User login
Malpractice Counsel
Stroke in a Young Man
A 26-year-old man presented to the ED with the chief complaint of mild right-sided weakness, paresthesias, and slurred speech. He stated the onset was sudden—approximately 30 minutes prior to arrival to the ED. The patient denied any previous similar symptoms and was otherwise in good health; he denied taking any medications. He drank alcohol socially, but denied smoking or illicit drug use.
On physical examination, his vital signs and oxygen saturation were normal. Pulmonary, cardiovascular, and abdominal examinations were also normal. The patient thought his speech was somewhat slurred, but the triage nurse and treating emergency physician (EP) had difficulty detecting any altered speech. He was noted to have mild (4+/5) right upper and lower extremity weakness; no facial droop was detected. The patient did have a mild pronator drift of the right upper extremity. Gait testing revealed a mild limp of the right lower extremity.
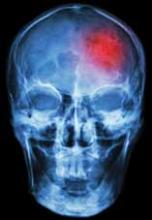
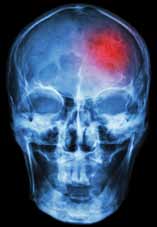
The EP consulted the hospitalist, and the patient was admitted to a monitored bed. The following morning, a brain magnetic resonance image revealed an ischemic stroke in the distribution of the left middle cerebral artery. The patient’s hospital course was uncomplicated, but at the time of discharge, he continued to have mild right-sided weakness and required the use of a cane.
The patient sued the hospital and the EP for negligence in failing to treat his condition in a timely manner and for not consulting a neurologist. The plaintiff’s attorneys argued the patient should have been given tissue plasminogen activator (tPA), which would have avoided the residual right-sided weakness. The defense denied negligence and argued the patient’s symptoms could have been due to several things for which tPA would have been an inappropriate treatment. A defense verdict was returned.
Discussion
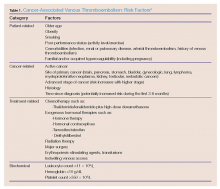
Stroke in young patients is relatively rare. With “young” defined as aged 18 to 45 years, this population accounts for approximately 2% to 12% of cerebral infarcts.1 In one nationwide US study of stroke in young adults, Ellis2 found that 4.9% of individuals experiencing a stroke in 2007 were between ages 18 and 44 years. Among this group, 78% experienced an ischemic stroke; 11.2% experienced a subarachnoid hemorrhage (SAH); and 10.8% had an intracerebral hemorrhage.2
While the clinical presentation of stroke in young adults is similar to that of older patients, the etiologies and risk factors are very different. In older patients, atherosclerosis is the major cause of ischemic stroke. In studies of young adults with ischemic stroke, cardioembolism was found to be the leading cause. Under this category, a patent foramen ovale (PFO) was considered a common cause, followed by atrial fibrillation, bacterial endocarditis, rheumatic heart disease, and atrial myxoma. There is, however, increasing controversy over the role of PFO as an etiology of stroke. Many investigators think its role has been overstated and is probably more of an incidental finding than a causal relationship.3 Patients with a suspected cardioembolic etiology will usually require an echocardiogram (with saline contrast or a “bubble study” for suspected PFO), cardiac monitoring, and a possible Holter monitor at the time of discharge (to detect paroxysmal arrhythmias).
Following cardioembolic etiologies, arterial dissection is the next most common category.4 In one study of patients aged 31 to 45 years old, arterial dissection was the most common cause of ischemic stroke.4 Clinical features suggesting dissection include a history of head or neck trauma (even minor trauma), headache or neck pain, and local neurological findings (eg, cranial nerve palsy or Horner syndrome).3 Unfortunately, only about 25% of patients volunteer a history of recent neck trauma. If a cervical or vertebral artery dissection is suspected, contrast enhanced magnetic resonance angiography (MRA) is the most sensitive and specific test, followed by carotid ultrasound and CT angiography.3
Traditional risk factors for stroke include hypertension and diabetes mellitus (DM). This is not true for younger adults that experience an ischemic stroke. Cigarette smoking is a very important risk factor for cerebrovascular accident in young adults; in addition, the more one smokes, the greater the risk. Other risk factors in young adults include history of migraine headaches (especially migraine with aura), pregnancy and the postpartum period, and illicit drug use.3
The defense’s argument that there are many causes of stroke in young adults that would be inappropriate for treatment with tPA, such as a PFO, carotid dissection or bacterial endocarditis, is absolutely true. Young patients need to be aggressively worked up for the etiology of their stroke, and may require additional testing, such as an MRA, echocardiogram, or Holter monitoring to determine the underlying cause of their stroke.
Obstruction Following Gastric Bypass Surgery
A 47-year-old woman presented to the ED complaining of severe back and abdominal pain. Onset had been gradual and began approximately 4 hours prior to arrival. She described the pain as crampy and constant. The patient had vomited twice; she denied diarrhea and had a normal bowel movement the previous day. She denied any vaginal or urinary complaints. Her past medical history was significant for hypertension and status post gastric bypass surgery 6 months prior. She had lost 42 pounds to date. She denied smoking or alcohol use.
The patient’s vital signs on physical examination were: blood pressure, 154/92 mm Hg; pulse, 106 beats/minute; respiratory rate, 18 breaths/minute; and temperature, 99˚F. Oxygen saturation was 96% on room air. The patient’s lungs were clear to auscultation bilaterally. The heart was mildly tachycardic, with a regular rhythm and without murmurs, rubs, or gallops. The abdominal examination revealed diffuse tenderness and involuntary guarding. There was no distention or rebound. Bowel sounds were present but hypoactive. Examination of the back revealed bilateral paraspinal muscle tenderness without costovertebral angle tenderness.
The EP ordered a CBC, BMP, serum lipase, and a urinalysis. The patient was given an intravenous (IV) bolus of 250 cc normal saline in addition to IV morphine 4 mg and IV ondansetron 4 mg. Her white blood cell (WBC) count was slightly elevated at 12.2 g/dL, with a normal differential. The remainder of the laboratory studies were normal, except for a serum bicarbonate of 22 mmol/L.
The patient stated she felt somewhat improved, but continued to have abdominal and back pain. The EP admitted her to the hospital for observation and pain control. She died the following day from a bowel obstruction. The family sued the EP for negligence in failing to order appropriate testing and for not consulting with specialists to diagnose the bowel obstruction, which is a known complication of gastric bypass surgery. The jury returned a verdict of $2.4 million against the EP.
Discussion
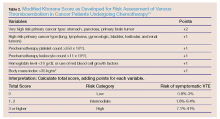
The frequency of bariatric surgery in the United States continues to increase, primarily due to its success with regard to weight loss, but also because of its demonstrated improvement in hypertension, obstructive sleep apnea, hyperlipidemia, and type 2 DM.1
Frequently, the term “gastric bypass surgery” is used interchangeably with bariatric surgery. However, the EP must realize these terms encompass multiple different operations. The four most common types of bariatric surgery in the United Stated are (1) adjustable gastric banding (AGB); (2) the Roux-en-Y gastric bypass (RYGB); (3) biliopancreatic diversion with duodenal switch (BPD-DS); and (4) vertical sleeve gastrectomy (VSG).2 (See the Table for a brief explanation of each type of procedure.)
Since each procedure has its own respective associated complications, it is important for the EP to know which the type of gastric bypass surgery the patient had. For example, leakage is much more frequent following RYGB than in gastric banding, while slippage and obstruction are the most common complications of gastric banding.3,4 It is also very helpful to know the specific type of procedure when discussing the case with the surgical consultant.
Based on a recent review of over 800,000 bariatric surgery patients, seven serious common complications following the surgery were identified.3 These included bleeding, leakage, obstruction, stomal ulceration, pulmonary embolism and respiratory complications, blood sugar disturbances (usually hypoglycemia and/or metabolic acidosis), and nutritional disturbances. While not all-inclusive, this list represents the most common serious complications of gastric bypass surgery.
The complaint of abdominal pain in a patient that has undergone bariatric surgery should be taken very seriously. In addition to determining the specific procedure performed and date, the patient should be questioned about vomiting, bowel movements, and the presence of blood in stool or vomit. Depending upon the degree of pain present, the patient may need to be given IV opioid analgesia to facilitate a thorough abdominal examination. A rectal examination should be performed to identify occult gastrointestinal bleeding.
These patients require laboratory testing, including CBC, BMP, and other laboratory evaluation as indicated by the history and physical examination. Early consultation with the bariatric surgeon is recommended. Many, if not most, patients with abdominal pain and vomiting will require imaging, usually a CT scan with contrast of the abdomen and pelvis. Because of the difficulty in interpreting the CT scan results in these patients, the bariatric surgeon will often want to personally review the films rather than rely solely on the interpretation by radiology services.
Unfortunately, the EP in this case did not appreciate the seriousness of the situation. The presence of severe abdominal pain, tenderness, guarding, mild tachycardia with leukocytosis, and metabolic acidosis all pointed to a more serious etiology than muscle spasm. This patient required IV fluids, analgesia, and imaging, as well as consultation with the bariatric surgeon.
- Chatzikonstantinou A, Wolf ME, Hennerici MG. Ischemic stroke in young adults: classification and risk factors. J Neurol. 2012;259(4):653-659.
- Ellis C. Stroke in young adults. Disabil Health J. 2010;3(3):222-224.
- Ferro JM, Massaro AR, Mas JL. Aetiological diagnosis of ischemic stroke in young adults. Lancet Neurol. 2010;9(11):1085-1096.
- Chan MT, Nadareishvili ZG, Norris JW; Canadian Stroke Consortium. Diagnostic strategies in young patients with ischemic stroke in Canada. Can J Neurol Sci. 2000;27(2):120-124.
- Buchwald H, Avidor Y, Braunwald E, et al. Bariatric surgery: a systematic review and meta-analysis. JAMA. 2004;292(14):1724-1737.
- Livingston EH. Patient guide: Endocrine and nutritional management after bariatric surgery: A patient’s guide. Hormone Health Network Web site. http://www.hormone.org/~/media/Hormone/Files/Patient%20Guides/Mens%20Health/PGBariatricSurgery_2014.pdf. Accessed December 17, 2014.
- Hussain A, El-Hasani S. Bariatric emergencies: current evidence and strategies of management. World J Emerg Surg. 2013;8(1):58.
- Campanille FC, Boru C, Rizzello M, et al. Acute complications after laparoscopic bariatric procedures: update for the general surgeon. Langenbecks Arch Surg. 2013;398(5):669-686
Stroke in a Young Man
A 26-year-old man presented to the ED with the chief complaint of mild right-sided weakness, paresthesias, and slurred speech. He stated the onset was sudden—approximately 30 minutes prior to arrival to the ED. The patient denied any previous similar symptoms and was otherwise in good health; he denied taking any medications. He drank alcohol socially, but denied smoking or illicit drug use.
On physical examination, his vital signs and oxygen saturation were normal. Pulmonary, cardiovascular, and abdominal examinations were also normal. The patient thought his speech was somewhat slurred, but the triage nurse and treating emergency physician (EP) had difficulty detecting any altered speech. He was noted to have mild (4+/5) right upper and lower extremity weakness; no facial droop was detected. The patient did have a mild pronator drift of the right upper extremity. Gait testing revealed a mild limp of the right lower extremity.
The EP consulted the hospitalist, and the patient was admitted to a monitored bed. The following morning, a brain magnetic resonance image revealed an ischemic stroke in the distribution of the left middle cerebral artery. The patient’s hospital course was uncomplicated, but at the time of discharge, he continued to have mild right-sided weakness and required the use of a cane.
The patient sued the hospital and the EP for negligence in failing to treat his condition in a timely manner and for not consulting a neurologist. The plaintiff’s attorneys argued the patient should have been given tissue plasminogen activator (tPA), which would have avoided the residual right-sided weakness. The defense denied negligence and argued the patient’s symptoms could have been due to several things for which tPA would have been an inappropriate treatment. A defense verdict was returned.
