User login
For MD-IQ use only
How best to diagnose and manage abdominal aortic aneurysms
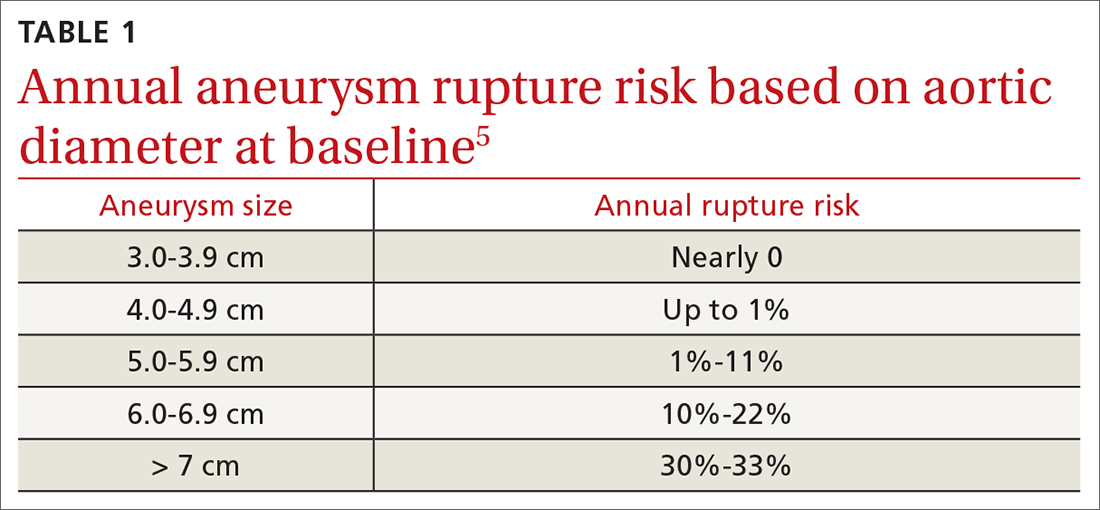
Ruptured abdominal aortic aneurysms (AAAs) caused about 6000 deaths annually in the United States between 2014 and 20201 and are associated with a pooled mortality rate of 81%.2 They result from a distinct degenerative process of the layers of the aortic wall.2 An AAA is defined as an abdominal aorta whose dilation is > 50% normal (more commonly, a diameter > 3 cm).3,4 The risk for rupture correlates closely with size; most ruptures occur in aneurysms > 5.5 cm3,4 (TABLE 15).
Most AAAs are asymptomatic and often go undetected until rupture, resulting in poor outcomes. Because of a low and declining prevalence of AAA and ruptured AAA in developed countries, screening recommendations target high-risk groups rather than the general population.4,6-8 This review summarizes risk factors, prevalence, and current evidence-based screening and management recommendations for AAA.
Who’s at risk?
Age is the most significant nonmodifiable risk factor, with AAA rupture uncommon in patients younger than 55 years.9 One retrospective study found the odds ratio (OR) for diagnosing AAA was 9.41 in adults ages 65 to 69 years (95% CI, 8.76-10.12; P < .0001) and 14.46 (95% CI, 13.45-15.55; P < .0001) in adults ages 70 to 74 years, compared to adults younger than 55 years.10
Smoking is the most potent modifiable risk factor for AAA. Among patients with AAA, > 90% have a history of smoking.4 The association between smoking and AAA is dose dependent, with an OR of 2.61 (95% CI, 2.47-2.74) in patients with a pack-per-year history < 5 years and 12.13 (95% CI, 11.66-12.61) in patients with a pack-per-year history > 35 years, compared to nonsmokers.10 The risk for AAA increases with smoking duration but decreases with cessation duration.4,10 Smoking cessation remains an important intervention, as active smokers have higher AAA rupture rates.11
Other risk factors for AAA include concomitant cardiovascular disease (CVD) such as coronary artery disease (CAD), cerebrovascular disease, atherosclerosis, dyslipidemia, and hypertension.10 Factors associated with reduced risk for AAA include African American race, Hispanic ethnicity, Asian ethnicity, diabetes, smoking cessation, consuming fruits and vegetables > 3 times per week, and exercising more than once per week.6,10
Prevalence declines but sex-based disparities in outcomes persist
The prevalence of AAA has declined in the United States and Europe in recent decades, correlating with declining rates of smoking.4,12 Reports published between 2011 and 2019 estimate that AAA prevalence in men older than 60 years has declined over time, with a prevalence of 1.2% to 3.3%.6 The prevalence of AAA has also decreased in women,6,13,14 estimated in 1 study to be as low as 0.74%.13 Similarly, deaths from ruptured AAA have declined markedly in the United States—by 70% between 1999 and 2016 according to 1 analysis.9
One striking difference in the male-female data is that although AAAs are more common in men, there is a 2- to 4-fold higher risk for rupture in women, who account for nearly half of all AAA-related deaths.9,10,15-17 The reasons for this heightened risk to women despite lower prevalence are not fully understood but are likely multifactorial and related to a general lack of screening for AAA in women, tendency for AAA to rupture at smaller diameters in women, rupture at an older age in women, and a history of worse surgical outcomes in women than men (though the gap in surgical outcomes appears to be closing).9,10,18
Continue to: While declines in AAA and AAA-related...
While declines in AAA and AAA-related death are largely attributed to lower smoking rates, other likely contributing factors include the implementation of screening programs, incidental detection during cross-sectional imaging, and improved surgical techniques and management of CV risk factors (eg, hypertension, hyperlipidemia).9,10
The benefits of screening older men
Randomized controlled trials (RCTs) have demonstrated the benefits of AAA screening programs. A meta-analysis of 4 populationbased RCTs of AAA screening in men ≥ 65 years demonstrated statistically significant reductions in AAA rupture (OR = 0.62; 95% CI, 0.55-0.70) and death from AAA (OR = 0.65; 95% CI, 0.57-0.74) over 12 to 15 years, with a number needed to screen (NNS) of 305 (95% CI, 248-411) to prevent 1 AAA-related death.18 The study also found screening decreases the rate of emergent surgeries for AAA (OR = 0.57; 95% CI, 0.48-0.68) while increasing the number of elective surgeries (OR = 1.44; 95% CI, 1.34-1.55) over 4 to 15 years.18
Only 1 study has demonstrated an improvement in all-cause mortality with screening programs, with a relatively small benefit (OR = 0.97; 95% CI, 0.94-0.99).19 Only 1 of the studies included women and, while underpowered, showed no difference in AAA-related death or rupture.20 Guidelines and recommendations of various countries and professional societies focus screening on subgroups at highest risk for AAA.4,6-8,18
Screening recommendations from USPSTF and others
The US Preventive Services Task Force (USPSTF) currently recommends one-time ultrasound screening for AAA in men ages 65 to 75 years who have ever smoked (commonly defined as having smoked > 100 cigarettes) in their lifetime.6 This grade “B” recommendation, initially made in 2005 and reaffirmed in the 2014 and 2019 USPSTF updates, recommends screening the highest-risk segment of the population (ie, older male smokers).
In men ages 65 to 75 years with no smoking history, rather than routine screening, the USPSTF recommends selectively offering screening based on the patient’s medical history, family history, risk factors, and personal values (with a “C” grade).6 The USPSTF continues to recommend against screening for AAA in women with no smoking history and no family history of AAA.6 According to the USPSTF, the evidence is insufficient to recommend for or against screening women ages 65 to 75 years who have ever smoked or have a family history of AAA (“I” statement).6
Continue to: One critique of the USPSTF recommendations
One critique of the USPSTF recommendations is that they fail to detect a significant portion of patients with AAA and AAA rupture. For example, in a retrospective analysis of 55,197 patients undergoing AAA repair, only 33% would have been detected by the USPSTF grade “B” recommendation to screen male smokers ages 65 to 75 years, and an analysis of AAA-related fatalities found 43% would be missed by USPSTF criteria.9,21
Screening guidelines from the Society for Vascular Surgery (SVS) are broader than those of the USPSTF, in an attempt to capture a larger percentage of the population at risk for AAA-related disease by extrapolating from epidemiologic data. The SVS guidelines include screening for women ages 65 to 75 years with a smoking history, screening men and women ages 65 to 75 years who have a first-degree relative with AAA, and consideration of screening patients older than 75 years if they are in good health and have a first-degree relative with AAA or a smoking history and have not been previously screened.4 However, these expanded recommendations are not supported by patient-oriented evidence.6
Attempts to broaden screening guidelines must be tempered by potential risks for harm, primarily overdiagnosis (ie, diagnosing AAAs that would not otherwise rise to clinical significance) and overtreatment (ie, resulting in unnecessary imaging, appointments, anxiety, or surgery). Negative psychological effects on quality of life after a diagnosis of AAA have not been shown to cause significant harm.6,18
A recent UK analysis found that screening programs for AAA in women modeled after those in men are not cost effective, with an NNS to prevent 1 death of 3900 in women vs 700 in men.15,18 Another recent trial of ultrasound screening in 5200 high-risk women ages 65 to 74 years found an AAA incidence of 0.29% (95% CI, 0.18%-0.48%) in which only 3 large aneurysms were identified.22
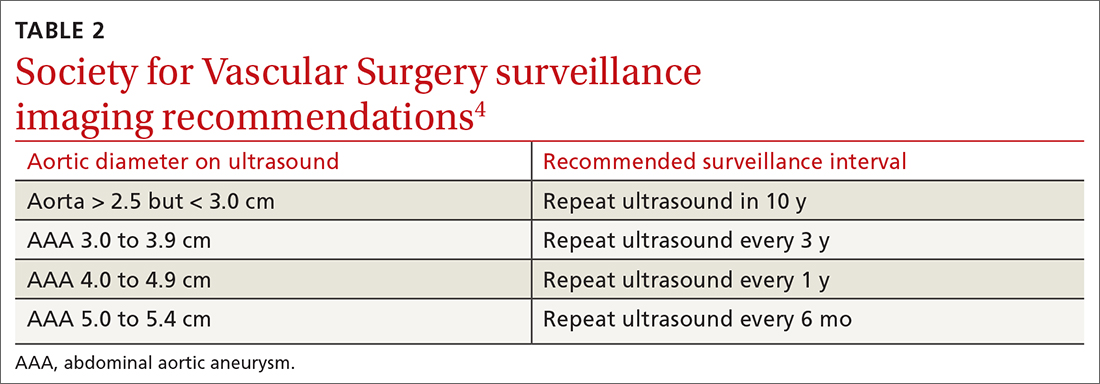
In the United States, rates of screening for AAA remain low.23 One study has shown electronic medical record–based reminders increased screening rates from 48% to 80%.24 Point-of-care bedside ultrasound performed by clinicians also could improve screening rates. Multiple studies have demonstrated that screening and diagnosis of AAA can be performed safely and effectively at the bedside by nonradiologists such as family physicians and emergency physicians.25-28 In 1 study, such exams added < 4 minutes to the patient encounter.26 Follow-up surveillance schedules for those identified as having a AAA are summarized in TABLE 2.4
Continue to: Management options
Management options: Immediate repair or surveillance?
After diagnosing AAA, important decisions must be made regarding management, including indications for surgical repair, appropriate follow-up surveillance, and medications for secondary prevention and cardiovascular risk reduction.
EVAR vs open repair
The 2 main surgical strategies for aneurysm repair are open repair and endovascular repair (EVAR). In the United States, EVAR is becoming the more common approach and was used to repair asymptomatic aneurysms in > 80% of patients and ruptured aneurysms in 50% of patients.6 There have been multiple RCTs assessing EVAR and open repair for large and small aneurysms.29-34 Findings across these studies consistently show EVAR is associated with lower immediate (ie, 30-day) morbidity and mortality but no longer-term survival benefit compared to open repair.
EVAR procedures require ongoing long-term surveillance for endovascular leakage and other complications, resulting in an increased need for re-intervention.31,33,35 For these reasons, the National Institute for Health and Care Excellence (NICE) guidelines suggest open repair as the preferred modality.7 However, SVS and the American College of Cardiology Foundation/American Heart Association guidance support either EVAR or open repair, noting that open repair may be preferable in patients unable to engage in long-term follow-up surveillance.36

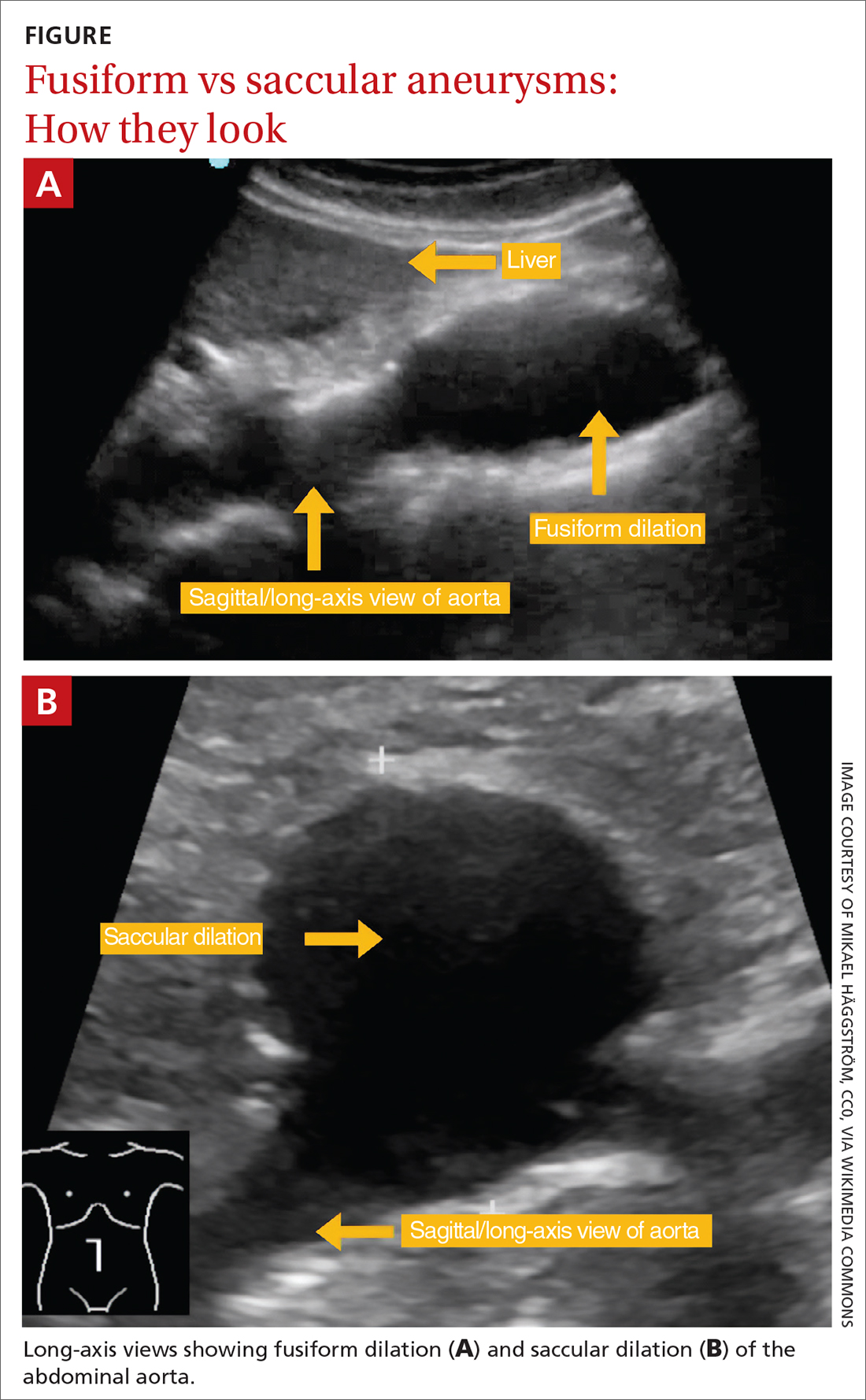
Indications for repair. In general, repair is indicated when an aneurysm reaches or exceeds 5.5 cm.4,7 Both SVS and NICE also recommend clinicians consider surgical repair of smaller, rapidly expanding aneurysms (> 1 cm over a 1-year period).4,7 Based on evidence suggesting a higher risk for rupture in women with smaller aneurysms,14,37 SVS recommends clinicians consider surgical repair in women with an AAA ≥ 5.0 cm. Several RCTs evaluating the benefits of immediate repair for smaller-sized aneurysms (4.0-5.5 cm) favored surveillance.38,39 Accepted indications for surgical repair are summarized in TABLE 3.4,7,34Surgical repair recommendations also are based on aneurysm morphology, which can be fusiform or saccular (FIGURE). More than 90% of AAAs are fusiform.40 Although saccular AAAs are less common, some studies suggest they are more prone to rupture than fusiform AAAs, and SVS guidelines suggest surgical repair of saccular aneurysms regardless of size.4,41,42
Perioperative and long-term risks. Both EVAR and open repair of AAA carry a high perioperative and long-term risk for death, as patients often have multiple comorbidities. A 2019 trial comparing EVAR to open repair with 14 years of follow-up reported death in 68% of patients in the EVAR group and 70% in the open repair group. 31 Among these deaths, 2.7% in the EVAR group and 3.7% in the open repair group were aneurysm related.31 The study also found a second surgical intervention was required in 19.8% of patients in the open repair group and 26.7% in the EVAR group.31
Continue to: When assessing perioperative risk...
When assessing perioperative risk, SVS guidelines recommend clinicians employ a shared decision-making approach with patients that incorporates Vascular Quality Initiative (VQI) mortality risk score.4 (VQI risk calculators are available at https://qxmd.com/vascular-study-group-new-england-decision-support-tools.43)
Medication management
Based on the close association of aortic aneurysm with atherosclerotic CVD (ASCVD), professional societies such as the European Society of Cardiology and European Atherosclerosis Society (ESC/EAS) have suggested aortic aneurysm is equivalent to ASCVD and should be managed medically in a similar manner to peripheral arterial disease.44 Indeed, many patients with AAA may have concomitant CAD or other arterial vascular diseases (eg, carotid, lower extremity).
Statins. In its guidelines, the ESC/EAS consider patients with AAA at “very high risk” for adverse CV events and suggest pharmacotherapy with high-intensity statins, adding ezetimibe or proprotein convertase subtilisin/kexin type 9 (PCSK9) inhibitors if needed, to reduce low-density lipoprotein cholesterol ≥ 50% from baseline, with a goal of < 55 mg/dL.44 Statin therapy additionally lowers all-cause postoperative mortality in patients undergoing AAA repair but does not affect the rate of aneurysm expansion.45
Aspirin and other anticoagulants. Although aspirin therapy may be indicated for the secondary prevention of other cardiovascular events that may coexist with AAA, it does not appear to affect the rate of growth or prevent rupture of aneurysms.46,47 In addition to aspirin, anticoagulants such as clopidogrel, enoxaparin, and warfarin are not recommended when the presence of AAA is the only indication.4
Other medications. Angiotensin-converting enzyme inhibitors, angiotensin receptor blockers, beta-blockers, and antibiotics (eg, doxycycline) have been studied as a treatment for AAA. However, none has shown benefit in reducing aneurysm growth or rupture and they are not recommended for that sole purpose.4,48
Metformin. There is a negative association between diabetes and AAA expansion and rupture. Several cohort studies have indicated that this may be an independent effect driven primarily by exposure to metformin. While it is not unreasonable to consider this another important indication for metformin use in patients with diabetes, RCT evidence has yet to establish a role for metformin in patients without diabetes who have AAA.48,49
ACKNOWLEDGEMENT
The authors thank Gwen Wilson, MLS, AHIP, for her assistance with the literature searches performed in the preparation of this manuscript.
CORRESPONDENCE
Nicholas LeFevre, MD, Family and Community Medicine, University of Missouri–Columbia School of Medicine, One Hospital Drive, M224 Medical Science Building, Columbia, MO 65212; [email protected]
1. CDC. Wide-ranging Online Data for Epidemiologic Research (WONDER) database. Accessed August 30, 2023. https://wonder.cdc.gov/ucd-icd10.html
2. Reimerink JJ, van der Laan MJ, Koelemay MJ, et al. Systematic review and meta-analysis of population-based mortality from ruptured abdominal aortic aneurysm. Br J Surg. 2013;100:1405-1413. doi: 10.1002/bjs.9235
3. Kent KC. Clinical practice. Abdominal aortic aneurysms. N Engl J Med. 2014;371:2101-2108. doi: 10.1056/NEJMcp1401430
4. Chaikof EL, Dalman RL, Eskandari MK, et al. The Society for Vascular Surgery practice guidelines on the care of patients with an abdominal aortic aneurysm. J Vasc Surg. 2018;67:2-77.e2. doi: 10.1016/j.jvs.2017.10.044
5. Moll FL, Powell JT, Fraedrich G, et al. Management of abdominal aortic aneurysms clinical practice guidelines of the European society for vascular surgery. Eur J Vasc Endovasc Surg. 2011;41 suppl 1:S1-S58. doi: 10.1016/j.ejvs.2010.09.011
6. Owens DK, Davidson KW, Krist AH, et al; US Preventive Services Task Force. Screening for abdominal aortic aneurysm: US Preventive Services Task Force recommendation statement. JAMA. 2019;322:2211-2218. doi: 10.1001/jama.2019.18928
7. National Institute for Health and Care Excellence. Abdominal aortic aneurysm: diagnosis and management. NICE guideline [NG156]. March 19, 2020. Accessed June 30, 2023. www.nice.org.uk/guidance/ng156/chapter/recommendations
8. Canadian Task Force on Preventive Health Care. Recommendations on screening for abdominal aortic aneurysm in primary care. CMAJ. 2017;189:E1137-E1145. doi: 10.1503/cmaj.170118
9. Abdulameer H, Al Taii H, Al-Kindi SG, et al. Epidemiology of fatal ruptured aortic aneurysms in the United States (1999-2016). J Vasc Surg. 2019;69:378-384.e2. doi: 10.1016/j.jvs.2018.03.435
10. Kent KC, Zwolak RM, Egorova NN, et al. Analysis of risk factors for abdominal aortic aneurysm in a cohort of more than 3 million individuals. J Vasc Surg. 2010;52:539-548. doi: 10.1016/j.jvs.2010.05.090
11. [No authors listed] Smoking, lung function and the prognosis of abdominal aortic aneurysm. The UK Small Aneurysm Trial Participants. Eur J Vasc Endovasc Surg. 2000;19:636-642. doi: 10.1053/ejvs.2000.1066
12. Oliver-Williams C, Sweeting MJ, Turton G, et al. Lessons learned about prevalence and growth rates of abdominal aortic aneurysms from a 25-year ultrasound population screening programme. Br J Surg. 2018;105:68-74. doi: 10.1002/bjs.10715
13. Ulug P, Powell JT, Sweeting MJ, et al. Meta-analysis of the current prevalence of screen-detected abdominal aortic aneurysm in women. Br J Surg. 2016;103:1097-1104. doi: 10.1002/bjs.10225
14. Chabok M, Nicolaides A, Aslam M, et al. Risk factors associated with increased prevalence of abdominal aortic aneurysm in women. Br J Surg. 2016;103:1132-1138. doi: 10.1002/bjs.10179
15. Sweeting, MJ, Masconi KL, Jones E, et al. Analysis of clinical benefit, harms, and cost-effectiveness of screening women for abdominal aortic aneurysm. Lancet. 2018;392:487-495. doi: 10.1016/S0140-6736(18)31222-4
16. Sweeting MJ, Thompson SG, Brown LC, et al; RESCAN collaborators. Meta-analysis of individual patient data to examine factors affecting growth and rupture of small abdominal aortic aneurysms. Br J Surg. 2012;99:655-665. doi: 10.1002/bjs.8707
17. Skibba AA, Evans JR, Hopkins SP, et al. Reconsidering gender relative to risk of rupture in the contemporary management of abdominal aortic aneurysms. J Vasc Surg. 2015;62:1429-1436. doi: 10.1016/j.jvs.2015.07.079
18. Guirguis-Blake JM, Beil TL, Senger CA, et al. Primary care screening for abdominal aortic aneurysm: updated evidence report and systematic review for the US Preventive Services Task Force. JAMA. 2019;322:2219-2238. doi: 10.1001/jama.2019.17021
19. Thompson SG, Ashton HA, Gao L, et al; Multicentre Aneurysm Screening Study (MASS) Group. Final follow-up of the Multicentre Aneurysm Screening Study (MASS) randomized trial of abdominal aortic aneurysm screening. Br J Surg. 2012;99:1649-1656. doi: 10.1002/bjs.8897
20. Ashton HA, Gao L, Kim LG, et al. Fifteen-year follow-up of a randomized clinical trial of ultrasonographic screening for abdominal aortic aneurysms. Br J Surg. 2007;94:696-701. doi: 10.1002/bjs.5780
21. Carnevale ML, Koleilat I, Lipsitz EC, et al. Extended screening guidelines for the diagnosis of abdominal aortic aneurysm. J Vasc Surg. 2020;72:1917-1926. doi: 10.1016/j.jvs.2020.03.047
22. Duncan A, Maslen C, Gibson C, et al. Ultrasound screening for abdominal aortic aneurysm in high-risk women. Br J Surg. 2021;108:1192-1198. doi: 10.1093/bjs/znab220
23. Shreibati JB, Baker LC, Hlatky MA, et al. Impact of the Screening Abdominal Aortic Aneurysms Very Efficiently (SAAAVE) Act on abdominal ultrasonography use among Medicare beneficiaries. Arch Intern Med. 2012;172:1456-1462. doi: 10.1001/archinternmed.2012.4268
24. Hye RJ, Smith AE, Wong GH, et al. Leveraging the electronic medical record to implement an abdominal aortic aneurysm screening program. J Vasc Surg. 2014;59:1535-1542. doi: 10.1016/j.jvs.2013.12.016
25. Rubano E, Mehta N, Caputo W, et al., Systematic review: emergency department bedside ultrasonography for diagnosing suspected abdominal aortic aneurysm. Acad Emerg Med. 2013. 20:128-138. doi: 10.1111/acem.12080
26. Blois B. Office-based ultrasound screening for abdominal aortic aneurysm. Can Fam Physician. 2012;58:e172-e178.
27. Arnold MJ, Jonas CE, Carter RE. Point-of-care ultrasonography. Am Fam Physician. 2020;101:275-285.
28. Nixon G, Blattner K, Muirhead J, et al. Point-of-care ultrasound for FAST and AAA in rural New Zealand: quality and impact on patient care. Rural Remote Health. 2019;19:5027. doi: 10.22605/RRH5027
29. Lederle FA, Wilson SE, Johnson GR, et al. Immediate repair compared with surveillance of small abdominal aortic aneurysms. N Engl J Med. 2002;346:1437-1444. doi: 10.1056/NEJMoa012573
30. Filardo G, Lederle FA, Ballard DJ, et al. Immediate open repair vs surveillance in patients with small abdominal aortic aneurysms: survival differences by aneurysm size. Mayo Clin Proc. 2013;88:910-919. doi: 10.1016/j.mayocp.2013.05.014
31. Lederle FA, Kyriakides TC, Stroupe KT, et al. Open versus endovascular repair of abdominal aortic aneurysm. N Engl J Med. 2019;380:2126-2135. doi: 10.1056/NEJMoa1715955
32. Patel R, Sweeting MJ, Powell JT, et al., Endovascular versus open repair of abdominal aortic aneurysm in 15-years’ follow-up of the UK endovascular aneurysm repair trial 1 (EVAR trial 1): a randomised controlled trial. Lancet. 2016;388:2366-2374. doi: 10.1016/S0140-6736(16)31135-7
33. van Schaik TG, Yeung KK, Verhagen HJ, et al. Long-term survival and secondary procedures after open or endovascular repair of abdominal aortic aneurysms. J Vasc Surg. 2017;66:1379-1389. doi: 10.1016/j.jvs.2017.05.122
34. Powell JT, Brady AR, Brown, LC, et al; United Kingdom Small Aneurysm Trial Participants. Long-term outcomes of immediate repair compared with surveillance of small abdominal aortic aneurysms. N Engl J Med. 2002;346:1445-1452. doi: 10.1056/NEJMoa013527
35. Paravastu SC, Jayarajasingam R, Cottam R, et al. Endovascular repair of abdominal aortic aneurysm. Cochrane Database Syst Rev. 2014:CD004178. doi: 10.1002/14651858.CD004178.pub2
36. Rooke TW, Hirsch AT, Misra S, et al. 2011 ACCF/AHA focused update of the guideline for the management of patients with peripheral artery disease (updating the 2005 guideline): a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2011;58:2020-2045. doi: 10.1016/j.jacc.2011.08.023
37. Bhak RH, Wininger M, Johnson GR, et al. Factors associated with small abdominal aortic aneurysm expansion rate. JAMA Surg. 2015;150:44-50. doi: 10.1001/jamasurg.2014.2025
38. Ouriel K, Clair DG, Kent KC, et al; Positive Impact of Endovascular Options for treating Aneurysms Early (PIVOTAL) Investigators. Endovascular repair compared with surveillance for patients with small abdominal aortic aneurysms. J Vasc Surg. 2010;51:1081-1087. doi: 10.1016/j.jvs.2009.10.113
39. Cao P, De Rango P, Verzini F, et al. Comparison of surveillance versus aortic endografting for small aneurysm repair (CAESAR): results from a randomised trial. Eur J Vasc Endovasc Surg. 2011;41:13-25. doi: 10.1016/j.ejvs.2010.08.026
40. Karthaus EG, Tong TML, Vahl A, et al; Dutch Society of Vascular Surgery, the Steering Committee of the Dutch Surgical Aneurysm Audit and the Dutch Institute for Clinical Auditing. Saccular abdominal aortic aneurysms: patient characteristics, clinical presentation, treatment, and outcomes in the Netherlands. Ann Surg. 2019;270:852-858. doi: 10.1097/SLA.0000000000003529
41. Nathan DP, Xu C, Pouch AM, et al. Increased wall stress of saccular versus fusiform aneurysms of the descending thoracic aorta. Ann Vasc Surg. 2011;25:1129-2237. doi: 10.1016/j.avsg.2011.07.008
42. Durojaye MS, Adeniyi TO, Alagbe OA. Multiple saccular aneurysms of the abdominal aorta: a case report and short review of risk factors for rupture on CT Scan. Ann Ib Postgrad Med. 2020;18:178-180.
43. Bertges DJ, Neal D, Schanzer A, et al. The Vascular Quality Initiative Cardiac Risk Index for prediction of myocardial infarction after vascular surgery. J Vasc Surg. 2016;64:1411-1421.e4. doi: 10.1016/j.jvs.2016.04.045
44. Mach F, Baigent C, Catapano AL, et al. 2019 ESC/EAS guidelines for the management of dyslipidaemias: lipid modification to reduce cardiovascular risk. Eur Heart J. 2020;41:111-188. doi: 10.1093/eurheartj/ehz455
45. Twine CP, Williams IM. Systematic review and meta-analysis of the effects of statin therapy on abdominal aortic aneurysms. Br J Surg. 2011;98:346-353. doi: 10.1002/bjs.7343
46. Arnett DK, Blumenthal RS, Albert MA, et al. 2019 ACC/AHA guideline on the primary prevention of cardiovascular disease: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation. 2019;140:e596-e646. doi: 10.1161/CIR.0000000000000678
47. Erbel R, Aboyans V, Boileau C, et al. 2014 ESC guidelines on the diagnosis and treatment of aortic diseases: document covering acute and chronic aortic diseases of the thoracic and abdominal aorta of the adult. The Task Force for the Diagnosis and Treatment of Aortic Diseases of the European Society of Cardiology (ESC). Eur Heart J. 2014;35:2873-2926. doi: 10.1093/eurheartj/ehu281
48. Lederle FA, Noorbaloochi S, Nugent S, et al. Multicentre study of abdominal aortic aneurysm measurement and enlargement. Br J Surg. 2015;102:1480-1487. doi: 10.1002/bjs.9895
49. Itoga NK, Rothenberg KA, Suarez P, et al. Metformin prescription status and abdominal aortic aneurysm disease progression in the U.S. veteran population. J Vasc Surg. 2019;69:710-716.e3. doi: 10.1016/j.jvs.2018.06.19
Ruptured abdominal aortic aneurysms (AAAs) caused about 6000 deaths annually in the United States between 2014 and 20201 and are associated with a pooled mortality rate of 81%.2 They result from a distinct degenerative process of the layers of the aortic wall.2 An AAA is defined as an abdominal aorta whose dilation is > 50% normal (more commonly, a diameter > 3 cm).3,4 The risk for rupture correlates closely with size; most ruptures occur in aneurysms > 5.5 cm3,4 (TABLE 15).

Most AAAs are asymptomatic and often go undetected until rupture, resulting in poor outcomes. Because of a low and declining prevalence of AAA and ruptured AAA in developed countries, screening recommendations target high-risk groups rather than the general population.4,6-8 This review summarizes risk factors, prevalence, and current evidence-based screening and management recommendations for AAA.
Who’s at risk?
Age is the most significant nonmodifiable risk factor, with AAA rupture uncommon in patients younger than 55 years.9 One retrospective study found the odds ratio (OR) for diagnosing AAA was 9.41 in adults ages 65 to 69 years (95% CI, 8.76-10.12; P < .0001) and 14.46 (95% CI, 13.45-15.55; P < .0001) in adults ages 70 to 74 years, compared to adults younger than 55 years.10
Smoking is the most potent modifiable risk factor for AAA. Among patients with AAA, > 90% have a history of smoking.4 The association between smoking and AAA is dose dependent, with an OR of 2.61 (95% CI, 2.47-2.74) in patients with a pack-per-year history < 5 years and 12.13 (95% CI, 11.66-12.61) in patients with a pack-per-year history > 35 years, compared to nonsmokers.10 The risk for AAA increases with smoking duration but decreases with cessation duration.4,10 Smoking cessation remains an important intervention, as active smokers have higher AAA rupture rates.11
Other risk factors for AAA include concomitant cardiovascular disease (CVD) such as coronary artery disease (CAD), cerebrovascular disease, atherosclerosis, dyslipidemia, and hypertension.10 Factors associated with reduced risk for AAA include African American race, Hispanic ethnicity, Asian ethnicity, diabetes, smoking cessation, consuming fruits and vegetables > 3 times per week, and exercising more than once per week.6,10
Prevalence declines but sex-based disparities in outcomes persist
The prevalence of AAA has declined in the United States and Europe in recent decades, correlating with declining rates of smoking.4,12 Reports published between 2011 and 2019 estimate that AAA prevalence in men older than 60 years has declined over time, with a prevalence of 1.2% to 3.3%.6 The prevalence of AAA has also decreased in women,6,13,14 estimated in 1 study to be as low as 0.74%.13 Similarly, deaths from ruptured AAA have declined markedly in the United States—by 70% between 1999 and 2016 according to 1 analysis.9
One striking difference in the male-female data is that although AAAs are more common in men, there is a 2- to 4-fold higher risk for rupture in women, who account for nearly half of all AAA-related deaths.9,10,15-17 The reasons for this heightened risk to women despite lower prevalence are not fully understood but are likely multifactorial and related to a general lack of screening for AAA in women, tendency for AAA to rupture at smaller diameters in women, rupture at an older age in women, and a history of worse surgical outcomes in women than men (though the gap in surgical outcomes appears to be closing).9,10,18
Continue to: While declines in AAA and AAA-related...
While declines in AAA and AAA-related death are largely attributed to lower smoking rates, other likely contributing factors include the implementation of screening programs, incidental detection during cross-sectional imaging, and improved surgical techniques and management of CV risk factors (eg, hypertension, hyperlipidemia).9,10
The benefits of screening older men
Randomized controlled trials (RCTs) have demonstrated the benefits of AAA screening programs. A meta-analysis of 4 populationbased RCTs of AAA screening in men ≥ 65 years demonstrated statistically significant reductions in AAA rupture (OR = 0.62; 95% CI, 0.55-0.70) and death from AAA (OR = 0.65; 95% CI, 0.57-0.74) over 12 to 15 years, with a number needed to screen (NNS) of 305 (95% CI, 248-411) to prevent 1 AAA-related death.18 The study also found screening decreases the rate of emergent surgeries for AAA (OR = 0.57; 95% CI, 0.48-0.68) while increasing the number of elective surgeries (OR = 1.44; 95% CI, 1.34-1.55) over 4 to 15 years.18
Only 1 study has demonstrated an improvement in all-cause mortality with screening programs, with a relatively small benefit (OR = 0.97; 95% CI, 0.94-0.99).19 Only 1 of the studies included women and, while underpowered, showed no difference in AAA-related death or rupture.20 Guidelines and recommendations of various countries and professional societies focus screening on subgroups at highest risk for AAA.4,6-8,18
Screening recommendations from USPSTF and others
The US Preventive Services Task Force (USPSTF) currently recommends one-time ultrasound screening for AAA in men ages 65 to 75 years who have ever smoked (commonly defined as having smoked > 100 cigarettes) in their lifetime.6 This grade “B” recommendation, initially made in 2005 and reaffirmed in the 2014 and 2019 USPSTF updates, recommends screening the highest-risk segment of the population (ie, older male smokers).
In men ages 65 to 75 years with no smoking history, rather than routine screening, the USPSTF recommends selectively offering screening based on the patient’s medical history, family history, risk factors, and personal values (with a “C” grade).6 The USPSTF continues to recommend against screening for AAA in women with no smoking history and no family history of AAA.6 According to the USPSTF, the evidence is insufficient to recommend for or against screening women ages 65 to 75 years who have ever smoked or have a family history of AAA (“I” statement).6
Continue to: One critique of the USPSTF recommendations
One critique of the USPSTF recommendations is that they fail to detect a significant portion of patients with AAA and AAA rupture. For example, in a retrospective analysis of 55,197 patients undergoing AAA repair, only 33% would have been detected by the USPSTF grade “B” recommendation to screen male smokers ages 65 to 75 years, and an analysis of AAA-related fatalities found 43% would be missed by USPSTF criteria.9,21
Screening guidelines from the Society for Vascular Surgery (SVS) are broader than those of the USPSTF, in an attempt to capture a larger percentage of the population at risk for AAA-related disease by extrapolating from epidemiologic data. The SVS guidelines include screening for women ages 65 to 75 years with a smoking history, screening men and women ages 65 to 75 years who have a first-degree relative with AAA, and consideration of screening patients older than 75 years if they are in good health and have a first-degree relative with AAA or a smoking history and have not been previously screened.4 However, these expanded recommendations are not supported by patient-oriented evidence.6
Attempts to broaden screening guidelines must be tempered by potential risks for harm, primarily overdiagnosis (ie, diagnosing AAAs that would not otherwise rise to clinical significance) and overtreatment (ie, resulting in unnecessary imaging, appointments, anxiety, or surgery). Negative psychological effects on quality of life after a diagnosis of AAA have not been shown to cause significant harm.6,18
A recent UK analysis found that screening programs for AAA in women modeled after those in men are not cost effective, with an NNS to prevent 1 death of 3900 in women vs 700 in men.15,18 Another recent trial of ultrasound screening in 5200 high-risk women ages 65 to 74 years found an AAA incidence of 0.29% (95% CI, 0.18%-0.48%) in which only 3 large aneurysms were identified.22
In the United States, rates of screening for AAA remain low.23 One study has shown electronic medical record–based reminders increased screening rates from 48% to 80%.24 Point-of-care bedside ultrasound performed by clinicians also could improve screening rates. Multiple studies have demonstrated that screening and diagnosis of AAA can be performed safely and effectively at the bedside by nonradiologists such as family physicians and emergency physicians.25-28 In 1 study, such exams added < 4 minutes to the patient encounter.26 Follow-up surveillance schedules for those identified as having a AAA are summarized in TABLE 2.4

Continue to: Management options
Management options: Immediate repair or surveillance?
After diagnosing AAA, important decisions must be made regarding management, including indications for surgical repair, appropriate follow-up surveillance, and medications for secondary prevention and cardiovascular risk reduction.
EVAR vs open repair
The 2 main surgical strategies for aneurysm repair are open repair and endovascular repair (EVAR). In the United States, EVAR is becoming the more common approach and was used to repair asymptomatic aneurysms in > 80% of patients and ruptured aneurysms in 50% of patients.6 There have been multiple RCTs assessing EVAR and open repair for large and small aneurysms.29-34 Findings across these studies consistently show EVAR is associated with lower immediate (ie, 30-day) morbidity and mortality but no longer-term survival benefit compared to open repair.
EVAR procedures require ongoing long-term surveillance for endovascular leakage and other complications, resulting in an increased need for re-intervention.31,33,35 For these reasons, the National Institute for Health and Care Excellence (NICE) guidelines suggest open repair as the preferred modality.7 However, SVS and the American College of Cardiology Foundation/American Heart Association guidance support either EVAR or open repair, noting that open repair may be preferable in patients unable to engage in long-term follow-up surveillance.36

