User login
Evaluation for Compromise of the Tank
Acute Onset of Severe Abdominal Pain
Evaluation for Leakiness of the Tank
In Vivo Micron-Scale Arthroscopic Imaging of Human Knee Osteoarthritis With Optical Coherence Tomography: Comparison With Magnetic Resonance Imaging and Arthroscopy
Gadolinium Warnings Reduce NSF
GAITHERSBURG, MD. — Black box warnings added to the labels of all gadolinium-based MRI contrast agents have reduced the number of reported nephrogenic systemic fibrosis events to almost none in the last year, according to Dr. James Kaiser.
“The numbers of new events have tapered dramatically, probably due to public awareness of the association of NSF [nephrogenic systemic fibrosis] with GBCA [gadolinium-based contrast agent] administration,” he said at a joint meeting of the Food and Drug Administration's Cardiovascular and Renal Drugs and Drug Safety and Risk Management advisory committees. Event dates are either the date of administration of contrast or the date of diagnosis of NSF.
The FDA began receiving reports of NSF possibly being linked to gadolinium-based contrast agents in 2006 when 194 event dates were reported.
This “probably reflects awareness of the medical community of the potential connection between GBCA administration and NSF and changes in radiologic practice,” said Dr. Kaiser of the FDA's office of surveillance and epidemiology. There were 128 reported events in 2007, 55 in 2008, and 6 in 2009 (through September).
In 2007, the FDA asked manufacturers to include a boxed warning on the product labels of all gadolinium-based contrast agents.
Five gadolinium-based contrast agents have been approved for use in the United States: Magnevist (gadopentetate dimeglumine), Omniscan (gadodiamide), OptiMARK (gadoversetamide), MultiHance (gadobenate dimeglumine) and ProHance (gadoteridol).
As of September 2009, 382 reports of NSF had been associated with Omniscan (GE HealthCare), 195 with Magnevist (Bayer HealthCare), 35 with OptiMARK (Covidien), 1 with MultiHance (Bracco Diagnostics), and 0 with ProHance (Bracco Diagnostics).
The FDA asked the committees to consider whether warning labels should continue to be grouped together as a class or if there was adequate evidence to single out agents that increase NSF risk.
“The majority of the group feels that at least two of the agents appear to be different from the other agents,” said Dr. Robert A. Harrington, who chairs the Cardiovascular and Renal Drugs Advisory Committee. The majority recommended the use of Omniscan and OptiMARK be contraindicated in patients with severe kidney dysfunction. However, there was uncertainty as to how to define severe kidney dysfunction.
There was less consensus on whether a third agent, Magnevist, might also warrant contraindication language.
GAITHERSBURG, MD. — Black box warnings added to the labels of all gadolinium-based MRI contrast agents have reduced the number of reported nephrogenic systemic fibrosis events to almost none in the last year, according to Dr. James Kaiser.
“The numbers of new events have tapered dramatically, probably due to public awareness of the association of NSF [nephrogenic systemic fibrosis] with GBCA [gadolinium-based contrast agent] administration,” he said at a joint meeting of the Food and Drug Administration's Cardiovascular and Renal Drugs and Drug Safety and Risk Management advisory committees. Event dates are either the date of administration of contrast or the date of diagnosis of NSF.
The FDA began receiving reports of NSF possibly being linked to gadolinium-based contrast agents in 2006 when 194 event dates were reported.
This “probably reflects awareness of the medical community of the potential connection between GBCA administration and NSF and changes in radiologic practice,” said Dr. Kaiser of the FDA's office of surveillance and epidemiology. There were 128 reported events in 2007, 55 in 2008, and 6 in 2009 (through September).
In 2007, the FDA asked manufacturers to include a boxed warning on the product labels of all gadolinium-based contrast agents.
Five gadolinium-based contrast agents have been approved for use in the United States: Magnevist (gadopentetate dimeglumine), Omniscan (gadodiamide), OptiMARK (gadoversetamide), MultiHance (gadobenate dimeglumine) and ProHance (gadoteridol).
As of September 2009, 382 reports of NSF had been associated with Omniscan (GE HealthCare), 195 with Magnevist (Bayer HealthCare), 35 with OptiMARK (Covidien), 1 with MultiHance (Bracco Diagnostics), and 0 with ProHance (Bracco Diagnostics).
The FDA asked the committees to consider whether warning labels should continue to be grouped together as a class or if there was adequate evidence to single out agents that increase NSF risk.
“The majority of the group feels that at least two of the agents appear to be different from the other agents,” said Dr. Robert A. Harrington, who chairs the Cardiovascular and Renal Drugs Advisory Committee. The majority recommended the use of Omniscan and OptiMARK be contraindicated in patients with severe kidney dysfunction. However, there was uncertainty as to how to define severe kidney dysfunction.
There was less consensus on whether a third agent, Magnevist, might also warrant contraindication language.
GAITHERSBURG, MD. — Black box warnings added to the labels of all gadolinium-based MRI contrast agents have reduced the number of reported nephrogenic systemic fibrosis events to almost none in the last year, according to Dr. James Kaiser.
“The numbers of new events have tapered dramatically, probably due to public awareness of the association of NSF [nephrogenic systemic fibrosis] with GBCA [gadolinium-based contrast agent] administration,” he said at a joint meeting of the Food and Drug Administration's Cardiovascular and Renal Drugs and Drug Safety and Risk Management advisory committees. Event dates are either the date of administration of contrast or the date of diagnosis of NSF.
The FDA began receiving reports of NSF possibly being linked to gadolinium-based contrast agents in 2006 when 194 event dates were reported.
This “probably reflects awareness of the medical community of the potential connection between GBCA administration and NSF and changes in radiologic practice,” said Dr. Kaiser of the FDA's office of surveillance and epidemiology. There were 128 reported events in 2007, 55 in 2008, and 6 in 2009 (through September).
In 2007, the FDA asked manufacturers to include a boxed warning on the product labels of all gadolinium-based contrast agents.
Five gadolinium-based contrast agents have been approved for use in the United States: Magnevist (gadopentetate dimeglumine), Omniscan (gadodiamide), OptiMARK (gadoversetamide), MultiHance (gadobenate dimeglumine) and ProHance (gadoteridol).
As of September 2009, 382 reports of NSF had been associated with Omniscan (GE HealthCare), 195 with Magnevist (Bayer HealthCare), 35 with OptiMARK (Covidien), 1 with MultiHance (Bracco Diagnostics), and 0 with ProHance (Bracco Diagnostics).
The FDA asked the committees to consider whether warning labels should continue to be grouped together as a class or if there was adequate evidence to single out agents that increase NSF risk.
“The majority of the group feels that at least two of the agents appear to be different from the other agents,” said Dr. Robert A. Harrington, who chairs the Cardiovascular and Renal Drugs Advisory Committee. The majority recommended the use of Omniscan and OptiMARK be contraindicated in patients with severe kidney dysfunction. However, there was uncertainty as to how to define severe kidney dysfunction.
There was less consensus on whether a third agent, Magnevist, might also warrant contraindication language.
CTA Is Tops for Evaluating Chest Pain in ED
SNOWMASS, COLO. — CT angiography has rapidly emerged as the most cost-effective imaging technique to exclude acute coronary syndrome in the emergency department.
The overall diagnostic accuracy of CT angiography (CTA) is essentially equivalent to that of SPECT myocardial perfusion imaging, its main competition. But CTA is the clear winner in terms of time to diagnosis and cost, Dr. Christopher M. Kramer said at a conference sponsored by the American College of Cardiology.