Discussion
Stroke in young patients is relatively rare. With “young” defined as aged 18 to 45 years, this population accounts for approximately 2% to 12% of cerebral infarcts.1 In one nationwide US study of stroke in young adults, Ellis2 found that 4.9% of individuals experiencing a stroke in 2007 were between ages 18 and 44 years. Among this group, 78% experienced an ischemic stroke; 11.2% experienced a subarachnoid hemorrhage (SAH); and 10.8% had an intracerebral hemorrhage.2
While the clinical presentation of stroke in young adults is similar to that of older patients, the etiologies and risk factors are very different. In older patients, atherosclerosis is the major cause of ischemic stroke. In studies of young adults with ischemic stroke, cardioembolism was found to be the leading cause. Under this category, a patent foramen ovale (PFO) was considered a common cause, followed by atrial fibrillation, bacterial endocarditis, rheumatic heart disease, and atrial myxoma. There is, however, increasing controversy over the role of PFO as an etiology of stroke. Many investigators think its role has been overstated and is probably more of an incidental finding than a causal relationship.3 Patients with a suspected cardioembolic etiology will usually require an echocardiogram (with saline contrast or a “bubble study” for suspected PFO), cardiac monitoring, and a possible Holter monitor at the time of discharge (to detect paroxysmal arrhythmias).
Following cardioembolic etiologies, arterial dissection is the next most common category.4 In one study of patients aged 31 to 45 years old, arterial dissection was the most common cause of ischemic stroke.4 Clinical features suggesting dissection include a history of head or neck trauma (even minor trauma), headache or neck pain, and local neurological findings (eg, cranial nerve palsy or Horner syndrome).3 Unfortunately, only about 25% of patients volunteer a history of recent neck trauma. If a cervical or vertebral artery dissection is suspected, contrast enhanced magnetic resonance angiography (MRA) is the most sensitive and specific test, followed by carotid ultrasound and CT angiography.3
Traditional risk factors for stroke include hypertension and diabetes mellitus (DM). This is not true for younger adults that experience an ischemic stroke. Cigarette smoking is a very important risk factor for cerebrovascular accident in young adults; in addition, the more one smokes, the greater the risk. Other risk factors in young adults include history of migraine headaches (especially migraine with aura), pregnancy and the postpartum period, and illicit drug use.3
The defense’s argument that there are many causes of stroke in young adults that would be inappropriate for treatment with tPA, such as a PFO, carotid dissection or bacterial endocarditis, is absolutely true. Young patients need to be aggressively worked up for the etiology of their stroke, and may require additional testing, such as an MRA, echocardiogram, or Holter monitoring to determine the underlying cause of their stroke.
Obstruction Following Gastric Bypass Surgery
A 47-year-old woman presented to the ED complaining of severe back and abdominal pain. Onset had been gradual and began approximately 4 hours prior to arrival. She described the pain as crampy and constant. The patient had vomited twice; she denied diarrhea and had a normal bowel movement the previous day. She denied any vaginal or urinary complaints. Her past medical history was significant for hypertension and status post gastric bypass surgery 6 months prior. She had lost 42 pounds to date. She denied smoking or alcohol use.
The patient’s vital signs on physical examination were: blood pressure, 154/92 mm Hg; pulse, 106 beats/minute; respiratory rate, 18 breaths/minute; and temperature, 99˚F. Oxygen saturation was 96% on room air. The patient’s lungs were clear to auscultation bilaterally. The heart was mildly tachycardic, with a regular rhythm and without murmurs, rubs, or gallops. The abdominal examination revealed diffuse tenderness and involuntary guarding. There was no distention or rebound. Bowel sounds were present but hypoactive. Examination of the back revealed bilateral paraspinal muscle tenderness without costovertebral angle tenderness.
The EP ordered a CBC, BMP, serum lipase, and a urinalysis. The patient was given an intravenous (IV) bolus of 250 cc normal saline in addition to IV morphine 4 mg and IV ondansetron 4 mg. Her white blood cell (WBC) count was slightly elevated at 12.2 g/dL, with a normal differential. The remainder of the laboratory studies were normal, except for a serum bicarbonate of 22 mmol/L.
The patient stated she felt somewhat improved, but continued to have abdominal and back pain. The EP admitted her to the hospital for observation and pain control. She died the following day from a bowel obstruction. The family sued the EP for negligence in failing to order appropriate testing and for not consulting with specialists to diagnose the bowel obstruction, which is a known complication of gastric bypass surgery. The jury returned a verdict of $2.4 million against the EP.
Discussion
The frequency of bariatric surgery in the United States continues to increase, primarily due to its success with regard to weight loss, but also because of its demonstrated improvement in hypertension, obstructive sleep apnea, hyperlipidemia, and type 2 DM.1
Frequently, the term “gastric bypass surgery” is used interchangeably with bariatric surgery. However, the EP must realize these terms encompass multiple different operations. The four most common types of bariatric surgery in the United Stated are (1) adjustable gastric banding (AGB); (2) the Roux-en-Y gastric bypass (RYGB); (3) biliopancreatic diversion with duodenal switch (BPD-DS); and (4) vertical sleeve gastrectomy (VSG).2 (See the Table for a brief explanation of each type of procedure.)
Since each procedure has its own respective associated complications, it is important for the EP to know which the type of gastric bypass surgery the patient had. For example, leakage is much more frequent following RYGB than in gastric banding, while slippage and obstruction are the most common complications of gastric banding.3,4 It is also very helpful to know the specific type of procedure when discussing the case with the surgical consultant.
Based on a recent review of over 800,000 bariatric surgery patients, seven serious common complications following the surgery were identified.3 These included bleeding, leakage, obstruction, stomal ulceration, pulmonary embolism and respiratory complications, blood sugar disturbances (usually hypoglycemia and/or metabolic acidosis), and nutritional disturbances. While not all-inclusive, this list represents the most common serious complications of gastric bypass surgery.
The complaint of abdominal pain in a patient that has undergone bariatric surgery should be taken very seriously. In addition to determining the specific procedure performed and date, the patient should be questioned about vomiting, bowel movements, and the presence of blood in stool or vomit. Depending upon the degree of pain present, the patient may need to be given IV opioid analgesia to facilitate a thorough abdominal examination. A rectal examination should be performed to identify occult gastrointestinal bleeding.
These patients require laboratory testing, including CBC, BMP, and other laboratory evaluation as indicated by the history and physical examination. Early consultation with the bariatric surgeon is recommended. Many, if not most, patients with abdominal pain and vomiting will require imaging, usually a CT scan with contrast of the abdomen and pelvis. Because of the difficulty in interpreting the CT scan results in these patients, the bariatric surgeon will often want to personally review the films rather than rely solely on the interpretation by radiology services.
Unfortunately, the EP in this case did not appreciate the seriousness of the situation. The presence of severe abdominal pain, tenderness, guarding, mild tachycardia with leukocytosis, and metabolic acidosis all pointed to a more serious etiology than muscle spasm. This patient required IV fluids, analgesia, and imaging, as well as consultation with the bariatric surgeon.
Stroke in a Young Man
A 26-year-old man presented to the ED with the chief complaint of mild right-sided weakness, paresthesias, and slurred speech. He stated the onset was sudden—approximately 30 minutes prior to arrival to the ED. The patient denied any previous similar symptoms and was otherwise in good health; he denied taking any medications. He drank alcohol socially, but denied smoking or illicit drug use.
On physical examination, his vital signs and oxygen saturation were normal. Pulmonary, cardiovascular, and abdominal examinations were also normal. The patient thought his speech was somewhat slurred, but the triage nurse and treating emergency physician (EP) had difficulty detecting any altered speech. He was noted to have mild (4+/5) right upper and lower extremity weakness; no facial droop was detected. The patient did have a mild pronator drift of the right upper extremity. Gait testing revealed a mild limp of the right lower extremity.
The EP consulted the hospitalist, and the patient was admitted to a monitored bed. The following morning, a brain magnetic resonance image revealed an ischemic stroke in the distribution of the left middle cerebral artery. The patient’s hospital course was uncomplicated, but at the time of discharge, he continued to have mild right-sided weakness and required the use of a cane.
The patient sued the hospital and the EP for negligence in failing to treat his condition in a timely manner and for not consulting a neurologist. The plaintiff’s attorneys argued the patient should have been given tissue plasminogen activator (tPA), which would have avoided the residual right-sided weakness. The defense denied negligence and argued the patient’s symptoms could have been due to several things for which tPA would have been an inappropriate treatment. A defense verdict was returned.
Discussion
Stroke in young patients is relatively rare. With “young” defined as aged 18 to 45 years, this population accounts for approximately 2% to 12% of cerebral infarcts.1 In one nationwide US study of stroke in young adults, Ellis2 found that 4.9% of individuals experiencing a stroke in 2007 were between ages 18 and 44 years. Among this group, 78% experienced an ischemic stroke; 11.2% experienced a subarachnoid hemorrhage (SAH); and 10.8% had an intracerebral hemorrhage.2
While the clinical presentation of stroke in young adults is similar to that of older patients, the etiologies and risk factors are very different. In older patients, atherosclerosis is the major cause of ischemic stroke. In studies of young adults with ischemic stroke, cardioembolism was found to be the leading cause. Under this category, a patent foramen ovale (PFO) was considered a common cause, followed by atrial fibrillation, bacterial endocarditis, rheumatic heart disease, and atrial myxoma. There is, however, increasing controversy over the role of PFO as an etiology of stroke. Many investigators think its role has been overstated and is probably more of an incidental finding than a causal relationship.3 Patients with a suspected cardioembolic etiology will usually require an echocardiogram (with saline contrast or a “bubble study” for suspected PFO), cardiac monitoring, and a possible Holter monitor at the time of discharge (to detect paroxysmal arrhythmias).
Following cardioembolic etiologies, arterial dissection is the next most common category.4 In one study of patients aged 31 to 45 years old, arterial dissection was the most common cause of ischemic stroke.4 Clinical features suggesting dissection include a history of head or neck trauma (even minor trauma), headache or neck pain, and local neurological findings (eg, cranial nerve palsy or Horner syndrome).3 Unfortunately, only about 25% of patients volunteer a history of recent neck trauma. If a cervical or vertebral artery dissection is suspected, contrast enhanced magnetic resonance angiography (MRA) is the most sensitive and specific test, followed by carotid ultrasound and CT angiography.3
Traditional risk factors for stroke include hypertension and diabetes mellitus (DM). This is not true for younger adults that experience an ischemic stroke. Cigarette smoking is a very important risk factor for cerebrovascular accident in young adults; in addition, the more one smokes, the greater the risk. Other risk factors in young adults include history of migraine headaches (especially migraine with aura), pregnancy and the postpartum period, and illicit drug use.3
The defense’s argument that there are many causes of stroke in young adults that would be inappropriate for treatment with tPA, such as a PFO, carotid dissection or bacterial endocarditis, is absolutely true. Young patients need to be aggressively worked up for the etiology of their stroke, and may require additional testing, such as an MRA, echocardiogram, or Holter monitoring to determine the underlying cause of their stroke.
Obstruction Following Gastric Bypass Surgery
A 47-year-old woman presented to the ED complaining of severe back and abdominal pain. Onset had been gradual and began approximately 4 hours prior to arrival. She described the pain as crampy and constant. The patient had vomited twice; she denied diarrhea and had a normal bowel movement the previous day. She denied any vaginal or urinary complaints. Her past medical history was significant for hypertension and status post gastric bypass surgery 6 months prior. She had lost 42 pounds to date. She denied smoking or alcohol use.
