User login
ACIP releases new dengue vaccine recommendations
The vaccine is only to be used for children aged 9-16 who live in endemic areas and who have evidence with a specific diagnostic test of prior dengue infection.
Dengue is a mosquito-borne virus found throughout the world, primarily in tropical or subtropical climates. Cases had steadily been increasing to 5.2 million in 2019, and the geographic distribution of cases is broadening with climate change and urbanization. About half of the world’s population is now at risk.
The dengue virus has four serotypes. The first infection may be mild or asymptomatic, but the second one can be life-threatening because of a phenomenon called antibody-dependent enhancement.
The lead author of the new recommendations is Gabriela Paz-Bailey, MD, PhD, division of vector-borne diseases, dengue branch, CDC. She told this news organization that, during the second infection, when there are “low levels of antibodies from that first infection, the antibodies help the virus get inside the cells. There the virus is not killed, and that results in increased viral load, and then that can result in more severe disease and the plasma leakage” syndrome, which can lead to shock, severe bleeding, and organ failure. The death rate for severe dengue is up to 13%.
Previous infection with Zika virus, common in the same areas where dengue is endemic, can also increase the risk for symptomatic and severe dengue for subsequent infections.
In the United States, Puerto Rico is the main focus of control efforts because 95% of domestic dengue cases originate there – almost 30,000 cases between 2010 and 2020, with 11,000 cases and 4,000 hospitalizations occurring in children between the ages of 10 and 19.
Because Aedes aegypti, the primary mosquito vector transmitting dengue, is resistant to all commonly used insecticides in Puerto Rico, preventive efforts have shifted from insecticides to vaccination.
Antibody tests prevaccination
The main concern with the Sanofi’s dengue vaccine is that it could act as an asymptomatic primary dengue infection, in effect priming the body for a severe reaction from antibody-dependent enhancement with a subsequent infection. That is why it’s critical that the vaccine only be given to children with evidence of prior disease.
Dr. Paz-Bailey said: “The CDC came up with recommendations of what the performance of the test used for prevaccination screening should be. And it was 98% specificity and 75% sensitivity. ... But no test by itself was found to have a specificity of 98%, and this is why we’re recommending the two-test algorithm,” in which two different assays are run off the same blood sample, drawn at a prevaccination visit.
If the child has evidence of prior dengue, they can proceed with vaccination to protect against recurrent infection. Dengvaxia is given as a series of three shots over 6 months. Vaccine efficacy is 82% – so not everyone is protected, and additionally, that protection declines over time.
There is concern that it will be difficult to achieve compliance with such a complex regimen. Dr. Paz-Bailey said, “But I think that the trust in vaccines that is highly prevalent for [Puerto] Rico and trusting the health care system, and sort of the importance that is assigned to dengue by providers and by parents because of previous outbreaks and previous experiences is going to help us.” She added, “I think that the COVID experience has been very revealing. And what we have learned is that Puerto Rico has a very strong health care system, a very strong network of vaccine providers. ... Coverage for COVID vaccine is higher than in other parts of the U.S.”
One of the interesting things about dengue is that the first infection can range from asymptomatic to life-threatening. The second infection is generally worse because of this antibody-dependent enhancement phenomenon. Eng Eong Ooi, MD, PhD, professor of microbiology and immunology, National University of Singapore, told this news organization, “After you have two infections, you seem to be protected quite well against the remaining two [serotypes]. The vaccine serves as another episode of infection in those who had prior dengue, so then any natural infections after the vaccination in the seropositive become like the outcome of a third or fourth infection.”
Vaccination alone will not solve dengue. Dr. Ooi said, “There’s not one method that would fully control dengue. You need both vaccines as well as control measures, whether it’s Wolbachia or something else. At the same time, I think we need antiviral drugs, because hitting this virus in just one part of its life cycle wouldn’t make a huge, lasting impact.” Dr. Ooi added that as “the spread of the virus and the population immunity drops, you’re actually now more vulnerable to dengue outbreaks when they do get introduced. So, suppressing transmission alone isn’t the answer. You also have to keep herd immunity levels high. So if we can reduce the virus transmission by controlling either mosquito population or transmission and at the same time vaccinate to keep the immunity levels high, then I think we have a chance of controlling dengue.”
Dr. Paz-Bailey concluded: “I do want to emphasize that we are excited about having these tools, because for years and years, we have had really limited options to prevent and control dengue. It’s an important addition to have the vaccine be approved to be used within the U.S., and it’s going to pave the road for future vaccines.”
Dr. Paz-Bailey and Dr. Ooi reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
The vaccine is only to be used for children aged 9-16 who live in endemic areas and who have evidence with a specific diagnostic test of prior dengue infection.
Dengue is a mosquito-borne virus found throughout the world, primarily in tropical or subtropical climates. Cases had steadily been increasing to 5.2 million in 2019, and the geographic distribution of cases is broadening with climate change and urbanization. About half of the world’s population is now at risk.
The dengue virus has four serotypes. The first infection may be mild or asymptomatic, but the second one can be life-threatening because of a phenomenon called antibody-dependent enhancement.
The lead author of the new recommendations is Gabriela Paz-Bailey, MD, PhD, division of vector-borne diseases, dengue branch, CDC. She told this news organization that, during the second infection, when there are “low levels of antibodies from that first infection, the antibodies help the virus get inside the cells. There the virus is not killed, and that results in increased viral load, and then that can result in more severe disease and the plasma leakage” syndrome, which can lead to shock, severe bleeding, and organ failure. The death rate for severe dengue is up to 13%.
Previous infection with Zika virus, common in the same areas where dengue is endemic, can also increase the risk for symptomatic and severe dengue for subsequent infections.
In the United States, Puerto Rico is the main focus of control efforts because 95% of domestic dengue cases originate there – almost 30,000 cases between 2010 and 2020, with 11,000 cases and 4,000 hospitalizations occurring in children between the ages of 10 and 19.
Because Aedes aegypti, the primary mosquito vector transmitting dengue, is resistant to all commonly used insecticides in Puerto Rico, preventive efforts have shifted from insecticides to vaccination.
Antibody tests prevaccination
The main concern with the Sanofi’s dengue vaccine is that it could act as an asymptomatic primary dengue infection, in effect priming the body for a severe reaction from antibody-dependent enhancement with a subsequent infection. That is why it’s critical that the vaccine only be given to children with evidence of prior disease.
Dr. Paz-Bailey said: “The CDC came up with recommendations of what the performance of the test used for prevaccination screening should be. And it was 98% specificity and 75% sensitivity. ... But no test by itself was found to have a specificity of 98%, and this is why we’re recommending the two-test algorithm,” in which two different assays are run off the same blood sample, drawn at a prevaccination visit.
If the child has evidence of prior dengue, they can proceed with vaccination to protect against recurrent infection. Dengvaxia is given as a series of three shots over 6 months. Vaccine efficacy is 82% – so not everyone is protected, and additionally, that protection declines over time.
There is concern that it will be difficult to achieve compliance with such a complex regimen. Dr. Paz-Bailey said, “But I think that the trust in vaccines that is highly prevalent for [Puerto] Rico and trusting the health care system, and sort of the importance that is assigned to dengue by providers and by parents because of previous outbreaks and previous experiences is going to help us.” She added, “I think that the COVID experience has been very revealing. And what we have learned is that Puerto Rico has a very strong health care system, a very strong network of vaccine providers. ... Coverage for COVID vaccine is higher than in other parts of the U.S.”
One of the interesting things about dengue is that the first infection can range from asymptomatic to life-threatening. The second infection is generally worse because of this antibody-dependent enhancement phenomenon. Eng Eong Ooi, MD, PhD, professor of microbiology and immunology, National University of Singapore, told this news organization, “After you have two infections, you seem to be protected quite well against the remaining two [serotypes]. The vaccine serves as another episode of infection in those who had prior dengue, so then any natural infections after the vaccination in the seropositive become like the outcome of a third or fourth infection.”
Vaccination alone will not solve dengue. Dr. Ooi said, “There’s not one method that would fully control dengue. You need both vaccines as well as control measures, whether it’s Wolbachia or something else. At the same time, I think we need antiviral drugs, because hitting this virus in just one part of its life cycle wouldn’t make a huge, lasting impact.” Dr. Ooi added that as “the spread of the virus and the population immunity drops, you’re actually now more vulnerable to dengue outbreaks when they do get introduced. So, suppressing transmission alone isn’t the answer. You also have to keep herd immunity levels high. So if we can reduce the virus transmission by controlling either mosquito population or transmission and at the same time vaccinate to keep the immunity levels high, then I think we have a chance of controlling dengue.”
Dr. Paz-Bailey concluded: “I do want to emphasize that we are excited about having these tools, because for years and years, we have had really limited options to prevent and control dengue. It’s an important addition to have the vaccine be approved to be used within the U.S., and it’s going to pave the road for future vaccines.”
Dr. Paz-Bailey and Dr. Ooi reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
The vaccine is only to be used for children aged 9-16 who live in endemic areas and who have evidence with a specific diagnostic test of prior dengue infection.
Dengue is a mosquito-borne virus found throughout the world, primarily in tropical or subtropical climates. Cases had steadily been increasing to 5.2 million in 2019, and the geographic distribution of cases is broadening with climate change and urbanization. About half of the world’s population is now at risk.
The dengue virus has four serotypes. The first infection may be mild or asymptomatic, but the second one can be life-threatening because of a phenomenon called antibody-dependent enhancement.
The lead author of the new recommendations is Gabriela Paz-Bailey, MD, PhD, division of vector-borne diseases, dengue branch, CDC. She told this news organization that, during the second infection, when there are “low levels of antibodies from that first infection, the antibodies help the virus get inside the cells. There the virus is not killed, and that results in increased viral load, and then that can result in more severe disease and the plasma leakage” syndrome, which can lead to shock, severe bleeding, and organ failure. The death rate for severe dengue is up to 13%.
Previous infection with Zika virus, common in the same areas where dengue is endemic, can also increase the risk for symptomatic and severe dengue for subsequent infections.
In the United States, Puerto Rico is the main focus of control efforts because 95% of domestic dengue cases originate there – almost 30,000 cases between 2010 and 2020, with 11,000 cases and 4,000 hospitalizations occurring in children between the ages of 10 and 19.
Because Aedes aegypti, the primary mosquito vector transmitting dengue, is resistant to all commonly used insecticides in Puerto Rico, preventive efforts have shifted from insecticides to vaccination.
Antibody tests prevaccination
The main concern with the Sanofi’s dengue vaccine is that it could act as an asymptomatic primary dengue infection, in effect priming the body for a severe reaction from antibody-dependent enhancement with a subsequent infection. That is why it’s critical that the vaccine only be given to children with evidence of prior disease.
Dr. Paz-Bailey said: “The CDC came up with recommendations of what the performance of the test used for prevaccination screening should be. And it was 98% specificity and 75% sensitivity. ... But no test by itself was found to have a specificity of 98%, and this is why we’re recommending the two-test algorithm,” in which two different assays are run off the same blood sample, drawn at a prevaccination visit.
If the child has evidence of prior dengue, they can proceed with vaccination to protect against recurrent infection. Dengvaxia is given as a series of three shots over 6 months. Vaccine efficacy is 82% – so not everyone is protected, and additionally, that protection declines over time.
There is concern that it will be difficult to achieve compliance with such a complex regimen. Dr. Paz-Bailey said, “But I think that the trust in vaccines that is highly prevalent for [Puerto] Rico and trusting the health care system, and sort of the importance that is assigned to dengue by providers and by parents because of previous outbreaks and previous experiences is going to help us.” She added, “I think that the COVID experience has been very revealing. And what we have learned is that Puerto Rico has a very strong health care system, a very strong network of vaccine providers. ... Coverage for COVID vaccine is higher than in other parts of the U.S.”
One of the interesting things about dengue is that the first infection can range from asymptomatic to life-threatening. The second infection is generally worse because of this antibody-dependent enhancement phenomenon. Eng Eong Ooi, MD, PhD, professor of microbiology and immunology, National University of Singapore, told this news organization, “After you have two infections, you seem to be protected quite well against the remaining two [serotypes]. The vaccine serves as another episode of infection in those who had prior dengue, so then any natural infections after the vaccination in the seropositive become like the outcome of a third or fourth infection.”
Vaccination alone will not solve dengue. Dr. Ooi said, “There’s not one method that would fully control dengue. You need both vaccines as well as control measures, whether it’s Wolbachia or something else. At the same time, I think we need antiviral drugs, because hitting this virus in just one part of its life cycle wouldn’t make a huge, lasting impact.” Dr. Ooi added that as “the spread of the virus and the population immunity drops, you’re actually now more vulnerable to dengue outbreaks when they do get introduced. So, suppressing transmission alone isn’t the answer. You also have to keep herd immunity levels high. So if we can reduce the virus transmission by controlling either mosquito population or transmission and at the same time vaccinate to keep the immunity levels high, then I think we have a chance of controlling dengue.”
Dr. Paz-Bailey concluded: “I do want to emphasize that we are excited about having these tools, because for years and years, we have had really limited options to prevent and control dengue. It’s an important addition to have the vaccine be approved to be used within the U.S., and it’s going to pave the road for future vaccines.”
Dr. Paz-Bailey and Dr. Ooi reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM MMWR RECOMMENDATIONS AND REPORTS
Clinician experience, life stressors drive HIV adherence, retention in new patients
A novel twist on the concept of “meeting people where they are” may hold the key to retaining new HIV patients, and even bringing the elusive goal of ending the AIDS epidemic a bit closer. While the concept commonly refers to community outreach and engagement, understanding patient experiences and expectations and personal life stressors in the actual clinic setting may improve overall outcomes, according to new research.
In fact,
“Medical science is not necessarily [at the forefront] of where we want to focus our efforts right now,” Emmanuel Guajardo, MD, lead study author and instructor of infectious diseases at Baylor College of Medicine, Houston, told this news organization.
Rather, “we need to focus on retention in care and adherence to medications. Doubling down on these efforts could really go a long way toward ending the HIV epidemic,” he said.
Study findings were published online Jan. 5, 2022, in AIDS and Behavior.
First time’s a charm
A total of 450 patients attending an HIV clinic in Houston were asked to complete a postvisit survey detailing their experience with the HIV clinician, as well as personal life stressors in the preceding 6 months. Study participants were predominantly non-Hispanic Black (54.2%) or Hispanic (30.7%) and mostly men who have sex with men (MSM), populations that mimic the patients seen at Dr. Guajardo’s clinic. Patients were given the option of survey completion while awaiting discharge, within 2 weeks at the clinic, or (as a last resort) by phone.
Overall scores were based on a composite of validated scales: patient experience scores were defined dichotomously (best experience, most positive experience vs. not the best experience), and life stressor events (death, relationship, economic) were assigned weighted scores based on life change impact (for example, death of a spouse received a score of 100 while moved/changed living location was assigned a score of 25).
“We found that patients who reported better initial experiences with their provider at the first visit were less likely to be lost to follow-up at 6 and 12 months,” explained Dr. Guajardo. “Having fewer life stressors at the first visit [was] also [protective].”
At 6 months, mean overall patient experience scores were 8.60 for those LTFU versus and 8.98 for those not LTFU (P = .011); corresponding mean scores at 12 months were 8.43 and 8.98 respectively (P = .001).
For the dichotomized scoring, patients reporting the best experience with the health care professional were significantly less likely to be LTFU at 6 months (adjusted odd ratio, 0.866; P = .038) and 12 months (aOR, 1.263; P = .029) versus those not reporting the best experience.
Mean life change scores appeared to portend patient drop-off; patients reporting more stressful life events were likelier to be LTFU at 6 months (mean life change score, 129 vs. 100 for those retained in care) and at 12 months (126 vs. 101).
Corresponding multivariate logistic regression models controlling for age, baseline CD4 cell count less than 200, and diagnosis of at least 3 months showed that patients with higher life stressor burdens were significantly more likely to be LTFU at both 6 months (aOR, 1.232, P = .037) and 12 months (aOR, 1.263, P = .029).
Approach matters
“The [study] really hits the nail on the head in terms of identifying a couple of these very salient issues that affect people’s care, especially concerning HIV,” Philip A. Chan, MD, infectious disease specialist and associate professor of medicine at Brown University, Providence, R.I, told this news organization.
“It highlights things that we see on the ground that can interfere with HIV care or [pre-exposure prophylaxis] care, just health care in general, certainly one’s relationship with the physician or provider, and also, you know, real-life stressors,” said Dr. Chan, who was not involved with the study.
Relationship building is especially important for historically underserved populations, a point that’s hardly lost on either Dr. Chan or Dr. Guajardo, who both pointed to higher levels of mistrust among certain patient populations because of their mistreatment by the health care system. The answer? Let the patient lead the initial discussion, allow them to feel comfortable and participate in their care in ways that are most beneficial to them.
“There’s so much miscommunication, misunderstanding, and stigma related to HIV out in the community. So, it’s important to really open the floor for whatever they want to talk about first, before I push any agenda on a new patient.” Dr. Guajardo said. Thereafter, he relies on open-ended questions such as ‘tell me about your sexual partners?’ or ‘what sort of sexual practices do you engage in?’
“At the end of the day, you just need someone dedicated, who can be respectful and listening and caring, and dedicate time to patients to help keep them in care, to listen, and to navigate our incredibly, incredibly complex health care system,” Dr. Chan added.
This study was partly supported by use of the facilities and resources of the Houston Veterans Affairs Center for Innovations in Quality, Effectiveness, and Safety and Harris Health System. Support for the study was also provided by the National Institute of Mental Health and the University of Texas MD Anderson Foundation Chair at Baylor College of Medicine. Dr. Guajardo and Dr. Chan disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
A novel twist on the concept of “meeting people where they are” may hold the key to retaining new HIV patients, and even bringing the elusive goal of ending the AIDS epidemic a bit closer. While the concept commonly refers to community outreach and engagement, understanding patient experiences and expectations and personal life stressors in the actual clinic setting may improve overall outcomes, according to new research.
In fact,
“Medical science is not necessarily [at the forefront] of where we want to focus our efforts right now,” Emmanuel Guajardo, MD, lead study author and instructor of infectious diseases at Baylor College of Medicine, Houston, told this news organization.
Rather, “we need to focus on retention in care and adherence to medications. Doubling down on these efforts could really go a long way toward ending the HIV epidemic,” he said.
Study findings were published online Jan. 5, 2022, in AIDS and Behavior.
First time’s a charm
A total of 450 patients attending an HIV clinic in Houston were asked to complete a postvisit survey detailing their experience with the HIV clinician, as well as personal life stressors in the preceding 6 months. Study participants were predominantly non-Hispanic Black (54.2%) or Hispanic (30.7%) and mostly men who have sex with men (MSM), populations that mimic the patients seen at Dr. Guajardo’s clinic. Patients were given the option of survey completion while awaiting discharge, within 2 weeks at the clinic, or (as a last resort) by phone.
Overall scores were based on a composite of validated scales: patient experience scores were defined dichotomously (best experience, most positive experience vs. not the best experience), and life stressor events (death, relationship, economic) were assigned weighted scores based on life change impact (for example, death of a spouse received a score of 100 while moved/changed living location was assigned a score of 25).
“We found that patients who reported better initial experiences with their provider at the first visit were less likely to be lost to follow-up at 6 and 12 months,” explained Dr. Guajardo. “Having fewer life stressors at the first visit [was] also [protective].”
At 6 months, mean overall patient experience scores were 8.60 for those LTFU versus and 8.98 for those not LTFU (P = .011); corresponding mean scores at 12 months were 8.43 and 8.98 respectively (P = .001).
For the dichotomized scoring, patients reporting the best experience with the health care professional were significantly less likely to be LTFU at 6 months (adjusted odd ratio, 0.866; P = .038) and 12 months (aOR, 1.263; P = .029) versus those not reporting the best experience.
Mean life change scores appeared to portend patient drop-off; patients reporting more stressful life events were likelier to be LTFU at 6 months (mean life change score, 129 vs. 100 for those retained in care) and at 12 months (126 vs. 101).
Corresponding multivariate logistic regression models controlling for age, baseline CD4 cell count less than 200, and diagnosis of at least 3 months showed that patients with higher life stressor burdens were significantly more likely to be LTFU at both 6 months (aOR, 1.232, P = .037) and 12 months (aOR, 1.263, P = .029).
Approach matters
“The [study] really hits the nail on the head in terms of identifying a couple of these very salient issues that affect people’s care, especially concerning HIV,” Philip A. Chan, MD, infectious disease specialist and associate professor of medicine at Brown University, Providence, R.I, told this news organization.
“It highlights things that we see on the ground that can interfere with HIV care or [pre-exposure prophylaxis] care, just health care in general, certainly one’s relationship with the physician or provider, and also, you know, real-life stressors,” said Dr. Chan, who was not involved with the study.
Relationship building is especially important for historically underserved populations, a point that’s hardly lost on either Dr. Chan or Dr. Guajardo, who both pointed to higher levels of mistrust among certain patient populations because of their mistreatment by the health care system. The answer? Let the patient lead the initial discussion, allow them to feel comfortable and participate in their care in ways that are most beneficial to them.
