask what the dataset is that’s training the tool that you may one day use.”
Convolutional neural network
In 2017, a seminal study published in Nature showed that for classification of melanoma and epidermal lesions, a type of AI used in image processing known as a convolutional neural network (CNN) was on par with dermatologists and outperformed the average. For epidermal lesions, the network was one standard deviation higher above the average for dermatologists, while for melanocytic lesions, the network was just below one standard deviation above the average of the dermatologists. A CNN “clearly can perform well because it works on a different level than how our brains work,” Dr. Patel said.
In a separate study, a CNN trained to recognize melanoma in dermoscopic images was compared to 58 international dermatologists with varying levels of dermoscopy experience; 29% were “beginners,” with less than 2 years of experience; 19% were “skilled,” with 2-5 years of experience; and 52% were “experts,” with at least 5 years of experience. The analysis consisted of two experiments: In level I, dermatologists classified lesions based on dermoscopy only. In level II, dermatologists were provided dermoscopy, clinical images, and additional clinical information, while the CNN was trained on images only. The researchers found that most dermatologists were outperformed by the CNN. “Physicians of all different levels of training and experience may benefit from assistance by a CNN’s image classification,” they concluded.
Gene expression profiling
Another aspect of AI is gene expression profiling (GEP), which Dr. Patel defined as the evaluation of frequency and intensity of genetic activity at once to create a global picture of cellular function. “It’s AI that uses machine learning to evaluate genetic expression to assess lesion behavior,” he explained.
One GEP test on the market is the Pigmented Lesion Assay (PLA) from DermTech, a noninvasive test that looks at the expression of two genes to predict if a lesion is malignant or not. “Based on their validation set, they have shown some impressive numbers,” with sensitivities above 90%, and published registry data that have shown higher sensitivities “and even specificities above 90%,” he said.
“On the surface, it looks like this would be a useful test,” Dr. Patel said. A study published in 2021 looked at the evidence of applying real-world evidence with this test to see if results held up. Based on the authors’ analysis, he noted, “you would need a sensitivity and specificity of 95% to yield a positivity rate of 9.5% for the PLA test, which is what has been reported in real-world use. So, there’s a disconnect somewhere and we are not quite there yet.” That may be a result of the dataset itself not being as uniform between the validation and the training datasets, he continued. Also, the expression of certain genes is different “if you don’t have a clean input variable” of what the test is being used for, he added.
“If you’re not mirroring the dataset, you’re not going to get clean data,” he said. “So, if you’re using this on younger patients or for sun-damaged lesional skin or nonmelanocytic lesions around sun-damaged areas, there are variable expressions that may not be accurately captured by that algorithm. This might help explain the real-world variation that we’re seeing.”
Another GEP test in use is the 31-Gene Expression Profile Test for Melanoma, which evaluates gene expressions in melanoma tumors and what the behavior of that tumor may be. The test has been available for more than a decade “and there is a lot of speculation about its use,” Dr. Patel said. “A recent paper attempted to come up with an algorithm of how to use this, but there’s a lot of concern about the endpoints of what changes in management might result from this test. That is what we need to be thinking about. There’s a lot of back and forth about this.”
In 2020, authors of a consensus statement on prognostic GEP in cutaneous melanoma concluded that before GEP testing is routinely used, the clinical benefit in the management of patients with melanoma should be established through further clinical investigation. Dr. Patel recommended the accompanying editorial on GEP in melanoma, written by Hensin Tsao, MD, PhD, and Warren H. Chan, MS, in JAMA Dermatology.
In Dr. Patel’s opinion, T1a melanomas (0.8 mm, nonulcerated) do not need routine GEP, but the GEP test may be useful in cases that are in the “gray zone,” such as those with T1b or some borderline T2a melanomas (> 0.8 mm, < 1.2mm, nonulcerated, but with high mitosis, etc.); patients with unique coexisting conditions such as pregnancy, and patients who may not tolerate sentinel lymph node biopsy (SLNB) or adjuvant therapy.
Echoing sentiments expressed in the JAMA Dermatology editorial, he advised dermatologists to “remember your training and know the data. GEP predicting survival is not the same as SLNB positive rate. GEP should not replace standard guidelines in T2a and higher melanomas. Nodal sampling remains part of all major guidelines and determines adjuvant therapy.”
He cited the characterization of GEP in the editorial as “a powerful technology” that heralds the age of personalized medicine, but it is not ready for ubiquitous use. Prospective studies and time will lead to highly accurate tools.”
Dr. Patel disclosed that he is chief medical officer for Lazarus AI.
FROM ODAC 2022
Fungating Mass on the Abdominal Wall
The Diagnosis: Basal Cell Carcinoma
Histopathology was consistent with fungating basal cell carcinoma (BCC). The nodules were comprised of syncytial basaloid cells with high nuclear to cytoplasmic ratios, numerous mitotic figures, fibromyxoid stroma, and peripheral nuclear palisading (Figure). Fortunately, no perineural or lymphovascular invasion was identified, and the margins of the specimen were negative. Despite the high-risk nature of giant BCC, the mass was solitary without notable local invasion, leaving it amendable to surgery. On follow-up, the patient has remained recurrence free, and her hemoglobin level has since stabilized.
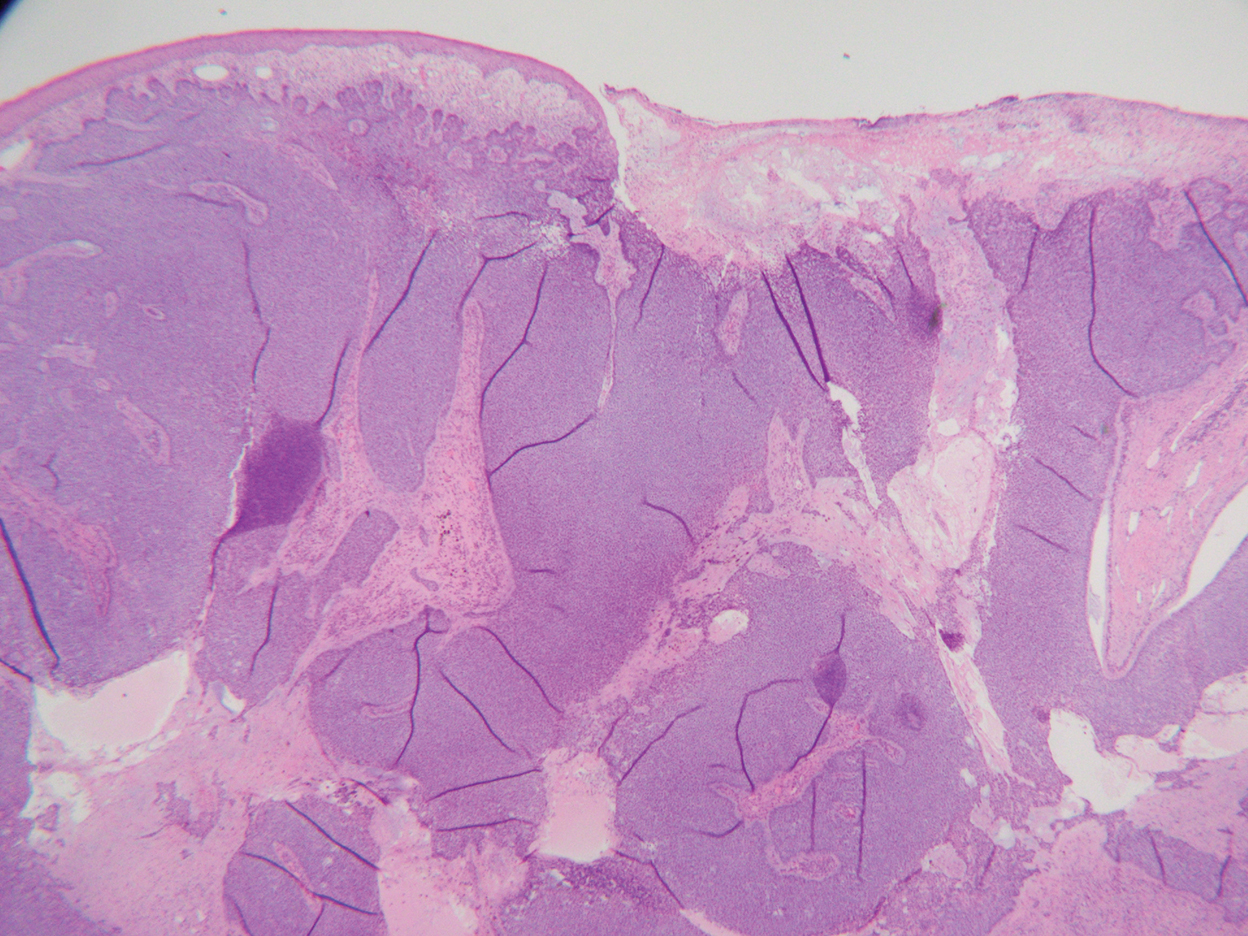
Skin cancer is the most common malignancy worldwide, and BCC accounts for more than 80% of nonmelanoma skin cancers in the United States. The incidence is on the rise due to the aging population and increasing cumulative skin exposure.1 Risk factors include both individual physical characteristics and environmental exposures. Individuals with lighter skin tones, red and blonde hair, and blue and green eyes are at an increased risk.2 UV radiation exposure is the most important cause of BCC.3 Chronic immunosuppression and exposure to arsenic, ionizing radiation, and psoralen plus UVA radiation also have been linked to the development of BCC.4-6 Basal cell carcinomas most commonly arise on sun-exposed areas such as the face, though more than 10% of cases appear on the trunk.7 Lesions characteristically remain localized, and growth rate is variable; however, when left untreated, BCCs have the potential to become locally destructive and difficult to treat.
Advanced BCCs are tumors that penetrate deeply into the skin. They often are not amenable to traditional therapy and/ or metastasize. Those that grow to a diameter greater than 5 cm, as in our patient, are known as giant BCCs. Only 0.5% to 1% of BCCs are giant BCCs8 ; they typically are more aggressive in nature with higher rates of local recurrence and metastasis. Individuals who develop giant BCCs either have had a delay in access to medical care or a history of BCC that was inadequately managed.9,10 During the COVID-19 pandemic, patient access to health care was substantially impacted during lockdowns. As in our patient, skin neoplasms and other medical conditions may present in later stages due to medical neglect.11,12 Metastasis is rare, even in advanced BCCs. A review of the literature from 1984 estimated that the incidence of metastasis of BCCs is 1 in 1000 to 35,000. Metastasis portends a poor prognosis with a median overall survival of 8 to 14 months.13 An updated review in 2013 found similar outcomes.14
The choice of management for BCCs depends on the risk for recurrence as well as individual patient factors. Characteristics such as tumor size, location, histology, whether it is a primary or recurrent lesion, and the presence of chronic skin disease determine the recurrence rate.15 The management of advanced BCCs often requires a multidisciplinary approach, as these neoplasms may not be amenable to local therapy without causing substantial morbidity. Mohs micrographic surgery is the treatment of choice for BCCs at high risk for recurrence.16 Standard surgical excision with postoperative margin assessment is acceptable when Mohs micrographic surgery is not available.17 Radiation therapy is an alternative for patients who are not candidates for surgery.18
Recently, improved understanding of the molecular pathogenesis of BCCs has led to the development of novel systemic therapies. The Hedgehog signaling pathway has been found to play a critical role in the development of most BCCs.19 Vismodegib and sonidegib are small-molecule inhibitors of the Hedgehog signaling pathway approved for the treatment of locally advanced and metastatic BCCs that are not amenable to surgery or radiation. Approximately 50% of advanced BCCs respond to these therapies; however, long-term treatment may be limited by intolerable side effects and the development of resistance.20 Basal cell carcinomas that spread to lymph nodes or distant sites are treated with traditional systemic therapy. Historically, conventional cytotoxic chemotherapies, such as platinum-containing regimens, were employed with limited benefit and notable morbidity.21
The differential diagnosis for our patient included several other cutaneous neoplasms. Squamous cell carcinoma is the second most common type of skin cancer. Similar to BCC, it can reach a substantial size if left untreated. Risk factors include chronic inflammation, exposure to radiation or chemical carcinogens, burns, human papillomavirus, and other chronic infections. Giant squamous cell carcinomas have high malignant potential and require imaging to assess the extent of invasion and for metastasis. Surgery typically is necessary for both staging and treatment. Adjuvant therapy also may be necessary.22,23
Internal malignant neoplasms rarely present as cutaneous metastases. Breast cancer, melanoma, and cancers of the upper respiratory tract most frequently metastasize to the skin. Although colorectal cancer (CRC) rarely metastasizes to the skin, it is an important cause of cutaneous metastasis due to its high incidence in the general population. When it does spread to the skin, CRC preferentially affects the abdominal wall. Lesions typically resemble the primary tumor but may appear anaplastic. The occurrence of cutaneous metastasis suggests latestage disease and carries a poor prognosis.24
Merkel cell carcinoma and melanoma are aggressive skin cancers with high mortality rates. The former is rarer but more lethal. Merkel cell carcinomas typically occur in elderly white men on sun-exposed areas of the skin. Tumors present as asymptomatic, rapidly expanding, blue-red, firm nodules. Immunosuppression and UV light exposure are notable risk factors.25 Of the 4 major subtypes of cutaneous melanoma, superficial spreading is the most common, followed by nodular, lentigo maligna, and acral lentiginous.26 Superficial spreading melanoma characteristically presents as an expanding asymmetric macule or thin plaque with irregular borders and variation in size and color (black, brown, or red). Nodular melanoma usually presents as symmetric in shape and color (amelanotic, black, or brown). Early recognition by both the patient and clinician is essential in preventing tumor growth and progression.27
Our patient’s presentation was highly concerning for cutaneous metastasis given her history of CRC. Furthermore, the finding of severe anemia was atypical for skin cancer and more characteristic of the prior malignancy. Imaging revealed a locally confined mass with no evidence of extension, lymph node involvement, or additional lesions. The diagnosis was clinched with histopathologic examination.
