User login
No weight gain or sexual dysfunction with first nonhormonal treatment for hot flashes
“Since the 2002 publication of initial findings of the [Women’s Health Initiative], women and clinicians have been much more interested in nonhormonal treatment options for moderate to severe vasomotor symptoms,” said Andrew M. Kauntiz, MD, at NAMS 2013.
“Because of this interest, we have seen extensive trials of SSRIs [selective serotonin reuptake inhibitors] and SNRIs [selective norepinephrin reuptake inhibitors] for the treatment of bothersome hot flashes. We recognize that these agents do have efficacy greater than placebo in the treatment of these distressing symptoms among menopausal women. However, a concern among women and their providers has been that SSRIs and SNRIs can cause unwanted weight gain as well as sexual side effects,” Dr. Kaunitz said.
Low-dose mesylate salt of paroxetine (Brisdelle), an SSRI, was FDA-approved in June 2013 as 7.5-mg paroxetine tablets—the first nonhormonal treatment for moderate to severe vasomotor symptoms associated with menopause.
Dr. Kaunitz and colleagues pooled the data from two Phase 3 randomized, double-blind, placebo-controlled trials that demonstrated reduced frequency and severity of vasomotor symptoms and favorable tolerability with the 7.5-mg paroxetine formulation.
They found no clinically meaningful or statistically significant changes from baseline in weight or sexual function among the paroxetine group (median weight 74.5 kg) versus placebo (75.8 kg). The median body mass index (BMI) was 27.9 kg/m2 among women using paroxetine versus 28.2 kg/m2 in the placebo group. The Arizona Sexual Experience Scale (ASEX) score was 59% in the paroxetine group versus 58% in the placebo group.
In addition, no significant difference between treatment groups was observed in the proportion of patients who had a gain in body weight of 7% or greater at weeks 4, 12, or 24. The rates of adverse events suggestive of sexual dysfunction were low and were similar in both treatment groups.
“What’s encouraging with our clinical trial findings is that it is the first nonhormonal treatment for menopausal vasomotor symptoms and does not cause weight gain or sexual side effects," said Dr. Kaunitz.
Listen to Dr. Kaunitz’s round-up of this new drug:
“Since the 2002 publication of initial findings of the [Women’s Health Initiative], women and clinicians have been much more interested in nonhormonal treatment options for moderate to severe vasomotor symptoms,” said Andrew M. Kauntiz, MD, at NAMS 2013.
“Because of this interest, we have seen extensive trials of SSRIs [selective serotonin reuptake inhibitors] and SNRIs [selective norepinephrin reuptake inhibitors] for the treatment of bothersome hot flashes. We recognize that these agents do have efficacy greater than placebo in the treatment of these distressing symptoms among menopausal women. However, a concern among women and their providers has been that SSRIs and SNRIs can cause unwanted weight gain as well as sexual side effects,” Dr. Kaunitz said.
Low-dose mesylate salt of paroxetine (Brisdelle), an SSRI, was FDA-approved in June 2013 as 7.5-mg paroxetine tablets—the first nonhormonal treatment for moderate to severe vasomotor symptoms associated with menopause.
Dr. Kaunitz and colleagues pooled the data from two Phase 3 randomized, double-blind, placebo-controlled trials that demonstrated reduced frequency and severity of vasomotor symptoms and favorable tolerability with the 7.5-mg paroxetine formulation.
They found no clinically meaningful or statistically significant changes from baseline in weight or sexual function among the paroxetine group (median weight 74.5 kg) versus placebo (75.8 kg). The median body mass index (BMI) was 27.9 kg/m2 among women using paroxetine versus 28.2 kg/m2 in the placebo group. The Arizona Sexual Experience Scale (ASEX) score was 59% in the paroxetine group versus 58% in the placebo group.
In addition, no significant difference between treatment groups was observed in the proportion of patients who had a gain in body weight of 7% or greater at weeks 4, 12, or 24. The rates of adverse events suggestive of sexual dysfunction were low and were similar in both treatment groups.
“What’s encouraging with our clinical trial findings is that it is the first nonhormonal treatment for menopausal vasomotor symptoms and does not cause weight gain or sexual side effects," said Dr. Kaunitz.
Listen to Dr. Kaunitz’s round-up of this new drug:
“Since the 2002 publication of initial findings of the [Women’s Health Initiative], women and clinicians have been much more interested in nonhormonal treatment options for moderate to severe vasomotor symptoms,” said Andrew M. Kauntiz, MD, at NAMS 2013.
“Because of this interest, we have seen extensive trials of SSRIs [selective serotonin reuptake inhibitors] and SNRIs [selective norepinephrin reuptake inhibitors] for the treatment of bothersome hot flashes. We recognize that these agents do have efficacy greater than placebo in the treatment of these distressing symptoms among menopausal women. However, a concern among women and their providers has been that SSRIs and SNRIs can cause unwanted weight gain as well as sexual side effects,” Dr. Kaunitz said.
Low-dose mesylate salt of paroxetine (Brisdelle), an SSRI, was FDA-approved in June 2013 as 7.5-mg paroxetine tablets—the first nonhormonal treatment for moderate to severe vasomotor symptoms associated with menopause.
Dr. Kaunitz and colleagues pooled the data from two Phase 3 randomized, double-blind, placebo-controlled trials that demonstrated reduced frequency and severity of vasomotor symptoms and favorable tolerability with the 7.5-mg paroxetine formulation.
They found no clinically meaningful or statistically significant changes from baseline in weight or sexual function among the paroxetine group (median weight 74.5 kg) versus placebo (75.8 kg). The median body mass index (BMI) was 27.9 kg/m2 among women using paroxetine versus 28.2 kg/m2 in the placebo group. The Arizona Sexual Experience Scale (ASEX) score was 59% in the paroxetine group versus 58% in the placebo group.
In addition, no significant difference between treatment groups was observed in the proportion of patients who had a gain in body weight of 7% or greater at weeks 4, 12, or 24. The rates of adverse events suggestive of sexual dysfunction were low and were similar in both treatment groups.
“What’s encouraging with our clinical trial findings is that it is the first nonhormonal treatment for menopausal vasomotor symptoms and does not cause weight gain or sexual side effects," said Dr. Kaunitz.
Listen to Dr. Kaunitz’s round-up of this new drug:
Ospemifene for dyspareunia in postmenopausal women is well tolerated
Oral ospemifene (Osphena) received FDA approval in February 2013 to treat moderate to severe dyspareunia that results from vulval and vaginal atrophy. This drug, a tissue-selective estrogen agonist/antagonist, is the first oral alternative to estrogen therapy, said Steven R. Goldstein, MD, who presented data on 12- and 52-week safety of ospemifene in Phase 2/3 placebo-controlled clinical trials at NAMS 2013.
Click on the audio player below to listen to Dr. Goldstein explain the available options for vulvar and vaginal atrophy and how oral ospemifene expands these options. In addition, he describes the tolerability and side effects of ospemifene that clinicians should be aware of.
Oral ospemifene (Osphena) received FDA approval in February 2013 to treat moderate to severe dyspareunia that results from vulval and vaginal atrophy. This drug, a tissue-selective estrogen agonist/antagonist, is the first oral alternative to estrogen therapy, said Steven R. Goldstein, MD, who presented data on 12- and 52-week safety of ospemifene in Phase 2/3 placebo-controlled clinical trials at NAMS 2013.
Click on the audio player below to listen to Dr. Goldstein explain the available options for vulvar and vaginal atrophy and how oral ospemifene expands these options. In addition, he describes the tolerability and side effects of ospemifene that clinicians should be aware of.
Oral ospemifene (Osphena) received FDA approval in February 2013 to treat moderate to severe dyspareunia that results from vulval and vaginal atrophy. This drug, a tissue-selective estrogen agonist/antagonist, is the first oral alternative to estrogen therapy, said Steven R. Goldstein, MD, who presented data on 12- and 52-week safety of ospemifene in Phase 2/3 placebo-controlled clinical trials at NAMS 2013.
Click on the audio player below to listen to Dr. Goldstein explain the available options for vulvar and vaginal atrophy and how oral ospemifene expands these options. In addition, he describes the tolerability and side effects of ospemifene that clinicians should be aware of.
Follow-up bone mineral density didn’t sharpen fracture risk assessment
Performing a second bone mineral density measurement 4 years after an initial measurement was "of little value" in refining the estimation of fracture risk in osteoporosis, according to a report published online Sept. 24 in JAMA.
The finding calls into question the current practice of performing serial bone mineral density (BMD) tests at even shorter 2-year intervals to enhance fracture risk assessment, the study investigators noted.
In a secondary analysis of data from the Framingham Osteoporosis Study, BMD change during a 4-year interval "provided little additional information beyond baseline BMD for the clinical management of osteoporosis," said Dr. Sarah D. Berry of the Institute for Aging Research, Hebrew SeniorLife, Boston, and her associates (JAMA 2013;310:1256-62).
The appropriate interval between BMD assessments remains controversial, and studies of the issue have yielded mixed results.
Studies that reported a strong association between BMD loss during a short interval and subsequent fractures typically focused on the small subgroup of patients who had accelerated bone deterioration. Other studies primarily involving patients with low or normal bone loss have reported only a weak association between BMD loss and later fractures, the investigators said.
To determine whether repeat BMD testing is useful, Dr. Berry and her colleagues examined the rate of hip and major osteoporotic fracture among 310 men and 492 women in the prospective, population-based Framingham cohort.
The study patients underwent an initial BMD test of the femoral neck in 1987-1999, at a mean age of 74.8 years. None was receiving treatment for osteoporosis at that time. The patients then had at least one repeat BMD test a mean of 3.7 years later (range, 2.4-6.0 years later). The study participants then were followed for approximately 12 years or until they died.
During follow-up, 113 study patients (14.1%) had one or more major osteoporotic fractures. There were 88 hip, 24 spine, 5 shoulder, and 33 forearm fractures.
BMD loss during the interval between the first and second BMD assessments was associated with subsequent fracture. However, assessment of such loss provided little clinical value beyond that of the initial BMD test.
"The second BMD measure resulted in a small proportion of individuals [being] reclassified as [at] high risk of hip or major osteoporotic fracture," Dr. Berry and her associates said. But it remains unclear whether such a small number of reclassifications justifies the current practice of performing repeat BMD tests every 2 years, they added.
"We conclude that repeating a BMD test after 4 years would rarely change the clinical management of osteoporosis based on risk scores of hip fracture," the researchers explained. "Individuals with the greatest changes in risk scores were those who would have already been classified at high risk based on [the initial] BMD and [their] clinical characteristics."
Although some experts suggest that short rescreening intervals are warranted in high-risk patients, "we found no difference in the association between BMD change and fracture when stratified by sex, age, BMI, weight loss, T score, or fracture risk score," the study authors noted.
"Despite our findings, we recognize that detecting BMD loss would have been paramount for the small numbers of individuals reclassified by a second BMD test who went on to experience a fracture," Dr. Berry and her colleagues said. For those patients, a repeat screening test would allow physicians to give osteoporosis medications and reduce fracture risk, even among patients 75 years or older.
However, for the clear majority of patients who show normal or only mild bone loss at an initial screening, further study is needed to predict which patients are likely to transition to high risk of fracture and would therefore benefit from repeat BMD testing.
The study was limited in that almost all the patients were white. The usefulness of repeated BMD screening might be different in other racial and ethnic populations, the investigators said.
The National Institutes of Health, the National Heart, Lung, and Blood Institute, and the Friends of Hebrew SeniorLife supported the study. Dr. Berry reported no relevant financial conflicts of interest; one of her associates reported ties to Amgen, Ammonett Pharma, Eli Lilly, Hologic, Merck Sharpe & Dohme, Novartis, and Roche.
Performing a second bone mineral density measurement 4 years after an initial measurement was "of little value" in refining the estimation of fracture risk in osteoporosis, according to a report published online Sept. 24 in JAMA.
The finding calls into question the current practice of performing serial bone mineral density (BMD) tests at even shorter 2-year intervals to enhance fracture risk assessment, the study investigators noted.
In a secondary analysis of data from the Framingham Osteoporosis Study, BMD change during a 4-year interval "provided little additional information beyond baseline BMD for the clinical management of osteoporosis," said Dr. Sarah D. Berry of the Institute for Aging Research, Hebrew SeniorLife, Boston, and her associates (JAMA 2013;310:1256-62).
The appropriate interval between BMD assessments remains controversial, and studies of the issue have yielded mixed results.
Studies that reported a strong association between BMD loss during a short interval and subsequent fractures typically focused on the small subgroup of patients who had accelerated bone deterioration. Other studies primarily involving patients with low or normal bone loss have reported only a weak association between BMD loss and later fractures, the investigators said.
To determine whether repeat BMD testing is useful, Dr. Berry and her colleagues examined the rate of hip and major osteoporotic fracture among 310 men and 492 women in the prospective, population-based Framingham cohort.
The study patients underwent an initial BMD test of the femoral neck in 1987-1999, at a mean age of 74.8 years. None was receiving treatment for osteoporosis at that time. The patients then had at least one repeat BMD test a mean of 3.7 years later (range, 2.4-6.0 years later). The study participants then were followed for approximately 12 years or until they died.
During follow-up, 113 study patients (14.1%) had one or more major osteoporotic fractures. There were 88 hip, 24 spine, 5 shoulder, and 33 forearm fractures.
BMD loss during the interval between the first and second BMD assessments was associated with subsequent fracture. However, assessment of such loss provided little clinical value beyond that of the initial BMD test.
"The second BMD measure resulted in a small proportion of individuals [being] reclassified as [at] high risk of hip or major osteoporotic fracture," Dr. Berry and her associates said. But it remains unclear whether such a small number of reclassifications justifies the current practice of performing repeat BMD tests every 2 years, they added.
"We conclude that repeating a BMD test after 4 years would rarely change the clinical management of osteoporosis based on risk scores of hip fracture," the researchers explained. "Individuals with the greatest changes in risk scores were those who would have already been classified at high risk based on [the initial] BMD and [their] clinical characteristics."
Although some experts suggest that short rescreening intervals are warranted in high-risk patients, "we found no difference in the association between BMD change and fracture when stratified by sex, age, BMI, weight loss, T score, or fracture risk score," the study authors noted.
"Despite our findings, we recognize that detecting BMD loss would have been paramount for the small numbers of individuals reclassified by a second BMD test who went on to experience a fracture," Dr. Berry and her colleagues said. For those patients, a repeat screening test would allow physicians to give osteoporosis medications and reduce fracture risk, even among patients 75 years or older.
However, for the clear majority of patients who show normal or only mild bone loss at an initial screening, further study is needed to predict which patients are likely to transition to high risk of fracture and would therefore benefit from repeat BMD testing.
The study was limited in that almost all the patients were white. The usefulness of repeated BMD screening might be different in other racial and ethnic populations, the investigators said.
The National Institutes of Health, the National Heart, Lung, and Blood Institute, and the Friends of Hebrew SeniorLife supported the study. Dr. Berry reported no relevant financial conflicts of interest; one of her associates reported ties to Amgen, Ammonett Pharma, Eli Lilly, Hologic, Merck Sharpe & Dohme, Novartis, and Roche.
Performing a second bone mineral density measurement 4 years after an initial measurement was "of little value" in refining the estimation of fracture risk in osteoporosis, according to a report published online Sept. 24 in JAMA.
The finding calls into question the current practice of performing serial bone mineral density (BMD) tests at even shorter 2-year intervals to enhance fracture risk assessment, the study investigators noted.
In a secondary analysis of data from the Framingham Osteoporosis Study, BMD change during a 4-year interval "provided little additional information beyond baseline BMD for the clinical management of osteoporosis," said Dr. Sarah D. Berry of the Institute for Aging Research, Hebrew SeniorLife, Boston, and her associates (JAMA 2013;310:1256-62).
The appropriate interval between BMD assessments remains controversial, and studies of the issue have yielded mixed results.
Studies that reported a strong association between BMD loss during a short interval and subsequent fractures typically focused on the small subgroup of patients who had accelerated bone deterioration. Other studies primarily involving patients with low or normal bone loss have reported only a weak association between BMD loss and later fractures, the investigators said.
To determine whether repeat BMD testing is useful, Dr. Berry and her colleagues examined the rate of hip and major osteoporotic fracture among 310 men and 492 women in the prospective, population-based Framingham cohort.
The study patients underwent an initial BMD test of the femoral neck in 1987-1999, at a mean age of 74.8 years. None was receiving treatment for osteoporosis at that time. The patients then had at least one repeat BMD test a mean of 3.7 years later (range, 2.4-6.0 years later). The study participants then were followed for approximately 12 years or until they died.
During follow-up, 113 study patients (14.1%) had one or more major osteoporotic fractures. There were 88 hip, 24 spine, 5 shoulder, and 33 forearm fractures.
BMD loss during the interval between the first and second BMD assessments was associated with subsequent fracture. However, assessment of such loss provided little clinical value beyond that of the initial BMD test.
"The second BMD measure resulted in a small proportion of individuals [being] reclassified as [at] high risk of hip or major osteoporotic fracture," Dr. Berry and her associates said. But it remains unclear whether such a small number of reclassifications justifies the current practice of performing repeat BMD tests every 2 years, they added.
"We conclude that repeating a BMD test after 4 years would rarely change the clinical management of osteoporosis based on risk scores of hip fracture," the researchers explained. "Individuals with the greatest changes in risk scores were those who would have already been classified at high risk based on [the initial] BMD and [their] clinical characteristics."
Although some experts suggest that short rescreening intervals are warranted in high-risk patients, "we found no difference in the association between BMD change and fracture when stratified by sex, age, BMI, weight loss, T score, or fracture risk score," the study authors noted.
