User login
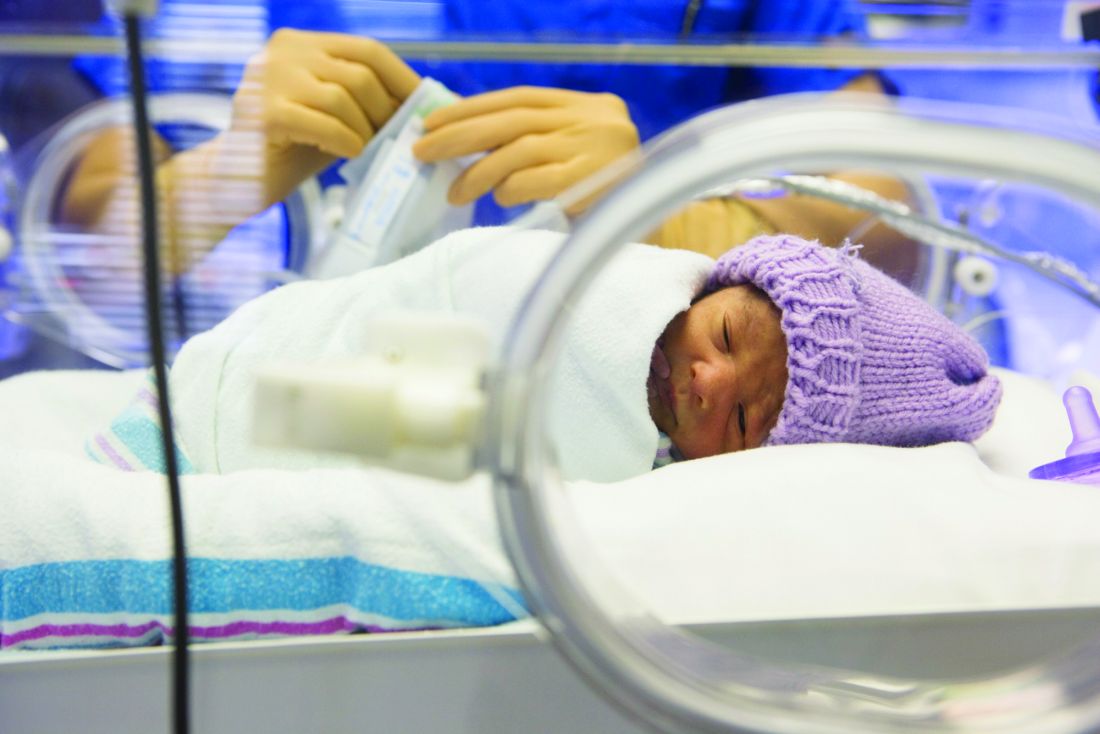
Axillary thermometry is the best choice for newborns
NASHVILLE, TENN. – Axillary thermometry outperformed both rectal and temporal artery thermometry in 205 newborns aged 12-72 hours in a study performed at the University of North Carolina at Chapel Hill.
The infants had two temperatures taken by each method over a period of 15 minutes, for a total of six readings per child and 1,230 measurements overall. Axillary thermometry proved both accurate and reliable. Rectal thermometry was accurate but less reliable, and temporal thermometry was reliable but less accurate.
Lead investigator Ketan Nadkarni, MD, a 3rd-year pediatrics resident, and his colleagues wanted to compare the three methods head-to-head to make sure axillary thermometers were okay to use in the nursery, and to see if it really was necessary to tell parents to use rectal thermometers; many are reluctant to use them. Plus, “there’s been a lot of controversy” in pediatrics “over the best way to measure temperature,” Dr. Nadkarni said at the Pediatric Hospital Medicine annual meeting.
“With our data, we think axillary is what we should continue to use in the newborn nursery,” he said. Some attending physicians still are hesitant to recommend axillary thermometers to new parents, but “all of the nurses are aware of” the study findings “and a lot of the residents are, too, so I think we are starting to move” in that direction.
The study had some unexpected findings as well: “The biggest surprise was how wide the distribution of rectal temperatures was. The distribution” around the mean “was way larger than we had thought, so [rectal thermometry was] not very reliable at all. Our study surprisingly exhibited suboptimal performance in terms of reliability,” for rectal thermometry, he said at the meeting, which was sponsored by the Society of Hospital Medicine, the American Academy of Pediatrics, and the Academic Pediatric Association.
Specifically, the average distance of any given rectal measurement from the mean rectal temperature of 98.3º F was 0.45º F. The second rectal temperature in the study sometimes varied a half a degree or more from the first taken shortly before, in the same infant.
The average distance of an axillary temperature from the axillary mean of 98.32º F was 0.32º F; for temporal thermometry it was 0.34º F from a mean of 98.55º F.
Another surprise was that temporal thermometry overestimated temperature by an average of about a quarter of a degree, compared with rectal readings. Even small overestimates could lead to unnecessary sepsis work-ups; “the last thing we want is to hospitalize these kids when they don’t need to be,” Dr. Nadkarni said.
The mean axillary and rectal temperatures, meanwhile, were only 0.02º F apart, which was not statistically significant. “Axillary was absolutely interchangeable with rectal in terms of accuracy,” he said.
The children were born at 37 weeks’ gestation or later, and were excluded if they had a temperature of 100.4º F or higher by any method. Rectal and axillary temperatures were taken with a Welch Allyn SureTemp Plus 690. Temple temperatures were taken with an Exergen TAT-2000c.
The investigators plan to run a similar trial in the ED with children up to 3 months old.
There was no external funding for the work, and Dr. Nadkarni had no relevant financial disclosures.
NASHVILLE, TENN. – Axillary thermometry outperformed both rectal and temporal artery thermometry in 205 newborns aged 12-72 hours in a study performed at the University of North Carolina at Chapel Hill.
The infants had two temperatures taken by each method over a period of 15 minutes, for a total of six readings per child and 1,230 measurements overall. Axillary thermometry proved both accurate and reliable. Rectal thermometry was accurate but less reliable, and temporal thermometry was reliable but less accurate.
Lead investigator Ketan Nadkarni, MD, a 3rd-year pediatrics resident, and his colleagues wanted to compare the three methods head-to-head to make sure axillary thermometers were okay to use in the nursery, and to see if it really was necessary to tell parents to use rectal thermometers; many are reluctant to use them. Plus, “there’s been a lot of controversy” in pediatrics “over the best way to measure temperature,” Dr. Nadkarni said at the Pediatric Hospital Medicine annual meeting.
“With our data, we think axillary is what we should continue to use in the newborn nursery,” he said. Some attending physicians still are hesitant to recommend axillary thermometers to new parents, but “all of the nurses are aware of” the study findings “and a lot of the residents are, too, so I think we are starting to move” in that direction.
The study had some unexpected findings as well: “The biggest surprise was how wide the distribution of rectal temperatures was. The distribution” around the mean “was way larger than we had thought, so [rectal thermometry was] not very reliable at all. Our study surprisingly exhibited suboptimal performance in terms of reliability,” for rectal thermometry, he said at the meeting, which was sponsored by the Society of Hospital Medicine, the American Academy of Pediatrics, and the Academic Pediatric Association.
Specifically, the average distance of any given rectal measurement from the mean rectal temperature of 98.3º F was 0.45º F. The second rectal temperature in the study sometimes varied a half a degree or more from the first taken shortly before, in the same infant.
The average distance of an axillary temperature from the axillary mean of 98.32º F was 0.32º F; for temporal thermometry it was 0.34º F from a mean of 98.55º F.
Another surprise was that temporal thermometry overestimated temperature by an average of about a quarter of a degree, compared with rectal readings. Even small overestimates could lead to unnecessary sepsis work-ups; “the last thing we want is to hospitalize these kids when they don’t need to be,” Dr. Nadkarni said.
The mean axillary and rectal temperatures, meanwhile, were only 0.02º F apart, which was not statistically significant. “Axillary was absolutely interchangeable with rectal in terms of accuracy,” he said.
The children were born at 37 weeks’ gestation or later, and were excluded if they had a temperature of 100.4º F or higher by any method. Rectal and axillary temperatures were taken with a Welch Allyn SureTemp Plus 690. Temple temperatures were taken with an Exergen TAT-2000c.
The investigators plan to run a similar trial in the ED with children up to 3 months old.
There was no external funding for the work, and Dr. Nadkarni had no relevant financial disclosures.
NASHVILLE, TENN. – Axillary thermometry outperformed both rectal and temporal artery thermometry in 205 newborns aged 12-72 hours in a study performed at the University of North Carolina at Chapel Hill.
The infants had two temperatures taken by each method over a period of 15 minutes, for a total of six readings per child and 1,230 measurements overall. Axillary thermometry proved both accurate and reliable. Rectal thermometry was accurate but less reliable, and temporal thermometry was reliable but less accurate.
Lead investigator Ketan Nadkarni, MD, a 3rd-year pediatrics resident, and his colleagues wanted to compare the three methods head-to-head to make sure axillary thermometers were okay to use in the nursery, and to see if it really was necessary to tell parents to use rectal thermometers; many are reluctant to use them. Plus, “there’s been a lot of controversy” in pediatrics “over the best way to measure temperature,” Dr. Nadkarni said at the Pediatric Hospital Medicine annual meeting.
“With our data, we think axillary is what we should continue to use in the newborn nursery,” he said. Some attending physicians still are hesitant to recommend axillary thermometers to new parents, but “all of the nurses are aware of” the study findings “and a lot of the residents are, too, so I think we are starting to move” in that direction.
The study had some unexpected findings as well: “The biggest surprise was how wide the distribution of rectal temperatures was. The distribution” around the mean “was way larger than we had thought, so [rectal thermometry was] not very reliable at all. Our study surprisingly exhibited suboptimal performance in terms of reliability,” for rectal thermometry, he said at the meeting, which was sponsored by the Society of Hospital Medicine, the American Academy of Pediatrics, and the Academic Pediatric Association.
Specifically, the average distance of any given rectal measurement from the mean rectal temperature of 98.3º F was 0.45º F. The second rectal temperature in the study sometimes varied a half a degree or more from the first taken shortly before, in the same infant.
The average distance of an axillary temperature from the axillary mean of 98.32º F was 0.32º F; for temporal thermometry it was 0.34º F from a mean of 98.55º F.
Another surprise was that temporal thermometry overestimated temperature by an average of about a quarter of a degree, compared with rectal readings. Even small overestimates could lead to unnecessary sepsis work-ups; “the last thing we want is to hospitalize these kids when they don’t need to be,” Dr. Nadkarni said.
The mean axillary and rectal temperatures, meanwhile, were only 0.02º F apart, which was not statistically significant. “Axillary was absolutely interchangeable with rectal in terms of accuracy,” he said.
The children were born at 37 weeks’ gestation or later, and were excluded if they had a temperature of 100.4º F or higher by any method. Rectal and axillary temperatures were taken with a Welch Allyn SureTemp Plus 690. Temple temperatures were taken with an Exergen TAT-2000c.
The investigators plan to run a similar trial in the ED with children up to 3 months old.
There was no external funding for the work, and Dr. Nadkarni had no relevant financial disclosures.
AT PHM 2017
Key clinical point:
Major finding: The average distance of an axillary temperature from the axillary mean of 98.32º F was only 0.32º F, while the average distance of any given rectal measurement from the mean rectal temperature of 98.3º F was 0.45º F, and for temporal thermometry it was 0.34º F from a mean of 98.55º F.
Data source: Head-to-head thermometry study in more than 200 infants aged 12-72 hours.
Disclosures: There was no outside funding, and Dr. Nadkarni had no relevant financial disclosures.
Cerebral palsy rate down in children born very or moderately preterm
, even in children who were born moderately preterm.
The researchers gathered data for cerebral palsy from the medical questionnaire, including information on head control, sitting, standing, walking, and quality of gait; trunk and limb tone; and any abnormal neurologic signs. Development was assessed using the second version of the 24-month Ages and Stages Questionnaire (ASQ), which is completed by parents.
Of 4,199 neonates born between 22 and 34 weeks’ gestation in 2011 enrolled in the EPIPAGE-2 study who lived until a median of 24.2 months corrected age, the rate of cerebral palsy dropped from 7% to 4% in those born between 24-26 and 27-31 weeks’ gestation, reported Véronique Pierrat, MD, PhD, of the Epidemiology and Biostatistics Sorbonne Paris Cité Research Center, INSERM, in Paris, and her associates. At 32-34 weeks’ gestation, the cerebral palsy rate was 1%. Only one child born at 22-23 weeks’ gestation lived beyond the neonatal period. Fewer than 1% of the children overall had severe auditory or visual impairment.
ASQ analysis was considered for 2,506 children, after excluding children with cerebral palsy, deafness or blindness, or severe congenital brain malformations. ASQ scores were below threshold in 50%, 41%, and 36% of children born at 24-26, 27-31, and 32-34 weeks’ gestation, respectively. “The domains most frequently scoring below threshold were communication and personal-social in all gestational age groups. Proportions of children scoring below the threshold in either of these domains decreased with increasing gestational age but still were 18% and 13%, respectively, at 32-34 weeks’ gestation,” the investigators said.
Only 1% or fewer of the children in this cohort had severe gastrointestinal or respiratory disabilities.
