User login
For MD-IQ use only
Coronavirus impact on medical education: Thoughts from two GI fellows’ perspectives
Introduction
We are living in an unprecedented time. During March 2020, in response to the COVID-19 (coronavirus disease 2019) outbreak, our institution removed all medical students from rotations with direct patient contact to prioritize their safety and well-being, following recommendations made by the Association of American Medical Colleges (AAMC).1 Similarly, we as gastroenterology fellows experienced an upheaval in our usual schedules and routines. Some of us were redeployed to other areas of the hospital, such as inpatient wards and emergency departments, to meet the needs of our patients and our health system. These changes were difficult, not only because we were practicing in different roles, but also because unknown situations commonly incite fear and anxiety.
Among the repercussions of the COVID-19 pandemic were the changes thrust upon medical students who suddenly found themselves without clinical exposure (both on core clerkships and electives) for the duration of the academic year.2 We too lost many of our educational and teaching opportunities as we adapted to our changing circumstances and new reality. Therefore, . We used the lessons we learned because of the changes in our own medical education to anticipate the best ways to provide learning opportunities for our students.
GI fellows’ experiences
The changes to our schedules and lack of in-person educational conferences seemingly happened overnight – the shock of being pulled from clinics, consults, and endoscopy left us feeling scared and lonely. We were quickly transitioned from knowing our roles and responsibilities as GI providers to taking over care for hospitalist patients as the “primary team,” working in the COVID emergency department (ED), and losing our clinic space. Redeployment to other clinical environments was anxiety-provoking. Self-doubt and fear were the most cited concerns as we asked ourselves: Do I remember enough general medicine to be an effective hospitalist? How do I place admission orders or perform a medication reconciliation on discharge? What can I expect in the COVID ED? Will I have to intubate someone? What about possible PPE shortages? Are my family members safe at home? Should I stay in a hotel? Do we have estimates on how long this will last?
Clinical schedules were reconfigured to consolidate the use of inpatient fellows and allow for reserves of fellows to be redeployed if needed. Schedules for the following 7 days were made just 48 hours prior to the start of each workweek. The anticipation and fear of the unknown were perhaps the hardest parts of the changes in our clinical learning environment. Little time was provided to make child care arrangements, coordinate with the schedules of significant others, or review topics and skills we might need in the next week that had gone unused for some time.
Our conference schedule was pared down considerably as fellows and attendings adjusted to their new responsibilities and a virtual platform for fellows’ education. While the transition to online lectures was seamless, the spirit of conference certainly changed. Impromptu questions and conversations that oftentimes arise organically during case conferences no longer occurred as virtual meetings do not offer the same space to foster these discussions as we awkwardly muted and unmuted ourselves. Participation in lectures seemed disjointed, which translated in some ways to less effective learning opportunities. Our involvement in endoscopy was also removed as only urgent cases were being performed and PPE conservation was of the utmost priority. This was especially concerning for third-year fellows on the cusp of graduation who would soon be independent practitioners without recent procedural practice. In general, the fellowship felt isolated and uncertain, which our program director addressed with weekly virtual COVID-19 “happy hour” updates.
GI fellows’ contribution
As our program encouraged us to come together during this time to support each other, we realized that while our clinical duties may look different during the COVID-19 crisis, our responsibility to learners was more important than ever. At many academic institutions, GI fellows are referred to as “the face of the division” owed in large part to our consistent presence on consult services and roles as teachers for medical students and residents who rotate with us. In an effort to assist the medical school’s charge to rapidly generate at-home curriculum for our students, we created an online curriculum for medical students to complete during the time they were previously scheduled to rotate with us on consults either as third- or fourth-year students.
We designed a series of interactive podcasts covering six topics that are commonly encountered issues on the GI consult service: upper GI bleeding, lower GI bleeding, biliary sepsis, acute pancreatitis, chronic diarrhea with a new diagnosis of inflammatory bowel disease, as well as cirrhosis and its associated complications.
Conclusion
The COVID-19 pandemic brought about significant change in the daily activities of GI fellows including new responsibilities and a great need for adaptation. We hope that the lessons the COVID-19 pandemic has taught us – to think of others and make our talents available to those who need them, to look for ways to adapt to challenges, to live in the present but focus on the future, and to spread creativity when able – will continue long after the curve has flattened.
References
1. Murphy B. American Medical Association website. https://www.ama-assn.org/residents-students/medical-school-life/online-learning-during-covid-19-tips-help-med-students. Apr 3, 2020.
2. Murphy B. American Medical Association website. https://www.ama-assn.org/delivering-care/public-health/covid-19-how-virus-impacting-medical-schools. Mar 20, 2020.
3. “H5P: Create, share and reuse interactive HTML5 content in your browser.” H5P website. https://h5p.org.
Dr. Bhavsar-Burke and Dr. Jansson-Knodell are GI fellows in the division of gastroenterology and hepatology, department of medicine, Indiana University, Indianapolis. The authors have no conflicts of interest.
Introduction
We are living in an unprecedented time. During March 2020, in response to the COVID-19 (coronavirus disease 2019) outbreak, our institution removed all medical students from rotations with direct patient contact to prioritize their safety and well-being, following recommendations made by the Association of American Medical Colleges (AAMC).1 Similarly, we as gastroenterology fellows experienced an upheaval in our usual schedules and routines. Some of us were redeployed to other areas of the hospital, such as inpatient wards and emergency departments, to meet the needs of our patients and our health system. These changes were difficult, not only because we were practicing in different roles, but also because unknown situations commonly incite fear and anxiety.
Among the repercussions of the COVID-19 pandemic were the changes thrust upon medical students who suddenly found themselves without clinical exposure (both on core clerkships and electives) for the duration of the academic year.2 We too lost many of our educational and teaching opportunities as we adapted to our changing circumstances and new reality. Therefore, . We used the lessons we learned because of the changes in our own medical education to anticipate the best ways to provide learning opportunities for our students.
GI fellows’ experiences
The changes to our schedules and lack of in-person educational conferences seemingly happened overnight – the shock of being pulled from clinics, consults, and endoscopy left us feeling scared and lonely. We were quickly transitioned from knowing our roles and responsibilities as GI providers to taking over care for hospitalist patients as the “primary team,” working in the COVID emergency department (ED), and losing our clinic space. Redeployment to other clinical environments was anxiety-provoking. Self-doubt and fear were the most cited concerns as we asked ourselves: Do I remember enough general medicine to be an effective hospitalist? How do I place admission orders or perform a medication reconciliation on discharge? What can I expect in the COVID ED? Will I have to intubate someone? What about possible PPE shortages? Are my family members safe at home? Should I stay in a hotel? Do we have estimates on how long this will last?
Clinical schedules were reconfigured to consolidate the use of inpatient fellows and allow for reserves of fellows to be redeployed if needed. Schedules for the following 7 days were made just 48 hours prior to the start of each workweek. The anticipation and fear of the unknown were perhaps the hardest parts of the changes in our clinical learning environment. Little time was provided to make child care arrangements, coordinate with the schedules of significant others, or review topics and skills we might need in the next week that had gone unused for some time.
Our conference schedule was pared down considerably as fellows and attendings adjusted to their new responsibilities and a virtual platform for fellows’ education. While the transition to online lectures was seamless, the spirit of conference certainly changed. Impromptu questions and conversations that oftentimes arise organically during case conferences no longer occurred as virtual meetings do not offer the same space to foster these discussions as we awkwardly muted and unmuted ourselves. Participation in lectures seemed disjointed, which translated in some ways to less effective learning opportunities. Our involvement in endoscopy was also removed as only urgent cases were being performed and PPE conservation was of the utmost priority. This was especially concerning for third-year fellows on the cusp of graduation who would soon be independent practitioners without recent procedural practice. In general, the fellowship felt isolated and uncertain, which our program director addressed with weekly virtual COVID-19 “happy hour” updates.
GI fellows’ contribution
As our program encouraged us to come together during this time to support each other, we realized that while our clinical duties may look different during the COVID-19 crisis, our responsibility to learners was more important than ever. At many academic institutions, GI fellows are referred to as “the face of the division” owed in large part to our consistent presence on consult services and roles as teachers for medical students and residents who rotate with us. In an effort to assist the medical school’s charge to rapidly generate at-home curriculum for our students, we created an online curriculum for medical students to complete during the time they were previously scheduled to rotate with us on consults either as third- or fourth-year students.
We designed a series of interactive podcasts covering six topics that are commonly encountered issues on the GI consult service: upper GI bleeding, lower GI bleeding, biliary sepsis, acute pancreatitis, chronic diarrhea with a new diagnosis of inflammatory bowel disease, as well as cirrhosis and its associated complications.


Conclusion
The COVID-19 pandemic brought about significant change in the daily activities of GI fellows including new responsibilities and a great need for adaptation. We hope that the lessons the COVID-19 pandemic has taught us – to think of others and make our talents available to those who need them, to look for ways to adapt to challenges, to live in the present but focus on the future, and to spread creativity when able – will continue long after the curve has flattened.
References
1. Murphy B. American Medical Association website. https://www.ama-assn.org/residents-students/medical-school-life/online-learning-during-covid-19-tips-help-med-students. Apr 3, 2020.
2. Murphy B. American Medical Association website. https://www.ama-assn.org/delivering-care/public-health/covid-19-how-virus-impacting-medical-schools. Mar 20, 2020.
3. “H5P: Create, share and reuse interactive HTML5 content in your browser.” H5P website. https://h5p.org.
Dr. Bhavsar-Burke and Dr. Jansson-Knodell are GI fellows in the division of gastroenterology and hepatology, department of medicine, Indiana University, Indianapolis. The authors have no conflicts of interest.
Introduction
We are living in an unprecedented time. During March 2020, in response to the COVID-19 (coronavirus disease 2019) outbreak, our institution removed all medical students from rotations with direct patient contact to prioritize their safety and well-being, following recommendations made by the Association of American Medical Colleges (AAMC).1 Similarly, we as gastroenterology fellows experienced an upheaval in our usual schedules and routines. Some of us were redeployed to other areas of the hospital, such as inpatient wards and emergency departments, to meet the needs of our patients and our health system. These changes were difficult, not only because we were practicing in different roles, but also because unknown situations commonly incite fear and anxiety.
Among the repercussions of the COVID-19 pandemic were the changes thrust upon medical students who suddenly found themselves without clinical exposure (both on core clerkships and electives) for the duration of the academic year.2 We too lost many of our educational and teaching opportunities as we adapted to our changing circumstances and new reality. Therefore, . We used the lessons we learned because of the changes in our own medical education to anticipate the best ways to provide learning opportunities for our students.
GI fellows’ experiences
The changes to our schedules and lack of in-person educational conferences seemingly happened overnight – the shock of being pulled from clinics, consults, and endoscopy left us feeling scared and lonely. We were quickly transitioned from knowing our roles and responsibilities as GI providers to taking over care for hospitalist patients as the “primary team,” working in the COVID emergency department (ED), and losing our clinic space. Redeployment to other clinical environments was anxiety-provoking. Self-doubt and fear were the most cited concerns as we asked ourselves: Do I remember enough general medicine to be an effective hospitalist? How do I place admission orders or perform a medication reconciliation on discharge? What can I expect in the COVID ED? Will I have to intubate someone? What about possible PPE shortages? Are my family members safe at home? Should I stay in a hotel? Do we have estimates on how long this will last?
Clinical schedules were reconfigured to consolidate the use of inpatient fellows and allow for reserves of fellows to be redeployed if needed. Schedules for the following 7 days were made just 48 hours prior to the start of each workweek. The anticipation and fear of the unknown were perhaps the hardest parts of the changes in our clinical learning environment. Little time was provided to make child care arrangements, coordinate with the schedules of significant others, or review topics and skills we might need in the next week that had gone unused for some time.
Our conference schedule was pared down considerably as fellows and attendings adjusted to their new responsibilities and a virtual platform for fellows’ education. While the transition to online lectures was seamless, the spirit of conference certainly changed. Impromptu questions and conversations that oftentimes arise organically during case conferences no longer occurred as virtual meetings do not offer the same space to foster these discussions as we awkwardly muted and unmuted ourselves. Participation in lectures seemed disjointed, which translated in some ways to less effective learning opportunities. Our involvement in endoscopy was also removed as only urgent cases were being performed and PPE conservation was of the utmost priority. This was especially concerning for third-year fellows on the cusp of graduation who would soon be independent practitioners without recent procedural practice. In general, the fellowship felt isolated and uncertain, which our program director addressed with weekly virtual COVID-19 “happy hour” updates.
GI fellows’ contribution
As our program encouraged us to come together during this time to support each other, we realized that while our clinical duties may look different during the COVID-19 crisis, our responsibility to learners was more important than ever. At many academic institutions, GI fellows are referred to as “the face of the division” owed in large part to our consistent presence on consult services and roles as teachers for medical students and residents who rotate with us. In an effort to assist the medical school’s charge to rapidly generate at-home curriculum for our students, we created an online curriculum for medical students to complete during the time they were previously scheduled to rotate with us on consults either as third- or fourth-year students.
We designed a series of interactive podcasts covering six topics that are commonly encountered issues on the GI consult service: upper GI bleeding, lower GI bleeding, biliary sepsis, acute pancreatitis, chronic diarrhea with a new diagnosis of inflammatory bowel disease, as well as cirrhosis and its associated complications.


Conclusion
The COVID-19 pandemic brought about significant change in the daily activities of GI fellows including new responsibilities and a great need for adaptation. We hope that the lessons the COVID-19 pandemic has taught us – to think of others and make our talents available to those who need them, to look for ways to adapt to challenges, to live in the present but focus on the future, and to spread creativity when able – will continue long after the curve has flattened.
References
1. Murphy B. American Medical Association website. https://www.ama-assn.org/residents-students/medical-school-life/online-learning-during-covid-19-tips-help-med-students. Apr 3, 2020.
2. Murphy B. American Medical Association website. https://www.ama-assn.org/delivering-care/public-health/covid-19-how-virus-impacting-medical-schools. Mar 20, 2020.
3. “H5P: Create, share and reuse interactive HTML5 content in your browser.” H5P website. https://h5p.org.
Dr. Bhavsar-Burke and Dr. Jansson-Knodell are GI fellows in the division of gastroenterology and hepatology, department of medicine, Indiana University, Indianapolis. The authors have no conflicts of interest.
Dermatology News welcomes new advisory board member
at University Hospital Saint-Louis in Paris, in the inflammatory diseases outpatient clinic, where he treats patients with severe psoriasis and other inflammatory chronic skin diseases.
He is a member of several dermatology specialty organizations, including the Société Française de Dermatologie, the European Academy of Dermatology, as well as the American Academy of Dermatology. His research interests are in immunology and inflammatory diseases; he also has a passion for art and history.
at University Hospital Saint-Louis in Paris, in the inflammatory diseases outpatient clinic, where he treats patients with severe psoriasis and other inflammatory chronic skin diseases.
He is a member of several dermatology specialty organizations, including the Société Française de Dermatologie, the European Academy of Dermatology, as well as the American Academy of Dermatology. His research interests are in immunology and inflammatory diseases; he also has a passion for art and history.
at University Hospital Saint-Louis in Paris, in the inflammatory diseases outpatient clinic, where he treats patients with severe psoriasis and other inflammatory chronic skin diseases.
He is a member of several dermatology specialty organizations, including the Société Française de Dermatologie, the European Academy of Dermatology, as well as the American Academy of Dermatology. His research interests are in immunology and inflammatory diseases; he also has a passion for art and history.
Choosing a career in health equity and health care policy
Dr. Anyane-Yeboa is a Commonwealth Fund Fellow in Minority Health Policy at Harvard University and a recent graduate of the Harvard T.H. Chan School of Public Health. She previously completed her gastroenterology fellowship at the University of Chicago. She will be an academic gastroenterologist at Massachusetts General Hospital starting in the fall of 2020.
How did your career pathway lead you to a career in health equity and policy?
I have been passionate about issues related to health equity, workforce diversity, and care of vulnerable populations since the early years of my career. For instance, as undergraduates my friends and I received a grant to start a program to provide mentorship for endangered youth in Boston. During my residency and chief residency, I advocated for increased resident diversity and created programs for underrepresented minority medical students to increase minority representation in medicine. During my gastroenterology fellowship, I remained passionate about the care of minority and underserved populations. During my second year of fellowship, I looked for advanced training opportunities where I could learn the skills to tackle health disparities in minority communities, and almost serendipitously came across the Commonwealth Fund Fellowship in Minority Health Policy. When I decided to apply for the fellowship, I knew that this would be a nontraditional path for most gastroenterology fellows, but the right path for me.
About the Commonwealth Fund Fellowship
The purpose of the Commonwealth Fund Fellowship in Minority Health Policy at Harvard University is to train the next generation of leaders in health care. The program is based at Harvard Medical School and supported by the Commonwealth Fund whose mission is to “provide affordable quality health care for all.” To date, the fellowship has trained more than 130 physicians who are advancing health care across the nation as leaders in public health, academic medicine, and health policy.
The fellowship is a year-long, full-time, degree-granting program. Fellows are eligible for a master’s in public health with a concentration in health management or health policy from the Harvard T.H. Chan School of Public Health or a master’s in public administration from the Harvard Kennedy School.
The fellowship program and experiences have been transformative for me. The structure of the program consists of visits to the Massachusetts Department of Public Health, the Boston Public Health Commission, and the Commonwealth Fund, as well as lectures, seminars, and journal club sessions with national leaders in public health, health policy, and health care delivery reform. Additional opportunities include one-on-one shadowing experiences with leaders in hospital administration at academic institutions in Boston and private meetings with leaders and staff at several government agencies in Washington, including the Centers for Medicaid & Medicare Services, the Office of Minority Health, the Food and Drug Administration, the Health Resources & Services Administration, and the National Institutes of Health.
The program has given me an opportunity to meet and learn from physicians who have chosen a variety of different career paths. Through the program I have had exposure to physicians in academic medicine, health care administration, health policy, and public service as well as those who have chosen a combination of clinical practice with any of the above. This experience has opened my eyes to the different possibilities for physician careers and has encouraged me to be open if new opportunities should arise.
As part of the fellowship, we also have regular meetings with Joan Reede, MD, MPH, who is the director of the fellowship and has been with the program since its inception; she is also the Dean of Diversity and Inclusion at Harvard Medical School. Dr. Reede is an incredibly wise, insightful, and caring mentor, but also a powerhouse in issues surrounding workforce diversity, mentorship, policy, care of underserved communities, and being an advocate for change. To have access to such a powerful individual who has dedicated her career to the mentorship of individuals like myself, who cares deeply about the impact of our careers, and who genuinely values each fellow almost as her own child is a unique gift that is hard to describe in words.
The Commonwealth Fund Fellowship also provides a large network of mentors and advisers. My direct mentor for the program is Monica Bharel, MD, MPH, who is a former Commonwealth Fund fellow and the current Commissioner of the Massachusetts Department of Public Health. However, I also have a wealth of other mentors and advisers in the alumni fellows, including Darrell Gray II, MD, MPH, a former fellow and gastroenterologist at the Ohio State University College of Medicine, as well as the other faculty associated with the program. I never imagined that I would have access to leaders in so many different sectors of health care and policy who are genuinely and passionately rooting for my success. In addition, my cofellows and I have created a uniquely special bond, and they will likely continue as my close network of peer advisers as I move forward throughout my career.
After the fellowship
I have no doubt that the Commonwealth Fund Fellowship will alter the trajectory of my career. It has already affected my career path in ways that I could not have anticipated years ago. The knowledge that I have gained in health care policy, innovation, and equity, as well as the networks that I have access to as a fellow, will be invaluable as I move forward. In terms of next steps, I will be working as an academic gastroenterologist; I will continue to lead initiatives, perform research, and participate in projects to elevate the voices of underserved communities and work toward health equity in gastroenterology. I am particularly passionate about ending disparities in colorectal cancer in minority communities and increasing awareness around minorities with inflammatory bowel disease.
I plan to work with health centers, city- and state-level organizations, and community partners to raise awareness around issues of equity in gastroenterology and develop interventions to create change. I will also work with local legislators and community-based organizations to advocate for policies that remove barriers to screening both locally and nationally. Further down the line, I am open to exploring careers in the public sector or health care administration if that is where my career takes me. The exposure that I had to these fields as part of the fellowship has shown me that it is possible to be a practicing gastroenterologist and simultaneously work in the public sector, health policy, or health care administration. If you are interested in applying to the Commonwealth Fund Fellowship in Minority Health Policy at Harvard University, please feel free to contact me at [email protected]. More information about the program and how to apply can be found at https://cff.hms.harvard.edu/.
Dr. Anyane-Yeboa is a Commonwealth Fund Fellow in Minority Health Policy at Harvard University and a recent graduate of the Harvard T.H. Chan School of Public Health. She previously completed her gastroenterology fellowship at the University of Chicago. She will be an academic gastroenterologist at Massachusetts General Hospital starting in the fall of 2020.
How did your career pathway lead you to a career in health equity and policy?
I have been passionate about issues related to health equity, workforce diversity, and care of vulnerable populations since the early years of my career. For instance, as undergraduates my friends and I received a grant to start a program to provide mentorship for endangered youth in Boston. During my residency and chief residency, I advocated for increased resident diversity and created programs for underrepresented minority medical students to increase minority representation in medicine. During my gastroenterology fellowship, I remained passionate about the care of minority and underserved populations. During my second year of fellowship, I looked for advanced training opportunities where I could learn the skills to tackle health disparities in minority communities, and almost serendipitously came across the Commonwealth Fund Fellowship in Minority Health Policy. When I decided to apply for the fellowship, I knew that this would be a nontraditional path for most gastroenterology fellows, but the right path for me.
About the Commonwealth Fund Fellowship
The purpose of the Commonwealth Fund Fellowship in Minority Health Policy at Harvard University is to train the next generation of leaders in health care. The program is based at Harvard Medical School and supported by the Commonwealth Fund whose mission is to “provide affordable quality health care for all.” To date, the fellowship has trained more than 130 physicians who are advancing health care across the nation as leaders in public health, academic medicine, and health policy.
The fellowship is a year-long, full-time, degree-granting program. Fellows are eligible for a master’s in public health with a concentration in health management or health policy from the Harvard T.H. Chan School of Public Health or a master’s in public administration from the Harvard Kennedy School.