- Rogers HW, Weinstock MA, Harris AR, et al. Incidence estimate of nonmelanoma skin cancer in the United States, 2006. Arch Dermatol. 2010;146:283-287.
- Lear JT, Tan BB, Smith AG, et al. Risk factors for basal cell carcinoma in the UK: case-control study in 806 patients. J R Soc Med. 1997; 90:371-374.
- Gallagher RP, Hill GB, Bajdik CD, et al. Sunlight exposure, pigmentary factors, and risk of nonmelanocytic skin cancer: I. basal cell carcinoma. Arch Dermatol. 1995;131:157-163.
- Guo HR, Yu HS, Hu H, et al. Arsenic in drinking water and skin cancers: cell-type specificity (Taiwan, ROC). Cancer Causes Control. 2001;12:909-916.
- Lichter MD, Karagas MR, Mott LA, et al; The New Hampshire Skin Cancer Study Group. Therapeutic ionizing radiation and the incidence of basal cell carcinoma and squamous cell carcinoma. Arch Dermatol. 2000;136:1007-1011.
- Nijsten TEC, Stern RS. The increased risk of skin cancer is persistent after discontinuation of psoralen plus ultraviolet A: a cohort study. J Invest Dermatol. 2003;121:252-258.
- Scrivener Y, Grosshans E, Cribier B. Variations of basal cell carcinomas according to gender, age, location and histopathological subtype. Br J Dermatol. 2002;147:41-47.
- Gualdi G, Monari P, Calzavara‐Pinton P, et al. When basal cell carcinomas became giant: an Italian multicenter study. Int J Dermatol. 2020;59:377-382.
- Randle HW, Roenigk RK, Brodland DG. Giant basal cell carcinoma (T3). who is at risk? Cancer. 1993;72:1624-1630.
- Archontaki M, Stavrianos SD, Korkolis DP, et al. Giant basal cell carcinoma: clinicopathological analysis of 51 cases and review of the literature. Anticancer Res. 2009;29:2655-2663.
- Shifat Ahmed SAK, Ajisola M, Azeem K, et al. Impact of the societal response to COVID-19 on access to healthcare for non-COVID-19 health issues in slum communities of Bangladesh, Kenya, Nigeria and Pakistan: results of pre-COVID and COVID-19 lockdown ssstakeholder engagements. BMJ Glob Health. 2020;5:E003042.
- Gomolin T, Cline A, Handler MZ. The danger of neglecting melanoma during the COVID-19 pandemic. J Dermatolog Treat. 2020;31:444-445.
- von Domarus H, Stevens PJ. Metastatic basal cell carcinoma. report of five cases and review of 170 cases in the literature. J Am Acad Dermatol. 1984;10:1043-1060.
- Wysong A, Aasi SZ, Tang JY. Update on metastatic basal cell carcinoma: a summary of published cases from 1981 through 2011. JAMA Dermatol. 2013;149:615-616.
- Bøgelund FS, Philipsen PA, Gniadecki R. Factors affecting the recurrence rate of basal cell carcinoma. Acta Derm Venereol. 2007;87:330-334.
- Mosterd K, Krekels GAM, Nieman FH, et al. Surgical excision versus Mohs’ micrographic surgery for primary and recurrent basal-cell carcinoma of the face: a prospective randomised controlled trial with 5-years’ follow-up. Lancet Oncol. 2008;9:1149-1156.
- Wetzig T, Woitek M, Eichhorn K, et al. Surgical excision of basal cell carcinoma with complete margin control: outcome at 5-year follow-up. Dermatology. 2010;220:363-369.
- Silverman MK, Kopf AW, Gladstein AH, et al. Recurrence rates of treated basal cell carcinomas. part 4: X-ray therapy. J Dermatol Surg Oncol. 1992;18:549-554.
- Tanese K, Emoto K, Kubota N, et al. Immunohistochemical visualization of the signature of activated Hedgehog signaling pathway in cutaneous epithelial tumors. J Dermatol. 2018;45:1181-1186.
- Basset-Séguin N, Hauschild A, Kunstfeld R, et al. Vismodegib in patients with advanced basal cell carcinoma: primary analysis of STEVIE, an international, open-label trial. Eur J Cancer. 2017;86:334-348.
- Carneiro BA, Watkin WG, Mehta UK, et al. Metastatic basal cell carcinoma: complete response to chemotherapy and associated pure red cell aplasia. Cancer Invest. 2006;24:396-400.
- Misiakos EP, Damaskou V, Koumarianou A, et al. A giant squamous cell carcinoma of the skin of the thoracic wall: a case report and review of the literature. J Med Case Rep. 2017;11:136.
- Wollina U, Bayyoud Y, Krönert C, et al. Giant epithelial malignancies (basal cell carcinoma, squamous cell carcinoma): a series of 20 tumors from a single center. J Cutan Aesthet Surg. 2012;5:12-19.
- Bittencourt MJS, Imbiriba AA, Oliveira OA, et al. Cutaneous metastasis of colorectal cancer. An Bras Dermatol. 2018;93:884-886.
- Heath M, Jaimes N, Lemos B, et al. Clinical characteristics of Merkel cell carcinoma at diagnosis in 195 patients: the AEIOU features. J Am Acad Dermatol. 2008;58:375-381.
- Buettner PG, Leiter U, Eigentler TK, et al. Development of prognostic factors and survival in cutaneous melanoma over 25 years: an analysis of the Central Malignant Melanoma Registry of the German Dermatological Society. Cancer. 2005;103:616-624.
- Klebanov N, Gunasekera N, Lin WM, et al. The clinical spectrum of cutaneous melanoma morphology. J Am Acad Dermatol. 2019; 80:178-188.e3.
The Diagnosis: Basal Cell Carcinoma
Histopathology was consistent with fungating basal cell carcinoma (BCC). The nodules were comprised of syncytial basaloid cells with high nuclear to cytoplasmic ratios, numerous mitotic figures, fibromyxoid stroma, and peripheral nuclear palisading (Figure). Fortunately, no perineural or lymphovascular invasion was identified, and the margins of the specimen were negative. Despite the high-risk nature of giant BCC, the mass was solitary without notable local invasion, leaving it amendable to surgery. On follow-up, the patient has remained recurrence free, and her hemoglobin level has since stabilized.

Skin cancer is the most common malignancy worldwide, and BCC accounts for more than 80% of nonmelanoma skin cancers in the United States. The incidence is on the rise due to the aging population and increasing cumulative skin exposure.1 Risk factors include both individual physical characteristics and environmental exposures. Individuals with lighter skin tones, red and blonde hair, and blue and green eyes are at an increased risk.2 UV radiation exposure is the most important cause of BCC.3 Chronic immunosuppression and exposure to arsenic, ionizing radiation, and psoralen plus UVA radiation also have been linked to the development of BCC.4-6 Basal cell carcinomas most commonly arise on sun-exposed areas such as the face, though more than 10% of cases appear on the trunk.7 Lesions characteristically remain localized, and growth rate is variable; however, when left untreated, BCCs have the potential to become locally destructive and difficult to treat.
Advanced BCCs are tumors that penetrate deeply into the skin. They often are not amenable to traditional therapy and/ or metastasize. Those that grow to a diameter greater than 5 cm, as in our patient, are known as giant BCCs. Only 0.5% to 1% of BCCs are giant BCCs8 ; they typically are more aggressive in nature with higher rates of local recurrence and metastasis. Individuals who develop giant BCCs either have had a delay in access to medical care or a history of BCC that was inadequately managed.9,10 During the COVID-19 pandemic, patient access to health care was substantially impacted during lockdowns. As in our patient, skin neoplasms and other medical conditions may present in later stages due to medical neglect.11,12 Metastasis is rare, even in advanced BCCs. A review of the literature from 1984 estimated that the incidence of metastasis of BCCs is 1 in 1000 to 35,000. Metastasis portends a poor prognosis with a median overall survival of 8 to 14 months.13 An updated review in 2013 found similar outcomes.14
The choice of management for BCCs depends on the risk for recurrence as well as individual patient factors. Characteristics such as tumor size, location, histology, whether it is a primary or recurrent lesion, and the presence of chronic skin disease determine the recurrence rate.15 The management of advanced BCCs often requires a multidisciplinary approach, as these neoplasms may not be amenable to local therapy without causing substantial morbidity. Mohs micrographic surgery is the treatment of choice for BCCs at high risk for recurrence.16 Standard surgical excision with postoperative margin assessment is acceptable when Mohs micrographic surgery is not available.17 Radiation therapy is an alternative for patients who are not candidates for surgery.18
Recently, improved understanding of the molecular pathogenesis of BCCs has led to the development of novel systemic therapies. The Hedgehog signaling pathway has been found to play a critical role in the development of most BCCs.19 Vismodegib and sonidegib are small-molecule inhibitors of the Hedgehog signaling pathway approved for the treatment of locally advanced and metastatic BCCs that are not amenable to surgery or radiation. Approximately 50% of advanced BCCs respond to these therapies; however, long-term treatment may be limited by intolerable side effects and the development of resistance.20 Basal cell carcinomas that spread to lymph nodes or distant sites are treated with traditional systemic therapy. Historically, conventional cytotoxic chemotherapies, such as platinum-containing regimens, were employed with limited benefit and notable morbidity.21
The differential diagnosis for our patient included several other cutaneous neoplasms. Squamous cell carcinoma is the second most common type of skin cancer. Similar to BCC, it can reach a substantial size if left untreated. Risk factors include chronic inflammation, exposure to radiation or chemical carcinogens, burns, human papillomavirus, and other chronic infections. Giant squamous cell carcinomas have high malignant potential and require imaging to assess the extent of invasion and for metastasis. Surgery typically is necessary for both staging and treatment. Adjuvant therapy also may be necessary.22,23
Internal malignant neoplasms rarely present as cutaneous metastases. Breast cancer, melanoma, and cancers of the upper respiratory tract most frequently metastasize to the skin. Although colorectal cancer (CRC) rarely metastasizes to the skin, it is an important cause of cutaneous metastasis due to its high incidence in the general population. When it does spread to the skin, CRC preferentially affects the abdominal wall. Lesions typically resemble the primary tumor but may appear anaplastic. The occurrence of cutaneous metastasis suggests latestage disease and carries a poor prognosis.24
Merkel cell carcinoma and melanoma are aggressive skin cancers with high mortality rates. The former is rarer but more lethal. Merkel cell carcinomas typically occur in elderly white men on sun-exposed areas of the skin. Tumors present as asymptomatic, rapidly expanding, blue-red, firm nodules. Immunosuppression and UV light exposure are notable risk factors.25 Of the 4 major subtypes of cutaneous melanoma, superficial spreading is the most common, followed by nodular, lentigo maligna, and acral lentiginous.26 Superficial spreading melanoma characteristically presents as an expanding asymmetric macule or thin plaque with irregular borders and variation in size and color (black, brown, or red). Nodular melanoma usually presents as symmetric in shape and color (amelanotic, black, or brown). Early recognition by both the patient and clinician is essential in preventing tumor growth and progression.27
Our patient’s presentation was highly concerning for cutaneous metastasis given her history of CRC. Furthermore, the finding of severe anemia was atypical for skin cancer and more characteristic of the prior malignancy. Imaging revealed a locally confined mass with no evidence of extension, lymph node involvement, or additional lesions. The diagnosis was clinched with histopathologic examination.
