User login
ACIP recommends LAIV as an option for all people with egg allergies
Live attenuated influenza vaccine (LAIV) is likely to be an option for all individuals with egg allergies, regardless of allergy severity, because the Centers for Disease Control and Prevention’s Advisory Committee on Immunization Practices voted to approve proposed amendments to the existing recommendations regarding administration of LAIV in individuals with egg allergies.
With 11 members voting “yes” and three choosing to abstain, the committee agreed to leave sections 1, 2, and 3 as they currently are, with a slight change to section 1 so that it will now include the latter half of recommendations that were previously placed under section 4. Therefore, section 1 of the recommendations for influenza vaccination of persons with egg allergy – which already states that “Regardless of a recipient’s allergy history, all vaccines should be administered in settings in which personnel and equipment for rapid recognition and treatment of anaphylaxis are available” – will now also include text stating that the vaccine should be administered in a medical setting and supervised by a health care provider with “experience in the recognition and management of severe allergic conditions,” or that such a medical professional should be “immediately available.”
Sections 2 and 3 will remain the same. Section 2 states that a previous allergic reaction to the influenza vaccine is a contraindication to ever receiving the vaccine again in the future. Section 3 states that individuals with egg allergy who have only experienced hives due to eggs still should be administered the influenza vaccine.
The committee voted to strike section 5 of the existing recommendations, which calls for a 30-minute postvaccination observation period. However, the committee still advises that health care providers monitor all patients, particularly adolescents, for 15 minutes immediately after vaccination, and that individuals should be seated or lying down “to decrease the risk for injury should syncopy occur.”
The CDC generally follows ACIP’s recommendations.
Live attenuated influenza vaccine (LAIV) is likely to be an option for all individuals with egg allergies, regardless of allergy severity, because the Centers for Disease Control and Prevention’s Advisory Committee on Immunization Practices voted to approve proposed amendments to the existing recommendations regarding administration of LAIV in individuals with egg allergies.
With 11 members voting “yes” and three choosing to abstain, the committee agreed to leave sections 1, 2, and 3 as they currently are, with a slight change to section 1 so that it will now include the latter half of recommendations that were previously placed under section 4. Therefore, section 1 of the recommendations for influenza vaccination of persons with egg allergy – which already states that “Regardless of a recipient’s allergy history, all vaccines should be administered in settings in which personnel and equipment for rapid recognition and treatment of anaphylaxis are available” – will now also include text stating that the vaccine should be administered in a medical setting and supervised by a health care provider with “experience in the recognition and management of severe allergic conditions,” or that such a medical professional should be “immediately available.”
Sections 2 and 3 will remain the same. Section 2 states that a previous allergic reaction to the influenza vaccine is a contraindication to ever receiving the vaccine again in the future. Section 3 states that individuals with egg allergy who have only experienced hives due to eggs still should be administered the influenza vaccine.
The committee voted to strike section 5 of the existing recommendations, which calls for a 30-minute postvaccination observation period. However, the committee still advises that health care providers monitor all patients, particularly adolescents, for 15 minutes immediately after vaccination, and that individuals should be seated or lying down “to decrease the risk for injury should syncopy occur.”
The CDC generally follows ACIP’s recommendations.
Live attenuated influenza vaccine (LAIV) is likely to be an option for all individuals with egg allergies, regardless of allergy severity, because the Centers for Disease Control and Prevention’s Advisory Committee on Immunization Practices voted to approve proposed amendments to the existing recommendations regarding administration of LAIV in individuals with egg allergies.
With 11 members voting “yes” and three choosing to abstain, the committee agreed to leave sections 1, 2, and 3 as they currently are, with a slight change to section 1 so that it will now include the latter half of recommendations that were previously placed under section 4. Therefore, section 1 of the recommendations for influenza vaccination of persons with egg allergy – which already states that “Regardless of a recipient’s allergy history, all vaccines should be administered in settings in which personnel and equipment for rapid recognition and treatment of anaphylaxis are available” – will now also include text stating that the vaccine should be administered in a medical setting and supervised by a health care provider with “experience in the recognition and management of severe allergic conditions,” or that such a medical professional should be “immediately available.”
Sections 2 and 3 will remain the same. Section 2 states that a previous allergic reaction to the influenza vaccine is a contraindication to ever receiving the vaccine again in the future. Section 3 states that individuals with egg allergy who have only experienced hives due to eggs still should be administered the influenza vaccine.
The committee voted to strike section 5 of the existing recommendations, which calls for a 30-minute postvaccination observation period. However, the committee still advises that health care providers monitor all patients, particularly adolescents, for 15 minutes immediately after vaccination, and that individuals should be seated or lying down “to decrease the risk for injury should syncopy occur.”
The CDC generally follows ACIP’s recommendations.
FROM AN ACIP MEETING
Treating influenza: A guide to antiviral safety in pregnancy
Oseltamivir and zanamivir are competitive inhibitors for the neuraminidase enzyme for the influenza virus. They block the surface receptor enzyme and prevent release of virus from the host cell, thus limiting propagation of the infection. These medications can be given as prophylaxis after exposure to influenza or can be given therapeutically for a suspected or confirmed infection. Oseltamivir is recommended for treatment of suspected or confirmed influenza infection in the special population of pregnant women, as the risk for complications of influenza is increased in this group.
Safety evidence
However, there are limited data on the safety and efficacy of the neuraminidase inhibitors in pregnancy. With respect to safety, there have been seven publications in the literature addressing the risk for major birth defects following treatment or prophylaxis with one or both of these products, with the majority of the published data relating to oseltamivir exposure.
In a review by Tanaka et al. in 2009, 90 pregnancies treated therapeutically with oseltamivir in the first trimester were reported to two teratogen information services in Japan; one major birth defect (1.1%) was reported (CMAJ. 2009 Jul 7;181[1-2]:55-8). A year later, Greer et al. published a retrospective chart review at a Texas hospital between 2003 and 2008. During that period, 137 pregnancies that involved a pharmacy record of dispensing of oseltamivir were identified. Of these, 18 were dispensed in the first trimester, and none were linked to a major birth defect outcome (Obstet Gynecol. 2010 Apr;115[4]:711-6).
A 2011 record linkage study in Sweden identified 86 pregnant women for whom oseltamivir (n=81) or zanamivir had been prescribed. Of these, four were linked to a major birth defect in the infant; however, only one of the four prescriptions had been filled in the first trimester (Pharmacoepidemiol Drug Saf. 2011 Oct;20[10]:1030-4). In 2013, Saito et al. reported on a case series gathered from 157 obstetric facilities in Japan. Among 156 infants born to women exposed to oseltamivir in the first trimester, 2 (1.3%) were reported to have a major congenital anomaly; there were no congenital malformations reported in the 15 first-trimester exposures to zanamivir (Am J Obstet Gynecol. 2013 Aug;209[2[:130.e1-9).
In 2014, a teratogen information service in the United Kingdom reported on eight first-trimester exposures to oseltamivir and 37 to zanamivir, with no major birth defects noted in either group (BJOG. 2014 Jun;121[7]:901-6). Additionally, a French prescription database study identified 49 pregnancies thought to be exposed to oseltamivir in the first trimester with one reported congenital anomaly (BJOG. 2014 Jun;121[7]:895-900).
Finally, the manufacturer of oseltamivir published a summary of pregnancies from global pharmacovigilance data accumulated through spontaneous reports and other studies between 2000 and 2012 (Pharmacoepidemiol Drug Saf. 2014 Oct;23[10]:1035-42). Outcomes were available for 1,875 infants. Among these, 81 (4.3%) had major birth defects. However, following case review, the authors indicated that only 11 of the defects (occurring in 9 infants) were biologically plausible based on the timing of the exposure to oseltamivir.
Efficacy examined
With respect to efficacy, two small studies have addressed the pharmacokinetics of oseltamivir in pregnancy to determine if the recommended dosages for nonpregnant individuals are appropriate for pregnancy.
In the earlier of the two studies, Greer et al. looked at the pharmacokinetics of oseltamivir in 30 pregnant women, 10 in each of the three trimesters, who were taking 75 mg of the drug either once or twice daily. Maternal samples were drawn before and after the first dose of oseltamivir. They found little evidence of differences across the three trimesters and concluded that the parent drug values were in the pharmacologic range for clinical efficacy (Am J Obstet Gynecol. 2011 Jun;204[6 Suppl 1]:S89-93).
In contrast, Pillai et al. enrolled a small sample of women being treated with oseltamivir; they evaluated pharmacokinetics for the active metabolite of oseltamivir following 48 or more hours of treatment in 29 pregnant and 35 nonpregnant women (Br J Clin Pharmacol. 2015 Nov;80[5]:1042-50). Significantly lower levels of the active metabolite were noted in the pregnant women, compared with nonpregnant women. The authors suggested that the physiologic changes of pregnancy, correlated with increased renal clearance, produced an approximate 30% lower exposure to the drug in the pregnant state. While they were not able to relate this to maternal or infant outcomes, this finding suggested that further work is needed to determine if dosing recommendations should be adjusted in pregnancy.
The current recommendation is that pregnant women or women within 2 weeks post partum be given oseltamivir for treatment of suspected or confirmed influenza regardless of trimester of pregnancy. The limited safety data that are currently available have not suggested an increased risk for major birth defects following treatment with this product. However, the data are sparse for oseltamivir and even more so for zanamivir. Larger studies focused on these treatments are needed.
