User login
For MD-IQ use only
Female cardiac advantage essentially lost after MI
Women are known to lag 5-10 years behind men in experiencing coronary heart disease (CHD), but new research suggests the gap narrows substantially following a myocardial infarction.
“Women lose a considerable portion, but not all, of their coronary and survival advantage – i.e., the lower event rates – after suffering a MI,” study author Sanne Peters, PhD, George Institute for Global Health, Imperial College London, said in an interview.
Previous studies of sex differences in event rates after a coronary event have produced mixed results and were primarily focused on mortality following MI. Importantly, the studies also lacked a control group without a history of CHD and, thus, were unable to provide a reference point for the disparity in event rates, she explained.
Using the MarketScan and Medicare databases, however, Dr. Peters and colleagues matched 339,890 U.S. adults hospitalized for an MI between January 2015 and December 2016 with 1,359,560 U.S. adults without a history of CHD.
Over a median 1.3 years follow-up, there were 12,518 MIs in the non-CHD group and 27,115 recurrent MIs in the MI group.
The age-standardized rate of MI per 1,000 person-years was 4.0 in women and 6.1 in men without a history of CHD, compared with 57.6 in women and 62.7 in men with a prior MI.
After multivariate adjustment, the women-to-men hazard ratio for MI was 0.64 (95% confidence interval, 0.62-0.67) in the non-CHD group and 0.94 (95% CI, 0.92-0.96) in the prior MI group, the authors reported Oct. 5 in the Journal of the American College of Cardiology
Additional results show the multivariate adjusted women-to-men hazard ratios for three other cardiovascular outcomes follow a similar pattern in the non-CHD and prior MI groups:
- CHD events: 0.53 (95% CI, 0.51-0.54) and 0.87 (95% CI, 0.85-0.89).
- Heart failure hospitalization: 0.93 (95% CI, 0.90-0.96) and 1.02 (95% CI, 1.00-1.04).
- All-cause mortality: 0.72 (95% CI, 0.71-0.73) and 0.90 (95% CI, 0.89-0.92).
“By including a control group of individuals without CHD, we demonstrated that the magnitude of the sex difference in cardiac event rates and survival is considerably smaller among those with prior MI than among those without a history of CHD,” Dr. Peters said.
Of note, the sex differences were consistent across age and race/ethnicity groups for all events, except for heart failure hospitalizations, where the adjusted hazard ratio for women vs. men age 80 years or older was 0.95 for those without a history of CHD (95% CI, 0.91-0.98) and 0.99 (95% CI, 0.96-1.02) for participants with a previous MI.
Dr. Peters said it’s not clear why the female advantage is attenuated post-MI but that one explanation is that women are less likely than men to receive guideline-recommended treatments and dosages or to adhere to prescribed therapies after MI hospitalization, which could put them at a higher risk of subsequent events and worse outcomes than men.
“Sex differences in pathophysiology of CHD and its complications may also explain, to some extent, why the rates of recurrent events are considerably more similar between the sexes than incident event rates,” she said. Compared with men, women have a higher incidence of MI with nonobstructive coronary artery disease and of heart failure with preserved ejection fraction, and evidence-based treatment options are more limited for both conditions.
“After people read this, I think the important thing to recognize is we need to push– as much as we can, with what meds we have, and what data we have – secondary prevention in these women,” Laxmi Mehta, MD, director of preventive cardiology and women’s cardiovascular health at Ohio State University, Columbus, said in an interview.
The lack of a female advantage post-MI should also elicit a “really meaningful conversation with our patients on shared decision-making of why they need to be on medications, remembering on our part to prescribe the medications, remembering to prescribe cardiac rehab, and also reminding our community we do need more data and need to investigate this further,” she said.
In an accompanying editorial, Nanette Wenger, MD, of Emory University, Atlanta, also points out that nonobstructive coronary disease is more common in women and, “yet, guideline-based therapies are those validated for obstructive coronary disease in a predominantly male population but, nonetheless, are applied for nonobstructive coronary disease.”
She advocates for aggressive evaluation and treatment for women with chest pain symptoms as well as early identification of women at risk for CHD, specifically those with metabolic syndrome, preeclampsia, hypertensive disorders of pregnancy, chronic inflammatory conditions, and high-risk race/ethnicity.
“Next, when coronary angiography is undertaken, particularly in younger women, an assiduous search for spontaneous coronary artery dissection and its appropriate management, as well as prompt and evidence-based interventions and medical therapies for an acute coronary event [are indicated],” Dr. Wenger wrote. “However, basic to improving outcomes for women is the elucidation of the optimal noninvasive techniques to identify microvascular disease, which could then enable delineation of appropriate preventive and therapeutic approaches.”
Dr. Peters is supported by a U.K. Medical Research Council Skills Development Fellowship. Dr. Mehta and Dr. Wenger disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
Women are known to lag 5-10 years behind men in experiencing coronary heart disease (CHD), but new research suggests the gap narrows substantially following a myocardial infarction.
“Women lose a considerable portion, but not all, of their coronary and survival advantage – i.e., the lower event rates – after suffering a MI,” study author Sanne Peters, PhD, George Institute for Global Health, Imperial College London, said in an interview.
Previous studies of sex differences in event rates after a coronary event have produced mixed results and were primarily focused on mortality following MI. Importantly, the studies also lacked a control group without a history of CHD and, thus, were unable to provide a reference point for the disparity in event rates, she explained.
Using the MarketScan and Medicare databases, however, Dr. Peters and colleagues matched 339,890 U.S. adults hospitalized for an MI between January 2015 and December 2016 with 1,359,560 U.S. adults without a history of CHD.
Over a median 1.3 years follow-up, there were 12,518 MIs in the non-CHD group and 27,115 recurrent MIs in the MI group.
The age-standardized rate of MI per 1,000 person-years was 4.0 in women and 6.1 in men without a history of CHD, compared with 57.6 in women and 62.7 in men with a prior MI.
After multivariate adjustment, the women-to-men hazard ratio for MI was 0.64 (95% confidence interval, 0.62-0.67) in the non-CHD group and 0.94 (95% CI, 0.92-0.96) in the prior MI group, the authors reported Oct. 5 in the Journal of the American College of Cardiology
Additional results show the multivariate adjusted women-to-men hazard ratios for three other cardiovascular outcomes follow a similar pattern in the non-CHD and prior MI groups:
- CHD events: 0.53 (95% CI, 0.51-0.54) and 0.87 (95% CI, 0.85-0.89).
- Heart failure hospitalization: 0.93 (95% CI, 0.90-0.96) and 1.02 (95% CI, 1.00-1.04).
- All-cause mortality: 0.72 (95% CI, 0.71-0.73) and 0.90 (95% CI, 0.89-0.92).
“By including a control group of individuals without CHD, we demonstrated that the magnitude of the sex difference in cardiac event rates and survival is considerably smaller among those with prior MI than among those without a history of CHD,” Dr. Peters said.
Of note, the sex differences were consistent across age and race/ethnicity groups for all events, except for heart failure hospitalizations, where the adjusted hazard ratio for women vs. men age 80 years or older was 0.95 for those without a history of CHD (95% CI, 0.91-0.98) and 0.99 (95% CI, 0.96-1.02) for participants with a previous MI.
Dr. Peters said it’s not clear why the female advantage is attenuated post-MI but that one explanation is that women are less likely than men to receive guideline-recommended treatments and dosages or to adhere to prescribed therapies after MI hospitalization, which could put them at a higher risk of subsequent events and worse outcomes than men.
“Sex differences in pathophysiology of CHD and its complications may also explain, to some extent, why the rates of recurrent events are considerably more similar between the sexes than incident event rates,” she said. Compared with men, women have a higher incidence of MI with nonobstructive coronary artery disease and of heart failure with preserved ejection fraction, and evidence-based treatment options are more limited for both conditions.
“After people read this, I think the important thing to recognize is we need to push– as much as we can, with what meds we have, and what data we have – secondary prevention in these women,” Laxmi Mehta, MD, director of preventive cardiology and women’s cardiovascular health at Ohio State University, Columbus, said in an interview.
The lack of a female advantage post-MI should also elicit a “really meaningful conversation with our patients on shared decision-making of why they need to be on medications, remembering on our part to prescribe the medications, remembering to prescribe cardiac rehab, and also reminding our community we do need more data and need to investigate this further,” she said.
In an accompanying editorial, Nanette Wenger, MD, of Emory University, Atlanta, also points out that nonobstructive coronary disease is more common in women and, “yet, guideline-based therapies are those validated for obstructive coronary disease in a predominantly male population but, nonetheless, are applied for nonobstructive coronary disease.”
She advocates for aggressive evaluation and treatment for women with chest pain symptoms as well as early identification of women at risk for CHD, specifically those with metabolic syndrome, preeclampsia, hypertensive disorders of pregnancy, chronic inflammatory conditions, and high-risk race/ethnicity.
“Next, when coronary angiography is undertaken, particularly in younger women, an assiduous search for spontaneous coronary artery dissection and its appropriate management, as well as prompt and evidence-based interventions and medical therapies for an acute coronary event [are indicated],” Dr. Wenger wrote. “However, basic to improving outcomes for women is the elucidation of the optimal noninvasive techniques to identify microvascular disease, which could then enable delineation of appropriate preventive and therapeutic approaches.”
Dr. Peters is supported by a U.K. Medical Research Council Skills Development Fellowship. Dr. Mehta and Dr. Wenger disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
Women are known to lag 5-10 years behind men in experiencing coronary heart disease (CHD), but new research suggests the gap narrows substantially following a myocardial infarction.
“Women lose a considerable portion, but not all, of their coronary and survival advantage – i.e., the lower event rates – after suffering a MI,” study author Sanne Peters, PhD, George Institute for Global Health, Imperial College London, said in an interview.
Previous studies of sex differences in event rates after a coronary event have produced mixed results and were primarily focused on mortality following MI. Importantly, the studies also lacked a control group without a history of CHD and, thus, were unable to provide a reference point for the disparity in event rates, she explained.
Using the MarketScan and Medicare databases, however, Dr. Peters and colleagues matched 339,890 U.S. adults hospitalized for an MI between January 2015 and December 2016 with 1,359,560 U.S. adults without a history of CHD.
Over a median 1.3 years follow-up, there were 12,518 MIs in the non-CHD group and 27,115 recurrent MIs in the MI group.
The age-standardized rate of MI per 1,000 person-years was 4.0 in women and 6.1 in men without a history of CHD, compared with 57.6 in women and 62.7 in men with a prior MI.
After multivariate adjustment, the women-to-men hazard ratio for MI was 0.64 (95% confidence interval, 0.62-0.67) in the non-CHD group and 0.94 (95% CI, 0.92-0.96) in the prior MI group, the authors reported Oct. 5 in the Journal of the American College of Cardiology
Additional results show the multivariate adjusted women-to-men hazard ratios for three other cardiovascular outcomes follow a similar pattern in the non-CHD and prior MI groups:
- CHD events: 0.53 (95% CI, 0.51-0.54) and 0.87 (95% CI, 0.85-0.89).
- Heart failure hospitalization: 0.93 (95% CI, 0.90-0.96) and 1.02 (95% CI, 1.00-1.04).
- All-cause mortality: 0.72 (95% CI, 0.71-0.73) and 0.90 (95% CI, 0.89-0.92).
“By including a control group of individuals without CHD, we demonstrated that the magnitude of the sex difference in cardiac event rates and survival is considerably smaller among those with prior MI than among those without a history of CHD,” Dr. Peters said.
Of note, the sex differences were consistent across age and race/ethnicity groups for all events, except for heart failure hospitalizations, where the adjusted hazard ratio for women vs. men age 80 years or older was 0.95 for those without a history of CHD (95% CI, 0.91-0.98) and 0.99 (95% CI, 0.96-1.02) for participants with a previous MI.
Dr. Peters said it’s not clear why the female advantage is attenuated post-MI but that one explanation is that women are less likely than men to receive guideline-recommended treatments and dosages or to adhere to prescribed therapies after MI hospitalization, which could put them at a higher risk of subsequent events and worse outcomes than men.
“Sex differences in pathophysiology of CHD and its complications may also explain, to some extent, why the rates of recurrent events are considerably more similar between the sexes than incident event rates,” she said. Compared with men, women have a higher incidence of MI with nonobstructive coronary artery disease and of heart failure with preserved ejection fraction, and evidence-based treatment options are more limited for both conditions.
“After people read this, I think the important thing to recognize is we need to push– as much as we can, with what meds we have, and what data we have – secondary prevention in these women,” Laxmi Mehta, MD, director of preventive cardiology and women’s cardiovascular health at Ohio State University, Columbus, said in an interview.
The lack of a female advantage post-MI should also elicit a “really meaningful conversation with our patients on shared decision-making of why they need to be on medications, remembering on our part to prescribe the medications, remembering to prescribe cardiac rehab, and also reminding our community we do need more data and need to investigate this further,” she said.
In an accompanying editorial, Nanette Wenger, MD, of Emory University, Atlanta, also points out that nonobstructive coronary disease is more common in women and, “yet, guideline-based therapies are those validated for obstructive coronary disease in a predominantly male population but, nonetheless, are applied for nonobstructive coronary disease.”
She advocates for aggressive evaluation and treatment for women with chest pain symptoms as well as early identification of women at risk for CHD, specifically those with metabolic syndrome, preeclampsia, hypertensive disorders of pregnancy, chronic inflammatory conditions, and high-risk race/ethnicity.
“Next, when coronary angiography is undertaken, particularly in younger women, an assiduous search for spontaneous coronary artery dissection and its appropriate management, as well as prompt and evidence-based interventions and medical therapies for an acute coronary event [are indicated],” Dr. Wenger wrote. “However, basic to improving outcomes for women is the elucidation of the optimal noninvasive techniques to identify microvascular disease, which could then enable delineation of appropriate preventive and therapeutic approaches.”
Dr. Peters is supported by a U.K. Medical Research Council Skills Development Fellowship. Dr. Mehta and Dr. Wenger disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
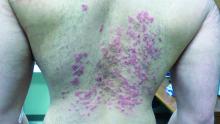
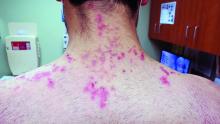
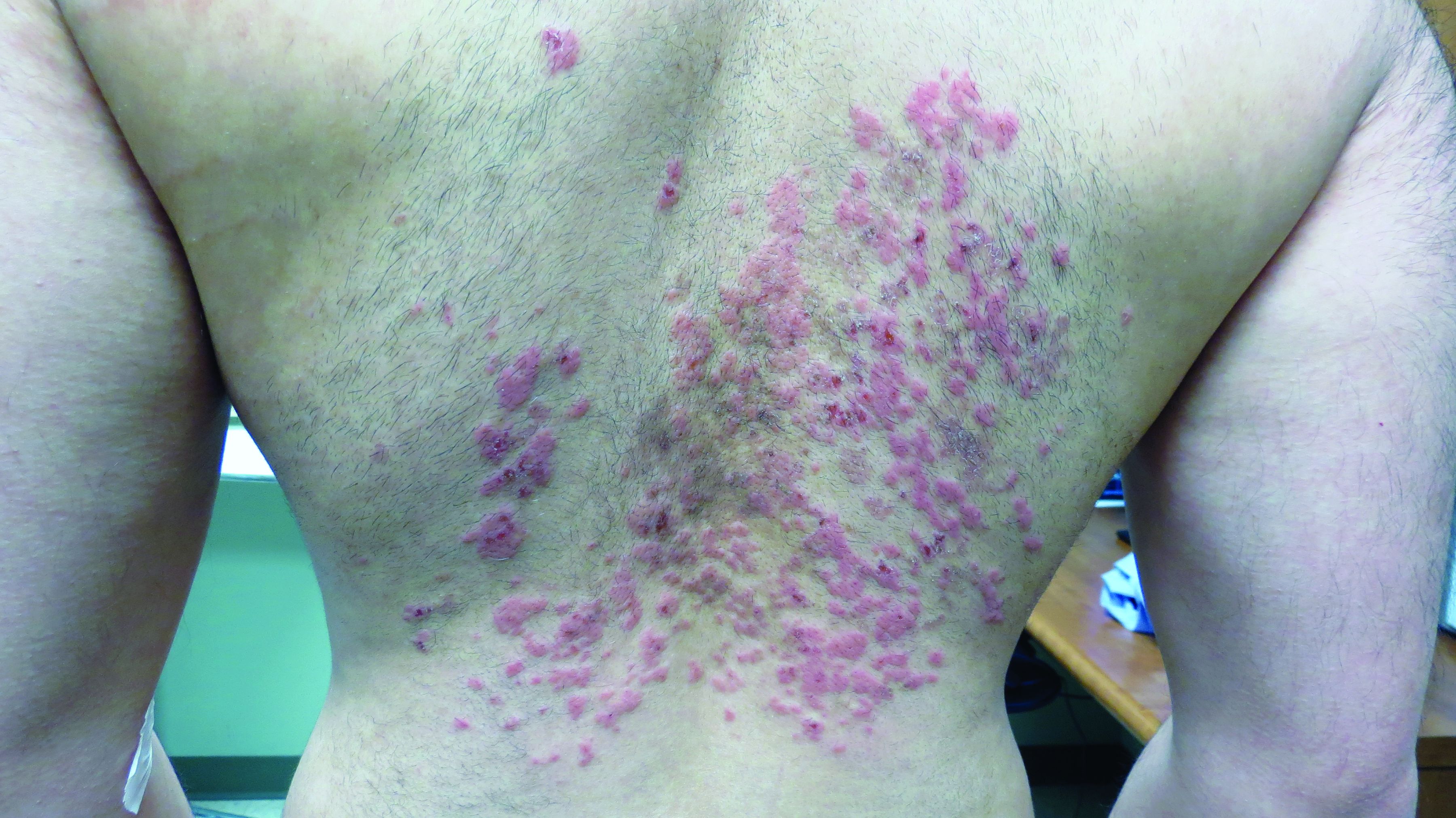
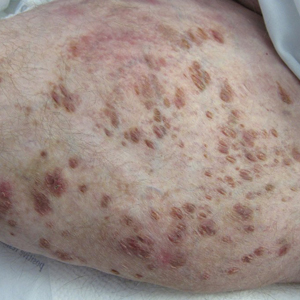
A 31-year-old with a 3-week history of a waxing and waning, mildly pruritic eruption on his neck, chest, and back
Prurigo pigmentosa is an inflammatory disorder of uncertain etiology characterized by the eruption of erythematous, markedly pruritic, urticaria-like papules and vesicles on the posterior neck, mid- to upper back, and chest. Crops of papules appear rapidly and then involute within days, leaving behind postinflammatory hyperpigmentation in a netlike configuration. New papules may appear prior to resolution of hyperpigmented macules, resulting in a mixed presentation of erythematous papules overlying reticulated hyperpigmentation.1
The condition was initially described in Japanese individuals, and to date, most cases have occurred in this population.2 However, the incidence of prurigo pigmentosa is increasing worldwide, including in the United States, which has led to the identification of several metabolic risk factors including diabetes mellitus, fasting, and dieting, with the common etiologic endpoint of ketosis.3With the increasing popularity of diets with strict carbohydrate limits, often with the goal of ketosis, dermatologists should be aware of the clinical appearance and common history of this rash to facilitate prompt diagnosis and treatment.
