User login
COVID-19 vaccine hesitancy ‘somewhat understandable,’ expert says
“I worry that vaccines are going to be sold like magic powder that we sprinkle across the land and make the virus go away,” Paul Offit, MD, said at the virtual American Academy of Pediatrics (AAP) 2020 National Conference. “That’s not true.”
according to Dr. Offit, director of the Vaccine Education Center and an attending physician in the Division of Infectious Diseases at Children’s Hospital of Philadelphia.
“I think we can get a vaccine that’s 75%-80% effective at preventing mild to moderate disease, but that means one of every four people can still get moderate to severe disease,” Dr. Offit continued.
And that’s if there is high uptake of the vaccine, which may not be the case. Recent polls have suggested there is considerable concern about the pending vaccines.
“It’s somewhat understandable,” Dr. Offitt acknowledged, especially given the “frightening” language used to describe vaccine development. Terms such as “warp speed” may suggest that haste might trump safety considerations. Before COVID-19, the fastest vaccine ever developed was for mumps, he said, with the virus isolated in 1963 and a commercial product available in 1967.
Addressing hesitancy in clinics
In a wide-ranging livestream plenary presentation, Dr. Offit, coinventor of a rotavirus vaccine, shed light on SARS-CoV-2 vaccine development and his impressions of vaccine hesitancy among patients and families. He also offered advice for how to reassure those skeptical of the safety and efficacy of any SARS-COV-2 vaccine, given the accelerated development process.
With more than 180 different vaccines in various stages of investigation, Dr. Offit called the effort to develop COVID-19 vaccines “unprecedented.” Part of that is a result of governments relieving pharmaceutical companies of much of the typical financial risk – which often climbs to hundreds of millions of dollars – by underwriting the costs of vaccine development to battle the pandemic-inducing virus, he said.
But this very swiftness is also stoking antivaccine sentiment. Dr. Offit, part of vaccine advisory groups for the National Institutes of Health and U.S. Food and Drug Administration, cited recent research reporting nearly half of American adults definitely or probably would not get a COVID-19 vaccine if it were available today.
“One way you convince skeptics is with data presented in a clear, compassionate, and compelling way,” he said.
“The other group is vaccine cynics, who are basically conspiracy theorists who believe pharmaceutical companies control the world, the government, the medical establishment. I think there’s no talking them down from this.”
Numerous strategies are being used in COVID-19 vaccine development, he noted, including messenger RNA, DNA, viral vectors, purified protein, and whole killed virus. Dr. Offit believes any candidates approved for distribution will likely be in the range of 75% effective at preventing mild to moderate symptoms.
But clinicians should be ready to face immediate questions of safety. “Even if this vaccination is given to 20,000 [trial participants] safely, that’s not 20 million,” Dr. Offit said. “Anyone could reasonably ask questions about if it causes rare, serious side effects.
“The good news is, there are systems in place,” such as adverse event reporting systems, to identify rare events, even those that occur in one in a million vaccine recipients. Reminding patients of that continued surveillance can be reassuring.
Another reassuring point is that COVID-19 vaccine trial participants have included people from many diverse populations, he said. But children, notably absent so far, should be added to trials immediately, Dr. Offit contends.
“This is going to be important when you consider strategies to get children universally back into school,” he said, which is a “critical issue” from both learning and wellness standpoints. “It breaks my heart that we’ve been unable to do this when other countries have.”
Transparency will be paramount
While presenting data transparently to patients is key in helping them accept COVID-19 vaccination, Dr. Offit said, he also believes “telling stories” can be just as effective, if not more so. When the varicella vaccine was approved in 1995, he said, the “uptake the first few years was pretty miserable” until public service messaging emphasized that some children die from chickenpox.
“Fear works,” he said. “You always worry about pushback of something being oversold, but hopefully we’re scared enough about this virus” to convince people that vaccination is wise. “I do think personal stories carry weight on both sides,” Dr. Offit said.
Mark Sawyer, MD, of University of California San Diego School of Medicine and Rady Children’s Hospital in San Diego, California, said Offit’s presentation offered important takeaways for clinicians about how to broach the topic of COVID-19 vaccination with patients and families.
“We need to communicate clearly and transparently to patients about what we do and don’t know” about the vaccines, Dr. Sawyer said in an interview. “We will know if they have common side effects, but we will not know about very rare side effects until we have used the vaccines for a while.
“We will know how well the vaccine works over the short-term, but we won’t know over the long term,” added Dr. Sawyer, a member of the AAP Committee on Infectious Diseases.
“We can reassure the community that SARS-CoV-2 vaccines are being evaluated in trials in the same way and with the same thoroughness as other vaccines have been,” he said. “That should give people confidence that shortcuts are not being taken with regard to safety and effectiveness evaluations.”
Dr. Offit and Dr. Sawyer have disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
“I worry that vaccines are going to be sold like magic powder that we sprinkle across the land and make the virus go away,” Paul Offit, MD, said at the virtual American Academy of Pediatrics (AAP) 2020 National Conference. “That’s not true.”
according to Dr. Offit, director of the Vaccine Education Center and an attending physician in the Division of Infectious Diseases at Children’s Hospital of Philadelphia.
“I think we can get a vaccine that’s 75%-80% effective at preventing mild to moderate disease, but that means one of every four people can still get moderate to severe disease,” Dr. Offit continued.
And that’s if there is high uptake of the vaccine, which may not be the case. Recent polls have suggested there is considerable concern about the pending vaccines.
“It’s somewhat understandable,” Dr. Offitt acknowledged, especially given the “frightening” language used to describe vaccine development. Terms such as “warp speed” may suggest that haste might trump safety considerations. Before COVID-19, the fastest vaccine ever developed was for mumps, he said, with the virus isolated in 1963 and a commercial product available in 1967.
Addressing hesitancy in clinics
In a wide-ranging livestream plenary presentation, Dr. Offit, coinventor of a rotavirus vaccine, shed light on SARS-CoV-2 vaccine development and his impressions of vaccine hesitancy among patients and families. He also offered advice for how to reassure those skeptical of the safety and efficacy of any SARS-COV-2 vaccine, given the accelerated development process.
With more than 180 different vaccines in various stages of investigation, Dr. Offit called the effort to develop COVID-19 vaccines “unprecedented.” Part of that is a result of governments relieving pharmaceutical companies of much of the typical financial risk – which often climbs to hundreds of millions of dollars – by underwriting the costs of vaccine development to battle the pandemic-inducing virus, he said.
But this very swiftness is also stoking antivaccine sentiment. Dr. Offit, part of vaccine advisory groups for the National Institutes of Health and U.S. Food and Drug Administration, cited recent research reporting nearly half of American adults definitely or probably would not get a COVID-19 vaccine if it were available today.
“One way you convince skeptics is with data presented in a clear, compassionate, and compelling way,” he said.
“The other group is vaccine cynics, who are basically conspiracy theorists who believe pharmaceutical companies control the world, the government, the medical establishment. I think there’s no talking them down from this.”
Numerous strategies are being used in COVID-19 vaccine development, he noted, including messenger RNA, DNA, viral vectors, purified protein, and whole killed virus. Dr. Offit believes any candidates approved for distribution will likely be in the range of 75% effective at preventing mild to moderate symptoms.
But clinicians should be ready to face immediate questions of safety. “Even if this vaccination is given to 20,000 [trial participants] safely, that’s not 20 million,” Dr. Offit said. “Anyone could reasonably ask questions about if it causes rare, serious side effects.
“The good news is, there are systems in place,” such as adverse event reporting systems, to identify rare events, even those that occur in one in a million vaccine recipients. Reminding patients of that continued surveillance can be reassuring.
Another reassuring point is that COVID-19 vaccine trial participants have included people from many diverse populations, he said. But children, notably absent so far, should be added to trials immediately, Dr. Offit contends.
“This is going to be important when you consider strategies to get children universally back into school,” he said, which is a “critical issue” from both learning and wellness standpoints. “It breaks my heart that we’ve been unable to do this when other countries have.”
Transparency will be paramount
While presenting data transparently to patients is key in helping them accept COVID-19 vaccination, Dr. Offit said, he also believes “telling stories” can be just as effective, if not more so. When the varicella vaccine was approved in 1995, he said, the “uptake the first few years was pretty miserable” until public service messaging emphasized that some children die from chickenpox.
“Fear works,” he said. “You always worry about pushback of something being oversold, but hopefully we’re scared enough about this virus” to convince people that vaccination is wise. “I do think personal stories carry weight on both sides,” Dr. Offit said.
Mark Sawyer, MD, of University of California San Diego School of Medicine and Rady Children’s Hospital in San Diego, California, said Offit’s presentation offered important takeaways for clinicians about how to broach the topic of COVID-19 vaccination with patients and families.
“We need to communicate clearly and transparently to patients about what we do and don’t know” about the vaccines, Dr. Sawyer said in an interview. “We will know if they have common side effects, but we will not know about very rare side effects until we have used the vaccines for a while.
“We will know how well the vaccine works over the short-term, but we won’t know over the long term,” added Dr. Sawyer, a member of the AAP Committee on Infectious Diseases.
“We can reassure the community that SARS-CoV-2 vaccines are being evaluated in trials in the same way and with the same thoroughness as other vaccines have been,” he said. “That should give people confidence that shortcuts are not being taken with regard to safety and effectiveness evaluations.”
Dr. Offit and Dr. Sawyer have disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
“I worry that vaccines are going to be sold like magic powder that we sprinkle across the land and make the virus go away,” Paul Offit, MD, said at the virtual American Academy of Pediatrics (AAP) 2020 National Conference. “That’s not true.”
according to Dr. Offit, director of the Vaccine Education Center and an attending physician in the Division of Infectious Diseases at Children’s Hospital of Philadelphia.
“I think we can get a vaccine that’s 75%-80% effective at preventing mild to moderate disease, but that means one of every four people can still get moderate to severe disease,” Dr. Offit continued.
And that’s if there is high uptake of the vaccine, which may not be the case. Recent polls have suggested there is considerable concern about the pending vaccines.
“It’s somewhat understandable,” Dr. Offitt acknowledged, especially given the “frightening” language used to describe vaccine development. Terms such as “warp speed” may suggest that haste might trump safety considerations. Before COVID-19, the fastest vaccine ever developed was for mumps, he said, with the virus isolated in 1963 and a commercial product available in 1967.
Addressing hesitancy in clinics
In a wide-ranging livestream plenary presentation, Dr. Offit, coinventor of a rotavirus vaccine, shed light on SARS-CoV-2 vaccine development and his impressions of vaccine hesitancy among patients and families. He also offered advice for how to reassure those skeptical of the safety and efficacy of any SARS-COV-2 vaccine, given the accelerated development process.
With more than 180 different vaccines in various stages of investigation, Dr. Offit called the effort to develop COVID-19 vaccines “unprecedented.” Part of that is a result of governments relieving pharmaceutical companies of much of the typical financial risk – which often climbs to hundreds of millions of dollars – by underwriting the costs of vaccine development to battle the pandemic-inducing virus, he said.
But this very swiftness is also stoking antivaccine sentiment. Dr. Offit, part of vaccine advisory groups for the National Institutes of Health and U.S. Food and Drug Administration, cited recent research reporting nearly half of American adults definitely or probably would not get a COVID-19 vaccine if it were available today.
“One way you convince skeptics is with data presented in a clear, compassionate, and compelling way,” he said.
“The other group is vaccine cynics, who are basically conspiracy theorists who believe pharmaceutical companies control the world, the government, the medical establishment. I think there’s no talking them down from this.”
Numerous strategies are being used in COVID-19 vaccine development, he noted, including messenger RNA, DNA, viral vectors, purified protein, and whole killed virus. Dr. Offit believes any candidates approved for distribution will likely be in the range of 75% effective at preventing mild to moderate symptoms.
But clinicians should be ready to face immediate questions of safety. “Even if this vaccination is given to 20,000 [trial participants] safely, that’s not 20 million,” Dr. Offit said. “Anyone could reasonably ask questions about if it causes rare, serious side effects.
“The good news is, there are systems in place,” such as adverse event reporting systems, to identify rare events, even those that occur in one in a million vaccine recipients. Reminding patients of that continued surveillance can be reassuring.
Another reassuring point is that COVID-19 vaccine trial participants have included people from many diverse populations, he said. But children, notably absent so far, should be added to trials immediately, Dr. Offit contends.
“This is going to be important when you consider strategies to get children universally back into school,” he said, which is a “critical issue” from both learning and wellness standpoints. “It breaks my heart that we’ve been unable to do this when other countries have.”
Transparency will be paramount
While presenting data transparently to patients is key in helping them accept COVID-19 vaccination, Dr. Offit said, he also believes “telling stories” can be just as effective, if not more so. When the varicella vaccine was approved in 1995, he said, the “uptake the first few years was pretty miserable” until public service messaging emphasized that some children die from chickenpox.
“Fear works,” he said. “You always worry about pushback of something being oversold, but hopefully we’re scared enough about this virus” to convince people that vaccination is wise. “I do think personal stories carry weight on both sides,” Dr. Offit said.
Mark Sawyer, MD, of University of California San Diego School of Medicine and Rady Children’s Hospital in San Diego, California, said Offit’s presentation offered important takeaways for clinicians about how to broach the topic of COVID-19 vaccination with patients and families.
“We need to communicate clearly and transparently to patients about what we do and don’t know” about the vaccines, Dr. Sawyer said in an interview. “We will know if they have common side effects, but we will not know about very rare side effects until we have used the vaccines for a while.
“We will know how well the vaccine works over the short-term, but we won’t know over the long term,” added Dr. Sawyer, a member of the AAP Committee on Infectious Diseases.
“We can reassure the community that SARS-CoV-2 vaccines are being evaluated in trials in the same way and with the same thoroughness as other vaccines have been,” he said. “That should give people confidence that shortcuts are not being taken with regard to safety and effectiveness evaluations.”
Dr. Offit and Dr. Sawyer have disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
Review finds mortality rates low in young pregnant women with SJS, TEN
Investigators who but higher rates of C-sections.
The systematic review found that early diagnosis and withdrawal of the causative medications, such as antiretrovirals, were beneficial.
While SJS and TEN have been reported in pregnant women, “the outcomes and treatment of these cases are poorly characterized in the literature,” noted Ajay N. Sharma, a medical student at the University of California, Irvine, and coauthors, who published their findings in the International Journal of Women’s Dermatology.
“Immune changes that occur during pregnancy create a relative state of immunosuppression, likely increasing the risk of these skin reactions,” Mr. Sharma said in an interview. Allopurinol, antiepileptic drugs, antibacterial sulfonamides, nevirapine, and oxicam NSAIDs are agents most often associated with SJS/TEN.
He and his coauthors conducted a systematic literature review to analyze the risk factors, outcomes, and treatment of SJS and TEN in pregnant patients and their newborns using PubMed and Cochrane data from September 2019. The review included 26 articles covering 177 pregnant patients with SJS or TEN. Affected women were fairly young, averaging 29.9 years of age and more than 24 weeks along in their pregnancy when they experienced a reaction.
The majority of cases (81.9%) involved SJS diagnoses. Investigators identified antiretroviral therapy (90% of all cases), antibiotics (3%), and gestational drugs (2%) as the most common causative agents. “Multiple large cohort studies included in our review specifically assessed outcomes in only pregnant patients with HIV, resulting in an overall distribution of offending medications biased toward antiretroviral therapy,” noted Mr. Sharma. Nevirapine, a staple antiretroviral in developing countries (the site of most studies in the review), emerged as the biggest causal agent linked to 75 cases; 1 case was linked to the antiretroviral drug efavirenz.
Approximately 85% of pregnant women in this review had HIV. However, the young patient population studied had few comorbidities and low transmission rates to the fetus. In the 94 cases where outcomes data were available, 98% of the mothers and 96% of the newborns survived. Two pregnant patients in this cohort died, one from septic shock secondary to a TEN superinfection, and the other from intracranial hemorrhage secondary to metastatic melanoma. Of the 94 fetuses, 4 died: 2 of sepsis after birth, 1 in utero with its mother, and there was 1 stillbirth.
“Withdrawal of the offending drug was enacted in every recorded case of SJS or TEN during pregnancy. This single intervention was adequate in 159 patients; no additional therapy was needed in these cases aside from standard wound care, fluid and electrolyte repletion, and pain control,” wrote the investigators. Clinicians administered antibiotics, fluid resuscitation, steroids, and intravenous immunoglobulin in patients needing further assistance.
The investigators also reported high rates of C-section – almost 50% – in this group of pregnant women.
Inconsistent reporting between studies limited results, Mr. Sharma and colleagues noted. “Not every report specified body surface area involvement, treatment regimen, maternal or fetal outcome, or delivery method. Although additional studies in the form of large-scale, randomized, clinical trials are needed to better delineate treatment, this systematic review provides a framework for managing this population.”
The study authors reported no conflicts of interest and no funding for the study.
SOURCE: Sharma AN et al. Int J Womens Dermatol. 2020 Apr 13;6(4):239-47.
Investigators who but higher rates of C-sections.