Dr. Chambers is professor of pediatrics and director of clinical research at Rady Children’s Hospital, and associate director of the Clinical and Translational Research Institute at the University of California, San Diego. She is director of MotherToBaby California, past president of the Organization of Teratology Information Specialists, and past president of the Teratology Society. She reported having no financial disclosures relevant to this column, but has received research funding Roche-Genentech and GlaxoSmithKline unrelated to antiviral medications. Email her at [email protected].
Oseltamivir and zanamivir are competitive inhibitors for the neuraminidase enzyme for the influenza virus. They block the surface receptor enzyme and prevent release of virus from the host cell, thus limiting propagation of the infection. These medications can be given as prophylaxis after exposure to influenza or can be given therapeutically for a suspected or confirmed infection. Oseltamivir is recommended for treatment of suspected or confirmed influenza infection in the special population of pregnant women, as the risk for complications of influenza is increased in this group.
Safety evidence
However, there are limited data on the safety and efficacy of the neuraminidase inhibitors in pregnancy. With respect to safety, there have been seven publications in the literature addressing the risk for major birth defects following treatment or prophylaxis with one or both of these products, with the majority of the published data relating to oseltamivir exposure.
In a review by Tanaka et al. in 2009, 90 pregnancies treated therapeutically with oseltamivir in the first trimester were reported to two teratogen information services in Japan; one major birth defect (1.1%) was reported (CMAJ. 2009 Jul 7;181[1-2]:55-8). A year later, Greer et al. published a retrospective chart review at a Texas hospital between 2003 and 2008. During that period, 137 pregnancies that involved a pharmacy record of dispensing of oseltamivir were identified. Of these, 18 were dispensed in the first trimester, and none were linked to a major birth defect outcome (Obstet Gynecol. 2010 Apr;115[4]:711-6).
A 2011 record linkage study in Sweden identified 86 pregnant women for whom oseltamivir (n=81) or zanamivir had been prescribed. Of these, four were linked to a major birth defect in the infant; however, only one of the four prescriptions had been filled in the first trimester (Pharmacoepidemiol Drug Saf. 2011 Oct;20[10]:1030-4). In 2013, Saito et al. reported on a case series gathered from 157 obstetric facilities in Japan. Among 156 infants born to women exposed to oseltamivir in the first trimester, 2 (1.3%) were reported to have a major congenital anomaly; there were no congenital malformations reported in the 15 first-trimester exposures to zanamivir (Am J Obstet Gynecol. 2013 Aug;209[2[:130.e1-9).
In 2014, a teratogen information service in the United Kingdom reported on eight first-trimester exposures to oseltamivir and 37 to zanamivir, with no major birth defects noted in either group (BJOG. 2014 Jun;121[7]:901-6). Additionally, a French prescription database study identified 49 pregnancies thought to be exposed to oseltamivir in the first trimester with one reported congenital anomaly (BJOG. 2014 Jun;121[7]:895-900).
Finally, the manufacturer of oseltamivir published a summary of pregnancies from global pharmacovigilance data accumulated through spontaneous reports and other studies between 2000 and 2012 (Pharmacoepidemiol Drug Saf. 2014 Oct;23[10]:1035-42). Outcomes were available for 1,875 infants. Among these, 81 (4.3%) had major birth defects. However, following case review, the authors indicated that only 11 of the defects (occurring in 9 infants) were biologically plausible based on the timing of the exposure to oseltamivir.
Efficacy examined
With respect to efficacy, two small studies have addressed the pharmacokinetics of oseltamivir in pregnancy to determine if the recommended dosages for nonpregnant individuals are appropriate for pregnancy.
In the earlier of the two studies, Greer et al. looked at the pharmacokinetics of oseltamivir in 30 pregnant women, 10 in each of the three trimesters, who were taking 75 mg of the drug either once or twice daily. Maternal samples were drawn before and after the first dose of oseltamivir. They found little evidence of differences across the three trimesters and concluded that the parent drug values were in the pharmacologic range for clinical efficacy (Am J Obstet Gynecol. 2011 Jun;204[6 Suppl 1]:S89-93).
In contrast, Pillai et al. enrolled a small sample of women being treated with oseltamivir; they evaluated pharmacokinetics for the active metabolite of oseltamivir following 48 or more hours of treatment in 29 pregnant and 35 nonpregnant women (Br J Clin Pharmacol. 2015 Nov;80[5]:1042-50). Significantly lower levels of the active metabolite were noted in the pregnant women, compared with nonpregnant women. The authors suggested that the physiologic changes of pregnancy, correlated with increased renal clearance, produced an approximate 30% lower exposure to the drug in the pregnant state. While they were not able to relate this to maternal or infant outcomes, this finding suggested that further work is needed to determine if dosing recommendations should be adjusted in pregnancy.
The current recommendation is that pregnant women or women within 2 weeks post partum be given oseltamivir for treatment of suspected or confirmed influenza regardless of trimester of pregnancy. The limited safety data that are currently available have not suggested an increased risk for major birth defects following treatment with this product. However, the data are sparse for oseltamivir and even more so for zanamivir. Larger studies focused on these treatments are needed.
Dr. Chambers is professor of pediatrics and director of clinical research at Rady Children’s Hospital, and associate director of the Clinical and Translational Research Institute at the University of California, San Diego. She is director of MotherToBaby California, past president of the Organization of Teratology Information Specialists, and past president of the Teratology Society. She reported having no financial disclosures relevant to this column, but has received research funding Roche-Genentech and GlaxoSmithKline unrelated to antiviral medications. Email her at [email protected].
Oseltamivir and zanamivir are competitive inhibitors for the neuraminidase enzyme for the influenza virus. They block the surface receptor enzyme and prevent release of virus from the host cell, thus limiting propagation of the infection. These medications can be given as prophylaxis after exposure to influenza or can be given therapeutically for a suspected or confirmed infection. Oseltamivir is recommended for treatment of suspected or confirmed influenza infection in the special population of pregnant women, as the risk for complications of influenza is increased in this group.
Safety evidence
However, there are limited data on the safety and efficacy of the neuraminidase inhibitors in pregnancy. With respect to safety, there have been seven publications in the literature addressing the risk for major birth defects following treatment or prophylaxis with one or both of these products, with the majority of the published data relating to oseltamivir exposure.
In a review by Tanaka et al. in 2009, 90 pregnancies treated therapeutically with oseltamivir in the first trimester were reported to two teratogen information services in Japan; one major birth defect (1.1%) was reported (CMAJ. 2009 Jul 7;181[1-2]:55-8). A year later, Greer et al. published a retrospective chart review at a Texas hospital between 2003 and 2008. During that period, 137 pregnancies that involved a pharmacy record of dispensing of oseltamivir were identified. Of these, 18 were dispensed in the first trimester, and none were linked to a major birth defect outcome (Obstet Gynecol. 2010 Apr;115[4]:711-6).
A 2011 record linkage study in Sweden identified 86 pregnant women for whom oseltamivir (n=81) or zanamivir had been prescribed. Of these, four were linked to a major birth defect in the infant; however, only one of the four prescriptions had been filled in the first trimester (Pharmacoepidemiol Drug Saf. 2011 Oct;20[10]:1030-4). In 2013, Saito et al. reported on a case series gathered from 157 obstetric facilities in Japan. Among 156 infants born to women exposed to oseltamivir in the first trimester, 2 (1.3%) were reported to have a major congenital anomaly; there were no congenital malformations reported in the 15 first-trimester exposures to zanamivir (Am J Obstet Gynecol. 2013 Aug;209[2[:130.e1-9).
In 2014, a teratogen information service in the United Kingdom reported on eight first-trimester exposures to oseltamivir and 37 to zanamivir, with no major birth defects noted in either group (BJOG. 2014 Jun;121[7]:901-6). Additionally, a French prescription database study identified 49 pregnancies thought to be exposed to oseltamivir in the first trimester with one reported congenital anomaly (BJOG. 2014 Jun;121[7]:895-900).
Finally, the manufacturer of oseltamivir published a summary of pregnancies from global pharmacovigilance data accumulated through spontaneous reports and other studies between 2000 and 2012 (Pharmacoepidemiol Drug Saf. 2014 Oct;23[10]:1035-42). Outcomes were available for 1,875 infants. Among these, 81 (4.3%) had major birth defects. However, following case review, the authors indicated that only 11 of the defects (occurring in 9 infants) were biologically plausible based on the timing of the exposure to oseltamivir.
Efficacy examined
With respect to efficacy, two small studies have addressed the pharmacokinetics of oseltamivir in pregnancy to determine if the recommended dosages for nonpregnant individuals are appropriate for pregnancy.
In the earlier of the two studies, Greer et al. looked at the pharmacokinetics of oseltamivir in 30 pregnant women, 10 in each of the three trimesters, who were taking 75 mg of the drug either once or twice daily. Maternal samples were drawn before and after the first dose of oseltamivir. They found little evidence of differences across the three trimesters and concluded that the parent drug values were in the pharmacologic range for clinical efficacy (Am J Obstet Gynecol. 2011 Jun;204[6 Suppl 1]:S89-93).
In contrast, Pillai et al. enrolled a small sample of women being treated with oseltamivir; they evaluated pharmacokinetics for the active metabolite of oseltamivir following 48 or more hours of treatment in 29 pregnant and 35 nonpregnant women (Br J Clin Pharmacol. 2015 Nov;80[5]:1042-50). Significantly lower levels of the active metabolite were noted in the pregnant women, compared with nonpregnant women. The authors suggested that the physiologic changes of pregnancy, correlated with increased renal clearance, produced an approximate 30% lower exposure to the drug in the pregnant state. While they were not able to relate this to maternal or infant outcomes, this finding suggested that further work is needed to determine if dosing recommendations should be adjusted in pregnancy.