The Diagnosis: Basal Cell Carcinoma
Histopathology was consistent with fungating basal cell carcinoma (BCC). The nodules were comprised of syncytial basaloid cells with high nuclear to cytoplasmic ratios, numerous mitotic figures, fibromyxoid stroma, and peripheral nuclear palisading (Figure). Fortunately, no perineural or lymphovascular invasion was identified, and the margins of the specimen were negative. Despite the high-risk nature of giant BCC, the mass was solitary without notable local invasion, leaving it amendable to surgery. On follow-up, the patient has remained recurrence free, and her hemoglobin level has since stabilized.

Skin cancer is the most common malignancy worldwide, and BCC accounts for more than 80% of nonmelanoma skin cancers in the United States. The incidence is on the rise due to the aging population and increasing cumulative skin exposure.1 Risk factors include both individual physical characteristics and environmental exposures. Individuals with lighter skin tones, red and blonde hair, and blue and green eyes are at an increased risk.2 UV radiation exposure is the most important cause of BCC.3 Chronic immunosuppression and exposure to arsenic, ionizing radiation, and psoralen plus UVA radiation also have been linked to the development of BCC.4-6 Basal cell carcinomas most commonly arise on sun-exposed areas such as the face, though more than 10% of cases appear on the trunk.7 Lesions characteristically remain localized, and growth rate is variable; however, when left untreated, BCCs have the potential to become locally destructive and difficult to treat.
Advanced BCCs are tumors that penetrate deeply into the skin. They often are not amenable to traditional therapy and/ or metastasize. Those that grow to a diameter greater than 5 cm, as in our patient, are known as giant BCCs. Only 0.5% to 1% of BCCs are giant BCCs8 ; they typically are more aggressive in nature with higher rates of local recurrence and metastasis. Individuals who develop giant BCCs either have had a delay in access to medical care or a history of BCC that was inadequately managed.9,10 During the COVID-19 pandemic, patient access to health care was substantially impacted during lockdowns. As in our patient, skin neoplasms and other medical conditions may present in later stages due to medical neglect.11,12 Metastasis is rare, even in advanced BCCs. A review of the literature from 1984 estimated that the incidence of metastasis of BCCs is 1 in 1000 to 35,000. Metastasis portends a poor prognosis with a median overall survival of 8 to 14 months.13 An updated review in 2013 found similar outcomes.14
The choice of management for BCCs depends on the risk for recurrence as well as individual patient factors. Characteristics such as tumor size, location, histology, whether it is a primary or recurrent lesion, and the presence of chronic skin disease determine the recurrence rate.15 The management of advanced BCCs often requires a multidisciplinary approach, as these neoplasms may not be amenable to local therapy without causing substantial morbidity. Mohs micrographic surgery is the treatment of choice for BCCs at high risk for recurrence.16 Standard surgical excision with postoperative margin assessment is acceptable when Mohs micrographic surgery is not available.17 Radiation therapy is an alternative for patients who are not candidates for surgery.18
Recently, improved understanding of the molecular pathogenesis of BCCs has led to the development of novel systemic therapies. The Hedgehog signaling pathway has been found to play a critical role in the development of most BCCs.19 Vismodegib and sonidegib are small-molecule inhibitors of the Hedgehog signaling pathway approved for the treatment of locally advanced and metastatic BCCs that are not amenable to surgery or radiation. Approximately 50% of advanced BCCs respond to these therapies; however, long-term treatment may be limited by intolerable side effects and the development of resistance.20 Basal cell carcinomas that spread to lymph nodes or distant sites are treated with traditional systemic therapy. Historically, conventional cytotoxic chemotherapies, such as platinum-containing regimens, were employed with limited benefit and notable morbidity.21
The differential diagnosis for our patient included several other cutaneous neoplasms. Squamous cell carcinoma is the second most common type of skin cancer. Similar to BCC, it can reach a substantial size if left untreated. Risk factors include chronic inflammation, exposure to radiation or chemical carcinogens, burns, human papillomavirus, and other chronic infections. Giant squamous cell carcinomas have high malignant potential and require imaging to assess the extent of invasion and for metastasis. Surgery typically is necessary for both staging and treatment. Adjuvant therapy also may be necessary.22,23
Internal malignant neoplasms rarely present as cutaneous metastases. Breast cancer, melanoma, and cancers of the upper respiratory tract most frequently metastasize to the skin. Although colorectal cancer (CRC) rarely metastasizes to the skin, it is an important cause of cutaneous metastasis due to its high incidence in the general population. When it does spread to the skin, CRC preferentially affects the abdominal wall. Lesions typically resemble the primary tumor but may appear anaplastic. The occurrence of cutaneous metastasis suggests latestage disease and carries a poor prognosis.24
Merkel cell carcinoma and melanoma are aggressive skin cancers with high mortality rates. The former is rarer but more lethal. Merkel cell carcinomas typically occur in elderly white men on sun-exposed areas of the skin. Tumors present as asymptomatic, rapidly expanding, blue-red, firm nodules. Immunosuppression and UV light exposure are notable risk factors.25 Of the 4 major subtypes of cutaneous melanoma, superficial spreading is the most common, followed by nodular, lentigo maligna, and acral lentiginous.26 Superficial spreading melanoma characteristically presents as an expanding asymmetric macule or thin plaque with irregular borders and variation in size and color (black, brown, or red). Nodular melanoma usually presents as symmetric in shape and color (amelanotic, black, or brown). Early recognition by both the patient and clinician is essential in preventing tumor growth and progression.27
Our patient’s presentation was highly concerning for cutaneous metastasis given her history of CRC. Furthermore, the finding of severe anemia was atypical for skin cancer and more characteristic of the prior malignancy. Imaging revealed a locally confined mass with no evidence of extension, lymph node involvement, or additional lesions. The diagnosis was clinched with histopathologic examination.
- Rogers HW, Weinstock MA, Harris AR, et al. Incidence estimate of nonmelanoma skin cancer in the United States, 2006. Arch Dermatol. 2010;146:283-287.
- Lear JT, Tan BB, Smith AG, et al. Risk factors for basal cell carcinoma in the UK: case-control study in 806 patients. J R Soc Med. 1997; 90:371-374.
- Gallagher RP, Hill GB, Bajdik CD, et al. Sunlight exposure, pigmentary factors, and risk of nonmelanocytic skin cancer: I. basal cell carcinoma. Arch Dermatol. 1995;131:157-163.
- Guo HR, Yu HS, Hu H, et al. Arsenic in drinking water and skin cancers: cell-type specificity (Taiwan, ROC). Cancer Causes Control. 2001;12:909-916.
- Lichter MD, Karagas MR, Mott LA, et al; The New Hampshire Skin Cancer Study Group. Therapeutic ionizing radiation and the incidence of basal cell carcinoma and squamous cell carcinoma. Arch Dermatol. 2000;136:1007-1011.
- Nijsten TEC, Stern RS. The increased risk of skin cancer is persistent after discontinuation of psoralen plus ultraviolet A: a cohort study. J Invest Dermatol. 2003;121:252-258.
- Scrivener Y, Grosshans E, Cribier B. Variations of basal cell carcinomas according to gender, age, location and histopathological subtype. Br J Dermatol. 2002;147:41-47.
- Gualdi G, Monari P, Calzavara‐Pinton P, et al. When basal cell carcinomas became giant: an Italian multicenter study. Int J Dermatol. 2020;59:377-382.
- Randle HW, Roenigk RK, Brodland DG. Giant basal cell carcinoma (T3). who is at risk? Cancer. 1993;72:1624-1630.
- Archontaki M, Stavrianos SD, Korkolis DP, et al. Giant basal cell carcinoma: clinicopathological analysis of 51 cases and review of the literature. Anticancer Res. 2009;29:2655-2663.
- Shifat Ahmed SAK, Ajisola M, Azeem K, et al. Impact of the societal response to COVID-19 on access to healthcare for non-COVID-19 health issues in slum communities of Bangladesh, Kenya, Nigeria and Pakistan: results of pre-COVID and COVID-19 lockdown ssstakeholder engagements. BMJ Glob Health. 2020;5:E003042.
- Gomolin T, Cline A, Handler MZ. The danger of neglecting melanoma during the COVID-19 pandemic. J Dermatolog Treat. 2020;31:444-445.
- von Domarus H, Stevens PJ. Metastatic basal cell carcinoma. report of five cases and review of 170 cases in the literature. J Am Acad Dermatol. 1984;10:1043-1060.
- Wysong A, Aasi SZ, Tang JY. Update on metastatic basal cell carcinoma: a summary of published cases from 1981 through 2011. JAMA Dermatol. 2013;149:615-616.
- Bøgelund FS, Philipsen PA, Gniadecki R. Factors affecting the recurrence rate of basal cell carcinoma. Acta Derm Venereol. 2007;87:330-334.
- Mosterd K, Krekels GAM, Nieman FH, et al. Surgical excision versus Mohs’ micrographic surgery for primary and recurrent basal-cell carcinoma of the face: a prospective randomised controlled trial with 5-years’ follow-up. Lancet Oncol. 2008;9:1149-1156.
- Wetzig T, Woitek M, Eichhorn K, et al. Surgical excision of basal cell carcinoma with complete margin control: outcome at 5-year follow-up. Dermatology. 2010;220:363-369.
- Silverman MK, Kopf AW, Gladstein AH, et al. Recurrence rates of treated basal cell carcinomas. part 4: X-ray therapy. J Dermatol Surg Oncol. 1992;18:549-554.
- Tanese K, Emoto K, Kubota N, et al. Immunohistochemical visualization of the signature of activated Hedgehog signaling pathway in cutaneous epithelial tumors. J Dermatol. 2018;45:1181-1186.
- Basset-Séguin N, Hauschild A, Kunstfeld R, et al. Vismodegib in patients with advanced basal cell carcinoma: primary analysis of STEVIE, an international, open-label trial. Eur J Cancer. 2017;86:334-348.
- Carneiro BA, Watkin WG, Mehta UK, et al. Metastatic basal cell carcinoma: complete response to chemotherapy and associated pure red cell aplasia. Cancer Invest. 2006;24:396-400.
- Misiakos EP, Damaskou V, Koumarianou A, et al. A giant squamous cell carcinoma of the skin of the thoracic wall: a case report and review of the literature. J Med Case Rep. 2017;11:136.
- Wollina U, Bayyoud Y, Krönert C, et al. Giant epithelial malignancies (basal cell carcinoma, squamous cell carcinoma): a series of 20 tumors from a single center. J Cutan Aesthet Surg. 2012;5:12-19.
- Bittencourt MJS, Imbiriba AA, Oliveira OA, et al. Cutaneous metastasis of colorectal cancer. An Bras Dermatol. 2018;93:884-886.
- Heath M, Jaimes N, Lemos B, et al. Clinical characteristics of Merkel cell carcinoma at diagnosis in 195 patients: the AEIOU features. J Am Acad Dermatol. 2008;58:375-381.
- Buettner PG, Leiter U, Eigentler TK, et al. Development of prognostic factors and survival in cutaneous melanoma over 25 years: an analysis of the Central Malignant Melanoma Registry of the German Dermatological Society. Cancer. 2005;103:616-624.