In a comparison of 1997 data and this 2011 data after adjustment for baseline characteristics in children born at 22-31 weeks’ gestation, survival increased by a mean 6% , and survival without neuromotor or sensory impairment by 7%; in children born at 24-31 weeks’ gestation, cerebral palsy decreased by a mean 3%. No statistically significant changes were found between the two periods for survival, survival without neuromotor or sensory disabilities, and rates of cerebral palsy in children born at 24 weeks’ gestation, but “noticeable improvements were seen at 25-26 weeks and, to a lesser extent, at 27-31 weeks,” Dr. Pierrat and her associates said. At 32-34 weeks’ gestation, the cerebral palsy rate dropped by 3%, but survival and survival without severe neuromotor or sensory impairment were similar between the two time periods.
In regard to the 2011 data, “the proportion of infants at risk of developmental delay was high, even for those born at 32-34 weeks’ gestation. Our results invite questioning perinatal strategies in France, and in countries with similar recommendations. However, improving outcomes at extremely low gestational age requires a complex change in philosophy of care and close cooperation not only between obstetricians and neonatologists, but also developmental specialists, parent associations, and policy makers,” Dr. Pierrat and her associates concluded.
The investigators said they had no relevant financial disclosures. The study was funded by the French Institute of Public Health Research/Institute of Public Health and several of its partners, the PREMUP Foundation, Fondation de France, and Fondation pour la Recherche Médicale.
, even in children who were born moderately preterm.
The researchers gathered data for cerebral palsy from the medical questionnaire, including information on head control, sitting, standing, walking, and quality of gait; trunk and limb tone; and any abnormal neurologic signs. Development was assessed using the second version of the 24-month Ages and Stages Questionnaire (ASQ), which is completed by parents.
Of 4,199 neonates born between 22 and 34 weeks’ gestation in 2011 enrolled in the EPIPAGE-2 study who lived until a median of 24.2 months corrected age, the rate of cerebral palsy dropped from 7% to 4% in those born between 24-26 and 27-31 weeks’ gestation, reported Véronique Pierrat, MD, PhD, of the Epidemiology and Biostatistics Sorbonne Paris Cité Research Center, INSERM, in Paris, and her associates. At 32-34 weeks’ gestation, the cerebral palsy rate was 1%. Only one child born at 22-23 weeks’ gestation lived beyond the neonatal period. Fewer than 1% of the children overall had severe auditory or visual impairment.
ASQ analysis was considered for 2,506 children, after excluding children with cerebral palsy, deafness or blindness, or severe congenital brain malformations. ASQ scores were below threshold in 50%, 41%, and 36% of children born at 24-26, 27-31, and 32-34 weeks’ gestation, respectively. “The domains most frequently scoring below threshold were communication and personal-social in all gestational age groups. Proportions of children scoring below the threshold in either of these domains decreased with increasing gestational age but still were 18% and 13%, respectively, at 32-34 weeks’ gestation,” the investigators said.
Only 1% or fewer of the children in this cohort had severe gastrointestinal or respiratory disabilities.
In a comparison of 1997 data and this 2011 data after adjustment for baseline characteristics in children born at 22-31 weeks’ gestation, survival increased by a mean 6% , and survival without neuromotor or sensory impairment by 7%; in children born at 24-31 weeks’ gestation, cerebral palsy decreased by a mean 3%. No statistically significant changes were found between the two periods for survival, survival without neuromotor or sensory disabilities, and rates of cerebral palsy in children born at 24 weeks’ gestation, but “noticeable improvements were seen at 25-26 weeks and, to a lesser extent, at 27-31 weeks,” Dr. Pierrat and her associates said. At 32-34 weeks’ gestation, the cerebral palsy rate dropped by 3%, but survival and survival without severe neuromotor or sensory impairment were similar between the two time periods.
In regard to the 2011 data, “the proportion of infants at risk of developmental delay was high, even for those born at 32-34 weeks’ gestation. Our results invite questioning perinatal strategies in France, and in countries with similar recommendations. However, improving outcomes at extremely low gestational age requires a complex change in philosophy of care and close cooperation not only between obstetricians and neonatologists, but also developmental specialists, parent associations, and policy makers,” Dr. Pierrat and her associates concluded.
The investigators said they had no relevant financial disclosures. The study was funded by the French Institute of Public Health Research/Institute of Public Health and several of its partners, the PREMUP Foundation, Fondation de France, and Fondation pour la Recherche Médicale.
, even in children who were born moderately preterm.
The researchers gathered data for cerebral palsy from the medical questionnaire, including information on head control, sitting, standing, walking, and quality of gait; trunk and limb tone; and any abnormal neurologic signs. Development was assessed using the second version of the 24-month Ages and Stages Questionnaire (ASQ), which is completed by parents.
Of 4,199 neonates born between 22 and 34 weeks’ gestation in 2011 enrolled in the EPIPAGE-2 study who lived until a median of 24.2 months corrected age, the rate of cerebral palsy dropped from 7% to 4% in those born between 24-26 and 27-31 weeks’ gestation, reported Véronique Pierrat, MD, PhD, of the Epidemiology and Biostatistics Sorbonne Paris Cité Research Center, INSERM, in Paris, and her associates. At 32-34 weeks’ gestation, the cerebral palsy rate was 1%. Only one child born at 22-23 weeks’ gestation lived beyond the neonatal period. Fewer than 1% of the children overall had severe auditory or visual impairment.
ASQ analysis was considered for 2,506 children, after excluding children with cerebral palsy, deafness or blindness, or severe congenital brain malformations. ASQ scores were below threshold in 50%, 41%, and 36% of children born at 24-26, 27-31, and 32-34 weeks’ gestation, respectively. “The domains most frequently scoring below threshold were communication and personal-social in all gestational age groups. Proportions of children scoring below the threshold in either of these domains decreased with increasing gestational age but still were 18% and 13%, respectively, at 32-34 weeks’ gestation,” the investigators said.
Only 1% or fewer of the children in this cohort had severe gastrointestinal or respiratory disabilities.
In a comparison of 1997 data and this 2011 data after adjustment for baseline characteristics in children born at 22-31 weeks’ gestation, survival increased by a mean 6% , and survival without neuromotor or sensory impairment by 7%; in children born at 24-31 weeks’ gestation, cerebral palsy decreased by a mean 3%. No statistically significant changes were found between the two periods for survival, survival without neuromotor or sensory disabilities, and rates of cerebral palsy in children born at 24 weeks’ gestation, but “noticeable improvements were seen at 25-26 weeks and, to a lesser extent, at 27-31 weeks,” Dr. Pierrat and her associates said. At 32-34 weeks’ gestation, the cerebral palsy rate dropped by 3%, but survival and survival without severe neuromotor or sensory impairment were similar between the two time periods.
In regard to the 2011 data, “the proportion of infants at risk of developmental delay was high, even for those born at 32-34 weeks’ gestation. Our results invite questioning perinatal strategies in France, and in countries with similar recommendations. However, improving outcomes at extremely low gestational age requires a complex change in philosophy of care and close cooperation not only between obstetricians and neonatologists, but also developmental specialists, parent associations, and policy makers,” Dr. Pierrat and her associates concluded.
The investigators said they had no relevant financial disclosures. The study was funded by the French Institute of Public Health Research/Institute of Public Health and several of its partners, the PREMUP Foundation, Fondation de France, and Fondation pour la Recherche Médicale.
FROM BMJ
Key clinical point: Cerebral palsy rates are down in children born very and moderately preterm, but the risk of developmental delay remains high.
Major finding: ASQ scores were below threshold in 50%, 41%, and 36% of children born at 24-26, 27-31, and 32-34 weeks’ gestation, respectively.
Data source: A national population study of 4,199 French neonates born between 22 and 34 weeks’ gestation in 2011.
Disclosures: The investigators said they had no relevant financial disclosures. The study was funded by the French Institute of Public Health Research/Institute of Public Health and several of its partners, the PREMUP Foundation, Fondation de France, and Fondation pour la Recherche Médicale.
Recommendations for infant sleep position are not being followed
Despite the American Academy of Pediatrics recommendation that parents place infants supine for sleeping, many mothers do not do so, according to the results of the Study of Attitudes and Factors Affecting Infant Care.
In 32 hospitals, 3,297 mothers were surveyed to determine their intentions, the actual infant sleeping position used, and the factors that affected their behaviors. Of those, 2,491 (77%) reported that they usually place their infants in supine position, but only 49% reported that they exclusively place their infants supine. In addition, 14% reported that they place infants on their sides and 8% prone, reported Eve R. Colson, MD, of the department of pediatrics at Yale University, New Haven, Conn., and her coauthors (Pediatrics. 2017. doi: 10.1542/peds.2017-0596).
Several other discrepancies in compliance with the AAP recommendation also were noted. African American mothers were more likely to intend to use prone position, compared with white mothers (adjusted odds ratio, 2.5; 95% confidence interval, 1.57-3.85). Those who did not complete high school were also more likely to intend to use the prone position (aOR, 2.1; 95% CI, 1.16-3.73). On the other hand, those who received recommendation-compliant advice from a doctor were less likely to place their infants in the prone (aOR, 0.6; 95% CI, 0.39-0.93) or side (aOR, 0.5; 95% CI, 0.36-0.67) positions.
“Of particular note, those who reported that their social norms supported placing the infant in the prone position were much more likely to do so, compared with those who felt that their social norms supported using only the supine position (aOR, 11.6; 95% CI, 7.24-18.7). And, most remarkably, those who had positive attitudes about the prone sleep position ... were more likely to choose the prone position (aOR, 130; 95% CI, 71.8-236),” the researchers wrote. These findings indicate that choices in infant sleeping position are directly influenced by attitudes toward the choice, subjective social norms, and perceptions about control.
“These beliefs persist and are potentially modifiable, so they should be considered an important part of any intervention to change practice,” Dr. Colson and her colleagues wrote.
The study was a nationally representative sample of mothers of infants aged 2-6 months. Although the data were taken from patient surveys, which could have been misreported, they are supported by the findings of other studies.
[polldaddy:{"method":"iframe","type":"survey","src":"//newspolls2017.polldaddy.com/s/recommendations-for-infant-sleep-position-are-not-being-followed?iframe=1"}]
“Maternal race and education continue to be factors associated with choice of infant sleeping position as does advice from a doctor. Factors that appear to be of equal or greater importance are those related to attitudes, subjective social norms, and perceived control, all of which can potentially be altered through educational interventions,” Dr. Colson and her colleagues concluded.
The Eunice Kennedy Shriver National Institute of Child Health and Human Development and the National Institutes of Health funded the study. The authors reported no financial disclosures.
Over the 10 years spanning 1994-2004, the sudden infant death syndrome rate in the United States fell by 53%, correlating with an increase in exclusive supine sleep from less than 10% to 78%. However, since then, rates of both supine sleep and SIDS death have remained stagnant.
To make progress in these areas, current data are needed on supine sleep to enhance understanding of how families make these decisions. Colson et al. provide exactly the kind of information we need to guide providers and public health officials in their efforts to help families maintain the safest sleep environments for their infants.
As a start, mothers who want to practice safe sleep need to be empowered to insist that other caregivers in their lives support their parenting decisions, because the study shows that mothers who feel that they have more control are more likely to use the recommended position. We also must look at how we can help change personal attitudes and societal norms in favor of supine sleep because these issues were found to be some of the strongest predictors of prone sleep position.
We, as health care providers, need to provide clear and consistent messaging in both word and behavior to help mothers make safe decisions for their infants.
Michael H. Goodstein, MD, is a neonatologist at WellSpan York (Pa.) Hospital. Barbara M. Ostfeld, PhD, is the program director of the SIDS Center of New Jersey at Rutgers University, New Brunswick. Their remarks accompanied the article by Colson et al. (Pediatrics 2017 Aug 21. doi: 10.1542/peds.2017-2068). Neither author reported any financial disclosures.
Over the 10 years spanning 1994-2004, the sudden infant death syndrome rate in the United States fell by 53%, correlating with an increase in exclusive supine sleep from less than 10% to 78%. However, since then, rates of both supine sleep and SIDS death have remained stagnant.
To make progress in these areas, current data are needed on supine sleep to enhance understanding of how families make these decisions. Colson et al. provide exactly the kind of information we need to guide providers and public health officials in their efforts to help families maintain the safest sleep environments for their infants.
As a start, mothers who want to practice safe sleep need to be empowered to insist that other caregivers in their lives support their parenting decisions, because the study shows that mothers who feel that they have more control are more likely to use the recommended position. We also must look at how we can help change personal attitudes and societal norms in favor of supine sleep because these issues were found to be some of the strongest predictors of prone sleep position.
We, as health care providers, need to provide clear and consistent messaging in both word and behavior to help mothers make safe decisions for their infants.
Michael H. Goodstein, MD, is a neonatologist at WellSpan York (Pa.) Hospital. Barbara M. Ostfeld, PhD, is the program director of the SIDS Center of New Jersey at Rutgers University, New Brunswick. Their remarks accompanied the article by Colson et al. (Pediatrics 2017 Aug 21. doi: 10.1542/peds.2017-2068). Neither author reported any financial disclosures.
Over the 10 years spanning 1994-2004, the sudden infant death syndrome rate in the United States fell by 53%, correlating with an increase in exclusive supine sleep from less than 10% to 78%. However, since then, rates of both supine sleep and SIDS death have remained stagnant.
To make progress in these areas, current data are needed on supine sleep to enhance understanding of how families make these decisions. Colson et al. provide exactly the kind of information we need to guide providers and public health officials in their efforts to help families maintain the safest sleep environments for their infants.
As a start, mothers who want to practice safe sleep need to be empowered to insist that other caregivers in their lives support their parenting decisions, because the study shows that mothers who feel that they have more control are more likely to use the recommended position. We also must look at how we can help change personal attitudes and societal norms in favor of supine sleep because these issues were found to be some of the strongest predictors of prone sleep position.