The current recommendation is that pregnant women or women within 2 weeks post partum be given oseltamivir for treatment of suspected or confirmed influenza regardless of trimester of pregnancy. The limited safety data that are currently available have not suggested an increased risk for major birth defects following treatment with this product. However, the data are sparse for oseltamivir and even more so for zanamivir. Larger studies focused on these treatments are needed.
Dr. Chambers is professor of pediatrics and director of clinical research at Rady Children’s Hospital, and associate director of the Clinical and Translational Research Institute at the University of California, San Diego. She is director of MotherToBaby California, past president of the Organization of Teratology Information Specialists, and past president of the Teratology Society. She reported having no financial disclosures relevant to this column, but has received research funding Roche-Genentech and GlaxoSmithKline unrelated to antiviral medications. Email her at [email protected].
U.S. flu activity at its highest level yet
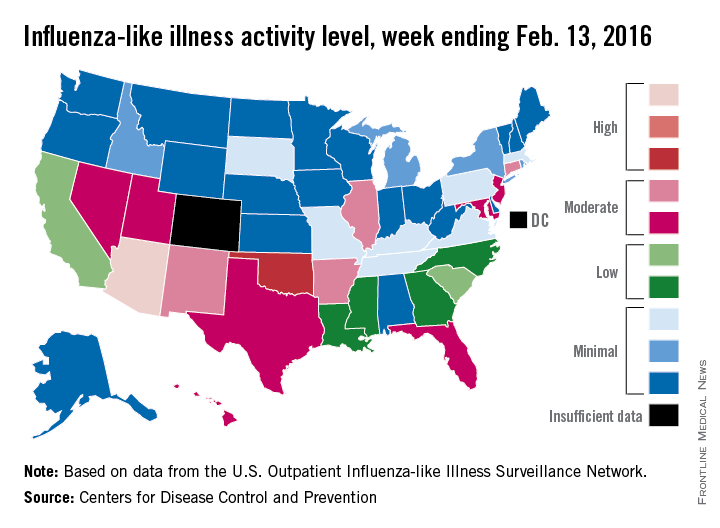
Activity of influenza-like illness (ILI) was at its highest level so far for week 18 of the 2015-2016 flu season, according to the Centers for Disease Control and Prevention.
That increased activity put Arizona and Puerto Rico at level 10 on the CDC’s 1-10 scale of ILI activity for the week ending Feb. 13. Joining them in the “high” range of activity was Oklahoma at level 8, the CDC reported Feb. 19.
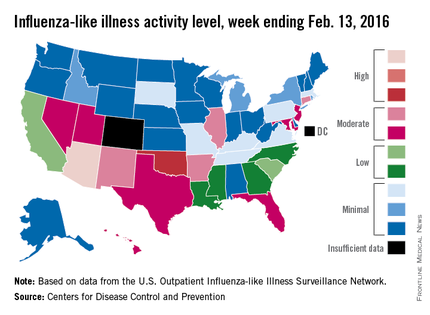
The percentage of visits for ILI in week 18 was 3.1%, higher than the national baseline of 2.1% and the highest level of the flu season. For the week, 30 states were at level 2 or higher on the CDC’s 1-10 scale, which is the highest number for the season. States in the “moderate” range were Arkansas, Connecticut, Illinois, and New Mexico at level 7 and Florida, Hawaii, Maryland, Nevada, New Jersey, Texas, and Utah at level 6. California and South Carolina (level 5) and Georgia, Louisiana, Mississippi, and North Carolina (level 4) took their place in the “low” range, data from the CDC’s Outpatient Influenza-like Illness Surveillance Network show.
Two ILI-related pediatric deaths were reported for the week – one of which occurred during the week ending Jan. 16 – bringing the total to 13 for the season. Three of those deaths occurred in Florida and two in California, with Arizona, Illinois, Louisiana, Michigan, Nevada, Puerto Rico, Tennessee, and Washington each reporting one death, the CDC said.
Since Oct. 1, 2015, the overall ILI-related hospitalization rate is 4.1 per 100,000 population, with 1,147 laboratory-confirmed flu hospitalizations reported. Among all hospitalizations, 71.1% were associated with influenza A, 26.4% with influenza B, 1.7% with influenza A and B coinfection, and 0.8% had no virus type information. Hospitalization data come from the Influenza Hospitalization Surveillance Network, which covers more than 70 counties in 10 states as well as 3 additional states.
Activity of influenza-like illness (ILI) was at its highest level so far for week 18 of the 2015-2016 flu season, according to the Centers for Disease Control and Prevention.
That increased activity put Arizona and Puerto Rico at level 10 on the CDC’s 1-10 scale of ILI activity for the week ending Feb. 13. Joining them in the “high” range of activity was Oklahoma at level 8, the CDC reported Feb. 19.

The percentage of visits for ILI in week 18 was 3.1%, higher than the national baseline of 2.1% and the highest level of the flu season. For the week, 30 states were at level 2 or higher on the CDC’s 1-10 scale, which is the highest number for the season. States in the “moderate” range were Arkansas, Connecticut, Illinois, and New Mexico at level 7 and Florida, Hawaii, Maryland, Nevada, New Jersey, Texas, and Utah at level 6. California and South Carolina (level 5) and Georgia, Louisiana, Mississippi, and North Carolina (level 4) took their place in the “low” range, data from the CDC’s Outpatient Influenza-like Illness Surveillance Network show.
Two ILI-related pediatric deaths were reported for the week – one of which occurred during the week ending Jan. 16 – bringing the total to 13 for the season. Three of those deaths occurred in Florida and two in California, with Arizona, Illinois, Louisiana, Michigan, Nevada, Puerto Rico, Tennessee, and Washington each reporting one death, the CDC said.
Since Oct. 1, 2015, the overall ILI-related hospitalization rate is 4.1 per 100,000 population, with 1,147 laboratory-confirmed flu hospitalizations reported. Among all hospitalizations, 71.1% were associated with influenza A, 26.4% with influenza B, 1.7% with influenza A and B coinfection, and 0.8% had no virus type information. Hospitalization data come from the Influenza Hospitalization Surveillance Network, which covers more than 70 counties in 10 states as well as 3 additional states.
Activity of influenza-like illness (ILI) was at its highest level so far for week 18 of the 2015-2016 flu season, according to the Centers for Disease Control and Prevention.
That increased activity put Arizona and Puerto Rico at level 10 on the CDC’s 1-10 scale of ILI activity for the week ending Feb. 13. Joining them in the “high” range of activity was Oklahoma at level 8, the CDC reported Feb. 19.

The percentage of visits for ILI in week 18 was 3.1%, higher than the national baseline of 2.1% and the highest level of the flu season. For the week, 30 states were at level 2 or higher on the CDC’s 1-10 scale, which is the highest number for the season. States in the “moderate” range were Arkansas, Connecticut, Illinois, and New Mexico at level 7 and Florida, Hawaii, Maryland, Nevada, New Jersey, Texas, and Utah at level 6. California and South Carolina (level 5) and Georgia, Louisiana, Mississippi, and North Carolina (level 4) took their place in the “low” range, data from the CDC’s Outpatient Influenza-like Illness Surveillance Network show.
Two ILI-related pediatric deaths were reported for the week – one of which occurred during the week ending Jan. 16 – bringing the total to 13 for the season. Three of those deaths occurred in Florida and two in California, with Arizona, Illinois, Louisiana, Michigan, Nevada, Puerto Rico, Tennessee, and Washington each reporting one death, the CDC said.
Since Oct. 1, 2015, the overall ILI-related hospitalization rate is 4.1 per 100,000 population, with 1,147 laboratory-confirmed flu hospitalizations reported. Among all hospitalizations, 71.1% were associated with influenza A, 26.4% with influenza B, 1.7% with influenza A and B coinfection, and 0.8% had no virus type information. Hospitalization data come from the Influenza Hospitalization Surveillance Network, which covers more than 70 counties in 10 states as well as 3 additional states.
Poverty promotes flu hospitalizations
Influenza-related hospitalizations were approximately twice as high among residents of areas where at least 20% of the population lived below the federal poverty level, compared with areas of less poverty, based on data from more than 27 million individuals in the United States.
Census and hospitalization data included 14 states and spanned two flu seasons (2010-2011 and 2011-2012). Overall, the incidence of flu-related hospitalizations was approximately 21 per 100,000 person-years in high-poverty areas, compared with approximately 11 per 100,000 person-years in census areas where less than 5% of the population lived below the poverty level. The data were consistent across all age groups and ethnicities.
Flu vaccination rates were inversely associated with poverty level, ranging from a high of 48% in high-income areas to a low of 35% in areas with the most poverty. This finding, however, was probably caused by lower vaccination rates among adults aged 65 years and older in higher-poverty areas, compared with high-income areas (80% vs. 94%) noted Dr. James L. Hadler of the Yale School of Public Health in New Haven, Conn., and colleagues.
“Enhanced influenza outreach to improve influenza vaccination coverage for persons living in poorer neighborhoods and efforts to increase use of antivirals by clinicians serving these neighborhoods could reduce poverty-related disparities in severe influenza outcomes,” the researchers noted.
