User login
First protocol on how to use lung ultrasound to triage COVID-19
The first protocol for the use of lung ultrasound to quantitatively and reproducibly assess the degree of lung involvement in patients suspected of having COVID-19 infection has been published by a team of Italian experts with experience using the technology on the front line.
Particularly in Spain and Italy — where the pandemic has struck hardest in Europe — hard-pressed clinicians seeking to quickly understand whether patients with seemingly mild disease could be harboring more serious lung involvement have increasingly relied upon lung ultrasound in the emergency room.
Now Libertario Demi, PhD, head of the ultrasound laboratory, University of Trento, Italy, and colleagues have developed a protocol, published online March 30 in the Journal of Ultrasound Medicine, to standardize practice.
Their research, which builds on previous work by the team, offers broad agreement with industry-led algorithms and emphasizes the use of wireless, handheld ultrasound devices, ideally consisting of a separate probe and tablet, to make sterilization easy.
Firms such as the Butterfly Network, Phillips, Clarius, GE Healthcare, and Siemens are among numerous companies that produce one or more such devices, including some that are completely integrated.
Not Universally Accepted
However, lung ultrasound is not yet universally accepted as a tool for diagnosing pneumonia in the context of COVID-19 and triaging patients.
The National Health Service in England does not even mention lung ultrasound in its radiology decision tool for suspected COVID-19, specifying instead chest X-ray as the first-line diagnostic imaging tool, with CT scanning in equivocal cases.
But Giovanni Volpicelli, MD, University Hospital San Luigi Gonzaga, Turin, Italy, who has previously described his experience to Medscape Medical News, says many patients with COVID-19 in his hospital presented with a negative chest X-ray but were found to have interstitial pneumonia on lung ultrasound.
Moreover, while CT scan remains the gold standard, the risk of nosocomial infection is more easily controlled if patients do not have to be transported to the radiology department but remain in the emergency room and instead undergo lung ultrasound there, he stressed.
Experts Share Experience of Lung Ultrasound in COVID-19
In developing and publishing their protocol, Demi, senior author of the article, and other colleagues from the heavily affected cities of Northern Italy, say their aim is “to share our experience and to propose a standardization with respect to the use of lung ultrasound in the management of COVID-19 patients.”
They reviewed an anonymized database of around 60,000 ultrasound images of confirmed COVID-19 cases and reviewers were blinded to patients’ clinical backgrounds.
For image acquisition, the authors recommend scanning 14 areas in each patient for 10 seconds, making the scans intercostal to cover the widest possible surface area.
They advise the use of a single focal point on the pleural line, which they write, optimizes the beam shape for observing the lung surface.
The authors also urge that the mechanical index (MI) be kept low because high MIs sustained for long periods “may result in damaging the lung.”
They also stress that cosmetic filters and modalities such as harmonic imaging, contrast, doppler, and compounding should be avoided, alongside saturation phenomena.
What Constitutes Intermediate Disease?
Once the images have been taken, they are scored on a 0-3 scale for each of the 14 areas, with no weighting on any individual area.
A score of 0 is given when the pleural line is continuous and regular, with the presence of A-lines, denoting that the lungs are unaffected.
An area is given a score of 3 when the scan shows dense and largely extended white lung tissue, with or without consolidations, indicating severe disease.
At both ends of this spectrum, there is agreement between the Italian protocol and an algorithm developed by the Butterfly Network.
However, the two differ when it comes to scoring intermediate cases. On the Butterfly algorithm, the suggestion is to look for B-lines, caused by fluid and cellular infiltration into the interstitium, and to weigh that against the need for supplementary oxygen.
The Italian team, in contrast, says a score of 1 is given when the pleural line is indented, with vertical areas of white visible below.
A score of 2 is given when the pleural line is broken, with small to large areas of consolidation and associated areas of white below.
Demi told Medscape Medical News that they did not refer to B-lines in their protocol as their visibility depends entirely on the imaging frequency and the probe used.
“This means that scoring on B-lines, people with different machines would give completely different scores for the same patient.”
He continued: “We prefer to refer to horizontal and vertical artifacts, and provide an analysis of the patterns, which is related to the physics of the interactions between the ultrasound waves and lung surface.”
In response, Mike Stone, MD, Legacy Emanuel Medical Center, Portland, Oregon, and director of education at Butterfly, said there appears to be wide variation in lung findings that “may or may not correlate with the severity of symptoms.”
He told Medscape Medical News it is “hard to know exactly if someone with pure B-lines will progress to serious illness or if someone with some subpleural consolidations will do well.”
A Negative Ultrasound Is the Most Useful
Volpicelli believes that, in any case, any patient with an intermediate pattern will require further diagnosis, such as other imaging modalities and blood exams, and the real role of lung ultrasound is in assessing patients at either end of the spectrum.
“In other words, there are situations where lung ultrasound can be considered definitive,” he told Medscape Medical News. “For instance, if I see a patient with mild signs of the disease, just fever, and I perform lung ultrasound and see nothing, lung ultrasound rules out pneumonia.”
“This patient may have COVID-19 of course, but they do not have pneumonia, and they can be treated at home, awaiting the result of the swab test. And this is useful because you can reduce the burden in the emergency department.”
Volpicelli continued: “On the other hand, there are patients with acute respiratory failure in respiratory distress. If the lung ultrasound is normal, you can rule out COVID-19 and you need to use other diagnostic procedures to understand the problem.”
“This is also very important for us because it’s crucial to be able to remove the patient from the isolation area and perform CT scan, chest radiography, and all the other diagnostic tools that we need.”
Are Wireless Machines Needed? Not Necessarily
With regard to the use of wireless technology, the Italian team says that “in the setting of COVID-19, wireless probes and tablets represent the most appropriate ultrasound equipment” because they can “easily be wrapped in single-use plastic covers, reducing the risk of contamination,” and making sterilization easy.
Stone suggests that integrated portable devices, however, are no more likely to cause cross-contamination than separate probes and tablets, as they can fit within a sterile sheath as a single unit.
Volpicelli, for his part, doesn’t like what he sees as undue focus on wireless devices for lung ultrasound in the COVID-19 protocols.
He is concerned that recommending them as the best approach may be sending out the wrong message, which could be very “dangerous” as people may then think they cannot perform this screening with standard ultrasound machines.
For him, the issue of cross contamination with standard lung ultrasound machines is “nonexistent. Cleaning the machine is quite easy and I do it hundreds of times per week.”
He does acknowledge, however, that if the lung ultrasound is performed under certain circumstances, for example when a patient is using a continuous positive airway pressure (CPAP) machine, “the risk of having the machine contaminated is a little bit higher.”
“In these situations...we have a more intensive cleaning procedure to avoid cross-contamination.”
He stressed: “Not all centers have wireless machines, whereas a normal machine is usually in all hospitals.”
“The advantages of using lung ultrasound [in COVID-19] are too great to be limited by something that is not important in my opinion,” he concluded.
Stone is director of education at the Butterfly Network. No other conflicts of interest were declared.
This article first appeared on Medscape.com.
The first protocol for the use of lung ultrasound to quantitatively and reproducibly assess the degree of lung involvement in patients suspected of having COVID-19 infection has been published by a team of Italian experts with experience using the technology on the front line.
Particularly in Spain and Italy — where the pandemic has struck hardest in Europe — hard-pressed clinicians seeking to quickly understand whether patients with seemingly mild disease could be harboring more serious lung involvement have increasingly relied upon lung ultrasound in the emergency room.
Now Libertario Demi, PhD, head of the ultrasound laboratory, University of Trento, Italy, and colleagues have developed a protocol, published online March 30 in the Journal of Ultrasound Medicine, to standardize practice.
Their research, which builds on previous work by the team, offers broad agreement with industry-led algorithms and emphasizes the use of wireless, handheld ultrasound devices, ideally consisting of a separate probe and tablet, to make sterilization easy.
Firms such as the Butterfly Network, Phillips, Clarius, GE Healthcare, and Siemens are among numerous companies that produce one or more such devices, including some that are completely integrated.
Not Universally Accepted
However, lung ultrasound is not yet universally accepted as a tool for diagnosing pneumonia in the context of COVID-19 and triaging patients.
The National Health Service in England does not even mention lung ultrasound in its radiology decision tool for suspected COVID-19, specifying instead chest X-ray as the first-line diagnostic imaging tool, with CT scanning in equivocal cases.
But Giovanni Volpicelli, MD, University Hospital San Luigi Gonzaga, Turin, Italy, who has previously described his experience to Medscape Medical News, says many patients with COVID-19 in his hospital presented with a negative chest X-ray but were found to have interstitial pneumonia on lung ultrasound.
Moreover, while CT scan remains the gold standard, the risk of nosocomial infection is more easily controlled if patients do not have to be transported to the radiology department but remain in the emergency room and instead undergo lung ultrasound there, he stressed.
Experts Share Experience of Lung Ultrasound in COVID-19
In developing and publishing their protocol, Demi, senior author of the article, and other colleagues from the heavily affected cities of Northern Italy, say their aim is “to share our experience and to propose a standardization with respect to the use of lung ultrasound in the management of COVID-19 patients.”
They reviewed an anonymized database of around 60,000 ultrasound images of confirmed COVID-19 cases and reviewers were blinded to patients’ clinical backgrounds.
For image acquisition, the authors recommend scanning 14 areas in each patient for 10 seconds, making the scans intercostal to cover the widest possible surface area.
They advise the use of a single focal point on the pleural line, which they write, optimizes the beam shape for observing the lung surface.
The authors also urge that the mechanical index (MI) be kept low because high MIs sustained for long periods “may result in damaging the lung.”
They also stress that cosmetic filters and modalities such as harmonic imaging, contrast, doppler, and compounding should be avoided, alongside saturation phenomena.
What Constitutes Intermediate Disease?
Once the images have been taken, they are scored on a 0-3 scale for each of the 14 areas, with no weighting on any individual area.
A score of 0 is given when the pleural line is continuous and regular, with the presence of A-lines, denoting that the lungs are unaffected.
An area is given a score of 3 when the scan shows dense and largely extended white lung tissue, with or without consolidations, indicating severe disease.
At both ends of this spectrum, there is agreement between the Italian protocol and an algorithm developed by the Butterfly Network.
However, the two differ when it comes to scoring intermediate cases. On the Butterfly algorithm, the suggestion is to look for B-lines, caused by fluid and cellular infiltration into the interstitium, and to weigh that against the need for supplementary oxygen.
The Italian team, in contrast, says a score of 1 is given when the pleural line is indented, with vertical areas of white visible below.
A score of 2 is given when the pleural line is broken, with small to large areas of consolidation and associated areas of white below.
Demi told Medscape Medical News that they did not refer to B-lines in their protocol as their visibility depends entirely on the imaging frequency and the probe used.
“This means that scoring on B-lines, people with different machines would give completely different scores for the same patient.”
He continued: “We prefer to refer to horizontal and vertical artifacts, and provide an analysis of the patterns, which is related to the physics of the interactions between the ultrasound waves and lung surface.”
In response, Mike Stone, MD, Legacy Emanuel Medical Center, Portland, Oregon, and director of education at Butterfly, said there appears to be wide variation in lung findings that “may or may not correlate with the severity of symptoms.”
He told Medscape Medical News it is “hard to know exactly if someone with pure B-lines will progress to serious illness or if someone with some subpleural consolidations will do well.”
A Negative Ultrasound Is the Most Useful
Volpicelli believes that, in any case, any patient with an intermediate pattern will require further diagnosis, such as other imaging modalities and blood exams, and the real role of lung ultrasound is in assessing patients at either end of the spectrum.
“In other words, there are situations where lung ultrasound can be considered definitive,” he told Medscape Medical News. “For instance, if I see a patient with mild signs of the disease, just fever, and I perform lung ultrasound and see nothing, lung ultrasound rules out pneumonia.”
“This patient may have COVID-19 of course, but they do not have pneumonia, and they can be treated at home, awaiting the result of the swab test. And this is useful because you can reduce the burden in the emergency department.”
Volpicelli continued: “On the other hand, there are patients with acute respiratory failure in respiratory distress. If the lung ultrasound is normal, you can rule out COVID-19 and you need to use other diagnostic procedures to understand the problem.”
“This is also very important for us because it’s crucial to be able to remove the patient from the isolation area and perform CT scan, chest radiography, and all the other diagnostic tools that we need.”
Are Wireless Machines Needed? Not Necessarily
With regard to the use of wireless technology, the Italian team says that “in the setting of COVID-19, wireless probes and tablets represent the most appropriate ultrasound equipment” because they can “easily be wrapped in single-use plastic covers, reducing the risk of contamination,” and making sterilization easy.
Stone suggests that integrated portable devices, however, are no more likely to cause cross-contamination than separate probes and tablets, as they can fit within a sterile sheath as a single unit.
Volpicelli, for his part, doesn’t like what he sees as undue focus on wireless devices for lung ultrasound in the COVID-19 protocols.
He is concerned that recommending them as the best approach may be sending out the wrong message, which could be very “dangerous” as people may then think they cannot perform this screening with standard ultrasound machines.
For him, the issue of cross contamination with standard lung ultrasound machines is “nonexistent. Cleaning the machine is quite easy and I do it hundreds of times per week.”
He does acknowledge, however, that if the lung ultrasound is performed under certain circumstances, for example when a patient is using a continuous positive airway pressure (CPAP) machine, “the risk of having the machine contaminated is a little bit higher.”
“In these situations...we have a more intensive cleaning procedure to avoid cross-contamination.”
He stressed: “Not all centers have wireless machines, whereas a normal machine is usually in all hospitals.”
“The advantages of using lung ultrasound [in COVID-19] are too great to be limited by something that is not important in my opinion,” he concluded.
Stone is director of education at the Butterfly Network. No other conflicts of interest were declared.
This article first appeared on Medscape.com.
The first protocol for the use of lung ultrasound to quantitatively and reproducibly assess the degree of lung involvement in patients suspected of having COVID-19 infection has been published by a team of Italian experts with experience using the technology on the front line.
Particularly in Spain and Italy — where the pandemic has struck hardest in Europe — hard-pressed clinicians seeking to quickly understand whether patients with seemingly mild disease could be harboring more serious lung involvement have increasingly relied upon lung ultrasound in the emergency room.
Now Libertario Demi, PhD, head of the ultrasound laboratory, University of Trento, Italy, and colleagues have developed a protocol, published online March 30 in the Journal of Ultrasound Medicine, to standardize practice.
Their research, which builds on previous work by the team, offers broad agreement with industry-led algorithms and emphasizes the use of wireless, handheld ultrasound devices, ideally consisting of a separate probe and tablet, to make sterilization easy.
Firms such as the Butterfly Network, Phillips, Clarius, GE Healthcare, and Siemens are among numerous companies that produce one or more such devices, including some that are completely integrated.
Not Universally Accepted
However, lung ultrasound is not yet universally accepted as a tool for diagnosing pneumonia in the context of COVID-19 and triaging patients.
The National Health Service in England does not even mention lung ultrasound in its radiology decision tool for suspected COVID-19, specifying instead chest X-ray as the first-line diagnostic imaging tool, with CT scanning in equivocal cases.
But Giovanni Volpicelli, MD, University Hospital San Luigi Gonzaga, Turin, Italy, who has previously described his experience to Medscape Medical News, says many patients with COVID-19 in his hospital presented with a negative chest X-ray but were found to have interstitial pneumonia on lung ultrasound.
Moreover, while CT scan remains the gold standard, the risk of nosocomial infection is more easily controlled if patients do not have to be transported to the radiology department but remain in the emergency room and instead undergo lung ultrasound there, he stressed.
Experts Share Experience of Lung Ultrasound in COVID-19
In developing and publishing their protocol, Demi, senior author of the article, and other colleagues from the heavily affected cities of Northern Italy, say their aim is “to share our experience and to propose a standardization with respect to the use of lung ultrasound in the management of COVID-19 patients.”
They reviewed an anonymized database of around 60,000 ultrasound images of confirmed COVID-19 cases and reviewers were blinded to patients’ clinical backgrounds.
For image acquisition, the authors recommend scanning 14 areas in each patient for 10 seconds, making the scans intercostal to cover the widest possible surface area.
They advise the use of a single focal point on the pleural line, which they write, optimizes the beam shape for observing the lung surface.
The authors also urge that the mechanical index (MI) be kept low because high MIs sustained for long periods “may result in damaging the lung.”
They also stress that cosmetic filters and modalities such as harmonic imaging, contrast, doppler, and compounding should be avoided, alongside saturation phenomena.
What Constitutes Intermediate Disease?
Once the images have been taken, they are scored on a 0-3 scale for each of the 14 areas, with no weighting on any individual area.
A score of 0 is given when the pleural line is continuous and regular, with the presence of A-lines, denoting that the lungs are unaffected.
An area is given a score of 3 when the scan shows dense and largely extended white lung tissue, with or without consolidations, indicating severe disease.
At both ends of this spectrum, there is agreement between the Italian protocol and an algorithm developed by the Butterfly Network.
However, the two differ when it comes to scoring intermediate cases. On the Butterfly algorithm, the suggestion is to look for B-lines, caused by fluid and cellular infiltration into the interstitium, and to weigh that against the need for supplementary oxygen.
The Italian team, in contrast, says a score of 1 is given when the pleural line is indented, with vertical areas of white visible below.
A score of 2 is given when the pleural line is broken, with small to large areas of consolidation and associated areas of white below.
Demi told Medscape Medical News that they did not refer to B-lines in their protocol as their visibility depends entirely on the imaging frequency and the probe used.
“This means that scoring on B-lines, people with different machines would give completely different scores for the same patient.”
He continued: “We prefer to refer to horizontal and vertical artifacts, and provide an analysis of the patterns, which is related to the physics of the interactions between the ultrasound waves and lung surface.”
In response, Mike Stone, MD, Legacy Emanuel Medical Center, Portland, Oregon, and director of education at Butterfly, said there appears to be wide variation in lung findings that “may or may not correlate with the severity of symptoms.”
He told Medscape Medical News it is “hard to know exactly if someone with pure B-lines will progress to serious illness or if someone with some subpleural consolidations will do well.”
A Negative Ultrasound Is the Most Useful
Volpicelli believes that, in any case, any patient with an intermediate pattern will require further diagnosis, such as other imaging modalities and blood exams, and the real role of lung ultrasound is in assessing patients at either end of the spectrum.
“In other words, there are situations where lung ultrasound can be considered definitive,” he told Medscape Medical News. “For instance, if I see a patient with mild signs of the disease, just fever, and I perform lung ultrasound and see nothing, lung ultrasound rules out pneumonia.”
“This patient may have COVID-19 of course, but they do not have pneumonia, and they can be treated at home, awaiting the result of the swab test. And this is useful because you can reduce the burden in the emergency department.”
Volpicelli continued: “On the other hand, there are patients with acute respiratory failure in respiratory distress. If the lung ultrasound is normal, you can rule out COVID-19 and you need to use other diagnostic procedures to understand the problem.”
“This is also very important for us because it’s crucial to be able to remove the patient from the isolation area and perform CT scan, chest radiography, and all the other diagnostic tools that we need.”
Are Wireless Machines Needed? Not Necessarily
With regard to the use of wireless technology, the Italian team says that “in the setting of COVID-19, wireless probes and tablets represent the most appropriate ultrasound equipment” because they can “easily be wrapped in single-use plastic covers, reducing the risk of contamination,” and making sterilization easy.
Stone suggests that integrated portable devices, however, are no more likely to cause cross-contamination than separate probes and tablets, as they can fit within a sterile sheath as a single unit.
Volpicelli, for his part, doesn’t like what he sees as undue focus on wireless devices for lung ultrasound in the COVID-19 protocols.
He is concerned that recommending them as the best approach may be sending out the wrong message, which could be very “dangerous” as people may then think they cannot perform this screening with standard ultrasound machines.
For him, the issue of cross contamination with standard lung ultrasound machines is “nonexistent. Cleaning the machine is quite easy and I do it hundreds of times per week.”
He does acknowledge, however, that if the lung ultrasound is performed under certain circumstances, for example when a patient is using a continuous positive airway pressure (CPAP) machine, “the risk of having the machine contaminated is a little bit higher.”
“In these situations...we have a more intensive cleaning procedure to avoid cross-contamination.”
He stressed: “Not all centers have wireless machines, whereas a normal machine is usually in all hospitals.”
“The advantages of using lung ultrasound [in COVID-19] are too great to be limited by something that is not important in my opinion,” he concluded.
Stone is director of education at the Butterfly Network. No other conflicts of interest were declared.
This article first appeared on Medscape.com.
Crisis counseling, not therapy, is what’s needed in the wake of COVID-19
In the wake of the attacks on the World Trade Center, the public mental health system in the New York City area mounted the largest mental health disaster response in history. I was New York City’s mental health commissioner at the time. We called the initiative Project Liberty and over 3 years obtained $137 million in funding from the Federal Emergency Management Agency (FEMA) to support it.