We, as health care providers, need to provide clear and consistent messaging in both word and behavior to help mothers make safe decisions for their infants.
Michael H. Goodstein, MD, is a neonatologist at WellSpan York (Pa.) Hospital. Barbara M. Ostfeld, PhD, is the program director of the SIDS Center of New Jersey at Rutgers University, New Brunswick. Their remarks accompanied the article by Colson et al. (Pediatrics 2017 Aug 21. doi: 10.1542/peds.2017-2068). Neither author reported any financial disclosures.
Despite the American Academy of Pediatrics recommendation that parents place infants supine for sleeping, many mothers do not do so, according to the results of the Study of Attitudes and Factors Affecting Infant Care.
In 32 hospitals, 3,297 mothers were surveyed to determine their intentions, the actual infant sleeping position used, and the factors that affected their behaviors. Of those, 2,491 (77%) reported that they usually place their infants in supine position, but only 49% reported that they exclusively place their infants supine. In addition, 14% reported that they place infants on their sides and 8% prone, reported Eve R. Colson, MD, of the department of pediatrics at Yale University, New Haven, Conn., and her coauthors (Pediatrics. 2017. doi: 10.1542/peds.2017-0596).
Several other discrepancies in compliance with the AAP recommendation also were noted. African American mothers were more likely to intend to use prone position, compared with white mothers (adjusted odds ratio, 2.5; 95% confidence interval, 1.57-3.85). Those who did not complete high school were also more likely to intend to use the prone position (aOR, 2.1; 95% CI, 1.16-3.73). On the other hand, those who received recommendation-compliant advice from a doctor were less likely to place their infants in the prone (aOR, 0.6; 95% CI, 0.39-0.93) or side (aOR, 0.5; 95% CI, 0.36-0.67) positions.
“Of particular note, those who reported that their social norms supported placing the infant in the prone position were much more likely to do so, compared with those who felt that their social norms supported using only the supine position (aOR, 11.6; 95% CI, 7.24-18.7). And, most remarkably, those who had positive attitudes about the prone sleep position ... were more likely to choose the prone position (aOR, 130; 95% CI, 71.8-236),” the researchers wrote. These findings indicate that choices in infant sleeping position are directly influenced by attitudes toward the choice, subjective social norms, and perceptions about control.
“These beliefs persist and are potentially modifiable, so they should be considered an important part of any intervention to change practice,” Dr. Colson and her colleagues wrote.
The study was a nationally representative sample of mothers of infants aged 2-6 months. Although the data were taken from patient surveys, which could have been misreported, they are supported by the findings of other studies.
[polldaddy:{"method":"iframe","type":"survey","src":"//newspolls2017.polldaddy.com/s/recommendations-for-infant-sleep-position-are-not-being-followed?iframe=1"}]
“Maternal race and education continue to be factors associated with choice of infant sleeping position as does advice from a doctor. Factors that appear to be of equal or greater importance are those related to attitudes, subjective social norms, and perceived control, all of which can potentially be altered through educational interventions,” Dr. Colson and her colleagues concluded.
The Eunice Kennedy Shriver National Institute of Child Health and Human Development and the National Institutes of Health funded the study. The authors reported no financial disclosures.
Despite the American Academy of Pediatrics recommendation that parents place infants supine for sleeping, many mothers do not do so, according to the results of the Study of Attitudes and Factors Affecting Infant Care.
In 32 hospitals, 3,297 mothers were surveyed to determine their intentions, the actual infant sleeping position used, and the factors that affected their behaviors. Of those, 2,491 (77%) reported that they usually place their infants in supine position, but only 49% reported that they exclusively place their infants supine. In addition, 14% reported that they place infants on their sides and 8% prone, reported Eve R. Colson, MD, of the department of pediatrics at Yale University, New Haven, Conn., and her coauthors (Pediatrics. 2017. doi: 10.1542/peds.2017-0596).
Several other discrepancies in compliance with the AAP recommendation also were noted. African American mothers were more likely to intend to use prone position, compared with white mothers (adjusted odds ratio, 2.5; 95% confidence interval, 1.57-3.85). Those who did not complete high school were also more likely to intend to use the prone position (aOR, 2.1; 95% CI, 1.16-3.73). On the other hand, those who received recommendation-compliant advice from a doctor were less likely to place their infants in the prone (aOR, 0.6; 95% CI, 0.39-0.93) or side (aOR, 0.5; 95% CI, 0.36-0.67) positions.
“Of particular note, those who reported that their social norms supported placing the infant in the prone position were much more likely to do so, compared with those who felt that their social norms supported using only the supine position (aOR, 11.6; 95% CI, 7.24-18.7). And, most remarkably, those who had positive attitudes about the prone sleep position ... were more likely to choose the prone position (aOR, 130; 95% CI, 71.8-236),” the researchers wrote. These findings indicate that choices in infant sleeping position are directly influenced by attitudes toward the choice, subjective social norms, and perceptions about control.
“These beliefs persist and are potentially modifiable, so they should be considered an important part of any intervention to change practice,” Dr. Colson and her colleagues wrote.
The study was a nationally representative sample of mothers of infants aged 2-6 months. Although the data were taken from patient surveys, which could have been misreported, they are supported by the findings of other studies.
[polldaddy:{"method":"iframe","type":"survey","src":"//newspolls2017.polldaddy.com/s/recommendations-for-infant-sleep-position-are-not-being-followed?iframe=1"}]
“Maternal race and education continue to be factors associated with choice of infant sleeping position as does advice from a doctor. Factors that appear to be of equal or greater importance are those related to attitudes, subjective social norms, and perceived control, all of which can potentially be altered through educational interventions,” Dr. Colson and her colleagues concluded.
The Eunice Kennedy Shriver National Institute of Child Health and Human Development and the National Institutes of Health funded the study. The authors reported no financial disclosures.
FROM PEDIATRICS
Key clinical point:
Major finding: Of the 3,297 mothers surveyed, 2,491 (77%) reported that they usually place their infants in supine position, but only 49% reported that they exclusively place their infants supine.
Data source: The Study of Attitudes and Factors Affecting Infant Care, involving 3,297 mothers.
Disclosures: The Eunice Kennedy Shriver National Institute of Child Health and Human Development and the National Institutes of Health funded the study. The authors reported no financial disclosures.
Alternative birthing practices increase risk of infection
All three have been associated with sporadic, serious neonatal infections.
The U.S. prevalence of water births – delivering a baby underwater – is currently unknown, but in the United Kingdom the practice is common. According to a 2015 National Health Service maternity survey, approximately 9% of women who underwent vaginal delivery opted for water birth (Arch Dis Child Fetal Neonatal Ed. 2016 Jul;101[4]:F357-65). Both the Royal College of Obstetricians and Gynaecologists and the Royal College of Midwives endorse this practice for healthy women with uncomplicated term pregnancies. According to a 2009 Cochrane Review, immersion during the first phase of labor reduces the use of epidural/spinal analgesia (Cochrane Database Syst Rev. 2009. doi: 10.1002/14651858.CD000111.pub3). The maternal benefits of delivery under water, though, have not been clearly defined.
Legionella pneumophila is an uncommon pathogen in children, but cases of neonatal Legionnaires’ disease have been reported after water birth. Two affected babies born in Arizona in 2016 were successfully treated and survived (MMWR Morb Mortal Wkly Rep. 2017. doi: 10.15585/mmwr.mm6622a4). A baby born in Texas in 2014 died of sepsis and respiratory failure (Emerg Infect Dis. 2015. doi: 10.3201/eid2101.140846). Canadian investigators have reported fatal disseminated herpes simplex virus infection in an infant after water birth; the mother had herpetic whitlow and a recent blister concerning for HSV on her thigh (J Pediatric Infect Dis Soc. 2017 May 16. doi: 10.1093/jpids/pix035).
Admittedly, each of these cases might have been prevented by adherence to recommended infection control practices, and the absolute risk of infection after water birth is unknown and likely to be small. Still, neither the American Academy of Pediatrics nor the American College of Obstetricians and Gynecologists currently recommend the practice. ACOG suggests that “births occur on land, not in water” and has called for well-designed, prospective studies of the maternal and perinatal benefits and risks associated with immersion during labor and delivery (Obstet Gynecol. 2016;128:1198-9).
Placentophagia – consuming the placenta after birth – has been promoted by celebrity moms, including Katherine Heigl and Kourtney Kardashian. Placenta can be cooked, blended raw into a smoothie, or dehydrated and encapsulated.
Proponents of placentophagia claim health benefits of this practice, including improved mood and energy, and increased breast milk production. There are few published data to support these claims. A recent case report suggests the practice has the potential to harm the baby. In June 2017, Oregon public health authorities described a neonate with recurrent episodes of group B streptococcal (GBS) bacteremia. An identical strain of GBS was cultured from capsules containing the mother’s dehydrated placenta – she had consumed six of the capsules daily beginning a few days after the baby’s birth. According to the Morbidity and Mortality Weekly Report communication, “no standards exist for processing placenta for consumption” and the “placenta encapsulation process does not eradicate infectious pathogens per se. … Placenta capsule ingestion should be avoided”(MMWR Morb Mortal Wkly Rep. 2017;66:677-8. doi: 10.15585/mmwr.mm6625a4).
Finally, the ritual practice of umbilical cord nonseverance or lotus birth deserves a mention. In a lotus birth, the umbilical cord is left uncut, allowing the placenta to remain attached to the baby until the cord dries and naturally separates, generally 3-10 days after delivery. Describing a spiritual connection between the baby and the placenta, proponents claim lotus birth promotes bonding and allows for a gentler transition between intra- and extrauterine life.
A review of PubMed turned up no formal studies of this practice, but case reports describe complications such as neonatal idiopathic hepatitis and neonatal sepsis. The Royal College of Obstetricians and Gynaecologists has issued a warning about lotus births, advising that babies be monitored closely for infection. RCOG spokesperson Dr. Patrick O’Brien said in a 2008 statement, “If left for a period of time after the birth, there is a risk of infection in the placenta which can consequently spread to the baby. The placenta is particularly prone to infection as it contains blood. Within a short time after birth, once the umbilical cord has stopped pulsating, the placenta has no circulation and is essentially dead tissue.”
Interestingly, a quick scan of Etsy, the popular e-commerce website, turned up a number of lotus birth kits for sale. These generally contain a decorative cloth bag as well as an herb mix containing lavender and eucalyptus to promote drying and mask the smell of the decomposing placenta.
In contrast, many pediatricians, me included, are not well informed about these practices and don’t routinely ask expectant moms about their plans. I propose that we can advocate for our patients-to-be by learning about these practices so that we can engage in an honest, respectful discussion about potential risks and benefits. For me, for now, the risks outweigh the benefits.
Dr. Bryant is a pediatrician specializing in infectious diseases at the University of Louisville (Ky.) and Norton Children’s Hospital, also in Louisville. She said she had no relevant financial disclosures. Email her at [email protected].
All three have been associated with sporadic, serious neonatal infections.
The U.S. prevalence of water births – delivering a baby underwater – is currently unknown, but in the United Kingdom the practice is common. According to a 2015 National Health Service maternity survey, approximately 9% of women who underwent vaginal delivery opted for water birth (Arch Dis Child Fetal Neonatal Ed. 2016 Jul;101[4]:F357-65). Both the Royal College of Obstetricians and Gynaecologists and the Royal College of Midwives endorse this practice for healthy women with uncomplicated term pregnancies. According to a 2009 Cochrane Review, immersion during the first phase of labor reduces the use of epidural/spinal analgesia (Cochrane Database Syst Rev. 2009. doi: 10.1002/14651858.CD000111.pub3). The maternal benefits of delivery under water, though, have not been clearly defined.
Legionella pneumophila is an uncommon pathogen in children, but cases of neonatal Legionnaires’ disease have been reported after water birth. Two affected babies born in Arizona in 2016 were successfully treated and survived (MMWR Morb Mortal Wkly Rep. 2017. doi: 10.15585/mmwr.mm6622a4). A baby born in Texas in 2014 died of sepsis and respiratory failure (Emerg Infect Dis. 2015. doi: 10.3201/eid2101.140846). Canadian investigators have reported fatal disseminated herpes simplex virus infection in an infant after water birth; the mother had herpetic whitlow and a recent blister concerning for HSV on her thigh (J Pediatric Infect Dis Soc. 2017 May 16. doi: 10.1093/jpids/pix035).
Admittedly, each of these cases might have been prevented by adherence to recommended infection control practices, and the absolute risk of infection after water birth is unknown and likely to be small. Still, neither the American Academy of Pediatrics nor the American College of Obstetricians and Gynecologists currently recommend the practice. ACOG suggests that “births occur on land, not in water” and has called for well-designed, prospective studies of the maternal and perinatal benefits and risks associated with immersion during labor and delivery (Obstet Gynecol. 2016;128:1198-9).
Placentophagia – consuming the placenta after birth – has been promoted by celebrity moms, including Katherine Heigl and Kourtney Kardashian. Placenta can be cooked, blended raw into a smoothie, or dehydrated and encapsulated.
Proponents of placentophagia claim health benefits of this practice, including improved mood and energy, and increased breast milk production. There are few published data to support these claims. A recent case report suggests the practice has the potential to harm the baby. In June 2017, Oregon public health authorities described a neonate with recurrent episodes of group B streptococcal (GBS) bacteremia. An identical strain of GBS was cultured from capsules containing the mother’s dehydrated placenta – she had consumed six of the capsules daily beginning a few days after the baby’s birth. According to the Morbidity and Mortality Weekly Report communication, “no standards exist for processing placenta for consumption” and the “placenta encapsulation process does not eradicate infectious pathogens per se. … Placenta capsule ingestion should be avoided”(MMWR Morb Mortal Wkly Rep. 2017;66:677-8. doi: 10.15585/mmwr.mm6625a4).