The findings were published in the Morbidity and Mortality Weekly Report (MMWR 2016;65[5]:101-5). Read the full article here.
Influenza-related hospitalizations were approximately twice as high among residents of areas where at least 20% of the population lived below the federal poverty level, compared with areas of less poverty, based on data from more than 27 million individuals in the United States.
Census and hospitalization data included 14 states and spanned two flu seasons (2010-2011 and 2011-2012). Overall, the incidence of flu-related hospitalizations was approximately 21 per 100,000 person-years in high-poverty areas, compared with approximately 11 per 100,000 person-years in census areas where less than 5% of the population lived below the poverty level. The data were consistent across all age groups and ethnicities.
Flu vaccination rates were inversely associated with poverty level, ranging from a high of 48% in high-income areas to a low of 35% in areas with the most poverty. This finding, however, was probably caused by lower vaccination rates among adults aged 65 years and older in higher-poverty areas, compared with high-income areas (80% vs. 94%) noted Dr. James L. Hadler of the Yale School of Public Health in New Haven, Conn., and colleagues.
“Enhanced influenza outreach to improve influenza vaccination coverage for persons living in poorer neighborhoods and efforts to increase use of antivirals by clinicians serving these neighborhoods could reduce poverty-related disparities in severe influenza outcomes,” the researchers noted.
The findings were published in the Morbidity and Mortality Weekly Report (MMWR 2016;65[5]:101-5). Read the full article here.
Influenza-related hospitalizations were approximately twice as high among residents of areas where at least 20% of the population lived below the federal poverty level, compared with areas of less poverty, based on data from more than 27 million individuals in the United States.
Census and hospitalization data included 14 states and spanned two flu seasons (2010-2011 and 2011-2012). Overall, the incidence of flu-related hospitalizations was approximately 21 per 100,000 person-years in high-poverty areas, compared with approximately 11 per 100,000 person-years in census areas where less than 5% of the population lived below the poverty level. The data were consistent across all age groups and ethnicities.
Flu vaccination rates were inversely associated with poverty level, ranging from a high of 48% in high-income areas to a low of 35% in areas with the most poverty. This finding, however, was probably caused by lower vaccination rates among adults aged 65 years and older in higher-poverty areas, compared with high-income areas (80% vs. 94%) noted Dr. James L. Hadler of the Yale School of Public Health in New Haven, Conn., and colleagues.
“Enhanced influenza outreach to improve influenza vaccination coverage for persons living in poorer neighborhoods and efforts to increase use of antivirals by clinicians serving these neighborhoods could reduce poverty-related disparities in severe influenza outcomes,” the researchers noted.
The findings were published in the Morbidity and Mortality Weekly Report (MMWR 2016;65[5]:101-5). Read the full article here.
FROM MMWR
Influenza linked to atrial fibrillation in large observational study
A diagnosis of influenza increased the risk of subsequent atrial fibrillation by about 18%, investigators reported online in Heart Rhythm.
Clinicians therefore should consider atrial fibrillation (AF) in patients with influenza-like symptoms who report palpitations or experience an ischemic stroke, said Dr. Ting-Yung Chang of Taipei Veterans General Hospital in Taiwan and his associates. Influenza vaccination might help prevent AF, and high-risk patients should be encouraged to receive the vaccination annually, they said. However, a large prospective study is needed to clarify whether influenza vaccination reduces the risk of AF and subsequent ischemic stroke and systemic thromboembolic events, they added.
Atrial fibrillation increases the risk of stroke about fivefold, triples the risk of heart failure, and doubles the chances of dementia and death, the researchers noted. Mounting evidence implicates inflammation and sympathetic nervous system dysregulation in the pathogenesis of AF, raising questions about whether influenza might underlie or contribute to some cases of AF. To explore relationships among AF, influenza, and influenza vaccination, the investigators analyzed data for 11,374 patients with AF who were enrolled in the Taiwan National Health Insurance Research Database between 2000 and 2010. They matched each patient with AF to four controls based on age, sex, enrollment date, and the Charlson comorbidity index (
Heart Rhythm. 2016 Feb. doi: 10.1016/j.hrthm.2016.01.026
).
Unvaccinated patients with influenza were 18% more likely to develop AF than unvaccinated patients without influenza (odds ratio, 1.18; 95% confidence interval, 1.01-1.38; P = .032), even after adjusting for demographic factors, medical history, and use of relevant health care services, the researchers reported. In contrast, vaccinated patients who later developed influenza were about as likely to develop AF as unvaccinated patients who did not develop influenza, both in the overall analysis and in subgroups stratified by age, sex, and comorbidities. Moreover, vaccinated patients without influenza were even less likely to develop AF than unvaccinated patients without influenza (OR, 0.88; 95% CI, 0.84-0.93; P less than .001).
The registry database excluded relevant data on smoking history, body mass index, and physical activity level, the researchers said. “Influenza infection was diagnosed using ICD-9 codes with concomitant use of antiviral agents, and was not further confirmed based on the results of viral culture with throat swab,” they added. “The diagnostic accuracy of influenza infection cannot be fully ascertained.”
The National Science Council and the Taipei Veterans General Hospital funded the study. The researchers had no disclosures.
The authors readily acknowledge the limitations of [this] large, observational study using an insurance database. Despite these admitted limitations, the authors should be commended on adding to the literature regarding modifiable risk factor reduction for the prevention of AF. Recently, a growing body of literature has examined this topic with several straightforward yet promising interventions identified. Weight loss, moderate exercise, and treatment for underlying obstructive sleep apnea have all been shown to reduce the risk of atrial fibrillation incidence or recurrence. Influenza vaccination could represent another simple, cost‐effective intervention to prevent AF. Although the flu vaccine is already recommended for many patient groups, this study suggests that there are even more potential public health benefits of the vaccine.
Dr. Bradley P. Knight is with the division of cardiology, department of medicine, at Northwestern University, Chicago. He had no disclosures. These comments were adapted from his editorial (Heart Rhythm. 2016 Feb. doi: 10.1016/j.hrthm.2016.01.025).
The authors readily acknowledge the limitations of [this] large, observational study using an insurance database. Despite these admitted limitations, the authors should be commended on adding to the literature regarding modifiable risk factor reduction for the prevention of AF. Recently, a growing body of literature has examined this topic with several straightforward yet promising interventions identified. Weight loss, moderate exercise, and treatment for underlying obstructive sleep apnea have all been shown to reduce the risk of atrial fibrillation incidence or recurrence. Influenza vaccination could represent another simple, cost‐effective intervention to prevent AF. Although the flu vaccine is already recommended for many patient groups, this study suggests that there are even more potential public health benefits of the vaccine.
Dr. Bradley P. Knight is with the division of cardiology, department of medicine, at Northwestern University, Chicago. He had no disclosures. These comments were adapted from his editorial (Heart Rhythm. 2016 Feb. doi: 10.1016/j.hrthm.2016.01.025).
The authors readily acknowledge the limitations of [this] large, observational study using an insurance database. Despite these admitted limitations, the authors should be commended on adding to the literature regarding modifiable risk factor reduction for the prevention of AF. Recently, a growing body of literature has examined this topic with several straightforward yet promising interventions identified. Weight loss, moderate exercise, and treatment for underlying obstructive sleep apnea have all been shown to reduce the risk of atrial fibrillation incidence or recurrence. Influenza vaccination could represent another simple, cost‐effective intervention to prevent AF. Although the flu vaccine is already recommended for many patient groups, this study suggests that there are even more potential public health benefits of the vaccine.
Dr. Bradley P. Knight is with the division of cardiology, department of medicine, at Northwestern University, Chicago. He had no disclosures. These comments were adapted from his editorial (Heart Rhythm. 2016 Feb. doi: 10.1016/j.hrthm.2016.01.025).
A diagnosis of influenza increased the risk of subsequent atrial fibrillation by about 18%, investigators reported online in Heart Rhythm.
Clinicians therefore should consider atrial fibrillation (AF) in patients with influenza-like symptoms who report palpitations or experience an ischemic stroke, said Dr. Ting-Yung Chang of Taipei Veterans General Hospital in Taiwan and his associates. Influenza vaccination might help prevent AF, and high-risk patients should be encouraged to receive the vaccination annually, they said. However, a large prospective study is needed to clarify whether influenza vaccination reduces the risk of AF and subsequent ischemic stroke and systemic thromboembolic events, they added.
Atrial fibrillation increases the risk of stroke about fivefold, triples the risk of heart failure, and doubles the chances of dementia and death, the researchers noted. Mounting evidence implicates inflammation and sympathetic nervous system dysregulation in the pathogenesis of AF, raising questions about whether influenza might underlie or contribute to some cases of AF. To explore relationships among AF, influenza, and influenza vaccination, the investigators analyzed data for 11,374 patients with AF who were enrolled in the Taiwan National Health Insurance Research Database between 2000 and 2010. They matched each patient with AF to four controls based on age, sex, enrollment date, and the Charlson comorbidity index (
Heart Rhythm. 2016 Feb. doi: 10.1016/j.hrthm.2016.01.026
).
Unvaccinated patients with influenza were 18% more likely to develop AF than unvaccinated patients without influenza (odds ratio, 1.18; 95% confidence interval, 1.01-1.38; P = .032), even after adjusting for demographic factors, medical history, and use of relevant health care services, the researchers reported. In contrast, vaccinated patients who later developed influenza were about as likely to develop AF as unvaccinated patients who did not develop influenza, both in the overall analysis and in subgroups stratified by age, sex, and comorbidities. Moreover, vaccinated patients without influenza were even less likely to develop AF than unvaccinated patients without influenza (OR, 0.88; 95% CI, 0.84-0.93; P less than .001).
