User login
STOP enforcing a 5-year rule for menopausal hormone therapy
Immediately after the worrisome initial findings of the Women’s Health Initiative (WHI) were published in July 2002,1 leading organizations and experts in menopausal medicine began advising practitioners to prescribe the “lowest dose of hormones for the shortest period of time.” News headlines that cited menopausal hormone therapy (HT) as a risk factor for myocardial infarction, venous thromboembolism (VTE), gall bladder disease, stroke, urinary incontinence, dementia, and cancers of the breast and lung fueled fear among the lay public and led to a burgeoning market for alternative therapies to address menopausal symptoms.2 Companies that marketed alternative therapies, including bioidentical hormones, often exaggerated the reported risks of menopausal HT and implied that their products were safe and effective, although supporting evidence was lacking.3
More than a decade later, despite a growing body of data reinforcing the safety and efficacy of HT for recently menopausal women,4 many medical professionals remain reluctant to prescribe HT—and when they do prescribe it, they push for a 5-year limit.4,5 This has led to needless suffering and reduced quality of life among thousands of women entering the menopausal transition.6,7
THE IMPORTANCE OF TARGETING HT TO THE APPROPRIATE POPULATION
Over the past decade, experts have conducted in-depth analyses of WHI findings and other contemporary data on the benefits and risks of HT. One fact is clear: The original reports and the way the data were portrayed in the media overstated the risks of HT in newly menopausal women.2,8 Reanalysis has shown that when HT is initiated within 10 years of menopause, the risks are few and generally are outweighed by benefits.9–11 When HT is initiated by women in their 60s and 70s, however, the reverse may be true.
HT is the best therapy for menopausal vasomotor symptoms and has a secondary benefit of preventing osteoporosis.12 HT also may offer cardiovascular benefits in younger menopausal women, although no appropriately powered randomized, clinical trial has yet confirmed this presumption.9,13
Related Article: In young hysterectomized women, does unopposed estrogen therapy increase overall survival? Andrew M. Kaunitz, MD (Examining the Evidence, October 2013)
HT AND BREAST CANCER: CONTEXT IS CRITICAL
The original WHI publication and the news reports that followed emphasized that women using combination estrogen-progestin HT experienced a 24% increase in the incidence of breast cancer, which became apparent in the fifth year of therapy.1 A closer look at the data reveals that the increased incidence of breast cancer reported in this arm of the WHI involved just 38 breast cancers per 10,000 women using HT per year, compared with 30 breast cancers per 10,000 women using placebo. The absolute risk increased by eight breast cancers per 10,000 women, or 0.08%, for each year of use. In the WHI, the 75% of women who were new users of HT actually had no increased risk of breast cancer (hazard ratio [HR], 1.06; 95% confidence interval [CI], 0.81–1.38).
Related Article: Osteoporosis treatment and breast cancer prevention: Two goals, one treatment? Robert L. Barbieri, MD (Editorial, November 2013)
It is important to put this degree of increased risk into perspective. An increase of 0.08% per year is less than one-tenth of a percentage point and is comparable to the risk of breast cancer that a woman accepts if she drinks alcohol regularly, allows herself to become overweight during perimenopause, or fails to exercise at least three times a week.14 Cumulative data from a number of observational studies suggest that the effect of estrogen alone (without a progestin) on breast cancer is even lower, and that estrogen can be taken for many years before any effect is seen. Indeed, among women receiving estrogen alone in the WHI, the risk of breast cancer did not increase. In fact, there was a statistically significant decrease in breast cancer in this population.
Related Article: USPSTF recommends tamoxifen or raloxifene to reduce breast cancer risk in high-risk patients (October 2013)
WHY A 5-YEAR LIMIT IS INAPPROPRIATE
As I explained above, the increase in the incidence of breast cancer observed in the estrogen-progestin arm of the WHI after 5 years represents an increase in the absolute risk of breast cancer of only 0.08% per year. Although HT carries other small potential risks, most experts agree that they are outweighed by the potential benefits among most perimenopausal women. Because an individual’s risks and benefits probably vary according to her personal and family history, clinicians can mitigate the risks, in part, by tailoring the dose, regimen, and route of delivery to the individual’s situation. The risk of VTE is greatest during the first year of HT and approaches background rates thereafter. The risk of stroke in newly menopausal women who initiated HT in the WHI was approximately 1/1,000.13
Health-care practitioners also can minimize the risks of HT by monitoring outcomes, such as blood pressure, unscheduled bleeding, and so on.15 It also may be helpful to counsel patients about interventions for other conditions that contribute to risk, including obesity, smoking, inactivity, hypertension, and hyperlipidemia.
Quality of life was largely ignored in the decade after publication of the initial WHI findings because it was thought that the lives saved by avoiding HT would justify some level of distress.6,7 There also was a presumption—promoted by advocates of natural products and alternative therapies—that interventions such as acupuncture, paced respiration, and herbal remedies were safe and effective at alleviating hot flashes, night sweats, mood swings, and sleep disruption. Complaints of vaginal dryness and dyspareunia from urogenital atrophy often were inadequately addressed because local estrogen was incorrectly thought to increase the risk of hormone-induced breast cancer. Rates of osteoporosis and hip fracture also have risen over the past decade as the protective effect of systemic HT for many women was lost.16
Although most postmenopausal women (60%) experience hot flashes for less than 7 years, as many as 15% report that hot flashes persist for 15 years or longer. The symptoms that can accompany hot flashes (including sweating, palpitations, apprehension, and anxiety) contribute to a woman’s discomfort, inconvenience, and distress, particularly when the hot flashes are frequent, and can be a significant contributor to sleep disturbance. Vasomotor symptoms adversely affect quality of life for 20% to 25% of women, primarily due to the physical discomfort and social embarrassment that they evoke—although night sweats and sleep disturbance also are reported to exert a negative impact.17–19
THE BOTTOM LINE
Nothing magical happens after 5 years of HT to increase a woman’s risk of breast cancer. Any cumulative effect of combination HT on the risk of breast cancer is gradual and small. It is not appropriate to demand that a patient stop HT after 5 years if it affords dramatic improvement in her quality of life, provided she has been correctly informed about potential risks and chooses to continue with therapy.
- Writing Group for the Women`s Health Initiative Investigators. Risks and benefits of estrogen and progestin in healthy postmenopausal women: Principal results of the Women`s Health Initiative randomized controlled trial. JAMA. 2002;288(3):321–333.
- Brown S. Shock, terror and controversy: how the media reacted to the Women’s Health Initiative. Climacteric. 2012;15(3):275–280.
- Bioidentical hormones. Med Lett Drugs Ther. 2010;52(1339):43–44.
- North American Menopause Society. The 2012 Hormone Therapy Position Statement of The North American Menopause Society. Menopause. 2012;19(3):257–271.
- Rossouw JE, Manson JE, Kaunitz AM, Anderson GL. Lessons learned from the Women’s Health Initiative trials of menopausal hormone therapy. Obstet Gynecol. 2013;121(1):172–176.
- Pines A, Sturdee DW, MacLennan AH. Quality of life and the role of menopausal hormone therapy. Climacteric. 2012;15(3):213–216.
- Burger HG, MacLennan AH, Huang K-E, Castelo-Branco C. Evidence-based assessment of the impact of the WHI on women’s health. Climacteric. 2012;15(3):281–287.
- Utian WH. NIH and WHI: Time for a mea culpa and steps beyond. Menopause. 2007;14(6):1056–1059.
- LaCroix AZ, Chlebowski RT, Manson JE, et al. Health outcomes after stopping conjugated equine estrogens among postmenopausal women with prior hysterectomy: A randomized controlled trial. JAMA. 2011;305(13):1305–1314.
- Stuenkel CA, Gass MLS, Manson JE, et al. A decade after the Women`s Health Initiative—The experts do agree. Menopause. 2012;19(8):846-847.
- Langer RD, Manson JE, Allison MA. Have we come full circle—or moved forward? The Women’s Health Initiative 10 years on. Climacteric. 2012;15(3):206–212.
- Gallagher JC, Levine JP. Preventing osteoporosis in symptomatic postmenopausal women. Menopause. 2011;18(1):109–118.
- Hodis HN, Mack WJ. Postmenopausal hormone therapy in clinical perspective. Menopause. 2007;14(5):944–957.
- Singletary SE. Rating the risk factors for breast cancer. Ann Surg. 2003;237(4):474–482.
- Archer DF, Oger E. Estrogen and progestogen effect on venous thromboembolism in menopausal women. Climacteric. 2012;15(3):235–240.
- Islam S, Liu Q, Chines A, Helzner E. Trend in incidence of osteoporosis-related fractures among 40- to 69-year-old women: Analysis of a large insurance claims database, 2000-2005. Menopause. 2009;16(1):77–83.
- Whiteman MK, Staropoli CA, Langenberg PW, McCarter RJ, Kjerulff KH, Flaws JA. Smoking, body mass and hot flashes in midlife women. Obstet Gynecol. 2003;101(2):264–272.
- Utian WH. Psychosocial and socioeconomic burden of vasomotor symptoms in menopause: A comprehensive review. Health Qual Life Outcomes. 2005;3:47.
- Hunter M, Rendall M. Bio-psycho-socio-cultural perspectives on menopause. Best Pract Res Clin Obstet Gynaecol. 2007;21(2):261–274.
Immediately after the worrisome initial findings of the Women’s Health Initiative (WHI) were published in July 2002,1 leading organizations and experts in menopausal medicine began advising practitioners to prescribe the “lowest dose of hormones for the shortest period of time.” News headlines that cited menopausal hormone therapy (HT) as a risk factor for myocardial infarction, venous thromboembolism (VTE), gall bladder disease, stroke, urinary incontinence, dementia, and cancers of the breast and lung fueled fear among the lay public and led to a burgeoning market for alternative therapies to address menopausal symptoms.2 Companies that marketed alternative therapies, including bioidentical hormones, often exaggerated the reported risks of menopausal HT and implied that their products were safe and effective, although supporting evidence was lacking.3
More than a decade later, despite a growing body of data reinforcing the safety and efficacy of HT for recently menopausal women,4 many medical professionals remain reluctant to prescribe HT—and when they do prescribe it, they push for a 5-year limit.4,5 This has led to needless suffering and reduced quality of life among thousands of women entering the menopausal transition.6,7
THE IMPORTANCE OF TARGETING HT TO THE APPROPRIATE POPULATION
Over the past decade, experts have conducted in-depth analyses of WHI findings and other contemporary data on the benefits and risks of HT. One fact is clear: The original reports and the way the data were portrayed in the media overstated the risks of HT in newly menopausal women.2,8 Reanalysis has shown that when HT is initiated within 10 years of menopause, the risks are few and generally are outweighed by benefits.9–11 When HT is initiated by women in their 60s and 70s, however, the reverse may be true.
HT is the best therapy for menopausal vasomotor symptoms and has a secondary benefit of preventing osteoporosis.12 HT also may offer cardiovascular benefits in younger menopausal women, although no appropriately powered randomized, clinical trial has yet confirmed this presumption.9,13
Related Article: In young hysterectomized women, does unopposed estrogen therapy increase overall survival? Andrew M. Kaunitz, MD (Examining the Evidence, October 2013)
HT AND BREAST CANCER: CONTEXT IS CRITICAL
The original WHI publication and the news reports that followed emphasized that women using combination estrogen-progestin HT experienced a 24% increase in the incidence of breast cancer, which became apparent in the fifth year of therapy.1 A closer look at the data reveals that the increased incidence of breast cancer reported in this arm of the WHI involved just 38 breast cancers per 10,000 women using HT per year, compared with 30 breast cancers per 10,000 women using placebo. The absolute risk increased by eight breast cancers per 10,000 women, or 0.08%, for each year of use. In the WHI, the 75% of women who were new users of HT actually had no increased risk of breast cancer (hazard ratio [HR], 1.06; 95% confidence interval [CI], 0.81–1.38).
Related Article: Osteoporosis treatment and breast cancer prevention: Two goals, one treatment? Robert L. Barbieri, MD (Editorial, November 2013)
It is important to put this degree of increased risk into perspective. An increase of 0.08% per year is less than one-tenth of a percentage point and is comparable to the risk of breast cancer that a woman accepts if she drinks alcohol regularly, allows herself to become overweight during perimenopause, or fails to exercise at least three times a week.14 Cumulative data from a number of observational studies suggest that the effect of estrogen alone (without a progestin) on breast cancer is even lower, and that estrogen can be taken for many years before any effect is seen. Indeed, among women receiving estrogen alone in the WHI, the risk of breast cancer did not increase. In fact, there was a statistically significant decrease in breast cancer in this population.
Related Article: USPSTF recommends tamoxifen or raloxifene to reduce breast cancer risk in high-risk patients (October 2013)
WHY A 5-YEAR LIMIT IS INAPPROPRIATE
As I explained above, the increase in the incidence of breast cancer observed in the estrogen-progestin arm of the WHI after 5 years represents an increase in the absolute risk of breast cancer of only 0.08% per year. Although HT carries other small potential risks, most experts agree that they are outweighed by the potential benefits among most perimenopausal women. Because an individual’s risks and benefits probably vary according to her personal and family history, clinicians can mitigate the risks, in part, by tailoring the dose, regimen, and route of delivery to the individual’s situation. The risk of VTE is greatest during the first year of HT and approaches background rates thereafter. The risk of stroke in newly menopausal women who initiated HT in the WHI was approximately 1/1,000.13
Health-care practitioners also can minimize the risks of HT by monitoring outcomes, such as blood pressure, unscheduled bleeding, and so on.15 It also may be helpful to counsel patients about interventions for other conditions that contribute to risk, including obesity, smoking, inactivity, hypertension, and hyperlipidemia.
Quality of life was largely ignored in the decade after publication of the initial WHI findings because it was thought that the lives saved by avoiding HT would justify some level of distress.6,7 There also was a presumption—promoted by advocates of natural products and alternative therapies—that interventions such as acupuncture, paced respiration, and herbal remedies were safe and effective at alleviating hot flashes, night sweats, mood swings, and sleep disruption. Complaints of vaginal dryness and dyspareunia from urogenital atrophy often were inadequately addressed because local estrogen was incorrectly thought to increase the risk of hormone-induced breast cancer. Rates of osteoporosis and hip fracture also have risen over the past decade as the protective effect of systemic HT for many women was lost.16
Although most postmenopausal women (60%) experience hot flashes for less than 7 years, as many as 15% report that hot flashes persist for 15 years or longer. The symptoms that can accompany hot flashes (including sweating, palpitations, apprehension, and anxiety) contribute to a woman’s discomfort, inconvenience, and distress, particularly when the hot flashes are frequent, and can be a significant contributor to sleep disturbance. Vasomotor symptoms adversely affect quality of life for 20% to 25% of women, primarily due to the physical discomfort and social embarrassment that they evoke—although night sweats and sleep disturbance also are reported to exert a negative impact.17–19
THE BOTTOM LINE
Nothing magical happens after 5 years of HT to increase a woman’s risk of breast cancer. Any cumulative effect of combination HT on the risk of breast cancer is gradual and small. It is not appropriate to demand that a patient stop HT after 5 years if it affords dramatic improvement in her quality of life, provided she has been correctly informed about potential risks and chooses to continue with therapy.
Immediately after the worrisome initial findings of the Women’s Health Initiative (WHI) were published in July 2002,1 leading organizations and experts in menopausal medicine began advising practitioners to prescribe the “lowest dose of hormones for the shortest period of time.” News headlines that cited menopausal hormone therapy (HT) as a risk factor for myocardial infarction, venous thromboembolism (VTE), gall bladder disease, stroke, urinary incontinence, dementia, and cancers of the breast and lung fueled fear among the lay public and led to a burgeoning market for alternative therapies to address menopausal symptoms.2 Companies that marketed alternative therapies, including bioidentical hormones, often exaggerated the reported risks of menopausal HT and implied that their products were safe and effective, although supporting evidence was lacking.3
More than a decade later, despite a growing body of data reinforcing the safety and efficacy of HT for recently menopausal women,4 many medical professionals remain reluctant to prescribe HT—and when they do prescribe it, they push for a 5-year limit.4,5 This has led to needless suffering and reduced quality of life among thousands of women entering the menopausal transition.6,7
THE IMPORTANCE OF TARGETING HT TO THE APPROPRIATE POPULATION
Over the past decade, experts have conducted in-depth analyses of WHI findings and other contemporary data on the benefits and risks of HT. One fact is clear: The original reports and the way the data were portrayed in the media overstated the risks of HT in newly menopausal women.2,8 Reanalysis has shown that when HT is initiated within 10 years of menopause, the risks are few and generally are outweighed by benefits.9–11 When HT is initiated by women in their 60s and 70s, however, the reverse may be true.
HT is the best therapy for menopausal vasomotor symptoms and has a secondary benefit of preventing osteoporosis.12 HT also may offer cardiovascular benefits in younger menopausal women, although no appropriately powered randomized, clinical trial has yet confirmed this presumption.9,13
Related Article: In young hysterectomized women, does unopposed estrogen therapy increase overall survival? Andrew M. Kaunitz, MD (Examining the Evidence, October 2013)
HT AND BREAST CANCER: CONTEXT IS CRITICAL
The original WHI publication and the news reports that followed emphasized that women using combination estrogen-progestin HT experienced a 24% increase in the incidence of breast cancer, which became apparent in the fifth year of therapy.1 A closer look at the data reveals that the increased incidence of breast cancer reported in this arm of the WHI involved just 38 breast cancers per 10,000 women using HT per year, compared with 30 breast cancers per 10,000 women using placebo. The absolute risk increased by eight breast cancers per 10,000 women, or 0.08%, for each year of use. In the WHI, the 75% of women who were new users of HT actually had no increased risk of breast cancer (hazard ratio [HR], 1.06; 95% confidence interval [CI], 0.81–1.38).
Related Article: Osteoporosis treatment and breast cancer prevention: Two goals, one treatment? Robert L. Barbieri, MD (Editorial, November 2013)
It is important to put this degree of increased risk into perspective. An increase of 0.08% per year is less than one-tenth of a percentage point and is comparable to the risk of breast cancer that a woman accepts if she drinks alcohol regularly, allows herself to become overweight during perimenopause, or fails to exercise at least three times a week.14 Cumulative data from a number of observational studies suggest that the effect of estrogen alone (without a progestin) on breast cancer is even lower, and that estrogen can be taken for many years before any effect is seen. Indeed, among women receiving estrogen alone in the WHI, the risk of breast cancer did not increase. In fact, there was a statistically significant decrease in breast cancer in this population.
Related Article: USPSTF recommends tamoxifen or raloxifene to reduce breast cancer risk in high-risk patients (October 2013)
WHY A 5-YEAR LIMIT IS INAPPROPRIATE
As I explained above, the increase in the incidence of breast cancer observed in the estrogen-progestin arm of the WHI after 5 years represents an increase in the absolute risk of breast cancer of only 0.08% per year. Although HT carries other small potential risks, most experts agree that they are outweighed by the potential benefits among most perimenopausal women. Because an individual’s risks and benefits probably vary according to her personal and family history, clinicians can mitigate the risks, in part, by tailoring the dose, regimen, and route of delivery to the individual’s situation. The risk of VTE is greatest during the first year of HT and approaches background rates thereafter. The risk of stroke in newly menopausal women who initiated HT in the WHI was approximately 1/1,000.13
Health-care practitioners also can minimize the risks of HT by monitoring outcomes, such as blood pressure, unscheduled bleeding, and so on.15 It also may be helpful to counsel patients about interventions for other conditions that contribute to risk, including obesity, smoking, inactivity, hypertension, and hyperlipidemia.
Quality of life was largely ignored in the decade after publication of the initial WHI findings because it was thought that the lives saved by avoiding HT would justify some level of distress.6,7 There also was a presumption—promoted by advocates of natural products and alternative therapies—that interventions such as acupuncture, paced respiration, and herbal remedies were safe and effective at alleviating hot flashes, night sweats, mood swings, and sleep disruption. Complaints of vaginal dryness and dyspareunia from urogenital atrophy often were inadequately addressed because local estrogen was incorrectly thought to increase the risk of hormone-induced breast cancer. Rates of osteoporosis and hip fracture also have risen over the past decade as the protective effect of systemic HT for many women was lost.16
Although most postmenopausal women (60%) experience hot flashes for less than 7 years, as many as 15% report that hot flashes persist for 15 years or longer. The symptoms that can accompany hot flashes (including sweating, palpitations, apprehension, and anxiety) contribute to a woman’s discomfort, inconvenience, and distress, particularly when the hot flashes are frequent, and can be a significant contributor to sleep disturbance. Vasomotor symptoms adversely affect quality of life for 20% to 25% of women, primarily due to the physical discomfort and social embarrassment that they evoke—although night sweats and sleep disturbance also are reported to exert a negative impact.17–19
THE BOTTOM LINE
Nothing magical happens after 5 years of HT to increase a woman’s risk of breast cancer. Any cumulative effect of combination HT on the risk of breast cancer is gradual and small. It is not appropriate to demand that a patient stop HT after 5 years if it affords dramatic improvement in her quality of life, provided she has been correctly informed about potential risks and chooses to continue with therapy.