Clinical exam with appropriate history is usually sufficient for diagnosis. However, biopsy with histopathologic analysis can be utilized to confirm atypical cases. Histopathologic findings depend on the stage of the lesion biopsied. The earliest finding is a shallow perivascular neutrophilic infiltrate, neutrophil exocytosis, and epidermal and superficial dermal edema. As lesions progress, the prominent findings include epidermal vesiculation with necrotic keratinocytes and a lichenoid infiltrate dominated by lymphocytes and eosinophils. In the final stages, lesions demonstrate variable parakeratosis and acanthosis, as well as prominent dermal melanophagia.1
Treatment of prurigo pigmentosa includes modification of the patient’s underlying health issues to avoid ketosis, and in the case of diet-induced ketosis, reinstitution of a more balanced diet with sufficient carbohydrates. In the case of the patient presented here, rash resolved 1 week following instruction to include more carbohydrates in his diet. For recalcitrant cases or those without a clear precipitating factor, the addition of oral antibiotics is often helpful. Tetracyclines or dapsone are typically employed, usually in courses of 1-2 months.3,4
Dr. Johnson is a PGY-4 dermatology resident at Carilion Clinic in Roanoke, Va. He provided the case and photos. Donna Bilu Martin, MD, is the editor of the column.
References
1. Boer A et al. Am J Dermatopathol. 2003 Apr;25(2):117-292.
2. Satter E et al. J Cutan Pathol. 2016 Oct;43(10):809-14.
3. Alshaya M et al. JAAD Case Rep. 2019 Jun 8;5(6):504-7.
4. Hartman M et al. Cutis. 2019 Mar;103(3):E10-3.
Prurigo pigmentosa is an inflammatory disorder of uncertain etiology characterized by the eruption of erythematous, markedly pruritic, urticaria-like papules and vesicles on the posterior neck, mid- to upper back, and chest. Crops of papules appear rapidly and then involute within days, leaving behind postinflammatory hyperpigmentation in a netlike configuration. New papules may appear prior to resolution of hyperpigmented macules, resulting in a mixed presentation of erythematous papules overlying reticulated hyperpigmentation.1
The condition was initially described in Japanese individuals, and to date, most cases have occurred in this population.2 However, the incidence of prurigo pigmentosa is increasing worldwide, including in the United States, which has led to the identification of several metabolic risk factors including diabetes mellitus, fasting, and dieting, with the common etiologic endpoint of ketosis.3With the increasing popularity of diets with strict carbohydrate limits, often with the goal of ketosis, dermatologists should be aware of the clinical appearance and common history of this rash to facilitate prompt diagnosis and treatment.
Clinical exam with appropriate history is usually sufficient for diagnosis. However, biopsy with histopathologic analysis can be utilized to confirm atypical cases. Histopathologic findings depend on the stage of the lesion biopsied. The earliest finding is a shallow perivascular neutrophilic infiltrate, neutrophil exocytosis, and epidermal and superficial dermal edema. As lesions progress, the prominent findings include epidermal vesiculation with necrotic keratinocytes and a lichenoid infiltrate dominated by lymphocytes and eosinophils. In the final stages, lesions demonstrate variable parakeratosis and acanthosis, as well as prominent dermal melanophagia.1
Treatment of prurigo pigmentosa includes modification of the patient’s underlying health issues to avoid ketosis, and in the case of diet-induced ketosis, reinstitution of a more balanced diet with sufficient carbohydrates. In the case of the patient presented here, rash resolved 1 week following instruction to include more carbohydrates in his diet. For recalcitrant cases or those without a clear precipitating factor, the addition of oral antibiotics is often helpful. Tetracyclines or dapsone are typically employed, usually in courses of 1-2 months.3,4
Dr. Johnson is a PGY-4 dermatology resident at Carilion Clinic in Roanoke, Va. He provided the case and photos. Donna Bilu Martin, MD, is the editor of the column.
References
1. Boer A et al. Am J Dermatopathol. 2003 Apr;25(2):117-292.
2. Satter E et al. J Cutan Pathol. 2016 Oct;43(10):809-14.
3. Alshaya M et al. JAAD Case Rep. 2019 Jun 8;5(6):504-7.
4. Hartman M et al. Cutis. 2019 Mar;103(3):E10-3.
Prurigo pigmentosa is an inflammatory disorder of uncertain etiology characterized by the eruption of erythematous, markedly pruritic, urticaria-like papules and vesicles on the posterior neck, mid- to upper back, and chest. Crops of papules appear rapidly and then involute within days, leaving behind postinflammatory hyperpigmentation in a netlike configuration. New papules may appear prior to resolution of hyperpigmented macules, resulting in a mixed presentation of erythematous papules overlying reticulated hyperpigmentation.1
The condition was initially described in Japanese individuals, and to date, most cases have occurred in this population.2 However, the incidence of prurigo pigmentosa is increasing worldwide, including in the United States, which has led to the identification of several metabolic risk factors including diabetes mellitus, fasting, and dieting, with the common etiologic endpoint of ketosis.3With the increasing popularity of diets with strict carbohydrate limits, often with the goal of ketosis, dermatologists should be aware of the clinical appearance and common history of this rash to facilitate prompt diagnosis and treatment.
Clinical exam with appropriate history is usually sufficient for diagnosis. However, biopsy with histopathologic analysis can be utilized to confirm atypical cases. Histopathologic findings depend on the stage of the lesion biopsied. The earliest finding is a shallow perivascular neutrophilic infiltrate, neutrophil exocytosis, and epidermal and superficial dermal edema. As lesions progress, the prominent findings include epidermal vesiculation with necrotic keratinocytes and a lichenoid infiltrate dominated by lymphocytes and eosinophils. In the final stages, lesions demonstrate variable parakeratosis and acanthosis, as well as prominent dermal melanophagia.1
Treatment of prurigo pigmentosa includes modification of the patient’s underlying health issues to avoid ketosis, and in the case of diet-induced ketosis, reinstitution of a more balanced diet with sufficient carbohydrates. In the case of the patient presented here, rash resolved 1 week following instruction to include more carbohydrates in his diet. For recalcitrant cases or those without a clear precipitating factor, the addition of oral antibiotics is often helpful. Tetracyclines or dapsone are typically employed, usually in courses of 1-2 months.3,4
Dr. Johnson is a PGY-4 dermatology resident at Carilion Clinic in Roanoke, Va. He provided the case and photos. Donna Bilu Martin, MD, is the editor of the column.
References
1. Boer A et al. Am J Dermatopathol. 2003 Apr;25(2):117-292.
2. Satter E et al. J Cutan Pathol. 2016 Oct;43(10):809-14.
3. Alshaya M et al. JAAD Case Rep. 2019 Jun 8;5(6):504-7.
4. Hartman M et al. Cutis. 2019 Mar;103(3):E10-3.
AHA issues new scientific statement on heart health for LGBTQ adults
Cardiovascular health should be routinely assessed and addressed in LGBTQ adults, the American Heart Association concluded in a new scientific statement.
“Among the most important takeaways from this scientific statement is the need for health care providers in clinical settings to routinely assess sexual orientation and gender identity,” Billy A. Caceres, PhD, RN, chair of the statement writing group, said in an interview.
“This will help health care providers engage LGBTQ patients in discussions about their heart health that account for the unique experiences of this population,” said Dr. Caceres, assistant professor at Columbia University, New York.
The statement was published online Oct. 8 in Circulation.
‘Invisible’ population
There are roughly 11 million LGBTQ adults in the United States, yet they are often “invisible in health care settings and cardiovascular research,” Dr. Caceres noted. The AHA scientific statement is the first from a national organization in the United States to comprehensively summarize the evidence on cardiovascular (CV) research in LGBTQ adults.
There is mounting evidence that LGBTQ adults experience worse CV health relative to their cisgender heterosexual peers. Disparities in CV health may be driven by unique psychosocial stressors in the LGBTQ individuals such as family rejection and anxiety of concealment of their sexual orientation or gender identity.
While there is limited information on the CV health of LGBTQ people, the writing group said providers should be aware of the following:
- LGBTQ adults are more likely to use tobacco than their cisgender heterosexual peers.
- Transgender adults may be less physically active than their cisgender counterparts. Gender-affirming care might play a role in promoting physical activity among transgender people.
- Transgender women may be at increased risk for heart disease because of behavioral and clinical factors (such as the use of gender-affirming hormones like estrogen).
- Transgender women and nonbinary persons are more likely to binge drink.
- Lesbian and bisexual women have a higher prevalence of obesity than heterosexual women do.
“We need to better understand how to support LGBTQ adults in optimizing their CV health. To do this, we will need rigorous research that examines potential explanations for the CV health disparities that have been observed in LGBTQ adults,” Dr. Caceres said.
He noted that research is also needed within the LGBTQ population among groups that might be at greater risk for heart disease, including racial- and ethnic-minority and low-income LGBTQ adults.
“Researchers should also design and test evidence-based interventions to promote the heart health of LGBTQ adults. This is an area that is greatly lacking within CV health research,” said Dr. Caceres.
Discrimination in health care
Discrimination against LGBTQ adults in health care settings also remains a problem, the authors noted.
The writing group cites data showing that nearly 56% of sexual-minority and 70% of gender-minority adults report having experienced some form of discrimination from clinicians, including the use of harsh/abusive language.
“Perhaps most alarming,” roughly 8% of sexual-minority and 25% of transgender individuals have been denied health care by clinicians, they noted.
“LGBTQ individuals are delaying primary care and preventative visits because there is a great fear of being treated differently. Being treated differently often means receiving inadequate or inferior care because of sexual orientation or gender identity,” Dr. Caceres said in a news release.
The writing group calls for greater emphasis on LGBTQ health issues in the education of all health care providers. Dr. Caceres said it’s “paramount to include content about LGBTQ health in clinical training and licensure requirements in order to address these cardiovascular health disparities.”
Traditionally, there has been very little LGBTQ-related content in health care professional education training. A 2018 online survey of students at 10 medical schools found that approximately 80% of students did not feel competent to provide care for transgender patients.
But that may soon change. In September 2020, the Accreditation Review Commission on Education for the Physician Assistant began requiring LGBTQ curricular content, the writing group notes.
The AHA scientific statement on LGBTQ was developed by the writing group on behalf of the AHA Council on Cardiovascular and Stroke Nursing, the Council on Hypertension, the Council on Lifestyle and Cardiometabolic Health, the Council on Peripheral Vascular Disease, and the Stroke Council.
A version of this article originally appeared on Medscape.com.
Cardiovascular health should be routinely assessed and addressed in LGBTQ adults, the American Heart Association concluded in a new scientific statement.
“Among the most important takeaways from this scientific statement is the need for health care providers in clinical settings to routinely assess sexual orientation and gender identity,” Billy A. Caceres, PhD, RN, chair of the statement writing group, said in an interview.
“This will help health care providers engage LGBTQ patients in discussions about their heart health that account for the unique experiences of this population,” said Dr. Caceres, assistant professor at Columbia University, New York.
The statement was published online Oct. 8 in Circulation.
‘Invisible’ population
There are roughly 11 million LGBTQ adults in the United States, yet they are often “invisible in health care settings and cardiovascular research,” Dr. Caceres noted. The AHA scientific statement is the first from a national organization in the United States to comprehensively summarize the evidence on cardiovascular (CV) research in LGBTQ adults.
There is mounting evidence that LGBTQ adults experience worse CV health relative to their cisgender heterosexual peers. Disparities in CV health may be driven by unique psychosocial stressors in the LGBTQ individuals such as family rejection and anxiety of concealment of their sexual orientation or gender identity.
While there is limited information on the CV health of LGBTQ people, the writing group said providers should be aware of the following:
- LGBTQ adults are more likely to use tobacco than their cisgender heterosexual peers.
- Transgender adults may be less physically active than their cisgender counterparts. Gender-affirming care might play a role in promoting physical activity among transgender people.
- Transgender women may be at increased risk for heart disease because of behavioral and clinical factors (such as the use of gender-affirming hormones like estrogen).
- Transgender women and nonbinary persons are more likely to binge drink.
- Lesbian and bisexual women have a higher prevalence of obesity than heterosexual women do.
“We need to better understand how to support LGBTQ adults in optimizing their CV health. To do this, we will need rigorous research that examines potential explanations for the CV health disparities that have been observed in LGBTQ adults,” Dr. Caceres said.
He noted that research is also needed within the LGBTQ population among groups that might be at greater risk for heart disease, including racial- and ethnic-minority and low-income LGBTQ adults.
“Researchers should also design and test evidence-based interventions to promote the heart health of LGBTQ adults. This is an area that is greatly lacking within CV health research,” said Dr. Caceres.
Discrimination in health care
Discrimination against LGBTQ adults in health care settings also remains a problem, the authors noted.
The writing group cites data showing that nearly 56% of sexual-minority and 70% of gender-minority adults report having experienced some form of discrimination from clinicians, including the use of harsh/abusive language.
“Perhaps most alarming,” roughly 8% of sexual-minority and 25% of transgender individuals have been denied health care by clinicians, they noted.
“LGBTQ individuals are delaying primary care and preventative visits because there is a great fear of being treated differently. Being treated differently often means receiving inadequate or inferior care because of sexual orientation or gender identity,” Dr. Caceres said in a news release.
The writing group calls for greater emphasis on LGBTQ health issues in the education of all health care providers. Dr. Caceres said it’s “paramount to include content about LGBTQ health in clinical training and licensure requirements in order to address these cardiovascular health disparities.”
Traditionally, there has been very little LGBTQ-related content in health care professional education training. A 2018 online survey of students at 10 medical schools found that approximately 80% of students did not feel competent to provide care for transgender patients.
But that may soon change. In September 2020, the Accreditation Review Commission on Education for the Physician Assistant began requiring LGBTQ curricular content, the writing group notes.
The AHA scientific statement on LGBTQ was developed by the writing group on behalf of the AHA Council on Cardiovascular and Stroke Nursing, the Council on Hypertension, the Council on Lifestyle and Cardiometabolic Health, the Council on Peripheral Vascular Disease, and the Stroke Council.
A version of this article originally appeared on Medscape.com.
Cardiovascular health should be routinely assessed and addressed in LGBTQ adults, the American Heart Association concluded in a new scientific statement.
“Among the most important takeaways from this scientific statement is the need for health care providers in clinical settings to routinely assess sexual orientation and gender identity,” Billy A. Caceres, PhD, RN, chair of the statement writing group, said in an interview.
“This will help health care providers engage LGBTQ patients in discussions about their heart health that account for the unique experiences of this population,” said Dr. Caceres, assistant professor at Columbia University, New York.
The statement was published online Oct. 8 in Circulation.
‘Invisible’ population
There are roughly 11 million LGBTQ adults in the United States, yet they are often “invisible in health care settings and cardiovascular research,” Dr. Caceres noted. The AHA scientific statement is the first from a national organization in the United States to comprehensively summarize the evidence on cardiovascular (CV) research in LGBTQ adults.
There is mounting evidence that LGBTQ adults experience worse CV health relative to their cisgender heterosexual peers. Disparities in CV health may be driven by unique psychosocial stressors in the LGBTQ individuals such as family rejection and anxiety of concealment of their sexual orientation or gender identity.
While there is limited information on the CV health of LGBTQ people, the writing group said providers should be aware of the following:
- LGBTQ adults are more likely to use tobacco than their cisgender heterosexual peers.
- Transgender adults may be less physically active than their cisgender counterparts. Gender-affirming care might play a role in promoting physical activity among transgender people.
- Transgender women may be at increased risk for heart disease because of behavioral and clinical factors (such as the use of gender-affirming hormones like estrogen).
- Transgender women and nonbinary persons are more likely to binge drink.
- Lesbian and bisexual women have a higher prevalence of obesity than heterosexual women do.
“We need to better understand how to support LGBTQ adults in optimizing their CV health. To do this, we will need rigorous research that examines potential explanations for the CV health disparities that have been observed in LGBTQ adults,” Dr. Caceres said.
He noted that research is also needed within the LGBTQ population among groups that might be at greater risk for heart disease, including racial- and ethnic-minority and low-income LGBTQ adults.
“Researchers should also design and test evidence-based interventions to promote the heart health of LGBTQ adults. This is an area that is greatly lacking within CV health research,” said Dr. Caceres.
Discrimination in health care
Discrimination against LGBTQ adults in health care settings also remains a problem, the authors noted.
The writing group cites data showing that nearly 56% of sexual-minority and 70% of gender-minority adults report having experienced some form of discrimination from clinicians, including the use of harsh/abusive language.
“Perhaps most alarming,” roughly 8% of sexual-minority and 25% of transgender individuals have been denied health care by clinicians, they noted.
“LGBTQ individuals are delaying primary care and preventative visits because there is a great fear of being treated differently. Being treated differently often means receiving inadequate or inferior care because of sexual orientation or gender identity,” Dr. Caceres said in a news release.
The writing group calls for greater emphasis on LGBTQ health issues in the education of all health care providers. Dr. Caceres said it’s “paramount to include content about LGBTQ health in clinical training and licensure requirements in order to address these cardiovascular health disparities.”
Traditionally, there has been very little LGBTQ-related content in health care professional education training. A 2018 online survey of students at 10 medical schools found that approximately 80% of students did not feel competent to provide care for transgender patients.
But that may soon change. In September 2020, the Accreditation Review Commission on Education for the Physician Assistant began requiring LGBTQ curricular content, the writing group notes.
The AHA scientific statement on LGBTQ was developed by the writing group on behalf of the AHA Council on Cardiovascular and Stroke Nursing, the Council on Hypertension, the Council on Lifestyle and Cardiometabolic Health, the Council on Peripheral Vascular Disease, and the Stroke Council.
A version of this article originally appeared on Medscape.com.
Hospital medicine, it’s time to vote
Whether physicians or advanced practice practitioners, we are the backbone of our nation’s network of acute care facilities, and on a daily basis, we see just about everything. We have valuable insight into how to improve our nation’s health care system, especially now, as our nation continues to battle COVID-19.
Our role, squarely on the front lines during this pandemic, has given us an important perspective that needs to be heard. We spend our days managing patients with complexity, coordinating with specialists and subspecialists, and advocating – at local, state, and national levels – so that our patients can more easily transition to their lives out of the hospital.
Our current polarized political climate makes it seem that individual voices will not make a difference. It is easy to feel frustrated and powerless. However, those in our specialty are actually in a perfect position to have an educated and influential say in how we move forward, not only about the immediate health crises, but also regarding future health care issues. That voice begins with voting.
Historically, physicians have had surprisingly low rates of voting. For example, a 2007 study found significantly lower rates of voting among physicians, compared with the general public.1 While physician voter turnout may have improved in the past decade, given the substantial changes in health care and the increasing amount of physician engagement in the public sphere, our participation should be greater still. Elected officials listen to, and follow up with, constituents who make their voices heard. Each of us can ensure that the health care policy priorities of our fast-growing specialty are addressed by mobilizing to the voting booth.
Candidates we elect shape our health care system for the future, directly impacting us and our patients. Cost, coverage, access to health care, the Centers for Medicare & Medicaid Services inpatient fee schedules, the ongoing pandemic response, surprise billing, use of telehealth, observation status, and the three-midnight rule are just a few of the issues most important to hospital medicine.
Therefore, we, the SHM Public Policy Committee, urge all of our colleagues, regardless of political sway, to make your voice heard this and every election henceforth. The first step is to register to vote, if you have not done so already.2 Next, exercise that privilege. Given the pandemic, this is not as simple a process as it has been in the past. Take the time to plan your approach to early voting, mail-in voting, or election day voting. Check your County Supervisor of Elections’ website for further information, including how to register, view candidate profiles, check your precinct, and request a mail-in ballot.
In addition to casting your vote, we encourage you to share your opinions and engage in dialogue about health care issues. Clinical fact can dispel rumor and misinformation, and daily experiences can personalize our patients’ health care stories and the impact laws and rules have on our ability to practice. We are part of a trusted profession and have a unique perspective; others need and want to hear it. They can only do that if we are part of the process. Arming yourself with information and voting are the first steps on the path of advocacy. Interpersonal advocacy can also be done on social media. For example, SHM has an active grassroots advocacy network on Twitter. Tag @SHMadvocacy in your tweets to share your thoughts with their network.