Chest pain accounts for more than 6 million ED visits annually, resulting in 1.24 million admissions for unstable angina/non–ST-elevation MI and another 330,000 for STEMI. Emergency physicians are eager for new ways to rapidly and reliably rule out acute coronary syndrome—and CTA has a lot to offer in this regard, said Dr. Kramer, professor of medicine and radiology and director of the cardiovascular imaging center at the University of Virginia, Charlottesville.
In the recent 16-center CT-STAT trial, now in press, 701 low-risk patients with chest pain and a nondiagnostic ECG in the ED were randomized to CTA or the hospital's standard protocol, which typically included serial biomarkers along with SPECT myocardial perfusion imaging (MPI).
Time to diagnosis averaged 6.3 hours in the group who received the standard work-up compared to 2.9 hours with CTA, a 53% reduction. Median costs to obtain a diagnosis were $3,158 with the standard protocol and $2,137 with CTA, a 38% reduction.
ACS was diagnosed in roughly 3% of patients in each study arm, and invasive coronary angiography was performed in 5%. No significant stenosis was found in 82% of patients who underwent CTA and 90% who had MPI.
The 6-month major adverse cardiac event rates in this low-risk CT-STAT population were 0.3% in the standard management group and 0.0% in the CTA arm.
SPECT MPI is at present slightly more widely available in EDs around the country than is CTA. The other two up and coming imaging methods with utility in the setting of chest pain and nondiagnostic ECG in the ED—cardiac MRI and contrast echocardiography perfusion—have only limited availability. In terms of accuracy, they're similar to MPI and CTA, said Dr. Kramer.
A recent pooled analysis of MPI in ED evaluation of chest pain that included nine studies and nearly 1,900 patients showed the imaging method had 98% sensitivity and 73% specificity for identifying acute coronary syndrome. On the basis of its ability to reduce hospital admissions, it has been estimated that MPI reduces hospital costs by about $800 per patient.
Dr. Kramer serves as a consultant to Siemens Medical Solutions and is the recipient of research grants from Astellas and GlaxoSmithKline.
CTA is the clear winner in terms of time to diagnosis and cost in the emergency department.
Source Dr. Kramer
SNOWMASS, COLO. — CT angiography has rapidly emerged as the most cost-effective imaging technique to exclude acute coronary syndrome in the emergency department.
The overall diagnostic accuracy of CT angiography (CTA) is essentially equivalent to that of SPECT myocardial perfusion imaging, its main competition. But CTA is the clear winner in terms of time to diagnosis and cost, Dr. Christopher M. Kramer said at a conference sponsored by the American College of Cardiology.
Chest pain accounts for more than 6 million ED visits annually, resulting in 1.24 million admissions for unstable angina/non–ST-elevation MI and another 330,000 for STEMI. Emergency physicians are eager for new ways to rapidly and reliably rule out acute coronary syndrome—and CTA has a lot to offer in this regard, said Dr. Kramer, professor of medicine and radiology and director of the cardiovascular imaging center at the University of Virginia, Charlottesville.
In the recent 16-center CT-STAT trial, now in press, 701 low-risk patients with chest pain and a nondiagnostic ECG in the ED were randomized to CTA or the hospital's standard protocol, which typically included serial biomarkers along with SPECT myocardial perfusion imaging (MPI).
Time to diagnosis averaged 6.3 hours in the group who received the standard work-up compared to 2.9 hours with CTA, a 53% reduction. Median costs to obtain a diagnosis were $3,158 with the standard protocol and $2,137 with CTA, a 38% reduction.
ACS was diagnosed in roughly 3% of patients in each study arm, and invasive coronary angiography was performed in 5%. No significant stenosis was found in 82% of patients who underwent CTA and 90% who had MPI.
The 6-month major adverse cardiac event rates in this low-risk CT-STAT population were 0.3% in the standard management group and 0.0% in the CTA arm.
SPECT MPI is at present slightly more widely available in EDs around the country than is CTA. The other two up and coming imaging methods with utility in the setting of chest pain and nondiagnostic ECG in the ED—cardiac MRI and contrast echocardiography perfusion—have only limited availability. In terms of accuracy, they're similar to MPI and CTA, said Dr. Kramer.
A recent pooled analysis of MPI in ED evaluation of chest pain that included nine studies and nearly 1,900 patients showed the imaging method had 98% sensitivity and 73% specificity for identifying acute coronary syndrome. On the basis of its ability to reduce hospital admissions, it has been estimated that MPI reduces hospital costs by about $800 per patient.
Dr. Kramer serves as a consultant to Siemens Medical Solutions and is the recipient of research grants from Astellas and GlaxoSmithKline.
CTA is the clear winner in terms of time to diagnosis and cost in the emergency department.
Source Dr. Kramer
SNOWMASS, COLO. — CT angiography has rapidly emerged as the most cost-effective imaging technique to exclude acute coronary syndrome in the emergency department.
The overall diagnostic accuracy of CT angiography (CTA) is essentially equivalent to that of SPECT myocardial perfusion imaging, its main competition. But CTA is the clear winner in terms of time to diagnosis and cost, Dr. Christopher M. Kramer said at a conference sponsored by the American College of Cardiology.
Chest pain accounts for more than 6 million ED visits annually, resulting in 1.24 million admissions for unstable angina/non–ST-elevation MI and another 330,000 for STEMI. Emergency physicians are eager for new ways to rapidly and reliably rule out acute coronary syndrome—and CTA has a lot to offer in this regard, said Dr. Kramer, professor of medicine and radiology and director of the cardiovascular imaging center at the University of Virginia, Charlottesville.
In the recent 16-center CT-STAT trial, now in press, 701 low-risk patients with chest pain and a nondiagnostic ECG in the ED were randomized to CTA or the hospital's standard protocol, which typically included serial biomarkers along with SPECT myocardial perfusion imaging (MPI).
Time to diagnosis averaged 6.3 hours in the group who received the standard work-up compared to 2.9 hours with CTA, a 53% reduction. Median costs to obtain a diagnosis were $3,158 with the standard protocol and $2,137 with CTA, a 38% reduction.
ACS was diagnosed in roughly 3% of patients in each study arm, and invasive coronary angiography was performed in 5%. No significant stenosis was found in 82% of patients who underwent CTA and 90% who had MPI.
The 6-month major adverse cardiac event rates in this low-risk CT-STAT population were 0.3% in the standard management group and 0.0% in the CTA arm.
SPECT MPI is at present slightly more widely available in EDs around the country than is CTA. The other two up and coming imaging methods with utility in the setting of chest pain and nondiagnostic ECG in the ED—cardiac MRI and contrast echocardiography perfusion—have only limited availability. In terms of accuracy, they're similar to MPI and CTA, said Dr. Kramer.
A recent pooled analysis of MPI in ED evaluation of chest pain that included nine studies and nearly 1,900 patients showed the imaging method had 98% sensitivity and 73% specificity for identifying acute coronary syndrome. On the basis of its ability to reduce hospital admissions, it has been estimated that MPI reduces hospital costs by about $800 per patient.
Dr. Kramer serves as a consultant to Siemens Medical Solutions and is the recipient of research grants from Astellas and GlaxoSmithKline.
CTA is the clear winner in terms of time to diagnosis and cost in the emergency department.
