User login
Diabetic Foot Ulcers: Life-Threatening Issue in Need of Help
The photo of the patient’s foot, sent from his campsite, included a cheeky note: “I remember you telling me that getting in trouble doing something was better than getting in trouble doing nothing. This lets me get out there and know that I have feedback.”
The “this” was the patient’s “foot selfie,” an approach that allows patients at a risk for diabetic foot ulcers (DFUs) to snap a picture and send it to their healthcare providers for evaluation.
This particular patient had an extensive history of previous wounds. Some had essentially kept him house-bound in the past, as he was afraid to get another one.
This time, however, he got an all-clear to keep on camping, “and we scheduled him in on the following Tuesday [for follow-up],” said the camper’s physician David G. Armstrong, DPM, MD, PhD, professor of surgery and neurological surgery, USC Keck School of Medicine, Los Angeles.
Dr. Armstrong is one of the researchers evaluating the concept of foot selfies. It’s a welcome advance, he and others said, and has been shown to help heal wounds and reverse pre-ulcer lesions. Research on foot selfies continues, but much more is needed to solve the issue of DFUs, diabetic foot infections (DFIs), and the high rates of reinfection, experts know.
Worldwide, about 18.6 million people have a DFU each year, including 1.6 million in the United States. About 50%-60% of ulcers become infected, with 20% of moderate to severe infections requiring amputation of the limb. The 5-year mortality rate for DFUs is 30%, but it climbs to 70% after amputation. While about 40% of ulcers heal within 12 weeks, 42% recur at the 1-year mark, setting up a vicious and costly cycle. Healthcare costs for patients with diabetes and DFUs are five times as high as costs for patients with diabetes but no DFUs. The per capita cost to treat a DFU in America is $17,500.
While the statistics paint a grim picture, progress is being made on several fronts:
- US Food and Drug Administration (FDA) guidance on the development of drugs for DFUs, under evaluation, is forthcoming.
- New treatments are under study.
- A multidisciplinary team approach is known to improve outcomes.
Anatomy of a DFU
When neuropathy develops in those with diabetes, they no longer have what Dr. Armstrong calls the “gift” of pain perception. “They can wear a hole in their foot like you and I wear a hole in our sock or shoe,” he said. “That hole is called a diabetic foot ulcer.”
A DFU is an open wound on the foot, often occurring when bleeding develops beneath a callus and then the callus wears away. Deeper tissues of the foot are then exposed.
About half of the DFUs get infected, hence the FDA guidance, said Dr. Armstrong, who is also founding president of the American Limb Preservation Society, which aims to eliminate preventable amputations within the next generation. Every 20 seconds, Dr. Armstrong said, someone in the world loses a leg due to diabetes.
FDA Guidance on Drug Development for DFIs
In October, the FDA issued draft guidance for industry to articulate the design of clinical trials for developing antibacterial drugs to treat DFIs without concomitant bone and joint involvement. Comments closed on December 18. Among the points in the guidance, which is nonbinding, are to include DFIs of varying depths and extent in phase 3 trials and ideally to include only those patients who have not had prior antibacterial treatment for the current DFI.
According to an FDA spokesperson, “The agency is working to finalize the guidance. However, a timeline for its release has not yet been established.”
The good news about the upcoming FDA guidance, Dr. Armstrong said, is that the agency has realized the importance of treating the infections. Fully one third of direct costs of care for diabetes are spent on the lower extremities, he said. Keeping patients out of the hospital, uninfected, and “keeping legs on bodies” are all important goals, he said.
Pharmaceutical firms need to understand that “you aren’t dealing with a normal ulcer,” said Andrew J.M. Boulton, MD, professor of medicine at the University of Manchester and physician consultant at the Manchester Royal Infirmary, Manchester, England, and a visiting professor at the University of Miami. For research, “the most important thing is to take account of off-loading the ulcers,” he said. “Most ulcers will heal if put in a boot.”
Dr. Boulton, like Dr. Armstrong, a long-time expert in the field, contended that pharma has not understood this concept and has wasted millions over the last three decades doing studies that were poorly designed and controlled.
Treatments: Current, Under Study
Currently, DFIs are treated with antimicrobial therapy, without or without debridement, along with a clinical assessment for ischemia. If ischemia is found, care progresses to wound care and off-loading devices, such as healing sandals. Clinicians then assess the likelihood of improved outcomes with revascularization based on operative risks and distribution of lower extremity artery disease and proceed depending on the likelihood. If osteomyelitis testing shows it is present, providers proceed to wound debridement, limb-sparing amputation, and prolonged antimicrobials, as needed.
More options are needed, Dr. Armstrong said.
Among the many approaches under study:
- DFUs can be accurately detected by applying artificial intelligence to the “foot selfie” images taken by patients on smartphones, research by Dr. and has found.
- After a phase 3 study of for DFUs originally intending to enroll 300 subjects was discontinued because of slow patient recruitment, an interim analysis was conducted on 44 participants. It showed a positive trend toward wound closure in the group receiving the injected gene therapy, VM202 (ENGENSIS), in their calf muscles. VM202 is a plasmid DNA-encoding human hepatocyte growth factor. While those in both the intervention and placebo groups showed wound-closing effects at month 6, in 23 patients with neuro-ischemic ulcers, the percentage of those reaching complete closure of the DFU was significantly higher in the treated group at months 3, 4, and 5 (P = .0391, .0391, and .0361, respectively). After excluding two outliers, the difference in months 3-6 became more significant (P = .03).
- An closed more DFUs than standard care after 12 weeks — 70% vs 34% (P = .00032). Of the 100 participants randomized, 50 per group, 42% of the treatment group and 56% of the control group experienced adverse events, with eight withdrawn due to serious adverse events (such as osteomyelitis).
- A closed more refractory DFUs over a 16-week study than standard sharp debridement, with 65% of water-treated ulcers healed but just 42% of the standard care group (P = .021, unadjusted).
- Researchers from UC Davis and VA Northern California Healthcare are evaluating timolol, a beta adrenergic receptor blocker already approved for topical administration for glaucoma, as a way to heal chronic DFUs faster. After demonstrating that the medication worked in animal models, researchers then launched a study to use it off-label for DFUs. While data are still being analyzed, researcher Roslyn (Rivkah) Isseroff, MD, of UC Davis and VA, said that data so far demonstrate that the timolol reduced transepidermal water loss in the healed wounds, and that is linked with a decrease in re-ulceration.
The Power of a Team
Multidisciplinary approaches to treatment are effective in reducing amputation, with one review of 33 studies finding the approach worked to decrease amputation in 94% of them. “The American Limb Preservation Society (ALPS) lists 30 programs,” said Dr. Armstrong, the founding president of the organization. “There may be as many as 100.”
Team compositions vary but usually include at least one medical specialty clinician, such as infectious disease, primary care, or endocrinology, and two or more specialty clinicians, such as vascular, podiatric, orthopedic, or plastic surgery. A shoe specialist is needed to prescribe and manage footwear. Other important team members include nutrition experts and behavioral health professionals to deal with associated depression.
Johns Hopkins’ Multidisciplinary Diabetic Foot and Wound Service launched in 2012 and includes vascular surgeons, surgical podiatrists, endocrinologists, wound care nurses, advanced practice staff, board-certified wound care specialists, orthopedic surgeons, infection disease experts, physical therapists, and certified orthotists.
“This interdisciplinary care model has been repeatedly validated by research as superior for limb salvage and wound healing,” said Nestoras Mathioudakis, MD, codirector of the service. “For instance, endocrinologists and diabetes educators are crucial for managing uncontrolled diabetes — a key factor in infection and delayed wound healing. Similarly, vascular surgeons play a vital role in addressing peripheral arterial disease to improve blood flow to the affected area.”
“Diabetic foot ulcers might require prolonged periods of specialized care, including meticulous wound management and off-loading, overseen by surgical podiatrists and wound care experts,” he said. “In cases where infection is present, particularly with multidrug resistant organisms or when standard antibiotics are contraindicated, the insight of an infectious disease specialist is invaluable.”
While the makeup of teams varies from location to location, he said “the hallmark of effective teams is their ability to comprehensively manage glycemic control, foot wounds, vascular disease, and infections.”
The power of teams, Dr. Armstrong said, is very much evident after his weekly “foot selfie rounds” conducted Mondays at 7 AM, with an “all feet on deck” approach. “Not a week goes by when we don’t stop a hospitalization,” he said of the team evaluating the photos, due to detecting issues early, while still in the manageable state.
Teams can trump technology, Dr. Armstrong said. A team of just a primary care doctor and a podiatrist can make a significant reduction in amputations, he said, just by a “Knock your socks off” approach. He reminds primary care doctors that observing the feet of their patients with diabetes can go a long way to reducing DFUs and the hospitalizations and amputations that can result.
Dr. Mathioudakis and Dr. Isseroff reported no disclosures. Dr. Boulton consults for Urgo Medical, Nevro Corporation, and AOT, Inc. Dr. Armstrong reported receiving consulting fees from Podimetrics; Molnlycke; Cardiovascular Systems, Inc.; Endo Pharmaceuticals; and Averitas Pharma (GRT US).
A version of this article first appeared on Medscape.com.
The photo of the patient’s foot, sent from his campsite, included a cheeky note: “I remember you telling me that getting in trouble doing something was better than getting in trouble doing nothing. This lets me get out there and know that I have feedback.”
The “this” was the patient’s “foot selfie,” an approach that allows patients at a risk for diabetic foot ulcers (DFUs) to snap a picture and send it to their healthcare providers for evaluation.
This particular patient had an extensive history of previous wounds. Some had essentially kept him house-bound in the past, as he was afraid to get another one.
This time, however, he got an all-clear to keep on camping, “and we scheduled him in on the following Tuesday [for follow-up],” said the camper’s physician David G. Armstrong, DPM, MD, PhD, professor of surgery and neurological surgery, USC Keck School of Medicine, Los Angeles.
Dr. Armstrong is one of the researchers evaluating the concept of foot selfies. It’s a welcome advance, he and others said, and has been shown to help heal wounds and reverse pre-ulcer lesions. Research on foot selfies continues, but much more is needed to solve the issue of DFUs, diabetic foot infections (DFIs), and the high rates of reinfection, experts know.
Worldwide, about 18.6 million people have a DFU each year, including 1.6 million in the United States. About 50%-60% of ulcers become infected, with 20% of moderate to severe infections requiring amputation of the limb. The 5-year mortality rate for DFUs is 30%, but it climbs to 70% after amputation. While about 40% of ulcers heal within 12 weeks, 42% recur at the 1-year mark, setting up a vicious and costly cycle. Healthcare costs for patients with diabetes and DFUs are five times as high as costs for patients with diabetes but no DFUs. The per capita cost to treat a DFU in America is $17,500.
While the statistics paint a grim picture, progress is being made on several fronts:
- US Food and Drug Administration (FDA) guidance on the development of drugs for DFUs, under evaluation, is forthcoming.
- New treatments are under study.
- A multidisciplinary team approach is known to improve outcomes.
Anatomy of a DFU
When neuropathy develops in those with diabetes, they no longer have what Dr. Armstrong calls the “gift” of pain perception. “They can wear a hole in their foot like you and I wear a hole in our sock or shoe,” he said. “That hole is called a diabetic foot ulcer.”
A DFU is an open wound on the foot, often occurring when bleeding develops beneath a callus and then the callus wears away. Deeper tissues of the foot are then exposed.
About half of the DFUs get infected, hence the FDA guidance, said Dr. Armstrong, who is also founding president of the American Limb Preservation Society, which aims to eliminate preventable amputations within the next generation. Every 20 seconds, Dr. Armstrong said, someone in the world loses a leg due to diabetes.
FDA Guidance on Drug Development for DFIs
In October, the FDA issued draft guidance for industry to articulate the design of clinical trials for developing antibacterial drugs to treat DFIs without concomitant bone and joint involvement. Comments closed on December 18. Among the points in the guidance, which is nonbinding, are to include DFIs of varying depths and extent in phase 3 trials and ideally to include only those patients who have not had prior antibacterial treatment for the current DFI.
According to an FDA spokesperson, “The agency is working to finalize the guidance. However, a timeline for its release has not yet been established.”
The good news about the upcoming FDA guidance, Dr. Armstrong said, is that the agency has realized the importance of treating the infections. Fully one third of direct costs of care for diabetes are spent on the lower extremities, he said. Keeping patients out of the hospital, uninfected, and “keeping legs on bodies” are all important goals, he said.
Pharmaceutical firms need to understand that “you aren’t dealing with a normal ulcer,” said Andrew J.M. Boulton, MD, professor of medicine at the University of Manchester and physician consultant at the Manchester Royal Infirmary, Manchester, England, and a visiting professor at the University of Miami. For research, “the most important thing is to take account of off-loading the ulcers,” he said. “Most ulcers will heal if put in a boot.”
Dr. Boulton, like Dr. Armstrong, a long-time expert in the field, contended that pharma has not understood this concept and has wasted millions over the last three decades doing studies that were poorly designed and controlled.
Treatments: Current, Under Study
Currently, DFIs are treated with antimicrobial therapy, without or without debridement, along with a clinical assessment for ischemia. If ischemia is found, care progresses to wound care and off-loading devices, such as healing sandals. Clinicians then assess the likelihood of improved outcomes with revascularization based on operative risks and distribution of lower extremity artery disease and proceed depending on the likelihood. If osteomyelitis testing shows it is present, providers proceed to wound debridement, limb-sparing amputation, and prolonged antimicrobials, as needed.
More options are needed, Dr. Armstrong said.
Among the many approaches under study:
- DFUs can be accurately detected by applying artificial intelligence to the “foot selfie” images taken by patients on smartphones, research by Dr. and has found.
- After a phase 3 study of for DFUs originally intending to enroll 300 subjects was discontinued because of slow patient recruitment, an interim analysis was conducted on 44 participants. It showed a positive trend toward wound closure in the group receiving the injected gene therapy, VM202 (ENGENSIS), in their calf muscles. VM202 is a plasmid DNA-encoding human hepatocyte growth factor. While those in both the intervention and placebo groups showed wound-closing effects at month 6, in 23 patients with neuro-ischemic ulcers, the percentage of those reaching complete closure of the DFU was significantly higher in the treated group at months 3, 4, and 5 (P = .0391, .0391, and .0361, respectively). After excluding two outliers, the difference in months 3-6 became more significant (P = .03).
- An closed more DFUs than standard care after 12 weeks — 70% vs 34% (P = .00032). Of the 100 participants randomized, 50 per group, 42% of the treatment group and 56% of the control group experienced adverse events, with eight withdrawn due to serious adverse events (such as osteomyelitis).
- A closed more refractory DFUs over a 16-week study than standard sharp debridement, with 65% of water-treated ulcers healed but just 42% of the standard care group (P = .021, unadjusted).
- Researchers from UC Davis and VA Northern California Healthcare are evaluating timolol, a beta adrenergic receptor blocker already approved for topical administration for glaucoma, as a way to heal chronic DFUs faster. After demonstrating that the medication worked in animal models, researchers then launched a study to use it off-label for DFUs. While data are still being analyzed, researcher Roslyn (Rivkah) Isseroff, MD, of UC Davis and VA, said that data so far demonstrate that the timolol reduced transepidermal water loss in the healed wounds, and that is linked with a decrease in re-ulceration.
The Power of a Team
Multidisciplinary approaches to treatment are effective in reducing amputation, with one review of 33 studies finding the approach worked to decrease amputation in 94% of them. “The American Limb Preservation Society (ALPS) lists 30 programs,” said Dr. Armstrong, the founding president of the organization. “There may be as many as 100.”
Team compositions vary but usually include at least one medical specialty clinician, such as infectious disease, primary care, or endocrinology, and two or more specialty clinicians, such as vascular, podiatric, orthopedic, or plastic surgery. A shoe specialist is needed to prescribe and manage footwear. Other important team members include nutrition experts and behavioral health professionals to deal with associated depression.
Johns Hopkins’ Multidisciplinary Diabetic Foot and Wound Service launched in 2012 and includes vascular surgeons, surgical podiatrists, endocrinologists, wound care nurses, advanced practice staff, board-certified wound care specialists, orthopedic surgeons, infection disease experts, physical therapists, and certified orthotists.
“This interdisciplinary care model has been repeatedly validated by research as superior for limb salvage and wound healing,” said Nestoras Mathioudakis, MD, codirector of the service. “For instance, endocrinologists and diabetes educators are crucial for managing uncontrolled diabetes — a key factor in infection and delayed wound healing. Similarly, vascular surgeons play a vital role in addressing peripheral arterial disease to improve blood flow to the affected area.”
“Diabetic foot ulcers might require prolonged periods of specialized care, including meticulous wound management and off-loading, overseen by surgical podiatrists and wound care experts,” he said. “In cases where infection is present, particularly with multidrug resistant organisms or when standard antibiotics are contraindicated, the insight of an infectious disease specialist is invaluable.”
While the makeup of teams varies from location to location, he said “the hallmark of effective teams is their ability to comprehensively manage glycemic control, foot wounds, vascular disease, and infections.”
The power of teams, Dr. Armstrong said, is very much evident after his weekly “foot selfie rounds” conducted Mondays at 7 AM, with an “all feet on deck” approach. “Not a week goes by when we don’t stop a hospitalization,” he said of the team evaluating the photos, due to detecting issues early, while still in the manageable state.
Teams can trump technology, Dr. Armstrong said. A team of just a primary care doctor and a podiatrist can make a significant reduction in amputations, he said, just by a “Knock your socks off” approach. He reminds primary care doctors that observing the feet of their patients with diabetes can go a long way to reducing DFUs and the hospitalizations and amputations that can result.
Dr. Mathioudakis and Dr. Isseroff reported no disclosures. Dr. Boulton consults for Urgo Medical, Nevro Corporation, and AOT, Inc. Dr. Armstrong reported receiving consulting fees from Podimetrics; Molnlycke; Cardiovascular Systems, Inc.; Endo Pharmaceuticals; and Averitas Pharma (GRT US).
A version of this article first appeared on Medscape.com.
The photo of the patient’s foot, sent from his campsite, included a cheeky note: “I remember you telling me that getting in trouble doing something was better than getting in trouble doing nothing. This lets me get out there and know that I have feedback.”
The “this” was the patient’s “foot selfie,” an approach that allows patients at a risk for diabetic foot ulcers (DFUs) to snap a picture and send it to their healthcare providers for evaluation.
This particular patient had an extensive history of previous wounds. Some had essentially kept him house-bound in the past, as he was afraid to get another one.
