User login
COVID-19 child case count now over 400,000
according to a new report from the American Academy of Pediatrics and the Children’s Hospital Association.
The 406,000 children who have tested positive for COVID-19 represent 9.1% of all cases reported so far by 49 states (New York does not provide age distribution), New York City, the District of Columbia, Puerto Rico, and Guam. Since the proportion of child cases also was 9.1% on Aug. 6, the most recent week is the first without an increase since tracking began in mid-April, the report shows.
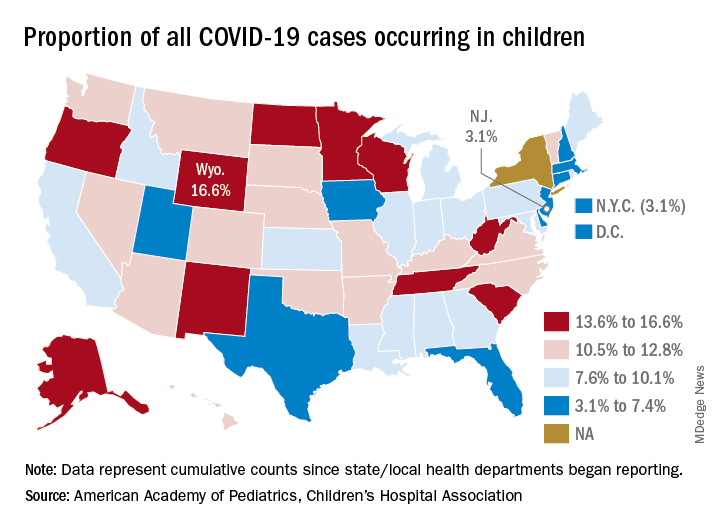
State-level data show that Wyoming has the highest percentage of child cases (16.6%) after Alabama changed its “definition of child case from 0-24 to 0-17 years, resulting in a downward revision of cumulative child cases,” the AAP and the CHA said. Alabama’s proportion of such cases dropped from 22.5% to 9.0%.
New Jersey had the lowest rate (3.1%) again this week, along with New York City, but both were up slightly from the week before, when New Jersey was at 2.9% and N.Y.C. was 3.0%. The only states, other than Alabama, that saw declines over the last week were Arkansas, Massachusetts, Mississippi, South Dakota, Texas, and West Virginia. Texas, however, has reported age for only 8% of its confirmed cases, the report noted.
The overall rate of child COVID-19 cases as of Aug. 13 was 538 per 100,000 children, up from 500.7 per 100,000 a week earlier. Arizona was again highest among the states with a rate of 1,254 per 100,000 (up from 1,206) and Vermont was lowest at 121, although Puerto Rico (114) and Guam (88) were lower still, the AAP/CHA data indicate.
For the nine states that report testing information for children, Arizona has the highest positivity rate at 18.3% and West Virginia has the lowest at 3.6%. Data on hospitalizations – available from 21 states and N.Y.C. – show that 3,849 children have been admitted, with rates varying from 0.2% of children in Hawaii to 8.8% in the Big Apple, according to the report.
More specific information on child cases, such as symptoms or underlying conditions, is not being provided by states at this time, the AAP and CHA pointed out.
according to a new report from the American Academy of Pediatrics and the Children’s Hospital Association.

The 406,000 children who have tested positive for COVID-19 represent 9.1% of all cases reported so far by 49 states (New York does not provide age distribution), New York City, the District of Columbia, Puerto Rico, and Guam. Since the proportion of child cases also was 9.1% on Aug. 6, the most recent week is the first without an increase since tracking began in mid-April, the report shows.
State-level data show that Wyoming has the highest percentage of child cases (16.6%) after Alabama changed its “definition of child case from 0-24 to 0-17 years, resulting in a downward revision of cumulative child cases,” the AAP and the CHA said. Alabama’s proportion of such cases dropped from 22.5% to 9.0%.
New Jersey had the lowest rate (3.1%) again this week, along with New York City, but both were up slightly from the week before, when New Jersey was at 2.9% and N.Y.C. was 3.0%. The only states, other than Alabama, that saw declines over the last week were Arkansas, Massachusetts, Mississippi, South Dakota, Texas, and West Virginia. Texas, however, has reported age for only 8% of its confirmed cases, the report noted.
The overall rate of child COVID-19 cases as of Aug. 13 was 538 per 100,000 children, up from 500.7 per 100,000 a week earlier. Arizona was again highest among the states with a rate of 1,254 per 100,000 (up from 1,206) and Vermont was lowest at 121, although Puerto Rico (114) and Guam (88) were lower still, the AAP/CHA data indicate.
For the nine states that report testing information for children, Arizona has the highest positivity rate at 18.3% and West Virginia has the lowest at 3.6%. Data on hospitalizations – available from 21 states and N.Y.C. – show that 3,849 children have been admitted, with rates varying from 0.2% of children in Hawaii to 8.8% in the Big Apple, according to the report.
More specific information on child cases, such as symptoms or underlying conditions, is not being provided by states at this time, the AAP and CHA pointed out.
according to a new report from the American Academy of Pediatrics and the Children’s Hospital Association.

The 406,000 children who have tested positive for COVID-19 represent 9.1% of all cases reported so far by 49 states (New York does not provide age distribution), New York City, the District of Columbia, Puerto Rico, and Guam. Since the proportion of child cases also was 9.1% on Aug. 6, the most recent week is the first without an increase since tracking began in mid-April, the report shows.
State-level data show that Wyoming has the highest percentage of child cases (16.6%) after Alabama changed its “definition of child case from 0-24 to 0-17 years, resulting in a downward revision of cumulative child cases,” the AAP and the CHA said. Alabama’s proportion of such cases dropped from 22.5% to 9.0%.
New Jersey had the lowest rate (3.1%) again this week, along with New York City, but both were up slightly from the week before, when New Jersey was at 2.9% and N.Y.C. was 3.0%. The only states, other than Alabama, that saw declines over the last week were Arkansas, Massachusetts, Mississippi, South Dakota, Texas, and West Virginia. Texas, however, has reported age for only 8% of its confirmed cases, the report noted.
The overall rate of child COVID-19 cases as of Aug. 13 was 538 per 100,000 children, up from 500.7 per 100,000 a week earlier. Arizona was again highest among the states with a rate of 1,254 per 100,000 (up from 1,206) and Vermont was lowest at 121, although Puerto Rico (114) and Guam (88) were lower still, the AAP/CHA data indicate.
For the nine states that report testing information for children, Arizona has the highest positivity rate at 18.3% and West Virginia has the lowest at 3.6%. Data on hospitalizations – available from 21 states and N.Y.C. – show that 3,849 children have been admitted, with rates varying from 0.2% of children in Hawaii to 8.8% in the Big Apple, according to the report.
More specific information on child cases, such as symptoms or underlying conditions, is not being provided by states at this time, the AAP and CHA pointed out.
COVID-19: A Dermatologist’s Experience From the US Epicenter
The 1918 H1N1 influenza pandemic was the most severe pandemic in recent history. Fifty to 100 million individuals died worldwide, with approximately 675,000 deaths in the United States.1-3 The fatality rate was approximately 2% and was highest during the second and third waves of the disease.4 At that time, there were no diagnostic tests for influenza infection, influenza vaccines, antiviral drugs, antibiotics to treat secondary bacterial infections, or mechanical ventilation. Some cities decided to close schools, limit public gatherings, self-isolate, and issue quarantine orders; the federal government took no central role.
The 1918 influenza pandemic seems far away in history, but my mother often tells me stories about her own grandmother who disliked shaking anyone’s hands and would worry when people coughed or sneezed around her. It sounded like she was overreacting. Now, we can better relate to her concerns. Life has changed dramatically.
In mid-February 2020, news spread that the coronavirus disease 2019 (COVID-19) had spread from Wuhan, China, to a number of countries in Asia and the Middle East. I was following the news with great sadness for those affected countries, especially for Iran, my country of origin, which had become an epicenter of COVID-19. We were not worried for ourselves in the United States. These infections seemed far away. However, once Italy became the new epicenter of COVID-19 with alarmingly high death rates, I grasped the inevitable reality: The novel coronavirus would not spare the United States and would not spare New York.
Then the virus arrived in New York City. On March 10, 2020, our hospital recommended using teledermatology instead of in-person visits in an attempt to keep patients safe in their own homes. Cases of COVID-19 were escalating, hospitals were filling up, health care workers were falling ill, and there was a shortage of health care staff and personal protective equipment (PPE). Dermatologists at various hospitals were asked to retrain to help care for COVID-19 patients.
On March 13, flights from Europe to the United States were suspended. A statewide stay-at-home order subsequently went into effect on March 22. It felt surreal. From March 23 on, various specialty physicians and nurses in our hospital volunteered to work as frontline staff in the newly prepared annex where patients with possible COVID-19 would arrive. My dermatology co-residents and I started working as frontline physicians. Everything we had heard from the countries affected first had become our reality. Our hospital, part of the largest public health care system in the nation, became a dedicated COVID-19 treatment center.
Large numbers of scared patients with symptoms of COVID-19 flooded the annex. We sent the majority of them home, unable to offer them even a diagnostic test, and advised them to stay isolated. We only had the capacity to test those who required hospital admission.
It broke my heart even more when my colleagues became patients. We often felt helpless, not being able to help every patient and not being able to help our infected colleagues.
Elective surgeries were suspended. Inpatient beds, including specialized intensive care unit beds, rapidly filled up with COVID-19 patients. To help with the surge of patients, our hospital added medical and intensive care unit beds. The hospital became surreal, the corridors eerily empty and silent while every bed was filled, and health care workers were rushing around the inpatient units.
Life quickly became filled with fears—worries about how sick the patients would be, how much we would be able to help them, whether we would have enough PPE, who among our friends or family might be infected next, and whether we might ourselves be next. As PPE became scarce, I desperately searched for some form of protective equipment. I hunted for protective masks, face shields, eye protection, and gowns. We had to reuse disposable N95 masks and face shields multiple times and disinfect them as best we could. Our attendings ordered any protective gear they could find for us. Nearly everything was sold out; the very few items remaining would not for arrive for months. I could have never imagined that I would be afraid of going to work, of not having the appropriate protective gear, and that any day might be my last because of my profession.
New York City had become the epicenter of COVID-19. The city, the country, and the world were in chaos. Hospitals were overflowing, and makeshift morgues were appearing outside of hospitals. Those who could fled the city. Despite warnings from experts, we were not prepared. The number of deaths was climbing rapidly. There was no clarity on who could be tested or how to get it done. It felt like a nightmare.
Social distancing was in place, nonessential businesses were shut down, street vendors disappeared, and people were advised to wear face coverings. People were afraid of each other, afraid of getting too close and catching the virus. New York City—The City That Never Sleeps—went into deep sleep. Every day brought ever greater numbers of infected patients and more deaths.
Every day at 7:00
After around 2 months of lockdown, New York City passed its peak, and the epicenter moved on. The current death toll (ie, confirmed deaths due to COVID-19) in New York stands at 18,836, while the reported death toll in the United States is 143,868, according to the Centers for Disease Control and Prevention. New York City has started a phased reopening to a new normal. Elective care has resumed, and people are leaving their homes again, eager to bring some sense of normalcy back into their lives.
I fear for those who will contract the virus in the next wave. I wonder what we will have learned.
Acknowledgment
The author wishes to thank Steven R. Feldman, MD, PhD (Winston-Salem, North Carolina), for his friendship and invaluable assistance with the conception and editing of this manuscript.
- Taubenberger JK. The origin and virulence of the 1918 “Spanish” influenza virus. Proc Am Philos Soc. 2006;150:86-112.
- Morens DM, Taubenberger JK. The mother of all pandemics is 100 years old (and going strong)! Am J Public Health. 2018;108:1449-1454.
- Johnson NPAS, Mueller J. Updating the accounts: global mortality of the 1918-1920 “Spanish” influenza pandemic. Bull Hist Med. 2002;76:105-115.
- Morens DM, Fauci AS. The 1918 influenza pandemic: insights for the 21st century. J Infect Dis. 2007;195:1018-1028.
The 1918 H1N1 influenza pandemic was the most severe pandemic in recent history. Fifty to 100 million individuals died worldwide, with approximately 675,000 deaths in the United States.1-3 The fatality rate was approximately 2% and was highest during the second and third waves of the disease.4 At that time, there were no diagnostic tests for influenza infection, influenza vaccines, antiviral drugs, antibiotics to treat secondary bacterial infections, or mechanical ventilation. Some cities decided to close schools, limit public gatherings, self-isolate, and issue quarantine orders; the federal government took no central role.
The 1918 influenza pandemic seems far away in history, but my mother often tells me stories about her own grandmother who disliked shaking anyone’s hands and would worry when people coughed or sneezed around her. It sounded like she was overreacting. Now, we can better relate to her concerns. Life has changed dramatically.
In mid-February 2020, news spread that the coronavirus disease 2019 (COVID-19) had spread from Wuhan, China, to a number of countries in Asia and the Middle East. I was following the news with great sadness for those affected countries, especially for Iran, my country of origin, which had become an epicenter of COVID-19. We were not worried for ourselves in the United States. These infections seemed far away. However, once Italy became the new epicenter of COVID-19 with alarmingly high death rates, I grasped the inevitable reality: The novel coronavirus would not spare the United States and would not spare New York.
Then the virus arrived in New York City. On March 10, 2020, our hospital recommended using teledermatology instead of in-person visits in an attempt to keep patients safe in their own homes. Cases of COVID-19 were escalating, hospitals were filling up, health care workers were falling ill, and there was a shortage of health care staff and personal protective equipment (PPE). Dermatologists at various hospitals were asked to retrain to help care for COVID-19 patients.
On March 13, flights from Europe to the United States were suspended. A statewide stay-at-home order subsequently went into effect on March 22. It felt surreal. From March 23 on, various specialty physicians and nurses in our hospital volunteered to work as frontline staff in the newly prepared annex where patients with possible COVID-19 would arrive. My dermatology co-residents and I started working as frontline physicians. Everything we had heard from the countries affected first had become our reality. Our hospital, part of the largest public health care system in the nation, became a dedicated COVID-19 treatment center.
Large numbers of scared patients with symptoms of COVID-19 flooded the annex. We sent the majority of them home, unable to offer them even a diagnostic test, and advised them to stay isolated. We only had the capacity to test those who required hospital admission.
It broke my heart even more when my colleagues became patients. We often felt helpless, not being able to help every patient and not being able to help our infected colleagues.
Elective surgeries were suspended. Inpatient beds, including specialized intensive care unit beds, rapidly filled up with COVID-19 patients. To help with the surge of patients, our hospital added medical and intensive care unit beds. The hospital became surreal, the corridors eerily empty and silent while every bed was filled, and health care workers were rushing around the inpatient units.
Life quickly became filled with fears—worries about how sick the patients would be, how much we would be able to help them, whether we would have enough PPE, who among our friends or family might be infected next, and whether we might ourselves be next. As PPE became scarce, I desperately searched for some form of protective equipment. I hunted for protective masks, face shields, eye protection, and gowns. We had to reuse disposable N95 masks and face shields multiple times and disinfect them as best we could. Our attendings ordered any protective gear they could find for us. Nearly everything was sold out; the very few items remaining would not for arrive for months. I could have never imagined that I would be afraid of going to work, of not having the appropriate protective gear, and that any day might be my last because of my profession.
New York City had become the epicenter of COVID-19. The city, the country, and the world were in chaos. Hospitals were overflowing, and makeshift morgues were appearing outside of hospitals. Those who could fled the city. Despite warnings from experts, we were not prepared. The number of deaths was climbing rapidly. There was no clarity on who could be tested or how to get it done. It felt like a nightmare.
Social distancing was in place, nonessential businesses were shut down, street vendors disappeared, and people were advised to wear face coverings. People were afraid of each other, afraid of getting too close and catching the virus. New York City—The City That Never Sleeps—went into deep sleep. Every day brought ever greater numbers of infected patients and more deaths.
Every day at 7:00
After around 2 months of lockdown, New York City passed its peak, and the epicenter moved on. The current death toll (ie, confirmed deaths due to COVID-19) in New York stands at 18,836, while the reported death toll in the United States is 143,868, according to the Centers for Disease Control and Prevention. New York City has started a phased reopening to a new normal. Elective care has resumed, and people are leaving their homes again, eager to bring some sense of normalcy back into their lives.
I fear for those who will contract the virus in the next wave. I wonder what we will have learned.
Acknowledgment
The author wishes to thank Steven R. Feldman, MD, PhD (Winston-Salem, North Carolina), for his friendship and invaluable assistance with the conception and editing of this manuscript.
The 1918 H1N1 influenza pandemic was the most severe pandemic in recent history. Fifty to 100 million individuals died worldwide, with approximately 675,000 deaths in the United States.1-3 The fatality rate was approximately 2% and was highest during the second and third waves of the disease.4 At that time, there were no diagnostic tests for influenza infection, influenza vaccines, antiviral drugs, antibiotics to treat secondary bacterial infections, or mechanical ventilation. Some cities decided to close schools, limit public gatherings, self-isolate, and issue quarantine orders; the federal government took no central role.
The 1918 influenza pandemic seems far away in history, but my mother often tells me stories about her own grandmother who disliked shaking anyone’s hands and would worry when people coughed or sneezed around her. It sounded like she was overreacting. Now, we can better relate to her concerns. Life has changed dramatically.
In mid-February 2020, news spread that the coronavirus disease 2019 (COVID-19) had spread from Wuhan, China, to a number of countries in Asia and the Middle East. I was following the news with great sadness for those affected countries, especially for Iran, my country of origin, which had become an epicenter of COVID-19. We were not worried for ourselves in the United States. These infections seemed far away. However, once Italy became the new epicenter of COVID-19 with alarmingly high death rates, I grasped the inevitable reality: The novel coronavirus would not spare the United States and would not spare New York.
Then the virus arrived in New York City. On March 10, 2020, our hospital recommended using teledermatology instead of in-person visits in an attempt to keep patients safe in their own homes. Cases of COVID-19 were escalating, hospitals were filling up, health care workers were falling ill, and there was a shortage of health care staff and personal protective equipment (PPE). Dermatologists at various hospitals were asked to retrain to help care for COVID-19 patients.
On March 13, flights from Europe to the United States were suspended. A statewide stay-at-home order subsequently went into effect on March 22. It felt surreal. From March 23 on, various specialty physicians and nurses in our hospital volunteered to work as frontline staff in the newly prepared annex where patients with possible COVID-19 would arrive. My dermatology co-residents and I started working as frontline physicians. Everything we had heard from the countries affected first had become our reality. Our hospital, part of the largest public health care system in the nation, became a dedicated COVID-19 treatment center.
Large numbers of scared patients with symptoms of COVID-19 flooded the annex. We sent the majority of them home, unable to offer them even a diagnostic test, and advised them to stay isolated. We only had the capacity to test those who required hospital admission.
It broke my heart even more when my colleagues became patients. We often felt helpless, not being able to help every patient and not being able to help our infected colleagues.
Elective surgeries were suspended. Inpatient beds, including specialized intensive care unit beds, rapidly filled up with COVID-19 patients. To help with the surge of patients, our hospital added medical and intensive care unit beds. The hospital became surreal, the corridors eerily empty and silent while every bed was filled, and health care workers were rushing around the inpatient units.
Life quickly became filled with fears—worries about how sick the patients would be, how much we would be able to help them, whether we would have enough PPE, who among our friends or family might be infected next, and whether we might ourselves be next. As PPE became scarce, I desperately searched for some form of protective equipment. I hunted for protective masks, face shields, eye protection, and gowns. We had to reuse disposable N95 masks and face shields multiple times and disinfect them as best we could. Our attendings ordered any protective gear they could find for us. Nearly everything was sold out; the very few items remaining would not for arrive for months. I could have never imagined that I would be afraid of going to work, of not having the appropriate protective gear, and that any day might be my last because of my profession.
New York City had become the epicenter of COVID-19. The city, the country, and the world were in chaos. Hospitals were overflowing, and makeshift morgues were appearing outside of hospitals. Those who could fled the city. Despite warnings from experts, we were not prepared. The number of deaths was climbing rapidly. There was no clarity on who could be tested or how to get it done. It felt like a nightmare.
Social distancing was in place, nonessential businesses were shut down, street vendors disappeared, and people were advised to wear face coverings. People were afraid of each other, afraid of getting too close and catching the virus. New York City—The City That Never Sleeps—went into deep sleep. Every day brought ever greater numbers of infected patients and more deaths.
Every day at 7:00
After around 2 months of lockdown, New York City passed its peak, and the epicenter moved on. The current death toll (ie, confirmed deaths due to COVID-19) in New York stands at 18,836, while the reported death toll in the United States is 143,868, according to the Centers for Disease Control and Prevention. New York City has started a phased reopening to a new normal. Elective care has resumed, and people are leaving their homes again, eager to bring some sense of normalcy back into their lives.
I fear for those who will contract the virus in the next wave. I wonder what we will have learned.