"Despite our findings, we recognize that detecting BMD loss would have been paramount for the small numbers of individuals reclassified by a second BMD test who went on to experience a fracture," Dr. Berry and her colleagues said. For those patients, a repeat screening test would allow physicians to give osteoporosis medications and reduce fracture risk, even among patients 75 years or older.
However, for the clear majority of patients who show normal or only mild bone loss at an initial screening, further study is needed to predict which patients are likely to transition to high risk of fracture and would therefore benefit from repeat BMD testing.
The study was limited in that almost all the patients were white. The usefulness of repeated BMD screening might be different in other racial and ethnic populations, the investigators said.
The National Institutes of Health, the National Heart, Lung, and Blood Institute, and the Friends of Hebrew SeniorLife supported the study. Dr. Berry reported no relevant financial conflicts of interest; one of her associates reported ties to Amgen, Ammonett Pharma, Eli Lilly, Hologic, Merck Sharpe & Dohme, Novartis, and Roche.
FROM JAMA
Major finding: A second bone mineral density test 4 years after an initial test was not useful in refining the prediction of major fractures and didn't change the clinical management of osteoporosis.
Data source: A secondary analysis of data from the prospective, population-based Framingham Osteoporosis Study involving 802 older men and women who underwent serial BMD testing and were followed for 12 years.
Disclosures: The National Institutes of Health, the National Heart, Lung, and Blood Institute, and the Friends of Hebrew SeniorLife supported the study. Dr. Berry reported no relevant financial conflicts of interest; one of her associates reported ties to Amgen, Ammonett Pharma, Eli Lilly, Hologic, Merck Sharpe & Dohme, Novartis, and Roche.
Female hair loss differs by age
SAN FRANCISCO – Three distinct stages of pattern hair loss in women are related to the age of onset, and are not necessarily androgen related.
Between puberty and age 40 years, hair miniaturization in females tends to be caused by androgenetic alopecia, a common hereditary thinning or balding induced by androgens in genetically susceptible people of both sexes. By contrast, women in their 60s or older may develop hair thinning from age-related, or "senescent" alopecia, which is distinct from androgenetic alopecia because senescent alopecia is not mediated by dihydrotestosterone, Dr. Vera H. Price said at the annual meeting of the Pacific Dermatologic Association.
However, a newly identified stage that often occurs between 45 and 55 years of age is gaining popularity in the lexicon of hair loss. In this stage, called "female pattern hair loss," the role of androgens is less clear-cut, and other hormonal and nonhormonal factors may play a role, said Dr. Price, professor of dermatology at the University of California, San Francisco.
All three stages show similar histopathology, with follicular downsizing, normal sebaceous glands, and no significant inflammation. However, recognizing and understanding the three stages help inform management, said Dr. Price.
Treatment with minoxidil is suitable for all three stages of hair loss, for example, but androgen blockade via off-label treatment with finasteride is not helpful in senescent alopecia. "I don’t use it after 60 years of age in women or men," she said.
Millions of men who have taken finasteride 5 mg/day for benign prostatic hypertrophy have not regrown scalp hair, evidence that senescent alopecia is not dihydrotestosterone mediated, she noted.
• Androgenetic alopecia. To screen for suspected androgenetic alopecia in women, check for menstrual irregularities, infertility, hirsutism, severe cystic acne, galactorrhea, and virilization. "If none are present, you do not have to do a single hormonal test" because it’s not androgenetic alopecia causing the hair loss, Dr. Price said.
If any one of those conditions is present, however, check levels of testosterone, dehydroepiandrosterone sulfate (DHEAS), and prolactin.
In all hair loss patients, get a complete blood count and check for normal levels of thyroid-stimulating hormone (TSH), ferritin, and 25-hydroxyvitamin D; the latter two are required for a normal hair cycle. Be sure to order the ferritin level specifically, because ordering "iron studies" won’t include a ferritin level, she added.
• Female pattern hair loss. The pathogenesis behind female pattern hair loss is not well understood. As women go through menopause, hair growth parameters change. The hair growth rate slows, the anagen/telogen ratio decreases, and hair diameters become smaller, Dr. Price explained.
Hormonal and molecular factors that may influence female pattern hair loss need to be better defined, including the possible roles of estrogen, estrogen receptor (ER)-beta, aromatase, 5-alpha-reductase, dihydrotestosterone, and androgen receptors, she said.
"When we understand that, we’ll understand this group a little more clearly," she added. "I’ve always been puzzled a little bit when the onset is in this age group."
• Senescent alopecia. "I call it ‘wisdom-related’ alopecia," quipped Dr. Price. The gene expression profiles of androgenetic alopecia and senescent alopecia differ. In the former, hair growth cycle genes are differentially expressed. In the latter, systemic senescent/aging genes are differentially expressed, suggesting these are two distinct disorders.
• Management. For any stage of hair loss, minoxidil (Rogaine) may help if used properly over an extended period of time, said Dr. Price. Apply the 2% or 5% solution every single day directly to a dry scalp, not right after a shower, and spread it gently across the scalp, she advised. Give minoxidil time to absorb without spraying, moussing, or blow-drying the hair while it absorbs. Once-daily minoxidil foam 5% also has been approved for women and is less oily and absorbed faster.
Of note, androgen blockade via off-label oral finasteride 1 mg/day for confirmed androgenetic alopecia is contraindicated in women who may be or may become pregnant because it will cause hypospadias in a male fetus, said Dr. Price. Prescribe concurrent oral contraception in premenopausal women or make sure the woman is postmenopausal when using finasteride.
"Do I use it in women? I do, but I’m very careful," she said. "They have to be post hysterectomy or post tubal ligation. I want to be certain that they’re not going to conceive."
In appropriate patients, minoxidil and finasteride could be used together if the patient can afford it, she added.
Spironolactone, an androgen receptor inhibitor, also has been used in a dose of 200 mg/day to retard hair thinning due to androgenetic alopecia. Data show that spironolactone 50-200 mg/day can be used successfully to treat acne and hirsutism, but there are no evidence-based studies showing that it helps hair regrowth.
"I use very little spironolactone for androgenetic alopecia," Dr. Price said. "It will not grow hair. I think it’s used a lot because people aren’t familiar with minoxidil or don’t know about using finasteride if there’s no possibility of pregnancy."
Dr. Price reported having financial associations with Allergan and Follica, neither of which was pertinent to this topic.
On Twitter @sherryboschert
SAN FRANCISCO – Three distinct stages of pattern hair loss in women are related to the age of onset, and are not necessarily androgen related.
Between puberty and age 40 years, hair miniaturization in females tends to be caused by androgenetic alopecia, a common hereditary thinning or balding induced by androgens in genetically susceptible people of both sexes. By contrast, women in their 60s or older may develop hair thinning from age-related, or "senescent" alopecia, which is distinct from androgenetic alopecia because senescent alopecia is not mediated by dihydrotestosterone, Dr. Vera H. Price said at the annual meeting of the Pacific Dermatologic Association.
However, a newly identified stage that often occurs between 45 and 55 years of age is gaining popularity in the lexicon of hair loss. In this stage, called "female pattern hair loss," the role of androgens is less clear-cut, and other hormonal and nonhormonal factors may play a role, said Dr. Price, professor of dermatology at the University of California, San Francisco.
All three stages show similar histopathology, with follicular downsizing, normal sebaceous glands, and no significant inflammation. However, recognizing and understanding the three stages help inform management, said Dr. Price.
Treatment with minoxidil is suitable for all three stages of hair loss, for example, but androgen blockade via off-label treatment with finasteride is not helpful in senescent alopecia. "I don’t use it after 60 years of age in women or men," she said.
Millions of men who have taken finasteride 5 mg/day for benign prostatic hypertrophy have not regrown scalp hair, evidence that senescent alopecia is not dihydrotestosterone mediated, she noted.
• Androgenetic alopecia. To screen for suspected androgenetic alopecia in women, check for menstrual irregularities, infertility, hirsutism, severe cystic acne, galactorrhea, and virilization. "If none are present, you do not have to do a single hormonal test" because it’s not androgenetic alopecia causing the hair loss, Dr. Price said.
If any one of those conditions is present, however, check levels of testosterone, dehydroepiandrosterone sulfate (DHEAS), and prolactin.
In all hair loss patients, get a complete blood count and check for normal levels of thyroid-stimulating hormone (TSH), ferritin, and 25-hydroxyvitamin D; the latter two are required for a normal hair cycle. Be sure to order the ferritin level specifically, because ordering "iron studies" won’t include a ferritin level, she added.
• Female pattern hair loss. The pathogenesis behind female pattern hair loss is not well understood. As women go through menopause, hair growth parameters change. The hair growth rate slows, the anagen/telogen ratio decreases, and hair diameters become smaller, Dr. Price explained.
Hormonal and molecular factors that may influence female pattern hair loss need to be better defined, including the possible roles of estrogen, estrogen receptor (ER)-beta, aromatase, 5-alpha-reductase, dihydrotestosterone, and androgen receptors, she said.
"When we understand that, we’ll understand this group a little more clearly," she added. "I’ve always been puzzled a little bit when the onset is in this age group."
• Senescent alopecia. "I call it ‘wisdom-related’ alopecia," quipped Dr. Price. The gene expression profiles of androgenetic alopecia and senescent alopecia differ. In the former, hair growth cycle genes are differentially expressed. In the latter, systemic senescent/aging genes are differentially expressed, suggesting these are two distinct disorders.
• Management. For any stage of hair loss, minoxidil (Rogaine) may help if used properly over an extended period of time, said Dr. Price. Apply the 2% or 5% solution every single day directly to a dry scalp, not right after a shower, and spread it gently across the scalp, she advised. Give minoxidil time to absorb without spraying, moussing, or blow-drying the hair while it absorbs. Once-daily minoxidil foam 5% also has been approved for women and is less oily and absorbed faster.
Of note, androgen blockade via off-label oral finasteride 1 mg/day for confirmed androgenetic alopecia is contraindicated in women who may be or may become pregnant because it will cause hypospadias in a male fetus, said Dr. Price. Prescribe concurrent oral contraception in premenopausal women or make sure the woman is postmenopausal when using finasteride.
"Do I use it in women? I do, but I’m very careful," she said. "They have to be post hysterectomy or post tubal ligation. I want to be certain that they’re not going to conceive."
In appropriate patients, minoxidil and finasteride could be used together if the patient can afford it, she added.
Spironolactone, an androgen receptor inhibitor, also has been used in a dose of 200 mg/day to retard hair thinning due to androgenetic alopecia. Data show that spironolactone 50-200 mg/day can be used successfully to treat acne and hirsutism, but there are no evidence-based studies showing that it helps hair regrowth.
"I use very little spironolactone for androgenetic alopecia," Dr. Price said. "It will not grow hair. I think it’s used a lot because people aren’t familiar with minoxidil or don’t know about using finasteride if there’s no possibility of pregnancy."
Dr. Price reported having financial associations with Allergan and Follica, neither of which was pertinent to this topic.
On Twitter @sherryboschert
SAN FRANCISCO – Three distinct stages of pattern hair loss in women are related to the age of onset, and are not necessarily androgen related.
Between puberty and age 40 years, hair miniaturization in females tends to be caused by androgenetic alopecia, a common hereditary thinning or balding induced by androgens in genetically susceptible people of both sexes. By contrast, women in their 60s or older may develop hair thinning from age-related, or "senescent" alopecia, which is distinct from androgenetic alopecia because senescent alopecia is not mediated by dihydrotestosterone, Dr. Vera H. Price said at the annual meeting of the Pacific Dermatologic Association.
However, a newly identified stage that often occurs between 45 and 55 years of age is gaining popularity in the lexicon of hair loss. In this stage, called "female pattern hair loss," the role of androgens is less clear-cut, and other hormonal and nonhormonal factors may play a role, said Dr. Price, professor of dermatology at the University of California, San Francisco.
All three stages show similar histopathology, with follicular downsizing, normal sebaceous glands, and no significant inflammation. However, recognizing and understanding the three stages help inform management, said Dr. Price.
Treatment with minoxidil is suitable for all three stages of hair loss, for example, but androgen blockade via off-label treatment with finasteride is not helpful in senescent alopecia. "I don’t use it after 60 years of age in women or men," she said.
Millions of men who have taken finasteride 5 mg/day for benign prostatic hypertrophy have not regrown scalp hair, evidence that senescent alopecia is not dihydrotestosterone mediated, she noted.
• Androgenetic alopecia. To screen for suspected androgenetic alopecia in women, check for menstrual irregularities, infertility, hirsutism, severe cystic acne, galactorrhea, and virilization. "If none are present, you do not have to do a single hormonal test" because it’s not androgenetic alopecia causing the hair loss, Dr. Price said.
If any one of those conditions is present, however, check levels of testosterone, dehydroepiandrosterone sulfate (DHEAS), and prolactin.
In all hair loss patients, get a complete blood count and check for normal levels of thyroid-stimulating hormone (TSH), ferritin, and 25-hydroxyvitamin D; the latter two are required for a normal hair cycle. Be sure to order the ferritin level specifically, because ordering "iron studies" won’t include a ferritin level, she added.
• Female pattern hair loss. The pathogenesis behind female pattern hair loss is not well understood. As women go through menopause, hair growth parameters change. The hair growth rate slows, the anagen/telogen ratio decreases, and hair diameters become smaller, Dr. Price explained.
Hormonal and molecular factors that may influence female pattern hair loss need to be better defined, including the possible roles of estrogen, estrogen receptor (ER)-beta, aromatase, 5-alpha-reductase, dihydrotestosterone, and androgen receptors, she said.
"When we understand that, we’ll understand this group a little more clearly," she added. "I’ve always been puzzled a little bit when the onset is in this age group."
• Senescent alopecia. "I call it ‘wisdom-related’ alopecia," quipped Dr. Price. The gene expression profiles of androgenetic alopecia and senescent alopecia differ. In the former, hair growth cycle genes are differentially expressed. In the latter, systemic senescent/aging genes are differentially expressed, suggesting these are two distinct disorders.
• Management. For any stage of hair loss, minoxidil (Rogaine) may help if used properly over an extended period of time, said Dr. Price. Apply the 2% or 5% solution every single day directly to a dry scalp, not right after a shower, and spread it gently across the scalp, she advised. Give minoxidil time to absorb without spraying, moussing, or blow-drying the hair while it absorbs. Once-daily minoxidil foam 5% also has been approved for women and is less oily and absorbed faster.
Of note, androgen blockade via off-label oral finasteride 1 mg/day for confirmed androgenetic alopecia is contraindicated in women who may be or may become pregnant because it will cause hypospadias in a male fetus, said Dr. Price. Prescribe concurrent oral contraception in premenopausal women or make sure the woman is postmenopausal when using finasteride.
"Do I use it in women? I do, but I’m very careful," she said. "They have to be post hysterectomy or post tubal ligation. I want to be certain that they’re not going to conceive."
In appropriate patients, minoxidil and finasteride could be used together if the patient can afford it, she added.
Spironolactone, an androgen receptor inhibitor, also has been used in a dose of 200 mg/day to retard hair thinning due to androgenetic alopecia. Data show that spironolactone 50-200 mg/day can be used successfully to treat acne and hirsutism, but there are no evidence-based studies showing that it helps hair regrowth.
"I use very little spironolactone for androgenetic alopecia," Dr. Price said. "It will not grow hair. I think it’s used a lot because people aren’t familiar with minoxidil or don’t know about using finasteride if there’s no possibility of pregnancy."
Dr. Price reported having financial associations with Allergan and Follica, neither of which was pertinent to this topic.
On Twitter @sherryboschert
EXPERT ANALYSIS FROM THE PDA ANNUAL MEETING
What is the gynecologist’s role in the care of BRCA previvors?
Angelina Jolie’s mother and maternal grandmother died of ovarian cancer; her mother’s sister died of breast cancer. After testing positive for a clinically significant BRCA mutation, Ms. Jolie became a “previvor,” a woman who is currently healthy but at very high risk for developing a life-threatening breast or ovarian malignancy. Advances in genetic testing will result in many more women being identified as BRCA previvors. These women face many daunting decisions concerning options for risk-reducing breast and pelvic surgery. We gynecologists need to be prepared to help shepherd them through this process.
Which of our patients should be tested for BRCA mutations?
Experts have provided complex guidance on who should be tested for a BRCA mutation.1 These recommendations include testing women with a personal history of:
• epithelial ovarian cancer
• fallopian tube cancer, or
• primary peritoneal cancer.
Women with a personal history of breast cancer at an early age also should consider being tested. If women with breast or ovarian cancer, or both, test positive for BRCA1 or BRCA2, then living family members can be offered testing.
Healthy women with a family history of breast and/or ovarian cancer also may benefit from genetic testing. My clinical experience is that, unless the family history is as dramatic as that of Ms. Jolie, it requires considerable time and expertise to assemble a valid extended-family history and provide an estimate of risk. This task may be best completed by a genetic counselor. A simple, Web-based BRCA risk-assessment tool is provided by Myriad Genetics.2
A clinically significant BRCA mutation is detected. What is your patient’s risk?
Unfortunately, women with BRCA mutations are at very high risk for breast and ovarian cancer.
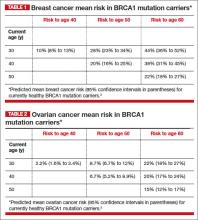
BRCA1. For patients with a BRCA1 mutation, the mean cumulative cancer risk to age 70 years is approximately 57% for breast cancer (95% confidence interval [CI], 47% to 66%) and 40% for ovarian cancer (95% CI, 35% to 46%).3
BRCA2. For patients with a BRCA2 mutation, the mean cumulative cancer risk to age 70 years is 49% for breast cancer (95% CI, 40% to 57%) and 18% for ovarian cancer (95% CI,13% to 23%). Tables 1 and 2 provide an approximation of the risk of developing breast or ovarian cancer for BRCA1 previvors aged 30, 40, and 50. The breast and ovarian cancer risk for BRCA2 previvors (not shown) is less than that for BRCA1 previvors.