“There’s so much miscommunication, misunderstanding, and stigma related to HIV out in the community. So, it’s important to really open the floor for whatever they want to talk about first, before I push any agenda on a new patient.” Dr. Guajardo said. Thereafter, he relies on open-ended questions such as ‘tell me about your sexual partners?’ or ‘what sort of sexual practices do you engage in?’
“At the end of the day, you just need someone dedicated, who can be respectful and listening and caring, and dedicate time to patients to help keep them in care, to listen, and to navigate our incredibly, incredibly complex health care system,” Dr. Chan added.
This study was partly supported by use of the facilities and resources of the Houston Veterans Affairs Center for Innovations in Quality, Effectiveness, and Safety and Harris Health System. Support for the study was also provided by the National Institute of Mental Health and the University of Texas MD Anderson Foundation Chair at Baylor College of Medicine. Dr. Guajardo and Dr. Chan disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
A novel twist on the concept of “meeting people where they are” may hold the key to retaining new HIV patients, and even bringing the elusive goal of ending the AIDS epidemic a bit closer. While the concept commonly refers to community outreach and engagement, understanding patient experiences and expectations and personal life stressors in the actual clinic setting may improve overall outcomes, according to new research.
In fact,
“Medical science is not necessarily [at the forefront] of where we want to focus our efforts right now,” Emmanuel Guajardo, MD, lead study author and instructor of infectious diseases at Baylor College of Medicine, Houston, told this news organization.
Rather, “we need to focus on retention in care and adherence to medications. Doubling down on these efforts could really go a long way toward ending the HIV epidemic,” he said.
Study findings were published online Jan. 5, 2022, in AIDS and Behavior.
First time’s a charm
A total of 450 patients attending an HIV clinic in Houston were asked to complete a postvisit survey detailing their experience with the HIV clinician, as well as personal life stressors in the preceding 6 months. Study participants were predominantly non-Hispanic Black (54.2%) or Hispanic (30.7%) and mostly men who have sex with men (MSM), populations that mimic the patients seen at Dr. Guajardo’s clinic. Patients were given the option of survey completion while awaiting discharge, within 2 weeks at the clinic, or (as a last resort) by phone.
Overall scores were based on a composite of validated scales: patient experience scores were defined dichotomously (best experience, most positive experience vs. not the best experience), and life stressor events (death, relationship, economic) were assigned weighted scores based on life change impact (for example, death of a spouse received a score of 100 while moved/changed living location was assigned a score of 25).
“We found that patients who reported better initial experiences with their provider at the first visit were less likely to be lost to follow-up at 6 and 12 months,” explained Dr. Guajardo. “Having fewer life stressors at the first visit [was] also [protective].”
At 6 months, mean overall patient experience scores were 8.60 for those LTFU versus and 8.98 for those not LTFU (P = .011); corresponding mean scores at 12 months were 8.43 and 8.98 respectively (P = .001).
For the dichotomized scoring, patients reporting the best experience with the health care professional were significantly less likely to be LTFU at 6 months (adjusted odd ratio, 0.866; P = .038) and 12 months (aOR, 1.263; P = .029) versus those not reporting the best experience.
Mean life change scores appeared to portend patient drop-off; patients reporting more stressful life events were likelier to be LTFU at 6 months (mean life change score, 129 vs. 100 for those retained in care) and at 12 months (126 vs. 101).
Corresponding multivariate logistic regression models controlling for age, baseline CD4 cell count less than 200, and diagnosis of at least 3 months showed that patients with higher life stressor burdens were significantly more likely to be LTFU at both 6 months (aOR, 1.232, P = .037) and 12 months (aOR, 1.263, P = .029).
Approach matters
“The [study] really hits the nail on the head in terms of identifying a couple of these very salient issues that affect people’s care, especially concerning HIV,” Philip A. Chan, MD, infectious disease specialist and associate professor of medicine at Brown University, Providence, R.I, told this news organization.
“It highlights things that we see on the ground that can interfere with HIV care or [pre-exposure prophylaxis] care, just health care in general, certainly one’s relationship with the physician or provider, and also, you know, real-life stressors,” said Dr. Chan, who was not involved with the study.
Relationship building is especially important for historically underserved populations, a point that’s hardly lost on either Dr. Chan or Dr. Guajardo, who both pointed to higher levels of mistrust among certain patient populations because of their mistreatment by the health care system. The answer? Let the patient lead the initial discussion, allow them to feel comfortable and participate in their care in ways that are most beneficial to them.
“There’s so much miscommunication, misunderstanding, and stigma related to HIV out in the community. So, it’s important to really open the floor for whatever they want to talk about first, before I push any agenda on a new patient.” Dr. Guajardo said. Thereafter, he relies on open-ended questions such as ‘tell me about your sexual partners?’ or ‘what sort of sexual practices do you engage in?’
“At the end of the day, you just need someone dedicated, who can be respectful and listening and caring, and dedicate time to patients to help keep them in care, to listen, and to navigate our incredibly, incredibly complex health care system,” Dr. Chan added.
This study was partly supported by use of the facilities and resources of the Houston Veterans Affairs Center for Innovations in Quality, Effectiveness, and Safety and Harris Health System. Support for the study was also provided by the National Institute of Mental Health and the University of Texas MD Anderson Foundation Chair at Baylor College of Medicine. Dr. Guajardo and Dr. Chan disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM AIDS AND BEHAVIOR
Common cold could protect against COVID-19, study says
, according to a small study published Jan. 10 in Nature Communications.
Previous studies have shown that T cells created from other coronaviruses can recognize SARS-CoV-2, the virus that causes COVID-19. In the new study, researchers at Imperial College London found that the presence of these T cells at the time of COVID-19 exposure could reduce the chance of getting infected.
The findings could provide a blueprint for a second-generation, universal vaccine to prevent infection from COVID-19 variants, including Omicron and ones that crop up later.
“Being exposed to SARS-CoV-2 virus doesn’t always result in infection, and we’ve been keen to understand why,” Rhia Kundu, PhD, the lead study author from Imperial’s National Heart and Lung Institute, said in a statement.
People with higher levels of T cells from the common cold were less likely to become infected with COVID-19, the researchers found.
“While this is an important discovery, it is only one form of protection, and I would stress that no one should rely on this alone,” Dr. Kundu said. “Instead, the best way to protect yourself against COVID-19 is to be fully vaccinated, including getting your booster dose.”
For the study, Dr. Kundu and colleagues analyzed blood samples from 52 people who lived with someone with confirmed COVID-19 in September 2020. Among the 26 people who didn’t contract COVID-19, there were “significantly higher levels” of preexisting T cells from common cold coronaviruses, as compared with the 26 people who did become infected.
The T cells researched in the study are considered “cross-reactive” and can recognize the proteins of SARS-CoV-2. They offer protection by targeting proteins inside the SARS-CoV-2 virus, rather than the spike proteins on the surface that allow the virus to invade cells.
The current COVID-19 vaccines target the spike proteins, which are more likely to mutate than internal proteins, the researchers wrote. The Omicron variant, for instance, has numerous mutations on spike proteins that may allow it to evade vaccines.
The data suggest that the next step of COVID-19 vaccine development could focus on internal proteins, the researchers said, which could provide lasting protection because T-cell responses persist longer than antibody responses that fade within a few months of vaccination.
“New vaccines that include these conserved, internal proteins would therefore induce broadly protective T-cell responses that should protect against current and future SARS-CoV-2 variants,” Ajit Lalvani, MD, the senior study author and director of Imperial’s respiratory infections health protection research unit, said in the statement.
But more research is needed, the authors said, noting that the study had a small sample size and lacked ethnic diversity, which puts limits on the research.
A version of this article first appeared on WebMD.com
, according to a small study published Jan. 10 in Nature Communications.
Previous studies have shown that T cells created from other coronaviruses can recognize SARS-CoV-2, the virus that causes COVID-19. In the new study, researchers at Imperial College London found that the presence of these T cells at the time of COVID-19 exposure could reduce the chance of getting infected.
The findings could provide a blueprint for a second-generation, universal vaccine to prevent infection from COVID-19 variants, including Omicron and ones that crop up later.
“Being exposed to SARS-CoV-2 virus doesn’t always result in infection, and we’ve been keen to understand why,” Rhia Kundu, PhD, the lead study author from Imperial’s National Heart and Lung Institute, said in a statement.
People with higher levels of T cells from the common cold were less likely to become infected with COVID-19, the researchers found.
“While this is an important discovery, it is only one form of protection, and I would stress that no one should rely on this alone,” Dr. Kundu said. “Instead, the best way to protect yourself against COVID-19 is to be fully vaccinated, including getting your booster dose.”
For the study, Dr. Kundu and colleagues analyzed blood samples from 52 people who lived with someone with confirmed COVID-19 in September 2020. Among the 26 people who didn’t contract COVID-19, there were “significantly higher levels” of preexisting T cells from common cold coronaviruses, as compared with the 26 people who did become infected.
The T cells researched in the study are considered “cross-reactive” and can recognize the proteins of SARS-CoV-2. They offer protection by targeting proteins inside the SARS-CoV-2 virus, rather than the spike proteins on the surface that allow the virus to invade cells.
The current COVID-19 vaccines target the spike proteins, which are more likely to mutate than internal proteins, the researchers wrote. The Omicron variant, for instance, has numerous mutations on spike proteins that may allow it to evade vaccines.
The data suggest that the next step of COVID-19 vaccine development could focus on internal proteins, the researchers said, which could provide lasting protection because T-cell responses persist longer than antibody responses that fade within a few months of vaccination.
“New vaccines that include these conserved, internal proteins would therefore induce broadly protective T-cell responses that should protect against current and future SARS-CoV-2 variants,” Ajit Lalvani, MD, the senior study author and director of Imperial’s respiratory infections health protection research unit, said in the statement.
But more research is needed, the authors said, noting that the study had a small sample size and lacked ethnic diversity, which puts limits on the research.
A version of this article first appeared on WebMD.com
, according to a small study published Jan. 10 in Nature Communications.
Previous studies have shown that T cells created from other coronaviruses can recognize SARS-CoV-2, the virus that causes COVID-19. In the new study, researchers at Imperial College London found that the presence of these T cells at the time of COVID-19 exposure could reduce the chance of getting infected.
The findings could provide a blueprint for a second-generation, universal vaccine to prevent infection from COVID-19 variants, including Omicron and ones that crop up later.
“Being exposed to SARS-CoV-2 virus doesn’t always result in infection, and we’ve been keen to understand why,” Rhia Kundu, PhD, the lead study author from Imperial’s National Heart and Lung Institute, said in a statement.
People with higher levels of T cells from the common cold were less likely to become infected with COVID-19, the researchers found.
“While this is an important discovery, it is only one form of protection, and I would stress that no one should rely on this alone,” Dr. Kundu said. “Instead, the best way to protect yourself against COVID-19 is to be fully vaccinated, including getting your booster dose.”
For the study, Dr. Kundu and colleagues analyzed blood samples from 52 people who lived with someone with confirmed COVID-19 in September 2020. Among the 26 people who didn’t contract COVID-19, there were “significantly higher levels” of preexisting T cells from common cold coronaviruses, as compared with the 26 people who did become infected.
The T cells researched in the study are considered “cross-reactive” and can recognize the proteins of SARS-CoV-2. They offer protection by targeting proteins inside the SARS-CoV-2 virus, rather than the spike proteins on the surface that allow the virus to invade cells.
The current COVID-19 vaccines target the spike proteins, which are more likely to mutate than internal proteins, the researchers wrote. The Omicron variant, for instance, has numerous mutations on spike proteins that may allow it to evade vaccines.
The data suggest that the next step of COVID-19 vaccine development could focus on internal proteins, the researchers said, which could provide lasting protection because T-cell responses persist longer than antibody responses that fade within a few months of vaccination.
“New vaccines that include these conserved, internal proteins would therefore induce broadly protective T-cell responses that should protect against current and future SARS-CoV-2 variants,” Ajit Lalvani, MD, the senior study author and director of Imperial’s respiratory infections health protection research unit, said in the statement.
But more research is needed, the authors said, noting that the study had a small sample size and lacked ethnic diversity, which puts limits on the research.
A version of this article first appeared on WebMD.com
Ranking seven COVID-19 antigen tests by ease of use: Report
Some COVID-19 rapid antigen home test kits are much easier to use than others, according to an analysis by ECRI, an independent, nonprofit patient safety organization.
None of the tests were rated as “excellent” in terms of usability and some had “noteworthy” usability concerns, the company said.
If a test is hard to use, “chances are that you may miss a step or not follow the right order, or contaminate the testing area and that can definitely influence the accuracy of the test and lead to a wrong test result,” Marcus Schabacker, MD, PhD, president and CEO of ECRI, told this news organization.
To gauge usability, ECRI used the “industry-standard” system usability scale (SUS), which rates products on a scale of 0 to 100 with 100 being the easiest to use.
More than 30 points separated the top and bottom tests analyzed. The top performer was On/Go, followed by CareStart and Flowflex.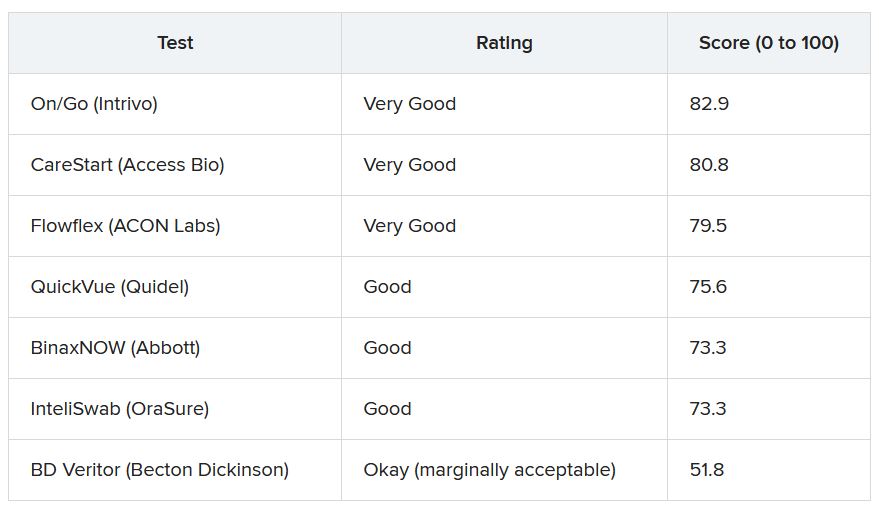
ECRI analysts found that some tests require particularly fine motor skills or have instructions with extremely small font size that may make it hard for older adults or people with complex health conditions to use the tests correctly.
“If you have a tremor from Parkinson’s, for example, or anything which won’t allow you to handle small items, you will have difficulties to do that test by yourself. That is the No. 1 concern we have,” Dr. Schabacker said.
“The second concern is readability, as all of these tests have relatively small instructions. One of them actually has doesn’t even have instructions – you have to download an app,” he noted.
Given demand and supply issues, Dr. Schabacker acknowledged that consumers might not have a choice in which test to use and may have to rely on whatever is available.
These tests are a “hot commodity right now,” he said. “If you have a choice, people should use the ones which are easiest to use, which is the On/Go, the CareStart, or the Flowflex.”
A version of this article first appeared on Medscape.com.
Some COVID-19 rapid antigen home test kits are much easier to use than others, according to an analysis by ECRI, an independent, nonprofit patient safety organization.
None of the tests were rated as “excellent” in terms of usability and some had “noteworthy” usability concerns, the company said.
If a test is hard to use, “chances are that you may miss a step or not follow the right order, or contaminate the testing area and that can definitely influence the accuracy of the test and lead to a wrong test result,” Marcus Schabacker, MD, PhD, president and CEO of ECRI, told this news organization.
To gauge usability, ECRI used the “industry-standard” system usability scale (SUS), which rates products on a scale of 0 to 100 with 100 being the easiest to use.
More than 30 points separated the top and bottom tests analyzed. The top performer was On/Go, followed by CareStart and Flowflex.
ECRI analysts found that some tests require particularly fine motor skills or have instructions with extremely small font size that may make it hard for older adults or people with complex health conditions to use the tests correctly.
“If you have a tremor from Parkinson’s, for example, or anything which won’t allow you to handle small items, you will have difficulties to do that test by yourself. That is the No. 1 concern we have,” Dr. Schabacker said.
“The second concern is readability, as all of these tests have relatively small instructions. One of them actually has doesn’t even have instructions – you have to download an app,” he noted.
Given demand and supply issues, Dr. Schabacker acknowledged that consumers might not have a choice in which test to use and may have to rely on whatever is available.
These tests are a “hot commodity right now,” he said. “If you have a choice, people should use the ones which are easiest to use, which is the On/Go, the CareStart, or the Flowflex.”
A version of this article first appeared on Medscape.com.
Some COVID-19 rapid antigen home test kits are much easier to use than others, according to an analysis by ECRI, an independent, nonprofit patient safety organization.
None of the tests were rated as “excellent” in terms of usability and some had “noteworthy” usability concerns, the company said.
If a test is hard to use, “chances are that you may miss a step or not follow the right order, or contaminate the testing area and that can definitely influence the accuracy of the test and lead to a wrong test result,” Marcus Schabacker, MD, PhD, president and CEO of ECRI, told this news organization.
To gauge usability, ECRI used the “industry-standard” system usability scale (SUS), which rates products on a scale of 0 to 100 with 100 being the easiest to use.
More than 30 points separated the top and bottom tests analyzed. The top performer was On/Go, followed by CareStart and Flowflex.
ECRI analysts found that some tests require particularly fine motor skills or have instructions with extremely small font size that may make it hard for older adults or people with complex health conditions to use the tests correctly.
“If you have a tremor from Parkinson’s, for example, or anything which won’t allow you to handle small items, you will have difficulties to do that test by yourself. That is the No. 1 concern we have,” Dr. Schabacker said.
“The second concern is readability, as all of these tests have relatively small instructions. One of them actually has doesn’t even have instructions – you have to download an app,” he noted.
Given demand and supply issues, Dr. Schabacker acknowledged that consumers might not have a choice in which test to use and may have to rely on whatever is available.
These tests are a “hot commodity right now,” he said. “If you have a choice, people should use the ones which are easiest to use, which is the On/Go, the CareStart, or the Flowflex.”
A version of this article first appeared on Medscape.com.
The etiology of acute otitis media in young children in recent years
Since the COVID-19 pandemic began, pediatricians have been seeing fewer cases of all respiratory illnesses, including acute otitis media (AOM). However, as I prepare this column, an uptick has commenced and likely will continue in an upward trajectory as we emerge from the pandemic into an endemic coronavirus era. Our group in Rochester, N.Y., has continued prospective studies of AOM throughout the pandemic. We found that nasopharyngeal colonization by Streptococcus pneumoniae (pneumococcus), Haemophilus influenzae, and Moraxella catarrhalis remained prevalent in our study cohort of children aged 6-36 months. However, with all the precautions of masking, social distancing, hand washing, and quick exclusion from day care when illness occurred, the frequency of detecting these common otopathogens decreased, as one might expect.1
Leading up to the pandemic, we had an abundance of data to characterize AOM etiology and found that the cause of AOM continues to change following the introduction of the 13-valent pneumococcal conjugate vaccine (PCV13, Prevnar 13). Our most recent report on otopathogen distribution and antibiotic susceptibility covered the years 2015-2019.2 A total of 589 children were enrolled prospectively and we collected 495 middle ear fluid samples (MEF) from 319 AOM cases using tympanocentesis. The frequency of isolates was H. influenzae (34%), pneumococcus (24%), and M. catarrhalis (15%). Beta-lactamase–positive H. influenzae strains were identified among 49% of the isolates, rendering them resistant to amoxicillin. PCV13 serotypes were infrequently isolated. However, we did isolate vaccine types (VTs) in some children from MEF, notably serotypes 19F, 19A, and 3. Non-PCV13 pneumococcus serotypes 35B, 23B, and 15B/C emerged as the most common serotypes. Amoxicillin resistance was identified among 25% of pneumococcal strains. Out of 16 antibiotics tested, 9 (56%) showed a significant increase in nonsusceptibility among pneumococcal isolates. 100% of M. catarrhalis isolates were beta-lactamase producers and therefore resistant to amoxicillin.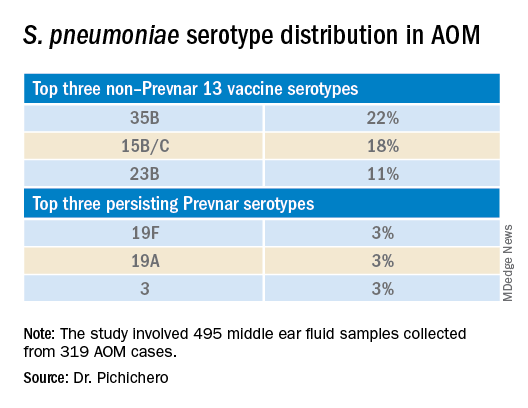
PCV13 has resulted in a decline in both invasive and noninvasive pneumococcal infections caused by strains expressing the 13 capsular serotypes included in the vaccine. However, the emergence of replacement serotypes occurred after introduction of PCV73,4 and continues to occur during the PCV13 era, as shown from the results presented here. Non-PCV13 serotypes accounted for more than 90% of MEF isolates during 2015-2019, with 35B, 21 and 23B being the most commonly isolated. Other emergent serotypes of potential importance were nonvaccine serotypes 15A, 15B, 15C, 23A and 11A. This is highly relevant because forthcoming higher-valency PCVs – PCV15 (manufactured by Merck) and PCV20 (manufactured by Pfizer) will not include many of the dominant capsular serotypes of pneumococcus strains causing AOM. Consequently, the impact of higher-valency PCVs on AOM will not be as great as was observed with the introduction of PCV7 or PCV13.