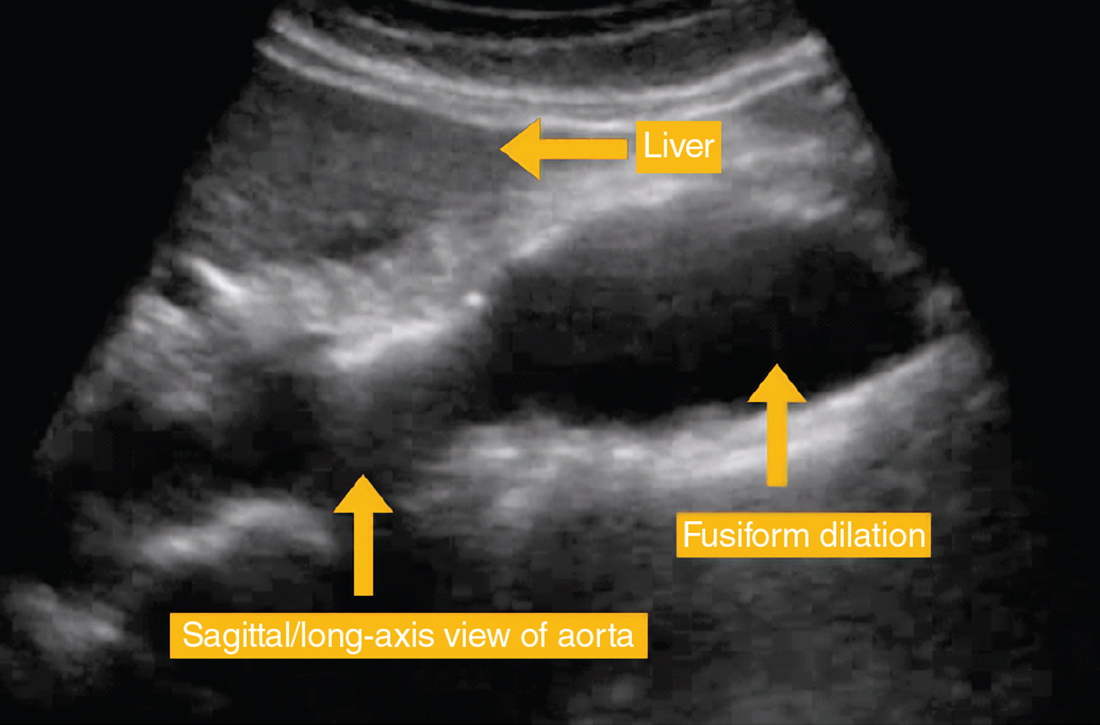
Indications for repair. In general, repair is indicated when an aneurysm reaches or exceeds 5.5 cm.4,7 Both SVS and NICE also recommend clinicians consider surgical repair of smaller, rapidly expanding aneurysms (> 1 cm over a 1-year period).4,7 Based on evidence suggesting a higher risk for rupture in women with smaller aneurysms,14,37 SVS recommends clinicians consider surgical repair in women with an AAA ≥ 5.0 cm. Several RCTs evaluating the benefits of immediate repair for smaller-sized aneurysms (4.0-5.5 cm) favored surveillance.38,39 Accepted indications for surgical repair are summarized in TABLE 3.4,7,34Surgical repair recommendations also are based on aneurysm morphology, which can be fusiform or saccular (FIGURE). More than 90% of AAAs are fusiform.40 Although saccular AAAs are less common, some studies suggest they are more prone to rupture than fusiform AAAs, and SVS guidelines suggest surgical repair of saccular aneurysms regardless of size.4,41,42

Perioperative and long-term risks. Both EVAR and open repair of AAA carry a high perioperative and long-term risk for death, as patients often have multiple comorbidities. A 2019 trial comparing EVAR to open repair with 14 years of follow-up reported death in 68% of patients in the EVAR group and 70% in the open repair group. 31 Among these deaths, 2.7% in the EVAR group and 3.7% in the open repair group were aneurysm related.31 The study also found a second surgical intervention was required in 19.8% of patients in the open repair group and 26.7% in the EVAR group.31
Continue to: When assessing perioperative risk...
When assessing perioperative risk, SVS guidelines recommend clinicians employ a shared decision-making approach with patients that incorporates Vascular Quality Initiative (VQI) mortality risk score.4 (VQI risk calculators are available at https://qxmd.com/vascular-study-group-new-england-decision-support-tools.43)
Medication management
Based on the close association of aortic aneurysm with atherosclerotic CVD (ASCVD), professional societies such as the European Society of Cardiology and European Atherosclerosis Society (ESC/EAS) have suggested aortic aneurysm is equivalent to ASCVD and should be managed medically in a similar manner to peripheral arterial disease.44 Indeed, many patients with AAA may have concomitant CAD or other arterial vascular diseases (eg, carotid, lower extremity).
Statins. In its guidelines, the ESC/EAS consider patients with AAA at “very high risk” for adverse CV events and suggest pharmacotherapy with high-intensity statins, adding ezetimibe or proprotein convertase subtilisin/kexin type 9 (PCSK9) inhibitors if needed, to reduce low-density lipoprotein cholesterol ≥ 50% from baseline, with a goal of < 55 mg/dL.44 Statin therapy additionally lowers all-cause postoperative mortality in patients undergoing AAA repair but does not affect the rate of aneurysm expansion.45
Aspirin and other anticoagulants. Although aspirin therapy may be indicated for the secondary prevention of other cardiovascular events that may coexist with AAA, it does not appear to affect the rate of growth or prevent rupture of aneurysms.46,47 In addition to aspirin, anticoagulants such as clopidogrel, enoxaparin, and warfarin are not recommended when the presence of AAA is the only indication.4
Other medications. Angiotensin-converting enzyme inhibitors, angiotensin receptor blockers, beta-blockers, and antibiotics (eg, doxycycline) have been studied as a treatment for AAA. However, none has shown benefit in reducing aneurysm growth or rupture and they are not recommended for that sole purpose.4,48
Metformin. There is a negative association between diabetes and AAA expansion and rupture. Several cohort studies have indicated that this may be an independent effect driven primarily by exposure to metformin. While it is not unreasonable to consider this another important indication for metformin use in patients with diabetes, RCT evidence has yet to establish a role for metformin in patients without diabetes who have AAA.48,49
ACKNOWLEDGEMENT
The authors thank Gwen Wilson, MLS, AHIP, for her assistance with the literature searches performed in the preparation of this manuscript.
CORRESPONDENCE
Nicholas LeFevre, MD, Family and Community Medicine, University of Missouri–Columbia School of Medicine, One Hospital Drive, M224 Medical Science Building, Columbia, MO 65212; [email protected]
Ruptured abdominal aortic aneurysms (AAAs) caused about 6000 deaths annually in the United States between 2014 and 20201 and are associated with a pooled mortality rate of 81%.2 They result from a distinct degenerative process of the layers of the aortic wall.2 An AAA is defined as an abdominal aorta whose dilation is > 50% normal (more commonly, a diameter > 3 cm).3,4 The risk for rupture correlates closely with size; most ruptures occur in aneurysms > 5.5 cm3,4 (TABLE 15).

Most AAAs are asymptomatic and often go undetected until rupture, resulting in poor outcomes. Because of a low and declining prevalence of AAA and ruptured AAA in developed countries, screening recommendations target high-risk groups rather than the general population.4,6-8 This review summarizes risk factors, prevalence, and current evidence-based screening and management recommendations for AAA.
Who’s at risk?
Age is the most significant nonmodifiable risk factor, with AAA rupture uncommon in patients younger than 55 years.9 One retrospective study found the odds ratio (OR) for diagnosing AAA was 9.41 in adults ages 65 to 69 years (95% CI, 8.76-10.12; P < .0001) and 14.46 (95% CI, 13.45-15.55; P < .0001) in adults ages 70 to 74 years, compared to adults younger than 55 years.10
Smoking is the most potent modifiable risk factor for AAA. Among patients with AAA, > 90% have a history of smoking.4 The association between smoking and AAA is dose dependent, with an OR of 2.61 (95% CI, 2.47-2.74) in patients with a pack-per-year history < 5 years and 12.13 (95% CI, 11.66-12.61) in patients with a pack-per-year history > 35 years, compared to nonsmokers.10 The risk for AAA increases with smoking duration but decreases with cessation duration.4,10 Smoking cessation remains an important intervention, as active smokers have higher AAA rupture rates.11
Other risk factors for AAA include concomitant cardiovascular disease (CVD) such as coronary artery disease (CAD), cerebrovascular disease, atherosclerosis, dyslipidemia, and hypertension.10 Factors associated with reduced risk for AAA include African American race, Hispanic ethnicity, Asian ethnicity, diabetes, smoking cessation, consuming fruits and vegetables > 3 times per week, and exercising more than once per week.6,10
Prevalence declines but sex-based disparities in outcomes persist
The prevalence of AAA has declined in the United States and Europe in recent decades, correlating with declining rates of smoking.4,12 Reports published between 2011 and 2019 estimate that AAA prevalence in men older than 60 years has declined over time, with a prevalence of 1.2% to 3.3%.6 The prevalence of AAA has also decreased in women,6,13,14 estimated in 1 study to be as low as 0.74%.13 Similarly, deaths from ruptured AAA have declined markedly in the United States—by 70% between 1999 and 2016 according to 1 analysis.9
One striking difference in the male-female data is that although AAAs are more common in men, there is a 2- to 4-fold higher risk for rupture in women, who account for nearly half of all AAA-related deaths.9,10,15-17 The reasons for this heightened risk to women despite lower prevalence are not fully understood but are likely multifactorial and related to a general lack of screening for AAA in women, tendency for AAA to rupture at smaller diameters in women, rupture at an older age in women, and a history of worse surgical outcomes in women than men (though the gap in surgical outcomes appears to be closing).9,10,18
Continue to: While declines in AAA and AAA-related...
While declines in AAA and AAA-related death are largely attributed to lower smoking rates, other likely contributing factors include the implementation of screening programs, incidental detection during cross-sectional imaging, and improved surgical techniques and management of CV risk factors (eg, hypertension, hyperlipidemia).9,10
The benefits of screening older men
Randomized controlled trials (RCTs) have demonstrated the benefits of AAA screening programs. A meta-analysis of 4 populationbased RCTs of AAA screening in men ≥ 65 years demonstrated statistically significant reductions in AAA rupture (OR = 0.62; 95% CI, 0.55-0.70) and death from AAA (OR = 0.65; 95% CI, 0.57-0.74) over 12 to 15 years, with a number needed to screen (NNS) of 305 (95% CI, 248-411) to prevent 1 AAA-related death.18 The study also found screening decreases the rate of emergent surgeries for AAA (OR = 0.57; 95% CI, 0.48-0.68) while increasing the number of elective surgeries (OR = 1.44; 95% CI, 1.34-1.55) over 4 to 15 years.18
Only 1 study has demonstrated an improvement in all-cause mortality with screening programs, with a relatively small benefit (OR = 0.97; 95% CI, 0.94-0.99).19 Only 1 of the studies included women and, while underpowered, showed no difference in AAA-related death or rupture.20 Guidelines and recommendations of various countries and professional societies focus screening on subgroups at highest risk for AAA.4,6-8,18
Screening recommendations from USPSTF and others
The US Preventive Services Task Force (USPSTF) currently recommends one-time ultrasound screening for AAA in men ages 65 to 75 years who have ever smoked (commonly defined as having smoked > 100 cigarettes) in their lifetime.6 This grade “B” recommendation, initially made in 2005 and reaffirmed in the 2014 and 2019 USPSTF updates, recommends screening the highest-risk segment of the population (ie, older male smokers).
In men ages 65 to 75 years with no smoking history, rather than routine screening, the USPSTF recommends selectively offering screening based on the patient’s medical history, family history, risk factors, and personal values (with a “C” grade).6 The USPSTF continues to recommend against screening for AAA in women with no smoking history and no family history of AAA.6 According to the USPSTF, the evidence is insufficient to recommend for or against screening women ages 65 to 75 years who have ever smoked or have a family history of AAA (“I” statement).6
Continue to: One critique of the USPSTF recommendations
One critique of the USPSTF recommendations is that they fail to detect a significant portion of patients with AAA and AAA rupture. For example, in a retrospective analysis of 55,197 patients undergoing AAA repair, only 33% would have been detected by the USPSTF grade “B” recommendation to screen male smokers ages 65 to 75 years, and an analysis of AAA-related fatalities found 43% would be missed by USPSTF criteria.9,21
Screening guidelines from the Society for Vascular Surgery (SVS) are broader than those of the USPSTF, in an attempt to capture a larger percentage of the population at risk for AAA-related disease by extrapolating from epidemiologic data. The SVS guidelines include screening for women ages 65 to 75 years with a smoking history, screening men and women ages 65 to 75 years who have a first-degree relative with AAA, and consideration of screening patients older than 75 years if they are in good health and have a first-degree relative with AAA or a smoking history and have not been previously screened.4 However, these expanded recommendations are not supported by patient-oriented evidence.6
Attempts to broaden screening guidelines must be tempered by potential risks for harm, primarily overdiagnosis (ie, diagnosing AAAs that would not otherwise rise to clinical significance) and overtreatment (ie, resulting in unnecessary imaging, appointments, anxiety, or surgery). Negative psychological effects on quality of life after a diagnosis of AAA have not been shown to cause significant harm.6,18
A recent UK analysis found that screening programs for AAA in women modeled after those in men are not cost effective, with an NNS to prevent 1 death of 3900 in women vs 700 in men.15,18 Another recent trial of ultrasound screening in 5200 high-risk women ages 65 to 74 years found an AAA incidence of 0.29% (95% CI, 0.18%-0.48%) in which only 3 large aneurysms were identified.22
In the United States, rates of screening for AAA remain low.23 One study has shown electronic medical record–based reminders increased screening rates from 48% to 80%.24 Point-of-care bedside ultrasound performed by clinicians also could improve screening rates. Multiple studies have demonstrated that screening and diagnosis of AAA can be performed safely and effectively at the bedside by nonradiologists such as family physicians and emergency physicians.25-28 In 1 study, such exams added < 4 minutes to the patient encounter.26 Follow-up surveillance schedules for those identified as having a AAA are summarized in TABLE 2.4

Continue to: Management options
Management options: Immediate repair or surveillance?
After diagnosing AAA, important decisions must be made regarding management, including indications for surgical repair, appropriate follow-up surveillance, and medications for secondary prevention and cardiovascular risk reduction.
EVAR vs open repair
The 2 main surgical strategies for aneurysm repair are open repair and endovascular repair (EVAR). In the United States, EVAR is becoming the more common approach and was used to repair asymptomatic aneurysms in > 80% of patients and ruptured aneurysms in 50% of patients.6 There have been multiple RCTs assessing EVAR and open repair for large and small aneurysms.29-34 Findings across these studies consistently show EVAR is associated with lower immediate (ie, 30-day) morbidity and mortality but no longer-term survival benefit compared to open repair.
EVAR procedures require ongoing long-term surveillance for endovascular leakage and other complications, resulting in an increased need for re-intervention.31,33,35 For these reasons, the National Institute for Health and Care Excellence (NICE) guidelines suggest open repair as the preferred modality.7 However, SVS and the American College of Cardiology Foundation/American Heart Association guidance support either EVAR or open repair, noting that open repair may be preferable in patients unable to engage in long-term follow-up surveillance.36

Indications for repair. In general, repair is indicated when an aneurysm reaches or exceeds 5.5 cm.4,7 Both SVS and NICE also recommend clinicians consider surgical repair of smaller, rapidly expanding aneurysms (> 1 cm over a 1-year period).4,7 Based on evidence suggesting a higher risk for rupture in women with smaller aneurysms,14,37 SVS recommends clinicians consider surgical repair in women with an AAA ≥ 5.0 cm. Several RCTs evaluating the benefits of immediate repair for smaller-sized aneurysms (4.0-5.5 cm) favored surveillance.38,39 Accepted indications for surgical repair are summarized in TABLE 3.4,7,34Surgical repair recommendations also are based on aneurysm morphology, which can be fusiform or saccular (FIGURE). More than 90% of AAAs are fusiform.40 Although saccular AAAs are less common, some studies suggest they are more prone to rupture than fusiform AAAs, and SVS guidelines suggest surgical repair of saccular aneurysms regardless of size.4,41,42