- Klebanov N, Gunasekera N, Lin WM, et al. The clinical spectrum of cutaneous melanoma morphology. J Am Acad Dermatol. 2019; 80:178-188.e3.
- Rogers HW, Weinstock MA, Harris AR, et al. Incidence estimate of nonmelanoma skin cancer in the United States, 2006. Arch Dermatol. 2010;146:283-287.
- Lear JT, Tan BB, Smith AG, et al. Risk factors for basal cell carcinoma in the UK: case-control study in 806 patients. J R Soc Med. 1997; 90:371-374.
- Gallagher RP, Hill GB, Bajdik CD, et al. Sunlight exposure, pigmentary factors, and risk of nonmelanocytic skin cancer: I. basal cell carcinoma. Arch Dermatol. 1995;131:157-163.
- Guo HR, Yu HS, Hu H, et al. Arsenic in drinking water and skin cancers: cell-type specificity (Taiwan, ROC). Cancer Causes Control. 2001;12:909-916.
- Lichter MD, Karagas MR, Mott LA, et al; The New Hampshire Skin Cancer Study Group. Therapeutic ionizing radiation and the incidence of basal cell carcinoma and squamous cell carcinoma. Arch Dermatol. 2000;136:1007-1011.
- Nijsten TEC, Stern RS. The increased risk of skin cancer is persistent after discontinuation of psoralen plus ultraviolet A: a cohort study. J Invest Dermatol. 2003;121:252-258.
- Scrivener Y, Grosshans E, Cribier B. Variations of basal cell carcinomas according to gender, age, location and histopathological subtype. Br J Dermatol. 2002;147:41-47.
- Gualdi G, Monari P, Calzavara‐Pinton P, et al. When basal cell carcinomas became giant: an Italian multicenter study. Int J Dermatol. 2020;59:377-382.
- Randle HW, Roenigk RK, Brodland DG. Giant basal cell carcinoma (T3). who is at risk? Cancer. 1993;72:1624-1630.
- Archontaki M, Stavrianos SD, Korkolis DP, et al. Giant basal cell carcinoma: clinicopathological analysis of 51 cases and review of the literature. Anticancer Res. 2009;29:2655-2663.
- Shifat Ahmed SAK, Ajisola M, Azeem K, et al. Impact of the societal response to COVID-19 on access to healthcare for non-COVID-19 health issues in slum communities of Bangladesh, Kenya, Nigeria and Pakistan: results of pre-COVID and COVID-19 lockdown ssstakeholder engagements. BMJ Glob Health. 2020;5:E003042.
- Gomolin T, Cline A, Handler MZ. The danger of neglecting melanoma during the COVID-19 pandemic. J Dermatolog Treat. 2020;31:444-445.
- von Domarus H, Stevens PJ. Metastatic basal cell carcinoma. report of five cases and review of 170 cases in the literature. J Am Acad Dermatol. 1984;10:1043-1060.
- Wysong A, Aasi SZ, Tang JY. Update on metastatic basal cell carcinoma: a summary of published cases from 1981 through 2011. JAMA Dermatol. 2013;149:615-616.
- Bøgelund FS, Philipsen PA, Gniadecki R. Factors affecting the recurrence rate of basal cell carcinoma. Acta Derm Venereol. 2007;87:330-334.
- Mosterd K, Krekels GAM, Nieman FH, et al. Surgical excision versus Mohs’ micrographic surgery for primary and recurrent basal-cell carcinoma of the face: a prospective randomised controlled trial with 5-years’ follow-up. Lancet Oncol. 2008;9:1149-1156.
- Wetzig T, Woitek M, Eichhorn K, et al. Surgical excision of basal cell carcinoma with complete margin control: outcome at 5-year follow-up. Dermatology. 2010;220:363-369.
- Silverman MK, Kopf AW, Gladstein AH, et al. Recurrence rates of treated basal cell carcinomas. part 4: X-ray therapy. J Dermatol Surg Oncol. 1992;18:549-554.
- Tanese K, Emoto K, Kubota N, et al. Immunohistochemical visualization of the signature of activated Hedgehog signaling pathway in cutaneous epithelial tumors. J Dermatol. 2018;45:1181-1186.
- Basset-Séguin N, Hauschild A, Kunstfeld R, et al. Vismodegib in patients with advanced basal cell carcinoma: primary analysis of STEVIE, an international, open-label trial. Eur J Cancer. 2017;86:334-348.
- Carneiro BA, Watkin WG, Mehta UK, et al. Metastatic basal cell carcinoma: complete response to chemotherapy and associated pure red cell aplasia. Cancer Invest. 2006;24:396-400.
- Misiakos EP, Damaskou V, Koumarianou A, et al. A giant squamous cell carcinoma of the skin of the thoracic wall: a case report and review of the literature. J Med Case Rep. 2017;11:136.
- Wollina U, Bayyoud Y, Krönert C, et al. Giant epithelial malignancies (basal cell carcinoma, squamous cell carcinoma): a series of 20 tumors from a single center. J Cutan Aesthet Surg. 2012;5:12-19.
- Bittencourt MJS, Imbiriba AA, Oliveira OA, et al. Cutaneous metastasis of colorectal cancer. An Bras Dermatol. 2018;93:884-886.
- Heath M, Jaimes N, Lemos B, et al. Clinical characteristics of Merkel cell carcinoma at diagnosis in 195 patients: the AEIOU features. J Am Acad Dermatol. 2008;58:375-381.
- Buettner PG, Leiter U, Eigentler TK, et al. Development of prognostic factors and survival in cutaneous melanoma over 25 years: an analysis of the Central Malignant Melanoma Registry of the German Dermatological Society. Cancer. 2005;103:616-624.
- Klebanov N, Gunasekera N, Lin WM, et al. The clinical spectrum of cutaneous melanoma morphology. J Am Acad Dermatol. 2019; 80:178-188.e3.
A 77-year-old woman was admitted to the hospital with anemia (hemoglobin, 5.2 g/dL [reference range, 12.0–15.5 g/dL]) and a rapidly growing abdominal wall mass. She had a history of stage IIA colon cancer (T3N0M0) that was treated 5 years prior with a partial colon resection and adjuvant chemotherapy. She initially noticed a red scaly lesion developing around a scar from a prior surgery that had been stable for years. Over the last 2 months, the lesion rapidly expanded and would intermittently bleed. Physical examination revealed a 13×10×4.5-cm, pink-red, nodular, firm mass over the patient’s right upper quadrant. Computed tomography revealed a mass limited to the skin and superficial tissue. General surgery was consulted for excision of the mass.
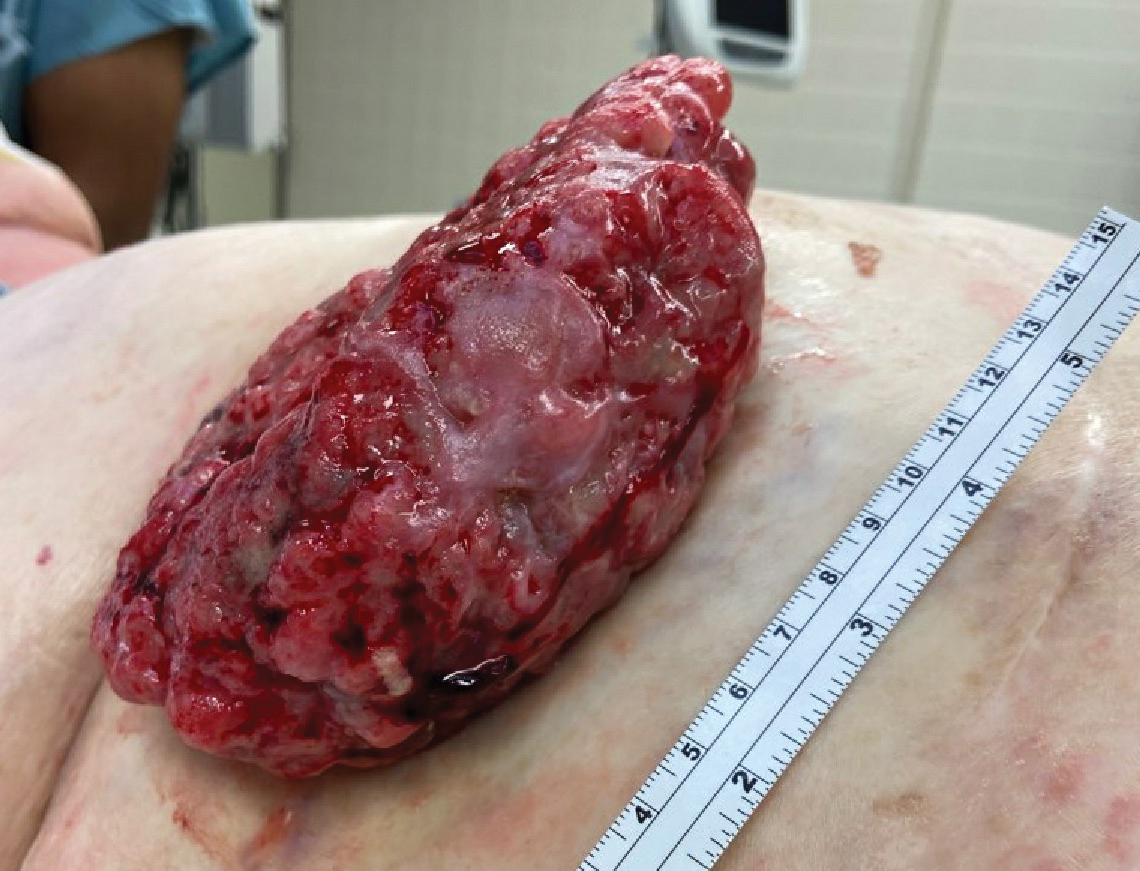
Indurated Violaceous Lesions on the Face, Trunk, and Legs
The Diagnosis: Kaposi Sarcoma
A punch biopsy of a lesion on the right side of the back revealed a diffuse, poorly circumscribed, spindle cell neoplasm of the papillary and reticular dermis with associated vascular and pseudovascular spaces distended by erythrocytes (Figure 1). Immunostaining was positive for human herpesvirus 8 (HHV-8)(Figure 2), ETS-related gene, CD31, and CD34 and negative for pan cytokeratin, confirming the diagnosis of Kaposi sarcoma (KS). Bacterial, fungal, and mycobacterial tissue cultures were negative. The patient was tested for HIV and referred to infectious disease and oncology. He subsequently was found to have HIV with a viral load greater than 1 million copies. He was started on antiretroviral therapy and Pneumocystis jirovecii pneumonia prophylaxis. Computed tomography of the chest, abdomen, and pelvis showed bilateral, multifocal, perihilar, flame-shaped consolidations suggestive of KS. The patient later disclosed having an intermittent dry cough of more than a year’s duration with occasional bright red blood per rectum after bowel movements. After workup, the patient was found to have cytomegalovirus esophagitis/gastritis and candidal esophagitis that were treated with valganciclovir and fluconazole, respectively.