The patient’s vital signs on physical examination were: blood pressure, 154/92 mm Hg; pulse, 106 beats/minute; respiratory rate, 18 breaths/minute; and temperature, 99˚F. Oxygen saturation was 96% on room air. The patient’s lungs were clear to auscultation bilaterally. The heart was mildly tachycardic, with a regular rhythm and without murmurs, rubs, or gallops. The abdominal examination revealed diffuse tenderness and involuntary guarding. There was no distention or rebound. Bowel sounds were present but hypoactive. Examination of the back revealed bilateral paraspinal muscle tenderness without costovertebral angle tenderness.
The EP ordered a CBC, BMP, serum lipase, and a urinalysis. The patient was given an intravenous (IV) bolus of 250 cc normal saline in addition to IV morphine 4 mg and IV ondansetron 4 mg. Her white blood cell (WBC) count was slightly elevated at 12.2 g/dL, with a normal differential. The remainder of the laboratory studies were normal, except for a serum bicarbonate of 22 mmol/L.
The patient stated she felt somewhat improved, but continued to have abdominal and back pain. The EP admitted her to the hospital for observation and pain control. She died the following day from a bowel obstruction. The family sued the EP for negligence in failing to order appropriate testing and for not consulting with specialists to diagnose the bowel obstruction, which is a known complication of gastric bypass surgery. The jury returned a verdict of $2.4 million against the EP.
Discussion
The frequency of bariatric surgery in the United States continues to increase, primarily due to its success with regard to weight loss, but also because of its demonstrated improvement in hypertension, obstructive sleep apnea, hyperlipidemia, and type 2 DM.1
Frequently, the term “gastric bypass surgery” is used interchangeably with bariatric surgery. However, the EP must realize these terms encompass multiple different operations. The four most common types of bariatric surgery in the United Stated are (1) adjustable gastric banding (AGB); (2) the Roux-en-Y gastric bypass (RYGB); (3) biliopancreatic diversion with duodenal switch (BPD-DS); and (4) vertical sleeve gastrectomy (VSG).2 (See the Table for a brief explanation of each type of procedure.)
Since each procedure has its own respective associated complications, it is important for the EP to know which the type of gastric bypass surgery the patient had. For example, leakage is much more frequent following RYGB than in gastric banding, while slippage and obstruction are the most common complications of gastric banding.3,4 It is also very helpful to know the specific type of procedure when discussing the case with the surgical consultant.
Based on a recent review of over 800,000 bariatric surgery patients, seven serious common complications following the surgery were identified.3 These included bleeding, leakage, obstruction, stomal ulceration, pulmonary embolism and respiratory complications, blood sugar disturbances (usually hypoglycemia and/or metabolic acidosis), and nutritional disturbances. While not all-inclusive, this list represents the most common serious complications of gastric bypass surgery.
The complaint of abdominal pain in a patient that has undergone bariatric surgery should be taken very seriously. In addition to determining the specific procedure performed and date, the patient should be questioned about vomiting, bowel movements, and the presence of blood in stool or vomit. Depending upon the degree of pain present, the patient may need to be given IV opioid analgesia to facilitate a thorough abdominal examination. A rectal examination should be performed to identify occult gastrointestinal bleeding.
These patients require laboratory testing, including CBC, BMP, and other laboratory evaluation as indicated by the history and physical examination. Early consultation with the bariatric surgeon is recommended. Many, if not most, patients with abdominal pain and vomiting will require imaging, usually a CT scan with contrast of the abdomen and pelvis. Because of the difficulty in interpreting the CT scan results in these patients, the bariatric surgeon will often want to personally review the films rather than rely solely on the interpretation by radiology services.
Unfortunately, the EP in this case did not appreciate the seriousness of the situation. The presence of severe abdominal pain, tenderness, guarding, mild tachycardia with leukocytosis, and metabolic acidosis all pointed to a more serious etiology than muscle spasm. This patient required IV fluids, analgesia, and imaging, as well as consultation with the bariatric surgeon.
- Chatzikonstantinou A, Wolf ME, Hennerici MG. Ischemic stroke in young adults: classification and risk factors. J Neurol. 2012;259(4):653-659.
- Ellis C. Stroke in young adults. Disabil Health J. 2010;3(3):222-224.
- Ferro JM, Massaro AR, Mas JL. Aetiological diagnosis of ischemic stroke in young adults. Lancet Neurol. 2010;9(11):1085-1096.
- Chan MT, Nadareishvili ZG, Norris JW; Canadian Stroke Consortium. Diagnostic strategies in young patients with ischemic stroke in Canada. Can J Neurol Sci. 2000;27(2):120-124.
- Buchwald H, Avidor Y, Braunwald E, et al. Bariatric surgery: a systematic review and meta-analysis. JAMA. 2004;292(14):1724-1737.
- Livingston EH. Patient guide: Endocrine and nutritional management after bariatric surgery: A patient’s guide. Hormone Health Network Web site. http://www.hormone.org/~/media/Hormone/Files/Patient%20Guides/Mens%20Health/PGBariatricSurgery_2014.pdf. Accessed December 17, 2014.
- Hussain A, El-Hasani S. Bariatric emergencies: current evidence and strategies of management. World J Emerg Surg. 2013;8(1):58.
- Campanille FC, Boru C, Rizzello M, et al. Acute complications after laparoscopic bariatric procedures: update for the general surgeon. Langenbecks Arch Surg. 2013;398(5):669-686
- Chatzikonstantinou A, Wolf ME, Hennerici MG. Ischemic stroke in young adults: classification and risk factors. J Neurol. 2012;259(4):653-659.
- Ellis C. Stroke in young adults. Disabil Health J. 2010;3(3):222-224.
- Ferro JM, Massaro AR, Mas JL. Aetiological diagnosis of ischemic stroke in young adults. Lancet Neurol. 2010;9(11):1085-1096.
- Chan MT, Nadareishvili ZG, Norris JW; Canadian Stroke Consortium. Diagnostic strategies in young patients with ischemic stroke in Canada. Can J Neurol Sci. 2000;27(2):120-124.
- Buchwald H, Avidor Y, Braunwald E, et al. Bariatric surgery: a systematic review and meta-analysis. JAMA. 2004;292(14):1724-1737.
- Livingston EH. Patient guide: Endocrine and nutritional management after bariatric surgery: A patient’s guide. Hormone Health Network Web site. http://www.hormone.org/~/media/Hormone/Files/Patient%20Guides/Mens%20Health/PGBariatricSurgery_2014.pdf. Accessed December 17, 2014.
- Hussain A, El-Hasani S. Bariatric emergencies: current evidence and strategies of management. World J Emerg Surg. 2013;8(1):58.
- Campanille FC, Boru C, Rizzello M, et al. Acute complications after laparoscopic bariatric procedures: update for the general surgeon. Langenbecks Arch Surg. 2013;398(5):669-686
Spontaneous, Chronic Expanding Posterior Thigh Hematoma Mimicking Soft-Tissue Sarcoma in a Morbidly Obese Pregnant Woman
Soft-tissue sarcomas are quite rare, with an annual incidence of 20 to 30 per 1,000,000 persons in the United States.1 Because of their heterogeneous presentation, they remain a diagnostic challenge and are often initially confused for more common, benign disorders.2 Chronic expanding hematoma, first described by Friedlander and colleagues3 in 1968, is a rare entity that is particularly difficult to distinguish from soft-tissue malignancy.3-5 Chronic expanding hematoma is defined as a hematoma that gradually expands over 1 month or longer, is absent of neoplastic change on histologic sections, and does not occur in the setting of coagulopathy.6
Typically associated with remote trauma, these lesions often present as a slowly growing mass on the anterior or lateral thigh, calf, or buttock.3-4,7-9 They have been reported to persist as long as 46 years, with sizes ranging from 3 to 55 cm in maximum diameter.7 On imaging, they have a cystic appearance with a dense fibrous capsule.7-8 Most cases resolve uneventfully after drainage or marginal excision, although some cases require repeated intervention.7 This case report describes a morbidly obese patient with a chronic expanding hematoma in the distal posterior thigh whose definitive treatment was delayed 6 months because of her pregnancy status and inability to lie prone for open biopsy. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
A 27-year-old morbidly obese woman, who was pregnant at 12 weeks gestation, was seen in an orthopedic oncology clinic with a 1-month history of a slowly growing, painful posterior thigh mass. She had no history of cancer or bleeding disorder, and denied a history of trauma or constitutional symptoms consistent with malignancy. Coagulation studies were normal. Magnetic resonance imaging (MRI) obtained 2 weeks prior in the emergency room showed a cystic lesion with mass-like components in the posterior compartment of the distal right thigh, measuring 17 cm longitudinally. The lesion was located adjacent to, but not involving, the sciatic nerve and femoral vasculature. On initial examination, the large soft-tissue mass was evident and moderately painful to palpation; no skin changes were noted, and the patient had a normal sensorimotor examination. Fine-needle aspiration was performed, which resulted in amorphous debris consistent with hematoma.
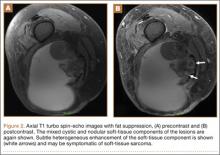
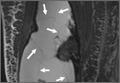
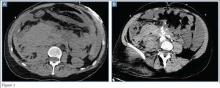
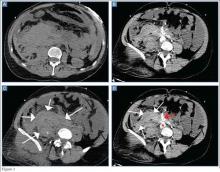
Repeat MRI 2 months later showed increased size of the lesion (9.5×10.5 cm axial, 22.0 cm craniocaudal). Although most findings of a more extensive imaging protocol, including precontrast and postcontrast sequences, were consistent with hematoma, the lesion also had several characteristics that indicated soft-tissue sarcoma. Specifically, findings suggestive of chronic hematoma included the hyperintense short tau inversion recovery (STIR) T1/T2 signal of the cystic component consistent with proteinaceous fluid and the low STIR TI/T2 signal of the periphery consistent with a rim of hemosiderin (Figure 1). Additionally, the cystic component of the lesion had multiple fine septations that are atypical for a hematoma (Figure 1), and several lymph nodes greater than 1.7 cm in short axis were noted in the anterior thigh and hemipelvis that were suspicious of metastatic lymphadenopathy. The encapsulated appearance of the lesion with a sharply defined margin and short transition zone were also reassuring findings for a benign lesion (Figures 1, 2A, 2B). However, several findings were identified that suggested soft-tissue sarcoma, including a nodular soft-tissue component on the medial wall of the lesion that had heterogeneous enhancement with contrast (Figure 2B). We, therefore, proceeded with ultrasound-guided core needle biopsy of the mass and cytologic sampling of the fluid components, which were again consistent with hematoma; no evidence of internal vascular flow was noted on Doppler ultrasound. Ultrasound-guided right inguinal lymph node biopsy was also performed and was negative for malignancy. Because of her large body habitus and pregnancy status, it was agreed that open biopsy should be delayed until after delivery to avoid placing the patient in a prone position.
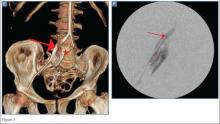
The patient visited the emergency room several times during the following months because of intermittent exacerbations of her lower extremity pain, swelling, and occasional paresthesias. About 6 months after initial presentation, repeat MRI again showed increased size of the mass (13.5×13.5 cm axial, 28 cm craniocaudal). There was also increased displacement of the adjacent neurovascular structures but no evidence of deep vein thrombosis. Because of concerns about the increased symptomatology of her thigh mass and possible sampling error of the previous biopsies, an elective cesarean section was performed at 35 weeks gestation. One week later, after clearance by her obstetrician, we proceeded with open biopsy of the mass in prone position. Initial sampling was negative for malignancy on frozen section; then, we expressed 1.75 L of brown fluid and solidified blood products, irrigated copiously, and placed a surgical drain. The permanent histologic specimens were again consistent with hematoma, and microbial cultures were negative. A week later, the patient accidentally removed her drain, and she presented with a fever (101°F) on postoperative day (POD) 15. Computed tomography showed reaccumulation of fluid; duplex ultrasound was negative. She was placed on cephalexin and underwent ultrasound-guided replacement of the drain with removal of an additional 750 mL fluid on POD 20. She drained an additional 150 to 200 mL/d for 1 month, with marked improvement in her leg swelling and knee range of motion. The drainage decreased during the next 3 weeks, and the drain was removed on POD 75.