The systematic review found that early diagnosis and withdrawal of the causative medications, such as antiretrovirals, were beneficial.
While SJS and TEN have been reported in pregnant women, “the outcomes and treatment of these cases are poorly characterized in the literature,” noted Ajay N. Sharma, a medical student at the University of California, Irvine, and coauthors, who published their findings in the International Journal of Women’s Dermatology.
“Immune changes that occur during pregnancy create a relative state of immunosuppression, likely increasing the risk of these skin reactions,” Mr. Sharma said in an interview. Allopurinol, antiepileptic drugs, antibacterial sulfonamides, nevirapine, and oxicam NSAIDs are agents most often associated with SJS/TEN.
He and his coauthors conducted a systematic literature review to analyze the risk factors, outcomes, and treatment of SJS and TEN in pregnant patients and their newborns using PubMed and Cochrane data from September 2019. The review included 26 articles covering 177 pregnant patients with SJS or TEN. Affected women were fairly young, averaging 29.9 years of age and more than 24 weeks along in their pregnancy when they experienced a reaction.
The majority of cases (81.9%) involved SJS diagnoses. Investigators identified antiretroviral therapy (90% of all cases), antibiotics (3%), and gestational drugs (2%) as the most common causative agents. “Multiple large cohort studies included in our review specifically assessed outcomes in only pregnant patients with HIV, resulting in an overall distribution of offending medications biased toward antiretroviral therapy,” noted Mr. Sharma. Nevirapine, a staple antiretroviral in developing countries (the site of most studies in the review), emerged as the biggest causal agent linked to 75 cases; 1 case was linked to the antiretroviral drug efavirenz.
Approximately 85% of pregnant women in this review had HIV. However, the young patient population studied had few comorbidities and low transmission rates to the fetus. In the 94 cases where outcomes data were available, 98% of the mothers and 96% of the newborns survived. Two pregnant patients in this cohort died, one from septic shock secondary to a TEN superinfection, and the other from intracranial hemorrhage secondary to metastatic melanoma. Of the 94 fetuses, 4 died: 2 of sepsis after birth, 1 in utero with its mother, and there was 1 stillbirth.
“Withdrawal of the offending drug was enacted in every recorded case of SJS or TEN during pregnancy. This single intervention was adequate in 159 patients; no additional therapy was needed in these cases aside from standard wound care, fluid and electrolyte repletion, and pain control,” wrote the investigators. Clinicians administered antibiotics, fluid resuscitation, steroids, and intravenous immunoglobulin in patients needing further assistance.
The investigators also reported high rates of C-section – almost 50% – in this group of pregnant women.
Inconsistent reporting between studies limited results, Mr. Sharma and colleagues noted. “Not every report specified body surface area involvement, treatment regimen, maternal or fetal outcome, or delivery method. Although additional studies in the form of large-scale, randomized, clinical trials are needed to better delineate treatment, this systematic review provides a framework for managing this population.”
The study authors reported no conflicts of interest and no funding for the study.
SOURCE: Sharma AN et al. Int J Womens Dermatol. 2020 Apr 13;6(4):239-47.
Investigators who but higher rates of C-sections.
The systematic review found that early diagnosis and withdrawal of the causative medications, such as antiretrovirals, were beneficial.
While SJS and TEN have been reported in pregnant women, “the outcomes and treatment of these cases are poorly characterized in the literature,” noted Ajay N. Sharma, a medical student at the University of California, Irvine, and coauthors, who published their findings in the International Journal of Women’s Dermatology.
“Immune changes that occur during pregnancy create a relative state of immunosuppression, likely increasing the risk of these skin reactions,” Mr. Sharma said in an interview. Allopurinol, antiepileptic drugs, antibacterial sulfonamides, nevirapine, and oxicam NSAIDs are agents most often associated with SJS/TEN.
He and his coauthors conducted a systematic literature review to analyze the risk factors, outcomes, and treatment of SJS and TEN in pregnant patients and their newborns using PubMed and Cochrane data from September 2019. The review included 26 articles covering 177 pregnant patients with SJS or TEN. Affected women were fairly young, averaging 29.9 years of age and more than 24 weeks along in their pregnancy when they experienced a reaction.
The majority of cases (81.9%) involved SJS diagnoses. Investigators identified antiretroviral therapy (90% of all cases), antibiotics (3%), and gestational drugs (2%) as the most common causative agents. “Multiple large cohort studies included in our review specifically assessed outcomes in only pregnant patients with HIV, resulting in an overall distribution of offending medications biased toward antiretroviral therapy,” noted Mr. Sharma. Nevirapine, a staple antiretroviral in developing countries (the site of most studies in the review), emerged as the biggest causal agent linked to 75 cases; 1 case was linked to the antiretroviral drug efavirenz.
Approximately 85% of pregnant women in this review had HIV. However, the young patient population studied had few comorbidities and low transmission rates to the fetus. In the 94 cases where outcomes data were available, 98% of the mothers and 96% of the newborns survived. Two pregnant patients in this cohort died, one from septic shock secondary to a TEN superinfection, and the other from intracranial hemorrhage secondary to metastatic melanoma. Of the 94 fetuses, 4 died: 2 of sepsis after birth, 1 in utero with its mother, and there was 1 stillbirth.
“Withdrawal of the offending drug was enacted in every recorded case of SJS or TEN during pregnancy. This single intervention was adequate in 159 patients; no additional therapy was needed in these cases aside from standard wound care, fluid and electrolyte repletion, and pain control,” wrote the investigators. Clinicians administered antibiotics, fluid resuscitation, steroids, and intravenous immunoglobulin in patients needing further assistance.
The investigators also reported high rates of C-section – almost 50% – in this group of pregnant women.
Inconsistent reporting between studies limited results, Mr. Sharma and colleagues noted. “Not every report specified body surface area involvement, treatment regimen, maternal or fetal outcome, or delivery method. Although additional studies in the form of large-scale, randomized, clinical trials are needed to better delineate treatment, this systematic review provides a framework for managing this population.”
The study authors reported no conflicts of interest and no funding for the study.
SOURCE: Sharma AN et al. Int J Womens Dermatol. 2020 Apr 13;6(4):239-47.
FROM THE INTERNATIONAL JOURNAL OF WOMEN’S DERMATOLOGY
One measure of child COVID-19 may be trending downward
After increasing for several weeks, the proportion of new COVID-19 cases occurring in children has dropped for the second week in a row, according to data in a new report from the American Academy of Pediatrics and the Children’s Hospital Association.
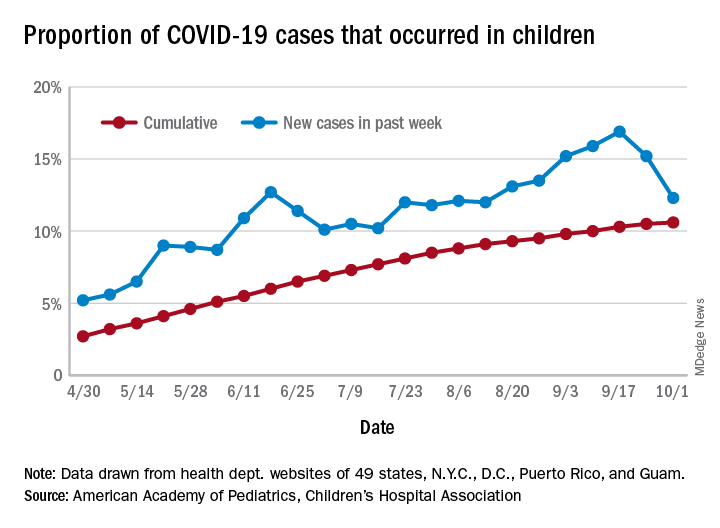
COVID-19 cases in children accounted for 12.3% of all new cases in the United States for the week ending Oct. 1, down from 15.2% the previous week. That measure had reached its highest point, 16.9%, just one week earlier (Sept. 17), the AAP and the CHA said in their weekly COVID-19 report.
based on data from the health departments of 49 states (New York does not provide ages on its website), as well as the District of Columbia, New York City, Puerto Rico, and Guam.
The child COVID-19 rate for the United States was 874 per 100,000 children as of Oct. 1, and that figure has doubled since the end of July. At the state level, the highest rates can be found in Tennessee (2,031.4 per 100,000), North Dakota (2,029.6), and South Carolina (2,002.6), with the lowest rates in Vermont (168.9), Maine (229.1), and New Hampshire (268.3), the AAP/CHA report shows.
The children of Wyoming make up the largest share, 22.4%, of any state’s COVID-19 cases, followed by North Dakota and Tennessee, both at 18.3%. New Jersey is lower than any other state at 3.9%, although New York City is a slightly lower 3.6%, the AAP and CHA said.
“The data are limited because the states differ in how they report the data, and it is unknown how many children have been infected but not tested. It is unclear how much of the increase in child cases is due to increased testing capacity,” the AAP said in an earlier statement.
After increasing for several weeks, the proportion of new COVID-19 cases occurring in children has dropped for the second week in a row, according to data in a new report from the American Academy of Pediatrics and the Children’s Hospital Association.

COVID-19 cases in children accounted for 12.3% of all new cases in the United States for the week ending Oct. 1, down from 15.2% the previous week. That measure had reached its highest point, 16.9%, just one week earlier (Sept. 17), the AAP and the CHA said in their weekly COVID-19 report.
based on data from the health departments of 49 states (New York does not provide ages on its website), as well as the District of Columbia, New York City, Puerto Rico, and Guam.
The child COVID-19 rate for the United States was 874 per 100,000 children as of Oct. 1, and that figure has doubled since the end of July. At the state level, the highest rates can be found in Tennessee (2,031.4 per 100,000), North Dakota (2,029.6), and South Carolina (2,002.6), with the lowest rates in Vermont (168.9), Maine (229.1), and New Hampshire (268.3), the AAP/CHA report shows.
The children of Wyoming make up the largest share, 22.4%, of any state’s COVID-19 cases, followed by North Dakota and Tennessee, both at 18.3%. New Jersey is lower than any other state at 3.9%, although New York City is a slightly lower 3.6%, the AAP and CHA said.
“The data are limited because the states differ in how they report the data, and it is unknown how many children have been infected but not tested. It is unclear how much of the increase in child cases is due to increased testing capacity,” the AAP said in an earlier statement.
After increasing for several weeks, the proportion of new COVID-19 cases occurring in children has dropped for the second week in a row, according to data in a new report from the American Academy of Pediatrics and the Children’s Hospital Association.

COVID-19 cases in children accounted for 12.3% of all new cases in the United States for the week ending Oct. 1, down from 15.2% the previous week. That measure had reached its highest point, 16.9%, just one week earlier (Sept. 17), the AAP and the CHA said in their weekly COVID-19 report.
based on data from the health departments of 49 states (New York does not provide ages on its website), as well as the District of Columbia, New York City, Puerto Rico, and Guam.
The child COVID-19 rate for the United States was 874 per 100,000 children as of Oct. 1, and that figure has doubled since the end of July. At the state level, the highest rates can be found in Tennessee (2,031.4 per 100,000), North Dakota (2,029.6), and South Carolina (2,002.6), with the lowest rates in Vermont (168.9), Maine (229.1), and New Hampshire (268.3), the AAP/CHA report shows.
The children of Wyoming make up the largest share, 22.4%, of any state’s COVID-19 cases, followed by North Dakota and Tennessee, both at 18.3%. New Jersey is lower than any other state at 3.9%, although New York City is a slightly lower 3.6%, the AAP and CHA said.
“The data are limited because the states differ in how they report the data, and it is unknown how many children have been infected but not tested. It is unclear how much of the increase in child cases is due to increased testing capacity,” the AAP said in an earlier statement.
CMS gives hospitals 14 weeks to start daily COVID, flu reports
The federal government is giving hospitals 14 weeks to comply with daily reporting requirements for COVID-19.
The Centers for Medicare & Medicaid Services will send letters on October 7 to all 6,200 hospitals that receive reimbursement from the two federal health programs informing them of how well they are doing now, said CMS Administrator Seema Verma on a press call.
Verma would not give an estimate on how many hospitals are currently not compliant. But Deborah Birx, MD, a member of the White House Coronavirus Task Force, said on the call that 86% of hospitals are currently reporting daily.
Federal officials on the call also announced that hospitals would have the option to begin reporting certain data on influenza starting October 19, but that it would become mandatory a few weeks later.
The reporting is important “to really ensure that we’re triangulating all data to understand where this epidemic is, how it’s moving through different populations, and ensuring that we’re meeting the needs of specific hospitals and communities,” Birx said.
The federal government began a new hospital reporting system in April but did not require hospitals to participate until it quietly issued guidance in mid-July informing facilities that they should no longer report to the Centers for Disease Control and Prevention (CDC).
The move perplexed many public health experts and epidemiologists, who expressed concern that asking hospitals to use a new data system during a pandemic could result in delays and lost information. The new HHS data collection site, HHS Protect, is being managed by a private contractor, not the CDC, which also raised alarms.
The final CMS rule issued in August went into effect immediately, without any chance for comment or revision. CMS said at the time that the pandemic was reason enough to skip over the normal bureaucratic process.
Hospitals were not pleased. But Verma claimed that since then CMS had been working with hospital organizations on enforcement.
“We’re going to do everything we can to facilitate reporting, including an enforcement timeline that will provide hospitals ample opportunity to come into compliance,” she said.
Hospitals that do not comply will get a notice every 3 weeks. Three weeks after the second notice, they’ll get weekly notices for a month, and a final termination notice at 14 weeks.
The Federation of American Hospitals (FAH), however, said their members were still not happy. “It is both inappropriate and frankly overkill for CMS to tie compliance with reporting to Medicare conditions of participation,” said FAH President and CEO Chip Kahn in a statement. He called the CMS proposal “sledgehammer enforcement,” and said that the continuing data request might weaken hospitals’ response to the pandemic because it would divert time and money away from patient care.
Rick Pollack, president and CEO of the American Hospital Association called the CMS rule an “overly heavy-handed approach that could jeopardize access to hospital care for all Americans.” He noted in a statement that barring hospitals from Medicare and Medicaid could harm beneficiaries and the effort to provide COVID care.
Pollack also noted that AHA has “observed errors in data processing and confusion about exactly what was being requested at the hospital, state, contractor, and federal level, and has worked diligently with the federal agencies to identify and correct those problems.”
The document that lays out U.S. Department of Health and Human Services (HHS) Protect reporting requirements were updated again on October 6 to add influenza data. The hospitals must report on total patients with laboratory-confirmed flu; previous day’s flu admissions; total ICU patients with lab-confirmed flu; total inpatients with either flu or COVID-19; and the previous day’s deaths for flu and COVID.
CDC Director Robert Redfield, MD, said on the press call that the new data will give the agency crucial hospital-level information and perhaps better estimates of the flu burden. Flu trends have been tracked using the CDC’s Influenza Hospitalization Surveillance Network (FluSurv-NET), which will not be replaced, Redfield said. But that network only tracks hospitalizations in 14 states and does not provide information in “nearly real-time,” he said.
Having the new data “will give us a true situational awareness of severe respiratory illness, provide local hospitalization trends, and help direct resources such as antiretrovirals to address potential increased impact of flu and COVID cocirculation,” Redfield said.
This article first appeared on Medscape.com.
The federal government is giving hospitals 14 weeks to comply with daily reporting requirements for COVID-19.
The Centers for Medicare & Medicaid Services will send letters on October 7 to all 6,200 hospitals that receive reimbursement from the two federal health programs informing them of how well they are doing now, said CMS Administrator Seema Verma on a press call.
Verma would not give an estimate on how many hospitals are currently not compliant. But Deborah Birx, MD, a member of the White House Coronavirus Task Force, said on the call that 86% of hospitals are currently reporting daily.
Federal officials on the call also announced that hospitals would have the option to begin reporting certain data on influenza starting October 19, but that it would become mandatory a few weeks later.
The reporting is important “to really ensure that we’re triangulating all data to understand where this epidemic is, how it’s moving through different populations, and ensuring that we’re meeting the needs of specific hospitals and communities,” Birx said.
The federal government began a new hospital reporting system in April but did not require hospitals to participate until it quietly issued guidance in mid-July informing facilities that they should no longer report to the Centers for Disease Control and Prevention (CDC).
The move perplexed many public health experts and epidemiologists, who expressed concern that asking hospitals to use a new data system during a pandemic could result in delays and lost information. The new HHS data collection site, HHS Protect, is being managed by a private contractor, not the CDC, which also raised alarms.
The final CMS rule issued in August went into effect immediately, without any chance for comment or revision. CMS said at the time that the pandemic was reason enough to skip over the normal bureaucratic process.
Hospitals were not pleased. But Verma claimed that since then CMS had been working with hospital organizations on enforcement.
“We’re going to do everything we can to facilitate reporting, including an enforcement timeline that will provide hospitals ample opportunity to come into compliance,” she said.
Hospitals that do not comply will get a notice every 3 weeks. Three weeks after the second notice, they’ll get weekly notices for a month, and a final termination notice at 14 weeks.
