User login
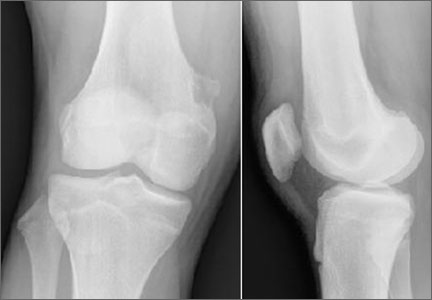
Rare Case of Dual Lesion: Nonossifying Fibroma and Osteochondroma
High-Altitude Illness
Patients participating in occupational and sports-related activities requiring ascent to high elevations are at risk of developing a range of high-altitude illnesses. Prompt recognition and treatment are paramount to improving outcomes and preventing life-threatening sequelae. High-elevation locations are the setting of many recreational activities for outdoor enthusiasts. As such, illnesses associated with high altitude may be encountered by those summiting peaks, traveling by air, or working in flight medicine or as part of an emergency rescue team. The altitude syndromes discussed in this review are acute mountain sickness (AMS), high-altitude cerebral edema (HACE), and high-altitude pulmonary edema (HAPE). While these conditions do not represent all altitude-related illnesses, they are the primary pathological processes for which physicians should be familiar when working with high-altitude populations.
Physiological Response to Altitude
The Lake Louise Criteria
Acute Mountain Sickness
Acute mountain sickness comprises a constellation of symptoms caused by the atmospheric changes at elevations above approximately 2,500 m. It is the most common form of high-altitude illness, affecting 25% of travelers at moderate altitude and 50% to 85% above 4,000 m.3
Symptoms
The onset of symptoms (eg, headache, anorexia, nausea, vomiting, weakness) may occur at 2,000 m in the setting of rapid ascent—most commonly at 6 to 12 hours, but onset can range from 1 hour to 2 days after ascent. If symptoms begin after 3 days, other diagnoses should be considered. Symptoms of AMS are generally worse after the first night of sleep at elevation. On physical examination, vital signs are usually normal, though postural hypotension and tachycardia are possible. Oxygen saturation may be markedly decreased after rapid ascent, and chest auscultation may reveal rales in 20% of patients.4 Peripheral and facial edema may also be present. Funduscopic examination may show venous tortuosity and dilation, and retinal hemorrhage is common in ascents over 4,800 m.
Differential Diagnosis
The differential diagnosis for AMS is broad and includes hypothermia, dehydration, exhaustion, subarachnoid hemorrhage, intracranial mass, carbon monoxide poisoning, alcohol hangover, intoxication, central nervous system infection and migraine. Risk factors for developing AMS are a previous history of altitude illness, rapid ascent, and lack of previous acclimatization. Interestingly, physical fitness does not protect a person from developing AMS.5
Mechanism of AMS
The true mechanism of AMS is uncertain, but it is clear that a fall in barometric pressure results in hypobaric hypoxia. This is thought to lead to an increased blood volume in the brain and increased cerebral blood flow, possibly precipitating an enlarged brain. A mechanism related to vasogenic edema has been proposed due to patients’ clinical improvement with dexamethasone therapy.6 Acute mountain sickness does appear to be related to overall fluid balance, as an increase in reninangiotensin, aldosterone, and antidiuretic hormone has been observed in patients with the condition. Elevation of these hormones is contrary to the appropriate physiological response of diuresis.
Treatment
Treatment of AMS begins with descent from elevation as soon as possible. Descent should be at least 500 m from the aggravating elevation. Patients should remain at least 1 to 2 days at this lower elevation before attempting reascent. If descent is not feasible, any further ascent should be delayed until symptoms have resolved.
Dexamethasone. This glucocorticoid has been used clinically with good success, although the mechanism of action in unclear. The initial dose is 8 mg followed by 4 mg every 6 hours.3
Acetazolamide. A carbonic anhydrase inhibitor, acetazolamide acts to temper symptoms by causing an acidosis that increases ventilation and prevents periodic breathing and hypoxia during sleep. The standard dose is 250 mg twice daily.3
Oxygen. Supplemental oxygen provided at 1 to 2 L/min via nasal cannula for 12 to 24 hours may help to improve symptoms. A portable hyperbaric oxygen (HBO) bag (eg, a Gamow bag) can be used to create an effective altitude of approximately 1,500 to 2,000 m inside the bag. The patient is placed completely within the bag, the zipper is sealed shut, and the bag is inflated with a foot pump. Treatment in such a chamber can be provided in 1-hour increments and repeated as needed. However, if descent is possible, use of the HBO chamber should not prevent or delay descent.
Ibuprofen. Compared to placebo, studies have shown ibuprofen 600 mg three times a day reduces the severity of AMS.7
Prevention
Strategies to prevent AMS are similar to those used to treat the condition. These include gradual ascent and prophylactic drug therapy.
Gradual Ascent. Gradual ascent is the primary strategy to prevent AMS. At altitudes above 3,000 m, each subsequent night should not be spent at an elevation 300 m higher than the previous night.
Acetazolamide. Pretreatment with acetazolamide is indicated for patients with a history of altitude illness or who anticipate an abrupt ascent (eg, rescue workers). Acetazolamide has been shown in multiple studies to be effective in the prevention of AMS.8 Adverse side effects of acetazolamide include paresthesias and increased urinary frequency; the drug may also make carbonated beverages taste flat. The preventive dose is 125 mg twice daily, and should be started the day before ascent.
Dexamethasone. In addition to treating AMS, dexamethasone may be taken as a preventive in doses of 2 mg every 6 hours or 4 mg twice daily.3 However, unlike acetazolamide, which acts to facilitate acclimatization, dexamethasone only prevents symptoms. Thus, cessation of the drug can result in rebound AMS symptoms, and prolonged use can result in adrenal suppression.3 Therefore, it should not be used for more than 10 days.
Sumatriptan and Gabapentin. In recent studies, sumatriptan and gabapentin haven shown benefit in preventing AMS, 9,10 but further study is needed before either of these drugs can be recommended.
Ginkgo Biloba. While ginkgo biloba has been touted as an effective preventive treatment, studies have shown no benefit to its use.8
Ibuprofen. ibuprofen 600 mg three times daily can be initiated the day prior to ascent, and has been shown to decrease the incidence of AMS.7
High-Altitude Cerebral Edema
Mechanism of HACE
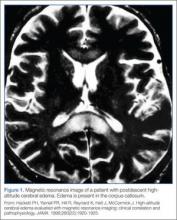
The exact mechanism of HACE is unclear. Magnetic resonance imaging of patients with the condition demonstrates cerebral edema primarily localized to the corpus callosum.11 These findings suggest an increased permeability in the blood-brain barrier, leading to vasogenic cerebral edema. Cases of death associated with HACE are the result of herniation. Fortunately, if the condition is recognized promptly and appropriate management is instituted, most patients will recover without permanent deficits.
Current recommendations for treating HACE are similar to treatment strategies for AMS.
Descent. A therapeutic priority, descent may prove challenging as the patient may be ataxic, have altered mental status, and have difficulty facilitating his or her own descent.
Oxygen. A portable HBO bag can be used to simulate descent until evacuation is possible. Supplemental oxygen should be applied immediately.
Dexamethasone. In treating HACE, dexamethasone may be administered at a loading dose of 8 mg, followed by 4 mg every 6 hours.3
Airway Management. If the patient has significantly altered mental status, appropriate airway management must be initiated.
High-Altitude Pulmonary Edema
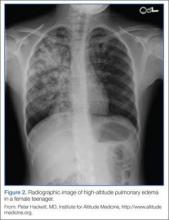
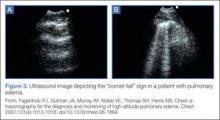
The most common cause of death from altitude illness is HAPE,12 a form of noncardiogenic pulmonary edema. This condition generally occurs at elevations above 3,000 m. Symptoms begin 2 to 5 days after ascent and progress in a typical pattern. A patient will initially experience a nonproductive cough and dyspnea at rest. The dyspnea worsens, and the cough becomes productive of pink, frothy sputum. Without medical intervention, lethargy, coma, and death may follow.
Symptoms of HAPE generally worsen following a night of sleep at elevation. Physical examination reveals crackles, tachycardia, tachypnea, and hypoxia. Diagnosis requires at least two of the following signs:
- Crackles or wheezing in at least one lung field
- Central cyanosis
- Tachypnea
- Tachycardia.
In addition to the above signs, at least two of the following symptoms must also be present:
- Dyspnea at rest
- Cough
- Weakness or decreased exercise performance
- Chest tightness
- Congestion.
Mechanism of HAPE
The mechanism of HAPE is better understood than that of AMS and HACE. In HAPE, high microvascular pressures in the lungs lead to elevated pulmonary vascular resistance and pulmonary artery pressure. Pulmonary edema ensues, but left ventricular function is preserved. Patients with a naturally low HVR, high pulmonary artery pressures at rest, preexisting pulmonary hypertension, or a previous history of HAPE are predisposed to developing the condition. Risk factors include heavy exertion, rapid ascent, cold, salt ingestion, and sleeping medications.
Treatment
Decent and warming of the patient as soon as possible, along with treatment outlined below, are essential.
Oxygen. Treatment of HAPE begins with supplemental oxygen to immediately lower pulmonary artery pressure. Oxygen should initially be administered at 4 to 6 L/min; if the patient improves clinically and can maintain oxygen saturations greater than 90%, oxygen may be decreased with a goal to maintain saturation above 90%.
Nifedipine. Following oxygen, descent, and warming, nifedipine can be used as an adjunctive therapy. The treatment dose for HAPE is 20 to 30 mg of the sustained release form every 12 hours.3
Salmeterol/Albuterol and Expiratory Positive Airway Pressure. The oral inhalers salmeterol or albuterol may be used for bronchodilation; however, there is little evidence to support their effectiveness in HAPE. Ventilation with expiratory positive airway pressure can be employed if available.
Prevention
For patients with a predisposition to HAPE, preventive measures should be considered prior to ascent. As with all forms of altitude illness, gradual ascent is the most effective prevention method available.
Phosphodiesterase Inhibitors. Phosphodiesterase inhibitors act via pulmonary vasodilation to prevent HAPE in some patients. Tadalafil at a dose of 10 mg twice daily or 20 mg once daily has been shown to reduce the incidence of HAPE.14 Alternatively, sildenafil 50 mg three times daily may be used.
Acetazolamide and β-Agonists. Although both acetazolamide and β-agonists such as albuterol have been theorized to aid in preventing HAPE, this has not been proven.15
Conclusion
Clinically, high-altitude illnesses range from subtle symptoms to severe, life threatening disease. Knowledge of these disease processes and clinical presentation prior to travel or work in a high-altitude setting is essential. Rapid recognition of symptoms and prompt, appropriate interventions, such as descent when necessary, can significantly improve the outcomes of these conditions.
Dr Haroutunian is an emergency physician, department of emergency medicine, Exempla St Joseph Hospital, Denver, Colorado. Dr Bono is professor and vice chairman, department of emergency medicine, Eastern Virginia Medical School, Norfolk.
- Hackett PH, Oelz O. The Lake Louise consensus on the definition and qualification of altitude illness. In: Sutton JR, Coates G, Houston CS, eds. Hypoxia and Mountain Medicine. Burlington, VT: Queen City Printers; 1992:327-330.
- Roach RC, Bärtch P, Hackett PH, Oelz O, and the Lake Louise AMS Scoring Consensus Committee. The Lake Louise Acute Mountain Sickness Scoring System. In: Hypoxia and Molecular Medicine. Proceedings of the 8th International Hypoxia Symposium. Burlington, VT: Queen City Printers; 1993:272-274.
- Eide RP 3rd, Asplund CA. Altitude illness: update on prevention and treatment. Curr Sports Med Rep. 2012;11(3):124-130.
- Milzman DP, Damergis JA, Napoli AM. Rapid ascent changes in vitals at altitude. Ann Emerg Med. 2008;51(4):536.
- Bärtsch P, Swenson ER. Clinical practice: Acute high-altitude illnesses. N Engl J Med. 2013;368(24):2294-2302.
- Hackett PH, Roach RC. Medical therapy of mountain illness. Ann Emerg Med. 1987;16(9):980-986.
- Lipman GS, Kanaan NC, Holck PS, Constance BB, Gertsch JH; PAINS Group. Ibuprofen prevents altitude illness: a randomized controlled trial for prevention of altitude illness with nonsteroidal anti-inflammatories. Ann Emerg Med. 2012;59(6): 484-490.
- Seupaul RA, Welch JL, Malka ST, Emmett TW. Pharmacologic prophylaxis for acute mountain sickness: a systematic shortcut review. Ann Emerg Med. 2012; 59(4):307-317.
- Jafarian S, Gorouhi F, Salimi S, Lotfi J. Sumatriptan for prevention of acute mountain sickness: randomized clinical trial. Ann Neurol. 2007;62(3):273-277.
- Jafarian S, Abolfazli R, Gorouhi F, Rezaie S, Lotfi J. Gabapentin for prevention of hypobaric hypoxia-induced headache: randomized double-blind clinical trial. J Neurol Neurosurg Psychiatry. 2008;79(3): 321-323.
- Hackett PH, Yarnell PR, Hill R, Reynard K, Heit J, McCormick J. High-altitude cerebral edema evaluated with magnetic resonance imaging: clinical correlation and pathophysiology. JAMA. 1998;280(22):1920-1925.
- Gallagher SA1, Hackett PH. High-altitude illness. Emerg Med Clin North Am. 2004;22(2):329-355.
- Fagenholz PJ, Gutman JA, Murray AF, Noble VE, Thomas SH, Harris NS. Chest ultrasonography for the diagnosis and monitoring of high-altitude pulmonary edema. Chest. 2007;131(4): 1013-1018.
- Leshem E1, Caine Y, Rosenberg E, Maaravi Y, Hermesh H, Schwartz E. Tadalafil and acetazolamide versus acetazolamide for the prevention of severe high-altitude illness. J Travel Med. 2012;19(5): 308-310.
- Schoene RB. Illnesses at high altitude. Chest. 2008;134(2):402-416.
Patients participating in occupational and sports-related activities requiring ascent to high elevations are at risk of developing a range of high-altitude illnesses. Prompt recognition and treatment are paramount to improving outcomes and preventing life-threatening sequelae. High-elevation locations are the setting of many recreational activities for outdoor enthusiasts. As such, illnesses associated with high altitude may be encountered by those summiting peaks, traveling by air, or working in flight medicine or as part of an emergency rescue team. The altitude syndromes discussed in this review are acute mountain sickness (AMS), high-altitude cerebral edema (HACE), and high-altitude pulmonary edema (HAPE). While these conditions do not represent all altitude-related illnesses, they are the primary pathological processes for which physicians should be familiar when working with high-altitude populations.
Physiological Response to Altitude
The Lake Louise Criteria
Acute Mountain Sickness
Acute mountain sickness comprises a constellation of symptoms caused by the atmospheric changes at elevations above approximately 2,500 m. It is the most common form of high-altitude illness, affecting 25% of travelers at moderate altitude and 50% to 85% above 4,000 m.3
Symptoms
The onset of symptoms (eg, headache, anorexia, nausea, vomiting, weakness) may occur at 2,000 m in the setting of rapid ascent—most commonly at 6 to 12 hours, but onset can range from 1 hour to 2 days after ascent. If symptoms begin after 3 days, other diagnoses should be considered. Symptoms of AMS are generally worse after the first night of sleep at elevation. On physical examination, vital signs are usually normal, though postural hypotension and tachycardia are possible. Oxygen saturation may be markedly decreased after rapid ascent, and chest auscultation may reveal rales in 20% of patients.4 Peripheral and facial edema may also be present. Funduscopic examination may show venous tortuosity and dilation, and retinal hemorrhage is common in ascents over 4,800 m.
Differential Diagnosis
The differential diagnosis for AMS is broad and includes hypothermia, dehydration, exhaustion, subarachnoid hemorrhage, intracranial mass, carbon monoxide poisoning, alcohol hangover, intoxication, central nervous system infection and migraine. Risk factors for developing AMS are a previous history of altitude illness, rapid ascent, and lack of previous acclimatization. Interestingly, physical fitness does not protect a person from developing AMS.5
Mechanism of AMS
The true mechanism of AMS is uncertain, but it is clear that a fall in barometric pressure results in hypobaric hypoxia. This is thought to lead to an increased blood volume in the brain and increased cerebral blood flow, possibly precipitating an enlarged brain. A mechanism related to vasogenic edema has been proposed due to patients’ clinical improvement with dexamethasone therapy.6 Acute mountain sickness does appear to be related to overall fluid balance, as an increase in reninangiotensin, aldosterone, and antidiuretic hormone has been observed in patients with the condition. Elevation of these hormones is contrary to the appropriate physiological response of diuresis.
Treatment
Treatment of AMS begins with descent from elevation as soon as possible. Descent should be at least 500 m from the aggravating elevation. Patients should remain at least 1 to 2 days at this lower elevation before attempting reascent. If descent is not feasible, any further ascent should be delayed until symptoms have resolved.
Dexamethasone. This glucocorticoid has been used clinically with good success, although the mechanism of action in unclear. The initial dose is 8 mg followed by 4 mg every 6 hours.3
Acetazolamide. A carbonic anhydrase inhibitor, acetazolamide acts to temper symptoms by causing an acidosis that increases ventilation and prevents periodic breathing and hypoxia during sleep. The standard dose is 250 mg twice daily.3
Oxygen. Supplemental oxygen provided at 1 to 2 L/min via nasal cannula for 12 to 24 hours may help to improve symptoms. A portable hyperbaric oxygen (HBO) bag (eg, a Gamow bag) can be used to create an effective altitude of approximately 1,500 to 2,000 m inside the bag. The patient is placed completely within the bag, the zipper is sealed shut, and the bag is inflated with a foot pump. Treatment in such a chamber can be provided in 1-hour increments and repeated as needed. However, if descent is possible, use of the HBO chamber should not prevent or delay descent.
Ibuprofen. Compared to placebo, studies have shown ibuprofen 600 mg three times a day reduces the severity of AMS.7
Prevention
Strategies to prevent AMS are similar to those used to treat the condition. These include gradual ascent and prophylactic drug therapy.
Gradual Ascent. Gradual ascent is the primary strategy to prevent AMS. At altitudes above 3,000 m, each subsequent night should not be spent at an elevation 300 m higher than the previous night.
Acetazolamide. Pretreatment with acetazolamide is indicated for patients with a history of altitude illness or who anticipate an abrupt ascent (eg, rescue workers). Acetazolamide has been shown in multiple studies to be effective in the prevention of AMS.8 Adverse side effects of acetazolamide include paresthesias and increased urinary frequency; the drug may also make carbonated beverages taste flat. The preventive dose is 125 mg twice daily, and should be started the day before ascent.