The fellowship program and experiences have been transformative for me. The structure of the program consists of visits to the Massachusetts Department of Public Health, the Boston Public Health Commission, and the Commonwealth Fund, as well as lectures, seminars, and journal club sessions with national leaders in public health, health policy, and health care delivery reform. Additional opportunities include one-on-one shadowing experiences with leaders in hospital administration at academic institutions in Boston and private meetings with leaders and staff at several government agencies in Washington, including the Centers for Medicaid & Medicare Services, the Office of Minority Health, the Food and Drug Administration, the Health Resources & Services Administration, and the National Institutes of Health.
The program has given me an opportunity to meet and learn from physicians who have chosen a variety of different career paths. Through the program I have had exposure to physicians in academic medicine, health care administration, health policy, and public service as well as those who have chosen a combination of clinical practice with any of the above. This experience has opened my eyes to the different possibilities for physician careers and has encouraged me to be open if new opportunities should arise.
As part of the fellowship, we also have regular meetings with Joan Reede, MD, MPH, who is the director of the fellowship and has been with the program since its inception; she is also the Dean of Diversity and Inclusion at Harvard Medical School. Dr. Reede is an incredibly wise, insightful, and caring mentor, but also a powerhouse in issues surrounding workforce diversity, mentorship, policy, care of underserved communities, and being an advocate for change. To have access to such a powerful individual who has dedicated her career to the mentorship of individuals like myself, who cares deeply about the impact of our careers, and who genuinely values each fellow almost as her own child is a unique gift that is hard to describe in words.
The Commonwealth Fund Fellowship also provides a large network of mentors and advisers. My direct mentor for the program is Monica Bharel, MD, MPH, who is a former Commonwealth Fund fellow and the current Commissioner of the Massachusetts Department of Public Health. However, I also have a wealth of other mentors and advisers in the alumni fellows, including Darrell Gray II, MD, MPH, a former fellow and gastroenterologist at the Ohio State University College of Medicine, as well as the other faculty associated with the program. I never imagined that I would have access to leaders in so many different sectors of health care and policy who are genuinely and passionately rooting for my success. In addition, my cofellows and I have created a uniquely special bond, and they will likely continue as my close network of peer advisers as I move forward throughout my career.
After the fellowship
I have no doubt that the Commonwealth Fund Fellowship will alter the trajectory of my career. It has already affected my career path in ways that I could not have anticipated years ago. The knowledge that I have gained in health care policy, innovation, and equity, as well as the networks that I have access to as a fellow, will be invaluable as I move forward. In terms of next steps, I will be working as an academic gastroenterologist; I will continue to lead initiatives, perform research, and participate in projects to elevate the voices of underserved communities and work toward health equity in gastroenterology. I am particularly passionate about ending disparities in colorectal cancer in minority communities and increasing awareness around minorities with inflammatory bowel disease.
I plan to work with health centers, city- and state-level organizations, and community partners to raise awareness around issues of equity in gastroenterology and develop interventions to create change. I will also work with local legislators and community-based organizations to advocate for policies that remove barriers to screening both locally and nationally. Further down the line, I am open to exploring careers in the public sector or health care administration if that is where my career takes me. The exposure that I had to these fields as part of the fellowship has shown me that it is possible to be a practicing gastroenterologist and simultaneously work in the public sector, health policy, or health care administration. If you are interested in applying to the Commonwealth Fund Fellowship in Minority Health Policy at Harvard University, please feel free to contact me at [email protected]. More information about the program and how to apply can be found at https://cff.hms.harvard.edu/.
Dr. Anyane-Yeboa is a Commonwealth Fund Fellow in Minority Health Policy at Harvard University and a recent graduate of the Harvard T.H. Chan School of Public Health. She previously completed her gastroenterology fellowship at the University of Chicago. She will be an academic gastroenterologist at Massachusetts General Hospital starting in the fall of 2020.
How did your career pathway lead you to a career in health equity and policy?
I have been passionate about issues related to health equity, workforce diversity, and care of vulnerable populations since the early years of my career. For instance, as undergraduates my friends and I received a grant to start a program to provide mentorship for endangered youth in Boston. During my residency and chief residency, I advocated for increased resident diversity and created programs for underrepresented minority medical students to increase minority representation in medicine. During my gastroenterology fellowship, I remained passionate about the care of minority and underserved populations. During my second year of fellowship, I looked for advanced training opportunities where I could learn the skills to tackle health disparities in minority communities, and almost serendipitously came across the Commonwealth Fund Fellowship in Minority Health Policy. When I decided to apply for the fellowship, I knew that this would be a nontraditional path for most gastroenterology fellows, but the right path for me.
About the Commonwealth Fund Fellowship
The purpose of the Commonwealth Fund Fellowship in Minority Health Policy at Harvard University is to train the next generation of leaders in health care. The program is based at Harvard Medical School and supported by the Commonwealth Fund whose mission is to “provide affordable quality health care for all.” To date, the fellowship has trained more than 130 physicians who are advancing health care across the nation as leaders in public health, academic medicine, and health policy.
The fellowship is a year-long, full-time, degree-granting program. Fellows are eligible for a master’s in public health with a concentration in health management or health policy from the Harvard T.H. Chan School of Public Health or a master’s in public administration from the Harvard Kennedy School.
The fellowship program and experiences have been transformative for me. The structure of the program consists of visits to the Massachusetts Department of Public Health, the Boston Public Health Commission, and the Commonwealth Fund, as well as lectures, seminars, and journal club sessions with national leaders in public health, health policy, and health care delivery reform. Additional opportunities include one-on-one shadowing experiences with leaders in hospital administration at academic institutions in Boston and private meetings with leaders and staff at several government agencies in Washington, including the Centers for Medicaid & Medicare Services, the Office of Minority Health, the Food and Drug Administration, the Health Resources & Services Administration, and the National Institutes of Health.
The program has given me an opportunity to meet and learn from physicians who have chosen a variety of different career paths. Through the program I have had exposure to physicians in academic medicine, health care administration, health policy, and public service as well as those who have chosen a combination of clinical practice with any of the above. This experience has opened my eyes to the different possibilities for physician careers and has encouraged me to be open if new opportunities should arise.
As part of the fellowship, we also have regular meetings with Joan Reede, MD, MPH, who is the director of the fellowship and has been with the program since its inception; she is also the Dean of Diversity and Inclusion at Harvard Medical School. Dr. Reede is an incredibly wise, insightful, and caring mentor, but also a powerhouse in issues surrounding workforce diversity, mentorship, policy, care of underserved communities, and being an advocate for change. To have access to such a powerful individual who has dedicated her career to the mentorship of individuals like myself, who cares deeply about the impact of our careers, and who genuinely values each fellow almost as her own child is a unique gift that is hard to describe in words.
The Commonwealth Fund Fellowship also provides a large network of mentors and advisers. My direct mentor for the program is Monica Bharel, MD, MPH, who is a former Commonwealth Fund fellow and the current Commissioner of the Massachusetts Department of Public Health. However, I also have a wealth of other mentors and advisers in the alumni fellows, including Darrell Gray II, MD, MPH, a former fellow and gastroenterologist at the Ohio State University College of Medicine, as well as the other faculty associated with the program. I never imagined that I would have access to leaders in so many different sectors of health care and policy who are genuinely and passionately rooting for my success. In addition, my cofellows and I have created a uniquely special bond, and they will likely continue as my close network of peer advisers as I move forward throughout my career.
After the fellowship
I have no doubt that the Commonwealth Fund Fellowship will alter the trajectory of my career. It has already affected my career path in ways that I could not have anticipated years ago. The knowledge that I have gained in health care policy, innovation, and equity, as well as the networks that I have access to as a fellow, will be invaluable as I move forward. In terms of next steps, I will be working as an academic gastroenterologist; I will continue to lead initiatives, perform research, and participate in projects to elevate the voices of underserved communities and work toward health equity in gastroenterology. I am particularly passionate about ending disparities in colorectal cancer in minority communities and increasing awareness around minorities with inflammatory bowel disease.
I plan to work with health centers, city- and state-level organizations, and community partners to raise awareness around issues of equity in gastroenterology and develop interventions to create change. I will also work with local legislators and community-based organizations to advocate for policies that remove barriers to screening both locally and nationally. Further down the line, I am open to exploring careers in the public sector or health care administration if that is where my career takes me. The exposure that I had to these fields as part of the fellowship has shown me that it is possible to be a practicing gastroenterologist and simultaneously work in the public sector, health policy, or health care administration. If you are interested in applying to the Commonwealth Fund Fellowship in Minority Health Policy at Harvard University, please feel free to contact me at [email protected]. More information about the program and how to apply can be found at https://cff.hms.harvard.edu/.
Severe Gingival Swelling and Erythema
The Diagnosis: Plasma Cell Gingivitis
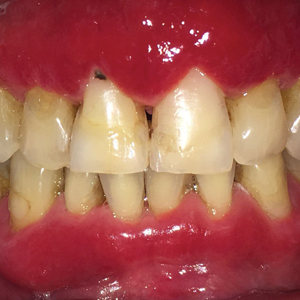
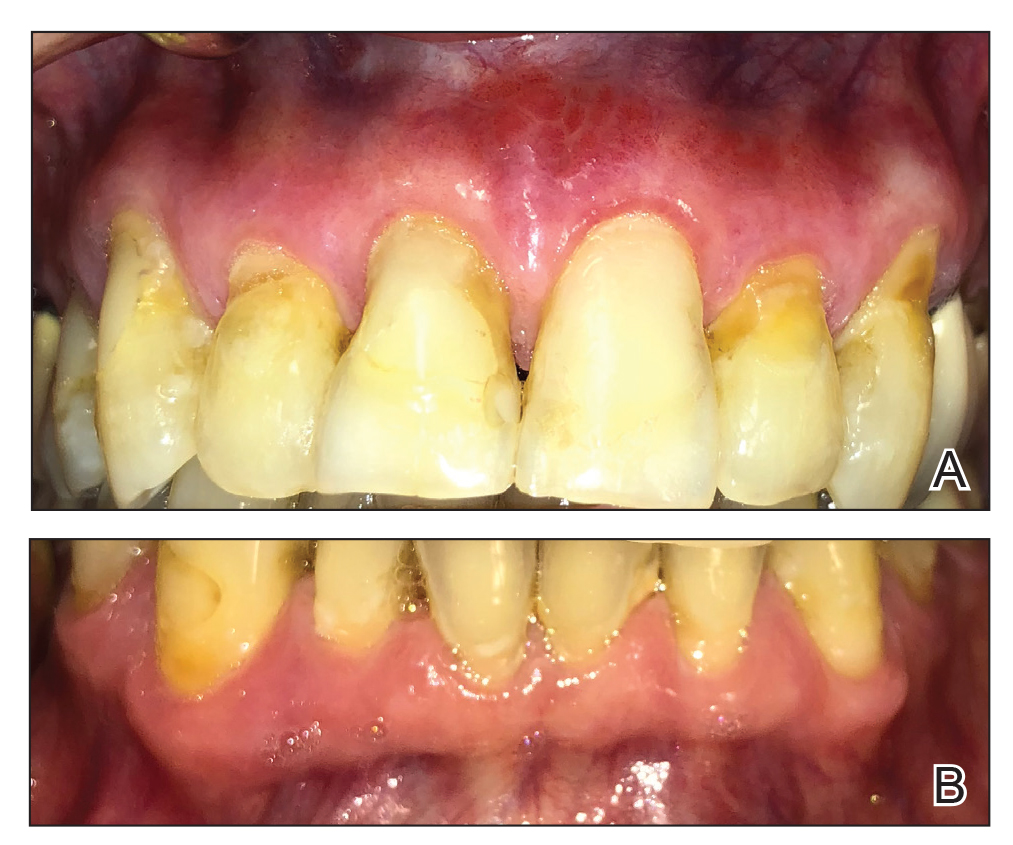
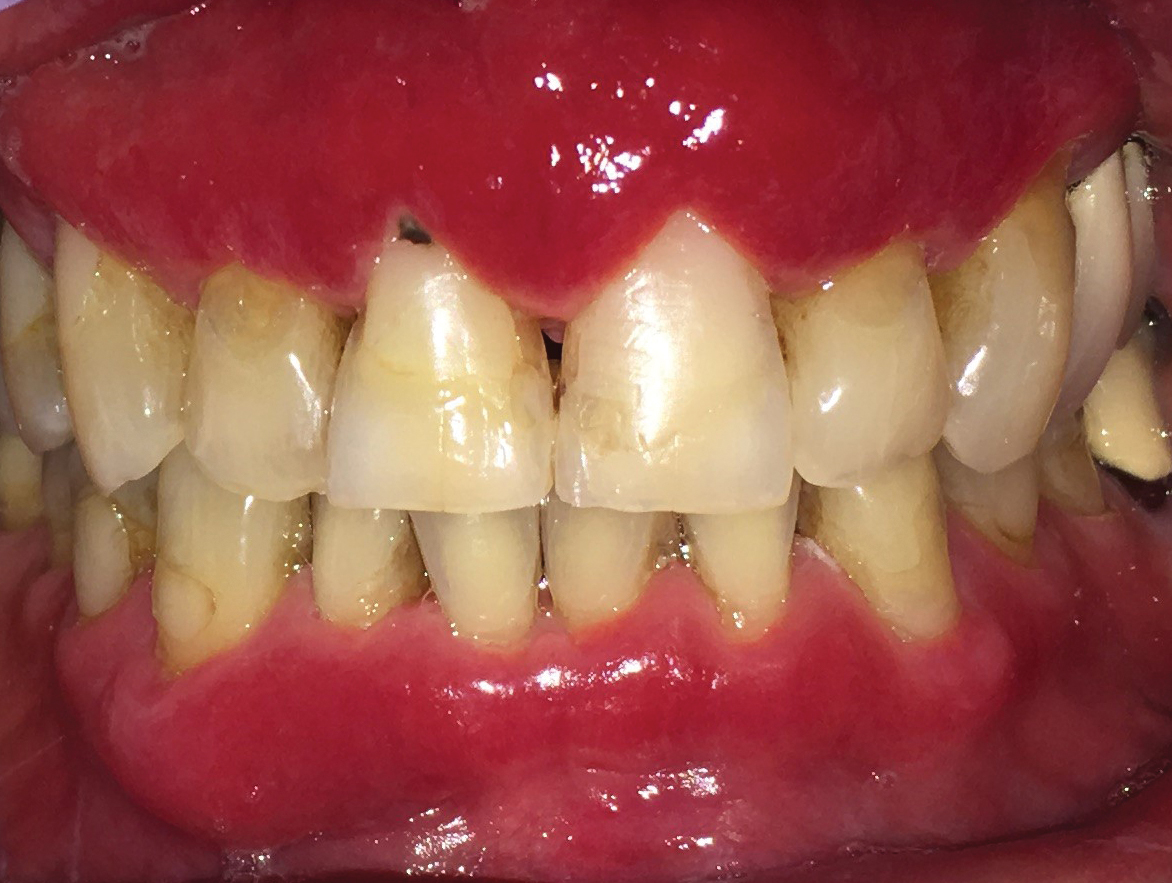
Microscopic analysis demonstrated an acanthotic stratified squamous epithelium with an edematous fibrous stroma containing dense perivascular infiltrates of plasma cells and lymphocytes (Figure 1). Immunohistochemical analysis with kappa, lambda, and CD79a immunostains indicated a polyclonal proliferation of plasma cells that excluded monoclonal plasma cell neoplasia (Figure 2). Direct immunofluorescence (DIF) was negative. Serum enzyme-linked immunosorbent assay for bullous pemphigoid 180 and 230 antibodies as well as desmoglein 1 and 3 antibodies was normal. The cumulative findings were consistent with plasma cell gingivitis (PCG). It was recommended that the patient avoid possible foods (eg, citrus) and oral hygiene products (eg, mint-flavored toothpaste) that could trigger PCG. With patient compliance to an elimination diet for 3 months, the condition resolved (Figure 3).
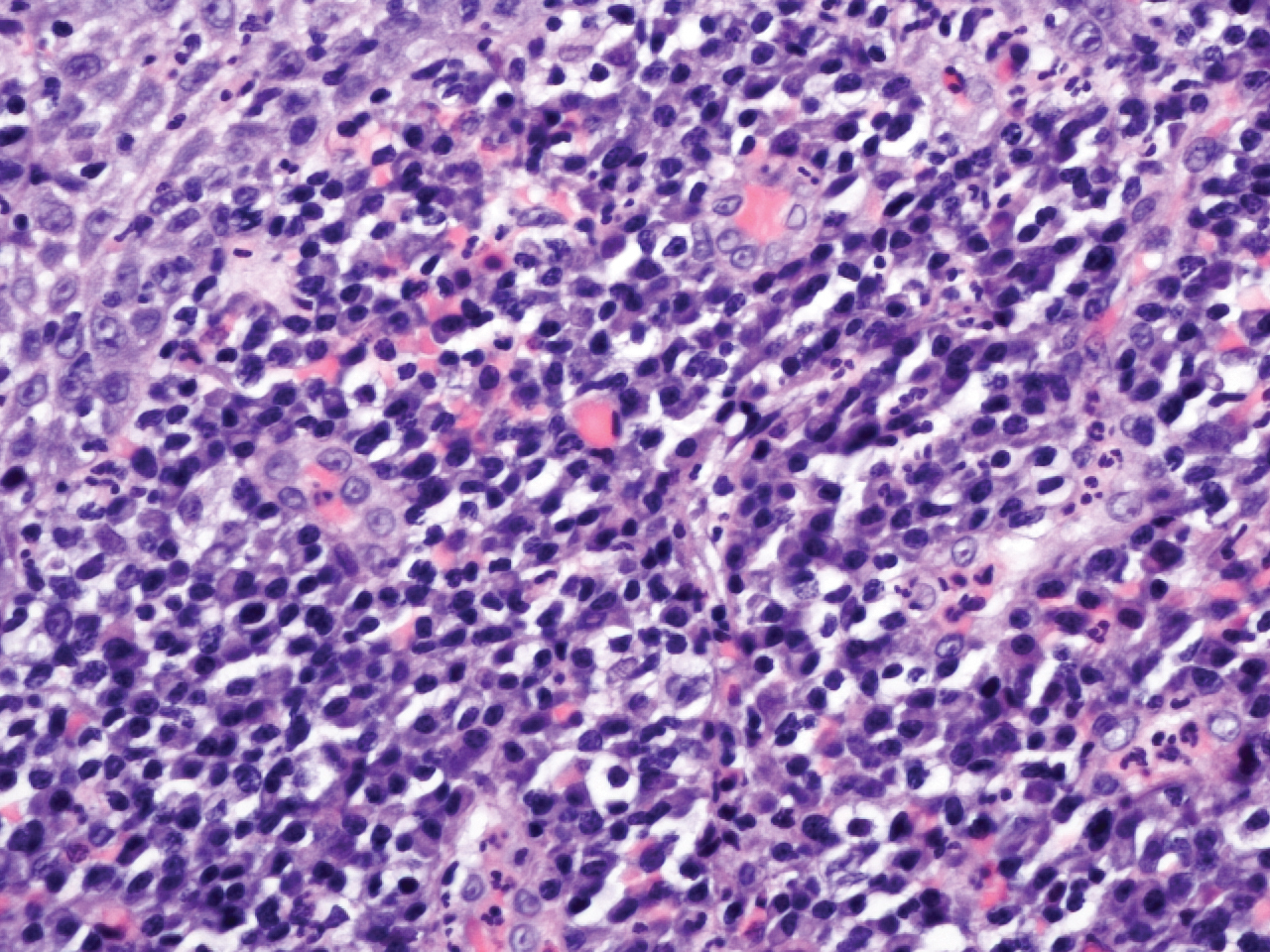
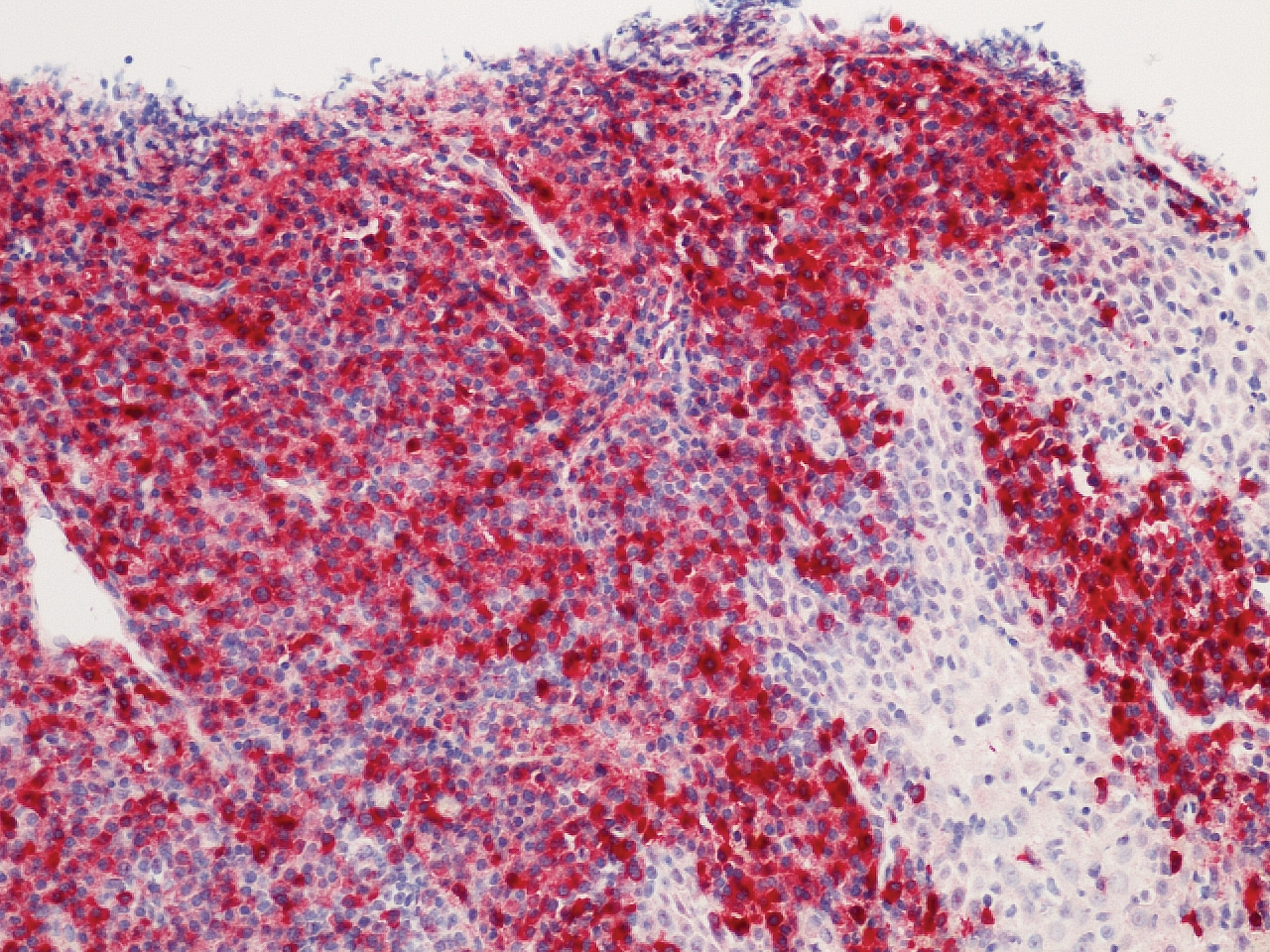
Plasma cell gingivitis is a rare condition characterized by generalized edema and erythema of the attached gingiva. It was described in the 1960s and classified into 3 types based on etiology: (1) hypersensitivity (most common), (2) neoplastic, and (3) PCG of unknown origin.1,2 Spices, herbs, and flavoring agents are implicated as potential triggers of hypersensitivity PCG, while neoplastic PCG is associated with monoclonal plasma cell neoplasms, such as multiple myeloma and extramedullary plasmacytoma.2,3 Histologically, a diffuse subepithelial infiltrate of a polyclonal mixture of plasma cells typically is observed in hypersensitivity PCG.3 The plasma cell infiltration in hypersensitivity PCG is a benign reactive process without known risk for development of plasma cell malignancy, but the presence of a notable number of plasma cells may require special tissue staining to rule out the possibility of associated neoplasia.2,3 There are no standardized protocols for management of PCG.4 Elimination of potential allergens, including flavored oral hygiene products, may result in resolution of hypersensitivity PCG lesions, as exemplified in our patient.1 Neoplastic PCG responds to treatment of the underlying malignancy.5 Topical, intralesional, and/or systemic steroids may be considered in symptomatic cases of PCG.4
Clinical presentation of PCG can mimic immune-mediated mucocutaneous diseases such as mucous membrane pemphigoid (MMP), pemphigus vulgaris (PV), and oral lichen planus; microscopic analysis is needed to establish the diagnosis.6 Mucous membrane pemphigoid is a chronic autoimmune blistering disease involving the mucous membranes with possible cutaneous involvement. It is characterized by a complement-mediated autoantibody process against one or several antigens in the epithelial basement membrane. The oral mucosa is involved in 85% of MMP patients, and 65% of patients experience complications involving the ocular conjunctiva. Intraorally, MMP typically manifests as painful erosions, ulcerations, desquamative gingivitis, and/or occasionally intact blisters. Ocular complications include conjunctivitis and corneal erosions that often scar, resulting in blindness in approximately 15% of patients with ocular involvement. Microscopic features of MMP classically exhibit subepithelial separation with a mixed inflammatory cell infiltrate on routine analysis and linear deposition of IgG, IgA, or C3 within the basement membrane zone on DIF. Treatment of MMP involves topical or systemic immunosuppressants to control symptoms, minimize complications, and alter disease progression.6
Pemphigus vulgaris is an autoimmune vesiculobullous disease that affects the oral mucosa with or without cutaneous involvement.7 Desmogleins 1 and 3, transmembrane glycoproteins of desmosomes that convene cell-to-cell adhesion, are identified as antigens in PV. Antibodies against these desmoglein proteins result in intraepithelial separation, which leads to blister formation.7 Oral manifestations of PV include mucosal erosions and ulcerations as well as desquamative gingivitis. Bullae rarely are seen in the oral cavity, as they tend to rupture, leaving nonhealing ulcerations.8 Histologically, PV is characterized by acantholysis of the suprabasal cell layers with an intact basement membrane zone on routine examination. The distinctive microscopic feature of PV is the detection of cell surface-bound IgG within the epidermis on DIF.7 Treatment of PV may include topical and/or systemic corticosteroids and other immunosuppressants. Rituximab, a monoclonal antibody, has been successful in the management of PV.8
Oral lichen planus is a T-cell mediated autoimmune condition that leads to subepithelial lymphocytic infiltration and excessive keratinocyte apoptosis.9 Women typically are affected more often than men, and 75% of patients also have cutaneous manifestations of the condition. Desquamation and/or erythema of the gingiva may be the initial manifestation of oral lichen planus.9 Other commonly involved sites include the buccal mucosa, tongue, and palate. Biopsy of affected tissues typically demonstrates degeneration of the basal cell layer with subjacent bandlike lymphocytic infiltration on routine staining. Linear fibrinogen at the basement membrane zone usually is observed on DIF. Topical corticosteroids are considered first-line therapy, but systemic therapy including corticosteroids, steroid-sparing agents, or immunomodulators may be used in severe cases.9
There are 3 variants of plasma cell neoplasms including multiple myeloma, medullary plasmacytoma (also known as solitary bone plasmacytoma), and extramedullary plasmacytoma (EMP).10 Extramedullary plasmacytoma, sometimes referred to as extraosseous plasmacytoma, is described as a solitary or multiple plasma cell neoplasm contained in the soft tissue. Its occurrence is rare, accounting for only 3% of plasma cell neoplasms. Approximately 90% of EMPs affect the head and neck region, and males are affected 4 times more often than females. The oral cavity is one of the sites of clinical presentation; the gingival tissue infrequently is affected. When EMP affects the gingiva, it can mimic any form of gingivitis as well as other benign inflammatory conditions, such as pyogenic granuloma. Biopsy is the gold standard diagnostic method for differentiating EMP from other conditions, and specific immunohistochemical stains are essential for the diagnosis. Extramedullary plasmacytoma has the best prognosis among plasma cell neoplasms, despite the risk for progression to multiple myeloma. Extramedullary plasmacytoma lesions are very sensitive to radiotherapy, and the 10-year survival rate is approximately 70%.10
- Sollecito TP, Greenberg MS. Plasma cell gingivitis: report of two cases. Oral Surg Oral Med Oral Pathol. 1992;73:690-693.