Acknowledgment
The author wishes to thank Steven R. Feldman, MD, PhD (Winston-Salem, North Carolina), for his friendship and invaluable assistance with the conception and editing of this manuscript.
- Taubenberger JK. The origin and virulence of the 1918 “Spanish” influenza virus. Proc Am Philos Soc. 2006;150:86-112.
- Morens DM, Taubenberger JK. The mother of all pandemics is 100 years old (and going strong)! Am J Public Health. 2018;108:1449-1454.
- Johnson NPAS, Mueller J. Updating the accounts: global mortality of the 1918-1920 “Spanish” influenza pandemic. Bull Hist Med. 2002;76:105-115.
- Morens DM, Fauci AS. The 1918 influenza pandemic: insights for the 21st century. J Infect Dis. 2007;195:1018-1028.
- Taubenberger JK. The origin and virulence of the 1918 “Spanish” influenza virus. Proc Am Philos Soc. 2006;150:86-112.
- Morens DM, Taubenberger JK. The mother of all pandemics is 100 years old (and going strong)! Am J Public Health. 2018;108:1449-1454.
- Johnson NPAS, Mueller J. Updating the accounts: global mortality of the 1918-1920 “Spanish” influenza pandemic. Bull Hist Med. 2002;76:105-115.
- Morens DM, Fauci AS. The 1918 influenza pandemic: insights for the 21st century. J Infect Dis. 2007;195:1018-1028.
Practice Points
- Coronavirus disease 2019 (COVID-19) can spread quickly, creating chaos in the health care system and leading to critical supply shortages within a short amount of time.
- Social distancing, quarantine, and isolation appear to be powerful tools in reducing the spread of COVID-19.
Severe obesity ups risk for death in younger men with COVID-19
In a large California health care plan, among patients with COVID-19, men aged 60 years and younger had a much higher risk of dying within 3 weeks of diagnosis if they had severe obesity as opposed to being of normal weight, independently of other risk factors.
reported Sara Y. Tartof, PhD, MPH, Kaiser Permanente Southern California, Pasadena, Calif., and coauthors.
The data “highlight the leading role of severe obesity over correlated risk factors, providing a target for early intervention,” they concluded in an article published online Aug. 12 in Annals of Internal Medicine.
This work adds to nearly 300 articles that have shown that severe obesity is associated with an increased risk for morbidity and mortality from COVID-19.
In an accompanying editorial, David A. Kass, MD, said: “Consistency of this new study and prior research should put to rest the contention that obesity is common in severe COVID-19 because it is common in the population.”
Rather, these findings show that “obesity is an important independent risk factor for serious COVID-19 disease,” he pointed out.
On the basis of this evidence, “arguably the hardest question to answer is: What is to be done?” wondered Kass, of Johns Hopkins University, Baltimore.
Although data consistently show that a body mass index >35 kg/m2 is predictive of major health risks, “weight reduction at that level of obesity is difficult and certainly is not achieved rapidly,” Dr. Kass stressed.
“Therefore ... social distancing; altering behaviors to reduce viral exposure and transmission, such as wearing masks; and instituting policies and health care approaches that recognize the potential effects of obesity should be implemented,” he emphasized. “These actions should help and are certainly doable.”
Similarly, Dr. Tartof and colleagues said their “findings also reveal the distressing collision of two pandemics: COVID-19 and obesity.
“As COVID-19 continues to spread unabated, we must focus our immediate efforts on containing the crisis at hand,” they urged.
However, the findings also “underscore the need for future collective efforts to combat the equally devastating, and potentially synergistic, force of the obesity epidemic.”
COVID-19 pandemic collides with obesity epidemic
Previous studies of obesity and COVID-19 were small, did not adjust for multiple confounders, or did not include nonhospitalized patients, Dr. Tartof and coauthors wrote.
Their study included 6,916 members of the Kaiser Permanente Southern California health care plan who were diagnosed with COVID-19 from Feb. 13 to May 2, 2020.
The researchers calculated the risk for death at 21 days after a COVID-19 diagnosis; findings were corrected for age, sex, race/ethnicity, smoking, myocardial infarction, heart failure, peripheral vascular disease, cerebrovascular disease, chronic pulmonary disease, renal disease, metastatic tumor or malignancy, other immune disease, hyperlipidemia, hypertension, asthma, organ transplant, and diabetes status.
On the basis of BMI, the patients were classified as being underweight, of normal weight, overweight, or as having class 1, 2, or 3 obesity. BMI of 18.5 to 24 kg/m2 is defined as normal weight.
Class 3 obesity, also called severe obesity, included moderately severe obesity (BMI, 40-44 kg/m2) and extremely severe obesity (≥45 kg/m2).
A little more than half of the patients were women (55%), and more than 50% were Hispanic (54%).
A total of 206 patients (3%) died within 21 days of being diagnosed with COVID-19; of these, 67% had been hospitalized, and 43% had been intubated.
Overall, the COVID-19 patients with moderately severe or extremely severe obesity had a 2.7-fold and 4.2-fold increased risk for death, respectively, within 3 weeks compared with patients of normal weight.
Patients in the other BMI categories did not have a significantly higher risk of dying during follow-up.
However, each decade of increasing age after age 40 was associated with a stepwise increased risk for death within 3 weeks of the COVID-19 diagnosis.
Risk stratified by age and sex
Further analysis showed that, “most strikingly,” among patients aged 60 and younger, those with moderately severe obesity and extremely severe obesity had significant 17-fold and 12-fold higher risks of dying during follow-up, respectively, compared with patients of normal weight, the researchers reported.
In patients older than 60, moderately severe obesity did not confer a significant increased risk for imminent death from COVID-19; extremely severe obesity conferred a smaller, threefold increased risk for this.
“Our finding that severe obesity, particularly among younger patients, eclipses the mortality risk posed by other obesity-related conditions, such as history of myocardial infarction (MI), diabetes, hypertension, or hyperlipidemia, suggests a significant pathophysiologic link between excess adiposity and severe COVID-19 illness,” the researchers noted.
This independent increased risk for death with severe obesity was seen in men but not in women.
Men with moderately severe and extremely severe obesity had significant 4.8-fold and 10-fold higher risks of dying within 3 weeks, respectively, compared with men of normal weight.
“That the risks are higher in younger patients is probably not because obesity is particularly damaging in this age group; it is more likely that other serious comorbidities that evolve later in life take over as dominant risk factors,” Dr. Kass suggested in his editorial.
“That males are particularly affected may reflect their greater visceral adiposity over females, given that this fat is notably proinflammatory and contributes to metabolic and vascular disease,” he added.
“As a cardiologist who studies heart failure,” Dr. Kass wrote, “I am struck by how many of the mechanisms that are mentioned in reviews of obesity risk and heart disease are also mentioned in reviews of obesity and COVID-19.”
The study was funded by Roche-Genentech. Kass has disclosed no relevant financial relationships. Disclosures of the authors are listed in the article.
A version of this article originally appeared on Medscape.com.
In a large California health care plan, among patients with COVID-19, men aged 60 years and younger had a much higher risk of dying within 3 weeks of diagnosis if they had severe obesity as opposed to being of normal weight, independently of other risk factors.
reported Sara Y. Tartof, PhD, MPH, Kaiser Permanente Southern California, Pasadena, Calif., and coauthors.
The data “highlight the leading role of severe obesity over correlated risk factors, providing a target for early intervention,” they concluded in an article published online Aug. 12 in Annals of Internal Medicine.
This work adds to nearly 300 articles that have shown that severe obesity is associated with an increased risk for morbidity and mortality from COVID-19.
In an accompanying editorial, David A. Kass, MD, said: “Consistency of this new study and prior research should put to rest the contention that obesity is common in severe COVID-19 because it is common in the population.”
Rather, these findings show that “obesity is an important independent risk factor for serious COVID-19 disease,” he pointed out.
On the basis of this evidence, “arguably the hardest question to answer is: What is to be done?” wondered Kass, of Johns Hopkins University, Baltimore.
Although data consistently show that a body mass index >35 kg/m2 is predictive of major health risks, “weight reduction at that level of obesity is difficult and certainly is not achieved rapidly,” Dr. Kass stressed.
“Therefore ... social distancing; altering behaviors to reduce viral exposure and transmission, such as wearing masks; and instituting policies and health care approaches that recognize the potential effects of obesity should be implemented,” he emphasized. “These actions should help and are certainly doable.”
Similarly, Dr. Tartof and colleagues said their “findings also reveal the distressing collision of two pandemics: COVID-19 and obesity.
“As COVID-19 continues to spread unabated, we must focus our immediate efforts on containing the crisis at hand,” they urged.
However, the findings also “underscore the need for future collective efforts to combat the equally devastating, and potentially synergistic, force of the obesity epidemic.”
COVID-19 pandemic collides with obesity epidemic
Previous studies of obesity and COVID-19 were small, did not adjust for multiple confounders, or did not include nonhospitalized patients, Dr. Tartof and coauthors wrote.
Their study included 6,916 members of the Kaiser Permanente Southern California health care plan who were diagnosed with COVID-19 from Feb. 13 to May 2, 2020.
The researchers calculated the risk for death at 21 days after a COVID-19 diagnosis; findings were corrected for age, sex, race/ethnicity, smoking, myocardial infarction, heart failure, peripheral vascular disease, cerebrovascular disease, chronic pulmonary disease, renal disease, metastatic tumor or malignancy, other immune disease, hyperlipidemia, hypertension, asthma, organ transplant, and diabetes status.
On the basis of BMI, the patients were classified as being underweight, of normal weight, overweight, or as having class 1, 2, or 3 obesity. BMI of 18.5 to 24 kg/m2 is defined as normal weight.
Class 3 obesity, also called severe obesity, included moderately severe obesity (BMI, 40-44 kg/m2) and extremely severe obesity (≥45 kg/m2).
A little more than half of the patients were women (55%), and more than 50% were Hispanic (54%).
A total of 206 patients (3%) died within 21 days of being diagnosed with COVID-19; of these, 67% had been hospitalized, and 43% had been intubated.
Overall, the COVID-19 patients with moderately severe or extremely severe obesity had a 2.7-fold and 4.2-fold increased risk for death, respectively, within 3 weeks compared with patients of normal weight.
Patients in the other BMI categories did not have a significantly higher risk of dying during follow-up.
However, each decade of increasing age after age 40 was associated with a stepwise increased risk for death within 3 weeks of the COVID-19 diagnosis.
Risk stratified by age and sex
Further analysis showed that, “most strikingly,” among patients aged 60 and younger, those with moderately severe obesity and extremely severe obesity had significant 17-fold and 12-fold higher risks of dying during follow-up, respectively, compared with patients of normal weight, the researchers reported.
In patients older than 60, moderately severe obesity did not confer a significant increased risk for imminent death from COVID-19; extremely severe obesity conferred a smaller, threefold increased risk for this.
“Our finding that severe obesity, particularly among younger patients, eclipses the mortality risk posed by other obesity-related conditions, such as history of myocardial infarction (MI), diabetes, hypertension, or hyperlipidemia, suggests a significant pathophysiologic link between excess adiposity and severe COVID-19 illness,” the researchers noted.
This independent increased risk for death with severe obesity was seen in men but not in women.
Men with moderately severe and extremely severe obesity had significant 4.8-fold and 10-fold higher risks of dying within 3 weeks, respectively, compared with men of normal weight.
“That the risks are higher in younger patients is probably not because obesity is particularly damaging in this age group; it is more likely that other serious comorbidities that evolve later in life take over as dominant risk factors,” Dr. Kass suggested in his editorial.
“That males are particularly affected may reflect their greater visceral adiposity over females, given that this fat is notably proinflammatory and contributes to metabolic and vascular disease,” he added.
“As a cardiologist who studies heart failure,” Dr. Kass wrote, “I am struck by how many of the mechanisms that are mentioned in reviews of obesity risk and heart disease are also mentioned in reviews of obesity and COVID-19.”
The study was funded by Roche-Genentech. Kass has disclosed no relevant financial relationships. Disclosures of the authors are listed in the article.
A version of this article originally appeared on Medscape.com.
In a large California health care plan, among patients with COVID-19, men aged 60 years and younger had a much higher risk of dying within 3 weeks of diagnosis if they had severe obesity as opposed to being of normal weight, independently of other risk factors.
reported Sara Y. Tartof, PhD, MPH, Kaiser Permanente Southern California, Pasadena, Calif., and coauthors.
The data “highlight the leading role of severe obesity over correlated risk factors, providing a target for early intervention,” they concluded in an article published online Aug. 12 in Annals of Internal Medicine.
This work adds to nearly 300 articles that have shown that severe obesity is associated with an increased risk for morbidity and mortality from COVID-19.
In an accompanying editorial, David A. Kass, MD, said: “Consistency of this new study and prior research should put to rest the contention that obesity is common in severe COVID-19 because it is common in the population.”
Rather, these findings show that “obesity is an important independent risk factor for serious COVID-19 disease,” he pointed out.
On the basis of this evidence, “arguably the hardest question to answer is: What is to be done?” wondered Kass, of Johns Hopkins University, Baltimore.
Although data consistently show that a body mass index >35 kg/m2 is predictive of major health risks, “weight reduction at that level of obesity is difficult and certainly is not achieved rapidly,” Dr. Kass stressed.
“Therefore ... social distancing; altering behaviors to reduce viral exposure and transmission, such as wearing masks; and instituting policies and health care approaches that recognize the potential effects of obesity should be implemented,” he emphasized. “These actions should help and are certainly doable.”
Similarly, Dr. Tartof and colleagues said their “findings also reveal the distressing collision of two pandemics: COVID-19 and obesity.
“As COVID-19 continues to spread unabated, we must focus our immediate efforts on containing the crisis at hand,” they urged.
However, the findings also “underscore the need for future collective efforts to combat the equally devastating, and potentially synergistic, force of the obesity epidemic.”
COVID-19 pandemic collides with obesity epidemic
Previous studies of obesity and COVID-19 were small, did not adjust for multiple confounders, or did not include nonhospitalized patients, Dr. Tartof and coauthors wrote.
Their study included 6,916 members of the Kaiser Permanente Southern California health care plan who were diagnosed with COVID-19 from Feb. 13 to May 2, 2020.
The researchers calculated the risk for death at 21 days after a COVID-19 diagnosis; findings were corrected for age, sex, race/ethnicity, smoking, myocardial infarction, heart failure, peripheral vascular disease, cerebrovascular disease, chronic pulmonary disease, renal disease, metastatic tumor or malignancy, other immune disease, hyperlipidemia, hypertension, asthma, organ transplant, and diabetes status.
On the basis of BMI, the patients were classified as being underweight, of normal weight, overweight, or as having class 1, 2, or 3 obesity. BMI of 18.5 to 24 kg/m2 is defined as normal weight.
Class 3 obesity, also called severe obesity, included moderately severe obesity (BMI, 40-44 kg/m2) and extremely severe obesity (≥45 kg/m2).
A little more than half of the patients were women (55%), and more than 50% were Hispanic (54%).
A total of 206 patients (3%) died within 21 days of being diagnosed with COVID-19; of these, 67% had been hospitalized, and 43% had been intubated.
Overall, the COVID-19 patients with moderately severe or extremely severe obesity had a 2.7-fold and 4.2-fold increased risk for death, respectively, within 3 weeks compared with patients of normal weight.
Patients in the other BMI categories did not have a significantly higher risk of dying during follow-up.
However, each decade of increasing age after age 40 was associated with a stepwise increased risk for death within 3 weeks of the COVID-19 diagnosis.
Risk stratified by age and sex
Further analysis showed that, “most strikingly,” among patients aged 60 and younger, those with moderately severe obesity and extremely severe obesity had significant 17-fold and 12-fold higher risks of dying during follow-up, respectively, compared with patients of normal weight, the researchers reported.
In patients older than 60, moderately severe obesity did not confer a significant increased risk for imminent death from COVID-19; extremely severe obesity conferred a smaller, threefold increased risk for this.
“Our finding that severe obesity, particularly among younger patients, eclipses the mortality risk posed by other obesity-related conditions, such as history of myocardial infarction (MI), diabetes, hypertension, or hyperlipidemia, suggests a significant pathophysiologic link between excess adiposity and severe COVID-19 illness,” the researchers noted.
This independent increased risk for death with severe obesity was seen in men but not in women.
Men with moderately severe and extremely severe obesity had significant 4.8-fold and 10-fold higher risks of dying within 3 weeks, respectively, compared with men of normal weight.
“That the risks are higher in younger patients is probably not because obesity is particularly damaging in this age group; it is more likely that other serious comorbidities that evolve later in life take over as dominant risk factors,” Dr. Kass suggested in his editorial.
“That males are particularly affected may reflect their greater visceral adiposity over females, given that this fat is notably proinflammatory and contributes to metabolic and vascular disease,” he added.
“As a cardiologist who studies heart failure,” Dr. Kass wrote, “I am struck by how many of the mechanisms that are mentioned in reviews of obesity risk and heart disease are also mentioned in reviews of obesity and COVID-19.”
The study was funded by Roche-Genentech. Kass has disclosed no relevant financial relationships. Disclosures of the authors are listed in the article.
A version of this article originally appeared on Medscape.com.
Pooled COVID-19 testing feasible, greatly reduces supply use
‘Straightforward, cost effective, and efficient’
Combining specimens from several low-risk inpatients in a single test for SARS-CoV-2 infection allowed hospital staff to stretch testing supplies and provide test results quickly for many more patients than they might have otherwise, researchers found.
“We believe this strategy conserved [personal protective equipment (PPE)], led to a marked reduction in staff and patient anxiety, and improved patient care,” wrote David Mastrianni, MD, and colleagues from Saratoga Hospital in Saratoga Springs, N.Y. “Our impression is that testing all admitted patients has also been reassuring to our community.”
The researchers published their findings July 20 in the Journal of Hospital Medicine.
“What was really important about this study was they were actually able to implement pooled testing after communication with the [Food and Drug Administration],” Samir S. Shah, MD, MSCE, SFHM, the journal’s editor-in-chief, said in an interview.
“Pooled testing combines samples from multiple people within a single test. The benefit is, if the test is negative [you know that] everyone whose sample was combined … is negative. So you’ve effectively tested anywhere from three to five people with the resources required for only one test,” Dr. Shah continued.
The challenge is that, if the test is positive, everyone in that testing group must be retested individually because one or more of them has the infection, said Dr. Shah, director of hospital medicine at Cincinnati Children’s Hospital Medical Center.
Dr. Mastrianni said early in the pandemic they started getting the “New York surge” at their hospital, located approximately 3 hours from New York City. They wanted to test all of the inpatients at their hospital for COVID-19 and they had a rapid in-house test that worked well, “but we just didn’t have enough cartridges, and we couldn’t get deliveries, and we started pooling.” In fact, they ran out of testing supplies at one point during the study but were able to replenish their supply in about a day, he noted.
For the current study, all patients admitted to the hospital, including those admitted for observation, underwent testing for SARS-CoV-2. Staff in the emergency department designated patients as low risk if they had no symptoms or other clinical evidence of COVID-19; those patients underwent pooled testing.
Patients with clinical evidence of COVID-19, such as respiratory symptoms or laboratory or radiographic findings consistent with infection, were considered high risk and were tested on an individual basis and thus excluded from the current analysis.
The pooled testing strategy required some patients to be held in the emergency department until there were three available for pooled testing. On several occasions when this was not practical, specimens from two patients were pooled.
Between April 17 and May 11, clinicians tested 530 patients via pooled testing using 179 cartridges (172 with swabs from three patients and 7 with swabs from two patients). There were four positive pooled tests, which necessitated the use of an additional 11 cartridges. Overall, the testing used 190 cartridges, which is 340 fewer than would have been used if all patients had been tested individually.
Among the low-risk patients, the positive rate was 0.8% (4/530). No patients from pools that were negative tested positive later during their hospitalization or developed evidence of the infection.
Team effort, flexibility needed
Dr. Mastrianni said he expected their study to find that pooled testing saved testing resources, but he “was surprised by the complexity of the logistics in the hospital, and how it really required getting everybody to work together. …There were a lot of details, and it really took a lot of teamwork.”
The nursing supervisor in the emergency department was in charge of the batch and coordinated with the laboratory, he explained. There were many moving parts to manage, including monitoring how many patients were being admitted, what their conditions were, whether they were high or low risk, and where they would house those patients as the emergency department became increasingly busy. “It’s a lot for them, but they’ve adapted really well,” Dr. Mastrianni said.
Pooling tests seems to work best for three to five patients at a time; larger batches increase the chance of having a positive test, and thus identifying the sick individual(s) becomes more challenging and expensive, Dr. Shah said.
“It’s a fine line between having a pool large enough that you save on testing supplies and testing costs but not having the pool so large that you dramatically increase your likelihood of having a positive test,” Dr. Shah said.
Hospitals will likely need to be flexible and adapt as the local positivity rate changes and supply levels vary, according to the authors.
“Pooled testing is mainly dependent on the COVID-19 positive rate in the population of interest in addition to the sensitivity of the [reverse transcriptase-polymerase chain reaction (RT-PCR)] method used for COVID-19 testing,” said Baha Abdalhamid, MD, PhD, of the department of pathology and microbiology at the University of Nebraska Medical Center in Omaha.