Through Project Liberty, New York established the Crisis Counseling Assistance and Training Program (CCP). And it didn’t take us long to realize that what affected people need following a disaster is not necessarily psychotherapy, as might be expected, but in fact crisis counseling, or helping impacted individuals and their families regain control of their anxieties and effectively respond to an immediate disaster. This proved true not only after 9/11 but also after other recent disasters, including hurricanes Katrina and Sandy. The mental health system must now step up again to assuage fears and anxieties—both individual and collective—around the rapidly spreading COVID-19 pandemic.
So, what is crisis counseling?
A person’s usual adaptive, problem-solving capabilities are often compromised after a disaster, but they are there, and if accessed, they can help those afflicted with mental symptoms following a crisis to mentally endure. thereby making it a different approach from traditional psychotherapy.
The five key concepts in crisis counseling are:
- It is strength-based, which means its foundation is rooted in the assumption that resilience and competence are innate human qualities.
- Crisis counseling also employs anonymity. Impacted individuals should not be diagnosed or labeled. As a result, there are no resulting medical records.
- The approach is outreach-oriented, in which counselors provide services out in the community rather than in traditional mental health settings. This occurs primarily in homes, community centers, and settings, as well as in disaster shelters.
- It is culturally attuned, whereby all staff appreciate and respect a community’s cultural beliefs, values, and primary language.
- It is aimed at supporting, not replacing, existing community support systems (eg, a crisis counselor supports but does not organize, deliver, or manage community recovery activities).
Crisis counselors are required to be licensed psychologists or have obtained a bachelor’s degree or higher in psychology, human services, or another health-related field. In other words, crisis counseling draws on a broad, though related, group of individuals. Before deployment into a disaster area, an applicant must complete the FEMA Crisis Counseling Assistance and Training, which is offered in the disaster area by the FEMA-funded CCP.
Crisis counselors provide trustworthy and actionable information about the disaster at hand and where to turn for resources and assistance. They assist with emotional support. And they aim to educate individuals, families, and communities about how to be resilient.
Crisis counseling, however, may not suffice for everyone impacted. We know that a person’s severity of response to a crisis is highly associated with the intensity and duration of exposure to the disaster (especially when it is life-threatening) and/or the degree of a person’s serious loss (of a loved one, home, job, health). We also know that previous trauma (eg, from childhood, domestic violence, or forced immigration) also predicts the gravity of the response to a current crisis. Which is why crisis counselors also are taught to identify those experiencing significant and persistent mental health and addiction problems because they need to be assisted, literally, in obtaining professional treatment.
Only in recent years has trauma been a recognized driver of stress, distress, and mental and addictive disorders. Until relatively recently, skill with, and access to, crisis counseling—and trauma-informed care—was rare among New York’s large and talented mental health professional community. Few had been trained in it in graduate school or practiced it because New York had been spared a disaster on par with 9/11. Following the attacks, Project Liberty’s programs served nearly 1.5 million affected individuals of very diverse ages, races, cultural backgrounds, and socioeconomic status. Their levels of “psychological distress,” the term we used and measured, ranged from low to very high.
The coronavirus pandemic now presents us with a tragically similar, catastrophic moment. The human consequences we face—psychologically, economically, and socially—are just beginning. But this time, the need is not just in New York but throughout our country.
We humans are resilient. We can bend the arc of crisis toward the light, to recovering our existing but overwhelmed capabilities. We can achieve this in a variety of ways. We can practice self-care. This isn’t an act of selfishness but is rather like putting on your own oxygen mask before trying to help your friend or loved one do the same. We can stay connected to the people we care about. We can eat well, get sufficient sleep, take a walk.
Identifying and pursuing practical goals is also important, like obtaining food, housing that is safe and reliable, transportation to where you need to go, and drawing upon financial and other resources that are issued in a disaster area. We can practice positive thinking and recall how we’ve mastered our troubles in the past; we can remind ourselves that “this too will pass.” Crises create an unusually opportune time for change and self-discovery. As Churchill said to the British people in the darkest moments of the start of World War II, “Never give up.”
Worthy of its own itemization are spiritual beliefs, faith—that however we think about a higher power (religious or secular), that power is on our side. Faith can comfort and sustain hope, particularly at a time when doubt about ourselves and humanity is triggered by disaster.
Maya Angelou’s words remind us at this moment of disaster: “...let us try to help before we have to offer therapy. That is to say, let’s see if we can’t prevent being ill by trying to offer a love of prevention before illness.”
Dr. Sederer is the former chief medical officer for the New York State Office of Mental Health and an adjunct professor in the Department of Epidemiology at the Columbia University School of Public Health. His latest book is The Addiction Solution: Treating Our Dependence on Opioids and Other Drugs.
This article first appeared on Medscape.com.
In the wake of the attacks on the World Trade Center, the public mental health system in the New York City area mounted the largest mental health disaster response in history. I was New York City’s mental health commissioner at the time. We called the initiative Project Liberty and over 3 years obtained $137 million in funding from the Federal Emergency Management Agency (FEMA) to support it.
Through Project Liberty, New York established the Crisis Counseling Assistance and Training Program (CCP). And it didn’t take us long to realize that what affected people need following a disaster is not necessarily psychotherapy, as might be expected, but in fact crisis counseling, or helping impacted individuals and their families regain control of their anxieties and effectively respond to an immediate disaster. This proved true not only after 9/11 but also after other recent disasters, including hurricanes Katrina and Sandy. The mental health system must now step up again to assuage fears and anxieties—both individual and collective—around the rapidly spreading COVID-19 pandemic.
So, what is crisis counseling?
A person’s usual adaptive, problem-solving capabilities are often compromised after a disaster, but they are there, and if accessed, they can help those afflicted with mental symptoms following a crisis to mentally endure. thereby making it a different approach from traditional psychotherapy.
The five key concepts in crisis counseling are:
- It is strength-based, which means its foundation is rooted in the assumption that resilience and competence are innate human qualities.
- Crisis counseling also employs anonymity. Impacted individuals should not be diagnosed or labeled. As a result, there are no resulting medical records.
- The approach is outreach-oriented, in which counselors provide services out in the community rather than in traditional mental health settings. This occurs primarily in homes, community centers, and settings, as well as in disaster shelters.
- It is culturally attuned, whereby all staff appreciate and respect a community’s cultural beliefs, values, and primary language.
- It is aimed at supporting, not replacing, existing community support systems (eg, a crisis counselor supports but does not organize, deliver, or manage community recovery activities).
Crisis counselors are required to be licensed psychologists or have obtained a bachelor’s degree or higher in psychology, human services, or another health-related field. In other words, crisis counseling draws on a broad, though related, group of individuals. Before deployment into a disaster area, an applicant must complete the FEMA Crisis Counseling Assistance and Training, which is offered in the disaster area by the FEMA-funded CCP.
Crisis counselors provide trustworthy and actionable information about the disaster at hand and where to turn for resources and assistance. They assist with emotional support. And they aim to educate individuals, families, and communities about how to be resilient.
Crisis counseling, however, may not suffice for everyone impacted. We know that a person’s severity of response to a crisis is highly associated with the intensity and duration of exposure to the disaster (especially when it is life-threatening) and/or the degree of a person’s serious loss (of a loved one, home, job, health). We also know that previous trauma (eg, from childhood, domestic violence, or forced immigration) also predicts the gravity of the response to a current crisis. Which is why crisis counselors also are taught to identify those experiencing significant and persistent mental health and addiction problems because they need to be assisted, literally, in obtaining professional treatment.
Only in recent years has trauma been a recognized driver of stress, distress, and mental and addictive disorders. Until relatively recently, skill with, and access to, crisis counseling—and trauma-informed care—was rare among New York’s large and talented mental health professional community. Few had been trained in it in graduate school or practiced it because New York had been spared a disaster on par with 9/11. Following the attacks, Project Liberty’s programs served nearly 1.5 million affected individuals of very diverse ages, races, cultural backgrounds, and socioeconomic status. Their levels of “psychological distress,” the term we used and measured, ranged from low to very high.
The coronavirus pandemic now presents us with a tragically similar, catastrophic moment. The human consequences we face—psychologically, economically, and socially—are just beginning. But this time, the need is not just in New York but throughout our country.
We humans are resilient. We can bend the arc of crisis toward the light, to recovering our existing but overwhelmed capabilities. We can achieve this in a variety of ways. We can practice self-care. This isn’t an act of selfishness but is rather like putting on your own oxygen mask before trying to help your friend or loved one do the same. We can stay connected to the people we care about. We can eat well, get sufficient sleep, take a walk.
Identifying and pursuing practical goals is also important, like obtaining food, housing that is safe and reliable, transportation to where you need to go, and drawing upon financial and other resources that are issued in a disaster area. We can practice positive thinking and recall how we’ve mastered our troubles in the past; we can remind ourselves that “this too will pass.” Crises create an unusually opportune time for change and self-discovery. As Churchill said to the British people in the darkest moments of the start of World War II, “Never give up.”
Worthy of its own itemization are spiritual beliefs, faith—that however we think about a higher power (religious or secular), that power is on our side. Faith can comfort and sustain hope, particularly at a time when doubt about ourselves and humanity is triggered by disaster.
Maya Angelou’s words remind us at this moment of disaster: “...let us try to help before we have to offer therapy. That is to say, let’s see if we can’t prevent being ill by trying to offer a love of prevention before illness.”
Dr. Sederer is the former chief medical officer for the New York State Office of Mental Health and an adjunct professor in the Department of Epidemiology at the Columbia University School of Public Health. His latest book is The Addiction Solution: Treating Our Dependence on Opioids and Other Drugs.
This article first appeared on Medscape.com.
In the wake of the attacks on the World Trade Center, the public mental health system in the New York City area mounted the largest mental health disaster response in history. I was New York City’s mental health commissioner at the time. We called the initiative Project Liberty and over 3 years obtained $137 million in funding from the Federal Emergency Management Agency (FEMA) to support it.
Through Project Liberty, New York established the Crisis Counseling Assistance and Training Program (CCP). And it didn’t take us long to realize that what affected people need following a disaster is not necessarily psychotherapy, as might be expected, but in fact crisis counseling, or helping impacted individuals and their families regain control of their anxieties and effectively respond to an immediate disaster. This proved true not only after 9/11 but also after other recent disasters, including hurricanes Katrina and Sandy. The mental health system must now step up again to assuage fears and anxieties—both individual and collective—around the rapidly spreading COVID-19 pandemic.
So, what is crisis counseling?
A person’s usual adaptive, problem-solving capabilities are often compromised after a disaster, but they are there, and if accessed, they can help those afflicted with mental symptoms following a crisis to mentally endure. thereby making it a different approach from traditional psychotherapy.
The five key concepts in crisis counseling are:
- It is strength-based, which means its foundation is rooted in the assumption that resilience and competence are innate human qualities.
- Crisis counseling also employs anonymity. Impacted individuals should not be diagnosed or labeled. As a result, there are no resulting medical records.
- The approach is outreach-oriented, in which counselors provide services out in the community rather than in traditional mental health settings. This occurs primarily in homes, community centers, and settings, as well as in disaster shelters.
- It is culturally attuned, whereby all staff appreciate and respect a community’s cultural beliefs, values, and primary language.
- It is aimed at supporting, not replacing, existing community support systems (eg, a crisis counselor supports but does not organize, deliver, or manage community recovery activities).
Crisis counselors are required to be licensed psychologists or have obtained a bachelor’s degree or higher in psychology, human services, or another health-related field. In other words, crisis counseling draws on a broad, though related, group of individuals. Before deployment into a disaster area, an applicant must complete the FEMA Crisis Counseling Assistance and Training, which is offered in the disaster area by the FEMA-funded CCP.
Crisis counselors provide trustworthy and actionable information about the disaster at hand and where to turn for resources and assistance. They assist with emotional support. And they aim to educate individuals, families, and communities about how to be resilient.
Crisis counseling, however, may not suffice for everyone impacted. We know that a person’s severity of response to a crisis is highly associated with the intensity and duration of exposure to the disaster (especially when it is life-threatening) and/or the degree of a person’s serious loss (of a loved one, home, job, health). We also know that previous trauma (eg, from childhood, domestic violence, or forced immigration) also predicts the gravity of the response to a current crisis. Which is why crisis counselors also are taught to identify those experiencing significant and persistent mental health and addiction problems because they need to be assisted, literally, in obtaining professional treatment.
Only in recent years has trauma been a recognized driver of stress, distress, and mental and addictive disorders. Until relatively recently, skill with, and access to, crisis counseling—and trauma-informed care—was rare among New York’s large and talented mental health professional community. Few had been trained in it in graduate school or practiced it because New York had been spared a disaster on par with 9/11. Following the attacks, Project Liberty’s programs served nearly 1.5 million affected individuals of very diverse ages, races, cultural backgrounds, and socioeconomic status. Their levels of “psychological distress,” the term we used and measured, ranged from low to very high.
The coronavirus pandemic now presents us with a tragically similar, catastrophic moment. The human consequences we face—psychologically, economically, and socially—are just beginning. But this time, the need is not just in New York but throughout our country.
We humans are resilient. We can bend the arc of crisis toward the light, to recovering our existing but overwhelmed capabilities. We can achieve this in a variety of ways. We can practice self-care. This isn’t an act of selfishness but is rather like putting on your own oxygen mask before trying to help your friend or loved one do the same. We can stay connected to the people we care about. We can eat well, get sufficient sleep, take a walk.
Identifying and pursuing practical goals is also important, like obtaining food, housing that is safe and reliable, transportation to where you need to go, and drawing upon financial and other resources that are issued in a disaster area. We can practice positive thinking and recall how we’ve mastered our troubles in the past; we can remind ourselves that “this too will pass.” Crises create an unusually opportune time for change and self-discovery. As Churchill said to the British people in the darkest moments of the start of World War II, “Never give up.”
Worthy of its own itemization are spiritual beliefs, faith—that however we think about a higher power (religious or secular), that power is on our side. Faith can comfort and sustain hope, particularly at a time when doubt about ourselves and humanity is triggered by disaster.
Maya Angelou’s words remind us at this moment of disaster: “...let us try to help before we have to offer therapy. That is to say, let’s see if we can’t prevent being ill by trying to offer a love of prevention before illness.”
Dr. Sederer is the former chief medical officer for the New York State Office of Mental Health and an adjunct professor in the Department of Epidemiology at the Columbia University School of Public Health. His latest book is The Addiction Solution: Treating Our Dependence on Opioids and Other Drugs.
This article first appeared on Medscape.com.
COVID-19 and surge capacity in U.S. hospitals
Background
As of April 2020, the United States is faced with the early stages of the coronavirus disease 2019 (COVID-19) pandemic. Experts predict up to 60% of the population will become infected with a fatality rate of 1% and a hospitalization rate of approximately 20%. Efforts to suppress viral spread have been unsuccessful as cases are reported in all 50 states, and fatalities are rising. Currently many American hospitals are ill-prepared for a significant increase in their census of critically ill and contagious patients, i.e., hospitals lack adequate surge capacity to safely handle a nationwide outbreak of COVID-19. As seen in other nations such as Italy, China, and Iran, this leads to rationing of life-saving health care and potentially preventable morbidity and mortality.
Introduction
Hospitals will be unable to provide the current standard of care to patients as the rate of infection with coronavirus disease 2019 (COVID-19) escalates. As of April 9, the World Health Organization has confirmed 1,539,118 cases and 89,998 deaths globally; and the Centers for Disease Control and Prevention has confirmed 435,941 cases and 14,865 deaths in the United States.1,2 Experts predict up to 60% of the population will eventually become infected with a fatality rate of about 1% and a hospitalization rate of approximately 20%.3,4
In the United States, with a population of 300 million people, this represents up to 180 million infected, 36 million requiring hospitalization, 11 million requiring intensive care, and 2 million fatalities over the duration of the pandemic. On March 13, President Donald Trump declared a state of national emergency, authorizing $50 billion dollars in emergency health care spending as well as asking every hospital in the country to immediately activate its emergency response plan. The use of isolation and quarantine may space out casualties over time, however high rates and volumes of hospitalizations are still expected.4,5
As the influx of patients afflicted with COVID-19 grows, needs will outstrip hospital resources forcing clinicians to ration beds and supplies. In Italy, China, and Iran, physicians are already faced with these difficult decisions. Antonio Pesenti, head of the Italian Lombardy regional crisis response unit, characterized the change in health care delivery: “We’re now being forced to set up intensive care treatment in corridors, in operating theaters, in recovery rooms. We’ve emptied entire hospital sections to make space for seriously sick people.”6
Surge capacity
Surge capacity is a hospital’s ability to adequately care for a significant influx of patients.7 Since 2011, the American College of Emergency Physicians has published guidelines calling for hospitals to have a surge capacity accounting for infectious disease outbreaks, and demands on supplies, personnel, and physical space.7 Even prior to the development of COVID-19, many hospitals faced emergency department crowding and strains on hospital capacity.8 The Organization for Economic Co-operation and Development (OECD) estimates hospital beds per 1,000 inhabitants at 2.77 for the USA, 3.18 for Italy, 4.34 for China, and 13.05 for Japan.9 Before COVID-19 many American hospitals had an insufficient number of beds. Now, in the initial phase of the pandemic, it is even more important to optimize surge capacity across the American health care system.
Requirements for COVID-19 preparation
To prepare for the increased number of seriously and critically ill patients, individual hospitals and regions must perform a needs assessment. The fundamental disease process of COVID-19 is a contagious viral pneumonia; treatment hinges on four major categories of intervention: spatial isolation (including physical space, beds, partitions, droplet precautions, food, water, and sanitation), oxygenation (including wall and portable oxygen, nasal canulae, and masks), mechanical ventilation (including ventilator machines, tubing, anesthetics, and reliable electrical power) and personnel (including physicians, nurses, technicians, and adequate personal protective equipment).10 In special circumstances and where available, extra corporeal membrane oxygenation may be considered.10 The necessary interventions are summarized in Table 1. 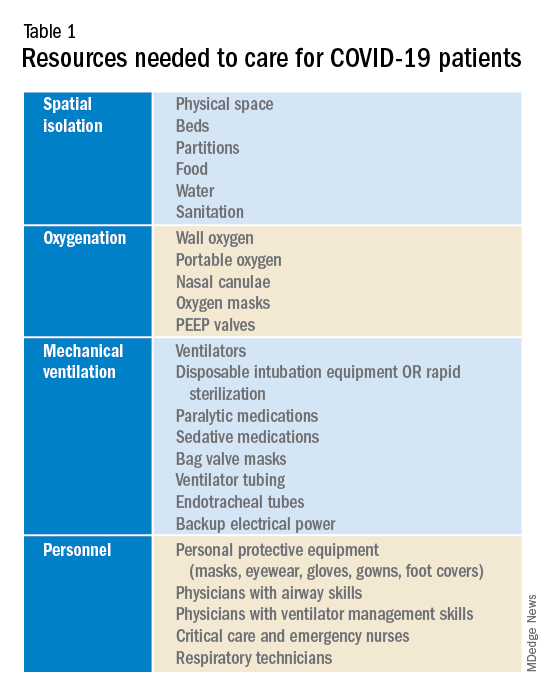
Emergency, critical care, nursing, and medical leadership should consider what sort of space, personnel, and supplies will be needed to care for a large volume of patients with contagious viral pneumonia at the same time as other hospital patients. Attention should also be given to potential need for morgue expansion. Hospitals must be proactive in procuring supplies and preparing for demands on beds and physical space. Specifically, logistics coordinators should start stockpiling ventilators, oxygen, respiratory equipment, and personal protective equipment. Reallocating supplies from other regions of the hospital such as operating rooms and ambulatory surgery centers may be considered. These resources, particularly ventilators and ventilator supplies, are already in disturbingly limited supply, and they are likely to be single most important limiting factor for survival rates. To prevent regional shortages, stockpiling efforts should ideally be aided by state and federal governments. The production and acquisition of ventilators should be immediately and significantly increased.
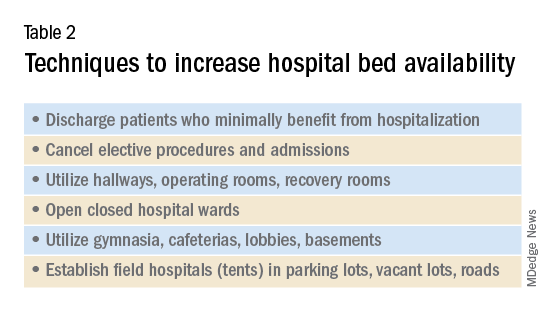
Hospitals must additionally prepare for demands for physical space and beds. Techniques to maximize space and bed availability (see Table 2) include discharging patients who do not require hospitalization, and canceling elective procedures and admissions. Additional methods would be to utilize unconventional preexisting spaces such as hallways, operating rooms, recovery rooms, hallways, closed hospital wards, basements, lobbies, cafeterias, and parking lots. Administrators should also consider establishing field hospitals or field wards, such as tents in open spaces and nearby roads. Medical care performed in unconventional environments will need to account for electricity, temperature control, oxygen delivery, and sanitation.
