User login
Injection beats pill for long-lasting HIV prevention
Injections of cabotegravir (ViiV Healthcare) given every other month are more effective in blocking HIV transmission than is the once-a-day combination of tenofovir disoproxil fumarate and emtricitabine (Truvada, Gilead Science), new data from the HPTN 083 trial show.
The findings “could transform the HIV prevention landscape for so many people,” said Megan Coleman, DNP, from Whitman-Walker Health in Washington, DC, who regularly prescribes Truvada as pre-exposure prophylaxis (PrEP).
At Whitman-Walker alone, about 3000 people were taking the pill in early 2020, but “for some people, taking a pill every day just isn’t a viable option,” said Coleman. “To have something that can support a patient’s choice and a patient’s ability to reduce their own risk of HIV is amazing.”
Final results from the trial — which looked at the drug in cisgender men and transgender women who have sex with men — were presented at the International AIDS Conference 2020.
Early Study Termination
Half of the 4566 study participants — from 43 sites in Africa, Asia, Latin America, and the United States — were younger than 30 years, 12.4% were transgender women, 29.7% were black, and 46.1% were Hispanic.
By design, ViiV Healthcare, the study sponsor, required that 50% of American participants be black to reflect the population at risk for HIV in the United States, said Raphael Landovitz, MD, from the UCLA David Geffen School of Medicine in Los Angeles, who is protocol chair for HPTN 083. In fact, 49.7% of the American cohort was black and 17.8% was Hispanic.
Patients randomized to the cabotegravir group received daily oral cabotegravir plus daily oral placebo for 5 weeks, to assess safety, followed by a cabotegravir injection at weeks 5 and 9 and every 2 months thereafter out to week 153 plus daily oral placebo. Patients randomized to the Truvada group received daily oral Truvada plus daily oral placebo for 5 weeks, followed by daily oral Truvada plus placebo injection, on the same schedule, out to week 153.
After the final injection, all participants continued on daily oral Truvada for 48 weeks.
The researchers expected to wait until 172 participants acquired HIV; they decided at the outset that this number would be sufficient to power a decision on whether or not cabotegravir injections are better than daily oral Truvada. But by May 2020, when 52 of the study participants had acquired HIV, the results were so lopsided in favor of cabotegravir that the trial was stopped. At that point, all participants were offered cabotegravir injections every 2 months.
Thirty-nine of the 52 (75%) new HIV infections occurred in the Truvada group. In fact, (hazard ratio, 0.34).
“This definitively establishes the superiority of cabotegravir,” said Landovitz.
He and his colleagues had been legitimately concerned that HIV acquisition would be so low in the trial that they wouldn’t be able to show how effective the injectable was. The success of Truvada PrEP has made it difficult to design prevention trials.
“We know that Truvada works extremely well, so the fact that we were able to show that cabotegravir in this population works better” is a powerful observation, said Landovitz. This is especially true because the rates of sexually transmitted infections — which are thought to increase risk for HIV transmission — were so high. Overall, 16.5% of the participants tested positive for syphilis during the trial, 13.3% tested positive for gonorrhea, and 21.1% tested positive for Chlamydia.
Five Surprising Seroconversions
Eleven of the 15 HIV infections in the cabotegravir group occurred in people who had received at least one injection. Three of these infections actually occurred during the first 5 weeks of the study when participants were taking oral cabotegravir, two occurred when participants chose to discontinue the injection and return to daily oral Truvada, and one occurred after a participant missed the injection for a prolonged period of time.
But five of the transmissions occurred in participants who appeared to be perfectly adherent.
Landovitz offered a number of possible reasons for this surprising finding.
“Number one could be that there’s something about these five particular individuals such that they grind up and eliminate the cabotegravir faster than other people, so an 8-week interval is too long for them,” he explained. “Another possibility, although pretty rare, is that there is a rare circulating virus that is intrinsically resistant to cabotegravir.”
Breakthrough HIV transmissions have been rare in people taking oral PrEP.
Disruptions caused by the COVID-19 pandemic have meant that the researchers don’t yet have the data on drug-resistant mutations or drug levels for these five participants, but they will.
“I suspect the truth is that there will never be a 100% failsafe HIV prevention mechanism,” said Landovitz.
“Impressive” Findings
The findings were greeted with excitement, although questions remain.
They are “impressive,” especially the data on black and Hispanic participants, said Paul Sax, MD, medical director of the Division of Infectious Diseases at Brigham and Women’s Hospital in Boston.
However, he said he is interested in the data showing that although participants in both groups gained weight during the study, there was early weight loss in the Truvada group, meaning that those in the cabotegravir group weighed more at the end of the study than those in the Truvada group.
“I’ve been watching the data on weight with integrase inhibitors,” he explained, including weight data specific to Truvada and to the combination of emtricitabine and tenofovir alafenamide (Descovy, Gilead). It looks like Truvada “has some sort of weight-suppressive effects. That’s going to be a thing we’re going to have to watch.”
Coleman said she is already thinking about patients at Whitman-Walker who might do well on cabotegravir and those who can start PrEP for the first time with this option.
“Not only would people probably switch to this option, but maybe people would be interested in starting a biomedical prevention approach that isn’t a pill every day,” she said. “It’s just exciting to have another option. Hopefully, in a few years, we’ll have implantable devices and rings; I can’t even imagine what all those brilliant minds are coming up with.”
But that’s still a ways off. First, cabotegravir has yet to be approved for HIV prevention, and ideally, eventually, there will be a way to determine if cabotegravir is safe for each patient that doesn’t involve a month of daily pills.
“We need to solve that problem because it’s so complicated to do an oral lead-in for a month or so,” said Carl Dieffenbach, PhD, director of the Division of AIDS at the National Institute of Allergy and Infectious Diseases, National Institutes of Health. “Otherwise it’s not going to be feasible.”
We need to make sure this gets licensed for men and women and transgender individuals.
Even with these positive data, Dieffenbach and other officials are not keen to have ViiV apply for licensing right away. Last October, Descovy was the second oral PrEP pill approved for HIV prevention, but only for use by gay men and transgender women — it hadn’t been well studied in cisgender women — causing an outcry. Now, officials are suggesting that ViiV not make the same mistake.
They are urging the company to hold off until data from the sister study of the medication in women — HPTN 084 — is completed in 2022.
“We need to make sure this gets licensed for men and women and transgender individuals,” Dieffenbach told Medscape Medical News. “We just need to give this a little more time and then build a plan with contingencies, so that if something happens, we still have collected all the safety data in women so we can say it’s safe.”
ViiV seems to be making such a plan.
“Our goal is to seek approval across all genders and we will work with the FDA and other regulatory agencies to map out a plan to achieve this goal,” said Kimberly Smith, MD, head of research and development at ViiV Healthcare.
The World Health Organization (WHO), meanwhile, doesn’t expect to change its guidelines on HIV prevention medications until data from HPTN 084 are reported.
“What’s important when we look at guidelines is that we also look across populations,” said Meg Doherty, coordinator of treatment and care in the Department of HIV/AIDS at WHO. “We’re waiting to know more about how cabotegravir works in women, because we certainly want to have prevention drugs that can be used in men and women at different age ranges and, ideally, during pregnancy.”
International AIDS Conference 2020: Abstracts OAXLB01. Presented July 8, 2020.
This article first appeared on Medscape.com.
Injections of cabotegravir (ViiV Healthcare) given every other month are more effective in blocking HIV transmission than is the once-a-day combination of tenofovir disoproxil fumarate and emtricitabine (Truvada, Gilead Science), new data from the HPTN 083 trial show.
The findings “could transform the HIV prevention landscape for so many people,” said Megan Coleman, DNP, from Whitman-Walker Health in Washington, DC, who regularly prescribes Truvada as pre-exposure prophylaxis (PrEP).
At Whitman-Walker alone, about 3000 people were taking the pill in early 2020, but “for some people, taking a pill every day just isn’t a viable option,” said Coleman. “To have something that can support a patient’s choice and a patient’s ability to reduce their own risk of HIV is amazing.”
Final results from the trial — which looked at the drug in cisgender men and transgender women who have sex with men — were presented at the International AIDS Conference 2020.
Early Study Termination
Half of the 4566 study participants — from 43 sites in Africa, Asia, Latin America, and the United States — were younger than 30 years, 12.4% were transgender women, 29.7% were black, and 46.1% were Hispanic.
By design, ViiV Healthcare, the study sponsor, required that 50% of American participants be black to reflect the population at risk for HIV in the United States, said Raphael Landovitz, MD, from the UCLA David Geffen School of Medicine in Los Angeles, who is protocol chair for HPTN 083. In fact, 49.7% of the American cohort was black and 17.8% was Hispanic.
Patients randomized to the cabotegravir group received daily oral cabotegravir plus daily oral placebo for 5 weeks, to assess safety, followed by a cabotegravir injection at weeks 5 and 9 and every 2 months thereafter out to week 153 plus daily oral placebo. Patients randomized to the Truvada group received daily oral Truvada plus daily oral placebo for 5 weeks, followed by daily oral Truvada plus placebo injection, on the same schedule, out to week 153.
After the final injection, all participants continued on daily oral Truvada for 48 weeks.
The researchers expected to wait until 172 participants acquired HIV; they decided at the outset that this number would be sufficient to power a decision on whether or not cabotegravir injections are better than daily oral Truvada. But by May 2020, when 52 of the study participants had acquired HIV, the results were so lopsided in favor of cabotegravir that the trial was stopped. At that point, all participants were offered cabotegravir injections every 2 months.
Thirty-nine of the 52 (75%) new HIV infections occurred in the Truvada group. In fact, (hazard ratio, 0.34).
“This definitively establishes the superiority of cabotegravir,” said Landovitz.
He and his colleagues had been legitimately concerned that HIV acquisition would be so low in the trial that they wouldn’t be able to show how effective the injectable was. The success of Truvada PrEP has made it difficult to design prevention trials.
“We know that Truvada works extremely well, so the fact that we were able to show that cabotegravir in this population works better” is a powerful observation, said Landovitz. This is especially true because the rates of sexually transmitted infections — which are thought to increase risk for HIV transmission — were so high. Overall, 16.5% of the participants tested positive for syphilis during the trial, 13.3% tested positive for gonorrhea, and 21.1% tested positive for Chlamydia.
Five Surprising Seroconversions
Eleven of the 15 HIV infections in the cabotegravir group occurred in people who had received at least one injection. Three of these infections actually occurred during the first 5 weeks of the study when participants were taking oral cabotegravir, two occurred when participants chose to discontinue the injection and return to daily oral Truvada, and one occurred after a participant missed the injection for a prolonged period of time.
But five of the transmissions occurred in participants who appeared to be perfectly adherent.
Landovitz offered a number of possible reasons for this surprising finding.
“Number one could be that there’s something about these five particular individuals such that they grind up and eliminate the cabotegravir faster than other people, so an 8-week interval is too long for them,” he explained. “Another possibility, although pretty rare, is that there is a rare circulating virus that is intrinsically resistant to cabotegravir.”
Breakthrough HIV transmissions have been rare in people taking oral PrEP.
Disruptions caused by the COVID-19 pandemic have meant that the researchers don’t yet have the data on drug-resistant mutations or drug levels for these five participants, but they will.
“I suspect the truth is that there will never be a 100% failsafe HIV prevention mechanism,” said Landovitz.
“Impressive” Findings
The findings were greeted with excitement, although questions remain.
They are “impressive,” especially the data on black and Hispanic participants, said Paul Sax, MD, medical director of the Division of Infectious Diseases at Brigham and Women’s Hospital in Boston.
However, he said he is interested in the data showing that although participants in both groups gained weight during the study, there was early weight loss in the Truvada group, meaning that those in the cabotegravir group weighed more at the end of the study than those in the Truvada group.
“I’ve been watching the data on weight with integrase inhibitors,” he explained, including weight data specific to Truvada and to the combination of emtricitabine and tenofovir alafenamide (Descovy, Gilead). It looks like Truvada “has some sort of weight-suppressive effects. That’s going to be a thing we’re going to have to watch.”
Coleman said she is already thinking about patients at Whitman-Walker who might do well on cabotegravir and those who can start PrEP for the first time with this option.
“Not only would people probably switch to this option, but maybe people would be interested in starting a biomedical prevention approach that isn’t a pill every day,” she said. “It’s just exciting to have another option. Hopefully, in a few years, we’ll have implantable devices and rings; I can’t even imagine what all those brilliant minds are coming up with.”
But that’s still a ways off. First, cabotegravir has yet to be approved for HIV prevention, and ideally, eventually, there will be a way to determine if cabotegravir is safe for each patient that doesn’t involve a month of daily pills.
“We need to solve that problem because it’s so complicated to do an oral lead-in for a month or so,” said Carl Dieffenbach, PhD, director of the Division of AIDS at the National Institute of Allergy and Infectious Diseases, National Institutes of Health. “Otherwise it’s not going to be feasible.”
We need to make sure this gets licensed for men and women and transgender individuals.
Even with these positive data, Dieffenbach and other officials are not keen to have ViiV apply for licensing right away. Last October, Descovy was the second oral PrEP pill approved for HIV prevention, but only for use by gay men and transgender women — it hadn’t been well studied in cisgender women — causing an outcry. Now, officials are suggesting that ViiV not make the same mistake.
They are urging the company to hold off until data from the sister study of the medication in women — HPTN 084 — is completed in 2022.
“We need to make sure this gets licensed for men and women and transgender individuals,” Dieffenbach told Medscape Medical News. “We just need to give this a little more time and then build a plan with contingencies, so that if something happens, we still have collected all the safety data in women so we can say it’s safe.”
ViiV seems to be making such a plan.
“Our goal is to seek approval across all genders and we will work with the FDA and other regulatory agencies to map out a plan to achieve this goal,” said Kimberly Smith, MD, head of research and development at ViiV Healthcare.
The World Health Organization (WHO), meanwhile, doesn’t expect to change its guidelines on HIV prevention medications until data from HPTN 084 are reported.
“What’s important when we look at guidelines is that we also look across populations,” said Meg Doherty, coordinator of treatment and care in the Department of HIV/AIDS at WHO. “We’re waiting to know more about how cabotegravir works in women, because we certainly want to have prevention drugs that can be used in men and women at different age ranges and, ideally, during pregnancy.”
International AIDS Conference 2020: Abstracts OAXLB01. Presented July 8, 2020.
This article first appeared on Medscape.com.
Injections of cabotegravir (ViiV Healthcare) given every other month are more effective in blocking HIV transmission than is the once-a-day combination of tenofovir disoproxil fumarate and emtricitabine (Truvada, Gilead Science), new data from the HPTN 083 trial show.
The findings “could transform the HIV prevention landscape for so many people,” said Megan Coleman, DNP, from Whitman-Walker Health in Washington, DC, who regularly prescribes Truvada as pre-exposure prophylaxis (PrEP).
At Whitman-Walker alone, about 3000 people were taking the pill in early 2020, but “for some people, taking a pill every day just isn’t a viable option,” said Coleman. “To have something that can support a patient’s choice and a patient’s ability to reduce their own risk of HIV is amazing.”
Final results from the trial — which looked at the drug in cisgender men and transgender women who have sex with men — were presented at the International AIDS Conference 2020.
Early Study Termination
Half of the 4566 study participants — from 43 sites in Africa, Asia, Latin America, and the United States — were younger than 30 years, 12.4% were transgender women, 29.7% were black, and 46.1% were Hispanic.
By design, ViiV Healthcare, the study sponsor, required that 50% of American participants be black to reflect the population at risk for HIV in the United States, said Raphael Landovitz, MD, from the UCLA David Geffen School of Medicine in Los Angeles, who is protocol chair for HPTN 083. In fact, 49.7% of the American cohort was black and 17.8% was Hispanic.
Patients randomized to the cabotegravir group received daily oral cabotegravir plus daily oral placebo for 5 weeks, to assess safety, followed by a cabotegravir injection at weeks 5 and 9 and every 2 months thereafter out to week 153 plus daily oral placebo. Patients randomized to the Truvada group received daily oral Truvada plus daily oral placebo for 5 weeks, followed by daily oral Truvada plus placebo injection, on the same schedule, out to week 153.
After the final injection, all participants continued on daily oral Truvada for 48 weeks.
The researchers expected to wait until 172 participants acquired HIV; they decided at the outset that this number would be sufficient to power a decision on whether or not cabotegravir injections are better than daily oral Truvada. But by May 2020, when 52 of the study participants had acquired HIV, the results were so lopsided in favor of cabotegravir that the trial was stopped. At that point, all participants were offered cabotegravir injections every 2 months.
Thirty-nine of the 52 (75%) new HIV infections occurred in the Truvada group. In fact, (hazard ratio, 0.34).
“This definitively establishes the superiority of cabotegravir,” said Landovitz.
He and his colleagues had been legitimately concerned that HIV acquisition would be so low in the trial that they wouldn’t be able to show how effective the injectable was. The success of Truvada PrEP has made it difficult to design prevention trials.
“We know that Truvada works extremely well, so the fact that we were able to show that cabotegravir in this population works better” is a powerful observation, said Landovitz. This is especially true because the rates of sexually transmitted infections — which are thought to increase risk for HIV transmission — were so high. Overall, 16.5% of the participants tested positive for syphilis during the trial, 13.3% tested positive for gonorrhea, and 21.1% tested positive for Chlamydia.
Five Surprising Seroconversions
Eleven of the 15 HIV infections in the cabotegravir group occurred in people who had received at least one injection. Three of these infections actually occurred during the first 5 weeks of the study when participants were taking oral cabotegravir, two occurred when participants chose to discontinue the injection and return to daily oral Truvada, and one occurred after a participant missed the injection for a prolonged period of time.
But five of the transmissions occurred in participants who appeared to be perfectly adherent.
Landovitz offered a number of possible reasons for this surprising finding.
“Number one could be that there’s something about these five particular individuals such that they grind up and eliminate the cabotegravir faster than other people, so an 8-week interval is too long for them,” he explained. “Another possibility, although pretty rare, is that there is a rare circulating virus that is intrinsically resistant to cabotegravir.”
Breakthrough HIV transmissions have been rare in people taking oral PrEP.
Disruptions caused by the COVID-19 pandemic have meant that the researchers don’t yet have the data on drug-resistant mutations or drug levels for these five participants, but they will.
“I suspect the truth is that there will never be a 100% failsafe HIV prevention mechanism,” said Landovitz.
“Impressive” Findings
The findings were greeted with excitement, although questions remain.
They are “impressive,” especially the data on black and Hispanic participants, said Paul Sax, MD, medical director of the Division of Infectious Diseases at Brigham and Women’s Hospital in Boston.
However, he said he is interested in the data showing that although participants in both groups gained weight during the study, there was early weight loss in the Truvada group, meaning that those in the cabotegravir group weighed more at the end of the study than those in the Truvada group.
“I’ve been watching the data on weight with integrase inhibitors,” he explained, including weight data specific to Truvada and to the combination of emtricitabine and tenofovir alafenamide (Descovy, Gilead). It looks like Truvada “has some sort of weight-suppressive effects. That’s going to be a thing we’re going to have to watch.”
Coleman said she is already thinking about patients at Whitman-Walker who might do well on cabotegravir and those who can start PrEP for the first time with this option.
“Not only would people probably switch to this option, but maybe people would be interested in starting a biomedical prevention approach that isn’t a pill every day,” she said. “It’s just exciting to have another option. Hopefully, in a few years, we’ll have implantable devices and rings; I can’t even imagine what all those brilliant minds are coming up with.”
But that’s still a ways off. First, cabotegravir has yet to be approved for HIV prevention, and ideally, eventually, there will be a way to determine if cabotegravir is safe for each patient that doesn’t involve a month of daily pills.
“We need to solve that problem because it’s so complicated to do an oral lead-in for a month or so,” said Carl Dieffenbach, PhD, director of the Division of AIDS at the National Institute of Allergy and Infectious Diseases, National Institutes of Health. “Otherwise it’s not going to be feasible.”
We need to make sure this gets licensed for men and women and transgender individuals.
Even with these positive data, Dieffenbach and other officials are not keen to have ViiV apply for licensing right away. Last October, Descovy was the second oral PrEP pill approved for HIV prevention, but only for use by gay men and transgender women — it hadn’t been well studied in cisgender women — causing an outcry. Now, officials are suggesting that ViiV not make the same mistake.
They are urging the company to hold off until data from the sister study of the medication in women — HPTN 084 — is completed in 2022.
“We need to make sure this gets licensed for men and women and transgender individuals,” Dieffenbach told Medscape Medical News. “We just need to give this a little more time and then build a plan with contingencies, so that if something happens, we still have collected all the safety data in women so we can say it’s safe.”
ViiV seems to be making such a plan.
“Our goal is to seek approval across all genders and we will work with the FDA and other regulatory agencies to map out a plan to achieve this goal,” said Kimberly Smith, MD, head of research and development at ViiV Healthcare.
The World Health Organization (WHO), meanwhile, doesn’t expect to change its guidelines on HIV prevention medications until data from HPTN 084 are reported.
“What’s important when we look at guidelines is that we also look across populations,” said Meg Doherty, coordinator of treatment and care in the Department of HIV/AIDS at WHO. “We’re waiting to know more about how cabotegravir works in women, because we certainly want to have prevention drugs that can be used in men and women at different age ranges and, ideally, during pregnancy.”
International AIDS Conference 2020: Abstracts OAXLB01. Presented July 8, 2020.
This article first appeared on Medscape.com.
Children rarely transmit SARS-CoV-2 within households
“Unlike with other viral respiratory infections, children do not seem to be a major vector of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) transmission, with most pediatric cases described inside familial clusters and no documentation of child-to-child or child-to-adult transmission,” said Klara M. Posfay-Barbe, MD, of the University of Geneva, Switzerland, and colleagues.
In a study published in Pediatrics, the researchers analyzed data from all COVID-19 patients younger than 16 years who were identified between March 10, 2020, and April 10, 2020, through a hospital surveillance network. Parents and household contacts were called for contact tracing.
In 31 of 39 (79%) households, at least one adult family member had a suspected or confirmed SARS-CoV-2 infection before onset of symptoms in the child. These findings support data from previous studies suggesting that children mainly become infected from adult family members rather than transmitting the virus to them, the researchers said
In only 3 of 39 (8%) households was the study child the first to develop symptoms. “Surprisingly, in 33% of households, symptomatic HHCs [household contacts] tested negative despite belonging to a familial cluster with confirmed SARS-CoV-2 cases, suggesting an underreporting of cases,” Dr. Posfay-Barbe and associates noted.
The findings were limited by several factors including potential underreporting of cases because those with mild or atypical presentations may not have sought medical care, and the inability to confirm child-to-adult transmission. The results were strengthened by the extensive contact tracing and very few individuals lost to follow-up, they said; however, more diagnostic screening and contact tracing are needed to improve understanding of household transmission of SARS-CoV-2, they concluded.
Resolving the issue of how much children contribute to transmission of SARS-CoV-2 is essential to making informed decisions about public health, including how to structure schools and child-care facility reopening, Benjamin Lee, MD, and William V. Raszka Jr., MD, both of the University of Vermont, Burlington, said in an accompanying editorial (Pediatrics. 2020 Jul 10. doi: 10.1542/peds/2020-004879).
The data in the current study support other studies of transmission among household contacts in China suggesting that, in most cases of childhood infections, “the child was not the source of infection and that children most frequently acquire COVID-19 from adults, rather than transmitting it to them,” they wrote.
In addition, the limited data on transmission of SARS-CoV-2 by children outside of the household show few cases of secondary infection from children identified with SARS-CoV-2 in school settings in studies from France and Australia, Dr. Lee and Dr. Raszka noted.
the editorialists wrote. “This would be another manner by which SARS-CoV2 differs drastically from influenza, for which school-based transmission is well recognized as a significant driver of epidemic disease and forms the basis for most evidence regarding school closures as public health strategy.”
“Therefore, serious consideration should be paid toward strategies that allow schools to remain open, even during periods of COVID-19 spread,” the editorialists concluded. “In doing so, we could minimize the potentially profound adverse social, developmental, and health costs that our children will continue to suffer until an effective treatment or vaccine can be developed and distributed or, failing that, until we reach herd immunity,” Dr. Lee and Dr. Raszka emphasized.
The study received no outside funding. The researchers and editorialists had no financial conflicts to disclose.
SOURCE: Posfay-Barbe KM et al. Pediatrics. 2020 Jul 10. doi: 10.1542/peds.2020-1576.
“Unlike with other viral respiratory infections, children do not seem to be a major vector of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) transmission, with most pediatric cases described inside familial clusters and no documentation of child-to-child or child-to-adult transmission,” said Klara M. Posfay-Barbe, MD, of the University of Geneva, Switzerland, and colleagues.
In a study published in Pediatrics, the researchers analyzed data from all COVID-19 patients younger than 16 years who were identified between March 10, 2020, and April 10, 2020, through a hospital surveillance network. Parents and household contacts were called for contact tracing.
In 31 of 39 (79%) households, at least one adult family member had a suspected or confirmed SARS-CoV-2 infection before onset of symptoms in the child. These findings support data from previous studies suggesting that children mainly become infected from adult family members rather than transmitting the virus to them, the researchers said
In only 3 of 39 (8%) households was the study child the first to develop symptoms. “Surprisingly, in 33% of households, symptomatic HHCs [household contacts] tested negative despite belonging to a familial cluster with confirmed SARS-CoV-2 cases, suggesting an underreporting of cases,” Dr. Posfay-Barbe and associates noted.
The findings were limited by several factors including potential underreporting of cases because those with mild or atypical presentations may not have sought medical care, and the inability to confirm child-to-adult transmission. The results were strengthened by the extensive contact tracing and very few individuals lost to follow-up, they said; however, more diagnostic screening and contact tracing are needed to improve understanding of household transmission of SARS-CoV-2, they concluded.
Resolving the issue of how much children contribute to transmission of SARS-CoV-2 is essential to making informed decisions about public health, including how to structure schools and child-care facility reopening, Benjamin Lee, MD, and William V. Raszka Jr., MD, both of the University of Vermont, Burlington, said in an accompanying editorial (Pediatrics. 2020 Jul 10. doi: 10.1542/peds/2020-004879).
The data in the current study support other studies of transmission among household contacts in China suggesting that, in most cases of childhood infections, “the child was not the source of infection and that children most frequently acquire COVID-19 from adults, rather than transmitting it to them,” they wrote.
In addition, the limited data on transmission of SARS-CoV-2 by children outside of the household show few cases of secondary infection from children identified with SARS-CoV-2 in school settings in studies from France and Australia, Dr. Lee and Dr. Raszka noted.
the editorialists wrote. “This would be another manner by which SARS-CoV2 differs drastically from influenza, for which school-based transmission is well recognized as a significant driver of epidemic disease and forms the basis for most evidence regarding school closures as public health strategy.”
“Therefore, serious consideration should be paid toward strategies that allow schools to remain open, even during periods of COVID-19 spread,” the editorialists concluded. “In doing so, we could minimize the potentially profound adverse social, developmental, and health costs that our children will continue to suffer until an effective treatment or vaccine can be developed and distributed or, failing that, until we reach herd immunity,” Dr. Lee and Dr. Raszka emphasized.
The study received no outside funding. The researchers and editorialists had no financial conflicts to disclose.
SOURCE: Posfay-Barbe KM et al. Pediatrics. 2020 Jul 10. doi: 10.1542/peds.2020-1576.
“Unlike with other viral respiratory infections, children do not seem to be a major vector of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) transmission, with most pediatric cases described inside familial clusters and no documentation of child-to-child or child-to-adult transmission,” said Klara M. Posfay-Barbe, MD, of the University of Geneva, Switzerland, and colleagues.
In a study published in Pediatrics, the researchers analyzed data from all COVID-19 patients younger than 16 years who were identified between March 10, 2020, and April 10, 2020, through a hospital surveillance network. Parents and household contacts were called for contact tracing.
In 31 of 39 (79%) households, at least one adult family member had a suspected or confirmed SARS-CoV-2 infection before onset of symptoms in the child. These findings support data from previous studies suggesting that children mainly become infected from adult family members rather than transmitting the virus to them, the researchers said
In only 3 of 39 (8%) households was the study child the first to develop symptoms. “Surprisingly, in 33% of households, symptomatic HHCs [household contacts] tested negative despite belonging to a familial cluster with confirmed SARS-CoV-2 cases, suggesting an underreporting of cases,” Dr. Posfay-Barbe and associates noted.
The findings were limited by several factors including potential underreporting of cases because those with mild or atypical presentations may not have sought medical care, and the inability to confirm child-to-adult transmission. The results were strengthened by the extensive contact tracing and very few individuals lost to follow-up, they said; however, more diagnostic screening and contact tracing are needed to improve understanding of household transmission of SARS-CoV-2, they concluded.
Resolving the issue of how much children contribute to transmission of SARS-CoV-2 is essential to making informed decisions about public health, including how to structure schools and child-care facility reopening, Benjamin Lee, MD, and William V. Raszka Jr., MD, both of the University of Vermont, Burlington, said in an accompanying editorial (Pediatrics. 2020 Jul 10. doi: 10.1542/peds/2020-004879).
The data in the current study support other studies of transmission among household contacts in China suggesting that, in most cases of childhood infections, “the child was not the source of infection and that children most frequently acquire COVID-19 from adults, rather than transmitting it to them,” they wrote.
In addition, the limited data on transmission of SARS-CoV-2 by children outside of the household show few cases of secondary infection from children identified with SARS-CoV-2 in school settings in studies from France and Australia, Dr. Lee and Dr. Raszka noted.
the editorialists wrote. “This would be another manner by which SARS-CoV2 differs drastically from influenza, for which school-based transmission is well recognized as a significant driver of epidemic disease and forms the basis for most evidence regarding school closures as public health strategy.”
“Therefore, serious consideration should be paid toward strategies that allow schools to remain open, even during periods of COVID-19 spread,” the editorialists concluded. “In doing so, we could minimize the potentially profound adverse social, developmental, and health costs that our children will continue to suffer until an effective treatment or vaccine can be developed and distributed or, failing that, until we reach herd immunity,” Dr. Lee and Dr. Raszka emphasized.
The study received no outside funding. The researchers and editorialists had no financial conflicts to disclose.
SOURCE: Posfay-Barbe KM et al. Pediatrics. 2020 Jul 10. doi: 10.1542/peds.2020-1576.