- Writing Group for the Women`s Health Initiative Investigators. Risks and benefits of estrogen and progestin in healthy postmenopausal women: Principal results of the Women`s Health Initiative randomized controlled trial. JAMA. 2002;288(3):321–333.
- Brown S. Shock, terror and controversy: how the media reacted to the Women’s Health Initiative. Climacteric. 2012;15(3):275–280.
- Bioidentical hormones. Med Lett Drugs Ther. 2010;52(1339):43–44.
- North American Menopause Society. The 2012 Hormone Therapy Position Statement of The North American Menopause Society. Menopause. 2012;19(3):257–271.
- Rossouw JE, Manson JE, Kaunitz AM, Anderson GL. Lessons learned from the Women’s Health Initiative trials of menopausal hormone therapy. Obstet Gynecol. 2013;121(1):172–176.
- Pines A, Sturdee DW, MacLennan AH. Quality of life and the role of menopausal hormone therapy. Climacteric. 2012;15(3):213–216.
- Burger HG, MacLennan AH, Huang K-E, Castelo-Branco C. Evidence-based assessment of the impact of the WHI on women’s health. Climacteric. 2012;15(3):281–287.
- Utian WH. NIH and WHI: Time for a mea culpa and steps beyond. Menopause. 2007;14(6):1056–1059.
- LaCroix AZ, Chlebowski RT, Manson JE, et al. Health outcomes after stopping conjugated equine estrogens among postmenopausal women with prior hysterectomy: A randomized controlled trial. JAMA. 2011;305(13):1305–1314.
- Stuenkel CA, Gass MLS, Manson JE, et al. A decade after the Women`s Health Initiative—The experts do agree. Menopause. 2012;19(8):846-847.
- Langer RD, Manson JE, Allison MA. Have we come full circle—or moved forward? The Women’s Health Initiative 10 years on. Climacteric. 2012;15(3):206–212.
- Gallagher JC, Levine JP. Preventing osteoporosis in symptomatic postmenopausal women. Menopause. 2011;18(1):109–118.
- Hodis HN, Mack WJ. Postmenopausal hormone therapy in clinical perspective. Menopause. 2007;14(5):944–957.
- Singletary SE. Rating the risk factors for breast cancer. Ann Surg. 2003;237(4):474–482.
- Archer DF, Oger E. Estrogen and progestogen effect on venous thromboembolism in menopausal women. Climacteric. 2012;15(3):235–240.
- Islam S, Liu Q, Chines A, Helzner E. Trend in incidence of osteoporosis-related fractures among 40- to 69-year-old women: Analysis of a large insurance claims database, 2000-2005. Menopause. 2009;16(1):77–83.
- Whiteman MK, Staropoli CA, Langenberg PW, McCarter RJ, Kjerulff KH, Flaws JA. Smoking, body mass and hot flashes in midlife women. Obstet Gynecol. 2003;101(2):264–272.
- Utian WH. Psychosocial and socioeconomic burden of vasomotor symptoms in menopause: A comprehensive review. Health Qual Life Outcomes. 2005;3:47.
- Hunter M, Rendall M. Bio-psycho-socio-cultural perspectives on menopause. Best Pract Res Clin Obstet Gynaecol. 2007;21(2):261–274.
- Writing Group for the Women`s Health Initiative Investigators. Risks and benefits of estrogen and progestin in healthy postmenopausal women: Principal results of the Women`s Health Initiative randomized controlled trial. JAMA. 2002;288(3):321–333.
- Brown S. Shock, terror and controversy: how the media reacted to the Women’s Health Initiative. Climacteric. 2012;15(3):275–280.
- Bioidentical hormones. Med Lett Drugs Ther. 2010;52(1339):43–44.
- North American Menopause Society. The 2012 Hormone Therapy Position Statement of The North American Menopause Society. Menopause. 2012;19(3):257–271.
- Rossouw JE, Manson JE, Kaunitz AM, Anderson GL. Lessons learned from the Women’s Health Initiative trials of menopausal hormone therapy. Obstet Gynecol. 2013;121(1):172–176.
- Pines A, Sturdee DW, MacLennan AH. Quality of life and the role of menopausal hormone therapy. Climacteric. 2012;15(3):213–216.
- Burger HG, MacLennan AH, Huang K-E, Castelo-Branco C. Evidence-based assessment of the impact of the WHI on women’s health. Climacteric. 2012;15(3):281–287.
- Utian WH. NIH and WHI: Time for a mea culpa and steps beyond. Menopause. 2007;14(6):1056–1059.
- LaCroix AZ, Chlebowski RT, Manson JE, et al. Health outcomes after stopping conjugated equine estrogens among postmenopausal women with prior hysterectomy: A randomized controlled trial. JAMA. 2011;305(13):1305–1314.
- Stuenkel CA, Gass MLS, Manson JE, et al. A decade after the Women`s Health Initiative—The experts do agree. Menopause. 2012;19(8):846-847.
- Langer RD, Manson JE, Allison MA. Have we come full circle—or moved forward? The Women’s Health Initiative 10 years on. Climacteric. 2012;15(3):206–212.
- Gallagher JC, Levine JP. Preventing osteoporosis in symptomatic postmenopausal women. Menopause. 2011;18(1):109–118.
- Hodis HN, Mack WJ. Postmenopausal hormone therapy in clinical perspective. Menopause. 2007;14(5):944–957.
- Singletary SE. Rating the risk factors for breast cancer. Ann Surg. 2003;237(4):474–482.
- Archer DF, Oger E. Estrogen and progestogen effect on venous thromboembolism in menopausal women. Climacteric. 2012;15(3):235–240.
- Islam S, Liu Q, Chines A, Helzner E. Trend in incidence of osteoporosis-related fractures among 40- to 69-year-old women: Analysis of a large insurance claims database, 2000-2005. Menopause. 2009;16(1):77–83.
- Whiteman MK, Staropoli CA, Langenberg PW, McCarter RJ, Kjerulff KH, Flaws JA. Smoking, body mass and hot flashes in midlife women. Obstet Gynecol. 2003;101(2):264–272.
- Utian WH. Psychosocial and socioeconomic burden of vasomotor symptoms in menopause: A comprehensive review. Health Qual Life Outcomes. 2005;3:47.
- Hunter M, Rendall M. Bio-psycho-socio-cultural perspectives on menopause. Best Pract Res Clin Obstet Gynaecol. 2007;21(2):261–274.
Screening yields long-term reduction in CRC mortality
SAN DIEGO – The mortality-reducing benefit of colorectal cancer screening persists long term, according to an updated analysis of the randomized Minnesota Fecal Occult Blood Trial.
Investigators led by Dr. Aasma Shaukat, a gastroenterologist with the University of Minnesota, Minneapolis, analyzed data for more than 46,000 participants aged 50-80 years who were assigned to screening with the fecal occult blood test (FOBT) or no screening.
After the first 18 years, the cumulative colorectal cancer mortality rate was reduced by one-third with annual screening and by one-fifth with biennial screening as compared with no screening, according to initially reported results (J. Natl. Cancer Inst. 1999;91:434-7).
The updated results, now after 30 years of follow-up – the longest of any such trial to date – showed that the reductions in risk with screening were essentially unchanged, Dr. Shaukat reported at the annual meeting of the American College of Gastroenterology.
"Screening for colon cancer using fecal occult blood testing consistently reduces colorectal cancer mortality..., and this effect is sustained through 30 years of follow-up," she commented. "This suggests the effect of polypectomy, because [if] fecal occult blood testing [detected only] early cancers, most of the benefit would be seen in the first 8-10 years. The sustained benefit suggests that these individuals underwent removal of polyps that would have turned into cancer and resulted in a death at 30 years."
In additional trial findings, the benefit of screening appeared to be greater for men than women and greater for those who began screening after the age of 60 years.
Session attendee Dr. Samir Gupta, of the University of California, San Diego, asked, "After the initial 10 or 15 years of protocolized screening, is it possible that screening was going on in the control group" and that might explain the lesser benefit in younger individuals, as they would have had more time to be screened.
"During the trial, through 1998, the screening in the control group was less than 5%, so there wasn’t much screening going on in the control group," Dr. Shaukat replied. However, "after the trial ended, we don’t have information on people’s screening behavior. And screening became popular, particularly in the last two decades. So there is a possibility that a large number of people that were in the control group underwent screening, and that’s diluting the effects that we would have otherwise seen."
She offered a few possible additional reasons for the smaller benefit in the younger group. "One is that group doesn’t have a higher risk of colon cancer and of dying from colon cancer, and hence, we are just not seeing the effect of screening," she said. "The second is that perhaps fecal occult blood testing is fairly insensitive in that age group; that’s something that needs to be explored further."
Session comoderator Dr. Jonathan A. Leighton of the Mayo Clinic in Scottsdale, Ariz., asked, "Why do you think there was that benefit in men over women?"
"That’s something that’s actually been shown in several natural history and modeling studies," Dr. Shaukat replied. "Men have a higher incidence of colon cancer and they have a higher risk of dying from colon cancer compared to women. So it bears to reason that we would see a larger effect of screening among men compared to women." That said, subgroup analyses should be considered hypothesis generating and require confirmation, she acknowledged.
Dr. Carol Burke of the Cleveland Clinic, who also attended the session, asked whether the screening benefit would have been even greater in analyses restricted to adherent patients.
"Compliance-adjusted estimates ... are a lot larger, to the magnitude of about a 40% reduction in colorectal cancer mortality," Dr. Shaukat replied.
"What effect do you think FIT [fecal immunochemical test] would have on the magnitude [of benefit] – similar for FIT or better because of adherence?" Dr. Burke further queried.
"These fecal occult blood tests were rehydrated, so their sensitivity is comparable to modern-day FIT. So we expect FIT to do the same if not better," Dr. Shaukat replied.
In the trial, 46,551 participants in the Minnesota Colon Cancer Control Study were assigned to annual or biennial screening or no screening between 1976 and 1992. Those with positive FOBT results underwent colonoscopy with polypectomy if needed. All groups had annual follow-up thereafter through 1998.
Adherence to screening was high in the trial, with 90% of participants in the screening groups completing at least one FOBT, and no difference between men and women, according to Dr. Shaukat.
At each screening, 10% of participants had positive results, and 83% of this subset overall underwent colonoscopy. The complication rate from colonoscopy was low, at less than 0.1%.
In the updated analysis, 71% of trial participants had died as ascertained from the National Death Index.
In intention-to-treat analyses, the risk of colorectal cancer mortality was a significant 32% lower in the group screened annually (relative risk, 0.68) and 22% lower in the group screened biennially (RR, 0.78) as compared with the nonscreened group, according to data reported at the meeting and recently published (N. Engl. J. Med. 2013;369:1106-14).
"We don’t know what happened after 1998," Dr. Shaukat reminded attendees. "At best, the effects that we are seeing might be dilute, and if truly the control group had remained unscreened, we would have seen perhaps larger differences."
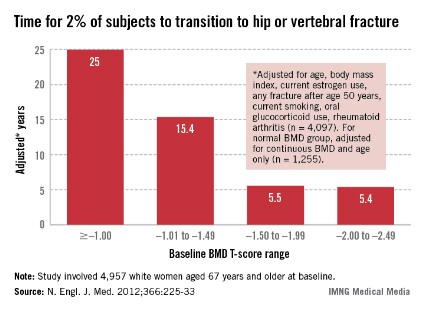
The absolute cumulative colorectal cancer mortality rates were 0.02 with annual screening, 0.02 with biennial screening, and 0.03 with no screening. "This separation [in curves] started at about 13 years of follow-up and persisted through 30 years of follow-up," she pointed out.
All-cause mortality was statistically indistinguishable between groups, although the trial was underpowered to assess this outcome.
In subgroup analyses, there was a near-significant interaction of screening with sex (P = .06), whereby the benefit was greater among men (RR, 0.62) than among women (RR, 0.83).
Additionally, among men, there was a significant interaction with age (P = .04), whereby screening was most beneficial among those 60-69 years old at baseline (RR, 0.46). The benefit among women appeared to be restricted to those who started screening at age 60 or later.
"We don’t have information on the incidence of colon cancer, and hence we can’t comment on right- versus left-sided colon cancer mortality," Dr. Shaukat noted.
Dr. Shaukat disclosed no conflicts of interest.
SAN DIEGO – The mortality-reducing benefit of colorectal cancer screening persists long term, according to an updated analysis of the randomized Minnesota Fecal Occult Blood Trial.
Investigators led by Dr. Aasma Shaukat, a gastroenterologist with the University of Minnesota, Minneapolis, analyzed data for more than 46,000 participants aged 50-80 years who were assigned to screening with the fecal occult blood test (FOBT) or no screening.
After the first 18 years, the cumulative colorectal cancer mortality rate was reduced by one-third with annual screening and by one-fifth with biennial screening as compared with no screening, according to initially reported results (J. Natl. Cancer Inst. 1999;91:434-7).
The updated results, now after 30 years of follow-up – the longest of any such trial to date – showed that the reductions in risk with screening were essentially unchanged, Dr. Shaukat reported at the annual meeting of the American College of Gastroenterology.
"Screening for colon cancer using fecal occult blood testing consistently reduces colorectal cancer mortality..., and this effect is sustained through 30 years of follow-up," she commented. "This suggests the effect of polypectomy, because [if] fecal occult blood testing [detected only] early cancers, most of the benefit would be seen in the first 8-10 years. The sustained benefit suggests that these individuals underwent removal of polyps that would have turned into cancer and resulted in a death at 30 years."
In additional trial findings, the benefit of screening appeared to be greater for men than women and greater for those who began screening after the age of 60 years.
Session attendee Dr. Samir Gupta, of the University of California, San Diego, asked, "After the initial 10 or 15 years of protocolized screening, is it possible that screening was going on in the control group" and that might explain the lesser benefit in younger individuals, as they would have had more time to be screened.
"During the trial, through 1998, the screening in the control group was less than 5%, so there wasn’t much screening going on in the control group," Dr. Shaukat replied. However, "after the trial ended, we don’t have information on people’s screening behavior. And screening became popular, particularly in the last two decades. So there is a possibility that a large number of people that were in the control group underwent screening, and that’s diluting the effects that we would have otherwise seen."
She offered a few possible additional reasons for the smaller benefit in the younger group. "One is that group doesn’t have a higher risk of colon cancer and of dying from colon cancer, and hence, we are just not seeing the effect of screening," she said. "The second is that perhaps fecal occult blood testing is fairly insensitive in that age group; that’s something that needs to be explored further."
Session comoderator Dr. Jonathan A. Leighton of the Mayo Clinic in Scottsdale, Ariz., asked, "Why do you think there was that benefit in men over women?"
"That’s something that’s actually been shown in several natural history and modeling studies," Dr. Shaukat replied. "Men have a higher incidence of colon cancer and they have a higher risk of dying from colon cancer compared to women. So it bears to reason that we would see a larger effect of screening among men compared to women." That said, subgroup analyses should be considered hypothesis generating and require confirmation, she acknowledged.
Dr. Carol Burke of the Cleveland Clinic, who also attended the session, asked whether the screening benefit would have been even greater in analyses restricted to adherent patients.
"Compliance-adjusted estimates ... are a lot larger, to the magnitude of about a 40% reduction in colorectal cancer mortality," Dr. Shaukat replied.
"What effect do you think FIT [fecal immunochemical test] would have on the magnitude [of benefit] – similar for FIT or better because of adherence?" Dr. Burke further queried.
"These fecal occult blood tests were rehydrated, so their sensitivity is comparable to modern-day FIT. So we expect FIT to do the same if not better," Dr. Shaukat replied.
In the trial, 46,551 participants in the Minnesota Colon Cancer Control Study were assigned to annual or biennial screening or no screening between 1976 and 1992. Those with positive FOBT results underwent colonoscopy with polypectomy if needed. All groups had annual follow-up thereafter through 1998.
Adherence to screening was high in the trial, with 90% of participants in the screening groups completing at least one FOBT, and no difference between men and women, according to Dr. Shaukat.
At each screening, 10% of participants had positive results, and 83% of this subset overall underwent colonoscopy. The complication rate from colonoscopy was low, at less than 0.1%.
In the updated analysis, 71% of trial participants had died as ascertained from the National Death Index.
In intention-to-treat analyses, the risk of colorectal cancer mortality was a significant 32% lower in the group screened annually (relative risk, 0.68) and 22% lower in the group screened biennially (RR, 0.78) as compared with the nonscreened group, according to data reported at the meeting and recently published (N. Engl. J. Med. 2013;369:1106-14).
"We don’t know what happened after 1998," Dr. Shaukat reminded attendees. "At best, the effects that we are seeing might be dilute, and if truly the control group had remained unscreened, we would have seen perhaps larger differences."
The absolute cumulative colorectal cancer mortality rates were 0.02 with annual screening, 0.02 with biennial screening, and 0.03 with no screening. "This separation [in curves] started at about 13 years of follow-up and persisted through 30 years of follow-up," she pointed out.
All-cause mortality was statistically indistinguishable between groups, although the trial was underpowered to assess this outcome.
In subgroup analyses, there was a near-significant interaction of screening with sex (P = .06), whereby the benefit was greater among men (RR, 0.62) than among women (RR, 0.83).
Additionally, among men, there was a significant interaction with age (P = .04), whereby screening was most beneficial among those 60-69 years old at baseline (RR, 0.46). The benefit among women appeared to be restricted to those who started screening at age 60 or later.
"We don’t have information on the incidence of colon cancer, and hence we can’t comment on right- versus left-sided colon cancer mortality," Dr. Shaukat noted.
Dr. Shaukat disclosed no conflicts of interest.
SAN DIEGO – The mortality-reducing benefit of colorectal cancer screening persists long term, according to an updated analysis of the randomized Minnesota Fecal Occult Blood Trial.
Investigators led by Dr. Aasma Shaukat, a gastroenterologist with the University of Minnesota, Minneapolis, analyzed data for more than 46,000 participants aged 50-80 years who were assigned to screening with the fecal occult blood test (FOBT) or no screening.
After the first 18 years, the cumulative colorectal cancer mortality rate was reduced by one-third with annual screening and by one-fifth with biennial screening as compared with no screening, according to initially reported results (J. Natl. Cancer Inst. 1999;91:434-7).
The updated results, now after 30 years of follow-up – the longest of any such trial to date – showed that the reductions in risk with screening were essentially unchanged, Dr. Shaukat reported at the annual meeting of the American College of Gastroenterology.
"Screening for colon cancer using fecal occult blood testing consistently reduces colorectal cancer mortality..., and this effect is sustained through 30 years of follow-up," she commented. "This suggests the effect of polypectomy, because [if] fecal occult blood testing [detected only] early cancers, most of the benefit would be seen in the first 8-10 years. The sustained benefit suggests that these individuals underwent removal of polyps that would have turned into cancer and resulted in a death at 30 years."
In additional trial findings, the benefit of screening appeared to be greater for men than women and greater for those who began screening after the age of 60 years.
Session attendee Dr. Samir Gupta, of the University of California, San Diego, asked, "After the initial 10 or 15 years of protocolized screening, is it possible that screening was going on in the control group" and that might explain the lesser benefit in younger individuals, as they would have had more time to be screened.
"During the trial, through 1998, the screening in the control group was less than 5%, so there wasn’t much screening going on in the control group," Dr. Shaukat replied. However, "after the trial ended, we don’t have information on people’s screening behavior. And screening became popular, particularly in the last two decades. So there is a possibility that a large number of people that were in the control group underwent screening, and that’s diluting the effects that we would have otherwise seen."
She offered a few possible additional reasons for the smaller benefit in the younger group. "One is that group doesn’t have a higher risk of colon cancer and of dying from colon cancer, and hence, we are just not seeing the effect of screening," she said. "The second is that perhaps fecal occult blood testing is fairly insensitive in that age group; that’s something that needs to be explored further."
Session comoderator Dr. Jonathan A. Leighton of the Mayo Clinic in Scottsdale, Ariz., asked, "Why do you think there was that benefit in men over women?"
"That’s something that’s actually been shown in several natural history and modeling studies," Dr. Shaukat replied. "Men have a higher incidence of colon cancer and they have a higher risk of dying from colon cancer compared to women. So it bears to reason that we would see a larger effect of screening among men compared to women." That said, subgroup analyses should be considered hypothesis generating and require confirmation, she acknowledged.
Dr. Carol Burke of the Cleveland Clinic, who also attended the session, asked whether the screening benefit would have been even greater in analyses restricted to adherent patients.
