User login
Patient with CKD: Contrast or no contrast?
A 67-year-old man with stage 3 chronic kidney disease (CKD) develops abdominal pain over 24 hours. He has had low grade fevers and nausea. He has a history of colon cancer and had a resection four years ago. Abdominal exam reveals tenderness to palpation, including rebound tenderness in his right lower quadrant. Labs: hemoglobin: 13; hematocrit: 39; white blood cells: 18,000; platelets: 333; blood urea nitrogen: 28; creatinine: 1.8 (estimated glomerular filtration rate: 37); sodium: 136; potassium: 3.9; bicarbonate: 24; chlorine: 105; and lipase: 10.
What testing would you recommend?
A) Ultrasound
B) Non contrast computed tomography (CT)
C) Contrast CT
D) MRI without gadolinium
The correct answer here is to get a contrast CT scan, as it will give you the most appropriate diagnostic information.
For years, we have hesitated to order contrast studies in our patients with CKD, for fear of causing contrast-induced nephrotoxicity. We might choose less helpful studies that avoid contrast, or might not obtain imaging that is needed. Over the years I have especially seen this in the case of avoiding computed tomography angiography (CTA) for evaluation of pulmonary embolus and choosing the much less useful ventilation/perfusion scan. The problem arises with the fact that patients with CKD are more likely to develop worsening renal function when they get sick.
Lee and colleagues performed an analysis of six retrospective studies involving a total of 55,963 participants. They found that patients with CKD receiving contrast material did not have an increased risk of deteriorating renal function compared with those without CKD (odds ratio, 1.07; 95% confidence interval, 0.98-1.17).1
The early studies reporting contrast-induced renal disease were in patients who received high osmolality contrast agents.2 Most patients now receive low osmolality agents, with less nephrotoxicity.3
Key points of guidelines
This year, the American College of Radiology and the National Kidney Foundation put out joint guidelines that helped clarify why there is a diminished concern for contrast-induced kidney disease in the modern era.4 Below are some of the key points of these guidelines:
- The risk of contrast-induced acute kidney injury (AKI) from intravenous iodinated contrast media is lower than previously thought.
- Necessary contrast material–enhanced CT without a suitable alternative should not be avoided solely on the basis of contrast-induced chronic kidney insufficiency risk.
- Contrast-induced AKI risk should be determined primarily by using CKD stage and AKI.
- Patients at high risk for contrast-induced kidney injury include those with recent AKI and those with estimated glomerular filtration rate (eGFR) less than 30 mL/min per 1.73 m2.
Data supporting guidelines
The data from several studies used to support these recommendations were impressive, showing just how low the risk for contrast-induced AKI is in most patients. In these studies, the risk of contrast-induced AKI has been estimated to be near 0% for patients with an eGFR greater than or equal to 45 and 0%-2% for patients with an eGFR of 30-44.5-7 This information and recommendations make imaging much easier. In most of our patients, we can get contrast studies when we need them. The group to be concerned about are patients with eGFRs less than 30. The guidelines single out this group as the patients where risk/benefit needs to be calculated before proceeding with the study, and to use prophylactic saline hydration in patients not undergoing dialysis.
Myth: Contrast-induced renal disease is common.
Dr. Paauw is professor of medicine in the division of general internal medicine at the University of Washington, Seattle, and he serves as third-year medical student clerkship director at the University of Washington. He is a member of the editorial advisory board of Internal Medicine News. Dr. Paauw has no conflicts to disclose. Contact him at [email protected].
References
1. Lee YC et al. Contrast-induced acute kidney injury among patients with chronic kidney disease undergoing imaging studies: A meta-analysis. Am J Roentgenol. 2019 Oct;213(4):728-35.
2. Luk L et al. Intravenous contrast-induced nephropathy: The rise and fall of a threatening idea. Adv Chronic Kidney Dis. 2017 May;24(3):169-75.
3. Goldfarb S et al. Low-osmolality contrast media and the risk of contrast-associated nephrotoxicity. Invest Radiol. 1993;28(Suppl 5):7-10.
4. Davenport MS, et al. Use of intravenous iodinated contrast media in patients with kidney disease: Consensus statements from the American College of Radiology and the National Kidney Foundation. Kidney Med. 2020 Jan 22;2(1):85-93.
5. Davenport MS et al. Contrast material–induced nephrotoxicity and intravenous low-osmolality iodinated contrast material. Radiology. 2013;267(1):94-105.
6. McDonald RJ et al. Intravenous contrast material–induced nephropathy: Causal or coincident phenomenon? Radiology. 2013;267(1):106-18.
7. McDonald JS et al. Risk of intravenous contrast material–mediated acute kidney injury: A propensity scorematched study stratified by baseline-estimated glomerular filtration rate. Radiology. 2014;271(1):65-73.
A 67-year-old man with stage 3 chronic kidney disease (CKD) develops abdominal pain over 24 hours. He has had low grade fevers and nausea. He has a history of colon cancer and had a resection four years ago. Abdominal exam reveals tenderness to palpation, including rebound tenderness in his right lower quadrant. Labs: hemoglobin: 13; hematocrit: 39; white blood cells: 18,000; platelets: 333; blood urea nitrogen: 28; creatinine: 1.8 (estimated glomerular filtration rate: 37); sodium: 136; potassium: 3.9; bicarbonate: 24; chlorine: 105; and lipase: 10.
What testing would you recommend?
A) Ultrasound
B) Non contrast computed tomography (CT)
C) Contrast CT
D) MRI without gadolinium
The correct answer here is to get a contrast CT scan, as it will give you the most appropriate diagnostic information.
For years, we have hesitated to order contrast studies in our patients with CKD, for fear of causing contrast-induced nephrotoxicity. We might choose less helpful studies that avoid contrast, or might not obtain imaging that is needed. Over the years I have especially seen this in the case of avoiding computed tomography angiography (CTA) for evaluation of pulmonary embolus and choosing the much less useful ventilation/perfusion scan. The problem arises with the fact that patients with CKD are more likely to develop worsening renal function when they get sick.
Lee and colleagues performed an analysis of six retrospective studies involving a total of 55,963 participants. They found that patients with CKD receiving contrast material did not have an increased risk of deteriorating renal function compared with those without CKD (odds ratio, 1.07; 95% confidence interval, 0.98-1.17).1
The early studies reporting contrast-induced renal disease were in patients who received high osmolality contrast agents.2 Most patients now receive low osmolality agents, with less nephrotoxicity.3
Key points of guidelines
This year, the American College of Radiology and the National Kidney Foundation put out joint guidelines that helped clarify why there is a diminished concern for contrast-induced kidney disease in the modern era.4 Below are some of the key points of these guidelines:
- The risk of contrast-induced acute kidney injury (AKI) from intravenous iodinated contrast media is lower than previously thought.
- Necessary contrast material–enhanced CT without a suitable alternative should not be avoided solely on the basis of contrast-induced chronic kidney insufficiency risk.
- Contrast-induced AKI risk should be determined primarily by using CKD stage and AKI.
- Patients at high risk for contrast-induced kidney injury include those with recent AKI and those with estimated glomerular filtration rate (eGFR) less than 30 mL/min per 1.73 m2.
Data supporting guidelines
The data from several studies used to support these recommendations were impressive, showing just how low the risk for contrast-induced AKI is in most patients. In these studies, the risk of contrast-induced AKI has been estimated to be near 0% for patients with an eGFR greater than or equal to 45 and 0%-2% for patients with an eGFR of 30-44.5-7 This information and recommendations make imaging much easier. In most of our patients, we can get contrast studies when we need them. The group to be concerned about are patients with eGFRs less than 30. The guidelines single out this group as the patients where risk/benefit needs to be calculated before proceeding with the study, and to use prophylactic saline hydration in patients not undergoing dialysis.
Myth: Contrast-induced renal disease is common.
Dr. Paauw is professor of medicine in the division of general internal medicine at the University of Washington, Seattle, and he serves as third-year medical student clerkship director at the University of Washington. He is a member of the editorial advisory board of Internal Medicine News. Dr. Paauw has no conflicts to disclose. Contact him at [email protected].
References
1. Lee YC et al. Contrast-induced acute kidney injury among patients with chronic kidney disease undergoing imaging studies: A meta-analysis. Am J Roentgenol. 2019 Oct;213(4):728-35.
2. Luk L et al. Intravenous contrast-induced nephropathy: The rise and fall of a threatening idea. Adv Chronic Kidney Dis. 2017 May;24(3):169-75.
3. Goldfarb S et al. Low-osmolality contrast media and the risk of contrast-associated nephrotoxicity. Invest Radiol. 1993;28(Suppl 5):7-10.
4. Davenport MS, et al. Use of intravenous iodinated contrast media in patients with kidney disease: Consensus statements from the American College of Radiology and the National Kidney Foundation. Kidney Med. 2020 Jan 22;2(1):85-93.
5. Davenport MS et al. Contrast material–induced nephrotoxicity and intravenous low-osmolality iodinated contrast material. Radiology. 2013;267(1):94-105.
6. McDonald RJ et al. Intravenous contrast material–induced nephropathy: Causal or coincident phenomenon? Radiology. 2013;267(1):106-18.
7. McDonald JS et al. Risk of intravenous contrast material–mediated acute kidney injury: A propensity scorematched study stratified by baseline-estimated glomerular filtration rate. Radiology. 2014;271(1):65-73.
A 67-year-old man with stage 3 chronic kidney disease (CKD) develops abdominal pain over 24 hours. He has had low grade fevers and nausea. He has a history of colon cancer and had a resection four years ago. Abdominal exam reveals tenderness to palpation, including rebound tenderness in his right lower quadrant. Labs: hemoglobin: 13; hematocrit: 39; white blood cells: 18,000; platelets: 333; blood urea nitrogen: 28; creatinine: 1.8 (estimated glomerular filtration rate: 37); sodium: 136; potassium: 3.9; bicarbonate: 24; chlorine: 105; and lipase: 10.
What testing would you recommend?
A) Ultrasound
B) Non contrast computed tomography (CT)
C) Contrast CT
D) MRI without gadolinium
The correct answer here is to get a contrast CT scan, as it will give you the most appropriate diagnostic information.
For years, we have hesitated to order contrast studies in our patients with CKD, for fear of causing contrast-induced nephrotoxicity. We might choose less helpful studies that avoid contrast, or might not obtain imaging that is needed. Over the years I have especially seen this in the case of avoiding computed tomography angiography (CTA) for evaluation of pulmonary embolus and choosing the much less useful ventilation/perfusion scan. The problem arises with the fact that patients with CKD are more likely to develop worsening renal function when they get sick.
Lee and colleagues performed an analysis of six retrospective studies involving a total of 55,963 participants. They found that patients with CKD receiving contrast material did not have an increased risk of deteriorating renal function compared with those without CKD (odds ratio, 1.07; 95% confidence interval, 0.98-1.17).1
The early studies reporting contrast-induced renal disease were in patients who received high osmolality contrast agents.2 Most patients now receive low osmolality agents, with less nephrotoxicity.3
Key points of guidelines
This year, the American College of Radiology and the National Kidney Foundation put out joint guidelines that helped clarify why there is a diminished concern for contrast-induced kidney disease in the modern era.4 Below are some of the key points of these guidelines:
- The risk of contrast-induced acute kidney injury (AKI) from intravenous iodinated contrast media is lower than previously thought.
- Necessary contrast material–enhanced CT without a suitable alternative should not be avoided solely on the basis of contrast-induced chronic kidney insufficiency risk.
- Contrast-induced AKI risk should be determined primarily by using CKD stage and AKI.
- Patients at high risk for contrast-induced kidney injury include those with recent AKI and those with estimated glomerular filtration rate (eGFR) less than 30 mL/min per 1.73 m2.
Data supporting guidelines
The data from several studies used to support these recommendations were impressive, showing just how low the risk for contrast-induced AKI is in most patients. In these studies, the risk of contrast-induced AKI has been estimated to be near 0% for patients with an eGFR greater than or equal to 45 and 0%-2% for patients with an eGFR of 30-44.5-7 This information and recommendations make imaging much easier. In most of our patients, we can get contrast studies when we need them. The group to be concerned about are patients with eGFRs less than 30. The guidelines single out this group as the patients where risk/benefit needs to be calculated before proceeding with the study, and to use prophylactic saline hydration in patients not undergoing dialysis.
Myth: Contrast-induced renal disease is common.
Dr. Paauw is professor of medicine in the division of general internal medicine at the University of Washington, Seattle, and he serves as third-year medical student clerkship director at the University of Washington. He is a member of the editorial advisory board of Internal Medicine News. Dr. Paauw has no conflicts to disclose. Contact him at [email protected].
References
1. Lee YC et al. Contrast-induced acute kidney injury among patients with chronic kidney disease undergoing imaging studies: A meta-analysis. Am J Roentgenol. 2019 Oct;213(4):728-35.
2. Luk L et al. Intravenous contrast-induced nephropathy: The rise and fall of a threatening idea. Adv Chronic Kidney Dis. 2017 May;24(3):169-75.
3. Goldfarb S et al. Low-osmolality contrast media and the risk of contrast-associated nephrotoxicity. Invest Radiol. 1993;28(Suppl 5):7-10.
4. Davenport MS, et al. Use of intravenous iodinated contrast media in patients with kidney disease: Consensus statements from the American College of Radiology and the National Kidney Foundation. Kidney Med. 2020 Jan 22;2(1):85-93.
5. Davenport MS et al. Contrast material–induced nephrotoxicity and intravenous low-osmolality iodinated contrast material. Radiology. 2013;267(1):94-105.
6. McDonald RJ et al. Intravenous contrast material–induced nephropathy: Causal or coincident phenomenon? Radiology. 2013;267(1):106-18.
7. McDonald JS et al. Risk of intravenous contrast material–mediated acute kidney injury: A propensity scorematched study stratified by baseline-estimated glomerular filtration rate. Radiology. 2014;271(1):65-73.
Finerenone’s heart benefits hold up in T2D patients without CVD
Finerenone, the first nonsteroidal mineralocorticoid receptor antagonist to complete a phase 3 trial, showed cardiovascular benefits in patients with type 2 diabetes and chronic kidney disease, regardless of whether they entered the study with a history of cardiovascular disease, in follow-up analyses of the FIDELIO-DKD trial, which included 5,674 patients.
“Finerenone demonstrated benefits for primary and secondary cardiovascular disease protection,” said Gerasimos Filippatos, MD, at the American Heart Association scientific sessions. Finerenone treatment cut the rate of cardiovascular death, nonfatal MI or stroke, or heart failure hospitalization, when compared with placebo, by a relative 15% among patients with a history of cardiovascular disease (CVD), and by a relative 14% in patients without this history, differences that met a statistical test for consistency. But the absolute, drug-associated increments in benefit over placebo differed between the two CVD subgroups because of a sharp underlying difference in event rates.
In contrast, the analyses reported by Dr. Filippatos and associates from the FIDELIO-DKD study showed significant heterogeneity based on the presence or absence of CVD for the study’s primary endpoint, a composite renal metric that tallied the combined rate of death from renal causes, renal failure, or a sustained drop in estimated glomerular filtration rate of at least 40%. Researchers enrolled patients into FIDELIO-DKD based on having type 2 diabetes (T2D) and chronic kidney disease (CKD). The prevalence of a history of CVD was 46%.
Among patients with a history of CVD, the composite adverse CVD outcome occurred at a rate of 8.5/100 patient-years in patients on placebo and in 7.18/100 patients years among those on finerenone during a median of 2.6 years of follow-up, a 1.32/100–patient-year absolute between-group difference. Among patients in a primary prevention setting, incident CVD event rates during follow-up were roughly half that in the secondary prevention patients. The upshot was that, in the placebo group, the rate was 3.92/100 patient- years, and in those on finerenone was 3.43/100 patient-years, a 0.49/100–patient-year absolute difference.
CVD history produced heterogeneity for the primary endpoint
In the analysis that focused on the study’s primary, renal endpoint, among patients identified as having CVD at study entry, the outcome occurred at a rate of 9.06/100 patient-years in the placebo subgroup and at a rate of 6.6/100 patient years in those who received finerenone, a significant 30% relative risk reduction and an absolute between-group difference of 2.46/100 patient-years.
In contrast, among patients without a CVD history, the composite renal endpoint occurred at a rate of 9.1/100 patient-years in the placebo patients and 8.42/100 patient-years in those on finerenone, a 6% relative risk reduction that was not significant, and a 0.68/100–patient-year absolute difference. This disparity in the primary event rate between the two treatment arms reached statistical significance (P = .016), the investigators reported in the published version of the report in Circulation that simultaneously appeared online.
“The totality of evidence suggests that finerenone could be used in patients with T2D with or without a history of CVD,” explained Dr. Filippatos in an interview. “The P-interaction for the composite kidney outcome is significant, but it is not corrected for multiple testing; therefore, it might be a false-chance finding and must be interpreted cautiously.
Furthermore, in another prespecified kidney composite outcome the results were consistent in patients with and without a history of CVD. In sum, all the FIDELIO-DKD analyses so far are “suggestive of a beneficial effect in patients without a history of CVD.”
Despite these patients receiving guideline directed therapies, “there remains a high unmet medical need in patients with T2D and CKD,” added Dr. Filippatos, professor of cardiology at the University of Athens. “We use multiple treatments for patients with heart failure, and we should use the same mindset for treating patients with T2D and CKD. The costs of dialysis and kidney transplant are very high, so it is important to consider options that slow progression of CKD in these patients.”
In FIDELIO-DKD, virtually all patients were on background therapy with a renin-angiotensin-system (RAS) inhibitor, so the trial’s results suggest that treatment should at least involve dual therapy with finerenone and a RAS inhibitor. Fewer than 5% were on background therapy with a sodium-glucose cotransporter 2 (SGLT2) inhibitor, a drug class recently established as another key agent for treating CKD in patients with T2D, setting up the prospect for triple therapy, although this approach has not yet undergone prospective testing.
Combining RAS inhibition, finerenone, and an SGLT2 inhibitor is “potentially a marriage made in diabetes heaven,” commented Deepak L. Bhatt, MD, a professor of medicine at Harvard Medical School, Boston, who has not participated in finerenone studies.
Finerenone looks better for safety
Regardless of subgroup analyses based on history of CVD, the findings from all patients enrolled in FIDELIO-DKD were positive for the both the primary renal outcome and key secondary outcome of composite CVD events. In the total randomized cohort, treatment with finerenone on top of optimized treatment with an ACE inhibitor or angiotensin receptor blocker (RAS inhibition) led to a significant 18% relative risk reduction, compared with placebo, for the primary renal endpoint, and a significant 14% relative drop in the key secondary CVD outcome. Those results were published in October in the New England Journal of Medicine.
For treating patients with T2D and CKD ,finerenone overall “looks like a major advance,” Dr. Bhatt said in an interview.
In addition to the positive efficacy results, several experts also focused on what they saw as superior safety of finerenone in the trial, compared with the historical safety of the steroidal mineralocorticoid receptor antagonists (MRAs) now in use: spironolactone and eplerenone.
“I’m a big believer in spironolactone, but it has issues with side effects, and eplerenone never seemed to catch on,” said Dr. Bhatt, who is also executive director of interventional cardiovascular programs at Brigham and Women’s Hospital in Boston.
“A lot of physicians like these MRAs, but acknowledge that side effects have kept these drugs from being used to the extent they should.” The existing MRAs, especially spironolactone, have become a key drug class for treating heart failure with reduced ejection fraction (and, some claim, for also treating heart failure with preserved ejection fraction), as well as treatment-resistant hypertension and primary aldosteronism. By design, FIDELIO-DKD did not enroll patients with heart failure because treatment with an MRA is indicated for those with heart failure with reduced ejection fraction.
The spironolactone adverse effect that generates the greatest concern is hyperkalemia. During his discussion of FIDELIO-DKD as designated discussant, Christoph Wanner, MD, noted a recent study in which the incidence of hyperkalemia severe enough to cause study discontinuation was 23% among patients treated with spironolactone for heart failure, which contrasts with the 2.3% rate in FIDELIO-DKD among finerenone recipients. This hyperkalemia incidence from finerenone also improved on the historical performance of other drugs, like aliskiren (Tekturna), said Dr. Wanner, professor and head of nephrology at the University of Würzburg (Germany).
The FIDELIO-DKD results place finerenone alongside the RAS- and SGLT2-inhibitor drug classes as appropriate treatments for most patients with T2D and CKD. “We have entered a new era of effective treatment for diabetic kidney disease,” Dr. Wanner declared.
“The overall safety profile of finerenone looked better, including hyperkalemia,” said Dr. Bhatt. “Hyperkalemia with spironolactone is not necessarily as bad as the perception. With careful monitoring of spironolactone, the hyperkalemia is manageable. But the perception is that it’s bad, and along with gynecomastia it’s a real killer.”
While some dismiss gynecomastia as a major concern (for men) with spironolactone treatment, “if medical students learn one thing about spironolactone, it’s that it can cause gynecomastia,” adding to the negative image that the approved MRAs carry, Dr. Bhatt said.
“The hyperkalemia was manageable. This is very important because of past problems with potassium when using spironolactone,” Dr. Filippatos said. Finerenone also looks “more cardiorenal protective” than the steroidal MRAs, exerting renal benefits in FIDELIO-DKD never previously described for a steroidal MRA.
Some of the uncertainty about the efficacy of finerenone in patients with a history of cardiovascular disease will lift when results are available in about another year from the FIGARO-DKD pivotal trial of finerenone, which enrolled more than 7,000 patients with T2D and CKD (entry criteria very similar to FIDELIO-CKD). A big difference is that FIGARO-DKD has a composite CVD event metric as its primary endpoint, and includes hospitalization for heart failure as one facet of the composite.
FIDELIO-DKD was sponsored by Bayer. Dr. Filippatos has been a lecturer on behalf of, served as a researcher for, or both for Bayer and also for Amgen, Boehringer Ingelheim, Medtronic, Novartis, Servier, and Vifor. Dr. Bhatt has received research funding from Bayer and also from several other companies, and he also is an adviser to several companies. Dr. Wanner has received honoraria from Bayer, and also from AstraZeneca, Boehringer Ingelheim, FMC, Gilead, GlaxoSmithKline, Lilly, and Merck.
Finerenone, the first nonsteroidal mineralocorticoid receptor antagonist to complete a phase 3 trial, showed cardiovascular benefits in patients with type 2 diabetes and chronic kidney disease, regardless of whether they entered the study with a history of cardiovascular disease, in follow-up analyses of the FIDELIO-DKD trial, which included 5,674 patients.
“Finerenone demonstrated benefits for primary and secondary cardiovascular disease protection,” said Gerasimos Filippatos, MD, at the American Heart Association scientific sessions. Finerenone treatment cut the rate of cardiovascular death, nonfatal MI or stroke, or heart failure hospitalization, when compared with placebo, by a relative 15% among patients with a history of cardiovascular disease (CVD), and by a relative 14% in patients without this history, differences that met a statistical test for consistency. But the absolute, drug-associated increments in benefit over placebo differed between the two CVD subgroups because of a sharp underlying difference in event rates.
In contrast, the analyses reported by Dr. Filippatos and associates from the FIDELIO-DKD study showed significant heterogeneity based on the presence or absence of CVD for the study’s primary endpoint, a composite renal metric that tallied the combined rate of death from renal causes, renal failure, or a sustained drop in estimated glomerular filtration rate of at least 40%. Researchers enrolled patients into FIDELIO-DKD based on having type 2 diabetes (T2D) and chronic kidney disease (CKD). The prevalence of a history of CVD was 46%.
Among patients with a history of CVD, the composite adverse CVD outcome occurred at a rate of 8.5/100 patient-years in patients on placebo and in 7.18/100 patients years among those on finerenone during a median of 2.6 years of follow-up, a 1.32/100–patient-year absolute between-group difference. Among patients in a primary prevention setting, incident CVD event rates during follow-up were roughly half that in the secondary prevention patients. The upshot was that, in the placebo group, the rate was 3.92/100 patient- years, and in those on finerenone was 3.43/100 patient-years, a 0.49/100–patient-year absolute difference.
CVD history produced heterogeneity for the primary endpoint
In the analysis that focused on the study’s primary, renal endpoint, among patients identified as having CVD at study entry, the outcome occurred at a rate of 9.06/100 patient-years in the placebo subgroup and at a rate of 6.6/100 patient years in those who received finerenone, a significant 30% relative risk reduction and an absolute between-group difference of 2.46/100 patient-years.
In contrast, among patients without a CVD history, the composite renal endpoint occurred at a rate of 9.1/100 patient-years in the placebo patients and 8.42/100 patient-years in those on finerenone, a 6% relative risk reduction that was not significant, and a 0.68/100–patient-year absolute difference. This disparity in the primary event rate between the two treatment arms reached statistical significance (P = .016), the investigators reported in the published version of the report in Circulation that simultaneously appeared online.
“The totality of evidence suggests that finerenone could be used in patients with T2D with or without a history of CVD,” explained Dr. Filippatos in an interview. “The P-interaction for the composite kidney outcome is significant, but it is not corrected for multiple testing; therefore, it might be a false-chance finding and must be interpreted cautiously.
Furthermore, in another prespecified kidney composite outcome the results were consistent in patients with and without a history of CVD. In sum, all the FIDELIO-DKD analyses so far are “suggestive of a beneficial effect in patients without a history of CVD.”
Despite these patients receiving guideline directed therapies, “there remains a high unmet medical need in patients with T2D and CKD,” added Dr. Filippatos, professor of cardiology at the University of Athens. “We use multiple treatments for patients with heart failure, and we should use the same mindset for treating patients with T2D and CKD. The costs of dialysis and kidney transplant are very high, so it is important to consider options that slow progression of CKD in these patients.”
In FIDELIO-DKD, virtually all patients were on background therapy with a renin-angiotensin-system (RAS) inhibitor, so the trial’s results suggest that treatment should at least involve dual therapy with finerenone and a RAS inhibitor. Fewer than 5% were on background therapy with a sodium-glucose cotransporter 2 (SGLT2) inhibitor, a drug class recently established as another key agent for treating CKD in patients with T2D, setting up the prospect for triple therapy, although this approach has not yet undergone prospective testing.
Combining RAS inhibition, finerenone, and an SGLT2 inhibitor is “potentially a marriage made in diabetes heaven,” commented Deepak L. Bhatt, MD, a professor of medicine at Harvard Medical School, Boston, who has not participated in finerenone studies.
Finerenone looks better for safety
Regardless of subgroup analyses based on history of CVD, the findings from all patients enrolled in FIDELIO-DKD were positive for the both the primary renal outcome and key secondary outcome of composite CVD events. In the total randomized cohort, treatment with finerenone on top of optimized treatment with an ACE inhibitor or angiotensin receptor blocker (RAS inhibition) led to a significant 18% relative risk reduction, compared with placebo, for the primary renal endpoint, and a significant 14% relative drop in the key secondary CVD outcome. Those results were published in October in the New England Journal of Medicine.
For treating patients with T2D and CKD ,finerenone overall “looks like a major advance,” Dr. Bhatt said in an interview.
In addition to the positive efficacy results, several experts also focused on what they saw as superior safety of finerenone in the trial, compared with the historical safety of the steroidal mineralocorticoid receptor antagonists (MRAs) now in use: spironolactone and eplerenone.
“I’m a big believer in spironolactone, but it has issues with side effects, and eplerenone never seemed to catch on,” said Dr. Bhatt, who is also executive director of interventional cardiovascular programs at Brigham and Women’s Hospital in Boston.
“A lot of physicians like these MRAs, but acknowledge that side effects have kept these drugs from being used to the extent they should.” The existing MRAs, especially spironolactone, have become a key drug class for treating heart failure with reduced ejection fraction (and, some claim, for also treating heart failure with preserved ejection fraction), as well as treatment-resistant hypertension and primary aldosteronism. By design, FIDELIO-DKD did not enroll patients with heart failure because treatment with an MRA is indicated for those with heart failure with reduced ejection fraction.
The spironolactone adverse effect that generates the greatest concern is hyperkalemia. During his discussion of FIDELIO-DKD as designated discussant, Christoph Wanner, MD, noted a recent study in which the incidence of hyperkalemia severe enough to cause study discontinuation was 23% among patients treated with spironolactone for heart failure, which contrasts with the 2.3% rate in FIDELIO-DKD among finerenone recipients. This hyperkalemia incidence from finerenone also improved on the historical performance of other drugs, like aliskiren (Tekturna), said Dr. Wanner, professor and head of nephrology at the University of Würzburg (Germany).
The FIDELIO-DKD results place finerenone alongside the RAS- and SGLT2-inhibitor drug classes as appropriate treatments for most patients with T2D and CKD. “We have entered a new era of effective treatment for diabetic kidney disease,” Dr. Wanner declared.
“The overall safety profile of finerenone looked better, including hyperkalemia,” said Dr. Bhatt. “Hyperkalemia with spironolactone is not necessarily as bad as the perception. With careful monitoring of spironolactone, the hyperkalemia is manageable. But the perception is that it’s bad, and along with gynecomastia it’s a real killer.”
While some dismiss gynecomastia as a major concern (for men) with spironolactone treatment, “if medical students learn one thing about spironolactone, it’s that it can cause gynecomastia,” adding to the negative image that the approved MRAs carry, Dr. Bhatt said.
“The hyperkalemia was manageable. This is very important because of past problems with potassium when using spironolactone,” Dr. Filippatos said. Finerenone also looks “more cardiorenal protective” than the steroidal MRAs, exerting renal benefits in FIDELIO-DKD never previously described for a steroidal MRA.
Some of the uncertainty about the efficacy of finerenone in patients with a history of cardiovascular disease will lift when results are available in about another year from the FIGARO-DKD pivotal trial of finerenone, which enrolled more than 7,000 patients with T2D and CKD (entry criteria very similar to FIDELIO-CKD). A big difference is that FIGARO-DKD has a composite CVD event metric as its primary endpoint, and includes hospitalization for heart failure as one facet of the composite.
FIDELIO-DKD was sponsored by Bayer. Dr. Filippatos has been a lecturer on behalf of, served as a researcher for, or both for Bayer and also for Amgen, Boehringer Ingelheim, Medtronic, Novartis, Servier, and Vifor. Dr. Bhatt has received research funding from Bayer and also from several other companies, and he also is an adviser to several companies. Dr. Wanner has received honoraria from Bayer, and also from AstraZeneca, Boehringer Ingelheim, FMC, Gilead, GlaxoSmithKline, Lilly, and Merck.
Finerenone, the first nonsteroidal mineralocorticoid receptor antagonist to complete a phase 3 trial, showed cardiovascular benefits in patients with type 2 diabetes and chronic kidney disease, regardless of whether they entered the study with a history of cardiovascular disease, in follow-up analyses of the FIDELIO-DKD trial, which included 5,674 patients.
“Finerenone demonstrated benefits for primary and secondary cardiovascular disease protection,” said Gerasimos Filippatos, MD, at the American Heart Association scientific sessions. Finerenone treatment cut the rate of cardiovascular death, nonfatal MI or stroke, or heart failure hospitalization, when compared with placebo, by a relative 15% among patients with a history of cardiovascular disease (CVD), and by a relative 14% in patients without this history, differences that met a statistical test for consistency. But the absolute, drug-associated increments in benefit over placebo differed between the two CVD subgroups because of a sharp underlying difference in event rates.
In contrast, the analyses reported by Dr. Filippatos and associates from the FIDELIO-DKD study showed significant heterogeneity based on the presence or absence of CVD for the study’s primary endpoint, a composite renal metric that tallied the combined rate of death from renal causes, renal failure, or a sustained drop in estimated glomerular filtration rate of at least 40%. Researchers enrolled patients into FIDELIO-DKD based on having type 2 diabetes (T2D) and chronic kidney disease (CKD). The prevalence of a history of CVD was 46%.
Among patients with a history of CVD, the composite adverse CVD outcome occurred at a rate of 8.5/100 patient-years in patients on placebo and in 7.18/100 patients years among those on finerenone during a median of 2.6 years of follow-up, a 1.32/100–patient-year absolute between-group difference. Among patients in a primary prevention setting, incident CVD event rates during follow-up were roughly half that in the secondary prevention patients. The upshot was that, in the placebo group, the rate was 3.92/100 patient- years, and in those on finerenone was 3.43/100 patient-years, a 0.49/100–patient-year absolute difference.
CVD history produced heterogeneity for the primary endpoint
In the analysis that focused on the study’s primary, renal endpoint, among patients identified as having CVD at study entry, the outcome occurred at a rate of 9.06/100 patient-years in the placebo subgroup and at a rate of 6.6/100 patient years in those who received finerenone, a significant 30% relative risk reduction and an absolute between-group difference of 2.46/100 patient-years.
In contrast, among patients without a CVD history, the composite renal endpoint occurred at a rate of 9.1/100 patient-years in the placebo patients and 8.42/100 patient-years in those on finerenone, a 6% relative risk reduction that was not significant, and a 0.68/100–patient-year absolute difference. This disparity in the primary event rate between the two treatment arms reached statistical significance (P = .016), the investigators reported in the published version of the report in Circulation that simultaneously appeared online.
“The totality of evidence suggests that finerenone could be used in patients with T2D with or without a history of CVD,” explained Dr. Filippatos in an interview. “The P-interaction for the composite kidney outcome is significant, but it is not corrected for multiple testing; therefore, it might be a false-chance finding and must be interpreted cautiously.
Furthermore, in another prespecified kidney composite outcome the results were consistent in patients with and without a history of CVD. In sum, all the FIDELIO-DKD analyses so far are “suggestive of a beneficial effect in patients without a history of CVD.”
Despite these patients receiving guideline directed therapies, “there remains a high unmet medical need in patients with T2D and CKD,” added Dr. Filippatos, professor of cardiology at the University of Athens. “We use multiple treatments for patients with heart failure, and we should use the same mindset for treating patients with T2D and CKD. The costs of dialysis and kidney transplant are very high, so it is important to consider options that slow progression of CKD in these patients.”
In FIDELIO-DKD, virtually all patients were on background therapy with a renin-angiotensin-system (RAS) inhibitor, so the trial’s results suggest that treatment should at least involve dual therapy with finerenone and a RAS inhibitor. Fewer than 5% were on background therapy with a sodium-glucose cotransporter 2 (SGLT2) inhibitor, a drug class recently established as another key agent for treating CKD in patients with T2D, setting up the prospect for triple therapy, although this approach has not yet undergone prospective testing.
Combining RAS inhibition, finerenone, and an SGLT2 inhibitor is “potentially a marriage made in diabetes heaven,” commented Deepak L. Bhatt, MD, a professor of medicine at Harvard Medical School, Boston, who has not participated in finerenone studies.
Finerenone looks better for safety
Regardless of subgroup analyses based on history of CVD, the findings from all patients enrolled in FIDELIO-DKD were positive for the both the primary renal outcome and key secondary outcome of composite CVD events. In the total randomized cohort, treatment with finerenone on top of optimized treatment with an ACE inhibitor or angiotensin receptor blocker (RAS inhibition) led to a significant 18% relative risk reduction, compared with placebo, for the primary renal endpoint, and a significant 14% relative drop in the key secondary CVD outcome. Those results were published in October in the New England Journal of Medicine.
For treating patients with T2D and CKD ,finerenone overall “looks like a major advance,” Dr. Bhatt said in an interview.
In addition to the positive efficacy results, several experts also focused on what they saw as superior safety of finerenone in the trial, compared with the historical safety of the steroidal mineralocorticoid receptor antagonists (MRAs) now in use: spironolactone and eplerenone.
“I’m a big believer in spironolactone, but it has issues with side effects, and eplerenone never seemed to catch on,” said Dr. Bhatt, who is also executive director of interventional cardiovascular programs at Brigham and Women’s Hospital in Boston.
“A lot of physicians like these MRAs, but acknowledge that side effects have kept these drugs from being used to the extent they should.” The existing MRAs, especially spironolactone, have become a key drug class for treating heart failure with reduced ejection fraction (and, some claim, for also treating heart failure with preserved ejection fraction), as well as treatment-resistant hypertension and primary aldosteronism. By design, FIDELIO-DKD did not enroll patients with heart failure because treatment with an MRA is indicated for those with heart failure with reduced ejection fraction.
The spironolactone adverse effect that generates the greatest concern is hyperkalemia. During his discussion of FIDELIO-DKD as designated discussant, Christoph Wanner, MD, noted a recent study in which the incidence of hyperkalemia severe enough to cause study discontinuation was 23% among patients treated with spironolactone for heart failure, which contrasts with the 2.3% rate in FIDELIO-DKD among finerenone recipients. This hyperkalemia incidence from finerenone also improved on the historical performance of other drugs, like aliskiren (Tekturna), said Dr. Wanner, professor and head of nephrology at the University of Würzburg (Germany).
The FIDELIO-DKD results place finerenone alongside the RAS- and SGLT2-inhibitor drug classes as appropriate treatments for most patients with T2D and CKD. “We have entered a new era of effective treatment for diabetic kidney disease,” Dr. Wanner declared.
“The overall safety profile of finerenone looked better, including hyperkalemia,” said Dr. Bhatt. “Hyperkalemia with spironolactone is not necessarily as bad as the perception. With careful monitoring of spironolactone, the hyperkalemia is manageable. But the perception is that it’s bad, and along with gynecomastia it’s a real killer.”
While some dismiss gynecomastia as a major concern (for men) with spironolactone treatment, “if medical students learn one thing about spironolactone, it’s that it can cause gynecomastia,” adding to the negative image that the approved MRAs carry, Dr. Bhatt said.
“The hyperkalemia was manageable. This is very important because of past problems with potassium when using spironolactone,” Dr. Filippatos said. Finerenone also looks “more cardiorenal protective” than the steroidal MRAs, exerting renal benefits in FIDELIO-DKD never previously described for a steroidal MRA.
Some of the uncertainty about the efficacy of finerenone in patients with a history of cardiovascular disease will lift when results are available in about another year from the FIGARO-DKD pivotal trial of finerenone, which enrolled more than 7,000 patients with T2D and CKD (entry criteria very similar to FIDELIO-CKD). A big difference is that FIGARO-DKD has a composite CVD event metric as its primary endpoint, and includes hospitalization for heart failure as one facet of the composite.
FIDELIO-DKD was sponsored by Bayer. Dr. Filippatos has been a lecturer on behalf of, served as a researcher for, or both for Bayer and also for Amgen, Boehringer Ingelheim, Medtronic, Novartis, Servier, and Vifor. Dr. Bhatt has received research funding from Bayer and also from several other companies, and he also is an adviser to several companies. Dr. Wanner has received honoraria from Bayer, and also from AstraZeneca, Boehringer Ingelheim, FMC, Gilead, GlaxoSmithKline, Lilly, and Merck.
FROM AHA 2020
Dapagliflozin Reduces Adverse Renal and Cardiovascular Events in Patients With Chronic Kidney Disease
Study Overview
Objective. To assess whether dapagliflozin added to guideline-recommended therapies is effective and safe over the long-term to reduce the rate of renal and cardiovascular events in patients across multiple chronic kidney disease (CKD) stages, with and without type 2 diabetes.
Design. The Dapagliflozin and Prevention of Adverse Outcomes in CKD (DAPA-CKD) trial (NCT03036150) was a randomized, double-blind, parallel-group, placebo-controlled, multicenter event-driven, clinical trial sponsored by Astra-Zeneca. It was conducted at 386 sites in 21 countries from February 2, 2017, to June 12, 2020. A recruitment period of 24 months and a total study duration of 45 months were initially planned. The primary efficacy analysis was based on the intention-to-treat population. This was the first randomized controlled trial designed to assess the effects of sodium-glucose co-transporter 2 (SGLT2) inhibitors on renal and cardiovascular outcomes in patients with CKD.
