User login
VIDEO: Don’t miss reservoirs when treating recurrent onychomycosis
WAILEA, HAWAII – Patients attribute every nail problem to nail fungus, but part of the problem with nails is concomitant tinea pedis, tinea corporis, and other reservoirs of infection, according to Neal Bhatia, MD, director of clinical dermatology at Therapeutics Clinical Research in San Diego.
Failure to locate and treat these other reservoirs can lead to recurrence of the nail infection, Dr. Bhatia said at the Hawaii Dermatology Seminar provided by Global Academy for Medical Education/Skin Disease Education Foundation.
“If we aren’t treating the skin as well as the nails, it is creating a reservoir effect that reaccumulates in the nail itself,” he said. Similarly, the nails can serve as a reservoir of infection for the skin.
“That makes it all the more important” to treat both skin and nails and “treat through the disease state,” he commented in a video interview. Cost can influence treatment decisions, and there is a role for both topical and systemic therapy, he said. However, “if there’s not adequate testing or if there’s not a good proof of the diagnosis, we are just losing out no matter what we use,” he cautioned.
Dr. Bhatia disclosed relationships with companies including Actavis, Aqua, Allergan, Anacor, Bayer, Biofrontera, Biopharmx, Dermira, Dusa, Exceltis, Ferndale, Foamix, Galderma, Intraderm, ISDIN, LaRoche-Posay, Leo, Novartis, Sanofi, and Valeant.
SDEF and this news organization are owned by the same parent company.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
WAILEA, HAWAII – Patients attribute every nail problem to nail fungus, but part of the problem with nails is concomitant tinea pedis, tinea corporis, and other reservoirs of infection, according to Neal Bhatia, MD, director of clinical dermatology at Therapeutics Clinical Research in San Diego.
Failure to locate and treat these other reservoirs can lead to recurrence of the nail infection, Dr. Bhatia said at the Hawaii Dermatology Seminar provided by Global Academy for Medical Education/Skin Disease Education Foundation.
“If we aren’t treating the skin as well as the nails, it is creating a reservoir effect that reaccumulates in the nail itself,” he said. Similarly, the nails can serve as a reservoir of infection for the skin.
“That makes it all the more important” to treat both skin and nails and “treat through the disease state,” he commented in a video interview. Cost can influence treatment decisions, and there is a role for both topical and systemic therapy, he said. However, “if there’s not adequate testing or if there’s not a good proof of the diagnosis, we are just losing out no matter what we use,” he cautioned.
Dr. Bhatia disclosed relationships with companies including Actavis, Aqua, Allergan, Anacor, Bayer, Biofrontera, Biopharmx, Dermira, Dusa, Exceltis, Ferndale, Foamix, Galderma, Intraderm, ISDIN, LaRoche-Posay, Leo, Novartis, Sanofi, and Valeant.
SDEF and this news organization are owned by the same parent company.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
WAILEA, HAWAII – Patients attribute every nail problem to nail fungus, but part of the problem with nails is concomitant tinea pedis, tinea corporis, and other reservoirs of infection, according to Neal Bhatia, MD, director of clinical dermatology at Therapeutics Clinical Research in San Diego.
Failure to locate and treat these other reservoirs can lead to recurrence of the nail infection, Dr. Bhatia said at the Hawaii Dermatology Seminar provided by Global Academy for Medical Education/Skin Disease Education Foundation.
“If we aren’t treating the skin as well as the nails, it is creating a reservoir effect that reaccumulates in the nail itself,” he said. Similarly, the nails can serve as a reservoir of infection for the skin.
“That makes it all the more important” to treat both skin and nails and “treat through the disease state,” he commented in a video interview. Cost can influence treatment decisions, and there is a role for both topical and systemic therapy, he said. However, “if there’s not adequate testing or if there’s not a good proof of the diagnosis, we are just losing out no matter what we use,” he cautioned.
Dr. Bhatia disclosed relationships with companies including Actavis, Aqua, Allergan, Anacor, Bayer, Biofrontera, Biopharmx, Dermira, Dusa, Exceltis, Ferndale, Foamix, Galderma, Intraderm, ISDIN, LaRoche-Posay, Leo, Novartis, Sanofi, and Valeant.
SDEF and this news organization are owned by the same parent company.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
AT SDEF HAWAII DERMATOLOGY SEMINAR
Survey shines new light on weighty comorbidity burden in adult atopic dermatitis
VIENNA – Newly enhanced appreciation of the profound burden of comorbidities associated with adult atopic dermatitis (AD) is provided by the Liberty AD-AWARE study, investigators said at a joint program of the International Eczema Council and the International Psoriasis Council held in conjunction with the annual congress of the European Academy of Dermatology and Venereology.
“I think the only reason we thought psoriasis is a systemic disease and atopic dermatitis is not is because people were researching it much more in psoriasis. I think atopic dermatitis will emerge as potentially more systemic than psoriasis, including the comorbidities. It’s just a matter of time before the evidence is put forth for atopic dermatitis,” predicted Emma Guttman-Yassky, MD, PhD, professor and vice chair of the department of dermatology at Mount Sinai School of Medicine in New York.
Dr. Guttman-Yassky noted that 85% of cases of AD begin before 5 years of age. Many cases resolve later in childhood, but for others it becomes a chronic lifelong condition. And while the burden of AD has been well characterized in the pediatric population, that’s not so in affected adults. This was the impetus for the Liberty AD-AWARE (Adults With Atopic Dermatitis Reporting on their Experience) study, an Internet-based cross-sectional survey of more than 1,500 adults with AD receiving their care from dermatologists at eight major U.S. academic medical centers.
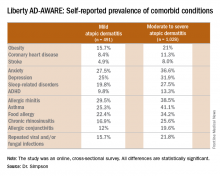
Eric L. Simpson, MD, a coinvestigator with Dr. Guttman-Yassky in Liberty AD-AWARE, observed that the study documented self-reported high rates of a range of psychiatric, cardiovascular, allergic, respiratory, and infectious diseases in participants. And while a cross-sectional study can’t establish causality, it’s important to appreciate that rates of these comorbidities were across the board significantly higher in the 1,028 patients with moderate to severe AD over the prior 12 months than in the 491 classified as having mild AD.
These associations between AD and mental health problems have been confirmed in other studies. For example, a recent analysis of data on more than 354,000 children and nearly 35,000 adults in the United States demonstrated that AD was independently associated with a 14% increased likelihood of attention-deficit/hyperactivity disorder in children and a 61% increased risk in adults. Those risks of ADHD rose far higher in individuals with severe AD and sleep disruption (Br J Dermatol. 2016 Nov;175[5]:920-9).
A number of theories have been put forth to explain these associations, including altered brain development stemming from early exposure to inflammatory cytokines or perhaps shared genetic predisposition, but Dr. Simpson proposed a simpler explanation which carries more optimistic implications.
“I suspect the mental health problems associated with adult atopic dermatitis are probably nonspecific sequelae of any chronic skin disorder involving severe itch and sleep disturbances,” said Dr. Simpson, professor of dermatology at Oregon Health & Science University, Portland.
Moreover, there is good reason to believe that novel therapies targeting inflammation more effectively than what’s been available to date may help improve mental health outcomes, as well as asthma in affected adults with AD, he added. He cited a phase IIb, randomized, double-blind, placebo-controlled study for which he was lead investigator. In this trial, 16 weeks of treatment with dupilumab, a first-in-class investigational blocker of the interleukin-4/interleukin-13 signaling pathway, not only resulted in significant reductions in itch and sleep problems, it also decreased anxiety and depression symptoms and improved multiple validated measures of health-related quality of life (J Am Acad Dermatol. 2016 Sep;75[3]:506-15).
Liberty AD-AWARE provides hints of the profound cumulative negative impact moderate to severe AD can have on a patient’s life course. Among the group with moderate to severe disease, 7.5% said AD had a large negative effect on their pursuit of an education, 10.7% said their disease had influenced their career choice “a lot/very much,” 13.3% were unemployed for reasons other than being retired or a student, and 17.1% reported an annual family income of less than $25,000. All these rates were multifold higher than in patients with mild AD in the study, which didn’t include a non-AD control group.
Dr. Guttman-Yassky observed that 42% of the moderate to severe AD group in Liberty AD-AWARE reported their current treatments were ineffective at controlling their disease, even though study participants were presumably receiving high-quality care at academic medical centers. Twenty-eight percent of patients with inadequately controlled AD had used phototherapy or an immunomodulatory drug within the past 7 days, underscoring the limitations of those forms of therapy in patients with more severe AD as well as the need for new and better treatments.
Dr. Guttman-Yassky has played a key role in the paradigm shift regarding understanding of the pathogenesis of AD as involving not just disordered skin barrier function but also immunologic impairment. She was senior author of a study that showed the nonlesional skin of patients with AD is characterized by high-level expression of inflammatory cytokines, whereas the nonlesional skin of psoriasis patients is not, an observation that serves to highlight the need for proactive treatments for AD (J Allergy Clin Immunol. 2011 Apr;127[4]:954-64.e1-4). Later, she and her coworkers demonstrated that AD is characterized by greater levels of T-cell activation among central and effector CD4+ and CD8+CLA+ and CD8+CLA– memory cell subsets (J Allergy Clin Immunol. 2015 Jul;136[1]:208-11).
More recently, she was also senior author of a landmark study that provides a mechanism to account for the reason AD patients would potentially have more comorbid illnesses than psoriasis patients. The investigators demonstrated that AD is accompanied by systemic expansion of transitional and chronically activated memory B cells, plasmablasts, and IgE-expressing memory B cells in both skin and blood. In other words, AD is characterized by a greater level of systemic immune activation, compared with psoriasis, where activated T cells are largely confined to the skin, and activated central memory B cells don’t figure prominently (J Allergy Clin Immunol. 2016 Jan;137[1]:118-29.e5).
The Liberty AD-AWARE study was sponsored by Sanofi and Regeneron. Dr. Simpson and Dr. Guttman-Yassky reported receiving research grants from and serving as consultants to those and other pharmaceutical companies.
VIENNA – Newly enhanced appreciation of the profound burden of comorbidities associated with adult atopic dermatitis (AD) is provided by the Liberty AD-AWARE study, investigators said at a joint program of the International Eczema Council and the International Psoriasis Council held in conjunction with the annual congress of the European Academy of Dermatology and Venereology.
“I think the only reason we thought psoriasis is a systemic disease and atopic dermatitis is not is because people were researching it much more in psoriasis. I think atopic dermatitis will emerge as potentially more systemic than psoriasis, including the comorbidities. It’s just a matter of time before the evidence is put forth for atopic dermatitis,” predicted Emma Guttman-Yassky, MD, PhD, professor and vice chair of the department of dermatology at Mount Sinai School of Medicine in New York.
Dr. Guttman-Yassky noted that 85% of cases of AD begin before 5 years of age. Many cases resolve later in childhood, but for others it becomes a chronic lifelong condition. And while the burden of AD has been well characterized in the pediatric population, that’s not so in affected adults. This was the impetus for the Liberty AD-AWARE (Adults With Atopic Dermatitis Reporting on their Experience) study, an Internet-based cross-sectional survey of more than 1,500 adults with AD receiving their care from dermatologists at eight major U.S. academic medical centers.
Eric L. Simpson, MD, a coinvestigator with Dr. Guttman-Yassky in Liberty AD-AWARE, observed that the study documented self-reported high rates of a range of psychiatric, cardiovascular, allergic, respiratory, and infectious diseases in participants. And while a cross-sectional study can’t establish causality, it’s important to appreciate that rates of these comorbidities were across the board significantly higher in the 1,028 patients with moderate to severe AD over the prior 12 months than in the 491 classified as having mild AD.
These associations between AD and mental health problems have been confirmed in other studies. For example, a recent analysis of data on more than 354,000 children and nearly 35,000 adults in the United States demonstrated that AD was independently associated with a 14% increased likelihood of attention-deficit/hyperactivity disorder in children and a 61% increased risk in adults. Those risks of ADHD rose far higher in individuals with severe AD and sleep disruption (Br J Dermatol. 2016 Nov;175[5]:920-9).
A number of theories have been put forth to explain these associations, including altered brain development stemming from early exposure to inflammatory cytokines or perhaps shared genetic predisposition, but Dr. Simpson proposed a simpler explanation which carries more optimistic implications.
“I suspect the mental health problems associated with adult atopic dermatitis are probably nonspecific sequelae of any chronic skin disorder involving severe itch and sleep disturbances,” said Dr. Simpson, professor of dermatology at Oregon Health & Science University, Portland.