Finally, the ritual practice of umbilical cord nonseverance or lotus birth deserves a mention. In a lotus birth, the umbilical cord is left uncut, allowing the placenta to remain attached to the baby until the cord dries and naturally separates, generally 3-10 days after delivery. Describing a spiritual connection between the baby and the placenta, proponents claim lotus birth promotes bonding and allows for a gentler transition between intra- and extrauterine life.
A review of PubMed turned up no formal studies of this practice, but case reports describe complications such as neonatal idiopathic hepatitis and neonatal sepsis. The Royal College of Obstetricians and Gynaecologists has issued a warning about lotus births, advising that babies be monitored closely for infection. RCOG spokesperson Dr. Patrick O’Brien said in a 2008 statement, “If left for a period of time after the birth, there is a risk of infection in the placenta which can consequently spread to the baby. The placenta is particularly prone to infection as it contains blood. Within a short time after birth, once the umbilical cord has stopped pulsating, the placenta has no circulation and is essentially dead tissue.”
Interestingly, a quick scan of Etsy, the popular e-commerce website, turned up a number of lotus birth kits for sale. These generally contain a decorative cloth bag as well as an herb mix containing lavender and eucalyptus to promote drying and mask the smell of the decomposing placenta.
In contrast, many pediatricians, me included, are not well informed about these practices and don’t routinely ask expectant moms about their plans. I propose that we can advocate for our patients-to-be by learning about these practices so that we can engage in an honest, respectful discussion about potential risks and benefits. For me, for now, the risks outweigh the benefits.
Dr. Bryant is a pediatrician specializing in infectious diseases at the University of Louisville (Ky.) and Norton Children’s Hospital, also in Louisville. She said she had no relevant financial disclosures. Email her at [email protected].
All three have been associated with sporadic, serious neonatal infections.
The U.S. prevalence of water births – delivering a baby underwater – is currently unknown, but in the United Kingdom the practice is common. According to a 2015 National Health Service maternity survey, approximately 9% of women who underwent vaginal delivery opted for water birth (Arch Dis Child Fetal Neonatal Ed. 2016 Jul;101[4]:F357-65). Both the Royal College of Obstetricians and Gynaecologists and the Royal College of Midwives endorse this practice for healthy women with uncomplicated term pregnancies. According to a 2009 Cochrane Review, immersion during the first phase of labor reduces the use of epidural/spinal analgesia (Cochrane Database Syst Rev. 2009. doi: 10.1002/14651858.CD000111.pub3). The maternal benefits of delivery under water, though, have not been clearly defined.
Legionella pneumophila is an uncommon pathogen in children, but cases of neonatal Legionnaires’ disease have been reported after water birth. Two affected babies born in Arizona in 2016 were successfully treated and survived (MMWR Morb Mortal Wkly Rep. 2017. doi: 10.15585/mmwr.mm6622a4). A baby born in Texas in 2014 died of sepsis and respiratory failure (Emerg Infect Dis. 2015. doi: 10.3201/eid2101.140846). Canadian investigators have reported fatal disseminated herpes simplex virus infection in an infant after water birth; the mother had herpetic whitlow and a recent blister concerning for HSV on her thigh (J Pediatric Infect Dis Soc. 2017 May 16. doi: 10.1093/jpids/pix035).
Admittedly, each of these cases might have been prevented by adherence to recommended infection control practices, and the absolute risk of infection after water birth is unknown and likely to be small. Still, neither the American Academy of Pediatrics nor the American College of Obstetricians and Gynecologists currently recommend the practice. ACOG suggests that “births occur on land, not in water” and has called for well-designed, prospective studies of the maternal and perinatal benefits and risks associated with immersion during labor and delivery (Obstet Gynecol. 2016;128:1198-9).
Placentophagia – consuming the placenta after birth – has been promoted by celebrity moms, including Katherine Heigl and Kourtney Kardashian. Placenta can be cooked, blended raw into a smoothie, or dehydrated and encapsulated.
Proponents of placentophagia claim health benefits of this practice, including improved mood and energy, and increased breast milk production. There are few published data to support these claims. A recent case report suggests the practice has the potential to harm the baby. In June 2017, Oregon public health authorities described a neonate with recurrent episodes of group B streptococcal (GBS) bacteremia. An identical strain of GBS was cultured from capsules containing the mother’s dehydrated placenta – she had consumed six of the capsules daily beginning a few days after the baby’s birth. According to the Morbidity and Mortality Weekly Report communication, “no standards exist for processing placenta for consumption” and the “placenta encapsulation process does not eradicate infectious pathogens per se. … Placenta capsule ingestion should be avoided”(MMWR Morb Mortal Wkly Rep. 2017;66:677-8. doi: 10.15585/mmwr.mm6625a4).
Finally, the ritual practice of umbilical cord nonseverance or lotus birth deserves a mention. In a lotus birth, the umbilical cord is left uncut, allowing the placenta to remain attached to the baby until the cord dries and naturally separates, generally 3-10 days after delivery. Describing a spiritual connection between the baby and the placenta, proponents claim lotus birth promotes bonding and allows for a gentler transition between intra- and extrauterine life.
A review of PubMed turned up no formal studies of this practice, but case reports describe complications such as neonatal idiopathic hepatitis and neonatal sepsis. The Royal College of Obstetricians and Gynaecologists has issued a warning about lotus births, advising that babies be monitored closely for infection. RCOG spokesperson Dr. Patrick O’Brien said in a 2008 statement, “If left for a period of time after the birth, there is a risk of infection in the placenta which can consequently spread to the baby. The placenta is particularly prone to infection as it contains blood. Within a short time after birth, once the umbilical cord has stopped pulsating, the placenta has no circulation and is essentially dead tissue.”
Interestingly, a quick scan of Etsy, the popular e-commerce website, turned up a number of lotus birth kits for sale. These generally contain a decorative cloth bag as well as an herb mix containing lavender and eucalyptus to promote drying and mask the smell of the decomposing placenta.
In contrast, many pediatricians, me included, are not well informed about these practices and don’t routinely ask expectant moms about their plans. I propose that we can advocate for our patients-to-be by learning about these practices so that we can engage in an honest, respectful discussion about potential risks and benefits. For me, for now, the risks outweigh the benefits.
Dr. Bryant is a pediatrician specializing in infectious diseases at the University of Louisville (Ky.) and Norton Children’s Hospital, also in Louisville. She said she had no relevant financial disclosures. Email her at [email protected].
Opening the door to gene editing?
In early August, in Portland, Ore. The scale and success of such experimentation with human embryos is unprecedented in the United States. Given the highly experimental nature of fertility clinics in the United States and abroad, many suggest that these findings open the door to designer babies. A careful read of the report, however, indicates that the door is still quite closed, perhaps cracked open just a little.
The research team used a new method of cutting the genome, called CRISPR-Cas9. CRISPR utilizes two key components that the team combined in a test tube together: a Cas9 protein that can cut the DNA and a synthetic RNA that can guide the protein to cut a 20-letter sequence in the human genome specifically. In these experiments, the Cas9-RNA protein was designed to cut a pathogenic mutation in the MYBPC3 gene, which can cause hypertrophic cardiomyopathy. The research team could not obtain human zygotes with this mutation on both copies of the genome (a rare homozygous genotype). Such zygotes would have the most severe phenotype and be the most compelling test case for CRISPR. Instead, they focused on gene editing heterozygous human zygotes that have one normal maternal copy of the MYBPC3 gene and one pathogenic paternal copy. The heterozygous zygotes were produced by the research team via in vitro fertilization (IVF) or intracytoplasmic sperm injection (ICSI) using sperm donated by males carrying the pathogenic mutation (Nature. 2017 Aug 2. doi: 10.1038/nature23305).
When researchers injected the Cas9-RNA protein targeting the mutation into already fertilized zygotes, they found that 67% of the resulting embryos had two normal copies of the MYBPC3 gene. Without gene editing, approximately 50% of the embryos would have two normal copies, because the male sperm donor would produce equal numbers of sperm with normal and pathogenic genotypes. Thus, editing likely corrected only about 17% of the embryos that would have otherwise had one pathogenic paternal mutation. Thirty-six percent of embryos had additional mutations from imprecise gene editing. Further, some of the gene edits and additional mutations were mosaic, meaning that the resulting embryo harbored many different genotypes.
To overcome these challenges, the research team precisely controlled the timing of CRISPR injection to coincide with fertilization. With controlled timing, gene editing was restricted to only the paternal pathogenic mutation, resulting in 72% of all injected embryos having two normal copies of the gene in all cells without any mosaicism. Whole genome sequencing revealed no additional mutations above the detection limit of the assay. Finally, preimplantation development proceeded normally to the blastocyst stage, suggesting that the edited embryos have no functional deficits from the procedure.
A surprising finding was that new sequences could not be put into the embryo. The research team had coinjected a synthetic DNA template that differed from the normal maternal copy, but never saw this sequence incorporated into any embryo. Instead, the zygote utilized the maternal copy of the gene with the normal sequence as a template for repairing the DNA cut in the paternal copy produced by CRISPR. The biology behind this repair process is poorly understood and has not been previously reported with other human cell types. These observations suggest that we cannot easily “write” our genome. Instead, our vocabulary is limited to what is already within either the maternal or paternal copy of the genome. In other words, designer babies are not around the corner. While preimplantation genetic diagnosis (PGD) is still currently the safest way to avoid passing on autosomal dominant mutations, these new findings could enable correction of such mutations within IVF embryos, resulting in a larger pool of embryos for IVF clinics to work with.
Apart from these technical challenges, the National Academies has not given a green light to implant edited human embryos. Instead, the organization calls for several requirements to be met, including “broad societal consensus” on the need for this type of intervention. While it is not clear whether or how consensus could be achieved, it is clear that scientists, clinicians, and patients will need help from the rest of society for this research to have an impact clinically.
Dr. Saha is assistant professor of biomedical engineering at the Wisconsin Institute for Discovery at the University of Wisconsin, Madison. His lab works on gene editing of human cells. He has patent filings through the Wisconsin Alumni Research Foundation on gene editing inventions.
In early August, in Portland, Ore. The scale and success of such experimentation with human embryos is unprecedented in the United States. Given the highly experimental nature of fertility clinics in the United States and abroad, many suggest that these findings open the door to designer babies. A careful read of the report, however, indicates that the door is still quite closed, perhaps cracked open just a little.
The research team used a new method of cutting the genome, called CRISPR-Cas9. CRISPR utilizes two key components that the team combined in a test tube together: a Cas9 protein that can cut the DNA and a synthetic RNA that can guide the protein to cut a 20-letter sequence in the human genome specifically. In these experiments, the Cas9-RNA protein was designed to cut a pathogenic mutation in the MYBPC3 gene, which can cause hypertrophic cardiomyopathy. The research team could not obtain human zygotes with this mutation on both copies of the genome (a rare homozygous genotype). Such zygotes would have the most severe phenotype and be the most compelling test case for CRISPR. Instead, they focused on gene editing heterozygous human zygotes that have one normal maternal copy of the MYBPC3 gene and one pathogenic paternal copy. The heterozygous zygotes were produced by the research team via in vitro fertilization (IVF) or intracytoplasmic sperm injection (ICSI) using sperm donated by males carrying the pathogenic mutation (Nature. 2017 Aug 2. doi: 10.1038/nature23305).
When researchers injected the Cas9-RNA protein targeting the mutation into already fertilized zygotes, they found that 67% of the resulting embryos had two normal copies of the MYBPC3 gene. Without gene editing, approximately 50% of the embryos would have two normal copies, because the male sperm donor would produce equal numbers of sperm with normal and pathogenic genotypes. Thus, editing likely corrected only about 17% of the embryos that would have otherwise had one pathogenic paternal mutation. Thirty-six percent of embryos had additional mutations from imprecise gene editing. Further, some of the gene edits and additional mutations were mosaic, meaning that the resulting embryo harbored many different genotypes.
To overcome these challenges, the research team precisely controlled the timing of CRISPR injection to coincide with fertilization. With controlled timing, gene editing was restricted to only the paternal pathogenic mutation, resulting in 72% of all injected embryos having two normal copies of the gene in all cells without any mosaicism. Whole genome sequencing revealed no additional mutations above the detection limit of the assay. Finally, preimplantation development proceeded normally to the blastocyst stage, suggesting that the edited embryos have no functional deficits from the procedure.
A surprising finding was that new sequences could not be put into the embryo. The research team had coinjected a synthetic DNA template that differed from the normal maternal copy, but never saw this sequence incorporated into any embryo. Instead, the zygote utilized the maternal copy of the gene with the normal sequence as a template for repairing the DNA cut in the paternal copy produced by CRISPR. The biology behind this repair process is poorly understood and has not been previously reported with other human cell types. These observations suggest that we cannot easily “write” our genome. Instead, our vocabulary is limited to what is already within either the maternal or paternal copy of the genome. In other words, designer babies are not around the corner. While preimplantation genetic diagnosis (PGD) is still currently the safest way to avoid passing on autosomal dominant mutations, these new findings could enable correction of such mutations within IVF embryos, resulting in a larger pool of embryos for IVF clinics to work with.
Apart from these technical challenges, the National Academies has not given a green light to implant edited human embryos. Instead, the organization calls for several requirements to be met, including “broad societal consensus” on the need for this type of intervention. While it is not clear whether or how consensus could be achieved, it is clear that scientists, clinicians, and patients will need help from the rest of society for this research to have an impact clinically.