Dexamethasone. In addition to treating AMS, dexamethasone may be taken as a preventive in doses of 2 mg every 6 hours or 4 mg twice daily.3 However, unlike acetazolamide, which acts to facilitate acclimatization, dexamethasone only prevents symptoms. Thus, cessation of the drug can result in rebound AMS symptoms, and prolonged use can result in adrenal suppression.3 Therefore, it should not be used for more than 10 days.
Sumatriptan and Gabapentin. In recent studies, sumatriptan and gabapentin haven shown benefit in preventing AMS, 9,10 but further study is needed before either of these drugs can be recommended.
Ginkgo Biloba. While ginkgo biloba has been touted as an effective preventive treatment, studies have shown no benefit to its use.8
Ibuprofen. ibuprofen 600 mg three times daily can be initiated the day prior to ascent, and has been shown to decrease the incidence of AMS.7
High-Altitude Cerebral Edema
Mechanism of HACE
The exact mechanism of HACE is unclear. Magnetic resonance imaging of patients with the condition demonstrates cerebral edema primarily localized to the corpus callosum.11 These findings suggest an increased permeability in the blood-brain barrier, leading to vasogenic cerebral edema. Cases of death associated with HACE are the result of herniation. Fortunately, if the condition is recognized promptly and appropriate management is instituted, most patients will recover without permanent deficits.
Current recommendations for treating HACE are similar to treatment strategies for AMS.
Descent. A therapeutic priority, descent may prove challenging as the patient may be ataxic, have altered mental status, and have difficulty facilitating his or her own descent.
Oxygen. A portable HBO bag can be used to simulate descent until evacuation is possible. Supplemental oxygen should be applied immediately.
Dexamethasone. In treating HACE, dexamethasone may be administered at a loading dose of 8 mg, followed by 4 mg every 6 hours.3
Airway Management. If the patient has significantly altered mental status, appropriate airway management must be initiated.
High-Altitude Pulmonary Edema
The most common cause of death from altitude illness is HAPE,12 a form of noncardiogenic pulmonary edema. This condition generally occurs at elevations above 3,000 m. Symptoms begin 2 to 5 days after ascent and progress in a typical pattern. A patient will initially experience a nonproductive cough and dyspnea at rest. The dyspnea worsens, and the cough becomes productive of pink, frothy sputum. Without medical intervention, lethargy, coma, and death may follow.
Symptoms of HAPE generally worsen following a night of sleep at elevation. Physical examination reveals crackles, tachycardia, tachypnea, and hypoxia. Diagnosis requires at least two of the following signs:
- Crackles or wheezing in at least one lung field
- Central cyanosis
- Tachypnea
- Tachycardia.
In addition to the above signs, at least two of the following symptoms must also be present:
- Dyspnea at rest
- Cough
- Weakness or decreased exercise performance
- Chest tightness
- Congestion.
Mechanism of HAPE
The mechanism of HAPE is better understood than that of AMS and HACE. In HAPE, high microvascular pressures in the lungs lead to elevated pulmonary vascular resistance and pulmonary artery pressure. Pulmonary edema ensues, but left ventricular function is preserved. Patients with a naturally low HVR, high pulmonary artery pressures at rest, preexisting pulmonary hypertension, or a previous history of HAPE are predisposed to developing the condition. Risk factors include heavy exertion, rapid ascent, cold, salt ingestion, and sleeping medications.
Treatment
Decent and warming of the patient as soon as possible, along with treatment outlined below, are essential.
Oxygen. Treatment of HAPE begins with supplemental oxygen to immediately lower pulmonary artery pressure. Oxygen should initially be administered at 4 to 6 L/min; if the patient improves clinically and can maintain oxygen saturations greater than 90%, oxygen may be decreased with a goal to maintain saturation above 90%.
Nifedipine. Following oxygen, descent, and warming, nifedipine can be used as an adjunctive therapy. The treatment dose for HAPE is 20 to 30 mg of the sustained release form every 12 hours.3
Salmeterol/Albuterol and Expiratory Positive Airway Pressure. The oral inhalers salmeterol or albuterol may be used for bronchodilation; however, there is little evidence to support their effectiveness in HAPE. Ventilation with expiratory positive airway pressure can be employed if available.
Prevention
For patients with a predisposition to HAPE, preventive measures should be considered prior to ascent. As with all forms of altitude illness, gradual ascent is the most effective prevention method available.
Phosphodiesterase Inhibitors. Phosphodiesterase inhibitors act via pulmonary vasodilation to prevent HAPE in some patients. Tadalafil at a dose of 10 mg twice daily or 20 mg once daily has been shown to reduce the incidence of HAPE.14 Alternatively, sildenafil 50 mg three times daily may be used.
Acetazolamide and β-Agonists. Although both acetazolamide and β-agonists such as albuterol have been theorized to aid in preventing HAPE, this has not been proven.15
Conclusion
Clinically, high-altitude illnesses range from subtle symptoms to severe, life threatening disease. Knowledge of these disease processes and clinical presentation prior to travel or work in a high-altitude setting is essential. Rapid recognition of symptoms and prompt, appropriate interventions, such as descent when necessary, can significantly improve the outcomes of these conditions.
Dr Haroutunian is an emergency physician, department of emergency medicine, Exempla St Joseph Hospital, Denver, Colorado. Dr Bono is professor and vice chairman, department of emergency medicine, Eastern Virginia Medical School, Norfolk.
Patients participating in occupational and sports-related activities requiring ascent to high elevations are at risk of developing a range of high-altitude illnesses. Prompt recognition and treatment are paramount to improving outcomes and preventing life-threatening sequelae. High-elevation locations are the setting of many recreational activities for outdoor enthusiasts. As such, illnesses associated with high altitude may be encountered by those summiting peaks, traveling by air, or working in flight medicine or as part of an emergency rescue team. The altitude syndromes discussed in this review are acute mountain sickness (AMS), high-altitude cerebral edema (HACE), and high-altitude pulmonary edema (HAPE). While these conditions do not represent all altitude-related illnesses, they are the primary pathological processes for which physicians should be familiar when working with high-altitude populations.
Physiological Response to Altitude
The Lake Louise Criteria
Acute Mountain Sickness
Acute mountain sickness comprises a constellation of symptoms caused by the atmospheric changes at elevations above approximately 2,500 m. It is the most common form of high-altitude illness, affecting 25% of travelers at moderate altitude and 50% to 85% above 4,000 m.3
Symptoms
The onset of symptoms (eg, headache, anorexia, nausea, vomiting, weakness) may occur at 2,000 m in the setting of rapid ascent—most commonly at 6 to 12 hours, but onset can range from 1 hour to 2 days after ascent. If symptoms begin after 3 days, other diagnoses should be considered. Symptoms of AMS are generally worse after the first night of sleep at elevation. On physical examination, vital signs are usually normal, though postural hypotension and tachycardia are possible. Oxygen saturation may be markedly decreased after rapid ascent, and chest auscultation may reveal rales in 20% of patients.4 Peripheral and facial edema may also be present. Funduscopic examination may show venous tortuosity and dilation, and retinal hemorrhage is common in ascents over 4,800 m.
Differential Diagnosis
The differential diagnosis for AMS is broad and includes hypothermia, dehydration, exhaustion, subarachnoid hemorrhage, intracranial mass, carbon monoxide poisoning, alcohol hangover, intoxication, central nervous system infection and migraine. Risk factors for developing AMS are a previous history of altitude illness, rapid ascent, and lack of previous acclimatization. Interestingly, physical fitness does not protect a person from developing AMS.5
Mechanism of AMS
The true mechanism of AMS is uncertain, but it is clear that a fall in barometric pressure results in hypobaric hypoxia. This is thought to lead to an increased blood volume in the brain and increased cerebral blood flow, possibly precipitating an enlarged brain. A mechanism related to vasogenic edema has been proposed due to patients’ clinical improvement with dexamethasone therapy.6 Acute mountain sickness does appear to be related to overall fluid balance, as an increase in reninangiotensin, aldosterone, and antidiuretic hormone has been observed in patients with the condition. Elevation of these hormones is contrary to the appropriate physiological response of diuresis.
Treatment
Treatment of AMS begins with descent from elevation as soon as possible. Descent should be at least 500 m from the aggravating elevation. Patients should remain at least 1 to 2 days at this lower elevation before attempting reascent. If descent is not feasible, any further ascent should be delayed until symptoms have resolved.
Dexamethasone. This glucocorticoid has been used clinically with good success, although the mechanism of action in unclear. The initial dose is 8 mg followed by 4 mg every 6 hours.3
Acetazolamide. A carbonic anhydrase inhibitor, acetazolamide acts to temper symptoms by causing an acidosis that increases ventilation and prevents periodic breathing and hypoxia during sleep. The standard dose is 250 mg twice daily.3
Oxygen. Supplemental oxygen provided at 1 to 2 L/min via nasal cannula for 12 to 24 hours may help to improve symptoms. A portable hyperbaric oxygen (HBO) bag (eg, a Gamow bag) can be used to create an effective altitude of approximately 1,500 to 2,000 m inside the bag. The patient is placed completely within the bag, the zipper is sealed shut, and the bag is inflated with a foot pump. Treatment in such a chamber can be provided in 1-hour increments and repeated as needed. However, if descent is possible, use of the HBO chamber should not prevent or delay descent.
Ibuprofen. Compared to placebo, studies have shown ibuprofen 600 mg three times a day reduces the severity of AMS.7
Prevention
Strategies to prevent AMS are similar to those used to treat the condition. These include gradual ascent and prophylactic drug therapy.
Gradual Ascent. Gradual ascent is the primary strategy to prevent AMS. At altitudes above 3,000 m, each subsequent night should not be spent at an elevation 300 m higher than the previous night.
Acetazolamide. Pretreatment with acetazolamide is indicated for patients with a history of altitude illness or who anticipate an abrupt ascent (eg, rescue workers). Acetazolamide has been shown in multiple studies to be effective in the prevention of AMS.8 Adverse side effects of acetazolamide include paresthesias and increased urinary frequency; the drug may also make carbonated beverages taste flat. The preventive dose is 125 mg twice daily, and should be started the day before ascent.
Dexamethasone. In addition to treating AMS, dexamethasone may be taken as a preventive in doses of 2 mg every 6 hours or 4 mg twice daily.3 However, unlike acetazolamide, which acts to facilitate acclimatization, dexamethasone only prevents symptoms. Thus, cessation of the drug can result in rebound AMS symptoms, and prolonged use can result in adrenal suppression.3 Therefore, it should not be used for more than 10 days.
Sumatriptan and Gabapentin. In recent studies, sumatriptan and gabapentin haven shown benefit in preventing AMS, 9,10 but further study is needed before either of these drugs can be recommended.
Ginkgo Biloba. While ginkgo biloba has been touted as an effective preventive treatment, studies have shown no benefit to its use.8
Ibuprofen. ibuprofen 600 mg three times daily can be initiated the day prior to ascent, and has been shown to decrease the incidence of AMS.7
High-Altitude Cerebral Edema
Mechanism of HACE
The exact mechanism of HACE is unclear. Magnetic resonance imaging of patients with the condition demonstrates cerebral edema primarily localized to the corpus callosum.11 These findings suggest an increased permeability in the blood-brain barrier, leading to vasogenic cerebral edema. Cases of death associated with HACE are the result of herniation. Fortunately, if the condition is recognized promptly and appropriate management is instituted, most patients will recover without permanent deficits.
Current recommendations for treating HACE are similar to treatment strategies for AMS.
Descent. A therapeutic priority, descent may prove challenging as the patient may be ataxic, have altered mental status, and have difficulty facilitating his or her own descent.
Oxygen. A portable HBO bag can be used to simulate descent until evacuation is possible. Supplemental oxygen should be applied immediately.
Dexamethasone. In treating HACE, dexamethasone may be administered at a loading dose of 8 mg, followed by 4 mg every 6 hours.3
Airway Management. If the patient has significantly altered mental status, appropriate airway management must be initiated.
High-Altitude Pulmonary Edema
The most common cause of death from altitude illness is HAPE,12 a form of noncardiogenic pulmonary edema. This condition generally occurs at elevations above 3,000 m. Symptoms begin 2 to 5 days after ascent and progress in a typical pattern. A patient will initially experience a nonproductive cough and dyspnea at rest. The dyspnea worsens, and the cough becomes productive of pink, frothy sputum. Without medical intervention, lethargy, coma, and death may follow.
Symptoms of HAPE generally worsen following a night of sleep at elevation. Physical examination reveals crackles, tachycardia, tachypnea, and hypoxia. Diagnosis requires at least two of the following signs:
- Crackles or wheezing in at least one lung field
- Central cyanosis
- Tachypnea
- Tachycardia.
In addition to the above signs, at least two of the following symptoms must also be present:
- Dyspnea at rest
- Cough
- Weakness or decreased exercise performance
- Chest tightness
- Congestion.
Mechanism of HAPE
The mechanism of HAPE is better understood than that of AMS and HACE. In HAPE, high microvascular pressures in the lungs lead to elevated pulmonary vascular resistance and pulmonary artery pressure. Pulmonary edema ensues, but left ventricular function is preserved. Patients with a naturally low HVR, high pulmonary artery pressures at rest, preexisting pulmonary hypertension, or a previous history of HAPE are predisposed to developing the condition. Risk factors include heavy exertion, rapid ascent, cold, salt ingestion, and sleeping medications.
Treatment
Decent and warming of the patient as soon as possible, along with treatment outlined below, are essential.
Oxygen. Treatment of HAPE begins with supplemental oxygen to immediately lower pulmonary artery pressure. Oxygen should initially be administered at 4 to 6 L/min; if the patient improves clinically and can maintain oxygen saturations greater than 90%, oxygen may be decreased with a goal to maintain saturation above 90%.
Nifedipine. Following oxygen, descent, and warming, nifedipine can be used as an adjunctive therapy. The treatment dose for HAPE is 20 to 30 mg of the sustained release form every 12 hours.3
Salmeterol/Albuterol and Expiratory Positive Airway Pressure. The oral inhalers salmeterol or albuterol may be used for bronchodilation; however, there is little evidence to support their effectiveness in HAPE. Ventilation with expiratory positive airway pressure can be employed if available.
Prevention
For patients with a predisposition to HAPE, preventive measures should be considered prior to ascent. As with all forms of altitude illness, gradual ascent is the most effective prevention method available.
Phosphodiesterase Inhibitors. Phosphodiesterase inhibitors act via pulmonary vasodilation to prevent HAPE in some patients. Tadalafil at a dose of 10 mg twice daily or 20 mg once daily has been shown to reduce the incidence of HAPE.14 Alternatively, sildenafil 50 mg three times daily may be used.
Acetazolamide and β-Agonists. Although both acetazolamide and β-agonists such as albuterol have been theorized to aid in preventing HAPE, this has not been proven.15
Conclusion
Clinically, high-altitude illnesses range from subtle symptoms to severe, life threatening disease. Knowledge of these disease processes and clinical presentation prior to travel or work in a high-altitude setting is essential. Rapid recognition of symptoms and prompt, appropriate interventions, such as descent when necessary, can significantly improve the outcomes of these conditions.
Dr Haroutunian is an emergency physician, department of emergency medicine, Exempla St Joseph Hospital, Denver, Colorado. Dr Bono is professor and vice chairman, department of emergency medicine, Eastern Virginia Medical School, Norfolk.
- Hackett PH, Oelz O. The Lake Louise consensus on the definition and qualification of altitude illness. In: Sutton JR, Coates G, Houston CS, eds. Hypoxia and Mountain Medicine. Burlington, VT: Queen City Printers; 1992:327-330.
- Roach RC, Bärtch P, Hackett PH, Oelz O, and the Lake Louise AMS Scoring Consensus Committee. The Lake Louise Acute Mountain Sickness Scoring System. In: Hypoxia and Molecular Medicine. Proceedings of the 8th International Hypoxia Symposium. Burlington, VT: Queen City Printers; 1993:272-274.
- Eide RP 3rd, Asplund CA. Altitude illness: update on prevention and treatment. Curr Sports Med Rep. 2012;11(3):124-130.
- Milzman DP, Damergis JA, Napoli AM. Rapid ascent changes in vitals at altitude. Ann Emerg Med. 2008;51(4):536.
- Bärtsch P, Swenson ER. Clinical practice: Acute high-altitude illnesses. N Engl J Med. 2013;368(24):2294-2302.
- Hackett PH, Roach RC. Medical therapy of mountain illness. Ann Emerg Med. 1987;16(9):980-986.
- Lipman GS, Kanaan NC, Holck PS, Constance BB, Gertsch JH; PAINS Group. Ibuprofen prevents altitude illness: a randomized controlled trial for prevention of altitude illness with nonsteroidal anti-inflammatories. Ann Emerg Med. 2012;59(6): 484-490.
- Seupaul RA, Welch JL, Malka ST, Emmett TW. Pharmacologic prophylaxis for acute mountain sickness: a systematic shortcut review. Ann Emerg Med. 2012; 59(4):307-317.
- Jafarian S, Gorouhi F, Salimi S, Lotfi J. Sumatriptan for prevention of acute mountain sickness: randomized clinical trial. Ann Neurol. 2007;62(3):273-277.
- Jafarian S, Abolfazli R, Gorouhi F, Rezaie S, Lotfi J. Gabapentin for prevention of hypobaric hypoxia-induced headache: randomized double-blind clinical trial. J Neurol Neurosurg Psychiatry. 2008;79(3): 321-323.
- Hackett PH, Yarnell PR, Hill R, Reynard K, Heit J, McCormick J. High-altitude cerebral edema evaluated with magnetic resonance imaging: clinical correlation and pathophysiology. JAMA. 1998;280(22):1920-1925.
- Gallagher SA1, Hackett PH. High-altitude illness. Emerg Med Clin North Am. 2004;22(2):329-355.
- Fagenholz PJ, Gutman JA, Murray AF, Noble VE, Thomas SH, Harris NS. Chest ultrasonography for the diagnosis and monitoring of high-altitude pulmonary edema. Chest. 2007;131(4): 1013-1018.
- Leshem E1, Caine Y, Rosenberg E, Maaravi Y, Hermesh H, Schwartz E. Tadalafil and acetazolamide versus acetazolamide for the prevention of severe high-altitude illness. J Travel Med. 2012;19(5): 308-310.
- Schoene RB. Illnesses at high altitude. Chest. 2008;134(2):402-416.
- Hackett PH, Oelz O. The Lake Louise consensus on the definition and qualification of altitude illness. In: Sutton JR, Coates G, Houston CS, eds. Hypoxia and Mountain Medicine. Burlington, VT: Queen City Printers; 1992:327-330.
- Roach RC, Bärtch P, Hackett PH, Oelz O, and the Lake Louise AMS Scoring Consensus Committee. The Lake Louise Acute Mountain Sickness Scoring System. In: Hypoxia and Molecular Medicine. Proceedings of the 8th International Hypoxia Symposium. Burlington, VT: Queen City Printers; 1993:272-274.
- Eide RP 3rd, Asplund CA. Altitude illness: update on prevention and treatment. Curr Sports Med Rep. 2012;11(3):124-130.