FROM PEDIATRICS
Risky business: Longer-course prophylactic perioperative antimicrobials
Background: National guidelines recommend that surgical prophylactic antimicrobials be initiated within 1 hour prior to incision and discontinued 24 hours postoperatively. However, the risks and benefits of longer duration of antimicrobials are uncertain.
Study design: Retrospective cohort study.
Setting: Veterans Affairs hospitals.
Synopsis: After stratification by type of surgery and adjustment for covariates, antibiotic prophylaxis greater than 24 hours was not associated with lower SSI risk.
However, the odds of postoperative AKI increased with each additional day of prophylaxis (adjusted odds ratios, 1.82; 95% confidence interval,1.54-2.16 and aOR, 1.79; 95% CI, 1.27-2.53) with longer than 72 hours prophylaxis for cardiac and noncardiac surgery, respectively). Similarly, C. difficile infections increased with each additional day beyond 24 hours (aOR, 3.65; 95% CI, 2.40-5.55 with more than 72 hours of use).
Bottom line: Each day of perioperative antimicrobial prophylaxis beyond 24 hours increases the risk for postoperative AKI or C. difficile infection without reducing the risk of surgical site infection.
Citation: Branch-Elliman W et al. Association of duration and type of surgical prophylaxis with antimicrobial-associated adverse events. JAMA Surg. 2019 Apr 24. doi: 10.1001/jamasurg.2019.0569.
Dr. Miller is a hospitalist at the University of Colorado at Denver, Aurora.
Background: National guidelines recommend that surgical prophylactic antimicrobials be initiated within 1 hour prior to incision and discontinued 24 hours postoperatively. However, the risks and benefits of longer duration of antimicrobials are uncertain.
Study design: Retrospective cohort study.
Setting: Veterans Affairs hospitals.
Synopsis: After stratification by type of surgery and adjustment for covariates, antibiotic prophylaxis greater than 24 hours was not associated with lower SSI risk.
However, the odds of postoperative AKI increased with each additional day of prophylaxis (adjusted odds ratios, 1.82; 95% confidence interval,1.54-2.16 and aOR, 1.79; 95% CI, 1.27-2.53) with longer than 72 hours prophylaxis for cardiac and noncardiac surgery, respectively). Similarly, C. difficile infections increased with each additional day beyond 24 hours (aOR, 3.65; 95% CI, 2.40-5.55 with more than 72 hours of use).
Bottom line: Each day of perioperative antimicrobial prophylaxis beyond 24 hours increases the risk for postoperative AKI or C. difficile infection without reducing the risk of surgical site infection.
Citation: Branch-Elliman W et al. Association of duration and type of surgical prophylaxis with antimicrobial-associated adverse events. JAMA Surg. 2019 Apr 24. doi: 10.1001/jamasurg.2019.0569.
Dr. Miller is a hospitalist at the University of Colorado at Denver, Aurora.
Background: National guidelines recommend that surgical prophylactic antimicrobials be initiated within 1 hour prior to incision and discontinued 24 hours postoperatively. However, the risks and benefits of longer duration of antimicrobials are uncertain.
Study design: Retrospective cohort study.
Setting: Veterans Affairs hospitals.
Synopsis: After stratification by type of surgery and adjustment for covariates, antibiotic prophylaxis greater than 24 hours was not associated with lower SSI risk.
However, the odds of postoperative AKI increased with each additional day of prophylaxis (adjusted odds ratios, 1.82; 95% confidence interval,1.54-2.16 and aOR, 1.79; 95% CI, 1.27-2.53) with longer than 72 hours prophylaxis for cardiac and noncardiac surgery, respectively). Similarly, C. difficile infections increased with each additional day beyond 24 hours (aOR, 3.65; 95% CI, 2.40-5.55 with more than 72 hours of use).
Bottom line: Each day of perioperative antimicrobial prophylaxis beyond 24 hours increases the risk for postoperative AKI or C. difficile infection without reducing the risk of surgical site infection.
Citation: Branch-Elliman W et al. Association of duration and type of surgical prophylaxis with antimicrobial-associated adverse events. JAMA Surg. 2019 Apr 24. doi: 10.1001/jamasurg.2019.0569.
Dr. Miller is a hospitalist at the University of Colorado at Denver, Aurora.
Even a few days of steroids may be risky, new study suggests
Extended use of corticosteroids for chronic inflammatory conditions puts patients at risk for serious adverse events (AEs), including cardiovascular disease, osteoporosis, cataracts, and diabetes. Now, a growing body of evidence suggests that even short bursts of these drugs are associated with serious risks.
Most recently, a population-based study of more than 2.6 million people found that taking corticosteroids for 14 days or less was associated with a substantially greater risk for gastrointestinal (GI) bleeding, sepsis, and heart failure, particularly within the first 30 days after therapy.
In the study, Tsung-Chieh Yao, MD, PhD, a professor in the division of allergy, asthma, and rheumatology in the department of pediatrics at Chang Gung Memorial Hospital in Taoyuan, Taiwan, and colleagues used a self-controlled case series to analyze data from Taiwan’s National Health Insurance Research Database of medical claims. They compared patients’ conditions in the period from 5 to 90 days before treatment to conditions from the periods from 5 to 30 days and from 31 to 90 days after therapy.
With a median duration of 3 days of treatment, the incidence rate ratios (IRRs) were 1.80 (95% confidence interval, 1.75-1.84) for GI bleeding, 1.99 (95% CI, 1.70-2.32) for sepsis, and 2.37 (95% CI, 2.13-2.63) for heart failure.
Given the findings, physicians should weigh the benefits against the risks of rare but potentially serious consequences of these anti-inflammatory drugs, according to the authors.
“After initiating patients on oral steroid bursts, physicians should be on the lookout for these severe adverse events, particularly within the first month after initiation of steroid therapy,” Dr. Yao said in an interview.
The findings were published online July 6 in Annals of Internal Medicine.
Of the 15,859,129 adult Asians in the Taiwanese database, the study included 2,623,327 adults aged 20-64 years who received single steroid bursts (14 days or less) between Jan. 1, 2013, and Dec. 31, 2015.
Almost 60% of the indications were for skin disorders, such as eczema and urticaria, and for respiratory tract infections, such as sinusitis and acute pharyngitis. Among specialties, dermatology, otolaryngology, family practice, internal medicine, and pediatrics accounted for 88% of prescriptions.
“Our findings are important for physicians and guideline developers because short-term use of oral corticosteroids is common and the real-world safety of this approach remains unclear,” the authors wrote. They acknowledged that the database did not provide information on such potential confounders as disease severity and lifestyle factors, nor did it include children and vulnerable individuals, which may limit the generalizability of the results.
The findings echo those of a 2017 cohort study conducted by researchers at the University of Michigan in Ann Arbor. That study, by Akbar K. Waljee, MD, assistant professor of gastroenterology, University of Michigan, Ann Arbor, and colleagues, included data on more than 1.5 million privately insured U.S. adults. The researchers included somewhat longer steroid bursts of up to 30 days’ duration and found that use of the drugs was associated with a greater than fivefold increased risk for sepsis, a more than threefold increased risk for venous thromboembolism, and a nearly twofold increased risk for fracture within 30 days of starting treatment.
Furthermore, the elevated risk persisted at prednisone-equivalent doses of less than 20 mg/d (IRR, 4.02 for sepsis, 3.61 for venous thromboembolism, and 1.83 for fracture; all P < .001).
The U.S. study also found that during the 3-year period from 2012 to 2014, more than 20% of patients were prescribed short-term oral corticosteroids.
“Both studies indicate that these short-term regimens are more common in the real world than was previously thought and are not risk free,” Dr. Yao said.
Recognition that corticosteroids are associated with adverse events has been building for decades, according to the authors of an editorial that accompanies the new study.
“However, we commonly use short corticosteroid ‘bursts’ for minor ailments despite a lack of evidence for meaningful benefit. We are now learning that bursts as short as 3 days may increase risk for serious AEs, even in young and healthy people,” wrote editorialists Beth I. Wallace, MD, of the Center for Clinical Management Research at the VA Ann Arbor Healthcare System and the Institute for Healthcare Policy and Innovation at Michigan Medicine, Ann Arbor, and Dr. Waljee, who led the 2017 study.
Dr. Wallace and Dr. Waljee drew parallels between corticosteroid bursts and other short-term regimens, such as of antibiotics and opiates, in which prescriber preference and sometimes patient pressure play a role. “All of these treatments have well-defined indications but can cause net harm when used. We can thus conceive of a corticosteroid stewardship model of targeted interventions that aims to reduce inappropriate prescribing,” they wrote.
In an interview, Dr. Wallace, a rheumatologist who prescribes oral steroids fairly frequently, noted that the Taiwan study is the first to investigate steroid bursts. “Up till now, these very short courses have flown under the radar. Clinicians very commonly prescribe short courses to help relieve symptoms of self-limited conditions like bronchitis, and we assume that because the exposure duration is short, the risks are low, especially for patients who are otherwise healthy.”
She warned that the data in the current study indicate that these short bursts – even at the lower end of the 1- to 2-week courses American physicians prescribe most often – carry small but real increases in risk for serious AEs. “And these increases were seen in young, healthy people, not just in people with preexisting conditions,” she said. “So, we might need to start thinking harder about how we are prescribing even these very short courses of steroids and try to use steroids only when their meaningful benefits really outweigh the risk.”
She noted that a patient with a chronic inflammatory condition such as rheumatoid arthritis may benefit substantially from short-term steroids to treat a disease flare. In that specific case, the benefits of short-term steroids may outweigh the risks, Dr. Wallace said.
But not everyone thinks a new strategy is needed. For Whitney A. High, MD, associate professor of dermatology and pathology at the University of Colorado at Denver, Aurora, the overprescribing of short-term corticosteroids is not a problem, and dermatologists are already exercising caution.
“I only prescribe these drugs short term to, at a guess, about 1 in 40 patients and only when a patient is miserable and quality of life is being seriously affected,” he said in an interview. “And that’s something that can’t be measured in a database study like the one from Taiwan but only in a risk-benefit analysis,” he said.
Furthermore, dermatologists have other drugs and technologies in their armamentarium, including topical steroids with occlusion or with wet wraps, phototherapy, phosphodiesterase inhibitors, calcipotriene, methotrexate and other immunosuppressive agents, and biologics. “In fact, many of these agents are specifically referred to as steroid-sparing,” Dr. High said.
Nor does he experience much pressure from patients to prescribe these drugs. “While occasionally I may encounter a patient who places pressure on me for oral steroids, it’s probably not nearly as frequently as providers in other fields are pressured to prescribe antibiotics or narcotics,” he said.
According to the Taiwanese researchers, the next step is to conduct more studies, including clinical trials, to determine optimal use of corticosteroids by monitoring adverse events. In the meantime, for practitioners such as Dr. Wallace and Dr. High, there is ample evidence from several recent studies of the harms of short-term corticosteroids, whereas the benefits for patients with self-limiting conditions remain uncertain. “This and other studies like it quite appropriately remind providers to avoid oral steroids when they’re not necessary and to seek alternatives where possible,” Dr. High said.
The study was supported by the National Health Research Institutes of Taiwan, the Ministry of Science and Technology of Taiwan, the Chang Gung Medical Foundation, and the Eunice Kennedy Shriver National Institute of Child Health and Human Development of the National Institutes of Health (NIH). Dr. Yao has disclosed no relevant financial relationships. Dr. Wu has received grants from GlaxoSmithKline outside the submitted work. The editorialists and Dr. High have disclosed no relevant financial relationships. Dr. Wallace received an NIH grant during the writing of the editorial.
A version of this article originally appeared on Medscape.com.
Extended use of corticosteroids for chronic inflammatory conditions puts patients at risk for serious adverse events (AEs), including cardiovascular disease, osteoporosis, cataracts, and diabetes. Now, a growing body of evidence suggests that even short bursts of these drugs are associated with serious risks.
Most recently, a population-based study of more than 2.6 million people found that taking corticosteroids for 14 days or less was associated with a substantially greater risk for gastrointestinal (GI) bleeding, sepsis, and heart failure, particularly within the first 30 days after therapy.
In the study, Tsung-Chieh Yao, MD, PhD, a professor in the division of allergy, asthma, and rheumatology in the department of pediatrics at Chang Gung Memorial Hospital in Taoyuan, Taiwan, and colleagues used a self-controlled case series to analyze data from Taiwan’s National Health Insurance Research Database of medical claims. They compared patients’ conditions in the period from 5 to 90 days before treatment to conditions from the periods from 5 to 30 days and from 31 to 90 days after therapy.
With a median duration of 3 days of treatment, the incidence rate ratios (IRRs) were 1.80 (95% confidence interval, 1.75-1.84) for GI bleeding, 1.99 (95% CI, 1.70-2.32) for sepsis, and 2.37 (95% CI, 2.13-2.63) for heart failure.
Given the findings, physicians should weigh the benefits against the risks of rare but potentially serious consequences of these anti-inflammatory drugs, according to the authors.
“After initiating patients on oral steroid bursts, physicians should be on the lookout for these severe adverse events, particularly within the first month after initiation of steroid therapy,” Dr. Yao said in an interview.
The findings were published online July 6 in Annals of Internal Medicine.
Of the 15,859,129 adult Asians in the Taiwanese database, the study included 2,623,327 adults aged 20-64 years who received single steroid bursts (14 days or less) between Jan. 1, 2013, and Dec. 31, 2015.
Almost 60% of the indications were for skin disorders, such as eczema and urticaria, and for respiratory tract infections, such as sinusitis and acute pharyngitis. Among specialties, dermatology, otolaryngology, family practice, internal medicine, and pediatrics accounted for 88% of prescriptions.
“Our findings are important for physicians and guideline developers because short-term use of oral corticosteroids is common and the real-world safety of this approach remains unclear,” the authors wrote. They acknowledged that the database did not provide information on such potential confounders as disease severity and lifestyle factors, nor did it include children and vulnerable individuals, which may limit the generalizability of the results.
The findings echo those of a 2017 cohort study conducted by researchers at the University of Michigan in Ann Arbor. That study, by Akbar K. Waljee, MD, assistant professor of gastroenterology, University of Michigan, Ann Arbor, and colleagues, included data on more than 1.5 million privately insured U.S. adults. The researchers included somewhat longer steroid bursts of up to 30 days’ duration and found that use of the drugs was associated with a greater than fivefold increased risk for sepsis, a more than threefold increased risk for venous thromboembolism, and a nearly twofold increased risk for fracture within 30 days of starting treatment.
Furthermore, the elevated risk persisted at prednisone-equivalent doses of less than 20 mg/d (IRR, 4.02 for sepsis, 3.61 for venous thromboembolism, and 1.83 for fracture; all P < .001).
The U.S. study also found that during the 3-year period from 2012 to 2014, more than 20% of patients were prescribed short-term oral corticosteroids.
“Both studies indicate that these short-term regimens are more common in the real world than was previously thought and are not risk free,” Dr. Yao said.
Recognition that corticosteroids are associated with adverse events has been building for decades, according to the authors of an editorial that accompanies the new study.
“However, we commonly use short corticosteroid ‘bursts’ for minor ailments despite a lack of evidence for meaningful benefit. We are now learning that bursts as short as 3 days may increase risk for serious AEs, even in young and healthy people,” wrote editorialists Beth I. Wallace, MD, of the Center for Clinical Management Research at the VA Ann Arbor Healthcare System and the Institute for Healthcare Policy and Innovation at Michigan Medicine, Ann Arbor, and Dr. Waljee, who led the 2017 study.
Dr. Wallace and Dr. Waljee drew parallels between corticosteroid bursts and other short-term regimens, such as of antibiotics and opiates, in which prescriber preference and sometimes patient pressure play a role. “All of these treatments have well-defined indications but can cause net harm when used. We can thus conceive of a corticosteroid stewardship model of targeted interventions that aims to reduce inappropriate prescribing,” they wrote.
In an interview, Dr. Wallace, a rheumatologist who prescribes oral steroids fairly frequently, noted that the Taiwan study is the first to investigate steroid bursts. “Up till now, these very short courses have flown under the radar. Clinicians very commonly prescribe short courses to help relieve symptoms of self-limited conditions like bronchitis, and we assume that because the exposure duration is short, the risks are low, especially for patients who are otherwise healthy.”
She warned that the data in the current study indicate that these short bursts – even at the lower end of the 1- to 2-week courses American physicians prescribe most often – carry small but real increases in risk for serious AEs. “And these increases were seen in young, healthy people, not just in people with preexisting conditions,” she said. “So, we might need to start thinking harder about how we are prescribing even these very short courses of steroids and try to use steroids only when their meaningful benefits really outweigh the risk.”
She noted that a patient with a chronic inflammatory condition such as rheumatoid arthritis may benefit substantially from short-term steroids to treat a disease flare. In that specific case, the benefits of short-term steroids may outweigh the risks, Dr. Wallace said.
But not everyone thinks a new strategy is needed. For Whitney A. High, MD, associate professor of dermatology and pathology at the University of Colorado at Denver, Aurora, the overprescribing of short-term corticosteroids is not a problem, and dermatologists are already exercising caution.
“I only prescribe these drugs short term to, at a guess, about 1 in 40 patients and only when a patient is miserable and quality of life is being seriously affected,” he said in an interview. “And that’s something that can’t be measured in a database study like the one from Taiwan but only in a risk-benefit analysis,” he said.
Furthermore, dermatologists have other drugs and technologies in their armamentarium, including topical steroids with occlusion or with wet wraps, phototherapy, phosphodiesterase inhibitors, calcipotriene, methotrexate and other immunosuppressive agents, and biologics. “In fact, many of these agents are specifically referred to as steroid-sparing,” Dr. High said.
Nor does he experience much pressure from patients to prescribe these drugs. “While occasionally I may encounter a patient who places pressure on me for oral steroids, it’s probably not nearly as frequently as providers in other fields are pressured to prescribe antibiotics or narcotics,” he said.
According to the Taiwanese researchers, the next step is to conduct more studies, including clinical trials, to determine optimal use of corticosteroids by monitoring adverse events. In the meantime, for practitioners such as Dr. Wallace and Dr. High, there is ample evidence from several recent studies of the harms of short-term corticosteroids, whereas the benefits for patients with self-limiting conditions remain uncertain. “This and other studies like it quite appropriately remind providers to avoid oral steroids when they’re not necessary and to seek alternatives where possible,” Dr. High said.
The study was supported by the National Health Research Institutes of Taiwan, the Ministry of Science and Technology of Taiwan, the Chang Gung Medical Foundation, and the Eunice Kennedy Shriver National Institute of Child Health and Human Development of the National Institutes of Health (NIH). Dr. Yao has disclosed no relevant financial relationships. Dr. Wu has received grants from GlaxoSmithKline outside the submitted work. The editorialists and Dr. High have disclosed no relevant financial relationships. Dr. Wallace received an NIH grant during the writing of the editorial.
A version of this article originally appeared on Medscape.com.
Extended use of corticosteroids for chronic inflammatory conditions puts patients at risk for serious adverse events (AEs), including cardiovascular disease, osteoporosis, cataracts, and diabetes. Now, a growing body of evidence suggests that even short bursts of these drugs are associated with serious risks.
Most recently, a population-based study of more than 2.6 million people found that taking corticosteroids for 14 days or less was associated with a substantially greater risk for gastrointestinal (GI) bleeding, sepsis, and heart failure, particularly within the first 30 days after therapy.
In the study, Tsung-Chieh Yao, MD, PhD, a professor in the division of allergy, asthma, and rheumatology in the department of pediatrics at Chang Gung Memorial Hospital in Taoyuan, Taiwan, and colleagues used a self-controlled case series to analyze data from Taiwan’s National Health Insurance Research Database of medical claims. They compared patients’ conditions in the period from 5 to 90 days before treatment to conditions from the periods from 5 to 30 days and from 31 to 90 days after therapy.
With a median duration of 3 days of treatment, the incidence rate ratios (IRRs) were 1.80 (95% confidence interval, 1.75-1.84) for GI bleeding, 1.99 (95% CI, 1.70-2.32) for sepsis, and 2.37 (95% CI, 2.13-2.63) for heart failure.
Given the findings, physicians should weigh the benefits against the risks of rare but potentially serious consequences of these anti-inflammatory drugs, according to the authors.
“After initiating patients on oral steroid bursts, physicians should be on the lookout for these severe adverse events, particularly within the first month after initiation of steroid therapy,” Dr. Yao said in an interview.
The findings were published online July 6 in Annals of Internal Medicine.
Of the 15,859,129 adult Asians in the Taiwanese database, the study included 2,623,327 adults aged 20-64 years who received single steroid bursts (14 days or less) between Jan. 1, 2013, and Dec. 31, 2015.
Almost 60% of the indications were for skin disorders, such as eczema and urticaria, and for respiratory tract infections, such as sinusitis and acute pharyngitis. Among specialties, dermatology, otolaryngology, family practice, internal medicine, and pediatrics accounted for 88% of prescriptions.
“Our findings are important for physicians and guideline developers because short-term use of oral corticosteroids is common and the real-world safety of this approach remains unclear,” the authors wrote. They acknowledged that the database did not provide information on such potential confounders as disease severity and lifestyle factors, nor did it include children and vulnerable individuals, which may limit the generalizability of the results.
The findings echo those of a 2017 cohort study conducted by researchers at the University of Michigan in Ann Arbor. That study, by Akbar K. Waljee, MD, assistant professor of gastroenterology, University of Michigan, Ann Arbor, and colleagues, included data on more than 1.5 million privately insured U.S. adults. The researchers included somewhat longer steroid bursts of up to 30 days’ duration and found that use of the drugs was associated with a greater than fivefold increased risk for sepsis, a more than threefold increased risk for venous thromboembolism, and a nearly twofold increased risk for fracture within 30 days of starting treatment.
Furthermore, the elevated risk persisted at prednisone-equivalent doses of less than 20 mg/d (IRR, 4.02 for sepsis, 3.61 for venous thromboembolism, and 1.83 for fracture; all P < .001).
The U.S. study also found that during the 3-year period from 2012 to 2014, more than 20% of patients were prescribed short-term oral corticosteroids.
“Both studies indicate that these short-term regimens are more common in the real world than was previously thought and are not risk free,” Dr. Yao said.
Recognition that corticosteroids are associated with adverse events has been building for decades, according to the authors of an editorial that accompanies the new study.
“However, we commonly use short corticosteroid ‘bursts’ for minor ailments despite a lack of evidence for meaningful benefit. We are now learning that bursts as short as 3 days may increase risk for serious AEs, even in young and healthy people,” wrote editorialists Beth I. Wallace, MD, of the Center for Clinical Management Research at the VA Ann Arbor Healthcare System and the Institute for Healthcare Policy and Innovation at Michigan Medicine, Ann Arbor, and Dr. Waljee, who led the 2017 study.
Dr. Wallace and Dr. Waljee drew parallels between corticosteroid bursts and other short-term regimens, such as of antibiotics and opiates, in which prescriber preference and sometimes patient pressure play a role. “All of these treatments have well-defined indications but can cause net harm when used. We can thus conceive of a corticosteroid stewardship model of targeted interventions that aims to reduce inappropriate prescribing,” they wrote.
In an interview, Dr. Wallace, a rheumatologist who prescribes oral steroids fairly frequently, noted that the Taiwan study is the first to investigate steroid bursts. “Up till now, these very short courses have flown under the radar. Clinicians very commonly prescribe short courses to help relieve symptoms of self-limited conditions like bronchitis, and we assume that because the exposure duration is short, the risks are low, especially for patients who are otherwise healthy.”
She warned that the data in the current study indicate that these short bursts – even at the lower end of the 1- to 2-week courses American physicians prescribe most often – carry small but real increases in risk for serious AEs. “And these increases were seen in young, healthy people, not just in people with preexisting conditions,” she said. “So, we might need to start thinking harder about how we are prescribing even these very short courses of steroids and try to use steroids only when their meaningful benefits really outweigh the risk.”
She noted that a patient with a chronic inflammatory condition such as rheumatoid arthritis may benefit substantially from short-term steroids to treat a disease flare. In that specific case, the benefits of short-term steroids may outweigh the risks, Dr. Wallace said.
But not everyone thinks a new strategy is needed. For Whitney A. High, MD, associate professor of dermatology and pathology at the University of Colorado at Denver, Aurora, the overprescribing of short-term corticosteroids is not a problem, and dermatologists are already exercising caution.
“I only prescribe these drugs short term to, at a guess, about 1 in 40 patients and only when a patient is miserable and quality of life is being seriously affected,” he said in an interview. “And that’s something that can’t be measured in a database study like the one from Taiwan but only in a risk-benefit analysis,” he said.
Furthermore, dermatologists have other drugs and technologies in their armamentarium, including topical steroids with occlusion or with wet wraps, phototherapy, phosphodiesterase inhibitors, calcipotriene, methotrexate and other immunosuppressive agents, and biologics. “In fact, many of these agents are specifically referred to as steroid-sparing,” Dr. High said.
Nor does he experience much pressure from patients to prescribe these drugs. “While occasionally I may encounter a patient who places pressure on me for oral steroids, it’s probably not nearly as frequently as providers in other fields are pressured to prescribe antibiotics or narcotics,” he said.
According to the Taiwanese researchers, the next step is to conduct more studies, including clinical trials, to determine optimal use of corticosteroids by monitoring adverse events. In the meantime, for practitioners such as Dr. Wallace and Dr. High, there is ample evidence from several recent studies of the harms of short-term corticosteroids, whereas the benefits for patients with self-limiting conditions remain uncertain. “This and other studies like it quite appropriately remind providers to avoid oral steroids when they’re not necessary and to seek alternatives where possible,” Dr. High said.
The study was supported by the National Health Research Institutes of Taiwan, the Ministry of Science and Technology of Taiwan, the Chang Gung Medical Foundation, and the Eunice Kennedy Shriver National Institute of Child Health and Human Development of the National Institutes of Health (NIH). Dr. Yao has disclosed no relevant financial relationships. Dr. Wu has received grants from GlaxoSmithKline outside the submitted work. The editorialists and Dr. High have disclosed no relevant financial relationships. Dr. Wallace received an NIH grant during the writing of the editorial.
A version of this article originally appeared on Medscape.com.
Retreatment of Hepatitis C Infection With Direct-Acting Antivirals
An estimated 3.5 million people in the US have chronic hepatitis C virus (HCV) infection, and between 10% and 20% of those developed cirrhosis over 20 to 30 years.1 There are at least 6 genotypes (GTs) of HCV, with GT1 being the most common in the US and previously one of the most difficult to treat.2,3 The goal of treatment is to achieve viral cure, called sustained virologic response (SVR) when HCV viral load remains undetectable several weeks after therapy completion. In the 2000s, pegylated interferon (pegIFN) and ribavirin (RBV) were the standard of care.2 For patients with GT1 infections, an SVR of 40 to 50% was commonly seen after 48 weeks of pegIFN/RBV regimens compared with 70 to 80% SVR for GT2 or GT3 after 24 weeks of pegIFN/RBV therapy.2 However, treatment has evolved rapidly (Table 1).2-17
In 2011, the US Food and Drug Administration (FDA) approved the protease inhibitors (PIs) boceprevir and telaprevir, which added a new class of agents with increased SVR for patients with GT1 infection; however, pegIFN and RBV were still needed for treatment.4 In addition, both PIs required multiple doses per day and strict adherence to an 8-hour schedule.4 Boceprevir required treatment with RBV and pegIFN for 48 weeks unless futility rule was met at 24 weeks of treatment (ie, viral load still detectable).4 The SVR in patients with GT1 infection improved to > 65% for patients in clinical trials.2 FDA approval of the direct-acting antivirals (DAAs) sofosbuvir and simeprevir in late 2013 decreased the usual duration of therapy to only 12 weeks with improved SVR rates 12 weeks posttherapy (SVR12) to 90% or higher.2,6,10
FDA approval of ledipasvir (LDV)/sofosbuvir (SOF) in October 2014 resulted in the first interferon-free all-oral regimen indicated for HCV GT1 infection.11 In December 2014, FDA approved a combination of paritaprevir, ritonavir, ombitasvir, and dasabuvir (PrOD).12 In 2015 GT-specific approvals were issued for daclastavir to be used with SOV for GT1 and GT3 and a combination similar to PrOD without dasabuvir (PrO) for GT4.13 In 2016, a combination of elbasvir (ERB) and grazoprevir (GZP) was approved for GT1 and GT4.14
In 2016, a pangenotypic DAA of SOF and velpatasvir (VEL) was approved.15 Most recently, combinations of SOF, VEL, and voxilaprevir (VOX), and glecaprevir (GLE) and pibrentasvir (PIB) were approved for patients with previous DAA treatment failures.7, 8,16,17 These oral regimens avoided the significant adverse events (AEs) associated with pegIFN and RBV (eg, thrombocytopenia, depression), were expected to improve treatment adherence and shorten duration of therapy.
The West Palm Beach Veterans Affairs has had a nurse practitioner (NP)-based HCV treatment clinic since the late 1990s. When PIs became available, a CPS started reviewing patient electronic health records (EHRs) and monitored response to therapy along with the NP to ensure discontinuation of therapy if futility criteria were met.7 Our unpublished experience showed SVR > 60% with both boceprevir and SOF regimens and > 90% with oral DAA regimens.
This review will provide the SVR rates for patients that needed retreatment for HCV infection since 2015 until December 2019. We treated all willing patients, beginning with the patients who had experienced failures with previous regimens. Patients first received education on HCV infection and treatment options in a group class then they were seen by the NP individually for specific education on treatment. The CPS reviewed the patient’s medical record to assess for appropriate therapy, possible drug-drug interactions and contraindications to therapy. In addition, patient outcomes (eg, viral load, AEs) were documented by the CPS in collaboration with the NP throughout treatment until viral load for SVR evaluation was obtained.
Methods
A retrospective EHR review of patients retreated from January 2015 to December 2019 was conducted. Data collected included age, sex, HCV GT, previous therapy, new medications prescribed, creatinine clearance, and achievement of SVR12. This retrospective review was approved by the facility’s scientific advisory committee as part of performance improvement efforts. Descriptive statistics are provided.
Results
Boceprevir
We treated 31 patients with boceprevir of which 3 met futility rule and 28 completed therapy. Eighteen of 28 responded (64%) to the treatment. The 10 patients who failed treatment were retreated with LDV/SOF, and all achieved SVR.