Finally, as advocates for our patients in health care, we can also help ensure their safety during this election, in particular regarding COVID-19. Some patients may not wish to engage us in politics, and we must respect their decision. Others may seek our counsel and we should provide it in an unbiased fashion. We can ask our patients if they have considered a safe voting plan, help patients review the alternatives to voting in person if desired, and inform those who wish to physically cast a vote on Election Day of how to mitigate the risk of in-person voting.
Every election is important and health care is front and center for a multitude of reasons. We who practice hospital medicine are integral to our communities and need to be more politically involved. This is our chance to share our voice through our vote, not just this year, but in future elections as well.
Ann Sheehy, MD, SFHM, is division chief of the Division of Hospital Medicine at the University of Wisconsin, Madison, and chair of the SHM Public Policy Committee. Other members of the SHM PPC include Marta Almli, MD; John Biebelhausen, MD; Robert Burke, MD, MS, FHM; George Cheely, MD; Hyung (Harry) Cho, MD, SFHM; Jennifer Cowart, MD, FHM; Suparna Dutta, MD, MS, MPH; Bradley Flansbaum, DO, MPH, MHM; Alain Folefack, MD; Rick Hilger MD SFHM; Melinda Johnson, MD; Sevan Karadolian, MD; Joshua D. Lenchus, DO, FACP, SFHM; Steve Phillipson, MD; Dahlia Rizk, DO; Kendall Rogers, MD, SFHM; Brett Stauffer, MD, MHS; Amit Vashist, MD, SFHM; Robert Zipper, MD, SFHM.
References
1. Grande D et al. Do doctors vote? J Gen Int Med. 2007 May;22(5):585-9.
2. How to register to vote, confirm or change your registration and get a voter registration card. https://www.usa.gov/voter-registration/.
Whether physicians or advanced practice practitioners, we are the backbone of our nation’s network of acute care facilities, and on a daily basis, we see just about everything. We have valuable insight into how to improve our nation’s health care system, especially now, as our nation continues to battle COVID-19.
Our role, squarely on the front lines during this pandemic, has given us an important perspective that needs to be heard. We spend our days managing patients with complexity, coordinating with specialists and subspecialists, and advocating – at local, state, and national levels – so that our patients can more easily transition to their lives out of the hospital.
Our current polarized political climate makes it seem that individual voices will not make a difference. It is easy to feel frustrated and powerless. However, those in our specialty are actually in a perfect position to have an educated and influential say in how we move forward, not only about the immediate health crises, but also regarding future health care issues. That voice begins with voting.
Historically, physicians have had surprisingly low rates of voting. For example, a 2007 study found significantly lower rates of voting among physicians, compared with the general public.1 While physician voter turnout may have improved in the past decade, given the substantial changes in health care and the increasing amount of physician engagement in the public sphere, our participation should be greater still. Elected officials listen to, and follow up with, constituents who make their voices heard. Each of us can ensure that the health care policy priorities of our fast-growing specialty are addressed by mobilizing to the voting booth.
Candidates we elect shape our health care system for the future, directly impacting us and our patients. Cost, coverage, access to health care, the Centers for Medicare & Medicaid Services inpatient fee schedules, the ongoing pandemic response, surprise billing, use of telehealth, observation status, and the three-midnight rule are just a few of the issues most important to hospital medicine.
Therefore, we, the SHM Public Policy Committee, urge all of our colleagues, regardless of political sway, to make your voice heard this and every election henceforth. The first step is to register to vote, if you have not done so already.2 Next, exercise that privilege. Given the pandemic, this is not as simple a process as it has been in the past. Take the time to plan your approach to early voting, mail-in voting, or election day voting. Check your County Supervisor of Elections’ website for further information, including how to register, view candidate profiles, check your precinct, and request a mail-in ballot.
In addition to casting your vote, we encourage you to share your opinions and engage in dialogue about health care issues. Clinical fact can dispel rumor and misinformation, and daily experiences can personalize our patients’ health care stories and the impact laws and rules have on our ability to practice. We are part of a trusted profession and have a unique perspective; others need and want to hear it. They can only do that if we are part of the process. Arming yourself with information and voting are the first steps on the path of advocacy. Interpersonal advocacy can also be done on social media. For example, SHM has an active grassroots advocacy network on Twitter. Tag @SHMadvocacy in your tweets to share your thoughts with their network.
Finally, as advocates for our patients in health care, we can also help ensure their safety during this election, in particular regarding COVID-19. Some patients may not wish to engage us in politics, and we must respect their decision. Others may seek our counsel and we should provide it in an unbiased fashion. We can ask our patients if they have considered a safe voting plan, help patients review the alternatives to voting in person if desired, and inform those who wish to physically cast a vote on Election Day of how to mitigate the risk of in-person voting.
Every election is important and health care is front and center for a multitude of reasons. We who practice hospital medicine are integral to our communities and need to be more politically involved. This is our chance to share our voice through our vote, not just this year, but in future elections as well.
Ann Sheehy, MD, SFHM, is division chief of the Division of Hospital Medicine at the University of Wisconsin, Madison, and chair of the SHM Public Policy Committee. Other members of the SHM PPC include Marta Almli, MD; John Biebelhausen, MD; Robert Burke, MD, MS, FHM; George Cheely, MD; Hyung (Harry) Cho, MD, SFHM; Jennifer Cowart, MD, FHM; Suparna Dutta, MD, MS, MPH; Bradley Flansbaum, DO, MPH, MHM; Alain Folefack, MD; Rick Hilger MD SFHM; Melinda Johnson, MD; Sevan Karadolian, MD; Joshua D. Lenchus, DO, FACP, SFHM; Steve Phillipson, MD; Dahlia Rizk, DO; Kendall Rogers, MD, SFHM; Brett Stauffer, MD, MHS; Amit Vashist, MD, SFHM; Robert Zipper, MD, SFHM.
References
1. Grande D et al. Do doctors vote? J Gen Int Med. 2007 May;22(5):585-9.
2. How to register to vote, confirm or change your registration and get a voter registration card. https://www.usa.gov/voter-registration/.
Whether physicians or advanced practice practitioners, we are the backbone of our nation’s network of acute care facilities, and on a daily basis, we see just about everything. We have valuable insight into how to improve our nation’s health care system, especially now, as our nation continues to battle COVID-19.
Our role, squarely on the front lines during this pandemic, has given us an important perspective that needs to be heard. We spend our days managing patients with complexity, coordinating with specialists and subspecialists, and advocating – at local, state, and national levels – so that our patients can more easily transition to their lives out of the hospital.
Our current polarized political climate makes it seem that individual voices will not make a difference. It is easy to feel frustrated and powerless. However, those in our specialty are actually in a perfect position to have an educated and influential say in how we move forward, not only about the immediate health crises, but also regarding future health care issues. That voice begins with voting.
Historically, physicians have had surprisingly low rates of voting. For example, a 2007 study found significantly lower rates of voting among physicians, compared with the general public.1 While physician voter turnout may have improved in the past decade, given the substantial changes in health care and the increasing amount of physician engagement in the public sphere, our participation should be greater still. Elected officials listen to, and follow up with, constituents who make their voices heard. Each of us can ensure that the health care policy priorities of our fast-growing specialty are addressed by mobilizing to the voting booth.
Candidates we elect shape our health care system for the future, directly impacting us and our patients. Cost, coverage, access to health care, the Centers for Medicare & Medicaid Services inpatient fee schedules, the ongoing pandemic response, surprise billing, use of telehealth, observation status, and the three-midnight rule are just a few of the issues most important to hospital medicine.
Therefore, we, the SHM Public Policy Committee, urge all of our colleagues, regardless of political sway, to make your voice heard this and every election henceforth. The first step is to register to vote, if you have not done so already.2 Next, exercise that privilege. Given the pandemic, this is not as simple a process as it has been in the past. Take the time to plan your approach to early voting, mail-in voting, or election day voting. Check your County Supervisor of Elections’ website for further information, including how to register, view candidate profiles, check your precinct, and request a mail-in ballot.
In addition to casting your vote, we encourage you to share your opinions and engage in dialogue about health care issues. Clinical fact can dispel rumor and misinformation, and daily experiences can personalize our patients’ health care stories and the impact laws and rules have on our ability to practice. We are part of a trusted profession and have a unique perspective; others need and want to hear it. They can only do that if we are part of the process. Arming yourself with information and voting are the first steps on the path of advocacy. Interpersonal advocacy can also be done on social media. For example, SHM has an active grassroots advocacy network on Twitter. Tag @SHMadvocacy in your tweets to share your thoughts with their network.
Finally, as advocates for our patients in health care, we can also help ensure their safety during this election, in particular regarding COVID-19. Some patients may not wish to engage us in politics, and we must respect their decision. Others may seek our counsel and we should provide it in an unbiased fashion. We can ask our patients if they have considered a safe voting plan, help patients review the alternatives to voting in person if desired, and inform those who wish to physically cast a vote on Election Day of how to mitigate the risk of in-person voting.
Every election is important and health care is front and center for a multitude of reasons. We who practice hospital medicine are integral to our communities and need to be more politically involved. This is our chance to share our voice through our vote, not just this year, but in future elections as well.
Ann Sheehy, MD, SFHM, is division chief of the Division of Hospital Medicine at the University of Wisconsin, Madison, and chair of the SHM Public Policy Committee. Other members of the SHM PPC include Marta Almli, MD; John Biebelhausen, MD; Robert Burke, MD, MS, FHM; George Cheely, MD; Hyung (Harry) Cho, MD, SFHM; Jennifer Cowart, MD, FHM; Suparna Dutta, MD, MS, MPH; Bradley Flansbaum, DO, MPH, MHM; Alain Folefack, MD; Rick Hilger MD SFHM; Melinda Johnson, MD; Sevan Karadolian, MD; Joshua D. Lenchus, DO, FACP, SFHM; Steve Phillipson, MD; Dahlia Rizk, DO; Kendall Rogers, MD, SFHM; Brett Stauffer, MD, MHS; Amit Vashist, MD, SFHM; Robert Zipper, MD, SFHM.
References
1. Grande D et al. Do doctors vote? J Gen Int Med. 2007 May;22(5):585-9.
2. How to register to vote, confirm or change your registration and get a voter registration card. https://www.usa.gov/voter-registration/.
Review finds mortality rates low in young pregnant women with SJS, TEN
Investigators who but higher rates of C-sections.
The systematic review found that early diagnosis and withdrawal of the causative medications, such as antiretrovirals, were beneficial.
While SJS and TEN have been reported in pregnant women, “the outcomes and treatment of these cases are poorly characterized in the literature,” noted Ajay N. Sharma, a medical student at the University of California, Irvine, and coauthors, who published their findings in the International Journal of Women’s Dermatology.
“Immune changes that occur during pregnancy create a relative state of immunosuppression, likely increasing the risk of these skin reactions,” Mr. Sharma said in an interview. Allopurinol, antiepileptic drugs, antibacterial sulfonamides, nevirapine, and oxicam NSAIDs are agents most often associated with SJS/TEN.
He and his coauthors conducted a systematic literature review to analyze the risk factors, outcomes, and treatment of SJS and TEN in pregnant patients and their newborns using PubMed and Cochrane data from September 2019. The review included 26 articles covering 177 pregnant patients with SJS or TEN. Affected women were fairly young, averaging 29.9 years of age and more than 24 weeks along in their pregnancy when they experienced a reaction.
The majority of cases (81.9%) involved SJS diagnoses. Investigators identified antiretroviral therapy (90% of all cases), antibiotics (3%), and gestational drugs (2%) as the most common causative agents. “Multiple large cohort studies included in our review specifically assessed outcomes in only pregnant patients with HIV, resulting in an overall distribution of offending medications biased toward antiretroviral therapy,” noted Mr. Sharma. Nevirapine, a staple antiretroviral in developing countries (the site of most studies in the review), emerged as the biggest causal agent linked to 75 cases; 1 case was linked to the antiretroviral drug efavirenz.
Approximately 85% of pregnant women in this review had HIV. However, the young patient population studied had few comorbidities and low transmission rates to the fetus. In the 94 cases where outcomes data were available, 98% of the mothers and 96% of the newborns survived. Two pregnant patients in this cohort died, one from septic shock secondary to a TEN superinfection, and the other from intracranial hemorrhage secondary to metastatic melanoma. Of the 94 fetuses, 4 died: 2 of sepsis after birth, 1 in utero with its mother, and there was 1 stillbirth.
“Withdrawal of the offending drug was enacted in every recorded case of SJS or TEN during pregnancy. This single intervention was adequate in 159 patients; no additional therapy was needed in these cases aside from standard wound care, fluid and electrolyte repletion, and pain control,” wrote the investigators. Clinicians administered antibiotics, fluid resuscitation, steroids, and intravenous immunoglobulin in patients needing further assistance.
The investigators also reported high rates of C-section – almost 50% – in this group of pregnant women.
Inconsistent reporting between studies limited results, Mr. Sharma and colleagues noted. “Not every report specified body surface area involvement, treatment regimen, maternal or fetal outcome, or delivery method. Although additional studies in the form of large-scale, randomized, clinical trials are needed to better delineate treatment, this systematic review provides a framework for managing this population.”
The study authors reported no conflicts of interest and no funding for the study.
SOURCE: Sharma AN et al. Int J Womens Dermatol. 2020 Apr 13;6(4):239-47.
Investigators who but higher rates of C-sections.
The systematic review found that early diagnosis and withdrawal of the causative medications, such as antiretrovirals, were beneficial.
While SJS and TEN have been reported in pregnant women, “the outcomes and treatment of these cases are poorly characterized in the literature,” noted Ajay N. Sharma, a medical student at the University of California, Irvine, and coauthors, who published their findings in the International Journal of Women’s Dermatology.
“Immune changes that occur during pregnancy create a relative state of immunosuppression, likely increasing the risk of these skin reactions,” Mr. Sharma said in an interview. Allopurinol, antiepileptic drugs, antibacterial sulfonamides, nevirapine, and oxicam NSAIDs are agents most often associated with SJS/TEN.
He and his coauthors conducted a systematic literature review to analyze the risk factors, outcomes, and treatment of SJS and TEN in pregnant patients and their newborns using PubMed and Cochrane data from September 2019. The review included 26 articles covering 177 pregnant patients with SJS or TEN. Affected women were fairly young, averaging 29.9 years of age and more than 24 weeks along in their pregnancy when they experienced a reaction.
The majority of cases (81.9%) involved SJS diagnoses. Investigators identified antiretroviral therapy (90% of all cases), antibiotics (3%), and gestational drugs (2%) as the most common causative agents. “Multiple large cohort studies included in our review specifically assessed outcomes in only pregnant patients with HIV, resulting in an overall distribution of offending medications biased toward antiretroviral therapy,” noted Mr. Sharma. Nevirapine, a staple antiretroviral in developing countries (the site of most studies in the review), emerged as the biggest causal agent linked to 75 cases; 1 case was linked to the antiretroviral drug efavirenz.
Approximately 85% of pregnant women in this review had HIV. However, the young patient population studied had few comorbidities and low transmission rates to the fetus. In the 94 cases where outcomes data were available, 98% of the mothers and 96% of the newborns survived. Two pregnant patients in this cohort died, one from septic shock secondary to a TEN superinfection, and the other from intracranial hemorrhage secondary to metastatic melanoma. Of the 94 fetuses, 4 died: 2 of sepsis after birth, 1 in utero with its mother, and there was 1 stillbirth.
“Withdrawal of the offending drug was enacted in every recorded case of SJS or TEN during pregnancy. This single intervention was adequate in 159 patients; no additional therapy was needed in these cases aside from standard wound care, fluid and electrolyte repletion, and pain control,” wrote the investigators. Clinicians administered antibiotics, fluid resuscitation, steroids, and intravenous immunoglobulin in patients needing further assistance.
The investigators also reported high rates of C-section – almost 50% – in this group of pregnant women.
Inconsistent reporting between studies limited results, Mr. Sharma and colleagues noted. “Not every report specified body surface area involvement, treatment regimen, maternal or fetal outcome, or delivery method. Although additional studies in the form of large-scale, randomized, clinical trials are needed to better delineate treatment, this systematic review provides a framework for managing this population.”
The study authors reported no conflicts of interest and no funding for the study.
SOURCE: Sharma AN et al. Int J Womens Dermatol. 2020 Apr 13;6(4):239-47.
Investigators who but higher rates of C-sections.
The systematic review found that early diagnosis and withdrawal of the causative medications, such as antiretrovirals, were beneficial.
While SJS and TEN have been reported in pregnant women, “the outcomes and treatment of these cases are poorly characterized in the literature,” noted Ajay N. Sharma, a medical student at the University of California, Irvine, and coauthors, who published their findings in the International Journal of Women’s Dermatology.
“Immune changes that occur during pregnancy create a relative state of immunosuppression, likely increasing the risk of these skin reactions,” Mr. Sharma said in an interview. Allopurinol, antiepileptic drugs, antibacterial sulfonamides, nevirapine, and oxicam NSAIDs are agents most often associated with SJS/TEN.
He and his coauthors conducted a systematic literature review to analyze the risk factors, outcomes, and treatment of SJS and TEN in pregnant patients and their newborns using PubMed and Cochrane data from September 2019. The review included 26 articles covering 177 pregnant patients with SJS or TEN. Affected women were fairly young, averaging 29.9 years of age and more than 24 weeks along in their pregnancy when they experienced a reaction.
The majority of cases (81.9%) involved SJS diagnoses. Investigators identified antiretroviral therapy (90% of all cases), antibiotics (3%), and gestational drugs (2%) as the most common causative agents. “Multiple large cohort studies included in our review specifically assessed outcomes in only pregnant patients with HIV, resulting in an overall distribution of offending medications biased toward antiretroviral therapy,” noted Mr. Sharma. Nevirapine, a staple antiretroviral in developing countries (the site of most studies in the review), emerged as the biggest causal agent linked to 75 cases; 1 case was linked to the antiretroviral drug efavirenz.
Approximately 85% of pregnant women in this review had HIV. However, the young patient population studied had few comorbidities and low transmission rates to the fetus. In the 94 cases where outcomes data were available, 98% of the mothers and 96% of the newborns survived. Two pregnant patients in this cohort died, one from septic shock secondary to a TEN superinfection, and the other from intracranial hemorrhage secondary to metastatic melanoma. Of the 94 fetuses, 4 died: 2 of sepsis after birth, 1 in utero with its mother, and there was 1 stillbirth.
“Withdrawal of the offending drug was enacted in every recorded case of SJS or TEN during pregnancy. This single intervention was adequate in 159 patients; no additional therapy was needed in these cases aside from standard wound care, fluid and electrolyte repletion, and pain control,” wrote the investigators. Clinicians administered antibiotics, fluid resuscitation, steroids, and intravenous immunoglobulin in patients needing further assistance.
The investigators also reported high rates of C-section – almost 50% – in this group of pregnant women.
Inconsistent reporting between studies limited results, Mr. Sharma and colleagues noted. “Not every report specified body surface area involvement, treatment regimen, maternal or fetal outcome, or delivery method. Although additional studies in the form of large-scale, randomized, clinical trials are needed to better delineate treatment, this systematic review provides a framework for managing this population.”
The study authors reported no conflicts of interest and no funding for the study.
SOURCE: Sharma AN et al. Int J Womens Dermatol. 2020 Apr 13;6(4):239-47.