Moreover, there is good reason to believe that novel therapies targeting inflammation more effectively than what’s been available to date may help improve mental health outcomes, as well as asthma in affected adults with AD, he added. He cited a phase IIb, randomized, double-blind, placebo-controlled study for which he was lead investigator. In this trial, 16 weeks of treatment with dupilumab, a first-in-class investigational blocker of the interleukin-4/interleukin-13 signaling pathway, not only resulted in significant reductions in itch and sleep problems, it also decreased anxiety and depression symptoms and improved multiple validated measures of health-related quality of life (J Am Acad Dermatol. 2016 Sep;75[3]:506-15).
Liberty AD-AWARE provides hints of the profound cumulative negative impact moderate to severe AD can have on a patient’s life course. Among the group with moderate to severe disease, 7.5% said AD had a large negative effect on their pursuit of an education, 10.7% said their disease had influenced their career choice “a lot/very much,” 13.3% were unemployed for reasons other than being retired or a student, and 17.1% reported an annual family income of less than $25,000. All these rates were multifold higher than in patients with mild AD in the study, which didn’t include a non-AD control group.
Dr. Guttman-Yassky observed that 42% of the moderate to severe AD group in Liberty AD-AWARE reported their current treatments were ineffective at controlling their disease, even though study participants were presumably receiving high-quality care at academic medical centers. Twenty-eight percent of patients with inadequately controlled AD had used phototherapy or an immunomodulatory drug within the past 7 days, underscoring the limitations of those forms of therapy in patients with more severe AD as well as the need for new and better treatments.
Dr. Guttman-Yassky has played a key role in the paradigm shift regarding understanding of the pathogenesis of AD as involving not just disordered skin barrier function but also immunologic impairment. She was senior author of a study that showed the nonlesional skin of patients with AD is characterized by high-level expression of inflammatory cytokines, whereas the nonlesional skin of psoriasis patients is not, an observation that serves to highlight the need for proactive treatments for AD (J Allergy Clin Immunol. 2011 Apr;127[4]:954-64.e1-4). Later, she and her coworkers demonstrated that AD is characterized by greater levels of T-cell activation among central and effector CD4+ and CD8+CLA+ and CD8+CLA– memory cell subsets (J Allergy Clin Immunol. 2015 Jul;136[1]:208-11).
More recently, she was also senior author of a landmark study that provides a mechanism to account for the reason AD patients would potentially have more comorbid illnesses than psoriasis patients. The investigators demonstrated that AD is accompanied by systemic expansion of transitional and chronically activated memory B cells, plasmablasts, and IgE-expressing memory B cells in both skin and blood. In other words, AD is characterized by a greater level of systemic immune activation, compared with psoriasis, where activated T cells are largely confined to the skin, and activated central memory B cells don’t figure prominently (J Allergy Clin Immunol. 2016 Jan;137[1]:118-29.e5).
The Liberty AD-AWARE study was sponsored by Sanofi and Regeneron. Dr. Simpson and Dr. Guttman-Yassky reported receiving research grants from and serving as consultants to those and other pharmaceutical companies.
VIENNA – Newly enhanced appreciation of the profound burden of comorbidities associated with adult atopic dermatitis (AD) is provided by the Liberty AD-AWARE study, investigators said at a joint program of the International Eczema Council and the International Psoriasis Council held in conjunction with the annual congress of the European Academy of Dermatology and Venereology.
“I think the only reason we thought psoriasis is a systemic disease and atopic dermatitis is not is because people were researching it much more in psoriasis. I think atopic dermatitis will emerge as potentially more systemic than psoriasis, including the comorbidities. It’s just a matter of time before the evidence is put forth for atopic dermatitis,” predicted Emma Guttman-Yassky, MD, PhD, professor and vice chair of the department of dermatology at Mount Sinai School of Medicine in New York.
Dr. Guttman-Yassky noted that 85% of cases of AD begin before 5 years of age. Many cases resolve later in childhood, but for others it becomes a chronic lifelong condition. And while the burden of AD has been well characterized in the pediatric population, that’s not so in affected adults. This was the impetus for the Liberty AD-AWARE (Adults With Atopic Dermatitis Reporting on their Experience) study, an Internet-based cross-sectional survey of more than 1,500 adults with AD receiving their care from dermatologists at eight major U.S. academic medical centers.
Eric L. Simpson, MD, a coinvestigator with Dr. Guttman-Yassky in Liberty AD-AWARE, observed that the study documented self-reported high rates of a range of psychiatric, cardiovascular, allergic, respiratory, and infectious diseases in participants. And while a cross-sectional study can’t establish causality, it’s important to appreciate that rates of these comorbidities were across the board significantly higher in the 1,028 patients with moderate to severe AD over the prior 12 months than in the 491 classified as having mild AD.
These associations between AD and mental health problems have been confirmed in other studies. For example, a recent analysis of data on more than 354,000 children and nearly 35,000 adults in the United States demonstrated that AD was independently associated with a 14% increased likelihood of attention-deficit/hyperactivity disorder in children and a 61% increased risk in adults. Those risks of ADHD rose far higher in individuals with severe AD and sleep disruption (Br J Dermatol. 2016 Nov;175[5]:920-9).
A number of theories have been put forth to explain these associations, including altered brain development stemming from early exposure to inflammatory cytokines or perhaps shared genetic predisposition, but Dr. Simpson proposed a simpler explanation which carries more optimistic implications.
“I suspect the mental health problems associated with adult atopic dermatitis are probably nonspecific sequelae of any chronic skin disorder involving severe itch and sleep disturbances,” said Dr. Simpson, professor of dermatology at Oregon Health & Science University, Portland.
Moreover, there is good reason to believe that novel therapies targeting inflammation more effectively than what’s been available to date may help improve mental health outcomes, as well as asthma in affected adults with AD, he added. He cited a phase IIb, randomized, double-blind, placebo-controlled study for which he was lead investigator. In this trial, 16 weeks of treatment with dupilumab, a first-in-class investigational blocker of the interleukin-4/interleukin-13 signaling pathway, not only resulted in significant reductions in itch and sleep problems, it also decreased anxiety and depression symptoms and improved multiple validated measures of health-related quality of life (J Am Acad Dermatol. 2016 Sep;75[3]:506-15).
Liberty AD-AWARE provides hints of the profound cumulative negative impact moderate to severe AD can have on a patient’s life course. Among the group with moderate to severe disease, 7.5% said AD had a large negative effect on their pursuit of an education, 10.7% said their disease had influenced their career choice “a lot/very much,” 13.3% were unemployed for reasons other than being retired or a student, and 17.1% reported an annual family income of less than $25,000. All these rates were multifold higher than in patients with mild AD in the study, which didn’t include a non-AD control group.
Dr. Guttman-Yassky observed that 42% of the moderate to severe AD group in Liberty AD-AWARE reported their current treatments were ineffective at controlling their disease, even though study participants were presumably receiving high-quality care at academic medical centers. Twenty-eight percent of patients with inadequately controlled AD had used phototherapy or an immunomodulatory drug within the past 7 days, underscoring the limitations of those forms of therapy in patients with more severe AD as well as the need for new and better treatments.
Dr. Guttman-Yassky has played a key role in the paradigm shift regarding understanding of the pathogenesis of AD as involving not just disordered skin barrier function but also immunologic impairment. She was senior author of a study that showed the nonlesional skin of patients with AD is characterized by high-level expression of inflammatory cytokines, whereas the nonlesional skin of psoriasis patients is not, an observation that serves to highlight the need for proactive treatments for AD (J Allergy Clin Immunol. 2011 Apr;127[4]:954-64.e1-4). Later, she and her coworkers demonstrated that AD is characterized by greater levels of T-cell activation among central and effector CD4+ and CD8+CLA+ and CD8+CLA– memory cell subsets (J Allergy Clin Immunol. 2015 Jul;136[1]:208-11).
More recently, she was also senior author of a landmark study that provides a mechanism to account for the reason AD patients would potentially have more comorbid illnesses than psoriasis patients. The investigators demonstrated that AD is accompanied by systemic expansion of transitional and chronically activated memory B cells, plasmablasts, and IgE-expressing memory B cells in both skin and blood. In other words, AD is characterized by a greater level of systemic immune activation, compared with psoriasis, where activated T cells are largely confined to the skin, and activated central memory B cells don’t figure prominently (J Allergy Clin Immunol. 2016 Jan;137[1]:118-29.e5).
The Liberty AD-AWARE study was sponsored by Sanofi and Regeneron. Dr. Simpson and Dr. Guttman-Yassky reported receiving research grants from and serving as consultants to those and other pharmaceutical companies.
EXPERT ANALYSIS FROM THE EADV CONGRESS
Novel oral antifungal headed to phase III for onychomycosis
VIENNA – A once-weekly oral antifungal drug known as VT-1161 will move into phase III clinical testing in 2017 based on its impressive performance in an interim analysis of a phase IIb study, Amir Tavakkol, PhD, reported at the annual congress of the European Academy of Dermatology and Venereology.
“We saw robust evidence of clinical and mycologic efficacy in patients with moderate to severe onychomycosis. This was true even in patients with nails considered very difficult to treat because of dermatophytomas and spikes, which are usually poor prognostic elements,” said Dr. Tavakkol, chief development officer at Viamet Pharmaceuticals in Durham, N.C., which is developing VT-1161.
He reported on 107 patients with toenail onychomycosis who enrolled in the phase IIb, double-blind, placebo-controlled RENOVATE (Restoring Nail: An Oral VT-1161 Tablet Evaluation) study. At enrollment they averaged 47% disease involvement of the big toenail, the target nail for the trial. Participants in the five study arms had an average of 4.2-5.0 affected toenails. Both the percentage of nail involvement and the number of diseased toenails were roughly twice as great as is typical in studies of topical antifungals, underscoring that participants in the VT-1161 trial had fairly severe onychomycosis.
Patients were assigned to one of five study arms: 300 mg of VT-1161 once weekly for 12 weeks, then 12 weeks of placebo; 600 mg of VT-1161 once weekly for 12 weeks, followed by 12 weeks of placebo; either 300 or 600 mg of the antifungal agent once weekly for the full 24 weeks; or 24 weeks of once-per-week placebo. Immediately prior to the formal start of the study, however, everyone received 14 days of a once-daily loading dose of VT-1161 or placebo at the dose they would subsequently take once weekly during the trial.
The primary outcome in the ongoing study is the percentage of patients with a complete cure, both mycologic and clinical, at 48 weeks. Those data aren’t in yet, but Dr. Tavakkol presented the results of the prespecified interim analysis at 24 weeks.
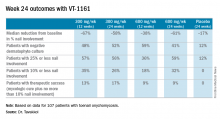
“Please keep in mind that this is only at most 24 weeks of therapy. Given the rate of nail growth at 1 mm per month when it is healthy, these are remarkable data. Ten percent or less nail involvement is basically 1-2 mm at the distal end. I believe a substantial percentage of these patients will reach clinical cure by 48 weeks,” he said.
VT-1161, a molecule with high selectivity for fungal CYP51, blocks the production of ergosterol, a key component of the fungal cell membrane, according to the company. It has no known drug interactions. At all doses studied in the trial, it was safe and well tolerated, with no abnormal liver function tests, no effect on bilirubin, and no change in QTc interval. Only 8 of the 107 patients reported adverse events deemed possibly related to the study drug by blinded investigators. No one dropped out of the study.
“VT-1161 is also being developed for recurrent vulvovaginal candidiasis. The results there are outstanding, too,” Dr. Tavakkol said.
The trial was funded by Viamet, where he is employed.
VIENNA – A once-weekly oral antifungal drug known as VT-1161 will move into phase III clinical testing in 2017 based on its impressive performance in an interim analysis of a phase IIb study, Amir Tavakkol, PhD, reported at the annual congress of the European Academy of Dermatology and Venereology.
“We saw robust evidence of clinical and mycologic efficacy in patients with moderate to severe onychomycosis. This was true even in patients with nails considered very difficult to treat because of dermatophytomas and spikes, which are usually poor prognostic elements,” said Dr. Tavakkol, chief development officer at Viamet Pharmaceuticals in Durham, N.C., which is developing VT-1161.
He reported on 107 patients with toenail onychomycosis who enrolled in the phase IIb, double-blind, placebo-controlled RENOVATE (Restoring Nail: An Oral VT-1161 Tablet Evaluation) study. At enrollment they averaged 47% disease involvement of the big toenail, the target nail for the trial. Participants in the five study arms had an average of 4.2-5.0 affected toenails. Both the percentage of nail involvement and the number of diseased toenails were roughly twice as great as is typical in studies of topical antifungals, underscoring that participants in the VT-1161 trial had fairly severe onychomycosis.