Setting and participants. This trial randomly assigned 4304 adult participants with CKD stages 2 to 4 (an estimated glomerular filtration rate [GFR] of 25 to 75 mL/min/1.73 m2 of body-surface area) and elevated urinary albumin excretion (urinary albumin-to-creatinine ratio of 200 to 5000, measured in mg of albumin per g of creatinine) to receive dapagliflozin (10 mg once daily) or placebo. Exclusion criteria included type 1 diabetes, polycystic kidney disease, lupus nephritis, antineutrophil cytoplasmic antibody–associated vasculitis, recent immunosuppressive therapy for primary or secondary kidney disease, New York Heart Association class IV congestive heart failure, myocardial infarction, unstable angina, stroke or transient ischemic attacks, or recent coronary revascularization or valvular repair/replacement. All participants received a stable dose of renin–angiotensin system inhibitor for 4 weeks prior to screening, and the vast majority received a maximum tolerated dose at enrollment. Randomization was monitored to ensure that at least 30% of participants recruited did not have diabetes and that no more than 10% had stage 2 CKD. Participants were randomly assigned to receive dapagliflozin (n = 2152) or matching placebo (n = 2152) to ensure a 1:1 ratio of the 2 regimens. Dapagliflozin and placebo had identical appearance and administration schedules. All participants and trial personnel (except members of the independent data monitoring committee) were unaware of the trial-group assignments. After randomization, in-person study visits were conducted at 2 weeks, at 2, 4, and 8 months, and at 4-month intervals thereafter.
Main outcome measures. The primary outcome was a composite of the first occurrence of either a sustained decline in the estimated GFR of at least 50%, end-stage kidney disease, or death from renal or cardiovascular causes. Secondary outcomes, in hierarchical order, were: (1) the composite kidney outcome of a sustained decline in the estimated GFR of at least 50%, end-stage kidney disease, or death from renal causes; (2) a composite cardiovascular outcome defined as hospitalization for heart failure or death from cardiovascular causes; and (3) death from any cause. All outcomes were assessed by time-to-event analyses.
Given the extensive prior experience with dapagliflozin, only selected adverse events were recorded. These included serious adverse events, adverse events resulting in the discontinuation of dapagliflozin or placebo, and adverse events of interest to dapagliflozin (eg, volume depletion symptoms, renal events, major hypoglycemia, fractures, diabetic ketoacidosis, events leading to higher risk of lower limb amputation, and lower limb amputations).
Main results. On March 26, 2020, the independent data monitoring committee recommended stopping the trial because of clear efficacy on the basis of 408 primary outcome events. The participants were 61.8 ± 12.1 years of age, and 1425 participants (33.1%) were female. The baseline mean estimated GFR was 43.1 ± 12.4 mL/min/1.73 m2, the median urinary albumin-to-creatinine ratio was 949, and 2906 participants (67.5%) had type 2 diabetes. Over a median of 2.4 years, a primary outcome event occurred in 197 participants (9.2%) in the dapagliflozin group and 312 (14.5%) in the placebo group (hazard ratio [HR], 0.61; 95% confidence interval [CI], 0.51-0.72; P < 0.001). The number of participants who needed to be treated during the trial period to prevent 1 primary outcome event was 19 (95% CI, 15-27). The beneficial effect of dapagliflozin compared with placebo was consistent across all 8 prespecified subgroups (ie, age, sex, race, geographic region, type 2 diabetes, estimated GFR, urinary albumin-to-creatinine ratio, and systolic blood pressure) for the primary outcome. The effects of dapagliflozin were similar in participants with type 2 diabetes and in those without type 2 diabetes.
The incidence of each secondary outcome was similarly lower in the dapagliflozin-treated group than in the placebo group. The HR for the composite kidney outcome of a sustained decline in the estimated GFR of at least 50%, end-stage kidney disease, or death from renal causes was 0.56 (95% CI, 0.45-0.68; P < 0.001), and the HR for the composite cardiovascular outcome of hospitalization for heart failure or death from cardiovascular causes was 0.71 (95% CI, 0.55-0.92; P = 0.009). Death occurred in 101 participants (4.7%) in the dapagliflozin group and 146 participants (6.8%) in the placebo group (HR, 0.69; 95% CI, 0.53-0.88; P = 0.004). The known safety profile of dapagliflozin was confirmed by the similar overall incidences of adverse events and serious adverse events in the dapagliflozin and placebo groups.
Conclusion. In patients with CKD, with or without type 2 diabetes, the risk of a composite of a sustained decline in the estimated GFR of at least 50%, end-stage kidney disease, or death from renal or cardiovascular causes was significantly lowered by dapagliflozin treatment.
Commentary
Although SGLT2 inhibitors were designed to reduce plasma glucose and hemoglobin A1c (HbA1c) by increasing urinary glucose excretion in a non-insulin-dependent fashion, an increasing number of clinical trials have demonstrated their possible cardiovascular and renal benefits that extend beyond glycemic control. In 2008, the US Food and Drug Administration (FDA) issued a guidance recommending the evaluation of long-term cardiovascular outcomes prior to approval and commercialization of new antidiabetic therapies to ensure minimum cardiovascular risks following the discovery of cardiovascular safety issues associated with antidiabetic compounds, including rosiglitazone, after drug approval. No one foresaw that this recommendation would lead to the discovery of new classes of antidiabetic drugs (glucagon-like peptide 1 [GLP1] and SGLT2 inhibitors) that improve cardiovascular outcomes. A series of clinical trials of SGLT2 inhibitors, including empagliflozin,1 canagliflozin,2 and dapagliflozin,3 showed a reduction in cardiovascular death and hospitalization due to heart failure among patients with type 2 diabetes. Furthermore, a meta-analysis from 2019 found that SGLT2 inhibitors reduced the risk of a composite of cardiovascular death or hospitalization for heart failure by 23% and the risk of progression of kidney failure by 45% in patients with diabetes.4 Thus, the strong and consistent evidence from these large and well-designed outcome trials led the American Diabetes Association in its most recent guidelines to recommend adding SGLT2 inhibitors to metformin for the treatment of patients with type 2 diabetes with or at high risk of atherosclerotic cardiovascular disease, heart failure, or CKD, regardless of baseline HbA1c levels or HbA1c target.5 As a result of the compelling effects of SGLT2 inhibitors on cardiovascular outcomes in diabetic patients, as well as increasing evidence that these clinical effects were independent of glycemic control, several subsequent trials were conducted to evaluate whether this new class of drugs may improve clinical outcomes in nondiabetic patients.
The Dapagliflozin and Prevention of Adverse Outcomes in Heart Failure (DAPA-HF) was the first clinical trial to investigate the effect of SGLT2 inhibitors on cardiovascular disease in nondiabetic patients. Findings from DAPA-HF showed that dapagliflozin reduced the risk of worsening heart failure or death from cardiovascular causes, independent of the presence of underlying diabetes. This initial finding resonates with a growing body of evidence6,7 that supports the use of SGLT2 inhibitors as an adjunctive therapy for heart failure in the absence of diabetes.
The Canagliflozin and Renal Events in Diabetes with Established Nephropathy Clinical Evaluation (CREDENCE) trial showed that long-term administration of canagliflozin conferred cardiovascular, as well as renal, protection in patients with type 2 diabetes with CKD.8 Similar to the protective effects on heart failure, the renal benefits of SGLT2 inhibitors appeared to be independent of their blood glucose-lowering effects. Thus, these recent discoveries led to the design of the DAPA-CKD trial to further assess the long-term efficacy and safety of the SGLT2 inhibitor dapagliflozin in patients with CKD precipitated by causes other than type 2 diabetes. Although diabetes is the most common cause for CKD, it nonetheless only accounts for 40% of all CKD etiologies. To date, the only classes of medication that have been shown to slow a decline in kidney function in patients with diabetes are angiotensin-converting enzyme (ACE) inhibitors and angiotensin receptor blockers (ARBs). Given that CKD is an important contributor to illness, is associated with diminished quality of life and reduced life expectancy, and increases health care costs, the findings of the DAPA-CKD trial are particularly significant as they show a renal benefit of dapagliflozin treatment across CKD stages that is independent of underlying diabetes. Therefore, SGLT2 inhibitors may offer a new and unique treatment option for millions of patients with CKD worldwide for whom ACE inhibitors and ARBs were otherwise the only treatments to prevent kidney failure. Moreover, with a number-needed-to-treat of 19 to prevent 1 composite renal vascular event over a period of 2.4 years, dapagliflozin requires a much lower number needed to treat compared to ACE inhibitors and ARBs in similar patients.
The trial has several limitations in study design. For example, the management of diabetes and hypertension were left to the discretion of each trial site, in keeping with local clinical practice and guidelines. It is unknown whether this variability in the management of comorbidities that impact kidney function had an effect on the study’s results. In addition, the trial was stopped early as a result of recommendations from an independent committee due to the demonstrated efficacy of dapagliflozin. This may have reduced the statistical power to assess some of the secondary outcomes. Finally, the authors discussed an initial dip in the estimated GFR after initiation of dapagliflozin treatment, similar to that observed in other SGLT2 inhibitor clinical trials. However, they were unable to ascertain the reversibility of this effect after the discontinuation of dapagliflozin because assessment of GFR was not completed after trial closure. Nonetheless, the authors specified that the reversibility of this initial estimated GFR dip had been assessed and observed in other clinical trials involving dapagliflozin.
The nonglycemic benefits of SGLT2 inhibitors, including improvement in renal outcomes, have strong implications for the future management of patients with CKD. If this indication is approved by the FDA and recommended by clinical guidelines, the ease of SGLT2 inhibitor prescription (eg, minimal drug-drug interaction, no titration), treatment administration (orally once daily), and safety profile may lead to wide use of SGLT2 inhibitors by generalists, nephrologists, and endocrinologists in preserving or improving renal outcomes in patients at risk for end-stage kidney disease. Given that SGLT2 inhibitors are a new class of pharmacologic therapeutics, patient education should include a discussion of the possible side effects, such as euglycemic ketoacidosis, genital and urinary tract infection, and foot and leg amputation. Finally, as Strandberg and colleagues reported in a recent commentary,9 the safety of SGLT2 inhibitors in older adults with multimorbidity, frailty, and polypharmacy remains unclear. Thus, future studies of SGLT2 inhibitors are needed to better evaluate their clinical effects in older adults.
Applications for Clinical Practice
This trial enrolled a dedicated patient population with CKD and demonstrated a benefit of dapagliflozin in reducing renal and cardiovascular outcomes, regardless of baseline diabetes status. These drugs (dapagliflozin as well as other SGLT2 inhibitors) will likely have a prominent role in future CKD management guidelines. Until then, several barriers remain before SGLT2 inhibitors can be widely used in clinical practice. Among these barriers are FDA approval for their use in patients with and without diabetes with an estimated GFR < 30 mL/min/1.73 m2 and lowering the costs of this class of drugs.
—Rachel Litke, MD, PhD
Icahn School of Medicine at Mount Sinai
Fred Ko, MD, MS
1. Zinman B, Wanner C, Lachin JM, et al. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med. 2015;373:2117-2128.
2. Neal B, Perkovic V, Matthews DR. Canagliflozin and cardiovascular and renal events in type 2 diabetes. N Engl J Med. 2017;377:2099.
3. Wiviott SD, Raz I, Bonaca MP, et al. Dapagliflozin and cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2019;380:347-357.
4. Zelniker TA, Wiviott SD, Raz I, Sabatine MS. SGLT-2 inhibitors for people with type 2 diabetes - Authors’ reply. Lancet. 2019;394:560-561.
5. American Diabetes Association 10. Cardiovascular disease and risk management: standards of medical care in diabetes-2020. Diabetes Care. 2020;43(Suppl 1):S111-S34.
6. Packer M, Anker SD, Butler J, et al. Cardiovascular and renal outcomes with empagliflozin in heart failure. N Engl J Med. 2020;383:1413-1424.
7. Zannad F, Ferreira JP, Pocock SJ, et al. SGLT2 inhibitors in patients with heart failure with reduced ejection fraction: a meta-analysis of the EMPEROR-Reduced and DAPA-HF trials. Lancet. 2020;396:819-829.
8. Perkovic V, Jardine MJ, Neal B, et al. Canagliflozin and renal outcomes in type 2 diabetes and nephropathy. N Engl J Med. 2019;380:2295-2306.
9. Strandberg TE, Petrovic M, Benetos A. SGLT-2 inhibitors for people with type 2 diabetes. Lancet. 2019;394:560.
Study Overview
Objective. To assess whether dapagliflozin added to guideline-recommended therapies is effective and safe over the long-term to reduce the rate of renal and cardiovascular events in patients across multiple chronic kidney disease (CKD) stages, with and without type 2 diabetes.
Design. The Dapagliflozin and Prevention of Adverse Outcomes in CKD (DAPA-CKD) trial (NCT03036150) was a randomized, double-blind, parallel-group, placebo-controlled, multicenter event-driven, clinical trial sponsored by Astra-Zeneca. It was conducted at 386 sites in 21 countries from February 2, 2017, to June 12, 2020. A recruitment period of 24 months and a total study duration of 45 months were initially planned. The primary efficacy analysis was based on the intention-to-treat population. This was the first randomized controlled trial designed to assess the effects of sodium-glucose co-transporter 2 (SGLT2) inhibitors on renal and cardiovascular outcomes in patients with CKD.
Setting and participants. This trial randomly assigned 4304 adult participants with CKD stages 2 to 4 (an estimated glomerular filtration rate [GFR] of 25 to 75 mL/min/1.73 m2 of body-surface area) and elevated urinary albumin excretion (urinary albumin-to-creatinine ratio of 200 to 5000, measured in mg of albumin per g of creatinine) to receive dapagliflozin (10 mg once daily) or placebo. Exclusion criteria included type 1 diabetes, polycystic kidney disease, lupus nephritis, antineutrophil cytoplasmic antibody–associated vasculitis, recent immunosuppressive therapy for primary or secondary kidney disease, New York Heart Association class IV congestive heart failure, myocardial infarction, unstable angina, stroke or transient ischemic attacks, or recent coronary revascularization or valvular repair/replacement. All participants received a stable dose of renin–angiotensin system inhibitor for 4 weeks prior to screening, and the vast majority received a maximum tolerated dose at enrollment. Randomization was monitored to ensure that at least 30% of participants recruited did not have diabetes and that no more than 10% had stage 2 CKD. Participants were randomly assigned to receive dapagliflozin (n = 2152) or matching placebo (n = 2152) to ensure a 1:1 ratio of the 2 regimens. Dapagliflozin and placebo had identical appearance and administration schedules. All participants and trial personnel (except members of the independent data monitoring committee) were unaware of the trial-group assignments. After randomization, in-person study visits were conducted at 2 weeks, at 2, 4, and 8 months, and at 4-month intervals thereafter.
Main outcome measures. The primary outcome was a composite of the first occurrence of either a sustained decline in the estimated GFR of at least 50%, end-stage kidney disease, or death from renal or cardiovascular causes. Secondary outcomes, in hierarchical order, were: (1) the composite kidney outcome of a sustained decline in the estimated GFR of at least 50%, end-stage kidney disease, or death from renal causes; (2) a composite cardiovascular outcome defined as hospitalization for heart failure or death from cardiovascular causes; and (3) death from any cause. All outcomes were assessed by time-to-event analyses.
Given the extensive prior experience with dapagliflozin, only selected adverse events were recorded. These included serious adverse events, adverse events resulting in the discontinuation of dapagliflozin or placebo, and adverse events of interest to dapagliflozin (eg, volume depletion symptoms, renal events, major hypoglycemia, fractures, diabetic ketoacidosis, events leading to higher risk of lower limb amputation, and lower limb amputations).
Main results. On March 26, 2020, the independent data monitoring committee recommended stopping the trial because of clear efficacy on the basis of 408 primary outcome events. The participants were 61.8 ± 12.1 years of age, and 1425 participants (33.1%) were female. The baseline mean estimated GFR was 43.1 ± 12.4 mL/min/1.73 m2, the median urinary albumin-to-creatinine ratio was 949, and 2906 participants (67.5%) had type 2 diabetes. Over a median of 2.4 years, a primary outcome event occurred in 197 participants (9.2%) in the dapagliflozin group and 312 (14.5%) in the placebo group (hazard ratio [HR], 0.61; 95% confidence interval [CI], 0.51-0.72; P < 0.001). The number of participants who needed to be treated during the trial period to prevent 1 primary outcome event was 19 (95% CI, 15-27). The beneficial effect of dapagliflozin compared with placebo was consistent across all 8 prespecified subgroups (ie, age, sex, race, geographic region, type 2 diabetes, estimated GFR, urinary albumin-to-creatinine ratio, and systolic blood pressure) for the primary outcome. The effects of dapagliflozin were similar in participants with type 2 diabetes and in those without type 2 diabetes.
The incidence of each secondary outcome was similarly lower in the dapagliflozin-treated group than in the placebo group. The HR for the composite kidney outcome of a sustained decline in the estimated GFR of at least 50%, end-stage kidney disease, or death from renal causes was 0.56 (95% CI, 0.45-0.68; P < 0.001), and the HR for the composite cardiovascular outcome of hospitalization for heart failure or death from cardiovascular causes was 0.71 (95% CI, 0.55-0.92; P = 0.009). Death occurred in 101 participants (4.7%) in the dapagliflozin group and 146 participants (6.8%) in the placebo group (HR, 0.69; 95% CI, 0.53-0.88; P = 0.004). The known safety profile of dapagliflozin was confirmed by the similar overall incidences of adverse events and serious adverse events in the dapagliflozin and placebo groups.
Conclusion. In patients with CKD, with or without type 2 diabetes, the risk of a composite of a sustained decline in the estimated GFR of at least 50%, end-stage kidney disease, or death from renal or cardiovascular causes was significantly lowered by dapagliflozin treatment.
Commentary
Although SGLT2 inhibitors were designed to reduce plasma glucose and hemoglobin A1c (HbA1c) by increasing urinary glucose excretion in a non-insulin-dependent fashion, an increasing number of clinical trials have demonstrated their possible cardiovascular and renal benefits that extend beyond glycemic control. In 2008, the US Food and Drug Administration (FDA) issued a guidance recommending the evaluation of long-term cardiovascular outcomes prior to approval and commercialization of new antidiabetic therapies to ensure minimum cardiovascular risks following the discovery of cardiovascular safety issues associated with antidiabetic compounds, including rosiglitazone, after drug approval. No one foresaw that this recommendation would lead to the discovery of new classes of antidiabetic drugs (glucagon-like peptide 1 [GLP1] and SGLT2 inhibitors) that improve cardiovascular outcomes. A series of clinical trials of SGLT2 inhibitors, including empagliflozin,1 canagliflozin,2 and dapagliflozin,3 showed a reduction in cardiovascular death and hospitalization due to heart failure among patients with type 2 diabetes. Furthermore, a meta-analysis from 2019 found that SGLT2 inhibitors reduced the risk of a composite of cardiovascular death or hospitalization for heart failure by 23% and the risk of progression of kidney failure by 45% in patients with diabetes.4 Thus, the strong and consistent evidence from these large and well-designed outcome trials led the American Diabetes Association in its most recent guidelines to recommend adding SGLT2 inhibitors to metformin for the treatment of patients with type 2 diabetes with or at high risk of atherosclerotic cardiovascular disease, heart failure, or CKD, regardless of baseline HbA1c levels or HbA1c target.5 As a result of the compelling effects of SGLT2 inhibitors on cardiovascular outcomes in diabetic patients, as well as increasing evidence that these clinical effects were independent of glycemic control, several subsequent trials were conducted to evaluate whether this new class of drugs may improve clinical outcomes in nondiabetic patients.
The Dapagliflozin and Prevention of Adverse Outcomes in Heart Failure (DAPA-HF) was the first clinical trial to investigate the effect of SGLT2 inhibitors on cardiovascular disease in nondiabetic patients. Findings from DAPA-HF showed that dapagliflozin reduced the risk of worsening heart failure or death from cardiovascular causes, independent of the presence of underlying diabetes. This initial finding resonates with a growing body of evidence6,7 that supports the use of SGLT2 inhibitors as an adjunctive therapy for heart failure in the absence of diabetes.
The Canagliflozin and Renal Events in Diabetes with Established Nephropathy Clinical Evaluation (CREDENCE) trial showed that long-term administration of canagliflozin conferred cardiovascular, as well as renal, protection in patients with type 2 diabetes with CKD.8 Similar to the protective effects on heart failure, the renal benefits of SGLT2 inhibitors appeared to be independent of their blood glucose-lowering effects. Thus, these recent discoveries led to the design of the DAPA-CKD trial to further assess the long-term efficacy and safety of the SGLT2 inhibitor dapagliflozin in patients with CKD precipitated by causes other than type 2 diabetes. Although diabetes is the most common cause for CKD, it nonetheless only accounts for 40% of all CKD etiologies. To date, the only classes of medication that have been shown to slow a decline in kidney function in patients with diabetes are angiotensin-converting enzyme (ACE) inhibitors and angiotensin receptor blockers (ARBs). Given that CKD is an important contributor to illness, is associated with diminished quality of life and reduced life expectancy, and increases health care costs, the findings of the DAPA-CKD trial are particularly significant as they show a renal benefit of dapagliflozin treatment across CKD stages that is independent of underlying diabetes. Therefore, SGLT2 inhibitors may offer a new and unique treatment option for millions of patients with CKD worldwide for whom ACE inhibitors and ARBs were otherwise the only treatments to prevent kidney failure. Moreover, with a number-needed-to-treat of 19 to prevent 1 composite renal vascular event over a period of 2.4 years, dapagliflozin requires a much lower number needed to treat compared to ACE inhibitors and ARBs in similar patients.
The trial has several limitations in study design. For example, the management of diabetes and hypertension were left to the discretion of each trial site, in keeping with local clinical practice and guidelines. It is unknown whether this variability in the management of comorbidities that impact kidney function had an effect on the study’s results. In addition, the trial was stopped early as a result of recommendations from an independent committee due to the demonstrated efficacy of dapagliflozin. This may have reduced the statistical power to assess some of the secondary outcomes. Finally, the authors discussed an initial dip in the estimated GFR after initiation of dapagliflozin treatment, similar to that observed in other SGLT2 inhibitor clinical trials. However, they were unable to ascertain the reversibility of this effect after the discontinuation of dapagliflozin because assessment of GFR was not completed after trial closure. Nonetheless, the authors specified that the reversibility of this initial estimated GFR dip had been assessed and observed in other clinical trials involving dapagliflozin.
The nonglycemic benefits of SGLT2 inhibitors, including improvement in renal outcomes, have strong implications for the future management of patients with CKD. If this indication is approved by the FDA and recommended by clinical guidelines, the ease of SGLT2 inhibitor prescription (eg, minimal drug-drug interaction, no titration), treatment administration (orally once daily), and safety profile may lead to wide use of SGLT2 inhibitors by generalists, nephrologists, and endocrinologists in preserving or improving renal outcomes in patients at risk for end-stage kidney disease. Given that SGLT2 inhibitors are a new class of pharmacologic therapeutics, patient education should include a discussion of the possible side effects, such as euglycemic ketoacidosis, genital and urinary tract infection, and foot and leg amputation. Finally, as Strandberg and colleagues reported in a recent commentary,9 the safety of SGLT2 inhibitors in older adults with multimorbidity, frailty, and polypharmacy remains unclear. Thus, future studies of SGLT2 inhibitors are needed to better evaluate their clinical effects in older adults.
Applications for Clinical Practice
This trial enrolled a dedicated patient population with CKD and demonstrated a benefit of dapagliflozin in reducing renal and cardiovascular outcomes, regardless of baseline diabetes status. These drugs (dapagliflozin as well as other SGLT2 inhibitors) will likely have a prominent role in future CKD management guidelines. Until then, several barriers remain before SGLT2 inhibitors can be widely used in clinical practice. Among these barriers are FDA approval for their use in patients with and without diabetes with an estimated GFR < 30 mL/min/1.73 m2 and lowering the costs of this class of drugs.
—Rachel Litke, MD, PhD
Icahn School of Medicine at Mount Sinai
Fred Ko, MD, MS
Study Overview
Objective. To assess whether dapagliflozin added to guideline-recommended therapies is effective and safe over the long-term to reduce the rate of renal and cardiovascular events in patients across multiple chronic kidney disease (CKD) stages, with and without type 2 diabetes.
Design. The Dapagliflozin and Prevention of Adverse Outcomes in CKD (DAPA-CKD) trial (NCT03036150) was a randomized, double-blind, parallel-group, placebo-controlled, multicenter event-driven, clinical trial sponsored by Astra-Zeneca. It was conducted at 386 sites in 21 countries from February 2, 2017, to June 12, 2020. A recruitment period of 24 months and a total study duration of 45 months were initially planned. The primary efficacy analysis was based on the intention-to-treat population. This was the first randomized controlled trial designed to assess the effects of sodium-glucose co-transporter 2 (SGLT2) inhibitors on renal and cardiovascular outcomes in patients with CKD.
Setting and participants. This trial randomly assigned 4304 adult participants with CKD stages 2 to 4 (an estimated glomerular filtration rate [GFR] of 25 to 75 mL/min/1.73 m2 of body-surface area) and elevated urinary albumin excretion (urinary albumin-to-creatinine ratio of 200 to 5000, measured in mg of albumin per g of creatinine) to receive dapagliflozin (10 mg once daily) or placebo. Exclusion criteria included type 1 diabetes, polycystic kidney disease, lupus nephritis, antineutrophil cytoplasmic antibody–associated vasculitis, recent immunosuppressive therapy for primary or secondary kidney disease, New York Heart Association class IV congestive heart failure, myocardial infarction, unstable angina, stroke or transient ischemic attacks, or recent coronary revascularization or valvular repair/replacement. All participants received a stable dose of renin–angiotensin system inhibitor for 4 weeks prior to screening, and the vast majority received a maximum tolerated dose at enrollment. Randomization was monitored to ensure that at least 30% of participants recruited did not have diabetes and that no more than 10% had stage 2 CKD. Participants were randomly assigned to receive dapagliflozin (n = 2152) or matching placebo (n = 2152) to ensure a 1:1 ratio of the 2 regimens. Dapagliflozin and placebo had identical appearance and administration schedules. All participants and trial personnel (except members of the independent data monitoring committee) were unaware of the trial-group assignments. After randomization, in-person study visits were conducted at 2 weeks, at 2, 4, and 8 months, and at 4-month intervals thereafter.
Main outcome measures. The primary outcome was a composite of the first occurrence of either a sustained decline in the estimated GFR of at least 50%, end-stage kidney disease, or death from renal or cardiovascular causes. Secondary outcomes, in hierarchical order, were: (1) the composite kidney outcome of a sustained decline in the estimated GFR of at least 50%, end-stage kidney disease, or death from renal causes; (2) a composite cardiovascular outcome defined as hospitalization for heart failure or death from cardiovascular causes; and (3) death from any cause. All outcomes were assessed by time-to-event analyses.
Given the extensive prior experience with dapagliflozin, only selected adverse events were recorded. These included serious adverse events, adverse events resulting in the discontinuation of dapagliflozin or placebo, and adverse events of interest to dapagliflozin (eg, volume depletion symptoms, renal events, major hypoglycemia, fractures, diabetic ketoacidosis, events leading to higher risk of lower limb amputation, and lower limb amputations).
Main results. On March 26, 2020, the independent data monitoring committee recommended stopping the trial because of clear efficacy on the basis of 408 primary outcome events. The participants were 61.8 ± 12.1 years of age, and 1425 participants (33.1%) were female. The baseline mean estimated GFR was 43.1 ± 12.4 mL/min/1.73 m2, the median urinary albumin-to-creatinine ratio was 949, and 2906 participants (67.5%) had type 2 diabetes. Over a median of 2.4 years, a primary outcome event occurred in 197 participants (9.2%) in the dapagliflozin group and 312 (14.5%) in the placebo group (hazard ratio [HR], 0.61; 95% confidence interval [CI], 0.51-0.72; P < 0.001). The number of participants who needed to be treated during the trial period to prevent 1 primary outcome event was 19 (95% CI, 15-27). The beneficial effect of dapagliflozin compared with placebo was consistent across all 8 prespecified subgroups (ie, age, sex, race, geographic region, type 2 diabetes, estimated GFR, urinary albumin-to-creatinine ratio, and systolic blood pressure) for the primary outcome. The effects of dapagliflozin were similar in participants with type 2 diabetes and in those without type 2 diabetes.
The incidence of each secondary outcome was similarly lower in the dapagliflozin-treated group than in the placebo group. The HR for the composite kidney outcome of a sustained decline in the estimated GFR of at least 50%, end-stage kidney disease, or death from renal causes was 0.56 (95% CI, 0.45-0.68; P < 0.001), and the HR for the composite cardiovascular outcome of hospitalization for heart failure or death from cardiovascular causes was 0.71 (95% CI, 0.55-0.92; P = 0.009). Death occurred in 101 participants (4.7%) in the dapagliflozin group and 146 participants (6.8%) in the placebo group (HR, 0.69; 95% CI, 0.53-0.88; P = 0.004). The known safety profile of dapagliflozin was confirmed by the similar overall incidences of adverse events and serious adverse events in the dapagliflozin and placebo groups.
Conclusion. In patients with CKD, with or without type 2 diabetes, the risk of a composite of a sustained decline in the estimated GFR of at least 50%, end-stage kidney disease, or death from renal or cardiovascular causes was significantly lowered by dapagliflozin treatment.
Commentary
Although SGLT2 inhibitors were designed to reduce plasma glucose and hemoglobin A1c (HbA1c) by increasing urinary glucose excretion in a non-insulin-dependent fashion, an increasing number of clinical trials have demonstrated their possible cardiovascular and renal benefits that extend beyond glycemic control. In 2008, the US Food and Drug Administration (FDA) issued a guidance recommending the evaluation of long-term cardiovascular outcomes prior to approval and commercialization of new antidiabetic therapies to ensure minimum cardiovascular risks following the discovery of cardiovascular safety issues associated with antidiabetic compounds, including rosiglitazone, after drug approval. No one foresaw that this recommendation would lead to the discovery of new classes of antidiabetic drugs (glucagon-like peptide 1 [GLP1] and SGLT2 inhibitors) that improve cardiovascular outcomes. A series of clinical trials of SGLT2 inhibitors, including empagliflozin,1 canagliflozin,2 and dapagliflozin,3 showed a reduction in cardiovascular death and hospitalization due to heart failure among patients with type 2 diabetes. Furthermore, a meta-analysis from 2019 found that SGLT2 inhibitors reduced the risk of a composite of cardiovascular death or hospitalization for heart failure by 23% and the risk of progression of kidney failure by 45% in patients with diabetes.4 Thus, the strong and consistent evidence from these large and well-designed outcome trials led the American Diabetes Association in its most recent guidelines to recommend adding SGLT2 inhibitors to metformin for the treatment of patients with type 2 diabetes with or at high risk of atherosclerotic cardiovascular disease, heart failure, or CKD, regardless of baseline HbA1c levels or HbA1c target.5 As a result of the compelling effects of SGLT2 inhibitors on cardiovascular outcomes in diabetic patients, as well as increasing evidence that these clinical effects were independent of glycemic control, several subsequent trials were conducted to evaluate whether this new class of drugs may improve clinical outcomes in nondiabetic patients.
The Dapagliflozin and Prevention of Adverse Outcomes in Heart Failure (DAPA-HF) was the first clinical trial to investigate the effect of SGLT2 inhibitors on cardiovascular disease in nondiabetic patients. Findings from DAPA-HF showed that dapagliflozin reduced the risk of worsening heart failure or death from cardiovascular causes, independent of the presence of underlying diabetes. This initial finding resonates with a growing body of evidence6,7 that supports the use of SGLT2 inhibitors as an adjunctive therapy for heart failure in the absence of diabetes.
The Canagliflozin and Renal Events in Diabetes with Established Nephropathy Clinical Evaluation (CREDENCE) trial showed that long-term administration of canagliflozin conferred cardiovascular, as well as renal, protection in patients with type 2 diabetes with CKD.8 Similar to the protective effects on heart failure, the renal benefits of SGLT2 inhibitors appeared to be independent of their blood glucose-lowering effects. Thus, these recent discoveries led to the design of the DAPA-CKD trial to further assess the long-term efficacy and safety of the SGLT2 inhibitor dapagliflozin in patients with CKD precipitated by causes other than type 2 diabetes. Although diabetes is the most common cause for CKD, it nonetheless only accounts for 40% of all CKD etiologies. To date, the only classes of medication that have been shown to slow a decline in kidney function in patients with diabetes are angiotensin-converting enzyme (ACE) inhibitors and angiotensin receptor blockers (ARBs). Given that CKD is an important contributor to illness, is associated with diminished quality of life and reduced life expectancy, and increases health care costs, the findings of the DAPA-CKD trial are particularly significant as they show a renal benefit of dapagliflozin treatment across CKD stages that is independent of underlying diabetes. Therefore, SGLT2 inhibitors may offer a new and unique treatment option for millions of patients with CKD worldwide for whom ACE inhibitors and ARBs were otherwise the only treatments to prevent kidney failure. Moreover, with a number-needed-to-treat of 19 to prevent 1 composite renal vascular event over a period of 2.4 years, dapagliflozin requires a much lower number needed to treat compared to ACE inhibitors and ARBs in similar patients.
The trial has several limitations in study design. For example, the management of diabetes and hypertension were left to the discretion of each trial site, in keeping with local clinical practice and guidelines. It is unknown whether this variability in the management of comorbidities that impact kidney function had an effect on the study’s results. In addition, the trial was stopped early as a result of recommendations from an independent committee due to the demonstrated efficacy of dapagliflozin. This may have reduced the statistical power to assess some of the secondary outcomes. Finally, the authors discussed an initial dip in the estimated GFR after initiation of dapagliflozin treatment, similar to that observed in other SGLT2 inhibitor clinical trials. However, they were unable to ascertain the reversibility of this effect after the discontinuation of dapagliflozin because assessment of GFR was not completed after trial closure. Nonetheless, the authors specified that the reversibility of this initial estimated GFR dip had been assessed and observed in other clinical trials involving dapagliflozin.
The nonglycemic benefits of SGLT2 inhibitors, including improvement in renal outcomes, have strong implications for the future management of patients with CKD. If this indication is approved by the FDA and recommended by clinical guidelines, the ease of SGLT2 inhibitor prescription (eg, minimal drug-drug interaction, no titration), treatment administration (orally once daily), and safety profile may lead to wide use of SGLT2 inhibitors by generalists, nephrologists, and endocrinologists in preserving or improving renal outcomes in patients at risk for end-stage kidney disease. Given that SGLT2 inhibitors are a new class of pharmacologic therapeutics, patient education should include a discussion of the possible side effects, such as euglycemic ketoacidosis, genital and urinary tract infection, and foot and leg amputation. Finally, as Strandberg and colleagues reported in a recent commentary,9 the safety of SGLT2 inhibitors in older adults with multimorbidity, frailty, and polypharmacy remains unclear. Thus, future studies of SGLT2 inhibitors are needed to better evaluate their clinical effects in older adults.
Applications for Clinical Practice
This trial enrolled a dedicated patient population with CKD and demonstrated a benefit of dapagliflozin in reducing renal and cardiovascular outcomes, regardless of baseline diabetes status. These drugs (dapagliflozin as well as other SGLT2 inhibitors) will likely have a prominent role in future CKD management guidelines. Until then, several barriers remain before SGLT2 inhibitors can be widely used in clinical practice. Among these barriers are FDA approval for their use in patients with and without diabetes with an estimated GFR < 30 mL/min/1.73 m2 and lowering the costs of this class of drugs.
—Rachel Litke, MD, PhD
Icahn School of Medicine at Mount Sinai
Fred Ko, MD, MS
1. Zinman B, Wanner C, Lachin JM, et al. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med. 2015;373:2117-2128.
2. Neal B, Perkovic V, Matthews DR. Canagliflozin and cardiovascular and renal events in type 2 diabetes. N Engl J Med. 2017;377:2099.
3. Wiviott SD, Raz I, Bonaca MP, et al. Dapagliflozin and cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2019;380:347-357.
4. Zelniker TA, Wiviott SD, Raz I, Sabatine MS. SGLT-2 inhibitors for people with type 2 diabetes - Authors’ reply. Lancet. 2019;394:560-561.
5. American Diabetes Association 10. Cardiovascular disease and risk management: standards of medical care in diabetes-2020. Diabetes Care. 2020;43(Suppl 1):S111-S34.
6. Packer M, Anker SD, Butler J, et al. Cardiovascular and renal outcomes with empagliflozin in heart failure. N Engl J Med. 2020;383:1413-1424.
7. Zannad F, Ferreira JP, Pocock SJ, et al. SGLT2 inhibitors in patients with heart failure with reduced ejection fraction: a meta-analysis of the EMPEROR-Reduced and DAPA-HF trials. Lancet. 2020;396:819-829.
8. Perkovic V, Jardine MJ, Neal B, et al. Canagliflozin and renal outcomes in type 2 diabetes and nephropathy. N Engl J Med. 2019;380:2295-2306.
9. Strandberg TE, Petrovic M, Benetos A. SGLT-2 inhibitors for people with type 2 diabetes. Lancet. 2019;394:560.
1. Zinman B, Wanner C, Lachin JM, et al. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med. 2015;373:2117-2128.
2. Neal B, Perkovic V, Matthews DR. Canagliflozin and cardiovascular and renal events in type 2 diabetes. N Engl J Med. 2017;377:2099.
3. Wiviott SD, Raz I, Bonaca MP, et al. Dapagliflozin and cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2019;380:347-357.
4. Zelniker TA, Wiviott SD, Raz I, Sabatine MS. SGLT-2 inhibitors for people with type 2 diabetes - Authors’ reply. Lancet. 2019;394:560-561.
5. American Diabetes Association 10. Cardiovascular disease and risk management: standards of medical care in diabetes-2020. Diabetes Care. 2020;43(Suppl 1):S111-S34.
6. Packer M, Anker SD, Butler J, et al. Cardiovascular and renal outcomes with empagliflozin in heart failure. N Engl J Med. 2020;383:1413-1424.
7. Zannad F, Ferreira JP, Pocock SJ, et al. SGLT2 inhibitors in patients with heart failure with reduced ejection fraction: a meta-analysis of the EMPEROR-Reduced and DAPA-HF trials. Lancet. 2020;396:819-829.
8. Perkovic V, Jardine MJ, Neal B, et al. Canagliflozin and renal outcomes in type 2 diabetes and nephropathy. N Engl J Med. 2019;380:2295-2306.
9. Strandberg TE, Petrovic M, Benetos A. SGLT-2 inhibitors for people with type 2 diabetes. Lancet. 2019;394:560.
New guidelines address diabetes management in kidney disease
A new guideline from the Kidney Disease: Improving Global Outcomes group addressing issues around diabetes management in patients with chronic kidney disease (CKD) has just been published in synopsis form in Annals of Internal Medicine.
The full guideline, including 12 recommendations and 48 practice points for clinicians caring for patients with diabetes and CKD, was published last month in Kidney International and on the KDIGO website.
More than 40% of people with diabetes develop CKD, and a significant number develop kidney failure requiring dialysis or transplant. This is the first guidance from KDIGO to address the comorbidity.
The new synopsis is aimed at primary care and nonnephrology specialist clinicians who manage patients with diabetes and CKD, in addition to nephrologists, first author Sankar D. Navaneethan, MD, said in an interview.
“Most of these patients are in the hands of primary care, endocrinology, and cardiology. We want to emphasize when they see patients with different severities of kidney disease [is] what are some of the things they have to be cognizant of,” said Dr. Navaneethan, professor of medicine and director of clinical research in the section of nephrology at Baylor College of Medicine, Houston.
The synopsis summarizes key recommendations from the larger guidance regarding comprehensive care needs, glycemic monitoring and targets, lifestyle interventions, glucose-lowering therapies, and educational/integrated care approaches.
It does not depart from prior diabetes guidelines, but it does provide advice for specific situations relevant to CKD, such as the limitations of hemoglobin A1c when estimated glomerular filtration rate (eGFR) drops below 30 mL/min per 1.73m2, and dietary protein consumption. It is based on published evidence up until February 2020.
For the nephrologist audience in particular, Dr. Navaneethan said, “we wanted to highlight team-based care, interacting with other specialists and working with them.”
“We [nephrologists] are more used to team-based care in dialysis patients. ... So we wanted to highlight that self-management programs and team-based care are important for empowering patients.”
“As nephrologists, we might not be comfortable starting patients on an SGLT2 [sodium-glucose cotransporter 2] inhibitor. We may need to reach out to our endocrinology or primary care colleagues and learn from them,” he explained.
RAS inhibitor use, smoking cessation, glycemic targets
Under “comprehensive care,” the guideline panel recommends treatment with an ACE inhibitor or an angiotensin II receptor blocker – renin-angiotensin system (RAS) blockade – for patients with diabetes, hypertension, and albuminuria (albumin-creatinine ratio >30 mg/g).
These medications should be titrated to the highest approved tolerated dose, with close monitoring of serum potassium and serum creatinine levels within 2-4 weeks of initiation or change in dose.
The document guides clinicians on that monitoring, as well as on RAS blockade use in patient subgroups, use of alternative agents, and mitigation of adverse effects.
Patients with diabetes and CKD who use tobacco should be advised to quit.
The group recommended A1c to monitor glycemic control in patients with diabetes and CKD not receiving dialysis.
However, when eGFR is below 30 mL/min per 1.73m2, A1c levels tend to be lower because of shortened erythrocyte lifespan, which interpretation should take into account. Continuous glucose monitoring can be used as an alternative because it is not affected by CKD.
Glycemic targets should be individualized depending on hypoglycemia risk, ranging from 6.5% to 8.0% for A1c or time in range of 70-180 mg/dL for continuous glucose monitoring readings.
SGLT2 inhibitors, metformin, and GLP-1 agonists
The panel also recommends treatment with both metformin and an SGLT2 inhibitor for patients with type 2 diabetes, CKD, and an eGFR ≥30 mL/min per 1.73m2.
For those who do not achieve glycemic targets or who cannot take those medications, a long-acting glucagonlike peptide–1 receptor agonist can be used instead.