The registry database excluded relevant data on smoking history, body mass index, and physical activity level, the researchers said. “Influenza infection was diagnosed using ICD-9 codes with concomitant use of antiviral agents, and was not further confirmed based on the results of viral culture with throat swab,” they added. “The diagnostic accuracy of influenza infection cannot be fully ascertained.”
The National Science Council and the Taipei Veterans General Hospital funded the study. The researchers had no disclosures.
A diagnosis of influenza increased the risk of subsequent atrial fibrillation by about 18%, investigators reported online in Heart Rhythm.
Clinicians therefore should consider atrial fibrillation (AF) in patients with influenza-like symptoms who report palpitations or experience an ischemic stroke, said Dr. Ting-Yung Chang of Taipei Veterans General Hospital in Taiwan and his associates. Influenza vaccination might help prevent AF, and high-risk patients should be encouraged to receive the vaccination annually, they said. However, a large prospective study is needed to clarify whether influenza vaccination reduces the risk of AF and subsequent ischemic stroke and systemic thromboembolic events, they added.
Atrial fibrillation increases the risk of stroke about fivefold, triples the risk of heart failure, and doubles the chances of dementia and death, the researchers noted. Mounting evidence implicates inflammation and sympathetic nervous system dysregulation in the pathogenesis of AF, raising questions about whether influenza might underlie or contribute to some cases of AF. To explore relationships among AF, influenza, and influenza vaccination, the investigators analyzed data for 11,374 patients with AF who were enrolled in the Taiwan National Health Insurance Research Database between 2000 and 2010. They matched each patient with AF to four controls based on age, sex, enrollment date, and the Charlson comorbidity index (
Heart Rhythm. 2016 Feb. doi: 10.1016/j.hrthm.2016.01.026
).
Unvaccinated patients with influenza were 18% more likely to develop AF than unvaccinated patients without influenza (odds ratio, 1.18; 95% confidence interval, 1.01-1.38; P = .032), even after adjusting for demographic factors, medical history, and use of relevant health care services, the researchers reported. In contrast, vaccinated patients who later developed influenza were about as likely to develop AF as unvaccinated patients who did not develop influenza, both in the overall analysis and in subgroups stratified by age, sex, and comorbidities. Moreover, vaccinated patients without influenza were even less likely to develop AF than unvaccinated patients without influenza (OR, 0.88; 95% CI, 0.84-0.93; P less than .001).
The registry database excluded relevant data on smoking history, body mass index, and physical activity level, the researchers said. “Influenza infection was diagnosed using ICD-9 codes with concomitant use of antiviral agents, and was not further confirmed based on the results of viral culture with throat swab,” they added. “The diagnostic accuracy of influenza infection cannot be fully ascertained.”
The National Science Council and the Taipei Veterans General Hospital funded the study. The researchers had no disclosures.
Key clinical point: Influenza might underlie some cases of atrial fibrillation.
Major finding: Among unvaccinated patients, an influenza diagnosis increased the odds of atrial fibrillation by 18% (OR, 1.18; P = .03).
Data source: An observational registry study of 11,374 patients with atrial fibrillation and 45,496 healthy controls.
Disclosures: The National Science Council and the Taipei Veterans General Hospital funded the study. The researchers had no disclosures.
U.S. flu activity steady in late January
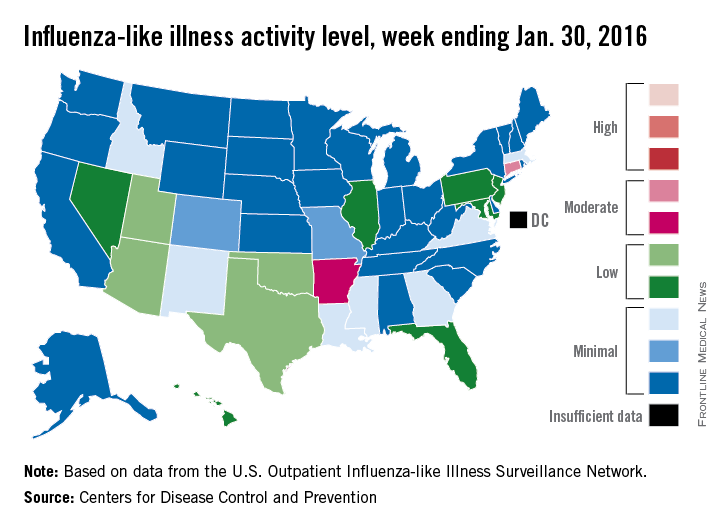
Overall influenza-like illness (ILI) activity underwent a small increase in the United States during week 16 of the 2015-2016 flu season, but Puerto Rico took a significant turn for the worse as activity there rose to the highest possible level, the Centers for Disease Control and Prevention reported Feb. 5.
Puerto Rico, which had been at level 8 on the CDC’s 1-10 scale of flu activity for the previous 3 weeks, jumped up to level 10 for the week ending Jan. 30, 2016. Meanwhile, the main measure for national flu activity, the proportion of outpatient visits for ILI, was 2.2% for the second week in a row, just above the national baseline of 2.1%, the CDC said.
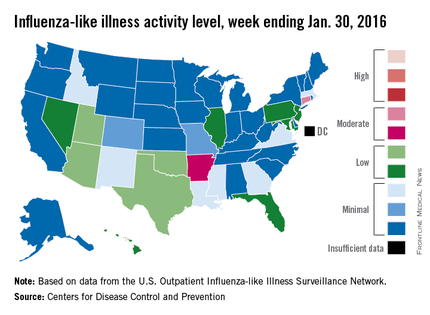
The state with the highest level of flu activity (level 7) for the week was Connecticut, with Arkansas the only other state in the “moderate” range at level 6. States in the “low” range were Florida, Hawaii, Illinois, Maryland, Nevada, New Jersey, and Pennsylvania at level 4 and Georgia, Idaho, Louisiana, Massachusetts, Mississippi, New Mexico, and Virginia at level 3. All told, there were 22 states with activity above level 1, according to data from the CDC’s Outpatient Influenza-like Illness Surveillance Network.
There were two influenza-associated pediatric deaths reported during week 16, although one death actually occurred during the week ending Jan. 16. There have been nine flu-associated pediatric deaths reported so far during the 2015-2016 season.
The hospitalization rate for the season so far is 2.6/100,000 population, up from 2.1/100,000 last week. Adults aged 65 years and older had the highest rate at 8.5/100,000, with children aged 0-4 years next at 3.8/100,000.
Overall influenza-like illness (ILI) activity underwent a small increase in the United States during week 16 of the 2015-2016 flu season, but Puerto Rico took a significant turn for the worse as activity there rose to the highest possible level, the Centers for Disease Control and Prevention reported Feb. 5.
Puerto Rico, which had been at level 8 on the CDC’s 1-10 scale of flu activity for the previous 3 weeks, jumped up to level 10 for the week ending Jan. 30, 2016. Meanwhile, the main measure for national flu activity, the proportion of outpatient visits for ILI, was 2.2% for the second week in a row, just above the national baseline of 2.1%, the CDC said.

The state with the highest level of flu activity (level 7) for the week was Connecticut, with Arkansas the only other state in the “moderate” range at level 6. States in the “low” range were Florida, Hawaii, Illinois, Maryland, Nevada, New Jersey, and Pennsylvania at level 4 and Georgia, Idaho, Louisiana, Massachusetts, Mississippi, New Mexico, and Virginia at level 3. All told, there were 22 states with activity above level 1, according to data from the CDC’s Outpatient Influenza-like Illness Surveillance Network.
There were two influenza-associated pediatric deaths reported during week 16, although one death actually occurred during the week ending Jan. 16. There have been nine flu-associated pediatric deaths reported so far during the 2015-2016 season.
The hospitalization rate for the season so far is 2.6/100,000 population, up from 2.1/100,000 last week. Adults aged 65 years and older had the highest rate at 8.5/100,000, with children aged 0-4 years next at 3.8/100,000.
Overall influenza-like illness (ILI) activity underwent a small increase in the United States during week 16 of the 2015-2016 flu season, but Puerto Rico took a significant turn for the worse as activity there rose to the highest possible level, the Centers for Disease Control and Prevention reported Feb. 5.
Puerto Rico, which had been at level 8 on the CDC’s 1-10 scale of flu activity for the previous 3 weeks, jumped up to level 10 for the week ending Jan. 30, 2016. Meanwhile, the main measure for national flu activity, the proportion of outpatient visits for ILI, was 2.2% for the second week in a row, just above the national baseline of 2.1%, the CDC said.

The state with the highest level of flu activity (level 7) for the week was Connecticut, with Arkansas the only other state in the “moderate” range at level 6. States in the “low” range were Florida, Hawaii, Illinois, Maryland, Nevada, New Jersey, and Pennsylvania at level 4 and Georgia, Idaho, Louisiana, Massachusetts, Mississippi, New Mexico, and Virginia at level 3. All told, there were 22 states with activity above level 1, according to data from the CDC’s Outpatient Influenza-like Illness Surveillance Network.
There were two influenza-associated pediatric deaths reported during week 16, although one death actually occurred during the week ending Jan. 16. There have been nine flu-associated pediatric deaths reported so far during the 2015-2016 season.