“Each laboratory and hospital needs to do their own validation testing because it is dependent on the positive rate of COVID-19,” added Dr. Abdalhamid, who was not involved in the current study.
It’s important for clinicians to “do a good history to find who’s high risk and who’s low risk,” Dr. Mastrianni said. Clinicians also need to remember that, although a patient may test negative initially, they may still have COVID-19, he warned. That test reflects a single point in time, and a patient could be infected and not yet be ill, so clinicians need to be alert to a change in the patient’s status.
Best for settings with low-risk individuals
“Pooled COVID-19 testing is a straightforward, cost-effective, and efficient approach,” Dr. Abdalhamid said. He and his colleagues found pooled testing could increase testing capability by 69% or more when the incidence rate of SARS-CoV-2 infection is 10% or lower.
He said the approach would be helpful in other settings “as long as the positive rate is equal to or less than 10%. Asymptomatic population or surveillance groups such as students, athletes, and military service members are [an] interesting population to test using pooling testing because we expect these populations to have low positive rates, which makes pooled testing ideal.”
Benefit outweighs risk
“There is risk of missing specimens with low concentration of the virus,” Dr. Abdalhamid cautioned. “These specimens might be missed due to the dilution factor of pooling [false-negative specimens]. We did not have a single false-negative specimen in our proof-of-concept study. In addition, there are practical approaches to deal with false-negative pooled specimens.
“The benefit definitely outweighs the risk of false-negative specimens because false-negative results rarely occur, if any. In addition, there is significant saving of time, reagents, and supplies in [a] pooled specimens approach as well as expansion of the test for higher number of patients,” Dr. Abdalhamid continued.
Dr. Mastrianni’s hospital currently has enough testing cartridges, but they are continuing to conduct pooled testing to conserve resources for the benefit of their own hospital and for the nation as a whole, he said.
The authors have disclosed no relevant financial relationships. Dr. Abdalhamid and Dr. Shah have disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
‘Straightforward, cost effective, and efficient’
‘Straightforward, cost effective, and efficient’
Combining specimens from several low-risk inpatients in a single test for SARS-CoV-2 infection allowed hospital staff to stretch testing supplies and provide test results quickly for many more patients than they might have otherwise, researchers found.
“We believe this strategy conserved [personal protective equipment (PPE)], led to a marked reduction in staff and patient anxiety, and improved patient care,” wrote David Mastrianni, MD, and colleagues from Saratoga Hospital in Saratoga Springs, N.Y. “Our impression is that testing all admitted patients has also been reassuring to our community.”
The researchers published their findings July 20 in the Journal of Hospital Medicine.
“What was really important about this study was they were actually able to implement pooled testing after communication with the [Food and Drug Administration],” Samir S. Shah, MD, MSCE, SFHM, the journal’s editor-in-chief, said in an interview.
“Pooled testing combines samples from multiple people within a single test. The benefit is, if the test is negative [you know that] everyone whose sample was combined … is negative. So you’ve effectively tested anywhere from three to five people with the resources required for only one test,” Dr. Shah continued.
The challenge is that, if the test is positive, everyone in that testing group must be retested individually because one or more of them has the infection, said Dr. Shah, director of hospital medicine at Cincinnati Children’s Hospital Medical Center.
Dr. Mastrianni said early in the pandemic they started getting the “New York surge” at their hospital, located approximately 3 hours from New York City. They wanted to test all of the inpatients at their hospital for COVID-19 and they had a rapid in-house test that worked well, “but we just didn’t have enough cartridges, and we couldn’t get deliveries, and we started pooling.” In fact, they ran out of testing supplies at one point during the study but were able to replenish their supply in about a day, he noted.
For the current study, all patients admitted to the hospital, including those admitted for observation, underwent testing for SARS-CoV-2. Staff in the emergency department designated patients as low risk if they had no symptoms or other clinical evidence of COVID-19; those patients underwent pooled testing.
Patients with clinical evidence of COVID-19, such as respiratory symptoms or laboratory or radiographic findings consistent with infection, were considered high risk and were tested on an individual basis and thus excluded from the current analysis.
The pooled testing strategy required some patients to be held in the emergency department until there were three available for pooled testing. On several occasions when this was not practical, specimens from two patients were pooled.
Between April 17 and May 11, clinicians tested 530 patients via pooled testing using 179 cartridges (172 with swabs from three patients and 7 with swabs from two patients). There were four positive pooled tests, which necessitated the use of an additional 11 cartridges. Overall, the testing used 190 cartridges, which is 340 fewer than would have been used if all patients had been tested individually.
Among the low-risk patients, the positive rate was 0.8% (4/530). No patients from pools that were negative tested positive later during their hospitalization or developed evidence of the infection.
Team effort, flexibility needed
Dr. Mastrianni said he expected their study to find that pooled testing saved testing resources, but he “was surprised by the complexity of the logistics in the hospital, and how it really required getting everybody to work together. …There were a lot of details, and it really took a lot of teamwork.”
The nursing supervisor in the emergency department was in charge of the batch and coordinated with the laboratory, he explained. There were many moving parts to manage, including monitoring how many patients were being admitted, what their conditions were, whether they were high or low risk, and where they would house those patients as the emergency department became increasingly busy. “It’s a lot for them, but they’ve adapted really well,” Dr. Mastrianni said.
Pooling tests seems to work best for three to five patients at a time; larger batches increase the chance of having a positive test, and thus identifying the sick individual(s) becomes more challenging and expensive, Dr. Shah said.
“It’s a fine line between having a pool large enough that you save on testing supplies and testing costs but not having the pool so large that you dramatically increase your likelihood of having a positive test,” Dr. Shah said.
Hospitals will likely need to be flexible and adapt as the local positivity rate changes and supply levels vary, according to the authors.
“Pooled testing is mainly dependent on the COVID-19 positive rate in the population of interest in addition to the sensitivity of the [reverse transcriptase-polymerase chain reaction (RT-PCR)] method used for COVID-19 testing,” said Baha Abdalhamid, MD, PhD, of the department of pathology and microbiology at the University of Nebraska Medical Center in Omaha.
“Each laboratory and hospital needs to do their own validation testing because it is dependent on the positive rate of COVID-19,” added Dr. Abdalhamid, who was not involved in the current study.
It’s important for clinicians to “do a good history to find who’s high risk and who’s low risk,” Dr. Mastrianni said. Clinicians also need to remember that, although a patient may test negative initially, they may still have COVID-19, he warned. That test reflects a single point in time, and a patient could be infected and not yet be ill, so clinicians need to be alert to a change in the patient’s status.
Best for settings with low-risk individuals
“Pooled COVID-19 testing is a straightforward, cost-effective, and efficient approach,” Dr. Abdalhamid said. He and his colleagues found pooled testing could increase testing capability by 69% or more when the incidence rate of SARS-CoV-2 infection is 10% or lower.
He said the approach would be helpful in other settings “as long as the positive rate is equal to or less than 10%. Asymptomatic population or surveillance groups such as students, athletes, and military service members are [an] interesting population to test using pooling testing because we expect these populations to have low positive rates, which makes pooled testing ideal.”
Benefit outweighs risk
“There is risk of missing specimens with low concentration of the virus,” Dr. Abdalhamid cautioned. “These specimens might be missed due to the dilution factor of pooling [false-negative specimens]. We did not have a single false-negative specimen in our proof-of-concept study. In addition, there are practical approaches to deal with false-negative pooled specimens.
“The benefit definitely outweighs the risk of false-negative specimens because false-negative results rarely occur, if any. In addition, there is significant saving of time, reagents, and supplies in [a] pooled specimens approach as well as expansion of the test for higher number of patients,” Dr. Abdalhamid continued.
Dr. Mastrianni’s hospital currently has enough testing cartridges, but they are continuing to conduct pooled testing to conserve resources for the benefit of their own hospital and for the nation as a whole, he said.
The authors have disclosed no relevant financial relationships. Dr. Abdalhamid and Dr. Shah have disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
Combining specimens from several low-risk inpatients in a single test for SARS-CoV-2 infection allowed hospital staff to stretch testing supplies and provide test results quickly for many more patients than they might have otherwise, researchers found.
“We believe this strategy conserved [personal protective equipment (PPE)], led to a marked reduction in staff and patient anxiety, and improved patient care,” wrote David Mastrianni, MD, and colleagues from Saratoga Hospital in Saratoga Springs, N.Y. “Our impression is that testing all admitted patients has also been reassuring to our community.”
The researchers published their findings July 20 in the Journal of Hospital Medicine.
“What was really important about this study was they were actually able to implement pooled testing after communication with the [Food and Drug Administration],” Samir S. Shah, MD, MSCE, SFHM, the journal’s editor-in-chief, said in an interview.
“Pooled testing combines samples from multiple people within a single test. The benefit is, if the test is negative [you know that] everyone whose sample was combined … is negative. So you’ve effectively tested anywhere from three to five people with the resources required for only one test,” Dr. Shah continued.
The challenge is that, if the test is positive, everyone in that testing group must be retested individually because one or more of them has the infection, said Dr. Shah, director of hospital medicine at Cincinnati Children’s Hospital Medical Center.
Dr. Mastrianni said early in the pandemic they started getting the “New York surge” at their hospital, located approximately 3 hours from New York City. They wanted to test all of the inpatients at their hospital for COVID-19 and they had a rapid in-house test that worked well, “but we just didn’t have enough cartridges, and we couldn’t get deliveries, and we started pooling.” In fact, they ran out of testing supplies at one point during the study but were able to replenish their supply in about a day, he noted.
For the current study, all patients admitted to the hospital, including those admitted for observation, underwent testing for SARS-CoV-2. Staff in the emergency department designated patients as low risk if they had no symptoms or other clinical evidence of COVID-19; those patients underwent pooled testing.
Patients with clinical evidence of COVID-19, such as respiratory symptoms or laboratory or radiographic findings consistent with infection, were considered high risk and were tested on an individual basis and thus excluded from the current analysis.
The pooled testing strategy required some patients to be held in the emergency department until there were three available for pooled testing. On several occasions when this was not practical, specimens from two patients were pooled.
Between April 17 and May 11, clinicians tested 530 patients via pooled testing using 179 cartridges (172 with swabs from three patients and 7 with swabs from two patients). There were four positive pooled tests, which necessitated the use of an additional 11 cartridges. Overall, the testing used 190 cartridges, which is 340 fewer than would have been used if all patients had been tested individually.
Among the low-risk patients, the positive rate was 0.8% (4/530). No patients from pools that were negative tested positive later during their hospitalization or developed evidence of the infection.
Team effort, flexibility needed
Dr. Mastrianni said he expected their study to find that pooled testing saved testing resources, but he “was surprised by the complexity of the logistics in the hospital, and how it really required getting everybody to work together. …There were a lot of details, and it really took a lot of teamwork.”
The nursing supervisor in the emergency department was in charge of the batch and coordinated with the laboratory, he explained. There were many moving parts to manage, including monitoring how many patients were being admitted, what their conditions were, whether they were high or low risk, and where they would house those patients as the emergency department became increasingly busy. “It’s a lot for them, but they’ve adapted really well,” Dr. Mastrianni said.
Pooling tests seems to work best for three to five patients at a time; larger batches increase the chance of having a positive test, and thus identifying the sick individual(s) becomes more challenging and expensive, Dr. Shah said.
“It’s a fine line between having a pool large enough that you save on testing supplies and testing costs but not having the pool so large that you dramatically increase your likelihood of having a positive test,” Dr. Shah said.
Hospitals will likely need to be flexible and adapt as the local positivity rate changes and supply levels vary, according to the authors.
“Pooled testing is mainly dependent on the COVID-19 positive rate in the population of interest in addition to the sensitivity of the [reverse transcriptase-polymerase chain reaction (RT-PCR)] method used for COVID-19 testing,” said Baha Abdalhamid, MD, PhD, of the department of pathology and microbiology at the University of Nebraska Medical Center in Omaha.
“Each laboratory and hospital needs to do their own validation testing because it is dependent on the positive rate of COVID-19,” added Dr. Abdalhamid, who was not involved in the current study.
It’s important for clinicians to “do a good history to find who’s high risk and who’s low risk,” Dr. Mastrianni said. Clinicians also need to remember that, although a patient may test negative initially, they may still have COVID-19, he warned. That test reflects a single point in time, and a patient could be infected and not yet be ill, so clinicians need to be alert to a change in the patient’s status.
Best for settings with low-risk individuals
“Pooled COVID-19 testing is a straightforward, cost-effective, and efficient approach,” Dr. Abdalhamid said. He and his colleagues found pooled testing could increase testing capability by 69% or more when the incidence rate of SARS-CoV-2 infection is 10% or lower.
He said the approach would be helpful in other settings “as long as the positive rate is equal to or less than 10%. Asymptomatic population or surveillance groups such as students, athletes, and military service members are [an] interesting population to test using pooling testing because we expect these populations to have low positive rates, which makes pooled testing ideal.”
Benefit outweighs risk
“There is risk of missing specimens with low concentration of the virus,” Dr. Abdalhamid cautioned. “These specimens might be missed due to the dilution factor of pooling [false-negative specimens]. We did not have a single false-negative specimen in our proof-of-concept study. In addition, there are practical approaches to deal with false-negative pooled specimens.
“The benefit definitely outweighs the risk of false-negative specimens because false-negative results rarely occur, if any. In addition, there is significant saving of time, reagents, and supplies in [a] pooled specimens approach as well as expansion of the test for higher number of patients,” Dr. Abdalhamid continued.
Dr. Mastrianni’s hospital currently has enough testing cartridges, but they are continuing to conduct pooled testing to conserve resources for the benefit of their own hospital and for the nation as a whole, he said.
The authors have disclosed no relevant financial relationships. Dr. Abdalhamid and Dr. Shah have disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
Pan-Pseudothrombocytopenia in COVID-19: A Harbinger for Lethal Arterial Thrombosis?
In late 2019 a new pandemic started in Wuhan, China, caused by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) due to its similarities with the virus responsible for the SARS outbreak of 2003. The disease manifestations are named coronavirus disease 2019 (COVID-19).1
Pseudothrombocytopenia, or platelet clumping, visualized on the peripheral blood smear, is a common cause for artificial thrombocytopenia laboratory reporting and is frequently attributed to laboratory artifact. In this case presentation, a critically ill patient with COVID-19 developed pan-pseudothrombocytopenia (ethylenediaminetetraacetic acid [EDTA], sodium citrate, and heparin tubes) just prior to his death from a ST-segment elevation myocardial infarction (STEMI) in the setting of therapeutic anticoagulation during a prolonged hospitalization. This case raises the possibility that pseudothrombocytopenia in the setting of COVID-19 critical illness may represent an ominous feature of COVID-19-associated coagulopathy (CAC). Furthermore, it prompts the question whether pseudothrombocytopenia in this setting is representative of increased platelet aggregation activity in vivo.
Case Presentation
A 50-year-old African American man who was diagnosed with COVID-19 3 days prior to admission presented to the emergency department of the W.G. (Bill) Hefner VA Medical Center in Salisbury, North Carolina, with worsening dyspnea and fever. His primary chronic medical problems included obesity (body mass index, 33), type 2 diabetes mellitus (hemoglobin A1c 2 months prior of 6.6%), migraine headaches, and obstructive sleep apnea. Shortly after presentation, his respiratory status declined, requiring intubation. He was admitted to the medical intensive care unit for further management.
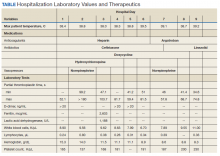
Notable findings at admission included > 20 mcg/mL FEU D-dimer (normal range, 0-0.56 mcg/mL FEU), 20.4 mg/dL C-reactive protein (normal range, < 1 mg/dL), 30 mm/h erythrocyte sedimentation rate (normal range, 0-25 mm/h), and 3.56 ng/mL procalcitonin (normal range, 0.05-1.99 ng/mL). Patient’s hemoglobin and platelet counts were normal. Empiric antimicrobial therapy was initiated with ceftriaxone (2 g IV daily) and doxycycline (100 mg IV twice daily) due to concern of superimposed infection in the setting of an elevated procalcitonin.
A heparin infusion was initiated (5,000 U IV bolus followed by continuous infusion with goal partial thromboplastin time [PTT] of 1.5x the upper limit of normal) on admission to treat CAC. Renal function worsened requiring intermittent renal replacement therapy on day 3. His lactate dehydrogenase was elevated to 1,188 U/L (normal range: 100-240 U/L) and ferritin was elevated to 2,603 ng/mL (normal range: 25-350 ng/mL) (Table). Initial neuromuscular blockade and prone positioning maneuvers were instituted to optimize oxygenation based on the latest literature for respiratory distress in the COVID-19 management.2
Intermittent norepinephrine infusion (5 mcg/min with a 2 mcg/min titration every 5 minutes as needed to maintain mean arterial pressure of > 65 mm Hg) was required for hemodynamic support throughout the patient’s course. Several therapies for COVID-19 were considered and were a reflection of the rapidly evolving literature during the care of patients with this disease. The patient originally received hydroxychloroquine (200 mg by mouth twice daily) in accordance with the US Department of Veterans Affairs (VA) institutional protocol between day 2 and day 4; however, hydroxychloroquine was stopped due to concerns of QTc prolongation. The patient also received 1 unit of convalescent plasma on day 6 after being enrolled in the expanded access program.3 The patient was not a candidate for remdesivir due to his unstable renal function and need for vasopressors. Finally, interleukin-6 inhibitors also were considered; however, the risk of superimposed infection precluded its use.
On day 7 antimicrobial therapy was transitioned to linezolid (600 mg IV twice daily) due to the persistence of fever and a portable chest radiograph revealing diffuse infiltrates throughout the bilateral lungs, worse compared with prior radiograph on day 5, suggesting a worsening of pneumonia. On day 12, the patient was transitioned to cefepime (1 gram IV daily) to broaden antimicrobial coverage and was continued thereafter. Blood cultures were negative throughout his hospitalization.
Given his worsening clinical scenario there was a question about whether or not the patient was still shedding virus for prognostic and therapeutic implications. Therefore, his SARS-CoV-2 test by polymerase chain reaction nasopharyngeal was positive again on day 18. On day 20, the patient developed leukocytosis, his fever persisted, and a portable chest radiograph revealed extensive bilateral pulmonary opacities with focal worsening in left lower base. Due to this constellation of findings, a vancomycin IV (1,500 mg once) was started for empirical treatment of hospital-acquired pneumonia. Sputum samples obtained on day 20 revealed Staphylococcus aureus on subsequent days.
From a hematologic perspective, on day 9 due to challenges to maintain a therapeutic level of anticoagulation with heparin infusion thought to be related to antithrombin deficiency, anticoagulation was changed to argatroban infusion (0.5 mcg/kg/min targeting a PTT of 70-105 seconds) for ongoing management of CAC. Although D-dimer was > 20 mcg/mL FEU on admission and on days 4 and 5, D-dimer trended down to 12.5 mcg/mL FEU on day 16.
Throughout the patient’s hospital stay, no significant bleeding was seen. Hemoglobin was 15.2 g/dL on admission, but anemia developed with a nadir of 6.5 g/dL, warranting transfusion of red blood cells on day 22. Platelet count was 165,000 per microliter on admission and remained within normal limits until platelet clumping was noted on day 15 laboratory collection.
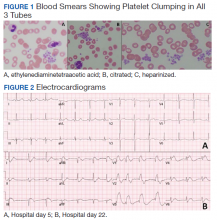
Hematology was consulted on day 20 to obtain an accurate platelet count. A peripheral blood smear from a sodium citrate containing tube was remarkable for prominent platelet clumping, particularly at the periphery of the slide (Figure 1). Platelet clumping was reproduced in samples containing EDTA and heparin. Other features of the peripheral blood smear included the presence of echinocytes with rare schistocytes. To investigate for presence of disseminated intravascular coagulation on day 22, fibrinogen was found to be mildly elevated at 538 mg/dL (normal range: 243-517 mg/dL) and a D-dimer value of 11.96 mcg/mL FEU.
On day 22, the patient’s ventilator requirements escalated to requiring 100% FiO2 and 10 cm H20 of positive end-expiratory pressure with mean arterial pressures in the 50 to 60 mm Hg range. Within 30 minutes an electrocardiogram (EKG) obtained revealed a STEMI (Figure 2). Troponin was measured at 0.65 ng/mL (normal range: 0.02-0.06 ng/mL). Just after an EKG was performed, the patient developed a ventricular fibrillation arrest and was unable to obtain return of spontaneous circulation. The patient was pronounced dead. The family declined an autopsy.