Clinical trial data are summarized for the SGLT2 inhibitor canagliflozin supporting its use in patients with CKD specifically, along with mitigation of adverse events. Last year, the Food and Drug Administration approved this agent to slow the progression of diabetic nephropathy based on the CREDENCE study.
Results from the DAPA-CKD trial showing CKD reduction with another SGLT2 inhibitor, dapagliflozin, were not available at the time the new document was written, nor was the recent study showing diabetic CKD benefit for the novel mineralocorticoid receptor antagonist finerenone, Dr. Navaneethan noted.
The panel determined that there is insufficient evidence for adding other glucose-lowering agents to insulin in patients with type 1 diabetes and CKD.
Lifestyle interventions: Dietary protein, sodium, and physical activity
Most of the dietary guidance for patients with diabetes and CKD is the same as for the general population, including a recommendation to eat a diet high in vegetables, fruits, whole grains, fiber, legumes, plant-based proteins, unsaturated fats, and nuts, and lower in processed meats, refined carbohydrates, and sweetened beverages.
However, the guideline details two key areas that differ, one with regard to protein intake and the other on sodium.
Although lower protein intake had been advised in the past for patients with CKD, clinical trial evidence has not shown protein restriction to reduce glomerular hyperfiltration or slow kidney disease progression.
Therefore, the same level recommended for the general population – 0.8 g/kg per day – is also advised for those with diabetes and CKD who are not on dialysis.
Those who are on dialysis can increase daily protein intake to 1.0-1.2 g/kg per day to offset catabolism and negative nitrogen imbalance.
Because kidney function decline is associated with sodium retention that can raise cardiovascular risk, sodium should be limited to less than 2 g/day (or less than 90 mmol or 5 g of sodium chloride per day).
The panel also recommended moderate-intensity physical activity for at least 150 minutes per week or to tolerance.
“We wanted to emphasize how important lifestyle is. It’s the foundation you want to build on. You can take medications without all these other things – exercise, diet, weight loss – but they won’t be nearly as effective,” Dr. Navaneethan commented.
Self-management education, team-based care
The final section of the synopsis advises that people with diabetes and CKD receive structured self-management educational programs, and that “policy makers and institutional decision-makers implement team-based, integrated care focused on risk evaluation and patient empowerment to provide comprehensive care in patients with diabetes and CKD.”
Despite limited data for those measures specifically in patients with diabetes and CKD, “the working group believed that well-informed patients would choose self-management as the cornerstone of any chronic care model; therefore, a high value was placed on the potential benefits of self-management education programs in persons with diabetes and CKD.”
And regarding team-based care, “despite a paucity of direct evidence, the working group judged that multidisciplinary integrated care for patients with diabetes and CKD would represent a good investment.”
The guidelines will likely be updated in the next 1-2 years, Dr. Navaneethan said in an interview.
Dr. Navaneethan has reported receiving consultancy fees from Bayer, Boehringer Ingelheim, Reata, and Tricida, and research support from Keryx.
A version of this article originally appeared on Medscape.com.
A new guideline from the Kidney Disease: Improving Global Outcomes group addressing issues around diabetes management in patients with chronic kidney disease (CKD) has just been published in synopsis form in Annals of Internal Medicine.
The full guideline, including 12 recommendations and 48 practice points for clinicians caring for patients with diabetes and CKD, was published last month in Kidney International and on the KDIGO website.
More than 40% of people with diabetes develop CKD, and a significant number develop kidney failure requiring dialysis or transplant. This is the first guidance from KDIGO to address the comorbidity.
The new synopsis is aimed at primary care and nonnephrology specialist clinicians who manage patients with diabetes and CKD, in addition to nephrologists, first author Sankar D. Navaneethan, MD, said in an interview.
“Most of these patients are in the hands of primary care, endocrinology, and cardiology. We want to emphasize when they see patients with different severities of kidney disease [is] what are some of the things they have to be cognizant of,” said Dr. Navaneethan, professor of medicine and director of clinical research in the section of nephrology at Baylor College of Medicine, Houston.
The synopsis summarizes key recommendations from the larger guidance regarding comprehensive care needs, glycemic monitoring and targets, lifestyle interventions, glucose-lowering therapies, and educational/integrated care approaches.
It does not depart from prior diabetes guidelines, but it does provide advice for specific situations relevant to CKD, such as the limitations of hemoglobin A1c when estimated glomerular filtration rate (eGFR) drops below 30 mL/min per 1.73m2, and dietary protein consumption. It is based on published evidence up until February 2020.
For the nephrologist audience in particular, Dr. Navaneethan said, “we wanted to highlight team-based care, interacting with other specialists and working with them.”
“We [nephrologists] are more used to team-based care in dialysis patients. ... So we wanted to highlight that self-management programs and team-based care are important for empowering patients.”
“As nephrologists, we might not be comfortable starting patients on an SGLT2 [sodium-glucose cotransporter 2] inhibitor. We may need to reach out to our endocrinology or primary care colleagues and learn from them,” he explained.
RAS inhibitor use, smoking cessation, glycemic targets
Under “comprehensive care,” the guideline panel recommends treatment with an ACE inhibitor or an angiotensin II receptor blocker – renin-angiotensin system (RAS) blockade – for patients with diabetes, hypertension, and albuminuria (albumin-creatinine ratio >30 mg/g).
These medications should be titrated to the highest approved tolerated dose, with close monitoring of serum potassium and serum creatinine levels within 2-4 weeks of initiation or change in dose.
The document guides clinicians on that monitoring, as well as on RAS blockade use in patient subgroups, use of alternative agents, and mitigation of adverse effects.
Patients with diabetes and CKD who use tobacco should be advised to quit.
The group recommended A1c to monitor glycemic control in patients with diabetes and CKD not receiving dialysis.
However, when eGFR is below 30 mL/min per 1.73m2, A1c levels tend to be lower because of shortened erythrocyte lifespan, which interpretation should take into account. Continuous glucose monitoring can be used as an alternative because it is not affected by CKD.
Glycemic targets should be individualized depending on hypoglycemia risk, ranging from 6.5% to 8.0% for A1c or time in range of 70-180 mg/dL for continuous glucose monitoring readings.
SGLT2 inhibitors, metformin, and GLP-1 agonists
The panel also recommends treatment with both metformin and an SGLT2 inhibitor for patients with type 2 diabetes, CKD, and an eGFR ≥30 mL/min per 1.73m2.
For those who do not achieve glycemic targets or who cannot take those medications, a long-acting glucagonlike peptide–1 receptor agonist can be used instead.
Clinical trial data are summarized for the SGLT2 inhibitor canagliflozin supporting its use in patients with CKD specifically, along with mitigation of adverse events. Last year, the Food and Drug Administration approved this agent to slow the progression of diabetic nephropathy based on the CREDENCE study.
Results from the DAPA-CKD trial showing CKD reduction with another SGLT2 inhibitor, dapagliflozin, were not available at the time the new document was written, nor was the recent study showing diabetic CKD benefit for the novel mineralocorticoid receptor antagonist finerenone, Dr. Navaneethan noted.
The panel determined that there is insufficient evidence for adding other glucose-lowering agents to insulin in patients with type 1 diabetes and CKD.
Lifestyle interventions: Dietary protein, sodium, and physical activity
Most of the dietary guidance for patients with diabetes and CKD is the same as for the general population, including a recommendation to eat a diet high in vegetables, fruits, whole grains, fiber, legumes, plant-based proteins, unsaturated fats, and nuts, and lower in processed meats, refined carbohydrates, and sweetened beverages.
However, the guideline details two key areas that differ, one with regard to protein intake and the other on sodium.
Although lower protein intake had been advised in the past for patients with CKD, clinical trial evidence has not shown protein restriction to reduce glomerular hyperfiltration or slow kidney disease progression.
Therefore, the same level recommended for the general population – 0.8 g/kg per day – is also advised for those with diabetes and CKD who are not on dialysis.
Those who are on dialysis can increase daily protein intake to 1.0-1.2 g/kg per day to offset catabolism and negative nitrogen imbalance.
Because kidney function decline is associated with sodium retention that can raise cardiovascular risk, sodium should be limited to less than 2 g/day (or less than 90 mmol or 5 g of sodium chloride per day).
The panel also recommended moderate-intensity physical activity for at least 150 minutes per week or to tolerance.
“We wanted to emphasize how important lifestyle is. It’s the foundation you want to build on. You can take medications without all these other things – exercise, diet, weight loss – but they won’t be nearly as effective,” Dr. Navaneethan commented.
Self-management education, team-based care
The final section of the synopsis advises that people with diabetes and CKD receive structured self-management educational programs, and that “policy makers and institutional decision-makers implement team-based, integrated care focused on risk evaluation and patient empowerment to provide comprehensive care in patients with diabetes and CKD.”
Despite limited data for those measures specifically in patients with diabetes and CKD, “the working group believed that well-informed patients would choose self-management as the cornerstone of any chronic care model; therefore, a high value was placed on the potential benefits of self-management education programs in persons with diabetes and CKD.”
And regarding team-based care, “despite a paucity of direct evidence, the working group judged that multidisciplinary integrated care for patients with diabetes and CKD would represent a good investment.”
The guidelines will likely be updated in the next 1-2 years, Dr. Navaneethan said in an interview.
Dr. Navaneethan has reported receiving consultancy fees from Bayer, Boehringer Ingelheim, Reata, and Tricida, and research support from Keryx.
A version of this article originally appeared on Medscape.com.
A new guideline from the Kidney Disease: Improving Global Outcomes group addressing issues around diabetes management in patients with chronic kidney disease (CKD) has just been published in synopsis form in Annals of Internal Medicine.
The full guideline, including 12 recommendations and 48 practice points for clinicians caring for patients with diabetes and CKD, was published last month in Kidney International and on the KDIGO website.
More than 40% of people with diabetes develop CKD, and a significant number develop kidney failure requiring dialysis or transplant. This is the first guidance from KDIGO to address the comorbidity.
The new synopsis is aimed at primary care and nonnephrology specialist clinicians who manage patients with diabetes and CKD, in addition to nephrologists, first author Sankar D. Navaneethan, MD, said in an interview.
“Most of these patients are in the hands of primary care, endocrinology, and cardiology. We want to emphasize when they see patients with different severities of kidney disease [is] what are some of the things they have to be cognizant of,” said Dr. Navaneethan, professor of medicine and director of clinical research in the section of nephrology at Baylor College of Medicine, Houston.
The synopsis summarizes key recommendations from the larger guidance regarding comprehensive care needs, glycemic monitoring and targets, lifestyle interventions, glucose-lowering therapies, and educational/integrated care approaches.
It does not depart from prior diabetes guidelines, but it does provide advice for specific situations relevant to CKD, such as the limitations of hemoglobin A1c when estimated glomerular filtration rate (eGFR) drops below 30 mL/min per 1.73m2, and dietary protein consumption. It is based on published evidence up until February 2020.
For the nephrologist audience in particular, Dr. Navaneethan said, “we wanted to highlight team-based care, interacting with other specialists and working with them.”
“We [nephrologists] are more used to team-based care in dialysis patients. ... So we wanted to highlight that self-management programs and team-based care are important for empowering patients.”
“As nephrologists, we might not be comfortable starting patients on an SGLT2 [sodium-glucose cotransporter 2] inhibitor. We may need to reach out to our endocrinology or primary care colleagues and learn from them,” he explained.
RAS inhibitor use, smoking cessation, glycemic targets
Under “comprehensive care,” the guideline panel recommends treatment with an ACE inhibitor or an angiotensin II receptor blocker – renin-angiotensin system (RAS) blockade – for patients with diabetes, hypertension, and albuminuria (albumin-creatinine ratio >30 mg/g).
These medications should be titrated to the highest approved tolerated dose, with close monitoring of serum potassium and serum creatinine levels within 2-4 weeks of initiation or change in dose.
The document guides clinicians on that monitoring, as well as on RAS blockade use in patient subgroups, use of alternative agents, and mitigation of adverse effects.
Patients with diabetes and CKD who use tobacco should be advised to quit.
The group recommended A1c to monitor glycemic control in patients with diabetes and CKD not receiving dialysis.
However, when eGFR is below 30 mL/min per 1.73m2, A1c levels tend to be lower because of shortened erythrocyte lifespan, which interpretation should take into account. Continuous glucose monitoring can be used as an alternative because it is not affected by CKD.
Glycemic targets should be individualized depending on hypoglycemia risk, ranging from 6.5% to 8.0% for A1c or time in range of 70-180 mg/dL for continuous glucose monitoring readings.
SGLT2 inhibitors, metformin, and GLP-1 agonists
The panel also recommends treatment with both metformin and an SGLT2 inhibitor for patients with type 2 diabetes, CKD, and an eGFR ≥30 mL/min per 1.73m2.
For those who do not achieve glycemic targets or who cannot take those medications, a long-acting glucagonlike peptide–1 receptor agonist can be used instead.
Clinical trial data are summarized for the SGLT2 inhibitor canagliflozin supporting its use in patients with CKD specifically, along with mitigation of adverse events. Last year, the Food and Drug Administration approved this agent to slow the progression of diabetic nephropathy based on the CREDENCE study.
Results from the DAPA-CKD trial showing CKD reduction with another SGLT2 inhibitor, dapagliflozin, were not available at the time the new document was written, nor was the recent study showing diabetic CKD benefit for the novel mineralocorticoid receptor antagonist finerenone, Dr. Navaneethan noted.
The panel determined that there is insufficient evidence for adding other glucose-lowering agents to insulin in patients with type 1 diabetes and CKD.
Lifestyle interventions: Dietary protein, sodium, and physical activity
Most of the dietary guidance for patients with diabetes and CKD is the same as for the general population, including a recommendation to eat a diet high in vegetables, fruits, whole grains, fiber, legumes, plant-based proteins, unsaturated fats, and nuts, and lower in processed meats, refined carbohydrates, and sweetened beverages.
However, the guideline details two key areas that differ, one with regard to protein intake and the other on sodium.
Although lower protein intake had been advised in the past for patients with CKD, clinical trial evidence has not shown protein restriction to reduce glomerular hyperfiltration or slow kidney disease progression.
Therefore, the same level recommended for the general population – 0.8 g/kg per day – is also advised for those with diabetes and CKD who are not on dialysis.
Those who are on dialysis can increase daily protein intake to 1.0-1.2 g/kg per day to offset catabolism and negative nitrogen imbalance.
Because kidney function decline is associated with sodium retention that can raise cardiovascular risk, sodium should be limited to less than 2 g/day (or less than 90 mmol or 5 g of sodium chloride per day).
The panel also recommended moderate-intensity physical activity for at least 150 minutes per week or to tolerance.
“We wanted to emphasize how important lifestyle is. It’s the foundation you want to build on. You can take medications without all these other things – exercise, diet, weight loss – but they won’t be nearly as effective,” Dr. Navaneethan commented.
Self-management education, team-based care
The final section of the synopsis advises that people with diabetes and CKD receive structured self-management educational programs, and that “policy makers and institutional decision-makers implement team-based, integrated care focused on risk evaluation and patient empowerment to provide comprehensive care in patients with diabetes and CKD.”
Despite limited data for those measures specifically in patients with diabetes and CKD, “the working group believed that well-informed patients would choose self-management as the cornerstone of any chronic care model; therefore, a high value was placed on the potential benefits of self-management education programs in persons with diabetes and CKD.”
And regarding team-based care, “despite a paucity of direct evidence, the working group judged that multidisciplinary integrated care for patients with diabetes and CKD would represent a good investment.”
The guidelines will likely be updated in the next 1-2 years, Dr. Navaneethan said in an interview.
Dr. Navaneethan has reported receiving consultancy fees from Bayer, Boehringer Ingelheim, Reata, and Tricida, and research support from Keryx.
A version of this article originally appeared on Medscape.com.
New eGFR equation ‘less biased’ by age, kidney function; some disagree
The European Kidney Function Consortium (EKFC) equation surpasses existing equations by “resulting in generally lower bias across the spectrum of age and kidney function,” its developers wrote in an article published online Nov. 9 in Annals of Internal Medicine.
“The new EKFC equation may have helpful properties and perform better in estimating GFR, compared with the current KDIGO [Kidney Disease: Improving Global Outcomes]-recommended equations,” they added.
The primary KDIGO-recommended equation in its most recent guideline was the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) equation, designed for adults, and a companion equation, the CKiD, covers children and adolescents.
“Key in our [new] equation is the adjustment for differences in serum creatinine generation between children and adults, or between men and women,” lead author Hans Pottel, PhD, KU Leuven (Belgium), said in an interview.
In an accompanying editorial, Andrew M. Levey, MD, and associates wrote: “We agree that a single eGFR equation that can be used in children and adults and performs well in the transition from adolescence to young adulthood is a worthy goal.”
“But the claim of equivalent or superior performance, compared with the CKD-EPI equation is not conclusive,” claimed Dr. Levey, who led the research team that developed the CKD-EPI equation, and coauthors.
Dr. Levey is professor of medicine at Tufts University, Boston.
What’s new is Q
Dr. Pottel and codevelopers devised what they call Q values: age- and sex-dependent median creatinine levels in normal individuals.
Q values act to “normalize or rescale creatinine before entering it into the equation, because we know that creatinine generation is different” based on factors that include age, sex, and muscle mass.
The EKFC equation extends the CKD-EPI equation and first eGFR equation by using Q values and applying across age ranges, like the full-age spectrum (FAS) equation, first reported in 2016 by a team led by Dr. Pottel.
“Although the FAS equation was designed to overcome the challenge in measuring GFR in patients transitioning from adolescence to adult nephrology care, it also underestimates GFR at low serum creatinine values and in patients with chronic kidney disease,” wrote Dr. Pottel and coauthors.
Hence, their intent to tweak the FAS equation to overcome this limitation and create the EKFC equation.
“The new equation combines the strengths of the CKD-EPI and FAS equations,” they woite.
However, “we acknowledge that lack of precision is still a major problem with all eGFR equations,” including the new EKFC, they added.
Editorialists dispute better performance of EKFC over CKD-EPI
In their editorial, Dr. Levey and coauthors noted the EKFC equations and other adapted equations in development “represent a conceptual advance over the FAS equations,” but they dispute the claims of better performance, compared with the CKD-EPI.
“We compared the performance of the EKFC and CKD-EPI equations in a different, large external validation population of Black and non-Black adults,” the external population used to validate the CKD-EPI equation, the editorialists reported.
The upshot was “our results did not confirm the author’s conclusions” about the EKFC equation.
In response, Dr. Pottel highlighted that the EKFC equation is currently not designed for use in Black patients.
“With its derivation and validation now reported in the new article, the EKFC equation is fully validated and ready for routine use in Whites,” he said. “We plan to evaluate and possibly fine tune our equation for its application in other ethnicities.”
Regarding the inferior performance, compared with the CKD-EPI equation in the non-Black population tested by the editorialists, Dr. Pottel cited “calibration issues for serum creatinine” that some experts have found in the datasets compiled by developers of the CKI-EPI equation that could limit the utility of these data.
Still room for improvement; app hopefully coming next year
Dr. Pottel and coauthors developed and validated the EKFC equation with data from 19,629 patients drawn from 13 cohorts. This included 11,251 patients from seven cohorts for development and internal validation, and 8378 from six cohorts for external validation. The EKFC effort received endorsement from the European Renal Association–European Dialysis and Transplant Association.
However, “We acknowledge that there is still room for improvement,” Dr. Pottel said.
Although the new report presents the EKFC equations (actually two slightly different equations depending on whether a patient’s serum creatinine is higher or lower than the relevant Q value), most potential users will likely find the equations easier to work with once they’re in an app form that allows someone to simply plug in age, sex, and serum creatinine level. That app currently doesn’t exist but is coming soon, promised Dr. Pottel.
“I hope to have an electronic tool by the beginning of 2021,” he said. “I have to find a programmer who can do this for me.”
The EKFC project has received no commercial funding. Dr. Pottel reported no relevant financial relationships. Dr. Levey has reported receiving research funding from AstraZeneca.
A version of this article originally appeared on Medscape.com.
The European Kidney Function Consortium (EKFC) equation surpasses existing equations by “resulting in generally lower bias across the spectrum of age and kidney function,” its developers wrote in an article published online Nov. 9 in Annals of Internal Medicine.
“The new EKFC equation may have helpful properties and perform better in estimating GFR, compared with the current KDIGO [Kidney Disease: Improving Global Outcomes]-recommended equations,” they added.
The primary KDIGO-recommended equation in its most recent guideline was the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) equation, designed for adults, and a companion equation, the CKiD, covers children and adolescents.
“Key in our [new] equation is the adjustment for differences in serum creatinine generation between children and adults, or between men and women,” lead author Hans Pottel, PhD, KU Leuven (Belgium), said in an interview.
In an accompanying editorial, Andrew M. Levey, MD, and associates wrote: “We agree that a single eGFR equation that can be used in children and adults and performs well in the transition from adolescence to young adulthood is a worthy goal.”
“But the claim of equivalent or superior performance, compared with the CKD-EPI equation is not conclusive,” claimed Dr. Levey, who led the research team that developed the CKD-EPI equation, and coauthors.
Dr. Levey is professor of medicine at Tufts University, Boston.
What’s new is Q
Dr. Pottel and codevelopers devised what they call Q values: age- and sex-dependent median creatinine levels in normal individuals.
Q values act to “normalize or rescale creatinine before entering it into the equation, because we know that creatinine generation is different” based on factors that include age, sex, and muscle mass.
The EKFC equation extends the CKD-EPI equation and first eGFR equation by using Q values and applying across age ranges, like the full-age spectrum (FAS) equation, first reported in 2016 by a team led by Dr. Pottel.
“Although the FAS equation was designed to overcome the challenge in measuring GFR in patients transitioning from adolescence to adult nephrology care, it also underestimates GFR at low serum creatinine values and in patients with chronic kidney disease,” wrote Dr. Pottel and coauthors.
Hence, their intent to tweak the FAS equation to overcome this limitation and create the EKFC equation.
“The new equation combines the strengths of the CKD-EPI and FAS equations,” they woite.
However, “we acknowledge that lack of precision is still a major problem with all eGFR equations,” including the new EKFC, they added.
Editorialists dispute better performance of EKFC over CKD-EPI
In their editorial, Dr. Levey and coauthors noted the EKFC equations and other adapted equations in development “represent a conceptual advance over the FAS equations,” but they dispute the claims of better performance, compared with the CKD-EPI.
“We compared the performance of the EKFC and CKD-EPI equations in a different, large external validation population of Black and non-Black adults,” the external population used to validate the CKD-EPI equation, the editorialists reported.
The upshot was “our results did not confirm the author’s conclusions” about the EKFC equation.
In response, Dr. Pottel highlighted that the EKFC equation is currently not designed for use in Black patients.
“With its derivation and validation now reported in the new article, the EKFC equation is fully validated and ready for routine use in Whites,” he said. “We plan to evaluate and possibly fine tune our equation for its application in other ethnicities.”
Regarding the inferior performance, compared with the CKD-EPI equation in the non-Black population tested by the editorialists, Dr. Pottel cited “calibration issues for serum creatinine” that some experts have found in the datasets compiled by developers of the CKI-EPI equation that could limit the utility of these data.
Still room for improvement; app hopefully coming next year
Dr. Pottel and coauthors developed and validated the EKFC equation with data from 19,629 patients drawn from 13 cohorts. This included 11,251 patients from seven cohorts for development and internal validation, and 8378 from six cohorts for external validation. The EKFC effort received endorsement from the European Renal Association–European Dialysis and Transplant Association.
However, “We acknowledge that there is still room for improvement,” Dr. Pottel said.
Although the new report presents the EKFC equations (actually two slightly different equations depending on whether a patient’s serum creatinine is higher or lower than the relevant Q value), most potential users will likely find the equations easier to work with once they’re in an app form that allows someone to simply plug in age, sex, and serum creatinine level. That app currently doesn’t exist but is coming soon, promised Dr. Pottel.
“I hope to have an electronic tool by the beginning of 2021,” he said. “I have to find a programmer who can do this for me.”
The EKFC project has received no commercial funding. Dr. Pottel reported no relevant financial relationships. Dr. Levey has reported receiving research funding from AstraZeneca.
A version of this article originally appeared on Medscape.com.
The European Kidney Function Consortium (EKFC) equation surpasses existing equations by “resulting in generally lower bias across the spectrum of age and kidney function,” its developers wrote in an article published online Nov. 9 in Annals of Internal Medicine.
“The new EKFC equation may have helpful properties and perform better in estimating GFR, compared with the current KDIGO [Kidney Disease: Improving Global Outcomes]-recommended equations,” they added.
The primary KDIGO-recommended equation in its most recent guideline was the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) equation, designed for adults, and a companion equation, the CKiD, covers children and adolescents.
“Key in our [new] equation is the adjustment for differences in serum creatinine generation between children and adults, or between men and women,” lead author Hans Pottel, PhD, KU Leuven (Belgium), said in an interview.
In an accompanying editorial, Andrew M. Levey, MD, and associates wrote: “We agree that a single eGFR equation that can be used in children and adults and performs well in the transition from adolescence to young adulthood is a worthy goal.”
“But the claim of equivalent or superior performance, compared with the CKD-EPI equation is not conclusive,” claimed Dr. Levey, who led the research team that developed the CKD-EPI equation, and coauthors.
Dr. Levey is professor of medicine at Tufts University, Boston.
What’s new is Q
Dr. Pottel and codevelopers devised what they call Q values: age- and sex-dependent median creatinine levels in normal individuals.
Q values act to “normalize or rescale creatinine before entering it into the equation, because we know that creatinine generation is different” based on factors that include age, sex, and muscle mass.
The EKFC equation extends the CKD-EPI equation and first eGFR equation by using Q values and applying across age ranges, like the full-age spectrum (FAS) equation, first reported in 2016 by a team led by Dr. Pottel.
“Although the FAS equation was designed to overcome the challenge in measuring GFR in patients transitioning from adolescence to adult nephrology care, it also underestimates GFR at low serum creatinine values and in patients with chronic kidney disease,” wrote Dr. Pottel and coauthors.
Hence, their intent to tweak the FAS equation to overcome this limitation and create the EKFC equation.
“The new equation combines the strengths of the CKD-EPI and FAS equations,” they woite.
However, “we acknowledge that lack of precision is still a major problem with all eGFR equations,” including the new EKFC, they added.
Editorialists dispute better performance of EKFC over CKD-EPI
In their editorial, Dr. Levey and coauthors noted the EKFC equations and other adapted equations in development “represent a conceptual advance over the FAS equations,” but they dispute the claims of better performance, compared with the CKD-EPI.
“We compared the performance of the EKFC and CKD-EPI equations in a different, large external validation population of Black and non-Black adults,” the external population used to validate the CKD-EPI equation, the editorialists reported.
The upshot was “our results did not confirm the author’s conclusions” about the EKFC equation.
In response, Dr. Pottel highlighted that the EKFC equation is currently not designed for use in Black patients.
“With its derivation and validation now reported in the new article, the EKFC equation is fully validated and ready for routine use in Whites,” he said. “We plan to evaluate and possibly fine tune our equation for its application in other ethnicities.”
Regarding the inferior performance, compared with the CKD-EPI equation in the non-Black population tested by the editorialists, Dr. Pottel cited “calibration issues for serum creatinine” that some experts have found in the datasets compiled by developers of the CKI-EPI equation that could limit the utility of these data.
Still room for improvement; app hopefully coming next year
Dr. Pottel and coauthors developed and validated the EKFC equation with data from 19,629 patients drawn from 13 cohorts. This included 11,251 patients from seven cohorts for development and internal validation, and 8378 from six cohorts for external validation. The EKFC effort received endorsement from the European Renal Association–European Dialysis and Transplant Association.
However, “We acknowledge that there is still room for improvement,” Dr. Pottel said.
Although the new report presents the EKFC equations (actually two slightly different equations depending on whether a patient’s serum creatinine is higher or lower than the relevant Q value), most potential users will likely find the equations easier to work with once they’re in an app form that allows someone to simply plug in age, sex, and serum creatinine level. That app currently doesn’t exist but is coming soon, promised Dr. Pottel.
“I hope to have an electronic tool by the beginning of 2021,” he said. “I have to find a programmer who can do this for me.”
The EKFC project has received no commercial funding. Dr. Pottel reported no relevant financial relationships. Dr. Levey has reported receiving research funding from AstraZeneca.
A version of this article originally appeared on Medscape.com.
How to assess and relieve that perplexing rashless itch
Pruritus, defined as a sensation that induces a desire to scratch1 and classified as acute or chronic (lasting > 6 weeks),2 is one of the most common complaints among primary care patients: Approximately 1% of ambulatory visits in the United States are linked to pruritus.3
Chronic pruritus impairs quality of life; its impact has been compared to that of chronic pain.4 Treatment should therefore be instituted promptly. Although this condition might appear benign, chronic pruritus can be a symptom of a serious condition, as we describe here. When persistent pruritus is refractory to treatment, systemic causes should be fully explored.
In this article, we discuss the pathogenesis and management of pruritus without skin eruption in the adult nonpregnant patient. We also present practice recommendations to help you determine whether your patient’s pruritus is indicative of a serious systemic condition.
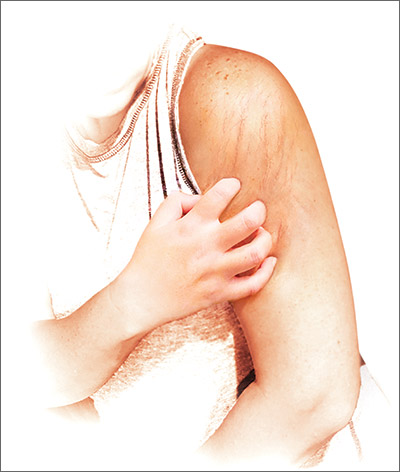
An incomplete understanding of the pathophysiology of pruritus
The pathophysiology of pruritus is not fully understood. It is generally recognized, however, that pruritus starts in the peripheral nerves located in the dermal–epidermal junction of the skin.5 The sensation is then transmitted along unmyelinated slow-conducting C fibers to the dorsal horn of the spinal cord.5,6 There are 2 types of C fibers that transmit the itch impulse6: A histamine-dependent type and a non-histamine-dependent type, which might explain why pruritus can be refractory to antihistamine treatment.6
Once the itch impulse has moved from the spinal cord, it travels along the spinothalamic tract up to the contralateral thalamus.1 From there, the impulse ascends to the cerebral cortex.1 In the cortex, the impulse triggers multiple areas of the brain, such as those responsible for sensation, motor function, reward, memory, and emotion.7
Several chemical mediators have been found to be peripheral and central inducers of pruritus: histamine, endogenous opioids, substance P, and serotonin.2 There are indications that certain receptors, such as mu-opioid receptors and kappa-opioid receptors, are key contributors to itch as well.2
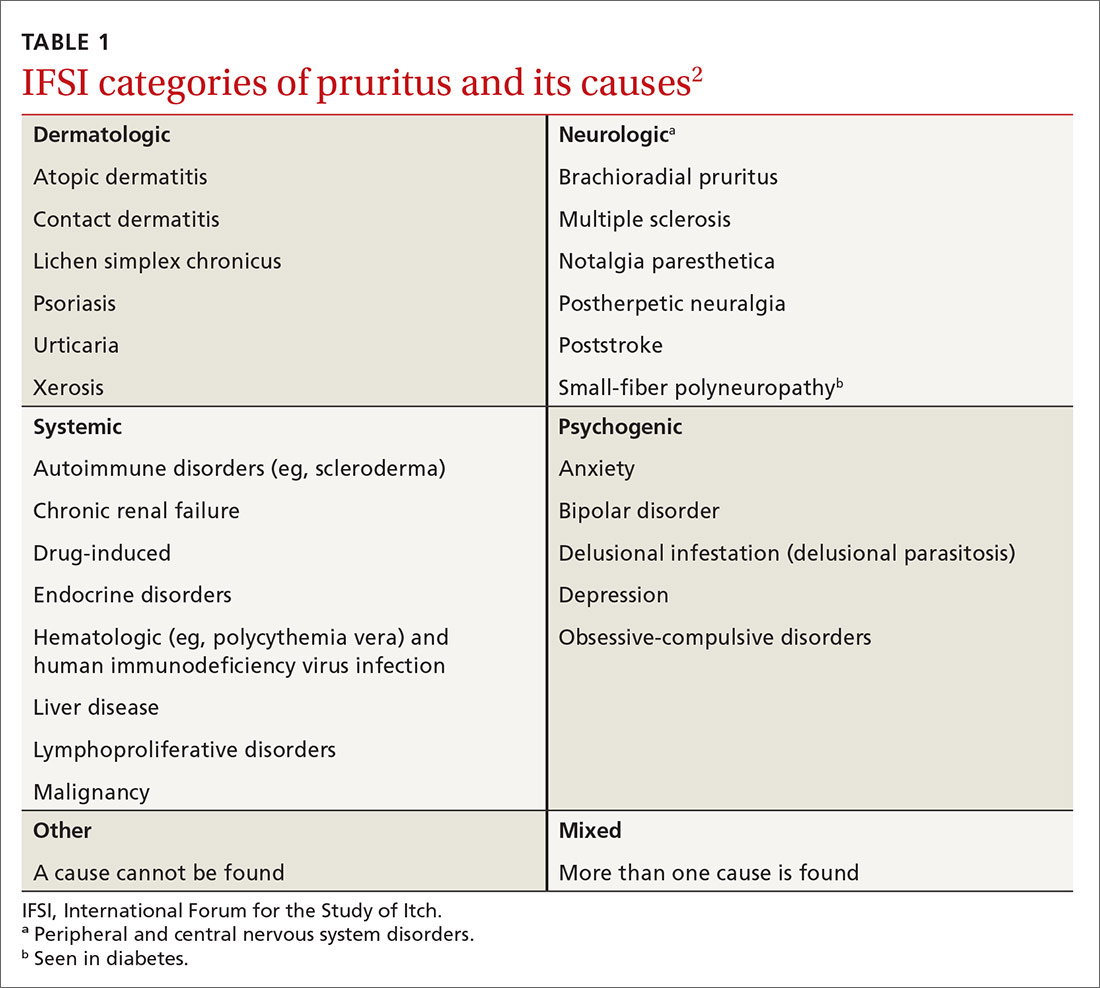
A diverse etiology
The International Forum for the Study of Itch (IFSI) has established 6 main categories of causes of pruritus(TABLE 1)2:
- dermatologic
- systemic
- neurologic
- psychogenic
- mixed
- other.
Continue to: In this review...
In this review, we focus on the work-up and management of 3 of those categories: systemic, neurologic, and psychogenic causes of pruritus.
Systemic causes
Research has shown that 14% to 24% of patients who seek the care of a dermatologist for chronic itch without skin lesions have a systemic illness.8
Renal disease. Approximately 40% of patients with end-stage renal disease who are on hemodialysis or peritoneal dialysis have uremic pruritus.2 The itch is mostly generalized but can be pronounced on the back. For most patients, the itch is worse at night, causing a major impact on quality of life.6
Liver disease. In hepatic disease, there is often impairment in the secretion of bile, which can lead to cholestatic pruritus.2 This condition commonly affects the hands and feet first; later, it becomes generalized.2 Cholestatic pruritus can be elicited by tight-fitting clothing. Relief is not achieved by scratching.9 This type of itch effects 70% of patients with primary biliary cirrhosis and 15% of patients with hepatitis C infection.9
Hematologic disorders. Pruritus is a hallmark symptom of polycythemia rubra vera. Almost 50% of patients with this disorder report pruritus that occurs after exposure to water9; aquagenic pruritus can precede the formal diagnosis of polycythemia rubra vera by years.2 It has been speculated that platelet aggregation in this disorder leads to release of serotonin and histamine, which, in turn, causes itch.9
Continue to: Endocrine disorders
Endocrine disorders. Approximately 4% to 11% of patients with thyrotoxicosis have pruritus.1 It has been suggested that vasodilation, increased skin temperature, and a decreased itch threshold from untreated Graves disease might be inciting factors.
Malignancy. In generalized chronic pruritus without a known cause, strongly consider the likelihood of underlying malignancy8,10; for 10% of these patients, their chronic pruritus is a paraneoplastic sign. Paraneoplastic pruritus is characterized as an itch that predates clinical onset, or occurs early in the course, of a malignancy.9 The condition is most strongly linked to cancers of the liver, gallbladder, biliary tract, hematologic system, and skin.11
Chronic pruritus affects 30% of patients with Hodgkin lymphoma.9 General pruritus can precede this diagnosis by months, even years.1 In Hodgkin lymphoma patients who are in remission, a return of pruritic symptoms can be a harbinger of recurrence.9
Neurologic causes
A recent study found that 8% to 15% of patients referred to a dermatology clinic for chronic pruritus without skin eruption had underlying neurologic pathology.12 Although the specific mechanisms of neuropathic itch are still poorly understood, it has been theorized that the itch emanates from neuronal damage, which can come from peripheral or central nervous system lesions.9
Brachioradial pruritus. There are divergent theories about the etiology of brachioradial pruritus. One hypothesis is that the condition is caused by cervical nerve-root impingement at the level of C5-C8 that leads to nerve damage2; another is that chronic exposure to sunlight causes injury to peripheral cutaneous nerves.2 Brachioradial pruritus is localized to the dorsolateral forearm; it can also involve the neck, back, shoulder, upper arm, and chest, unilaterally and bilaterally. This pruritus can be intermittent and become worse upon exposure to sunlight.2
Continue to: Notalgia paresthetica
Notalgia paresthetica. This condition might also cause neuropathic pruritus as a consequence of nerve impingement. The itch of notalgia paresthesia is located on the skin, medial to the scapular border on the upper or mid-back.2 It has been postulated that the itch is caused by nerve entrapment of the posterior rami of spinal nerves arising from T2-T6.9 However, another theory suggests that the itch is caused by damage to peripheral nerves.9 The itch of notalgia paresthetica can wax and wane.2
Poststroke pruritus. Brain lesions, most often caused by stroke, can cause neuropathic itch. One of the best-known syndromes related to poststroke itch is Wallenberg syndrome (ischemia from a lateral medullary infarction), which typically presents with itch, thermalgic hypoesthesia of the face, cerebellar dysfunction, nausea, and vomiting.7
Shingles. More than one-half of patients who develop postherpetic neuralgia as a consequence of a herpes zoster infection also develop neuropathic pruritus.9 It is thought that postherpetic pruritus shares a comparable pathophysiology with postherpetic neuralgia, in which neurons involved in itch stimuli become damaged.7
Diabetes mellitus. Pruritus from diabetes can be classified as systemic or neuropathic. Diabetes is one of the most common causes of small-fiber polyneuropathy, which can cause neuropathic pruritus.13
Multiple sclerosis. Central nervous system lesions that affect sensory pathways can lead to neuropathic itch in multiple sclerosis. Patients can have severe episodes of generalized pruritus. It has been hypothesized that the neuropathic itch in multiple sclerosis is induced by activation of artificial synapses in demyelinated areas.2
Continue to: Psychogenic pruritus
Psychogenic pruritus
Chronic pruritus can be a comorbidity of psychiatric illness. A retrospective study found that pruritus occurs in 32% to 42% of psychiatric inpatients.14 Depression, anxiety, bipolar disorders, obsessive–compulsive disorders, somatoform disorders, psychosis, and substance abuse all have a strong link to psychogenic excoriation.15 Psychogenic excoriation, which can cause secondary skin lesions, occurs in psychiatric patients who excessively pick and scratch normal skin because they perceive an itch sensation or have a delusion of infestation.2 Affected skin can be marked by scattered crusted lesions (FIGURE) anywhere on the body that the patient can reach—most commonly, the extremities.2
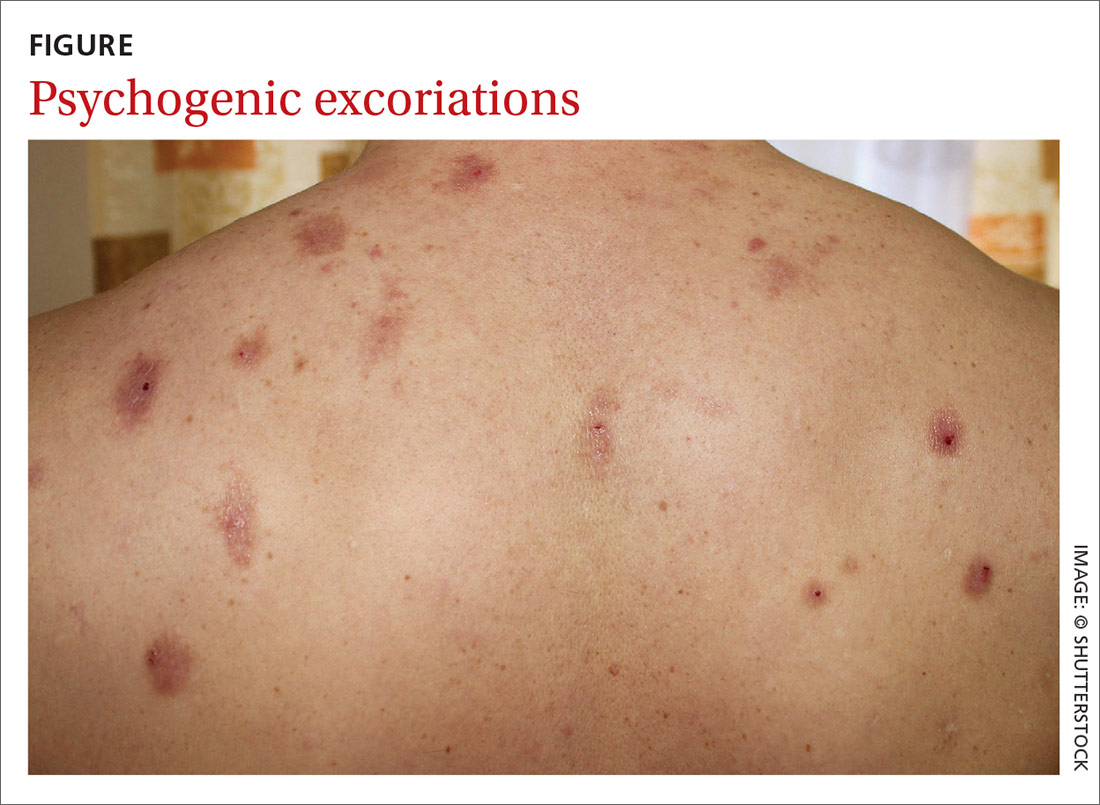
Delusion of infestation. Patients with a delusion of infestation have a strong belief that their body is infected by some kind of insect or microorganism.16 Before a diagnosis of delusion of infestation can be made, other organic causes must be excluded, including withdrawal from such substances as cocaine, amphetamines, and alcohol.16 Patients with a delusion of infestation can have, and maintain, a symptomatic response with continuing use of an atypical antipsychotic agent, including risperidone and olanzapine.17
Evaluation and diagnostic work-up
A thorough medical history, review of systems, medication review, social history, and family history are important when evaluating a patient with chronic pruritus.18 These items can be valuable in formulating a differential diagnosis, even before a physical examination.
Physical examination. The physical exam should include detailed inspection of the entire skin and hair18; such a comprehensive physical exam can determine whether the source of the itch is cutaneous.7 This, in turn, can help further narrow the differential diagnosis. It is crucial that the physical exam include palpation of the liver, spleen, lymph nodes, and thyroid for organomegaly,8 which could indicate a serious systemic condition, such as lymphoma.
The ice-pack sign—in which an ice pack is applied to the pruritic area, the patient experiences immediate relief of pruritus, and the itch returns soon after the ice pack is removed—is considered pathognomonic for brachioradial pruritus.19
Continue to: Chronic pruritus with abnormal findings...
Chronic pruritus with abnormal findings on the physical exam should prompt an initial work-up.18 Also consider an initial work-up for a patient with chronic pruritus whose symptom has not been relieved with conservative treatment.18
Laboratory testing. The initial laboratory work-up could include any of the following evaluations: complete blood count, measurement of thyroid-stimulating hormone, comprehensive metabolic panel (liver function, renal function, and the serum glucose level) and the erythrocyte sedimentation rate (TABLE 2).18 If warranted by the evaluation and physical exam, blood work can also include serologic studies for human immunodeficiency virus infection and hepatitis.17
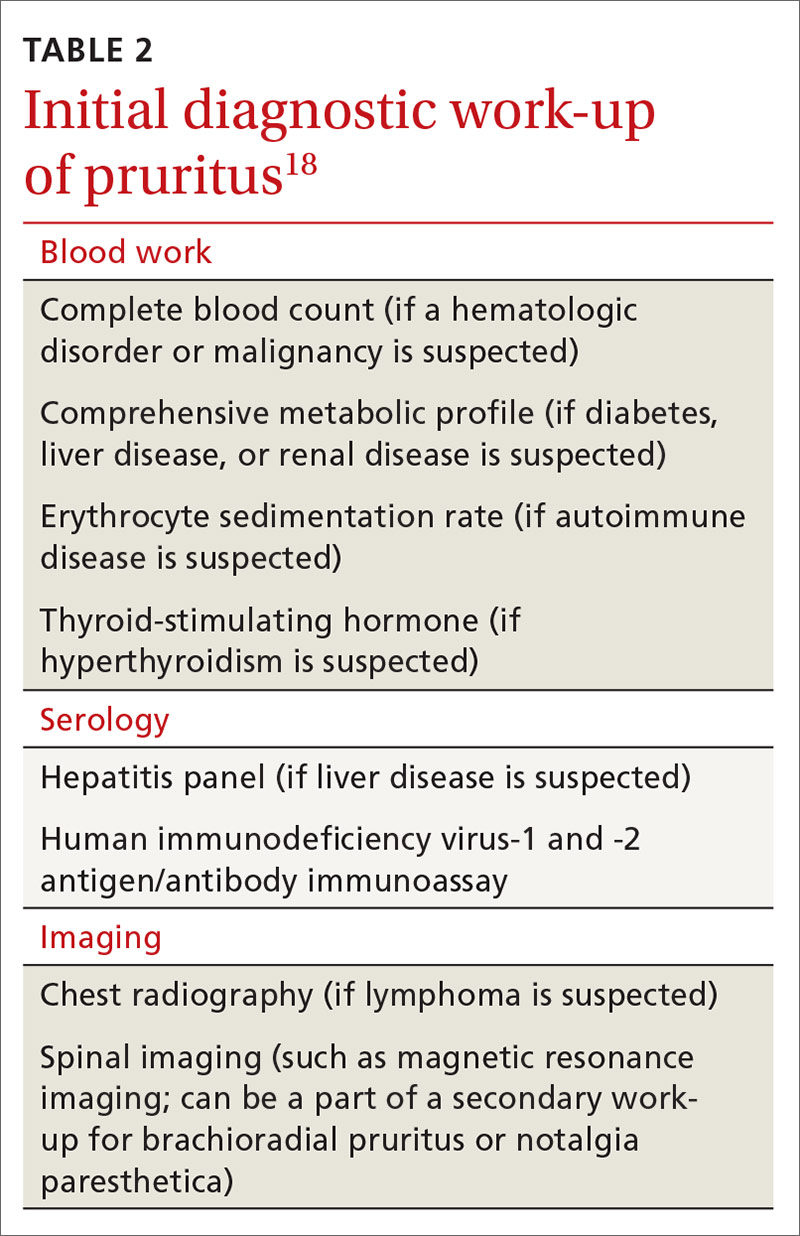
Imaging. Chest radiography should be performed if there is suspicion of malignancy, such as lymphoma.7 Although brachioradial pruritus and notalgia paresthetica have been postulated to be caused by impingement of spinal nerves, obtaining spinal imaging, such as magnetic resonance imaging, as part of the initial work-up is not recommended; because spinal images might not show evidence of spinal disease, obtaining spinal imaging is not a requirement before treating brachioradial pruritus and notalgia paresthetica. Do consider spinal imaging, however, for patients in whom brachioradial pruritus or notalgia paresthetica is suspected and conservative treatment has not produced a response.
Treatment: Nondrug approaches, topicals, systemic agents
Start conservatively. Treatment of pruritus should begin with behavior modification and nonpharmacotherapeutic options (TABLE 38). Educate the patient that scratching might cause secondary skin lesions; empowering them with that knowledge is sometimes enough to help break the scratching cycling—especially if the patient combines behavior modification with proper skin hydration with an emollient. To prevent secondary skin lesions through involuntary scratching, consider recommending that lesions be covered with an occlusive dressing or protective clothing.13