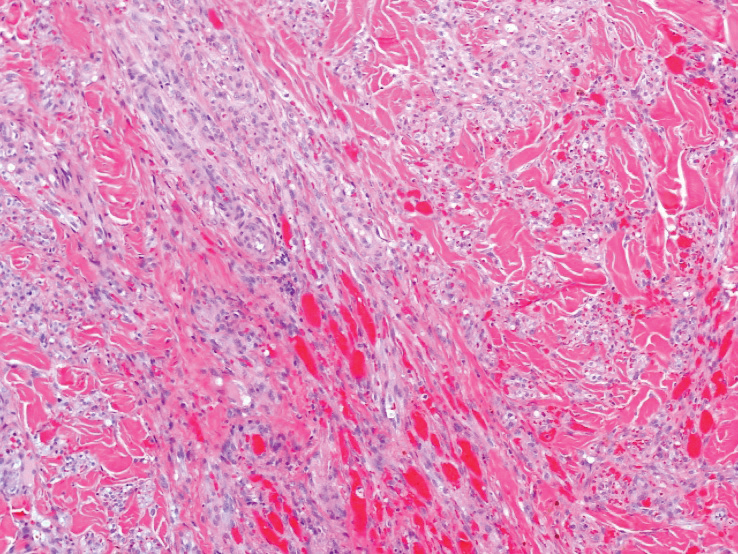
Kaposi sarcoma is an angioproliferative, AIDSdefining disease associated with HHV-8. There are 4 types of KS as defined by the populations they affect. AIDS-associated KS occurs in individuals with HIV, as seen in our patient. It often is accompanied by extensive mucocutaneous and visceral lesions, as well as systemic symptoms such as fever, weight loss, and diarrhea.1 Classic KS is a variant that presents in older men of Mediterranean, Eastern European, and South American descent. Cutaneous lesions typically are distributed on the lower extremities.2,3 Endemic (African) KS is seen in HIV-negative children and young adults in equatorial Africa. It most commonly affects the lower extremities or lymph nodes and usually follows a more aggressive course.2 Lastly, iatrogenic KS is associated with immunosuppressive medications or conditions, such as organ transplantation, chemotherapy, and rheumatologic disorders.3,4
Kaposi sarcoma commonly presents as violaceous or dark red macules, patches, papules, plaques, and nodules on various parts of the body (Figure 3). Lesions typically begin as macules and progress into plaques or nodules. Our patient presented as a deceptively healthy young man with lesions at various stages of development. In addition to the skin and oral mucosa, the lungs, lymph nodes, and gastrointestinal tract commonly are involved in AIDS-associated KS.5 Patients may experience symptoms of internal involvement, including bleeding, hematochezia, odynophagia, or dyspnea.
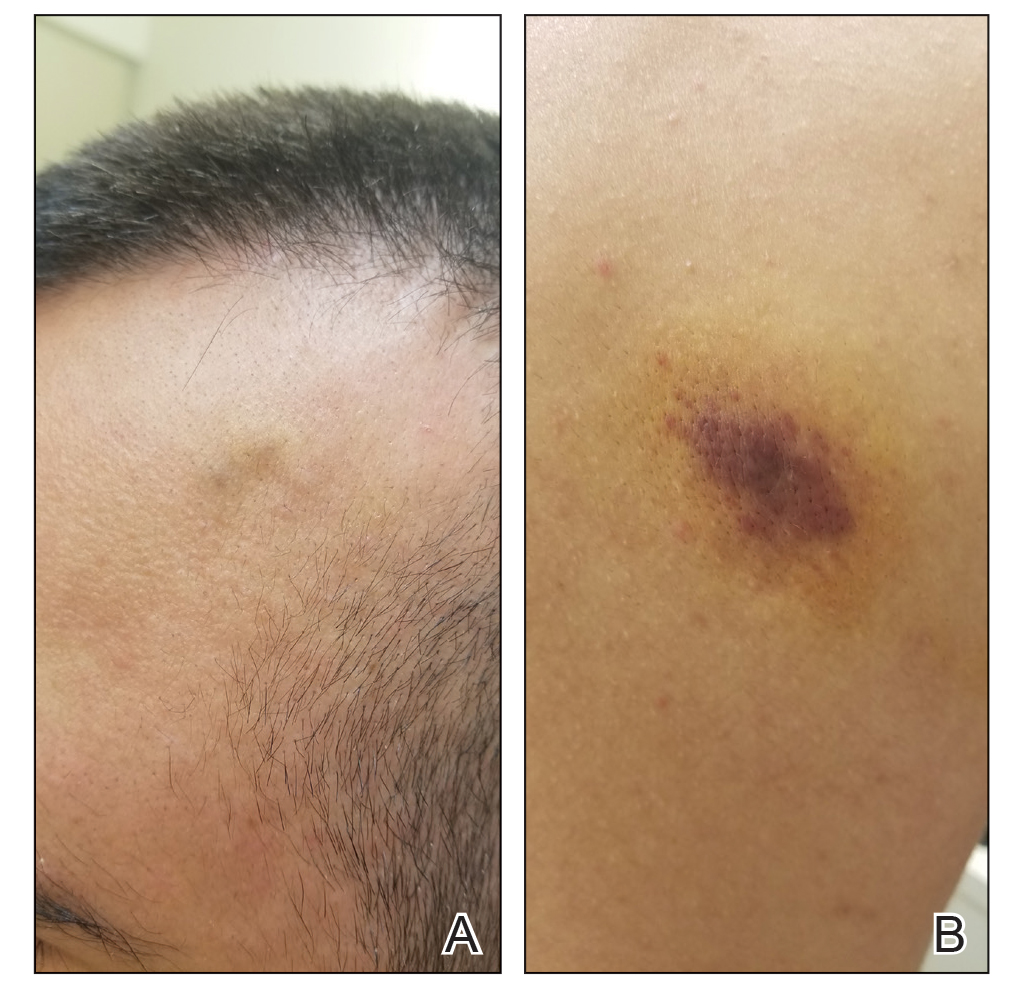
The differential diagnosis includes conditions that can mimic KS, including bacillary angiomatosis, angioinvasive fungal disease, sarcoid, and other malignancies. A skin biopsy is the gold standard for definitive diagnosis of KS. Histopathology shows a vascular proliferation in the dermis and spindle cell proliferation.6 Kaposi sarcoma stains positively for factor VIII–related antigen, CD31, and CD34.2 Additionally, staining for HHV-8 gene products, such as latency-associated nuclear antigen 1, is helpful in differentiating KS from other conditions.7
In HIV-associated KS, the mainstay of treatment is initiation of highly active antiretroviral therapy. Typically, as the CD4 count rises with treatment, the tumor burden classic KS, effective treatment options include recurrent cryotherapy or intralesional chemotherapeutics, such as vincristine, for localized lesions; for widespread disease, pegylated liposomal doxorubicin or radiation have been found to be effective options. Lastly, for patients with iatrogenic KS, reducing immunosuppressive medications is a reasonable first step in management. If this does not yield adequate improvement, transitioning from calcineurin inhibitors (eg, cyclosporine) to proliferation signal inhibitors (eg, sirolimus) may lead to resolution.7
- Friedman-Kien AE, Saltzman BR. Clinical manifestations of classical, endemic African, and epidemic AIDS-associated Kaposi’s sarcoma. J Am Acad Dermatol. 1990;22:1237-1250.
- Radu O, Pantanowitz L. Kaposi sarcoma. Arch Pathol Lab Med. 2013;137:289-294.
- Vangipuram R, Tyring SK. Epidemiology of Kaposi sarcoma: review and description of the nonepidemic variant. Int J Dermatol. 2019;58:538-542.
- Klepp O, Dahl O, Stenwig JT. Association of Kaposi’s sarcoma and prior immunosuppressive therapy. a 5‐year material of Kaposi’s sarcoma in Norway. Cancer. 1978;42:2626-2630.
- Lemlich G, Schwam L, Lebwohl M. Kaposi’s sarcoma and acquired immunodeficiency syndrome: postmortem findings in twenty-four cases. J Am Acad Dermatol. 1987;16:319-325.
- Kaposi sarcoma. Nat Rev Dis Primers. 2019;5:10.
- Curtiss P, Strazzulla LC, Friedman-Kien AE. An update on Kaposi’s sarcoma: epidemiology, pathogenesis and treatment. Dermatol Ther. 2016;6:465-470.
The Diagnosis: Kaposi Sarcoma
A punch biopsy of a lesion on the right side of the back revealed a diffuse, poorly circumscribed, spindle cell neoplasm of the papillary and reticular dermis with associated vascular and pseudovascular spaces distended by erythrocytes (Figure 1). Immunostaining was positive for human herpesvirus 8 (HHV-8)(Figure 2), ETS-related gene, CD31, and CD34 and negative for pan cytokeratin, confirming the diagnosis of Kaposi sarcoma (KS). Bacterial, fungal, and mycobacterial tissue cultures were negative. The patient was tested for HIV and referred to infectious disease and oncology. He subsequently was found to have HIV with a viral load greater than 1 million copies. He was started on antiretroviral therapy and Pneumocystis jirovecii pneumonia prophylaxis. Computed tomography of the chest, abdomen, and pelvis showed bilateral, multifocal, perihilar, flame-shaped consolidations suggestive of KS. The patient later disclosed having an intermittent dry cough of more than a year’s duration with occasional bright red blood per rectum after bowel movements. After workup, the patient was found to have cytomegalovirus esophagitis/gastritis and candidal esophagitis that were treated with valganciclovir and fluconazole, respectively.

Kaposi sarcoma is an angioproliferative, AIDSdefining disease associated with HHV-8. There are 4 types of KS as defined by the populations they affect. AIDS-associated KS occurs in individuals with HIV, as seen in our patient. It often is accompanied by extensive mucocutaneous and visceral lesions, as well as systemic symptoms such as fever, weight loss, and diarrhea.1 Classic KS is a variant that presents in older men of Mediterranean, Eastern European, and South American descent. Cutaneous lesions typically are distributed on the lower extremities.2,3 Endemic (African) KS is seen in HIV-negative children and young adults in equatorial Africa. It most commonly affects the lower extremities or lymph nodes and usually follows a more aggressive course.2 Lastly, iatrogenic KS is associated with immunosuppressive medications or conditions, such as organ transplantation, chemotherapy, and rheumatologic disorders.3,4

Kaposi sarcoma commonly presents as violaceous or dark red macules, patches, papules, plaques, and nodules on various parts of the body (Figure 3). Lesions typically begin as macules and progress into plaques or nodules. Our patient presented as a deceptively healthy young man with lesions at various stages of development. In addition to the skin and oral mucosa, the lungs, lymph nodes, and gastrointestinal tract commonly are involved in AIDS-associated KS.5 Patients may experience symptoms of internal involvement, including bleeding, hematochezia, odynophagia, or dyspnea.

The differential diagnosis includes conditions that can mimic KS, including bacillary angiomatosis, angioinvasive fungal disease, sarcoid, and other malignancies. A skin biopsy is the gold standard for definitive diagnosis of KS. Histopathology shows a vascular proliferation in the dermis and spindle cell proliferation.6 Kaposi sarcoma stains positively for factor VIII–related antigen, CD31, and CD34.2 Additionally, staining for HHV-8 gene products, such as latency-associated nuclear antigen 1, is helpful in differentiating KS from other conditions.7
In HIV-associated KS, the mainstay of treatment is initiation of highly active antiretroviral therapy. Typically, as the CD4 count rises with treatment, the tumor burden classic KS, effective treatment options include recurrent cryotherapy or intralesional chemotherapeutics, such as vincristine, for localized lesions; for widespread disease, pegylated liposomal doxorubicin or radiation have been found to be effective options. Lastly, for patients with iatrogenic KS, reducing immunosuppressive medications is a reasonable first step in management. If this does not yield adequate improvement, transitioning from calcineurin inhibitors (eg, cyclosporine) to proliferation signal inhibitors (eg, sirolimus) may lead to resolution.7
The Diagnosis: Kaposi Sarcoma
A punch biopsy of a lesion on the right side of the back revealed a diffuse, poorly circumscribed, spindle cell neoplasm of the papillary and reticular dermis with associated vascular and pseudovascular spaces distended by erythrocytes (Figure 1). Immunostaining was positive for human herpesvirus 8 (HHV-8)(Figure 2), ETS-related gene, CD31, and CD34 and negative for pan cytokeratin, confirming the diagnosis of Kaposi sarcoma (KS). Bacterial, fungal, and mycobacterial tissue cultures were negative. The patient was tested for HIV and referred to infectious disease and oncology. He subsequently was found to have HIV with a viral load greater than 1 million copies. He was started on antiretroviral therapy and Pneumocystis jirovecii pneumonia prophylaxis. Computed tomography of the chest, abdomen, and pelvis showed bilateral, multifocal, perihilar, flame-shaped consolidations suggestive of KS. The patient later disclosed having an intermittent dry cough of more than a year’s duration with occasional bright red blood per rectum after bowel movements. After workup, the patient was found to have cytomegalovirus esophagitis/gastritis and candidal esophagitis that were treated with valganciclovir and fluconazole, respectively.