Options for risk-reducing surgery
Risk-reducing breast and pelvic surgery markedly lessens the risk of developing cancer in the breast and ovary, decreasing the risk of mortality not only from breast cancer and ovarian cancer but from all causes.4
How many women choose risk-reducing surgery, and why? In a cohort of 306 healthy BRCA mutation carriers in the Netherlands, 10 years of follow-up revealed that 75% underwent risk-reducing pelvic surgery, and 50% underwent risk-reducing mastectomy.5 The age of the woman and her desire to preserve child-bearing strongly influenced the decision to undergo prophylactic surgery and the timing of the surgery.
Following risk-reducing surgery, most women are satisfied with their choice. In one follow-up study, the authors reported that women who had difficulty arriving at a decision were less satisfied with their choice to have risk-reducing surgery than women who arrived at their decision with confidence.6
Risk-reducing breast surgery: What’s involved?
As can be seen in Tables 1 and 2, the risk of your 30-year-old patient with a BRCA1 mutation developing breast cancer throughout her 30s is much greater than her risk of developing ovarian cancer. For this patient, bilateral total mastectomy is the standard risk-reducing procedure because subcutaneous mastectomy may leave islands of glandular tissue that may serve as a nidus for cancer development. However, both nipple-sparing and skin-sparing mastectomy have been reported to be successful in most cases, and may be favored by many women.8,9 Following risk-reducing breast surgery, most women can immediately begin multistage breast reconstruction.
Ms. Jolie underwent a “nipple delay” surgery 2 weeks before her mastectomy. This procedure involves lifting a portion of the nipple skin off the underlying breast to stimulate the nipple to start drawing its blood flow from the surrounding skin rather than the underlying tissue. In addition, during the nipple delay procedure a biopsy of the tissue from beneath the nipple is performed to ensure that no cancer is present.
Risk-reducing pelvic surgery: Essential steps
The standard operation to reduce the risk of ovarian cancer in BRCA mutation carriers is a bilateral salpingo-oophorectomy (BSO). Additional steps should be performed to assess for distant disease, including:
• careful assessment of all peritoneal surfaces
• pelvic washing for cytology
• omental biopsy
• cytologic smear of the diaphragm.
The fallopian tube should be resected in its entirety, but cornual resection is not necessary. Care should be taken to remove the entire ovary and avoid leaving small remnants of the ovary on the proximal vascular pedicles. Consideration should be given to performing a hysterectomy at the time of risk-reducing BSO. If the uterus is removed, estrogen-only hormone therapy (HT) can be prescribed. Estrogen-only HT causes fewer adverse events than combination estrogen-progestin therapy.
Two-stage pelvic surgery approach
Recent research indicates that high-grade serous tumors caused by BRCA mutations often originate in the distal half of the fallopian tube and then progress to the ovary. Serous tumors also may arise from the ovary or peritoneum.10 Building on this finding (that in BRCA previvors serous tumors often begin in the distal fallopian tube), some experts now recommend that a two-stage risk-reducing pelvic surgery option be offered to women with BRCA mutations. No large clinical trials have been reported using the two-stage approach to risk-reducing pelvic surgery, but published case series suggest that it is a plausible approach.
Stage 1: Ovary preservation. In Stage 1 surgery, the fallopian tubes are removed through a laparoscopic approach, but the ovaries are preserved. Some experts also recommend removal of the fallopian tube–peritoneum–ovarian junction.11,12 Stage 1 surgery reduces the risk of developing a cancer that originates in the fallopian tube and preserves ovarian estradiol and progesterone secretion, thereby avoiding premature menopause.
Stage 2: Ovary removal. Years later, as late as age 50, Stage 2 surgery is performed, and the ovaries are removed laparoscopically.
In the Stage 2 operation, consideration should be given to performing a hysterectomy. Since this second surgery will make the woman menopausal, concomitant hysterectomy would permit estrogen-only HT.
It should be noted that, for BRCA previvors, removing the ovaries at an early age is associated with a reduced breast cancer risk. The two-stage surgical approach does not offer this advantage of breast cancer risk reduction.
Appropriate gyn follow-up after risk-reducing BsO
Consider HT or estrogen-progestin contraceptives. For young, premenopausal women who undergo risk-reducing BSO, HT or estrogen-progestin contraceptives will help to reduce the risk of hot flashes, sleep disturbance, and symptoms of vaginal dryness. Although there is a theoretical concern that estrogen and progestin treatment may increase the risk of breast cancer, many experts are comfortable with prescribing estrogen and progestins to young BRCA carriers following risk-reducing BSO.
If your young, premenopausal patient underwent risk-reducing BSO but declines HT, a bone density test should be obtained within 1 or 2 years of surgery; you can offer nonhormonal treatment for her menopausal symptoms.
After undergoing risk-reducing BSO, patients should be prescribed weight-bearing exercise, vitamin D 600 U daily, and calcium supplements. In addition, because of a 5% risk of developing a peritoneal cancer, provide an annual gynecologic exam with CA-125 measurement and pelvic sonography every 6 months.13
Appropriate surveillance for women who forego risk-reducing surgery
This surveillance strategy may be helpful in detecting cancer at an early stage:
1. Beginning at age 25: Perform clinical breast exam once or twice per year
2. Beginning at age 25, or based on individualized assessment influenced by the earliest age of onset of cancer in the family: Alternate breast magnetic resonance imaging and mammography every 6 months
3. Beginning at age 35, or 10 years before the earliest age of onset of ovarian cancer in the family: Perform pelvic examination, CA-125 measurement, and pelvic sonography every 6 months. In women with BRCA mutations, estrogen-progestin contraceptive use may reduce the risk of ovarian cancer without increasing the risk of breast cancer.7
Her genetics can put your patient at risk. You are in a position to help protect her.
Imagine prematurely losing your mother, mother’s sister, and maternal grandmother to breast and ovarian cancers. Each funeral for a young relative resurrects memories of previous painful losses. The children and adults worry, “Who will be the next to die?” The early identification of families with BRCA mutations offers the hope of options to reduce premature death, preserving the quality of life and keeping families intact, so they can gather together for enjoyable holidays, not funerals.
Tell us what you think, at [email protected]. Please include your name and city and state.
1. National Comprehensive Cancer Network. Ge-netic/Familial High-Risk Assessment: Breast and Ovarian. Version 4.2013. http://www.nccn.org/professionals/physician_gls/pdf/genetics_screening.pdf. Accessed August 20, 2013.
2. Myriad Genetic Laboratories. BRCA risk calculator. http://www.myriadpro.com/brca-risk-calcu lator/calc.html. Accessed August 20, 2013.
3.Chen S, Parmigiani G. Meta-analysis of BRCA1 and BRCA2 penetrance. J Clin Oncol. 2007;25(11):1329–1333.
4. Domchek SM, Friebel TM, Singer CF, et al. As-sociation of risk-reducing surgery in BRCA1 or BRCA2 mutation carriers with cancer risk and mortality. JAMA. 2010;304(9):967–975.
5. Skytte AB, Gerdes AM, Andresen MK, et al. Risk-reducing mastectomy and salpingo-oophorectomy in unaffected BRCA mutation carriers: Uptake and timing. Clin Genet. 2010;77(4):342–349.
6. Westin SN, Sun CC, Lu KH, et al. Satisfaction with ovarian carcinoma risk-reduction strategies among women at high risk for breast and ovarian carcinoma. Cancer. 2011;117(12):2659–2667.
7. Iodice S, Barile M, Rotmensz N, et al. Oral contraceptive use and breast or ovarian cancer risk in BRCA1/2 carriers: A meta-analysis. Eur J Cancer. 2010;46(12):2275–2284.
8. Garwood ER, Moore D, Ewing C, et al. Total skin-sparing mastectomy: Complications and local recurrence rates in 2 cohorts of patients. Ann Surg. 2009;249(1):26–32.
9. Sacchini V, Pinotti JA, Barros A, et al. Nipple-sparing mastectomy for breast cancer and risk reduction: Oncologic or technical problem? J Am Coll Surg. 2006;203(5):704–714.
10. Yates MS, Meyer LA, Deavers MT, et al. Microscopic and early-stage ovarian cancers in BRCA1/2 mutation carriers: Building a model for early BRCA-associated tumorigenesis. Cancer Prev Res (Phila). 2011;4(4):463–470.
11. Leblanc E, Narducci F, Farre I, et al. Radical fimbriectomy: a reasonable temporary risk-reducing surgery for selected women with a germ line mutation of BRCA 1 or 2 genes? Rationale and preliminary development. Gynecol Oncol. 2011;121(3):472–476.
12. Seidman JD, Yemelyanova A, Zaino RJ, Kurman RJ. The fallopian tube-peritoneal junction: A potential site of carcinogenesis. Int J Gynecol Pathol. 2011;30(1):4–11.
13. Chapman JS, Powell CB, McLennan J, et al. Surveillance of survivors: follow-up after risk-reducing salpingo-oophorectomy in BRCA1/2 mutation carriers. Gynecol Oncol. 2011;122(2):339–343.
Angelina Jolie’s mother and maternal grandmother died of ovarian cancer; her mother’s sister died of breast cancer. After testing positive for a clinically significant BRCA mutation, Ms. Jolie became a “previvor,” a woman who is currently healthy but at very high risk for developing a life-threatening breast or ovarian malignancy. Advances in genetic testing will result in many more women being identified as BRCA previvors. These women face many daunting decisions concerning options for risk-reducing breast and pelvic surgery. We gynecologists need to be prepared to help shepherd them through this process.
Which of our patients should be tested for BRCA mutations?
Experts have provided complex guidance on who should be tested for a BRCA mutation.1 These recommendations include testing women with a personal history of:
• epithelial ovarian cancer
• fallopian tube cancer, or
• primary peritoneal cancer.
Women with a personal history of breast cancer at an early age also should consider being tested. If women with breast or ovarian cancer, or both, test positive for BRCA1 or BRCA2, then living family members can be offered testing.
Healthy women with a family history of breast and/or ovarian cancer also may benefit from genetic testing. My clinical experience is that, unless the family history is as dramatic as that of Ms. Jolie, it requires considerable time and expertise to assemble a valid extended-family history and provide an estimate of risk. This task may be best completed by a genetic counselor. A simple, Web-based BRCA risk-assessment tool is provided by Myriad Genetics.2
A clinically significant BRCA mutation is detected. What is your patient’s risk?
Unfortunately, women with BRCA mutations are at very high risk for breast and ovarian cancer.
BRCA1. For patients with a BRCA1 mutation, the mean cumulative cancer risk to age 70 years is approximately 57% for breast cancer (95% confidence interval [CI], 47% to 66%) and 40% for ovarian cancer (95% CI, 35% to 46%).3
BRCA2. For patients with a BRCA2 mutation, the mean cumulative cancer risk to age 70 years is 49% for breast cancer (95% CI, 40% to 57%) and 18% for ovarian cancer (95% CI,13% to 23%). Tables 1 and 2 provide an approximation of the risk of developing breast or ovarian cancer for BRCA1 previvors aged 30, 40, and 50. The breast and ovarian cancer risk for BRCA2 previvors (not shown) is less than that for BRCA1 previvors.
Options for risk-reducing surgery
Risk-reducing breast and pelvic surgery markedly lessens the risk of developing cancer in the breast and ovary, decreasing the risk of mortality not only from breast cancer and ovarian cancer but from all causes.4
How many women choose risk-reducing surgery, and why? In a cohort of 306 healthy BRCA mutation carriers in the Netherlands, 10 years of follow-up revealed that 75% underwent risk-reducing pelvic surgery, and 50% underwent risk-reducing mastectomy.5 The age of the woman and her desire to preserve child-bearing strongly influenced the decision to undergo prophylactic surgery and the timing of the surgery.
Following risk-reducing surgery, most women are satisfied with their choice. In one follow-up study, the authors reported that women who had difficulty arriving at a decision were less satisfied with their choice to have risk-reducing surgery than women who arrived at their decision with confidence.6
Risk-reducing breast surgery: What’s involved?
As can be seen in Tables 1 and 2, the risk of your 30-year-old patient with a BRCA1 mutation developing breast cancer throughout her 30s is much greater than her risk of developing ovarian cancer. For this patient, bilateral total mastectomy is the standard risk-reducing procedure because subcutaneous mastectomy may leave islands of glandular tissue that may serve as a nidus for cancer development. However, both nipple-sparing and skin-sparing mastectomy have been reported to be successful in most cases, and may be favored by many women.8,9 Following risk-reducing breast surgery, most women can immediately begin multistage breast reconstruction.
Ms. Jolie underwent a “nipple delay” surgery 2 weeks before her mastectomy. This procedure involves lifting a portion of the nipple skin off the underlying breast to stimulate the nipple to start drawing its blood flow from the surrounding skin rather than the underlying tissue. In addition, during the nipple delay procedure a biopsy of the tissue from beneath the nipple is performed to ensure that no cancer is present.
Risk-reducing pelvic surgery: Essential steps
The standard operation to reduce the risk of ovarian cancer in BRCA mutation carriers is a bilateral salpingo-oophorectomy (BSO). Additional steps should be performed to assess for distant disease, including:
• careful assessment of all peritoneal surfaces
• pelvic washing for cytology
• omental biopsy
• cytologic smear of the diaphragm.
The fallopian tube should be resected in its entirety, but cornual resection is not necessary. Care should be taken to remove the entire ovary and avoid leaving small remnants of the ovary on the proximal vascular pedicles. Consideration should be given to performing a hysterectomy at the time of risk-reducing BSO. If the uterus is removed, estrogen-only hormone therapy (HT) can be prescribed. Estrogen-only HT causes fewer adverse events than combination estrogen-progestin therapy.
Two-stage pelvic surgery approach
Recent research indicates that high-grade serous tumors caused by BRCA mutations often originate in the distal half of the fallopian tube and then progress to the ovary. Serous tumors also may arise from the ovary or peritoneum.10 Building on this finding (that in BRCA previvors serous tumors often begin in the distal fallopian tube), some experts now recommend that a two-stage risk-reducing pelvic surgery option be offered to women with BRCA mutations. No large clinical trials have been reported using the two-stage approach to risk-reducing pelvic surgery, but published case series suggest that it is a plausible approach.
Stage 1: Ovary preservation. In Stage 1 surgery, the fallopian tubes are removed through a laparoscopic approach, but the ovaries are preserved. Some experts also recommend removal of the fallopian tube–peritoneum–ovarian junction.11,12 Stage 1 surgery reduces the risk of developing a cancer that originates in the fallopian tube and preserves ovarian estradiol and progesterone secretion, thereby avoiding premature menopause.
Stage 2: Ovary removal. Years later, as late as age 50, Stage 2 surgery is performed, and the ovaries are removed laparoscopically.
In the Stage 2 operation, consideration should be given to performing a hysterectomy. Since this second surgery will make the woman menopausal, concomitant hysterectomy would permit estrogen-only HT.
It should be noted that, for BRCA previvors, removing the ovaries at an early age is associated with a reduced breast cancer risk. The two-stage surgical approach does not offer this advantage of breast cancer risk reduction.
Appropriate gyn follow-up after risk-reducing BsO
Consider HT or estrogen-progestin contraceptives. For young, premenopausal women who undergo risk-reducing BSO, HT or estrogen-progestin contraceptives will help to reduce the risk of hot flashes, sleep disturbance, and symptoms of vaginal dryness. Although there is a theoretical concern that estrogen and progestin treatment may increase the risk of breast cancer, many experts are comfortable with prescribing estrogen and progestins to young BRCA carriers following risk-reducing BSO.
If your young, premenopausal patient underwent risk-reducing BSO but declines HT, a bone density test should be obtained within 1 or 2 years of surgery; you can offer nonhormonal treatment for her menopausal symptoms.
After undergoing risk-reducing BSO, patients should be prescribed weight-bearing exercise, vitamin D 600 U daily, and calcium supplements. In addition, because of a 5% risk of developing a peritoneal cancer, provide an annual gynecologic exam with CA-125 measurement and pelvic sonography every 6 months.13
Appropriate surveillance for women who forego risk-reducing surgery
This surveillance strategy may be helpful in detecting cancer at an early stage:
1. Beginning at age 25: Perform clinical breast exam once or twice per year
2. Beginning at age 25, or based on individualized assessment influenced by the earliest age of onset of cancer in the family: Alternate breast magnetic resonance imaging and mammography every 6 months
3. Beginning at age 35, or 10 years before the earliest age of onset of ovarian cancer in the family: Perform pelvic examination, CA-125 measurement, and pelvic sonography every 6 months. In women with BRCA mutations, estrogen-progestin contraceptive use may reduce the risk of ovarian cancer without increasing the risk of breast cancer.7
Her genetics can put your patient at risk. You are in a position to help protect her.
Imagine prematurely losing your mother, mother’s sister, and maternal grandmother to breast and ovarian cancers. Each funeral for a young relative resurrects memories of previous painful losses. The children and adults worry, “Who will be the next to die?” The early identification of families with BRCA mutations offers the hope of options to reduce premature death, preserving the quality of life and keeping families intact, so they can gather together for enjoyable holidays, not funerals.
Tell us what you think, at [email protected]. Please include your name and city and state.
Angelina Jolie’s mother and maternal grandmother died of ovarian cancer; her mother’s sister died of breast cancer. After testing positive for a clinically significant BRCA mutation, Ms. Jolie became a “previvor,” a woman who is currently healthy but at very high risk for developing a life-threatening breast or ovarian malignancy. Advances in genetic testing will result in many more women being identified as BRCA previvors. These women face many daunting decisions concerning options for risk-reducing breast and pelvic surgery. We gynecologists need to be prepared to help shepherd them through this process.