Discussion
Pseudothrombocytopenia, or platelet clumping (agglutination), is estimated to be present in up to 2% of hospitalized patients.4 Pseudothrombocytopenia was found to be the root cause of thrombocytopenia hematology consultations in up to 4% of hospitalized patients.5 The etiology is commonly ascribed to EDTA inducing a conformational change in the GpIIb-IIIa platelet complex, rendering it susceptible to binding of autoantibodies, which cause subsequent platelet agglutination.6 In most cases (83%), the use of a non-EDTA anticoagulant, such as sodium citrate, resolves the platelet agglutination and allows for accurate platelet count reporting.4 Pseudothrombocytopenia in most cases is considered an in vitro finding without clinical relevance.7 However, in this patient’s case, his pan-pseudothrombocytopenia was temporally associated with an arterial occlusive event (STEMI) leading to his demise despite therapeutic anticoagulation in the setting of CAC. This temporal association raises the possibility that pseudothrombocytopenia seen on the peripheral blood smear is an accurate representation of in vivo activity.
Pseudothrombocytopenia has been associated with sepsis from bacterial and viral causes as well as autoimmune and medication effect.4,8-10 Li and colleagues reported transient EDTA-dependent pseudothrombocytopenia in a patient with COVID-19 infection; however, platelet clumping resolved with use of a citrate tube, and the EDTA-dependent pseudothrombocytopenia phenomenon resolved with patient recovery.11 The frequency of COVID-19-related pseudothrombocytopenia is currently unknown.
Although the understanding of COVID-19-associated CAC continues to evolve, it seems that initial reports support the idea that hemostatic dysfunction tends to more thrombosis than to bleeding.12 Rather than overt disseminated intravascular coagulation with reduced fibrinogen and bleeding, CAC is more closely associated with blood clotting, as demonstrated by autopsy studies revealing microvascular thrombosis in the lungs.13 The D-dimer test has been identified as the most useful biomarker by the International Society of Thrombosis and Hemostasis to screen for CAC and stratify patients who warrant admission or closer monitoring.12 Other identified features of CAC include prolonged prothrombin time and thrombocytopenia.12
There have been varying clinical approaches to CAC management. A retrospective review found that prophylactic heparin doses were associated with improved mortality in those with elevated D-dimer > 3.0 mg/L.14 There continues to be a diversity of varying clinical approaches with many medical centers advocating for an intensified prophylactic twice daily low molecular-weight heparin compared with others advocating for full therapeutic dose anticoagulation for patients with elevated D-dimer.15 This patient was treated aggressively with full-dose anticoagulation, and despite his having a down-trend in D-dimer, he suffered a lethal arterial thrombosis in the form of a STEMI.
Varatharajah and Rajah
Conclusions
This patient’s case highlights the presence of pan-pseudothrombocytopenia despite the use of a sodium citrate and heparin containing tube in a COVID-19 infection with multiorgan dysfunction. This developed 1 week prior to the patient suffering a STEMI despite therapeutic anticoagulation. Although the exact nature of CAC remains to be worked out, it is possible that platelet agglutination/clumping seen on the peripheral blood smear is representative of in vivo activity and serves as a harbinger for worsening thrombosis. The frequency of such phenomenon and efficacy of further interventions has yet to be explored.
1. World Health Organization. Naming the coronavirus disease (COVID-19) and the virus that causes it. https://www.who.int/emergencies/diseases/novel-coronavirus-2019/technical-guidance/naming-the-coronavirus-disease-(COVID-2019)-and-the-virus-that-causes-it. Accessed July 15, 2020.
2. Ghelichkhani P, Esmaeili M. Prone position in management of COVID-19 patients; a commentary. Arch Acad Emerg Med. 2020;8(1):e48. Published 2020 April 11.
3. National Library of Medicine, Clinicaltrials.gov. Expanded access to convalescent plasma for the treatment of patients with COVID-19. NCT04338360. https://clinicaltrials.gov/ct2/show/nct04338360. Update April 20, 2020. Accessed July 15, 2020.
4. Tan GC, Stalling M, Dennis G, Nunez M, Kahwash SB. Pseudothrombocytopenia due to platelet clumping: a case report and brief review of the literature. Case Rep Hematol. 2016;2016:3036476. doi:10.1155/2016/3036476
5. Boxer M, Biuso TJ. Etiologies of thrombocytopenia in the community hospital: the experience of 1 hematologist. Am J Med. 2020;133(5):e183-e186. doi:10.1016/j.amjmed.2019.10.027
6. Fiorin F, Steffan A, Pradella P, Bizzaro N, Potenza R, De Angelis V. IgG platelet antibodies in EDTA-dependent pseudothrombocytopenia bind to platelet membrane glycoprotein IIb. Am J Clin Pathol. 1998;110(2):178-183. doi:10.1093/ajcp/110.2.178
7. Nagler M, Keller P, Siegrist S, Alberio L. A case of EDTA-Dependent pseudothrombocytopenia: simple recognition of an underdiagnosed and misleading phenomenon. BMC Clin Pathol. 2014;14:19. doi:10.1186/1472-6890-14-19
8. Mori M, Kudo H, Yoshitake S, Ito K, Shinguu C, Noguchi T. Transient EDTA-dependent pseudothrombocytopenia in a patient with sepsis. Intensive Care Med. 2000;26(2):218-220. doi:10.1007/s001340050050.
9. Choe W-H, Cho Y-U, Chae J-D, Kim S-H. 2013. Pseudothrombocytopenia or platelet clumping as a possible cause of low platelet count in patients with viral infection: a case series from single institution focusing on hepatitis A virus infection. Int J Lab Hematol. 2013;35(1):70-76. doi:10.1111/j.1751-553x.2012.01466.
10. Hsieh AT, Chao TY, Chen YC. Pseudothrombocytopenia associated with infectious mononucleosis. Arch Pathol Lab Med. 2003;127(1):e17-e18. doi:10.1043/0003-9985(2003)1272.0.CO;2
11. Li H, Wang B, Ning L, Luo Y, Xiang S. Transient appearance of EDTA dependent pseudothrombocytopenia in a patient with 2019 novel coronavirus pneumonia [published online ahead of print, 2020 May 5]. Platelets. 2020;1-2. doi:10.1080/09537104.2020.1760231
12. Thachil J, Tang N, Gando S, et al. ISTH interim guidance on recognition and management of coagulopathy in COVID-19. J Thromb Haemost. 2020;18(5):1023-1026. doi:10.1111/jth.14810
13. Magro C, Mulvey JJ, Berlin D, et al. Complement associated microvascular injury and thrombosis in the pathogenesis of severe COVID-19 infection: a report of five cases. Transl Res. 2020;220:1-13. doi:10.1016/j.trsl.2020.04.007
14. Tang N, Bai H, Chen X, Gong J, Li D, Sun Z. Anticoagulant treatment is associated with decreased mortality in severe coronavirus disease 2019 patients with coagulopathy. J Thromb Haemost. 2020;18(5):1094-1099. doi:10.1111/jth.14817
15. Connors JM, Levy JH. COVID-19 and its implications for thrombosis and anticoagulation. Blood. 2020;125(23):2033-2040. doi.org/10.1182/blood.2020006000.
16. Varatharajah N, Rajah S. Microthrombotic complications of COVID-19 are likely due to embolism of circulating endothelial derived ultralarge von Willebrand factor (eULVWF) Decorated-Platelet Strings. Fed Pract. 2020;37(6):258-259. doi:10.12788/fp.0001
17. Bernardo A, Ball C, Nolasco L, Choi H, Moake JL, Dong JF. Platelets adhered to endothelial cell-bound ultra-large von Willebrand factor strings support leukocyte tethering and rolling under high shear stress. J Thromb Haemost. 2005;3(3):562-570. doi:10.1111/j.1538-7836.2005.01122.x
In late 2019 a new pandemic started in Wuhan, China, caused by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) due to its similarities with the virus responsible for the SARS outbreak of 2003. The disease manifestations are named coronavirus disease 2019 (COVID-19).1
Pseudothrombocytopenia, or platelet clumping, visualized on the peripheral blood smear, is a common cause for artificial thrombocytopenia laboratory reporting and is frequently attributed to laboratory artifact. In this case presentation, a critically ill patient with COVID-19 developed pan-pseudothrombocytopenia (ethylenediaminetetraacetic acid [EDTA], sodium citrate, and heparin tubes) just prior to his death from a ST-segment elevation myocardial infarction (STEMI) in the setting of therapeutic anticoagulation during a prolonged hospitalization. This case raises the possibility that pseudothrombocytopenia in the setting of COVID-19 critical illness may represent an ominous feature of COVID-19-associated coagulopathy (CAC). Furthermore, it prompts the question whether pseudothrombocytopenia in this setting is representative of increased platelet aggregation activity in vivo.
Case Presentation
A 50-year-old African American man who was diagnosed with COVID-19 3 days prior to admission presented to the emergency department of the W.G. (Bill) Hefner VA Medical Center in Salisbury, North Carolina, with worsening dyspnea and fever. His primary chronic medical problems included obesity (body mass index, 33), type 2 diabetes mellitus (hemoglobin A1c 2 months prior of 6.6%), migraine headaches, and obstructive sleep apnea. Shortly after presentation, his respiratory status declined, requiring intubation. He was admitted to the medical intensive care unit for further management.
Notable findings at admission included > 20 mcg/mL FEU D-dimer (normal range, 0-0.56 mcg/mL FEU), 20.4 mg/dL C-reactive protein (normal range, < 1 mg/dL), 30 mm/h erythrocyte sedimentation rate (normal range, 0-25 mm/h), and 3.56 ng/mL procalcitonin (normal range, 0.05-1.99 ng/mL). Patient’s hemoglobin and platelet counts were normal. Empiric antimicrobial therapy was initiated with ceftriaxone (2 g IV daily) and doxycycline (100 mg IV twice daily) due to concern of superimposed infection in the setting of an elevated procalcitonin.
A heparin infusion was initiated (5,000 U IV bolus followed by continuous infusion with goal partial thromboplastin time [PTT] of 1.5x the upper limit of normal) on admission to treat CAC. Renal function worsened requiring intermittent renal replacement therapy on day 3. His lactate dehydrogenase was elevated to 1,188 U/L (normal range: 100-240 U/L) and ferritin was elevated to 2,603 ng/mL (normal range: 25-350 ng/mL) (Table). Initial neuromuscular blockade and prone positioning maneuvers were instituted to optimize oxygenation based on the latest literature for respiratory distress in the COVID-19 management.2
Intermittent norepinephrine infusion (5 mcg/min with a 2 mcg/min titration every 5 minutes as needed to maintain mean arterial pressure of > 65 mm Hg) was required for hemodynamic support throughout the patient’s course. Several therapies for COVID-19 were considered and were a reflection of the rapidly evolving literature during the care of patients with this disease. The patient originally received hydroxychloroquine (200 mg by mouth twice daily) in accordance with the US Department of Veterans Affairs (VA) institutional protocol between day 2 and day 4; however, hydroxychloroquine was stopped due to concerns of QTc prolongation. The patient also received 1 unit of convalescent plasma on day 6 after being enrolled in the expanded access program.3 The patient was not a candidate for remdesivir due to his unstable renal function and need for vasopressors. Finally, interleukin-6 inhibitors also were considered; however, the risk of superimposed infection precluded its use.
On day 7 antimicrobial therapy was transitioned to linezolid (600 mg IV twice daily) due to the persistence of fever and a portable chest radiograph revealing diffuse infiltrates throughout the bilateral lungs, worse compared with prior radiograph on day 5, suggesting a worsening of pneumonia. On day 12, the patient was transitioned to cefepime (1 gram IV daily) to broaden antimicrobial coverage and was continued thereafter. Blood cultures were negative throughout his hospitalization.
Given his worsening clinical scenario there was a question about whether or not the patient was still shedding virus for prognostic and therapeutic implications. Therefore, his SARS-CoV-2 test by polymerase chain reaction nasopharyngeal was positive again on day 18. On day 20, the patient developed leukocytosis, his fever persisted, and a portable chest radiograph revealed extensive bilateral pulmonary opacities with focal worsening in left lower base. Due to this constellation of findings, a vancomycin IV (1,500 mg once) was started for empirical treatment of hospital-acquired pneumonia. Sputum samples obtained on day 20 revealed Staphylococcus aureus on subsequent days.
From a hematologic perspective, on day 9 due to challenges to maintain a therapeutic level of anticoagulation with heparin infusion thought to be related to antithrombin deficiency, anticoagulation was changed to argatroban infusion (0.5 mcg/kg/min targeting a PTT of 70-105 seconds) for ongoing management of CAC. Although D-dimer was > 20 mcg/mL FEU on admission and on days 4 and 5, D-dimer trended down to 12.5 mcg/mL FEU on day 16.
Throughout the patient’s hospital stay, no significant bleeding was seen. Hemoglobin was 15.2 g/dL on admission, but anemia developed with a nadir of 6.5 g/dL, warranting transfusion of red blood cells on day 22. Platelet count was 165,000 per microliter on admission and remained within normal limits until platelet clumping was noted on day 15 laboratory collection.
Hematology was consulted on day 20 to obtain an accurate platelet count. A peripheral blood smear from a sodium citrate containing tube was remarkable for prominent platelet clumping, particularly at the periphery of the slide (Figure 1). Platelet clumping was reproduced in samples containing EDTA and heparin. Other features of the peripheral blood smear included the presence of echinocytes with rare schistocytes. To investigate for presence of disseminated intravascular coagulation on day 22, fibrinogen was found to be mildly elevated at 538 mg/dL (normal range: 243-517 mg/dL) and a D-dimer value of 11.96 mcg/mL FEU.
On day 22, the patient’s ventilator requirements escalated to requiring 100% FiO2 and 10 cm H20 of positive end-expiratory pressure with mean arterial pressures in the 50 to 60 mm Hg range. Within 30 minutes an electrocardiogram (EKG) obtained revealed a STEMI (Figure 2). Troponin was measured at 0.65 ng/mL (normal range: 0.02-0.06 ng/mL). Just after an EKG was performed, the patient developed a ventricular fibrillation arrest and was unable to obtain return of spontaneous circulation. The patient was pronounced dead. The family declined an autopsy.
Discussion
Pseudothrombocytopenia, or platelet clumping (agglutination), is estimated to be present in up to 2% of hospitalized patients.4 Pseudothrombocytopenia was found to be the root cause of thrombocytopenia hematology consultations in up to 4% of hospitalized patients.5 The etiology is commonly ascribed to EDTA inducing a conformational change in the GpIIb-IIIa platelet complex, rendering it susceptible to binding of autoantibodies, which cause subsequent platelet agglutination.6 In most cases (83%), the use of a non-EDTA anticoagulant, such as sodium citrate, resolves the platelet agglutination and allows for accurate platelet count reporting.4 Pseudothrombocytopenia in most cases is considered an in vitro finding without clinical relevance.7 However, in this patient’s case, his pan-pseudothrombocytopenia was temporally associated with an arterial occlusive event (STEMI) leading to his demise despite therapeutic anticoagulation in the setting of CAC. This temporal association raises the possibility that pseudothrombocytopenia seen on the peripheral blood smear is an accurate representation of in vivo activity.
Pseudothrombocytopenia has been associated with sepsis from bacterial and viral causes as well as autoimmune and medication effect.4,8-10 Li and colleagues reported transient EDTA-dependent pseudothrombocytopenia in a patient with COVID-19 infection; however, platelet clumping resolved with use of a citrate tube, and the EDTA-dependent pseudothrombocytopenia phenomenon resolved with patient recovery.11 The frequency of COVID-19-related pseudothrombocytopenia is currently unknown.
Although the understanding of COVID-19-associated CAC continues to evolve, it seems that initial reports support the idea that hemostatic dysfunction tends to more thrombosis than to bleeding.12 Rather than overt disseminated intravascular coagulation with reduced fibrinogen and bleeding, CAC is more closely associated with blood clotting, as demonstrated by autopsy studies revealing microvascular thrombosis in the lungs.13 The D-dimer test has been identified as the most useful biomarker by the International Society of Thrombosis and Hemostasis to screen for CAC and stratify patients who warrant admission or closer monitoring.12 Other identified features of CAC include prolonged prothrombin time and thrombocytopenia.12
There have been varying clinical approaches to CAC management. A retrospective review found that prophylactic heparin doses were associated with improved mortality in those with elevated D-dimer > 3.0 mg/L.14 There continues to be a diversity of varying clinical approaches with many medical centers advocating for an intensified prophylactic twice daily low molecular-weight heparin compared with others advocating for full therapeutic dose anticoagulation for patients with elevated D-dimer.15 This patient was treated aggressively with full-dose anticoagulation, and despite his having a down-trend in D-dimer, he suffered a lethal arterial thrombosis in the form of a STEMI.
Varatharajah and Rajah
Conclusions
This patient’s case highlights the presence of pan-pseudothrombocytopenia despite the use of a sodium citrate and heparin containing tube in a COVID-19 infection with multiorgan dysfunction. This developed 1 week prior to the patient suffering a STEMI despite therapeutic anticoagulation. Although the exact nature of CAC remains to be worked out, it is possible that platelet agglutination/clumping seen on the peripheral blood smear is representative of in vivo activity and serves as a harbinger for worsening thrombosis. The frequency of such phenomenon and efficacy of further interventions has yet to be explored.
In late 2019 a new pandemic started in Wuhan, China, caused by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) due to its similarities with the virus responsible for the SARS outbreak of 2003. The disease manifestations are named coronavirus disease 2019 (COVID-19).1
Pseudothrombocytopenia, or platelet clumping, visualized on the peripheral blood smear, is a common cause for artificial thrombocytopenia laboratory reporting and is frequently attributed to laboratory artifact. In this case presentation, a critically ill patient with COVID-19 developed pan-pseudothrombocytopenia (ethylenediaminetetraacetic acid [EDTA], sodium citrate, and heparin tubes) just prior to his death from a ST-segment elevation myocardial infarction (STEMI) in the setting of therapeutic anticoagulation during a prolonged hospitalization. This case raises the possibility that pseudothrombocytopenia in the setting of COVID-19 critical illness may represent an ominous feature of COVID-19-associated coagulopathy (CAC). Furthermore, it prompts the question whether pseudothrombocytopenia in this setting is representative of increased platelet aggregation activity in vivo.
Case Presentation
A 50-year-old African American man who was diagnosed with COVID-19 3 days prior to admission presented to the emergency department of the W.G. (Bill) Hefner VA Medical Center in Salisbury, North Carolina, with worsening dyspnea and fever. His primary chronic medical problems included obesity (body mass index, 33), type 2 diabetes mellitus (hemoglobin A1c 2 months prior of 6.6%), migraine headaches, and obstructive sleep apnea. Shortly after presentation, his respiratory status declined, requiring intubation. He was admitted to the medical intensive care unit for further management.
Notable findings at admission included > 20 mcg/mL FEU D-dimer (normal range, 0-0.56 mcg/mL FEU), 20.4 mg/dL C-reactive protein (normal range, < 1 mg/dL), 30 mm/h erythrocyte sedimentation rate (normal range, 0-25 mm/h), and 3.56 ng/mL procalcitonin (normal range, 0.05-1.99 ng/mL). Patient’s hemoglobin and platelet counts were normal. Empiric antimicrobial therapy was initiated with ceftriaxone (2 g IV daily) and doxycycline (100 mg IV twice daily) due to concern of superimposed infection in the setting of an elevated procalcitonin.
A heparin infusion was initiated (5,000 U IV bolus followed by continuous infusion with goal partial thromboplastin time [PTT] of 1.5x the upper limit of normal) on admission to treat CAC. Renal function worsened requiring intermittent renal replacement therapy on day 3. His lactate dehydrogenase was elevated to 1,188 U/L (normal range: 100-240 U/L) and ferritin was elevated to 2,603 ng/mL (normal range: 25-350 ng/mL) (Table). Initial neuromuscular blockade and prone positioning maneuvers were instituted to optimize oxygenation based on the latest literature for respiratory distress in the COVID-19 management.2
Intermittent norepinephrine infusion (5 mcg/min with a 2 mcg/min titration every 5 minutes as needed to maintain mean arterial pressure of > 65 mm Hg) was required for hemodynamic support throughout the patient’s course. Several therapies for COVID-19 were considered and were a reflection of the rapidly evolving literature during the care of patients with this disease. The patient originally received hydroxychloroquine (200 mg by mouth twice daily) in accordance with the US Department of Veterans Affairs (VA) institutional protocol between day 2 and day 4; however, hydroxychloroquine was stopped due to concerns of QTc prolongation. The patient also received 1 unit of convalescent plasma on day 6 after being enrolled in the expanded access program.3 The patient was not a candidate for remdesivir due to his unstable renal function and need for vasopressors. Finally, interleukin-6 inhibitors also were considered; however, the risk of superimposed infection precluded its use.
On day 7 antimicrobial therapy was transitioned to linezolid (600 mg IV twice daily) due to the persistence of fever and a portable chest radiograph revealing diffuse infiltrates throughout the bilateral lungs, worse compared with prior radiograph on day 5, suggesting a worsening of pneumonia. On day 12, the patient was transitioned to cefepime (1 gram IV daily) to broaden antimicrobial coverage and was continued thereafter. Blood cultures were negative throughout his hospitalization.