Source Dr. Kramer
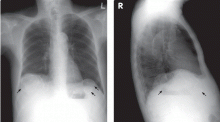
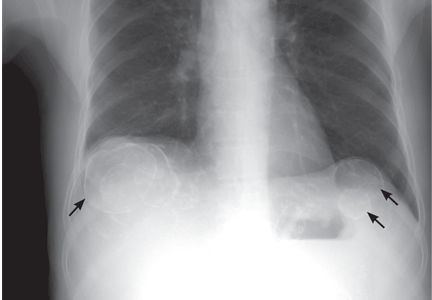
Calcified cysts in the upper abdomen
Q: Which is the most plausible diagnosis?
- Nephrolithiasis
- Hydatid cyst
- Polycystic kidney and liver disease
- Metastatic calcification
- Intra-abdominal abscesses
- Cystadenoma
A: This patient has polycystic kidney disease. He first came to our hospital 21 years ago because of upper gastrointestinal bleeding, and he was found to have multiple cystic lesions. He was diagnosed as having polycystic kidney disease with multiple hepatic cysts.
About 15 years ago, he had several episodes of gross hematuria with right flank pain, which were attributed to bleeding from the cysts. Because his renal function was deteriorating, he started regular hemodialysis at that time. He underwent parathyroidectomy for secondary hyperparathyroidism 2 years ago.
The differential diagnosis of calcified cystic lesions in the upper abdomen includes infectious diseases (eg, chronic intra-abdominal abscess, echinococcosis, cysticercosis), neoplasms (eg, cystadenomas, cystadenocarcinomas), organized hematomas, liver cysts, renal cysts, gallstones, renal stones, calcified lymph nodes, and vessel calcification.
Autosomal dominant polycystic kidney disease is the most common inherited renal disease.1 Typically, patients with this disease have a positive family history, although about 5% to 10% do not.1,3 (Our patient did not.)
The diagnosis is suggested by family history and imaging, with typical findings including large kidneys and extensive cysts scattered throughout both kidneys.1–3 The history, epidemiologic data, and the character of the cystic lesions help in making the final diagnosis.
In patients at risk, the presence of at least two renal cysts (unilateral or bilateral) at age 15 to 30, of at least two cysts in each kidney at age 30 to 59, and of at least four cysts in each kidney at age 60 or older is regarded as sufficient to establish the diagnosis.1–3
Approximately 80% of patients with autosomal dominant polycystic kidney disease also have hepatic cysts.1,3 Cyst calcifications occur in about 25% of patients, either as a residual effect of intracystic hemorrhage or as a consequence of secondary hyperparathyroidism.4,5
- Braun WE. Autosomal dominant polycystic kidney disease: emerging concepts of pathogenesis and new treatments. Cleve Clin J Med 2009; 76:97–104.
- Torres VE, Bennett WM. Diagnosis of and screening for autosomal dominant polycystic kidney disease. In:Rose BD, editor: UpToDate. Waltham, MA: UpToDate, 2009.
- Grantham JJ. Clinical practice. Autosomal dominant polycystic kidney disease. N Engl J Med 2008; 359:1477–1485.
- Coffin B, Hadengue A, Degos F, Benhamou JP. Calcified hepatic and renal cysts in adult dominant polycystic kidney disease. Dig Dis Sci 1990; 35:1172–1175.
- Levine E, Grantham JJ. Calcified renal stones and cyst calcifications in autosomal dominant polycystic kidney disease: clinical and CT study in 84 patients. AJR Am J Roentgenol 1992; 159:77–81.
Q: Which is the most plausible diagnosis?
- Nephrolithiasis
- Hydatid cyst
- Polycystic kidney and liver disease
- Metastatic calcification
- Intra-abdominal abscesses
- Cystadenoma
A: This patient has polycystic kidney disease. He first came to our hospital 21 years ago because of upper gastrointestinal bleeding, and he was found to have multiple cystic lesions. He was diagnosed as having polycystic kidney disease with multiple hepatic cysts.
About 15 years ago, he had several episodes of gross hematuria with right flank pain, which were attributed to bleeding from the cysts. Because his renal function was deteriorating, he started regular hemodialysis at that time. He underwent parathyroidectomy for secondary hyperparathyroidism 2 years ago.
The differential diagnosis of calcified cystic lesions in the upper abdomen includes infectious diseases (eg, chronic intra-abdominal abscess, echinococcosis, cysticercosis), neoplasms (eg, cystadenomas, cystadenocarcinomas), organized hematomas, liver cysts, renal cysts, gallstones, renal stones, calcified lymph nodes, and vessel calcification.
Autosomal dominant polycystic kidney disease is the most common inherited renal disease.1 Typically, patients with this disease have a positive family history, although about 5% to 10% do not.1,3 (Our patient did not.)
The diagnosis is suggested by family history and imaging, with typical findings including large kidneys and extensive cysts scattered throughout both kidneys.1–3 The history, epidemiologic data, and the character of the cystic lesions help in making the final diagnosis.
In patients at risk, the presence of at least two renal cysts (unilateral or bilateral) at age 15 to 30, of at least two cysts in each kidney at age 30 to 59, and of at least four cysts in each kidney at age 60 or older is regarded as sufficient to establish the diagnosis.1–3
Approximately 80% of patients with autosomal dominant polycystic kidney disease also have hepatic cysts.1,3 Cyst calcifications occur in about 25% of patients, either as a residual effect of intracystic hemorrhage or as a consequence of secondary hyperparathyroidism.4,5
Q: Which is the most plausible diagnosis?
- Nephrolithiasis
- Hydatid cyst
- Polycystic kidney and liver disease
- Metastatic calcification
- Intra-abdominal abscesses
- Cystadenoma
A: This patient has polycystic kidney disease. He first came to our hospital 21 years ago because of upper gastrointestinal bleeding, and he was found to have multiple cystic lesions. He was diagnosed as having polycystic kidney disease with multiple hepatic cysts.
About 15 years ago, he had several episodes of gross hematuria with right flank pain, which were attributed to bleeding from the cysts. Because his renal function was deteriorating, he started regular hemodialysis at that time. He underwent parathyroidectomy for secondary hyperparathyroidism 2 years ago.
The differential diagnosis of calcified cystic lesions in the upper abdomen includes infectious diseases (eg, chronic intra-abdominal abscess, echinococcosis, cysticercosis), neoplasms (eg, cystadenomas, cystadenocarcinomas), organized hematomas, liver cysts, renal cysts, gallstones, renal stones, calcified lymph nodes, and vessel calcification.
Autosomal dominant polycystic kidney disease is the most common inherited renal disease.1 Typically, patients with this disease have a positive family history, although about 5% to 10% do not.1,3 (Our patient did not.)
The diagnosis is suggested by family history and imaging, with typical findings including large kidneys and extensive cysts scattered throughout both kidneys.1–3 The history, epidemiologic data, and the character of the cystic lesions help in making the final diagnosis.
In patients at risk, the presence of at least two renal cysts (unilateral or bilateral) at age 15 to 30, of at least two cysts in each kidney at age 30 to 59, and of at least four cysts in each kidney at age 60 or older is regarded as sufficient to establish the diagnosis.1–3
Approximately 80% of patients with autosomal dominant polycystic kidney disease also have hepatic cysts.1,3 Cyst calcifications occur in about 25% of patients, either as a residual effect of intracystic hemorrhage or as a consequence of secondary hyperparathyroidism.4,5
- Braun WE. Autosomal dominant polycystic kidney disease: emerging concepts of pathogenesis and new treatments. Cleve Clin J Med 2009; 76:97–104.