This time, however, he got an all-clear to keep on camping, “and we scheduled him in on the following Tuesday [for follow-up],” said the camper’s physician David G. Armstrong, DPM, MD, PhD, professor of surgery and neurological surgery, USC Keck School of Medicine, Los Angeles.
Dr. Armstrong is one of the researchers evaluating the concept of foot selfies. It’s a welcome advance, he and others said, and has been shown to help heal wounds and reverse pre-ulcer lesions. Research on foot selfies continues, but much more is needed to solve the issue of DFUs, diabetic foot infections (DFIs), and the high rates of reinfection, experts know.
Worldwide, about 18.6 million people have a DFU each year, including 1.6 million in the United States. About 50%-60% of ulcers become infected, with 20% of moderate to severe infections requiring amputation of the limb. The 5-year mortality rate for DFUs is 30%, but it climbs to 70% after amputation. While about 40% of ulcers heal within 12 weeks, 42% recur at the 1-year mark, setting up a vicious and costly cycle. Healthcare costs for patients with diabetes and DFUs are five times as high as costs for patients with diabetes but no DFUs. The per capita cost to treat a DFU in America is $17,500.
While the statistics paint a grim picture, progress is being made on several fronts:
- US Food and Drug Administration (FDA) guidance on the development of drugs for DFUs, under evaluation, is forthcoming.
- New treatments are under study.
- A multidisciplinary team approach is known to improve outcomes.
Anatomy of a DFU
When neuropathy develops in those with diabetes, they no longer have what Dr. Armstrong calls the “gift” of pain perception. “They can wear a hole in their foot like you and I wear a hole in our sock or shoe,” he said. “That hole is called a diabetic foot ulcer.”
A DFU is an open wound on the foot, often occurring when bleeding develops beneath a callus and then the callus wears away. Deeper tissues of the foot are then exposed.
About half of the DFUs get infected, hence the FDA guidance, said Dr. Armstrong, who is also founding president of the American Limb Preservation Society, which aims to eliminate preventable amputations within the next generation. Every 20 seconds, Dr. Armstrong said, someone in the world loses a leg due to diabetes.
FDA Guidance on Drug Development for DFIs
In October, the FDA issued draft guidance for industry to articulate the design of clinical trials for developing antibacterial drugs to treat DFIs without concomitant bone and joint involvement. Comments closed on December 18. Among the points in the guidance, which is nonbinding, are to include DFIs of varying depths and extent in phase 3 trials and ideally to include only those patients who have not had prior antibacterial treatment for the current DFI.
According to an FDA spokesperson, “The agency is working to finalize the guidance. However, a timeline for its release has not yet been established.”
The good news about the upcoming FDA guidance, Dr. Armstrong said, is that the agency has realized the importance of treating the infections. Fully one third of direct costs of care for diabetes are spent on the lower extremities, he said. Keeping patients out of the hospital, uninfected, and “keeping legs on bodies” are all important goals, he said.
Pharmaceutical firms need to understand that “you aren’t dealing with a normal ulcer,” said Andrew J.M. Boulton, MD, professor of medicine at the University of Manchester and physician consultant at the Manchester Royal Infirmary, Manchester, England, and a visiting professor at the University of Miami. For research, “the most important thing is to take account of off-loading the ulcers,” he said. “Most ulcers will heal if put in a boot.”
Dr. Boulton, like Dr. Armstrong, a long-time expert in the field, contended that pharma has not understood this concept and has wasted millions over the last three decades doing studies that were poorly designed and controlled.
Treatments: Current, Under Study
Currently, DFIs are treated with antimicrobial therapy, without or without debridement, along with a clinical assessment for ischemia. If ischemia is found, care progresses to wound care and off-loading devices, such as healing sandals. Clinicians then assess the likelihood of improved outcomes with revascularization based on operative risks and distribution of lower extremity artery disease and proceed depending on the likelihood. If osteomyelitis testing shows it is present, providers proceed to wound debridement, limb-sparing amputation, and prolonged antimicrobials, as needed.
More options are needed, Dr. Armstrong said.
Among the many approaches under study:
- DFUs can be accurately detected by applying artificial intelligence to the “foot selfie” images taken by patients on smartphones, research by Dr. and has found.
- After a phase 3 study of for DFUs originally intending to enroll 300 subjects was discontinued because of slow patient recruitment, an interim analysis was conducted on 44 participants. It showed a positive trend toward wound closure in the group receiving the injected gene therapy, VM202 (ENGENSIS), in their calf muscles. VM202 is a plasmid DNA-encoding human hepatocyte growth factor. While those in both the intervention and placebo groups showed wound-closing effects at month 6, in 23 patients with neuro-ischemic ulcers, the percentage of those reaching complete closure of the DFU was significantly higher in the treated group at months 3, 4, and 5 (P = .0391, .0391, and .0361, respectively). After excluding two outliers, the difference in months 3-6 became more significant (P = .03).
- An closed more DFUs than standard care after 12 weeks — 70% vs 34% (P = .00032). Of the 100 participants randomized, 50 per group, 42% of the treatment group and 56% of the control group experienced adverse events, with eight withdrawn due to serious adverse events (such as osteomyelitis).
- A closed more refractory DFUs over a 16-week study than standard sharp debridement, with 65% of water-treated ulcers healed but just 42% of the standard care group (P = .021, unadjusted).
- Researchers from UC Davis and VA Northern California Healthcare are evaluating timolol, a beta adrenergic receptor blocker already approved for topical administration for glaucoma, as a way to heal chronic DFUs faster. After demonstrating that the medication worked in animal models, researchers then launched a study to use it off-label for DFUs. While data are still being analyzed, researcher Roslyn (Rivkah) Isseroff, MD, of UC Davis and VA, said that data so far demonstrate that the timolol reduced transepidermal water loss in the healed wounds, and that is linked with a decrease in re-ulceration.
The Power of a Team
Multidisciplinary approaches to treatment are effective in reducing amputation, with one review of 33 studies finding the approach worked to decrease amputation in 94% of them. “The American Limb Preservation Society (ALPS) lists 30 programs,” said Dr. Armstrong, the founding president of the organization. “There may be as many as 100.”
Team compositions vary but usually include at least one medical specialty clinician, such as infectious disease, primary care, or endocrinology, and two or more specialty clinicians, such as vascular, podiatric, orthopedic, or plastic surgery. A shoe specialist is needed to prescribe and manage footwear. Other important team members include nutrition experts and behavioral health professionals to deal with associated depression.
Johns Hopkins’ Multidisciplinary Diabetic Foot and Wound Service launched in 2012 and includes vascular surgeons, surgical podiatrists, endocrinologists, wound care nurses, advanced practice staff, board-certified wound care specialists, orthopedic surgeons, infection disease experts, physical therapists, and certified orthotists.
“This interdisciplinary care model has been repeatedly validated by research as superior for limb salvage and wound healing,” said Nestoras Mathioudakis, MD, codirector of the service. “For instance, endocrinologists and diabetes educators are crucial for managing uncontrolled diabetes — a key factor in infection and delayed wound healing. Similarly, vascular surgeons play a vital role in addressing peripheral arterial disease to improve blood flow to the affected area.”
“Diabetic foot ulcers might require prolonged periods of specialized care, including meticulous wound management and off-loading, overseen by surgical podiatrists and wound care experts,” he said. “In cases where infection is present, particularly with multidrug resistant organisms or when standard antibiotics are contraindicated, the insight of an infectious disease specialist is invaluable.”
While the makeup of teams varies from location to location, he said “the hallmark of effective teams is their ability to comprehensively manage glycemic control, foot wounds, vascular disease, and infections.”
The power of teams, Dr. Armstrong said, is very much evident after his weekly “foot selfie rounds” conducted Mondays at 7 AM, with an “all feet on deck” approach. “Not a week goes by when we don’t stop a hospitalization,” he said of the team evaluating the photos, due to detecting issues early, while still in the manageable state.
Teams can trump technology, Dr. Armstrong said. A team of just a primary care doctor and a podiatrist can make a significant reduction in amputations, he said, just by a “Knock your socks off” approach. He reminds primary care doctors that observing the feet of their patients with diabetes can go a long way to reducing DFUs and the hospitalizations and amputations that can result.
Dr. Mathioudakis and Dr. Isseroff reported no disclosures. Dr. Boulton consults for Urgo Medical, Nevro Corporation, and AOT, Inc. Dr. Armstrong reported receiving consulting fees from Podimetrics; Molnlycke; Cardiovascular Systems, Inc.; Endo Pharmaceuticals; and Averitas Pharma (GRT US).
A version of this article first appeared on Medscape.com.
4 Years In, a Sobering Look at Long COVID Progress
Four years ago in the spring of 2020, physicians and patients coined the term “long COVID” to describe a form of the viral infection from which recovery seemed impossible. (And the old nickname “long-haulers” seems so quaint now.)
What started as a pandemic that killed nearly 3 million people globally in 2020 alone would turn into a chronic disease causing a long list of symptoms — from extreme fatigue, to brain fog, tremors, nausea, headaches, rapid heartbeat, and more.
Today, 6.4% of Americans report symptoms of long COVID, and many have never recovered.
Still, we’ve come a long way, although there’s much we don’t understand about the condition. At the very least, physicians have a greater understanding that long COVID exists and can cause serious long-term symptoms.
While physicians may not have a blanket diagnostic tool that works for all patients with long COVID, they have refined existing tests for more accurate results, said Nisha Viswanathan, MD, director of the University of California Los Angeles Long COVID Program at UCLA Health.
Also, a range of new treatments, now undergoing clinical trials, have emerged that have proved effective in managing long COVID symptoms.
Catecholamine testing, for example, is now commonly used to diagnose long COVID, particularly in those who have dysautonomia, a condition caused by dysfunction of the autonomic nervous system and marked by dizziness, low blood pressure, nausea, and brain fog.
Very high levels of the neurotransmitter, for example, were shown to indicate long COVID in a January 2021 study published in the journal Clinical Medicine.
Certain biomarkers have also been shown indicative of the condition, including low serotonin levels. A study published this year in Cell found lower serotonin levels in patients with long COVID driven by low levels of circulating SARS-CoV-2, the virus that causes the condition.
Still, said Dr. Viswanathan, long COVID is a disease diagnosed by figuring out what a patient does not have — by ruling out other causes — rather than what they do. “It’s still a moving target,” she said, meaning that the disease is always changing based on the variant of acute COVID.
Promising Treatments Have Emerged
Dysautonomia, and especially the associated brain fog, fatigue, and dizziness, are now common conditions. As a result, physicians have gotten better at treating them. The vagus nerve is the main nerve of the parasympathetic nervous system that controls everything from digestion to mental health. A February 2022 pilot study suggested a link between vagus nerve dysfunction and some long COVID symptoms.
Vagus nerve stimulation is one form of treatment which involves using a device to stimulate the vagus nerve with electrical impulses. Dr. Viswanathan has been using the treatment in patients with fatigue, brain fog, anxiety, and depression — results, she contends, have been positive.
“This is something tangible that we can offer to patients,” she said.
Curative treatments for long COVID remain elusive, but doctors have many more tools for symptom management than before, said Ziyad Al-Aly, MD, a global expert on long COVID and chief of research and development at the Veterans Affairs St. Louis Health Care System.
For example, physicians are using beta-blockers to treat postural tachycardia syndrome (POTS), a symptom of long COVID that happens when the heart rate increases rapidly after someone stands up or lies down. Beta-blockers, such as the off-label medication ivabradine, have been used clinically to control heart rate, according to a March 2022 study published in the journal HeartRhythm Case Reports.
“It’s not a cure, but beta-blockers can help patients manage their symptoms,” said Dr. Al-Aly.
Additionally, some patients respond well to low-dose naltrexone for the treatment of extreme fatigue associated with long COVID. A January 2024 article in the journal Clinical Therapeutics found that fatigue symptoms improved in patients taking the medication.
Dr. Al-Aly said doctors treating patients with long COVID are getting better at pinpointing the phenotype or manifestation of the condition and diagnosing a treatment accordingly. Treating long COVID fatigue is not the same as treating POTS or symptoms of headache and joint pain.
It’s still all about the management of symptoms and doctors lack any US Food and Drug Administration–approved medications specifically for the condition.
Clinical Trials Exploring New Therapies
Still, a number of large clinical trials currently underway may change that, said David F. Putrino, PhD, who runs the long COVID clinic at Mount Sinai Health System in New York City.
Two clinical trials headed by Dr. Putrino’s lab are looking into repurposing two HIV antivirals to see whether they affect the levels of circulating SARS-CoV-2 virus in the body that may cause long COVID. The hope is that the antivirals Truvada and maraviroc can reduce the «reactivation of latent virus» that, said Dr. Putrino, causes lingering long COVID symptoms.
Ongoing trials are looking into the promise of SARS-CoV-2 monoclonal antibodies, produced from cells made by cloning a unique white blood cell, as a treatment option. The trials are investigating whether these antibodies may similarly target viral reservoirs that are causing persistence of symptoms in some patients.
Other trials are underway through the National Institutes of Health (NIH) RECOVER initiative in which more than 17,000 patients are enrolled, the largest study of its kind, said Grace McComsey, MD.
Dr. McComsey, who leads the study at University Hospitals Health System in Cleveland, said that after following patients for up to 4 years researchers have gathered “a massive repository of information” they hope will help scientists crack the code of this very complex disease.
She and other RECOVER researchers have recently published studies on a variety of findings, reporting in February, for example, that COVID infections may trigger other autoimmune diseases such as rheumatoid arthritis and type 2 diabetes. Another recent finding showed that people with HIV are at a higher risk for complications due to acute COVID-19.
Lack of Urgency Holds Back Progress
Still, others like Dr. Al-Aly and Dr. Putrino felt that the initiative isn’t moving fast enough. Dr. Al-Aly said that the NIH needs to “get its act together” and do more for long COVID. In the future, he said that we need to double down on our efforts to expand funding and increase urgency to better understand the mechanism of disease, risk factors, and treatments, as well as societal and economic implications.
“We did trials for COVID-19 vaccines at warp speed, but we’re doing trials for long COVID at a snail’s pace,” he said.
Dr. Al-Aly is concerned about the chronic nature of the disease and how it affects patients down the line. His large-scale study published last month in the journal Science looked specifically at chronic fatigue syndrome triggered by the infection and its long-term impact on patients.
He’s concerned about the practical implications for people who are weighted down with symptoms for multiple years.
“Being fatigued and ill for a few months is one thing, but being at home for 5 years is a totally different ballgame.”
A version of this article first appeared on Medscape.com.
Four years ago in the spring of 2020, physicians and patients coined the term “long COVID” to describe a form of the viral infection from which recovery seemed impossible. (And the old nickname “long-haulers” seems so quaint now.)
What started as a pandemic that killed nearly 3 million people globally in 2020 alone would turn into a chronic disease causing a long list of symptoms — from extreme fatigue, to brain fog, tremors, nausea, headaches, rapid heartbeat, and more.
Today, 6.4% of Americans report symptoms of long COVID, and many have never recovered.
Still, we’ve come a long way, although there’s much we don’t understand about the condition. At the very least, physicians have a greater understanding that long COVID exists and can cause serious long-term symptoms.
While physicians may not have a blanket diagnostic tool that works for all patients with long COVID, they have refined existing tests for more accurate results, said Nisha Viswanathan, MD, director of the University of California Los Angeles Long COVID Program at UCLA Health.
Also, a range of new treatments, now undergoing clinical trials, have emerged that have proved effective in managing long COVID symptoms.
Catecholamine testing, for example, is now commonly used to diagnose long COVID, particularly in those who have dysautonomia, a condition caused by dysfunction of the autonomic nervous system and marked by dizziness, low blood pressure, nausea, and brain fog.
Very high levels of the neurotransmitter, for example, were shown to indicate long COVID in a January 2021 study published in the journal Clinical Medicine.
Certain biomarkers have also been shown indicative of the condition, including low serotonin levels. A study published this year in Cell found lower serotonin levels in patients with long COVID driven by low levels of circulating SARS-CoV-2, the virus that causes the condition.
Still, said Dr. Viswanathan, long COVID is a disease diagnosed by figuring out what a patient does not have — by ruling out other causes — rather than what they do. “It’s still a moving target,” she said, meaning that the disease is always changing based on the variant of acute COVID.
Promising Treatments Have Emerged
Dysautonomia, and especially the associated brain fog, fatigue, and dizziness, are now common conditions. As a result, physicians have gotten better at treating them. The vagus nerve is the main nerve of the parasympathetic nervous system that controls everything from digestion to mental health. A February 2022 pilot study suggested a link between vagus nerve dysfunction and some long COVID symptoms.
Vagus nerve stimulation is one form of treatment which involves using a device to stimulate the vagus nerve with electrical impulses. Dr. Viswanathan has been using the treatment in patients with fatigue, brain fog, anxiety, and depression — results, she contends, have been positive.
“This is something tangible that we can offer to patients,” she said.
Curative treatments for long COVID remain elusive, but doctors have many more tools for symptom management than before, said Ziyad Al-Aly, MD, a global expert on long COVID and chief of research and development at the Veterans Affairs St. Louis Health Care System.
For example, physicians are using beta-blockers to treat postural tachycardia syndrome (POTS), a symptom of long COVID that happens when the heart rate increases rapidly after someone stands up or lies down. Beta-blockers, such as the off-label medication ivabradine, have been used clinically to control heart rate, according to a March 2022 study published in the journal HeartRhythm Case Reports.
“It’s not a cure, but beta-blockers can help patients manage their symptoms,” said Dr. Al-Aly.
Additionally, some patients respond well to low-dose naltrexone for the treatment of extreme fatigue associated with long COVID. A January 2024 article in the journal Clinical Therapeutics found that fatigue symptoms improved in patients taking the medication.
Dr. Al-Aly said doctors treating patients with long COVID are getting better at pinpointing the phenotype or manifestation of the condition and diagnosing a treatment accordingly. Treating long COVID fatigue is not the same as treating POTS or symptoms of headache and joint pain.
It’s still all about the management of symptoms and doctors lack any US Food and Drug Administration–approved medications specifically for the condition.
Clinical Trials Exploring New Therapies
Still, a number of large clinical trials currently underway may change that, said David F. Putrino, PhD, who runs the long COVID clinic at Mount Sinai Health System in New York City.
Two clinical trials headed by Dr. Putrino’s lab are looking into repurposing two HIV antivirals to see whether they affect the levels of circulating SARS-CoV-2 virus in the body that may cause long COVID. The hope is that the antivirals Truvada and maraviroc can reduce the «reactivation of latent virus» that, said Dr. Putrino, causes lingering long COVID symptoms.