- Milzman DP, Damergis JA, Napoli AM. Rapid ascent changes in vitals at altitude. Ann Emerg Med. 2008;51(4):536.
- Bärtsch P, Swenson ER. Clinical practice: Acute high-altitude illnesses. N Engl J Med. 2013;368(24):2294-2302.
- Hackett PH, Roach RC. Medical therapy of mountain illness. Ann Emerg Med. 1987;16(9):980-986.
- Lipman GS, Kanaan NC, Holck PS, Constance BB, Gertsch JH; PAINS Group. Ibuprofen prevents altitude illness: a randomized controlled trial for prevention of altitude illness with nonsteroidal anti-inflammatories. Ann Emerg Med. 2012;59(6): 484-490.
- Seupaul RA, Welch JL, Malka ST, Emmett TW. Pharmacologic prophylaxis for acute mountain sickness: a systematic shortcut review. Ann Emerg Med. 2012; 59(4):307-317.
- Jafarian S, Gorouhi F, Salimi S, Lotfi J. Sumatriptan for prevention of acute mountain sickness: randomized clinical trial. Ann Neurol. 2007;62(3):273-277.
- Jafarian S, Abolfazli R, Gorouhi F, Rezaie S, Lotfi J. Gabapentin for prevention of hypobaric hypoxia-induced headache: randomized double-blind clinical trial. J Neurol Neurosurg Psychiatry. 2008;79(3): 321-323.
- Hackett PH, Yarnell PR, Hill R, Reynard K, Heit J, McCormick J. High-altitude cerebral edema evaluated with magnetic resonance imaging: clinical correlation and pathophysiology. JAMA. 1998;280(22):1920-1925.
- Gallagher SA1, Hackett PH. High-altitude illness. Emerg Med Clin North Am. 2004;22(2):329-355.
- Fagenholz PJ, Gutman JA, Murray AF, Noble VE, Thomas SH, Harris NS. Chest ultrasonography for the diagnosis and monitoring of high-altitude pulmonary edema. Chest. 2007;131(4): 1013-1018.
- Leshem E1, Caine Y, Rosenberg E, Maaravi Y, Hermesh H, Schwartz E. Tadalafil and acetazolamide versus acetazolamide for the prevention of severe high-altitude illness. J Travel Med. 2012;19(5): 308-310.
- Schoene RB. Illnesses at high altitude. Chest. 2008;134(2):402-416.
Emergency Imaging: What is the suspected diagnosis? Is additional imaging necessary, and if so, why?
A 25-year-old man with no significant past medical history presented with low-back pain that radiated down into his right thigh. The patient stated the pain began 1 week earlier when he was lifting weights and had increased in severity to the point where he was no longer able to walk or stand up straight. He had taken nonprescription nonsteroidal anti-inflammatory drugs but received no significant relief.
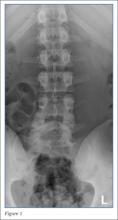
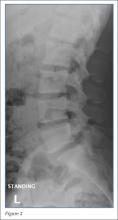
Radiographs of the lumbosacral spine were obtained; representative anteroposterior (AP) and lateral images are shown above (Figures 1 and 2).
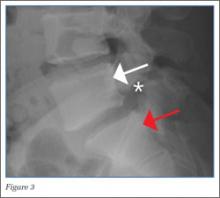
The lateral view of the lumbar spine demonstrates mild anterolisthesis of L5 on S1 with the posterior cortex of L5 (white arrow, Figure 3) anterior to the posterior cortex of S1 (red arrow, Figure 3). Normally, the posterior cortices of the adjacent vertebral bodies should align. Lucency is also noted in the region of the pars interarticularis (white asterisk, Figure 3). The combination of anterolisthesis and this lucency in a young patient suggests the diagnosis of spondylolysis (pars defect).
The pathophysiology of spondylolysis is still uncertain. Two theories have been proposed—underlying dysplastic pars interarticularis versus repetitive microtrauma resulting in stress factors are the two proposed underlying mechanism. If patients are genetically predisposed, underlying dysplasia probably contributes to the pathology, while microtrauma triggers the actual defect.2 Most patients respond well with conservative management.
When evaluating for spondylolysis, AP, lateral, 45-degree right and left oblique views, and collimated lateral views of the lumbosacral spine should be obtained. With this five-view study, up to 96.5% of pars defect can be identified.
In the general population, if spondylolysis is suspected and radiographs are negative, magnetic resonance imaging, computed tomography, and/or single-photon emission computed tomography bone scintigraphy can be used for further evaluation.4,5 In this case, the diagnosis was made based on radiographic imaging, and the patient was discharged with a scheduled follow-up with an orthopedic surgeon.
Dr Salama is a resident of radiology, resident of radiology, New York-Presbyterian Hospital/Weill Cornell Medical Center, New York. Dr Belfi is an assistant professor of radiology, Weill Cornell Medical College New York; and an assistant attending radiologist, New York-Presbyterian Hospital/Weill Cornell Medical Center, New York. Dr Hentel is an associate professor of clinical radiology, Weill Cornell Medical College, New York. He is also chief of emergency/musculoskeletal imaging and executive vice-chairman for the department of radiology, New York-Presbyterian Hospital/Weill Cornell Medical Center. He is associate editor, imaging, of the EMERGENCY MEDICINE editorial board.
- Belfi LM, Ortiz AO, Katz DS. Computed tomography evaluation of spondylolysis and spondylolisthesis in asymptomatic patients. Spine (Phila Pa 1976). 2006;31(24):E907-E910. doi:10.1097/01.brs.0000245947.31473.0a.
- Foreman P, Griessenauer CJ, Watanabe K, et al. L5 spondylolysis/spondylolisthesis: a comprehensive review with an anatomic focus. Childs Nerv Syst. 2013;29(2):209-216. doi:10.1007/s00381-012-1942-2.
- Amato M, Totty WG, Gilula LA. Spondylolysis of the lumbar spine: demonstration of defects and laminal fragmentation. Radiology. 1984;153(3):627-629.
- Saraste H, Nilsson B, Broström LA, et al. Relationship between radiological and clinical variables in spondylolysis. Int Orthop. 1984;8(3):163-174. doi:10.1007/BF00269912.
- Lee JH, Ehara S, Tamakawa Y, Shimamura T. Spondylolysis of the upper lumbar spine: Radiological features. Clin Imaging. 1999;23(6):389-393. doi:10.1016/S0899-7071(99)00158-8.
A 25-year-old man with no significant past medical history presented with low-back pain that radiated down into his right thigh. The patient stated the pain began 1 week earlier when he was lifting weights and had increased in severity to the point where he was no longer able to walk or stand up straight. He had taken nonprescription nonsteroidal anti-inflammatory drugs but received no significant relief.
Radiographs of the lumbosacral spine were obtained; representative anteroposterior (AP) and lateral images are shown above (Figures 1 and 2).
The lateral view of the lumbar spine demonstrates mild anterolisthesis of L5 on S1 with the posterior cortex of L5 (white arrow, Figure 3) anterior to the posterior cortex of S1 (red arrow, Figure 3). Normally, the posterior cortices of the adjacent vertebral bodies should align. Lucency is also noted in the region of the pars interarticularis (white asterisk, Figure 3). The combination of anterolisthesis and this lucency in a young patient suggests the diagnosis of spondylolysis (pars defect).
The pathophysiology of spondylolysis is still uncertain. Two theories have been proposed—underlying dysplastic pars interarticularis versus repetitive microtrauma resulting in stress factors are the two proposed underlying mechanism. If patients are genetically predisposed, underlying dysplasia probably contributes to the pathology, while microtrauma triggers the actual defect.2 Most patients respond well with conservative management.
When evaluating for spondylolysis, AP, lateral, 45-degree right and left oblique views, and collimated lateral views of the lumbosacral spine should be obtained. With this five-view study, up to 96.5% of pars defect can be identified.
In the general population, if spondylolysis is suspected and radiographs are negative, magnetic resonance imaging, computed tomography, and/or single-photon emission computed tomography bone scintigraphy can be used for further evaluation.4,5 In this case, the diagnosis was made based on radiographic imaging, and the patient was discharged with a scheduled follow-up with an orthopedic surgeon.
Dr Salama is a resident of radiology, resident of radiology, New York-Presbyterian Hospital/Weill Cornell Medical Center, New York. Dr Belfi is an assistant professor of radiology, Weill Cornell Medical College New York; and an assistant attending radiologist, New York-Presbyterian Hospital/Weill Cornell Medical Center, New York. Dr Hentel is an associate professor of clinical radiology, Weill Cornell Medical College, New York. He is also chief of emergency/musculoskeletal imaging and executive vice-chairman for the department of radiology, New York-Presbyterian Hospital/Weill Cornell Medical Center. He is associate editor, imaging, of the EMERGENCY MEDICINE editorial board.
A 25-year-old man with no significant past medical history presented with low-back pain that radiated down into his right thigh. The patient stated the pain began 1 week earlier when he was lifting weights and had increased in severity to the point where he was no longer able to walk or stand up straight. He had taken nonprescription nonsteroidal anti-inflammatory drugs but received no significant relief.
Radiographs of the lumbosacral spine were obtained; representative anteroposterior (AP) and lateral images are shown above (Figures 1 and 2).
The lateral view of the lumbar spine demonstrates mild anterolisthesis of L5 on S1 with the posterior cortex of L5 (white arrow, Figure 3) anterior to the posterior cortex of S1 (red arrow, Figure 3). Normally, the posterior cortices of the adjacent vertebral bodies should align. Lucency is also noted in the region of the pars interarticularis (white asterisk, Figure 3). The combination of anterolisthesis and this lucency in a young patient suggests the diagnosis of spondylolysis (pars defect).
The pathophysiology of spondylolysis is still uncertain. Two theories have been proposed—underlying dysplastic pars interarticularis versus repetitive microtrauma resulting in stress factors are the two proposed underlying mechanism. If patients are genetically predisposed, underlying dysplasia probably contributes to the pathology, while microtrauma triggers the actual defect.2 Most patients respond well with conservative management.
When evaluating for spondylolysis, AP, lateral, 45-degree right and left oblique views, and collimated lateral views of the lumbosacral spine should be obtained. With this five-view study, up to 96.5% of pars defect can be identified.
In the general population, if spondylolysis is suspected and radiographs are negative, magnetic resonance imaging, computed tomography, and/or single-photon emission computed tomography bone scintigraphy can be used for further evaluation.4,5 In this case, the diagnosis was made based on radiographic imaging, and the patient was discharged with a scheduled follow-up with an orthopedic surgeon.
Dr Salama is a resident of radiology, resident of radiology, New York-Presbyterian Hospital/Weill Cornell Medical Center, New York. Dr Belfi is an assistant professor of radiology, Weill Cornell Medical College New York; and an assistant attending radiologist, New York-Presbyterian Hospital/Weill Cornell Medical Center, New York. Dr Hentel is an associate professor of clinical radiology, Weill Cornell Medical College, New York. He is also chief of emergency/musculoskeletal imaging and executive vice-chairman for the department of radiology, New York-Presbyterian Hospital/Weill Cornell Medical Center. He is associate editor, imaging, of the EMERGENCY MEDICINE editorial board.
- Belfi LM, Ortiz AO, Katz DS. Computed tomography evaluation of spondylolysis and spondylolisthesis in asymptomatic patients. Spine (Phila Pa 1976). 2006;31(24):E907-E910. doi:10.1097/01.brs.0000245947.31473.0a.
- Foreman P, Griessenauer CJ, Watanabe K, et al. L5 spondylolysis/spondylolisthesis: a comprehensive review with an anatomic focus. Childs Nerv Syst. 2013;29(2):209-216. doi:10.1007/s00381-012-1942-2.
- Amato M, Totty WG, Gilula LA. Spondylolysis of the lumbar spine: demonstration of defects and laminal fragmentation. Radiology. 1984;153(3):627-629.
- Saraste H, Nilsson B, Broström LA, et al. Relationship between radiological and clinical variables in spondylolysis. Int Orthop. 1984;8(3):163-174. doi:10.1007/BF00269912.
- Lee JH, Ehara S, Tamakawa Y, Shimamura T. Spondylolysis of the upper lumbar spine: Radiological features. Clin Imaging. 1999;23(6):389-393. doi:10.1016/S0899-7071(99)00158-8.
- Belfi LM, Ortiz AO, Katz DS. Computed tomography evaluation of spondylolysis and spondylolisthesis in asymptomatic patients. Spine (Phila Pa 1976). 2006;31(24):E907-E910. doi:10.1097/01.brs.0000245947.31473.0a.
- Foreman P, Griessenauer CJ, Watanabe K, et al. L5 spondylolysis/spondylolisthesis: a comprehensive review with an anatomic focus. Childs Nerv Syst. 2013;29(2):209-216. doi:10.1007/s00381-012-1942-2.
- Amato M, Totty WG, Gilula LA. Spondylolysis of the lumbar spine: demonstration of defects and laminal fragmentation. Radiology. 1984;153(3):627-629.
- Saraste H, Nilsson B, Broström LA, et al. Relationship between radiological and clinical variables in spondylolysis. Int Orthop. 1984;8(3):163-174. doi:10.1007/BF00269912.
- Lee JH, Ehara S, Tamakawa Y, Shimamura T. Spondylolysis of the upper lumbar spine: Radiological features. Clin Imaging. 1999;23(6):389-393. doi:10.1016/S0899-7071(99)00158-8.
Case Report: Headache in a Postpartum Patient With Essential Thrombocytosis
Case
At presentation, the patient was in no apparent distress and had normal vital signs, including normal blood pressure. On physical examination, she was alert and oriented, with unremarkable head, neck, fundoscopic, cardiac, pulmonary, skin, and neurological examinations. Laboratory results were only notable for a platelet count of 677 x 109/L. The remainder of the complete blood count, chemistry, and coagulation panels was all within normal limits. A noncontrast computed tomography (CT) of the brain was unremarkable. She was treated symptomatically with ketorolac, diphenhydramine, and metoclopramide, but received no relief.
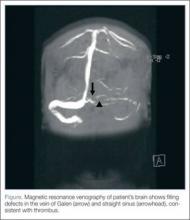
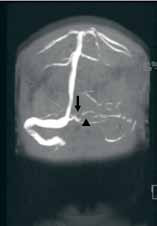
Based on the patient’s history of ET and postpartum state, there was a high suspicion for cerebral venous thrombosis (CVT). Magnetic resonance imaging/magnetic resonance venography (MRI/MRV) of the brain was obtained, which revealed near-complete thrombosis of the vein of Galen and straight sinus (Figure). Neurology and hematology services were consulted emergently, and the patient was started on a heparin drip and admitted to the neurosurgical intensive care unit. Hydroxyurea was also given for cytoreduction, and platelets normalized to 305 x 109/L within 1 day of treatment. The patient’s neurological examination remained nonfocal, her headache gradually resolved, and she was discharged on hospital day 3 on oral anticoagulants.
Discussion
Essential thrombocytosis is a chronic myeloproliferative disorder characterized by clonal proliferation of the megakaryocyte cell line due to a defect in a pluripotent hematopoietic stem cell.1 The estimated annual incidence is 2.5 per 100,000 individuals.2 It affects patients of both genders, most commonly between ages 50 to 70 years.3
Up to 25% of patients with ET experience neurological complications, mainly occlusive strokes, chronic headache, and dizziness.4 Since pregnancy also increases thrombogenicity, and CVT occurs in 12 per 100,000 deliveries,5 it should be considered in any pregnant or postpartum patient with neurological symptoms.
Diagnosis of ET
Essential thrombocytosis is a diagnosis of exclusion, and no clinical or laboratory finding is diagnostic.1 Although patients with ET are at increased risk for bleeding, these events usually involve mucosal and cutaneous sites and are clinically insignificant. Thrombotic complications account for the majority of the morbidity and mortality associated with ET and can be both arterial and venous. In one case series, roughly 50% patients with ET experienced a thrombotic event within 9 years from diagnosis.6 Thrombotic complications are seen most commonly in patients younger than age 55 years at diagnosis. Common sites of thrombosis include the deep veins of the lower extremities, pulmonary vessels, hepatic vein, portal vein, digital microvasculature, and placenta.1
Despite the high rate of thrombotic complications in ET, there is little data on neurological complications and few reports of CVT in patients with ET.7-9 In one chart review of 70 patients with ET, researchers identified 18 patients who developed neurological complications,4 the most common of which was cerebrovascular accident/transient ischemic attack. Only one patient in the series had a CVT. The majority of patients with neurological events were female and no clinical or laboratory parameter predicted the risk of neurological event.
Thrombocytosis
Thrombocytosis is classified as either primary/essential or secondary/reactive. The majority of thrombocytosis is secondary/reactive and is caused by a variety of inflammatory conditions, such as infection and malignancy. Unlike ET, secondary thrombocytosis is not associated with any bleeding or thrombotic complications and does not require direct treatment.1
Treatment of ET
The decision to treat ET is based on clinical parameters and is usually reserved for patients at high-risk of thrombosis, including those with history of thrombosis or platelets >1,500 x 109/L.1 The first-line agent for treatment of essential thrombocytosis, hydroxyurea has been shown to decrease thrombotic episodes.10 Other cytoreductive agents include anagrilide and interferon-α, the use of which is considered safe in pregnancy.1
Cerebral Venous Thrombosis
Cerebral venous thrombosis is a rare but potentially fatal condition. Patients may present with headache (95%), seizures (47%), and vision changes (41%), and may or may not have focal or generalized neurological deficits.11 Thrombosis can lead to venous obstruction, resulting in increased intracranial pressure and ultimately cerebral herniation and death. Predisposing risk factors for CVT include thrombophilic and procoagulant disorders, maxillofacial infections, trauma, malignancy, and vasculitides. Among female patients with CVT, studies show approximately 50% were on oral contraceptives and 20% were pregnant or in the postpartum period at the time of the event. Nearly half of patients with CVT have multiple risk factors.12
Diagnosis of CVT
Diagnosis of CVT requires a high clinical suspicion. Lumbar puncture usually reveals high opening pressure with normal cerebrospinal fluid analysis.13 The classic finding of CVT on a contrast-enhanced CT scan is the “empty delta sign,” which is a central hypointensity within the superior sagittal sinus secondary due to slow/absent flow surrounded by contrast enhancement in a triangular shape. An ischemic infarction crossing arterial boundaries or near a venous sinus—with or without a hemorrhagic component—is also suggestive of CVT. However, CT scans are read as normal or indeterminate in up to 30% patients.14 Moreover, while CT venography may visualize the cerebral venous system, there is a false-negative rate of 25%.15 Therefore, MRI with MRV is the gold standard for diagnosis.