Dr. Saha is assistant professor of biomedical engineering at the Wisconsin Institute for Discovery at the University of Wisconsin, Madison. His lab works on gene editing of human cells. He has patent filings through the Wisconsin Alumni Research Foundation on gene editing inventions.
In early August, in Portland, Ore. The scale and success of such experimentation with human embryos is unprecedented in the United States. Given the highly experimental nature of fertility clinics in the United States and abroad, many suggest that these findings open the door to designer babies. A careful read of the report, however, indicates that the door is still quite closed, perhaps cracked open just a little.
The research team used a new method of cutting the genome, called CRISPR-Cas9. CRISPR utilizes two key components that the team combined in a test tube together: a Cas9 protein that can cut the DNA and a synthetic RNA that can guide the protein to cut a 20-letter sequence in the human genome specifically. In these experiments, the Cas9-RNA protein was designed to cut a pathogenic mutation in the MYBPC3 gene, which can cause hypertrophic cardiomyopathy. The research team could not obtain human zygotes with this mutation on both copies of the genome (a rare homozygous genotype). Such zygotes would have the most severe phenotype and be the most compelling test case for CRISPR. Instead, they focused on gene editing heterozygous human zygotes that have one normal maternal copy of the MYBPC3 gene and one pathogenic paternal copy. The heterozygous zygotes were produced by the research team via in vitro fertilization (IVF) or intracytoplasmic sperm injection (ICSI) using sperm donated by males carrying the pathogenic mutation (Nature. 2017 Aug 2. doi: 10.1038/nature23305).
When researchers injected the Cas9-RNA protein targeting the mutation into already fertilized zygotes, they found that 67% of the resulting embryos had two normal copies of the MYBPC3 gene. Without gene editing, approximately 50% of the embryos would have two normal copies, because the male sperm donor would produce equal numbers of sperm with normal and pathogenic genotypes. Thus, editing likely corrected only about 17% of the embryos that would have otherwise had one pathogenic paternal mutation. Thirty-six percent of embryos had additional mutations from imprecise gene editing. Further, some of the gene edits and additional mutations were mosaic, meaning that the resulting embryo harbored many different genotypes.
To overcome these challenges, the research team precisely controlled the timing of CRISPR injection to coincide with fertilization. With controlled timing, gene editing was restricted to only the paternal pathogenic mutation, resulting in 72% of all injected embryos having two normal copies of the gene in all cells without any mosaicism. Whole genome sequencing revealed no additional mutations above the detection limit of the assay. Finally, preimplantation development proceeded normally to the blastocyst stage, suggesting that the edited embryos have no functional deficits from the procedure.
A surprising finding was that new sequences could not be put into the embryo. The research team had coinjected a synthetic DNA template that differed from the normal maternal copy, but never saw this sequence incorporated into any embryo. Instead, the zygote utilized the maternal copy of the gene with the normal sequence as a template for repairing the DNA cut in the paternal copy produced by CRISPR. The biology behind this repair process is poorly understood and has not been previously reported with other human cell types. These observations suggest that we cannot easily “write” our genome. Instead, our vocabulary is limited to what is already within either the maternal or paternal copy of the genome. In other words, designer babies are not around the corner. While preimplantation genetic diagnosis (PGD) is still currently the safest way to avoid passing on autosomal dominant mutations, these new findings could enable correction of such mutations within IVF embryos, resulting in a larger pool of embryos for IVF clinics to work with.
Apart from these technical challenges, the National Academies has not given a green light to implant edited human embryos. Instead, the organization calls for several requirements to be met, including “broad societal consensus” on the need for this type of intervention. While it is not clear whether or how consensus could be achieved, it is clear that scientists, clinicians, and patients will need help from the rest of society for this research to have an impact clinically.
Dr. Saha is assistant professor of biomedical engineering at the Wisconsin Institute for Discovery at the University of Wisconsin, Madison. His lab works on gene editing of human cells. He has patent filings through the Wisconsin Alumni Research Foundation on gene editing inventions.
Researchers identify ‘congenital NAD deficiency disorders’
Mutations that disrupt de novo synthesis of nicotinamide adenine dinucleotide (NAD) were associated with multiple congenital malformations in humans and mice, and supplementing niacin during gestation prevented these malformations in mice, new research suggests.
The malformations include vertebral defects, anal atresia, cardiac defects, tracheoesophageal fistula, renal anomalies, and limb abnormalities (VACTERL), “a nonrandom combination of congenital defects without a known cause,” wrote Hongjun Shi, PhD, of Victor Chang Cardiac Research Institute, New South Wales, Australia, and colleagues (N Engl J Med. 2017;377:544-52).
Numerous genetic and environmental factors can potentially cause NAD deficiency during gestation and the investigators suggested collectively referring to the resulting malformations as “congenital NAD deficiency disorders.”
Congenital defects can occur together in newborns more often than would be expected by chance, but “in many such cases, it has proved difficult to identify a genetic cause,” the investigators noted. Using genomic sequencing, they looked for possible pathogenic gene variants within four unrelated families in which a person was born with multiple congenital malformations. Next, they evaluated the function of the variants by testing in vitro enzyme activity and measuring relevant plasma metabolites. Finally, they used the CRISPR (clustered regularly interspaced short palindromic repeats)–Cas9 system to create mouse models with similar variants.
This approach identified variants in two genes encoding enzymes of the kynurenine pathway: 3-hydroxyanthranilic acid 3,4-dioxygenase (HAAO) and kynureninase (KYNU). Three patients had homozygous variants associated with loss-of-function changes in these proteins. A fourth patient had heterozygous variants in the gene encoding KYNU.
“The mutant enzymes had greatly reduced activity in vitro,” the researchers wrote. Patients had decreased circulating levels of NAD, which tryptophan synthesizes through the kynurenine pathway. Notably, mouse embryos lacking the mouse equivalents of HAAO or KYNU also had congenital defects associated with NAD deficiency. Preventing NAD deficiency during gestation averted these defects in mice.
“The NAD de novo synthesis pathway catabolizes tryptophan,” the researchers added. “Although metabolite levels upstream of the block are elevated, and the metabolites have postnatal functions, we found that it is the deficiency in embryonic NAD, downstream of the block, that is disrupting embryogenesis.”
The study was supported by the Australian and New South Wales governments and foundations. The investigators reported having no other financial disclosures.
Shi et al. report that a deficiency of nicotinamide adenine dinucleotide (NAD) causes congenital malformations, suggesting that interventions to raise NAD levels during fetal and early postnatal development might further reduce the incidence of congenital anomalies.
Regardless of how NAD depletion leads to congenital malformations (whether by compromising the detection of DNA damage by PARP proteins, reducing the supply of nucleotides, or both), dietary supplementation with NAD precursors merits further study. At high doses, niacin can cause flushing and gastrointestinal symptoms, but it has few side effects at lower doses.
Nicotinamide mononucleotide, nicotinamide riboside, and nicotinamide itself are better tolerated than niacin and are generally considered to be safe as dietary supplements, but the doses of NAD precursors required to reduce the risk of congenital malformations in humans are not known. Also unknown is the extent to which raising dietary levels of NAD would limit cognitive impairment in infants with congenital malformations.
Matthew G. Vander Heiden, MD, PhD, is with the Massachusetts Institute of Technology, Cambridge, Mass., and the Dana Farber Cancer Center, Boston. He reported receiving personal fees from Agios Pharmaceuticals and Aeglea Biotherapeutics outside the submitted work. These comments are adapted from an editorial (N Engl J Med. 2007;377:509-11).
Shi et al. report that a deficiency of nicotinamide adenine dinucleotide (NAD) causes congenital malformations, suggesting that interventions to raise NAD levels during fetal and early postnatal development might further reduce the incidence of congenital anomalies.
Regardless of how NAD depletion leads to congenital malformations (whether by compromising the detection of DNA damage by PARP proteins, reducing the supply of nucleotides, or both), dietary supplementation with NAD precursors merits further study. At high doses, niacin can cause flushing and gastrointestinal symptoms, but it has few side effects at lower doses.
Nicotinamide mononucleotide, nicotinamide riboside, and nicotinamide itself are better tolerated than niacin and are generally considered to be safe as dietary supplements, but the doses of NAD precursors required to reduce the risk of congenital malformations in humans are not known. Also unknown is the extent to which raising dietary levels of NAD would limit cognitive impairment in infants with congenital malformations.
Matthew G. Vander Heiden, MD, PhD, is with the Massachusetts Institute of Technology, Cambridge, Mass., and the Dana Farber Cancer Center, Boston. He reported receiving personal fees from Agios Pharmaceuticals and Aeglea Biotherapeutics outside the submitted work. These comments are adapted from an editorial (N Engl J Med. 2007;377:509-11).
Shi et al. report that a deficiency of nicotinamide adenine dinucleotide (NAD) causes congenital malformations, suggesting that interventions to raise NAD levels during fetal and early postnatal development might further reduce the incidence of congenital anomalies.
Regardless of how NAD depletion leads to congenital malformations (whether by compromising the detection of DNA damage by PARP proteins, reducing the supply of nucleotides, or both), dietary supplementation with NAD precursors merits further study. At high doses, niacin can cause flushing and gastrointestinal symptoms, but it has few side effects at lower doses.
Nicotinamide mononucleotide, nicotinamide riboside, and nicotinamide itself are better tolerated than niacin and are generally considered to be safe as dietary supplements, but the doses of NAD precursors required to reduce the risk of congenital malformations in humans are not known. Also unknown is the extent to which raising dietary levels of NAD would limit cognitive impairment in infants with congenital malformations.
Matthew G. Vander Heiden, MD, PhD, is with the Massachusetts Institute of Technology, Cambridge, Mass., and the Dana Farber Cancer Center, Boston. He reported receiving personal fees from Agios Pharmaceuticals and Aeglea Biotherapeutics outside the submitted work. These comments are adapted from an editorial (N Engl J Med. 2007;377:509-11).
Mutations that disrupt de novo synthesis of nicotinamide adenine dinucleotide (NAD) were associated with multiple congenital malformations in humans and mice, and supplementing niacin during gestation prevented these malformations in mice, new research suggests.
The malformations include vertebral defects, anal atresia, cardiac defects, tracheoesophageal fistula, renal anomalies, and limb abnormalities (VACTERL), “a nonrandom combination of congenital defects without a known cause,” wrote Hongjun Shi, PhD, of Victor Chang Cardiac Research Institute, New South Wales, Australia, and colleagues (N Engl J Med. 2017;377:544-52).
Numerous genetic and environmental factors can potentially cause NAD deficiency during gestation and the investigators suggested collectively referring to the resulting malformations as “congenital NAD deficiency disorders.”
Congenital defects can occur together in newborns more often than would be expected by chance, but “in many such cases, it has proved difficult to identify a genetic cause,” the investigators noted. Using genomic sequencing, they looked for possible pathogenic gene variants within four unrelated families in which a person was born with multiple congenital malformations. Next, they evaluated the function of the variants by testing in vitro enzyme activity and measuring relevant plasma metabolites. Finally, they used the CRISPR (clustered regularly interspaced short palindromic repeats)–Cas9 system to create mouse models with similar variants.
This approach identified variants in two genes encoding enzymes of the kynurenine pathway: 3-hydroxyanthranilic acid 3,4-dioxygenase (HAAO) and kynureninase (KYNU). Three patients had homozygous variants associated with loss-of-function changes in these proteins. A fourth patient had heterozygous variants in the gene encoding KYNU.
“The mutant enzymes had greatly reduced activity in vitro,” the researchers wrote. Patients had decreased circulating levels of NAD, which tryptophan synthesizes through the kynurenine pathway. Notably, mouse embryos lacking the mouse equivalents of HAAO or KYNU also had congenital defects associated with NAD deficiency. Preventing NAD deficiency during gestation averted these defects in mice.
“The NAD de novo synthesis pathway catabolizes tryptophan,” the researchers added. “Although metabolite levels upstream of the block are elevated, and the metabolites have postnatal functions, we found that it is the deficiency in embryonic NAD, downstream of the block, that is disrupting embryogenesis.”
The study was supported by the Australian and New South Wales governments and foundations. The investigators reported having no other financial disclosures.
Mutations that disrupt de novo synthesis of nicotinamide adenine dinucleotide (NAD) were associated with multiple congenital malformations in humans and mice, and supplementing niacin during gestation prevented these malformations in mice, new research suggests.
The malformations include vertebral defects, anal atresia, cardiac defects, tracheoesophageal fistula, renal anomalies, and limb abnormalities (VACTERL), “a nonrandom combination of congenital defects without a known cause,” wrote Hongjun Shi, PhD, of Victor Chang Cardiac Research Institute, New South Wales, Australia, and colleagues (N Engl J Med. 2017;377:544-52).
Numerous genetic and environmental factors can potentially cause NAD deficiency during gestation and the investigators suggested collectively referring to the resulting malformations as “congenital NAD deficiency disorders.”
Congenital defects can occur together in newborns more often than would be expected by chance, but “in many such cases, it has proved difficult to identify a genetic cause,” the investigators noted. Using genomic sequencing, they looked for possible pathogenic gene variants within four unrelated families in which a person was born with multiple congenital malformations. Next, they evaluated the function of the variants by testing in vitro enzyme activity and measuring relevant plasma metabolites. Finally, they used the CRISPR (clustered regularly interspaced short palindromic repeats)–Cas9 system to create mouse models with similar variants.