"Compliance-adjusted estimates ... are a lot larger, to the magnitude of about a 40% reduction in colorectal cancer mortality," Dr. Shaukat replied.
"What effect do you think FIT [fecal immunochemical test] would have on the magnitude [of benefit] – similar for FIT or better because of adherence?" Dr. Burke further queried.
"These fecal occult blood tests were rehydrated, so their sensitivity is comparable to modern-day FIT. So we expect FIT to do the same if not better," Dr. Shaukat replied.
In the trial, 46,551 participants in the Minnesota Colon Cancer Control Study were assigned to annual or biennial screening or no screening between 1976 and 1992. Those with positive FOBT results underwent colonoscopy with polypectomy if needed. All groups had annual follow-up thereafter through 1998.
Adherence to screening was high in the trial, with 90% of participants in the screening groups completing at least one FOBT, and no difference between men and women, according to Dr. Shaukat.
At each screening, 10% of participants had positive results, and 83% of this subset overall underwent colonoscopy. The complication rate from colonoscopy was low, at less than 0.1%.
In the updated analysis, 71% of trial participants had died as ascertained from the National Death Index.
In intention-to-treat analyses, the risk of colorectal cancer mortality was a significant 32% lower in the group screened annually (relative risk, 0.68) and 22% lower in the group screened biennially (RR, 0.78) as compared with the nonscreened group, according to data reported at the meeting and recently published (N. Engl. J. Med. 2013;369:1106-14).
"We don’t know what happened after 1998," Dr. Shaukat reminded attendees. "At best, the effects that we are seeing might be dilute, and if truly the control group had remained unscreened, we would have seen perhaps larger differences."
The absolute cumulative colorectal cancer mortality rates were 0.02 with annual screening, 0.02 with biennial screening, and 0.03 with no screening. "This separation [in curves] started at about 13 years of follow-up and persisted through 30 years of follow-up," she pointed out.
All-cause mortality was statistically indistinguishable between groups, although the trial was underpowered to assess this outcome.
In subgroup analyses, there was a near-significant interaction of screening with sex (P = .06), whereby the benefit was greater among men (RR, 0.62) than among women (RR, 0.83).
Additionally, among men, there was a significant interaction with age (P = .04), whereby screening was most beneficial among those 60-69 years old at baseline (RR, 0.46). The benefit among women appeared to be restricted to those who started screening at age 60 or later.
"We don’t have information on the incidence of colon cancer, and hence we can’t comment on right- versus left-sided colon cancer mortality," Dr. Shaukat noted.
Dr. Shaukat disclosed no conflicts of interest.
AT THE ACG ANNUAL MEETING
Major finding: After 30 years, participants who had been screened annually and biennially had respective 32% and 22% reductions in colorectal cancer mortality relative to nonscreened peers.
Data source: A randomized trial of fecal occult blood testing among 46,551 individuals aged 50-80 years (the Minnesota Colon Cancer Control Study).
Disclosures: Dr. Shaukat disclosed no conflicts of interest.
Osteoporosis treatment and breast cancer prevention: Two goals, one treatment?
CASE: ACTIVE, HEALTHY, OSTEOPOROTIC, AND AT-RISK FOR BREAST CANCER
Your patient, a 58-year-old, G0 healthy and active woman underwent early menopause at 44 years. She recently had a bone mineral density test, results of which showed a T-score of –2.8 at the spine and –2.5 at the hip. She is taking vitamin D and calcium. She is an exceptionally active woman who plays tennis and walks more than 5 miles daily. Two years earlier, at age 56, she had abnormal mammography results, and a breast biopsy revealed atypical hyperplasia. Her mother has a history of Paget disease of the breast.
Prior to selecting a bone medicine to treat this woman’s osteoporosis, should you assess her risk of breast cancer?
In this editorial, I make the argument that, yes, you should assess this patient’s risk of breast cancer prior to selecting treatment for her osteoporosis, as her level of breast cancer risk can help determine the optimal osteoporosis therapy.
An expert panel convened by the American Society of Clinical Oncology noted that far too few American women are being assessed for their risk of breast cancer, and too few American women at increased risk for breast cancer are being offered pharmacologic preventive therapy.1 One small step to increase the frequency of risk assessment and counseling regarding preventive therapy is to assess breast cancer risk in women with osteoporosis to help guide the selection of a bone medicine.
Options for osteoporosis treatment
Most experts recommend that menopausal women with osteoporosis (T-score ≤–2.5) should be offered a bone medicine in addition to the standard prescription therapy of vitamin D and calcium, exercise, and smoking cessation.
The most commonly prescribed bone medicines are bisphosphonates, estrogen, and raloxifene. Following the publication of the Women’s Health Initiative (WHI) in 2002, the use of estrogen and raloxifene for the treatment of osteoporosis decreased significantly. Both the bisphosphonates and estrogen are believed to be slightly more effective at increasing bone density than raloxifene, but there are no direct head-to-head, large-scale fracture-reduction studies comparing these agents.2 In one large retrospective database study, women taking alendronate or raloxifene were reported to have similar vertebral and nonvertebral fracture rates.3
Unlike the bisphosphonates and estrogen, raloxifene has been demonstrated to reduce the risk of breast cancer by about 50%. For women with newly diagnosed osteoporosis and an above-average risk of developing breast cancer, raloxifene may represent an optimal pharmacologic intervention. But, how would you know if your patient with newly diagnosed osteoporosis is at increased risk for breast cancer?
Assessing breast cancer risk
The Gail breast cancer risk assessment tool is often used in clinical practice to identify women who are at above-average risk for breast cancer (defined as a 5-year risk of developing breast cancer ≥1.66%).4 For the patient described in the opening case, the Gail tool predicts that her 5-year risk of breast cancer is 6%, compared with 1.7% for a woman of the same age who is at average risk. In addition, the Gail tool predicts that for the woman in this case, her lifetime risk of breast cancer, to age 90, is 30.1%, compared with 9.5% for a woman of the same age who is at average risk.
The woman in this case is clearly at increased risk for breast cancer. What are her choices for reducing her risk of developing breast cancer?
Options for preventing breast cancer
There are many strategies to prevent breast cancer, including lifestyle interventions, pharmacotherapy, and mastectomy. Lifestyle interventions that may reduce the risk of breast cancer in postmenopausal women include: maintain a body weight in the normal range,5 reduce or eliminate the consumption of alcoholic beverages,6 exercise daily,7 and quit smoking.8
Mastectomy has been demonstrated to reduce the risk of breast cancer in women at very high risk (BRCA positive), but it is seldom used in women at moderate risk for breast cancer.
Pharmacologic interventions for the prevention of breast cancer include tamoxifen, raloxifene, and exemestane.1 All three agents reduce the risk of breast cancer by about 50%. In fact, the US Preventive Services Task Force (USPSTF) recently recommended the use of tamoxifen or raloxifene to reduce breast cancer risk in patients at high risk. (See “USPSTF recommends tamoxifen or raloxifene to reduce breast cancer risk in high-risk patients”.)
Exemestane is an aromatase inhibitor that causes bone loss. Consequently, this agent would not be an optimal choice for use in a woman with osteoporosis. Like raloxifene, tamoxifen is thought to increase bone density and decrease the risk of osteoporotic fracture.9,10 Consequently, for a woman with osteoporosis, with an elevated risk of breast cancer, raloxifene or tamoxifen could be prescribed with the dual goals of reducing the risk of osteoporoticfracture and reducing the risk of breast cancer.
Two good options: Raloxifene and tamoxifen
Raloxifene and tamoxifen are both good choices for treating osteoporosis in women at high risk for breast cancer. For women with a uterus, raloxifene is the preferred agent because tamoxifen can cause endometrial cancer. For women without a uterus, either raloxifene or tamoxifen could be utilized.
What is the benefit-to-risk ratio for these agents?
Dr. Gabriel Hortobagyi and Dr. Powel Brown have provided a snapshot of the pros and cons of using raloxifene and tamoxifen for breast cancer prophylaxis by estimating benefits and risks in 1,000 women treated for 5 years with an additional 2 years of follow-up (7 years of observation).11 In their analysis it was assumed that the women had a 5-year risk of developing breast cancer of 4% (the mean risk for “high risk” subjects entered into the STAR P-2 Trial).
They calculated that after 7 years of observation, treating 1,000 women with tamoxifen 20 mg daily for 5 years will prevent 20 invasive and 20 noninvasive breast cancers and cause 2.25 endometrial cancers and 3.3 thromboembolic events. Treating 1,000 women with raloxifene 60 mg daily for 5 years will prevent15 invasive and 16 noninvasive breast cancers and cause no cases of endometrial cancer and 2.47 thromboembolic events. They concluded that for these major events, tamoxifen caused 40 beneficial events and 5.55 adverse events for a benefit-to-risk ratio of approximately 7. Raloxifene caused 31 beneficial events and 2.47 adverse events for a benefit-to-risk ratio of approximately 13.
Be aware of treatment-specific adverse effects
Raloxifene and tamoxifen treatment cause different patterns of symptom side effects and gynecologic problems. Tamoxifen treatment results in more vasomotor symptoms, leg cramps, and bladder control problems than treatment with raloxifene. Raloxifene is associated with more dyspareunia, lower libido, and more vaginal dryness, weight gain, and musculoskeletal problems than tamoxifen.12
Tamoxifen treatment results in more problems with leiomyomata, endometriosis, endometrial polyps, and endometrial cancer than treatment with raloxifene.13 In turn, this results in more gynecologic surgical procedures, such as endometrial biopsy, oophorectomy, laparoscopy, and hysteroscopy being performed on women taking tamoxifen than on women taking raloxifene. In the largest clinical trial, adherence to treatment was greater for raloxifene than tamoxifen.12 For women with an intact uterus, raloxifene is likely the better choice for breast cancer prevention.
CASE: I WOULD RECOMMEND RALOXIFENE TO THE CASE PATIENT
For the woman presented in this case—who has osteoporosis and a 5-year risk for breast cancer of 6%, as well as an intact uterus—a 5-year course of raloxifene would be an appropriate treatment both to reduce her risk of breast cancer and to treat her osteoporosis.
To achieve the goal of the American Society of Clinical Oncology to increase the use of chemoprevention in women at increased risk of breast cancer, ObGyns will need to take a lead role in assessing our patients for breast cancer risk and counseling them about chemopreventive options.
We want to hear from you! Tell us what you think.
- Visvanathan K, Hurley P, Bantug E, et al. Use of pharmacologic interventions for breast cancer risk reduction: American Society for Clinical Oncology clinical practice guideline. J Clin Oncol. 2013;31(23):2942–2962.
- Luckey M, Kagan R, Greenspan S, et al. Once-weekly alendronate 70 mg and raloxifene 60 mg daily in the treatment of postmenopausal osteoporosis. Menopause. 2004;11(4):405–415.
- Foster SA, Shi N, Curkendall S, et al. Fractures in women treated with raloxifene or alendronate: A retrospective database analysis. BMC Women’s Health. 2013;13:15–24.
- National Cancer Institute. Breast Cancer Risk Assessment Tool. http://www.cancer.gov/bcrisktool/. Updated May 16, 2011. Accessed October 21, 2013.
- van den Brandt PA, Spiegelman D, Yaun SS, et al. Pooled analysis of prospective cohort studies on height, weight, and breast cancer risk. Am J Epidemiol. 2000;152(6):514–527.
- Bagnardi V, Rota M, Botteri E, et al. Light alcohol drinking and cancer: A meta-analysis. Ann Oncol. 2013;24(5):301–308.
- Lynch BM, Neilson HK, Friedenreich CM. Physical activity and breast cancer prevention. Recent Results Cancer Res. 2011;186:13–42.
- Gaudet MM, Gapstur SM, Sun J, Diver WR, Hannan LM, Thun MJ. Active smoking and breast cancer risk: Original cohort data and meta-analysis. J Natl Cancer Inst. 2013;105(8):515–525.
- Fisher B, Costantino JP, Wickerham DL, et al. Tamoxifen for the prevention of breast cancer: Current status of the National Surgical Adjuvant Breast and Bowel Project P-1 study. J Natl Cancer Inst. 2005;97(22):1652–1662.
- Cooke AL, Metge C, Lix L, Prior HJ, Leslie WD. Tamoxifen use and osteoporotic fracture risk: A population-based analysis. J Clin Oncol. 2008;26(32):5227–5232.
- Hortobagyi GN, Brown PH. Two good choices to prevent breast cancer: great taste, less filling. Cancer Prev Res (Phila). 2010;3(6):681–685.
- Land SR, Wickerham DL, Costantino JP, et al. Patient-reported symptoms and quality of life during treatment with tamoxifen or raloxifene for breast cancer prevention. JAMA. 2006;295(23):2742–2751.
- Runowicz CD, Costantino JP, Wickerham L, et al. Gynecologic conditions in participants in the NSABP breast cancer prevention study of tamoxifen and raloxifene (STAR). Am J Obstet Gynecol. 2011;205(6):535.e1–e5.
CASE: ACTIVE, HEALTHY, OSTEOPOROTIC, AND AT-RISK FOR BREAST CANCER
Your patient, a 58-year-old, G0 healthy and active woman underwent early menopause at 44 years. She recently had a bone mineral density test, results of which showed a T-score of –2.8 at the spine and –2.5 at the hip. She is taking vitamin D and calcium. She is an exceptionally active woman who plays tennis and walks more than 5 miles daily. Two years earlier, at age 56, she had abnormal mammography results, and a breast biopsy revealed atypical hyperplasia. Her mother has a history of Paget disease of the breast.
Prior to selecting a bone medicine to treat this woman’s osteoporosis, should you assess her risk of breast cancer?
In this editorial, I make the argument that, yes, you should assess this patient’s risk of breast cancer prior to selecting treatment for her osteoporosis, as her level of breast cancer risk can help determine the optimal osteoporosis therapy.
An expert panel convened by the American Society of Clinical Oncology noted that far too few American women are being assessed for their risk of breast cancer, and too few American women at increased risk for breast cancer are being offered pharmacologic preventive therapy.1 One small step to increase the frequency of risk assessment and counseling regarding preventive therapy is to assess breast cancer risk in women with osteoporosis to help guide the selection of a bone medicine.
Options for osteoporosis treatment
Most experts recommend that menopausal women with osteoporosis (T-score ≤–2.5) should be offered a bone medicine in addition to the standard prescription therapy of vitamin D and calcium, exercise, and smoking cessation.
The most commonly prescribed bone medicines are bisphosphonates, estrogen, and raloxifene. Following the publication of the Women’s Health Initiative (WHI) in 2002, the use of estrogen and raloxifene for the treatment of osteoporosis decreased significantly. Both the bisphosphonates and estrogen are believed to be slightly more effective at increasing bone density than raloxifene, but there are no direct head-to-head, large-scale fracture-reduction studies comparing these agents.2 In one large retrospective database study, women taking alendronate or raloxifene were reported to have similar vertebral and nonvertebral fracture rates.3
Unlike the bisphosphonates and estrogen, raloxifene has been demonstrated to reduce the risk of breast cancer by about 50%. For women with newly diagnosed osteoporosis and an above-average risk of developing breast cancer, raloxifene may represent an optimal pharmacologic intervention. But, how would you know if your patient with newly diagnosed osteoporosis is at increased risk for breast cancer?
Assessing breast cancer risk
The Gail breast cancer risk assessment tool is often used in clinical practice to identify women who are at above-average risk for breast cancer (defined as a 5-year risk of developing breast cancer ≥1.66%).4 For the patient described in the opening case, the Gail tool predicts that her 5-year risk of breast cancer is 6%, compared with 1.7% for a woman of the same age who is at average risk. In addition, the Gail tool predicts that for the woman in this case, her lifetime risk of breast cancer, to age 90, is 30.1%, compared with 9.5% for a woman of the same age who is at average risk.
The woman in this case is clearly at increased risk for breast cancer. What are her choices for reducing her risk of developing breast cancer?
Options for preventing breast cancer
There are many strategies to prevent breast cancer, including lifestyle interventions, pharmacotherapy, and mastectomy. Lifestyle interventions that may reduce the risk of breast cancer in postmenopausal women include: maintain a body weight in the normal range,5 reduce or eliminate the consumption of alcoholic beverages,6 exercise daily,7 and quit smoking.8
Mastectomy has been demonstrated to reduce the risk of breast cancer in women at very high risk (BRCA positive), but it is seldom used in women at moderate risk for breast cancer.
Pharmacologic interventions for the prevention of breast cancer include tamoxifen, raloxifene, and exemestane.1 All three agents reduce the risk of breast cancer by about 50%. In fact, the US Preventive Services Task Force (USPSTF) recently recommended the use of tamoxifen or raloxifene to reduce breast cancer risk in patients at high risk. (See “USPSTF recommends tamoxifen or raloxifene to reduce breast cancer risk in high-risk patients”.)
Exemestane is an aromatase inhibitor that causes bone loss. Consequently, this agent would not be an optimal choice for use in a woman with osteoporosis. Like raloxifene, tamoxifen is thought to increase bone density and decrease the risk of osteoporotic fracture.9,10 Consequently, for a woman with osteoporosis, with an elevated risk of breast cancer, raloxifene or tamoxifen could be prescribed with the dual goals of reducing the risk of osteoporoticfracture and reducing the risk of breast cancer.
Two good options: Raloxifene and tamoxifen
Raloxifene and tamoxifen are both good choices for treating osteoporosis in women at high risk for breast cancer. For women with a uterus, raloxifene is the preferred agent because tamoxifen can cause endometrial cancer. For women without a uterus, either raloxifene or tamoxifen could be utilized.
What is the benefit-to-risk ratio for these agents?
Dr. Gabriel Hortobagyi and Dr. Powel Brown have provided a snapshot of the pros and cons of using raloxifene and tamoxifen for breast cancer prophylaxis by estimating benefits and risks in 1,000 women treated for 5 years with an additional 2 years of follow-up (7 years of observation).11 In their analysis it was assumed that the women had a 5-year risk of developing breast cancer of 4% (the mean risk for “high risk” subjects entered into the STAR P-2 Trial).
They calculated that after 7 years of observation, treating 1,000 women with tamoxifen 20 mg daily for 5 years will prevent 20 invasive and 20 noninvasive breast cancers and cause 2.25 endometrial cancers and 3.3 thromboembolic events. Treating 1,000 women with raloxifene 60 mg daily for 5 years will prevent15 invasive and 16 noninvasive breast cancers and cause no cases of endometrial cancer and 2.47 thromboembolic events. They concluded that for these major events, tamoxifen caused 40 beneficial events and 5.55 adverse events for a benefit-to-risk ratio of approximately 7. Raloxifene caused 31 beneficial events and 2.47 adverse events for a benefit-to-risk ratio of approximately 13.
Be aware of treatment-specific adverse effects
Raloxifene and tamoxifen treatment cause different patterns of symptom side effects and gynecologic problems. Tamoxifen treatment results in more vasomotor symptoms, leg cramps, and bladder control problems than treatment with raloxifene. Raloxifene is associated with more dyspareunia, lower libido, and more vaginal dryness, weight gain, and musculoskeletal problems than tamoxifen.12
Tamoxifen treatment results in more problems with leiomyomata, endometriosis, endometrial polyps, and endometrial cancer than treatment with raloxifene.13 In turn, this results in more gynecologic surgical procedures, such as endometrial biopsy, oophorectomy, laparoscopy, and hysteroscopy being performed on women taking tamoxifen than on women taking raloxifene. In the largest clinical trial, adherence to treatment was greater for raloxifene than tamoxifen.12 For women with an intact uterus, raloxifene is likely the better choice for breast cancer prevention.
CASE: I WOULD RECOMMEND RALOXIFENE TO THE CASE PATIENT
For the woman presented in this case—who has osteoporosis and a 5-year risk for breast cancer of 6%, as well as an intact uterus—a 5-year course of raloxifene would be an appropriate treatment both to reduce her risk of breast cancer and to treat her osteoporosis.
To achieve the goal of the American Society of Clinical Oncology to increase the use of chemoprevention in women at increased risk of breast cancer, ObGyns will need to take a lead role in assessing our patients for breast cancer risk and counseling them about chemopreventive options.
We want to hear from you! Tell us what you think.
CASE: ACTIVE, HEALTHY, OSTEOPOROTIC, AND AT-RISK FOR BREAST CANCER
Your patient, a 58-year-old, G0 healthy and active woman underwent early menopause at 44 years. She recently had a bone mineral density test, results of which showed a T-score of –2.8 at the spine and –2.5 at the hip. She is taking vitamin D and calcium. She is an exceptionally active woman who plays tennis and walks more than 5 miles daily. Two years earlier, at age 56, she had abnormal mammography results, and a breast biopsy revealed atypical hyperplasia. Her mother has a history of Paget disease of the breast.
Prior to selecting a bone medicine to treat this woman’s osteoporosis, should you assess her risk of breast cancer?