Pregnancy and CVT
As previously noted, pregnant or postpartum patients are at an increased risk of developing CVT. During pregnancy, decreased levels of protein S inhibitors and increased levels of fibrinogen, clotting factors, and protein C inhibitors lead to increased thrombogenicity.16 Older maternal age, pregnancy-related hypertension, peripartum infections, and cesarean delivery also increase the risk of CVT.16
Patients with CVT associated with pregnancy have more acute onset of symptoms and neurological findings, and 2% to 10% less mortality than patients with CVT secondary to other etiologies. Cerebral venous thrombosis should be considered in the differential diagnosis of any pregnant or postpartum patient presenting with neurological symptoms.
Treatment of CVT
Regardless of underlying etiology or symptom duration, all patients with CVT should receive anticoagulation therapy. Unfractionated or low-molecular weight heparin has been shown to decrease thrombus propagation, increase the rate of recanalization, and improve long-term outcomes—even in the presence of intracranial hemorrhage.17 In the 30% to 40% of patients with CVT who present with intracranial hemorrhage, anticoagulation decreases mortality and is not associated with new or enlarged bleeding.17-19 Patients who continue to deteriorate despite anticoagulation therapy may benefit from endovascular thrombolysis or decompressive hemicraniectomy.20 In a study of patients with CVT, 80% had full recovery, 6% had minor disability, and 14% had a poor outcome.12
Conclusion
This case describes a patient with two risk factors for CVT, a life-threatening condition. It should be considered in patients with ET and/or pregnant and postpartum patients presenting with headache or other neurological symptoms. Magnetic resonance venography is the diagnostic imaging of choice as the CVT may not be visible on CT. All patients with thrombosis and ET should receive anticoagulation therapy and hydroxyurea to prevent cerebral herniation and death.
Dr Canders is a resident, department of emergency medicine, David Geffen School of Medicine, University of California, Los Angeles. Dr Taira is a clinical assistant professor, department of emergency medicine, Los Angeles County Hospital/University of Southern California, Los Angeles.
Disclosure: The authors report no financial disclosures or conflicts of interests related to this article.
- Schafer AI. Thrombocytosis and thrombocythemia. Blood Rev. 2001;15(4):159-166.
- Mesa RA, Silverstein MN, Jacobsen SJ, Wollan PC, Tefferi A. Population-based incidence and survival figures in essential thrombocythemia and agnogenic myeloid metaplasia: an Olmsted County Study, 1976-1995. Am J Hematol. 1999;61():10-15.
- Tefferi A. Recent progress in the pathogenesis and management of essential thrombocythemia. Leuk Res. 2001;25(5):369-377.
- Kesler A, Ellis MH, Manor Y, Gadoth N, Lishner M. Neurological complications of essential thrombocytosis (ET). Acta Neurol Scand. 2000;102(5):299-302.
- Cantú C, Barinagarrementeria F. Cerebral venous thrombosis associated with pregnancy and puerperium: Review of 67 cases. Stroke. 1993;24(12):1880-1884.
- Bazzan M, Tamponi G, Schinco P, Vaccarino A, Foli C, Gallone G, et al. Thrombosis-free survival and life expectancy in 187 consecutive patients with essential thrombocythemia. Ann Hematol. 1999;78(12):539-543.
- Walther EU, Tiecks FP, Haberl RL. Cranial sinus thrombosis associated with essential thrombocythemia followed by heparin-associated thrombocytopenia. Neurology. 1996;47(1):300-301.
- McDonald TD, Tatemichi TK, Kranzler SJ, Chi L, Hilal SK, Mohr JP. Thrombosis of the superior sagittal sinus associated with essential thrombocytosis followed by MRI during anticoagulant therapy. Neurology. 1989;39(11):1554-1555.
- Iob I, Scanarini M, Andrioli GC, Pardatscher K. Thrombosis of the superior sagittal sinus associated with idiopathic thrombocytosis. Surg Neurol. 1979;11(6):439-41.
- Cortelazzo S, Finazzi G, Ruggeri M, Vestri O, Galli M, Rodeghiero F, et al. Hydroxyurea for patients with essential thrombocythemia and a high risk of thrombosis. N Engl J Med. 1995;332(17):1132-1136.
- de Bruijn SF, de Haan RJ, Stam J. Clinical features and prognostic factors of cerebral venous sinus thrombosis in a prospective series of 59 patients. For The Cerebral Venous Sinus Thrombosis Study Group. J Neurol Neurosurg Psychiatry. 2001;70(1):105-108.
- Ferro JM, Canhão P, Stam J, Bousser MG, Barinagarrementeria F; ISCVT Investigators. Prognosis of cerebral vein and dural sinus thrombosis: results of the International Study on Cerebral Vein and Dural Sinus Thrombosis (ISCVT). Stroke. 2004;35(3):664-670.
- DeLashaw MR, Vizioli TL, Jr, Counselman FL. Headache and seizure in a young woman postpartum. J Emerg Med. 2005;29(3):289-293.
- Bousser MG. Cerebral venous thrombosis: diagnosis and management. J Neurol. 2000;247(4):252-258.
- Grover M. Cerebral venous thrombosis as a cause of acute headache. J Am Board Fam Pract. 2004;17(4):295-298.
- Martinelli I, Sacchi E, Landi G, Taioli E, Duca F, Mannucci PM. High risk of cerebral venous thrombosis in carriers of prothrombic-gene mutation and in users of oral contraceptives. N Engl J Med. 1988;338(25):1793-1797.
- Star M, Flaster M. Advances and controversies in the management of cerebral venous thrombosis. Neurol Clin. 2013;31(3):765-783.
- Einhäupl KM, Villringer A, Meister W, Mehraein S, Garner C, Pellkofer M, et al. Heparin treatment in sinus venous thrombosis. Lancet. 1991;338(8767):597-600.
- Preter M, Tzourio C, Ameri A, Bousser MG. Long-term prognosis in cerebral venous thrombosis: follow-up of 77 patients. Stroke 1996;27(2):243-246.
- Coutinho JM, Stam J. How to treat cerebral venous and sinus thrombosis. J Thromb Haemost. 2010;8(5):877-883.
Case
At presentation, the patient was in no apparent distress and had normal vital signs, including normal blood pressure. On physical examination, she was alert and oriented, with unremarkable head, neck, fundoscopic, cardiac, pulmonary, skin, and neurological examinations. Laboratory results were only notable for a platelet count of 677 x 109/L. The remainder of the complete blood count, chemistry, and coagulation panels was all within normal limits. A noncontrast computed tomography (CT) of the brain was unremarkable. She was treated symptomatically with ketorolac, diphenhydramine, and metoclopramide, but received no relief.
Based on the patient’s history of ET and postpartum state, there was a high suspicion for cerebral venous thrombosis (CVT). Magnetic resonance imaging/magnetic resonance venography (MRI/MRV) of the brain was obtained, which revealed near-complete thrombosis of the vein of Galen and straight sinus (Figure). Neurology and hematology services were consulted emergently, and the patient was started on a heparin drip and admitted to the neurosurgical intensive care unit. Hydroxyurea was also given for cytoreduction, and platelets normalized to 305 x 109/L within 1 day of treatment. The patient’s neurological examination remained nonfocal, her headache gradually resolved, and she was discharged on hospital day 3 on oral anticoagulants.
Discussion
Essential thrombocytosis is a chronic myeloproliferative disorder characterized by clonal proliferation of the megakaryocyte cell line due to a defect in a pluripotent hematopoietic stem cell.1 The estimated annual incidence is 2.5 per 100,000 individuals.2 It affects patients of both genders, most commonly between ages 50 to 70 years.3
Up to 25% of patients with ET experience neurological complications, mainly occlusive strokes, chronic headache, and dizziness.4 Since pregnancy also increases thrombogenicity, and CVT occurs in 12 per 100,000 deliveries,5 it should be considered in any pregnant or postpartum patient with neurological symptoms.
Diagnosis of ET
Essential thrombocytosis is a diagnosis of exclusion, and no clinical or laboratory finding is diagnostic.1 Although patients with ET are at increased risk for bleeding, these events usually involve mucosal and cutaneous sites and are clinically insignificant. Thrombotic complications account for the majority of the morbidity and mortality associated with ET and can be both arterial and venous. In one case series, roughly 50% patients with ET experienced a thrombotic event within 9 years from diagnosis.6 Thrombotic complications are seen most commonly in patients younger than age 55 years at diagnosis. Common sites of thrombosis include the deep veins of the lower extremities, pulmonary vessels, hepatic vein, portal vein, digital microvasculature, and placenta.1
Despite the high rate of thrombotic complications in ET, there is little data on neurological complications and few reports of CVT in patients with ET.7-9 In one chart review of 70 patients with ET, researchers identified 18 patients who developed neurological complications,4 the most common of which was cerebrovascular accident/transient ischemic attack. Only one patient in the series had a CVT. The majority of patients with neurological events were female and no clinical or laboratory parameter predicted the risk of neurological event.
Thrombocytosis
Thrombocytosis is classified as either primary/essential or secondary/reactive. The majority of thrombocytosis is secondary/reactive and is caused by a variety of inflammatory conditions, such as infection and malignancy. Unlike ET, secondary thrombocytosis is not associated with any bleeding or thrombotic complications and does not require direct treatment.1
Treatment of ET
The decision to treat ET is based on clinical parameters and is usually reserved for patients at high-risk of thrombosis, including those with history of thrombosis or platelets >1,500 x 109/L.1 The first-line agent for treatment of essential thrombocytosis, hydroxyurea has been shown to decrease thrombotic episodes.10 Other cytoreductive agents include anagrilide and interferon-α, the use of which is considered safe in pregnancy.1
Cerebral Venous Thrombosis
Cerebral venous thrombosis is a rare but potentially fatal condition. Patients may present with headache (95%), seizures (47%), and vision changes (41%), and may or may not have focal or generalized neurological deficits.11 Thrombosis can lead to venous obstruction, resulting in increased intracranial pressure and ultimately cerebral herniation and death. Predisposing risk factors for CVT include thrombophilic and procoagulant disorders, maxillofacial infections, trauma, malignancy, and vasculitides. Among female patients with CVT, studies show approximately 50% were on oral contraceptives and 20% were pregnant or in the postpartum period at the time of the event. Nearly half of patients with CVT have multiple risk factors.12
Diagnosis of CVT
Diagnosis of CVT requires a high clinical suspicion. Lumbar puncture usually reveals high opening pressure with normal cerebrospinal fluid analysis.13 The classic finding of CVT on a contrast-enhanced CT scan is the “empty delta sign,” which is a central hypointensity within the superior sagittal sinus secondary due to slow/absent flow surrounded by contrast enhancement in a triangular shape. An ischemic infarction crossing arterial boundaries or near a venous sinus—with or without a hemorrhagic component—is also suggestive of CVT. However, CT scans are read as normal or indeterminate in up to 30% patients.14 Moreover, while CT venography may visualize the cerebral venous system, there is a false-negative rate of 25%.15 Therefore, MRI with MRV is the gold standard for diagnosis.
Pregnancy and CVT
As previously noted, pregnant or postpartum patients are at an increased risk of developing CVT. During pregnancy, decreased levels of protein S inhibitors and increased levels of fibrinogen, clotting factors, and protein C inhibitors lead to increased thrombogenicity.16 Older maternal age, pregnancy-related hypertension, peripartum infections, and cesarean delivery also increase the risk of CVT.16
Patients with CVT associated with pregnancy have more acute onset of symptoms and neurological findings, and 2% to 10% less mortality than patients with CVT secondary to other etiologies. Cerebral venous thrombosis should be considered in the differential diagnosis of any pregnant or postpartum patient presenting with neurological symptoms.
Treatment of CVT
Regardless of underlying etiology or symptom duration, all patients with CVT should receive anticoagulation therapy. Unfractionated or low-molecular weight heparin has been shown to decrease thrombus propagation, increase the rate of recanalization, and improve long-term outcomes—even in the presence of intracranial hemorrhage.17 In the 30% to 40% of patients with CVT who present with intracranial hemorrhage, anticoagulation decreases mortality and is not associated with new or enlarged bleeding.17-19 Patients who continue to deteriorate despite anticoagulation therapy may benefit from endovascular thrombolysis or decompressive hemicraniectomy.20 In a study of patients with CVT, 80% had full recovery, 6% had minor disability, and 14% had a poor outcome.12
Conclusion
This case describes a patient with two risk factors for CVT, a life-threatening condition. It should be considered in patients with ET and/or pregnant and postpartum patients presenting with headache or other neurological symptoms. Magnetic resonance venography is the diagnostic imaging of choice as the CVT may not be visible on CT. All patients with thrombosis and ET should receive anticoagulation therapy and hydroxyurea to prevent cerebral herniation and death.
Dr Canders is a resident, department of emergency medicine, David Geffen School of Medicine, University of California, Los Angeles. Dr Taira is a clinical assistant professor, department of emergency medicine, Los Angeles County Hospital/University of Southern California, Los Angeles.
Disclosure: The authors report no financial disclosures or conflicts of interests related to this article.
Case
At presentation, the patient was in no apparent distress and had normal vital signs, including normal blood pressure. On physical examination, she was alert and oriented, with unremarkable head, neck, fundoscopic, cardiac, pulmonary, skin, and neurological examinations. Laboratory results were only notable for a platelet count of 677 x 109/L. The remainder of the complete blood count, chemistry, and coagulation panels was all within normal limits. A noncontrast computed tomography (CT) of the brain was unremarkable. She was treated symptomatically with ketorolac, diphenhydramine, and metoclopramide, but received no relief.
Based on the patient’s history of ET and postpartum state, there was a high suspicion for cerebral venous thrombosis (CVT). Magnetic resonance imaging/magnetic resonance venography (MRI/MRV) of the brain was obtained, which revealed near-complete thrombosis of the vein of Galen and straight sinus (Figure). Neurology and hematology services were consulted emergently, and the patient was started on a heparin drip and admitted to the neurosurgical intensive care unit. Hydroxyurea was also given for cytoreduction, and platelets normalized to 305 x 109/L within 1 day of treatment. The patient’s neurological examination remained nonfocal, her headache gradually resolved, and she was discharged on hospital day 3 on oral anticoagulants.
Discussion
Essential thrombocytosis is a chronic myeloproliferative disorder characterized by clonal proliferation of the megakaryocyte cell line due to a defect in a pluripotent hematopoietic stem cell.1 The estimated annual incidence is 2.5 per 100,000 individuals.2 It affects patients of both genders, most commonly between ages 50 to 70 years.3
Up to 25% of patients with ET experience neurological complications, mainly occlusive strokes, chronic headache, and dizziness.4 Since pregnancy also increases thrombogenicity, and CVT occurs in 12 per 100,000 deliveries,5 it should be considered in any pregnant or postpartum patient with neurological symptoms.
Diagnosis of ET
Essential thrombocytosis is a diagnosis of exclusion, and no clinical or laboratory finding is diagnostic.1 Although patients with ET are at increased risk for bleeding, these events usually involve mucosal and cutaneous sites and are clinically insignificant. Thrombotic complications account for the majority of the morbidity and mortality associated with ET and can be both arterial and venous. In one case series, roughly 50% patients with ET experienced a thrombotic event within 9 years from diagnosis.6 Thrombotic complications are seen most commonly in patients younger than age 55 years at diagnosis. Common sites of thrombosis include the deep veins of the lower extremities, pulmonary vessels, hepatic vein, portal vein, digital microvasculature, and placenta.1
Despite the high rate of thrombotic complications in ET, there is little data on neurological complications and few reports of CVT in patients with ET.7-9 In one chart review of 70 patients with ET, researchers identified 18 patients who developed neurological complications,4 the most common of which was cerebrovascular accident/transient ischemic attack. Only one patient in the series had a CVT. The majority of patients with neurological events were female and no clinical or laboratory parameter predicted the risk of neurological event.
Thrombocytosis
Thrombocytosis is classified as either primary/essential or secondary/reactive. The majority of thrombocytosis is secondary/reactive and is caused by a variety of inflammatory conditions, such as infection and malignancy. Unlike ET, secondary thrombocytosis is not associated with any bleeding or thrombotic complications and does not require direct treatment.1
Treatment of ET
The decision to treat ET is based on clinical parameters and is usually reserved for patients at high-risk of thrombosis, including those with history of thrombosis or platelets >1,500 x 109/L.1 The first-line agent for treatment of essential thrombocytosis, hydroxyurea has been shown to decrease thrombotic episodes.10 Other cytoreductive agents include anagrilide and interferon-α, the use of which is considered safe in pregnancy.1
Cerebral Venous Thrombosis
Cerebral venous thrombosis is a rare but potentially fatal condition. Patients may present with headache (95%), seizures (47%), and vision changes (41%), and may or may not have focal or generalized neurological deficits.11 Thrombosis can lead to venous obstruction, resulting in increased intracranial pressure and ultimately cerebral herniation and death. Predisposing risk factors for CVT include thrombophilic and procoagulant disorders, maxillofacial infections, trauma, malignancy, and vasculitides. Among female patients with CVT, studies show approximately 50% were on oral contraceptives and 20% were pregnant or in the postpartum period at the time of the event. Nearly half of patients with CVT have multiple risk factors.12
Diagnosis of CVT
Diagnosis of CVT requires a high clinical suspicion. Lumbar puncture usually reveals high opening pressure with normal cerebrospinal fluid analysis.13 The classic finding of CVT on a contrast-enhanced CT scan is the “empty delta sign,” which is a central hypointensity within the superior sagittal sinus secondary due to slow/absent flow surrounded by contrast enhancement in a triangular shape. An ischemic infarction crossing arterial boundaries or near a venous sinus—with or without a hemorrhagic component—is also suggestive of CVT. However, CT scans are read as normal or indeterminate in up to 30% patients.14 Moreover, while CT venography may visualize the cerebral venous system, there is a false-negative rate of 25%.15 Therefore, MRI with MRV is the gold standard for diagnosis.
Pregnancy and CVT
As previously noted, pregnant or postpartum patients are at an increased risk of developing CVT. During pregnancy, decreased levels of protein S inhibitors and increased levels of fibrinogen, clotting factors, and protein C inhibitors lead to increased thrombogenicity.16 Older maternal age, pregnancy-related hypertension, peripartum infections, and cesarean delivery also increase the risk of CVT.16
Patients with CVT associated with pregnancy have more acute onset of symptoms and neurological findings, and 2% to 10% less mortality than patients with CVT secondary to other etiologies. Cerebral venous thrombosis should be considered in the differential diagnosis of any pregnant or postpartum patient presenting with neurological symptoms.
Treatment of CVT
Regardless of underlying etiology or symptom duration, all patients with CVT should receive anticoagulation therapy. Unfractionated or low-molecular weight heparin has been shown to decrease thrombus propagation, increase the rate of recanalization, and improve long-term outcomes—even in the presence of intracranial hemorrhage.17 In the 30% to 40% of patients with CVT who present with intracranial hemorrhage, anticoagulation decreases mortality and is not associated with new or enlarged bleeding.17-19 Patients who continue to deteriorate despite anticoagulation therapy may benefit from endovascular thrombolysis or decompressive hemicraniectomy.20 In a study of patients with CVT, 80% had full recovery, 6% had minor disability, and 14% had a poor outcome.12
Conclusion
This case describes a patient with two risk factors for CVT, a life-threatening condition. It should be considered in patients with ET and/or pregnant and postpartum patients presenting with headache or other neurological symptoms. Magnetic resonance venography is the diagnostic imaging of choice as the CVT may not be visible on CT. All patients with thrombosis and ET should receive anticoagulation therapy and hydroxyurea to prevent cerebral herniation and death.