Sofosbuvir
A total of 53 patients were treated with SOF, RBV, and pegIFN for 12 weeks. Forty-one achieved SVR (77%). Of the 12 who failed therapy, all have been retreated and achieved SVR (Table 2).
Interferon-Free DAA Oral Regimens
More than 900 patients have been treated with interferon-free regimens since 2015 and outcomes were documented for > 800 patients. The SVR rates by GT were as follows: GT1 639 of 676 (95%); GT2 76 of 79 (96%); GT3 40 of 48 (83%); and GT4 6 of 6 (100%). Eighty-four percent of patients had GT1 infection. The median age of patient was 62 years, 72% were treatment naïve, and 35% having cirrhosis (based on liver biopsy or FIB4 score).18
Of 48 treatment failures, 30 patients were retreated; the rest of the patients were lost to follow-up (n = 9) or unable to receive retreatment (n = 9) mainly due to decompensated cirrhosis or liver cancer and short life expectancy. The median age of patient in this retreatment group was 62 years, 62% had cirrhosis, and most were infected with GT1. The average creatinine clearance was 73 mL/min. Twenty-two patients who failed therapy with ledipasvir/SOF were retreated (Table 3). A total of 13 patients out of the 19 tested eventually achieved SVR (68%). Four of the patients who had treatment failure again had GT1 infection and the other 2 GT3. All had cirrhosis.
Thirty-five patients were treated with PrOD, and 32 achieved SVR (91%). All 3 patients were retreated. One patient each achieved SVR with ERB/GZP, SOF/VEL and SOF/VEL/VOX. Fifty patients were treated with ERB/GZP and 45 achieved SVR (90%). All 5 treatment failures were retreated. Four achieved SVR and 1 was lost to follow-up (Table 4). Overall, of 30 patients who were retreated after failure with an all-oral DAA regimen, 27 patients had SVR values available and 21 achieved it (78%).
Discussion
Overall SVR was very high for patients who received oral treatment for HCV infection. A low number of patients failed therapy and were retreated. Patients who failed therapy again were similar in age but were more likely to have cirrhosis when compared with the overall interferon-free treated group. Thus, prompt treatment after HCV detection and before disease progression may improve treatment outcomes. Achieving SVR has been shown to improve fibrosis, portal hypertension, splenomegaly and cirrhosis, and reduce the risk of hepatocellular carcinoma by 70% and liver-related mortality by 90%.19-21/
Patients who failed therapy primarily had GT1—the most prevalent GT treated. A higher prevalence of GT1 is expected since it is the most common GT in the US.6 However, disease progression occurs more rapidly in those with GT3 and is more difficult to treat.22 The overall response rate was lower with this GT (83%) in this report, with only 1 of 3 patients retreated achieving an SVR.
Similar results are documented in retreatment trials.23 In the POLARIS-1 trial, treatment with SOF/VEL/VOX resulted in an overall response rate of 96% but only 91% for patients with GT3, compared with 95 to 100% for GTs 1, 2, or 4.23 In the current report, only 1 patient (GT1) failed retreatment with SOF/VEL/VOX. At this time, there are no clear treatment options for this patient. However, patients who fail GLE/PIB (none so far in the current report) may be able to receive SOF/VEL/VOX.24 In a small study, 29 of 31 patients achieved SVR with SOF/VEL/VOX after GLE/PIB failure (12 of 13 GT1 and 17 of 18 GT3).24
Limitations
This review was an observational, nonrandomized design, and only 1 medical center was involved. These results may not be applicable to other patient populations without a clinic set up with routine follow-ups to encourage adherence and completion of therapy.
Conclusions
Treatment of HCV infection has improved significantly over the past 10 years. Use of DAAs results in SVR for > 90% of patients, especially if the disease had not progressed to cirrhosis. Failure after retreatment for HCV infection was rare as well. Given that cirrhosis seems to increase the chance of treatment failure, it is imperative to identify candidates for treatment before the infection has progressed to cirrhosis. Patients infected with GT3 in particular should be more aggressively identified and treated.
Acknowledgments
The authors thank Nick P. Becky, PharmD, for his contributions to the identification of patients needing treatment for their HCV infection and review of initial manuscript information.
1. Centers for Disease Control and Prevention. Viral hepatitis: hepatitis C information. https://www.cdc.gov/hepatitis/hcv/index.htm. Reviewed April 14, 2020. Accessed June 16, 2020.
2. American Association for the Study of Liver Disease, Infectious Diseases Society of America. HCV Guidance: Recommendations for Testing, Managing, and Treating Hepatitis C. https://www.hcvguidelines.org. Accessed June 16, 2020.
3. Lingala S, Ghany MG. Natural history of hepatitis C. Gastroenterol Clin N Am. 2015;44(4):717-734. doi:10.1016/j.gtc.2015.07.003
4. Foote BS, Spooner LM, Belliveau PP. Boceprevir: a protease inhibitor for the treatment of chronic hepatitis C. Ann Pharmacother. 2011;45(9):1085-1093. doi:10.1345/aph.1P744
5. Kayali Z, Schmidt WN. Finally sofosbuvir: an oral anti-HCV drug with wide performance capability. Pharmgenomics Pers Med. 2014:7:387-398. doi:10.2147/PGPM.S52629
6. Falade-Nwulis O, Suarez-Cuervo C, Nelson DR, Fried MW, Segal JB, Sulkowski MS. Oral direct-acting agent therapy for hepatitis C virus infection: a systematic review. Ann Intern Med. 2017;166(9):637-648. doi:10.7326/M16-2575
7. Carrion AF, Martin P. Glecaprevir + pibrentasvir for treatment of hepatitis C. Expert Opinion Pharmacother. 2018;19(4):413-419. doi:10.1080/14656566.2018.1444030
8. Chahine EB, Kelley D, Childs-Kean LM. Sofosbuvir/velpatasvir/voxilaprevir: a pan-genotypic direct-acting antiviral combination for hepatitis C. Ann Pharmacother. 2018;52(4):352-363. doi:10.1177/1060028017741508
9. Lagasca AM, Kan VL. Hepatitis C treatment at a Veteran Affairs medical center after the availability of direct-acting agents: things are looking up. Clin Infect Dis. 2015:61(8):1347-1349. doi:10.1093/cid/civ573
10. Sovaldi (sofosbuvir) [package insert]. Foster City, CA: Gilead Sciences Inc; 2017.
11. Harvoni (ledipasvir and sofosbuvir) [package insert]. Foster City, CA: Gilead Sciences Inc; 2017.
12. Viekira Pak (ombitasvir, paritaprevir and ritonavir; dasabuvir) [package insert]. North Chicago, IL: AbbVie Inc; 2018.
13. Technivie (ombitasvir, paritaprevir and ritonavir) [package insert]. North Chicago, IL: AbbVie Inc; 2018.
14. Zepatier (elbasvir and grazoprevir) [package insert]. Whitehouse Station, NJ: Merck & Co Inc; 2018.
15. Epclusa (sofosbuvir and velpatasvir) [package insert]. Foster City, CA: Gilead Sciences Inc; 2017.
16. Mavyret (glecaprevir and pibrentasvir) [package insert]. North Chicago, IL: AbbVie Inc; 2019.
17. Vosevi (sofosbuvir, velpatasvir and voxilaprevir) [package insert]. Foster City, CA: Gilead Sciences Inc; 2017.
18. Vallet-Pichard A, Mallet V, Nalpas V, et al. FIB-4: an inexpensive and accurate marker of fibrosis in HCV infection. Comparison with liver biopsy and FibroTest. Hepatology. 2017;46(1):32-36. doi:10.1002/hep.21669
19. Morgan RL, Baack B, Smith BD, Yartel A, Pitasi M, Falck-Ytter Y. Eradication of hepatitis C virus infection and the development of hepatocellular carcinoma: a meta-analysis of observational studies. Ann Intern Med. 2013;158(5, pt 1):329-337. doi:10.7326/0003-4819-158-5-201303050-00005
20. van der Meer AJ, Veldt BJ, Feld JJ, et al. Association between sustained virological response and all-cause mortality among patients with chronic hepatitis C and advanced hepatic fibrosis. JAMA. 2012;308(24):2584-2593. doi:10.1001/jama.2012.144878
21. Veldt BJ, Heathcote EJ, Wedemeyer H, et al. Sustained virologic response and clinical outcomes in patients with chronic hepatitis C and advanced fibrosis. Ann Intern Med. 2007;147(10):677-684. doi:10.7326/0003-4819-147-10-200711200-00003
22. Chen A, Patel K, Naggie S. Genotype 3 infection: the last stand of hepatitis C virus. Drugs. 2017;77(2):131-144. doi:10.1007/s40265-016-0685-x
23. Bourlière M, Gordon SC, Flamm SL, et al; POLARIS-1 and POLARIS-4 Investigators. Sofosbuvir, velpatasvir and voxilaprevir for previously treated HCV infection. N Engl J Med. 2017;376(22):2136-2146. doi:10.1056/NEJMoa1613512
24. Pearlman B, Perrys M, Hinds A. Sofosbuvir/velpatasvir/voxilaprevir for previous treatment failures with glecaprevir/pibrentasvir in chronic hepatitis C infection. Am J Gastroenterol. 2019;114(9):1550-1552. doi:10.14309/ajg.0000000000000248
An estimated 3.5 million people in the US have chronic hepatitis C virus (HCV) infection, and between 10% and 20% of those developed cirrhosis over 20 to 30 years.1 There are at least 6 genotypes (GTs) of HCV, with GT1 being the most common in the US and previously one of the most difficult to treat.2,3 The goal of treatment is to achieve viral cure, called sustained virologic response (SVR) when HCV viral load remains undetectable several weeks after therapy completion. In the 2000s, pegylated interferon (pegIFN) and ribavirin (RBV) were the standard of care.2 For patients with GT1 infections, an SVR of 40 to 50% was commonly seen after 48 weeks of pegIFN/RBV regimens compared with 70 to 80% SVR for GT2 or GT3 after 24 weeks of pegIFN/RBV therapy.2 However, treatment has evolved rapidly (Table 1).2-17
In 2011, the US Food and Drug Administration (FDA) approved the protease inhibitors (PIs) boceprevir and telaprevir, which added a new class of agents with increased SVR for patients with GT1 infection; however, pegIFN and RBV were still needed for treatment.4 In addition, both PIs required multiple doses per day and strict adherence to an 8-hour schedule.4 Boceprevir required treatment with RBV and pegIFN for 48 weeks unless futility rule was met at 24 weeks of treatment (ie, viral load still detectable).4 The SVR in patients with GT1 infection improved to > 65% for patients in clinical trials.2 FDA approval of the direct-acting antivirals (DAAs) sofosbuvir and simeprevir in late 2013 decreased the usual duration of therapy to only 12 weeks with improved SVR rates 12 weeks posttherapy (SVR12) to 90% or higher.2,6,10
FDA approval of ledipasvir (LDV)/sofosbuvir (SOF) in October 2014 resulted in the first interferon-free all-oral regimen indicated for HCV GT1 infection.11 In December 2014, FDA approved a combination of paritaprevir, ritonavir, ombitasvir, and dasabuvir (PrOD).12 In 2015 GT-specific approvals were issued for daclastavir to be used with SOV for GT1 and GT3 and a combination similar to PrOD without dasabuvir (PrO) for GT4.13 In 2016, a combination of elbasvir (ERB) and grazoprevir (GZP) was approved for GT1 and GT4.14
In 2016, a pangenotypic DAA of SOF and velpatasvir (VEL) was approved.15 Most recently, combinations of SOF, VEL, and voxilaprevir (VOX), and glecaprevir (GLE) and pibrentasvir (PIB) were approved for patients with previous DAA treatment failures.7, 8,16,17 These oral regimens avoided the significant adverse events (AEs) associated with pegIFN and RBV (eg, thrombocytopenia, depression), were expected to improve treatment adherence and shorten duration of therapy.
The West Palm Beach Veterans Affairs has had a nurse practitioner (NP)-based HCV treatment clinic since the late 1990s. When PIs became available, a CPS started reviewing patient electronic health records (EHRs) and monitored response to therapy along with the NP to ensure discontinuation of therapy if futility criteria were met.7 Our unpublished experience showed SVR > 60% with both boceprevir and SOF regimens and > 90% with oral DAA regimens.
This review will provide the SVR rates for patients that needed retreatment for HCV infection since 2015 until December 2019. We treated all willing patients, beginning with the patients who had experienced failures with previous regimens. Patients first received education on HCV infection and treatment options in a group class then they were seen by the NP individually for specific education on treatment. The CPS reviewed the patient’s medical record to assess for appropriate therapy, possible drug-drug interactions and contraindications to therapy. In addition, patient outcomes (eg, viral load, AEs) were documented by the CPS in collaboration with the NP throughout treatment until viral load for SVR evaluation was obtained.
Methods
A retrospective EHR review of patients retreated from January 2015 to December 2019 was conducted. Data collected included age, sex, HCV GT, previous therapy, new medications prescribed, creatinine clearance, and achievement of SVR12. This retrospective review was approved by the facility’s scientific advisory committee as part of performance improvement efforts. Descriptive statistics are provided.
Results
Boceprevir
We treated 31 patients with boceprevir of which 3 met futility rule and 28 completed therapy. Eighteen of 28 responded (64%) to the treatment. The 10 patients who failed treatment were retreated with LDV/SOF, and all achieved SVR.
Sofosbuvir
A total of 53 patients were treated with SOF, RBV, and pegIFN for 12 weeks. Forty-one achieved SVR (77%). Of the 12 who failed therapy, all have been retreated and achieved SVR (Table 2).
Interferon-Free DAA Oral Regimens
More than 900 patients have been treated with interferon-free regimens since 2015 and outcomes were documented for > 800 patients. The SVR rates by GT were as follows: GT1 639 of 676 (95%); GT2 76 of 79 (96%); GT3 40 of 48 (83%); and GT4 6 of 6 (100%). Eighty-four percent of patients had GT1 infection. The median age of patient was 62 years, 72% were treatment naïve, and 35% having cirrhosis (based on liver biopsy or FIB4 score).18
Of 48 treatment failures, 30 patients were retreated; the rest of the patients were lost to follow-up (n = 9) or unable to receive retreatment (n = 9) mainly due to decompensated cirrhosis or liver cancer and short life expectancy. The median age of patient in this retreatment group was 62 years, 62% had cirrhosis, and most were infected with GT1. The average creatinine clearance was 73 mL/min. Twenty-two patients who failed therapy with ledipasvir/SOF were retreated (Table 3). A total of 13 patients out of the 19 tested eventually achieved SVR (68%). Four of the patients who had treatment failure again had GT1 infection and the other 2 GT3. All had cirrhosis.
Thirty-five patients were treated with PrOD, and 32 achieved SVR (91%). All 3 patients were retreated. One patient each achieved SVR with ERB/GZP, SOF/VEL and SOF/VEL/VOX. Fifty patients were treated with ERB/GZP and 45 achieved SVR (90%). All 5 treatment failures were retreated. Four achieved SVR and 1 was lost to follow-up (Table 4). Overall, of 30 patients who were retreated after failure with an all-oral DAA regimen, 27 patients had SVR values available and 21 achieved it (78%).
Discussion
Overall SVR was very high for patients who received oral treatment for HCV infection. A low number of patients failed therapy and were retreated. Patients who failed therapy again were similar in age but were more likely to have cirrhosis when compared with the overall interferon-free treated group. Thus, prompt treatment after HCV detection and before disease progression may improve treatment outcomes. Achieving SVR has been shown to improve fibrosis, portal hypertension, splenomegaly and cirrhosis, and reduce the risk of hepatocellular carcinoma by 70% and liver-related mortality by 90%.19-21/
Patients who failed therapy primarily had GT1—the most prevalent GT treated. A higher prevalence of GT1 is expected since it is the most common GT in the US.6 However, disease progression occurs more rapidly in those with GT3 and is more difficult to treat.22 The overall response rate was lower with this GT (83%) in this report, with only 1 of 3 patients retreated achieving an SVR.
Similar results are documented in retreatment trials.23 In the POLARIS-1 trial, treatment with SOF/VEL/VOX resulted in an overall response rate of 96% but only 91% for patients with GT3, compared with 95 to 100% for GTs 1, 2, or 4.23 In the current report, only 1 patient (GT1) failed retreatment with SOF/VEL/VOX. At this time, there are no clear treatment options for this patient. However, patients who fail GLE/PIB (none so far in the current report) may be able to receive SOF/VEL/VOX.24 In a small study, 29 of 31 patients achieved SVR with SOF/VEL/VOX after GLE/PIB failure (12 of 13 GT1 and 17 of 18 GT3).24
Limitations
This review was an observational, nonrandomized design, and only 1 medical center was involved. These results may not be applicable to other patient populations without a clinic set up with routine follow-ups to encourage adherence and completion of therapy.
Conclusions
Treatment of HCV infection has improved significantly over the past 10 years. Use of DAAs results in SVR for > 90% of patients, especially if the disease had not progressed to cirrhosis. Failure after retreatment for HCV infection was rare as well. Given that cirrhosis seems to increase the chance of treatment failure, it is imperative to identify candidates for treatment before the infection has progressed to cirrhosis. Patients infected with GT3 in particular should be more aggressively identified and treated.
Acknowledgments
The authors thank Nick P. Becky, PharmD, for his contributions to the identification of patients needing treatment for their HCV infection and review of initial manuscript information.
An estimated 3.5 million people in the US have chronic hepatitis C virus (HCV) infection, and between 10% and 20% of those developed cirrhosis over 20 to 30 years.1 There are at least 6 genotypes (GTs) of HCV, with GT1 being the most common in the US and previously one of the most difficult to treat.2,3 The goal of treatment is to achieve viral cure, called sustained virologic response (SVR) when HCV viral load remains undetectable several weeks after therapy completion. In the 2000s, pegylated interferon (pegIFN) and ribavirin (RBV) were the standard of care.2 For patients with GT1 infections, an SVR of 40 to 50% was commonly seen after 48 weeks of pegIFN/RBV regimens compared with 70 to 80% SVR for GT2 or GT3 after 24 weeks of pegIFN/RBV therapy.2 However, treatment has evolved rapidly (Table 1).2-17
In 2011, the US Food and Drug Administration (FDA) approved the protease inhibitors (PIs) boceprevir and telaprevir, which added a new class of agents with increased SVR for patients with GT1 infection; however, pegIFN and RBV were still needed for treatment.4 In addition, both PIs required multiple doses per day and strict adherence to an 8-hour schedule.4 Boceprevir required treatment with RBV and pegIFN for 48 weeks unless futility rule was met at 24 weeks of treatment (ie, viral load still detectable).4 The SVR in patients with GT1 infection improved to > 65% for patients in clinical trials.2 FDA approval of the direct-acting antivirals (DAAs) sofosbuvir and simeprevir in late 2013 decreased the usual duration of therapy to only 12 weeks with improved SVR rates 12 weeks posttherapy (SVR12) to 90% or higher.2,6,10
FDA approval of ledipasvir (LDV)/sofosbuvir (SOF) in October 2014 resulted in the first interferon-free all-oral regimen indicated for HCV GT1 infection.11 In December 2014, FDA approved a combination of paritaprevir, ritonavir, ombitasvir, and dasabuvir (PrOD).12 In 2015 GT-specific approvals were issued for daclastavir to be used with SOV for GT1 and GT3 and a combination similar to PrOD without dasabuvir (PrO) for GT4.13 In 2016, a combination of elbasvir (ERB) and grazoprevir (GZP) was approved for GT1 and GT4.14
In 2016, a pangenotypic DAA of SOF and velpatasvir (VEL) was approved.15 Most recently, combinations of SOF, VEL, and voxilaprevir (VOX), and glecaprevir (GLE) and pibrentasvir (PIB) were approved for patients with previous DAA treatment failures.7, 8,16,17 These oral regimens avoided the significant adverse events (AEs) associated with pegIFN and RBV (eg, thrombocytopenia, depression), were expected to improve treatment adherence and shorten duration of therapy.
The West Palm Beach Veterans Affairs has had a nurse practitioner (NP)-based HCV treatment clinic since the late 1990s. When PIs became available, a CPS started reviewing patient electronic health records (EHRs) and monitored response to therapy along with the NP to ensure discontinuation of therapy if futility criteria were met.7 Our unpublished experience showed SVR > 60% with both boceprevir and SOF regimens and > 90% with oral DAA regimens.
This review will provide the SVR rates for patients that needed retreatment for HCV infection since 2015 until December 2019. We treated all willing patients, beginning with the patients who had experienced failures with previous regimens. Patients first received education on HCV infection and treatment options in a group class then they were seen by the NP individually for specific education on treatment. The CPS reviewed the patient’s medical record to assess for appropriate therapy, possible drug-drug interactions and contraindications to therapy. In addition, patient outcomes (eg, viral load, AEs) were documented by the CPS in collaboration with the NP throughout treatment until viral load for SVR evaluation was obtained.
Methods
A retrospective EHR review of patients retreated from January 2015 to December 2019 was conducted. Data collected included age, sex, HCV GT, previous therapy, new medications prescribed, creatinine clearance, and achievement of SVR12. This retrospective review was approved by the facility’s scientific advisory committee as part of performance improvement efforts. Descriptive statistics are provided.
Results
Boceprevir
We treated 31 patients with boceprevir of which 3 met futility rule and 28 completed therapy. Eighteen of 28 responded (64%) to the treatment. The 10 patients who failed treatment were retreated with LDV/SOF, and all achieved SVR.
Sofosbuvir
A total of 53 patients were treated with SOF, RBV, and pegIFN for 12 weeks. Forty-one achieved SVR (77%). Of the 12 who failed therapy, all have been retreated and achieved SVR (Table 2).
Interferon-Free DAA Oral Regimens
More than 900 patients have been treated with interferon-free regimens since 2015 and outcomes were documented for > 800 patients. The SVR rates by GT were as follows: GT1 639 of 676 (95%); GT2 76 of 79 (96%); GT3 40 of 48 (83%); and GT4 6 of 6 (100%). Eighty-four percent of patients had GT1 infection. The median age of patient was 62 years, 72% were treatment naïve, and 35% having cirrhosis (based on liver biopsy or FIB4 score).18
Of 48 treatment failures, 30 patients were retreated; the rest of the patients were lost to follow-up (n = 9) or unable to receive retreatment (n = 9) mainly due to decompensated cirrhosis or liver cancer and short life expectancy. The median age of patient in this retreatment group was 62 years, 62% had cirrhosis, and most were infected with GT1. The average creatinine clearance was 73 mL/min. Twenty-two patients who failed therapy with ledipasvir/SOF were retreated (Table 3). A total of 13 patients out of the 19 tested eventually achieved SVR (68%). Four of the patients who had treatment failure again had GT1 infection and the other 2 GT3. All had cirrhosis.
Thirty-five patients were treated with PrOD, and 32 achieved SVR (91%). All 3 patients were retreated. One patient each achieved SVR with ERB/GZP, SOF/VEL and SOF/VEL/VOX. Fifty patients were treated with ERB/GZP and 45 achieved SVR (90%). All 5 treatment failures were retreated. Four achieved SVR and 1 was lost to follow-up (Table 4). Overall, of 30 patients who were retreated after failure with an all-oral DAA regimen, 27 patients had SVR values available and 21 achieved it (78%).
Discussion
Overall SVR was very high for patients who received oral treatment for HCV infection. A low number of patients failed therapy and were retreated. Patients who failed therapy again were similar in age but were more likely to have cirrhosis when compared with the overall interferon-free treated group. Thus, prompt treatment after HCV detection and before disease progression may improve treatment outcomes. Achieving SVR has been shown to improve fibrosis, portal hypertension, splenomegaly and cirrhosis, and reduce the risk of hepatocellular carcinoma by 70% and liver-related mortality by 90%.19-21/
Patients who failed therapy primarily had GT1—the most prevalent GT treated. A higher prevalence of GT1 is expected since it is the most common GT in the US.6 However, disease progression occurs more rapidly in those with GT3 and is more difficult to treat.22 The overall response rate was lower with this GT (83%) in this report, with only 1 of 3 patients retreated achieving an SVR.
Similar results are documented in retreatment trials.23 In the POLARIS-1 trial, treatment with SOF/VEL/VOX resulted in an overall response rate of 96% but only 91% for patients with GT3, compared with 95 to 100% for GTs 1, 2, or 4.23 In the current report, only 1 patient (GT1) failed retreatment with SOF/VEL/VOX. At this time, there are no clear treatment options for this patient. However, patients who fail GLE/PIB (none so far in the current report) may be able to receive SOF/VEL/VOX.24 In a small study, 29 of 31 patients achieved SVR with SOF/VEL/VOX after GLE/PIB failure (12 of 13 GT1 and 17 of 18 GT3).24
Limitations
This review was an observational, nonrandomized design, and only 1 medical center was involved. These results may not be applicable to other patient populations without a clinic set up with routine follow-ups to encourage adherence and completion of therapy.
Conclusions
Treatment of HCV infection has improved significantly over the past 10 years. Use of DAAs results in SVR for > 90% of patients, especially if the disease had not progressed to cirrhosis. Failure after retreatment for HCV infection was rare as well. Given that cirrhosis seems to increase the chance of treatment failure, it is imperative to identify candidates for treatment before the infection has progressed to cirrhosis. Patients infected with GT3 in particular should be more aggressively identified and treated.
Acknowledgments
The authors thank Nick P. Becky, PharmD, for his contributions to the identification of patients needing treatment for their HCV infection and review of initial manuscript information.
1. Centers for Disease Control and Prevention. Viral hepatitis: hepatitis C information. https://www.cdc.gov/hepatitis/hcv/index.htm. Reviewed April 14, 2020. Accessed June 16, 2020.
2. American Association for the Study of Liver Disease, Infectious Diseases Society of America. HCV Guidance: Recommendations for Testing, Managing, and Treating Hepatitis C. https://www.hcvguidelines.org. Accessed June 16, 2020.
3. Lingala S, Ghany MG. Natural history of hepatitis C. Gastroenterol Clin N Am. 2015;44(4):717-734. doi:10.1016/j.gtc.2015.07.003
4. Foote BS, Spooner LM, Belliveau PP. Boceprevir: a protease inhibitor for the treatment of chronic hepatitis C. Ann Pharmacother. 2011;45(9):1085-1093. doi:10.1345/aph.1P744
5. Kayali Z, Schmidt WN. Finally sofosbuvir: an oral anti-HCV drug with wide performance capability. Pharmgenomics Pers Med. 2014:7:387-398. doi:10.2147/PGPM.S52629
6. Falade-Nwulis O, Suarez-Cuervo C, Nelson DR, Fried MW, Segal JB, Sulkowski MS. Oral direct-acting agent therapy for hepatitis C virus infection: a systematic review. Ann Intern Med. 2017;166(9):637-648. doi:10.7326/M16-2575
7. Carrion AF, Martin P. Glecaprevir + pibrentasvir for treatment of hepatitis C. Expert Opinion Pharmacother. 2018;19(4):413-419. doi:10.1080/14656566.2018.1444030
8. Chahine EB, Kelley D, Childs-Kean LM. Sofosbuvir/velpatasvir/voxilaprevir: a pan-genotypic direct-acting antiviral combination for hepatitis C. Ann Pharmacother. 2018;52(4):352-363. doi:10.1177/1060028017741508
9. Lagasca AM, Kan VL. Hepatitis C treatment at a Veteran Affairs medical center after the availability of direct-acting agents: things are looking up. Clin Infect Dis. 2015:61(8):1347-1349. doi:10.1093/cid/civ573
10. Sovaldi (sofosbuvir) [package insert]. Foster City, CA: Gilead Sciences Inc; 2017.
11. Harvoni (ledipasvir and sofosbuvir) [package insert]. Foster City, CA: Gilead Sciences Inc; 2017.
12. Viekira Pak (ombitasvir, paritaprevir and ritonavir; dasabuvir) [package insert]. North Chicago, IL: AbbVie Inc; 2018.
13. Technivie (ombitasvir, paritaprevir and ritonavir) [package insert]. North Chicago, IL: AbbVie Inc; 2018.
14. Zepatier (elbasvir and grazoprevir) [package insert]. Whitehouse Station, NJ: Merck & Co Inc; 2018.
15. Epclusa (sofosbuvir and velpatasvir) [package insert]. Foster City, CA: Gilead Sciences Inc; 2017.
16. Mavyret (glecaprevir and pibrentasvir) [package insert]. North Chicago, IL: AbbVie Inc; 2019.
17. Vosevi (sofosbuvir, velpatasvir and voxilaprevir) [package insert]. Foster City, CA: Gilead Sciences Inc; 2017.
18. Vallet-Pichard A, Mallet V, Nalpas V, et al. FIB-4: an inexpensive and accurate marker of fibrosis in HCV infection. Comparison with liver biopsy and FibroTest. Hepatology. 2017;46(1):32-36. doi:10.1002/hep.21669
19. Morgan RL, Baack B, Smith BD, Yartel A, Pitasi M, Falck-Ytter Y. Eradication of hepatitis C virus infection and the development of hepatocellular carcinoma: a meta-analysis of observational studies. Ann Intern Med. 2013;158(5, pt 1):329-337. doi:10.7326/0003-4819-158-5-201303050-00005
20. van der Meer AJ, Veldt BJ, Feld JJ, et al. Association between sustained virological response and all-cause mortality among patients with chronic hepatitis C and advanced hepatic fibrosis. JAMA. 2012;308(24):2584-2593. doi:10.1001/jama.2012.144878
21. Veldt BJ, Heathcote EJ, Wedemeyer H, et al. Sustained virologic response and clinical outcomes in patients with chronic hepatitis C and advanced fibrosis. Ann Intern Med. 2007;147(10):677-684. doi:10.7326/0003-4819-147-10-200711200-00003
22. Chen A, Patel K, Naggie S. Genotype 3 infection: the last stand of hepatitis C virus. Drugs. 2017;77(2):131-144. doi:10.1007/s40265-016-0685-x
23. Bourlière M, Gordon SC, Flamm SL, et al; POLARIS-1 and POLARIS-4 Investigators. Sofosbuvir, velpatasvir and voxilaprevir for previously treated HCV infection. N Engl J Med. 2017;376(22):2136-2146. doi:10.1056/NEJMoa1613512
24. Pearlman B, Perrys M, Hinds A. Sofosbuvir/velpatasvir/voxilaprevir for previous treatment failures with glecaprevir/pibrentasvir in chronic hepatitis C infection. Am J Gastroenterol. 2019;114(9):1550-1552. doi:10.14309/ajg.0000000000000248
1. Centers for Disease Control and Prevention. Viral hepatitis: hepatitis C information. https://www.cdc.gov/hepatitis/hcv/index.htm. Reviewed April 14, 2020. Accessed June 16, 2020.