In this editorial, I make the argument that, yes, you should assess this patient’s risk of breast cancer prior to selecting treatment for her osteoporosis, as her level of breast cancer risk can help determine the optimal osteoporosis therapy.
An expert panel convened by the American Society of Clinical Oncology noted that far too few American women are being assessed for their risk of breast cancer, and too few American women at increased risk for breast cancer are being offered pharmacologic preventive therapy.1 One small step to increase the frequency of risk assessment and counseling regarding preventive therapy is to assess breast cancer risk in women with osteoporosis to help guide the selection of a bone medicine.
Options for osteoporosis treatment
Most experts recommend that menopausal women with osteoporosis (T-score ≤–2.5) should be offered a bone medicine in addition to the standard prescription therapy of vitamin D and calcium, exercise, and smoking cessation.
The most commonly prescribed bone medicines are bisphosphonates, estrogen, and raloxifene. Following the publication of the Women’s Health Initiative (WHI) in 2002, the use of estrogen and raloxifene for the treatment of osteoporosis decreased significantly. Both the bisphosphonates and estrogen are believed to be slightly more effective at increasing bone density than raloxifene, but there are no direct head-to-head, large-scale fracture-reduction studies comparing these agents.2 In one large retrospective database study, women taking alendronate or raloxifene were reported to have similar vertebral and nonvertebral fracture rates.3
Unlike the bisphosphonates and estrogen, raloxifene has been demonstrated to reduce the risk of breast cancer by about 50%. For women with newly diagnosed osteoporosis and an above-average risk of developing breast cancer, raloxifene may represent an optimal pharmacologic intervention. But, how would you know if your patient with newly diagnosed osteoporosis is at increased risk for breast cancer?
Assessing breast cancer risk
The Gail breast cancer risk assessment tool is often used in clinical practice to identify women who are at above-average risk for breast cancer (defined as a 5-year risk of developing breast cancer ≥1.66%).4 For the patient described in the opening case, the Gail tool predicts that her 5-year risk of breast cancer is 6%, compared with 1.7% for a woman of the same age who is at average risk. In addition, the Gail tool predicts that for the woman in this case, her lifetime risk of breast cancer, to age 90, is 30.1%, compared with 9.5% for a woman of the same age who is at average risk.
The woman in this case is clearly at increased risk for breast cancer. What are her choices for reducing her risk of developing breast cancer?
Options for preventing breast cancer
There are many strategies to prevent breast cancer, including lifestyle interventions, pharmacotherapy, and mastectomy. Lifestyle interventions that may reduce the risk of breast cancer in postmenopausal women include: maintain a body weight in the normal range,5 reduce or eliminate the consumption of alcoholic beverages,6 exercise daily,7 and quit smoking.8
Mastectomy has been demonstrated to reduce the risk of breast cancer in women at very high risk (BRCA positive), but it is seldom used in women at moderate risk for breast cancer.
Pharmacologic interventions for the prevention of breast cancer include tamoxifen, raloxifene, and exemestane.1 All three agents reduce the risk of breast cancer by about 50%. In fact, the US Preventive Services Task Force (USPSTF) recently recommended the use of tamoxifen or raloxifene to reduce breast cancer risk in patients at high risk. (See “USPSTF recommends tamoxifen or raloxifene to reduce breast cancer risk in high-risk patients”.)
Exemestane is an aromatase inhibitor that causes bone loss. Consequently, this agent would not be an optimal choice for use in a woman with osteoporosis. Like raloxifene, tamoxifen is thought to increase bone density and decrease the risk of osteoporotic fracture.9,10 Consequently, for a woman with osteoporosis, with an elevated risk of breast cancer, raloxifene or tamoxifen could be prescribed with the dual goals of reducing the risk of osteoporoticfracture and reducing the risk of breast cancer.
Two good options: Raloxifene and tamoxifen
Raloxifene and tamoxifen are both good choices for treating osteoporosis in women at high risk for breast cancer. For women with a uterus, raloxifene is the preferred agent because tamoxifen can cause endometrial cancer. For women without a uterus, either raloxifene or tamoxifen could be utilized.
What is the benefit-to-risk ratio for these agents?
Dr. Gabriel Hortobagyi and Dr. Powel Brown have provided a snapshot of the pros and cons of using raloxifene and tamoxifen for breast cancer prophylaxis by estimating benefits and risks in 1,000 women treated for 5 years with an additional 2 years of follow-up (7 years of observation).11 In their analysis it was assumed that the women had a 5-year risk of developing breast cancer of 4% (the mean risk for “high risk” subjects entered into the STAR P-2 Trial).
They calculated that after 7 years of observation, treating 1,000 women with tamoxifen 20 mg daily for 5 years will prevent 20 invasive and 20 noninvasive breast cancers and cause 2.25 endometrial cancers and 3.3 thromboembolic events. Treating 1,000 women with raloxifene 60 mg daily for 5 years will prevent15 invasive and 16 noninvasive breast cancers and cause no cases of endometrial cancer and 2.47 thromboembolic events. They concluded that for these major events, tamoxifen caused 40 beneficial events and 5.55 adverse events for a benefit-to-risk ratio of approximately 7. Raloxifene caused 31 beneficial events and 2.47 adverse events for a benefit-to-risk ratio of approximately 13.
Be aware of treatment-specific adverse effects
Raloxifene and tamoxifen treatment cause different patterns of symptom side effects and gynecologic problems. Tamoxifen treatment results in more vasomotor symptoms, leg cramps, and bladder control problems than treatment with raloxifene. Raloxifene is associated with more dyspareunia, lower libido, and more vaginal dryness, weight gain, and musculoskeletal problems than tamoxifen.12
Tamoxifen treatment results in more problems with leiomyomata, endometriosis, endometrial polyps, and endometrial cancer than treatment with raloxifene.13 In turn, this results in more gynecologic surgical procedures, such as endometrial biopsy, oophorectomy, laparoscopy, and hysteroscopy being performed on women taking tamoxifen than on women taking raloxifene. In the largest clinical trial, adherence to treatment was greater for raloxifene than tamoxifen.12 For women with an intact uterus, raloxifene is likely the better choice for breast cancer prevention.
CASE: I WOULD RECOMMEND RALOXIFENE TO THE CASE PATIENT
For the woman presented in this case—who has osteoporosis and a 5-year risk for breast cancer of 6%, as well as an intact uterus—a 5-year course of raloxifene would be an appropriate treatment both to reduce her risk of breast cancer and to treat her osteoporosis.
To achieve the goal of the American Society of Clinical Oncology to increase the use of chemoprevention in women at increased risk of breast cancer, ObGyns will need to take a lead role in assessing our patients for breast cancer risk and counseling them about chemopreventive options.
We want to hear from you! Tell us what you think.
- Visvanathan K, Hurley P, Bantug E, et al. Use of pharmacologic interventions for breast cancer risk reduction: American Society for Clinical Oncology clinical practice guideline. J Clin Oncol. 2013;31(23):2942–2962.
- Luckey M, Kagan R, Greenspan S, et al. Once-weekly alendronate 70 mg and raloxifene 60 mg daily in the treatment of postmenopausal osteoporosis. Menopause. 2004;11(4):405–415.
- Foster SA, Shi N, Curkendall S, et al. Fractures in women treated with raloxifene or alendronate: A retrospective database analysis. BMC Women’s Health. 2013;13:15–24.
- National Cancer Institute. Breast Cancer Risk Assessment Tool. http://www.cancer.gov/bcrisktool/. Updated May 16, 2011. Accessed October 21, 2013.
- van den Brandt PA, Spiegelman D, Yaun SS, et al. Pooled analysis of prospective cohort studies on height, weight, and breast cancer risk. Am J Epidemiol. 2000;152(6):514–527.
- Bagnardi V, Rota M, Botteri E, et al. Light alcohol drinking and cancer: A meta-analysis. Ann Oncol. 2013;24(5):301–308.
- Lynch BM, Neilson HK, Friedenreich CM. Physical activity and breast cancer prevention. Recent Results Cancer Res. 2011;186:13–42.
- Gaudet MM, Gapstur SM, Sun J, Diver WR, Hannan LM, Thun MJ. Active smoking and breast cancer risk: Original cohort data and meta-analysis. J Natl Cancer Inst. 2013;105(8):515–525.
- Fisher B, Costantino JP, Wickerham DL, et al. Tamoxifen for the prevention of breast cancer: Current status of the National Surgical Adjuvant Breast and Bowel Project P-1 study. J Natl Cancer Inst. 2005;97(22):1652–1662.
- Cooke AL, Metge C, Lix L, Prior HJ, Leslie WD. Tamoxifen use and osteoporotic fracture risk: A population-based analysis. J Clin Oncol. 2008;26(32):5227–5232.
- Hortobagyi GN, Brown PH. Two good choices to prevent breast cancer: great taste, less filling. Cancer Prev Res (Phila). 2010;3(6):681–685.
- Land SR, Wickerham DL, Costantino JP, et al. Patient-reported symptoms and quality of life during treatment with tamoxifen or raloxifene for breast cancer prevention. JAMA. 2006;295(23):2742–2751.
- Runowicz CD, Costantino JP, Wickerham L, et al. Gynecologic conditions in participants in the NSABP breast cancer prevention study of tamoxifen and raloxifene (STAR). Am J Obstet Gynecol. 2011;205(6):535.e1–e5.
- Visvanathan K, Hurley P, Bantug E, et al. Use of pharmacologic interventions for breast cancer risk reduction: American Society for Clinical Oncology clinical practice guideline. J Clin Oncol. 2013;31(23):2942–2962.
- Luckey M, Kagan R, Greenspan S, et al. Once-weekly alendronate 70 mg and raloxifene 60 mg daily in the treatment of postmenopausal osteoporosis. Menopause. 2004;11(4):405–415.
- Foster SA, Shi N, Curkendall S, et al. Fractures in women treated with raloxifene or alendronate: A retrospective database analysis. BMC Women’s Health. 2013;13:15–24.
- National Cancer Institute. Breast Cancer Risk Assessment Tool. http://www.cancer.gov/bcrisktool/. Updated May 16, 2011. Accessed October 21, 2013.
- van den Brandt PA, Spiegelman D, Yaun SS, et al. Pooled analysis of prospective cohort studies on height, weight, and breast cancer risk. Am J Epidemiol. 2000;152(6):514–527.
- Bagnardi V, Rota M, Botteri E, et al. Light alcohol drinking and cancer: A meta-analysis. Ann Oncol. 2013;24(5):301–308.
- Lynch BM, Neilson HK, Friedenreich CM. Physical activity and breast cancer prevention. Recent Results Cancer Res. 2011;186:13–42.
- Gaudet MM, Gapstur SM, Sun J, Diver WR, Hannan LM, Thun MJ. Active smoking and breast cancer risk: Original cohort data and meta-analysis. J Natl Cancer Inst. 2013;105(8):515–525.
- Fisher B, Costantino JP, Wickerham DL, et al. Tamoxifen for the prevention of breast cancer: Current status of the National Surgical Adjuvant Breast and Bowel Project P-1 study. J Natl Cancer Inst. 2005;97(22):1652–1662.
- Cooke AL, Metge C, Lix L, Prior HJ, Leslie WD. Tamoxifen use and osteoporotic fracture risk: A population-based analysis. J Clin Oncol. 2008;26(32):5227–5232.
- Hortobagyi GN, Brown PH. Two good choices to prevent breast cancer: great taste, less filling. Cancer Prev Res (Phila). 2010;3(6):681–685.
- Land SR, Wickerham DL, Costantino JP, et al. Patient-reported symptoms and quality of life during treatment with tamoxifen or raloxifene for breast cancer prevention. JAMA. 2006;295(23):2742–2751.
- Runowicz CD, Costantino JP, Wickerham L, et al. Gynecologic conditions in participants in the NSABP breast cancer prevention study of tamoxifen and raloxifene (STAR). Am J Obstet Gynecol. 2011;205(6):535.e1–e5.
Hormone therapy’s protection against endometrial cancer persists in Women’s Health Initiative follow-up study
AMSTERDAM – The continuous use of estrogen and progestin protects against the development of endometrial cancer in postmenopausal women, according to extended follow-up findings from the seminal Women’s Health Initiative randomized, placebo-controlled trial.
After a median of 13.2 years’ follow-up, there were 35% fewer endometrial cancers among women given combined estrogen and progestin vs. placebo (hazard ratio, 0.65; P = .007). A total of 66 women treated with the hormone therapy (HT) and 95 given placebo had developed endometrial cancer, yielding annual incidences of 0.06% and 0.10%, respectively.
"We do not feel that this effect on endometrial cancer changes the overall balance of risk and benefit of estrogen plus progestin," Dr. Rowan T. Chlebowski stated at the multidisciplinary European cancer congresses.
Dr. Chlebowski of the Los Angeles Biomedical Research Institute at Harbor-UCLA Medical Center in Torrance, added that the original conclusion of the WHI study remains: "Estrogen plus progestin should not be used for chronic disease risk reduction."
Between 1993 and 1998, 16,608 women aged 50-79 years with intact uteri were enrolled into the Women’s Health Initiative randomized, controlled clinical trial of hormone therapy at 40 clinical centers in the United States. The study was halted early as it found an excess of cardiovascular diseases and breast cancer among women given the HT vs. placebo. However, there was a 17% decrease in the risk for endometrial cancer in women given HT, as well as reductions in colorectal cancer and hip fracture (JAMA 2002;288:321-33).
Women who had participated in the study were recontacted to obtain their consent to look at the impact of the HT on their risk of endometrial cancer, with 12,788 (83%) surviving women giving their consent. Of these women, 6,545 had received continuous oral treatment with conjugated equine estrogen (0.625 mg/day) and medroxyprogesterone acetate (2.5 mg/day), and the remaining 6,243 had received placebo.
The findings now reported represented a median of 5.6 years of treatment data and a further 8.2 years of additional follow-up. Analysis revealed that the major difference emerged after the treatment was stopped, with 41 vs. 65 cases reported cases postintervention (HR, 0.59; P = .008).
Dr. Chlebowski reported that in the endometrial cancers that did occur, there were fewer poorly differentiated or anaplastic tumors (HR, 0.51) and fewer cases of regional or distant disease (HR, 0.43) in the HT than placebo group.
Subgroup analyses found that there was a similar effect of the estrogen plus progestin influence on endometrial cancer risk generally, even when body mass index was taken into account. BMI is a known risk factor for endometrial cancer, with risk increasing with increasing body weight.
There were 5 deaths from endometrial cancer in the HT group and 11 in the placebo group, but this difference was not statistically significant (HR, 0.42).
Dr. Marcia Hall, a consultant medical oncologist at the Mount Vernon Cancer Centre in greater London, provided independent comment on the results. "The combination of estrogen plus progestin does indeed protect against endometrial cancer," she said, noting that the results are in line with those of observational studies, such as the U.K.’s Million Women Study (Lancet 2007;369:1703-10).
There are women who may still benefit greatly from HT, Dr. Hall maintained. This includes premenopausal women who have had a hysterectomies, oophorectomies, or chemical castrations for other conditions; women experiencing menopausal symptoms, and those who may be at higher risk for bone diseases, such as osteoporosis and enter the menopause early. "Continuous estrogen, perhaps at the lowest dose possible, is a moderately safe drug in these conditions and situations," Dr. Hall said. She suggested that a low estrogen dose could perhaps be combined with a different progestin and mode of administration, such as levonorgestrel delivered by the intrauterine system (Mirena).
The latest WHI findings "should provoke further exploration of the role of progestins in the prevention of endometrial and possibly colorectal cancers," Dr. Hall concluded. "I hope it may also allow us to rethink the management of endometrial cancer in a population with high-risk comorbidities."
The U.S. National Institutes of Health sponsored the WHI. Dr. Chlebowski has acted as a consultant to Pfizer, Novartis, and Amgen, and has received honorarium from Novartis. Dr. Hall had no relevant conflicts of interest.
AMSTERDAM – The continuous use of estrogen and progestin protects against the development of endometrial cancer in postmenopausal women, according to extended follow-up findings from the seminal Women’s Health Initiative randomized, placebo-controlled trial.
After a median of 13.2 years’ follow-up, there were 35% fewer endometrial cancers among women given combined estrogen and progestin vs. placebo (hazard ratio, 0.65; P = .007). A total of 66 women treated with the hormone therapy (HT) and 95 given placebo had developed endometrial cancer, yielding annual incidences of 0.06% and 0.10%, respectively.
"We do not feel that this effect on endometrial cancer changes the overall balance of risk and benefit of estrogen plus progestin," Dr. Rowan T. Chlebowski stated at the multidisciplinary European cancer congresses.
Dr. Chlebowski of the Los Angeles Biomedical Research Institute at Harbor-UCLA Medical Center in Torrance, added that the original conclusion of the WHI study remains: "Estrogen plus progestin should not be used for chronic disease risk reduction."
Between 1993 and 1998, 16,608 women aged 50-79 years with intact uteri were enrolled into the Women’s Health Initiative randomized, controlled clinical trial of hormone therapy at 40 clinical centers in the United States. The study was halted early as it found an excess of cardiovascular diseases and breast cancer among women given the HT vs. placebo. However, there was a 17% decrease in the risk for endometrial cancer in women given HT, as well as reductions in colorectal cancer and hip fracture (JAMA 2002;288:321-33).
Women who had participated in the study were recontacted to obtain their consent to look at the impact of the HT on their risk of endometrial cancer, with 12,788 (83%) surviving women giving their consent. Of these women, 6,545 had received continuous oral treatment with conjugated equine estrogen (0.625 mg/day) and medroxyprogesterone acetate (2.5 mg/day), and the remaining 6,243 had received placebo.
The findings now reported represented a median of 5.6 years of treatment data and a further 8.2 years of additional follow-up. Analysis revealed that the major difference emerged after the treatment was stopped, with 41 vs. 65 cases reported cases postintervention (HR, 0.59; P = .008).
Dr. Chlebowski reported that in the endometrial cancers that did occur, there were fewer poorly differentiated or anaplastic tumors (HR, 0.51) and fewer cases of regional or distant disease (HR, 0.43) in the HT than placebo group.
Subgroup analyses found that there was a similar effect of the estrogen plus progestin influence on endometrial cancer risk generally, even when body mass index was taken into account. BMI is a known risk factor for endometrial cancer, with risk increasing with increasing body weight.
There were 5 deaths from endometrial cancer in the HT group and 11 in the placebo group, but this difference was not statistically significant (HR, 0.42).
Dr. Marcia Hall, a consultant medical oncologist at the Mount Vernon Cancer Centre in greater London, provided independent comment on the results. "The combination of estrogen plus progestin does indeed protect against endometrial cancer," she said, noting that the results are in line with those of observational studies, such as the U.K.’s Million Women Study (Lancet 2007;369:1703-10).
There are women who may still benefit greatly from HT, Dr. Hall maintained. This includes premenopausal women who have had a hysterectomies, oophorectomies, or chemical castrations for other conditions; women experiencing menopausal symptoms, and those who may be at higher risk for bone diseases, such as osteoporosis and enter the menopause early. "Continuous estrogen, perhaps at the lowest dose possible, is a moderately safe drug in these conditions and situations," Dr. Hall said. She suggested that a low estrogen dose could perhaps be combined with a different progestin and mode of administration, such as levonorgestrel delivered by the intrauterine system (Mirena).
The latest WHI findings "should provoke further exploration of the role of progestins in the prevention of endometrial and possibly colorectal cancers," Dr. Hall concluded. "I hope it may also allow us to rethink the management of endometrial cancer in a population with high-risk comorbidities."
The U.S. National Institutes of Health sponsored the WHI. Dr. Chlebowski has acted as a consultant to Pfizer, Novartis, and Amgen, and has received honorarium from Novartis. Dr. Hall had no relevant conflicts of interest.
AMSTERDAM – The continuous use of estrogen and progestin protects against the development of endometrial cancer in postmenopausal women, according to extended follow-up findings from the seminal Women’s Health Initiative randomized, placebo-controlled trial.
After a median of 13.2 years’ follow-up, there were 35% fewer endometrial cancers among women given combined estrogen and progestin vs. placebo (hazard ratio, 0.65; P = .007). A total of 66 women treated with the hormone therapy (HT) and 95 given placebo had developed endometrial cancer, yielding annual incidences of 0.06% and 0.10%, respectively.
"We do not feel that this effect on endometrial cancer changes the overall balance of risk and benefit of estrogen plus progestin," Dr. Rowan T. Chlebowski stated at the multidisciplinary European cancer congresses.
Dr. Chlebowski of the Los Angeles Biomedical Research Institute at Harbor-UCLA Medical Center in Torrance, added that the original conclusion of the WHI study remains: "Estrogen plus progestin should not be used for chronic disease risk reduction."
