User login
U.S. flu activity continues to drop, but still widespread
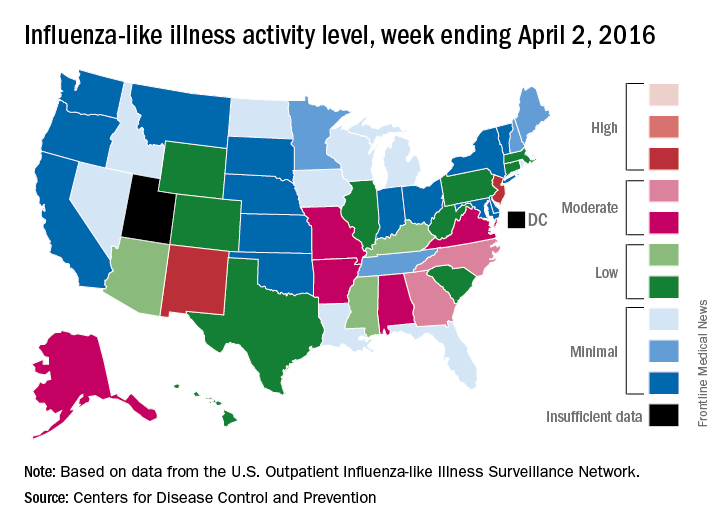
A third straight week of reduced influenza-like illness (ILI) left the U.S. with no states at the highest level of ILI activity for the first time since early February, according to the Centers for Disease Control and Prevention.
The states with the highest activity for the week ending April 2, 2016, were New Jersey and New Mexico, and both were at level 8 on the CDC’s 1-10 scale, putting them on the low end of the “high” range. States in the “moderate” range were Georgia and North Carolina at level 7 and Alabama, Alaska, Arkansas, Missouri, and Virginia at level 6, according to a report from the CDC’s Influenza-like Illness Surveillance Network (ILINet).
The proportion of outpatient visits for ILI was 2.4% for the week, down from 2.9% the week before but still above the national baseline of 2.1%, the CDC said. The CDC also reported a cumulative rate of 24.4 laboratory-confirmed influenza-associated hospitalizations per 100,000 population for the 2015-16 flu season.
There were seven flu-related pediatric deaths reported – all of them occurring during earlier weeks. So far, 40 flu-related pediatric deaths have been reported during the 2015-2016 season, with California having the highest number (9). The CDC said 7.4% of all deaths reported through the 122 Cities Mortality Reporting System were due to pneumonia and influenza. This percentage was above the epidemic threshold of 7.1% for week 13 of the flu season.
A third straight week of reduced influenza-like illness (ILI) left the U.S. with no states at the highest level of ILI activity for the first time since early February, according to the Centers for Disease Control and Prevention.
The states with the highest activity for the week ending April 2, 2016, were New Jersey and New Mexico, and both were at level 8 on the CDC’s 1-10 scale, putting them on the low end of the “high” range. States in the “moderate” range were Georgia and North Carolina at level 7 and Alabama, Alaska, Arkansas, Missouri, and Virginia at level 6, according to a report from the CDC’s Influenza-like Illness Surveillance Network (ILINet).
The proportion of outpatient visits for ILI was 2.4% for the week, down from 2.9% the week before but still above the national baseline of 2.1%, the CDC said. The CDC also reported a cumulative rate of 24.4 laboratory-confirmed influenza-associated hospitalizations per 100,000 population for the 2015-16 flu season.
There were seven flu-related pediatric deaths reported – all of them occurring during earlier weeks. So far, 40 flu-related pediatric deaths have been reported during the 2015-2016 season, with California having the highest number (9). The CDC said 7.4% of all deaths reported through the 122 Cities Mortality Reporting System were due to pneumonia and influenza. This percentage was above the epidemic threshold of 7.1% for week 13 of the flu season.
A third straight week of reduced influenza-like illness (ILI) left the U.S. with no states at the highest level of ILI activity for the first time since early February, according to the Centers for Disease Control and Prevention.
The states with the highest activity for the week ending April 2, 2016, were New Jersey and New Mexico, and both were at level 8 on the CDC’s 1-10 scale, putting them on the low end of the “high” range. States in the “moderate” range were Georgia and North Carolina at level 7 and Alabama, Alaska, Arkansas, Missouri, and Virginia at level 6, according to a report from the CDC’s Influenza-like Illness Surveillance Network (ILINet).
The proportion of outpatient visits for ILI was 2.4% for the week, down from 2.9% the week before but still above the national baseline of 2.1%, the CDC said. The CDC also reported a cumulative rate of 24.4 laboratory-confirmed influenza-associated hospitalizations per 100,000 population for the 2015-16 flu season.
There were seven flu-related pediatric deaths reported – all of them occurring during earlier weeks. So far, 40 flu-related pediatric deaths have been reported during the 2015-2016 season, with California having the highest number (9). The CDC said 7.4% of all deaths reported through the 122 Cities Mortality Reporting System were due to pneumonia and influenza. This percentage was above the epidemic threshold of 7.1% for week 13 of the flu season.
U.S. flu activity continues downward trend
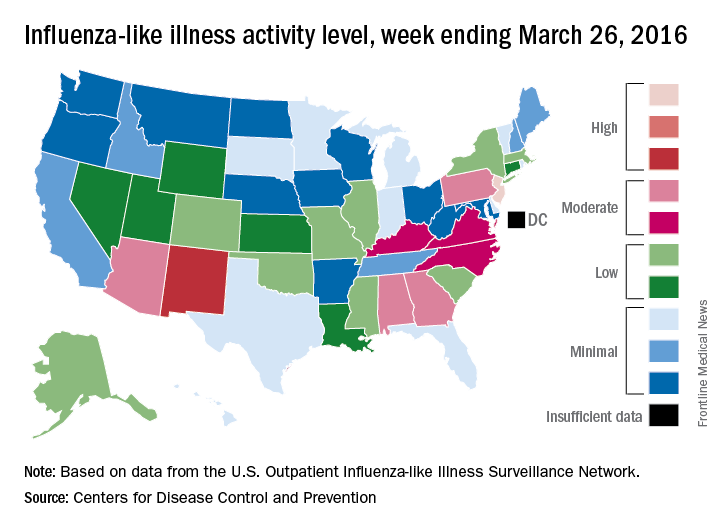
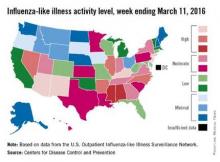
Influenza-like illness (ILI) activity in the United States continued to drop during the week ending March 26, 2016, with only one state still at the highest level, according to the Centers for Disease Control and Prevention.
That one state was New Jersey, which was at level 10 on the CDC’s 1-10 scale of ILI activity. One state at level 10 was down from three states the week before and seven states 2 weeks earlier. Also down for a second consecutive week was the proportion of outpatient visits for ILI, which was 2.9% for the most recent week, compared with 3.2% the previous week and a season high of 3.7% for the week ending March 12, the CDC’s Influenza-like Illness Surveillance Network (ILINet) reported.
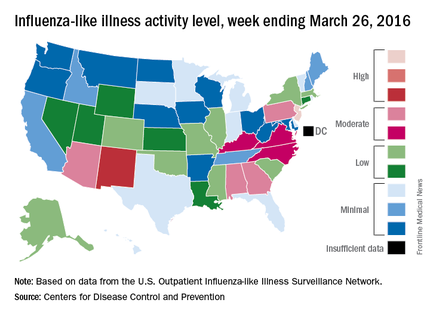
The only other state in the “high” range for the week ending March 26 was New Mexico at level 8. States in the “moderate” range were Alabama, Arizona, Georgia, and Pennsylvania at level 7 and Kentucky, North Carolina, and Virginia at level 6, according to data from ILINet.