Discussion
The presence of a hematoma in the extremities is usually a straightforward diagnosis. However, the unusual circumstances of this case highlight all the indications for investigation for possible soft-tissue sarcoma when a patient presents with what appears to be a benign condition.
Hematomas are rare in the absence of trauma or coagulopathy, with chronic expansion of hematomas rarer still.4,7,10-11 The patient had no evidence of coagulopathy because of her ability to have an uncomplicated pregnancy and elective cesarean section. She denied a history of trauma, and the location of her hematoma at the posterior distal thigh is an uncommon site of injury. In this setting, fine-needle aspiration and serial imaging to assess for progressive increase in lesion size were indicated to rule out malignancy.2
MRI is the gold-standard imaging modality for distinguishing soft-tissue masses from hematomas.5,12-14 Unlike the typical appearance of a hematoma, sarcomas of the soft-tissue extremities are often complex cystic lesions with multiple septations, internal soft-tissue components, and relatively ill-defined margins.15-17 However, as a hematoma becomes chronic, it can develop a fibrinous capsule, and the contents can manifest an atypical, heterogeneous appearance from scattered, progressive accumulation of blood products that is essentially indistinguishable from sarcomas on imaging.5
Because of the expansion of the hematoma and the atypical appearance of the mass on imaging, repeated core biopsy and, eventually, open biopsy were indicated, despite a preliminary negative diagnosis based on fine-needle aspiration. This resulted from the possibility of sampling error that is particularly relevant to cystic sarcomas, because only portions of the mass may be composed of malignant cells.2 An unusual aspect of this case is the regional lymphadenopathy noted on MRI, because regional lymphatic spread is a known mechanism of metastasis in soft-tissue sarcomas.18 However, the inguinal biopsies showed a chronic inflammatory infiltrate and were negative for malignancy, and enlarged nodes were not seen on imaging several months later. It is possible that the lymphadenopathy resulted from an unrelated process; alternatively, it may have been secondary to impaired lymphatic drainage because of mass effect from the hematoma, which also caused temporary lower extremity swelling.
The distal posterior thigh is an unreported location for a chronic expanding hematoma. Our patient developed slowly progressive lower-limb swelling and, eventually, paresthesias because of displacement of the neurovasculature, an unusual sequela that was recently reported in a similar case of an acute spontaneous hematoma in a patient on warfarin.19 Rupture of a Baker cyst is a possible inciting factor in our patient, although the proximal location of the lesion and the clearly defined tissue plane on MRI between the hematoma and the popliteal region make this unlikely. Finally, the patient’s lesion showed no evidence of vascular flow on Doppler ultrasonography, although giant hematomas secondary to popliteal aneurysm rupture have been reported.20-22
Conclusion
This case highlights the features of a chronic expanding hematoma that can suggest soft-tissue sarcoma and shows the recommended diagnostic steps to differentiate the 2 conditions. This case also describes an unreported location for a chronic expanding hematoma with resulting progressive neurovascular displacement caused by mass effect. We recommend careful monitoring of patients with similarly expansile lesions in this region for signs of neurovascular compromise.
1. O’Sullivan B, Pisters PW. Staging and prognostic factor evaluation in soft tissue sarcoma. Surg Oncol Clin N Am. 2003;12(2):333-353.
2. Rougraff B. The diagnosis and management of soft tissue sarcomas of the extremities in the adult. Curr Probl Cancer. 1999;23(1):1-50.
3. Friedlander HL, Bump RG. Chronic expanding hematoma of the calf. A case report. J Bone Joint Surg Am. 1968;50(6):1237-1241.
4. Liu CW, Kuo CL, Tsai TY, Lin LC, Wu CC. Massive gluteal mass mimicking sarcoma: chronic expanding hematoma. Formosan J Musculoskeletal Disord. 2011;2(3):106-108.
5. Taieb S, Penel N, Vanseymortier L, Ceugnart L. Soft tissue sarcomas or intramuscular haematomas? Eur J Radiol. 2009;72(1):44-49.
6. Reid JD, Kommareddi S, Lankerani M, Park MC. Chronic expanding hematomas. A clinicopathologic entity. JAMA. 1980;244(21):2441-2442.
7. Okada K, Sugiyama T, Kato H, Tani T. Chronic expanding hematoma mimicking soft tissue neoplasm. J Clin Oncol. 2001;19(11):2971-2972.
8. Negoro K, Uchida K, Yayama T, Kokubo Y, Baba H. Chronic expanding hematoma of the thigh. Joint Bone Spine. 2012;79(2):192-194.
9. Goddard MS, Vakil JJ, McCarthy EF, Khanuja HS. Chronic expanding hematoma of the lateral thigh and massive bony destruction after a failed total hip arthroplasty. J Arthroplasty. 2011;26(2):338.e13-.e15.
10. Radford DM, Schuh ME, Nambisan RN, Karakousis CP. Pseudo-tumor of the calf. Eur J Surg Oncol. 1993;19(3):300-301.
11. Mann HA, Hilton A, Goddard NJ, Smith MA, Holloway B, Lee CA. Synovial sarcoma mimicking haemophilic pseudotumour. Sarcoma. 2006;2006:27212.
12. Kransdorf MJ, Murphey MD. Radiologic evaluation of soft-tissue masses: a current perspective. AJR Am J Roentgenol. 2000;175(3):575-587.
13. Vanel D, Verstraete KL, Shapeero LG. Primary tumors of the musculoskeletal system. Radiol Clin North Am. 1997;35(1):213-237.
14. Siegel MJ. Magnetic resonance imaging of musculoskeletal soft tissue masses. Radiol Clin North Am. 2001;39(4):701-720.
15. O’Connor EE, Dixon LB, Peabody T, Stacy GS. MRI of cystic and soft-tissue masses of the shoulder joint. AJR Am J Roentgenol. 2004;183(1):39-47.
16. Bermejo A, De Bustamante TD, Martinez A, Carrera R, Zabia E, Manjon P. MR imaging in the evaluation of cystic-appearing soft-tissue masses of the extremities. Radiographics. 2013;33(3):833-855.
17. Morrison C, Wakely PE Jr, Ashman CJ, Lemley D, Theil K. Cystic synovial sarcoma. Ann Diagn Pathol. 2001;5(1):48-56.
18. Eilber FC, Rosen G, Nelson SD, et al. High-grade extremity soft tissue sarcomas: factors predictive of local recurrence and its effect on morbidity and mortality. Ann Surg. 2003;237(2):218-226.
19. Kuo CH. Peripheral neuropathy and lower limb swelling caused by a giant popliteal fossa hematoma. Neurol Sci. 2012;33(2):475-476.
20. Reijnen MM, de Rhoter W, Zeebregts CJ. Treatment of a symptomatic popliteal pseudoaneurysm using a stent-graft and ultrasound-guided evacuation of the haematoma. Emerg Radiol. 2009;16(2):167-169.
21. Rossi FH, Veith FJ, Lipsitz EC, Izukawa NM, Oliveira LA, Silva DG. Giant femoropopliteal artery aneurysm and vein rupture. Vascular. 2004;12(4):263-265.
22. Lamoca LM, Alerany MB, Hernando LL. Endovascular therapy for a ruptured popliteal aneurysm. Catheter Cardiovasc Interv. 2010;75(3):427-429.
Soft-tissue sarcomas are quite rare, with an annual incidence of 20 to 30 per 1,000,000 persons in the United States.1 Because of their heterogeneous presentation, they remain a diagnostic challenge and are often initially confused for more common, benign disorders.2 Chronic expanding hematoma, first described by Friedlander and colleagues3 in 1968, is a rare entity that is particularly difficult to distinguish from soft-tissue malignancy.3-5 Chronic expanding hematoma is defined as a hematoma that gradually expands over 1 month or longer, is absent of neoplastic change on histologic sections, and does not occur in the setting of coagulopathy.6
Typically associated with remote trauma, these lesions often present as a slowly growing mass on the anterior or lateral thigh, calf, or buttock.3-4,7-9 They have been reported to persist as long as 46 years, with sizes ranging from 3 to 55 cm in maximum diameter.7 On imaging, they have a cystic appearance with a dense fibrous capsule.7-8 Most cases resolve uneventfully after drainage or marginal excision, although some cases require repeated intervention.7 This case report describes a morbidly obese patient with a chronic expanding hematoma in the distal posterior thigh whose definitive treatment was delayed 6 months because of her pregnancy status and inability to lie prone for open biopsy. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
A 27-year-old morbidly obese woman, who was pregnant at 12 weeks gestation, was seen in an orthopedic oncology clinic with a 1-month history of a slowly growing, painful posterior thigh mass. She had no history of cancer or bleeding disorder, and denied a history of trauma or constitutional symptoms consistent with malignancy. Coagulation studies were normal. Magnetic resonance imaging (MRI) obtained 2 weeks prior in the emergency room showed a cystic lesion with mass-like components in the posterior compartment of the distal right thigh, measuring 17 cm longitudinally. The lesion was located adjacent to, but not involving, the sciatic nerve and femoral vasculature. On initial examination, the large soft-tissue mass was evident and moderately painful to palpation; no skin changes were noted, and the patient had a normal sensorimotor examination. Fine-needle aspiration was performed, which resulted in amorphous debris consistent with hematoma.
Repeat MRI 2 months later showed increased size of the lesion (9.5×10.5 cm axial, 22.0 cm craniocaudal). Although most findings of a more extensive imaging protocol, including precontrast and postcontrast sequences, were consistent with hematoma, the lesion also had several characteristics that indicated soft-tissue sarcoma. Specifically, findings suggestive of chronic hematoma included the hyperintense short tau inversion recovery (STIR) T1/T2 signal of the cystic component consistent with proteinaceous fluid and the low STIR TI/T2 signal of the periphery consistent with a rim of hemosiderin (Figure 1). Additionally, the cystic component of the lesion had multiple fine septations that are atypical for a hematoma (Figure 1), and several lymph nodes greater than 1.7 cm in short axis were noted in the anterior thigh and hemipelvis that were suspicious of metastatic lymphadenopathy. The encapsulated appearance of the lesion with a sharply defined margin and short transition zone were also reassuring findings for a benign lesion (Figures 1, 2A, 2B). However, several findings were identified that suggested soft-tissue sarcoma, including a nodular soft-tissue component on the medial wall of the lesion that had heterogeneous enhancement with contrast (Figure 2B). We, therefore, proceeded with ultrasound-guided core needle biopsy of the mass and cytologic sampling of the fluid components, which were again consistent with hematoma; no evidence of internal vascular flow was noted on Doppler ultrasound. Ultrasound-guided right inguinal lymph node biopsy was also performed and was negative for malignancy. Because of her large body habitus and pregnancy status, it was agreed that open biopsy should be delayed until after delivery to avoid placing the patient in a prone position.
The patient visited the emergency room several times during the following months because of intermittent exacerbations of her lower extremity pain, swelling, and occasional paresthesias. About 6 months after initial presentation, repeat MRI again showed increased size of the mass (13.5×13.5 cm axial, 28 cm craniocaudal). There was also increased displacement of the adjacent neurovascular structures but no evidence of deep vein thrombosis. Because of concerns about the increased symptomatology of her thigh mass and possible sampling error of the previous biopsies, an elective cesarean section was performed at 35 weeks gestation. One week later, after clearance by her obstetrician, we proceeded with open biopsy of the mass in prone position. Initial sampling was negative for malignancy on frozen section; then, we expressed 1.75 L of brown fluid and solidified blood products, irrigated copiously, and placed a surgical drain. The permanent histologic specimens were again consistent with hematoma, and microbial cultures were negative. A week later, the patient accidentally removed her drain, and she presented with a fever (101°F) on postoperative day (POD) 15. Computed tomography showed reaccumulation of fluid; duplex ultrasound was negative. She was placed on cephalexin and underwent ultrasound-guided replacement of the drain with removal of an additional 750 mL fluid on POD 20. She drained an additional 150 to 200 mL/d for 1 month, with marked improvement in her leg swelling and knee range of motion. The drainage decreased during the next 3 weeks, and the drain was removed on POD 75.