Patients were assigned to one of five study arms: 300 mg of VT-1161 once weekly for 12 weeks, then 12 weeks of placebo; 600 mg of VT-1161 once weekly for 12 weeks, followed by 12 weeks of placebo; either 300 or 600 mg of the antifungal agent once weekly for the full 24 weeks; or 24 weeks of once-per-week placebo. Immediately prior to the formal start of the study, however, everyone received 14 days of a once-daily loading dose of VT-1161 or placebo at the dose they would subsequently take once weekly during the trial.
The primary outcome in the ongoing study is the percentage of patients with a complete cure, both mycologic and clinical, at 48 weeks. Those data aren’t in yet, but Dr. Tavakkol presented the results of the prespecified interim analysis at 24 weeks.
“Please keep in mind that this is only at most 24 weeks of therapy. Given the rate of nail growth at 1 mm per month when it is healthy, these are remarkable data. Ten percent or less nail involvement is basically 1-2 mm at the distal end. I believe a substantial percentage of these patients will reach clinical cure by 48 weeks,” he said.
VT-1161, a molecule with high selectivity for fungal CYP51, blocks the production of ergosterol, a key component of the fungal cell membrane, according to the company. It has no known drug interactions. At all doses studied in the trial, it was safe and well tolerated, with no abnormal liver function tests, no effect on bilirubin, and no change in QTc interval. Only 8 of the 107 patients reported adverse events deemed possibly related to the study drug by blinded investigators. No one dropped out of the study.
“VT-1161 is also being developed for recurrent vulvovaginal candidiasis. The results there are outstanding, too,” Dr. Tavakkol said.
The trial was funded by Viamet, where he is employed.
VIENNA – A once-weekly oral antifungal drug known as VT-1161 will move into phase III clinical testing in 2017 based on its impressive performance in an interim analysis of a phase IIb study, Amir Tavakkol, PhD, reported at the annual congress of the European Academy of Dermatology and Venereology.
“We saw robust evidence of clinical and mycologic efficacy in patients with moderate to severe onychomycosis. This was true even in patients with nails considered very difficult to treat because of dermatophytomas and spikes, which are usually poor prognostic elements,” said Dr. Tavakkol, chief development officer at Viamet Pharmaceuticals in Durham, N.C., which is developing VT-1161.
He reported on 107 patients with toenail onychomycosis who enrolled in the phase IIb, double-blind, placebo-controlled RENOVATE (Restoring Nail: An Oral VT-1161 Tablet Evaluation) study. At enrollment they averaged 47% disease involvement of the big toenail, the target nail for the trial. Participants in the five study arms had an average of 4.2-5.0 affected toenails. Both the percentage of nail involvement and the number of diseased toenails were roughly twice as great as is typical in studies of topical antifungals, underscoring that participants in the VT-1161 trial had fairly severe onychomycosis.
Patients were assigned to one of five study arms: 300 mg of VT-1161 once weekly for 12 weeks, then 12 weeks of placebo; 600 mg of VT-1161 once weekly for 12 weeks, followed by 12 weeks of placebo; either 300 or 600 mg of the antifungal agent once weekly for the full 24 weeks; or 24 weeks of once-per-week placebo. Immediately prior to the formal start of the study, however, everyone received 14 days of a once-daily loading dose of VT-1161 or placebo at the dose they would subsequently take once weekly during the trial.
The primary outcome in the ongoing study is the percentage of patients with a complete cure, both mycologic and clinical, at 48 weeks. Those data aren’t in yet, but Dr. Tavakkol presented the results of the prespecified interim analysis at 24 weeks.
“Please keep in mind that this is only at most 24 weeks of therapy. Given the rate of nail growth at 1 mm per month when it is healthy, these are remarkable data. Ten percent or less nail involvement is basically 1-2 mm at the distal end. I believe a substantial percentage of these patients will reach clinical cure by 48 weeks,” he said.
VT-1161, a molecule with high selectivity for fungal CYP51, blocks the production of ergosterol, a key component of the fungal cell membrane, according to the company. It has no known drug interactions. At all doses studied in the trial, it was safe and well tolerated, with no abnormal liver function tests, no effect on bilirubin, and no change in QTc interval. Only 8 of the 107 patients reported adverse events deemed possibly related to the study drug by blinded investigators. No one dropped out of the study.
“VT-1161 is also being developed for recurrent vulvovaginal candidiasis. The results there are outstanding, too,” Dr. Tavakkol said.
The trial was funded by Viamet, where he is employed.
AT THE EADV CONGRESS
Key clinical point:
Major finding: Among onychomycosis patients with an average 47% target toenail involvement at baseline, 35% had improved to no more than 10% nail involvement at 24 weeks after 12 weeks of once-weekly VT-1161 followed by 12 weeks of placebo.
Data source: A double-blind, randomized, phase IIb clinical trial involving 107 patients with toenail onychomycosis.
Disclosures: The trial was funded by Viamet Pharmaceuticals, where the study presenter is employed.
Survey finds high rate of misdiagnosed fungal infections
Fungal skin infections may be missed or misdiagnosed by many dermatologists, according to the results of a survey published online in the Journal of the American Academy of Dermatology.
For the interactive survey, conducted during a session on fungal infections at the 2016 Orlando Dermatology Aesthetic and Clinical Conference, board-certified dermatologists viewed 13 clinical images (which included other conditions such as secondary syphilis and pityriasis rosea) and were asked via an audience response system whether or not they thought the case was a fungal skin infection. In only 1 of the 13 cases presented did 90% of the dermatologists correctly categorize the case as either a dermatomycosis or not, reported Ramsin Joseph Yadgar of George Washington University in Washington, D.C., and colleagues.
Although most cases (8 of 13) “were appropriately categorized by more than 50% of the audience, this percentage decreased as accuracy of categorization increased,” they wrote. “For example, in only 4 of the 13 cases did audience members accurately categorize the cases with more than 75% accuracy,” they said (J Am Acad Dermatol. 2016 Nov 11. pii: S0190-9622[16]30883-0. doi: 10.1016/j.jaad.2016.09.041).
“Dermatology is full of doppelgangers,” Dr. Friedman, director of the residency program and of translational research in the department of dermatology at George Washington University, said in an interview.
“While we [dermatologists] pride ourselves on our visual prowess, there are many skin diseases which do not follow the textbook and can be quite protean in their presentations,” he said.
The variability in presentation makes diagnosing fungal infections especially challenging, he noted. “Fungal infections of the skin can have many clinical flavors and can infect skin, hair and nails. Also, inappropriate treatment can obscure the appearance of the infection, and the fact that there are multiple other conditions that can look like these [fungal] infections makes proper identification difficult.”
Although the results were limited by several factors including possible selection bias, lack of measurable response rate, and small sample size, the findings highlight how easy it can be to miss a diagnosis of fungal infection, “which can result in inappropriate therapy, worsening of symptoms, and even additional skin and soft-tissue infections,” the researchers wrote.
“Keep an open mind and cast a wider differential,” to help catch fungal infections, and use all the dermatologic tools, including slide preps, cultures, and biopsies, Dr. Friedman said. Better diagnostic tools and improved training for clinicians outside of dermatology also could reduce the misdiagnosis of fungal infections, he added. “Many of these patients are misdiagnosed in the emergency department, urgent care, or primary care settings,” and delayed treatment increases associated morbidity, he said.
Mr. Yadgar, Dr. Friedman, and another coauthor, Neal Bhatia, MD, of Therapeutics Clinical Research, San Diego, Calif., had no financial conflicts to disclose. There was no funding source.
Fungal skin infections may be missed or misdiagnosed by many dermatologists, according to the results of a survey published online in the Journal of the American Academy of Dermatology.
For the interactive survey, conducted during a session on fungal infections at the 2016 Orlando Dermatology Aesthetic and Clinical Conference, board-certified dermatologists viewed 13 clinical images (which included other conditions such as secondary syphilis and pityriasis rosea) and were asked via an audience response system whether or not they thought the case was a fungal skin infection. In only 1 of the 13 cases presented did 90% of the dermatologists correctly categorize the case as either a dermatomycosis or not, reported Ramsin Joseph Yadgar of George Washington University in Washington, D.C., and colleagues.
Although most cases (8 of 13) “were appropriately categorized by more than 50% of the audience, this percentage decreased as accuracy of categorization increased,” they wrote. “For example, in only 4 of the 13 cases did audience members accurately categorize the cases with more than 75% accuracy,” they said (J Am Acad Dermatol. 2016 Nov 11. pii: S0190-9622[16]30883-0. doi: 10.1016/j.jaad.2016.09.041).
“Dermatology is full of doppelgangers,” Dr. Friedman, director of the residency program and of translational research in the department of dermatology at George Washington University, said in an interview.
“While we [dermatologists] pride ourselves on our visual prowess, there are many skin diseases which do not follow the textbook and can be quite protean in their presentations,” he said.
The variability in presentation makes diagnosing fungal infections especially challenging, he noted. “Fungal infections of the skin can have many clinical flavors and can infect skin, hair and nails. Also, inappropriate treatment can obscure the appearance of the infection, and the fact that there are multiple other conditions that can look like these [fungal] infections makes proper identification difficult.”
Although the results were limited by several factors including possible selection bias, lack of measurable response rate, and small sample size, the findings highlight how easy it can be to miss a diagnosis of fungal infection, “which can result in inappropriate therapy, worsening of symptoms, and even additional skin and soft-tissue infections,” the researchers wrote.
“Keep an open mind and cast a wider differential,” to help catch fungal infections, and use all the dermatologic tools, including slide preps, cultures, and biopsies, Dr. Friedman said. Better diagnostic tools and improved training for clinicians outside of dermatology also could reduce the misdiagnosis of fungal infections, he added. “Many of these patients are misdiagnosed in the emergency department, urgent care, or primary care settings,” and delayed treatment increases associated morbidity, he said.
Mr. Yadgar, Dr. Friedman, and another coauthor, Neal Bhatia, MD, of Therapeutics Clinical Research, San Diego, Calif., had no financial conflicts to disclose. There was no funding source.
Fungal skin infections may be missed or misdiagnosed by many dermatologists, according to the results of a survey published online in the Journal of the American Academy of Dermatology.
For the interactive survey, conducted during a session on fungal infections at the 2016 Orlando Dermatology Aesthetic and Clinical Conference, board-certified dermatologists viewed 13 clinical images (which included other conditions such as secondary syphilis and pityriasis rosea) and were asked via an audience response system whether or not they thought the case was a fungal skin infection. In only 1 of the 13 cases presented did 90% of the dermatologists correctly categorize the case as either a dermatomycosis or not, reported Ramsin Joseph Yadgar of George Washington University in Washington, D.C., and colleagues.
Although most cases (8 of 13) “were appropriately categorized by more than 50% of the audience, this percentage decreased as accuracy of categorization increased,” they wrote. “For example, in only 4 of the 13 cases did audience members accurately categorize the cases with more than 75% accuracy,” they said (J Am Acad Dermatol. 2016 Nov 11. pii: S0190-9622[16]30883-0. doi: 10.1016/j.jaad.2016.09.041).
“Dermatology is full of doppelgangers,” Dr. Friedman, director of the residency program and of translational research in the department of dermatology at George Washington University, said in an interview.
“While we [dermatologists] pride ourselves on our visual prowess, there are many skin diseases which do not follow the textbook and can be quite protean in their presentations,” he said.
The variability in presentation makes diagnosing fungal infections especially challenging, he noted. “Fungal infections of the skin can have many clinical flavors and can infect skin, hair and nails. Also, inappropriate treatment can obscure the appearance of the infection, and the fact that there are multiple other conditions that can look like these [fungal] infections makes proper identification difficult.”
Although the results were limited by several factors including possible selection bias, lack of measurable response rate, and small sample size, the findings highlight how easy it can be to miss a diagnosis of fungal infection, “which can result in inappropriate therapy, worsening of symptoms, and even additional skin and soft-tissue infections,” the researchers wrote.
“Keep an open mind and cast a wider differential,” to help catch fungal infections, and use all the dermatologic tools, including slide preps, cultures, and biopsies, Dr. Friedman said. Better diagnostic tools and improved training for clinicians outside of dermatology also could reduce the misdiagnosis of fungal infections, he added. “Many of these patients are misdiagnosed in the emergency department, urgent care, or primary care settings,” and delayed treatment increases associated morbidity, he said.