Of special interest, 22% of pneumococcus isolates from MEF were serotype 35B, making it the most prevalent. Recently we reported a significant rise in antibiotic nonsusceptibility in Spn isolates, contributed mainly by serotype 35B5 and we have been studying how 35B strains transitioned from commensal to otopathogen in children.6 Because serotype 35B strains are increasingly prevalent and often antibiotic resistant, absence of this serotype from PCV15 and PCV20 is cause for concern.
The frequency of isolation of H. influenzae and M. catarrhalis has remained stable across the PCV13 era as the No. 1 and No. 3 pathogens. Similarly, the production of beta-lactamase among strains causing AOM has remained stable at close to 50% and 100%, respectively. Use of amoxicillin, either high dose or standard dose, would not be expected to kill these bacteria.
Our study design has limitations. The population is derived from a predominantly middle-class, suburban population of children in upstate New York and may not be representative of other types of populations in the United States. The children are 6-36 months old, the age when most AOM occurs. MEF samples that were culture negative for bacteria were not further tested by polymerase chain reaction methods.
Dr. Pichichero is a specialist in pediatric infectious diseases, Center for Infectious Diseases and Immunology, and director of the Research Institute, at Rochester (N.Y.) General Hospital. He has no conflicts of interest to declare.
References
1. Kaur R et al. Front Pediatr. 2021;9:722483.
2. Kaur R et al. Euro J Clin Microbiol Infect Dis. 2021;41:37-44
3. Pelton SI et al. Pediatr Infect Disease J. 2004;23:1015-22.
4. Farrell DJ et al. Pediatr Infect Disease J. 2007;26:123-8..
5. Kaur R et al. Clin Infect Dis 2021;72(5):797-805.
6. Fuji N et al. Front Cell Infect Microbiol. 2021;11:744742.
Since the COVID-19 pandemic began, pediatricians have been seeing fewer cases of all respiratory illnesses, including acute otitis media (AOM). However, as I prepare this column, an uptick has commenced and likely will continue in an upward trajectory as we emerge from the pandemic into an endemic coronavirus era. Our group in Rochester, N.Y., has continued prospective studies of AOM throughout the pandemic. We found that nasopharyngeal colonization by Streptococcus pneumoniae (pneumococcus), Haemophilus influenzae, and Moraxella catarrhalis remained prevalent in our study cohort of children aged 6-36 months. However, with all the precautions of masking, social distancing, hand washing, and quick exclusion from day care when illness occurred, the frequency of detecting these common otopathogens decreased, as one might expect.1
Leading up to the pandemic, we had an abundance of data to characterize AOM etiology and found that the cause of AOM continues to change following the introduction of the 13-valent pneumococcal conjugate vaccine (PCV13, Prevnar 13). Our most recent report on otopathogen distribution and antibiotic susceptibility covered the years 2015-2019.2 A total of 589 children were enrolled prospectively and we collected 495 middle ear fluid samples (MEF) from 319 AOM cases using tympanocentesis. The frequency of isolates was H. influenzae (34%), pneumococcus (24%), and M. catarrhalis (15%). Beta-lactamase–positive H. influenzae strains were identified among 49% of the isolates, rendering them resistant to amoxicillin. PCV13 serotypes were infrequently isolated. However, we did isolate vaccine types (VTs) in some children from MEF, notably serotypes 19F, 19A, and 3. Non-PCV13 pneumococcus serotypes 35B, 23B, and 15B/C emerged as the most common serotypes. Amoxicillin resistance was identified among 25% of pneumococcal strains. Out of 16 antibiotics tested, 9 (56%) showed a significant increase in nonsusceptibility among pneumococcal isolates. 100% of M. catarrhalis isolates were beta-lactamase producers and therefore resistant to amoxicillin.
PCV13 has resulted in a decline in both invasive and noninvasive pneumococcal infections caused by strains expressing the 13 capsular serotypes included in the vaccine. However, the emergence of replacement serotypes occurred after introduction of PCV73,4 and continues to occur during the PCV13 era, as shown from the results presented here. Non-PCV13 serotypes accounted for more than 90% of MEF isolates during 2015-2019, with 35B, 21 and 23B being the most commonly isolated. Other emergent serotypes of potential importance were nonvaccine serotypes 15A, 15B, 15C, 23A and 11A. This is highly relevant because forthcoming higher-valency PCVs – PCV15 (manufactured by Merck) and PCV20 (manufactured by Pfizer) will not include many of the dominant capsular serotypes of pneumococcus strains causing AOM. Consequently, the impact of higher-valency PCVs on AOM will not be as great as was observed with the introduction of PCV7 or PCV13.
Of special interest, 22% of pneumococcus isolates from MEF were serotype 35B, making it the most prevalent. Recently we reported a significant rise in antibiotic nonsusceptibility in Spn isolates, contributed mainly by serotype 35B5 and we have been studying how 35B strains transitioned from commensal to otopathogen in children.6 Because serotype 35B strains are increasingly prevalent and often antibiotic resistant, absence of this serotype from PCV15 and PCV20 is cause for concern.
The frequency of isolation of H. influenzae and M. catarrhalis has remained stable across the PCV13 era as the No. 1 and No. 3 pathogens. Similarly, the production of beta-lactamase among strains causing AOM has remained stable at close to 50% and 100%, respectively. Use of amoxicillin, either high dose or standard dose, would not be expected to kill these bacteria.
Our study design has limitations. The population is derived from a predominantly middle-class, suburban population of children in upstate New York and may not be representative of other types of populations in the United States. The children are 6-36 months old, the age when most AOM occurs. MEF samples that were culture negative for bacteria were not further tested by polymerase chain reaction methods.
Dr. Pichichero is a specialist in pediatric infectious diseases, Center for Infectious Diseases and Immunology, and director of the Research Institute, at Rochester (N.Y.) General Hospital. He has no conflicts of interest to declare.
References
1. Kaur R et al. Front Pediatr. 2021;9:722483.
2. Kaur R et al. Euro J Clin Microbiol Infect Dis. 2021;41:37-44
3. Pelton SI et al. Pediatr Infect Disease J. 2004;23:1015-22.
4. Farrell DJ et al. Pediatr Infect Disease J. 2007;26:123-8..
5. Kaur R et al. Clin Infect Dis 2021;72(5):797-805.
6. Fuji N et al. Front Cell Infect Microbiol. 2021;11:744742.
Since the COVID-19 pandemic began, pediatricians have been seeing fewer cases of all respiratory illnesses, including acute otitis media (AOM). However, as I prepare this column, an uptick has commenced and likely will continue in an upward trajectory as we emerge from the pandemic into an endemic coronavirus era. Our group in Rochester, N.Y., has continued prospective studies of AOM throughout the pandemic. We found that nasopharyngeal colonization by Streptococcus pneumoniae (pneumococcus), Haemophilus influenzae, and Moraxella catarrhalis remained prevalent in our study cohort of children aged 6-36 months. However, with all the precautions of masking, social distancing, hand washing, and quick exclusion from day care when illness occurred, the frequency of detecting these common otopathogens decreased, as one might expect.1
Leading up to the pandemic, we had an abundance of data to characterize AOM etiology and found that the cause of AOM continues to change following the introduction of the 13-valent pneumococcal conjugate vaccine (PCV13, Prevnar 13). Our most recent report on otopathogen distribution and antibiotic susceptibility covered the years 2015-2019.2 A total of 589 children were enrolled prospectively and we collected 495 middle ear fluid samples (MEF) from 319 AOM cases using tympanocentesis. The frequency of isolates was H. influenzae (34%), pneumococcus (24%), and M. catarrhalis (15%). Beta-lactamase–positive H. influenzae strains were identified among 49% of the isolates, rendering them resistant to amoxicillin. PCV13 serotypes were infrequently isolated. However, we did isolate vaccine types (VTs) in some children from MEF, notably serotypes 19F, 19A, and 3. Non-PCV13 pneumococcus serotypes 35B, 23B, and 15B/C emerged as the most common serotypes. Amoxicillin resistance was identified among 25% of pneumococcal strains. Out of 16 antibiotics tested, 9 (56%) showed a significant increase in nonsusceptibility among pneumococcal isolates. 100% of M. catarrhalis isolates were beta-lactamase producers and therefore resistant to amoxicillin.
PCV13 has resulted in a decline in both invasive and noninvasive pneumococcal infections caused by strains expressing the 13 capsular serotypes included in the vaccine. However, the emergence of replacement serotypes occurred after introduction of PCV73,4 and continues to occur during the PCV13 era, as shown from the results presented here. Non-PCV13 serotypes accounted for more than 90% of MEF isolates during 2015-2019, with 35B, 21 and 23B being the most commonly isolated. Other emergent serotypes of potential importance were nonvaccine serotypes 15A, 15B, 15C, 23A and 11A. This is highly relevant because forthcoming higher-valency PCVs – PCV15 (manufactured by Merck) and PCV20 (manufactured by Pfizer) will not include many of the dominant capsular serotypes of pneumococcus strains causing AOM. Consequently, the impact of higher-valency PCVs on AOM will not be as great as was observed with the introduction of PCV7 or PCV13.
Of special interest, 22% of pneumococcus isolates from MEF were serotype 35B, making it the most prevalent. Recently we reported a significant rise in antibiotic nonsusceptibility in Spn isolates, contributed mainly by serotype 35B5 and we have been studying how 35B strains transitioned from commensal to otopathogen in children.6 Because serotype 35B strains are increasingly prevalent and often antibiotic resistant, absence of this serotype from PCV15 and PCV20 is cause for concern.
The frequency of isolation of H. influenzae and M. catarrhalis has remained stable across the PCV13 era as the No. 1 and No. 3 pathogens. Similarly, the production of beta-lactamase among strains causing AOM has remained stable at close to 50% and 100%, respectively. Use of amoxicillin, either high dose or standard dose, would not be expected to kill these bacteria.
Our study design has limitations. The population is derived from a predominantly middle-class, suburban population of children in upstate New York and may not be representative of other types of populations in the United States. The children are 6-36 months old, the age when most AOM occurs. MEF samples that were culture negative for bacteria were not further tested by polymerase chain reaction methods.
Dr. Pichichero is a specialist in pediatric infectious diseases, Center for Infectious Diseases and Immunology, and director of the Research Institute, at Rochester (N.Y.) General Hospital. He has no conflicts of interest to declare.
References
1. Kaur R et al. Front Pediatr. 2021;9:722483.
2. Kaur R et al. Euro J Clin Microbiol Infect Dis. 2021;41:37-44
3. Pelton SI et al. Pediatr Infect Disease J. 2004;23:1015-22.
4. Farrell DJ et al. Pediatr Infect Disease J. 2007;26:123-8..
5. Kaur R et al. Clin Infect Dis 2021;72(5):797-805.
6. Fuji N et al. Front Cell Infect Microbiol. 2021;11:744742.
Children and COVID: New cases and hospital admissions skyrocket
, according to the American Academy of Pediatrics and the Children’s Hospital Association.
The total for the week of Dec. 31 to Jan. 6 – the highest since the pandemic began – was an increase of 78% over the previous week (325,000) and 192% higher than just 2 weeks before (199,000), the AAP and CHA said in their weekly COVID-19 report. No region of the country was spared, as all four saw at least 50,000 more cases than the week before, but the increase was largest in the West and smallest in the Midwest.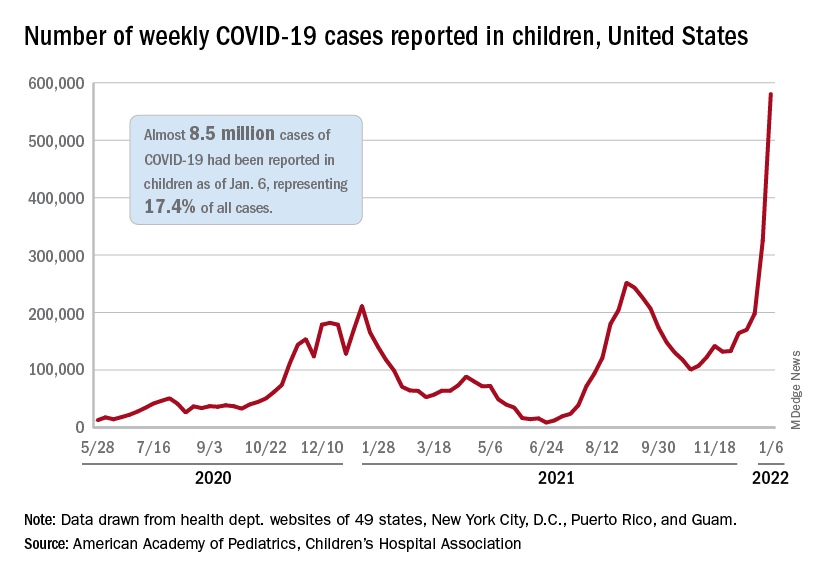
“Nearly 8.5 million children have tested positive for COVID-19 since the onset of the pandemic; nearly 11% of these cases have been added in the past 2 weeks,” the AAP said.
The situation is the same for hospitalizations. On Dec. 15, the daily rate of new admissions for children aged 0-17 years was 0.26 per 100,000, and by Jan. 7 it had more than quadrupled to 1.15 per 100,000, the Centers for Disease Control and Prevention reported. Before Omicron, the highest rate was 0.47 per 100,000 on Sept. 4, 2021.
The number of children occupying inpatient beds who had laboratory-confirmed COVID-19 went from 2,343 on Jan. 2 to 3,476 on Jan. 9, a jump of more than 48% in just 1 week. Texas had more hospitalized children (392) than any other state on Jan. 9, with California (339) and New York (313) the only other states over 300, according to data from the Department of Health & Human Services.
For vaccinations. however, the situation is definitely not the same. The number of children added to the ranks of those with at least one dose of COVID-19 vaccine was down in early 2022 (Jan. 3-9) for both 5- to 11-year-olds (–8.2%) and 16- to 17-year-olds (–12.2%) but higher among those aged 12-15 (12.2%), compared with the previous week (Dec. 27 to Jan. 2), the CDC said on its COVID Data Tracker.
Cumulative figures show that 26.3% of all children aged 5-11 had received at least one dose of vaccine and 17.2% were fully vaccinated as of Jan. 10, compared with 62.2% and 52.0% of 12- to 15-year-olds and 68.5% and 58.1% of those aged 16-17. Altogether, over 23.8 million children in those three age groups have received at least one dose and almost 18.6 million are fully vaccinated, the CDC said.
, according to the American Academy of Pediatrics and the Children’s Hospital Association.
The total for the week of Dec. 31 to Jan. 6 – the highest since the pandemic began – was an increase of 78% over the previous week (325,000) and 192% higher than just 2 weeks before (199,000), the AAP and CHA said in their weekly COVID-19 report. No region of the country was spared, as all four saw at least 50,000 more cases than the week before, but the increase was largest in the West and smallest in the Midwest.
“Nearly 8.5 million children have tested positive for COVID-19 since the onset of the pandemic; nearly 11% of these cases have been added in the past 2 weeks,” the AAP said.
The situation is the same for hospitalizations. On Dec. 15, the daily rate of new admissions for children aged 0-17 years was 0.26 per 100,000, and by Jan. 7 it had more than quadrupled to 1.15 per 100,000, the Centers for Disease Control and Prevention reported. Before Omicron, the highest rate was 0.47 per 100,000 on Sept. 4, 2021.
The number of children occupying inpatient beds who had laboratory-confirmed COVID-19 went from 2,343 on Jan. 2 to 3,476 on Jan. 9, a jump of more than 48% in just 1 week. Texas had more hospitalized children (392) than any other state on Jan. 9, with California (339) and New York (313) the only other states over 300, according to data from the Department of Health & Human Services.
For vaccinations. however, the situation is definitely not the same. The number of children added to the ranks of those with at least one dose of COVID-19 vaccine was down in early 2022 (Jan. 3-9) for both 5- to 11-year-olds (–8.2%) and 16- to 17-year-olds (–12.2%) but higher among those aged 12-15 (12.2%), compared with the previous week (Dec. 27 to Jan. 2), the CDC said on its COVID Data Tracker.
Cumulative figures show that 26.3% of all children aged 5-11 had received at least one dose of vaccine and 17.2% were fully vaccinated as of Jan. 10, compared with 62.2% and 52.0% of 12- to 15-year-olds and 68.5% and 58.1% of those aged 16-17. Altogether, over 23.8 million children in those three age groups have received at least one dose and almost 18.6 million are fully vaccinated, the CDC said.
, according to the American Academy of Pediatrics and the Children’s Hospital Association.
The total for the week of Dec. 31 to Jan. 6 – the highest since the pandemic began – was an increase of 78% over the previous week (325,000) and 192% higher than just 2 weeks before (199,000), the AAP and CHA said in their weekly COVID-19 report. No region of the country was spared, as all four saw at least 50,000 more cases than the week before, but the increase was largest in the West and smallest in the Midwest.
“Nearly 8.5 million children have tested positive for COVID-19 since the onset of the pandemic; nearly 11% of these cases have been added in the past 2 weeks,” the AAP said.
The situation is the same for hospitalizations. On Dec. 15, the daily rate of new admissions for children aged 0-17 years was 0.26 per 100,000, and by Jan. 7 it had more than quadrupled to 1.15 per 100,000, the Centers for Disease Control and Prevention reported. Before Omicron, the highest rate was 0.47 per 100,000 on Sept. 4, 2021.
The number of children occupying inpatient beds who had laboratory-confirmed COVID-19 went from 2,343 on Jan. 2 to 3,476 on Jan. 9, a jump of more than 48% in just 1 week. Texas had more hospitalized children (392) than any other state on Jan. 9, with California (339) and New York (313) the only other states over 300, according to data from the Department of Health & Human Services.
For vaccinations. however, the situation is definitely not the same. The number of children added to the ranks of those with at least one dose of COVID-19 vaccine was down in early 2022 (Jan. 3-9) for both 5- to 11-year-olds (–8.2%) and 16- to 17-year-olds (–12.2%) but higher among those aged 12-15 (12.2%), compared with the previous week (Dec. 27 to Jan. 2), the CDC said on its COVID Data Tracker.
Cumulative figures show that 26.3% of all children aged 5-11 had received at least one dose of vaccine and 17.2% were fully vaccinated as of Jan. 10, compared with 62.2% and 52.0% of 12- to 15-year-olds and 68.5% and 58.1% of those aged 16-17. Altogether, over 23.8 million children in those three age groups have received at least one dose and almost 18.6 million are fully vaccinated, the CDC said.
CDC: More kids hospitalized with COVID since pandemic began
Hospital admissions of U.S. children younger than 5 – the only group ineligible for vaccination – have reached their peak since the start of the pandemic, according to new data from the Centers for Disease Control and Prevention.
CDC Director Rochelle Walensky, MD, said the higher numbers show the importance of vaccination for all eligible groups.
“This is the highest number of pediatric hospitalizations we’ve seen throughout the pandemic, which we said about Delta until now,” she said at a CDC briefing Friday. “This very well may be that there are just more cases out there, and our children are more vulnerable when they have more cases surrounding them.”
Despite the skyrocketing admissions, hospitalizations are still relatively low for children, she said. The hospitalization rate for children under 5 is 4 in 100,000, and it’s about 1 in 100,000 in children 5-17.
Dr. Walensky said not all children are being hospitalized for COVID-19 – some are admitted for unrelated issues and test positive but don’t have symptoms.
“We are still learning more about the severity of Omicron in children,” she said, noting that just over 50% of children 12-18 are fully vaccinated, while only 16% of those ages 5-11 are fully vaccinated.
Friday’s teleconference was the first CDC briefing in several months and comes on the heels of recent guideline updates for testing and isolation that have left the American public dumbfounded. When asked why the briefing was held, Dr. Walensky said there had been interest in hearing more from the CDC, saying, “I anticipate this will be the first of many briefings.”
She also defended the confusing guideline changes, saying, “We’re in an unprecedented time with the speed of Omicron cases rising. … This is hard, and I am committed to continuing to improve as we learn more about the science and communicate that to you.”
A version of this article first appeared on WebMD.com.
Hospital admissions of U.S. children younger than 5 – the only group ineligible for vaccination – have reached their peak since the start of the pandemic, according to new data from the Centers for Disease Control and Prevention.
CDC Director Rochelle Walensky, MD, said the higher numbers show the importance of vaccination for all eligible groups.
“This is the highest number of pediatric hospitalizations we’ve seen throughout the pandemic, which we said about Delta until now,” she said at a CDC briefing Friday. “This very well may be that there are just more cases out there, and our children are more vulnerable when they have more cases surrounding them.”
Despite the skyrocketing admissions, hospitalizations are still relatively low for children, she said. The hospitalization rate for children under 5 is 4 in 100,000, and it’s about 1 in 100,000 in children 5-17.
Dr. Walensky said not all children are being hospitalized for COVID-19 – some are admitted for unrelated issues and test positive but don’t have symptoms.