FROM THE INTERNATIONAL JOURNAL OF WOMEN’S DERMATOLOGY
2020 has been quite a year
I remember New Year’s Day 2020, full of hope and wonderment of what the year would bring. I was coming into the Society of Hospital Medicine as the incoming President, taking the 2020 reins in the organization’s 20th year. It would be a year of transitioning to a new CEO, reinvigorating our membership engagement efforts, and renewing a strategic plan for forward progress into the next decade. It would be a year chock full of travel, speaking engagements, and meetings with thousands of hospitalists around the globe.
What I didn’t know is that we would soon face the grim reality that the long-voiced concern of infectious disease experts and epidemiologists would come true. That our colleagues and friends and families would be infected, hospitalized, and die from this new disease, for which there were no good, effective treatments. That our ability to come together as a nation to implement basic infection control and epidemiologic practices would be fractured, uncoordinated, and ineffective. That within 6 months of the first case on U.S. soil, we would witness 5,270,000 people being infected from the disease, and 167,000 dying from it. And that the stunning toll of the disease would ripple into every nook and cranny of our society, from the economy to the fabric of our families and to the mental and physical health of all of our citizens.
However, what I couldn’t have known on this past New Year’s Day is how incredibly resilient and innovative our hospital medicine society and community would be to not only endure this new way of working and living, but also to find ways to improve upon how we care for all patients, despite COVID-19. What I couldn’t have known is how hospitalists would pivot to new arenas of care settings, including the EDs, ICUs, “COVID units,” and telehealth – flawlessly and seamlessly filling care gaps that would otherwise be catastrophically unfilled.
What I couldn’t have known is how we would be willing to come back into work, day after day, to care for our patients, despite the risks to ourselves and our families. What I couldn’t have known is how hospitalists would come together as a community to network and share knowledge in unprecedented ways, both humbly and proactively – knowing that we would not have all the answers but that we probably had better answers than most. What I couldn’t have known is that the SHM staff would pivot our entire SHM team away from previous “staple” offerings (e.g., live meetings) to virtual learning and network opportunities, which would be attended at rates higher than ever seen before, including live webinars, HMX exchanges, and e-learnings. What I couldn’t have known is that we would figure out, in a matter of weeks, what treatments were and were not effective for our patients and get those treatments to them despite the difficulties. And what I couldn’t have known is how much prouder I would be, more than ever before, to tell people: “I am a hospitalist.”
I took my son to the dentist recently, and when we were just about to leave, the dentist asked: “What do you do for a living?” and I stated: “I am a hospitalist.” He slowly breathed in and replied: “Oh … wow … you have really seen things …” Yes, we have.
So, is 2020 shaping up as expected? Absolutely not! But I am more inspired, humbled, and motivated than ever to proudly serve SHM with more energy and enthusiasm than I would have dreamed on New Year’s Day. And even if we can’t see each other in person (as we so naively planned), through virtual meetings (national, regional, and chapter), webinars, social media, and other listening modes, we will still be able to connect as a community and share ideas and issues as we muddle through the remainder of 2020 and beyond. We need each other more than ever before, and I am so proud to be a part of this SHM family.
Dr. Scheurer is chief quality officer and professor of medicine at the Medical University of South Carolina, Charleston. She is president of SHM.
I remember New Year’s Day 2020, full of hope and wonderment of what the year would bring. I was coming into the Society of Hospital Medicine as the incoming President, taking the 2020 reins in the organization’s 20th year. It would be a year of transitioning to a new CEO, reinvigorating our membership engagement efforts, and renewing a strategic plan for forward progress into the next decade. It would be a year chock full of travel, speaking engagements, and meetings with thousands of hospitalists around the globe.
What I didn’t know is that we would soon face the grim reality that the long-voiced concern of infectious disease experts and epidemiologists would come true. That our colleagues and friends and families would be infected, hospitalized, and die from this new disease, for which there were no good, effective treatments. That our ability to come together as a nation to implement basic infection control and epidemiologic practices would be fractured, uncoordinated, and ineffective. That within 6 months of the first case on U.S. soil, we would witness 5,270,000 people being infected from the disease, and 167,000 dying from it. And that the stunning toll of the disease would ripple into every nook and cranny of our society, from the economy to the fabric of our families and to the mental and physical health of all of our citizens.
However, what I couldn’t have known on this past New Year’s Day is how incredibly resilient and innovative our hospital medicine society and community would be to not only endure this new way of working and living, but also to find ways to improve upon how we care for all patients, despite COVID-19. What I couldn’t have known is how hospitalists would pivot to new arenas of care settings, including the EDs, ICUs, “COVID units,” and telehealth – flawlessly and seamlessly filling care gaps that would otherwise be catastrophically unfilled.
What I couldn’t have known is how we would be willing to come back into work, day after day, to care for our patients, despite the risks to ourselves and our families. What I couldn’t have known is how hospitalists would come together as a community to network and share knowledge in unprecedented ways, both humbly and proactively – knowing that we would not have all the answers but that we probably had better answers than most. What I couldn’t have known is that the SHM staff would pivot our entire SHM team away from previous “staple” offerings (e.g., live meetings) to virtual learning and network opportunities, which would be attended at rates higher than ever seen before, including live webinars, HMX exchanges, and e-learnings. What I couldn’t have known is that we would figure out, in a matter of weeks, what treatments were and were not effective for our patients and get those treatments to them despite the difficulties. And what I couldn’t have known is how much prouder I would be, more than ever before, to tell people: “I am a hospitalist.”
I took my son to the dentist recently, and when we were just about to leave, the dentist asked: “What do you do for a living?” and I stated: “I am a hospitalist.” He slowly breathed in and replied: “Oh … wow … you have really seen things …” Yes, we have.
So, is 2020 shaping up as expected? Absolutely not! But I am more inspired, humbled, and motivated than ever to proudly serve SHM with more energy and enthusiasm than I would have dreamed on New Year’s Day. And even if we can’t see each other in person (as we so naively planned), through virtual meetings (national, regional, and chapter), webinars, social media, and other listening modes, we will still be able to connect as a community and share ideas and issues as we muddle through the remainder of 2020 and beyond. We need each other more than ever before, and I am so proud to be a part of this SHM family.
Dr. Scheurer is chief quality officer and professor of medicine at the Medical University of South Carolina, Charleston. She is president of SHM.
I remember New Year’s Day 2020, full of hope and wonderment of what the year would bring. I was coming into the Society of Hospital Medicine as the incoming President, taking the 2020 reins in the organization’s 20th year. It would be a year of transitioning to a new CEO, reinvigorating our membership engagement efforts, and renewing a strategic plan for forward progress into the next decade. It would be a year chock full of travel, speaking engagements, and meetings with thousands of hospitalists around the globe.
What I didn’t know is that we would soon face the grim reality that the long-voiced concern of infectious disease experts and epidemiologists would come true. That our colleagues and friends and families would be infected, hospitalized, and die from this new disease, for which there were no good, effective treatments. That our ability to come together as a nation to implement basic infection control and epidemiologic practices would be fractured, uncoordinated, and ineffective. That within 6 months of the first case on U.S. soil, we would witness 5,270,000 people being infected from the disease, and 167,000 dying from it. And that the stunning toll of the disease would ripple into every nook and cranny of our society, from the economy to the fabric of our families and to the mental and physical health of all of our citizens.
However, what I couldn’t have known on this past New Year’s Day is how incredibly resilient and innovative our hospital medicine society and community would be to not only endure this new way of working and living, but also to find ways to improve upon how we care for all patients, despite COVID-19. What I couldn’t have known is how hospitalists would pivot to new arenas of care settings, including the EDs, ICUs, “COVID units,” and telehealth – flawlessly and seamlessly filling care gaps that would otherwise be catastrophically unfilled.
What I couldn’t have known is how we would be willing to come back into work, day after day, to care for our patients, despite the risks to ourselves and our families. What I couldn’t have known is how hospitalists would come together as a community to network and share knowledge in unprecedented ways, both humbly and proactively – knowing that we would not have all the answers but that we probably had better answers than most. What I couldn’t have known is that the SHM staff would pivot our entire SHM team away from previous “staple” offerings (e.g., live meetings) to virtual learning and network opportunities, which would be attended at rates higher than ever seen before, including live webinars, HMX exchanges, and e-learnings. What I couldn’t have known is that we would figure out, in a matter of weeks, what treatments were and were not effective for our patients and get those treatments to them despite the difficulties. And what I couldn’t have known is how much prouder I would be, more than ever before, to tell people: “I am a hospitalist.”
I took my son to the dentist recently, and when we were just about to leave, the dentist asked: “What do you do for a living?” and I stated: “I am a hospitalist.” He slowly breathed in and replied: “Oh … wow … you have really seen things …” Yes, we have.
So, is 2020 shaping up as expected? Absolutely not! But I am more inspired, humbled, and motivated than ever to proudly serve SHM with more energy and enthusiasm than I would have dreamed on New Year’s Day. And even if we can’t see each other in person (as we so naively planned), through virtual meetings (national, regional, and chapter), webinars, social media, and other listening modes, we will still be able to connect as a community and share ideas and issues as we muddle through the remainder of 2020 and beyond. We need each other more than ever before, and I am so proud to be a part of this SHM family.
Dr. Scheurer is chief quality officer and professor of medicine at the Medical University of South Carolina, Charleston. She is president of SHM.
Psychosocial resilience associated with better cardiovascular health in Blacks
Resilience might deserve targeting
Increased psychosocial resilience, which captures a sense of purpose, optimism, and life-coping strategies, correlates with improved cardiovascular (CV) health in Black Americans, according to a study that might hold a key for identifying new strategies for CV disease prevention.
“Our findings highlight the importance of individual psychosocial factors that promote cardiovascular health among Black adults, traditionally considered to be a high-risk population,” according to a team of authors collaborating on a study produced by the Morehouse-Emory Cardiovascular Center for Health Equity in Atlanta.
Studies associating psychosocial resilience with improved health outcomes have been published before. In a 12-study review of this concept, it was emphasized that resilience is a dynamic process, not a personality trait, and has shown promise as a target of efforts to relieve the burden of disease (Johnston MC et al. Psychosomatics 2015;56:168-80).
In this study, which received partial support from the American Heart Association, psychosocial resilience was evaluated at both the individual level and at the community level among 389 Black adults living in Atlanta. The senior author was Tené T. Lewis, PhD, of the department of epidemiology at Emory’s Rollins School of Public Health (Circ Cardiovasc Qual Outcomes 2020 Oct 7;13:3006638).
Psychosocial resilience was calculated across the domains of environmental mastery, purpose of life, optimism, coping, and lack of depression with standardized tests, such as the Life Orientation Test-Revised questionnaire for optimism and the Ryff Scales of Psychological Well-Being for the domains of environmental mastery and purpose of life. A composite score for psychosocial resilience was reached by calculating the median score across the measured domains.
Patients with high psychosocial resilience, defined as a composite score above the median, or low resilience, defined as a lower score, were then compared for CV health based on the AHA’s Life’s Simple 7 (LS7) score.
LS7 scores incorporate measures for exercise, diet, smoking history, blood pressure, glucose, cholesterol, and body mass index. Composite LS7 scores range from 0 to 14. Prior work cited by the authors have associated each 1-unit increase in LS7 score with a 13% lower risk of CVD.
As a continuous variable for CV risk at the individual level, each higher standard-deviation increment in the composite psychosocial resilience score was associated with a highly significant 0.42-point increase in LS7 score (P < .001) for study participants. In other words, increasing resilience predicted lower CV risk scores.
Resilience was also calculated at the community level by looking at census tract-level rates of CV mortality and morbidity relative to socioeconomic status. Again, high CV resilience, defined as scores above the median, were compared with lower scores across neighborhoods with similar median household income. As a continuous variable in this analysis, each higher standard-deviation increment in the resilience score was associated with a 0.27-point increase in LS7 score (P = .01).
After adjustment for sociodemographic factors, the association between psychosocial resilience and CV health remained significant for both the individual and community calculations, according to the authors. When examined jointly, high individual psychosocial resilience remained independently associated with improved CV health, but living in a high-resilience neighborhood was not an independent predictor.
When evaluated individually, each of the domains in the psychosocial resistance score were positively correlated with higher LS7 scores, meaning lower CV risk. The strongest associations on a statistical level were low depressive symptoms (P = .001), environmental mastery (P = .006), and purpose in life (P = .009).
The impact of high psychosocial resistance scores was greatest in Black adults living in low-resilience neighborhoods. Among these subjects, high resilience was associated with a nearly 1-point increase in LS7 score relative to low resilience (8.38 vs. 7.42). This was unexpected, but it “is consistent with some broader conceptual literature that posits that individual psychosocial resilience matters more under conditions of adversity,” the authors reported.
Understanding disparities is key
Black race has repeatedly been associated with an increased risk of CV events, but this study is valuable for providing a fresh perspective on the potential reasons, according to the authors of an accompanying editorial, Amber E. Johnson, MD, and Jared Magnani, MD, who are both affiliated with the division of cardiology at the University of Pittsburgh (Circ Cardiovasc Qual Outcomes 2020 Oct 7. doi: 10.1161/CIRCOUTCOMES.120.007357.
“Clinicians increasingly recognize that race-based disparities do not stem inherently from race; instead, the disparities stem from the underlying social determinations of health,” they wrote, citing such variables as unequal access to pay and acceptable living conditions “and the structural racism that perpetuates them.”
They agreed with the authors that promotion of psychosocial resilience among Black people living in communities with poor CV health has the potential to improve CV outcomes, but they warned that this is complex. Although they contend that resilience techniques can be taught, they cautioned there might be limitations if the underlying factors associated with poor psychosocial resilience remain unchanged.
“Thus, the superficial application of positive psychology strategies is likely insufficient to bring parity to CV health outcomes,” they wrote, concluding that strategies to promote health equity would negate the need for interventions to bolster resilience.
Studies that focus on Black adults and cardiovascular health, including this investigation into the role of psychosocial factors “are much needed and very welcome,” said Harlan M. Krumholz, MD, a cardiologist and professor in the Institute for Social and Policy Studies at Yale University, New Haven, Conn.
He sees a broad array of potential directions of research.
“The study opens many questions about whether the resilience can be strengthened by interventions; whether addressing structural racism could reduce the need for such resilience, and whether this association is specific to Black adults in an urban center or is generally present in other settings and in other populations,” Dr. Krumholz said.
An effort is now needed to determine “whether this is a marker or a mediator of cardiovascular health,” he added.
In either case, resilience is a potentially important factor for understanding racial disparities in CV-disease prevalence and outcomes, according to the authors of the accompanying editorial and Dr. Krumholz.
SOURCE: Kim JH et al. Circ Cardiovasc Qual Outcomes. 2020 Oct 7;13:e006638.
Resilience might deserve targeting
Resilience might deserve targeting
Increased psychosocial resilience, which captures a sense of purpose, optimism, and life-coping strategies, correlates with improved cardiovascular (CV) health in Black Americans, according to a study that might hold a key for identifying new strategies for CV disease prevention.
“Our findings highlight the importance of individual psychosocial factors that promote cardiovascular health among Black adults, traditionally considered to be a high-risk population,” according to a team of authors collaborating on a study produced by the Morehouse-Emory Cardiovascular Center for Health Equity in Atlanta.
Studies associating psychosocial resilience with improved health outcomes have been published before. In a 12-study review of this concept, it was emphasized that resilience is a dynamic process, not a personality trait, and has shown promise as a target of efforts to relieve the burden of disease (Johnston MC et al. Psychosomatics 2015;56:168-80).
In this study, which received partial support from the American Heart Association, psychosocial resilience was evaluated at both the individual level and at the community level among 389 Black adults living in Atlanta. The senior author was Tené T. Lewis, PhD, of the department of epidemiology at Emory’s Rollins School of Public Health (Circ Cardiovasc Qual Outcomes 2020 Oct 7;13:3006638).
Psychosocial resilience was calculated across the domains of environmental mastery, purpose of life, optimism, coping, and lack of depression with standardized tests, such as the Life Orientation Test-Revised questionnaire for optimism and the Ryff Scales of Psychological Well-Being for the domains of environmental mastery and purpose of life. A composite score for psychosocial resilience was reached by calculating the median score across the measured domains.
Patients with high psychosocial resilience, defined as a composite score above the median, or low resilience, defined as a lower score, were then compared for CV health based on the AHA’s Life’s Simple 7 (LS7) score.
LS7 scores incorporate measures for exercise, diet, smoking history, blood pressure, glucose, cholesterol, and body mass index. Composite LS7 scores range from 0 to 14. Prior work cited by the authors have associated each 1-unit increase in LS7 score with a 13% lower risk of CVD.
As a continuous variable for CV risk at the individual level, each higher standard-deviation increment in the composite psychosocial resilience score was associated with a highly significant 0.42-point increase in LS7 score (P < .001) for study participants. In other words, increasing resilience predicted lower CV risk scores.
Resilience was also calculated at the community level by looking at census tract-level rates of CV mortality and morbidity relative to socioeconomic status. Again, high CV resilience, defined as scores above the median, were compared with lower scores across neighborhoods with similar median household income. As a continuous variable in this analysis, each higher standard-deviation increment in the resilience score was associated with a 0.27-point increase in LS7 score (P = .01).
After adjustment for sociodemographic factors, the association between psychosocial resilience and CV health remained significant for both the individual and community calculations, according to the authors. When examined jointly, high individual psychosocial resilience remained independently associated with improved CV health, but living in a high-resilience neighborhood was not an independent predictor.
When evaluated individually, each of the domains in the psychosocial resistance score were positively correlated with higher LS7 scores, meaning lower CV risk. The strongest associations on a statistical level were low depressive symptoms (P = .001), environmental mastery (P = .006), and purpose in life (P = .009).
The impact of high psychosocial resistance scores was greatest in Black adults living in low-resilience neighborhoods. Among these subjects, high resilience was associated with a nearly 1-point increase in LS7 score relative to low resilience (8.38 vs. 7.42). This was unexpected, but it “is consistent with some broader conceptual literature that posits that individual psychosocial resilience matters more under conditions of adversity,” the authors reported.
Understanding disparities is key
Black race has repeatedly been associated with an increased risk of CV events, but this study is valuable for providing a fresh perspective on the potential reasons, according to the authors of an accompanying editorial, Amber E. Johnson, MD, and Jared Magnani, MD, who are both affiliated with the division of cardiology at the University of Pittsburgh (Circ Cardiovasc Qual Outcomes 2020 Oct 7. doi: 10.1161/CIRCOUTCOMES.120.007357.
“Clinicians increasingly recognize that race-based disparities do not stem inherently from race; instead, the disparities stem from the underlying social determinations of health,” they wrote, citing such variables as unequal access to pay and acceptable living conditions “and the structural racism that perpetuates them.”
They agreed with the authors that promotion of psychosocial resilience among Black people living in communities with poor CV health has the potential to improve CV outcomes, but they warned that this is complex. Although they contend that resilience techniques can be taught, they cautioned there might be limitations if the underlying factors associated with poor psychosocial resilience remain unchanged.
“Thus, the superficial application of positive psychology strategies is likely insufficient to bring parity to CV health outcomes,” they wrote, concluding that strategies to promote health equity would negate the need for interventions to bolster resilience.
Studies that focus on Black adults and cardiovascular health, including this investigation into the role of psychosocial factors “are much needed and very welcome,” said Harlan M. Krumholz, MD, a cardiologist and professor in the Institute for Social and Policy Studies at Yale University, New Haven, Conn.
He sees a broad array of potential directions of research.
“The study opens many questions about whether the resilience can be strengthened by interventions; whether addressing structural racism could reduce the need for such resilience, and whether this association is specific to Black adults in an urban center or is generally present in other settings and in other populations,” Dr. Krumholz said.
An effort is now needed to determine “whether this is a marker or a mediator of cardiovascular health,” he added.