Mr. Yadgar, Dr. Friedman, and another coauthor, Neal Bhatia, MD, of Therapeutics Clinical Research, San Diego, Calif., had no financial conflicts to disclose. There was no funding source.
FROM JAAD
Key clinical point: Fungal infections may often be missed or misdiagnosed by dermatologists.
Major finding: In 1 of 13 cases did 90% of an audience of dermatologists correctly categorize the condition.
Data source: A survey of board-certified dermatologists, asked whether or not 13 clinical images were a fungal infection or not, during a session on fungal infections at a dermatology meeting.
Disclosures: The research team had no relevant financial conflicts to disclose.
Empirical micafungin falls short for treating suspected fungal infection in the ICU
MILAN – Empirical antifungal treatment did not improve the rate of survival free of invasive fungal infection among high-risk colonized patients in the intensive care unit, based on results from the EMPIRICUS randomized controlled trial.
Trial participants were 260 nonneutropenic, nontransplanted critically ill patients with ICU-acquired sepsis, Candida colonization of at least one site, and multiple organ failure who were exposed to broad-spectrum antibacterial agents. They were randomized to 14 days of empirical treatment with micafungin (Mycamine, 100 mg once daily) or placebo.
By day 28, about two-thirds of patients overall remained alive and free of proven invasive fungal infection, with no significant difference between groups, according to data reported at the annual congress of the European Society of Intensive Care Medicine and simultaneously published online (JAMA. 2016 Oct 5. doi: 10.1001/jama.2016.14655). Results were similar in subsets of patients having established risk factors for candidemia.
The EMPIRICUS (Empirical Antifungal Treatment in ICUs) findings add to data from other studies suggesting that, in this patient population, sepsis is seldom a result of invasive fungal infection and Candida colonization status is not helpful for guiding treatment, according to the researchers, who were led by Dr. Jean-Francois Timsit of Inserm/Paris Diderot University and department of medical intensive care and infectious diseases, Hôpital Bichat-Claude-Bernard, Paris.
“Altogether, these results call into question the routine use of systematic surveillance for Candida colonization. Besides sparing unnecessary use of health care resources, it may also avoid inducing resistances to antifungals,” they maintain. “Whether this trial closes 3 decades of clinical research on Candida colonization deserves consideration.”
Patients were recruited to EMPIRICUS from 19 ICUs in France. On average, study participants had three Candida-colonized sites.
A modified intent-to-treat analysis showed that, by day 28 after enrollment, 68% of patients in the micafungin group and 60.2% in the placebo group were alive and free of invasive fungal infection, a nonsignificant difference.
Findings were similar in the subset of patients having high serum levels of (1-3)-beta-D-glucan and in the subset of patients having high Sepsis-Related Organ Failure Assessment (SOFA) scores – both risk factors for candidemia – and regardless of the number of colonized sites.
In analyses of secondary outcomes, empirical micafungin was associated with a lower rate of new invasive fungal infection when compared with placebo (3% vs. 12%; P = .008), but the rate of mortality was statistically indistinguishable (30% vs. 29.7%).
The groups were statistically indistinguishable with respect to the number of organ failure–free days and the rate of ventilator-acquired pneumonia.
Dr. Timsit disclosed that he receives lecture fees from Gilead, Pfizer, Merck, and Astellas; research grants to his university and research organization from Astellas, Gilead, Merck, and Pfizer companies; a consultancy honorarium from Bayer; and personal fees from Abbott for scientific board participation; additionally, he disclosed participation on a scientific committee of epidemiological studies organized by Astellas and Merck companies outside the submitted work. Astellas provided a research grant to the Grenoble Alpes University Hospital based on the final study protocol. The study was sponsored by the University of Grenoble 1/Albert Michallon University Hospital. The University of Grenoble provided compensation to the participating hospitals and universities for extra costs associated with the study.
Taken together, findings from EMPIRICUS and similar trials suggest that empirical antifungal treatment may reduce rates of invasive infection in critically ill patients, but does not improve survival.
These findings highlight two emerging themes in critical care medicine – less is more and targeted therapies are important when treating invasive fungal infection. In particular, the safety and efficacy of the newer antifungal agents are driving greater empirical use, yet this practice increases the cost of care and may contribute to antifungal resistance.
Guidelines have been implemented for empirical treatment of Candida and serial surveillance, yet there are no conclusive mortality benefits for this approach. Data have not ruled out the possibility that some subgroups of patients may see a survival benefit but, in light of the situation, guidelines concerning empirical treatment and surveillance should be revisited.
Like other prophylactic interventions, the risks and potential benefits of empirical echinocandin therapy for critically ill, immune-competent patients in the ICU need to be studied. Novel biomarkers or clinical risk assessment algorithms may help in identifying those patients who are at highest risk of infection-related morbidity and mortality and would benefit most from targeted preventive therapies.
Trishul Siddharthan, MD, Petros C. Karakousis, MD, and William Checkley, MD, PhD, are with the Johns Hopkins University in Baltimore. They made their remarks in an accompanying editorial in JAMA (2016 Oct 5. doi: 10.1001/jama.2016.13801).
Taken together, findings from EMPIRICUS and similar trials suggest that empirical antifungal treatment may reduce rates of invasive infection in critically ill patients, but does not improve survival.
These findings highlight two emerging themes in critical care medicine – less is more and targeted therapies are important when treating invasive fungal infection. In particular, the safety and efficacy of the newer antifungal agents are driving greater empirical use, yet this practice increases the cost of care and may contribute to antifungal resistance.
Guidelines have been implemented for empirical treatment of Candida and serial surveillance, yet there are no conclusive mortality benefits for this approach. Data have not ruled out the possibility that some subgroups of patients may see a survival benefit but, in light of the situation, guidelines concerning empirical treatment and surveillance should be revisited.
Like other prophylactic interventions, the risks and potential benefits of empirical echinocandin therapy for critically ill, immune-competent patients in the ICU need to be studied. Novel biomarkers or clinical risk assessment algorithms may help in identifying those patients who are at highest risk of infection-related morbidity and mortality and would benefit most from targeted preventive therapies.
Trishul Siddharthan, MD, Petros C. Karakousis, MD, and William Checkley, MD, PhD, are with the Johns Hopkins University in Baltimore. They made their remarks in an accompanying editorial in JAMA (2016 Oct 5. doi: 10.1001/jama.2016.13801).
Taken together, findings from EMPIRICUS and similar trials suggest that empirical antifungal treatment may reduce rates of invasive infection in critically ill patients, but does not improve survival.
These findings highlight two emerging themes in critical care medicine – less is more and targeted therapies are important when treating invasive fungal infection. In particular, the safety and efficacy of the newer antifungal agents are driving greater empirical use, yet this practice increases the cost of care and may contribute to antifungal resistance.
Guidelines have been implemented for empirical treatment of Candida and serial surveillance, yet there are no conclusive mortality benefits for this approach. Data have not ruled out the possibility that some subgroups of patients may see a survival benefit but, in light of the situation, guidelines concerning empirical treatment and surveillance should be revisited.
Like other prophylactic interventions, the risks and potential benefits of empirical echinocandin therapy for critically ill, immune-competent patients in the ICU need to be studied. Novel biomarkers or clinical risk assessment algorithms may help in identifying those patients who are at highest risk of infection-related morbidity and mortality and would benefit most from targeted preventive therapies.
Trishul Siddharthan, MD, Petros C. Karakousis, MD, and William Checkley, MD, PhD, are with the Johns Hopkins University in Baltimore. They made their remarks in an accompanying editorial in JAMA (2016 Oct 5. doi: 10.1001/jama.2016.13801).
MILAN – Empirical antifungal treatment did not improve the rate of survival free of invasive fungal infection among high-risk colonized patients in the intensive care unit, based on results from the EMPIRICUS randomized controlled trial.
Trial participants were 260 nonneutropenic, nontransplanted critically ill patients with ICU-acquired sepsis, Candida colonization of at least one site, and multiple organ failure who were exposed to broad-spectrum antibacterial agents. They were randomized to 14 days of empirical treatment with micafungin (Mycamine, 100 mg once daily) or placebo.
By day 28, about two-thirds of patients overall remained alive and free of proven invasive fungal infection, with no significant difference between groups, according to data reported at the annual congress of the European Society of Intensive Care Medicine and simultaneously published online (JAMA. 2016 Oct 5. doi: 10.1001/jama.2016.14655). Results were similar in subsets of patients having established risk factors for candidemia.
The EMPIRICUS (Empirical Antifungal Treatment in ICUs) findings add to data from other studies suggesting that, in this patient population, sepsis is seldom a result of invasive fungal infection and Candida colonization status is not helpful for guiding treatment, according to the researchers, who were led by Dr. Jean-Francois Timsit of Inserm/Paris Diderot University and department of medical intensive care and infectious diseases, Hôpital Bichat-Claude-Bernard, Paris.
“Altogether, these results call into question the routine use of systematic surveillance for Candida colonization. Besides sparing unnecessary use of health care resources, it may also avoid inducing resistances to antifungals,” they maintain. “Whether this trial closes 3 decades of clinical research on Candida colonization deserves consideration.”
Patients were recruited to EMPIRICUS from 19 ICUs in France. On average, study participants had three Candida-colonized sites.
A modified intent-to-treat analysis showed that, by day 28 after enrollment, 68% of patients in the micafungin group and 60.2% in the placebo group were alive and free of invasive fungal infection, a nonsignificant difference.
Findings were similar in the subset of patients having high serum levels of (1-3)-beta-D-glucan and in the subset of patients having high Sepsis-Related Organ Failure Assessment (SOFA) scores – both risk factors for candidemia – and regardless of the number of colonized sites.
In analyses of secondary outcomes, empirical micafungin was associated with a lower rate of new invasive fungal infection when compared with placebo (3% vs. 12%; P = .008), but the rate of mortality was statistically indistinguishable (30% vs. 29.7%).
The groups were statistically indistinguishable with respect to the number of organ failure–free days and the rate of ventilator-acquired pneumonia.
Dr. Timsit disclosed that he receives lecture fees from Gilead, Pfizer, Merck, and Astellas; research grants to his university and research organization from Astellas, Gilead, Merck, and Pfizer companies; a consultancy honorarium from Bayer; and personal fees from Abbott for scientific board participation; additionally, he disclosed participation on a scientific committee of epidemiological studies organized by Astellas and Merck companies outside the submitted work. Astellas provided a research grant to the Grenoble Alpes University Hospital based on the final study protocol. The study was sponsored by the University of Grenoble 1/Albert Michallon University Hospital. The University of Grenoble provided compensation to the participating hospitals and universities for extra costs associated with the study.
MILAN – Empirical antifungal treatment did not improve the rate of survival free of invasive fungal infection among high-risk colonized patients in the intensive care unit, based on results from the EMPIRICUS randomized controlled trial.
Trial participants were 260 nonneutropenic, nontransplanted critically ill patients with ICU-acquired sepsis, Candida colonization of at least one site, and multiple organ failure who were exposed to broad-spectrum antibacterial agents. They were randomized to 14 days of empirical treatment with micafungin (Mycamine, 100 mg once daily) or placebo.
By day 28, about two-thirds of patients overall remained alive and free of proven invasive fungal infection, with no significant difference between groups, according to data reported at the annual congress of the European Society of Intensive Care Medicine and simultaneously published online (JAMA. 2016 Oct 5. doi: 10.1001/jama.2016.14655). Results were similar in subsets of patients having established risk factors for candidemia.
The EMPIRICUS (Empirical Antifungal Treatment in ICUs) findings add to data from other studies suggesting that, in this patient population, sepsis is seldom a result of invasive fungal infection and Candida colonization status is not helpful for guiding treatment, according to the researchers, who were led by Dr. Jean-Francois Timsit of Inserm/Paris Diderot University and department of medical intensive care and infectious diseases, Hôpital Bichat-Claude-Bernard, Paris.
“Altogether, these results call into question the routine use of systematic surveillance for Candida colonization. Besides sparing unnecessary use of health care resources, it may also avoid inducing resistances to antifungals,” they maintain. “Whether this trial closes 3 decades of clinical research on Candida colonization deserves consideration.”
Patients were recruited to EMPIRICUS from 19 ICUs in France. On average, study participants had three Candida-colonized sites.
A modified intent-to-treat analysis showed that, by day 28 after enrollment, 68% of patients in the micafungin group and 60.2% in the placebo group were alive and free of invasive fungal infection, a nonsignificant difference.
Findings were similar in the subset of patients having high serum levels of (1-3)-beta-D-glucan and in the subset of patients having high Sepsis-Related Organ Failure Assessment (SOFA) scores – both risk factors for candidemia – and regardless of the number of colonized sites.