Ongoing trials are looking into the promise of SARS-CoV-2 monoclonal antibodies, produced from cells made by cloning a unique white blood cell, as a treatment option. The trials are investigating whether these antibodies may similarly target viral reservoirs that are causing persistence of symptoms in some patients.
Other trials are underway through the National Institutes of Health (NIH) RECOVER initiative in which more than 17,000 patients are enrolled, the largest study of its kind, said Grace McComsey, MD.
Dr. McComsey, who leads the study at University Hospitals Health System in Cleveland, said that after following patients for up to 4 years researchers have gathered “a massive repository of information” they hope will help scientists crack the code of this very complex disease.
She and other RECOVER researchers have recently published studies on a variety of findings, reporting in February, for example, that COVID infections may trigger other autoimmune diseases such as rheumatoid arthritis and type 2 diabetes. Another recent finding showed that people with HIV are at a higher risk for complications due to acute COVID-19.
Lack of Urgency Holds Back Progress
Still, others like Dr. Al-Aly and Dr. Putrino felt that the initiative isn’t moving fast enough. Dr. Al-Aly said that the NIH needs to “get its act together” and do more for long COVID. In the future, he said that we need to double down on our efforts to expand funding and increase urgency to better understand the mechanism of disease, risk factors, and treatments, as well as societal and economic implications.
“We did trials for COVID-19 vaccines at warp speed, but we’re doing trials for long COVID at a snail’s pace,” he said.
Dr. Al-Aly is concerned about the chronic nature of the disease and how it affects patients down the line. His large-scale study published last month in the journal Science looked specifically at chronic fatigue syndrome triggered by the infection and its long-term impact on patients.
He’s concerned about the practical implications for people who are weighted down with symptoms for multiple years.
“Being fatigued and ill for a few months is one thing, but being at home for 5 years is a totally different ballgame.”
A version of this article first appeared on Medscape.com.
Four years ago in the spring of 2020, physicians and patients coined the term “long COVID” to describe a form of the viral infection from which recovery seemed impossible. (And the old nickname “long-haulers” seems so quaint now.)
What started as a pandemic that killed nearly 3 million people globally in 2020 alone would turn into a chronic disease causing a long list of symptoms — from extreme fatigue, to brain fog, tremors, nausea, headaches, rapid heartbeat, and more.
Today, 6.4% of Americans report symptoms of long COVID, and many have never recovered.
Still, we’ve come a long way, although there’s much we don’t understand about the condition. At the very least, physicians have a greater understanding that long COVID exists and can cause serious long-term symptoms.
While physicians may not have a blanket diagnostic tool that works for all patients with long COVID, they have refined existing tests for more accurate results, said Nisha Viswanathan, MD, director of the University of California Los Angeles Long COVID Program at UCLA Health.
Also, a range of new treatments, now undergoing clinical trials, have emerged that have proved effective in managing long COVID symptoms.
Catecholamine testing, for example, is now commonly used to diagnose long COVID, particularly in those who have dysautonomia, a condition caused by dysfunction of the autonomic nervous system and marked by dizziness, low blood pressure, nausea, and brain fog.
Very high levels of the neurotransmitter, for example, were shown to indicate long COVID in a January 2021 study published in the journal Clinical Medicine.
Certain biomarkers have also been shown indicative of the condition, including low serotonin levels. A study published this year in Cell found lower serotonin levels in patients with long COVID driven by low levels of circulating SARS-CoV-2, the virus that causes the condition.
Still, said Dr. Viswanathan, long COVID is a disease diagnosed by figuring out what a patient does not have — by ruling out other causes — rather than what they do. “It’s still a moving target,” she said, meaning that the disease is always changing based on the variant of acute COVID.
Promising Treatments Have Emerged
Dysautonomia, and especially the associated brain fog, fatigue, and dizziness, are now common conditions. As a result, physicians have gotten better at treating them. The vagus nerve is the main nerve of the parasympathetic nervous system that controls everything from digestion to mental health. A February 2022 pilot study suggested a link between vagus nerve dysfunction and some long COVID symptoms.
Vagus nerve stimulation is one form of treatment which involves using a device to stimulate the vagus nerve with electrical impulses. Dr. Viswanathan has been using the treatment in patients with fatigue, brain fog, anxiety, and depression — results, she contends, have been positive.
“This is something tangible that we can offer to patients,” she said.
Curative treatments for long COVID remain elusive, but doctors have many more tools for symptom management than before, said Ziyad Al-Aly, MD, a global expert on long COVID and chief of research and development at the Veterans Affairs St. Louis Health Care System.
For example, physicians are using beta-blockers to treat postural tachycardia syndrome (POTS), a symptom of long COVID that happens when the heart rate increases rapidly after someone stands up or lies down. Beta-blockers, such as the off-label medication ivabradine, have been used clinically to control heart rate, according to a March 2022 study published in the journal HeartRhythm Case Reports.
“It’s not a cure, but beta-blockers can help patients manage their symptoms,” said Dr. Al-Aly.
Additionally, some patients respond well to low-dose naltrexone for the treatment of extreme fatigue associated with long COVID. A January 2024 article in the journal Clinical Therapeutics found that fatigue symptoms improved in patients taking the medication.
Dr. Al-Aly said doctors treating patients with long COVID are getting better at pinpointing the phenotype or manifestation of the condition and diagnosing a treatment accordingly. Treating long COVID fatigue is not the same as treating POTS or symptoms of headache and joint pain.
It’s still all about the management of symptoms and doctors lack any US Food and Drug Administration–approved medications specifically for the condition.
Clinical Trials Exploring New Therapies
Still, a number of large clinical trials currently underway may change that, said David F. Putrino, PhD, who runs the long COVID clinic at Mount Sinai Health System in New York City.
Two clinical trials headed by Dr. Putrino’s lab are looking into repurposing two HIV antivirals to see whether they affect the levels of circulating SARS-CoV-2 virus in the body that may cause long COVID. The hope is that the antivirals Truvada and maraviroc can reduce the «reactivation of latent virus» that, said Dr. Putrino, causes lingering long COVID symptoms.
Ongoing trials are looking into the promise of SARS-CoV-2 monoclonal antibodies, produced from cells made by cloning a unique white blood cell, as a treatment option. The trials are investigating whether these antibodies may similarly target viral reservoirs that are causing persistence of symptoms in some patients.
Other trials are underway through the National Institutes of Health (NIH) RECOVER initiative in which more than 17,000 patients are enrolled, the largest study of its kind, said Grace McComsey, MD.
Dr. McComsey, who leads the study at University Hospitals Health System in Cleveland, said that after following patients for up to 4 years researchers have gathered “a massive repository of information” they hope will help scientists crack the code of this very complex disease.
She and other RECOVER researchers have recently published studies on a variety of findings, reporting in February, for example, that COVID infections may trigger other autoimmune diseases such as rheumatoid arthritis and type 2 diabetes. Another recent finding showed that people with HIV are at a higher risk for complications due to acute COVID-19.
Lack of Urgency Holds Back Progress
Still, others like Dr. Al-Aly and Dr. Putrino felt that the initiative isn’t moving fast enough. Dr. Al-Aly said that the NIH needs to “get its act together” and do more for long COVID. In the future, he said that we need to double down on our efforts to expand funding and increase urgency to better understand the mechanism of disease, risk factors, and treatments, as well as societal and economic implications.
“We did trials for COVID-19 vaccines at warp speed, but we’re doing trials for long COVID at a snail’s pace,” he said.
Dr. Al-Aly is concerned about the chronic nature of the disease and how it affects patients down the line. His large-scale study published last month in the journal Science looked specifically at chronic fatigue syndrome triggered by the infection and its long-term impact on patients.
He’s concerned about the practical implications for people who are weighted down with symptoms for multiple years.
“Being fatigued and ill for a few months is one thing, but being at home for 5 years is a totally different ballgame.”
A version of this article first appeared on Medscape.com.
Recently Immunized Febrile Infants Have Low Infection Risk
TOPLINE:
METHODOLOGY:
- Researchers evaluated 508 infants aged 6-12 weeks who presented with a fever of 38 °C or greater at two US military academic emergency departments (EDs) over a span of 4 years.
- The infants were categorized as “recently immunized” if they had received immunizations within 72 hours before ED presentation and “not recently immunized” if they had not. Among the 508 infants, 114 were immunized recently.
- The primary outcome was the prevalence of a serious bacterial infection (SBI), categorized into IBI and non-IBI on the basis of culture and radiography findings.
TAKEAWAY:
- The prevalence of SBI was 3.5% in the recently immunized febrile infants and 13.7% in not recently immunized febrile infants.
- Among the recently immunized infants, the prevalence of SBI was lower in those immunized within the first 24 hours than those immunized more than 24 hours before ED presentation (2% vs 14.3%, respectively).
- Almost all identified SBI cases were of urinary tract infection (UTI), with the only non-UTI case being pneumonia in an infant who exhibited respiratory symptoms within 24 hours of receiving immunization.
IN PRACTICE:
Physicians should discuss the possibilities of a less invasive approach for evaluating recently immunized febrile infants. The study findings support the general recommendation to obtain a urinalysis for all recently immunized infants over 60 days presenting with fever, including those presenting less than 24 hours post immunization.
SOURCE:
This study, led by Kyla Casey, MD, Department of Emergency Medicine, Naval Medical Center San Diego, was published online in The American Journal of Emergency Medicine.
LIMITATIONS:
The small sample size and retrospective design might have resulted in an overestimation of outcomes like IBIs within 24 hours after immunization. As the study was conducted in a specific clinical setting with febrile infants from military medical centers, the findings may have limited generalizability. Moreover, the inclusion of premature infants without age correction for prematurity could have impacted the prevalence of IBIs. Factors like missing vaccination history, healthcare referral patterns, and immunization practices in the military system may have introduced bias.
DISCLOSURE:
This research did not receive any specific grant from funding agencies in the public, commercial, or not for profit sectors. The authors had no conflicts of interest to disclose.
A version of this article appeared on Medscape.com.
TOPLINE:
METHODOLOGY:
- Researchers evaluated 508 infants aged 6-12 weeks who presented with a fever of 38 °C or greater at two US military academic emergency departments (EDs) over a span of 4 years.
- The infants were categorized as “recently immunized” if they had received immunizations within 72 hours before ED presentation and “not recently immunized” if they had not. Among the 508 infants, 114 were immunized recently.
- The primary outcome was the prevalence of a serious bacterial infection (SBI), categorized into IBI and non-IBI on the basis of culture and radiography findings.
TAKEAWAY:
- The prevalence of SBI was 3.5% in the recently immunized febrile infants and 13.7% in not recently immunized febrile infants.
- Among the recently immunized infants, the prevalence of SBI was lower in those immunized within the first 24 hours than those immunized more than 24 hours before ED presentation (2% vs 14.3%, respectively).
- Almost all identified SBI cases were of urinary tract infection (UTI), with the only non-UTI case being pneumonia in an infant who exhibited respiratory symptoms within 24 hours of receiving immunization.
IN PRACTICE:
Physicians should discuss the possibilities of a less invasive approach for evaluating recently immunized febrile infants. The study findings support the general recommendation to obtain a urinalysis for all recently immunized infants over 60 days presenting with fever, including those presenting less than 24 hours post immunization.
SOURCE:
This study, led by Kyla Casey, MD, Department of Emergency Medicine, Naval Medical Center San Diego, was published online in The American Journal of Emergency Medicine.
LIMITATIONS:
The small sample size and retrospective design might have resulted in an overestimation of outcomes like IBIs within 24 hours after immunization. As the study was conducted in a specific clinical setting with febrile infants from military medical centers, the findings may have limited generalizability. Moreover, the inclusion of premature infants without age correction for prematurity could have impacted the prevalence of IBIs. Factors like missing vaccination history, healthcare referral patterns, and immunization practices in the military system may have introduced bias.
DISCLOSURE:
This research did not receive any specific grant from funding agencies in the public, commercial, or not for profit sectors. The authors had no conflicts of interest to disclose.
A version of this article appeared on Medscape.com.
TOPLINE:
METHODOLOGY:
- Researchers evaluated 508 infants aged 6-12 weeks who presented with a fever of 38 °C or greater at two US military academic emergency departments (EDs) over a span of 4 years.
- The infants were categorized as “recently immunized” if they had received immunizations within 72 hours before ED presentation and “not recently immunized” if they had not. Among the 508 infants, 114 were immunized recently.
- The primary outcome was the prevalence of a serious bacterial infection (SBI), categorized into IBI and non-IBI on the basis of culture and radiography findings.
TAKEAWAY:
- The prevalence of SBI was 3.5% in the recently immunized febrile infants and 13.7% in not recently immunized febrile infants.
- Among the recently immunized infants, the prevalence of SBI was lower in those immunized within the first 24 hours than those immunized more than 24 hours before ED presentation (2% vs 14.3%, respectively).
- Almost all identified SBI cases were of urinary tract infection (UTI), with the only non-UTI case being pneumonia in an infant who exhibited respiratory symptoms within 24 hours of receiving immunization.
IN PRACTICE:
Physicians should discuss the possibilities of a less invasive approach for evaluating recently immunized febrile infants. The study findings support the general recommendation to obtain a urinalysis for all recently immunized infants over 60 days presenting with fever, including those presenting less than 24 hours post immunization.
SOURCE:
This study, led by Kyla Casey, MD, Department of Emergency Medicine, Naval Medical Center San Diego, was published online in The American Journal of Emergency Medicine.
LIMITATIONS:
The small sample size and retrospective design might have resulted in an overestimation of outcomes like IBIs within 24 hours after immunization. As the study was conducted in a specific clinical setting with febrile infants from military medical centers, the findings may have limited generalizability. Moreover, the inclusion of premature infants without age correction for prematurity could have impacted the prevalence of IBIs. Factors like missing vaccination history, healthcare referral patterns, and immunization practices in the military system may have introduced bias.
DISCLOSURE:
This research did not receive any specific grant from funding agencies in the public, commercial, or not for profit sectors. The authors had no conflicts of interest to disclose.
A version of this article appeared on Medscape.com.
D-Mannose as UTI Treatment Offers No Benefit
TOPLINE:
A natural sugar used to treat recurring urinary tract infections (rUTIs) did not reduce future episodes, outpatient visits, the use of antibiotics, or symptoms compared with a placebo, according to a new study.
METHODOLOGY:
- D-Mannose is recommended as a natural alternative to antibiotics and sold as a dietary supplement; research showing the efficacy of D-mannose in treating UTIs is mixed.
- The double-blind, randomized controlled trial followed 598 women older than 18 years (median age, 61.3; range 18.2-93.5 years) with a history of rUTIs over 6 months from nearly 100 primary care clinics in the United Kingdom.
- Patients took 2 g of D-mannose or placebo powder daily and recorded their symptoms using a daily diary or through responses to health surveys, weekly questionnaires, and phone calls.
- Researchers checked medical records for urine culture results, antibiotic prescriptions, hospitalizations, and outpatient visits for UTIs.
TAKEAWAY:
- Approximately 51% of participants who took D-mannose and 55.7% of those who took a placebo contacted a healthcare professional reporting a UTI (relative risk, 0.92; 95% CI, 0.80-1.05; P = .22).
- Women in both groups reported similar durations of “moderately bad” or “worse” symptoms, and the number of antibiotic courses, instances of clinically suspected UTIs, and hospital admissions were similar between the two groups.
- Some studies have reported that synthetic mannosides are promising alternatives to D-mannose.
IN PRACTICE:
“D-Mannose should not be recommended to prevent future episodes of medically attended UTI in women with recurrent UTI in primary care,” the study authors wrote.
SOURCE:
The study was led by Gail Hayward, DPhil, associate professor at the Nuffield Department of Primary Care Health Sciences at the University of Oxford in England, and was published online in JAMA Internal Medicine.
LIMITATIONS:
Some participants may have taken less than 2 mg/d or skipped days. Because researchers used powder rather than capsules, dosing could have been inconsistent. Researchers did not obtain a microbiologic confirmation for each rUTI. A small percentage of women were taking antibiotics.
DISCLOSURES:
The study was funded by the National Institute for Health and Care Research (NIHR) School for Primary Care Research and the NIHR Oxford Biomedical Research Centre. Various authors reported receiving support from the NIHR Health Protection Research Unit on Healthcare-Associated Infections and Antimicrobial Resistance and were NIHR investigators.
A version of this article first appeared on Medscape.com.
TOPLINE:
A natural sugar used to treat recurring urinary tract infections (rUTIs) did not reduce future episodes, outpatient visits, the use of antibiotics, or symptoms compared with a placebo, according to a new study.
METHODOLOGY:
- D-Mannose is recommended as a natural alternative to antibiotics and sold as a dietary supplement; research showing the efficacy of D-mannose in treating UTIs is mixed.
- The double-blind, randomized controlled trial followed 598 women older than 18 years (median age, 61.3; range 18.2-93.5 years) with a history of rUTIs over 6 months from nearly 100 primary care clinics in the United Kingdom.
- Patients took 2 g of D-mannose or placebo powder daily and recorded their symptoms using a daily diary or through responses to health surveys, weekly questionnaires, and phone calls.
- Researchers checked medical records for urine culture results, antibiotic prescriptions, hospitalizations, and outpatient visits for UTIs.
TAKEAWAY:
- Approximately 51% of participants who took D-mannose and 55.7% of those who took a placebo contacted a healthcare professional reporting a UTI (relative risk, 0.92; 95% CI, 0.80-1.05; P = .22).
- Women in both groups reported similar durations of “moderately bad” or “worse” symptoms, and the number of antibiotic courses, instances of clinically suspected UTIs, and hospital admissions were similar between the two groups.
- Some studies have reported that synthetic mannosides are promising alternatives to D-mannose.
IN PRACTICE:
“D-Mannose should not be recommended to prevent future episodes of medically attended UTI in women with recurrent UTI in primary care,” the study authors wrote.
SOURCE:
The study was led by Gail Hayward, DPhil, associate professor at the Nuffield Department of Primary Care Health Sciences at the University of Oxford in England, and was published online in JAMA Internal Medicine.
LIMITATIONS:
Some participants may have taken less than 2 mg/d or skipped days. Because researchers used powder rather than capsules, dosing could have been inconsistent. Researchers did not obtain a microbiologic confirmation for each rUTI. A small percentage of women were taking antibiotics.