Stress has been shown to make chronic itch worse; therefore, stress-reduction activities, such as exercise, meditation, and yoga, might be helpful.20 For patients in whom pruritus has a psychological component, referral to a psychiatrist or psychologist might be therapeutic.
Continue to: When a patient complains...
When a patient complains of severe pruritus at first presentation, consider pharmacotherapy in conjunction with nonpharmacotherapeutic options. Several of the more effective topical therapies for pruritusa are listed in TABLE 4.20 Well-known systemic agents for this purpose are reviewed below and listed in TABLE 5.7
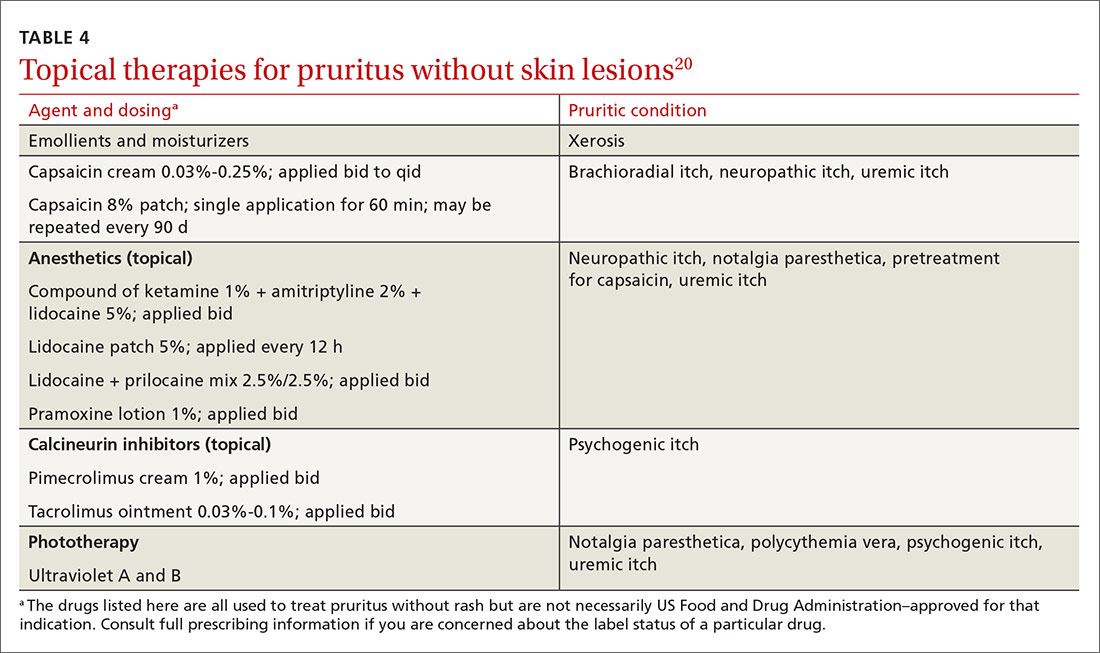
Systemic treatment
Antihistamines. A staple in the treatment of pruritus for many years, antihistamines are not effective for all causes; however, they are effective in treating paraneoplastic pruritus.20 First-generation antihistamines, with their sedating effect, can be useful for patients who experience generalized pruritus at night.20