Dr Canders is a resident, department of emergency medicine, David Geffen School of Medicine, University of California, Los Angeles. Dr Taira is a clinical assistant professor, department of emergency medicine, Los Angeles County Hospital/University of Southern California, Los Angeles.
Disclosure: The authors report no financial disclosures or conflicts of interests related to this article.
- Schafer AI. Thrombocytosis and thrombocythemia. Blood Rev. 2001;15(4):159-166.
- Mesa RA, Silverstein MN, Jacobsen SJ, Wollan PC, Tefferi A. Population-based incidence and survival figures in essential thrombocythemia and agnogenic myeloid metaplasia: an Olmsted County Study, 1976-1995. Am J Hematol. 1999;61():10-15.
- Tefferi A. Recent progress in the pathogenesis and management of essential thrombocythemia. Leuk Res. 2001;25(5):369-377.
- Kesler A, Ellis MH, Manor Y, Gadoth N, Lishner M. Neurological complications of essential thrombocytosis (ET). Acta Neurol Scand. 2000;102(5):299-302.
- Cantú C, Barinagarrementeria F. Cerebral venous thrombosis associated with pregnancy and puerperium: Review of 67 cases. Stroke. 1993;24(12):1880-1884.
- Bazzan M, Tamponi G, Schinco P, Vaccarino A, Foli C, Gallone G, et al. Thrombosis-free survival and life expectancy in 187 consecutive patients with essential thrombocythemia. Ann Hematol. 1999;78(12):539-543.
- Walther EU, Tiecks FP, Haberl RL. Cranial sinus thrombosis associated with essential thrombocythemia followed by heparin-associated thrombocytopenia. Neurology. 1996;47(1):300-301.
- McDonald TD, Tatemichi TK, Kranzler SJ, Chi L, Hilal SK, Mohr JP. Thrombosis of the superior sagittal sinus associated with essential thrombocytosis followed by MRI during anticoagulant therapy. Neurology. 1989;39(11):1554-1555.
- Iob I, Scanarini M, Andrioli GC, Pardatscher K. Thrombosis of the superior sagittal sinus associated with idiopathic thrombocytosis. Surg Neurol. 1979;11(6):439-41.
- Cortelazzo S, Finazzi G, Ruggeri M, Vestri O, Galli M, Rodeghiero F, et al. Hydroxyurea for patients with essential thrombocythemia and a high risk of thrombosis. N Engl J Med. 1995;332(17):1132-1136.
- de Bruijn SF, de Haan RJ, Stam J. Clinical features and prognostic factors of cerebral venous sinus thrombosis in a prospective series of 59 patients. For The Cerebral Venous Sinus Thrombosis Study Group. J Neurol Neurosurg Psychiatry. 2001;70(1):105-108.
- Ferro JM, Canhão P, Stam J, Bousser MG, Barinagarrementeria F; ISCVT Investigators. Prognosis of cerebral vein and dural sinus thrombosis: results of the International Study on Cerebral Vein and Dural Sinus Thrombosis (ISCVT). Stroke. 2004;35(3):664-670.
- DeLashaw MR, Vizioli TL, Jr, Counselman FL. Headache and seizure in a young woman postpartum. J Emerg Med. 2005;29(3):289-293.
- Bousser MG. Cerebral venous thrombosis: diagnosis and management. J Neurol. 2000;247(4):252-258.
- Grover M. Cerebral venous thrombosis as a cause of acute headache. J Am Board Fam Pract. 2004;17(4):295-298.
- Martinelli I, Sacchi E, Landi G, Taioli E, Duca F, Mannucci PM. High risk of cerebral venous thrombosis in carriers of prothrombic-gene mutation and in users of oral contraceptives. N Engl J Med. 1988;338(25):1793-1797.
- Star M, Flaster M. Advances and controversies in the management of cerebral venous thrombosis. Neurol Clin. 2013;31(3):765-783.
- Einhäupl KM, Villringer A, Meister W, Mehraein S, Garner C, Pellkofer M, et al. Heparin treatment in sinus venous thrombosis. Lancet. 1991;338(8767):597-600.
- Preter M, Tzourio C, Ameri A, Bousser MG. Long-term prognosis in cerebral venous thrombosis: follow-up of 77 patients. Stroke 1996;27(2):243-246.
- Coutinho JM, Stam J. How to treat cerebral venous and sinus thrombosis. J Thromb Haemost. 2010;8(5):877-883.
- Schafer AI. Thrombocytosis and thrombocythemia. Blood Rev. 2001;15(4):159-166.
- Mesa RA, Silverstein MN, Jacobsen SJ, Wollan PC, Tefferi A. Population-based incidence and survival figures in essential thrombocythemia and agnogenic myeloid metaplasia: an Olmsted County Study, 1976-1995. Am J Hematol. 1999;61():10-15.
- Tefferi A. Recent progress in the pathogenesis and management of essential thrombocythemia. Leuk Res. 2001;25(5):369-377.
- Kesler A, Ellis MH, Manor Y, Gadoth N, Lishner M. Neurological complications of essential thrombocytosis (ET). Acta Neurol Scand. 2000;102(5):299-302.
- Cantú C, Barinagarrementeria F. Cerebral venous thrombosis associated with pregnancy and puerperium: Review of 67 cases. Stroke. 1993;24(12):1880-1884.
- Bazzan M, Tamponi G, Schinco P, Vaccarino A, Foli C, Gallone G, et al. Thrombosis-free survival and life expectancy in 187 consecutive patients with essential thrombocythemia. Ann Hematol. 1999;78(12):539-543.
- Walther EU, Tiecks FP, Haberl RL. Cranial sinus thrombosis associated with essential thrombocythemia followed by heparin-associated thrombocytopenia. Neurology. 1996;47(1):300-301.
- McDonald TD, Tatemichi TK, Kranzler SJ, Chi L, Hilal SK, Mohr JP. Thrombosis of the superior sagittal sinus associated with essential thrombocytosis followed by MRI during anticoagulant therapy. Neurology. 1989;39(11):1554-1555.
- Iob I, Scanarini M, Andrioli GC, Pardatscher K. Thrombosis of the superior sagittal sinus associated with idiopathic thrombocytosis. Surg Neurol. 1979;11(6):439-41.
- Cortelazzo S, Finazzi G, Ruggeri M, Vestri O, Galli M, Rodeghiero F, et al. Hydroxyurea for patients with essential thrombocythemia and a high risk of thrombosis. N Engl J Med. 1995;332(17):1132-1136.
- de Bruijn SF, de Haan RJ, Stam J. Clinical features and prognostic factors of cerebral venous sinus thrombosis in a prospective series of 59 patients. For The Cerebral Venous Sinus Thrombosis Study Group. J Neurol Neurosurg Psychiatry. 2001;70(1):105-108.
- Ferro JM, Canhão P, Stam J, Bousser MG, Barinagarrementeria F; ISCVT Investigators. Prognosis of cerebral vein and dural sinus thrombosis: results of the International Study on Cerebral Vein and Dural Sinus Thrombosis (ISCVT). Stroke. 2004;35(3):664-670.
- DeLashaw MR, Vizioli TL, Jr, Counselman FL. Headache and seizure in a young woman postpartum. J Emerg Med. 2005;29(3):289-293.
- Bousser MG. Cerebral venous thrombosis: diagnosis and management. J Neurol. 2000;247(4):252-258.
- Grover M. Cerebral venous thrombosis as a cause of acute headache. J Am Board Fam Pract. 2004;17(4):295-298.
- Martinelli I, Sacchi E, Landi G, Taioli E, Duca F, Mannucci PM. High risk of cerebral venous thrombosis in carriers of prothrombic-gene mutation and in users of oral contraceptives. N Engl J Med. 1988;338(25):1793-1797.
- Star M, Flaster M. Advances and controversies in the management of cerebral venous thrombosis. Neurol Clin. 2013;31(3):765-783.
- Einhäupl KM, Villringer A, Meister W, Mehraein S, Garner C, Pellkofer M, et al. Heparin treatment in sinus venous thrombosis. Lancet. 1991;338(8767):597-600.
- Preter M, Tzourio C, Ameri A, Bousser MG. Long-term prognosis in cerebral venous thrombosis: follow-up of 77 patients. Stroke 1996;27(2):243-246.
- Coutinho JM, Stam J. How to treat cerebral venous and sinus thrombosis. J Thromb Haemost. 2010;8(5):877-883.
An 18-year-old woman with hepatic cysts
An 18-year-old woman presents with 3 days of epigastric abdominal pain, with no fever or constitutional symptoms. She was born in the United States and reports yearly trips since age 3 to her family’s farm in a rural area of Mexico, where she is exposed to dogs and horses.
Ultrasonography reveals two large hepatic cysts measuring 5.8 × 6.8 × 5.4 cm and 5.3 × 4.9 × 7 cm, with thickened walls and internal debris (Figure 1). The debris moves to dependent areas when the patient is asked to move onto her side.
Laboratory values at the time of presentation are as follows:
- White blood cell count 11.9 × 109/L (reference range 4.5–11.0), with 20% eosinophils
- Alkaline phosphatase 116 U/L (30–100)
- Total protein 7.3 g/dL (6.0–8.0)
- Albumin 4.3 g/dL (3.5–5.0)
- Aspartate aminotransferase (AST) 19 U/L (10–40)
- Alanine aminotransferase (ALT) 18 U/L (5–40)
- Total bilirubin 0.2 mg/dL (0.3–1.2)
- Direct bilirubin 0.1 mg/dL (0.1–0.3)
- Echinococcus antibody (IgG) testing is positive.
CYSTIC ECHINOCOCCOSIS
The two clinically relevant species of Echinococcus that cause human infection are E granulosus (in cystic echinococcosis) and E multilocularis (in alveolar echinococcosis). Based on clinical and radiographic findings, hepatic hydatid disease from cystic echinococcosis can usually be differentiated from the alveolar form.
E granulosus is a parasitic tapeworm that requires an intermediate host (sheep, goats, cows) and a definite host (dogs, foxes, and related species) for its life cycle. Humans become infected when they ingest food contaminated with feces that contain the eggs of the tapeworm or when they handle carnivorous animals, usually dogs, and accidentally ingest the tapeworm eggs. Once ingested, the egg releases an oncosphere that penetrates the intestinal wall, enters the circulation, and develops into a cyst, most often in the liver and the lungs.1 Human-to-human transmission does not occur.2
Hydatid cysts grow slowly, at a rate of 1 to 50 mm per year,3 so most patients remain asymptomatic for several years. Symptoms occur when a cyst ruptures or impinges on structures.3 Fever and constitutional symptoms usually occur only if there is rupture or bacterial superinfection of the cyst. Tests of liver function tend to be normal unless a cyst obstructs biliary flow. Eosinophilia occurs in 25% of patients.1 Eosinophilia along with the abrupt onset of abdominal pain suggests cyst rupture.
Making the diagnosis
Diagnosis is made by characteristic ultrasonographic findings and by serologic testing. Antibody assays for Echinococcus include indirect hemagglutination, enzyme-linked immunosorbent assay, and latex agglutination. However, these serologic antibody assays for immunoglobulin G cross-react to different echinococcal species as well as to other helminthic infections. Specific serologic studies such as an enzyme-linked immunosorbent assay for E multilocularis are available to confirm the species of Echinococcus but are only used to distinguish cystic echinococcosis from alveolar echinococcosis.
Treatment options
Treatment options include surgery, percutaneous procedures, drug therapy, and observation.
Currently, there is no clear consensus on treatment. To guide treatment decisions, the World Health Organization Informal Working Group on Echinococcosis (WHO-IWGE) recommends management of hepatic hydatid cysts based on classification, size, symptoms, location, and available resources.3
Two percutaneous treatments are aspiration, injection, and re-aspiration to destroy the germinal matrix, and percutaneous therapy to destroy the endocyst. Percutaneous aspiration, injection, and re-aspiration is increasingly used as the first-line treatment for single or easily accessible cysts and for patients who cannot undergo surgery. Surgery is considered for multiple cysts, large cysts, and cysts not easily accessible with a percutaneous technique.3 Complication rates and length of hospital stay with percutaneous aspiration are lower than with surgery.4 Observation is recommended for small, asymptomatic, inactive cysts.
Leakage of cyst contents during surgical or percutaneous intervention or spontaneous rupture can cause a recurrence,5 and anaphylaxis is a potential complication of cyst rupture.1 For this reason, giving oral albendazole (Albenza) is recommended before any intervention. Sterilization of the cyst contents with a protoscolicidal agent (20% NaCl) before evacuation of cyst contents is also standard practice.
The rate of cyst recurrence is 16.2% with open surgery and 3.5% with percutaneous intervention.6 A higher incidence of recurrence in patients who undergo surgical cystectomy likely reflects the more complicated and active nature of the cysts in patients who undergo surgery.6
Albendazole is the drug of choice for hepatic hydatid disease.3 The optimal duration of treatment is unclear but should be guided by a combination of clinical response, medication side effects, serologic titers, and imaging. The most common adverse effects of albendazole are hepatotoxicity, abdominal pain, and nausea.
OUR PATIENT’S DIAGNOSIS AND TREATMENT
In our patient, ultrasonography confirmed the diagnosis of cystic echinococcosis by the finding of active anechoic cysts with echogenic internal debris and with a well-delineated cyst wall. The WHO-IWGE classification was CE1, ie, active anechoic cysts with internal echogenic debris.
Our patient underwent surgical rather than percutaneous cystectomy because of concern about possible cyst leakage, since she had presented with the acute onset of pain and eosinophilia. We were also concerned about the subdiaphragmatic location of the larger cyst, which could have been difficult to reach percutaneously.
Open total pericystectomy involved opening the cyst cavity, sterilizing the contents with 20% NaCl, evacuating the cyst contents, and removing the cyst tissue. Two large cysts were excised and sent for histologic examination, which confirmed E granulosus. Percutaneous aspiration was necessary 4 months later because of a recurrence, and albendazole 400 mg twice daily was continued for another 5 months. Ultrasonography 3 years later showed no evidence of echinococcal cysts, and her antibody titers remain undetectable.
- McManus DP, Gray DJ, Zhang W, Yang Y. Diagnosis, treatment, and management of echinococcosis. BMJ 2012; 344:e3866.
- McManus DP, Zhang W, Li J, Bartley PB. Echinococcosis. Lancet 2003; 362:1295–1304.
- Brunetti E, Kern P, Vuitton DA; Writing Panel for the WHO-IWGE. Expert consensus for the diagnosis and treatment of cystic and alveolar echinococcosis in humans. Acta Trop 2010; 114:1–16.
- Khuroo MS, Wani NA, Javid G, et al. Percutaneous drainage compared with surgery for hepatic hydatid cysts. N Engl J Med 1997; 337:881–887.
- Kayaalp C, Sengul N, Akoglu M. Importance of cyst content in hydatid liver surgery. Arch Surg 2002; 137:159–163.
- Yagci G, Ustunsoz B, Kaymakcioglu N, et al. Results of surgical, laparoscopic, and percutaneous treatment for hydatid disease of the liver: 10 years experience with 355 patients. World J Surg 2005; 29:1670–1679.
An 18-year-old woman presents with 3 days of epigastric abdominal pain, with no fever or constitutional symptoms. She was born in the United States and reports yearly trips since age 3 to her family’s farm in a rural area of Mexico, where she is exposed to dogs and horses.
Ultrasonography reveals two large hepatic cysts measuring 5.8 × 6.8 × 5.4 cm and 5.3 × 4.9 × 7 cm, with thickened walls and internal debris (Figure 1). The debris moves to dependent areas when the patient is asked to move onto her side.
Laboratory values at the time of presentation are as follows:
- White blood cell count 11.9 × 109/L (reference range 4.5–11.0), with 20% eosinophils
- Alkaline phosphatase 116 U/L (30–100)
- Total protein 7.3 g/dL (6.0–8.0)
- Albumin 4.3 g/dL (3.5–5.0)
- Aspartate aminotransferase (AST) 19 U/L (10–40)
- Alanine aminotransferase (ALT) 18 U/L (5–40)
- Total bilirubin 0.2 mg/dL (0.3–1.2)
- Direct bilirubin 0.1 mg/dL (0.1–0.3)
- Echinococcus antibody (IgG) testing is positive.
CYSTIC ECHINOCOCCOSIS
The two clinically relevant species of Echinococcus that cause human infection are E granulosus (in cystic echinococcosis) and E multilocularis (in alveolar echinococcosis). Based on clinical and radiographic findings, hepatic hydatid disease from cystic echinococcosis can usually be differentiated from the alveolar form.
E granulosus is a parasitic tapeworm that requires an intermediate host (sheep, goats, cows) and a definite host (dogs, foxes, and related species) for its life cycle. Humans become infected when they ingest food contaminated with feces that contain the eggs of the tapeworm or when they handle carnivorous animals, usually dogs, and accidentally ingest the tapeworm eggs. Once ingested, the egg releases an oncosphere that penetrates the intestinal wall, enters the circulation, and develops into a cyst, most often in the liver and the lungs.1 Human-to-human transmission does not occur.2
Hydatid cysts grow slowly, at a rate of 1 to 50 mm per year,3 so most patients remain asymptomatic for several years. Symptoms occur when a cyst ruptures or impinges on structures.3 Fever and constitutional symptoms usually occur only if there is rupture or bacterial superinfection of the cyst. Tests of liver function tend to be normal unless a cyst obstructs biliary flow. Eosinophilia occurs in 25% of patients.1 Eosinophilia along with the abrupt onset of abdominal pain suggests cyst rupture.
Making the diagnosis
Diagnosis is made by characteristic ultrasonographic findings and by serologic testing. Antibody assays for Echinococcus include indirect hemagglutination, enzyme-linked immunosorbent assay, and latex agglutination. However, these serologic antibody assays for immunoglobulin G cross-react to different echinococcal species as well as to other helminthic infections. Specific serologic studies such as an enzyme-linked immunosorbent assay for E multilocularis are available to confirm the species of Echinococcus but are only used to distinguish cystic echinococcosis from alveolar echinococcosis.
Treatment options
Treatment options include surgery, percutaneous procedures, drug therapy, and observation.