- Gargiulo AV, Ladone JA, Ladone PA, et al. Case report: plasma cell gingivitis A. CDS Rev. 1995;88:22-23.
- Abhishek K, Rashmi J. Plasma cell gingivitis associated with inflammatory cheilitis: a report on a rare case. Ethiop J Health Sci. 2013;23:183-187.
- Arduino PG, D'Aiuto F, Cavallito C, et al. Professional oral hygiene as a therapeutic option for pediatric patients with plasma cell gingivitis: preliminary results of a prospective case series. J Periodontol. 2011;82:1670-1675.
- Nayak A, Nayak MT. Multiple myeloma with an unusual oral presentation. J Exp Ther Oncol. 2016;11:199-206.
- Xu HH, Werth VP, Parisi E, et al. Mucous membrane pemphigoid. Dent Clin North Am. 2013;57:611-630.
- Hammers CM, Stanley JR. Mechanisms of disease: pemphigus and bullous pemphigoid. Ann Rev Pathol. 2016;11:75-97.
- Cizenski JD, Michel P, Watson IT, et al. Spectrum of orocutaneous disease associations: immune-mediated conditions. J Am Acad Dermatol. 2017;77:795-806.
- Stoopler ET, Sollecito TP. Recurrent gingival and oral mucosal lesions. JAMA. 2014;312:1794-1795.
- Nair SK, Faizuddin M, Jayanthi D, et al. Extramedullary plasmacytoma of gingiva and soft tissue in neck. J Clin Diagn Res. 2014;8:ZD16-ZD18.
The Diagnosis: Plasma Cell Gingivitis
Microscopic analysis demonstrated an acanthotic stratified squamous epithelium with an edematous fibrous stroma containing dense perivascular infiltrates of plasma cells and lymphocytes (Figure 1). Immunohistochemical analysis with kappa, lambda, and CD79a immunostains indicated a polyclonal proliferation of plasma cells that excluded monoclonal plasma cell neoplasia (Figure 2). Direct immunofluorescence (DIF) was negative. Serum enzyme-linked immunosorbent assay for bullous pemphigoid 180 and 230 antibodies as well as desmoglein 1 and 3 antibodies was normal. The cumulative findings were consistent with plasma cell gingivitis (PCG). It was recommended that the patient avoid possible foods (eg, citrus) and oral hygiene products (eg, mint-flavored toothpaste) that could trigger PCG. With patient compliance to an elimination diet for 3 months, the condition resolved (Figure 3).



Plasma cell gingivitis is a rare condition characterized by generalized edema and erythema of the attached gingiva. It was described in the 1960s and classified into 3 types based on etiology: (1) hypersensitivity (most common), (2) neoplastic, and (3) PCG of unknown origin.1,2 Spices, herbs, and flavoring agents are implicated as potential triggers of hypersensitivity PCG, while neoplastic PCG is associated with monoclonal plasma cell neoplasms, such as multiple myeloma and extramedullary plasmacytoma.2,3 Histologically, a diffuse subepithelial infiltrate of a polyclonal mixture of plasma cells typically is observed in hypersensitivity PCG.3 The plasma cell infiltration in hypersensitivity PCG is a benign reactive process without known risk for development of plasma cell malignancy, but the presence of a notable number of plasma cells may require special tissue staining to rule out the possibility of associated neoplasia.2,3 There are no standardized protocols for management of PCG.4 Elimination of potential allergens, including flavored oral hygiene products, may result in resolution of hypersensitivity PCG lesions, as exemplified in our patient.1 Neoplastic PCG responds to treatment of the underlying malignancy.5 Topical, intralesional, and/or systemic steroids may be considered in symptomatic cases of PCG.4
Clinical presentation of PCG can mimic immune-mediated mucocutaneous diseases such as mucous membrane pemphigoid (MMP), pemphigus vulgaris (PV), and oral lichen planus; microscopic analysis is needed to establish the diagnosis.6 Mucous membrane pemphigoid is a chronic autoimmune blistering disease involving the mucous membranes with possible cutaneous involvement. It is characterized by a complement-mediated autoantibody process against one or several antigens in the epithelial basement membrane. The oral mucosa is involved in 85% of MMP patients, and 65% of patients experience complications involving the ocular conjunctiva. Intraorally, MMP typically manifests as painful erosions, ulcerations, desquamative gingivitis, and/or occasionally intact blisters. Ocular complications include conjunctivitis and corneal erosions that often scar, resulting in blindness in approximately 15% of patients with ocular involvement. Microscopic features of MMP classically exhibit subepithelial separation with a mixed inflammatory cell infiltrate on routine analysis and linear deposition of IgG, IgA, or C3 within the basement membrane zone on DIF. Treatment of MMP involves topical or systemic immunosuppressants to control symptoms, minimize complications, and alter disease progression.6
Pemphigus vulgaris is an autoimmune vesiculobullous disease that affects the oral mucosa with or without cutaneous involvement.7 Desmogleins 1 and 3, transmembrane glycoproteins of desmosomes that convene cell-to-cell adhesion, are identified as antigens in PV. Antibodies against these desmoglein proteins result in intraepithelial separation, which leads to blister formation.7 Oral manifestations of PV include mucosal erosions and ulcerations as well as desquamative gingivitis. Bullae rarely are seen in the oral cavity, as they tend to rupture, leaving nonhealing ulcerations.8 Histologically, PV is characterized by acantholysis of the suprabasal cell layers with an intact basement membrane zone on routine examination. The distinctive microscopic feature of PV is the detection of cell surface-bound IgG within the epidermis on DIF.7 Treatment of PV may include topical and/or systemic corticosteroids and other immunosuppressants. Rituximab, a monoclonal antibody, has been successful in the management of PV.8
Oral lichen planus is a T-cell mediated autoimmune condition that leads to subepithelial lymphocytic infiltration and excessive keratinocyte apoptosis.9 Women typically are affected more often than men, and 75% of patients also have cutaneous manifestations of the condition. Desquamation and/or erythema of the gingiva may be the initial manifestation of oral lichen planus.9 Other commonly involved sites include the buccal mucosa, tongue, and palate. Biopsy of affected tissues typically demonstrates degeneration of the basal cell layer with subjacent bandlike lymphocytic infiltration on routine staining. Linear fibrinogen at the basement membrane zone usually is observed on DIF. Topical corticosteroids are considered first-line therapy, but systemic therapy including corticosteroids, steroid-sparing agents, or immunomodulators may be used in severe cases.9
There are 3 variants of plasma cell neoplasms including multiple myeloma, medullary plasmacytoma (also known as solitary bone plasmacytoma), and extramedullary plasmacytoma (EMP).10 Extramedullary plasmacytoma, sometimes referred to as extraosseous plasmacytoma, is described as a solitary or multiple plasma cell neoplasm contained in the soft tissue. Its occurrence is rare, accounting for only 3% of plasma cell neoplasms. Approximately 90% of EMPs affect the head and neck region, and males are affected 4 times more often than females. The oral cavity is one of the sites of clinical presentation; the gingival tissue infrequently is affected. When EMP affects the gingiva, it can mimic any form of gingivitis as well as other benign inflammatory conditions, such as pyogenic granuloma. Biopsy is the gold standard diagnostic method for differentiating EMP from other conditions, and specific immunohistochemical stains are essential for the diagnosis. Extramedullary plasmacytoma has the best prognosis among plasma cell neoplasms, despite the risk for progression to multiple myeloma. Extramedullary plasmacytoma lesions are very sensitive to radiotherapy, and the 10-year survival rate is approximately 70%.10
The Diagnosis: Plasma Cell Gingivitis
Microscopic analysis demonstrated an acanthotic stratified squamous epithelium with an edematous fibrous stroma containing dense perivascular infiltrates of plasma cells and lymphocytes (Figure 1). Immunohistochemical analysis with kappa, lambda, and CD79a immunostains indicated a polyclonal proliferation of plasma cells that excluded monoclonal plasma cell neoplasia (Figure 2). Direct immunofluorescence (DIF) was negative. Serum enzyme-linked immunosorbent assay for bullous pemphigoid 180 and 230 antibodies as well as desmoglein 1 and 3 antibodies was normal. The cumulative findings were consistent with plasma cell gingivitis (PCG). It was recommended that the patient avoid possible foods (eg, citrus) and oral hygiene products (eg, mint-flavored toothpaste) that could trigger PCG. With patient compliance to an elimination diet for 3 months, the condition resolved (Figure 3).



Plasma cell gingivitis is a rare condition characterized by generalized edema and erythema of the attached gingiva. It was described in the 1960s and classified into 3 types based on etiology: (1) hypersensitivity (most common), (2) neoplastic, and (3) PCG of unknown origin.1,2 Spices, herbs, and flavoring agents are implicated as potential triggers of hypersensitivity PCG, while neoplastic PCG is associated with monoclonal plasma cell neoplasms, such as multiple myeloma and extramedullary plasmacytoma.2,3 Histologically, a diffuse subepithelial infiltrate of a polyclonal mixture of plasma cells typically is observed in hypersensitivity PCG.3 The plasma cell infiltration in hypersensitivity PCG is a benign reactive process without known risk for development of plasma cell malignancy, but the presence of a notable number of plasma cells may require special tissue staining to rule out the possibility of associated neoplasia.2,3 There are no standardized protocols for management of PCG.4 Elimination of potential allergens, including flavored oral hygiene products, may result in resolution of hypersensitivity PCG lesions, as exemplified in our patient.1 Neoplastic PCG responds to treatment of the underlying malignancy.5 Topical, intralesional, and/or systemic steroids may be considered in symptomatic cases of PCG.4
Clinical presentation of PCG can mimic immune-mediated mucocutaneous diseases such as mucous membrane pemphigoid (MMP), pemphigus vulgaris (PV), and oral lichen planus; microscopic analysis is needed to establish the diagnosis.6 Mucous membrane pemphigoid is a chronic autoimmune blistering disease involving the mucous membranes with possible cutaneous involvement. It is characterized by a complement-mediated autoantibody process against one or several antigens in the epithelial basement membrane. The oral mucosa is involved in 85% of MMP patients, and 65% of patients experience complications involving the ocular conjunctiva. Intraorally, MMP typically manifests as painful erosions, ulcerations, desquamative gingivitis, and/or occasionally intact blisters. Ocular complications include conjunctivitis and corneal erosions that often scar, resulting in blindness in approximately 15% of patients with ocular involvement. Microscopic features of MMP classically exhibit subepithelial separation with a mixed inflammatory cell infiltrate on routine analysis and linear deposition of IgG, IgA, or C3 within the basement membrane zone on DIF. Treatment of MMP involves topical or systemic immunosuppressants to control symptoms, minimize complications, and alter disease progression.6
Pemphigus vulgaris is an autoimmune vesiculobullous disease that affects the oral mucosa with or without cutaneous involvement.7 Desmogleins 1 and 3, transmembrane glycoproteins of desmosomes that convene cell-to-cell adhesion, are identified as antigens in PV. Antibodies against these desmoglein proteins result in intraepithelial separation, which leads to blister formation.7 Oral manifestations of PV include mucosal erosions and ulcerations as well as desquamative gingivitis. Bullae rarely are seen in the oral cavity, as they tend to rupture, leaving nonhealing ulcerations.8 Histologically, PV is characterized by acantholysis of the suprabasal cell layers with an intact basement membrane zone on routine examination. The distinctive microscopic feature of PV is the detection of cell surface-bound IgG within the epidermis on DIF.7 Treatment of PV may include topical and/or systemic corticosteroids and other immunosuppressants. Rituximab, a monoclonal antibody, has been successful in the management of PV.8
Oral lichen planus is a T-cell mediated autoimmune condition that leads to subepithelial lymphocytic infiltration and excessive keratinocyte apoptosis.9 Women typically are affected more often than men, and 75% of patients also have cutaneous manifestations of the condition. Desquamation and/or erythema of the gingiva may be the initial manifestation of oral lichen planus.9 Other commonly involved sites include the buccal mucosa, tongue, and palate. Biopsy of affected tissues typically demonstrates degeneration of the basal cell layer with subjacent bandlike lymphocytic infiltration on routine staining. Linear fibrinogen at the basement membrane zone usually is observed on DIF. Topical corticosteroids are considered first-line therapy, but systemic therapy including corticosteroids, steroid-sparing agents, or immunomodulators may be used in severe cases.9
There are 3 variants of plasma cell neoplasms including multiple myeloma, medullary plasmacytoma (also known as solitary bone plasmacytoma), and extramedullary plasmacytoma (EMP).10 Extramedullary plasmacytoma, sometimes referred to as extraosseous plasmacytoma, is described as a solitary or multiple plasma cell neoplasm contained in the soft tissue. Its occurrence is rare, accounting for only 3% of plasma cell neoplasms. Approximately 90% of EMPs affect the head and neck region, and males are affected 4 times more often than females. The oral cavity is one of the sites of clinical presentation; the gingival tissue infrequently is affected. When EMP affects the gingiva, it can mimic any form of gingivitis as well as other benign inflammatory conditions, such as pyogenic granuloma. Biopsy is the gold standard diagnostic method for differentiating EMP from other conditions, and specific immunohistochemical stains are essential for the diagnosis. Extramedullary plasmacytoma has the best prognosis among plasma cell neoplasms, despite the risk for progression to multiple myeloma. Extramedullary plasmacytoma lesions are very sensitive to radiotherapy, and the 10-year survival rate is approximately 70%.10
- Sollecito TP, Greenberg MS. Plasma cell gingivitis: report of two cases. Oral Surg Oral Med Oral Pathol. 1992;73:690-693.
- Gargiulo AV, Ladone JA, Ladone PA, et al. Case report: plasma cell gingivitis A. CDS Rev. 1995;88:22-23.
- Abhishek K, Rashmi J. Plasma cell gingivitis associated with inflammatory cheilitis: a report on a rare case. Ethiop J Health Sci. 2013;23:183-187.
- Arduino PG, D'Aiuto F, Cavallito C, et al. Professional oral hygiene as a therapeutic option for pediatric patients with plasma cell gingivitis: preliminary results of a prospective case series. J Periodontol. 2011;82:1670-1675.
- Nayak A, Nayak MT. Multiple myeloma with an unusual oral presentation. J Exp Ther Oncol. 2016;11:199-206.
- Xu HH, Werth VP, Parisi E, et al. Mucous membrane pemphigoid. Dent Clin North Am. 2013;57:611-630.
- Hammers CM, Stanley JR. Mechanisms of disease: pemphigus and bullous pemphigoid. Ann Rev Pathol. 2016;11:75-97.
- Cizenski JD, Michel P, Watson IT, et al. Spectrum of orocutaneous disease associations: immune-mediated conditions. J Am Acad Dermatol. 2017;77:795-806.
- Stoopler ET, Sollecito TP. Recurrent gingival and oral mucosal lesions. JAMA. 2014;312:1794-1795.
- Nair SK, Faizuddin M, Jayanthi D, et al. Extramedullary plasmacytoma of gingiva and soft tissue in neck. J Clin Diagn Res. 2014;8:ZD16-ZD18.
- Sollecito TP, Greenberg MS. Plasma cell gingivitis: report of two cases. Oral Surg Oral Med Oral Pathol. 1992;73:690-693.
- Gargiulo AV, Ladone JA, Ladone PA, et al. Case report: plasma cell gingivitis A. CDS Rev. 1995;88:22-23.
- Abhishek K, Rashmi J. Plasma cell gingivitis associated with inflammatory cheilitis: a report on a rare case. Ethiop J Health Sci. 2013;23:183-187.
- Arduino PG, D'Aiuto F, Cavallito C, et al. Professional oral hygiene as a therapeutic option for pediatric patients with plasma cell gingivitis: preliminary results of a prospective case series. J Periodontol. 2011;82:1670-1675.
- Nayak A, Nayak MT. Multiple myeloma with an unusual oral presentation. J Exp Ther Oncol. 2016;11:199-206.
- Xu HH, Werth VP, Parisi E, et al. Mucous membrane pemphigoid. Dent Clin North Am. 2013;57:611-630.
- Hammers CM, Stanley JR. Mechanisms of disease: pemphigus and bullous pemphigoid. Ann Rev Pathol. 2016;11:75-97.
- Cizenski JD, Michel P, Watson IT, et al. Spectrum of orocutaneous disease associations: immune-mediated conditions. J Am Acad Dermatol. 2017;77:795-806.