Conclusion
To minimize unnecessary loss of life and suffering, hospitals must expand their surge capacities in preparation for the predictable rise in demand for health care resources related to COVID-19. Numerous hospitals, particularly those that serve low-income and underserved communities, operate with a narrow financial margin.11 Independently preparing for the surge capacity needed to face COVID-19 may be infeasible for several hospitals. As a result, many health care systems will rely on government aid during this period for financial and material support. To maximize preparedness and response, hospitals should ask for and receive aid from the Federal Emergency Management Agency (FEMA), American Red Cross, state governments, and the military; these resources should be mobilized now.
Dr. Blumenberg, Dr. Noble, and Dr. Hendrickson are based in the department of emergency medicine & toxicology, Oregon Health and Science University, Portland.
References
1. Coronavirus disease 2019 (COVID-19) situation report – 60. 2020 Mar 19.
2. Coronavirus disease 2019 (COVID-19) Cases in the U.S. CDC. 2020 Apr 8.
3. Li Q et al. Early transmission dynamics in Wuhan, China, of novel coronavirus–infected pneumonia. N Engl J Med. 2020 Jan. doi: 10.1056/NEJMoa2001316.
4. Anderson RM et al. How will country-based mitigation measures influence the course of the COVID-19 epidemic? Lancet. 2020 Mar. doi: 10.1016/S0140-6736(20)30567-5.
5. Fraser C et al. Factors that make an infectious disease outbreak controllable. Proc Natl Acad Sci U S A. 2004;101(16):6146-51. doi: 10.1073/pnas.0307506101.
6. Mackenzie J and Balmer C. Italy locks down millions as its coronavirus deaths jump. Reuters. 2020 Mar 9.
7. Health care system surge capacity recognition, preparedness, and response. Ann Emerg Med. 2012;59(3):240-1. doi: 10.1016/j.annemergmed.2011.11.030.
8. Pitts SR et al. A cross-sectional study of emergency department boarding practices in the United States. Acad Emerg Med. 2014;21(5):497-503. doi: 10.1111/acem.12375.
9. Health at a Glance 2019. OECD; 2019. doi: 10.1787/4dd50c09-en.
10. Murthy S et al. Care for critically ill patients with COVID-19. JAMA. 2020 Mar. doi: 10.1001/jama.2020.3633.
11. Ly DP et al. The association between hospital margins, quality of care, and closure or other change in operating status. J Gen Intern Med. 2011;26(11):1291-6. doi: 10.1007/s11606-011-1815-5.
Background
As of April 2020, the United States is faced with the early stages of the coronavirus disease 2019 (COVID-19) pandemic. Experts predict up to 60% of the population will become infected with a fatality rate of 1% and a hospitalization rate of approximately 20%. Efforts to suppress viral spread have been unsuccessful as cases are reported in all 50 states, and fatalities are rising. Currently many American hospitals are ill-prepared for a significant increase in their census of critically ill and contagious patients, i.e., hospitals lack adequate surge capacity to safely handle a nationwide outbreak of COVID-19. As seen in other nations such as Italy, China, and Iran, this leads to rationing of life-saving health care and potentially preventable morbidity and mortality.
Introduction
Hospitals will be unable to provide the current standard of care to patients as the rate of infection with coronavirus disease 2019 (COVID-19) escalates. As of April 9, the World Health Organization has confirmed 1,539,118 cases and 89,998 deaths globally; and the Centers for Disease Control and Prevention has confirmed 435,941 cases and 14,865 deaths in the United States.1,2 Experts predict up to 60% of the population will eventually become infected with a fatality rate of about 1% and a hospitalization rate of approximately 20%.3,4
In the United States, with a population of 300 million people, this represents up to 180 million infected, 36 million requiring hospitalization, 11 million requiring intensive care, and 2 million fatalities over the duration of the pandemic. On March 13, President Donald Trump declared a state of national emergency, authorizing $50 billion dollars in emergency health care spending as well as asking every hospital in the country to immediately activate its emergency response plan. The use of isolation and quarantine may space out casualties over time, however high rates and volumes of hospitalizations are still expected.4,5
As the influx of patients afflicted with COVID-19 grows, needs will outstrip hospital resources forcing clinicians to ration beds and supplies. In Italy, China, and Iran, physicians are already faced with these difficult decisions. Antonio Pesenti, head of the Italian Lombardy regional crisis response unit, characterized the change in health care delivery: “We’re now being forced to set up intensive care treatment in corridors, in operating theaters, in recovery rooms. We’ve emptied entire hospital sections to make space for seriously sick people.”6
Surge capacity
Surge capacity is a hospital’s ability to adequately care for a significant influx of patients.7 Since 2011, the American College of Emergency Physicians has published guidelines calling for hospitals to have a surge capacity accounting for infectious disease outbreaks, and demands on supplies, personnel, and physical space.7 Even prior to the development of COVID-19, many hospitals faced emergency department crowding and strains on hospital capacity.8 The Organization for Economic Co-operation and Development (OECD) estimates hospital beds per 1,000 inhabitants at 2.77 for the USA, 3.18 for Italy, 4.34 for China, and 13.05 for Japan.9 Before COVID-19 many American hospitals had an insufficient number of beds. Now, in the initial phase of the pandemic, it is even more important to optimize surge capacity across the American health care system.
Requirements for COVID-19 preparation
To prepare for the increased number of seriously and critically ill patients, individual hospitals and regions must perform a needs assessment. The fundamental disease process of COVID-19 is a contagious viral pneumonia; treatment hinges on four major categories of intervention: spatial isolation (including physical space, beds, partitions, droplet precautions, food, water, and sanitation), oxygenation (including wall and portable oxygen, nasal canulae, and masks), mechanical ventilation (including ventilator machines, tubing, anesthetics, and reliable electrical power) and personnel (including physicians, nurses, technicians, and adequate personal protective equipment).10 In special circumstances and where available, extra corporeal membrane oxygenation may be considered.10 The necessary interventions are summarized in Table 1. 
Emergency, critical care, nursing, and medical leadership should consider what sort of space, personnel, and supplies will be needed to care for a large volume of patients with contagious viral pneumonia at the same time as other hospital patients. Attention should also be given to potential need for morgue expansion. Hospitals must be proactive in procuring supplies and preparing for demands on beds and physical space. Specifically, logistics coordinators should start stockpiling ventilators, oxygen, respiratory equipment, and personal protective equipment. Reallocating supplies from other regions of the hospital such as operating rooms and ambulatory surgery centers may be considered. These resources, particularly ventilators and ventilator supplies, are already in disturbingly limited supply, and they are likely to be single most important limiting factor for survival rates. To prevent regional shortages, stockpiling efforts should ideally be aided by state and federal governments. The production and acquisition of ventilators should be immediately and significantly increased.
Hospitals must additionally prepare for demands for physical space and beds. Techniques to maximize space and bed availability (see Table 2) include discharging patients who do not require hospitalization, and canceling elective procedures and admissions. Additional methods would be to utilize unconventional preexisting spaces such as hallways, operating rooms, recovery rooms, hallways, closed hospital wards, basements, lobbies, cafeterias, and parking lots. Administrators should also consider establishing field hospitals or field wards, such as tents in open spaces and nearby roads. Medical care performed in unconventional environments will need to account for electricity, temperature control, oxygen delivery, and sanitation.

Conclusion
To minimize unnecessary loss of life and suffering, hospitals must expand their surge capacities in preparation for the predictable rise in demand for health care resources related to COVID-19. Numerous hospitals, particularly those that serve low-income and underserved communities, operate with a narrow financial margin.11 Independently preparing for the surge capacity needed to face COVID-19 may be infeasible for several hospitals. As a result, many health care systems will rely on government aid during this period for financial and material support. To maximize preparedness and response, hospitals should ask for and receive aid from the Federal Emergency Management Agency (FEMA), American Red Cross, state governments, and the military; these resources should be mobilized now.
Dr. Blumenberg, Dr. Noble, and Dr. Hendrickson are based in the department of emergency medicine & toxicology, Oregon Health and Science University, Portland.
References
1. Coronavirus disease 2019 (COVID-19) situation report – 60. 2020 Mar 19.
2. Coronavirus disease 2019 (COVID-19) Cases in the U.S. CDC. 2020 Apr 8.
3. Li Q et al. Early transmission dynamics in Wuhan, China, of novel coronavirus–infected pneumonia. N Engl J Med. 2020 Jan. doi: 10.1056/NEJMoa2001316.
4. Anderson RM et al. How will country-based mitigation measures influence the course of the COVID-19 epidemic? Lancet. 2020 Mar. doi: 10.1016/S0140-6736(20)30567-5.
5. Fraser C et al. Factors that make an infectious disease outbreak controllable. Proc Natl Acad Sci U S A. 2004;101(16):6146-51. doi: 10.1073/pnas.0307506101.
6. Mackenzie J and Balmer C. Italy locks down millions as its coronavirus deaths jump. Reuters. 2020 Mar 9.
7. Health care system surge capacity recognition, preparedness, and response. Ann Emerg Med. 2012;59(3):240-1. doi: 10.1016/j.annemergmed.2011.11.030.
8. Pitts SR et al. A cross-sectional study of emergency department boarding practices in the United States. Acad Emerg Med. 2014;21(5):497-503. doi: 10.1111/acem.12375.
9. Health at a Glance 2019. OECD; 2019. doi: 10.1787/4dd50c09-en.
10. Murthy S et al. Care for critically ill patients with COVID-19. JAMA. 2020 Mar. doi: 10.1001/jama.2020.3633.
11. Ly DP et al. The association between hospital margins, quality of care, and closure or other change in operating status. J Gen Intern Med. 2011;26(11):1291-6. doi: 10.1007/s11606-011-1815-5.
Background
As of April 2020, the United States is faced with the early stages of the coronavirus disease 2019 (COVID-19) pandemic. Experts predict up to 60% of the population will become infected with a fatality rate of 1% and a hospitalization rate of approximately 20%. Efforts to suppress viral spread have been unsuccessful as cases are reported in all 50 states, and fatalities are rising. Currently many American hospitals are ill-prepared for a significant increase in their census of critically ill and contagious patients, i.e., hospitals lack adequate surge capacity to safely handle a nationwide outbreak of COVID-19. As seen in other nations such as Italy, China, and Iran, this leads to rationing of life-saving health care and potentially preventable morbidity and mortality.
Introduction
Hospitals will be unable to provide the current standard of care to patients as the rate of infection with coronavirus disease 2019 (COVID-19) escalates. As of April 9, the World Health Organization has confirmed 1,539,118 cases and 89,998 deaths globally; and the Centers for Disease Control and Prevention has confirmed 435,941 cases and 14,865 deaths in the United States.1,2 Experts predict up to 60% of the population will eventually become infected with a fatality rate of about 1% and a hospitalization rate of approximately 20%.3,4
In the United States, with a population of 300 million people, this represents up to 180 million infected, 36 million requiring hospitalization, 11 million requiring intensive care, and 2 million fatalities over the duration of the pandemic. On March 13, President Donald Trump declared a state of national emergency, authorizing $50 billion dollars in emergency health care spending as well as asking every hospital in the country to immediately activate its emergency response plan. The use of isolation and quarantine may space out casualties over time, however high rates and volumes of hospitalizations are still expected.4,5
As the influx of patients afflicted with COVID-19 grows, needs will outstrip hospital resources forcing clinicians to ration beds and supplies. In Italy, China, and Iran, physicians are already faced with these difficult decisions. Antonio Pesenti, head of the Italian Lombardy regional crisis response unit, characterized the change in health care delivery: “We’re now being forced to set up intensive care treatment in corridors, in operating theaters, in recovery rooms. We’ve emptied entire hospital sections to make space for seriously sick people.”6
Surge capacity
Surge capacity is a hospital’s ability to adequately care for a significant influx of patients.7 Since 2011, the American College of Emergency Physicians has published guidelines calling for hospitals to have a surge capacity accounting for infectious disease outbreaks, and demands on supplies, personnel, and physical space.7 Even prior to the development of COVID-19, many hospitals faced emergency department crowding and strains on hospital capacity.8 The Organization for Economic Co-operation and Development (OECD) estimates hospital beds per 1,000 inhabitants at 2.77 for the USA, 3.18 for Italy, 4.34 for China, and 13.05 for Japan.9 Before COVID-19 many American hospitals had an insufficient number of beds. Now, in the initial phase of the pandemic, it is even more important to optimize surge capacity across the American health care system.
Requirements for COVID-19 preparation
To prepare for the increased number of seriously and critically ill patients, individual hospitals and regions must perform a needs assessment. The fundamental disease process of COVID-19 is a contagious viral pneumonia; treatment hinges on four major categories of intervention: spatial isolation (including physical space, beds, partitions, droplet precautions, food, water, and sanitation), oxygenation (including wall and portable oxygen, nasal canulae, and masks), mechanical ventilation (including ventilator machines, tubing, anesthetics, and reliable electrical power) and personnel (including physicians, nurses, technicians, and adequate personal protective equipment).10 In special circumstances and where available, extra corporeal membrane oxygenation may be considered.10 The necessary interventions are summarized in Table 1. 
Emergency, critical care, nursing, and medical leadership should consider what sort of space, personnel, and supplies will be needed to care for a large volume of patients with contagious viral pneumonia at the same time as other hospital patients. Attention should also be given to potential need for morgue expansion. Hospitals must be proactive in procuring supplies and preparing for demands on beds and physical space. Specifically, logistics coordinators should start stockpiling ventilators, oxygen, respiratory equipment, and personal protective equipment. Reallocating supplies from other regions of the hospital such as operating rooms and ambulatory surgery centers may be considered. These resources, particularly ventilators and ventilator supplies, are already in disturbingly limited supply, and they are likely to be single most important limiting factor for survival rates. To prevent regional shortages, stockpiling efforts should ideally be aided by state and federal governments. The production and acquisition of ventilators should be immediately and significantly increased.
Hospitals must additionally prepare for demands for physical space and beds. Techniques to maximize space and bed availability (see Table 2) include discharging patients who do not require hospitalization, and canceling elective procedures and admissions. Additional methods would be to utilize unconventional preexisting spaces such as hallways, operating rooms, recovery rooms, hallways, closed hospital wards, basements, lobbies, cafeterias, and parking lots. Administrators should also consider establishing field hospitals or field wards, such as tents in open spaces and nearby roads. Medical care performed in unconventional environments will need to account for electricity, temperature control, oxygen delivery, and sanitation.

Conclusion
To minimize unnecessary loss of life and suffering, hospitals must expand their surge capacities in preparation for the predictable rise in demand for health care resources related to COVID-19. Numerous hospitals, particularly those that serve low-income and underserved communities, operate with a narrow financial margin.11 Independently preparing for the surge capacity needed to face COVID-19 may be infeasible for several hospitals. As a result, many health care systems will rely on government aid during this period for financial and material support. To maximize preparedness and response, hospitals should ask for and receive aid from the Federal Emergency Management Agency (FEMA), American Red Cross, state governments, and the military; these resources should be mobilized now.
Dr. Blumenberg, Dr. Noble, and Dr. Hendrickson are based in the department of emergency medicine & toxicology, Oregon Health and Science University, Portland.
References
1. Coronavirus disease 2019 (COVID-19) situation report – 60. 2020 Mar 19.
2. Coronavirus disease 2019 (COVID-19) Cases in the U.S. CDC. 2020 Apr 8.
3. Li Q et al. Early transmission dynamics in Wuhan, China, of novel coronavirus–infected pneumonia. N Engl J Med. 2020 Jan. doi: 10.1056/NEJMoa2001316.
4. Anderson RM et al. How will country-based mitigation measures influence the course of the COVID-19 epidemic? Lancet. 2020 Mar. doi: 10.1016/S0140-6736(20)30567-5.
5. Fraser C et al. Factors that make an infectious disease outbreak controllable. Proc Natl Acad Sci U S A. 2004;101(16):6146-51. doi: 10.1073/pnas.0307506101.
6. Mackenzie J and Balmer C. Italy locks down millions as its coronavirus deaths jump. Reuters. 2020 Mar 9.
7. Health care system surge capacity recognition, preparedness, and response. Ann Emerg Med. 2012;59(3):240-1. doi: 10.1016/j.annemergmed.2011.11.030.
8. Pitts SR et al. A cross-sectional study of emergency department boarding practices in the United States. Acad Emerg Med. 2014;21(5):497-503. doi: 10.1111/acem.12375.
9. Health at a Glance 2019. OECD; 2019. doi: 10.1787/4dd50c09-en.
10. Murthy S et al. Care for critically ill patients with COVID-19. JAMA. 2020 Mar. doi: 10.1001/jama.2020.3633.
11. Ly DP et al. The association between hospital margins, quality of care, and closure or other change in operating status. J Gen Intern Med. 2011;26(11):1291-6. doi: 10.1007/s11606-011-1815-5.
See acute hepatitis? Consider COVID-19, N.Y. case suggests
A woman presented to the emergency department with high liver enzyme levels and dark urine. She developed fever on day 2 of care, and then tested positive for the new coronavirus, researchers at Northwell Health, in Hempstead, New York, report.
The authors say the case, published online in the American Journal of Gastroenterology, is the first documented instance of a patient with COVID-19 presenting with acute hepatitis as the primary symptom before developing respiratory symptoms.
Prior data show that the most common early indications of COVID-19 are respiratory symptoms with fever, shortness of breath, sore throat, and cough, and with imaging results consistent with pneumonia. However, liver enzyme abnormalities are not uncommon in the disease course.
“In patients who are now presenting with acute hepatitis, people need to think of COVID,” senior author David Bernstein, MD, chief of the Division of Hepatology at Northwell Health, told Medscape Medical News.
In addition to Bernstein, Praneet Wander, MD, also in Northwell’s hepatology division, and Marcia Epstein, MD, with Northwell’s Department of Infectious Disease, authored the case report.
Bernstein said Northwell currently has the largest number of COVID-19 cases in the nation and that many patients are presenting with abnormal liver test results and COVID-19 symptoms.
He said that anecdotally, colleagues elsewhere in the United States are also reporting the connection.
“It seems to be that the liver enzyme elevations are part and parcel of this disease,” he said.
Case Details
According to the case report, the 59-year-old woman, who lives alone, came to the emergency department with a chief complaint of dark urine. She was given a face mask and was isolated, per protocol.
“She denied cough, sore throat, shortness of breath, diarrhea, nausea, vomiting or abdominal pain,” the authors wrote. She denied having been in contact with someone who was sick.
She had well-controlled HIV, and recent outpatient liver test results were normal. Eighteen hours after she came to the ED, she was admitted, owing to concern regarding rising liver enzyme levels in conjunction with her being HIV positive.
On presentation, her temperature was 98.9° F. There were no skin indications, lungs were normal, and “there was no jaundice, right upper quadrant tenderness, hepatomegaly or splenomegaly.”
Liver enzyme levels were as follows: aspartate aminotransferase (AST), 1230 (IU/L); alanine aminotransferase (ALT), 697 IU/L (normal for both is < 50 IU/L); alkaline phosphatase, 141 IU/L (normal, < 125 IU/L).
The patient tested negative for hepatitis A, B, C, E, cytomegalovirus, and Epstein-Barr virus. A respiratory viral panel and autoimmune markers were normal.
Fever Appeared on Day 2
She was admitted, and 18 hours after she came to the ED, she developed a fever of 102.2° F. A chest x-ray showed interstitial opacities in both lungs.
Nasopharyngeal samples were taken, and polymerase chain reaction test results were positive for the novel coronavirus. The patient was placed on 3 L of oxygen.
On post admission day 4, a 5-day course of hydroxychloroquine (200 mg twice a day) was initiated.
The patient was discharged to home on hospital day 8. The serum bilirubin level was 0.6 mg/dL; AST, 114 IU/L; ALT, 227 IU/L; and alkaline phosphatase, 259 IU/L.
According to Bernstein, it’s hard to tell in what order COVID-19 symptoms occur because people are staying home with other complaints. They may only present to the emergency department after they develop more typical COVID-19 symptoms, such as shortness of breath.
In this case, the patient noticed a darkening of her urine, “but if she had come the next day, she would have had fever. I think we just happened to catch it early,” Bernstein said.
He added that he saw no connection between the underlying HIV and her liver abnormalities or COVID-19 diagnosis.
Bernstein notes that most COVID-19 patients are not admitted, and he said he worries that a COVID-19 test might not be on the radar of providers in the outpatient setting when a patient presents with elevated liver enzymes levels.
If elevated liver enzyme levels can predict disease course, the information could alter how and where the disease is treated, Bernstein said.
“This is a first report. We’re really right now in the beginning of learning,” he said.
This article first appeared on Medscape.com.
A woman presented to the emergency department with high liver enzyme levels and dark urine. She developed fever on day 2 of care, and then tested positive for the new coronavirus, researchers at Northwell Health, in Hempstead, New York, report.