The Federation of American Hospitals (FAH), however, said their members were still not happy. “It is both inappropriate and frankly overkill for CMS to tie compliance with reporting to Medicare conditions of participation,” said FAH President and CEO Chip Kahn in a statement. He called the CMS proposal “sledgehammer enforcement,” and said that the continuing data request might weaken hospitals’ response to the pandemic because it would divert time and money away from patient care.
Rick Pollack, president and CEO of the American Hospital Association called the CMS rule an “overly heavy-handed approach that could jeopardize access to hospital care for all Americans.” He noted in a statement that barring hospitals from Medicare and Medicaid could harm beneficiaries and the effort to provide COVID care.
Pollack also noted that AHA has “observed errors in data processing and confusion about exactly what was being requested at the hospital, state, contractor, and federal level, and has worked diligently with the federal agencies to identify and correct those problems.”
The document that lays out U.S. Department of Health and Human Services (HHS) Protect reporting requirements were updated again on October 6 to add influenza data. The hospitals must report on total patients with laboratory-confirmed flu; previous day’s flu admissions; total ICU patients with lab-confirmed flu; total inpatients with either flu or COVID-19; and the previous day’s deaths for flu and COVID.
CDC Director Robert Redfield, MD, said on the press call that the new data will give the agency crucial hospital-level information and perhaps better estimates of the flu burden. Flu trends have been tracked using the CDC’s Influenza Hospitalization Surveillance Network (FluSurv-NET), which will not be replaced, Redfield said. But that network only tracks hospitalizations in 14 states and does not provide information in “nearly real-time,” he said.
Having the new data “will give us a true situational awareness of severe respiratory illness, provide local hospitalization trends, and help direct resources such as antiretrovirals to address potential increased impact of flu and COVID cocirculation,” Redfield said.
This article first appeared on Medscape.com.
The federal government is giving hospitals 14 weeks to comply with daily reporting requirements for COVID-19.
The Centers for Medicare & Medicaid Services will send letters on October 7 to all 6,200 hospitals that receive reimbursement from the two federal health programs informing them of how well they are doing now, said CMS Administrator Seema Verma on a press call.
Verma would not give an estimate on how many hospitals are currently not compliant. But Deborah Birx, MD, a member of the White House Coronavirus Task Force, said on the call that 86% of hospitals are currently reporting daily.
Federal officials on the call also announced that hospitals would have the option to begin reporting certain data on influenza starting October 19, but that it would become mandatory a few weeks later.
The reporting is important “to really ensure that we’re triangulating all data to understand where this epidemic is, how it’s moving through different populations, and ensuring that we’re meeting the needs of specific hospitals and communities,” Birx said.
The federal government began a new hospital reporting system in April but did not require hospitals to participate until it quietly issued guidance in mid-July informing facilities that they should no longer report to the Centers for Disease Control and Prevention (CDC).
The move perplexed many public health experts and epidemiologists, who expressed concern that asking hospitals to use a new data system during a pandemic could result in delays and lost information. The new HHS data collection site, HHS Protect, is being managed by a private contractor, not the CDC, which also raised alarms.
The final CMS rule issued in August went into effect immediately, without any chance for comment or revision. CMS said at the time that the pandemic was reason enough to skip over the normal bureaucratic process.
Hospitals were not pleased. But Verma claimed that since then CMS had been working with hospital organizations on enforcement.
“We’re going to do everything we can to facilitate reporting, including an enforcement timeline that will provide hospitals ample opportunity to come into compliance,” she said.
Hospitals that do not comply will get a notice every 3 weeks. Three weeks after the second notice, they’ll get weekly notices for a month, and a final termination notice at 14 weeks.
The Federation of American Hospitals (FAH), however, said their members were still not happy. “It is both inappropriate and frankly overkill for CMS to tie compliance with reporting to Medicare conditions of participation,” said FAH President and CEO Chip Kahn in a statement. He called the CMS proposal “sledgehammer enforcement,” and said that the continuing data request might weaken hospitals’ response to the pandemic because it would divert time and money away from patient care.
Rick Pollack, president and CEO of the American Hospital Association called the CMS rule an “overly heavy-handed approach that could jeopardize access to hospital care for all Americans.” He noted in a statement that barring hospitals from Medicare and Medicaid could harm beneficiaries and the effort to provide COVID care.
Pollack also noted that AHA has “observed errors in data processing and confusion about exactly what was being requested at the hospital, state, contractor, and federal level, and has worked diligently with the federal agencies to identify and correct those problems.”
The document that lays out U.S. Department of Health and Human Services (HHS) Protect reporting requirements were updated again on October 6 to add influenza data. The hospitals must report on total patients with laboratory-confirmed flu; previous day’s flu admissions; total ICU patients with lab-confirmed flu; total inpatients with either flu or COVID-19; and the previous day’s deaths for flu and COVID.
CDC Director Robert Redfield, MD, said on the press call that the new data will give the agency crucial hospital-level information and perhaps better estimates of the flu burden. Flu trends have been tracked using the CDC’s Influenza Hospitalization Surveillance Network (FluSurv-NET), which will not be replaced, Redfield said. But that network only tracks hospitalizations in 14 states and does not provide information in “nearly real-time,” he said.
Having the new data “will give us a true situational awareness of severe respiratory illness, provide local hospitalization trends, and help direct resources such as antiretrovirals to address potential increased impact of flu and COVID cocirculation,” Redfield said.
This article first appeared on Medscape.com.
Antibiotics or appendectomy? Both good options
Patients given antibiotics for appendicitis fared no worse in quality of life, at least in the short term, than did patients whose appendix was removed, according to a large, randomized, nonblinded, noninferiority study published online Oct. 5 in The New England Journal of Medicine.
One expert says the body of data, including this trial, indicates that the best appendicitis treatment now comes down to individual patients and choice.
David Flum, MD, director of the Surgical Outcomes Research Center at the University of Washington in Seattle, and colleagues conducted the Comparison of Outcomes of Antibiotic Drugs and Appendectomy (CODA) trial, which compared a 10-day course of antibiotics with appendectomy for patients with appendicitis at 25 US centers.
Although some may interpret the study as praising the potential role of antibiotics, the author of an accompanying editorial warns against rushing to antibiotics, even during a pandemic when hospital resources may be strained.
In the study of 1552 adults (414 with an appendicolith), 776 were randomly assigned to the antibiotics group and 776 to appendectomy (96% of whom underwent a laparoscopic procedure).
After 30 days, antibiotics were found to be noninferior to appendectomy, the standard of treatment for 120 years, as determined on the basis of 30-day scores for the European Quality of Life–5 Dimensions (EQ-5D) questionnaire (mean difference, 0.01 points; 95% CI, −0.001 to 0.03).
EQ-5D at 30 days was chosen as the primary endpoint because it has been validated as an overall measure of health after appendicitis treatment and the 30-day time frame mimics the typical recovery period for appendectomy, Flum and colleagues explain.
Some results favored appendectomy
However, editorialist Danny Jacobs, MD, MPH, president of Oregon Health and Science University in Portland, points out that about a third (29%) of the patients in the antibiotics group had undergone appendectomy by 90 days.
Appendicolith, a well-established potential complication, he acknowledges, was the main driver of the need for surgery (41% with that complication needed appendectomy), but it was not the sole reason.
Complications were more common in the antibiotics group than in the appendectomy group (8.1 vs 3.5 per 100 participants; rate ratio, 2.28; 95% CI, 1.30 – 3.98). The rate of serious adverse events was 4.0 per 100 participants in the antibiotics group and 3.0 per 100 participants in the appendectomy group (rate ratio, 1.29; 95% CI, 0.67 – 2.50). Additionally, the number of emergency department visits was nearly three times higher in the antibiotics group, and more time was spent in the hospital by that group, Jacobs points out.
He notes that the article mentions circumstances such as the COVID-19 pandemic may figure into consideration when weighing antibiotics against appendectomy. But he warns that there also may be a danger of treatment bias in vulnerable populations and that COVID-19 has highlighted disparities in care overall.
“It will be important to ensure that some people, in particular vulnerable populations, are not offered antibiotic therapy preferentially or without adequate education regarding the longer-term implications,” Jacobs writes.
Flum told Medscape Medical News he agrees with Jacobs that the potential for bias is important.
“We should all be worried that new healthcare options won’t be equally applied,” he said.
But he and his coauthors offer an alternative view of the results of the study.
“In the antibiotics group,” they write, “more than 7 in 10 participants avoided surgery, many were treated on an outpatient basis, and participants and caregivers missed less time at work than with appendectomy.”
Flum said, “[T]hat’s going to be attractive to some patients. Not all, but some.”
Douglas Smink, MD, MPH, chief of surgery at Brigham and Women’s Faulkner Hospital in Boston, told Medscape Medical News that he sees this study as an argument for surgery remaining the go-to option for appendicitis, unless there is a safety reason for not performing the surgery.
Patients come in and want their appendix out immediately, he said, and surgery offers a quick option with short length of stay and few complications.
Additionally, he said, if patients are told that, with antibiotics, “there’s a 1 in 3 chance you’re going to need [an appendectomy] in the next 3 months, I think most people would say, ‘Just take it out then,’ ” he said.
Can research decide which is best?
The controversy has been well studied. But with no clear answer in any of the studies about whether appendectomy or use of antibiotics is better, should the current study put the research to rest?
Flum told Medscape Medical News that this study, which is three times the size of the next-largest study, makes clear “there are choices.”
Previous trials in Europe “did not move the needle” on the issue, he said, “in part because they didn’t include the patients who typically get appendectomies.”
He said their team tried to build on those studies and include “typical patients in typical hospitals with typical appendicitis” and found that both surgery and antibiotics are safe and have advantages and disadvantages, depending on the patient.
Smink says one thing that has been definitively answered with this trial is that patients with appendicolith are “more likely to fail with antibiotics.”
Previous trials have excluded patients with appendicolith, and this one did not.
“That’s something we’ve not really known for sure but we’ve assumed,” he said.
But now, Smink says, he thinks the research on the topic has gone about as far as it can go.
He notes that none of the trials has shown antibiotics to be better than appendectomy. “I have a hard time believing we are going to find anything different if we did another study like this. This is a really well-done one,” he said.
“If the best you can do is show noninferiority, which is where we are with these studies on appendicitis, you’re always going to have both options, which is great for patients and doctors,” he said.
The study was funded by the Patient-Centered Outcomes Research Institute. The original article lists the authors’ relevant financial relationships. Jacobs and Smink reported no such relationships.
This article first appeared on Medscape.com.
Patients given antibiotics for appendicitis fared no worse in quality of life, at least in the short term, than did patients whose appendix was removed, according to a large, randomized, nonblinded, noninferiority study published online Oct. 5 in The New England Journal of Medicine.
One expert says the body of data, including this trial, indicates that the best appendicitis treatment now comes down to individual patients and choice.
David Flum, MD, director of the Surgical Outcomes Research Center at the University of Washington in Seattle, and colleagues conducted the Comparison of Outcomes of Antibiotic Drugs and Appendectomy (CODA) trial, which compared a 10-day course of antibiotics with appendectomy for patients with appendicitis at 25 US centers.
Although some may interpret the study as praising the potential role of antibiotics, the author of an accompanying editorial warns against rushing to antibiotics, even during a pandemic when hospital resources may be strained.
In the study of 1552 adults (414 with an appendicolith), 776 were randomly assigned to the antibiotics group and 776 to appendectomy (96% of whom underwent a laparoscopic procedure).
After 30 days, antibiotics were found to be noninferior to appendectomy, the standard of treatment for 120 years, as determined on the basis of 30-day scores for the European Quality of Life–5 Dimensions (EQ-5D) questionnaire (mean difference, 0.01 points; 95% CI, −0.001 to 0.03).
EQ-5D at 30 days was chosen as the primary endpoint because it has been validated as an overall measure of health after appendicitis treatment and the 30-day time frame mimics the typical recovery period for appendectomy, Flum and colleagues explain.
Some results favored appendectomy
However, editorialist Danny Jacobs, MD, MPH, president of Oregon Health and Science University in Portland, points out that about a third (29%) of the patients in the antibiotics group had undergone appendectomy by 90 days.
Appendicolith, a well-established potential complication, he acknowledges, was the main driver of the need for surgery (41% with that complication needed appendectomy), but it was not the sole reason.
Complications were more common in the antibiotics group than in the appendectomy group (8.1 vs 3.5 per 100 participants; rate ratio, 2.28; 95% CI, 1.30 – 3.98). The rate of serious adverse events was 4.0 per 100 participants in the antibiotics group and 3.0 per 100 participants in the appendectomy group (rate ratio, 1.29; 95% CI, 0.67 – 2.50). Additionally, the number of emergency department visits was nearly three times higher in the antibiotics group, and more time was spent in the hospital by that group, Jacobs points out.
He notes that the article mentions circumstances such as the COVID-19 pandemic may figure into consideration when weighing antibiotics against appendectomy. But he warns that there also may be a danger of treatment bias in vulnerable populations and that COVID-19 has highlighted disparities in care overall.
“It will be important to ensure that some people, in particular vulnerable populations, are not offered antibiotic therapy preferentially or without adequate education regarding the longer-term implications,” Jacobs writes.
Flum told Medscape Medical News he agrees with Jacobs that the potential for bias is important.
“We should all be worried that new healthcare options won’t be equally applied,” he said.
But he and his coauthors offer an alternative view of the results of the study.
“In the antibiotics group,” they write, “more than 7 in 10 participants avoided surgery, many were treated on an outpatient basis, and participants and caregivers missed less time at work than with appendectomy.”
Flum said, “[T]hat’s going to be attractive to some patients. Not all, but some.”
Douglas Smink, MD, MPH, chief of surgery at Brigham and Women’s Faulkner Hospital in Boston, told Medscape Medical News that he sees this study as an argument for surgery remaining the go-to option for appendicitis, unless there is a safety reason for not performing the surgery.
Patients come in and want their appendix out immediately, he said, and surgery offers a quick option with short length of stay and few complications.
Additionally, he said, if patients are told that, with antibiotics, “there’s a 1 in 3 chance you’re going to need [an appendectomy] in the next 3 months, I think most people would say, ‘Just take it out then,’ ” he said.
Can research decide which is best?
The controversy has been well studied. But with no clear answer in any of the studies about whether appendectomy or use of antibiotics is better, should the current study put the research to rest?
Flum told Medscape Medical News that this study, which is three times the size of the next-largest study, makes clear “there are choices.”
Previous trials in Europe “did not move the needle” on the issue, he said, “in part because they didn’t include the patients who typically get appendectomies.”
He said their team tried to build on those studies and include “typical patients in typical hospitals with typical appendicitis” and found that both surgery and antibiotics are safe and have advantages and disadvantages, depending on the patient.
Smink says one thing that has been definitively answered with this trial is that patients with appendicolith are “more likely to fail with antibiotics.”
Previous trials have excluded patients with appendicolith, and this one did not.
“That’s something we’ve not really known for sure but we’ve assumed,” he said.
But now, Smink says, he thinks the research on the topic has gone about as far as it can go.
He notes that none of the trials has shown antibiotics to be better than appendectomy. “I have a hard time believing we are going to find anything different if we did another study like this. This is a really well-done one,” he said.
“If the best you can do is show noninferiority, which is where we are with these studies on appendicitis, you’re always going to have both options, which is great for patients and doctors,” he said.
The study was funded by the Patient-Centered Outcomes Research Institute. The original article lists the authors’ relevant financial relationships. Jacobs and Smink reported no such relationships.
This article first appeared on Medscape.com.
Patients given antibiotics for appendicitis fared no worse in quality of life, at least in the short term, than did patients whose appendix was removed, according to a large, randomized, nonblinded, noninferiority study published online Oct. 5 in The New England Journal of Medicine.
One expert says the body of data, including this trial, indicates that the best appendicitis treatment now comes down to individual patients and choice.
David Flum, MD, director of the Surgical Outcomes Research Center at the University of Washington in Seattle, and colleagues conducted the Comparison of Outcomes of Antibiotic Drugs and Appendectomy (CODA) trial, which compared a 10-day course of antibiotics with appendectomy for patients with appendicitis at 25 US centers.
Although some may interpret the study as praising the potential role of antibiotics, the author of an accompanying editorial warns against rushing to antibiotics, even during a pandemic when hospital resources may be strained.
In the study of 1552 adults (414 with an appendicolith), 776 were randomly assigned to the antibiotics group and 776 to appendectomy (96% of whom underwent a laparoscopic procedure).
After 30 days, antibiotics were found to be noninferior to appendectomy, the standard of treatment for 120 years, as determined on the basis of 30-day scores for the European Quality of Life–5 Dimensions (EQ-5D) questionnaire (mean difference, 0.01 points; 95% CI, −0.001 to 0.03).
EQ-5D at 30 days was chosen as the primary endpoint because it has been validated as an overall measure of health after appendicitis treatment and the 30-day time frame mimics the typical recovery period for appendectomy, Flum and colleagues explain.
Some results favored appendectomy
However, editorialist Danny Jacobs, MD, MPH, president of Oregon Health and Science University in Portland, points out that about a third (29%) of the patients in the antibiotics group had undergone appendectomy by 90 days.