- Stoopler ET, Sollecito TP. Recurrent gingival and oral mucosal lesions. JAMA. 2014;312:1794-1795.
- Nair SK, Faizuddin M, Jayanthi D, et al. Extramedullary plasmacytoma of gingiva and soft tissue in neck. J Clin Diagn Res. 2014;8:ZD16-ZD18.
A 62-year-old man presented to an oral medicine specialist with gingival inflammation of at least 1 year's duration. He reported mild discomfort when consuming spicy foods and denied associated extraoral lesions. His medical history revealed hypertension, hypothyroidism, and psoriasis. Medications included lisinopril 10 mg and levothyroxine 100 µg daily. No known drug allergies were reported. His family and social history were noncontributory, and a detailed review of systems was unremarkable. Extraoral examination revealed no lymphadenopathy, salivary gland enlargement, or thyromegaly. Intraoral examination revealed diffuse enlargement of the maxillary and mandibular gingiva accompanied by severe erythema and bleeding on provocation. A 3-mm punch biopsy of the gingiva was performed for routine analysis and direct immunofluorescence.
Disseminated Erythema Induratum in a Patient With a History of Tuberculosis
To the Editor:
Erythema induratum, also known as nodular vasculitis, is a panniculitis that usually affects the lower extremities in middle-aged women. Classically, it has been described as a delayed-type hypersensitivity reaction to Mycobacterium tuberculosis, also known as a tuberculid.1,2 Other infections, however, also have been implicated as causes of erythema induratum, including bacillus Calmette-Guérin (BCG), the attenuated form of Mycobacterium bovis, which commonly is used for tuberculosis vaccination. Medications also may cause erythema induratum. The characteristic distribution of the nodules on the posterior calves helps to distinguish erythema induratum from other panniculitides. A PubMed search of articles indexed for MEDLINE using the term disseminated erythema induratum revealed few case reports documenting nodules on the arms, thighs, or chest, and only 1 case report of disseminated erythema induratum.3-8 We describe a rare combination of disseminated erythema induratum in a patient with remote exposure to tuberculosis and recent BCG exposure.
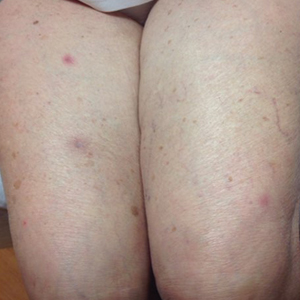
An 88-year-old woman presented for evaluation of violaceous, minimally tender, nonulcerated, subcutaneous nodules on the legs, arms, and trunk of several weeks’ duration (Figure 1). She had a remote history of tuberculosis as a child, prior to the advent of modern antituberculosis regimens. Her medical history also included hypertension, breast cancer treated with lymph node dissection, gastroesophageal reflux disease, and bladder cancer treated with intravesical BCG 10 years prior to the onset of the nodules. She reported minimal coughing and a 25-lb weight loss over the last year, but she denied night sweats, fever, or chills.
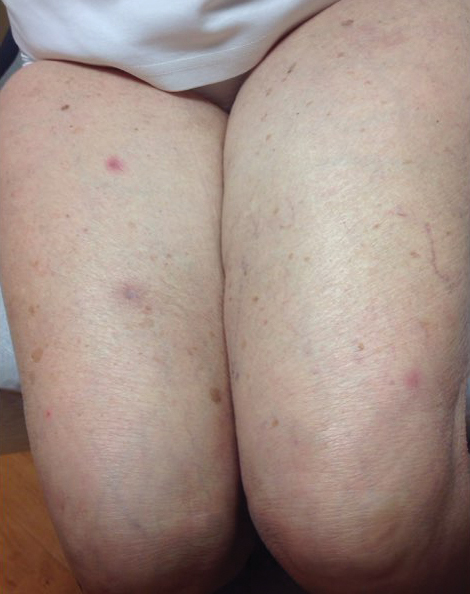
Workup included a biopsy, which showed a dense inflammatory infiltrate within the septae and lobules of the subcutaneous tissue (Figure 2A). Foci of necrosis were seen within the fat lobules (Figure 2B). The histologic diagnosis was erythema induratum. Tissue cultures for bacteria, fungi, and atypical mycobacteria were negative. Mycobacterium tuberculosis polymerase chain reaction (PCR) analysis also was negative. An IFN-γ release assay test was positive for infection with M tuberculosis, suggesting that the erythema induratum was due to tuberculosis rather than BCG exposure. A chest radiograph demonstrated a 22-mm nodule in the left lung (unchanged from a prior film) and a new 10-mm nodule in the left upper lobe.
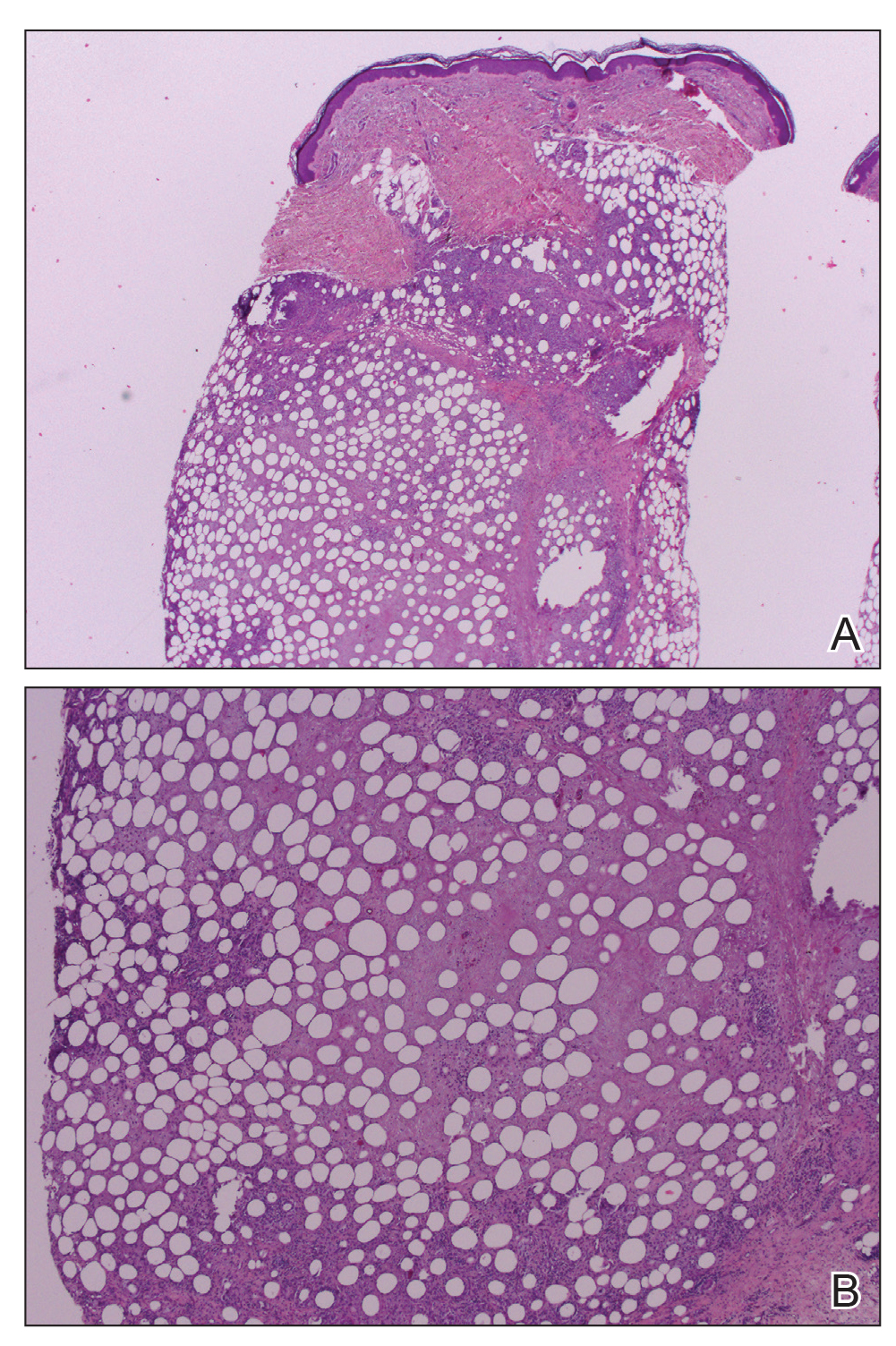
The patient was referred to an infectious disease specialist who concurred that the erythema induratum and the new lung nodule likely represented a reactivation of tuberculosis. Sputum samples were found to be smear and culture negative for mycobacteria, but due to high clinical suspicion, she was started on a 4-drug tuberculosis regimen of isoniazid, rifampin, pyrazinamide, and ethambutol. Some lesions had started to improve prior to the institution of therapy; after initiation of treatment, all lesions resolved within 4 weeks of starting treatment without recurrence.
Erythema induratum was first described by Bazin9 in 1861. The disorder usually occurs in middle-aged women and is characterized by violaceous ulcerative plaques that classically present on the lower extremities, especially the calves. When the eruption occurs due to a nontuberculous etiology, the term nodular vasculitis is used.1,5 The distinction largely is historical, as most dermatologists today recognize erythema induratum and nodular vasculitis to be the same entity. Examples of nontuberculous causes include infections such as Nocardia, Pseudomonas, Fusarium, or other Mycobacterium species.10 Medications such as propylthiouracil also have been implicated.11 The classification of erythema induratum as a tuberculid suggests that the nodules are a reaction pattern rather than a primary infection, though the term tuberculid may be imprecise. The differential diagnosis of violaceous nodules on the lower extremities and trunk is broad and includes erythema nodosum, cutaneous polyarteritis nodosa, pancreatic panniculitis, subcutaneous T-cell lymphoma, and lupus profundus.1,11,12
Histologically, lesions classically demonstrate a mostly lobular panniculitis with varying degrees of septal fibrosis and focal necrosis. Neutrophils may predominate early, while adipocyte necrosis, epithelioid histiocytes, multinucleated giant cells, and lymphocytes may be found in older lesions. The presence of vasculitis as a requisite diagnostic criterion remains controversial.1,12
The incidence of erythema induratum has decreased since multidrug tuberculosis treatment has become more widespread.3 Our case displayed the disseminated variant of erythema induratum, an even rarer clinical entity.8 Interestingly, our patient had a history of tuberculosis and exposure to BCG prior to the development of lesions. Case reports have documented erythema induratum after BCG exposure but less frequently than in cases associated with tuberculosis.3,13
The use of BCG vaccines has necessitated the need for a more precise method of determining tuberculosis activity. The tuberculin skin test reacts positively with a history of BCG exposure, rendering it an inadequate test in a patient who is suspected of having an active or latent M tuberculosis infection.13,14 IFN-γ release assays are more specific in detecting latent or active tuberculosis than the tuberculin skin test. Such assays use early secretory antigenic target 6 and cultured filtrate protein 10 as antigens to determine sensitization to M tuberculosis.13,15 These antigens are not produced by BCG or Mycobacterium avium; however, other mycobacteria such as Mycobacterium marinum, Mycobacterium kansasii, and some strains of M bovis produce the aforementioned antigens, and exposure to these microbes may be confounding.13 Importantly, positive IFN-γ release assay results also have been documented after BCG exposure but occur at a much lower frequency than for tuberculosis.15 Thus, the combination of the positive IFN-γ release assay and new chest radiograph nodule in our patient provided strong evidence of reactivated tuberculosis as the precipitating cause of her skin disease.
Despite her negative PCR study, our patient’s presentation remains consistent with the diagnosis of disseminated erythema induratum.13,15 The value of PCR studies in establishing the diagnosis remains to be determined. Case reports have described positive PCR results detecting M tuberculosis in panniculitic nodules, suggesting that trace amounts of the organism are present in lesional tissue despite the negative culture result and immunostains.1 Tuberculid reactions, including lichen scrofulosorum, papulonecrotic tuberculid, and erythema induratum, historically are defined by the lack of positive cultures and immunostains, making positive PCR results difficult to reconcile pathophysiologically.1,13 Therefore, use of the term tuberculid altogether as a descriptor for pathogenesis of this disease may need to be avoided.16 Postulated explanations for the relationship of tuberculid diseases and negative cultures and immunostains include the presence of a small number of bacilli that escape routine laboratory detection, early destruction of organisms, or a reaction to circulating M tuberculosis fragments.2 Regardless, until the pathophysiology of erythema induratum has been fully elucidated, the value of PCR remains unclear.
Disseminated erythema induratum, an exceptionally rare variant of panniculitis, may be seen in patients with a remote history of M tuberculosis exposure and/or recent therapeutic BCG exposure. It is imperative to rule out active tuberculosis, especially in elderly patients whose disease predated the advent of modern antituberculosis therapy. Using an IFN-γ release assay in addition to chest radiographs and other clinical stigmata allows differentiation of the etiology of erythema induratum in those patients with tuberculosis who also were treated with BCG.
- Mascaro JM, Basalga E. Erythema induratum of Bazin. Dermatol Clin. 2008;28:439-445.
- Lighter J, Tse DB, Li Y, et al. Erythema induratum of Bazin in a child: evidence for a cell-mediated hyper-response to Mycobacterium tuberculosis. Pediatr Infect Dis J. 2009;28:326-328.
- Inoue T, Fukumoto T, Ansai S, et al. Erythema induratum of Bazin in an infant after bacilli Calmette-Guerin vaccination. J Dermatol. 2006;33:268-272.
- Degonda Halter M, Nebiker P, Hug B, et al. Atypical erythema induratum Bazin with tuberculous osteomyelitis. Internist. 2006;47:853-856.
- Gilchrist H, Patterson JW. Erythema nodosum and erythema induratum (nodular vasculitis): diagnosis and management. Dermatol Ther. 2010;23:320-327.
- Sharma S, Sehgal VN, Bhattacharya SN, et al. Clinicopathologic spectrum of cutaneous tuberculosis: a retrospective analysis of 165 Indians. Am J Dermatopathol. 2015;37:444-450.
- Sethuraman G, Ramesh V. Cutaneous tuberculosis in children. Pediatr Dermatol. 2013;30:7-16.
- Teramura K, Fujimoto N, Nakanishi G, et al. Disseminated erythema induratum of Bazin. Eur J Dermatol. 2014;24:697-698.
- Bazin E. Extrait des Lecons Théoretiques et Cliniques sur le Scrofule. 2nd ed. Paris, France: Delhaye; 1861.
- Campbell SM, Winkelmann RR, Sammons DL. Erythema induratum caused by Mycobacterium chelonei in an immunocompetent patient. J Clin Aesthet Dermatol. 2013;6:38-40.
- Patterson JW. Panniculitis. In: Bolognia JL, Jorizzo J, Rapini RP, et al, eds. Dermatology. Barcelona, Spain: Mosby Elsevier; 2012:1641-1662.
- Segura S, Pujol R, Trinidade F, et al. Vasculitis in erythema induratum of Bazin: a histopathologic study of 101 biopsy specimens from 86 patients. J Am Acad Dermatol. 2008;59:839-851.
- Vera-Kellet C, Peters L, Elwood K, et al. Usefulness of interferon-γ release assays in the diagnosis of erythema induratum. Arch Dermatol. 2011;147:949-952.
- Prajapati V, Steed M, Grewal P, et al. Erythema induratum: case series illustrating the utility of the interferon-γ release assay in determining the association with tuberculosis. J Cutan Med Surg. 2013;17:S6-S11.
- Sim JH, Whang KU. Application of the QuantiFERON-Gold TB test in erythema induratum. J Dermatolog Treat. 2014;25:260-263.
- Wiebels D, Turnbull K, Steinkraus V, et al. Erythema induratum Bazin.”tuberculid” or tuberculosis? [in German]. Hautarzt. 2007;58:237-240.
To the Editor:
Erythema induratum, also known as nodular vasculitis, is a panniculitis that usually affects the lower extremities in middle-aged women. Classically, it has been described as a delayed-type hypersensitivity reaction to Mycobacterium tuberculosis, also known as a tuberculid.1,2 Other infections, however, also have been implicated as causes of erythema induratum, including bacillus Calmette-Guérin (BCG), the attenuated form of Mycobacterium bovis, which commonly is used for tuberculosis vaccination. Medications also may cause erythema induratum. The characteristic distribution of the nodules on the posterior calves helps to distinguish erythema induratum from other panniculitides. A PubMed search of articles indexed for MEDLINE using the term disseminated erythema induratum revealed few case reports documenting nodules on the arms, thighs, or chest, and only 1 case report of disseminated erythema induratum.3-8 We describe a rare combination of disseminated erythema induratum in a patient with remote exposure to tuberculosis and recent BCG exposure.
An 88-year-old woman presented for evaluation of violaceous, minimally tender, nonulcerated, subcutaneous nodules on the legs, arms, and trunk of several weeks’ duration (Figure 1). She had a remote history of tuberculosis as a child, prior to the advent of modern antituberculosis regimens. Her medical history also included hypertension, breast cancer treated with lymph node dissection, gastroesophageal reflux disease, and bladder cancer treated with intravesical BCG 10 years prior to the onset of the nodules. She reported minimal coughing and a 25-lb weight loss over the last year, but she denied night sweats, fever, or chills.

Workup included a biopsy, which showed a dense inflammatory infiltrate within the septae and lobules of the subcutaneous tissue (Figure 2A). Foci of necrosis were seen within the fat lobules (Figure 2B). The histologic diagnosis was erythema induratum. Tissue cultures for bacteria, fungi, and atypical mycobacteria were negative. Mycobacterium tuberculosis polymerase chain reaction (PCR) analysis also was negative. An IFN-γ release assay test was positive for infection with M tuberculosis, suggesting that the erythema induratum was due to tuberculosis rather than BCG exposure. A chest radiograph demonstrated a 22-mm nodule in the left lung (unchanged from a prior film) and a new 10-mm nodule in the left upper lobe.

The patient was referred to an infectious disease specialist who concurred that the erythema induratum and the new lung nodule likely represented a reactivation of tuberculosis. Sputum samples were found to be smear and culture negative for mycobacteria, but due to high clinical suspicion, she was started on a 4-drug tuberculosis regimen of isoniazid, rifampin, pyrazinamide, and ethambutol. Some lesions had started to improve prior to the institution of therapy; after initiation of treatment, all lesions resolved within 4 weeks of starting treatment without recurrence.
Erythema induratum was first described by Bazin9 in 1861. The disorder usually occurs in middle-aged women and is characterized by violaceous ulcerative plaques that classically present on the lower extremities, especially the calves. When the eruption occurs due to a nontuberculous etiology, the term nodular vasculitis is used.1,5 The distinction largely is historical, as most dermatologists today recognize erythema induratum and nodular vasculitis to be the same entity. Examples of nontuberculous causes include infections such as Nocardia, Pseudomonas, Fusarium, or other Mycobacterium species.10 Medications such as propylthiouracil also have been implicated.11 The classification of erythema induratum as a tuberculid suggests that the nodules are a reaction pattern rather than a primary infection, though the term tuberculid may be imprecise. The differential diagnosis of violaceous nodules on the lower extremities and trunk is broad and includes erythema nodosum, cutaneous polyarteritis nodosa, pancreatic panniculitis, subcutaneous T-cell lymphoma, and lupus profundus.1,11,12
Histologically, lesions classically demonstrate a mostly lobular panniculitis with varying degrees of septal fibrosis and focal necrosis. Neutrophils may predominate early, while adipocyte necrosis, epithelioid histiocytes, multinucleated giant cells, and lymphocytes may be found in older lesions. The presence of vasculitis as a requisite diagnostic criterion remains controversial.1,12
The incidence of erythema induratum has decreased since multidrug tuberculosis treatment has become more widespread.3 Our case displayed the disseminated variant of erythema induratum, an even rarer clinical entity.8 Interestingly, our patient had a history of tuberculosis and exposure to BCG prior to the development of lesions. Case reports have documented erythema induratum after BCG exposure but less frequently than in cases associated with tuberculosis.3,13
The use of BCG vaccines has necessitated the need for a more precise method of determining tuberculosis activity. The tuberculin skin test reacts positively with a history of BCG exposure, rendering it an inadequate test in a patient who is suspected of having an active or latent M tuberculosis infection.13,14 IFN-γ release assays are more specific in detecting latent or active tuberculosis than the tuberculin skin test. Such assays use early secretory antigenic target 6 and cultured filtrate protein 10 as antigens to determine sensitization to M tuberculosis.13,15 These antigens are not produced by BCG or Mycobacterium avium; however, other mycobacteria such as Mycobacterium marinum, Mycobacterium kansasii, and some strains of M bovis produce the aforementioned antigens, and exposure to these microbes may be confounding.13 Importantly, positive IFN-γ release assay results also have been documented after BCG exposure but occur at a much lower frequency than for tuberculosis.15 Thus, the combination of the positive IFN-γ release assay and new chest radiograph nodule in our patient provided strong evidence of reactivated tuberculosis as the precipitating cause of her skin disease.
Despite her negative PCR study, our patient’s presentation remains consistent with the diagnosis of disseminated erythema induratum.13,15 The value of PCR studies in establishing the diagnosis remains to be determined. Case reports have described positive PCR results detecting M tuberculosis in panniculitic nodules, suggesting that trace amounts of the organism are present in lesional tissue despite the negative culture result and immunostains.1 Tuberculid reactions, including lichen scrofulosorum, papulonecrotic tuberculid, and erythema induratum, historically are defined by the lack of positive cultures and immunostains, making positive PCR results difficult to reconcile pathophysiologically.1,13 Therefore, use of the term tuberculid altogether as a descriptor for pathogenesis of this disease may need to be avoided.16 Postulated explanations for the relationship of tuberculid diseases and negative cultures and immunostains include the presence of a small number of bacilli that escape routine laboratory detection, early destruction of organisms, or a reaction to circulating M tuberculosis fragments.2 Regardless, until the pathophysiology of erythema induratum has been fully elucidated, the value of PCR remains unclear.
Disseminated erythema induratum, an exceptionally rare variant of panniculitis, may be seen in patients with a remote history of M tuberculosis exposure and/or recent therapeutic BCG exposure. It is imperative to rule out active tuberculosis, especially in elderly patients whose disease predated the advent of modern antituberculosis therapy. Using an IFN-γ release assay in addition to chest radiographs and other clinical stigmata allows differentiation of the etiology of erythema induratum in those patients with tuberculosis who also were treated with BCG.