DISCLOSURES:
The study was funded by the National Institute for Health and Care Research (NIHR) School for Primary Care Research and the NIHR Oxford Biomedical Research Centre. Various authors reported receiving support from the NIHR Health Protection Research Unit on Healthcare-Associated Infections and Antimicrobial Resistance and were NIHR investigators.
A version of this article first appeared on Medscape.com.
TOPLINE:
A natural sugar used to treat recurring urinary tract infections (rUTIs) did not reduce future episodes, outpatient visits, the use of antibiotics, or symptoms compared with a placebo, according to a new study.
METHODOLOGY:
- D-Mannose is recommended as a natural alternative to antibiotics and sold as a dietary supplement; research showing the efficacy of D-mannose in treating UTIs is mixed.
- The double-blind, randomized controlled trial followed 598 women older than 18 years (median age, 61.3; range 18.2-93.5 years) with a history of rUTIs over 6 months from nearly 100 primary care clinics in the United Kingdom.
- Patients took 2 g of D-mannose or placebo powder daily and recorded their symptoms using a daily diary or through responses to health surveys, weekly questionnaires, and phone calls.
- Researchers checked medical records for urine culture results, antibiotic prescriptions, hospitalizations, and outpatient visits for UTIs.
TAKEAWAY:
- Approximately 51% of participants who took D-mannose and 55.7% of those who took a placebo contacted a healthcare professional reporting a UTI (relative risk, 0.92; 95% CI, 0.80-1.05; P = .22).
- Women in both groups reported similar durations of “moderately bad” or “worse” symptoms, and the number of antibiotic courses, instances of clinically suspected UTIs, and hospital admissions were similar between the two groups.
- Some studies have reported that synthetic mannosides are promising alternatives to D-mannose.
IN PRACTICE:
“D-Mannose should not be recommended to prevent future episodes of medically attended UTI in women with recurrent UTI in primary care,” the study authors wrote.
SOURCE:
The study was led by Gail Hayward, DPhil, associate professor at the Nuffield Department of Primary Care Health Sciences at the University of Oxford in England, and was published online in JAMA Internal Medicine.
LIMITATIONS:
Some participants may have taken less than 2 mg/d or skipped days. Because researchers used powder rather than capsules, dosing could have been inconsistent. Researchers did not obtain a microbiologic confirmation for each rUTI. A small percentage of women were taking antibiotics.
DISCLOSURES:
The study was funded by the National Institute for Health and Care Research (NIHR) School for Primary Care Research and the NIHR Oxford Biomedical Research Centre. Various authors reported receiving support from the NIHR Health Protection Research Unit on Healthcare-Associated Infections and Antimicrobial Resistance and were NIHR investigators.
A version of this article first appeared on Medscape.com.
Worldwide Uptick in Invasive Group A Streptococcus Disease Post Pandemic — What Should We Know?
Invasive group A streptococcus (iGAS) infections are rare (4-9 cases/100,000 US population annually) but potentially devastating (approximately 2,300 deaths annually in US), and affect all ages. Cases increase in winter-spring, paralleling the “season” of increased noninvasive GAS, e.g., pharyngitis and scarlet fever. iGAS case rates are lower in children than adults. That said, one well-known pediatric iGAS scenario has been deep cellulitis and necrotizing fasciitis during the healing phase of varicella. Other forms of iGAS include bacteremia, pneumonia (particularly when empyema is present), lymphangitis, erysipelas, and toxic shock syndrome. iGAS can occur with/after influenza but has also occurred concurrently with other viral respiratory infections.
Persons with underlying conditions (cancer or immune compromised status; chronic diseases of the heart, kidney or lung; diabetes mellitus) are at higher risk. Other subpopulations at risk for iGAS are illicit drug users, the elderly, homeless persons, nursing home residents, American Indian persons, and Alaska Native persons. Most experts feel that highly toxigenic strains of GAS are responsible for most iGAS. Indeed, most iGAS isolates produce (sometimes hyper-produce) superantigens that cause exaggerated innate immune responses, higher levels of inflammation, and often times tissue destruction, e.g., “flesh eating bacteria.” And who can forget that Jim Henson, creator of the Muppets, died of iGAS?
But why discuss iGAS in 2024? The pattern for iGAS has fluctuated more than usual in the last decade. So much so that the recent upsurge has caught the collective eye of the lay press. So, patients and friends may have questions about why and how iGAS is increasing lately. The bottom line is that no one knows for sure. However, the most recent 2 years of uptick may reflect GAS circulating at relatively high levels even when taking into account that GAS season occurs in winter-spring most years. Yet it seems likely that additional factors may have played a role in the fluctuations noted this past decade, e.g., temporary changes in societal behavior, a new GAS strain with over two dozen mutations, and possibly rapid waning of protection against GAS exotoxins.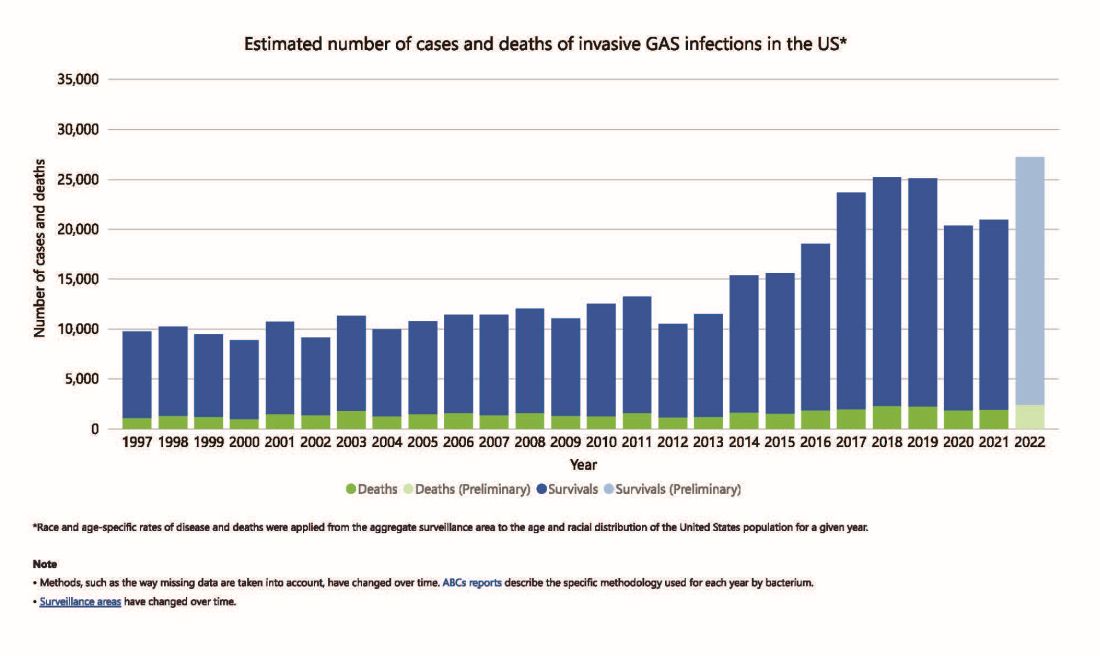
Social Behavior Factor
The SARS-CoV-2 pandemic brought extremes of disease and death to the world and dramatic changes in social behavior. A byproduct was dramatic decreases in nearly all infectious diseases, with numerous reports of near absence of many respiratory and gastrointestinal viruses in the 2020-2021 seasons. Interestingly, we did not see a drop in human rhinovirus infections, justifying its nickname as the cockroach of viruses. Reports also emerged about drops in bacterial diseases during 2020-2021 (although not so much for STIs), including noninvasive and invasive GAS disease, and also GAS-associated deaths (lowest since 2016).1 The drop in iGAS during social restrictions makes sense because GAS is spread by direct contact with infected persons or their secretions, and social contact had dramatically decreased particularly in the first 6 months of the pandemic.
However, since 2022 and the return to “normal” social behaviors, both viral diseases (e.g., RSV, influenza, and Norovirus), and some bacterial diseases have rebounded. That said, something else must be contributing, because iGAS rates had increased 4-5 years pre pandemic. In fact, the fluctuating pattern included “normal” annual rates in the early 2000s rising in ~2015 followed by the explainable pandemic drop (by nearly 25%), and not-too-unexpected 2-year postpandemic rise. But interestingly enough, the rebound is higher than might be expected for iGAS and children were overrepresented in first year’s rise (2022 rate for pediatric iGAS was the highest since 1997) while those older than 65 were overrepresented in second year (2023).1
Emergence of M1UK
One potential factor for the prepandemic rise in iGAS infections worldwide is the emergence and worldwide spread of a new GAS emm type variant designated M1UK.2 GAS isolates can be typed into categories designated as emm types based on DNA sequence. There are more than 240 emm types, with 6 being most common — M1, M3, and M28 (each up to 20% of GAS isolates) and M12, M82, and M89 (each up to 10%). M1, M3 and M28 have also been particularly associated with invasive disease. While emm types vary year to year and region by region, the overall emm type distribution among iGAS isolates in the United States had not been unusual since the turn of the century and the US M1 strain was the same as that which had been predominant worldwide (designated M1GLOBAL). This new M1UK sublineage had emerged around 2010 and had been increasing pre pandemic. The M1UK sequence contained a specific set of 27 SNPs (single nucleoside polymorphisms, i.e., single base mutations) and was associated with an uptick in scarlet fever in the United Kingdom starting around 2010. Its prevalence increased up to around 2015 while spreading internationally. It also had enhanced expression of SpeA, a phage-encoded superantigen. Some of the M1UK mutations also appear to alter GAS metabolic processes to allow better survival (better “fitness”) compared with other GAS. So, a more virulent hardier GAS had arisen and seems a reasonable candidate for contributing to the increased iGAS rates.
Waning Antibody to GAS As Potential Factor in Rebound
No consensus exists on correlates of protection from iGAS. However, adults seem to have less noninvasive GAS than children. One potential reason is that frequent GAS re-exposure, regardless of whether disease results, likely boosts anti-GAS antibodies. Pandemic social restrictions temporarily prevented such boosts. In children with developing antibody repertoires, anti-GAS antibodies may have waned below protective levels faster during a year without frequent boosting. Thus, children were iGAS susceptible soon after pandemic restrictions were dropped (2022). Increased iGAS rates in the elderly in 2023 may have occurred because of diminished GAS exposures accelerating immune senescence with anti-GAS antibodies dropping, but less quickly than in children. These speculations are simply hypotheses until future studies can test them.
All that said, how do we use information on increased iGAS in our daily practices? In addition to standard preventive strategies for viral coinfections (e.g., varicella and influenza vaccine), reminding families about rigorous attention to wound care is the one high-risk scenario we have not yet discussed. During 2024, a time of expected increased prevalence of iGAS, early wound care needs to be fastidious. Further, share warning signs with families (e.g., rapidly expanding painful erythema), “streaks” ascending from extremity wounds, fever and a highly painful wound, darkening almost purple color within cellulitis or soft tissue infection, or loss of sensation in the middle of an otherwise painful soft tissue infection. These presentations require immediate medical attention.
If such a patient presents, the Centers for Disease Control and Prevention (CDC) recommends admission along with blood and, where possible, wound cultures. If in the context of pneumonia with pleural effusion, culturing pleural fluid is also important. Remember, leading edge cultures are not often positive for GAS, seemingly because GAS exotoxins are found at erythema’s leading edge, not the bacteria. The bacteria are somewhere more central in the inflammatory process. Despite not being prominent among recent iGAS cases, another scenario that could sneak up on you is the infected surgical wound as nascent iGAS.
Finally, remember that nationally increasing numbers of iGAS isolates are resistant to erythromycin and clindamycin, the latter usually recommended to reduce tissue damage in iGAS.3 So, it is important to be aware of susceptibility patterns in your locale and consider an ID consultation. My hope is that you do not see an iGAS case this year, but we all need to remain alert. With a high index of suspicion and rapid diagnosis, you can minimize long-term sequelae and potential fatalities.
While it is too early to tell how the rest of 2024 will turn out, preliminary indications are that GAS is circulating at higher than usual levels (30%-35% GAS positive throat swabs in early April 2024 in Kansas City area) and iGAS rates will likely also be relatively high, particularly if Ontario, Canada, data are any indication.4 
Dr. Harrison is professor of pediatrics and pediatric infectious diseases at Children’s Mercy Hospitals and Clinics, Kansas City, Mo. He said he had no relevant financial disclosures. Email him at [email protected].
References
1. Current Group A Strep Activity, Centers for Disease Control and Prevention. April 2024. CDC webpage on current invasive GAS disease. April 2024.
2. Li Y et al. Expansion of Invasive Group A Streptococcus M1UK Lineage in Active Bacterial Core Surveillance, United States, 2019-2021 Emerg Infect Dis. 2023;29(10):2116-2120. doi: 10.3201/eid2910.230675.
3. Andreoni F et al. Clindamycin Affects Group A Streptococcus Virulence Factors and Improves Clinical Outcome. J Infect Dis. 2017 Jan 15;215(2):269-277. doi: 10.1093/infdis/jiw229.
4. Group A Streptococcal Disease, Invasive (iGAS), Public Health Ontario.
Invasive group A streptococcus (iGAS) infections are rare (4-9 cases/100,000 US population annually) but potentially devastating (approximately 2,300 deaths annually in US), and affect all ages. Cases increase in winter-spring, paralleling the “season” of increased noninvasive GAS, e.g., pharyngitis and scarlet fever. iGAS case rates are lower in children than adults. That said, one well-known pediatric iGAS scenario has been deep cellulitis and necrotizing fasciitis during the healing phase of varicella. Other forms of iGAS include bacteremia, pneumonia (particularly when empyema is present), lymphangitis, erysipelas, and toxic shock syndrome. iGAS can occur with/after influenza but has also occurred concurrently with other viral respiratory infections.
Persons with underlying conditions (cancer or immune compromised status; chronic diseases of the heart, kidney or lung; diabetes mellitus) are at higher risk. Other subpopulations at risk for iGAS are illicit drug users, the elderly, homeless persons, nursing home residents, American Indian persons, and Alaska Native persons. Most experts feel that highly toxigenic strains of GAS are responsible for most iGAS. Indeed, most iGAS isolates produce (sometimes hyper-produce) superantigens that cause exaggerated innate immune responses, higher levels of inflammation, and often times tissue destruction, e.g., “flesh eating bacteria.” And who can forget that Jim Henson, creator of the Muppets, died of iGAS?
But why discuss iGAS in 2024? The pattern for iGAS has fluctuated more than usual in the last decade. So much so that the recent upsurge has caught the collective eye of the lay press. So, patients and friends may have questions about why and how iGAS is increasing lately. The bottom line is that no one knows for sure. However, the most recent 2 years of uptick may reflect GAS circulating at relatively high levels even when taking into account that GAS season occurs in winter-spring most years. Yet it seems likely that additional factors may have played a role in the fluctuations noted this past decade, e.g., temporary changes in societal behavior, a new GAS strain with over two dozen mutations, and possibly rapid waning of protection against GAS exotoxins.
Social Behavior Factor
The SARS-CoV-2 pandemic brought extremes of disease and death to the world and dramatic changes in social behavior. A byproduct was dramatic decreases in nearly all infectious diseases, with numerous reports of near absence of many respiratory and gastrointestinal viruses in the 2020-2021 seasons. Interestingly, we did not see a drop in human rhinovirus infections, justifying its nickname as the cockroach of viruses. Reports also emerged about drops in bacterial diseases during 2020-2021 (although not so much for STIs), including noninvasive and invasive GAS disease, and also GAS-associated deaths (lowest since 2016).1 The drop in iGAS during social restrictions makes sense because GAS is spread by direct contact with infected persons or their secretions, and social contact had dramatically decreased particularly in the first 6 months of the pandemic.
However, since 2022 and the return to “normal” social behaviors, both viral diseases (e.g., RSV, influenza, and Norovirus), and some bacterial diseases have rebounded. That said, something else must be contributing, because iGAS rates had increased 4-5 years pre pandemic. In fact, the fluctuating pattern included “normal” annual rates in the early 2000s rising in ~2015 followed by the explainable pandemic drop (by nearly 25%), and not-too-unexpected 2-year postpandemic rise. But interestingly enough, the rebound is higher than might be expected for iGAS and children were overrepresented in first year’s rise (2022 rate for pediatric iGAS was the highest since 1997) while those older than 65 were overrepresented in second year (2023).1
Emergence of M1UK
One potential factor for the prepandemic rise in iGAS infections worldwide is the emergence and worldwide spread of a new GAS emm type variant designated M1UK.2 GAS isolates can be typed into categories designated as emm types based on DNA sequence. There are more than 240 emm types, with 6 being most common — M1, M3, and M28 (each up to 20% of GAS isolates) and M12, M82, and M89 (each up to 10%). M1, M3 and M28 have also been particularly associated with invasive disease. While emm types vary year to year and region by region, the overall emm type distribution among iGAS isolates in the United States had not been unusual since the turn of the century and the US M1 strain was the same as that which had been predominant worldwide (designated M1GLOBAL). This new M1UK sublineage had emerged around 2010 and had been increasing pre pandemic. The M1UK sequence contained a specific set of 27 SNPs (single nucleoside polymorphisms, i.e., single base mutations) and was associated with an uptick in scarlet fever in the United Kingdom starting around 2010. Its prevalence increased up to around 2015 while spreading internationally. It also had enhanced expression of SpeA, a phage-encoded superantigen. Some of the M1UK mutations also appear to alter GAS metabolic processes to allow better survival (better “fitness”) compared with other GAS. So, a more virulent hardier GAS had arisen and seems a reasonable candidate for contributing to the increased iGAS rates.
Waning Antibody to GAS As Potential Factor in Rebound
No consensus exists on correlates of protection from iGAS. However, adults seem to have less noninvasive GAS than children. One potential reason is that frequent GAS re-exposure, regardless of whether disease results, likely boosts anti-GAS antibodies. Pandemic social restrictions temporarily prevented such boosts. In children with developing antibody repertoires, anti-GAS antibodies may have waned below protective levels faster during a year without frequent boosting. Thus, children were iGAS susceptible soon after pandemic restrictions were dropped (2022). Increased iGAS rates in the elderly in 2023 may have occurred because of diminished GAS exposures accelerating immune senescence with anti-GAS antibodies dropping, but less quickly than in children. These speculations are simply hypotheses until future studies can test them.