2. American Association for the Study of Liver Disease, Infectious Diseases Society of America. HCV Guidance: Recommendations for Testing, Managing, and Treating Hepatitis C. https://www.hcvguidelines.org. Accessed June 16, 2020.
3. Lingala S, Ghany MG. Natural history of hepatitis C. Gastroenterol Clin N Am. 2015;44(4):717-734. doi:10.1016/j.gtc.2015.07.003
4. Foote BS, Spooner LM, Belliveau PP. Boceprevir: a protease inhibitor for the treatment of chronic hepatitis C. Ann Pharmacother. 2011;45(9):1085-1093. doi:10.1345/aph.1P744
5. Kayali Z, Schmidt WN. Finally sofosbuvir: an oral anti-HCV drug with wide performance capability. Pharmgenomics Pers Med. 2014:7:387-398. doi:10.2147/PGPM.S52629
6. Falade-Nwulis O, Suarez-Cuervo C, Nelson DR, Fried MW, Segal JB, Sulkowski MS. Oral direct-acting agent therapy for hepatitis C virus infection: a systematic review. Ann Intern Med. 2017;166(9):637-648. doi:10.7326/M16-2575
7. Carrion AF, Martin P. Glecaprevir + pibrentasvir for treatment of hepatitis C. Expert Opinion Pharmacother. 2018;19(4):413-419. doi:10.1080/14656566.2018.1444030
8. Chahine EB, Kelley D, Childs-Kean LM. Sofosbuvir/velpatasvir/voxilaprevir: a pan-genotypic direct-acting antiviral combination for hepatitis C. Ann Pharmacother. 2018;52(4):352-363. doi:10.1177/1060028017741508
9. Lagasca AM, Kan VL. Hepatitis C treatment at a Veteran Affairs medical center after the availability of direct-acting agents: things are looking up. Clin Infect Dis. 2015:61(8):1347-1349. doi:10.1093/cid/civ573
10. Sovaldi (sofosbuvir) [package insert]. Foster City, CA: Gilead Sciences Inc; 2017.
11. Harvoni (ledipasvir and sofosbuvir) [package insert]. Foster City, CA: Gilead Sciences Inc; 2017.
12. Viekira Pak (ombitasvir, paritaprevir and ritonavir; dasabuvir) [package insert]. North Chicago, IL: AbbVie Inc; 2018.
13. Technivie (ombitasvir, paritaprevir and ritonavir) [package insert]. North Chicago, IL: AbbVie Inc; 2018.
14. Zepatier (elbasvir and grazoprevir) [package insert]. Whitehouse Station, NJ: Merck & Co Inc; 2018.
15. Epclusa (sofosbuvir and velpatasvir) [package insert]. Foster City, CA: Gilead Sciences Inc; 2017.
16. Mavyret (glecaprevir and pibrentasvir) [package insert]. North Chicago, IL: AbbVie Inc; 2019.
17. Vosevi (sofosbuvir, velpatasvir and voxilaprevir) [package insert]. Foster City, CA: Gilead Sciences Inc; 2017.
18. Vallet-Pichard A, Mallet V, Nalpas V, et al. FIB-4: an inexpensive and accurate marker of fibrosis in HCV infection. Comparison with liver biopsy and FibroTest. Hepatology. 2017;46(1):32-36. doi:10.1002/hep.21669
19. Morgan RL, Baack B, Smith BD, Yartel A, Pitasi M, Falck-Ytter Y. Eradication of hepatitis C virus infection and the development of hepatocellular carcinoma: a meta-analysis of observational studies. Ann Intern Med. 2013;158(5, pt 1):329-337. doi:10.7326/0003-4819-158-5-201303050-00005
20. van der Meer AJ, Veldt BJ, Feld JJ, et al. Association between sustained virological response and all-cause mortality among patients with chronic hepatitis C and advanced hepatic fibrosis. JAMA. 2012;308(24):2584-2593. doi:10.1001/jama.2012.144878
21. Veldt BJ, Heathcote EJ, Wedemeyer H, et al. Sustained virologic response and clinical outcomes in patients with chronic hepatitis C and advanced fibrosis. Ann Intern Med. 2007;147(10):677-684. doi:10.7326/0003-4819-147-10-200711200-00003
22. Chen A, Patel K, Naggie S. Genotype 3 infection: the last stand of hepatitis C virus. Drugs. 2017;77(2):131-144. doi:10.1007/s40265-016-0685-x
23. Bourlière M, Gordon SC, Flamm SL, et al; POLARIS-1 and POLARIS-4 Investigators. Sofosbuvir, velpatasvir and voxilaprevir for previously treated HCV infection. N Engl J Med. 2017;376(22):2136-2146. doi:10.1056/NEJMoa1613512
24. Pearlman B, Perrys M, Hinds A. Sofosbuvir/velpatasvir/voxilaprevir for previous treatment failures with glecaprevir/pibrentasvir in chronic hepatitis C infection. Am J Gastroenterol. 2019;114(9):1550-1552. doi:10.14309/ajg.0000000000000248
International medical graduates facing challenges amid COVID-19
International medical graduates (IMGs) constitute more than 24% of the total percentage of active physicians, 30% of active psychiatrists, and 33% of psychiatry residents in the United States.1 IMGs serve in various medical specialties and provide medical care to socioeconomically disadvantaged patients in underserved communities.2 Evidence suggests that patient outcomes among elderly patients admitted in U.S. hospitals for those treated by IMGs were on par with outcomes of U.S. graduates. Moreover, patients who were treated by IMGs had a lower mortality rates.3
IMGs trained in the United States make considerable contributions to psychiatry and have been very successful as educators, researchers, and leaders. Over the last 3 decades, for example, three American Psychiatric Association (APA) presidents and one past president of the American Academy of Child and Adolescent Psychiatry were IMGs. Many of them also hold department chair positions at many academic institutions.4,5
In short, IMGs are an important part of the U.S. health care system – particularly in psychiatry.
In addition to participating in psychiatry residency programs, IMG physicians are heavily represented in subspecialties, including geriatric psychiatry (45%), addiction psychiatry (42%), child and adolescent psychiatry (36%), psychosomatic medicine (32%), and forensic psychiatry (25%).6 IMG trainees face multiple challenges that begin as they transition to psychiatry residency in the United States, including understanding the American health care system, electronic medical records and documentation, and evidence-based medicine. In addition, they need to adapt to cultural changes, and work on language barriers, communication skills, and social isolation.7,8 Training programs account for these challenges and proactively take essential steps to facilitate the transition of IMGs into the U.S. system.9,10
As training programs prepare for the new academic year starting from July 2020 and continue to provide educational experiences to current trainees, the COVID-19 pandemic has brought additional challenges for the training programs. The gravity of the novel coronavirus pandemic continues to deepen, causing immense fear and uncertainty globally. An APA poll of more than 1,000 adults conducted early in the pandemic showed that about 40% of Americans were anxious about becoming seriously ill or dying with COVID-19. Nearly half of the respondents (48%) were anxious about the possibility of getting COVID-19, and even more (62%) were anxious about the possibility of their loved ones getting infected by this virus. Also, one-third of Americans reported a serious impact on their mental health.
Furthermore, the ailing economy and increasing unemployment are raising financial concerns for individuals and families. This pandemic also has had an impact on our patients’ sleep hygiene, relationships with their loved ones, and consumption of alcohol or other drugs/substances.11 Deteriorating mental health raises concerns about increased suicide risk as a secondary consequence.12
Physicians and other frontline teams who are taking care of these patients and their families continue to provide unexcelled, compassionate care in these unprecedented times. Selfless care continues despite awareness of the high probability of getting exposed to the virus and spreading it further to family members. Physicians involved in direct patient care for COVID-19 patients are at high risk for demoralization, burnout, depression, and anxiety.13
Struggles experienced by IMGs
On the personal front, IMGs often struggle with multiple stressors, such as lack of social support, ethnic-minority prejudice, and the need to understand financial structures such as mortgages in the new countries even after extended periods of residence.14 This virus has killed many health care professionals, including physicians around the world. There was a report of suicide by an emergency medicine physician who was treating patients with COVID-19 and ended up contracting the virus. That news was devastating and overwhelming for everyone, especially health care clinicians. It also adds to the stress and worries of IMGs who are still on nonimmigrant visas.
Bigger concerns exist if there is a demise of a nonimmigrant IMG and the implications of that loss for dependent families – who might face deportation. Even for those who were recently granted permanent residency status, worries about limited support systems and financial hardships to their families can be stressors.
Also, a large number of IMGs represent the geographical area where the pandemic began. Fortunately, the World Health Organization has taken a firm stance against possible discrimination by calling for global solidarity in these times. Furthermore, the WHO has emphasized the importance of referring to the disease caused by SARS-CoV-2 as “COVID-19” only – and not by the name of a particular country or city.15 Despite those official positions, people continue to express racially discriminatory opinions related to the virus, and those comments are not only disturbing to IMGs, they also are demoralizing.
Travel restrictions
In addition to the worries that IMGs might have about their own health and that of their families residing with them, the well-being of their extended families, including their aging parents back in their countries of origin, is unsettling as well. It is even more unnerving during the pandemic because the Centers for Disease Control and Prevention and the State Department advised avoiding all international travel at this time. Under these circumstances, IMGs are concerned about travel to their countries of origin in the event of a family emergency and the quarantine protocols in place, at both the country of origin and at residences.
Immigration issues
The U.S. administration temporarily suspended all immigration for 60 days, starting from April 2020. Recently, an executive order was signed suspending entry in the country on several visas, including the J-1 and the H1-B. Those are two categories that allow physicians to train and work in the United States.
IMGs in the United States reside and practice here under different types of immigrant and nonimmigrant visas (J-1, H1-B). This year, the Match results coincided with the timeline of those new immigration restrictions. Many IMGs are currently in the process of renewing their H1-B visas. They are worried because their visas will expire in the coming months. During the pandemic, U.S. Citizenship and Immigration Services suspended routine visa services and premium processing for visa renewals. This halt led to a delay in visa processing for graduating residents in June and practicing physicians seeking visa renewal. Those delays add to personal stress, and furthermore, distract these immigrant physicians from fighting this pandemic.
Another complication is that rules for J-1 visa holders have changed so that trainees must return to their countries of origin for at least 2 years after completing their training. If they decide to continue practicing medicine in the United States, they need a specific type of J-1 waiver and must gain a pathway to be a lawful permanent resident (Green Card). Many IMGs who are on waiver positions might not be able to treat patients ailing from COVID-19 to the full extent because waivers restrict them to practicing only in certain identified health systems.
IMGs who are coming from a country such India have to wait for more than 11 years after completing their accredited training to get permanent residency because of backlog for the permanent residency process.16 While waiting for a Green Card, they must continue to work on an H1-B visa, which requires periodic renewal.
Potential impact on training
Non-U.S. citizen IMGs accounted for 13% of the total of first-year positions in the 2020 Match. They will start medical training in residency programs in the United States in the coming months. The numbers for psychiatry residency matches are higher; about 16% of total first-year positions are filled by non-U.S. IMGs.17 At this time, when they should be celebrating their successful Match after many years of hard work and persistence, there is increased anxiety. They wonder whether they will be able to enter the United States to begin their training program on time. Their concerns are multifold, but the main concern is related to uncertainties around getting visas on time. With the recent executive order in place, physicians only working actively with COVID-19 patients will be able to enter the country on visas. As mental health concerns continue to rise during these times, incoming residents might not be able to start training if they are out of the country.
Furthermore, because of travel/air restrictions, there are worries about whether physicians will be able to get flights to the United States, given the lockdown in many countries around the world. Conversely, IMGs who will be graduating from residency and fellowship programs this summer and have accepted new positions also are dealing with similar uncertainty. Their new jobs will require visa processing, and the current scenario provides limited insight, so far, about whether they will be able to start their respective jobs or whether they will have to return to their home countries until their visa processing is completed.
The American Medical Association has advised the Secretary of State and acting Secretary of Homeland Security to expedite physician workforce expansion in an effort to meet the growing need for health care services during this pandemic.18 It is encouraging that, recently, the State Department declared that visa processing will continue for medical professionals and that cases would be expedited for those who meet the criteria. However, the requirement for in-person interviews remains for individuals who are seeking a U.S. visa outside the country.
As residency programs are trying their best to continue to provide educational experiences to trainees during this phase, if psychiatry residents are placed on quarantine because of either getting exposed or contracting the illness, there is a possibility that they might need to extend their training. This would bring another challenge for IMGs, requiring them to extend their visas to complete their training. Future J-1 waiver jobs could be compromised.
Investment in physician wellness critical
Psychiatrists, along with other health care workers, are front-line soldiers in the fight against COVID-19. All physicians are at high risk for demoralization, burnout, depression, anxiety, and suicide. It is of utmost importance that we invest immediately in physicians’ wellness. As noted, significant numbers of psychiatrists are IMGs who are dealing with additional challenges while responding to the pandemic. There are certain challenges for IMGs, such as the well-being of their extended families in other countries, and travel bans put in place because of the pandemic. Those issues are not easy to resolve. However, addressing visa issues and providing support to their families in the event that something happens to physicians during the pandemic would be reassuring and would help alleviate additional stress. Those kinds of actions also would allow immigrant physicians to focus on clinical work and to improve their overall well-being. Given the health risks and numerous other insecurities that go along with living amid a pandemic, IMGs should not have the additional pressure of visa uncertainty.
Public health crises such as COVID-19 are associated with increased rates of anxiety,19 depression,20 illicit substance use,21 and an increased rate of suicide.22 Patients with serious mental illness might be among the hardest hit both physically and mentally during the pandemic.23 Even in the absence of a pandemic, there is already a shortage of psychiatrists at the national level, and it is expected that this shortage will grow in the future. Rural and underserved areas are expected to experience the physician deficit more acutely.24
The pandemic is likely to resolve gradually and unpredictably – and might recur along the way over the next 1-2 years. However, the psychiatrist shortage will escalate more, as the mental health needs in the United States increase further in coming months. We need psychiatrists now more than ever, and it will be crucial that prospective residents, graduating residents, and fellows are able to come on board to join the American health care system promptly. In addition to national-level interventions, residency programs, potential employers, and communities must be aware of and do whatever they can to address the challenges faced by IMGs during these times.
Dr. Raman Baweja is affiliated with the department of psychiatry and behavioral health at Penn State University, Hershey. He has no conflicts of interest. Dr. Verma is affiliated with Rogers Behavioral Health in Kenosha County, Wis., and the department of psychiatry and behavioral health at Rosalind Franklin University of Medicine and Science in North Chicago. She has no conflicts of interest. Dr. Ritika Baweja is affiliated with the department of psychiatry and behavioral health at Penn State. Dr. Ritika Baweja is the spouse of Dr. Raman Baweja. Dr. Adam is affiliated with the department of psychiatry at the University of Missouri, Columbia.
References
1. American Psychiatric Association. Navigating psychiatry residency in the United States. A Guide for IMG Physicians.
2. Berg S. 5 IMG physicians who speak up for patients and fellow doctors. American Medical Association. 2019 Oct 22.
3. Tsugawa Y et al. BMJ. 2017 Feb 3;256. doi: 10.1136/bmj.j273.
4. Gogineni RR et al. Child Adolesc Psychiatr Clin N Am. 2010 Oct 1;19(4):833-53.
5. Majeed MH et al. Academic Psychiatry. 2017 Dec 1;41(6):849-51.
6. Brotherton SE and Etzel SI. JAMA. 2018 Sep 11;320(10):1051-70.
7. Sockalingam S et al. Acad Psychiatry. 2012 Jul 1;36(4):277-81.
8. Singareddy R et al. Acad Psychiatry. 2008 Jul-Aug;32(4):343-4.
9. Kramer MN. Acad Psychiatry. 2005 Jul-Aug;29(3):322-4.
10. Rao NR and Kotapati VP. Pathways for success in academic medicine for an international medical graduate: Challenges and opportunities. In “Roberts Academic Medicine Handbook” 2020. Springer:163-70.
11. American Psychiatric Association. New poll: COVID-19 impacting mental well-being: Americans feeling anxious, especially for loved ones; older adults are less anxious. 2020 Mar 25.
12. Reger MA et al. JAMA Psychiatry. 2020 Apr 10. doi: 10.1001/jamapsychiatry.2020.1060.
13. Lai J et al. JAMA Netw Open. 2020 Mar 23;3(3):e203976-e203976. doi: 10.1001/jamanetworkopen.2020.3976.
14. Kalra G et al. Acad Psychiatry. 2012 Jul;36(4):323-9.
15. WHO best practices for the naming of new human infectious diseases. World Health Organization. 2015.
16. U.S. Department of State. Bureau of Consular Affairs. Visa Bulletin for March 2020.
17. National Resident Matching Program® (NRMP®). Thousands of medical students and graduates celebrate NRMP Match results.
18. American Medical Association. AMA: U.S. should open visas to international physicians amid COVID-19. AMA press release. 2020 Mar 25.
19. McKay D et al. J Anxiety Disord. 2020 Jun;73:02233. doi: 10.1016/j.janxdis.2020.102233.
20. Tang W et al. J Affect Disord. 2020 May 13;274:1-7.
21. Collins F et al. NIH Director’s Blog. NIH.gov. 2020 Apr 21.
22. Reger M et al. JAMA Psychiatry. 2020 Apr 10. doi: 10.1001/jamapsychiatry.2020.1060.
23. Druss BG. JAMA Psychiatry. 2020 Apr 3. doi: 10.1001/jamapsychiatry.2020.0894.
24. American Association of Medical Colleges. “The complexities of physician supply and demand: Projections from 2018-2033.” 2020 Jun.
International medical graduates (IMGs) constitute more than 24% of the total percentage of active physicians, 30% of active psychiatrists, and 33% of psychiatry residents in the United States.1 IMGs serve in various medical specialties and provide medical care to socioeconomically disadvantaged patients in underserved communities.2 Evidence suggests that patient outcomes among elderly patients admitted in U.S. hospitals for those treated by IMGs were on par with outcomes of U.S. graduates. Moreover, patients who were treated by IMGs had a lower mortality rates.3
IMGs trained in the United States make considerable contributions to psychiatry and have been very successful as educators, researchers, and leaders. Over the last 3 decades, for example, three American Psychiatric Association (APA) presidents and one past president of the American Academy of Child and Adolescent Psychiatry were IMGs. Many of them also hold department chair positions at many academic institutions.4,5
In short, IMGs are an important part of the U.S. health care system – particularly in psychiatry.
In addition to participating in psychiatry residency programs, IMG physicians are heavily represented in subspecialties, including geriatric psychiatry (45%), addiction psychiatry (42%), child and adolescent psychiatry (36%), psychosomatic medicine (32%), and forensic psychiatry (25%).6 IMG trainees face multiple challenges that begin as they transition to psychiatry residency in the United States, including understanding the American health care system, electronic medical records and documentation, and evidence-based medicine. In addition, they need to adapt to cultural changes, and work on language barriers, communication skills, and social isolation.7,8 Training programs account for these challenges and proactively take essential steps to facilitate the transition of IMGs into the U.S. system.9,10
As training programs prepare for the new academic year starting from July 2020 and continue to provide educational experiences to current trainees, the COVID-19 pandemic has brought additional challenges for the training programs. The gravity of the novel coronavirus pandemic continues to deepen, causing immense fear and uncertainty globally. An APA poll of more than 1,000 adults conducted early in the pandemic showed that about 40% of Americans were anxious about becoming seriously ill or dying with COVID-19. Nearly half of the respondents (48%) were anxious about the possibility of getting COVID-19, and even more (62%) were anxious about the possibility of their loved ones getting infected by this virus. Also, one-third of Americans reported a serious impact on their mental health.
Furthermore, the ailing economy and increasing unemployment are raising financial concerns for individuals and families. This pandemic also has had an impact on our patients’ sleep hygiene, relationships with their loved ones, and consumption of alcohol or other drugs/substances.11 Deteriorating mental health raises concerns about increased suicide risk as a secondary consequence.12
Physicians and other frontline teams who are taking care of these patients and their families continue to provide unexcelled, compassionate care in these unprecedented times. Selfless care continues despite awareness of the high probability of getting exposed to the virus and spreading it further to family members. Physicians involved in direct patient care for COVID-19 patients are at high risk for demoralization, burnout, depression, and anxiety.13
Struggles experienced by IMGs
On the personal front, IMGs often struggle with multiple stressors, such as lack of social support, ethnic-minority prejudice, and the need to understand financial structures such as mortgages in the new countries even after extended periods of residence.14 This virus has killed many health care professionals, including physicians around the world. There was a report of suicide by an emergency medicine physician who was treating patients with COVID-19 and ended up contracting the virus. That news was devastating and overwhelming for everyone, especially health care clinicians. It also adds to the stress and worries of IMGs who are still on nonimmigrant visas.
Bigger concerns exist if there is a demise of a nonimmigrant IMG and the implications of that loss for dependent families – who might face deportation. Even for those who were recently granted permanent residency status, worries about limited support systems and financial hardships to their families can be stressors.
Also, a large number of IMGs represent the geographical area where the pandemic began. Fortunately, the World Health Organization has taken a firm stance against possible discrimination by calling for global solidarity in these times. Furthermore, the WHO has emphasized the importance of referring to the disease caused by SARS-CoV-2 as “COVID-19” only – and not by the name of a particular country or city.15 Despite those official positions, people continue to express racially discriminatory opinions related to the virus, and those comments are not only disturbing to IMGs, they also are demoralizing.
Travel restrictions
In addition to the worries that IMGs might have about their own health and that of their families residing with them, the well-being of their extended families, including their aging parents back in their countries of origin, is unsettling as well. It is even more unnerving during the pandemic because the Centers for Disease Control and Prevention and the State Department advised avoiding all international travel at this time. Under these circumstances, IMGs are concerned about travel to their countries of origin in the event of a family emergency and the quarantine protocols in place, at both the country of origin and at residences.
Immigration issues
The U.S. administration temporarily suspended all immigration for 60 days, starting from April 2020. Recently, an executive order was signed suspending entry in the country on several visas, including the J-1 and the H1-B. Those are two categories that allow physicians to train and work in the United States.
IMGs in the United States reside and practice here under different types of immigrant and nonimmigrant visas (J-1, H1-B). This year, the Match results coincided with the timeline of those new immigration restrictions. Many IMGs are currently in the process of renewing their H1-B visas. They are worried because their visas will expire in the coming months. During the pandemic, U.S. Citizenship and Immigration Services suspended routine visa services and premium processing for visa renewals. This halt led to a delay in visa processing for graduating residents in June and practicing physicians seeking visa renewal. Those delays add to personal stress, and furthermore, distract these immigrant physicians from fighting this pandemic.
Another complication is that rules for J-1 visa holders have changed so that trainees must return to their countries of origin for at least 2 years after completing their training. If they decide to continue practicing medicine in the United States, they need a specific type of J-1 waiver and must gain a pathway to be a lawful permanent resident (Green Card). Many IMGs who are on waiver positions might not be able to treat patients ailing from COVID-19 to the full extent because waivers restrict them to practicing only in certain identified health systems.
IMGs who are coming from a country such India have to wait for more than 11 years after completing their accredited training to get permanent residency because of backlog for the permanent residency process.16 While waiting for a Green Card, they must continue to work on an H1-B visa, which requires periodic renewal.
Potential impact on training
Non-U.S. citizen IMGs accounted for 13% of the total of first-year positions in the 2020 Match. They will start medical training in residency programs in the United States in the coming months. The numbers for psychiatry residency matches are higher; about 16% of total first-year positions are filled by non-U.S. IMGs.17 At this time, when they should be celebrating their successful Match after many years of hard work and persistence, there is increased anxiety. They wonder whether they will be able to enter the United States to begin their training program on time. Their concerns are multifold, but the main concern is related to uncertainties around getting visas on time. With the recent executive order in place, physicians only working actively with COVID-19 patients will be able to enter the country on visas. As mental health concerns continue to rise during these times, incoming residents might not be able to start training if they are out of the country.
Furthermore, because of travel/air restrictions, there are worries about whether physicians will be able to get flights to the United States, given the lockdown in many countries around the world. Conversely, IMGs who will be graduating from residency and fellowship programs this summer and have accepted new positions also are dealing with similar uncertainty. Their new jobs will require visa processing, and the current scenario provides limited insight, so far, about whether they will be able to start their respective jobs or whether they will have to return to their home countries until their visa processing is completed.
The American Medical Association has advised the Secretary of State and acting Secretary of Homeland Security to expedite physician workforce expansion in an effort to meet the growing need for health care services during this pandemic.18 It is encouraging that, recently, the State Department declared that visa processing will continue for medical professionals and that cases would be expedited for those who meet the criteria. However, the requirement for in-person interviews remains for individuals who are seeking a U.S. visa outside the country.
As residency programs are trying their best to continue to provide educational experiences to trainees during this phase, if psychiatry residents are placed on quarantine because of either getting exposed or contracting the illness, there is a possibility that they might need to extend their training. This would bring another challenge for IMGs, requiring them to extend their visas to complete their training. Future J-1 waiver jobs could be compromised.
Investment in physician wellness critical
Psychiatrists, along with other health care workers, are front-line soldiers in the fight against COVID-19. All physicians are at high risk for demoralization, burnout, depression, anxiety, and suicide. It is of utmost importance that we invest immediately in physicians’ wellness. As noted, significant numbers of psychiatrists are IMGs who are dealing with additional challenges while responding to the pandemic. There are certain challenges for IMGs, such as the well-being of their extended families in other countries, and travel bans put in place because of the pandemic. Those issues are not easy to resolve. However, addressing visa issues and providing support to their families in the event that something happens to physicians during the pandemic would be reassuring and would help alleviate additional stress. Those kinds of actions also would allow immigrant physicians to focus on clinical work and to improve their overall well-being. Given the health risks and numerous other insecurities that go along with living amid a pandemic, IMGs should not have the additional pressure of visa uncertainty.
Public health crises such as COVID-19 are associated with increased rates of anxiety,19 depression,20 illicit substance use,21 and an increased rate of suicide.22 Patients with serious mental illness might be among the hardest hit both physically and mentally during the pandemic.23 Even in the absence of a pandemic, there is already a shortage of psychiatrists at the national level, and it is expected that this shortage will grow in the future. Rural and underserved areas are expected to experience the physician deficit more acutely.24
The pandemic is likely to resolve gradually and unpredictably – and might recur along the way over the next 1-2 years. However, the psychiatrist shortage will escalate more, as the mental health needs in the United States increase further in coming months. We need psychiatrists now more than ever, and it will be crucial that prospective residents, graduating residents, and fellows are able to come on board to join the American health care system promptly. In addition to national-level interventions, residency programs, potential employers, and communities must be aware of and do whatever they can to address the challenges faced by IMGs during these times.
Dr. Raman Baweja is affiliated with the department of psychiatry and behavioral health at Penn State University, Hershey. He has no conflicts of interest. Dr. Verma is affiliated with Rogers Behavioral Health in Kenosha County, Wis., and the department of psychiatry and behavioral health at Rosalind Franklin University of Medicine and Science in North Chicago. She has no conflicts of interest. Dr. Ritika Baweja is affiliated with the department of psychiatry and behavioral health at Penn State. Dr. Ritika Baweja is the spouse of Dr. Raman Baweja. Dr. Adam is affiliated with the department of psychiatry at the University of Missouri, Columbia.
References
1. American Psychiatric Association. Navigating psychiatry residency in the United States. A Guide for IMG Physicians.
2. Berg S. 5 IMG physicians who speak up for patients and fellow doctors. American Medical Association. 2019 Oct 22.
3. Tsugawa Y et al. BMJ. 2017 Feb 3;256. doi: 10.1136/bmj.j273.
4. Gogineni RR et al. Child Adolesc Psychiatr Clin N Am. 2010 Oct 1;19(4):833-53.
5. Majeed MH et al. Academic Psychiatry. 2017 Dec 1;41(6):849-51.
6. Brotherton SE and Etzel SI. JAMA. 2018 Sep 11;320(10):1051-70.
7. Sockalingam S et al. Acad Psychiatry. 2012 Jul 1;36(4):277-81.
8. Singareddy R et al. Acad Psychiatry. 2008 Jul-Aug;32(4):343-4.
9. Kramer MN. Acad Psychiatry. 2005 Jul-Aug;29(3):322-4.
10. Rao NR and Kotapati VP. Pathways for success in academic medicine for an international medical graduate: Challenges and opportunities. In “Roberts Academic Medicine Handbook” 2020. Springer:163-70.
11. American Psychiatric Association. New poll: COVID-19 impacting mental well-being: Americans feeling anxious, especially for loved ones; older adults are less anxious. 2020 Mar 25.
12. Reger MA et al. JAMA Psychiatry. 2020 Apr 10. doi: 10.1001/jamapsychiatry.2020.1060.
13. Lai J et al. JAMA Netw Open. 2020 Mar 23;3(3):e203976-e203976. doi: 10.1001/jamanetworkopen.2020.3976.
14. Kalra G et al. Acad Psychiatry. 2012 Jul;36(4):323-9.
15. WHO best practices for the naming of new human infectious diseases. World Health Organization. 2015.
16. U.S. Department of State. Bureau of Consular Affairs. Visa Bulletin for March 2020.
17. National Resident Matching Program® (NRMP®). Thousands of medical students and graduates celebrate NRMP Match results.
18. American Medical Association. AMA: U.S. should open visas to international physicians amid COVID-19. AMA press release. 2020 Mar 25.
19. McKay D et al. J Anxiety Disord. 2020 Jun;73:02233. doi: 10.1016/j.janxdis.2020.102233.
20. Tang W et al. J Affect Disord. 2020 May 13;274:1-7.
21. Collins F et al. NIH Director’s Blog. NIH.gov. 2020 Apr 21.
22. Reger M et al. JAMA Psychiatry. 2020 Apr 10. doi: 10.1001/jamapsychiatry.2020.1060.
23. Druss BG. JAMA Psychiatry. 2020 Apr 3. doi: 10.1001/jamapsychiatry.2020.0894.
24. American Association of Medical Colleges. “The complexities of physician supply and demand: Projections from 2018-2033.” 2020 Jun.