Between 1993 and 1998, 16,608 women aged 50-79 years with intact uteri were enrolled into the Women’s Health Initiative randomized, controlled clinical trial of hormone therapy at 40 clinical centers in the United States. The study was halted early as it found an excess of cardiovascular diseases and breast cancer among women given the HT vs. placebo. However, there was a 17% decrease in the risk for endometrial cancer in women given HT, as well as reductions in colorectal cancer and hip fracture (JAMA 2002;288:321-33).
Women who had participated in the study were recontacted to obtain their consent to look at the impact of the HT on their risk of endometrial cancer, with 12,788 (83%) surviving women giving their consent. Of these women, 6,545 had received continuous oral treatment with conjugated equine estrogen (0.625 mg/day) and medroxyprogesterone acetate (2.5 mg/day), and the remaining 6,243 had received placebo.
The findings now reported represented a median of 5.6 years of treatment data and a further 8.2 years of additional follow-up. Analysis revealed that the major difference emerged after the treatment was stopped, with 41 vs. 65 cases reported cases postintervention (HR, 0.59; P = .008).
Dr. Chlebowski reported that in the endometrial cancers that did occur, there were fewer poorly differentiated or anaplastic tumors (HR, 0.51) and fewer cases of regional or distant disease (HR, 0.43) in the HT than placebo group.
Subgroup analyses found that there was a similar effect of the estrogen plus progestin influence on endometrial cancer risk generally, even when body mass index was taken into account. BMI is a known risk factor for endometrial cancer, with risk increasing with increasing body weight.
There were 5 deaths from endometrial cancer in the HT group and 11 in the placebo group, but this difference was not statistically significant (HR, 0.42).
Dr. Marcia Hall, a consultant medical oncologist at the Mount Vernon Cancer Centre in greater London, provided independent comment on the results. "The combination of estrogen plus progestin does indeed protect against endometrial cancer," she said, noting that the results are in line with those of observational studies, such as the U.K.’s Million Women Study (Lancet 2007;369:1703-10).
There are women who may still benefit greatly from HT, Dr. Hall maintained. This includes premenopausal women who have had a hysterectomies, oophorectomies, or chemical castrations for other conditions; women experiencing menopausal symptoms, and those who may be at higher risk for bone diseases, such as osteoporosis and enter the menopause early. "Continuous estrogen, perhaps at the lowest dose possible, is a moderately safe drug in these conditions and situations," Dr. Hall said. She suggested that a low estrogen dose could perhaps be combined with a different progestin and mode of administration, such as levonorgestrel delivered by the intrauterine system (Mirena).
The latest WHI findings "should provoke further exploration of the role of progestins in the prevention of endometrial and possibly colorectal cancers," Dr. Hall concluded. "I hope it may also allow us to rethink the management of endometrial cancer in a population with high-risk comorbidities."
The U.S. National Institutes of Health sponsored the WHI. Dr. Chlebowski has acted as a consultant to Pfizer, Novartis, and Amgen, and has received honorarium from Novartis. Dr. Hall had no relevant conflicts of interest.
AT THE EUROPEAN CANCER CONGRESS 2013
Major finding: HT was associated with a 35% reduction in the risk for endometrial cancer (P = .007) when compared with placebo.
Data source: The Women’s Health Initiative, randomized, double-blind clinical trial of 16,608 postmenopausal women treated with hormone therapy or placebo.
Disclosures: The National Institutes of Health sponsored the WHI. Dr. Chlebowski has acted as a consultant to Pfizer, Novartis, and Amgen, and has received honorarium from Novartis. Dr. Hall had no relevant conflicts of interest.
Breast cancer hormone therapy may affect cognitive function
SAN FRANCISCO – Patients with breast cancer who received hormone therapy were over seven times more likely to show cognitive decline as were untreated patients after controlling for other factors, based on a prospective study of 81 patients.
Further, objective results on neuropsychological testing tended to back up patients’ complaints of cognitive difficulties.
Hormone therapy may be a risk factor for cognitive deficits, and interventional studies should be designed to focus on this group of patients, Dr. Hope S. Rugo and her associates recommended in a poster presentation at a breast cancer symposium sponsored by the American Society of Clinical Oncology.
The study collected neuropsychological test results and patient reports before treatment and at several points after starting hormone therapy (22 patients), chemotherapy (14), or chemotherapy followed by hormone therapy (33), and in a control group of 12 untreated patients.
Compared with baseline results, nearly 25% of patients had cognitive decline on neuropsychological testing after 5 months. (Among treated patients, this occurred 1 month after ending chemotherapy or 5 months after starting hormone therapy.) Nearly 35% had cognitive declines at 9 months of follow-up, and 30% had cognitive declines after 18 months.
"Decline in cognitive function is common in patients receiving adjuvant therapy for early-stage breast cancer," concluded Dr. Rugo, director of the Breast Oncology Clinical Trials Program at the University of California, San Francisco. "Ongoing hormone therapy appears to be a risk factor for worse cognitive function."
Other factors that did not predict cognitive decline in the multivariate analysis included age, education level, average estradiol level over time, and estimated verbal IQ at baseline.
Separate univariate conditional logistic regression analyses found that hormone therapy predicted cognitive decline with an odds ratio of 5, but chemotherapy, radiation therapy, average fatigue over time, and average depression over time were not predictive of cognitive decline at any point.
The study enrolled women aged 35-80 years with early-stage breast cancer. Those who underwent adjuvant therapy received 3-4 months of chemotherapy alone, 5 years of hormone therapy alone, or both.
A separate analysis in the same study looked at how well subjective patient reports correlated with objective measures on neuropsychological tests. Dr. Lara Heflin and her associates found significant cross-section correlations between patient-reported cognitive problems and psychological distress and fatigue.
After researchers controlled for the influence of depression and fatigue, however, significant relationships remained between patients’ perceived cognitive functioning and measurable cognitive decline from baseline (pretreatment) to the first follow-up. Patients whose scores indicated memory decline were more likely to perceive memory problems, and patients whose scores on letter fluency declined were more likely to perceive problems with verbal fluency.
"Patients who self-report cognitive problems may indeed be experiencing cognitive decline, and their self-report should not simply be attributed to fatigue or to psychological factors such as anxiety and depression," concluded Dr. Heflin, a visiting professor of psychology at New Mexico Highlands University, Las Vegas.
Patients in the study had no prior chemotherapy or central nervous system radiation and no history of major psychiatric illness, serious head injury, neurologic disease, drug or alcohol abuse, or significant medical illness. The median age was 54 years, and 78% of patients were white. Patient characteristics were similar between groups.
The symposium was cosponsored by the American Society of Breast Disease, the American Society of Breast Surgeons, the National Consortium of Breast Centers, the Society of Surgical Oncology, and the American Society for Radiation Oncology.
The National Institutes of Health funded the study. Dr. Rugo reported having financial associations with Merck, Novartis, and Pfizer.
On Twitter @sherryboschert
While the relationship between chemotherapy and neurocognitive changes is increasingly well described, cognitive changes associated with hormone therapy for breast cancer has proven to be somewhat complex. Chemotherapy can confound this. There are questions about different reports of outcomes for different drugs, for selective estrogen-receptor modulators and aromatase inhibitors, as well as how menopausal status, prior oophorectomy, and hormone therapy all affect cognitive changes in women.

|
|
The important points of this study are that there are measurements at baseline, 1 month, and 18 months for a longitudinal picture. In addition, both comprehensive neuropsychological testing and patient-reported outcomes are available at each of those time points.
Previous studies have assessed these relationships, including a small study of memory impairment with anastrozole vs. tamoxifen in 31 patients treated for a minimum of 3 months. There was no difference between groups in depression, anxiety and fatigue, but researchers found that those on anastrozole had significantly poorer performance on learning and memory measures than those taking tamoxifen (Menopause 2007;14:995-8).
Another small substudy compared 94 patients enrolled in the ATAC (Arimidex, Tamoxifen Alone or in Combination) study to 35 noncancer controls. There were no differences in working memory, attention and visual memory, but those on endocrine therapy had poorer performance on tests of verbal memory and processing speed compared with controls.
The IBIS II (International Breast Cancer Intervention II) prevention study comparing anastrozole vs. placebo showed no overall difference in cognitive function with the addition of anastrozole. At 6 months, those on anastrozole had poorer memory, but this finding resolved by 24 months.
In a subset of 179 patients in the TEAM (Tamoxifen Exemestane Adjuvant Multinational) phase III adjuvant study, comparing exemestane with tamoxifen then exemestane, there was little change from baseline to 1 year in all domains of cognitive function with the addition of exemestane.
A substudy done from the BIG 1-98 (Breast International Group 1-98) trial indicated significant improvement in cognitive function from year 5 of therapy to year 6 after cessation of hormone therapies. Interestingly, perceived cognition did not change in the patients.
This finding corroborates similar results from a prospective, longitudinal study of cognitive complaints and neuropsychological testing for verbal memory, psychomotor speed, and executive functioning in 189 patients who were beginning hormone therapy at the University of California, Los Angeles. (J. Natl. Cancer Inst. 2013;105:791-801).
While the relationship between chemotherapy and neurocognitive changes is increasingly well described, cognitive changes associated with hormone therapy for breast cancer has proven to be somewhat complex. Chemotherapy can confound this. There are questions about different reports of outcomes for different drugs, for selective estrogen-receptor modulators and aromatase inhibitors, as well as how menopausal status, prior oophorectomy, and hormone therapy all affect cognitive changes in women.

|
|
The important points of this study are that there are measurements at baseline, 1 month, and 18 months for a longitudinal picture. In addition, both comprehensive neuropsychological testing and patient-reported outcomes are available at each of those time points.
Previous studies have assessed these relationships, including a small study of memory impairment with anastrozole vs. tamoxifen in 31 patients treated for a minimum of 3 months. There was no difference between groups in depression, anxiety and fatigue, but researchers found that those on anastrozole had significantly poorer performance on learning and memory measures than those taking tamoxifen (Menopause 2007;14:995-8).
Another small substudy compared 94 patients enrolled in the ATAC (Arimidex, Tamoxifen Alone or in Combination) study to 35 noncancer controls. There were no differences in working memory, attention and visual memory, but those on endocrine therapy had poorer performance on tests of verbal memory and processing speed compared with controls.
The IBIS II (International Breast Cancer Intervention II) prevention study comparing anastrozole vs. placebo showed no overall difference in cognitive function with the addition of anastrozole. At 6 months, those on anastrozole had poorer memory, but this finding resolved by 24 months.
In a subset of 179 patients in the TEAM (Tamoxifen Exemestane Adjuvant Multinational) phase III adjuvant study, comparing exemestane with tamoxifen then exemestane, there was little change from baseline to 1 year in all domains of cognitive function with the addition of exemestane.
A substudy done from the BIG 1-98 (Breast International Group 1-98) trial indicated significant improvement in cognitive function from year 5 of therapy to year 6 after cessation of hormone therapies. Interestingly, perceived cognition did not change in the patients.
This finding corroborates similar results from a prospective, longitudinal study of cognitive complaints and neuropsychological testing for verbal memory, psychomotor speed, and executive functioning in 189 patients who were beginning hormone therapy at the University of California, Los Angeles. (J. Natl. Cancer Inst. 2013;105:791-801).
While the relationship between chemotherapy and neurocognitive changes is increasingly well described, cognitive changes associated with hormone therapy for breast cancer has proven to be somewhat complex. Chemotherapy can confound this. There are questions about different reports of outcomes for different drugs, for selective estrogen-receptor modulators and aromatase inhibitors, as well as how menopausal status, prior oophorectomy, and hormone therapy all affect cognitive changes in women.

|
|
The important points of this study are that there are measurements at baseline, 1 month, and 18 months for a longitudinal picture. In addition, both comprehensive neuropsychological testing and patient-reported outcomes are available at each of those time points.
Previous studies have assessed these relationships, including a small study of memory impairment with anastrozole vs. tamoxifen in 31 patients treated for a minimum of 3 months. There was no difference between groups in depression, anxiety and fatigue, but researchers found that those on anastrozole had significantly poorer performance on learning and memory measures than those taking tamoxifen (Menopause 2007;14:995-8).
Another small substudy compared 94 patients enrolled in the ATAC (Arimidex, Tamoxifen Alone or in Combination) study to 35 noncancer controls. There were no differences in working memory, attention and visual memory, but those on endocrine therapy had poorer performance on tests of verbal memory and processing speed compared with controls.
The IBIS II (International Breast Cancer Intervention II) prevention study comparing anastrozole vs. placebo showed no overall difference in cognitive function with the addition of anastrozole. At 6 months, those on anastrozole had poorer memory, but this finding resolved by 24 months.
In a subset of 179 patients in the TEAM (Tamoxifen Exemestane Adjuvant Multinational) phase III adjuvant study, comparing exemestane with tamoxifen then exemestane, there was little change from baseline to 1 year in all domains of cognitive function with the addition of exemestane.
A substudy done from the BIG 1-98 (Breast International Group 1-98) trial indicated significant improvement in cognitive function from year 5 of therapy to year 6 after cessation of hormone therapies. Interestingly, perceived cognition did not change in the patients.
This finding corroborates similar results from a prospective, longitudinal study of cognitive complaints and neuropsychological testing for verbal memory, psychomotor speed, and executive functioning in 189 patients who were beginning hormone therapy at the University of California, Los Angeles. (J. Natl. Cancer Inst. 2013;105:791-801).
SAN FRANCISCO – Patients with breast cancer who received hormone therapy were over seven times more likely to show cognitive decline as were untreated patients after controlling for other factors, based on a prospective study of 81 patients.
Further, objective results on neuropsychological testing tended to back up patients’ complaints of cognitive difficulties.
Hormone therapy may be a risk factor for cognitive deficits, and interventional studies should be designed to focus on this group of patients, Dr. Hope S. Rugo and her associates recommended in a poster presentation at a breast cancer symposium sponsored by the American Society of Clinical Oncology.
The study collected neuropsychological test results and patient reports before treatment and at several points after starting hormone therapy (22 patients), chemotherapy (14), or chemotherapy followed by hormone therapy (33), and in a control group of 12 untreated patients.
Compared with baseline results, nearly 25% of patients had cognitive decline on neuropsychological testing after 5 months. (Among treated patients, this occurred 1 month after ending chemotherapy or 5 months after starting hormone therapy.) Nearly 35% had cognitive declines at 9 months of follow-up, and 30% had cognitive declines after 18 months.
"Decline in cognitive function is common in patients receiving adjuvant therapy for early-stage breast cancer," concluded Dr. Rugo, director of the Breast Oncology Clinical Trials Program at the University of California, San Francisco. "Ongoing hormone therapy appears to be a risk factor for worse cognitive function."
Other factors that did not predict cognitive decline in the multivariate analysis included age, education level, average estradiol level over time, and estimated verbal IQ at baseline.
Separate univariate conditional logistic regression analyses found that hormone therapy predicted cognitive decline with an odds ratio of 5, but chemotherapy, radiation therapy, average fatigue over time, and average depression over time were not predictive of cognitive decline at any point.
The study enrolled women aged 35-80 years with early-stage breast cancer. Those who underwent adjuvant therapy received 3-4 months of chemotherapy alone, 5 years of hormone therapy alone, or both.
A separate analysis in the same study looked at how well subjective patient reports correlated with objective measures on neuropsychological tests. Dr. Lara Heflin and her associates found significant cross-section correlations between patient-reported cognitive problems and psychological distress and fatigue.
After researchers controlled for the influence of depression and fatigue, however, significant relationships remained between patients’ perceived cognitive functioning and measurable cognitive decline from baseline (pretreatment) to the first follow-up. Patients whose scores indicated memory decline were more likely to perceive memory problems, and patients whose scores on letter fluency declined were more likely to perceive problems with verbal fluency.
"Patients who self-report cognitive problems may indeed be experiencing cognitive decline, and their self-report should not simply be attributed to fatigue or to psychological factors such as anxiety and depression," concluded Dr. Heflin, a visiting professor of psychology at New Mexico Highlands University, Las Vegas.
Patients in the study had no prior chemotherapy or central nervous system radiation and no history of major psychiatric illness, serious head injury, neurologic disease, drug or alcohol abuse, or significant medical illness. The median age was 54 years, and 78% of patients were white. Patient characteristics were similar between groups.
The symposium was cosponsored by the American Society of Breast Disease, the American Society of Breast Surgeons, the National Consortium of Breast Centers, the Society of Surgical Oncology, and the American Society for Radiation Oncology.
The National Institutes of Health funded the study. Dr. Rugo reported having financial associations with Merck, Novartis, and Pfizer.
On Twitter @sherryboschert
SAN FRANCISCO – Patients with breast cancer who received hormone therapy were over seven times more likely to show cognitive decline as were untreated patients after controlling for other factors, based on a prospective study of 81 patients.
Further, objective results on neuropsychological testing tended to back up patients’ complaints of cognitive difficulties.
Hormone therapy may be a risk factor for cognitive deficits, and interventional studies should be designed to focus on this group of patients, Dr. Hope S. Rugo and her associates recommended in a poster presentation at a breast cancer symposium sponsored by the American Society of Clinical Oncology.
The study collected neuropsychological test results and patient reports before treatment and at several points after starting hormone therapy (22 patients), chemotherapy (14), or chemotherapy followed by hormone therapy (33), and in a control group of 12 untreated patients.
Compared with baseline results, nearly 25% of patients had cognitive decline on neuropsychological testing after 5 months. (Among treated patients, this occurred 1 month after ending chemotherapy or 5 months after starting hormone therapy.) Nearly 35% had cognitive declines at 9 months of follow-up, and 30% had cognitive declines after 18 months.
"Decline in cognitive function is common in patients receiving adjuvant therapy for early-stage breast cancer," concluded Dr. Rugo, director of the Breast Oncology Clinical Trials Program at the University of California, San Francisco. "Ongoing hormone therapy appears to be a risk factor for worse cognitive function."
Other factors that did not predict cognitive decline in the multivariate analysis included age, education level, average estradiol level over time, and estimated verbal IQ at baseline.
Separate univariate conditional logistic regression analyses found that hormone therapy predicted cognitive decline with an odds ratio of 5, but chemotherapy, radiation therapy, average fatigue over time, and average depression over time were not predictive of cognitive decline at any point.
The study enrolled women aged 35-80 years with early-stage breast cancer. Those who underwent adjuvant therapy received 3-4 months of chemotherapy alone, 5 years of hormone therapy alone, or both.
A separate analysis in the same study looked at how well subjective patient reports correlated with objective measures on neuropsychological tests. Dr. Lara Heflin and her associates found significant cross-section correlations between patient-reported cognitive problems and psychological distress and fatigue.
After researchers controlled for the influence of depression and fatigue, however, significant relationships remained between patients’ perceived cognitive functioning and measurable cognitive decline from baseline (pretreatment) to the first follow-up. Patients whose scores indicated memory decline were more likely to perceive memory problems, and patients whose scores on letter fluency declined were more likely to perceive problems with verbal fluency.
"Patients who self-report cognitive problems may indeed be experiencing cognitive decline, and their self-report should not simply be attributed to fatigue or to psychological factors such as anxiety and depression," concluded Dr. Heflin, a visiting professor of psychology at New Mexico Highlands University, Las Vegas.
Patients in the study had no prior chemotherapy or central nervous system radiation and no history of major psychiatric illness, serious head injury, neurologic disease, drug or alcohol abuse, or significant medical illness. The median age was 54 years, and 78% of patients were white. Patient characteristics were similar between groups.
The symposium was cosponsored by the American Society of Breast Disease, the American Society of Breast Surgeons, the National Consortium of Breast Centers, the Society of Surgical Oncology, and the American Society for Radiation Oncology.
The National Institutes of Health funded the study. Dr. Rugo reported having financial associations with Merck, Novartis, and Pfizer.
On Twitter @sherryboschert
AT THE ASCO BREAST CANCER SYMPOSIUM
Major finding: The odds of any decline in cognitive function were significantly higher in patients on hormone therapy (OR, 7.7), compared with untreated patients in a multivariate conditional logistic regression analysis.
Data source: Prospective longitudinal study of 81 patients with breast cancer treated with hormone therapy (22 patients), chemotherapy (14), chemotherapy followed by hormone therapy (33), or no therapy (12).
Disclosures: NIH funded the study. Dr. Rugo reported having financial associations with Merck, Novartis, and Pfizer.
In the hormone therapy fight, bioidenticals come out swinging
With seemingly ageless celebrities touting the benefits of bioidentical, compounded hormones, and the cloud of the Women’s Health Initiative still obscuring the view, both physicians and patients want to know the truth about natural hormone replacement.
But as in so many areas of life, the truth can be many things to many people, Dr. Jan L. Shifren shared at the annual meeting of the North American Menopause Society (NAMS).