Which of our patients should be tested for BRCA mutations?
Experts have provided complex guidance on who should be tested for a BRCA mutation.1 These recommendations include testing women with a personal history of:
• epithelial ovarian cancer
• fallopian tube cancer, or
• primary peritoneal cancer.
Women with a personal history of breast cancer at an early age also should consider being tested. If women with breast or ovarian cancer, or both, test positive for BRCA1 or BRCA2, then living family members can be offered testing.
Healthy women with a family history of breast and/or ovarian cancer also may benefit from genetic testing. My clinical experience is that, unless the family history is as dramatic as that of Ms. Jolie, it requires considerable time and expertise to assemble a valid extended-family history and provide an estimate of risk. This task may be best completed by a genetic counselor. A simple, Web-based BRCA risk-assessment tool is provided by Myriad Genetics.2
A clinically significant BRCA mutation is detected. What is your patient’s risk?
Unfortunately, women with BRCA mutations are at very high risk for breast and ovarian cancer.
BRCA1. For patients with a BRCA1 mutation, the mean cumulative cancer risk to age 70 years is approximately 57% for breast cancer (95% confidence interval [CI], 47% to 66%) and 40% for ovarian cancer (95% CI, 35% to 46%).3
BRCA2. For patients with a BRCA2 mutation, the mean cumulative cancer risk to age 70 years is 49% for breast cancer (95% CI, 40% to 57%) and 18% for ovarian cancer (95% CI,13% to 23%). Tables 1 and 2 provide an approximation of the risk of developing breast or ovarian cancer for BRCA1 previvors aged 30, 40, and 50. The breast and ovarian cancer risk for BRCA2 previvors (not shown) is less than that for BRCA1 previvors.
Options for risk-reducing surgery
Risk-reducing breast and pelvic surgery markedly lessens the risk of developing cancer in the breast and ovary, decreasing the risk of mortality not only from breast cancer and ovarian cancer but from all causes.4
How many women choose risk-reducing surgery, and why? In a cohort of 306 healthy BRCA mutation carriers in the Netherlands, 10 years of follow-up revealed that 75% underwent risk-reducing pelvic surgery, and 50% underwent risk-reducing mastectomy.5 The age of the woman and her desire to preserve child-bearing strongly influenced the decision to undergo prophylactic surgery and the timing of the surgery.
Following risk-reducing surgery, most women are satisfied with their choice. In one follow-up study, the authors reported that women who had difficulty arriving at a decision were less satisfied with their choice to have risk-reducing surgery than women who arrived at their decision with confidence.6
Risk-reducing breast surgery: What’s involved?
As can be seen in Tables 1 and 2, the risk of your 30-year-old patient with a BRCA1 mutation developing breast cancer throughout her 30s is much greater than her risk of developing ovarian cancer. For this patient, bilateral total mastectomy is the standard risk-reducing procedure because subcutaneous mastectomy may leave islands of glandular tissue that may serve as a nidus for cancer development. However, both nipple-sparing and skin-sparing mastectomy have been reported to be successful in most cases, and may be favored by many women.8,9 Following risk-reducing breast surgery, most women can immediately begin multistage breast reconstruction.
Ms. Jolie underwent a “nipple delay” surgery 2 weeks before her mastectomy. This procedure involves lifting a portion of the nipple skin off the underlying breast to stimulate the nipple to start drawing its blood flow from the surrounding skin rather than the underlying tissue. In addition, during the nipple delay procedure a biopsy of the tissue from beneath the nipple is performed to ensure that no cancer is present.
Risk-reducing pelvic surgery: Essential steps
The standard operation to reduce the risk of ovarian cancer in BRCA mutation carriers is a bilateral salpingo-oophorectomy (BSO). Additional steps should be performed to assess for distant disease, including:
• careful assessment of all peritoneal surfaces
• pelvic washing for cytology
• omental biopsy
• cytologic smear of the diaphragm.
The fallopian tube should be resected in its entirety, but cornual resection is not necessary. Care should be taken to remove the entire ovary and avoid leaving small remnants of the ovary on the proximal vascular pedicles. Consideration should be given to performing a hysterectomy at the time of risk-reducing BSO. If the uterus is removed, estrogen-only hormone therapy (HT) can be prescribed. Estrogen-only HT causes fewer adverse events than combination estrogen-progestin therapy.
Two-stage pelvic surgery approach
Recent research indicates that high-grade serous tumors caused by BRCA mutations often originate in the distal half of the fallopian tube and then progress to the ovary. Serous tumors also may arise from the ovary or peritoneum.10 Building on this finding (that in BRCA previvors serous tumors often begin in the distal fallopian tube), some experts now recommend that a two-stage risk-reducing pelvic surgery option be offered to women with BRCA mutations. No large clinical trials have been reported using the two-stage approach to risk-reducing pelvic surgery, but published case series suggest that it is a plausible approach.
Stage 1: Ovary preservation. In Stage 1 surgery, the fallopian tubes are removed through a laparoscopic approach, but the ovaries are preserved. Some experts also recommend removal of the fallopian tube–peritoneum–ovarian junction.11,12 Stage 1 surgery reduces the risk of developing a cancer that originates in the fallopian tube and preserves ovarian estradiol and progesterone secretion, thereby avoiding premature menopause.
Stage 2: Ovary removal. Years later, as late as age 50, Stage 2 surgery is performed, and the ovaries are removed laparoscopically.
In the Stage 2 operation, consideration should be given to performing a hysterectomy. Since this second surgery will make the woman menopausal, concomitant hysterectomy would permit estrogen-only HT.
It should be noted that, for BRCA previvors, removing the ovaries at an early age is associated with a reduced breast cancer risk. The two-stage surgical approach does not offer this advantage of breast cancer risk reduction.
Appropriate gyn follow-up after risk-reducing BsO
Consider HT or estrogen-progestin contraceptives. For young, premenopausal women who undergo risk-reducing BSO, HT or estrogen-progestin contraceptives will help to reduce the risk of hot flashes, sleep disturbance, and symptoms of vaginal dryness. Although there is a theoretical concern that estrogen and progestin treatment may increase the risk of breast cancer, many experts are comfortable with prescribing estrogen and progestins to young BRCA carriers following risk-reducing BSO.
If your young, premenopausal patient underwent risk-reducing BSO but declines HT, a bone density test should be obtained within 1 or 2 years of surgery; you can offer nonhormonal treatment for her menopausal symptoms.
After undergoing risk-reducing BSO, patients should be prescribed weight-bearing exercise, vitamin D 600 U daily, and calcium supplements. In addition, because of a 5% risk of developing a peritoneal cancer, provide an annual gynecologic exam with CA-125 measurement and pelvic sonography every 6 months.13
Appropriate surveillance for women who forego risk-reducing surgery
This surveillance strategy may be helpful in detecting cancer at an early stage:
1. Beginning at age 25: Perform clinical breast exam once or twice per year
2. Beginning at age 25, or based on individualized assessment influenced by the earliest age of onset of cancer in the family: Alternate breast magnetic resonance imaging and mammography every 6 months
3. Beginning at age 35, or 10 years before the earliest age of onset of ovarian cancer in the family: Perform pelvic examination, CA-125 measurement, and pelvic sonography every 6 months. In women with BRCA mutations, estrogen-progestin contraceptive use may reduce the risk of ovarian cancer without increasing the risk of breast cancer.7
Her genetics can put your patient at risk. You are in a position to help protect her.
Imagine prematurely losing your mother, mother’s sister, and maternal grandmother to breast and ovarian cancers. Each funeral for a young relative resurrects memories of previous painful losses. The children and adults worry, “Who will be the next to die?” The early identification of families with BRCA mutations offers the hope of options to reduce premature death, preserving the quality of life and keeping families intact, so they can gather together for enjoyable holidays, not funerals.
Tell us what you think, at [email protected]. Please include your name and city and state.
1. National Comprehensive Cancer Network. Ge-netic/Familial High-Risk Assessment: Breast and Ovarian. Version 4.2013. http://www.nccn.org/professionals/physician_gls/pdf/genetics_screening.pdf. Accessed August 20, 2013.
2. Myriad Genetic Laboratories. BRCA risk calculator. http://www.myriadpro.com/brca-risk-calcu lator/calc.html. Accessed August 20, 2013.
3.Chen S, Parmigiani G. Meta-analysis of BRCA1 and BRCA2 penetrance. J Clin Oncol. 2007;25(11):1329–1333.
4. Domchek SM, Friebel TM, Singer CF, et al. As-sociation of risk-reducing surgery in BRCA1 or BRCA2 mutation carriers with cancer risk and mortality. JAMA. 2010;304(9):967–975.
5. Skytte AB, Gerdes AM, Andresen MK, et al. Risk-reducing mastectomy and salpingo-oophorectomy in unaffected BRCA mutation carriers: Uptake and timing. Clin Genet. 2010;77(4):342–349.
6. Westin SN, Sun CC, Lu KH, et al. Satisfaction with ovarian carcinoma risk-reduction strategies among women at high risk for breast and ovarian carcinoma. Cancer. 2011;117(12):2659–2667.
7. Iodice S, Barile M, Rotmensz N, et al. Oral contraceptive use and breast or ovarian cancer risk in BRCA1/2 carriers: A meta-analysis. Eur J Cancer. 2010;46(12):2275–2284.
8. Garwood ER, Moore D, Ewing C, et al. Total skin-sparing mastectomy: Complications and local recurrence rates in 2 cohorts of patients. Ann Surg. 2009;249(1):26–32.
9. Sacchini V, Pinotti JA, Barros A, et al. Nipple-sparing mastectomy for breast cancer and risk reduction: Oncologic or technical problem? J Am Coll Surg. 2006;203(5):704–714.
10. Yates MS, Meyer LA, Deavers MT, et al. Microscopic and early-stage ovarian cancers in BRCA1/2 mutation carriers: Building a model for early BRCA-associated tumorigenesis. Cancer Prev Res (Phila). 2011;4(4):463–470.
11. Leblanc E, Narducci F, Farre I, et al. Radical fimbriectomy: a reasonable temporary risk-reducing surgery for selected women with a germ line mutation of BRCA 1 or 2 genes? Rationale and preliminary development. Gynecol Oncol. 2011;121(3):472–476.
12. Seidman JD, Yemelyanova A, Zaino RJ, Kurman RJ. The fallopian tube-peritoneal junction: A potential site of carcinogenesis. Int J Gynecol Pathol. 2011;30(1):4–11.
13. Chapman JS, Powell CB, McLennan J, et al. Surveillance of survivors: follow-up after risk-reducing salpingo-oophorectomy in BRCA1/2 mutation carriers. Gynecol Oncol. 2011;122(2):339–343.
1. National Comprehensive Cancer Network. Ge-netic/Familial High-Risk Assessment: Breast and Ovarian. Version 4.2013. http://www.nccn.org/professionals/physician_gls/pdf/genetics_screening.pdf. Accessed August 20, 2013.
2. Myriad Genetic Laboratories. BRCA risk calculator. http://www.myriadpro.com/brca-risk-calcu lator/calc.html. Accessed August 20, 2013.
3.Chen S, Parmigiani G. Meta-analysis of BRCA1 and BRCA2 penetrance. J Clin Oncol. 2007;25(11):1329–1333.
4. Domchek SM, Friebel TM, Singer CF, et al. As-sociation of risk-reducing surgery in BRCA1 or BRCA2 mutation carriers with cancer risk and mortality. JAMA. 2010;304(9):967–975.
5. Skytte AB, Gerdes AM, Andresen MK, et al. Risk-reducing mastectomy and salpingo-oophorectomy in unaffected BRCA mutation carriers: Uptake and timing. Clin Genet. 2010;77(4):342–349.
6. Westin SN, Sun CC, Lu KH, et al. Satisfaction with ovarian carcinoma risk-reduction strategies among women at high risk for breast and ovarian carcinoma. Cancer. 2011;117(12):2659–2667.
7. Iodice S, Barile M, Rotmensz N, et al. Oral contraceptive use and breast or ovarian cancer risk in BRCA1/2 carriers: A meta-analysis. Eur J Cancer. 2010;46(12):2275–2284.
8. Garwood ER, Moore D, Ewing C, et al. Total skin-sparing mastectomy: Complications and local recurrence rates in 2 cohorts of patients. Ann Surg. 2009;249(1):26–32.
9. Sacchini V, Pinotti JA, Barros A, et al. Nipple-sparing mastectomy for breast cancer and risk reduction: Oncologic or technical problem? J Am Coll Surg. 2006;203(5):704–714.
10. Yates MS, Meyer LA, Deavers MT, et al. Microscopic and early-stage ovarian cancers in BRCA1/2 mutation carriers: Building a model for early BRCA-associated tumorigenesis. Cancer Prev Res (Phila). 2011;4(4):463–470.
11. Leblanc E, Narducci F, Farre I, et al. Radical fimbriectomy: a reasonable temporary risk-reducing surgery for selected women with a germ line mutation of BRCA 1 or 2 genes? Rationale and preliminary development. Gynecol Oncol. 2011;121(3):472–476.
12. Seidman JD, Yemelyanova A, Zaino RJ, Kurman RJ. The fallopian tube-peritoneal junction: A potential site of carcinogenesis. Int J Gynecol Pathol. 2011;30(1):4–11.
13. Chapman JS, Powell CB, McLennan J, et al. Surveillance of survivors: follow-up after risk-reducing salpingo-oophorectomy in BRCA1/2 mutation carriers. Gynecol Oncol. 2011;122(2):339–343.
Switching nonadherent to denosumab improved bone density
SAN FRANCISCO – Switching to denosumab boosted bone mineral density more than did switching to ibandronate or risedronate in high-risk osteoporosis patients who were not adherent to oral bisphosphonate therapy, an assessment of data from 1,576 patients in two studies found.
The open-label studies included postmenopausal women who previously had been prescribed oral bisphosphonate therapy for osteoporosis but had either stopped the drug or were insufficiently adherent to therapy, by a score of less than six on the Osteoporosis-Specific Morisky Medication Adherence Scale.
One study randomized patients to denosumab (Prolia) 60 mg subcutaneously every 6 months or ibandronate (Boniva) 150 mg orally every month.
The other study randomized patients to the same denosumab regimen or risedronate (Actonel) 150 mg orally per month, taken as a 75-mg tablet on each of two consecutive days.
BMD measurements on 1,576 women in the studies showed significantly greater increases at 12 months at the hip, femoral neck, and spine in patients receiving denosumab, compared with either of the oral bisphosphonates, Dr. Christopher Recknor and his associates reported at the annual meeting of the Endocrine Society.
Bone density in patients on denosumab increased at the hip by about 2.3% in one study and 2% in the other, increased at the femoral neck by roughly 1.7% in one study and 1.5% in the other, and increased at the spine by 4% in one study and 3.5% in the other. In comparison, BMDs in patients on ibandronate increased by roughly 1.1% at the hip, 0.7% at the femoral neck, and 2% at the spine. BMDs in patients on risedronate increased by about 0.4% at the hip and 1% at the spine but decreased by 0.1% at the femoral neck.
The changes in bone density did not differ significantly between the 33% of patients who had had a prior fragility fracture and patients with no history of fragility fracture, with one exception: The gains in femoral neck BMD from denosumab compared with ibandronate were significantly larger in patients with a prior fracture, compared with those with no prior fracture, reported Dr. Recknor, medical director of the United Osteoporosis Centers, Gainesville, Ga.
In patients with a prior fragility fracture, femoral neck BMD increased by about 2.2% on denosumab and by 0.1% on ibandronate, compared with gains in patients with no prior fracture of 1.5% on denosumab and 1% on ibandronate.
The study defined fragility fractures as those not involving the skull, facial bones, fingers, and toes and not associated with severe trauma or pathological fractures.
In the lower-risk patients without a prior fracture, "that is where Boniva does not seem to work very well" in comparison with switching to another oral bisphosphonate, Dr. Recknor said in an interview. For high-risk patients with a prior fracture, however, "I’m even more convinced that if I’ve got somebody who’s at high risk and they’re noncompliant" with bisphosphonate therapy, "giving Prolia would be the way to go."
Even if the bisphosphonate regimen is switched to monthly dosing to try to improve adherence, "the bone density increases are just not going to be that great," he said.
BMDs at baseline did not differ significantly between treatment groups or between patients with or without a prior fragility fracture.
Infections, though not serious ones, were reported in 24% of patients on denosumab in each study and in 19% of patients on ibandronate and 21% of patients on risedronate. There were no cases of atypical femoral fracture, delayed fracture healing, or osteonecrosis of the jaw.
The study was funded by Amgen, which markets denosumab, and by GlaxoSmithKline. Dr. Recknor disclosed ties with Amgen, Novartis Pharmaceuticals, Ion Med Systems, Eli Lilly, Merck, and UCB. Some coinvestigators were employees of Amgen and others reported financial ties with these companies and/or Warner Chilcott, which markets risedronate; Roche Pharmaceuticals, which markets ibandronate through Genentech; Tarsa, Wyeth, Takeda, Nycomed, Servier, and Pfizer.
On Twitter @sherryboschert
SAN FRANCISCO – Switching to denosumab boosted bone mineral density more than did switching to ibandronate or risedronate in high-risk osteoporosis patients who were not adherent to oral bisphosphonate therapy, an assessment of data from 1,576 patients in two studies found.
The open-label studies included postmenopausal women who previously had been prescribed oral bisphosphonate therapy for osteoporosis but had either stopped the drug or were insufficiently adherent to therapy, by a score of less than six on the Osteoporosis-Specific Morisky Medication Adherence Scale.