Given his worsening clinical scenario there was a question about whether or not the patient was still shedding virus for prognostic and therapeutic implications. Therefore, his SARS-CoV-2 test by polymerase chain reaction nasopharyngeal was positive again on day 18. On day 20, the patient developed leukocytosis, his fever persisted, and a portable chest radiograph revealed extensive bilateral pulmonary opacities with focal worsening in left lower base. Due to this constellation of findings, a vancomycin IV (1,500 mg once) was started for empirical treatment of hospital-acquired pneumonia. Sputum samples obtained on day 20 revealed Staphylococcus aureus on subsequent days.
From a hematologic perspective, on day 9 due to challenges to maintain a therapeutic level of anticoagulation with heparin infusion thought to be related to antithrombin deficiency, anticoagulation was changed to argatroban infusion (0.5 mcg/kg/min targeting a PTT of 70-105 seconds) for ongoing management of CAC. Although D-dimer was > 20 mcg/mL FEU on admission and on days 4 and 5, D-dimer trended down to 12.5 mcg/mL FEU on day 16.
Throughout the patient’s hospital stay, no significant bleeding was seen. Hemoglobin was 15.2 g/dL on admission, but anemia developed with a nadir of 6.5 g/dL, warranting transfusion of red blood cells on day 22. Platelet count was 165,000 per microliter on admission and remained within normal limits until platelet clumping was noted on day 15 laboratory collection.
Hematology was consulted on day 20 to obtain an accurate platelet count. A peripheral blood smear from a sodium citrate containing tube was remarkable for prominent platelet clumping, particularly at the periphery of the slide (Figure 1). Platelet clumping was reproduced in samples containing EDTA and heparin. Other features of the peripheral blood smear included the presence of echinocytes with rare schistocytes. To investigate for presence of disseminated intravascular coagulation on day 22, fibrinogen was found to be mildly elevated at 538 mg/dL (normal range: 243-517 mg/dL) and a D-dimer value of 11.96 mcg/mL FEU.
On day 22, the patient’s ventilator requirements escalated to requiring 100% FiO2 and 10 cm H20 of positive end-expiratory pressure with mean arterial pressures in the 50 to 60 mm Hg range. Within 30 minutes an electrocardiogram (EKG) obtained revealed a STEMI (Figure 2). Troponin was measured at 0.65 ng/mL (normal range: 0.02-0.06 ng/mL). Just after an EKG was performed, the patient developed a ventricular fibrillation arrest and was unable to obtain return of spontaneous circulation. The patient was pronounced dead. The family declined an autopsy.
Discussion
Pseudothrombocytopenia, or platelet clumping (agglutination), is estimated to be present in up to 2% of hospitalized patients.4 Pseudothrombocytopenia was found to be the root cause of thrombocytopenia hematology consultations in up to 4% of hospitalized patients.5 The etiology is commonly ascribed to EDTA inducing a conformational change in the GpIIb-IIIa platelet complex, rendering it susceptible to binding of autoantibodies, which cause subsequent platelet agglutination.6 In most cases (83%), the use of a non-EDTA anticoagulant, such as sodium citrate, resolves the platelet agglutination and allows for accurate platelet count reporting.4 Pseudothrombocytopenia in most cases is considered an in vitro finding without clinical relevance.7 However, in this patient’s case, his pan-pseudothrombocytopenia was temporally associated with an arterial occlusive event (STEMI) leading to his demise despite therapeutic anticoagulation in the setting of CAC. This temporal association raises the possibility that pseudothrombocytopenia seen on the peripheral blood smear is an accurate representation of in vivo activity.
Pseudothrombocytopenia has been associated with sepsis from bacterial and viral causes as well as autoimmune and medication effect.4,8-10 Li and colleagues reported transient EDTA-dependent pseudothrombocytopenia in a patient with COVID-19 infection; however, platelet clumping resolved with use of a citrate tube, and the EDTA-dependent pseudothrombocytopenia phenomenon resolved with patient recovery.11 The frequency of COVID-19-related pseudothrombocytopenia is currently unknown.
Although the understanding of COVID-19-associated CAC continues to evolve, it seems that initial reports support the idea that hemostatic dysfunction tends to more thrombosis than to bleeding.12 Rather than overt disseminated intravascular coagulation with reduced fibrinogen and bleeding, CAC is more closely associated with blood clotting, as demonstrated by autopsy studies revealing microvascular thrombosis in the lungs.13 The D-dimer test has been identified as the most useful biomarker by the International Society of Thrombosis and Hemostasis to screen for CAC and stratify patients who warrant admission or closer monitoring.12 Other identified features of CAC include prolonged prothrombin time and thrombocytopenia.12
There have been varying clinical approaches to CAC management. A retrospective review found that prophylactic heparin doses were associated with improved mortality in those with elevated D-dimer > 3.0 mg/L.14 There continues to be a diversity of varying clinical approaches with many medical centers advocating for an intensified prophylactic twice daily low molecular-weight heparin compared with others advocating for full therapeutic dose anticoagulation for patients with elevated D-dimer.15 This patient was treated aggressively with full-dose anticoagulation, and despite his having a down-trend in D-dimer, he suffered a lethal arterial thrombosis in the form of a STEMI.
Varatharajah and Rajah
Conclusions
This patient’s case highlights the presence of pan-pseudothrombocytopenia despite the use of a sodium citrate and heparin containing tube in a COVID-19 infection with multiorgan dysfunction. This developed 1 week prior to the patient suffering a STEMI despite therapeutic anticoagulation. Although the exact nature of CAC remains to be worked out, it is possible that platelet agglutination/clumping seen on the peripheral blood smear is representative of in vivo activity and serves as a harbinger for worsening thrombosis. The frequency of such phenomenon and efficacy of further interventions has yet to be explored.
1. World Health Organization. Naming the coronavirus disease (COVID-19) and the virus that causes it. https://www.who.int/emergencies/diseases/novel-coronavirus-2019/technical-guidance/naming-the-coronavirus-disease-(COVID-2019)-and-the-virus-that-causes-it. Accessed July 15, 2020.
2. Ghelichkhani P, Esmaeili M. Prone position in management of COVID-19 patients; a commentary. Arch Acad Emerg Med. 2020;8(1):e48. Published 2020 April 11.
3. National Library of Medicine, Clinicaltrials.gov. Expanded access to convalescent plasma for the treatment of patients with COVID-19. NCT04338360. https://clinicaltrials.gov/ct2/show/nct04338360. Update April 20, 2020. Accessed July 15, 2020.
4. Tan GC, Stalling M, Dennis G, Nunez M, Kahwash SB. Pseudothrombocytopenia due to platelet clumping: a case report and brief review of the literature. Case Rep Hematol. 2016;2016:3036476. doi:10.1155/2016/3036476
5. Boxer M, Biuso TJ. Etiologies of thrombocytopenia in the community hospital: the experience of 1 hematologist. Am J Med. 2020;133(5):e183-e186. doi:10.1016/j.amjmed.2019.10.027
6. Fiorin F, Steffan A, Pradella P, Bizzaro N, Potenza R, De Angelis V. IgG platelet antibodies in EDTA-dependent pseudothrombocytopenia bind to platelet membrane glycoprotein IIb. Am J Clin Pathol. 1998;110(2):178-183. doi:10.1093/ajcp/110.2.178
7. Nagler M, Keller P, Siegrist S, Alberio L. A case of EDTA-Dependent pseudothrombocytopenia: simple recognition of an underdiagnosed and misleading phenomenon. BMC Clin Pathol. 2014;14:19. doi:10.1186/1472-6890-14-19
8. Mori M, Kudo H, Yoshitake S, Ito K, Shinguu C, Noguchi T. Transient EDTA-dependent pseudothrombocytopenia in a patient with sepsis. Intensive Care Med. 2000;26(2):218-220. doi:10.1007/s001340050050.
9. Choe W-H, Cho Y-U, Chae J-D, Kim S-H. 2013. Pseudothrombocytopenia or platelet clumping as a possible cause of low platelet count in patients with viral infection: a case series from single institution focusing on hepatitis A virus infection. Int J Lab Hematol. 2013;35(1):70-76. doi:10.1111/j.1751-553x.2012.01466.
10. Hsieh AT, Chao TY, Chen YC. Pseudothrombocytopenia associated with infectious mononucleosis. Arch Pathol Lab Med. 2003;127(1):e17-e18. doi:10.1043/0003-9985(2003)1272.0.CO;2
11. Li H, Wang B, Ning L, Luo Y, Xiang S. Transient appearance of EDTA dependent pseudothrombocytopenia in a patient with 2019 novel coronavirus pneumonia [published online ahead of print, 2020 May 5]. Platelets. 2020;1-2. doi:10.1080/09537104.2020.1760231
12. Thachil J, Tang N, Gando S, et al. ISTH interim guidance on recognition and management of coagulopathy in COVID-19. J Thromb Haemost. 2020;18(5):1023-1026. doi:10.1111/jth.14810
13. Magro C, Mulvey JJ, Berlin D, et al. Complement associated microvascular injury and thrombosis in the pathogenesis of severe COVID-19 infection: a report of five cases. Transl Res. 2020;220:1-13. doi:10.1016/j.trsl.2020.04.007
14. Tang N, Bai H, Chen X, Gong J, Li D, Sun Z. Anticoagulant treatment is associated with decreased mortality in severe coronavirus disease 2019 patients with coagulopathy. J Thromb Haemost. 2020;18(5):1094-1099. doi:10.1111/jth.14817
15. Connors JM, Levy JH. COVID-19 and its implications for thrombosis and anticoagulation. Blood. 2020;125(23):2033-2040. doi.org/10.1182/blood.2020006000.
16. Varatharajah N, Rajah S. Microthrombotic complications of COVID-19 are likely due to embolism of circulating endothelial derived ultralarge von Willebrand factor (eULVWF) Decorated-Platelet Strings. Fed Pract. 2020;37(6):258-259. doi:10.12788/fp.0001
17. Bernardo A, Ball C, Nolasco L, Choi H, Moake JL, Dong JF. Platelets adhered to endothelial cell-bound ultra-large von Willebrand factor strings support leukocyte tethering and rolling under high shear stress. J Thromb Haemost. 2005;3(3):562-570. doi:10.1111/j.1538-7836.2005.01122.x
1. World Health Organization. Naming the coronavirus disease (COVID-19) and the virus that causes it. https://www.who.int/emergencies/diseases/novel-coronavirus-2019/technical-guidance/naming-the-coronavirus-disease-(COVID-2019)-and-the-virus-that-causes-it. Accessed July 15, 2020.
2. Ghelichkhani P, Esmaeili M. Prone position in management of COVID-19 patients; a commentary. Arch Acad Emerg Med. 2020;8(1):e48. Published 2020 April 11.
3. National Library of Medicine, Clinicaltrials.gov. Expanded access to convalescent plasma for the treatment of patients with COVID-19. NCT04338360. https://clinicaltrials.gov/ct2/show/nct04338360. Update April 20, 2020. Accessed July 15, 2020.
4. Tan GC, Stalling M, Dennis G, Nunez M, Kahwash SB. Pseudothrombocytopenia due to platelet clumping: a case report and brief review of the literature. Case Rep Hematol. 2016;2016:3036476. doi:10.1155/2016/3036476
5. Boxer M, Biuso TJ. Etiologies of thrombocytopenia in the community hospital: the experience of 1 hematologist. Am J Med. 2020;133(5):e183-e186. doi:10.1016/j.amjmed.2019.10.027
6. Fiorin F, Steffan A, Pradella P, Bizzaro N, Potenza R, De Angelis V. IgG platelet antibodies in EDTA-dependent pseudothrombocytopenia bind to platelet membrane glycoprotein IIb. Am J Clin Pathol. 1998;110(2):178-183. doi:10.1093/ajcp/110.2.178
7. Nagler M, Keller P, Siegrist S, Alberio L. A case of EDTA-Dependent pseudothrombocytopenia: simple recognition of an underdiagnosed and misleading phenomenon. BMC Clin Pathol. 2014;14:19. doi:10.1186/1472-6890-14-19
8. Mori M, Kudo H, Yoshitake S, Ito K, Shinguu C, Noguchi T. Transient EDTA-dependent pseudothrombocytopenia in a patient with sepsis. Intensive Care Med. 2000;26(2):218-220. doi:10.1007/s001340050050.
9. Choe W-H, Cho Y-U, Chae J-D, Kim S-H. 2013. Pseudothrombocytopenia or platelet clumping as a possible cause of low platelet count in patients with viral infection: a case series from single institution focusing on hepatitis A virus infection. Int J Lab Hematol. 2013;35(1):70-76. doi:10.1111/j.1751-553x.2012.01466.
10. Hsieh AT, Chao TY, Chen YC. Pseudothrombocytopenia associated with infectious mononucleosis. Arch Pathol Lab Med. 2003;127(1):e17-e18. doi:10.1043/0003-9985(2003)1272.0.CO;2
11. Li H, Wang B, Ning L, Luo Y, Xiang S. Transient appearance of EDTA dependent pseudothrombocytopenia in a patient with 2019 novel coronavirus pneumonia [published online ahead of print, 2020 May 5]. Platelets. 2020;1-2. doi:10.1080/09537104.2020.1760231
12. Thachil J, Tang N, Gando S, et al. ISTH interim guidance on recognition and management of coagulopathy in COVID-19. J Thromb Haemost. 2020;18(5):1023-1026. doi:10.1111/jth.14810
13. Magro C, Mulvey JJ, Berlin D, et al. Complement associated microvascular injury and thrombosis in the pathogenesis of severe COVID-19 infection: a report of five cases. Transl Res. 2020;220:1-13. doi:10.1016/j.trsl.2020.04.007
14. Tang N, Bai H, Chen X, Gong J, Li D, Sun Z. Anticoagulant treatment is associated with decreased mortality in severe coronavirus disease 2019 patients with coagulopathy. J Thromb Haemost. 2020;18(5):1094-1099. doi:10.1111/jth.14817
15. Connors JM, Levy JH. COVID-19 and its implications for thrombosis and anticoagulation. Blood. 2020;125(23):2033-2040. doi.org/10.1182/blood.2020006000.
16. Varatharajah N, Rajah S. Microthrombotic complications of COVID-19 are likely due to embolism of circulating endothelial derived ultralarge von Willebrand factor (eULVWF) Decorated-Platelet Strings. Fed Pract. 2020;37(6):258-259. doi:10.12788/fp.0001
17. Bernardo A, Ball C, Nolasco L, Choi H, Moake JL, Dong JF. Platelets adhered to endothelial cell-bound ultra-large von Willebrand factor strings support leukocyte tethering and rolling under high shear stress. J Thromb Haemost. 2005;3(3):562-570. doi:10.1111/j.1538-7836.2005.01122.x
All Hands on Deck: The Federal Health Care Response to the COVID-19 National Emergency
A torrent of blame has deluged the administration’s management of the pandemic. There is though one part of the government that deserves the praise of the nation for its response to this public health crisis—the federal health care system. In this column, we discuss the ways in which the Veterans Health Administration (VHA), the Department of Defense (DoD), and the US Public Health Service (PHS) Commissioned Corps especially have bravely and generously responded to the medical emergency of COVID-19 in the US.
Four missions drive the US Department of Veterans Affairs (VA). Though the fourth of these missions usually is in the background, it has risen to the forefront during the pandemic. To put the fourth mission in its proper perspective, we first should review the other 3 charges given to the largest integrated health care system in the country.
The first mission is to provide the highest quality care possible for the more than 9 million veterans enrolled in that system at each of the 1,255 VHA locations. The second mission is to ensure that the Veterans Benefits Administration delivers the full range of benefits that veterans earned through their service. These including funding for education, loans for homes, and many other types of support that assist service men and women to be successful in their transition from military to civilian life. The third mission is to honor the commitment of those who fought for their country unto death. The National Cemeteries Administration oversees 142 national cemeteries where veterans are buried with dignity and remembered with gratitude for their uniformed service. The purpose of these 3 internally focused missions is to provide a safety net for eligible veterans from the day they separate from the military until the hour they pass from this earth.
The fourth mission is different. This mission looks outside the military family to the civilian world. Its goal is to bolster the ability of the nation as a whole to handle wars, terrorism, national emergencies, and natural disasters. It does this through emergency response plans that preserve the integrity of the 3 other missions to veterans while enhancing the capacity of local and state governments to manage the threat of these public health, safety, or security crises.1
At the same time the VA was aggressively mounting a defense against the threat COVID-19 posed to the other missions, it also launched the fourth mission. In announcing these actions in April 2020, VA Secretary Robert Wilke succinctly summarized the need to balance the fourth mission with the other 3. “VA is committed to helping the nation in this effort to combat COVID-19. Helping veterans is our first mission, but in many locations across the country we’re helping states and local communities. VA is in this fight not only for the millions of veterans we serve each day; we’re in the fight for the people of the United States.”2
During the 2009 H1N1 pandemic I saw firsthand how VA disaster preparedness and emergency training were far superior to many academic and community health care systems. Given VA’s detailed and drilled crisis response plans, its specialized expertise in public health disasters, and its immense resources, it is no wonder that as the virus stretched civilian health care systems, some states turned to the VA for help. At my Albuquerque, New Mexico, VA medical center, 5 medical surgical beds and 3 intensive care beds were opened to the Indian Health Service overwhelmed with cases of COVID-19 in the hard-hit Navajo Nation. In New Jersey where Federal Practitioner is published, the fourth mission reached out to the state-run veterans homes as 90 VA nurses and gerontologists were deployed to 2 of its veterans facilities where close to 150 veterans have died.3 State veterans homes in Massachusetts, Pennsylvania, Alabama, and many other states have received supplies, including direly needed testing and personal protective equipment, staff, technology, and training.4
In July, VA published an impressive summary of fourth mission activities, which I encourage you to read. When you are look at this site, remember with a moment of silent appreciation all the altruistic and courageous VA clinical and administrative staff who volunteered for these assignments many of which put them directly in harm’s way.5
The VA is not alone in answering the call of COVID-19. In March, despite the grave risk to their health, their life, and their families, the USNS Comfort was deployed to New York City to help with its COVID-19 response while the USNS Mercy assisted in the efforts in Los Angeles. More recently, the military deployed > 700 Military Health System medical and support professionals to support COVID-19 operations in both Texas and California. Brooke Army Medical Center in San Antonio has taken on a handful of civilian patients with COVID-19 and increase its level I trauma cases as local hospitals have strained under the caseload.6
For the PHS Commissioned Corps its first mission is to serve as “America’s health responders.”7 This pandemic has intensified the extant health inequities in our country and compounded them with racial injustice and economic disparity. Thus, it is important to recognize that the very purpose of the PHS is to “fight disease, conduct research, and care for patients in underserved communities across the nation.”8 More than 3,900 PHS officers have been deployed nationally and internationally in COVID-19 clinical strike teams. Early in the pandemic the clinical response teams were deployed to a long-term care facility in Kirkland, Washington; convention center-based hospitals in New York City, Detroit, Michigan, and Washington DC, and Navajo Nation facilities. PHS officers also are providing clinical guidance at Bureau of Prison facilities for infection control and personal protective equipment training.
We know that there are many more examples of heroic service by federal health care professionals and staff than we could locate or celebrate in this brief column. Readers of this journal are well aware of the near constant criticism of the VA and calls for privatization,9 the inadequate funding of the PHS,10 and the recent downsizing of DoD health care11 that threatens to undermine its core functions. The pandemic has powerfully demonstrated that degrading the ability of federal health care to agilely and masterfully mobilize in the event of a public health disaster endangers not just veterans and the military but the health and well-being of a nation, particularly its most vulnerable citizens.
1. US Department of Veterans Affairs. About VA: VA mission statement. https://www.va.gov/about_va. Updated April 8, 2020. Accessed August 3, 2020.
2. US Department of Veterans Affairs, Office of Public and Intergovernmental Affairs. VA announces ‘Fourth Mission’ actions to help America respond to COVID-19. https://www.va.gov/opa/pressrel/pressrelease.cfm?id=5420. Published April 14, 2020. Accessed August 3, 2020.
3. Dyer J. COVID-19 strikes hard at state-run veterans nursing homes. https://www.mdedge.com/fedprac/article/221098/coronavirus-updates/covid-19-strikes-hard-state-run-veterans-nursing-homes. Published April 21, 2020. Accessed August 3, 2020.
4. Leigh D. Coronavirus news: VA secretary addresses COVID-19 deaths among veterans in the tri-state. https://abc7ny.com/va-secretary-veteran-covid-19-deaths-nursing-homes-veterans-memorial-home/6227770. Published June 3, 2020. Accessed August 3, 2020.
5. US Department of Veterans Affairs, Veterans Health Administration. VA Fourth Mission Summary. https://www.va.gov/health/coronavirus/statesupport.asp. Updated August 3, 2020. Accessed August 3, 2020.
6. Sanchez E. BAMC adapts to support greater San Antonio community during COVID-19 pandemic. https://www.health.mil/News/Articles/2020/07/15/BAMC-adapts-to-support-greater-San-Antonio-community-during-COVID-19-pandemic. Published July 17, 2020. Accessed August 3, 2020.
7. US Public Health Service. Commissioned Corps of the U.S. Public Health Service: America’s health responders. https://www.usphs.gov/default.aspx. Accessed August 3, 2020.