To the Editor:
Erythema induratum, also known as nodular vasculitis, is a panniculitis that usually affects the lower extremities in middle-aged women. Classically, it has been described as a delayed-type hypersensitivity reaction to Mycobacterium tuberculosis, also known as a tuberculid.1,2 Other infections, however, also have been implicated as causes of erythema induratum, including bacillus Calmette-Guérin (BCG), the attenuated form of Mycobacterium bovis, which commonly is used for tuberculosis vaccination. Medications also may cause erythema induratum. The characteristic distribution of the nodules on the posterior calves helps to distinguish erythema induratum from other panniculitides. A PubMed search of articles indexed for MEDLINE using the term disseminated erythema induratum revealed few case reports documenting nodules on the arms, thighs, or chest, and only 1 case report of disseminated erythema induratum.3-8 We describe a rare combination of disseminated erythema induratum in a patient with remote exposure to tuberculosis and recent BCG exposure.
An 88-year-old woman presented for evaluation of violaceous, minimally tender, nonulcerated, subcutaneous nodules on the legs, arms, and trunk of several weeks’ duration (Figure 1). She had a remote history of tuberculosis as a child, prior to the advent of modern antituberculosis regimens. Her medical history also included hypertension, breast cancer treated with lymph node dissection, gastroesophageal reflux disease, and bladder cancer treated with intravesical BCG 10 years prior to the onset of the nodules. She reported minimal coughing and a 25-lb weight loss over the last year, but she denied night sweats, fever, or chills.

Workup included a biopsy, which showed a dense inflammatory infiltrate within the septae and lobules of the subcutaneous tissue (Figure 2A). Foci of necrosis were seen within the fat lobules (Figure 2B). The histologic diagnosis was erythema induratum. Tissue cultures for bacteria, fungi, and atypical mycobacteria were negative. Mycobacterium tuberculosis polymerase chain reaction (PCR) analysis also was negative. An IFN-γ release assay test was positive for infection with M tuberculosis, suggesting that the erythema induratum was due to tuberculosis rather than BCG exposure. A chest radiograph demonstrated a 22-mm nodule in the left lung (unchanged from a prior film) and a new 10-mm nodule in the left upper lobe.

The patient was referred to an infectious disease specialist who concurred that the erythema induratum and the new lung nodule likely represented a reactivation of tuberculosis. Sputum samples were found to be smear and culture negative for mycobacteria, but due to high clinical suspicion, she was started on a 4-drug tuberculosis regimen of isoniazid, rifampin, pyrazinamide, and ethambutol. Some lesions had started to improve prior to the institution of therapy; after initiation of treatment, all lesions resolved within 4 weeks of starting treatment without recurrence.
Erythema induratum was first described by Bazin9 in 1861. The disorder usually occurs in middle-aged women and is characterized by violaceous ulcerative plaques that classically present on the lower extremities, especially the calves. When the eruption occurs due to a nontuberculous etiology, the term nodular vasculitis is used.1,5 The distinction largely is historical, as most dermatologists today recognize erythema induratum and nodular vasculitis to be the same entity. Examples of nontuberculous causes include infections such as Nocardia, Pseudomonas, Fusarium, or other Mycobacterium species.10 Medications such as propylthiouracil also have been implicated.11 The classification of erythema induratum as a tuberculid suggests that the nodules are a reaction pattern rather than a primary infection, though the term tuberculid may be imprecise. The differential diagnosis of violaceous nodules on the lower extremities and trunk is broad and includes erythema nodosum, cutaneous polyarteritis nodosa, pancreatic panniculitis, subcutaneous T-cell lymphoma, and lupus profundus.1,11,12
Histologically, lesions classically demonstrate a mostly lobular panniculitis with varying degrees of septal fibrosis and focal necrosis. Neutrophils may predominate early, while adipocyte necrosis, epithelioid histiocytes, multinucleated giant cells, and lymphocytes may be found in older lesions. The presence of vasculitis as a requisite diagnostic criterion remains controversial.1,12
The incidence of erythema induratum has decreased since multidrug tuberculosis treatment has become more widespread.3 Our case displayed the disseminated variant of erythema induratum, an even rarer clinical entity.8 Interestingly, our patient had a history of tuberculosis and exposure to BCG prior to the development of lesions. Case reports have documented erythema induratum after BCG exposure but less frequently than in cases associated with tuberculosis.3,13
The use of BCG vaccines has necessitated the need for a more precise method of determining tuberculosis activity. The tuberculin skin test reacts positively with a history of BCG exposure, rendering it an inadequate test in a patient who is suspected of having an active or latent M tuberculosis infection.13,14 IFN-γ release assays are more specific in detecting latent or active tuberculosis than the tuberculin skin test. Such assays use early secretory antigenic target 6 and cultured filtrate protein 10 as antigens to determine sensitization to M tuberculosis.13,15 These antigens are not produced by BCG or Mycobacterium avium; however, other mycobacteria such as Mycobacterium marinum, Mycobacterium kansasii, and some strains of M bovis produce the aforementioned antigens, and exposure to these microbes may be confounding.13 Importantly, positive IFN-γ release assay results also have been documented after BCG exposure but occur at a much lower frequency than for tuberculosis.15 Thus, the combination of the positive IFN-γ release assay and new chest radiograph nodule in our patient provided strong evidence of reactivated tuberculosis as the precipitating cause of her skin disease.
Despite her negative PCR study, our patient’s presentation remains consistent with the diagnosis of disseminated erythema induratum.13,15 The value of PCR studies in establishing the diagnosis remains to be determined. Case reports have described positive PCR results detecting M tuberculosis in panniculitic nodules, suggesting that trace amounts of the organism are present in lesional tissue despite the negative culture result and immunostains.1 Tuberculid reactions, including lichen scrofulosorum, papulonecrotic tuberculid, and erythema induratum, historically are defined by the lack of positive cultures and immunostains, making positive PCR results difficult to reconcile pathophysiologically.1,13 Therefore, use of the term tuberculid altogether as a descriptor for pathogenesis of this disease may need to be avoided.16 Postulated explanations for the relationship of tuberculid diseases and negative cultures and immunostains include the presence of a small number of bacilli that escape routine laboratory detection, early destruction of organisms, or a reaction to circulating M tuberculosis fragments.2 Regardless, until the pathophysiology of erythema induratum has been fully elucidated, the value of PCR remains unclear.
Disseminated erythema induratum, an exceptionally rare variant of panniculitis, may be seen in patients with a remote history of M tuberculosis exposure and/or recent therapeutic BCG exposure. It is imperative to rule out active tuberculosis, especially in elderly patients whose disease predated the advent of modern antituberculosis therapy. Using an IFN-γ release assay in addition to chest radiographs and other clinical stigmata allows differentiation of the etiology of erythema induratum in those patients with tuberculosis who also were treated with BCG.
- Mascaro JM, Basalga E. Erythema induratum of Bazin. Dermatol Clin. 2008;28:439-445.
- Lighter J, Tse DB, Li Y, et al. Erythema induratum of Bazin in a child: evidence for a cell-mediated hyper-response to Mycobacterium tuberculosis. Pediatr Infect Dis J. 2009;28:326-328.
- Inoue T, Fukumoto T, Ansai S, et al. Erythema induratum of Bazin in an infant after bacilli Calmette-Guerin vaccination. J Dermatol. 2006;33:268-272.
- Degonda Halter M, Nebiker P, Hug B, et al. Atypical erythema induratum Bazin with tuberculous osteomyelitis. Internist. 2006;47:853-856.
- Gilchrist H, Patterson JW. Erythema nodosum and erythema induratum (nodular vasculitis): diagnosis and management. Dermatol Ther. 2010;23:320-327.
- Sharma S, Sehgal VN, Bhattacharya SN, et al. Clinicopathologic spectrum of cutaneous tuberculosis: a retrospective analysis of 165 Indians. Am J Dermatopathol. 2015;37:444-450.
- Sethuraman G, Ramesh V. Cutaneous tuberculosis in children. Pediatr Dermatol. 2013;30:7-16.
- Teramura K, Fujimoto N, Nakanishi G, et al. Disseminated erythema induratum of Bazin. Eur J Dermatol. 2014;24:697-698.
- Bazin E. Extrait des Lecons Théoretiques et Cliniques sur le Scrofule. 2nd ed. Paris, France: Delhaye; 1861.
- Campbell SM, Winkelmann RR, Sammons DL. Erythema induratum caused by Mycobacterium chelonei in an immunocompetent patient. J Clin Aesthet Dermatol. 2013;6:38-40.
- Patterson JW. Panniculitis. In: Bolognia JL, Jorizzo J, Rapini RP, et al, eds. Dermatology. Barcelona, Spain: Mosby Elsevier; 2012:1641-1662.
- Segura S, Pujol R, Trinidade F, et al. Vasculitis in erythema induratum of Bazin: a histopathologic study of 101 biopsy specimens from 86 patients. J Am Acad Dermatol. 2008;59:839-851.
- Vera-Kellet C, Peters L, Elwood K, et al. Usefulness of interferon-γ release assays in the diagnosis of erythema induratum. Arch Dermatol. 2011;147:949-952.
- Prajapati V, Steed M, Grewal P, et al. Erythema induratum: case series illustrating the utility of the interferon-γ release assay in determining the association with tuberculosis. J Cutan Med Surg. 2013;17:S6-S11.
- Sim JH, Whang KU. Application of the QuantiFERON-Gold TB test in erythema induratum. J Dermatolog Treat. 2014;25:260-263.
- Wiebels D, Turnbull K, Steinkraus V, et al. Erythema induratum Bazin.”tuberculid” or tuberculosis? [in German]. Hautarzt. 2007;58:237-240.
- Mascaro JM, Basalga E. Erythema induratum of Bazin. Dermatol Clin. 2008;28:439-445.
- Lighter J, Tse DB, Li Y, et al. Erythema induratum of Bazin in a child: evidence for a cell-mediated hyper-response to Mycobacterium tuberculosis. Pediatr Infect Dis J. 2009;28:326-328.
- Inoue T, Fukumoto T, Ansai S, et al. Erythema induratum of Bazin in an infant after bacilli Calmette-Guerin vaccination. J Dermatol. 2006;33:268-272.
- Degonda Halter M, Nebiker P, Hug B, et al. Atypical erythema induratum Bazin with tuberculous osteomyelitis. Internist. 2006;47:853-856.
- Gilchrist H, Patterson JW. Erythema nodosum and erythema induratum (nodular vasculitis): diagnosis and management. Dermatol Ther. 2010;23:320-327.
- Sharma S, Sehgal VN, Bhattacharya SN, et al. Clinicopathologic spectrum of cutaneous tuberculosis: a retrospective analysis of 165 Indians. Am J Dermatopathol. 2015;37:444-450.
- Sethuraman G, Ramesh V. Cutaneous tuberculosis in children. Pediatr Dermatol. 2013;30:7-16.
- Teramura K, Fujimoto N, Nakanishi G, et al. Disseminated erythema induratum of Bazin. Eur J Dermatol. 2014;24:697-698.
- Bazin E. Extrait des Lecons Théoretiques et Cliniques sur le Scrofule. 2nd ed. Paris, France: Delhaye; 1861.
- Campbell SM, Winkelmann RR, Sammons DL. Erythema induratum caused by Mycobacterium chelonei in an immunocompetent patient. J Clin Aesthet Dermatol. 2013;6:38-40.
- Patterson JW. Panniculitis. In: Bolognia JL, Jorizzo J, Rapini RP, et al, eds. Dermatology. Barcelona, Spain: Mosby Elsevier; 2012:1641-1662.
- Segura S, Pujol R, Trinidade F, et al. Vasculitis in erythema induratum of Bazin: a histopathologic study of 101 biopsy specimens from 86 patients. J Am Acad Dermatol. 2008;59:839-851.
- Vera-Kellet C, Peters L, Elwood K, et al. Usefulness of interferon-γ release assays in the diagnosis of erythema induratum. Arch Dermatol. 2011;147:949-952.
- Prajapati V, Steed M, Grewal P, et al. Erythema induratum: case series illustrating the utility of the interferon-γ release assay in determining the association with tuberculosis. J Cutan Med Surg. 2013;17:S6-S11.
- Sim JH, Whang KU. Application of the QuantiFERON-Gold TB test in erythema induratum. J Dermatolog Treat. 2014;25:260-263.
- Wiebels D, Turnbull K, Steinkraus V, et al. Erythema induratum Bazin.”tuberculid” or tuberculosis? [in German]. Hautarzt. 2007;58:237-240.
Practice Points
- Erythema induratum is an uncommon panniculitis attributed to a delayed-type hypersensitivity reaction, classically to Mycobacterium tuberculosis.
- The workup for such patients with exposure to both M tuberculosis and bacillus Calmette-Guérin should include IFN-11γ release assays.
- Clinicians should be aware of the disseminated variant of erythema induratum and the laboratory testing needed to establish a cause and help direct treatment.
Renal denervation response similar regardless of CV risks, comorbidities
In a new analysis of international registry data, renal denervation resulted in similar reduced blood pressure levels in patients with varying high-risk comorbidities and across a range of cardiovascular risk scores.
At 3 years, 24-hour systolic BP was reduced by an average of –8.9 mm Hg overall, with slightly higher or lower readings seen in those with higher cardiovascular risk scores (–10.4 mm Hg) and 65 years or older (–10.2 mm Hg). Similar reductions were seen in those with resistant hypertension (–8.7 mm Hg), diabetes (–8.6 mm Hg), isolated systolic hypertension (–10.1 mm Hg), chronic kidney disease (–10.1 mm Hg), or atrial fibrillation (–10.0 mm Hg).
“In the largest international registry of its kind, the efficacy of renal denervation was similar in patients with and without baseline conditions associated with increased sympathetic activity and irrespective of ASCVD [atherosclerotic cardiovascular disease] risk,” first author Felix Mahfoud, MD, said in an interview.
Dr. Mahfoud, from University Hospital of Saarland, Homburg, Germany, and colleagues published their analysis in the Journal of the American College of Cardiology.
The article reported a post hoc analysis of data from the Global SYMPLICITY Registry (GSR), an international, Medtronic-funded effort that includes 2,652 patients with uncontrolled hypertension treated with a Symplicity denervation system. Data were obtained from 196 centers in 45 countries.
“Blood pressure reductions were durable and sustained to 3 years and the rates of new-onset, end-stage renal disease and elevation in serum creatinine levels were very low in patients at high and low [cardiovascular] risk,” reported Dr. Mahfoud.
As expected, adverse event rates were higher for patients with higher baseline cardiovascular risk. “Elevated rates were also seen in patients with [atrial fibrillation] and diabetes, identifying these subgroups who might derive even greater clinical benefit from improved BP control using renal denervation,” said Dr. Mahfoud.
Asked which patients might be optimal candidates for renal denervation, Dr. Mahfoud recommended the technology for “patients with uncontrolled hypertension on medication, patients with nonadherence, unwillingness, or intolerability to medication, and patients with combined systolic and diastolic hypertension.”
Analyses limited by incomplete data
Stephen C. Textor, MD, has concerns over the amount of missing data in the GSR database and its continued use as a repository of information on renal denervation.
“I am a bit lukewarm on this paper in part because of the nature of the registry data they’re using,” he added in an interview. “The problem I see is that the registry is not terribly uniform as to what information they collect on each patient, not terribly uniform in terms of how the procedure is performed, and not terribly uniform on how they follow up patients.”
Indeed, the post hoc subgroup analyses represent only a limited subset because of incomplete data, added Dr. Textor, a nephrologist at the Cleveland Clinic in Rochester, Minn.
“Remarkably, only 504 [patients] had “matched” data for office [systolic BP] levels at the time points defined in the report,” he wrote in an editorial comment accompanying the registry report (J Am Coll Cardiol. 2020 Jun 16;75[23]:2889-91).
Similarly, the researchers were able to calculate baseline atherosclerotic cardiovascular risk scores in only 1,485 patients (56% of total), primarily because of missing cholesterol measurements.
“They simply did these paired comparison that may have included a couple hundred cases, and on average, there were no differences in response, but what I would have liked to see is a multivariate analysis, where you have all the data on everybody and look at what are the factors that impact response?” Dr. Textor said in the interview.
“They really couldn’t do that because they just, they’re just too many holes in the data,” he added.
On the bright side, Dr. Textor noted that, while the impact overall on systolic BP was “modest,” the standard deviations in some cases were large, indicating that some people had large reductions of systolic BP of more than 30-40 mm Hg.
“There is a belief out there that there are some people that really benefit from this, but how to identify them has been the question,” Dr. Textor said.
Enthusiasm for renal denervation plummeted after results from the SYMPLICITY HTN-3 showed the procedure failing to meet its efficacy endpoint in resistant hypertension. The procedure was associated with a 14–mm Hg fall in systolic BP, compared with an 11–mm Hg drop in the “sham” control group (N Engl J Med. 2014 Apr 10;370:1393-401). However, post hoc analysis of the trial revealed significant shortcomings in design and execution.
No renal denervation device is approved in the United States. The Symplicity device used in this registry is approved in the European Union.
In early 2020, the Food and Drug Administration promised a rigorous review of new renal denervation trials. Subsequently, primary results from the SPYRAL HTN-OFF MED pivotal trial were presented at the annual meeting of the American College of Cardiology in March and showed promising efficacy.
SPYRAL HTN OFF-MED was designed in collaboration with the FDA to obtain meaningful evidence of whether renal denervation performed with the Symplicity Spyral multielectrode catheter (Medtronic Vascular) could reduce BP in patients not taking antihypertensive medication.
Dr. Mahfoud reported he has received speaking honoraria from Medtronic and ReCor. Two other authors are employees of Medtronic. Dr. Textor reported no relationships relevant to the contents of this paper. The Global SYMPLICITY Registry is funded by Medtronic Vascular.
SOURCE: Mahfoud F et al. J Am Coll Cardiol. 2020 June 16;75:2879-88.
This article was updated 6/16/20.
In a new analysis of international registry data, renal denervation resulted in similar reduced blood pressure levels in patients with varying high-risk comorbidities and across a range of cardiovascular risk scores.
At 3 years, 24-hour systolic BP was reduced by an average of –8.9 mm Hg overall, with slightly higher or lower readings seen in those with higher cardiovascular risk scores (–10.4 mm Hg) and 65 years or older (–10.2 mm Hg). Similar reductions were seen in those with resistant hypertension (–8.7 mm Hg), diabetes (–8.6 mm Hg), isolated systolic hypertension (–10.1 mm Hg), chronic kidney disease (–10.1 mm Hg), or atrial fibrillation (–10.0 mm Hg).
“In the largest international registry of its kind, the efficacy of renal denervation was similar in patients with and without baseline conditions associated with increased sympathetic activity and irrespective of ASCVD [atherosclerotic cardiovascular disease] risk,” first author Felix Mahfoud, MD, said in an interview.
Dr. Mahfoud, from University Hospital of Saarland, Homburg, Germany, and colleagues published their analysis in the Journal of the American College of Cardiology.
The article reported a post hoc analysis of data from the Global SYMPLICITY Registry (GSR), an international, Medtronic-funded effort that includes 2,652 patients with uncontrolled hypertension treated with a Symplicity denervation system. Data were obtained from 196 centers in 45 countries.
“Blood pressure reductions were durable and sustained to 3 years and the rates of new-onset, end-stage renal disease and elevation in serum creatinine levels were very low in patients at high and low [cardiovascular] risk,” reported Dr. Mahfoud.
As expected, adverse event rates were higher for patients with higher baseline cardiovascular risk. “Elevated rates were also seen in patients with [atrial fibrillation] and diabetes, identifying these subgroups who might derive even greater clinical benefit from improved BP control using renal denervation,” said Dr. Mahfoud.
Asked which patients might be optimal candidates for renal denervation, Dr. Mahfoud recommended the technology for “patients with uncontrolled hypertension on medication, patients with nonadherence, unwillingness, or intolerability to medication, and patients with combined systolic and diastolic hypertension.”
Analyses limited by incomplete data
Stephen C. Textor, MD, has concerns over the amount of missing data in the GSR database and its continued use as a repository of information on renal denervation.
“I am a bit lukewarm on this paper in part because of the nature of the registry data they’re using,” he added in an interview. “The problem I see is that the registry is not terribly uniform as to what information they collect on each patient, not terribly uniform in terms of how the procedure is performed, and not terribly uniform on how they follow up patients.”
Indeed, the post hoc subgroup analyses represent only a limited subset because of incomplete data, added Dr. Textor, a nephrologist at the Cleveland Clinic in Rochester, Minn.
“Remarkably, only 504 [patients] had “matched” data for office [systolic BP] levels at the time points defined in the report,” he wrote in an editorial comment accompanying the registry report (J Am Coll Cardiol. 2020 Jun 16;75[23]:2889-91).
Similarly, the researchers were able to calculate baseline atherosclerotic cardiovascular risk scores in only 1,485 patients (56% of total), primarily because of missing cholesterol measurements.
“They simply did these paired comparison that may have included a couple hundred cases, and on average, there were no differences in response, but what I would have liked to see is a multivariate analysis, where you have all the data on everybody and look at what are the factors that impact response?” Dr. Textor said in the interview.
“They really couldn’t do that because they just, they’re just too many holes in the data,” he added.
On the bright side, Dr. Textor noted that, while the impact overall on systolic BP was “modest,” the standard deviations in some cases were large, indicating that some people had large reductions of systolic BP of more than 30-40 mm Hg.
“There is a belief out there that there are some people that really benefit from this, but how to identify them has been the question,” Dr. Textor said.
Enthusiasm for renal denervation plummeted after results from the SYMPLICITY HTN-3 showed the procedure failing to meet its efficacy endpoint in resistant hypertension. The procedure was associated with a 14–mm Hg fall in systolic BP, compared with an 11–mm Hg drop in the “sham” control group (N Engl J Med. 2014 Apr 10;370:1393-401). However, post hoc analysis of the trial revealed significant shortcomings in design and execution.
No renal denervation device is approved in the United States. The Symplicity device used in this registry is approved in the European Union.
In early 2020, the Food and Drug Administration promised a rigorous review of new renal denervation trials. Subsequently, primary results from the SPYRAL HTN-OFF MED pivotal trial were presented at the annual meeting of the American College of Cardiology in March and showed promising efficacy.
SPYRAL HTN OFF-MED was designed in collaboration with the FDA to obtain meaningful evidence of whether renal denervation performed with the Symplicity Spyral multielectrode catheter (Medtronic Vascular) could reduce BP in patients not taking antihypertensive medication.