Discussion
The presence of a hematoma in the extremities is usually a straightforward diagnosis. However, the unusual circumstances of this case highlight all the indications for investigation for possible soft-tissue sarcoma when a patient presents with what appears to be a benign condition.
Hematomas are rare in the absence of trauma or coagulopathy, with chronic expansion of hematomas rarer still.4,7,10-11 The patient had no evidence of coagulopathy because of her ability to have an uncomplicated pregnancy and elective cesarean section. She denied a history of trauma, and the location of her hematoma at the posterior distal thigh is an uncommon site of injury. In this setting, fine-needle aspiration and serial imaging to assess for progressive increase in lesion size were indicated to rule out malignancy.2
MRI is the gold-standard imaging modality for distinguishing soft-tissue masses from hematomas.5,12-14 Unlike the typical appearance of a hematoma, sarcomas of the soft-tissue extremities are often complex cystic lesions with multiple septations, internal soft-tissue components, and relatively ill-defined margins.15-17 However, as a hematoma becomes chronic, it can develop a fibrinous capsule, and the contents can manifest an atypical, heterogeneous appearance from scattered, progressive accumulation of blood products that is essentially indistinguishable from sarcomas on imaging.5
Because of the expansion of the hematoma and the atypical appearance of the mass on imaging, repeated core biopsy and, eventually, open biopsy were indicated, despite a preliminary negative diagnosis based on fine-needle aspiration. This resulted from the possibility of sampling error that is particularly relevant to cystic sarcomas, because only portions of the mass may be composed of malignant cells.2 An unusual aspect of this case is the regional lymphadenopathy noted on MRI, because regional lymphatic spread is a known mechanism of metastasis in soft-tissue sarcomas.18 However, the inguinal biopsies showed a chronic inflammatory infiltrate and were negative for malignancy, and enlarged nodes were not seen on imaging several months later. It is possible that the lymphadenopathy resulted from an unrelated process; alternatively, it may have been secondary to impaired lymphatic drainage because of mass effect from the hematoma, which also caused temporary lower extremity swelling.
The distal posterior thigh is an unreported location for a chronic expanding hematoma. Our patient developed slowly progressive lower-limb swelling and, eventually, paresthesias because of displacement of the neurovasculature, an unusual sequela that was recently reported in a similar case of an acute spontaneous hematoma in a patient on warfarin.19 Rupture of a Baker cyst is a possible inciting factor in our patient, although the proximal location of the lesion and the clearly defined tissue plane on MRI between the hematoma and the popliteal region make this unlikely. Finally, the patient’s lesion showed no evidence of vascular flow on Doppler ultrasonography, although giant hematomas secondary to popliteal aneurysm rupture have been reported.20-22
Conclusion
This case highlights the features of a chronic expanding hematoma that can suggest soft-tissue sarcoma and shows the recommended diagnostic steps to differentiate the 2 conditions. This case also describes an unreported location for a chronic expanding hematoma with resulting progressive neurovascular displacement caused by mass effect. We recommend careful monitoring of patients with similarly expansile lesions in this region for signs of neurovascular compromise.
Soft-tissue sarcomas are quite rare, with an annual incidence of 20 to 30 per 1,000,000 persons in the United States.1 Because of their heterogeneous presentation, they remain a diagnostic challenge and are often initially confused for more common, benign disorders.2 Chronic expanding hematoma, first described by Friedlander and colleagues3 in 1968, is a rare entity that is particularly difficult to distinguish from soft-tissue malignancy.3-5 Chronic expanding hematoma is defined as a hematoma that gradually expands over 1 month or longer, is absent of neoplastic change on histologic sections, and does not occur in the setting of coagulopathy.6
Typically associated with remote trauma, these lesions often present as a slowly growing mass on the anterior or lateral thigh, calf, or buttock.3-4,7-9 They have been reported to persist as long as 46 years, with sizes ranging from 3 to 55 cm in maximum diameter.7 On imaging, they have a cystic appearance with a dense fibrous capsule.7-8 Most cases resolve uneventfully after drainage or marginal excision, although some cases require repeated intervention.7 This case report describes a morbidly obese patient with a chronic expanding hematoma in the distal posterior thigh whose definitive treatment was delayed 6 months because of her pregnancy status and inability to lie prone for open biopsy. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
A 27-year-old morbidly obese woman, who was pregnant at 12 weeks gestation, was seen in an orthopedic oncology clinic with a 1-month history of a slowly growing, painful posterior thigh mass. She had no history of cancer or bleeding disorder, and denied a history of trauma or constitutional symptoms consistent with malignancy. Coagulation studies were normal. Magnetic resonance imaging (MRI) obtained 2 weeks prior in the emergency room showed a cystic lesion with mass-like components in the posterior compartment of the distal right thigh, measuring 17 cm longitudinally. The lesion was located adjacent to, but not involving, the sciatic nerve and femoral vasculature. On initial examination, the large soft-tissue mass was evident and moderately painful to palpation; no skin changes were noted, and the patient had a normal sensorimotor examination. Fine-needle aspiration was performed, which resulted in amorphous debris consistent with hematoma.
Repeat MRI 2 months later showed increased size of the lesion (9.5×10.5 cm axial, 22.0 cm craniocaudal). Although most findings of a more extensive imaging protocol, including precontrast and postcontrast sequences, were consistent with hematoma, the lesion also had several characteristics that indicated soft-tissue sarcoma. Specifically, findings suggestive of chronic hematoma included the hyperintense short tau inversion recovery (STIR) T1/T2 signal of the cystic component consistent with proteinaceous fluid and the low STIR TI/T2 signal of the periphery consistent with a rim of hemosiderin (Figure 1). Additionally, the cystic component of the lesion had multiple fine septations that are atypical for a hematoma (Figure 1), and several lymph nodes greater than 1.7 cm in short axis were noted in the anterior thigh and hemipelvis that were suspicious of metastatic lymphadenopathy. The encapsulated appearance of the lesion with a sharply defined margin and short transition zone were also reassuring findings for a benign lesion (Figures 1, 2A, 2B). However, several findings were identified that suggested soft-tissue sarcoma, including a nodular soft-tissue component on the medial wall of the lesion that had heterogeneous enhancement with contrast (Figure 2B). We, therefore, proceeded with ultrasound-guided core needle biopsy of the mass and cytologic sampling of the fluid components, which were again consistent with hematoma; no evidence of internal vascular flow was noted on Doppler ultrasound. Ultrasound-guided right inguinal lymph node biopsy was also performed and was negative for malignancy. Because of her large body habitus and pregnancy status, it was agreed that open biopsy should be delayed until after delivery to avoid placing the patient in a prone position.
The patient visited the emergency room several times during the following months because of intermittent exacerbations of her lower extremity pain, swelling, and occasional paresthesias. About 6 months after initial presentation, repeat MRI again showed increased size of the mass (13.5×13.5 cm axial, 28 cm craniocaudal). There was also increased displacement of the adjacent neurovascular structures but no evidence of deep vein thrombosis. Because of concerns about the increased symptomatology of her thigh mass and possible sampling error of the previous biopsies, an elective cesarean section was performed at 35 weeks gestation. One week later, after clearance by her obstetrician, we proceeded with open biopsy of the mass in prone position. Initial sampling was negative for malignancy on frozen section; then, we expressed 1.75 L of brown fluid and solidified blood products, irrigated copiously, and placed a surgical drain. The permanent histologic specimens were again consistent with hematoma, and microbial cultures were negative. A week later, the patient accidentally removed her drain, and she presented with a fever (101°F) on postoperative day (POD) 15. Computed tomography showed reaccumulation of fluid; duplex ultrasound was negative. She was placed on cephalexin and underwent ultrasound-guided replacement of the drain with removal of an additional 750 mL fluid on POD 20. She drained an additional 150 to 200 mL/d for 1 month, with marked improvement in her leg swelling and knee range of motion. The drainage decreased during the next 3 weeks, and the drain was removed on POD 75.
Discussion
The presence of a hematoma in the extremities is usually a straightforward diagnosis. However, the unusual circumstances of this case highlight all the indications for investigation for possible soft-tissue sarcoma when a patient presents with what appears to be a benign condition.
Hematomas are rare in the absence of trauma or coagulopathy, with chronic expansion of hematomas rarer still.4,7,10-11 The patient had no evidence of coagulopathy because of her ability to have an uncomplicated pregnancy and elective cesarean section. She denied a history of trauma, and the location of her hematoma at the posterior distal thigh is an uncommon site of injury. In this setting, fine-needle aspiration and serial imaging to assess for progressive increase in lesion size were indicated to rule out malignancy.2
MRI is the gold-standard imaging modality for distinguishing soft-tissue masses from hematomas.5,12-14 Unlike the typical appearance of a hematoma, sarcomas of the soft-tissue extremities are often complex cystic lesions with multiple septations, internal soft-tissue components, and relatively ill-defined margins.15-17 However, as a hematoma becomes chronic, it can develop a fibrinous capsule, and the contents can manifest an atypical, heterogeneous appearance from scattered, progressive accumulation of blood products that is essentially indistinguishable from sarcomas on imaging.5
Because of the expansion of the hematoma and the atypical appearance of the mass on imaging, repeated core biopsy and, eventually, open biopsy were indicated, despite a preliminary negative diagnosis based on fine-needle aspiration. This resulted from the possibility of sampling error that is particularly relevant to cystic sarcomas, because only portions of the mass may be composed of malignant cells.2 An unusual aspect of this case is the regional lymphadenopathy noted on MRI, because regional lymphatic spread is a known mechanism of metastasis in soft-tissue sarcomas.18 However, the inguinal biopsies showed a chronic inflammatory infiltrate and were negative for malignancy, and enlarged nodes were not seen on imaging several months later. It is possible that the lymphadenopathy resulted from an unrelated process; alternatively, it may have been secondary to impaired lymphatic drainage because of mass effect from the hematoma, which also caused temporary lower extremity swelling.
The distal posterior thigh is an unreported location for a chronic expanding hematoma. Our patient developed slowly progressive lower-limb swelling and, eventually, paresthesias because of displacement of the neurovasculature, an unusual sequela that was recently reported in a similar case of an acute spontaneous hematoma in a patient on warfarin.19 Rupture of a Baker cyst is a possible inciting factor in our patient, although the proximal location of the lesion and the clearly defined tissue plane on MRI between the hematoma and the popliteal region make this unlikely. Finally, the patient’s lesion showed no evidence of vascular flow on Doppler ultrasonography, although giant hematomas secondary to popliteal aneurysm rupture have been reported.20-22
Conclusion
This case highlights the features of a chronic expanding hematoma that can suggest soft-tissue sarcoma and shows the recommended diagnostic steps to differentiate the 2 conditions. This case also describes an unreported location for a chronic expanding hematoma with resulting progressive neurovascular displacement caused by mass effect. We recommend careful monitoring of patients with similarly expansile lesions in this region for signs of neurovascular compromise.
1. O’Sullivan B, Pisters PW. Staging and prognostic factor evaluation in soft tissue sarcoma. Surg Oncol Clin N Am. 2003;12(2):333-353.
2. Rougraff B. The diagnosis and management of soft tissue sarcomas of the extremities in the adult. Curr Probl Cancer. 1999;23(1):1-50.