In either case, resilience is a potentially important factor for understanding racial disparities in CV-disease prevalence and outcomes, according to the authors of the accompanying editorial and Dr. Krumholz.
SOURCE: Kim JH et al. Circ Cardiovasc Qual Outcomes. 2020 Oct 7;13:e006638.
Increased psychosocial resilience, which captures a sense of purpose, optimism, and life-coping strategies, correlates with improved cardiovascular (CV) health in Black Americans, according to a study that might hold a key for identifying new strategies for CV disease prevention.
“Our findings highlight the importance of individual psychosocial factors that promote cardiovascular health among Black adults, traditionally considered to be a high-risk population,” according to a team of authors collaborating on a study produced by the Morehouse-Emory Cardiovascular Center for Health Equity in Atlanta.
Studies associating psychosocial resilience with improved health outcomes have been published before. In a 12-study review of this concept, it was emphasized that resilience is a dynamic process, not a personality trait, and has shown promise as a target of efforts to relieve the burden of disease (Johnston MC et al. Psychosomatics 2015;56:168-80).
In this study, which received partial support from the American Heart Association, psychosocial resilience was evaluated at both the individual level and at the community level among 389 Black adults living in Atlanta. The senior author was Tené T. Lewis, PhD, of the department of epidemiology at Emory’s Rollins School of Public Health (Circ Cardiovasc Qual Outcomes 2020 Oct 7;13:3006638).
Psychosocial resilience was calculated across the domains of environmental mastery, purpose of life, optimism, coping, and lack of depression with standardized tests, such as the Life Orientation Test-Revised questionnaire for optimism and the Ryff Scales of Psychological Well-Being for the domains of environmental mastery and purpose of life. A composite score for psychosocial resilience was reached by calculating the median score across the measured domains.
Patients with high psychosocial resilience, defined as a composite score above the median, or low resilience, defined as a lower score, were then compared for CV health based on the AHA’s Life’s Simple 7 (LS7) score.
LS7 scores incorporate measures for exercise, diet, smoking history, blood pressure, glucose, cholesterol, and body mass index. Composite LS7 scores range from 0 to 14. Prior work cited by the authors have associated each 1-unit increase in LS7 score with a 13% lower risk of CVD.
As a continuous variable for CV risk at the individual level, each higher standard-deviation increment in the composite psychosocial resilience score was associated with a highly significant 0.42-point increase in LS7 score (P < .001) for study participants. In other words, increasing resilience predicted lower CV risk scores.
Resilience was also calculated at the community level by looking at census tract-level rates of CV mortality and morbidity relative to socioeconomic status. Again, high CV resilience, defined as scores above the median, were compared with lower scores across neighborhoods with similar median household income. As a continuous variable in this analysis, each higher standard-deviation increment in the resilience score was associated with a 0.27-point increase in LS7 score (P = .01).
After adjustment for sociodemographic factors, the association between psychosocial resilience and CV health remained significant for both the individual and community calculations, according to the authors. When examined jointly, high individual psychosocial resilience remained independently associated with improved CV health, but living in a high-resilience neighborhood was not an independent predictor.
When evaluated individually, each of the domains in the psychosocial resistance score were positively correlated with higher LS7 scores, meaning lower CV risk. The strongest associations on a statistical level were low depressive symptoms (P = .001), environmental mastery (P = .006), and purpose in life (P = .009).
The impact of high psychosocial resistance scores was greatest in Black adults living in low-resilience neighborhoods. Among these subjects, high resilience was associated with a nearly 1-point increase in LS7 score relative to low resilience (8.38 vs. 7.42). This was unexpected, but it “is consistent with some broader conceptual literature that posits that individual psychosocial resilience matters more under conditions of adversity,” the authors reported.
Understanding disparities is key
Black race has repeatedly been associated with an increased risk of CV events, but this study is valuable for providing a fresh perspective on the potential reasons, according to the authors of an accompanying editorial, Amber E. Johnson, MD, and Jared Magnani, MD, who are both affiliated with the division of cardiology at the University of Pittsburgh (Circ Cardiovasc Qual Outcomes 2020 Oct 7. doi: 10.1161/CIRCOUTCOMES.120.007357.
“Clinicians increasingly recognize that race-based disparities do not stem inherently from race; instead, the disparities stem from the underlying social determinations of health,” they wrote, citing such variables as unequal access to pay and acceptable living conditions “and the structural racism that perpetuates them.”
They agreed with the authors that promotion of psychosocial resilience among Black people living in communities with poor CV health has the potential to improve CV outcomes, but they warned that this is complex. Although they contend that resilience techniques can be taught, they cautioned there might be limitations if the underlying factors associated with poor psychosocial resilience remain unchanged.
“Thus, the superficial application of positive psychology strategies is likely insufficient to bring parity to CV health outcomes,” they wrote, concluding that strategies to promote health equity would negate the need for interventions to bolster resilience.
Studies that focus on Black adults and cardiovascular health, including this investigation into the role of psychosocial factors “are much needed and very welcome,” said Harlan M. Krumholz, MD, a cardiologist and professor in the Institute for Social and Policy Studies at Yale University, New Haven, Conn.
He sees a broad array of potential directions of research.
“The study opens many questions about whether the resilience can be strengthened by interventions; whether addressing structural racism could reduce the need for such resilience, and whether this association is specific to Black adults in an urban center or is generally present in other settings and in other populations,” Dr. Krumholz said.
An effort is now needed to determine “whether this is a marker or a mediator of cardiovascular health,” he added.
In either case, resilience is a potentially important factor for understanding racial disparities in CV-disease prevalence and outcomes, according to the authors of the accompanying editorial and Dr. Krumholz.
SOURCE: Kim JH et al. Circ Cardiovasc Qual Outcomes. 2020 Oct 7;13:e006638.
FROM CIRCULATION: CARDIOVASCULAR QUALITY AND OUTCOMES
The ally in the waiting room
Improving communication with patients’ loved ones
We think of a patient’s recovery happening in multiple locations – in a hospital room or a rehabilitation facility, for example. But many clinicians may not consider the opportunity to aid healing that lies in the waiting room.
The waiting room is where a patient’s loved ones often are and they, sometimes more than anyone, can unlock the path to a patient’s quicker recovery. Friends and family can offer encouragement, as they have an existing bond of trust that can help if a patient needs reinforcement to take their medications or follow other health care advice. But if loved ones are going to help patients, they need help from clinicians. Beyond being potential allies, they are also hurting, experiencing worry or confusion in a world of medical jargon.
The coronavirus changes the relationship of patients and their loved ones, as patients are often isolated or limited in the number of visitors they are allowed to see. A smartphone replaces the smiling faces of friends and relatives at their bedside, and a text is a poor substitute for a hug.
The Hospitalist asked some experienced hospitalists for insight on how best to communicate with patients’ loved ones to improve outcomes for all, medically and emotionally.
Team approach
“Patients feel isolated, terrified, and vulnerable but still need an advocate in the hospital, so daily communication with a patient’s loved one is important to give a sense that the patient is looked after,” said Kari Esbensen, MD, PhD, a hospitalist and palliative care expert at Emory University Hospital Midtown, Atlanta.
Glenn Rosenbluth, MD, a pediatric hospitalist and director, quality and safety programs, at the University of California, San Francisco, Benioff Children’s Hospital, agreed. He said that the most important thing is to communicate, period.
“We fall into this pattern of ‘out of sight, out of mind,’ ” he said. “We need to take the extra step to find out who a patient’s loved ones are. If it is a clinical visit, ask the patient, or maybe get the information from a caseworker, or just pay attention to who is dropping in to see the patient. Having a second person available to jot down notes, or having a handy list of questions – it all helps the patient. We forget that sometimes it can seem like a whirlwind for the patient when they are hurting. We have to remember that a loved one is important to a patient’s care team and we need to include them, empower them, and show that we want to hear their voices.”
Dr. Esbensen said it is critical to start off on the right foot when communicating with a patient’s loved one, especially during the current pandemic.
“With COVID-19, the most important thing is to speak honestly, to say hope for the best but prepare for the worst-case scenario,” Dr. Esbensen said. “We’ve seen that conditions can shift dramatically in short periods of time. The loved one needs to have a sense of the positive and negative possibilities. Families tend to lack understanding of the changes in the patient that are caused by COVID-19. The patient can come out of the hospital debilitated, very different than when they entered the hospital, and we need to warn people close to them about this. Unrealistic expectations need to be guarded against if a patient’s loved ones are going to help.”
Perhaps the best form of communication with a patient’s loved ones is an often-forgotten skill: listening.
“Get an idea from the patient’s loved ones of what the issues are, as well as their idea of what they think of the disease and how it spreads,” Dr. Esbensen said. “Sometimes they are right on target but sometimes there are misinterpretations and we need to help them understand it better. It’s not a ‘one-size-fits-all’ speech that we should give, but try to say, ‘tell me what you think is going on, what you think you’ve heard, and what you’re worried about,’ and learn what is most important to the patient. Start on those terms and adapt; this way you can correct and address what makes them most fearful, which can be different for each loved one. For some, the concern could be that they have children or other vulnerable people in the house. Finding out these other issues is important.”
Venkatrao Medarametla, MD, SFHM, medical director for hospital medicine at Baystate Medical Center, Springfield, Mass., emphasized that, in a time when hospitalists are being pulled in every direction, it is easy to lose your attention.
“It’s very important that family members know you’re present with them,” he said. “This can be an emotional time and they need empathy. It’s very easy for our list of tasks to get in the way of communicating, including with our body language.”
Dr. Medarametla said one of the reasons to communicate with patients’ loved ones is to calm them – a patient’s relatives or their friends may not be under your medical care, but they are still human beings.
“A lot of people just want information and want to be helpful, but we also need to realize that, while we are caring for many patients, this one person is the patient they are focused on,” said Laura Nell Hodo, MD, a pediatric hospitalist at Kravis Children’s Hospital at Mount Sinai in New York. “Don’t rush, and if you know that a patient’s loved one needs more time, make sure it can be found – if not then, at least later on the phone. Fifteen to 20 minutes may be what’s needed, and you can’t shortchange them.”
Dr. Hodo said that a patient’s loved ones often do not realize it is possible to receive phone calls from hospitalists. “We need to remind them that they can get in touch with us. We have to remember how helpless they can feel and how they want to understand what is happening in the hospital.”
For medical adherence issues, sometimes it is best to communicate with the patient and loved one at the same time, Dr. Hodo advised. “Whether it’s for medication or postdischarge exercises, if they both receive the information together it can reinforce adherence. But you also need to remember that the patient may only want a loved one told about certain things, or possibly nothing at all. We need to make sure we understand the patient’s wishes, regardless of whether we think a person close to them can be an ally or not.”
Dr. Esbensen also noted that a loved one can give hospitalists important clues to the emotional components of a patient’s care.
“I remember a patient whose wife told me how he worked in a garage, how he was strong and did not want people to think he was a weak guy just because of what was happening to him,” Dr. Esbensen said. “I didn’t know that he felt he might be perceived in this way. I mentioned to him how I learned he was a good mechanic and he perked up and felt seen in a different light. These things make a difference.”
But when is the best time to speak with a patient’s loved ones? Since much communication is done via phone during the pandemic, there are different philosophies.
“We had a debate among colleagues to see how each of us did it,” Dr. Esbensen said. “Some try to call after each patient encounter, while they are outside the room and it’s fresh in their mind, but others find it better to make the call after their rounds, to give the person their full attention. Most of the time I try to do it that way.”
She noted that, in the current environment, a phone call may be better than a face-to-face conversation with patients’ loved ones.
“We’re covered in so much gear to protect us from the coronavirus that it can feel like a great distance exists between us and the person with whom we’re speaking,” she said. “It’s strange, but the phone can make the conversation seem more relaxed and may get people to open up more.”
Even when they leave
All the hospitalists affirmed that loved ones can make a big difference for the patient through all aspects of care. Long after a patient returns home, the support of loved ones can have a profound impact in speeding healing and improving long-term outcomes.
Dr. Esbensen said COVID-19 and other serious illnesses can leave a patient needing support, and maybe a “push” when feeling low keeps them from adhering to medical advice.
“It’s not just in the hospital but after discharge,” she said. “A person offering support can really help patients throughout their journey, and much success in recovering from illness occurs after the transition home. Having the support of that one person a patient trusts can be critical.”
Dr. Hodo believes that the coronavirus pandemic could forever change the way hospitalists communicate with patients and their loved ones.
“I work in pediatrics and we know serious medical decisions can’t be made without guardians or parents,” she said. “But in adult medicine doctors may not automatically ask the patient about calling someone for input on decision-making. With COVID, you cannot assume a patient is on their own, because there are protocols keeping people from physically being present in the patient’s room. My experience from working in adult coronavirus units is that the thinking about the loved ones’ role in patient care – and communication with them – might just change. … At least, I hope so.”
Quick takeaways for hospitalists
- Get beyond personal protective equipment. A conversation with a patient’s loved one might be easier to achieve via phone, without all the protective gear in the way.
- Encourage adherence. Speaking with patients and loved ones together may be more effective. They may reach agreement quicker on how best to adhere to medical advice.
- Loved ones offer clues. They might give you a better sense of a patient’s worries, or help you to connect better with those in your care.
- Be present. You have a long to-do list but do not let empathy fall off it, even if you feel overwhelmed.
Improving communication with patients’ loved ones
Improving communication with patients’ loved ones
We think of a patient’s recovery happening in multiple locations – in a hospital room or a rehabilitation facility, for example. But many clinicians may not consider the opportunity to aid healing that lies in the waiting room.
The waiting room is where a patient’s loved ones often are and they, sometimes more than anyone, can unlock the path to a patient’s quicker recovery. Friends and family can offer encouragement, as they have an existing bond of trust that can help if a patient needs reinforcement to take their medications or follow other health care advice. But if loved ones are going to help patients, they need help from clinicians. Beyond being potential allies, they are also hurting, experiencing worry or confusion in a world of medical jargon.
The coronavirus changes the relationship of patients and their loved ones, as patients are often isolated or limited in the number of visitors they are allowed to see. A smartphone replaces the smiling faces of friends and relatives at their bedside, and a text is a poor substitute for a hug.
The Hospitalist asked some experienced hospitalists for insight on how best to communicate with patients’ loved ones to improve outcomes for all, medically and emotionally.
Team approach
“Patients feel isolated, terrified, and vulnerable but still need an advocate in the hospital, so daily communication with a patient’s loved one is important to give a sense that the patient is looked after,” said Kari Esbensen, MD, PhD, a hospitalist and palliative care expert at Emory University Hospital Midtown, Atlanta.
Glenn Rosenbluth, MD, a pediatric hospitalist and director, quality and safety programs, at the University of California, San Francisco, Benioff Children’s Hospital, agreed. He said that the most important thing is to communicate, period.
“We fall into this pattern of ‘out of sight, out of mind,’ ” he said. “We need to take the extra step to find out who a patient’s loved ones are. If it is a clinical visit, ask the patient, or maybe get the information from a caseworker, or just pay attention to who is dropping in to see the patient. Having a second person available to jot down notes, or having a handy list of questions – it all helps the patient. We forget that sometimes it can seem like a whirlwind for the patient when they are hurting. We have to remember that a loved one is important to a patient’s care team and we need to include them, empower them, and show that we want to hear their voices.”
Dr. Esbensen said it is critical to start off on the right foot when communicating with a patient’s loved one, especially during the current pandemic.
“With COVID-19, the most important thing is to speak honestly, to say hope for the best but prepare for the worst-case scenario,” Dr. Esbensen said. “We’ve seen that conditions can shift dramatically in short periods of time. The loved one needs to have a sense of the positive and negative possibilities. Families tend to lack understanding of the changes in the patient that are caused by COVID-19. The patient can come out of the hospital debilitated, very different than when they entered the hospital, and we need to warn people close to them about this. Unrealistic expectations need to be guarded against if a patient’s loved ones are going to help.”
Perhaps the best form of communication with a patient’s loved ones is an often-forgotten skill: listening.
“Get an idea from the patient’s loved ones of what the issues are, as well as their idea of what they think of the disease and how it spreads,” Dr. Esbensen said. “Sometimes they are right on target but sometimes there are misinterpretations and we need to help them understand it better. It’s not a ‘one-size-fits-all’ speech that we should give, but try to say, ‘tell me what you think is going on, what you think you’ve heard, and what you’re worried about,’ and learn what is most important to the patient. Start on those terms and adapt; this way you can correct and address what makes them most fearful, which can be different for each loved one. For some, the concern could be that they have children or other vulnerable people in the house. Finding out these other issues is important.”
Venkatrao Medarametla, MD, SFHM, medical director for hospital medicine at Baystate Medical Center, Springfield, Mass., emphasized that, in a time when hospitalists are being pulled in every direction, it is easy to lose your attention.
“It’s very important that family members know you’re present with them,” he said. “This can be an emotional time and they need empathy. It’s very easy for our list of tasks to get in the way of communicating, including with our body language.”
Dr. Medarametla said one of the reasons to communicate with patients’ loved ones is to calm them – a patient’s relatives or their friends may not be under your medical care, but they are still human beings.
“A lot of people just want information and want to be helpful, but we also need to realize that, while we are caring for many patients, this one person is the patient they are focused on,” said Laura Nell Hodo, MD, a pediatric hospitalist at Kravis Children’s Hospital at Mount Sinai in New York. “Don’t rush, and if you know that a patient’s loved one needs more time, make sure it can be found – if not then, at least later on the phone. Fifteen to 20 minutes may be what’s needed, and you can’t shortchange them.”
Dr. Hodo said that a patient’s loved ones often do not realize it is possible to receive phone calls from hospitalists. “We need to remind them that they can get in touch with us. We have to remember how helpless they can feel and how they want to understand what is happening in the hospital.”
For medical adherence issues, sometimes it is best to communicate with the patient and loved one at the same time, Dr. Hodo advised. “Whether it’s for medication or postdischarge exercises, if they both receive the information together it can reinforce adherence. But you also need to remember that the patient may only want a loved one told about certain things, or possibly nothing at all. We need to make sure we understand the patient’s wishes, regardless of whether we think a person close to them can be an ally or not.”
Dr. Esbensen also noted that a loved one can give hospitalists important clues to the emotional components of a patient’s care.
“I remember a patient whose wife told me how he worked in a garage, how he was strong and did not want people to think he was a weak guy just because of what was happening to him,” Dr. Esbensen said. “I didn’t know that he felt he might be perceived in this way. I mentioned to him how I learned he was a good mechanic and he perked up and felt seen in a different light. These things make a difference.”
But when is the best time to speak with a patient’s loved ones? Since much communication is done via phone during the pandemic, there are different philosophies.
“We had a debate among colleagues to see how each of us did it,” Dr. Esbensen said. “Some try to call after each patient encounter, while they are outside the room and it’s fresh in their mind, but others find it better to make the call after their rounds, to give the person their full attention. Most of the time I try to do it that way.”
She noted that, in the current environment, a phone call may be better than a face-to-face conversation with patients’ loved ones.
“We’re covered in so much gear to protect us from the coronavirus that it can feel like a great distance exists between us and the person with whom we’re speaking,” she said. “It’s strange, but the phone can make the conversation seem more relaxed and may get people to open up more.”
Even when they leave
All the hospitalists affirmed that loved ones can make a big difference for the patient through all aspects of care. Long after a patient returns home, the support of loved ones can have a profound impact in speeding healing and improving long-term outcomes.
Dr. Esbensen said COVID-19 and other serious illnesses can leave a patient needing support, and maybe a “push” when feeling low keeps them from adhering to medical advice.