- Torres VE, Bennett WM. Diagnosis of and screening for autosomal dominant polycystic kidney disease. In:Rose BD, editor: UpToDate. Waltham, MA: UpToDate, 2009.
- Grantham JJ. Clinical practice. Autosomal dominant polycystic kidney disease. N Engl J Med 2008; 359:1477–1485.
- Coffin B, Hadengue A, Degos F, Benhamou JP. Calcified hepatic and renal cysts in adult dominant polycystic kidney disease. Dig Dis Sci 1990; 35:1172–1175.
- Levine E, Grantham JJ. Calcified renal stones and cyst calcifications in autosomal dominant polycystic kidney disease: clinical and CT study in 84 patients. AJR Am J Roentgenol 1992; 159:77–81.
- Braun WE. Autosomal dominant polycystic kidney disease: emerging concepts of pathogenesis and new treatments. Cleve Clin J Med 2009; 76:97–104.
- Torres VE, Bennett WM. Diagnosis of and screening for autosomal dominant polycystic kidney disease. In:Rose BD, editor: UpToDate. Waltham, MA: UpToDate, 2009.
- Grantham JJ. Clinical practice. Autosomal dominant polycystic kidney disease. N Engl J Med 2008; 359:1477–1485.
- Coffin B, Hadengue A, Degos F, Benhamou JP. Calcified hepatic and renal cysts in adult dominant polycystic kidney disease. Dig Dis Sci 1990; 35:1172–1175.
- Levine E, Grantham JJ. Calcified renal stones and cyst calcifications in autosomal dominant polycystic kidney disease: clinical and CT study in 84 patients. AJR Am J Roentgenol 1992; 159:77–81.
Sorting through the recent controversies in breast cancer screening
Editor’s Note: This commentary, written by members of the Cleveland Clinic Breast Cancer Screening Task Force, was not independently peer-reviewed.
In November 2009, the US Preventive Services Task Force (USPSTF) announced its new guidelines for breast cancer screening—and created an instant controversy by suggesting that fewer screening tests be done.1
The November 2009 update recommended that most women wait until age 50 to get their first screening mammogram instead of getting it at age 40, that they get a mammogram every other year instead of every year, and that physicians not teach their patients breast self-examination anymore. However, on December 4, 2009, the USPSTF members voted to modify the recommendation for women under age 50, stating that the decision to start screening mammography every 2 years should be individualized, taking into account the patient’s preferences after being apprised of the possible benefits and harms.2
Various professional and advocacy groups have reacted differently to the new guidelines, and as a result, women are unsure about the optimal screening for breast cancer.
NEW GUIDELINES ARE BASED ON TWO STUDIES
The USPSTF commissioned two studies, which it used to formulate the new recommendations.3,4 Its goal was to evaluate the current evidence for the efficacy of several screening tests and schedules in reducing breast cancer mortality rates.
An updated systematic review
Nelson et al3 performed a systematic review of studies of the benefit and harm of screening with mammography, clinical breast examination, and breast self-examination.
Screening mammography continued to demonstrate a reduction in deaths due to breast cancer. The risk reduction ranged from 14% to 32% in women age 50 to 69. Similarly, it was calculated to reduce the incidence of deaths due to breast cancer by 15% in women age 39 to 49. However, this younger age group has a relatively low incidence of breast cancer, and therefore, according to this analysis, 556 women need to undergo one round of screening to detect one case of invasive breast cancer, and 1,904 women need to be offered screening (over several rounds, which varied by trial) to prevent one breast cancer death.3
Most of the harm of screening in the 39-to-49-year age category was due to false-positive results, which were more common in this group than in older women. The authors calculated that after every round of screening mammography, about 84 of every 1,000 women in the younger age category need additional imaging and about 9 need a biopsy. The issue of overdiagnosis (detection of cancers that would have never been a problem in one’s lifetime) was not specifically addressed for this age category, and in different studies, estimates of overdiagnosis rates for all age groups varied widely, from less than 1% to 30%.
Beyond age 70, the authors reported the data insufficient for evaluating the benefit and harm of screening mammography.
Breast self-examination was found to offer no benefit, based largely on two randomized studies, one in St. Petersburg, Russia,5 and the other in Shanghai, China,6 both places where screening mammography was not routinely offered. These studies and one observational study in the United States7 failed to show a reduction in breast cancer mortality rates with breast self-examination.
Clinical breast examination (ie, by a health care provider) lacked sufficient data to draw conclusions.
A study based on statistical models of mammography
Mandelblatt et al4 used statistical modeling to estimate the effect of mammographic screening at various ages and at different intervals.
The authors used six statistical models previously shown to give similar qualitative estimates of the contribution of screening in reducing breast cancer mortality rates. They estimated the number of mammograms required relative to the number of cancers detected, the number of breast cancer deaths prevented, and the harms (false-positive mammograms, unnecessary biopsies, and overdiagnosis) incurred with 20 different screening strategies, ie, screening with different starting and stopping ages and at intervals of either 1 or 2 years.
They estimated that screening every other year would achieve most of the benefit of screening every year, with less harm. Looking at the different strategies and models, on average, biennial screening would, by their calculations, achieve about 81% of the mortality reduction achieved with annual screening. Compared with screening women ages 50 to 69 only, extending screening to women age 40 to 49 would reduce the cancer mortality rate by 3% more, while extending it up to age 79 would reduce it by another 7% to 8%.
In terms of harm, the models predicted more false-positive studies if screening were started before age 50 and if it were done annually rather than biennially. They also predicted that more unnecessary biopsies would be done with annual screening than with screening every 2 years. The models suggested that the risk for overdiagnosis was higher in older age groups because of higher rates of death from causes other than breast cancer, and that the overdiagnosis rate was also somewhat higher with annual than with 2-year screening.
WHAT WOULD LESS SCREENING MEAN?
Our practice has been to initiate annual screening with mammography at age 40 and to continue as long as the patient’s life expectancy is at least 10 years.
According to the models used by Mandelblatt et al,4 screening 1,000 women every year, starting at age 40 and continuing until age 84, would result in 177 to 227 life-years gained compared with no screening. In contrast, screening only women age 50 to 74 and only every other year (as advocated in the new guidelines) would entail about one-third the number of mammograms but would result in fewer life-years gained per 1,000 women screened: between 96 and 128. If we take the mean of the estimates from the six models, adherence to the new screening guidelines would be estimated to result in about 79 fewer life-years gained for every 1,000 women screened. On the other hand, each woman screened would need to undergo about 25 fewer screening mammograms in her lifetime.4
KEY POINTS ABOUT BREAST CANCER SCREENING
Together, these studies demonstrate several points about breast cancer screening.
Importantly, randomized controlled trials and model analyses continue to show that screening mammography reduces the breast cancer mortality rate.
The studies and models also reinforce the concept that those at greatest risk get the most benefit from screening. Because the incidence of breast cancer rises with age, the probability of a true-positive result is higher in women over age 50 than it is in younger women, and, therefore, the screening test performs better.
On the other hand, women at high risk of dying of other causes, such as those over age 75, achieve less benefit from screening, as some of the cancers detected in this manner may not contribute to their death even if they are not detected early.
Screening is therefore best targeted at people who are healthy but who are at sufficient risk for the disease in question to justify the screening.