Three flu-related pediatric deaths were reported to CDC during the week, but two occurred the previous week and one occurred in February. That brings the total of flu-related pediatric deaths to 33 for the 2015-2016 influenza season, the CDC said. However, 7.7% of all deaths reported through the 122 Cities Mortality Reporting System were due to pneumonia and influenza. This percentage was above the epidemic threshold of 7.2%.
The CDC also reported a cumulative rate for the season of 21.4 laboratory-confirmed influenza-associated hospitalizations per 100,000 population. The highest rate of hospitalization was among adults aged 65 years or older (54.5 per 100,000 population), followed by adults aged 50-64 (31.4 per 100,000 population) and children aged 0-4 years (29.3 per 100,000 population).
Influenza-like illness (ILI) activity in the United States continued to drop during the week ending March 26, 2016, with only one state still at the highest level, according to the Centers for Disease Control and Prevention.
That one state was New Jersey, which was at level 10 on the CDC’s 1-10 scale of ILI activity. One state at level 10 was down from three states the week before and seven states 2 weeks earlier. Also down for a second consecutive week was the proportion of outpatient visits for ILI, which was 2.9% for the most recent week, compared with 3.2% the previous week and a season high of 3.7% for the week ending March 12, the CDC’s Influenza-like Illness Surveillance Network (ILINet) reported.

The only other state in the “high” range for the week ending March 26 was New Mexico at level 8. States in the “moderate” range were Alabama, Arizona, Georgia, and Pennsylvania at level 7 and Kentucky, North Carolina, and Virginia at level 6, according to data from ILINet.
Three flu-related pediatric deaths were reported to CDC during the week, but two occurred the previous week and one occurred in February. That brings the total of flu-related pediatric deaths to 33 for the 2015-2016 influenza season, the CDC said. However, 7.7% of all deaths reported through the 122 Cities Mortality Reporting System were due to pneumonia and influenza. This percentage was above the epidemic threshold of 7.2%.
The CDC also reported a cumulative rate for the season of 21.4 laboratory-confirmed influenza-associated hospitalizations per 100,000 population. The highest rate of hospitalization was among adults aged 65 years or older (54.5 per 100,000 population), followed by adults aged 50-64 (31.4 per 100,000 population) and children aged 0-4 years (29.3 per 100,000 population).
Influenza-like illness (ILI) activity in the United States continued to drop during the week ending March 26, 2016, with only one state still at the highest level, according to the Centers for Disease Control and Prevention.
That one state was New Jersey, which was at level 10 on the CDC’s 1-10 scale of ILI activity. One state at level 10 was down from three states the week before and seven states 2 weeks earlier. Also down for a second consecutive week was the proportion of outpatient visits for ILI, which was 2.9% for the most recent week, compared with 3.2% the previous week and a season high of 3.7% for the week ending March 12, the CDC’s Influenza-like Illness Surveillance Network (ILINet) reported.

The only other state in the “high” range for the week ending March 26 was New Mexico at level 8. States in the “moderate” range were Alabama, Arizona, Georgia, and Pennsylvania at level 7 and Kentucky, North Carolina, and Virginia at level 6, according to data from ILINet.
Three flu-related pediatric deaths were reported to CDC during the week, but two occurred the previous week and one occurred in February. That brings the total of flu-related pediatric deaths to 33 for the 2015-2016 influenza season, the CDC said. However, 7.7% of all deaths reported through the 122 Cities Mortality Reporting System were due to pneumonia and influenza. This percentage was above the epidemic threshold of 7.2%.
The CDC also reported a cumulative rate for the season of 21.4 laboratory-confirmed influenza-associated hospitalizations per 100,000 population. The highest rate of hospitalization was among adults aged 65 years or older (54.5 per 100,000 population), followed by adults aged 50-64 (31.4 per 100,000 population) and children aged 0-4 years (29.3 per 100,000 population).
U.S. flu activity may be waning
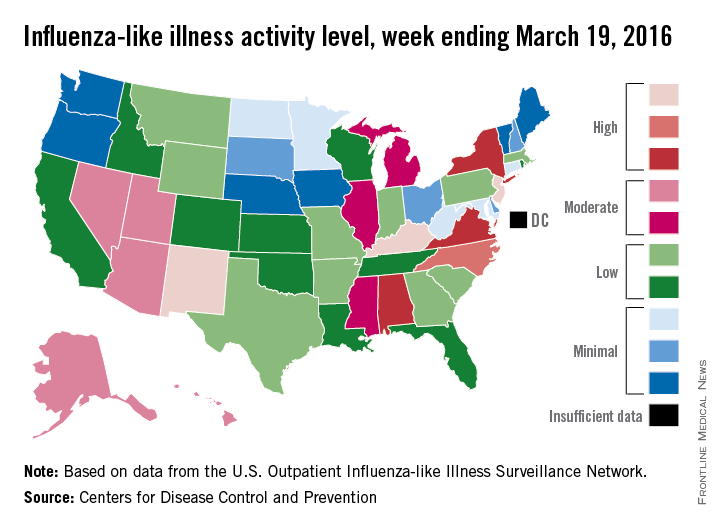
The 2015-2016 U.S. flu season may have reached its peak. The proportion of outpatient visits for influenza-like illness (ILI) dropped to 3.2% for the week ending March 19, according to the Centers for Disease Control and Prevention.
The drop came after 9 consecutive weeks without a decrease, as the proportion of outpatient visits for ILI topped out at 3.7%, the CDC reported. The national baseline is 2.1%.
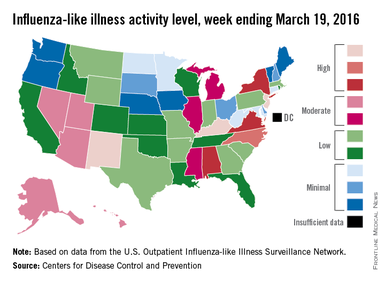
For the week ending March 19, three states – Kentucky, New Jersey, and New Mexico – were at level 10 on the CDC’s 1-10 scale of ILI activity, compared with seven the week before. Other states in the “high” range for the week were North Carolina at level 9 and Alabama, New York, and Virginia at level 8, according to data from the CDC’s Influenza-like Illness Surveillance Network (ILINet).
The CDC also reported a cumulative rate of 18.2 laboratory-confirmed influenza-associated hospitalizations per 100,000 population for the 2015-2016 flu season.
Two flu-related pediatric deaths were reported during the most recent week, one of which occurred during the week ending March 5. That brings the total to 30 reported for the 2015-2016 season. For the three previous flu seasons, the pediatric death totals were 148 (2014-2015), 111 (2013-2014), and 171 (2012-2013), according to the CDC report.
The 2015-2016 U.S. flu season may have reached its peak. The proportion of outpatient visits for influenza-like illness (ILI) dropped to 3.2% for the week ending March 19, according to the Centers for Disease Control and Prevention.
The drop came after 9 consecutive weeks without a decrease, as the proportion of outpatient visits for ILI topped out at 3.7%, the CDC reported. The national baseline is 2.1%.

For the week ending March 19, three states – Kentucky, New Jersey, and New Mexico – were at level 10 on the CDC’s 1-10 scale of ILI activity, compared with seven the week before. Other states in the “high” range for the week were North Carolina at level 9 and Alabama, New York, and Virginia at level 8, according to data from the CDC’s Influenza-like Illness Surveillance Network (ILINet).