International medical graduates (IMGs) constitute more than 24% of the total percentage of active physicians, 30% of active psychiatrists, and 33% of psychiatry residents in the United States.1 IMGs serve in various medical specialties and provide medical care to socioeconomically disadvantaged patients in underserved communities.2 Evidence suggests that patient outcomes among elderly patients admitted in U.S. hospitals for those treated by IMGs were on par with outcomes of U.S. graduates. Moreover, patients who were treated by IMGs had a lower mortality rates.3
IMGs trained in the United States make considerable contributions to psychiatry and have been very successful as educators, researchers, and leaders. Over the last 3 decades, for example, three American Psychiatric Association (APA) presidents and one past president of the American Academy of Child and Adolescent Psychiatry were IMGs. Many of them also hold department chair positions at many academic institutions.4,5
In short, IMGs are an important part of the U.S. health care system – particularly in psychiatry.
In addition to participating in psychiatry residency programs, IMG physicians are heavily represented in subspecialties, including geriatric psychiatry (45%), addiction psychiatry (42%), child and adolescent psychiatry (36%), psychosomatic medicine (32%), and forensic psychiatry (25%).6 IMG trainees face multiple challenges that begin as they transition to psychiatry residency in the United States, including understanding the American health care system, electronic medical records and documentation, and evidence-based medicine. In addition, they need to adapt to cultural changes, and work on language barriers, communication skills, and social isolation.7,8 Training programs account for these challenges and proactively take essential steps to facilitate the transition of IMGs into the U.S. system.9,10
As training programs prepare for the new academic year starting from July 2020 and continue to provide educational experiences to current trainees, the COVID-19 pandemic has brought additional challenges for the training programs. The gravity of the novel coronavirus pandemic continues to deepen, causing immense fear and uncertainty globally. An APA poll of more than 1,000 adults conducted early in the pandemic showed that about 40% of Americans were anxious about becoming seriously ill or dying with COVID-19. Nearly half of the respondents (48%) were anxious about the possibility of getting COVID-19, and even more (62%) were anxious about the possibility of their loved ones getting infected by this virus. Also, one-third of Americans reported a serious impact on their mental health.
Furthermore, the ailing economy and increasing unemployment are raising financial concerns for individuals and families. This pandemic also has had an impact on our patients’ sleep hygiene, relationships with their loved ones, and consumption of alcohol or other drugs/substances.11 Deteriorating mental health raises concerns about increased suicide risk as a secondary consequence.12
Physicians and other frontline teams who are taking care of these patients and their families continue to provide unexcelled, compassionate care in these unprecedented times. Selfless care continues despite awareness of the high probability of getting exposed to the virus and spreading it further to family members. Physicians involved in direct patient care for COVID-19 patients are at high risk for demoralization, burnout, depression, and anxiety.13
Struggles experienced by IMGs
On the personal front, IMGs often struggle with multiple stressors, such as lack of social support, ethnic-minority prejudice, and the need to understand financial structures such as mortgages in the new countries even after extended periods of residence.14 This virus has killed many health care professionals, including physicians around the world. There was a report of suicide by an emergency medicine physician who was treating patients with COVID-19 and ended up contracting the virus. That news was devastating and overwhelming for everyone, especially health care clinicians. It also adds to the stress and worries of IMGs who are still on nonimmigrant visas.
Bigger concerns exist if there is a demise of a nonimmigrant IMG and the implications of that loss for dependent families – who might face deportation. Even for those who were recently granted permanent residency status, worries about limited support systems and financial hardships to their families can be stressors.
Also, a large number of IMGs represent the geographical area where the pandemic began. Fortunately, the World Health Organization has taken a firm stance against possible discrimination by calling for global solidarity in these times. Furthermore, the WHO has emphasized the importance of referring to the disease caused by SARS-CoV-2 as “COVID-19” only – and not by the name of a particular country or city.15 Despite those official positions, people continue to express racially discriminatory opinions related to the virus, and those comments are not only disturbing to IMGs, they also are demoralizing.
Travel restrictions
In addition to the worries that IMGs might have about their own health and that of their families residing with them, the well-being of their extended families, including their aging parents back in their countries of origin, is unsettling as well. It is even more unnerving during the pandemic because the Centers for Disease Control and Prevention and the State Department advised avoiding all international travel at this time. Under these circumstances, IMGs are concerned about travel to their countries of origin in the event of a family emergency and the quarantine protocols in place, at both the country of origin and at residences.
Immigration issues
The U.S. administration temporarily suspended all immigration for 60 days, starting from April 2020. Recently, an executive order was signed suspending entry in the country on several visas, including the J-1 and the H1-B. Those are two categories that allow physicians to train and work in the United States.
IMGs in the United States reside and practice here under different types of immigrant and nonimmigrant visas (J-1, H1-B). This year, the Match results coincided with the timeline of those new immigration restrictions. Many IMGs are currently in the process of renewing their H1-B visas. They are worried because their visas will expire in the coming months. During the pandemic, U.S. Citizenship and Immigration Services suspended routine visa services and premium processing for visa renewals. This halt led to a delay in visa processing for graduating residents in June and practicing physicians seeking visa renewal. Those delays add to personal stress, and furthermore, distract these immigrant physicians from fighting this pandemic.
Another complication is that rules for J-1 visa holders have changed so that trainees must return to their countries of origin for at least 2 years after completing their training. If they decide to continue practicing medicine in the United States, they need a specific type of J-1 waiver and must gain a pathway to be a lawful permanent resident (Green Card). Many IMGs who are on waiver positions might not be able to treat patients ailing from COVID-19 to the full extent because waivers restrict them to practicing only in certain identified health systems.
IMGs who are coming from a country such India have to wait for more than 11 years after completing their accredited training to get permanent residency because of backlog for the permanent residency process.16 While waiting for a Green Card, they must continue to work on an H1-B visa, which requires periodic renewal.
Potential impact on training
Non-U.S. citizen IMGs accounted for 13% of the total of first-year positions in the 2020 Match. They will start medical training in residency programs in the United States in the coming months. The numbers for psychiatry residency matches are higher; about 16% of total first-year positions are filled by non-U.S. IMGs.17 At this time, when they should be celebrating their successful Match after many years of hard work and persistence, there is increased anxiety. They wonder whether they will be able to enter the United States to begin their training program on time. Their concerns are multifold, but the main concern is related to uncertainties around getting visas on time. With the recent executive order in place, physicians only working actively with COVID-19 patients will be able to enter the country on visas. As mental health concerns continue to rise during these times, incoming residents might not be able to start training if they are out of the country.
Furthermore, because of travel/air restrictions, there are worries about whether physicians will be able to get flights to the United States, given the lockdown in many countries around the world. Conversely, IMGs who will be graduating from residency and fellowship programs this summer and have accepted new positions also are dealing with similar uncertainty. Their new jobs will require visa processing, and the current scenario provides limited insight, so far, about whether they will be able to start their respective jobs or whether they will have to return to their home countries until their visa processing is completed.
The American Medical Association has advised the Secretary of State and acting Secretary of Homeland Security to expedite physician workforce expansion in an effort to meet the growing need for health care services during this pandemic.18 It is encouraging that, recently, the State Department declared that visa processing will continue for medical professionals and that cases would be expedited for those who meet the criteria. However, the requirement for in-person interviews remains for individuals who are seeking a U.S. visa outside the country.
As residency programs are trying their best to continue to provide educational experiences to trainees during this phase, if psychiatry residents are placed on quarantine because of either getting exposed or contracting the illness, there is a possibility that they might need to extend their training. This would bring another challenge for IMGs, requiring them to extend their visas to complete their training. Future J-1 waiver jobs could be compromised.
Investment in physician wellness critical
Psychiatrists, along with other health care workers, are front-line soldiers in the fight against COVID-19. All physicians are at high risk for demoralization, burnout, depression, anxiety, and suicide. It is of utmost importance that we invest immediately in physicians’ wellness. As noted, significant numbers of psychiatrists are IMGs who are dealing with additional challenges while responding to the pandemic. There are certain challenges for IMGs, such as the well-being of their extended families in other countries, and travel bans put in place because of the pandemic. Those issues are not easy to resolve. However, addressing visa issues and providing support to their families in the event that something happens to physicians during the pandemic would be reassuring and would help alleviate additional stress. Those kinds of actions also would allow immigrant physicians to focus on clinical work and to improve their overall well-being. Given the health risks and numerous other insecurities that go along with living amid a pandemic, IMGs should not have the additional pressure of visa uncertainty.
Public health crises such as COVID-19 are associated with increased rates of anxiety,19 depression,20 illicit substance use,21 and an increased rate of suicide.22 Patients with serious mental illness might be among the hardest hit both physically and mentally during the pandemic.23 Even in the absence of a pandemic, there is already a shortage of psychiatrists at the national level, and it is expected that this shortage will grow in the future. Rural and underserved areas are expected to experience the physician deficit more acutely.24
The pandemic is likely to resolve gradually and unpredictably – and might recur along the way over the next 1-2 years. However, the psychiatrist shortage will escalate more, as the mental health needs in the United States increase further in coming months. We need psychiatrists now more than ever, and it will be crucial that prospective residents, graduating residents, and fellows are able to come on board to join the American health care system promptly. In addition to national-level interventions, residency programs, potential employers, and communities must be aware of and do whatever they can to address the challenges faced by IMGs during these times.
Dr. Raman Baweja is affiliated with the department of psychiatry and behavioral health at Penn State University, Hershey. He has no conflicts of interest. Dr. Verma is affiliated with Rogers Behavioral Health in Kenosha County, Wis., and the department of psychiatry and behavioral health at Rosalind Franklin University of Medicine and Science in North Chicago. She has no conflicts of interest. Dr. Ritika Baweja is affiliated with the department of psychiatry and behavioral health at Penn State. Dr. Ritika Baweja is the spouse of Dr. Raman Baweja. Dr. Adam is affiliated with the department of psychiatry at the University of Missouri, Columbia.
References
1. American Psychiatric Association. Navigating psychiatry residency in the United States. A Guide for IMG Physicians.
2. Berg S. 5 IMG physicians who speak up for patients and fellow doctors. American Medical Association. 2019 Oct 22.
3. Tsugawa Y et al. BMJ. 2017 Feb 3;256. doi: 10.1136/bmj.j273.
4. Gogineni RR et al. Child Adolesc Psychiatr Clin N Am. 2010 Oct 1;19(4):833-53.
5. Majeed MH et al. Academic Psychiatry. 2017 Dec 1;41(6):849-51.
6. Brotherton SE and Etzel SI. JAMA. 2018 Sep 11;320(10):1051-70.
7. Sockalingam S et al. Acad Psychiatry. 2012 Jul 1;36(4):277-81.
8. Singareddy R et al. Acad Psychiatry. 2008 Jul-Aug;32(4):343-4.
9. Kramer MN. Acad Psychiatry. 2005 Jul-Aug;29(3):322-4.
10. Rao NR and Kotapati VP. Pathways for success in academic medicine for an international medical graduate: Challenges and opportunities. In “Roberts Academic Medicine Handbook” 2020. Springer:163-70.
11. American Psychiatric Association. New poll: COVID-19 impacting mental well-being: Americans feeling anxious, especially for loved ones; older adults are less anxious. 2020 Mar 25.
12. Reger MA et al. JAMA Psychiatry. 2020 Apr 10. doi: 10.1001/jamapsychiatry.2020.1060.
13. Lai J et al. JAMA Netw Open. 2020 Mar 23;3(3):e203976-e203976. doi: 10.1001/jamanetworkopen.2020.3976.
14. Kalra G et al. Acad Psychiatry. 2012 Jul;36(4):323-9.
15. WHO best practices for the naming of new human infectious diseases. World Health Organization. 2015.
16. U.S. Department of State. Bureau of Consular Affairs. Visa Bulletin for March 2020.
17. National Resident Matching Program® (NRMP®). Thousands of medical students and graduates celebrate NRMP Match results.
18. American Medical Association. AMA: U.S. should open visas to international physicians amid COVID-19. AMA press release. 2020 Mar 25.
19. McKay D et al. J Anxiety Disord. 2020 Jun;73:02233. doi: 10.1016/j.janxdis.2020.102233.
20. Tang W et al. J Affect Disord. 2020 May 13;274:1-7.
21. Collins F et al. NIH Director’s Blog. NIH.gov. 2020 Apr 21.
22. Reger M et al. JAMA Psychiatry. 2020 Apr 10. doi: 10.1001/jamapsychiatry.2020.1060.
23. Druss BG. JAMA Psychiatry. 2020 Apr 3. doi: 10.1001/jamapsychiatry.2020.0894.
24. American Association of Medical Colleges. “The complexities of physician supply and demand: Projections from 2018-2033.” 2020 Jun.
‘Doc, can I get a mask exemption?’
As more jurisdictions mandate facial coverings in public, questions have arisen about whether it’s safe for everyone – including those with lung disease – to wear masks.
To address these issues, Medscape spoke with the chief medical officer of the American Lung Association, Dr. Albert Rizzo.
The CDC recommendations on mask wearing say, “Cloth face coverings should not be placed on young children under age 2, anyone who has trouble breathing, or is unconscious, incapacitated, or otherwise unable to remove the mask without assistance.” Does this language suggest that there indeed is a subset of the adult population with lung disease who shouldn’t wear masks?
It makes sense to say that if it makes you uncomfortable to wear a mask because it affects your breathing, you should think twice about getting in a situation where you would have to wear a mask.
I’ve told many of my high-risk patients, “The best way to avoid getting COVID-19 is to stay home and stay away from sick people, especially if you feel that you are not going to be able to wear a mask or facial covering of some sort.”
The reason that some people have trouble with a mask is that they haven’t tried the right style of mask – by that I mean how tightly it fits and the material it’s made out of. Sometimes it really is just that people with lung disease don’t like to have anything covering their faces. Many of these patients feel better where there is air blowing across their faces – they will have a fan blowing even in the middle of winter because they feel more comfortable.
I won’t say it’s all in their heads, but sometimes it’s a matter of desensitizing themselves to wearing a mask. I liken it to people who have sleep apnea. We often have to desensitize them to wearing a mask for sleeping. We tell them to put it on while they are watching TV — don’t hook it up to anything yet, just get used to having something on your face.
I’ve told my patients the same thing about masks for COVID-19. Put on the mask, see how it feels. If you become uncomfortable breathing with it on, take it off, but maybe you can handle it for a half hour or 45 minutes. Find out how much time you have for a trip to the grocery store based on how comfortable you are wearing it at home.
It’s a matter of training the patient, giving them options of how to get comfortable with it, and then making them realize that they have to weigh the benefits and risks of wearing the mask and feeling out of breath versus going out in public and being potentially exposed to coronavirus. And the bottom line is, anybody who is wearing a mask and starts to feel uncomfortable, they can take the mask off.
You mentioned different types of masks. Is there a type of mask that is typically more breathable that clinicians can recommend to patients with lung disease?
First, I remind patients who think they will have trouble breathing with a mask on that they are choosing a mask not so much to protect themselves – that would take an N95 mask to filter out the virus. The mask is worn so that when they cough or drink or speak, they aren’t sending respiratory droplets out into the environment. Even when we speak, respiratory droplets can easily go out as far as 6 feet, or further with coughing or sneezing. With facial coverings, we try to keep those respiratory droplets from getting out and infecting others.
So when choosing a mask, you don’t have to worry as much about a tight-fitting mask. I recommend a loose-fitting mask that covers the nose and mouth and isn’t going to fall off but isn’t so tight around the ears and neck to make them feel uncomfortable. Even though it doesn’t really protect the wearer, it is cutting down on the ability to breathe in droplets – maybe not microscopic particles, but it’s better than nothing.
Is a face shield a reasonable alternative for someone who feels they can’t breathe with a mask on?
Yes. I’m surprised that face shields don’t get more attention. I’ve tried them out, and they are actually more comfortable than masks. They do impede the spilling out of droplets into the public, but they are not as close fitting to the face as a mask. If you want to protect others, the face shield should be adequate. It is not as good at preventing you from breathing in viral particles.
Some people have claimed that wearing a mask makes them hyperventilate and feel like they are going to pass out, or the mask causes them to become hypoxic. Are these valid concerns?
We get two questions about masks from patients who feel that they are short of breath or are worried about wearing a mask. One is whether their oxygen level is dropping. It’s usually not that. It’s usually because they feel that the mask is an impediment to getting air in. Their oxygen levels are stable.
The other question is whether the mask causes CO2 retention. For the mask to trap enough exhaled CO2 and for us to breathe enough of that CO2 back in to raise our CO2 level, it has to be a pretty tight-fitting mask. With the type of masks we are suggesting that people wear, that’s very unlikely to occur.
What can clinicians do to reassure patients with some type of lung disease that they can safely wear masks?
There are a few things they can do right in the office. Have them put the mask on for a few minutes and make sure they feel comfortable with it. With an oximeter, patients can see that their oxygen levels don’t change when they are breathing through the mask for a period of time.
You can’t really measure CO2 retention that easily, but most patients with chronic obstructive pulmonary disease or pulmonary fibrosis don’t have an elevated CO2 at baseline. A little more education is helpful in those situations. In most cases, they aren’t going to retain enough CO2 to have problems wearing a mask.
Only a small percentage of patients with lung disease are CO2 retainers, and many of those patients are being seen by pulmonary specialists. Those are the patients you might want to be more cautious with, to make sure they aren’t wearing anything that is tight fitting or that makes them work harder to breathe. It’s not that the mask is causing CO2 retention, but the increased work of breathing may make it harder to exhale the CO2.
Does a mask interfere with supplemental oxygen in any way?
Supplemental oxygen is typically supplied through a nasal cannula, so 100% oxygen is still getting to the nasal passages and entrained down into the airway, so it shouldn’t be a problem.
Some of the resistance to wearing masks has come from people with asthma. Is it safe for patients with asthma to wear masks, or should these patients be exempt from wearing masks?
In general, the breathing of people with mild asthma, both young and old, should not be impeded by the wearing of facial coverings. The concerns about oxygen and carbon dioxide among patients with more severe lung disease should not play a role in asthma.
Since younger adults with COVID-19 seem to have fewer or no symptoms and may actually be carrying the virus unknowingly, this should be the main population who should wear masks to prevent transmission to others.
Exemptions for mask wearing for mild asthma should be discouraged and dealt with on a case-by-case basis if there is a particular concern for that individual.
How do you respond if a patient asks you for a formal medical exemption to wearing a mask?
We’ve been asked to do a lot of letter writing for patients around going back to work, as well as the issue of wearing masks. The discussion usually revolves around trying to avoid going somewhere where you would have to wear a mask if it makes you feel uncomfortable.
I do not recommend automatically exempting individuals from wearing masks, even many of my pulmonary patients. There needs to be an understanding by the patient regarding the purpose of the mask and the overall advice to stay out of situations where social distancing is not being practiced. If you can take the time to discuss options as mentioned above – mask styles, desensitization, etc – the patient usually understands and will try wearing a mask.
On a case-by-case basis, some individuals may need to be exempted, but I feel this is a small number. I prefer my high-risk (older, chronic disease, etc) patients do everything they can to avoid infection – handwashing, mask wearing, and socially distancing.
They should also realize that even with a note, it is not going to help if they are in the middle of the grocery store and someone confronts them about not wearing a mask. It may help as they enter a store that says “masks required” and they can show it to someone monitoring the door. But I’m not really sure in what situations having that note is going to be helpful if confrontations occur.
Patients are also asking how safe is it for them to go back to work and be out in public. I tell them, nothing is going to be 100% safe. Until we have an effective vaccine, we are all going to have to weigh the potential risks of going to an area where social distancing isn’t maintained, people aren’t wearing face masks, and you can’t wash your hands as much as you’d like to. That’s going to be a struggle for all of us to get back out into situations where people interact socially.
Albert A. Rizzo, MD, is chief medical officer for the American Lung Association, chief of the Section of Pulmonary and Critical Care Medicine at the Christiana Care Health System in Newark, Delaware, and a member of Christiana Care Pulmonary Associates. He is board certified in internal medicine, pulmonary medicine, critical care medicine, and sleep medicine and is a clinical assistant professor of medicine at Thomas Jefferson University Medical School, Philadelphia.
This article first appeared on Medscape.com.
As more jurisdictions mandate facial coverings in public, questions have arisen about whether it’s safe for everyone – including those with lung disease – to wear masks.
To address these issues, Medscape spoke with the chief medical officer of the American Lung Association, Dr. Albert Rizzo.
The CDC recommendations on mask wearing say, “Cloth face coverings should not be placed on young children under age 2, anyone who has trouble breathing, or is unconscious, incapacitated, or otherwise unable to remove the mask without assistance.” Does this language suggest that there indeed is a subset of the adult population with lung disease who shouldn’t wear masks?
It makes sense to say that if it makes you uncomfortable to wear a mask because it affects your breathing, you should think twice about getting in a situation where you would have to wear a mask.
I’ve told many of my high-risk patients, “The best way to avoid getting COVID-19 is to stay home and stay away from sick people, especially if you feel that you are not going to be able to wear a mask or facial covering of some sort.”
The reason that some people have trouble with a mask is that they haven’t tried the right style of mask – by that I mean how tightly it fits and the material it’s made out of. Sometimes it really is just that people with lung disease don’t like to have anything covering their faces. Many of these patients feel better where there is air blowing across their faces – they will have a fan blowing even in the middle of winter because they feel more comfortable.
I won’t say it’s all in their heads, but sometimes it’s a matter of desensitizing themselves to wearing a mask. I liken it to people who have sleep apnea. We often have to desensitize them to wearing a mask for sleeping. We tell them to put it on while they are watching TV — don’t hook it up to anything yet, just get used to having something on your face.
I’ve told my patients the same thing about masks for COVID-19. Put on the mask, see how it feels. If you become uncomfortable breathing with it on, take it off, but maybe you can handle it for a half hour or 45 minutes. Find out how much time you have for a trip to the grocery store based on how comfortable you are wearing it at home.
It’s a matter of training the patient, giving them options of how to get comfortable with it, and then making them realize that they have to weigh the benefits and risks of wearing the mask and feeling out of breath versus going out in public and being potentially exposed to coronavirus. And the bottom line is, anybody who is wearing a mask and starts to feel uncomfortable, they can take the mask off.
You mentioned different types of masks. Is there a type of mask that is typically more breathable that clinicians can recommend to patients with lung disease?
First, I remind patients who think they will have trouble breathing with a mask on that they are choosing a mask not so much to protect themselves – that would take an N95 mask to filter out the virus. The mask is worn so that when they cough or drink or speak, they aren’t sending respiratory droplets out into the environment. Even when we speak, respiratory droplets can easily go out as far as 6 feet, or further with coughing or sneezing. With facial coverings, we try to keep those respiratory droplets from getting out and infecting others.
So when choosing a mask, you don’t have to worry as much about a tight-fitting mask. I recommend a loose-fitting mask that covers the nose and mouth and isn’t going to fall off but isn’t so tight around the ears and neck to make them feel uncomfortable. Even though it doesn’t really protect the wearer, it is cutting down on the ability to breathe in droplets – maybe not microscopic particles, but it’s better than nothing.
Is a face shield a reasonable alternative for someone who feels they can’t breathe with a mask on?
Yes. I’m surprised that face shields don’t get more attention. I’ve tried them out, and they are actually more comfortable than masks. They do impede the spilling out of droplets into the public, but they are not as close fitting to the face as a mask. If you want to protect others, the face shield should be adequate. It is not as good at preventing you from breathing in viral particles.
Some people have claimed that wearing a mask makes them hyperventilate and feel like they are going to pass out, or the mask causes them to become hypoxic. Are these valid concerns?
We get two questions about masks from patients who feel that they are short of breath or are worried about wearing a mask. One is whether their oxygen level is dropping. It’s usually not that. It’s usually because they feel that the mask is an impediment to getting air in. Their oxygen levels are stable.
The other question is whether the mask causes CO2 retention. For the mask to trap enough exhaled CO2 and for us to breathe enough of that CO2 back in to raise our CO2 level, it has to be a pretty tight-fitting mask. With the type of masks we are suggesting that people wear, that’s very unlikely to occur.
What can clinicians do to reassure patients with some type of lung disease that they can safely wear masks?
There are a few things they can do right in the office. Have them put the mask on for a few minutes and make sure they feel comfortable with it. With an oximeter, patients can see that their oxygen levels don’t change when they are breathing through the mask for a period of time.
You can’t really measure CO2 retention that easily, but most patients with chronic obstructive pulmonary disease or pulmonary fibrosis don’t have an elevated CO2 at baseline. A little more education is helpful in those situations. In most cases, they aren’t going to retain enough CO2 to have problems wearing a mask.
Only a small percentage of patients with lung disease are CO2 retainers, and many of those patients are being seen by pulmonary specialists. Those are the patients you might want to be more cautious with, to make sure they aren’t wearing anything that is tight fitting or that makes them work harder to breathe. It’s not that the mask is causing CO2 retention, but the increased work of breathing may make it harder to exhale the CO2.
Does a mask interfere with supplemental oxygen in any way?
Supplemental oxygen is typically supplied through a nasal cannula, so 100% oxygen is still getting to the nasal passages and entrained down into the airway, so it shouldn’t be a problem.
Some of the resistance to wearing masks has come from people with asthma. Is it safe for patients with asthma to wear masks, or should these patients be exempt from wearing masks?
In general, the breathing of people with mild asthma, both young and old, should not be impeded by the wearing of facial coverings. The concerns about oxygen and carbon dioxide among patients with more severe lung disease should not play a role in asthma.
Since younger adults with COVID-19 seem to have fewer or no symptoms and may actually be carrying the virus unknowingly, this should be the main population who should wear masks to prevent transmission to others.
Exemptions for mask wearing for mild asthma should be discouraged and dealt with on a case-by-case basis if there is a particular concern for that individual.
How do you respond if a patient asks you for a formal medical exemption to wearing a mask?
We’ve been asked to do a lot of letter writing for patients around going back to work, as well as the issue of wearing masks. The discussion usually revolves around trying to avoid going somewhere where you would have to wear a mask if it makes you feel uncomfortable.
I do not recommend automatically exempting individuals from wearing masks, even many of my pulmonary patients. There needs to be an understanding by the patient regarding the purpose of the mask and the overall advice to stay out of situations where social distancing is not being practiced. If you can take the time to discuss options as mentioned above – mask styles, desensitization, etc – the patient usually understands and will try wearing a mask.
On a case-by-case basis, some individuals may need to be exempted, but I feel this is a small number. I prefer my high-risk (older, chronic disease, etc) patients do everything they can to avoid infection – handwashing, mask wearing, and socially distancing.
They should also realize that even with a note, it is not going to help if they are in the middle of the grocery store and someone confronts them about not wearing a mask. It may help as they enter a store that says “masks required” and they can show it to someone monitoring the door. But I’m not really sure in what situations having that note is going to be helpful if confrontations occur.
Patients are also asking how safe is it for them to go back to work and be out in public. I tell them, nothing is going to be 100% safe. Until we have an effective vaccine, we are all going to have to weigh the potential risks of going to an area where social distancing isn’t maintained, people aren’t wearing face masks, and you can’t wash your hands as much as you’d like to. That’s going to be a struggle for all of us to get back out into situations where people interact socially.
Albert A. Rizzo, MD, is chief medical officer for the American Lung Association, chief of the Section of Pulmonary and Critical Care Medicine at the Christiana Care Health System in Newark, Delaware, and a member of Christiana Care Pulmonary Associates. He is board certified in internal medicine, pulmonary medicine, critical care medicine, and sleep medicine and is a clinical assistant professor of medicine at Thomas Jefferson University Medical School, Philadelphia.
This article first appeared on Medscape.com.
As more jurisdictions mandate facial coverings in public, questions have arisen about whether it’s safe for everyone – including those with lung disease – to wear masks.
To address these issues, Medscape spoke with the chief medical officer of the American Lung Association, Dr. Albert Rizzo.
The CDC recommendations on mask wearing say, “Cloth face coverings should not be placed on young children under age 2, anyone who has trouble breathing, or is unconscious, incapacitated, or otherwise unable to remove the mask without assistance.” Does this language suggest that there indeed is a subset of the adult population with lung disease who shouldn’t wear masks?
It makes sense to say that if it makes you uncomfortable to wear a mask because it affects your breathing, you should think twice about getting in a situation where you would have to wear a mask.
I’ve told many of my high-risk patients, “The best way to avoid getting COVID-19 is to stay home and stay away from sick people, especially if you feel that you are not going to be able to wear a mask or facial covering of some sort.”
The reason that some people have trouble with a mask is that they haven’t tried the right style of mask – by that I mean how tightly it fits and the material it’s made out of. Sometimes it really is just that people with lung disease don’t like to have anything covering their faces. Many of these patients feel better where there is air blowing across their faces – they will have a fan blowing even in the middle of winter because they feel more comfortable.
I won’t say it’s all in their heads, but sometimes it’s a matter of desensitizing themselves to wearing a mask. I liken it to people who have sleep apnea. We often have to desensitize them to wearing a mask for sleeping. We tell them to put it on while they are watching TV — don’t hook it up to anything yet, just get used to having something on your face.
I’ve told my patients the same thing about masks for COVID-19. Put on the mask, see how it feels. If you become uncomfortable breathing with it on, take it off, but maybe you can handle it for a half hour or 45 minutes. Find out how much time you have for a trip to the grocery store based on how comfortable you are wearing it at home.
It’s a matter of training the patient, giving them options of how to get comfortable with it, and then making them realize that they have to weigh the benefits and risks of wearing the mask and feeling out of breath versus going out in public and being potentially exposed to coronavirus. And the bottom line is, anybody who is wearing a mask and starts to feel uncomfortable, they can take the mask off.
You mentioned different types of masks. Is there a type of mask that is typically more breathable that clinicians can recommend to patients with lung disease?
First, I remind patients who think they will have trouble breathing with a mask on that they are choosing a mask not so much to protect themselves – that would take an N95 mask to filter out the virus. The mask is worn so that when they cough or drink or speak, they aren’t sending respiratory droplets out into the environment. Even when we speak, respiratory droplets can easily go out as far as 6 feet, or further with coughing or sneezing. With facial coverings, we try to keep those respiratory droplets from getting out and infecting others.