The authors say the case, published online in the American Journal of Gastroenterology, is the first documented instance of a patient with COVID-19 presenting with acute hepatitis as the primary symptom before developing respiratory symptoms.
Prior data show that the most common early indications of COVID-19 are respiratory symptoms with fever, shortness of breath, sore throat, and cough, and with imaging results consistent with pneumonia. However, liver enzyme abnormalities are not uncommon in the disease course.
“In patients who are now presenting with acute hepatitis, people need to think of COVID,” senior author David Bernstein, MD, chief of the Division of Hepatology at Northwell Health, told Medscape Medical News.
In addition to Bernstein, Praneet Wander, MD, also in Northwell’s hepatology division, and Marcia Epstein, MD, with Northwell’s Department of Infectious Disease, authored the case report.
Bernstein said Northwell currently has the largest number of COVID-19 cases in the nation and that many patients are presenting with abnormal liver test results and COVID-19 symptoms.
He said that anecdotally, colleagues elsewhere in the United States are also reporting the connection.
“It seems to be that the liver enzyme elevations are part and parcel of this disease,” he said.
Case Details
According to the case report, the 59-year-old woman, who lives alone, came to the emergency department with a chief complaint of dark urine. She was given a face mask and was isolated, per protocol.
“She denied cough, sore throat, shortness of breath, diarrhea, nausea, vomiting or abdominal pain,” the authors wrote. She denied having been in contact with someone who was sick.
She had well-controlled HIV, and recent outpatient liver test results were normal. Eighteen hours after she came to the ED, she was admitted, owing to concern regarding rising liver enzyme levels in conjunction with her being HIV positive.
On presentation, her temperature was 98.9° F. There were no skin indications, lungs were normal, and “there was no jaundice, right upper quadrant tenderness, hepatomegaly or splenomegaly.”
Liver enzyme levels were as follows: aspartate aminotransferase (AST), 1230 (IU/L); alanine aminotransferase (ALT), 697 IU/L (normal for both is < 50 IU/L); alkaline phosphatase, 141 IU/L (normal, < 125 IU/L).
The patient tested negative for hepatitis A, B, C, E, cytomegalovirus, and Epstein-Barr virus. A respiratory viral panel and autoimmune markers were normal.
Fever Appeared on Day 2
She was admitted, and 18 hours after she came to the ED, she developed a fever of 102.2° F. A chest x-ray showed interstitial opacities in both lungs.
Nasopharyngeal samples were taken, and polymerase chain reaction test results were positive for the novel coronavirus. The patient was placed on 3 L of oxygen.
On post admission day 4, a 5-day course of hydroxychloroquine (200 mg twice a day) was initiated.
The patient was discharged to home on hospital day 8. The serum bilirubin level was 0.6 mg/dL; AST, 114 IU/L; ALT, 227 IU/L; and alkaline phosphatase, 259 IU/L.
According to Bernstein, it’s hard to tell in what order COVID-19 symptoms occur because people are staying home with other complaints. They may only present to the emergency department after they develop more typical COVID-19 symptoms, such as shortness of breath.
In this case, the patient noticed a darkening of her urine, “but if she had come the next day, she would have had fever. I think we just happened to catch it early,” Bernstein said.
He added that he saw no connection between the underlying HIV and her liver abnormalities or COVID-19 diagnosis.
Bernstein notes that most COVID-19 patients are not admitted, and he said he worries that a COVID-19 test might not be on the radar of providers in the outpatient setting when a patient presents with elevated liver enzymes levels.
If elevated liver enzyme levels can predict disease course, the information could alter how and where the disease is treated, Bernstein said.
“This is a first report. We’re really right now in the beginning of learning,” he said.
This article first appeared on Medscape.com.
A woman presented to the emergency department with high liver enzyme levels and dark urine. She developed fever on day 2 of care, and then tested positive for the new coronavirus, researchers at Northwell Health, in Hempstead, New York, report.
The authors say the case, published online in the American Journal of Gastroenterology, is the first documented instance of a patient with COVID-19 presenting with acute hepatitis as the primary symptom before developing respiratory symptoms.
Prior data show that the most common early indications of COVID-19 are respiratory symptoms with fever, shortness of breath, sore throat, and cough, and with imaging results consistent with pneumonia. However, liver enzyme abnormalities are not uncommon in the disease course.
“In patients who are now presenting with acute hepatitis, people need to think of COVID,” senior author David Bernstein, MD, chief of the Division of Hepatology at Northwell Health, told Medscape Medical News.
In addition to Bernstein, Praneet Wander, MD, also in Northwell’s hepatology division, and Marcia Epstein, MD, with Northwell’s Department of Infectious Disease, authored the case report.
Bernstein said Northwell currently has the largest number of COVID-19 cases in the nation and that many patients are presenting with abnormal liver test results and COVID-19 symptoms.
He said that anecdotally, colleagues elsewhere in the United States are also reporting the connection.
“It seems to be that the liver enzyme elevations are part and parcel of this disease,” he said.
Case Details
According to the case report, the 59-year-old woman, who lives alone, came to the emergency department with a chief complaint of dark urine. She was given a face mask and was isolated, per protocol.
“She denied cough, sore throat, shortness of breath, diarrhea, nausea, vomiting or abdominal pain,” the authors wrote. She denied having been in contact with someone who was sick.
She had well-controlled HIV, and recent outpatient liver test results were normal. Eighteen hours after she came to the ED, she was admitted, owing to concern regarding rising liver enzyme levels in conjunction with her being HIV positive.
On presentation, her temperature was 98.9° F. There were no skin indications, lungs were normal, and “there was no jaundice, right upper quadrant tenderness, hepatomegaly or splenomegaly.”
Liver enzyme levels were as follows: aspartate aminotransferase (AST), 1230 (IU/L); alanine aminotransferase (ALT), 697 IU/L (normal for both is < 50 IU/L); alkaline phosphatase, 141 IU/L (normal, < 125 IU/L).
The patient tested negative for hepatitis A, B, C, E, cytomegalovirus, and Epstein-Barr virus. A respiratory viral panel and autoimmune markers were normal.
Fever Appeared on Day 2
She was admitted, and 18 hours after she came to the ED, she developed a fever of 102.2° F. A chest x-ray showed interstitial opacities in both lungs.
Nasopharyngeal samples were taken, and polymerase chain reaction test results were positive for the novel coronavirus. The patient was placed on 3 L of oxygen.
On post admission day 4, a 5-day course of hydroxychloroquine (200 mg twice a day) was initiated.
The patient was discharged to home on hospital day 8. The serum bilirubin level was 0.6 mg/dL; AST, 114 IU/L; ALT, 227 IU/L; and alkaline phosphatase, 259 IU/L.
According to Bernstein, it’s hard to tell in what order COVID-19 symptoms occur because people are staying home with other complaints. They may only present to the emergency department after they develop more typical COVID-19 symptoms, such as shortness of breath.
In this case, the patient noticed a darkening of her urine, “but if she had come the next day, she would have had fever. I think we just happened to catch it early,” Bernstein said.
He added that he saw no connection between the underlying HIV and her liver abnormalities or COVID-19 diagnosis.
Bernstein notes that most COVID-19 patients are not admitted, and he said he worries that a COVID-19 test might not be on the radar of providers in the outpatient setting when a patient presents with elevated liver enzymes levels.
If elevated liver enzyme levels can predict disease course, the information could alter how and where the disease is treated, Bernstein said.
“This is a first report. We’re really right now in the beginning of learning,” he said.
This article first appeared on Medscape.com.
COVID-19: A guide to making telepsychiatry work
Changes prompted by social distancing could last beyond the pandemic
As the coronavirus pandemic persists, insurers and the federal government are making it easier for mental health professionals to deliver safe and effective psychiatric services to patients via Zoom, FaceTime, and other conferencing tools. Many psychiatrists, meanwhile, are embracing telepsychiatry for the first time – in some cases with urgency.
Jay H. Shore, MD, MPH, said in an interview that mental health providers at his medical center have gone entirely virtual in recent weeks.
“The genie is out of the bottle on this,” said Dr. Shore, director of telemedicine at the Helen and Arthur E. Johnson Depression Center and director of telemedicine programming for the department of psychiatry at the University of Colorado at Denver, Aurora. He thinks this is the beginning of a new era that will last beyond the pandemic. “There’s going to be a much wider and diffuse acceptance of telemedicine as we go forward,” he added.
Dr. Shore and several colleagues from across the country offered several tips about factors to consider while learning to use telepsychiatry as a treatment tool.
To start, Dr. Shore advised reviewing the American Psychiatric Association’s Telepsychiatry Practice Guidelines and its Telepsychiatry Toolkit, which include dozens of brief videos about topics such as room lighting and managing the content process.
Another resource is the joint APA–American Academy of Child and Adolescent Psychiatry Telepsychiatry Toolkit, said Shabana Khan, MD, an assistant professor and director of telemedicine for the department of child and adolescent psychiatry at New York University Langone Health.
One of the challenges is managing emergencies long distance. If a patient experiences a mental health emergency in a psychiatrist’s office, the clinician can call 911 or direct staff to seek help. “When they’re at their house,” said Dr. Shore, “it’s a little different.”
Staff members are not present at home offices, for example, and the patient might live in a different city and therefore have a different 911 system. “It’s important to know your protocol about how you plan to handle these emergencies before you start working with the patient,” Dr. Shore said.
Another tip is to ask staff to perform a test session to work out the technical kinks before the first patient appointment. “They can make the connection and make sure there’s a video signal with adequate quality,” Dr. Shore said. Failing to conduct a test run can lead to spending several minutes of a session trying to help patients figure out how to make video conferencing work properly.
“You can spend a lot of time acting as IT support,” he said.
It is important to ensure that virtual visits are not interrupted by technical glitches, Daniel Bristow, MD, said in an interview. If possible, hardwire your laptop or computer to an ethernet cable, said Dr. Bristow, president of the Oregon Psychiatric Physicians Association, the state’s branch of the APA. “This will lead to fewer fluctuations that you could see by using wifi,” said Dr. Bristow, who practices in Portland.
“Initially, I assumed that those with psychotic symptoms might struggle more. But I have been surprised at how well some patients have done,” said Andrew J. McLean, MD, MPH, clinical professor and chair of the department of psychiatry and behavioral science at the University of North Dakota, Grand Forks.
However, it might help to provide additional coaching to those patients, said Dr. Bristow. He offers a warning to these patients: “If you feel like you’re getting messages over the TV, my talking to you may make you feel worse.” However, “in every case, the patient was able to say, ‘I know you’re real.’ One patient even said: ‘I’ve heard these voices from my TV for years. But I know you’re a doctor, and you’re in an office trying to help me.’ ”
Dr. Shore thinks that video meetings have the potential to help psychiatrists and patients form better personal connections than in-person meetings. Patients with anxiety or PTSD, for example, “may feel safer since they’re in their own space, and they have a greater sense of control over the session than being in somebody’s office,” he said.
Dr. Khan agreed. “Some children, such as those with a significant trauma history or with significant anxiety, may feel more comfortable with this modality and may open up more during video sessions,” she said. In addition, “the distance that telepsychiatry provides may also enhance feelings of confidentiality and reduce potential stigma that may be associated with seeking mental health care.”
When it comes to using videoconferencing to treat children, take advantage of interactive features that are available, said Katherine Nguyen Williams, PhD. Zoom’s HIPAA-compliant health care software, for example, offers a “share screen” capability. “It allows for easy interactive activities,” said Dr. Nguyen Williams, director of strategic development and clinical innovation at Rady Children’s Hospital’s department of psychiatry at the University of California, San Diego. “Clinicians can play tic-tac-toe on the screen with the young patients, and they can work on cognitive-behavioral therapy worksheets together on the digital screen. Clinicians can even show a mindfulness video to the patient while actively coaching and giving feedback to the patient as they practice diaphragmatic breathing while viewing the video.
“There are so many more options for making virtual therapy as interactive as face-to-face therapy,” said Dr. Nguyen Williams, who also is an associate clinical professor at the university. “This is the key to getting and keeping the patient engaged in telepsychiatry.”
Despite the many positive aspects of using telepsychiatry as a treatment tool, some negative factors must be considered. “You lose some of the nuances, subtleties in terms of expression, movement, smell, etc.,” said Dr. McLean. “Also, there are rare instances where a part of a physical examination would be appropriate, which also is precluded.”
Videoconferencing software might allow the clinician to zoom in to take a closer look at a patient to look for subtle movements and tremors, Dr. McLean said. And, he added, he has asked nursing staff to check for particular signs and symptoms during visits and to describe them to him. “Still,” Dr. McLean said, “this does not take the place of being there.”
Dr. Shore suggested several other practical considerations. For example, while on a screen, keep the home environment as professional as the office would be, he said. Be clear with family members about the importance of not interrupting and make sure that privacy is maintained. The message should be: “I’m working from home, and I’m not available during these hours,” Dr. Shore said. “You need to be aware that, during this time, I need this for clinical work.”
Dr. Shore reported serving as chief medical officer of AccessCare Services, and receiving royalties from American Psychiatric Association Publishing and Springer. He also is coauthor with Peter Yellowlees, MD, of “Telepsychiatry and Health Technologies: A Guide for Mental Health Professionals” (Arlington, Va.: American Psychiatric Association Publishing, 2018). Dr. Khan and Dr. McLean reported no relevant disclosures. Dr. Bristow reported relationships with MCG Health and Insight + Regroup Telehealth.
For more details about using telepsychiatry in the time of COVID-19, listen to the April 8 Psychcast Masterclass lecture by Dr. Shore.
Changes prompted by social distancing could last beyond the pandemic
Changes prompted by social distancing could last beyond the pandemic
As the coronavirus pandemic persists, insurers and the federal government are making it easier for mental health professionals to deliver safe and effective psychiatric services to patients via Zoom, FaceTime, and other conferencing tools. Many psychiatrists, meanwhile, are embracing telepsychiatry for the first time – in some cases with urgency.
Jay H. Shore, MD, MPH, said in an interview that mental health providers at his medical center have gone entirely virtual in recent weeks.
“The genie is out of the bottle on this,” said Dr. Shore, director of telemedicine at the Helen and Arthur E. Johnson Depression Center and director of telemedicine programming for the department of psychiatry at the University of Colorado at Denver, Aurora. He thinks this is the beginning of a new era that will last beyond the pandemic. “There’s going to be a much wider and diffuse acceptance of telemedicine as we go forward,” he added.
Dr. Shore and several colleagues from across the country offered several tips about factors to consider while learning to use telepsychiatry as a treatment tool.
To start, Dr. Shore advised reviewing the American Psychiatric Association’s Telepsychiatry Practice Guidelines and its Telepsychiatry Toolkit, which include dozens of brief videos about topics such as room lighting and managing the content process.
Another resource is the joint APA–American Academy of Child and Adolescent Psychiatry Telepsychiatry Toolkit, said Shabana Khan, MD, an assistant professor and director of telemedicine for the department of child and adolescent psychiatry at New York University Langone Health.
One of the challenges is managing emergencies long distance. If a patient experiences a mental health emergency in a psychiatrist’s office, the clinician can call 911 or direct staff to seek help. “When they’re at their house,” said Dr. Shore, “it’s a little different.”
Staff members are not present at home offices, for example, and the patient might live in a different city and therefore have a different 911 system. “It’s important to know your protocol about how you plan to handle these emergencies before you start working with the patient,” Dr. Shore said.
Another tip is to ask staff to perform a test session to work out the technical kinks before the first patient appointment. “They can make the connection and make sure there’s a video signal with adequate quality,” Dr. Shore said. Failing to conduct a test run can lead to spending several minutes of a session trying to help patients figure out how to make video conferencing work properly.
“You can spend a lot of time acting as IT support,” he said.
It is important to ensure that virtual visits are not interrupted by technical glitches, Daniel Bristow, MD, said in an interview. If possible, hardwire your laptop or computer to an ethernet cable, said Dr. Bristow, president of the Oregon Psychiatric Physicians Association, the state’s branch of the APA. “This will lead to fewer fluctuations that you could see by using wifi,” said Dr. Bristow, who practices in Portland.
“Initially, I assumed that those with psychotic symptoms might struggle more. But I have been surprised at how well some patients have done,” said Andrew J. McLean, MD, MPH, clinical professor and chair of the department of psychiatry and behavioral science at the University of North Dakota, Grand Forks.
However, it might help to provide additional coaching to those patients, said Dr. Bristow. He offers a warning to these patients: “If you feel like you’re getting messages over the TV, my talking to you may make you feel worse.” However, “in every case, the patient was able to say, ‘I know you’re real.’ One patient even said: ‘I’ve heard these voices from my TV for years. But I know you’re a doctor, and you’re in an office trying to help me.’ ”
Dr. Shore thinks that video meetings have the potential to help psychiatrists and patients form better personal connections than in-person meetings. Patients with anxiety or PTSD, for example, “may feel safer since they’re in their own space, and they have a greater sense of control over the session than being in somebody’s office,” he said.
Dr. Khan agreed. “Some children, such as those with a significant trauma history or with significant anxiety, may feel more comfortable with this modality and may open up more during video sessions,” she said. In addition, “the distance that telepsychiatry provides may also enhance feelings of confidentiality and reduce potential stigma that may be associated with seeking mental health care.”
When it comes to using videoconferencing to treat children, take advantage of interactive features that are available, said Katherine Nguyen Williams, PhD. Zoom’s HIPAA-compliant health care software, for example, offers a “share screen” capability. “It allows for easy interactive activities,” said Dr. Nguyen Williams, director of strategic development and clinical innovation at Rady Children’s Hospital’s department of psychiatry at the University of California, San Diego. “Clinicians can play tic-tac-toe on the screen with the young patients, and they can work on cognitive-behavioral therapy worksheets together on the digital screen. Clinicians can even show a mindfulness video to the patient while actively coaching and giving feedback to the patient as they practice diaphragmatic breathing while viewing the video.
“There are so many more options for making virtual therapy as interactive as face-to-face therapy,” said Dr. Nguyen Williams, who also is an associate clinical professor at the university. “This is the key to getting and keeping the patient engaged in telepsychiatry.”
Despite the many positive aspects of using telepsychiatry as a treatment tool, some negative factors must be considered. “You lose some of the nuances, subtleties in terms of expression, movement, smell, etc.,” said Dr. McLean. “Also, there are rare instances where a part of a physical examination would be appropriate, which also is precluded.”
Videoconferencing software might allow the clinician to zoom in to take a closer look at a patient to look for subtle movements and tremors, Dr. McLean said. And, he added, he has asked nursing staff to check for particular signs and symptoms during visits and to describe them to him. “Still,” Dr. McLean said, “this does not take the place of being there.”
Dr. Shore suggested several other practical considerations. For example, while on a screen, keep the home environment as professional as the office would be, he said. Be clear with family members about the importance of not interrupting and make sure that privacy is maintained. The message should be: “I’m working from home, and I’m not available during these hours,” Dr. Shore said. “You need to be aware that, during this time, I need this for clinical work.”
Dr. Shore reported serving as chief medical officer of AccessCare Services, and receiving royalties from American Psychiatric Association Publishing and Springer. He also is coauthor with Peter Yellowlees, MD, of “Telepsychiatry and Health Technologies: A Guide for Mental Health Professionals” (Arlington, Va.: American Psychiatric Association Publishing, 2018). Dr. Khan and Dr. McLean reported no relevant disclosures. Dr. Bristow reported relationships with MCG Health and Insight + Regroup Telehealth.
For more details about using telepsychiatry in the time of COVID-19, listen to the April 8 Psychcast Masterclass lecture by Dr. Shore.
As the coronavirus pandemic persists, insurers and the federal government are making it easier for mental health professionals to deliver safe and effective psychiatric services to patients via Zoom, FaceTime, and other conferencing tools. Many psychiatrists, meanwhile, are embracing telepsychiatry for the first time – in some cases with urgency.
Jay H. Shore, MD, MPH, said in an interview that mental health providers at his medical center have gone entirely virtual in recent weeks.
“The genie is out of the bottle on this,” said Dr. Shore, director of telemedicine at the Helen and Arthur E. Johnson Depression Center and director of telemedicine programming for the department of psychiatry at the University of Colorado at Denver, Aurora. He thinks this is the beginning of a new era that will last beyond the pandemic. “There’s going to be a much wider and diffuse acceptance of telemedicine as we go forward,” he added.
Dr. Shore and several colleagues from across the country offered several tips about factors to consider while learning to use telepsychiatry as a treatment tool.
To start, Dr. Shore advised reviewing the American Psychiatric Association’s Telepsychiatry Practice Guidelines and its Telepsychiatry Toolkit, which include dozens of brief videos about topics such as room lighting and managing the content process.
Another resource is the joint APA–American Academy of Child and Adolescent Psychiatry Telepsychiatry Toolkit, said Shabana Khan, MD, an assistant professor and director of telemedicine for the department of child and adolescent psychiatry at New York University Langone Health.