“We are still learning more about the severity of Omicron in children,” she said, noting that just over 50% of children 12-18 are fully vaccinated, while only 16% of those ages 5-11 are fully vaccinated.
Friday’s teleconference was the first CDC briefing in several months and comes on the heels of recent guideline updates for testing and isolation that have left the American public dumbfounded. When asked why the briefing was held, Dr. Walensky said there had been interest in hearing more from the CDC, saying, “I anticipate this will be the first of many briefings.”
She also defended the confusing guideline changes, saying, “We’re in an unprecedented time with the speed of Omicron cases rising. … This is hard, and I am committed to continuing to improve as we learn more about the science and communicate that to you.”
A version of this article first appeared on WebMD.com.
Hospital admissions of U.S. children younger than 5 – the only group ineligible for vaccination – have reached their peak since the start of the pandemic, according to new data from the Centers for Disease Control and Prevention.
CDC Director Rochelle Walensky, MD, said the higher numbers show the importance of vaccination for all eligible groups.
“This is the highest number of pediatric hospitalizations we’ve seen throughout the pandemic, which we said about Delta until now,” she said at a CDC briefing Friday. “This very well may be that there are just more cases out there, and our children are more vulnerable when they have more cases surrounding them.”
Despite the skyrocketing admissions, hospitalizations are still relatively low for children, she said. The hospitalization rate for children under 5 is 4 in 100,000, and it’s about 1 in 100,000 in children 5-17.
Dr. Walensky said not all children are being hospitalized for COVID-19 – some are admitted for unrelated issues and test positive but don’t have symptoms.
“We are still learning more about the severity of Omicron in children,” she said, noting that just over 50% of children 12-18 are fully vaccinated, while only 16% of those ages 5-11 are fully vaccinated.
Friday’s teleconference was the first CDC briefing in several months and comes on the heels of recent guideline updates for testing and isolation that have left the American public dumbfounded. When asked why the briefing was held, Dr. Walensky said there had been interest in hearing more from the CDC, saying, “I anticipate this will be the first of many briefings.”
She also defended the confusing guideline changes, saying, “We’re in an unprecedented time with the speed of Omicron cases rising. … This is hard, and I am committed to continuing to improve as we learn more about the science and communicate that to you.”
A version of this article first appeared on WebMD.com.
Pediatric antibiotic prescriptions plummeted in pandemic
Antibiotic prescribing in pediatric primary care decreased dramatically when the COVID-19 pandemic hit, and new research indicates that drop was sustained through June of 2021.
Lauren Dutcher, MD, with the division of infectious diseases at Hospital of the University of Pennsylvania in Philadelphia, led a study of 27 pediatric primary care practices in the United States. Encounters from Jan. 1, 2018, through June 30, 2021, were included.
Researchers found a 72.7% drop in antibiotic prescriptions when they compared prepandemic April 2019 through December 2019 with the same period in 2020.
Prescriptions remained at the lower levels, primarily driven by reductions in respiratory tract infection (RTI) encounters, and began to rise only in April of 2021, the authors write.
Findings were published online Jan. 11 in Pediatrics.
Researchers report there were 69,327 antibiotic prescriptions from April through December in 2019 and 18,935 antibiotic prescriptions during the same months in 2020.
“The reduction in prescriptions at visits for respiratory tract infection (RTI) accounted for 87.3% of this decrease,” the authors write.
Both prescribing and acute non–COVID-19 respiratory tract infection diagnoses decreased.
Researchers conclude reductions in viral RTI transmission likely played a large role in reduced RTI pediatric visits and antibiotic prescriptions.
Dr. Dutcher told this publication the reduction was likely caused by a combination of less viral transmission of respiratory infections, helped in part by masking and distancing, but also avoidance of health care in the pandemic.
She said the data reinforce the need for appropriate prescribing.
“Antibiotic prescribing is really heavily driven by respiratory infections so this should continue to clue providers in on how frequently that can be unnecessary,“ she said.
Dr. Dutcher said there was probably a reduction in secondary bacterial infections as well as the viral infections.
The research is more comprehensive than some other previous studies, the authors write.
“Although other studies demonstrated early reductions in RTIs and antibiotic prescribing during the COVID-19 pandemic, to our knowledge, this is the first study to demonstrate a sustained decrease in antibiotic prescribing in pediatric primary care throughout 2020 and early 2021,” they write.
The findings also suggest benefits of preventive measures during the pandemic, the authors say.
“Our data suggest that reducing community viral RTI transmission through social distancing and masking corresponds with a reduction in antibiotic prescribing,” they write.
Kao-Ping Chua, MD, a pediatrician and an assistant professor of pediatrics at the University of Michigan in Ann Arbor, said the reductions indicate one of two things is happening: either children aren’t getting sick as often during the pandemic or they are getting sick, but not coming in.
But if they were sick and not coming in, the expectation would be that they would show up in large numbers in emergency departments from untreated infections, he said.
“We just haven’t seen that,” he said.
He said one of the main points the authors make is that masks, distancing, and hand washing may be keeping kids from diseases beyond COVID-19.
He said longer-term data will be needed to show if the trend highlighted in this paper lasts, given children have now returned to school and pediatricians started to see lots of respiratory syncytial virus (RSV) cases this summer.
Anecdotally, he said, he has been prescribing more antibiotics of late for presentations such as ear infections.
Dr. Dutcher said that, though her team doesn’t have data yet since the end of the study period, she agreed that anecdotally it is likely that the prescriptions have been on the rise since June.
Dr. Chua said the reduction in visits also reduces the chance that a physician will be tempted to give in to families’ demands to prescribe an antibiotic.
“Every visit for a sick child represents an opportunity to inappropriately prescribe antibiotics,” Dr. Chua said. Dr. Chua’s own research has found that up to one-quarter of pediatric and adult antibiotic prescriptions are unnecessary.
This work was supported by a Centers for Disease Control and Prevention cooperative agreement, Epicenters for the Prevention of Healthcare Associated Infections. Dr. Dutcher and Dr. Chua had no relevant financial disclosures.
This article was updated 1/11/22.
Antibiotic prescribing in pediatric primary care decreased dramatically when the COVID-19 pandemic hit, and new research indicates that drop was sustained through June of 2021.
Lauren Dutcher, MD, with the division of infectious diseases at Hospital of the University of Pennsylvania in Philadelphia, led a study of 27 pediatric primary care practices in the United States. Encounters from Jan. 1, 2018, through June 30, 2021, were included.
Researchers found a 72.7% drop in antibiotic prescriptions when they compared prepandemic April 2019 through December 2019 with the same period in 2020.
Prescriptions remained at the lower levels, primarily driven by reductions in respiratory tract infection (RTI) encounters, and began to rise only in April of 2021, the authors write.
Findings were published online Jan. 11 in Pediatrics.
Researchers report there were 69,327 antibiotic prescriptions from April through December in 2019 and 18,935 antibiotic prescriptions during the same months in 2020.
“The reduction in prescriptions at visits for respiratory tract infection (RTI) accounted for 87.3% of this decrease,” the authors write.
Both prescribing and acute non–COVID-19 respiratory tract infection diagnoses decreased.
Researchers conclude reductions in viral RTI transmission likely played a large role in reduced RTI pediatric visits and antibiotic prescriptions.
Dr. Dutcher told this publication the reduction was likely caused by a combination of less viral transmission of respiratory infections, helped in part by masking and distancing, but also avoidance of health care in the pandemic.
She said the data reinforce the need for appropriate prescribing.
“Antibiotic prescribing is really heavily driven by respiratory infections so this should continue to clue providers in on how frequently that can be unnecessary,“ she said.
Dr. Dutcher said there was probably a reduction in secondary bacterial infections as well as the viral infections.
The research is more comprehensive than some other previous studies, the authors write.
“Although other studies demonstrated early reductions in RTIs and antibiotic prescribing during the COVID-19 pandemic, to our knowledge, this is the first study to demonstrate a sustained decrease in antibiotic prescribing in pediatric primary care throughout 2020 and early 2021,” they write.
The findings also suggest benefits of preventive measures during the pandemic, the authors say.
“Our data suggest that reducing community viral RTI transmission through social distancing and masking corresponds with a reduction in antibiotic prescribing,” they write.
Kao-Ping Chua, MD, a pediatrician and an assistant professor of pediatrics at the University of Michigan in Ann Arbor, said the reductions indicate one of two things is happening: either children aren’t getting sick as often during the pandemic or they are getting sick, but not coming in.
But if they were sick and not coming in, the expectation would be that they would show up in large numbers in emergency departments from untreated infections, he said.
“We just haven’t seen that,” he said.
He said one of the main points the authors make is that masks, distancing, and hand washing may be keeping kids from diseases beyond COVID-19.
He said longer-term data will be needed to show if the trend highlighted in this paper lasts, given children have now returned to school and pediatricians started to see lots of respiratory syncytial virus (RSV) cases this summer.
Anecdotally, he said, he has been prescribing more antibiotics of late for presentations such as ear infections.
Dr. Dutcher said that, though her team doesn’t have data yet since the end of the study period, she agreed that anecdotally it is likely that the prescriptions have been on the rise since June.
Dr. Chua said the reduction in visits also reduces the chance that a physician will be tempted to give in to families’ demands to prescribe an antibiotic.
“Every visit for a sick child represents an opportunity to inappropriately prescribe antibiotics,” Dr. Chua said. Dr. Chua’s own research has found that up to one-quarter of pediatric and adult antibiotic prescriptions are unnecessary.
This work was supported by a Centers for Disease Control and Prevention cooperative agreement, Epicenters for the Prevention of Healthcare Associated Infections. Dr. Dutcher and Dr. Chua had no relevant financial disclosures.
This article was updated 1/11/22.
Antibiotic prescribing in pediatric primary care decreased dramatically when the COVID-19 pandemic hit, and new research indicates that drop was sustained through June of 2021.
Lauren Dutcher, MD, with the division of infectious diseases at Hospital of the University of Pennsylvania in Philadelphia, led a study of 27 pediatric primary care practices in the United States. Encounters from Jan. 1, 2018, through June 30, 2021, were included.
Researchers found a 72.7% drop in antibiotic prescriptions when they compared prepandemic April 2019 through December 2019 with the same period in 2020.
Prescriptions remained at the lower levels, primarily driven by reductions in respiratory tract infection (RTI) encounters, and began to rise only in April of 2021, the authors write.
Findings were published online Jan. 11 in Pediatrics.
Researchers report there were 69,327 antibiotic prescriptions from April through December in 2019 and 18,935 antibiotic prescriptions during the same months in 2020.
“The reduction in prescriptions at visits for respiratory tract infection (RTI) accounted for 87.3% of this decrease,” the authors write.
Both prescribing and acute non–COVID-19 respiratory tract infection diagnoses decreased.
Researchers conclude reductions in viral RTI transmission likely played a large role in reduced RTI pediatric visits and antibiotic prescriptions.
Dr. Dutcher told this publication the reduction was likely caused by a combination of less viral transmission of respiratory infections, helped in part by masking and distancing, but also avoidance of health care in the pandemic.
She said the data reinforce the need for appropriate prescribing.
“Antibiotic prescribing is really heavily driven by respiratory infections so this should continue to clue providers in on how frequently that can be unnecessary,“ she said.
Dr. Dutcher said there was probably a reduction in secondary bacterial infections as well as the viral infections.
The research is more comprehensive than some other previous studies, the authors write.
“Although other studies demonstrated early reductions in RTIs and antibiotic prescribing during the COVID-19 pandemic, to our knowledge, this is the first study to demonstrate a sustained decrease in antibiotic prescribing in pediatric primary care throughout 2020 and early 2021,” they write.
The findings also suggest benefits of preventive measures during the pandemic, the authors say.
“Our data suggest that reducing community viral RTI transmission through social distancing and masking corresponds with a reduction in antibiotic prescribing,” they write.
Kao-Ping Chua, MD, a pediatrician and an assistant professor of pediatrics at the University of Michigan in Ann Arbor, said the reductions indicate one of two things is happening: either children aren’t getting sick as often during the pandemic or they are getting sick, but not coming in.
But if they were sick and not coming in, the expectation would be that they would show up in large numbers in emergency departments from untreated infections, he said.
“We just haven’t seen that,” he said.
He said one of the main points the authors make is that masks, distancing, and hand washing may be keeping kids from diseases beyond COVID-19.
He said longer-term data will be needed to show if the trend highlighted in this paper lasts, given children have now returned to school and pediatricians started to see lots of respiratory syncytial virus (RSV) cases this summer.
Anecdotally, he said, he has been prescribing more antibiotics of late for presentations such as ear infections.
Dr. Dutcher said that, though her team doesn’t have data yet since the end of the study period, she agreed that anecdotally it is likely that the prescriptions have been on the rise since June.
Dr. Chua said the reduction in visits also reduces the chance that a physician will be tempted to give in to families’ demands to prescribe an antibiotic.
“Every visit for a sick child represents an opportunity to inappropriately prescribe antibiotics,” Dr. Chua said. Dr. Chua’s own research has found that up to one-quarter of pediatric and adult antibiotic prescriptions are unnecessary.
This work was supported by a Centers for Disease Control and Prevention cooperative agreement, Epicenters for the Prevention of Healthcare Associated Infections. Dr. Dutcher and Dr. Chua had no relevant financial disclosures.
This article was updated 1/11/22.
FROM PEDIATRICS
A COVID-19 Clinical Management Committee to Standardize Care in a 2-Hospital System
From the Department of Medicine (Drs. Meisenberg, Muganlinskaya, Sharma, Amjadi, Arnold, Barnes, Clance, Khalil, Miller, Mooradian, O’Connell, Patel, Press, Samaras, Shanmugam, Tavadze, and Thompson), Department of Pharmacy (Drs. Jiang, Jarawan, Sheth, and Trinh), Department of Nursing (Dr. Ohnmacht), and Department of Women and Children’s Services (Dr. Raji), Luminis Health, Annapolis, MD, and Lanham, MD.
Objective: The COVID-19 pandemic has been a challenge for hospital medical staffs worldwide due to high volumes of patients acutely ill with novel syndromes and prevailing uncertainty regarding optimum supportive and therapeutic interventions. Additionally, the response to this crisis was driven by a plethora of nontraditional information sources, such as email chains, websites, non–peer-reviewed preprints, and press releases. Care patterns became idiosyncratic and often incorporated unproven interventions driven by these nontraditional information sources. This report evaluates the efforts of a health system to create and empower a multidisciplinary committee to develop, implement, and monitor evidence-based, standardized protocols for patients with COVID-19.
Methods: This report describes the composition of the committee, its scope, and its important interactions with the health system pharmacy and therapeutics committee, research teams, and other work groups planning other aspects of COVID-19 management. It illustrates how the committee was used to demonstrate for trainees the process and value of critically examining evidence, even in a chaotic environment.
Results: Data show successful interventions in reducing excessive ordering of certain laboratory tests, reduction of nonrecommended therapies, and rapid uptake of evidence-based or guidelines-supported interventions.
Conclusions: A multidisciplinary committee dedicated solely to planning, implementing, and monitoring standard approaches that eventually became evidence-based decision-making led to an improved focus on treatment options and outcomes for COVID-19 patients. Data presented illustrate the attainable success that is both adaptable and suitable for similar emergencies in the future.
Keywords: COVID-19; clinical management; pharmacy and therapeutics; treatment; therapy.
The COVID-19 pandemic has spread to nearly all countries, carrying with it high morbidity, mortality, and severe impacts on both well-developed and less-well-developed health systems. Media reports of chaos within overwhelmed hospitals have been prominent.1,2 As of January 5, 2022, SARS-CoV-2 has infected more than 295 million people globally and directly caused the death of more than 5.4 million,3 though this number is likely an undercount even in countries with well-developed mortality tracking.4
Throughout the COVID-19 pandemic, hospital-based medical teams have been confronted with a flood of severely ill patients with novel syndromes. Initially, there were no standards for therapy or supportive care except for treatments borrowed from similar syndromes. In the setting of high volumes, high acuity, and public dismay, it is unsurprising that the usual deliberative methods for weighing evidence and initiating interventions were often pushed aside in favor of the solace of active intervention.5 In this milieu of limited evidence, there was a lamentable, if understandable, tendency to seek guidance from “nontraditional” sources,6 including email chains from colleagues, hospital websites, non–peer-reviewed manuscripts, advanced publication by medical journals,7 and nonscientific media presentations. In many localities, practitioners responded in idiosyncratic ways. For example, findings of high cytokine levels in COVID-19,8 along with reports of in-vitro antiviral activity with drugs like hydroxychloroquine against both SARS9 and SARS-CoV-2,10 drove laboratory test ordering and therapeutic interventions, respectively, carving shortcuts into the traditional clinical trial–dependent standards. Clinical trial results eventually emerged.11COVID-19 created a clinical dilemma for hospital medical staffs in terms of how to organize, standardize, and rapidly adapt to a flood of new information. In this report, we describe how 1 health system responded to these challenges by forming a COVID-19 Clinical Management Committee (CCMC) and empowering this interdisciplinary team to review evidence, create and adjust order sets, educate practitioners, oversee care, and collaborate across teams addressing other aspects of the COVID-19 response.
Program Overview
Health System Description
Luminis Health is a health system with 2 acute care hospitals that was formed in 2019 just before the start of the pandemic. Anne Arundel Medical Center (hospital A) is a 385-bed teaching hospital in Annapolis, MD. It has more than 23 000 discharges annually. Patients with COVID-19 were cared for by either an internal medicine teaching service or nonteaching hospitalist services on cohorted nursing units. Doctor’s Community Medical Center, in Lanham, MD (hospital B), is a 206-bed acute care hospital with more than 10 350 annual discharges. COVID-19 patients were cared for by hospitalist groups, initially in noncohorted units with transition to cohorted nursing units after a few months. The medical staffs are generally distinct, with different leadership structures, though the Luminis Health Department of Medicine has oversight responsibilities at both hospitals. More than 47 physicians attended COVID-19 patients at hospital A (with medical residents) and 30 individual physicians at hospital B, respectively, including intensivists. The nursing and pharmacy staffs are distinct, but there is a shared oversight Pharmacy and Therapeutics (P&T) Committee.
The 2 hospitals had distinct electronic medical records (EMR) until January 2021, when hospital B adopted the same EMR as hospital A (Epic).
Mission and Formation of CCMC
In order to coordinate the therapeutic approach across the health system, it was important for the CCMC to be designated by the health system P&T committee as an official subcommittee so that decisions on restrictions of medications and/or new or revised order sets could be rapidly initiated across the system without waiting for the subsequent P&T meetings. The full committee retained oversight of the CCMC. Some P&T members were also on the CCMC.
The committee reviewed new reports in medical journals and prepublication servers and consulted recommendations of professional societies, such as the National Institutes of Health (NIH) COVID-19 guidelines, Infectious Diseases Society of America, Society of Critical Care Medicine, and US Food and Drug Administration (FDA) Emergency Use Authorizations (EUA), among other sources.
Composition of the CCMC
Physician leaders from both hospitals in the following specialties were solicited for participation: critical care, epidemiology, hospital medicine (internal medicine), emergency medicine, infectious diseases, nephrology, women and children’s services, and medical informatics. Specialists in other areas, such as hematology, were invited for topic-specific discussions. Hospital pharmacists with different specialties and nursing leadership were essential contributors. The committee members were expected to use various communication channels to inform frontline clinicians of new care standards and the existence of new order sets, which were embedded in the EMR.
Clinical Research
An important connection for the CCMC was with theCOVID-19 clinical research team. Three members of the research team were also members of the CCMC. All new study proposals for therapeutics were discussed with the CCMC as they were being considered by the research team. In this way, feedback on the feasibility and acceptance of new study opportunities could be discussed with the CCMC. Occasionally, CCMC decisions impacted clinical research accrual strategies. For example, new data from randomized trials about tocilizumab1,2 demonstrated benefits in some subsets of patients and resulted in a recommendation for use by the NIH guideline committee in these populations.1 The CCMC quickly adopted this recommendation, which required a reprioritization of clinical research enrollment for studies testing other immune-modulating agents. This important dialogue was mediated within the CCMC.
Guideline Distribution, Reinforcement, and Platform for Feedback
New guidelines were disseminated to clinicians via daily brief patient huddles held on COVID units, with participation by nursing and pharmacy, and by weekly meetings with hospitalist leaders and frontline hospital physicians. Order sets and guidelines were maintained on the intranet. Adherence was reinforced by unit-based and central pharmacists. Order sets, including admission order sets, could be created only by designated informatics personnel, thus enforcing standardization. Feedback on the utility of the order sets was obtained during the weekly meetings or huddles, as described above. To ensure a sense of transparency, physicians who had interest in commenting on a particular therapy, or who wished to discuss a particular manuscript, news article, or website, were invited to attend CCMC meetings.
Scope of CCMC
In order to be effective and timely, we limited the scope of our work to the report to the inpatient therapeutic environment, allowing other committees to work on other aspects of the pandemic response. In addition to issuing guidance and creating order sets to direct clinical practice, the CCMC also monitored COVID-19 therapeutic shortages15,16 and advised on prioritization of such treatments as convalescent plasma, remdesivir (prioritization and duration of therapy, 5 vs 10 days), baricitinib, and tocilizumab, depending upon the location of the patient (critical care or not). The CCMC was not involved in the management of non–COVID-19 shortages brought about by supply chain deficiencies.