All that said, how do we use information on increased iGAS in our daily practices? In addition to standard preventive strategies for viral coinfections (e.g., varicella and influenza vaccine), reminding families about rigorous attention to wound care is the one high-risk scenario we have not yet discussed. During 2024, a time of expected increased prevalence of iGAS, early wound care needs to be fastidious. Further, share warning signs with families (e.g., rapidly expanding painful erythema), “streaks” ascending from extremity wounds, fever and a highly painful wound, darkening almost purple color within cellulitis or soft tissue infection, or loss of sensation in the middle of an otherwise painful soft tissue infection. These presentations require immediate medical attention.
If such a patient presents, the Centers for Disease Control and Prevention (CDC) recommends admission along with blood and, where possible, wound cultures. If in the context of pneumonia with pleural effusion, culturing pleural fluid is also important. Remember, leading edge cultures are not often positive for GAS, seemingly because GAS exotoxins are found at erythema’s leading edge, not the bacteria. The bacteria are somewhere more central in the inflammatory process. Despite not being prominent among recent iGAS cases, another scenario that could sneak up on you is the infected surgical wound as nascent iGAS.
Finally, remember that nationally increasing numbers of iGAS isolates are resistant to erythromycin and clindamycin, the latter usually recommended to reduce tissue damage in iGAS.3 So, it is important to be aware of susceptibility patterns in your locale and consider an ID consultation. My hope is that you do not see an iGAS case this year, but we all need to remain alert. With a high index of suspicion and rapid diagnosis, you can minimize long-term sequelae and potential fatalities.
While it is too early to tell how the rest of 2024 will turn out, preliminary indications are that GAS is circulating at higher than usual levels (30%-35% GAS positive throat swabs in early April 2024 in Kansas City area) and iGAS rates will likely also be relatively high, particularly if Ontario, Canada, data are any indication.4 
Dr. Harrison is professor of pediatrics and pediatric infectious diseases at Children’s Mercy Hospitals and Clinics, Kansas City, Mo. He said he had no relevant financial disclosures. Email him at [email protected].
References
1. Current Group A Strep Activity, Centers for Disease Control and Prevention. April 2024. CDC webpage on current invasive GAS disease. April 2024.
2. Li Y et al. Expansion of Invasive Group A Streptococcus M1UK Lineage in Active Bacterial Core Surveillance, United States, 2019-2021 Emerg Infect Dis. 2023;29(10):2116-2120. doi: 10.3201/eid2910.230675.
3. Andreoni F et al. Clindamycin Affects Group A Streptococcus Virulence Factors and Improves Clinical Outcome. J Infect Dis. 2017 Jan 15;215(2):269-277. doi: 10.1093/infdis/jiw229.
4. Group A Streptococcal Disease, Invasive (iGAS), Public Health Ontario.
Invasive group A streptococcus (iGAS) infections are rare (4-9 cases/100,000 US population annually) but potentially devastating (approximately 2,300 deaths annually in US), and affect all ages. Cases increase in winter-spring, paralleling the “season” of increased noninvasive GAS, e.g., pharyngitis and scarlet fever. iGAS case rates are lower in children than adults. That said, one well-known pediatric iGAS scenario has been deep cellulitis and necrotizing fasciitis during the healing phase of varicella. Other forms of iGAS include bacteremia, pneumonia (particularly when empyema is present), lymphangitis, erysipelas, and toxic shock syndrome. iGAS can occur with/after influenza but has also occurred concurrently with other viral respiratory infections.
Persons with underlying conditions (cancer or immune compromised status; chronic diseases of the heart, kidney or lung; diabetes mellitus) are at higher risk. Other subpopulations at risk for iGAS are illicit drug users, the elderly, homeless persons, nursing home residents, American Indian persons, and Alaska Native persons. Most experts feel that highly toxigenic strains of GAS are responsible for most iGAS. Indeed, most iGAS isolates produce (sometimes hyper-produce) superantigens that cause exaggerated innate immune responses, higher levels of inflammation, and often times tissue destruction, e.g., “flesh eating bacteria.” And who can forget that Jim Henson, creator of the Muppets, died of iGAS?
But why discuss iGAS in 2024? The pattern for iGAS has fluctuated more than usual in the last decade. So much so that the recent upsurge has caught the collective eye of the lay press. So, patients and friends may have questions about why and how iGAS is increasing lately. The bottom line is that no one knows for sure. However, the most recent 2 years of uptick may reflect GAS circulating at relatively high levels even when taking into account that GAS season occurs in winter-spring most years. Yet it seems likely that additional factors may have played a role in the fluctuations noted this past decade, e.g., temporary changes in societal behavior, a new GAS strain with over two dozen mutations, and possibly rapid waning of protection against GAS exotoxins.
Social Behavior Factor
The SARS-CoV-2 pandemic brought extremes of disease and death to the world and dramatic changes in social behavior. A byproduct was dramatic decreases in nearly all infectious diseases, with numerous reports of near absence of many respiratory and gastrointestinal viruses in the 2020-2021 seasons. Interestingly, we did not see a drop in human rhinovirus infections, justifying its nickname as the cockroach of viruses. Reports also emerged about drops in bacterial diseases during 2020-2021 (although not so much for STIs), including noninvasive and invasive GAS disease, and also GAS-associated deaths (lowest since 2016).1 The drop in iGAS during social restrictions makes sense because GAS is spread by direct contact with infected persons or their secretions, and social contact had dramatically decreased particularly in the first 6 months of the pandemic.
However, since 2022 and the return to “normal” social behaviors, both viral diseases (e.g., RSV, influenza, and Norovirus), and some bacterial diseases have rebounded. That said, something else must be contributing, because iGAS rates had increased 4-5 years pre pandemic. In fact, the fluctuating pattern included “normal” annual rates in the early 2000s rising in ~2015 followed by the explainable pandemic drop (by nearly 25%), and not-too-unexpected 2-year postpandemic rise. But interestingly enough, the rebound is higher than might be expected for iGAS and children were overrepresented in first year’s rise (2022 rate for pediatric iGAS was the highest since 1997) while those older than 65 were overrepresented in second year (2023).1
Emergence of M1UK
One potential factor for the prepandemic rise in iGAS infections worldwide is the emergence and worldwide spread of a new GAS emm type variant designated M1UK.2 GAS isolates can be typed into categories designated as emm types based on DNA sequence. There are more than 240 emm types, with 6 being most common — M1, M3, and M28 (each up to 20% of GAS isolates) and M12, M82, and M89 (each up to 10%). M1, M3 and M28 have also been particularly associated with invasive disease. While emm types vary year to year and region by region, the overall emm type distribution among iGAS isolates in the United States had not been unusual since the turn of the century and the US M1 strain was the same as that which had been predominant worldwide (designated M1GLOBAL). This new M1UK sublineage had emerged around 2010 and had been increasing pre pandemic. The M1UK sequence contained a specific set of 27 SNPs (single nucleoside polymorphisms, i.e., single base mutations) and was associated with an uptick in scarlet fever in the United Kingdom starting around 2010. Its prevalence increased up to around 2015 while spreading internationally. It also had enhanced expression of SpeA, a phage-encoded superantigen. Some of the M1UK mutations also appear to alter GAS metabolic processes to allow better survival (better “fitness”) compared with other GAS. So, a more virulent hardier GAS had arisen and seems a reasonable candidate for contributing to the increased iGAS rates.
Waning Antibody to GAS As Potential Factor in Rebound
No consensus exists on correlates of protection from iGAS. However, adults seem to have less noninvasive GAS than children. One potential reason is that frequent GAS re-exposure, regardless of whether disease results, likely boosts anti-GAS antibodies. Pandemic social restrictions temporarily prevented such boosts. In children with developing antibody repertoires, anti-GAS antibodies may have waned below protective levels faster during a year without frequent boosting. Thus, children were iGAS susceptible soon after pandemic restrictions were dropped (2022). Increased iGAS rates in the elderly in 2023 may have occurred because of diminished GAS exposures accelerating immune senescence with anti-GAS antibodies dropping, but less quickly than in children. These speculations are simply hypotheses until future studies can test them.
All that said, how do we use information on increased iGAS in our daily practices? In addition to standard preventive strategies for viral coinfections (e.g., varicella and influenza vaccine), reminding families about rigorous attention to wound care is the one high-risk scenario we have not yet discussed. During 2024, a time of expected increased prevalence of iGAS, early wound care needs to be fastidious. Further, share warning signs with families (e.g., rapidly expanding painful erythema), “streaks” ascending from extremity wounds, fever and a highly painful wound, darkening almost purple color within cellulitis or soft tissue infection, or loss of sensation in the middle of an otherwise painful soft tissue infection. These presentations require immediate medical attention.
If such a patient presents, the Centers for Disease Control and Prevention (CDC) recommends admission along with blood and, where possible, wound cultures. If in the context of pneumonia with pleural effusion, culturing pleural fluid is also important. Remember, leading edge cultures are not often positive for GAS, seemingly because GAS exotoxins are found at erythema’s leading edge, not the bacteria. The bacteria are somewhere more central in the inflammatory process. Despite not being prominent among recent iGAS cases, another scenario that could sneak up on you is the infected surgical wound as nascent iGAS.
Finally, remember that nationally increasing numbers of iGAS isolates are resistant to erythromycin and clindamycin, the latter usually recommended to reduce tissue damage in iGAS.3 So, it is important to be aware of susceptibility patterns in your locale and consider an ID consultation. My hope is that you do not see an iGAS case this year, but we all need to remain alert. With a high index of suspicion and rapid diagnosis, you can minimize long-term sequelae and potential fatalities.
While it is too early to tell how the rest of 2024 will turn out, preliminary indications are that GAS is circulating at higher than usual levels (30%-35% GAS positive throat swabs in early April 2024 in Kansas City area) and iGAS rates will likely also be relatively high, particularly if Ontario, Canada, data are any indication.4 
Dr. Harrison is professor of pediatrics and pediatric infectious diseases at Children’s Mercy Hospitals and Clinics, Kansas City, Mo. He said he had no relevant financial disclosures. Email him at [email protected].
References
1. Current Group A Strep Activity, Centers for Disease Control and Prevention. April 2024. CDC webpage on current invasive GAS disease. April 2024.
2. Li Y et al. Expansion of Invasive Group A Streptococcus M1UK Lineage in Active Bacterial Core Surveillance, United States, 2019-2021 Emerg Infect Dis. 2023;29(10):2116-2120. doi: 10.3201/eid2910.230675.
3. Andreoni F et al. Clindamycin Affects Group A Streptococcus Virulence Factors and Improves Clinical Outcome. J Infect Dis. 2017 Jan 15;215(2):269-277. doi: 10.1093/infdis/jiw229.
4. Group A Streptococcal Disease, Invasive (iGAS), Public Health Ontario.
Antibiotics of Little Benefit in Lower Respiratory Tract Infection
Antibiotics had no measurable effect on the severity or duration of coughs due to acute lower respiratory tract infection (LRTI, or acute bronchitis), a large prospective study found.
In fact, those receiving an antibiotic in the primary- and urgent-care setting had a small but significant increase in overall length of illness (17.5 vs 15.9 days; P = .05) — largely because patients with longer illness before the index visit were more likely to receive these drugs. The study adds further support for reducing the prescription of antibiotics for LRTIs.
“Importantly, the pathogen data demonstrated that the length of time until illness resolution for those with bacterial infection was the same as for those not receiving an antibiotic versus those receiving one (17.3 vs 17.4 days),” researchers led by Daniel J. Merenstein, MD, a professor and director of research programs, family medicine, at Georgetown University Medical Center in Washington, wrote in the Journal of General Internal Medicine (doi: 10.1007/s11606-024-08758-y).
Patients believed an antibiotic would shorten their illness by an average of about 4 days, from 13.4 days to 9.7 days, whereas the average duration of all coughs was more than 2 weeks regardless of pathogen type or receipt of an antibiotic.
“Patients had unrealistic expectations regarding the duration of LRTI and the effect of antibiotics, which should be the target of antibiotic stewardship efforts,” the group wrote.
LRTIs can, however, be dangerous, with 3%-5% progressing to pneumonia, “but not everyone has easy access at an initial visit to an x-ray, which may be the reason clinicians still give antibiotics without any other evidence of a bacterial infection,” Dr. Merenstein said in a news release. “Patients have come to expect antibiotics for a cough, even if it doesn’t help. Basic symptom-relieving medications plus time bring a resolution to most people’s infections.”
The authors noted that cough is the most common reason for an ambulatory care visit, accounting for 2.7 million outpatient visits and more than 4 million emergency department visits annually.
Risks
Overuse of antibiotics can result in dizziness, nausea, diarrhea, and rash, along with a roughly 4% chance of serious adverse effects including anaphylaxis; Stevens-Johnson syndrome, a serious skin and mucous membrane disorder; and Clostridioides difficile-associated diarrhea.
An estimated half of all antibiotic prescriptions for acute respiratory conditions are unnecessary. Before the COVID-19 pandemic, antibiotics were prescribed about 70% of the time for a diagnosis of uncomplicated cough and LRTI. The viral pandemic did not change this practice according to a meta-analysis of 130 studies showing that 78% of COVID-19 patients were prescribed an antibiotic.
The study
The study looked at a cohort of 718 patients, with a mean age of 38.9 years, 65.3% female, of whom 207 received an antibiotic and 511 did not. Of those with baseline data, 29% had an antibiotic prescribed at baseline, the most common (in 85%) being amoxicillin-clavulanate, azithromycin, doxycycline, and amoxicillin. Antibiotics had no effect on the duration or overall severity of cough in viral, bacterial, or mixed infections. Receipt of an antibiotic did, however, reduce the likelihood of a follow-up visit: 14.1% vs 8.2% (adjusted odds ratio, 0.47; 95% confidence interval, 0.26-0.84) — perhaps because it removed the motivation for seeking another consultation. Antibiotic recipients were more likely to receive a systemic corticosteroid (31.9% vs 4.5%, P <.001) and were also more likely to receive an albuterol inhaler (22.7% vs 7.6%, P <.001).
Jeffrey A. Linder, MD, MPH, a primary care physician and chief of internal medicine and geriatrics at Northwestern University Feinberg School of Medicine in Chicago, agrees that in the vast majority of LRTIs — usually acute bronchitis — antibiotics do not speed the healing process. “Forty years of research show that antibiotics do not make acute bronchitis go away any faster,” Dr. Linder, who was not involved in the current study, said in an interview. “There’s even growing evidence that a lot of pneumonia is viral as well, and 10 or 20 years from now we may often not be giving antibiotics for pneumonia because we’ll be able to see better if it’s caused by a virus.”
A large 2018 review by Dr. Linder and associates reported that 46% of antibiotics were prescribed without any infection-related diagnosis code and 20% without an office visit.
Dr. Linder routinely informs patients requesting an antibiotic about the risks of putting an ineffective chemical into their body. “I stress that it can cause rash and other allergic reactions, and even promote C diff infection,” he said. “And I also say it messes with the good bacteria in the microbiome, and they usually come around.”
Patients need to know, Dr. Linder added, that the normal course of healing the respiratory tract after acute bronchitis takes weeks. While a wet cough with sputum or phlegm will last a few days, it’s replaced with a dry annoying cough that persists for up to 3 weeks. “As long as they’re feeling generally better, that cough is normal,” he said. “A virus has run roughshod over their airways and they need a long time to heal and the cough is part of the healing process. Think how long it takes to heal a cut on a finger.”
In an era of escalating antimicrobial resistance fueled by antibiotic overuse, it’s become increasingly important to reserve antibiotics for necessary cases. According to a recent World Health Organization call to action, “Uncontrolled antimicrobial resistance is expected to lower life expectancy and lead to unprecedented health expenditure and economic losses.”
That said, there is important clinical work to be done to determine if there is a limited role for antibiotics in patients with cough, perhaps based on age and baseline severity. “Serious cough symptoms and how to treat them properly needs to be studied more, perhaps in a randomized clinical trial as this study was observational and there haven’t been any randomized trials looking at this issue since about 2012,” Dr. Merenstein said.
This research was funded by the Agency for Healthcare Research and Quality. The authors have no conflicts of interest to declare. Dr. Linder reported stock ownership in pharmaceutical companies but none that make antibiotics or other infectious disease drugs.
Antibiotics had no measurable effect on the severity or duration of coughs due to acute lower respiratory tract infection (LRTI, or acute bronchitis), a large prospective study found.
In fact, those receiving an antibiotic in the primary- and urgent-care setting had a small but significant increase in overall length of illness (17.5 vs 15.9 days; P = .05) — largely because patients with longer illness before the index visit were more likely to receive these drugs. The study adds further support for reducing the prescription of antibiotics for LRTIs.
“Importantly, the pathogen data demonstrated that the length of time until illness resolution for those with bacterial infection was the same as for those not receiving an antibiotic versus those receiving one (17.3 vs 17.4 days),” researchers led by Daniel J. Merenstein, MD, a professor and director of research programs, family medicine, at Georgetown University Medical Center in Washington, wrote in the Journal of General Internal Medicine (doi: 10.1007/s11606-024-08758-y).
Patients believed an antibiotic would shorten their illness by an average of about 4 days, from 13.4 days to 9.7 days, whereas the average duration of all coughs was more than 2 weeks regardless of pathogen type or receipt of an antibiotic.
“Patients had unrealistic expectations regarding the duration of LRTI and the effect of antibiotics, which should be the target of antibiotic stewardship efforts,” the group wrote.
LRTIs can, however, be dangerous, with 3%-5% progressing to pneumonia, “but not everyone has easy access at an initial visit to an x-ray, which may be the reason clinicians still give antibiotics without any other evidence of a bacterial infection,” Dr. Merenstein said in a news release. “Patients have come to expect antibiotics for a cough, even if it doesn’t help. Basic symptom-relieving medications plus time bring a resolution to most people’s infections.”
The authors noted that cough is the most common reason for an ambulatory care visit, accounting for 2.7 million outpatient visits and more than 4 million emergency department visits annually.
Risks
Overuse of antibiotics can result in dizziness, nausea, diarrhea, and rash, along with a roughly 4% chance of serious adverse effects including anaphylaxis; Stevens-Johnson syndrome, a serious skin and mucous membrane disorder; and Clostridioides difficile-associated diarrhea.
An estimated half of all antibiotic prescriptions for acute respiratory conditions are unnecessary. Before the COVID-19 pandemic, antibiotics were prescribed about 70% of the time for a diagnosis of uncomplicated cough and LRTI. The viral pandemic did not change this practice according to a meta-analysis of 130 studies showing that 78% of COVID-19 patients were prescribed an antibiotic.