"For a woman with bothersome menopausal symptoms and concerns about potential hormone therapy [HT] risks, ‘bioHT’ may mean a formulation of hormones with all of the benefits and no risks, as it’s ‘natural’ and contains no package insert with a black box warning," said Dr. Shifren, director of the menopause program at Massachusetts General Hospital, Boston. "For a bioHT practitioner, it may mean a way of helping menopausal women disenfranchised with the medical establishment, while providing a steady source of income. For a clinician or pharmacist practicing within the current standard of care, an FDA inspector, or a lawyer for a women with endometrial cancer associated with its use, bioHT may mean something quite different!"
Bioidentical hormones are typically plant-based compounds with a molecular structure identical to that of the corresponding human hormone. Dosing is individual and based on a woman’s salivary hormone levels. Prescriptions are pharmacist compounded, typically in a topical preparation, but sometimes in an oral tablet or injected pellet.
All of this may sound reasonable at first glance, Dr. Shifren said. But science and money just keep getting in the way of the celebrity endorsements.
"Hormone therapy should be guided by symptoms. There are no data to support the use of blood or saliva measurements to improve treatment efficacy," Dr. Shifren said. Serum hormone levels constantly fluctuate – not only within the menstrual cycle, but also within a single day, "so it’s impossible to ‘match’ any individual woman’s ‘ideal’ hormone levels."
Additionally, Dr. Shifren said, salivary levels don’t even correspond to serum levels.
Safety is one of the biggest problems with such compounds. Because their manufacture is in the hands of a single individual, there’s no quality oversight. Women can’t count on getting the correct prescribed dose of hormone – and sometimes not even close to it.
A recent study commissioned by MORE magazine examined the exact hormone content in 12 bioHT prescriptions, which were filled by 12 pharmacies. Two pharmacies were retail stores and 10 were online companies.
Flora Research Labs analyzed the medications with mass spectrometry. The values varied widely: 96%-260% of the prescribed estrogen dose and 60%-80% of the prescribed progesterone dose.
"None of these would have met FDA standards," Dr. Shifren said. "The purity, bioavailability, and dose-to-dose consistency of any given prescription is an unknown."
Concern about the safety of conventional HT frequently drives women to seek out what they consider a more natural alternative. Although much more data have emerged, the original furor over the Women’s Health Initiative still casts HT in a negative light for many, Dr. Shifren said.
For women in good health, who start combination estrogen/progesterone HT early in menopause, at the lowest effective dose, the increased risks of cardiovascular disease and cancer are negligible. A 2010 British study of 15,710 cases and almost 60,000 controls found that low-dose transdermal HT had no effect on stroke risk. High-dose patch estrogen increased the risk by 89%, and both low- and high-dose oral estrogen increased the risk by 69%; however, each of those increases translated to an absolute increase of only about 1 stroke per year (BMJ 2010;340:c2519 [doi: 10.1136/bmj.c2519]).
A 2008 French study of almost 81,000 women found no significant associations between breast cancer and route of HT administration. However, the study did conclude that the combination of estrogen and progesterone was probably the safest, with a risk ratio of 1.0. Estrogen plus dydrogesterone resulted in a nonsignificant risk increase (relative risk, 1.16). Estrogen in combination with other progestogens carried a significant 69% risk increase (Breast Cancer Res. Treat. 2008;107:103-11).
Finally, Dr. Shifren said, it’s just about impossible to take profit out of the picture. Compounding HT sales are reaching more than $2 billion per year now.
"Many practitioners who prescribe compounded hormones also sell them, or benefit financially from relationships with compounders. This poses a potential conflict of interest and violates standards of professional ethical conduct," she said.
Dr. Shifren provides research consulting for New England Research Institutes.
With seemingly ageless celebrities touting the benefits of bioidentical, compounded hormones, and the cloud of the Women’s Health Initiative still obscuring the view, both physicians and patients want to know the truth about natural hormone replacement.
But as in so many areas of life, the truth can be many things to many people, Dr. Jan L. Shifren shared at the annual meeting of the North American Menopause Society (NAMS).
"For a woman with bothersome menopausal symptoms and concerns about potential hormone therapy [HT] risks, ‘bioHT’ may mean a formulation of hormones with all of the benefits and no risks, as it’s ‘natural’ and contains no package insert with a black box warning," said Dr. Shifren, director of the menopause program at Massachusetts General Hospital, Boston. "For a bioHT practitioner, it may mean a way of helping menopausal women disenfranchised with the medical establishment, while providing a steady source of income. For a clinician or pharmacist practicing within the current standard of care, an FDA inspector, or a lawyer for a women with endometrial cancer associated with its use, bioHT may mean something quite different!"
Bioidentical hormones are typically plant-based compounds with a molecular structure identical to that of the corresponding human hormone. Dosing is individual and based on a woman’s salivary hormone levels. Prescriptions are pharmacist compounded, typically in a topical preparation, but sometimes in an oral tablet or injected pellet.
All of this may sound reasonable at first glance, Dr. Shifren said. But science and money just keep getting in the way of the celebrity endorsements.
"Hormone therapy should be guided by symptoms. There are no data to support the use of blood or saliva measurements to improve treatment efficacy," Dr. Shifren said. Serum hormone levels constantly fluctuate – not only within the menstrual cycle, but also within a single day, "so it’s impossible to ‘match’ any individual woman’s ‘ideal’ hormone levels."
Additionally, Dr. Shifren said, salivary levels don’t even correspond to serum levels.
Safety is one of the biggest problems with such compounds. Because their manufacture is in the hands of a single individual, there’s no quality oversight. Women can’t count on getting the correct prescribed dose of hormone – and sometimes not even close to it.
A recent study commissioned by MORE magazine examined the exact hormone content in 12 bioHT prescriptions, which were filled by 12 pharmacies. Two pharmacies were retail stores and 10 were online companies.
Flora Research Labs analyzed the medications with mass spectrometry. The values varied widely: 96%-260% of the prescribed estrogen dose and 60%-80% of the prescribed progesterone dose.
"None of these would have met FDA standards," Dr. Shifren said. "The purity, bioavailability, and dose-to-dose consistency of any given prescription is an unknown."
Concern about the safety of conventional HT frequently drives women to seek out what they consider a more natural alternative. Although much more data have emerged, the original furor over the Women’s Health Initiative still casts HT in a negative light for many, Dr. Shifren said.
For women in good health, who start combination estrogen/progesterone HT early in menopause, at the lowest effective dose, the increased risks of cardiovascular disease and cancer are negligible. A 2010 British study of 15,710 cases and almost 60,000 controls found that low-dose transdermal HT had no effect on stroke risk. High-dose patch estrogen increased the risk by 89%, and both low- and high-dose oral estrogen increased the risk by 69%; however, each of those increases translated to an absolute increase of only about 1 stroke per year (BMJ 2010;340:c2519 [doi: 10.1136/bmj.c2519]).
A 2008 French study of almost 81,000 women found no significant associations between breast cancer and route of HT administration. However, the study did conclude that the combination of estrogen and progesterone was probably the safest, with a risk ratio of 1.0. Estrogen plus dydrogesterone resulted in a nonsignificant risk increase (relative risk, 1.16). Estrogen in combination with other progestogens carried a significant 69% risk increase (Breast Cancer Res. Treat. 2008;107:103-11).
Finally, Dr. Shifren said, it’s just about impossible to take profit out of the picture. Compounding HT sales are reaching more than $2 billion per year now.
"Many practitioners who prescribe compounded hormones also sell them, or benefit financially from relationships with compounders. This poses a potential conflict of interest and violates standards of professional ethical conduct," she said.
Dr. Shifren provides research consulting for New England Research Institutes.
With seemingly ageless celebrities touting the benefits of bioidentical, compounded hormones, and the cloud of the Women’s Health Initiative still obscuring the view, both physicians and patients want to know the truth about natural hormone replacement.
But as in so many areas of life, the truth can be many things to many people, Dr. Jan L. Shifren shared at the annual meeting of the North American Menopause Society (NAMS).
"For a woman with bothersome menopausal symptoms and concerns about potential hormone therapy [HT] risks, ‘bioHT’ may mean a formulation of hormones with all of the benefits and no risks, as it’s ‘natural’ and contains no package insert with a black box warning," said Dr. Shifren, director of the menopause program at Massachusetts General Hospital, Boston. "For a bioHT practitioner, it may mean a way of helping menopausal women disenfranchised with the medical establishment, while providing a steady source of income. For a clinician or pharmacist practicing within the current standard of care, an FDA inspector, or a lawyer for a women with endometrial cancer associated with its use, bioHT may mean something quite different!"
Bioidentical hormones are typically plant-based compounds with a molecular structure identical to that of the corresponding human hormone. Dosing is individual and based on a woman’s salivary hormone levels. Prescriptions are pharmacist compounded, typically in a topical preparation, but sometimes in an oral tablet or injected pellet.
All of this may sound reasonable at first glance, Dr. Shifren said. But science and money just keep getting in the way of the celebrity endorsements.
"Hormone therapy should be guided by symptoms. There are no data to support the use of blood or saliva measurements to improve treatment efficacy," Dr. Shifren said. Serum hormone levels constantly fluctuate – not only within the menstrual cycle, but also within a single day, "so it’s impossible to ‘match’ any individual woman’s ‘ideal’ hormone levels."
Additionally, Dr. Shifren said, salivary levels don’t even correspond to serum levels.
Safety is one of the biggest problems with such compounds. Because their manufacture is in the hands of a single individual, there’s no quality oversight. Women can’t count on getting the correct prescribed dose of hormone – and sometimes not even close to it.
A recent study commissioned by MORE magazine examined the exact hormone content in 12 bioHT prescriptions, which were filled by 12 pharmacies. Two pharmacies were retail stores and 10 were online companies.
Flora Research Labs analyzed the medications with mass spectrometry. The values varied widely: 96%-260% of the prescribed estrogen dose and 60%-80% of the prescribed progesterone dose.
"None of these would have met FDA standards," Dr. Shifren said. "The purity, bioavailability, and dose-to-dose consistency of any given prescription is an unknown."
Concern about the safety of conventional HT frequently drives women to seek out what they consider a more natural alternative. Although much more data have emerged, the original furor over the Women’s Health Initiative still casts HT in a negative light for many, Dr. Shifren said.
For women in good health, who start combination estrogen/progesterone HT early in menopause, at the lowest effective dose, the increased risks of cardiovascular disease and cancer are negligible. A 2010 British study of 15,710 cases and almost 60,000 controls found that low-dose transdermal HT had no effect on stroke risk. High-dose patch estrogen increased the risk by 89%, and both low- and high-dose oral estrogen increased the risk by 69%; however, each of those increases translated to an absolute increase of only about 1 stroke per year (BMJ 2010;340:c2519 [doi: 10.1136/bmj.c2519]).
A 2008 French study of almost 81,000 women found no significant associations between breast cancer and route of HT administration. However, the study did conclude that the combination of estrogen and progesterone was probably the safest, with a risk ratio of 1.0. Estrogen plus dydrogesterone resulted in a nonsignificant risk increase (relative risk, 1.16). Estrogen in combination with other progestogens carried a significant 69% risk increase (Breast Cancer Res. Treat. 2008;107:103-11).
Finally, Dr. Shifren said, it’s just about impossible to take profit out of the picture. Compounding HT sales are reaching more than $2 billion per year now.
"Many practitioners who prescribe compounded hormones also sell them, or benefit financially from relationships with compounders. This poses a potential conflict of interest and violates standards of professional ethical conduct," she said.
Dr. Shifren provides research consulting for New England Research Institutes.
EXPERT ANALYSIS FROM THE NAMS 2013 ANNUAL MEETING
Bone mineral density identifies fracture risk in women over 65
Bone mineral density testing is an effective means of identifying women aged 65 years and older who are at high risk of fracture, according to Jane A. Cauley, Dr.P.H., an epidemiologist at the University of Pittsburgh.
All women aged 65 and over should have a BMD test. In a presentation at the annual meeting of the North American Menopause Society, she related the findings of a 2012 study conducted to determine when the test should be repeated. In that study of 4,957 women aged 67 years and older at baseline who underwent baseline T-score testing, the mean interval until 2% of participants had a hip or clinical vertebral fracture was 25 years for women whose baseline bone mineral density (BMD) T-score was greater than or equal to –1.00, 15.4 years for those whose T-score range was –1.01 to –1.49, 5.5 years for those whose T-score range was –1.50 to –1.99, and 5.4 years for those whose T-score was –2.00 to –2.49 (N. Engl. J. Med. 2012;366:225-33).
Also, in women with BMD T-scores greater than –1.50, osteoporosis developed in less than 10% during 15 years of follow-up.
Based on these findings, Dr. Cauley suggested that an initial BMD test should be recommended for all women aged 65 years and older, and that physicians should reassess the testing interval based on the initial result. If the T-score declines, so should the length of the testing interval.
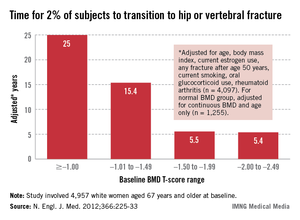
Bone mineral density testing is an effective means of identifying women aged 65 years and older who are at high risk of fracture, according to Jane A. Cauley, Dr.P.H., an epidemiologist at the University of Pittsburgh.
All women aged 65 and over should have a BMD test. In a presentation at the annual meeting of the North American Menopause Society, she related the findings of a 2012 study conducted to determine when the test should be repeated. In that study of 4,957 women aged 67 years and older at baseline who underwent baseline T-score testing, the mean interval until 2% of participants had a hip or clinical vertebral fracture was 25 years for women whose baseline bone mineral density (BMD) T-score was greater than or equal to –1.00, 15.4 years for those whose T-score range was –1.01 to –1.49, 5.5 years for those whose T-score range was –1.50 to –1.99, and 5.4 years for those whose T-score was –2.00 to –2.49 (N. Engl. J. Med. 2012;366:225-33).
Also, in women with BMD T-scores greater than –1.50, osteoporosis developed in less than 10% during 15 years of follow-up.
Based on these findings, Dr. Cauley suggested that an initial BMD test should be recommended for all women aged 65 years and older, and that physicians should reassess the testing interval based on the initial result. If the T-score declines, so should the length of the testing interval.

Bone mineral density testing is an effective means of identifying women aged 65 years and older who are at high risk of fracture, according to Jane A. Cauley, Dr.P.H., an epidemiologist at the University of Pittsburgh.
All women aged 65 and over should have a BMD test. In a presentation at the annual meeting of the North American Menopause Society, she related the findings of a 2012 study conducted to determine when the test should be repeated. In that study of 4,957 women aged 67 years and older at baseline who underwent baseline T-score testing, the mean interval until 2% of participants had a hip or clinical vertebral fracture was 25 years for women whose baseline bone mineral density (BMD) T-score was greater than or equal to –1.00, 15.4 years for those whose T-score range was –1.01 to –1.49, 5.5 years for those whose T-score range was –1.50 to –1.99, and 5.4 years for those whose T-score was –2.00 to –2.49 (N. Engl. J. Med. 2012;366:225-33).
Also, in women with BMD T-scores greater than –1.50, osteoporosis developed in less than 10% during 15 years of follow-up.
Based on these findings, Dr. Cauley suggested that an initial BMD test should be recommended for all women aged 65 years and older, and that physicians should reassess the testing interval based on the initial result. If the T-score declines, so should the length of the testing interval.

In young hysterectomized women, does unopposed estrogen therapy increase overall survival?
During the 1990s, more than 90% of hysterectomized women aged 50 to 59 years used ET following the procedure. When the initial findings of the WHI were published in 2002, they prompted many women to refuse or discontinue ET—despite the fact that the initial findings concerned the use of estrogen and progestin in combination in women with an intact uterus. Today, only some 30% of women use ET after hysterectomy.
When findings from the WHI estrogen-only arm were eventually published, they revealed that ET reduces mortality among women 50 to 59 years old, compared with placebo. Although most of the reduction in mortality relates to fewer deaths from coronary heart disease, a decline in deaths from breast cancer also was seen.2,3
Sarrel and colleagues calculated the excess mortality among US women aged 50 to 59 that could have been prevented by ET during the decade from 2002 through 2011. Their estimates ranged from approximately 19,000 deaths to as many as 92,000 deaths.
By calling attention to the negative health consequences of estrogen avoidance in young hysterectomized women, Sarrel and colleagues have performed a valuable public service.
Plethora of WHI data may have contributed to confusion
The WHI clinical trials have produced numerous analyses in various subsets of women. The sheer volume of data may be daunting in some cases, and likely has led to a failure to distinguish between estrogen-only and estrogen-progestin therapy, which have very different safety profiles.
Further, some clinicians and many patients have overlooked the fact that the risk-benefit profile of hormone therapy (both estrogen-only and estrogen-progestin therapy) is more favorable in younger, recently menopausal women than it is in older women.
I encounter evidence of this unwarranted fear of ET in my practice, with highly symptomatic, recently menopausal women who are appropriate candidates for hormone therapy electing to refuse the most effective treatment for menopausal symptoms.
Of course, hormone therapy, like all medications, has risks as well as benefits. For example, oral ET increases the risk of venous thrombosis and stroke, and long-term use of estrogen-progestin therapy increases the risk of breast cancer. However, the overblown fears of estrogen therapy have caused many appropriate candidates to miss out on symptom relief, prevention of osteoporosis, and treatment of symptomatic genital atrophy.
What this evidence means for practice
This provocative report demonstrates that wholesale avoidance of hormone therapy can have important negative public health consequence.
--Andrew M. Kaunitz, MD
We want to hear from you! Tell us what you think.
1. Rossouw JE, Anderson GL, Prentice RL, et al; Writing Group for the Women’s Health Initiative Investigators. Risks and benefits of estrogen plus progestin in healthy postmenopausal women: principal results From the Women’s Health Initiative randomized controlled trial. JAMA. 2002;288(3):321–333.
2. LaCroix AZ, Chlebowski RT, Manson JE, et al; WHI Investigators. Health outcomes after stopping conjugated equine estrogens among postmenopausal women with prior hysterectomy: a randomized controlled trial. JAMA. 2011;305(13):1305–1314.
3. Anderson GL, Chlebowski RI, Aragaki AK, et al. Conjugated equine estrogen and breast cancer incidence and mortality in postmenopausal women with hysterectomy: extended follow-up of the Women’s Health Initiative randomized placebo-controlled trial. Lancet Oncol. 2012; 13(5):476–486.
During the 1990s, more than 90% of hysterectomized women aged 50 to 59 years used ET following the procedure. When the initial findings of the WHI were published in 2002, they prompted many women to refuse or discontinue ET—despite the fact that the initial findings concerned the use of estrogen and progestin in combination in women with an intact uterus. Today, only some 30% of women use ET after hysterectomy.
When findings from the WHI estrogen-only arm were eventually published, they revealed that ET reduces mortality among women 50 to 59 years old, compared with placebo. Although most of the reduction in mortality relates to fewer deaths from coronary heart disease, a decline in deaths from breast cancer also was seen.2,3
Sarrel and colleagues calculated the excess mortality among US women aged 50 to 59 that could have been prevented by ET during the decade from 2002 through 2011. Their estimates ranged from approximately 19,000 deaths to as many as 92,000 deaths.
By calling attention to the negative health consequences of estrogen avoidance in young hysterectomized women, Sarrel and colleagues have performed a valuable public service.
Plethora of WHI data may have contributed to confusion
The WHI clinical trials have produced numerous analyses in various subsets of women. The sheer volume of data may be daunting in some cases, and likely has led to a failure to distinguish between estrogen-only and estrogen-progestin therapy, which have very different safety profiles.
Further, some clinicians and many patients have overlooked the fact that the risk-benefit profile of hormone therapy (both estrogen-only and estrogen-progestin therapy) is more favorable in younger, recently menopausal women than it is in older women.
I encounter evidence of this unwarranted fear of ET in my practice, with highly symptomatic, recently menopausal women who are appropriate candidates for hormone therapy electing to refuse the most effective treatment for menopausal symptoms.
Of course, hormone therapy, like all medications, has risks as well as benefits. For example, oral ET increases the risk of venous thrombosis and stroke, and long-term use of estrogen-progestin therapy increases the risk of breast cancer. However, the overblown fears of estrogen therapy have caused many appropriate candidates to miss out on symptom relief, prevention of osteoporosis, and treatment of symptomatic genital atrophy.
What this evidence means for practice
This provocative report demonstrates that wholesale avoidance of hormone therapy can have important negative public health consequence.
--Andrew M. Kaunitz, MD
We want to hear from you! Tell us what you think.
During the 1990s, more than 90% of hysterectomized women aged 50 to 59 years used ET following the procedure. When the initial findings of the WHI were published in 2002, they prompted many women to refuse or discontinue ET—despite the fact that the initial findings concerned the use of estrogen and progestin in combination in women with an intact uterus. Today, only some 30% of women use ET after hysterectomy.