Perioperative and long-term risks. Both EVAR and open repair of AAA carry a high perioperative and long-term risk for death, as patients often have multiple comorbidities. A 2019 trial comparing EVAR to open repair with 14 years of follow-up reported death in 68% of patients in the EVAR group and 70% in the open repair group. 31 Among these deaths, 2.7% in the EVAR group and 3.7% in the open repair group were aneurysm related.31 The study also found a second surgical intervention was required in 19.8% of patients in the open repair group and 26.7% in the EVAR group.31
Continue to: When assessing perioperative risk...
When assessing perioperative risk, SVS guidelines recommend clinicians employ a shared decision-making approach with patients that incorporates Vascular Quality Initiative (VQI) mortality risk score.4 (VQI risk calculators are available at https://qxmd.com/vascular-study-group-new-england-decision-support-tools.43)
Medication management
Based on the close association of aortic aneurysm with atherosclerotic CVD (ASCVD), professional societies such as the European Society of Cardiology and European Atherosclerosis Society (ESC/EAS) have suggested aortic aneurysm is equivalent to ASCVD and should be managed medically in a similar manner to peripheral arterial disease.44 Indeed, many patients with AAA may have concomitant CAD or other arterial vascular diseases (eg, carotid, lower extremity).
Statins. In its guidelines, the ESC/EAS consider patients with AAA at “very high risk” for adverse CV events and suggest pharmacotherapy with high-intensity statins, adding ezetimibe or proprotein convertase subtilisin/kexin type 9 (PCSK9) inhibitors if needed, to reduce low-density lipoprotein cholesterol ≥ 50% from baseline, with a goal of < 55 mg/dL.44 Statin therapy additionally lowers all-cause postoperative mortality in patients undergoing AAA repair but does not affect the rate of aneurysm expansion.45
Aspirin and other anticoagulants. Although aspirin therapy may be indicated for the secondary prevention of other cardiovascular events that may coexist with AAA, it does not appear to affect the rate of growth or prevent rupture of aneurysms.46,47 In addition to aspirin, anticoagulants such as clopidogrel, enoxaparin, and warfarin are not recommended when the presence of AAA is the only indication.4
Other medications. Angiotensin-converting enzyme inhibitors, angiotensin receptor blockers, beta-blockers, and antibiotics (eg, doxycycline) have been studied as a treatment for AAA. However, none has shown benefit in reducing aneurysm growth or rupture and they are not recommended for that sole purpose.4,48
Metformin. There is a negative association between diabetes and AAA expansion and rupture. Several cohort studies have indicated that this may be an independent effect driven primarily by exposure to metformin. While it is not unreasonable to consider this another important indication for metformin use in patients with diabetes, RCT evidence has yet to establish a role for metformin in patients without diabetes who have AAA.48,49
ACKNOWLEDGEMENT
The authors thank Gwen Wilson, MLS, AHIP, for her assistance with the literature searches performed in the preparation of this manuscript.
CORRESPONDENCE
Nicholas LeFevre, MD, Family and Community Medicine, University of Missouri–Columbia School of Medicine, One Hospital Drive, M224 Medical Science Building, Columbia, MO 65212; [email protected]
1. CDC. Wide-ranging Online Data for Epidemiologic Research (WONDER) database. Accessed August 30, 2023. https://wonder.cdc.gov/ucd-icd10.html
2. Reimerink JJ, van der Laan MJ, Koelemay MJ, et al. Systematic review and meta-analysis of population-based mortality from ruptured abdominal aortic aneurysm. Br J Surg. 2013;100:1405-1413. doi: 10.1002/bjs.9235
3. Kent KC. Clinical practice. Abdominal aortic aneurysms. N Engl J Med. 2014;371:2101-2108. doi: 10.1056/NEJMcp1401430
4. Chaikof EL, Dalman RL, Eskandari MK, et al. The Society for Vascular Surgery practice guidelines on the care of patients with an abdominal aortic aneurysm. J Vasc Surg. 2018;67:2-77.e2. doi: 10.1016/j.jvs.2017.10.044
5. Moll FL, Powell JT, Fraedrich G, et al. Management of abdominal aortic aneurysms clinical practice guidelines of the European society for vascular surgery. Eur J Vasc Endovasc Surg. 2011;41 suppl 1:S1-S58. doi: 10.1016/j.ejvs.2010.09.011
6. Owens DK, Davidson KW, Krist AH, et al; US Preventive Services Task Force. Screening for abdominal aortic aneurysm: US Preventive Services Task Force recommendation statement. JAMA. 2019;322:2211-2218. doi: 10.1001/jama.2019.18928
7. National Institute for Health and Care Excellence. Abdominal aortic aneurysm: diagnosis and management. NICE guideline [NG156]. March 19, 2020. Accessed June 30, 2023. www.nice.org.uk/guidance/ng156/chapter/recommendations
8. Canadian Task Force on Preventive Health Care. Recommendations on screening for abdominal aortic aneurysm in primary care. CMAJ. 2017;189:E1137-E1145. doi: 10.1503/cmaj.170118
9. Abdulameer H, Al Taii H, Al-Kindi SG, et al. Epidemiology of fatal ruptured aortic aneurysms in the United States (1999-2016). J Vasc Surg. 2019;69:378-384.e2. doi: 10.1016/j.jvs.2018.03.435
10. Kent KC, Zwolak RM, Egorova NN, et al. Analysis of risk factors for abdominal aortic aneurysm in a cohort of more than 3 million individuals. J Vasc Surg. 2010;52:539-548. doi: 10.1016/j.jvs.2010.05.090
11. [No authors listed] Smoking, lung function and the prognosis of abdominal aortic aneurysm. The UK Small Aneurysm Trial Participants. Eur J Vasc Endovasc Surg. 2000;19:636-642. doi: 10.1053/ejvs.2000.1066
12. Oliver-Williams C, Sweeting MJ, Turton G, et al. Lessons learned about prevalence and growth rates of abdominal aortic aneurysms from a 25-year ultrasound population screening programme. Br J Surg. 2018;105:68-74. doi: 10.1002/bjs.10715
13. Ulug P, Powell JT, Sweeting MJ, et al. Meta-analysis of the current prevalence of screen-detected abdominal aortic aneurysm in women. Br J Surg. 2016;103:1097-1104. doi: 10.1002/bjs.10225
14. Chabok M, Nicolaides A, Aslam M, et al. Risk factors associated with increased prevalence of abdominal aortic aneurysm in women. Br J Surg. 2016;103:1132-1138. doi: 10.1002/bjs.10179
15. Sweeting, MJ, Masconi KL, Jones E, et al. Analysis of clinical benefit, harms, and cost-effectiveness of screening women for abdominal aortic aneurysm. Lancet. 2018;392:487-495. doi: 10.1016/S0140-6736(18)31222-4
16. Sweeting MJ, Thompson SG, Brown LC, et al; RESCAN collaborators. Meta-analysis of individual patient data to examine factors affecting growth and rupture of small abdominal aortic aneurysms. Br J Surg. 2012;99:655-665. doi: 10.1002/bjs.8707
17. Skibba AA, Evans JR, Hopkins SP, et al. Reconsidering gender relative to risk of rupture in the contemporary management of abdominal aortic aneurysms. J Vasc Surg. 2015;62:1429-1436. doi: 10.1016/j.jvs.2015.07.079
18. Guirguis-Blake JM, Beil TL, Senger CA, et al. Primary care screening for abdominal aortic aneurysm: updated evidence report and systematic review for the US Preventive Services Task Force. JAMA. 2019;322:2219-2238. doi: 10.1001/jama.2019.17021
19. Thompson SG, Ashton HA, Gao L, et al; Multicentre Aneurysm Screening Study (MASS) Group. Final follow-up of the Multicentre Aneurysm Screening Study (MASS) randomized trial of abdominal aortic aneurysm screening. Br J Surg. 2012;99:1649-1656. doi: 10.1002/bjs.8897
20. Ashton HA, Gao L, Kim LG, et al. Fifteen-year follow-up of a randomized clinical trial of ultrasonographic screening for abdominal aortic aneurysms. Br J Surg. 2007;94:696-701. doi: 10.1002/bjs.5780
21. Carnevale ML, Koleilat I, Lipsitz EC, et al. Extended screening guidelines for the diagnosis of abdominal aortic aneurysm. J Vasc Surg. 2020;72:1917-1926. doi: 10.1016/j.jvs.2020.03.047
22. Duncan A, Maslen C, Gibson C, et al. Ultrasound screening for abdominal aortic aneurysm in high-risk women. Br J Surg. 2021;108:1192-1198. doi: 10.1093/bjs/znab220
23. Shreibati JB, Baker LC, Hlatky MA, et al. Impact of the Screening Abdominal Aortic Aneurysms Very Efficiently (SAAAVE) Act on abdominal ultrasonography use among Medicare beneficiaries. Arch Intern Med. 2012;172:1456-1462. doi: 10.1001/archinternmed.2012.4268
24. Hye RJ, Smith AE, Wong GH, et al. Leveraging the electronic medical record to implement an abdominal aortic aneurysm screening program. J Vasc Surg. 2014;59:1535-1542. doi: 10.1016/j.jvs.2013.12.016
25. Rubano E, Mehta N, Caputo W, et al., Systematic review: emergency department bedside ultrasonography for diagnosing suspected abdominal aortic aneurysm. Acad Emerg Med. 2013. 20:128-138. doi: 10.1111/acem.12080
26. Blois B. Office-based ultrasound screening for abdominal aortic aneurysm. Can Fam Physician. 2012;58:e172-e178.
27. Arnold MJ, Jonas CE, Carter RE. Point-of-care ultrasonography. Am Fam Physician. 2020;101:275-285.
28. Nixon G, Blattner K, Muirhead J, et al. Point-of-care ultrasound for FAST and AAA in rural New Zealand: quality and impact on patient care. Rural Remote Health. 2019;19:5027. doi: 10.22605/RRH5027
29. Lederle FA, Wilson SE, Johnson GR, et al. Immediate repair compared with surveillance of small abdominal aortic aneurysms. N Engl J Med. 2002;346:1437-1444. doi: 10.1056/NEJMoa012573
30. Filardo G, Lederle FA, Ballard DJ, et al. Immediate open repair vs surveillance in patients with small abdominal aortic aneurysms: survival differences by aneurysm size. Mayo Clin Proc. 2013;88:910-919. doi: 10.1016/j.mayocp.2013.05.014
31. Lederle FA, Kyriakides TC, Stroupe KT, et al. Open versus endovascular repair of abdominal aortic aneurysm. N Engl J Med. 2019;380:2126-2135. doi: 10.1056/NEJMoa1715955
32. Patel R, Sweeting MJ, Powell JT, et al., Endovascular versus open repair of abdominal aortic aneurysm in 15-years’ follow-up of the UK endovascular aneurysm repair trial 1 (EVAR trial 1): a randomised controlled trial. Lancet. 2016;388:2366-2374. doi: 10.1016/S0140-6736(16)31135-7
33. van Schaik TG, Yeung KK, Verhagen HJ, et al. Long-term survival and secondary procedures after open or endovascular repair of abdominal aortic aneurysms. J Vasc Surg. 2017;66:1379-1389. doi: 10.1016/j.jvs.2017.05.122
34. Powell JT, Brady AR, Brown, LC, et al; United Kingdom Small Aneurysm Trial Participants. Long-term outcomes of immediate repair compared with surveillance of small abdominal aortic aneurysms. N Engl J Med. 2002;346:1445-1452. doi: 10.1056/NEJMoa013527
35. Paravastu SC, Jayarajasingam R, Cottam R, et al. Endovascular repair of abdominal aortic aneurysm. Cochrane Database Syst Rev. 2014:CD004178. doi: 10.1002/14651858.CD004178.pub2
36. Rooke TW, Hirsch AT, Misra S, et al. 2011 ACCF/AHA focused update of the guideline for the management of patients with peripheral artery disease (updating the 2005 guideline): a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2011;58:2020-2045. doi: 10.1016/j.jacc.2011.08.023
37. Bhak RH, Wininger M, Johnson GR, et al. Factors associated with small abdominal aortic aneurysm expansion rate. JAMA Surg. 2015;150:44-50. doi: 10.1001/jamasurg.2014.2025
38. Ouriel K, Clair DG, Kent KC, et al; Positive Impact of Endovascular Options for treating Aneurysms Early (PIVOTAL) Investigators. Endovascular repair compared with surveillance for patients with small abdominal aortic aneurysms. J Vasc Surg. 2010;51:1081-1087. doi: 10.1016/j.jvs.2009.10.113
39. Cao P, De Rango P, Verzini F, et al. Comparison of surveillance versus aortic endografting for small aneurysm repair (CAESAR): results from a randomised trial. Eur J Vasc Endovasc Surg. 2011;41:13-25. doi: 10.1016/j.ejvs.2010.08.026
40. Karthaus EG, Tong TML, Vahl A, et al; Dutch Society of Vascular Surgery, the Steering Committee of the Dutch Surgical Aneurysm Audit and the Dutch Institute for Clinical Auditing. Saccular abdominal aortic aneurysms: patient characteristics, clinical presentation, treatment, and outcomes in the Netherlands. Ann Surg. 2019;270:852-858. doi: 10.1097/SLA.0000000000003529
41. Nathan DP, Xu C, Pouch AM, et al. Increased wall stress of saccular versus fusiform aneurysms of the descending thoracic aorta. Ann Vasc Surg. 2011;25:1129-2237. doi: 10.1016/j.avsg.2011.07.008
42. Durojaye MS, Adeniyi TO, Alagbe OA. Multiple saccular aneurysms of the abdominal aorta: a case report and short review of risk factors for rupture on CT Scan. Ann Ib Postgrad Med. 2020;18:178-180.
43. Bertges DJ, Neal D, Schanzer A, et al. The Vascular Quality Initiative Cardiac Risk Index for prediction of myocardial infarction after vascular surgery. J Vasc Surg. 2016;64:1411-1421.e4. doi: 10.1016/j.jvs.2016.04.045
44. Mach F, Baigent C, Catapano AL, et al. 2019 ESC/EAS guidelines for the management of dyslipidaemias: lipid modification to reduce cardiovascular risk. Eur Heart J. 2020;41:111-188. doi: 10.1093/eurheartj/ehz455
45. Twine CP, Williams IM. Systematic review and meta-analysis of the effects of statin therapy on abdominal aortic aneurysms. Br J Surg. 2011;98:346-353. doi: 10.1002/bjs.7343
46. Arnett DK, Blumenthal RS, Albert MA, et al. 2019 ACC/AHA guideline on the primary prevention of cardiovascular disease: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation. 2019;140:e596-e646. doi: 10.1161/CIR.0000000000000678
47. Erbel R, Aboyans V, Boileau C, et al. 2014 ESC guidelines on the diagnosis and treatment of aortic diseases: document covering acute and chronic aortic diseases of the thoracic and abdominal aorta of the adult. The Task Force for the Diagnosis and Treatment of Aortic Diseases of the European Society of Cardiology (ESC). Eur Heart J. 2014;35:2873-2926. doi: 10.1093/eurheartj/ehu281
48. Lederle FA, Noorbaloochi S, Nugent S, et al. Multicentre study of abdominal aortic aneurysm measurement and enlargement. Br J Surg. 2015;102:1480-1487. doi: 10.1002/bjs.9895
49. Itoga NK, Rothenberg KA, Suarez P, et al. Metformin prescription status and abdominal aortic aneurysm disease progression in the U.S. veteran population. J Vasc Surg. 2019;69:710-716.e3. doi: 10.1016/j.jvs.2018.06.19
1. CDC. Wide-ranging Online Data for Epidemiologic Research (WONDER) database. Accessed August 30, 2023. https://wonder.cdc.gov/ucd-icd10.html
2. Reimerink JJ, van der Laan MJ, Koelemay MJ, et al. Systematic review and meta-analysis of population-based mortality from ruptured abdominal aortic aneurysm. Br J Surg. 2013;100:1405-1413. doi: 10.1002/bjs.9235
3. Kent KC. Clinical practice. Abdominal aortic aneurysms. N Engl J Med. 2014;371:2101-2108. doi: 10.1056/NEJMcp1401430
4. Chaikof EL, Dalman RL, Eskandari MK, et al. The Society for Vascular Surgery practice guidelines on the care of patients with an abdominal aortic aneurysm. J Vasc Surg. 2018;67:2-77.e2. doi: 10.1016/j.jvs.2017.10.044
5. Moll FL, Powell JT, Fraedrich G, et al. Management of abdominal aortic aneurysms clinical practice guidelines of the European society for vascular surgery. Eur J Vasc Endovasc Surg. 2011;41 suppl 1:S1-S58. doi: 10.1016/j.ejvs.2010.09.011
6. Owens DK, Davidson KW, Krist AH, et al; US Preventive Services Task Force. Screening for abdominal aortic aneurysm: US Preventive Services Task Force recommendation statement. JAMA. 2019;322:2211-2218. doi: 10.1001/jama.2019.18928
7. National Institute for Health and Care Excellence. Abdominal aortic aneurysm: diagnosis and management. NICE guideline [NG156]. March 19, 2020. Accessed June 30, 2023. www.nice.org.uk/guidance/ng156/chapter/recommendations
8. Canadian Task Force on Preventive Health Care. Recommendations on screening for abdominal aortic aneurysm in primary care. CMAJ. 2017;189:E1137-E1145. doi: 10.1503/cmaj.170118
9. Abdulameer H, Al Taii H, Al-Kindi SG, et al. Epidemiology of fatal ruptured aortic aneurysms in the United States (1999-2016). J Vasc Surg. 2019;69:378-384.e2. doi: 10.1016/j.jvs.2018.03.435
10. Kent KC, Zwolak RM, Egorova NN, et al. Analysis of risk factors for abdominal aortic aneurysm in a cohort of more than 3 million individuals. J Vasc Surg. 2010;52:539-548. doi: 10.1016/j.jvs.2010.05.090
11. [No authors listed] Smoking, lung function and the prognosis of abdominal aortic aneurysm. The UK Small Aneurysm Trial Participants. Eur J Vasc Endovasc Surg. 2000;19:636-642. doi: 10.1053/ejvs.2000.1066
12. Oliver-Williams C, Sweeting MJ, Turton G, et al. Lessons learned about prevalence and growth rates of abdominal aortic aneurysms from a 25-year ultrasound population screening programme. Br J Surg. 2018;105:68-74. doi: 10.1002/bjs.10715
13. Ulug P, Powell JT, Sweeting MJ, et al. Meta-analysis of the current prevalence of screen-detected abdominal aortic aneurysm in women. Br J Surg. 2016;103:1097-1104. doi: 10.1002/bjs.10225
14. Chabok M, Nicolaides A, Aslam M, et al. Risk factors associated with increased prevalence of abdominal aortic aneurysm in women. Br J Surg. 2016;103:1132-1138. doi: 10.1002/bjs.10179
15. Sweeting, MJ, Masconi KL, Jones E, et al. Analysis of clinical benefit, harms, and cost-effectiveness of screening women for abdominal aortic aneurysm. Lancet. 2018;392:487-495. doi: 10.1016/S0140-6736(18)31222-4
16. Sweeting MJ, Thompson SG, Brown LC, et al; RESCAN collaborators. Meta-analysis of individual patient data to examine factors affecting growth and rupture of small abdominal aortic aneurysms. Br J Surg. 2012;99:655-665. doi: 10.1002/bjs.8707
17. Skibba AA, Evans JR, Hopkins SP, et al. Reconsidering gender relative to risk of rupture in the contemporary management of abdominal aortic aneurysms. J Vasc Surg. 2015;62:1429-1436. doi: 10.1016/j.jvs.2015.07.079
18. Guirguis-Blake JM, Beil TL, Senger CA, et al. Primary care screening for abdominal aortic aneurysm: updated evidence report and systematic review for the US Preventive Services Task Force. JAMA. 2019;322:2219-2238. doi: 10.1001/jama.2019.17021
19. Thompson SG, Ashton HA, Gao L, et al; Multicentre Aneurysm Screening Study (MASS) Group. Final follow-up of the Multicentre Aneurysm Screening Study (MASS) randomized trial of abdominal aortic aneurysm screening. Br J Surg. 2012;99:1649-1656. doi: 10.1002/bjs.8897
20. Ashton HA, Gao L, Kim LG, et al. Fifteen-year follow-up of a randomized clinical trial of ultrasonographic screening for abdominal aortic aneurysms. Br J Surg. 2007;94:696-701. doi: 10.1002/bjs.5780
21. Carnevale ML, Koleilat I, Lipsitz EC, et al. Extended screening guidelines for the diagnosis of abdominal aortic aneurysm. J Vasc Surg. 2020;72:1917-1926. doi: 10.1016/j.jvs.2020.03.047
22. Duncan A, Maslen C, Gibson C, et al. Ultrasound screening for abdominal aortic aneurysm in high-risk women. Br J Surg. 2021;108:1192-1198. doi: 10.1093/bjs/znab220
23. Shreibati JB, Baker LC, Hlatky MA, et al. Impact of the Screening Abdominal Aortic Aneurysms Very Efficiently (SAAAVE) Act on abdominal ultrasonography use among Medicare beneficiaries. Arch Intern Med. 2012;172:1456-1462. doi: 10.1001/archinternmed.2012.4268
24. Hye RJ, Smith AE, Wong GH, et al. Leveraging the electronic medical record to implement an abdominal aortic aneurysm screening program. J Vasc Surg. 2014;59:1535-1542. doi: 10.1016/j.jvs.2013.12.016
25. Rubano E, Mehta N, Caputo W, et al., Systematic review: emergency department bedside ultrasonography for diagnosing suspected abdominal aortic aneurysm. Acad Emerg Med. 2013. 20:128-138. doi: 10.1111/acem.12080
26. Blois B. Office-based ultrasound screening for abdominal aortic aneurysm. Can Fam Physician. 2012;58:e172-e178.
27. Arnold MJ, Jonas CE, Carter RE. Point-of-care ultrasonography. Am Fam Physician. 2020;101:275-285.
28. Nixon G, Blattner K, Muirhead J, et al. Point-of-care ultrasound for FAST and AAA in rural New Zealand: quality and impact on patient care. Rural Remote Health. 2019;19:5027. doi: 10.22605/RRH5027
29. Lederle FA, Wilson SE, Johnson GR, et al. Immediate repair compared with surveillance of small abdominal aortic aneurysms. N Engl J Med. 2002;346:1437-1444. doi: 10.1056/NEJMoa012573
30. Filardo G, Lederle FA, Ballard DJ, et al. Immediate open repair vs surveillance in patients with small abdominal aortic aneurysms: survival differences by aneurysm size. Mayo Clin Proc. 2013;88:910-919. doi: 10.1016/j.mayocp.2013.05.014
31. Lederle FA, Kyriakides TC, Stroupe KT, et al. Open versus endovascular repair of abdominal aortic aneurysm. N Engl J Med. 2019;380:2126-2135. doi: 10.1056/NEJMoa1715955
32. Patel R, Sweeting MJ, Powell JT, et al., Endovascular versus open repair of abdominal aortic aneurysm in 15-years’ follow-up of the UK endovascular aneurysm repair trial 1 (EVAR trial 1): a randomised controlled trial. Lancet. 2016;388:2366-2374. doi: 10.1016/S0140-6736(16)31135-7
33. van Schaik TG, Yeung KK, Verhagen HJ, et al. Long-term survival and secondary procedures after open or endovascular repair of abdominal aortic aneurysms. J Vasc Surg. 2017;66:1379-1389. doi: 10.1016/j.jvs.2017.05.122
34. Powell JT, Brady AR, Brown, LC, et al; United Kingdom Small Aneurysm Trial Participants. Long-term outcomes of immediate repair compared with surveillance of small abdominal aortic aneurysms. N Engl J Med. 2002;346:1445-1452. doi: 10.1056/NEJMoa013527
35. Paravastu SC, Jayarajasingam R, Cottam R, et al. Endovascular repair of abdominal aortic aneurysm. Cochrane Database Syst Rev. 2014:CD004178. doi: 10.1002/14651858.CD004178.pub2
36. Rooke TW, Hirsch AT, Misra S, et al. 2011 ACCF/AHA focused update of the guideline for the management of patients with peripheral artery disease (updating the 2005 guideline): a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2011;58:2020-2045. doi: 10.1016/j.jacc.2011.08.023
37. Bhak RH, Wininger M, Johnson GR, et al. Factors associated with small abdominal aortic aneurysm expansion rate. JAMA Surg. 2015;150:44-50. doi: 10.1001/jamasurg.2014.2025
38. Ouriel K, Clair DG, Kent KC, et al; Positive Impact of Endovascular Options for treating Aneurysms Early (PIVOTAL) Investigators. Endovascular repair compared with surveillance for patients with small abdominal aortic aneurysms. J Vasc Surg. 2010;51:1081-1087. doi: 10.1016/j.jvs.2009.10.113
39. Cao P, De Rango P, Verzini F, et al. Comparison of surveillance versus aortic endografting for small aneurysm repair (CAESAR): results from a randomised trial. Eur J Vasc Endovasc Surg. 2011;41:13-25. doi: 10.1016/j.ejvs.2010.08.026
40. Karthaus EG, Tong TML, Vahl A, et al; Dutch Society of Vascular Surgery, the Steering Committee of the Dutch Surgical Aneurysm Audit and the Dutch Institute for Clinical Auditing. Saccular abdominal aortic aneurysms: patient characteristics, clinical presentation, treatment, and outcomes in the Netherlands. Ann Surg. 2019;270:852-858. doi: 10.1097/SLA.0000000000003529
41. Nathan DP, Xu C, Pouch AM, et al. Increased wall stress of saccular versus fusiform aneurysms of the descending thoracic aorta. Ann Vasc Surg. 2011;25:1129-2237. doi: 10.1016/j.avsg.2011.07.008
42. Durojaye MS, Adeniyi TO, Alagbe OA. Multiple saccular aneurysms of the abdominal aorta: a case report and short review of risk factors for rupture on CT Scan. Ann Ib Postgrad Med. 2020;18:178-180.
43. Bertges DJ, Neal D, Schanzer A, et al. The Vascular Quality Initiative Cardiac Risk Index for prediction of myocardial infarction after vascular surgery. J Vasc Surg. 2016;64:1411-1421.e4. doi: 10.1016/j.jvs.2016.04.045
44. Mach F, Baigent C, Catapano AL, et al. 2019 ESC/EAS guidelines for the management of dyslipidaemias: lipid modification to reduce cardiovascular risk. Eur Heart J. 2020;41:111-188. doi: 10.1093/eurheartj/ehz455
45. Twine CP, Williams IM. Systematic review and meta-analysis of the effects of statin therapy on abdominal aortic aneurysms. Br J Surg. 2011;98:346-353. doi: 10.1002/bjs.7343
46. Arnett DK, Blumenthal RS, Albert MA, et al. 2019 ACC/AHA guideline on the primary prevention of cardiovascular disease: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation. 2019;140:e596-e646. doi: 10.1161/CIR.0000000000000678
47. Erbel R, Aboyans V, Boileau C, et al. 2014 ESC guidelines on the diagnosis and treatment of aortic diseases: document covering acute and chronic aortic diseases of the thoracic and abdominal aorta of the adult. The Task Force for the Diagnosis and Treatment of Aortic Diseases of the European Society of Cardiology (ESC). Eur Heart J. 2014;35:2873-2926. doi: 10.1093/eurheartj/ehu281
48. Lederle FA, Noorbaloochi S, Nugent S, et al. Multicentre study of abdominal aortic aneurysm measurement and enlargement. Br J Surg. 2015;102:1480-1487. doi: 10.1002/bjs.9895
49. Itoga NK, Rothenberg KA, Suarez P, et al. Metformin prescription status and abdominal aortic aneurysm disease progression in the U.S. veteran population. J Vasc Surg. 2019;69:710-716.e3. doi: 10.1016/j.jvs.2018.06.19
PRACTICE RECOMMENDATIONS
› Perform a one-time abdominal aortic aneurysm (AAA) screening ultrasound in men ages 65 to 75 years who have ever smoked. B
› Consider performing a one-time AAA screening ultrasound in women ages 65 to 75 years who have ever smoked. C
› Prescribe high-intensity statin therapy for men and women with atherosclerotic AAA. A
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
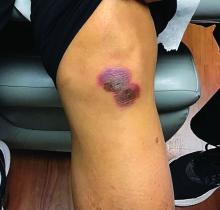
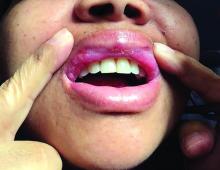
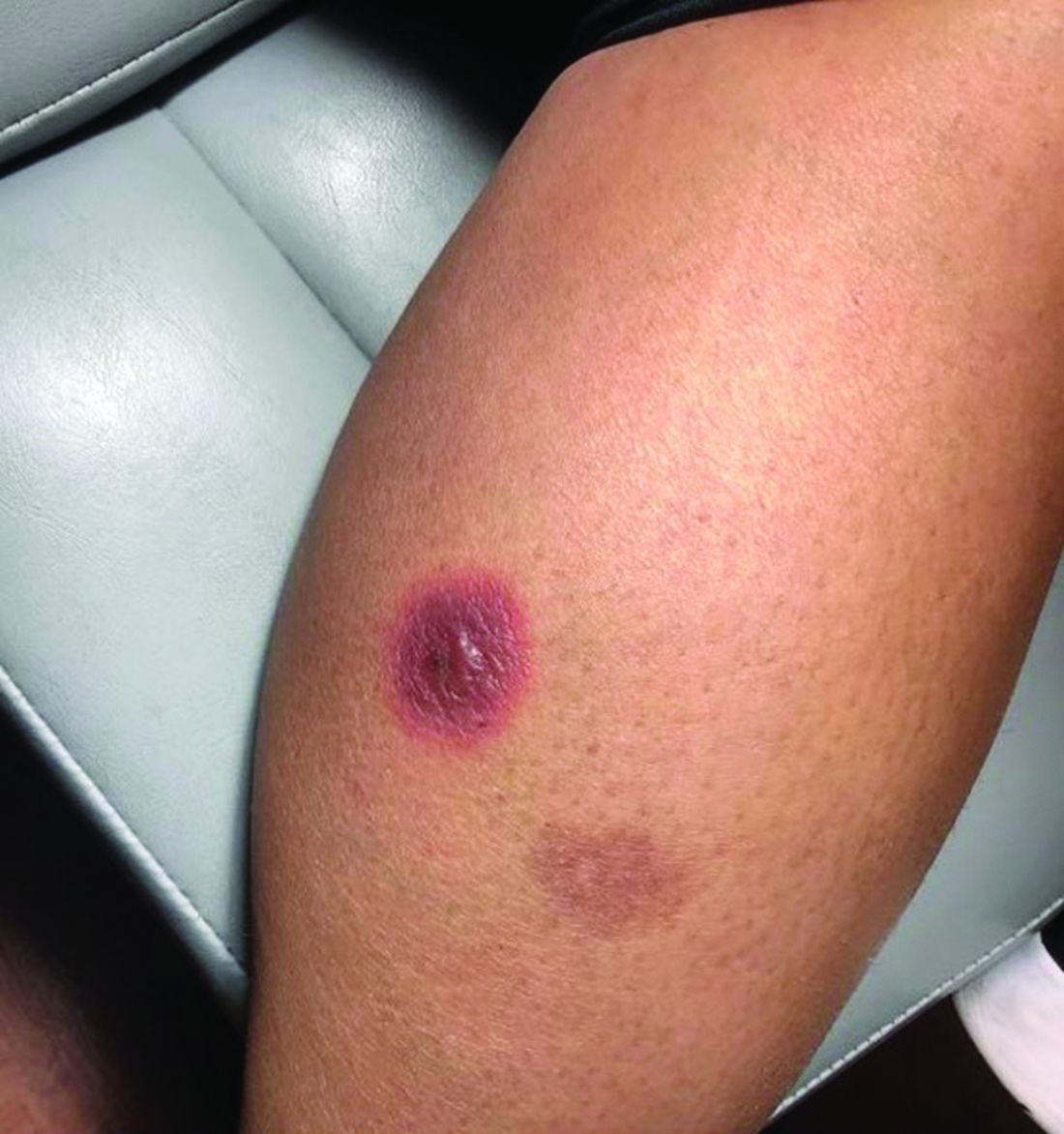
A 42-year-old woman presented with a few days of erosions on her buccal mucosa, tongue, and soft palate
in which lesions present in the same location upon repeated intake of the offending drug. The lesions typically present within 30 minutes to 8 hours of administration of the drug. These reactions can be considered allergic or pseudo-allergic, in which case, there is no notable adaptive immune response. CD8+ T cells appear to play a role in the epidermal injury via release of interferons and interactions with other inflammatory cells.
There are numerous drugs that can precipitate these findings. NSAIDs; antibiotics, such as tetracyclines, sulfonamides; and phenytoin are common offenders. In the case of our patient, naproxen was the offending medication.
The classic presentation of FDE features annular, erythematous to violaceous macules on the skin or mucosa that can be asymptomatic or can produce burning, pain, or pruritus. The most common locations include the trunk and extremities, but the palms, soles, face, scalp, and mucosa can also be impacted. The oral mucosa seems to be the most common mucosal location. Intravenous administration of a drug is associated with more severe symptoms. Systemic symptoms are typically absent, and the eruption may initially be in one location, but may appear elsewhere upon repeated exposure to the offending medication.
The differential diagnosis includes arthropod bite reactions, urticaria, and erythema multiforme. Although FDEs are typically a clinical diagnosis, the histopathology will commonly show a vacuolar interface dermatitis. Furthermore, a variety of immune cells can be found, including neutrophilic, eosinophilic, and lymphocytic infiltrate. A combination of two or more histological patterns often favors the diagnosis of FDE.
Steroid creams can be prescribed to decrease the inflammatory reaction and improve symptoms; however, the definitive treatment of this condition is cessation of the offending agent. Postinflammatory hyperpigmentation is a common symptom after resolution of the condition, and it may take months to fade away. Further darkening can be prevented by practicing sun safety measures such as wearing sunblock, covering the affected areas, and avoiding prolonged sun exposure.
This case and the photos were submitted by Lucas Shapiro, BS, of Nova Southeastern University College of Osteopathic Medicine, Fort Lauderdale, Fla., and Igor Chaplik, DO, Aesthetix Dermatology, Fort Lauderdale. The column was edited by Donna Bilu Martin, MD.
Dr. Bilu Martin is a board-certified dermatologist in private practice at Premier Dermatology, MD, in Aventura, Fla. More diagnostic cases are available at mdedge.com/dermatology. To submit a case for possible publication, send an email to [email protected].
References
Shaker G et al. Cureus. 2022 Aug 23;14(8):e28299.
Srivastava R et al. Indian J Dent. 2015 Apr-Jun;6(2):103-6.
Weyers W, Metze D. Dermatol Pract Concept. 2011 Jan 31;1(1):33-47.
in which lesions present in the same location upon repeated intake of the offending drug. The lesions typically present within 30 minutes to 8 hours of administration of the drug. These reactions can be considered allergic or pseudo-allergic, in which case, there is no notable adaptive immune response. CD8+ T cells appear to play a role in the epidermal injury via release of interferons and interactions with other inflammatory cells.
There are numerous drugs that can precipitate these findings. NSAIDs; antibiotics, such as tetracyclines, sulfonamides; and phenytoin are common offenders. In the case of our patient, naproxen was the offending medication.
The classic presentation of FDE features annular, erythematous to violaceous macules on the skin or mucosa that can be asymptomatic or can produce burning, pain, or pruritus. The most common locations include the trunk and extremities, but the palms, soles, face, scalp, and mucosa can also be impacted. The oral mucosa seems to be the most common mucosal location. Intravenous administration of a drug is associated with more severe symptoms. Systemic symptoms are typically absent, and the eruption may initially be in one location, but may appear elsewhere upon repeated exposure to the offending medication.
The differential diagnosis includes arthropod bite reactions, urticaria, and erythema multiforme. Although FDEs are typically a clinical diagnosis, the histopathology will commonly show a vacuolar interface dermatitis. Furthermore, a variety of immune cells can be found, including neutrophilic, eosinophilic, and lymphocytic infiltrate. A combination of two or more histological patterns often favors the diagnosis of FDE.
Steroid creams can be prescribed to decrease the inflammatory reaction and improve symptoms; however, the definitive treatment of this condition is cessation of the offending agent. Postinflammatory hyperpigmentation is a common symptom after resolution of the condition, and it may take months to fade away. Further darkening can be prevented by practicing sun safety measures such as wearing sunblock, covering the affected areas, and avoiding prolonged sun exposure.
This case and the photos were submitted by Lucas Shapiro, BS, of Nova Southeastern University College of Osteopathic Medicine, Fort Lauderdale, Fla., and Igor Chaplik, DO, Aesthetix Dermatology, Fort Lauderdale. The column was edited by Donna Bilu Martin, MD.
Dr. Bilu Martin is a board-certified dermatologist in private practice at Premier Dermatology, MD, in Aventura, Fla. More diagnostic cases are available at mdedge.com/dermatology. To submit a case for possible publication, send an email to [email protected].
References
Shaker G et al. Cureus. 2022 Aug 23;14(8):e28299.
Srivastava R et al. Indian J Dent. 2015 Apr-Jun;6(2):103-6.
Weyers W, Metze D. Dermatol Pract Concept. 2011 Jan 31;1(1):33-47.
in which lesions present in the same location upon repeated intake of the offending drug. The lesions typically present within 30 minutes to 8 hours of administration of the drug. These reactions can be considered allergic or pseudo-allergic, in which case, there is no notable adaptive immune response. CD8+ T cells appear to play a role in the epidermal injury via release of interferons and interactions with other inflammatory cells.
There are numerous drugs that can precipitate these findings. NSAIDs; antibiotics, such as tetracyclines, sulfonamides; and phenytoin are common offenders. In the case of our patient, naproxen was the offending medication.
The classic presentation of FDE features annular, erythematous to violaceous macules on the skin or mucosa that can be asymptomatic or can produce burning, pain, or pruritus. The most common locations include the trunk and extremities, but the palms, soles, face, scalp, and mucosa can also be impacted. The oral mucosa seems to be the most common mucosal location. Intravenous administration of a drug is associated with more severe symptoms. Systemic symptoms are typically absent, and the eruption may initially be in one location, but may appear elsewhere upon repeated exposure to the offending medication.
The differential diagnosis includes arthropod bite reactions, urticaria, and erythema multiforme. Although FDEs are typically a clinical diagnosis, the histopathology will commonly show a vacuolar interface dermatitis. Furthermore, a variety of immune cells can be found, including neutrophilic, eosinophilic, and lymphocytic infiltrate. A combination of two or more histological patterns often favors the diagnosis of FDE.
Steroid creams can be prescribed to decrease the inflammatory reaction and improve symptoms; however, the definitive treatment of this condition is cessation of the offending agent. Postinflammatory hyperpigmentation is a common symptom after resolution of the condition, and it may take months to fade away. Further darkening can be prevented by practicing sun safety measures such as wearing sunblock, covering the affected areas, and avoiding prolonged sun exposure.
This case and the photos were submitted by Lucas Shapiro, BS, of Nova Southeastern University College of Osteopathic Medicine, Fort Lauderdale, Fla., and Igor Chaplik, DO, Aesthetix Dermatology, Fort Lauderdale. The column was edited by Donna Bilu Martin, MD.
Dr. Bilu Martin is a board-certified dermatologist in private practice at Premier Dermatology, MD, in Aventura, Fla. More diagnostic cases are available at mdedge.com/dermatology. To submit a case for possible publication, send an email to [email protected].
References
Shaker G et al. Cureus. 2022 Aug 23;14(8):e28299.
Srivastava R et al. Indian J Dent. 2015 Apr-Jun;6(2):103-6.
Weyers W, Metze D. Dermatol Pract Concept. 2011 Jan 31;1(1):33-47.
Can these salt substitutes prevent complications of hypertension?
ILLUSTRATIVE CASE
A 47-year-old man in generally good health presents to a family medicine clinic for a well visit. He does not use tobacco products and had a benign colonoscopy last year. He reports walking for 30 minutes 3 to 4 times per week for exercise, althoug h he has gained 3 lbs over the past 2 years. He has no family history of early coronary artery disease, but his father and older brother have hypertension. His mother has a history of diabetes and hyperlipidemia.
The patient’s physical exam is unremarkable except for an elevated BP reading of 151/82 mm Hg. A review of his chart indicates he has had multiple elevated readings in the past that have ranged from 132/72 mm Hg to 139/89 mm Hg. The patient is interested in antihypertensive treatment but wants to know if modifying his diet and replacing his regular table salt with a salt substitute will lower his high BP. What can you recommend?
Hypertension is a leading cause of CV morbidity and mortality worldwide and is linked to increased dietary sodium intake. An estimated 1.28 billion people worldwide have hypertension; however, more than half of cases are undiagnosed.2The US Preventive Services Task Force recommends screening for hypertension in adults older than 18 years and confirming elevated measurements conducted in a nonclinical setting before starting medication (grade “A”).3
Cut-points for the diagnosis of hypertension vary. The American Academy of Family Physicians, 4 the Eighth Joint National Committee (JNC 8), 5 the International Society of Hypertension, 6 and the European Society of Cardiology 7 use ≥ 140 mm Hg systolic BP (SBP) or ≥ 90 mm Hg diastolic BP (DBP) to define hypertension. The American College of Cardiology/American Heart Association guidelines use ≥ 130/80 mm Hg. 8
When treating patients with hypertension, primary care physicians often recommend lifestyle modifications such as the
Systematic reviews have shown a measurable improvement in BP with sodium reduction and potassium substitution. 10-12 More importantly, high-quality evidence demonstrates a decreased risk for CV disease, kidney disease, and all-cause mortality with lower dietary sodium intake. 13 Previous studies have shown that potassium-enriched salt substitutes lower BP, but their impact on CV morbidity and mortality is not well defined. Although lowering BP is associated with improved clinical impact, there is a lack of patient-oriented evidence that demonstrates improvement in CV disease and mortality.
The Salt Substitute and Stroke Study (SSaSS), published in 2021, demonstrated the protective effect of salt substitution against stroke, other CV events, and death. 14 Furthermore, this 5-year, cluster-randomized controlled trial of 20,995 participants across 600 villages in China demonstrated reduced CV mortality and BP reduction similar to standard pharmacologic treatment. Prior to SSaSS, 17 randomized controlled trials demonstrated a BP-lowering effect of salt substitutes but did not directly study the impact on clinical outcomes. 13
Continue to: In this 2022 systematic review...
In this 2022 systematic review and meta-analysis, 1 Yin et al evaluated 21 trials, including SSaSS, for the effect of salt substitutes on BP and other clinical outcomes, and the generalizability of the study results to diverse populations. The systematic review included parallel-group, step-wedge, and cluster-randomized controlled trials reporting the effect of salt substitutes on BP or clinical outcomes.
STUDY SUMMARY
Salt substitutes reduced BP across diverse populations
This systematic review and meta-analysis reviewed existing literature for randomized controlled trials investigating the effects of potassium-enriched salt substitutes on clinical outcomes for patients without kidney disease. The most commonly used salt substitute was potassium chloride, at 25% to 65% potassium.
The systematic review identified 21 trials comprising 31,949 study participants from 15 different countries with 1 to 60 months’ duration. Meta-analyses were performed using 19 trials for BP outcomes and 5 trials for vascular outcomes. Eleven trials were rated as having low risk for bias, 8 were deemed to have some concern, and 2 were rated as high risk for bias. Comparisons of data excluding studies with high risk for bias yielded results similar to comparisons of all studies.
The meta-analysis of 19 trials demonstrated reduced SBP (–4.6 mm Hg; 95% CI, –6.1 to –3.1) and DBP (–1.6 mm Hg; 95% CI, –2.4 to –0.8) in participants using potassium-enriched salt substitutes. However, the authors noted substantial heterogeneity among the studies (I 2 > 70%) for both SBP and DBP outcomes. Although there were no subgroup differences for age, sex, hypertension history, or other biomarkers, outcome differences were associated with trial duration, baseline potassium intake, and composition of the salt substitute.
Potassium-enriched salt substitutes were associated with reduced total mortality (risk ratio [RR] = 0.89; 95% CI, 0.85-0.94), CV mortality (RR = 0.87; 95% CI, 0.81-0.94), and CV events (RR = 0.89; 95% CI, 0.85-0.94). In a meta-regression, each 10% reduction in the sodium content of the salt substitute was associated with a 1.5–mm Hg greater reduction in SBP (95% CI, –3.0 to –0.03) and a 1.0–mm Hg greater reduction in DBP (95% CI, –1.8 to –0.1). However, the authors suggest interpreting meta-regression results with caution.
Continue to: Only 2 of the studes...
Only 2 of the studies in the systematic review explicitly reported the adverse effect of hyperkalemia, and there was no statistical difference in events between randomized groups. Eight other studies reported no serious adverse events related to hyperkalemia , and 11 studies did not report on the risk for hyperkalemia.
WHAT’S NEW
High-quality data demonstrate beneficial outcomes
Previous observational and interventional studies demonstrated a BP-lowering effect of salt substitutes, but limited data with poor-quality evidence existed for the impact of salt substitutes on clinical outcomes such as mortality and CV events. This systematic review and meta-analysis suggests that potassium-supplemented salt may reduce BP and secondarily reduce the risk for CV events, CV mortality, and total mortality, without clear harmful effects reported.
CAVEATS
Some patient populations, comorbidities excluded from study
The study did not include patients with kidney disease or those taking potassium-sparing diuretics. Furthermore, the available data do not include primary prevention participants.
Subgroup analyses should be interpreted with caution due to the small number of trials available for individual subgroups. In addition, funnel plot asymmetry for studies reporting DBP suggests at least some effect of publication bias for that outcome.
Although BP reduction due to salt substitutes may be small at an individual level, these levels of reduction may be important at a population level.
CHALLENGES TO IMPLEMENTATION
For appropriate patients, no challenges anticipated
There are no significant challenges to implementing conclusions from this study in the primary care setting. Family physicians should be able to recommend potassium-enriched salt substitutes to patients with hypertension who are not at risk for hyperkalemia, including those with kidney disease, on potassium-sparing diuretics, or with a history of hyperkalemia/hyperkalemic conditions. Salt substitutes, including potassium-enriched salts, are readily available in stores.
1. Yin X, Rodgers A, Perkovic A, et al. Effects of salt substitutes on clinical outcomes: a systematic review and meta-analysis. Heart. 2022;108:1608-1615. doi: 10.1136/heartjnl-2022-321332
2. NCD Risk Factor Collaboration (NCD-RisC). Worldwide trends in hypertension prevalence and progress in treatment and control from 1990 to 2019: a pooled analysis of 1201 population-representative studies with 104 million participants. Lancet. 2021;398:957-980. doi: 10.1016/S0140-6736(21)01330-1
3. USPSTF. Hypertension in adults: screening. Final recommendation statement. Published April 27, 2021. Accessed September 18, 2023. www.uspreventiveservicestaskforce.org/uspstf/recommendation/hypertension-in-adults-screening
4. Coles S, Fisher L, Lin KW, et al. Blood pressure targets in adults with hypertension: a clinical practice guideline from the AAFP. Published November 4, 2022. Accessed September 18, 2023. www.aafp.org/dam/AAFP/documents/journals/afp/AAFPHypertensionGuideline.pdf
5. James PA, Oparil S, Carter BL, et al. 2014 evidence-based guideline for the management of high blood pressure in adults: report from the panel members appointed to the Eighth Joint National Committee (JNC 8). JAMA. 2014;311:507-520. doi: 10.1001/jama. 2013.284427
6. Unger T, Borgi C, Charchar F, et al. 2020 International Society of Hypertension global hypertension practice guidelines. Hypertension. 2020;75:1334-1357. doi: 10.1161/HYPERTENSIONAHA.120.15026
7. Mancia G, Kreutz R, Brunstrom M, et al; the Task Force for the Management of Arterial Hypertension of the European Society of Hypertension. 2023 ESH Guidelines for the management of arterial hypertension. Endorsed by the European Renal Association (ERA) and the International Society of Hypertension (ISH). J Hypertens. 2023; Jun 21. doi: 10.1097/HJH.0000000000003480
8. Whelton PK, Carey RM, Aronow WS, et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA Guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Hypertension. 2018;71:e13-e115. 10.1161/HYP.0000000000000065
9. National Center for Health Statistics. National Ambulatory Medical Care Survey: 2014 state and national summary tables. Accessed June 27, 2023. www.cdc.gov/nchs/data/ahcd/namcs_summary/2014_namcs_web_tables.pdf
10. Huang L, Trieu K, Yoshimura S, et al. Effect of dose and duration of reduction in dietary sodium on blood pressure levels: systematic review and meta-analysis of randomised trials. BMJ. 2020;368:m315. doi: 10.1136/bmj.m315
11. Filippini T, Violi F, D’Amico R, et al. The effect of potassium supplementation on blood pressure in hypertensive subjects: a systematic review and meta-analysis. Int J Cardiol. 2017;230:127-135. doi: 10.1016/j.ijcard.2016.12.048
12. Brand A, Visser ME, Schoonees A, et al. Replacing salt with low-sodium salt substitutes (LSSS) for cardiovascular health in adults, children and pregnant women. Cochrane Database Syst Rev. 2022;8:CD015207. doi: 10.1002/14651858.CD015207
13. He FJ, Tan M, Ma Y, et al. Salt reduction to prevent hypertension and cardiovascular disease: JACC state-of-the-art review. J Am Coll Cardiol. 2020;75:632-647. doi: 10.1016/j.jacc.2019.11.055
14. Neal B, Wu Y, Feng X, et al. Effect of salt substitution on cardiovascular events and death. N Engl J Med. 2021;385:1067-1077. doi: 10.1056/NEJMoa2105675
ILLUSTRATIVE CASE
A 47-year-old man in generally good health presents to a family medicine clinic for a well visit. He does not use tobacco products and had a benign colonoscopy last year. He reports walking for 30 minutes 3 to 4 times per week for exercise, althoug h he has gained 3 lbs over the past 2 years. He has no family history of early coronary artery disease, but his father and older brother have hypertension. His mother has a history of diabetes and hyperlipidemia.
The patient’s physical exam is unremarkable except for an elevated BP reading of 151/82 mm Hg. A review of his chart indicates he has had multiple elevated readings in the past that have ranged from 132/72 mm Hg to 139/89 mm Hg. The patient is interested in antihypertensive treatment but wants to know if modifying his diet and replacing his regular table salt with a salt substitute will lower his high BP. What can you recommend?
Hypertension is a leading cause of CV morbidity and mortality worldwide and is linked to increased dietary sodium intake. An estimated 1.28 billion people worldwide have hypertension; however, more than half of cases are undiagnosed.2The US Preventive Services Task Force recommends screening for hypertension in adults older than 18 years and confirming elevated measurements conducted in a nonclinical setting before starting medication (grade “A”).3
Cut-points for the diagnosis of hypertension vary. The American Academy of Family Physicians, 4 the Eighth Joint National Committee (JNC 8), 5 the International Society of Hypertension, 6 and the European Society of Cardiology 7 use ≥ 140 mm Hg systolic BP (SBP) or ≥ 90 mm Hg diastolic BP (DBP) to define hypertension. The American College of Cardiology/American Heart Association guidelines use ≥ 130/80 mm Hg. 8
When treating patients with hypertension, primary care physicians often recommend lifestyle modifications such as the
Systematic reviews have shown a measurable improvement in BP with sodium reduction and potassium substitution. 10-12 More importantly, high-quality evidence demonstrates a decreased risk for CV disease, kidney disease, and all-cause mortality with lower dietary sodium intake. 13 Previous studies have shown that potassium-enriched salt substitutes lower BP, but their impact on CV morbidity and mortality is not well defined. Although lowering BP is associated with improved clinical impact, there is a lack of patient-oriented evidence that demonstrates improvement in CV disease and mortality.
The Salt Substitute and Stroke Study (SSaSS), published in 2021, demonstrated the protective effect of salt substitution against stroke, other CV events, and death. 14 Furthermore, this 5-year, cluster-randomized controlled trial of 20,995 participants across 600 villages in China demonstrated reduced CV mortality and BP reduction similar to standard pharmacologic treatment. Prior to SSaSS, 17 randomized controlled trials demonstrated a BP-lowering effect of salt substitutes but did not directly study the impact on clinical outcomes. 13
Continue to: In this 2022 systematic review...
In this 2022 systematic review and meta-analysis, 1 Yin et al evaluated 21 trials, including SSaSS, for the effect of salt substitutes on BP and other clinical outcomes, and the generalizability of the study results to diverse populations. The systematic review included parallel-group, step-wedge, and cluster-randomized controlled trials reporting the effect of salt substitutes on BP or clinical outcomes.
STUDY SUMMARY
Salt substitutes reduced BP across diverse populations
This systematic review and meta-analysis reviewed existing literature for randomized controlled trials investigating the effects of potassium-enriched salt substitutes on clinical outcomes for patients without kidney disease. The most commonly used salt substitute was potassium chloride, at 25% to 65% potassium.
The systematic review identified 21 trials comprising 31,949 study participants from 15 different countries with 1 to 60 months’ duration. Meta-analyses were performed using 19 trials for BP outcomes and 5 trials for vascular outcomes. Eleven trials were rated as having low risk for bias, 8 were deemed to have some concern, and 2 were rated as high risk for bias. Comparisons of data excluding studies with high risk for bias yielded results similar to comparisons of all studies.
The meta-analysis of 19 trials demonstrated reduced SBP (–4.6 mm Hg; 95% CI, –6.1 to –3.1) and DBP (–1.6 mm Hg; 95% CI, –2.4 to –0.8) in participants using potassium-enriched salt substitutes. However, the authors noted substantial heterogeneity among the studies (I 2 > 70%) for both SBP and DBP outcomes. Although there were no subgroup differences for age, sex, hypertension history, or other biomarkers, outcome differences were associated with trial duration, baseline potassium intake, and composition of the salt substitute.
Potassium-enriched salt substitutes were associated with reduced total mortality (risk ratio [RR] = 0.89; 95% CI, 0.85-0.94), CV mortality (RR = 0.87; 95% CI, 0.81-0.94), and CV events (RR = 0.89; 95% CI, 0.85-0.94). In a meta-regression, each 10% reduction in the sodium content of the salt substitute was associated with a 1.5–mm Hg greater reduction in SBP (95% CI, –3.0 to –0.03) and a 1.0–mm Hg greater reduction in DBP (95% CI, –1.8 to –0.1). However, the authors suggest interpreting meta-regression results with caution.
Continue to: Only 2 of the studes...
Only 2 of the studies in the systematic review explicitly reported the adverse effect of hyperkalemia, and there was no statistical difference in events between randomized groups. Eight other studies reported no serious adverse events related to hyperkalemia , and 11 studies did not report on the risk for hyperkalemia.
WHAT’S NEW
High-quality data demonstrate beneficial outcomes
Previous observational and interventional studies demonstrated a BP-lowering effect of salt substitutes, but limited data with poor-quality evidence existed for the impact of salt substitutes on clinical outcomes such as mortality and CV events. This systematic review and meta-analysis suggests that potassium-supplemented salt may reduce BP and secondarily reduce the risk for CV events, CV mortality, and total mortality, without clear harmful effects reported.
CAVEATS
Some patient populations, comorbidities excluded from study
The study did not include patients with kidney disease or those taking potassium-sparing diuretics. Furthermore, the available data do not include primary prevention participants.
Subgroup analyses should be interpreted with caution due to the small number of trials available for individual subgroups. In addition, funnel plot asymmetry for studies reporting DBP suggests at least some effect of publication bias for that outcome.
Although BP reduction due to salt substitutes may be small at an individual level, these levels of reduction may be important at a population level.
CHALLENGES TO IMPLEMENTATION
For appropriate patients, no challenges anticipated
There are no significant challenges to implementing conclusions from this study in the primary care setting. Family physicians should be able to recommend potassium-enriched salt substitutes to patients with hypertension who are not at risk for hyperkalemia, including those with kidney disease, on potassium-sparing diuretics, or with a history of hyperkalemia/hyperkalemic conditions. Salt substitutes, including potassium-enriched salts, are readily available in stores.
ILLUSTRATIVE CASE
A 47-year-old man in generally good health presents to a family medicine clinic for a well visit. He does not use tobacco products and had a benign colonoscopy last year. He reports walking for 30 minutes 3 to 4 times per week for exercise, althoug h he has gained 3 lbs over the past 2 years. He has no family history of early coronary artery disease, but his father and older brother have hypertension. His mother has a history of diabetes and hyperlipidemia.
The patient’s physical exam is unremarkable except for an elevated BP reading of 151/82 mm Hg. A review of his chart indicates he has had multiple elevated readings in the past that have ranged from 132/72 mm Hg to 139/89 mm Hg. The patient is interested in antihypertensive treatment but wants to know if modifying his diet and replacing his regular table salt with a salt substitute will lower his high BP. What can you recommend?
Hypertension is a leading cause of CV morbidity and mortality worldwide and is linked to increased dietary sodium intake. An estimated 1.28 billion people worldwide have hypertension; however, more than half of cases are undiagnosed.2The US Preventive Services Task Force recommends screening for hypertension in adults older than 18 years and confirming elevated measurements conducted in a nonclinical setting before starting medication (grade “A”).3
Cut-points for the diagnosis of hypertension vary. The American Academy of Family Physicians, 4 the Eighth Joint National Committee (JNC 8), 5 the International Society of Hypertension, 6 and the European Society of Cardiology 7 use ≥ 140 mm Hg systolic BP (SBP) or ≥ 90 mm Hg diastolic BP (DBP) to define hypertension. The American College of Cardiology/American Heart Association guidelines use ≥ 130/80 mm Hg. 8
When treating patients with hypertension, primary care physicians often recommend lifestyle modifications such as the
Systematic reviews have shown a measurable improvement in BP with sodium reduction and potassium substitution. 10-12 More importantly, high-quality evidence demonstrates a decreased risk for CV disease, kidney disease, and all-cause mortality with lower dietary sodium intake. 13 Previous studies have shown that potassium-enriched salt substitutes lower BP, but their impact on CV morbidity and mortality is not well defined. Although lowering BP is associated with improved clinical impact, there is a lack of patient-oriented evidence that demonstrates improvement in CV disease and mortality.
The Salt Substitute and Stroke Study (SSaSS), published in 2021, demonstrated the protective effect of salt substitution against stroke, other CV events, and death. 14 Furthermore, this 5-year, cluster-randomized controlled trial of 20,995 participants across 600 villages in China demonstrated reduced CV mortality and BP reduction similar to standard pharmacologic treatment. Prior to SSaSS, 17 randomized controlled trials demonstrated a BP-lowering effect of salt substitutes but did not directly study the impact on clinical outcomes. 13
Continue to: In this 2022 systematic review...
In this 2022 systematic review and meta-analysis, 1 Yin et al evaluated 21 trials, including SSaSS, for the effect of salt substitutes on BP and other clinical outcomes, and the generalizability of the study results to diverse populations. The systematic review included parallel-group, step-wedge, and cluster-randomized controlled trials reporting the effect of salt substitutes on BP or clinical outcomes.
STUDY SUMMARY
Salt substitutes reduced BP across diverse populations
This systematic review and meta-analysis reviewed existing literature for randomized controlled trials investigating the effects of potassium-enriched salt substitutes on clinical outcomes for patients without kidney disease. The most commonly used salt substitute was potassium chloride, at 25% to 65% potassium.
The systematic review identified 21 trials comprising 31,949 study participants from 15 different countries with 1 to 60 months’ duration. Meta-analyses were performed using 19 trials for BP outcomes and 5 trials for vascular outcomes. Eleven trials were rated as having low risk for bias, 8 were deemed to have some concern, and 2 were rated as high risk for bias. Comparisons of data excluding studies with high risk for bias yielded results similar to comparisons of all studies.
The meta-analysis of 19 trials demonstrated reduced SBP (–4.6 mm Hg; 95% CI, –6.1 to –3.1) and DBP (–1.6 mm Hg; 95% CI, –2.4 to –0.8) in participants using potassium-enriched salt substitutes. However, the authors noted substantial heterogeneity among the studies (I 2 > 70%) for both SBP and DBP outcomes. Although there were no subgroup differences for age, sex, hypertension history, or other biomarkers, outcome differences were associated with trial duration, baseline potassium intake, and composition of the salt substitute.
Potassium-enriched salt substitutes were associated with reduced total mortality (risk ratio [RR] = 0.89; 95% CI, 0.85-0.94), CV mortality (RR = 0.87; 95% CI, 0.81-0.94), and CV events (RR = 0.89; 95% CI, 0.85-0.94). In a meta-regression, each 10% reduction in the sodium content of the salt substitute was associated with a 1.5–mm Hg greater reduction in SBP (95% CI, –3.0 to –0.03) and a 1.0–mm Hg greater reduction in DBP (95% CI, –1.8 to –0.1). However, the authors suggest interpreting meta-regression results with caution.
Continue to: Only 2 of the studes...
Only 2 of the studies in the systematic review explicitly reported the adverse effect of hyperkalemia, and there was no statistical difference in events between randomized groups. Eight other studies reported no serious adverse events related to hyperkalemia , and 11 studies did not report on the risk for hyperkalemia.
WHAT’S NEW
High-quality data demonstrate beneficial outcomes
Previous observational and interventional studies demonstrated a BP-lowering effect of salt substitutes, but limited data with poor-quality evidence existed for the impact of salt substitutes on clinical outcomes such as mortality and CV events. This systematic review and meta-analysis suggests that potassium-supplemented salt may reduce BP and secondarily reduce the risk for CV events, CV mortality, and total mortality, without clear harmful effects reported.
CAVEATS
Some patient populations, comorbidities excluded from study
The study did not include patients with kidney disease or those taking potassium-sparing diuretics. Furthermore, the available data do not include primary prevention participants.
Subgroup analyses should be interpreted with caution due to the small number of trials available for individual subgroups. In addition, funnel plot asymmetry for studies reporting DBP suggests at least some effect of publication bias for that outcome.
Although BP reduction due to salt substitutes may be small at an individual level, these levels of reduction may be important at a population level.
CHALLENGES TO IMPLEMENTATION
For appropriate patients, no challenges anticipated
There are no significant challenges to implementing conclusions from this study in the primary care setting. Family physicians should be able to recommend potassium-enriched salt substitutes to patients with hypertension who are not at risk for hyperkalemia, including those with kidney disease, on potassium-sparing diuretics, or with a history of hyperkalemia/hyperkalemic conditions. Salt substitutes, including potassium-enriched salts, are readily available in stores.