CLEVELAND CLINIC’S POSITION
In December 2009, the Cleveland Clinic Breast Cancer Screening Task Force, a multidisciplinary panel of breast cancer experts, breast radiologists, and primary care providers, convened to review the literature and set forth institutional recommendations for breast cancer screening for healthy women. The authors of this paper are members of this task force. Our consensus recommendations:
- We continue to recommend annual mammography for most healthy women over age 40.
- Screening every other year is an option for older postmenopausal women, as they are likely to achieve most of the benefit of annual screening with this schedule.
- We agree with the USPSTF finding that there are insufficient data to provide evidenced-based recommendations regarding the benefits and harms of clinical breast examination. However, breast examination was done as part of the screening in many of the randomized trials of mammography and cannot easily be separated from mammography. Therefore, we believe that careful examination of the breasts remains an important consideration in the general physical examination.
- The USPSTF recommendation not to teach breast self-examination was based on studies that probably do not apply to the US population. Therefore, we continue to recommend that women be familiar with their breasts and report any changes to their physicians.
How we reached these conclusions
The task force discussions focused heavily on at what age mammography should be started and how often it should be done. In addition to an in-depth review of the studies on which the USPSTF recommendations were based, we considered a review posted on the Society of Breast Imaging (SBI) Web site.8
A key point from the SBI’s review is that although breast cancer occurs less often in women under age 50, approximately 1 in 69 women are diagnosed with invasive cancer when in their 40s. Some—probably a minority— have a family history of breast cancer and thus warrant earlier screening on that basis.
Breast cancer is, therefore, an important public health concern for women ages 40 to 49. While mammography is an imperfect test, it has a demonstrated ability to find cancers at an earlier stage in this age group. The SBI statement also summarized data suggesting that the 40-to-49-year age group would experience significantly fewer lives saved by screening if the mammography interval were increased from once a year to every other year (ie, by approximately one-half—from 36% of deaths prevented with annual screening to 18% deaths prevented with screening every other year).
Screening every other year is also expected to result in fewer lives saved in women ages 50 to 69 (39% of deaths prevented by biennial screening instead of 44% to 46% with annual screening). However, this proportion of deaths prevented with more frequent (ie, annual) screening is smaller than in the younger age group. Breast cancers that arise before menopause are considered biologically more aggressive, so the longer the interval between screening tests, the lower the likelihood of detecting some of these potentially more lethal cancers.
We believe, for several reasons, that the randomized trials may have underestimated the benefit of mammography. The trials included in the USPSTF studies did not use modern mammographic techniques such as digital mammography. Some of the trials used single-view mammography, which may be less sensitive. Also, the rate of compliance with screening in these randomized trials was only about 70%, which would lead to an underestimation of the number of lives saved with mammography screening. Yet in spite of these limitations, the data continue to show a reduction in breast cancer deaths in all age categories studied.
Other issues the task force considered
Harms of screening are acceptable. We agree that the need for additional imaging or possibly breast biopsy is an acceptable consequence of screening for most women, especially when weighed against the potential benefit of improving survival. Nelson et al3 briefly discussed the risk of inducing other cancers through radiation exposure, and any such risk appears to be low enough that it is overshadowed by the reduction in the breast cancer mortality rate achieved from screening.
The USPSTF studies did not address the issue of cost, which is another potential harm of screening. However, screening mammography is relatively inexpensive compared with other potentially life-saving screening tests.
Our position differs from that of the American College of Physicians (ACP), which has endorsed the USPSTF recommendation for reduced breast cancer screening. The USPSTF has been a leading group in providing practice recommendations based on high-level evidence predominantly from randomized controlled clinical trials, and its recommendations have been consistently followed by the ACP and many of its members, including Cleveland Clinic physicians. It is, therefore, not without considerable discussion that we have come to our consensus.
Evidence for less screening was not compelling. One of our concerns about the new USPSTF recommendations is that the changes are based largely on a model analysis of the efficiency of different screening strategies rather than on randomized controlled trials comparing different strategies. We did not find this level of present evidence to be sufficiently compelling to make a change in our practice that may result in loss of lives from breast cancer.
Screening guidelines will continue to change over time as technology improves and new data are introduced. In the future, risk-assessment strategies such as incorporating genetic profiles may allow us to use factors more predictive than age to target our screening population.
While we continue to strive for better means of early detection and cancer prevention, the Cleveland Clinic task force is currently recommending yearly screening with mammography and breast examination for most women, starting at age 40.
- US Preventive Services Task Force. Screening for breast cancer: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med 2009; 151:716–726.
- US Preventive Services Task Force (USPSTF). Recommendation statement from USPSTF: screening for breast cancer. Medscape. http://www.medscape.com/viewarticle/714016. Accessed 12/28/2009.
- Nelson HD, Tyne K, Naik A, et al. Screening for breast cancer: an update for the U.S. Preventive Services Task Force. Ann Intern Med 2009; 151:727–737.
- Mandelblatt JS, Cronin KA, Bailey S, et al. Effects of mammography screening under different screening schedules: model estimates of potential benefits and harms. Ann Intern Med 2009; 151:738–747.
- Semiglazov VF, Manikhas AG, Moiseenko VM, et al. Results of a prospective randomized investigation [Russia (St. Petersburg)/WHO] to evaluate the significance of self-examination for the early detection of breast cancer [in Russian]. Vopr Onkol 2003; 49:434–441. Cited by Nelson et al (see reference 3, above).
- Thomas DB, Gao DL, Ray RM, et al. Randomized trial of breast self-examination in Shanghai: final results. J Natl Cancer Inst 2002; 94:1445–1457. Cited by Nelson et al (see reference 3, above).
- Tu SP, Reisch LM, Taplin SH, Kreuter W, Elmore JG. Breast self-examination: self-reported frequency, quality, and associated outcomes. J Cancer Educ 2006; 21:175–181. Cited by Nelson et al (see reference 3, above).
- Berg WA, Hendrick E, Kopans DB, Smith RA. Frequently asked questions about mammography and the USPSTF recommendations: a guide for practitioners. Society of Breast Imaging. http://www.sbi-online.org/associations/8199/files/Detailed_Response_to_USPSTF_Guidelines-12-11-09-Berg.pdf. Accessed 12/28/2009.
Editor’s Note: This commentary, written by members of the Cleveland Clinic Breast Cancer Screening Task Force, was not independently peer-reviewed.
In November 2009, the US Preventive Services Task Force (USPSTF) announced its new guidelines for breast cancer screening—and created an instant controversy by suggesting that fewer screening tests be done.1
The November 2009 update recommended that most women wait until age 50 to get their first screening mammogram instead of getting it at age 40, that they get a mammogram every other year instead of every year, and that physicians not teach their patients breast self-examination anymore. However, on December 4, 2009, the USPSTF members voted to modify the recommendation for women under age 50, stating that the decision to start screening mammography every 2 years should be individualized, taking into account the patient’s preferences after being apprised of the possible benefits and harms.2
Various professional and advocacy groups have reacted differently to the new guidelines, and as a result, women are unsure about the optimal screening for breast cancer.
NEW GUIDELINES ARE BASED ON TWO STUDIES
The USPSTF commissioned two studies, which it used to formulate the new recommendations.3,4 Its goal was to evaluate the current evidence for the efficacy of several screening tests and schedules in reducing breast cancer mortality rates.
An updated systematic review
Nelson et al3 performed a systematic review of studies of the benefit and harm of screening with mammography, clinical breast examination, and breast self-examination.