This approach identified variants in two genes encoding enzymes of the kynurenine pathway: 3-hydroxyanthranilic acid 3,4-dioxygenase (HAAO) and kynureninase (KYNU). Three patients had homozygous variants associated with loss-of-function changes in these proteins. A fourth patient had heterozygous variants in the gene encoding KYNU.
“The mutant enzymes had greatly reduced activity in vitro,” the researchers wrote. Patients had decreased circulating levels of NAD, which tryptophan synthesizes through the kynurenine pathway. Notably, mouse embryos lacking the mouse equivalents of HAAO or KYNU also had congenital defects associated with NAD deficiency. Preventing NAD deficiency during gestation averted these defects in mice.
“The NAD de novo synthesis pathway catabolizes tryptophan,” the researchers added. “Although metabolite levels upstream of the block are elevated, and the metabolites have postnatal functions, we found that it is the deficiency in embryonic NAD, downstream of the block, that is disrupting embryogenesis.”
The study was supported by the Australian and New South Wales governments and foundations. The investigators reported having no other financial disclosures.
FROM THE NEW ENGLAND JOURNAL OF MEDICINE
Key clinical point:
Major finding: Major congenital defects affecting unrelated families were associated with variants in genes encoding 3-hydroxyanthranilic acid 3,4-dioxygenase (HAAO) and kynureninase (KYNU).
Data source: Genomic sequencing of four unrelated families in which a person was born with multiple congenital malformations, plus in vitro measurements of enzyme activity and plasma metabolites and studies of mouse models created with the CRISPR–Cas9 system.
Disclosures: The study was supported by the Australian and New South Wales governments and foundations. The investigators reported having no other financial disclosures.
Mobile messages support safe sleep practices
Mobile health interventions targeting mothers of healthy newborns significantly improved safe sleep practices, compared with controls, in a randomized trial of 1,600 mothers published online July 25 in JAMA.
Despite the success of the Back to Sleep campaign in reducing rates of sudden infant death syndrome, approximately 3,500 infant deaths due to SIDS, accidental suffocation, or strangulation in bed occurred in 2014, wrote Rachel Y. Moon, MD, of the University of Virginia, Charlottesville, and her colleagues (JAMA. 2017;318:351-9).
A total of 1,263 mothers completed the study, and mothers who received the mobile messages about safe sleep were significantly more likely than those who received the control messages to engage in safe sleep practices, including placing babies on their backs (89% vs. 80%), sharing a room without cosleeping (83% vs. 70%), avoiding the use of soft bedding (79% vs. 68%), and use of pacifiers (69% vs. 60%). The initial nursing quality intervention alone had no significant impact on any of the safe sleep practices, the researchers noted.
The results were limited by several factors, including the 21% lost to follow up and lack of data on adverse events and clinical outcomes, the researchers said. However, the results suggest that mobile messages could be cost effective and easily implemented by hospitals.
“Furthermore, because the rates of opening and viewing messages in this study were consistently higher than 50%, and almost all adults now have cell phones or email access, it is likely that this type of intervention would be feasible and well received by parents,” Dr. Moon and her associates added. “Whether widespread implementation is feasible or if it reduces sudden and unexpected infant death rates remains to be studied.”
“The messages and videos were timed to address challenges and questions that arise at specific time points; therefore, providing this additional information to parents at critical times may have been important in assuaging concerns about adherence to recommended practices. Furthermore, receiving frequent videos and email or text messages may have served as a virtual support system for mothers, reinforcing safe parental practices,” Dr. Moon and her associates noted.
The researchers had no financial conflicts to disclose. The study was supported in part by the National Institute of Child Health and Human Development and by the CJ Foundation for SIDS.
In this study by Dr. Moon and her associates, new mothers who received both the nursing educational intervention and mobile intervention for safe sleep reported the highest percentages for adhering to safe sleep practices, and moms who received the safe sleep mobile intervention alone had the second-highest percentages.
However, the study was underpowered and too short termed to determine whether this intervention actually will reduce the occurrence of SIDS.
Limitations of this study include that the mothers who did not respond at follow-up were more likely to be younger, black, single, and less educated – all risk factors for SIDS. The study also was restricted to healthy term infants, and preterm babies are another high-risk SIDS group.
Nonetheless, the fact that this study chose to use multifaceted approaches was promising, combining “health messaging, education of health care professionals, and interventions aimed at reducing barriers to safe sleep practices for infant caregivers.” Whatever interventions are tried, they “need to be adapted for implementation among the highest-risk groups such as non-Hispanic black, American Indian, and Alaskan Native mothers and families because these are the populations with the highest rates of SIDS and sleep-related infant death.”
Carrie K. Shapiro-Mendoza, PhD, MPH is affiliated with the division of reproductive health at the Centers for Disease Control and Prevention in Atlanta, Georgia. She commented in an editorial accompanying the report by Moon et al. (JAMA. 2017;318:336-8). Dr. Shapiro-Mendoza had no financial conflicts to disclose.
In this study by Dr. Moon and her associates, new mothers who received both the nursing educational intervention and mobile intervention for safe sleep reported the highest percentages for adhering to safe sleep practices, and moms who received the safe sleep mobile intervention alone had the second-highest percentages.
However, the study was underpowered and too short termed to determine whether this intervention actually will reduce the occurrence of SIDS.
Limitations of this study include that the mothers who did not respond at follow-up were more likely to be younger, black, single, and less educated – all risk factors for SIDS. The study also was restricted to healthy term infants, and preterm babies are another high-risk SIDS group.
Nonetheless, the fact that this study chose to use multifaceted approaches was promising, combining “health messaging, education of health care professionals, and interventions aimed at reducing barriers to safe sleep practices for infant caregivers.” Whatever interventions are tried, they “need to be adapted for implementation among the highest-risk groups such as non-Hispanic black, American Indian, and Alaskan Native mothers and families because these are the populations with the highest rates of SIDS and sleep-related infant death.”
Carrie K. Shapiro-Mendoza, PhD, MPH is affiliated with the division of reproductive health at the Centers for Disease Control and Prevention in Atlanta, Georgia. She commented in an editorial accompanying the report by Moon et al. (JAMA. 2017;318:336-8). Dr. Shapiro-Mendoza had no financial conflicts to disclose.
In this study by Dr. Moon and her associates, new mothers who received both the nursing educational intervention and mobile intervention for safe sleep reported the highest percentages for adhering to safe sleep practices, and moms who received the safe sleep mobile intervention alone had the second-highest percentages.
However, the study was underpowered and too short termed to determine whether this intervention actually will reduce the occurrence of SIDS.
Limitations of this study include that the mothers who did not respond at follow-up were more likely to be younger, black, single, and less educated – all risk factors for SIDS. The study also was restricted to healthy term infants, and preterm babies are another high-risk SIDS group.
Nonetheless, the fact that this study chose to use multifaceted approaches was promising, combining “health messaging, education of health care professionals, and interventions aimed at reducing barriers to safe sleep practices for infant caregivers.” Whatever interventions are tried, they “need to be adapted for implementation among the highest-risk groups such as non-Hispanic black, American Indian, and Alaskan Native mothers and families because these are the populations with the highest rates of SIDS and sleep-related infant death.”
Carrie K. Shapiro-Mendoza, PhD, MPH is affiliated with the division of reproductive health at the Centers for Disease Control and Prevention in Atlanta, Georgia. She commented in an editorial accompanying the report by Moon et al. (JAMA. 2017;318:336-8). Dr. Shapiro-Mendoza had no financial conflicts to disclose.
Mobile health interventions targeting mothers of healthy newborns significantly improved safe sleep practices, compared with controls, in a randomized trial of 1,600 mothers published online July 25 in JAMA.
Despite the success of the Back to Sleep campaign in reducing rates of sudden infant death syndrome, approximately 3,500 infant deaths due to SIDS, accidental suffocation, or strangulation in bed occurred in 2014, wrote Rachel Y. Moon, MD, of the University of Virginia, Charlottesville, and her colleagues (JAMA. 2017;318:351-9).
A total of 1,263 mothers completed the study, and mothers who received the mobile messages about safe sleep were significantly more likely than those who received the control messages to engage in safe sleep practices, including placing babies on their backs (89% vs. 80%), sharing a room without cosleeping (83% vs. 70%), avoiding the use of soft bedding (79% vs. 68%), and use of pacifiers (69% vs. 60%). The initial nursing quality intervention alone had no significant impact on any of the safe sleep practices, the researchers noted.
The results were limited by several factors, including the 21% lost to follow up and lack of data on adverse events and clinical outcomes, the researchers said. However, the results suggest that mobile messages could be cost effective and easily implemented by hospitals.
“Furthermore, because the rates of opening and viewing messages in this study were consistently higher than 50%, and almost all adults now have cell phones or email access, it is likely that this type of intervention would be feasible and well received by parents,” Dr. Moon and her associates added. “Whether widespread implementation is feasible or if it reduces sudden and unexpected infant death rates remains to be studied.”
“The messages and videos were timed to address challenges and questions that arise at specific time points; therefore, providing this additional information to parents at critical times may have been important in assuaging concerns about adherence to recommended practices. Furthermore, receiving frequent videos and email or text messages may have served as a virtual support system for mothers, reinforcing safe parental practices,” Dr. Moon and her associates noted.
The researchers had no financial conflicts to disclose. The study was supported in part by the National Institute of Child Health and Human Development and by the CJ Foundation for SIDS.
Mobile health interventions targeting mothers of healthy newborns significantly improved safe sleep practices, compared with controls, in a randomized trial of 1,600 mothers published online July 25 in JAMA.
Despite the success of the Back to Sleep campaign in reducing rates of sudden infant death syndrome, approximately 3,500 infant deaths due to SIDS, accidental suffocation, or strangulation in bed occurred in 2014, wrote Rachel Y. Moon, MD, of the University of Virginia, Charlottesville, and her colleagues (JAMA. 2017;318:351-9).
A total of 1,263 mothers completed the study, and mothers who received the mobile messages about safe sleep were significantly more likely than those who received the control messages to engage in safe sleep practices, including placing babies on their backs (89% vs. 80%), sharing a room without cosleeping (83% vs. 70%), avoiding the use of soft bedding (79% vs. 68%), and use of pacifiers (69% vs. 60%). The initial nursing quality intervention alone had no significant impact on any of the safe sleep practices, the researchers noted.
The results were limited by several factors, including the 21% lost to follow up and lack of data on adverse events and clinical outcomes, the researchers said. However, the results suggest that mobile messages could be cost effective and easily implemented by hospitals.
“Furthermore, because the rates of opening and viewing messages in this study were consistently higher than 50%, and almost all adults now have cell phones or email access, it is likely that this type of intervention would be feasible and well received by parents,” Dr. Moon and her associates added. “Whether widespread implementation is feasible or if it reduces sudden and unexpected infant death rates remains to be studied.”
“The messages and videos were timed to address challenges and questions that arise at specific time points; therefore, providing this additional information to parents at critical times may have been important in assuaging concerns about adherence to recommended practices. Furthermore, receiving frequent videos and email or text messages may have served as a virtual support system for mothers, reinforcing safe parental practices,” Dr. Moon and her associates noted.
The researchers had no financial conflicts to disclose. The study was supported in part by the National Institute of Child Health and Human Development and by the CJ Foundation for SIDS.
FROM JAMA
Key clinical point: Email and text messages effectively communicated safe infant sleep practices to mothers.
Major finding: Overall,
Data source: The data come from a randomized trial of 1,600 mothers with healthy newborns.
Disclosures: The researchers had no financial conflicts to disclose. The study was supported in part by the National Institute of Child Health and Human Development and by the CJ Foundation for SIDS.
Extreme caffeine use in early pregnancy risks offspring behavioral disorders
, Susanne Hvolgaard Mikkelsen, PhD, of Aarhus (Denmark) University and her associates said.
“Caffeine metabolism is delayed during pregnancy, allowing longer opportunities for absorption. Caffeine increases dopamine levels by slowing down the rate of dopamine reabsorption, and this dysfunction of the dopaminergic system is thought to explain part of the association between caffeine consumption and offspring behavioral disorders,” the investigators said.
From 1996 to 2002, Danish physicians recruited women during their first pregnancy visit to participate in a study evaluating daily coffee and tea consumption during pregnancy, with follow-up of the children at age 11 years using the Danish National Birth Cohort. A follow-up questionnaire, including the Strength and Difficulties Questionnaire, was completed by the children, their parents, and their teachers; the results were paired with data on coffee and tea consumption by the mothers of 47,491 children. At 15 weeks’ gestation, 12% of the women had consumed more than three cups/day of coffee, and 3% ingested eight or more cups/day of coffee; 19% of the women drank more than three cups/day of tea, and 4% drank eight or more cups/day of tea. The amount of coffee and tea consumed at 30 weeks’ gestation was only slightly less.
The percentage of children predicted to have “possible/probable” ADHD was 5%, conduct-oppositional disorders was 7%, anxiety-depressive disorders was 7%, and any psychiatric disorder was 14%.
A statistically significant association was found between maternal consumption of eight or more cups of coffee at 15 weeks’ gestation and greater risk of ADHD (a risk ratio [RR] of 1.47), conduct-oppositional disorder (RR, 1.22), and any psychiatric disorder (RR, 1.23). A statistically significant link was found between maternal consumption of eight or more cups of tea at 15 weeks’ gestation and greater risk of conduct-oppositional disorder (RR, 1.21). There was a statistically significant association between maternal consumption of eight or more cups of tea at 15 weeks’ gestation and a higher risk of anxiety-depressive disorder (RR, 1.28), but not for coffee. These significant relationships were not found again at 30 weeks’ gestation.