The CDC also reported a cumulative rate of 18.2 laboratory-confirmed influenza-associated hospitalizations per 100,000 population for the 2015-2016 flu season.
Two flu-related pediatric deaths were reported during the most recent week, one of which occurred during the week ending March 5. That brings the total to 30 reported for the 2015-2016 season. For the three previous flu seasons, the pediatric death totals were 148 (2014-2015), 111 (2013-2014), and 171 (2012-2013), according to the CDC report.
The 2015-2016 U.S. flu season may have reached its peak. The proportion of outpatient visits for influenza-like illness (ILI) dropped to 3.2% for the week ending March 19, according to the Centers for Disease Control and Prevention.
The drop came after 9 consecutive weeks without a decrease, as the proportion of outpatient visits for ILI topped out at 3.7%, the CDC reported. The national baseline is 2.1%.

For the week ending March 19, three states – Kentucky, New Jersey, and New Mexico – were at level 10 on the CDC’s 1-10 scale of ILI activity, compared with seven the week before. Other states in the “high” range for the week were North Carolina at level 9 and Alabama, New York, and Virginia at level 8, according to data from the CDC’s Influenza-like Illness Surveillance Network (ILINet).
The CDC also reported a cumulative rate of 18.2 laboratory-confirmed influenza-associated hospitalizations per 100,000 population for the 2015-2016 flu season.
Two flu-related pediatric deaths were reported during the most recent week, one of which occurred during the week ending March 5. That brings the total to 30 reported for the 2015-2016 season. For the three previous flu seasons, the pediatric death totals were 148 (2014-2015), 111 (2013-2014), and 171 (2012-2013), according to the CDC report.
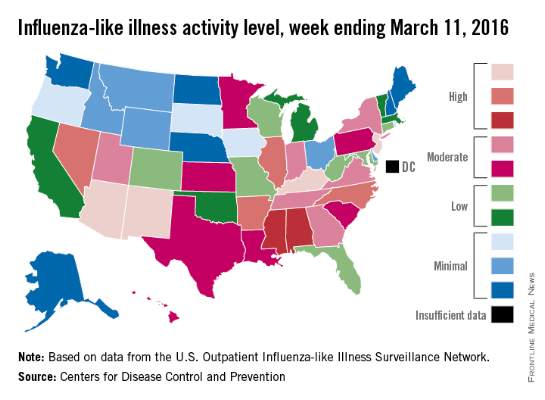
U.S. flu activity: Another week, another increase
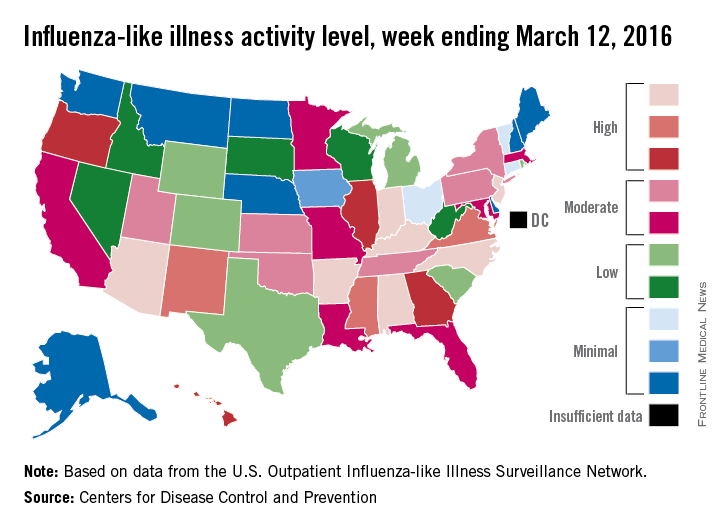
Influenza-like illness (ILI) activity in the U.S. showed no signs of slowing down during the week ending March 12, 2016, as the number of states at the highest level increased to seven, compared with four the previous week, according to the Centers for Disease Control and Prevention.
The seven states at level 10 on the CDC’s 1-10 scale of ILI activity were Alabama, Arizona, Arkansas, Indiana, Kentucky, New Jersey, and North Carolina. Other states in the “high” range for the week were Mississippi, New Mexico, and Virginia at level 9 and Georgia, Hawaii, Illinois, and Oregon at level 8, the CDC’s Influenza-like Illness Surveillance Network (ILINet) reported.
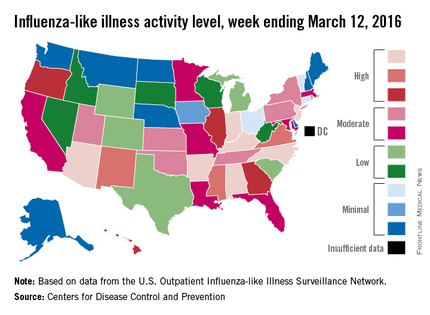
The proportion of outpatient visits for ILI was 3.7% for the week, up from 3.5% the previous week and another new high for the season. The national baseline is 2.1%. The geographic spread of influenza in 40 states and Puerto Rico was reported as widespread, the CDC said.
There were eight flu-related pediatric deaths reported to the CDC, of which only one occurred during the week ending March 12. For the season so far, a total of 28 flu-related pediatric deaths have been reported in 14 states and Puerto Rico.
Since Oct. 1, 2015, 4,006 laboratory-confirmed flu-associated hospitalizations have been reported to the CDC’s Influenza Hospitalization Surveillance Network, which covers more than 70 counties in a group of 10 Emerging Infections Program states plus four additional states. The overall hospitalization rate for the season is 14.5 per 100,000 population, with the highest rate occurring in adults aged 65 years and older (37.2 per 100,000), followed by adults aged 50-64 (21.3) and children aged 0-4 years (20.9), the CDC report noted.
Influenza-like illness (ILI) activity in the U.S. showed no signs of slowing down during the week ending March 12, 2016, as the number of states at the highest level increased to seven, compared with four the previous week, according to the Centers for Disease Control and Prevention.
The seven states at level 10 on the CDC’s 1-10 scale of ILI activity were Alabama, Arizona, Arkansas, Indiana, Kentucky, New Jersey, and North Carolina. Other states in the “high” range for the week were Mississippi, New Mexico, and Virginia at level 9 and Georgia, Hawaii, Illinois, and Oregon at level 8, the CDC’s Influenza-like Illness Surveillance Network (ILINet) reported.

The proportion of outpatient visits for ILI was 3.7% for the week, up from 3.5% the previous week and another new high for the season. The national baseline is 2.1%. The geographic spread of influenza in 40 states and Puerto Rico was reported as widespread, the CDC said.
There were eight flu-related pediatric deaths reported to the CDC, of which only one occurred during the week ending March 12. For the season so far, a total of 28 flu-related pediatric deaths have been reported in 14 states and Puerto Rico.
Since Oct. 1, 2015, 4,006 laboratory-confirmed flu-associated hospitalizations have been reported to the CDC’s Influenza Hospitalization Surveillance Network, which covers more than 70 counties in a group of 10 Emerging Infections Program states plus four additional states. The overall hospitalization rate for the season is 14.5 per 100,000 population, with the highest rate occurring in adults aged 65 years and older (37.2 per 100,000), followed by adults aged 50-64 (21.3) and children aged 0-4 years (20.9), the CDC report noted.
Influenza-like illness (ILI) activity in the U.S. showed no signs of slowing down during the week ending March 12, 2016, as the number of states at the highest level increased to seven, compared with four the previous week, according to the Centers for Disease Control and Prevention.