“It’s not just in the hospital but after discharge,” she said. “A person offering support can really help patients throughout their journey, and much success in recovering from illness occurs after the transition home. Having the support of that one person a patient trusts can be critical.”
Dr. Hodo believes that the coronavirus pandemic could forever change the way hospitalists communicate with patients and their loved ones.
“I work in pediatrics and we know serious medical decisions can’t be made without guardians or parents,” she said. “But in adult medicine doctors may not automatically ask the patient about calling someone for input on decision-making. With COVID, you cannot assume a patient is on their own, because there are protocols keeping people from physically being present in the patient’s room. My experience from working in adult coronavirus units is that the thinking about the loved ones’ role in patient care – and communication with them – might just change. … At least, I hope so.”
Quick takeaways for hospitalists
- Get beyond personal protective equipment. A conversation with a patient’s loved one might be easier to achieve via phone, without all the protective gear in the way.
- Encourage adherence. Speaking with patients and loved ones together may be more effective. They may reach agreement quicker on how best to adhere to medical advice.
- Loved ones offer clues. They might give you a better sense of a patient’s worries, or help you to connect better with those in your care.
- Be present. You have a long to-do list but do not let empathy fall off it, even if you feel overwhelmed.
We think of a patient’s recovery happening in multiple locations – in a hospital room or a rehabilitation facility, for example. But many clinicians may not consider the opportunity to aid healing that lies in the waiting room.
The waiting room is where a patient’s loved ones often are and they, sometimes more than anyone, can unlock the path to a patient’s quicker recovery. Friends and family can offer encouragement, as they have an existing bond of trust that can help if a patient needs reinforcement to take their medications or follow other health care advice. But if loved ones are going to help patients, they need help from clinicians. Beyond being potential allies, they are also hurting, experiencing worry or confusion in a world of medical jargon.
The coronavirus changes the relationship of patients and their loved ones, as patients are often isolated or limited in the number of visitors they are allowed to see. A smartphone replaces the smiling faces of friends and relatives at their bedside, and a text is a poor substitute for a hug.
The Hospitalist asked some experienced hospitalists for insight on how best to communicate with patients’ loved ones to improve outcomes for all, medically and emotionally.
Team approach
“Patients feel isolated, terrified, and vulnerable but still need an advocate in the hospital, so daily communication with a patient’s loved one is important to give a sense that the patient is looked after,” said Kari Esbensen, MD, PhD, a hospitalist and palliative care expert at Emory University Hospital Midtown, Atlanta.
Glenn Rosenbluth, MD, a pediatric hospitalist and director, quality and safety programs, at the University of California, San Francisco, Benioff Children’s Hospital, agreed. He said that the most important thing is to communicate, period.
“We fall into this pattern of ‘out of sight, out of mind,’ ” he said. “We need to take the extra step to find out who a patient’s loved ones are. If it is a clinical visit, ask the patient, or maybe get the information from a caseworker, or just pay attention to who is dropping in to see the patient. Having a second person available to jot down notes, or having a handy list of questions – it all helps the patient. We forget that sometimes it can seem like a whirlwind for the patient when they are hurting. We have to remember that a loved one is important to a patient’s care team and we need to include them, empower them, and show that we want to hear their voices.”
Dr. Esbensen said it is critical to start off on the right foot when communicating with a patient’s loved one, especially during the current pandemic.
“With COVID-19, the most important thing is to speak honestly, to say hope for the best but prepare for the worst-case scenario,” Dr. Esbensen said. “We’ve seen that conditions can shift dramatically in short periods of time. The loved one needs to have a sense of the positive and negative possibilities. Families tend to lack understanding of the changes in the patient that are caused by COVID-19. The patient can come out of the hospital debilitated, very different than when they entered the hospital, and we need to warn people close to them about this. Unrealistic expectations need to be guarded against if a patient’s loved ones are going to help.”
Perhaps the best form of communication with a patient’s loved ones is an often-forgotten skill: listening.
“Get an idea from the patient’s loved ones of what the issues are, as well as their idea of what they think of the disease and how it spreads,” Dr. Esbensen said. “Sometimes they are right on target but sometimes there are misinterpretations and we need to help them understand it better. It’s not a ‘one-size-fits-all’ speech that we should give, but try to say, ‘tell me what you think is going on, what you think you’ve heard, and what you’re worried about,’ and learn what is most important to the patient. Start on those terms and adapt; this way you can correct and address what makes them most fearful, which can be different for each loved one. For some, the concern could be that they have children or other vulnerable people in the house. Finding out these other issues is important.”
Venkatrao Medarametla, MD, SFHM, medical director for hospital medicine at Baystate Medical Center, Springfield, Mass., emphasized that, in a time when hospitalists are being pulled in every direction, it is easy to lose your attention.
“It’s very important that family members know you’re present with them,” he said. “This can be an emotional time and they need empathy. It’s very easy for our list of tasks to get in the way of communicating, including with our body language.”
Dr. Medarametla said one of the reasons to communicate with patients’ loved ones is to calm them – a patient’s relatives or their friends may not be under your medical care, but they are still human beings.
“A lot of people just want information and want to be helpful, but we also need to realize that, while we are caring for many patients, this one person is the patient they are focused on,” said Laura Nell Hodo, MD, a pediatric hospitalist at Kravis Children’s Hospital at Mount Sinai in New York. “Don’t rush, and if you know that a patient’s loved one needs more time, make sure it can be found – if not then, at least later on the phone. Fifteen to 20 minutes may be what’s needed, and you can’t shortchange them.”
Dr. Hodo said that a patient’s loved ones often do not realize it is possible to receive phone calls from hospitalists. “We need to remind them that they can get in touch with us. We have to remember how helpless they can feel and how they want to understand what is happening in the hospital.”
For medical adherence issues, sometimes it is best to communicate with the patient and loved one at the same time, Dr. Hodo advised. “Whether it’s for medication or postdischarge exercises, if they both receive the information together it can reinforce adherence. But you also need to remember that the patient may only want a loved one told about certain things, or possibly nothing at all. We need to make sure we understand the patient’s wishes, regardless of whether we think a person close to them can be an ally or not.”
Dr. Esbensen also noted that a loved one can give hospitalists important clues to the emotional components of a patient’s care.
“I remember a patient whose wife told me how he worked in a garage, how he was strong and did not want people to think he was a weak guy just because of what was happening to him,” Dr. Esbensen said. “I didn’t know that he felt he might be perceived in this way. I mentioned to him how I learned he was a good mechanic and he perked up and felt seen in a different light. These things make a difference.”
But when is the best time to speak with a patient’s loved ones? Since much communication is done via phone during the pandemic, there are different philosophies.
“We had a debate among colleagues to see how each of us did it,” Dr. Esbensen said. “Some try to call after each patient encounter, while they are outside the room and it’s fresh in their mind, but others find it better to make the call after their rounds, to give the person their full attention. Most of the time I try to do it that way.”
She noted that, in the current environment, a phone call may be better than a face-to-face conversation with patients’ loved ones.
“We’re covered in so much gear to protect us from the coronavirus that it can feel like a great distance exists between us and the person with whom we’re speaking,” she said. “It’s strange, but the phone can make the conversation seem more relaxed and may get people to open up more.”
Even when they leave
All the hospitalists affirmed that loved ones can make a big difference for the patient through all aspects of care. Long after a patient returns home, the support of loved ones can have a profound impact in speeding healing and improving long-term outcomes.
Dr. Esbensen said COVID-19 and other serious illnesses can leave a patient needing support, and maybe a “push” when feeling low keeps them from adhering to medical advice.
“It’s not just in the hospital but after discharge,” she said. “A person offering support can really help patients throughout their journey, and much success in recovering from illness occurs after the transition home. Having the support of that one person a patient trusts can be critical.”
Dr. Hodo believes that the coronavirus pandemic could forever change the way hospitalists communicate with patients and their loved ones.
“I work in pediatrics and we know serious medical decisions can’t be made without guardians or parents,” she said. “But in adult medicine doctors may not automatically ask the patient about calling someone for input on decision-making. With COVID, you cannot assume a patient is on their own, because there are protocols keeping people from physically being present in the patient’s room. My experience from working in adult coronavirus units is that the thinking about the loved ones’ role in patient care – and communication with them – might just change. … At least, I hope so.”
Quick takeaways for hospitalists
- Get beyond personal protective equipment. A conversation with a patient’s loved one might be easier to achieve via phone, without all the protective gear in the way.
- Encourage adherence. Speaking with patients and loved ones together may be more effective. They may reach agreement quicker on how best to adhere to medical advice.
- Loved ones offer clues. They might give you a better sense of a patient’s worries, or help you to connect better with those in your care.
- Be present. You have a long to-do list but do not let empathy fall off it, even if you feel overwhelmed.
Locus Minoris Resistentiae: Mycobacterium chelonae in Striae Distensae
To the Editor:
Immunosuppressed patients are at particular risk for disseminated mycobacterial infections. A locus minoris resistentiae offers less resistance to the infectious spread of these microorganisms. We present a case of Mycobacterium chelonae infection preferentially involving striae distensae.
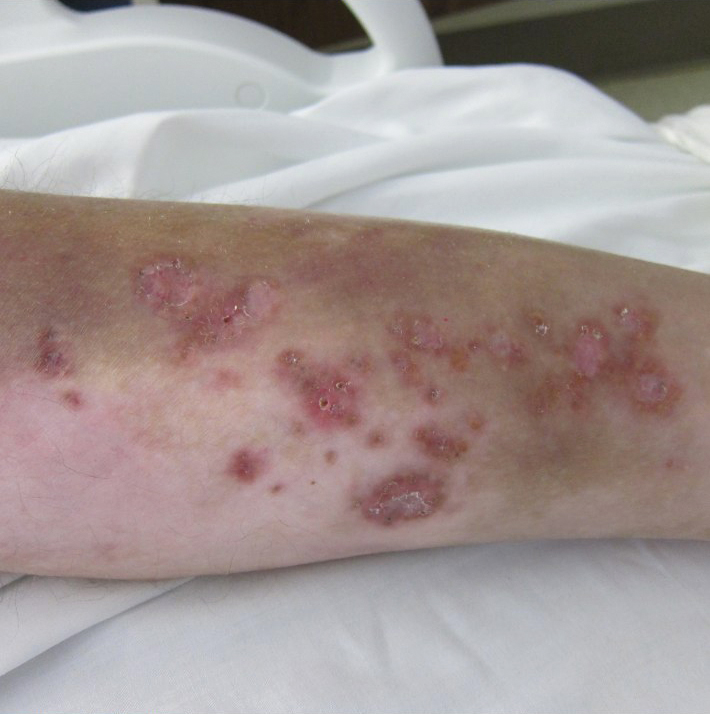
A 30-year-old man with chronic eosinophilic pneumonia requiring high-dose corticosteroid therapy presented with widespread skin lesions. He reported no history of cutaneous trauma or aquatic activities. Physical examination revealed the patient was markedly cushingoid with generalized cutaneous atrophy and widespread striae. Multiple erythematous papules surrounded a large ulceration on the dorsal aspect of the left hand. Depressed erythematous plaques and several small crusted erosions extended up the left lower leg (Figure 1) to the knee. Strikingly, numerous brown and pink papules and small plaques on the left thigh were primarily confined within striae (Figure 2).
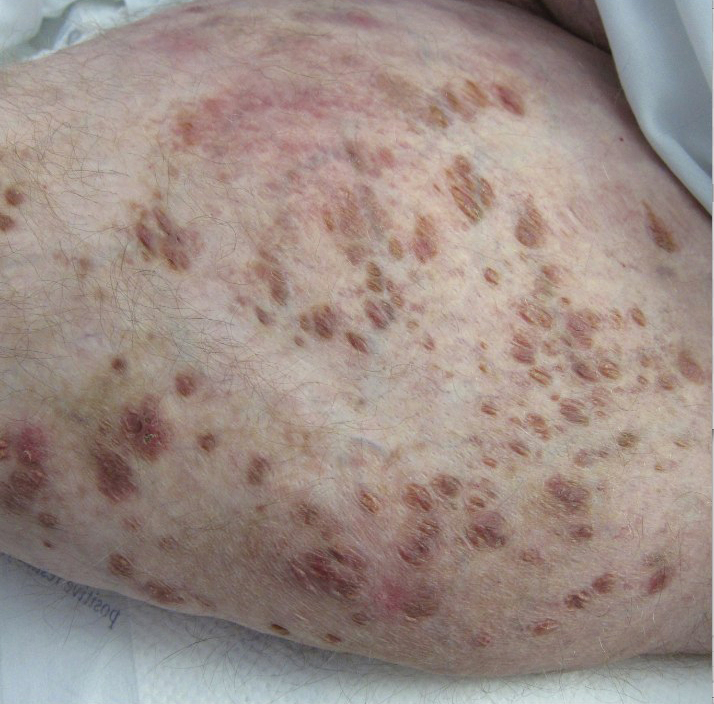
A biopsy of the left thigh revealed granulomatous inflammation (Figure 3) with numerous acid-fast bacilli (Figure 4). Broad-spectrum coverage for fast-growing acid-fast bacilli with amikacin, ceftriaxone, levofloxacin, and clarithromycin was initiated with steady improvement of the eruption. Tissue culture subsequently grew Mycobacterium abscessus-chelonae, and therapy was narrowed to clarithromycin and moxifloxacin.
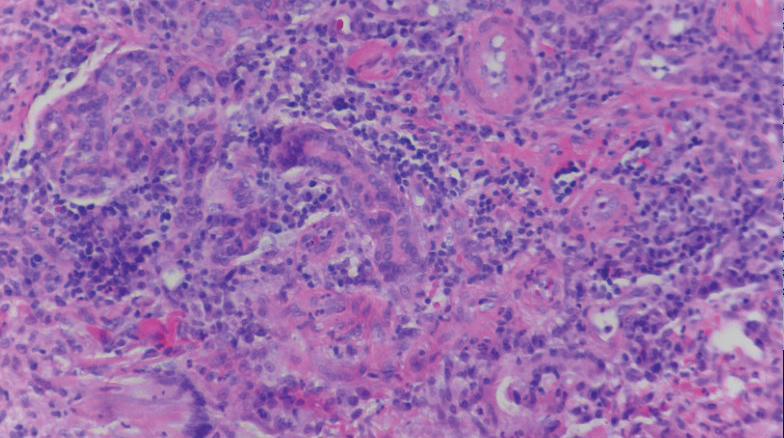
Mycobacterium chelonae is a rapidly growing mycobacteria isolated from soil and water worldwide, and human skin is a commensal organism. Cutaneous infections have been associated with traumatic injury, tattooing, surgery, cosmetic procedures, vascular access sites, and acupuncture.1 Most cases of cutaneous M chelonae infection begin as a violaceous nodule. Over weeks to months, the localized infection progresses to multiple papules, nodules, draining abscesses, or ulcers. Infections tend to disseminate in immunosuppressed patients, and granulomatous inflammation may not be seen.1
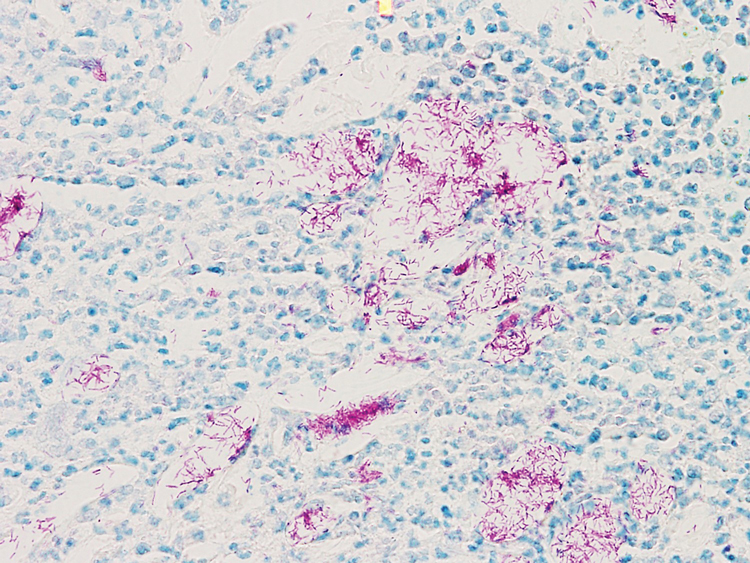
Atypical mycobacterial infection occurring within striae distensae is an example of locus minoris resistentiae—a place of less resistance. Wolf et al2 hypothesized that locus minoris resistentiae could explain the occurrence of an isotopic response or the occurrence of a new skin disorder at the site of a previously healed skin condition. They suggested that the same site could be affected by 2 unrelated diseases at different times due to an inherited or acquired susceptibility in the area.2 Herpes zoster serves as a primary example of Wolf phenomenon, as numerous conditions including granuloma annulare, pseudolymphoma, Bowen disease, and acne have reportedly emerged in its wake.3
Although locus minoris resistentiae does not specifically involve traumatized skin, it must be distinguished from the Koebner phenomenon, characterized by the appearance of isomorphic lesions in areas of otherwise healthy skin subjected to cutaneous injury, as well as the pseudo-Koebner phenomenon, a similar process involving infectious agents.3
In our patient, striae distensae represented areas of increased predisposition to infection. The catabolic effect of high corticosteroid levels on fibroblast activity decreased collagen deposition in the dermal matrix, leading to the formation of linear bands of atrophic skin.4 The elastic fiber network in striae distensae is reduced and reorganized compared to normal skin, in which an intertwining elastic system forms a continuum from the dermoepidermal junction to the deep dermis.5 The number of vertically oriented fibrillin microfibrils subjacent to the dermoepidermal junction and elastin fibers in the papillary dermis is comparatively diminished such that the elastin and fibrillin fibers in the deep dermis run more horizontally compared to normal skin.4 Consequently, collagen alignment in striae distensae demonstrates more anisotropy, or directionally dependent variability, and the dermal matrix is looser and more floccular than the surrounding skin.6 These alterations of dermal architecture likely provide a mechanical advantage for intradermal spread of M chelonae within striae.
Other dermatoses have been observed to occur within striae distensae, specifically leukemia cutis, urticarial vasculitis, lupus erythematosus, keloids, linear focal elastosis, chronic graft-vs-host disease, psoriasis, gestational pemphigoid, and vitiligo.7 Given the dissimilarities of these conditions, the distinctive milieu of striae must provide an invitation—a locus minoris resistentiae—for secondary pathology.
Chronic corticosteroid use leads to both immunosuppression and striae distensae, effectively creating a perfect storm for an atypical mycobacterial skin infection demonstrating locus minoris resistentiae. The immunosuppressed state makes patients more susceptible to infection, and striae distensae may serve as a conduit for the offending organisms.
Acknowledgments—We are indebted to Letty Peterson, MD (Vidalia, Georgia), for her referral of this case, and to Stephen Mullins, MD (Augusta, Georgia), for his dermatopathology services.
- Hay RJ. Mycobacterium chelonae—a growing problem in soft tissue infection. Cur Opin Infect Dis. 2009;22:99-101.
- Wolf R, Brenner S, Ruocco V, et al. Isotopic response. Int J Dermatol. 1995;34:341-348.
- Medeiros do Santos Camargo C, Brotas AM, Ramos-e-Silva M, et al. Isomorphic phenomenon of Koebner: facts and controversies. Clin Dermatol. 2013;31:741-749.
- Watson REB, Parry EJ, Humphries JD, et al. Fibrillin microfibrils are reduced in skin exhibiting striae distensae. Br J Dermatol. 1998;138:931-397.
- Bertin C, A Lopes-DaCunha, Nkengne A, et al. Striae distensae are characterized by distinct microstructural features as measured by non-invasive methods in vivo. Skin Res Technol. 2014;20:81-86.