In analyses of secondary outcomes, empirical micafungin was associated with a lower rate of new invasive fungal infection when compared with placebo (3% vs. 12%; P = .008), but the rate of mortality was statistically indistinguishable (30% vs. 29.7%).
The groups were statistically indistinguishable with respect to the number of organ failure–free days and the rate of ventilator-acquired pneumonia.
Dr. Timsit disclosed that he receives lecture fees from Gilead, Pfizer, Merck, and Astellas; research grants to his university and research organization from Astellas, Gilead, Merck, and Pfizer companies; a consultancy honorarium from Bayer; and personal fees from Abbott for scientific board participation; additionally, he disclosed participation on a scientific committee of epidemiological studies organized by Astellas and Merck companies outside the submitted work. Astellas provided a research grant to the Grenoble Alpes University Hospital based on the final study protocol. The study was sponsored by the University of Grenoble 1/Albert Michallon University Hospital. The University of Grenoble provided compensation to the participating hospitals and universities for extra costs associated with the study.
FROM ESICM CONGRESS 2016
Key clinical point:
Major finding: The day 28 invasive fungal infection–free survival was 68% with empirical micafungin and 60.2% with placebo, a nonsignificant difference.
Data source: A randomized controlled trial among 260 critically ill patients with ICU-acquired sepsis, Candida colonization, and multiple organ failure who were exposed to broad-spectrum antibacterial agents (EMPIRICUS trial).
Disclosures: Dr. Timsit disclosed that he receives lecture fees from Gilead, Pfizer, Merck, and Astellas; research grants to his university and research organization from Astellas, Gilead, Merck, and Pfizer companies; a consultancy honorarium from Bayer; and personal fees from Abbott for scientific board participation; additionally, he discloses participation on a scientific committee of epidemiological studies organized by Astellas and Merck companies outside the submitted work. Astellas provided a research grant to the Grenoble Alpes University Hospital based on the final study protocol. The study was sponsored by the University of Grenoble 1/Albert Michallon University Hospital. The University of Grenoble provided compensation to the participating hospitals and universities for extra costs associated with the study.
Empiric warfarin adjustment cut drug-drug interactions with antimicrobials
BOSTON – A medication management strategy to minimize the effect of drug-drug interactions (DDIs) between warfarin and common antimicrobials resulted in significantly greater time within therapeutic range for anticoagulation, as well as a numerically smaller, but nonsignificant, number of bleeding events.
After implementation of a comprehensive inpatient and postdischarge guideline to manage DDIs between warfarin and 16 antibiotics, antivirals, or antifungal medications, patients’ in-hospital time within therapeutic range (TWTR) increased to 72% from 50% preimplementation (P = .043). Warfarin TWTR also improved across care transitions after the guidelines were implemented, rising to 70% from 46% (P = .012). No bleeding events occurred in the group studied after the guidelines were instituted, compared with four events in the comparator preguidelines group (P = .11).
Nghi Ha, PharmD, MPH, and his collaborators sought to determine whether formalizing a process to manage potentially dangerous antimicrobial-warfarin DDIs made a difference in achieving more TWTR for patients, as determined by international normalized ratio (INR) values. Dr. Ha, a clinical pharmacist at University of Michigan Health System, Ann Arbor, presented the results during a poster session at the annual meeting of the American Society for Microbiology.
Secondary outcome measures studied by Dr. Ha and his associates included the incidence of thrombosis or major bleeding events, as well as tracking documentation of medications and the anticoagulation plan in progress and discharge notes.
Patients were included if they were at least 18 years old, and if they were on 3 days or more of an antimicrobial with potential for DDI with warfarin. Patients who were also newly on other medications with the potential for significant DDI with warfarin were excluded to minimize the potential for confounding.
Dr. Ha and his collaborators characterized the study as a retrospective, single-center, quasi-experimental design of a pharmacist-run anticoagulation service. The study examined endpoints before and after implementation of comprehensive guidelines, and included 78 preguideline and 31 postguideline patients.
The guidelines drafted by the investigators and tested in their study included empiric adjustment of warfarin dosing for patients who were placed on an antibiotic with high potential to increase INR. These included many azoles and sulfamethoxazole/trimethoprim, for which initial warfarin doses were empirically reduced 20%-30% for patients whose INRs were therapeutic at the start of antimicrobial therapy. For ciprofloxacin, erythromycin, clarithromycin, and isoniazid, the guidelines recommended initial empiric warfarin dose reductions of 10%-15%.
Patients whose INRs were subtherapeutic at the beginning of therapy and who received these antimicrobials were continued on their maintenance warfarin dosing, but were monitored for rising INRs over the first 48 hours, for consideration of dosing adjustment as needed. Individuals with supratherapeutic INRs at the beginning of antimicrobial dosing had their warfarin doses reduced or held by a more aggressive algorithm based on their initial INR, and based on the potential of the antimicrobial to increase INR.
On discharge, patients were either reverted to their previous warfarin regimen if they had been stable on that regimen, or had their inpatient warfarin dosing increased by 10%-20%.
Drugs that were deemed to have moderate potential to increase INR included doxycycline, levofloxacin, moxifloxacin, quinupristin/dalfopristin, telaprevir, boceprevir, and simeprevir. For these medications, the protocol recommended no initial dose adjustment, but recommended monitoring of INR to consider a dose reduction if needed. On hospital discharge, patients who had been on these medications were to resume their previous warfarin dosing.
Antimicrobials with potential to decrease INR included nafcillin, for which the protocol recommended empiric warfarin dose increases of 25%-50%, starting 3-5 days after nafcillin was begun. Patients on rifampin or rifabutin were to increase their warfarin by 20%-30%, also 3-5 days after beginning the antibiotics. Patients on ritonavir alone, or any protease inhibitor given for HIV along with ritonavir, were closely monitored, but no empiric dosing adjustments were made.
Patients with initial subtherapeutic INRs had dosing increased by 30%-50% for nafcillin and 20%-30% for rifampin and rifabutin. A stepped algorithm for dose adjustment or withholding was also developed for these medications to treat patients with initial supratherapeutic INRs. Patients on these medications were to resume their previous warfarin dosing, with monitoring and adjustment if they had not been previously stable.
Documentation of antimicrobial-warfarin DDI in the anticoagulation service discharge summary improved significantly once the guidelines were implemented (40% compared with 14%, P = .02). There was not a significant improvement in DDI documentation in daily progress notes.
The comprehensive intervention included the formulation of guidelines and requirements to document the medication interaction in the medical chart. Other interventions included training for clinical pharmacists and the development of pocket cards and flyers to educate team members about the new guidelines. The electronic health record had triggers built and implemented to prompt consideration of warfarin/antimicrobial DDIs as well.
Dr. Ha and his coauthors noted that the uncontrolled nature of the pre-post study design was one limitation of the study. Also, the real-world design of the study meant that investigators could not control for diet, comorbidities, and other factors that have the potential to affect INR. “Implementing a process to identify high-risk antimicrobial-warfarin DDIs and provide guidelines for empiric warfarin dose adjustment … can improve INR time within therapeutic range,” noted Dr. Ha and his coauthors.
The study authors reported no external sources of funding and no conflicts of interest.
On Twitter @karioakes
BOSTON – A medication management strategy to minimize the effect of drug-drug interactions (DDIs) between warfarin and common antimicrobials resulted in significantly greater time within therapeutic range for anticoagulation, as well as a numerically smaller, but nonsignificant, number of bleeding events.
After implementation of a comprehensive inpatient and postdischarge guideline to manage DDIs between warfarin and 16 antibiotics, antivirals, or antifungal medications, patients’ in-hospital time within therapeutic range (TWTR) increased to 72% from 50% preimplementation (P = .043). Warfarin TWTR also improved across care transitions after the guidelines were implemented, rising to 70% from 46% (P = .012). No bleeding events occurred in the group studied after the guidelines were instituted, compared with four events in the comparator preguidelines group (P = .11).
Nghi Ha, PharmD, MPH, and his collaborators sought to determine whether formalizing a process to manage potentially dangerous antimicrobial-warfarin DDIs made a difference in achieving more TWTR for patients, as determined by international normalized ratio (INR) values. Dr. Ha, a clinical pharmacist at University of Michigan Health System, Ann Arbor, presented the results during a poster session at the annual meeting of the American Society for Microbiology.
Secondary outcome measures studied by Dr. Ha and his associates included the incidence of thrombosis or major bleeding events, as well as tracking documentation of medications and the anticoagulation plan in progress and discharge notes.
Patients were included if they were at least 18 years old, and if they were on 3 days or more of an antimicrobial with potential for DDI with warfarin. Patients who were also newly on other medications with the potential for significant DDI with warfarin were excluded to minimize the potential for confounding.
Dr. Ha and his collaborators characterized the study as a retrospective, single-center, quasi-experimental design of a pharmacist-run anticoagulation service. The study examined endpoints before and after implementation of comprehensive guidelines, and included 78 preguideline and 31 postguideline patients.
The guidelines drafted by the investigators and tested in their study included empiric adjustment of warfarin dosing for patients who were placed on an antibiotic with high potential to increase INR. These included many azoles and sulfamethoxazole/trimethoprim, for which initial warfarin doses were empirically reduced 20%-30% for patients whose INRs were therapeutic at the start of antimicrobial therapy. For ciprofloxacin, erythromycin, clarithromycin, and isoniazid, the guidelines recommended initial empiric warfarin dose reductions of 10%-15%.
Patients whose INRs were subtherapeutic at the beginning of therapy and who received these antimicrobials were continued on their maintenance warfarin dosing, but were monitored for rising INRs over the first 48 hours, for consideration of dosing adjustment as needed. Individuals with supratherapeutic INRs at the beginning of antimicrobial dosing had their warfarin doses reduced or held by a more aggressive algorithm based on their initial INR, and based on the potential of the antimicrobial to increase INR.
On discharge, patients were either reverted to their previous warfarin regimen if they had been stable on that regimen, or had their inpatient warfarin dosing increased by 10%-20%.
Drugs that were deemed to have moderate potential to increase INR included doxycycline, levofloxacin, moxifloxacin, quinupristin/dalfopristin, telaprevir, boceprevir, and simeprevir. For these medications, the protocol recommended no initial dose adjustment, but recommended monitoring of INR to consider a dose reduction if needed. On hospital discharge, patients who had been on these medications were to resume their previous warfarin dosing.
Antimicrobials with potential to decrease INR included nafcillin, for which the protocol recommended empiric warfarin dose increases of 25%-50%, starting 3-5 days after nafcillin was begun. Patients on rifampin or rifabutin were to increase their warfarin by 20%-30%, also 3-5 days after beginning the antibiotics. Patients on ritonavir alone, or any protease inhibitor given for HIV along with ritonavir, were closely monitored, but no empiric dosing adjustments were made.
Patients with initial subtherapeutic INRs had dosing increased by 30%-50% for nafcillin and 20%-30% for rifampin and rifabutin. A stepped algorithm for dose adjustment or withholding was also developed for these medications to treat patients with initial supratherapeutic INRs. Patients on these medications were to resume their previous warfarin dosing, with monitoring and adjustment if they had not been previously stable.
Documentation of antimicrobial-warfarin DDI in the anticoagulation service discharge summary improved significantly once the guidelines were implemented (40% compared with 14%, P = .02). There was not a significant improvement in DDI documentation in daily progress notes.
The comprehensive intervention included the formulation of guidelines and requirements to document the medication interaction in the medical chart. Other interventions included training for clinical pharmacists and the development of pocket cards and flyers to educate team members about the new guidelines. The electronic health record had triggers built and implemented to prompt consideration of warfarin/antimicrobial DDIs as well.
Dr. Ha and his coauthors noted that the uncontrolled nature of the pre-post study design was one limitation of the study. Also, the real-world design of the study meant that investigators could not control for diet, comorbidities, and other factors that have the potential to affect INR. “Implementing a process to identify high-risk antimicrobial-warfarin DDIs and provide guidelines for empiric warfarin dose adjustment … can improve INR time within therapeutic range,” noted Dr. Ha and his coauthors.
The study authors reported no external sources of funding and no conflicts of interest.
On Twitter @karioakes
BOSTON – A medication management strategy to minimize the effect of drug-drug interactions (DDIs) between warfarin and common antimicrobials resulted in significantly greater time within therapeutic range for anticoagulation, as well as a numerically smaller, but nonsignificant, number of bleeding events.