Kaposi sarcoma is an angioproliferative, AIDSdefining disease associated with HHV-8. There are 4 types of KS as defined by the populations they affect. AIDS-associated KS occurs in individuals with HIV, as seen in our patient. It often is accompanied by extensive mucocutaneous and visceral lesions, as well as systemic symptoms such as fever, weight loss, and diarrhea.1 Classic KS is a variant that presents in older men of Mediterranean, Eastern European, and South American descent. Cutaneous lesions typically are distributed on the lower extremities.2,3 Endemic (African) KS is seen in HIV-negative children and young adults in equatorial Africa. It most commonly affects the lower extremities or lymph nodes and usually follows a more aggressive course.2 Lastly, iatrogenic KS is associated with immunosuppressive medications or conditions, such as organ transplantation, chemotherapy, and rheumatologic disorders.3,4

Kaposi sarcoma commonly presents as violaceous or dark red macules, patches, papules, plaques, and nodules on various parts of the body (Figure 3). Lesions typically begin as macules and progress into plaques or nodules. Our patient presented as a deceptively healthy young man with lesions at various stages of development. In addition to the skin and oral mucosa, the lungs, lymph nodes, and gastrointestinal tract commonly are involved in AIDS-associated KS.5 Patients may experience symptoms of internal involvement, including bleeding, hematochezia, odynophagia, or dyspnea.

The differential diagnosis includes conditions that can mimic KS, including bacillary angiomatosis, angioinvasive fungal disease, sarcoid, and other malignancies. A skin biopsy is the gold standard for definitive diagnosis of KS. Histopathology shows a vascular proliferation in the dermis and spindle cell proliferation.6 Kaposi sarcoma stains positively for factor VIII–related antigen, CD31, and CD34.2 Additionally, staining for HHV-8 gene products, such as latency-associated nuclear antigen 1, is helpful in differentiating KS from other conditions.7
In HIV-associated KS, the mainstay of treatment is initiation of highly active antiretroviral therapy. Typically, as the CD4 count rises with treatment, the tumor burden classic KS, effective treatment options include recurrent cryotherapy or intralesional chemotherapeutics, such as vincristine, for localized lesions; for widespread disease, pegylated liposomal doxorubicin or radiation have been found to be effective options. Lastly, for patients with iatrogenic KS, reducing immunosuppressive medications is a reasonable first step in management. If this does not yield adequate improvement, transitioning from calcineurin inhibitors (eg, cyclosporine) to proliferation signal inhibitors (eg, sirolimus) may lead to resolution.7
- Friedman-Kien AE, Saltzman BR. Clinical manifestations of classical, endemic African, and epidemic AIDS-associated Kaposi’s sarcoma. J Am Acad Dermatol. 1990;22:1237-1250.
- Radu O, Pantanowitz L. Kaposi sarcoma. Arch Pathol Lab Med. 2013;137:289-294.
- Vangipuram R, Tyring SK. Epidemiology of Kaposi sarcoma: review and description of the nonepidemic variant. Int J Dermatol. 2019;58:538-542.
- Klepp O, Dahl O, Stenwig JT. Association of Kaposi’s sarcoma and prior immunosuppressive therapy. a 5‐year material of Kaposi’s sarcoma in Norway. Cancer. 1978;42:2626-2630.
- Lemlich G, Schwam L, Lebwohl M. Kaposi’s sarcoma and acquired immunodeficiency syndrome: postmortem findings in twenty-four cases. J Am Acad Dermatol. 1987;16:319-325.
- Kaposi sarcoma. Nat Rev Dis Primers. 2019;5:10.
- Curtiss P, Strazzulla LC, Friedman-Kien AE. An update on Kaposi’s sarcoma: epidemiology, pathogenesis and treatment. Dermatol Ther. 2016;6:465-470.
- Friedman-Kien AE, Saltzman BR. Clinical manifestations of classical, endemic African, and epidemic AIDS-associated Kaposi’s sarcoma. J Am Acad Dermatol. 1990;22:1237-1250.
- Radu O, Pantanowitz L. Kaposi sarcoma. Arch Pathol Lab Med. 2013;137:289-294.
- Vangipuram R, Tyring SK. Epidemiology of Kaposi sarcoma: review and description of the nonepidemic variant. Int J Dermatol. 2019;58:538-542.
- Klepp O, Dahl O, Stenwig JT. Association of Kaposi’s sarcoma and prior immunosuppressive therapy. a 5‐year material of Kaposi’s sarcoma in Norway. Cancer. 1978;42:2626-2630.
- Lemlich G, Schwam L, Lebwohl M. Kaposi’s sarcoma and acquired immunodeficiency syndrome: postmortem findings in twenty-four cases. J Am Acad Dermatol. 1987;16:319-325.
- Kaposi sarcoma. Nat Rev Dis Primers. 2019;5:10.
- Curtiss P, Strazzulla LC, Friedman-Kien AE. An update on Kaposi’s sarcoma: epidemiology, pathogenesis and treatment. Dermatol Ther. 2016;6:465-470.
A 25-year-old man with no notable medical history presented to the dermatology clinic with growing selfdescribed cysts on the face, trunk, and legs of 6 months’ duration. The lesions started as bruiselike discolorations and progressed to become firm nodules and inflamed masses. Some were minimally itchy and sensitive to touch, but there was no history of bleeding or drainage. The patient denied any new or recent environmental or animal exposures, use of illicit drugs, or travel correlating with the rash onset. He denied any prior treatments. He reported being in his normal state of health and was not taking any medications. Physical examination revealed indurated, violaceous, purpuric subcutaneous nodules, plaques, and masses on the forehead, cheek (top), jaw, flank, axillae (bottom), and back.
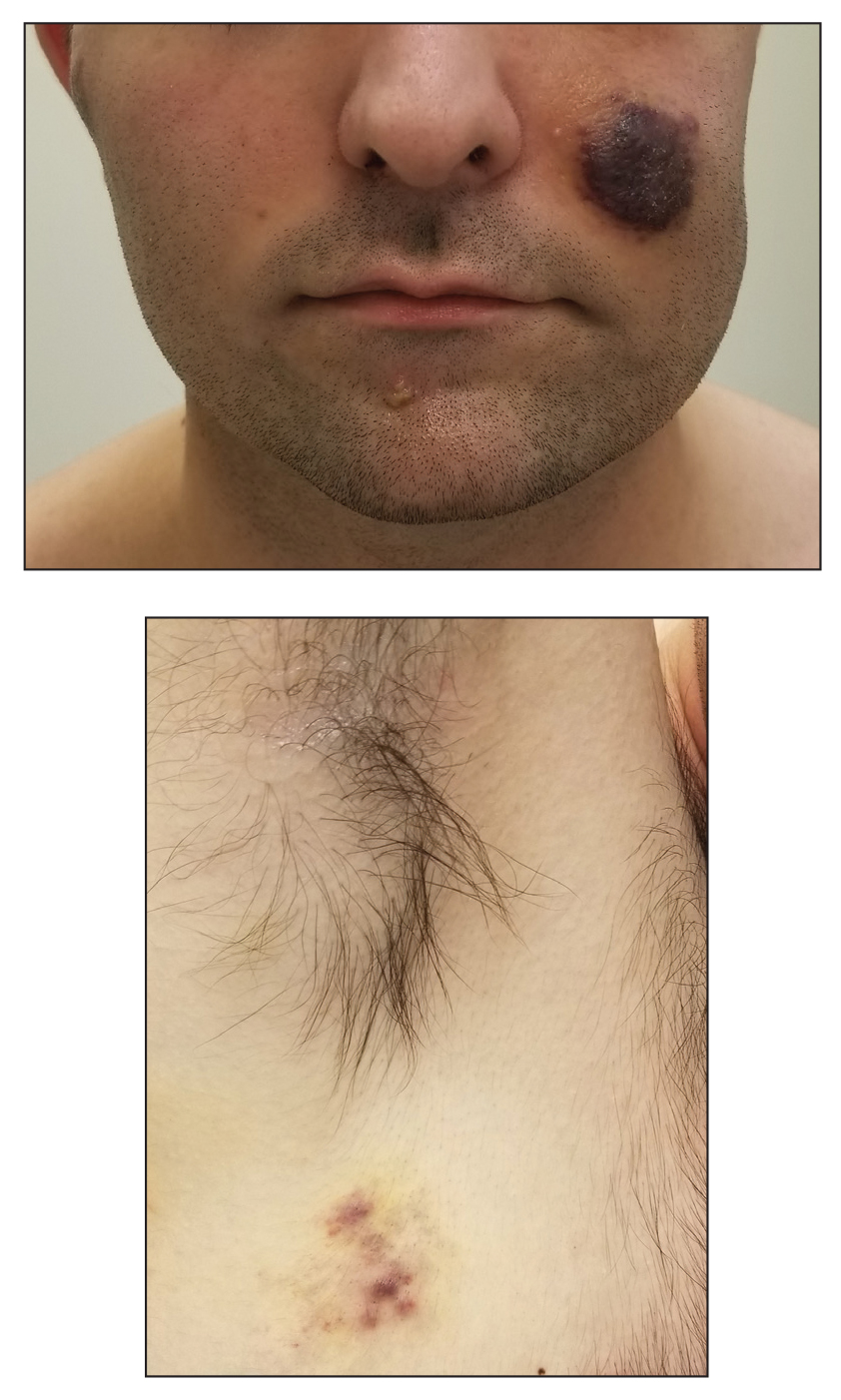
Erythematous Indurated Nodule on the Forehead
The Diagnosis: Dermatofibrosarcoma Protuberans
Histopathologic examination showed a dermal tumor composed of spindle cells in a storiform arrangement (Figure 1). Immunohistochemistry demonstrated positive CD34 staining of the tumoral cells (Figure 2). Clinical review, histopathologic examination, and immunohistochemistry confirmed a diagnosis of dermatofibrosarcoma protuberans (DFSP). The patient underwent Mohs micrographic surgery (MMS) with clear margins after 3 stages, followed by repair with a rotation flap. No evidence of recurrence was found at 4-year follow-up.