So when choosing a mask, you don’t have to worry as much about a tight-fitting mask. I recommend a loose-fitting mask that covers the nose and mouth and isn’t going to fall off but isn’t so tight around the ears and neck to make them feel uncomfortable. Even though it doesn’t really protect the wearer, it is cutting down on the ability to breathe in droplets – maybe not microscopic particles, but it’s better than nothing.
Is a face shield a reasonable alternative for someone who feels they can’t breathe with a mask on?
Yes. I’m surprised that face shields don’t get more attention. I’ve tried them out, and they are actually more comfortable than masks. They do impede the spilling out of droplets into the public, but they are not as close fitting to the face as a mask. If you want to protect others, the face shield should be adequate. It is not as good at preventing you from breathing in viral particles.
Some people have claimed that wearing a mask makes them hyperventilate and feel like they are going to pass out, or the mask causes them to become hypoxic. Are these valid concerns?
We get two questions about masks from patients who feel that they are short of breath or are worried about wearing a mask. One is whether their oxygen level is dropping. It’s usually not that. It’s usually because they feel that the mask is an impediment to getting air in. Their oxygen levels are stable.
The other question is whether the mask causes CO2 retention. For the mask to trap enough exhaled CO2 and for us to breathe enough of that CO2 back in to raise our CO2 level, it has to be a pretty tight-fitting mask. With the type of masks we are suggesting that people wear, that’s very unlikely to occur.
What can clinicians do to reassure patients with some type of lung disease that they can safely wear masks?
There are a few things they can do right in the office. Have them put the mask on for a few minutes and make sure they feel comfortable with it. With an oximeter, patients can see that their oxygen levels don’t change when they are breathing through the mask for a period of time.
You can’t really measure CO2 retention that easily, but most patients with chronic obstructive pulmonary disease or pulmonary fibrosis don’t have an elevated CO2 at baseline. A little more education is helpful in those situations. In most cases, they aren’t going to retain enough CO2 to have problems wearing a mask.
Only a small percentage of patients with lung disease are CO2 retainers, and many of those patients are being seen by pulmonary specialists. Those are the patients you might want to be more cautious with, to make sure they aren’t wearing anything that is tight fitting or that makes them work harder to breathe. It’s not that the mask is causing CO2 retention, but the increased work of breathing may make it harder to exhale the CO2.
Does a mask interfere with supplemental oxygen in any way?
Supplemental oxygen is typically supplied through a nasal cannula, so 100% oxygen is still getting to the nasal passages and entrained down into the airway, so it shouldn’t be a problem.
Some of the resistance to wearing masks has come from people with asthma. Is it safe for patients with asthma to wear masks, or should these patients be exempt from wearing masks?
In general, the breathing of people with mild asthma, both young and old, should not be impeded by the wearing of facial coverings. The concerns about oxygen and carbon dioxide among patients with more severe lung disease should not play a role in asthma.
Since younger adults with COVID-19 seem to have fewer or no symptoms and may actually be carrying the virus unknowingly, this should be the main population who should wear masks to prevent transmission to others.
Exemptions for mask wearing for mild asthma should be discouraged and dealt with on a case-by-case basis if there is a particular concern for that individual.
How do you respond if a patient asks you for a formal medical exemption to wearing a mask?
We’ve been asked to do a lot of letter writing for patients around going back to work, as well as the issue of wearing masks. The discussion usually revolves around trying to avoid going somewhere where you would have to wear a mask if it makes you feel uncomfortable.
I do not recommend automatically exempting individuals from wearing masks, even many of my pulmonary patients. There needs to be an understanding by the patient regarding the purpose of the mask and the overall advice to stay out of situations where social distancing is not being practiced. If you can take the time to discuss options as mentioned above – mask styles, desensitization, etc – the patient usually understands and will try wearing a mask.
On a case-by-case basis, some individuals may need to be exempted, but I feel this is a small number. I prefer my high-risk (older, chronic disease, etc) patients do everything they can to avoid infection – handwashing, mask wearing, and socially distancing.
They should also realize that even with a note, it is not going to help if they are in the middle of the grocery store and someone confronts them about not wearing a mask. It may help as they enter a store that says “masks required” and they can show it to someone monitoring the door. But I’m not really sure in what situations having that note is going to be helpful if confrontations occur.
Patients are also asking how safe is it for them to go back to work and be out in public. I tell them, nothing is going to be 100% safe. Until we have an effective vaccine, we are all going to have to weigh the potential risks of going to an area where social distancing isn’t maintained, people aren’t wearing face masks, and you can’t wash your hands as much as you’d like to. That’s going to be a struggle for all of us to get back out into situations where people interact socially.
Albert A. Rizzo, MD, is chief medical officer for the American Lung Association, chief of the Section of Pulmonary and Critical Care Medicine at the Christiana Care Health System in Newark, Delaware, and a member of Christiana Care Pulmonary Associates. He is board certified in internal medicine, pulmonary medicine, critical care medicine, and sleep medicine and is a clinical assistant professor of medicine at Thomas Jefferson University Medical School, Philadelphia.
This article first appeared on Medscape.com.
Eczema Herpeticum in a Patient With Hailey-Hailey Disease Confounded by Coexistent Psoriasis
To the Editor:
Hailey-Hailey disease (HHD), also known as benign familial pemphigus, is an uncommon autosomal-dominant skin disease.1 Defects in the ATPase type 2C member 1 gene, ATP2C1, result in abnormal intracellular epidermal adherence, and patients experience recurring blisters in skin folds. Longitudinal white streaks of the fingernails also may be present.1 The illness does not appear until puberty and is heightened by the second or third decade of life. Family history often suggests the presence of disease.2 Misdiagnosis of HHD occurs because of a wide spectrum of presentations. The presence of superimposed infections and carcinomas may both obscure and exacerbate this disease.2
Herpes simplex viruse types 1 and 2 (HSV-1 and HSV-2) are DNA viruses that cause common recurrent diseases. Usually, HSV-1 is associated with infection of the mouth and HSV-2 is associated with infection of the genitalia.3 Longitudinal cutaneous lesions manifest as grouped vesicles on an erythematous base. Tzanck smear of herpetic vesicles will reveal the presence of multinucleated giant cells. A direct fluorescent antibody technique also may be used to confirm the diagnosis.3
Erythrodermic HHD disease is a rare condition; moreover, there are only a few reported cases with coexistence of HHD and HSV in the literature.3-6 We report a rare presentation of erythrodermic HHD and coexistent psoriasis with HSV superinfection.
A 69-year-old man presented to an outpatient dermatology clinic for evaluation and treatment of a rash on the scalp, face, back, and lower legs. The patient confirmed a dandruff diagnosis on the scalp and face as well as psoriasis on the trunk and extremities for the last 45 years. He described a history of successful treatment with topical agents and UV light therapy. A family history revealed that the patient’s father and 1 of 2 siblings had a similar rash and “skin problems.” The patient had a medical history of thyroid cancer treated with radiation treatment and a partial thyroidectomy 35 years prior to the current presentation as well as incompletely treated chronic hepatitis C.
A search of medical records revealed a punch biopsy from the posterior neck that demonstrated an acantholytic dyskeratosis with suprabasal acantholysis. Clinicians were unable to differentiate if it was Darier disease (DAR) or HHD. Treatment of the patient’s seborrheic dermatitis and acantholytic disorder was successful at that time with ketoconazole shampoo, ketoconazole cream, desonide cream, and triamcinolone cream. The patient remained stable for 5 years before presenting again to the dermatology clinic for worsening rash despite topical therapies.
At the current presentation, physical examination at the outpatient dermatology clinic revealed few scaly, erythematous, eroded papules distributed on the mid-back; erythematous greasy scaling on the scalp, face, and chest; and pink scaly plaques with white-silvery scale on the anterior lower legs. Histopathology of a specimen from the right mid-back demonstrated acantholysis with suprabasal clefting, hyperkeratosis, and parakeratosis with no dyskeratotic cells identified. The pathologic differential diagnosis included primary acantholytic processes including Grover disease, DAR, HHD, and pemphigus. Pathology from the right shin demonstrated acanthosis, confluent parakeratosis with associated decreased granular cell layer and collections of neutrophils within the stratum corneum, spongiosis, and superficial dermal perivascular chronic inflammation with focal exocytosis and dilated blood vessels in the papillary dermis. The clinical and pathological diagnosis on the lower legs was consistent with psoriasis. Diagnoses of seborrheic dermatitis, psoriasis on the lower legs, and HHD vs DAR on the back and chest were made. The patient was instructed to continue ketoconazole shampoo, ketoconazole cream, and desonide for seborrheic dermatitis; fluocinonide ointment 0.05% to the lower legs for psoriasis; and triamcinolone cream and a bland moisturizer to the back and chest for HHD.
Over the ensuing months, the rash worsened with erythema and scaling affecting more than half of the body surface area. Topical corticosteroids and bland emollients resulted in minimal success. Biologics and acitretin were considered for the psoriasiform dermatitis but avoided due to the patient’s medical history of thyroid cancer and chronic hepatitis C infection. Because the patient described prior success with UV light therapy for psoriasis, he requested light therapy. A subsequent trial of narrowband UVB light therapy initially improved some of the psoriasiform dermatitis on the trunk and extremities; however, after 4 weeks of treatment, the patient described pain in some of the skin and felt he was burned by minimal exposure to light therapy on one particular visit, which caused him to stop light therapy.
Approximately 2 weeks later, the patient presented to the emergency department stating his psoriasis was infected; he was diagnosed with psoriasis with secondary cellulitis and received intravenous vancomycin and piperacillin-tazobactam, with bacterial cultures demonstrating Corynebacterium and methicillin-resistant Staphylococcus aureus. Some improvement was noted in the patient’s skin after antibiotics were initiated, but he continued to describe worsening “burning and pain” throughout the psoriasis lesions. The patient’s care was transferred to the Veterans Affairs hospital where a dermatology inpatient consultation was placed.
Our initial dermatologic examination revealed generalized scaly erythroderma on the neck, trunk, and extremities, sparing the face, palms, and soles (Figure 1). Multiple crusted and intact vesicles also were present overlying the erythematous plaques on the chest, back, and proximal extremities, most grouped in clusters. The patient endorsed new symptoms of pain and burning. Tzanck smear from the abdomen along with shave biopsies from the left flank and right abdomen were performed, and intravenous acyclovir was initiated immediately after these procedures.
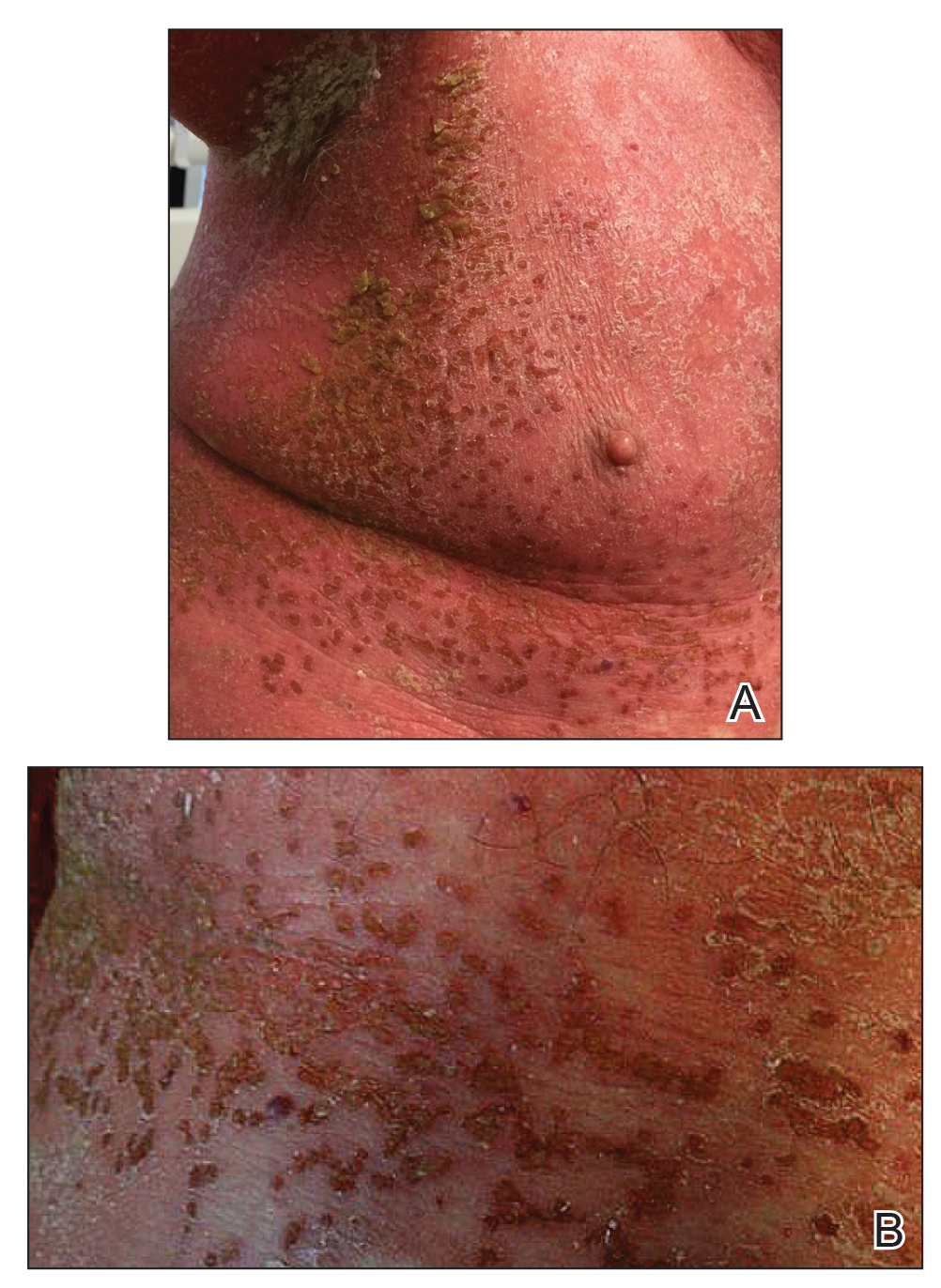
Viral cultures were taken but were incorrectly processed by the laboratory. Tzanck smear showed severe acute inflammation with numerous neutrophils, multinucleated giant cells with viral nuclear changes, and positive immunostaining for HSV and negative immunostaining for herpes zoster. Both pathology specimens revealed an intense acute mixed, mainly neutrophilic, inflammatory infiltrate extending into the deeper dermis as well as distorted and necrotic hair follicles, some of which displayed multinucleated epithelial cells with margination of chromatin that were positive for both HSV-1 and HSV-2 and negative for herpes zoster (Figure 2). The positivity of both HSV strains might represent co-infection or could be a cross-reaction of antibodies used in immunohistochemistry to the HSV antigens. There was acantholysis surrounding the ulceration and extending through the full thickness of the epidermis with a dilapidated brick wall pattern (Figure 3) as well as negative immunohistochemical staining for HSV-1 and HSV-2 antigens. The clinical and histological picture together, along with prior clinical and pathological reports, confirmed the diagnoses of acute erythrodermic HHD with HSV superinfection.
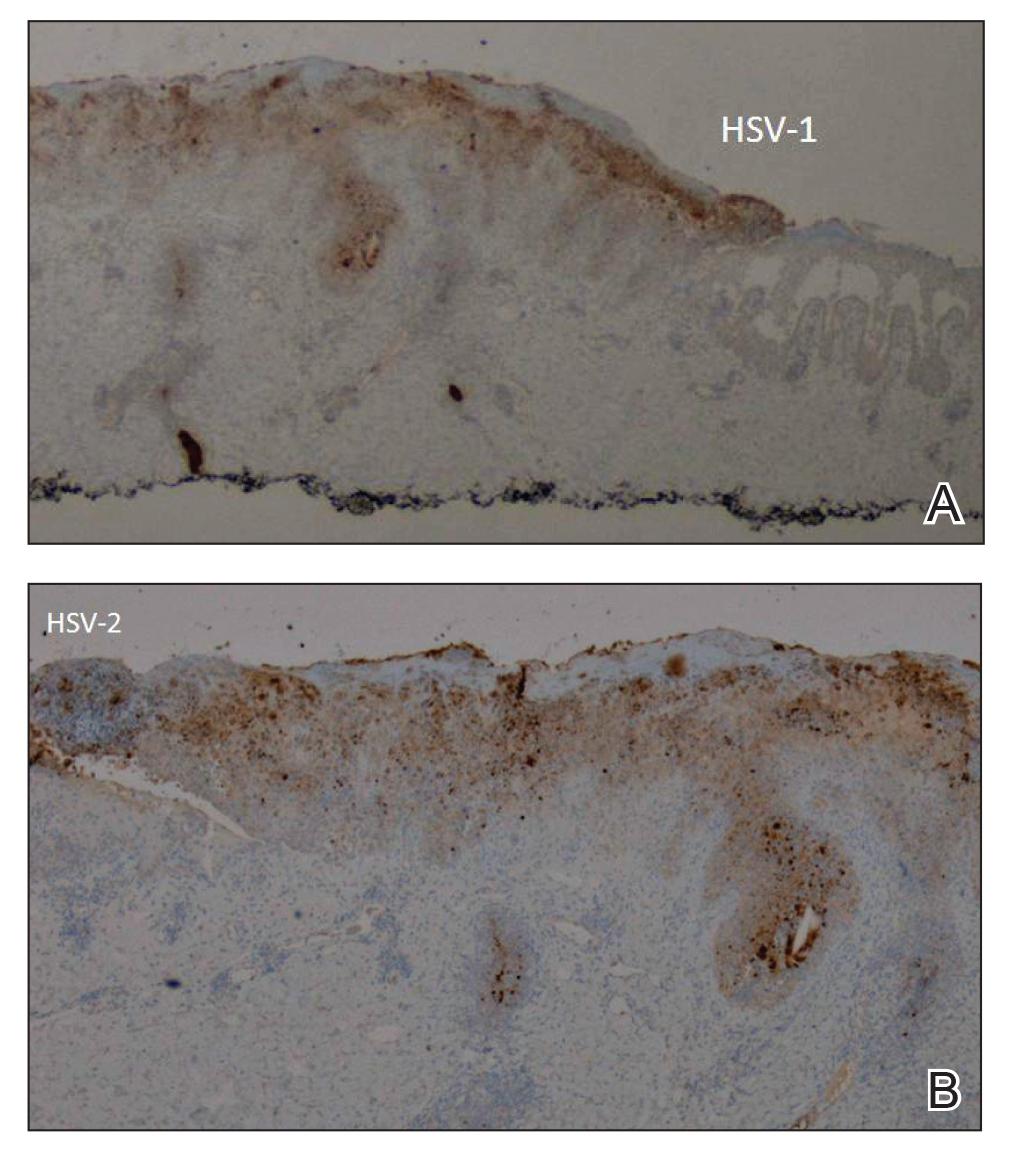
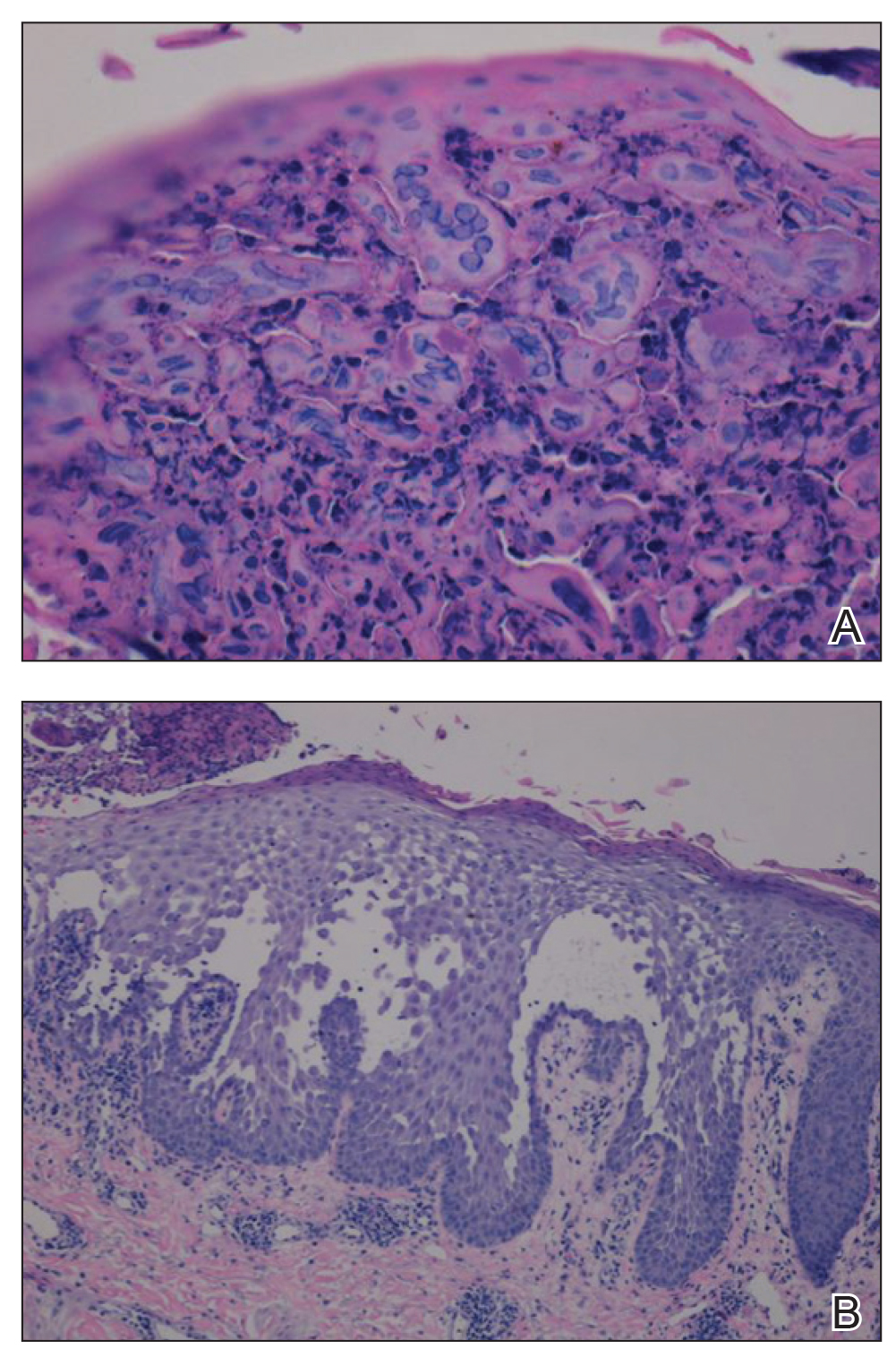
The patient’s condition and pain improved within 24 hours on intravenous acyclovir. On the third day, his lesions were resolving and symptoms improved, so he was transitioned to oral acyclovir and discharged from the hospital. Follow-up in the dermatology outpatient clinic 1 week later revealed that all vesicles and papules had cleared, but the patient was still erythrodermic. Because HHD cannot always be distinguished histologically from other forms of pemphigus but yields a negative immunofluorescence, direct immunofluorescence and indirect immunofluorescence were obtained upon patient follow-up in the clinic and were both negative. Hepatitis C viral loads were undetectable. Consultations to gastroenterology and oncology teams were placed for consideration of systemic agents, and the patient was initiated on oral acitretin 25 mg daily, along with clobetasol as adjuvant therapy for any residual skin plaques. The laboratory results were closely monitored. Within 4 weeks after starting acitretin, the patient’s erythroderma had completely resolved. The patient has remained stable since then, except for one episode of secondary Staphylococcus infection that cleared on oral antibiotics. The patient remains stable and clear on oral acitretin 25 mg daily, with concomitant desonide cream and fluocinonide ointment as needed.
Hailey-Hailey disease is characterized by recurrent episodes of erythema, blisters, and plaques localized to intertriginous and perianal areas.1,2 Patients display a spectrum of lesions that vary in severity.8 Typical histologic examination reveals a dilapidated brick wall appearance. Pathology of well-developed lesions will show suprabasal acantholysis with minimal dyskeratosis.2
The generalized form of HHD is an extremely rare variant of the disease.10 Generalized HHD may resemble acute hypersensitivity reaction, erythema multiforme, and toxic epidermal necrolysis.1 Chronic diseases, such as psoriasis (as in this patient), also may contribute to a clinically confusing picture.8 Hailey-Hailey disease and psoriasis are thought to occasionally koebnerize (isomorphic response) to areas of trauma.16 Our patient experienced widespread erythematous papules and plaques not restricted to skin folds. His skin lesions continued to worsen over several months progressing to erythroderma. The presence of suprabasal acantholysis in a dilapidated brick wall pattern, along with the patient’s history, prior pathology reports, clinical picture, and negative direct immunofluorescence and indirect immunofluorescence studies helped to confirm the diagnosis of erythrodermic HHD.
Hailey-Hailey disease is caused by heterozygous mutations in the ATP2C1 gene on chromosome 3q21-24 coding for a Golgi ATPase called SPCA1 (secretory pathway calcium/manganese-ATPase).9 Subsequent disturbances in cytosolic-Golgi calcium concentrations interfere with epidermal keratinocyte adherence resulting in acantholytic disease. Studies of interfamilial and intrafamilial mutations fail to pinpoint a common mutation pattern among patients with generalized phenotypes,9 which further supports theories that intrinsic or extrinsic factors such as friction, heat, radiation, contact allergens, and infection affect the severity of HHD disease and not the type of mutation.3,9
Generalization of HHD is likely caused by nonspecific triggers in an already genetically disturbed epidermis.10 Interrupted epithelial function exposes skin to infections that exacerbate the underlying disease. Superimposing bacterial infections are commonly reported in HHD. Staphylococcus, Streptococcus, and Candida species colonize the skin and aggravate the disease.11 Much less commonly, HSV superinfection can complicate HHD.3-7 No data are currently available about the frequency or incidence of Herpesviridae in HHD.7 Some studies suggest that UVB light therapy can be an exacerbating factor in DAR and some but not all HHD patients,12,13 while other case reports14,15 document clinically improved responses using phototherapy for patients with HHD. Clinicians should remain suspicious and evaluate for HSV infection in refractory or sudden exacerbation of HHD.7 Furthermore, coexistent psoriasis and HHD also is a rare entity but has been described,8 which illustrates the importance of not attributing all skin manifestations to a previously diagnosed disorder but instead keeping an open mind in case new dermatologic conditions present themselves at a later time.
We present a rare case of erythrodermic HHD and coexistent psoriasis with HSV superinfection. We hope to draw awareness to this association of generalized HHD with both HSV and psoriasis to help clinicians make the correct diagnosis promptly in similar cases in the future.
- Chave TA, Milligan A. Acute generalized Hailey-Hailey disease. Clin Exp Dermatol. 2002;27:290-292.
- Mohr MR, Erdag G, Shada Al, et al. Two patients with Hailey-Hailey disease, multiple primary melanomas, and other cancers. Arch Dermatol. 2011;147:211-215.
- Lee GM, Kim YM, Lee SY, et al. A case of eczema herpeticum with Hailey-Hailey Disease. Ann Dermatol. 2009;21:311-314.
- Zaim MT, Bickers DR. Herpes simplex associated with Hailey-Hailey disease. J Am Acad Dermatol. 1987;17:701-702.
- Peppiatt T, Keefe M, White JE. Hailey-Hailey disease-exacerbation by herpes simplex virus and patch tests. Clin Exp Dermatol. 2006;17:201-202.
- Almeida L, Grossman ME. Benign familial pemphigus complicated by herpes simplex virus. Cutis. 1989;44:261-262.
- Nikkels AF, Delvenne P, Herfs M, et al. Occult herpes simplex virus colonization of bullous dermatitides. Am J Clin Dermatol. 2008;9:163-168.
- Chao SC, Lee JY, Wu MC, et al. A novel splice mutation in the ATP2C1 gene in a woman with concomitant psoriasis vulgaris and disseminated Hailey-Hailey disease. Int J Dermatol. 2012;51:947-951.
- Ikeda S, Shigihara T, Mayuzumi N, et al. Mutations of ATP2C1 in Japanese patients with Hailey-Hailey disease: intrafamilial and interfamilial phenotype variations and lack of correlation with mutation patterns. J Invest Dermatol. 2001;117:1654-1656.
- Marsch W, Stuttgen G. Generalized Hailey-Hailey disease. Br J Dermatol. 1978;99:553-559.
- Friedman-Birnbaum R, Haim S, Marcus S. Generalized familial benign chronic pemphigus. Dermatologica. 1980;161:112-115.
- Richard G, Linse R, Harth W. Hailey-Hailey disease. early detection of heterozygotes by an ultraviolet provocation tests—clinical relevance of the method. Hautarzt. 1993;44:376-379.
- Mayuzumi N, Ikeda S, Kawada H, et al. Effects of ultraviolet B irradiation, proinflammatory cytokines and raised extracellular calcium concentration on the expression of ATP2A2 and ATP2C1. Br J Dermatol. 2005;152:697-701.
- Vanderbeck KA, Giroux L, Murugan NJ, et al. Combined therapeutic use of oral alitretinoin and narrowband ultraviolet-B therapy in the treatment of Hailey-Hailey disease. Dermatol Rep. 2014;6:5604.
- Mizuno K, Hamada T, Hasimoto T, et al. Successful treatment with narrow-band UVB therapy for a case of generalized Hailey-Hailey disease with a novel splice-site mutation in ATP2C1 gene. Dermatol Ther. 2014;27:233-235.
- Thappa DM. The isomorphic phenomenon of Koebner. Indian J Dermatol Venereol Leprol. 2004;70:187-189.
To the Editor:
Hailey-Hailey disease (HHD), also known as benign familial pemphigus, is an uncommon autosomal-dominant skin disease.1 Defects in the ATPase type 2C member 1 gene, ATP2C1, result in abnormal intracellular epidermal adherence, and patients experience recurring blisters in skin folds. Longitudinal white streaks of the fingernails also may be present.1 The illness does not appear until puberty and is heightened by the second or third decade of life. Family history often suggests the presence of disease.2 Misdiagnosis of HHD occurs because of a wide spectrum of presentations. The presence of superimposed infections and carcinomas may both obscure and exacerbate this disease.2
Herpes simplex viruse types 1 and 2 (HSV-1 and HSV-2) are DNA viruses that cause common recurrent diseases. Usually, HSV-1 is associated with infection of the mouth and HSV-2 is associated with infection of the genitalia.3 Longitudinal cutaneous lesions manifest as grouped vesicles on an erythematous base. Tzanck smear of herpetic vesicles will reveal the presence of multinucleated giant cells. A direct fluorescent antibody technique also may be used to confirm the diagnosis.3
Erythrodermic HHD disease is a rare condition; moreover, there are only a few reported cases with coexistence of HHD and HSV in the literature.3-6 We report a rare presentation of erythrodermic HHD and coexistent psoriasis with HSV superinfection.