The hospitalization rate for the season so far is 2.6/100,000 population, up from 2.1/100,000 last week. Adults aged 65 years and older had the highest rate at 8.5/100,000, with children aged 0-4 years next at 3.8/100,000.
Majority of children aged 6-23 months are not vaccinated for flu
Less than half of children aged 6-23 months are vaccinated for influenza in the United States, according to an analysis of data obtained via the 2003-2013 National Immunization Survey.
The researchers analyzed providers’ reports of influenza vaccinations, received as one or two doses by children aged 6-23 months. The age group studied is at highest risk of influenza-related complications and was the first group of children for which the Advisory Committee on Immunization Practices recommended influenza vaccination, regardless of an individual’s medical condition.
A child’s age was defined by his or her age on Nov. 1 of each influenza season under study. Two full calendar years of data files were combined to enable analysis of full influenza seasons, which cover parts of 2 consecutive calendar years. The percentages of children requiring two doses to be considered fully vaccinated were based on the dosage recommendations for each flu season.
Overall, flu vaccination coverage increased, reaching 45% in the 2011-2012 flu season, up from 5% during the 2002-2003 flu season. Within each racial/ethnic group examined, influenza vaccination coverage also grew; however, lower percentages of non-Hispanic black children and Hispanic children were vaccinated than of non-Hispanic white children during all 10 of the flu seasons studied. Coverage ranged from 24% in Mississippi to 72% in Massachusetts.
“Despite the increase, the majority of children 6-23 months in the United States were not fully vaccinated against influenza,” said Tammy A. Santibanez, Ph.D., of the Centers for Disease Control and Prevention, and her colleagues.
Among other findings consistent throughout each flu season examined was that full influenza vaccination coverage was higher among children requiring only one dose of a flu vaccine, compared with those requiring two doses of a flu vaccine.
“Prevention of influenza among infants and young children is a public health priority because of their high risk for influenza-related complications,” wrote Dr. Santibanez and her colleagues. “Appropriate implementation of evidence-based strategies is needed to increase the percentage of children who are fully vaccinated.”
Read the study in Pediatrics (doi: 10.1542/peds.2015.3280).
Less than half of children aged 6-23 months are vaccinated for influenza in the United States, according to an analysis of data obtained via the 2003-2013 National Immunization Survey.
The researchers analyzed providers’ reports of influenza vaccinations, received as one or two doses by children aged 6-23 months. The age group studied is at highest risk of influenza-related complications and was the first group of children for which the Advisory Committee on Immunization Practices recommended influenza vaccination, regardless of an individual’s medical condition.
A child’s age was defined by his or her age on Nov. 1 of each influenza season under study. Two full calendar years of data files were combined to enable analysis of full influenza seasons, which cover parts of 2 consecutive calendar years. The percentages of children requiring two doses to be considered fully vaccinated were based on the dosage recommendations for each flu season.
Overall, flu vaccination coverage increased, reaching 45% in the 2011-2012 flu season, up from 5% during the 2002-2003 flu season. Within each racial/ethnic group examined, influenza vaccination coverage also grew; however, lower percentages of non-Hispanic black children and Hispanic children were vaccinated than of non-Hispanic white children during all 10 of the flu seasons studied. Coverage ranged from 24% in Mississippi to 72% in Massachusetts.
“Despite the increase, the majority of children 6-23 months in the United States were not fully vaccinated against influenza,” said Tammy A. Santibanez, Ph.D., of the Centers for Disease Control and Prevention, and her colleagues.
Among other findings consistent throughout each flu season examined was that full influenza vaccination coverage was higher among children requiring only one dose of a flu vaccine, compared with those requiring two doses of a flu vaccine.
“Prevention of influenza among infants and young children is a public health priority because of their high risk for influenza-related complications,” wrote Dr. Santibanez and her colleagues. “Appropriate implementation of evidence-based strategies is needed to increase the percentage of children who are fully vaccinated.”
Read the study in Pediatrics (doi: 10.1542/peds.2015.3280).
Less than half of children aged 6-23 months are vaccinated for influenza in the United States, according to an analysis of data obtained via the 2003-2013 National Immunization Survey.
The researchers analyzed providers’ reports of influenza vaccinations, received as one or two doses by children aged 6-23 months. The age group studied is at highest risk of influenza-related complications and was the first group of children for which the Advisory Committee on Immunization Practices recommended influenza vaccination, regardless of an individual’s medical condition.
A child’s age was defined by his or her age on Nov. 1 of each influenza season under study. Two full calendar years of data files were combined to enable analysis of full influenza seasons, which cover parts of 2 consecutive calendar years. The percentages of children requiring two doses to be considered fully vaccinated were based on the dosage recommendations for each flu season.
Overall, flu vaccination coverage increased, reaching 45% in the 2011-2012 flu season, up from 5% during the 2002-2003 flu season. Within each racial/ethnic group examined, influenza vaccination coverage also grew; however, lower percentages of non-Hispanic black children and Hispanic children were vaccinated than of non-Hispanic white children during all 10 of the flu seasons studied. Coverage ranged from 24% in Mississippi to 72% in Massachusetts.
“Despite the increase, the majority of children 6-23 months in the United States were not fully vaccinated against influenza,” said Tammy A. Santibanez, Ph.D., of the Centers for Disease Control and Prevention, and her colleagues.
Among other findings consistent throughout each flu season examined was that full influenza vaccination coverage was higher among children requiring only one dose of a flu vaccine, compared with those requiring two doses of a flu vaccine.
“Prevention of influenza among infants and young children is a public health priority because of their high risk for influenza-related complications,” wrote Dr. Santibanez and her colleagues. “Appropriate implementation of evidence-based strategies is needed to increase the percentage of children who are fully vaccinated.”
Read the study in Pediatrics (doi: 10.1542/peds.2015.3280).
FROM PEDIATRICS
U.S. flu activity continues slow increase
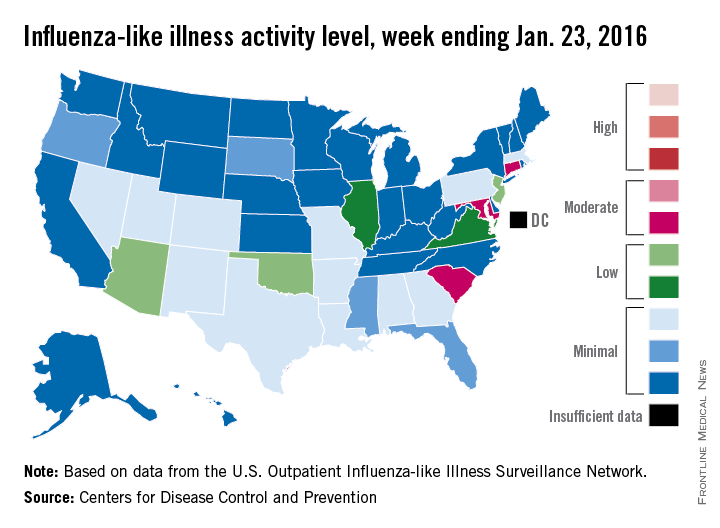
The proportion of U.S. outpatient visits for influenza-like illness (ILI) crept up from 2.1% to 2.2% for the week ending Jan. 23 – week 15 of the 2015-2016 flu season, the Centers for Disease Control and Prevention reported.
That increase put the proportion of visits involving ILI back above the national baseline level of 2.1% for the first time since the week ending Jan. 2, when it reached a season-high 2.8%, the CDC said.
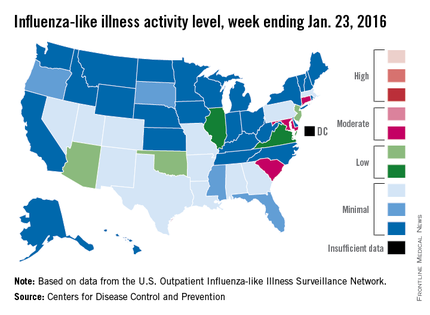
Puerto Rico was the nation’s influenza hotspot at level 8 on the CDC’s 1-10 scale of ILI activity, but the states with the most activity were Connecticut, Maryland, and South Carolina, which were categorized in the “moderate” range at level 6. Connecticut was up from level 2 the week before; the other two states were unchanged.
There were five states in the “low” range: Arizona, New Jersey, and Oklahoma at level 5 and Illinois and Virginia at level 4. Arizona (down from level 7) and Oklahoma (up from level 3) were the biggest movers in this group from the previous week. There were 12 states at level 3 and four states at level 2, putting the total at 24 states at level 2 or higher, according to data from the CDC’s Outpatient Influenza-like Illness Surveillance Network.
There were no pediatric deaths reported in week 15, keeping the total for the season at seven, the CDC said, which is well below the number of deaths reported by week 15 in each of the last three flu seasons.
The ILI hospitalization rate for the season so far is 2.1 per 100,000 population, with 575 laboratory-confirmed, influenza-associated hospitalizations reported between Oct. 1, 2015, and Jan. 23, 2016. The highest hospitalization rates are among adults aged 65 years and older (7.0 per 100,000 population) and children aged 0-4 years (3.4 per 100,000), the CDC reported.
The proportion of U.S. outpatient visits for influenza-like illness (ILI) crept up from 2.1% to 2.2% for the week ending Jan. 23 – week 15 of the 2015-2016 flu season, the Centers for Disease Control and Prevention reported.
That increase put the proportion of visits involving ILI back above the national baseline level of 2.1% for the first time since the week ending Jan. 2, when it reached a season-high 2.8%, the CDC said.