8. Kim EJ, Marrast L, Conigliaro J. COVID-19: magnifying the effect of health disparities. J Gen Intern Med . 2020;35(8):2441-2442. doi:10.1007/s11606-020-05881-4
9. Gordon S, Craven J. The best health system to react to COVID-19. The American Prospect. March 20, 2020. https://prospect.org/coronavirus/the-best-health-system-to-react-to-covid-19. Accessed August 1, 2020.
10. Lessons from the COVID-19 pandemic: it’s time to invest in public health. Fed Pract . 2020;37(suppl 3):S8-S11.
11. Wright O, Zuegel K. COVID-19 shows why military health care shouldn’t be downsized. https://www.militarytimes.com/opinion/commentary/2020/03/31/covid-19-shows-why-military-health-care-shouldnt-be-downsized. Published March 31, 2020. Accessed August 1,2020.
A torrent of blame has deluged the administration’s management of the pandemic. There is though one part of the government that deserves the praise of the nation for its response to this public health crisis—the federal health care system. In this column, we discuss the ways in which the Veterans Health Administration (VHA), the Department of Defense (DoD), and the US Public Health Service (PHS) Commissioned Corps especially have bravely and generously responded to the medical emergency of COVID-19 in the US.
Four missions drive the US Department of Veterans Affairs (VA). Though the fourth of these missions usually is in the background, it has risen to the forefront during the pandemic. To put the fourth mission in its proper perspective, we first should review the other 3 charges given to the largest integrated health care system in the country.
The first mission is to provide the highest quality care possible for the more than 9 million veterans enrolled in that system at each of the 1,255 VHA locations. The second mission is to ensure that the Veterans Benefits Administration delivers the full range of benefits that veterans earned through their service. These including funding for education, loans for homes, and many other types of support that assist service men and women to be successful in their transition from military to civilian life. The third mission is to honor the commitment of those who fought for their country unto death. The National Cemeteries Administration oversees 142 national cemeteries where veterans are buried with dignity and remembered with gratitude for their uniformed service. The purpose of these 3 internally focused missions is to provide a safety net for eligible veterans from the day they separate from the military until the hour they pass from this earth.
The fourth mission is different. This mission looks outside the military family to the civilian world. Its goal is to bolster the ability of the nation as a whole to handle wars, terrorism, national emergencies, and natural disasters. It does this through emergency response plans that preserve the integrity of the 3 other missions to veterans while enhancing the capacity of local and state governments to manage the threat of these public health, safety, or security crises.1
At the same time the VA was aggressively mounting a defense against the threat COVID-19 posed to the other missions, it also launched the fourth mission. In announcing these actions in April 2020, VA Secretary Robert Wilke succinctly summarized the need to balance the fourth mission with the other 3. “VA is committed to helping the nation in this effort to combat COVID-19. Helping veterans is our first mission, but in many locations across the country we’re helping states and local communities. VA is in this fight not only for the millions of veterans we serve each day; we’re in the fight for the people of the United States.”2
During the 2009 H1N1 pandemic I saw firsthand how VA disaster preparedness and emergency training were far superior to many academic and community health care systems. Given VA’s detailed and drilled crisis response plans, its specialized expertise in public health disasters, and its immense resources, it is no wonder that as the virus stretched civilian health care systems, some states turned to the VA for help. At my Albuquerque, New Mexico, VA medical center, 5 medical surgical beds and 3 intensive care beds were opened to the Indian Health Service overwhelmed with cases of COVID-19 in the hard-hit Navajo Nation. In New Jersey where Federal Practitioner is published, the fourth mission reached out to the state-run veterans homes as 90 VA nurses and gerontologists were deployed to 2 of its veterans facilities where close to 150 veterans have died.3 State veterans homes in Massachusetts, Pennsylvania, Alabama, and many other states have received supplies, including direly needed testing and personal protective equipment, staff, technology, and training.4
In July, VA published an impressive summary of fourth mission activities, which I encourage you to read. When you are look at this site, remember with a moment of silent appreciation all the altruistic and courageous VA clinical and administrative staff who volunteered for these assignments many of which put them directly in harm’s way.5
The VA is not alone in answering the call of COVID-19. In March, despite the grave risk to their health, their life, and their families, the USNS Comfort was deployed to New York City to help with its COVID-19 response while the USNS Mercy assisted in the efforts in Los Angeles. More recently, the military deployed > 700 Military Health System medical and support professionals to support COVID-19 operations in both Texas and California. Brooke Army Medical Center in San Antonio has taken on a handful of civilian patients with COVID-19 and increase its level I trauma cases as local hospitals have strained under the caseload.6
For the PHS Commissioned Corps its first mission is to serve as “America’s health responders.”7 This pandemic has intensified the extant health inequities in our country and compounded them with racial injustice and economic disparity. Thus, it is important to recognize that the very purpose of the PHS is to “fight disease, conduct research, and care for patients in underserved communities across the nation.”8 More than 3,900 PHS officers have been deployed nationally and internationally in COVID-19 clinical strike teams. Early in the pandemic the clinical response teams were deployed to a long-term care facility in Kirkland, Washington; convention center-based hospitals in New York City, Detroit, Michigan, and Washington DC, and Navajo Nation facilities. PHS officers also are providing clinical guidance at Bureau of Prison facilities for infection control and personal protective equipment training.
We know that there are many more examples of heroic service by federal health care professionals and staff than we could locate or celebrate in this brief column. Readers of this journal are well aware of the near constant criticism of the VA and calls for privatization,9 the inadequate funding of the PHS,10 and the recent downsizing of DoD health care11 that threatens to undermine its core functions. The pandemic has powerfully demonstrated that degrading the ability of federal health care to agilely and masterfully mobilize in the event of a public health disaster endangers not just veterans and the military but the health and well-being of a nation, particularly its most vulnerable citizens.
A torrent of blame has deluged the administration’s management of the pandemic. There is though one part of the government that deserves the praise of the nation for its response to this public health crisis—the federal health care system. In this column, we discuss the ways in which the Veterans Health Administration (VHA), the Department of Defense (DoD), and the US Public Health Service (PHS) Commissioned Corps especially have bravely and generously responded to the medical emergency of COVID-19 in the US.
Four missions drive the US Department of Veterans Affairs (VA). Though the fourth of these missions usually is in the background, it has risen to the forefront during the pandemic. To put the fourth mission in its proper perspective, we first should review the other 3 charges given to the largest integrated health care system in the country.
The first mission is to provide the highest quality care possible for the more than 9 million veterans enrolled in that system at each of the 1,255 VHA locations. The second mission is to ensure that the Veterans Benefits Administration delivers the full range of benefits that veterans earned through their service. These including funding for education, loans for homes, and many other types of support that assist service men and women to be successful in their transition from military to civilian life. The third mission is to honor the commitment of those who fought for their country unto death. The National Cemeteries Administration oversees 142 national cemeteries where veterans are buried with dignity and remembered with gratitude for their uniformed service. The purpose of these 3 internally focused missions is to provide a safety net for eligible veterans from the day they separate from the military until the hour they pass from this earth.
The fourth mission is different. This mission looks outside the military family to the civilian world. Its goal is to bolster the ability of the nation as a whole to handle wars, terrorism, national emergencies, and natural disasters. It does this through emergency response plans that preserve the integrity of the 3 other missions to veterans while enhancing the capacity of local and state governments to manage the threat of these public health, safety, or security crises.1
At the same time the VA was aggressively mounting a defense against the threat COVID-19 posed to the other missions, it also launched the fourth mission. In announcing these actions in April 2020, VA Secretary Robert Wilke succinctly summarized the need to balance the fourth mission with the other 3. “VA is committed to helping the nation in this effort to combat COVID-19. Helping veterans is our first mission, but in many locations across the country we’re helping states and local communities. VA is in this fight not only for the millions of veterans we serve each day; we’re in the fight for the people of the United States.”2
During the 2009 H1N1 pandemic I saw firsthand how VA disaster preparedness and emergency training were far superior to many academic and community health care systems. Given VA’s detailed and drilled crisis response plans, its specialized expertise in public health disasters, and its immense resources, it is no wonder that as the virus stretched civilian health care systems, some states turned to the VA for help. At my Albuquerque, New Mexico, VA medical center, 5 medical surgical beds and 3 intensive care beds were opened to the Indian Health Service overwhelmed with cases of COVID-19 in the hard-hit Navajo Nation. In New Jersey where Federal Practitioner is published, the fourth mission reached out to the state-run veterans homes as 90 VA nurses and gerontologists were deployed to 2 of its veterans facilities where close to 150 veterans have died.3 State veterans homes in Massachusetts, Pennsylvania, Alabama, and many other states have received supplies, including direly needed testing and personal protective equipment, staff, technology, and training.4
In July, VA published an impressive summary of fourth mission activities, which I encourage you to read. When you are look at this site, remember with a moment of silent appreciation all the altruistic and courageous VA clinical and administrative staff who volunteered for these assignments many of which put them directly in harm’s way.5
The VA is not alone in answering the call of COVID-19. In March, despite the grave risk to their health, their life, and their families, the USNS Comfort was deployed to New York City to help with its COVID-19 response while the USNS Mercy assisted in the efforts in Los Angeles. More recently, the military deployed > 700 Military Health System medical and support professionals to support COVID-19 operations in both Texas and California. Brooke Army Medical Center in San Antonio has taken on a handful of civilian patients with COVID-19 and increase its level I trauma cases as local hospitals have strained under the caseload.6
For the PHS Commissioned Corps its first mission is to serve as “America’s health responders.”7 This pandemic has intensified the extant health inequities in our country and compounded them with racial injustice and economic disparity. Thus, it is important to recognize that the very purpose of the PHS is to “fight disease, conduct research, and care for patients in underserved communities across the nation.”8 More than 3,900 PHS officers have been deployed nationally and internationally in COVID-19 clinical strike teams. Early in the pandemic the clinical response teams were deployed to a long-term care facility in Kirkland, Washington; convention center-based hospitals in New York City, Detroit, Michigan, and Washington DC, and Navajo Nation facilities. PHS officers also are providing clinical guidance at Bureau of Prison facilities for infection control and personal protective equipment training.
We know that there are many more examples of heroic service by federal health care professionals and staff than we could locate or celebrate in this brief column. Readers of this journal are well aware of the near constant criticism of the VA and calls for privatization,9 the inadequate funding of the PHS,10 and the recent downsizing of DoD health care11 that threatens to undermine its core functions. The pandemic has powerfully demonstrated that degrading the ability of federal health care to agilely and masterfully mobilize in the event of a public health disaster endangers not just veterans and the military but the health and well-being of a nation, particularly its most vulnerable citizens.
1. US Department of Veterans Affairs. About VA: VA mission statement. https://www.va.gov/about_va. Updated April 8, 2020. Accessed August 3, 2020.
2. US Department of Veterans Affairs, Office of Public and Intergovernmental Affairs. VA announces ‘Fourth Mission’ actions to help America respond to COVID-19. https://www.va.gov/opa/pressrel/pressrelease.cfm?id=5420. Published April 14, 2020. Accessed August 3, 2020.
3. Dyer J. COVID-19 strikes hard at state-run veterans nursing homes. https://www.mdedge.com/fedprac/article/221098/coronavirus-updates/covid-19-strikes-hard-state-run-veterans-nursing-homes. Published April 21, 2020. Accessed August 3, 2020.
4. Leigh D. Coronavirus news: VA secretary addresses COVID-19 deaths among veterans in the tri-state. https://abc7ny.com/va-secretary-veteran-covid-19-deaths-nursing-homes-veterans-memorial-home/6227770. Published June 3, 2020. Accessed August 3, 2020.
5. US Department of Veterans Affairs, Veterans Health Administration. VA Fourth Mission Summary. https://www.va.gov/health/coronavirus/statesupport.asp. Updated August 3, 2020. Accessed August 3, 2020.
6. Sanchez E. BAMC adapts to support greater San Antonio community during COVID-19 pandemic. https://www.health.mil/News/Articles/2020/07/15/BAMC-adapts-to-support-greater-San-Antonio-community-during-COVID-19-pandemic. Published July 17, 2020. Accessed August 3, 2020.
7. US Public Health Service. Commissioned Corps of the U.S. Public Health Service: America’s health responders. https://www.usphs.gov/default.aspx. Accessed August 3, 2020.
8. Kim EJ, Marrast L, Conigliaro J. COVID-19: magnifying the effect of health disparities. J Gen Intern Med . 2020;35(8):2441-2442. doi:10.1007/s11606-020-05881-4
9. Gordon S, Craven J. The best health system to react to COVID-19. The American Prospect. March 20, 2020. https://prospect.org/coronavirus/the-best-health-system-to-react-to-covid-19. Accessed August 1, 2020.
10. Lessons from the COVID-19 pandemic: it’s time to invest in public health. Fed Pract . 2020;37(suppl 3):S8-S11.
11. Wright O, Zuegel K. COVID-19 shows why military health care shouldn’t be downsized. https://www.militarytimes.com/opinion/commentary/2020/03/31/covid-19-shows-why-military-health-care-shouldnt-be-downsized. Published March 31, 2020. Accessed August 1,2020.
1. US Department of Veterans Affairs. About VA: VA mission statement. https://www.va.gov/about_va. Updated April 8, 2020. Accessed August 3, 2020.
2. US Department of Veterans Affairs, Office of Public and Intergovernmental Affairs. VA announces ‘Fourth Mission’ actions to help America respond to COVID-19. https://www.va.gov/opa/pressrel/pressrelease.cfm?id=5420. Published April 14, 2020. Accessed August 3, 2020.
3. Dyer J. COVID-19 strikes hard at state-run veterans nursing homes. https://www.mdedge.com/fedprac/article/221098/coronavirus-updates/covid-19-strikes-hard-state-run-veterans-nursing-homes. Published April 21, 2020. Accessed August 3, 2020.
4. Leigh D. Coronavirus news: VA secretary addresses COVID-19 deaths among veterans in the tri-state. https://abc7ny.com/va-secretary-veteran-covid-19-deaths-nursing-homes-veterans-memorial-home/6227770. Published June 3, 2020. Accessed August 3, 2020.
5. US Department of Veterans Affairs, Veterans Health Administration. VA Fourth Mission Summary. https://www.va.gov/health/coronavirus/statesupport.asp. Updated August 3, 2020. Accessed August 3, 2020.
6. Sanchez E. BAMC adapts to support greater San Antonio community during COVID-19 pandemic. https://www.health.mil/News/Articles/2020/07/15/BAMC-adapts-to-support-greater-San-Antonio-community-during-COVID-19-pandemic. Published July 17, 2020. Accessed August 3, 2020.
7. US Public Health Service. Commissioned Corps of the U.S. Public Health Service: America’s health responders. https://www.usphs.gov/default.aspx. Accessed August 3, 2020.
8. Kim EJ, Marrast L, Conigliaro J. COVID-19: magnifying the effect of health disparities. J Gen Intern Med . 2020;35(8):2441-2442. doi:10.1007/s11606-020-05881-4
9. Gordon S, Craven J. The best health system to react to COVID-19. The American Prospect. March 20, 2020. https://prospect.org/coronavirus/the-best-health-system-to-react-to-covid-19. Accessed August 1, 2020.
10. Lessons from the COVID-19 pandemic: it’s time to invest in public health. Fed Pract . 2020;37(suppl 3):S8-S11.
11. Wright O, Zuegel K. COVID-19 shows why military health care shouldn’t be downsized. https://www.militarytimes.com/opinion/commentary/2020/03/31/covid-19-shows-why-military-health-care-shouldnt-be-downsized. Published March 31, 2020. Accessed August 1,2020.
Tales of the Pandemic
After learning about coronavirus disease 2019 (COVID-19) on the news, we were all aware that it would eventually affect our lives and our dermatology practices. However, once the COVID-19 pandemic arrived in the United States, we were under a shelter-in-place order, schools were shut, and most businesses were closed within a few weeks.
As dermatologists, we were considered essential workers, and our offices could remain open. However, as the numbers of cases accelerated in New York City—the global epicenter of the pandemic—and we approached our peak, I closed down my practice, except for emergencies.
One of the first medical challenges dermatologists faced in the early days of the COVID-19 pandemic was the proper management of our psoriasis patients. The major concern was that patients on biologics and other immunomodulatory therapies might be at an increased risk for COVID-19 infection and increased morbidity if affected. I received a multitude of telephone calls from patients taking these therapies who expressed high levels of concern and anxiety and were looking for direction as to whether they should continue their medications.
Early on, several of our professional societies provided guidelines regarding the use of systemic immunosuppressive agents during the COVID-19 pandemic. On April 15, 2020, the American Academy of Dermatology (AAD) advised, “Dermatologists must delicately balance the risk of immunosuppression with the risk of disease flare requiring urgent intervention with patient-specific risks.”1 The AAD strongly recommended that patients should not stop their ongoing systemic immunosuppressive therapy without consulting their physicians. The AAD’s guidance provided specific recommendations for the following groups: (1) patients on systemic immunosuppressive agents who have not tested positive or exhibited signs/symptoms of COVID-19, (2) patients on systemic immunosuppressive agents who have tested positive for COVID-19 or exhibit signs/symptoms of COVID-19, (3) patients who have halted systemic immunosuppressive therapy after testing positive for COVID-19 (in whom it recommended physicians could reinitiate treatment), and (4) patients being considered for systemic immunosuppressive agents.1
The National Psoriasis Foundation (NPF) also recognized the need for additional guidelines for health care providers and patients on managing psoriatic disease during the COVID-19 pandemic. In June 2020, the NPF formed a COVID-19 Task Force, which released its own recommendations for adult and pediatric patients with psoriatic disease.2 Similar to the AAD, the NPF COVID-19 Task Force recommended that patients do not stop biologic or oral therapies for psoriasis during the current health crisis, stating the following: “While some uncertainties remain, initial data suggest that the benefit of continuing treatments for psoriatic diseases outweighs the hypothetical risks associated with immune modulating treatment of poor COVID-19–related outcomes for most patients.” Individuals in high-risk groups were advised to consult their health care providers regarding whether they should continue or alter therapy during the pandemic, and the clinical decision would be guided by the specific treatment regimen; the patient’s age, disease characteristics, and underlying medical conditions; or any particular concerns. Additionally, the task force emphasized that patients with psoriatic disease should continue to follow common sense measures to lower the risk of becoming infected with COVID-19, including practicing physical distancing, wearing face coverings in public settings, and washing their hands regularly.2
We remain in the midst of the COVID-19 pandemic with no true guidance as to the future course and impact of the infection. It is important to realize that our understanding of the coronavirus and its impact on our patients is constantly evolving. I encourage all providers to stay current with updates on clinical guidelines. In addition, we should pay attention to the myriad of clinical trials and registries now underway, as they may provide more insight into optimal clinical management in these challenging times.
Most importantly, stay safe!
- American Academy of Dermatology. Guidance on the use of medications during COVID-19 outbreak. https://assets.ctfassets.net/1ny4yoiyrqia/PicgNuD0IpYd9MSOwab47/5e6d85324e7b5aafed45dde0ac4ea21e/Guidance_on_medications_AHTF_approved_April_15.pdf. Updated April 15, 2020. Accessed July 27, 2020.
- National Psoriasis Foundation. NPF forms COVID-19 Task Force. https://www.psoriasis.org/advance/coronavirus. Updated July 7, 2020. Accessed July 27, 2020.
After learning about coronavirus disease 2019 (COVID-19) on the news, we were all aware that it would eventually affect our lives and our dermatology practices. However, once the COVID-19 pandemic arrived in the United States, we were under a shelter-in-place order, schools were shut, and most businesses were closed within a few weeks.
As dermatologists, we were considered essential workers, and our offices could remain open. However, as the numbers of cases accelerated in New York City—the global epicenter of the pandemic—and we approached our peak, I closed down my practice, except for emergencies.
One of the first medical challenges dermatologists faced in the early days of the COVID-19 pandemic was the proper management of our psoriasis patients. The major concern was that patients on biologics and other immunomodulatory therapies might be at an increased risk for COVID-19 infection and increased morbidity if affected. I received a multitude of telephone calls from patients taking these therapies who expressed high levels of concern and anxiety and were looking for direction as to whether they should continue their medications.