Appendicolith, a well-established potential complication, he acknowledges, was the main driver of the need for surgery (41% with that complication needed appendectomy), but it was not the sole reason.
Complications were more common in the antibiotics group than in the appendectomy group (8.1 vs 3.5 per 100 participants; rate ratio, 2.28; 95% CI, 1.30 – 3.98). The rate of serious adverse events was 4.0 per 100 participants in the antibiotics group and 3.0 per 100 participants in the appendectomy group (rate ratio, 1.29; 95% CI, 0.67 – 2.50). Additionally, the number of emergency department visits was nearly three times higher in the antibiotics group, and more time was spent in the hospital by that group, Jacobs points out.
He notes that the article mentions circumstances such as the COVID-19 pandemic may figure into consideration when weighing antibiotics against appendectomy. But he warns that there also may be a danger of treatment bias in vulnerable populations and that COVID-19 has highlighted disparities in care overall.
“It will be important to ensure that some people, in particular vulnerable populations, are not offered antibiotic therapy preferentially or without adequate education regarding the longer-term implications,” Jacobs writes.
Flum told Medscape Medical News he agrees with Jacobs that the potential for bias is important.
“We should all be worried that new healthcare options won’t be equally applied,” he said.
But he and his coauthors offer an alternative view of the results of the study.
“In the antibiotics group,” they write, “more than 7 in 10 participants avoided surgery, many were treated on an outpatient basis, and participants and caregivers missed less time at work than with appendectomy.”
Flum said, “[T]hat’s going to be attractive to some patients. Not all, but some.”
Douglas Smink, MD, MPH, chief of surgery at Brigham and Women’s Faulkner Hospital in Boston, told Medscape Medical News that he sees this study as an argument for surgery remaining the go-to option for appendicitis, unless there is a safety reason for not performing the surgery.
Patients come in and want their appendix out immediately, he said, and surgery offers a quick option with short length of stay and few complications.
Additionally, he said, if patients are told that, with antibiotics, “there’s a 1 in 3 chance you’re going to need [an appendectomy] in the next 3 months, I think most people would say, ‘Just take it out then,’ ” he said.
Can research decide which is best?
The controversy has been well studied. But with no clear answer in any of the studies about whether appendectomy or use of antibiotics is better, should the current study put the research to rest?
Flum told Medscape Medical News that this study, which is three times the size of the next-largest study, makes clear “there are choices.”
Previous trials in Europe “did not move the needle” on the issue, he said, “in part because they didn’t include the patients who typically get appendectomies.”
He said their team tried to build on those studies and include “typical patients in typical hospitals with typical appendicitis” and found that both surgery and antibiotics are safe and have advantages and disadvantages, depending on the patient.
Smink says one thing that has been definitively answered with this trial is that patients with appendicolith are “more likely to fail with antibiotics.”
Previous trials have excluded patients with appendicolith, and this one did not.
“That’s something we’ve not really known for sure but we’ve assumed,” he said.
But now, Smink says, he thinks the research on the topic has gone about as far as it can go.
He notes that none of the trials has shown antibiotics to be better than appendectomy. “I have a hard time believing we are going to find anything different if we did another study like this. This is a really well-done one,” he said.
“If the best you can do is show noninferiority, which is where we are with these studies on appendicitis, you’re always going to have both options, which is great for patients and doctors,” he said.
The study was funded by the Patient-Centered Outcomes Research Institute. The original article lists the authors’ relevant financial relationships. Jacobs and Smink reported no such relationships.
This article first appeared on Medscape.com.
FDA posts COVID vaccine guidance amid White House pushback
while medical and trade associations called for a thorough review of any such product before approval.
The FDA took the unusual step of posting background materials much earlier than usual for its planned Oct. 22 advisory committee meeting on potential vaccines for COVID-19. The FDA also on Tuesday afternoon released a new guidance document, expanding on a previous set of recommendations the agency released in June.
In the new guidance document, FDA officials outline what will be required for even a limited clearance, known as an emergency use authorization (EUA), for a COVID-19 vaccine.
“Data from phase 3 studies should include a median follow-up duration of at least 2 months after completion of the full vaccination regimen to help provide adequate information to assess a vaccine’s benefit-risk profile,” the FDA said in the document.
FDA staff have emphasized the higher bar that drugmakers and regulators face in considering approval of a COVID-19 vaccine.
“Vaccines are complex biological products, and an EUA for a COVID-19 vaccine may allow for rapid and widespread deployment for administration of the vaccine to millions of individuals, including healthy people,” the agency staff said in the briefing documents.
The FDA’s briefing document for the Oct. 22 meeting appears to be markedly at odds with the claim Trump made in a video Monday night, in which he told the American public that “vaccines are coming momentarily.”
Trump, who is in a tightly contested presidential race against Democratic candidate Joe Biden, has repeatedly made claims of the potential arrival of COVID vaccines that are at odds with timelines offered with guarded optimism by experts in infectious diseases.
But based on these new guidelines from the FDA, it appears that the White House may now endorse the FDA’s stance, according to a Wall Street Journal report based on “people familiar with the matter.”
The publication reports that the White House, which has yet to officially comment, “endorsed the U.S. Food and Drug Administration’s plans for assessing whether a Covid-19 vaccine should be given widely, casting aside objections to requirements that would likely mean a shot won’t be cleared until after Election Day, people familiar with the matter said.”
Anthony S. Fauci, MD, director of the National Institute of Allergy and Infectious Diseases, on Monday night said during a virtual appearance at the twenty-first annual New Yorker Festival that there could be evidence as early as November or December about whether one of the vaccines now in testing will work out. He declared himself to have “cautious optimism” about potential rollout of vaccines as early as late 2020 or early 2021.
Peter Lurie, MD, MPH, who earlier served as the FDA’s associate commissioner for public health strategy and analysis, described the agency’s release of the briefing document as being a positive development.
News organizations, including the New York Times, have reported that the White House had sought to block the FDA from releasing further instructions for companies developing COVID-19 vaccines. The Associated Press on Tuesday said that a senior Trump administration official confirmed that the White House had blocked earlier FDA plans to formally publish the safety guidelines based on the 2-month data requirement, arguing that there was “no clinical or medical reason” for it.
“It is an encouraging sign that, despite opposition from the White House, the Food and Drug Administration has effectively published guidelines for emergency release of a vaccine for COVID-19 by disclosing the advice it has been providing to individual sponsors,” said Dr. Lurie, who is now executive director and president of the Center for Science in the Public Interest.
In a news release, he said the White House had sought to keep the FDA guidance under wraps “so it could maintain the public fiction that a safe and effective vaccine could be available before Election Day or even so that it could force emergency authorization of a vaccine with more limited follow-up.”
“Even the pharmaceutical industry has been clamoring for the release of these guidelines. We all want a safe and effective vaccine to end the pandemic, and we want it sooner rather than later,” Dr. Lurie said. “But we can’t afford for the Trump administration to bungle vaccine review the way they’ve bungled nearly every other aspect of its pandemic response.”
Tuesday also saw a flood of statements in support of FDA officials, including tweets from the chief executive of Pfizer, which is among the leaders in the race to develop a COVID-19 vaccine. Pfizer’s Albert Bourla, DVM, PhD, said that the FDA’s “public servants are known for their high integrity and scientific expertise and we have full faith in their ability to set appropriate standards for the approval of a COVID vaccine or treatment.”
The American Medical Association on Tuesday announced a public webinar on Wednesday where its president, Susan R. Bailey, MD, will discuss the COVID-19 vaccine review process with Peter Marks, MD, PhD, director of the Center for Biologics Evaluation and Research at the FDA. The AMA described this webinar as part of work “to restore trust in science and science-based decision-making among policymakers and the public.”
“To ensure media and the physician community are continuously informed about the federal review process for COVID-19 vaccine candidates, the AMA will host a webinar series to gain fact-based insights from the nation’s highest-ranking subject matter experts working to protect the health of the public,” the organization said in announcing the webinar.
In a statement, leaders of the Association of American Medical Colleges said that the FDA’s Vaccines and Related Biological Products Advisory Committee should evaluate any COVID-19 candidate vaccines prior to the FDA issuing an EUA.
“Full approval of a new vaccine or biologic requires demonstration of safety and effectiveness through a process that includes evaluation by the VRBPAC. Their recommendations are considered by FDA staff who ultimately have the authority to approve the new product,” said AAMC chief scientific officer Ross McKinney Jr, MD, and AAMC CEO David J. Skorton, MD, in the statement.
Thomas M. File Jr., MD, president of the Infectious Diseases Society of America, said in a statement that his association again asked the White House to “follow medical and scientific expertise in efforts to combat COVID-19.”
“It is imperative that a vaccine be approved on the basis of FDA’s quality standards and that its safety and efficacy are established before it is authorized,” Dr. File said. “A vaccine that has been approved with speed, rather than safety and efficacy, at the forefront will compound the challenges posed by this pandemic. FDA guidelines for approval that set standards the American people can trust are essential to the success of a vaccine.”
Stephen J. Ubl, chief executive of the Pharmaceutical Research and Manufacturers of America, said in a statement that his association “supports any efforts by FDA to provide clarifying guidance and we have engaged with the agency to support bringing greater transparency to the review process for COVID-19 vaccines.”
“To help address this public health crisis, our companies have also taken unprecedented steps to share vaccine clinical trial protocols and data in real time,” Mr. Ubl said. “We welcome the agency’s efforts to instill confidence in the rigorous safety of these potential vaccines.”
On Oct. 1, Michelle McMurry-Heath, MD, PhD, president and chief executive of the Biotechnology Innovation Organization, released publicly her letter urging Department of Health & Human Services Secretary Alex Azar to “publicly release all new guidance” related to a COVID-19 vaccine. Such a move would bolster public confidence in the vaccine, she said.
“We cannot allow a lack of transparency to undermine confidence in the vaccine development process. The public must have full faith in the scientific process and the rigor of FDA’s regulatory oversight if we are to end the pandemic,” she wrote in the Oct. 1 letter to Azar. “Releasing any additional guidance on granting emergency use authorization for a vaccine will go a long way in accomplishing this critical goal.”
This article first appeared on Medscape.com.
while medical and trade associations called for a thorough review of any such product before approval.
The FDA took the unusual step of posting background materials much earlier than usual for its planned Oct. 22 advisory committee meeting on potential vaccines for COVID-19. The FDA also on Tuesday afternoon released a new guidance document, expanding on a previous set of recommendations the agency released in June.
In the new guidance document, FDA officials outline what will be required for even a limited clearance, known as an emergency use authorization (EUA), for a COVID-19 vaccine.
“Data from phase 3 studies should include a median follow-up duration of at least 2 months after completion of the full vaccination regimen to help provide adequate information to assess a vaccine’s benefit-risk profile,” the FDA said in the document.
FDA staff have emphasized the higher bar that drugmakers and regulators face in considering approval of a COVID-19 vaccine.
“Vaccines are complex biological products, and an EUA for a COVID-19 vaccine may allow for rapid and widespread deployment for administration of the vaccine to millions of individuals, including healthy people,” the agency staff said in the briefing documents.
The FDA’s briefing document for the Oct. 22 meeting appears to be markedly at odds with the claim Trump made in a video Monday night, in which he told the American public that “vaccines are coming momentarily.”
Trump, who is in a tightly contested presidential race against Democratic candidate Joe Biden, has repeatedly made claims of the potential arrival of COVID vaccines that are at odds with timelines offered with guarded optimism by experts in infectious diseases.
But based on these new guidelines from the FDA, it appears that the White House may now endorse the FDA’s stance, according to a Wall Street Journal report based on “people familiar with the matter.”
The publication reports that the White House, which has yet to officially comment, “endorsed the U.S. Food and Drug Administration’s plans for assessing whether a Covid-19 vaccine should be given widely, casting aside objections to requirements that would likely mean a shot won’t be cleared until after Election Day, people familiar with the matter said.”
Anthony S. Fauci, MD, director of the National Institute of Allergy and Infectious Diseases, on Monday night said during a virtual appearance at the twenty-first annual New Yorker Festival that there could be evidence as early as November or December about whether one of the vaccines now in testing will work out. He declared himself to have “cautious optimism” about potential rollout of vaccines as early as late 2020 or early 2021.
Peter Lurie, MD, MPH, who earlier served as the FDA’s associate commissioner for public health strategy and analysis, described the agency’s release of the briefing document as being a positive development.
News organizations, including the New York Times, have reported that the White House had sought to block the FDA from releasing further instructions for companies developing COVID-19 vaccines. The Associated Press on Tuesday said that a senior Trump administration official confirmed that the White House had blocked earlier FDA plans to formally publish the safety guidelines based on the 2-month data requirement, arguing that there was “no clinical or medical reason” for it.
“It is an encouraging sign that, despite opposition from the White House, the Food and Drug Administration has effectively published guidelines for emergency release of a vaccine for COVID-19 by disclosing the advice it has been providing to individual sponsors,” said Dr. Lurie, who is now executive director and president of the Center for Science in the Public Interest.
In a news release, he said the White House had sought to keep the FDA guidance under wraps “so it could maintain the public fiction that a safe and effective vaccine could be available before Election Day or even so that it could force emergency authorization of a vaccine with more limited follow-up.”
“Even the pharmaceutical industry has been clamoring for the release of these guidelines. We all want a safe and effective vaccine to end the pandemic, and we want it sooner rather than later,” Dr. Lurie said. “But we can’t afford for the Trump administration to bungle vaccine review the way they’ve bungled nearly every other aspect of its pandemic response.”
Tuesday also saw a flood of statements in support of FDA officials, including tweets from the chief executive of Pfizer, which is among the leaders in the race to develop a COVID-19 vaccine. Pfizer’s Albert Bourla, DVM, PhD, said that the FDA’s “public servants are known for their high integrity and scientific expertise and we have full faith in their ability to set appropriate standards for the approval of a COVID vaccine or treatment.”
The American Medical Association on Tuesday announced a public webinar on Wednesday where its president, Susan R. Bailey, MD, will discuss the COVID-19 vaccine review process with Peter Marks, MD, PhD, director of the Center for Biologics Evaluation and Research at the FDA. The AMA described this webinar as part of work “to restore trust in science and science-based decision-making among policymakers and the public.”
“To ensure media and the physician community are continuously informed about the federal review process for COVID-19 vaccine candidates, the AMA will host a webinar series to gain fact-based insights from the nation’s highest-ranking subject matter experts working to protect the health of the public,” the organization said in announcing the webinar.
In a statement, leaders of the Association of American Medical Colleges said that the FDA’s Vaccines and Related Biological Products Advisory Committee should evaluate any COVID-19 candidate vaccines prior to the FDA issuing an EUA.
“Full approval of a new vaccine or biologic requires demonstration of safety and effectiveness through a process that includes evaluation by the VRBPAC. Their recommendations are considered by FDA staff who ultimately have the authority to approve the new product,” said AAMC chief scientific officer Ross McKinney Jr, MD, and AAMC CEO David J. Skorton, MD, in the statement.
Thomas M. File Jr., MD, president of the Infectious Diseases Society of America, said in a statement that his association again asked the White House to “follow medical and scientific expertise in efforts to combat COVID-19.”
“It is imperative that a vaccine be approved on the basis of FDA’s quality standards and that its safety and efficacy are established before it is authorized,” Dr. File said. “A vaccine that has been approved with speed, rather than safety and efficacy, at the forefront will compound the challenges posed by this pandemic. FDA guidelines for approval that set standards the American people can trust are essential to the success of a vaccine.”
Stephen J. Ubl, chief executive of the Pharmaceutical Research and Manufacturers of America, said in a statement that his association “supports any efforts by FDA to provide clarifying guidance and we have engaged with the agency to support bringing greater transparency to the review process for COVID-19 vaccines.”
“To help address this public health crisis, our companies have also taken unprecedented steps to share vaccine clinical trial protocols and data in real time,” Mr. Ubl said. “We welcome the agency’s efforts to instill confidence in the rigorous safety of these potential vaccines.”
On Oct. 1, Michelle McMurry-Heath, MD, PhD, president and chief executive of the Biotechnology Innovation Organization, released publicly her letter urging Department of Health & Human Services Secretary Alex Azar to “publicly release all new guidance” related to a COVID-19 vaccine. Such a move would bolster public confidence in the vaccine, she said.
“We cannot allow a lack of transparency to undermine confidence in the vaccine development process. The public must have full faith in the scientific process and the rigor of FDA’s regulatory oversight if we are to end the pandemic,” she wrote in the Oct. 1 letter to Azar. “Releasing any additional guidance on granting emergency use authorization for a vaccine will go a long way in accomplishing this critical goal.”
This article first appeared on Medscape.com.
while medical and trade associations called for a thorough review of any such product before approval.
The FDA took the unusual step of posting background materials much earlier than usual for its planned Oct. 22 advisory committee meeting on potential vaccines for COVID-19. The FDA also on Tuesday afternoon released a new guidance document, expanding on a previous set of recommendations the agency released in June.