Table 1 shows some aspects of the health system pandemic-response planning and the committee workforce that undertook that work. Though many items were out of scope for the CCMC, members of the CCMC did participate in the planning work of these other committees and therefore stayed connected to this complementary work.
A Teaching Opportunity About Making Thoughtful Choices
Another important feature of the CCMC was the contributions of residents from both pharmacy and internal medicine. The purpose and operations of the committee were recognized as an opportunity to involve learners in a curriculum based on Kern’s 6-step approach.17 Though the problem identification and general needs assessment were easily defined, the targeted needs assessment, extracted from individual and group interviews with learners and the committee members, pointed at the need to learn how to assess and analyze a rapidly growing body of literature on several relevant clinical aspects of SARS-CoV-2 and COVID-19. To achieve goals and objectives, residents were assigned to present current literature on a particular intervention during a committee meeting, specifically commenting on the merit or deficiencies of the study design, the strength of the data, and applicability to the local context with a recommendation. Prior to the presentations, the residents worked with faculty to identify the best studies or systematic analyses with potential to alter current practices. We thus used the CCMC process as a teaching tool about evidence-based medicine and the dilemma of clinical equipoise. This was imperative, since trainees thrust into the COVID-19 response have often keenly observed a movement away from deliberative decision-making.18 Indeed, including residents in the process of deliberative responses to COVID-19 addresses a recent call to adjust medical education during COVID-19 to “adapt curriculum to current issues in real time.”19
Interventions and Therapies Considered
Table 2 shows the topics reviewed by the CCMC. By the time of the first meeting, nonstandardization of care was already a source of concern for clinicians. Dialogue often continued outside of the formal meetings. Many topics were considered more than once as new guidance developed, changes to EUAs occurred, and new data or new publicity arose.
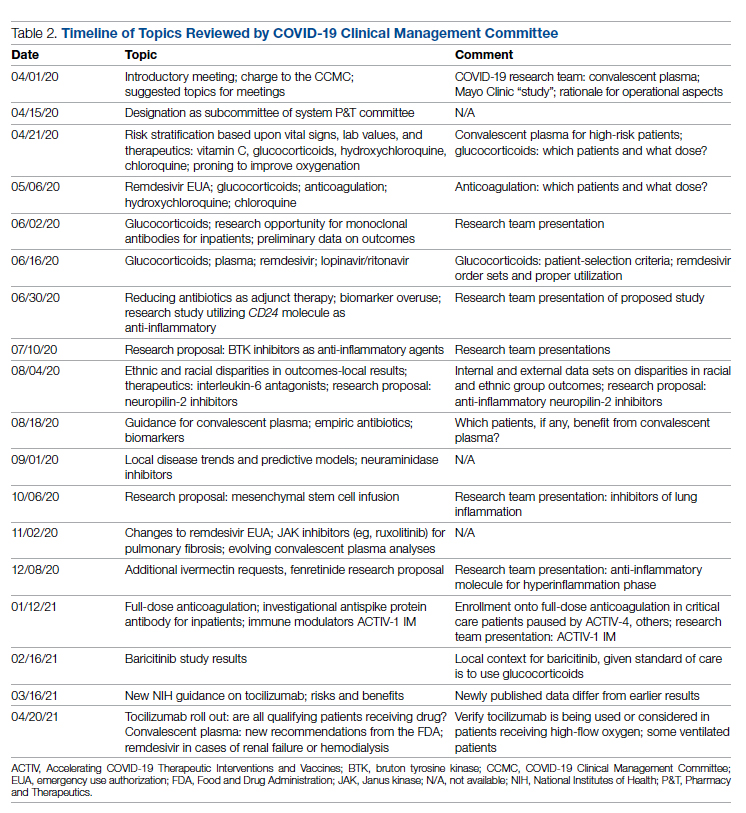
Methods
The Human Protections Administrator determined that this work constituted “quality improvement, and not research” and was therefore exempt from institutional review board review.
Quantitative Analysis
All admitted patients from March 10, 2020, through April 20, 2021, were considered in the quantitative aspects of this report except as noted. Patients diagnosed with COVID-19 were identified by searching our internal data base using diagnostic codes. Patient admissions with the following diagnostic codes were included (prior to April 1, 2020): J12.89, J20.8, J40, J22, J98.8, J80, each with the additional code of B97.29. After April 1, 2020, the guideline for coding COVID-19 was U07.1.
Descriptive statistics were used to measure utilization rates of certain medications and laboratory tests of interest over time. These data were adjusted for number of unique admissions. In a few cases, not all data elements were available from both hospitals due to differences in the EMR.
Case fatality rate was calculated based upon whether the patient died or was admitted to inpatient hospice as a result of COVID-19. Four patients transferred out of hospital A and 18 transferred out of hospital B were censored from case-fatality-rate determination.
Figure 1 shows the number of admissions for each acute care hospital in the health system and the combined COVID-19 case-fatality rate over time.
Results
A total of 5955 consecutive COVID-19 patients admitted from March 10, 2020, through April 30, 2021, were analyzed. Patients with International Statistical Classification of Diseases, Tenth Revision codes J12.89. J20.8, J40, J22, J98.8, J80, each with the additional code of B97.29 (or the code UO7.1 after April 1, 2020), were included in the analysis. The median age of admitted patients was 65 years (range 19-91 years). Using the NIH classification system for severity,20 the distribution of severity during the first 24 hours after the time of hospital admission was as follows: asymptomatic/presymptomatic, 0.5%; mild illness, 5.3%; moderate illness, 37.1%; severe illness, 55.5%; and critical illness, 1.1%.
The impact of the CCMC can be estimated by looking at care patterns over time. Since the work of the CCMC was aimed at influencing and standardizing physician ordering and therapy choices through order set creation and other forms of oversight, we measured the use of the CCMC-approved order sets at both hospitals and the use of certain laboratory tests and therapies that the CCMC sought either to limit or increase. These counts were adjusted for number of unique COVID-19 admissions. But the limits of the case collection tool meant it also collected cases that were not eligible for some of the interventions. For example, COVID-19 admissions without hypoxemia would not have been eligible for remdesivir or glucocorticoids. When admitted, some patients were already on steroids for other medical indications and did not receive the prescribed dexamethasone dose that we measured in pharmacy databases. Similarly, a few patients were hospitalized for indications unrelated to COVID-19, such as surgery or childbirth, and were found to be SARS-CoV-2-positive on routine screening.
Figure 2 shows the utilization of CCMC-approved standard COVID-19 admission order sets as a proportion of all COVID-19 admissions over time. The trend reveals a modest increase in usage (R2 = 0.34), but these data do not reflect the progressive build of content into order sets over time. One of the goals of the order sets was to standardize and reduce the ordering of certain biomarkers: C-reactive protein, serum ferritin, and D-dimer, which were ordered frequently in many early patients. Orders for these 3 laboratory tests are combined and expressed as an average number of labs per COVID-19 admission in Figure 2. A downward trend, with an R2 value of 0.65, is suggestive of impact from the order sets, though other explanations are possible.
Medication guidance was also a goal of the CCMC, simultaneously discouraging poorly supported interventions and driving uptake of the recommended evidence-based interventions in appropriate patients. Figure 3 shows the utilization pattern for some drugs of interest over the course of the pandemic, specifically the proportion of patients receiving at least 1 dose of medication among all COVID-19 admissions by month. (Data for hospital B was excluded from this analysis because it did not include all admitted patients.)
Hydroxychloroquine, which enjoyed a wave of popularity early on during the pandemic, was a target of successful order stewardship through the CCMC. Use of hydroxychloroquine as a COVID-19 therapeutic option after the first 2 months of the pandemic stopped, and subsequent use at low levels likely represented continuation therapy for outpatients who took hydroxychloroquine for rheumatologic indications.
Dexamethasone, as used in the RECOVERY trial,21 had a swift uptake among physicians after it was incorporated into order sets and its use encouraged. Similarly, uptake was immediate for remdesivir when, in May 2020, preliminary reports showed at least some benefits, confirmed by later analysis,22 and it received an FDA EUA.
Our data also show successful stewardship of the interleukin-6 antagonist toclilizumab, which was discouraged early on by the CCMC due to lack of data or negative results. But in March 2021, with new studies releasing data12,13 and new recommendations14 for its use in some subsets of patients with COVID-19, this drug was encouraged in appropriate subsets. A new order set with qualifying indications was prepared by the CCMC and new educational efforts made to encourage its use in appropriate patients.
Ivermectin was nonformulary at the start of the pandemic. This drug enjoyed much publicity from media sources and was promoted by certain physicians and on websites,23 based on in-vitro activity against coronaviruses. Eventually, the World Health Organization24 and the FDA25 found it necessary to issue advisory statements to the public against its use outside of clinical trials. The CCMC had requests from physicians to incorporate ivermectin but declined to add it to the formulary and recommended not approving nonformulary requests due to lack of data. As a result, ivermectin was not used at either hospital.
Discussion
COVID-19 represents many challenges to health systems all over the world. For Luminis Health, the high volume of acutely ill patients with novel syndromes was a particular challenge for the hospital-based care teams. A flood of information from preprints, press releases, preliminary reports, and many other nontraditional sources made deliberative management decisions difficult for individual physicians. Much commentary has appeared around the phenomenon but with less practical advice about how to make day-to-day care decisions at a time of scientific uncertainty and intense pressure to intervene.26,27 The CCMC was designed to overcome the information management dilemma. The need to coordinate, standardize, and oversee care was necessary given the large number of physicians who cared for COVID-19 patients on inpatient services.
It should be noted that creating order sets and issuing guidance is necessary, but not sufficient, to achieve our goals of being updated and consistent. This is especially true with large cadres of health care workers attending COVID-19 patients. Guidelines and recommendations were reinforced by unit-based oversight and stewardship from pharmacy and other leaders who constituted the CCMC.
The reduction in COVID-19 mortality over time experienced in this health care system was not unique and cannot necessarily be attributed to standardization of care. Similar improvements in mortality have been reported at many US hospitals in aggregate.28 Many other factors, including changes in patient characteristics, may be responsible for reduction in mortality over time.
Throughout this report we have relied upon an implicit assumption that standardization of medical therapeutics is desirable and leads to better outcomes as compared with allowing unlimited empiricism by individual physicians, either consultants or hospitalists. Our program represents a single health system with 2 acute care hospitals located 25 miles apart and which thus were similarly impacted by the different phases of the pandemic. Generalizability to health systems either smaller or larger, or in different geographical areas, has not been established. Data limitations have already been discussed.
We did not measure user satisfaction with the program either from physicians or nurses. However, the high rate of compliance suggests general agreement with the content and process.
We cannot definitively ascribe reduction in utilization of some nonrecommended treatments and increased utilization of the recommended therapies to the work of the CCMC. Individual physicians may have made these adjustments on their own or under the influence of other sources.
Finally, it should be noted that the mission to rapidly respond to data from well-conducted trials might be thwarted by too rigid a process or a committee’s lack of a sense of urgency. Organizing a committee and then empowering it to act is no guarantee of success; commitment to the mission is.
Conclusion
COVID-19 represented a challenge to medical staffs everywhere, inundating them with high volumes of acutely ill patients presenting with unfamiliar syndromes. Initial responses were characterized by idiosyncratic management approaches based on nontraditional sources of opinion and influences.
This report describes how a complex medical system brought order and standardization through a deliberative, but urgent, multidisciplinary committee with responsibility for planning, implementing, and monitoring standard approaches that eventually became evidence based. The composition of the committee and its scope of influence, limited to inpatient management, were important elements of success, allowing for better focus on the many treatment decisions. The important connection between the management committee and the system P&T committee, the clinical research effort, and teaching programs in both medicine and pharmacy are offered as exemplars of coordination. The data presented show success in achieving standardized, guideline-directed care. The approach is adoptable and suitable for similar emergencies in the future.
Acknowledgments: The authors thank Gary Scabis, Kip Waite, John Moxley, Angela Clubb, and David Woodley for their assistance in gathering data. We express appreciation and admiration for all our colleagues at the bedside.
Corresponding author: Barry R. Meisenberg, MD, Department of Medicine, Luminis Health, 2001 Medical Pkwy, Annapolis, MD 21401; [email protected].
Financial disclosures: None.
1. Gettleman J, Raj S, Kumar H. India’s health system cracks under the strain as coronavirus cases surge. The New York Times. April 22, 2021. https://www.nytimes.com/2021/04/21/world/asia/india-coronavirus-oxygen.html
2. Rappleye H, Lehren AW, Strickler L, Fitzpatrick S. ‘This system is doomed’: doctors, nurses sound off in NBC News coronavirus survey. NBC News. March 20, 2020. https://www.nbcnews.com/news/us-news/system-doomed-doctors-nurses-sound-nbc-news-coronavirus-survey-n1164841
3. Johns Hopkins Coronavirus Resource Center. Accessed January 5, 2022. https://coronavirus.jhu.edu/map.html
4. Fineberg HV. The toll of COVID-19. JAMA. 2020;324(15):1502-1503. doi:10.1001/jama.2020.20019
5. Meisenberg BR. Medical staffs response to COVID-19 ‘data’: have we misplaced our skeptic’s eye? Am J Med. 2021;134(2):151-152. doi:10.1016/j.amjmed.2020.09.013
6. McMahon JH, Lydeamore MH, Stewardson AJ. Bringing evidence from press release to the clinic in the era of COVID-19. J Antimicrob Chemother. 2021;76(3):547-549. doi:10.1093/jac/dkaa506
7. Rubin EJ, Baden LR, Morrissey S, Campion EW. Medical journals and the 2019-nCoV outbreak. N Engl J Med. 2020;382(9):866. doi:10.1056/NEJMe2001329
8. Liu F, Li L, Xu M, et al. Prognostic value of interleukin-6, C-reactive protein, and procalcitonin in patients with COVID-19. J Clin Virol. 2020;127:104370. doi:10.1016/j.jcv.2020.104370
9. Vincent MJ, Bergeron E, Benjannet S, et al. Chloroquine is a potent inhibitor of SARS coronavirus infection and spread. Virol J. 2005;2:69. doi:10.1186/1743-422X-2-69
10. Wang M, Cao R, Zhang L, et al. Remdesivir and chloroquine effectively inhibit the recently emerged novel coronavirus (2019-nCoV) in vitro. Cell Res. 2020;30:269-271. doi:10.1038/s41422-020-0282-0
11. RECOVERY Collaborative Group. Effect of hydroxychloroquine in hospitalized patients with Covid-19. N Engl J Med. 2020;383:2030-2040. doi:10.1056/NEJMoa2022926
12. RECOVERY Collaborative Group. Tocilizumab in patients admitted to hospital with COVID-19 (RECOVERY): preliminary results of a randomised, controlled, open-label, platform trial [preprint]. February 11, 2021. doi:10.1101/2021.02.11.21249258 https://www.medrxiv.org/content/10.1101/2021.02.11.21249258v1
13. REMAP-CAP Investigators. Interleukin-6 receptor antagonists in critically ill patients with COVID-19. N Engl J Med. 2021;384(16):1491-1502. doi:10.1056/NEJMoa2100433
14. National Institutes of Health. COVID-19 treatment guidelines: interleukin-6 inhibitors. https://www.covid19treatmentguidelines.nih.gov/immunomodulators/interleukin-6-inhibitors/
15. Deana C, Vetrugno L, Tonizzo A, et al. Drug supply during COVID-19 pandemic: remember not to run with your tank empty. Hosp Pharm. 2021;56(5):405-407. doi:10.1177/0018578720931749
16. Choe J, Crane M, Greene J, et al. The Pandemic and the Supply Chain: Addressing Gaps in Pharmaceutical Production and Distribution. Johns Hopkins University, November 2020. https://www.jhsph.edu/research/affiliated-programs/johns-hopkins-drug-access-and-affordability-initiative/publications/Pandemic_Supply_Chain.pdf
17. Kern DE. Overview: a six-step approach to curriculum development. In: Kern DE, Thornton PA, Hughes MT, eds. Curriculum Development for Medical Education: A Six-Step Approach. 3rd ed. Johns Hopkins University Press; 2016.
18. Rice TW, Janz DR. In defense of evidence-based medicine for the treatment of COVID-19 acute respiratory distress syndrome. Ann Am Thorac Soc. 2020;17(7):787-789. doi:10.1513/AnnalsATS.202004-325IP
19. Lucey CR, Johnston SC. The transformational effects of COVID-19 on medical education. JAMA. 2020;324(11):1033-1034. doi:10.1001/jama.2020.14136
20. National Institutes of Health. COVID-19 treatment guidelines: clinical spectrum of SARS-CoV-2 infection. https://www.covid19treatmentguidelines.nih.gov/overview/clinical-spectrum/
21. RECOVERY Collaborative Group. Dexamethasone in hospitalized patients with Covid-19. N Engl J Med. 2021;384:693-704. doi:10.1056/NEJMoa2021436
22. Beigel JH, Tomashek KM, Dodd LE, et al. Remdesivir for the treatment of Covid-19—final report. N Engl J Med. 2020;383:1813-1826. doi:10.1056/NEJMoa2007764
23. Jiminez D. Ivermectin and Covid-19: how a cheap antiparasitic became political. April 19, 2021. https://www.pharmaceutical-technology.com/features/ivermectin-covid-19-antiparasitic-political/
24. World Health Organization. WHO advises that ivermectin only be used to treat COVID-19 within clinical trials. March 31, 2021. https://www.who.int/news-room/feature-stories/detail/who-advises-that-ivermectin-only-be-used-to-treat-covid-19-within-clinical-trials
25. U.S. Food and Drug Administration. Why you should not use ivermectin to treat or prevent COVID-19. March 5, 2021. https://www.fda.gov/consumers/consumer-updates/why-you-should-not-use-ivermectin-treat-or-prevent-covid-19
26. Seymour CW, McCreary EK, Stegenga J. Sensible medicine-balancing intervention and inaction during the COVID-19 pandemic. JAMA. 2020;324(18):1827-1828. doi:10.1001/jama.2020.20271
27. Flanagin A, Fontanarosa PB, Bauchner H. Preprints involving medical research—do the benefits outweigh the challenges? JAMA. 2020;324(18):1840-1843. doi:10.1001/jama.2020.20674
28. Asch DA, Shells NE, Islam N, et al. Variation in US hospital mortality rates for patients admitted with COVID-19 during the first 6 months of the pandemic. JAMA Intern Med. 2021;181(4):471-478. doi:10.1001/jamainternmed.2020.8193
From the Department of Medicine (Drs. Meisenberg, Muganlinskaya, Sharma, Amjadi, Arnold, Barnes, Clance, Khalil, Miller, Mooradian, O’Connell, Patel, Press, Samaras, Shanmugam, Tavadze, and Thompson), Department of Pharmacy (Drs. Jiang, Jarawan, Sheth, and Trinh), Department of Nursing (Dr. Ohnmacht), and Department of Women and Children’s Services (Dr. Raji), Luminis Health, Annapolis, MD, and Lanham, MD.
Objective: The COVID-19 pandemic has been a challenge for hospital medical staffs worldwide due to high volumes of patients acutely ill with novel syndromes and prevailing uncertainty regarding optimum supportive and therapeutic interventions. Additionally, the response to this crisis was driven by a plethora of nontraditional information sources, such as email chains, websites, non–peer-reviewed preprints, and press releases. Care patterns became idiosyncratic and often incorporated unproven interventions driven by these nontraditional information sources. This report evaluates the efforts of a health system to create and empower a multidisciplinary committee to develop, implement, and monitor evidence-based, standardized protocols for patients with COVID-19.
Methods: This report describes the composition of the committee, its scope, and its important interactions with the health system pharmacy and therapeutics committee, research teams, and other work groups planning other aspects of COVID-19 management. It illustrates how the committee was used to demonstrate for trainees the process and value of critically examining evidence, even in a chaotic environment.
Results: Data show successful interventions in reducing excessive ordering of certain laboratory tests, reduction of nonrecommended therapies, and rapid uptake of evidence-based or guidelines-supported interventions.
Conclusions: A multidisciplinary committee dedicated solely to planning, implementing, and monitoring standard approaches that eventually became evidence-based decision-making led to an improved focus on treatment options and outcomes for COVID-19 patients. Data presented illustrate the attainable success that is both adaptable and suitable for similar emergencies in the future.
Keywords: COVID-19; clinical management; pharmacy and therapeutics; treatment; therapy.