A 69-year-old man presented to an outpatient dermatology clinic for evaluation and treatment of a rash on the scalp, face, back, and lower legs. The patient confirmed a dandruff diagnosis on the scalp and face as well as psoriasis on the trunk and extremities for the last 45 years. He described a history of successful treatment with topical agents and UV light therapy. A family history revealed that the patient’s father and 1 of 2 siblings had a similar rash and “skin problems.” The patient had a medical history of thyroid cancer treated with radiation treatment and a partial thyroidectomy 35 years prior to the current presentation as well as incompletely treated chronic hepatitis C.
A search of medical records revealed a punch biopsy from the posterior neck that demonstrated an acantholytic dyskeratosis with suprabasal acantholysis. Clinicians were unable to differentiate if it was Darier disease (DAR) or HHD. Treatment of the patient’s seborrheic dermatitis and acantholytic disorder was successful at that time with ketoconazole shampoo, ketoconazole cream, desonide cream, and triamcinolone cream. The patient remained stable for 5 years before presenting again to the dermatology clinic for worsening rash despite topical therapies.
At the current presentation, physical examination at the outpatient dermatology clinic revealed few scaly, erythematous, eroded papules distributed on the mid-back; erythematous greasy scaling on the scalp, face, and chest; and pink scaly plaques with white-silvery scale on the anterior lower legs. Histopathology of a specimen from the right mid-back demonstrated acantholysis with suprabasal clefting, hyperkeratosis, and parakeratosis with no dyskeratotic cells identified. The pathologic differential diagnosis included primary acantholytic processes including Grover disease, DAR, HHD, and pemphigus. Pathology from the right shin demonstrated acanthosis, confluent parakeratosis with associated decreased granular cell layer and collections of neutrophils within the stratum corneum, spongiosis, and superficial dermal perivascular chronic inflammation with focal exocytosis and dilated blood vessels in the papillary dermis. The clinical and pathological diagnosis on the lower legs was consistent with psoriasis. Diagnoses of seborrheic dermatitis, psoriasis on the lower legs, and HHD vs DAR on the back and chest were made. The patient was instructed to continue ketoconazole shampoo, ketoconazole cream, and desonide for seborrheic dermatitis; fluocinonide ointment 0.05% to the lower legs for psoriasis; and triamcinolone cream and a bland moisturizer to the back and chest for HHD.
Over the ensuing months, the rash worsened with erythema and scaling affecting more than half of the body surface area. Topical corticosteroids and bland emollients resulted in minimal success. Biologics and acitretin were considered for the psoriasiform dermatitis but avoided due to the patient’s medical history of thyroid cancer and chronic hepatitis C infection. Because the patient described prior success with UV light therapy for psoriasis, he requested light therapy. A subsequent trial of narrowband UVB light therapy initially improved some of the psoriasiform dermatitis on the trunk and extremities; however, after 4 weeks of treatment, the patient described pain in some of the skin and felt he was burned by minimal exposure to light therapy on one particular visit, which caused him to stop light therapy.
Approximately 2 weeks later, the patient presented to the emergency department stating his psoriasis was infected; he was diagnosed with psoriasis with secondary cellulitis and received intravenous vancomycin and piperacillin-tazobactam, with bacterial cultures demonstrating Corynebacterium and methicillin-resistant Staphylococcus aureus. Some improvement was noted in the patient’s skin after antibiotics were initiated, but he continued to describe worsening “burning and pain” throughout the psoriasis lesions. The patient’s care was transferred to the Veterans Affairs hospital where a dermatology inpatient consultation was placed.
Our initial dermatologic examination revealed generalized scaly erythroderma on the neck, trunk, and extremities, sparing the face, palms, and soles (Figure 1). Multiple crusted and intact vesicles also were present overlying the erythematous plaques on the chest, back, and proximal extremities, most grouped in clusters. The patient endorsed new symptoms of pain and burning. Tzanck smear from the abdomen along with shave biopsies from the left flank and right abdomen were performed, and intravenous acyclovir was initiated immediately after these procedures.

Viral cultures were taken but were incorrectly processed by the laboratory. Tzanck smear showed severe acute inflammation with numerous neutrophils, multinucleated giant cells with viral nuclear changes, and positive immunostaining for HSV and negative immunostaining for herpes zoster. Both pathology specimens revealed an intense acute mixed, mainly neutrophilic, inflammatory infiltrate extending into the deeper dermis as well as distorted and necrotic hair follicles, some of which displayed multinucleated epithelial cells with margination of chromatin that were positive for both HSV-1 and HSV-2 and negative for herpes zoster (Figure 2). The positivity of both HSV strains might represent co-infection or could be a cross-reaction of antibodies used in immunohistochemistry to the HSV antigens. There was acantholysis surrounding the ulceration and extending through the full thickness of the epidermis with a dilapidated brick wall pattern (Figure 3) as well as negative immunohistochemical staining for HSV-1 and HSV-2 antigens. The clinical and histological picture together, along with prior clinical and pathological reports, confirmed the diagnoses of acute erythrodermic HHD with HSV superinfection.


The patient’s condition and pain improved within 24 hours on intravenous acyclovir. On the third day, his lesions were resolving and symptoms improved, so he was transitioned to oral acyclovir and discharged from the hospital. Follow-up in the dermatology outpatient clinic 1 week later revealed that all vesicles and papules had cleared, but the patient was still erythrodermic. Because HHD cannot always be distinguished histologically from other forms of pemphigus but yields a negative immunofluorescence, direct immunofluorescence and indirect immunofluorescence were obtained upon patient follow-up in the clinic and were both negative. Hepatitis C viral loads were undetectable. Consultations to gastroenterology and oncology teams were placed for consideration of systemic agents, and the patient was initiated on oral acitretin 25 mg daily, along with clobetasol as adjuvant therapy for any residual skin plaques. The laboratory results were closely monitored. Within 4 weeks after starting acitretin, the patient’s erythroderma had completely resolved. The patient has remained stable since then, except for one episode of secondary Staphylococcus infection that cleared on oral antibiotics. The patient remains stable and clear on oral acitretin 25 mg daily, with concomitant desonide cream and fluocinonide ointment as needed.
Hailey-Hailey disease is characterized by recurrent episodes of erythema, blisters, and plaques localized to intertriginous and perianal areas.1,2 Patients display a spectrum of lesions that vary in severity.8 Typical histologic examination reveals a dilapidated brick wall appearance. Pathology of well-developed lesions will show suprabasal acantholysis with minimal dyskeratosis.2
The generalized form of HHD is an extremely rare variant of the disease.10 Generalized HHD may resemble acute hypersensitivity reaction, erythema multiforme, and toxic epidermal necrolysis.1 Chronic diseases, such as psoriasis (as in this patient), also may contribute to a clinically confusing picture.8 Hailey-Hailey disease and psoriasis are thought to occasionally koebnerize (isomorphic response) to areas of trauma.16 Our patient experienced widespread erythematous papules and plaques not restricted to skin folds. His skin lesions continued to worsen over several months progressing to erythroderma. The presence of suprabasal acantholysis in a dilapidated brick wall pattern, along with the patient’s history, prior pathology reports, clinical picture, and negative direct immunofluorescence and indirect immunofluorescence studies helped to confirm the diagnosis of erythrodermic HHD.
Hailey-Hailey disease is caused by heterozygous mutations in the ATP2C1 gene on chromosome 3q21-24 coding for a Golgi ATPase called SPCA1 (secretory pathway calcium/manganese-ATPase).9 Subsequent disturbances in cytosolic-Golgi calcium concentrations interfere with epidermal keratinocyte adherence resulting in acantholytic disease. Studies of interfamilial and intrafamilial mutations fail to pinpoint a common mutation pattern among patients with generalized phenotypes,9 which further supports theories that intrinsic or extrinsic factors such as friction, heat, radiation, contact allergens, and infection affect the severity of HHD disease and not the type of mutation.3,9
Generalization of HHD is likely caused by nonspecific triggers in an already genetically disturbed epidermis.10 Interrupted epithelial function exposes skin to infections that exacerbate the underlying disease. Superimposing bacterial infections are commonly reported in HHD. Staphylococcus, Streptococcus, and Candida species colonize the skin and aggravate the disease.11 Much less commonly, HSV superinfection can complicate HHD.3-7 No data are currently available about the frequency or incidence of Herpesviridae in HHD.7 Some studies suggest that UVB light therapy can be an exacerbating factor in DAR and some but not all HHD patients,12,13 while other case reports14,15 document clinically improved responses using phototherapy for patients with HHD. Clinicians should remain suspicious and evaluate for HSV infection in refractory or sudden exacerbation of HHD.7 Furthermore, coexistent psoriasis and HHD also is a rare entity but has been described,8 which illustrates the importance of not attributing all skin manifestations to a previously diagnosed disorder but instead keeping an open mind in case new dermatologic conditions present themselves at a later time.
We present a rare case of erythrodermic HHD and coexistent psoriasis with HSV superinfection. We hope to draw awareness to this association of generalized HHD with both HSV and psoriasis to help clinicians make the correct diagnosis promptly in similar cases in the future.
To the Editor:
Hailey-Hailey disease (HHD), also known as benign familial pemphigus, is an uncommon autosomal-dominant skin disease.1 Defects in the ATPase type 2C member 1 gene, ATP2C1, result in abnormal intracellular epidermal adherence, and patients experience recurring blisters in skin folds. Longitudinal white streaks of the fingernails also may be present.1 The illness does not appear until puberty and is heightened by the second or third decade of life. Family history often suggests the presence of disease.2 Misdiagnosis of HHD occurs because of a wide spectrum of presentations. The presence of superimposed infections and carcinomas may both obscure and exacerbate this disease.2
Herpes simplex viruse types 1 and 2 (HSV-1 and HSV-2) are DNA viruses that cause common recurrent diseases. Usually, HSV-1 is associated with infection of the mouth and HSV-2 is associated with infection of the genitalia.3 Longitudinal cutaneous lesions manifest as grouped vesicles on an erythematous base. Tzanck smear of herpetic vesicles will reveal the presence of multinucleated giant cells. A direct fluorescent antibody technique also may be used to confirm the diagnosis.3
Erythrodermic HHD disease is a rare condition; moreover, there are only a few reported cases with coexistence of HHD and HSV in the literature.3-6 We report a rare presentation of erythrodermic HHD and coexistent psoriasis with HSV superinfection.
A 69-year-old man presented to an outpatient dermatology clinic for evaluation and treatment of a rash on the scalp, face, back, and lower legs. The patient confirmed a dandruff diagnosis on the scalp and face as well as psoriasis on the trunk and extremities for the last 45 years. He described a history of successful treatment with topical agents and UV light therapy. A family history revealed that the patient’s father and 1 of 2 siblings had a similar rash and “skin problems.” The patient had a medical history of thyroid cancer treated with radiation treatment and a partial thyroidectomy 35 years prior to the current presentation as well as incompletely treated chronic hepatitis C.
A search of medical records revealed a punch biopsy from the posterior neck that demonstrated an acantholytic dyskeratosis with suprabasal acantholysis. Clinicians were unable to differentiate if it was Darier disease (DAR) or HHD. Treatment of the patient’s seborrheic dermatitis and acantholytic disorder was successful at that time with ketoconazole shampoo, ketoconazole cream, desonide cream, and triamcinolone cream. The patient remained stable for 5 years before presenting again to the dermatology clinic for worsening rash despite topical therapies.
At the current presentation, physical examination at the outpatient dermatology clinic revealed few scaly, erythematous, eroded papules distributed on the mid-back; erythematous greasy scaling on the scalp, face, and chest; and pink scaly plaques with white-silvery scale on the anterior lower legs. Histopathology of a specimen from the right mid-back demonstrated acantholysis with suprabasal clefting, hyperkeratosis, and parakeratosis with no dyskeratotic cells identified. The pathologic differential diagnosis included primary acantholytic processes including Grover disease, DAR, HHD, and pemphigus. Pathology from the right shin demonstrated acanthosis, confluent parakeratosis with associated decreased granular cell layer and collections of neutrophils within the stratum corneum, spongiosis, and superficial dermal perivascular chronic inflammation with focal exocytosis and dilated blood vessels in the papillary dermis. The clinical and pathological diagnosis on the lower legs was consistent with psoriasis. Diagnoses of seborrheic dermatitis, psoriasis on the lower legs, and HHD vs DAR on the back and chest were made. The patient was instructed to continue ketoconazole shampoo, ketoconazole cream, and desonide for seborrheic dermatitis; fluocinonide ointment 0.05% to the lower legs for psoriasis; and triamcinolone cream and a bland moisturizer to the back and chest for HHD.
Over the ensuing months, the rash worsened with erythema and scaling affecting more than half of the body surface area. Topical corticosteroids and bland emollients resulted in minimal success. Biologics and acitretin were considered for the psoriasiform dermatitis but avoided due to the patient’s medical history of thyroid cancer and chronic hepatitis C infection. Because the patient described prior success with UV light therapy for psoriasis, he requested light therapy. A subsequent trial of narrowband UVB light therapy initially improved some of the psoriasiform dermatitis on the trunk and extremities; however, after 4 weeks of treatment, the patient described pain in some of the skin and felt he was burned by minimal exposure to light therapy on one particular visit, which caused him to stop light therapy.
Approximately 2 weeks later, the patient presented to the emergency department stating his psoriasis was infected; he was diagnosed with psoriasis with secondary cellulitis and received intravenous vancomycin and piperacillin-tazobactam, with bacterial cultures demonstrating Corynebacterium and methicillin-resistant Staphylococcus aureus. Some improvement was noted in the patient’s skin after antibiotics were initiated, but he continued to describe worsening “burning and pain” throughout the psoriasis lesions. The patient’s care was transferred to the Veterans Affairs hospital where a dermatology inpatient consultation was placed.
Our initial dermatologic examination revealed generalized scaly erythroderma on the neck, trunk, and extremities, sparing the face, palms, and soles (Figure 1). Multiple crusted and intact vesicles also were present overlying the erythematous plaques on the chest, back, and proximal extremities, most grouped in clusters. The patient endorsed new symptoms of pain and burning. Tzanck smear from the abdomen along with shave biopsies from the left flank and right abdomen were performed, and intravenous acyclovir was initiated immediately after these procedures.

Viral cultures were taken but were incorrectly processed by the laboratory. Tzanck smear showed severe acute inflammation with numerous neutrophils, multinucleated giant cells with viral nuclear changes, and positive immunostaining for HSV and negative immunostaining for herpes zoster. Both pathology specimens revealed an intense acute mixed, mainly neutrophilic, inflammatory infiltrate extending into the deeper dermis as well as distorted and necrotic hair follicles, some of which displayed multinucleated epithelial cells with margination of chromatin that were positive for both HSV-1 and HSV-2 and negative for herpes zoster (Figure 2). The positivity of both HSV strains might represent co-infection or could be a cross-reaction of antibodies used in immunohistochemistry to the HSV antigens. There was acantholysis surrounding the ulceration and extending through the full thickness of the epidermis with a dilapidated brick wall pattern (Figure 3) as well as negative immunohistochemical staining for HSV-1 and HSV-2 antigens. The clinical and histological picture together, along with prior clinical and pathological reports, confirmed the diagnoses of acute erythrodermic HHD with HSV superinfection.


The patient’s condition and pain improved within 24 hours on intravenous acyclovir. On the third day, his lesions were resolving and symptoms improved, so he was transitioned to oral acyclovir and discharged from the hospital. Follow-up in the dermatology outpatient clinic 1 week later revealed that all vesicles and papules had cleared, but the patient was still erythrodermic. Because HHD cannot always be distinguished histologically from other forms of pemphigus but yields a negative immunofluorescence, direct immunofluorescence and indirect immunofluorescence were obtained upon patient follow-up in the clinic and were both negative. Hepatitis C viral loads were undetectable. Consultations to gastroenterology and oncology teams were placed for consideration of systemic agents, and the patient was initiated on oral acitretin 25 mg daily, along with clobetasol as adjuvant therapy for any residual skin plaques. The laboratory results were closely monitored. Within 4 weeks after starting acitretin, the patient’s erythroderma had completely resolved. The patient has remained stable since then, except for one episode of secondary Staphylococcus infection that cleared on oral antibiotics. The patient remains stable and clear on oral acitretin 25 mg daily, with concomitant desonide cream and fluocinonide ointment as needed.
Hailey-Hailey disease is characterized by recurrent episodes of erythema, blisters, and plaques localized to intertriginous and perianal areas.1,2 Patients display a spectrum of lesions that vary in severity.8 Typical histologic examination reveals a dilapidated brick wall appearance. Pathology of well-developed lesions will show suprabasal acantholysis with minimal dyskeratosis.2
The generalized form of HHD is an extremely rare variant of the disease.10 Generalized HHD may resemble acute hypersensitivity reaction, erythema multiforme, and toxic epidermal necrolysis.1 Chronic diseases, such as psoriasis (as in this patient), also may contribute to a clinically confusing picture.8 Hailey-Hailey disease and psoriasis are thought to occasionally koebnerize (isomorphic response) to areas of trauma.16 Our patient experienced widespread erythematous papules and plaques not restricted to skin folds. His skin lesions continued to worsen over several months progressing to erythroderma. The presence of suprabasal acantholysis in a dilapidated brick wall pattern, along with the patient’s history, prior pathology reports, clinical picture, and negative direct immunofluorescence and indirect immunofluorescence studies helped to confirm the diagnosis of erythrodermic HHD.
Hailey-Hailey disease is caused by heterozygous mutations in the ATP2C1 gene on chromosome 3q21-24 coding for a Golgi ATPase called SPCA1 (secretory pathway calcium/manganese-ATPase).9 Subsequent disturbances in cytosolic-Golgi calcium concentrations interfere with epidermal keratinocyte adherence resulting in acantholytic disease. Studies of interfamilial and intrafamilial mutations fail to pinpoint a common mutation pattern among patients with generalized phenotypes,9 which further supports theories that intrinsic or extrinsic factors such as friction, heat, radiation, contact allergens, and infection affect the severity of HHD disease and not the type of mutation.3,9
Generalization of HHD is likely caused by nonspecific triggers in an already genetically disturbed epidermis.10 Interrupted epithelial function exposes skin to infections that exacerbate the underlying disease. Superimposing bacterial infections are commonly reported in HHD. Staphylococcus, Streptococcus, and Candida species colonize the skin and aggravate the disease.11 Much less commonly, HSV superinfection can complicate HHD.3-7 No data are currently available about the frequency or incidence of Herpesviridae in HHD.7 Some studies suggest that UVB light therapy can be an exacerbating factor in DAR and some but not all HHD patients,12,13 while other case reports14,15 document clinically improved responses using phototherapy for patients with HHD. Clinicians should remain suspicious and evaluate for HSV infection in refractory or sudden exacerbation of HHD.7 Furthermore, coexistent psoriasis and HHD also is a rare entity but has been described,8 which illustrates the importance of not attributing all skin manifestations to a previously diagnosed disorder but instead keeping an open mind in case new dermatologic conditions present themselves at a later time.
We present a rare case of erythrodermic HHD and coexistent psoriasis with HSV superinfection. We hope to draw awareness to this association of generalized HHD with both HSV and psoriasis to help clinicians make the correct diagnosis promptly in similar cases in the future.
- Chave TA, Milligan A. Acute generalized Hailey-Hailey disease. Clin Exp Dermatol. 2002;27:290-292.
- Mohr MR, Erdag G, Shada Al, et al. Two patients with Hailey-Hailey disease, multiple primary melanomas, and other cancers. Arch Dermatol. 2011;147:211-215.
- Lee GM, Kim YM, Lee SY, et al. A case of eczema herpeticum with Hailey-Hailey Disease. Ann Dermatol. 2009;21:311-314.
- Zaim MT, Bickers DR. Herpes simplex associated with Hailey-Hailey disease. J Am Acad Dermatol. 1987;17:701-702.
- Peppiatt T, Keefe M, White JE. Hailey-Hailey disease-exacerbation by herpes simplex virus and patch tests. Clin Exp Dermatol. 2006;17:201-202.
- Almeida L, Grossman ME. Benign familial pemphigus complicated by herpes simplex virus. Cutis. 1989;44:261-262.
- Nikkels AF, Delvenne P, Herfs M, et al. Occult herpes simplex virus colonization of bullous dermatitides. Am J Clin Dermatol. 2008;9:163-168.
- Chao SC, Lee JY, Wu MC, et al. A novel splice mutation in the ATP2C1 gene in a woman with concomitant psoriasis vulgaris and disseminated Hailey-Hailey disease. Int J Dermatol. 2012;51:947-951.
- Ikeda S, Shigihara T, Mayuzumi N, et al. Mutations of ATP2C1 in Japanese patients with Hailey-Hailey disease: intrafamilial and interfamilial phenotype variations and lack of correlation with mutation patterns. J Invest Dermatol. 2001;117:1654-1656.
- Marsch W, Stuttgen G. Generalized Hailey-Hailey disease. Br J Dermatol. 1978;99:553-559.
- Friedman-Birnbaum R, Haim S, Marcus S. Generalized familial benign chronic pemphigus. Dermatologica. 1980;161:112-115.
- Richard G, Linse R, Harth W. Hailey-Hailey disease. early detection of heterozygotes by an ultraviolet provocation tests—clinical relevance of the method. Hautarzt. 1993;44:376-379.
- Mayuzumi N, Ikeda S, Kawada H, et al. Effects of ultraviolet B irradiation, proinflammatory cytokines and raised extracellular calcium concentration on the expression of ATP2A2 and ATP2C1. Br J Dermatol. 2005;152:697-701.
- Vanderbeck KA, Giroux L, Murugan NJ, et al. Combined therapeutic use of oral alitretinoin and narrowband ultraviolet-B therapy in the treatment of Hailey-Hailey disease. Dermatol Rep. 2014;6:5604.
- Mizuno K, Hamada T, Hasimoto T, et al. Successful treatment with narrow-band UVB therapy for a case of generalized Hailey-Hailey disease with a novel splice-site mutation in ATP2C1 gene. Dermatol Ther. 2014;27:233-235.
- Thappa DM. The isomorphic phenomenon of Koebner. Indian J Dermatol Venereol Leprol. 2004;70:187-189.
- Chave TA, Milligan A. Acute generalized Hailey-Hailey disease. Clin Exp Dermatol. 2002;27:290-292.
- Mohr MR, Erdag G, Shada Al, et al. Two patients with Hailey-Hailey disease, multiple primary melanomas, and other cancers. Arch Dermatol. 2011;147:211-215.
- Lee GM, Kim YM, Lee SY, et al. A case of eczema herpeticum with Hailey-Hailey Disease. Ann Dermatol. 2009;21:311-314.
- Zaim MT, Bickers DR. Herpes simplex associated with Hailey-Hailey disease. J Am Acad Dermatol. 1987;17:701-702.
- Peppiatt T, Keefe M, White JE. Hailey-Hailey disease-exacerbation by herpes simplex virus and patch tests. Clin Exp Dermatol. 2006;17:201-202.
- Almeida L, Grossman ME. Benign familial pemphigus complicated by herpes simplex virus. Cutis. 1989;44:261-262.
- Nikkels AF, Delvenne P, Herfs M, et al. Occult herpes simplex virus colonization of bullous dermatitides. Am J Clin Dermatol. 2008;9:163-168.
- Chao SC, Lee JY, Wu MC, et al. A novel splice mutation in the ATP2C1 gene in a woman with concomitant psoriasis vulgaris and disseminated Hailey-Hailey disease. Int J Dermatol. 2012;51:947-951.
- Ikeda S, Shigihara T, Mayuzumi N, et al. Mutations of ATP2C1 in Japanese patients with Hailey-Hailey disease: intrafamilial and interfamilial phenotype variations and lack of correlation with mutation patterns. J Invest Dermatol. 2001;117:1654-1656.
- Marsch W, Stuttgen G. Generalized Hailey-Hailey disease. Br J Dermatol. 1978;99:553-559.
- Friedman-Birnbaum R, Haim S, Marcus S. Generalized familial benign chronic pemphigus. Dermatologica. 1980;161:112-115.
- Richard G, Linse R, Harth W. Hailey-Hailey disease. early detection of heterozygotes by an ultraviolet provocation tests—clinical relevance of the method. Hautarzt. 1993;44:376-379.
- Mayuzumi N, Ikeda S, Kawada H, et al. Effects of ultraviolet B irradiation, proinflammatory cytokines and raised extracellular calcium concentration on the expression of ATP2A2 and ATP2C1. Br J Dermatol. 2005;152:697-701.
- Vanderbeck KA, Giroux L, Murugan NJ, et al. Combined therapeutic use of oral alitretinoin and narrowband ultraviolet-B therapy in the treatment of Hailey-Hailey disease. Dermatol Rep. 2014;6:5604.
- Mizuno K, Hamada T, Hasimoto T, et al. Successful treatment with narrow-band UVB therapy for a case of generalized Hailey-Hailey disease with a novel splice-site mutation in ATP2C1 gene. Dermatol Ther. 2014;27:233-235.
- Thappa DM. The isomorphic phenomenon of Koebner. Indian J Dermatol Venereol Leprol. 2004;70:187-189.
Practice Points
- Misdiagnosis of Hailey-Hailey disease (HHD) occurs because of a wide spectrum of presentations.
- Hailey-Hailey disease and psoriasis are thought to occasionally koebnerize (isomorphic response) to areas of trauma.
- Clinicians should remain suspicious and evaluate for herpes simplex virus infection in refractory or sudden exacerbation of HHD.
New Insights Into the Dermatology Residency Application Process Amid the COVID-19 Pandemic
Residency application is an arduous experience for many medical students. The National Resident Matching Program reported that US medical school seniors who matched into dermatology applied to a median of 90 programs and attended 9 interviews in 2019.1 High application and interview travel costs are a disadvantage for applicants from lower socioeconomic backgrounds. We propose that the coronavirus disease 2019 (COVID-19) pandemic should serve as a call to action for dermatology to update and promote a more equitable, time-effective, and cost-efficient residency interview process.
In light of COVID-19, dermatology residency program directors have recommended a holistic application review process, taking into consideration “disparities in strength of applications due to lack of opportunity for students with smaller home programs or in areas more affected by this crisis.”2 However, in a 2018 survey of 180 dermatology faculty members, 80% stated that time spent reviewing residency applications was already excessive.3 The Association of American Medical Colleges reported that for medical student applicants with US Medical Licensing Examination Step 1 scores lower than 237 or higher than 251, the value added by submitting one additional application beyond means of 43 (95% confidence interval [CI], 34-53) and 34 (95% CI, 28-41), respectively, is reduced relative to the value added by each application before reaching the point of diminishing returns.4 Therefore, we suggest limiting the number of applications per applicant to the upper bounds of the CI for the lower US Medical Licensing Examination Step 1 score (53), facilitating a more detailed review of fewer applications by each program and limiting student expenses.
On May 7, 2020, the Association of American Medical Colleges made a statement strongly encouraging medical school and teaching hospital faculty to conduct interviews through videoconferencing.5 Videoconferencing interviews (VCIs) minimize travel-associated health risks, providing a more equitable structure for applicants and programs in areas disproportionately impacted by the pandemic. In the 2018 survey of dermatology faculty members, only 11% believed that applicants interviewing virtually received equal consideration to those interviewing in person; a solution to this problem would be to mandate that all applicants use VCIs during the COVID-19 pandemic.3 This coming year, residency programs may elect to replace in-person interviews with VCIs, or they may utilize VCIs as screening tools to narrow down the applicant pool and for students to rank their preferred programs prior to an in-person interview. By inviting fewer applicants for in-person interviews, travel-associated health risks, financial costs, and missed educational activities would be minimized. Given that many medical students have had academic activities cancelled or postponed due to COVID-19, student opportunities for live clinical experiences should be maximized.
As programs plan for future application cycles beyond COVID-19, they must work to balance competing interests. Videoconferencing interviews allow for improved access to interviewing for applicants of lower socioeconomic classes, improved geographic mobility of applicants, and increased flexibility in accommodating faculty schedules with reduced time away from patient care and research; however, with VCIs one may lose the personal element that comes from the in-person interview, including interactions among applicants, faculty, current residents, and staff on the day of interview, as well as the departmental tour. Additionally, the quality of VCIs may be diminished by technical difficulties and the possibility of distractions, making standardization of the interview experience for applicants challenging.
The COVID-19 pandemic will surely leave its mark on the residency application cycle. We must take time now to collaborate and brainstorm creative solutions to maximize the equity and efficiency of the application process for both residency programs and students. We welcome reader feedback on these ideas and other possible solutions in the form of Letters to the Editor.
- National Resident Matching Program. Results of the 2019 NRMP Applicant Survey by Preferred Specialty and Applicant Type. Washington, DC: National Resident Matching Program; 2019. https://mk0nrmp3oyqui6wqfm.kinstacdn.com/wp-content/uploads/2019/06/Applicant-Survey-Report-2019.pdf. Accessed June 22, 2020.
- Association of American Medical Colleges. Specialty response to COVID-19: dermatology residency program director consensus statement on 2020-21 application cycle. https://aamc-orange.global.ssl.fastly.net/production/media/filer_
public/0f/7b/0f7b547e-65b5-4d93-8247-951206e7f726/updated_dermatology_program_director_
statement_on_2020-21_application_cycle_.pdf. Updated June 1, 2020. Accessed June 24, 2020. - Rojek NW, Shinkai K, Fett N. Dermatology faculty and residents’ perspectives on the dermatology residency application process: a nationwide survey. J Am Acad Dermatol. 2018;79:157-159.
- Association of American Medical Colleges. Apply smart: data to consider when applying to residency. https://www.students-residents.aamc.org/applying-residency/filteredresult/apply-smart-data-consider-when-applying-residency/. Accessed June 22, 2020.