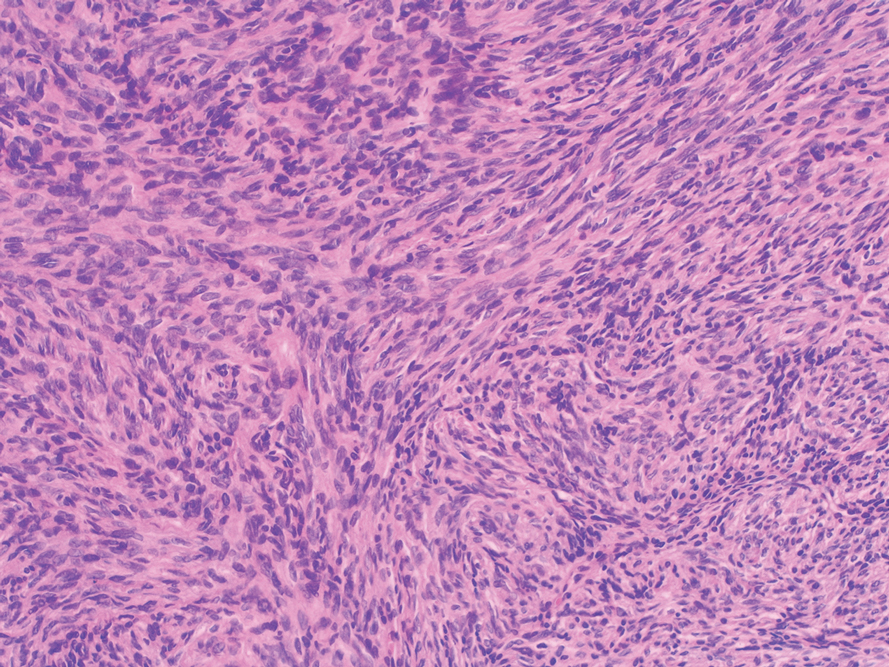
Dermatofibrosarcoma protuberans is a rare low-grade sarcoma of fibroblast origin with an annual incidence of 0.8 to 5 cases per million individuals.1 It typically presents in patients aged 30 to 50 years on the trunk, scalp, or proximal extremities as an asymptomatic, flesh-colored, erythematous or brown, indurated plaque or nodule.2 Due to its variable presentation, these lesions often may be misdiagnosed as lipomas or epidermoid cysts, preventing proper targeted treatment. Therefore, suspicious enlarging indurated nodules require a lower threshold for biopsy.1
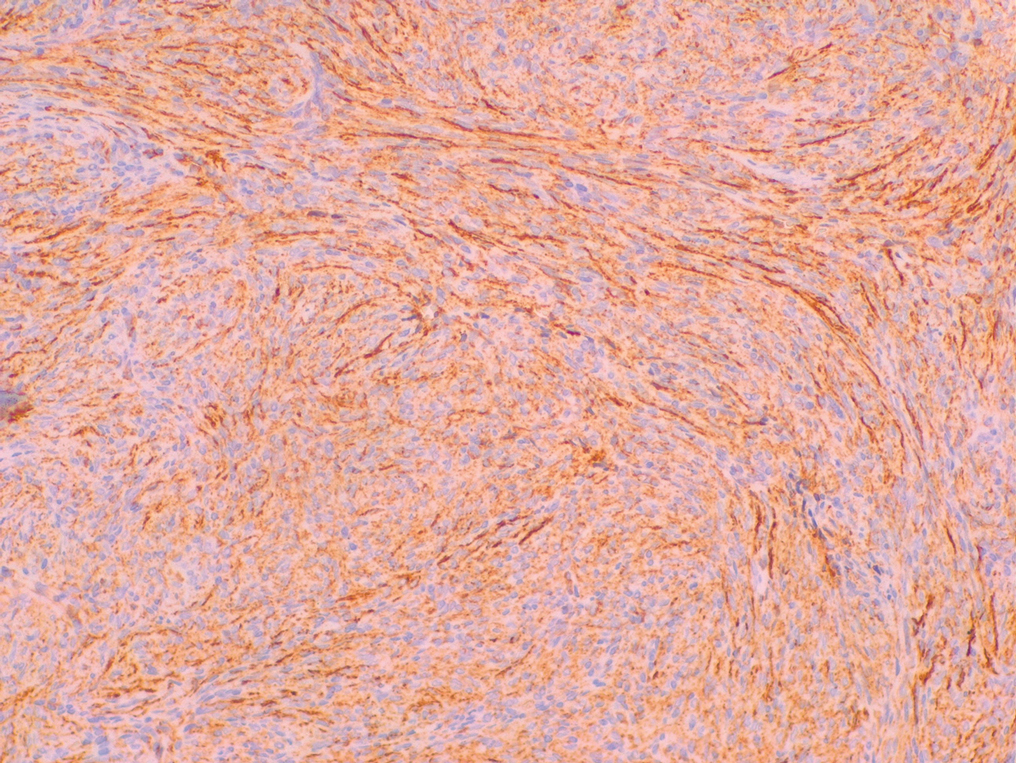
A definitive diagnosis of DFSP is achieved after a biopsy and histopathologic evaluation. Hematoxylin and eosin staining typically shows diffuse infiltration of the dermis and the subcutaneous fat by densely packed, cytologic, relatively uniform, spindle-shaped tumor cells arranged in a characteristic storiform shape. Tumor cells are spread along the septae of the subcutaneous fatty tissue.3 Immunohistochemistry is characterized by positive CD34 and negative factor XIIIa, with rare exceptions.
The differential diagnosis includes lipoma, epidermoid cyst, plexiform fibrohistiocytic tumor, and malignant peripheral nerve sheath tumor.3 Positive CD34 immunostaining, negative S-100 staining, and a storiform pattern of spindle cells can assist in differentiating DFSP from these possible differential diagnoses; lesions of these other entities are characterized by different pathologic findings. Lipomas are composed of fat tissue, epidermoid cysts have epithelial-lined cysts filled with keratin, plexiform fibrohistiocytic tumors have plexiform rays of fibrous tissue extending into fat with negative CD34 staining, and malignant peripheral nerve sheath tumors have fleshy variegated masses involving the peripheral nerve trunks with partial S-100 staining.4-7 Additional evaluation to confirm DFSP can be accomplished by analysis of tumor samples by fluorescence in situ hybridization or reverse transcriptase–polymerase chain reaction to detect chromosomal translocations and fusion gene transcripts, as chromosomal translocations may be found in more than 90% of cases.3
Early diagnosis of DFSP is beneficial, as it can help prevent recurrence as well as metastasis. Studies have attempted to document the risk for recurrence as well as metastasis based on characteristic features and treatment strategies of DFSP. In a study of 186 patients, 3 had metastatic disease to the lungs, the most common site of metastasis.8 These 3 patients had fibrosarcomatous transformation within DFSP, emphasizing the importance of detailing this finding early in the diagnosis, as it was characterized by a higher degree of cellularity, cytologic atypia, mitotic activity, and negative CD34 immunostaining.9 In patients with suspected metastasis, lymph node ultrasonography, chest radiography, and computed tomography may be utilized.3
When treating DFSP, the goal is complete removal of the tumor with clear margins. Mohs micrographic surgery, modified MMS, and wide local excision (WLE) with 2- to 4-cm margins are appropriate treatment options, though MMS is the treatment of choice. A study comparing MMS and WLE demonstrated 3% and 30.8% recurrence rates, respectively.8 In MMS, complete margin evaluation on microscopy is performed after each stage to ensure negative surgical margins. The presence of positive surgical margins elicits continued resection until the margins are clear.10,11
Other treatment modalities may be considered for patients with DFSP. Molecular therapy with imatinib, an oral tyrosine kinase inhibitor targeting platelet-derived growth factor–regulated expression, can be utilized for inoperable tumors; however, additional clinical trials are required to ensure efficacy.3 Surgical removal of the possible remaining tumor is still recommended after molecular therapy. Radiotherapy is an additional method of treatment that may be used for inoperable tumors.3
Dermatofibrosarcoma protuberans is a rare lowgrade sarcoma of fibroblast origin that typically does not metastasize but often has notable subclinical extension and recurrence. Differentiating DFSP from other tumors often may be difficult. A protuberant, flesh-colored, slowgrowing, and asymptomatic lesion often may be confused with lipomas or epidermoid cysts; therefore, biopsies with immunohistostaining for suspicious lesions is required.12 Mohs micrographic surgery has evolved as the treatment of choice for this tumor, though WLE and new targeted molecular therapies still are considered. Proper diagnosis and treatment of DFSP is paramount in preventing future morbidity.
- Benoit A, Aycock J, Milam D, et al. Dermatofibrosarcoma protuberans of the forehead with extensive subclinical spread. Dermatol Surg. 2016;42:261-264. doi:10.1097/DSS.0000000000000604
- Khachemoune A, Barkoe D, Braun M, et al. Dermatofibrosarcoma protuberans of the forehead and scalp with involvement of the outer calvarial plate: multistaged repair with the use of skin expanders. Dermatol Surg. 2005;31:115-119. doi:10.1111/j.1524-4725.2005.31021
- Saiag P, Grob J-J, Lebbe C, et al. Diagnosis and treatment of dermatofibrosarcoma protuberans. European consensus-based interdisciplinary guideline. Eur J Cancer. 2015;51:2604-2608. doi:10.1016/j.ejca.2015.06.108
- Charifa A, Badri T. Lipomas, pathology. StatPearls. StatPearls Publishing; 2020.
- Zito PM, Scharf R. Cyst, epidermoid (sebaceous cyst). StatPearls. StatPearls Publishing; 2020.
- Taher A, Pushpanathan C. Plexiform fibrohistiocytic tumor: a brief review. Arch Pathol Lab Med. 2007;131:1135-1138. doi:10.5858 /2007-131-1135-PFTABR
- Rodriguez FJ, Folpe AL, Giannini C, et al. Pathology of peripheral nerve sheath tumors: diagnostic overview and update on selected diagnostic problems. Acta Neuropathol. 2012;123:295-319. doi:10.1007 /s00401-012-0954-z
- Lowe GC, Onajin O, Baum CL, et al. A comparison of Mohs micrographic surgery and wide local excision for treatment of dermatofibrosarcoma protuberans with long-term follow-up: the Mayo Clinic experience. Dermatol Surg. 2017;43:98-106. doi:10.1097/DSS.0000000000000910
- Rouhani P, Fletcher CDM, Devesa SS, et al. Cutaneous soft tissue sarcoma incidence patterns in the U.S.: an analysis of 12,114 cases. Cancer. 2008;113:616-627. doi:10.1002/cncr.23571
- Ratner D, Thomas CO, Johnson TM, et al. Mohs micrographic surgery for the treatment of dermatofibrosarcoma protuberans. results of a multiinstitutional series with an analysis of the extent of microscopic spread. J Am Acad Dermatol. 1997;37:600-613. doi:10.1016/s0190 -9622(97)70179-8
- Buck DW, Kim JYS, Alam M, et al. Multidisciplinary approach to the management of dermatofibrosarcoma protuberans. J Am Acad Dermatol. 2012;67:861-866. doi:10.1016/j.jaad.2012.01.039
- Shih P-Y, Chen C-H, Kuo T-T, et al. Deep dermatofibrosarcoma protuberans: a pitfall in the ultrasonographic diagnosis of lipoma -like subcutaneous lesions. Dermatologica Sinica. 2010;28:32-35. doi:10.1016/S1027-8117(10)60005-5
The Diagnosis: Dermatofibrosarcoma Protuberans
Histopathologic examination showed a dermal tumor composed of spindle cells in a storiform arrangement (Figure 1). Immunohistochemistry demonstrated positive CD34 staining of the tumoral cells (Figure 2). Clinical review, histopathologic examination, and immunohistochemistry confirmed a diagnosis of dermatofibrosarcoma protuberans (DFSP). The patient underwent Mohs micrographic surgery (MMS) with clear margins after 3 stages, followed by repair with a rotation flap. No evidence of recurrence was found at 4-year follow-up.

Dermatofibrosarcoma protuberans is a rare low-grade sarcoma of fibroblast origin with an annual incidence of 0.8 to 5 cases per million individuals.1 It typically presents in patients aged 30 to 50 years on the trunk, scalp, or proximal extremities as an asymptomatic, flesh-colored, erythematous or brown, indurated plaque or nodule.2 Due to its variable presentation, these lesions often may be misdiagnosed as lipomas or epidermoid cysts, preventing proper targeted treatment. Therefore, suspicious enlarging indurated nodules require a lower threshold for biopsy.1

A definitive diagnosis of DFSP is achieved after a biopsy and histopathologic evaluation. Hematoxylin and eosin staining typically shows diffuse infiltration of the dermis and the subcutaneous fat by densely packed, cytologic, relatively uniform, spindle-shaped tumor cells arranged in a characteristic storiform shape. Tumor cells are spread along the septae of the subcutaneous fatty tissue.3 Immunohistochemistry is characterized by positive CD34 and negative factor XIIIa, with rare exceptions.
The differential diagnosis includes lipoma, epidermoid cyst, plexiform fibrohistiocytic tumor, and malignant peripheral nerve sheath tumor.3 Positive CD34 immunostaining, negative S-100 staining, and a storiform pattern of spindle cells can assist in differentiating DFSP from these possible differential diagnoses; lesions of these other entities are characterized by different pathologic findings. Lipomas are composed of fat tissue, epidermoid cysts have epithelial-lined cysts filled with keratin, plexiform fibrohistiocytic tumors have plexiform rays of fibrous tissue extending into fat with negative CD34 staining, and malignant peripheral nerve sheath tumors have fleshy variegated masses involving the peripheral nerve trunks with partial S-100 staining.4-7 Additional evaluation to confirm DFSP can be accomplished by analysis of tumor samples by fluorescence in situ hybridization or reverse transcriptase–polymerase chain reaction to detect chromosomal translocations and fusion gene transcripts, as chromosomal translocations may be found in more than 90% of cases.3
Early diagnosis of DFSP is beneficial, as it can help prevent recurrence as well as metastasis. Studies have attempted to document the risk for recurrence as well as metastasis based on characteristic features and treatment strategies of DFSP. In a study of 186 patients, 3 had metastatic disease to the lungs, the most common site of metastasis.8 These 3 patients had fibrosarcomatous transformation within DFSP, emphasizing the importance of detailing this finding early in the diagnosis, as it was characterized by a higher degree of cellularity, cytologic atypia, mitotic activity, and negative CD34 immunostaining.9 In patients with suspected metastasis, lymph node ultrasonography, chest radiography, and computed tomography may be utilized.3
When treating DFSP, the goal is complete removal of the tumor with clear margins. Mohs micrographic surgery, modified MMS, and wide local excision (WLE) with 2- to 4-cm margins are appropriate treatment options, though MMS is the treatment of choice. A study comparing MMS and WLE demonstrated 3% and 30.8% recurrence rates, respectively.8 In MMS, complete margin evaluation on microscopy is performed after each stage to ensure negative surgical margins. The presence of positive surgical margins elicits continued resection until the margins are clear.10,11
Other treatment modalities may be considered for patients with DFSP. Molecular therapy with imatinib, an oral tyrosine kinase inhibitor targeting platelet-derived growth factor–regulated expression, can be utilized for inoperable tumors; however, additional clinical trials are required to ensure efficacy.3 Surgical removal of the possible remaining tumor is still recommended after molecular therapy. Radiotherapy is an additional method of treatment that may be used for inoperable tumors.3
Dermatofibrosarcoma protuberans is a rare lowgrade sarcoma of fibroblast origin that typically does not metastasize but often has notable subclinical extension and recurrence. Differentiating DFSP from other tumors often may be difficult. A protuberant, flesh-colored, slowgrowing, and asymptomatic lesion often may be confused with lipomas or epidermoid cysts; therefore, biopsies with immunohistostaining for suspicious lesions is required.12 Mohs micrographic surgery has evolved as the treatment of choice for this tumor, though WLE and new targeted molecular therapies still are considered. Proper diagnosis and treatment of DFSP is paramount in preventing future morbidity.