One of the challenges is managing emergencies long distance. If a patient experiences a mental health emergency in a psychiatrist’s office, the clinician can call 911 or direct staff to seek help. “When they’re at their house,” said Dr. Shore, “it’s a little different.”
Staff members are not present at home offices, for example, and the patient might live in a different city and therefore have a different 911 system. “It’s important to know your protocol about how you plan to handle these emergencies before you start working with the patient,” Dr. Shore said.
Another tip is to ask staff to perform a test session to work out the technical kinks before the first patient appointment. “They can make the connection and make sure there’s a video signal with adequate quality,” Dr. Shore said. Failing to conduct a test run can lead to spending several minutes of a session trying to help patients figure out how to make video conferencing work properly.
“You can spend a lot of time acting as IT support,” he said.
It is important to ensure that virtual visits are not interrupted by technical glitches, Daniel Bristow, MD, said in an interview. If possible, hardwire your laptop or computer to an ethernet cable, said Dr. Bristow, president of the Oregon Psychiatric Physicians Association, the state’s branch of the APA. “This will lead to fewer fluctuations that you could see by using wifi,” said Dr. Bristow, who practices in Portland.
“Initially, I assumed that those with psychotic symptoms might struggle more. But I have been surprised at how well some patients have done,” said Andrew J. McLean, MD, MPH, clinical professor and chair of the department of psychiatry and behavioral science at the University of North Dakota, Grand Forks.
However, it might help to provide additional coaching to those patients, said Dr. Bristow. He offers a warning to these patients: “If you feel like you’re getting messages over the TV, my talking to you may make you feel worse.” However, “in every case, the patient was able to say, ‘I know you’re real.’ One patient even said: ‘I’ve heard these voices from my TV for years. But I know you’re a doctor, and you’re in an office trying to help me.’ ”
Dr. Shore thinks that video meetings have the potential to help psychiatrists and patients form better personal connections than in-person meetings. Patients with anxiety or PTSD, for example, “may feel safer since they’re in their own space, and they have a greater sense of control over the session than being in somebody’s office,” he said.
Dr. Khan agreed. “Some children, such as those with a significant trauma history or with significant anxiety, may feel more comfortable with this modality and may open up more during video sessions,” she said. In addition, “the distance that telepsychiatry provides may also enhance feelings of confidentiality and reduce potential stigma that may be associated with seeking mental health care.”
When it comes to using videoconferencing to treat children, take advantage of interactive features that are available, said Katherine Nguyen Williams, PhD. Zoom’s HIPAA-compliant health care software, for example, offers a “share screen” capability. “It allows for easy interactive activities,” said Dr. Nguyen Williams, director of strategic development and clinical innovation at Rady Children’s Hospital’s department of psychiatry at the University of California, San Diego. “Clinicians can play tic-tac-toe on the screen with the young patients, and they can work on cognitive-behavioral therapy worksheets together on the digital screen. Clinicians can even show a mindfulness video to the patient while actively coaching and giving feedback to the patient as they practice diaphragmatic breathing while viewing the video.
“There are so many more options for making virtual therapy as interactive as face-to-face therapy,” said Dr. Nguyen Williams, who also is an associate clinical professor at the university. “This is the key to getting and keeping the patient engaged in telepsychiatry.”
Despite the many positive aspects of using telepsychiatry as a treatment tool, some negative factors must be considered. “You lose some of the nuances, subtleties in terms of expression, movement, smell, etc.,” said Dr. McLean. “Also, there are rare instances where a part of a physical examination would be appropriate, which also is precluded.”
Videoconferencing software might allow the clinician to zoom in to take a closer look at a patient to look for subtle movements and tremors, Dr. McLean said. And, he added, he has asked nursing staff to check for particular signs and symptoms during visits and to describe them to him. “Still,” Dr. McLean said, “this does not take the place of being there.”
Dr. Shore suggested several other practical considerations. For example, while on a screen, keep the home environment as professional as the office would be, he said. Be clear with family members about the importance of not interrupting and make sure that privacy is maintained. The message should be: “I’m working from home, and I’m not available during these hours,” Dr. Shore said. “You need to be aware that, during this time, I need this for clinical work.”
Dr. Shore reported serving as chief medical officer of AccessCare Services, and receiving royalties from American Psychiatric Association Publishing and Springer. He also is coauthor with Peter Yellowlees, MD, of “Telepsychiatry and Health Technologies: A Guide for Mental Health Professionals” (Arlington, Va.: American Psychiatric Association Publishing, 2018). Dr. Khan and Dr. McLean reported no relevant disclosures. Dr. Bristow reported relationships with MCG Health and Insight + Regroup Telehealth.
For more details about using telepsychiatry in the time of COVID-19, listen to the April 8 Psychcast Masterclass lecture by Dr. Shore.
COVID-19: Dramatic changes to telepsychiatry rules and regs
In the wake of the coronavirus pandemic,
Under the 1135 emergency waiver, Medicare has expanded telehealth services to include patients across the country – not just in rural areas or under other limited conditions, as was previously the case. In addition, there’s now a waiver to the Ryan Haight Act that allows the prescribing of controlled substances via telemedicine.
Peter Yellowlees, MD, from University of California, Davis, reported that outpatient service at his center was converted to an almost 100% telepsychiatry service from mid- to late March.
He and John Torous, MD, director of digital psychiatry at Beth Israel Deaconess Medical Center, Boston, led a free webinar late last month sponsored by the Substance Abuse and Mental Health Services Administration (SAMHSA).
During the hour-long event, they answered questions and offered tips on changes in licensure, patient safety, new prescribing rules, and equipment needed.
“Clinicians need to be aware of these changes so they can ensure they are reaching as many people as possible and taking advantage of the reduced barriers to offering safe and effective video visits,” Dr. Torous said in an interview.
‘This is huge’
The new 1135 waiver “basically says CMS will pay for any patient on Medicare who is seen by video by any provider who is correctly licensed in any state in this country,” Dr. Yellowlees told webinar attendees.
“You don’t need to be licensed in the state where the patient is if the patient is on Medicare. This opens up a huge number of patients we can now see on video,” he said. “And you can bill at normal Medicare rates for whatever you normally get for your in-person patients.”
Although this temporary rule only applies to Medicare and not to private insurers, or to patients on Medicaid, “these are really big changes. This is huge,” Dr. Torous said.
Previously, the “originating site” rule stated that, for the most part, clinicians had to be licensed in the state where the patient was located and not where the physician was stationed.
Asked about college students receiving mental health care who were in school in the psychiatrist’s area but are now back home in a state where the clinician doesn’t have a license, Dr. Yellowlees said that scenario could be a bit “tricky.”
“Most of those patients probably aren’t on Medicare. Legally, you [usually] can’t see them on video if they have private insurance or Medicaid. So, hopefully you can give them a 3-month supply of medication and then recommend they see a local provider,” he said.
Still, all states have their own rules, Dr. Yellowlees said. He and Dr. Torous noted that the Federation of State Medical Boards has a “very up-to-date” listing of policies at FSMB.org, all of which are organized by state. In addition, the American Psychiatric Association provides a telepsychiatry toolkit on its website.
Ryan Haight Act and prescribing
Physicians are now permitted to prescribe medication to patients assessed via telemedicine.
For those with substance use disorders, the U.S. Drug Enforcement Administration has announced a new waiver for the Ryan Haight Online Pharmacy Consumer Protection Act.
The waiver states that “practitioners in all areas of the United States may issue prescriptions for all schedule II-V controlled substances” – as long as it’s for a legitimate medical purpose; real-time, two-way interactive communication with patients has been used; and the clinician “is acting in accordance with applicable Federal and State laws.”
“It’s now possible to prescribe all the normal psychiatric drugs but also benzodiazepines, stimulants, and potentially narcotics over telepsychiatry,” even at a first visit via video, Dr. Yellowlees said.
However, he noted at this point the waiver is current for only 60 days. “This isn’t a permanent condition. It could be extended or even shortened at any given time.”
In addition, SAMHSA has relaxed some of its own regulations regarding telehealth and opioid treatment programs. An FAQ section on the organization’s website provides guidance for providing methadone and buprenorphine treatment.
“Some of the previous regulations will probably be put back in place later on, but the new changes are helpful now,” Dr. Yellowlees said.
Simple equipment needed
Regarding equipment, Dr. Yellowlees noted that the most important component is just a laptop, tablet, or smartphone – for the clinician and for the patient.
“You don’t need fancy new technology with a separate camera or microphone,” he said. However, it might be worth investing in a little better system down the line, he added.
Simple platforms that can be used to meet virtually with patients include FaceTime, Google Hangouts, and Skype.
Although some of these (such as FaceTime) are not HIPAA compliant, “that’s okay for now” under the new rules, Dr. Yellowlees said. While the health system/commercial version of Skype is compliant, the normal consumer-downloaded version is not, he noted.
“I would still strongly suggest using HIPAA-compliant video-conferencing programs in the long run,” he added.
Either way, it’s important for various safety practices to be put into place. For example, clinicians should be careful because the consumer version of Skype can show names of patients who were previously spoken with.
A business associate agreement (BAA) is something that HIPAA-compliant video systems will offer and which should be signed. It’s an agreement that “you’ll be, essentially, looking through a tunnel at the persona at the other end, and the company cannot get inside the tunnel and watch you while you’re having your interview,” said Dr. Yellowlees.
“There are multiple videoconferencing systems around that you can use,” he added. “The three major ones are from Zoom, Vidyo, and VSee, but there are probably 40 or 50 more.”
“There are a lot out there, and we’re certainly not endorsing any one of them,” Dr. Torous added.
When evaluating potential programs, Dr. Yellowlees suggested looking at Yelp-style reviews or telemedicine review sites, or talk with colleagues.
“Basically, you want systems that offer high-definition video quality and the ability to ‘lock’ and ‘unlock’ the rooms. And you want it to have an app so mobile devices can use it,” he said.
Phone vs. video
Some patients, especially older ones, may be resistant to the idea of video chats, preferring to talk via telephone instead.
“If you can use video, it’s better to do that if you can, especially when setting up the systems are relatively simple,” Dr. Yellowlees said, adding that it might just be an issue of patients needing help to get started.
However, “for some people, this is a barrier that we have to respect,” Dr. Torous said.
Either way, clinicians should check the American Medical Association’s website for information about coding for both video and phone visits.
Asked whether a clinician needs written consent from patients for conducting telepsychiatry visits, Dr. Yellowlees said it’s important to check state-by-state rules. For example, California allows a verbal consent.
In many cases, “simply jot down a note that consent was given and how” and write down the address where the patient is located at time of visit, such as for their home, he said.
If a patient wants to conduct a telehealth session while in their car, Dr. Yellowlees suggested getting the address of the parking lot. For safety, clinicians also are advised asking for the cell phone number of the patient as well as that of a loved one.
Vital signs
When it comes to checking vital signs, Dr. Yellowlees suggested asking patients to purchase an inexpensive blood pressure (BP) monitor, thermometer, etc, prior to an appointment.
“Ask them to do a BP test on video and show you the readings. For the AIMS [Abnormal Involuntary Movement Scale] test, or to check for tardive dyskinesia, instruct patients to come close to the camera to show movement.”
In addition, most psychiatric rating scales are available online, which patients can fill out before a telehealth visit. The Serious Mental Illness (SMI) Adviser mobile app also includes several of these scales, Dr. Torous noted.
Overall, “there have been dramatic changes in the rules and regulations governing [telepsychiatry] that, for the next 60 days, make it easier to offer telehealth to patients,” Dr. Torous said.
Therefore, all psychiatrists need to “get on board,” as soon as possible, Dr. Yellowlees added.
The webinar was funded in part by a grant from SAMHSA.
A version of this article originally appeared on Medscape.com.
In the wake of the coronavirus pandemic,
Under the 1135 emergency waiver, Medicare has expanded telehealth services to include patients across the country – not just in rural areas or under other limited conditions, as was previously the case. In addition, there’s now a waiver to the Ryan Haight Act that allows the prescribing of controlled substances via telemedicine.
Peter Yellowlees, MD, from University of California, Davis, reported that outpatient service at his center was converted to an almost 100% telepsychiatry service from mid- to late March.
He and John Torous, MD, director of digital psychiatry at Beth Israel Deaconess Medical Center, Boston, led a free webinar late last month sponsored by the Substance Abuse and Mental Health Services Administration (SAMHSA).
During the hour-long event, they answered questions and offered tips on changes in licensure, patient safety, new prescribing rules, and equipment needed.
“Clinicians need to be aware of these changes so they can ensure they are reaching as many people as possible and taking advantage of the reduced barriers to offering safe and effective video visits,” Dr. Torous said in an interview.
‘This is huge’
The new 1135 waiver “basically says CMS will pay for any patient on Medicare who is seen by video by any provider who is correctly licensed in any state in this country,” Dr. Yellowlees told webinar attendees.
“You don’t need to be licensed in the state where the patient is if the patient is on Medicare. This opens up a huge number of patients we can now see on video,” he said. “And you can bill at normal Medicare rates for whatever you normally get for your in-person patients.”
Although this temporary rule only applies to Medicare and not to private insurers, or to patients on Medicaid, “these are really big changes. This is huge,” Dr. Torous said.
Previously, the “originating site” rule stated that, for the most part, clinicians had to be licensed in the state where the patient was located and not where the physician was stationed.
Asked about college students receiving mental health care who were in school in the psychiatrist’s area but are now back home in a state where the clinician doesn’t have a license, Dr. Yellowlees said that scenario could be a bit “tricky.”
“Most of those patients probably aren’t on Medicare. Legally, you [usually] can’t see them on video if they have private insurance or Medicaid. So, hopefully you can give them a 3-month supply of medication and then recommend they see a local provider,” he said.
Still, all states have their own rules, Dr. Yellowlees said. He and Dr. Torous noted that the Federation of State Medical Boards has a “very up-to-date” listing of policies at FSMB.org, all of which are organized by state. In addition, the American Psychiatric Association provides a telepsychiatry toolkit on its website.
Ryan Haight Act and prescribing
Physicians are now permitted to prescribe medication to patients assessed via telemedicine.
For those with substance use disorders, the U.S. Drug Enforcement Administration has announced a new waiver for the Ryan Haight Online Pharmacy Consumer Protection Act.
The waiver states that “practitioners in all areas of the United States may issue prescriptions for all schedule II-V controlled substances” – as long as it’s for a legitimate medical purpose; real-time, two-way interactive communication with patients has been used; and the clinician “is acting in accordance with applicable Federal and State laws.”
“It’s now possible to prescribe all the normal psychiatric drugs but also benzodiazepines, stimulants, and potentially narcotics over telepsychiatry,” even at a first visit via video, Dr. Yellowlees said.
However, he noted at this point the waiver is current for only 60 days. “This isn’t a permanent condition. It could be extended or even shortened at any given time.”
In addition, SAMHSA has relaxed some of its own regulations regarding telehealth and opioid treatment programs. An FAQ section on the organization’s website provides guidance for providing methadone and buprenorphine treatment.
“Some of the previous regulations will probably be put back in place later on, but the new changes are helpful now,” Dr. Yellowlees said.
Simple equipment needed
Regarding equipment, Dr. Yellowlees noted that the most important component is just a laptop, tablet, or smartphone – for the clinician and for the patient.
“You don’t need fancy new technology with a separate camera or microphone,” he said. However, it might be worth investing in a little better system down the line, he added.
Simple platforms that can be used to meet virtually with patients include FaceTime, Google Hangouts, and Skype.
Although some of these (such as FaceTime) are not HIPAA compliant, “that’s okay for now” under the new rules, Dr. Yellowlees said. While the health system/commercial version of Skype is compliant, the normal consumer-downloaded version is not, he noted.
“I would still strongly suggest using HIPAA-compliant video-conferencing programs in the long run,” he added.
Either way, it’s important for various safety practices to be put into place. For example, clinicians should be careful because the consumer version of Skype can show names of patients who were previously spoken with.
A business associate agreement (BAA) is something that HIPAA-compliant video systems will offer and which should be signed. It’s an agreement that “you’ll be, essentially, looking through a tunnel at the persona at the other end, and the company cannot get inside the tunnel and watch you while you’re having your interview,” said Dr. Yellowlees.
“There are multiple videoconferencing systems around that you can use,” he added. “The three major ones are from Zoom, Vidyo, and VSee, but there are probably 40 or 50 more.”
“There are a lot out there, and we’re certainly not endorsing any one of them,” Dr. Torous added.
When evaluating potential programs, Dr. Yellowlees suggested looking at Yelp-style reviews or telemedicine review sites, or talk with colleagues.
“Basically, you want systems that offer high-definition video quality and the ability to ‘lock’ and ‘unlock’ the rooms. And you want it to have an app so mobile devices can use it,” he said.
Phone vs. video
Some patients, especially older ones, may be resistant to the idea of video chats, preferring to talk via telephone instead.
“If you can use video, it’s better to do that if you can, especially when setting up the systems are relatively simple,” Dr. Yellowlees said, adding that it might just be an issue of patients needing help to get started.
However, “for some people, this is a barrier that we have to respect,” Dr. Torous said.
Either way, clinicians should check the American Medical Association’s website for information about coding for both video and phone visits.
Asked whether a clinician needs written consent from patients for conducting telepsychiatry visits, Dr. Yellowlees said it’s important to check state-by-state rules. For example, California allows a verbal consent.
In many cases, “simply jot down a note that consent was given and how” and write down the address where the patient is located at time of visit, such as for their home, he said.
If a patient wants to conduct a telehealth session while in their car, Dr. Yellowlees suggested getting the address of the parking lot. For safety, clinicians also are advised asking for the cell phone number of the patient as well as that of a loved one.
Vital signs
When it comes to checking vital signs, Dr. Yellowlees suggested asking patients to purchase an inexpensive blood pressure (BP) monitor, thermometer, etc, prior to an appointment.
“Ask them to do a BP test on video and show you the readings. For the AIMS [Abnormal Involuntary Movement Scale] test, or to check for tardive dyskinesia, instruct patients to come close to the camera to show movement.”
In addition, most psychiatric rating scales are available online, which patients can fill out before a telehealth visit. The Serious Mental Illness (SMI) Adviser mobile app also includes several of these scales, Dr. Torous noted.
Overall, “there have been dramatic changes in the rules and regulations governing [telepsychiatry] that, for the next 60 days, make it easier to offer telehealth to patients,” Dr. Torous said.
Therefore, all psychiatrists need to “get on board,” as soon as possible, Dr. Yellowlees added.
The webinar was funded in part by a grant from SAMHSA.
A version of this article originally appeared on Medscape.com.
In the wake of the coronavirus pandemic,
Under the 1135 emergency waiver, Medicare has expanded telehealth services to include patients across the country – not just in rural areas or under other limited conditions, as was previously the case. In addition, there’s now a waiver to the Ryan Haight Act that allows the prescribing of controlled substances via telemedicine.
Peter Yellowlees, MD, from University of California, Davis, reported that outpatient service at his center was converted to an almost 100% telepsychiatry service from mid- to late March.
He and John Torous, MD, director of digital psychiatry at Beth Israel Deaconess Medical Center, Boston, led a free webinar late last month sponsored by the Substance Abuse and Mental Health Services Administration (SAMHSA).
During the hour-long event, they answered questions and offered tips on changes in licensure, patient safety, new prescribing rules, and equipment needed.
“Clinicians need to be aware of these changes so they can ensure they are reaching as many people as possible and taking advantage of the reduced barriers to offering safe and effective video visits,” Dr. Torous said in an interview.
‘This is huge’
The new 1135 waiver “basically says CMS will pay for any patient on Medicare who is seen by video by any provider who is correctly licensed in any state in this country,” Dr. Yellowlees told webinar attendees.
“You don’t need to be licensed in the state where the patient is if the patient is on Medicare. This opens up a huge number of patients we can now see on video,” he said. “And you can bill at normal Medicare rates for whatever you normally get for your in-person patients.”
Although this temporary rule only applies to Medicare and not to private insurers, or to patients on Medicaid, “these are really big changes. This is huge,” Dr. Torous said.
Previously, the “originating site” rule stated that, for the most part, clinicians had to be licensed in the state where the patient was located and not where the physician was stationed.
Asked about college students receiving mental health care who were in school in the psychiatrist’s area but are now back home in a state where the clinician doesn’t have a license, Dr. Yellowlees said that scenario could be a bit “tricky.”
“Most of those patients probably aren’t on Medicare. Legally, you [usually] can’t see them on video if they have private insurance or Medicaid. So, hopefully you can give them a 3-month supply of medication and then recommend they see a local provider,” he said.
Still, all states have their own rules, Dr. Yellowlees said. He and Dr. Torous noted that the Federation of State Medical Boards has a “very up-to-date” listing of policies at FSMB.org, all of which are organized by state. In addition, the American Psychiatric Association provides a telepsychiatry toolkit on its website.
Ryan Haight Act and prescribing
Physicians are now permitted to prescribe medication to patients assessed via telemedicine.
For those with substance use disorders, the U.S. Drug Enforcement Administration has announced a new waiver for the Ryan Haight Online Pharmacy Consumer Protection Act.