- Elsaie ML, Baumann LS, Elsaaiee LT. Striae distensae (stretch marks) and different modalities of therapy: an update. Dermatol Surg. 2009;35:563-573.
- Liu CI, Hsu CH. Leukemia cutis at the site of striae distensae: an isotopic response? Int J Dermatol. 1995;34:341-348.
To the Editor:
Immunosuppressed patients are at particular risk for disseminated mycobacterial infections. A locus minoris resistentiae offers less resistance to the infectious spread of these microorganisms. We present a case of Mycobacterium chelonae infection preferentially involving striae distensae.

A 30-year-old man with chronic eosinophilic pneumonia requiring high-dose corticosteroid therapy presented with widespread skin lesions. He reported no history of cutaneous trauma or aquatic activities. Physical examination revealed the patient was markedly cushingoid with generalized cutaneous atrophy and widespread striae. Multiple erythematous papules surrounded a large ulceration on the dorsal aspect of the left hand. Depressed erythematous plaques and several small crusted erosions extended up the left lower leg (Figure 1) to the knee. Strikingly, numerous brown and pink papules and small plaques on the left thigh were primarily confined within striae (Figure 2).

A biopsy of the left thigh revealed granulomatous inflammation (Figure 3) with numerous acid-fast bacilli (Figure 4). Broad-spectrum coverage for fast-growing acid-fast bacilli with amikacin, ceftriaxone, levofloxacin, and clarithromycin was initiated with steady improvement of the eruption. Tissue culture subsequently grew Mycobacterium abscessus-chelonae, and therapy was narrowed to clarithromycin and moxifloxacin.

Mycobacterium chelonae is a rapidly growing mycobacteria isolated from soil and water worldwide, and human skin is a commensal organism. Cutaneous infections have been associated with traumatic injury, tattooing, surgery, cosmetic procedures, vascular access sites, and acupuncture.1 Most cases of cutaneous M chelonae infection begin as a violaceous nodule. Over weeks to months, the localized infection progresses to multiple papules, nodules, draining abscesses, or ulcers. Infections tend to disseminate in immunosuppressed patients, and granulomatous inflammation may not be seen.1

Atypical mycobacterial infection occurring within striae distensae is an example of locus minoris resistentiae—a place of less resistance. Wolf et al2 hypothesized that locus minoris resistentiae could explain the occurrence of an isotopic response or the occurrence of a new skin disorder at the site of a previously healed skin condition. They suggested that the same site could be affected by 2 unrelated diseases at different times due to an inherited or acquired susceptibility in the area.2 Herpes zoster serves as a primary example of Wolf phenomenon, as numerous conditions including granuloma annulare, pseudolymphoma, Bowen disease, and acne have reportedly emerged in its wake.3
Although locus minoris resistentiae does not specifically involve traumatized skin, it must be distinguished from the Koebner phenomenon, characterized by the appearance of isomorphic lesions in areas of otherwise healthy skin subjected to cutaneous injury, as well as the pseudo-Koebner phenomenon, a similar process involving infectious agents.3
In our patient, striae distensae represented areas of increased predisposition to infection. The catabolic effect of high corticosteroid levels on fibroblast activity decreased collagen deposition in the dermal matrix, leading to the formation of linear bands of atrophic skin.4 The elastic fiber network in striae distensae is reduced and reorganized compared to normal skin, in which an intertwining elastic system forms a continuum from the dermoepidermal junction to the deep dermis.5 The number of vertically oriented fibrillin microfibrils subjacent to the dermoepidermal junction and elastin fibers in the papillary dermis is comparatively diminished such that the elastin and fibrillin fibers in the deep dermis run more horizontally compared to normal skin.4 Consequently, collagen alignment in striae distensae demonstrates more anisotropy, or directionally dependent variability, and the dermal matrix is looser and more floccular than the surrounding skin.6 These alterations of dermal architecture likely provide a mechanical advantage for intradermal spread of M chelonae within striae.
Other dermatoses have been observed to occur within striae distensae, specifically leukemia cutis, urticarial vasculitis, lupus erythematosus, keloids, linear focal elastosis, chronic graft-vs-host disease, psoriasis, gestational pemphigoid, and vitiligo.7 Given the dissimilarities of these conditions, the distinctive milieu of striae must provide an invitation—a locus minoris resistentiae—for secondary pathology.
Chronic corticosteroid use leads to both immunosuppression and striae distensae, effectively creating a perfect storm for an atypical mycobacterial skin infection demonstrating locus minoris resistentiae. The immunosuppressed state makes patients more susceptible to infection, and striae distensae may serve as a conduit for the offending organisms.
Acknowledgments—We are indebted to Letty Peterson, MD (Vidalia, Georgia), for her referral of this case, and to Stephen Mullins, MD (Augusta, Georgia), for his dermatopathology services.
To the Editor:
Immunosuppressed patients are at particular risk for disseminated mycobacterial infections. A locus minoris resistentiae offers less resistance to the infectious spread of these microorganisms. We present a case of Mycobacterium chelonae infection preferentially involving striae distensae.

A 30-year-old man with chronic eosinophilic pneumonia requiring high-dose corticosteroid therapy presented with widespread skin lesions. He reported no history of cutaneous trauma or aquatic activities. Physical examination revealed the patient was markedly cushingoid with generalized cutaneous atrophy and widespread striae. Multiple erythematous papules surrounded a large ulceration on the dorsal aspect of the left hand. Depressed erythematous plaques and several small crusted erosions extended up the left lower leg (Figure 1) to the knee. Strikingly, numerous brown and pink papules and small plaques on the left thigh were primarily confined within striae (Figure 2).

A biopsy of the left thigh revealed granulomatous inflammation (Figure 3) with numerous acid-fast bacilli (Figure 4). Broad-spectrum coverage for fast-growing acid-fast bacilli with amikacin, ceftriaxone, levofloxacin, and clarithromycin was initiated with steady improvement of the eruption. Tissue culture subsequently grew Mycobacterium abscessus-chelonae, and therapy was narrowed to clarithromycin and moxifloxacin.

Mycobacterium chelonae is a rapidly growing mycobacteria isolated from soil and water worldwide, and human skin is a commensal organism. Cutaneous infections have been associated with traumatic injury, tattooing, surgery, cosmetic procedures, vascular access sites, and acupuncture.1 Most cases of cutaneous M chelonae infection begin as a violaceous nodule. Over weeks to months, the localized infection progresses to multiple papules, nodules, draining abscesses, or ulcers. Infections tend to disseminate in immunosuppressed patients, and granulomatous inflammation may not be seen.1

Atypical mycobacterial infection occurring within striae distensae is an example of locus minoris resistentiae—a place of less resistance. Wolf et al2 hypothesized that locus minoris resistentiae could explain the occurrence of an isotopic response or the occurrence of a new skin disorder at the site of a previously healed skin condition. They suggested that the same site could be affected by 2 unrelated diseases at different times due to an inherited or acquired susceptibility in the area.2 Herpes zoster serves as a primary example of Wolf phenomenon, as numerous conditions including granuloma annulare, pseudolymphoma, Bowen disease, and acne have reportedly emerged in its wake.3
Although locus minoris resistentiae does not specifically involve traumatized skin, it must be distinguished from the Koebner phenomenon, characterized by the appearance of isomorphic lesions in areas of otherwise healthy skin subjected to cutaneous injury, as well as the pseudo-Koebner phenomenon, a similar process involving infectious agents.3
In our patient, striae distensae represented areas of increased predisposition to infection. The catabolic effect of high corticosteroid levels on fibroblast activity decreased collagen deposition in the dermal matrix, leading to the formation of linear bands of atrophic skin.4 The elastic fiber network in striae distensae is reduced and reorganized compared to normal skin, in which an intertwining elastic system forms a continuum from the dermoepidermal junction to the deep dermis.5 The number of vertically oriented fibrillin microfibrils subjacent to the dermoepidermal junction and elastin fibers in the papillary dermis is comparatively diminished such that the elastin and fibrillin fibers in the deep dermis run more horizontally compared to normal skin.4 Consequently, collagen alignment in striae distensae demonstrates more anisotropy, or directionally dependent variability, and the dermal matrix is looser and more floccular than the surrounding skin.6 These alterations of dermal architecture likely provide a mechanical advantage for intradermal spread of M chelonae within striae.
Other dermatoses have been observed to occur within striae distensae, specifically leukemia cutis, urticarial vasculitis, lupus erythematosus, keloids, linear focal elastosis, chronic graft-vs-host disease, psoriasis, gestational pemphigoid, and vitiligo.7 Given the dissimilarities of these conditions, the distinctive milieu of striae must provide an invitation—a locus minoris resistentiae—for secondary pathology.
Chronic corticosteroid use leads to both immunosuppression and striae distensae, effectively creating a perfect storm for an atypical mycobacterial skin infection demonstrating locus minoris resistentiae. The immunosuppressed state makes patients more susceptible to infection, and striae distensae may serve as a conduit for the offending organisms.
Acknowledgments—We are indebted to Letty Peterson, MD (Vidalia, Georgia), for her referral of this case, and to Stephen Mullins, MD (Augusta, Georgia), for his dermatopathology services.
- Hay RJ. Mycobacterium chelonae—a growing problem in soft tissue infection. Cur Opin Infect Dis. 2009;22:99-101.
- Wolf R, Brenner S, Ruocco V, et al. Isotopic response. Int J Dermatol. 1995;34:341-348.
- Medeiros do Santos Camargo C, Brotas AM, Ramos-e-Silva M, et al. Isomorphic phenomenon of Koebner: facts and controversies. Clin Dermatol. 2013;31:741-749.
- Watson REB, Parry EJ, Humphries JD, et al. Fibrillin microfibrils are reduced in skin exhibiting striae distensae. Br J Dermatol. 1998;138:931-397.
- Bertin C, A Lopes-DaCunha, Nkengne A, et al. Striae distensae are characterized by distinct microstructural features as measured by non-invasive methods in vivo. Skin Res Technol. 2014;20:81-86.
- Elsaie ML, Baumann LS, Elsaaiee LT. Striae distensae (stretch marks) and different modalities of therapy: an update. Dermatol Surg. 2009;35:563-573.
- Liu CI, Hsu CH. Leukemia cutis at the site of striae distensae: an isotopic response? Int J Dermatol. 1995;34:341-348.
- Hay RJ. Mycobacterium chelonae—a growing problem in soft tissue infection. Cur Opin Infect Dis. 2009;22:99-101.
- Wolf R, Brenner S, Ruocco V, et al. Isotopic response. Int J Dermatol. 1995;34:341-348.
- Medeiros do Santos Camargo C, Brotas AM, Ramos-e-Silva M, et al. Isomorphic phenomenon of Koebner: facts and controversies. Clin Dermatol. 2013;31:741-749.
- Watson REB, Parry EJ, Humphries JD, et al. Fibrillin microfibrils are reduced in skin exhibiting striae distensae. Br J Dermatol. 1998;138:931-397.
- Bertin C, A Lopes-DaCunha, Nkengne A, et al. Striae distensae are characterized by distinct microstructural features as measured by non-invasive methods in vivo. Skin Res Technol. 2014;20:81-86.
- Elsaie ML, Baumann LS, Elsaaiee LT. Striae distensae (stretch marks) and different modalities of therapy: an update. Dermatol Surg. 2009;35:563-573.
- Liu CI, Hsu CH. Leukemia cutis at the site of striae distensae: an isotopic response? Int J Dermatol. 1995;34:341-348.
Practice Points
- Striae distensae, seen frequently in the setting of chronic corticosteroid use, are at an increased risk for localized infection, particularly in immunocompromised patients. There should be a low threshold to biopsy striae distensae that demonstrate morphologic evolution.
- The Koebner reaction, also known as an isomorphic response, refers to the appearance of certain dermatoses in previously healthy skin subjected to cutaneous injury.
- Locus minoris resistentiae is an isotropic response that characterizes the presentation of a new dermatosis within an area previously affected by an unrelated skin condition that has healed.
How Kodak Gold contributed to bias in dermatology
A recent review looked at all articles describing skin manifestations associated with COVID-19 – 46 articles with a total of 130 clinical images – and found none that documented dermatologic conditions in people with dark skin. This was despite the disproportionate incidence of the disease in Black, Latino, and Native American/American Indian populations of color.
What’s going on? Temitayo Ogunleye, MD, an assistant professor of dermatology at the University of Pennsylvania, Philadelphia, spoke with lead investigator Jenna Lester, MD, a dermatologist and director of the Skin of Color Clinic at University of California, San Francisco, about the implications of her research.
Dr. Ogunleye: What prompted you to do this study in the first place?
Dr. Lester: It was actually driven by frustration. While we’re recognizing these COVID-related dermatologic manifestations, we’re still trying to figure out their clinical significance. We’ve learned what changes could be an early sign of infection and what might occur in people who are otherwise asymptomatic and should be tested. But in the process, we’re leaving out the group of people – dark-skinned populations – who are most heavily impacted by COVID. This is an injustice to the people who could benefit the most if found to have an early, visible sign of the disease on their skin. For example, pernio-like lesions and erythema, both of which have been seen in COVID patients, are harder to identify in darker skin.
As dermatologists, we know that the skin is the biggest organ and one that has the advantage of being visible. We partner with patients because they can see what we can see. We can explain what skin changes mean, and having examples to show them is really powerful. Because doctors typically respond best to numbers, I recognized that we needed data to prove that this lack of representation of persons of color in our COVID documentation was in fact true.
Can you tell us the key findings from your research?
We included any article that described the cutaneous manifestations of COVID – and also included a photograph – and was published over a period of 5 months. Then we categorized each image using the Fitzpatrick Skin Type Scale. We found that A handful, about 6%, depicted skin changes in patients with Fitzpatrick type IV skin.
Were you surprised by the findings?
No. I was not surprised at all, for a couple of reasons. First, many of the referrals that I had been getting to evaluate possible COVID manifestations were from primary care doctors. And as we both know, erythema, hyperpigmentation, and discoloration can be difficult for even a trained dermatologist to pick up on. So if these patients with skin changes potentially suggestive of COVID are presenting to their primary care doctor who could not determine what they were, perhaps because they had never seen them in someone with darker skin, it means those same clinicians are not likely to document these rashes. I suspect that a lot of these photos were sourced from primary care.
The other reason for my lack of surprise is that I have looked at this issue before. In a study I did in 2019 looking at images in dermatology textbooks, my coauthors and I found that there was a pretty dramatic underrepresentation of skin of color overall. Another analysis of images in core dermatology textbooks, published earlier this year by one of your colleagues at the University of Pennsylvania, Jules Lipoff, MD, showed that the percentage of images of dark skin ranged from as low as 4% to a high of about 18%.
So I wonder whether that makes us less likely to look for things in patients with darker skin, except for those conditions that we’re taught are more common in people with darker skin, like keloids, vitiligo, or certain types of hair loss. I wonder whether we just don’t think of other conditions because all the photos we ever see of psoriasis or rosacea, for example, are of people with lighter skin.
You bring up a good point about the cyclical nature of this process. If you don’t see skin conditions in darker skin, then you don’t know what to look for and so you never look for it.
Exactly. What if a Black patient says, for example: “This looks different on my skin; do you think this could be related to COVID?” And their doctor looks at their skin and says no. Not because it isn’t, but because they haven’t seen that before. Then they don’t take a picture of it and we’ll never know what that might have been.
The disturbing thing I have found is that there is an overrepresentation of dark skin in images of sexually transmitted infections. So based on what you are taught, you begin to create these powerful cognitive biases in your brain as a clinician and you start to put people in categories: “This person is more likely to get this because I’ve seen a lot of photos of it.”
Take the example of a 40ish Black woman with a cough who is presumed to have sarcoidosis, because we’ve all been taught that. But what we have not been taught, at least not as definitively, is that the highest prevalence of sarcoidosis is in Nordic countries, where there are not many Black people.
So these loops teach you things that don’t necessarily represent reality. We are taught to recognize patterns, but the patterns that we’ve created are not necessarily valid and carry the biases of the people who decided they were important for us to learn.
So the underrepresentation of persons of color in images depicting skin changes in COVID-19 is, in your perspective, a continuation of this pattern of bias?
I definitely think it is. It’s important to understand the history of photography and the development of color film in order to contextualize that question. Kodak Gold was one of the first color films made. To assist photographers, the company developed a Shirley card, which could be used for color balancing in developing this film. That card, which was distributed to film development labs across the country, depicted a fair-skinned White woman as the standard for how people should appear in the photograph. Film technology was built around this idea.
As a result, people with darker skin were never portrayed accurately and did not have a lot of detail to their features or their skin. A number of famous photographers boycotted by not using the film.
But it wasn’t until chocolate manufacturers and the furniture industries decided that Kodak Gold film was not showing their brown products in enough detail that Kodak was forced to change.
It took these big industries saying “we’re not going to use your film anymore” to spur the development of multicultural Shirley cards which included images of Asian, White, and Black women (a Latina was added later). That didn’t happen until 1995. By then, digital photography had already started to take off. Unfortunately, that advance built off of the original color film and still harbored some of the same issues.
So in addition to concerns about clinicians not recognizing a skin change in a darker-skinned person, if they do recognize it and do decide to photograph it, it just might not come out right. So then it’s possible that clinicians just decide to stop taking photos.
This is another example of structural racism – things that are just baked into the system about which people are unaware. I fear we’ll continue to perpetuate these unconscious biases with the development of augmented intelligence or various algorithms.
I think that this history – which I was unaware of – highlights what happens when White skin becomes the standard. It certainly explains the issue we’ve both seen of clinicians not recognizing erythema on dark skin.
That’s a big one. And I think it’s a big one because it’s a shared presenting sign of a lot of different rashes. It can also be a sign of a dermatologic emergency that requires rapid recognition and treatment. Erythema can be very subtle, especially in skin of color. It is one of the things that we use to grade severity of psoriasis, and as a result of it not being appreciated in people with darker skin, those patients have not been included in a lot of the clinical trials.
There has even been discussion of whether erythema is something we should deem to be an important finding in people with darker skin.
Maybe one of the problems is terminology. Erythema connotes pink or red coloration, and that does not really show up on dark skin as overtly.
Exactly. Emollient use is also different in people of color. So the “classic” scales of psoriasis often aren’t present in someone who uses a lot of lotion.
In addition to the different clinical appearance of many common skin conditions, cultural practices might be different in different groups, which may also alter your differential diagnoses and treatment recommendations – for instance, moisturizer use, shampooing frequency, or use of different hair styling products.
I totally agree. Hair is another big one. Identifying broken hairs, short hairs, texture changes, and variability in the size of the hair shaft is different in coiled, Black hair and is not really applicable to people with more textured hair patterns. People of different ethnicities and cultures do different things. Standards that we traditionally use, such as the hair-pull test, don’t work quite as well in different groups.
I think that speaks to the changes that we also need to make in educating both dermatologists and nondermatologists in diagnostic criteria and clinical findings.
Dermatology is the second least diverse specialty. And that means our experts – faculty, lecturers, mentors – are not likely to be people of color. You don’t know what you don’t know. You only have your own experiences. If you don’t have people who can explain why something may be different for a different group of people, you don’t have an opportunity to broaden your understanding. You and I noticed because we know what it’s like to have this type of hair. But it’s not something that other people who don’t share the same hair type would have the same perspective on.
It is even more reason to make sure that our dermatology workforce mirrors our population. About 3% of the dermatologists in the United States are Black; the numbers are equally bad for Latino and indigenous dermatologists. If you don’t have enough people in your immediate environment who can help broaden your perspective, that becomes a problem that can harm patients.
If we don’t have a diverse group of people in the field who are contributing to the literature, changing some of the ways that we think about practice, then everyone is just going to keep doing the wrong thing.