The COVID-19 pandemic has spread to nearly all countries, carrying with it high morbidity, mortality, and severe impacts on both well-developed and less-well-developed health systems. Media reports of chaos within overwhelmed hospitals have been prominent.1,2 As of January 5, 2022, SARS-CoV-2 has infected more than 295 million people globally and directly caused the death of more than 5.4 million,3 though this number is likely an undercount even in countries with well-developed mortality tracking.4
Throughout the COVID-19 pandemic, hospital-based medical teams have been confronted with a flood of severely ill patients with novel syndromes. Initially, there were no standards for therapy or supportive care except for treatments borrowed from similar syndromes. In the setting of high volumes, high acuity, and public dismay, it is unsurprising that the usual deliberative methods for weighing evidence and initiating interventions were often pushed aside in favor of the solace of active intervention.5 In this milieu of limited evidence, there was a lamentable, if understandable, tendency to seek guidance from “nontraditional” sources,6 including email chains from colleagues, hospital websites, non–peer-reviewed manuscripts, advanced publication by medical journals,7 and nonscientific media presentations. In many localities, practitioners responded in idiosyncratic ways. For example, findings of high cytokine levels in COVID-19,8 along with reports of in-vitro antiviral activity with drugs like hydroxychloroquine against both SARS9 and SARS-CoV-2,10 drove laboratory test ordering and therapeutic interventions, respectively, carving shortcuts into the traditional clinical trial–dependent standards. Clinical trial results eventually emerged.11COVID-19 created a clinical dilemma for hospital medical staffs in terms of how to organize, standardize, and rapidly adapt to a flood of new information. In this report, we describe how 1 health system responded to these challenges by forming a COVID-19 Clinical Management Committee (CCMC) and empowering this interdisciplinary team to review evidence, create and adjust order sets, educate practitioners, oversee care, and collaborate across teams addressing other aspects of the COVID-19 response.
Program Overview
Health System Description
Luminis Health is a health system with 2 acute care hospitals that was formed in 2019 just before the start of the pandemic. Anne Arundel Medical Center (hospital A) is a 385-bed teaching hospital in Annapolis, MD. It has more than 23 000 discharges annually. Patients with COVID-19 were cared for by either an internal medicine teaching service or nonteaching hospitalist services on cohorted nursing units. Doctor’s Community Medical Center, in Lanham, MD (hospital B), is a 206-bed acute care hospital with more than 10 350 annual discharges. COVID-19 patients were cared for by hospitalist groups, initially in noncohorted units with transition to cohorted nursing units after a few months. The medical staffs are generally distinct, with different leadership structures, though the Luminis Health Department of Medicine has oversight responsibilities at both hospitals. More than 47 physicians attended COVID-19 patients at hospital A (with medical residents) and 30 individual physicians at hospital B, respectively, including intensivists. The nursing and pharmacy staffs are distinct, but there is a shared oversight Pharmacy and Therapeutics (P&T) Committee.
The 2 hospitals had distinct electronic medical records (EMR) until January 2021, when hospital B adopted the same EMR as hospital A (Epic).
Mission and Formation of CCMC
In order to coordinate the therapeutic approach across the health system, it was important for the CCMC to be designated by the health system P&T committee as an official subcommittee so that decisions on restrictions of medications and/or new or revised order sets could be rapidly initiated across the system without waiting for the subsequent P&T meetings. The full committee retained oversight of the CCMC. Some P&T members were also on the CCMC.
The committee reviewed new reports in medical journals and prepublication servers and consulted recommendations of professional societies, such as the National Institutes of Health (NIH) COVID-19 guidelines, Infectious Diseases Society of America, Society of Critical Care Medicine, and US Food and Drug Administration (FDA) Emergency Use Authorizations (EUA), among other sources.
Composition of the CCMC
Physician leaders from both hospitals in the following specialties were solicited for participation: critical care, epidemiology, hospital medicine (internal medicine), emergency medicine, infectious diseases, nephrology, women and children’s services, and medical informatics. Specialists in other areas, such as hematology, were invited for topic-specific discussions. Hospital pharmacists with different specialties and nursing leadership were essential contributors. The committee members were expected to use various communication channels to inform frontline clinicians of new care standards and the existence of new order sets, which were embedded in the EMR.
Clinical Research
An important connection for the CCMC was with theCOVID-19 clinical research team. Three members of the research team were also members of the CCMC. All new study proposals for therapeutics were discussed with the CCMC as they were being considered by the research team. In this way, feedback on the feasibility and acceptance of new study opportunities could be discussed with the CCMC. Occasionally, CCMC decisions impacted clinical research accrual strategies. For example, new data from randomized trials about tocilizumab1,2 demonstrated benefits in some subsets of patients and resulted in a recommendation for use by the NIH guideline committee in these populations.1 The CCMC quickly adopted this recommendation, which required a reprioritization of clinical research enrollment for studies testing other immune-modulating agents. This important dialogue was mediated within the CCMC.
Guideline Distribution, Reinforcement, and Platform for Feedback
New guidelines were disseminated to clinicians via daily brief patient huddles held on COVID units, with participation by nursing and pharmacy, and by weekly meetings with hospitalist leaders and frontline hospital physicians. Order sets and guidelines were maintained on the intranet. Adherence was reinforced by unit-based and central pharmacists. Order sets, including admission order sets, could be created only by designated informatics personnel, thus enforcing standardization. Feedback on the utility of the order sets was obtained during the weekly meetings or huddles, as described above. To ensure a sense of transparency, physicians who had interest in commenting on a particular therapy, or who wished to discuss a particular manuscript, news article, or website, were invited to attend CCMC meetings.
Scope of CCMC
In order to be effective and timely, we limited the scope of our work to the report to the inpatient therapeutic environment, allowing other committees to work on other aspects of the pandemic response. In addition to issuing guidance and creating order sets to direct clinical practice, the CCMC also monitored COVID-19 therapeutic shortages15,16 and advised on prioritization of such treatments as convalescent plasma, remdesivir (prioritization and duration of therapy, 5 vs 10 days), baricitinib, and tocilizumab, depending upon the location of the patient (critical care or not). The CCMC was not involved in the management of non–COVID-19 shortages brought about by supply chain deficiencies.
Table 1 shows some aspects of the health system pandemic-response planning and the committee workforce that undertook that work. Though many items were out of scope for the CCMC, members of the CCMC did participate in the planning work of these other committees and therefore stayed connected to this complementary work.

A Teaching Opportunity About Making Thoughtful Choices
Another important feature of the CCMC was the contributions of residents from both pharmacy and internal medicine. The purpose and operations of the committee were recognized as an opportunity to involve learners in a curriculum based on Kern’s 6-step approach.17 Though the problem identification and general needs assessment were easily defined, the targeted needs assessment, extracted from individual and group interviews with learners and the committee members, pointed at the need to learn how to assess and analyze a rapidly growing body of literature on several relevant clinical aspects of SARS-CoV-2 and COVID-19. To achieve goals and objectives, residents were assigned to present current literature on a particular intervention during a committee meeting, specifically commenting on the merit or deficiencies of the study design, the strength of the data, and applicability to the local context with a recommendation. Prior to the presentations, the residents worked with faculty to identify the best studies or systematic analyses with potential to alter current practices. We thus used the CCMC process as a teaching tool about evidence-based medicine and the dilemma of clinical equipoise. This was imperative, since trainees thrust into the COVID-19 response have often keenly observed a movement away from deliberative decision-making.18 Indeed, including residents in the process of deliberative responses to COVID-19 addresses a recent call to adjust medical education during COVID-19 to “adapt curriculum to current issues in real time.”19
Interventions and Therapies Considered
Table 2 shows the topics reviewed by the CCMC. By the time of the first meeting, nonstandardization of care was already a source of concern for clinicians. Dialogue often continued outside of the formal meetings. Many topics were considered more than once as new guidance developed, changes to EUAs occurred, and new data or new publicity arose.

Methods
The Human Protections Administrator determined that this work constituted “quality improvement, and not research” and was therefore exempt from institutional review board review.
Quantitative Analysis
All admitted patients from March 10, 2020, through April 20, 2021, were considered in the quantitative aspects of this report except as noted. Patients diagnosed with COVID-19 were identified by searching our internal data base using diagnostic codes. Patient admissions with the following diagnostic codes were included (prior to April 1, 2020): J12.89, J20.8, J40, J22, J98.8, J80, each with the additional code of B97.29. After April 1, 2020, the guideline for coding COVID-19 was U07.1.
Descriptive statistics were used to measure utilization rates of certain medications and laboratory tests of interest over time. These data were adjusted for number of unique admissions. In a few cases, not all data elements were available from both hospitals due to differences in the EMR.
Case fatality rate was calculated based upon whether the patient died or was admitted to inpatient hospice as a result of COVID-19. Four patients transferred out of hospital A and 18 transferred out of hospital B were censored from case-fatality-rate determination.
Figure 1 shows the number of admissions for each acute care hospital in the health system and the combined COVID-19 case-fatality rate over time.

Results
A total of 5955 consecutive COVID-19 patients admitted from March 10, 2020, through April 30, 2021, were analyzed. Patients with International Statistical Classification of Diseases, Tenth Revision codes J12.89. J20.8, J40, J22, J98.8, J80, each with the additional code of B97.29 (or the code UO7.1 after April 1, 2020), were included in the analysis. The median age of admitted patients was 65 years (range 19-91 years). Using the NIH classification system for severity,20 the distribution of severity during the first 24 hours after the time of hospital admission was as follows: asymptomatic/presymptomatic, 0.5%; mild illness, 5.3%; moderate illness, 37.1%; severe illness, 55.5%; and critical illness, 1.1%.
The impact of the CCMC can be estimated by looking at care patterns over time. Since the work of the CCMC was aimed at influencing and standardizing physician ordering and therapy choices through order set creation and other forms of oversight, we measured the use of the CCMC-approved order sets at both hospitals and the use of certain laboratory tests and therapies that the CCMC sought either to limit or increase. These counts were adjusted for number of unique COVID-19 admissions. But the limits of the case collection tool meant it also collected cases that were not eligible for some of the interventions. For example, COVID-19 admissions without hypoxemia would not have been eligible for remdesivir or glucocorticoids. When admitted, some patients were already on steroids for other medical indications and did not receive the prescribed dexamethasone dose that we measured in pharmacy databases. Similarly, a few patients were hospitalized for indications unrelated to COVID-19, such as surgery or childbirth, and were found to be SARS-CoV-2-positive on routine screening.
Figure 2 shows the utilization of CCMC-approved standard COVID-19 admission order sets as a proportion of all COVID-19 admissions over time. The trend reveals a modest increase in usage (R2 = 0.34), but these data do not reflect the progressive build of content into order sets over time. One of the goals of the order sets was to standardize and reduce the ordering of certain biomarkers: C-reactive protein, serum ferritin, and D-dimer, which were ordered frequently in many early patients. Orders for these 3 laboratory tests are combined and expressed as an average number of labs per COVID-19 admission in Figure 2. A downward trend, with an R2 value of 0.65, is suggestive of impact from the order sets, though other explanations are possible.

Medication guidance was also a goal of the CCMC, simultaneously discouraging poorly supported interventions and driving uptake of the recommended evidence-based interventions in appropriate patients. Figure 3 shows the utilization pattern for some drugs of interest over the course of the pandemic, specifically the proportion of patients receiving at least 1 dose of medication among all COVID-19 admissions by month. (Data for hospital B was excluded from this analysis because it did not include all admitted patients.)

Hydroxychloroquine, which enjoyed a wave of popularity early on during the pandemic, was a target of successful order stewardship through the CCMC. Use of hydroxychloroquine as a COVID-19 therapeutic option after the first 2 months of the pandemic stopped, and subsequent use at low levels likely represented continuation therapy for outpatients who took hydroxychloroquine for rheumatologic indications.
Dexamethasone, as used in the RECOVERY trial,21 had a swift uptake among physicians after it was incorporated into order sets and its use encouraged. Similarly, uptake was immediate for remdesivir when, in May 2020, preliminary reports showed at least some benefits, confirmed by later analysis,22 and it received an FDA EUA.
Our data also show successful stewardship of the interleukin-6 antagonist toclilizumab, which was discouraged early on by the CCMC due to lack of data or negative results. But in March 2021, with new studies releasing data12,13 and new recommendations14 for its use in some subsets of patients with COVID-19, this drug was encouraged in appropriate subsets. A new order set with qualifying indications was prepared by the CCMC and new educational efforts made to encourage its use in appropriate patients.
Ivermectin was nonformulary at the start of the pandemic. This drug enjoyed much publicity from media sources and was promoted by certain physicians and on websites,23 based on in-vitro activity against coronaviruses. Eventually, the World Health Organization24 and the FDA25 found it necessary to issue advisory statements to the public against its use outside of clinical trials. The CCMC had requests from physicians to incorporate ivermectin but declined to add it to the formulary and recommended not approving nonformulary requests due to lack of data. As a result, ivermectin was not used at either hospital.
Discussion
COVID-19 represents many challenges to health systems all over the world. For Luminis Health, the high volume of acutely ill patients with novel syndromes was a particular challenge for the hospital-based care teams. A flood of information from preprints, press releases, preliminary reports, and many other nontraditional sources made deliberative management decisions difficult for individual physicians. Much commentary has appeared around the phenomenon but with less practical advice about how to make day-to-day care decisions at a time of scientific uncertainty and intense pressure to intervene.26,27 The CCMC was designed to overcome the information management dilemma. The need to coordinate, standardize, and oversee care was necessary given the large number of physicians who cared for COVID-19 patients on inpatient services.
It should be noted that creating order sets and issuing guidance is necessary, but not sufficient, to achieve our goals of being updated and consistent. This is especially true with large cadres of health care workers attending COVID-19 patients. Guidelines and recommendations were reinforced by unit-based oversight and stewardship from pharmacy and other leaders who constituted the CCMC.
The reduction in COVID-19 mortality over time experienced in this health care system was not unique and cannot necessarily be attributed to standardization of care. Similar improvements in mortality have been reported at many US hospitals in aggregate.28 Many other factors, including changes in patient characteristics, may be responsible for reduction in mortality over time.
Throughout this report we have relied upon an implicit assumption that standardization of medical therapeutics is desirable and leads to better outcomes as compared with allowing unlimited empiricism by individual physicians, either consultants or hospitalists. Our program represents a single health system with 2 acute care hospitals located 25 miles apart and which thus were similarly impacted by the different phases of the pandemic. Generalizability to health systems either smaller or larger, or in different geographical areas, has not been established. Data limitations have already been discussed.
We did not measure user satisfaction with the program either from physicians or nurses. However, the high rate of compliance suggests general agreement with the content and process.
We cannot definitively ascribe reduction in utilization of some nonrecommended treatments and increased utilization of the recommended therapies to the work of the CCMC. Individual physicians may have made these adjustments on their own or under the influence of other sources.
Finally, it should be noted that the mission to rapidly respond to data from well-conducted trials might be thwarted by too rigid a process or a committee’s lack of a sense of urgency. Organizing a committee and then empowering it to act is no guarantee of success; commitment to the mission is.
Conclusion
COVID-19 represented a challenge to medical staffs everywhere, inundating them with high volumes of acutely ill patients presenting with unfamiliar syndromes. Initial responses were characterized by idiosyncratic management approaches based on nontraditional sources of opinion and influences.
This report describes how a complex medical system brought order and standardization through a deliberative, but urgent, multidisciplinary committee with responsibility for planning, implementing, and monitoring standard approaches that eventually became evidence based. The composition of the committee and its scope of influence, limited to inpatient management, were important elements of success, allowing for better focus on the many treatment decisions. The important connection between the management committee and the system P&T committee, the clinical research effort, and teaching programs in both medicine and pharmacy are offered as exemplars of coordination. The data presented show success in achieving standardized, guideline-directed care. The approach is adoptable and suitable for similar emergencies in the future.
Acknowledgments: The authors thank Gary Scabis, Kip Waite, John Moxley, Angela Clubb, and David Woodley for their assistance in gathering data. We express appreciation and admiration for all our colleagues at the bedside.
Corresponding author: Barry R. Meisenberg, MD, Department of Medicine, Luminis Health, 2001 Medical Pkwy, Annapolis, MD 21401; [email protected].
Financial disclosures: None.
From the Department of Medicine (Drs. Meisenberg, Muganlinskaya, Sharma, Amjadi, Arnold, Barnes, Clance, Khalil, Miller, Mooradian, O’Connell, Patel, Press, Samaras, Shanmugam, Tavadze, and Thompson), Department of Pharmacy (Drs. Jiang, Jarawan, Sheth, and Trinh), Department of Nursing (Dr. Ohnmacht), and Department of Women and Children’s Services (Dr. Raji), Luminis Health, Annapolis, MD, and Lanham, MD.
Objective: The COVID-19 pandemic has been a challenge for hospital medical staffs worldwide due to high volumes of patients acutely ill with novel syndromes and prevailing uncertainty regarding optimum supportive and therapeutic interventions. Additionally, the response to this crisis was driven by a plethora of nontraditional information sources, such as email chains, websites, non–peer-reviewed preprints, and press releases. Care patterns became idiosyncratic and often incorporated unproven interventions driven by these nontraditional information sources. This report evaluates the efforts of a health system to create and empower a multidisciplinary committee to develop, implement, and monitor evidence-based, standardized protocols for patients with COVID-19.
Methods: This report describes the composition of the committee, its scope, and its important interactions with the health system pharmacy and therapeutics committee, research teams, and other work groups planning other aspects of COVID-19 management. It illustrates how the committee was used to demonstrate for trainees the process and value of critically examining evidence, even in a chaotic environment.
Results: Data show successful interventions in reducing excessive ordering of certain laboratory tests, reduction of nonrecommended therapies, and rapid uptake of evidence-based or guidelines-supported interventions.
Conclusions: A multidisciplinary committee dedicated solely to planning, implementing, and monitoring standard approaches that eventually became evidence-based decision-making led to an improved focus on treatment options and outcomes for COVID-19 patients. Data presented illustrate the attainable success that is both adaptable and suitable for similar emergencies in the future.
Keywords: COVID-19; clinical management; pharmacy and therapeutics; treatment; therapy.
The COVID-19 pandemic has spread to nearly all countries, carrying with it high morbidity, mortality, and severe impacts on both well-developed and less-well-developed health systems. Media reports of chaos within overwhelmed hospitals have been prominent.1,2 As of January 5, 2022, SARS-CoV-2 has infected more than 295 million people globally and directly caused the death of more than 5.4 million,3 though this number is likely an undercount even in countries with well-developed mortality tracking.4
Throughout the COVID-19 pandemic, hospital-based medical teams have been confronted with a flood of severely ill patients with novel syndromes. Initially, there were no standards for therapy or supportive care except for treatments borrowed from similar syndromes. In the setting of high volumes, high acuity, and public dismay, it is unsurprising that the usual deliberative methods for weighing evidence and initiating interventions were often pushed aside in favor of the solace of active intervention.5 In this milieu of limited evidence, there was a lamentable, if understandable, tendency to seek guidance from “nontraditional” sources,6 including email chains from colleagues, hospital websites, non–peer-reviewed manuscripts, advanced publication by medical journals,7 and nonscientific media presentations. In many localities, practitioners responded in idiosyncratic ways. For example, findings of high cytokine levels in COVID-19,8 along with reports of in-vitro antiviral activity with drugs like hydroxychloroquine against both SARS9 and SARS-CoV-2,10 drove laboratory test ordering and therapeutic interventions, respectively, carving shortcuts into the traditional clinical trial–dependent standards. Clinical trial results eventually emerged.11COVID-19 created a clinical dilemma for hospital medical staffs in terms of how to organize, standardize, and rapidly adapt to a flood of new information. In this report, we describe how 1 health system responded to these challenges by forming a COVID-19 Clinical Management Committee (CCMC) and empowering this interdisciplinary team to review evidence, create and adjust order sets, educate practitioners, oversee care, and collaborate across teams addressing other aspects of the COVID-19 response.
Program Overview
Health System Description
Luminis Health is a health system with 2 acute care hospitals that was formed in 2019 just before the start of the pandemic. Anne Arundel Medical Center (hospital A) is a 385-bed teaching hospital in Annapolis, MD. It has more than 23 000 discharges annually. Patients with COVID-19 were cared for by either an internal medicine teaching service or nonteaching hospitalist services on cohorted nursing units. Doctor’s Community Medical Center, in Lanham, MD (hospital B), is a 206-bed acute care hospital with more than 10 350 annual discharges. COVID-19 patients were cared for by hospitalist groups, initially in noncohorted units with transition to cohorted nursing units after a few months. The medical staffs are generally distinct, with different leadership structures, though the Luminis Health Department of Medicine has oversight responsibilities at both hospitals. More than 47 physicians attended COVID-19 patients at hospital A (with medical residents) and 30 individual physicians at hospital B, respectively, including intensivists. The nursing and pharmacy staffs are distinct, but there is a shared oversight Pharmacy and Therapeutics (P&T) Committee.
The 2 hospitals had distinct electronic medical records (EMR) until January 2021, when hospital B adopted the same EMR as hospital A (Epic).
Mission and Formation of CCMC
In order to coordinate the therapeutic approach across the health system, it was important for the CCMC to be designated by the health system P&T committee as an official subcommittee so that decisions on restrictions of medications and/or new or revised order sets could be rapidly initiated across the system without waiting for the subsequent P&T meetings. The full committee retained oversight of the CCMC. Some P&T members were also on the CCMC.
The committee reviewed new reports in medical journals and prepublication servers and consulted recommendations of professional societies, such as the National Institutes of Health (NIH) COVID-19 guidelines, Infectious Diseases Society of America, Society of Critical Care Medicine, and US Food and Drug Administration (FDA) Emergency Use Authorizations (EUA), among other sources.