The study
The study looked at a cohort of 718 patients, with a mean age of 38.9 years, 65.3% female, of whom 207 received an antibiotic and 511 did not. Of those with baseline data, 29% had an antibiotic prescribed at baseline, the most common (in 85%) being amoxicillin-clavulanate, azithromycin, doxycycline, and amoxicillin. Antibiotics had no effect on the duration or overall severity of cough in viral, bacterial, or mixed infections. Receipt of an antibiotic did, however, reduce the likelihood of a follow-up visit: 14.1% vs 8.2% (adjusted odds ratio, 0.47; 95% confidence interval, 0.26-0.84) — perhaps because it removed the motivation for seeking another consultation. Antibiotic recipients were more likely to receive a systemic corticosteroid (31.9% vs 4.5%, P <.001) and were also more likely to receive an albuterol inhaler (22.7% vs 7.6%, P <.001).
Jeffrey A. Linder, MD, MPH, a primary care physician and chief of internal medicine and geriatrics at Northwestern University Feinberg School of Medicine in Chicago, agrees that in the vast majority of LRTIs — usually acute bronchitis — antibiotics do not speed the healing process. “Forty years of research show that antibiotics do not make acute bronchitis go away any faster,” Dr. Linder, who was not involved in the current study, said in an interview. “There’s even growing evidence that a lot of pneumonia is viral as well, and 10 or 20 years from now we may often not be giving antibiotics for pneumonia because we’ll be able to see better if it’s caused by a virus.”
A large 2018 review by Dr. Linder and associates reported that 46% of antibiotics were prescribed without any infection-related diagnosis code and 20% without an office visit.
Dr. Linder routinely informs patients requesting an antibiotic about the risks of putting an ineffective chemical into their body. “I stress that it can cause rash and other allergic reactions, and even promote C diff infection,” he said. “And I also say it messes with the good bacteria in the microbiome, and they usually come around.”
Patients need to know, Dr. Linder added, that the normal course of healing the respiratory tract after acute bronchitis takes weeks. While a wet cough with sputum or phlegm will last a few days, it’s replaced with a dry annoying cough that persists for up to 3 weeks. “As long as they’re feeling generally better, that cough is normal,” he said. “A virus has run roughshod over their airways and they need a long time to heal and the cough is part of the healing process. Think how long it takes to heal a cut on a finger.”
In an era of escalating antimicrobial resistance fueled by antibiotic overuse, it’s become increasingly important to reserve antibiotics for necessary cases. According to a recent World Health Organization call to action, “Uncontrolled antimicrobial resistance is expected to lower life expectancy and lead to unprecedented health expenditure and economic losses.”
That said, there is important clinical work to be done to determine if there is a limited role for antibiotics in patients with cough, perhaps based on age and baseline severity. “Serious cough symptoms and how to treat them properly needs to be studied more, perhaps in a randomized clinical trial as this study was observational and there haven’t been any randomized trials looking at this issue since about 2012,” Dr. Merenstein said.
This research was funded by the Agency for Healthcare Research and Quality. The authors have no conflicts of interest to declare. Dr. Linder reported stock ownership in pharmaceutical companies but none that make antibiotics or other infectious disease drugs.
Antibiotics had no measurable effect on the severity or duration of coughs due to acute lower respiratory tract infection (LRTI, or acute bronchitis), a large prospective study found.
In fact, those receiving an antibiotic in the primary- and urgent-care setting had a small but significant increase in overall length of illness (17.5 vs 15.9 days; P = .05) — largely because patients with longer illness before the index visit were more likely to receive these drugs. The study adds further support for reducing the prescription of antibiotics for LRTIs.
“Importantly, the pathogen data demonstrated that the length of time until illness resolution for those with bacterial infection was the same as for those not receiving an antibiotic versus those receiving one (17.3 vs 17.4 days),” researchers led by Daniel J. Merenstein, MD, a professor and director of research programs, family medicine, at Georgetown University Medical Center in Washington, wrote in the Journal of General Internal Medicine (doi: 10.1007/s11606-024-08758-y).
Patients believed an antibiotic would shorten their illness by an average of about 4 days, from 13.4 days to 9.7 days, whereas the average duration of all coughs was more than 2 weeks regardless of pathogen type or receipt of an antibiotic.
“Patients had unrealistic expectations regarding the duration of LRTI and the effect of antibiotics, which should be the target of antibiotic stewardship efforts,” the group wrote.
LRTIs can, however, be dangerous, with 3%-5% progressing to pneumonia, “but not everyone has easy access at an initial visit to an x-ray, which may be the reason clinicians still give antibiotics without any other evidence of a bacterial infection,” Dr. Merenstein said in a news release. “Patients have come to expect antibiotics for a cough, even if it doesn’t help. Basic symptom-relieving medications plus time bring a resolution to most people’s infections.”
The authors noted that cough is the most common reason for an ambulatory care visit, accounting for 2.7 million outpatient visits and more than 4 million emergency department visits annually.
Risks
Overuse of antibiotics can result in dizziness, nausea, diarrhea, and rash, along with a roughly 4% chance of serious adverse effects including anaphylaxis; Stevens-Johnson syndrome, a serious skin and mucous membrane disorder; and Clostridioides difficile-associated diarrhea.
An estimated half of all antibiotic prescriptions for acute respiratory conditions are unnecessary. Before the COVID-19 pandemic, antibiotics were prescribed about 70% of the time for a diagnosis of uncomplicated cough and LRTI. The viral pandemic did not change this practice according to a meta-analysis of 130 studies showing that 78% of COVID-19 patients were prescribed an antibiotic.
The study
The study looked at a cohort of 718 patients, with a mean age of 38.9 years, 65.3% female, of whom 207 received an antibiotic and 511 did not. Of those with baseline data, 29% had an antibiotic prescribed at baseline, the most common (in 85%) being amoxicillin-clavulanate, azithromycin, doxycycline, and amoxicillin. Antibiotics had no effect on the duration or overall severity of cough in viral, bacterial, or mixed infections. Receipt of an antibiotic did, however, reduce the likelihood of a follow-up visit: 14.1% vs 8.2% (adjusted odds ratio, 0.47; 95% confidence interval, 0.26-0.84) — perhaps because it removed the motivation for seeking another consultation. Antibiotic recipients were more likely to receive a systemic corticosteroid (31.9% vs 4.5%, P <.001) and were also more likely to receive an albuterol inhaler (22.7% vs 7.6%, P <.001).
Jeffrey A. Linder, MD, MPH, a primary care physician and chief of internal medicine and geriatrics at Northwestern University Feinberg School of Medicine in Chicago, agrees that in the vast majority of LRTIs — usually acute bronchitis — antibiotics do not speed the healing process. “Forty years of research show that antibiotics do not make acute bronchitis go away any faster,” Dr. Linder, who was not involved in the current study, said in an interview. “There’s even growing evidence that a lot of pneumonia is viral as well, and 10 or 20 years from now we may often not be giving antibiotics for pneumonia because we’ll be able to see better if it’s caused by a virus.”
A large 2018 review by Dr. Linder and associates reported that 46% of antibiotics were prescribed without any infection-related diagnosis code and 20% without an office visit.
Dr. Linder routinely informs patients requesting an antibiotic about the risks of putting an ineffective chemical into their body. “I stress that it can cause rash and other allergic reactions, and even promote C diff infection,” he said. “And I also say it messes with the good bacteria in the microbiome, and they usually come around.”
Patients need to know, Dr. Linder added, that the normal course of healing the respiratory tract after acute bronchitis takes weeks. While a wet cough with sputum or phlegm will last a few days, it’s replaced with a dry annoying cough that persists for up to 3 weeks. “As long as they’re feeling generally better, that cough is normal,” he said. “A virus has run roughshod over their airways and they need a long time to heal and the cough is part of the healing process. Think how long it takes to heal a cut on a finger.”
In an era of escalating antimicrobial resistance fueled by antibiotic overuse, it’s become increasingly important to reserve antibiotics for necessary cases. According to a recent World Health Organization call to action, “Uncontrolled antimicrobial resistance is expected to lower life expectancy and lead to unprecedented health expenditure and economic losses.”
That said, there is important clinical work to be done to determine if there is a limited role for antibiotics in patients with cough, perhaps based on age and baseline severity. “Serious cough symptoms and how to treat them properly needs to be studied more, perhaps in a randomized clinical trial as this study was observational and there haven’t been any randomized trials looking at this issue since about 2012,” Dr. Merenstein said.
This research was funded by the Agency for Healthcare Research and Quality. The authors have no conflicts of interest to declare. Dr. Linder reported stock ownership in pharmaceutical companies but none that make antibiotics or other infectious disease drugs.
FROM JOURNAL OF GENERAL INTERNAL MEDICINE
European Scientists Assess Avian Flu Pandemic Risk
As avian influenza continues to spread among wild bird populations in the European Union (EU), scientists have described a wide range of factors that could drive the virus to spread efficiently among humans, thereby increasing its pandemic potential.
Although transmission of avian influenza A(H5N1) from infected birds to humans is rare, “new strains carrying potential mutations for mammalian adaptation” could occur, according to a report issued on April 3 by the European Centre for Disease Prevention and Control and the European Food Safety Authority. The analysis identified a threat of strains currently circulating outside Europe that could enter the EU and the wider European Economic Area (EEA).
“If avian A(H5N1) influenza viruses acquire the ability to spread efficiently among humans, large-scale transmission could occur due to the lack of immune defenses against H5 viruses in humans,” the report warned.
Evolution of Avian Influenza Remains Hard to Predict
However, despite many occurrences of human exposure to avian influenza since 2020, “no symptomatic or productive infection in a human has been identified in the EU/EEA,” the scientists stated. Furthermore, after almost three decades of human exposure to the A(H5N1) virus of the Gs/GD lineage, the virus has not yet acquired the mutations required for airborne transmissibility between humans. However, it remains “difficult to predict the evolutionary direction the virus will take in the future,” the scientists assessed.
“Clearly, humans are being exposed in the current USA cattle outbreak,” Professor James Wood, infectious disease epidemiologist at the University of Cambridge, United Kingdom, told this news organization. “But, arguably, what is more significant is how few cases there have been with this virus lineage and its close relatives, despite massive global exposures over the last 3 years. All diagnosed human cases seem to have been singletons, with no evidence of human-to-human transmission.”
Ian Jones, professor of virology at the University of Reading, United Kingdom, sees no evidence of an imminent spillover of avian influenza from birds. But he told this news organization: “The trouble is, the clock resets every minute. Every time the virus has come out of a bird and gone somewhere, the clock is reset. So you can never say that just because it hasn’t happened since whenever, it’s never going to happen.”
Preventive Measures Recommended
The European report recommended a range of cautionary measures that included enhanced surveillance, access to rapid diagnostics, and sharing of genetic sequence data. It urged EU authorities to work together, adopting a One Health perspective, to limit the exposure of mammals, including humans, to avian influenza viruses.
Sarah Pitt, a microbiologist at the University of Brighton, United Kingdom, said the emphasis on authorities taking a One Health approach was sound. “You’re looking at humans, animals, plants, and the environment and how they’re all closely interacted,” she told this news organization. “Putting all those things together is actually going to be good for human health. So they’ve mentioned One Health a lot and I’m sure that’s on purpose because it’s the latest buzzword, and presumably it’s a way of getting governments to take it seriously.”
Overall, Dr. Pitt believes the document is designed to move zoonotic infectious diseases a bit higher up the agenda. “They should have been higher up the agenda before COVID,” she said.
The report also called for consideration of preventative measures, such as vaccination of poultry flocks.
Overall, Dr. Jones assesses the European report as “a reworking of what’s been pretty well covered over the years.” Despite extensive work by scientists in the field, he said: “I’m not sure we’re any better at predicting an emerging virus than we’ve ever been. I would point out that we didn’t spot SARS-CoV-2 coming, even though we had SARS-CoV-1 a few years earlier. Nobody spotted the 2009 pandemic from influenza, even though there was a lot of surveillance around at the time.”
A version of this article appeared on Medscape.com.
As avian influenza continues to spread among wild bird populations in the European Union (EU), scientists have described a wide range of factors that could drive the virus to spread efficiently among humans, thereby increasing its pandemic potential.
Although transmission of avian influenza A(H5N1) from infected birds to humans is rare, “new strains carrying potential mutations for mammalian adaptation” could occur, according to a report issued on April 3 by the European Centre for Disease Prevention and Control and the European Food Safety Authority. The analysis identified a threat of strains currently circulating outside Europe that could enter the EU and the wider European Economic Area (EEA).
“If avian A(H5N1) influenza viruses acquire the ability to spread efficiently among humans, large-scale transmission could occur due to the lack of immune defenses against H5 viruses in humans,” the report warned.
Evolution of Avian Influenza Remains Hard to Predict
However, despite many occurrences of human exposure to avian influenza since 2020, “no symptomatic or productive infection in a human has been identified in the EU/EEA,” the scientists stated. Furthermore, after almost three decades of human exposure to the A(H5N1) virus of the Gs/GD lineage, the virus has not yet acquired the mutations required for airborne transmissibility between humans. However, it remains “difficult to predict the evolutionary direction the virus will take in the future,” the scientists assessed.
“Clearly, humans are being exposed in the current USA cattle outbreak,” Professor James Wood, infectious disease epidemiologist at the University of Cambridge, United Kingdom, told this news organization. “But, arguably, what is more significant is how few cases there have been with this virus lineage and its close relatives, despite massive global exposures over the last 3 years. All diagnosed human cases seem to have been singletons, with no evidence of human-to-human transmission.”
Ian Jones, professor of virology at the University of Reading, United Kingdom, sees no evidence of an imminent spillover of avian influenza from birds. But he told this news organization: “The trouble is, the clock resets every minute. Every time the virus has come out of a bird and gone somewhere, the clock is reset. So you can never say that just because it hasn’t happened since whenever, it’s never going to happen.”
Preventive Measures Recommended
The European report recommended a range of cautionary measures that included enhanced surveillance, access to rapid diagnostics, and sharing of genetic sequence data. It urged EU authorities to work together, adopting a One Health perspective, to limit the exposure of mammals, including humans, to avian influenza viruses.
Sarah Pitt, a microbiologist at the University of Brighton, United Kingdom, said the emphasis on authorities taking a One Health approach was sound. “You’re looking at humans, animals, plants, and the environment and how they’re all closely interacted,” she told this news organization. “Putting all those things together is actually going to be good for human health. So they’ve mentioned One Health a lot and I’m sure that’s on purpose because it’s the latest buzzword, and presumably it’s a way of getting governments to take it seriously.”
Overall, Dr. Pitt believes the document is designed to move zoonotic infectious diseases a bit higher up the agenda. “They should have been higher up the agenda before COVID,” she said.
The report also called for consideration of preventative measures, such as vaccination of poultry flocks.
Overall, Dr. Jones assesses the European report as “a reworking of what’s been pretty well covered over the years.” Despite extensive work by scientists in the field, he said: “I’m not sure we’re any better at predicting an emerging virus than we’ve ever been. I would point out that we didn’t spot SARS-CoV-2 coming, even though we had SARS-CoV-1 a few years earlier. Nobody spotted the 2009 pandemic from influenza, even though there was a lot of surveillance around at the time.”
A version of this article appeared on Medscape.com.
As avian influenza continues to spread among wild bird populations in the European Union (EU), scientists have described a wide range of factors that could drive the virus to spread efficiently among humans, thereby increasing its pandemic potential.
Although transmission of avian influenza A(H5N1) from infected birds to humans is rare, “new strains carrying potential mutations for mammalian adaptation” could occur, according to a report issued on April 3 by the European Centre for Disease Prevention and Control and the European Food Safety Authority. The analysis identified a threat of strains currently circulating outside Europe that could enter the EU and the wider European Economic Area (EEA).
“If avian A(H5N1) influenza viruses acquire the ability to spread efficiently among humans, large-scale transmission could occur due to the lack of immune defenses against H5 viruses in humans,” the report warned.
Evolution of Avian Influenza Remains Hard to Predict
However, despite many occurrences of human exposure to avian influenza since 2020, “no symptomatic or productive infection in a human has been identified in the EU/EEA,” the scientists stated. Furthermore, after almost three decades of human exposure to the A(H5N1) virus of the Gs/GD lineage, the virus has not yet acquired the mutations required for airborne transmissibility between humans. However, it remains “difficult to predict the evolutionary direction the virus will take in the future,” the scientists assessed.
“Clearly, humans are being exposed in the current USA cattle outbreak,” Professor James Wood, infectious disease epidemiologist at the University of Cambridge, United Kingdom, told this news organization. “But, arguably, what is more significant is how few cases there have been with this virus lineage and its close relatives, despite massive global exposures over the last 3 years. All diagnosed human cases seem to have been singletons, with no evidence of human-to-human transmission.”
Ian Jones, professor of virology at the University of Reading, United Kingdom, sees no evidence of an imminent spillover of avian influenza from birds. But he told this news organization: “The trouble is, the clock resets every minute. Every time the virus has come out of a bird and gone somewhere, the clock is reset. So you can never say that just because it hasn’t happened since whenever, it’s never going to happen.”
Preventive Measures Recommended
The European report recommended a range of cautionary measures that included enhanced surveillance, access to rapid diagnostics, and sharing of genetic sequence data. It urged EU authorities to work together, adopting a One Health perspective, to limit the exposure of mammals, including humans, to avian influenza viruses.
Sarah Pitt, a microbiologist at the University of Brighton, United Kingdom, said the emphasis on authorities taking a One Health approach was sound. “You’re looking at humans, animals, plants, and the environment and how they’re all closely interacted,” she told this news organization. “Putting all those things together is actually going to be good for human health. So they’ve mentioned One Health a lot and I’m sure that’s on purpose because it’s the latest buzzword, and presumably it’s a way of getting governments to take it seriously.”
Overall, Dr. Pitt believes the document is designed to move zoonotic infectious diseases a bit higher up the agenda. “They should have been higher up the agenda before COVID,” she said.
The report also called for consideration of preventative measures, such as vaccination of poultry flocks.
Overall, Dr. Jones assesses the European report as “a reworking of what’s been pretty well covered over the years.” Despite extensive work by scientists in the field, he said: “I’m not sure we’re any better at predicting an emerging virus than we’ve ever been. I would point out that we didn’t spot SARS-CoV-2 coming, even though we had SARS-CoV-1 a few years earlier. Nobody spotted the 2009 pandemic from influenza, even though there was a lot of surveillance around at the time.”
A version of this article appeared on Medscape.com.