3. Friedlander HL, Bump RG. Chronic expanding hematoma of the calf. A case report. J Bone Joint Surg Am. 1968;50(6):1237-1241.
4. Liu CW, Kuo CL, Tsai TY, Lin LC, Wu CC. Massive gluteal mass mimicking sarcoma: chronic expanding hematoma. Formosan J Musculoskeletal Disord. 2011;2(3):106-108.
5. Taieb S, Penel N, Vanseymortier L, Ceugnart L. Soft tissue sarcomas or intramuscular haematomas? Eur J Radiol. 2009;72(1):44-49.
6. Reid JD, Kommareddi S, Lankerani M, Park MC. Chronic expanding hematomas. A clinicopathologic entity. JAMA. 1980;244(21):2441-2442.
7. Okada K, Sugiyama T, Kato H, Tani T. Chronic expanding hematoma mimicking soft tissue neoplasm. J Clin Oncol. 2001;19(11):2971-2972.
8. Negoro K, Uchida K, Yayama T, Kokubo Y, Baba H. Chronic expanding hematoma of the thigh. Joint Bone Spine. 2012;79(2):192-194.
9. Goddard MS, Vakil JJ, McCarthy EF, Khanuja HS. Chronic expanding hematoma of the lateral thigh and massive bony destruction after a failed total hip arthroplasty. J Arthroplasty. 2011;26(2):338.e13-.e15.
10. Radford DM, Schuh ME, Nambisan RN, Karakousis CP. Pseudo-tumor of the calf. Eur J Surg Oncol. 1993;19(3):300-301.
11. Mann HA, Hilton A, Goddard NJ, Smith MA, Holloway B, Lee CA. Synovial sarcoma mimicking haemophilic pseudotumour. Sarcoma. 2006;2006:27212.
12. Kransdorf MJ, Murphey MD. Radiologic evaluation of soft-tissue masses: a current perspective. AJR Am J Roentgenol. 2000;175(3):575-587.
13. Vanel D, Verstraete KL, Shapeero LG. Primary tumors of the musculoskeletal system. Radiol Clin North Am. 1997;35(1):213-237.
14. Siegel MJ. Magnetic resonance imaging of musculoskeletal soft tissue masses. Radiol Clin North Am. 2001;39(4):701-720.
15. O’Connor EE, Dixon LB, Peabody T, Stacy GS. MRI of cystic and soft-tissue masses of the shoulder joint. AJR Am J Roentgenol. 2004;183(1):39-47.
16. Bermejo A, De Bustamante TD, Martinez A, Carrera R, Zabia E, Manjon P. MR imaging in the evaluation of cystic-appearing soft-tissue masses of the extremities. Radiographics. 2013;33(3):833-855.
17. Morrison C, Wakely PE Jr, Ashman CJ, Lemley D, Theil K. Cystic synovial sarcoma. Ann Diagn Pathol. 2001;5(1):48-56.
18. Eilber FC, Rosen G, Nelson SD, et al. High-grade extremity soft tissue sarcomas: factors predictive of local recurrence and its effect on morbidity and mortality. Ann Surg. 2003;237(2):218-226.
19. Kuo CH. Peripheral neuropathy and lower limb swelling caused by a giant popliteal fossa hematoma. Neurol Sci. 2012;33(2):475-476.
20. Reijnen MM, de Rhoter W, Zeebregts CJ. Treatment of a symptomatic popliteal pseudoaneurysm using a stent-graft and ultrasound-guided evacuation of the haematoma. Emerg Radiol. 2009;16(2):167-169.
21. Rossi FH, Veith FJ, Lipsitz EC, Izukawa NM, Oliveira LA, Silva DG. Giant femoropopliteal artery aneurysm and vein rupture. Vascular. 2004;12(4):263-265.
22. Lamoca LM, Alerany MB, Hernando LL. Endovascular therapy for a ruptured popliteal aneurysm. Catheter Cardiovasc Interv. 2010;75(3):427-429.
1. O’Sullivan B, Pisters PW. Staging and prognostic factor evaluation in soft tissue sarcoma. Surg Oncol Clin N Am. 2003;12(2):333-353.
2. Rougraff B. The diagnosis and management of soft tissue sarcomas of the extremities in the adult. Curr Probl Cancer. 1999;23(1):1-50.
3. Friedlander HL, Bump RG. Chronic expanding hematoma of the calf. A case report. J Bone Joint Surg Am. 1968;50(6):1237-1241.
4. Liu CW, Kuo CL, Tsai TY, Lin LC, Wu CC. Massive gluteal mass mimicking sarcoma: chronic expanding hematoma. Formosan J Musculoskeletal Disord. 2011;2(3):106-108.
5. Taieb S, Penel N, Vanseymortier L, Ceugnart L. Soft tissue sarcomas or intramuscular haematomas? Eur J Radiol. 2009;72(1):44-49.
6. Reid JD, Kommareddi S, Lankerani M, Park MC. Chronic expanding hematomas. A clinicopathologic entity. JAMA. 1980;244(21):2441-2442.
7. Okada K, Sugiyama T, Kato H, Tani T. Chronic expanding hematoma mimicking soft tissue neoplasm. J Clin Oncol. 2001;19(11):2971-2972.
8. Negoro K, Uchida K, Yayama T, Kokubo Y, Baba H. Chronic expanding hematoma of the thigh. Joint Bone Spine. 2012;79(2):192-194.
9. Goddard MS, Vakil JJ, McCarthy EF, Khanuja HS. Chronic expanding hematoma of the lateral thigh and massive bony destruction after a failed total hip arthroplasty. J Arthroplasty. 2011;26(2):338.e13-.e15.
10. Radford DM, Schuh ME, Nambisan RN, Karakousis CP. Pseudo-tumor of the calf. Eur J Surg Oncol. 1993;19(3):300-301.
11. Mann HA, Hilton A, Goddard NJ, Smith MA, Holloway B, Lee CA. Synovial sarcoma mimicking haemophilic pseudotumour. Sarcoma. 2006;2006:27212.
12. Kransdorf MJ, Murphey MD. Radiologic evaluation of soft-tissue masses: a current perspective. AJR Am J Roentgenol. 2000;175(3):575-587.
13. Vanel D, Verstraete KL, Shapeero LG. Primary tumors of the musculoskeletal system. Radiol Clin North Am. 1997;35(1):213-237.
14. Siegel MJ. Magnetic resonance imaging of musculoskeletal soft tissue masses. Radiol Clin North Am. 2001;39(4):701-720.
15. O’Connor EE, Dixon LB, Peabody T, Stacy GS. MRI of cystic and soft-tissue masses of the shoulder joint. AJR Am J Roentgenol. 2004;183(1):39-47.
16. Bermejo A, De Bustamante TD, Martinez A, Carrera R, Zabia E, Manjon P. MR imaging in the evaluation of cystic-appearing soft-tissue masses of the extremities. Radiographics. 2013;33(3):833-855.
17. Morrison C, Wakely PE Jr, Ashman CJ, Lemley D, Theil K. Cystic synovial sarcoma. Ann Diagn Pathol. 2001;5(1):48-56.
18. Eilber FC, Rosen G, Nelson SD, et al. High-grade extremity soft tissue sarcomas: factors predictive of local recurrence and its effect on morbidity and mortality. Ann Surg. 2003;237(2):218-226.
19. Kuo CH. Peripheral neuropathy and lower limb swelling caused by a giant popliteal fossa hematoma. Neurol Sci. 2012;33(2):475-476.
20. Reijnen MM, de Rhoter W, Zeebregts CJ. Treatment of a symptomatic popliteal pseudoaneurysm using a stent-graft and ultrasound-guided evacuation of the haematoma. Emerg Radiol. 2009;16(2):167-169.
21. Rossi FH, Veith FJ, Lipsitz EC, Izukawa NM, Oliveira LA, Silva DG. Giant femoropopliteal artery aneurysm and vein rupture. Vascular. 2004;12(4):263-265.
22. Lamoca LM, Alerany MB, Hernando LL. Endovascular therapy for a ruptured popliteal aneurysm. Catheter Cardiovasc Interv. 2010;75(3):427-429.
Office-Based Rapid Prototyping in Orthopedic Surgery: A Novel Planning Technique and Review of the Literature
Three-dimensional (3-D) printing is a rapidly evolving technology with both medical and nonmedical applications.1,2 Rapid prototyping involves creating a physical model of human tissue from a 3-D computer-generated rendering.3 The method relies on export of Digital Imaging and Communications in Medicine (DICOM)–based computed tomography (CT) or magnetic resonance imaging (MRI) data into standard triangular language (STL) format. Reducing CT or MRI slice thickness increases resolution of the final model.2 Five types of rapid prototyping exist: STL, selective laser sintering, fused deposition modeling, multijet modeling, and 3-D printing.
Most implant manufacturers can produce a 3-D model based on surgeon-provided DICOM images. The ability to produce anatomical models in an office-based setting is a more recent development. Three-dimensional modeling may allow for more accurate and extensive preoperative planning than radiographic examination alone does, and may even allow surgeons to perform procedures as part of preoperative preparation. This can allow for early recognition of unanticipated intraoperative problems or of the need for special techniques and implants that would not have been otherwise available, all of which may ultimately reduce operative time.
The breadth of applications for office-based 3-D prototyping is not well described in the orthopedic surgery literature. In this article, we describe 7 cases of complex orthopedic disorders that were surgically treated after preoperative planning in which use of a 3-D printer allowed for “mock” surgery before the actual procedures. In 3 of the cases, the models were made by the implant manufacturers. Working with these models prompted us to buy a 3-D printer (Fortus 250; Stratasys, Eden Prairie, Minnesota) for in-office use. In the other 4 cases, we used this printer to create our own models. As indicated in the manufacturer’s literature, the printer uses fused deposition modeling, which builds a model layer by layer by heating thermoplastic material to a semi-liquid state and extruding it according to computer-controlled pathways.
We present preoperative images, preoperative 3-D modeling, and intraoperative and postoperative images along with brief case descriptions (Table). The patients provided written informed consent for print and electronic publication of these case reports.
Case Reports
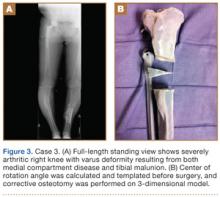
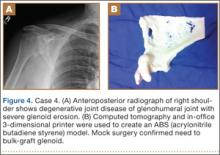
Case 1
A 28-year-old woman with a history of spondyloepiphyseal dysplasia presented to our clinic with bilateral hip pain. About 8 years earlier, she had undergone bilateral proximal and distal femoral osteotomies. Her function had initially improved, but over the 2 to 3 years before presentation she began having more pain and stiffness with activity. At time of initial evaluation, she was able to walk only 1 to 2 blocks and had difficulty getting in and out of a car and up out of a seated position.
On physical examination, the patient was 3 feet 10 inches tall and weighed 77 pounds. She ambulated with decreased stance phase on both lower extremities and had developed a significant amount of increased forward pelvic inclination and increased lumbar lordosis. Both hips and thighs had multiple healed scars from prior surgeries and pin tracts. Range of motion (ROM) on both sides was restricted to 85° of flexion, 10° of internal rotation, 15° of external rotation, and 15° of abduction.
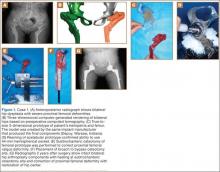
Plain radiographs showed advanced degenerative joint disease (DJD) of both hips with dysplastic acetabuli and evidence of healed osteotomies (Figure 1). Femoral deformities, noted bilaterally, consisted of marked valgus proximally and varus distally. Preoperative CT was used to create a 3-D model of the pelvis and femur. The model was created by the same implant manufacturer that produced the final components (Depuy, Warsaw, Indiana). Corrective femoral osteotomy was performed on the model to allow for design and use of a custom implant, while the modeled pelvis confirmed the ability to reproduce the normal hip center with a 44-mm conventional hemispherical socket.