In the new guidance document, FDA officials outline what will be required for even a limited clearance, known as an emergency use authorization (EUA), for a COVID-19 vaccine.
“Data from phase 3 studies should include a median follow-up duration of at least 2 months after completion of the full vaccination regimen to help provide adequate information to assess a vaccine’s benefit-risk profile,” the FDA said in the document.
FDA staff have emphasized the higher bar that drugmakers and regulators face in considering approval of a COVID-19 vaccine.
“Vaccines are complex biological products, and an EUA for a COVID-19 vaccine may allow for rapid and widespread deployment for administration of the vaccine to millions of individuals, including healthy people,” the agency staff said in the briefing documents.
The FDA’s briefing document for the Oct. 22 meeting appears to be markedly at odds with the claim Trump made in a video Monday night, in which he told the American public that “vaccines are coming momentarily.”
Trump, who is in a tightly contested presidential race against Democratic candidate Joe Biden, has repeatedly made claims of the potential arrival of COVID vaccines that are at odds with timelines offered with guarded optimism by experts in infectious diseases.
But based on these new guidelines from the FDA, it appears that the White House may now endorse the FDA’s stance, according to a Wall Street Journal report based on “people familiar with the matter.”
The publication reports that the White House, which has yet to officially comment, “endorsed the U.S. Food and Drug Administration’s plans for assessing whether a Covid-19 vaccine should be given widely, casting aside objections to requirements that would likely mean a shot won’t be cleared until after Election Day, people familiar with the matter said.”
Anthony S. Fauci, MD, director of the National Institute of Allergy and Infectious Diseases, on Monday night said during a virtual appearance at the twenty-first annual New Yorker Festival that there could be evidence as early as November or December about whether one of the vaccines now in testing will work out. He declared himself to have “cautious optimism” about potential rollout of vaccines as early as late 2020 or early 2021.
Peter Lurie, MD, MPH, who earlier served as the FDA’s associate commissioner for public health strategy and analysis, described the agency’s release of the briefing document as being a positive development.
News organizations, including the New York Times, have reported that the White House had sought to block the FDA from releasing further instructions for companies developing COVID-19 vaccines. The Associated Press on Tuesday said that a senior Trump administration official confirmed that the White House had blocked earlier FDA plans to formally publish the safety guidelines based on the 2-month data requirement, arguing that there was “no clinical or medical reason” for it.
“It is an encouraging sign that, despite opposition from the White House, the Food and Drug Administration has effectively published guidelines for emergency release of a vaccine for COVID-19 by disclosing the advice it has been providing to individual sponsors,” said Dr. Lurie, who is now executive director and president of the Center for Science in the Public Interest.
In a news release, he said the White House had sought to keep the FDA guidance under wraps “so it could maintain the public fiction that a safe and effective vaccine could be available before Election Day or even so that it could force emergency authorization of a vaccine with more limited follow-up.”
“Even the pharmaceutical industry has been clamoring for the release of these guidelines. We all want a safe and effective vaccine to end the pandemic, and we want it sooner rather than later,” Dr. Lurie said. “But we can’t afford for the Trump administration to bungle vaccine review the way they’ve bungled nearly every other aspect of its pandemic response.”
Tuesday also saw a flood of statements in support of FDA officials, including tweets from the chief executive of Pfizer, which is among the leaders in the race to develop a COVID-19 vaccine. Pfizer’s Albert Bourla, DVM, PhD, said that the FDA’s “public servants are known for their high integrity and scientific expertise and we have full faith in their ability to set appropriate standards for the approval of a COVID vaccine or treatment.”
The American Medical Association on Tuesday announced a public webinar on Wednesday where its president, Susan R. Bailey, MD, will discuss the COVID-19 vaccine review process with Peter Marks, MD, PhD, director of the Center for Biologics Evaluation and Research at the FDA. The AMA described this webinar as part of work “to restore trust in science and science-based decision-making among policymakers and the public.”
“To ensure media and the physician community are continuously informed about the federal review process for COVID-19 vaccine candidates, the AMA will host a webinar series to gain fact-based insights from the nation’s highest-ranking subject matter experts working to protect the health of the public,” the organization said in announcing the webinar.
In a statement, leaders of the Association of American Medical Colleges said that the FDA’s Vaccines and Related Biological Products Advisory Committee should evaluate any COVID-19 candidate vaccines prior to the FDA issuing an EUA.
“Full approval of a new vaccine or biologic requires demonstration of safety and effectiveness through a process that includes evaluation by the VRBPAC. Their recommendations are considered by FDA staff who ultimately have the authority to approve the new product,” said AAMC chief scientific officer Ross McKinney Jr, MD, and AAMC CEO David J. Skorton, MD, in the statement.
Thomas M. File Jr., MD, president of the Infectious Diseases Society of America, said in a statement that his association again asked the White House to “follow medical and scientific expertise in efforts to combat COVID-19.”
“It is imperative that a vaccine be approved on the basis of FDA’s quality standards and that its safety and efficacy are established before it is authorized,” Dr. File said. “A vaccine that has been approved with speed, rather than safety and efficacy, at the forefront will compound the challenges posed by this pandemic. FDA guidelines for approval that set standards the American people can trust are essential to the success of a vaccine.”
Stephen J. Ubl, chief executive of the Pharmaceutical Research and Manufacturers of America, said in a statement that his association “supports any efforts by FDA to provide clarifying guidance and we have engaged with the agency to support bringing greater transparency to the review process for COVID-19 vaccines.”
“To help address this public health crisis, our companies have also taken unprecedented steps to share vaccine clinical trial protocols and data in real time,” Mr. Ubl said. “We welcome the agency’s efforts to instill confidence in the rigorous safety of these potential vaccines.”
On Oct. 1, Michelle McMurry-Heath, MD, PhD, president and chief executive of the Biotechnology Innovation Organization, released publicly her letter urging Department of Health & Human Services Secretary Alex Azar to “publicly release all new guidance” related to a COVID-19 vaccine. Such a move would bolster public confidence in the vaccine, she said.
“We cannot allow a lack of transparency to undermine confidence in the vaccine development process. The public must have full faith in the scientific process and the rigor of FDA’s regulatory oversight if we are to end the pandemic,” she wrote in the Oct. 1 letter to Azar. “Releasing any additional guidance on granting emergency use authorization for a vaccine will go a long way in accomplishing this critical goal.”
This article first appeared on Medscape.com.
Experts assess infection risks for patients on biologics
In a new review, a group of infectious disease experts have summarized and made recommendations about recent findings regarding infections that can occur during treatment with an evolving set of targeted and biologic therapies for rheumatoid arthritis and psoriatic arthritis.
“We claim for the need for multicenter registries and multidisciplinary approaches, for new vaccines trials in RA and PsA, and for better defining when and how biologics can be restarted after severe infections,” lead author Olivier Lortholary, MD, of the Institut Pasteur in Paris, and his coauthors wrote in Annals of the Rheumatic Diseases.
“The take-home message is that different DMARDs [disease-modifying antirheumatic drugs], in many ways, are very similar,” said coauthor Kevin L. Winthrop, MD, MPH, professor of public health and ophthalmology at Oregon Health & Science University, Portland, in an interview. “They all have fairly similar risks when it comes to ‘classical’ or routine bacterial infections. But when you talk about opportunistic infections, you start seeing the differences between these drugs.”
The experts began by addressing the current view of the infectious risk of biologic therapies, citing a recent meta-analysis in which standard (odds ratio, 1.31; 95% confidence interval, 1.09-1.58) and high (OR, 1.90; 95% CI, 1.50-2.39) doses of biologics were associated with increased risk of serious infection. They also noted that the ‘healthy drug survivor effect’ tends to confound long-term extensions of randomized clinical trials involving biologics.
“That is largely because people who are more likely to do well or have proven themselves to do well with that infection, they tend to stay in [trials] and stay on drugs,” Dr. Winthrop said. “The ones who develop infections are more likely to drop out. You see this survival of the fittest-type situation, where healthy users dominate a cohort over time. That’s why you see incidence rates decreasing.”
In response, Arthur Kavanaugh, MD, professor of medicine in the division of rheumatology, allergy, and immunology at the University of California, San Diego, and the director of the Center for Innovative Therapy there, backed the idea of a general ‘depletion of the susceptibles’ but warned doctors to evaluate each patient and situation accordingly. “Providers need to be vigilant throughout for common infections, rarer infections, and infections at greatest risk for the individual patient based on factors like comorbidities and concomitant medications,” he said in an interview.
When considering restarting a biologic in a patient who recently suffered a serious infection, the experts prescribed no general rule and noted that it will “depend on the type of infection, on the mechanism of action of the drug, on the other available drugs for the considered disease and, of course, on the willingness of the patients to restart a drug possibly having [given] him/her a side effect.”
Assessing infection risk related to various inhibitors
Regarding infections caused by TNF-alpha inhibitors (TNFIs), the experts acknowledged a broad increase in risk for mycobacterial and fungal infections, especially tuberculosis and histoplasmosis. They added that patients on TNFIs are more prone to developing pneumonia and soft tissue infections, while smaller studies have indicated a higher risk of listeriosis, legionellosis, herpes zoster (HZ), and reactivation of chronic hepatitis B virus infection.
As for recommendations, they endorsed discontinuing TNFIs when a serious infection occurs and not restarting until after treatment and clinical response. Patients should be screened for latent tuberculosis infection (LTBI) before starting the drug, and anti-TB drugs should be presented to patients with LTBI so they do not progress to active TB.
Regarding other biologics, they cited several studies indicating that IL-6 inhibitors can increase infection risks in RA patients at a rate similar to TNFIs. Among the most common infections were pneumonia and cellulitis. In addition, although PsA patients on IL-17 inhibitors have a dose-dependent risk of mild to moderate mucocutaneous candidiasis, there was no increased risk of serious opportunistic infections like TB.
In assessing JAK inhibitors, they cited a pooled analysis that indicated pneumonia and skin and soft-tissue infections as the most common and noted the high incidence of HZ, compared with other infections. They added that abatacept (Orencia) did not appear to increase risk of infections in RA patients, such as HZ, dermatomycosis, candidiasis, or endemic mycoses. Those same patients did not see an increased overall infection risk after treatment with rituximab (Rituxan), and clinical trials containing treatment with apremilast (Otezla) reported a rare occurrence of serious infections.
Recommendation-wise, they endorsed screening for LTBI before starting IL-6 inhibitors and antiviral prophylaxis with acyclovir in particularly at-risk patients on JAK inhibitors. Age-appropriate influenza vaccinations were also recommended for rituximab, because of the development of rituximab-induced hypogammaglobulinemia.
Prediction and prevention
When it comes to predicting infections in patients on biologics, the experts wrote that it “remains a challenge.” The potential effects of pretreatment underlying disease, the lack of validated biomarkers, and the relatively low rate of infections all combine to stymie prediction. That said, they acknowledged ongoing efforts in monitoring lymphocyte subpopulation counts and immunoglobin levels, as well as a clinical score called the RABBIT Risk Score for Infections, which was validated in two separate cohorts.
“As Yogi Berra said, predictions are hard, especially about the future,” Dr. Kavanaugh said. “Discussions with your patient are always important.”
In regard to overall prevention, they acknowledged that most of their recommendations are of low evidence, except for antiviral prophylaxis for hepatitis B patients on rituximab and the aforementioned LTBI therapy in patients on TNFIs. Broadly, they advocated for all RA and PsA patients to receive a full infectious disease evaluation before the start of targeted and biologic therapies.
They also addressed vaccinations, recommending an evaluation of the patient’s immunization history and potentially planning a catch-up schedule for those in need of the influenza vaccine, a diphtheria-tetanus-pertussis booster, or the pneumococcal vaccine. More broadly, they stated that “a better response is expected if [non-live] vaccination is performed before the introduction of immunosuppressive drugs.” They added that live vaccines should be administered as soon as possible.
What rheumatologists can do
“So how do you mitigate risk?” Dr. Winthrop asked. “You have to be able to predict the risk, see what’s modifiable, and try to act on it. A lot of the risk of infection has more to do with the patient than the therapy.
“You try to minimize what you’re doing to the patient, particularly around steroids,” he said. “And then you think about screening and vaccinations. Rheumatologists need to be involved in those conversations because they’re the ones who know how these drugs interact with vaccines. A lot of the drugs might dumb down vaccine responses. Be sure to consider that and give the vaccines at times that will optimize their immunogenicity and likely efficacy.”
“Thankfully, infections are not that common,” Dr. Kavanaugh said. “Rheumatologists depend on data from trials, but more safety data comes from registry data and personal and shared experience.”
The authors declared no potential conflicts of interest.
SOURCE: Lortholary O et al. Ann Rheum Dis. 2020 Sep 22. doi: 10.1136/annrheumdis-2020-217092.
In a new review, a group of infectious disease experts have summarized and made recommendations about recent findings regarding infections that can occur during treatment with an evolving set of targeted and biologic therapies for rheumatoid arthritis and psoriatic arthritis.
“We claim for the need for multicenter registries and multidisciplinary approaches, for new vaccines trials in RA and PsA, and for better defining when and how biologics can be restarted after severe infections,” lead author Olivier Lortholary, MD, of the Institut Pasteur in Paris, and his coauthors wrote in Annals of the Rheumatic Diseases.
“The take-home message is that different DMARDs [disease-modifying antirheumatic drugs], in many ways, are very similar,” said coauthor Kevin L. Winthrop, MD, MPH, professor of public health and ophthalmology at Oregon Health & Science University, Portland, in an interview. “They all have fairly similar risks when it comes to ‘classical’ or routine bacterial infections. But when you talk about opportunistic infections, you start seeing the differences between these drugs.”
The experts began by addressing the current view of the infectious risk of biologic therapies, citing a recent meta-analysis in which standard (odds ratio, 1.31; 95% confidence interval, 1.09-1.58) and high (OR, 1.90; 95% CI, 1.50-2.39) doses of biologics were associated with increased risk of serious infection. They also noted that the ‘healthy drug survivor effect’ tends to confound long-term extensions of randomized clinical trials involving biologics.
“That is largely because people who are more likely to do well or have proven themselves to do well with that infection, they tend to stay in [trials] and stay on drugs,” Dr. Winthrop said. “The ones who develop infections are more likely to drop out. You see this survival of the fittest-type situation, where healthy users dominate a cohort over time. That’s why you see incidence rates decreasing.”
In response, Arthur Kavanaugh, MD, professor of medicine in the division of rheumatology, allergy, and immunology at the University of California, San Diego, and the director of the Center for Innovative Therapy there, backed the idea of a general ‘depletion of the susceptibles’ but warned doctors to evaluate each patient and situation accordingly. “Providers need to be vigilant throughout for common infections, rarer infections, and infections at greatest risk for the individual patient based on factors like comorbidities and concomitant medications,” he said in an interview.
When considering restarting a biologic in a patient who recently suffered a serious infection, the experts prescribed no general rule and noted that it will “depend on the type of infection, on the mechanism of action of the drug, on the other available drugs for the considered disease and, of course, on the willingness of the patients to restart a drug possibly having [given] him/her a side effect.”
Assessing infection risk related to various inhibitors
Regarding infections caused by TNF-alpha inhibitors (TNFIs), the experts acknowledged a broad increase in risk for mycobacterial and fungal infections, especially tuberculosis and histoplasmosis. They added that patients on TNFIs are more prone to developing pneumonia and soft tissue infections, while smaller studies have indicated a higher risk of listeriosis, legionellosis, herpes zoster (HZ), and reactivation of chronic hepatitis B virus infection.
As for recommendations, they endorsed discontinuing TNFIs when a serious infection occurs and not restarting until after treatment and clinical response. Patients should be screened for latent tuberculosis infection (LTBI) before starting the drug, and anti-TB drugs should be presented to patients with LTBI so they do not progress to active TB.
Regarding other biologics, they cited several studies indicating that IL-6 inhibitors can increase infection risks in RA patients at a rate similar to TNFIs. Among the most common infections were pneumonia and cellulitis. In addition, although PsA patients on IL-17 inhibitors have a dose-dependent risk of mild to moderate mucocutaneous candidiasis, there was no increased risk of serious opportunistic infections like TB.
In assessing JAK inhibitors, they cited a pooled analysis that indicated pneumonia and skin and soft-tissue infections as the most common and noted the high incidence of HZ, compared with other infections. They added that abatacept (Orencia) did not appear to increase risk of infections in RA patients, such as HZ, dermatomycosis, candidiasis, or endemic mycoses. Those same patients did not see an increased overall infection risk after treatment with rituximab (Rituxan), and clinical trials containing treatment with apremilast (Otezla) reported a rare occurrence of serious infections.
Recommendation-wise, they endorsed screening for LTBI before starting IL-6 inhibitors and antiviral prophylaxis with acyclovir in particularly at-risk patients on JAK inhibitors. Age-appropriate influenza vaccinations were also recommended for rituximab, because of the development of rituximab-induced hypogammaglobulinemia.