Currently, there is no clear consensus on treatment. To guide treatment decisions, the World Health Organization Informal Working Group on Echinococcosis (WHO-IWGE) recommends management of hepatic hydatid cysts based on classification, size, symptoms, location, and available resources.3
Two percutaneous treatments are aspiration, injection, and re-aspiration to destroy the germinal matrix, and percutaneous therapy to destroy the endocyst. Percutaneous aspiration, injection, and re-aspiration is increasingly used as the first-line treatment for single or easily accessible cysts and for patients who cannot undergo surgery. Surgery is considered for multiple cysts, large cysts, and cysts not easily accessible with a percutaneous technique.3 Complication rates and length of hospital stay with percutaneous aspiration are lower than with surgery.4 Observation is recommended for small, asymptomatic, inactive cysts.
Leakage of cyst contents during surgical or percutaneous intervention or spontaneous rupture can cause a recurrence,5 and anaphylaxis is a potential complication of cyst rupture.1 For this reason, giving oral albendazole (Albenza) is recommended before any intervention. Sterilization of the cyst contents with a protoscolicidal agent (20% NaCl) before evacuation of cyst contents is also standard practice.
The rate of cyst recurrence is 16.2% with open surgery and 3.5% with percutaneous intervention.6 A higher incidence of recurrence in patients who undergo surgical cystectomy likely reflects the more complicated and active nature of the cysts in patients who undergo surgery.6
Albendazole is the drug of choice for hepatic hydatid disease.3 The optimal duration of treatment is unclear but should be guided by a combination of clinical response, medication side effects, serologic titers, and imaging. The most common adverse effects of albendazole are hepatotoxicity, abdominal pain, and nausea.
OUR PATIENT’S DIAGNOSIS AND TREATMENT
In our patient, ultrasonography confirmed the diagnosis of cystic echinococcosis by the finding of active anechoic cysts with echogenic internal debris and with a well-delineated cyst wall. The WHO-IWGE classification was CE1, ie, active anechoic cysts with internal echogenic debris.
Our patient underwent surgical rather than percutaneous cystectomy because of concern about possible cyst leakage, since she had presented with the acute onset of pain and eosinophilia. We were also concerned about the subdiaphragmatic location of the larger cyst, which could have been difficult to reach percutaneously.
Open total pericystectomy involved opening the cyst cavity, sterilizing the contents with 20% NaCl, evacuating the cyst contents, and removing the cyst tissue. Two large cysts were excised and sent for histologic examination, which confirmed E granulosus. Percutaneous aspiration was necessary 4 months later because of a recurrence, and albendazole 400 mg twice daily was continued for another 5 months. Ultrasonography 3 years later showed no evidence of echinococcal cysts, and her antibody titers remain undetectable.
An 18-year-old woman presents with 3 days of epigastric abdominal pain, with no fever or constitutional symptoms. She was born in the United States and reports yearly trips since age 3 to her family’s farm in a rural area of Mexico, where she is exposed to dogs and horses.
Ultrasonography reveals two large hepatic cysts measuring 5.8 × 6.8 × 5.4 cm and 5.3 × 4.9 × 7 cm, with thickened walls and internal debris (Figure 1). The debris moves to dependent areas when the patient is asked to move onto her side.
Laboratory values at the time of presentation are as follows:
- White blood cell count 11.9 × 109/L (reference range 4.5–11.0), with 20% eosinophils
- Alkaline phosphatase 116 U/L (30–100)
- Total protein 7.3 g/dL (6.0–8.0)
- Albumin 4.3 g/dL (3.5–5.0)
- Aspartate aminotransferase (AST) 19 U/L (10–40)
- Alanine aminotransferase (ALT) 18 U/L (5–40)
- Total bilirubin 0.2 mg/dL (0.3–1.2)
- Direct bilirubin 0.1 mg/dL (0.1–0.3)
- Echinococcus antibody (IgG) testing is positive.
CYSTIC ECHINOCOCCOSIS
The two clinically relevant species of Echinococcus that cause human infection are E granulosus (in cystic echinococcosis) and E multilocularis (in alveolar echinococcosis). Based on clinical and radiographic findings, hepatic hydatid disease from cystic echinococcosis can usually be differentiated from the alveolar form.
E granulosus is a parasitic tapeworm that requires an intermediate host (sheep, goats, cows) and a definite host (dogs, foxes, and related species) for its life cycle. Humans become infected when they ingest food contaminated with feces that contain the eggs of the tapeworm or when they handle carnivorous animals, usually dogs, and accidentally ingest the tapeworm eggs. Once ingested, the egg releases an oncosphere that penetrates the intestinal wall, enters the circulation, and develops into a cyst, most often in the liver and the lungs.1 Human-to-human transmission does not occur.2
Hydatid cysts grow slowly, at a rate of 1 to 50 mm per year,3 so most patients remain asymptomatic for several years. Symptoms occur when a cyst ruptures or impinges on structures.3 Fever and constitutional symptoms usually occur only if there is rupture or bacterial superinfection of the cyst. Tests of liver function tend to be normal unless a cyst obstructs biliary flow. Eosinophilia occurs in 25% of patients.1 Eosinophilia along with the abrupt onset of abdominal pain suggests cyst rupture.
Making the diagnosis
Diagnosis is made by characteristic ultrasonographic findings and by serologic testing. Antibody assays for Echinococcus include indirect hemagglutination, enzyme-linked immunosorbent assay, and latex agglutination. However, these serologic antibody assays for immunoglobulin G cross-react to different echinococcal species as well as to other helminthic infections. Specific serologic studies such as an enzyme-linked immunosorbent assay for E multilocularis are available to confirm the species of Echinococcus but are only used to distinguish cystic echinococcosis from alveolar echinococcosis.
Treatment options
Treatment options include surgery, percutaneous procedures, drug therapy, and observation.
Currently, there is no clear consensus on treatment. To guide treatment decisions, the World Health Organization Informal Working Group on Echinococcosis (WHO-IWGE) recommends management of hepatic hydatid cysts based on classification, size, symptoms, location, and available resources.3
Two percutaneous treatments are aspiration, injection, and re-aspiration to destroy the germinal matrix, and percutaneous therapy to destroy the endocyst. Percutaneous aspiration, injection, and re-aspiration is increasingly used as the first-line treatment for single or easily accessible cysts and for patients who cannot undergo surgery. Surgery is considered for multiple cysts, large cysts, and cysts not easily accessible with a percutaneous technique.3 Complication rates and length of hospital stay with percutaneous aspiration are lower than with surgery.4 Observation is recommended for small, asymptomatic, inactive cysts.
Leakage of cyst contents during surgical or percutaneous intervention or spontaneous rupture can cause a recurrence,5 and anaphylaxis is a potential complication of cyst rupture.1 For this reason, giving oral albendazole (Albenza) is recommended before any intervention. Sterilization of the cyst contents with a protoscolicidal agent (20% NaCl) before evacuation of cyst contents is also standard practice.
The rate of cyst recurrence is 16.2% with open surgery and 3.5% with percutaneous intervention.6 A higher incidence of recurrence in patients who undergo surgical cystectomy likely reflects the more complicated and active nature of the cysts in patients who undergo surgery.6
Albendazole is the drug of choice for hepatic hydatid disease.3 The optimal duration of treatment is unclear but should be guided by a combination of clinical response, medication side effects, serologic titers, and imaging. The most common adverse effects of albendazole are hepatotoxicity, abdominal pain, and nausea.
OUR PATIENT’S DIAGNOSIS AND TREATMENT
In our patient, ultrasonography confirmed the diagnosis of cystic echinococcosis by the finding of active anechoic cysts with echogenic internal debris and with a well-delineated cyst wall. The WHO-IWGE classification was CE1, ie, active anechoic cysts with internal echogenic debris.
Our patient underwent surgical rather than percutaneous cystectomy because of concern about possible cyst leakage, since she had presented with the acute onset of pain and eosinophilia. We were also concerned about the subdiaphragmatic location of the larger cyst, which could have been difficult to reach percutaneously.
Open total pericystectomy involved opening the cyst cavity, sterilizing the contents with 20% NaCl, evacuating the cyst contents, and removing the cyst tissue. Two large cysts were excised and sent for histologic examination, which confirmed E granulosus. Percutaneous aspiration was necessary 4 months later because of a recurrence, and albendazole 400 mg twice daily was continued for another 5 months. Ultrasonography 3 years later showed no evidence of echinococcal cysts, and her antibody titers remain undetectable.
- McManus DP, Gray DJ, Zhang W, Yang Y. Diagnosis, treatment, and management of echinococcosis. BMJ 2012; 344:e3866.
- McManus DP, Zhang W, Li J, Bartley PB. Echinococcosis. Lancet 2003; 362:1295–1304.
- Brunetti E, Kern P, Vuitton DA; Writing Panel for the WHO-IWGE. Expert consensus for the diagnosis and treatment of cystic and alveolar echinococcosis in humans. Acta Trop 2010; 114:1–16.
- Khuroo MS, Wani NA, Javid G, et al. Percutaneous drainage compared with surgery for hepatic hydatid cysts. N Engl J Med 1997; 337:881–887.
- Kayaalp C, Sengul N, Akoglu M. Importance of cyst content in hydatid liver surgery. Arch Surg 2002; 137:159–163.
- Yagci G, Ustunsoz B, Kaymakcioglu N, et al. Results of surgical, laparoscopic, and percutaneous treatment for hydatid disease of the liver: 10 years experience with 355 patients. World J Surg 2005; 29:1670–1679.
- McManus DP, Gray DJ, Zhang W, Yang Y. Diagnosis, treatment, and management of echinococcosis. BMJ 2012; 344:e3866.
- McManus DP, Zhang W, Li J, Bartley PB. Echinococcosis. Lancet 2003; 362:1295–1304.
- Brunetti E, Kern P, Vuitton DA; Writing Panel for the WHO-IWGE. Expert consensus for the diagnosis and treatment of cystic and alveolar echinococcosis in humans. Acta Trop 2010; 114:1–16.
- Khuroo MS, Wani NA, Javid G, et al. Percutaneous drainage compared with surgery for hepatic hydatid cysts. N Engl J Med 1997; 337:881–887.
- Kayaalp C, Sengul N, Akoglu M. Importance of cyst content in hydatid liver surgery. Arch Surg 2002; 137:159–163.
- Yagci G, Ustunsoz B, Kaymakcioglu N, et al. Results of surgical, laparoscopic, and percutaneous treatment for hydatid disease of the liver: 10 years experience with 355 patients. World J Surg 2005; 29:1670–1679.
Syncope during a pharmacologic nuclear stress test
A 60-year-old woman was referred for pharmacologic nuclear stress testing before treatment for breast cancer. She had hypertension, diabetes mellitus, coronary artery disease, and a remote history of stroke, and she was taking clonidine (Catapres), labetalol (Normodyne, Trandate), furosemide (Lasix), hydralazine, valsartan (Diovan), insulin, and the aspirin-dipyridamole combination Aggrenox. Her vital signs and electrocardiogram before the stress test were normal.
The stress test was started with a standard protocol of adenosine (Adenoscan) infused intravenously over 4 minutes. For the first 2 minutes, she was stable and had no symptoms, but then sinus pauses and second-degree atrioventricular block type 2 developed, after which her heart stopped beating (Figure 1). The infusion was immediately stopped, but she became unresponsive and remained pulseless.
Cardiopulmonary resuscitation was started, aminophylline 100 mg was given intravenously, and she regained a pulse and blood pressure within a few minutes. She was then transferred to the emergency room, where she returned to her baseline clinical and neurologic status without symptoms.
AN UNRECOGNIZED DRUG INTERACTION
Asystole occurred in this patient because of the interaction of intravenous adenosine with the dipyridamole in the medication Aggrenox. Although adenosine, given during pharmacologic stress testing, is known to interact with various medications, the potential for this interaction may be overlooked if the culprit is present in a combination drug. Aggrenox is commonly given for secondary stroke prevention and should be discontinued before pharmacologic nuclear stress testing.
Pharmacologic stress testing involves two commonly used stress agents, adenosine and regadenoson (Lexiscan), which cause coronary vasodilation through their action on A2A receptors in the heart. Coronary vasodilation results in flow heterogeneity in the region of a stenotic artery, which can be detected with nuclear perfusion agents. In addition, adenosine has a short-lived effect on the A1 receptors that block atrioventricular conduction.1
Dipyridamole (Persantine) is contraindicated when either adenosine or regadenoson is used. Dipyridamole enhances the effect of exogenous and endogenous adenosine by inhibiting its uptake by cardiac cells, thus enhancing the action of these coronary vasodilators.2 Atrioventricular block is common during adenosine stress testing but is transient because adenosine has a short half-life (< 10 seconds), and complete heart block or asystole, as seen in this patient, is rare. Giving intravenous adenosine or regadenoson to patients on dipyridamole may have a marked effect on adenosine receptors, so that profound bradycardia and even asystole leading to cardiac collapse may occur. No data are available on the specific interaction of dipyridamole and regadenoson.
Even though the pharmacodynamics of the interaction between dipyridamole and adenosine are known,3 few reports are available detailing serious adverse events. The contraindication to pharmacologic stress testing in patients taking dipyridamole is noted in the American Society of Nuclear Cardiology Guidelines for stress protocols,4 which advise discontinuing dipyridamole-containing drugs at least 48 hours before the use of adenosine or regadenoson. Similarly, the American Heart Association guidelines5 for the management of supraventricular tachycardia recommend an initial dose of 3 mg of adenosine rather than 6 mg in patients who have been taking dipyridamole.
The dose of aminophylline for reversing the adverse effects of adenosine or regadenoson is 50 to 250 mg intravenously over 30 to 60 seconds. But since these adverse effects are short-lived once the infusion is stopped, aminophylline is usually given only if the adverse effects are severe, as in this patient.
Pharmacologic nuclear stress testing with adenosine receptor agonists (eg, adenosine or regadenoson) is contraindicated in patients taking dipyridamole or the combination pill Aggrenox because of the potential for profound bradyarrhythmias or asystole.
- Zoghbi GJ, Iskandrian AE. Selective adenosine agonists and myocardial perfusion imaging. J Nucl Cardiol 2012; 19:126–141.
- Lerman BB, Wesley RC, Belardinelli L. Electrophysiologic effects of dipyridamole on atrioventricular nodal conduction and supraventricular tachycardia. Role of endogenous adenosine. Circulation 1989; 80:1536–1543.
- Biaggioni I, Onrot J, Hollister AS, Robertson D. Cardiovascular effects of adenosine infusion in man and their modulation by dipyridamole. Life Sci 1986; 39:2229–2236.
- Henzlova MJ, Cerqueira MD, Mahmarian JJ, Yao SS; Quality Assurance Committee of the American Society of Nuclear Cardiology. Stress protocols and tracers. J Nucl Cardiol 2006; 13:e80–e90.
- ECC Committee, Subcommittees and Task Forces of the American Heart Association. 2005 American Heart Association guidelines for cardiopulmonary resuscitation and emergency cardiovascular care. Part 7.3: management of symptomatic bradycardia and tachycardia. Circulation 2005; 112(suppl 24):IV67–IV77.
A 60-year-old woman was referred for pharmacologic nuclear stress testing before treatment for breast cancer. She had hypertension, diabetes mellitus, coronary artery disease, and a remote history of stroke, and she was taking clonidine (Catapres), labetalol (Normodyne, Trandate), furosemide (Lasix), hydralazine, valsartan (Diovan), insulin, and the aspirin-dipyridamole combination Aggrenox. Her vital signs and electrocardiogram before the stress test were normal.
The stress test was started with a standard protocol of adenosine (Adenoscan) infused intravenously over 4 minutes. For the first 2 minutes, she was stable and had no symptoms, but then sinus pauses and second-degree atrioventricular block type 2 developed, after which her heart stopped beating (Figure 1). The infusion was immediately stopped, but she became unresponsive and remained pulseless.
Cardiopulmonary resuscitation was started, aminophylline 100 mg was given intravenously, and she regained a pulse and blood pressure within a few minutes. She was then transferred to the emergency room, where she returned to her baseline clinical and neurologic status without symptoms.
AN UNRECOGNIZED DRUG INTERACTION
Asystole occurred in this patient because of the interaction of intravenous adenosine with the dipyridamole in the medication Aggrenox. Although adenosine, given during pharmacologic stress testing, is known to interact with various medications, the potential for this interaction may be overlooked if the culprit is present in a combination drug. Aggrenox is commonly given for secondary stroke prevention and should be discontinued before pharmacologic nuclear stress testing.
Pharmacologic stress testing involves two commonly used stress agents, adenosine and regadenoson (Lexiscan), which cause coronary vasodilation through their action on A2A receptors in the heart. Coronary vasodilation results in flow heterogeneity in the region of a stenotic artery, which can be detected with nuclear perfusion agents. In addition, adenosine has a short-lived effect on the A1 receptors that block atrioventricular conduction.1
Dipyridamole (Persantine) is contraindicated when either adenosine or regadenoson is used. Dipyridamole enhances the effect of exogenous and endogenous adenosine by inhibiting its uptake by cardiac cells, thus enhancing the action of these coronary vasodilators.2 Atrioventricular block is common during adenosine stress testing but is transient because adenosine has a short half-life (< 10 seconds), and complete heart block or asystole, as seen in this patient, is rare. Giving intravenous adenosine or regadenoson to patients on dipyridamole may have a marked effect on adenosine receptors, so that profound bradycardia and even asystole leading to cardiac collapse may occur. No data are available on the specific interaction of dipyridamole and regadenoson.
Even though the pharmacodynamics of the interaction between dipyridamole and adenosine are known,3 few reports are available detailing serious adverse events. The contraindication to pharmacologic stress testing in patients taking dipyridamole is noted in the American Society of Nuclear Cardiology Guidelines for stress protocols,4 which advise discontinuing dipyridamole-containing drugs at least 48 hours before the use of adenosine or regadenoson. Similarly, the American Heart Association guidelines5 for the management of supraventricular tachycardia recommend an initial dose of 3 mg of adenosine rather than 6 mg in patients who have been taking dipyridamole.
The dose of aminophylline for reversing the adverse effects of adenosine or regadenoson is 50 to 250 mg intravenously over 30 to 60 seconds. But since these adverse effects are short-lived once the infusion is stopped, aminophylline is usually given only if the adverse effects are severe, as in this patient.
Pharmacologic nuclear stress testing with adenosine receptor agonists (eg, adenosine or regadenoson) is contraindicated in patients taking dipyridamole or the combination pill Aggrenox because of the potential for profound bradyarrhythmias or asystole.
A 60-year-old woman was referred for pharmacologic nuclear stress testing before treatment for breast cancer. She had hypertension, diabetes mellitus, coronary artery disease, and a remote history of stroke, and she was taking clonidine (Catapres), labetalol (Normodyne, Trandate), furosemide (Lasix), hydralazine, valsartan (Diovan), insulin, and the aspirin-dipyridamole combination Aggrenox. Her vital signs and electrocardiogram before the stress test were normal.
The stress test was started with a standard protocol of adenosine (Adenoscan) infused intravenously over 4 minutes. For the first 2 minutes, she was stable and had no symptoms, but then sinus pauses and second-degree atrioventricular block type 2 developed, after which her heart stopped beating (Figure 1). The infusion was immediately stopped, but she became unresponsive and remained pulseless.