The seven states at level 10 on the CDC’s 1-10 scale of ILI activity were Alabama, Arizona, Arkansas, Indiana, Kentucky, New Jersey, and North Carolina. Other states in the “high” range for the week were Mississippi, New Mexico, and Virginia at level 9 and Georgia, Hawaii, Illinois, and Oregon at level 8, the CDC’s Influenza-like Illness Surveillance Network (ILINet) reported.

The proportion of outpatient visits for ILI was 3.7% for the week, up from 3.5% the previous week and another new high for the season. The national baseline is 2.1%. The geographic spread of influenza in 40 states and Puerto Rico was reported as widespread, the CDC said.
There were eight flu-related pediatric deaths reported to the CDC, of which only one occurred during the week ending March 12. For the season so far, a total of 28 flu-related pediatric deaths have been reported in 14 states and Puerto Rico.
Since Oct. 1, 2015, 4,006 laboratory-confirmed flu-associated hospitalizations have been reported to the CDC’s Influenza Hospitalization Surveillance Network, which covers more than 70 counties in a group of 10 Emerging Infections Program states plus four additional states. The overall hospitalization rate for the season is 14.5 per 100,000 population, with the highest rate occurring in adults aged 65 years and older (37.2 per 100,000), followed by adults aged 50-64 (21.3) and children aged 0-4 years (20.9), the CDC report noted.
Flu vaccination found safe in surgical patients
Immunizing surgical patients against seasonal influenza before they are discharged from the hospital appears safe and is a sound strategy for expanding vaccine coverage, especially among people at high risk, according to a report published online March 14 in Annals of Internal Medicine.
All health care contacts, including hospitalizations, are considered excellent opportunities for influenza vaccination, and current recommendations advise that eligible inpatients receive the immunization before discharge. However, surgical patients don’t often get the flu vaccine before they leave the hospital, likely because of concerns that potential adverse effects like fever and myalgia could be falsely attributed to surgical complications. This would lead to unnecessary patient evaluations and could interfere with postsurgical care, said Sara Y. Tartof, Ph.D., and her associates in the department of research and evaluation, Kaiser Permanente Southern California, Pasadena.
“Although this concern is understandable, few clinical data support it,” they noted.
“To provide clinical evidence that would either substantiate or refute” these concerns about perioperative flu vaccination, the investigators analyzed data in the electronic health records for 81,647 surgeries. All the study participants were deemed eligible for flu vaccination. They were socioeconomically and ethnically diverse, ranged in age from 6 months to 106 years, and underwent surgery at 14 hospitals during three consecutive flu seasons. Operations included general, cardiac, eye, dermatologic, ENT, neurologic, ob.gyn., oral/maxillofacial, orthopedic, plastic, podiatric, urologic, and vascular procedures.
Patients received a flu vaccine in 6,420 hospital stays for surgery – only 15% of 42,777 eligible hospitalizations – usually on the day of discharge. (The remaining 38,870 patients either had been vaccinated before hospital admission or were vaccinated more than a week after discharge and were not included in further analyses.)
Compared with eligible patients who didn’t receive a flu vaccine during hospitalization for surgery, those who did showed no increased risk for subsequent inpatient visits, ED visits, or clinical work-ups for infection. Patients who received the flu vaccine before discharge showed a minimally increased risk for outpatient visits during the week following hospitalization, but this was considered unlikely “to translate into substantial clinical impact,” especially when balanced against the benefit of immunization, Dr. Tartof and her associates said (Ann Intern Med. 2016 Mar 14. doi: 10.7326/M15-1667).
Giving the flu vaccine during a surgical hospitalization “is an opportunity to protect a high-risk population,” because surgery patients tend to be of an age, and to have comorbid conditions, that raise their risk for flu complications. In addition, previous research has reported that 39%-46% of adults hospitalized for influenza-related disease in a given year had been hospitalized during the preceding autumn, indicating that recent hospitalization also raises the risk for flu complications, the investigators said.
“Our data support the rationale for increasing vaccination rates among surgical inpatients,” they said.
This study was funded by the U.S. Centers for Disease Control and Prevention through the Vaccine Safety Datalink program. Dr. Tartof reported receiving grants from Merck outside of this work; two of her associates reported receiving grants from Novartis and GlaxoSmithKline outside of this work.
Immunizing surgical patients against seasonal influenza before they are discharged from the hospital appears safe and is a sound strategy for expanding vaccine coverage, especially among people at high risk, according to a report published online March 14 in Annals of Internal Medicine.
All health care contacts, including hospitalizations, are considered excellent opportunities for influenza vaccination, and current recommendations advise that eligible inpatients receive the immunization before discharge. However, surgical patients don’t often get the flu vaccine before they leave the hospital, likely because of concerns that potential adverse effects like fever and myalgia could be falsely attributed to surgical complications. This would lead to unnecessary patient evaluations and could interfere with postsurgical care, said Sara Y. Tartof, Ph.D., and her associates in the department of research and evaluation, Kaiser Permanente Southern California, Pasadena.
“Although this concern is understandable, few clinical data support it,” they noted.
“To provide clinical evidence that would either substantiate or refute” these concerns about perioperative flu vaccination, the investigators analyzed data in the electronic health records for 81,647 surgeries. All the study participants were deemed eligible for flu vaccination. They were socioeconomically and ethnically diverse, ranged in age from 6 months to 106 years, and underwent surgery at 14 hospitals during three consecutive flu seasons. Operations included general, cardiac, eye, dermatologic, ENT, neurologic, ob.gyn., oral/maxillofacial, orthopedic, plastic, podiatric, urologic, and vascular procedures.
Patients received a flu vaccine in 6,420 hospital stays for surgery – only 15% of 42,777 eligible hospitalizations – usually on the day of discharge. (The remaining 38,870 patients either had been vaccinated before hospital admission or were vaccinated more than a week after discharge and were not included in further analyses.)
Compared with eligible patients who didn’t receive a flu vaccine during hospitalization for surgery, those who did showed no increased risk for subsequent inpatient visits, ED visits, or clinical work-ups for infection. Patients who received the flu vaccine before discharge showed a minimally increased risk for outpatient visits during the week following hospitalization, but this was considered unlikely “to translate into substantial clinical impact,” especially when balanced against the benefit of immunization, Dr. Tartof and her associates said (Ann Intern Med. 2016 Mar 14. doi: 10.7326/M15-1667).
Giving the flu vaccine during a surgical hospitalization “is an opportunity to protect a high-risk population,” because surgery patients tend to be of an age, and to have comorbid conditions, that raise their risk for flu complications. In addition, previous research has reported that 39%-46% of adults hospitalized for influenza-related disease in a given year had been hospitalized during the preceding autumn, indicating that recent hospitalization also raises the risk for flu complications, the investigators said.
“Our data support the rationale for increasing vaccination rates among surgical inpatients,” they said.
This study was funded by the U.S. Centers for Disease Control and Prevention through the Vaccine Safety Datalink program. Dr. Tartof reported receiving grants from Merck outside of this work; two of her associates reported receiving grants from Novartis and GlaxoSmithKline outside of this work.
Immunizing surgical patients against seasonal influenza before they are discharged from the hospital appears safe and is a sound strategy for expanding vaccine coverage, especially among people at high risk, according to a report published online March 14 in Annals of Internal Medicine.
All health care contacts, including hospitalizations, are considered excellent opportunities for influenza vaccination, and current recommendations advise that eligible inpatients receive the immunization before discharge. However, surgical patients don’t often get the flu vaccine before they leave the hospital, likely because of concerns that potential adverse effects like fever and myalgia could be falsely attributed to surgical complications. This would lead to unnecessary patient evaluations and could interfere with postsurgical care, said Sara Y. Tartof, Ph.D., and her associates in the department of research and evaluation, Kaiser Permanente Southern California, Pasadena.