When findings from the WHI estrogen-only arm were eventually published, they revealed that ET reduces mortality among women 50 to 59 years old, compared with placebo. Although most of the reduction in mortality relates to fewer deaths from coronary heart disease, a decline in deaths from breast cancer also was seen.2,3
Sarrel and colleagues calculated the excess mortality among US women aged 50 to 59 that could have been prevented by ET during the decade from 2002 through 2011. Their estimates ranged from approximately 19,000 deaths to as many as 92,000 deaths.
By calling attention to the negative health consequences of estrogen avoidance in young hysterectomized women, Sarrel and colleagues have performed a valuable public service.
Plethora of WHI data may have contributed to confusion
The WHI clinical trials have produced numerous analyses in various subsets of women. The sheer volume of data may be daunting in some cases, and likely has led to a failure to distinguish between estrogen-only and estrogen-progestin therapy, which have very different safety profiles.
Further, some clinicians and many patients have overlooked the fact that the risk-benefit profile of hormone therapy (both estrogen-only and estrogen-progestin therapy) is more favorable in younger, recently menopausal women than it is in older women.
I encounter evidence of this unwarranted fear of ET in my practice, with highly symptomatic, recently menopausal women who are appropriate candidates for hormone therapy electing to refuse the most effective treatment for menopausal symptoms.
Of course, hormone therapy, like all medications, has risks as well as benefits. For example, oral ET increases the risk of venous thrombosis and stroke, and long-term use of estrogen-progestin therapy increases the risk of breast cancer. However, the overblown fears of estrogen therapy have caused many appropriate candidates to miss out on symptom relief, prevention of osteoporosis, and treatment of symptomatic genital atrophy.
What this evidence means for practice
This provocative report demonstrates that wholesale avoidance of hormone therapy can have important negative public health consequence.
--Andrew M. Kaunitz, MD
We want to hear from you! Tell us what you think.
1. Rossouw JE, Anderson GL, Prentice RL, et al; Writing Group for the Women’s Health Initiative Investigators. Risks and benefits of estrogen plus progestin in healthy postmenopausal women: principal results From the Women’s Health Initiative randomized controlled trial. JAMA. 2002;288(3):321–333.
2. LaCroix AZ, Chlebowski RT, Manson JE, et al; WHI Investigators. Health outcomes after stopping conjugated equine estrogens among postmenopausal women with prior hysterectomy: a randomized controlled trial. JAMA. 2011;305(13):1305–1314.
3. Anderson GL, Chlebowski RI, Aragaki AK, et al. Conjugated equine estrogen and breast cancer incidence and mortality in postmenopausal women with hysterectomy: extended follow-up of the Women’s Health Initiative randomized placebo-controlled trial. Lancet Oncol. 2012; 13(5):476–486.
1. Rossouw JE, Anderson GL, Prentice RL, et al; Writing Group for the Women’s Health Initiative Investigators. Risks and benefits of estrogen plus progestin in healthy postmenopausal women: principal results From the Women’s Health Initiative randomized controlled trial. JAMA. 2002;288(3):321–333.
2. LaCroix AZ, Chlebowski RT, Manson JE, et al; WHI Investigators. Health outcomes after stopping conjugated equine estrogens among postmenopausal women with prior hysterectomy: a randomized controlled trial. JAMA. 2011;305(13):1305–1314.
3. Anderson GL, Chlebowski RI, Aragaki AK, et al. Conjugated equine estrogen and breast cancer incidence and mortality in postmenopausal women with hysterectomy: extended follow-up of the Women’s Health Initiative randomized placebo-controlled trial. Lancet Oncol. 2012; 13(5):476–486.
Your postmenopausal patient reports a history of migraine
Brought to you by the menopause experts
| Andrew M. Kaunitz, MD |
| JoAnn V. Pinkerton, MD |
| James A. Simon, MD |
Disclosures
Dr. Kaunitz reports that his institution receives grant or research support from Bayer, Teva, Medical Diagnostic Laboratories, and Noven, and that he is a consultant to Bayer, Actavis, and Teva.
Dr. Pinkerton reports that her institution receives consulting fees from Pfizer, DepoMed, Shionogi, and Noven and multicenter research fees from DepoMed, Endoceutics, and Bionova.
Dr. Simon reports being a consultant to or on the advisory boards of Abbott Laboratories, Agile Therapeutics, Amgen, Ascend Therapeutics, BioSante, Depomed, Lelo, MD Therapeutics, Meda Pharmaceuticals, Merck, Noven, Novo Nordisk, Novogyne, Pfizer, Shionogi, Shippan Point Advisors LLC, Slate Pharmaceuticals, Sprout Pharmaceuticals, Teva, Warner Chilcott, and Watson. He also reports receiving (currently or in the past year) grant/research support from BioSante, EndoCeutics, Novo Nordisk, Novogyne, Palatin Technologies, Teva, and Warner Chilcott. He reports serving on the speakers bureaus of Amgen, Merck, Novartis, Noven, Novo Nordisk, Novogyne, Teva, and Warner Chilcott. Dr. Simon is currently the Chief Medical Officer for Sprout Pharmaceuticals.
CASE: Menopausal symptoms and a history of migraine with aura
Your new patient is a 52-year-old woman (G2P2) who reports a long history of two types of migraine: menstrually related migraine without aura and nonmenstrually related migraine with aura (usually involving visual scotomata). Other than the history of migraine, her health is good. Now postmenopausal, she has been referred to you by her primary care physician (PCP) for management of severe vasomotor symptoms and sleep disturbance.
Because of this patient’s history of migraine, her PCP declined to prescribe oral contraceptives (OCs) in the past over concern of increasing her risk of stroke. For her vasomotor symptoms, her PCP prescribed a trial of venlafaxine (Effexor) 75 mg daily, but her orgasms, which always had been difficult to achieve, became impossible. In addition, the patient began to perspire heavily unrelated to her hot flashes. As a result, she describes her mood as “terrible,” her energy level as “miniscule,” and she reports losing interest in sex completely (“I am just too tired”). She and her referring physician wonder whether it would be safe to try hormone therapy (HT).
A physical examination, including funduscopic assessment, reveals no abnormalities. Her blood pressure is 126/70 mm Hg, and blood chemistry results, including C-reactive protein, 25-hydroxy vitamin D, a complete blood count, and lipid profile, are all normal.
Would you offer this patient the option of HT?
Migraine affects roughly twice as many women as men.1 During the reproductive years, rapid fluctuations in ovarian hormones—both increases at midcycle and, to a greater extent, decreases during the premenstrual phase—are believed to be migraine “triggers.” Women who experience menstrually related migraine before menopause typically have an increased risk of migraine during perimenopause, with a significant reduction of migraine symptoms following menopause.2
Side effects of SSRIs and SNRIs
Most providers are aware that selective serotonin reuptake inhibitors (SSRIs) cause sexual side effects in as many as 80% of users. There is a dose-related pattern to reports of sexual problems among SSRI users, with higher doses causing more problems. The most common sexual symptoms associated with SSRIs are delayed ejaculation and absent or delayed orgasm.3
What is not as widely known is that even serotonin-norepinephrine reuptake inhibitors (SNRIs) can cause sexual dysfunction. For example, in a prospective, multicenter study from Spain involving more than
1,000 outpatients, all of whom were taking an antidepressant, the overall rate of sexual dysfunction was 59%.4 Sexual dysfunction was most common among users of SSRIs and venlafaxine, an SNRI.5
Another common side effect of venlafaxine, sweating, is unrelated to hot flashes.5 So two of this patient’s concerns—orgasmic difficulties and profuse sweating unrelated to hot flashes—may have been caused or worsened by her antidepressant.
Another nonhormonal option
In June 2013, the US Food and Drug Administration (FDA) approved paroxetine mesylate (Brisdelle), an SSRI, for the treatment of moderate to severe menopausal vasomotor symptoms. Because the drug is a strong CYP2D6 inhibitor, it should not be given to women taking another medication that is metabolized by CYP2D6—most notably, tamoxifen.
Preliminary data on this drug suggest that, at the recommended dose (7.5 mg/d), it has no effect on sexual function.6 For this reason, it is another option for our patient to consider.
Related article: The gynecologist's role in managing menstrual migraine Anne H. Calhoun, MD
Migraine and the risk of stroke
Migraine with aura has been associated with an increased risk of stroke and other cerebral vascular events,7 and that risk is further elevated in patients treated with OCs.8 Although migraine without aura also may be associated with an elevated risk of stroke, OCs do not further increase that risk.
Some practitioners assume that the data on the risk of stroke associated with OC use also applies to hormone therapy, but there is no evidence that HT, in which doses of estrogen are far lower than in OCs, increases the risk of stroke in migraineurs to any greater degree than would be expected in unselected populations (ie, as noted in the Women’s Health Initiative, Nurses Health Study, or other large investigations). Therefore, HT would be an appropriate option for this patient if her very slight risk of stroke on HT would be acceptable to the practitioner and patient.
Choosing an HT formulation
Consider the pharmacokinetic profile. Many oral estrogen HT products have rapid-release characteristics that make them likely to contribute to rapid rises and falls in the user’s estrogen level. Oral estrogens also are associated with procoagulant properties that may increase the risk of thrombosis and thromboembolism. Nonoral estrogens do not appear to increase these risks.13
Nonoral estrogens (patches, gels, sprays, lotions, and vaginal rings) provide a more stable pharmacokinetic profile, as do some oral products with controlled-release properties.
As for progestins, some formulations (medroxyprogesterone acetate) tend to cause vasoconstriction, whereas others (micronized progesterone) tend to be vasodilators. Whether these properties affect the rate of migraine or risk of stroke is unclear.
My management approach for this patient
In the absence of any systematic data on the use of HT in this clinical setting, and without any concrete suggestions from migraine experts, I would take the following three-step approach:
1. I would begin with a low-dose nonoral estradiol formulation, prescribing it without a progestin even in a woman who still has a uterus. My aim: to determine the lowest effective dose of HT for this particular patient. I would follow the patient on this dose for 3 months.
JoAnn V. Pinkerton, MD:
Another goal is to determine whether transdermal estradiol increases headaches. Before settling on a therapy, however, I would ask how long this patient has been postmenopausal, how long she has been experiencing vasomotor symptoms, and how severe those symptoms are. For example, is she having 7 or 8 hot flashes per day and waking from night sweats once or twice per night? I also would ask her how long she remained on the venlafaxine. The additional information would allow me to fine-tune her treatment.
2. If this formulation is tolerated, I would add micronized progesterone (oral or vaginal) for endometrial protection.
JoAnn V. Pinkerton, MD:
I would give oral progesterone if it is FDA approved for postmenopausal use, vaginal progesterone if it isn’t.
3. I would follow the patient’s clinical response—specifically, her vasomotor symptoms and rate of migraine with or without aura.
Hormone therapy is one option for postmenopausal migraineurs with bothersome vasomotor symptoms
Many women with a history of migraine move into menopause expecting their condition to improve, says headache expert Anne H. Calhoun, MD, a founder of the Carolina Headache Institute in Chapel Hill, North Carolina.
“Over the years, these women have heard that things get better with menopause.”
For women with a history of episodic migraine, that expectation is realistic, Calhoun says. “But for women with chronic migraine, who may experience a low-grade headache on a daily, or almost daily basis, with 10 or 12 severe headaches in a month, things usually get worse after menopause because the sleep issues of menopause are superimposed on the migraine.”
Dr. Calhoun observes that hormone therapy (HT) has never been contraindicated in women with migraine, although many neurologists are hesitant to prescribe any hormones for this population.
Before prescribing HT to a postmenopausal migraineur, Dr. Calhoun considers a range of variables, including sleep patterns, current medications, anxiety, frequency and severity of vasomotor symptoms, and any other problems the patient may be experiencing.
“It’s basically the same assessment as with any postmenopausal patient—to determine whether HT is a reasonable option,” she says.
And when she determines that HT is appropriate, “I almost exclusively use transdermal HT. I also am more likely to prescribe continuous use of a transdermal patch or skin gel, as I want to achieve very consistent hormone levels, day in and day out,” she says.
My bottom line
No systematic data on the use of HT in migraineurs has been published. In the absence of such data, some practitioners have extrapolated data on the use of OCs in this population and decline to prescribe HT to women with migraine. However, HT and OCs are vastly different in formulation, dose, and risks. Rather than make assumptions on the basis of irrelevant data, we should conduct studies of HT use in migraineurs.
Related article: Update on Menopause Andrew M. Kaunitz, MD (June 2013)
Women who have menstrually related migraine typically have an increased risk of migraine during perimenopause and a significant reduction in migraine following menopause. If hot flashes are bothersome, these women certainly can use HT. I recommend prescribing HT in a continuous fashion that maintains stable hormone levels in the blood, as fluctuating hormones tend to trigger migraines.
Andrew M. Kaunitz, MD:
I would just add that transdermal estradiol is preferred, to be given at the lowest effective dose.
Do you have a troubling case in menopause? Suggest it to the expert panel: [email protected]. They may address your management dilemma in a future issue.
We want to hear from you! Send us your Letter to the Editor
1. Shuster LT, Faubion SS. Sood R, Casey PM. Hormonal manipulation strategies in the management of menstrual migraine and other hormonally related headaches. Curr Neurol Neurosci Rep. 2011;11(2):131–138.
2. Loder E, Rizzoli P, Golub J. Hormonal management of migraine associated with menses and the menopause: a clinical review. Headache. 2007;47(2):329–340.
3. Balon R. SSRI-associated sexual dysfunction. Am J Psychiatry. 2006;163:1504–1509.
4. Taylor MJ. Strategies for managing antidepressant-induced sexual dysfunction: a review. Curr Psychiatry Rep. 2006;8(6):431–436.
5. Effexor [package insert]. New York, NY: Pfizer; 2012.
6. Brisdelle [package insert]. Miami, FL: Noven Therapeutics; 2013.
7. Etminan M, Takkouche B, Isorna FC, Samli A. Risk of ischaemic stroke in people with migraine: systematic review and meta-analysis of observational studies. BMJ. 2005;330(7482):63–65.
8. Becker WJ. Use of oral contraceptives in patients with migraine. Neurology. 1999;53(4 Suppl 1):S19–S25.
9. WHO Collaborative Study of Cardiovascular Disease and Steroid Hormone Contraception. Ischaemic stroke and combined oral contraceptives: results of an international, multicentre, case-control study. Lancet. 1996;348(9026): 498–505.
10. Petitti DB, Sidney S, Bernstein A, Wolf S, Quesenberry C, Ziel HK. Stroke in users of low-dose oral contraceptives. N Engl J Med. 1996;335(1):8-15.
11. Marjoribanks J, Farquhar C, Roberts H, Lethaby A. Long-term hormone therapy for perimenopausal and postmenopausal women. Cochrane Database Syst Rev. 2012;7:CD004143. doi: 10.1002/14651858.CD004143.pub4
12. Renoux C, Dell’aniello S, Garbe E, Suissa S. Transdermal and oral hormone replacement therapy and the risk of stroke: a nested case-control study. BMJ. 2010;340:c2519.
13. Kaunitz AM. Update on Menopause. OBG Manag. 2013;25(6):36–43, 49.
Brought to you by the menopause experts
| Andrew M. Kaunitz, MD |
| JoAnn V. Pinkerton, MD |
| James A. Simon, MD |
Disclosures
Dr. Kaunitz reports that his institution receives grant or research support from Bayer, Teva, Medical Diagnostic Laboratories, and Noven, and that he is a consultant to Bayer, Actavis, and Teva.
Dr. Pinkerton reports that her institution receives consulting fees from Pfizer, DepoMed, Shionogi, and Noven and multicenter research fees from DepoMed, Endoceutics, and Bionova.
Dr. Simon reports being a consultant to or on the advisory boards of Abbott Laboratories, Agile Therapeutics, Amgen, Ascend Therapeutics, BioSante, Depomed, Lelo, MD Therapeutics, Meda Pharmaceuticals, Merck, Noven, Novo Nordisk, Novogyne, Pfizer, Shionogi, Shippan Point Advisors LLC, Slate Pharmaceuticals, Sprout Pharmaceuticals, Teva, Warner Chilcott, and Watson. He also reports receiving (currently or in the past year) grant/research support from BioSante, EndoCeutics, Novo Nordisk, Novogyne, Palatin Technologies, Teva, and Warner Chilcott. He reports serving on the speakers bureaus of Amgen, Merck, Novartis, Noven, Novo Nordisk, Novogyne, Teva, and Warner Chilcott. Dr. Simon is currently the Chief Medical Officer for Sprout Pharmaceuticals.
CASE: Menopausal symptoms and a history of migraine with aura
Your new patient is a 52-year-old woman (G2P2) who reports a long history of two types of migraine: menstrually related migraine without aura and nonmenstrually related migraine with aura (usually involving visual scotomata). Other than the history of migraine, her health is good. Now postmenopausal, she has been referred to you by her primary care physician (PCP) for management of severe vasomotor symptoms and sleep disturbance.
Because of this patient’s history of migraine, her PCP declined to prescribe oral contraceptives (OCs) in the past over concern of increasing her risk of stroke. For her vasomotor symptoms, her PCP prescribed a trial of venlafaxine (Effexor) 75 mg daily, but her orgasms, which always had been difficult to achieve, became impossible. In addition, the patient began to perspire heavily unrelated to her hot flashes. As a result, she describes her mood as “terrible,” her energy level as “miniscule,” and she reports losing interest in sex completely (“I am just too tired”). She and her referring physician wonder whether it would be safe to try hormone therapy (HT).
A physical examination, including funduscopic assessment, reveals no abnormalities. Her blood pressure is 126/70 mm Hg, and blood chemistry results, including C-reactive protein, 25-hydroxy vitamin D, a complete blood count, and lipid profile, are all normal.
Would you offer this patient the option of HT?
Migraine affects roughly twice as many women as men.1 During the reproductive years, rapid fluctuations in ovarian hormones—both increases at midcycle and, to a greater extent, decreases during the premenstrual phase—are believed to be migraine “triggers.” Women who experience menstrually related migraine before menopause typically have an increased risk of migraine during perimenopause, with a significant reduction of migraine symptoms following menopause.2
Side effects of SSRIs and SNRIs
Most providers are aware that selective serotonin reuptake inhibitors (SSRIs) cause sexual side effects in as many as 80% of users. There is a dose-related pattern to reports of sexual problems among SSRI users, with higher doses causing more problems. The most common sexual symptoms associated with SSRIs are delayed ejaculation and absent or delayed orgasm.3
What is not as widely known is that even serotonin-norepinephrine reuptake inhibitors (SNRIs) can cause sexual dysfunction. For example, in a prospective, multicenter study from Spain involving more than
1,000 outpatients, all of whom were taking an antidepressant, the overall rate of sexual dysfunction was 59%.4 Sexual dysfunction was most common among users of SSRIs and venlafaxine, an SNRI.5
Another common side effect of venlafaxine, sweating, is unrelated to hot flashes.5 So two of this patient’s concerns—orgasmic difficulties and profuse sweating unrelated to hot flashes—may have been caused or worsened by her antidepressant.
Another nonhormonal option
In June 2013, the US Food and Drug Administration (FDA) approved paroxetine mesylate (Brisdelle), an SSRI, for the treatment of moderate to severe menopausal vasomotor symptoms. Because the drug is a strong CYP2D6 inhibitor, it should not be given to women taking another medication that is metabolized by CYP2D6—most notably, tamoxifen.
Preliminary data on this drug suggest that, at the recommended dose (7.5 mg/d), it has no effect on sexual function.6 For this reason, it is another option for our patient to consider.
Related article: The gynecologist's role in managing menstrual migraine Anne H. Calhoun, MD
Migraine and the risk of stroke
Migraine with aura has been associated with an increased risk of stroke and other cerebral vascular events,7 and that risk is further elevated in patients treated with OCs.8 Although migraine without aura also may be associated with an elevated risk of stroke, OCs do not further increase that risk.
Some practitioners assume that the data on the risk of stroke associated with OC use also applies to hormone therapy, but there is no evidence that HT, in which doses of estrogen are far lower than in OCs, increases the risk of stroke in migraineurs to any greater degree than would be expected in unselected populations (ie, as noted in the Women’s Health Initiative, Nurses Health Study, or other large investigations). Therefore, HT would be an appropriate option for this patient if her very slight risk of stroke on HT would be acceptable to the practitioner and patient.
Choosing an HT formulation
Consider the pharmacokinetic profile. Many oral estrogen HT products have rapid-release characteristics that make them likely to contribute to rapid rises and falls in the user’s estrogen level. Oral estrogens also are associated with procoagulant properties that may increase the risk of thrombosis and thromboembolism. Nonoral estrogens do not appear to increase these risks.13
Nonoral estrogens (patches, gels, sprays, lotions, and vaginal rings) provide a more stable pharmacokinetic profile, as do some oral products with controlled-release properties.
As for progestins, some formulations (medroxyprogesterone acetate) tend to cause vasoconstriction, whereas others (micronized progesterone) tend to be vasodilators. Whether these properties affect the rate of migraine or risk of stroke is unclear.