Anticonvulsants. Gabapentin and pregabalin are analogs of the neurotransmitter gamma-aminobutyric acid.20 This drug class is helpful in neuropathic pruritus specifically caused by impingements, such as brachioradial pruritus and notalgia paresthetica.20 In addition, of all systemic therapies used to treat uremic pruritus, gabapentin has, in clinical trials, most consistently been found effective for uremic pruritus.6 (Note: Use renal dosing of gabapentin in patients with renal failure.)
Antidepressants. Selective serotonin reuptake inhibitors (SSRIs; eg, fluvoxamine, paroxetine, and sertraline) might cause itch to subside by increasing the serotonin level, which, in turn, works to decrease inflammatory substances that cause itch.7 SSRIs have been used to treat patients with psychogenic pruritus, cholestatic pruritus, and paraneoplastic pruritus.7
Tricyclic antidepressants (eg, amitriptyline and doxepin) lessen the itch by antagonizing histamine receptors and through anticholinergic mechanisms. Tricyclics are best used in the treatment of psychogenic and nocturnal itch.7
Continue to: Mirtazapine...
Mirtazapine, a tetracyclic antidepressant, works in patients with uremic pruritus, psychogenic pruritus, cholestatic pruritus, and paraneoplastic pruritus.1
Substance P antagonist. Aprepitant, a neurokinin receptor I antagonist, is a newer agent that inhibits binding of the itch mediator substance P to the neurokinin receptor. The drug has been found helpful in patients with drug-induced, paraneoplastic, and brachioradial pruritus.7
Opioid-receptor agents. Naltrexone, as a mu opioid-receptor antagonist, has shown promise as a treatment for uremic pruritus and cholestatic pruritus. Nalfurafine, a kappa opioid-receptor agonist, is emerging as a possible therapy for uremic pruritus.7
Bile-acid sequestrants. A few small studies have shown that treatment with a bile-acid sequestrant, such as cholestyramine and ursodiol, induces moderate improvement in symptoms in patients with cholestatic pruritus.21
CORRESPONDENCE
Matasha Russell, MD, Department of Family and Community Medicine, The University of Texas Health Science Center at Houston, McGovern Medical School, 6431 Fannin Street, JJL 324, Houston, TX 77030; [email protected].
1. Tarikci N, E, S, et al. Pruritus in systemic diseases: a review of etiological factors and new treatment modalities. ScientificWorldJournal. 2015;2015:803752.
2. Yosipovitch G, Bernhard JD. Clinical practice. Chronic pruritus. N Engl J Med. 2013;368:1625-1634.
3. Silverberg JI, Kantor RW, Dalal P. A comprehensive conceptual model of the experience of chronic itch in adults. Am J Clin Dermatol. 2018;19:759-769.
4. Matterne U, Apfelbacher CJ, Vogelgsang L, et al. Incidence and determinants of chronic pruritus: a population based cohort study. Acta Derm Venereol. 2013;93:532-537.
5. Moses S. Pruritus. Am Fam Physician. 2003;68:1135-1142.
6. Combs SA, Teixeira JP, Germain MJ. Pruritus in kidney disease. Semin Nephrol. 2015;35:383-391.
7. Shevchenko A, Valdes-Rodriguez R, Yosipovitch G. Causes, pathophysiology, and treatment of pruritus in the mature patient. Clin Dermatol. 2018;36:140-151.
8. Reamy BV, Bunt C. A diagnostic approach to pruritus. Am Fam Physician. 2011;84:195-202.
9. M. Current concepts of pathophysiology, epidemiology and classification of pruritus. Srp Arh Celok Lek. 2014;142:106-112.
10. Fett N, Haynes K, Propert KJ, et al. Five-year malignancy incidence in patients with chronic pruritus: a population-based cohort study aimed at limiting unnecessary screening practices. J Am Acad Dermatol. 2014;70:651-658.
11. Larson VA, Tang O, S, et al. Association between itch and cancer in 16,925 patients with pruritus: experience at a tertiary care center. J Am Acad Dermatol. 2019;80:931-937.
12. Rosen JD, Fostini AC, Chan YH, et al. Cross-sectional study of clinical distinctions between neuropathic and inflammatory pruritus. J Am Acad Dermatol. 2018;79:1143-1144.
13. Oaklander AL. Neuropathic itch. Semin Cutan Med Surg. 2011;30:87-92.
14. Ferm I, Sterner M, Wallengren J. Somatic and psychiatric comorbidity in patients with chronic pruritus. Acta Derm Venereol. 2010;90:395-400.
15. Jafferany M, Davari ME. Itch and psyche: psychiatric aspects of pruritus. Int J Dermatol. 2019;58:3-23.
16. Koo J, Lebwohl A. Psychodermatology: the mind and skin connection. Am Fam Physician. 2001;64:1873-1878.
17. Bewley AP, Lepping P, Freudenmann RW, et al. Delusional parasitosis: time to call it delusional infestation. Br J Dermatol.2010;163:1-2.
18. Clerc C-J, Misery L. A literature review of senile pruritus: from diagnosis to treatment. Acta Derm Venereol. 2017;97:433-440.
19. Bernhard JD, Bordeaux JS. Medical pearl: the ice-pack sign in brachioradial pruritus. J Am Acad Dermatol. 2005;52:1073.
20. Sanders KM, Nattkemper LA, Yosipovitch G. Advances in understanding itching and scratching: a new era of targeted treatments [version 1]. F1000Res. 2016;5 F1000 Faculty Rev–2042.
21. Hegade VS, Kendrick SFW, Dobbins RL, et al. Effect of ileal bile acid transporter inhibitor GSK2330672 on pruritus in primary biliary cholangitis: a double-blind, randomised, placebo-controlled, crossover, phase 2a study. Lancet. 2017;389:1114-1123.
Pruritus, defined as a sensation that induces a desire to scratch1 and classified as acute or chronic (lasting > 6 weeks),2 is one of the most common complaints among primary care patients: Approximately 1% of ambulatory visits in the United States are linked to pruritus.3
Chronic pruritus impairs quality of life; its impact has been compared to that of chronic pain.4 Treatment should therefore be instituted promptly. Although this condition might appear benign, chronic pruritus can be a symptom of a serious condition, as we describe here. When persistent pruritus is refractory to treatment, systemic causes should be fully explored.
In this article, we discuss the pathogenesis and management of pruritus without skin eruption in the adult nonpregnant patient. We also present practice recommendations to help you determine whether your patient’s pruritus is indicative of a serious systemic condition.