“Although this concern is understandable, few clinical data support it,” they noted.
“To provide clinical evidence that would either substantiate or refute” these concerns about perioperative flu vaccination, the investigators analyzed data in the electronic health records for 81,647 surgeries. All the study participants were deemed eligible for flu vaccination. They were socioeconomically and ethnically diverse, ranged in age from 6 months to 106 years, and underwent surgery at 14 hospitals during three consecutive flu seasons. Operations included general, cardiac, eye, dermatologic, ENT, neurologic, ob.gyn., oral/maxillofacial, orthopedic, plastic, podiatric, urologic, and vascular procedures.
Patients received a flu vaccine in 6,420 hospital stays for surgery – only 15% of 42,777 eligible hospitalizations – usually on the day of discharge. (The remaining 38,870 patients either had been vaccinated before hospital admission or were vaccinated more than a week after discharge and were not included in further analyses.)
Compared with eligible patients who didn’t receive a flu vaccine during hospitalization for surgery, those who did showed no increased risk for subsequent inpatient visits, ED visits, or clinical work-ups for infection. Patients who received the flu vaccine before discharge showed a minimally increased risk for outpatient visits during the week following hospitalization, but this was considered unlikely “to translate into substantial clinical impact,” especially when balanced against the benefit of immunization, Dr. Tartof and her associates said (Ann Intern Med. 2016 Mar 14. doi: 10.7326/M15-1667).
Giving the flu vaccine during a surgical hospitalization “is an opportunity to protect a high-risk population,” because surgery patients tend to be of an age, and to have comorbid conditions, that raise their risk for flu complications. In addition, previous research has reported that 39%-46% of adults hospitalized for influenza-related disease in a given year had been hospitalized during the preceding autumn, indicating that recent hospitalization also raises the risk for flu complications, the investigators said.
“Our data support the rationale for increasing vaccination rates among surgical inpatients,” they said.
This study was funded by the U.S. Centers for Disease Control and Prevention through the Vaccine Safety Datalink program. Dr. Tartof reported receiving grants from Merck outside of this work; two of her associates reported receiving grants from Novartis and GlaxoSmithKline outside of this work.
FROM ANNALS OF INTERNAL MEDICINE
Key clinical point: Immunizing surgical patients against seasonal influenza before they leave the hospital appears safe.
Major finding: Patients received a flu vaccine in only 6,420 hospital stays for surgery, comprising only 15% of the patient hospitalizations that were eligible.
Data source: A retrospective cohort study involving 81,647 surgeries at 14 California hospitals during three consecutive flu seasons.
Disclosures: This study was funded by the U.S. Centers for Disease Control and Prevention through the Vaccine Safety Datalink program. Dr. Tartof reported receiving grants from Merck outside of this work; two of her associates reported receiving grants from Novartis and GlaxoSmithKline outside of this work.
Flu activity reaches another new season high
There were four states at the highest level of influenza-like illness (ILI) activity for the week, more than any other week of the 2015-2016 flu season, according to the Centers for Disease Control and Prevention.
Arizona, Kentucky, New Jersey, and New Mexico, along with Puerto Rico, were all at level 10 on the CDC’s 1-10 scale of ILI activity for the week ending March 5, 2016. Other states in the “high” range were Arkansas, Illinois, Nevada, and North Carolina at level 9 and Alabama and Mississippi at level 8, the CDC reported.
The proportion of outpatient visits for ILI was 3.5% for the week, the highest of the season so far and well above the national baseline of 2.1%. In addition to the 10 states and Puerto Rico with ILI levels in the high range, there were 13 states in the “moderate” range (6 or 7 on the 1-10 scale) and 12 states in the “low” range (4 or 5), with a total of 44 states at level 2 or higher, data from the CDC’s Outpatient Influenza-like Illness Surveillance Network (ILINet) show.
Two flu-related pediatric deaths were reported during week 21 of the flu season, although both occurred earlier: one during the week ending Feb. 13 and one during the week ending Feb. 27. There have been a total of 20 pediatric deaths reported for the 2015-2016 season, with Florida (four deaths), California, (three), Arizona (two), and Nevada (two) the only states reporting more than one, the CDC report noted.
The continued rise in ILI activity is somewhat unusual. The only season out of the previous 10 with a peak later than the current one was 2011-2012, which peaked at only 2.4% on the week ending March 17, 2012. The earliest of the bunch came during the 2009-2010 season, which peaked at 7.7% during the week ending Oct. 24, 2009, according to ILINet data.
There were four states at the highest level of influenza-like illness (ILI) activity for the week, more than any other week of the 2015-2016 flu season, according to the Centers for Disease Control and Prevention.
Arizona, Kentucky, New Jersey, and New Mexico, along with Puerto Rico, were all at level 10 on the CDC’s 1-10 scale of ILI activity for the week ending March 5, 2016. Other states in the “high” range were Arkansas, Illinois, Nevada, and North Carolina at level 9 and Alabama and Mississippi at level 8, the CDC reported.
The proportion of outpatient visits for ILI was 3.5% for the week, the highest of the season so far and well above the national baseline of 2.1%. In addition to the 10 states and Puerto Rico with ILI levels in the high range, there were 13 states in the “moderate” range (6 or 7 on the 1-10 scale) and 12 states in the “low” range (4 or 5), with a total of 44 states at level 2 or higher, data from the CDC’s Outpatient Influenza-like Illness Surveillance Network (ILINet) show.
Two flu-related pediatric deaths were reported during week 21 of the flu season, although both occurred earlier: one during the week ending Feb. 13 and one during the week ending Feb. 27. There have been a total of 20 pediatric deaths reported for the 2015-2016 season, with Florida (four deaths), California, (three), Arizona (two), and Nevada (two) the only states reporting more than one, the CDC report noted.
The continued rise in ILI activity is somewhat unusual. The only season out of the previous 10 with a peak later than the current one was 2011-2012, which peaked at only 2.4% on the week ending March 17, 2012. The earliest of the bunch came during the 2009-2010 season, which peaked at 7.7% during the week ending Oct. 24, 2009, according to ILINet data.
There were four states at the highest level of influenza-like illness (ILI) activity for the week, more than any other week of the 2015-2016 flu season, according to the Centers for Disease Control and Prevention.
Arizona, Kentucky, New Jersey, and New Mexico, along with Puerto Rico, were all at level 10 on the CDC’s 1-10 scale of ILI activity for the week ending March 5, 2016. Other states in the “high” range were Arkansas, Illinois, Nevada, and North Carolina at level 9 and Alabama and Mississippi at level 8, the CDC reported.
The proportion of outpatient visits for ILI was 3.5% for the week, the highest of the season so far and well above the national baseline of 2.1%. In addition to the 10 states and Puerto Rico with ILI levels in the high range, there were 13 states in the “moderate” range (6 or 7 on the 1-10 scale) and 12 states in the “low” range (4 or 5), with a total of 44 states at level 2 or higher, data from the CDC’s Outpatient Influenza-like Illness Surveillance Network (ILINet) show.
Two flu-related pediatric deaths were reported during week 21 of the flu season, although both occurred earlier: one during the week ending Feb. 13 and one during the week ending Feb. 27. There have been a total of 20 pediatric deaths reported for the 2015-2016 season, with Florida (four deaths), California, (three), Arizona (two), and Nevada (two) the only states reporting more than one, the CDC report noted.
The continued rise in ILI activity is somewhat unusual. The only season out of the previous 10 with a peak later than the current one was 2011-2012, which peaked at only 2.4% on the week ending March 17, 2012. The earliest of the bunch came during the 2009-2010 season, which peaked at 7.7% during the week ending Oct. 24, 2009, according to ILINet data.