The waiver states that “practitioners in all areas of the United States may issue prescriptions for all schedule II-V controlled substances” – as long as it’s for a legitimate medical purpose; real-time, two-way interactive communication with patients has been used; and the clinician “is acting in accordance with applicable Federal and State laws.”
“It’s now possible to prescribe all the normal psychiatric drugs but also benzodiazepines, stimulants, and potentially narcotics over telepsychiatry,” even at a first visit via video, Dr. Yellowlees said.
However, he noted at this point the waiver is current for only 60 days. “This isn’t a permanent condition. It could be extended or even shortened at any given time.”
In addition, SAMHSA has relaxed some of its own regulations regarding telehealth and opioid treatment programs. An FAQ section on the organization’s website provides guidance for providing methadone and buprenorphine treatment.
“Some of the previous regulations will probably be put back in place later on, but the new changes are helpful now,” Dr. Yellowlees said.
Simple equipment needed
Regarding equipment, Dr. Yellowlees noted that the most important component is just a laptop, tablet, or smartphone – for the clinician and for the patient.
“You don’t need fancy new technology with a separate camera or microphone,” he said. However, it might be worth investing in a little better system down the line, he added.
Simple platforms that can be used to meet virtually with patients include FaceTime, Google Hangouts, and Skype.
Although some of these (such as FaceTime) are not HIPAA compliant, “that’s okay for now” under the new rules, Dr. Yellowlees said. While the health system/commercial version of Skype is compliant, the normal consumer-downloaded version is not, he noted.
“I would still strongly suggest using HIPAA-compliant video-conferencing programs in the long run,” he added.
Either way, it’s important for various safety practices to be put into place. For example, clinicians should be careful because the consumer version of Skype can show names of patients who were previously spoken with.
A business associate agreement (BAA) is something that HIPAA-compliant video systems will offer and which should be signed. It’s an agreement that “you’ll be, essentially, looking through a tunnel at the persona at the other end, and the company cannot get inside the tunnel and watch you while you’re having your interview,” said Dr. Yellowlees.
“There are multiple videoconferencing systems around that you can use,” he added. “The three major ones are from Zoom, Vidyo, and VSee, but there are probably 40 or 50 more.”
“There are a lot out there, and we’re certainly not endorsing any one of them,” Dr. Torous added.
When evaluating potential programs, Dr. Yellowlees suggested looking at Yelp-style reviews or telemedicine review sites, or talk with colleagues.
“Basically, you want systems that offer high-definition video quality and the ability to ‘lock’ and ‘unlock’ the rooms. And you want it to have an app so mobile devices can use it,” he said.
Phone vs. video
Some patients, especially older ones, may be resistant to the idea of video chats, preferring to talk via telephone instead.
“If you can use video, it’s better to do that if you can, especially when setting up the systems are relatively simple,” Dr. Yellowlees said, adding that it might just be an issue of patients needing help to get started.
However, “for some people, this is a barrier that we have to respect,” Dr. Torous said.
Either way, clinicians should check the American Medical Association’s website for information about coding for both video and phone visits.
Asked whether a clinician needs written consent from patients for conducting telepsychiatry visits, Dr. Yellowlees said it’s important to check state-by-state rules. For example, California allows a verbal consent.
In many cases, “simply jot down a note that consent was given and how” and write down the address where the patient is located at time of visit, such as for their home, he said.
If a patient wants to conduct a telehealth session while in their car, Dr. Yellowlees suggested getting the address of the parking lot. For safety, clinicians also are advised asking for the cell phone number of the patient as well as that of a loved one.
Vital signs
When it comes to checking vital signs, Dr. Yellowlees suggested asking patients to purchase an inexpensive blood pressure (BP) monitor, thermometer, etc, prior to an appointment.
“Ask them to do a BP test on video and show you the readings. For the AIMS [Abnormal Involuntary Movement Scale] test, or to check for tardive dyskinesia, instruct patients to come close to the camera to show movement.”
In addition, most psychiatric rating scales are available online, which patients can fill out before a telehealth visit. The Serious Mental Illness (SMI) Adviser mobile app also includes several of these scales, Dr. Torous noted.
Overall, “there have been dramatic changes in the rules and regulations governing [telepsychiatry] that, for the next 60 days, make it easier to offer telehealth to patients,” Dr. Torous said.
Therefore, all psychiatrists need to “get on board,” as soon as possible, Dr. Yellowlees added.
The webinar was funded in part by a grant from SAMHSA.
A version of this article originally appeared on Medscape.com.
Comorbidities the rule in New York’s COVID-19 deaths
In New York state, just over 86% of reported COVID-19 deaths involved at least one comorbidity, according to the state’s department of health.
As of midnight on April 6, there had been 5,489 fatalities caused by COVID-19 in the state, of which 86.2% (4,732) had at least one underlying condition, the New York State Department of Health reported April 7 on its COVID-19 tracker.
The leading comorbidity, seen in 55.4% of all deaths, was hypertension. In comparison, a recent estimate from the U.S. Department of Health & Human Services put the prevalence of high blood pressure at about 45% in the overall adult population.
In New York, the rest of the 10 most common comorbidities in COVID-19 fatalities were diabetes (37.3%), hyperlipidemia (18.5%), coronary artery disease (12.4%), renal disease (11.0%), dementia (9.1%), chronic obstructive pulmonary disease (8.3%), cancer (8.1%), atrial fibrillation (7.1%), and heart failure (7.1%), the NYSDOH said.
Other data on the tracker site show that 63% of all deaths involved a patient who was aged 70 years or older and that 61% of COVID-19 patients who have died in New York were male and 38.8% were female (sex unknown for 0.2%). Among all individuals who have tested positive, 54.8% were male and 44.6% were female (sex unknown for 0.6%).
As of the end of day on April 6, a total of 340,058 persons had been tested in the state and 40.8% (138,863) were positive for the SARS-CoV-2 virus. By county, the highest positive rates are in New York City: Queens at 57.4%, Brooklyn at 52.4%, and the Bronx at 52.3%, according to the NYSDOH.
In New York state, just over 86% of reported COVID-19 deaths involved at least one comorbidity, according to the state’s department of health.

As of midnight on April 6, there had been 5,489 fatalities caused by COVID-19 in the state, of which 86.2% (4,732) had at least one underlying condition, the New York State Department of Health reported April 7 on its COVID-19 tracker.
The leading comorbidity, seen in 55.4% of all deaths, was hypertension. In comparison, a recent estimate from the U.S. Department of Health & Human Services put the prevalence of high blood pressure at about 45% in the overall adult population.
In New York, the rest of the 10 most common comorbidities in COVID-19 fatalities were diabetes (37.3%), hyperlipidemia (18.5%), coronary artery disease (12.4%), renal disease (11.0%), dementia (9.1%), chronic obstructive pulmonary disease (8.3%), cancer (8.1%), atrial fibrillation (7.1%), and heart failure (7.1%), the NYSDOH said.
Other data on the tracker site show that 63% of all deaths involved a patient who was aged 70 years or older and that 61% of COVID-19 patients who have died in New York were male and 38.8% were female (sex unknown for 0.2%). Among all individuals who have tested positive, 54.8% were male and 44.6% were female (sex unknown for 0.6%).
As of the end of day on April 6, a total of 340,058 persons had been tested in the state and 40.8% (138,863) were positive for the SARS-CoV-2 virus. By county, the highest positive rates are in New York City: Queens at 57.4%, Brooklyn at 52.4%, and the Bronx at 52.3%, according to the NYSDOH.
In New York state, just over 86% of reported COVID-19 deaths involved at least one comorbidity, according to the state’s department of health.

As of midnight on April 6, there had been 5,489 fatalities caused by COVID-19 in the state, of which 86.2% (4,732) had at least one underlying condition, the New York State Department of Health reported April 7 on its COVID-19 tracker.
The leading comorbidity, seen in 55.4% of all deaths, was hypertension. In comparison, a recent estimate from the U.S. Department of Health & Human Services put the prevalence of high blood pressure at about 45% in the overall adult population.
In New York, the rest of the 10 most common comorbidities in COVID-19 fatalities were diabetes (37.3%), hyperlipidemia (18.5%), coronary artery disease (12.4%), renal disease (11.0%), dementia (9.1%), chronic obstructive pulmonary disease (8.3%), cancer (8.1%), atrial fibrillation (7.1%), and heart failure (7.1%), the NYSDOH said.
Other data on the tracker site show that 63% of all deaths involved a patient who was aged 70 years or older and that 61% of COVID-19 patients who have died in New York were male and 38.8% were female (sex unknown for 0.2%). Among all individuals who have tested positive, 54.8% were male and 44.6% were female (sex unknown for 0.6%).
As of the end of day on April 6, a total of 340,058 persons had been tested in the state and 40.8% (138,863) were positive for the SARS-CoV-2 virus. By county, the highest positive rates are in New York City: Queens at 57.4%, Brooklyn at 52.4%, and the Bronx at 52.3%, according to the NYSDOH.
SARS-CoV-2 escapes cotton, surgical masks of infected
June 9, 2020 — Editor’s note: The study on which this news story is based has been retracted by the journal. The retraction notice can be found here.
according to Seongman Bae, MD, of the University of Ulsan College of Medicine in Seoul, South Korea, and associates.
The report was published in Annals of Internal Medicine.
Because the COVID-19 pandemic has caused a shortage of N95 and surgical masks, cotton masks have gained interest as a substitute, as surgical masks have been shown to effectively filter influenza virus, the researchers wrote. However, the size of and concentrations of SARS-CoV-2 in aerosols generated during coughing are unknown.
To compare the effectiveness of cotton and surgical masks, a group of patients infected with SARS-CoV-2 coughed into petri dishes while wearing no mask, a surgical mask, and a cotton mask. The mask surfaces were swabbed afterward to assess viral positivity on the mask itself.
The median nasopharyngeal and saliva viral load was 5.66 log copies/mL and 4.00 log copies/mL, respectively. The median viral loads after coughing was 2.56 log copies/mL without a mask, 2.42 log copies/mL with a surgical mask, and 1.85 log copies/mL with a cotton mask. All outer surfaces of the mask were positive for SARS-CoV-2, while most inner surfaces were negative.
The investigators acknowledged that the test did not include N95 masks and does not reflect the actual infection transmission, and that they didn’t know whether cotton or surgical masks shorten the travel distance of droplets while coughing.
“Further study is needed to recommend whether face masks decrease transmission of virus from asymptomatic individuals or those with suspected COVID-19 who are not coughing,” they added.
The study was funded by a grant from the government-wide R&D Fund Project for Infectious Disease Research. The investigators reported that they had no conflicts of interest.
SOURCE: Bae S et al. Ann Intern Med. 2020 Apr 6. doi: 10.7326/M20-1342.
Correction, 4/9/20: The headline of an earlier version of this article misstated a finding of this study. Whether cotton and surgical masks can block transmission was not investigated.
June 9, 2020 — Editor’s note: The study on which this news story is based has been retracted by the journal. The retraction notice can be found here.
according to Seongman Bae, MD, of the University of Ulsan College of Medicine in Seoul, South Korea, and associates.
The report was published in Annals of Internal Medicine.
Because the COVID-19 pandemic has caused a shortage of N95 and surgical masks, cotton masks have gained interest as a substitute, as surgical masks have been shown to effectively filter influenza virus, the researchers wrote. However, the size of and concentrations of SARS-CoV-2 in aerosols generated during coughing are unknown.
To compare the effectiveness of cotton and surgical masks, a group of patients infected with SARS-CoV-2 coughed into petri dishes while wearing no mask, a surgical mask, and a cotton mask. The mask surfaces were swabbed afterward to assess viral positivity on the mask itself.
The median nasopharyngeal and saliva viral load was 5.66 log copies/mL and 4.00 log copies/mL, respectively. The median viral loads after coughing was 2.56 log copies/mL without a mask, 2.42 log copies/mL with a surgical mask, and 1.85 log copies/mL with a cotton mask. All outer surfaces of the mask were positive for SARS-CoV-2, while most inner surfaces were negative.
The investigators acknowledged that the test did not include N95 masks and does not reflect the actual infection transmission, and that they didn’t know whether cotton or surgical masks shorten the travel distance of droplets while coughing.
“Further study is needed to recommend whether face masks decrease transmission of virus from asymptomatic individuals or those with suspected COVID-19 who are not coughing,” they added.
The study was funded by a grant from the government-wide R&D Fund Project for Infectious Disease Research. The investigators reported that they had no conflicts of interest.
SOURCE: Bae S et al. Ann Intern Med. 2020 Apr 6. doi: 10.7326/M20-1342.
Correction, 4/9/20: The headline of an earlier version of this article misstated a finding of this study. Whether cotton and surgical masks can block transmission was not investigated.
June 9, 2020 — Editor’s note: The study on which this news story is based has been retracted by the journal. The retraction notice can be found here.
according to Seongman Bae, MD, of the University of Ulsan College of Medicine in Seoul, South Korea, and associates.
The report was published in Annals of Internal Medicine.
Because the COVID-19 pandemic has caused a shortage of N95 and surgical masks, cotton masks have gained interest as a substitute, as surgical masks have been shown to effectively filter influenza virus, the researchers wrote. However, the size of and concentrations of SARS-CoV-2 in aerosols generated during coughing are unknown.
To compare the effectiveness of cotton and surgical masks, a group of patients infected with SARS-CoV-2 coughed into petri dishes while wearing no mask, a surgical mask, and a cotton mask. The mask surfaces were swabbed afterward to assess viral positivity on the mask itself.
The median nasopharyngeal and saliva viral load was 5.66 log copies/mL and 4.00 log copies/mL, respectively. The median viral loads after coughing was 2.56 log copies/mL without a mask, 2.42 log copies/mL with a surgical mask, and 1.85 log copies/mL with a cotton mask. All outer surfaces of the mask were positive for SARS-CoV-2, while most inner surfaces were negative.
The investigators acknowledged that the test did not include N95 masks and does not reflect the actual infection transmission, and that they didn’t know whether cotton or surgical masks shorten the travel distance of droplets while coughing.
“Further study is needed to recommend whether face masks decrease transmission of virus from asymptomatic individuals or those with suspected COVID-19 who are not coughing,” they added.
The study was funded by a grant from the government-wide R&D Fund Project for Infectious Disease Research. The investigators reported that they had no conflicts of interest.
SOURCE: Bae S et al. Ann Intern Med. 2020 Apr 6. doi: 10.7326/M20-1342.
Correction, 4/9/20: The headline of an earlier version of this article misstated a finding of this study. Whether cotton and surgical masks can block transmission was not investigated.
FROM ANNALS OF INTERNAL MEDICINE
Infected Bronchogenic Cyst With Left Atrial, Pulmonary Artery, and Esophageal Compression
Bronchogenic cyst is a rare foregut malformation that typically presents during the second decade of life that arises due to aberrant development from the tracheobronchial tree.1 Mediastinal bronchogenic cyst is the most common primary cystic lesion of the mediastinum, and bronchogenic cysts of the mediastinum represent 18% of all primary mediastinal malformations.2 Patients with mediastinal bronchogenic cysts may present with symptoms of cough, dyspnea, or wheezing if there is encroachment on surrounding structures.
Rarely, bronchogenic cysts can become infected. Definitive treatment of bronchogenic cysts is surgical excision; however, endobronchial ultrasound (EBUS)-guided drainage also can be employed. EBUS-guided drainage may be used when the cyst cannot be distinguished from solid mass on computed tomography (CT) images, to relieve symptomatic compression of surrounding structures, or to provide a histologic or microbial diagnosis in cases where surgical excision is not immediately available. We present the first-ever described case of bronchogenic cyst infected with Actinomyces, diagnosed by EBUS-guided drainage as well as a review of the literature regarding infected bronchogenic cysts and management of cysts affecting mediastinal structures.
Case Presentation
A 57-year-old African American male presented with a 4-day history of continuous, sharp, substernal chest pain accompanied by dyspnea. Additionally, the patient reported progressive dysphagia to solids. The posteroanterior view of a chest X-ray showed a widened mediastinum with splaying of the carina. A contrast-enhanced CT of the chest showed a large, middle mediastinal mass of heterogenous density measuring 7.3. × 7.0 × 6.0 cm with compression of the right pulmonary artery, left atria, superior vena cava and esophagus (Figure 1).
The mass demonstrated neither clear fluid-fluid level nor rounded structure with a distinct wall and uniform attenuation consistent with pure cystic structure and, in fact, was concerning for malignant process, such as lymphoma. Due to the malignancy concern and the findings of significant compression of surrounding mediastinal structures, the decision was made to proceed with bronchoscopy and EBUS-guided transbronchial needle aspiration (EBUS-TBNA) to assist in diagnosis and potentially provide symptomatic relief.
Under general anesthesia a P160 Olympus bronchoscope was advanced into the tracheobronchial tree; bronchoscopy with airway inspection revealed splayed carina with obtuse angle but was otherwise unremarkable. Next, an EBUS P160 fiber optic Olympus bronchoscope was advanced; ultrasound demonstrated a cystic structure. The EBUS-TBNA of cystic structure yielded 20 mL of brown, purulent fluid with decompression bringing pulmonary artery in ultrasound field (Figure 2). Rapid on-site cytology was performed with no preliminary findings of malignancy. The fluid was then sent for cytology and microbiologic evaluation.
Following EBUS-guided aspiration, the patient reported significant improvement in chest pain, dyspnea, and dysphagia. A repeat chest CT demonstrated decrease in mass size to 5.9 × 5.5 × 4.6 cm with relief of the compression of the right pulmonary artery and decreased mass effect on the carina (Figure 3). Pathology ultimately demonstrated no evidence of malignancy but did demonstrate filamentous material with sulfur granules and anthracotic pigment suggestive of Actinomyces infection (Figure 4).
The patient was placed on amoxicillin/clavulanate 875 mg to 125 mg twice daily for 4 weeks based on antibiotic susceptibility testing to prevent progression to mediastinitis related to Actinomyces infection. The duration of therapy was extrapolated from treatment regimens described in case series of cervicofacial and abdominal Actinomyces infections.3 Thoracic surgery evaluation for definitive excision of cyst was recommended after the patient completed his course of antibiotics.
The patient underwent dental evaluation to identify the source of Actinomyces infection but there appeared to be no odontogenic source. The patient also had extensive skin survey with no findings of overt source of Actinomyces and CT abdomen/pelvis also identified no abscess that could be a potential source. He subsequently underwent thoracoscopic resection with pathology demonstrating a fibrous cyst wall lined with ciliated columnar epithelium consistent with diagnosis of bronchogenic cyst (Figure 5).
Discussion
Bronchogenic cysts can present at birth or later in life; patients may be asymptomatic for decades prior to discovery.4 Cysts located in the mediastinum can cause compression of the trachea and esophagus and cause cough, dyspnea, chest pain, and dysphagia.5 More life-threatening complications include infection, tracheal compression, malignant transformation, superior vena cava syndrome, or spontaneous rupture into the airway.6,7
Infection can occasionally occur, and various bacterial etiologies have been described. Hernandez-Solis and colleagues describe 12 cases of superinfected bronchogenic cysts with Staphylococcus aureus and Pseudomonas aeroginosa, the most commonly described organisms.8 Casal and colleagues describe a case of α-hemolytic Streptococci treated with amoxicillin.9 Liman and colleagues describe 2 cases of bronchogenic cyst infected with Mycobacterium and cite an additional case report by Lin and colleagues similarly infected by Mycobacterium.10,11 Only 1 case was identified to have direct bronchial communication as a potential source of introduction of infection into bronchogenic cyst. In other cases, potential sources of infection were not identified, though it was postulated that direct ventilation could be a potential source of inoculation.
Surgical resection of mediastinal bronchogenic cysts has traditionally been considered the definitive treatment of choice.12,13 However, bronchogenic cysts may sometimes be difficult to differentiate from soft tissue tumors by chest CT, especially in cases of cysts with nonserous fluid. In particular, cysts that are infected are likely to have increased density and high attenuation on imaging; therefore, surgical excision may be delayed until diagnosis is made.14 Due to low complication rates, EBUS is increasingly used in the diagnosis and therapeutic management of bronchogenic cysts as an alternative to surgery, particularly for those who are symptomatic.15,16 Ultrasound guidance can allow for complete aspiration of the cyst, causing complete collapse of the cystic space and can facilitate adhesion between the mucosal surfaces lining the cavity and reduce recurrence.17 Nonetheless, bronchogenic cysts that are found to be infected, recur, or have a malignant component should be resected for definitive treatment.18
The mass discovered on our patient’s imaging appeared to have heterogenous attenuation consistent with malignancy rather than homogenous attenuation surrounded by a clearly demarcated wall consistent with a cystic structure; therefore, EBUS-TBNA was initially pursued and yielded an expedited diagnosis of the first-ever described bronchogenic cyst with Actinomyces superinfection as well as dramatic symptomatic relief of compression of surrounding mediastinal structures, particularly of the right pulmonary artery. As this is a congenital malformation, the patient was likely asymptomatic until the cyst became infected, after which he likely experience cyst growth with subsequent encroachment of surrounding mediastinal structures. Additionally, identification of pathogen by TBNA allowed for treatment before surgical excision, possibly avoiding accidental spread of pathogen intraoperatively.