Composition of the CCMC
Physician leaders from both hospitals in the following specialties were solicited for participation: critical care, epidemiology, hospital medicine (internal medicine), emergency medicine, infectious diseases, nephrology, women and children’s services, and medical informatics. Specialists in other areas, such as hematology, were invited for topic-specific discussions. Hospital pharmacists with different specialties and nursing leadership were essential contributors. The committee members were expected to use various communication channels to inform frontline clinicians of new care standards and the existence of new order sets, which were embedded in the EMR.
Clinical Research
An important connection for the CCMC was with theCOVID-19 clinical research team. Three members of the research team were also members of the CCMC. All new study proposals for therapeutics were discussed with the CCMC as they were being considered by the research team. In this way, feedback on the feasibility and acceptance of new study opportunities could be discussed with the CCMC. Occasionally, CCMC decisions impacted clinical research accrual strategies. For example, new data from randomized trials about tocilizumab1,2 demonstrated benefits in some subsets of patients and resulted in a recommendation for use by the NIH guideline committee in these populations.1 The CCMC quickly adopted this recommendation, which required a reprioritization of clinical research enrollment for studies testing other immune-modulating agents. This important dialogue was mediated within the CCMC.
Guideline Distribution, Reinforcement, and Platform for Feedback
New guidelines were disseminated to clinicians via daily brief patient huddles held on COVID units, with participation by nursing and pharmacy, and by weekly meetings with hospitalist leaders and frontline hospital physicians. Order sets and guidelines were maintained on the intranet. Adherence was reinforced by unit-based and central pharmacists. Order sets, including admission order sets, could be created only by designated informatics personnel, thus enforcing standardization. Feedback on the utility of the order sets was obtained during the weekly meetings or huddles, as described above. To ensure a sense of transparency, physicians who had interest in commenting on a particular therapy, or who wished to discuss a particular manuscript, news article, or website, were invited to attend CCMC meetings.
Scope of CCMC
In order to be effective and timely, we limited the scope of our work to the report to the inpatient therapeutic environment, allowing other committees to work on other aspects of the pandemic response. In addition to issuing guidance and creating order sets to direct clinical practice, the CCMC also monitored COVID-19 therapeutic shortages15,16 and advised on prioritization of such treatments as convalescent plasma, remdesivir (prioritization and duration of therapy, 5 vs 10 days), baricitinib, and tocilizumab, depending upon the location of the patient (critical care or not). The CCMC was not involved in the management of non–COVID-19 shortages brought about by supply chain deficiencies.
Table 1 shows some aspects of the health system pandemic-response planning and the committee workforce that undertook that work. Though many items were out of scope for the CCMC, members of the CCMC did participate in the planning work of these other committees and therefore stayed connected to this complementary work.

A Teaching Opportunity About Making Thoughtful Choices
Another important feature of the CCMC was the contributions of residents from both pharmacy and internal medicine. The purpose and operations of the committee were recognized as an opportunity to involve learners in a curriculum based on Kern’s 6-step approach.17 Though the problem identification and general needs assessment were easily defined, the targeted needs assessment, extracted from individual and group interviews with learners and the committee members, pointed at the need to learn how to assess and analyze a rapidly growing body of literature on several relevant clinical aspects of SARS-CoV-2 and COVID-19. To achieve goals and objectives, residents were assigned to present current literature on a particular intervention during a committee meeting, specifically commenting on the merit or deficiencies of the study design, the strength of the data, and applicability to the local context with a recommendation. Prior to the presentations, the residents worked with faculty to identify the best studies or systematic analyses with potential to alter current practices. We thus used the CCMC process as a teaching tool about evidence-based medicine and the dilemma of clinical equipoise. This was imperative, since trainees thrust into the COVID-19 response have often keenly observed a movement away from deliberative decision-making.18 Indeed, including residents in the process of deliberative responses to COVID-19 addresses a recent call to adjust medical education during COVID-19 to “adapt curriculum to current issues in real time.”19
Interventions and Therapies Considered
Table 2 shows the topics reviewed by the CCMC. By the time of the first meeting, nonstandardization of care was already a source of concern for clinicians. Dialogue often continued outside of the formal meetings. Many topics were considered more than once as new guidance developed, changes to EUAs occurred, and new data or new publicity arose.

Methods
The Human Protections Administrator determined that this work constituted “quality improvement, and not research” and was therefore exempt from institutional review board review.
Quantitative Analysis
All admitted patients from March 10, 2020, through April 20, 2021, were considered in the quantitative aspects of this report except as noted. Patients diagnosed with COVID-19 were identified by searching our internal data base using diagnostic codes. Patient admissions with the following diagnostic codes were included (prior to April 1, 2020): J12.89, J20.8, J40, J22, J98.8, J80, each with the additional code of B97.29. After April 1, 2020, the guideline for coding COVID-19 was U07.1.
Descriptive statistics were used to measure utilization rates of certain medications and laboratory tests of interest over time. These data were adjusted for number of unique admissions. In a few cases, not all data elements were available from both hospitals due to differences in the EMR.
Case fatality rate was calculated based upon whether the patient died or was admitted to inpatient hospice as a result of COVID-19. Four patients transferred out of hospital A and 18 transferred out of hospital B were censored from case-fatality-rate determination.
Figure 1 shows the number of admissions for each acute care hospital in the health system and the combined COVID-19 case-fatality rate over time.

Results
A total of 5955 consecutive COVID-19 patients admitted from March 10, 2020, through April 30, 2021, were analyzed. Patients with International Statistical Classification of Diseases, Tenth Revision codes J12.89. J20.8, J40, J22, J98.8, J80, each with the additional code of B97.29 (or the code UO7.1 after April 1, 2020), were included in the analysis. The median age of admitted patients was 65 years (range 19-91 years). Using the NIH classification system for severity,20 the distribution of severity during the first 24 hours after the time of hospital admission was as follows: asymptomatic/presymptomatic, 0.5%; mild illness, 5.3%; moderate illness, 37.1%; severe illness, 55.5%; and critical illness, 1.1%.
The impact of the CCMC can be estimated by looking at care patterns over time. Since the work of the CCMC was aimed at influencing and standardizing physician ordering and therapy choices through order set creation and other forms of oversight, we measured the use of the CCMC-approved order sets at both hospitals and the use of certain laboratory tests and therapies that the CCMC sought either to limit or increase. These counts were adjusted for number of unique COVID-19 admissions. But the limits of the case collection tool meant it also collected cases that were not eligible for some of the interventions. For example, COVID-19 admissions without hypoxemia would not have been eligible for remdesivir or glucocorticoids. When admitted, some patients were already on steroids for other medical indications and did not receive the prescribed dexamethasone dose that we measured in pharmacy databases. Similarly, a few patients were hospitalized for indications unrelated to COVID-19, such as surgery or childbirth, and were found to be SARS-CoV-2-positive on routine screening.
Figure 2 shows the utilization of CCMC-approved standard COVID-19 admission order sets as a proportion of all COVID-19 admissions over time. The trend reveals a modest increase in usage (R2 = 0.34), but these data do not reflect the progressive build of content into order sets over time. One of the goals of the order sets was to standardize and reduce the ordering of certain biomarkers: C-reactive protein, serum ferritin, and D-dimer, which were ordered frequently in many early patients. Orders for these 3 laboratory tests are combined and expressed as an average number of labs per COVID-19 admission in Figure 2. A downward trend, with an R2 value of 0.65, is suggestive of impact from the order sets, though other explanations are possible.

Medication guidance was also a goal of the CCMC, simultaneously discouraging poorly supported interventions and driving uptake of the recommended evidence-based interventions in appropriate patients. Figure 3 shows the utilization pattern for some drugs of interest over the course of the pandemic, specifically the proportion of patients receiving at least 1 dose of medication among all COVID-19 admissions by month. (Data for hospital B was excluded from this analysis because it did not include all admitted patients.)

Hydroxychloroquine, which enjoyed a wave of popularity early on during the pandemic, was a target of successful order stewardship through the CCMC. Use of hydroxychloroquine as a COVID-19 therapeutic option after the first 2 months of the pandemic stopped, and subsequent use at low levels likely represented continuation therapy for outpatients who took hydroxychloroquine for rheumatologic indications.
Dexamethasone, as used in the RECOVERY trial,21 had a swift uptake among physicians after it was incorporated into order sets and its use encouraged. Similarly, uptake was immediate for remdesivir when, in May 2020, preliminary reports showed at least some benefits, confirmed by later analysis,22 and it received an FDA EUA.
Our data also show successful stewardship of the interleukin-6 antagonist toclilizumab, which was discouraged early on by the CCMC due to lack of data or negative results. But in March 2021, with new studies releasing data12,13 and new recommendations14 for its use in some subsets of patients with COVID-19, this drug was encouraged in appropriate subsets. A new order set with qualifying indications was prepared by the CCMC and new educational efforts made to encourage its use in appropriate patients.
Ivermectin was nonformulary at the start of the pandemic. This drug enjoyed much publicity from media sources and was promoted by certain physicians and on websites,23 based on in-vitro activity against coronaviruses. Eventually, the World Health Organization24 and the FDA25 found it necessary to issue advisory statements to the public against its use outside of clinical trials. The CCMC had requests from physicians to incorporate ivermectin but declined to add it to the formulary and recommended not approving nonformulary requests due to lack of data. As a result, ivermectin was not used at either hospital.
Discussion
COVID-19 represents many challenges to health systems all over the world. For Luminis Health, the high volume of acutely ill patients with novel syndromes was a particular challenge for the hospital-based care teams. A flood of information from preprints, press releases, preliminary reports, and many other nontraditional sources made deliberative management decisions difficult for individual physicians. Much commentary has appeared around the phenomenon but with less practical advice about how to make day-to-day care decisions at a time of scientific uncertainty and intense pressure to intervene.26,27 The CCMC was designed to overcome the information management dilemma. The need to coordinate, standardize, and oversee care was necessary given the large number of physicians who cared for COVID-19 patients on inpatient services.
It should be noted that creating order sets and issuing guidance is necessary, but not sufficient, to achieve our goals of being updated and consistent. This is especially true with large cadres of health care workers attending COVID-19 patients. Guidelines and recommendations were reinforced by unit-based oversight and stewardship from pharmacy and other leaders who constituted the CCMC.
The reduction in COVID-19 mortality over time experienced in this health care system was not unique and cannot necessarily be attributed to standardization of care. Similar improvements in mortality have been reported at many US hospitals in aggregate.28 Many other factors, including changes in patient characteristics, may be responsible for reduction in mortality over time.
Throughout this report we have relied upon an implicit assumption that standardization of medical therapeutics is desirable and leads to better outcomes as compared with allowing unlimited empiricism by individual physicians, either consultants or hospitalists. Our program represents a single health system with 2 acute care hospitals located 25 miles apart and which thus were similarly impacted by the different phases of the pandemic. Generalizability to health systems either smaller or larger, or in different geographical areas, has not been established. Data limitations have already been discussed.
We did not measure user satisfaction with the program either from physicians or nurses. However, the high rate of compliance suggests general agreement with the content and process.
We cannot definitively ascribe reduction in utilization of some nonrecommended treatments and increased utilization of the recommended therapies to the work of the CCMC. Individual physicians may have made these adjustments on their own or under the influence of other sources.
Finally, it should be noted that the mission to rapidly respond to data from well-conducted trials might be thwarted by too rigid a process or a committee’s lack of a sense of urgency. Organizing a committee and then empowering it to act is no guarantee of success; commitment to the mission is.
Conclusion
COVID-19 represented a challenge to medical staffs everywhere, inundating them with high volumes of acutely ill patients presenting with unfamiliar syndromes. Initial responses were characterized by idiosyncratic management approaches based on nontraditional sources of opinion and influences.
This report describes how a complex medical system brought order and standardization through a deliberative, but urgent, multidisciplinary committee with responsibility for planning, implementing, and monitoring standard approaches that eventually became evidence based. The composition of the committee and its scope of influence, limited to inpatient management, were important elements of success, allowing for better focus on the many treatment decisions. The important connection between the management committee and the system P&T committee, the clinical research effort, and teaching programs in both medicine and pharmacy are offered as exemplars of coordination. The data presented show success in achieving standardized, guideline-directed care. The approach is adoptable and suitable for similar emergencies in the future.
Acknowledgments: The authors thank Gary Scabis, Kip Waite, John Moxley, Angela Clubb, and David Woodley for their assistance in gathering data. We express appreciation and admiration for all our colleagues at the bedside.
Corresponding author: Barry R. Meisenberg, MD, Department of Medicine, Luminis Health, 2001 Medical Pkwy, Annapolis, MD 21401; [email protected].
Financial disclosures: None.
1. Gettleman J, Raj S, Kumar H. India’s health system cracks under the strain as coronavirus cases surge. The New York Times. April 22, 2021. https://www.nytimes.com/2021/04/21/world/asia/india-coronavirus-oxygen.html
2. Rappleye H, Lehren AW, Strickler L, Fitzpatrick S. ‘This system is doomed’: doctors, nurses sound off in NBC News coronavirus survey. NBC News. March 20, 2020. https://www.nbcnews.com/news/us-news/system-doomed-doctors-nurses-sound-nbc-news-coronavirus-survey-n1164841
3. Johns Hopkins Coronavirus Resource Center. Accessed January 5, 2022. https://coronavirus.jhu.edu/map.html
4. Fineberg HV. The toll of COVID-19. JAMA. 2020;324(15):1502-1503. doi:10.1001/jama.2020.20019
5. Meisenberg BR. Medical staffs response to COVID-19 ‘data’: have we misplaced our skeptic’s eye? Am J Med. 2021;134(2):151-152. doi:10.1016/j.amjmed.2020.09.013
6. McMahon JH, Lydeamore MH, Stewardson AJ. Bringing evidence from press release to the clinic in the era of COVID-19. J Antimicrob Chemother. 2021;76(3):547-549. doi:10.1093/jac/dkaa506
7. Rubin EJ, Baden LR, Morrissey S, Campion EW. Medical journals and the 2019-nCoV outbreak. N Engl J Med. 2020;382(9):866. doi:10.1056/NEJMe2001329
8. Liu F, Li L, Xu M, et al. Prognostic value of interleukin-6, C-reactive protein, and procalcitonin in patients with COVID-19. J Clin Virol. 2020;127:104370. doi:10.1016/j.jcv.2020.104370
9. Vincent MJ, Bergeron E, Benjannet S, et al. Chloroquine is a potent inhibitor of SARS coronavirus infection and spread. Virol J. 2005;2:69. doi:10.1186/1743-422X-2-69
10. Wang M, Cao R, Zhang L, et al. Remdesivir and chloroquine effectively inhibit the recently emerged novel coronavirus (2019-nCoV) in vitro. Cell Res. 2020;30:269-271. doi:10.1038/s41422-020-0282-0
11. RECOVERY Collaborative Group. Effect of hydroxychloroquine in hospitalized patients with Covid-19. N Engl J Med. 2020;383:2030-2040. doi:10.1056/NEJMoa2022926
12. RECOVERY Collaborative Group. Tocilizumab in patients admitted to hospital with COVID-19 (RECOVERY): preliminary results of a randomised, controlled, open-label, platform trial [preprint]. February 11, 2021. doi:10.1101/2021.02.11.21249258 https://www.medrxiv.org/content/10.1101/2021.02.11.21249258v1
13. REMAP-CAP Investigators. Interleukin-6 receptor antagonists in critically ill patients with COVID-19. N Engl J Med. 2021;384(16):1491-1502. doi:10.1056/NEJMoa2100433
14. National Institutes of Health. COVID-19 treatment guidelines: interleukin-6 inhibitors. https://www.covid19treatmentguidelines.nih.gov/immunomodulators/interleukin-6-inhibitors/
15. Deana C, Vetrugno L, Tonizzo A, et al. Drug supply during COVID-19 pandemic: remember not to run with your tank empty. Hosp Pharm. 2021;56(5):405-407. doi:10.1177/0018578720931749
16. Choe J, Crane M, Greene J, et al. The Pandemic and the Supply Chain: Addressing Gaps in Pharmaceutical Production and Distribution. Johns Hopkins University, November 2020. https://www.jhsph.edu/research/affiliated-programs/johns-hopkins-drug-access-and-affordability-initiative/publications/Pandemic_Supply_Chain.pdf
17. Kern DE. Overview: a six-step approach to curriculum development. In: Kern DE, Thornton PA, Hughes MT, eds. Curriculum Development for Medical Education: A Six-Step Approach. 3rd ed. Johns Hopkins University Press; 2016.
18. Rice TW, Janz DR. In defense of evidence-based medicine for the treatment of COVID-19 acute respiratory distress syndrome. Ann Am Thorac Soc. 2020;17(7):787-789. doi:10.1513/AnnalsATS.202004-325IP
19. Lucey CR, Johnston SC. The transformational effects of COVID-19 on medical education. JAMA. 2020;324(11):1033-1034. doi:10.1001/jama.2020.14136
20. National Institutes of Health. COVID-19 treatment guidelines: clinical spectrum of SARS-CoV-2 infection. https://www.covid19treatmentguidelines.nih.gov/overview/clinical-spectrum/
21. RECOVERY Collaborative Group. Dexamethasone in hospitalized patients with Covid-19. N Engl J Med. 2021;384:693-704. doi:10.1056/NEJMoa2021436
22. Beigel JH, Tomashek KM, Dodd LE, et al. Remdesivir for the treatment of Covid-19—final report. N Engl J Med. 2020;383:1813-1826. doi:10.1056/NEJMoa2007764
23. Jiminez D. Ivermectin and Covid-19: how a cheap antiparasitic became political. April 19, 2021. https://www.pharmaceutical-technology.com/features/ivermectin-covid-19-antiparasitic-political/
24. World Health Organization. WHO advises that ivermectin only be used to treat COVID-19 within clinical trials. March 31, 2021. https://www.who.int/news-room/feature-stories/detail/who-advises-that-ivermectin-only-be-used-to-treat-covid-19-within-clinical-trials
25. U.S. Food and Drug Administration. Why you should not use ivermectin to treat or prevent COVID-19. March 5, 2021. https://www.fda.gov/consumers/consumer-updates/why-you-should-not-use-ivermectin-treat-or-prevent-covid-19
26. Seymour CW, McCreary EK, Stegenga J. Sensible medicine-balancing intervention and inaction during the COVID-19 pandemic. JAMA. 2020;324(18):1827-1828. doi:10.1001/jama.2020.20271
27. Flanagin A, Fontanarosa PB, Bauchner H. Preprints involving medical research—do the benefits outweigh the challenges? JAMA. 2020;324(18):1840-1843. doi:10.1001/jama.2020.20674
28. Asch DA, Shells NE, Islam N, et al. Variation in US hospital mortality rates for patients admitted with COVID-19 during the first 6 months of the pandemic. JAMA Intern Med. 2021;181(4):471-478. doi:10.1001/jamainternmed.2020.8193
1. Gettleman J, Raj S, Kumar H. India’s health system cracks under the strain as coronavirus cases surge. The New York Times. April 22, 2021. https://www.nytimes.com/2021/04/21/world/asia/india-coronavirus-oxygen.html
2. Rappleye H, Lehren AW, Strickler L, Fitzpatrick S. ‘This system is doomed’: doctors, nurses sound off in NBC News coronavirus survey. NBC News. March 20, 2020. https://www.nbcnews.com/news/us-news/system-doomed-doctors-nurses-sound-nbc-news-coronavirus-survey-n1164841
3. Johns Hopkins Coronavirus Resource Center. Accessed January 5, 2022. https://coronavirus.jhu.edu/map.html
4. Fineberg HV. The toll of COVID-19. JAMA. 2020;324(15):1502-1503. doi:10.1001/jama.2020.20019
5. Meisenberg BR. Medical staffs response to COVID-19 ‘data’: have we misplaced our skeptic’s eye? Am J Med. 2021;134(2):151-152. doi:10.1016/j.amjmed.2020.09.013
6. McMahon JH, Lydeamore MH, Stewardson AJ. Bringing evidence from press release to the clinic in the era of COVID-19. J Antimicrob Chemother. 2021;76(3):547-549. doi:10.1093/jac/dkaa506
7. Rubin EJ, Baden LR, Morrissey S, Campion EW. Medical journals and the 2019-nCoV outbreak. N Engl J Med. 2020;382(9):866. doi:10.1056/NEJMe2001329
8. Liu F, Li L, Xu M, et al. Prognostic value of interleukin-6, C-reactive protein, and procalcitonin in patients with COVID-19. J Clin Virol. 2020;127:104370. doi:10.1016/j.jcv.2020.104370
9. Vincent MJ, Bergeron E, Benjannet S, et al. Chloroquine is a potent inhibitor of SARS coronavirus infection and spread. Virol J. 2005;2:69. doi:10.1186/1743-422X-2-69
10. Wang M, Cao R, Zhang L, et al. Remdesivir and chloroquine effectively inhibit the recently emerged novel coronavirus (2019-nCoV) in vitro. Cell Res. 2020;30:269-271. doi:10.1038/s41422-020-0282-0
11. RECOVERY Collaborative Group. Effect of hydroxychloroquine in hospitalized patients with Covid-19. N Engl J Med. 2020;383:2030-2040. doi:10.1056/NEJMoa2022926
12. RECOVERY Collaborative Group. Tocilizumab in patients admitted to hospital with COVID-19 (RECOVERY): preliminary results of a randomised, controlled, open-label, platform trial [preprint]. February 11, 2021. doi:10.1101/2021.02.11.21249258 https://www.medrxiv.org/content/10.1101/2021.02.11.21249258v1
13. REMAP-CAP Investigators. Interleukin-6 receptor antagonists in critically ill patients with COVID-19. N Engl J Med. 2021;384(16):1491-1502. doi:10.1056/NEJMoa2100433
14. National Institutes of Health. COVID-19 treatment guidelines: interleukin-6 inhibitors. https://www.covid19treatmentguidelines.nih.gov/immunomodulators/interleukin-6-inhibitors/
15. Deana C, Vetrugno L, Tonizzo A, et al. Drug supply during COVID-19 pandemic: remember not to run with your tank empty. Hosp Pharm. 2021;56(5):405-407. doi:10.1177/0018578720931749
16. Choe J, Crane M, Greene J, et al. The Pandemic and the Supply Chain: Addressing Gaps in Pharmaceutical Production and Distribution. Johns Hopkins University, November 2020. https://www.jhsph.edu/research/affiliated-programs/johns-hopkins-drug-access-and-affordability-initiative/publications/Pandemic_Supply_Chain.pdf
17. Kern DE. Overview: a six-step approach to curriculum development. In: Kern DE, Thornton PA, Hughes MT, eds. Curriculum Development for Medical Education: A Six-Step Approach. 3rd ed. Johns Hopkins University Press; 2016.