Screening mammography continued to demonstrate a reduction in deaths due to breast cancer. The risk reduction ranged from 14% to 32% in women age 50 to 69. Similarly, it was calculated to reduce the incidence of deaths due to breast cancer by 15% in women age 39 to 49. However, this younger age group has a relatively low incidence of breast cancer, and therefore, according to this analysis, 556 women need to undergo one round of screening to detect one case of invasive breast cancer, and 1,904 women need to be offered screening (over several rounds, which varied by trial) to prevent one breast cancer death.3
Most of the harm of screening in the 39-to-49-year age category was due to false-positive results, which were more common in this group than in older women. The authors calculated that after every round of screening mammography, about 84 of every 1,000 women in the younger age category need additional imaging and about 9 need a biopsy. The issue of overdiagnosis (detection of cancers that would have never been a problem in one’s lifetime) was not specifically addressed for this age category, and in different studies, estimates of overdiagnosis rates for all age groups varied widely, from less than 1% to 30%.
Beyond age 70, the authors reported the data insufficient for evaluating the benefit and harm of screening mammography.
Breast self-examination was found to offer no benefit, based largely on two randomized studies, one in St. Petersburg, Russia,5 and the other in Shanghai, China,6 both places where screening mammography was not routinely offered. These studies and one observational study in the United States7 failed to show a reduction in breast cancer mortality rates with breast self-examination.
Clinical breast examination (ie, by a health care provider) lacked sufficient data to draw conclusions.
A study based on statistical models of mammography
Mandelblatt et al4 used statistical modeling to estimate the effect of mammographic screening at various ages and at different intervals.
The authors used six statistical models previously shown to give similar qualitative estimates of the contribution of screening in reducing breast cancer mortality rates. They estimated the number of mammograms required relative to the number of cancers detected, the number of breast cancer deaths prevented, and the harms (false-positive mammograms, unnecessary biopsies, and overdiagnosis) incurred with 20 different screening strategies, ie, screening with different starting and stopping ages and at intervals of either 1 or 2 years.
They estimated that screening every other year would achieve most of the benefit of screening every year, with less harm. Looking at the different strategies and models, on average, biennial screening would, by their calculations, achieve about 81% of the mortality reduction achieved with annual screening. Compared with screening women ages 50 to 69 only, extending screening to women age 40 to 49 would reduce the cancer mortality rate by 3% more, while extending it up to age 79 would reduce it by another 7% to 8%.
In terms of harm, the models predicted more false-positive studies if screening were started before age 50 and if it were done annually rather than biennially. They also predicted that more unnecessary biopsies would be done with annual screening than with screening every 2 years. The models suggested that the risk for overdiagnosis was higher in older age groups because of higher rates of death from causes other than breast cancer, and that the overdiagnosis rate was also somewhat higher with annual than with 2-year screening.
WHAT WOULD LESS SCREENING MEAN?
Our practice has been to initiate annual screening with mammography at age 40 and to continue as long as the patient’s life expectancy is at least 10 years.
According to the models used by Mandelblatt et al,4 screening 1,000 women every year, starting at age 40 and continuing until age 84, would result in 177 to 227 life-years gained compared with no screening. In contrast, screening only women age 50 to 74 and only every other year (as advocated in the new guidelines) would entail about one-third the number of mammograms but would result in fewer life-years gained per 1,000 women screened: between 96 and 128. If we take the mean of the estimates from the six models, adherence to the new screening guidelines would be estimated to result in about 79 fewer life-years gained for every 1,000 women screened. On the other hand, each woman screened would need to undergo about 25 fewer screening mammograms in her lifetime.4
KEY POINTS ABOUT BREAST CANCER SCREENING
Together, these studies demonstrate several points about breast cancer screening.
Importantly, randomized controlled trials and model analyses continue to show that screening mammography reduces the breast cancer mortality rate.
The studies and models also reinforce the concept that those at greatest risk get the most benefit from screening. Because the incidence of breast cancer rises with age, the probability of a true-positive result is higher in women over age 50 than it is in younger women, and, therefore, the screening test performs better.
On the other hand, women at high risk of dying of other causes, such as those over age 75, achieve less benefit from screening, as some of the cancers detected in this manner may not contribute to their death even if they are not detected early.
Screening is therefore best targeted at people who are healthy but who are at sufficient risk for the disease in question to justify the screening.
CLEVELAND CLINIC’S POSITION
In December 2009, the Cleveland Clinic Breast Cancer Screening Task Force, a multidisciplinary panel of breast cancer experts, breast radiologists, and primary care providers, convened to review the literature and set forth institutional recommendations for breast cancer screening for healthy women. The authors of this paper are members of this task force. Our consensus recommendations:
- We continue to recommend annual mammography for most healthy women over age 40.
- Screening every other year is an option for older postmenopausal women, as they are likely to achieve most of the benefit of annual screening with this schedule.
- We agree with the USPSTF finding that there are insufficient data to provide evidenced-based recommendations regarding the benefits and harms of clinical breast examination. However, breast examination was done as part of the screening in many of the randomized trials of mammography and cannot easily be separated from mammography. Therefore, we believe that careful examination of the breasts remains an important consideration in the general physical examination.
- The USPSTF recommendation not to teach breast self-examination was based on studies that probably do not apply to the US population. Therefore, we continue to recommend that women be familiar with their breasts and report any changes to their physicians.
How we reached these conclusions
The task force discussions focused heavily on at what age mammography should be started and how often it should be done. In addition to an in-depth review of the studies on which the USPSTF recommendations were based, we considered a review posted on the Society of Breast Imaging (SBI) Web site.8
A key point from the SBI’s review is that although breast cancer occurs less often in women under age 50, approximately 1 in 69 women are diagnosed with invasive cancer when in their 40s. Some—probably a minority— have a family history of breast cancer and thus warrant earlier screening on that basis.
Breast cancer is, therefore, an important public health concern for women ages 40 to 49. While mammography is an imperfect test, it has a demonstrated ability to find cancers at an earlier stage in this age group. The SBI statement also summarized data suggesting that the 40-to-49-year age group would experience significantly fewer lives saved by screening if the mammography interval were increased from once a year to every other year (ie, by approximately one-half—from 36% of deaths prevented with annual screening to 18% deaths prevented with screening every other year).
Screening every other year is also expected to result in fewer lives saved in women ages 50 to 69 (39% of deaths prevented by biennial screening instead of 44% to 46% with annual screening). However, this proportion of deaths prevented with more frequent (ie, annual) screening is smaller than in the younger age group. Breast cancers that arise before menopause are considered biologically more aggressive, so the longer the interval between screening tests, the lower the likelihood of detecting some of these potentially more lethal cancers.
We believe, for several reasons, that the randomized trials may have underestimated the benefit of mammography. The trials included in the USPSTF studies did not use modern mammographic techniques such as digital mammography. Some of the trials used single-view mammography, which may be less sensitive. Also, the rate of compliance with screening in these randomized trials was only about 70%, which would lead to an underestimation of the number of lives saved with mammography screening. Yet in spite of these limitations, the data continue to show a reduction in breast cancer deaths in all age categories studied.
Other issues the task force considered
Harms of screening are acceptable. We agree that the need for additional imaging or possibly breast biopsy is an acceptable consequence of screening for most women, especially when weighed against the potential benefit of improving survival. Nelson et al3 briefly discussed the risk of inducing other cancers through radiation exposure, and any such risk appears to be low enough that it is overshadowed by the reduction in the breast cancer mortality rate achieved from screening.