Early on, several of our professional societies provided guidelines regarding the use of systemic immunosuppressive agents during the COVID-19 pandemic. On April 15, 2020, the American Academy of Dermatology (AAD) advised, “Dermatologists must delicately balance the risk of immunosuppression with the risk of disease flare requiring urgent intervention with patient-specific risks.”1 The AAD strongly recommended that patients should not stop their ongoing systemic immunosuppressive therapy without consulting their physicians. The AAD’s guidance provided specific recommendations for the following groups: (1) patients on systemic immunosuppressive agents who have not tested positive or exhibited signs/symptoms of COVID-19, (2) patients on systemic immunosuppressive agents who have tested positive for COVID-19 or exhibit signs/symptoms of COVID-19, (3) patients who have halted systemic immunosuppressive therapy after testing positive for COVID-19 (in whom it recommended physicians could reinitiate treatment), and (4) patients being considered for systemic immunosuppressive agents.1
The National Psoriasis Foundation (NPF) also recognized the need for additional guidelines for health care providers and patients on managing psoriatic disease during the COVID-19 pandemic. In June 2020, the NPF formed a COVID-19 Task Force, which released its own recommendations for adult and pediatric patients with psoriatic disease.2 Similar to the AAD, the NPF COVID-19 Task Force recommended that patients do not stop biologic or oral therapies for psoriasis during the current health crisis, stating the following: “While some uncertainties remain, initial data suggest that the benefit of continuing treatments for psoriatic diseases outweighs the hypothetical risks associated with immune modulating treatment of poor COVID-19–related outcomes for most patients.” Individuals in high-risk groups were advised to consult their health care providers regarding whether they should continue or alter therapy during the pandemic, and the clinical decision would be guided by the specific treatment regimen; the patient’s age, disease characteristics, and underlying medical conditions; or any particular concerns. Additionally, the task force emphasized that patients with psoriatic disease should continue to follow common sense measures to lower the risk of becoming infected with COVID-19, including practicing physical distancing, wearing face coverings in public settings, and washing their hands regularly.2
We remain in the midst of the COVID-19 pandemic with no true guidance as to the future course and impact of the infection. It is important to realize that our understanding of the coronavirus and its impact on our patients is constantly evolving. I encourage all providers to stay current with updates on clinical guidelines. In addition, we should pay attention to the myriad of clinical trials and registries now underway, as they may provide more insight into optimal clinical management in these challenging times.
Most importantly, stay safe!
After learning about coronavirus disease 2019 (COVID-19) on the news, we were all aware that it would eventually affect our lives and our dermatology practices. However, once the COVID-19 pandemic arrived in the United States, we were under a shelter-in-place order, schools were shut, and most businesses were closed within a few weeks.
As dermatologists, we were considered essential workers, and our offices could remain open. However, as the numbers of cases accelerated in New York City—the global epicenter of the pandemic—and we approached our peak, I closed down my practice, except for emergencies.
One of the first medical challenges dermatologists faced in the early days of the COVID-19 pandemic was the proper management of our psoriasis patients. The major concern was that patients on biologics and other immunomodulatory therapies might be at an increased risk for COVID-19 infection and increased morbidity if affected. I received a multitude of telephone calls from patients taking these therapies who expressed high levels of concern and anxiety and were looking for direction as to whether they should continue their medications.
Early on, several of our professional societies provided guidelines regarding the use of systemic immunosuppressive agents during the COVID-19 pandemic. On April 15, 2020, the American Academy of Dermatology (AAD) advised, “Dermatologists must delicately balance the risk of immunosuppression with the risk of disease flare requiring urgent intervention with patient-specific risks.”1 The AAD strongly recommended that patients should not stop their ongoing systemic immunosuppressive therapy without consulting their physicians. The AAD’s guidance provided specific recommendations for the following groups: (1) patients on systemic immunosuppressive agents who have not tested positive or exhibited signs/symptoms of COVID-19, (2) patients on systemic immunosuppressive agents who have tested positive for COVID-19 or exhibit signs/symptoms of COVID-19, (3) patients who have halted systemic immunosuppressive therapy after testing positive for COVID-19 (in whom it recommended physicians could reinitiate treatment), and (4) patients being considered for systemic immunosuppressive agents.1
The National Psoriasis Foundation (NPF) also recognized the need for additional guidelines for health care providers and patients on managing psoriatic disease during the COVID-19 pandemic. In June 2020, the NPF formed a COVID-19 Task Force, which released its own recommendations for adult and pediatric patients with psoriatic disease.2 Similar to the AAD, the NPF COVID-19 Task Force recommended that patients do not stop biologic or oral therapies for psoriasis during the current health crisis, stating the following: “While some uncertainties remain, initial data suggest that the benefit of continuing treatments for psoriatic diseases outweighs the hypothetical risks associated with immune modulating treatment of poor COVID-19–related outcomes for most patients.” Individuals in high-risk groups were advised to consult their health care providers regarding whether they should continue or alter therapy during the pandemic, and the clinical decision would be guided by the specific treatment regimen; the patient’s age, disease characteristics, and underlying medical conditions; or any particular concerns. Additionally, the task force emphasized that patients with psoriatic disease should continue to follow common sense measures to lower the risk of becoming infected with COVID-19, including practicing physical distancing, wearing face coverings in public settings, and washing their hands regularly.2
We remain in the midst of the COVID-19 pandemic with no true guidance as to the future course and impact of the infection. It is important to realize that our understanding of the coronavirus and its impact on our patients is constantly evolving. I encourage all providers to stay current with updates on clinical guidelines. In addition, we should pay attention to the myriad of clinical trials and registries now underway, as they may provide more insight into optimal clinical management in these challenging times.
Most importantly, stay safe!
- American Academy of Dermatology. Guidance on the use of medications during COVID-19 outbreak. https://assets.ctfassets.net/1ny4yoiyrqia/PicgNuD0IpYd9MSOwab47/5e6d85324e7b5aafed45dde0ac4ea21e/Guidance_on_medications_AHTF_approved_April_15.pdf. Updated April 15, 2020. Accessed July 27, 2020.
- National Psoriasis Foundation. NPF forms COVID-19 Task Force. https://www.psoriasis.org/advance/coronavirus. Updated July 7, 2020. Accessed July 27, 2020.
- American Academy of Dermatology. Guidance on the use of medications during COVID-19 outbreak. https://assets.ctfassets.net/1ny4yoiyrqia/PicgNuD0IpYd9MSOwab47/5e6d85324e7b5aafed45dde0ac4ea21e/Guidance_on_medications_AHTF_approved_April_15.pdf. Updated April 15, 2020. Accessed July 27, 2020.
- National Psoriasis Foundation. NPF forms COVID-19 Task Force. https://www.psoriasis.org/advance/coronavirus. Updated July 7, 2020. Accessed July 27, 2020.
Utilization of Telehealth Services During the COVID-19 Pandemic
In 2017, lawmakers and insurers in the state of Texas approved the use of telehealth services in times of crisis.1 During the coronavirus disease 2019 (COVID-19) pandemic, our clinic has used telemedicine to provide remote care to dermatology patients. We posit that the quick introduction and implementation of telemedicine during this time of need will change the way we practice dermatology in the future.
At the University of Texas Medical Branch in Galveston, Texas, we primarily have used 2 forms of telemedicine during the COVID-19 pandemic: live face-to-face video communication (our institution primarily uses FaceTime), and a combination of telephone calls with store-and-forward images. All dermatology services at our institution were converted to telemedicine visits, and in-person office visits were only done if deemed necessary after triage by telemedicine in April and May 2020. This strategy removed the necessity for patients to leave their homes for their appointments, which not only saved them travel costs and time but also reduced the potential spread of COVID-19. Since this time, the clinic has reopened for in-person visits; however, patients still have the option to schedule a telehealth appointment if they prefer. Many patients still select the telehealth option for the above reasons.
Although routine skin checks were not always possible by video and/or store-and-forward images, telemedicine worked very well for follow-up visits, especially isotretinoin follow-ups. During the COVID-19 outbreak, iPLEDGE (https://www.ipledgeprogram.com/iPledgeUI/home.u) rapidly adapted to the use of telemedicine and even began to allow home pregnancy tests to be entered into the iPLEDGE system by health care providers. Isotretinoin follow-ups are especially useful for patients who do not require laboratory monitoring at the visit. Patients are easily evaluated, screened for side effects, and continued on their treatment if no concerns are found during the telemedicine visit. Patients who require laboratory monitoring are still able to schedule tests at our clinics or at free-standing laboratories near their homes without having an in-office dermatology appointment. At-home pregnancy tests are still being utilized as an option for patients electing for telehealth follow-ups. This strategy is both health conscious by protecting the patient from exposure to COVID-19 at a testing center and cost-effective, especially for our uninsured patients, while still meeting the safety check for iPLEDGE.
Additionally, we utilized store-and-forward telemedicine for hospital consultations. If the patient’s condition can easily be diagnosed by viewing unedited clinical images remotely, the clinician can further decrease the risk of COVID-19 spread and exposure by providing the consultation and treatment recommendations by telephone. In cases in which a diagnosis could not be made by reviewing clinical photographs remotely, an in-person visit would be done. We continue to use this strategy for our confirmed COVID-positive hospital consultations to help protect our faculty and residents and decrease the use of personal protective equipment. We propose this model could be instituted for patients admitted to hospitals without access to dermatology consultations. Store-and-forward photographs of worrisome lesions and rashes also can be used to triage visits. For example, a patient with a new-onset keratoacanthoma and a history of nonmelanoma skin cancer contacted our clinic during the pandemic and sent store-and-forward images for review. The patient was triaged by a telemedicine visit and was then brought into the clinic for biopsy based on his clinical photographs and history. Patients also have requested prescriptions for bimatoprost and tretinoin via telehealth, a service that many medical spas and online telehealth companies provide already but was not offered at our practice until now.
Telemedicine also has potentially helped decrease the number of patients going to urgent care clinics for dermatology-related issues. Additionally, we have utilized one provider per day to be the “on-call” dermatologist who would be doing telemedicine appointments for patients with new-onset conditions. This strategy not only minimized possible patient exposure to COVID-19 but also helped preserve resources at urgent care clinics and emergency departments, which currently are inundated with patients. Since we have reopened for in-person visits, we have been unable to sustain an on-call dermatologist for telemedicine but may re-employ this strategy in the future.
The unique experience of practicing medicine during a pandemic has and will affect the way we practice moving forward. The way telemedicine has been quickly and easily implemented by the health care community during the COVID-19 pandemic has taught our dermatologists the value of this method of health care delivery. We will likely continue to use telemedicine after the pandemic has been contained. Telemedicine has the potential to expand access to care to rural and underserved areas, hospitals without on-call dermatologists, and homebound patients. We also may be better able to provide isotretinoin to our patients who have deferred treatment due to difficulty with transportation to the monthly visits. Store-and-forward images could help patients referred to dermatology avoid long wait times for obvious skin cancers that would benefit from early treatment. Telemedicine visits also could potentially improve attendance for patients who forget about their appointment by calling them after they miss their scheduled appointment time and complete a telehealth encounter on the same day instead, which could help recover costs of no-show appointments for clinics.
It is still unclear how private insurance companies will adapt to the new use of telemedicine, but we hope they follow the lead of Medicare, which released a statement on March 6, 2020, supporting the implementation of telehealth services.2 Although Medicare has made adjustments to allow for equal reimbursement for telehealth appointments, private insurance companies still vary greatly. Many practices are struggling and some remained open despite shelter-in-place orders, but we propose telemedicine may be a safer alternative for patients and providers during the current health crisis that would keep billable services in place. It is still uncertain whether the laws enacted to make telemedicine accessible during this time will hold after COVID-19 is contained, but we are hopeful that living through the pandemic will bring some positive benefit to our practice and the patients we serve.
- Texas laws and regulations relating to telemedicine. Texas Medical Association website. https://www.texmed.org/Template.aspx?id=47554. Updated March 19, 2020. Accessed July 14, 2020.
- Centers for Medicare & Medicaid Services. President Trump expands telehealth benefits for Medicare beneficiaries during COVID 19 outbreak. https://www.cms.gov/newsroom/press-releases/president-trump-expands-telehealth-benefits-medicare-beneficiaries-during-covid-19-outbreak. Published March 17, 2020. Accessed July 14, 2020.
In 2017, lawmakers and insurers in the state of Texas approved the use of telehealth services in times of crisis.1 During the coronavirus disease 2019 (COVID-19) pandemic, our clinic has used telemedicine to provide remote care to dermatology patients. We posit that the quick introduction and implementation of telemedicine during this time of need will change the way we practice dermatology in the future.
At the University of Texas Medical Branch in Galveston, Texas, we primarily have used 2 forms of telemedicine during the COVID-19 pandemic: live face-to-face video communication (our institution primarily uses FaceTime), and a combination of telephone calls with store-and-forward images. All dermatology services at our institution were converted to telemedicine visits, and in-person office visits were only done if deemed necessary after triage by telemedicine in April and May 2020. This strategy removed the necessity for patients to leave their homes for their appointments, which not only saved them travel costs and time but also reduced the potential spread of COVID-19. Since this time, the clinic has reopened for in-person visits; however, patients still have the option to schedule a telehealth appointment if they prefer. Many patients still select the telehealth option for the above reasons.
Although routine skin checks were not always possible by video and/or store-and-forward images, telemedicine worked very well for follow-up visits, especially isotretinoin follow-ups. During the COVID-19 outbreak, iPLEDGE (https://www.ipledgeprogram.com/iPledgeUI/home.u) rapidly adapted to the use of telemedicine and even began to allow home pregnancy tests to be entered into the iPLEDGE system by health care providers. Isotretinoin follow-ups are especially useful for patients who do not require laboratory monitoring at the visit. Patients are easily evaluated, screened for side effects, and continued on their treatment if no concerns are found during the telemedicine visit. Patients who require laboratory monitoring are still able to schedule tests at our clinics or at free-standing laboratories near their homes without having an in-office dermatology appointment. At-home pregnancy tests are still being utilized as an option for patients electing for telehealth follow-ups. This strategy is both health conscious by protecting the patient from exposure to COVID-19 at a testing center and cost-effective, especially for our uninsured patients, while still meeting the safety check for iPLEDGE.
Additionally, we utilized store-and-forward telemedicine for hospital consultations. If the patient’s condition can easily be diagnosed by viewing unedited clinical images remotely, the clinician can further decrease the risk of COVID-19 spread and exposure by providing the consultation and treatment recommendations by telephone. In cases in which a diagnosis could not be made by reviewing clinical photographs remotely, an in-person visit would be done. We continue to use this strategy for our confirmed COVID-positive hospital consultations to help protect our faculty and residents and decrease the use of personal protective equipment. We propose this model could be instituted for patients admitted to hospitals without access to dermatology consultations. Store-and-forward photographs of worrisome lesions and rashes also can be used to triage visits. For example, a patient with a new-onset keratoacanthoma and a history of nonmelanoma skin cancer contacted our clinic during the pandemic and sent store-and-forward images for review. The patient was triaged by a telemedicine visit and was then brought into the clinic for biopsy based on his clinical photographs and history. Patients also have requested prescriptions for bimatoprost and tretinoin via telehealth, a service that many medical spas and online telehealth companies provide already but was not offered at our practice until now.
Telemedicine also has potentially helped decrease the number of patients going to urgent care clinics for dermatology-related issues. Additionally, we have utilized one provider per day to be the “on-call” dermatologist who would be doing telemedicine appointments for patients with new-onset conditions. This strategy not only minimized possible patient exposure to COVID-19 but also helped preserve resources at urgent care clinics and emergency departments, which currently are inundated with patients. Since we have reopened for in-person visits, we have been unable to sustain an on-call dermatologist for telemedicine but may re-employ this strategy in the future.
The unique experience of practicing medicine during a pandemic has and will affect the way we practice moving forward. The way telemedicine has been quickly and easily implemented by the health care community during the COVID-19 pandemic has taught our dermatologists the value of this method of health care delivery. We will likely continue to use telemedicine after the pandemic has been contained. Telemedicine has the potential to expand access to care to rural and underserved areas, hospitals without on-call dermatologists, and homebound patients. We also may be better able to provide isotretinoin to our patients who have deferred treatment due to difficulty with transportation to the monthly visits. Store-and-forward images could help patients referred to dermatology avoid long wait times for obvious skin cancers that would benefit from early treatment. Telemedicine visits also could potentially improve attendance for patients who forget about their appointment by calling them after they miss their scheduled appointment time and complete a telehealth encounter on the same day instead, which could help recover costs of no-show appointments for clinics.
It is still unclear how private insurance companies will adapt to the new use of telemedicine, but we hope they follow the lead of Medicare, which released a statement on March 6, 2020, supporting the implementation of telehealth services.2 Although Medicare has made adjustments to allow for equal reimbursement for telehealth appointments, private insurance companies still vary greatly. Many practices are struggling and some remained open despite shelter-in-place orders, but we propose telemedicine may be a safer alternative for patients and providers during the current health crisis that would keep billable services in place. It is still uncertain whether the laws enacted to make telemedicine accessible during this time will hold after COVID-19 is contained, but we are hopeful that living through the pandemic will bring some positive benefit to our practice and the patients we serve.
In 2017, lawmakers and insurers in the state of Texas approved the use of telehealth services in times of crisis.1 During the coronavirus disease 2019 (COVID-19) pandemic, our clinic has used telemedicine to provide remote care to dermatology patients. We posit that the quick introduction and implementation of telemedicine during this time of need will change the way we practice dermatology in the future.
At the University of Texas Medical Branch in Galveston, Texas, we primarily have used 2 forms of telemedicine during the COVID-19 pandemic: live face-to-face video communication (our institution primarily uses FaceTime), and a combination of telephone calls with store-and-forward images. All dermatology services at our institution were converted to telemedicine visits, and in-person office visits were only done if deemed necessary after triage by telemedicine in April and May 2020. This strategy removed the necessity for patients to leave their homes for their appointments, which not only saved them travel costs and time but also reduced the potential spread of COVID-19. Since this time, the clinic has reopened for in-person visits; however, patients still have the option to schedule a telehealth appointment if they prefer. Many patients still select the telehealth option for the above reasons.
Although routine skin checks were not always possible by video and/or store-and-forward images, telemedicine worked very well for follow-up visits, especially isotretinoin follow-ups. During the COVID-19 outbreak, iPLEDGE (https://www.ipledgeprogram.com/iPledgeUI/home.u) rapidly adapted to the use of telemedicine and even began to allow home pregnancy tests to be entered into the iPLEDGE system by health care providers. Isotretinoin follow-ups are especially useful for patients who do not require laboratory monitoring at the visit. Patients are easily evaluated, screened for side effects, and continued on their treatment if no concerns are found during the telemedicine visit. Patients who require laboratory monitoring are still able to schedule tests at our clinics or at free-standing laboratories near their homes without having an in-office dermatology appointment. At-home pregnancy tests are still being utilized as an option for patients electing for telehealth follow-ups. This strategy is both health conscious by protecting the patient from exposure to COVID-19 at a testing center and cost-effective, especially for our uninsured patients, while still meeting the safety check for iPLEDGE.
Additionally, we utilized store-and-forward telemedicine for hospital consultations. If the patient’s condition can easily be diagnosed by viewing unedited clinical images remotely, the clinician can further decrease the risk of COVID-19 spread and exposure by providing the consultation and treatment recommendations by telephone. In cases in which a diagnosis could not be made by reviewing clinical photographs remotely, an in-person visit would be done. We continue to use this strategy for our confirmed COVID-positive hospital consultations to help protect our faculty and residents and decrease the use of personal protective equipment. We propose this model could be instituted for patients admitted to hospitals without access to dermatology consultations. Store-and-forward photographs of worrisome lesions and rashes also can be used to triage visits. For example, a patient with a new-onset keratoacanthoma and a history of nonmelanoma skin cancer contacted our clinic during the pandemic and sent store-and-forward images for review. The patient was triaged by a telemedicine visit and was then brought into the clinic for biopsy based on his clinical photographs and history. Patients also have requested prescriptions for bimatoprost and tretinoin via telehealth, a service that many medical spas and online telehealth companies provide already but was not offered at our practice until now.
Telemedicine also has potentially helped decrease the number of patients going to urgent care clinics for dermatology-related issues. Additionally, we have utilized one provider per day to be the “on-call” dermatologist who would be doing telemedicine appointments for patients with new-onset conditions. This strategy not only minimized possible patient exposure to COVID-19 but also helped preserve resources at urgent care clinics and emergency departments, which currently are inundated with patients. Since we have reopened for in-person visits, we have been unable to sustain an on-call dermatologist for telemedicine but may re-employ this strategy in the future.
The unique experience of practicing medicine during a pandemic has and will affect the way we practice moving forward. The way telemedicine has been quickly and easily implemented by the health care community during the COVID-19 pandemic has taught our dermatologists the value of this method of health care delivery. We will likely continue to use telemedicine after the pandemic has been contained. Telemedicine has the potential to expand access to care to rural and underserved areas, hospitals without on-call dermatologists, and homebound patients. We also may be better able to provide isotretinoin to our patients who have deferred treatment due to difficulty with transportation to the monthly visits. Store-and-forward images could help patients referred to dermatology avoid long wait times for obvious skin cancers that would benefit from early treatment. Telemedicine visits also could potentially improve attendance for patients who forget about their appointment by calling them after they miss their scheduled appointment time and complete a telehealth encounter on the same day instead, which could help recover costs of no-show appointments for clinics.
It is still unclear how private insurance companies will adapt to the new use of telemedicine, but we hope they follow the lead of Medicare, which released a statement on March 6, 2020, supporting the implementation of telehealth services.2 Although Medicare has made adjustments to allow for equal reimbursement for telehealth appointments, private insurance companies still vary greatly. Many practices are struggling and some remained open despite shelter-in-place orders, but we propose telemedicine may be a safer alternative for patients and providers during the current health crisis that would keep billable services in place. It is still uncertain whether the laws enacted to make telemedicine accessible during this time will hold after COVID-19 is contained, but we are hopeful that living through the pandemic will bring some positive benefit to our practice and the patients we serve.