Puerto Rico was the nation’s influenza hotspot at level 8 on the CDC’s 1-10 scale of ILI activity, but the states with the most activity were Connecticut, Maryland, and South Carolina, which were categorized in the “moderate” range at level 6. Connecticut was up from level 2 the week before; the other two states were unchanged.
There were five states in the “low” range: Arizona, New Jersey, and Oklahoma at level 5 and Illinois and Virginia at level 4. Arizona (down from level 7) and Oklahoma (up from level 3) were the biggest movers in this group from the previous week. There were 12 states at level 3 and four states at level 2, putting the total at 24 states at level 2 or higher, according to data from the CDC’s Outpatient Influenza-like Illness Surveillance Network.
There were no pediatric deaths reported in week 15, keeping the total for the season at seven, the CDC said, which is well below the number of deaths reported by week 15 in each of the last three flu seasons.
The ILI hospitalization rate for the season so far is 2.1 per 100,000 population, with 575 laboratory-confirmed, influenza-associated hospitalizations reported between Oct. 1, 2015, and Jan. 23, 2016. The highest hospitalization rates are among adults aged 65 years and older (7.0 per 100,000 population) and children aged 0-4 years (3.4 per 100,000), the CDC reported.
The proportion of U.S. outpatient visits for influenza-like illness (ILI) crept up from 2.1% to 2.2% for the week ending Jan. 23 – week 15 of the 2015-2016 flu season, the Centers for Disease Control and Prevention reported.
That increase put the proportion of visits involving ILI back above the national baseline level of 2.1% for the first time since the week ending Jan. 2, when it reached a season-high 2.8%, the CDC said.

Puerto Rico was the nation’s influenza hotspot at level 8 on the CDC’s 1-10 scale of ILI activity, but the states with the most activity were Connecticut, Maryland, and South Carolina, which were categorized in the “moderate” range at level 6. Connecticut was up from level 2 the week before; the other two states were unchanged.
There were five states in the “low” range: Arizona, New Jersey, and Oklahoma at level 5 and Illinois and Virginia at level 4. Arizona (down from level 7) and Oklahoma (up from level 3) were the biggest movers in this group from the previous week. There were 12 states at level 3 and four states at level 2, putting the total at 24 states at level 2 or higher, according to data from the CDC’s Outpatient Influenza-like Illness Surveillance Network.
There were no pediatric deaths reported in week 15, keeping the total for the season at seven, the CDC said, which is well below the number of deaths reported by week 15 in each of the last three flu seasons.
The ILI hospitalization rate for the season so far is 2.1 per 100,000 population, with 575 laboratory-confirmed, influenza-associated hospitalizations reported between Oct. 1, 2015, and Jan. 23, 2016. The highest hospitalization rates are among adults aged 65 years and older (7.0 per 100,000 population) and children aged 0-4 years (3.4 per 100,000), the CDC reported.
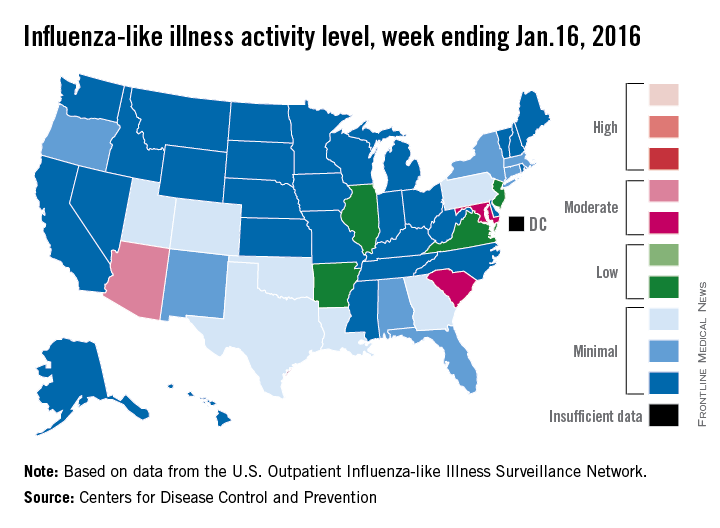
U.S. flu activity picks up slightly
There were no states in the “high” range of influenza-like illness (ILI) activity during week 14 of the 2015-2016 flu season, but there were more states with elevated levels, compared with the previous week, the Centers for Disease Control and Prevention reported.
Arizona had the highest activity (level 7) for the week ending Jan. 16, with Maryland and South Carolina (level 6) the only other states in the “moderate” range of ILI activity. There were 21 states at level 2 or higher, up from 18 the week before, but still below the peak of 24 that was seen 2 weeks ago. The overall proportion of outpatient visits for ILI was 2.1% for week 14, which was up from 2% for week 13 but was right at the national baseline, according to data from the CDC’s Outpatient Influenza-like Illness Surveillance Network.
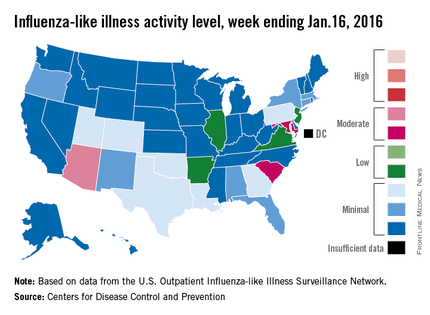
There were no influenza-related pediatric deaths reported for week 14, so the number of total deaths remains at seven for the season, which is well below the week-14 totals for each of the previous three seasons. There have been 494 laboratory-confirmed influenza-associated hospitalizations reported in the 13 states of the CDC’s Influenza Hospitalization Surveillance Network through week 14, for an overall hospitalization rate of 1.8/100,000 population, the CDC said.
Among all hospitalizations, 65.4% were associated with influenza A, 28.7% with influenza B, 3.2% with influenza A and B coinfection, and 2.6% had no virus type information, the CDC report noted.
There were no states in the “high” range of influenza-like illness (ILI) activity during week 14 of the 2015-2016 flu season, but there were more states with elevated levels, compared with the previous week, the Centers for Disease Control and Prevention reported.
Arizona had the highest activity (level 7) for the week ending Jan. 16, with Maryland and South Carolina (level 6) the only other states in the “moderate” range of ILI activity. There were 21 states at level 2 or higher, up from 18 the week before, but still below the peak of 24 that was seen 2 weeks ago. The overall proportion of outpatient visits for ILI was 2.1% for week 14, which was up from 2% for week 13 but was right at the national baseline, according to data from the CDC’s Outpatient Influenza-like Illness Surveillance Network.

There were no influenza-related pediatric deaths reported for week 14, so the number of total deaths remains at seven for the season, which is well below the week-14 totals for each of the previous three seasons. There have been 494 laboratory-confirmed influenza-associated hospitalizations reported in the 13 states of the CDC’s Influenza Hospitalization Surveillance Network through week 14, for an overall hospitalization rate of 1.8/100,000 population, the CDC said.
Among all hospitalizations, 65.4% were associated with influenza A, 28.7% with influenza B, 3.2% with influenza A and B coinfection, and 2.6% had no virus type information, the CDC report noted.
There were no states in the “high” range of influenza-like illness (ILI) activity during week 14 of the 2015-2016 flu season, but there were more states with elevated levels, compared with the previous week, the Centers for Disease Control and Prevention reported.
Arizona had the highest activity (level 7) for the week ending Jan. 16, with Maryland and South Carolina (level 6) the only other states in the “moderate” range of ILI activity. There were 21 states at level 2 or higher, up from 18 the week before, but still below the peak of 24 that was seen 2 weeks ago. The overall proportion of outpatient visits for ILI was 2.1% for week 14, which was up from 2% for week 13 but was right at the national baseline, according to data from the CDC’s Outpatient Influenza-like Illness Surveillance Network.

There were no influenza-related pediatric deaths reported for week 14, so the number of total deaths remains at seven for the season, which is well below the week-14 totals for each of the previous three seasons. There have been 494 laboratory-confirmed influenza-associated hospitalizations reported in the 13 states of the CDC’s Influenza Hospitalization Surveillance Network through week 14, for an overall hospitalization rate of 1.8/100,000 population, the CDC said.
Among all hospitalizations, 65.4% were associated with influenza A, 28.7% with influenza B, 3.2% with influenza A and B coinfection, and 2.6% had no virus type information, the CDC report noted.
Gene signatures aid diagnosis of acute respiratory infection etiology
Pathogen-specific host gene expression patterns accurately discriminated most noninfectious from infectious illnesses, and bacterial from viral causes of acute respiratory infection (ARI) in an observational study conducted in acute care settings.
The findings could have important implications for combating inappropriate antibiotic use and emerging antibiotic resistance, Dr. Ephraim L. Tsalik of the department of medicine at Duke University, Durham, N.C., and his colleagues reported online Jan. 20 in Science Translational Medicine.
The investigators analyzed peripheral whole-blood gene expression from 273 subjects with community-onset viral ARI (115 subjects), bacterial ARI (70 subjects), or noninfectious illness (88 subjects) who were seen in an emergency department, and from 44 healthy control subjects. Classifiers for bacterial ARI, viral ARI, and noninfectious causes of illness were developed, and were 87% accurate overall (Sci Transl Med. 2016;8[322]:322ra11. doi/ 10.1126/scitranslmed.aad6873).