The USPSTF studies did not address the issue of cost, which is another potential harm of screening. However, screening mammography is relatively inexpensive compared with other potentially life-saving screening tests.
Our position differs from that of the American College of Physicians (ACP), which has endorsed the USPSTF recommendation for reduced breast cancer screening. The USPSTF has been a leading group in providing practice recommendations based on high-level evidence predominantly from randomized controlled clinical trials, and its recommendations have been consistently followed by the ACP and many of its members, including Cleveland Clinic physicians. It is, therefore, not without considerable discussion that we have come to our consensus.
Evidence for less screening was not compelling. One of our concerns about the new USPSTF recommendations is that the changes are based largely on a model analysis of the efficiency of different screening strategies rather than on randomized controlled trials comparing different strategies. We did not find this level of present evidence to be sufficiently compelling to make a change in our practice that may result in loss of lives from breast cancer.
Screening guidelines will continue to change over time as technology improves and new data are introduced. In the future, risk-assessment strategies such as incorporating genetic profiles may allow us to use factors more predictive than age to target our screening population.
While we continue to strive for better means of early detection and cancer prevention, the Cleveland Clinic task force is currently recommending yearly screening with mammography and breast examination for most women, starting at age 40.
Editor’s Note: This commentary, written by members of the Cleveland Clinic Breast Cancer Screening Task Force, was not independently peer-reviewed.
In November 2009, the US Preventive Services Task Force (USPSTF) announced its new guidelines for breast cancer screening—and created an instant controversy by suggesting that fewer screening tests be done.1
The November 2009 update recommended that most women wait until age 50 to get their first screening mammogram instead of getting it at age 40, that they get a mammogram every other year instead of every year, and that physicians not teach their patients breast self-examination anymore. However, on December 4, 2009, the USPSTF members voted to modify the recommendation for women under age 50, stating that the decision to start screening mammography every 2 years should be individualized, taking into account the patient’s preferences after being apprised of the possible benefits and harms.2
Various professional and advocacy groups have reacted differently to the new guidelines, and as a result, women are unsure about the optimal screening for breast cancer.
NEW GUIDELINES ARE BASED ON TWO STUDIES
The USPSTF commissioned two studies, which it used to formulate the new recommendations.3,4 Its goal was to evaluate the current evidence for the efficacy of several screening tests and schedules in reducing breast cancer mortality rates.
An updated systematic review
Nelson et al3 performed a systematic review of studies of the benefit and harm of screening with mammography, clinical breast examination, and breast self-examination.
Screening mammography continued to demonstrate a reduction in deaths due to breast cancer. The risk reduction ranged from 14% to 32% in women age 50 to 69. Similarly, it was calculated to reduce the incidence of deaths due to breast cancer by 15% in women age 39 to 49. However, this younger age group has a relatively low incidence of breast cancer, and therefore, according to this analysis, 556 women need to undergo one round of screening to detect one case of invasive breast cancer, and 1,904 women need to be offered screening (over several rounds, which varied by trial) to prevent one breast cancer death.3
Most of the harm of screening in the 39-to-49-year age category was due to false-positive results, which were more common in this group than in older women. The authors calculated that after every round of screening mammography, about 84 of every 1,000 women in the younger age category need additional imaging and about 9 need a biopsy. The issue of overdiagnosis (detection of cancers that would have never been a problem in one’s lifetime) was not specifically addressed for this age category, and in different studies, estimates of overdiagnosis rates for all age groups varied widely, from less than 1% to 30%.
Beyond age 70, the authors reported the data insufficient for evaluating the benefit and harm of screening mammography.
Breast self-examination was found to offer no benefit, based largely on two randomized studies, one in St. Petersburg, Russia,5 and the other in Shanghai, China,6 both places where screening mammography was not routinely offered. These studies and one observational study in the United States7 failed to show a reduction in breast cancer mortality rates with breast self-examination.
Clinical breast examination (ie, by a health care provider) lacked sufficient data to draw conclusions.
A study based on statistical models of mammography
Mandelblatt et al4 used statistical modeling to estimate the effect of mammographic screening at various ages and at different intervals.
The authors used six statistical models previously shown to give similar qualitative estimates of the contribution of screening in reducing breast cancer mortality rates. They estimated the number of mammograms required relative to the number of cancers detected, the number of breast cancer deaths prevented, and the harms (false-positive mammograms, unnecessary biopsies, and overdiagnosis) incurred with 20 different screening strategies, ie, screening with different starting and stopping ages and at intervals of either 1 or 2 years.
They estimated that screening every other year would achieve most of the benefit of screening every year, with less harm. Looking at the different strategies and models, on average, biennial screening would, by their calculations, achieve about 81% of the mortality reduction achieved with annual screening. Compared with screening women ages 50 to 69 only, extending screening to women age 40 to 49 would reduce the cancer mortality rate by 3% more, while extending it up to age 79 would reduce it by another 7% to 8%.
In terms of harm, the models predicted more false-positive studies if screening were started before age 50 and if it were done annually rather than biennially. They also predicted that more unnecessary biopsies would be done with annual screening than with screening every 2 years. The models suggested that the risk for overdiagnosis was higher in older age groups because of higher rates of death from causes other than breast cancer, and that the overdiagnosis rate was also somewhat higher with annual than with 2-year screening.
WHAT WOULD LESS SCREENING MEAN?
Our practice has been to initiate annual screening with mammography at age 40 and to continue as long as the patient’s life expectancy is at least 10 years.
According to the models used by Mandelblatt et al,4 screening 1,000 women every year, starting at age 40 and continuing until age 84, would result in 177 to 227 life-years gained compared with no screening. In contrast, screening only women age 50 to 74 and only every other year (as advocated in the new guidelines) would entail about one-third the number of mammograms but would result in fewer life-years gained per 1,000 women screened: between 96 and 128. If we take the mean of the estimates from the six models, adherence to the new screening guidelines would be estimated to result in about 79 fewer life-years gained for every 1,000 women screened. On the other hand, each woman screened would need to undergo about 25 fewer screening mammograms in her lifetime.4
KEY POINTS ABOUT BREAST CANCER SCREENING
Together, these studies demonstrate several points about breast cancer screening.
Importantly, randomized controlled trials and model analyses continue to show that screening mammography reduces the breast cancer mortality rate.
The studies and models also reinforce the concept that those at greatest risk get the most benefit from screening. Because the incidence of breast cancer rises with age, the probability of a true-positive result is higher in women over age 50 than it is in younger women, and, therefore, the screening test performs better.
On the other hand, women at high risk of dying of other causes, such as those over age 75, achieve less benefit from screening, as some of the cancers detected in this manner may not contribute to their death even if they are not detected early.
Screening is therefore best targeted at people who are healthy but who are at sufficient risk for the disease in question to justify the screening.
CLEVELAND CLINIC’S POSITION
In December 2009, the Cleveland Clinic Breast Cancer Screening Task Force, a multidisciplinary panel of breast cancer experts, breast radiologists, and primary care providers, convened to review the literature and set forth institutional recommendations for breast cancer screening for healthy women. The authors of this paper are members of this task force. Our consensus recommendations:
- We continue to recommend annual mammography for most healthy women over age 40.
- Screening every other year is an option for older postmenopausal women, as they are likely to achieve most of the benefit of annual screening with this schedule.