After surgery, the patient was able to ambulate without a limp and return to work. Her hip ROM was pain-free passively and actively with flexion to 100°, internal rotation to 35°, external rotation to 20°, and abduction to 30°.
Case 2
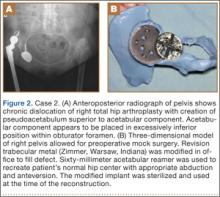
A 48-year-old woman with a history of Crowe IV hip dysplasia presented to our clinic with a chronically dislocated right total hip arthroplasty (THA) (Figure 2). Her initial THA was revised 1 year later because of acetabular component failure. Two years later, she was diagnosed with a deep periprosthetic infection, which was ultimately treated with 2-stage reimplantation. She subsequently dislocated and underwent re-revision of the S-ROM body and stem (DePuy Synthes, Warsaw, Indiana). At a visit after that revision, she was noted to be chronically dislocated, and was sent to our clinic for further management.
Preoperative radiographs showed a right uncemented THA with the femoral head dislocated toward the false acetabulum, retained hardware, and an old ununited trochanteric fragment. Both the femoral and acetabular components appeared well-fixed, though the acetabular component was positioned inferior, toward the obturator foramen.
Preoperative CT with metal artifact subtraction was used to create a 3-D model of the residual bony pelvis. The model was made by an implant manufacturer (Zimmer, Warsaw, Indiana). The shape of the superior defect was amenable to reconstruction using a modified revision trabecular metal socket. The pelvic model was reamed to accept a conventional hemispherical socket. The defect was reamed to accept a modified revision trabecular metal socket. The real implant was fashioned before surgery and was sterilized to avoid the need for intraoperative modification. Use of the preoperative model significantly reduced the time that would have been needed to modify the implant during actual surgery.
The patient’s right THA was revised. At time of surgery, the modified revision trabecular metal acetabular component was noted to seat appropriately in the superior defect. The true acetabulum was reestablished, and a hemispherical socket was placed with multiple screws. The 2 components were then unitized using cement in the same manner as would be done with an off-the-shelf augment.
Case 3
A 57-year-old man presented with a 10-year history of right knee pain. About 30 years before presentation at our clinic, he was treated for an open right tibia fracture sustained in a motorcycle accident. He had been treated nonsurgically, with injections, but they failed to provide sustained relief.
Preoperative radiographs showed severe advanced DJD in conjunction with an extra-articular posttraumatic varus tibial shaft deformity (Figure 3). An implant manufacturer (Zimmer) used a CT scan to create a model of the deformity. The resultant center of rotation angle was calculated using preoperative images and conventional techniques for deformity correction, and a lateral closing-wedge osteotomy was performed on the CT-based model. The initial attempt at deformity correction was slightly excessive, and the amount of resected bone slightly thicker than the calculated wedge, resulting in a valgus deformity. This error was noted, and the decision was made to recut a new model with a slight amount of residual varus that could be corrected during the final knee arthroplasty procedure.
Corrective osteotomy was performed with a lateral plate. Six months later, the patient had no residual pain, and CT confirmed union at the osteotomy site and a slight amount of residual varus. The patient then underwent routine total knee arthroplasty (TKA) using an abbreviated keel to avoid the need for removal of the previously placed hardware. The varus deformity was completely corrected.
Case 4
A 73-year-old man had a history of shoulder pain dating back to his childhood. Despite treatment with nonsteroidal anti-inflammatory drugs, physical therapy, and injections, his debilitating pain persisted. Physical examination revealed limited ROM and an intact rotator cuff.
Plain radiographs showed severe DJD of the glenohumeral joint (Figure 4). Severe erosions of the glenoid were noted, prompting further workup with CT, which showed significant bone loss, particularly along the posterior margin of the glenoid. We used our 3-D printer to create a model of the scapula from CT images. The model was then reamed in the usual fashion to accept a 3-pegged glenoid component. On placement of a trial implant, a large deficiency was seen posteriorly. We thought the size and location of the defect made it amenable to grafting using the patient’s humeral head.
The patient elected to undergo right total shoulder arthroplasty. During the procedure, the glenoid defect was found to be identical to what was encountered with the model before surgery. A portion of the patient’s humeral head was then fashioned to fit the defect, and was secured with three 2.7-mm screws, after provisional fixation using 2.0-mm Kirschner wires. The screws were countersunk, and the graft was contoured by hand to match the previous reaming. A 3-pegged 52-mm glenoid component was then cemented into position with excellent stability.
Case 5
A 64-year-old man presented to our clinic with left hip pain 40 years after THA. The original procedure was performed for resolved proximal femoral osteomyelitis. Plain radiographs showed a loose cemented McKee-Farrar hip arthroplasty (Figure 5). Because of the elevated position of the acetabular component relative to the native hip center, CT was used to determine the amount of femoral bone loss.
We used our 3-D printer to create a model and tried to recreate the native hip center with conventional off-the-shelf implants. A 50-mm hemispherical socket trial was placed in the appropriate location, along with a trabecular metal augment trial to provide extended coverage over the superolateral portion of the socket. Noted between the socket and the augment was a large gap; a substantial amount of cement would have been needed to unitize the construct. We thought a custom acetabular component would avoid the need for cement. In addition, given the patient’s small stature, the conventional acetabular component would allow a head only 32 mm in diameter. With a custom implant, the head could be enlarged to 36 mm, providing improved ROM and stability.
The patient underwent revision left hip arthroplasty using a custom acetabular component. A 3-D model available at time of surgery was used to aid implant placement.
Case 6
A 23-year-old man with multiple hereditary exostoses presented to our clinic with a painful mass in the left calf. Plain radiographs showed extensive osteochondromatosis involving the left proximal tibiofibular joint (Figure 6). The exostosis extended posteromedially, displacing the arterial trifurcation. MRI showed a small cartilage cap without evidence of malignant transformation.
CT angiogram allowed the vasculature to be modeled along with the deformity. A 3-D model was fabricated. The model included the entire proximal tibiofibular joint, as well as the anterior tibial, peroneal, and posterior tibial arteries. Cautious intralesional resection was recommended because of the proximity to all 3 vessels.
The patient underwent tumor resection through a longitudinal posterior approach. The interval between the medial and lateral heads of the gastrocnemius muscles was developed to expose the underlying soleus muscle. The soleus was split longitudinally from its hiatus to the inferior portion of the exostosis. This allowed for identification of the trifurcation and the tibial nerve, which were protected. Osteotomes were used to resect the mass at its base, the edges were carefully trimmed, and bone wax was placed over the defect. Anterior and lateral to this mass was another large mass (under the soleus muscle), which was also transected using an osteotome. The gastrocnemius and soleus muscles were then reflected off the fibula in order to remove 2 other exostoses, beneath the neck and head of the fibula.
Case 7
A 71-year-old man with a history of idiopathic lymphedema and peripheral neuropathy presented to our clinic with a left cavovarus foot deformity and a history of recurrent neuropathic foot ulcers (Figure 7). Physical examination revealed a callus over the lateral aspect of the base of the fifth metatarsal. Preoperative radiograph showed evidence of prior triple arthrodesis with a cavovarus foot deformity. CT scan was used to create a 3-D model of the foot. The model was then used to identify an appropriate location for lateral midtarsal and calcaneal closing-wedge osteotomies.
The patient underwent midfoot and hindfoot surgical correction. At surgery, the lateral closing-wedge osteotomies were performed according to the preoperative model. Radiographs 1 year after surgery showed correction of the forefoot varus.
Discussion
Three-dimensional printing for medical applications of anatomical modeling is not a new concept.1,3,4 Its use has been reported for a variety of applications in orthopedic surgery, including the printing of porous and metallic surfaces5 and bone-tissue engineering.6-9 Rapid prototyping for medical application was first reported in 1990 when a CT-based model was used to create a cranial bone.10 Reports of using the technique are becoming more widespread, particularly in the dental and maxillofacial literature, which includes reports on a variety of applications, including patient-specific drill guides, splints, and implants.11-14 The ability to perform mock surgery in advance of an actual procedure provides an invaluable opportunity to anticipate potential intraoperative problems, reduce operative time, and improve the accuracy of reconstruction.
Office-based rapid prototyping that uses an in-house 3-D printer is a novel application of this technology. It allows for creation of a patient-specific model for preoperative planning purposes. We are unaware of any other reports demonstrating the breadth and utility of office-based rapid prototyping in orthopedic surgery. For general reference, a printer similar to ours requires an initial investment of $52,000 to $56,000. This cost generally covers the printer, printer base cabinet, installation, training, and printer software (different from the 3-D modeling software), plus a 1-year warranty. A service agreement costs about $4000 annually. Printer and model supply expenses depend on the material used for the model (eg, ABS [acrylonitrile butadiene styrene]) and on the size and complexity of the 3-D models created. Average time to generate an appropriately formatted 3-D printing file is about 1 hour, though times can vary largely, according to amount of metal artifact subtraction necessary and the experience of the software user. For the rare, extremely complex deformities that require a significant amount of metal artifact subtraction, file preparation times can exceed 3 or 4 hours. We think these preparation times will decrease as communication between radiology file export format and modeling software ultimately allows for metal artifact subtraction images to function within the modeling software environment. Once an appropriately formatted file has been created, typical printing times vary according to the size of the to-be-modeled bone. For a hemipelvis, printing time is 30 to 40 hours; printing that is started on a Friday afternoon will be complete by Monday morning.
There are few reports of rapid prototyping in orthopedic surgery. In 2003, Minns and colleagues15 used a 3-D model in the planning of a tibial resection for TKA. They found the model to be accurate at time of surgery, resulting in appropriate tibial coverage by a conventional meniscal-bearing implant. Munjal and colleagues16 reported on 10 complex failed hip arthroplasty cases in which patients had revision surgery after preoperative planning using 3-D modeling techniques. The authors found that, in 8 of the 10 cases, conventional classification systems of bone loss were inaccurate in comparison with the prototype. Four cases required reconstruction with a custom triflange when conventional implants were not deemed reasonable based on the pelvic model. Tam and colleagues17 reported using a 3-D prototype as an aid in surgical planning for resection of a scapular osteochondroma in a 6-year-old patient. They found the rapid prototype to be useful at time of resection—similar to what we found with 1 patient (case 6). Adding contrast media to our patient’s scan allowed for 3-D visualization of the lesion and the encased vasculature. Fu and colleagues18 reported using a patient-specific drill template to insert anterior transpedicular screws. They constructed 24 prototypes of a formalin-preserved cervical vertebra to create a patient-specific biocompatible drill template for use in correcting multilevel cervical instability. They found the technique to be highly reproducible and accurate. Zein and colleagues19 used a rapid prototype of 3 consecutive human livers to preoperatively identify the vascular and biliary tract anatomy. They reported a high degree of accuracy—mean dimensional errors of less than 4 mm for the entire model and 1.3 mm for the vascular diameter.
The models created by implant manufacturers in this series were used to perform “mock” surgery before the actual procedures. Working with these models prompted us to buy our own 3-D printer. The learning curve can be steep, but commercially available 3-D printers allow for prompt in-office production of high-quality realistic prototypes at relatively low per-case cost (Figure 8). Three-dimensional modeling allows surgeons to assess the accuracy of their original surgical plans and, if necessary, correct them before surgery. Although computer-aided design models are useful, the ability to “perform surgery preoperatively” adds another element to surgeons’ understanding of the potential issues that may arise. Also, an in-office printer can help improve surgeons’ understanding and control over the process by which images are translated from radiographic file to 3-D model. Disadvantages of an in-office system include start-up and maintenance costs, office space requirements, and a significant learning curve for software and hardware applications. In addition, creation of 3-D models requires close interaction with radiologists who can provide appropriately formatted DICOM images, as metal artifact subtraction can be challenging. We think that, as image formatting and software capabilities are continually refined, this technology will become an invaluable part of multiple subspecialties across orthopedic surgery, with potentially infinite clinical, educational, and research applications.