Hepatitis E Vaccine Shows Long-Term Efficacy
The hepatitis E virus (HEV) is among the leading global causes of acute viral hepatitis. Molecular studies of HEV strains have identified four main genotypes. Genotypes 1 and 2 are limited to humans and are transmitted through contaminated water in resource-limited countries, mainly in Asia. Genotypes 3 and 4 are zoonotic, causing sporadic indigenous hepatitis E in nearly all countries.
Each year, approximately 20 million HEV infections occur worldwide, resulting in around 3.3 million symptomatic infections and 70,000 deaths. Despite this toll, HEV infection remains underestimated, and Western countries are likely not immune to the virus. To date, two recombinant vaccines against hepatitis E, based on genotype 1, have been developed and approved in China, but further studies are needed to determine the duration of vaccination protection.
Ten-Year Results
This study is an extension of a randomized, double-blind, placebo-controlled phase 3 clinical trial of the Hecolin hepatitis E vaccine that was conducted in Dongtai County, Jiangsu, China. In the initial trial, healthy adults aged 16-65 years were recruited, stratified by age and sex, and randomly assigned in a 1:1 ratio to receive three doses of intramuscular hepatitis E vaccine or placebo at months 0, 1, and 6.
A hepatitis E surveillance system, including 205 clinical sentinels covering the entire study region, was established before the study began and maintained for 10 years after vaccination to identify individuals with suspected hepatitis. In addition, an external control cohort was formed to assess vaccine efficacy. The primary endpoint was the vaccine’s efficacy in preventing confirmed hepatitis E occurring at least 30 days after the administration of the third vaccine dose.
Follow-up occurred every 3 months. Participants with hepatitis symptoms for 3 days or more underwent alanine aminotransferase (ALT) concentration measurement. Patients with ALT concentrations ≥ 2.5 times the upper limit of normal were considered to have acute hepatitis. A diagnosis of HEV-confirmed infection was made for patients with acute hepatitis presenting with at least two of the following markers: Presence of HEV RNA, presence of positive anti-HEV immunoglobulin (Ig) M antibodies, and at least fourfold increase in anti-HEV IgG concentrations.
For the efficacy analysis, a Poisson regression model was used to estimate the relative risk and its 95% CI of incidence between groups. Incidence was reported as the number of patients with hepatitis E per 10,000 person-years.
Immunogenicity persistence was assessed by measuring anti-HEV IgG in participants. Serum samples were collected at months 0, 7, 13, 19, 31, 43, 55, 79, and 103 for Qingdao district participants and at months 0, 7, 19, 31, 43, 67, and 91 for Anfeng district participants.
Efficacy and Duration
The follow-up period extended from 2007 to 2017. In total, 97,356 participants completed the three-dose regimen and were included in the per-protocol population (48,693 in the vaccine group and 48,663 in the placebo group), and 178,236 residents from the study region participated in the external control cohort. During the study period, 90 cases of hepatitis E were identified, with 13 in the vaccine group (0.2 per 10,000 person-years) and 77 in the placebo group (1.4 per 10,000 person-years). This indicated a vaccine efficacy of 86.6% in the per-protocol analysis.
In the subgroups evaluated for immunogenicity persistence, among those who were initially seronegative and received three doses of hepatitis E vaccine, 254 out of 291 vaccinated participants (87.3%) in Qingdao after 8.5 years and 1270 (73.0%) out of 1740 vaccinated participants in Anfeng after 7.5 years maintained detectable antibody concentrations.
The identification of infections despite vaccination is notable, especially with eight cases occurring beyond the fourth year following the last dose. This information is crucial for understanding potential immunity decline over time and highlights the importance of exploring various vaccination strategies to optimize protection.
An ongoing phase 4 clinical trial in Bangladesh, exploring different administration schedules and target populations, could help optimize vaccination strategies. The remarkable efficacy (100%) observed over a 30-month period for the two-dose schedule (doses are administered 1 month apart) is promising.
The observation of higher IgG antibody avidity in participants with infections despite vaccination underscores the importance of robust antibody responses to mitigate disease severity and duration. Several study limitations, such as lack of data on deaths and emigrations, a single-center study design, predominance of genotype 4 infections, and the risk for bias in the external control cohort, should be acknowledged.
In conclusion, this study provides compelling evidence of sustained protection of the hepatitis E vaccine over a decade. The observed persistence of induced antibodies for at least 8.5 years supports the long-term efficacy of the vaccine. Diverse global trials, further investigation into the impact of natural infections on vaccine-induced antibodies, and confirmation of inter-genotypic protection are needed.
This story was translated from JIM, which is part of the Medscape professional network, using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article appeared on Medscape.com.
The hepatitis E virus (HEV) is among the leading global causes of acute viral hepatitis. Molecular studies of HEV strains have identified four main genotypes. Genotypes 1 and 2 are limited to humans and are transmitted through contaminated water in resource-limited countries, mainly in Asia. Genotypes 3 and 4 are zoonotic, causing sporadic indigenous hepatitis E in nearly all countries.
Each year, approximately 20 million HEV infections occur worldwide, resulting in around 3.3 million symptomatic infections and 70,000 deaths. Despite this toll, HEV infection remains underestimated, and Western countries are likely not immune to the virus. To date, two recombinant vaccines against hepatitis E, based on genotype 1, have been developed and approved in China, but further studies are needed to determine the duration of vaccination protection.
Ten-Year Results
This study is an extension of a randomized, double-blind, placebo-controlled phase 3 clinical trial of the Hecolin hepatitis E vaccine that was conducted in Dongtai County, Jiangsu, China. In the initial trial, healthy adults aged 16-65 years were recruited, stratified by age and sex, and randomly assigned in a 1:1 ratio to receive three doses of intramuscular hepatitis E vaccine or placebo at months 0, 1, and 6.
A hepatitis E surveillance system, including 205 clinical sentinels covering the entire study region, was established before the study began and maintained for 10 years after vaccination to identify individuals with suspected hepatitis. In addition, an external control cohort was formed to assess vaccine efficacy. The primary endpoint was the vaccine’s efficacy in preventing confirmed hepatitis E occurring at least 30 days after the administration of the third vaccine dose.
Follow-up occurred every 3 months. Participants with hepatitis symptoms for 3 days or more underwent alanine aminotransferase (ALT) concentration measurement. Patients with ALT concentrations ≥ 2.5 times the upper limit of normal were considered to have acute hepatitis. A diagnosis of HEV-confirmed infection was made for patients with acute hepatitis presenting with at least two of the following markers: Presence of HEV RNA, presence of positive anti-HEV immunoglobulin (Ig) M antibodies, and at least fourfold increase in anti-HEV IgG concentrations.
For the efficacy analysis, a Poisson regression model was used to estimate the relative risk and its 95% CI of incidence between groups. Incidence was reported as the number of patients with hepatitis E per 10,000 person-years.
Immunogenicity persistence was assessed by measuring anti-HEV IgG in participants. Serum samples were collected at months 0, 7, 13, 19, 31, 43, 55, 79, and 103 for Qingdao district participants and at months 0, 7, 19, 31, 43, 67, and 91 for Anfeng district participants.
Efficacy and Duration
The follow-up period extended from 2007 to 2017. In total, 97,356 participants completed the three-dose regimen and were included in the per-protocol population (48,693 in the vaccine group and 48,663 in the placebo group), and 178,236 residents from the study region participated in the external control cohort. During the study period, 90 cases of hepatitis E were identified, with 13 in the vaccine group (0.2 per 10,000 person-years) and 77 in the placebo group (1.4 per 10,000 person-years). This indicated a vaccine efficacy of 86.6% in the per-protocol analysis.
In the subgroups evaluated for immunogenicity persistence, among those who were initially seronegative and received three doses of hepatitis E vaccine, 254 out of 291 vaccinated participants (87.3%) in Qingdao after 8.5 years and 1270 (73.0%) out of 1740 vaccinated participants in Anfeng after 7.5 years maintained detectable antibody concentrations.
The identification of infections despite vaccination is notable, especially with eight cases occurring beyond the fourth year following the last dose. This information is crucial for understanding potential immunity decline over time and highlights the importance of exploring various vaccination strategies to optimize protection.
An ongoing phase 4 clinical trial in Bangladesh, exploring different administration schedules and target populations, could help optimize vaccination strategies. The remarkable efficacy (100%) observed over a 30-month period for the two-dose schedule (doses are administered 1 month apart) is promising.
The observation of higher IgG antibody avidity in participants with infections despite vaccination underscores the importance of robust antibody responses to mitigate disease severity and duration. Several study limitations, such as lack of data on deaths and emigrations, a single-center study design, predominance of genotype 4 infections, and the risk for bias in the external control cohort, should be acknowledged.
In conclusion, this study provides compelling evidence of sustained protection of the hepatitis E vaccine over a decade. The observed persistence of induced antibodies for at least 8.5 years supports the long-term efficacy of the vaccine. Diverse global trials, further investigation into the impact of natural infections on vaccine-induced antibodies, and confirmation of inter-genotypic protection are needed.
This story was translated from JIM, which is part of the Medscape professional network, using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article appeared on Medscape.com.
The hepatitis E virus (HEV) is among the leading global causes of acute viral hepatitis. Molecular studies of HEV strains have identified four main genotypes. Genotypes 1 and 2 are limited to humans and are transmitted through contaminated water in resource-limited countries, mainly in Asia. Genotypes 3 and 4 are zoonotic, causing sporadic indigenous hepatitis E in nearly all countries.
Each year, approximately 20 million HEV infections occur worldwide, resulting in around 3.3 million symptomatic infections and 70,000 deaths. Despite this toll, HEV infection remains underestimated, and Western countries are likely not immune to the virus. To date, two recombinant vaccines against hepatitis E, based on genotype 1, have been developed and approved in China, but further studies are needed to determine the duration of vaccination protection.
Ten-Year Results
This study is an extension of a randomized, double-blind, placebo-controlled phase 3 clinical trial of the Hecolin hepatitis E vaccine that was conducted in Dongtai County, Jiangsu, China. In the initial trial, healthy adults aged 16-65 years were recruited, stratified by age and sex, and randomly assigned in a 1:1 ratio to receive three doses of intramuscular hepatitis E vaccine or placebo at months 0, 1, and 6.
A hepatitis E surveillance system, including 205 clinical sentinels covering the entire study region, was established before the study began and maintained for 10 years after vaccination to identify individuals with suspected hepatitis. In addition, an external control cohort was formed to assess vaccine efficacy. The primary endpoint was the vaccine’s efficacy in preventing confirmed hepatitis E occurring at least 30 days after the administration of the third vaccine dose.
Follow-up occurred every 3 months. Participants with hepatitis symptoms for 3 days or more underwent alanine aminotransferase (ALT) concentration measurement. Patients with ALT concentrations ≥ 2.5 times the upper limit of normal were considered to have acute hepatitis. A diagnosis of HEV-confirmed infection was made for patients with acute hepatitis presenting with at least two of the following markers: Presence of HEV RNA, presence of positive anti-HEV immunoglobulin (Ig) M antibodies, and at least fourfold increase in anti-HEV IgG concentrations.
For the efficacy analysis, a Poisson regression model was used to estimate the relative risk and its 95% CI of incidence between groups. Incidence was reported as the number of patients with hepatitis E per 10,000 person-years.
Immunogenicity persistence was assessed by measuring anti-HEV IgG in participants. Serum samples were collected at months 0, 7, 13, 19, 31, 43, 55, 79, and 103 for Qingdao district participants and at months 0, 7, 19, 31, 43, 67, and 91 for Anfeng district participants.
Efficacy and Duration
The follow-up period extended from 2007 to 2017. In total, 97,356 participants completed the three-dose regimen and were included in the per-protocol population (48,693 in the vaccine group and 48,663 in the placebo group), and 178,236 residents from the study region participated in the external control cohort. During the study period, 90 cases of hepatitis E were identified, with 13 in the vaccine group (0.2 per 10,000 person-years) and 77 in the placebo group (1.4 per 10,000 person-years). This indicated a vaccine efficacy of 86.6% in the per-protocol analysis.
In the subgroups evaluated for immunogenicity persistence, among those who were initially seronegative and received three doses of hepatitis E vaccine, 254 out of 291 vaccinated participants (87.3%) in Qingdao after 8.5 years and 1270 (73.0%) out of 1740 vaccinated participants in Anfeng after 7.5 years maintained detectable antibody concentrations.
The identification of infections despite vaccination is notable, especially with eight cases occurring beyond the fourth year following the last dose. This information is crucial for understanding potential immunity decline over time and highlights the importance of exploring various vaccination strategies to optimize protection.
An ongoing phase 4 clinical trial in Bangladesh, exploring different administration schedules and target populations, could help optimize vaccination strategies. The remarkable efficacy (100%) observed over a 30-month period for the two-dose schedule (doses are administered 1 month apart) is promising.
The observation of higher IgG antibody avidity in participants with infections despite vaccination underscores the importance of robust antibody responses to mitigate disease severity and duration. Several study limitations, such as lack of data on deaths and emigrations, a single-center study design, predominance of genotype 4 infections, and the risk for bias in the external control cohort, should be acknowledged.
In conclusion, this study provides compelling evidence of sustained protection of the hepatitis E vaccine over a decade. The observed persistence of induced antibodies for at least 8.5 years supports the long-term efficacy of the vaccine. Diverse global trials, further investigation into the impact of natural infections on vaccine-induced antibodies, and confirmation of inter-genotypic protection are needed.
This story was translated from JIM, which is part of the Medscape professional network, using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article appeared on Medscape.com.
Is It Time to Stop Using the Term AIDS?
The acronym AIDS is redundant, loaded with stigma, and potentially harmful, according to a group of specialists who suggest replacing the term with “advanced HIV.”
Mexico, and colleagues.
People generally associate the acronym AIDS with patients who have no available treatment options and a short life expectancy, said Dr. Núñez. That mischaracterization may affect treatment decisions by patients and clinicians and could result in exaggerated infection-control measures.
Using the HIV/AIDS combination erroneously implies equivalence and can mislead the public and clinicians, which the authors explained in their Viewpoint article published in The Lancet HIV.
Original Reason for the Term
AIDS, which stands for acquired immunodeficiency syndrome, was coined in 1982 by the US Centers for Disease Control and Prevention (CDC) to name a disease with an unknown cause that affected people with weakened cell-mediated immunity.
“When HIV was found to be the cause of the disease (labeled HIV in 1986), the term AIDS, strictly speaking, became unnecessary,” Dr. Núñez said.
AIDS was originally intended as a case definition for surveillance purposes, and treatment decisions were based on whether patients met the case definition for AIDS, he pointed out.
“The fact that some people still do so in this day and age shows that this is not only unhelpful, but misleading and even harmful,” he noted. Without the label AIDS, clinicians can focus on whether and for how long people have been on treatment, whether they recently switched treatment, and other factors that will help determine appropriate care.
Some Organizations Removed AIDS From Their Names
Some organizations have already removed AIDS from their names. For example, the International AIDS Society–USA, which issues guidelines on antiretroviral treatment, changed its name to the International Antiviral Society–USA.
In 2017, the name of AIDS.gov was changed to HIV.gov. In its explanation, the group wrote, “Today, people with HIV who are diagnosed early, linked to care, start antiretroviral therapy, and take it as prescribed can achieve life-long viral suppression that prevents HIV infection from progressing to AIDS.”
A different view on the term AIDS comes from Greg Millett, MPH, vice president at the Foundation for AIDS Research (amfAR) and the director of amfAR’s Public Policy Office.
Although he believes that AIDS is an anachronistic term, as a researcher for more than 30 years in the field; a policy director in Washington; a scientist; and a person living with HIV, “it feels like a distinction without a difference. At least from where I sit, there are far more pressing issues that we’re facing as an HIV community,” Millett shared.
For instance, “we’re seeing that global, as well as domestic, HIV funding is in, by far, the most precarious position that I’ve ever seen in the field. Calling it AIDS or HIV makes no difference in trying to alleviate that jeopardy,” he said.
Millett also said that the stigma and persecution and, in some cases, criminalization of people living with HIV or AIDS is pervasive and won’t go away with a name change, which is a point the authors also acknowledged.
“We need to focus on the social determinants of health,” he said. “That is the thing that is going to move the needle among people living with HIV, not nomenclature.”
Millett likens the argument to the one between Black and African American. “As a Black American, I remember fierce debates in the early ‘90s over whether we should be called African Americans or Blacks. Some argued that African American carried greater dignity and would help with self-esteem and address inequities by emphasizing that we are American. Many others said that it doesn’t make a difference.”
“It is clear that being called African American has not fixed intractable issues like poverty, structural racism, or inequities in incarceration,” he pointed out.
End the Epidemic, Not the Name
The authors misinterpret the impact of the term on stigma, said James W. Curran, MD, MPH, dean emeritus of the Rollins School of Public Health and professor of epidemiology and global health at Emory University, both in Atlanta, Georgia. The term AIDS “is more likely attributed to the fatal nature of the infection itself,” without treatment, he explained, and the mode of transmission, exacerbated by homophobia.
“The term has been in widespread use for 40 years and recognized worldwide,” Dr. Curran, who led the nation’s efforts in the battle against HIV and AIDS at the CDC for 15 years before joining Emory as dean, said.
He also worries about the continued trajectory of lives lost: “Over 35 million people worldwide have perished from HIV/AIDS, including over 500,000 per year now.”
Meanwhile, “global programs such as PEPFAR [the US President’s Emergency Plan for AIDS Relief] are under fire and threatened by Congress as no longer necessary. Removing AIDS from the terminology may add to confusion,” making people think “that the epidemic is over,” he said.
Although the authors argue that keeping the term may cause harm, eliminating it might worsen a different kind of harm. “There is a risk that abolishing the term will further de-emphasize the importance of the problem, with no significant impact on stigma,” Dr. Curran added.
A version of this article appeared on Medscape.com.
The acronym AIDS is redundant, loaded with stigma, and potentially harmful, according to a group of specialists who suggest replacing the term with “advanced HIV.”
Mexico, and colleagues.
People generally associate the acronym AIDS with patients who have no available treatment options and a short life expectancy, said Dr. Núñez. That mischaracterization may affect treatment decisions by patients and clinicians and could result in exaggerated infection-control measures.
Using the HIV/AIDS combination erroneously implies equivalence and can mislead the public and clinicians, which the authors explained in their Viewpoint article published in The Lancet HIV.
Original Reason for the Term
AIDS, which stands for acquired immunodeficiency syndrome, was coined in 1982 by the US Centers for Disease Control and Prevention (CDC) to name a disease with an unknown cause that affected people with weakened cell-mediated immunity.