One study randomized patients to denosumab (Prolia) 60 mg subcutaneously every 6 months or ibandronate (Boniva) 150 mg orally every month.
The other study randomized patients to the same denosumab regimen or risedronate (Actonel) 150 mg orally per month, taken as a 75-mg tablet on each of two consecutive days.
BMD measurements on 1,576 women in the studies showed significantly greater increases at 12 months at the hip, femoral neck, and spine in patients receiving denosumab, compared with either of the oral bisphosphonates, Dr. Christopher Recknor and his associates reported at the annual meeting of the Endocrine Society.
Bone density in patients on denosumab increased at the hip by about 2.3% in one study and 2% in the other, increased at the femoral neck by roughly 1.7% in one study and 1.5% in the other, and increased at the spine by 4% in one study and 3.5% in the other. In comparison, BMDs in patients on ibandronate increased by roughly 1.1% at the hip, 0.7% at the femoral neck, and 2% at the spine. BMDs in patients on risedronate increased by about 0.4% at the hip and 1% at the spine but decreased by 0.1% at the femoral neck.
The changes in bone density did not differ significantly between the 33% of patients who had had a prior fragility fracture and patients with no history of fragility fracture, with one exception: The gains in femoral neck BMD from denosumab compared with ibandronate were significantly larger in patients with a prior fracture, compared with those with no prior fracture, reported Dr. Recknor, medical director of the United Osteoporosis Centers, Gainesville, Ga.
In patients with a prior fragility fracture, femoral neck BMD increased by about 2.2% on denosumab and by 0.1% on ibandronate, compared with gains in patients with no prior fracture of 1.5% on denosumab and 1% on ibandronate.
The study defined fragility fractures as those not involving the skull, facial bones, fingers, and toes and not associated with severe trauma or pathological fractures.
In the lower-risk patients without a prior fracture, "that is where Boniva does not seem to work very well" in comparison with switching to another oral bisphosphonate, Dr. Recknor said in an interview. For high-risk patients with a prior fracture, however, "I’m even more convinced that if I’ve got somebody who’s at high risk and they’re noncompliant" with bisphosphonate therapy, "giving Prolia would be the way to go."
Even if the bisphosphonate regimen is switched to monthly dosing to try to improve adherence, "the bone density increases are just not going to be that great," he said.
BMDs at baseline did not differ significantly between treatment groups or between patients with or without a prior fragility fracture.
Infections, though not serious ones, were reported in 24% of patients on denosumab in each study and in 19% of patients on ibandronate and 21% of patients on risedronate. There were no cases of atypical femoral fracture, delayed fracture healing, or osteonecrosis of the jaw.
The study was funded by Amgen, which markets denosumab, and by GlaxoSmithKline. Dr. Recknor disclosed ties with Amgen, Novartis Pharmaceuticals, Ion Med Systems, Eli Lilly, Merck, and UCB. Some coinvestigators were employees of Amgen and others reported financial ties with these companies and/or Warner Chilcott, which markets risedronate; Roche Pharmaceuticals, which markets ibandronate through Genentech; Tarsa, Wyeth, Takeda, Nycomed, Servier, and Pfizer.
On Twitter @sherryboschert
SAN FRANCISCO – Switching to denosumab boosted bone mineral density more than did switching to ibandronate or risedronate in high-risk osteoporosis patients who were not adherent to oral bisphosphonate therapy, an assessment of data from 1,576 patients in two studies found.
The open-label studies included postmenopausal women who previously had been prescribed oral bisphosphonate therapy for osteoporosis but had either stopped the drug or were insufficiently adherent to therapy, by a score of less than six on the Osteoporosis-Specific Morisky Medication Adherence Scale.
One study randomized patients to denosumab (Prolia) 60 mg subcutaneously every 6 months or ibandronate (Boniva) 150 mg orally every month.
The other study randomized patients to the same denosumab regimen or risedronate (Actonel) 150 mg orally per month, taken as a 75-mg tablet on each of two consecutive days.
BMD measurements on 1,576 women in the studies showed significantly greater increases at 12 months at the hip, femoral neck, and spine in patients receiving denosumab, compared with either of the oral bisphosphonates, Dr. Christopher Recknor and his associates reported at the annual meeting of the Endocrine Society.
Bone density in patients on denosumab increased at the hip by about 2.3% in one study and 2% in the other, increased at the femoral neck by roughly 1.7% in one study and 1.5% in the other, and increased at the spine by 4% in one study and 3.5% in the other. In comparison, BMDs in patients on ibandronate increased by roughly 1.1% at the hip, 0.7% at the femoral neck, and 2% at the spine. BMDs in patients on risedronate increased by about 0.4% at the hip and 1% at the spine but decreased by 0.1% at the femoral neck.
The changes in bone density did not differ significantly between the 33% of patients who had had a prior fragility fracture and patients with no history of fragility fracture, with one exception: The gains in femoral neck BMD from denosumab compared with ibandronate were significantly larger in patients with a prior fracture, compared with those with no prior fracture, reported Dr. Recknor, medical director of the United Osteoporosis Centers, Gainesville, Ga.
In patients with a prior fragility fracture, femoral neck BMD increased by about 2.2% on denosumab and by 0.1% on ibandronate, compared with gains in patients with no prior fracture of 1.5% on denosumab and 1% on ibandronate.
The study defined fragility fractures as those not involving the skull, facial bones, fingers, and toes and not associated with severe trauma or pathological fractures.
In the lower-risk patients without a prior fracture, "that is where Boniva does not seem to work very well" in comparison with switching to another oral bisphosphonate, Dr. Recknor said in an interview. For high-risk patients with a prior fracture, however, "I’m even more convinced that if I’ve got somebody who’s at high risk and they’re noncompliant" with bisphosphonate therapy, "giving Prolia would be the way to go."
Even if the bisphosphonate regimen is switched to monthly dosing to try to improve adherence, "the bone density increases are just not going to be that great," he said.
BMDs at baseline did not differ significantly between treatment groups or between patients with or without a prior fragility fracture.
Infections, though not serious ones, were reported in 24% of patients on denosumab in each study and in 19% of patients on ibandronate and 21% of patients on risedronate. There were no cases of atypical femoral fracture, delayed fracture healing, or osteonecrosis of the jaw.
The study was funded by Amgen, which markets denosumab, and by GlaxoSmithKline. Dr. Recknor disclosed ties with Amgen, Novartis Pharmaceuticals, Ion Med Systems, Eli Lilly, Merck, and UCB. Some coinvestigators were employees of Amgen and others reported financial ties with these companies and/or Warner Chilcott, which markets risedronate; Roche Pharmaceuticals, which markets ibandronate through Genentech; Tarsa, Wyeth, Takeda, Nycomed, Servier, and Pfizer.
On Twitter @sherryboschert
AT ENDO 2013
Major finding: Bone density increased at 1 year by 2.3% and 2% in the hip, 1.7% and 1.5% at the femoral neck, and 4% and 3.5% at the spine on denosumab compared with 1.1% at the hip, 0.7% at the femoral neck, and 2% at the spine on ibandronate and 0.4% at the hip and 0.4% at the spine with slightly decreased density at the femoral neck on risedronate.
Data source: Secondary analysis of data from two open-label, randomized studies of 1,576 postmenopausal women who had been insufficiently adherent to oral bisphosphonate therapy.
Disclosures: The study was funded by Amgen, which markets denosumab, and by GlaxoSmithKline. Dr. Recknor disclosed ties with Amgen, Novartis Pharmaceuticals, Ion Med Systems, Eli Lilly, Merck, and UCB. Some coinvestigators were employees of Amgen and others reported financial ties with these companies and/or Warner Chilcott, which markets risedronate; Roche Pharmaceuticals, which markets ibandronate through Genentech; Tarsa, Wyeth, Takeda, Nycomed, Servier, and Pfizer.
Studies speak volumes about brain changes and cognition in women
BOSTON – Women who complain about cognitive problems after menopause might have increases rather than decreases in volume of certain brain regions, reported investigators at the Alzheimer’s Association International Conference 2013.
Brain imaging studies of 48 women in the early years of menopause showed that those with subjective cognitive complaints had significantly greater volumes in certain regions of the brain than did noncomplainers, including the right posterior cingulate gyrus (P less than .04), the right transverse temporal cortex (P less than .03), The left caudal middle frontal gyrus, and the right paracentral gyrus (P less than .05 for both), said Lilia Zurkovsky, Ph.D., a postdoctoral fellow from the Center for Cognitive Medicine at Vanderbilt University Medical Center in Nashville, Tenn.
The findings appear to run counter to those of another study (Neurology 2006;12:67: 834-42) showing that older adults with cognitive complaints had smaller volumes in temporal and frontal areas, compared with healthy age-matched controls, Dr. Zurkovsky acknowledged. She noted, however, that her group studied postmenopausal women in their 50s and 60s, whereas the earlier study included men. In addition, the mean age for each group in that study was above 70 years.
Combined with recent findings pointing to subjective cognitive and/or memory complaints as possible early signs of Alzheimer’s disease, the studies hint at a possible pattern of change as women age.
"If we look at all the data together, it would seem as though in early middle age, 50- to 60-year old individuals with complaints may be having some sort of compensatory mechanism. That’s speculation; all we know is that they have this increase in volume before they have a decrease in volume as shown by other papers," Dr. Zurkovsky said.
She and her colleagues scanned the participants with T1-weight magnetic resonance imaging with a 3 Tesla magnet, and measured cognitive complaints with the Cognitive Complaint Index (CCI) and five surveys with 120 total questions about the individual’s perceptions of cognitive abilities since menopause.
Brain changes in healthy postmenopausal women were the focus of a second, unrelated study also reported at AAIC2013.
Australian investigators followed participants in the Womens Healthy Ageing Project, a longitudinal study of the menopausal transition among cognitively normal women. The investigators looked at MRI brain scans conducted in 2002 and 2012, and brain-amyloid imaging studies with 18-F florbetaben positron emission tomography (FBB-PET). Florbetaben is an investigational amyloid imaging agent, similar to florbetapir (Amyvid).
They found that among the 26 women who had both FBB-PET and the two MRI scans spaced a decade apart, a significant decline was found over time in hippocampal volume, but not in gray matter volume. There was also a nonsignificant trend correlating higher levels of FBB uptake, as measured by a standardized uptake value ratio (SUVR) with greater declines in hippocampal volume.
"Those who were in the higher tertile of florbetaben SUVR have had an 18%-19% decrement in hippocampal volume over that 10-year period," said lead author Paul Yates, Ph.D., a neuroscientist and imaging research fellow at Austin Health in Heidelberg, Australia.
Although these findings are preliminary and the changes observed were at the trend level only, they suggest that people with intermediate levels of uptake of an amyloid imaging agent might be at increased risk for Alzheimer’s disease, he said.
Dr. Zurkovsky’s study is supported by funding from the National Institute on Aging, Vanderbilt University, and the University of Vermont, Burlington. Dr. Yates’s study is supported by the National Ageing Research Institute at the University of Melbourne, with additional support from Bayer and Piramal Imaging. Neither Dr. Zurkovsky nor Dr. Yates reported having financial disclosures.
BOSTON – Women who complain about cognitive problems after menopause might have increases rather than decreases in volume of certain brain regions, reported investigators at the Alzheimer’s Association International Conference 2013.
Brain imaging studies of 48 women in the early years of menopause showed that those with subjective cognitive complaints had significantly greater volumes in certain regions of the brain than did noncomplainers, including the right posterior cingulate gyrus (P less than .04), the right transverse temporal cortex (P less than .03), The left caudal middle frontal gyrus, and the right paracentral gyrus (P less than .05 for both), said Lilia Zurkovsky, Ph.D., a postdoctoral fellow from the Center for Cognitive Medicine at Vanderbilt University Medical Center in Nashville, Tenn.
The findings appear to run counter to those of another study (Neurology 2006;12:67: 834-42) showing that older adults with cognitive complaints had smaller volumes in temporal and frontal areas, compared with healthy age-matched controls, Dr. Zurkovsky acknowledged. She noted, however, that her group studied postmenopausal women in their 50s and 60s, whereas the earlier study included men. In addition, the mean age for each group in that study was above 70 years.
Combined with recent findings pointing to subjective cognitive and/or memory complaints as possible early signs of Alzheimer’s disease, the studies hint at a possible pattern of change as women age.
"If we look at all the data together, it would seem as though in early middle age, 50- to 60-year old individuals with complaints may be having some sort of compensatory mechanism. That’s speculation; all we know is that they have this increase in volume before they have a decrease in volume as shown by other papers," Dr. Zurkovsky said.
She and her colleagues scanned the participants with T1-weight magnetic resonance imaging with a 3 Tesla magnet, and measured cognitive complaints with the Cognitive Complaint Index (CCI) and five surveys with 120 total questions about the individual’s perceptions of cognitive abilities since menopause.
Brain changes in healthy postmenopausal women were the focus of a second, unrelated study also reported at AAIC2013.
Australian investigators followed participants in the Womens Healthy Ageing Project, a longitudinal study of the menopausal transition among cognitively normal women. The investigators looked at MRI brain scans conducted in 2002 and 2012, and brain-amyloid imaging studies with 18-F florbetaben positron emission tomography (FBB-PET). Florbetaben is an investigational amyloid imaging agent, similar to florbetapir (Amyvid).
They found that among the 26 women who had both FBB-PET and the two MRI scans spaced a decade apart, a significant decline was found over time in hippocampal volume, but not in gray matter volume. There was also a nonsignificant trend correlating higher levels of FBB uptake, as measured by a standardized uptake value ratio (SUVR) with greater declines in hippocampal volume.
"Those who were in the higher tertile of florbetaben SUVR have had an 18%-19% decrement in hippocampal volume over that 10-year period," said lead author Paul Yates, Ph.D., a neuroscientist and imaging research fellow at Austin Health in Heidelberg, Australia.
Although these findings are preliminary and the changes observed were at the trend level only, they suggest that people with intermediate levels of uptake of an amyloid imaging agent might be at increased risk for Alzheimer’s disease, he said.
Dr. Zurkovsky’s study is supported by funding from the National Institute on Aging, Vanderbilt University, and the University of Vermont, Burlington. Dr. Yates’s study is supported by the National Ageing Research Institute at the University of Melbourne, with additional support from Bayer and Piramal Imaging. Neither Dr. Zurkovsky nor Dr. Yates reported having financial disclosures.
BOSTON – Women who complain about cognitive problems after menopause might have increases rather than decreases in volume of certain brain regions, reported investigators at the Alzheimer’s Association International Conference 2013.
Brain imaging studies of 48 women in the early years of menopause showed that those with subjective cognitive complaints had significantly greater volumes in certain regions of the brain than did noncomplainers, including the right posterior cingulate gyrus (P less than .04), the right transverse temporal cortex (P less than .03), The left caudal middle frontal gyrus, and the right paracentral gyrus (P less than .05 for both), said Lilia Zurkovsky, Ph.D., a postdoctoral fellow from the Center for Cognitive Medicine at Vanderbilt University Medical Center in Nashville, Tenn.
The findings appear to run counter to those of another study (Neurology 2006;12:67: 834-42) showing that older adults with cognitive complaints had smaller volumes in temporal and frontal areas, compared with healthy age-matched controls, Dr. Zurkovsky acknowledged. She noted, however, that her group studied postmenopausal women in their 50s and 60s, whereas the earlier study included men. In addition, the mean age for each group in that study was above 70 years.
Combined with recent findings pointing to subjective cognitive and/or memory complaints as possible early signs of Alzheimer’s disease, the studies hint at a possible pattern of change as women age.
"If we look at all the data together, it would seem as though in early middle age, 50- to 60-year old individuals with complaints may be having some sort of compensatory mechanism. That’s speculation; all we know is that they have this increase in volume before they have a decrease in volume as shown by other papers," Dr. Zurkovsky said.
She and her colleagues scanned the participants with T1-weight magnetic resonance imaging with a 3 Tesla magnet, and measured cognitive complaints with the Cognitive Complaint Index (CCI) and five surveys with 120 total questions about the individual’s perceptions of cognitive abilities since menopause.
Brain changes in healthy postmenopausal women were the focus of a second, unrelated study also reported at AAIC2013.
Australian investigators followed participants in the Womens Healthy Ageing Project, a longitudinal study of the menopausal transition among cognitively normal women. The investigators looked at MRI brain scans conducted in 2002 and 2012, and brain-amyloid imaging studies with 18-F florbetaben positron emission tomography (FBB-PET). Florbetaben is an investigational amyloid imaging agent, similar to florbetapir (Amyvid).
They found that among the 26 women who had both FBB-PET and the two MRI scans spaced a decade apart, a significant decline was found over time in hippocampal volume, but not in gray matter volume. There was also a nonsignificant trend correlating higher levels of FBB uptake, as measured by a standardized uptake value ratio (SUVR) with greater declines in hippocampal volume.
"Those who were in the higher tertile of florbetaben SUVR have had an 18%-19% decrement in hippocampal volume over that 10-year period," said lead author Paul Yates, Ph.D., a neuroscientist and imaging research fellow at Austin Health in Heidelberg, Australia.
Although these findings are preliminary and the changes observed were at the trend level only, they suggest that people with intermediate levels of uptake of an amyloid imaging agent might be at increased risk for Alzheimer’s disease, he said.
Dr. Zurkovsky’s study is supported by funding from the National Institute on Aging, Vanderbilt University, and the University of Vermont, Burlington. Dr. Yates’s study is supported by the National Ageing Research Institute at the University of Melbourne, with additional support from Bayer and Piramal Imaging. Neither Dr. Zurkovsky nor Dr. Yates reported having financial disclosures.
AT AAIC2013
Major finding: Postmenopausal women with subjective cognitive complaints had significantly larger volumes of some brain regions, compared with women without subjective cognitive complaints.