After implementation of a comprehensive inpatient and postdischarge guideline to manage DDIs between warfarin and 16 antibiotics, antivirals, or antifungal medications, patients’ in-hospital time within therapeutic range (TWTR) increased to 72% from 50% preimplementation (P = .043). Warfarin TWTR also improved across care transitions after the guidelines were implemented, rising to 70% from 46% (P = .012). No bleeding events occurred in the group studied after the guidelines were instituted, compared with four events in the comparator preguidelines group (P = .11).
Nghi Ha, PharmD, MPH, and his collaborators sought to determine whether formalizing a process to manage potentially dangerous antimicrobial-warfarin DDIs made a difference in achieving more TWTR for patients, as determined by international normalized ratio (INR) values. Dr. Ha, a clinical pharmacist at University of Michigan Health System, Ann Arbor, presented the results during a poster session at the annual meeting of the American Society for Microbiology.
Secondary outcome measures studied by Dr. Ha and his associates included the incidence of thrombosis or major bleeding events, as well as tracking documentation of medications and the anticoagulation plan in progress and discharge notes.
Patients were included if they were at least 18 years old, and if they were on 3 days or more of an antimicrobial with potential for DDI with warfarin. Patients who were also newly on other medications with the potential for significant DDI with warfarin were excluded to minimize the potential for confounding.
Dr. Ha and his collaborators characterized the study as a retrospective, single-center, quasi-experimental design of a pharmacist-run anticoagulation service. The study examined endpoints before and after implementation of comprehensive guidelines, and included 78 preguideline and 31 postguideline patients.
The guidelines drafted by the investigators and tested in their study included empiric adjustment of warfarin dosing for patients who were placed on an antibiotic with high potential to increase INR. These included many azoles and sulfamethoxazole/trimethoprim, for which initial warfarin doses were empirically reduced 20%-30% for patients whose INRs were therapeutic at the start of antimicrobial therapy. For ciprofloxacin, erythromycin, clarithromycin, and isoniazid, the guidelines recommended initial empiric warfarin dose reductions of 10%-15%.
Patients whose INRs were subtherapeutic at the beginning of therapy and who received these antimicrobials were continued on their maintenance warfarin dosing, but were monitored for rising INRs over the first 48 hours, for consideration of dosing adjustment as needed. Individuals with supratherapeutic INRs at the beginning of antimicrobial dosing had their warfarin doses reduced or held by a more aggressive algorithm based on their initial INR, and based on the potential of the antimicrobial to increase INR.
On discharge, patients were either reverted to their previous warfarin regimen if they had been stable on that regimen, or had their inpatient warfarin dosing increased by 10%-20%.
Drugs that were deemed to have moderate potential to increase INR included doxycycline, levofloxacin, moxifloxacin, quinupristin/dalfopristin, telaprevir, boceprevir, and simeprevir. For these medications, the protocol recommended no initial dose adjustment, but recommended monitoring of INR to consider a dose reduction if needed. On hospital discharge, patients who had been on these medications were to resume their previous warfarin dosing.
Antimicrobials with potential to decrease INR included nafcillin, for which the protocol recommended empiric warfarin dose increases of 25%-50%, starting 3-5 days after nafcillin was begun. Patients on rifampin or rifabutin were to increase their warfarin by 20%-30%, also 3-5 days after beginning the antibiotics. Patients on ritonavir alone, or any protease inhibitor given for HIV along with ritonavir, were closely monitored, but no empiric dosing adjustments were made.
Patients with initial subtherapeutic INRs had dosing increased by 30%-50% for nafcillin and 20%-30% for rifampin and rifabutin. A stepped algorithm for dose adjustment or withholding was also developed for these medications to treat patients with initial supratherapeutic INRs. Patients on these medications were to resume their previous warfarin dosing, with monitoring and adjustment if they had not been previously stable.
Documentation of antimicrobial-warfarin DDI in the anticoagulation service discharge summary improved significantly once the guidelines were implemented (40% compared with 14%, P = .02). There was not a significant improvement in DDI documentation in daily progress notes.
The comprehensive intervention included the formulation of guidelines and requirements to document the medication interaction in the medical chart. Other interventions included training for clinical pharmacists and the development of pocket cards and flyers to educate team members about the new guidelines. The electronic health record had triggers built and implemented to prompt consideration of warfarin/antimicrobial DDIs as well.
Dr. Ha and his coauthors noted that the uncontrolled nature of the pre-post study design was one limitation of the study. Also, the real-world design of the study meant that investigators could not control for diet, comorbidities, and other factors that have the potential to affect INR. “Implementing a process to identify high-risk antimicrobial-warfarin DDIs and provide guidelines for empiric warfarin dose adjustment … can improve INR time within therapeutic range,” noted Dr. Ha and his coauthors.
The study authors reported no external sources of funding and no conflicts of interest.
On Twitter @karioakes
AT ASM MICROBE 2016
Key clinical point: Clinical guidelines with empiric warfarin adjustments improved time within therapeutic range (TWTR) for inpatients on antimicrobials.
Major finding: In-hospital TWTR increased to 72% from 50% before implementation of clinical guidelines (P = .043).
Data source: Retrospective, single-center study of inpatients on warfarin and antimicrobial with potential for DDI before (n = 78) and after (n = 31) implementation of a comprehensive clinical guideline.
Disclosures: The study investigators reported no outside sources of funding and no disclosures.
PCR assay quicker but less sensitive at penicilliosis diagnosis
A real-time PCR assay was effective at rapidly diagnosing penicilliosis caused by Talaromyces marneffei, according to Thuy Le, MD, and her associates.
Sensitivity of the assay was better when samples were collected from plasma prior to antifungal therapy. In a group of 27 HIV-infected patients from whom samples were collected prior to antifungal therapy, the assay detected the T. marneffei MP1 gene in 19 samples, while in a group of 23 HIV-infected patients from whom samples were collected within 48 hours of antifungal therapy, the assay successfully detected the MP1 gene in 12 samples.
In an additional sample of 20 HIV-infected patients without penicilliosis, the assay found no signals of the T. marneffei MP1 gene in any of the tested plasma samples, giving a specificity of 100%. All testing was completed within 5-6 hours, significantly less than the 5 days needed for Bactec system testing.
“This real-time PCR assay should not replace the need for conventional microbiology methods in diagnosing penicilliosis. However, in conjunction with culturing, it can be used as a rapid rule-in test that can make a significant difference in patient management by allowing antifungal therapy to begin sooner, particularly in patients without skin lesions, and has the potential to improve the outcomes of T. marneffei–infected patients,” the investigators concluded.
Find the full study in Mycoses (doi: 10.1111/myc.12530).
A real-time PCR assay was effective at rapidly diagnosing penicilliosis caused by Talaromyces marneffei, according to Thuy Le, MD, and her associates.
Sensitivity of the assay was better when samples were collected from plasma prior to antifungal therapy. In a group of 27 HIV-infected patients from whom samples were collected prior to antifungal therapy, the assay detected the T. marneffei MP1 gene in 19 samples, while in a group of 23 HIV-infected patients from whom samples were collected within 48 hours of antifungal therapy, the assay successfully detected the MP1 gene in 12 samples.
In an additional sample of 20 HIV-infected patients without penicilliosis, the assay found no signals of the T. marneffei MP1 gene in any of the tested plasma samples, giving a specificity of 100%. All testing was completed within 5-6 hours, significantly less than the 5 days needed for Bactec system testing.
“This real-time PCR assay should not replace the need for conventional microbiology methods in diagnosing penicilliosis. However, in conjunction with culturing, it can be used as a rapid rule-in test that can make a significant difference in patient management by allowing antifungal therapy to begin sooner, particularly in patients without skin lesions, and has the potential to improve the outcomes of T. marneffei–infected patients,” the investigators concluded.
Find the full study in Mycoses (doi: 10.1111/myc.12530).
A real-time PCR assay was effective at rapidly diagnosing penicilliosis caused by Talaromyces marneffei, according to Thuy Le, MD, and her associates.
Sensitivity of the assay was better when samples were collected from plasma prior to antifungal therapy. In a group of 27 HIV-infected patients from whom samples were collected prior to antifungal therapy, the assay detected the T. marneffei MP1 gene in 19 samples, while in a group of 23 HIV-infected patients from whom samples were collected within 48 hours of antifungal therapy, the assay successfully detected the MP1 gene in 12 samples.
In an additional sample of 20 HIV-infected patients without penicilliosis, the assay found no signals of the T. marneffei MP1 gene in any of the tested plasma samples, giving a specificity of 100%. All testing was completed within 5-6 hours, significantly less than the 5 days needed for Bactec system testing.
“This real-time PCR assay should not replace the need for conventional microbiology methods in diagnosing penicilliosis. However, in conjunction with culturing, it can be used as a rapid rule-in test that can make a significant difference in patient management by allowing antifungal therapy to begin sooner, particularly in patients without skin lesions, and has the potential to improve the outcomes of T. marneffei–infected patients,” the investigators concluded.
Find the full study in Mycoses (doi: 10.1111/myc.12530).
FROM MYCOSES
Mycobiome much more diverse in children than in adults
The normal fungal communities that inhabit healthy skin are much more diverse in children than adults, a new study has discovered.
That diversity dwindles, however, around puberty, when the lipophilic taxa Malassezia surges in abundance. This is probably mediated by the increase in sebaceous gland activation and sebum composition that occurs around sexual maturity, Jay-Hyun Jo, PhD, wrote (J Invest Dermatol. 2016 Jul 28; doi: 10.1016/j.jid.2016.05.130).
The diversity of the childhood mycobiome may also play into the larger prevalence of fungal skin diseases in children, wrote Dr. Jo of the National Cancer Institute.
“Several fungal skin infections (dermatophytoses), such as tinea capitis and tinea corporis, are more frequently seen in children. This epidemiological dichotomy in fungal infections may relate to the physiologic characteristics of younger skin, which appears more permissive to colonization by diverse fungi.”
The researchers used the fungal internal transcribed spacer–1 (ITS1) sequence to pinpoint the taxonomic details of the mycobiome of 14 healthy children and 19 healthy adults. They looked at samples from 10 sites on each subject: the external auditory canal, forehead, occiput, retroauricular crease, back, manubrium, antecubital fossa, inguinal crease, volar forearm, and nares.
Malassezia monopolized the adult samples, constituting 80%-99% of the communities on each skin site. In children, however, Malassezia was much less common, comprising 35%-76% of the samples of each site.
However, children boasted a much more diverse mycobiome. Other constituents included members of the Ascomycota, Aspergillus, Epicoccum, and Phoma taxae. Ascomycota species were found on 40% of samples from children, compared with 9.5% of samples from adults. Children also played host to communities of Epicoccum, Cladosporium, and Cryptococcus.
There were individual variations in diversity, however, the authors noted. “For clinical samples from children, decreased diversity was correlated with increased relative abundance of Malassezia, especially on sebaceous sites. Given the predominance of Malassezia on sebaceous skin, it is possible that reduction in diversity was attributed to relative overexpansion of Malassezia.”
The team also discovered gender differences in the mycobiome of children. The sebaceous skin sites of boys were much more likely to host species of Epicoccum and Cryptococcus. Girls showed an early enrichment of Malassezia. “These results suggested that gender may affect mycobiome structures during sexual maturation.”
“Since Malassezia is an obligatory lipophilic fungus, differential Malassezia abundance might be due to the full activation of sebaceous glands during puberty,” they theorized. “Therefore, it would be intriguing to identify the sebaceous gland activity and sebum signatures during childhood in conjunction with sequence-based mycobiome analysis.”
The National Institutes of Health funded the study. Dr. Jo had no financial disclosures.
On Twitter @Alz_Gal
The normal fungal communities that inhabit healthy skin are much more diverse in children than adults, a new study has discovered.
That diversity dwindles, however, around puberty, when the lipophilic taxa Malassezia surges in abundance. This is probably mediated by the increase in sebaceous gland activation and sebum composition that occurs around sexual maturity, Jay-Hyun Jo, PhD, wrote (J Invest Dermatol. 2016 Jul 28; doi: 10.1016/j.jid.2016.05.130).
The diversity of the childhood mycobiome may also play into the larger prevalence of fungal skin diseases in children, wrote Dr. Jo of the National Cancer Institute.
“Several fungal skin infections (dermatophytoses), such as tinea capitis and tinea corporis, are more frequently seen in children. This epidemiological dichotomy in fungal infections may relate to the physiologic characteristics of younger skin, which appears more permissive to colonization by diverse fungi.”