“The period of greatest prenatal brain development is 10-26 weeks after conception,which may explain our results suggesting the fetus to be more vulnerable to caffeine in first and second trimester, compared with the third trimester,” the researchers said.
There was no information available about caffeine consumption by chocolate, energy drinks, or medication; the measure of cola consumption also was not taken into account. Confounding by genetics or socioeconomic factors may affect these findings, Dr. Mikkelsen and her associates said.
Read more at (J Pediatr. 2017 Jul 18. doi: 10.1016/j.jpeds.2017.06.051).
, Susanne Hvolgaard Mikkelsen, PhD, of Aarhus (Denmark) University and her associates said.
“Caffeine metabolism is delayed during pregnancy, allowing longer opportunities for absorption. Caffeine increases dopamine levels by slowing down the rate of dopamine reabsorption, and this dysfunction of the dopaminergic system is thought to explain part of the association between caffeine consumption and offspring behavioral disorders,” the investigators said.
From 1996 to 2002, Danish physicians recruited women during their first pregnancy visit to participate in a study evaluating daily coffee and tea consumption during pregnancy, with follow-up of the children at age 11 years using the Danish National Birth Cohort. A follow-up questionnaire, including the Strength and Difficulties Questionnaire, was completed by the children, their parents, and their teachers; the results were paired with data on coffee and tea consumption by the mothers of 47,491 children. At 15 weeks’ gestation, 12% of the women had consumed more than three cups/day of coffee, and 3% ingested eight or more cups/day of coffee; 19% of the women drank more than three cups/day of tea, and 4% drank eight or more cups/day of tea. The amount of coffee and tea consumed at 30 weeks’ gestation was only slightly less.
The percentage of children predicted to have “possible/probable” ADHD was 5%, conduct-oppositional disorders was 7%, anxiety-depressive disorders was 7%, and any psychiatric disorder was 14%.
A statistically significant association was found between maternal consumption of eight or more cups of coffee at 15 weeks’ gestation and greater risk of ADHD (a risk ratio [RR] of 1.47), conduct-oppositional disorder (RR, 1.22), and any psychiatric disorder (RR, 1.23). A statistically significant link was found between maternal consumption of eight or more cups of tea at 15 weeks’ gestation and greater risk of conduct-oppositional disorder (RR, 1.21). There was a statistically significant association between maternal consumption of eight or more cups of tea at 15 weeks’ gestation and a higher risk of anxiety-depressive disorder (RR, 1.28), but not for coffee. These significant relationships were not found again at 30 weeks’ gestation.
“The period of greatest prenatal brain development is 10-26 weeks after conception,which may explain our results suggesting the fetus to be more vulnerable to caffeine in first and second trimester, compared with the third trimester,” the researchers said.
There was no information available about caffeine consumption by chocolate, energy drinks, or medication; the measure of cola consumption also was not taken into account. Confounding by genetics or socioeconomic factors may affect these findings, Dr. Mikkelsen and her associates said.
Read more at (J Pediatr. 2017 Jul 18. doi: 10.1016/j.jpeds.2017.06.051).
, Susanne Hvolgaard Mikkelsen, PhD, of Aarhus (Denmark) University and her associates said.
“Caffeine metabolism is delayed during pregnancy, allowing longer opportunities for absorption. Caffeine increases dopamine levels by slowing down the rate of dopamine reabsorption, and this dysfunction of the dopaminergic system is thought to explain part of the association between caffeine consumption and offspring behavioral disorders,” the investigators said.
From 1996 to 2002, Danish physicians recruited women during their first pregnancy visit to participate in a study evaluating daily coffee and tea consumption during pregnancy, with follow-up of the children at age 11 years using the Danish National Birth Cohort. A follow-up questionnaire, including the Strength and Difficulties Questionnaire, was completed by the children, their parents, and their teachers; the results were paired with data on coffee and tea consumption by the mothers of 47,491 children. At 15 weeks’ gestation, 12% of the women had consumed more than three cups/day of coffee, and 3% ingested eight or more cups/day of coffee; 19% of the women drank more than three cups/day of tea, and 4% drank eight or more cups/day of tea. The amount of coffee and tea consumed at 30 weeks’ gestation was only slightly less.
The percentage of children predicted to have “possible/probable” ADHD was 5%, conduct-oppositional disorders was 7%, anxiety-depressive disorders was 7%, and any psychiatric disorder was 14%.
A statistically significant association was found between maternal consumption of eight or more cups of coffee at 15 weeks’ gestation and greater risk of ADHD (a risk ratio [RR] of 1.47), conduct-oppositional disorder (RR, 1.22), and any psychiatric disorder (RR, 1.23). A statistically significant link was found between maternal consumption of eight or more cups of tea at 15 weeks’ gestation and greater risk of conduct-oppositional disorder (RR, 1.21). There was a statistically significant association between maternal consumption of eight or more cups of tea at 15 weeks’ gestation and a higher risk of anxiety-depressive disorder (RR, 1.28), but not for coffee. These significant relationships were not found again at 30 weeks’ gestation.
“The period of greatest prenatal brain development is 10-26 weeks after conception,which may explain our results suggesting the fetus to be more vulnerable to caffeine in first and second trimester, compared with the third trimester,” the researchers said.
There was no information available about caffeine consumption by chocolate, energy drinks, or medication; the measure of cola consumption also was not taken into account. Confounding by genetics or socioeconomic factors may affect these findings, Dr. Mikkelsen and her associates said.
Read more at (J Pediatr. 2017 Jul 18. doi: 10.1016/j.jpeds.2017.06.051).
FROM THE JOURNAL OF PEDIATRICS
Procalcitonin-guided decision making found best in neonatal suspected sepsis
, according to an investigator-initiated, superiority and noninferiority, multicenter, randomized controlled intervention study.
In the Neonatal Procalcitonin Intervention Study (NeoPInS) study aimed to reduce the duration of antibiotic treatment, 1,710 neonates who were already receiving antibiotic therapy were enrolled in 18 hospitals in Canada, the Czech Republic, the Netherlands, and Switzerland, and randomized to procalcitonin-guided decision making or standard care. Some patients were excluded from the larger initial population because of congenital malformation, surgery in the first week of life, or a lack of parental consent, among other reasons.
The intention-to-treat population included 866 neonates in the procalcitonin group whose median treatment duration was 55 hours (95% confidence interval [CI], 50.5-60.0) and 844 in the standard group whose median treatment duration was 65 hours (95% CI, 63.0-69.0; P less than .0001), with a median difference of –9.9. The per-protocol population saw an even greater median difference of –12.2, with a treatment duration of 52 hours in 745 neonates assigned to procalcitonin-guided decision making (95% CI, 48.2-56.0) and a duration of 64 hours in 663 neonates assigned to standard care (95% CI, 61.0-68.1; P less than .0001).
Length of hospital stay also was significantly shorter in both procalcitonin groups, according to the researchers.
One neonate in the standard group died from the consequences of severe perinatal asphyxia. In each procalcitonin group, five (0.6% and 0.7%, respectively) had a suspected reinfection, compared with three in each of the standard groups (0.5% and 0.6%, respectively). None of the neonates suspected of reinfection had a culture-proven bacteria infection.
“In this era of globally increasing antibiotic resistance rates ... every dose of antimicrobial therapy counts in the emergence of antimicrobial resistance and in changing the human microbiome,” the investigators wrote, “and other evidence suggests that changes in the microbiome in early life are particularly important in shaping the individual’s immune system and future health.”
The primary outcomes of the study were duration of treatment and reinfection or death in the first month of life, with a secondary outcome of length of hospital stay. Noninferiority for reinfection and death could not be shown because there were too few of these events.
“Procalcitonin-guided decision making led to a significant reduction in duration of empirical antibiotic therapy and hospital stay in term and near-term neonates with suspected early-onset sepsis, with a low rate of reinfections and with no study-related mortality. Combining serial procalcitonin measurements with initial assessment based on perinatal risk factors, the neonate’s clinical signs and symptoms, and conventional laboratory variables support antimicrobial stewardship and help physicians to decide to discontinue antibiotic treatment sooner in neonates classified as having low or moderate risk of infection,” Dr. Stocker, Dr. van Herk, and their associates said.
A limitation was that results cannot be extrapolated to other populations. The neonates in this study all had easy and low-threshold access to care, so the results may not apply to low-income or low-access areas, they said.
Length of hospital stay results may be affected by other conditions developed by the neonates. “It would be interesting to analyze duration of hospital stay separately for neonates with or without other reasons for hospital admission, but this was not possible in our study design,” the investigators said.
The authors reported no relevant financial disclosures. The Thrasher Foundation, the NutsOhra Foundation, and the Sophia Foundation for Scientific Research funded the study.
Dr. Stocker, Dr. Herk, and their coauthors “are to be commended for conducting this pragmatic and international, multicenter trial.” When withholding antibiotics, especially from neonates who are susceptible to infection, safety is key. In this “landmark study done in neonates,” the authors determined that procalcitonin can be safely used to guide decision making in neonates at low risk of sepsis. Using procalcitonin in a clinical algorithm also was effective, reducing the duration of antibiotic therapy by around 20% and the length of hospital stay by around 5%.
Although the biomarker is the most accurate diagnostic tool currently available, it should be used with caution. On its own, it is not enough to determine if a neonate is in need of continued antibiotic therapy. It should be used in conjunction with clinical examination and reasoning on the part of the physician. In addition, the cutoffs that have been determined for adults are too low for neonates. “Nevertheless, if the cutoff is adapted accordingly and embedded in a reasonable clinical algorithm – as done in the NeoPInS study – procalcitonin can and should be used to improve antibiotic stewardship.
“Procalcitonin-guided antibiotic de-escalation therapy is evidence based and state of the art for antibiotic therapy for suspected and proven bacterial infection in different clinical settings and different acuity of infections.”
Philipp Schuetz, MD, and Beat Mueller, MD, are of the University of Basel, Aarau, Switzerland. Dr. Schuetz received support from BRAHMS/Thermofisher and bioMérieux to attend meetings, fulfill speaking engagements, and for unrestricted research grants related to procalcitonin. Dr. Mueller has received research support, as well as consulting fees and speakers’ honoraria, from BRAHMS/Thermofisher and bioMérieux, related to procalcitonin. They made these remarks in a commentary accompanying Dr. Stocker’s and Dr. van Herk’s report (Lancet. 2017 Jul 12. doi: 10.1016/S0140-6736[17]31628-8).
Dr. Stocker, Dr. Herk, and their coauthors “are to be commended for conducting this pragmatic and international, multicenter trial.” When withholding antibiotics, especially from neonates who are susceptible to infection, safety is key. In this “landmark study done in neonates,” the authors determined that procalcitonin can be safely used to guide decision making in neonates at low risk of sepsis. Using procalcitonin in a clinical algorithm also was effective, reducing the duration of antibiotic therapy by around 20% and the length of hospital stay by around 5%.
Although the biomarker is the most accurate diagnostic tool currently available, it should be used with caution. On its own, it is not enough to determine if a neonate is in need of continued antibiotic therapy. It should be used in conjunction with clinical examination and reasoning on the part of the physician. In addition, the cutoffs that have been determined for adults are too low for neonates. “Nevertheless, if the cutoff is adapted accordingly and embedded in a reasonable clinical algorithm – as done in the NeoPInS study – procalcitonin can and should be used to improve antibiotic stewardship.
“Procalcitonin-guided antibiotic de-escalation therapy is evidence based and state of the art for antibiotic therapy for suspected and proven bacterial infection in different clinical settings and different acuity of infections.”
Philipp Schuetz, MD, and Beat Mueller, MD, are of the University of Basel, Aarau, Switzerland. Dr. Schuetz received support from BRAHMS/Thermofisher and bioMérieux to attend meetings, fulfill speaking engagements, and for unrestricted research grants related to procalcitonin. Dr. Mueller has received research support, as well as consulting fees and speakers’ honoraria, from BRAHMS/Thermofisher and bioMérieux, related to procalcitonin. They made these remarks in a commentary accompanying Dr. Stocker’s and Dr. van Herk’s report (Lancet. 2017 Jul 12. doi: 10.1016/S0140-6736[17]31628-8).
Dr. Stocker, Dr. Herk, and their coauthors “are to be commended for conducting this pragmatic and international, multicenter trial.” When withholding antibiotics, especially from neonates who are susceptible to infection, safety is key. In this “landmark study done in neonates,” the authors determined that procalcitonin can be safely used to guide decision making in neonates at low risk of sepsis. Using procalcitonin in a clinical algorithm also was effective, reducing the duration of antibiotic therapy by around 20% and the length of hospital stay by around 5%.
Although the biomarker is the most accurate diagnostic tool currently available, it should be used with caution. On its own, it is not enough to determine if a neonate is in need of continued antibiotic therapy. It should be used in conjunction with clinical examination and reasoning on the part of the physician. In addition, the cutoffs that have been determined for adults are too low for neonates. “Nevertheless, if the cutoff is adapted accordingly and embedded in a reasonable clinical algorithm – as done in the NeoPInS study – procalcitonin can and should be used to improve antibiotic stewardship.
“Procalcitonin-guided antibiotic de-escalation therapy is evidence based and state of the art for antibiotic therapy for suspected and proven bacterial infection in different clinical settings and different acuity of infections.”