An incomplete understanding of the pathophysiology of pruritus
The pathophysiology of pruritus is not fully understood. It is generally recognized, however, that pruritus starts in the peripheral nerves located in the dermal–epidermal junction of the skin.5 The sensation is then transmitted along unmyelinated slow-conducting C fibers to the dorsal horn of the spinal cord.5,6 There are 2 types of C fibers that transmit the itch impulse6: A histamine-dependent type and a non-histamine-dependent type, which might explain why pruritus can be refractory to antihistamine treatment.6
Once the itch impulse has moved from the spinal cord, it travels along the spinothalamic tract up to the contralateral thalamus.1 From there, the impulse ascends to the cerebral cortex.1 In the cortex, the impulse triggers multiple areas of the brain, such as those responsible for sensation, motor function, reward, memory, and emotion.7
Several chemical mediators have been found to be peripheral and central inducers of pruritus: histamine, endogenous opioids, substance P, and serotonin.2 There are indications that certain receptors, such as mu-opioid receptors and kappa-opioid receptors, are key contributors to itch as well.2

A diverse etiology
The International Forum for the Study of Itch (IFSI) has established 6 main categories of causes of pruritus(TABLE 1)2:
- dermatologic
- systemic
- neurologic
- psychogenic
- mixed
- other.
Continue to: In this review...
In this review, we focus on the work-up and management of 3 of those categories: systemic, neurologic, and psychogenic causes of pruritus.
Systemic causes
Research has shown that 14% to 24% of patients who seek the care of a dermatologist for chronic itch without skin lesions have a systemic illness.8
Renal disease. Approximately 40% of patients with end-stage renal disease who are on hemodialysis or peritoneal dialysis have uremic pruritus.2 The itch is mostly generalized but can be pronounced on the back. For most patients, the itch is worse at night, causing a major impact on quality of life.6
Liver disease. In hepatic disease, there is often impairment in the secretion of bile, which can lead to cholestatic pruritus.2 This condition commonly affects the hands and feet first; later, it becomes generalized.2 Cholestatic pruritus can be elicited by tight-fitting clothing. Relief is not achieved by scratching.9 This type of itch effects 70% of patients with primary biliary cirrhosis and 15% of patients with hepatitis C infection.9
Hematologic disorders. Pruritus is a hallmark symptom of polycythemia rubra vera. Almost 50% of patients with this disorder report pruritus that occurs after exposure to water9; aquagenic pruritus can precede the formal diagnosis of polycythemia rubra vera by years.2 It has been speculated that platelet aggregation in this disorder leads to release of serotonin and histamine, which, in turn, causes itch.9
Continue to: Endocrine disorders
Endocrine disorders. Approximately 4% to 11% of patients with thyrotoxicosis have pruritus.1 It has been suggested that vasodilation, increased skin temperature, and a decreased itch threshold from untreated Graves disease might be inciting factors.
Malignancy. In generalized chronic pruritus without a known cause, strongly consider the likelihood of underlying malignancy8,10; for 10% of these patients, their chronic pruritus is a paraneoplastic sign. Paraneoplastic pruritus is characterized as an itch that predates clinical onset, or occurs early in the course, of a malignancy.9 The condition is most strongly linked to cancers of the liver, gallbladder, biliary tract, hematologic system, and skin.11
Chronic pruritus affects 30% of patients with Hodgkin lymphoma.9 General pruritus can precede this diagnosis by months, even years.1 In Hodgkin lymphoma patients who are in remission, a return of pruritic symptoms can be a harbinger of recurrence.9
Neurologic causes
A recent study found that 8% to 15% of patients referred to a dermatology clinic for chronic pruritus without skin eruption had underlying neurologic pathology.12 Although the specific mechanisms of neuropathic itch are still poorly understood, it has been theorized that the itch emanates from neuronal damage, which can come from peripheral or central nervous system lesions.9
Brachioradial pruritus. There are divergent theories about the etiology of brachioradial pruritus. One hypothesis is that the condition is caused by cervical nerve-root impingement at the level of C5-C8 that leads to nerve damage2; another is that chronic exposure to sunlight causes injury to peripheral cutaneous nerves.2 Brachioradial pruritus is localized to the dorsolateral forearm; it can also involve the neck, back, shoulder, upper arm, and chest, unilaterally and bilaterally. This pruritus can be intermittent and become worse upon exposure to sunlight.2
Continue to: Notalgia paresthetica
Notalgia paresthetica. This condition might also cause neuropathic pruritus as a consequence of nerve impingement. The itch of notalgia paresthesia is located on the skin, medial to the scapular border on the upper or mid-back.2 It has been postulated that the itch is caused by nerve entrapment of the posterior rami of spinal nerves arising from T2-T6.9 However, another theory suggests that the itch is caused by damage to peripheral nerves.9 The itch of notalgia paresthetica can wax and wane.2
Poststroke pruritus. Brain lesions, most often caused by stroke, can cause neuropathic itch. One of the best-known syndromes related to poststroke itch is Wallenberg syndrome (ischemia from a lateral medullary infarction), which typically presents with itch, thermalgic hypoesthesia of the face, cerebellar dysfunction, nausea, and vomiting.7
Shingles. More than one-half of patients who develop postherpetic neuralgia as a consequence of a herpes zoster infection also develop neuropathic pruritus.9 It is thought that postherpetic pruritus shares a comparable pathophysiology with postherpetic neuralgia, in which neurons involved in itch stimuli become damaged.7
Diabetes mellitus. Pruritus from diabetes can be classified as systemic or neuropathic. Diabetes is one of the most common causes of small-fiber polyneuropathy, which can cause neuropathic pruritus.13
Multiple sclerosis. Central nervous system lesions that affect sensory pathways can lead to neuropathic itch in multiple sclerosis. Patients can have severe episodes of generalized pruritus. It has been hypothesized that the neuropathic itch in multiple sclerosis is induced by activation of artificial synapses in demyelinated areas.2
Continue to: Psychogenic pruritus
Psychogenic pruritus
Chronic pruritus can be a comorbidity of psychiatric illness. A retrospective study found that pruritus occurs in 32% to 42% of psychiatric inpatients.14 Depression, anxiety, bipolar disorders, obsessive–compulsive disorders, somatoform disorders, psychosis, and substance abuse all have a strong link to psychogenic excoriation.15 Psychogenic excoriation, which can cause secondary skin lesions, occurs in psychiatric patients who excessively pick and scratch normal skin because they perceive an itch sensation or have a delusion of infestation.2 Affected skin can be marked by scattered crusted lesions (FIGURE) anywhere on the body that the patient can reach—most commonly, the extremities.2

Delusion of infestation. Patients with a delusion of infestation have a strong belief that their body is infected by some kind of insect or microorganism.16 Before a diagnosis of delusion of infestation can be made, other organic causes must be excluded, including withdrawal from such substances as cocaine, amphetamines, and alcohol.16 Patients with a delusion of infestation can have, and maintain, a symptomatic response with continuing use of an atypical antipsychotic agent, including risperidone and olanzapine.17
Evaluation and diagnostic work-up
A thorough medical history, review of systems, medication review, social history, and family history are important when evaluating a patient with chronic pruritus.18 These items can be valuable in formulating a differential diagnosis, even before a physical examination.
Physical examination. The physical exam should include detailed inspection of the entire skin and hair18; such a comprehensive physical exam can determine whether the source of the itch is cutaneous.7 This, in turn, can help further narrow the differential diagnosis. It is crucial that the physical exam include palpation of the liver, spleen, lymph nodes, and thyroid for organomegaly,8 which could indicate a serious systemic condition, such as lymphoma.
The ice-pack sign—in which an ice pack is applied to the pruritic area, the patient experiences immediate relief of pruritus, and the itch returns soon after the ice pack is removed—is considered pathognomonic for brachioradial pruritus.19
Continue to: Chronic pruritus with abnormal findings...
Chronic pruritus with abnormal findings on the physical exam should prompt an initial work-up.18 Also consider an initial work-up for a patient with chronic pruritus whose symptom has not been relieved with conservative treatment.18
Laboratory testing. The initial laboratory work-up could include any of the following evaluations: complete blood count, measurement of thyroid-stimulating hormone, comprehensive metabolic panel (liver function, renal function, and the serum glucose level) and the erythrocyte sedimentation rate (TABLE 2).18 If warranted by the evaluation and physical exam, blood work can also include serologic studies for human immunodeficiency virus infection and hepatitis.17

Imaging. Chest radiography should be performed if there is suspicion of malignancy, such as lymphoma.7 Although brachioradial pruritus and notalgia paresthetica have been postulated to be caused by impingement of spinal nerves, obtaining spinal imaging, such as magnetic resonance imaging, as part of the initial work-up is not recommended; because spinal images might not show evidence of spinal disease, obtaining spinal imaging is not a requirement before treating brachioradial pruritus and notalgia paresthetica. Do consider spinal imaging, however, for patients in whom brachioradial pruritus or notalgia paresthetica is suspected and conservative treatment has not produced a response.
Treatment: Nondrug approaches, topicals, systemic agents
Start conservatively. Treatment of pruritus should begin with behavior modification and nonpharmacotherapeutic options (TABLE 38). Educate the patient that scratching might cause secondary skin lesions; empowering them with that knowledge is sometimes enough to help break the scratching cycling—especially if the patient combines behavior modification with proper skin hydration with an emollient. To prevent secondary skin lesions through involuntary scratching, consider recommending that lesions be covered with an occlusive dressing or protective clothing.13

Stress has been shown to make chronic itch worse; therefore, stress-reduction activities, such as exercise, meditation, and yoga, might be helpful.20 For patients in whom pruritus has a psychological component, referral to a psychiatrist or psychologist might be therapeutic.
Continue to: When a patient complains...
When a patient complains of severe pruritus at first presentation, consider pharmacotherapy in conjunction with nonpharmacotherapeutic options. Several of the more effective topical therapies for pruritusa are listed in TABLE 4.20 Well-known systemic agents for this purpose are reviewed below and listed in TABLE 5.7

Systemic treatment
Antihistamines. A staple in the treatment of pruritus for many years, antihistamines are not effective for all causes; however, they are effective in treating paraneoplastic pruritus.20 First-generation antihistamines, with their sedating effect, can be useful for patients who experience generalized pruritus at night.20

Anticonvulsants. Gabapentin and pregabalin are analogs of the neurotransmitter gamma-aminobutyric acid.20 This drug class is helpful in neuropathic pruritus specifically caused by impingements, such as brachioradial pruritus and notalgia paresthetica.20 In addition, of all systemic therapies used to treat uremic pruritus, gabapentin has, in clinical trials, most consistently been found effective for uremic pruritus.6 (Note: Use renal dosing of gabapentin in patients with renal failure.)
Antidepressants. Selective serotonin reuptake inhibitors (SSRIs; eg, fluvoxamine, paroxetine, and sertraline) might cause itch to subside by increasing the serotonin level, which, in turn, works to decrease inflammatory substances that cause itch.7 SSRIs have been used to treat patients with psychogenic pruritus, cholestatic pruritus, and paraneoplastic pruritus.7
Tricyclic antidepressants (eg, amitriptyline and doxepin) lessen the itch by antagonizing histamine receptors and through anticholinergic mechanisms. Tricyclics are best used in the treatment of psychogenic and nocturnal itch.7
Continue to: Mirtazapine...
Mirtazapine, a tetracyclic antidepressant, works in patients with uremic pruritus, psychogenic pruritus, cholestatic pruritus, and paraneoplastic pruritus.1
Substance P antagonist. Aprepitant, a neurokinin receptor I antagonist, is a newer agent that inhibits binding of the itch mediator substance P to the neurokinin receptor. The drug has been found helpful in patients with drug-induced, paraneoplastic, and brachioradial pruritus.7
Opioid-receptor agents. Naltrexone, as a mu opioid-receptor antagonist, has shown promise as a treatment for uremic pruritus and cholestatic pruritus. Nalfurafine, a kappa opioid-receptor agonist, is emerging as a possible therapy for uremic pruritus.7
Bile-acid sequestrants. A few small studies have shown that treatment with a bile-acid sequestrant, such as cholestyramine and ursodiol, induces moderate improvement in symptoms in patients with cholestatic pruritus.21
CORRESPONDENCE
Matasha Russell, MD, Department of Family and Community Medicine, The University of Texas Health Science Center at Houston, McGovern Medical School, 6431 Fannin Street, JJL 324, Houston, TX 77030; [email protected].
Pruritus, defined as a sensation that induces a desire to scratch1 and classified as acute or chronic (lasting > 6 weeks),2 is one of the most common complaints among primary care patients: Approximately 1% of ambulatory visits in the United States are linked to pruritus.3
Chronic pruritus impairs quality of life; its impact has been compared to that of chronic pain.4 Treatment should therefore be instituted promptly. Although this condition might appear benign, chronic pruritus can be a symptom of a serious condition, as we describe here. When persistent pruritus is refractory to treatment, systemic causes should be fully explored.
In this article, we discuss the pathogenesis and management of pruritus without skin eruption in the adult nonpregnant patient. We also present practice recommendations to help you determine whether your patient’s pruritus is indicative of a serious systemic condition.

An incomplete understanding of the pathophysiology of pruritus
The pathophysiology of pruritus is not fully understood. It is generally recognized, however, that pruritus starts in the peripheral nerves located in the dermal–epidermal junction of the skin.5 The sensation is then transmitted along unmyelinated slow-conducting C fibers to the dorsal horn of the spinal cord.5,6 There are 2 types of C fibers that transmit the itch impulse6: A histamine-dependent type and a non-histamine-dependent type, which might explain why pruritus can be refractory to antihistamine treatment.6
Once the itch impulse has moved from the spinal cord, it travels along the spinothalamic tract up to the contralateral thalamus.1 From there, the impulse ascends to the cerebral cortex.1 In the cortex, the impulse triggers multiple areas of the brain, such as those responsible for sensation, motor function, reward, memory, and emotion.7
Several chemical mediators have been found to be peripheral and central inducers of pruritus: histamine, endogenous opioids, substance P, and serotonin.2 There are indications that certain receptors, such as mu-opioid receptors and kappa-opioid receptors, are key contributors to itch as well.2

A diverse etiology
The International Forum for the Study of Itch (IFSI) has established 6 main categories of causes of pruritus(TABLE 1)2:
- dermatologic
- systemic
- neurologic
- psychogenic
- mixed
- other.
Continue to: In this review...
In this review, we focus on the work-up and management of 3 of those categories: systemic, neurologic, and psychogenic causes of pruritus.
Systemic causes
Research has shown that 14% to 24% of patients who seek the care of a dermatologist for chronic itch without skin lesions have a systemic illness.8
Renal disease. Approximately 40% of patients with end-stage renal disease who are on hemodialysis or peritoneal dialysis have uremic pruritus.2 The itch is mostly generalized but can be pronounced on the back. For most patients, the itch is worse at night, causing a major impact on quality of life.6
Liver disease. In hepatic disease, there is often impairment in the secretion of bile, which can lead to cholestatic pruritus.2 This condition commonly affects the hands and feet first; later, it becomes generalized.2 Cholestatic pruritus can be elicited by tight-fitting clothing. Relief is not achieved by scratching.9 This type of itch effects 70% of patients with primary biliary cirrhosis and 15% of patients with hepatitis C infection.9
Hematologic disorders. Pruritus is a hallmark symptom of polycythemia rubra vera. Almost 50% of patients with this disorder report pruritus that occurs after exposure to water9; aquagenic pruritus can precede the formal diagnosis of polycythemia rubra vera by years.2 It has been speculated that platelet aggregation in this disorder leads to release of serotonin and histamine, which, in turn, causes itch.9
Continue to: Endocrine disorders
Endocrine disorders. Approximately 4% to 11% of patients with thyrotoxicosis have pruritus.1 It has been suggested that vasodilation, increased skin temperature, and a decreased itch threshold from untreated Graves disease might be inciting factors.
Malignancy. In generalized chronic pruritus without a known cause, strongly consider the likelihood of underlying malignancy8,10; for 10% of these patients, their chronic pruritus is a paraneoplastic sign. Paraneoplastic pruritus is characterized as an itch that predates clinical onset, or occurs early in the course, of a malignancy.9 The condition is most strongly linked to cancers of the liver, gallbladder, biliary tract, hematologic system, and skin.11
Chronic pruritus affects 30% of patients with Hodgkin lymphoma.9 General pruritus can precede this diagnosis by months, even years.1 In Hodgkin lymphoma patients who are in remission, a return of pruritic symptoms can be a harbinger of recurrence.9
Neurologic causes
A recent study found that 8% to 15% of patients referred to a dermatology clinic for chronic pruritus without skin eruption had underlying neurologic pathology.12 Although the specific mechanisms of neuropathic itch are still poorly understood, it has been theorized that the itch emanates from neuronal damage, which can come from peripheral or central nervous system lesions.9
Brachioradial pruritus. There are divergent theories about the etiology of brachioradial pruritus. One hypothesis is that the condition is caused by cervical nerve-root impingement at the level of C5-C8 that leads to nerve damage2; another is that chronic exposure to sunlight causes injury to peripheral cutaneous nerves.2 Brachioradial pruritus is localized to the dorsolateral forearm; it can also involve the neck, back, shoulder, upper arm, and chest, unilaterally and bilaterally. This pruritus can be intermittent and become worse upon exposure to sunlight.2
Continue to: Notalgia paresthetica
Notalgia paresthetica. This condition might also cause neuropathic pruritus as a consequence of nerve impingement. The itch of notalgia paresthesia is located on the skin, medial to the scapular border on the upper or mid-back.2 It has been postulated that the itch is caused by nerve entrapment of the posterior rami of spinal nerves arising from T2-T6.9 However, another theory suggests that the itch is caused by damage to peripheral nerves.9 The itch of notalgia paresthetica can wax and wane.2
Poststroke pruritus. Brain lesions, most often caused by stroke, can cause neuropathic itch. One of the best-known syndromes related to poststroke itch is Wallenberg syndrome (ischemia from a lateral medullary infarction), which typically presents with itch, thermalgic hypoesthesia of the face, cerebellar dysfunction, nausea, and vomiting.7
Shingles. More than one-half of patients who develop postherpetic neuralgia as a consequence of a herpes zoster infection also develop neuropathic pruritus.9 It is thought that postherpetic pruritus shares a comparable pathophysiology with postherpetic neuralgia, in which neurons involved in itch stimuli become damaged.7
Diabetes mellitus. Pruritus from diabetes can be classified as systemic or neuropathic. Diabetes is one of the most common causes of small-fiber polyneuropathy, which can cause neuropathic pruritus.13
Multiple sclerosis. Central nervous system lesions that affect sensory pathways can lead to neuropathic itch in multiple sclerosis. Patients can have severe episodes of generalized pruritus. It has been hypothesized that the neuropathic itch in multiple sclerosis is induced by activation of artificial synapses in demyelinated areas.2
Continue to: Psychogenic pruritus
Psychogenic pruritus
Chronic pruritus can be a comorbidity of psychiatric illness. A retrospective study found that pruritus occurs in 32% to 42% of psychiatric inpatients.14 Depression, anxiety, bipolar disorders, obsessive–compulsive disorders, somatoform disorders, psychosis, and substance abuse all have a strong link to psychogenic excoriation.15 Psychogenic excoriation, which can cause secondary skin lesions, occurs in psychiatric patients who excessively pick and scratch normal skin because they perceive an itch sensation or have a delusion of infestation.2 Affected skin can be marked by scattered crusted lesions (FIGURE) anywhere on the body that the patient can reach—most commonly, the extremities.2

Delusion of infestation. Patients with a delusion of infestation have a strong belief that their body is infected by some kind of insect or microorganism.16 Before a diagnosis of delusion of infestation can be made, other organic causes must be excluded, including withdrawal from such substances as cocaine, amphetamines, and alcohol.16 Patients with a delusion of infestation can have, and maintain, a symptomatic response with continuing use of an atypical antipsychotic agent, including risperidone and olanzapine.17
Evaluation and diagnostic work-up
A thorough medical history, review of systems, medication review, social history, and family history are important when evaluating a patient with chronic pruritus.18 These items can be valuable in formulating a differential diagnosis, even before a physical examination.
Physical examination. The physical exam should include detailed inspection of the entire skin and hair18; such a comprehensive physical exam can determine whether the source of the itch is cutaneous.7 This, in turn, can help further narrow the differential diagnosis. It is crucial that the physical exam include palpation of the liver, spleen, lymph nodes, and thyroid for organomegaly,8 which could indicate a serious systemic condition, such as lymphoma.
The ice-pack sign—in which an ice pack is applied to the pruritic area, the patient experiences immediate relief of pruritus, and the itch returns soon after the ice pack is removed—is considered pathognomonic for brachioradial pruritus.19
Continue to: Chronic pruritus with abnormal findings...
Chronic pruritus with abnormal findings on the physical exam should prompt an initial work-up.18 Also consider an initial work-up for a patient with chronic pruritus whose symptom has not been relieved with conservative treatment.18
Laboratory testing. The initial laboratory work-up could include any of the following evaluations: complete blood count, measurement of thyroid-stimulating hormone, comprehensive metabolic panel (liver function, renal function, and the serum glucose level) and the erythrocyte sedimentation rate (TABLE 2).18 If warranted by the evaluation and physical exam, blood work can also include serologic studies for human immunodeficiency virus infection and hepatitis.17

Imaging. Chest radiography should be performed if there is suspicion of malignancy, such as lymphoma.7 Although brachioradial pruritus and notalgia paresthetica have been postulated to be caused by impingement of spinal nerves, obtaining spinal imaging, such as magnetic resonance imaging, as part of the initial work-up is not recommended; because spinal images might not show evidence of spinal disease, obtaining spinal imaging is not a requirement before treating brachioradial pruritus and notalgia paresthetica. Do consider spinal imaging, however, for patients in whom brachioradial pruritus or notalgia paresthetica is suspected and conservative treatment has not produced a response.
Treatment: Nondrug approaches, topicals, systemic agents
Start conservatively. Treatment of pruritus should begin with behavior modification and nonpharmacotherapeutic options (TABLE 38). Educate the patient that scratching might cause secondary skin lesions; empowering them with that knowledge is sometimes enough to help break the scratching cycling—especially if the patient combines behavior modification with proper skin hydration with an emollient. To prevent secondary skin lesions through involuntary scratching, consider recommending that lesions be covered with an occlusive dressing or protective clothing.13

Stress has been shown to make chronic itch worse; therefore, stress-reduction activities, such as exercise, meditation, and yoga, might be helpful.20 For patients in whom pruritus has a psychological component, referral to a psychiatrist or psychologist might be therapeutic.
Continue to: When a patient complains...
When a patient complains of severe pruritus at first presentation, consider pharmacotherapy in conjunction with nonpharmacotherapeutic options. Several of the more effective topical therapies for pruritusa are listed in TABLE 4.20 Well-known systemic agents for this purpose are reviewed below and listed in TABLE 5.7

Systemic treatment
Antihistamines. A staple in the treatment of pruritus for many years, antihistamines are not effective for all causes; however, they are effective in treating paraneoplastic pruritus.20 First-generation antihistamines, with their sedating effect, can be useful for patients who experience generalized pruritus at night.20