The researchers used the fungal internal transcribed spacer–1 (ITS1) sequence to pinpoint the taxonomic details of the mycobiome of 14 healthy children and 19 healthy adults. They looked at samples from 10 sites on each subject: the external auditory canal, forehead, occiput, retroauricular crease, back, manubrium, antecubital fossa, inguinal crease, volar forearm, and nares.
Malassezia monopolized the adult samples, constituting 80%-99% of the communities on each skin site. In children, however, Malassezia was much less common, comprising 35%-76% of the samples of each site.
However, children boasted a much more diverse mycobiome. Other constituents included members of the Ascomycota, Aspergillus, Epicoccum, and Phoma taxae. Ascomycota species were found on 40% of samples from children, compared with 9.5% of samples from adults. Children also played host to communities of Epicoccum, Cladosporium, and Cryptococcus.
There were individual variations in diversity, however, the authors noted. “For clinical samples from children, decreased diversity was correlated with increased relative abundance of Malassezia, especially on sebaceous sites. Given the predominance of Malassezia on sebaceous skin, it is possible that reduction in diversity was attributed to relative overexpansion of Malassezia.”
The team also discovered gender differences in the mycobiome of children. The sebaceous skin sites of boys were much more likely to host species of Epicoccum and Cryptococcus. Girls showed an early enrichment of Malassezia. “These results suggested that gender may affect mycobiome structures during sexual maturation.”
“Since Malassezia is an obligatory lipophilic fungus, differential Malassezia abundance might be due to the full activation of sebaceous glands during puberty,” they theorized. “Therefore, it would be intriguing to identify the sebaceous gland activity and sebum signatures during childhood in conjunction with sequence-based mycobiome analysis.”
The National Institutes of Health funded the study. Dr. Jo had no financial disclosures.
On Twitter @Alz_Gal
The normal fungal communities that inhabit healthy skin are much more diverse in children than adults, a new study has discovered.
That diversity dwindles, however, around puberty, when the lipophilic taxa Malassezia surges in abundance. This is probably mediated by the increase in sebaceous gland activation and sebum composition that occurs around sexual maturity, Jay-Hyun Jo, PhD, wrote (J Invest Dermatol. 2016 Jul 28; doi: 10.1016/j.jid.2016.05.130).
The diversity of the childhood mycobiome may also play into the larger prevalence of fungal skin diseases in children, wrote Dr. Jo of the National Cancer Institute.
“Several fungal skin infections (dermatophytoses), such as tinea capitis and tinea corporis, are more frequently seen in children. This epidemiological dichotomy in fungal infections may relate to the physiologic characteristics of younger skin, which appears more permissive to colonization by diverse fungi.”
The researchers used the fungal internal transcribed spacer–1 (ITS1) sequence to pinpoint the taxonomic details of the mycobiome of 14 healthy children and 19 healthy adults. They looked at samples from 10 sites on each subject: the external auditory canal, forehead, occiput, retroauricular crease, back, manubrium, antecubital fossa, inguinal crease, volar forearm, and nares.
Malassezia monopolized the adult samples, constituting 80%-99% of the communities on each skin site. In children, however, Malassezia was much less common, comprising 35%-76% of the samples of each site.
However, children boasted a much more diverse mycobiome. Other constituents included members of the Ascomycota, Aspergillus, Epicoccum, and Phoma taxae. Ascomycota species were found on 40% of samples from children, compared with 9.5% of samples from adults. Children also played host to communities of Epicoccum, Cladosporium, and Cryptococcus.
There were individual variations in diversity, however, the authors noted. “For clinical samples from children, decreased diversity was correlated with increased relative abundance of Malassezia, especially on sebaceous sites. Given the predominance of Malassezia on sebaceous skin, it is possible that reduction in diversity was attributed to relative overexpansion of Malassezia.”
The team also discovered gender differences in the mycobiome of children. The sebaceous skin sites of boys were much more likely to host species of Epicoccum and Cryptococcus. Girls showed an early enrichment of Malassezia. “These results suggested that gender may affect mycobiome structures during sexual maturation.”
“Since Malassezia is an obligatory lipophilic fungus, differential Malassezia abundance might be due to the full activation of sebaceous glands during puberty,” they theorized. “Therefore, it would be intriguing to identify the sebaceous gland activity and sebum signatures during childhood in conjunction with sequence-based mycobiome analysis.”
The National Institutes of Health funded the study. Dr. Jo had no financial disclosures.
On Twitter @Alz_Gal
FROM THE JOURNAL OF INVESTIGATIVE DERMATOLOGY
Key clinical point: The mycobiome of children is much more diverse than that of adults.
Major finding: Malassezia species comprised 80%-99% the adult mycobiome, while numerous other taxae were found on children’s skin.
Data source: The taxonomic analysis comprised 19 healthy adults and 14 healthy children.
Disclosures: The National Institutes of Health funded the study. Dr. Jo had no financial disclosures.
Candida auris in Venezuela outbreak is triazole-resistant, opportunistic
BOSTON – An investigation into 18 nosocomial Candida auris infections at a tertiary care center in Venezuela showed that isolates of the emerging fungal pathogen obtained during the outbreak were resistant to fluconazole and voriconazole. However, the isolates were intermediately susceptible to amphotericin B and susceptible to 5-fluorocitosine, and demonstrated high susceptibility to the candin antifungal anidulafungin.
Dr. Belinda Calvo, an infectious disease specialist at the University of Maracaibo, Venezuela, and her collaborators reported these findings, related to a 2012-2013 C. auris outbreak at the hospital. Dr. Calvo and her coinvestigators noted that other invasive C. auris outbreaks have been reported in India, Korea, and South Africa, but that “the real prevalence of this organism may be underestimated,” since common rapid microbial identification techniques may misidentify the species.
In a poster session at the annual meeting of the American Society of Microbiology, Dr. Calvo and her collaborators reported that the 18 patients involved in the Venezuelan outbreak were critically ill, of whom 11 were pediatric, and all had central venous catheter placement. All but two of the pediatric patients were neonates, and all had serious underlying morbidities; several had significant congenital anomalies. The median patient age was 26 days (range, 2 days to 72 years), reflecting the high number of neonates affected. One of the adult patients had esophageal carcinoma. Overall, 10/18 patients (56%) had undergone surgical procedures, and all had received antibiotics.
As has been reported in other C. auris outbreaks, isolates from blood cultures of affected individuals were initially reported as C. haemulonii by the Vitek 2 C automated microbial identification system. Molecular identification was completed by sequencing the internal transcribed spacer (ITS) of the rDNA gene, with analysis aided by the National Institutes of Health’s GenBank and the Netherland’s CBS Fungal Diversity Centre , in order to confirm the identity of the fungal isolates as C. auris. Dr. Calvo and her associates were able to generate a dendrogram of the 18 isolates, showing high clonality, a trait shared with other nosocomial C. auris outbreaks.
Susceptibility testing of the C. auris cultured from blood samples of the affected patients showed that fluconazole had a minimum inhibitory concentration to inhibit the growth of 50% of the organisms (MIC50) of greater than 64 mcg/mL. For fluconazole, the MIC90, range, and geometric mean were all also above 64 mcg/mL, indicating a high level of resistance. For voriconazole, the MICs, range, and mean were all 4 mcg/mL. For amphotericin B, the MIC50 was 1 mcg/mL, the MIC90 was 2 mcg/mL, the range was 1-2, and the geometric mean was 1.414 mcg/mL.
The high number of pediatric patients affected, as well as early pathogen identification with speedy and appropriate antifungal therapy and prompt removal of central venous catheters, likely contributed to the relatively low 30-day crude mortality rate of 28%, said Dr. Calvo and her coauthors.
“C. auris should be considered an emergent multiresistant species,” wrote Dr. Calbo and her collaborators, noting that the opportunistic pathogen has a “high potential for nosocomial horizontal transmission.”
In June 2016, the Centers for Disease Control issued a clinical alert to U.S. healthcare facilities regarding the global emergence of invasive infections caused by C. auris.
The study authors reported no external sources of funding and no conflicts of interest.
On Twitter @karioakes
BOSTON – An investigation into 18 nosocomial Candida auris infections at a tertiary care center in Venezuela showed that isolates of the emerging fungal pathogen obtained during the outbreak were resistant to fluconazole and voriconazole. However, the isolates were intermediately susceptible to amphotericin B and susceptible to 5-fluorocitosine, and demonstrated high susceptibility to the candin antifungal anidulafungin.
Dr. Belinda Calvo, an infectious disease specialist at the University of Maracaibo, Venezuela, and her collaborators reported these findings, related to a 2012-2013 C. auris outbreak at the hospital. Dr. Calvo and her coinvestigators noted that other invasive C. auris outbreaks have been reported in India, Korea, and South Africa, but that “the real prevalence of this organism may be underestimated,” since common rapid microbial identification techniques may misidentify the species.
In a poster session at the annual meeting of the American Society of Microbiology, Dr. Calvo and her collaborators reported that the 18 patients involved in the Venezuelan outbreak were critically ill, of whom 11 were pediatric, and all had central venous catheter placement. All but two of the pediatric patients were neonates, and all had serious underlying morbidities; several had significant congenital anomalies. The median patient age was 26 days (range, 2 days to 72 years), reflecting the high number of neonates affected. One of the adult patients had esophageal carcinoma. Overall, 10/18 patients (56%) had undergone surgical procedures, and all had received antibiotics.
As has been reported in other C. auris outbreaks, isolates from blood cultures of affected individuals were initially reported as C. haemulonii by the Vitek 2 C automated microbial identification system. Molecular identification was completed by sequencing the internal transcribed spacer (ITS) of the rDNA gene, with analysis aided by the National Institutes of Health’s GenBank and the Netherland’s CBS Fungal Diversity Centre , in order to confirm the identity of the fungal isolates as C. auris. Dr. Calvo and her associates were able to generate a dendrogram of the 18 isolates, showing high clonality, a trait shared with other nosocomial C. auris outbreaks.
Susceptibility testing of the C. auris cultured from blood samples of the affected patients showed that fluconazole had a minimum inhibitory concentration to inhibit the growth of 50% of the organisms (MIC50) of greater than 64 mcg/mL. For fluconazole, the MIC90, range, and geometric mean were all also above 64 mcg/mL, indicating a high level of resistance. For voriconazole, the MICs, range, and mean were all 4 mcg/mL. For amphotericin B, the MIC50 was 1 mcg/mL, the MIC90 was 2 mcg/mL, the range was 1-2, and the geometric mean was 1.414 mcg/mL.
The high number of pediatric patients affected, as well as early pathogen identification with speedy and appropriate antifungal therapy and prompt removal of central venous catheters, likely contributed to the relatively low 30-day crude mortality rate of 28%, said Dr. Calvo and her coauthors.
“C. auris should be considered an emergent multiresistant species,” wrote Dr. Calbo and her collaborators, noting that the opportunistic pathogen has a “high potential for nosocomial horizontal transmission.”
In June 2016, the Centers for Disease Control issued a clinical alert to U.S. healthcare facilities regarding the global emergence of invasive infections caused by C. auris.
The study authors reported no external sources of funding and no conflicts of interest.
On Twitter @karioakes
BOSTON – An investigation into 18 nosocomial Candida auris infections at a tertiary care center in Venezuela showed that isolates of the emerging fungal pathogen obtained during the outbreak were resistant to fluconazole and voriconazole. However, the isolates were intermediately susceptible to amphotericin B and susceptible to 5-fluorocitosine, and demonstrated high susceptibility to the candin antifungal anidulafungin.
Dr. Belinda Calvo, an infectious disease specialist at the University of Maracaibo, Venezuela, and her collaborators reported these findings, related to a 2012-2013 C. auris outbreak at the hospital. Dr. Calvo and her coinvestigators noted that other invasive C. auris outbreaks have been reported in India, Korea, and South Africa, but that “the real prevalence of this organism may be underestimated,” since common rapid microbial identification techniques may misidentify the species.
In a poster session at the annual meeting of the American Society of Microbiology, Dr. Calvo and her collaborators reported that the 18 patients involved in the Venezuelan outbreak were critically ill, of whom 11 were pediatric, and all had central venous catheter placement. All but two of the pediatric patients were neonates, and all had serious underlying morbidities; several had significant congenital anomalies. The median patient age was 26 days (range, 2 days to 72 years), reflecting the high number of neonates affected. One of the adult patients had esophageal carcinoma. Overall, 10/18 patients (56%) had undergone surgical procedures, and all had received antibiotics.