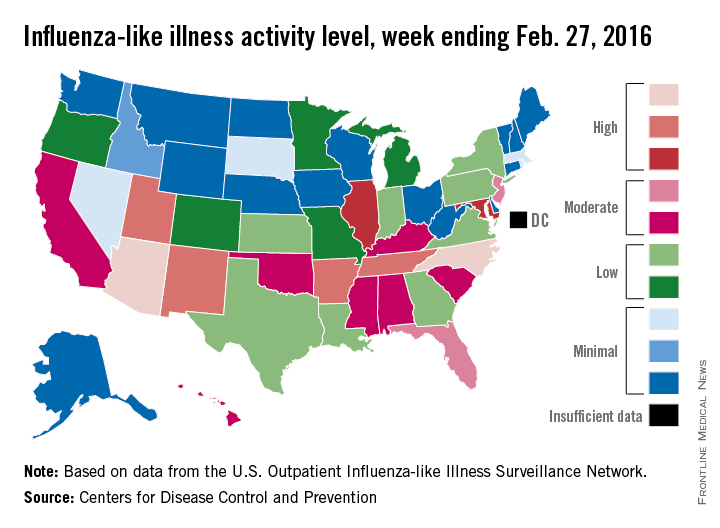
U.S. flu activity falls for first time since early January
Influenza-like illness (ILI) activity in the 2015-2016 U.S. flu season declined for the first time since early January, according to the Centers for Disease Control and Prevention.
The proportion of outpatient visits for ILI was reported at 3.2% for the week ending Feb. 20, but the CDC has adjusted that figure to 3.3%, which makes the 3.2% reported for this most recent week (week 20 of the season, ending Feb. 27, 2016) a decrease from the week before.
Despite that drop, two states were at level 10 on the CDC’s 1-10 scale of ILI activity for the first time this season. Arizona had already reached level 10, and joining it there last week was North Carolina, moving up from level 8 the week before. Other states in the “high” range of activity were Arkansas, New Mexico, Tennessee, and Utah at level 9, and Illinois and Maryland at level 8, the CDC reported March 4. Puerto Rico, which had been at level 10 for several weeks, moved down to level 8.
States in the “moderate” range of activity for the week ending Feb. 27 were Florida and New Jersey at level 7 and Alabama, California, Hawaii, Kentucky, Mississippi, Oklahoma, and South Carolina at level 6. Altogether, there were 35 states at level 2 or higher, according to data from the CDC’s Outpatient Influenza-like Illness Surveillance Network.
Four pediatric ILI-related deaths were reported to the CDC during week 20, but three actually occurred during week 19. There have been 18 ILI-related deaths so far during the 2015-2016 season, the CDC said.
Influenza-like illness (ILI) activity in the 2015-2016 U.S. flu season declined for the first time since early January, according to the Centers for Disease Control and Prevention.
The proportion of outpatient visits for ILI was reported at 3.2% for the week ending Feb. 20, but the CDC has adjusted that figure to 3.3%, which makes the 3.2% reported for this most recent week (week 20 of the season, ending Feb. 27, 2016) a decrease from the week before.
Despite that drop, two states were at level 10 on the CDC’s 1-10 scale of ILI activity for the first time this season. Arizona had already reached level 10, and joining it there last week was North Carolina, moving up from level 8 the week before. Other states in the “high” range of activity were Arkansas, New Mexico, Tennessee, and Utah at level 9, and Illinois and Maryland at level 8, the CDC reported March 4. Puerto Rico, which had been at level 10 for several weeks, moved down to level 8.
States in the “moderate” range of activity for the week ending Feb. 27 were Florida and New Jersey at level 7 and Alabama, California, Hawaii, Kentucky, Mississippi, Oklahoma, and South Carolina at level 6. Altogether, there were 35 states at level 2 or higher, according to data from the CDC’s Outpatient Influenza-like Illness Surveillance Network.
Four pediatric ILI-related deaths were reported to the CDC during week 20, but three actually occurred during week 19. There have been 18 ILI-related deaths so far during the 2015-2016 season, the CDC said.
Influenza-like illness (ILI) activity in the 2015-2016 U.S. flu season declined for the first time since early January, according to the Centers for Disease Control and Prevention.
The proportion of outpatient visits for ILI was reported at 3.2% for the week ending Feb. 20, but the CDC has adjusted that figure to 3.3%, which makes the 3.2% reported for this most recent week (week 20 of the season, ending Feb. 27, 2016) a decrease from the week before.
Despite that drop, two states were at level 10 on the CDC’s 1-10 scale of ILI activity for the first time this season. Arizona had already reached level 10, and joining it there last week was North Carolina, moving up from level 8 the week before. Other states in the “high” range of activity were Arkansas, New Mexico, Tennessee, and Utah at level 9, and Illinois and Maryland at level 8, the CDC reported March 4. Puerto Rico, which had been at level 10 for several weeks, moved down to level 8.
States in the “moderate” range of activity for the week ending Feb. 27 were Florida and New Jersey at level 7 and Alabama, California, Hawaii, Kentucky, Mississippi, Oklahoma, and South Carolina at level 6. Altogether, there were 35 states at level 2 or higher, according to data from the CDC’s Outpatient Influenza-like Illness Surveillance Network.
Four pediatric ILI-related deaths were reported to the CDC during week 20, but three actually occurred during week 19. There have been 18 ILI-related deaths so far during the 2015-2016 season, the CDC said.
Vaccines committee approves recommended influenza strains for 2016-2017 vaccine
The Food and Drug Administration’s Vaccines and Related Biological Products Advisory Committee unanimously approved recommendations regarding the trivalent and quadrivalent influenza vaccines to be distributed during the 2016-2017 flu season.
The 14-member committee voted that the components of the trivalent influenza vaccine for the upcoming flu season should include an A/California/7/2009 (H1N1) pdm09-like virus; an A/Hong Kong/4801/2014 (H3N2)-like virus; and a B/Brisbane/60/2008-like virus of the B/Victoria lineage.
Additionally, the quadrivalent influenza vaccine should include a B/Phuket/3073/2013-like virus of the B/Yamagata lineage as “the second influenza B strain in the vaccine.”
All four components correspond with the recommendations of the World Health Organization, which announced its proposed components for influenza vaccines in the Northern Hemisphere on Feb. 25.
“I’m comfortable trying to follow the footprint of the virus we’ve seen today, quite elegantly put out in front of us,” said committee member Dr. Sarah Long, professor of pediatrics at Drexel University in Philadelphia, adding that she was “very pleased with what happened in the last year” regarding the predictions of dominant virus strains and the effectiveness of the eventual vaccine.
The proposed vaccine for next season differs from the one distributed during the 2015-16 flu season. While both vaccines contain the identical California strain of influenza A and the Phuket strain of influenza B, the 2015-2016 vaccine included an A/Switzerland/9715293/2013 (H3N2)-like virus in its trivalent form, and a B/Brisbane/60/2008-like virus of the B/Victoria lineage in its quadrivalent form.
“Timely vaccine supply requires close collaboration and communication between multiple stakeholders to ensure sufficient provision of [a] well-matched vaccine,” said Matthew Downham, Ph.D., associate director of biopharmaceutical development, AstraZeneca. Timely strain selection will ensure “vaccine availability and usage” for the most widespread and effective coverage.
While the FDA is not obligated to follow the recommendations of the Vaccines and Related Biological Products Advisory Committee, it generally does.
None of the committee members reported any relevant financial disclosures, nor were there any waivers for conflicts of interest.