Dr. Mahfoud reported he has received speaking honoraria from Medtronic and ReCor. Two other authors are employees of Medtronic. Dr. Textor reported no relationships relevant to the contents of this paper. The Global SYMPLICITY Registry is funded by Medtronic Vascular.
SOURCE: Mahfoud F et al. J Am Coll Cardiol. 2020 June 16;75:2879-88.
This article was updated 6/16/20.
In a new analysis of international registry data, renal denervation resulted in similar reduced blood pressure levels in patients with varying high-risk comorbidities and across a range of cardiovascular risk scores.
At 3 years, 24-hour systolic BP was reduced by an average of –8.9 mm Hg overall, with slightly higher or lower readings seen in those with higher cardiovascular risk scores (–10.4 mm Hg) and 65 years or older (–10.2 mm Hg). Similar reductions were seen in those with resistant hypertension (–8.7 mm Hg), diabetes (–8.6 mm Hg), isolated systolic hypertension (–10.1 mm Hg), chronic kidney disease (–10.1 mm Hg), or atrial fibrillation (–10.0 mm Hg).
“In the largest international registry of its kind, the efficacy of renal denervation was similar in patients with and without baseline conditions associated with increased sympathetic activity and irrespective of ASCVD [atherosclerotic cardiovascular disease] risk,” first author Felix Mahfoud, MD, said in an interview.
Dr. Mahfoud, from University Hospital of Saarland, Homburg, Germany, and colleagues published their analysis in the Journal of the American College of Cardiology.
The article reported a post hoc analysis of data from the Global SYMPLICITY Registry (GSR), an international, Medtronic-funded effort that includes 2,652 patients with uncontrolled hypertension treated with a Symplicity denervation system. Data were obtained from 196 centers in 45 countries.
“Blood pressure reductions were durable and sustained to 3 years and the rates of new-onset, end-stage renal disease and elevation in serum creatinine levels were very low in patients at high and low [cardiovascular] risk,” reported Dr. Mahfoud.
As expected, adverse event rates were higher for patients with higher baseline cardiovascular risk. “Elevated rates were also seen in patients with [atrial fibrillation] and diabetes, identifying these subgroups who might derive even greater clinical benefit from improved BP control using renal denervation,” said Dr. Mahfoud.
Asked which patients might be optimal candidates for renal denervation, Dr. Mahfoud recommended the technology for “patients with uncontrolled hypertension on medication, patients with nonadherence, unwillingness, or intolerability to medication, and patients with combined systolic and diastolic hypertension.”
Analyses limited by incomplete data
Stephen C. Textor, MD, has concerns over the amount of missing data in the GSR database and its continued use as a repository of information on renal denervation.
“I am a bit lukewarm on this paper in part because of the nature of the registry data they’re using,” he added in an interview. “The problem I see is that the registry is not terribly uniform as to what information they collect on each patient, not terribly uniform in terms of how the procedure is performed, and not terribly uniform on how they follow up patients.”
Indeed, the post hoc subgroup analyses represent only a limited subset because of incomplete data, added Dr. Textor, a nephrologist at the Cleveland Clinic in Rochester, Minn.
“Remarkably, only 504 [patients] had “matched” data for office [systolic BP] levels at the time points defined in the report,” he wrote in an editorial comment accompanying the registry report (J Am Coll Cardiol. 2020 Jun 16;75[23]:2889-91).
Similarly, the researchers were able to calculate baseline atherosclerotic cardiovascular risk scores in only 1,485 patients (56% of total), primarily because of missing cholesterol measurements.
“They simply did these paired comparison that may have included a couple hundred cases, and on average, there were no differences in response, but what I would have liked to see is a multivariate analysis, where you have all the data on everybody and look at what are the factors that impact response?” Dr. Textor said in the interview.
“They really couldn’t do that because they just, they’re just too many holes in the data,” he added.
On the bright side, Dr. Textor noted that, while the impact overall on systolic BP was “modest,” the standard deviations in some cases were large, indicating that some people had large reductions of systolic BP of more than 30-40 mm Hg.
“There is a belief out there that there are some people that really benefit from this, but how to identify them has been the question,” Dr. Textor said.
Enthusiasm for renal denervation plummeted after results from the SYMPLICITY HTN-3 showed the procedure failing to meet its efficacy endpoint in resistant hypertension. The procedure was associated with a 14–mm Hg fall in systolic BP, compared with an 11–mm Hg drop in the “sham” control group (N Engl J Med. 2014 Apr 10;370:1393-401). However, post hoc analysis of the trial revealed significant shortcomings in design and execution.
No renal denervation device is approved in the United States. The Symplicity device used in this registry is approved in the European Union.
In early 2020, the Food and Drug Administration promised a rigorous review of new renal denervation trials. Subsequently, primary results from the SPYRAL HTN-OFF MED pivotal trial were presented at the annual meeting of the American College of Cardiology in March and showed promising efficacy.
SPYRAL HTN OFF-MED was designed in collaboration with the FDA to obtain meaningful evidence of whether renal denervation performed with the Symplicity Spyral multielectrode catheter (Medtronic Vascular) could reduce BP in patients not taking antihypertensive medication.
Dr. Mahfoud reported he has received speaking honoraria from Medtronic and ReCor. Two other authors are employees of Medtronic. Dr. Textor reported no relationships relevant to the contents of this paper. The Global SYMPLICITY Registry is funded by Medtronic Vascular.
SOURCE: Mahfoud F et al. J Am Coll Cardiol. 2020 June 16;75:2879-88.
This article was updated 6/16/20.
FROM THE JOURNAL OF THE AMERICAN COLLEGE OF CARDIOLOGY
For COVID-19 plus diabetes, glycemic control tops treatment list
Optimizing glycemic control “is the key to overall treatment in people with diabetes and COVID-19,” said Antonio Ceriello, MD, during a June 5 webinar sponsored by Harvard Medical School, Boston.
Dr. Ceriello, a research consultant with the Italian Ministry of Health, IRCCS Multi-Medica, Milan, highlighted a recent study that examined the association of blood glucose control and outcomes in COVID-19 patients with preexisting type 2 diabetes.
Among 7,000 cases of COVID-19, type 2 diabetes correlated with a higher death rate. However, those with well-controlled blood glucose (upper limit ≤10 mmol/L) had a survival rate of 98.9%, compared with just 11% among those with poorly controlled blood glucose (upper limit >10 mmol/L), a reduction in risk of 86% (adjusted hazard ratio, 0.14; Cell Metab. 2020 May 1. doi: 10.1016/j.cmet.2020.04.021).
Clinicians should also consider the possible side effects of hypoglycemic agents in the evolution of this disease. This is true of all patients, not just diabetes patients, Dr. Ceriello said. “We have data showing that ... hyperglycemia contributes directly to worsening the prognosis of COVID-19 independent of the presence of diabetes.”
One study found that the glycosylation of ACE-2 played an important role in allowing cellular entry of the virus (Am J Physiol Endocrinol Metab. 2020 Mar 31;318:E736-41). “This is something that could be related to hyperglycemia,” he added.
Another risk factor is thrombosis, a clear contributor to death rates in COVID-19. Research on thrombosis incidence in COVID-19 patients with diabetes reported higher levels of D-dimer levels in people with diabetes, especially among those who couldn’t manage their disease.
Tying all of these factors together, Dr. Ceriello discussed how ACE-2 glycosylation, in combination with other factors in SARS-CoV-2 infection, could lead to hyperglycemia, thrombosis, and subsequently multiorgan damage in diabetes patients.
Other research has associated higher HbA1c levels (mean HbA1c, 7.5%) with higher mortality risk in COVID-19 patients, said another speaker, Linong Ji, MD, director for endocrinology and metabolism at Peking University People’s Hospital, Beijing, and director of Peking University’s Diabetes Center. Proper guidance is key to ensuring early detection of hyperglycemic crisis in people with diabetes, advised Dr. Ji.
Global management of diabetes in SARS-CoV-2 patients is “quite challenging,” given that most patients don’t have their diabetes under control, said host and moderator A. Enrique Caballero, MD, an endocrinologist/investigator in the division of endocrinology, diabetes, and hypertension and division of global health equity at Brigham and Women’s Hospital, Boston. “They are not meeting treatment targets for cholesterol or glucose control. So we’re not managing optimal care. And now on top of this, we have COVID-19.”
Optimizing glycemic control “is the key to overall treatment in people with diabetes and COVID-19,” said Antonio Ceriello, MD, during a June 5 webinar sponsored by Harvard Medical School, Boston.
Dr. Ceriello, a research consultant with the Italian Ministry of Health, IRCCS Multi-Medica, Milan, highlighted a recent study that examined the association of blood glucose control and outcomes in COVID-19 patients with preexisting type 2 diabetes.
Among 7,000 cases of COVID-19, type 2 diabetes correlated with a higher death rate. However, those with well-controlled blood glucose (upper limit ≤10 mmol/L) had a survival rate of 98.9%, compared with just 11% among those with poorly controlled blood glucose (upper limit >10 mmol/L), a reduction in risk of 86% (adjusted hazard ratio, 0.14; Cell Metab. 2020 May 1. doi: 10.1016/j.cmet.2020.04.021).
Clinicians should also consider the possible side effects of hypoglycemic agents in the evolution of this disease. This is true of all patients, not just diabetes patients, Dr. Ceriello said. “We have data showing that ... hyperglycemia contributes directly to worsening the prognosis of COVID-19 independent of the presence of diabetes.”
One study found that the glycosylation of ACE-2 played an important role in allowing cellular entry of the virus (Am J Physiol Endocrinol Metab. 2020 Mar 31;318:E736-41). “This is something that could be related to hyperglycemia,” he added.
Another risk factor is thrombosis, a clear contributor to death rates in COVID-19. Research on thrombosis incidence in COVID-19 patients with diabetes reported higher levels of D-dimer levels in people with diabetes, especially among those who couldn’t manage their disease.
Tying all of these factors together, Dr. Ceriello discussed how ACE-2 glycosylation, in combination with other factors in SARS-CoV-2 infection, could lead to hyperglycemia, thrombosis, and subsequently multiorgan damage in diabetes patients.
Other research has associated higher HbA1c levels (mean HbA1c, 7.5%) with higher mortality risk in COVID-19 patients, said another speaker, Linong Ji, MD, director for endocrinology and metabolism at Peking University People’s Hospital, Beijing, and director of Peking University’s Diabetes Center. Proper guidance is key to ensuring early detection of hyperglycemic crisis in people with diabetes, advised Dr. Ji.
Global management of diabetes in SARS-CoV-2 patients is “quite challenging,” given that most patients don’t have their diabetes under control, said host and moderator A. Enrique Caballero, MD, an endocrinologist/investigator in the division of endocrinology, diabetes, and hypertension and division of global health equity at Brigham and Women’s Hospital, Boston. “They are not meeting treatment targets for cholesterol or glucose control. So we’re not managing optimal care. And now on top of this, we have COVID-19.”
Optimizing glycemic control “is the key to overall treatment in people with diabetes and COVID-19,” said Antonio Ceriello, MD, during a June 5 webinar sponsored by Harvard Medical School, Boston.
Dr. Ceriello, a research consultant with the Italian Ministry of Health, IRCCS Multi-Medica, Milan, highlighted a recent study that examined the association of blood glucose control and outcomes in COVID-19 patients with preexisting type 2 diabetes.
Among 7,000 cases of COVID-19, type 2 diabetes correlated with a higher death rate. However, those with well-controlled blood glucose (upper limit ≤10 mmol/L) had a survival rate of 98.9%, compared with just 11% among those with poorly controlled blood glucose (upper limit >10 mmol/L), a reduction in risk of 86% (adjusted hazard ratio, 0.14; Cell Metab. 2020 May 1. doi: 10.1016/j.cmet.2020.04.021).
Clinicians should also consider the possible side effects of hypoglycemic agents in the evolution of this disease. This is true of all patients, not just diabetes patients, Dr. Ceriello said. “We have data showing that ... hyperglycemia contributes directly to worsening the prognosis of COVID-19 independent of the presence of diabetes.”
One study found that the glycosylation of ACE-2 played an important role in allowing cellular entry of the virus (Am J Physiol Endocrinol Metab. 2020 Mar 31;318:E736-41). “This is something that could be related to hyperglycemia,” he added.
Another risk factor is thrombosis, a clear contributor to death rates in COVID-19. Research on thrombosis incidence in COVID-19 patients with diabetes reported higher levels of D-dimer levels in people with diabetes, especially among those who couldn’t manage their disease.
Tying all of these factors together, Dr. Ceriello discussed how ACE-2 glycosylation, in combination with other factors in SARS-CoV-2 infection, could lead to hyperglycemia, thrombosis, and subsequently multiorgan damage in diabetes patients.
Other research has associated higher HbA1c levels (mean HbA1c, 7.5%) with higher mortality risk in COVID-19 patients, said another speaker, Linong Ji, MD, director for endocrinology and metabolism at Peking University People’s Hospital, Beijing, and director of Peking University’s Diabetes Center. Proper guidance is key to ensuring early detection of hyperglycemic crisis in people with diabetes, advised Dr. Ji.
Global management of diabetes in SARS-CoV-2 patients is “quite challenging,” given that most patients don’t have their diabetes under control, said host and moderator A. Enrique Caballero, MD, an endocrinologist/investigator in the division of endocrinology, diabetes, and hypertension and division of global health equity at Brigham and Women’s Hospital, Boston. “They are not meeting treatment targets for cholesterol or glucose control. So we’re not managing optimal care. And now on top of this, we have COVID-19.”
High-frequency spinal cord stimulation eases painful diabetic neuropathy
For patients with painful diabetic neuropathy that doesn’t resolve with standard treatment, use of a 10-kHz spinal cord stimulation device may relieve pain and improve sensation, initial results of a large randomized controlled trial suggest.
Some 79% of patients had substantial pain relief 3 months after starting treatment, compared with 5% of patients managed with conventional medical treatment, according to results of SENZA-PDN, which investigators say is the largest-ever randomized, controlled trial of spinal cord stimulation for managing painful diabetic neuropathy.
Although this was not a comparative trial, investigator Erika Petersen, MD, said in an interview that results seen with the 10-kHz spinal cord stimulator (Nevro Corp.) exceed what has been seen in previous studies of spinal cord stimulation devices operating at lower frequencies, where response rates have been in the 40%-55% range.
New option for front line providers?
“My overall takeaway here is that these initial 3-month results are very promising,” said Dr. Petersen, who is Director of Functional & Restorative Neurosurgery and Neuromodulation at the University of Arkansas for Medical Sciences in Little Rock.
Patient-perceived numbness and sensory assessments by investigators also improved following implantation of the spinal cord stimulator, according to Dr. Peterson, who added that measurements of sleep and activity also seemed to improve in these patients with painful diabetic neuropathy.
“Spinal cord stimulation has been established for chronic back and leg pain, but being able to innovate in this population with diabetic neuropathy is really something that we anticipate will improve quality of life and functional benefit for a large number of patients who currently have been stuck with the options that are currently available,” Dr. Petersen said in an interview.
Natalie H. Strand, MD, assistant professor of pain medicine at Mayo Clinic, Scottsdale, Ariz., said that while the findings of this randomized study may require corroboration, they do suggest that this neuromodulation device may provide another option for front line diabetes providers when patients have persistent pain despite appropriately medication management.
“These patients are probably under-referred to interventional pain specialists,” said Dr. Strand in an interview. “The primary care physicians and endocrinologists may not think of neuromodulation as an appropriate treatment, and they may not know that it can be so effective.”
“Anything that we can add as physicians to help decrease the burden of diabetes is going to be very impactful,” Dr. Strand added. “While this is focused on pain, what we’re really trying to treat is the entire patient – improve their quality of life and make diabetes more manageable.”
Nearly 80% of treated patients responded at 3 months
The SENZA-PDN study results were presented as a late-breaking poster presentation at the virtual annual scientific sessions of the American Diabetes Association. Those results included 103 patients randomized to conventional medical management alone, and 113 who received medical management plus the spinal cord stimulator, which Dr. Strand described as a minimally invasive, reversibly implanted epidural device designed to stimulate the spinal cord and reverse pain sensations.
The median age was about 61 years and roughly two-thirds were male. All patients had to have lower extremity pain with an average intensity of at least 5 out of 10 cm on the visual analog scale (VAS) at enrollment, according to published inclusion criteria for the study (NCT03228420).
Three months after device implantation, 75 out of 95 evaluable patients (79%) had a response, defined as 50% or greater pain relief plus no worsening of neurological deficit related to painful diabetic neuropathy. By contrast, only 5 of 94 medically managed patients (5%) met those response criteria (P < 0.001), according to reported data.
The mean VAS score in the device group dropped from 7.6 at baseline to 2.4 at 1 month and 1.7 at 3 months, data show. In the medical management group, mean VAS scores were 7.0 at baseline, 6.7 at 1 month, and 6.5 at 3 months.
Sensory assessment of monofilament and pinprick perception, performed by investigators at 3 months, indicated a 72% improvement in the device arm versus 7% improvement in the medical management arm, while analysis of patient-drawn diagrams additionally suggested improvement in perceived numbness, according to investigators.
Quality-of-life improvements related to sleep and activity were also apparent at 3 months in the device group, Dr. Petersen said, with investigators noting substantial reductions in trouble falling asleep because of pain and awakening due to pain. Likewise, data at this initial report suggested improvements in 6-minute walk test that were apparent in the device group but not the medical management group.
While the spinal cord stimulator under investigation is already approved by the U.S. Food and Drug Administration, Dr. Petersen said a lack of data specific to painful diabetic neuropathy has been a hurdle to insurance coverage for some patients.
“I’ve had patients who clearly have every suggestion that they match the characteristics of our research population here, but the insurance will decline the procedure as being experimental,” she said. “My hope is that randomized, controlled trial results in a research study such as this is something that will improve the access of the therapy to patients who would not be able to afford it without having insurance cover the procedure.”
Follow-up of the study will continue for 24 months and will include assessment of health economics and use of pain medication, Dr. Petersen said.
The SENZA-PDN study is funded by Nevro Corp. Dr. Petersen said that she receives research funding and consulting fees from Nevro Corp. and other device manufacturers. Dr. Strand said she had no disclosures related to the research.
SOURCE: Petersen E. ADA 2020, Late-breaking poster 31-LB.
For patients with painful diabetic neuropathy that doesn’t resolve with standard treatment, use of a 10-kHz spinal cord stimulation device may relieve pain and improve sensation, initial results of a large randomized controlled trial suggest.
Some 79% of patients had substantial pain relief 3 months after starting treatment, compared with 5% of patients managed with conventional medical treatment, according to results of SENZA-PDN, which investigators say is the largest-ever randomized, controlled trial of spinal cord stimulation for managing painful diabetic neuropathy.
Although this was not a comparative trial, investigator Erika Petersen, MD, said in an interview that results seen with the 10-kHz spinal cord stimulator (Nevro Corp.) exceed what has been seen in previous studies of spinal cord stimulation devices operating at lower frequencies, where response rates have been in the 40%-55% range.
New option for front line providers?
“My overall takeaway here is that these initial 3-month results are very promising,” said Dr. Petersen, who is Director of Functional & Restorative Neurosurgery and Neuromodulation at the University of Arkansas for Medical Sciences in Little Rock.
Patient-perceived numbness and sensory assessments by investigators also improved following implantation of the spinal cord stimulator, according to Dr. Peterson, who added that measurements of sleep and activity also seemed to improve in these patients with painful diabetic neuropathy.
“Spinal cord stimulation has been established for chronic back and leg pain, but being able to innovate in this population with diabetic neuropathy is really something that we anticipate will improve quality of life and functional benefit for a large number of patients who currently have been stuck with the options that are currently available,” Dr. Petersen said in an interview.
Natalie H. Strand, MD, assistant professor of pain medicine at Mayo Clinic, Scottsdale, Ariz., said that while the findings of this randomized study may require corroboration, they do suggest that this neuromodulation device may provide another option for front line diabetes providers when patients have persistent pain despite appropriately medication management.
“These patients are probably under-referred to interventional pain specialists,” said Dr. Strand in an interview. “The primary care physicians and endocrinologists may not think of neuromodulation as an appropriate treatment, and they may not know that it can be so effective.”
“Anything that we can add as physicians to help decrease the burden of diabetes is going to be very impactful,” Dr. Strand added. “While this is focused on pain, what we’re really trying to treat is the entire patient – improve their quality of life and make diabetes more manageable.”
Nearly 80% of treated patients responded at 3 months
The SENZA-PDN study results were presented as a late-breaking poster presentation at the virtual annual scientific sessions of the American Diabetes Association. Those results included 103 patients randomized to conventional medical management alone, and 113 who received medical management plus the spinal cord stimulator, which Dr. Strand described as a minimally invasive, reversibly implanted epidural device designed to stimulate the spinal cord and reverse pain sensations.
The median age was about 61 years and roughly two-thirds were male. All patients had to have lower extremity pain with an average intensity of at least 5 out of 10 cm on the visual analog scale (VAS) at enrollment, according to published inclusion criteria for the study (NCT03228420).
Three months after device implantation, 75 out of 95 evaluable patients (79%) had a response, defined as 50% or greater pain relief plus no worsening of neurological deficit related to painful diabetic neuropathy. By contrast, only 5 of 94 medically managed patients (5%) met those response criteria (P < 0.001), according to reported data.
The mean VAS score in the device group dropped from 7.6 at baseline to 2.4 at 1 month and 1.7 at 3 months, data show. In the medical management group, mean VAS scores were 7.0 at baseline, 6.7 at 1 month, and 6.5 at 3 months.
Sensory assessment of monofilament and pinprick perception, performed by investigators at 3 months, indicated a 72% improvement in the device arm versus 7% improvement in the medical management arm, while analysis of patient-drawn diagrams additionally suggested improvement in perceived numbness, according to investigators.
Quality-of-life improvements related to sleep and activity were also apparent at 3 months in the device group, Dr. Petersen said, with investigators noting substantial reductions in trouble falling asleep because of pain and awakening due to pain. Likewise, data at this initial report suggested improvements in 6-minute walk test that were apparent in the device group but not the medical management group.
While the spinal cord stimulator under investigation is already approved by the U.S. Food and Drug Administration, Dr. Petersen said a lack of data specific to painful diabetic neuropathy has been a hurdle to insurance coverage for some patients.
“I’ve had patients who clearly have every suggestion that they match the characteristics of our research population here, but the insurance will decline the procedure as being experimental,” she said. “My hope is that randomized, controlled trial results in a research study such as this is something that will improve the access of the therapy to patients who would not be able to afford it without having insurance cover the procedure.”