Data source: Prospective study of 48 postmenopausal women in their 50s and 60s; and a prospective, longitudinal study of healthy postmenopausal women, 26 of whom had MRI brain scans a decade apart, and brain amyloid imaging.
Disclosures: Dr. Zurkovsky’s study is supported by funding from the National Institute on Aging, Vanderbilt University, and the University of Vermont, Burlington. Dr. Yates’s study is supported by the National Ageing Research Institute at the University of Melbourne, with additional support from Bayer and Piramal Imaging. Neither Dr. Zurkovsky nor Dr. Yates reported having financial disclosures.
Is there a link between impaired mobility and urinary incontinence in elderly, community-dwelling women?
Urinary incontinence affects more than one-third of women aged 70 years or older. As the authors of this study point out, urinary incontinence is comparable to other chronic conditions, such as hypertension and diabetes mellitus, in its impact on quality of life, and is a common reason for institutionalization.
The risk of urinary incontinence increases with age. In elderly women, it is often mixed (ie, having both urge- and stress-related components) and associated with functional impairments, including reduced mobility. It is thought that the association between incontinence and functional impairment is related primarily to the urge component:
- Women with impaired mobility take longer to reach the toilet, increasing the risk of leakage when the urge to urinate is strong
- Women with urge incontinence may be more likely to limit their activities so that they are always near a toilet.
Cognitive impairment may also play a role, affecting motor skills and bladder control.
To better understand why advancing age is linked with urinary incontinence, Fritel and colleagues studied a population of 1,942 urban-dwelling French women aged 75 to 85 years (mean age, 79.3 years; mean body mass index, 25.9 kg/m2).
Details of the study
Investigators assessed the frequency and quantity of urine leaks, the impact of urinary incontinence on daily life, and the participants’ mobility and balance. Data on urinary incontinence were collected via a self-administered questionnaire (the International Consultation on Incontinence Questionnaire–Short Form). Motor-related physical function was assessed using standardized balance and gait tests.
Urinary incontinence was reported by 42% of participants. Of these women, 57% reported daily urine leakage, with mixed incontinence found to be more prevalent than urge incontinence, which was more prevalent than stress incontinence. Overall, women with urinary incontinence reported that its impact on daily life was mild. Among those with mixed or urge incontinence, limitations in mobility and balance were correlated significantly with the severity of incontinence.
What this evidence means for practice
These findings support earlier data suggesting that impaired mobility can promote urge and mixed urinary incontinence. Because there also is a possibility that cerebral deterioration in aging women causes gait and balance problems that increase the likelihood of urge incontinence, the authors advise against the use of anticholinergic agents in elderly women, as these drugs can impair cognitive function. Another take-home message from this French report is that we should counsel elderly patients about the importance of maintaining balance and mobility through exercise, physical therapy, or other strategies.
Andrew M. Kaunitz, MD
Urinary incontinence affects more than one-third of women aged 70 years or older. As the authors of this study point out, urinary incontinence is comparable to other chronic conditions, such as hypertension and diabetes mellitus, in its impact on quality of life, and is a common reason for institutionalization.
The risk of urinary incontinence increases with age. In elderly women, it is often mixed (ie, having both urge- and stress-related components) and associated with functional impairments, including reduced mobility. It is thought that the association between incontinence and functional impairment is related primarily to the urge component:
- Women with impaired mobility take longer to reach the toilet, increasing the risk of leakage when the urge to urinate is strong
- Women with urge incontinence may be more likely to limit their activities so that they are always near a toilet.
Cognitive impairment may also play a role, affecting motor skills and bladder control.
To better understand why advancing age is linked with urinary incontinence, Fritel and colleagues studied a population of 1,942 urban-dwelling French women aged 75 to 85 years (mean age, 79.3 years; mean body mass index, 25.9 kg/m2).
Details of the study
Investigators assessed the frequency and quantity of urine leaks, the impact of urinary incontinence on daily life, and the participants’ mobility and balance. Data on urinary incontinence were collected via a self-administered questionnaire (the International Consultation on Incontinence Questionnaire–Short Form). Motor-related physical function was assessed using standardized balance and gait tests.
Urinary incontinence was reported by 42% of participants. Of these women, 57% reported daily urine leakage, with mixed incontinence found to be more prevalent than urge incontinence, which was more prevalent than stress incontinence. Overall, women with urinary incontinence reported that its impact on daily life was mild. Among those with mixed or urge incontinence, limitations in mobility and balance were correlated significantly with the severity of incontinence.
What this evidence means for practice
These findings support earlier data suggesting that impaired mobility can promote urge and mixed urinary incontinence. Because there also is a possibility that cerebral deterioration in aging women causes gait and balance problems that increase the likelihood of urge incontinence, the authors advise against the use of anticholinergic agents in elderly women, as these drugs can impair cognitive function. Another take-home message from this French report is that we should counsel elderly patients about the importance of maintaining balance and mobility through exercise, physical therapy, or other strategies.
Andrew M. Kaunitz, MD
Urinary incontinence affects more than one-third of women aged 70 years or older. As the authors of this study point out, urinary incontinence is comparable to other chronic conditions, such as hypertension and diabetes mellitus, in its impact on quality of life, and is a common reason for institutionalization.
The risk of urinary incontinence increases with age. In elderly women, it is often mixed (ie, having both urge- and stress-related components) and associated with functional impairments, including reduced mobility. It is thought that the association between incontinence and functional impairment is related primarily to the urge component:
- Women with impaired mobility take longer to reach the toilet, increasing the risk of leakage when the urge to urinate is strong
- Women with urge incontinence may be more likely to limit their activities so that they are always near a toilet.
Cognitive impairment may also play a role, affecting motor skills and bladder control.
To better understand why advancing age is linked with urinary incontinence, Fritel and colleagues studied a population of 1,942 urban-dwelling French women aged 75 to 85 years (mean age, 79.3 years; mean body mass index, 25.9 kg/m2).
Details of the study
Investigators assessed the frequency and quantity of urine leaks, the impact of urinary incontinence on daily life, and the participants’ mobility and balance. Data on urinary incontinence were collected via a self-administered questionnaire (the International Consultation on Incontinence Questionnaire–Short Form). Motor-related physical function was assessed using standardized balance and gait tests.
Urinary incontinence was reported by 42% of participants. Of these women, 57% reported daily urine leakage, with mixed incontinence found to be more prevalent than urge incontinence, which was more prevalent than stress incontinence. Overall, women with urinary incontinence reported that its impact on daily life was mild. Among those with mixed or urge incontinence, limitations in mobility and balance were correlated significantly with the severity of incontinence.
What this evidence means for practice
These findings support earlier data suggesting that impaired mobility can promote urge and mixed urinary incontinence. Because there also is a possibility that cerebral deterioration in aging women causes gait and balance problems that increase the likelihood of urge incontinence, the authors advise against the use of anticholinergic agents in elderly women, as these drugs can impair cognitive function. Another take-home message from this French report is that we should counsel elderly patients about the importance of maintaining balance and mobility through exercise, physical therapy, or other strategies.
Andrew M. Kaunitz, MD
Crisco is an effective vaginal lubricant
June 2013
Crisco is an effective vaginal lubricant
I’ve been in ObGyn practice since 1976—and have recommended Crisco to my patients as a vaginal lubricant for just about as long. I laughed at the mention of storing Crisco in a crystal jar because it sounded like one of my ideas.
Many of my patients use Crisco prior to exercise and other activities because it tends to protect them from irritation. I also have used Crisco as a base for some sexual lubricants that I have compounded, and it seems to work very well.
Another novel remedy: I often recommend cold milk to relieve vaginal irritations and to boost the effect of topical steroids prior to their use—I even patented the treatment and am in the process of licensing it to a consumer company. I previously had a product on the market for treating diaper rash, sore breasts from nursing, irritation from exercise, and other complaints using the same technology and patent.
I now have my patients wet and then freeze a panty liner, apply skim milk to its surface, and wear it until it is no longer cold. It provides great relief from irritation, hair removal, herpetic attacks, and so on.
Stephen M. Renzin, MD
Larchmont, New York
There are better lubricants than Crisco!
The suggestion of Crisco as a vaginal lubricant was distasteful. There are so many products on the market; even olive oil is a better alternative. Crisco stains, gets hot when placed on the body, and is messy. I doubt our male colleagues would apply it to their bodies.
Lisa Riha, DNP, FNP-BC
Yorktown, Virginia
Migraine drug relieves vasomotor symptoms
In over 30 years of practice, I have found that the old migraine drug Bellergal‑S (belladonna, ergotamine, and phenobarbital) works very well to relieve vasomotor symptoms and insomnia. It is clearly inappropriate for women with heart disease, and the brand is no longer made, but it can be compounded, as necessary.
Tanja Todd, MD
Germantown, Tennessee
Dr. Barbieri responds
I appreciate the observations of Dr. Renzin and Dr. Todd regarding the use of cold milk to treat vaginal irritation and a compounded version of Bellergal-S to treat vasomotor symptoms and migraine headache.
In regard to Dr. Riha’s criticism of the use of Crisco as a vaginal lubricant, I agree that there are no large-scale clinical trials comparing the effects of Crisco versus an alternative agent in women. However, many postmenopausal patients with vaginal symptoms report improvement with Crisco.
Outpatient vaginal hysterectomy places undue burden on the family
In her commentary on the study of outpatient vaginal hysterectomy, Dr. Rosanne Kho did not discuss the impact that postoperative care of these patients can have on the family. It is a tremendous challenge for the family to care for a woman who has undergone vaginal hysterectomy. I had two friends, both of whom had excellent support at home, who experienced complications. One had a breakdown of the vaginal cuff and bled. The family transported her to the emergency room (ER), where packing was applied. She was ultimately returned to the operating room and given 2 U of red blood cells, followed by a 3-day hospital stay.
The other friend developed a pulmonary embolus on postoperative day 5, as she had been sedentary due to pain. She was taken to the ER, given heparin, and hospitalized for 3 days. She is now on long-term anticoagulation therapy.
Lisa Riha, DNP, FNP-BC
Yorktown, Virginia
“UPDATE ON MENOPAUSE”
ANDREW M. KAUNITZ, MD (JUNE 2013)
Two questions on menopause management
I have two questions about Dr. Andrew Kaunitz’s Update on Menopause:
- How should I manage a 70-year-old patient who has been taking hormone therapy (HT) for 20 years (transdermal estradiol plus progesterone) and is doing well with stable health? Can Dr. Kaunitz offer any guidelines on dosing, weaning, or maintaining the status quo until the HT is medically contraindicated?
- Does ospemifene have other benefits, such as breast protection and bone health? The package insert, other articles, and my pharmaceutical rep say nothing about additional sites of action. If the drug offers nothing besides protection against vaginal atrophy, is it worth the risk of venous thromboembolism (VTE)?
Maureen O’Regan, MD
Arlington, Virginia
Dr. Kaunitz responds
Dr. O’Regan thoughtfully raises two clinical questions relevant to menopausal practice: management of extended use of HT, and selection of appropriate treatment for symptomatic genital atrophy.
I have several patients in their 70s who are ongoing users of HT. Regrettably, no data from randomized trials are available to provide guidance to providers or patients about the benefits or risks of extended HT. Because the patient Dr. O’Regan describes is taking estradiol and progesterone, she presumably has an intact uterus. We know that the risk of breast cancer increases with increasing duration of estrogen-progestin therapy, so it is important to counsel long-term users proactively about this concern and encourage them to keep up to date with screening mammography.
Even at age 70, there is a substantial risk that vasomotor symptoms will return if HT is discontinued, and it is not clear whether tapering the dose of HT offers any advantages over abrupt discontinuation in this regard.1 Given that age is an independent risk factor for VTE, I agree that transdermal estradiol is more appropriate than oral estrogen in a 70-year-old woman.
In my practice, I encourage older HT users to consider trying a lower dose. If bothersome vasomotor symptoms do not recur, I either continue with a very low dose (especially if the patient has risk factors for osteoporosis) or ultimately discontinue hormone therapy.
Although we know that the selective estrogen receptor modulators (SERMs) tamoxifen and raloxifene provide protection against osteoporosis and breast cancer, I am not aware that ospemifene has been studied in regard to these outcomes. For most of my patients with symptomatic genital atrophy, I plan to continue recommending vaginal estrogen (creams, ring, or tablets). For symptomatic women who would prefer oral therapy, however, ospemifene should prove to be a welcome new option.
Reference
- North American Menopause Society. Position statement: The 2012 hormone therapy position statement of the North American Menopause Society. Menopause. 2012;19(3):257–271.
Another technique for resolving shoulder dystocia
I find another maneuver useful for a shoulder dystocia emergency: I locate the axilla of the anterior shoulder and place my index finger or index and middle finger in the axilla from the posterior aspect and gently rotate the anterior shoulder anteriorly (adduction), thereby reducing the diameter of the shoulders. This maneuver has an effect similar to that of Rubin’s maneuver: It reduces pressure on the shoulder under the pubic bone by applying traction posteriorly toward the maternal perineum.
A key point: To avoid injury, do not apply pressure into the pit of the axilla.
Daniel Sacks, MD
West Palm Beach, Florida
My first maneuver: Deliver the posterior shoulder
I agree with Dr. Barbieri that delivering the posterior shoulder is the preferable method of resolving a shoulder dystocia emergency. If the posterior arm is fully extended, then by applying pressure in the cubital fossa and pushing it posteriorly, one might facilitate flexion of the arm and make it easier to reach the forearm and follow it to the wrist, finally grasping and pulling it. A large mediolateral episiotomy is essential.
This procedure also can be applied during cesarean delivery for a macrosomic fetus. Delivering the posterior shoulder will facilitate the delivery and reduce the risk of extending the incision laterally into the uterine vessels.
Raymond Michael, MD
Marshall, Minnesota
Help the baby “deliver itself”
I first attempt to elevate the head, the opposite approach to what everyone else suggests. This maneuver causes the posterior shoulder to move past the plane of the pubic symphysis and helps disengage the anterior shoulder. Then, with or without suprapubic pressure, I rotate the posterior shoulder anteriorly while ensuring that the “turning of the screw” keeps this shoulder moving anteriorly in front of the plane of the pubic symphysis, and the baby usually just delivers itself.
This maneuver has yet to fail, so I have not had to move on to other techniques, which have usually been performed before I am called.
Robert Graebe, MD
Long Branch, New Jersey
Article on shoulder dystocia prompted anxious memory
The May issue of OBG Management was superb, with great information about cervical management, menopause, cesarean delivery, and more. But the article about shoulder dystocia gave me anxiety because it took me back to the one time I experienced this frightening emergency. After my patient had had a normal pregnancy and uneventful labor, it happened…and the instant “Oh no!” moment of fear. That moment was followed by immediate recall and focused, determined implementation of necessary maneuvers to remedy the matter as soon as possible.
I am happy to report that the baby (just under 7 lb at birth) is now grown and doing quite well.
Melody T. McCloud, MD
Atlanta, Georgia
Dr. Barbieri responds
I appreciate the pearls provided by Dr. Sacks, Dr. Michael, and Dr. Graebe. We thank them for sharing their clinical expertise with the OBG Management community.
As Dr. McCloud reports, a severe shoulder dystocia is a particularly frightening event, forever etched on the memory of the obstetrician. As she attests, a quick response involving a relentlessly rehearsed series of interventions calms the nerves and is the secret to successful resolution of this obstetric emergency.
The robot is unnecessary for benign hysterectomy
The physicians in my practice work in a 210-bed hospital in a sparsely populated state. We don’t have the privilege of using a robot for surgery, so we began performing total laparoscopic hysterectomy (TLH) without the robot about 3 years ago. A few of our earlier cases took as long as 3 hours to complete, but we now are able to perform TLH on almost any benign condition in 30 to 90 minutes.
We have avoided the need to convert to open laparotomy in more than 100 consecutive cases, and have performed TLH in uteri as large as 850 g, as well as in women with stage 4 endometriosis.
The partners in my practice who perform TLH did not find the procedure difficult to learn. Even with laparoscopic suture closure of the vaginal apex, we feel that the robot is unnecessary for laparoscopic hysterectomy for benign conditions.
Our overall abdominal hysterectomy rate for the past 5 years is about 12%, by the way.
Philip Wagner, MD
Cheyenne, Wyoming
June 2013
Crisco is an effective vaginal lubricant
I’ve been in ObGyn practice since 1976—and have recommended Crisco to my patients as a vaginal lubricant for just about as long. I laughed at the mention of storing Crisco in a crystal jar because it sounded like one of my ideas.
Many of my patients use Crisco prior to exercise and other activities because it tends to protect them from irritation. I also have used Crisco as a base for some sexual lubricants that I have compounded, and it seems to work very well.
Another novel remedy: I often recommend cold milk to relieve vaginal irritations and to boost the effect of topical steroids prior to their use—I even patented the treatment and am in the process of licensing it to a consumer company. I previously had a product on the market for treating diaper rash, sore breasts from nursing, irritation from exercise, and other complaints using the same technology and patent.
I now have my patients wet and then freeze a panty liner, apply skim milk to its surface, and wear it until it is no longer cold. It provides great relief from irritation, hair removal, herpetic attacks, and so on.
Stephen M. Renzin, MD
Larchmont, New York
There are better lubricants than Crisco!
The suggestion of Crisco as a vaginal lubricant was distasteful. There are so many products on the market; even olive oil is a better alternative. Crisco stains, gets hot when placed on the body, and is messy. I doubt our male colleagues would apply it to their bodies.
Lisa Riha, DNP, FNP-BC
Yorktown, Virginia
Migraine drug relieves vasomotor symptoms
In over 30 years of practice, I have found that the old migraine drug Bellergal‑S (belladonna, ergotamine, and phenobarbital) works very well to relieve vasomotor symptoms and insomnia. It is clearly inappropriate for women with heart disease, and the brand is no longer made, but it can be compounded, as necessary.