Cardiopulmonary resuscitation was started, aminophylline 100 mg was given intravenously, and she regained a pulse and blood pressure within a few minutes. She was then transferred to the emergency room, where she returned to her baseline clinical and neurologic status without symptoms.
AN UNRECOGNIZED DRUG INTERACTION
Asystole occurred in this patient because of the interaction of intravenous adenosine with the dipyridamole in the medication Aggrenox. Although adenosine, given during pharmacologic stress testing, is known to interact with various medications, the potential for this interaction may be overlooked if the culprit is present in a combination drug. Aggrenox is commonly given for secondary stroke prevention and should be discontinued before pharmacologic nuclear stress testing.
Pharmacologic stress testing involves two commonly used stress agents, adenosine and regadenoson (Lexiscan), which cause coronary vasodilation through their action on A2A receptors in the heart. Coronary vasodilation results in flow heterogeneity in the region of a stenotic artery, which can be detected with nuclear perfusion agents. In addition, adenosine has a short-lived effect on the A1 receptors that block atrioventricular conduction.1
Dipyridamole (Persantine) is contraindicated when either adenosine or regadenoson is used. Dipyridamole enhances the effect of exogenous and endogenous adenosine by inhibiting its uptake by cardiac cells, thus enhancing the action of these coronary vasodilators.2 Atrioventricular block is common during adenosine stress testing but is transient because adenosine has a short half-life (< 10 seconds), and complete heart block or asystole, as seen in this patient, is rare. Giving intravenous adenosine or regadenoson to patients on dipyridamole may have a marked effect on adenosine receptors, so that profound bradycardia and even asystole leading to cardiac collapse may occur. No data are available on the specific interaction of dipyridamole and regadenoson.
Even though the pharmacodynamics of the interaction between dipyridamole and adenosine are known,3 few reports are available detailing serious adverse events. The contraindication to pharmacologic stress testing in patients taking dipyridamole is noted in the American Society of Nuclear Cardiology Guidelines for stress protocols,4 which advise discontinuing dipyridamole-containing drugs at least 48 hours before the use of adenosine or regadenoson. Similarly, the American Heart Association guidelines5 for the management of supraventricular tachycardia recommend an initial dose of 3 mg of adenosine rather than 6 mg in patients who have been taking dipyridamole.
The dose of aminophylline for reversing the adverse effects of adenosine or regadenoson is 50 to 250 mg intravenously over 30 to 60 seconds. But since these adverse effects are short-lived once the infusion is stopped, aminophylline is usually given only if the adverse effects are severe, as in this patient.
Pharmacologic nuclear stress testing with adenosine receptor agonists (eg, adenosine or regadenoson) is contraindicated in patients taking dipyridamole or the combination pill Aggrenox because of the potential for profound bradyarrhythmias or asystole.
- Zoghbi GJ, Iskandrian AE. Selective adenosine agonists and myocardial perfusion imaging. J Nucl Cardiol 2012; 19:126–141.
- Lerman BB, Wesley RC, Belardinelli L. Electrophysiologic effects of dipyridamole on atrioventricular nodal conduction and supraventricular tachycardia. Role of endogenous adenosine. Circulation 1989; 80:1536–1543.
- Biaggioni I, Onrot J, Hollister AS, Robertson D. Cardiovascular effects of adenosine infusion in man and their modulation by dipyridamole. Life Sci 1986; 39:2229–2236.
- Henzlova MJ, Cerqueira MD, Mahmarian JJ, Yao SS; Quality Assurance Committee of the American Society of Nuclear Cardiology. Stress protocols and tracers. J Nucl Cardiol 2006; 13:e80–e90.
- ECC Committee, Subcommittees and Task Forces of the American Heart Association. 2005 American Heart Association guidelines for cardiopulmonary resuscitation and emergency cardiovascular care. Part 7.3: management of symptomatic bradycardia and tachycardia. Circulation 2005; 112(suppl 24):IV67–IV77.
- Zoghbi GJ, Iskandrian AE. Selective adenosine agonists and myocardial perfusion imaging. J Nucl Cardiol 2012; 19:126–141.
- Lerman BB, Wesley RC, Belardinelli L. Electrophysiologic effects of dipyridamole on atrioventricular nodal conduction and supraventricular tachycardia. Role of endogenous adenosine. Circulation 1989; 80:1536–1543.
- Biaggioni I, Onrot J, Hollister AS, Robertson D. Cardiovascular effects of adenosine infusion in man and their modulation by dipyridamole. Life Sci 1986; 39:2229–2236.
- Henzlova MJ, Cerqueira MD, Mahmarian JJ, Yao SS; Quality Assurance Committee of the American Society of Nuclear Cardiology. Stress protocols and tracers. J Nucl Cardiol 2006; 13:e80–e90.
- ECC Committee, Subcommittees and Task Forces of the American Heart Association. 2005 American Heart Association guidelines for cardiopulmonary resuscitation and emergency cardiovascular care. Part 7.3: management of symptomatic bradycardia and tachycardia. Circulation 2005; 112(suppl 24):IV67–IV77.
Stress cardiac magnetic resonance feasible and prognostic in obese patients
Stress cardiac magnetic resonance is highly feasible and prognostically useful in obese patients, a population in which stress imaging methods are limited, according to a study of nearly 300 patients reported online in JACC: Cardiovascular Imaging on April 9.
Dr. Ravi V. Shah of Harvard Medical School, Boston, and his colleagues, said stress echocardiography and nuclear perfusion can be challenging in obese patients, and PET has issues around ionizing radiation and cost, but the use of stress cardiac magnetic resonance had also been limited by concerns about claustrophobia and safety monitoring.
In this feasibility study in 285 patients with a mean body mass index of 35.4 kg/m2, the primary outcome was a composite of cardiac death or MI (MACE). During a mean follow-up of 2.1 years, 19 patients died, 7 from cardiovascular causes.
The incidence of MACE increased with both inducible ischemia and late gadolinium enhancement (LGE). The patients with no evidence of inducible ischemia, infarction, or LGE had a very low annualized MACE rate of 0.3%, while those who had no inducible ischemia who did have LGE had a rate of 2.4%. The MACE rate jumped significantly, to 6.3%, in patients with inducible ischemia and no LGE, and further yet in patients with evidence of both to 6.7%.
Diabetes, age, prior MI, prior revascularization, and reduced left ventricular ejection fraction were all associated with MACE in the study.
The investigators noted that only 13 (5%) of patients failed to complete the study protocol because of claustrophobia, intolerance to the stress agent, or poor gating, and sedation was required in 19 (7%) of patients.
However diagnostic-quality imaging was achieved in more than 89% of patients (JACC Cardiovasc. Imaging 2014 [dx.doi.org/10.1016/j.jcmg.2013.11.011]).
These results "confirm, in an obese population, that inducible ischemia and LGE by stress perfusion CMR are robust markers of risk even in those patients without a clinical history of prior infarction," the authors concluded.
Researchers declared grants and awards from the American Heart Association, the National Institutes of Health and the Alberta Heritage Foundation for Medical Research, and one author declared research support from Astellas Pharma US.
Stress SPECT imaging has been problematic in obese patients, often yielding poor quality images. This study by Dr. Shah and his coworkers clearly shows the prognostic value of stress cardiac magnetic resonance in obese individuals.
Those patients with absence of both ischemia and late gadolinium enhancement had an excellent prognosis during follow-up. It should be pointed out that PET myocardial perfusion imaging is also effective in obese patients with a similar event-free survival in those with normal studies. As the authors note, however, PET imaging is associated with some radiation exposure, whereas CMR imaging has no radiation exposure to the patient.
CMR use in this setting is limited, however, as some patients require sedation because of claustrophobia, and the technique is not readily available to all cardiac imaging laboratories.
Dr. George A. Beller is chief of the cardiovascular division at the University of Virginia Health System in Charlottesville. He has no financial conflicts of interest.
Stress SPECT imaging has been problematic in obese patients, often yielding poor quality images. This study by Dr. Shah and his coworkers clearly shows the prognostic value of stress cardiac magnetic resonance in obese individuals.
Those patients with absence of both ischemia and late gadolinium enhancement had an excellent prognosis during follow-up. It should be pointed out that PET myocardial perfusion imaging is also effective in obese patients with a similar event-free survival in those with normal studies. As the authors note, however, PET imaging is associated with some radiation exposure, whereas CMR imaging has no radiation exposure to the patient.
CMR use in this setting is limited, however, as some patients require sedation because of claustrophobia, and the technique is not readily available to all cardiac imaging laboratories.
Dr. George A. Beller is chief of the cardiovascular division at the University of Virginia Health System in Charlottesville. He has no financial conflicts of interest.
Stress SPECT imaging has been problematic in obese patients, often yielding poor quality images. This study by Dr. Shah and his coworkers clearly shows the prognostic value of stress cardiac magnetic resonance in obese individuals.
Those patients with absence of both ischemia and late gadolinium enhancement had an excellent prognosis during follow-up. It should be pointed out that PET myocardial perfusion imaging is also effective in obese patients with a similar event-free survival in those with normal studies. As the authors note, however, PET imaging is associated with some radiation exposure, whereas CMR imaging has no radiation exposure to the patient.
CMR use in this setting is limited, however, as some patients require sedation because of claustrophobia, and the technique is not readily available to all cardiac imaging laboratories.
Dr. George A. Beller is chief of the cardiovascular division at the University of Virginia Health System in Charlottesville. He has no financial conflicts of interest.
Stress cardiac magnetic resonance is highly feasible and prognostically useful in obese patients, a population in which stress imaging methods are limited, according to a study of nearly 300 patients reported online in JACC: Cardiovascular Imaging on April 9.
Dr. Ravi V. Shah of Harvard Medical School, Boston, and his colleagues, said stress echocardiography and nuclear perfusion can be challenging in obese patients, and PET has issues around ionizing radiation and cost, but the use of stress cardiac magnetic resonance had also been limited by concerns about claustrophobia and safety monitoring.
In this feasibility study in 285 patients with a mean body mass index of 35.4 kg/m2, the primary outcome was a composite of cardiac death or MI (MACE). During a mean follow-up of 2.1 years, 19 patients died, 7 from cardiovascular causes.
The incidence of MACE increased with both inducible ischemia and late gadolinium enhancement (LGE). The patients with no evidence of inducible ischemia, infarction, or LGE had a very low annualized MACE rate of 0.3%, while those who had no inducible ischemia who did have LGE had a rate of 2.4%. The MACE rate jumped significantly, to 6.3%, in patients with inducible ischemia and no LGE, and further yet in patients with evidence of both to 6.7%.
Diabetes, age, prior MI, prior revascularization, and reduced left ventricular ejection fraction were all associated with MACE in the study.
The investigators noted that only 13 (5%) of patients failed to complete the study protocol because of claustrophobia, intolerance to the stress agent, or poor gating, and sedation was required in 19 (7%) of patients.
However diagnostic-quality imaging was achieved in more than 89% of patients (JACC Cardiovasc. Imaging 2014 [dx.doi.org/10.1016/j.jcmg.2013.11.011]).
These results "confirm, in an obese population, that inducible ischemia and LGE by stress perfusion CMR are robust markers of risk even in those patients without a clinical history of prior infarction," the authors concluded.
Researchers declared grants and awards from the American Heart Association, the National Institutes of Health and the Alberta Heritage Foundation for Medical Research, and one author declared research support from Astellas Pharma US.
Stress cardiac magnetic resonance is highly feasible and prognostically useful in obese patients, a population in which stress imaging methods are limited, according to a study of nearly 300 patients reported online in JACC: Cardiovascular Imaging on April 9.
Dr. Ravi V. Shah of Harvard Medical School, Boston, and his colleagues, said stress echocardiography and nuclear perfusion can be challenging in obese patients, and PET has issues around ionizing radiation and cost, but the use of stress cardiac magnetic resonance had also been limited by concerns about claustrophobia and safety monitoring.
In this feasibility study in 285 patients with a mean body mass index of 35.4 kg/m2, the primary outcome was a composite of cardiac death or MI (MACE). During a mean follow-up of 2.1 years, 19 patients died, 7 from cardiovascular causes.
The incidence of MACE increased with both inducible ischemia and late gadolinium enhancement (LGE). The patients with no evidence of inducible ischemia, infarction, or LGE had a very low annualized MACE rate of 0.3%, while those who had no inducible ischemia who did have LGE had a rate of 2.4%. The MACE rate jumped significantly, to 6.3%, in patients with inducible ischemia and no LGE, and further yet in patients with evidence of both to 6.7%.
Diabetes, age, prior MI, prior revascularization, and reduced left ventricular ejection fraction were all associated with MACE in the study.
The investigators noted that only 13 (5%) of patients failed to complete the study protocol because of claustrophobia, intolerance to the stress agent, or poor gating, and sedation was required in 19 (7%) of patients.
However diagnostic-quality imaging was achieved in more than 89% of patients (JACC Cardiovasc. Imaging 2014 [dx.doi.org/10.1016/j.jcmg.2013.11.011]).
These results "confirm, in an obese population, that inducible ischemia and LGE by stress perfusion CMR are robust markers of risk even in those patients without a clinical history of prior infarction," the authors concluded.
Researchers declared grants and awards from the American Heart Association, the National Institutes of Health and the Alberta Heritage Foundation for Medical Research, and one author declared research support from Astellas Pharma US.
FROM JACC CARDIOVASCULAR IMAGING
Major finding: Obese individuals with no evidence of inducible ischemia or LGE in stress CMR are at significantly lower annualized risk of MACE (0.3%), compared with those with evidence of both (6.7%).
Data source: Cohort study in 285 patients with a mean body mass index of 35.4 kg/m2.
Disclosures: Researchers declared grants and awards from the American Heart Association, the National Institutes of Health, and the Alberta Heritage Foundation for Medical Research; one author declared research support from Astellas Pharma US.
An intravenous drug user with persistent dyspnea and lung infiltrates
A 58-year-old-man with a history of intravenous drug abuse, chronic hepatitis C, and anxiety presented to our emergency department twice in 4 weeks with progressive dyspnea and night sweats. He was a nonsmoker and had been an electrician for 15 years.
The first time he came in, chest radiography revealed bilateral reticulonodular infiltrates in the lung bases. He was treated with intravenous ceftriaxone (Rocephin) and azithromycin (Zithromax) for presumed community-acquired pneumonia and was then sent home on a 10-day course of oral amoxicillin-clavulanate (Augmentin). The antibiotics did not improve his symptoms, and 3 weeks later he presented again to the emergency department.
On his second presentation, he was in respiratory distress (oxygen saturation 78% on room air) and was afebrile and tachypneic. Physical examination revealed numerous injection marks or “tracks” on the skin of both arms, and auscultation revealed diminished intensity of breath sounds in both lung bases.
Repeat chest radiography demonstrated that the infiltrates were still there. Computed tomography was ordered and showed mild centrilobular emphysematous changes in both lungs, bibasilar opacifications, and a mass-like lesion (3.3 × 1.9 cm) in the right lower lobe (Figure 1).
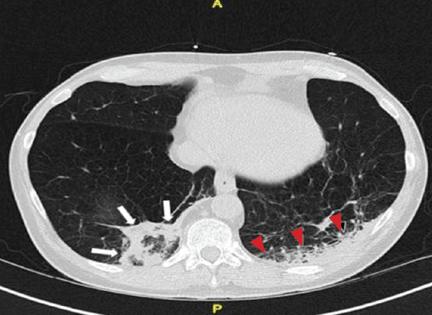
He subsequently underwent bronchoscopy, which showed no endobronchial abnormalities. Transbronchial lung biopsy was performed, and histopathologic analysis of the specimen (Figure 2) revealed rodlike, birefringent crystals under polarized light, with an extensive foreign-body giant-cell reaction outside pulmonary capillaries, suggestive of intravascular pulmonary talcosis. Blood and sputum cultures were negative for pathologic organisms. Bronchoalveolar lavage samples were negative for pathologic organisms and malignant cells.
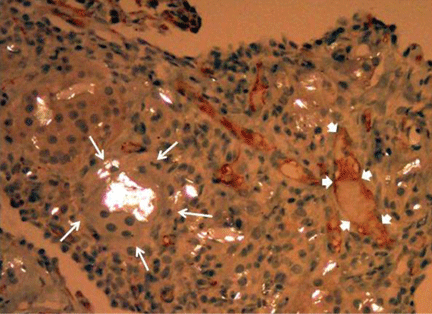
On further questioning, the patient revealed that he intravenously injected various drugs intended for oral use, such as crushed meperidine (Demerol), methylphenidate (Ritalin), and methadone tablets.
Pulmonary function tests indicated a severe obstructive pattern. The predicted forced expiratory volume in the first second of expiration (FEV1) was 25%, and the ratio of FEV1 to forced vital capacity was 27%.
Transthoracic echocardiography revealed mild pulmonary hypertension with a right ventricular systolic pressure of 28 mm Hg at rest.
Based on the results of the histologic examination, a diagnosis of intravascular pulmonary talcosis was made. Antibiotics were discontinued, and treatment with albuterol and ipratropium bromide (Combivent) inhalers was started. The patient remained oxygen-dependent at the time of hospital discharge.
INTRAVASCULAR PULMONARY TALCOSIS
Intravascular pulmonary talcosis is seen predominantly in those who chronically inject intravenous drugs intended for oral use.1,2
Many oral medications contain talc as a filler and lubricant to prevent the tablet from sticking to equipment during the manufacturing process. When oral medications containing talc are crushed, dissolved in water, and injected intravenously, the talc crystals and other particles lodge in the pulmonary vascular bed, resulting in microscopic pulmonary embolizations.
Over time, these particles migrate to the pulmonary interstitium and incite a foreign-body granulomatous reaction, which may be associated with progressive pulmonary fibrosis. The severity of this immune reaction and fibrosis may vary; hence, some patients remain asymptomatic, whereas some present with dyspnea from extensive fibrosis and pulmonary hypertension.
Persistent dyspnea along with persistent infiltrates on chest imaging in an intravenous drug abuser should prompt suspicion for intravascular pulmonary talcosis as well as consideration of other diagnoses, such as pneumonia, malignancy, and septic pulmonary emboli.
There is no established treatment for intravascular pulmonary talcosis; treatment is often supportive. A few studies and case reports have indicated varied success with systemic and inhaled corticosteroids.3–5 In extreme cases, lung transplantation may be necessary; however, this would require a comprehensive psychiatric assessment to minimize the risk of addiction relapse after transplantation.
- Arnett EN, Battle WE, Russo JV, Roberts WC. Intravenous injection of talc-containing drugs intended for oral use. A cause of pulmonary granulomatosis and pulmonary hypertension. Am J Med 1976; 60:711–718.
- Griffith CC, Raval JS, Nichols L. Intravascular talcosis due to intravenous drug use is an underrecognized cause of pulmonary hypertension. Pulm Med 2012; 2012:617531.