As has been reported in other C. auris outbreaks, isolates from blood cultures of affected individuals were initially reported as C. haemulonii by the Vitek 2 C automated microbial identification system. Molecular identification was completed by sequencing the internal transcribed spacer (ITS) of the rDNA gene, with analysis aided by the National Institutes of Health’s GenBank and the Netherland’s CBS Fungal Diversity Centre , in order to confirm the identity of the fungal isolates as C. auris. Dr. Calvo and her associates were able to generate a dendrogram of the 18 isolates, showing high clonality, a trait shared with other nosocomial C. auris outbreaks.
Susceptibility testing of the C. auris cultured from blood samples of the affected patients showed that fluconazole had a minimum inhibitory concentration to inhibit the growth of 50% of the organisms (MIC50) of greater than 64 mcg/mL. For fluconazole, the MIC90, range, and geometric mean were all also above 64 mcg/mL, indicating a high level of resistance. For voriconazole, the MICs, range, and mean were all 4 mcg/mL. For amphotericin B, the MIC50 was 1 mcg/mL, the MIC90 was 2 mcg/mL, the range was 1-2, and the geometric mean was 1.414 mcg/mL.
The high number of pediatric patients affected, as well as early pathogen identification with speedy and appropriate antifungal therapy and prompt removal of central venous catheters, likely contributed to the relatively low 30-day crude mortality rate of 28%, said Dr. Calvo and her coauthors.
“C. auris should be considered an emergent multiresistant species,” wrote Dr. Calbo and her collaborators, noting that the opportunistic pathogen has a “high potential for nosocomial horizontal transmission.”
In June 2016, the Centers for Disease Control issued a clinical alert to U.S. healthcare facilities regarding the global emergence of invasive infections caused by C. auris.
The study authors reported no external sources of funding and no conflicts of interest.
On Twitter @karioakes
AT ASM 2016
Key clinical point: Isolates in an outbreak of nosocomially acquired Candida auris were fluconazole-resistant.
Major finding: All C. auris isolates were resistant to fluconazole, with geometric mean minimum inhibitory concentrations greater than 64 mcg/mL.
Data source: Retrospective, single-center study of 18 pediatric and adult patients with C. auris infections at a tertiary care center in Venezuela.
Disclosures: The study investigators reported no outside sources of funding and no disclosures.
New IDSA aspergillosis guidelines endorse galactomannan for diagnosis
New aspergillosis guidelines from the Infectious Diseases Society of America recommend serum and bronchoalveolar lavage galactomannan as a marker for the diagnosis of invasive Aspergillus in adult and pediatric patients who have hematologic malignancies or have undergone hematopoietic stem cell transplants.
Serial monitoring of serum galactomannan (GM) is also useful to monitor disease progression, therapeutic response, and prognosis in hematologic malignancy and hematopoietic stem cell transplant (HSCT) patients who have elevated baseline GM (Clin Infect Dis. 2016 Jun 29. doi: 10.1093/cid/ciw326).
Serum beta-D-glucan assays also are recommended for diagnosing invasive Aspergillus (IA) in high-risk hematologic malignancy and allogeneic HSCT patients, although these tests are not very specific for the infection.
The advice illustrates the Society’s emphasis on early diagnosis in its new guidelines, which supplant the group’s 2008 guidance. There are almost 100 recommendations covering – in depth – the management of invasive, allergic, and chronic Aspergillus infections in all their manifestations. It’s a step-by-step, how-to manual for handling the problem.
“Aspergillosis mortality rates have decreased significantly in recent years, but there is still significant mortality from the infection, and we have a ways to go. We felt that early diagnosis was key, which is why it’s such an important part of these guidelines,” said lead author Thomas Patterson, MD, chief of the Division of Infectious Diseases at the University of Texas Health Science Center, San Antonio. He highlighted the most important developments in a recent interview.
“We know a lot more since 2008 about the benefits of using biomarkers like GM in bronchoalveolar lavage samples, which could be highly useful for diagnosis. However, biomarkers have not been as well validated for biologic response and are not recommended” in most cases for monitoring how well patients are doing. Also, “biomarkers are not as useful in solid organ transplants; we discuss that” in the guidelines, Dr. Patterson said.
The society came out against routine polymerase chain reaction (PCR) testing of blood samples for diagnosis. Although there has been a lot of work on the technique, the evidence isn’t strong enough yet to establish overall clinical benefit, but there is emerging evidence for the diagnostic use of PCR in conjunction with radiologic findings.
For treatment, voriconazole remains the go-to drug, but the guidelines make room for more recently approved therapies. “We now have isavuconazole, which may be better tolerated,” but it’s recommended only as an alternative to voriconazole because evidence comes mostly from a single clinical trial, he said.
Posaconazole extended-release tablets are strongly recommended as prophylaxis based on high-quality evidence from studies in neutropenic patients. Posaconazole extended-release tablets result in significantly higher antifungal blood levels than those seen with voriconazole, and “it certainly has been useful in some patients”; however, posaconazole is not approved for primary therapy in the United States, Dr. Patterson said.
A large clinical trial that tested voriconazole plus an echinocandin against voriconazole alone found that in patients diagnosed using serum galactomannan – especially those with hematologic malignancies – outcomes were better with the combination. “The panel felt combinations could be considered in some patients” but didn’t recommend them for routine use because [again,] there’s not strong evidence,” he said.
For now, it seems that higher-risk patients with hematologic malignancies and those with more widespread disease might be the ones who benefit most from combination therapy.
“We also discussed allergic and saprophytic diseases. We know that some patients with allergic bronchopulmonary aspergillosis will respond to antifungal therapy, and perhaps reduce their need for steroids, so that’s now part of the suggestions, as well,” he said.
The IDSA funded the work. Dr. Patterson receives research funding from Astellas, Merck, and Revolution Medicines, and has been an adviser to numerous drug companies.
New aspergillosis guidelines from the Infectious Diseases Society of America recommend serum and bronchoalveolar lavage galactomannan as a marker for the diagnosis of invasive Aspergillus in adult and pediatric patients who have hematologic malignancies or have undergone hematopoietic stem cell transplants.
Serial monitoring of serum galactomannan (GM) is also useful to monitor disease progression, therapeutic response, and prognosis in hematologic malignancy and hematopoietic stem cell transplant (HSCT) patients who have elevated baseline GM (Clin Infect Dis. 2016 Jun 29. doi: 10.1093/cid/ciw326).
Serum beta-D-glucan assays also are recommended for diagnosing invasive Aspergillus (IA) in high-risk hematologic malignancy and allogeneic HSCT patients, although these tests are not very specific for the infection.
The advice illustrates the Society’s emphasis on early diagnosis in its new guidelines, which supplant the group’s 2008 guidance. There are almost 100 recommendations covering – in depth – the management of invasive, allergic, and chronic Aspergillus infections in all their manifestations. It’s a step-by-step, how-to manual for handling the problem.
“Aspergillosis mortality rates have decreased significantly in recent years, but there is still significant mortality from the infection, and we have a ways to go. We felt that early diagnosis was key, which is why it’s such an important part of these guidelines,” said lead author Thomas Patterson, MD, chief of the Division of Infectious Diseases at the University of Texas Health Science Center, San Antonio. He highlighted the most important developments in a recent interview.
“We know a lot more since 2008 about the benefits of using biomarkers like GM in bronchoalveolar lavage samples, which could be highly useful for diagnosis. However, biomarkers have not been as well validated for biologic response and are not recommended” in most cases for monitoring how well patients are doing. Also, “biomarkers are not as useful in solid organ transplants; we discuss that” in the guidelines, Dr. Patterson said.
The society came out against routine polymerase chain reaction (PCR) testing of blood samples for diagnosis. Although there has been a lot of work on the technique, the evidence isn’t strong enough yet to establish overall clinical benefit, but there is emerging evidence for the diagnostic use of PCR in conjunction with radiologic findings.
For treatment, voriconazole remains the go-to drug, but the guidelines make room for more recently approved therapies. “We now have isavuconazole, which may be better tolerated,” but it’s recommended only as an alternative to voriconazole because evidence comes mostly from a single clinical trial, he said.
Posaconazole extended-release tablets are strongly recommended as prophylaxis based on high-quality evidence from studies in neutropenic patients. Posaconazole extended-release tablets result in significantly higher antifungal blood levels than those seen with voriconazole, and “it certainly has been useful in some patients”; however, posaconazole is not approved for primary therapy in the United States, Dr. Patterson said.
A large clinical trial that tested voriconazole plus an echinocandin against voriconazole alone found that in patients diagnosed using serum galactomannan – especially those with hematologic malignancies – outcomes were better with the combination. “The panel felt combinations could be considered in some patients” but didn’t recommend them for routine use because [again,] there’s not strong evidence,” he said.
For now, it seems that higher-risk patients with hematologic malignancies and those with more widespread disease might be the ones who benefit most from combination therapy.
“We also discussed allergic and saprophytic diseases. We know that some patients with allergic bronchopulmonary aspergillosis will respond to antifungal therapy, and perhaps reduce their need for steroids, so that’s now part of the suggestions, as well,” he said.
The IDSA funded the work. Dr. Patterson receives research funding from Astellas, Merck, and Revolution Medicines, and has been an adviser to numerous drug companies.
New aspergillosis guidelines from the Infectious Diseases Society of America recommend serum and bronchoalveolar lavage galactomannan as a marker for the diagnosis of invasive Aspergillus in adult and pediatric patients who have hematologic malignancies or have undergone hematopoietic stem cell transplants.
Serial monitoring of serum galactomannan (GM) is also useful to monitor disease progression, therapeutic response, and prognosis in hematologic malignancy and hematopoietic stem cell transplant (HSCT) patients who have elevated baseline GM (Clin Infect Dis. 2016 Jun 29. doi: 10.1093/cid/ciw326).
Serum beta-D-glucan assays also are recommended for diagnosing invasive Aspergillus (IA) in high-risk hematologic malignancy and allogeneic HSCT patients, although these tests are not very specific for the infection.
The advice illustrates the Society’s emphasis on early diagnosis in its new guidelines, which supplant the group’s 2008 guidance. There are almost 100 recommendations covering – in depth – the management of invasive, allergic, and chronic Aspergillus infections in all their manifestations. It’s a step-by-step, how-to manual for handling the problem.
“Aspergillosis mortality rates have decreased significantly in recent years, but there is still significant mortality from the infection, and we have a ways to go. We felt that early diagnosis was key, which is why it’s such an important part of these guidelines,” said lead author Thomas Patterson, MD, chief of the Division of Infectious Diseases at the University of Texas Health Science Center, San Antonio. He highlighted the most important developments in a recent interview.
“We know a lot more since 2008 about the benefits of using biomarkers like GM in bronchoalveolar lavage samples, which could be highly useful for diagnosis. However, biomarkers have not been as well validated for biologic response and are not recommended” in most cases for monitoring how well patients are doing. Also, “biomarkers are not as useful in solid organ transplants; we discuss that” in the guidelines, Dr. Patterson said.
The society came out against routine polymerase chain reaction (PCR) testing of blood samples for diagnosis. Although there has been a lot of work on the technique, the evidence isn’t strong enough yet to establish overall clinical benefit, but there is emerging evidence for the diagnostic use of PCR in conjunction with radiologic findings.
For treatment, voriconazole remains the go-to drug, but the guidelines make room for more recently approved therapies. “We now have isavuconazole, which may be better tolerated,” but it’s recommended only as an alternative to voriconazole because evidence comes mostly from a single clinical trial, he said.
Posaconazole extended-release tablets are strongly recommended as prophylaxis based on high-quality evidence from studies in neutropenic patients. Posaconazole extended-release tablets result in significantly higher antifungal blood levels than those seen with voriconazole, and “it certainly has been useful in some patients”; however, posaconazole is not approved for primary therapy in the United States, Dr. Patterson said.
A large clinical trial that tested voriconazole plus an echinocandin against voriconazole alone found that in patients diagnosed using serum galactomannan – especially those with hematologic malignancies – outcomes were better with the combination. “The panel felt combinations could be considered in some patients” but didn’t recommend them for routine use because [again,] there’s not strong evidence,” he said.
For now, it seems that higher-risk patients with hematologic malignancies and those with more widespread disease might be the ones who benefit most from combination therapy.
“We also discussed allergic and saprophytic diseases. We know that some patients with allergic bronchopulmonary aspergillosis will respond to antifungal therapy, and perhaps reduce their need for steroids, so that’s now part of the suggestions, as well,” he said.
The IDSA funded the work. Dr. Patterson receives research funding from Astellas, Merck, and Revolution Medicines, and has been an adviser to numerous drug companies.
FROM CLINICAL INFECTIOUS DISEASES