My management approach for this patient
In the absence of any systematic data on the use of HT in this clinical setting, and without any concrete suggestions from migraine experts, I would take the following three-step approach:
1. I would begin with a low-dose nonoral estradiol formulation, prescribing it without a progestin even in a woman who still has a uterus. My aim: to determine the lowest effective dose of HT for this particular patient. I would follow the patient on this dose for 3 months.
JoAnn V. Pinkerton, MD:
Another goal is to determine whether transdermal estradiol increases headaches. Before settling on a therapy, however, I would ask how long this patient has been postmenopausal, how long she has been experiencing vasomotor symptoms, and how severe those symptoms are. For example, is she having 7 or 8 hot flashes per day and waking from night sweats once or twice per night? I also would ask her how long she remained on the venlafaxine. The additional information would allow me to fine-tune her treatment.
2. If this formulation is tolerated, I would add micronized progesterone (oral or vaginal) for endometrial protection.
JoAnn V. Pinkerton, MD:
I would give oral progesterone if it is FDA approved for postmenopausal use, vaginal progesterone if it isn’t.
3. I would follow the patient’s clinical response—specifically, her vasomotor symptoms and rate of migraine with or without aura.
Hormone therapy is one option for postmenopausal migraineurs with bothersome vasomotor symptoms
Many women with a history of migraine move into menopause expecting their condition to improve, says headache expert Anne H. Calhoun, MD, a founder of the Carolina Headache Institute in Chapel Hill, North Carolina.
“Over the years, these women have heard that things get better with menopause.”
For women with a history of episodic migraine, that expectation is realistic, Calhoun says. “But for women with chronic migraine, who may experience a low-grade headache on a daily, or almost daily basis, with 10 or 12 severe headaches in a month, things usually get worse after menopause because the sleep issues of menopause are superimposed on the migraine.”
Dr. Calhoun observes that hormone therapy (HT) has never been contraindicated in women with migraine, although many neurologists are hesitant to prescribe any hormones for this population.
Before prescribing HT to a postmenopausal migraineur, Dr. Calhoun considers a range of variables, including sleep patterns, current medications, anxiety, frequency and severity of vasomotor symptoms, and any other problems the patient may be experiencing.
“It’s basically the same assessment as with any postmenopausal patient—to determine whether HT is a reasonable option,” she says.
And when she determines that HT is appropriate, “I almost exclusively use transdermal HT. I also am more likely to prescribe continuous use of a transdermal patch or skin gel, as I want to achieve very consistent hormone levels, day in and day out,” she says.
My bottom line
No systematic data on the use of HT in migraineurs has been published. In the absence of such data, some practitioners have extrapolated data on the use of OCs in this population and decline to prescribe HT to women with migraine. However, HT and OCs are vastly different in formulation, dose, and risks. Rather than make assumptions on the basis of irrelevant data, we should conduct studies of HT use in migraineurs.
Related article: Update on Menopause Andrew M. Kaunitz, MD (June 2013)
Women who have menstrually related migraine typically have an increased risk of migraine during perimenopause and a significant reduction in migraine following menopause. If hot flashes are bothersome, these women certainly can use HT. I recommend prescribing HT in a continuous fashion that maintains stable hormone levels in the blood, as fluctuating hormones tend to trigger migraines.
Andrew M. Kaunitz, MD:
I would just add that transdermal estradiol is preferred, to be given at the lowest effective dose.
Do you have a troubling case in menopause? Suggest it to the expert panel: [email protected]. They may address your management dilemma in a future issue.
We want to hear from you! Send us your Letter to the Editor
Brought to you by the menopause experts
| Andrew M. Kaunitz, MD |
| JoAnn V. Pinkerton, MD |
| James A. Simon, MD |
Disclosures
Dr. Kaunitz reports that his institution receives grant or research support from Bayer, Teva, Medical Diagnostic Laboratories, and Noven, and that he is a consultant to Bayer, Actavis, and Teva.
Dr. Pinkerton reports that her institution receives consulting fees from Pfizer, DepoMed, Shionogi, and Noven and multicenter research fees from DepoMed, Endoceutics, and Bionova.
Dr. Simon reports being a consultant to or on the advisory boards of Abbott Laboratories, Agile Therapeutics, Amgen, Ascend Therapeutics, BioSante, Depomed, Lelo, MD Therapeutics, Meda Pharmaceuticals, Merck, Noven, Novo Nordisk, Novogyne, Pfizer, Shionogi, Shippan Point Advisors LLC, Slate Pharmaceuticals, Sprout Pharmaceuticals, Teva, Warner Chilcott, and Watson. He also reports receiving (currently or in the past year) grant/research support from BioSante, EndoCeutics, Novo Nordisk, Novogyne, Palatin Technologies, Teva, and Warner Chilcott. He reports serving on the speakers bureaus of Amgen, Merck, Novartis, Noven, Novo Nordisk, Novogyne, Teva, and Warner Chilcott. Dr. Simon is currently the Chief Medical Officer for Sprout Pharmaceuticals.
CASE: Menopausal symptoms and a history of migraine with aura
Your new patient is a 52-year-old woman (G2P2) who reports a long history of two types of migraine: menstrually related migraine without aura and nonmenstrually related migraine with aura (usually involving visual scotomata). Other than the history of migraine, her health is good. Now postmenopausal, she has been referred to you by her primary care physician (PCP) for management of severe vasomotor symptoms and sleep disturbance.
Because of this patient’s history of migraine, her PCP declined to prescribe oral contraceptives (OCs) in the past over concern of increasing her risk of stroke. For her vasomotor symptoms, her PCP prescribed a trial of venlafaxine (Effexor) 75 mg daily, but her orgasms, which always had been difficult to achieve, became impossible. In addition, the patient began to perspire heavily unrelated to her hot flashes. As a result, she describes her mood as “terrible,” her energy level as “miniscule,” and she reports losing interest in sex completely (“I am just too tired”). She and her referring physician wonder whether it would be safe to try hormone therapy (HT).
A physical examination, including funduscopic assessment, reveals no abnormalities. Her blood pressure is 126/70 mm Hg, and blood chemistry results, including C-reactive protein, 25-hydroxy vitamin D, a complete blood count, and lipid profile, are all normal.
Would you offer this patient the option of HT?
Migraine affects roughly twice as many women as men.1 During the reproductive years, rapid fluctuations in ovarian hormones—both increases at midcycle and, to a greater extent, decreases during the premenstrual phase—are believed to be migraine “triggers.” Women who experience menstrually related migraine before menopause typically have an increased risk of migraine during perimenopause, with a significant reduction of migraine symptoms following menopause.2
Side effects of SSRIs and SNRIs
Most providers are aware that selective serotonin reuptake inhibitors (SSRIs) cause sexual side effects in as many as 80% of users. There is a dose-related pattern to reports of sexual problems among SSRI users, with higher doses causing more problems. The most common sexual symptoms associated with SSRIs are delayed ejaculation and absent or delayed orgasm.3
What is not as widely known is that even serotonin-norepinephrine reuptake inhibitors (SNRIs) can cause sexual dysfunction. For example, in a prospective, multicenter study from Spain involving more than
1,000 outpatients, all of whom were taking an antidepressant, the overall rate of sexual dysfunction was 59%.4 Sexual dysfunction was most common among users of SSRIs and venlafaxine, an SNRI.5
Another common side effect of venlafaxine, sweating, is unrelated to hot flashes.5 So two of this patient’s concerns—orgasmic difficulties and profuse sweating unrelated to hot flashes—may have been caused or worsened by her antidepressant.
Another nonhormonal option
In June 2013, the US Food and Drug Administration (FDA) approved paroxetine mesylate (Brisdelle), an SSRI, for the treatment of moderate to severe menopausal vasomotor symptoms. Because the drug is a strong CYP2D6 inhibitor, it should not be given to women taking another medication that is metabolized by CYP2D6—most notably, tamoxifen.
Preliminary data on this drug suggest that, at the recommended dose (7.5 mg/d), it has no effect on sexual function.6 For this reason, it is another option for our patient to consider.
Related article: The gynecologist's role in managing menstrual migraine Anne H. Calhoun, MD
Migraine and the risk of stroke
Migraine with aura has been associated with an increased risk of stroke and other cerebral vascular events,7 and that risk is further elevated in patients treated with OCs.8 Although migraine without aura also may be associated with an elevated risk of stroke, OCs do not further increase that risk.
Some practitioners assume that the data on the risk of stroke associated with OC use also applies to hormone therapy, but there is no evidence that HT, in which doses of estrogen are far lower than in OCs, increases the risk of stroke in migraineurs to any greater degree than would be expected in unselected populations (ie, as noted in the Women’s Health Initiative, Nurses Health Study, or other large investigations). Therefore, HT would be an appropriate option for this patient if her very slight risk of stroke on HT would be acceptable to the practitioner and patient.
Choosing an HT formulation
Consider the pharmacokinetic profile. Many oral estrogen HT products have rapid-release characteristics that make them likely to contribute to rapid rises and falls in the user’s estrogen level. Oral estrogens also are associated with procoagulant properties that may increase the risk of thrombosis and thromboembolism. Nonoral estrogens do not appear to increase these risks.13
Nonoral estrogens (patches, gels, sprays, lotions, and vaginal rings) provide a more stable pharmacokinetic profile, as do some oral products with controlled-release properties.
As for progestins, some formulations (medroxyprogesterone acetate) tend to cause vasoconstriction, whereas others (micronized progesterone) tend to be vasodilators. Whether these properties affect the rate of migraine or risk of stroke is unclear.
My management approach for this patient
In the absence of any systematic data on the use of HT in this clinical setting, and without any concrete suggestions from migraine experts, I would take the following three-step approach:
1. I would begin with a low-dose nonoral estradiol formulation, prescribing it without a progestin even in a woman who still has a uterus. My aim: to determine the lowest effective dose of HT for this particular patient. I would follow the patient on this dose for 3 months.
JoAnn V. Pinkerton, MD:
Another goal is to determine whether transdermal estradiol increases headaches. Before settling on a therapy, however, I would ask how long this patient has been postmenopausal, how long she has been experiencing vasomotor symptoms, and how severe those symptoms are. For example, is she having 7 or 8 hot flashes per day and waking from night sweats once or twice per night? I also would ask her how long she remained on the venlafaxine. The additional information would allow me to fine-tune her treatment.
2. If this formulation is tolerated, I would add micronized progesterone (oral or vaginal) for endometrial protection.
JoAnn V. Pinkerton, MD:
I would give oral progesterone if it is FDA approved for postmenopausal use, vaginal progesterone if it isn’t.
3. I would follow the patient’s clinical response—specifically, her vasomotor symptoms and rate of migraine with or without aura.
Hormone therapy is one option for postmenopausal migraineurs with bothersome vasomotor symptoms
Many women with a history of migraine move into menopause expecting their condition to improve, says headache expert Anne H. Calhoun, MD, a founder of the Carolina Headache Institute in Chapel Hill, North Carolina.
“Over the years, these women have heard that things get better with menopause.”
For women with a history of episodic migraine, that expectation is realistic, Calhoun says. “But for women with chronic migraine, who may experience a low-grade headache on a daily, or almost daily basis, with 10 or 12 severe headaches in a month, things usually get worse after menopause because the sleep issues of menopause are superimposed on the migraine.”
Dr. Calhoun observes that hormone therapy (HT) has never been contraindicated in women with migraine, although many neurologists are hesitant to prescribe any hormones for this population.
Before prescribing HT to a postmenopausal migraineur, Dr. Calhoun considers a range of variables, including sleep patterns, current medications, anxiety, frequency and severity of vasomotor symptoms, and any other problems the patient may be experiencing.
“It’s basically the same assessment as with any postmenopausal patient—to determine whether HT is a reasonable option,” she says.
And when she determines that HT is appropriate, “I almost exclusively use transdermal HT. I also am more likely to prescribe continuous use of a transdermal patch or skin gel, as I want to achieve very consistent hormone levels, day in and day out,” she says.
My bottom line
No systematic data on the use of HT in migraineurs has been published. In the absence of such data, some practitioners have extrapolated data on the use of OCs in this population and decline to prescribe HT to women with migraine. However, HT and OCs are vastly different in formulation, dose, and risks. Rather than make assumptions on the basis of irrelevant data, we should conduct studies of HT use in migraineurs.
Related article: Update on Menopause Andrew M. Kaunitz, MD (June 2013)
Women who have menstrually related migraine typically have an increased risk of migraine during perimenopause and a significant reduction in migraine following menopause. If hot flashes are bothersome, these women certainly can use HT. I recommend prescribing HT in a continuous fashion that maintains stable hormone levels in the blood, as fluctuating hormones tend to trigger migraines.
Andrew M. Kaunitz, MD:
I would just add that transdermal estradiol is preferred, to be given at the lowest effective dose.
Do you have a troubling case in menopause? Suggest it to the expert panel: [email protected]. They may address your management dilemma in a future issue.
We want to hear from you! Send us your Letter to the Editor
1. Shuster LT, Faubion SS. Sood R, Casey PM. Hormonal manipulation strategies in the management of menstrual migraine and other hormonally related headaches. Curr Neurol Neurosci Rep. 2011;11(2):131–138.
2. Loder E, Rizzoli P, Golub J. Hormonal management of migraine associated with menses and the menopause: a clinical review. Headache. 2007;47(2):329–340.
3. Balon R. SSRI-associated sexual dysfunction. Am J Psychiatry. 2006;163:1504–1509.
4. Taylor MJ. Strategies for managing antidepressant-induced sexual dysfunction: a review. Curr Psychiatry Rep. 2006;8(6):431–436.
5. Effexor [package insert]. New York, NY: Pfizer; 2012.
6. Brisdelle [package insert]. Miami, FL: Noven Therapeutics; 2013.
7. Etminan M, Takkouche B, Isorna FC, Samli A. Risk of ischaemic stroke in people with migraine: systematic review and meta-analysis of observational studies. BMJ. 2005;330(7482):63–65.
8. Becker WJ. Use of oral contraceptives in patients with migraine. Neurology. 1999;53(4 Suppl 1):S19–S25.
9. WHO Collaborative Study of Cardiovascular Disease and Steroid Hormone Contraception. Ischaemic stroke and combined oral contraceptives: results of an international, multicentre, case-control study. Lancet. 1996;348(9026): 498–505.
10. Petitti DB, Sidney S, Bernstein A, Wolf S, Quesenberry C, Ziel HK. Stroke in users of low-dose oral contraceptives. N Engl J Med. 1996;335(1):8-15.
11. Marjoribanks J, Farquhar C, Roberts H, Lethaby A. Long-term hormone therapy for perimenopausal and postmenopausal women. Cochrane Database Syst Rev. 2012;7:CD004143. doi: 10.1002/14651858.CD004143.pub4
12. Renoux C, Dell’aniello S, Garbe E, Suissa S. Transdermal and oral hormone replacement therapy and the risk of stroke: a nested case-control study. BMJ. 2010;340:c2519.
13. Kaunitz AM. Update on Menopause. OBG Manag. 2013;25(6):36–43, 49.
1. Shuster LT, Faubion SS. Sood R, Casey PM. Hormonal manipulation strategies in the management of menstrual migraine and other hormonally related headaches. Curr Neurol Neurosci Rep. 2011;11(2):131–138.
2. Loder E, Rizzoli P, Golub J. Hormonal management of migraine associated with menses and the menopause: a clinical review. Headache. 2007;47(2):329–340.
3. Balon R. SSRI-associated sexual dysfunction. Am J Psychiatry. 2006;163:1504–1509.
4. Taylor MJ. Strategies for managing antidepressant-induced sexual dysfunction: a review. Curr Psychiatry Rep. 2006;8(6):431–436.
5. Effexor [package insert]. New York, NY: Pfizer; 2012.
6. Brisdelle [package insert]. Miami, FL: Noven Therapeutics; 2013.
7. Etminan M, Takkouche B, Isorna FC, Samli A. Risk of ischaemic stroke in people with migraine: systematic review and meta-analysis of observational studies. BMJ. 2005;330(7482):63–65.
8. Becker WJ. Use of oral contraceptives in patients with migraine. Neurology. 1999;53(4 Suppl 1):S19–S25.
9. WHO Collaborative Study of Cardiovascular Disease and Steroid Hormone Contraception. Ischaemic stroke and combined oral contraceptives: results of an international, multicentre, case-control study. Lancet. 1996;348(9026): 498–505.
10. Petitti DB, Sidney S, Bernstein A, Wolf S, Quesenberry C, Ziel HK. Stroke in users of low-dose oral contraceptives. N Engl J Med. 1996;335(1):8-15.
11. Marjoribanks J, Farquhar C, Roberts H, Lethaby A. Long-term hormone therapy for perimenopausal and postmenopausal women. Cochrane Database Syst Rev. 2012;7:CD004143. doi: 10.1002/14651858.CD004143.pub4
12. Renoux C, Dell’aniello S, Garbe E, Suissa S. Transdermal and oral hormone replacement therapy and the risk of stroke: a nested case-control study. BMJ. 2010;340:c2519.
13. Kaunitz AM. Update on Menopause. OBG Manag. 2013;25(6):36–43, 49.
New option for treating menopausal vasomotor symptoms receives FDA approval
A conjugated estrogen/bazedoxifene formulation (Duavee) was approved by the FDA in early October to treat moderate-to-severe menopausal vasomotor symptoms and prevent menopausal osteoporosis in women with an intact uterus.
“Endometrial safety studies up to 1 year in length suggest that the SERM (bazedoxifene) component of this combination formulation prevents the elevated risk of endometrial hyperplasia associated with estrogen-alone treatment,” said Andrew M. Kaunitz, MD.
“[The new drug] is an alternative to hormone therapy in women with a uterus who are experiencing bothersome vasomotor symptoms in menopause,” concluded JoAnn V. Pinkerton, MD, in a NAMS presentation entitled, “Beyond hormone therapy: Innovative options for treatment of hot flashes.” To hear Dr. Pinkerton describe how much relief from their symptoms (specifically hot flash frequency and severity) patients can expect, as well as advice on patient selection, click on the audio player below.
“In clinical trials, the most commonly reported side effects [of Duavee] included muscle spasms, nausea, diarrhea, upset stomach, abdominal pain, throat pain, dizziness, and neck pain,” Dr. Kaunitz reported.
The manufacturer’s (Pfizer) Web site indicates that Duavee will be available in the first quarter of 2014.
A conjugated estrogen/bazedoxifene formulation (Duavee) was approved by the FDA in early October to treat moderate-to-severe menopausal vasomotor symptoms and prevent menopausal osteoporosis in women with an intact uterus.
“Endometrial safety studies up to 1 year in length suggest that the SERM (bazedoxifene) component of this combination formulation prevents the elevated risk of endometrial hyperplasia associated with estrogen-alone treatment,” said Andrew M. Kaunitz, MD.
“[The new drug] is an alternative to hormone therapy in women with a uterus who are experiencing bothersome vasomotor symptoms in menopause,” concluded JoAnn V. Pinkerton, MD, in a NAMS presentation entitled, “Beyond hormone therapy: Innovative options for treatment of hot flashes.” To hear Dr. Pinkerton describe how much relief from their symptoms (specifically hot flash frequency and severity) patients can expect, as well as advice on patient selection, click on the audio player below.
“In clinical trials, the most commonly reported side effects [of Duavee] included muscle spasms, nausea, diarrhea, upset stomach, abdominal pain, throat pain, dizziness, and neck pain,” Dr. Kaunitz reported.
The manufacturer’s (Pfizer) Web site indicates that Duavee will be available in the first quarter of 2014.
A conjugated estrogen/bazedoxifene formulation (Duavee) was approved by the FDA in early October to treat moderate-to-severe menopausal vasomotor symptoms and prevent menopausal osteoporosis in women with an intact uterus.
“Endometrial safety studies up to 1 year in length suggest that the SERM (bazedoxifene) component of this combination formulation prevents the elevated risk of endometrial hyperplasia associated with estrogen-alone treatment,” said Andrew M. Kaunitz, MD.
“[The new drug] is an alternative to hormone therapy in women with a uterus who are experiencing bothersome vasomotor symptoms in menopause,” concluded JoAnn V. Pinkerton, MD, in a NAMS presentation entitled, “Beyond hormone therapy: Innovative options for treatment of hot flashes.” To hear Dr. Pinkerton describe how much relief from their symptoms (specifically hot flash frequency and severity) patients can expect, as well as advice on patient selection, click on the audio player below.
“In clinical trials, the most commonly reported side effects [of Duavee] included muscle spasms, nausea, diarrhea, upset stomach, abdominal pain, throat pain, dizziness, and neck pain,” Dr. Kaunitz reported.
The manufacturer’s (Pfizer) Web site indicates that Duavee will be available in the first quarter of 2014.