The Food and Drug Administration’s Vaccines and Related Biological Products Advisory Committee unanimously approved recommendations regarding the trivalent and quadrivalent influenza vaccines to be distributed during the 2016-2017 flu season.
The 14-member committee voted that the components of the trivalent influenza vaccine for the upcoming flu season should include an A/California/7/2009 (H1N1) pdm09-like virus; an A/Hong Kong/4801/2014 (H3N2)-like virus; and a B/Brisbane/60/2008-like virus of the B/Victoria lineage.
Additionally, the quadrivalent influenza vaccine should include a B/Phuket/3073/2013-like virus of the B/Yamagata lineage as “the second influenza B strain in the vaccine.”
All four components correspond with the recommendations of the World Health Organization, which announced its proposed components for influenza vaccines in the Northern Hemisphere on Feb. 25.
“I’m comfortable trying to follow the footprint of the virus we’ve seen today, quite elegantly put out in front of us,” said committee member Dr. Sarah Long, professor of pediatrics at Drexel University in Philadelphia, adding that she was “very pleased with what happened in the last year” regarding the predictions of dominant virus strains and the effectiveness of the eventual vaccine.
The proposed vaccine for next season differs from the one distributed during the 2015-16 flu season. While both vaccines contain the identical California strain of influenza A and the Phuket strain of influenza B, the 2015-2016 vaccine included an A/Switzerland/9715293/2013 (H3N2)-like virus in its trivalent form, and a B/Brisbane/60/2008-like virus of the B/Victoria lineage in its quadrivalent form.
“Timely vaccine supply requires close collaboration and communication between multiple stakeholders to ensure sufficient provision of [a] well-matched vaccine,” said Matthew Downham, Ph.D., associate director of biopharmaceutical development, AstraZeneca. Timely strain selection will ensure “vaccine availability and usage” for the most widespread and effective coverage.
While the FDA is not obligated to follow the recommendations of the Vaccines and Related Biological Products Advisory Committee, it generally does.
None of the committee members reported any relevant financial disclosures, nor were there any waivers for conflicts of interest.
The Food and Drug Administration’s Vaccines and Related Biological Products Advisory Committee unanimously approved recommendations regarding the trivalent and quadrivalent influenza vaccines to be distributed during the 2016-2017 flu season.
The 14-member committee voted that the components of the trivalent influenza vaccine for the upcoming flu season should include an A/California/7/2009 (H1N1) pdm09-like virus; an A/Hong Kong/4801/2014 (H3N2)-like virus; and a B/Brisbane/60/2008-like virus of the B/Victoria lineage.
Additionally, the quadrivalent influenza vaccine should include a B/Phuket/3073/2013-like virus of the B/Yamagata lineage as “the second influenza B strain in the vaccine.”
All four components correspond with the recommendations of the World Health Organization, which announced its proposed components for influenza vaccines in the Northern Hemisphere on Feb. 25.
“I’m comfortable trying to follow the footprint of the virus we’ve seen today, quite elegantly put out in front of us,” said committee member Dr. Sarah Long, professor of pediatrics at Drexel University in Philadelphia, adding that she was “very pleased with what happened in the last year” regarding the predictions of dominant virus strains and the effectiveness of the eventual vaccine.
The proposed vaccine for next season differs from the one distributed during the 2015-16 flu season. While both vaccines contain the identical California strain of influenza A and the Phuket strain of influenza B, the 2015-2016 vaccine included an A/Switzerland/9715293/2013 (H3N2)-like virus in its trivalent form, and a B/Brisbane/60/2008-like virus of the B/Victoria lineage in its quadrivalent form.
“Timely vaccine supply requires close collaboration and communication between multiple stakeholders to ensure sufficient provision of [a] well-matched vaccine,” said Matthew Downham, Ph.D., associate director of biopharmaceutical development, AstraZeneca. Timely strain selection will ensure “vaccine availability and usage” for the most widespread and effective coverage.
While the FDA is not obligated to follow the recommendations of the Vaccines and Related Biological Products Advisory Committee, it generally does.
None of the committee members reported any relevant financial disclosures, nor were there any waivers for conflicts of interest.
FROM AN FDA ADVISORY COMMITTEE MEETING
U.S. flu activity continues steady climb
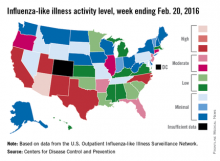
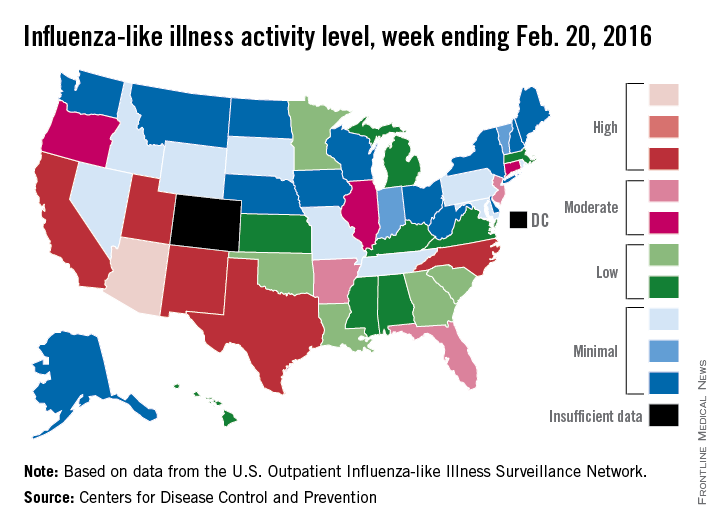
Influenza-like illness (ILI) activity reached a new national high for the 2015-2016 flu season during the week ending Feb. 20, according to the Centers for Disease Control and Prevention.
Nationwide, the proportion of outpatient visits for ILI was 3.2%, up from 3.1% the week before and well over the national baseline of 2.1%. Arizona and Puerto Rico were still at level 10 on the CDC’s 1-10 scale for ILI activity, and they were joined in the “high” range of activity by California, New Mexico, North Carolina, Texas, and Utah, which were all at level 8, the CDC reported Feb. 26.
States in the “moderate” range of activity for week 19 of the 2015-2016 flu season (week 7 of calendar year 2016) were Arkansas, Florida, and New Jersey at level 7 and Connecticut, Illinois, and Oregon at level 6. There were 13 states in the “low” range of activity – five at level 5 and eight states at level 4 – and 24 states in the “minimal” range, of which 13 were at level 1. Colorado and the District of Columbia had insufficient data to determine activity level, according to data from the CDC’s Outpatient Influenza-like Illness Surveillance Network.
For the week, one flu-related pediatric death, associated with an influenza B virus, was reported to the CDC, bringing the total to 14 for the season. The only states reporting more that one death are California (two) and Florida (three), the CDC said. The average number of deaths for the three previous flu seasons is over 143.
During week 19, a total of 18,844 respiratory specimens were tested, 13.8% of which were positive: 76.1% for influenza A and 23.9% for influenza B. Since Oct. 1, 2015, 4.2% of specimens have tested positive for influenza, with a 70% to 30% split between influenza A and B, the CDC report showed.
Influenza-like illness (ILI) activity reached a new national high for the 2015-2016 flu season during the week ending Feb. 20, according to the Centers for Disease Control and Prevention.
Nationwide, the proportion of outpatient visits for ILI was 3.2%, up from 3.1% the week before and well over the national baseline of 2.1%. Arizona and Puerto Rico were still at level 10 on the CDC’s 1-10 scale for ILI activity, and they were joined in the “high” range of activity by California, New Mexico, North Carolina, Texas, and Utah, which were all at level 8, the CDC reported Feb. 26.