“When HIV was found to be the cause of the disease (labeled HIV in 1986), the term AIDS, strictly speaking, became unnecessary,” Dr. Núñez said.
AIDS was originally intended as a case definition for surveillance purposes, and treatment decisions were based on whether patients met the case definition for AIDS, he pointed out.
“The fact that some people still do so in this day and age shows that this is not only unhelpful, but misleading and even harmful,” he noted. Without the label AIDS, clinicians can focus on whether and for how long people have been on treatment, whether they recently switched treatment, and other factors that will help determine appropriate care.
Some Organizations Removed AIDS From Their Names
Some organizations have already removed AIDS from their names. For example, the International AIDS Society–USA, which issues guidelines on antiretroviral treatment, changed its name to the International Antiviral Society–USA.
In 2017, the name of AIDS.gov was changed to HIV.gov. In its explanation, the group wrote, “Today, people with HIV who are diagnosed early, linked to care, start antiretroviral therapy, and take it as prescribed can achieve life-long viral suppression that prevents HIV infection from progressing to AIDS.”
A different view on the term AIDS comes from Greg Millett, MPH, vice president at the Foundation for AIDS Research (amfAR) and the director of amfAR’s Public Policy Office.
Although he believes that AIDS is an anachronistic term, as a researcher for more than 30 years in the field; a policy director in Washington; a scientist; and a person living with HIV, “it feels like a distinction without a difference. At least from where I sit, there are far more pressing issues that we’re facing as an HIV community,” Millett shared.
For instance, “we’re seeing that global, as well as domestic, HIV funding is in, by far, the most precarious position that I’ve ever seen in the field. Calling it AIDS or HIV makes no difference in trying to alleviate that jeopardy,” he said.
Millett also said that the stigma and persecution and, in some cases, criminalization of people living with HIV or AIDS is pervasive and won’t go away with a name change, which is a point the authors also acknowledged.
“We need to focus on the social determinants of health,” he said. “That is the thing that is going to move the needle among people living with HIV, not nomenclature.”
Millett likens the argument to the one between Black and African American. “As a Black American, I remember fierce debates in the early ‘90s over whether we should be called African Americans or Blacks. Some argued that African American carried greater dignity and would help with self-esteem and address inequities by emphasizing that we are American. Many others said that it doesn’t make a difference.”
“It is clear that being called African American has not fixed intractable issues like poverty, structural racism, or inequities in incarceration,” he pointed out.
End the Epidemic, Not the Name
The authors misinterpret the impact of the term on stigma, said James W. Curran, MD, MPH, dean emeritus of the Rollins School of Public Health and professor of epidemiology and global health at Emory University, both in Atlanta, Georgia. The term AIDS “is more likely attributed to the fatal nature of the infection itself,” without treatment, he explained, and the mode of transmission, exacerbated by homophobia.
“The term has been in widespread use for 40 years and recognized worldwide,” Dr. Curran, who led the nation’s efforts in the battle against HIV and AIDS at the CDC for 15 years before joining Emory as dean, said.
He also worries about the continued trajectory of lives lost: “Over 35 million people worldwide have perished from HIV/AIDS, including over 500,000 per year now.”
Meanwhile, “global programs such as PEPFAR [the US President’s Emergency Plan for AIDS Relief] are under fire and threatened by Congress as no longer necessary. Removing AIDS from the terminology may add to confusion,” making people think “that the epidemic is over,” he said.
Although the authors argue that keeping the term may cause harm, eliminating it might worsen a different kind of harm. “There is a risk that abolishing the term will further de-emphasize the importance of the problem, with no significant impact on stigma,” Dr. Curran added.
A version of this article appeared on Medscape.com.
The acronym AIDS is redundant, loaded with stigma, and potentially harmful, according to a group of specialists who suggest replacing the term with “advanced HIV.”
Mexico, and colleagues.
People generally associate the acronym AIDS with patients who have no available treatment options and a short life expectancy, said Dr. Núñez. That mischaracterization may affect treatment decisions by patients and clinicians and could result in exaggerated infection-control measures.
Using the HIV/AIDS combination erroneously implies equivalence and can mislead the public and clinicians, which the authors explained in their Viewpoint article published in The Lancet HIV.
Original Reason for the Term
AIDS, which stands for acquired immunodeficiency syndrome, was coined in 1982 by the US Centers for Disease Control and Prevention (CDC) to name a disease with an unknown cause that affected people with weakened cell-mediated immunity.
“When HIV was found to be the cause of the disease (labeled HIV in 1986), the term AIDS, strictly speaking, became unnecessary,” Dr. Núñez said.
AIDS was originally intended as a case definition for surveillance purposes, and treatment decisions were based on whether patients met the case definition for AIDS, he pointed out.
“The fact that some people still do so in this day and age shows that this is not only unhelpful, but misleading and even harmful,” he noted. Without the label AIDS, clinicians can focus on whether and for how long people have been on treatment, whether they recently switched treatment, and other factors that will help determine appropriate care.
Some Organizations Removed AIDS From Their Names
Some organizations have already removed AIDS from their names. For example, the International AIDS Society–USA, which issues guidelines on antiretroviral treatment, changed its name to the International Antiviral Society–USA.
In 2017, the name of AIDS.gov was changed to HIV.gov. In its explanation, the group wrote, “Today, people with HIV who are diagnosed early, linked to care, start antiretroviral therapy, and take it as prescribed can achieve life-long viral suppression that prevents HIV infection from progressing to AIDS.”
A different view on the term AIDS comes from Greg Millett, MPH, vice president at the Foundation for AIDS Research (amfAR) and the director of amfAR’s Public Policy Office.
Although he believes that AIDS is an anachronistic term, as a researcher for more than 30 years in the field; a policy director in Washington; a scientist; and a person living with HIV, “it feels like a distinction without a difference. At least from where I sit, there are far more pressing issues that we’re facing as an HIV community,” Millett shared.
For instance, “we’re seeing that global, as well as domestic, HIV funding is in, by far, the most precarious position that I’ve ever seen in the field. Calling it AIDS or HIV makes no difference in trying to alleviate that jeopardy,” he said.
Millett also said that the stigma and persecution and, in some cases, criminalization of people living with HIV or AIDS is pervasive and won’t go away with a name change, which is a point the authors also acknowledged.
“We need to focus on the social determinants of health,” he said. “That is the thing that is going to move the needle among people living with HIV, not nomenclature.”
Millett likens the argument to the one between Black and African American. “As a Black American, I remember fierce debates in the early ‘90s over whether we should be called African Americans or Blacks. Some argued that African American carried greater dignity and would help with self-esteem and address inequities by emphasizing that we are American. Many others said that it doesn’t make a difference.”
“It is clear that being called African American has not fixed intractable issues like poverty, structural racism, or inequities in incarceration,” he pointed out.
End the Epidemic, Not the Name
The authors misinterpret the impact of the term on stigma, said James W. Curran, MD, MPH, dean emeritus of the Rollins School of Public Health and professor of epidemiology and global health at Emory University, both in Atlanta, Georgia. The term AIDS “is more likely attributed to the fatal nature of the infection itself,” without treatment, he explained, and the mode of transmission, exacerbated by homophobia.
“The term has been in widespread use for 40 years and recognized worldwide,” Dr. Curran, who led the nation’s efforts in the battle against HIV and AIDS at the CDC for 15 years before joining Emory as dean, said.
He also worries about the continued trajectory of lives lost: “Over 35 million people worldwide have perished from HIV/AIDS, including over 500,000 per year now.”
Meanwhile, “global programs such as PEPFAR [the US President’s Emergency Plan for AIDS Relief] are under fire and threatened by Congress as no longer necessary. Removing AIDS from the terminology may add to confusion,” making people think “that the epidemic is over,” he said.
Although the authors argue that keeping the term may cause harm, eliminating it might worsen a different kind of harm. “There is a risk that abolishing the term will further de-emphasize the importance of the problem, with no significant impact on stigma,” Dr. Curran added.
A version of this article appeared on Medscape.com.
FROM THE LANCET HIV
Tuberculosis Prevention Brings Economic Gains, Says WHO
A new study conducted by the World Health Organization (WHO) suggests that in addition to providing significant improvements in public health, investment in the diagnosis and prevention of tuberculosis also generates economic benefits.
According to a survey conducted by governments and researchers from Brazil, Georgia, Kenya, and South Africa, even modest increases in funding for measures against tuberculosis can bring gains. Every $1 invested produces returns of as much as $39, it found.
The findings may remind governments and policymakers about the importance of investing in public health policies. According to the WHO, the study “provides strong economic arguments” about the true costs of tuberculosis and proves the benefits of increasing funding to accelerate the diagnosis and preventive treatment of the disease.
UN Targets Tuberculosis
In September 2023, during the last meeting of the United Nations General Assembly, following a widespread worsening of disease indicators because of the COVID-19 pandemic, world leaders signed a declaration committing to the expansion of efforts to combat tuberculosis during the next 5 years. The current WHO study was developed to provide a road map for the implementation of key measures against the disease.
The survey highlights two fundamental actions: The expansion of screening, especially in populations considered more vulnerable, and the provision of tuberculosis preventive treatment (TPT), which entails administering drugs to people who have been exposed to the disease but have not yet developed it.
“TPT is a proven and effective intervention to prevent the development of tuberculosis among exposed people, reducing the risk of developing the disease by about 60%-90% compared with individuals who did not receive it,” the document emphasized.
Investments Yield Returns
To achieve the necessary coverage levels, the study estimated that Brazil would need to increase annual per capita investment by $0.28 (about R$1.41) between 2024 and 2050. Brazilian society, in turn, would receive a return of $11 (R$55.27) for every dollar invested.
For South Africa, whose per capita investment increase is estimated at $1.10 per year, the return would be even more significant: $39 for every dollar allocated.
The WHO emphasized that funding for combating the disease is much lower than the value of the damage it causes to nations. “Tuberculosis has high costs for society. Only a small proportion of these costs go directly to the health system (ranging from 1.7% in South Africa to 7.8% in Kenya). Most are costs for patients and society.”
The study projected that between 2024 and 2050, the total cost of tuberculosis to Brazilian society would be $81.2 billion, with an average annual value of $3.01 billion. This figure represents, in 2024, 0.16% of the country’s gross domestic product.
Achieving screening and preventive treatment goals in Brazil would lead to a reduction of as much as 18% in the national incidence of the disease, as well as 1.9 million fewer deaths, between 2024 and 2050.
Although treatable and preventable, tuberculosis remains the leading cause of death from infectious agents worldwide. It is estimated that over 1.3 million people died from the disease in 2022.
The document provides the “health and economic justification for investing in evidence-based interventions recommended by WHO in tuberculosis screening and prevention,” according to WHO Director-General Tedros Adhanom Ghebreyesus, PhD.
“Today we have the knowledge, tools, and political commitment that can end this age-old disease that continues to be one of the leading causes of death from infectious diseases worldwide,” he added.
Emerging Concerns
Although the WHO highlighted the global increase in access to tuberculosis diagnosis and treatment in 2022, which coincided with the recovery of healthcare systems in several countries after the beginning of the pandemic, it emphasized that the implementation of preventive treatment for exposed individuals and high-vulnerability populations remains slow.
Another concern is the increase in drug resistance. Multidrug-resistant tuberculosis is considered a public health crisis. It is estimated that about 410,000 people had multidrug-resistant tuberculosis or rifampicin-resistant tuberculosis in 2022, but only two of every five patients had access to treatment.This story was translated from the Medscape Portuguese edition using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication.
A version of this article appeared on Medscape.com .
A new study conducted by the World Health Organization (WHO) suggests that in addition to providing significant improvements in public health, investment in the diagnosis and prevention of tuberculosis also generates economic benefits.
According to a survey conducted by governments and researchers from Brazil, Georgia, Kenya, and South Africa, even modest increases in funding for measures against tuberculosis can bring gains. Every $1 invested produces returns of as much as $39, it found.
The findings may remind governments and policymakers about the importance of investing in public health policies. According to the WHO, the study “provides strong economic arguments” about the true costs of tuberculosis and proves the benefits of increasing funding to accelerate the diagnosis and preventive treatment of the disease.
UN Targets Tuberculosis
In September 2023, during the last meeting of the United Nations General Assembly, following a widespread worsening of disease indicators because of the COVID-19 pandemic, world leaders signed a declaration committing to the expansion of efforts to combat tuberculosis during the next 5 years. The current WHO study was developed to provide a road map for the implementation of key measures against the disease.
The survey highlights two fundamental actions: The expansion of screening, especially in populations considered more vulnerable, and the provision of tuberculosis preventive treatment (TPT), which entails administering drugs to people who have been exposed to the disease but have not yet developed it.
“TPT is a proven and effective intervention to prevent the development of tuberculosis among exposed people, reducing the risk of developing the disease by about 60%-90% compared with individuals who did not receive it,” the document emphasized.
Investments Yield Returns
To achieve the necessary coverage levels, the study estimated that Brazil would need to increase annual per capita investment by $0.28 (about R$1.41) between 2024 and 2050. Brazilian society, in turn, would receive a return of $11 (R$55.27) for every dollar invested.
For South Africa, whose per capita investment increase is estimated at $1.10 per year, the return would be even more significant: $39 for every dollar allocated.
The WHO emphasized that funding for combating the disease is much lower than the value of the damage it causes to nations. “Tuberculosis has high costs for society. Only a small proportion of these costs go directly to the health system (ranging from 1.7% in South Africa to 7.8% in Kenya). Most are costs for patients and society.”
The study projected that between 2024 and 2050, the total cost of tuberculosis to Brazilian society would be $81.2 billion, with an average annual value of $3.01 billion. This figure represents, in 2024, 0.16% of the country’s gross domestic product.
Achieving screening and preventive treatment goals in Brazil would lead to a reduction of as much as 18% in the national incidence of the disease, as well as 1.9 million fewer deaths, between 2024 and 2050.
Although treatable and preventable, tuberculosis remains the leading cause of death from infectious agents worldwide. It is estimated that over 1.3 million people died from the disease in 2022.
The document provides the “health and economic justification for investing in evidence-based interventions recommended by WHO in tuberculosis screening and prevention,” according to WHO Director-General Tedros Adhanom Ghebreyesus, PhD.
“Today we have the knowledge, tools, and political commitment that can end this age-old disease that continues to be one of the leading causes of death from infectious diseases worldwide,” he added.
Emerging Concerns
Although the WHO highlighted the global increase in access to tuberculosis diagnosis and treatment in 2022, which coincided with the recovery of healthcare systems in several countries after the beginning of the pandemic, it emphasized that the implementation of preventive treatment for exposed individuals and high-vulnerability populations remains slow.
Another concern is the increase in drug resistance. Multidrug-resistant tuberculosis is considered a public health crisis. It is estimated that about 410,000 people had multidrug-resistant tuberculosis or rifampicin-resistant tuberculosis in 2022, but only two of every five patients had access to treatment.This story was translated from the Medscape Portuguese edition using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication.
A version of this article appeared on Medscape.com .
A new study conducted by the World Health Organization (WHO) suggests that in addition to providing significant improvements in public health, investment in the diagnosis and prevention of tuberculosis also generates economic benefits.
According to a survey conducted by governments and researchers from Brazil, Georgia, Kenya, and South Africa, even modest increases in funding for measures against tuberculosis can bring gains. Every $1 invested produces returns of as much as $39, it found.
The findings may remind governments and policymakers about the importance of investing in public health policies. According to the WHO, the study “provides strong economic arguments” about the true costs of tuberculosis and proves the benefits of increasing funding to accelerate the diagnosis and preventive treatment of the disease.
UN Targets Tuberculosis
In September 2023, during the last meeting of the United Nations General Assembly, following a widespread worsening of disease indicators because of the COVID-19 pandemic, world leaders signed a declaration committing to the expansion of efforts to combat tuberculosis during the next 5 years. The current WHO study was developed to provide a road map for the implementation of key measures against the disease.
The survey highlights two fundamental actions: The expansion of screening, especially in populations considered more vulnerable, and the provision of tuberculosis preventive treatment (TPT), which entails administering drugs to people who have been exposed to the disease but have not yet developed it.
“TPT is a proven and effective intervention to prevent the development of tuberculosis among exposed people, reducing the risk of developing the disease by about 60%-90% compared with individuals who did not receive it,” the document emphasized.
Investments Yield Returns
To achieve the necessary coverage levels, the study estimated that Brazil would need to increase annual per capita investment by $0.28 (about R$1.41) between 2024 and 2050. Brazilian society, in turn, would receive a return of $11 (R$55.27) for every dollar invested.
For South Africa, whose per capita investment increase is estimated at $1.10 per year, the return would be even more significant: $39 for every dollar allocated.
The WHO emphasized that funding for combating the disease is much lower than the value of the damage it causes to nations. “Tuberculosis has high costs for society. Only a small proportion of these costs go directly to the health system (ranging from 1.7% in South Africa to 7.8% in Kenya). Most are costs for patients and society.”
The study projected that between 2024 and 2050, the total cost of tuberculosis to Brazilian society would be $81.2 billion, with an average annual value of $3.01 billion. This figure represents, in 2024, 0.16% of the country’s gross domestic product.
Achieving screening and preventive treatment goals in Brazil would lead to a reduction of as much as 18% in the national incidence of the disease, as well as 1.9 million fewer deaths, between 2024 and 2050.
Although treatable and preventable, tuberculosis remains the leading cause of death from infectious agents worldwide. It is estimated that over 1.3 million people died from the disease in 2022.
The document provides the “health and economic justification for investing in evidence-based interventions recommended by WHO in tuberculosis screening and prevention,” according to WHO Director-General Tedros Adhanom Ghebreyesus, PhD.
“Today we have the knowledge, tools, and political commitment that can end this age-old disease that continues to be one of the leading causes of death from infectious diseases worldwide,” he added.
Emerging Concerns
Although the WHO highlighted the global increase in access to tuberculosis diagnosis and treatment in 2022, which coincided with the recovery of healthcare systems in several countries after the beginning of the pandemic, it emphasized that the implementation of preventive treatment for exposed individuals and high-vulnerability populations remains slow.
Another concern is the increase in drug resistance. Multidrug-resistant tuberculosis is considered a public health crisis. It is estimated that about 410,000 people had multidrug-resistant tuberculosis or rifampicin-resistant tuberculosis in 2022, but only two of every five patients had access to treatment.This story was translated from the Medscape Portuguese edition using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication.
A version of this article appeared on Medscape.com .