Follow-up of the study will continue for 24 months and will include assessment of health economics and use of pain medication, Dr. Petersen said.
The SENZA-PDN study is funded by Nevro Corp. Dr. Petersen said that she receives research funding and consulting fees from Nevro Corp. and other device manufacturers. Dr. Strand said she had no disclosures related to the research.
SOURCE: Petersen E. ADA 2020, Late-breaking poster 31-LB.
For patients with painful diabetic neuropathy that doesn’t resolve with standard treatment, use of a 10-kHz spinal cord stimulation device may relieve pain and improve sensation, initial results of a large randomized controlled trial suggest.
Some 79% of patients had substantial pain relief 3 months after starting treatment, compared with 5% of patients managed with conventional medical treatment, according to results of SENZA-PDN, which investigators say is the largest-ever randomized, controlled trial of spinal cord stimulation for managing painful diabetic neuropathy.
Although this was not a comparative trial, investigator Erika Petersen, MD, said in an interview that results seen with the 10-kHz spinal cord stimulator (Nevro Corp.) exceed what has been seen in previous studies of spinal cord stimulation devices operating at lower frequencies, where response rates have been in the 40%-55% range.
New option for front line providers?
“My overall takeaway here is that these initial 3-month results are very promising,” said Dr. Petersen, who is Director of Functional & Restorative Neurosurgery and Neuromodulation at the University of Arkansas for Medical Sciences in Little Rock.
Patient-perceived numbness and sensory assessments by investigators also improved following implantation of the spinal cord stimulator, according to Dr. Peterson, who added that measurements of sleep and activity also seemed to improve in these patients with painful diabetic neuropathy.
“Spinal cord stimulation has been established for chronic back and leg pain, but being able to innovate in this population with diabetic neuropathy is really something that we anticipate will improve quality of life and functional benefit for a large number of patients who currently have been stuck with the options that are currently available,” Dr. Petersen said in an interview.
Natalie H. Strand, MD, assistant professor of pain medicine at Mayo Clinic, Scottsdale, Ariz., said that while the findings of this randomized study may require corroboration, they do suggest that this neuromodulation device may provide another option for front line diabetes providers when patients have persistent pain despite appropriately medication management.
“These patients are probably under-referred to interventional pain specialists,” said Dr. Strand in an interview. “The primary care physicians and endocrinologists may not think of neuromodulation as an appropriate treatment, and they may not know that it can be so effective.”
“Anything that we can add as physicians to help decrease the burden of diabetes is going to be very impactful,” Dr. Strand added. “While this is focused on pain, what we’re really trying to treat is the entire patient – improve their quality of life and make diabetes more manageable.”
Nearly 80% of treated patients responded at 3 months
The SENZA-PDN study results were presented as a late-breaking poster presentation at the virtual annual scientific sessions of the American Diabetes Association. Those results included 103 patients randomized to conventional medical management alone, and 113 who received medical management plus the spinal cord stimulator, which Dr. Strand described as a minimally invasive, reversibly implanted epidural device designed to stimulate the spinal cord and reverse pain sensations.
The median age was about 61 years and roughly two-thirds were male. All patients had to have lower extremity pain with an average intensity of at least 5 out of 10 cm on the visual analog scale (VAS) at enrollment, according to published inclusion criteria for the study (NCT03228420).
Three months after device implantation, 75 out of 95 evaluable patients (79%) had a response, defined as 50% or greater pain relief plus no worsening of neurological deficit related to painful diabetic neuropathy. By contrast, only 5 of 94 medically managed patients (5%) met those response criteria (P < 0.001), according to reported data.
The mean VAS score in the device group dropped from 7.6 at baseline to 2.4 at 1 month and 1.7 at 3 months, data show. In the medical management group, mean VAS scores were 7.0 at baseline, 6.7 at 1 month, and 6.5 at 3 months.
Sensory assessment of monofilament and pinprick perception, performed by investigators at 3 months, indicated a 72% improvement in the device arm versus 7% improvement in the medical management arm, while analysis of patient-drawn diagrams additionally suggested improvement in perceived numbness, according to investigators.
Quality-of-life improvements related to sleep and activity were also apparent at 3 months in the device group, Dr. Petersen said, with investigators noting substantial reductions in trouble falling asleep because of pain and awakening due to pain. Likewise, data at this initial report suggested improvements in 6-minute walk test that were apparent in the device group but not the medical management group.
While the spinal cord stimulator under investigation is already approved by the U.S. Food and Drug Administration, Dr. Petersen said a lack of data specific to painful diabetic neuropathy has been a hurdle to insurance coverage for some patients.
“I’ve had patients who clearly have every suggestion that they match the characteristics of our research population here, but the insurance will decline the procedure as being experimental,” she said. “My hope is that randomized, controlled trial results in a research study such as this is something that will improve the access of the therapy to patients who would not be able to afford it without having insurance cover the procedure.”
Follow-up of the study will continue for 24 months and will include assessment of health economics and use of pain medication, Dr. Petersen said.
The SENZA-PDN study is funded by Nevro Corp. Dr. Petersen said that she receives research funding and consulting fees from Nevro Corp. and other device manufacturers. Dr. Strand said she had no disclosures related to the research.
SOURCE: Petersen E. ADA 2020, Late-breaking poster 31-LB.
FROM ADA 2020
Fighting COVID and police brutality, medical teams take to streets to treat protesters
Amid clouds of choking tear gas, booming flash-bang grenades and other “riot control agents,” volunteer medics plunged into street protests over the past weeks to help the injured – sometimes rushing to the front lines as soon as their hospital shifts ended.
Known as “street medics,” these unorthodox teams of nursing students, veterinarians, doctors, trauma surgeons, security guards, ski patrollers, nurses, wilderness EMTs, and off-the-clock ambulance workers poured water – not milk – into the eyes of tear-gassed protesters. They stanched bleeding wounds and plucked disoriented teenagers from clouds of gas, entering dangerous corners where on-duty emergency health responders may fear to go.
So donning cloth masks to protect against the virus – plus helmets, makeshift shields and other gear to guard against rubber bullets, projectiles and tear gas – the volunteer medics organized themselves into a web of first responders to care for people on the streets. They showed up early, set up first-aid stations, established transportation networks and covered their arms, helmets and backpacks with crosses made of red duct tape, to signify that they were medics. Some stayed late into the night past curfews until every protester had left.
Iris Butler, a 21-year-old certified nursing assistant who works in a nursing home, decided to offer her skills after seeing a man injured by a rubber bullet on her first night at the Denver protests. She showed up as a medic every night thereafter. She didn’t see it as a choice.
“I am working full time and basically being at the protest after getting straight off of work,” said Butler, who is black. That’s tiring, she added, but so is being a black woman in America.
After going out as a medic on her own, she soon met other volunteers. Together they used text-message chains to organize their efforts. One night, she responded to a man who had been shot with a rubber bullet in the chest; she said his torso had turned blue and purple from the impact. She also provided aid after a shooting near the protest left someone in critical condition.
“It’s hard, but bills need to be paid and justice needs to be served,” she said.
The street medic movement traces its roots, in part, to the 1960s protests, as well as the American Indian Movement and the Black Panther Party. Denver Action Medic Network offers a 20-hour training course that prepares them to treat patients in conflicts with police and large crowds; a four-hour session is offered to medical professionals as “bridge” training.
Since the coronavirus pandemic began, the Denver Action Medic Network has added new training guidelines: Don’t go to protests if sick or in contact with those who are infected; wear a mask; give people lots of space and use hand sanitizer. Jordan Garcia, a 39-year-old medic for over 20 years who works with the network of veteran street medics, said they also warn medics about the increased risk of transmission because of protesters coughing from tear gas, and urge them to get tested for the virus after the protests.
The number of volunteer medics swelled after George Floyd’s May 25 killing in Minneapolis. In Denver alone, at least 40 people reached out to the Denver Action Medic Network for training.
On June 3, Dr. Rupa Marya, an associate professor of medicine at the University of California,San Francisco, and the co-founder of the Do No Harm Coalition, which runs street medic training in the Bay Area, hosted a national webinar attended by over 3,000 medical professionals to provide the bridge training to be a street medic. In her online bio, Marya describes the coalition as “an organization of over 450 health workers committed to structural change” in addressing health problems.
“When we see suffering, that’s where we go,” Marya said. “And right now that suffering is happening on the streets.”
In the recent Denver protests, street medics responded to major head, face and eye injuries among protesters from what are sometimes described as “kinetic impact projectiles” or “less-than-lethal” bullets shot at protesters, along with tear-gas and flash-bang stun grenade canisters that either hit them or exploded in their faces.
Garcia, who by day works for an immigrant rights nonprofit, said that these weapons are not designed to be shot directly at people.
“We’re seeing police use these less-lethal weapons in lethal ways, and that is pretty upsetting,” Garcia said about the recent protests.
Denver police Chief Paul Pazen promised to make changes, including banning chokeholds and requiring SWAT teams to turn on their body cameras. Last week, a federal judge also issued a temporary injunction to stop Denver police from using tear gas and other less-than-lethal weapons in response to a class action lawsuit, in which a medic stated he was shot multiple times by police with pepper balls while treating patients. (Last week in North Carolina police were recorded destroying medic stations.)
Denver street medic Kevin Connell, a 30-year-old emergency room nurse, said he was hit with pepper balls in the back of his medic vest – which was clearly marked by red crosses – while treating a patient. He showed up to the Denver protests every night he did not have to work, he said, wearing a Kevlar medic vest, protective goggles and a homemade gas mask fashioned from a water bottle. As a member of the Denver Action Medic Network, Connell also served at the Standing Rock protests in North Dakota in a dispute over the building of the Dakota Access Pipeline.
“I mean, as bad as it sounds, it was only tear gas, pepper balls and rubber bullets that were being fired on us,” Connell said of his recent experience in Denver. “When I was at Standing Rock, they were using high-powered water hoses even when it was, like, freezing cold. … So I think the police here had a little bit more restraint.”
Still, first-time street medic Aj Mossman, a 31-year-old Denver emergency medical technician studying for nursing school, was shocked to be tear-gassed and struck in the back of the leg with a flash grenade while treating a protester on May 30. Mossman still has a large leg bruise.
The following night, Mossman, who uses the pronoun they, brought more protective gear, but said they are still having difficulty processing what felt like a war zone.
“I thought I understood what my black friends went through. I thought I understood what the black community went through,” said Mossman, who is white. “But I had absolutely no idea how violent the police were and how little they cared about who they hurt.”
For Butler, serving as a medic with others from various walks of life was inspiring. “They’re also out there to protect black and brown bodies. And that’s amazing,” she said. “That’s just a beautiful sight.”
This article originally appeared on Kaiser Health News, which is a nonprofit national health policy news service. It is an editorially independent program of the Henry J. Kaiser Family Foundation that is not affiliated with Kaiser Permanente.
Amid clouds of choking tear gas, booming flash-bang grenades and other “riot control agents,” volunteer medics plunged into street protests over the past weeks to help the injured – sometimes rushing to the front lines as soon as their hospital shifts ended.
Known as “street medics,” these unorthodox teams of nursing students, veterinarians, doctors, trauma surgeons, security guards, ski patrollers, nurses, wilderness EMTs, and off-the-clock ambulance workers poured water – not milk – into the eyes of tear-gassed protesters. They stanched bleeding wounds and plucked disoriented teenagers from clouds of gas, entering dangerous corners where on-duty emergency health responders may fear to go.
So donning cloth masks to protect against the virus – plus helmets, makeshift shields and other gear to guard against rubber bullets, projectiles and tear gas – the volunteer medics organized themselves into a web of first responders to care for people on the streets. They showed up early, set up first-aid stations, established transportation networks and covered their arms, helmets and backpacks with crosses made of red duct tape, to signify that they were medics. Some stayed late into the night past curfews until every protester had left.
Iris Butler, a 21-year-old certified nursing assistant who works in a nursing home, decided to offer her skills after seeing a man injured by a rubber bullet on her first night at the Denver protests. She showed up as a medic every night thereafter. She didn’t see it as a choice.
“I am working full time and basically being at the protest after getting straight off of work,” said Butler, who is black. That’s tiring, she added, but so is being a black woman in America.
After going out as a medic on her own, she soon met other volunteers. Together they used text-message chains to organize their efforts. One night, she responded to a man who had been shot with a rubber bullet in the chest; she said his torso had turned blue and purple from the impact. She also provided aid after a shooting near the protest left someone in critical condition.
“It’s hard, but bills need to be paid and justice needs to be served,” she said.
The street medic movement traces its roots, in part, to the 1960s protests, as well as the American Indian Movement and the Black Panther Party. Denver Action Medic Network offers a 20-hour training course that prepares them to treat patients in conflicts with police and large crowds; a four-hour session is offered to medical professionals as “bridge” training.
Since the coronavirus pandemic began, the Denver Action Medic Network has added new training guidelines: Don’t go to protests if sick or in contact with those who are infected; wear a mask; give people lots of space and use hand sanitizer. Jordan Garcia, a 39-year-old medic for over 20 years who works with the network of veteran street medics, said they also warn medics about the increased risk of transmission because of protesters coughing from tear gas, and urge them to get tested for the virus after the protests.
The number of volunteer medics swelled after George Floyd’s May 25 killing in Minneapolis. In Denver alone, at least 40 people reached out to the Denver Action Medic Network for training.
On June 3, Dr. Rupa Marya, an associate professor of medicine at the University of California,San Francisco, and the co-founder of the Do No Harm Coalition, which runs street medic training in the Bay Area, hosted a national webinar attended by over 3,000 medical professionals to provide the bridge training to be a street medic. In her online bio, Marya describes the coalition as “an organization of over 450 health workers committed to structural change” in addressing health problems.
“When we see suffering, that’s where we go,” Marya said. “And right now that suffering is happening on the streets.”
In the recent Denver protests, street medics responded to major head, face and eye injuries among protesters from what are sometimes described as “kinetic impact projectiles” or “less-than-lethal” bullets shot at protesters, along with tear-gas and flash-bang stun grenade canisters that either hit them or exploded in their faces.
Garcia, who by day works for an immigrant rights nonprofit, said that these weapons are not designed to be shot directly at people.
“We’re seeing police use these less-lethal weapons in lethal ways, and that is pretty upsetting,” Garcia said about the recent protests.
Denver police Chief Paul Pazen promised to make changes, including banning chokeholds and requiring SWAT teams to turn on their body cameras. Last week, a federal judge also issued a temporary injunction to stop Denver police from using tear gas and other less-than-lethal weapons in response to a class action lawsuit, in which a medic stated he was shot multiple times by police with pepper balls while treating patients. (Last week in North Carolina police were recorded destroying medic stations.)
Denver street medic Kevin Connell, a 30-year-old emergency room nurse, said he was hit with pepper balls in the back of his medic vest – which was clearly marked by red crosses – while treating a patient. He showed up to the Denver protests every night he did not have to work, he said, wearing a Kevlar medic vest, protective goggles and a homemade gas mask fashioned from a water bottle. As a member of the Denver Action Medic Network, Connell also served at the Standing Rock protests in North Dakota in a dispute over the building of the Dakota Access Pipeline.
“I mean, as bad as it sounds, it was only tear gas, pepper balls and rubber bullets that were being fired on us,” Connell said of his recent experience in Denver. “When I was at Standing Rock, they were using high-powered water hoses even when it was, like, freezing cold. … So I think the police here had a little bit more restraint.”
Still, first-time street medic Aj Mossman, a 31-year-old Denver emergency medical technician studying for nursing school, was shocked to be tear-gassed and struck in the back of the leg with a flash grenade while treating a protester on May 30. Mossman still has a large leg bruise.
The following night, Mossman, who uses the pronoun they, brought more protective gear, but said they are still having difficulty processing what felt like a war zone.
“I thought I understood what my black friends went through. I thought I understood what the black community went through,” said Mossman, who is white. “But I had absolutely no idea how violent the police were and how little they cared about who they hurt.”
For Butler, serving as a medic with others from various walks of life was inspiring. “They’re also out there to protect black and brown bodies. And that’s amazing,” she said. “That’s just a beautiful sight.”
This article originally appeared on Kaiser Health News, which is a nonprofit national health policy news service. It is an editorially independent program of the Henry J. Kaiser Family Foundation that is not affiliated with Kaiser Permanente.
Amid clouds of choking tear gas, booming flash-bang grenades and other “riot control agents,” volunteer medics plunged into street protests over the past weeks to help the injured – sometimes rushing to the front lines as soon as their hospital shifts ended.
Known as “street medics,” these unorthodox teams of nursing students, veterinarians, doctors, trauma surgeons, security guards, ski patrollers, nurses, wilderness EMTs, and off-the-clock ambulance workers poured water – not milk – into the eyes of tear-gassed protesters. They stanched bleeding wounds and plucked disoriented teenagers from clouds of gas, entering dangerous corners where on-duty emergency health responders may fear to go.
So donning cloth masks to protect against the virus – plus helmets, makeshift shields and other gear to guard against rubber bullets, projectiles and tear gas – the volunteer medics organized themselves into a web of first responders to care for people on the streets. They showed up early, set up first-aid stations, established transportation networks and covered their arms, helmets and backpacks with crosses made of red duct tape, to signify that they were medics. Some stayed late into the night past curfews until every protester had left.
Iris Butler, a 21-year-old certified nursing assistant who works in a nursing home, decided to offer her skills after seeing a man injured by a rubber bullet on her first night at the Denver protests. She showed up as a medic every night thereafter. She didn’t see it as a choice.
“I am working full time and basically being at the protest after getting straight off of work,” said Butler, who is black. That’s tiring, she added, but so is being a black woman in America.
After going out as a medic on her own, she soon met other volunteers. Together they used text-message chains to organize their efforts. One night, she responded to a man who had been shot with a rubber bullet in the chest; she said his torso had turned blue and purple from the impact. She also provided aid after a shooting near the protest left someone in critical condition.
“It’s hard, but bills need to be paid and justice needs to be served,” she said.
The street medic movement traces its roots, in part, to the 1960s protests, as well as the American Indian Movement and the Black Panther Party. Denver Action Medic Network offers a 20-hour training course that prepares them to treat patients in conflicts with police and large crowds; a four-hour session is offered to medical professionals as “bridge” training.
Since the coronavirus pandemic began, the Denver Action Medic Network has added new training guidelines: Don’t go to protests if sick or in contact with those who are infected; wear a mask; give people lots of space and use hand sanitizer. Jordan Garcia, a 39-year-old medic for over 20 years who works with the network of veteran street medics, said they also warn medics about the increased risk of transmission because of protesters coughing from tear gas, and urge them to get tested for the virus after the protests.
The number of volunteer medics swelled after George Floyd’s May 25 killing in Minneapolis. In Denver alone, at least 40 people reached out to the Denver Action Medic Network for training.
On June 3, Dr. Rupa Marya, an associate professor of medicine at the University of California,San Francisco, and the co-founder of the Do No Harm Coalition, which runs street medic training in the Bay Area, hosted a national webinar attended by over 3,000 medical professionals to provide the bridge training to be a street medic. In her online bio, Marya describes the coalition as “an organization of over 450 health workers committed to structural change” in addressing health problems.
“When we see suffering, that’s where we go,” Marya said. “And right now that suffering is happening on the streets.”
In the recent Denver protests, street medics responded to major head, face and eye injuries among protesters from what are sometimes described as “kinetic impact projectiles” or “less-than-lethal” bullets shot at protesters, along with tear-gas and flash-bang stun grenade canisters that either hit them or exploded in their faces.
Garcia, who by day works for an immigrant rights nonprofit, said that these weapons are not designed to be shot directly at people.
“We’re seeing police use these less-lethal weapons in lethal ways, and that is pretty upsetting,” Garcia said about the recent protests.
Denver police Chief Paul Pazen promised to make changes, including banning chokeholds and requiring SWAT teams to turn on their body cameras. Last week, a federal judge also issued a temporary injunction to stop Denver police from using tear gas and other less-than-lethal weapons in response to a class action lawsuit, in which a medic stated he was shot multiple times by police with pepper balls while treating patients. (Last week in North Carolina police were recorded destroying medic stations.)
Denver street medic Kevin Connell, a 30-year-old emergency room nurse, said he was hit with pepper balls in the back of his medic vest – which was clearly marked by red crosses – while treating a patient. He showed up to the Denver protests every night he did not have to work, he said, wearing a Kevlar medic vest, protective goggles and a homemade gas mask fashioned from a water bottle. As a member of the Denver Action Medic Network, Connell also served at the Standing Rock protests in North Dakota in a dispute over the building of the Dakota Access Pipeline.
“I mean, as bad as it sounds, it was only tear gas, pepper balls and rubber bullets that were being fired on us,” Connell said of his recent experience in Denver. “When I was at Standing Rock, they were using high-powered water hoses even when it was, like, freezing cold. … So I think the police here had a little bit more restraint.”
Still, first-time street medic Aj Mossman, a 31-year-old Denver emergency medical technician studying for nursing school, was shocked to be tear-gassed and struck in the back of the leg with a flash grenade while treating a protester on May 30. Mossman still has a large leg bruise.
The following night, Mossman, who uses the pronoun they, brought more protective gear, but said they are still having difficulty processing what felt like a war zone.
“I thought I understood what my black friends went through. I thought I understood what the black community went through,” said Mossman, who is white. “But I had absolutely no idea how violent the police were and how little they cared about who they hurt.”
For Butler, serving as a medic with others from various walks of life was inspiring. “They’re also out there to protect black and brown bodies. And that’s amazing,” she said. “That’s just a beautiful sight.”
This article originally appeared on Kaiser Health News, which is a nonprofit national health policy news service. It is an editorially independent program of the Henry J. Kaiser Family Foundation that is not affiliated with Kaiser Permanente.