1. Yin X, Rodgers A, Perkovic A, et al. Effects of salt substitutes on clinical outcomes: a systematic review and meta-analysis. Heart. 2022;108:1608-1615. doi: 10.1136/heartjnl-2022-321332
2. NCD Risk Factor Collaboration (NCD-RisC). Worldwide trends in hypertension prevalence and progress in treatment and control from 1990 to 2019: a pooled analysis of 1201 population-representative studies with 104 million participants. Lancet. 2021;398:957-980. doi: 10.1016/S0140-6736(21)01330-1
3. USPSTF. Hypertension in adults: screening. Final recommendation statement. Published April 27, 2021. Accessed September 18, 2023. www.uspreventiveservicestaskforce.org/uspstf/recommendation/hypertension-in-adults-screening
4. Coles S, Fisher L, Lin KW, et al. Blood pressure targets in adults with hypertension: a clinical practice guideline from the AAFP. Published November 4, 2022. Accessed September 18, 2023. www.aafp.org/dam/AAFP/documents/journals/afp/AAFPHypertensionGuideline.pdf
5. James PA, Oparil S, Carter BL, et al. 2014 evidence-based guideline for the management of high blood pressure in adults: report from the panel members appointed to the Eighth Joint National Committee (JNC 8). JAMA. 2014;311:507-520. doi: 10.1001/jama. 2013.284427
6. Unger T, Borgi C, Charchar F, et al. 2020 International Society of Hypertension global hypertension practice guidelines. Hypertension. 2020;75:1334-1357. doi: 10.1161/HYPERTENSIONAHA.120.15026
7. Mancia G, Kreutz R, Brunstrom M, et al; the Task Force for the Management of Arterial Hypertension of the European Society of Hypertension. 2023 ESH Guidelines for the management of arterial hypertension. Endorsed by the European Renal Association (ERA) and the International Society of Hypertension (ISH). J Hypertens. 2023; Jun 21. doi: 10.1097/HJH.0000000000003480
8. Whelton PK, Carey RM, Aronow WS, et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA Guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Hypertension. 2018;71:e13-e115. 10.1161/HYP.0000000000000065
9. National Center for Health Statistics. National Ambulatory Medical Care Survey: 2014 state and national summary tables. Accessed June 27, 2023. www.cdc.gov/nchs/data/ahcd/namcs_summary/2014_namcs_web_tables.pdf
10. Huang L, Trieu K, Yoshimura S, et al. Effect of dose and duration of reduction in dietary sodium on blood pressure levels: systematic review and meta-analysis of randomised trials. BMJ. 2020;368:m315. doi: 10.1136/bmj.m315
11. Filippini T, Violi F, D’Amico R, et al. The effect of potassium supplementation on blood pressure in hypertensive subjects: a systematic review and meta-analysis. Int J Cardiol. 2017;230:127-135. doi: 10.1016/j.ijcard.2016.12.048
12. Brand A, Visser ME, Schoonees A, et al. Replacing salt with low-sodium salt substitutes (LSSS) for cardiovascular health in adults, children and pregnant women. Cochrane Database Syst Rev. 2022;8:CD015207. doi: 10.1002/14651858.CD015207
13. He FJ, Tan M, Ma Y, et al. Salt reduction to prevent hypertension and cardiovascular disease: JACC state-of-the-art review. J Am Coll Cardiol. 2020;75:632-647. doi: 10.1016/j.jacc.2019.11.055
14. Neal B, Wu Y, Feng X, et al. Effect of salt substitution on cardiovascular events and death. N Engl J Med. 2021;385:1067-1077. doi: 10.1056/NEJMoa2105675
1. Yin X, Rodgers A, Perkovic A, et al. Effects of salt substitutes on clinical outcomes: a systematic review and meta-analysis. Heart. 2022;108:1608-1615. doi: 10.1136/heartjnl-2022-321332
2. NCD Risk Factor Collaboration (NCD-RisC). Worldwide trends in hypertension prevalence and progress in treatment and control from 1990 to 2019: a pooled analysis of 1201 population-representative studies with 104 million participants. Lancet. 2021;398:957-980. doi: 10.1016/S0140-6736(21)01330-1
3. USPSTF. Hypertension in adults: screening. Final recommendation statement. Published April 27, 2021. Accessed September 18, 2023. www.uspreventiveservicestaskforce.org/uspstf/recommendation/hypertension-in-adults-screening
4. Coles S, Fisher L, Lin KW, et al. Blood pressure targets in adults with hypertension: a clinical practice guideline from the AAFP. Published November 4, 2022. Accessed September 18, 2023. www.aafp.org/dam/AAFP/documents/journals/afp/AAFPHypertensionGuideline.pdf
5. James PA, Oparil S, Carter BL, et al. 2014 evidence-based guideline for the management of high blood pressure in adults: report from the panel members appointed to the Eighth Joint National Committee (JNC 8). JAMA. 2014;311:507-520. doi: 10.1001/jama. 2013.284427
6. Unger T, Borgi C, Charchar F, et al. 2020 International Society of Hypertension global hypertension practice guidelines. Hypertension. 2020;75:1334-1357. doi: 10.1161/HYPERTENSIONAHA.120.15026
7. Mancia G, Kreutz R, Brunstrom M, et al; the Task Force for the Management of Arterial Hypertension of the European Society of Hypertension. 2023 ESH Guidelines for the management of arterial hypertension. Endorsed by the European Renal Association (ERA) and the International Society of Hypertension (ISH). J Hypertens. 2023; Jun 21. doi: 10.1097/HJH.0000000000003480
8. Whelton PK, Carey RM, Aronow WS, et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA Guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Hypertension. 2018;71:e13-e115. 10.1161/HYP.0000000000000065
9. National Center for Health Statistics. National Ambulatory Medical Care Survey: 2014 state and national summary tables. Accessed June 27, 2023. www.cdc.gov/nchs/data/ahcd/namcs_summary/2014_namcs_web_tables.pdf
10. Huang L, Trieu K, Yoshimura S, et al. Effect of dose and duration of reduction in dietary sodium on blood pressure levels: systematic review and meta-analysis of randomised trials. BMJ. 2020;368:m315. doi: 10.1136/bmj.m315
11. Filippini T, Violi F, D’Amico R, et al. The effect of potassium supplementation on blood pressure in hypertensive subjects: a systematic review and meta-analysis. Int J Cardiol. 2017;230:127-135. doi: 10.1016/j.ijcard.2016.12.048
12. Brand A, Visser ME, Schoonees A, et al. Replacing salt with low-sodium salt substitutes (LSSS) for cardiovascular health in adults, children and pregnant women. Cochrane Database Syst Rev. 2022;8:CD015207. doi: 10.1002/14651858.CD015207
13. He FJ, Tan M, Ma Y, et al. Salt reduction to prevent hypertension and cardiovascular disease: JACC state-of-the-art review. J Am Coll Cardiol. 2020;75:632-647. doi: 10.1016/j.jacc.2019.11.055
14. Neal B, Wu Y, Feng X, et al. Effect of salt substitution on cardiovascular events and death. N Engl J Med. 2021;385:1067-1077. doi: 10.1056/NEJMoa2105675
PRACTICE CHANGER
Consider recommending potassium-enriched salt substitutes for appropriate patients with hypertension to reduce blood pressure (BP) and risk for related cardiovascular (CV) events or mortality.
STRENGTH OF RECOMMENDATION
A: Based on a systematic review and meta-analysis of controlled trials. 1
Yin X, Rodgers A, Perkovic A, et al. Effects of salt substitutes on clinical outcomes: a systematic review and meta-analysis. Heart . 2022;108:1608-1615. doi: 10.1136/heartjnl-2022-321332
Feeling salty about our sodium intake
The World Health Organization (WHO) recently released its inaugural report on the devastating global effects of hypertension, including recommendations for combatting this “silent killer.”1 Notable in the 276-page report is the emphasis on improving access to antihypertensive medications, in part through team-based care and simple evidence-based protocols. This strategy is not surprising given that in clinical medicine we focus on the “high-risk” strategy for prevention—ie, identify people at increased risk for an adverse health outcome (in this case, cardiovascular disease events) and offer them medication to reduce that risk.2
As part of the high-risk strategy, we also counsel at the individual level about lifestyle modifications—but unfortunately, we tend not to get very far. Given the substantial evidence demonstrating its benefits, a low-sodium DASH (Dietary Approaches to Stop Hypertension) eating plan is one of the lifestyle recommendations we make for our patients with hypertension.3,4 The DASH part of the diet involves getting our patients to eat more fruits, vegetables, and whole grains and limit sugar and saturated fats. To achieve the low-sodium part, we might counsel against added table salt, but mostly we discourage consumption of canned and other foods that are commercially processed, packaged, and prepared, because that’s the source of more than 70% of our sodium intake.5 It’s not difficult to understand why real-world uptake of the low-sodium DASH eating plan is low.6
This issue of The Journal of Family Practice features a PURL that supports a much more prominent role for salt substitutes in our counseling recommendations.7 Potassium-enriched salt substitutes not only lower blood pressure (BP) but also reduce the risk for cardiovascular events and death.8 They are widely available, and while more expensive per ounce than regular salt (sodium chloride), are still affordable.
Still, encouraging salt substitution with one patient at a time is relying on the high-risk strategy, with its inherently limited potential.2 An alternative is the population strategy. For hypertension, that would mean doing something for the entire population that would lead to a downward shift in the distribution of BP.2 The shift does not have to be large. We’ve known for more than 3 decades that just a 2–mm Hg reduction in the population’s average systolic BP would reduce stroke mortality by about 6%, coronary heart disease mortality by 4%, and total mortality by 3%.9 A 5–mm Hg reduction more than doubles those benefits. We are talking about tens of thousands fewer patients with heart disease and stroke each year and billions of dollars in health care cost savings.
Reducing our nation’s sodium intake, a quintessential population approach, has proven difficult. Our average daily sodium intake is about 3600 mg.10 Guidance on sodium reduction from the US Food and Drug Administration (targeted to industry) has aimed to reduce Americans’ average sodium intake to 3000 mg/d over the short term, fully acknowledging that the recommended sodium limit is 2300 mg/d.11 We’ve got a long way to go.
Might salt substitution at the population level be a way to simultaneously reduce our sodium intake and increase our potassium intake?12 The closest I found to a populationwide substitution study was a cluster randomized trial conducted in 6 villages in Peru.13 In a stepped-wedge design, households had 25% of their regular salt replaced with potassium salt. Small shops, bakeries, community kitchens, and food vendors also had salt replacement. The intention-to-treat analysis showed a small reduction in systolic BP (1.3 mm Hg) among those with hypertension at baseline (n = 428) and a 51% reduced incidence of developing hypertension among the other 1891 participants over the 4673 person-years of follow-up.
I found this study interesting and its results compelling, leading me to wonder: In the United States, where most of our sodium comes from the food industry, should we replace even a small amount of the sodium in processed foods with potassium? We’re not getting there with DASH alone.
1. World Health Organization. Global report on hypertension: the race against a silent killer. Published September 19, 2023. Accessed September 29, 2023. www.who.int/publications/i/item/9789240081062
2. Rose G. Sick individuals and sick populations. Int J Epidemiol. 2001;30:427-432. doi: 10.1093/ije/30.3.427
3. Chiavaroli L, Viguiliouk E, Nishi SK, et al. DASH dietary pattern and cardiometabolic outcomes: an umbrella review of systematic reviews and meta-analyses. Nutrients. 2019;11:338. doi: 10.3390/nu11020338
4. Saneei P, Salehi-Abargouei A, Esmaillzadeh A, et al. Influence of Dietary Approaches to Stop Hypertension (DASH) diet on blood pressure: a systematic review and meta-analysis on randomized controlled trials. Nutr Metab Cardiovasc Dis. 2014;24:1253-1261. doi: 10.1016/j.numecd.2014.06.008
5. Harnack LJ, Cogswell ME, Shikany JM, et al. Sources of sodium in US adults from 3 geographic regions. Circulation. 2017;135:1775-1783. doi: 10.1161/CIRCULATIONAHA.116.024446
6. Mellen PB, Gao SK, Vitolins MZ, et al. Deteriorating dietary habits among adults with hypertension: DASH dietary accordance, NHANES 1988-1994 and 1999-2004. Arch Intern Med. 2008;168:308-314. doi: 10.1001/archinternmed.2007.119
7. Chang ET, Powell R, Reese T. Can potassium-enriched salt substitutes prevent complications of hypertension? J Fam Pract. 2023;72:342-344. doi: 10.12788/jfp.0667
8. Yin X, Rodgers A, Perkovic A, et al. Effects of salt substitutes on clinical outcomes: a systematic review and meta-analysis. Heart. 2022;108:1608-1615. doi: 10.1136/heartjnl-2022-321332
9. Whelton PK, He J, Appel LJ, et al; National High Blood Pressure Education Program Coordinating Committee. Primary prevention of hypertension: clinical and public health advisory from The National High Blood Pressure Education Program. JAMA. 2002;288:1882-1888. doi: 10.1001/jama.288.15.1882
10. Cogswell ME, Loria CM, Terry AL, et al. Estimated 24-Hour urinary sodium and potassium excretion in US adults. JAMA. 2018;319:1209-1220. doi: 1001/jama.2018.1156
11. FDA. Guidance for industry: voluntary sodium reduction goals. Published October 2021. Accessed September 28, 2023. www.fda.gov/regulatory-information/search-fda-guidance-documents/guidance-industry-voluntary-sodium-reduction-goals
12. Nissaisorakarn V, Ormseth G, Earle W, et al. Less sodium, more potassium, or both: population-wide strategies to prevent hypertension. Am J Physiol Renal Physiol. 2023;325:F99-F104. doi: 10.1152/ajprenal.00007.202
13. Bernabe-Ortiz A, Sal Y Rosas VG, Ponce-Lucero V, et al. Effect of salt substitution on community-wide blood pressure and hypertension incidence. Nat Med. 2020;26:374-378. doi: 10.1038/s41591-020-0754-2
The World Health Organization (WHO) recently released its inaugural report on the devastating global effects of hypertension, including recommendations for combatting this “silent killer.”1 Notable in the 276-page report is the emphasis on improving access to antihypertensive medications, in part through team-based care and simple evidence-based protocols. This strategy is not surprising given that in clinical medicine we focus on the “high-risk” strategy for prevention—ie, identify people at increased risk for an adverse health outcome (in this case, cardiovascular disease events) and offer them medication to reduce that risk.2
As part of the high-risk strategy, we also counsel at the individual level about lifestyle modifications—but unfortunately, we tend not to get very far. Given the substantial evidence demonstrating its benefits, a low-sodium DASH (Dietary Approaches to Stop Hypertension) eating plan is one of the lifestyle recommendations we make for our patients with hypertension.3,4 The DASH part of the diet involves getting our patients to eat more fruits, vegetables, and whole grains and limit sugar and saturated fats. To achieve the low-sodium part, we might counsel against added table salt, but mostly we discourage consumption of canned and other foods that are commercially processed, packaged, and prepared, because that’s the source of more than 70% of our sodium intake.5 It’s not difficult to understand why real-world uptake of the low-sodium DASH eating plan is low.6
This issue of The Journal of Family Practice features a PURL that supports a much more prominent role for salt substitutes in our counseling recommendations.7 Potassium-enriched salt substitutes not only lower blood pressure (BP) but also reduce the risk for cardiovascular events and death.8 They are widely available, and while more expensive per ounce than regular salt (sodium chloride), are still affordable.
Still, encouraging salt substitution with one patient at a time is relying on the high-risk strategy, with its inherently limited potential.2 An alternative is the population strategy. For hypertension, that would mean doing something for the entire population that would lead to a downward shift in the distribution of BP.2 The shift does not have to be large. We’ve known for more than 3 decades that just a 2–mm Hg reduction in the population’s average systolic BP would reduce stroke mortality by about 6%, coronary heart disease mortality by 4%, and total mortality by 3%.9 A 5–mm Hg reduction more than doubles those benefits. We are talking about tens of thousands fewer patients with heart disease and stroke each year and billions of dollars in health care cost savings.
Reducing our nation’s sodium intake, a quintessential population approach, has proven difficult. Our average daily sodium intake is about 3600 mg.10 Guidance on sodium reduction from the US Food and Drug Administration (targeted to industry) has aimed to reduce Americans’ average sodium intake to 3000 mg/d over the short term, fully acknowledging that the recommended sodium limit is 2300 mg/d.11 We’ve got a long way to go.
Might salt substitution at the population level be a way to simultaneously reduce our sodium intake and increase our potassium intake?12 The closest I found to a populationwide substitution study was a cluster randomized trial conducted in 6 villages in Peru.13 In a stepped-wedge design, households had 25% of their regular salt replaced with potassium salt. Small shops, bakeries, community kitchens, and food vendors also had salt replacement. The intention-to-treat analysis showed a small reduction in systolic BP (1.3 mm Hg) among those with hypertension at baseline (n = 428) and a 51% reduced incidence of developing hypertension among the other 1891 participants over the 4673 person-years of follow-up.
I found this study interesting and its results compelling, leading me to wonder: In the United States, where most of our sodium comes from the food industry, should we replace even a small amount of the sodium in processed foods with potassium? We’re not getting there with DASH alone.
The World Health Organization (WHO) recently released its inaugural report on the devastating global effects of hypertension, including recommendations for combatting this “silent killer.”1 Notable in the 276-page report is the emphasis on improving access to antihypertensive medications, in part through team-based care and simple evidence-based protocols. This strategy is not surprising given that in clinical medicine we focus on the “high-risk” strategy for prevention—ie, identify people at increased risk for an adverse health outcome (in this case, cardiovascular disease events) and offer them medication to reduce that risk.2
As part of the high-risk strategy, we also counsel at the individual level about lifestyle modifications—but unfortunately, we tend not to get very far. Given the substantial evidence demonstrating its benefits, a low-sodium DASH (Dietary Approaches to Stop Hypertension) eating plan is one of the lifestyle recommendations we make for our patients with hypertension.3,4 The DASH part of the diet involves getting our patients to eat more fruits, vegetables, and whole grains and limit sugar and saturated fats. To achieve the low-sodium part, we might counsel against added table salt, but mostly we discourage consumption of canned and other foods that are commercially processed, packaged, and prepared, because that’s the source of more than 70% of our sodium intake.5 It’s not difficult to understand why real-world uptake of the low-sodium DASH eating plan is low.6
This issue of The Journal of Family Practice features a PURL that supports a much more prominent role for salt substitutes in our counseling recommendations.7 Potassium-enriched salt substitutes not only lower blood pressure (BP) but also reduce the risk for cardiovascular events and death.8 They are widely available, and while more expensive per ounce than regular salt (sodium chloride), are still affordable.
Still, encouraging salt substitution with one patient at a time is relying on the high-risk strategy, with its inherently limited potential.2 An alternative is the population strategy. For hypertension, that would mean doing something for the entire population that would lead to a downward shift in the distribution of BP.2 The shift does not have to be large. We’ve known for more than 3 decades that just a 2–mm Hg reduction in the population’s average systolic BP would reduce stroke mortality by about 6%, coronary heart disease mortality by 4%, and total mortality by 3%.9 A 5–mm Hg reduction more than doubles those benefits. We are talking about tens of thousands fewer patients with heart disease and stroke each year and billions of dollars in health care cost savings.
Reducing our nation’s sodium intake, a quintessential population approach, has proven difficult. Our average daily sodium intake is about 3600 mg.10 Guidance on sodium reduction from the US Food and Drug Administration (targeted to industry) has aimed to reduce Americans’ average sodium intake to 3000 mg/d over the short term, fully acknowledging that the recommended sodium limit is 2300 mg/d.11 We’ve got a long way to go.
Might salt substitution at the population level be a way to simultaneously reduce our sodium intake and increase our potassium intake?12 The closest I found to a populationwide substitution study was a cluster randomized trial conducted in 6 villages in Peru.13 In a stepped-wedge design, households had 25% of their regular salt replaced with potassium salt. Small shops, bakeries, community kitchens, and food vendors also had salt replacement. The intention-to-treat analysis showed a small reduction in systolic BP (1.3 mm Hg) among those with hypertension at baseline (n = 428) and a 51% reduced incidence of developing hypertension among the other 1891 participants over the 4673 person-years of follow-up.
I found this study interesting and its results compelling, leading me to wonder: In the United States, where most of our sodium comes from the food industry, should we replace even a small amount of the sodium in processed foods with potassium? We’re not getting there with DASH alone.
1. World Health Organization. Global report on hypertension: the race against a silent killer. Published September 19, 2023. Accessed September 29, 2023. www.who.int/publications/i/item/9789240081062
2. Rose G. Sick individuals and sick populations. Int J Epidemiol. 2001;30:427-432. doi: 10.1093/ije/30.3.427
3. Chiavaroli L, Viguiliouk E, Nishi SK, et al. DASH dietary pattern and cardiometabolic outcomes: an umbrella review of systematic reviews and meta-analyses. Nutrients. 2019;11:338. doi: 10.3390/nu11020338
4. Saneei P, Salehi-Abargouei A, Esmaillzadeh A, et al. Influence of Dietary Approaches to Stop Hypertension (DASH) diet on blood pressure: a systematic review and meta-analysis on randomized controlled trials. Nutr Metab Cardiovasc Dis. 2014;24:1253-1261. doi: 10.1016/j.numecd.2014.06.008
5. Harnack LJ, Cogswell ME, Shikany JM, et al. Sources of sodium in US adults from 3 geographic regions. Circulation. 2017;135:1775-1783. doi: 10.1161/CIRCULATIONAHA.116.024446
6. Mellen PB, Gao SK, Vitolins MZ, et al. Deteriorating dietary habits among adults with hypertension: DASH dietary accordance, NHANES 1988-1994 and 1999-2004. Arch Intern Med. 2008;168:308-314. doi: 10.1001/archinternmed.2007.119
7. Chang ET, Powell R, Reese T. Can potassium-enriched salt substitutes prevent complications of hypertension? J Fam Pract. 2023;72:342-344. doi: 10.12788/jfp.0667
8. Yin X, Rodgers A, Perkovic A, et al. Effects of salt substitutes on clinical outcomes: a systematic review and meta-analysis. Heart. 2022;108:1608-1615. doi: 10.1136/heartjnl-2022-321332
9. Whelton PK, He J, Appel LJ, et al; National High Blood Pressure Education Program Coordinating Committee. Primary prevention of hypertension: clinical and public health advisory from The National High Blood Pressure Education Program. JAMA. 2002;288:1882-1888. doi: 10.1001/jama.288.15.1882
10. Cogswell ME, Loria CM, Terry AL, et al. Estimated 24-Hour urinary sodium and potassium excretion in US adults. JAMA. 2018;319:1209-1220. doi: 1001/jama.2018.1156
11. FDA. Guidance for industry: voluntary sodium reduction goals. Published October 2021. Accessed September 28, 2023. www.fda.gov/regulatory-information/search-fda-guidance-documents/guidance-industry-voluntary-sodium-reduction-goals
12. Nissaisorakarn V, Ormseth G, Earle W, et al. Less sodium, more potassium, or both: population-wide strategies to prevent hypertension. Am J Physiol Renal Physiol. 2023;325:F99-F104. doi: 10.1152/ajprenal.00007.202
13. Bernabe-Ortiz A, Sal Y Rosas VG, Ponce-Lucero V, et al. Effect of salt substitution on community-wide blood pressure and hypertension incidence. Nat Med. 2020;26:374-378. doi: 10.1038/s41591-020-0754-2
1. World Health Organization. Global report on hypertension: the race against a silent killer. Published September 19, 2023. Accessed September 29, 2023. www.who.int/publications/i/item/9789240081062
2. Rose G. Sick individuals and sick populations. Int J Epidemiol. 2001;30:427-432. doi: 10.1093/ije/30.3.427
3. Chiavaroli L, Viguiliouk E, Nishi SK, et al. DASH dietary pattern and cardiometabolic outcomes: an umbrella review of systematic reviews and meta-analyses. Nutrients. 2019;11:338. doi: 10.3390/nu11020338
4. Saneei P, Salehi-Abargouei A, Esmaillzadeh A, et al. Influence of Dietary Approaches to Stop Hypertension (DASH) diet on blood pressure: a systematic review and meta-analysis on randomized controlled trials. Nutr Metab Cardiovasc Dis. 2014;24:1253-1261. doi: 10.1016/j.numecd.2014.06.008
5. Harnack LJ, Cogswell ME, Shikany JM, et al. Sources of sodium in US adults from 3 geographic regions. Circulation. 2017;135:1775-1783. doi: 10.1161/CIRCULATIONAHA.116.024446
6. Mellen PB, Gao SK, Vitolins MZ, et al. Deteriorating dietary habits among adults with hypertension: DASH dietary accordance, NHANES 1988-1994 and 1999-2004. Arch Intern Med. 2008;168:308-314. doi: 10.1001/archinternmed.2007.119
7. Chang ET, Powell R, Reese T. Can potassium-enriched salt substitutes prevent complications of hypertension? J Fam Pract. 2023;72:342-344. doi: 10.12788/jfp.0667
8. Yin X, Rodgers A, Perkovic A, et al. Effects of salt substitutes on clinical outcomes: a systematic review and meta-analysis. Heart. 2022;108:1608-1615. doi: 10.1136/heartjnl-2022-321332
9. Whelton PK, He J, Appel LJ, et al; National High Blood Pressure Education Program Coordinating Committee. Primary prevention of hypertension: clinical and public health advisory from The National High Blood Pressure Education Program. JAMA. 2002;288:1882-1888. doi: 10.1001/jama.288.15.1882
10. Cogswell ME, Loria CM, Terry AL, et al. Estimated 24-Hour urinary sodium and potassium excretion in US adults. JAMA. 2018;319:1209-1220. doi: 1001/jama.2018.1156
11. FDA. Guidance for industry: voluntary sodium reduction goals. Published October 2021. Accessed September 28, 2023. www.fda.gov/regulatory-information/search-fda-guidance-documents/guidance-industry-voluntary-sodium-reduction-goals
12. Nissaisorakarn V, Ormseth G, Earle W, et al. Less sodium, more potassium, or both: population-wide strategies to prevent hypertension. Am J Physiol Renal Physiol. 2023;325:F99-F104. doi: 10.1152/ajprenal.00007.202
13. Bernabe-Ortiz A, Sal Y Rosas VG, Ponce-Lucero V, et al. Effect of salt substitution on community-wide blood pressure and hypertension incidence. Nat Med. 2020;26:374-378. doi: 10.1038/s41591-020-0754-2
52-year-old man • intermittent fevers • recently received second dose of COVID-19 vaccine • tremors in all 4 extremities • Dx?
THE CASE
A 52-year-old man sought care at the emergency department for intermittent fevers that started within 6 days of receiving his second dose of the BNT162b2 mRNA COVID-19 vaccine (Pfizer/BioNTech). After an unremarkable work-up, he was discharged home. Six days later, he returned to the emergency department with a fever of 102 °F and new-onset, progressive tremors in all 4 of his extremities.
The patient had a history of rheumatoid arthritis, for which he was taking oral methotrexate 15 mg once weekly and golimumab 50 mg SQ once monthly, and atrial fibrillation. He’d also had mechanical aortic and mitral valves implanted and was taking warfarin (9 mg/d on weekdays, 6 mg/d on Saturday and Sunday). Aside from his fever, his vital signs were normal. He also had horizontal nystagmus (chronically present) and diffuse tremors/myoclonic movements throughout his upper and lower extremities. The tremors were present at rest and worsened with intention/activity, which affected the patient’s ability to walk and perform activities of daily living.
He was admitted the next day to the family medicine service for further evaluation. Neurology and infectious disease consultations were requested, and a broad initial work-up was undertaken. Hyperreflexia was present in all of his extremities, but his neurologic examination was otherwise normal. Initial laboratory tests demonstrated leukocytosis and elevated liver transaminases. His international normalized ratio (INR) and prothrombin time (PT) also were elevated (> 8 [goal, 2.5-3.5 for mechanical heart valves] and > 90 seconds [normal range, 9.7-13.0 seconds], respectively), thus his warfarin was held and oral vitamin K was started (initial dose of 2.5 mg, which was increased to 5 mg when his INR did not decrease enough).
By Day 2, his INR and PT had normalized enough to reinitiate his warfarin dosing. Results from the viral antibody and polymerase chain reaction testing indicated the presence of cytomegalovirus (CMV) infection with viremia; blood cultures for bacterial infection were negative. Brain magnetic resonance imaging was ordered and identified a small, acute left-side cerebellar stroke. Lumbar puncture also was ordered but deferred until his INR was below 1.5 (on Day 8), at which point it confirmed the absence of CMV or herpes simplex virus in his central nervous system.
THE DIAGNOSIS
The patient started oral valganciclovir 900 mg twice daily to ameliorate his tremors, but he did not tolerate it well, vomiting after dosing. He was switched to IV ganciclovir 5 mg/kg every 12 hours; however, his tremors were not improving, leading the team to suspect an etiology other than viral infection. A presumptive diagnosis of autoimmune movement disorder was made, and serum tests were ordered; the results were positive for antiphospholipid antibodies, including anticardiolipin and anti-ß2 glycoprotein-I antibodies. A final diagnosis of autoimmune antiphospholipid antibody syndrome (APS)–related movement disorder1 with coagulopathy was reached, and the patient was started on methylprednisolone 1 g/d IV.
We suspected the CMV viremia was reactivated by the COVID-19 vaccine and caused the APS that led to the movement disorder, coagulopathy, and likely, the thrombotic cerebellar stroke. The case was reported to the Vaccine Adverse Event Reporting System (VAERS).2
DISCUSSION
Continue to: The development of antiphospholipid antibodies...
The development of antiphospholipid antibodies has been independently associated with rheumatoid arthritis,5 COVID-19,6 and CMV infection,7 as well as with vaccination for influenza and tetanus.8 There also are reports of antiphospholipid antibodies occurring in patients who have received adenovirus-vectored and mRNA COVID-19 vaccines.9-11
Movement disorders occurring with APS are unusual, with approximately 1.3% to 4.5% of patients with APS demonstrating this manifestation.12 One of multiple autoimmune-related movement disorders, APS-related movement disorder is most commonly associated with systemic lupus erythematosus (SLE), although it can occur outside an SLE diagnosis.4
While APS-related movement disorder occurs with the presence of antiphospholipid antibodies, the pathogenesis of the movement disorder is unclear.4 Patients are typically young women, and the associated movements are choreiform. The condition often occurs with coagulopathy and arterial thrombosis.4 Psychiatric manifestations also can occur, including changes in behavior—up to and including psychosis.4
Evidence of COVID-19 vaccination reactivating herpesviruses exists, although it is rare and usually does not cause serious health outcomes.13 The annual incidence of reactivation related to vaccination is estimated to be 0.7 per 100,000 for varicella zoster virus and 0.03 per 100,000 for herpes simplex virus.13 The literature also suggests that the occurrence of Bell palsy—the onset of which may be related to the reactivation of a latent virus—may increase in relation to particular COVID-19 vaccines.14,15 Although there is no confirmed explanation for these reactivation events at this time, different theories related to altering the focus of immune cells from latent disease to the newly generated antigen have been suggested.16
To date, reactivation has not been demonstrated with CMV specifically. However, based on the literature reviewed here on the reactivation of herpesviruses and the temporal relationship to infection in our patient, we propose that the BNT162b2 mRNA vaccination reactivated his CMV infection and led to his APS-related movement disorder.
Continue to: Treatment is focused on resolved the autoimmune condition
Treatment is focused on resolving the autoimmune condition, usually with corticosteroids. Longer-term treatment of the movement disorder with antiepileptics such as carbamazepine and valproic acid may be necessary.4
Our patient received methylprednisolone IV 1 g/d for 3 days and responded quickly to the treatment. He was discharged to a post-acute rehabilitation hospital on Day 16 with a plan for 21 days of antiviral treatment for an acute CMV infection, 1 month of oral steroid taper for the APS, and continued warfarin treatment. This regimen resulted in complete resolution of his movement disorder and negative testing of antiphospholipid antibodies 16 days after he was discharged from the hospital.
THE TAKEAWAY
This case illustrates the possible reactivation of a herpesvirus (CMV) related to COVID-19 vaccination, as well as the development of APS-related movement disorder and coagulopathy related to acute CMV infection with viremia. Vaccination for the COVID-19 virus is seen as the best intervention available for preventing serious illness and death associated with COVID-19 infection. Thus, it is important to be aware of these unusual events when vaccinating large populations. This case also demonstrates the need to understand the interplay of immune status and possible disorders associated with autoimmune conditions. Keeping an open mind when evaluating patients with post-vaccination complaints is beneficial—especially given the volume of distrust and misinformation associated with COVID-19 vaccination.
CORRESPONDENCE
Aaron Lear, MD, MSc, CAQ, Cleveland Clinic Akron General Center for Family Medicine, 1 Akron General Avenue, Building 301, Akron, OH 44307; [email protected]
1. Martino D, Chew N-K, Mir P, et al. Atypical movement disorders in antiphospholipid syndrome. 2006;21:944-949. doi: 10.1002/mds.20842
2. Vaccine Adverse Event Reporting System. Accessed February 9, 2022. vaers.hhs.gov
3. Duarte-García A, Pham MM, Crowson CS, et al. The epidemiology of antiphospholipid syndrome: a population-based Study. Arthritis Rheumatol. 2019;71:1545-1552. doi: 10.1002/art.40901
4. Baizabal-Carvallo JF, Jankovic J. Autoimmune and paraneoplastic movement disorders: an update. J Neurol Sci. 2018;385:175-184. doi: 10.1016/j.jns.2017.12.035
5. O’Leary RE, Hsiao JL, Worswick SD. Antiphospholipid syndrome in a patient with rheumatoid arthritis. Cutis. 2017;99:E21-E24.
6. Taha M, Samavati L. Antiphospholipid antibodies in COVID-19: a meta-analysis and systematic review. RMD Open. 2021;7:e001580. doi: 10.1136/rmdopen-2021-001580
7. Nakayama T, Akahoshi M, Irino K, et al. Transient antiphospholipid syndrome associated with primary cytomegalovirus infection: a case report and literature review. Case Rep Rheumatol. 2014;2014:27154. doi: 10.1155/2014/271548
8. Cruz-Tapias P, Blank M, Anaya J-M, et al. Infections and vaccines in the etiology of antiphospholipid syndrome. Curr Opin Rheumatol. 2012;24:389-393. doi: 10.1097/BOR.0b013e32835448b8
9. Schultz NH, Sørvoll IH, Michelsen AE, et al. Thrombosis and thrombocytopenia after ChAdOx1 nCoV-19 vaccination. N Engl J Med. 2021;384:2124-2130. doi: 10.1056/nejmoa2104882
10. Cimolai N. Untangling the intricacies of infection, thrombosis, vaccination, and antiphospholipid antibodies for COVID-19. SN Compr Clin Med. 2021;3:2093-2108. doi: 10.1007/s42399-021-00992-3
11. Jinno S, Naka I, Nakazawa T. Catastrophic antiphospholipid syndrome complicated with essential thrombocythaemia after COVID-19 vaccination: in search of the underlying mechanism. Rheumatol Adv Pract. 2021;5:rkab096. doi: 10.1093/rap/rkab096
12. Ricarte IF, Dutra LA, Abrantes FF, et al. Neurologic manifestations of antiphospholipid syndrome. Lupus. 2018;27:1404-1414. doi: 10.1177/0961203318776110
13. Gringeri M, Battini V, Cammarata G, et al. Herpes zoster and simplex reactivation following COVID-19 vaccination: new insights from a vaccine adverse event reporting system (VAERS) database analysis. Expert Rev Vaccines. 2022;21:675-684. doi: 10.1080/14760584.2022.2044799
14. Cirillo N, Doan R. The association between COVID-19 vaccination and Bell’s palsy. Lancet Infect Dis. 2022;22:5-6. doi: 10.1016/s1473-3099(21)00467-9
15. Poudel S, Nepali P, Baniya S, et al. Bell’s palsy as a possible complication of mRNA-1273 (Moderna) vaccine against COVID-19. Ann Med Surg (Lond). 2022;78:103897. doi: 10.1016/j.amsu.2022.103897
16. Furer V, Zisman D, Kibari A, et al. Herpes zoster following BNT162b2 mRNA COVID-19 vaccination in patients with autoimmune inflammatory rheumatic diseases: a case series. Rheumatology (Oxford). 2021;60:SI90-SI95. doi: 10.1093/rheumatology/keab345
THE CASE
A 52-year-old man sought care at the emergency department for intermittent fevers that started within 6 days of receiving his second dose of the BNT162b2 mRNA COVID-19 vaccine (Pfizer/BioNTech). After an unremarkable work-up, he was discharged home. Six days later, he returned to the emergency department with a fever of 102 °F and new-onset, progressive tremors in all 4 of his extremities.
The patient had a history of rheumatoid arthritis, for which he was taking oral methotrexate 15 mg once weekly and golimumab 50 mg SQ once monthly, and atrial fibrillation. He’d also had mechanical aortic and mitral valves implanted and was taking warfarin (9 mg/d on weekdays, 6 mg/d on Saturday and Sunday). Aside from his fever, his vital signs were normal. He also had horizontal nystagmus (chronically present) and diffuse tremors/myoclonic movements throughout his upper and lower extremities. The tremors were present at rest and worsened with intention/activity, which affected the patient’s ability to walk and perform activities of daily living.
He was admitted the next day to the family medicine service for further evaluation. Neurology and infectious disease consultations were requested, and a broad initial work-up was undertaken. Hyperreflexia was present in all of his extremities, but his neurologic examination was otherwise normal. Initial laboratory tests demonstrated leukocytosis and elevated liver transaminases. His international normalized ratio (INR) and prothrombin time (PT) also were elevated (> 8 [goal, 2.5-3.5 for mechanical heart valves] and > 90 seconds [normal range, 9.7-13.0 seconds], respectively), thus his warfarin was held and oral vitamin K was started (initial dose of 2.5 mg, which was increased to 5 mg when his INR did not decrease enough).
By Day 2, his INR and PT had normalized enough to reinitiate his warfarin dosing. Results from the viral antibody and polymerase chain reaction testing indicated the presence of cytomegalovirus (CMV) infection with viremia; blood cultures for bacterial infection were negative. Brain magnetic resonance imaging was ordered and identified a small, acute left-side cerebellar stroke. Lumbar puncture also was ordered but deferred until his INR was below 1.5 (on Day 8), at which point it confirmed the absence of CMV or herpes simplex virus in his central nervous system.
THE DIAGNOSIS
The patient started oral valganciclovir 900 mg twice daily to ameliorate his tremors, but he did not tolerate it well, vomiting after dosing. He was switched to IV ganciclovir 5 mg/kg every 12 hours; however, his tremors were not improving, leading the team to suspect an etiology other than viral infection. A presumptive diagnosis of autoimmune movement disorder was made, and serum tests were ordered; the results were positive for antiphospholipid antibodies, including anticardiolipin and anti-ß2 glycoprotein-I antibodies. A final diagnosis of autoimmune antiphospholipid antibody syndrome (APS)–related movement disorder1 with coagulopathy was reached, and the patient was started on methylprednisolone 1 g/d IV.
We suspected the CMV viremia was reactivated by the COVID-19 vaccine and caused the APS that led to the movement disorder, coagulopathy, and likely, the thrombotic cerebellar stroke. The case was reported to the Vaccine Adverse Event Reporting System (VAERS).2
DISCUSSION
Continue to: The development of antiphospholipid antibodies...
The development of antiphospholipid antibodies has been independently associated with rheumatoid arthritis,5 COVID-19,6 and CMV infection,7 as well as with vaccination for influenza and tetanus.8 There also are reports of antiphospholipid antibodies occurring in patients who have received adenovirus-vectored and mRNA COVID-19 vaccines.9-11
Movement disorders occurring with APS are unusual, with approximately 1.3% to 4.5% of patients with APS demonstrating this manifestation.12 One of multiple autoimmune-related movement disorders, APS-related movement disorder is most commonly associated with systemic lupus erythematosus (SLE), although it can occur outside an SLE diagnosis.4
While APS-related movement disorder occurs with the presence of antiphospholipid antibodies, the pathogenesis of the movement disorder is unclear.4 Patients are typically young women, and the associated movements are choreiform. The condition often occurs with coagulopathy and arterial thrombosis.4 Psychiatric manifestations also can occur, including changes in behavior—up to and including psychosis.4
Evidence of COVID-19 vaccination reactivating herpesviruses exists, although it is rare and usually does not cause serious health outcomes.13 The annual incidence of reactivation related to vaccination is estimated to be 0.7 per 100,000 for varicella zoster virus and 0.03 per 100,000 for herpes simplex virus.13 The literature also suggests that the occurrence of Bell palsy—the onset of which may be related to the reactivation of a latent virus—may increase in relation to particular COVID-19 vaccines.14,15 Although there is no confirmed explanation for these reactivation events at this time, different theories related to altering the focus of immune cells from latent disease to the newly generated antigen have been suggested.16
To date, reactivation has not been demonstrated with CMV specifically. However, based on the literature reviewed here on the reactivation of herpesviruses and the temporal relationship to infection in our patient, we propose that the BNT162b2 mRNA vaccination reactivated his CMV infection and led to his APS-related movement disorder.
Continue to: Treatment is focused on resolved the autoimmune condition
Treatment is focused on resolving the autoimmune condition, usually with corticosteroids. Longer-term treatment of the movement disorder with antiepileptics such as carbamazepine and valproic acid may be necessary.4
Our patient received methylprednisolone IV 1 g/d for 3 days and responded quickly to the treatment. He was discharged to a post-acute rehabilitation hospital on Day 16 with a plan for 21 days of antiviral treatment for an acute CMV infection, 1 month of oral steroid taper for the APS, and continued warfarin treatment. This regimen resulted in complete resolution of his movement disorder and negative testing of antiphospholipid antibodies 16 days after he was discharged from the hospital.
THE TAKEAWAY
This case illustrates the possible reactivation of a herpesvirus (CMV) related to COVID-19 vaccination, as well as the development of APS-related movement disorder and coagulopathy related to acute CMV infection with viremia. Vaccination for the COVID-19 virus is seen as the best intervention available for preventing serious illness and death associated with COVID-19 infection. Thus, it is important to be aware of these unusual events when vaccinating large populations. This case also demonstrates the need to understand the interplay of immune status and possible disorders associated with autoimmune conditions. Keeping an open mind when evaluating patients with post-vaccination complaints is beneficial—especially given the volume of distrust and misinformation associated with COVID-19 vaccination.
CORRESPONDENCE
Aaron Lear, MD, MSc, CAQ, Cleveland Clinic Akron General Center for Family Medicine, 1 Akron General Avenue, Building 301, Akron, OH 44307; [email protected]
THE CASE
A 52-year-old man sought care at the emergency department for intermittent fevers that started within 6 days of receiving his second dose of the BNT162b2 mRNA COVID-19 vaccine (Pfizer/BioNTech). After an unremarkable work-up, he was discharged home. Six days later, he returned to the emergency department with a fever of 102 °F and new-onset, progressive tremors in all 4 of his extremities.
The patient had a history of rheumatoid arthritis, for which he was taking oral methotrexate 15 mg once weekly and golimumab 50 mg SQ once monthly, and atrial fibrillation. He’d also had mechanical aortic and mitral valves implanted and was taking warfarin (9 mg/d on weekdays, 6 mg/d on Saturday and Sunday). Aside from his fever, his vital signs were normal. He also had horizontal nystagmus (chronically present) and diffuse tremors/myoclonic movements throughout his upper and lower extremities. The tremors were present at rest and worsened with intention/activity, which affected the patient’s ability to walk and perform activities of daily living.
He was admitted the next day to the family medicine service for further evaluation. Neurology and infectious disease consultations were requested, and a broad initial work-up was undertaken. Hyperreflexia was present in all of his extremities, but his neurologic examination was otherwise normal. Initial laboratory tests demonstrated leukocytosis and elevated liver transaminases. His international normalized ratio (INR) and prothrombin time (PT) also were elevated (> 8 [goal, 2.5-3.5 for mechanical heart valves] and > 90 seconds [normal range, 9.7-13.0 seconds], respectively), thus his warfarin was held and oral vitamin K was started (initial dose of 2.5 mg, which was increased to 5 mg when his INR did not decrease enough).
By Day 2, his INR and PT had normalized enough to reinitiate his warfarin dosing. Results from the viral antibody and polymerase chain reaction testing indicated the presence of cytomegalovirus (CMV) infection with viremia; blood cultures for bacterial infection were negative. Brain magnetic resonance imaging was ordered and identified a small, acute left-side cerebellar stroke. Lumbar puncture also was ordered but deferred until his INR was below 1.5 (on Day 8), at which point it confirmed the absence of CMV or herpes simplex virus in his central nervous system.
THE DIAGNOSIS
The patient started oral valganciclovir 900 mg twice daily to ameliorate his tremors, but he did not tolerate it well, vomiting after dosing. He was switched to IV ganciclovir 5 mg/kg every 12 hours; however, his tremors were not improving, leading the team to suspect an etiology other than viral infection. A presumptive diagnosis of autoimmune movement disorder was made, and serum tests were ordered; the results were positive for antiphospholipid antibodies, including anticardiolipin and anti-ß2 glycoprotein-I antibodies. A final diagnosis of autoimmune antiphospholipid antibody syndrome (APS)–related movement disorder1 with coagulopathy was reached, and the patient was started on methylprednisolone 1 g/d IV.
We suspected the CMV viremia was reactivated by the COVID-19 vaccine and caused the APS that led to the movement disorder, coagulopathy, and likely, the thrombotic cerebellar stroke. The case was reported to the Vaccine Adverse Event Reporting System (VAERS).2
DISCUSSION
Continue to: The development of antiphospholipid antibodies...
The development of antiphospholipid antibodies has been independently associated with rheumatoid arthritis,5 COVID-19,6 and CMV infection,7 as well as with vaccination for influenza and tetanus.8 There also are reports of antiphospholipid antibodies occurring in patients who have received adenovirus-vectored and mRNA COVID-19 vaccines.9-11
Movement disorders occurring with APS are unusual, with approximately 1.3% to 4.5% of patients with APS demonstrating this manifestation.12 One of multiple autoimmune-related movement disorders, APS-related movement disorder is most commonly associated with systemic lupus erythematosus (SLE), although it can occur outside an SLE diagnosis.4
While APS-related movement disorder occurs with the presence of antiphospholipid antibodies, the pathogenesis of the movement disorder is unclear.4 Patients are typically young women, and the associated movements are choreiform. The condition often occurs with coagulopathy and arterial thrombosis.4 Psychiatric manifestations also can occur, including changes in behavior—up to and including psychosis.4
Evidence of COVID-19 vaccination reactivating herpesviruses exists, although it is rare and usually does not cause serious health outcomes.13 The annual incidence of reactivation related to vaccination is estimated to be 0.7 per 100,000 for varicella zoster virus and 0.03 per 100,000 for herpes simplex virus.13 The literature also suggests that the occurrence of Bell palsy—the onset of which may be related to the reactivation of a latent virus—may increase in relation to particular COVID-19 vaccines.14,15 Although there is no confirmed explanation for these reactivation events at this time, different theories related to altering the focus of immune cells from latent disease to the newly generated antigen have been suggested.16
To date, reactivation has not been demonstrated with CMV specifically. However, based on the literature reviewed here on the reactivation of herpesviruses and the temporal relationship to infection in our patient, we propose that the BNT162b2 mRNA vaccination reactivated his CMV infection and led to his APS-related movement disorder.
Continue to: Treatment is focused on resolved the autoimmune condition
Treatment is focused on resolving the autoimmune condition, usually with corticosteroids. Longer-term treatment of the movement disorder with antiepileptics such as carbamazepine and valproic acid may be necessary.4
Our patient received methylprednisolone IV 1 g/d for 3 days and responded quickly to the treatment. He was discharged to a post-acute rehabilitation hospital on Day 16 with a plan for 21 days of antiviral treatment for an acute CMV infection, 1 month of oral steroid taper for the APS, and continued warfarin treatment. This regimen resulted in complete resolution of his movement disorder and negative testing of antiphospholipid antibodies 16 days after he was discharged from the hospital.
THE TAKEAWAY
This case illustrates the possible reactivation of a herpesvirus (CMV) related to COVID-19 vaccination, as well as the development of APS-related movement disorder and coagulopathy related to acute CMV infection with viremia. Vaccination for the COVID-19 virus is seen as the best intervention available for preventing serious illness and death associated with COVID-19 infection. Thus, it is important to be aware of these unusual events when vaccinating large populations. This case also demonstrates the need to understand the interplay of immune status and possible disorders associated with autoimmune conditions. Keeping an open mind when evaluating patients with post-vaccination complaints is beneficial—especially given the volume of distrust and misinformation associated with COVID-19 vaccination.
CORRESPONDENCE
Aaron Lear, MD, MSc, CAQ, Cleveland Clinic Akron General Center for Family Medicine, 1 Akron General Avenue, Building 301, Akron, OH 44307; [email protected]
1. Martino D, Chew N-K, Mir P, et al. Atypical movement disorders in antiphospholipid syndrome. 2006;21:944-949. doi: 10.1002/mds.20842
2. Vaccine Adverse Event Reporting System. Accessed February 9, 2022. vaers.hhs.gov
3. Duarte-García A, Pham MM, Crowson CS, et al. The epidemiology of antiphospholipid syndrome: a population-based Study. Arthritis Rheumatol. 2019;71:1545-1552. doi: 10.1002/art.40901
4. Baizabal-Carvallo JF, Jankovic J. Autoimmune and paraneoplastic movement disorders: an update. J Neurol Sci. 2018;385:175-184. doi: 10.1016/j.jns.2017.12.035
5. O’Leary RE, Hsiao JL, Worswick SD. Antiphospholipid syndrome in a patient with rheumatoid arthritis. Cutis. 2017;99:E21-E24.
6. Taha M, Samavati L. Antiphospholipid antibodies in COVID-19: a meta-analysis and systematic review. RMD Open. 2021;7:e001580. doi: 10.1136/rmdopen-2021-001580
7. Nakayama T, Akahoshi M, Irino K, et al. Transient antiphospholipid syndrome associated with primary cytomegalovirus infection: a case report and literature review. Case Rep Rheumatol. 2014;2014:27154. doi: 10.1155/2014/271548
8. Cruz-Tapias P, Blank M, Anaya J-M, et al. Infections and vaccines in the etiology of antiphospholipid syndrome. Curr Opin Rheumatol. 2012;24:389-393. doi: 10.1097/BOR.0b013e32835448b8
9. Schultz NH, Sørvoll IH, Michelsen AE, et al. Thrombosis and thrombocytopenia after ChAdOx1 nCoV-19 vaccination. N Engl J Med. 2021;384:2124-2130. doi: 10.1056/nejmoa2104882
10. Cimolai N. Untangling the intricacies of infection, thrombosis, vaccination, and antiphospholipid antibodies for COVID-19. SN Compr Clin Med. 2021;3:2093-2108. doi: 10.1007/s42399-021-00992-3
11. Jinno S, Naka I, Nakazawa T. Catastrophic antiphospholipid syndrome complicated with essential thrombocythaemia after COVID-19 vaccination: in search of the underlying mechanism. Rheumatol Adv Pract. 2021;5:rkab096. doi: 10.1093/rap/rkab096
12. Ricarte IF, Dutra LA, Abrantes FF, et al. Neurologic manifestations of antiphospholipid syndrome. Lupus. 2018;27:1404-1414. doi: 10.1177/0961203318776110
13. Gringeri M, Battini V, Cammarata G, et al. Herpes zoster and simplex reactivation following COVID-19 vaccination: new insights from a vaccine adverse event reporting system (VAERS) database analysis. Expert Rev Vaccines. 2022;21:675-684. doi: 10.1080/14760584.2022.2044799
14. Cirillo N, Doan R. The association between COVID-19 vaccination and Bell’s palsy. Lancet Infect Dis. 2022;22:5-6. doi: 10.1016/s1473-3099(21)00467-9
15. Poudel S, Nepali P, Baniya S, et al. Bell’s palsy as a possible complication of mRNA-1273 (Moderna) vaccine against COVID-19. Ann Med Surg (Lond). 2022;78:103897. doi: 10.1016/j.amsu.2022.103897
16. Furer V, Zisman D, Kibari A, et al. Herpes zoster following BNT162b2 mRNA COVID-19 vaccination in patients with autoimmune inflammatory rheumatic diseases: a case series. Rheumatology (Oxford). 2021;60:SI90-SI95. doi: 10.1093/rheumatology/keab345
1. Martino D, Chew N-K, Mir P, et al. Atypical movement disorders in antiphospholipid syndrome. 2006;21:944-949. doi: 10.1002/mds.20842
2. Vaccine Adverse Event Reporting System. Accessed February 9, 2022. vaers.hhs.gov
3. Duarte-García A, Pham MM, Crowson CS, et al. The epidemiology of antiphospholipid syndrome: a population-based Study. Arthritis Rheumatol. 2019;71:1545-1552. doi: 10.1002/art.40901
4. Baizabal-Carvallo JF, Jankovic J. Autoimmune and paraneoplastic movement disorders: an update. J Neurol Sci. 2018;385:175-184. doi: 10.1016/j.jns.2017.12.035
5. O’Leary RE, Hsiao JL, Worswick SD. Antiphospholipid syndrome in a patient with rheumatoid arthritis. Cutis. 2017;99:E21-E24.
6. Taha M, Samavati L. Antiphospholipid antibodies in COVID-19: a meta-analysis and systematic review. RMD Open. 2021;7:e001580. doi: 10.1136/rmdopen-2021-001580
7. Nakayama T, Akahoshi M, Irino K, et al. Transient antiphospholipid syndrome associated with primary cytomegalovirus infection: a case report and literature review. Case Rep Rheumatol. 2014;2014:27154. doi: 10.1155/2014/271548
8. Cruz-Tapias P, Blank M, Anaya J-M, et al. Infections and vaccines in the etiology of antiphospholipid syndrome. Curr Opin Rheumatol. 2012;24:389-393. doi: 10.1097/BOR.0b013e32835448b8
9. Schultz NH, Sørvoll IH, Michelsen AE, et al. Thrombosis and thrombocytopenia after ChAdOx1 nCoV-19 vaccination. N Engl J Med. 2021;384:2124-2130. doi: 10.1056/nejmoa2104882
10. Cimolai N. Untangling the intricacies of infection, thrombosis, vaccination, and antiphospholipid antibodies for COVID-19. SN Compr Clin Med. 2021;3:2093-2108. doi: 10.1007/s42399-021-00992-3
11. Jinno S, Naka I, Nakazawa T. Catastrophic antiphospholipid syndrome complicated with essential thrombocythaemia after COVID-19 vaccination: in search of the underlying mechanism. Rheumatol Adv Pract. 2021;5:rkab096. doi: 10.1093/rap/rkab096
12. Ricarte IF, Dutra LA, Abrantes FF, et al. Neurologic manifestations of antiphospholipid syndrome. Lupus. 2018;27:1404-1414. doi: 10.1177/0961203318776110
13. Gringeri M, Battini V, Cammarata G, et al. Herpes zoster and simplex reactivation following COVID-19 vaccination: new insights from a vaccine adverse event reporting system (VAERS) database analysis. Expert Rev Vaccines. 2022;21:675-684. doi: 10.1080/14760584.2022.2044799
14. Cirillo N, Doan R. The association between COVID-19 vaccination and Bell’s palsy. Lancet Infect Dis. 2022;22:5-6. doi: 10.1016/s1473-3099(21)00467-9
15. Poudel S, Nepali P, Baniya S, et al. Bell’s palsy as a possible complication of mRNA-1273 (Moderna) vaccine against COVID-19. Ann Med Surg (Lond). 2022;78:103897. doi: 10.1016/j.amsu.2022.103897
16. Furer V, Zisman D, Kibari A, et al. Herpes zoster following BNT162b2 mRNA COVID-19 vaccination in patients with autoimmune inflammatory rheumatic diseases: a case series. Rheumatology (Oxford). 2021;60:SI90-SI95. doi: 10.1093/rheumatology/keab345
► Intermittent fevers
► Recently received second dose of COVID-19 vaccine
► Tremors in all 4 extremities
Inadequate sleep & obesity: Breaking the vicious cycle
Sleep is fundamental to overall health and longevity, with the average person spending about one-third of their life sleeping.1 Adequate sleep is critical for optimal cognition, memory consolidation, mood regulation, metabolism, appetite regulation, and immune and hormone functioning. According to the American Academy of Sleep Medicine and the Sleep Research Society, adults should sleep at least 7 hours per night on a regular basis “to promote optimal health.”2 Yet, between 2013 and 2020, only about 65% of adults in the United States were meeting this amount.3 Insufficient sleep is associated with an increased risk for chronic health conditions, including obesity, diabetes, cardiovascular diseases, and even premature death.4