- Association of American Medical Colleges. Conducting interviews during the coronavirus pandemic. https://www.aamc.org/what-we-do/mission-areas/medical-education/conducting-interviews-during-coronavirus-pandemic/. Published May 7, 2020. Accessed June 22, 2020.
Residency application is an arduous experience for many medical students. The National Resident Matching Program reported that US medical school seniors who matched into dermatology applied to a median of 90 programs and attended 9 interviews in 2019.1 High application and interview travel costs are a disadvantage for applicants from lower socioeconomic backgrounds. We propose that the coronavirus disease 2019 (COVID-19) pandemic should serve as a call to action for dermatology to update and promote a more equitable, time-effective, and cost-efficient residency interview process.
In light of COVID-19, dermatology residency program directors have recommended a holistic application review process, taking into consideration “disparities in strength of applications due to lack of opportunity for students with smaller home programs or in areas more affected by this crisis.”2 However, in a 2018 survey of 180 dermatology faculty members, 80% stated that time spent reviewing residency applications was already excessive.3 The Association of American Medical Colleges reported that for medical student applicants with US Medical Licensing Examination Step 1 scores lower than 237 or higher than 251, the value added by submitting one additional application beyond means of 43 (95% confidence interval [CI], 34-53) and 34 (95% CI, 28-41), respectively, is reduced relative to the value added by each application before reaching the point of diminishing returns.4 Therefore, we suggest limiting the number of applications per applicant to the upper bounds of the CI for the lower US Medical Licensing Examination Step 1 score (53), facilitating a more detailed review of fewer applications by each program and limiting student expenses.
On May 7, 2020, the Association of American Medical Colleges made a statement strongly encouraging medical school and teaching hospital faculty to conduct interviews through videoconferencing.5 Videoconferencing interviews (VCIs) minimize travel-associated health risks, providing a more equitable structure for applicants and programs in areas disproportionately impacted by the pandemic. In the 2018 survey of dermatology faculty members, only 11% believed that applicants interviewing virtually received equal consideration to those interviewing in person; a solution to this problem would be to mandate that all applicants use VCIs during the COVID-19 pandemic.3 This coming year, residency programs may elect to replace in-person interviews with VCIs, or they may utilize VCIs as screening tools to narrow down the applicant pool and for students to rank their preferred programs prior to an in-person interview. By inviting fewer applicants for in-person interviews, travel-associated health risks, financial costs, and missed educational activities would be minimized. Given that many medical students have had academic activities cancelled or postponed due to COVID-19, student opportunities for live clinical experiences should be maximized.
As programs plan for future application cycles beyond COVID-19, they must work to balance competing interests. Videoconferencing interviews allow for improved access to interviewing for applicants of lower socioeconomic classes, improved geographic mobility of applicants, and increased flexibility in accommodating faculty schedules with reduced time away from patient care and research; however, with VCIs one may lose the personal element that comes from the in-person interview, including interactions among applicants, faculty, current residents, and staff on the day of interview, as well as the departmental tour. Additionally, the quality of VCIs may be diminished by technical difficulties and the possibility of distractions, making standardization of the interview experience for applicants challenging.
The COVID-19 pandemic will surely leave its mark on the residency application cycle. We must take time now to collaborate and brainstorm creative solutions to maximize the equity and efficiency of the application process for both residency programs and students. We welcome reader feedback on these ideas and other possible solutions in the form of Letters to the Editor.
Residency application is an arduous experience for many medical students. The National Resident Matching Program reported that US medical school seniors who matched into dermatology applied to a median of 90 programs and attended 9 interviews in 2019.1 High application and interview travel costs are a disadvantage for applicants from lower socioeconomic backgrounds. We propose that the coronavirus disease 2019 (COVID-19) pandemic should serve as a call to action for dermatology to update and promote a more equitable, time-effective, and cost-efficient residency interview process.
In light of COVID-19, dermatology residency program directors have recommended a holistic application review process, taking into consideration “disparities in strength of applications due to lack of opportunity for students with smaller home programs or in areas more affected by this crisis.”2 However, in a 2018 survey of 180 dermatology faculty members, 80% stated that time spent reviewing residency applications was already excessive.3 The Association of American Medical Colleges reported that for medical student applicants with US Medical Licensing Examination Step 1 scores lower than 237 or higher than 251, the value added by submitting one additional application beyond means of 43 (95% confidence interval [CI], 34-53) and 34 (95% CI, 28-41), respectively, is reduced relative to the value added by each application before reaching the point of diminishing returns.4 Therefore, we suggest limiting the number of applications per applicant to the upper bounds of the CI for the lower US Medical Licensing Examination Step 1 score (53), facilitating a more detailed review of fewer applications by each program and limiting student expenses.
On May 7, 2020, the Association of American Medical Colleges made a statement strongly encouraging medical school and teaching hospital faculty to conduct interviews through videoconferencing.5 Videoconferencing interviews (VCIs) minimize travel-associated health risks, providing a more equitable structure for applicants and programs in areas disproportionately impacted by the pandemic. In the 2018 survey of dermatology faculty members, only 11% believed that applicants interviewing virtually received equal consideration to those interviewing in person; a solution to this problem would be to mandate that all applicants use VCIs during the COVID-19 pandemic.3 This coming year, residency programs may elect to replace in-person interviews with VCIs, or they may utilize VCIs as screening tools to narrow down the applicant pool and for students to rank their preferred programs prior to an in-person interview. By inviting fewer applicants for in-person interviews, travel-associated health risks, financial costs, and missed educational activities would be minimized. Given that many medical students have had academic activities cancelled or postponed due to COVID-19, student opportunities for live clinical experiences should be maximized.
As programs plan for future application cycles beyond COVID-19, they must work to balance competing interests. Videoconferencing interviews allow for improved access to interviewing for applicants of lower socioeconomic classes, improved geographic mobility of applicants, and increased flexibility in accommodating faculty schedules with reduced time away from patient care and research; however, with VCIs one may lose the personal element that comes from the in-person interview, including interactions among applicants, faculty, current residents, and staff on the day of interview, as well as the departmental tour. Additionally, the quality of VCIs may be diminished by technical difficulties and the possibility of distractions, making standardization of the interview experience for applicants challenging.
The COVID-19 pandemic will surely leave its mark on the residency application cycle. We must take time now to collaborate and brainstorm creative solutions to maximize the equity and efficiency of the application process for both residency programs and students. We welcome reader feedback on these ideas and other possible solutions in the form of Letters to the Editor.
- National Resident Matching Program. Results of the 2019 NRMP Applicant Survey by Preferred Specialty and Applicant Type. Washington, DC: National Resident Matching Program; 2019. https://mk0nrmp3oyqui6wqfm.kinstacdn.com/wp-content/uploads/2019/06/Applicant-Survey-Report-2019.pdf. Accessed June 22, 2020.
- Association of American Medical Colleges. Specialty response to COVID-19: dermatology residency program director consensus statement on 2020-21 application cycle. https://aamc-orange.global.ssl.fastly.net/production/media/filer_
public/0f/7b/0f7b547e-65b5-4d93-8247-951206e7f726/updated_dermatology_program_director_
statement_on_2020-21_application_cycle_.pdf. Updated June 1, 2020. Accessed June 24, 2020. - Rojek NW, Shinkai K, Fett N. Dermatology faculty and residents’ perspectives on the dermatology residency application process: a nationwide survey. J Am Acad Dermatol. 2018;79:157-159.
- Association of American Medical Colleges. Apply smart: data to consider when applying to residency. https://www.students-residents.aamc.org/applying-residency/filteredresult/apply-smart-data-consider-when-applying-residency/. Accessed June 22, 2020.
- Association of American Medical Colleges. Conducting interviews during the coronavirus pandemic. https://www.aamc.org/what-we-do/mission-areas/medical-education/conducting-interviews-during-coronavirus-pandemic/. Published May 7, 2020. Accessed June 22, 2020.
- National Resident Matching Program. Results of the 2019 NRMP Applicant Survey by Preferred Specialty and Applicant Type. Washington, DC: National Resident Matching Program; 2019. https://mk0nrmp3oyqui6wqfm.kinstacdn.com/wp-content/uploads/2019/06/Applicant-Survey-Report-2019.pdf. Accessed June 22, 2020.
- Association of American Medical Colleges. Specialty response to COVID-19: dermatology residency program director consensus statement on 2020-21 application cycle. https://aamc-orange.global.ssl.fastly.net/production/media/filer_
public/0f/7b/0f7b547e-65b5-4d93-8247-951206e7f726/updated_dermatology_program_director_
statement_on_2020-21_application_cycle_.pdf. Updated June 1, 2020. Accessed June 24, 2020. - Rojek NW, Shinkai K, Fett N. Dermatology faculty and residents’ perspectives on the dermatology residency application process: a nationwide survey. J Am Acad Dermatol. 2018;79:157-159.
- Association of American Medical Colleges. Apply smart: data to consider when applying to residency. https://www.students-residents.aamc.org/applying-residency/filteredresult/apply-smart-data-consider-when-applying-residency/. Accessed June 22, 2020.
- Association of American Medical Colleges. Conducting interviews during the coronavirus pandemic. https://www.aamc.org/what-we-do/mission-areas/medical-education/conducting-interviews-during-coronavirus-pandemic/. Published May 7, 2020. Accessed June 22, 2020.
Practice Points
- We propose that the coronavirus disease 2019 pandemic should serve as a call to action for dermatology to update and promote a more equitable, time-effective, and cost-efficient residency interview process.
- A limitation on the number of applications per candidate may lower expenses and allow for a more holistic review process by residency programs.
- The benefits and challenges of videoconferencing interviews must be weighed as residency programs decide on their continued use beyond this application cycle.
Rapid Screening of Invasive Fungal Infections in the Hospital Setting Using the (1,3)-β-D-glucan Assay
Practice Gap
Invasive fungal infections are a leading cause of morbidity and mortality among neutropenic, immunocompromised, and critically ill patients. Candida species are the most common cause of fungemia, with portals of entry into the bloodstream including the gastrointestinal tract, contaminated intravascular catheters, and localized foci of infection.1 Diagnosis of invasive candidiasis remains challenging due to an absence of specific clinical signs and symptoms, varying from a mild fever that is unresponsive to antibiotics to florid sepsis. When present, clinical clues may include chorioretinitis; muscle abscesses; and skin eruptions, characteristically with Candida tropicalis. Cutaneous manifestations of disseminated Candida infections appear in only 13% of affected patients.1 The lesions typically present as 5- to 10-mm pink dermal papules or painless pustules on an erythematous base and may be singular, localized, or diffuse in distribution. Body regions normally involved are the trunk, arms, and legs, rarely the head and neck.1 Cutaneous lesions often develop at a time when patients are febrile, are not responding to antibiotics, and are clinically deteriorating.
A 15-year-old adolescent boy with pre–B-cell acute lymphoblastic leukemia was admitted with febrile neutropenia for presumed septic shock secondary to an unknown infectious etiology. The patient was started on broad-spectrum intravenous antibiotics, and blood cultures were obtained. On the second day of hospitalization, he developed approximately 10 to 15 discrete, 3- to 6-mm, pink to violaceous papules scattered on the chest and arms (Figure 1). Over several hours, the number of lesions increased to more than 50 with involvement of the legs (Figure 2). A punch biopsy of lesional skin from the left dorsal wrist demonstrated a circumscribed abscess of yeast in the papillary dermis, which was highlighted by periodic acid–Schiff staining with minimal associated inflammation (Figure 3). Blood and tissue cultures persistently grew C tropicalis. The patient was started on intravenous liposomal amphotericin B but died on day 5 of hospitalization after developing endocarditis.
Early and reliable diagnosis of Candida species fungemia is of critical importance to successful treatment, particularly with the emergence of multidrug-resistant strains such as Candida auris.2 In patients with apparent cutaneous manifestations, a lesional punch biopsy for culture and histopathologic evaluation is recommended in addition to blood culture; however, organisms may or may not be present in large numbers, and they may be difficult to identify on routine hematoxylin and eosin–stained tissue sections. To enhance the likelihood of highlighting the fungus within the sample, the pathologist must be made aware of the presumptive diagnosis of disseminated candidiasis so that special techniques can be utilized, such as periodic acid–Schiff stain.
Although positive blood culture is the gold standard for candidemia diagnosis, only 30% to 50% of patients with disseminated candidiasis had positive blood cultures at autopsy.1 Another study showed the sensitivity of blood culture for the detection of invasive fungal infection to be as low as 8.3%.3 In cases with positive blood cultures, the median time to positivity is 2 to 3 days, but it can take as long as 8 days, thus limiting its clinical utility in acutely ill patients.4 Given the low sensitivity and prolonged time required for culture growth of most fungal organisms, novel assays for rapid, non–culture-based diagnosis of systemic fungal infections hold substantial clinical promise moving forward.
The Technique
One of the more promising non–culture-based fungal diagnostic methodologies is an antigen assay based on the detection of serum (1,3)-β-D-glucan (BDG), a major cell wall constituent of most pathogenic fungi. This assay is not specific for Candida species and can be positive for Aspergillosis species, Fusarium species, Coccidioides immitis, Histoplasma capsulatum, and Pneumocystis jirovecii pneumonia, among others; therefore, it functions as a general biomarker for fungi in the bloodstream.4,5 (1,3)-β-D-glucan assay can be useful as an adjunct for blood cultures and punch biopsy, especially when cultures are negative or the results remain outstanding. The results of the BDG assay are available in less than 24 hours at minimal cost, and the test is approved by the US Food and Drug Administration for use as an aid in invasive fungal disease diagnosis. In a meta-analysis of 11 studies, BDG sensitivity was 75%.4 In a study based on autopsy cases from 6 years, BDG specificity was 98.4% with positive and negative predictive values of 86.7% and 97.1%, respectively.3 Optimal results were achieved when 2 consecutive tests were positive.4 The serum assay output is based on spectrophotometer readings, which are converted to BDG concentrations (negative, <60 pg/mL; indeterminate, 60–79 pg/mL; positive ≥80 pg/mL).5 Although we cannot be certain, utilizing the BDG assay in our patient may have led to earlier treatment and a better outcome.
A disadvantage of the BDG assay is the potential for false-positive results, which have been reported in lung transplant recipients with respiratory mold colonization and patients with other systemic bacterial infections.4 False-positive results also have been associated with use of ampicillin-clavulanate and piperacillin-tazobactam antibiotics and human blood products, hemodialysis, and severe mucositis, thus reaffirming the importance of judicious interpretation of BDG assay results by the clinician.4,6 There also is a potential for false-negative results, as the BDG assay does not detect certain fungal species such as Cryptococcus species and Blastomyces dermatitidis, which produce very low levels of BDG, or zygomycetes (Absidia, Mucor, and Rizopus species), which are not known to produce BDG.6
Practice Implications
In the setting of invasive fungal infections, a high degree of clinical suspicion is paramount due to the often subtle nature of cutaneous manifestations. A positive BDG assay can be used to identify high-risk patients for empiric antifungal therapy, prompting early intervention and improved outcomes in these acutely ill patients. The BDG assay’s excellent negative predictive value is useful in ruling out invasive Candida infections and may justify stopping unnecessary empiric antifungal therapy.4 For the dermatology hospitalist, incorporation of the BDG assay as a noninvasive screening tool may allow for more rapid initiation of appropriate antifungal therapy while awaiting confirmatory skin biopsy or culture results in disseminated candidemia and other invasive fungal infections.
- Mays SR, Bogle MA, Bodey GP. Cutaneous fungal infections in the oncology patient: recognition and management. Am J Clin Dermatol. 2006;7:31-43.
- Candida auris. Centers for Disease Control and Prevention website. https://www.cdc.gov/fungal/candida-auris/. Updated May 15, 2020. Accessed July 10, 2020.
- Obayashi T, Negishi K, Suzuki T, et al. Reappraisal of the serum (1,3)-β-D-glucan assay for the diagnosis of invasive fungal infections—a study based on autopsy cases from 6 years. Clin Infect Dis. 2008;46:1864-1870.
- Clancy CJ, Nguyen MH. Finding the “missing 50%” of invasive candidiasis: how nonculture diagnostics will improve understanding of disease spectrum and transform patient care. Clin Infect Dis. 2013;56:1284-1292.
- McCarthy MW, Petraitiene R, Walsh TJ. Translational development and application of (1→3)-β-d-glucan for diagnosis and therapeutic monitoring of invasive mycoses [published online May 24, 2017]. Int J Mol Sci. doi:10.3390/ijms18061124.
- Beta-D glucan assay. MiraVista Diagnostics website. https://miravistalabs.com/medical-fungal-infection-testing/antigen-detection/beta-d-glucan-test/. Accessed June 5, 2020.
Practice Gap
Invasive fungal infections are a leading cause of morbidity and mortality among neutropenic, immunocompromised, and critically ill patients. Candida species are the most common cause of fungemia, with portals of entry into the bloodstream including the gastrointestinal tract, contaminated intravascular catheters, and localized foci of infection.1 Diagnosis of invasive candidiasis remains challenging due to an absence of specific clinical signs and symptoms, varying from a mild fever that is unresponsive to antibiotics to florid sepsis. When present, clinical clues may include chorioretinitis; muscle abscesses; and skin eruptions, characteristically with Candida tropicalis. Cutaneous manifestations of disseminated Candida infections appear in only 13% of affected patients.1 The lesions typically present as 5- to 10-mm pink dermal papules or painless pustules on an erythematous base and may be singular, localized, or diffuse in distribution. Body regions normally involved are the trunk, arms, and legs, rarely the head and neck.1 Cutaneous lesions often develop at a time when patients are febrile, are not responding to antibiotics, and are clinically deteriorating.
A 15-year-old adolescent boy with pre–B-cell acute lymphoblastic leukemia was admitted with febrile neutropenia for presumed septic shock secondary to an unknown infectious etiology. The patient was started on broad-spectrum intravenous antibiotics, and blood cultures were obtained. On the second day of hospitalization, he developed approximately 10 to 15 discrete, 3- to 6-mm, pink to violaceous papules scattered on the chest and arms (Figure 1). Over several hours, the number of lesions increased to more than 50 with involvement of the legs (Figure 2). A punch biopsy of lesional skin from the left dorsal wrist demonstrated a circumscribed abscess of yeast in the papillary dermis, which was highlighted by periodic acid–Schiff staining with minimal associated inflammation (Figure 3). Blood and tissue cultures persistently grew C tropicalis. The patient was started on intravenous liposomal amphotericin B but died on day 5 of hospitalization after developing endocarditis.



Early and reliable diagnosis of Candida species fungemia is of critical importance to successful treatment, particularly with the emergence of multidrug-resistant strains such as Candida auris.2 In patients with apparent cutaneous manifestations, a lesional punch biopsy for culture and histopathologic evaluation is recommended in addition to blood culture; however, organisms may or may not be present in large numbers, and they may be difficult to identify on routine hematoxylin and eosin–stained tissue sections. To enhance the likelihood of highlighting the fungus within the sample, the pathologist must be made aware of the presumptive diagnosis of disseminated candidiasis so that special techniques can be utilized, such as periodic acid–Schiff stain.
Although positive blood culture is the gold standard for candidemia diagnosis, only 30% to 50% of patients with disseminated candidiasis had positive blood cultures at autopsy.1 Another study showed the sensitivity of blood culture for the detection of invasive fungal infection to be as low as 8.3%.3 In cases with positive blood cultures, the median time to positivity is 2 to 3 days, but it can take as long as 8 days, thus limiting its clinical utility in acutely ill patients.4 Given the low sensitivity and prolonged time required for culture growth of most fungal organisms, novel assays for rapid, non–culture-based diagnosis of systemic fungal infections hold substantial clinical promise moving forward.
The Technique
One of the more promising non–culture-based fungal diagnostic methodologies is an antigen assay based on the detection of serum (1,3)-β-D-glucan (BDG), a major cell wall constituent of most pathogenic fungi. This assay is not specific for Candida species and can be positive for Aspergillosis species, Fusarium species, Coccidioides immitis, Histoplasma capsulatum, and Pneumocystis jirovecii pneumonia, among others; therefore, it functions as a general biomarker for fungi in the bloodstream.4,5 (1,3)-β-D-glucan assay can be useful as an adjunct for blood cultures and punch biopsy, especially when cultures are negative or the results remain outstanding. The results of the BDG assay are available in less than 24 hours at minimal cost, and the test is approved by the US Food and Drug Administration for use as an aid in invasive fungal disease diagnosis. In a meta-analysis of 11 studies, BDG sensitivity was 75%.4 In a study based on autopsy cases from 6 years, BDG specificity was 98.4% with positive and negative predictive values of 86.7% and 97.1%, respectively.3 Optimal results were achieved when 2 consecutive tests were positive.4 The serum assay output is based on spectrophotometer readings, which are converted to BDG concentrations (negative, <60 pg/mL; indeterminate, 60–79 pg/mL; positive ≥80 pg/mL).5 Although we cannot be certain, utilizing the BDG assay in our patient may have led to earlier treatment and a better outcome.
A disadvantage of the BDG assay is the potential for false-positive results, which have been reported in lung transplant recipients with respiratory mold colonization and patients with other systemic bacterial infections.4 False-positive results also have been associated with use of ampicillin-clavulanate and piperacillin-tazobactam antibiotics and human blood products, hemodialysis, and severe mucositis, thus reaffirming the importance of judicious interpretation of BDG assay results by the clinician.4,6 There also is a potential for false-negative results, as the BDG assay does not detect certain fungal species such as Cryptococcus species and Blastomyces dermatitidis, which produce very low levels of BDG, or zygomycetes (Absidia, Mucor, and Rizopus species), which are not known to produce BDG.6
Practice Implications
In the setting of invasive fungal infections, a high degree of clinical suspicion is paramount due to the often subtle nature of cutaneous manifestations. A positive BDG assay can be used to identify high-risk patients for empiric antifungal therapy, prompting early intervention and improved outcomes in these acutely ill patients. The BDG assay’s excellent negative predictive value is useful in ruling out invasive Candida infections and may justify stopping unnecessary empiric antifungal therapy.4 For the dermatology hospitalist, incorporation of the BDG assay as a noninvasive screening tool may allow for more rapid initiation of appropriate antifungal therapy while awaiting confirmatory skin biopsy or culture results in disseminated candidemia and other invasive fungal infections.
Practice Gap
Invasive fungal infections are a leading cause of morbidity and mortality among neutropenic, immunocompromised, and critically ill patients. Candida species are the most common cause of fungemia, with portals of entry into the bloodstream including the gastrointestinal tract, contaminated intravascular catheters, and localized foci of infection.1 Diagnosis of invasive candidiasis remains challenging due to an absence of specific clinical signs and symptoms, varying from a mild fever that is unresponsive to antibiotics to florid sepsis. When present, clinical clues may include chorioretinitis; muscle abscesses; and skin eruptions, characteristically with Candida tropicalis. Cutaneous manifestations of disseminated Candida infections appear in only 13% of affected patients.1 The lesions typically present as 5- to 10-mm pink dermal papules or painless pustules on an erythematous base and may be singular, localized, or diffuse in distribution. Body regions normally involved are the trunk, arms, and legs, rarely the head and neck.1 Cutaneous lesions often develop at a time when patients are febrile, are not responding to antibiotics, and are clinically deteriorating.
A 15-year-old adolescent boy with pre–B-cell acute lymphoblastic leukemia was admitted with febrile neutropenia for presumed septic shock secondary to an unknown infectious etiology. The patient was started on broad-spectrum intravenous antibiotics, and blood cultures were obtained. On the second day of hospitalization, he developed approximately 10 to 15 discrete, 3- to 6-mm, pink to violaceous papules scattered on the chest and arms (Figure 1). Over several hours, the number of lesions increased to more than 50 with involvement of the legs (Figure 2). A punch biopsy of lesional skin from the left dorsal wrist demonstrated a circumscribed abscess of yeast in the papillary dermis, which was highlighted by periodic acid–Schiff staining with minimal associated inflammation (Figure 3). Blood and tissue cultures persistently grew C tropicalis. The patient was started on intravenous liposomal amphotericin B but died on day 5 of hospitalization after developing endocarditis.



Early and reliable diagnosis of Candida species fungemia is of critical importance to successful treatment, particularly with the emergence of multidrug-resistant strains such as Candida auris.2 In patients with apparent cutaneous manifestations, a lesional punch biopsy for culture and histopathologic evaluation is recommended in addition to blood culture; however, organisms may or may not be present in large numbers, and they may be difficult to identify on routine hematoxylin and eosin–stained tissue sections. To enhance the likelihood of highlighting the fungus within the sample, the pathologist must be made aware of the presumptive diagnosis of disseminated candidiasis so that special techniques can be utilized, such as periodic acid–Schiff stain.
Although positive blood culture is the gold standard for candidemia diagnosis, only 30% to 50% of patients with disseminated candidiasis had positive blood cultures at autopsy.1 Another study showed the sensitivity of blood culture for the detection of invasive fungal infection to be as low as 8.3%.3 In cases with positive blood cultures, the median time to positivity is 2 to 3 days, but it can take as long as 8 days, thus limiting its clinical utility in acutely ill patients.4 Given the low sensitivity and prolonged time required for culture growth of most fungal organisms, novel assays for rapid, non–culture-based diagnosis of systemic fungal infections hold substantial clinical promise moving forward.
The Technique
One of the more promising non–culture-based fungal diagnostic methodologies is an antigen assay based on the detection of serum (1,3)-β-D-glucan (BDG), a major cell wall constituent of most pathogenic fungi. This assay is not specific for Candida species and can be positive for Aspergillosis species, Fusarium species, Coccidioides immitis, Histoplasma capsulatum, and Pneumocystis jirovecii pneumonia, among others; therefore, it functions as a general biomarker for fungi in the bloodstream.4,5 (1,3)-β-D-glucan assay can be useful as an adjunct for blood cultures and punch biopsy, especially when cultures are negative or the results remain outstanding. The results of the BDG assay are available in less than 24 hours at minimal cost, and the test is approved by the US Food and Drug Administration for use as an aid in invasive fungal disease diagnosis. In a meta-analysis of 11 studies, BDG sensitivity was 75%.4 In a study based on autopsy cases from 6 years, BDG specificity was 98.4% with positive and negative predictive values of 86.7% and 97.1%, respectively.3 Optimal results were achieved when 2 consecutive tests were positive.4 The serum assay output is based on spectrophotometer readings, which are converted to BDG concentrations (negative, <60 pg/mL; indeterminate, 60–79 pg/mL; positive ≥80 pg/mL).5 Although we cannot be certain, utilizing the BDG assay in our patient may have led to earlier treatment and a better outcome.
A disadvantage of the BDG assay is the potential for false-positive results, which have been reported in lung transplant recipients with respiratory mold colonization and patients with other systemic bacterial infections.4 False-positive results also have been associated with use of ampicillin-clavulanate and piperacillin-tazobactam antibiotics and human blood products, hemodialysis, and severe mucositis, thus reaffirming the importance of judicious interpretation of BDG assay results by the clinician.4,6 There also is a potential for false-negative results, as the BDG assay does not detect certain fungal species such as Cryptococcus species and Blastomyces dermatitidis, which produce very low levels of BDG, or zygomycetes (Absidia, Mucor, and Rizopus species), which are not known to produce BDG.6
Practice Implications
In the setting of invasive fungal infections, a high degree of clinical suspicion is paramount due to the often subtle nature of cutaneous manifestations. A positive BDG assay can be used to identify high-risk patients for empiric antifungal therapy, prompting early intervention and improved outcomes in these acutely ill patients. The BDG assay’s excellent negative predictive value is useful in ruling out invasive Candida infections and may justify stopping unnecessary empiric antifungal therapy.4 For the dermatology hospitalist, incorporation of the BDG assay as a noninvasive screening tool may allow for more rapid initiation of appropriate antifungal therapy while awaiting confirmatory skin biopsy or culture results in disseminated candidemia and other invasive fungal infections.
- Mays SR, Bogle MA, Bodey GP. Cutaneous fungal infections in the oncology patient: recognition and management. Am J Clin Dermatol. 2006;7:31-43.
- Candida auris. Centers for Disease Control and Prevention website. https://www.cdc.gov/fungal/candida-auris/. Updated May 15, 2020. Accessed July 10, 2020.
- Obayashi T, Negishi K, Suzuki T, et al. Reappraisal of the serum (1,3)-β-D-glucan assay for the diagnosis of invasive fungal infections—a study based on autopsy cases from 6 years. Clin Infect Dis. 2008;46:1864-1870.
- Clancy CJ, Nguyen MH. Finding the “missing 50%” of invasive candidiasis: how nonculture diagnostics will improve understanding of disease spectrum and transform patient care. Clin Infect Dis. 2013;56:1284-1292.
- McCarthy MW, Petraitiene R, Walsh TJ. Translational development and application of (1→3)-β-d-glucan for diagnosis and therapeutic monitoring of invasive mycoses [published online May 24, 2017]. Int J Mol Sci. doi:10.3390/ijms18061124.
- Beta-D glucan assay. MiraVista Diagnostics website. https://miravistalabs.com/medical-fungal-infection-testing/antigen-detection/beta-d-glucan-test/. Accessed June 5, 2020.
- Mays SR, Bogle MA, Bodey GP. Cutaneous fungal infections in the oncology patient: recognition and management. Am J Clin Dermatol. 2006;7:31-43.
- Candida auris. Centers for Disease Control and Prevention website. https://www.cdc.gov/fungal/candida-auris/. Updated May 15, 2020. Accessed July 10, 2020.
- Obayashi T, Negishi K, Suzuki T, et al. Reappraisal of the serum (1,3)-β-D-glucan assay for the diagnosis of invasive fungal infections—a study based on autopsy cases from 6 years. Clin Infect Dis. 2008;46:1864-1870.
- Clancy CJ, Nguyen MH. Finding the “missing 50%” of invasive candidiasis: how nonculture diagnostics will improve understanding of disease spectrum and transform patient care. Clin Infect Dis. 2013;56:1284-1292.
- McCarthy MW, Petraitiene R, Walsh TJ. Translational development and application of (1→3)-β-d-glucan for diagnosis and therapeutic monitoring of invasive mycoses [published online May 24, 2017]. Int J Mol Sci. doi:10.3390/ijms18061124.
- Beta-D glucan assay. MiraVista Diagnostics website. https://miravistalabs.com/medical-fungal-infection-testing/antigen-detection/beta-d-glucan-test/. Accessed June 5, 2020.