Tanja Todd, MD
Germantown, Tennessee
Dr. Barbieri responds
I appreciate the observations of Dr. Renzin and Dr. Todd regarding the use of cold milk to treat vaginal irritation and a compounded version of Bellergal-S to treat vasomotor symptoms and migraine headache.
In regard to Dr. Riha’s criticism of the use of Crisco as a vaginal lubricant, I agree that there are no large-scale clinical trials comparing the effects of Crisco versus an alternative agent in women. However, many postmenopausal patients with vaginal symptoms report improvement with Crisco.
Outpatient vaginal hysterectomy places undue burden on the family
In her commentary on the study of outpatient vaginal hysterectomy, Dr. Rosanne Kho did not discuss the impact that postoperative care of these patients can have on the family. It is a tremendous challenge for the family to care for a woman who has undergone vaginal hysterectomy. I had two friends, both of whom had excellent support at home, who experienced complications. One had a breakdown of the vaginal cuff and bled. The family transported her to the emergency room (ER), where packing was applied. She was ultimately returned to the operating room and given 2 U of red blood cells, followed by a 3-day hospital stay.
The other friend developed a pulmonary embolus on postoperative day 5, as she had been sedentary due to pain. She was taken to the ER, given heparin, and hospitalized for 3 days. She is now on long-term anticoagulation therapy.
Lisa Riha, DNP, FNP-BC
Yorktown, Virginia
“UPDATE ON MENOPAUSE”
ANDREW M. KAUNITZ, MD (JUNE 2013)
Two questions on menopause management
I have two questions about Dr. Andrew Kaunitz’s Update on Menopause:
- How should I manage a 70-year-old patient who has been taking hormone therapy (HT) for 20 years (transdermal estradiol plus progesterone) and is doing well with stable health? Can Dr. Kaunitz offer any guidelines on dosing, weaning, or maintaining the status quo until the HT is medically contraindicated?
- Does ospemifene have other benefits, such as breast protection and bone health? The package insert, other articles, and my pharmaceutical rep say nothing about additional sites of action. If the drug offers nothing besides protection against vaginal atrophy, is it worth the risk of venous thromboembolism (VTE)?
Maureen O’Regan, MD
Arlington, Virginia
Dr. Kaunitz responds
Dr. O’Regan thoughtfully raises two clinical questions relevant to menopausal practice: management of extended use of HT, and selection of appropriate treatment for symptomatic genital atrophy.
I have several patients in their 70s who are ongoing users of HT. Regrettably, no data from randomized trials are available to provide guidance to providers or patients about the benefits or risks of extended HT. Because the patient Dr. O’Regan describes is taking estradiol and progesterone, she presumably has an intact uterus. We know that the risk of breast cancer increases with increasing duration of estrogen-progestin therapy, so it is important to counsel long-term users proactively about this concern and encourage them to keep up to date with screening mammography.
Even at age 70, there is a substantial risk that vasomotor symptoms will return if HT is discontinued, and it is not clear whether tapering the dose of HT offers any advantages over abrupt discontinuation in this regard.1 Given that age is an independent risk factor for VTE, I agree that transdermal estradiol is more appropriate than oral estrogen in a 70-year-old woman.
In my practice, I encourage older HT users to consider trying a lower dose. If bothersome vasomotor symptoms do not recur, I either continue with a very low dose (especially if the patient has risk factors for osteoporosis) or ultimately discontinue hormone therapy.
Although we know that the selective estrogen receptor modulators (SERMs) tamoxifen and raloxifene provide protection against osteoporosis and breast cancer, I am not aware that ospemifene has been studied in regard to these outcomes. For most of my patients with symptomatic genital atrophy, I plan to continue recommending vaginal estrogen (creams, ring, or tablets). For symptomatic women who would prefer oral therapy, however, ospemifene should prove to be a welcome new option.
Reference
- North American Menopause Society. Position statement: The 2012 hormone therapy position statement of the North American Menopause Society. Menopause. 2012;19(3):257–271.
Another technique for resolving shoulder dystocia
I find another maneuver useful for a shoulder dystocia emergency: I locate the axilla of the anterior shoulder and place my index finger or index and middle finger in the axilla from the posterior aspect and gently rotate the anterior shoulder anteriorly (adduction), thereby reducing the diameter of the shoulders. This maneuver has an effect similar to that of Rubin’s maneuver: It reduces pressure on the shoulder under the pubic bone by applying traction posteriorly toward the maternal perineum.
A key point: To avoid injury, do not apply pressure into the pit of the axilla.
Daniel Sacks, MD
West Palm Beach, Florida
My first maneuver: Deliver the posterior shoulder
I agree with Dr. Barbieri that delivering the posterior shoulder is the preferable method of resolving a shoulder dystocia emergency. If the posterior arm is fully extended, then by applying pressure in the cubital fossa and pushing it posteriorly, one might facilitate flexion of the arm and make it easier to reach the forearm and follow it to the wrist, finally grasping and pulling it. A large mediolateral episiotomy is essential.
This procedure also can be applied during cesarean delivery for a macrosomic fetus. Delivering the posterior shoulder will facilitate the delivery and reduce the risk of extending the incision laterally into the uterine vessels.
Raymond Michael, MD
Marshall, Minnesota
Help the baby “deliver itself”
I first attempt to elevate the head, the opposite approach to what everyone else suggests. This maneuver causes the posterior shoulder to move past the plane of the pubic symphysis and helps disengage the anterior shoulder. Then, with or without suprapubic pressure, I rotate the posterior shoulder anteriorly while ensuring that the “turning of the screw” keeps this shoulder moving anteriorly in front of the plane of the pubic symphysis, and the baby usually just delivers itself.
This maneuver has yet to fail, so I have not had to move on to other techniques, which have usually been performed before I am called.
Robert Graebe, MD
Long Branch, New Jersey
Article on shoulder dystocia prompted anxious memory
The May issue of OBG Management was superb, with great information about cervical management, menopause, cesarean delivery, and more. But the article about shoulder dystocia gave me anxiety because it took me back to the one time I experienced this frightening emergency. After my patient had had a normal pregnancy and uneventful labor, it happened…and the instant “Oh no!” moment of fear. That moment was followed by immediate recall and focused, determined implementation of necessary maneuvers to remedy the matter as soon as possible.
I am happy to report that the baby (just under 7 lb at birth) is now grown and doing quite well.
Melody T. McCloud, MD
Atlanta, Georgia
Dr. Barbieri responds
I appreciate the pearls provided by Dr. Sacks, Dr. Michael, and Dr. Graebe. We thank them for sharing their clinical expertise with the OBG Management community.
As Dr. McCloud reports, a severe shoulder dystocia is a particularly frightening event, forever etched on the memory of the obstetrician. As she attests, a quick response involving a relentlessly rehearsed series of interventions calms the nerves and is the secret to successful resolution of this obstetric emergency.
The robot is unnecessary for benign hysterectomy
The physicians in my practice work in a 210-bed hospital in a sparsely populated state. We don’t have the privilege of using a robot for surgery, so we began performing total laparoscopic hysterectomy (TLH) without the robot about 3 years ago. A few of our earlier cases took as long as 3 hours to complete, but we now are able to perform TLH on almost any benign condition in 30 to 90 minutes.
We have avoided the need to convert to open laparotomy in more than 100 consecutive cases, and have performed TLH in uteri as large as 850 g, as well as in women with stage 4 endometriosis.
The partners in my practice who perform TLH did not find the procedure difficult to learn. Even with laparoscopic suture closure of the vaginal apex, we feel that the robot is unnecessary for laparoscopic hysterectomy for benign conditions.
Our overall abdominal hysterectomy rate for the past 5 years is about 12%, by the way.
Philip Wagner, MD
Cheyenne, Wyoming
June 2013
Crisco is an effective vaginal lubricant
I’ve been in ObGyn practice since 1976—and have recommended Crisco to my patients as a vaginal lubricant for just about as long. I laughed at the mention of storing Crisco in a crystal jar because it sounded like one of my ideas.
Many of my patients use Crisco prior to exercise and other activities because it tends to protect them from irritation. I also have used Crisco as a base for some sexual lubricants that I have compounded, and it seems to work very well.
Another novel remedy: I often recommend cold milk to relieve vaginal irritations and to boost the effect of topical steroids prior to their use—I even patented the treatment and am in the process of licensing it to a consumer company. I previously had a product on the market for treating diaper rash, sore breasts from nursing, irritation from exercise, and other complaints using the same technology and patent.
I now have my patients wet and then freeze a panty liner, apply skim milk to its surface, and wear it until it is no longer cold. It provides great relief from irritation, hair removal, herpetic attacks, and so on.
Stephen M. Renzin, MD
Larchmont, New York
There are better lubricants than Crisco!
The suggestion of Crisco as a vaginal lubricant was distasteful. There are so many products on the market; even olive oil is a better alternative. Crisco stains, gets hot when placed on the body, and is messy. I doubt our male colleagues would apply it to their bodies.
Lisa Riha, DNP, FNP-BC
Yorktown, Virginia
Migraine drug relieves vasomotor symptoms
In over 30 years of practice, I have found that the old migraine drug Bellergal‑S (belladonna, ergotamine, and phenobarbital) works very well to relieve vasomotor symptoms and insomnia. It is clearly inappropriate for women with heart disease, and the brand is no longer made, but it can be compounded, as necessary.
Tanja Todd, MD
Germantown, Tennessee
Dr. Barbieri responds
I appreciate the observations of Dr. Renzin and Dr. Todd regarding the use of cold milk to treat vaginal irritation and a compounded version of Bellergal-S to treat vasomotor symptoms and migraine headache.
In regard to Dr. Riha’s criticism of the use of Crisco as a vaginal lubricant, I agree that there are no large-scale clinical trials comparing the effects of Crisco versus an alternative agent in women. However, many postmenopausal patients with vaginal symptoms report improvement with Crisco.
Outpatient vaginal hysterectomy places undue burden on the family
In her commentary on the study of outpatient vaginal hysterectomy, Dr. Rosanne Kho did not discuss the impact that postoperative care of these patients can have on the family. It is a tremendous challenge for the family to care for a woman who has undergone vaginal hysterectomy. I had two friends, both of whom had excellent support at home, who experienced complications. One had a breakdown of the vaginal cuff and bled. The family transported her to the emergency room (ER), where packing was applied. She was ultimately returned to the operating room and given 2 U of red blood cells, followed by a 3-day hospital stay.
The other friend developed a pulmonary embolus on postoperative day 5, as she had been sedentary due to pain. She was taken to the ER, given heparin, and hospitalized for 3 days. She is now on long-term anticoagulation therapy.
Lisa Riha, DNP, FNP-BC
Yorktown, Virginia
“UPDATE ON MENOPAUSE”
ANDREW M. KAUNITZ, MD (JUNE 2013)
Two questions on menopause management
I have two questions about Dr. Andrew Kaunitz’s Update on Menopause:
- How should I manage a 70-year-old patient who has been taking hormone therapy (HT) for 20 years (transdermal estradiol plus progesterone) and is doing well with stable health? Can Dr. Kaunitz offer any guidelines on dosing, weaning, or maintaining the status quo until the HT is medically contraindicated?
- Does ospemifene have other benefits, such as breast protection and bone health? The package insert, other articles, and my pharmaceutical rep say nothing about additional sites of action. If the drug offers nothing besides protection against vaginal atrophy, is it worth the risk of venous thromboembolism (VTE)?
Maureen O’Regan, MD
Arlington, Virginia
Dr. Kaunitz responds
Dr. O’Regan thoughtfully raises two clinical questions relevant to menopausal practice: management of extended use of HT, and selection of appropriate treatment for symptomatic genital atrophy.
I have several patients in their 70s who are ongoing users of HT. Regrettably, no data from randomized trials are available to provide guidance to providers or patients about the benefits or risks of extended HT. Because the patient Dr. O’Regan describes is taking estradiol and progesterone, she presumably has an intact uterus. We know that the risk of breast cancer increases with increasing duration of estrogen-progestin therapy, so it is important to counsel long-term users proactively about this concern and encourage them to keep up to date with screening mammography.
Even at age 70, there is a substantial risk that vasomotor symptoms will return if HT is discontinued, and it is not clear whether tapering the dose of HT offers any advantages over abrupt discontinuation in this regard.1 Given that age is an independent risk factor for VTE, I agree that transdermal estradiol is more appropriate than oral estrogen in a 70-year-old woman.
In my practice, I encourage older HT users to consider trying a lower dose. If bothersome vasomotor symptoms do not recur, I either continue with a very low dose (especially if the patient has risk factors for osteoporosis) or ultimately discontinue hormone therapy.
Although we know that the selective estrogen receptor modulators (SERMs) tamoxifen and raloxifene provide protection against osteoporosis and breast cancer, I am not aware that ospemifene has been studied in regard to these outcomes. For most of my patients with symptomatic genital atrophy, I plan to continue recommending vaginal estrogen (creams, ring, or tablets). For symptomatic women who would prefer oral therapy, however, ospemifene should prove to be a welcome new option.
Reference
- North American Menopause Society. Position statement: The 2012 hormone therapy position statement of the North American Menopause Society. Menopause. 2012;19(3):257–271.
Another technique for resolving shoulder dystocia
I find another maneuver useful for a shoulder dystocia emergency: I locate the axilla of the anterior shoulder and place my index finger or index and middle finger in the axilla from the posterior aspect and gently rotate the anterior shoulder anteriorly (adduction), thereby reducing the diameter of the shoulders. This maneuver has an effect similar to that of Rubin’s maneuver: It reduces pressure on the shoulder under the pubic bone by applying traction posteriorly toward the maternal perineum.
A key point: To avoid injury, do not apply pressure into the pit of the axilla.
Daniel Sacks, MD
West Palm Beach, Florida
My first maneuver: Deliver the posterior shoulder
I agree with Dr. Barbieri that delivering the posterior shoulder is the preferable method of resolving a shoulder dystocia emergency. If the posterior arm is fully extended, then by applying pressure in the cubital fossa and pushing it posteriorly, one might facilitate flexion of the arm and make it easier to reach the forearm and follow it to the wrist, finally grasping and pulling it. A large mediolateral episiotomy is essential.
This procedure also can be applied during cesarean delivery for a macrosomic fetus. Delivering the posterior shoulder will facilitate the delivery and reduce the risk of extending the incision laterally into the uterine vessels.
Raymond Michael, MD
Marshall, Minnesota
Help the baby “deliver itself”
I first attempt to elevate the head, the opposite approach to what everyone else suggests. This maneuver causes the posterior shoulder to move past the plane of the pubic symphysis and helps disengage the anterior shoulder. Then, with or without suprapubic pressure, I rotate the posterior shoulder anteriorly while ensuring that the “turning of the screw” keeps this shoulder moving anteriorly in front of the plane of the pubic symphysis, and the baby usually just delivers itself.
This maneuver has yet to fail, so I have not had to move on to other techniques, which have usually been performed before I am called.
Robert Graebe, MD
Long Branch, New Jersey
Article on shoulder dystocia prompted anxious memory
The May issue of OBG Management was superb, with great information about cervical management, menopause, cesarean delivery, and more. But the article about shoulder dystocia gave me anxiety because it took me back to the one time I experienced this frightening emergency. After my patient had had a normal pregnancy and uneventful labor, it happened…and the instant “Oh no!” moment of fear. That moment was followed by immediate recall and focused, determined implementation of necessary maneuvers to remedy the matter as soon as possible.
I am happy to report that the baby (just under 7 lb at birth) is now grown and doing quite well.
Melody T. McCloud, MD
Atlanta, Georgia
Dr. Barbieri responds
I appreciate the pearls provided by Dr. Sacks, Dr. Michael, and Dr. Graebe. We thank them for sharing their clinical expertise with the OBG Management community.
As Dr. McCloud reports, a severe shoulder dystocia is a particularly frightening event, forever etched on the memory of the obstetrician. As she attests, a quick response involving a relentlessly rehearsed series of interventions calms the nerves and is the secret to successful resolution of this obstetric emergency.
The robot is unnecessary for benign hysterectomy
The physicians in my practice work in a 210-bed hospital in a sparsely populated state. We don’t have the privilege of using a robot for surgery, so we began performing total laparoscopic hysterectomy (TLH) without the robot about 3 years ago. A few of our earlier cases took as long as 3 hours to complete, but we now are able to perform TLH on almost any benign condition in 30 to 90 minutes.
We have avoided the need to convert to open laparotomy in more than 100 consecutive cases, and have performed TLH in uteri as large as 850 g, as well as in women with stage 4 endometriosis.
The partners in my practice who perform TLH did not find the procedure difficult to learn. Even with laparoscopic suture closure of the vaginal apex, we feel that the robot is unnecessary for laparoscopic hysterectomy for benign conditions.
Our overall abdominal hysterectomy rate for the past 5 years is about 12%, by the way.
Philip Wagner, MD
Cheyenne, Wyoming
Analysis: Estrogen therapy after hysterectomy may have saved lives
Thousands of women in their 50s who had a hysterectomy may have died prematurely since 2002 because they did not use estrogen-only therapy, according to a mathematical analysis of data from the Women’s Health Initiative.
The use of estrogen therapy (ET) has been on a steady decline since 2002, when the Women’s Health Initiative (WHI) halted its trial of estrogen plus progestin due to adverse events, which sent shockwaves among women and the medical community. The therapy’s decline has continued even after recent WHI studies showed mortality benefits from estrogen therapy.
"We felt a sense of urgency about this project," said Dr. David L. Katz, director of the Yale University Prevention Research Center at Griffin Hospital, Derby, Conn., who developed the formula for the analysis.