In a population-based longitudinal study of sleep disorders, short sleep duration was associated with increased body mass index (BMI), low blood levels of leptin, and high ghrelin levels.5 In addition to physical impairments, poor sleep can impair cognitive performance and lead to vehicular accidents and increased accidents at work.4 The potential economic impact that this may have is significant, and includes increased costs and loss of productivity in the workplace.6
Many factors may contribute to short sleep duration: environment, mental and physical condition, and social influences such as occupation, family responsibilities, travel, group activities, and personal care. Furthermore, the rapidly evolving and developing media, communication, and entertainment industries are already strongly implicated in poor sleep quality and quantity, both contributing to excessive daytime sleepiness.7 Poor sleep quality is most notable in modern societies, and it correlates with the increasing prevalence of obesity, likely due to sleep’s effect on food consumption and physical activity.8 Optimizing a person’s sleep will improve overall health and longevity by inhibiting the development of chronic disease.
How insufficient sleep raises the risk for obesity
Not only is sleep beneficial for brain health, memory, learning, and growth, its effect on food consumption and physical activity likely correlates with the increased prevalence of obesity in modern society. Yet the optimal amount of sleep is controversial, and current recommendations of 7 or more hours of sleep per night for adults are derived from expert panels only.2 The recommended sleep duration for children is longer, and it varies by age.9 The quality of sleep and its impact on neuroendocrine hormones, not just the quantity of sleep, needs to be factored into these recommendations.
Sleep restriction activates the orexigenic system via the hormones leptin and ghrelin. These hormones control the food reward system, essentially increasing hunger and food intake. Leptin, created by white adipose tissue, is responsible for satiety and decreased food consumption.10 Ghrelin, made by oxyntic glands in the stomach, is responsible for the sensation of hunger.
In a 2004 study by Spiegel et al,11 leptin and ghrelin levels were measured during 2 days of sleep restriction (4 hours in bed) and sleep extension (10 hours in bed). Sleep restriction was associated with a decrease in leptin levels and an increase in ghrelin levels. The researchers reported that participants experienced an increase in hunger and appetite—especially for calorie-dense foods with high carbohydrate content.
Although research design has limitations with predominantly self-reported sleep data, studies have shown that short sleep time leads to increased food intake by increasing hunger signals and craving of unhealthy foods, and by providing more opportunities to eat while awake. It also may lead to decreased physical activity, creating a sedentary lifestyle that further encourages obesity.8 Reduced sleep is even correlated to decreased efficacy of weight-loss treatments.12
Continue to: Other sleep characteristics weakly correlated with obesity
Other sleep characteristics weakly correlated with obesity are sleep variability, timing, efficiency, quality, and daytime napping.8 Sleep variability causes dysregulation of eating patterns, leading to increased food intake. A shift to later sleep and waking times often results in higher consumption of calories after 8
Poor sleep efficiency and quality decreases N3-stage (deep non-REM) sleep, affects the autonomic nervous system, and has been associated with increased abdominal obesity. Daytime napping, which can cause irregular circadian rhythms and sleep schedules, is associated with increased obesity.15 Thus, each component of sleep needs to be assessed to promote optimal regulation of the orexigenic system.
Another study showed that inadequate sleep not only promotes unhealthy lifestyle habits that can lead to obesity but also decreases the ability to lose weight.16 This small study with 10 overweight patients provided its subjects with a controlled caloric intake over 2 weeks. Patients spent two 14-day periods 3 months apart in the laboratory, divided into 2 time-in-bed arms of 8.5 and 5.5 hours per night. Neuroendocrine changes caused by decreased sleep were associated with a significant lean body mass loss while conserving energy-dense fat.16 This study highlights the importance of sleep hygiene counseling when developing a weight-management plan with patients.
Sleep, and its many components, play an integral role in the prevention and treatment of obesity.17 Poor sleep will increase the risk for obesity and hinder its treatment. Therefore, sleep quality and duration are vital components of obesity management.
The sleep–obesity link in children and the elderly
Childhood obesity is linked to several chronic diseases in adulthood, including type 2 diabetes, cardiovascular disease, nonalcoholic fatty liver disease, asthma, and obstructive sleep apnea (OSA).18 According to 2017-2018 NHANES (National Health and Nutrition Examination Surveys) data, obesity (BMI ≥ 95th percentile) prevalence among children and adolescents was reported at 19.3% and severe obesity (BMI ≥ 120% of the 95th percentile) at 6.1%. Pediatric overweight prevalence (≥ 85th percentile and < 95th percentile) was 16.1%.19
Continue to: Although poor sleep is associated...
Although poor sleep is associated with increased risk for obesity, there is no proven cause-effect relationship.20 Nutrition and physical activity have been identified as 2 critical factors in childhood obesity, but sleep health also needs to be investigated. Shorter sleep duration is strongly associated with the development of obesity. Furthermore, children with obesity are more likely to have shorter sleep duration.21 A short sleep duration alters plasma levels of insulin, low-density lipoprotein, and high-sensitivity C-reactive protein. It is associated with lower diet quality, an increased intake of nutrient-poor foods, and a lower intake of vegetables and fruits.22 Recent studies have shown that interventions to promote earlier bedtimes can improve sleep duration in children.
Older adults have many sleeping issues, including insomnia, circadian rhythm sleep-wake disorders, sleep-related movement disorders, and sleep-breathing disorders. Additionally, the older population has increased sleep latency, decreased sleep efficiency and total sleep time, decreased REM sleep, more frequent nighttime awakenings, and more daytime napping.23 The increased sleep disturbance with age is mainly related to higher risk factors for sleep disorders than the aging process itself. Sleeping 5 or fewer hours is associated with an increased risk for obesity and central abdominal fat compared with those who sleep 7 to 8 hours per night.24 Similar to children and youth, older adults also show a strong correlation between inadequate sleep and obesity.24
The consequence: A vicious cycle
Obesity in turn leads to shorter sleep duration and more disruptions. This negatively affects the orexigenic system, and the resulting hormonal derangement promotes worsening obesity. It is a cycle of poor sleep causing obesity and obesity causing poor sleep. Insomnia, in combination with shorter (and longer) sleep times, also has been linked with obesity.25 These patients experience more daytime sleepiness, fatigue, and nighttime sleep disturbances, all correlated with decreased quality of life and higher prevalence of medical comorbidities.8,26 Additional comorbidities secondary to obesity, including gastroesophageal reflux, depression, and asthma, also have been linked to sleep disturbances.8
OSA is a common sleep complication associated with obesity. With the increasing prevalence of obesity, the prevalence of OSA is rising.8,27 Factors that heighten the risk for OSA are male sex, age 40 to 70 years, postmenopausal status, elevated BMI, and craniofacial and upper airway abnormality.28 However, the US Preventive Services Task Force found insufficient evidence to screen for or treat OSA in asymptomatic adults.28 Signs and symptoms of OSA include nighttime awakenings with choking, loud snoring, and feeling unrefreshed after sleep.29
OSA is caused by the intermittent narrowing and obstruction of the pharyngeal airway due to anatomical and structural irregularities or neuromuscular impairments. Untreated OSA is associated with cardiovascular disease and cardiac arrhythmias such as atrial fibrillation. Even with this correlation between obesity and sleep, it is estimated that 80% of OSA remains undiagnosed.30 Approximately half of primary care clinicians do not screen at-risk patients for OSA, and 90% do not use validated OSA screening tools.31 Screening tools that have been validated are the STOP, STOP-BANG, Epworth Sleepiness Scale, and 4-Variable Screening Tool. However, the US Department of Veterans Affairs and the US Department of Defense have a more recent guideline recommending STOP as an easier-to-administer screen for OSA.32 A positive result with a screening tool should be confirmed with polysomnography.32
Continue to: Intervention for OSA
Intervention for OSA. The longest randomized controlled study to date, Sleep AHEAD, evaluated over a period of 10 years the effect of weight loss on OSA severity achieved with either an intensive lifestyle intervention (ILI) or with diabetes support and education (DSE).33 OSA severity is rated on an Apnea-Hypopnea Index (AHI), with scores reflecting the number of sleep apnea events per hour. This study demonstrated that weight loss was associated with decreased OSA severity. At 4-year follow-up, the greater the weight loss with ILI intervention, the lower the patients’ OSA severity scores. The study found an average decrease in AHI of 0.68 events per hour for every kilogram of weight loss in the ILI group (P < .0001).33,34 Over the follow-up visits, the ILI participants had 7.4 events per hour, a more significantly reduced AHI than the DSE participants (P < .0001).33,34
Additionally, a small cohort of study participants achieved OSA remission (ILI, 34.4%; DSE, 22.2%), indicated by a low AHI score (< 5 events per hour). At the conclusion of the study, OSA severity decreased to a greater degree with ILI intervention.33,34
Alcohol and drug use can negatively influence sleep patterns and obesity. Higher alcohol consumption is associated with poorer sleep quality and higher chances of developing short sleep duration and snoring.35 Alcohol, a muscle relaxant, causes upper airway narrowing and reduced tongue muscle tone, thereby increasing snoring and OSA as demonstrated by increased AHI on polysomnography after alcohol intake. Alcohol also changes sleep architecture by increasing slow-wave sleep, decreasing REM sleep duration, and increasing sleep arousal in the second half of the night.36 Disrupted circadian rhythm after alcohol consumption was correlated with increased adenosine neurotransmitters derived from ethanol metabolism.37 Alcohol dependence may be related to other psychiatric symptoms, and chronic alcohol use eventually alters sleep mechanisms leading to persistent insomnia, further perpetuating adverse outcomes such as suicidal ideation.36 There are positive associations between beer drinking and measures of abdominal adiposity in men, and “the combination of short sleep duration [and] disinhibited eating … is associated with greater alcohol intake and excess weight.”38
Therefore, counsel patients to avoid alcohol since it is a modifiable risk factor with pervasive adverse health effects.
Many drugs have a profound effect on sleep patterns. Illicit drug use in particular can affect the brain’s neurotransmitter serotonin system. For example, ecstasy users have an increased risk for OSA.39 People with cocaine and heroin use disorder tend to have more sleep-maintenance insomnia.40
Continue to: In contrast, those with alcohol...
In contrast, those with alcohol or cannabis use disorder tend to have more sleep-onset insomnia.40 Not only do illicit drugs interrupt sleep, but daily tobacco use also has been correlated with increased insomnia and shorter sleep duration since nicotine is a stimulant.41
Insomnia is commonly treated with sedative antidepressants and hypnotics—eg, mirtazapine and olanzapine—that contribute to weight gain.42 In addition, other common pharmaceuticals used for sleep disorders, such as diphenhydramine, have sedative properties and tend to lead to weight gain.43 Because so many medications affect sleep and weight, carefully review patients’ medication lists and switch offending agents to weight-neutral drugs if possible.
Treatment and tools to improve sleep in patients with obesity
Given the strong correlation between obesity and sleep disorders, validated screening tools should be used to assess sleep quality, including onset and potential symptoms associated with poor sleep (TABLE 144). For weight management to succeed in patients with obesity, it is crucial to address sleep in addition to nutrition and physical activity.17,45
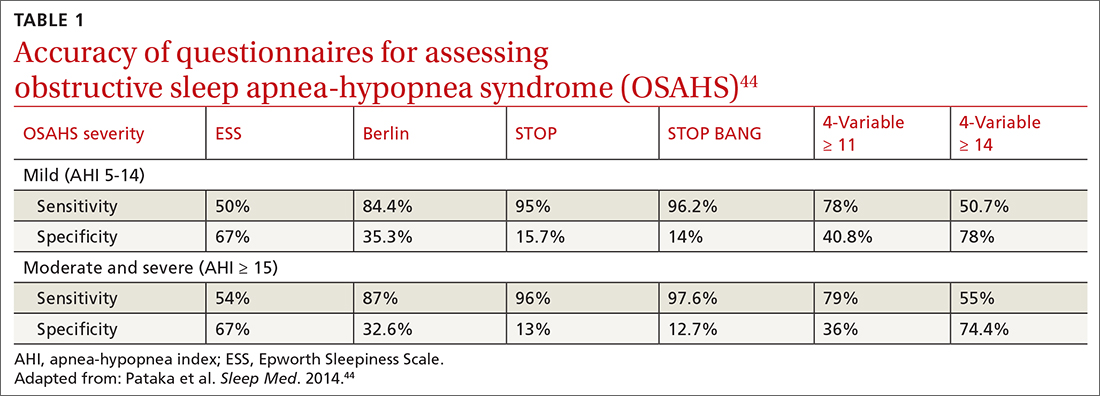
Physical activity has many benefits to overall health, especially for chronic diseases such as type 2 diabetes and hypertension. The Centers for Disease Control and Prevention recommends at least 150 minutes of moderate-intensity aerobic activity or 75 minutes of vigorous-intensity aerobic exercise per week in addition to muscle-strengthening activities 2 or more days per week.46 However, approximately 300 minutes of moderate-
Physical activity and diet in combination are vital, but diet restriction has a more substantial effect on weight loss than physical activity alone.48 Still, physical activity is essential in helping maintain and prevent weight regain.
Continue to: Nonpharmacologic interventions
Nonpharmacologic interventions include promoting greater sleep quality and quantity by emphasizing good sleep hygiene practices. Developing a practical and effective bedtime routine, creating a quiet sleep environment, and practicing healthy daily habits are essential components to sleep hygiene(TABLE 249,50). Relaxation techniques and cognitive behavioral therapy (CBT) also can help. CBT for insomnia (CBT-I) is the first-line intervention for chronic insomnia.51 Sleep restriction is a type of CBT used to treat insomnia, encouraging short-term sleep loss in the hopes of improving insomnia. A trial by Logue et al showed that patients with overweight and obesity randomized to undergo CBT with better sleep hygiene (nonpharmacologic) interventions had a greater mean weight loss percentage (5% vs 2%; P = .04) than did those who received CBT alone.52
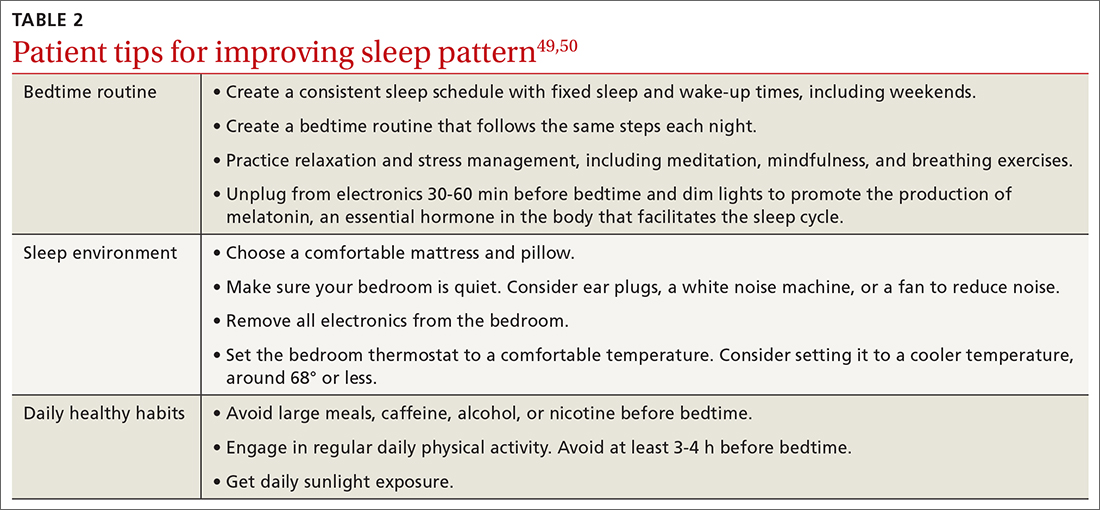
Eastern medicine including herbal interventions lack evidence of efficacy and safety. Further studies need to be done on the effects that chamomile, kava, valerian root (Valeriana officinalis), tryptophan, and Wu Ling (from mycelia Xylaria nigripes) might have on sleep.53
Proceed cautiously with medication. The American College of Physicians recommends a shared decision-making approach when considering pharmacologic therapy for chronic insomnia and the American Academy of Sleep Medicine (AASM) offers guidance on options.51,54 However, the evidence behind AASM sleep pharmacologic recommendations is weak, implying a lesser degree of confidence in the outcome and, therefore, in its appropriateness. Thus, it falls upon the clinician and patient to weigh the benefits and burdens of the pharmacologic treatments of insomnia. If indicated, medications suggested to treat sleep onset and sleep maintenance insomnia are eszopiclone, zolpidem, and temazepam. Zaleplon, triazolam, and ramelteon may improve sleep initiation. Suvorexant and doxepin are used for sleep-maintenance insomnia.54 Exploring patient preferences, cost of treatment, health care options, and available resources should all be considered.
CORRESPONDENCE
Ecler Ercole Jaqua, MD, MBA, FAAFP, AGSF, FACLM, DipABOM, Loma Linda University Health, 25455 Barton Road, Suite 206A, Loma Linda, CA 92354; [email protected]
1. Aminoff MJ, Boller F, Swaab DF. We spend about one-third of our life either sleeping or attempting to do so. Handb Clin Neurol. 2011;98:vii. doi: 10.1016/B978-0-444-52006-7.00047-2
2. Watson NF, Badr MS, Belenky G, et al. Recommended amount of sleep for a healthy adult: a joint consensus statement of the American Academy of Sleep Medicine and Sleep Research Society. Sleep. 2015;38:843-844. doi: 10.5665/sleep.4716
3. CDC. Sleep and sleep disorders, adults. Accessed September 21, 2023. www.cdc.gov/sleep/data-and-statistics/adults.html
4. Chattu VK, Manzar MD, Kumary S. The global problem of insufficient sleep and its serious public health implications. Healthcare (Basel). 2019;7:1. doi: 10.3390/healthcare7010001
5. Taheri S, Lin L, Austin D, et al. Short sleep duration is associated with reduced leptin, elevated ghrelin, and increased body mass index. PLoS Med. 2004;1:e62. doi: 10.1371/journal.pmed.0010062
6. Hafner M, Stepanek M, Taylor J, et al. Why sleep matters—the economic costs of insufficient sleep. Rand Health Q. 2017;6:11.
7. Hisler G, Twenge JM, Krizan Z. Associations between screen time and short sleep duration among adolescents varies by media type: evidence from a cohort study. Sleep Med. 2020;66:92-102. doi: 10.1016/j.sleep.2019.08.007
8. Ogilvie RP, Patel SR. The epidemiology of sleep and obesity. Sleep Health. 2017;3:383-388. doi: 10.1016/j.sleh.2017.07.013
9. CDC. Sleep and sleep disorders: How much sleep do I need? Accessed September 21, 2023. www.cdc.gov/sleep/about_sleep/how_much_sleep.html
10. van Egmond LT, Meth EMS, Engström J, et al. Effects of acute sleep loss on leptin, ghrelin, and adiponectin in adults with healthy weight and obesity: a laboratory study. Obesity (Silver Spring). 2023;31:635-641. doi: 10.1002/oby.23616
11. Spiegel K, Tasali E, Penev P, et al. Brief communication: sleep curtailment in healthy young men is associated with decreased leptin levels, elevated ghrelin levels, and increased hunger and appetite. Ann Intern Med. 2004;141:846-850. doi: 10.7326/0003-4819-141-11-200412070-00008
12. Antza C, Kostopoulos G, Mostafa S, et al. The links between sleep duration, obesity and type 2 diabetes mellitus. J Endocrinol. 2021;252:125-141. doi: 10.1530/JOE-21-0155
13. Baron KG, Reid KJ, Kern AS, et al. Role of sleep timing in caloric intake and BMI. Obesity (Silver Spring). 2011;19:1374-1381. doi: 10.1038/oby.2011.100
14. Liu XY, Zheng CL, Xu C, et al. Nighttime snacking is associated with risk of obesity and hyperglycemia in adults: a cross-sectional survey from Chinese adult teachers J Biomed Res. 2017;31:541-547. doi: 10.7555/JBR.31.20160083
15. Cai Z, Yang Y, Zhang J, et al. The relationship between daytime napping and obesity: a systematic review and meta-analysis. Sci Rep. 2023.13:12124. doi: 10.1038/s41598-023-37883-7
16. Nedeltcheva AV, Kilkus JM, Imperial J, et al. Insufficient sleep undermines dietary efforts to reduce adiposity. Ann Intern Med. 2010;153:435-441. doi: 10.7326/0003-4819-153-7-201010050-00006
17. Chaput JP, Tremblay A. Adequate sleep to improve the treatment of obesity. CMAJ. 2012;184:1975-1976. doi: 10.1503/cmaj.120876
18. Kelsey MM, Zaepfel A, Bjornstad P, et al. Age-related consequences of childhood obesity. Gerontology. 2014;60:222-228. doi: 10.1159/000356023
19. Fryar CD, Carroll MD, Afful J. Prevalence of overweight, obesity, and severe obesity among children and adolescents aged 2-19 years: United States, 1963-1965 through 2017-2018. National Center for Health Statistics Health E-Stats. Updated January 29, 2021. Accessed September 21, 2021. www.cdc.gov/nchs/data/hestat/obesity-child-17-18/overweight-obesity-child-H.pdf
20. Fatima Y, Doi SAR, Mamun AA. Sleep quality and obesity in young subjects: a meta-analysis. Obes Rev. 2016;17:1154-1166. doi: 10.1111/obr.12444
21. Gohil A, Hannon TS. Poor sleep and obesity: concurrent epidemics in adolescent youth. Front Endocrinol. 2018;9:364. doi: 10.3389/fendo.2018.00364
22. Golley RK, Maher CA, Matricciani L, et al. Sleep duration or bedtime? Exploring the association between sleep timing behaviour, diet and BMI in children and adolescents. Int J Obes (Lond). 2013;37:546-551. doi: 10.1038/ijo.2012.212
23. Alessi CA. Sleep issues. In: Harper GM, Lyons WL, Potter JF, eds. Geriatrics Review Syllabus (GRS 10). Updated January 2021. Accessed August 29, 2023. http://geriatricscareonline.org
24. Patel SR, Blackwell T, Redline S, et al. The association between sleep duration and obesity in older adults. Int J Obes (Lond). 2008;32:1825-1834. doi: 10.1038/ijo.2008.198
25. Cai GH, Theorell-Haglöw J, Janson C, et al. Insomnia symptoms and sleep duration and their combined effects in relation to associations with obesity and central obesity. Sleep Med. 2018;46:81-87. doi: 10.1016/j.sleep.2018.03.009
26. Beccuti G, Pannain S. Sleep and obesity. Curr Opin Clin Nutr Metab Care. 2011;14:402-412. doi: 10.1097/MCO.0b013 e3283479109
27. Franklin KA, Lindberg E. Obstructive sleep apnea is a common disorder in the population–a review on the epidemiology of sleep apnea. J Thorac Dis. 2015;7:1311-1322. doi: 10.3978/j.issn.2072-1439.2015.06.11
28. USPSTF. Bibbins-Domingo K, Grossman DC, Curry SJ, et al. Screening for obstructive sleep apnea in adults: US Preventive Services Task Force recommendation statement. JAMA. 2017;317:407-414. doi: 10.1001/jama.2016.20325
29. Goyal M, Johnson J. Obstructive sleep apnea diagnosis and management. Mo Med. 2017;114:120-124.
30. American Academy of Sleep Medicine. Hidden health crisis costing America billions: underdiagnosing and undertreating obstructive sleep apnea draining healthcare system. 2016. Accessed September 25, 2023. https://aasm.org/wp-content/uploads/2017/10/sleep-apnea-economic-crisis.pdf
31. Devaraj, NK. Knowledge, attitude, and practice regarding obstructive sleep apnea among primary care physicians. Sleep Breath. 2020;24:1581-1590. doi: 10.1007/s11325-020-02040-1
32. Mysliwiec V, Martin JL, Ulmer CS, et al. The management of chronic insomnia disorder and obstructive sleep apnea: synopsis of the 2019 U.S. Department of Veterans Affairs and U.S. Department of Defense Clinical Practice Guidelines. Ann Intern Med. 2020;172:325-336. doi: 10.7326/M19-3575
33. Kuna ST, Reboussin DM, Strotmeyer ES, et al. Effects of weight loss on obstructive sleep apnea severity. Ten-year results of the Sleep AHEAD study. Am J Respir Crit Care Med. 2021;203:221-229. doi: 10.1164/rccm.201912-2511OC
34. St-Onge MP, Tasali E. Weight loss is integral to obstructive sleep apnea management. Ten-year follow-up in Sleep AHEAD. Am J Respir Crit Care Med. 2021;203:161-162. doi: 10.1164/rccm.202007-2906ED
35. Zheng D, Yuan X, Ma C, et al. Alcohol consumption and sleep quality: a community-based study. Public Health Nutr. 2021;24:4851-4858. doi: 10.1017/S1368980020004553
36. Chakravorty S, Chaudhary NS, Brower KJ. Alcohol dependence and its relationship with insomnia and other sleep disorders. Alcohol Clin Exp Res. 2016;40:2271-2282. doi: 10.1111/acer.13217
37. Elmenhorst EM, Elmenhorst D, Benderoth S, et al. Cognitive impairments by alcohol and sleep deprivation indicate trait characteristics and a potential role for adenosine A1 receptors. Proc Natl Acad Sci U S A. 2018;115:8009-8014. doi: 10.1073/pnas.1803770115
38. Traversy G, Chaput JP. Alcohol consumption and obesity: an update. Curr Obes Rep. 2015;4:122-130. doi: 10.1007/s13679-014-0129-4
39. McCann UD, Sgambati FP, Schwartz AR, et al. Sleep apnea in young abstinent recreational MDMA (“ecstasy”) consumers. Neurology. 2009;73:2011-2017. doi: 10.1212/WNL.0b013e3181c51a62
40. Grau-López L, Grau-López L, Daigre C, et al. Insomnia symptoms in patients with substance use disorders during detoxification and associated clinical features. Front Psychiatry. 2020;11:540022. doi: 10.3389/fpsyt.2020.540022
41. Boehm MA, Lei QM, Lloyd RM, et al. Depression, anxiety, and tobacco use: overlapping impediments to sleep in a national sample of college students. J Am Coll Health. 2016;64:565-574. doi: 10.1080/07448481.2016.1205073
42. Gracious BL, Meyer AE. Psychotropic-induced weight gain and potential pharmacologic treatment strategies. Psychiatry (Edgmont). 2005;2:36-42.
43. Ratliff JC, Barber JA, Palmese LB, et al. Association of prescription H1 antihistamine use with obesity: results from the National Health and Nutrition Examination Survey. Obesity (Silver Spring). 2010;18:2398-2400. doi: 10.1038/oby.2010.176
44. Pataka A, Daskalopoulou E, Kalamaras G, et al. Evaluation of five different questionnaires for assessing sleep apnea syndrome in a sleep clinic. Sleep Med. 2014;15:776-781. doi: 10.1016/j.sleep.2014.03.012
45. Kline CE, Chasens ER, Bizhanova Z, et al. The association between sleep health and weight change during a 12-month behavioral weight loss intervention. Int J Obes (Lond). 2021;45:639-649. doi: 10.1038/s41366-020-00728-8
46. CDC. How much physical activity do adults need? Accessed August 23, 2023. www.cdc.gov/physicalactivity/basics/adults/index.htm
47. Flack KD, Hays HM, Moreland J, et al. Exercise for weight loss: further evaluating energy compensation with exercise. Med Sci Sports Exerc. 2020;52:2466-2475. doi: 10.1249/MSS.0000000000002376
48. Swift DL, Johannsen NM, Lavie CJ, et al. The role of exercise and physical activity in weight loss and maintenance. Prog Cardiovasc Dis. 2014;56:441-447. doi: 10.1016/j.pcad.2013.09.012
49. Irish LA, Kline CE, Gunn HE, et al. The role of sleep hygiene in promoting public health: a review of empirical evidence. Sleep Med Rev. 2015;22:23-36. doi: 10.1016/j.smrv.2014.10.001
50. CDC. Tips for better sleep. 2022. Accessed August 4, 2023. www.cdc.gov/sleep/about_sleep/sleep_hygiene.html
51. Qaseem A, Kansagara D, Forciea MA, et al. Management of chronic insomnia disorder in adults: a clinical practice guideline from the American College of Physicians. Ann Intern Med. 2016;165:125-133. doi: 10.7326/M15-2175
52. Logue EE, Bourguet CC, Palmieri PA, et al. The better weight-better sleep study: a pilot intervention in primary care. Am J Health Behav. 2012;36:319-334. doi: 10.5993/AJHB.36.3.4
53. Leach MJ, Page AT. Herbal medicine for insomnia: a systematic review and meta-analysis. Sleep Med Rev. 2015;24:1-12. doi: 10.1016/j.smrv.2014.12.003
54. Sateia MJ, Buysse DJ, Krystal AD, et al. Clinical practice guideline for the pharmacologic treatment of chronic insomnia in adults: an American Academy of Sleep Medicine clinical practice guideline. J Clin Sleep Med. 2017;13:307-349. doi: 10.5664/jcsm.6470
Sleep is fundamental to overall health and longevity, with the average person spending about one-third of their life sleeping.1 Adequate sleep is critical for optimal cognition, memory consolidation, mood regulation, metabolism, appetite regulation, and immune and hormone functioning. According to the American Academy of Sleep Medicine and the Sleep Research Society, adults should sleep at least 7 hours per night on a regular basis “to promote optimal health.”2 Yet, between 2013 and 2020, only about 65% of adults in the United States were meeting this amount.3 Insufficient sleep is associated with an increased risk for chronic health conditions, including obesity, diabetes, cardiovascular diseases, and even premature death.4