18. Rice TW, Janz DR. In defense of evidence-based medicine for the treatment of COVID-19 acute respiratory distress syndrome. Ann Am Thorac Soc. 2020;17(7):787-789. doi:10.1513/AnnalsATS.202004-325IP
19. Lucey CR, Johnston SC. The transformational effects of COVID-19 on medical education. JAMA. 2020;324(11):1033-1034. doi:10.1001/jama.2020.14136
20. National Institutes of Health. COVID-19 treatment guidelines: clinical spectrum of SARS-CoV-2 infection. https://www.covid19treatmentguidelines.nih.gov/overview/clinical-spectrum/
21. RECOVERY Collaborative Group. Dexamethasone in hospitalized patients with Covid-19. N Engl J Med. 2021;384:693-704. doi:10.1056/NEJMoa2021436
22. Beigel JH, Tomashek KM, Dodd LE, et al. Remdesivir for the treatment of Covid-19—final report. N Engl J Med. 2020;383:1813-1826. doi:10.1056/NEJMoa2007764
23. Jiminez D. Ivermectin and Covid-19: how a cheap antiparasitic became political. April 19, 2021. https://www.pharmaceutical-technology.com/features/ivermectin-covid-19-antiparasitic-political/
24. World Health Organization. WHO advises that ivermectin only be used to treat COVID-19 within clinical trials. March 31, 2021. https://www.who.int/news-room/feature-stories/detail/who-advises-that-ivermectin-only-be-used-to-treat-covid-19-within-clinical-trials
25. U.S. Food and Drug Administration. Why you should not use ivermectin to treat or prevent COVID-19. March 5, 2021. https://www.fda.gov/consumers/consumer-updates/why-you-should-not-use-ivermectin-treat-or-prevent-covid-19
26. Seymour CW, McCreary EK, Stegenga J. Sensible medicine-balancing intervention and inaction during the COVID-19 pandemic. JAMA. 2020;324(18):1827-1828. doi:10.1001/jama.2020.20271
27. Flanagin A, Fontanarosa PB, Bauchner H. Preprints involving medical research—do the benefits outweigh the challenges? JAMA. 2020;324(18):1840-1843. doi:10.1001/jama.2020.20674
28. Asch DA, Shells NE, Islam N, et al. Variation in US hospital mortality rates for patients admitted with COVID-19 during the first 6 months of the pandemic. JAMA Intern Med. 2021;181(4):471-478. doi:10.1001/jamainternmed.2020.8193
Neonatal sepsis: WHO-recommended Rx needs a major rethink
First-line treatment of neonatal sepsis in low- and middle-income countries (LMICs) with ampicillin-gentamicin – as recommended by the World Health Organization – needs to be reassessed, a retrospective, observational cohort study suggests. Rates of resistance to this particular antibiotic combination are extremely high in LMICs, and this treatment is unlikely to save many neonatal patients, according to the study’s results.
“The WHO guidelines are over 10 years old, and they are actually based on high-income country data, whereas data reported from low-income countries are reported by private labs, and they do not cater to the lower socioeconomic groups within these countries, which is important data to capture,” Timothy Walsh, MD, University of Oxford, United Kingdom, told this news organization.
“The main take-home message from our data is that ampicillin-gentamicin doesn’t work for most of the Gram-negative isolates we tested, and while there are alternatives, their use is confounded by [a lack of] financial support,” he added.
The study was published online in The Lancet Infectious Diseases.
BARNARDS study
In this substudy of the Burden of Antibiotic Resistance in Neonates from Developing Societies (BARNARDS) study, investigators focused on the effectiveness of antibiotic therapies after taking into account the high prevalence of pathogen resistance to ampicillin-gentamicin. Participating countries included Bangladesh, Ethiopia, India, Nigeria, Pakistan, Rwanda, and South Africa.
“Blood samples were obtained from neonates presenting with clinical signs of sepsis,” the authors note, “and WGS [whole-genome sequencing] and MICs [minimum inhibitory concentrations] for antibiotic treatment were determined for bacterial isolates from culture-confirmed sepsis.” Between Nov. 2015 and Feb. 2018, 36,285 neonates were enrolled into the main BARNARDS study, of whom 9,874 had clinically diagnosed sepsis and 5,749 had antibiotic data.
A total of 2,483 neonates had culture-confirmed sepsis, and WGS data were available for 457 isolates taken from 442 neonates. Slightly over three-quarters of the 5,749 neonates who had antibiotic data received first-line ampicillin-gentamicin. The other three most commonly prescribed antibiotic combinations were ceftazidime-amikacin, piperacillin-tazobactam-amikacin, and amoxicillin-clavulanate-amikacin.
Neonates treated with ceftazidime-amikacin had a 68% lower reported mortality than those treated with ampicillin-gentamicin at an adjusted hazard ratio of 0.32 (95% confidence interval, 0.14-0.72; P = .006), the investigators report. In contrast, no significant differences in mortality rates were reported for neonates treated with amoxicillin-clavulanate-amikacin or piperacillin-tazobactam-amikacin compared to those treated with ampicillin-gentamicin.
Investigators were careful to suggest that mortality effects associated with the different antibiotic combinations might have been confounded by either country-specific effects or underreporting of mortality, as a large proportion of neonates who were treated with ampicillin-gentamicin were followed for fewer than 10 days. However, in an unreported aspect of the same study, neonatal mortality from sepsis dropped by over 50% in two federally funded sites in Nigeria that changed their treatment from the WHO-recommended ampicillin-gentamicin regimen to ceftazidime-amikacin – which Dr. Walsh suggested was an endorsement of ceftazidime-amikacin over ampicillin-gentamicin if ever there was one.
Gram-negative resistance
In looking at resistance patterns to the antibiotic combinations used in these countries, investigators found that almost all Gram-negative isolates tested were “overwhelmingly resistant” to ampicillin, and over 70% of them were resistant to gentamicin as well. Extremely high resistance rates were also found against Staphylococcus spp, which are regarded as intrinsically resistant to ampicillin, rendering it basically useless in this particular treatment setting.
Amikacin had much lower level of resistance, with only about 26% of Gram-negative isolates showing resistance. In terms of coverage against Gram-negative isolates, the lowest level of coverage was provided by ampicillin-gentamicin at slightly over 28%, compared with about 73% for amoxicillin-clavulanate-amikacin, 77% for ceftazidime-amikacin, and 80% for piperacillin-tazobactam-amikacin.
In contrast, “Gram-positive isolates generally had reduced levels of resistance,” the authors state. As Dr. Walsh noted, the consortium also did an analysis assessing how much the antibiotic combinations cost and how much payment was deferred to the parents. For example, in Nigeria, the entire cost of treatment is passed down to the parents, “so if they are earning, say, $5.00 a day and the infant needs ceftazidime-amikacin, where the cost per dose is about $6.00 or $7.00 a day, parents can’t afford it,” Dr. Walsh observed.
This part of the conversation, he added, tends to get lost in many studies of antibiotic resistance in LMICs, which is a critical omission, because in many instances, the choice of treatment does come down to affordability. “It’s all very well for the WHO to sit there and say, ampicillin-gentamicin is perfect, but the combination actually doesn’t work in over 70% of the Gram-negative bacteria we looked at in these countries,” Dr. Walsh emphasized.
“The fact is that we have to be a lot more internationally engaged as to what’s actually happening in poorer populations, because unless we do, neonates are going to continue to die,” he said.
Editorial commentary
Commenting on the findings, lead editorialist Luregn Schlapbach, MD, PhD, of University Children’s Hospital Zurich, Switzerland, pointed out that the study has a number of limitations, including a high rate of dropouts from follow-up. This could possibly result in underestimation of neonatal mortality as well as country-specific biases. Nevertheless, Dr. Schlapbach feels that the integration of sequential clinical, genomic, microbiologic, drug, and cost data across a large network in LMIC settings is “exceptional” and will serve to inform “urgently needed” clinical trials in the field of neonatal sepsis.
“At present, increasing global antibiotic resistance is threatening progress against neonatal sepsis, prompting urgency to develop improved measures to effectively prevent and treat life-threatening infections in this high-risk group,” Dr. Schlapbach and colleagues write.
“The findings from the BARNARDS study call for randomized trials comparing mortality benefit and cost efficiency of different antibiotic combinations and management algorithms to safely reduce unnecessary antibiotic exposure for neonatal sepsis,” the editorialists concluded.
The authors and editorialists have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
First-line treatment of neonatal sepsis in low- and middle-income countries (LMICs) with ampicillin-gentamicin – as recommended by the World Health Organization – needs to be reassessed, a retrospective, observational cohort study suggests. Rates of resistance to this particular antibiotic combination are extremely high in LMICs, and this treatment is unlikely to save many neonatal patients, according to the study’s results.
“The WHO guidelines are over 10 years old, and they are actually based on high-income country data, whereas data reported from low-income countries are reported by private labs, and they do not cater to the lower socioeconomic groups within these countries, which is important data to capture,” Timothy Walsh, MD, University of Oxford, United Kingdom, told this news organization.
“The main take-home message from our data is that ampicillin-gentamicin doesn’t work for most of the Gram-negative isolates we tested, and while there are alternatives, their use is confounded by [a lack of] financial support,” he added.
The study was published online in The Lancet Infectious Diseases.
BARNARDS study
In this substudy of the Burden of Antibiotic Resistance in Neonates from Developing Societies (BARNARDS) study, investigators focused on the effectiveness of antibiotic therapies after taking into account the high prevalence of pathogen resistance to ampicillin-gentamicin. Participating countries included Bangladesh, Ethiopia, India, Nigeria, Pakistan, Rwanda, and South Africa.
“Blood samples were obtained from neonates presenting with clinical signs of sepsis,” the authors note, “and WGS [whole-genome sequencing] and MICs [minimum inhibitory concentrations] for antibiotic treatment were determined for bacterial isolates from culture-confirmed sepsis.” Between Nov. 2015 and Feb. 2018, 36,285 neonates were enrolled into the main BARNARDS study, of whom 9,874 had clinically diagnosed sepsis and 5,749 had antibiotic data.
A total of 2,483 neonates had culture-confirmed sepsis, and WGS data were available for 457 isolates taken from 442 neonates. Slightly over three-quarters of the 5,749 neonates who had antibiotic data received first-line ampicillin-gentamicin. The other three most commonly prescribed antibiotic combinations were ceftazidime-amikacin, piperacillin-tazobactam-amikacin, and amoxicillin-clavulanate-amikacin.
Neonates treated with ceftazidime-amikacin had a 68% lower reported mortality than those treated with ampicillin-gentamicin at an adjusted hazard ratio of 0.32 (95% confidence interval, 0.14-0.72; P = .006), the investigators report. In contrast, no significant differences in mortality rates were reported for neonates treated with amoxicillin-clavulanate-amikacin or piperacillin-tazobactam-amikacin compared to those treated with ampicillin-gentamicin.
Investigators were careful to suggest that mortality effects associated with the different antibiotic combinations might have been confounded by either country-specific effects or underreporting of mortality, as a large proportion of neonates who were treated with ampicillin-gentamicin were followed for fewer than 10 days. However, in an unreported aspect of the same study, neonatal mortality from sepsis dropped by over 50% in two federally funded sites in Nigeria that changed their treatment from the WHO-recommended ampicillin-gentamicin regimen to ceftazidime-amikacin – which Dr. Walsh suggested was an endorsement of ceftazidime-amikacin over ampicillin-gentamicin if ever there was one.
Gram-negative resistance
In looking at resistance patterns to the antibiotic combinations used in these countries, investigators found that almost all Gram-negative isolates tested were “overwhelmingly resistant” to ampicillin, and over 70% of them were resistant to gentamicin as well. Extremely high resistance rates were also found against Staphylococcus spp, which are regarded as intrinsically resistant to ampicillin, rendering it basically useless in this particular treatment setting.
Amikacin had much lower level of resistance, with only about 26% of Gram-negative isolates showing resistance. In terms of coverage against Gram-negative isolates, the lowest level of coverage was provided by ampicillin-gentamicin at slightly over 28%, compared with about 73% for amoxicillin-clavulanate-amikacin, 77% for ceftazidime-amikacin, and 80% for piperacillin-tazobactam-amikacin.
In contrast, “Gram-positive isolates generally had reduced levels of resistance,” the authors state. As Dr. Walsh noted, the consortium also did an analysis assessing how much the antibiotic combinations cost and how much payment was deferred to the parents. For example, in Nigeria, the entire cost of treatment is passed down to the parents, “so if they are earning, say, $5.00 a day and the infant needs ceftazidime-amikacin, where the cost per dose is about $6.00 or $7.00 a day, parents can’t afford it,” Dr. Walsh observed.
This part of the conversation, he added, tends to get lost in many studies of antibiotic resistance in LMICs, which is a critical omission, because in many instances, the choice of treatment does come down to affordability. “It’s all very well for the WHO to sit there and say, ampicillin-gentamicin is perfect, but the combination actually doesn’t work in over 70% of the Gram-negative bacteria we looked at in these countries,” Dr. Walsh emphasized.
“The fact is that we have to be a lot more internationally engaged as to what’s actually happening in poorer populations, because unless we do, neonates are going to continue to die,” he said.
Editorial commentary
Commenting on the findings, lead editorialist Luregn Schlapbach, MD, PhD, of University Children’s Hospital Zurich, Switzerland, pointed out that the study has a number of limitations, including a high rate of dropouts from follow-up. This could possibly result in underestimation of neonatal mortality as well as country-specific biases. Nevertheless, Dr. Schlapbach feels that the integration of sequential clinical, genomic, microbiologic, drug, and cost data across a large network in LMIC settings is “exceptional” and will serve to inform “urgently needed” clinical trials in the field of neonatal sepsis.
“At present, increasing global antibiotic resistance is threatening progress against neonatal sepsis, prompting urgency to develop improved measures to effectively prevent and treat life-threatening infections in this high-risk group,” Dr. Schlapbach and colleagues write.
“The findings from the BARNARDS study call for randomized trials comparing mortality benefit and cost efficiency of different antibiotic combinations and management algorithms to safely reduce unnecessary antibiotic exposure for neonatal sepsis,” the editorialists concluded.
The authors and editorialists have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
First-line treatment of neonatal sepsis in low- and middle-income countries (LMICs) with ampicillin-gentamicin – as recommended by the World Health Organization – needs to be reassessed, a retrospective, observational cohort study suggests. Rates of resistance to this particular antibiotic combination are extremely high in LMICs, and this treatment is unlikely to save many neonatal patients, according to the study’s results.
“The WHO guidelines are over 10 years old, and they are actually based on high-income country data, whereas data reported from low-income countries are reported by private labs, and they do not cater to the lower socioeconomic groups within these countries, which is important data to capture,” Timothy Walsh, MD, University of Oxford, United Kingdom, told this news organization.
“The main take-home message from our data is that ampicillin-gentamicin doesn’t work for most of the Gram-negative isolates we tested, and while there are alternatives, their use is confounded by [a lack of] financial support,” he added.
The study was published online in The Lancet Infectious Diseases.
BARNARDS study
In this substudy of the Burden of Antibiotic Resistance in Neonates from Developing Societies (BARNARDS) study, investigators focused on the effectiveness of antibiotic therapies after taking into account the high prevalence of pathogen resistance to ampicillin-gentamicin. Participating countries included Bangladesh, Ethiopia, India, Nigeria, Pakistan, Rwanda, and South Africa.
“Blood samples were obtained from neonates presenting with clinical signs of sepsis,” the authors note, “and WGS [whole-genome sequencing] and MICs [minimum inhibitory concentrations] for antibiotic treatment were determined for bacterial isolates from culture-confirmed sepsis.” Between Nov. 2015 and Feb. 2018, 36,285 neonates were enrolled into the main BARNARDS study, of whom 9,874 had clinically diagnosed sepsis and 5,749 had antibiotic data.
A total of 2,483 neonates had culture-confirmed sepsis, and WGS data were available for 457 isolates taken from 442 neonates. Slightly over three-quarters of the 5,749 neonates who had antibiotic data received first-line ampicillin-gentamicin. The other three most commonly prescribed antibiotic combinations were ceftazidime-amikacin, piperacillin-tazobactam-amikacin, and amoxicillin-clavulanate-amikacin.
Neonates treated with ceftazidime-amikacin had a 68% lower reported mortality than those treated with ampicillin-gentamicin at an adjusted hazard ratio of 0.32 (95% confidence interval, 0.14-0.72; P = .006), the investigators report. In contrast, no significant differences in mortality rates were reported for neonates treated with amoxicillin-clavulanate-amikacin or piperacillin-tazobactam-amikacin compared to those treated with ampicillin-gentamicin.
Investigators were careful to suggest that mortality effects associated with the different antibiotic combinations might have been confounded by either country-specific effects or underreporting of mortality, as a large proportion of neonates who were treated with ampicillin-gentamicin were followed for fewer than 10 days. However, in an unreported aspect of the same study, neonatal mortality from sepsis dropped by over 50% in two federally funded sites in Nigeria that changed their treatment from the WHO-recommended ampicillin-gentamicin regimen to ceftazidime-amikacin – which Dr. Walsh suggested was an endorsement of ceftazidime-amikacin over ampicillin-gentamicin if ever there was one.
Gram-negative resistance
In looking at resistance patterns to the antibiotic combinations used in these countries, investigators found that almost all Gram-negative isolates tested were “overwhelmingly resistant” to ampicillin, and over 70% of them were resistant to gentamicin as well. Extremely high resistance rates were also found against Staphylococcus spp, which are regarded as intrinsically resistant to ampicillin, rendering it basically useless in this particular treatment setting.
Amikacin had much lower level of resistance, with only about 26% of Gram-negative isolates showing resistance. In terms of coverage against Gram-negative isolates, the lowest level of coverage was provided by ampicillin-gentamicin at slightly over 28%, compared with about 73% for amoxicillin-clavulanate-amikacin, 77% for ceftazidime-amikacin, and 80% for piperacillin-tazobactam-amikacin.
In contrast, “Gram-positive isolates generally had reduced levels of resistance,” the authors state. As Dr. Walsh noted, the consortium also did an analysis assessing how much the antibiotic combinations cost and how much payment was deferred to the parents. For example, in Nigeria, the entire cost of treatment is passed down to the parents, “so if they are earning, say, $5.00 a day and the infant needs ceftazidime-amikacin, where the cost per dose is about $6.00 or $7.00 a day, parents can’t afford it,” Dr. Walsh observed.
This part of the conversation, he added, tends to get lost in many studies of antibiotic resistance in LMICs, which is a critical omission, because in many instances, the choice of treatment does come down to affordability. “It’s all very well for the WHO to sit there and say, ampicillin-gentamicin is perfect, but the combination actually doesn’t work in over 70% of the Gram-negative bacteria we looked at in these countries,” Dr. Walsh emphasized.
“The fact is that we have to be a lot more internationally engaged as to what’s actually happening in poorer populations, because unless we do, neonates are going to continue to die,” he said.
Editorial commentary
Commenting on the findings, lead editorialist Luregn Schlapbach, MD, PhD, of University Children’s Hospital Zurich, Switzerland, pointed out that the study has a number of limitations, including a high rate of dropouts from follow-up. This could possibly result in underestimation of neonatal mortality as well as country-specific biases. Nevertheless, Dr. Schlapbach feels that the integration of sequential clinical, genomic, microbiologic, drug, and cost data across a large network in LMIC settings is “exceptional” and will serve to inform “urgently needed” clinical trials in the field of neonatal sepsis.
“At present, increasing global antibiotic resistance is threatening progress against neonatal sepsis, prompting urgency to develop improved measures to effectively prevent and treat life-threatening infections in this high-risk group,” Dr. Schlapbach and colleagues write.
“The findings from the BARNARDS study call for randomized trials comparing mortality benefit and cost efficiency of different antibiotic combinations and management algorithms to safely reduce unnecessary antibiotic exposure for neonatal sepsis,” the editorialists concluded.
The authors and editorialists have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.