- Chau CH, Yew WW, Lee J. Inhaled budesonide in the treatment of talc-induced pulmonary granulomatosis. Respiration 2003; 70:439.
- Gysbrechts C, Michiels E, Verbeken E, et al. Interstitial lung disease more than 40 years after a 5 year occupational exposure to talc. Eur Respir J 1998; 11:1412–1415.
- Marchiori E, Lourenço S, Gasparetto TD, Zanetti G, Mano CM, Nobre LF. Pulmonary talcosis: imaging findings. Lung 2010; 188:165–171.
A 58-year-old-man with a history of intravenous drug abuse, chronic hepatitis C, and anxiety presented to our emergency department twice in 4 weeks with progressive dyspnea and night sweats. He was a nonsmoker and had been an electrician for 15 years.
The first time he came in, chest radiography revealed bilateral reticulonodular infiltrates in the lung bases. He was treated with intravenous ceftriaxone (Rocephin) and azithromycin (Zithromax) for presumed community-acquired pneumonia and was then sent home on a 10-day course of oral amoxicillin-clavulanate (Augmentin). The antibiotics did not improve his symptoms, and 3 weeks later he presented again to the emergency department.
On his second presentation, he was in respiratory distress (oxygen saturation 78% on room air) and was afebrile and tachypneic. Physical examination revealed numerous injection marks or “tracks” on the skin of both arms, and auscultation revealed diminished intensity of breath sounds in both lung bases.
Repeat chest radiography demonstrated that the infiltrates were still there. Computed tomography was ordered and showed mild centrilobular emphysematous changes in both lungs, bibasilar opacifications, and a mass-like lesion (3.3 × 1.9 cm) in the right lower lobe (Figure 1).

He subsequently underwent bronchoscopy, which showed no endobronchial abnormalities. Transbronchial lung biopsy was performed, and histopathologic analysis of the specimen (Figure 2) revealed rodlike, birefringent crystals under polarized light, with an extensive foreign-body giant-cell reaction outside pulmonary capillaries, suggestive of intravascular pulmonary talcosis. Blood and sputum cultures were negative for pathologic organisms. Bronchoalveolar lavage samples were negative for pathologic organisms and malignant cells.

On further questioning, the patient revealed that he intravenously injected various drugs intended for oral use, such as crushed meperidine (Demerol), methylphenidate (Ritalin), and methadone tablets.
Pulmonary function tests indicated a severe obstructive pattern. The predicted forced expiratory volume in the first second of expiration (FEV1) was 25%, and the ratio of FEV1 to forced vital capacity was 27%.
Transthoracic echocardiography revealed mild pulmonary hypertension with a right ventricular systolic pressure of 28 mm Hg at rest.
Based on the results of the histologic examination, a diagnosis of intravascular pulmonary talcosis was made. Antibiotics were discontinued, and treatment with albuterol and ipratropium bromide (Combivent) inhalers was started. The patient remained oxygen-dependent at the time of hospital discharge.
INTRAVASCULAR PULMONARY TALCOSIS
Intravascular pulmonary talcosis is seen predominantly in those who chronically inject intravenous drugs intended for oral use.1,2
Many oral medications contain talc as a filler and lubricant to prevent the tablet from sticking to equipment during the manufacturing process. When oral medications containing talc are crushed, dissolved in water, and injected intravenously, the talc crystals and other particles lodge in the pulmonary vascular bed, resulting in microscopic pulmonary embolizations.
Over time, these particles migrate to the pulmonary interstitium and incite a foreign-body granulomatous reaction, which may be associated with progressive pulmonary fibrosis. The severity of this immune reaction and fibrosis may vary; hence, some patients remain asymptomatic, whereas some present with dyspnea from extensive fibrosis and pulmonary hypertension.
Persistent dyspnea along with persistent infiltrates on chest imaging in an intravenous drug abuser should prompt suspicion for intravascular pulmonary talcosis as well as consideration of other diagnoses, such as pneumonia, malignancy, and septic pulmonary emboli.
There is no established treatment for intravascular pulmonary talcosis; treatment is often supportive. A few studies and case reports have indicated varied success with systemic and inhaled corticosteroids.3–5 In extreme cases, lung transplantation may be necessary; however, this would require a comprehensive psychiatric assessment to minimize the risk of addiction relapse after transplantation.
A 58-year-old-man with a history of intravenous drug abuse, chronic hepatitis C, and anxiety presented to our emergency department twice in 4 weeks with progressive dyspnea and night sweats. He was a nonsmoker and had been an electrician for 15 years.
The first time he came in, chest radiography revealed bilateral reticulonodular infiltrates in the lung bases. He was treated with intravenous ceftriaxone (Rocephin) and azithromycin (Zithromax) for presumed community-acquired pneumonia and was then sent home on a 10-day course of oral amoxicillin-clavulanate (Augmentin). The antibiotics did not improve his symptoms, and 3 weeks later he presented again to the emergency department.
On his second presentation, he was in respiratory distress (oxygen saturation 78% on room air) and was afebrile and tachypneic. Physical examination revealed numerous injection marks or “tracks” on the skin of both arms, and auscultation revealed diminished intensity of breath sounds in both lung bases.
Repeat chest radiography demonstrated that the infiltrates were still there. Computed tomography was ordered and showed mild centrilobular emphysematous changes in both lungs, bibasilar opacifications, and a mass-like lesion (3.3 × 1.9 cm) in the right lower lobe (Figure 1).

He subsequently underwent bronchoscopy, which showed no endobronchial abnormalities. Transbronchial lung biopsy was performed, and histopathologic analysis of the specimen (Figure 2) revealed rodlike, birefringent crystals under polarized light, with an extensive foreign-body giant-cell reaction outside pulmonary capillaries, suggestive of intravascular pulmonary talcosis. Blood and sputum cultures were negative for pathologic organisms. Bronchoalveolar lavage samples were negative for pathologic organisms and malignant cells.

On further questioning, the patient revealed that he intravenously injected various drugs intended for oral use, such as crushed meperidine (Demerol), methylphenidate (Ritalin), and methadone tablets.
Pulmonary function tests indicated a severe obstructive pattern. The predicted forced expiratory volume in the first second of expiration (FEV1) was 25%, and the ratio of FEV1 to forced vital capacity was 27%.
Transthoracic echocardiography revealed mild pulmonary hypertension with a right ventricular systolic pressure of 28 mm Hg at rest.
Based on the results of the histologic examination, a diagnosis of intravascular pulmonary talcosis was made. Antibiotics were discontinued, and treatment with albuterol and ipratropium bromide (Combivent) inhalers was started. The patient remained oxygen-dependent at the time of hospital discharge.
INTRAVASCULAR PULMONARY TALCOSIS
Intravascular pulmonary talcosis is seen predominantly in those who chronically inject intravenous drugs intended for oral use.1,2
Many oral medications contain talc as a filler and lubricant to prevent the tablet from sticking to equipment during the manufacturing process. When oral medications containing talc are crushed, dissolved in water, and injected intravenously, the talc crystals and other particles lodge in the pulmonary vascular bed, resulting in microscopic pulmonary embolizations.
Over time, these particles migrate to the pulmonary interstitium and incite a foreign-body granulomatous reaction, which may be associated with progressive pulmonary fibrosis. The severity of this immune reaction and fibrosis may vary; hence, some patients remain asymptomatic, whereas some present with dyspnea from extensive fibrosis and pulmonary hypertension.
Persistent dyspnea along with persistent infiltrates on chest imaging in an intravenous drug abuser should prompt suspicion for intravascular pulmonary talcosis as well as consideration of other diagnoses, such as pneumonia, malignancy, and septic pulmonary emboli.
There is no established treatment for intravascular pulmonary talcosis; treatment is often supportive. A few studies and case reports have indicated varied success with systemic and inhaled corticosteroids.3–5 In extreme cases, lung transplantation may be necessary; however, this would require a comprehensive psychiatric assessment to minimize the risk of addiction relapse after transplantation.
- Arnett EN, Battle WE, Russo JV, Roberts WC. Intravenous injection of talc-containing drugs intended for oral use. A cause of pulmonary granulomatosis and pulmonary hypertension. Am J Med 1976; 60:711–718.
- Griffith CC, Raval JS, Nichols L. Intravascular talcosis due to intravenous drug use is an underrecognized cause of pulmonary hypertension. Pulm Med 2012; 2012:617531.
- Chau CH, Yew WW, Lee J. Inhaled budesonide in the treatment of talc-induced pulmonary granulomatosis. Respiration 2003; 70:439.
- Gysbrechts C, Michiels E, Verbeken E, et al. Interstitial lung disease more than 40 years after a 5 year occupational exposure to talc. Eur Respir J 1998; 11:1412–1415.
- Marchiori E, Lourenço S, Gasparetto TD, Zanetti G, Mano CM, Nobre LF. Pulmonary talcosis: imaging findings. Lung 2010; 188:165–171.
- Arnett EN, Battle WE, Russo JV, Roberts WC. Intravenous injection of talc-containing drugs intended for oral use. A cause of pulmonary granulomatosis and pulmonary hypertension. Am J Med 1976; 60:711–718.
- Griffith CC, Raval JS, Nichols L. Intravascular talcosis due to intravenous drug use is an underrecognized cause of pulmonary hypertension. Pulm Med 2012; 2012:617531.
- Chau CH, Yew WW, Lee J. Inhaled budesonide in the treatment of talc-induced pulmonary granulomatosis. Respiration 2003; 70:439.
- Gysbrechts C, Michiels E, Verbeken E, et al. Interstitial lung disease more than 40 years after a 5 year occupational exposure to talc. Eur Respir J 1998; 11:1412–1415.
- Marchiori E, Lourenço S, Gasparetto TD, Zanetti G, Mano CM, Nobre LF. Pulmonary talcosis: imaging findings. Lung 2010; 188:165–171.
Pediatric Orthopedic Imaging: More Isn’t Always Better
Three excellent instructional cases from Dr. Lawrence Wells and colleagues from
the Children’s Hospital of Philadelphia follow in this E-Focus on Imaging in Pediatric Orthopedics of the February issue of The American Journal of Orthopedics (AJO). These cases highlight the important role of imaging in the practice of pediatric orthopedics, particularly its usefulness in problem solving for conditions that are difficult to diagnose clinically. Given the wide array of imaging techniques currently available, there is a tendency for surgeons to over-investigate. But more isn’t always better.
For example, while magnetic resonance (MR) imaging has the well-known advantages of avoidance of the potential hazards of ionizing radiation, multiplanar imaging capability, and superior soft-tissue contrast and resolution, the relatively long time period for acquisition of MR images make it relatively user-unfriendly for imaging in children. Movement artifacts can be a big problem, leading to image degradation and interpretation difficulties. For young children, having to administer heavy sedation or general anesthesia often negates the benefits of this diagnostic technique. Multidetector computed tomography (CT) produces images of excellent quality and resolution, particularly of bone. However, the price to pay for the thinner contiguous slices that enable production of the beautiful reformatted 2-dimensional sagittal and coronal images, and the stunning 3-dimensional
(3D) images, is a markedly increased radiation dose to the young patient.
It appears that the solution lies in a return to basic principles of good clinical practice. As illustrated by these 3 pediatric orthopedic cases in this month’s AJO, formulating a provisional diagnosis and short list of differential diagnoses starts with a well-taken and detailed clinical history and a meticulous physical examination. Simple hematologic investigations should be interpreted in light of the clinical findings. Imaging should be reserved for problem solving and should not be considered as a screening tool. There must be an imaging plan that aims to
address the following questions: Is there a lesion? If so, what and where exactly is it? And how can I best treat this patient’s condition—in this respect, is imaging really necessary?
For orthopedic problems, the time-honored radiograph still remains the initial imaging investigation in today’s practice. Too often, more expensive and advanced imaging modalities are requested first, even when the diagnosis can be made on
the basis of the plain film. This is poor clinical practice, and it reflects a lack of training and common sense. Radiographs are readily available, technically easy to perform, and give an overview of bone and joint lesions. It is the imaging investigation of choice for the detection of fractures and dislocations and also for the diagnosis of bone tumors and many other bone conditions. CT should be considered a supplementary examination to radiographs and is helpful when radiographs are equivocal or findings are subtle. CT is particularly suited for complex skeletal anatomy, for example, the spine, scapula, pelvis, and hindfoot.
In pediatric patients, reconstructed 3D CT images are useful for sorting out congenital spinal deformities.
For children and adolescents, ultrasonography can be used in place of MR imaging for many indications, particularly for assessing superficial structures such as tendons, muscles, ligaments, blood vessels, and other soft tissues. However, performing musculoskeletal ultrasonography well entails a rather long and steep
learning curve before technical expertise can be achieved. More advanced techniques such as MR imaging, nuclear medicine imaging, and imaging-guided interventional procedures should be used sparingly.
In fact, less may be better. If in doubt, pause before asking for more imaging and do consult your friendly neighborhood musculoskeletal radiologist.
Three excellent instructional cases from Dr. Lawrence Wells and colleagues from
the Children’s Hospital of Philadelphia follow in this E-Focus on Imaging in Pediatric Orthopedics of the February issue of The American Journal of Orthopedics (AJO). These cases highlight the important role of imaging in the practice of pediatric orthopedics, particularly its usefulness in problem solving for conditions that are difficult to diagnose clinically. Given the wide array of imaging techniques currently available, there is a tendency for surgeons to over-investigate. But more isn’t always better.
For example, while magnetic resonance (MR) imaging has the well-known advantages of avoidance of the potential hazards of ionizing radiation, multiplanar imaging capability, and superior soft-tissue contrast and resolution, the relatively long time period for acquisition of MR images make it relatively user-unfriendly for imaging in children. Movement artifacts can be a big problem, leading to image degradation and interpretation difficulties. For young children, having to administer heavy sedation or general anesthesia often negates the benefits of this diagnostic technique. Multidetector computed tomography (CT) produces images of excellent quality and resolution, particularly of bone. However, the price to pay for the thinner contiguous slices that enable production of the beautiful reformatted 2-dimensional sagittal and coronal images, and the stunning 3-dimensional
(3D) images, is a markedly increased radiation dose to the young patient.
It appears that the solution lies in a return to basic principles of good clinical practice. As illustrated by these 3 pediatric orthopedic cases in this month’s AJO, formulating a provisional diagnosis and short list of differential diagnoses starts with a well-taken and detailed clinical history and a meticulous physical examination. Simple hematologic investigations should be interpreted in light of the clinical findings. Imaging should be reserved for problem solving and should not be considered as a screening tool. There must be an imaging plan that aims to
address the following questions: Is there a lesion? If so, what and where exactly is it? And how can I best treat this patient’s condition—in this respect, is imaging really necessary?
For orthopedic problems, the time-honored radiograph still remains the initial imaging investigation in today’s practice. Too often, more expensive and advanced imaging modalities are requested first, even when the diagnosis can be made on
the basis of the plain film. This is poor clinical practice, and it reflects a lack of training and common sense. Radiographs are readily available, technically easy to perform, and give an overview of bone and joint lesions. It is the imaging investigation of choice for the detection of fractures and dislocations and also for the diagnosis of bone tumors and many other bone conditions. CT should be considered a supplementary examination to radiographs and is helpful when radiographs are equivocal or findings are subtle. CT is particularly suited for complex skeletal anatomy, for example, the spine, scapula, pelvis, and hindfoot.
In pediatric patients, reconstructed 3D CT images are useful for sorting out congenital spinal deformities.
For children and adolescents, ultrasonography can be used in place of MR imaging for many indications, particularly for assessing superficial structures such as tendons, muscles, ligaments, blood vessels, and other soft tissues. However, performing musculoskeletal ultrasonography well entails a rather long and steep
learning curve before technical expertise can be achieved. More advanced techniques such as MR imaging, nuclear medicine imaging, and imaging-guided interventional procedures should be used sparingly.
In fact, less may be better. If in doubt, pause before asking for more imaging and do consult your friendly neighborhood musculoskeletal radiologist.
Three excellent instructional cases from Dr. Lawrence Wells and colleagues from
the Children’s Hospital of Philadelphia follow in this E-Focus on Imaging in Pediatric Orthopedics of the February issue of The American Journal of Orthopedics (AJO). These cases highlight the important role of imaging in the practice of pediatric orthopedics, particularly its usefulness in problem solving for conditions that are difficult to diagnose clinically. Given the wide array of imaging techniques currently available, there is a tendency for surgeons to over-investigate. But more isn’t always better.
For example, while magnetic resonance (MR) imaging has the well-known advantages of avoidance of the potential hazards of ionizing radiation, multiplanar imaging capability, and superior soft-tissue contrast and resolution, the relatively long time period for acquisition of MR images make it relatively user-unfriendly for imaging in children. Movement artifacts can be a big problem, leading to image degradation and interpretation difficulties. For young children, having to administer heavy sedation or general anesthesia often negates the benefits of this diagnostic technique. Multidetector computed tomography (CT) produces images of excellent quality and resolution, particularly of bone. However, the price to pay for the thinner contiguous slices that enable production of the beautiful reformatted 2-dimensional sagittal and coronal images, and the stunning 3-dimensional
(3D) images, is a markedly increased radiation dose to the young patient.
It appears that the solution lies in a return to basic principles of good clinical practice. As illustrated by these 3 pediatric orthopedic cases in this month’s AJO, formulating a provisional diagnosis and short list of differential diagnoses starts with a well-taken and detailed clinical history and a meticulous physical examination. Simple hematologic investigations should be interpreted in light of the clinical findings. Imaging should be reserved for problem solving and should not be considered as a screening tool. There must be an imaging plan that aims to
address the following questions: Is there a lesion? If so, what and where exactly is it? And how can I best treat this patient’s condition—in this respect, is imaging really necessary?
For orthopedic problems, the time-honored radiograph still remains the initial imaging investigation in today’s practice. Too often, more expensive and advanced imaging modalities are requested first, even when the diagnosis can be made on
the basis of the plain film. This is poor clinical practice, and it reflects a lack of training and common sense. Radiographs are readily available, technically easy to perform, and give an overview of bone and joint lesions. It is the imaging investigation of choice for the detection of fractures and dislocations and also for the diagnosis of bone tumors and many other bone conditions. CT should be considered a supplementary examination to radiographs and is helpful when radiographs are equivocal or findings are subtle. CT is particularly suited for complex skeletal anatomy, for example, the spine, scapula, pelvis, and hindfoot.
In pediatric patients, reconstructed 3D CT images are useful for sorting out congenital spinal deformities.
For children and adolescents, ultrasonography can be used in place of MR imaging for many indications, particularly for assessing superficial structures such as tendons, muscles, ligaments, blood vessels, and other soft tissues. However, performing musculoskeletal ultrasonography well entails a rather long and steep
learning curve before technical expertise can be achieved. More advanced techniques such as MR imaging, nuclear medicine imaging, and imaging-guided interventional procedures should be used sparingly.
In fact, less may be better. If in doubt, pause before asking for more imaging and do consult your friendly neighborhood musculoskeletal radiologist.