"Our calculation is simple and robust, and there was really nothing aggressive about our assumptions. The urgency we feel is in getting the word out about the fact that women were dying every year as a result of unwillingness to talk about estrogen therapy," he said in an interview.
"The Mortality Toll of Estrogen Avoidance," part of the title of the study, is a mathematical analysis of the 2011 WHI-ET (Women’s Health Initiative Estrogen-Alone Trial) data, showing that a minimum of 18,600 and as many as 91,600 excess deaths occurred between 2002 and 2011 among hysterectomized women aged 50-59 years due to ET avoidance (Am. J. Public Health 2013 [doi: 10.2105/AJPH.2013.301295]).
In the 1990s, more than 90% of women in their 50s who had a hysterectomy used ET. It was the standard treatment. Research has consistently shown that ET is cardioprotective and bone protective, and relieves menopausal symptoms.
But all that came to a screeching halt in July 2002, when the WHI published the results of the Estrogen Plus Progestin Trial, and terminated the study because of the adverse effects of the therapy, which was the combination drug Prempro. The results were quickly generalized to all forms of hormone therapy, including ET, the authors of the analysis said.
In less than 2 years, half of the women who were using systemic hormone therapy stopped the treatment. Compared with 2001, use of oral estrogen-only among women aged 50-59 years with no uterus dropped by almost 60% in 2004, 71% by 2006, and 79% in 2010 and 2011, the authors noted.
The decline continued despite the positive findings of WHI-ET, first published in 2004, then in 2011, showing that the absolute total mortality risk was reduced by 13 per 10,000 women per year among hysterectomized women aged 50-59 years who were using estrogen during the 10-year follow-up (JAMA 2011;305:1305-14).
"I said to everyone that this is the most important paper in the last 10 years," said Dr. Philip M. Sarrel, one of the authors of the analysis, and emeritus professor of obstetrics and gynecology and psychiatry at Yale University, New Haven, Conn. "I said it’s got to have an impact. It hit the news, and 24 hours later it was gone. There was no impact. They came out and said here’s a lifesaving set of data, and the message just wasn’t heard."
The authors offered several reasons for why the study did not gain traction, but they pointed to clear communication as one of the main pitfalls.
"We’re not criticizing the WHI investigators," Dr. Sarrel said in an interview. "We’re critical of how the nuanced findings were presented."
"We believe that a mortality toll will better communicate the meaning and significance of the WHI-ET findings to women, health care providers, and the media," the authors wrote.
Despite repeated requests, WHI investigators said they were not available to comment.
For their analysis, the researchers used the WHI’s 13 per 10,000 women per year as a point estimate for the mortality burden associated with not using estrogen among this specific group of women. Dr. Katz developed a formula that would apply the excess mortality in women aged 50-59 years who had a hysterectomy to the entire population of comparable women in the United States.
There were more than 49,000 excess deaths over 10 years when the researchers applied the lower estimate for hysterectomy rate in the population. The extreme low estimate showed nearly 22,700 deaths; a higher estimated rate showed almost 59,500 excess deaths, and the extreme high estimate approximately 91,600.
They also calculated the mortality toll of estrogen avoidance for women whose ovaries were retained. When the lower hysterectomy estimates were applied, the sum of excess mortality for both groups was 40,300, the low-end estimate was 18,600, the higher estimate 48,800, and the high-end estimate 75,100.
The range of excess deaths was estimated to be approximately 40,300-48,800, when the researchers used the best available point estimate values with year-by-year adjustment, and adjustment for differential rates of estrogen use with and without retaining ovaries at hysterectomy.
"If you choose to believe that the formula is correct, then this is a very impressive paper," said Dr. David M. Jaspan, a pelvic surgeon at Einstein Medical Center in Philadelphia, who was not involved in the study. "Others may say you’re looking at a paper where the authors came up with their own calculation using ‘extrapolated’ data to generate their own results, so it’s garbage in, garbage out.
"In my opinion, this paper was successful in making the reader think about what we’re doing and think about the data we have, and think, ‘Are we extrapolating the information to patients who do not fit the WHI model?’ This paper should allow the reader to think about the postmenopausal patient population as individuals rather than all postmenopausal women as a group," he said in an interview.
The study authors emphasized that they were not being prescriptive, and that discussions about hormone therapy should be individualized.
"We’re not saying that the WHI harmed anybody," said Dr. Katz. "The only reason we know that there’s a survival advantage with estrogen is because of the WHI. What we’re lamenting is the oversimplified translation of WHI findings into headlines, which have characterized hormone replacement as all bad. Where medicine meets media, we have lumped together baby and bathwater. That’s the problem we’re trying to fix."
The study had several limitations. The estimates may be lower than they actually are because some of the hysterectomies are now done laparoscopically outside of the hospitals and were not taken into account in the calculations. The authors noted that they used the decline in use of oral ET-only for their estimates. They also did not include transdermal ET use, which was included in the WHI studies, and is found to be more effective than oral estrogen in preventing cardiovascular events. Meanwhile, the use of vaginal estrogen has increased between 2001 and 2009, but its effect on mortality needs further study, they said.
Dr. Katz had no disclosures. Dr. Sarrel has been a medical consultant for Noven Therapeutics, which is the maker of transdermal estrogen patches. Dr. Jaspan had no relevant disclosures. The article was funded by the Centers for Disease Control and Prevention.
[email protected]
On Twitter @naseemsmiller
Thousands of women in their 50s who had a hysterectomy may have died prematurely since 2002 because they did not use estrogen-only therapy, according to a mathematical analysis of data from the Women’s Health Initiative.
The use of estrogen therapy (ET) has been on a steady decline since 2002, when the Women’s Health Initiative (WHI) halted its trial of estrogen plus progestin due to adverse events, which sent shockwaves among women and the medical community. The therapy’s decline has continued even after recent WHI studies showed mortality benefits from estrogen therapy.
"We felt a sense of urgency about this project," said Dr. David L. Katz, director of the Yale University Prevention Research Center at Griffin Hospital, Derby, Conn., who developed the formula for the analysis.
"Our calculation is simple and robust, and there was really nothing aggressive about our assumptions. The urgency we feel is in getting the word out about the fact that women were dying every year as a result of unwillingness to talk about estrogen therapy," he said in an interview.
"The Mortality Toll of Estrogen Avoidance," part of the title of the study, is a mathematical analysis of the 2011 WHI-ET (Women’s Health Initiative Estrogen-Alone Trial) data, showing that a minimum of 18,600 and as many as 91,600 excess deaths occurred between 2002 and 2011 among hysterectomized women aged 50-59 years due to ET avoidance (Am. J. Public Health 2013 [doi: 10.2105/AJPH.2013.301295]).
In the 1990s, more than 90% of women in their 50s who had a hysterectomy used ET. It was the standard treatment. Research has consistently shown that ET is cardioprotective and bone protective, and relieves menopausal symptoms.
But all that came to a screeching halt in July 2002, when the WHI published the results of the Estrogen Plus Progestin Trial, and terminated the study because of the adverse effects of the therapy, which was the combination drug Prempro. The results were quickly generalized to all forms of hormone therapy, including ET, the authors of the analysis said.
In less than 2 years, half of the women who were using systemic hormone therapy stopped the treatment. Compared with 2001, use of oral estrogen-only among women aged 50-59 years with no uterus dropped by almost 60% in 2004, 71% by 2006, and 79% in 2010 and 2011, the authors noted.
The decline continued despite the positive findings of WHI-ET, first published in 2004, then in 2011, showing that the absolute total mortality risk was reduced by 13 per 10,000 women per year among hysterectomized women aged 50-59 years who were using estrogen during the 10-year follow-up (JAMA 2011;305:1305-14).
"I said to everyone that this is the most important paper in the last 10 years," said Dr. Philip M. Sarrel, one of the authors of the analysis, and emeritus professor of obstetrics and gynecology and psychiatry at Yale University, New Haven, Conn. "I said it’s got to have an impact. It hit the news, and 24 hours later it was gone. There was no impact. They came out and said here’s a lifesaving set of data, and the message just wasn’t heard."
The authors offered several reasons for why the study did not gain traction, but they pointed to clear communication as one of the main pitfalls.
"We’re not criticizing the WHI investigators," Dr. Sarrel said in an interview. "We’re critical of how the nuanced findings were presented."
"We believe that a mortality toll will better communicate the meaning and significance of the WHI-ET findings to women, health care providers, and the media," the authors wrote.
Despite repeated requests, WHI investigators said they were not available to comment.
For their analysis, the researchers used the WHI’s 13 per 10,000 women per year as a point estimate for the mortality burden associated with not using estrogen among this specific group of women. Dr. Katz developed a formula that would apply the excess mortality in women aged 50-59 years who had a hysterectomy to the entire population of comparable women in the United States.
There were more than 49,000 excess deaths over 10 years when the researchers applied the lower estimate for hysterectomy rate in the population. The extreme low estimate showed nearly 22,700 deaths; a higher estimated rate showed almost 59,500 excess deaths, and the extreme high estimate approximately 91,600.
They also calculated the mortality toll of estrogen avoidance for women whose ovaries were retained. When the lower hysterectomy estimates were applied, the sum of excess mortality for both groups was 40,300, the low-end estimate was 18,600, the higher estimate 48,800, and the high-end estimate 75,100.
The range of excess deaths was estimated to be approximately 40,300-48,800, when the researchers used the best available point estimate values with year-by-year adjustment, and adjustment for differential rates of estrogen use with and without retaining ovaries at hysterectomy.
"If you choose to believe that the formula is correct, then this is a very impressive paper," said Dr. David M. Jaspan, a pelvic surgeon at Einstein Medical Center in Philadelphia, who was not involved in the study. "Others may say you’re looking at a paper where the authors came up with their own calculation using ‘extrapolated’ data to generate their own results, so it’s garbage in, garbage out.
"In my opinion, this paper was successful in making the reader think about what we’re doing and think about the data we have, and think, ‘Are we extrapolating the information to patients who do not fit the WHI model?’ This paper should allow the reader to think about the postmenopausal patient population as individuals rather than all postmenopausal women as a group," he said in an interview.
The study authors emphasized that they were not being prescriptive, and that discussions about hormone therapy should be individualized.
"We’re not saying that the WHI harmed anybody," said Dr. Katz. "The only reason we know that there’s a survival advantage with estrogen is because of the WHI. What we’re lamenting is the oversimplified translation of WHI findings into headlines, which have characterized hormone replacement as all bad. Where medicine meets media, we have lumped together baby and bathwater. That’s the problem we’re trying to fix."
The study had several limitations. The estimates may be lower than they actually are because some of the hysterectomies are now done laparoscopically outside of the hospitals and were not taken into account in the calculations. The authors noted that they used the decline in use of oral ET-only for their estimates. They also did not include transdermal ET use, which was included in the WHI studies, and is found to be more effective than oral estrogen in preventing cardiovascular events. Meanwhile, the use of vaginal estrogen has increased between 2001 and 2009, but its effect on mortality needs further study, they said.
Dr. Katz had no disclosures. Dr. Sarrel has been a medical consultant for Noven Therapeutics, which is the maker of transdermal estrogen patches. Dr. Jaspan had no relevant disclosures. The article was funded by the Centers for Disease Control and Prevention.
[email protected]
On Twitter @naseemsmiller
Thousands of women in their 50s who had a hysterectomy may have died prematurely since 2002 because they did not use estrogen-only therapy, according to a mathematical analysis of data from the Women’s Health Initiative.
The use of estrogen therapy (ET) has been on a steady decline since 2002, when the Women’s Health Initiative (WHI) halted its trial of estrogen plus progestin due to adverse events, which sent shockwaves among women and the medical community. The therapy’s decline has continued even after recent WHI studies showed mortality benefits from estrogen therapy.
"We felt a sense of urgency about this project," said Dr. David L. Katz, director of the Yale University Prevention Research Center at Griffin Hospital, Derby, Conn., who developed the formula for the analysis.
"Our calculation is simple and robust, and there was really nothing aggressive about our assumptions. The urgency we feel is in getting the word out about the fact that women were dying every year as a result of unwillingness to talk about estrogen therapy," he said in an interview.
"The Mortality Toll of Estrogen Avoidance," part of the title of the study, is a mathematical analysis of the 2011 WHI-ET (Women’s Health Initiative Estrogen-Alone Trial) data, showing that a minimum of 18,600 and as many as 91,600 excess deaths occurred between 2002 and 2011 among hysterectomized women aged 50-59 years due to ET avoidance (Am. J. Public Health 2013 [doi: 10.2105/AJPH.2013.301295]).
In the 1990s, more than 90% of women in their 50s who had a hysterectomy used ET. It was the standard treatment. Research has consistently shown that ET is cardioprotective and bone protective, and relieves menopausal symptoms.
But all that came to a screeching halt in July 2002, when the WHI published the results of the Estrogen Plus Progestin Trial, and terminated the study because of the adverse effects of the therapy, which was the combination drug Prempro. The results were quickly generalized to all forms of hormone therapy, including ET, the authors of the analysis said.
In less than 2 years, half of the women who were using systemic hormone therapy stopped the treatment. Compared with 2001, use of oral estrogen-only among women aged 50-59 years with no uterus dropped by almost 60% in 2004, 71% by 2006, and 79% in 2010 and 2011, the authors noted.
The decline continued despite the positive findings of WHI-ET, first published in 2004, then in 2011, showing that the absolute total mortality risk was reduced by 13 per 10,000 women per year among hysterectomized women aged 50-59 years who were using estrogen during the 10-year follow-up (JAMA 2011;305:1305-14).
"I said to everyone that this is the most important paper in the last 10 years," said Dr. Philip M. Sarrel, one of the authors of the analysis, and emeritus professor of obstetrics and gynecology and psychiatry at Yale University, New Haven, Conn. "I said it’s got to have an impact. It hit the news, and 24 hours later it was gone. There was no impact. They came out and said here’s a lifesaving set of data, and the message just wasn’t heard."
The authors offered several reasons for why the study did not gain traction, but they pointed to clear communication as one of the main pitfalls.
"We’re not criticizing the WHI investigators," Dr. Sarrel said in an interview. "We’re critical of how the nuanced findings were presented."
"We believe that a mortality toll will better communicate the meaning and significance of the WHI-ET findings to women, health care providers, and the media," the authors wrote.
Despite repeated requests, WHI investigators said they were not available to comment.
For their analysis, the researchers used the WHI’s 13 per 10,000 women per year as a point estimate for the mortality burden associated with not using estrogen among this specific group of women. Dr. Katz developed a formula that would apply the excess mortality in women aged 50-59 years who had a hysterectomy to the entire population of comparable women in the United States.
There were more than 49,000 excess deaths over 10 years when the researchers applied the lower estimate for hysterectomy rate in the population. The extreme low estimate showed nearly 22,700 deaths; a higher estimated rate showed almost 59,500 excess deaths, and the extreme high estimate approximately 91,600.
They also calculated the mortality toll of estrogen avoidance for women whose ovaries were retained. When the lower hysterectomy estimates were applied, the sum of excess mortality for both groups was 40,300, the low-end estimate was 18,600, the higher estimate 48,800, and the high-end estimate 75,100.
The range of excess deaths was estimated to be approximately 40,300-48,800, when the researchers used the best available point estimate values with year-by-year adjustment, and adjustment for differential rates of estrogen use with and without retaining ovaries at hysterectomy.
"If you choose to believe that the formula is correct, then this is a very impressive paper," said Dr. David M. Jaspan, a pelvic surgeon at Einstein Medical Center in Philadelphia, who was not involved in the study. "Others may say you’re looking at a paper where the authors came up with their own calculation using ‘extrapolated’ data to generate their own results, so it’s garbage in, garbage out.
"In my opinion, this paper was successful in making the reader think about what we’re doing and think about the data we have, and think, ‘Are we extrapolating the information to patients who do not fit the WHI model?’ This paper should allow the reader to think about the postmenopausal patient population as individuals rather than all postmenopausal women as a group," he said in an interview.
The study authors emphasized that they were not being prescriptive, and that discussions about hormone therapy should be individualized.
"We’re not saying that the WHI harmed anybody," said Dr. Katz. "The only reason we know that there’s a survival advantage with estrogen is because of the WHI. What we’re lamenting is the oversimplified translation of WHI findings into headlines, which have characterized hormone replacement as all bad. Where medicine meets media, we have lumped together baby and bathwater. That’s the problem we’re trying to fix."
The study had several limitations. The estimates may be lower than they actually are because some of the hysterectomies are now done laparoscopically outside of the hospitals and were not taken into account in the calculations. The authors noted that they used the decline in use of oral ET-only for their estimates. They also did not include transdermal ET use, which was included in the WHI studies, and is found to be more effective than oral estrogen in preventing cardiovascular events. Meanwhile, the use of vaginal estrogen has increased between 2001 and 2009, but its effect on mortality needs further study, they said.
Dr. Katz had no disclosures. Dr. Sarrel has been a medical consultant for Noven Therapeutics, which is the maker of transdermal estrogen patches. Dr. Jaspan had no relevant disclosures. The article was funded by the Centers for Disease Control and Prevention.
[email protected]
On Twitter @naseemsmiller
FROM THE AMERICAN JOURNAL OF PUBLIC HEALTH
Major finding: A minimum of 18,600 and as many as 91,600 excess deaths occurred between 2002 and 2011 among hysterectomized women aged 50-59 years due to estrogen therapy avoidance
Data source: The 2011 Women’s Health Initiative Estrogen-Only trial
Disclosures: Dr. Katz had no disclosures. Dr. Sarrel has been a medical consultant for Noven Therapeutics, which is the maker of transdermal estrogen patches. Dr. Jaspan had no relevant disclosures. The article was funded by the Centers for Disease Control and Prevention.