- We agree with the USPSTF finding that there are insufficient data to provide evidenced-based recommendations regarding the benefits and harms of clinical breast examination. However, breast examination was done as part of the screening in many of the randomized trials of mammography and cannot easily be separated from mammography. Therefore, we believe that careful examination of the breasts remains an important consideration in the general physical examination.
- The USPSTF recommendation not to teach breast self-examination was based on studies that probably do not apply to the US population. Therefore, we continue to recommend that women be familiar with their breasts and report any changes to their physicians.
How we reached these conclusions
The task force discussions focused heavily on at what age mammography should be started and how often it should be done. In addition to an in-depth review of the studies on which the USPSTF recommendations were based, we considered a review posted on the Society of Breast Imaging (SBI) Web site.8
A key point from the SBI’s review is that although breast cancer occurs less often in women under age 50, approximately 1 in 69 women are diagnosed with invasive cancer when in their 40s. Some—probably a minority— have a family history of breast cancer and thus warrant earlier screening on that basis.
Breast cancer is, therefore, an important public health concern for women ages 40 to 49. While mammography is an imperfect test, it has a demonstrated ability to find cancers at an earlier stage in this age group. The SBI statement also summarized data suggesting that the 40-to-49-year age group would experience significantly fewer lives saved by screening if the mammography interval were increased from once a year to every other year (ie, by approximately one-half—from 36% of deaths prevented with annual screening to 18% deaths prevented with screening every other year).
Screening every other year is also expected to result in fewer lives saved in women ages 50 to 69 (39% of deaths prevented by biennial screening instead of 44% to 46% with annual screening). However, this proportion of deaths prevented with more frequent (ie, annual) screening is smaller than in the younger age group. Breast cancers that arise before menopause are considered biologically more aggressive, so the longer the interval between screening tests, the lower the likelihood of detecting some of these potentially more lethal cancers.
We believe, for several reasons, that the randomized trials may have underestimated the benefit of mammography. The trials included in the USPSTF studies did not use modern mammographic techniques such as digital mammography. Some of the trials used single-view mammography, which may be less sensitive. Also, the rate of compliance with screening in these randomized trials was only about 70%, which would lead to an underestimation of the number of lives saved with mammography screening. Yet in spite of these limitations, the data continue to show a reduction in breast cancer deaths in all age categories studied.
Other issues the task force considered
Harms of screening are acceptable. We agree that the need for additional imaging or possibly breast biopsy is an acceptable consequence of screening for most women, especially when weighed against the potential benefit of improving survival. Nelson et al3 briefly discussed the risk of inducing other cancers through radiation exposure, and any such risk appears to be low enough that it is overshadowed by the reduction in the breast cancer mortality rate achieved from screening.
The USPSTF studies did not address the issue of cost, which is another potential harm of screening. However, screening mammography is relatively inexpensive compared with other potentially life-saving screening tests.
Our position differs from that of the American College of Physicians (ACP), which has endorsed the USPSTF recommendation for reduced breast cancer screening. The USPSTF has been a leading group in providing practice recommendations based on high-level evidence predominantly from randomized controlled clinical trials, and its recommendations have been consistently followed by the ACP and many of its members, including Cleveland Clinic physicians. It is, therefore, not without considerable discussion that we have come to our consensus.
Evidence for less screening was not compelling. One of our concerns about the new USPSTF recommendations is that the changes are based largely on a model analysis of the efficiency of different screening strategies rather than on randomized controlled trials comparing different strategies. We did not find this level of present evidence to be sufficiently compelling to make a change in our practice that may result in loss of lives from breast cancer.
Screening guidelines will continue to change over time as technology improves and new data are introduced. In the future, risk-assessment strategies such as incorporating genetic profiles may allow us to use factors more predictive than age to target our screening population.
While we continue to strive for better means of early detection and cancer prevention, the Cleveland Clinic task force is currently recommending yearly screening with mammography and breast examination for most women, starting at age 40.
- US Preventive Services Task Force. Screening for breast cancer: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med 2009; 151:716–726.
- US Preventive Services Task Force (USPSTF). Recommendation statement from USPSTF: screening for breast cancer. Medscape. http://www.medscape.com/viewarticle/714016. Accessed 12/28/2009.
- Nelson HD, Tyne K, Naik A, et al. Screening for breast cancer: an update for the U.S. Preventive Services Task Force. Ann Intern Med 2009; 151:727–737.
- Mandelblatt JS, Cronin KA, Bailey S, et al. Effects of mammography screening under different screening schedules: model estimates of potential benefits and harms. Ann Intern Med 2009; 151:738–747.
- Semiglazov VF, Manikhas AG, Moiseenko VM, et al. Results of a prospective randomized investigation [Russia (St. Petersburg)/WHO] to evaluate the significance of self-examination for the early detection of breast cancer [in Russian]. Vopr Onkol 2003; 49:434–441. Cited by Nelson et al (see reference 3, above).
- Thomas DB, Gao DL, Ray RM, et al. Randomized trial of breast self-examination in Shanghai: final results. J Natl Cancer Inst 2002; 94:1445–1457. Cited by Nelson et al (see reference 3, above).
- Tu SP, Reisch LM, Taplin SH, Kreuter W, Elmore JG. Breast self-examination: self-reported frequency, quality, and associated outcomes. J Cancer Educ 2006; 21:175–181. Cited by Nelson et al (see reference 3, above).
- Berg WA, Hendrick E, Kopans DB, Smith RA. Frequently asked questions about mammography and the USPSTF recommendations: a guide for practitioners. Society of Breast Imaging. http://www.sbi-online.org/associations/8199/files/Detailed_Response_to_USPSTF_Guidelines-12-11-09-Berg.pdf. Accessed 12/28/2009.
- US Preventive Services Task Force. Screening for breast cancer: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med 2009; 151:716–726.
- US Preventive Services Task Force (USPSTF). Recommendation statement from USPSTF: screening for breast cancer. Medscape. http://www.medscape.com/viewarticle/714016. Accessed 12/28/2009.
- Nelson HD, Tyne K, Naik A, et al. Screening for breast cancer: an update for the U.S. Preventive Services Task Force. Ann Intern Med 2009; 151:727–737.
- Mandelblatt JS, Cronin KA, Bailey S, et al. Effects of mammography screening under different screening schedules: model estimates of potential benefits and harms. Ann Intern Med 2009; 151:738–747.
- Semiglazov VF, Manikhas AG, Moiseenko VM, et al. Results of a prospective randomized investigation [Russia (St. Petersburg)/WHO] to evaluate the significance of self-examination for the early detection of breast cancer [in Russian]. Vopr Onkol 2003; 49:434–441. Cited by Nelson et al (see reference 3, above).
- Thomas DB, Gao DL, Ray RM, et al. Randomized trial of breast self-examination in Shanghai: final results. J Natl Cancer Inst 2002; 94:1445–1457. Cited by Nelson et al (see reference 3, above).
- Tu SP, Reisch LM, Taplin SH, Kreuter W, Elmore JG. Breast self-examination: self-reported frequency, quality, and associated outcomes. J Cancer Educ 2006; 21:175–181. Cited by Nelson et al (see reference 3, above).
- Berg WA, Hendrick E, Kopans DB, Smith RA. Frequently asked questions about mammography and the USPSTF recommendations: a guide for practitioners. Society of Breast Imaging. http://www.sbi-online.org/associations/8199/files/Detailed_Response_to_USPSTF_Guidelines-12-11-09-Berg.pdf. Accessed 12/28/2009.