Conclusions
Our case adds to the literature on the use of EBUS-TBNA as a diagnostic and therapeutic modality for bronchogenic cyst. While cases of mediastinitis and pleural effusion following EBUS-guided aspiration of bronchogenic cysts have been reported, complications are extremely rare.19 EBUS is increasingly favored as a means of immediate diagnosis and treatment in cases where CT imaging may not overtly suggest cystic structure and in patients experiencing compression of critical mediastinal structures.
1. Weber T, Roth TC, Beshay M, Herrmann P, Stein R, Schmid RA. Video-assisted thoracoscopic surgery of mediastinal bronchogenic cysts in adults: a single-center experience. Ann Thorac Surg. 2004;78(3):987-991.
2. Martinod E, Pons F, Azorin J, et al. Thoracoscopic excision of mediastinal bronchogenic cysts: results in 20 cases. Ann Thorac Surg. 2000;69(5):1525-1528.
3. Könönen E, Wade WG. Actinomyces and related organisms in human infections. Clin Microbiol Rev. 2015;28(2):419-442.
4. Ribet ME, Copin MC, Gosselin BH. Bronchogenic cysts of the lung. Ann Thorac Surg. 1996;61(6):1636-1640.
5. Guillem P, Porte H, Marquette CH, Wurtz A. Progressive dysphonia and acute respiratory failure: revealing a bronchogenic cyst. Eur J Cardiothorac Surg. 1997;12(6):925-927.
6. McAdams HP, Kirejczyk WM, Rosado-de-Christenson ML, Matsumoto S. Bronchogenic cyst: imaging features with clinical and histopathologic correlation. Radiology. 2000;217(2):441-446.
7. Rammohan G, Berger HW, Lajam F, Buhain WJ. Superior vena cava syndrome caused by bronchogenic cyst. Chest. 1975;68(4):599-601.
8. Hernández-Solís A, Cruz-Ortiz H, Gutiérrez-Díaz Ceballos ME, Cicero-Sabido R. Quistes broncogénicos. Importancia de la infección en adultos. Estudio de 12 casos [Bronchogenic cysts. Importance of infection in adults. Study of 12 cases]. Cir Cir. 2015;83(2):112-116.
9. Casal RF, Jimenez CA, Mehran RJ, et al. Infected mediastinal bronchogenic cyst successfully treated by endobronchial ultrasound-guided fine-needle aspiration. Ann Thorac Surg. 2010;90(4):e52-e53.
10. Liman ST, Dogan Y, Topcu S, Karabulut N, Demirkan N, Keser Z. Mycobacterial infection of intraparenchymal bronchogenic cysts. Respir Med. 2006;100(11):2060-2062.
11. Lin SH, Lee LN, Chang YL, Lee YC, Ding LW, Hsueh PR. Infected bronchogenic cyst due to Mycobacterium avium in an immunocompetent patient. J Infect. 2005;51(3):e131-e133.
12. Gharagozloo F, Dausmann MJ, McReynolds SD, Sanderson DR, Helmers RA. Recurrent bronchogenic pseudocyst 24 years after incomplete excision. Report of a case. Chest. 1995;108(3):880-883.
13. Bolton JW, Shahian DM. Asymptomatic bronchogenic cysts: what is the best management? Ann Thorac Surg. 1992;53(6):1134-1137.
14. Sarper A, Ayten A, Golbasi I, Demircan A, Isin E. Bronchogenic cyst. Tex Heart Inst J. 2003;30(2):105-108.
15. Varela-Lema L, Fernández-Villar A, Ruano-Ravina A. Effectiveness and safety of endobronchial ultrasound-transbronchial needle aspiration: a systematic review. Eur Respir J. 2009;33(5):1156-1164.
16. Maturu VN, Dhooria S, Agarwal R. Efficacy and safety of transbronchial needle aspiration in diagnosis and treatment of mediastinal bronchogenic cysts: systematic review of case reports. J Bronchology Interv Pulmonol. 2015;22(3):195-203.
17. Galluccio G, Lucantoni G. Mediastinal bronchogenic cyst’s recurrence treated with EBUS-FNA with a long-term follow-up. Eur J Cardiothorac Surg. 2006;29(4):627-629.
18. Lee DH, Park CK, Kum DY, Kim JB, Hwang I. Clinical characteristics and management of intrathoracic bronchogenic cysts: a single center experience. Korean J Thorac Cardiovasc Surg. 2011;44(4):279-284.
19. Onuki T, Kuramochi M, Inagaki M. Mediastinitis of bronchogenic cyst caused by endobronchial ultrasound-guided transbronchial needle aspiration. Respirol Case Rep. 2014;2(2):73-75.
Bronchogenic cyst is a rare foregut malformation that typically presents during the second decade of life that arises due to aberrant development from the tracheobronchial tree.1 Mediastinal bronchogenic cyst is the most common primary cystic lesion of the mediastinum, and bronchogenic cysts of the mediastinum represent 18% of all primary mediastinal malformations.2 Patients with mediastinal bronchogenic cysts may present with symptoms of cough, dyspnea, or wheezing if there is encroachment on surrounding structures.
Rarely, bronchogenic cysts can become infected. Definitive treatment of bronchogenic cysts is surgical excision; however, endobronchial ultrasound (EBUS)-guided drainage also can be employed. EBUS-guided drainage may be used when the cyst cannot be distinguished from solid mass on computed tomography (CT) images, to relieve symptomatic compression of surrounding structures, or to provide a histologic or microbial diagnosis in cases where surgical excision is not immediately available. We present the first-ever described case of bronchogenic cyst infected with Actinomyces, diagnosed by EBUS-guided drainage as well as a review of the literature regarding infected bronchogenic cysts and management of cysts affecting mediastinal structures.
Case Presentation
A 57-year-old African American male presented with a 4-day history of continuous, sharp, substernal chest pain accompanied by dyspnea. Additionally, the patient reported progressive dysphagia to solids. The posteroanterior view of a chest X-ray showed a widened mediastinum with splaying of the carina. A contrast-enhanced CT of the chest showed a large, middle mediastinal mass of heterogenous density measuring 7.3. × 7.0 × 6.0 cm with compression of the right pulmonary artery, left atria, superior vena cava and esophagus (Figure 1).
The mass demonstrated neither clear fluid-fluid level nor rounded structure with a distinct wall and uniform attenuation consistent with pure cystic structure and, in fact, was concerning for malignant process, such as lymphoma. Due to the malignancy concern and the findings of significant compression of surrounding mediastinal structures, the decision was made to proceed with bronchoscopy and EBUS-guided transbronchial needle aspiration (EBUS-TBNA) to assist in diagnosis and potentially provide symptomatic relief.
Under general anesthesia a P160 Olympus bronchoscope was advanced into the tracheobronchial tree; bronchoscopy with airway inspection revealed splayed carina with obtuse angle but was otherwise unremarkable. Next, an EBUS P160 fiber optic Olympus bronchoscope was advanced; ultrasound demonstrated a cystic structure. The EBUS-TBNA of cystic structure yielded 20 mL of brown, purulent fluid with decompression bringing pulmonary artery in ultrasound field (Figure 2). Rapid on-site cytology was performed with no preliminary findings of malignancy. The fluid was then sent for cytology and microbiologic evaluation.
Following EBUS-guided aspiration, the patient reported significant improvement in chest pain, dyspnea, and dysphagia. A repeat chest CT demonstrated decrease in mass size to 5.9 × 5.5 × 4.6 cm with relief of the compression of the right pulmonary artery and decreased mass effect on the carina (Figure 3). Pathology ultimately demonstrated no evidence of malignancy but did demonstrate filamentous material with sulfur granules and anthracotic pigment suggestive of Actinomyces infection (Figure 4).
The patient was placed on amoxicillin/clavulanate 875 mg to 125 mg twice daily for 4 weeks based on antibiotic susceptibility testing to prevent progression to mediastinitis related to Actinomyces infection. The duration of therapy was extrapolated from treatment regimens described in case series of cervicofacial and abdominal Actinomyces infections.3 Thoracic surgery evaluation for definitive excision of cyst was recommended after the patient completed his course of antibiotics.
The patient underwent dental evaluation to identify the source of Actinomyces infection but there appeared to be no odontogenic source. The patient also had extensive skin survey with no findings of overt source of Actinomyces and CT abdomen/pelvis also identified no abscess that could be a potential source. He subsequently underwent thoracoscopic resection with pathology demonstrating a fibrous cyst wall lined with ciliated columnar epithelium consistent with diagnosis of bronchogenic cyst (Figure 5).
Discussion
Bronchogenic cysts can present at birth or later in life; patients may be asymptomatic for decades prior to discovery.4 Cysts located in the mediastinum can cause compression of the trachea and esophagus and cause cough, dyspnea, chest pain, and dysphagia.5 More life-threatening complications include infection, tracheal compression, malignant transformation, superior vena cava syndrome, or spontaneous rupture into the airway.6,7
Infection can occasionally occur, and various bacterial etiologies have been described. Hernandez-Solis and colleagues describe 12 cases of superinfected bronchogenic cysts with Staphylococcus aureus and Pseudomonas aeroginosa, the most commonly described organisms.8 Casal and colleagues describe a case of α-hemolytic Streptococci treated with amoxicillin.9 Liman and colleagues describe 2 cases of bronchogenic cyst infected with Mycobacterium and cite an additional case report by Lin and colleagues similarly infected by Mycobacterium.10,11 Only 1 case was identified to have direct bronchial communication as a potential source of introduction of infection into bronchogenic cyst. In other cases, potential sources of infection were not identified, though it was postulated that direct ventilation could be a potential source of inoculation.
Surgical resection of mediastinal bronchogenic cysts has traditionally been considered the definitive treatment of choice.12,13 However, bronchogenic cysts may sometimes be difficult to differentiate from soft tissue tumors by chest CT, especially in cases of cysts with nonserous fluid. In particular, cysts that are infected are likely to have increased density and high attenuation on imaging; therefore, surgical excision may be delayed until diagnosis is made.14 Due to low complication rates, EBUS is increasingly used in the diagnosis and therapeutic management of bronchogenic cysts as an alternative to surgery, particularly for those who are symptomatic.15,16 Ultrasound guidance can allow for complete aspiration of the cyst, causing complete collapse of the cystic space and can facilitate adhesion between the mucosal surfaces lining the cavity and reduce recurrence.17 Nonetheless, bronchogenic cysts that are found to be infected, recur, or have a malignant component should be resected for definitive treatment.18
The mass discovered on our patient’s imaging appeared to have heterogenous attenuation consistent with malignancy rather than homogenous attenuation surrounded by a clearly demarcated wall consistent with a cystic structure; therefore, EBUS-TBNA was initially pursued and yielded an expedited diagnosis of the first-ever described bronchogenic cyst with Actinomyces superinfection as well as dramatic symptomatic relief of compression of surrounding mediastinal structures, particularly of the right pulmonary artery. As this is a congenital malformation, the patient was likely asymptomatic until the cyst became infected, after which he likely experience cyst growth with subsequent encroachment of surrounding mediastinal structures. Additionally, identification of pathogen by TBNA allowed for treatment before surgical excision, possibly avoiding accidental spread of pathogen intraoperatively.
Conclusions
Our case adds to the literature on the use of EBUS-TBNA as a diagnostic and therapeutic modality for bronchogenic cyst. While cases of mediastinitis and pleural effusion following EBUS-guided aspiration of bronchogenic cysts have been reported, complications are extremely rare.19 EBUS is increasingly favored as a means of immediate diagnosis and treatment in cases where CT imaging may not overtly suggest cystic structure and in patients experiencing compression of critical mediastinal structures.
Bronchogenic cyst is a rare foregut malformation that typically presents during the second decade of life that arises due to aberrant development from the tracheobronchial tree.1 Mediastinal bronchogenic cyst is the most common primary cystic lesion of the mediastinum, and bronchogenic cysts of the mediastinum represent 18% of all primary mediastinal malformations.2 Patients with mediastinal bronchogenic cysts may present with symptoms of cough, dyspnea, or wheezing if there is encroachment on surrounding structures.
Rarely, bronchogenic cysts can become infected. Definitive treatment of bronchogenic cysts is surgical excision; however, endobronchial ultrasound (EBUS)-guided drainage also can be employed. EBUS-guided drainage may be used when the cyst cannot be distinguished from solid mass on computed tomography (CT) images, to relieve symptomatic compression of surrounding structures, or to provide a histologic or microbial diagnosis in cases where surgical excision is not immediately available. We present the first-ever described case of bronchogenic cyst infected with Actinomyces, diagnosed by EBUS-guided drainage as well as a review of the literature regarding infected bronchogenic cysts and management of cysts affecting mediastinal structures.
Case Presentation
A 57-year-old African American male presented with a 4-day history of continuous, sharp, substernal chest pain accompanied by dyspnea. Additionally, the patient reported progressive dysphagia to solids. The posteroanterior view of a chest X-ray showed a widened mediastinum with splaying of the carina. A contrast-enhanced CT of the chest showed a large, middle mediastinal mass of heterogenous density measuring 7.3. × 7.0 × 6.0 cm with compression of the right pulmonary artery, left atria, superior vena cava and esophagus (Figure 1).
The mass demonstrated neither clear fluid-fluid level nor rounded structure with a distinct wall and uniform attenuation consistent with pure cystic structure and, in fact, was concerning for malignant process, such as lymphoma. Due to the malignancy concern and the findings of significant compression of surrounding mediastinal structures, the decision was made to proceed with bronchoscopy and EBUS-guided transbronchial needle aspiration (EBUS-TBNA) to assist in diagnosis and potentially provide symptomatic relief.
Under general anesthesia a P160 Olympus bronchoscope was advanced into the tracheobronchial tree; bronchoscopy with airway inspection revealed splayed carina with obtuse angle but was otherwise unremarkable. Next, an EBUS P160 fiber optic Olympus bronchoscope was advanced; ultrasound demonstrated a cystic structure. The EBUS-TBNA of cystic structure yielded 20 mL of brown, purulent fluid with decompression bringing pulmonary artery in ultrasound field (Figure 2). Rapid on-site cytology was performed with no preliminary findings of malignancy. The fluid was then sent for cytology and microbiologic evaluation.
Following EBUS-guided aspiration, the patient reported significant improvement in chest pain, dyspnea, and dysphagia. A repeat chest CT demonstrated decrease in mass size to 5.9 × 5.5 × 4.6 cm with relief of the compression of the right pulmonary artery and decreased mass effect on the carina (Figure 3). Pathology ultimately demonstrated no evidence of malignancy but did demonstrate filamentous material with sulfur granules and anthracotic pigment suggestive of Actinomyces infection (Figure 4).
The patient was placed on amoxicillin/clavulanate 875 mg to 125 mg twice daily for 4 weeks based on antibiotic susceptibility testing to prevent progression to mediastinitis related to Actinomyces infection. The duration of therapy was extrapolated from treatment regimens described in case series of cervicofacial and abdominal Actinomyces infections.3 Thoracic surgery evaluation for definitive excision of cyst was recommended after the patient completed his course of antibiotics.
The patient underwent dental evaluation to identify the source of Actinomyces infection but there appeared to be no odontogenic source. The patient also had extensive skin survey with no findings of overt source of Actinomyces and CT abdomen/pelvis also identified no abscess that could be a potential source. He subsequently underwent thoracoscopic resection with pathology demonstrating a fibrous cyst wall lined with ciliated columnar epithelium consistent with diagnosis of bronchogenic cyst (Figure 5).
Discussion
Bronchogenic cysts can present at birth or later in life; patients may be asymptomatic for decades prior to discovery.4 Cysts located in the mediastinum can cause compression of the trachea and esophagus and cause cough, dyspnea, chest pain, and dysphagia.5 More life-threatening complications include infection, tracheal compression, malignant transformation, superior vena cava syndrome, or spontaneous rupture into the airway.6,7
Infection can occasionally occur, and various bacterial etiologies have been described. Hernandez-Solis and colleagues describe 12 cases of superinfected bronchogenic cysts with Staphylococcus aureus and Pseudomonas aeroginosa, the most commonly described organisms.8 Casal and colleagues describe a case of α-hemolytic Streptococci treated with amoxicillin.9 Liman and colleagues describe 2 cases of bronchogenic cyst infected with Mycobacterium and cite an additional case report by Lin and colleagues similarly infected by Mycobacterium.10,11 Only 1 case was identified to have direct bronchial communication as a potential source of introduction of infection into bronchogenic cyst. In other cases, potential sources of infection were not identified, though it was postulated that direct ventilation could be a potential source of inoculation.
Surgical resection of mediastinal bronchogenic cysts has traditionally been considered the definitive treatment of choice.12,13 However, bronchogenic cysts may sometimes be difficult to differentiate from soft tissue tumors by chest CT, especially in cases of cysts with nonserous fluid. In particular, cysts that are infected are likely to have increased density and high attenuation on imaging; therefore, surgical excision may be delayed until diagnosis is made.14 Due to low complication rates, EBUS is increasingly used in the diagnosis and therapeutic management of bronchogenic cysts as an alternative to surgery, particularly for those who are symptomatic.15,16 Ultrasound guidance can allow for complete aspiration of the cyst, causing complete collapse of the cystic space and can facilitate adhesion between the mucosal surfaces lining the cavity and reduce recurrence.17 Nonetheless, bronchogenic cysts that are found to be infected, recur, or have a malignant component should be resected for definitive treatment.18
The mass discovered on our patient’s imaging appeared to have heterogenous attenuation consistent with malignancy rather than homogenous attenuation surrounded by a clearly demarcated wall consistent with a cystic structure; therefore, EBUS-TBNA was initially pursued and yielded an expedited diagnosis of the first-ever described bronchogenic cyst with Actinomyces superinfection as well as dramatic symptomatic relief of compression of surrounding mediastinal structures, particularly of the right pulmonary artery. As this is a congenital malformation, the patient was likely asymptomatic until the cyst became infected, after which he likely experience cyst growth with subsequent encroachment of surrounding mediastinal structures. Additionally, identification of pathogen by TBNA allowed for treatment before surgical excision, possibly avoiding accidental spread of pathogen intraoperatively.
Conclusions
Our case adds to the literature on the use of EBUS-TBNA as a diagnostic and therapeutic modality for bronchogenic cyst. While cases of mediastinitis and pleural effusion following EBUS-guided aspiration of bronchogenic cysts have been reported, complications are extremely rare.19 EBUS is increasingly favored as a means of immediate diagnosis and treatment in cases where CT imaging may not overtly suggest cystic structure and in patients experiencing compression of critical mediastinal structures.
1. Weber T, Roth TC, Beshay M, Herrmann P, Stein R, Schmid RA. Video-assisted thoracoscopic surgery of mediastinal bronchogenic cysts in adults: a single-center experience. Ann Thorac Surg. 2004;78(3):987-991.
2. Martinod E, Pons F, Azorin J, et al. Thoracoscopic excision of mediastinal bronchogenic cysts: results in 20 cases. Ann Thorac Surg. 2000;69(5):1525-1528.
3. Könönen E, Wade WG. Actinomyces and related organisms in human infections. Clin Microbiol Rev. 2015;28(2):419-442.
4. Ribet ME, Copin MC, Gosselin BH. Bronchogenic cysts of the lung. Ann Thorac Surg. 1996;61(6):1636-1640.
5. Guillem P, Porte H, Marquette CH, Wurtz A. Progressive dysphonia and acute respiratory failure: revealing a bronchogenic cyst. Eur J Cardiothorac Surg. 1997;12(6):925-927.
6. McAdams HP, Kirejczyk WM, Rosado-de-Christenson ML, Matsumoto S. Bronchogenic cyst: imaging features with clinical and histopathologic correlation. Radiology. 2000;217(2):441-446.
7. Rammohan G, Berger HW, Lajam F, Buhain WJ. Superior vena cava syndrome caused by bronchogenic cyst. Chest. 1975;68(4):599-601.
8. Hernández-Solís A, Cruz-Ortiz H, Gutiérrez-Díaz Ceballos ME, Cicero-Sabido R. Quistes broncogénicos. Importancia de la infección en adultos. Estudio de 12 casos [Bronchogenic cysts. Importance of infection in adults. Study of 12 cases]. Cir Cir. 2015;83(2):112-116.
9. Casal RF, Jimenez CA, Mehran RJ, et al. Infected mediastinal bronchogenic cyst successfully treated by endobronchial ultrasound-guided fine-needle aspiration. Ann Thorac Surg. 2010;90(4):e52-e53.
10. Liman ST, Dogan Y, Topcu S, Karabulut N, Demirkan N, Keser Z. Mycobacterial infection of intraparenchymal bronchogenic cysts. Respir Med. 2006;100(11):2060-2062.