The Diagnosis: Dermatofibrosarcoma Protuberans
Histopathologic examination showed a dermal tumor composed of spindle cells in a storiform arrangement (Figure 1). Immunohistochemistry demonstrated positive CD34 staining of the tumoral cells (Figure 2). Clinical review, histopathologic examination, and immunohistochemistry confirmed a diagnosis of dermatofibrosarcoma protuberans (DFSP). The patient underwent Mohs micrographic surgery (MMS) with clear margins after 3 stages, followed by repair with a rotation flap. No evidence of recurrence was found at 4-year follow-up.

Dermatofibrosarcoma protuberans is a rare low-grade sarcoma of fibroblast origin with an annual incidence of 0.8 to 5 cases per million individuals.1 It typically presents in patients aged 30 to 50 years on the trunk, scalp, or proximal extremities as an asymptomatic, flesh-colored, erythematous or brown, indurated plaque or nodule.2 Due to its variable presentation, these lesions often may be misdiagnosed as lipomas or epidermoid cysts, preventing proper targeted treatment. Therefore, suspicious enlarging indurated nodules require a lower threshold for biopsy.1

A definitive diagnosis of DFSP is achieved after a biopsy and histopathologic evaluation. Hematoxylin and eosin staining typically shows diffuse infiltration of the dermis and the subcutaneous fat by densely packed, cytologic, relatively uniform, spindle-shaped tumor cells arranged in a characteristic storiform shape. Tumor cells are spread along the septae of the subcutaneous fatty tissue.3 Immunohistochemistry is characterized by positive CD34 and negative factor XIIIa, with rare exceptions.
The differential diagnosis includes lipoma, epidermoid cyst, plexiform fibrohistiocytic tumor, and malignant peripheral nerve sheath tumor.3 Positive CD34 immunostaining, negative S-100 staining, and a storiform pattern of spindle cells can assist in differentiating DFSP from these possible differential diagnoses; lesions of these other entities are characterized by different pathologic findings. Lipomas are composed of fat tissue, epidermoid cysts have epithelial-lined cysts filled with keratin, plexiform fibrohistiocytic tumors have plexiform rays of fibrous tissue extending into fat with negative CD34 staining, and malignant peripheral nerve sheath tumors have fleshy variegated masses involving the peripheral nerve trunks with partial S-100 staining.4-7 Additional evaluation to confirm DFSP can be accomplished by analysis of tumor samples by fluorescence in situ hybridization or reverse transcriptase–polymerase chain reaction to detect chromosomal translocations and fusion gene transcripts, as chromosomal translocations may be found in more than 90% of cases.3
Early diagnosis of DFSP is beneficial, as it can help prevent recurrence as well as metastasis. Studies have attempted to document the risk for recurrence as well as metastasis based on characteristic features and treatment strategies of DFSP. In a study of 186 patients, 3 had metastatic disease to the lungs, the most common site of metastasis.8 These 3 patients had fibrosarcomatous transformation within DFSP, emphasizing the importance of detailing this finding early in the diagnosis, as it was characterized by a higher degree of cellularity, cytologic atypia, mitotic activity, and negative CD34 immunostaining.9 In patients with suspected metastasis, lymph node ultrasonography, chest radiography, and computed tomography may be utilized.3
When treating DFSP, the goal is complete removal of the tumor with clear margins. Mohs micrographic surgery, modified MMS, and wide local excision (WLE) with 2- to 4-cm margins are appropriate treatment options, though MMS is the treatment of choice. A study comparing MMS and WLE demonstrated 3% and 30.8% recurrence rates, respectively.8 In MMS, complete margin evaluation on microscopy is performed after each stage to ensure negative surgical margins. The presence of positive surgical margins elicits continued resection until the margins are clear.10,11
Other treatment modalities may be considered for patients with DFSP. Molecular therapy with imatinib, an oral tyrosine kinase inhibitor targeting platelet-derived growth factor–regulated expression, can be utilized for inoperable tumors; however, additional clinical trials are required to ensure efficacy.3 Surgical removal of the possible remaining tumor is still recommended after molecular therapy. Radiotherapy is an additional method of treatment that may be used for inoperable tumors.3
Dermatofibrosarcoma protuberans is a rare lowgrade sarcoma of fibroblast origin that typically does not metastasize but often has notable subclinical extension and recurrence. Differentiating DFSP from other tumors often may be difficult. A protuberant, flesh-colored, slowgrowing, and asymptomatic lesion often may be confused with lipomas or epidermoid cysts; therefore, biopsies with immunohistostaining for suspicious lesions is required.12 Mohs micrographic surgery has evolved as the treatment of choice for this tumor, though WLE and new targeted molecular therapies still are considered. Proper diagnosis and treatment of DFSP is paramount in preventing future morbidity.
- Benoit A, Aycock J, Milam D, et al. Dermatofibrosarcoma protuberans of the forehead with extensive subclinical spread. Dermatol Surg. 2016;42:261-264. doi:10.1097/DSS.0000000000000604
- Khachemoune A, Barkoe D, Braun M, et al. Dermatofibrosarcoma protuberans of the forehead and scalp with involvement of the outer calvarial plate: multistaged repair with the use of skin expanders. Dermatol Surg. 2005;31:115-119. doi:10.1111/j.1524-4725.2005.31021
- Saiag P, Grob J-J, Lebbe C, et al. Diagnosis and treatment of dermatofibrosarcoma protuberans. European consensus-based interdisciplinary guideline. Eur J Cancer. 2015;51:2604-2608. doi:10.1016/j.ejca.2015.06.108
- Charifa A, Badri T. Lipomas, pathology. StatPearls. StatPearls Publishing; 2020.
- Zito PM, Scharf R. Cyst, epidermoid (sebaceous cyst). StatPearls. StatPearls Publishing; 2020.
- Taher A, Pushpanathan C. Plexiform fibrohistiocytic tumor: a brief review. Arch Pathol Lab Med. 2007;131:1135-1138. doi:10.5858 /2007-131-1135-PFTABR
- Rodriguez FJ, Folpe AL, Giannini C, et al. Pathology of peripheral nerve sheath tumors: diagnostic overview and update on selected diagnostic problems. Acta Neuropathol. 2012;123:295-319. doi:10.1007 /s00401-012-0954-z
- Lowe GC, Onajin O, Baum CL, et al. A comparison of Mohs micrographic surgery and wide local excision for treatment of dermatofibrosarcoma protuberans with long-term follow-up: the Mayo Clinic experience. Dermatol Surg. 2017;43:98-106. doi:10.1097/DSS.0000000000000910
- Rouhani P, Fletcher CDM, Devesa SS, et al. Cutaneous soft tissue sarcoma incidence patterns in the U.S.: an analysis of 12,114 cases. Cancer. 2008;113:616-627. doi:10.1002/cncr.23571
- Ratner D, Thomas CO, Johnson TM, et al. Mohs micrographic surgery for the treatment of dermatofibrosarcoma protuberans. results of a multiinstitutional series with an analysis of the extent of microscopic spread. J Am Acad Dermatol. 1997;37:600-613. doi:10.1016/s0190 -9622(97)70179-8
- Buck DW, Kim JYS, Alam M, et al. Multidisciplinary approach to the management of dermatofibrosarcoma protuberans. J Am Acad Dermatol. 2012;67:861-866. doi:10.1016/j.jaad.2012.01.039
- Shih P-Y, Chen C-H, Kuo T-T, et al. Deep dermatofibrosarcoma protuberans: a pitfall in the ultrasonographic diagnosis of lipoma -like subcutaneous lesions. Dermatologica Sinica. 2010;28:32-35. doi:10.1016/S1027-8117(10)60005-5
- Benoit A, Aycock J, Milam D, et al. Dermatofibrosarcoma protuberans of the forehead with extensive subclinical spread. Dermatol Surg. 2016;42:261-264. doi:10.1097/DSS.0000000000000604
- Khachemoune A, Barkoe D, Braun M, et al. Dermatofibrosarcoma protuberans of the forehead and scalp with involvement of the outer calvarial plate: multistaged repair with the use of skin expanders. Dermatol Surg. 2005;31:115-119. doi:10.1111/j.1524-4725.2005.31021
- Saiag P, Grob J-J, Lebbe C, et al. Diagnosis and treatment of dermatofibrosarcoma protuberans. European consensus-based interdisciplinary guideline. Eur J Cancer. 2015;51:2604-2608. doi:10.1016/j.ejca.2015.06.108
- Charifa A, Badri T. Lipomas, pathology. StatPearls. StatPearls Publishing; 2020.
- Zito PM, Scharf R. Cyst, epidermoid (sebaceous cyst). StatPearls. StatPearls Publishing; 2020.
- Taher A, Pushpanathan C. Plexiform fibrohistiocytic tumor: a brief review. Arch Pathol Lab Med. 2007;131:1135-1138. doi:10.5858 /2007-131-1135-PFTABR
- Rodriguez FJ, Folpe AL, Giannini C, et al. Pathology of peripheral nerve sheath tumors: diagnostic overview and update on selected diagnostic problems. Acta Neuropathol. 2012;123:295-319. doi:10.1007 /s00401-012-0954-z
- Lowe GC, Onajin O, Baum CL, et al. A comparison of Mohs micrographic surgery and wide local excision for treatment of dermatofibrosarcoma protuberans with long-term follow-up: the Mayo Clinic experience. Dermatol Surg. 2017;43:98-106. doi:10.1097/DSS.0000000000000910
- Rouhani P, Fletcher CDM, Devesa SS, et al. Cutaneous soft tissue sarcoma incidence patterns in the U.S.: an analysis of 12,114 cases. Cancer. 2008;113:616-627. doi:10.1002/cncr.23571
- Ratner D, Thomas CO, Johnson TM, et al. Mohs micrographic surgery for the treatment of dermatofibrosarcoma protuberans. results of a multiinstitutional series with an analysis of the extent of microscopic spread. J Am Acad Dermatol. 1997;37:600-613. doi:10.1016/s0190 -9622(97)70179-8
- Buck DW, Kim JYS, Alam M, et al. Multidisciplinary approach to the management of dermatofibrosarcoma protuberans. J Am Acad Dermatol. 2012;67:861-866. doi:10.1016/j.jaad.2012.01.039
- Shih P-Y, Chen C-H, Kuo T-T, et al. Deep dermatofibrosarcoma protuberans: a pitfall in the ultrasonographic diagnosis of lipoma -like subcutaneous lesions. Dermatologica Sinica. 2010;28:32-35. doi:10.1016/S1027-8117(10)60005-5
A 39-year-old man presented with an enlarging asymptomatic nodule on the forehead of more than 3 years’ duration. Physical examination revealed a 3.4×2.3-cm, indurated, firm, erythematous nodule on the frontotemporal scalp. The patient denied any history of trauma to the area.