States in the “moderate” range of activity for week 19 of the 2015-2016 flu season (week 7 of calendar year 2016) were Arkansas, Florida, and New Jersey at level 7 and Connecticut, Illinois, and Oregon at level 6. There were 13 states in the “low” range of activity – five at level 5 and eight states at level 4 – and 24 states in the “minimal” range, of which 13 were at level 1. Colorado and the District of Columbia had insufficient data to determine activity level, according to data from the CDC’s Outpatient Influenza-like Illness Surveillance Network.
For the week, one flu-related pediatric death, associated with an influenza B virus, was reported to the CDC, bringing the total to 14 for the season. The only states reporting more that one death are California (two) and Florida (three), the CDC said. The average number of deaths for the three previous flu seasons is over 143.
During week 19, a total of 18,844 respiratory specimens were tested, 13.8% of which were positive: 76.1% for influenza A and 23.9% for influenza B. Since Oct. 1, 2015, 4.2% of specimens have tested positive for influenza, with a 70% to 30% split between influenza A and B, the CDC report showed.
Influenza-like illness (ILI) activity reached a new national high for the 2015-2016 flu season during the week ending Feb. 20, according to the Centers for Disease Control and Prevention.
Nationwide, the proportion of outpatient visits for ILI was 3.2%, up from 3.1% the week before and well over the national baseline of 2.1%. Arizona and Puerto Rico were still at level 10 on the CDC’s 1-10 scale for ILI activity, and they were joined in the “high” range of activity by California, New Mexico, North Carolina, Texas, and Utah, which were all at level 8, the CDC reported Feb. 26.
States in the “moderate” range of activity for week 19 of the 2015-2016 flu season (week 7 of calendar year 2016) were Arkansas, Florida, and New Jersey at level 7 and Connecticut, Illinois, and Oregon at level 6. There were 13 states in the “low” range of activity – five at level 5 and eight states at level 4 – and 24 states in the “minimal” range, of which 13 were at level 1. Colorado and the District of Columbia had insufficient data to determine activity level, according to data from the CDC’s Outpatient Influenza-like Illness Surveillance Network.
For the week, one flu-related pediatric death, associated with an influenza B virus, was reported to the CDC, bringing the total to 14 for the season. The only states reporting more that one death are California (two) and Florida (three), the CDC said. The average number of deaths for the three previous flu seasons is over 143.
During week 19, a total of 18,844 respiratory specimens were tested, 13.8% of which were positive: 76.1% for influenza A and 23.9% for influenza B. Since Oct. 1, 2015, 4.2% of specimens have tested positive for influenza, with a 70% to 30% split between influenza A and B, the CDC report showed.
2015-2016 flu vaccine 59% effective, CDC says
The overall rate of effectiveness for the 2015-2016 season’s influenza vaccine is 59%, according to preliminary data shared at a Centers for Disease Control and Prevention’s Advisory Committee on Immunization Practices meeting.
“This means that getting a flu vaccine this season reduced the risk of having to go to the doctor because of flu by nearly 60%,” Dr. Joseph Bresee – who heads the CDC’s Epidemiology and Prevention Branch – stated in a press release. “It’s good news and underscores the importance and the benefit of both annual and ongoing vaccination efforts this season.”
These findings are based on data collected by the U.S. Flu Vaccine Effectiveness Network between November 2, 2015, and February 12, 2016. A 59% effectiveness rate would be roughly in line with what previous years’ vaccines have rated when similar strains of influenza have been prevalent, according to the CDC.
Against the H1N1 strain, which was “responsible for most flu illness this season,” the influenza vaccine was 51% effective. Dr. Bresee noted that the CDC has received reports of unvaccinated individuals this season dying or falling seriously ill because of infection from the H1N1 strain.
The vaccine was 76% effective against all influenza B virus strains, and 79% effective against the B/Yamagata virus strains. There are not enough data at this time, however, to determine the vaccine’s effectiveness against B/Victoria or H3N2 strains.
The CDC cautions that, because the current influenza season is still weeks away from being over, the final rate of vaccine effectiveness for 2015-2016 may change once all the data are analyzed. On average, influenza seasons last 13 weeks.
“Flu activity this season started a bit later and has been lower so far than we’ve seen during the previous three seasons, but activity is still on the upswing and expected to continue for several weeks,” Dr. Bresee stated.
The overall rate of effectiveness for the 2015-2016 season’s influenza vaccine is 59%, according to preliminary data shared at a Centers for Disease Control and Prevention’s Advisory Committee on Immunization Practices meeting.
“This means that getting a flu vaccine this season reduced the risk of having to go to the doctor because of flu by nearly 60%,” Dr. Joseph Bresee – who heads the CDC’s Epidemiology and Prevention Branch – stated in a press release. “It’s good news and underscores the importance and the benefit of both annual and ongoing vaccination efforts this season.”
These findings are based on data collected by the U.S. Flu Vaccine Effectiveness Network between November 2, 2015, and February 12, 2016. A 59% effectiveness rate would be roughly in line with what previous years’ vaccines have rated when similar strains of influenza have been prevalent, according to the CDC.
Against the H1N1 strain, which was “responsible for most flu illness this season,” the influenza vaccine was 51% effective. Dr. Bresee noted that the CDC has received reports of unvaccinated individuals this season dying or falling seriously ill because of infection from the H1N1 strain.
The vaccine was 76% effective against all influenza B virus strains, and 79% effective against the B/Yamagata virus strains. There are not enough data at this time, however, to determine the vaccine’s effectiveness against B/Victoria or H3N2 strains.
The CDC cautions that, because the current influenza season is still weeks away from being over, the final rate of vaccine effectiveness for 2015-2016 may change once all the data are analyzed. On average, influenza seasons last 13 weeks.
“Flu activity this season started a bit later and has been lower so far than we’ve seen during the previous three seasons, but activity is still on the upswing and expected to continue for several weeks,” Dr. Bresee stated.
The overall rate of effectiveness for the 2015-2016 season’s influenza vaccine is 59%, according to preliminary data shared at a Centers for Disease Control and Prevention’s Advisory Committee on Immunization Practices meeting.
“This means that getting a flu vaccine this season reduced the risk of having to go to the doctor because of flu by nearly 60%,” Dr. Joseph Bresee – who heads the CDC’s Epidemiology and Prevention Branch – stated in a press release. “It’s good news and underscores the importance and the benefit of both annual and ongoing vaccination efforts this season.”
These findings are based on data collected by the U.S. Flu Vaccine Effectiveness Network between November 2, 2015, and February 12, 2016. A 59% effectiveness rate would be roughly in line with what previous years’ vaccines have rated when similar strains of influenza have been prevalent, according to the CDC.
Against the H1N1 strain, which was “responsible for most flu illness this season,” the influenza vaccine was 51% effective. Dr. Bresee noted that the CDC has received reports of unvaccinated individuals this season dying or falling seriously ill because of infection from the H1N1 strain.
The vaccine was 76% effective against all influenza B virus strains, and 79% effective against the B/Yamagata virus strains. There are not enough data at this time, however, to determine the vaccine’s effectiveness against B/Victoria or H3N2 strains.
The CDC cautions that, because the current influenza season is still weeks away from being over, the final rate of vaccine effectiveness for 2015-2016 may change once all the data are analyzed. On average, influenza seasons last 13 weeks.
“Flu activity this season started a bit later and has been lower so far than we’ve seen during the previous three seasons, but activity is still on the upswing and expected to continue for several weeks,” Dr. Bresee stated.
FROM AN ACIP MEETING