We need to build our evidence and pay more attention to the racial breakdown of patients included in studies. We are recognizing that we have blind spots but we don’t have people or data that can change that. If what we are publishing overrepresents a certain group, how will we ever learn to do things differently?
I recognize the fact that this is not a new idea. Others have worked on these issues for a long time. Your colleague, Susan Taylor, MD, a professor at University of Pennsylvania and a founder of the Skin of Color Society, used her energy and frustration about this issue and published a whole textbook on it: “Dermatology for Skin of Color.” When I tried to publish my first paper on this issue, I got a lot of pushback, but I’m happy that now it’s something that everyone is talking about. So just in the span of a couple of years, the acceptance of this idea has changed dramatically.
I hope that this is the start of sustainable, systemic changes that can have a real impact.
Let me ask a technical question. Is the excuse of not knowing how to photograph darker skin just that – an excuse? Or is that concern truly valid?
That’s a great question. There are certain techniques that one can employ. And yes, it can be more challenging if you are not used to taking photos of people with darker skin, but it certainly can be learned.
I think that an intelligent group of people who have faced things before that felt insurmountable could continue to push to figure out how to do it. But it is something that does require skills, and part of it is because there’s this bias built into the camera, so you have to make up for that.
I think every single dermatologist has that ability, and you just have to know that it’s worthwhile to do because your patient is that valuable.
A version of this article originally appeared on Medscape.com.
A recent review looked at all articles describing skin manifestations associated with COVID-19 – 46 articles with a total of 130 clinical images – and found none that documented dermatologic conditions in people with dark skin. This was despite the disproportionate incidence of the disease in Black, Latino, and Native American/American Indian populations of color.
What’s going on? Temitayo Ogunleye, MD, an assistant professor of dermatology at the University of Pennsylvania, Philadelphia, spoke with lead investigator Jenna Lester, MD, a dermatologist and director of the Skin of Color Clinic at University of California, San Francisco, about the implications of her research.
Dr. Ogunleye: What prompted you to do this study in the first place?
Dr. Lester: It was actually driven by frustration. While we’re recognizing these COVID-related dermatologic manifestations, we’re still trying to figure out their clinical significance. We’ve learned what changes could be an early sign of infection and what might occur in people who are otherwise asymptomatic and should be tested. But in the process, we’re leaving out the group of people – dark-skinned populations – who are most heavily impacted by COVID. This is an injustice to the people who could benefit the most if found to have an early, visible sign of the disease on their skin. For example, pernio-like lesions and erythema, both of which have been seen in COVID patients, are harder to identify in darker skin.
As dermatologists, we know that the skin is the biggest organ and one that has the advantage of being visible. We partner with patients because they can see what we can see. We can explain what skin changes mean, and having examples to show them is really powerful. Because doctors typically respond best to numbers, I recognized that we needed data to prove that this lack of representation of persons of color in our COVID documentation was in fact true.
Can you tell us the key findings from your research?
We included any article that described the cutaneous manifestations of COVID – and also included a photograph – and was published over a period of 5 months. Then we categorized each image using the Fitzpatrick Skin Type Scale. We found that A handful, about 6%, depicted skin changes in patients with Fitzpatrick type IV skin.
Were you surprised by the findings?
No. I was not surprised at all, for a couple of reasons. First, many of the referrals that I had been getting to evaluate possible COVID manifestations were from primary care doctors. And as we both know, erythema, hyperpigmentation, and discoloration can be difficult for even a trained dermatologist to pick up on. So if these patients with skin changes potentially suggestive of COVID are presenting to their primary care doctor who could not determine what they were, perhaps because they had never seen them in someone with darker skin, it means those same clinicians are not likely to document these rashes. I suspect that a lot of these photos were sourced from primary care.
The other reason for my lack of surprise is that I have looked at this issue before. In a study I did in 2019 looking at images in dermatology textbooks, my coauthors and I found that there was a pretty dramatic underrepresentation of skin of color overall. Another analysis of images in core dermatology textbooks, published earlier this year by one of your colleagues at the University of Pennsylvania, Jules Lipoff, MD, showed that the percentage of images of dark skin ranged from as low as 4% to a high of about 18%.
So I wonder whether that makes us less likely to look for things in patients with darker skin, except for those conditions that we’re taught are more common in people with darker skin, like keloids, vitiligo, or certain types of hair loss. I wonder whether we just don’t think of other conditions because all the photos we ever see of psoriasis or rosacea, for example, are of people with lighter skin.
You bring up a good point about the cyclical nature of this process. If you don’t see skin conditions in darker skin, then you don’t know what to look for and so you never look for it.
Exactly. What if a Black patient says, for example: “This looks different on my skin; do you think this could be related to COVID?” And their doctor looks at their skin and says no. Not because it isn’t, but because they haven’t seen that before. Then they don’t take a picture of it and we’ll never know what that might have been.
The disturbing thing I have found is that there is an overrepresentation of dark skin in images of sexually transmitted infections. So based on what you are taught, you begin to create these powerful cognitive biases in your brain as a clinician and you start to put people in categories: “This person is more likely to get this because I’ve seen a lot of photos of it.”
Take the example of a 40ish Black woman with a cough who is presumed to have sarcoidosis, because we’ve all been taught that. But what we have not been taught, at least not as definitively, is that the highest prevalence of sarcoidosis is in Nordic countries, where there are not many Black people.
So these loops teach you things that don’t necessarily represent reality. We are taught to recognize patterns, but the patterns that we’ve created are not necessarily valid and carry the biases of the people who decided they were important for us to learn.
So the underrepresentation of persons of color in images depicting skin changes in COVID-19 is, in your perspective, a continuation of this pattern of bias?
I definitely think it is. It’s important to understand the history of photography and the development of color film in order to contextualize that question. Kodak Gold was one of the first color films made. To assist photographers, the company developed a Shirley card, which could be used for color balancing in developing this film. That card, which was distributed to film development labs across the country, depicted a fair-skinned White woman as the standard for how people should appear in the photograph. Film technology was built around this idea.
As a result, people with darker skin were never portrayed accurately and did not have a lot of detail to their features or their skin. A number of famous photographers boycotted by not using the film.
But it wasn’t until chocolate manufacturers and the furniture industries decided that Kodak Gold film was not showing their brown products in enough detail that Kodak was forced to change.
It took these big industries saying “we’re not going to use your film anymore” to spur the development of multicultural Shirley cards which included images of Asian, White, and Black women (a Latina was added later). That didn’t happen until 1995. By then, digital photography had already started to take off. Unfortunately, that advance built off of the original color film and still harbored some of the same issues.
So in addition to concerns about clinicians not recognizing a skin change in a darker-skinned person, if they do recognize it and do decide to photograph it, it just might not come out right. So then it’s possible that clinicians just decide to stop taking photos.
This is another example of structural racism – things that are just baked into the system about which people are unaware. I fear we’ll continue to perpetuate these unconscious biases with the development of augmented intelligence or various algorithms.
I think that this history – which I was unaware of – highlights what happens when White skin becomes the standard. It certainly explains the issue we’ve both seen of clinicians not recognizing erythema on dark skin.
That’s a big one. And I think it’s a big one because it’s a shared presenting sign of a lot of different rashes. It can also be a sign of a dermatologic emergency that requires rapid recognition and treatment. Erythema can be very subtle, especially in skin of color. It is one of the things that we use to grade severity of psoriasis, and as a result of it not being appreciated in people with darker skin, those patients have not been included in a lot of the clinical trials.
There has even been discussion of whether erythema is something we should deem to be an important finding in people with darker skin.
Maybe one of the problems is terminology. Erythema connotes pink or red coloration, and that does not really show up on dark skin as overtly.
Exactly. Emollient use is also different in people of color. So the “classic” scales of psoriasis often aren’t present in someone who uses a lot of lotion.
In addition to the different clinical appearance of many common skin conditions, cultural practices might be different in different groups, which may also alter your differential diagnoses and treatment recommendations – for instance, moisturizer use, shampooing frequency, or use of different hair styling products.
I totally agree. Hair is another big one. Identifying broken hairs, short hairs, texture changes, and variability in the size of the hair shaft is different in coiled, Black hair and is not really applicable to people with more textured hair patterns. People of different ethnicities and cultures do different things. Standards that we traditionally use, such as the hair-pull test, don’t work quite as well in different groups.
I think that speaks to the changes that we also need to make in educating both dermatologists and nondermatologists in diagnostic criteria and clinical findings.
Dermatology is the second least diverse specialty. And that means our experts – faculty, lecturers, mentors – are not likely to be people of color. You don’t know what you don’t know. You only have your own experiences. If you don’t have people who can explain why something may be different for a different group of people, you don’t have an opportunity to broaden your understanding. You and I noticed because we know what it’s like to have this type of hair. But it’s not something that other people who don’t share the same hair type would have the same perspective on.
It is even more reason to make sure that our dermatology workforce mirrors our population. About 3% of the dermatologists in the United States are Black; the numbers are equally bad for Latino and indigenous dermatologists. If you don’t have enough people in your immediate environment who can help broaden your perspective, that becomes a problem that can harm patients.
If we don’t have a diverse group of people in the field who are contributing to the literature, changing some of the ways that we think about practice, then everyone is just going to keep doing the wrong thing.
We need to build our evidence and pay more attention to the racial breakdown of patients included in studies. We are recognizing that we have blind spots but we don’t have people or data that can change that. If what we are publishing overrepresents a certain group, how will we ever learn to do things differently?
I recognize the fact that this is not a new idea. Others have worked on these issues for a long time. Your colleague, Susan Taylor, MD, a professor at University of Pennsylvania and a founder of the Skin of Color Society, used her energy and frustration about this issue and published a whole textbook on it: “Dermatology for Skin of Color.” When I tried to publish my first paper on this issue, I got a lot of pushback, but I’m happy that now it’s something that everyone is talking about. So just in the span of a couple of years, the acceptance of this idea has changed dramatically.
I hope that this is the start of sustainable, systemic changes that can have a real impact.
Let me ask a technical question. Is the excuse of not knowing how to photograph darker skin just that – an excuse? Or is that concern truly valid?
That’s a great question. There are certain techniques that one can employ. And yes, it can be more challenging if you are not used to taking photos of people with darker skin, but it certainly can be learned.
I think that an intelligent group of people who have faced things before that felt insurmountable could continue to push to figure out how to do it. But it is something that does require skills, and part of it is because there’s this bias built into the camera, so you have to make up for that.
I think every single dermatologist has that ability, and you just have to know that it’s worthwhile to do because your patient is that valuable.
A version of this article originally appeared on Medscape.com.
A recent review looked at all articles describing skin manifestations associated with COVID-19 – 46 articles with a total of 130 clinical images – and found none that documented dermatologic conditions in people with dark skin. This was despite the disproportionate incidence of the disease in Black, Latino, and Native American/American Indian populations of color.
What’s going on? Temitayo Ogunleye, MD, an assistant professor of dermatology at the University of Pennsylvania, Philadelphia, spoke with lead investigator Jenna Lester, MD, a dermatologist and director of the Skin of Color Clinic at University of California, San Francisco, about the implications of her research.
Dr. Ogunleye: What prompted you to do this study in the first place?
Dr. Lester: It was actually driven by frustration. While we’re recognizing these COVID-related dermatologic manifestations, we’re still trying to figure out their clinical significance. We’ve learned what changes could be an early sign of infection and what might occur in people who are otherwise asymptomatic and should be tested. But in the process, we’re leaving out the group of people – dark-skinned populations – who are most heavily impacted by COVID. This is an injustice to the people who could benefit the most if found to have an early, visible sign of the disease on their skin. For example, pernio-like lesions and erythema, both of which have been seen in COVID patients, are harder to identify in darker skin.
As dermatologists, we know that the skin is the biggest organ and one that has the advantage of being visible. We partner with patients because they can see what we can see. We can explain what skin changes mean, and having examples to show them is really powerful. Because doctors typically respond best to numbers, I recognized that we needed data to prove that this lack of representation of persons of color in our COVID documentation was in fact true.
Can you tell us the key findings from your research?
We included any article that described the cutaneous manifestations of COVID – and also included a photograph – and was published over a period of 5 months. Then we categorized each image using the Fitzpatrick Skin Type Scale. We found that A handful, about 6%, depicted skin changes in patients with Fitzpatrick type IV skin.
Were you surprised by the findings?
No. I was not surprised at all, for a couple of reasons. First, many of the referrals that I had been getting to evaluate possible COVID manifestations were from primary care doctors. And as we both know, erythema, hyperpigmentation, and discoloration can be difficult for even a trained dermatologist to pick up on. So if these patients with skin changes potentially suggestive of COVID are presenting to their primary care doctor who could not determine what they were, perhaps because they had never seen them in someone with darker skin, it means those same clinicians are not likely to document these rashes. I suspect that a lot of these photos were sourced from primary care.
The other reason for my lack of surprise is that I have looked at this issue before. In a study I did in 2019 looking at images in dermatology textbooks, my coauthors and I found that there was a pretty dramatic underrepresentation of skin of color overall. Another analysis of images in core dermatology textbooks, published earlier this year by one of your colleagues at the University of Pennsylvania, Jules Lipoff, MD, showed that the percentage of images of dark skin ranged from as low as 4% to a high of about 18%.
So I wonder whether that makes us less likely to look for things in patients with darker skin, except for those conditions that we’re taught are more common in people with darker skin, like keloids, vitiligo, or certain types of hair loss. I wonder whether we just don’t think of other conditions because all the photos we ever see of psoriasis or rosacea, for example, are of people with lighter skin.
You bring up a good point about the cyclical nature of this process. If you don’t see skin conditions in darker skin, then you don’t know what to look for and so you never look for it.
Exactly. What if a Black patient says, for example: “This looks different on my skin; do you think this could be related to COVID?” And their doctor looks at their skin and says no. Not because it isn’t, but because they haven’t seen that before. Then they don’t take a picture of it and we’ll never know what that might have been.
The disturbing thing I have found is that there is an overrepresentation of dark skin in images of sexually transmitted infections. So based on what you are taught, you begin to create these powerful cognitive biases in your brain as a clinician and you start to put people in categories: “This person is more likely to get this because I’ve seen a lot of photos of it.”
Take the example of a 40ish Black woman with a cough who is presumed to have sarcoidosis, because we’ve all been taught that. But what we have not been taught, at least not as definitively, is that the highest prevalence of sarcoidosis is in Nordic countries, where there are not many Black people.
So these loops teach you things that don’t necessarily represent reality. We are taught to recognize patterns, but the patterns that we’ve created are not necessarily valid and carry the biases of the people who decided they were important for us to learn.
So the underrepresentation of persons of color in images depicting skin changes in COVID-19 is, in your perspective, a continuation of this pattern of bias?
I definitely think it is. It’s important to understand the history of photography and the development of color film in order to contextualize that question. Kodak Gold was one of the first color films made. To assist photographers, the company developed a Shirley card, which could be used for color balancing in developing this film. That card, which was distributed to film development labs across the country, depicted a fair-skinned White woman as the standard for how people should appear in the photograph. Film technology was built around this idea.
As a result, people with darker skin were never portrayed accurately and did not have a lot of detail to their features or their skin. A number of famous photographers boycotted by not using the film.
But it wasn’t until chocolate manufacturers and the furniture industries decided that Kodak Gold film was not showing their brown products in enough detail that Kodak was forced to change.
It took these big industries saying “we’re not going to use your film anymore” to spur the development of multicultural Shirley cards which included images of Asian, White, and Black women (a Latina was added later). That didn’t happen until 1995. By then, digital photography had already started to take off. Unfortunately, that advance built off of the original color film and still harbored some of the same issues.
So in addition to concerns about clinicians not recognizing a skin change in a darker-skinned person, if they do recognize it and do decide to photograph it, it just might not come out right. So then it’s possible that clinicians just decide to stop taking photos.
This is another example of structural racism – things that are just baked into the system about which people are unaware. I fear we’ll continue to perpetuate these unconscious biases with the development of augmented intelligence or various algorithms.
I think that this history – which I was unaware of – highlights what happens when White skin becomes the standard. It certainly explains the issue we’ve both seen of clinicians not recognizing erythema on dark skin.
That’s a big one. And I think it’s a big one because it’s a shared presenting sign of a lot of different rashes. It can also be a sign of a dermatologic emergency that requires rapid recognition and treatment. Erythema can be very subtle, especially in skin of color. It is one of the things that we use to grade severity of psoriasis, and as a result of it not being appreciated in people with darker skin, those patients have not been included in a lot of the clinical trials.
There has even been discussion of whether erythema is something we should deem to be an important finding in people with darker skin.
Maybe one of the problems is terminology. Erythema connotes pink or red coloration, and that does not really show up on dark skin as overtly.
Exactly. Emollient use is also different in people of color. So the “classic” scales of psoriasis often aren’t present in someone who uses a lot of lotion.
In addition to the different clinical appearance of many common skin conditions, cultural practices might be different in different groups, which may also alter your differential diagnoses and treatment recommendations – for instance, moisturizer use, shampooing frequency, or use of different hair styling products.
I totally agree. Hair is another big one. Identifying broken hairs, short hairs, texture changes, and variability in the size of the hair shaft is different in coiled, Black hair and is not really applicable to people with more textured hair patterns. People of different ethnicities and cultures do different things. Standards that we traditionally use, such as the hair-pull test, don’t work quite as well in different groups.
I think that speaks to the changes that we also need to make in educating both dermatologists and nondermatologists in diagnostic criteria and clinical findings.
Dermatology is the second least diverse specialty. And that means our experts – faculty, lecturers, mentors – are not likely to be people of color. You don’t know what you don’t know. You only have your own experiences. If you don’t have people who can explain why something may be different for a different group of people, you don’t have an opportunity to broaden your understanding. You and I noticed because we know what it’s like to have this type of hair. But it’s not something that other people who don’t share the same hair type would have the same perspective on.
It is even more reason to make sure that our dermatology workforce mirrors our population. About 3% of the dermatologists in the United States are Black; the numbers are equally bad for Latino and indigenous dermatologists. If you don’t have enough people in your immediate environment who can help broaden your perspective, that becomes a problem that can harm patients.
If we don’t have a diverse group of people in the field who are contributing to the literature, changing some of the ways that we think about practice, then everyone is just going to keep doing the wrong thing.
We need to build our evidence and pay more attention to the racial breakdown of patients included in studies. We are recognizing that we have blind spots but we don’t have people or data that can change that. If what we are publishing overrepresents a certain group, how will we ever learn to do things differently?
I recognize the fact that this is not a new idea. Others have worked on these issues for a long time. Your colleague, Susan Taylor, MD, a professor at University of Pennsylvania and a founder of the Skin of Color Society, used her energy and frustration about this issue and published a whole textbook on it: “Dermatology for Skin of Color.” When I tried to publish my first paper on this issue, I got a lot of pushback, but I’m happy that now it’s something that everyone is talking about. So just in the span of a couple of years, the acceptance of this idea has changed dramatically.
I hope that this is the start of sustainable, systemic changes that can have a real impact.
Let me ask a technical question. Is the excuse of not knowing how to photograph darker skin just that – an excuse? Or is that concern truly valid?
That’s a great question. There are certain techniques that one can employ. And yes, it can be more challenging if you are not used to taking photos of people with darker skin, but it certainly can be learned.
I think that an intelligent group of people who have faced things before that felt insurmountable could continue to push to figure out how to do it. But it is something that does require skills, and part of it is because there’s this bias built into the camera, so you have to make up for that.
I think every single dermatologist has that ability, and you just have to know that it’s worthwhile to do because your patient is that valuable.
A version of this article originally appeared on Medscape.com.