- Texas laws and regulations relating to telemedicine. Texas Medical Association website. https://www.texmed.org/Template.aspx?id=47554. Updated March 19, 2020. Accessed July 14, 2020.
- Centers for Medicare & Medicaid Services. President Trump expands telehealth benefits for Medicare beneficiaries during COVID 19 outbreak. https://www.cms.gov/newsroom/press-releases/president-trump-expands-telehealth-benefits-medicare-beneficiaries-during-covid-19-outbreak. Published March 17, 2020. Accessed July 14, 2020.
- Texas laws and regulations relating to telemedicine. Texas Medical Association website. https://www.texmed.org/Template.aspx?id=47554. Updated March 19, 2020. Accessed July 14, 2020.
- Centers for Medicare & Medicaid Services. President Trump expands telehealth benefits for Medicare beneficiaries during COVID 19 outbreak. https://www.cms.gov/newsroom/press-releases/president-trump-expands-telehealth-benefits-medicare-beneficiaries-during-covid-19-outbreak. Published March 17, 2020. Accessed July 14, 2020.
Practice Points
- Telehealth can increase access to dermatologic care for both inpatient hospital consultations and outpatient clinic visits, especially in areas lacking dermatologists.
- With the current iPLEDGE accommodations for coronavirus disease 19, we have been able to treat patients who live 3 hours away and cannot travel for monthly isotretinoin visits.
- Telehealth allows our providers to better triage benign vs potentially malignant conditions to schedule patients in a more appropriate time frame.
Developing COVID-19 hospital protocols during the pandemic
As hospitalists and other physicians at the University of Texas at Austin considered how to treat COVID-19 patients in the early weeks of the pandemic, one question they had to consider was: What about convalescent plasma?
All they had to go on were small case series in Ebola, SARS, and MERS and a few small, nonrandomized COVID-19 studies showing a possible benefit and minimal risk, but the evidence was only “toward the middle or bottom” of the evidence pyramid, said Johanna Busch, MD, of the department of internal medicine at Dell Medical Center at the university.
The center’s COVID-19 committee asked a few of its members – infectious disease and internal medicine physicians – to analyze the literature and other factors. In the end, the committee – which meets regularly and also includes pulmonology–critical care experts, nursing experts, and others – recommended using convalescent plasma because of the evidence and the available supply. But in subsequent meetings, as the pandemic surged in the South and the supply dwindled, the committee changed its recommendation for convalescent plasma to more limited use, she said during the virtual annual meeting of the Society of Hospital Medicine.
“It’s all about teamwork,” said W. Michael Brode, MD, of the department of internal medicine at Dell. “The interprofessional team members know their roles and have shared expectations because they have a common understanding of the protocol.” It’s okay to deviate from the protocol, he said, as long as the language exists to communicate these deviations.
“Maybe the approach is more important than the actual content,” he said.
What Dr. Brode and Dr. Busch described was in large part a fine-tuning of communication – being available to communicate in real time and being aware of when certain specialists should be contacted – for instance, to determine at what oxygenation level internal medicine staff should get in touch with the pulmonary–critical care team.
Dr. Brode said that the groundwork is laid for productive meetings, with agendas announced ahead of time and readings assigned and presenters ready with near-finished products at meeting time, “with a clear path for operationalizing it.”
“We don’t want people kind of riffing off the top of their heads,” he said.
Committee members are encouraged to be as specific as possible when giving input into COVID-19 care decisions, he said.
“We’re so used to dealing with uncertainty, but that doesn’t really help when we’re trying to make tough decisions,” Dr. Brode said. They might be asked, “What are you going to write in your consult note template?” or “It’s 1:00 a.m. and your intern’s panicked and calling you – what are you going to tell them to do over the phone?”
The recommendations have to go into writing and are incorporated into the electronic medical record, a process that required some workarounds, he said. He also noted that the committee learned early on that they should assume that no one reads the e-mails – especially after being off for a period of time – so they likely won’t digest updates on an email-by-email basis.
“We quickly learned,” Dr. Brode said, “that this information needs to live on a Web site or [be] linked to the most up-to-date version in a cloud-sharing platform.”
In a question-and-answer discussion, session viewers expressed enthusiasm for the presenters’ one-page summary of protocols – much more, they said, and it could feel overwhelming.
Dr. Busch and Dr. Brode were asked how standardized order sets for COVID patients could be justified without comparison to a control group that didn’t use the standard order set.
Dr. Busch responded that, while there was no controlled trial, the order sets they use have evolved based on experience.
“At the beginning, we were following every inflammatory marker known to mankind, and then we realized as we gained more experience with COVID and COVID patients that some of those markers were not really informing any of our clinical decisions,” she said. “Obviously, as literature comes out we may reevaluate what goes into that standard order set and how frequently we follow labs.”
Dr. Brode said the context – a pandemic – has to be considered.
“In an ideal world, we could show that the intervention is superior through a randomized fashion with a control group, but really our thought process behind it is just, what is the default?” he said. “I looked at the order sets [as] not that they’re going to be dictating care, but it’s really like the guardrails of what’s reasonable. And when you’re in the middle of a surge, what is usually reasonable and easiest is what is going to be done.”
Dr. Busch and Dr. Brode reported no relevant financial relationships.
As hospitalists and other physicians at the University of Texas at Austin considered how to treat COVID-19 patients in the early weeks of the pandemic, one question they had to consider was: What about convalescent plasma?
All they had to go on were small case series in Ebola, SARS, and MERS and a few small, nonrandomized COVID-19 studies showing a possible benefit and minimal risk, but the evidence was only “toward the middle or bottom” of the evidence pyramid, said Johanna Busch, MD, of the department of internal medicine at Dell Medical Center at the university.
The center’s COVID-19 committee asked a few of its members – infectious disease and internal medicine physicians – to analyze the literature and other factors. In the end, the committee – which meets regularly and also includes pulmonology–critical care experts, nursing experts, and others – recommended using convalescent plasma because of the evidence and the available supply. But in subsequent meetings, as the pandemic surged in the South and the supply dwindled, the committee changed its recommendation for convalescent plasma to more limited use, she said during the virtual annual meeting of the Society of Hospital Medicine.
“It’s all about teamwork,” said W. Michael Brode, MD, of the department of internal medicine at Dell. “The interprofessional team members know their roles and have shared expectations because they have a common understanding of the protocol.” It’s okay to deviate from the protocol, he said, as long as the language exists to communicate these deviations.
“Maybe the approach is more important than the actual content,” he said.
What Dr. Brode and Dr. Busch described was in large part a fine-tuning of communication – being available to communicate in real time and being aware of when certain specialists should be contacted – for instance, to determine at what oxygenation level internal medicine staff should get in touch with the pulmonary–critical care team.
Dr. Brode said that the groundwork is laid for productive meetings, with agendas announced ahead of time and readings assigned and presenters ready with near-finished products at meeting time, “with a clear path for operationalizing it.”
“We don’t want people kind of riffing off the top of their heads,” he said.
Committee members are encouraged to be as specific as possible when giving input into COVID-19 care decisions, he said.
“We’re so used to dealing with uncertainty, but that doesn’t really help when we’re trying to make tough decisions,” Dr. Brode said. They might be asked, “What are you going to write in your consult note template?” or “It’s 1:00 a.m. and your intern’s panicked and calling you – what are you going to tell them to do over the phone?”
The recommendations have to go into writing and are incorporated into the electronic medical record, a process that required some workarounds, he said. He also noted that the committee learned early on that they should assume that no one reads the e-mails – especially after being off for a period of time – so they likely won’t digest updates on an email-by-email basis.
“We quickly learned,” Dr. Brode said, “that this information needs to live on a Web site or [be] linked to the most up-to-date version in a cloud-sharing platform.”
In a question-and-answer discussion, session viewers expressed enthusiasm for the presenters’ one-page summary of protocols – much more, they said, and it could feel overwhelming.
Dr. Busch and Dr. Brode were asked how standardized order sets for COVID patients could be justified without comparison to a control group that didn’t use the standard order set.
Dr. Busch responded that, while there was no controlled trial, the order sets they use have evolved based on experience.
“At the beginning, we were following every inflammatory marker known to mankind, and then we realized as we gained more experience with COVID and COVID patients that some of those markers were not really informing any of our clinical decisions,” she said. “Obviously, as literature comes out we may reevaluate what goes into that standard order set and how frequently we follow labs.”
Dr. Brode said the context – a pandemic – has to be considered.
“In an ideal world, we could show that the intervention is superior through a randomized fashion with a control group, but really our thought process behind it is just, what is the default?” he said. “I looked at the order sets [as] not that they’re going to be dictating care, but it’s really like the guardrails of what’s reasonable. And when you’re in the middle of a surge, what is usually reasonable and easiest is what is going to be done.”
Dr. Busch and Dr. Brode reported no relevant financial relationships.
As hospitalists and other physicians at the University of Texas at Austin considered how to treat COVID-19 patients in the early weeks of the pandemic, one question they had to consider was: What about convalescent plasma?
All they had to go on were small case series in Ebola, SARS, and MERS and a few small, nonrandomized COVID-19 studies showing a possible benefit and minimal risk, but the evidence was only “toward the middle or bottom” of the evidence pyramid, said Johanna Busch, MD, of the department of internal medicine at Dell Medical Center at the university.
The center’s COVID-19 committee asked a few of its members – infectious disease and internal medicine physicians – to analyze the literature and other factors. In the end, the committee – which meets regularly and also includes pulmonology–critical care experts, nursing experts, and others – recommended using convalescent plasma because of the evidence and the available supply. But in subsequent meetings, as the pandemic surged in the South and the supply dwindled, the committee changed its recommendation for convalescent plasma to more limited use, she said during the virtual annual meeting of the Society of Hospital Medicine.
“It’s all about teamwork,” said W. Michael Brode, MD, of the department of internal medicine at Dell. “The interprofessional team members know their roles and have shared expectations because they have a common understanding of the protocol.” It’s okay to deviate from the protocol, he said, as long as the language exists to communicate these deviations.
“Maybe the approach is more important than the actual content,” he said.
What Dr. Brode and Dr. Busch described was in large part a fine-tuning of communication – being available to communicate in real time and being aware of when certain specialists should be contacted – for instance, to determine at what oxygenation level internal medicine staff should get in touch with the pulmonary–critical care team.
Dr. Brode said that the groundwork is laid for productive meetings, with agendas announced ahead of time and readings assigned and presenters ready with near-finished products at meeting time, “with a clear path for operationalizing it.”
“We don’t want people kind of riffing off the top of their heads,” he said.
Committee members are encouraged to be as specific as possible when giving input into COVID-19 care decisions, he said.
“We’re so used to dealing with uncertainty, but that doesn’t really help when we’re trying to make tough decisions,” Dr. Brode said. They might be asked, “What are you going to write in your consult note template?” or “It’s 1:00 a.m. and your intern’s panicked and calling you – what are you going to tell them to do over the phone?”
The recommendations have to go into writing and are incorporated into the electronic medical record, a process that required some workarounds, he said. He also noted that the committee learned early on that they should assume that no one reads the e-mails – especially after being off for a period of time – so they likely won’t digest updates on an email-by-email basis.
“We quickly learned,” Dr. Brode said, “that this information needs to live on a Web site or [be] linked to the most up-to-date version in a cloud-sharing platform.”
In a question-and-answer discussion, session viewers expressed enthusiasm for the presenters’ one-page summary of protocols – much more, they said, and it could feel overwhelming.
Dr. Busch and Dr. Brode were asked how standardized order sets for COVID patients could be justified without comparison to a control group that didn’t use the standard order set.
Dr. Busch responded that, while there was no controlled trial, the order sets they use have evolved based on experience.
“At the beginning, we were following every inflammatory marker known to mankind, and then we realized as we gained more experience with COVID and COVID patients that some of those markers were not really informing any of our clinical decisions,” she said. “Obviously, as literature comes out we may reevaluate what goes into that standard order set and how frequently we follow labs.”
Dr. Brode said the context – a pandemic – has to be considered.
“In an ideal world, we could show that the intervention is superior through a randomized fashion with a control group, but really our thought process behind it is just, what is the default?” he said. “I looked at the order sets [as] not that they’re going to be dictating care, but it’s really like the guardrails of what’s reasonable. And when you’re in the middle of a surge, what is usually reasonable and easiest is what is going to be done.”
Dr. Busch and Dr. Brode reported no relevant financial relationships.
FROM HM20 VIRTUAL
COVID-19 cases in children nearly doubled in just 4 weeks
The cumulative number of new COVID-19 cases among children in the United States jumped by 90% during a recent 4-week period, according to a report that confirms children are not immune to the coronavirus.
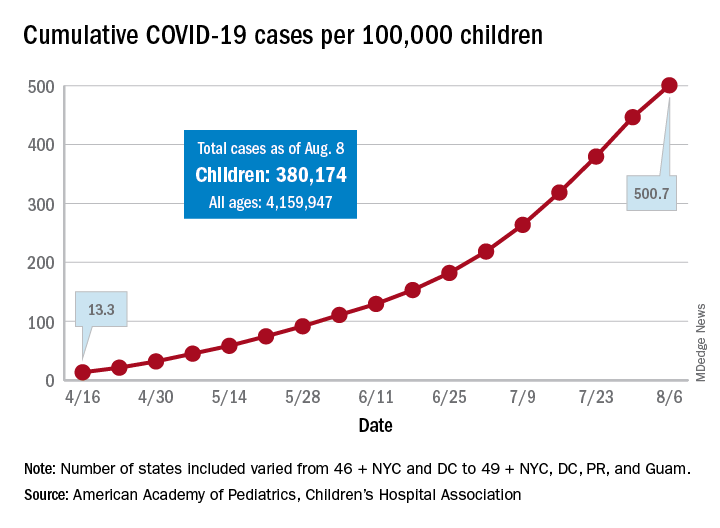
“In areas with rapid community spread, it’s likely that more children will also be infected, and these data show that,” Sally Goza, MD, president of the American Academy of Pediatrics, said in a written statement. “I urge people to wear cloth face coverings and be diligent in social distancing and hand-washing. It is up to us to make the difference, community by community.”
The joint report from the AAP and the Children’s Hospital Association draws on data from state and local health departments in 49 states, New York City, the District of Columbia, Puerto Rico, and Guam.
The cumulative number of COVID-19 cases in children as of Aug. 6, 2020, was 380,174, and that number is 90% higher – an increase of 179,990 cases – than the total on July 9, just 4 weeks earlier, the two organizations said in the report.
and 27 states out of 47 with available data now report that over 10% of their cases were children, with Wyoming the highest at 16.5% and New Jersey the lowest at 2.9%, the report data show.
Alabama has a higher percentage of 22.5%, but the state has been reporting cases in individuals aged 0-24 years as child cases since May 7. The report’s findings are somewhat limited by differences in reporting among the states and by “gaps in the data they are reporting [that affect] how the data can be interpreted,” the AAP said in its statement.
The cumulative number of cases per 100,000 children has risen from 13.3 in mid-April, when the total number was 9,259 cases, to 500.7 per 100,000 as of Aug. 6, and there are now 21 states, along with the District of Columbia, reporting a rate of over 500 cases per 100,000 children. Arizona has the highest rate at 1,206.4, followed by South Carolina (1,074.4) and Tennessee (1,050.8), the AAP and the CHA said.
In New York City, the early epicenter of the pandemic, the 390.5 cases per 100,000 children have been reported, and in New Jersey, which joined New York in the initial surge of cases, the number is 269.5. As of Aug. 6, Hawaii had the fewest cases of any state at 91.2 per 100,000, according to the report.
Children continue to represent a very low proportion of COVID-19 deaths, “but as case counts rise across the board, that is likely to impact more children with severe illness as well,” Sean O’Leary, MD, MPH, vice chair of the AAP’s committee on infectious diseases, said in the AAP statement.
It is possible that “some of the increase in numbers of cases in children could be due to more testing. Early in the pandemic, testing only occurred for the sickest individuals. Now that there is more testing capacity … the numbers reflect a broader slice of the population, including children who may have mild or few symptoms,” the AAP suggested.
This article was updated on 8/17/2020.
The cumulative number of new COVID-19 cases among children in the United States jumped by 90% during a recent 4-week period, according to a report that confirms children are not immune to the coronavirus.

“In areas with rapid community spread, it’s likely that more children will also be infected, and these data show that,” Sally Goza, MD, president of the American Academy of Pediatrics, said in a written statement. “I urge people to wear cloth face coverings and be diligent in social distancing and hand-washing. It is up to us to make the difference, community by community.”
The joint report from the AAP and the Children’s Hospital Association draws on data from state and local health departments in 49 states, New York City, the District of Columbia, Puerto Rico, and Guam.
The cumulative number of COVID-19 cases in children as of Aug. 6, 2020, was 380,174, and that number is 90% higher – an increase of 179,990 cases – than the total on July 9, just 4 weeks earlier, the two organizations said in the report.
and 27 states out of 47 with available data now report that over 10% of their cases were children, with Wyoming the highest at 16.5% and New Jersey the lowest at 2.9%, the report data show.
Alabama has a higher percentage of 22.5%, but the state has been reporting cases in individuals aged 0-24 years as child cases since May 7. The report’s findings are somewhat limited by differences in reporting among the states and by “gaps in the data they are reporting [that affect] how the data can be interpreted,” the AAP said in its statement.
The cumulative number of cases per 100,000 children has risen from 13.3 in mid-April, when the total number was 9,259 cases, to 500.7 per 100,000 as of Aug. 6, and there are now 21 states, along with the District of Columbia, reporting a rate of over 500 cases per 100,000 children. Arizona has the highest rate at 1,206.4, followed by South Carolina (1,074.4) and Tennessee (1,050.8), the AAP and the CHA said.
In New York City, the early epicenter of the pandemic, the 390.5 cases per 100,000 children have been reported, and in New Jersey, which joined New York in the initial surge of cases, the number is 269.5. As of Aug. 6, Hawaii had the fewest cases of any state at 91.2 per 100,000, according to the report.
Children continue to represent a very low proportion of COVID-19 deaths, “but as case counts rise across the board, that is likely to impact more children with severe illness as well,” Sean O’Leary, MD, MPH, vice chair of the AAP’s committee on infectious diseases, said in the AAP statement.
It is possible that “some of the increase in numbers of cases in children could be due to more testing. Early in the pandemic, testing only occurred for the sickest individuals. Now that there is more testing capacity … the numbers reflect a broader slice of the population, including children who may have mild or few symptoms,” the AAP suggested.
This article was updated on 8/17/2020.
The cumulative number of new COVID-19 cases among children in the United States jumped by 90% during a recent 4-week period, according to a report that confirms children are not immune to the coronavirus.

“In areas with rapid community spread, it’s likely that more children will also be infected, and these data show that,” Sally Goza, MD, president of the American Academy of Pediatrics, said in a written statement. “I urge people to wear cloth face coverings and be diligent in social distancing and hand-washing. It is up to us to make the difference, community by community.”
The joint report from the AAP and the Children’s Hospital Association draws on data from state and local health departments in 49 states, New York City, the District of Columbia, Puerto Rico, and Guam.
The cumulative number of COVID-19 cases in children as of Aug. 6, 2020, was 380,174, and that number is 90% higher – an increase of 179,990 cases – than the total on July 9, just 4 weeks earlier, the two organizations said in the report.
and 27 states out of 47 with available data now report that over 10% of their cases were children, with Wyoming the highest at 16.5% and New Jersey the lowest at 2.9%, the report data show.
Alabama has a higher percentage of 22.5%, but the state has been reporting cases in individuals aged 0-24 years as child cases since May 7. The report’s findings are somewhat limited by differences in reporting among the states and by “gaps in the data they are reporting [that affect] how the data can be interpreted,” the AAP said in its statement.
The cumulative number of cases per 100,000 children has risen from 13.3 in mid-April, when the total number was 9,259 cases, to 500.7 per 100,000 as of Aug. 6, and there are now 21 states, along with the District of Columbia, reporting a rate of over 500 cases per 100,000 children. Arizona has the highest rate at 1,206.4, followed by South Carolina (1,074.4) and Tennessee (1,050.8), the AAP and the CHA said.
In New York City, the early epicenter of the pandemic, the 390.5 cases per 100,000 children have been reported, and in New Jersey, which joined New York in the initial surge of cases, the number is 269.5. As of Aug. 6, Hawaii had the fewest cases of any state at 91.2 per 100,000, according to the report.
Children continue to represent a very low proportion of COVID-19 deaths, “but as case counts rise across the board, that is likely to impact more children with severe illness as well,” Sean O’Leary, MD, MPH, vice chair of the AAP’s committee on infectious diseases, said in the AAP statement.
It is possible that “some of the increase in numbers of cases in children could be due to more testing. Early in the pandemic, testing only occurred for the sickest individuals. Now that there is more testing capacity … the numbers reflect a broader slice of the population, including children who may have mild or few symptoms,” the AAP suggested.
This article was updated on 8/17/2020.