Prediction and prevention
When it comes to predicting infections in patients on biologics, the experts wrote that it “remains a challenge.” The potential effects of pretreatment underlying disease, the lack of validated biomarkers, and the relatively low rate of infections all combine to stymie prediction. That said, they acknowledged ongoing efforts in monitoring lymphocyte subpopulation counts and immunoglobin levels, as well as a clinical score called the RABBIT Risk Score for Infections, which was validated in two separate cohorts.
“As Yogi Berra said, predictions are hard, especially about the future,” Dr. Kavanaugh said. “Discussions with your patient are always important.”
In regard to overall prevention, they acknowledged that most of their recommendations are of low evidence, except for antiviral prophylaxis for hepatitis B patients on rituximab and the aforementioned LTBI therapy in patients on TNFIs. Broadly, they advocated for all RA and PsA patients to receive a full infectious disease evaluation before the start of targeted and biologic therapies.
They also addressed vaccinations, recommending an evaluation of the patient’s immunization history and potentially planning a catch-up schedule for those in need of the influenza vaccine, a diphtheria-tetanus-pertussis booster, or the pneumococcal vaccine. More broadly, they stated that “a better response is expected if [non-live] vaccination is performed before the introduction of immunosuppressive drugs.” They added that live vaccines should be administered as soon as possible.
What rheumatologists can do
“So how do you mitigate risk?” Dr. Winthrop asked. “You have to be able to predict the risk, see what’s modifiable, and try to act on it. A lot of the risk of infection has more to do with the patient than the therapy.
“You try to minimize what you’re doing to the patient, particularly around steroids,” he said. “And then you think about screening and vaccinations. Rheumatologists need to be involved in those conversations because they’re the ones who know how these drugs interact with vaccines. A lot of the drugs might dumb down vaccine responses. Be sure to consider that and give the vaccines at times that will optimize their immunogenicity and likely efficacy.”
“Thankfully, infections are not that common,” Dr. Kavanaugh said. “Rheumatologists depend on data from trials, but more safety data comes from registry data and personal and shared experience.”
The authors declared no potential conflicts of interest.
SOURCE: Lortholary O et al. Ann Rheum Dis. 2020 Sep 22. doi: 10.1136/annrheumdis-2020-217092.
In a new review, a group of infectious disease experts have summarized and made recommendations about recent findings regarding infections that can occur during treatment with an evolving set of targeted and biologic therapies for rheumatoid arthritis and psoriatic arthritis.
“We claim for the need for multicenter registries and multidisciplinary approaches, for new vaccines trials in RA and PsA, and for better defining when and how biologics can be restarted after severe infections,” lead author Olivier Lortholary, MD, of the Institut Pasteur in Paris, and his coauthors wrote in Annals of the Rheumatic Diseases.
“The take-home message is that different DMARDs [disease-modifying antirheumatic drugs], in many ways, are very similar,” said coauthor Kevin L. Winthrop, MD, MPH, professor of public health and ophthalmology at Oregon Health & Science University, Portland, in an interview. “They all have fairly similar risks when it comes to ‘classical’ or routine bacterial infections. But when you talk about opportunistic infections, you start seeing the differences between these drugs.”
The experts began by addressing the current view of the infectious risk of biologic therapies, citing a recent meta-analysis in which standard (odds ratio, 1.31; 95% confidence interval, 1.09-1.58) and high (OR, 1.90; 95% CI, 1.50-2.39) doses of biologics were associated with increased risk of serious infection. They also noted that the ‘healthy drug survivor effect’ tends to confound long-term extensions of randomized clinical trials involving biologics.
“That is largely because people who are more likely to do well or have proven themselves to do well with that infection, they tend to stay in [trials] and stay on drugs,” Dr. Winthrop said. “The ones who develop infections are more likely to drop out. You see this survival of the fittest-type situation, where healthy users dominate a cohort over time. That’s why you see incidence rates decreasing.”
In response, Arthur Kavanaugh, MD, professor of medicine in the division of rheumatology, allergy, and immunology at the University of California, San Diego, and the director of the Center for Innovative Therapy there, backed the idea of a general ‘depletion of the susceptibles’ but warned doctors to evaluate each patient and situation accordingly. “Providers need to be vigilant throughout for common infections, rarer infections, and infections at greatest risk for the individual patient based on factors like comorbidities and concomitant medications,” he said in an interview.
When considering restarting a biologic in a patient who recently suffered a serious infection, the experts prescribed no general rule and noted that it will “depend on the type of infection, on the mechanism of action of the drug, on the other available drugs for the considered disease and, of course, on the willingness of the patients to restart a drug possibly having [given] him/her a side effect.”
Assessing infection risk related to various inhibitors
Regarding infections caused by TNF-alpha inhibitors (TNFIs), the experts acknowledged a broad increase in risk for mycobacterial and fungal infections, especially tuberculosis and histoplasmosis. They added that patients on TNFIs are more prone to developing pneumonia and soft tissue infections, while smaller studies have indicated a higher risk of listeriosis, legionellosis, herpes zoster (HZ), and reactivation of chronic hepatitis B virus infection.
As for recommendations, they endorsed discontinuing TNFIs when a serious infection occurs and not restarting until after treatment and clinical response. Patients should be screened for latent tuberculosis infection (LTBI) before starting the drug, and anti-TB drugs should be presented to patients with LTBI so they do not progress to active TB.
Regarding other biologics, they cited several studies indicating that IL-6 inhibitors can increase infection risks in RA patients at a rate similar to TNFIs. Among the most common infections were pneumonia and cellulitis. In addition, although PsA patients on IL-17 inhibitors have a dose-dependent risk of mild to moderate mucocutaneous candidiasis, there was no increased risk of serious opportunistic infections like TB.
In assessing JAK inhibitors, they cited a pooled analysis that indicated pneumonia and skin and soft-tissue infections as the most common and noted the high incidence of HZ, compared with other infections. They added that abatacept (Orencia) did not appear to increase risk of infections in RA patients, such as HZ, dermatomycosis, candidiasis, or endemic mycoses. Those same patients did not see an increased overall infection risk after treatment with rituximab (Rituxan), and clinical trials containing treatment with apremilast (Otezla) reported a rare occurrence of serious infections.
Recommendation-wise, they endorsed screening for LTBI before starting IL-6 inhibitors and antiviral prophylaxis with acyclovir in particularly at-risk patients on JAK inhibitors. Age-appropriate influenza vaccinations were also recommended for rituximab, because of the development of rituximab-induced hypogammaglobulinemia.
Prediction and prevention
When it comes to predicting infections in patients on biologics, the experts wrote that it “remains a challenge.” The potential effects of pretreatment underlying disease, the lack of validated biomarkers, and the relatively low rate of infections all combine to stymie prediction. That said, they acknowledged ongoing efforts in monitoring lymphocyte subpopulation counts and immunoglobin levels, as well as a clinical score called the RABBIT Risk Score for Infections, which was validated in two separate cohorts.
“As Yogi Berra said, predictions are hard, especially about the future,” Dr. Kavanaugh said. “Discussions with your patient are always important.”
In regard to overall prevention, they acknowledged that most of their recommendations are of low evidence, except for antiviral prophylaxis for hepatitis B patients on rituximab and the aforementioned LTBI therapy in patients on TNFIs. Broadly, they advocated for all RA and PsA patients to receive a full infectious disease evaluation before the start of targeted and biologic therapies.
They also addressed vaccinations, recommending an evaluation of the patient’s immunization history and potentially planning a catch-up schedule for those in need of the influenza vaccine, a diphtheria-tetanus-pertussis booster, or the pneumococcal vaccine. More broadly, they stated that “a better response is expected if [non-live] vaccination is performed before the introduction of immunosuppressive drugs.” They added that live vaccines should be administered as soon as possible.
What rheumatologists can do
“So how do you mitigate risk?” Dr. Winthrop asked. “You have to be able to predict the risk, see what’s modifiable, and try to act on it. A lot of the risk of infection has more to do with the patient than the therapy.
“You try to minimize what you’re doing to the patient, particularly around steroids,” he said. “And then you think about screening and vaccinations. Rheumatologists need to be involved in those conversations because they’re the ones who know how these drugs interact with vaccines. A lot of the drugs might dumb down vaccine responses. Be sure to consider that and give the vaccines at times that will optimize their immunogenicity and likely efficacy.”
“Thankfully, infections are not that common,” Dr. Kavanaugh said. “Rheumatologists depend on data from trials, but more safety data comes from registry data and personal and shared experience.”
The authors declared no potential conflicts of interest.
SOURCE: Lortholary O et al. Ann Rheum Dis. 2020 Sep 22. doi: 10.1136/annrheumdis-2020-217092.
FROM ANNALS OF THE RHEUMATIC DISEASES
CDC flips, acknowledges aerosol spread of COVID-19
The information reiterates, however, that “COVID-19 is thought to spread mainly through close contact from person to person, including between people who are physically near each other (within about 6 feet). People who are infected but do not show symptoms can also spread the virus to others.”
In a statement to the media, the CDC said, “Today’s update acknowledges the existence of some published reports showing limited, uncommon circumstances where people with COVID-19 infected others who were more than 6 feet away or shortly after the COVID-19–positive person left an area. In these instances, transmission occurred in poorly ventilated and enclosed spaces that often involved activities that caused heavier breathing, like singing or exercise. Such environments and activities may contribute to the buildup of virus-carrying particles.”
“This is HUGE and been long delayed. But glad it’s now CDC official,” tweeted Eric Feigl-Ding, MD, an epidemiologist and health economist at Harvard University, Boston on Oct. 5.
The CDC announcement follows an abrupt flip-flop on information last month surrounding the aerosol spread of the virus.
Information deleted from website last month
On September 18, the CDC had added to its existing guidance that the virus is spread “through respiratory droplets or small particles, such as those in aerosols, produced when an infected person coughs, sneezes, sings, talks, or breathes. These particles can be inhaled into the nose, mouth, airways, and lungs and cause infection.”
The CDC then deleted that guidance on Sept. 21, saying it was a draft update released in error.
A key element of the now-deleted guidance said, “this is thought to be the main way the virus spreads.”
The information updated today reverses the now-deleted guidance and says aerosol transmission is not the main way the virus spreads.
It states that people who are within 6 feet of a person with COVID-19 or have direct contact with that person have the greatest risk of infection.
The CDC reiterated in the statement to the media today, “People can protect themselves from the virus that causes COVID-19 by staying at least 6 feet away from others, wearing a mask that covers their nose and mouth, washing their hands frequently, cleaning touched surfaces often, and staying home when sick.”
Among the journals that have published evidence on aerosol spread is Clinical Infectious Diseases, which, on July 6, published the paper, “It Is Time to Address Airborne Transmission of Coronavirus Disease 2019,” which was supported by 239 scientists.
The authors wrote, “there is significant potential for inhalation exposure to viruses in microscopic respiratory droplets (microdroplets) at short to medium distances (up to several meters, or room scale).”
Aerosols and airborne transmission “are the only way to explain super-spreader events we are seeing,” said Kimberly Prather, PhD, an atmospheric chemist at the University of California at San Diego, in an interview Oct. 5 with the Washington Post.
Dr. Prather added that, once aerosolization is acknowledged, this becomes a “fixable” problem through proper ventilation.
“Wear masks at all times indoors when others are present,” Dr. Prather said. But when inside, she said, there’s no such thing as a completely safe social distance.
This article first appeared on Medscape.com.
The information reiterates, however, that “COVID-19 is thought to spread mainly through close contact from person to person, including between people who are physically near each other (within about 6 feet). People who are infected but do not show symptoms can also spread the virus to others.”
In a statement to the media, the CDC said, “Today’s update acknowledges the existence of some published reports showing limited, uncommon circumstances where people with COVID-19 infected others who were more than 6 feet away or shortly after the COVID-19–positive person left an area. In these instances, transmission occurred in poorly ventilated and enclosed spaces that often involved activities that caused heavier breathing, like singing or exercise. Such environments and activities may contribute to the buildup of virus-carrying particles.”
“This is HUGE and been long delayed. But glad it’s now CDC official,” tweeted Eric Feigl-Ding, MD, an epidemiologist and health economist at Harvard University, Boston on Oct. 5.
The CDC announcement follows an abrupt flip-flop on information last month surrounding the aerosol spread of the virus.
Information deleted from website last month
On September 18, the CDC had added to its existing guidance that the virus is spread “through respiratory droplets or small particles, such as those in aerosols, produced when an infected person coughs, sneezes, sings, talks, or breathes. These particles can be inhaled into the nose, mouth, airways, and lungs and cause infection.”
The CDC then deleted that guidance on Sept. 21, saying it was a draft update released in error.
A key element of the now-deleted guidance said, “this is thought to be the main way the virus spreads.”
The information updated today reverses the now-deleted guidance and says aerosol transmission is not the main way the virus spreads.
It states that people who are within 6 feet of a person with COVID-19 or have direct contact with that person have the greatest risk of infection.
The CDC reiterated in the statement to the media today, “People can protect themselves from the virus that causes COVID-19 by staying at least 6 feet away from others, wearing a mask that covers their nose and mouth, washing their hands frequently, cleaning touched surfaces often, and staying home when sick.”
Among the journals that have published evidence on aerosol spread is Clinical Infectious Diseases, which, on July 6, published the paper, “It Is Time to Address Airborne Transmission of Coronavirus Disease 2019,” which was supported by 239 scientists.
The authors wrote, “there is significant potential for inhalation exposure to viruses in microscopic respiratory droplets (microdroplets) at short to medium distances (up to several meters, or room scale).”
Aerosols and airborne transmission “are the only way to explain super-spreader events we are seeing,” said Kimberly Prather, PhD, an atmospheric chemist at the University of California at San Diego, in an interview Oct. 5 with the Washington Post.
Dr. Prather added that, once aerosolization is acknowledged, this becomes a “fixable” problem through proper ventilation.
“Wear masks at all times indoors when others are present,” Dr. Prather said. But when inside, she said, there’s no such thing as a completely safe social distance.
This article first appeared on Medscape.com.
The information reiterates, however, that “COVID-19 is thought to spread mainly through close contact from person to person, including between people who are physically near each other (within about 6 feet). People who are infected but do not show symptoms can also spread the virus to others.”
In a statement to the media, the CDC said, “Today’s update acknowledges the existence of some published reports showing limited, uncommon circumstances where people with COVID-19 infected others who were more than 6 feet away or shortly after the COVID-19–positive person left an area. In these instances, transmission occurred in poorly ventilated and enclosed spaces that often involved activities that caused heavier breathing, like singing or exercise. Such environments and activities may contribute to the buildup of virus-carrying particles.”
“This is HUGE and been long delayed. But glad it’s now CDC official,” tweeted Eric Feigl-Ding, MD, an epidemiologist and health economist at Harvard University, Boston on Oct. 5.
The CDC announcement follows an abrupt flip-flop on information last month surrounding the aerosol spread of the virus.
Information deleted from website last month
On September 18, the CDC had added to its existing guidance that the virus is spread “through respiratory droplets or small particles, such as those in aerosols, produced when an infected person coughs, sneezes, sings, talks, or breathes. These particles can be inhaled into the nose, mouth, airways, and lungs and cause infection.”
The CDC then deleted that guidance on Sept. 21, saying it was a draft update released in error.
A key element of the now-deleted guidance said, “this is thought to be the main way the virus spreads.”
The information updated today reverses the now-deleted guidance and says aerosol transmission is not the main way the virus spreads.
It states that people who are within 6 feet of a person with COVID-19 or have direct contact with that person have the greatest risk of infection.
The CDC reiterated in the statement to the media today, “People can protect themselves from the virus that causes COVID-19 by staying at least 6 feet away from others, wearing a mask that covers their nose and mouth, washing their hands frequently, cleaning touched surfaces often, and staying home when sick.”
Among the journals that have published evidence on aerosol spread is Clinical Infectious Diseases, which, on July 6, published the paper, “It Is Time to Address Airborne Transmission of Coronavirus Disease 2019,” which was supported by 239 scientists.
The authors wrote, “there is significant potential for inhalation exposure to viruses in microscopic respiratory droplets (microdroplets) at short to medium distances (up to several meters, or room scale).”
Aerosols and airborne transmission “are the only way to explain super-spreader events we are seeing,” said Kimberly Prather, PhD, an atmospheric chemist at the University of California at San Diego, in an interview Oct. 5 with the Washington Post.
Dr. Prather added that, once aerosolization is acknowledged, this becomes a “fixable” problem through proper ventilation.
“Wear masks at all times indoors when others are present,” Dr. Prather said. But when inside, she said, there’s no such thing as a completely safe social distance.
This article first appeared on Medscape.com.
Substance use tied to increased COVID-19 risk
Substance use disorders (SUD), particularly opioid addiction and smoking, are tied to an increased risk for COVID-19 and serious adverse outcomes including hospitalization and death, new research suggests.
A study funded by the National Institutes of Health assessed electronic health records of more than 73 million patients in the United States. Although only 10.3% of the participants had an SUD, “they represented 15.6% of the COVID-19 cases,” the investigators reported.
In addition, those with a recent diagnosis of SUD were eight times more likely to develop COVID-19 versus those without such a diagnosis. For specific SUDs, the greatest risk was for those with an opioid addiction followed by those who were addicted to cigarettes.
coinvestigator Nora Volkow, MD, director of the National Institute on Drug Abuse, said in a press release.