In a population-based longitudinal study of sleep disorders, short sleep duration was associated with increased body mass index (BMI), low blood levels of leptin, and high ghrelin levels.5 In addition to physical impairments, poor sleep can impair cognitive performance and lead to vehicular accidents and increased accidents at work.4 The potential economic impact that this may have is significant, and includes increased costs and loss of productivity in the workplace.6
Many factors may contribute to short sleep duration: environment, mental and physical condition, and social influences such as occupation, family responsibilities, travel, group activities, and personal care. Furthermore, the rapidly evolving and developing media, communication, and entertainment industries are already strongly implicated in poor sleep quality and quantity, both contributing to excessive daytime sleepiness.7 Poor sleep quality is most notable in modern societies, and it correlates with the increasing prevalence of obesity, likely due to sleep’s effect on food consumption and physical activity.8 Optimizing a person’s sleep will improve overall health and longevity by inhibiting the development of chronic disease.
How insufficient sleep raises the risk for obesity
Not only is sleep beneficial for brain health, memory, learning, and growth, its effect on food consumption and physical activity likely correlates with the increased prevalence of obesity in modern society. Yet the optimal amount of sleep is controversial, and current recommendations of 7 or more hours of sleep per night for adults are derived from expert panels only.2 The recommended sleep duration for children is longer, and it varies by age.9 The quality of sleep and its impact on neuroendocrine hormones, not just the quantity of sleep, needs to be factored into these recommendations.
Sleep restriction activates the orexigenic system via the hormones leptin and ghrelin. These hormones control the food reward system, essentially increasing hunger and food intake. Leptin, created by white adipose tissue, is responsible for satiety and decreased food consumption.10 Ghrelin, made by oxyntic glands in the stomach, is responsible for the sensation of hunger.
In a 2004 study by Spiegel et al,11 leptin and ghrelin levels were measured during 2 days of sleep restriction (4 hours in bed) and sleep extension (10 hours in bed). Sleep restriction was associated with a decrease in leptin levels and an increase in ghrelin levels. The researchers reported that participants experienced an increase in hunger and appetite—especially for calorie-dense foods with high carbohydrate content.
Although research design has limitations with predominantly self-reported sleep data, studies have shown that short sleep time leads to increased food intake by increasing hunger signals and craving of unhealthy foods, and by providing more opportunities to eat while awake. It also may lead to decreased physical activity, creating a sedentary lifestyle that further encourages obesity.8 Reduced sleep is even correlated to decreased efficacy of weight-loss treatments.12
Continue to: Other sleep characteristics weakly correlated with obesity
Other sleep characteristics weakly correlated with obesity are sleep variability, timing, efficiency, quality, and daytime napping.8 Sleep variability causes dysregulation of eating patterns, leading to increased food intake. A shift to later sleep and waking times often results in higher consumption of calories after 8
Poor sleep efficiency and quality decreases N3-stage (deep non-REM) sleep, affects the autonomic nervous system, and has been associated with increased abdominal obesity. Daytime napping, which can cause irregular circadian rhythms and sleep schedules, is associated with increased obesity.15 Thus, each component of sleep needs to be assessed to promote optimal regulation of the orexigenic system.
Another study showed that inadequate sleep not only promotes unhealthy lifestyle habits that can lead to obesity but also decreases the ability to lose weight.16 This small study with 10 overweight patients provided its subjects with a controlled caloric intake over 2 weeks. Patients spent two 14-day periods 3 months apart in the laboratory, divided into 2 time-in-bed arms of 8.5 and 5.5 hours per night. Neuroendocrine changes caused by decreased sleep were associated with a significant lean body mass loss while conserving energy-dense fat.16 This study highlights the importance of sleep hygiene counseling when developing a weight-management plan with patients.
Sleep, and its many components, play an integral role in the prevention and treatment of obesity.17 Poor sleep will increase the risk for obesity and hinder its treatment. Therefore, sleep quality and duration are vital components of obesity management.
The sleep–obesity link in children and the elderly
Childhood obesity is linked to several chronic diseases in adulthood, including type 2 diabetes, cardiovascular disease, nonalcoholic fatty liver disease, asthma, and obstructive sleep apnea (OSA).18 According to 2017-2018 NHANES (National Health and Nutrition Examination Surveys) data, obesity (BMI ≥ 95th percentile) prevalence among children and adolescents was reported at 19.3% and severe obesity (BMI ≥ 120% of the 95th percentile) at 6.1%. Pediatric overweight prevalence (≥ 85th percentile and < 95th percentile) was 16.1%.19
Continue to: Although poor sleep is associated...
Although poor sleep is associated with increased risk for obesity, there is no proven cause-effect relationship.20 Nutrition and physical activity have been identified as 2 critical factors in childhood obesity, but sleep health also needs to be investigated. Shorter sleep duration is strongly associated with the development of obesity. Furthermore, children with obesity are more likely to have shorter sleep duration.21 A short sleep duration alters plasma levels of insulin, low-density lipoprotein, and high-sensitivity C-reactive protein. It is associated with lower diet quality, an increased intake of nutrient-poor foods, and a lower intake of vegetables and fruits.22 Recent studies have shown that interventions to promote earlier bedtimes can improve sleep duration in children.
Older adults have many sleeping issues, including insomnia, circadian rhythm sleep-wake disorders, sleep-related movement disorders, and sleep-breathing disorders. Additionally, the older population has increased sleep latency, decreased sleep efficiency and total sleep time, decreased REM sleep, more frequent nighttime awakenings, and more daytime napping.23 The increased sleep disturbance with age is mainly related to higher risk factors for sleep disorders than the aging process itself. Sleeping 5 or fewer hours is associated with an increased risk for obesity and central abdominal fat compared with those who sleep 7 to 8 hours per night.24 Similar to children and youth, older adults also show a strong correlation between inadequate sleep and obesity.24
The consequence: A vicious cycle
Obesity in turn leads to shorter sleep duration and more disruptions. This negatively affects the orexigenic system, and the resulting hormonal derangement promotes worsening obesity. It is a cycle of poor sleep causing obesity and obesity causing poor sleep. Insomnia, in combination with shorter (and longer) sleep times, also has been linked with obesity.25 These patients experience more daytime sleepiness, fatigue, and nighttime sleep disturbances, all correlated with decreased quality of life and higher prevalence of medical comorbidities.8,26 Additional comorbidities secondary to obesity, including gastroesophageal reflux, depression, and asthma, also have been linked to sleep disturbances.8
OSA is a common sleep complication associated with obesity. With the increasing prevalence of obesity, the prevalence of OSA is rising.8,27 Factors that heighten the risk for OSA are male sex, age 40 to 70 years, postmenopausal status, elevated BMI, and craniofacial and upper airway abnormality.28 However, the US Preventive Services Task Force found insufficient evidence to screen for or treat OSA in asymptomatic adults.28 Signs and symptoms of OSA include nighttime awakenings with choking, loud snoring, and feeling unrefreshed after sleep.29
OSA is caused by the intermittent narrowing and obstruction of the pharyngeal airway due to anatomical and structural irregularities or neuromuscular impairments. Untreated OSA is associated with cardiovascular disease and cardiac arrhythmias such as atrial fibrillation. Even with this correlation between obesity and sleep, it is estimated that 80% of OSA remains undiagnosed.30 Approximately half of primary care clinicians do not screen at-risk patients for OSA, and 90% do not use validated OSA screening tools.31 Screening tools that have been validated are the STOP, STOP-BANG, Epworth Sleepiness Scale, and 4-Variable Screening Tool. However, the US Department of Veterans Affairs and the US Department of Defense have a more recent guideline recommending STOP as an easier-to-administer screen for OSA.32 A positive result with a screening tool should be confirmed with polysomnography.32
Continue to: Intervention for OSA
Intervention for OSA. The longest randomized controlled study to date, Sleep AHEAD, evaluated over a period of 10 years the effect of weight loss on OSA severity achieved with either an intensive lifestyle intervention (ILI) or with diabetes support and education (DSE).33 OSA severity is rated on an Apnea-Hypopnea Index (AHI), with scores reflecting the number of sleep apnea events per hour. This study demonstrated that weight loss was associated with decreased OSA severity. At 4-year follow-up, the greater the weight loss with ILI intervention, the lower the patients’ OSA severity scores. The study found an average decrease in AHI of 0.68 events per hour for every kilogram of weight loss in the ILI group (P < .0001).33,34 Over the follow-up visits, the ILI participants had 7.4 events per hour, a more significantly reduced AHI than the DSE participants (P < .0001).33,34
Additionally, a small cohort of study participants achieved OSA remission (ILI, 34.4%; DSE, 22.2%), indicated by a low AHI score (< 5 events per hour). At the conclusion of the study, OSA severity decreased to a greater degree with ILI intervention.33,34
Alcohol and drug use can negatively influence sleep patterns and obesity. Higher alcohol consumption is associated with poorer sleep quality and higher chances of developing short sleep duration and snoring.35 Alcohol, a muscle relaxant, causes upper airway narrowing and reduced tongue muscle tone, thereby increasing snoring and OSA as demonstrated by increased AHI on polysomnography after alcohol intake. Alcohol also changes sleep architecture by increasing slow-wave sleep, decreasing REM sleep duration, and increasing sleep arousal in the second half of the night.36 Disrupted circadian rhythm after alcohol consumption was correlated with increased adenosine neurotransmitters derived from ethanol metabolism.37 Alcohol dependence may be related to other psychiatric symptoms, and chronic alcohol use eventually alters sleep mechanisms leading to persistent insomnia, further perpetuating adverse outcomes such as suicidal ideation.36 There are positive associations between beer drinking and measures of abdominal adiposity in men, and “the combination of short sleep duration [and] disinhibited eating … is associated with greater alcohol intake and excess weight.”38
Therefore, counsel patients to avoid alcohol since it is a modifiable risk factor with pervasive adverse health effects.
Many drugs have a profound effect on sleep patterns. Illicit drug use in particular can affect the brain’s neurotransmitter serotonin system. For example, ecstasy users have an increased risk for OSA.39 People with cocaine and heroin use disorder tend to have more sleep-maintenance insomnia.40
Continue to: In contrast, those with alcohol...
In contrast, those with alcohol or cannabis use disorder tend to have more sleep-onset insomnia.40 Not only do illicit drugs interrupt sleep, but daily tobacco use also has been correlated with increased insomnia and shorter sleep duration since nicotine is a stimulant.41
Insomnia is commonly treated with sedative antidepressants and hypnotics—eg, mirtazapine and olanzapine—that contribute to weight gain.42 In addition, other common pharmaceuticals used for sleep disorders, such as diphenhydramine, have sedative properties and tend to lead to weight gain.43 Because so many medications affect sleep and weight, carefully review patients’ medication lists and switch offending agents to weight-neutral drugs if possible.
Treatment and tools to improve sleep in patients with obesity
Given the strong correlation between obesity and sleep disorders, validated screening tools should be used to assess sleep quality, including onset and potential symptoms associated with poor sleep (TABLE 144). For weight management to succeed in patients with obesity, it is crucial to address sleep in addition to nutrition and physical activity.17,45