Ethical considerations in nutrition support because of provider bias
Case:
A 37-year-old woman presents with severe emaciation (body mass index, 9.4 kg/m2) because of chronic severe avoidant/restrictive food intake disorder. She had asked for parenteral nutrition (PN) for several years, whenever her providers pushed her to accept nutrition support, as she had experienced extreme distress because of presumed gastroparesis with enteral feeds or any time she tried to eat. All of her many physicians refused the request for PN on the basis that her intestine was believed to be functioning and her symptoms were functional, so they insisted on tube feeding. The medical team was angered by the request for PN, and very concerned that providing it would support her belief that she could not eat, which they likened to a delusion. They opined that refusal of appropriate therapy (enteral nutrition) did not constitute an indication for inappropriate therapy (PN). They also deemed her to have capacity, so her refusal of tube feeding was honored. She continued to deteriorate, and because of her inability to travel, along with financial and insurance-related issues, was unable to seek alternative care providers. The family provided access to highly credible external consultants, and begged that her providers initiate PN as a life-saving measure. Both were declined. She was taken by her family to the emergency department when she began to have difficulty ambulating and increasing confusion. In recognition of the severity of her starvation, she was to be admitted to the critical care unit. With minimal monitoring while awaiting transfer from the emergency department overnight, she developed severe hypoglycemia and sustained cardiac arrest. Although spontaneous circulation was resumed, she sustained anoxic brain injury, and died after removal of life-sustaining treatment.
Ethical considerations
This case illustrates how the practice of caring for certain patients may come with deep unconscious determinants and conflicts of expectation – the duty to treat can be unclear in cases of refractory eating disorders. Multiple clinical teams were angry at the patient and her family for requesting PN and refused external input.
Although other eating disorders have received more attention, there is little research specific to avoidant/restrictive food intake disorder. There is some consensus that someone at a very low weight because of anorexia nervosa cannot, by definition, have decisional capacity with regard to feeding. Certainly, reviews cite cognitive dysfunction as a common finding, far worse during starvation, in patients with anorexia nervosa,1,2 and nourishment over objection has been advised.3 Further, it is known that gastric dysfunction occurs with some frequency in the presence of starvation in patients with eating disorders.4 Moreover, the potential risks of PN should be contextualized and compared with the certainty of death in someone this starved. Finally, if the patient’s refusal to eat or be tube fed were a delusion, which is by definition “fixed,” refusing to provide PN, and allowing further starvation, would not be expected to have benefit in resolution of the delusion.
Issues related to nourishment can be highly emotive – from “starving to death” on the one hand and “force feeding” on the other. Delivery of adequate nutrition and hydration is considered a basic human right, and must be offered as part of basic care. At the same time, we have observed that the request for nutrition support creates severe moral distress and anger among clinicians treating patients with eating disorders or with fatal illness. Does a delusion preclude feeding, even if by less than ideal means? How should a physician react to feeding treatments they deem excessive or unnecessary? Does a treating team have a duty to consider input from specialists with expertise specific to the patient when such conflict occurs between the patient/family and the treating team? Speculation exists that onset of anorexia nervosa may be linked to a postinfectious condition – a post–viral disease brain reprogramming.5,6 Would an organic explanation change our attitude toward patients with eating disorders?
Medicine’s emotive harms
Clinicians hold more negative attitudes toward certain patients – our implicit bias. It has been suggested that nice patients may be preferred by clinicians and therefore receive more humanistic care.7 Clinicians hold more negative attitudes toward patients with eating disorders than toward other patients. Cases of starvation caused by eating disorders are often seen by clinicians as a form of deviance, which provokes a visceral reaction of anger and frustration. These reactions have been associated with patients’ lack of improvement and personality pathology and with clinicians’ stigmatizing beliefs and inexperience.8 One could argue that this type of unconscious partiality may be worse than intentional harm.
Families and patients often request a treatment as a way to exert their agency. We clinicians may experience ethical dissonance as a result, whether because of ego or because the desired treatment is less favorable (for example, parenteral vs. enteral nutrition). Should maintaining clinical obstinance overrule patient and family autonomy, particularly in the face of the availability of life-saving intervention, even if less desirable than other standard treatments?
Should the physicians have better considered the relative risk of PN? What is the true potential harm? Would it benefit the patient or family? While PN’s benefit is usually life prolongation, it is not without risk of infection, potential mucosal atrophy of the unused gut, hepatic dysfunction, high cost, and an increased complexity of care. However, the incidence of blood stream infections in hospitalized patients receiving PN is only 1 episode for every 100 patient-days of treatment.9 On the other hand, weight regain is a significant determinant of success for treating eating disorders.10 Does the small risk of line-related sepsis, unlikely to be fatal, outweigh the certainty of death from starvation? What is the source of providers’ anger toward such patients? Even when providers feel any hope of improved outcome to be unreasonable, does refusal to provide nourishment, even if less than ideally, improve the likelihood the family will “come to grips” with the situation? Is there an obligation to consider our contribution to the emotional harm to the family because of our refusal, especially if coupled with anger?
Duty of life-saving care
Treating a competent patient without consent is unlawful. Autonomy is the dominant ethical principle, and a mentally competent person has the right to refuse consent to medical treatment for any reason, even when that decision may lead to death. Authors urge that patient lives should not be intentionally shortened, including the withholding of life-prolonging medical treatments or interventions.11,12 Although starvation can compromise capacity, whether patients with severe starvation have truly lost their mental competence and right to self-determination is debated.13 Do physicians have a duty to provide nutrition support by whatever route a patient will accept as a life-saving measure or at least until nutritional stability and improved mental status can be attained?
Next steps
Despite potential concerns clinicians may have over the risks and disadvantages of PN, reeducation of clinician emotional responses toward providing it is needed. As illustrated by this case study, there are likely situations, not fitting the norm, when PN is warranted as a life-saving measure. An awareness of implicit bias we may experience is paramount in all situations. Case-by-case multidisciplinary evaluations are warranted based on guidelines from professional organizations,14 alongside core ethical principles, when considering nutrition support.
References
1. Guillaume S et al. Psychol Med. 2015 Dec;45(16):3377-91.
2. Katzman DK et al. Semin Clin Neuropsychiatry. 2001 Apr;6(2):146-52.
3. Elzakkers IF et al. Int J Eat Disord. 2014 Dec;47(8):845-52.
4. Robinson PH et al. Gut. 1988 Apr;29(4):458-64.
5. Breithaupt L et al. JAMA Psychiatry. 2019 Apr 24;76(8):800-9.
6. Sokol MS. J Child Adolesc Psychopharmacol. 2000;10(2):133-45.
7. Detsky AS, Baerlocher MO. JAMA. 2011 Jul;306(1):94-5.
8. Thompson-Brenner H et al. Psychiatr Serv. 2012 Jan;63(1):73-8.
9. Fonseca G et al. JPEN J Parenter Enteral Nutr. 2018 Jan;42(1):171-5.
10. National Collaborating Centre for Mental Health. In: Eating disorders: Core interventions in the treatment and management of anorexia nervosa, bulimia nervosa and related eating disorders. Leicester, United Kingdom: British Psychological Society, 2004.
11. Keown J. Leg Stud. 2000 Mar;20(1):66-84.
12. Sayers GM et al. Postgrad Med J. 2006 Feb;82(964):79-83.
13. Miller I. BioSocieties. 2017;12:89-108.
14. A.S.P.E.N. Ethics Position Paper Task Force; Barrocas A et al. Nutr Clin Pract. 2010 Dec;25(6):672-9.
Dr. Anderson (@dochitect) is a clinical fellow in geriatric medicine at the University of California, San Francisco; Dr. Seres (@davidseres1) is an associate professor of medicine in the Institute of Human Nutrition, director of medical nutrition, and associate clinical ethicist at Columbia University Irving Medical Center, New York. They have no funding sources to declare and no conflicts of interest.
Case:
A 37-year-old woman presents with severe emaciation (body mass index, 9.4 kg/m2) because of chronic severe avoidant/restrictive food intake disorder. She had asked for parenteral nutrition (PN) for several years, whenever her providers pushed her to accept nutrition support, as she had experienced extreme distress because of presumed gastroparesis with enteral feeds or any time she tried to eat. All of her many physicians refused the request for PN on the basis that her intestine was believed to be functioning and her symptoms were functional, so they insisted on tube feeding. The medical team was angered by the request for PN, and very concerned that providing it would support her belief that she could not eat, which they likened to a delusion. They opined that refusal of appropriate therapy (enteral nutrition) did not constitute an indication for inappropriate therapy (PN). They also deemed her to have capacity, so her refusal of tube feeding was honored. She continued to deteriorate, and because of her inability to travel, along with financial and insurance-related issues, was unable to seek alternative care providers. The family provided access to highly credible external consultants, and begged that her providers initiate PN as a life-saving measure. Both were declined. She was taken by her family to the emergency department when she began to have difficulty ambulating and increasing confusion. In recognition of the severity of her starvation, she was to be admitted to the critical care unit. With minimal monitoring while awaiting transfer from the emergency department overnight, she developed severe hypoglycemia and sustained cardiac arrest. Although spontaneous circulation was resumed, she sustained anoxic brain injury, and died after removal of life-sustaining treatment.
Ethical considerations
This case illustrates how the practice of caring for certain patients may come with deep unconscious determinants and conflicts of expectation – the duty to treat can be unclear in cases of refractory eating disorders. Multiple clinical teams were angry at the patient and her family for requesting PN and refused external input.
Although other eating disorders have received more attention, there is little research specific to avoidant/restrictive food intake disorder. There is some consensus that someone at a very low weight because of anorexia nervosa cannot, by definition, have decisional capacity with regard to feeding. Certainly, reviews cite cognitive dysfunction as a common finding, far worse during starvation, in patients with anorexia nervosa,1,2 and nourishment over objection has been advised.3 Further, it is known that gastric dysfunction occurs with some frequency in the presence of starvation in patients with eating disorders.4 Moreover, the potential risks of PN should be contextualized and compared with the certainty of death in someone this starved. Finally, if the patient’s refusal to eat or be tube fed were a delusion, which is by definition “fixed,” refusing to provide PN, and allowing further starvation, would not be expected to have benefit in resolution of the delusion.
Issues related to nourishment can be highly emotive – from “starving to death” on the one hand and “force feeding” on the other. Delivery of adequate nutrition and hydration is considered a basic human right, and must be offered as part of basic care. At the same time, we have observed that the request for nutrition support creates severe moral distress and anger among clinicians treating patients with eating disorders or with fatal illness. Does a delusion preclude feeding, even if by less than ideal means? How should a physician react to feeding treatments they deem excessive or unnecessary? Does a treating team have a duty to consider input from specialists with expertise specific to the patient when such conflict occurs between the patient/family and the treating team? Speculation exists that onset of anorexia nervosa may be linked to a postinfectious condition – a post–viral disease brain reprogramming.5,6 Would an organic explanation change our attitude toward patients with eating disorders?
Medicine’s emotive harms
Clinicians hold more negative attitudes toward certain patients – our implicit bias. It has been suggested that nice patients may be preferred by clinicians and therefore receive more humanistic care.7 Clinicians hold more negative attitudes toward patients with eating disorders than toward other patients. Cases of starvation caused by eating disorders are often seen by clinicians as a form of deviance, which provokes a visceral reaction of anger and frustration. These reactions have been associated with patients’ lack of improvement and personality pathology and with clinicians’ stigmatizing beliefs and inexperience.8 One could argue that this type of unconscious partiality may be worse than intentional harm.
Families and patients often request a treatment as a way to exert their agency. We clinicians may experience ethical dissonance as a result, whether because of ego or because the desired treatment is less favorable (for example, parenteral vs. enteral nutrition). Should maintaining clinical obstinance overrule patient and family autonomy, particularly in the face of the availability of life-saving intervention, even if less desirable than other standard treatments?
Should the physicians have better considered the relative risk of PN? What is the true potential harm? Would it benefit the patient or family? While PN’s benefit is usually life prolongation, it is not without risk of infection, potential mucosal atrophy of the unused gut, hepatic dysfunction, high cost, and an increased complexity of care. However, the incidence of blood stream infections in hospitalized patients receiving PN is only 1 episode for every 100 patient-days of treatment.9 On the other hand, weight regain is a significant determinant of success for treating eating disorders.10 Does the small risk of line-related sepsis, unlikely to be fatal, outweigh the certainty of death from starvation? What is the source of providers’ anger toward such patients? Even when providers feel any hope of improved outcome to be unreasonable, does refusal to provide nourishment, even if less than ideally, improve the likelihood the family will “come to grips” with the situation? Is there an obligation to consider our contribution to the emotional harm to the family because of our refusal, especially if coupled with anger?
Duty of life-saving care
Treating a competent patient without consent is unlawful. Autonomy is the dominant ethical principle, and a mentally competent person has the right to refuse consent to medical treatment for any reason, even when that decision may lead to death. Authors urge that patient lives should not be intentionally shortened, including the withholding of life-prolonging medical treatments or interventions.11,12 Although starvation can compromise capacity, whether patients with severe starvation have truly lost their mental competence and right to self-determination is debated.13 Do physicians have a duty to provide nutrition support by whatever route a patient will accept as a life-saving measure or at least until nutritional stability and improved mental status can be attained?
Next steps
Despite potential concerns clinicians may have over the risks and disadvantages of PN, reeducation of clinician emotional responses toward providing it is needed. As illustrated by this case study, there are likely situations, not fitting the norm, when PN is warranted as a life-saving measure. An awareness of implicit bias we may experience is paramount in all situations. Case-by-case multidisciplinary evaluations are warranted based on guidelines from professional organizations,14 alongside core ethical principles, when considering nutrition support.
References
1. Guillaume S et al. Psychol Med. 2015 Dec;45(16):3377-91.
2. Katzman DK et al. Semin Clin Neuropsychiatry. 2001 Apr;6(2):146-52.
3. Elzakkers IF et al. Int J Eat Disord. 2014 Dec;47(8):845-52.
4. Robinson PH et al. Gut. 1988 Apr;29(4):458-64.
5. Breithaupt L et al. JAMA Psychiatry. 2019 Apr 24;76(8):800-9.
6. Sokol MS. J Child Adolesc Psychopharmacol. 2000;10(2):133-45.
7. Detsky AS, Baerlocher MO. JAMA. 2011 Jul;306(1):94-5.
8. Thompson-Brenner H et al. Psychiatr Serv. 2012 Jan;63(1):73-8.
9. Fonseca G et al. JPEN J Parenter Enteral Nutr. 2018 Jan;42(1):171-5.
10. National Collaborating Centre for Mental Health. In: Eating disorders: Core interventions in the treatment and management of anorexia nervosa, bulimia nervosa and related eating disorders. Leicester, United Kingdom: British Psychological Society, 2004.
11. Keown J. Leg Stud. 2000 Mar;20(1):66-84.
12. Sayers GM et al. Postgrad Med J. 2006 Feb;82(964):79-83.
13. Miller I. BioSocieties. 2017;12:89-108.
14. A.S.P.E.N. Ethics Position Paper Task Force; Barrocas A et al. Nutr Clin Pract. 2010 Dec;25(6):672-9.
Dr. Anderson (@dochitect) is a clinical fellow in geriatric medicine at the University of California, San Francisco; Dr. Seres (@davidseres1) is an associate professor of medicine in the Institute of Human Nutrition, director of medical nutrition, and associate clinical ethicist at Columbia University Irving Medical Center, New York. They have no funding sources to declare and no conflicts of interest.
Case:
A 37-year-old woman presents with severe emaciation (body mass index, 9.4 kg/m2) because of chronic severe avoidant/restrictive food intake disorder. She had asked for parenteral nutrition (PN) for several years, whenever her providers pushed her to accept nutrition support, as she had experienced extreme distress because of presumed gastroparesis with enteral feeds or any time she tried to eat. All of her many physicians refused the request for PN on the basis that her intestine was believed to be functioning and her symptoms were functional, so they insisted on tube feeding. The medical team was angered by the request for PN, and very concerned that providing it would support her belief that she could not eat, which they likened to a delusion. They opined that refusal of appropriate therapy (enteral nutrition) did not constitute an indication for inappropriate therapy (PN). They also deemed her to have capacity, so her refusal of tube feeding was honored. She continued to deteriorate, and because of her inability to travel, along with financial and insurance-related issues, was unable to seek alternative care providers. The family provided access to highly credible external consultants, and begged that her providers initiate PN as a life-saving measure. Both were declined. She was taken by her family to the emergency department when she began to have difficulty ambulating and increasing confusion. In recognition of the severity of her starvation, she was to be admitted to the critical care unit. With minimal monitoring while awaiting transfer from the emergency department overnight, she developed severe hypoglycemia and sustained cardiac arrest. Although spontaneous circulation was resumed, she sustained anoxic brain injury, and died after removal of life-sustaining treatment.
Ethical considerations
This case illustrates how the practice of caring for certain patients may come with deep unconscious determinants and conflicts of expectation – the duty to treat can be unclear in cases of refractory eating disorders. Multiple clinical teams were angry at the patient and her family for requesting PN and refused external input.
Although other eating disorders have received more attention, there is little research specific to avoidant/restrictive food intake disorder. There is some consensus that someone at a very low weight because of anorexia nervosa cannot, by definition, have decisional capacity with regard to feeding. Certainly, reviews cite cognitive dysfunction as a common finding, far worse during starvation, in patients with anorexia nervosa,1,2 and nourishment over objection has been advised.3 Further, it is known that gastric dysfunction occurs with some frequency in the presence of starvation in patients with eating disorders.4 Moreover, the potential risks of PN should be contextualized and compared with the certainty of death in someone this starved. Finally, if the patient’s refusal to eat or be tube fed were a delusion, which is by definition “fixed,” refusing to provide PN, and allowing further starvation, would not be expected to have benefit in resolution of the delusion.
Issues related to nourishment can be highly emotive – from “starving to death” on the one hand and “force feeding” on the other. Delivery of adequate nutrition and hydration is considered a basic human right, and must be offered as part of basic care. At the same time, we have observed that the request for nutrition support creates severe moral distress and anger among clinicians treating patients with eating disorders or with fatal illness. Does a delusion preclude feeding, even if by less than ideal means? How should a physician react to feeding treatments they deem excessive or unnecessary? Does a treating team have a duty to consider input from specialists with expertise specific to the patient when such conflict occurs between the patient/family and the treating team? Speculation exists that onset of anorexia nervosa may be linked to a postinfectious condition – a post–viral disease brain reprogramming.5,6 Would an organic explanation change our attitude toward patients with eating disorders?
Medicine’s emotive harms
Clinicians hold more negative attitudes toward certain patients – our implicit bias. It has been suggested that nice patients may be preferred by clinicians and therefore receive more humanistic care.7 Clinicians hold more negative attitudes toward patients with eating disorders than toward other patients. Cases of starvation caused by eating disorders are often seen by clinicians as a form of deviance, which provokes a visceral reaction of anger and frustration. These reactions have been associated with patients’ lack of improvement and personality pathology and with clinicians’ stigmatizing beliefs and inexperience.8 One could argue that this type of unconscious partiality may be worse than intentional harm.
Families and patients often request a treatment as a way to exert their agency. We clinicians may experience ethical dissonance as a result, whether because of ego or because the desired treatment is less favorable (for example, parenteral vs. enteral nutrition). Should maintaining clinical obstinance overrule patient and family autonomy, particularly in the face of the availability of life-saving intervention, even if less desirable than other standard treatments?
Should the physicians have better considered the relative risk of PN? What is the true potential harm? Would it benefit the patient or family? While PN’s benefit is usually life prolongation, it is not without risk of infection, potential mucosal atrophy of the unused gut, hepatic dysfunction, high cost, and an increased complexity of care. However, the incidence of blood stream infections in hospitalized patients receiving PN is only 1 episode for every 100 patient-days of treatment.9 On the other hand, weight regain is a significant determinant of success for treating eating disorders.10 Does the small risk of line-related sepsis, unlikely to be fatal, outweigh the certainty of death from starvation? What is the source of providers’ anger toward such patients? Even when providers feel any hope of improved outcome to be unreasonable, does refusal to provide nourishment, even if less than ideally, improve the likelihood the family will “come to grips” with the situation? Is there an obligation to consider our contribution to the emotional harm to the family because of our refusal, especially if coupled with anger?
Duty of life-saving care
Treating a competent patient without consent is unlawful. Autonomy is the dominant ethical principle, and a mentally competent person has the right to refuse consent to medical treatment for any reason, even when that decision may lead to death. Authors urge that patient lives should not be intentionally shortened, including the withholding of life-prolonging medical treatments or interventions.11,12 Although starvation can compromise capacity, whether patients with severe starvation have truly lost their mental competence and right to self-determination is debated.13 Do physicians have a duty to provide nutrition support by whatever route a patient will accept as a life-saving measure or at least until nutritional stability and improved mental status can be attained?
Next steps
Despite potential concerns clinicians may have over the risks and disadvantages of PN, reeducation of clinician emotional responses toward providing it is needed. As illustrated by this case study, there are likely situations, not fitting the norm, when PN is warranted as a life-saving measure. An awareness of implicit bias we may experience is paramount in all situations. Case-by-case multidisciplinary evaluations are warranted based on guidelines from professional organizations,14 alongside core ethical principles, when considering nutrition support.
References
1. Guillaume S et al. Psychol Med. 2015 Dec;45(16):3377-91.
2. Katzman DK et al. Semin Clin Neuropsychiatry. 2001 Apr;6(2):146-52.
3. Elzakkers IF et al. Int J Eat Disord. 2014 Dec;47(8):845-52.
4. Robinson PH et al. Gut. 1988 Apr;29(4):458-64.
5. Breithaupt L et al. JAMA Psychiatry. 2019 Apr 24;76(8):800-9.
6. Sokol MS. J Child Adolesc Psychopharmacol. 2000;10(2):133-45.
7. Detsky AS, Baerlocher MO. JAMA. 2011 Jul;306(1):94-5.
8. Thompson-Brenner H et al. Psychiatr Serv. 2012 Jan;63(1):73-8.
9. Fonseca G et al. JPEN J Parenter Enteral Nutr. 2018 Jan;42(1):171-5.
10. National Collaborating Centre for Mental Health. In: Eating disorders: Core interventions in the treatment and management of anorexia nervosa, bulimia nervosa and related eating disorders. Leicester, United Kingdom: British Psychological Society, 2004.
11. Keown J. Leg Stud. 2000 Mar;20(1):66-84.
12. Sayers GM et al. Postgrad Med J. 2006 Feb;82(964):79-83.
13. Miller I. BioSocieties. 2017;12:89-108.
14. A.S.P.E.N. Ethics Position Paper Task Force; Barrocas A et al. Nutr Clin Pract. 2010 Dec;25(6):672-9.
Dr. Anderson (@dochitect) is a clinical fellow in geriatric medicine at the University of California, San Francisco; Dr. Seres (@davidseres1) is an associate professor of medicine in the Institute of Human Nutrition, director of medical nutrition, and associate clinical ethicist at Columbia University Irving Medical Center, New York. They have no funding sources to declare and no conflicts of interest.