Anticonvulsants. Gabapentin and pregabalin are analogs of the neurotransmitter gamma-aminobutyric acid.20 This drug class is helpful in neuropathic pruritus specifically caused by impingements, such as brachioradial pruritus and notalgia paresthetica.20 In addition, of all systemic therapies used to treat uremic pruritus, gabapentin has, in clinical trials, most consistently been found effective for uremic pruritus.6 (Note: Use renal dosing of gabapentin in patients with renal failure.)
Antidepressants. Selective serotonin reuptake inhibitors (SSRIs; eg, fluvoxamine, paroxetine, and sertraline) might cause itch to subside by increasing the serotonin level, which, in turn, works to decrease inflammatory substances that cause itch.7 SSRIs have been used to treat patients with psychogenic pruritus, cholestatic pruritus, and paraneoplastic pruritus.7
Tricyclic antidepressants (eg, amitriptyline and doxepin) lessen the itch by antagonizing histamine receptors and through anticholinergic mechanisms. Tricyclics are best used in the treatment of psychogenic and nocturnal itch.7
Continue to: Mirtazapine...
Mirtazapine, a tetracyclic antidepressant, works in patients with uremic pruritus, psychogenic pruritus, cholestatic pruritus, and paraneoplastic pruritus.1
Substance P antagonist. Aprepitant, a neurokinin receptor I antagonist, is a newer agent that inhibits binding of the itch mediator substance P to the neurokinin receptor. The drug has been found helpful in patients with drug-induced, paraneoplastic, and brachioradial pruritus.7
Opioid-receptor agents. Naltrexone, as a mu opioid-receptor antagonist, has shown promise as a treatment for uremic pruritus and cholestatic pruritus. Nalfurafine, a kappa opioid-receptor agonist, is emerging as a possible therapy for uremic pruritus.7
Bile-acid sequestrants. A few small studies have shown that treatment with a bile-acid sequestrant, such as cholestyramine and ursodiol, induces moderate improvement in symptoms in patients with cholestatic pruritus.21
CORRESPONDENCE
Matasha Russell, MD, Department of Family and Community Medicine, The University of Texas Health Science Center at Houston, McGovern Medical School, 6431 Fannin Street, JJL 324, Houston, TX 77030; [email protected].
1. Tarikci N, E, S, et al. Pruritus in systemic diseases: a review of etiological factors and new treatment modalities. ScientificWorldJournal. 2015;2015:803752.
2. Yosipovitch G, Bernhard JD. Clinical practice. Chronic pruritus. N Engl J Med. 2013;368:1625-1634.
3. Silverberg JI, Kantor RW, Dalal P. A comprehensive conceptual model of the experience of chronic itch in adults. Am J Clin Dermatol. 2018;19:759-769.
4. Matterne U, Apfelbacher CJ, Vogelgsang L, et al. Incidence and determinants of chronic pruritus: a population based cohort study. Acta Derm Venereol. 2013;93:532-537.
5. Moses S. Pruritus. Am Fam Physician. 2003;68:1135-1142.
6. Combs SA, Teixeira JP, Germain MJ. Pruritus in kidney disease. Semin Nephrol. 2015;35:383-391.
7. Shevchenko A, Valdes-Rodriguez R, Yosipovitch G. Causes, pathophysiology, and treatment of pruritus in the mature patient. Clin Dermatol. 2018;36:140-151.
8. Reamy BV, Bunt C. A diagnostic approach to pruritus. Am Fam Physician. 2011;84:195-202.
9. M. Current concepts of pathophysiology, epidemiology and classification of pruritus. Srp Arh Celok Lek. 2014;142:106-112.
10. Fett N, Haynes K, Propert KJ, et al. Five-year malignancy incidence in patients with chronic pruritus: a population-based cohort study aimed at limiting unnecessary screening practices. J Am Acad Dermatol. 2014;70:651-658.
11. Larson VA, Tang O, S, et al. Association between itch and cancer in 16,925 patients with pruritus: experience at a tertiary care center. J Am Acad Dermatol. 2019;80:931-937.
12. Rosen JD, Fostini AC, Chan YH, et al. Cross-sectional study of clinical distinctions between neuropathic and inflammatory pruritus. J Am Acad Dermatol. 2018;79:1143-1144.
13. Oaklander AL. Neuropathic itch. Semin Cutan Med Surg. 2011;30:87-92.
14. Ferm I, Sterner M, Wallengren J. Somatic and psychiatric comorbidity in patients with chronic pruritus. Acta Derm Venereol. 2010;90:395-400.
15. Jafferany M, Davari ME. Itch and psyche: psychiatric aspects of pruritus. Int J Dermatol. 2019;58:3-23.
16. Koo J, Lebwohl A. Psychodermatology: the mind and skin connection. Am Fam Physician. 2001;64:1873-1878.
17. Bewley AP, Lepping P, Freudenmann RW, et al. Delusional parasitosis: time to call it delusional infestation. Br J Dermatol.2010;163:1-2.
18. Clerc C-J, Misery L. A literature review of senile pruritus: from diagnosis to treatment. Acta Derm Venereol. 2017;97:433-440.
19. Bernhard JD, Bordeaux JS. Medical pearl: the ice-pack sign in brachioradial pruritus. J Am Acad Dermatol. 2005;52:1073.
20. Sanders KM, Nattkemper LA, Yosipovitch G. Advances in understanding itching and scratching: a new era of targeted treatments [version 1]. F1000Res. 2016;5 F1000 Faculty Rev–2042.
21. Hegade VS, Kendrick SFW, Dobbins RL, et al. Effect of ileal bile acid transporter inhibitor GSK2330672 on pruritus in primary biliary cholangitis: a double-blind, randomised, placebo-controlled, crossover, phase 2a study. Lancet. 2017;389:1114-1123.
1. Tarikci N, E, S, et al. Pruritus in systemic diseases: a review of etiological factors and new treatment modalities. ScientificWorldJournal. 2015;2015:803752.
2. Yosipovitch G, Bernhard JD. Clinical practice. Chronic pruritus. N Engl J Med. 2013;368:1625-1634.
3. Silverberg JI, Kantor RW, Dalal P. A comprehensive conceptual model of the experience of chronic itch in adults. Am J Clin Dermatol. 2018;19:759-769.
4. Matterne U, Apfelbacher CJ, Vogelgsang L, et al. Incidence and determinants of chronic pruritus: a population based cohort study. Acta Derm Venereol. 2013;93:532-537.
5. Moses S. Pruritus. Am Fam Physician. 2003;68:1135-1142.
6. Combs SA, Teixeira JP, Germain MJ. Pruritus in kidney disease. Semin Nephrol. 2015;35:383-391.
7. Shevchenko A, Valdes-Rodriguez R, Yosipovitch G. Causes, pathophysiology, and treatment of pruritus in the mature patient. Clin Dermatol. 2018;36:140-151.
8. Reamy BV, Bunt C. A diagnostic approach to pruritus. Am Fam Physician. 2011;84:195-202.
9. M. Current concepts of pathophysiology, epidemiology and classification of pruritus. Srp Arh Celok Lek. 2014;142:106-112.
10. Fett N, Haynes K, Propert KJ, et al. Five-year malignancy incidence in patients with chronic pruritus: a population-based cohort study aimed at limiting unnecessary screening practices. J Am Acad Dermatol. 2014;70:651-658.
11. Larson VA, Tang O, S, et al. Association between itch and cancer in 16,925 patients with pruritus: experience at a tertiary care center. J Am Acad Dermatol. 2019;80:931-937.
12. Rosen JD, Fostini AC, Chan YH, et al. Cross-sectional study of clinical distinctions between neuropathic and inflammatory pruritus. J Am Acad Dermatol. 2018;79:1143-1144.
13. Oaklander AL. Neuropathic itch. Semin Cutan Med Surg. 2011;30:87-92.
14. Ferm I, Sterner M, Wallengren J. Somatic and psychiatric comorbidity in patients with chronic pruritus. Acta Derm Venereol. 2010;90:395-400.
15. Jafferany M, Davari ME. Itch and psyche: psychiatric aspects of pruritus. Int J Dermatol. 2019;58:3-23.
16. Koo J, Lebwohl A. Psychodermatology: the mind and skin connection. Am Fam Physician. 2001;64:1873-1878.
17. Bewley AP, Lepping P, Freudenmann RW, et al. Delusional parasitosis: time to call it delusional infestation. Br J Dermatol.2010;163:1-2.
18. Clerc C-J, Misery L. A literature review of senile pruritus: from diagnosis to treatment. Acta Derm Venereol. 2017;97:433-440.
19. Bernhard JD, Bordeaux JS. Medical pearl: the ice-pack sign in brachioradial pruritus. J Am Acad Dermatol. 2005;52:1073.
20. Sanders KM, Nattkemper LA, Yosipovitch G. Advances in understanding itching and scratching: a new era of targeted treatments [version 1]. F1000Res. 2016;5 F1000 Faculty Rev–2042.
21. Hegade VS, Kendrick SFW, Dobbins RL, et al. Effect of ileal bile acid transporter inhibitor GSK2330672 on pruritus in primary biliary cholangitis: a double-blind, randomised, placebo-controlled, crossover, phase 2a study. Lancet. 2017;389:1114-1123.
PRACTICE RECOMMENDATIONS
› Undertake a diagnostic work-up for systemic causes of pruritus in patients who have a chronic, generalized itch and abnormal findings on physical examination. C
› Prescribe gabapentin for its effectiveness in treating pruritus caused by uremic and neurologic itch. B
› Consider prescribing one of the bile-acid sequestrants in patients with cholestatic pruritus because these agents can provide moderate relief of the symptom. B
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Novel drug slows progression of diabetic kidney disease
For patients with diabetic kidney disease, finerenone, an agent from a new class of selective, nonsteroidal mineralocorticoid receptor antagonists, led to significant reductions in combined adverse renal outcomes and in combined adverse cardiovascular outcomes in the pivotal FIDELIO-DKD trial.
And the safety results showed a good level of tolerability. The rate of hyperkalemia was higher with finerenone than with placebo, but the rate of drug discontinuations for elevated potassium was lower than that seen with spironolactone, a steroidal mineralocorticoid receptor antagonist (MRA).
“An ideal drug would cause no hyperkalemia, but the absolute risk we saw is a fraction of what we see with spironolactone in this vulnerable patient population,” said Rajiv Agarwal, MD, from Indiana in Indianapolis, during a press briefing.
After a median follow-up of 2.6 years, finerenone was associated with a 3.4% absolute reduction in the rate of combined adverse renal events, the study’s primary end point, which comprised kidney failure, renal death, and a drop in estimated glomerular filtration rate (eGFR) of at least 40% from baseline. This produced a significant relative risk reduction of 18%, with a number needed to treat of 32 to prevent one of these events, Dr. Agarwal reported at Kidney Week 2020. Findings from the FIDELIO-DKD trial were published simultaneously in the New England Journal of Medicine.
Finerenone was also associated with an absolute 2.4% reduction in the rate of combined adverse cardiovascular events, the study’s “key secondary end point,” which included cardiovascular death, nonfatal MI, nonfatal stroke, and hospitalization for heart failure. This translated into a significant relative risk reduction of 14% and a number needed to treat of 42 to prevent one of these events.
FIDELIO-DKD assessed 5,734 patients with type 2 diabetes and chronic kidney disease from more than 1,000 sites in 48 countries, including the United States, from 2015 to 2018. In the study cohort, average age was slightly more than 65 years, average baseline systolic blood pressure was 138 mm Hg, average duration of diabetes was nearly 17 years, average baseline glycated hemoglobin (A1c) was 7.7%, and fewer than 5% of patients were Black, 25% were Asian, and about 63% were White.
A suggestion of less severe hyperkalemia
Finerenone was well tolerated by the participants, and the findings suggest that it caused less clinically meaningful hyperkalemia than spironolactone, the most established and widely used MRA.
Like all MRA drugs, finerenone led to an increase in serum potassium in all patient subgroups – in this case 0.2 mmol/L – unlike placebo, said Dr. Agarwal.
The overall incidence of hyperkalemia was 16% in the 2,827 evaluable patients in the finerenone group and 8% in the 2,831 evaluable patients in the placebo group. Fewer than 10% of patients in the trial received a potassium-binding agent.
The rate of hyperkalemia leading to treatment discontinuation was higher in the finerenone group than in the placebo group (2.3% vs. 0.9%).
That 2.3% rate is 10 times lower than the 23.0% rate of hyperkalemia-related treatment discontinuation in patients who received spironolactone and no potassium-binding agent, said Dr. Agarwal, citing a previous study he was involved with.
He hypothesized that finerenone might cause less clinically meaningful hyperkalemia because it creates no active metabolites that linger in the body, whereas spironolactone produces active metabolites with a half life of about 1 week.
“The risk for hyperkalemia is clearly increased with finerenone compared with placebo, and in the absence of head-to-head studies, it’s hard to know how it compares with spironolactone or eplerenone [Inspra],” the other agents in the MRA class, said Mikhail N. Kosiborod, MD, from the University of Missouri–Kansas City.
“The rates of hyperkalemia observed in FIDELIO-DKD were overall comparable to what we would expect from eplerenone. But the rate of serious hyperkalemia was quite low with finerenone, which is reassuring,” Dr. Kosiborod said in an interview.
And the adverse-effect profile showed that finerenone “is as safe as you could expect from an MRA,” said Janani Rangaswami, MD, from the Einstein Medical Center in Philadelphia.
The rate of hyperkalemia should be interpreted in the context of the high risk the enrolled patients faced, given that they all had moderate to severe diabetic kidney disease with albuminuria and, in some cases, eGFR rates as low as 25 mL/min per 1.73m2, she explained. In addition, all patients were on maximally tolerated treatment with either an angiotensin-converting–enzyme inhibitor or an angiotensin receptor blocker to inhibit the renin angiotensin system (RAS).
“Considering this background, it’s a very acceptable adverse-event profile,” Dr. Rangaswami said in an interview.
Renal drugs that could work together
More than 99% of patients in FIDELIO-DKD were on an RAS inhibitor, but fewer than 5% were on a sodium glucose cotransporter 2 (SGLT2) inhibitor at baseline, and fewer than 10% started on this drug class during the course of the study.
Despite that, both Dr. Kosiborod and Dr. Rangaswami are enthusiastic about the prospect of using the three drugs in combination to maximize renal and cardiovascular benefits in FIDELIO-DKD–type patients. Recent results from the CREDENCE study of canagliflozin (Invokana) and from the DAPA-CKD study of dapagluflozin (Farxiga) have established SGLT2 inhibitors – at least those two – as key agents for patients with chronic kidney disease.
Dual treatment with an RAS inhibitor and an SGLT2 inhibitor is “clearly established” for patients with diabetic kidney disease, said Dr. Agarwal.
“After CREDENCE, DAPA-CKD, and now FIDELIO-DKD, we need to seriously consider triple therapy as the future of treatment for diabetic kidney disease to prevent both cardiovascular and kidney complications,” said Dr. Kosiborod. The approach will mimic the multidrug therapy that’s now standard for patients with heart failure with reduced ejection fraction (HFrEF). But he cautioned that this triple combination needs further testing.
“Triple therapy will be the standard of care” for patients with diabetic kidney disease, Dr. Rangaswami agreed, but she cautioned that she would not currently expand the target population for finerenone to patients without type 2 diabetes or to patients without the level of albuminuria required for entry into FIDELIO-DKD: at least 30 mg/g of creatinine per day. And patients with HFrEF were excluded from FIDELIO-DKD, so that limitation on finerenone use should remain for the time being, she added.
Dr. Rangaswami said she is optimistic about the potential efficacy of finerenone added to an SGLT2 inhibitor because of the likelihood that the two drug classes work in different but complementary ways. SGLT2 inhibitors seem to exert their renal protective effects largely through hemodynamic effects, whereas it is likely that finerenone exerts its effects largely as an anti-inflammatory and antifibrotic agent, she speculated. The FIDELIO-DKD results appear to rule out any major effect of finerenone on blood pressure lowering because average systolic pressure fell by only about 2 mm Hg in the treatment group.
“The benefits of finerenone for cardiorenal outcomes are substantial and clinically meaningful,” Dr. Kosiborod said. “We cannot assume that other MRAs, such as spironolactone, provide similar benefits,” he cautioned, but the results are “very good news for patients with type 2 diabetes and chronic kidney disease. We now have another effective intervention with a different mechanism of action.”
FIDELIO-DKD was sponsored by Bayer, the company developing finerenone (BAY 94-8862). Dr. Agarwal has been a consultant to and has received honoraria from Bayer and from several other companies. Dr. Kosiborod has been a consultant to Bayer and to AstraZeneca, Boehringer Ingelheim, Jansse, Merck, and Vifor and has received research funding from AstraZeneca and Boehringer Ingelheim. Dr. Rangaswami has disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
For patients with diabetic kidney disease, finerenone, an agent from a new class of selective, nonsteroidal mineralocorticoid receptor antagonists, led to significant reductions in combined adverse renal outcomes and in combined adverse cardiovascular outcomes in the pivotal FIDELIO-DKD trial.
And the safety results showed a good level of tolerability. The rate of hyperkalemia was higher with finerenone than with placebo, but the rate of drug discontinuations for elevated potassium was lower than that seen with spironolactone, a steroidal mineralocorticoid receptor antagonist (MRA).
“An ideal drug would cause no hyperkalemia, but the absolute risk we saw is a fraction of what we see with spironolactone in this vulnerable patient population,” said Rajiv Agarwal, MD, from Indiana in Indianapolis, during a press briefing.
After a median follow-up of 2.6 years, finerenone was associated with a 3.4% absolute reduction in the rate of combined adverse renal events, the study’s primary end point, which comprised kidney failure, renal death, and a drop in estimated glomerular filtration rate (eGFR) of at least 40% from baseline. This produced a significant relative risk reduction of 18%, with a number needed to treat of 32 to prevent one of these events, Dr. Agarwal reported at Kidney Week 2020. Findings from the FIDELIO-DKD trial were published simultaneously in the New England Journal of Medicine.
Finerenone was also associated with an absolute 2.4% reduction in the rate of combined adverse cardiovascular events, the study’s “key secondary end point,” which included cardiovascular death, nonfatal MI, nonfatal stroke, and hospitalization for heart failure. This translated into a significant relative risk reduction of 14% and a number needed to treat of 42 to prevent one of these events.
FIDELIO-DKD assessed 5,734 patients with type 2 diabetes and chronic kidney disease from more than 1,000 sites in 48 countries, including the United States, from 2015 to 2018. In the study cohort, average age was slightly more than 65 years, average baseline systolic blood pressure was 138 mm Hg, average duration of diabetes was nearly 17 years, average baseline glycated hemoglobin (A1c) was 7.7%, and fewer than 5% of patients were Black, 25% were Asian, and about 63% were White.
A suggestion of less severe hyperkalemia
Finerenone was well tolerated by the participants, and the findings suggest that it caused less clinically meaningful hyperkalemia than spironolactone, the most established and widely used MRA.
Like all MRA drugs, finerenone led to an increase in serum potassium in all patient subgroups – in this case 0.2 mmol/L – unlike placebo, said Dr. Agarwal.
The overall incidence of hyperkalemia was 16% in the 2,827 evaluable patients in the finerenone group and 8% in the 2,831 evaluable patients in the placebo group. Fewer than 10% of patients in the trial received a potassium-binding agent.
The rate of hyperkalemia leading to treatment discontinuation was higher in the finerenone group than in the placebo group (2.3% vs. 0.9%).
That 2.3% rate is 10 times lower than the 23.0% rate of hyperkalemia-related treatment discontinuation in patients who received spironolactone and no potassium-binding agent, said Dr. Agarwal, citing a previous study he was involved with.
He hypothesized that finerenone might cause less clinically meaningful hyperkalemia because it creates no active metabolites that linger in the body, whereas spironolactone produces active metabolites with a half life of about 1 week.
“The risk for hyperkalemia is clearly increased with finerenone compared with placebo, and in the absence of head-to-head studies, it’s hard to know how it compares with spironolactone or eplerenone [Inspra],” the other agents in the MRA class, said Mikhail N. Kosiborod, MD, from the University of Missouri–Kansas City.
“The rates of hyperkalemia observed in FIDELIO-DKD were overall comparable to what we would expect from eplerenone. But the rate of serious hyperkalemia was quite low with finerenone, which is reassuring,” Dr. Kosiborod said in an interview.
And the adverse-effect profile showed that finerenone “is as safe as you could expect from an MRA,” said Janani Rangaswami, MD, from the Einstein Medical Center in Philadelphia.
The rate of hyperkalemia should be interpreted in the context of the high risk the enrolled patients faced, given that they all had moderate to severe diabetic kidney disease with albuminuria and, in some cases, eGFR rates as low as 25 mL/min per 1.73m2, she explained. In addition, all patients were on maximally tolerated treatment with either an angiotensin-converting–enzyme inhibitor or an angiotensin receptor blocker to inhibit the renin angiotensin system (RAS).
“Considering this background, it’s a very acceptable adverse-event profile,” Dr. Rangaswami said in an interview.
Renal drugs that could work together
More than 99% of patients in FIDELIO-DKD were on an RAS inhibitor, but fewer than 5% were on a sodium glucose cotransporter 2 (SGLT2) inhibitor at baseline, and fewer than 10% started on this drug class during the course of the study.
Despite that, both Dr. Kosiborod and Dr. Rangaswami are enthusiastic about the prospect of using the three drugs in combination to maximize renal and cardiovascular benefits in FIDELIO-DKD–type patients. Recent results from the CREDENCE study of canagliflozin (Invokana) and from the DAPA-CKD study of dapagluflozin (Farxiga) have established SGLT2 inhibitors – at least those two – as key agents for patients with chronic kidney disease.
Dual treatment with an RAS inhibitor and an SGLT2 inhibitor is “clearly established” for patients with diabetic kidney disease, said Dr. Agarwal.
“After CREDENCE, DAPA-CKD, and now FIDELIO-DKD, we need to seriously consider triple therapy as the future of treatment for diabetic kidney disease to prevent both cardiovascular and kidney complications,” said Dr. Kosiborod. The approach will mimic the multidrug therapy that’s now standard for patients with heart failure with reduced ejection fraction (HFrEF). But he cautioned that this triple combination needs further testing.
“Triple therapy will be the standard of care” for patients with diabetic kidney disease, Dr. Rangaswami agreed, but she cautioned that she would not currently expand the target population for finerenone to patients without type 2 diabetes or to patients without the level of albuminuria required for entry into FIDELIO-DKD: at least 30 mg/g of creatinine per day. And patients with HFrEF were excluded from FIDELIO-DKD, so that limitation on finerenone use should remain for the time being, she added.
Dr. Rangaswami said she is optimistic about the potential efficacy of finerenone added to an SGLT2 inhibitor because of the likelihood that the two drug classes work in different but complementary ways. SGLT2 inhibitors seem to exert their renal protective effects largely through hemodynamic effects, whereas it is likely that finerenone exerts its effects largely as an anti-inflammatory and antifibrotic agent, she speculated. The FIDELIO-DKD results appear to rule out any major effect of finerenone on blood pressure lowering because average systolic pressure fell by only about 2 mm Hg in the treatment group.
“The benefits of finerenone for cardiorenal outcomes are substantial and clinically meaningful,” Dr. Kosiborod said. “We cannot assume that other MRAs, such as spironolactone, provide similar benefits,” he cautioned, but the results are “very good news for patients with type 2 diabetes and chronic kidney disease. We now have another effective intervention with a different mechanism of action.”
FIDELIO-DKD was sponsored by Bayer, the company developing finerenone (BAY 94-8862). Dr. Agarwal has been a consultant to and has received honoraria from Bayer and from several other companies. Dr. Kosiborod has been a consultant to Bayer and to AstraZeneca, Boehringer Ingelheim, Jansse, Merck, and Vifor and has received research funding from AstraZeneca and Boehringer Ingelheim. Dr. Rangaswami has disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
For patients with diabetic kidney disease, finerenone, an agent from a new class of selective, nonsteroidal mineralocorticoid receptor antagonists, led to significant reductions in combined adverse renal outcomes and in combined adverse cardiovascular outcomes in the pivotal FIDELIO-DKD trial.
And the safety results showed a good level of tolerability. The rate of hyperkalemia was higher with finerenone than with placebo, but the rate of drug discontinuations for elevated potassium was lower than that seen with spironolactone, a steroidal mineralocorticoid receptor antagonist (MRA).
“An ideal drug would cause no hyperkalemia, but the absolute risk we saw is a fraction of what we see with spironolactone in this vulnerable patient population,” said Rajiv Agarwal, MD, from Indiana in Indianapolis, during a press briefing.
After a median follow-up of 2.6 years, finerenone was associated with a 3.4% absolute reduction in the rate of combined adverse renal events, the study’s primary end point, which comprised kidney failure, renal death, and a drop in estimated glomerular filtration rate (eGFR) of at least 40% from baseline. This produced a significant relative risk reduction of 18%, with a number needed to treat of 32 to prevent one of these events, Dr. Agarwal reported at Kidney Week 2020. Findings from the FIDELIO-DKD trial were published simultaneously in the New England Journal of Medicine.
Finerenone was also associated with an absolute 2.4% reduction in the rate of combined adverse cardiovascular events, the study’s “key secondary end point,” which included cardiovascular death, nonfatal MI, nonfatal stroke, and hospitalization for heart failure. This translated into a significant relative risk reduction of 14% and a number needed to treat of 42 to prevent one of these events.
FIDELIO-DKD assessed 5,734 patients with type 2 diabetes and chronic kidney disease from more than 1,000 sites in 48 countries, including the United States, from 2015 to 2018. In the study cohort, average age was slightly more than 65 years, average baseline systolic blood pressure was 138 mm Hg, average duration of diabetes was nearly 17 years, average baseline glycated hemoglobin (A1c) was 7.7%, and fewer than 5% of patients were Black, 25% were Asian, and about 63% were White.
A suggestion of less severe hyperkalemia
Finerenone was well tolerated by the participants, and the findings suggest that it caused less clinically meaningful hyperkalemia than spironolactone, the most established and widely used MRA.
Like all MRA drugs, finerenone led to an increase in serum potassium in all patient subgroups – in this case 0.2 mmol/L – unlike placebo, said Dr. Agarwal.
The overall incidence of hyperkalemia was 16% in the 2,827 evaluable patients in the finerenone group and 8% in the 2,831 evaluable patients in the placebo group. Fewer than 10% of patients in the trial received a potassium-binding agent.
The rate of hyperkalemia leading to treatment discontinuation was higher in the finerenone group than in the placebo group (2.3% vs. 0.9%).
That 2.3% rate is 10 times lower than the 23.0% rate of hyperkalemia-related treatment discontinuation in patients who received spironolactone and no potassium-binding agent, said Dr. Agarwal, citing a previous study he was involved with.
He hypothesized that finerenone might cause less clinically meaningful hyperkalemia because it creates no active metabolites that linger in the body, whereas spironolactone produces active metabolites with a half life of about 1 week.
“The risk for hyperkalemia is clearly increased with finerenone compared with placebo, and in the absence of head-to-head studies, it’s hard to know how it compares with spironolactone or eplerenone [Inspra],” the other agents in the MRA class, said Mikhail N. Kosiborod, MD, from the University of Missouri–Kansas City.
“The rates of hyperkalemia observed in FIDELIO-DKD were overall comparable to what we would expect from eplerenone. But the rate of serious hyperkalemia was quite low with finerenone, which is reassuring,” Dr. Kosiborod said in an interview.
And the adverse-effect profile showed that finerenone “is as safe as you could expect from an MRA,” said Janani Rangaswami, MD, from the Einstein Medical Center in Philadelphia.
The rate of hyperkalemia should be interpreted in the context of the high risk the enrolled patients faced, given that they all had moderate to severe diabetic kidney disease with albuminuria and, in some cases, eGFR rates as low as 25 mL/min per 1.73m2, she explained. In addition, all patients were on maximally tolerated treatment with either an angiotensin-converting–enzyme inhibitor or an angiotensin receptor blocker to inhibit the renin angiotensin system (RAS).
“Considering this background, it’s a very acceptable adverse-event profile,” Dr. Rangaswami said in an interview.
Renal drugs that could work together
More than 99% of patients in FIDELIO-DKD were on an RAS inhibitor, but fewer than 5% were on a sodium glucose cotransporter 2 (SGLT2) inhibitor at baseline, and fewer than 10% started on this drug class during the course of the study.
Despite that, both Dr. Kosiborod and Dr. Rangaswami are enthusiastic about the prospect of using the three drugs in combination to maximize renal and cardiovascular benefits in FIDELIO-DKD–type patients. Recent results from the CREDENCE study of canagliflozin (Invokana) and from the DAPA-CKD study of dapagluflozin (Farxiga) have established SGLT2 inhibitors – at least those two – as key agents for patients with chronic kidney disease.
Dual treatment with an RAS inhibitor and an SGLT2 inhibitor is “clearly established” for patients with diabetic kidney disease, said Dr. Agarwal.
“After CREDENCE, DAPA-CKD, and now FIDELIO-DKD, we need to seriously consider triple therapy as the future of treatment for diabetic kidney disease to prevent both cardiovascular and kidney complications,” said Dr. Kosiborod. The approach will mimic the multidrug therapy that’s now standard for patients with heart failure with reduced ejection fraction (HFrEF). But he cautioned that this triple combination needs further testing.
“Triple therapy will be the standard of care” for patients with diabetic kidney disease, Dr. Rangaswami agreed, but she cautioned that she would not currently expand the target population for finerenone to patients without type 2 diabetes or to patients without the level of albuminuria required for entry into FIDELIO-DKD: at least 30 mg/g of creatinine per day. And patients with HFrEF were excluded from FIDELIO-DKD, so that limitation on finerenone use should remain for the time being, she added.
Dr. Rangaswami said she is optimistic about the potential efficacy of finerenone added to an SGLT2 inhibitor because of the likelihood that the two drug classes work in different but complementary ways. SGLT2 inhibitors seem to exert their renal protective effects largely through hemodynamic effects, whereas it is likely that finerenone exerts its effects largely as an anti-inflammatory and antifibrotic agent, she speculated. The FIDELIO-DKD results appear to rule out any major effect of finerenone on blood pressure lowering because average systolic pressure fell by only about 2 mm Hg in the treatment group.
“The benefits of finerenone for cardiorenal outcomes are substantial and clinically meaningful,” Dr. Kosiborod said. “We cannot assume that other MRAs, such as spironolactone, provide similar benefits,” he cautioned, but the results are “very good news for patients with type 2 diabetes and chronic kidney disease. We now have another effective intervention with a different mechanism of action.”
FIDELIO-DKD was sponsored by Bayer, the company developing finerenone (BAY 94-8862). Dr. Agarwal has been a consultant to and has received honoraria from Bayer and from several other companies. Dr. Kosiborod has been a consultant to Bayer and to AstraZeneca, Boehringer Ingelheim, Jansse, Merck, and Vifor and has received research funding from AstraZeneca and Boehringer Ingelheim. Dr. Rangaswami has disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
FROM KIDNEY WEEK
PICC lines often used inappropriately in advanced CKD patients
Background: PICC insertion is associated with risk for venous thrombosis and stenosis. National guidelines recommend avoiding PICC lines in patients with CKD stage 3b (glomerular filtration rate less than 45 mL/min per 1.73 m2) in order to preserve venous integrity for future creation of arteriovenous fistula, which is the ideal vascular access for hemodialysis.
Study design: Prospective cohort.
Setting: 52 hospitals in Michigan.
Synopsis: Data obtained from inpatients within the Michigan Hospital Medicine Safety Consortium between 2013 and 2016 showed that, of 20,545 total PICCs placed, 23% were placed in patients with a glomerular filtration rate less than 45 mL/min per 1.73 m2, and 3.2% were placed in those receiving dialysis. PICC placement in advanced CKD was more common in the ICU than in the ward setting, and placement more frequently utilized multilumen instead of single-lumen catheters. PICC-related complications were not more common in advanced CKD but were more often seen in the ICU and with multilumen PICCs. About one-quarter of PICCs were used for durations of less than 5 days.
The study is limited by lack of data in a subset of patients who had no documented GFR (2.7%) or missing covariate data (2.7%). The inability to ascertain other clinical information, such as nephrology approval of PICC, functional AV fistula or other hemodialysis access, or PICC complications after discharge further limit the findings.
Hospitalists should first decide if a PICC line is truly indicated, and if so, carefully weigh the risks and benefits of PICC placement in patients with advanced CKD.
Bottom line: PICC placement is common and often inappropriate in hospitalized patients with advanced CKD.
Citation: Paje D et al. Use of peripherally inserted central catheters in patients with advanced chronic kidney disease A prospective cohort study. Ann Intern Med. 2019 Jun 4;171:10-8.
Dr. Hageman is a hospitalist at Vanderbilt University Medical Center, Nashville, Tenn.
Background: PICC insertion is associated with risk for venous thrombosis and stenosis. National guidelines recommend avoiding PICC lines in patients with CKD stage 3b (glomerular filtration rate less than 45 mL/min per 1.73 m2) in order to preserve venous integrity for future creation of arteriovenous fistula, which is the ideal vascular access for hemodialysis.
Study design: Prospective cohort.
Setting: 52 hospitals in Michigan.
Synopsis: Data obtained from inpatients within the Michigan Hospital Medicine Safety Consortium between 2013 and 2016 showed that, of 20,545 total PICCs placed, 23% were placed in patients with a glomerular filtration rate less than 45 mL/min per 1.73 m2, and 3.2% were placed in those receiving dialysis. PICC placement in advanced CKD was more common in the ICU than in the ward setting, and placement more frequently utilized multilumen instead of single-lumen catheters. PICC-related complications were not more common in advanced CKD but were more often seen in the ICU and with multilumen PICCs. About one-quarter of PICCs were used for durations of less than 5 days.
The study is limited by lack of data in a subset of patients who had no documented GFR (2.7%) or missing covariate data (2.7%). The inability to ascertain other clinical information, such as nephrology approval of PICC, functional AV fistula or other hemodialysis access, or PICC complications after discharge further limit the findings.
Hospitalists should first decide if a PICC line is truly indicated, and if so, carefully weigh the risks and benefits of PICC placement in patients with advanced CKD.
Bottom line: PICC placement is common and often inappropriate in hospitalized patients with advanced CKD.
Citation: Paje D et al. Use of peripherally inserted central catheters in patients with advanced chronic kidney disease A prospective cohort study. Ann Intern Med. 2019 Jun 4;171:10-8.
Dr. Hageman is a hospitalist at Vanderbilt University Medical Center, Nashville, Tenn.
Background: PICC insertion is associated with risk for venous thrombosis and stenosis. National guidelines recommend avoiding PICC lines in patients with CKD stage 3b (glomerular filtration rate less than 45 mL/min per 1.73 m2) in order to preserve venous integrity for future creation of arteriovenous fistula, which is the ideal vascular access for hemodialysis.
Study design: Prospective cohort.
Setting: 52 hospitals in Michigan.
Synopsis: Data obtained from inpatients within the Michigan Hospital Medicine Safety Consortium between 2013 and 2016 showed that, of 20,545 total PICCs placed, 23% were placed in patients with a glomerular filtration rate less than 45 mL/min per 1.73 m2, and 3.2% were placed in those receiving dialysis. PICC placement in advanced CKD was more common in the ICU than in the ward setting, and placement more frequently utilized multilumen instead of single-lumen catheters. PICC-related complications were not more common in advanced CKD but were more often seen in the ICU and with multilumen PICCs. About one-quarter of PICCs were used for durations of less than 5 days.
The study is limited by lack of data in a subset of patients who had no documented GFR (2.7%) or missing covariate data (2.7%). The inability to ascertain other clinical information, such as nephrology approval of PICC, functional AV fistula or other hemodialysis access, or PICC complications after discharge further limit the findings.
Hospitalists should first decide if a PICC line is truly indicated, and if so, carefully weigh the risks and benefits of PICC placement in patients with advanced CKD.
Bottom line: PICC placement is common and often inappropriate in hospitalized patients with advanced CKD.
Citation: Paje D et al. Use of peripherally inserted central catheters in patients with advanced chronic kidney disease A prospective cohort study. Ann Intern Med. 2019 Jun 4;171:10-8.
Dr. Hageman is a hospitalist at Vanderbilt University Medical Center, Nashville, Tenn.
Retrospective Review on the Safety and Efficacy of Direct Oral Anticoagulants Compared With Warfarin in Patients With Cirrhosis
Coagulation in patients with cirrhosis is a complicated area of evolving research. Patients with cirrhosis were originally thought to be naturally anticoagulated due to the decreased production of clotting factors and platelets, combined with an increased international normalized ratio (INR).1 New data have shown that patients with cirrhosis are at a concomitant risk of bleeding and thrombosis due to increased platelet aggregation, decreased fibrinolysis, and decreased production of natural anticoagulants such as protein C and antithrombin.1 Traditionally, patients with cirrhosis needing anticoagulation therapy for comorbid conditions, such as nonvalvular atrial fibrillation (NVAF) or venous thromboembolism (VTE) were placed on warfarin therapy. Managing warfarin in patients with cirrhosis poses a challenge to clinicians due to the many food and drug interactions, narrow therapeutic index, and complications with maintaining a therapeutic INR.1
Direct oral anticoagulants (DOACs) have several benefits over warfarin therapy, including convenience, decreased monitoring, decreased drug and dietary restrictions, and faster onset of action.2 Conversely, DOACs undergo extensive hepatic metabolism giving rise to concerns about supratherapeutic drug levels and increased bleeding rates in patients with liver dysfunction.1 Consequently, patients with cirrhosis were excluded from the pivotal trials establishing DOACs for NVAF and VTE treatment. Exclusion of these patients in major clinical trials alongside the challenges of managing warfarin warrant an evaluation of the efficacy and safety of DOACs in patients with cirrhosis.
Recent retrospective studies have examined the use of DOACs in patients with cirrhosis and found favorable results. A retrospective chart review by Intagliata and colleagues consisting of 39 patients with cirrhosis using either a DOAC or warfarin found similar rates of all-cause bleeding and major bleeding between the 2 groups.3 A retrospective cohort study by Hum and colleagues consisting of 45 patients with cirrhosis compared the use of DOACs with warfarin or low-molecular weight heparin (LMWH).4 Hum and colleagues found patients prescribed a DOAC had significantly fewer major bleeding events than did patients using warfarin or LMWH.4 The largest retrospective cohort study consisted of 233 patients with chronic liver disease and found no differences among all-cause bleeding and major bleeding rates between patients using DOACs compared with those of patients using warfarin.5
The purpose of this research is to evaluate the safety and efficacy of DOACs in veteran patients with cirrhosis compared with patients using warfarin.
Methods
A retrospective single-center chart review was conducted at the Michael E. DeBakey Veterans Affairs Medical Center (MEDVAMC) in Houston, Texas, between October 31, 2014 and October 31, 2018. Patients included in the study were adults aged ≥ 18 years with a diagnosis of cirrhosis and prescribed any of the following oral anticoagulants: apixaban, dabigatran, edoxaban, rivaroxaban, or warfarin. Patients prescribed apixaban, dabigatran, edoxaban, or rivaroxaban were collectively grouped into the DOAC group, while patients prescribed warfarin were classified as the standard of care comparator group.
A diagnosis of cirrhosis was confirmed using a combination of the codes from the ninth and tenth editions of the International Classification of Diseases (ICD) for cirrhosis, documentation of diagnostic confirmation by clinicians from the gastroenterology or hepatology services, and positive liver biopsy result. Liver function tests, liver ultrasound results, and FibroSure biomarker assays were used to aid in confirming the diagnosis of cirrhosis but were not considered definitive. Patients were excluded from the trial if they had indications for anticoagulation other than NVAF and VTE and/or were prescribed triple antithrombotic therapy (dual antiplatelet therapy plus an anticoagulant). Patients who switched anticoagulant therapy during the trial period (ie, switched from warfarin to a DOAC) were also excluded from the analysis.
Patient demographic characteristics that were collected included weight; body mass index (BMI); etiology of cirrhosis; Child-Turcotte-Pugh, Model for End-Stage Liver Disease (MELD), and CHA2DS2-VASc score; concomitant antiplatelet, nonsteroidal anti-inflammatory drug (NSAID), proton pump inhibitor (PPI), and histamine-2 receptor antagonist
Two patient lists were used to identify patients for inclusion in the warfarin arm. The first patient list was generated using the US Department of Veterans Affairs (VA) Cirrhosis Tracker, which identified patients with an ICD-9/10 code for cirrhosis and an INR laboratory value. Patients generated from the VA Cirrhosis Tracker with an INR > 1.5 were screened for a warfarin prescription and then evaluated for full study inclusion. The second patient list was generated using the VA Advanced Liver Disease Dashboard which identified patients with ICD-9/10 codes for advanced liver disease and an active warfarin prescription. Patients with an active warfarin prescription were then evaluated for full study inclusion. A single patient list was generated to identify patients for inclusion in the DOAC arm. This patient list was generated using the VA DOAC dashboard, which identified patients with an active DOAC prescription and an ICD-9/10 code for cirrhosis. Patients with an ICD-9/10 code for cirrhosis and prescribed a DOAC were screened for full study inclusion. Patient data were collected from the MEDVAMC Computerized Patient Record System (CPRS) electronic health record (EHR). The research study was approved by the Baylor College of Medicine Institutional Review Board and the VA Office of Research and Development.
Outcomes
The primary endpoint for the study was all-cause bleeding. The secondary endpoints for the study were major bleeding and failed efficacy. Major bleeding was defined using the International Society on Thrombosis and Haemostasis (ISTH) 2005 definition: fatal bleeding, symptomatic bleeding in a critical organ area (ie, intracranial, intraspinal, intraocular, retroperitoneal, intraarticular, pericardial, or intramuscular with compartment syndrome), or bleeding causing a fall in hemoglobin level of > 2 g/dL or leading to the transfusion of ≥ 2 units of red cells.6 Failed efficacy was a combination endpoint that included development of VTE, stroke, myocardial infarction (MI), and/or death. A prespecified subgroup analysis was conducted at the end of the study period to analyze trends in the DOAC and warfarin groups with respect to all-cause bleeding. All-cause bleeding risk was stratified by weight, BMI, Child-Turcotte-Pugh score, MELD score, presence of gastric and/or esophageal varices, active malignancies, percentage of time within therapeutic INR range in the warfarin group, indications for anticoagulation, and antiplatelet, NSAID, PPI, and H2RA therapy.
Statistical Analysis
Data were analyzed using descriptive and inferential statistics. Continuous data were analyzed using the Student t test, and categorical data were analyzed using the Fisher exact test. Previous studies determined an all-cause bleeding rate of 10 to 17% for warfarin compared with 5% for DOACs.7,8 To detect a 12% difference in the all-cause bleeding rate between DOACs and warfarin, 212 patients would be needed to achieve 80% power at an α level of 0.05.
Results
A total of 170 patients were screened, and after applying inclusion and exclusion criteria, 79 patients were enrolled in the study (Figure). The DOAC group included 42 patients, and the warfarin group included 37 patients. In the DOAC group, 69.1% (n = 29) of patients were taking apixaban, 21.4% (n = 9) rivaroxaban, and 9.5% (n = 4) dabigatran. There were no patients prescribed edoxaban during the study period.
Baseline characteristics were similar between the 2 groups except for Child-Turcotte-Pugh score, MELD score, mean INR, and number of days on anticoagulation therapy (Table 1). Most of the patients were male (98.7%), and the mean age was 71 years. The most common causes of cirrhosis were viral (29.1%), nonalcoholic fatty liver disease (NAFLD) (24.1%), multiple causes (22.8%), and alcohol (21.5%). Sixty-two patients (78.5%) had a NVAF indication for anticoagulation. The average CHA2DS2-VASc score was 3.7. Aspirin was prescribed in 51.9% (n = 41) of patients, and PPIs were prescribed in 48.1% (n = 38) of patients. At inclusion, esophageal varices were present in 13 patients and active malignancies were present in 6 patients.
Statistically significant differences in baseline characteristics were found between mean INR, Child-Turcotte-Pugh scores, MELD scores, and number of days on anticoagulant therapy. The mean INR was 1.3 in the DOAC group compared with 2.1 in the warfarin group (P = .0001). Eighty-one percent (n = 34) of patients in the DOAC group had a Child-Turcotte-Pugh score of A compared with 43.2% (n = 16) of patients in the warfarin group (P = .0009). Eight patients in the DOAC group had a Child-Turcotte-Pugh score of B compared with 19 patients in the warfarin group (P = .004). The mean MELD score was 9.4 in the DOAC group compared with 16.3 in the warfarin group (P = .0001). The mean days on anticoagulant therapy was 500.4 days for the DOAC group compared with 1,652.4 days for the warfarin group (P = .0001).
Safety Outcome
The primary outcome comparing all-cause bleeding rates between patients on DOACs compared with warfarin are listed in Table 2. With respect to the primary outcome, 7 (16.7%) patients on DOACs experienced a bleeding event compared with 8 (21.6%) patients on warfarin (P = .77). No statistically significant differences were detected between the DOAC and warfarin groups with respect to all-cause bleeding. Seven bleeding events occurred in the DOAC group; 1 met the qualification for major bleeding with a suspected gastrointestinal (GI) bleed.6 The other 6 bleeding episodes in the DOAC group consisted of hematoma, epistaxis, hematuria, and hematochezia. Eight bleeding events occurred in the warfarin group; 2 met the qualification for major bleeding with an intracranial hemorrhage and upper GI bleed.6 The other 6 bleeding episodes in the warfarin group consisted of epistaxis, bleeding gums, hematuria, and hematochezia. There were no statistically significant differences between the rates of major bleeding and nonmajor bleeding between the DOAC and warfarin groups.
Efficacy Outcomes
There were 3 events in the DOAC group and 3 events in the warfarin group (P = .99). In the DOAC group, 2 patients experienced a pulmonary embolism, and 1 patient experienced a MI. In the warfarin group, 3 patients died (end-stage heart failure, unknown cause due to death at an outside hospital, and sepsis/organ failure). There were no statistically significant differences between the composite endpoint of failed efficacy or the individual endpoints of VTE, stroke, MI, and death.
Subgroup Analysis
A prespecified subgroup analysis was conducted to determine risk factors for all-cause bleeding within each treatment group (Table 3). No significant trends were observed in the following risk factors: Child-Turcotte-Pugh score, indication for anticoagulation, use of NSAIDs, PPIs or H2RAs, presence of gastric or esophageal varices, active malignancies, and time within therapeutic INR range in the warfarin group. Patients with bleeding events had slightly increased weight and BMI vs patients without bleeding events. Within the warfarin group, patients with bleeding events had slightly elevated MELD scores compared to patients without bleeding events. There was an equal balance of patients prescribed aspirin therapy between the groups with and without bleeding events. Overall, no significant risk factors were identified for all-cause bleeding.
Discussion
Initially, patients with cirrhosis were excluded from DOAC trials due to concerns for increased bleeding risk with hepatically eliminated medications. New retrospective research has concluded that in patients with cirrhosis, DOACs have similar or lower bleeding rates when compared directly to warfarin.9,10
In this study, no statistically significant differences were detected between the primary and secondary outcomes of all-cause bleeding, major bleeding, or failed efficacy. Subgroup analysis did not identify any significant risk factors with respect to all-cause bleeding among patients in the DOAC and warfarin groups. To meet 80% power, 212 patients needed to be enrolled in the study; however, only 79 patients were enrolled, and power was not met. The results of this study should be interpreted cautiously as hypothesis-generating due to the small sample size. Strengths of this study include similar baseline characteristics between the DOAC and warfarin groups, 4-year length of retrospective data review, and availability of both inpatient and outpatient EHR limiting the amount of missing data points.
Baseline characteristics were similar between the groups except for mean INR, Child-Turcotte-Pugh score, MELD score, and number of days on anticoagulation therapy. The difference in mean INR between groups is expected as patients in the warfarin group have a goal INR of 2 to 3 to maintain therapeutic efficacy and safety. INR is not used as a marker of efficacy or safety with DOACs; therefore, a consistent elevation in INR is not expected. Child- Turcotte-Pugh scores are calculated using INR levels.11 When calculating the score, patients with an INR < 1.7 receive 1 point; patients with an INR between 1.7 and 2.3 receive 2 points.11 Therefore, patients in the warfarin group will have artificially inflated Child-Turcotte-Pugh scores as this group has goal INR levels of 2 to 3. This makes Child-Turcotte-Pugh scores unreliable markers of disease severity in patients using warfarin therapy. When the INR scores for patients prescribed warfarin were replaced with values < 1.7, the statistical difference disappeared between the warfarin and DOAC groups. The same effect is seen on MELD scores for patients prescribed warfarin therapy. The MELD score is calculated using INR levels.12 MELD scores also will be artificially elevated in patients prescribed warfarin therapy due to the INR elevation to between 2 and 3. When MELD scores for patients prescribed warfarin were replaced with values similar to those in the DOAC group, the statistical difference disappeared between the warfarin and DOAC groups.
The last statistically significant difference was found in number of days on anticoagulant therapy. This difference was expected as warfarin is the standard of care for anticoagulation treatment in patients with cirrhosis. The first DOAC, dabigatran, was not approved by the US Food and Drug Administration until 2010.13 DOACs have only recently been used in patients with cirrhosis accounting for the statistically significant difference in days on anticoagulation therapy between the warfarin and DOAC groups.
Limitations
The inability to meet power or evaluate adherence and appropriate renal dose adjustments for DOACs limited this study. This study was conducted at a single center in a predominantly male veteran population and therefore may not be generalizable to other populations. A majority of patients in the DOAC group were prescribed apixaban (69.1%), which may have affected the overall rate of major bleeding in the DOAC group. Pivotal trials of apixaban have shown a consistent decreased risk of major bleeding in patients with NVAF or VTE when compared with warfarin.14,15 Therefore, the results of this study may not be generalizable to all DOACs.
An inherent limitation of this study was the inability to collect data verifying adherence in the DOAC group. However, in the warfarin group, percentage of time within the therapeutic INR range of 2 to 3 was collected. While not a direct marker of adherence, this does allow for limited evaluation of therapeutic efficacy and safety within the warfarin group. Last, proper dosing of DOACs in patients with and without adequate renal function was not evaluated in this study.
Conclusions
The results of this study are consistent with other retrospective research and literature reviews. There were no statistically significant differences identified between the rates of all-cause bleeding, major bleeding, and failed efficacy between the DOAC and warfarin groups. DOACs may be a safe alternative to warfarin in patients with cirrhosis requiring anticoagulation for NVAF or VTE, but large randomized trials are required to confirm these results.
1. Qamar A, Vaduganathan M, Greenberger NJ, Giugliano RP. Oral anticoagulation in patients with liver disease. J Am Coll Cardiol. 2018;71(19):2162-2175. doi:10.1016/j.jacc.2018.03.023
2. Priyanka P, Kupec JT, Krafft M, Shah NA, Reynolds GJ. Newer oral anticoagulants in the treatment of acute portal vein thrombosis in patients with and without cirrhosis. Int J Hepatol. 2018;2018:8432781. Published 2018 Jun 5. doi:10.1155/2018/8432781
3. Intagliata NM, Henry ZH, Maitland H, et al. Direct oral anticoagulants in cirrhosis patients pose similar risks of bleeding when compared to traditional anticoagulation. Dig Dis Sci. 2016;61(6):1721-1727. doi:10.1007/s10620-015-4012-2
4. Hum J, Shatzel JJ, Jou JH, Deloughery TG. The efficacy and safety of direct oral anticoagulants vs traditional anticoagulants in cirrhosis. Eur J Haematol. 2017;98(4):393-397. doi:10.1111/ejh.12844
5. Goriacko P, Veltri KT. Safety of direct oral anticoagulants vs warfarin in patients with chronic liver disease and atrial fibrillation. Eur J Haematol. 2018;100(5):488-493. doi:10.1111/ejh.13045
6. Schulman S, Kearon C; Subcommittee on Control of Anticoagulation of the Scientific and Standardization Committee of the International Society on Thrombosis and Haemostasis. Definition of major bleeding in clinical investigations of antihemostatic medicinal products in non-surgical patients. J Thromb Haemost. 2005;3(4):692-694. doi:10.1111/j.1538-7836.2005.01204.x
7. Rubboli A, Becattini C, Verheugt FW. Incidence, clinical impact and risk of bleeding during oral anticoagulation therapy. World J Cardiol. 2011;3(11):351-358. doi:10.4330/wjc.v3.i11.351
8. Ruff CT, Giugliano RP, Braunwald E, et al. Comparison of the efficacy and safety of new oral anticoagulants with warfarin in patients with atrial fibrillation: a meta-analysis of randomised trials. Lancet. 2014;383(9921):955-962. doi:10.1016/S0140-6736(13)62343-0
9. Hoolwerf EW, Kraaijpoel N, Büller HR, van Es N. Direct oral anticoagulants in patients with liver cirrhosis: A systematic review. Thromb Res. 2018;170:102-108. doi:10.1016/j.thromres.2018.08.011
10. Steuber TD, Howard ML, Nisly SA. Direct oral anticoagulants in chronic liver disease. Ann Pharmacother. 2019;53(10):1042-1049. doi:10.1177/1060028019841582
11. Janevska D, Chaloska-Ivanova V, Janevski V. Hepatocellular carcinoma: risk factors, diagnosis and treatment. Open Access Maced J Med Sci. 2015;3(4):732-736. doi:10.3889/oamjms.2015.111
12. Singal AK, Kamath PS. Model for End-Stage Liver Disease. J Clin Exp Hepatol. 2013;3(1):50-60. doi:10.1016/j.jceh.2012.11.002
13. Joppa SA, Salciccioli J, Adamski J, et al. A practical review of the emerging direct anticoagulants, laboratory monitoring, and reversal agents. J Clin Med. 2018;7(2):29. Published 2018 Feb 11. doi:10.3390/jcm7020029
14. Granger CB, Alexander JH, McMurray JJ, et al. Apixaban versus warfarin in patients with atrial fibrillation. N Engl J Med. 2011;365(11):981-992. doi:10.1056/NEJMoa1107039
15. Agnelli G, Buller HR, Cohen A, et al. Oral apixaban for the treatment of acute venous thromboembolism. N Engl J Med. 2013;369(9):799-808. doi:10.1056/NEJMoa1302507