“Bacterial ARI was identified in 83% of patients and excluded in 94% without bacterial infection. Viral ARI was identified in 90% and excluded in 92% of cases. Using the noninfectious illness classifier, infection was excluded in 86% of cases,” they wrote.
The classifiers were more accurate than procalcitonin – a widely used biomarker with some specificity for bacterial infection (86% vs. 78% accuracy in 238 available samples), and three published classifiers of bacterial vs. viral infection, and were validated in five publicly available data sets, they noted.
The gene signature patterns identified in the course of this study mark an important step toward development of a rapid blood test that could be used in clinics to guide appropriate treatment for ARIs, the investigators said.
Precision treatment of viruses
More precise ways to distinguish infections could reduce unnecessary antibiotic use and lead to more precise treatment of viruses, senior author Dr. Geoffrey S. Ginsburg, director of Duke’s Center for Applied Genomics & Precision Medicine, said in a press statement.
“Right now, we can give patients [oseltamivir] Tamiflu to help them recover from an influenza infection, but for most viral infections, the treatment is fluids and rest until it resolves. In the next 5-10 years, we will likely see new antiviral medications for common bugs like respiratory syncytial virus and even rhinovirus, and guiding treatment choices will be even more important,” he added.
Senior author Dr. Christopher W. Woods, also of Duke University, further explained in an interview that the findings are particularly exciting because “there isn’t much out there that accomplishes what we’ve done. So just about any level of accuracy is an improvement.” Further, he said the test is “a tool to aid in diagnosis, used in conjunction with the patient’s symptoms, examination, and other testing. So an imperfect test is okay, because it does not stand alone.”
Next steps include putting the assay on a testing platform that can be used at the point of care, and validating the findings in all populations, including infants, the elderly, and across ethnic groups, he said.
“The work is ongoing, and we expect to have results available within the course of an outpatient visit in the near future,” Dr. Woods, also a professor of medicine and global health, added, noting that efforts also are underway to “expand the repertoire of this approach to many different types of viral and bacterial infections and also to fungal infections, and to address the challenges of critically ill patients in intensive care units.”
This study was supported by the U.S. Defense Advanced Research Projects Agency, the National Institutes of Health, the Agency for Healthcare Research and Quality, the U.S. Department of Veterans Affairs Office of Research and Development, and an in-kind contribution from bioMérieux. The authors reported having no relevant competing interests.
Pathogen-specific host gene expression patterns accurately discriminated most noninfectious from infectious illnesses, and bacterial from viral causes of acute respiratory infection (ARI) in an observational study conducted in acute care settings.
The findings could have important implications for combating inappropriate antibiotic use and emerging antibiotic resistance, Dr. Ephraim L. Tsalik of the department of medicine at Duke University, Durham, N.C., and his colleagues reported online Jan. 20 in Science Translational Medicine.
The investigators analyzed peripheral whole-blood gene expression from 273 subjects with community-onset viral ARI (115 subjects), bacterial ARI (70 subjects), or noninfectious illness (88 subjects) who were seen in an emergency department, and from 44 healthy control subjects. Classifiers for bacterial ARI, viral ARI, and noninfectious causes of illness were developed, and were 87% accurate overall (Sci Transl Med. 2016;8[322]:322ra11. doi/ 10.1126/scitranslmed.aad6873).
“Bacterial ARI was identified in 83% of patients and excluded in 94% without bacterial infection. Viral ARI was identified in 90% and excluded in 92% of cases. Using the noninfectious illness classifier, infection was excluded in 86% of cases,” they wrote.
The classifiers were more accurate than procalcitonin – a widely used biomarker with some specificity for bacterial infection (86% vs. 78% accuracy in 238 available samples), and three published classifiers of bacterial vs. viral infection, and were validated in five publicly available data sets, they noted.
The gene signature patterns identified in the course of this study mark an important step toward development of a rapid blood test that could be used in clinics to guide appropriate treatment for ARIs, the investigators said.
Precision treatment of viruses
More precise ways to distinguish infections could reduce unnecessary antibiotic use and lead to more precise treatment of viruses, senior author Dr. Geoffrey S. Ginsburg, director of Duke’s Center for Applied Genomics & Precision Medicine, said in a press statement.
“Right now, we can give patients [oseltamivir] Tamiflu to help them recover from an influenza infection, but for most viral infections, the treatment is fluids and rest until it resolves. In the next 5-10 years, we will likely see new antiviral medications for common bugs like respiratory syncytial virus and even rhinovirus, and guiding treatment choices will be even more important,” he added.
Senior author Dr. Christopher W. Woods, also of Duke University, further explained in an interview that the findings are particularly exciting because “there isn’t much out there that accomplishes what we’ve done. So just about any level of accuracy is an improvement.” Further, he said the test is “a tool to aid in diagnosis, used in conjunction with the patient’s symptoms, examination, and other testing. So an imperfect test is okay, because it does not stand alone.”
Next steps include putting the assay on a testing platform that can be used at the point of care, and validating the findings in all populations, including infants, the elderly, and across ethnic groups, he said.
“The work is ongoing, and we expect to have results available within the course of an outpatient visit in the near future,” Dr. Woods, also a professor of medicine and global health, added, noting that efforts also are underway to “expand the repertoire of this approach to many different types of viral and bacterial infections and also to fungal infections, and to address the challenges of critically ill patients in intensive care units.”
This study was supported by the U.S. Defense Advanced Research Projects Agency, the National Institutes of Health, the Agency for Healthcare Research and Quality, the U.S. Department of Veterans Affairs Office of Research and Development, and an in-kind contribution from bioMérieux. The authors reported having no relevant competing interests.
Pathogen-specific host gene expression patterns accurately discriminated most noninfectious from infectious illnesses, and bacterial from viral causes of acute respiratory infection (ARI) in an observational study conducted in acute care settings.
The findings could have important implications for combating inappropriate antibiotic use and emerging antibiotic resistance, Dr. Ephraim L. Tsalik of the department of medicine at Duke University, Durham, N.C., and his colleagues reported online Jan. 20 in Science Translational Medicine.
The investigators analyzed peripheral whole-blood gene expression from 273 subjects with community-onset viral ARI (115 subjects), bacterial ARI (70 subjects), or noninfectious illness (88 subjects) who were seen in an emergency department, and from 44 healthy control subjects. Classifiers for bacterial ARI, viral ARI, and noninfectious causes of illness were developed, and were 87% accurate overall (Sci Transl Med. 2016;8[322]:322ra11. doi/ 10.1126/scitranslmed.aad6873).
“Bacterial ARI was identified in 83% of patients and excluded in 94% without bacterial infection. Viral ARI was identified in 90% and excluded in 92% of cases. Using the noninfectious illness classifier, infection was excluded in 86% of cases,” they wrote.
The classifiers were more accurate than procalcitonin – a widely used biomarker with some specificity for bacterial infection (86% vs. 78% accuracy in 238 available samples), and three published classifiers of bacterial vs. viral infection, and were validated in five publicly available data sets, they noted.
The gene signature patterns identified in the course of this study mark an important step toward development of a rapid blood test that could be used in clinics to guide appropriate treatment for ARIs, the investigators said.
Precision treatment of viruses
More precise ways to distinguish infections could reduce unnecessary antibiotic use and lead to more precise treatment of viruses, senior author Dr. Geoffrey S. Ginsburg, director of Duke’s Center for Applied Genomics & Precision Medicine, said in a press statement.
“Right now, we can give patients [oseltamivir] Tamiflu to help them recover from an influenza infection, but for most viral infections, the treatment is fluids and rest until it resolves. In the next 5-10 years, we will likely see new antiviral medications for common bugs like respiratory syncytial virus and even rhinovirus, and guiding treatment choices will be even more important,” he added.
Senior author Dr. Christopher W. Woods, also of Duke University, further explained in an interview that the findings are particularly exciting because “there isn’t much out there that accomplishes what we’ve done. So just about any level of accuracy is an improvement.” Further, he said the test is “a tool to aid in diagnosis, used in conjunction with the patient’s symptoms, examination, and other testing. So an imperfect test is okay, because it does not stand alone.”
Next steps include putting the assay on a testing platform that can be used at the point of care, and validating the findings in all populations, including infants, the elderly, and across ethnic groups, he said.
“The work is ongoing, and we expect to have results available within the course of an outpatient visit in the near future,” Dr. Woods, also a professor of medicine and global health, added, noting that efforts also are underway to “expand the repertoire of this approach to many different types of viral and bacterial infections and also to fungal infections, and to address the challenges of critically ill patients in intensive care units.”
This study was supported by the U.S. Defense Advanced Research Projects Agency, the National Institutes of Health, the Agency for Healthcare Research and Quality, the U.S. Department of Veterans Affairs Office of Research and Development, and an in-kind contribution from bioMérieux. The authors reported having no relevant competing interests.
FROM SCIENCE TRANSLATIONAL MEDICINE
Key clinical point: Pathogen-specific host gene expression patterns accurately discriminated most noninfectious from infectious illnesses, and bacterial from viral causes of acute respiratory infection (ARI) in an observational study.
Major finding: Classifiers for bacterial ARI, viral ARI, and noninfectious causes of illness were 87% accurate overall.
Data source: An observational cohort study involving 273 patients and 44 controls.
Disclosures: This study was supported by the U.S. Defense Advanced Research Projects Agency, the National Institutes of Health, the Agency for Healthcare Research and Quality, the U.S. Department of Veterans Affairs Office of Research and Development, and an in-kind contribution from bioMérieux. The authors reported having no relevant competing interests..