It may also be harder for individuals with addiction to access health care services for a variety of reasons, including low socioeconomic status or stigma, she said in an interview.
Dr. Volkow said she has encountered patients with medical emergencies who refuse to seek treatment at the emergency department because of previous experiences where they have been mistreated and encountered discrimination, and “that’s really very tragic.”
The findings were published online Sept. 14 in Molecular Psychiatry.
Is nicotine protective?
Dr. Volkow, her fellow senior author Rong Xu, PhD, Case Western Reserve University, Cleveland, and their team conducted the study because data released before the pandemic showed a significant increase in opioid overdose in 2019. “We were in an opioid crisis where we again saw an increase in mortality associated with overdose – and then COVID comes along. So the question was how are people who are already struggling faring? And if they were getting infected [with the coronavirus], what happened to them?”
Patients with SUDs have multiple medical comorbidities that are known risk factors for COVID-19, Dr. Volkow noted.
However, the only specific SUD that has been previously studied in this context is tobacco use disorder, she said. A report from Chinese investigators released early in the pandemic showed that smokers were more likely to be infected by coronavirus and more likely to die from COVID-19.
Interestingly, a cross-sectional study published in April suggested that smoking may be protective against COVID, and Dr. Volkow noted that a clinical study currently being conducted in France is assessing whether wearing a nicotine patch has the potential to prevent the virus.
“That’s very different from looking at a chronic smoker,” she pointed out. “It’s a potential that nicotine as a chemical [could be] a preventive measure as opposed to saying smoking will prevent you from getting COVID.”
Patients with SUDs, said Dr. Volkow, “are likely to be at greater risk because of the effects of drugs in the metabolic system and the interfering with oxygenation in the pulmonary vessels.”
The retrospective case-control study included EHR data from 73.1 million patients. In the study population, 54% were women, 55% were White, 10% Black, 2% Asian, 1% Hispanic/Latino, and the others were classified as other or unknown.
EHRs were collected through June 15 at 360 hospitals in all 50 states and were deidentified to ensure privacy. SUDs included alcohol, tobacco, cannabis, opioid, and cocaine.
Racial disparities
Results showed that about 7.5 million participants had a previous SUD diagnosis; of these, 722,370 had been diagnosed within the past year.
Tobacco use disorder was the most common diagnosis (n = 6,414,580), followed by alcohol (1,264,990), cannabis (490,420), opioid (471,520), and cocaine (222,680).
In addition, 12,030 (60% women) were diagnosed with COVID-19 and 1,880 had both COVID-19 and an SUD.
Adjusted analyses revealed that those who had a recent diagnosis of SUD were at a significantly greater increased risk for COVID-19 than individuals without an SUD (adjusted odds ratio, 8.7; 95% confidence interval, 8.4-9.0; P < 10–30).
This increased risk was greatest in participants with opioid use disorder (aOR, 10.2; 95% CI, 9.1-11.5; P < 10–30), followed by those with tobacco use disorder (aOR, 8.2; 95% CI, 7.9 - 8.5; P < 10–30).
Alcohol, cocaine, and cannabis had aORs of 7.7, 6.5, and 5.3, respectively. The aOR for lifetime SUD and COVID-19 was 1.5.
Among all patients with COVID-19, hospitalization rates were significantly greater in those with an SUD (43.8%) versus those without (30.1%), as were death rates at 9.6% versus 6.6%, respectively.
Race was a significant risk factor. Black patients with a recent SUD diagnosis were twice as likely as White patients to develop COVID-19 (aOR, 2.2; P < 10–30), and those specifically with opioid use disorder were four times more likely to develop the disease (aOR, 4.2 P < 10–25).
Black patients with both COVID-19 and lifetime SUD also had greater hospitalization and death rates versus their White peers (50.7% vs. 35.2% and 13% vs. 8.6%, respectively).
“This surprised me,” Dr. Volkow noted. “You can see the emergence of the racial disparities even under these conditions of really negative outcomes.”
Vulnerable populations
Cancer; obesity; HIV; diabetes; cardiovascular disease; and chronic kidney, liver, and lung diseases, which are all risk factors for COVID-19, were more prevalent in the group of patients with a recent SUD diagnosis versus those without.
In addition, asthma, type 2 diabetes, hypertension, obesity, and chronic kidney disease were more prevalent in the Black patents with a recent SUD than in the White patients.
Overall, the findings “identify individuals with SUD as a vulnerable population, especially African Americans with SUDs, who are at significantly increased risk for COVID-19 and its adverse outcomes,” the investigators wrote.
The results also highlight “the need to screen and treat individuals with SUD as part of the strategy to control the pandemic while ensuring no disparities in access to healthcare support,” they added.
Dr. Volkow noted that “marginalization” often occurs for individuals with addiction, making it more difficult for them to access health care services.
“It is incumbent upon clinicians to meet the unique challenges of caring for this vulnerable population, just as they would any other high-risk group,” she said.
“Patients should not just be treated for COVID, but should also be provided with treatment for their substance use disorder,” Dr. Volkow added.
‘Pretty convincing’
Andrew J. Saxon, MD, professor in the department of psychiatry and behavioral sciences at the University of Washington, Seattle, called the findings interesting.
“I found it pretty convincing that people who have substance use disorders are probably at higher risk for getting COVID-19 infection and more complications once they are infected,” he said.
Dr. Saxon, who was not involved with the research, is also director of the Center of Excellence in Substance Addiction Treatment and Education and is a member of the American Psychiatric Association’s Council on Addiction Psychiatry.
He noted that an important point from the study was not just about a patient having an SUD being at increased risk for COVID-19 “and a more severe disease trajectory.” Other factors associated with having an SUD, such as increased comorbidities, also likely play a part.
Dr. Saxon agreed that the ongoing opioid epidemic combined with the pandemic led to a “perfect storm” of problems.
“We were making slow but some progress getting more people the medications they need [to treat opioid use disorder], but the pandemic coming along disrupted those efforts. A lot of health care entities had to shut down for a while, seeing patients only remotely,” which led to barriers as many clinicians needed to learn how to proceed using telehealth options, said Dr. Saxon.
Universal screening?
Asked whether physicians should screen all patients for SUDs, Dr. Saxon said it’s a complicated question.
“Screening for tobacco and alcohol has a really good evidence base and practices should be doing that. The stigma is there but it’s a lot less than with illegal substances,” he said.
Screening for illegal substances or misuse of prescription substances may not be a good idea in health care settings “when it’s something they can’t do anything about. If you’re going to screen, you would have to have either referral processes in place or treatment available in your facility,” Dr. Saxon said.
Opioid use disorder is “especially amenable to treatment in a primary care or health care setting with prescribers,” he noted.
However, stimulant or cannabis use disorders “require fairly intensive behavioral interventions that are not easy to deliver in many health care settings. And we don›t have the workforce trained up to provide those treatments as widely as they should be,” said Dr. Saxon.
“Unless there’s some way to treat the issue, what’s the point of screening for it? That just creates frustration for patients and clinicians, as well,” he said. “It’s something we’re moving toward but we’re not quite there yet.”
The report authors and Dr. Saxon have disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
Substance use disorders (SUD), particularly opioid addiction and smoking, are tied to an increased risk for COVID-19 and serious adverse outcomes including hospitalization and death, new research suggests.
A study funded by the National Institutes of Health assessed electronic health records of more than 73 million patients in the United States. Although only 10.3% of the participants had an SUD, “they represented 15.6% of the COVID-19 cases,” the investigators reported.
In addition, those with a recent diagnosis of SUD were eight times more likely to develop COVID-19 versus those without such a diagnosis. For specific SUDs, the greatest risk was for those with an opioid addiction followed by those who were addicted to cigarettes.
coinvestigator Nora Volkow, MD, director of the National Institute on Drug Abuse, said in a press release.
It may also be harder for individuals with addiction to access health care services for a variety of reasons, including low socioeconomic status or stigma, she said in an interview.
Dr. Volkow said she has encountered patients with medical emergencies who refuse to seek treatment at the emergency department because of previous experiences where they have been mistreated and encountered discrimination, and “that’s really very tragic.”
The findings were published online Sept. 14 in Molecular Psychiatry.
Is nicotine protective?
Dr. Volkow, her fellow senior author Rong Xu, PhD, Case Western Reserve University, Cleveland, and their team conducted the study because data released before the pandemic showed a significant increase in opioid overdose in 2019. “We were in an opioid crisis where we again saw an increase in mortality associated with overdose – and then COVID comes along. So the question was how are people who are already struggling faring? And if they were getting infected [with the coronavirus], what happened to them?”
Patients with SUDs have multiple medical comorbidities that are known risk factors for COVID-19, Dr. Volkow noted.
However, the only specific SUD that has been previously studied in this context is tobacco use disorder, she said. A report from Chinese investigators released early in the pandemic showed that smokers were more likely to be infected by coronavirus and more likely to die from COVID-19.
Interestingly, a cross-sectional study published in April suggested that smoking may be protective against COVID, and Dr. Volkow noted that a clinical study currently being conducted in France is assessing whether wearing a nicotine patch has the potential to prevent the virus.
“That’s very different from looking at a chronic smoker,” she pointed out. “It’s a potential that nicotine as a chemical [could be] a preventive measure as opposed to saying smoking will prevent you from getting COVID.”
Patients with SUDs, said Dr. Volkow, “are likely to be at greater risk because of the effects of drugs in the metabolic system and the interfering with oxygenation in the pulmonary vessels.”
The retrospective case-control study included EHR data from 73.1 million patients. In the study population, 54% were women, 55% were White, 10% Black, 2% Asian, 1% Hispanic/Latino, and the others were classified as other or unknown.
EHRs were collected through June 15 at 360 hospitals in all 50 states and were deidentified to ensure privacy. SUDs included alcohol, tobacco, cannabis, opioid, and cocaine.
Racial disparities
Results showed that about 7.5 million participants had a previous SUD diagnosis; of these, 722,370 had been diagnosed within the past year.
Tobacco use disorder was the most common diagnosis (n = 6,414,580), followed by alcohol (1,264,990), cannabis (490,420), opioid (471,520), and cocaine (222,680).
In addition, 12,030 (60% women) were diagnosed with COVID-19 and 1,880 had both COVID-19 and an SUD.
Adjusted analyses revealed that those who had a recent diagnosis of SUD were at a significantly greater increased risk for COVID-19 than individuals without an SUD (adjusted odds ratio, 8.7; 95% confidence interval, 8.4-9.0; P < 10–30).
This increased risk was greatest in participants with opioid use disorder (aOR, 10.2; 95% CI, 9.1-11.5; P < 10–30), followed by those with tobacco use disorder (aOR, 8.2; 95% CI, 7.9 - 8.5; P < 10–30).
Alcohol, cocaine, and cannabis had aORs of 7.7, 6.5, and 5.3, respectively. The aOR for lifetime SUD and COVID-19 was 1.5.
Among all patients with COVID-19, hospitalization rates were significantly greater in those with an SUD (43.8%) versus those without (30.1%), as were death rates at 9.6% versus 6.6%, respectively.
Race was a significant risk factor. Black patients with a recent SUD diagnosis were twice as likely as White patients to develop COVID-19 (aOR, 2.2; P < 10–30), and those specifically with opioid use disorder were four times more likely to develop the disease (aOR, 4.2 P < 10–25).
Black patients with both COVID-19 and lifetime SUD also had greater hospitalization and death rates versus their White peers (50.7% vs. 35.2% and 13% vs. 8.6%, respectively).
“This surprised me,” Dr. Volkow noted. “You can see the emergence of the racial disparities even under these conditions of really negative outcomes.”
Vulnerable populations
Cancer; obesity; HIV; diabetes; cardiovascular disease; and chronic kidney, liver, and lung diseases, which are all risk factors for COVID-19, were more prevalent in the group of patients with a recent SUD diagnosis versus those without.
In addition, asthma, type 2 diabetes, hypertension, obesity, and chronic kidney disease were more prevalent in the Black patents with a recent SUD than in the White patients.
Overall, the findings “identify individuals with SUD as a vulnerable population, especially African Americans with SUDs, who are at significantly increased risk for COVID-19 and its adverse outcomes,” the investigators wrote.
The results also highlight “the need to screen and treat individuals with SUD as part of the strategy to control the pandemic while ensuring no disparities in access to healthcare support,” they added.
Dr. Volkow noted that “marginalization” often occurs for individuals with addiction, making it more difficult for them to access health care services.
“It is incumbent upon clinicians to meet the unique challenges of caring for this vulnerable population, just as they would any other high-risk group,” she said.
“Patients should not just be treated for COVID, but should also be provided with treatment for their substance use disorder,” Dr. Volkow added.
‘Pretty convincing’
Andrew J. Saxon, MD, professor in the department of psychiatry and behavioral sciences at the University of Washington, Seattle, called the findings interesting.
“I found it pretty convincing that people who have substance use disorders are probably at higher risk for getting COVID-19 infection and more complications once they are infected,” he said.
Dr. Saxon, who was not involved with the research, is also director of the Center of Excellence in Substance Addiction Treatment and Education and is a member of the American Psychiatric Association’s Council on Addiction Psychiatry.
He noted that an important point from the study was not just about a patient having an SUD being at increased risk for COVID-19 “and a more severe disease trajectory.” Other factors associated with having an SUD, such as increased comorbidities, also likely play a part.
Dr. Saxon agreed that the ongoing opioid epidemic combined with the pandemic led to a “perfect storm” of problems.
“We were making slow but some progress getting more people the medications they need [to treat opioid use disorder], but the pandemic coming along disrupted those efforts. A lot of health care entities had to shut down for a while, seeing patients only remotely,” which led to barriers as many clinicians needed to learn how to proceed using telehealth options, said Dr. Saxon.
Universal screening?
Asked whether physicians should screen all patients for SUDs, Dr. Saxon said it’s a complicated question.
“Screening for tobacco and alcohol has a really good evidence base and practices should be doing that. The stigma is there but it’s a lot less than with illegal substances,” he said.
Screening for illegal substances or misuse of prescription substances may not be a good idea in health care settings “when it’s something they can’t do anything about. If you’re going to screen, you would have to have either referral processes in place or treatment available in your facility,” Dr. Saxon said.
Opioid use disorder is “especially amenable to treatment in a primary care or health care setting with prescribers,” he noted.
However, stimulant or cannabis use disorders “require fairly intensive behavioral interventions that are not easy to deliver in many health care settings. And we don›t have the workforce trained up to provide those treatments as widely as they should be,” said Dr. Saxon.
“Unless there’s some way to treat the issue, what’s the point of screening for it? That just creates frustration for patients and clinicians, as well,” he said. “It’s something we’re moving toward but we’re not quite there yet.”
The report authors and Dr. Saxon have disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
Substance use disorders (SUD), particularly opioid addiction and smoking, are tied to an increased risk for COVID-19 and serious adverse outcomes including hospitalization and death, new research suggests.
A study funded by the National Institutes of Health assessed electronic health records of more than 73 million patients in the United States. Although only 10.3% of the participants had an SUD, “they represented 15.6% of the COVID-19 cases,” the investigators reported.
In addition, those with a recent diagnosis of SUD were eight times more likely to develop COVID-19 versus those without such a diagnosis. For specific SUDs, the greatest risk was for those with an opioid addiction followed by those who were addicted to cigarettes.
coinvestigator Nora Volkow, MD, director of the National Institute on Drug Abuse, said in a press release.
It may also be harder for individuals with addiction to access health care services for a variety of reasons, including low socioeconomic status or stigma, she said in an interview.
Dr. Volkow said she has encountered patients with medical emergencies who refuse to seek treatment at the emergency department because of previous experiences where they have been mistreated and encountered discrimination, and “that’s really very tragic.”
The findings were published online Sept. 14 in Molecular Psychiatry.
Is nicotine protective?
Dr. Volkow, her fellow senior author Rong Xu, PhD, Case Western Reserve University, Cleveland, and their team conducted the study because data released before the pandemic showed a significant increase in opioid overdose in 2019. “We were in an opioid crisis where we again saw an increase in mortality associated with overdose – and then COVID comes along. So the question was how are people who are already struggling faring? And if they were getting infected [with the coronavirus], what happened to them?”
Patients with SUDs have multiple medical comorbidities that are known risk factors for COVID-19, Dr. Volkow noted.
However, the only specific SUD that has been previously studied in this context is tobacco use disorder, she said. A report from Chinese investigators released early in the pandemic showed that smokers were more likely to be infected by coronavirus and more likely to die from COVID-19.
Interestingly, a cross-sectional study published in April suggested that smoking may be protective against COVID, and Dr. Volkow noted that a clinical study currently being conducted in France is assessing whether wearing a nicotine patch has the potential to prevent the virus.
“That’s very different from looking at a chronic smoker,” she pointed out. “It’s a potential that nicotine as a chemical [could be] a preventive measure as opposed to saying smoking will prevent you from getting COVID.”