11. Lin SH, Lee LN, Chang YL, Lee YC, Ding LW, Hsueh PR. Infected bronchogenic cyst due to Mycobacterium avium in an immunocompetent patient. J Infect. 2005;51(3):e131-e133.
12. Gharagozloo F, Dausmann MJ, McReynolds SD, Sanderson DR, Helmers RA. Recurrent bronchogenic pseudocyst 24 years after incomplete excision. Report of a case. Chest. 1995;108(3):880-883.
13. Bolton JW, Shahian DM. Asymptomatic bronchogenic cysts: what is the best management? Ann Thorac Surg. 1992;53(6):1134-1137.
14. Sarper A, Ayten A, Golbasi I, Demircan A, Isin E. Bronchogenic cyst. Tex Heart Inst J. 2003;30(2):105-108.
15. Varela-Lema L, Fernández-Villar A, Ruano-Ravina A. Effectiveness and safety of endobronchial ultrasound-transbronchial needle aspiration: a systematic review. Eur Respir J. 2009;33(5):1156-1164.
16. Maturu VN, Dhooria S, Agarwal R. Efficacy and safety of transbronchial needle aspiration in diagnosis and treatment of mediastinal bronchogenic cysts: systematic review of case reports. J Bronchology Interv Pulmonol. 2015;22(3):195-203.
17. Galluccio G, Lucantoni G. Mediastinal bronchogenic cyst’s recurrence treated with EBUS-FNA with a long-term follow-up. Eur J Cardiothorac Surg. 2006;29(4):627-629.
18. Lee DH, Park CK, Kum DY, Kim JB, Hwang I. Clinical characteristics and management of intrathoracic bronchogenic cysts: a single center experience. Korean J Thorac Cardiovasc Surg. 2011;44(4):279-284.
19. Onuki T, Kuramochi M, Inagaki M. Mediastinitis of bronchogenic cyst caused by endobronchial ultrasound-guided transbronchial needle aspiration. Respirol Case Rep. 2014;2(2):73-75.
1. Weber T, Roth TC, Beshay M, Herrmann P, Stein R, Schmid RA. Video-assisted thoracoscopic surgery of mediastinal bronchogenic cysts in adults: a single-center experience. Ann Thorac Surg. 2004;78(3):987-991.
2. Martinod E, Pons F, Azorin J, et al. Thoracoscopic excision of mediastinal bronchogenic cysts: results in 20 cases. Ann Thorac Surg. 2000;69(5):1525-1528.
3. Könönen E, Wade WG. Actinomyces and related organisms in human infections. Clin Microbiol Rev. 2015;28(2):419-442.
4. Ribet ME, Copin MC, Gosselin BH. Bronchogenic cysts of the lung. Ann Thorac Surg. 1996;61(6):1636-1640.
5. Guillem P, Porte H, Marquette CH, Wurtz A. Progressive dysphonia and acute respiratory failure: revealing a bronchogenic cyst. Eur J Cardiothorac Surg. 1997;12(6):925-927.
6. McAdams HP, Kirejczyk WM, Rosado-de-Christenson ML, Matsumoto S. Bronchogenic cyst: imaging features with clinical and histopathologic correlation. Radiology. 2000;217(2):441-446.
7. Rammohan G, Berger HW, Lajam F, Buhain WJ. Superior vena cava syndrome caused by bronchogenic cyst. Chest. 1975;68(4):599-601.
8. Hernández-Solís A, Cruz-Ortiz H, Gutiérrez-Díaz Ceballos ME, Cicero-Sabido R. Quistes broncogénicos. Importancia de la infección en adultos. Estudio de 12 casos [Bronchogenic cysts. Importance of infection in adults. Study of 12 cases]. Cir Cir. 2015;83(2):112-116.
9. Casal RF, Jimenez CA, Mehran RJ, et al. Infected mediastinal bronchogenic cyst successfully treated by endobronchial ultrasound-guided fine-needle aspiration. Ann Thorac Surg. 2010;90(4):e52-e53.
10. Liman ST, Dogan Y, Topcu S, Karabulut N, Demirkan N, Keser Z. Mycobacterial infection of intraparenchymal bronchogenic cysts. Respir Med. 2006;100(11):2060-2062.
11. Lin SH, Lee LN, Chang YL, Lee YC, Ding LW, Hsueh PR. Infected bronchogenic cyst due to Mycobacterium avium in an immunocompetent patient. J Infect. 2005;51(3):e131-e133.
12. Gharagozloo F, Dausmann MJ, McReynolds SD, Sanderson DR, Helmers RA. Recurrent bronchogenic pseudocyst 24 years after incomplete excision. Report of a case. Chest. 1995;108(3):880-883.
13. Bolton JW, Shahian DM. Asymptomatic bronchogenic cysts: what is the best management? Ann Thorac Surg. 1992;53(6):1134-1137.
14. Sarper A, Ayten A, Golbasi I, Demircan A, Isin E. Bronchogenic cyst. Tex Heart Inst J. 2003;30(2):105-108.
15. Varela-Lema L, Fernández-Villar A, Ruano-Ravina A. Effectiveness and safety of endobronchial ultrasound-transbronchial needle aspiration: a systematic review. Eur Respir J. 2009;33(5):1156-1164.
16. Maturu VN, Dhooria S, Agarwal R. Efficacy and safety of transbronchial needle aspiration in diagnosis and treatment of mediastinal bronchogenic cysts: systematic review of case reports. J Bronchology Interv Pulmonol. 2015;22(3):195-203.
17. Galluccio G, Lucantoni G. Mediastinal bronchogenic cyst’s recurrence treated with EBUS-FNA with a long-term follow-up. Eur J Cardiothorac Surg. 2006;29(4):627-629.
18. Lee DH, Park CK, Kum DY, Kim JB, Hwang I. Clinical characteristics and management of intrathoracic bronchogenic cysts: a single center experience. Korean J Thorac Cardiovasc Surg. 2011;44(4):279-284.
19. Onuki T, Kuramochi M, Inagaki M. Mediastinitis of bronchogenic cyst caused by endobronchial ultrasound-guided transbronchial needle aspiration. Respirol Case Rep. 2014;2(2):73-75.
‘The kids will be all right,’ won’t they?
Pediatric patients and COVID-19
The coronavirus disease 2019 (COVID-19) pandemic affects us in many ways. Pediatric patients, interestingly, are largely unaffected clinically by this disease. Less than 1% of documented infections occur in children under 10 years old, according to a review of over 72,000 cases from China.1 In that review, most children were asymptomatic or had mild illness, only three required intensive care, and only one death had been reported as of March 10, 2020. This is in stark contrast to the shocking morbidity and mortality statistics we are becoming all too familiar with on the adult side.
From a social standpoint, however, our pediatric patients’ lives have been turned upside down. Their schedules and routines upended, their education and friendships interrupted, and many are likely experiencing real anxiety and fear.2 For countless children, school is a major source of social, emotional, and nutritional support that has been cut off. Some will lose parents, grandparents, or other loved ones to this disease. Parents will lose jobs and will be unable to afford necessities. Pediatric patients will experience delays of procedures or treatments because of the pandemic. Some have projected that rates of child abuse will increase as has been reported during natural disasters.3
Pediatricians around the country are coming together to tackle these issues in creative ways, including the rapid expansion of virtual/telehealth programs. The school systems are developing strategies to deliver online content, and even food, to their students’ homes. Hopefully these tactics will mitigate some of the potential effects on the mental and physical well-being of these patients.
How about my kids? Will they be all right? I am lucky that my husband and I will have jobs throughout this ordeal. Unfortunately, given my role as a hospitalist and my husband’s as a pulmonary/critical care physician, these same jobs that will keep our kids nourished and supported pose the greatest threat to them. As health care workers, we are worried about protecting our families, which may include vulnerable members. The Spanish health ministry announced that medical professionals account for approximately one in eight documented COVID-19 infections in Spain.4 With inadequate supplies of personal protective equipment (PPE) in our own nation, we are concerned that our statistics could be similar.
There are multiple strategies to protect ourselves and our families during this difficult time. First, appropriate PPE is essential and integrity with the process must be maintained always. Hospital leaders can protect us by tirelessly working to acquire PPE. In Grand Rapids, Mich., our health system has partnered with multiple local manufacturing companies, including Steelcase, who are producing PPE for our workforce.5 Leaders can diligently update their system’s PPE recommendations to be in line with the latest CDC recommendations and disseminate the information regularly. Hospitalists should frequently check with their Infection Prevention department to make sure they understand if there have been any changes to the recommendations. Innovative solutions for sterilization of PPE, stethoscopes, badges and other equipment, such as with the use of UV boxes or hydrogen peroxide vapor,6 should be explored to minimize contamination. Hospitalists should bring a set of clothes and shoes to change into upon arrival to work and to change out of prior to leaving the hospital.
We must also keep our heads strong. Currently the anxiety amongst physicians is palpable but there is solidarity. Hospital leaders must ensure that hospitalists have easy access to free mental health resources, such as virtual counseling. Wellness teams must rise to the occasion with innovative tactics to support us. For example, Spectrum Health’s wellness team is sponsoring a blog where physicians can discuss COVID-19–related challenges openly. Hospitalist leaders should ensure that there is a structure for debriefing after critical incidents, which are sure to increase in frequency. Email lists and discussion boards sponsored by professional society also provide a collaborative venue for some of these discussions. We must take advantage of these resources and communicate with each other.
For me, in the end it comes back to the kids. My kids and most pediatric patients are not likely to be hospitalized from COVID-19, but they are also not immune to the toll that fighting this pandemic will take on our families. We took an oath to protect our patients, but what do we owe to our own children? At a minimum we can optimize how we protect ourselves every day, both physically and mentally. As we come together as a strong community to fight this pandemic, in addition to saving lives, we are working to ensure that, in the end, the kids will be all right.
Dr. Hadley is chief of pediatric hospital medicine at Spectrum Health/Helen DeVos Children’s Hospital in Grand Rapids, Mich., and clinical assistant professor at Michigan State University, East Lansing.
References
1. Wu Z, McGoogan JM. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: Summary of a report of 72 314 cases from the Chinese Center for Disease Control and Prevention. JAMA. 2020 Feb 24. doi: 10.1001/jama.2020.2648.
2. Hagan JF Jr; American Academy of Pediatrics Committee on Psychosocial Aspects of Child and Family Health; Task Force on Terrorism. Psychosocial implications of disaster or terrorism on children: A guide for the pediatrician. Pediatrics. 2005;116(3):787-795.
3. Gearhart S et al. The impact of natural disasters on domestic violence: An analysis of reports of simple assault in Florida (1997-2007). Violence Gend. 2018 Jun. doi: 10.1089/vio.2017.0077.
4. Minder R, Peltier E. Virus knocks thousands of health workers out of action in Europe. The New York Times. March 24, 2020.
5. McVicar B. West Michigan businesses hustle to produce medical supplies amid coronavirus pandemic. MLive. March 25, 2020.
6. Kenney PA et al. Hydrogen Peroxide Vapor sterilization of N95 respirators for reuse. medRxiv preprint. 2020 Mar. doi: 10.1101/2020.03.24.20041087.
Pediatric patients and COVID-19
Pediatric patients and COVID-19
The coronavirus disease 2019 (COVID-19) pandemic affects us in many ways. Pediatric patients, interestingly, are largely unaffected clinically by this disease. Less than 1% of documented infections occur in children under 10 years old, according to a review of over 72,000 cases from China.1 In that review, most children were asymptomatic or had mild illness, only three required intensive care, and only one death had been reported as of March 10, 2020. This is in stark contrast to the shocking morbidity and mortality statistics we are becoming all too familiar with on the adult side.
From a social standpoint, however, our pediatric patients’ lives have been turned upside down. Their schedules and routines upended, their education and friendships interrupted, and many are likely experiencing real anxiety and fear.2 For countless children, school is a major source of social, emotional, and nutritional support that has been cut off. Some will lose parents, grandparents, or other loved ones to this disease. Parents will lose jobs and will be unable to afford necessities. Pediatric patients will experience delays of procedures or treatments because of the pandemic. Some have projected that rates of child abuse will increase as has been reported during natural disasters.3
Pediatricians around the country are coming together to tackle these issues in creative ways, including the rapid expansion of virtual/telehealth programs. The school systems are developing strategies to deliver online content, and even food, to their students’ homes. Hopefully these tactics will mitigate some of the potential effects on the mental and physical well-being of these patients.
How about my kids? Will they be all right? I am lucky that my husband and I will have jobs throughout this ordeal. Unfortunately, given my role as a hospitalist and my husband’s as a pulmonary/critical care physician, these same jobs that will keep our kids nourished and supported pose the greatest threat to them. As health care workers, we are worried about protecting our families, which may include vulnerable members. The Spanish health ministry announced that medical professionals account for approximately one in eight documented COVID-19 infections in Spain.4 With inadequate supplies of personal protective equipment (PPE) in our own nation, we are concerned that our statistics could be similar.
There are multiple strategies to protect ourselves and our families during this difficult time. First, appropriate PPE is essential and integrity with the process must be maintained always. Hospital leaders can protect us by tirelessly working to acquire PPE. In Grand Rapids, Mich., our health system has partnered with multiple local manufacturing companies, including Steelcase, who are producing PPE for our workforce.5 Leaders can diligently update their system’s PPE recommendations to be in line with the latest CDC recommendations and disseminate the information regularly. Hospitalists should frequently check with their Infection Prevention department to make sure they understand if there have been any changes to the recommendations. Innovative solutions for sterilization of PPE, stethoscopes, badges and other equipment, such as with the use of UV boxes or hydrogen peroxide vapor,6 should be explored to minimize contamination. Hospitalists should bring a set of clothes and shoes to change into upon arrival to work and to change out of prior to leaving the hospital.
We must also keep our heads strong. Currently the anxiety amongst physicians is palpable but there is solidarity. Hospital leaders must ensure that hospitalists have easy access to free mental health resources, such as virtual counseling. Wellness teams must rise to the occasion with innovative tactics to support us. For example, Spectrum Health’s wellness team is sponsoring a blog where physicians can discuss COVID-19–related challenges openly. Hospitalist leaders should ensure that there is a structure for debriefing after critical incidents, which are sure to increase in frequency. Email lists and discussion boards sponsored by professional society also provide a collaborative venue for some of these discussions. We must take advantage of these resources and communicate with each other.
For me, in the end it comes back to the kids. My kids and most pediatric patients are not likely to be hospitalized from COVID-19, but they are also not immune to the toll that fighting this pandemic will take on our families. We took an oath to protect our patients, but what do we owe to our own children? At a minimum we can optimize how we protect ourselves every day, both physically and mentally. As we come together as a strong community to fight this pandemic, in addition to saving lives, we are working to ensure that, in the end, the kids will be all right.
Dr. Hadley is chief of pediatric hospital medicine at Spectrum Health/Helen DeVos Children’s Hospital in Grand Rapids, Mich., and clinical assistant professor at Michigan State University, East Lansing.
References
1. Wu Z, McGoogan JM. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: Summary of a report of 72 314 cases from the Chinese Center for Disease Control and Prevention. JAMA. 2020 Feb 24. doi: 10.1001/jama.2020.2648.
2. Hagan JF Jr; American Academy of Pediatrics Committee on Psychosocial Aspects of Child and Family Health; Task Force on Terrorism. Psychosocial implications of disaster or terrorism on children: A guide for the pediatrician. Pediatrics. 2005;116(3):787-795.
3. Gearhart S et al. The impact of natural disasters on domestic violence: An analysis of reports of simple assault in Florida (1997-2007). Violence Gend. 2018 Jun. doi: 10.1089/vio.2017.0077.
4. Minder R, Peltier E. Virus knocks thousands of health workers out of action in Europe. The New York Times. March 24, 2020.
5. McVicar B. West Michigan businesses hustle to produce medical supplies amid coronavirus pandemic. MLive. March 25, 2020.
6. Kenney PA et al. Hydrogen Peroxide Vapor sterilization of N95 respirators for reuse. medRxiv preprint. 2020 Mar. doi: 10.1101/2020.03.24.20041087.
The coronavirus disease 2019 (COVID-19) pandemic affects us in many ways. Pediatric patients, interestingly, are largely unaffected clinically by this disease. Less than 1% of documented infections occur in children under 10 years old, according to a review of over 72,000 cases from China.1 In that review, most children were asymptomatic or had mild illness, only three required intensive care, and only one death had been reported as of March 10, 2020. This is in stark contrast to the shocking morbidity and mortality statistics we are becoming all too familiar with on the adult side.
From a social standpoint, however, our pediatric patients’ lives have been turned upside down. Their schedules and routines upended, their education and friendships interrupted, and many are likely experiencing real anxiety and fear.2 For countless children, school is a major source of social, emotional, and nutritional support that has been cut off. Some will lose parents, grandparents, or other loved ones to this disease. Parents will lose jobs and will be unable to afford necessities. Pediatric patients will experience delays of procedures or treatments because of the pandemic. Some have projected that rates of child abuse will increase as has been reported during natural disasters.3
Pediatricians around the country are coming together to tackle these issues in creative ways, including the rapid expansion of virtual/telehealth programs. The school systems are developing strategies to deliver online content, and even food, to their students’ homes. Hopefully these tactics will mitigate some of the potential effects on the mental and physical well-being of these patients.
How about my kids? Will they be all right? I am lucky that my husband and I will have jobs throughout this ordeal. Unfortunately, given my role as a hospitalist and my husband’s as a pulmonary/critical care physician, these same jobs that will keep our kids nourished and supported pose the greatest threat to them. As health care workers, we are worried about protecting our families, which may include vulnerable members. The Spanish health ministry announced that medical professionals account for approximately one in eight documented COVID-19 infections in Spain.4 With inadequate supplies of personal protective equipment (PPE) in our own nation, we are concerned that our statistics could be similar.
There are multiple strategies to protect ourselves and our families during this difficult time. First, appropriate PPE is essential and integrity with the process must be maintained always. Hospital leaders can protect us by tirelessly working to acquire PPE. In Grand Rapids, Mich., our health system has partnered with multiple local manufacturing companies, including Steelcase, who are producing PPE for our workforce.5 Leaders can diligently update their system’s PPE recommendations to be in line with the latest CDC recommendations and disseminate the information regularly. Hospitalists should frequently check with their Infection Prevention department to make sure they understand if there have been any changes to the recommendations. Innovative solutions for sterilization of PPE, stethoscopes, badges and other equipment, such as with the use of UV boxes or hydrogen peroxide vapor,6 should be explored to minimize contamination. Hospitalists should bring a set of clothes and shoes to change into upon arrival to work and to change out of prior to leaving the hospital.
We must also keep our heads strong. Currently the anxiety amongst physicians is palpable but there is solidarity. Hospital leaders must ensure that hospitalists have easy access to free mental health resources, such as virtual counseling. Wellness teams must rise to the occasion with innovative tactics to support us. For example, Spectrum Health’s wellness team is sponsoring a blog where physicians can discuss COVID-19–related challenges openly. Hospitalist leaders should ensure that there is a structure for debriefing after critical incidents, which are sure to increase in frequency. Email lists and discussion boards sponsored by professional society also provide a collaborative venue for some of these discussions. We must take advantage of these resources and communicate with each other.
For me, in the end it comes back to the kids. My kids and most pediatric patients are not likely to be hospitalized from COVID-19, but they are also not immune to the toll that fighting this pandemic will take on our families. We took an oath to protect our patients, but what do we owe to our own children? At a minimum we can optimize how we protect ourselves every day, both physically and mentally. As we come together as a strong community to fight this pandemic, in addition to saving lives, we are working to ensure that, in the end, the kids will be all right.
Dr. Hadley is chief of pediatric hospital medicine at Spectrum Health/Helen DeVos Children’s Hospital in Grand Rapids, Mich., and clinical assistant professor at Michigan State University, East Lansing.
References
1. Wu Z, McGoogan JM. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: Summary of a report of 72 314 cases from the Chinese Center for Disease Control and Prevention. JAMA. 2020 Feb 24. doi: 10.1001/jama.2020.2648.
2. Hagan JF Jr; American Academy of Pediatrics Committee on Psychosocial Aspects of Child and Family Health; Task Force on Terrorism. Psychosocial implications of disaster or terrorism on children: A guide for the pediatrician. Pediatrics. 2005;116(3):787-795.
3. Gearhart S et al. The impact of natural disasters on domestic violence: An analysis of reports of simple assault in Florida (1997-2007). Violence Gend. 2018 Jun. doi: 10.1089/vio.2017.0077.
4. Minder R, Peltier E. Virus knocks thousands of health workers out of action in Europe. The New York Times. March 24, 2020.
5. McVicar B. West Michigan businesses hustle to produce medical supplies amid coronavirus pandemic. MLive. March 25, 2020.
6. Kenney PA et al. Hydrogen Peroxide Vapor sterilization of N95 respirators for reuse. medRxiv preprint. 2020 Mar. doi: 10.1101/2020.03.24.20041087.