Philipp Schuetz, MD, and Beat Mueller, MD, are of the University of Basel, Aarau, Switzerland. Dr. Schuetz received support from BRAHMS/Thermofisher and bioMérieux to attend meetings, fulfill speaking engagements, and for unrestricted research grants related to procalcitonin. Dr. Mueller has received research support, as well as consulting fees and speakers’ honoraria, from BRAHMS/Thermofisher and bioMérieux, related to procalcitonin. They made these remarks in a commentary accompanying Dr. Stocker’s and Dr. van Herk’s report (Lancet. 2017 Jul 12. doi: 10.1016/S0140-6736[17]31628-8).
, according to an investigator-initiated, superiority and noninferiority, multicenter, randomized controlled intervention study.
In the Neonatal Procalcitonin Intervention Study (NeoPInS) study aimed to reduce the duration of antibiotic treatment, 1,710 neonates who were already receiving antibiotic therapy were enrolled in 18 hospitals in Canada, the Czech Republic, the Netherlands, and Switzerland, and randomized to procalcitonin-guided decision making or standard care. Some patients were excluded from the larger initial population because of congenital malformation, surgery in the first week of life, or a lack of parental consent, among other reasons.
The intention-to-treat population included 866 neonates in the procalcitonin group whose median treatment duration was 55 hours (95% confidence interval [CI], 50.5-60.0) and 844 in the standard group whose median treatment duration was 65 hours (95% CI, 63.0-69.0; P less than .0001), with a median difference of –9.9. The per-protocol population saw an even greater median difference of –12.2, with a treatment duration of 52 hours in 745 neonates assigned to procalcitonin-guided decision making (95% CI, 48.2-56.0) and a duration of 64 hours in 663 neonates assigned to standard care (95% CI, 61.0-68.1; P less than .0001).
Length of hospital stay also was significantly shorter in both procalcitonin groups, according to the researchers.
One neonate in the standard group died from the consequences of severe perinatal asphyxia. In each procalcitonin group, five (0.6% and 0.7%, respectively) had a suspected reinfection, compared with three in each of the standard groups (0.5% and 0.6%, respectively). None of the neonates suspected of reinfection had a culture-proven bacteria infection.
“In this era of globally increasing antibiotic resistance rates ... every dose of antimicrobial therapy counts in the emergence of antimicrobial resistance and in changing the human microbiome,” the investigators wrote, “and other evidence suggests that changes in the microbiome in early life are particularly important in shaping the individual’s immune system and future health.”
The primary outcomes of the study were duration of treatment and reinfection or death in the first month of life, with a secondary outcome of length of hospital stay. Noninferiority for reinfection and death could not be shown because there were too few of these events.
“Procalcitonin-guided decision making led to a significant reduction in duration of empirical antibiotic therapy and hospital stay in term and near-term neonates with suspected early-onset sepsis, with a low rate of reinfections and with no study-related mortality. Combining serial procalcitonin measurements with initial assessment based on perinatal risk factors, the neonate’s clinical signs and symptoms, and conventional laboratory variables support antimicrobial stewardship and help physicians to decide to discontinue antibiotic treatment sooner in neonates classified as having low or moderate risk of infection,” Dr. Stocker, Dr. van Herk, and their associates said.
A limitation was that results cannot be extrapolated to other populations. The neonates in this study all had easy and low-threshold access to care, so the results may not apply to low-income or low-access areas, they said.
Length of hospital stay results may be affected by other conditions developed by the neonates. “It would be interesting to analyze duration of hospital stay separately for neonates with or without other reasons for hospital admission, but this was not possible in our study design,” the investigators said.
The authors reported no relevant financial disclosures. The Thrasher Foundation, the NutsOhra Foundation, and the Sophia Foundation for Scientific Research funded the study.
, according to an investigator-initiated, superiority and noninferiority, multicenter, randomized controlled intervention study.
In the Neonatal Procalcitonin Intervention Study (NeoPInS) study aimed to reduce the duration of antibiotic treatment, 1,710 neonates who were already receiving antibiotic therapy were enrolled in 18 hospitals in Canada, the Czech Republic, the Netherlands, and Switzerland, and randomized to procalcitonin-guided decision making or standard care. Some patients were excluded from the larger initial population because of congenital malformation, surgery in the first week of life, or a lack of parental consent, among other reasons.
The intention-to-treat population included 866 neonates in the procalcitonin group whose median treatment duration was 55 hours (95% confidence interval [CI], 50.5-60.0) and 844 in the standard group whose median treatment duration was 65 hours (95% CI, 63.0-69.0; P less than .0001), with a median difference of –9.9. The per-protocol population saw an even greater median difference of –12.2, with a treatment duration of 52 hours in 745 neonates assigned to procalcitonin-guided decision making (95% CI, 48.2-56.0) and a duration of 64 hours in 663 neonates assigned to standard care (95% CI, 61.0-68.1; P less than .0001).
Length of hospital stay also was significantly shorter in both procalcitonin groups, according to the researchers.
One neonate in the standard group died from the consequences of severe perinatal asphyxia. In each procalcitonin group, five (0.6% and 0.7%, respectively) had a suspected reinfection, compared with three in each of the standard groups (0.5% and 0.6%, respectively). None of the neonates suspected of reinfection had a culture-proven bacteria infection.
“In this era of globally increasing antibiotic resistance rates ... every dose of antimicrobial therapy counts in the emergence of antimicrobial resistance and in changing the human microbiome,” the investigators wrote, “and other evidence suggests that changes in the microbiome in early life are particularly important in shaping the individual’s immune system and future health.”
The primary outcomes of the study were duration of treatment and reinfection or death in the first month of life, with a secondary outcome of length of hospital stay. Noninferiority for reinfection and death could not be shown because there were too few of these events.
“Procalcitonin-guided decision making led to a significant reduction in duration of empirical antibiotic therapy and hospital stay in term and near-term neonates with suspected early-onset sepsis, with a low rate of reinfections and with no study-related mortality. Combining serial procalcitonin measurements with initial assessment based on perinatal risk factors, the neonate’s clinical signs and symptoms, and conventional laboratory variables support antimicrobial stewardship and help physicians to decide to discontinue antibiotic treatment sooner in neonates classified as having low or moderate risk of infection,” Dr. Stocker, Dr. van Herk, and their associates said.
A limitation was that results cannot be extrapolated to other populations. The neonates in this study all had easy and low-threshold access to care, so the results may not apply to low-income or low-access areas, they said.
Length of hospital stay results may be affected by other conditions developed by the neonates. “It would be interesting to analyze duration of hospital stay separately for neonates with or without other reasons for hospital admission, but this was not possible in our study design,” the investigators said.
The authors reported no relevant financial disclosures. The Thrasher Foundation, the NutsOhra Foundation, and the Sophia Foundation for Scientific Research funded the study.
FROM THE LANCET
Key clinical point: Procalcitonin-guided decision making had a significant impact on duration of antibiotic treatment and hospital stay in pediatric patients with suspected early-onset sepsis.
Major finding: The median differences in antibiotic treatment duration between procalcitonin groups and standard care groups were –9.9 and –12.1 hours in intention-to-treat and per protocol study populations.
Data source: An investigator-initiated, superiority and noninferiority, multicenter, randomized controlled intervention study (NeoPInS) of 1,710 neonates with suspected early-stage sepsis.
Disclosures: The authors reported no relevant financial disclosures. The Thrasher Foundation, the NutsOhra Foundation, and the Sophia Foundation for Scientific Research funded the study.
Early neuroimaging essential for Zika-exposed neonates
DENVER – The experience gleaned at ground zero of the Brazilian Zika virus epidemic drives home a clinical imperative: every neonate whose pregnant mother has presumed or confirmed Zika infection needs to undergo prompt neuroimaging, even if head circumference at birth is normal, Vanessa van der Linden, MD, said at the annual meeting of the Teratology Society.
Dr. van der Linden, a pediatric neurologist at the Association for Assistance of Disabled Children in Recife, Brazil, has done pioneering work in characterizing the recently recognized congenital Zika syndrome. She was the lead author of the first report of infants who had laboratory evidence of congenital Zika infection and normal head circumference at birth but who developed poor head growth and microcephaly later in infancy.
Comprehensive multispecialty medical and developmental follow-up documented that 10 of 13 infants had dysphagia, 7 had epilepsy, 3 had chorioretinal abnormalities, all 13 had hypertonia, and 12 had pyramidal and extrapyramidal signs with dystonia (MMWR. 2016 Dec 2;65[47]:1343-8).
In another recent publication, Dr. van der Linden and her coinvestigators described classic congenital Zika syndrome with microcephaly at birth as simply the tip of the Zika virus iceberg. In their retrospective review of 77 infants exposed to Zika in utero, 9 had microcephaly at birth, 7 developed microcephaly postnatally, and 3 didn’t have microcephaly at all. Those with microcephaly at birth showed the traditional neuroimaging findings of congenital Zika syndrome, including reduced brain volume, ventriculomegaly, subcortical calcifications, corpus callosum abnormalities, and an enlarged extra-axial space.
Those who subsequently developed microcephaly later in infancy showed most of the same neuroimaging abnormalities. The three infants who remained normocephalic displayed calcifications in the cortico-subcortical junction, asymmetric frontal polymicrogyria, delayed myelination, and milder ventriculomegaly than in the other two groups (AJNR Am J Neuroradiol. 2017 Jul;38[7]:1427-34).
The rehabilitation center where Dr. van der Linden and her colleagues are currently following roughly 200 children with congenital Zika syndrome is in the state of Pernambuco, which was particularly hard hit by the Zika epidemic.
She reported having no relevant financial disclosures.
DENVER – The experience gleaned at ground zero of the Brazilian Zika virus epidemic drives home a clinical imperative: every neonate whose pregnant mother has presumed or confirmed Zika infection needs to undergo prompt neuroimaging, even if head circumference at birth is normal, Vanessa van der Linden, MD, said at the annual meeting of the Teratology Society.
Dr. van der Linden, a pediatric neurologist at the Association for Assistance of Disabled Children in Recife, Brazil, has done pioneering work in characterizing the recently recognized congenital Zika syndrome. She was the lead author of the first report of infants who had laboratory evidence of congenital Zika infection and normal head circumference at birth but who developed poor head growth and microcephaly later in infancy.
Comprehensive multispecialty medical and developmental follow-up documented that 10 of 13 infants had dysphagia, 7 had epilepsy, 3 had chorioretinal abnormalities, all 13 had hypertonia, and 12 had pyramidal and extrapyramidal signs with dystonia (MMWR. 2016 Dec 2;65[47]:1343-8).
In another recent publication, Dr. van der Linden and her coinvestigators described classic congenital Zika syndrome with microcephaly at birth as simply the tip of the Zika virus iceberg. In their retrospective review of 77 infants exposed to Zika in utero, 9 had microcephaly at birth, 7 developed microcephaly postnatally, and 3 didn’t have microcephaly at all. Those with microcephaly at birth showed the traditional neuroimaging findings of congenital Zika syndrome, including reduced brain volume, ventriculomegaly, subcortical calcifications, corpus callosum abnormalities, and an enlarged extra-axial space.
Those who subsequently developed microcephaly later in infancy showed most of the same neuroimaging abnormalities. The three infants who remained normocephalic displayed calcifications in the cortico-subcortical junction, asymmetric frontal polymicrogyria, delayed myelination, and milder ventriculomegaly than in the other two groups (AJNR Am J Neuroradiol. 2017 Jul;38[7]:1427-34).
The rehabilitation center where Dr. van der Linden and her colleagues are currently following roughly 200 children with congenital Zika syndrome is in the state of Pernambuco, which was particularly hard hit by the Zika epidemic.
She reported having no relevant financial disclosures.
DENVER – The experience gleaned at ground zero of the Brazilian Zika virus epidemic drives home a clinical imperative: every neonate whose pregnant mother has presumed or confirmed Zika infection needs to undergo prompt neuroimaging, even if head circumference at birth is normal, Vanessa van der Linden, MD, said at the annual meeting of the Teratology Society.
Dr. van der Linden, a pediatric neurologist at the Association for Assistance of Disabled Children in Recife, Brazil, has done pioneering work in characterizing the recently recognized congenital Zika syndrome. She was the lead author of the first report of infants who had laboratory evidence of congenital Zika infection and normal head circumference at birth but who developed poor head growth and microcephaly later in infancy.
Comprehensive multispecialty medical and developmental follow-up documented that 10 of 13 infants had dysphagia, 7 had epilepsy, 3 had chorioretinal abnormalities, all 13 had hypertonia, and 12 had pyramidal and extrapyramidal signs with dystonia (MMWR. 2016 Dec 2;65[47]:1343-8).
In another recent publication, Dr. van der Linden and her coinvestigators described classic congenital Zika syndrome with microcephaly at birth as simply the tip of the Zika virus iceberg. In their retrospective review of 77 infants exposed to Zika in utero, 9 had microcephaly at birth, 7 developed microcephaly postnatally, and 3 didn’t have microcephaly at all. Those with microcephaly at birth showed the traditional neuroimaging findings of congenital Zika syndrome, including reduced brain volume, ventriculomegaly, subcortical calcifications, corpus callosum abnormalities, and an enlarged extra-axial space.
Those who subsequently developed microcephaly later in infancy showed most of the same neuroimaging abnormalities. The three infants who remained normocephalic displayed calcifications in the cortico-subcortical junction, asymmetric frontal polymicrogyria, delayed myelination, and milder ventriculomegaly than in the other two groups (AJNR Am J Neuroradiol. 2017 Jul;38[7]:1427-34).
The rehabilitation center where Dr. van der Linden and her colleagues are currently following roughly 200 children with congenital Zika syndrome is in the state of Pernambuco, which was particularly hard hit by the Zika epidemic.
She reported having no relevant financial disclosures.
EXPERT ANALYSIS FROM TERATOLOGY SOCIETY 2017