Patients with SUDs, said Dr. Volkow, “are likely to be at greater risk because of the effects of drugs in the metabolic system and the interfering with oxygenation in the pulmonary vessels.”
The retrospective case-control study included EHR data from 73.1 million patients. In the study population, 54% were women, 55% were White, 10% Black, 2% Asian, 1% Hispanic/Latino, and the others were classified as other or unknown.
EHRs were collected through June 15 at 360 hospitals in all 50 states and were deidentified to ensure privacy. SUDs included alcohol, tobacco, cannabis, opioid, and cocaine.
Racial disparities
Results showed that about 7.5 million participants had a previous SUD diagnosis; of these, 722,370 had been diagnosed within the past year.
Tobacco use disorder was the most common diagnosis (n = 6,414,580), followed by alcohol (1,264,990), cannabis (490,420), opioid (471,520), and cocaine (222,680).
In addition, 12,030 (60% women) were diagnosed with COVID-19 and 1,880 had both COVID-19 and an SUD.
Adjusted analyses revealed that those who had a recent diagnosis of SUD were at a significantly greater increased risk for COVID-19 than individuals without an SUD (adjusted odds ratio, 8.7; 95% confidence interval, 8.4-9.0; P < 10–30).
This increased risk was greatest in participants with opioid use disorder (aOR, 10.2; 95% CI, 9.1-11.5; P < 10–30), followed by those with tobacco use disorder (aOR, 8.2; 95% CI, 7.9 - 8.5; P < 10–30).
Alcohol, cocaine, and cannabis had aORs of 7.7, 6.5, and 5.3, respectively. The aOR for lifetime SUD and COVID-19 was 1.5.
Among all patients with COVID-19, hospitalization rates were significantly greater in those with an SUD (43.8%) versus those without (30.1%), as were death rates at 9.6% versus 6.6%, respectively.
Race was a significant risk factor. Black patients with a recent SUD diagnosis were twice as likely as White patients to develop COVID-19 (aOR, 2.2; P < 10–30), and those specifically with opioid use disorder were four times more likely to develop the disease (aOR, 4.2 P < 10–25).
Black patients with both COVID-19 and lifetime SUD also had greater hospitalization and death rates versus their White peers (50.7% vs. 35.2% and 13% vs. 8.6%, respectively).
“This surprised me,” Dr. Volkow noted. “You can see the emergence of the racial disparities even under these conditions of really negative outcomes.”
Vulnerable populations
Cancer; obesity; HIV; diabetes; cardiovascular disease; and chronic kidney, liver, and lung diseases, which are all risk factors for COVID-19, were more prevalent in the group of patients with a recent SUD diagnosis versus those without.
In addition, asthma, type 2 diabetes, hypertension, obesity, and chronic kidney disease were more prevalent in the Black patents with a recent SUD than in the White patients.
Overall, the findings “identify individuals with SUD as a vulnerable population, especially African Americans with SUDs, who are at significantly increased risk for COVID-19 and its adverse outcomes,” the investigators wrote.
The results also highlight “the need to screen and treat individuals with SUD as part of the strategy to control the pandemic while ensuring no disparities in access to healthcare support,” they added.
Dr. Volkow noted that “marginalization” often occurs for individuals with addiction, making it more difficult for them to access health care services.
“It is incumbent upon clinicians to meet the unique challenges of caring for this vulnerable population, just as they would any other high-risk group,” she said.
“Patients should not just be treated for COVID, but should also be provided with treatment for their substance use disorder,” Dr. Volkow added.
‘Pretty convincing’
Andrew J. Saxon, MD, professor in the department of psychiatry and behavioral sciences at the University of Washington, Seattle, called the findings interesting.
“I found it pretty convincing that people who have substance use disorders are probably at higher risk for getting COVID-19 infection and more complications once they are infected,” he said.
Dr. Saxon, who was not involved with the research, is also director of the Center of Excellence in Substance Addiction Treatment and Education and is a member of the American Psychiatric Association’s Council on Addiction Psychiatry.
He noted that an important point from the study was not just about a patient having an SUD being at increased risk for COVID-19 “and a more severe disease trajectory.” Other factors associated with having an SUD, such as increased comorbidities, also likely play a part.
Dr. Saxon agreed that the ongoing opioid epidemic combined with the pandemic led to a “perfect storm” of problems.
“We were making slow but some progress getting more people the medications they need [to treat opioid use disorder], but the pandemic coming along disrupted those efforts. A lot of health care entities had to shut down for a while, seeing patients only remotely,” which led to barriers as many clinicians needed to learn how to proceed using telehealth options, said Dr. Saxon.
Universal screening?
Asked whether physicians should screen all patients for SUDs, Dr. Saxon said it’s a complicated question.
“Screening for tobacco and alcohol has a really good evidence base and practices should be doing that. The stigma is there but it’s a lot less than with illegal substances,” he said.
Screening for illegal substances or misuse of prescription substances may not be a good idea in health care settings “when it’s something they can’t do anything about. If you’re going to screen, you would have to have either referral processes in place or treatment available in your facility,” Dr. Saxon said.
Opioid use disorder is “especially amenable to treatment in a primary care or health care setting with prescribers,” he noted.
However, stimulant or cannabis use disorders “require fairly intensive behavioral interventions that are not easy to deliver in many health care settings. And we don›t have the workforce trained up to provide those treatments as widely as they should be,” said Dr. Saxon.
“Unless there’s some way to treat the issue, what’s the point of screening for it? That just creates frustration for patients and clinicians, as well,” he said. “It’s something we’re moving toward but we’re not quite there yet.”
The report authors and Dr. Saxon have disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
Minorities bear brunt of pediatric COVID-19 cases
Black and Hispanic children comprised significantly more cases of COVID-19, compared with White children, based on data from a large, cross-sectional study of 1,000 cases.
“Data regarding disparities in SARS-CoV-2 infection and outcomes have been, thus far, mostly limited to adults,” wrote Monika K. Goyal, MD, of Children’s National Hospital, Washington, and colleagues. “Additional data further suggest that low socioeconomic status may further exacerbate health outcomes for racial and ethnic minorities.”
In a study published in Pediatrics, the researchers conducted a cross-sectional analysis of 1,000 children from a registry of non–acutely ill pediatric patients seen at a drive-through and walk-up COVID-19 test site.
Minority, socioeconomic status affect pediatric outcomes too
The median age of the study population was 8 years, and approximately half were male.
The researchers also examined the association of median family income (MFI) using census block group estimates data from the American Community Survey (2014–2018) to represent socioeconomic status.
Infection rates were significantly higher among children in the lowest three quartiles of MFI (24%, 27%, and 38% for quartiles 3, 2, and 1, respectively), compared with the highest quartile of MFI (9%).
After adjusting for age, sex, and MFI, Hispanic children were six times more likely and non-Hispanic Black children were twice as likely to test positive for COVID-19 than non-Hispanic White children (adjusted odds ratios, 6.3 and 2.3, respectively).
The study findings were limited by several factors including the use of clinician-reported ethnicity and thus potential for misclassification, the researchers noted. In addition, the socioeconomic and racial disparities may be underestimated because these groups have less access to primary care, and the study did not allow for confounding variables including housing conditions or occupancy.
“Although it was beyond the scope of this study to understand the causes for these differential rates of infection, the causes may be multifactorial, including, but not limited to, structural factors, poorer access to health care, limited resources, and bias and discrimination,” the researchers noted. In addition, the high infection rate among minority children may be impacted by parents who are less able to telework, find child care, or avoid public transportation, Dr. Goyal and associates wrote.
Future research should address “the modifiable reasons for these observed disparities as well as their differential impact in terms of SARS-CoV-2–related morbidity and mortality outcomes to mitigate the spread of infection and its health effects,” they concluded.
How to help
“This study is important because we need to understand which groups of children are at highest risk for SARS-CoV-2 infection in order to maximize efforts for screening, allocating resources, and prioritizing vaccine administration,” Karalyn Kinsella, MD, a pediatrician in private practice in Cheshire, Conn., said in an interview.
Dr. Kinsella said she was not surprised at the higher infection rates in general in minorities and low socioeconomic groups. “We already knew that adult COVID-19 rates were higher for people in certain racial/ethnic groups and those with socioeconomic disadvantages; however, I was shocked by the percentages. That is a huge burden for a population that already has disparities in health outcomes.”
“As the authors cite, this was not a research study of why these groups were more likely to be COVID-19 positive, but they speculated that crowded living conditions, multigenerational families living together, and many minorities being essential workers unable to work from home,” said Dr. Kinsella. Additional factors contributing to higher infection rates may include limited access to care, transportation issues, insurance coverage, schedule challenges, and fear of deportation. Some of these problems might be addressed by coming into communities in mobile vans, visiting community health centers and schools with free educational materials, using masks and hand sanitizer, and offering free access to testing.
“Future studies could confirm the cause of this discrepancy, as well as study community-based interventions and their outcomes,” Dr. Kinsella said. In the meantime, a take-home message for clinicians is the need to prioritize screening, resources, and vaccines to reflect the higher rates of SARS-CoV-2 infections in children from disadvantaged racial and socioeconomic backgrounds.
The study received no outside funding. The researchers had no financial conflicts to disclose, but lead author Dr. Goyal is a member of the Pediatrics editorial board. Dr. Kinsella had no financial conflicts to disclose, but serves on the Pediatric News editorial advisory board.
SOURCE: Goyal MK et al. Pediatrics. 2020 Sep 24. doi: 10.1542/peds.2020-009951.
Black and Hispanic children comprised significantly more cases of COVID-19, compared with White children, based on data from a large, cross-sectional study of 1,000 cases.
“Data regarding disparities in SARS-CoV-2 infection and outcomes have been, thus far, mostly limited to adults,” wrote Monika K. Goyal, MD, of Children’s National Hospital, Washington, and colleagues. “Additional data further suggest that low socioeconomic status may further exacerbate health outcomes for racial and ethnic minorities.”
In a study published in Pediatrics, the researchers conducted a cross-sectional analysis of 1,000 children from a registry of non–acutely ill pediatric patients seen at a drive-through and walk-up COVID-19 test site.
Minority, socioeconomic status affect pediatric outcomes too
The median age of the study population was 8 years, and approximately half were male.
The researchers also examined the association of median family income (MFI) using census block group estimates data from the American Community Survey (2014–2018) to represent socioeconomic status.
Infection rates were significantly higher among children in the lowest three quartiles of MFI (24%, 27%, and 38% for quartiles 3, 2, and 1, respectively), compared with the highest quartile of MFI (9%).
After adjusting for age, sex, and MFI, Hispanic children were six times more likely and non-Hispanic Black children were twice as likely to test positive for COVID-19 than non-Hispanic White children (adjusted odds ratios, 6.3 and 2.3, respectively).
The study findings were limited by several factors including the use of clinician-reported ethnicity and thus potential for misclassification, the researchers noted. In addition, the socioeconomic and racial disparities may be underestimated because these groups have less access to primary care, and the study did not allow for confounding variables including housing conditions or occupancy.
“Although it was beyond the scope of this study to understand the causes for these differential rates of infection, the causes may be multifactorial, including, but not limited to, structural factors, poorer access to health care, limited resources, and bias and discrimination,” the researchers noted. In addition, the high infection rate among minority children may be impacted by parents who are less able to telework, find child care, or avoid public transportation, Dr. Goyal and associates wrote.
Future research should address “the modifiable reasons for these observed disparities as well as their differential impact in terms of SARS-CoV-2–related morbidity and mortality outcomes to mitigate the spread of infection and its health effects,” they concluded.
How to help
“This study is important because we need to understand which groups of children are at highest risk for SARS-CoV-2 infection in order to maximize efforts for screening, allocating resources, and prioritizing vaccine administration,” Karalyn Kinsella, MD, a pediatrician in private practice in Cheshire, Conn., said in an interview.
Dr. Kinsella said she was not surprised at the higher infection rates in general in minorities and low socioeconomic groups. “We already knew that adult COVID-19 rates were higher for people in certain racial/ethnic groups and those with socioeconomic disadvantages; however, I was shocked by the percentages. That is a huge burden for a population that already has disparities in health outcomes.”
“As the authors cite, this was not a research study of why these groups were more likely to be COVID-19 positive, but they speculated that crowded living conditions, multigenerational families living together, and many minorities being essential workers unable to work from home,” said Dr. Kinsella. Additional factors contributing to higher infection rates may include limited access to care, transportation issues, insurance coverage, schedule challenges, and fear of deportation. Some of these problems might be addressed by coming into communities in mobile vans, visiting community health centers and schools with free educational materials, using masks and hand sanitizer, and offering free access to testing.
“Future studies could confirm the cause of this discrepancy, as well as study community-based interventions and their outcomes,” Dr. Kinsella said. In the meantime, a take-home message for clinicians is the need to prioritize screening, resources, and vaccines to reflect the higher rates of SARS-CoV-2 infections in children from disadvantaged racial and socioeconomic backgrounds.
The study received no outside funding. The researchers had no financial conflicts to disclose, but lead author Dr. Goyal is a member of the Pediatrics editorial board. Dr. Kinsella had no financial conflicts to disclose, but serves on the Pediatric News editorial advisory board.
SOURCE: Goyal MK et al. Pediatrics. 2020 Sep 24. doi: 10.1542/peds.2020-009951.
Black and Hispanic children comprised significantly more cases of COVID-19, compared with White children, based on data from a large, cross-sectional study of 1,000 cases.
“Data regarding disparities in SARS-CoV-2 infection and outcomes have been, thus far, mostly limited to adults,” wrote Monika K. Goyal, MD, of Children’s National Hospital, Washington, and colleagues. “Additional data further suggest that low socioeconomic status may further exacerbate health outcomes for racial and ethnic minorities.”
In a study published in Pediatrics, the researchers conducted a cross-sectional analysis of 1,000 children from a registry of non–acutely ill pediatric patients seen at a drive-through and walk-up COVID-19 test site.
Minority, socioeconomic status affect pediatric outcomes too
The median age of the study population was 8 years, and approximately half were male.
The researchers also examined the association of median family income (MFI) using census block group estimates data from the American Community Survey (2014–2018) to represent socioeconomic status.
Infection rates were significantly higher among children in the lowest three quartiles of MFI (24%, 27%, and 38% for quartiles 3, 2, and 1, respectively), compared with the highest quartile of MFI (9%).
After adjusting for age, sex, and MFI, Hispanic children were six times more likely and non-Hispanic Black children were twice as likely to test positive for COVID-19 than non-Hispanic White children (adjusted odds ratios, 6.3 and 2.3, respectively).
The study findings were limited by several factors including the use of clinician-reported ethnicity and thus potential for misclassification, the researchers noted. In addition, the socioeconomic and racial disparities may be underestimated because these groups have less access to primary care, and the study did not allow for confounding variables including housing conditions or occupancy.
“Although it was beyond the scope of this study to understand the causes for these differential rates of infection, the causes may be multifactorial, including, but not limited to, structural factors, poorer access to health care, limited resources, and bias and discrimination,” the researchers noted. In addition, the high infection rate among minority children may be impacted by parents who are less able to telework, find child care, or avoid public transportation, Dr. Goyal and associates wrote.
Future research should address “the modifiable reasons for these observed disparities as well as their differential impact in terms of SARS-CoV-2–related morbidity and mortality outcomes to mitigate the spread of infection and its health effects,” they concluded.
How to help
“This study is important because we need to understand which groups of children are at highest risk for SARS-CoV-2 infection in order to maximize efforts for screening, allocating resources, and prioritizing vaccine administration,” Karalyn Kinsella, MD, a pediatrician in private practice in Cheshire, Conn., said in an interview.
Dr. Kinsella said she was not surprised at the higher infection rates in general in minorities and low socioeconomic groups. “We already knew that adult COVID-19 rates were higher for people in certain racial/ethnic groups and those with socioeconomic disadvantages; however, I was shocked by the percentages. That is a huge burden for a population that already has disparities in health outcomes.”
“As the authors cite, this was not a research study of why these groups were more likely to be COVID-19 positive, but they speculated that crowded living conditions, multigenerational families living together, and many minorities being essential workers unable to work from home,” said Dr. Kinsella. Additional factors contributing to higher infection rates may include limited access to care, transportation issues, insurance coverage, schedule challenges, and fear of deportation. Some of these problems might be addressed by coming into communities in mobile vans, visiting community health centers and schools with free educational materials, using masks and hand sanitizer, and offering free access to testing.
“Future studies could confirm the cause of this discrepancy, as well as study community-based interventions and their outcomes,” Dr. Kinsella said. In the meantime, a take-home message for clinicians is the need to prioritize screening, resources, and vaccines to reflect the higher rates of SARS-CoV-2 infections in children from disadvantaged racial and socioeconomic backgrounds.
The study received no outside funding. The researchers had no financial conflicts to disclose, but lead author Dr. Goyal is a member of the Pediatrics editorial board. Dr. Kinsella had no financial conflicts to disclose, but serves on the Pediatric News editorial advisory board.
SOURCE: Goyal MK et al. Pediatrics. 2020 Sep 24. doi: 10.1542/peds.2020-009951.
FROM PEDIATRICS