Physical activity has many benefits to overall health, especially for chronic diseases such as type 2 diabetes and hypertension. The Centers for Disease Control and Prevention recommends at least 150 minutes of moderate-intensity aerobic activity or 75 minutes of vigorous-intensity aerobic exercise per week in addition to muscle-strengthening activities 2 or more days per week.46 However, approximately 300 minutes of moderate-
Physical activity and diet in combination are vital, but diet restriction has a more substantial effect on weight loss than physical activity alone.48 Still, physical activity is essential in helping maintain and prevent weight regain.
Continue to: Nonpharmacologic interventions
Nonpharmacologic interventions include promoting greater sleep quality and quantity by emphasizing good sleep hygiene practices. Developing a practical and effective bedtime routine, creating a quiet sleep environment, and practicing healthy daily habits are essential components to sleep hygiene(TABLE 249,50). Relaxation techniques and cognitive behavioral therapy (CBT) also can help. CBT for insomnia (CBT-I) is the first-line intervention for chronic insomnia.51 Sleep restriction is a type of CBT used to treat insomnia, encouraging short-term sleep loss in the hopes of improving insomnia. A trial by Logue et al showed that patients with overweight and obesity randomized to undergo CBT with better sleep hygiene (nonpharmacologic) interventions had a greater mean weight loss percentage (5% vs 2%; P = .04) than did those who received CBT alone.52

Eastern medicine including herbal interventions lack evidence of efficacy and safety. Further studies need to be done on the effects that chamomile, kava, valerian root (Valeriana officinalis), tryptophan, and Wu Ling (from mycelia Xylaria nigripes) might have on sleep.53
Proceed cautiously with medication. The American College of Physicians recommends a shared decision-making approach when considering pharmacologic therapy for chronic insomnia and the American Academy of Sleep Medicine (AASM) offers guidance on options.51,54 However, the evidence behind AASM sleep pharmacologic recommendations is weak, implying a lesser degree of confidence in the outcome and, therefore, in its appropriateness. Thus, it falls upon the clinician and patient to weigh the benefits and burdens of the pharmacologic treatments of insomnia. If indicated, medications suggested to treat sleep onset and sleep maintenance insomnia are eszopiclone, zolpidem, and temazepam. Zaleplon, triazolam, and ramelteon may improve sleep initiation. Suvorexant and doxepin are used for sleep-maintenance insomnia.54 Exploring patient preferences, cost of treatment, health care options, and available resources should all be considered.
CORRESPONDENCE
Ecler Ercole Jaqua, MD, MBA, FAAFP, AGSF, FACLM, DipABOM, Loma Linda University Health, 25455 Barton Road, Suite 206A, Loma Linda, CA 92354; [email protected]
Sleep is fundamental to overall health and longevity, with the average person spending about one-third of their life sleeping.1 Adequate sleep is critical for optimal cognition, memory consolidation, mood regulation, metabolism, appetite regulation, and immune and hormone functioning. According to the American Academy of Sleep Medicine and the Sleep Research Society, adults should sleep at least 7 hours per night on a regular basis “to promote optimal health.”2 Yet, between 2013 and 2020, only about 65% of adults in the United States were meeting this amount.3 Insufficient sleep is associated with an increased risk for chronic health conditions, including obesity, diabetes, cardiovascular diseases, and even premature death.4

In a population-based longitudinal study of sleep disorders, short sleep duration was associated with increased body mass index (BMI), low blood levels of leptin, and high ghrelin levels.5 In addition to physical impairments, poor sleep can impair cognitive performance and lead to vehicular accidents and increased accidents at work.4 The potential economic impact that this may have is significant, and includes increased costs and loss of productivity in the workplace.6
Many factors may contribute to short sleep duration: environment, mental and physical condition, and social influences such as occupation, family responsibilities, travel, group activities, and personal care. Furthermore, the rapidly evolving and developing media, communication, and entertainment industries are already strongly implicated in poor sleep quality and quantity, both contributing to excessive daytime sleepiness.7 Poor sleep quality is most notable in modern societies, and it correlates with the increasing prevalence of obesity, likely due to sleep’s effect on food consumption and physical activity.8 Optimizing a person’s sleep will improve overall health and longevity by inhibiting the development of chronic disease.
How insufficient sleep raises the risk for obesity
Not only is sleep beneficial for brain health, memory, learning, and growth, its effect on food consumption and physical activity likely correlates with the increased prevalence of obesity in modern society. Yet the optimal amount of sleep is controversial, and current recommendations of 7 or more hours of sleep per night for adults are derived from expert panels only.2 The recommended sleep duration for children is longer, and it varies by age.9 The quality of sleep and its impact on neuroendocrine hormones, not just the quantity of sleep, needs to be factored into these recommendations.
Sleep restriction activates the orexigenic system via the hormones leptin and ghrelin. These hormones control the food reward system, essentially increasing hunger and food intake. Leptin, created by white adipose tissue, is responsible for satiety and decreased food consumption.10 Ghrelin, made by oxyntic glands in the stomach, is responsible for the sensation of hunger.
In a 2004 study by Spiegel et al,11 leptin and ghrelin levels were measured during 2 days of sleep restriction (4 hours in bed) and sleep extension (10 hours in bed). Sleep restriction was associated with a decrease in leptin levels and an increase in ghrelin levels. The researchers reported that participants experienced an increase in hunger and appetite—especially for calorie-dense foods with high carbohydrate content.
Although research design has limitations with predominantly self-reported sleep data, studies have shown that short sleep time leads to increased food intake by increasing hunger signals and craving of unhealthy foods, and by providing more opportunities to eat while awake. It also may lead to decreased physical activity, creating a sedentary lifestyle that further encourages obesity.8 Reduced sleep is even correlated to decreased efficacy of weight-loss treatments.12
Continue to: Other sleep characteristics weakly correlated with obesity
Other sleep characteristics weakly correlated with obesity are sleep variability, timing, efficiency, quality, and daytime napping.8 Sleep variability causes dysregulation of eating patterns, leading to increased food intake. A shift to later sleep and waking times often results in higher consumption of calories after 8
Poor sleep efficiency and quality decreases N3-stage (deep non-REM) sleep, affects the autonomic nervous system, and has been associated with increased abdominal obesity. Daytime napping, which can cause irregular circadian rhythms and sleep schedules, is associated with increased obesity.15 Thus, each component of sleep needs to be assessed to promote optimal regulation of the orexigenic system.
Another study showed that inadequate sleep not only promotes unhealthy lifestyle habits that can lead to obesity but also decreases the ability to lose weight.16 This small study with 10 overweight patients provided its subjects with a controlled caloric intake over 2 weeks. Patients spent two 14-day periods 3 months apart in the laboratory, divided into 2 time-in-bed arms of 8.5 and 5.5 hours per night. Neuroendocrine changes caused by decreased sleep were associated with a significant lean body mass loss while conserving energy-dense fat.16 This study highlights the importance of sleep hygiene counseling when developing a weight-management plan with patients.
Sleep, and its many components, play an integral role in the prevention and treatment of obesity.17 Poor sleep will increase the risk for obesity and hinder its treatment. Therefore, sleep quality and duration are vital components of obesity management.
The sleep–obesity link in children and the elderly
Childhood obesity is linked to several chronic diseases in adulthood, including type 2 diabetes, cardiovascular disease, nonalcoholic fatty liver disease, asthma, and obstructive sleep apnea (OSA).18 According to 2017-2018 NHANES (National Health and Nutrition Examination Surveys) data, obesity (BMI ≥ 95th percentile) prevalence among children and adolescents was reported at 19.3% and severe obesity (BMI ≥ 120% of the 95th percentile) at 6.1%. Pediatric overweight prevalence (≥ 85th percentile and < 95th percentile) was 16.1%.19
Continue to: Although poor sleep is associated...
Although poor sleep is associated with increased risk for obesity, there is no proven cause-effect relationship.20 Nutrition and physical activity have been identified as 2 critical factors in childhood obesity, but sleep health also needs to be investigated. Shorter sleep duration is strongly associated with the development of obesity. Furthermore, children with obesity are more likely to have shorter sleep duration.21 A short sleep duration alters plasma levels of insulin, low-density lipoprotein, and high-sensitivity C-reactive protein. It is associated with lower diet quality, an increased intake of nutrient-poor foods, and a lower intake of vegetables and fruits.22 Recent studies have shown that interventions to promote earlier bedtimes can improve sleep duration in children.
Older adults have many sleeping issues, including insomnia, circadian rhythm sleep-wake disorders, sleep-related movement disorders, and sleep-breathing disorders. Additionally, the older population has increased sleep latency, decreased sleep efficiency and total sleep time, decreased REM sleep, more frequent nighttime awakenings, and more daytime napping.23 The increased sleep disturbance with age is mainly related to higher risk factors for sleep disorders than the aging process itself. Sleeping 5 or fewer hours is associated with an increased risk for obesity and central abdominal fat compared with those who sleep 7 to 8 hours per night.24 Similar to children and youth, older adults also show a strong correlation between inadequate sleep and obesity.24
The consequence: A vicious cycle
Obesity in turn leads to shorter sleep duration and more disruptions. This negatively affects the orexigenic system, and the resulting hormonal derangement promotes worsening obesity. It is a cycle of poor sleep causing obesity and obesity causing poor sleep. Insomnia, in combination with shorter (and longer) sleep times, also has been linked with obesity.25 These patients experience more daytime sleepiness, fatigue, and nighttime sleep disturbances, all correlated with decreased quality of life and higher prevalence of medical comorbidities.8,26 Additional comorbidities secondary to obesity, including gastroesophageal reflux, depression, and asthma, also have been linked to sleep disturbances.8
OSA is a common sleep complication associated with obesity. With the increasing prevalence of obesity, the prevalence of OSA is rising.8,27 Factors that heighten the risk for OSA are male sex, age 40 to 70 years, postmenopausal status, elevated BMI, and craniofacial and upper airway abnormality.28 However, the US Preventive Services Task Force found insufficient evidence to screen for or treat OSA in asymptomatic adults.28 Signs and symptoms of OSA include nighttime awakenings with choking, loud snoring, and feeling unrefreshed after sleep.29
OSA is caused by the intermittent narrowing and obstruction of the pharyngeal airway due to anatomical and structural irregularities or neuromuscular impairments. Untreated OSA is associated with cardiovascular disease and cardiac arrhythmias such as atrial fibrillation. Even with this correlation between obesity and sleep, it is estimated that 80% of OSA remains undiagnosed.30 Approximately half of primary care clinicians do not screen at-risk patients for OSA, and 90% do not use validated OSA screening tools.31 Screening tools that have been validated are the STOP, STOP-BANG, Epworth Sleepiness Scale, and 4-Variable Screening Tool. However, the US Department of Veterans Affairs and the US Department of Defense have a more recent guideline recommending STOP as an easier-to-administer screen for OSA.32 A positive result with a screening tool should be confirmed with polysomnography.32
Continue to: Intervention for OSA
Intervention for OSA. The longest randomized controlled study to date, Sleep AHEAD, evaluated over a period of 10 years the effect of weight loss on OSA severity achieved with either an intensive lifestyle intervention (ILI) or with diabetes support and education (DSE).33 OSA severity is rated on an Apnea-Hypopnea Index (AHI), with scores reflecting the number of sleep apnea events per hour. This study demonstrated that weight loss was associated with decreased OSA severity. At 4-year follow-up, the greater the weight loss with ILI intervention, the lower the patients’ OSA severity scores. The study found an average decrease in AHI of 0.68 events per hour for every kilogram of weight loss in the ILI group (P < .0001).33,34 Over the follow-up visits, the ILI participants had 7.4 events per hour, a more significantly reduced AHI than the DSE participants (P < .0001).33,34
Additionally, a small cohort of study participants achieved OSA remission (ILI, 34.4%; DSE, 22.2%), indicated by a low AHI score (< 5 events per hour). At the conclusion of the study, OSA severity decreased to a greater degree with ILI intervention.33,34
Alcohol and drug use can negatively influence sleep patterns and obesity. Higher alcohol consumption is associated with poorer sleep quality and higher chances of developing short sleep duration and snoring.35 Alcohol, a muscle relaxant, causes upper airway narrowing and reduced tongue muscle tone, thereby increasing snoring and OSA as demonstrated by increased AHI on polysomnography after alcohol intake. Alcohol also changes sleep architecture by increasing slow-wave sleep, decreasing REM sleep duration, and increasing sleep arousal in the second half of the night.36 Disrupted circadian rhythm after alcohol consumption was correlated with increased adenosine neurotransmitters derived from ethanol metabolism.37 Alcohol dependence may be related to other psychiatric symptoms, and chronic alcohol use eventually alters sleep mechanisms leading to persistent insomnia, further perpetuating adverse outcomes such as suicidal ideation.36 There are positive associations between beer drinking and measures of abdominal adiposity in men, and “the combination of short sleep duration [and] disinhibited eating … is associated with greater alcohol intake and excess weight.”38
Therefore, counsel patients to avoid alcohol since it is a modifiable risk factor with pervasive adverse health effects.
Many drugs have a profound effect on sleep patterns. Illicit drug use in particular can affect the brain’s neurotransmitter serotonin system. For example, ecstasy users have an increased risk for OSA.39 People with cocaine and heroin use disorder tend to have more sleep-maintenance insomnia.40
Continue to: In contrast, those with alcohol...
In contrast, those with alcohol or cannabis use disorder tend to have more sleep-onset insomnia.40 Not only do illicit drugs interrupt sleep, but daily tobacco use also has been correlated with increased insomnia and shorter sleep duration since nicotine is a stimulant.41
Insomnia is commonly treated with sedative antidepressants and hypnotics—eg, mirtazapine and olanzapine—that contribute to weight gain.42 In addition, other common pharmaceuticals used for sleep disorders, such as diphenhydramine, have sedative properties and tend to lead to weight gain.43 Because so many medications affect sleep and weight, carefully review patients’ medication lists and switch offending agents to weight-neutral drugs if possible.
Treatment and tools to improve sleep in patients with obesity
Given the strong correlation between obesity and sleep disorders, validated screening tools should be used to assess sleep quality, including onset and potential symptoms associated with poor sleep (TABLE 144). For weight management to succeed in patients with obesity, it is crucial to address sleep in addition to nutrition and physical activity.17,45

Physical activity has many benefits to overall health, especially for chronic diseases such as type 2 diabetes and hypertension. The Centers for Disease Control and Prevention recommends at least 150 minutes of moderate-intensity aerobic activity or 75 minutes of vigorous-intensity aerobic exercise per week in addition to muscle-strengthening activities 2 or more days per week.46 However, approximately 300 minutes of moderate-
Physical activity and diet in combination are vital, but diet restriction has a more substantial effect on weight loss than physical activity alone.48 Still, physical activity is essential in helping maintain and prevent weight regain.
Continue to: Nonpharmacologic interventions
Nonpharmacologic interventions include promoting greater sleep quality and quantity by emphasizing good sleep hygiene practices. Developing a practical and effective bedtime routine, creating a quiet sleep environment, and practicing healthy daily habits are essential components to sleep hygiene(TABLE 249,50). Relaxation techniques and cognitive behavioral therapy (CBT) also can help. CBT for insomnia (CBT-I) is the first-line intervention for chronic insomnia.51 Sleep restriction is a type of CBT used to treat insomnia, encouraging short-term sleep loss in the hopes of improving insomnia. A trial by Logue et al showed that patients with overweight and obesity randomized to undergo CBT with better sleep hygiene (nonpharmacologic) interventions had a greater mean weight loss percentage (5% vs 2%; P = .04) than did those who received CBT alone.52