Coagulation in patients with cirrhosis is a complicated area of evolving research. Patients with cirrhosis were originally thought to be naturally anticoagulated due to the decreased production of clotting factors and platelets, combined with an increased international normalized ratio (INR).1 New data have shown that patients with cirrhosis are at a concomitant risk of bleeding and thrombosis due to increased platelet aggregation, decreased fibrinolysis, and decreased production of natural anticoagulants such as protein C and antithrombin.1 Traditionally, patients with cirrhosis needing anticoagulation therapy for comorbid conditions, such as nonvalvular atrial fibrillation (NVAF) or venous thromboembolism (VTE) were placed on warfarin therapy. Managing warfarin in patients with cirrhosis poses a challenge to clinicians due to the many food and drug interactions, narrow therapeutic index, and complications with maintaining a therapeutic INR.1
Direct oral anticoagulants (DOACs) have several benefits over warfarin therapy, including convenience, decreased monitoring, decreased drug and dietary restrictions, and faster onset of action.2 Conversely, DOACs undergo extensive hepatic metabolism giving rise to concerns about supratherapeutic drug levels and increased bleeding rates in patients with liver dysfunction.1 Consequently, patients with cirrhosis were excluded from the pivotal trials establishing DOACs for NVAF and VTE treatment. Exclusion of these patients in major clinical trials alongside the challenges of managing warfarin warrant an evaluation of the efficacy and safety of DOACs in patients with cirrhosis.
Recent retrospective studies have examined the use of DOACs in patients with cirrhosis and found favorable results. A retrospective chart review by Intagliata and colleagues consisting of 39 patients with cirrhosis using either a DOAC or warfarin found similar rates of all-cause bleeding and major bleeding between the 2 groups.3 A retrospective cohort study by Hum and colleagues consisting of 45 patients with cirrhosis compared the use of DOACs with warfarin or low-molecular weight heparin (LMWH).4 Hum and colleagues found patients prescribed a DOAC had significantly fewer major bleeding events than did patients using warfarin or LMWH.4 The largest retrospective cohort study consisted of 233 patients with chronic liver disease and found no differences among all-cause bleeding and major bleeding rates between patients using DOACs compared with those of patients using warfarin.5
The purpose of this research is to evaluate the safety and efficacy of DOACs in veteran patients with cirrhosis compared with patients using warfarin.
Methods
A retrospective single-center chart review was conducted at the Michael E. DeBakey Veterans Affairs Medical Center (MEDVAMC) in Houston, Texas, between October 31, 2014 and October 31, 2018. Patients included in the study were adults aged ≥ 18 years with a diagnosis of cirrhosis and prescribed any of the following oral anticoagulants: apixaban, dabigatran, edoxaban, rivaroxaban, or warfarin. Patients prescribed apixaban, dabigatran, edoxaban, or rivaroxaban were collectively grouped into the DOAC group, while patients prescribed warfarin were classified as the standard of care comparator group.
A diagnosis of cirrhosis was confirmed using a combination of the codes from the ninth and tenth editions of the International Classification of Diseases (ICD) for cirrhosis, documentation of diagnostic confirmation by clinicians from the gastroenterology or hepatology services, and positive liver biopsy result. Liver function tests, liver ultrasound results, and FibroSure biomarker assays were used to aid in confirming the diagnosis of cirrhosis but were not considered definitive. Patients were excluded from the trial if they had indications for anticoagulation other than NVAF and VTE and/or were prescribed triple antithrombotic therapy (dual antiplatelet therapy plus an anticoagulant). Patients who switched anticoagulant therapy during the trial period (ie, switched from warfarin to a DOAC) were also excluded from the analysis.
Patient demographic characteristics that were collected included weight; body mass index (BMI); etiology of cirrhosis; Child-Turcotte-Pugh, Model for End-Stage Liver Disease (MELD), and CHA2DS2-VASc score; concomitant antiplatelet, nonsteroidal anti-inflammatory drug (NSAID), proton pump inhibitor (PPI), and histamine-2 receptor antagonist
Two patient lists were used to identify patients for inclusion in the warfarin arm. The first patient list was generated using the US Department of Veterans Affairs (VA) Cirrhosis Tracker, which identified patients with an ICD-9/10 code for cirrhosis and an INR laboratory value. Patients generated from the VA Cirrhosis Tracker with an INR > 1.5 were screened for a warfarin prescription and then evaluated for full study inclusion. The second patient list was generated using the VA Advanced Liver Disease Dashboard which identified patients with ICD-9/10 codes for advanced liver disease and an active warfarin prescription. Patients with an active warfarin prescription were then evaluated for full study inclusion. A single patient list was generated to identify patients for inclusion in the DOAC arm. This patient list was generated using the VA DOAC dashboard, which identified patients with an active DOAC prescription and an ICD-9/10 code for cirrhosis. Patients with an ICD-9/10 code for cirrhosis and prescribed a DOAC were screened for full study inclusion. Patient data were collected from the MEDVAMC Computerized Patient Record System (CPRS) electronic health record (EHR). The research study was approved by the Baylor College of Medicine Institutional Review Board and the VA Office of Research and Development.
Outcomes
The primary endpoint for the study was all-cause bleeding. The secondary endpoints for the study were major bleeding and failed efficacy. Major bleeding was defined using the International Society on Thrombosis and Haemostasis (ISTH) 2005 definition: fatal bleeding, symptomatic bleeding in a critical organ area (ie, intracranial, intraspinal, intraocular, retroperitoneal, intraarticular, pericardial, or intramuscular with compartment syndrome), or bleeding causing a fall in hemoglobin level of > 2 g/dL or leading to the transfusion of ≥ 2 units of red cells.6 Failed efficacy was a combination endpoint that included development of VTE, stroke, myocardial infarction (MI), and/or death. A prespecified subgroup analysis was conducted at the end of the study period to analyze trends in the DOAC and warfarin groups with respect to all-cause bleeding. All-cause bleeding risk was stratified by weight, BMI, Child-Turcotte-Pugh score, MELD score, presence of gastric and/or esophageal varices, active malignancies, percentage of time within therapeutic INR range in the warfarin group, indications for anticoagulation, and antiplatelet, NSAID, PPI, and H2RA therapy.
Statistical Analysis
Data were analyzed using descriptive and inferential statistics. Continuous data were analyzed using the Student t test, and categorical data were analyzed using the Fisher exact test. Previous studies determined an all-cause bleeding rate of 10 to 17% for warfarin compared with 5% for DOACs.7,8 To detect a 12% difference in the all-cause bleeding rate between DOACs and warfarin, 212 patients would be needed to achieve 80% power at an α level of 0.05.
Results
A total of 170 patients were screened, and after applying inclusion and exclusion criteria, 79 patients were enrolled in the study (Figure). The DOAC group included 42 patients, and the warfarin group included 37 patients. In the DOAC group, 69.1% (n = 29) of patients were taking apixaban, 21.4% (n = 9) rivaroxaban, and 9.5% (n = 4) dabigatran. There were no patients prescribed edoxaban during the study period.
Baseline characteristics were similar between the 2 groups except for Child-Turcotte-Pugh score, MELD score, mean INR, and number of days on anticoagulation therapy (Table 1). Most of the patients were male (98.7%), and the mean age was 71 years. The most common causes of cirrhosis were viral (29.1%), nonalcoholic fatty liver disease (NAFLD) (24.1%), multiple causes (22.8%), and alcohol (21.5%). Sixty-two patients (78.5%) had a NVAF indication for anticoagulation. The average CHA2DS2-VASc score was 3.7. Aspirin was prescribed in 51.9% (n = 41) of patients, and PPIs were prescribed in 48.1% (n = 38) of patients. At inclusion, esophageal varices were present in 13 patients and active malignancies were present in 6 patients.
Statistically significant differences in baseline characteristics were found between mean INR, Child-Turcotte-Pugh scores, MELD scores, and number of days on anticoagulant therapy. The mean INR was 1.3 in the DOAC group compared with 2.1 in the warfarin group (P = .0001). Eighty-one percent (n = 34) of patients in the DOAC group had a Child-Turcotte-Pugh score of A compared with 43.2% (n = 16) of patients in the warfarin group (P = .0009). Eight patients in the DOAC group had a Child-Turcotte-Pugh score of B compared with 19 patients in the warfarin group (P = .004). The mean MELD score was 9.4 in the DOAC group compared with 16.3 in the warfarin group (P = .0001). The mean days on anticoagulant therapy was 500.4 days for the DOAC group compared with 1,652.4 days for the warfarin group (P = .0001).
Safety Outcome
The primary outcome comparing all-cause bleeding rates between patients on DOACs compared with warfarin are listed in Table 2. With respect to the primary outcome, 7 (16.7%) patients on DOACs experienced a bleeding event compared with 8 (21.6%) patients on warfarin (P = .77). No statistically significant differences were detected between the DOAC and warfarin groups with respect to all-cause bleeding. Seven bleeding events occurred in the DOAC group; 1 met the qualification for major bleeding with a suspected gastrointestinal (GI) bleed.6 The other 6 bleeding episodes in the DOAC group consisted of hematoma, epistaxis, hematuria, and hematochezia. Eight bleeding events occurred in the warfarin group; 2 met the qualification for major bleeding with an intracranial hemorrhage and upper GI bleed.6 The other 6 bleeding episodes in the warfarin group consisted of epistaxis, bleeding gums, hematuria, and hematochezia. There were no statistically significant differences between the rates of major bleeding and nonmajor bleeding between the DOAC and warfarin groups.
Efficacy Outcomes
There were 3 events in the DOAC group and 3 events in the warfarin group (P = .99). In the DOAC group, 2 patients experienced a pulmonary embolism, and 1 patient experienced a MI. In the warfarin group, 3 patients died (end-stage heart failure, unknown cause due to death at an outside hospital, and sepsis/organ failure). There were no statistically significant differences between the composite endpoint of failed efficacy or the individual endpoints of VTE, stroke, MI, and death.
Subgroup Analysis
A prespecified subgroup analysis was conducted to determine risk factors for all-cause bleeding within each treatment group (Table 3). No significant trends were observed in the following risk factors: Child-Turcotte-Pugh score, indication for anticoagulation, use of NSAIDs, PPIs or H2RAs, presence of gastric or esophageal varices, active malignancies, and time within therapeutic INR range in the warfarin group. Patients with bleeding events had slightly increased weight and BMI vs patients without bleeding events. Within the warfarin group, patients with bleeding events had slightly elevated MELD scores compared to patients without bleeding events. There was an equal balance of patients prescribed aspirin therapy between the groups with and without bleeding events. Overall, no significant risk factors were identified for all-cause bleeding.
Discussion
Initially, patients with cirrhosis were excluded from DOAC trials due to concerns for increased bleeding risk with hepatically eliminated medications. New retrospective research has concluded that in patients with cirrhosis, DOACs have similar or lower bleeding rates when compared directly to warfarin.9,10
In this study, no statistically significant differences were detected between the primary and secondary outcomes of all-cause bleeding, major bleeding, or failed efficacy. Subgroup analysis did not identify any significant risk factors with respect to all-cause bleeding among patients in the DOAC and warfarin groups. To meet 80% power, 212 patients needed to be enrolled in the study; however, only 79 patients were enrolled, and power was not met. The results of this study should be interpreted cautiously as hypothesis-generating due to the small sample size. Strengths of this study include similar baseline characteristics between the DOAC and warfarin groups, 4-year length of retrospective data review, and availability of both inpatient and outpatient EHR limiting the amount of missing data points.
Baseline characteristics were similar between the groups except for mean INR, Child-Turcotte-Pugh score, MELD score, and number of days on anticoagulation therapy. The difference in mean INR between groups is expected as patients in the warfarin group have a goal INR of 2 to 3 to maintain therapeutic efficacy and safety. INR is not used as a marker of efficacy or safety with DOACs; therefore, a consistent elevation in INR is not expected. Child- Turcotte-Pugh scores are calculated using INR levels.11 When calculating the score, patients with an INR < 1.7 receive 1 point; patients with an INR between 1.7 and 2.3 receive 2 points.11 Therefore, patients in the warfarin group will have artificially inflated Child-Turcotte-Pugh scores as this group has goal INR levels of 2 to 3. This makes Child-Turcotte-Pugh scores unreliable markers of disease severity in patients using warfarin therapy. When the INR scores for patients prescribed warfarin were replaced with values < 1.7, the statistical difference disappeared between the warfarin and DOAC groups. The same effect is seen on MELD scores for patients prescribed warfarin therapy. The MELD score is calculated using INR levels.12 MELD scores also will be artificially elevated in patients prescribed warfarin therapy due to the INR elevation to between 2 and 3. When MELD scores for patients prescribed warfarin were replaced with values similar to those in the DOAC group, the statistical difference disappeared between the warfarin and DOAC groups.
The last statistically significant difference was found in number of days on anticoagulant therapy. This difference was expected as warfarin is the standard of care for anticoagulation treatment in patients with cirrhosis. The first DOAC, dabigatran, was not approved by the US Food and Drug Administration until 2010.13 DOACs have only recently been used in patients with cirrhosis accounting for the statistically significant difference in days on anticoagulation therapy between the warfarin and DOAC groups.
Limitations
The inability to meet power or evaluate adherence and appropriate renal dose adjustments for DOACs limited this study. This study was conducted at a single center in a predominantly male veteran population and therefore may not be generalizable to other populations. A majority of patients in the DOAC group were prescribed apixaban (69.1%), which may have affected the overall rate of major bleeding in the DOAC group. Pivotal trials of apixaban have shown a consistent decreased risk of major bleeding in patients with NVAF or VTE when compared with warfarin.14,15 Therefore, the results of this study may not be generalizable to all DOACs.
An inherent limitation of this study was the inability to collect data verifying adherence in the DOAC group. However, in the warfarin group, percentage of time within the therapeutic INR range of 2 to 3 was collected. While not a direct marker of adherence, this does allow for limited evaluation of therapeutic efficacy and safety within the warfarin group. Last, proper dosing of DOACs in patients with and without adequate renal function was not evaluated in this study.
Conclusions
The results of this study are consistent with other retrospective research and literature reviews. There were no statistically significant differences identified between the rates of all-cause bleeding, major bleeding, and failed efficacy between the DOAC and warfarin groups. DOACs may be a safe alternative to warfarin in patients with cirrhosis requiring anticoagulation for NVAF or VTE, but large randomized trials are required to confirm these results.
Coagulation in patients with cirrhosis is a complicated area of evolving research. Patients with cirrhosis were originally thought to be naturally anticoagulated due to the decreased production of clotting factors and platelets, combined with an increased international normalized ratio (INR).1 New data have shown that patients with cirrhosis are at a concomitant risk of bleeding and thrombosis due to increased platelet aggregation, decreased fibrinolysis, and decreased production of natural anticoagulants such as protein C and antithrombin.1 Traditionally, patients with cirrhosis needing anticoagulation therapy for comorbid conditions, such as nonvalvular atrial fibrillation (NVAF) or venous thromboembolism (VTE) were placed on warfarin therapy. Managing warfarin in patients with cirrhosis poses a challenge to clinicians due to the many food and drug interactions, narrow therapeutic index, and complications with maintaining a therapeutic INR.1
Direct oral anticoagulants (DOACs) have several benefits over warfarin therapy, including convenience, decreased monitoring, decreased drug and dietary restrictions, and faster onset of action.2 Conversely, DOACs undergo extensive hepatic metabolism giving rise to concerns about supratherapeutic drug levels and increased bleeding rates in patients with liver dysfunction.1 Consequently, patients with cirrhosis were excluded from the pivotal trials establishing DOACs for NVAF and VTE treatment. Exclusion of these patients in major clinical trials alongside the challenges of managing warfarin warrant an evaluation of the efficacy and safety of DOACs in patients with cirrhosis.
Recent retrospective studies have examined the use of DOACs in patients with cirrhosis and found favorable results. A retrospective chart review by Intagliata and colleagues consisting of 39 patients with cirrhosis using either a DOAC or warfarin found similar rates of all-cause bleeding and major bleeding between the 2 groups.3 A retrospective cohort study by Hum and colleagues consisting of 45 patients with cirrhosis compared the use of DOACs with warfarin or low-molecular weight heparin (LMWH).4 Hum and colleagues found patients prescribed a DOAC had significantly fewer major bleeding events than did patients using warfarin or LMWH.4 The largest retrospective cohort study consisted of 233 patients with chronic liver disease and found no differences among all-cause bleeding and major bleeding rates between patients using DOACs compared with those of patients using warfarin.5
The purpose of this research is to evaluate the safety and efficacy of DOACs in veteran patients with cirrhosis compared with patients using warfarin.
Methods
A retrospective single-center chart review was conducted at the Michael E. DeBakey Veterans Affairs Medical Center (MEDVAMC) in Houston, Texas, between October 31, 2014 and October 31, 2018. Patients included in the study were adults aged ≥ 18 years with a diagnosis of cirrhosis and prescribed any of the following oral anticoagulants: apixaban, dabigatran, edoxaban, rivaroxaban, or warfarin. Patients prescribed apixaban, dabigatran, edoxaban, or rivaroxaban were collectively grouped into the DOAC group, while patients prescribed warfarin were classified as the standard of care comparator group.
A diagnosis of cirrhosis was confirmed using a combination of the codes from the ninth and tenth editions of the International Classification of Diseases (ICD) for cirrhosis, documentation of diagnostic confirmation by clinicians from the gastroenterology or hepatology services, and positive liver biopsy result. Liver function tests, liver ultrasound results, and FibroSure biomarker assays were used to aid in confirming the diagnosis of cirrhosis but were not considered definitive. Patients were excluded from the trial if they had indications for anticoagulation other than NVAF and VTE and/or were prescribed triple antithrombotic therapy (dual antiplatelet therapy plus an anticoagulant). Patients who switched anticoagulant therapy during the trial period (ie, switched from warfarin to a DOAC) were also excluded from the analysis.
Patient demographic characteristics that were collected included weight; body mass index (BMI); etiology of cirrhosis; Child-Turcotte-Pugh, Model for End-Stage Liver Disease (MELD), and CHA2DS2-VASc score; concomitant antiplatelet, nonsteroidal anti-inflammatory drug (NSAID), proton pump inhibitor (PPI), and histamine-2 receptor antagonist
Two patient lists were used to identify patients for inclusion in the warfarin arm. The first patient list was generated using the US Department of Veterans Affairs (VA) Cirrhosis Tracker, which identified patients with an ICD-9/10 code for cirrhosis and an INR laboratory value. Patients generated from the VA Cirrhosis Tracker with an INR > 1.5 were screened for a warfarin prescription and then evaluated for full study inclusion. The second patient list was generated using the VA Advanced Liver Disease Dashboard which identified patients with ICD-9/10 codes for advanced liver disease and an active warfarin prescription. Patients with an active warfarin prescription were then evaluated for full study inclusion. A single patient list was generated to identify patients for inclusion in the DOAC arm. This patient list was generated using the VA DOAC dashboard, which identified patients with an active DOAC prescription and an ICD-9/10 code for cirrhosis. Patients with an ICD-9/10 code for cirrhosis and prescribed a DOAC were screened for full study inclusion. Patient data were collected from the MEDVAMC Computerized Patient Record System (CPRS) electronic health record (EHR). The research study was approved by the Baylor College of Medicine Institutional Review Board and the VA Office of Research and Development.
Outcomes
The primary endpoint for the study was all-cause bleeding. The secondary endpoints for the study were major bleeding and failed efficacy. Major bleeding was defined using the International Society on Thrombosis and Haemostasis (ISTH) 2005 definition: fatal bleeding, symptomatic bleeding in a critical organ area (ie, intracranial, intraspinal, intraocular, retroperitoneal, intraarticular, pericardial, or intramuscular with compartment syndrome), or bleeding causing a fall in hemoglobin level of > 2 g/dL or leading to the transfusion of ≥ 2 units of red cells.6 Failed efficacy was a combination endpoint that included development of VTE, stroke, myocardial infarction (MI), and/or death. A prespecified subgroup analysis was conducted at the end of the study period to analyze trends in the DOAC and warfarin groups with respect to all-cause bleeding. All-cause bleeding risk was stratified by weight, BMI, Child-Turcotte-Pugh score, MELD score, presence of gastric and/or esophageal varices, active malignancies, percentage of time within therapeutic INR range in the warfarin group, indications for anticoagulation, and antiplatelet, NSAID, PPI, and H2RA therapy.
Statistical Analysis
Data were analyzed using descriptive and inferential statistics. Continuous data were analyzed using the Student t test, and categorical data were analyzed using the Fisher exact test. Previous studies determined an all-cause bleeding rate of 10 to 17% for warfarin compared with 5% for DOACs.7,8 To detect a 12% difference in the all-cause bleeding rate between DOACs and warfarin, 212 patients would be needed to achieve 80% power at an α level of 0.05.
Results
A total of 170 patients were screened, and after applying inclusion and exclusion criteria, 79 patients were enrolled in the study (Figure). The DOAC group included 42 patients, and the warfarin group included 37 patients. In the DOAC group, 69.1% (n = 29) of patients were taking apixaban, 21.4% (n = 9) rivaroxaban, and 9.5% (n = 4) dabigatran. There were no patients prescribed edoxaban during the study period.
Baseline characteristics were similar between the 2 groups except for Child-Turcotte-Pugh score, MELD score, mean INR, and number of days on anticoagulation therapy (Table 1). Most of the patients were male (98.7%), and the mean age was 71 years. The most common causes of cirrhosis were viral (29.1%), nonalcoholic fatty liver disease (NAFLD) (24.1%), multiple causes (22.8%), and alcohol (21.5%). Sixty-two patients (78.5%) had a NVAF indication for anticoagulation. The average CHA2DS2-VASc score was 3.7. Aspirin was prescribed in 51.9% (n = 41) of patients, and PPIs were prescribed in 48.1% (n = 38) of patients. At inclusion, esophageal varices were present in 13 patients and active malignancies were present in 6 patients.
Statistically significant differences in baseline characteristics were found between mean INR, Child-Turcotte-Pugh scores, MELD scores, and number of days on anticoagulant therapy. The mean INR was 1.3 in the DOAC group compared with 2.1 in the warfarin group (P = .0001). Eighty-one percent (n = 34) of patients in the DOAC group had a Child-Turcotte-Pugh score of A compared with 43.2% (n = 16) of patients in the warfarin group (P = .0009). Eight patients in the DOAC group had a Child-Turcotte-Pugh score of B compared with 19 patients in the warfarin group (P = .004). The mean MELD score was 9.4 in the DOAC group compared with 16.3 in the warfarin group (P = .0001). The mean days on anticoagulant therapy was 500.4 days for the DOAC group compared with 1,652.4 days for the warfarin group (P = .0001).
Safety Outcome
The primary outcome comparing all-cause bleeding rates between patients on DOACs compared with warfarin are listed in Table 2. With respect to the primary outcome, 7 (16.7%) patients on DOACs experienced a bleeding event compared with 8 (21.6%) patients on warfarin (P = .77). No statistically significant differences were detected between the DOAC and warfarin groups with respect to all-cause bleeding. Seven bleeding events occurred in the DOAC group; 1 met the qualification for major bleeding with a suspected gastrointestinal (GI) bleed.6 The other 6 bleeding episodes in the DOAC group consisted of hematoma, epistaxis, hematuria, and hematochezia. Eight bleeding events occurred in the warfarin group; 2 met the qualification for major bleeding with an intracranial hemorrhage and upper GI bleed.6 The other 6 bleeding episodes in the warfarin group consisted of epistaxis, bleeding gums, hematuria, and hematochezia. There were no statistically significant differences between the rates of major bleeding and nonmajor bleeding between the DOAC and warfarin groups.
Efficacy Outcomes
There were 3 events in the DOAC group and 3 events in the warfarin group (P = .99). In the DOAC group, 2 patients experienced a pulmonary embolism, and 1 patient experienced a MI. In the warfarin group, 3 patients died (end-stage heart failure, unknown cause due to death at an outside hospital, and sepsis/organ failure). There were no statistically significant differences between the composite endpoint of failed efficacy or the individual endpoints of VTE, stroke, MI, and death.
Subgroup Analysis
A prespecified subgroup analysis was conducted to determine risk factors for all-cause bleeding within each treatment group (Table 3). No significant trends were observed in the following risk factors: Child-Turcotte-Pugh score, indication for anticoagulation, use of NSAIDs, PPIs or H2RAs, presence of gastric or esophageal varices, active malignancies, and time within therapeutic INR range in the warfarin group. Patients with bleeding events had slightly increased weight and BMI vs patients without bleeding events. Within the warfarin group, patients with bleeding events had slightly elevated MELD scores compared to patients without bleeding events. There was an equal balance of patients prescribed aspirin therapy between the groups with and without bleeding events. Overall, no significant risk factors were identified for all-cause bleeding.
Discussion
Initially, patients with cirrhosis were excluded from DOAC trials due to concerns for increased bleeding risk with hepatically eliminated medications. New retrospective research has concluded that in patients with cirrhosis, DOACs have similar or lower bleeding rates when compared directly to warfarin.9,10
In this study, no statistically significant differences were detected between the primary and secondary outcomes of all-cause bleeding, major bleeding, or failed efficacy. Subgroup analysis did not identify any significant risk factors with respect to all-cause bleeding among patients in the DOAC and warfarin groups. To meet 80% power, 212 patients needed to be enrolled in the study; however, only 79 patients were enrolled, and power was not met. The results of this study should be interpreted cautiously as hypothesis-generating due to the small sample size. Strengths of this study include similar baseline characteristics between the DOAC and warfarin groups, 4-year length of retrospective data review, and availability of both inpatient and outpatient EHR limiting the amount of missing data points.
Baseline characteristics were similar between the groups except for mean INR, Child-Turcotte-Pugh score, MELD score, and number of days on anticoagulation therapy. The difference in mean INR between groups is expected as patients in the warfarin group have a goal INR of 2 to 3 to maintain therapeutic efficacy and safety. INR is not used as a marker of efficacy or safety with DOACs; therefore, a consistent elevation in INR is not expected. Child- Turcotte-Pugh scores are calculated using INR levels.11 When calculating the score, patients with an INR < 1.7 receive 1 point; patients with an INR between 1.7 and 2.3 receive 2 points.11 Therefore, patients in the warfarin group will have artificially inflated Child-Turcotte-Pugh scores as this group has goal INR levels of 2 to 3. This makes Child-Turcotte-Pugh scores unreliable markers of disease severity in patients using warfarin therapy. When the INR scores for patients prescribed warfarin were replaced with values < 1.7, the statistical difference disappeared between the warfarin and DOAC groups. The same effect is seen on MELD scores for patients prescribed warfarin therapy. The MELD score is calculated using INR levels.12 MELD scores also will be artificially elevated in patients prescribed warfarin therapy due to the INR elevation to between 2 and 3. When MELD scores for patients prescribed warfarin were replaced with values similar to those in the DOAC group, the statistical difference disappeared between the warfarin and DOAC groups.
The last statistically significant difference was found in number of days on anticoagulant therapy. This difference was expected as warfarin is the standard of care for anticoagulation treatment in patients with cirrhosis. The first DOAC, dabigatran, was not approved by the US Food and Drug Administration until 2010.13 DOACs have only recently been used in patients with cirrhosis accounting for the statistically significant difference in days on anticoagulation therapy between the warfarin and DOAC groups.
Limitations
The inability to meet power or evaluate adherence and appropriate renal dose adjustments for DOACs limited this study. This study was conducted at a single center in a predominantly male veteran population and therefore may not be generalizable to other populations. A majority of patients in the DOAC group were prescribed apixaban (69.1%), which may have affected the overall rate of major bleeding in the DOAC group. Pivotal trials of apixaban have shown a consistent decreased risk of major bleeding in patients with NVAF or VTE when compared with warfarin.14,15 Therefore, the results of this study may not be generalizable to all DOACs.
An inherent limitation of this study was the inability to collect data verifying adherence in the DOAC group. However, in the warfarin group, percentage of time within the therapeutic INR range of 2 to 3 was collected. While not a direct marker of adherence, this does allow for limited evaluation of therapeutic efficacy and safety within the warfarin group. Last, proper dosing of DOACs in patients with and without adequate renal function was not evaluated in this study.
Conclusions
The results of this study are consistent with other retrospective research and literature reviews. There were no statistically significant differences identified between the rates of all-cause bleeding, major bleeding, and failed efficacy between the DOAC and warfarin groups. DOACs may be a safe alternative to warfarin in patients with cirrhosis requiring anticoagulation for NVAF or VTE, but large randomized trials are required to confirm these results.
1. Qamar A, Vaduganathan M, Greenberger NJ, Giugliano RP. Oral anticoagulation in patients with liver disease. J Am Coll Cardiol. 2018;71(19):2162-2175. doi:10.1016/j.jacc.2018.03.023
2. Priyanka P, Kupec JT, Krafft M, Shah NA, Reynolds GJ. Newer oral anticoagulants in the treatment of acute portal vein thrombosis in patients with and without cirrhosis. Int J Hepatol. 2018;2018:8432781. Published 2018 Jun 5. doi:10.1155/2018/8432781
3. Intagliata NM, Henry ZH, Maitland H, et al. Direct oral anticoagulants in cirrhosis patients pose similar risks of bleeding when compared to traditional anticoagulation. Dig Dis Sci. 2016;61(6):1721-1727. doi:10.1007/s10620-015-4012-2
4. Hum J, Shatzel JJ, Jou JH, Deloughery TG. The efficacy and safety of direct oral anticoagulants vs traditional anticoagulants in cirrhosis. Eur J Haematol. 2017;98(4):393-397. doi:10.1111/ejh.12844
5. Goriacko P, Veltri KT. Safety of direct oral anticoagulants vs warfarin in patients with chronic liver disease and atrial fibrillation. Eur J Haematol. 2018;100(5):488-493. doi:10.1111/ejh.13045
6. Schulman S, Kearon C; Subcommittee on Control of Anticoagulation of the Scientific and Standardization Committee of the International Society on Thrombosis and Haemostasis. Definition of major bleeding in clinical investigations of antihemostatic medicinal products in non-surgical patients. J Thromb Haemost. 2005;3(4):692-694. doi:10.1111/j.1538-7836.2005.01204.x
7. Rubboli A, Becattini C, Verheugt FW. Incidence, clinical impact and risk of bleeding during oral anticoagulation therapy. World J Cardiol. 2011;3(11):351-358. doi:10.4330/wjc.v3.i11.351
8. Ruff CT, Giugliano RP, Braunwald E, et al. Comparison of the efficacy and safety of new oral anticoagulants with warfarin in patients with atrial fibrillation: a meta-analysis of randomised trials. Lancet. 2014;383(9921):955-962. doi:10.1016/S0140-6736(13)62343-0
9. Hoolwerf EW, Kraaijpoel N, Büller HR, van Es N. Direct oral anticoagulants in patients with liver cirrhosis: A systematic review. Thromb Res. 2018;170:102-108. doi:10.1016/j.thromres.2018.08.011
10. Steuber TD, Howard ML, Nisly SA. Direct oral anticoagulants in chronic liver disease. Ann Pharmacother. 2019;53(10):1042-1049. doi:10.1177/1060028019841582
11. Janevska D, Chaloska-Ivanova V, Janevski V. Hepatocellular carcinoma: risk factors, diagnosis and treatment. Open Access Maced J Med Sci. 2015;3(4):732-736. doi:10.3889/oamjms.2015.111
12. Singal AK, Kamath PS. Model for End-Stage Liver Disease. J Clin Exp Hepatol. 2013;3(1):50-60. doi:10.1016/j.jceh.2012.11.002
13. Joppa SA, Salciccioli J, Adamski J, et al. A practical review of the emerging direct anticoagulants, laboratory monitoring, and reversal agents. J Clin Med. 2018;7(2):29. Published 2018 Feb 11. doi:10.3390/jcm7020029
14. Granger CB, Alexander JH, McMurray JJ, et al. Apixaban versus warfarin in patients with atrial fibrillation. N Engl J Med. 2011;365(11):981-992. doi:10.1056/NEJMoa1107039
15. Agnelli G, Buller HR, Cohen A, et al. Oral apixaban for the treatment of acute venous thromboembolism. N Engl J Med. 2013;369(9):799-808. doi:10.1056/NEJMoa1302507
1. Qamar A, Vaduganathan M, Greenberger NJ, Giugliano RP. Oral anticoagulation in patients with liver disease. J Am Coll Cardiol. 2018;71(19):2162-2175. doi:10.1016/j.jacc.2018.03.023
2. Priyanka P, Kupec JT, Krafft M, Shah NA, Reynolds GJ. Newer oral anticoagulants in the treatment of acute portal vein thrombosis in patients with and without cirrhosis. Int J Hepatol. 2018;2018:8432781. Published 2018 Jun 5. doi:10.1155/2018/8432781
3. Intagliata NM, Henry ZH, Maitland H, et al. Direct oral anticoagulants in cirrhosis patients pose similar risks of bleeding when compared to traditional anticoagulation. Dig Dis Sci. 2016;61(6):1721-1727. doi:10.1007/s10620-015-4012-2
4. Hum J, Shatzel JJ, Jou JH, Deloughery TG. The efficacy and safety of direct oral anticoagulants vs traditional anticoagulants in cirrhosis. Eur J Haematol. 2017;98(4):393-397. doi:10.1111/ejh.12844
5. Goriacko P, Veltri KT. Safety of direct oral anticoagulants vs warfarin in patients with chronic liver disease and atrial fibrillation. Eur J Haematol. 2018;100(5):488-493. doi:10.1111/ejh.13045
6. Schulman S, Kearon C; Subcommittee on Control of Anticoagulation of the Scientific and Standardization Committee of the International Society on Thrombosis and Haemostasis. Definition of major bleeding in clinical investigations of antihemostatic medicinal products in non-surgical patients. J Thromb Haemost. 2005;3(4):692-694. doi:10.1111/j.1538-7836.2005.01204.x
7. Rubboli A, Becattini C, Verheugt FW. Incidence, clinical impact and risk of bleeding during oral anticoagulation therapy. World J Cardiol. 2011;3(11):351-358. doi:10.4330/wjc.v3.i11.351
8. Ruff CT, Giugliano RP, Braunwald E, et al. Comparison of the efficacy and safety of new oral anticoagulants with warfarin in patients with atrial fibrillation: a meta-analysis of randomised trials. Lancet. 2014;383(9921):955-962. doi:10.1016/S0140-6736(13)62343-0
9. Hoolwerf EW, Kraaijpoel N, Büller HR, van Es N. Direct oral anticoagulants in patients with liver cirrhosis: A systematic review. Thromb Res. 2018;170:102-108. doi:10.1016/j.thromres.2018.08.011
10. Steuber TD, Howard ML, Nisly SA. Direct oral anticoagulants in chronic liver disease. Ann Pharmacother. 2019;53(10):1042-1049. doi:10.1177/1060028019841582
11. Janevska D, Chaloska-Ivanova V, Janevski V. Hepatocellular carcinoma: risk factors, diagnosis and treatment. Open Access Maced J Med Sci. 2015;3(4):732-736. doi:10.3889/oamjms.2015.111
12. Singal AK, Kamath PS. Model for End-Stage Liver Disease. J Clin Exp Hepatol. 2013;3(1):50-60. doi:10.1016/j.jceh.2012.11.002
13. Joppa SA, Salciccioli J, Adamski J, et al. A practical review of the emerging direct anticoagulants, laboratory monitoring, and reversal agents. J Clin Med. 2018;7(2):29. Published 2018 Feb 11. doi:10.3390/jcm7020029
14. Granger CB, Alexander JH, McMurray JJ, et al. Apixaban versus warfarin in patients with atrial fibrillation. N Engl J Med. 2011;365(11):981-992. doi:10.1056/NEJMoa1107039
15. Agnelli G, Buller HR, Cohen A, et al. Oral apixaban for the treatment of acute venous thromboembolism. N Engl J Med. 2013;369(9):799-808. doi:10.1056/NEJMoa1302507
Dapagliflozin’s CKD performance sends heart failure messages
The DAPA-CKD trial results, which proved dapagliflozin’s efficacy for slowing chronic kidney disease progression in patients selected for signs of worsening renal function, also have important messages for cardiologists, especially heart failure physicians.
Those messages include findings that were “consistent” with the results of the earlier DAPA-HF trial, which tested the same sodium-glucose transporter 2 (SGLT2) inhibitor in patients selected for having heart failure with reduced ejection fraction (HFrEF). In addition, a specific action of dapagliflozin (Farxiga) on the patients in DAPA-CKD, which enrolled patients based on markers of chronic kidney disease (CKD), was prevention of first and recurrent heart failure hospitalizations, John J.V. McMurray, MD, said at the virtual annual scientific meeting of the Heart Failure Society of America, further highlighting the role that dapagliflozin has in reducing both heart failure and renal events.
What DAPA-CKD means for heart failure
The main findings from the DAPA-CKD trial, published in September in the New England Journal of Medicine, included as a secondary outcome the combined rate of death from cardiovascular causes or hospitalization for heart failure (HHF). Treatment with dapagliflozin linked with a significant 29% relative reduction in this endpoint, compared with placebo-treated patients. At the HFSA meeting, Dr. McMurray reported for the first time the specific HHF numbers, a prespecified secondary endpoint for the study.
Patients on dapagliflozin had 37 total HHF events (1.7%), including both first-time and subsequent hospitalizations, while patients in the placebo arm had a total of 71 HHF events (3.3%) during the study’s median 2.4 years of follow-up, an absolute reduction of 1.6% that translated into a relative risk reduction of 49%.
The HHF findings from DAPA-CKD importantly showed that SGLT2 inhibition in patients with signs of renal dysfunction “will not only slow progression of kidney disease but will also reduce the risk of developing heart failure, crucially in patients with or without type 2 diabetes,” explained Dr. McMurray in an interview. “Cardiologists often consult in the kidney wards and advise on management of patients with chronic kidney disease, even those without heart failure.”
The DAPA-CKD findings carry another important message for heart failure management regarding the minimum level of renal function a patient can have and still safely receive dapagliflozin or possibly another agent from the same SGLT2 inhibitor class. In DAPA-CKD, patients safely received dapagliflozin with an estimated glomerular filtration rate (eGFR) as low as 25 mL/min per 1.73 m2; 14% of enrolled patients had an eGFR of 25-29 mL/min per 1.73 m2.
“Typically, about 40%-50% of patients with heart failure have chronic kidney disease,” which makes this safety finding important to clinicians who care for heart failure patients, but it’s also important for any patient who might be a candidate for dapagliflozin or another drug from its class. “We had no strong evidence before this trial that SGLT2 inhibition could reduce hard renal endpoints,” specifically need for chronic dialysis, renal transplant, or renal death, “in patients with or without diabetes,” Dr. McMurray said.
DAPA-CKD grows the pool of eligible heart failure patients
A further consequence of the DAPA-CKD findings is that when, as expected, regulatory bodies give dapagliflozin an indication for treating the types of CKD patients enrolled in the trial, it will functionally expand this treatment to an even larger swath of heart failure patients who currently don’t qualify for this treatment, specifically patients with CKD who also have heart failure with preserved ejection fraction (HFpEF). On Oct. 2, 2020, the Food and Drug Administration fast-tracked dapagliflozin for the CKD indication by granting it Breakthrough Therapy Designation based on the DAPA-CKD results.
Results first reported in 2019 from the DAPA-HF trial led to dapagliflozin receiving a labeled indication for treating HFrEF, the types of heart failure patients enrolled in the trial. Direct evidence on the efficacy of SGLT2 inhibitors for patients with HFpEF will not be available until results from a few trials now in progress become available during the next 12 months.
In the meantime, nearly half of patients with HFpEF also have CKD, noted Dr. McMurray, and another large portion of HFpEF patients have type 2 diabetes and hence qualify for SGLT2 inhibitor treatment that way. “Obviously, we would like to know specifically about heart failure outcomes in patients with HFpEF” on SGLT2 inhibitor treatment, he acknowledged. But the recent approval of dapagliflozin for patients with HFrEF and the likely indication coming soon for treating CKD means that the number of patients with heart failure who are not eligible for SGLT2 inhibitor treatment is dwindling down to some extent.
New DAPA-HF results show no drug, device interactions
In a separate session at the HFSA virtual meeting, Dr. McMurray and several collaborators on the DAPA-HF trial presented results from some new analyses. Dr. McMurray looked at the impact of dapagliflozin treatment on the primary endpoint when patients were stratified by the diuretic dosage they received at study entry. The results showed that “the benefits from dapagliflozin were irrespective of the use of background diuretic therapy or the diuretic dose,” he reported. Study findings also showed that roughly three-quarters of patients in the study had no change in their diuretic dosage during the course of the trial, that the fraction of patients who had an increase in their dosage was about the same as those whose diuretic dosage decreased, and that this pattern was similar in both the patients on dapagliflozin and in those randomized to placebo.
Another set of new analyses from DAPA-HF looked at the impact on dapagliflozin efficacy of background medical and device therapies for heart failure, as well as background diabetes therapies. The findings showed no signal of an interaction with background therapies. “The effects of dapagliflozin are incremental and complimentary to conventional therapies for HFrEF,” concluded Lars Kober, MD, a professor and heart failure physician at Copenhagen University Hospital.
DAPA-CKD was funded by AstraZeneca, the company that markets dapagliflozin (Farxiga). Dr. McMurray’s employer, Glasgow University, has received payments from AstraZeneca and several other companies to compensate for his time overseeing various clinical trials. Dr. Kober has received honoraria for speaking on behalf of several companies including AstraZeneca.
The DAPA-CKD trial results, which proved dapagliflozin’s efficacy for slowing chronic kidney disease progression in patients selected for signs of worsening renal function, also have important messages for cardiologists, especially heart failure physicians.
Those messages include findings that were “consistent” with the results of the earlier DAPA-HF trial, which tested the same sodium-glucose transporter 2 (SGLT2) inhibitor in patients selected for having heart failure with reduced ejection fraction (HFrEF). In addition, a specific action of dapagliflozin (Farxiga) on the patients in DAPA-CKD, which enrolled patients based on markers of chronic kidney disease (CKD), was prevention of first and recurrent heart failure hospitalizations, John J.V. McMurray, MD, said at the virtual annual scientific meeting of the Heart Failure Society of America, further highlighting the role that dapagliflozin has in reducing both heart failure and renal events.
What DAPA-CKD means for heart failure
The main findings from the DAPA-CKD trial, published in September in the New England Journal of Medicine, included as a secondary outcome the combined rate of death from cardiovascular causes or hospitalization for heart failure (HHF). Treatment with dapagliflozin linked with a significant 29% relative reduction in this endpoint, compared with placebo-treated patients. At the HFSA meeting, Dr. McMurray reported for the first time the specific HHF numbers, a prespecified secondary endpoint for the study.
Patients on dapagliflozin had 37 total HHF events (1.7%), including both first-time and subsequent hospitalizations, while patients in the placebo arm had a total of 71 HHF events (3.3%) during the study’s median 2.4 years of follow-up, an absolute reduction of 1.6% that translated into a relative risk reduction of 49%.
The HHF findings from DAPA-CKD importantly showed that SGLT2 inhibition in patients with signs of renal dysfunction “will not only slow progression of kidney disease but will also reduce the risk of developing heart failure, crucially in patients with or without type 2 diabetes,” explained Dr. McMurray in an interview. “Cardiologists often consult in the kidney wards and advise on management of patients with chronic kidney disease, even those without heart failure.”
The DAPA-CKD findings carry another important message for heart failure management regarding the minimum level of renal function a patient can have and still safely receive dapagliflozin or possibly another agent from the same SGLT2 inhibitor class. In DAPA-CKD, patients safely received dapagliflozin with an estimated glomerular filtration rate (eGFR) as low as 25 mL/min per 1.73 m2; 14% of enrolled patients had an eGFR of 25-29 mL/min per 1.73 m2.
“Typically, about 40%-50% of patients with heart failure have chronic kidney disease,” which makes this safety finding important to clinicians who care for heart failure patients, but it’s also important for any patient who might be a candidate for dapagliflozin or another drug from its class. “We had no strong evidence before this trial that SGLT2 inhibition could reduce hard renal endpoints,” specifically need for chronic dialysis, renal transplant, or renal death, “in patients with or without diabetes,” Dr. McMurray said.
DAPA-CKD grows the pool of eligible heart failure patients
A further consequence of the DAPA-CKD findings is that when, as expected, regulatory bodies give dapagliflozin an indication for treating the types of CKD patients enrolled in the trial, it will functionally expand this treatment to an even larger swath of heart failure patients who currently don’t qualify for this treatment, specifically patients with CKD who also have heart failure with preserved ejection fraction (HFpEF). On Oct. 2, 2020, the Food and Drug Administration fast-tracked dapagliflozin for the CKD indication by granting it Breakthrough Therapy Designation based on the DAPA-CKD results.
Results first reported in 2019 from the DAPA-HF trial led to dapagliflozin receiving a labeled indication for treating HFrEF, the types of heart failure patients enrolled in the trial. Direct evidence on the efficacy of SGLT2 inhibitors for patients with HFpEF will not be available until results from a few trials now in progress become available during the next 12 months.
In the meantime, nearly half of patients with HFpEF also have CKD, noted Dr. McMurray, and another large portion of HFpEF patients have type 2 diabetes and hence qualify for SGLT2 inhibitor treatment that way. “Obviously, we would like to know specifically about heart failure outcomes in patients with HFpEF” on SGLT2 inhibitor treatment, he acknowledged. But the recent approval of dapagliflozin for patients with HFrEF and the likely indication coming soon for treating CKD means that the number of patients with heart failure who are not eligible for SGLT2 inhibitor treatment is dwindling down to some extent.
New DAPA-HF results show no drug, device interactions
In a separate session at the HFSA virtual meeting, Dr. McMurray and several collaborators on the DAPA-HF trial presented results from some new analyses. Dr. McMurray looked at the impact of dapagliflozin treatment on the primary endpoint when patients were stratified by the diuretic dosage they received at study entry. The results showed that “the benefits from dapagliflozin were irrespective of the use of background diuretic therapy or the diuretic dose,” he reported. Study findings also showed that roughly three-quarters of patients in the study had no change in their diuretic dosage during the course of the trial, that the fraction of patients who had an increase in their dosage was about the same as those whose diuretic dosage decreased, and that this pattern was similar in both the patients on dapagliflozin and in those randomized to placebo.
Another set of new analyses from DAPA-HF looked at the impact on dapagliflozin efficacy of background medical and device therapies for heart failure, as well as background diabetes therapies. The findings showed no signal of an interaction with background therapies. “The effects of dapagliflozin are incremental and complimentary to conventional therapies for HFrEF,” concluded Lars Kober, MD, a professor and heart failure physician at Copenhagen University Hospital.
DAPA-CKD was funded by AstraZeneca, the company that markets dapagliflozin (Farxiga). Dr. McMurray’s employer, Glasgow University, has received payments from AstraZeneca and several other companies to compensate for his time overseeing various clinical trials. Dr. Kober has received honoraria for speaking on behalf of several companies including AstraZeneca.
The DAPA-CKD trial results, which proved dapagliflozin’s efficacy for slowing chronic kidney disease progression in patients selected for signs of worsening renal function, also have important messages for cardiologists, especially heart failure physicians.
Those messages include findings that were “consistent” with the results of the earlier DAPA-HF trial, which tested the same sodium-glucose transporter 2 (SGLT2) inhibitor in patients selected for having heart failure with reduced ejection fraction (HFrEF). In addition, a specific action of dapagliflozin (Farxiga) on the patients in DAPA-CKD, which enrolled patients based on markers of chronic kidney disease (CKD), was prevention of first and recurrent heart failure hospitalizations, John J.V. McMurray, MD, said at the virtual annual scientific meeting of the Heart Failure Society of America, further highlighting the role that dapagliflozin has in reducing both heart failure and renal events.
What DAPA-CKD means for heart failure
The main findings from the DAPA-CKD trial, published in September in the New England Journal of Medicine, included as a secondary outcome the combined rate of death from cardiovascular causes or hospitalization for heart failure (HHF). Treatment with dapagliflozin linked with a significant 29% relative reduction in this endpoint, compared with placebo-treated patients. At the HFSA meeting, Dr. McMurray reported for the first time the specific HHF numbers, a prespecified secondary endpoint for the study.
Patients on dapagliflozin had 37 total HHF events (1.7%), including both first-time and subsequent hospitalizations, while patients in the placebo arm had a total of 71 HHF events (3.3%) during the study’s median 2.4 years of follow-up, an absolute reduction of 1.6% that translated into a relative risk reduction of 49%.
The HHF findings from DAPA-CKD importantly showed that SGLT2 inhibition in patients with signs of renal dysfunction “will not only slow progression of kidney disease but will also reduce the risk of developing heart failure, crucially in patients with or without type 2 diabetes,” explained Dr. McMurray in an interview. “Cardiologists often consult in the kidney wards and advise on management of patients with chronic kidney disease, even those without heart failure.”
The DAPA-CKD findings carry another important message for heart failure management regarding the minimum level of renal function a patient can have and still safely receive dapagliflozin or possibly another agent from the same SGLT2 inhibitor class. In DAPA-CKD, patients safely received dapagliflozin with an estimated glomerular filtration rate (eGFR) as low as 25 mL/min per 1.73 m2; 14% of enrolled patients had an eGFR of 25-29 mL/min per 1.73 m2.
“Typically, about 40%-50% of patients with heart failure have chronic kidney disease,” which makes this safety finding important to clinicians who care for heart failure patients, but it’s also important for any patient who might be a candidate for dapagliflozin or another drug from its class. “We had no strong evidence before this trial that SGLT2 inhibition could reduce hard renal endpoints,” specifically need for chronic dialysis, renal transplant, or renal death, “in patients with or without diabetes,” Dr. McMurray said.
DAPA-CKD grows the pool of eligible heart failure patients
A further consequence of the DAPA-CKD findings is that when, as expected, regulatory bodies give dapagliflozin an indication for treating the types of CKD patients enrolled in the trial, it will functionally expand this treatment to an even larger swath of heart failure patients who currently don’t qualify for this treatment, specifically patients with CKD who also have heart failure with preserved ejection fraction (HFpEF). On Oct. 2, 2020, the Food and Drug Administration fast-tracked dapagliflozin for the CKD indication by granting it Breakthrough Therapy Designation based on the DAPA-CKD results.
Results first reported in 2019 from the DAPA-HF trial led to dapagliflozin receiving a labeled indication for treating HFrEF, the types of heart failure patients enrolled in the trial. Direct evidence on the efficacy of SGLT2 inhibitors for patients with HFpEF will not be available until results from a few trials now in progress become available during the next 12 months.
In the meantime, nearly half of patients with HFpEF also have CKD, noted Dr. McMurray, and another large portion of HFpEF patients have type 2 diabetes and hence qualify for SGLT2 inhibitor treatment that way. “Obviously, we would like to know specifically about heart failure outcomes in patients with HFpEF” on SGLT2 inhibitor treatment, he acknowledged. But the recent approval of dapagliflozin for patients with HFrEF and the likely indication coming soon for treating CKD means that the number of patients with heart failure who are not eligible for SGLT2 inhibitor treatment is dwindling down to some extent.
New DAPA-HF results show no drug, device interactions
In a separate session at the HFSA virtual meeting, Dr. McMurray and several collaborators on the DAPA-HF trial presented results from some new analyses. Dr. McMurray looked at the impact of dapagliflozin treatment on the primary endpoint when patients were stratified by the diuretic dosage they received at study entry. The results showed that “the benefits from dapagliflozin were irrespective of the use of background diuretic therapy or the diuretic dose,” he reported. Study findings also showed that roughly three-quarters of patients in the study had no change in their diuretic dosage during the course of the trial, that the fraction of patients who had an increase in their dosage was about the same as those whose diuretic dosage decreased, and that this pattern was similar in both the patients on dapagliflozin and in those randomized to placebo.
Another set of new analyses from DAPA-HF looked at the impact on dapagliflozin efficacy of background medical and device therapies for heart failure, as well as background diabetes therapies. The findings showed no signal of an interaction with background therapies. “The effects of dapagliflozin are incremental and complimentary to conventional therapies for HFrEF,” concluded Lars Kober, MD, a professor and heart failure physician at Copenhagen University Hospital.
DAPA-CKD was funded by AstraZeneca, the company that markets dapagliflozin (Farxiga). Dr. McMurray’s employer, Glasgow University, has received payments from AstraZeneca and several other companies to compensate for his time overseeing various clinical trials. Dr. Kober has received honoraria for speaking on behalf of several companies including AstraZeneca.
FROM HFSA 2020