Eastern medicine including herbal interventions lack evidence of efficacy and safety. Further studies need to be done on the effects that chamomile, kava, valerian root (Valeriana officinalis), tryptophan, and Wu Ling (from mycelia Xylaria nigripes) might have on sleep.53
Proceed cautiously with medication. The American College of Physicians recommends a shared decision-making approach when considering pharmacologic therapy for chronic insomnia and the American Academy of Sleep Medicine (AASM) offers guidance on options.51,54 However, the evidence behind AASM sleep pharmacologic recommendations is weak, implying a lesser degree of confidence in the outcome and, therefore, in its appropriateness. Thus, it falls upon the clinician and patient to weigh the benefits and burdens of the pharmacologic treatments of insomnia. If indicated, medications suggested to treat sleep onset and sleep maintenance insomnia are eszopiclone, zolpidem, and temazepam. Zaleplon, triazolam, and ramelteon may improve sleep initiation. Suvorexant and doxepin are used for sleep-maintenance insomnia.54 Exploring patient preferences, cost of treatment, health care options, and available resources should all be considered.
CORRESPONDENCE
Ecler Ercole Jaqua, MD, MBA, FAAFP, AGSF, FACLM, DipABOM, Loma Linda University Health, 25455 Barton Road, Suite 206A, Loma Linda, CA 92354; [email protected]
1. Aminoff MJ, Boller F, Swaab DF. We spend about one-third of our life either sleeping or attempting to do so. Handb Clin Neurol. 2011;98:vii. doi: 10.1016/B978-0-444-52006-7.00047-2
2. Watson NF, Badr MS, Belenky G, et al. Recommended amount of sleep for a healthy adult: a joint consensus statement of the American Academy of Sleep Medicine and Sleep Research Society. Sleep. 2015;38:843-844. doi: 10.5665/sleep.4716
3. CDC. Sleep and sleep disorders, adults. Accessed September 21, 2023. www.cdc.gov/sleep/data-and-statistics/adults.html
4. Chattu VK, Manzar MD, Kumary S. The global problem of insufficient sleep and its serious public health implications. Healthcare (Basel). 2019;7:1. doi: 10.3390/healthcare7010001
5. Taheri S, Lin L, Austin D, et al. Short sleep duration is associated with reduced leptin, elevated ghrelin, and increased body mass index. PLoS Med. 2004;1:e62. doi: 10.1371/journal.pmed.0010062
6. Hafner M, Stepanek M, Taylor J, et al. Why sleep matters—the economic costs of insufficient sleep. Rand Health Q. 2017;6:11.
7. Hisler G, Twenge JM, Krizan Z. Associations between screen time and short sleep duration among adolescents varies by media type: evidence from a cohort study. Sleep Med. 2020;66:92-102. doi: 10.1016/j.sleep.2019.08.007
8. Ogilvie RP, Patel SR. The epidemiology of sleep and obesity. Sleep Health. 2017;3:383-388. doi: 10.1016/j.sleh.2017.07.013
9. CDC. Sleep and sleep disorders: How much sleep do I need? Accessed September 21, 2023. www.cdc.gov/sleep/about_sleep/how_much_sleep.html
10. van Egmond LT, Meth EMS, Engström J, et al. Effects of acute sleep loss on leptin, ghrelin, and adiponectin in adults with healthy weight and obesity: a laboratory study. Obesity (Silver Spring). 2023;31:635-641. doi: 10.1002/oby.23616
11. Spiegel K, Tasali E, Penev P, et al. Brief communication: sleep curtailment in healthy young men is associated with decreased leptin levels, elevated ghrelin levels, and increased hunger and appetite. Ann Intern Med. 2004;141:846-850. doi: 10.7326/0003-4819-141-11-200412070-00008
12. Antza C, Kostopoulos G, Mostafa S, et al. The links between sleep duration, obesity and type 2 diabetes mellitus. J Endocrinol. 2021;252:125-141. doi: 10.1530/JOE-21-0155
13. Baron KG, Reid KJ, Kern AS, et al. Role of sleep timing in caloric intake and BMI. Obesity (Silver Spring). 2011;19:1374-1381. doi: 10.1038/oby.2011.100
14. Liu XY, Zheng CL, Xu C, et al. Nighttime snacking is associated with risk of obesity and hyperglycemia in adults: a cross-sectional survey from Chinese adult teachers J Biomed Res. 2017;31:541-547. doi: 10.7555/JBR.31.20160083
15. Cai Z, Yang Y, Zhang J, et al. The relationship between daytime napping and obesity: a systematic review and meta-analysis. Sci Rep. 2023.13:12124. doi: 10.1038/s41598-023-37883-7
16. Nedeltcheva AV, Kilkus JM, Imperial J, et al. Insufficient sleep undermines dietary efforts to reduce adiposity. Ann Intern Med. 2010;153:435-441. doi: 10.7326/0003-4819-153-7-201010050-00006
17. Chaput JP, Tremblay A. Adequate sleep to improve the treatment of obesity. CMAJ. 2012;184:1975-1976. doi: 10.1503/cmaj.120876
18. Kelsey MM, Zaepfel A, Bjornstad P, et al. Age-related consequences of childhood obesity. Gerontology. 2014;60:222-228. doi: 10.1159/000356023
19. Fryar CD, Carroll MD, Afful J. Prevalence of overweight, obesity, and severe obesity among children and adolescents aged 2-19 years: United States, 1963-1965 through 2017-2018. National Center for Health Statistics Health E-Stats. Updated January 29, 2021. Accessed September 21, 2021. www.cdc.gov/nchs/data/hestat/obesity-child-17-18/overweight-obesity-child-H.pdf
20. Fatima Y, Doi SAR, Mamun AA. Sleep quality and obesity in young subjects: a meta-analysis. Obes Rev. 2016;17:1154-1166. doi: 10.1111/obr.12444
21. Gohil A, Hannon TS. Poor sleep and obesity: concurrent epidemics in adolescent youth. Front Endocrinol. 2018;9:364. doi: 10.3389/fendo.2018.00364
22. Golley RK, Maher CA, Matricciani L, et al. Sleep duration or bedtime? Exploring the association between sleep timing behaviour, diet and BMI in children and adolescents. Int J Obes (Lond). 2013;37:546-551. doi: 10.1038/ijo.2012.212
23. Alessi CA. Sleep issues. In: Harper GM, Lyons WL, Potter JF, eds. Geriatrics Review Syllabus (GRS 10). Updated January 2021. Accessed August 29, 2023. http://geriatricscareonline.org
24. Patel SR, Blackwell T, Redline S, et al. The association between sleep duration and obesity in older adults. Int J Obes (Lond). 2008;32:1825-1834. doi: 10.1038/ijo.2008.198
25. Cai GH, Theorell-Haglöw J, Janson C, et al. Insomnia symptoms and sleep duration and their combined effects in relation to associations with obesity and central obesity. Sleep Med. 2018;46:81-87. doi: 10.1016/j.sleep.2018.03.009
26. Beccuti G, Pannain S. Sleep and obesity. Curr Opin Clin Nutr Metab Care. 2011;14:402-412. doi: 10.1097/MCO.0b013 e3283479109
27. Franklin KA, Lindberg E. Obstructive sleep apnea is a common disorder in the population–a review on the epidemiology of sleep apnea. J Thorac Dis. 2015;7:1311-1322. doi: 10.3978/j.issn.2072-1439.2015.06.11
28. USPSTF. Bibbins-Domingo K, Grossman DC, Curry SJ, et al. Screening for obstructive sleep apnea in adults: US Preventive Services Task Force recommendation statement. JAMA. 2017;317:407-414. doi: 10.1001/jama.2016.20325
29. Goyal M, Johnson J. Obstructive sleep apnea diagnosis and management. Mo Med. 2017;114:120-124.
30. American Academy of Sleep Medicine. Hidden health crisis costing America billions: underdiagnosing and undertreating obstructive sleep apnea draining healthcare system. 2016. Accessed September 25, 2023. https://aasm.org/wp-content/uploads/2017/10/sleep-apnea-economic-crisis.pdf
31. Devaraj, NK. Knowledge, attitude, and practice regarding obstructive sleep apnea among primary care physicians. Sleep Breath. 2020;24:1581-1590. doi: 10.1007/s11325-020-02040-1
32. Mysliwiec V, Martin JL, Ulmer CS, et al. The management of chronic insomnia disorder and obstructive sleep apnea: synopsis of the 2019 U.S. Department of Veterans Affairs and U.S. Department of Defense Clinical Practice Guidelines. Ann Intern Med. 2020;172:325-336. doi: 10.7326/M19-3575
33. Kuna ST, Reboussin DM, Strotmeyer ES, et al. Effects of weight loss on obstructive sleep apnea severity. Ten-year results of the Sleep AHEAD study. Am J Respir Crit Care Med. 2021;203:221-229. doi: 10.1164/rccm.201912-2511OC
34. St-Onge MP, Tasali E. Weight loss is integral to obstructive sleep apnea management. Ten-year follow-up in Sleep AHEAD. Am J Respir Crit Care Med. 2021;203:161-162. doi: 10.1164/rccm.202007-2906ED
35. Zheng D, Yuan X, Ma C, et al. Alcohol consumption and sleep quality: a community-based study. Public Health Nutr. 2021;24:4851-4858. doi: 10.1017/S1368980020004553
36. Chakravorty S, Chaudhary NS, Brower KJ. Alcohol dependence and its relationship with insomnia and other sleep disorders. Alcohol Clin Exp Res. 2016;40:2271-2282. doi: 10.1111/acer.13217
37. Elmenhorst EM, Elmenhorst D, Benderoth S, et al. Cognitive impairments by alcohol and sleep deprivation indicate trait characteristics and a potential role for adenosine A1 receptors. Proc Natl Acad Sci U S A. 2018;115:8009-8014. doi: 10.1073/pnas.1803770115
38. Traversy G, Chaput JP. Alcohol consumption and obesity: an update. Curr Obes Rep. 2015;4:122-130. doi: 10.1007/s13679-014-0129-4
39. McCann UD, Sgambati FP, Schwartz AR, et al. Sleep apnea in young abstinent recreational MDMA (“ecstasy”) consumers. Neurology. 2009;73:2011-2017. doi: 10.1212/WNL.0b013e3181c51a62
40. Grau-López L, Grau-López L, Daigre C, et al. Insomnia symptoms in patients with substance use disorders during detoxification and associated clinical features. Front Psychiatry. 2020;11:540022. doi: 10.3389/fpsyt.2020.540022
41. Boehm MA, Lei QM, Lloyd RM, et al. Depression, anxiety, and tobacco use: overlapping impediments to sleep in a national sample of college students. J Am Coll Health. 2016;64:565-574. doi: 10.1080/07448481.2016.1205073
42. Gracious BL, Meyer AE. Psychotropic-induced weight gain and potential pharmacologic treatment strategies. Psychiatry (Edgmont). 2005;2:36-42.
43. Ratliff JC, Barber JA, Palmese LB, et al. Association of prescription H1 antihistamine use with obesity: results from the National Health and Nutrition Examination Survey. Obesity (Silver Spring). 2010;18:2398-2400. doi: 10.1038/oby.2010.176
44. Pataka A, Daskalopoulou E, Kalamaras G, et al. Evaluation of five different questionnaires for assessing sleep apnea syndrome in a sleep clinic. Sleep Med. 2014;15:776-781. doi: 10.1016/j.sleep.2014.03.012
45. Kline CE, Chasens ER, Bizhanova Z, et al. The association between sleep health and weight change during a 12-month behavioral weight loss intervention. Int J Obes (Lond). 2021;45:639-649. doi: 10.1038/s41366-020-00728-8
46. CDC. How much physical activity do adults need? Accessed August 23, 2023. www.cdc.gov/physicalactivity/basics/adults/index.htm
47. Flack KD, Hays HM, Moreland J, et al. Exercise for weight loss: further evaluating energy compensation with exercise. Med Sci Sports Exerc. 2020;52:2466-2475. doi: 10.1249/MSS.0000000000002376
48. Swift DL, Johannsen NM, Lavie CJ, et al. The role of exercise and physical activity in weight loss and maintenance. Prog Cardiovasc Dis. 2014;56:441-447. doi: 10.1016/j.pcad.2013.09.012
49. Irish LA, Kline CE, Gunn HE, et al. The role of sleep hygiene in promoting public health: a review of empirical evidence. Sleep Med Rev. 2015;22:23-36. doi: 10.1016/j.smrv.2014.10.001
50. CDC. Tips for better sleep. 2022. Accessed August 4, 2023. www.cdc.gov/sleep/about_sleep/sleep_hygiene.html
51. Qaseem A, Kansagara D, Forciea MA, et al. Management of chronic insomnia disorder in adults: a clinical practice guideline from the American College of Physicians. Ann Intern Med. 2016;165:125-133. doi: 10.7326/M15-2175
52. Logue EE, Bourguet CC, Palmieri PA, et al. The better weight-better sleep study: a pilot intervention in primary care. Am J Health Behav. 2012;36:319-334. doi: 10.5993/AJHB.36.3.4
53. Leach MJ, Page AT. Herbal medicine for insomnia: a systematic review and meta-analysis. Sleep Med Rev. 2015;24:1-12. doi: 10.1016/j.smrv.2014.12.003
54. Sateia MJ, Buysse DJ, Krystal AD, et al. Clinical practice guideline for the pharmacologic treatment of chronic insomnia in adults: an American Academy of Sleep Medicine clinical practice guideline. J Clin Sleep Med. 2017;13:307-349. doi: 10.5664/jcsm.6470
1. Aminoff MJ, Boller F, Swaab DF. We spend about one-third of our life either sleeping or attempting to do so. Handb Clin Neurol. 2011;98:vii. doi: 10.1016/B978-0-444-52006-7.00047-2
2. Watson NF, Badr MS, Belenky G, et al. Recommended amount of sleep for a healthy adult: a joint consensus statement of the American Academy of Sleep Medicine and Sleep Research Society. Sleep. 2015;38:843-844. doi: 10.5665/sleep.4716
3. CDC. Sleep and sleep disorders, adults. Accessed September 21, 2023. www.cdc.gov/sleep/data-and-statistics/adults.html
4. Chattu VK, Manzar MD, Kumary S. The global problem of insufficient sleep and its serious public health implications. Healthcare (Basel). 2019;7:1. doi: 10.3390/healthcare7010001
5. Taheri S, Lin L, Austin D, et al. Short sleep duration is associated with reduced leptin, elevated ghrelin, and increased body mass index. PLoS Med. 2004;1:e62. doi: 10.1371/journal.pmed.0010062
6. Hafner M, Stepanek M, Taylor J, et al. Why sleep matters—the economic costs of insufficient sleep. Rand Health Q. 2017;6:11.
7. Hisler G, Twenge JM, Krizan Z. Associations between screen time and short sleep duration among adolescents varies by media type: evidence from a cohort study. Sleep Med. 2020;66:92-102. doi: 10.1016/j.sleep.2019.08.007
8. Ogilvie RP, Patel SR. The epidemiology of sleep and obesity. Sleep Health. 2017;3:383-388. doi: 10.1016/j.sleh.2017.07.013
9. CDC. Sleep and sleep disorders: How much sleep do I need? Accessed September 21, 2023. www.cdc.gov/sleep/about_sleep/how_much_sleep.html
10. van Egmond LT, Meth EMS, Engström J, et al. Effects of acute sleep loss on leptin, ghrelin, and adiponectin in adults with healthy weight and obesity: a laboratory study. Obesity (Silver Spring). 2023;31:635-641. doi: 10.1002/oby.23616
11. Spiegel K, Tasali E, Penev P, et al. Brief communication: sleep curtailment in healthy young men is associated with decreased leptin levels, elevated ghrelin levels, and increased hunger and appetite. Ann Intern Med. 2004;141:846-850. doi: 10.7326/0003-4819-141-11-200412070-00008
12. Antza C, Kostopoulos G, Mostafa S, et al. The links between sleep duration, obesity and type 2 diabetes mellitus. J Endocrinol. 2021;252:125-141. doi: 10.1530/JOE-21-0155
13. Baron KG, Reid KJ, Kern AS, et al. Role of sleep timing in caloric intake and BMI. Obesity (Silver Spring). 2011;19:1374-1381. doi: 10.1038/oby.2011.100
14. Liu XY, Zheng CL, Xu C, et al. Nighttime snacking is associated with risk of obesity and hyperglycemia in adults: a cross-sectional survey from Chinese adult teachers J Biomed Res. 2017;31:541-547. doi: 10.7555/JBR.31.20160083
15. Cai Z, Yang Y, Zhang J, et al. The relationship between daytime napping and obesity: a systematic review and meta-analysis. Sci Rep. 2023.13:12124. doi: 10.1038/s41598-023-37883-7
16. Nedeltcheva AV, Kilkus JM, Imperial J, et al. Insufficient sleep undermines dietary efforts to reduce adiposity. Ann Intern Med. 2010;153:435-441. doi: 10.7326/0003-4819-153-7-201010050-00006
17. Chaput JP, Tremblay A. Adequate sleep to improve the treatment of obesity. CMAJ. 2012;184:1975-1976. doi: 10.1503/cmaj.120876
18. Kelsey MM, Zaepfel A, Bjornstad P, et al. Age-related consequences of childhood obesity. Gerontology. 2014;60:222-228. doi: 10.1159/000356023
19. Fryar CD, Carroll MD, Afful J. Prevalence of overweight, obesity, and severe obesity among children and adolescents aged 2-19 years: United States, 1963-1965 through 2017-2018. National Center for Health Statistics Health E-Stats. Updated January 29, 2021. Accessed September 21, 2021. www.cdc.gov/nchs/data/hestat/obesity-child-17-18/overweight-obesity-child-H.pdf
20. Fatima Y, Doi SAR, Mamun AA. Sleep quality and obesity in young subjects: a meta-analysis. Obes Rev. 2016;17:1154-1166. doi: 10.1111/obr.12444
21. Gohil A, Hannon TS. Poor sleep and obesity: concurrent epidemics in adolescent youth. Front Endocrinol. 2018;9:364. doi: 10.3389/fendo.2018.00364
22. Golley RK, Maher CA, Matricciani L, et al. Sleep duration or bedtime? Exploring the association between sleep timing behaviour, diet and BMI in children and adolescents. Int J Obes (Lond). 2013;37:546-551. doi: 10.1038/ijo.2012.212
23. Alessi CA. Sleep issues. In: Harper GM, Lyons WL, Potter JF, eds. Geriatrics Review Syllabus (GRS 10). Updated January 2021. Accessed August 29, 2023. http://geriatricscareonline.org
24. Patel SR, Blackwell T, Redline S, et al. The association between sleep duration and obesity in older adults. Int J Obes (Lond). 2008;32:1825-1834. doi: 10.1038/ijo.2008.198
25. Cai GH, Theorell-Haglöw J, Janson C, et al. Insomnia symptoms and sleep duration and their combined effects in relation to associations with obesity and central obesity. Sleep Med. 2018;46:81-87. doi: 10.1016/j.sleep.2018.03.009
26. Beccuti G, Pannain S. Sleep and obesity. Curr Opin Clin Nutr Metab Care. 2011;14:402-412. doi: 10.1097/MCO.0b013 e3283479109
27. Franklin KA, Lindberg E. Obstructive sleep apnea is a common disorder in the population–a review on the epidemiology of sleep apnea. J Thorac Dis. 2015;7:1311-1322. doi: 10.3978/j.issn.2072-1439.2015.06.11
28. USPSTF. Bibbins-Domingo K, Grossman DC, Curry SJ, et al. Screening for obstructive sleep apnea in adults: US Preventive Services Task Force recommendation statement. JAMA. 2017;317:407-414. doi: 10.1001/jama.2016.20325
29. Goyal M, Johnson J. Obstructive sleep apnea diagnosis and management. Mo Med. 2017;114:120-124.
30. American Academy of Sleep Medicine. Hidden health crisis costing America billions: underdiagnosing and undertreating obstructive sleep apnea draining healthcare system. 2016. Accessed September 25, 2023. https://aasm.org/wp-content/uploads/2017/10/sleep-apnea-economic-crisis.pdf
31. Devaraj, NK. Knowledge, attitude, and practice regarding obstructive sleep apnea among primary care physicians. Sleep Breath. 2020;24:1581-1590. doi: 10.1007/s11325-020-02040-1
32. Mysliwiec V, Martin JL, Ulmer CS, et al. The management of chronic insomnia disorder and obstructive sleep apnea: synopsis of the 2019 U.S. Department of Veterans Affairs and U.S. Department of Defense Clinical Practice Guidelines. Ann Intern Med. 2020;172:325-336. doi: 10.7326/M19-3575
33. Kuna ST, Reboussin DM, Strotmeyer ES, et al. Effects of weight loss on obstructive sleep apnea severity. Ten-year results of the Sleep AHEAD study. Am J Respir Crit Care Med. 2021;203:221-229. doi: 10.1164/rccm.201912-2511OC
34. St-Onge MP, Tasali E. Weight loss is integral to obstructive sleep apnea management. Ten-year follow-up in Sleep AHEAD. Am J Respir Crit Care Med. 2021;203:161-162. doi: 10.1164/rccm.202007-2906ED
35. Zheng D, Yuan X, Ma C, et al. Alcohol consumption and sleep quality: a community-based study. Public Health Nutr. 2021;24:4851-4858. doi: 10.1017/S1368980020004553
36. Chakravorty S, Chaudhary NS, Brower KJ. Alcohol dependence and its relationship with insomnia and other sleep disorders. Alcohol Clin Exp Res. 2016;40:2271-2282. doi: 10.1111/acer.13217
37. Elmenhorst EM, Elmenhorst D, Benderoth S, et al. Cognitive impairments by alcohol and sleep deprivation indicate trait characteristics and a potential role for adenosine A1 receptors. Proc Natl Acad Sci U S A. 2018;115:8009-8014. doi: 10.1073/pnas.1803770115
38. Traversy G, Chaput JP. Alcohol consumption and obesity: an update. Curr Obes Rep. 2015;4:122-130. doi: 10.1007/s13679-014-0129-4
39. McCann UD, Sgambati FP, Schwartz AR, et al. Sleep apnea in young abstinent recreational MDMA (“ecstasy”) consumers. Neurology. 2009;73:2011-2017. doi: 10.1212/WNL.0b013e3181c51a62
40. Grau-López L, Grau-López L, Daigre C, et al. Insomnia symptoms in patients with substance use disorders during detoxification and associated clinical features. Front Psychiatry. 2020;11:540022. doi: 10.3389/fpsyt.2020.540022
41. Boehm MA, Lei QM, Lloyd RM, et al. Depression, anxiety, and tobacco use: overlapping impediments to sleep in a national sample of college students. J Am Coll Health. 2016;64:565-574. doi: 10.1080/07448481.2016.1205073
42. Gracious BL, Meyer AE. Psychotropic-induced weight gain and potential pharmacologic treatment strategies. Psychiatry (Edgmont). 2005;2:36-42.
43. Ratliff JC, Barber JA, Palmese LB, et al. Association of prescription H1 antihistamine use with obesity: results from the National Health and Nutrition Examination Survey. Obesity (Silver Spring). 2010;18:2398-2400. doi: 10.1038/oby.2010.176
44. Pataka A, Daskalopoulou E, Kalamaras G, et al. Evaluation of five different questionnaires for assessing sleep apnea syndrome in a sleep clinic. Sleep Med. 2014;15:776-781. doi: 10.1016/j.sleep.2014.03.012
45. Kline CE, Chasens ER, Bizhanova Z, et al. The association between sleep health and weight change during a 12-month behavioral weight loss intervention. Int J Obes (Lond). 2021;45:639-649. doi: 10.1038/s41366-020-00728-8
46. CDC. How much physical activity do adults need? Accessed August 23, 2023. www.cdc.gov/physicalactivity/basics/adults/index.htm
47. Flack KD, Hays HM, Moreland J, et al. Exercise for weight loss: further evaluating energy compensation with exercise. Med Sci Sports Exerc. 2020;52:2466-2475. doi: 10.1249/MSS.0000000000002376
48. Swift DL, Johannsen NM, Lavie CJ, et al. The role of exercise and physical activity in weight loss and maintenance. Prog Cardiovasc Dis. 2014;56:441-447. doi: 10.1016/j.pcad.2013.09.012
49. Irish LA, Kline CE, Gunn HE, et al. The role of sleep hygiene in promoting public health: a review of empirical evidence. Sleep Med Rev. 2015;22:23-36. doi: 10.1016/j.smrv.2014.10.001
50. CDC. Tips for better sleep. 2022. Accessed August 4, 2023. www.cdc.gov/sleep/about_sleep/sleep_hygiene.html
51. Qaseem A, Kansagara D, Forciea MA, et al. Management of chronic insomnia disorder in adults: a clinical practice guideline from the American College of Physicians. Ann Intern Med. 2016;165:125-133. doi: 10.7326/M15-2175
52. Logue EE, Bourguet CC, Palmieri PA, et al. The better weight-better sleep study: a pilot intervention in primary care. Am J Health Behav. 2012;36:319-334. doi: 10.5993/AJHB.36.3.4
53. Leach MJ, Page AT. Herbal medicine for insomnia: a systematic review and meta-analysis. Sleep Med Rev. 2015;24:1-12. doi: 10.1016/j.smrv.2014.12.003
54. Sateia MJ, Buysse DJ, Krystal AD, et al. Clinical practice guideline for the pharmacologic treatment of chronic insomnia in adults: an American Academy of Sleep Medicine clinical practice guideline. J Clin Sleep Med. 2017;13:307-349. doi: 10.5664/jcsm.6470
PRACTICE RECOMMENDATIONS
› Consider cognitive behaviorial therapy for insomnia (CBT-I) first-line treatment for insomnia. A
› Carefully review patients’ medication lists, as many pharmaceuticals can affect weight and sleep. C
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
FDA denies approval for patisiran in ATTR cardiomyopathy, despite panel nod
the company has announced.
ATTR amyloidosis is an underdiagnosed, rapidly progressive, debilitating, fatal disease caused by misfolded TTR proteins, which accumulate as amyloid deposits in various parts of the body, including the heart.
In September, the FDA Cardiovascular and Renal Drugs Advisory Committee voted 9 to 3 that the benefits of patisiran outweigh the risks for the treatment of ATTR amyloidosis cardiomyopathy on the basis of the results of the APOLLO-B phase 3 study.
However, many panel members questioned whether the benefits are clinically meaningful – a view shared by the FDA in a complete response letter (CRL) the FDA sent to Alnylam.
According to the company, the FDA indicated in the letter that the clinical meaningfulness of patisiran’s treatment effects for the cardiomyopathy of ATTR amyloidosis have “not been established,” and therefore, the supplemental new drug application for patisiran “could not be approved in its present form.”
The FDA did not identify any issues with respect to clinical safety, study conduct, drug quality, or manufacturing.
Nonetheless, as a result of the CRL, the company said it will no longer pursue an expanded indication for patisiran in cardiomyopathy of ATTR amyloidosis in the United States.
The company said it will continue to make patisiran available for patients with cardiomyopathy of ATTR amyloidosis who are enrolled in the open-label extension period of the APOLLO-B study and the patisiran expanded access protocol.
The company also said it will continue to focus on the HELIOS-B phase 3 study of vutrisiran, an investigational RNAi therapeutic in development for the treatment of cardiomyopathy of ATTR amyloidosis.
“We remain confident in the HELIOS-B phase 3 study of vutrisiran and look forward to sharing topline results in early 2024. If successful, we believe vutrisiran will offer convenient, quarterly subcutaneous dosing with a therapeutic profile that may potentially include cardiovascular outcome benefits,” Alnylam CEO Yvonne Greenstreet, MBChB, said in the statement.
Intravenously administered patisiran is already approved in the United States and Canada for the treatment of polyneuropathy of hereditary ATTR amyloidosis in adults.
A version of this article first appeared on Medscape.com.
the company has announced.
ATTR amyloidosis is an underdiagnosed, rapidly progressive, debilitating, fatal disease caused by misfolded TTR proteins, which accumulate as amyloid deposits in various parts of the body, including the heart.
In September, the FDA Cardiovascular and Renal Drugs Advisory Committee voted 9 to 3 that the benefits of patisiran outweigh the risks for the treatment of ATTR amyloidosis cardiomyopathy on the basis of the results of the APOLLO-B phase 3 study.
However, many panel members questioned whether the benefits are clinically meaningful – a view shared by the FDA in a complete response letter (CRL) the FDA sent to Alnylam.
According to the company, the FDA indicated in the letter that the clinical meaningfulness of patisiran’s treatment effects for the cardiomyopathy of ATTR amyloidosis have “not been established,” and therefore, the supplemental new drug application for patisiran “could not be approved in its present form.”
The FDA did not identify any issues with respect to clinical safety, study conduct, drug quality, or manufacturing.
Nonetheless, as a result of the CRL, the company said it will no longer pursue an expanded indication for patisiran in cardiomyopathy of ATTR amyloidosis in the United States.
The company said it will continue to make patisiran available for patients with cardiomyopathy of ATTR amyloidosis who are enrolled in the open-label extension period of the APOLLO-B study and the patisiran expanded access protocol.
The company also said it will continue to focus on the HELIOS-B phase 3 study of vutrisiran, an investigational RNAi therapeutic in development for the treatment of cardiomyopathy of ATTR amyloidosis.
“We remain confident in the HELIOS-B phase 3 study of vutrisiran and look forward to sharing topline results in early 2024. If successful, we believe vutrisiran will offer convenient, quarterly subcutaneous dosing with a therapeutic profile that may potentially include cardiovascular outcome benefits,” Alnylam CEO Yvonne Greenstreet, MBChB, said in the statement.
Intravenously administered patisiran is already approved in the United States and Canada for the treatment of polyneuropathy of hereditary ATTR amyloidosis in adults.
A version of this article first appeared on Medscape.com.
the company has announced.
ATTR amyloidosis is an underdiagnosed, rapidly progressive, debilitating, fatal disease caused by misfolded TTR proteins, which accumulate as amyloid deposits in various parts of the body, including the heart.
In September, the FDA Cardiovascular and Renal Drugs Advisory Committee voted 9 to 3 that the benefits of patisiran outweigh the risks for the treatment of ATTR amyloidosis cardiomyopathy on the basis of the results of the APOLLO-B phase 3 study.
However, many panel members questioned whether the benefits are clinically meaningful – a view shared by the FDA in a complete response letter (CRL) the FDA sent to Alnylam.
According to the company, the FDA indicated in the letter that the clinical meaningfulness of patisiran’s treatment effects for the cardiomyopathy of ATTR amyloidosis have “not been established,” and therefore, the supplemental new drug application for patisiran “could not be approved in its present form.”
The FDA did not identify any issues with respect to clinical safety, study conduct, drug quality, or manufacturing.
Nonetheless, as a result of the CRL, the company said it will no longer pursue an expanded indication for patisiran in cardiomyopathy of ATTR amyloidosis in the United States.
The company said it will continue to make patisiran available for patients with cardiomyopathy of ATTR amyloidosis who are enrolled in the open-label extension period of the APOLLO-B study and the patisiran expanded access protocol.
The company also said it will continue to focus on the HELIOS-B phase 3 study of vutrisiran, an investigational RNAi therapeutic in development for the treatment of cardiomyopathy of ATTR amyloidosis.
“We remain confident in the HELIOS-B phase 3 study of vutrisiran and look forward to sharing topline results in early 2024. If successful, we believe vutrisiran will offer convenient, quarterly subcutaneous dosing with a therapeutic profile that may potentially include cardiovascular outcome benefits,” Alnylam CEO Yvonne Greenstreet, MBChB, said in the statement.
Intravenously administered patisiran is already approved in the United States and Canada for the treatment of polyneuropathy of hereditary ATTR amyloidosis in adults.
A version of this article first appeared on Medscape.com.
Another day in the ED: Walking the line between empathy and desensitization
Patient after patient, emergency medicine physicians experience highs and lows, sometimes minutes apart. “It might be another Tuesday for us, but for the patient in that dramatic life moment on that day, it’s everything,” said Charissa Pacella, MD, chief of emergency medicine at UPMC Presbyterian in Pittsburgh.
Emergency department (ED) physicians frequently encounter fatal situations, feel frustration when they can’t save a person, and constantly see patients in distress. How do physicians weather the emotional storm of life in the ED with both their mental health and empathy intact?
Reserve time for emotions
Dr. Pacella, who has been practicing emergency medicine for 22 years, also serves in a leadership role for Physicians for Physicians, a confidential peer support program at UPMC for doctors struggling with the impact of adverse events and the stress they face. She said it’s essential to know how to compartmentalize and focus on the task at hand, but later revisit emotions from a personal perspective.
“We all separate our cognitive and leadership roles from our emotional response in the moment,” she said. “Everybody is just focused on doing the next right thing. And often it’s not until sometime later when you sit down or go home or maybe even going in for your next shift that it really hits you in a more emotional way.”
If you try to avoid or skip over this part of the process by shoving the emotions down and ignoring them, Dr. Pacella said, you leave out a crucial part of the care process. And over the course of a career, you’ll risk losing empathy and the human connection that most doctors went into medicine for, she told this news organization.
Connect with your colleagues
Physicians supporting each other is crucial, said Dr Pacella. And luckily, she added, connection tends to be a strength of the specialty.
“As emergency medicine physicians, we share a lot in common, and part of it is what brought us to choose this specialty in the first place. You picked it because there’s something appealing to you about the unknown. It’s a unique role, a unique environment, and a unique relationship you have with patients and being able to connect with colleagues,” she said.
Lisa Williford, MD, emergency medicine specialist at Texas Health Harris Methodist Hospital in Fort Worth, said her 14-year career has taught her that no matter how focused a medical professional can stay in the moment, emotions are happening at some level.
“During a level 1 trauma, there are a lot of people in the room – trauma surgeons, residents, nurses, x-ray techs, respiratory therapy, anesthesia – and every one of us is having emotions. It’s not realistic to think anyone is avoiding it.”
But beyond simply recognizing a shared experience, it’s important to talk to each other. It’s not just about how you’re feeling, but also what you do to help manage that emotional load.
“I’d say that more of us, especially since COVID, are learning that actually getting a therapist is a good thing, having a life coach is a good thing,” said Dr. Williford. Accepting mental health care and learning how to manage it is also a good thing.
Accept unpredictability
You may think you know how a difficult situation will affect you, but that assumption can put you in a vulnerable position. Dr. Pacella said she’s learned that for most physicians, a stress response to a critical incident often has less to do with the type of event and more about who is involved or your past experiences.
“I have reacted in a very emotional way at moments that I would never have expected or predicted,” said Dr. Pacella. “And it’s not always because of some awful event. It’s usually because of some emotional connection or trigger embedded in that encounter.”
For example, she said, you may have had a past case as an emergency physician where the outcome was not favorable, or the patient involved may remind you of yourself or someone you love.
“It might not necessarily be a horrible thing happening to a young, healthy person that triggers someone; it might be a minor problem involving a patient you, for whatever reason, identify with,” she said. “Or you may have had a similar patient where things didn’t go well for them. It’s just highly variable, even for an individual.”
Just as you can’t know what medical issues you’ll face in a day, you can’t predict how you’ll react. Approach each scenario with the knowledge that you may veer off emotional course – and prepare accordingly.
Bring mental wellness to the forefront of training
Dr. Williford, who also serves as regional director for ScribeNest, a doctor-operated company that trains medical scribes who are on the path to becoming medical professionals, said she feels strongly about bringing this conversation to the younger generation.
“For me, nobody at the med school level or residency level taught or talked about how to compartmentalize and cope with the traumatic experiences that we saw,” she said. “Only in the last decade have we started teaching our residents and medical students about burnout and resilience.
“I say things like, ‘Hey, we just witnessed an 18-year-old in cardiac arrest. We did CPR for an hour and didn’t get him back. And then you witnessed me tell his mom, who wailed. And then we turned around and treated an ankle sprain. Let’s sit down and talk about how jarring that all is and how nobody else experiences these things.’
“We have this expectation that our physicians know how to move on and connect with each new patient with care and empathy, but we have to tell our future doctors the actual steps we take to be able to do that.”
Seasoned physicians can lead the way for the next generation and turn the tide toward the normalization of talking about these struggles. By making it part of training, it becomes part of a physician’s skill set.
“With a happy, healthy career, we can pay it forward to the next generation and teach them how to be better than we were,” said Dr. Williford.
A version of this article appeared on Medscape.com.
Patient after patient, emergency medicine physicians experience highs and lows, sometimes minutes apart. “It might be another Tuesday for us, but for the patient in that dramatic life moment on that day, it’s everything,” said Charissa Pacella, MD, chief of emergency medicine at UPMC Presbyterian in Pittsburgh.
Emergency department (ED) physicians frequently encounter fatal situations, feel frustration when they can’t save a person, and constantly see patients in distress. How do physicians weather the emotional storm of life in the ED with both their mental health and empathy intact?
Reserve time for emotions
Dr. Pacella, who has been practicing emergency medicine for 22 years, also serves in a leadership role for Physicians for Physicians, a confidential peer support program at UPMC for doctors struggling with the impact of adverse events and the stress they face. She said it’s essential to know how to compartmentalize and focus on the task at hand, but later revisit emotions from a personal perspective.
“We all separate our cognitive and leadership roles from our emotional response in the moment,” she said. “Everybody is just focused on doing the next right thing. And often it’s not until sometime later when you sit down or go home or maybe even going in for your next shift that it really hits you in a more emotional way.”
If you try to avoid or skip over this part of the process by shoving the emotions down and ignoring them, Dr. Pacella said, you leave out a crucial part of the care process. And over the course of a career, you’ll risk losing empathy and the human connection that most doctors went into medicine for, she told this news organization.
Connect with your colleagues
Physicians supporting each other is crucial, said Dr Pacella. And luckily, she added, connection tends to be a strength of the specialty.
“As emergency medicine physicians, we share a lot in common, and part of it is what brought us to choose this specialty in the first place. You picked it because there’s something appealing to you about the unknown. It’s a unique role, a unique environment, and a unique relationship you have with patients and being able to connect with colleagues,” she said.
Lisa Williford, MD, emergency medicine specialist at Texas Health Harris Methodist Hospital in Fort Worth, said her 14-year career has taught her that no matter how focused a medical professional can stay in the moment, emotions are happening at some level.
“During a level 1 trauma, there are a lot of people in the room – trauma surgeons, residents, nurses, x-ray techs, respiratory therapy, anesthesia – and every one of us is having emotions. It’s not realistic to think anyone is avoiding it.”
But beyond simply recognizing a shared experience, it’s important to talk to each other. It’s not just about how you’re feeling, but also what you do to help manage that emotional load.
“I’d say that more of us, especially since COVID, are learning that actually getting a therapist is a good thing, having a life coach is a good thing,” said Dr. Williford. Accepting mental health care and learning how to manage it is also a good thing.
Accept unpredictability
You may think you know how a difficult situation will affect you, but that assumption can put you in a vulnerable position. Dr. Pacella said she’s learned that for most physicians, a stress response to a critical incident often has less to do with the type of event and more about who is involved or your past experiences.
“I have reacted in a very emotional way at moments that I would never have expected or predicted,” said Dr. Pacella. “And it’s not always because of some awful event. It’s usually because of some emotional connection or trigger embedded in that encounter.”
For example, she said, you may have had a past case as an emergency physician where the outcome was not favorable, or the patient involved may remind you of yourself or someone you love.
“It might not necessarily be a horrible thing happening to a young, healthy person that triggers someone; it might be a minor problem involving a patient you, for whatever reason, identify with,” she said. “Or you may have had a similar patient where things didn’t go well for them. It’s just highly variable, even for an individual.”
Just as you can’t know what medical issues you’ll face in a day, you can’t predict how you’ll react. Approach each scenario with the knowledge that you may veer off emotional course – and prepare accordingly.
Bring mental wellness to the forefront of training
Dr. Williford, who also serves as regional director for ScribeNest, a doctor-operated company that trains medical scribes who are on the path to becoming medical professionals, said she feels strongly about bringing this conversation to the younger generation.
“For me, nobody at the med school level or residency level taught or talked about how to compartmentalize and cope with the traumatic experiences that we saw,” she said. “Only in the last decade have we started teaching our residents and medical students about burnout and resilience.
“I say things like, ‘Hey, we just witnessed an 18-year-old in cardiac arrest. We did CPR for an hour and didn’t get him back. And then you witnessed me tell his mom, who wailed. And then we turned around and treated an ankle sprain. Let’s sit down and talk about how jarring that all is and how nobody else experiences these things.’
“We have this expectation that our physicians know how to move on and connect with each new patient with care and empathy, but we have to tell our future doctors the actual steps we take to be able to do that.”
Seasoned physicians can lead the way for the next generation and turn the tide toward the normalization of talking about these struggles. By making it part of training, it becomes part of a physician’s skill set.
“With a happy, healthy career, we can pay it forward to the next generation and teach them how to be better than we were,” said Dr. Williford.
A version of this article appeared on Medscape.com.
Patient after patient, emergency medicine physicians experience highs and lows, sometimes minutes apart. “It might be another Tuesday for us, but for the patient in that dramatic life moment on that day, it’s everything,” said Charissa Pacella, MD, chief of emergency medicine at UPMC Presbyterian in Pittsburgh.
Emergency department (ED) physicians frequently encounter fatal situations, feel frustration when they can’t save a person, and constantly see patients in distress. How do physicians weather the emotional storm of life in the ED with both their mental health and empathy intact?
Reserve time for emotions
Dr. Pacella, who has been practicing emergency medicine for 22 years, also serves in a leadership role for Physicians for Physicians, a confidential peer support program at UPMC for doctors struggling with the impact of adverse events and the stress they face. She said it’s essential to know how to compartmentalize and focus on the task at hand, but later revisit emotions from a personal perspective.
“We all separate our cognitive and leadership roles from our emotional response in the moment,” she said. “Everybody is just focused on doing the next right thing. And often it’s not until sometime later when you sit down or go home or maybe even going in for your next shift that it really hits you in a more emotional way.”
If you try to avoid or skip over this part of the process by shoving the emotions down and ignoring them, Dr. Pacella said, you leave out a crucial part of the care process. And over the course of a career, you’ll risk losing empathy and the human connection that most doctors went into medicine for, she told this news organization.
Connect with your colleagues
Physicians supporting each other is crucial, said Dr Pacella. And luckily, she added, connection tends to be a strength of the specialty.
“As emergency medicine physicians, we share a lot in common, and part of it is what brought us to choose this specialty in the first place. You picked it because there’s something appealing to you about the unknown. It’s a unique role, a unique environment, and a unique relationship you have with patients and being able to connect with colleagues,” she said.
Lisa Williford, MD, emergency medicine specialist at Texas Health Harris Methodist Hospital in Fort Worth, said her 14-year career has taught her that no matter how focused a medical professional can stay in the moment, emotions are happening at some level.
“During a level 1 trauma, there are a lot of people in the room – trauma surgeons, residents, nurses, x-ray techs, respiratory therapy, anesthesia – and every one of us is having emotions. It’s not realistic to think anyone is avoiding it.”
But beyond simply recognizing a shared experience, it’s important to talk to each other. It’s not just about how you’re feeling, but also what you do to help manage that emotional load.
“I’d say that more of us, especially since COVID, are learning that actually getting a therapist is a good thing, having a life coach is a good thing,” said Dr. Williford. Accepting mental health care and learning how to manage it is also a good thing.
Accept unpredictability
You may think you know how a difficult situation will affect you, but that assumption can put you in a vulnerable position. Dr. Pacella said she’s learned that for most physicians, a stress response to a critical incident often has less to do with the type of event and more about who is involved or your past experiences.
“I have reacted in a very emotional way at moments that I would never have expected or predicted,” said Dr. Pacella. “And it’s not always because of some awful event. It’s usually because of some emotional connection or trigger embedded in that encounter.”
For example, she said, you may have had a past case as an emergency physician where the outcome was not favorable, or the patient involved may remind you of yourself or someone you love.
“It might not necessarily be a horrible thing happening to a young, healthy person that triggers someone; it might be a minor problem involving a patient you, for whatever reason, identify with,” she said. “Or you may have had a similar patient where things didn’t go well for them. It’s just highly variable, even for an individual.”
Just as you can’t know what medical issues you’ll face in a day, you can’t predict how you’ll react. Approach each scenario with the knowledge that you may veer off emotional course – and prepare accordingly.
Bring mental wellness to the forefront of training
Dr. Williford, who also serves as regional director for ScribeNest, a doctor-operated company that trains medical scribes who are on the path to becoming medical professionals, said she feels strongly about bringing this conversation to the younger generation.
“For me, nobody at the med school level or residency level taught or talked about how to compartmentalize and cope with the traumatic experiences that we saw,” she said. “Only in the last decade have we started teaching our residents and medical students about burnout and resilience.
“I say things like, ‘Hey, we just witnessed an 18-year-old in cardiac arrest. We did CPR for an hour and didn’t get him back. And then you witnessed me tell his mom, who wailed. And then we turned around and treated an ankle sprain. Let’s sit down and talk about how jarring that all is and how nobody else experiences these things.’
“We have this expectation that our physicians know how to move on and connect with each new patient with care and empathy, but we have to tell our future doctors the actual steps we take to be able to do that.”
Seasoned physicians can lead the way for the next generation and turn the tide toward the normalization of talking about these struggles. By making it part of training, it becomes part of a physician’s skill set.
“With a happy, healthy career, we can pay it forward to the next generation and teach them how to be better than we were,” said Dr. Williford.
A version of this article appeared on Medscape.com.
Don’t fear POTS: Tips for diagnosis and treatment
This transcript has been edited for clarity.
Michelle L. O’Donoghue, MD, MPH: I’m here in Amsterdam at the European Society of Cardiology (ESC) Congress 2023. Joining me for a great discussion is my friend Dr. Pam Taub, who is a cardiologist and a professor of medicine at UC San Diego. She has a particular interest in postural orthostatic tachycardia syndrome (POTS), so that’s what we’ll be talking about today.
Thanks for joining me, Pam. When we think about POTS, for those who are not familiar with the term, what does it actually mean and how do you diagnose it?
No tilt table required
Pam R. Taub, MD: That’s typically associated with symptoms such as lightheadedness, dizziness, and cognitive difficulties such as brain fog. The diagnosis can be made by tilt-table testing, but it can also be made in the office with simple orthostats.
In my clinic, I have people lie down for 3-5 minutes. At the end of that period, you get a heart rate and blood pressure. Then you have them stand up for 3-5 minutes and then get heart rate and blood pressure, and you look at the differences. If the heart rate goes up by 30 points – so maybe they’re 80 beats/min when they’re lying down and when they stand up, it goes to 110 beats/min – that’s POTS, so very objective criteria. Typically, these people don’t have what we call orthostatic hypotension, where there is a significant decrease in the blood pressure. It’s more a heart rate issue.
Dr. O’Donoghue: How symptomatically do they usually present?
Dr. Taub: It’s a spectrum. Some people have mild symptoms. After they’re in the upright position for maybe 10 minutes, they get symptoms. There are some people who, when they go from a lying to standing position, they’re extremely symptomatic and can’t really do any activities. There are some people that are even wheelchair-bound because the symptoms are so debilitating. There’s a wide spectrum.
Dr. O’Donoghue: There has been more discussion, I feel like, about the rising prevalence of POTS as a diagnosis, and in particular since the COVID pandemic. What’s our understanding of the relationship between COVID and POTS and what the mechanism might be?
Dr. Taub: We’ve known that POTS can be triggered by a viral infection. Before COVID, we knew that in certain individuals that we think have an underlying genetic predisposition, usually some autoimmune substrate, when they get certain types of infections, whether it’s influenza or mononucleosis, they get POTS.
Typically, when they get an infection, they start getting deconditioned. They don’t feel well, so they’re on bed rest. When they get long periods of bed rest, when they start to become active, they start to have overactivation of their sympathetic nervous system, and they have a large amount of cardiovascular deconditioning. It’s a cycle that is often triggered after an infection.
A huge increase of POTS has been seen after COVID-19 because we had so many people exposed to this virus. With COVID-19, there is a period where people don’t feel great and they are getting bed rest, so they’re getting deconditioned. We’ve seen so many patients referred for post-COVID POTS and also long COVID or the post-acute sequelae of COVID-19, where POTS is a part of that presentation.
Female sex and autoimmune conditions
Dr. O’Donoghue: We know that POTS seems to disproportionately affect women. Is that understood? Is it thought that that’s related to the perhaps the autoimmune component of that illness?
Dr. Taub: Yes. The theory is because women tend to have more autoimmune conditions, that’s why they’re more predisposed. There’s a large amount of genetic susceptibility. For instance, we know that there’s an association between POTS and conditions like Ehlers-Danlos syndrome and between POTS and mast cell activation. Some of those conditions are more prevalent in women as well.
Dr. O’Donoghue: I feel like many physicians don’t know how to manage POTS, and they’re actually a little fearful perhaps to take it on. Fortunately, there have been a growing number of POTS clinics with specialists that focus on that area. For the average practitioner who maybe can’t refer to a POTS clinic, how should they approach that?
Dr. Taub: The first thing is its diagnosis. When someone tells you that they have symptoms of orthostatic intolerance – so, activities that involve standing – you need to first have that on your differential diagnosis. You can make the diagnosis in the office with orthostats. You don’t need a tilt table. It’s sometimes helpful if you’re unsure about the diagnosis, but you can make the diagnosis.
Many times, you’re finding people that have very mild symptoms. You can treat that with some good lifestyle recommendations, such as increased hydration, increasing salt in their diet, and compression. And the exercise component is really important.
Many people with POTS are told to go exercise, go for a run, or go for a walk. That’s incorrect, because these people have symptoms when they’re in the upright position. The type of exercise they need to do initially is exercise in the lying or seated position – so exercises like rowing or a seated bike, and strength training. As they start to feel better, then they can do upright exercise.
You should never tell a person that has POTS to just initially start with upright exercise, because they’re going to feel so much worse and then they’re never going to want to exercise. It’s really important to give them the right exercise recommendations. I find that for many of these mild cases, if they do the right exercise and engage in the right lifestyle strategies, they get better.
Compression wear and drug therapy
Dr. O’Donoghue: When it comes to compression stockings, do you usually start with a particular length?
Dr. Taub: It’s interesting. There are many different compression stockings, medical grade. Through patients with POTS, I’ve gotten feedback on certain types of athletic wear that have built-in compression, and that’s a little bit easier for people to wear every day because they can do their errands and it doesn’t look like they’re wearing medical-grade compression stockings.
Basically, I’ve collected all the different recommendations that patients say help, and I give them a list. The medical-grade compression stockings sometimes are very challenging to put on, and sometimes people just need light compression or even just socks. Any kind of compression is going to help.
Dr. O’Donoghue: That’s a great tip, because I know there are many patients who refuse to wear the compression stockings. If there’s a fashionable alternative, that’s always good to reach for.
Dr. Taub: Another thing that patients have told me is that abdominal compression is also very helpful. There are many commercially available abdominal compression options, like shapewear. Many patients with POTS use that and that helps, too.
Dr. O’Donoghue: Good. For those patients with POTS that is refractory to the measures you’ve already discussed, what are the next steps after that?
Dr. Taub: Pharmacotherapy is very synergistic with lifestyle, and there are many different pharmacotherapy options. One of the first things that you want to think about is lowering that heart rate. The reason people feel horrible is because their heart rate is usually very high when they’re upright. If they’re upright for long periods of time and they’re having very high heart rates, they’re going to get really tired because it’s like they’re exercising for hours when they’re upright.
Heart rate lowering is the cornerstone of therapy. Traditionally, we’ve used beta-blockers for heart rate lowering. The problem is they also lower blood pressure. They can also cause fatigue, so not the ideal agent for patients with POTS.
One of the clinical trials that I led was with a drug called ivabradine, which selectively works on the SA node and decreases heart rate without affecting blood pressure. What’s really elegant about ivabradine is it has a more potent effect when the heart rate is higher. When the patient is standing, it’s going to have a more potent effect on heart rate lowering. It’s really well tolerated in patients with POTS. In our study, we showed an improvement in quality of life metrics. That’s one of the first-line drugs that I use for patients with POTS.
The other thing is some of them will also have a concomitant lowering of blood pressure. You can think about medications that increase blood pressure, like midodrine, fludrocortisone, and droxidopa. Sometimes that combination of a heart rate-lowering medication and a medication that increases blood pressure really works well.
Dr. O’Donoghue: That’s very helpful. I think that those kinds of practical tips are the ones that practitioners really want to reach for, because they need to have that algorithm in their mind to take on this condition. Thanks again for walking us through that.
I think it’s a very interesting space, and there’s more that we’re going to be learning over the next few years as we further flesh out these post-COVID cases and what we learn from that as well.
Dr. Taub: There are many clinical trials now starting in POTS, so it’s exciting.
Dr. O’Donoghue: Absolutely. Thank you again for joining me today. Signing off, this is Dr Michelle O’Donoghue.
Dr. O’Donoghue is a cardiologist at Brigham and Women’s Hospital and senior investigator with the TIMI Study Group. A strong believer in evidence-based medicine, she relishes discussions about the published literature. A native Canadian, Dr. O’Donoghue loves spending time outdoors with her family but admits with shame that she’s never strapped on hockey skates. She disclosed ties with Amgen, AstraZeneca Pharmaceuticals LP, CVS Minute Clinic, Eisai, GlaxoSmithKline, Janssen Pharmaceuticals, Merck, Novartis, and The Medicines Company. Dr. Taub is professor of Medicine, University of California San Diego Health, La Jolla. She disclosed ties with Amgen, Bayer, Boehringer Ingelheim, Medtronic, Merck, Novartis, Novo Nordisk, and Sanofi.
A version of this article appeared on Medscape.com.
This transcript has been edited for clarity.
Michelle L. O’Donoghue, MD, MPH: I’m here in Amsterdam at the European Society of Cardiology (ESC) Congress 2023. Joining me for a great discussion is my friend Dr. Pam Taub, who is a cardiologist and a professor of medicine at UC San Diego. She has a particular interest in postural orthostatic tachycardia syndrome (POTS), so that’s what we’ll be talking about today.
Thanks for joining me, Pam. When we think about POTS, for those who are not familiar with the term, what does it actually mean and how do you diagnose it?
No tilt table required
Pam R. Taub, MD: That’s typically associated with symptoms such as lightheadedness, dizziness, and cognitive difficulties such as brain fog. The diagnosis can be made by tilt-table testing, but it can also be made in the office with simple orthostats.
In my clinic, I have people lie down for 3-5 minutes. At the end of that period, you get a heart rate and blood pressure. Then you have them stand up for 3-5 minutes and then get heart rate and blood pressure, and you look at the differences. If the heart rate goes up by 30 points – so maybe they’re 80 beats/min when they’re lying down and when they stand up, it goes to 110 beats/min – that’s POTS, so very objective criteria. Typically, these people don’t have what we call orthostatic hypotension, where there is a significant decrease in the blood pressure. It’s more a heart rate issue.
Dr. O’Donoghue: How symptomatically do they usually present?
Dr. Taub: It’s a spectrum. Some people have mild symptoms. After they’re in the upright position for maybe 10 minutes, they get symptoms. There are some people who, when they go from a lying to standing position, they’re extremely symptomatic and can’t really do any activities. There are some people that are even wheelchair-bound because the symptoms are so debilitating. There’s a wide spectrum.
Dr. O’Donoghue: There has been more discussion, I feel like, about the rising prevalence of POTS as a diagnosis, and in particular since the COVID pandemic. What’s our understanding of the relationship between COVID and POTS and what the mechanism might be?
Dr. Taub: We’ve known that POTS can be triggered by a viral infection. Before COVID, we knew that in certain individuals that we think have an underlying genetic predisposition, usually some autoimmune substrate, when they get certain types of infections, whether it’s influenza or mononucleosis, they get POTS.
Typically, when they get an infection, they start getting deconditioned. They don’t feel well, so they’re on bed rest. When they get long periods of bed rest, when they start to become active, they start to have overactivation of their sympathetic nervous system, and they have a large amount of cardiovascular deconditioning. It’s a cycle that is often triggered after an infection.
A huge increase of POTS has been seen after COVID-19 because we had so many people exposed to this virus. With COVID-19, there is a period where people don’t feel great and they are getting bed rest, so they’re getting deconditioned. We’ve seen so many patients referred for post-COVID POTS and also long COVID or the post-acute sequelae of COVID-19, where POTS is a part of that presentation.
Female sex and autoimmune conditions
Dr. O’Donoghue: We know that POTS seems to disproportionately affect women. Is that understood? Is it thought that that’s related to the perhaps the autoimmune component of that illness?
Dr. Taub: Yes. The theory is because women tend to have more autoimmune conditions, that’s why they’re more predisposed. There’s a large amount of genetic susceptibility. For instance, we know that there’s an association between POTS and conditions like Ehlers-Danlos syndrome and between POTS and mast cell activation. Some of those conditions are more prevalent in women as well.
Dr. O’Donoghue: I feel like many physicians don’t know how to manage POTS, and they’re actually a little fearful perhaps to take it on. Fortunately, there have been a growing number of POTS clinics with specialists that focus on that area. For the average practitioner who maybe can’t refer to a POTS clinic, how should they approach that?
Dr. Taub: The first thing is its diagnosis. When someone tells you that they have symptoms of orthostatic intolerance – so, activities that involve standing – you need to first have that on your differential diagnosis. You can make the diagnosis in the office with orthostats. You don’t need a tilt table. It’s sometimes helpful if you’re unsure about the diagnosis, but you can make the diagnosis.
Many times, you’re finding people that have very mild symptoms. You can treat that with some good lifestyle recommendations, such as increased hydration, increasing salt in their diet, and compression. And the exercise component is really important.
Many people with POTS are told to go exercise, go for a run, or go for a walk. That’s incorrect, because these people have symptoms when they’re in the upright position. The type of exercise they need to do initially is exercise in the lying or seated position – so exercises like rowing or a seated bike, and strength training. As they start to feel better, then they can do upright exercise.
You should never tell a person that has POTS to just initially start with upright exercise, because they’re going to feel so much worse and then they’re never going to want to exercise. It’s really important to give them the right exercise recommendations. I find that for many of these mild cases, if they do the right exercise and engage in the right lifestyle strategies, they get better.
Compression wear and drug therapy
Dr. O’Donoghue: When it comes to compression stockings, do you usually start with a particular length?
Dr. Taub: It’s interesting. There are many different compression stockings, medical grade. Through patients with POTS, I’ve gotten feedback on certain types of athletic wear that have built-in compression, and that’s a little bit easier for people to wear every day because they can do their errands and it doesn’t look like they’re wearing medical-grade compression stockings.
Basically, I’ve collected all the different recommendations that patients say help, and I give them a list. The medical-grade compression stockings sometimes are very challenging to put on, and sometimes people just need light compression or even just socks. Any kind of compression is going to help.
Dr. O’Donoghue: That’s a great tip, because I know there are many patients who refuse to wear the compression stockings. If there’s a fashionable alternative, that’s always good to reach for.
Dr. Taub: Another thing that patients have told me is that abdominal compression is also very helpful. There are many commercially available abdominal compression options, like shapewear. Many patients with POTS use that and that helps, too.
Dr. O’Donoghue: Good. For those patients with POTS that is refractory to the measures you’ve already discussed, what are the next steps after that?
Dr. Taub: Pharmacotherapy is very synergistic with lifestyle, and there are many different pharmacotherapy options. One of the first things that you want to think about is lowering that heart rate. The reason people feel horrible is because their heart rate is usually very high when they’re upright. If they’re upright for long periods of time and they’re having very high heart rates, they’re going to get really tired because it’s like they’re exercising for hours when they’re upright.
Heart rate lowering is the cornerstone of therapy. Traditionally, we’ve used beta-blockers for heart rate lowering. The problem is they also lower blood pressure. They can also cause fatigue, so not the ideal agent for patients with POTS.
One of the clinical trials that I led was with a drug called ivabradine, which selectively works on the SA node and decreases heart rate without affecting blood pressure. What’s really elegant about ivabradine is it has a more potent effect when the heart rate is higher. When the patient is standing, it’s going to have a more potent effect on heart rate lowering. It’s really well tolerated in patients with POTS. In our study, we showed an improvement in quality of life metrics. That’s one of the first-line drugs that I use for patients with POTS.
The other thing is some of them will also have a concomitant lowering of blood pressure. You can think about medications that increase blood pressure, like midodrine, fludrocortisone, and droxidopa. Sometimes that combination of a heart rate-lowering medication and a medication that increases blood pressure really works well.
Dr. O’Donoghue: That’s very helpful. I think that those kinds of practical tips are the ones that practitioners really want to reach for, because they need to have that algorithm in their mind to take on this condition. Thanks again for walking us through that.
I think it’s a very interesting space, and there’s more that we’re going to be learning over the next few years as we further flesh out these post-COVID cases and what we learn from that as well.
Dr. Taub: There are many clinical trials now starting in POTS, so it’s exciting.
Dr. O’Donoghue: Absolutely. Thank you again for joining me today. Signing off, this is Dr Michelle O’Donoghue.
Dr. O’Donoghue is a cardiologist at Brigham and Women’s Hospital and senior investigator with the TIMI Study Group. A strong believer in evidence-based medicine, she relishes discussions about the published literature. A native Canadian, Dr. O’Donoghue loves spending time outdoors with her family but admits with shame that she’s never strapped on hockey skates. She disclosed ties with Amgen, AstraZeneca Pharmaceuticals LP, CVS Minute Clinic, Eisai, GlaxoSmithKline, Janssen Pharmaceuticals, Merck, Novartis, and The Medicines Company. Dr. Taub is professor of Medicine, University of California San Diego Health, La Jolla. She disclosed ties with Amgen, Bayer, Boehringer Ingelheim, Medtronic, Merck, Novartis, Novo Nordisk, and Sanofi.
A version of this article appeared on Medscape.com.
This transcript has been edited for clarity.
Michelle L. O’Donoghue, MD, MPH: I’m here in Amsterdam at the European Society of Cardiology (ESC) Congress 2023. Joining me for a great discussion is my friend Dr. Pam Taub, who is a cardiologist and a professor of medicine at UC San Diego. She has a particular interest in postural orthostatic tachycardia syndrome (POTS), so that’s what we’ll be talking about today.
Thanks for joining me, Pam. When we think about POTS, for those who are not familiar with the term, what does it actually mean and how do you diagnose it?
No tilt table required
Pam R. Taub, MD: That’s typically associated with symptoms such as lightheadedness, dizziness, and cognitive difficulties such as brain fog. The diagnosis can be made by tilt-table testing, but it can also be made in the office with simple orthostats.
In my clinic, I have people lie down for 3-5 minutes. At the end of that period, you get a heart rate and blood pressure. Then you have them stand up for 3-5 minutes and then get heart rate and blood pressure, and you look at the differences. If the heart rate goes up by 30 points – so maybe they’re 80 beats/min when they’re lying down and when they stand up, it goes to 110 beats/min – that’s POTS, so very objective criteria. Typically, these people don’t have what we call orthostatic hypotension, where there is a significant decrease in the blood pressure. It’s more a heart rate issue.
Dr. O’Donoghue: How symptomatically do they usually present?
Dr. Taub: It’s a spectrum. Some people have mild symptoms. After they’re in the upright position for maybe 10 minutes, they get symptoms. There are some people who, when they go from a lying to standing position, they’re extremely symptomatic and can’t really do any activities. There are some people that are even wheelchair-bound because the symptoms are so debilitating. There’s a wide spectrum.
Dr. O’Donoghue: There has been more discussion, I feel like, about the rising prevalence of POTS as a diagnosis, and in particular since the COVID pandemic. What’s our understanding of the relationship between COVID and POTS and what the mechanism might be?
Dr. Taub: We’ve known that POTS can be triggered by a viral infection. Before COVID, we knew that in certain individuals that we think have an underlying genetic predisposition, usually some autoimmune substrate, when they get certain types of infections, whether it’s influenza or mononucleosis, they get POTS.
Typically, when they get an infection, they start getting deconditioned. They don’t feel well, so they’re on bed rest. When they get long periods of bed rest, when they start to become active, they start to have overactivation of their sympathetic nervous system, and they have a large amount of cardiovascular deconditioning. It’s a cycle that is often triggered after an infection.
A huge increase of POTS has been seen after COVID-19 because we had so many people exposed to this virus. With COVID-19, there is a period where people don’t feel great and they are getting bed rest, so they’re getting deconditioned. We’ve seen so many patients referred for post-COVID POTS and also long COVID or the post-acute sequelae of COVID-19, where POTS is a part of that presentation.
Female sex and autoimmune conditions
Dr. O’Donoghue: We know that POTS seems to disproportionately affect women. Is that understood? Is it thought that that’s related to the perhaps the autoimmune component of that illness?
Dr. Taub: Yes. The theory is because women tend to have more autoimmune conditions, that’s why they’re more predisposed. There’s a large amount of genetic susceptibility. For instance, we know that there’s an association between POTS and conditions like Ehlers-Danlos syndrome and between POTS and mast cell activation. Some of those conditions are more prevalent in women as well.
Dr. O’Donoghue: I feel like many physicians don’t know how to manage POTS, and they’re actually a little fearful perhaps to take it on. Fortunately, there have been a growing number of POTS clinics with specialists that focus on that area. For the average practitioner who maybe can’t refer to a POTS clinic, how should they approach that?
Dr. Taub: The first thing is its diagnosis. When someone tells you that they have symptoms of orthostatic intolerance – so, activities that involve standing – you need to first have that on your differential diagnosis. You can make the diagnosis in the office with orthostats. You don’t need a tilt table. It’s sometimes helpful if you’re unsure about the diagnosis, but you can make the diagnosis.
Many times, you’re finding people that have very mild symptoms. You can treat that with some good lifestyle recommendations, such as increased hydration, increasing salt in their diet, and compression. And the exercise component is really important.
Many people with POTS are told to go exercise, go for a run, or go for a walk. That’s incorrect, because these people have symptoms when they’re in the upright position. The type of exercise they need to do initially is exercise in the lying or seated position – so exercises like rowing or a seated bike, and strength training. As they start to feel better, then they can do upright exercise.
You should never tell a person that has POTS to just initially start with upright exercise, because they’re going to feel so much worse and then they’re never going to want to exercise. It’s really important to give them the right exercise recommendations. I find that for many of these mild cases, if they do the right exercise and engage in the right lifestyle strategies, they get better.
Compression wear and drug therapy
Dr. O’Donoghue: When it comes to compression stockings, do you usually start with a particular length?
Dr. Taub: It’s interesting. There are many different compression stockings, medical grade. Through patients with POTS, I’ve gotten feedback on certain types of athletic wear that have built-in compression, and that’s a little bit easier for people to wear every day because they can do their errands and it doesn’t look like they’re wearing medical-grade compression stockings.
Basically, I’ve collected all the different recommendations that patients say help, and I give them a list. The medical-grade compression stockings sometimes are very challenging to put on, and sometimes people just need light compression or even just socks. Any kind of compression is going to help.
Dr. O’Donoghue: That’s a great tip, because I know there are many patients who refuse to wear the compression stockings. If there’s a fashionable alternative, that’s always good to reach for.
Dr. Taub: Another thing that patients have told me is that abdominal compression is also very helpful. There are many commercially available abdominal compression options, like shapewear. Many patients with POTS use that and that helps, too.
Dr. O’Donoghue: Good. For those patients with POTS that is refractory to the measures you’ve already discussed, what are the next steps after that?
Dr. Taub: Pharmacotherapy is very synergistic with lifestyle, and there are many different pharmacotherapy options. One of the first things that you want to think about is lowering that heart rate. The reason people feel horrible is because their heart rate is usually very high when they’re upright. If they’re upright for long periods of time and they’re having very high heart rates, they’re going to get really tired because it’s like they’re exercising for hours when they’re upright.
Heart rate lowering is the cornerstone of therapy. Traditionally, we’ve used beta-blockers for heart rate lowering. The problem is they also lower blood pressure. They can also cause fatigue, so not the ideal agent for patients with POTS.
One of the clinical trials that I led was with a drug called ivabradine, which selectively works on the SA node and decreases heart rate without affecting blood pressure. What’s really elegant about ivabradine is it has a more potent effect when the heart rate is higher. When the patient is standing, it’s going to have a more potent effect on heart rate lowering. It’s really well tolerated in patients with POTS. In our study, we showed an improvement in quality of life metrics. That’s one of the first-line drugs that I use for patients with POTS.
The other thing is some of them will also have a concomitant lowering of blood pressure. You can think about medications that increase blood pressure, like midodrine, fludrocortisone, and droxidopa. Sometimes that combination of a heart rate-lowering medication and a medication that increases blood pressure really works well.
Dr. O’Donoghue: That’s very helpful. I think that those kinds of practical tips are the ones that practitioners really want to reach for, because they need to have that algorithm in their mind to take on this condition. Thanks again for walking us through that.
I think it’s a very interesting space, and there’s more that we’re going to be learning over the next few years as we further flesh out these post-COVID cases and what we learn from that as well.
Dr. Taub: There are many clinical trials now starting in POTS, so it’s exciting.
Dr. O’Donoghue: Absolutely. Thank you again for joining me today. Signing off, this is Dr Michelle O’Donoghue.
Dr. O’Donoghue is a cardiologist at Brigham and Women’s Hospital and senior investigator with the TIMI Study Group. A strong believer in evidence-based medicine, she relishes discussions about the published literature. A native Canadian, Dr. O’Donoghue loves spending time outdoors with her family but admits with shame that she’s never strapped on hockey skates. She disclosed ties with Amgen, AstraZeneca Pharmaceuticals LP, CVS Minute Clinic, Eisai, GlaxoSmithKline, Janssen Pharmaceuticals, Merck, Novartis, and The Medicines Company. Dr. Taub is professor of Medicine, University of California San Diego Health, La Jolla. She disclosed ties with Amgen, Bayer, Boehringer Ingelheim, Medtronic, Merck, Novartis, Novo Nordisk, and Sanofi.
A version of this article appeared on Medscape.com.
Lead pollutants as harmful to health as particulate matter
in a presentation to the World Bank. Their work was published in The Lancet Planetary Health.
As Mr. Larsen and Mr. Sánchez-Triana report, the economic consequences of increased exposure to lead are already immense, especially in low- and middle-income countries (LMICs). The study was financed by the Korea Green Growth Trust Fund and the World Bank’s Pollution Management and Environmental Health Program.
Intellectual, cardiovascular effects
“It is a very important publication that affects all of us,” pediatrician Stephan Böse-O’Reilly, MD, of the Institute and Polyclinic for Occupational, Social, and Environmental Health at Ludwig Maximilian University Hospital in Munich, Germany, said in an interview. “The study, the results of which I think are very reliable, shows that elevated levels of lead in the blood have a much more drastic effect on children’s intelligence than we previously thought.”
It is well known that lead affects the antenatal and postnatal cognitive development of children, Dr. Böse-O’Reilly explained. But the extent of this effect has quite clearly been underestimated before now.
On the other hand, Mr. Larsen and Mr. Sánchez-Triana’s work could prove that lead may lead to more cardiovascular diseases in adulthood. “We already knew that increased exposure to lead increased the risk of high blood pressure and, as a result, mortality,” said Dr. Böse-O’Reilly. “This study now very clearly shows that the risk of arteriosclerosis, for example, also increases through lead exposure.”
Figures from 2019
“For the first time, to our knowledge, we aimed to estimate the global burden and cost of IQ loss and cardiovascular disease mortality from lead exposure,” wrote Mr. Larsen and Mr. Sánchez-Triana. For their calculations, the scientists used blood lead level estimates from the Global Burden of Diseases, Injuries, and Risk Factors Study (GBD) 2019.
They estimated IQ loss in children younger than 5 years using the internationally recognized blood lead level–IQ loss function. The researchers subsequently estimated the cost of this IQ loss based on the loss in lifetime income, presented as cost in U.S. dollars and percentage of gross domestic product (GDP).
Mr. Larsen and Mr. Sánchez-Triana estimated cardiovascular deaths caused by lead exposure in adults aged 25 years or older using a model that captures the effects of lead exposure on cardiovascular disease mortality that is mediated through mechanisms other than hypertension.
Finally, they used the statistical life expectancy to estimate the welfare cost of premature mortality, also presented as cost in U.S. dollars and percentage of GDP. All estimates were calculated according to the World Bank income classification for 2019.
Millions of deaths
As reported by Mr. Larsen and Mr. Sánchez-Triana, children younger than 5 years lost an estimated 765 million IQ points worldwide because of lead exposure in this period. In 2019, 5,545,000 adults died from cardiovascular diseases caused by lead exposure. The scientists recorded 729 million of the IQ points lost (95.3%) and 5,004,000 (90.2%) of the deaths as occurring in LMICs.
The IQ loss here was nearly 80% higher than a previous estimate, wrote Mr. Larsen and Mr. Sánchez-Triana. The number of cardiovascular disease deaths they determined was six times higher than the GBD 2019 estimate.
“These are results with which the expert societies, especially the German Society of Pediatrics and Adolescent Medicine and the German Cardiac Society, and the corresponding professional associations need to concern themselves,” said Dr. Böse-O’Reilly.
Although blood lead concentrations have declined substantially since the phase-out of leaded gasoline, especially in Western countries, lead still represents a major health issue because it stays in the bones for decades.
European situation moderate
“We need a broad discussion on questions such as whether lead levels should be included in prophylactic assessments in certain age groups, what blood level is even tolerable, and in what situation medicinal therapy with chelating agents would possibly be appropriate,” said Dr. Böse-O’Reilly.
“Of course, we cannot answer these questions on the basis of one individual study,” he added. “However, the work in question definitely illustrates how dangerous lead can be and that we need further research into the actual burden and the best preventive measures.”
In this respect, the situation in Europe is still comparatively moderate. “Globally, lead exposure has risen in recent years,” said Dr. Böse-O’Reilly. According to an investigation by the Planet Earth Foundation, outside of the European Union, lead can increasingly be found in toys, spices, and cooking utensils, for example.
“Especially in lower-income countries, there is a lack of consumer protection or a good monitoring program like we have here in the EU,” said Dr. Böse-O’Reilly. In these countries, lead is sometimes added to spices by unscrupulous retailers to make the color more intense or to simply add to its weight to gain more profit.
Recycling lead-acid batteries or other electrical waste, often transferred to poorer countries, constitutes a large problem. “In general, children in Germany have a blood lead level of less than 1 mcg/dL,” explained Dr. Böse-O’Reilly. “In some regions of Indonesia, where these recycling factories are located, more than 50% of children have levels of more than 20 mcg/dL.”
Particulate matter
According to Mr. Larsen and Mr. Sánchez-Triana, the global cost of increased lead exposure was around $6 trillion USD in 2019, which was equivalent to 6.9% of global GDP. About 77% of the cost ($4.62 trillion USD) comprised the welfare costs of cardiovascular disease mortality, and 23% ($1.38 trillion USD) comprised the present value of future income losses because of IQ loss in children.
“Our findings suggest that global lead exposure has health and economic costs on par with PM2.5 air pollution,” wrote the authors. This places lead as an environmental risk factor on par with particulate matter and above that of air pollution from solid fuels, ahead of unsafe drinking water, unhygienic sanitation, or insufficient handwashing.
“This finding is in contrast to that of GBD 2019, which ranked lead exposure as a distant fourth environmental risk factor, due to not accounting for IQ loss in children – other than idiopathic developmental intellectual disability in a small subset of children – and reporting a substantially lower estimate of adult cardiovascular disease mortality,” wrote Mr. Larsen and Mr. Sánchez-Triana.
“A central implication for future research and policy is that LMICs bear an extraordinarily large share of the health and cost burden of lead exposure,” wrote the authors. Consequently, improved quality of blood lead level measurements and identification of sources containing lead are urgently needed there.
Improved recycling methods
Dr. Böse-O’Reilly would like an increased focus on children. “If children’s cognitive skills are lost, this of course has a long-term effect on a country’s economic position,” he said. “Precisely that which LMICs actually need for their development is being stripped from them.
“We should think long and hard about whether we really need to send so much of our electrical waste and so many old cars to poorer countries, where they are incorrectly recycled,” he warned. “We should at least give the LMICs the support necessary for them to be able to process lead-containing products in the future so that less lead makes it into the environment.
“Through these global cycles, we all contribute a lot toward the worldwide lead burden,” Dr. Böse-O’Reilly said. “In my opinion, the German Supply Chain Act is therefore definitely sensible. Not only does it protect our own economy, but it also protects the health of people in other countries.”
This article was translated from Medscape’s German Edition. A version of this article appeared on Medscape.com.
in a presentation to the World Bank. Their work was published in The Lancet Planetary Health.
As Mr. Larsen and Mr. Sánchez-Triana report, the economic consequences of increased exposure to lead are already immense, especially in low- and middle-income countries (LMICs). The study was financed by the Korea Green Growth Trust Fund and the World Bank’s Pollution Management and Environmental Health Program.
Intellectual, cardiovascular effects
“It is a very important publication that affects all of us,” pediatrician Stephan Böse-O’Reilly, MD, of the Institute and Polyclinic for Occupational, Social, and Environmental Health at Ludwig Maximilian University Hospital in Munich, Germany, said in an interview. “The study, the results of which I think are very reliable, shows that elevated levels of lead in the blood have a much more drastic effect on children’s intelligence than we previously thought.”
It is well known that lead affects the antenatal and postnatal cognitive development of children, Dr. Böse-O’Reilly explained. But the extent of this effect has quite clearly been underestimated before now.
On the other hand, Mr. Larsen and Mr. Sánchez-Triana’s work could prove that lead may lead to more cardiovascular diseases in adulthood. “We already knew that increased exposure to lead increased the risk of high blood pressure and, as a result, mortality,” said Dr. Böse-O’Reilly. “This study now very clearly shows that the risk of arteriosclerosis, for example, also increases through lead exposure.”
Figures from 2019
“For the first time, to our knowledge, we aimed to estimate the global burden and cost of IQ loss and cardiovascular disease mortality from lead exposure,” wrote Mr. Larsen and Mr. Sánchez-Triana. For their calculations, the scientists used blood lead level estimates from the Global Burden of Diseases, Injuries, and Risk Factors Study (GBD) 2019.
They estimated IQ loss in children younger than 5 years using the internationally recognized blood lead level–IQ loss function. The researchers subsequently estimated the cost of this IQ loss based on the loss in lifetime income, presented as cost in U.S. dollars and percentage of gross domestic product (GDP).
Mr. Larsen and Mr. Sánchez-Triana estimated cardiovascular deaths caused by lead exposure in adults aged 25 years or older using a model that captures the effects of lead exposure on cardiovascular disease mortality that is mediated through mechanisms other than hypertension.
Finally, they used the statistical life expectancy to estimate the welfare cost of premature mortality, also presented as cost in U.S. dollars and percentage of GDP. All estimates were calculated according to the World Bank income classification for 2019.
Millions of deaths
As reported by Mr. Larsen and Mr. Sánchez-Triana, children younger than 5 years lost an estimated 765 million IQ points worldwide because of lead exposure in this period. In 2019, 5,545,000 adults died from cardiovascular diseases caused by lead exposure. The scientists recorded 729 million of the IQ points lost (95.3%) and 5,004,000 (90.2%) of the deaths as occurring in LMICs.
The IQ loss here was nearly 80% higher than a previous estimate, wrote Mr. Larsen and Mr. Sánchez-Triana. The number of cardiovascular disease deaths they determined was six times higher than the GBD 2019 estimate.
“These are results with which the expert societies, especially the German Society of Pediatrics and Adolescent Medicine and the German Cardiac Society, and the corresponding professional associations need to concern themselves,” said Dr. Böse-O’Reilly.
Although blood lead concentrations have declined substantially since the phase-out of leaded gasoline, especially in Western countries, lead still represents a major health issue because it stays in the bones for decades.
European situation moderate
“We need a broad discussion on questions such as whether lead levels should be included in prophylactic assessments in certain age groups, what blood level is even tolerable, and in what situation medicinal therapy with chelating agents would possibly be appropriate,” said Dr. Böse-O’Reilly.
“Of course, we cannot answer these questions on the basis of one individual study,” he added. “However, the work in question definitely illustrates how dangerous lead can be and that we need further research into the actual burden and the best preventive measures.”
In this respect, the situation in Europe is still comparatively moderate. “Globally, lead exposure has risen in recent years,” said Dr. Böse-O’Reilly. According to an investigation by the Planet Earth Foundation, outside of the European Union, lead can increasingly be found in toys, spices, and cooking utensils, for example.
“Especially in lower-income countries, there is a lack of consumer protection or a good monitoring program like we have here in the EU,” said Dr. Böse-O’Reilly. In these countries, lead is sometimes added to spices by unscrupulous retailers to make the color more intense or to simply add to its weight to gain more profit.
Recycling lead-acid batteries or other electrical waste, often transferred to poorer countries, constitutes a large problem. “In general, children in Germany have a blood lead level of less than 1 mcg/dL,” explained Dr. Böse-O’Reilly. “In some regions of Indonesia, where these recycling factories are located, more than 50% of children have levels of more than 20 mcg/dL.”
Particulate matter
According to Mr. Larsen and Mr. Sánchez-Triana, the global cost of increased lead exposure was around $6 trillion USD in 2019, which was equivalent to 6.9% of global GDP. About 77% of the cost ($4.62 trillion USD) comprised the welfare costs of cardiovascular disease mortality, and 23% ($1.38 trillion USD) comprised the present value of future income losses because of IQ loss in children.
“Our findings suggest that global lead exposure has health and economic costs on par with PM2.5 air pollution,” wrote the authors. This places lead as an environmental risk factor on par with particulate matter and above that of air pollution from solid fuels, ahead of unsafe drinking water, unhygienic sanitation, or insufficient handwashing.
“This finding is in contrast to that of GBD 2019, which ranked lead exposure as a distant fourth environmental risk factor, due to not accounting for IQ loss in children – other than idiopathic developmental intellectual disability in a small subset of children – and reporting a substantially lower estimate of adult cardiovascular disease mortality,” wrote Mr. Larsen and Mr. Sánchez-Triana.
“A central implication for future research and policy is that LMICs bear an extraordinarily large share of the health and cost burden of lead exposure,” wrote the authors. Consequently, improved quality of blood lead level measurements and identification of sources containing lead are urgently needed there.
Improved recycling methods
Dr. Böse-O’Reilly would like an increased focus on children. “If children’s cognitive skills are lost, this of course has a long-term effect on a country’s economic position,” he said. “Precisely that which LMICs actually need for their development is being stripped from them.
“We should think long and hard about whether we really need to send so much of our electrical waste and so many old cars to poorer countries, where they are incorrectly recycled,” he warned. “We should at least give the LMICs the support necessary for them to be able to process lead-containing products in the future so that less lead makes it into the environment.
“Through these global cycles, we all contribute a lot toward the worldwide lead burden,” Dr. Böse-O’Reilly said. “In my opinion, the German Supply Chain Act is therefore definitely sensible. Not only does it protect our own economy, but it also protects the health of people in other countries.”
This article was translated from Medscape’s German Edition. A version of this article appeared on Medscape.com.
in a presentation to the World Bank. Their work was published in The Lancet Planetary Health.
As Mr. Larsen and Mr. Sánchez-Triana report, the economic consequences of increased exposure to lead are already immense, especially in low- and middle-income countries (LMICs). The study was financed by the Korea Green Growth Trust Fund and the World Bank’s Pollution Management and Environmental Health Program.
Intellectual, cardiovascular effects
“It is a very important publication that affects all of us,” pediatrician Stephan Böse-O’Reilly, MD, of the Institute and Polyclinic for Occupational, Social, and Environmental Health at Ludwig Maximilian University Hospital in Munich, Germany, said in an interview. “The study, the results of which I think are very reliable, shows that elevated levels of lead in the blood have a much more drastic effect on children’s intelligence than we previously thought.”
It is well known that lead affects the antenatal and postnatal cognitive development of children, Dr. Böse-O’Reilly explained. But the extent of this effect has quite clearly been underestimated before now.
On the other hand, Mr. Larsen and Mr. Sánchez-Triana’s work could prove that lead may lead to more cardiovascular diseases in adulthood. “We already knew that increased exposure to lead increased the risk of high blood pressure and, as a result, mortality,” said Dr. Böse-O’Reilly. “This study now very clearly shows that the risk of arteriosclerosis, for example, also increases through lead exposure.”
Figures from 2019
“For the first time, to our knowledge, we aimed to estimate the global burden and cost of IQ loss and cardiovascular disease mortality from lead exposure,” wrote Mr. Larsen and Mr. Sánchez-Triana. For their calculations, the scientists used blood lead level estimates from the Global Burden of Diseases, Injuries, and Risk Factors Study (GBD) 2019.
They estimated IQ loss in children younger than 5 years using the internationally recognized blood lead level–IQ loss function. The researchers subsequently estimated the cost of this IQ loss based on the loss in lifetime income, presented as cost in U.S. dollars and percentage of gross domestic product (GDP).
Mr. Larsen and Mr. Sánchez-Triana estimated cardiovascular deaths caused by lead exposure in adults aged 25 years or older using a model that captures the effects of lead exposure on cardiovascular disease mortality that is mediated through mechanisms other than hypertension.
Finally, they used the statistical life expectancy to estimate the welfare cost of premature mortality, also presented as cost in U.S. dollars and percentage of GDP. All estimates were calculated according to the World Bank income classification for 2019.
Millions of deaths
As reported by Mr. Larsen and Mr. Sánchez-Triana, children younger than 5 years lost an estimated 765 million IQ points worldwide because of lead exposure in this period. In 2019, 5,545,000 adults died from cardiovascular diseases caused by lead exposure. The scientists recorded 729 million of the IQ points lost (95.3%) and 5,004,000 (90.2%) of the deaths as occurring in LMICs.
The IQ loss here was nearly 80% higher than a previous estimate, wrote Mr. Larsen and Mr. Sánchez-Triana. The number of cardiovascular disease deaths they determined was six times higher than the GBD 2019 estimate.
“These are results with which the expert societies, especially the German Society of Pediatrics and Adolescent Medicine and the German Cardiac Society, and the corresponding professional associations need to concern themselves,” said Dr. Böse-O’Reilly.
Although blood lead concentrations have declined substantially since the phase-out of leaded gasoline, especially in Western countries, lead still represents a major health issue because it stays in the bones for decades.
European situation moderate
“We need a broad discussion on questions such as whether lead levels should be included in prophylactic assessments in certain age groups, what blood level is even tolerable, and in what situation medicinal therapy with chelating agents would possibly be appropriate,” said Dr. Böse-O’Reilly.
“Of course, we cannot answer these questions on the basis of one individual study,” he added. “However, the work in question definitely illustrates how dangerous lead can be and that we need further research into the actual burden and the best preventive measures.”
In this respect, the situation in Europe is still comparatively moderate. “Globally, lead exposure has risen in recent years,” said Dr. Böse-O’Reilly. According to an investigation by the Planet Earth Foundation, outside of the European Union, lead can increasingly be found in toys, spices, and cooking utensils, for example.
“Especially in lower-income countries, there is a lack of consumer protection or a good monitoring program like we have here in the EU,” said Dr. Böse-O’Reilly. In these countries, lead is sometimes added to spices by unscrupulous retailers to make the color more intense or to simply add to its weight to gain more profit.
Recycling lead-acid batteries or other electrical waste, often transferred to poorer countries, constitutes a large problem. “In general, children in Germany have a blood lead level of less than 1 mcg/dL,” explained Dr. Böse-O’Reilly. “In some regions of Indonesia, where these recycling factories are located, more than 50% of children have levels of more than 20 mcg/dL.”
Particulate matter
According to Mr. Larsen and Mr. Sánchez-Triana, the global cost of increased lead exposure was around $6 trillion USD in 2019, which was equivalent to 6.9% of global GDP. About 77% of the cost ($4.62 trillion USD) comprised the welfare costs of cardiovascular disease mortality, and 23% ($1.38 trillion USD) comprised the present value of future income losses because of IQ loss in children.
“Our findings suggest that global lead exposure has health and economic costs on par with PM2.5 air pollution,” wrote the authors. This places lead as an environmental risk factor on par with particulate matter and above that of air pollution from solid fuels, ahead of unsafe drinking water, unhygienic sanitation, or insufficient handwashing.
“This finding is in contrast to that of GBD 2019, which ranked lead exposure as a distant fourth environmental risk factor, due to not accounting for IQ loss in children – other than idiopathic developmental intellectual disability in a small subset of children – and reporting a substantially lower estimate of adult cardiovascular disease mortality,” wrote Mr. Larsen and Mr. Sánchez-Triana.
“A central implication for future research and policy is that LMICs bear an extraordinarily large share of the health and cost burden of lead exposure,” wrote the authors. Consequently, improved quality of blood lead level measurements and identification of sources containing lead are urgently needed there.
Improved recycling methods
Dr. Böse-O’Reilly would like an increased focus on children. “If children’s cognitive skills are lost, this of course has a long-term effect on a country’s economic position,” he said. “Precisely that which LMICs actually need for their development is being stripped from them.
“We should think long and hard about whether we really need to send so much of our electrical waste and so many old cars to poorer countries, where they are incorrectly recycled,” he warned. “We should at least give the LMICs the support necessary for them to be able to process lead-containing products in the future so that less lead makes it into the environment.
“Through these global cycles, we all contribute a lot toward the worldwide lead burden,” Dr. Böse-O’Reilly said. “In my opinion, the German Supply Chain Act is therefore definitely sensible. Not only does it protect our own economy, but it also protects the health of people in other countries.”
This article was translated from Medscape’s German Edition. A version of this article appeared on Medscape.com.
FROM THE LANCET PLANETARY HEALTH