1. McGurk M, Amis AA, Potamianos P, Goodger NM. Rapid prototyping techniques for anatomical modelling in medicine. Ann R Coll Surg Engl. 1997;79(3):169-174.
2. Webb PA. A review of rapid prototyping (RP) techniques in the medical and biomedical sector. J Med Eng Technol. 2000;24(4):149-153.
3. Esses SJ, Berman P, Bloom AI, Sosna J. Clinical applications of physical 3D models derived from MDCT data and created by rapid prototyping. AJR Am J Roentgenol. 2011;196(6):W683-W688.
4. Torres K, Staśkiewicz G, Śnieżyński M, Drop A, Maciejewski R. Application of rapid prototyping techniques for modelling of anatomical structures in medical training and education. Folia Morphol. 2011;70(1):1-4.
5. Melican MC, Zimmerman MC, Dhillon MS, Ponnambalam AR, Curodeau A, Parsons JR. Three-dimensional printing and porous metallic surfaces: a new orthopedic application. J Biomed Mater Res. 2001;55(2):194-202.
6. Butscher A, Bohner M, Hofmann S, Gauckler L, Müller R. Structural and material approaches to bone tissue engineering in powder-based three-dimensional printing. Acta Biomater. 2011;7(3):907-920.
7. Ciocca L, De Crescenzio F, Fantini M, Scotti R. CAD/CAM and rapid prototyped scaffold construction for bone regenerative medicine and surgical transfer of virtual planning: a pilot study. Comput Med Imaging Graph. 2009;33(1):58-62.
8. Leukers B, Gülkan H, Irsen SH, et al. Hydroxyapatite scaffolds for bone tissue engineering made by 3D printing. J Mater Sci Mater Med. 2005;16(12):1121-1124.
9. Seitz H, Rieder W, Irsen S, Leukers B, Tille C. Three-dimensional printing of porous ceramic scaffolds for bone tissue engineering. J Biomed Mater Res B Appl Biomater. 2005;74(2):782-788.
10. Mankovich NJ, Cheeseman AM, Stoker NG. The display of three-dimensional anatomy with stereolithographic models. J Digit Imaging. 1990;3(3):200-203.
11. Flügge TV, Nelson K, Schmelzeisen R, Metzger MC. Three-dimensional plotting and printing of an implant drilling guide: simplifying guided implant surgery. J Oral Maxillofac Surg. 2013;71(8):1340-1346.
12. Goiato MC, Santos MR, Pesqueira AA, Moreno A, dos Santos DM, Haddad MF. Prototyping for surgical and prosthetic treatment. J Craniofac Surg. 2011;22(3):914-917.
13. Metzger MC, Hohlweg-Majert B, Schwarz U, Teschner M, Hammer B, Schmelzeisen R. Manufacturing splints for orthognathic surgery using a three-dimensional printer. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2008;105(2):e1-e7.
14. Robiony M, Salvo I, Costa F, et al. Virtual reality surgical planning for maxillofacial distraction osteogenesis: the role of reverse engineering rapid prototyping and cooperative work. J Oral Maxillofac Surg. 2007;65(6):1198-1208.
15. Minns RJ, Bibb R, Banks R, Sutton RA. The use of a reconstructed three-dimensional solid model from CT to aid the surgical management of a total knee arthroplasty: a case study. Med Eng Phys. 2003;25(6):523-526.
16. Munjal S, Leopold SS, Kornreich D, Shott S, Finn HA. CT-generated 3-dimensional models for complex acetabular reconstruction. J Arthroplasty. 2000;15(5):644-653.
17. Tam MD, Laycock SD, Bell D, Chojnowski A. 3-D printout of a DICOM file to aid surgical planning in a 6 year old patient with a large scapular osteochondroma complicating congenital diaphyseal aclasia. J Radiol Case Rep. 2012;6(1):31-37.
18. Fu M, Lin L, Kong X, et al. Construction and accuracy assessment of patient-specific biocompatible drill template for cervical anterior transpedicular screw (ATPS) insertion: an in vitro study. PLoS One. 2013;8(1):e53580.
19. Zein NN, Hanouneh IA, Bishop PD, et al. Three-dimensional print of a liver for preoperative planning in living donor liver transplantation. Liver Transpl. 2013;19(12):1304-1310.
Three-dimensional (3-D) printing is a rapidly evolving technology with both medical and nonmedical applications.1,2 Rapid prototyping involves creating a physical model of human tissue from a 3-D computer-generated rendering.3 The method relies on export of Digital Imaging and Communications in Medicine (DICOM)–based computed tomography (CT) or magnetic resonance imaging (MRI) data into standard triangular language (STL) format. Reducing CT or MRI slice thickness increases resolution of the final model.2 Five types of rapid prototyping exist: STL, selective laser sintering, fused deposition modeling, multijet modeling, and 3-D printing.
Most implant manufacturers can produce a 3-D model based on surgeon-provided DICOM images. The ability to produce anatomical models in an office-based setting is a more recent development. Three-dimensional modeling may allow for more accurate and extensive preoperative planning than radiographic examination alone does, and may even allow surgeons to perform procedures as part of preoperative preparation. This can allow for early recognition of unanticipated intraoperative problems or of the need for special techniques and implants that would not have been otherwise available, all of which may ultimately reduce operative time.
The breadth of applications for office-based 3-D prototyping is not well described in the orthopedic surgery literature. In this article, we describe 7 cases of complex orthopedic disorders that were surgically treated after preoperative planning in which use of a 3-D printer allowed for “mock” surgery before the actual procedures. In 3 of the cases, the models were made by the implant manufacturers. Working with these models prompted us to buy a 3-D printer (Fortus 250; Stratasys, Eden Prairie, Minnesota) for in-office use. In the other 4 cases, we used this printer to create our own models. As indicated in the manufacturer’s literature, the printer uses fused deposition modeling, which builds a model layer by layer by heating thermoplastic material to a semi-liquid state and extruding it according to computer-controlled pathways.
We present preoperative images, preoperative 3-D modeling, and intraoperative and postoperative images along with brief case descriptions (Table). The patients provided written informed consent for print and electronic publication of these case reports.
Case Reports
Case 1
A 28-year-old woman with a history of spondyloepiphyseal dysplasia presented to our clinic with bilateral hip pain. About 8 years earlier, she had undergone bilateral proximal and distal femoral osteotomies. Her function had initially improved, but over the 2 to 3 years before presentation she began having more pain and stiffness with activity. At time of initial evaluation, she was able to walk only 1 to 2 blocks and had difficulty getting in and out of a car and up out of a seated position.
On physical examination, the patient was 3 feet 10 inches tall and weighed 77 pounds. She ambulated with decreased stance phase on both lower extremities and had developed a significant amount of increased forward pelvic inclination and increased lumbar lordosis. Both hips and thighs had multiple healed scars from prior surgeries and pin tracts. Range of motion (ROM) on both sides was restricted to 85° of flexion, 10° of internal rotation, 15° of external rotation, and 15° of abduction.
Plain radiographs showed advanced degenerative joint disease (DJD) of both hips with dysplastic acetabuli and evidence of healed osteotomies (Figure 1). Femoral deformities, noted bilaterally, consisted of marked valgus proximally and varus distally. Preoperative CT was used to create a 3-D model of the pelvis and femur. The model was created by the same implant manufacturer that produced the final components (Depuy, Warsaw, Indiana). Corrective femoral osteotomy was performed on the model to allow for design and use of a custom implant, while the modeled pelvis confirmed the ability to reproduce the normal hip center with a 44-mm conventional hemispherical socket.
After surgery, the patient was able to ambulate without a limp and return to work. Her hip ROM was pain-free passively and actively with flexion to 100°, internal rotation to 35°, external rotation to 20°, and abduction to 30°.
Case 2
A 48-year-old woman with a history of Crowe IV hip dysplasia presented to our clinic with a chronically dislocated right total hip arthroplasty (THA) (Figure 2). Her initial THA was revised 1 year later because of acetabular component failure. Two years later, she was diagnosed with a deep periprosthetic infection, which was ultimately treated with 2-stage reimplantation. She subsequently dislocated and underwent re-revision of the S-ROM body and stem (DePuy Synthes, Warsaw, Indiana). At a visit after that revision, she was noted to be chronically dislocated, and was sent to our clinic for further management.
Preoperative radiographs showed a right uncemented THA with the femoral head dislocated toward the false acetabulum, retained hardware, and an old ununited trochanteric fragment. Both the femoral and acetabular components appeared well-fixed, though the acetabular component was positioned inferior, toward the obturator foramen.
Preoperative CT with metal artifact subtraction was used to create a 3-D model of the residual bony pelvis. The model was made by an implant manufacturer (Zimmer, Warsaw, Indiana). The shape of the superior defect was amenable to reconstruction using a modified revision trabecular metal socket. The pelvic model was reamed to accept a conventional hemispherical socket. The defect was reamed to accept a modified revision trabecular metal socket. The real implant was fashioned before surgery and was sterilized to avoid the need for intraoperative modification. Use of the preoperative model significantly reduced the time that would have been needed to modify the implant during actual surgery.
The patient’s right THA was revised. At time of surgery, the modified revision trabecular metal acetabular component was noted to seat appropriately in the superior defect. The true acetabulum was reestablished, and a hemispherical socket was placed with multiple screws. The 2 components were then unitized using cement in the same manner as would be done with an off-the-shelf augment.
Case 3
A 57-year-old man presented with a 10-year history of right knee pain. About 30 years before presentation at our clinic, he was treated for an open right tibia fracture sustained in a motorcycle accident. He had been treated nonsurgically, with injections, but they failed to provide sustained relief.
Preoperative radiographs showed severe advanced DJD in conjunction with an extra-articular posttraumatic varus tibial shaft deformity (Figure 3). An implant manufacturer (Zimmer) used a CT scan to create a model of the deformity. The resultant center of rotation angle was calculated using preoperative images and conventional techniques for deformity correction, and a lateral closing-wedge osteotomy was performed on the CT-based model. The initial attempt at deformity correction was slightly excessive, and the amount of resected bone slightly thicker than the calculated wedge, resulting in a valgus deformity. This error was noted, and the decision was made to recut a new model with a slight amount of residual varus that could be corrected during the final knee arthroplasty procedure.
Corrective osteotomy was performed with a lateral plate. Six months later, the patient had no residual pain, and CT confirmed union at the osteotomy site and a slight amount of residual varus. The patient then underwent routine total knee arthroplasty (TKA) using an abbreviated keel to avoid the need for removal of the previously placed hardware. The varus deformity was completely corrected.
Case 4
A 73-year-old man had a history of shoulder pain dating back to his childhood. Despite treatment with nonsteroidal anti-inflammatory drugs, physical therapy, and injections, his debilitating pain persisted. Physical examination revealed limited ROM and an intact rotator cuff.
Plain radiographs showed severe DJD of the glenohumeral joint (Figure 4). Severe erosions of the glenoid were noted, prompting further workup with CT, which showed significant bone loss, particularly along the posterior margin of the glenoid. We used our 3-D printer to create a model of the scapula from CT images. The model was then reamed in the usual fashion to accept a 3-pegged glenoid component. On placement of a trial implant, a large deficiency was seen posteriorly. We thought the size and location of the defect made it amenable to grafting using the patient’s humeral head.
The patient elected to undergo right total shoulder arthroplasty. During the procedure, the glenoid defect was found to be identical to what was encountered with the model before surgery. A portion of the patient’s humeral head was then fashioned to fit the defect, and was secured with three 2.7-mm screws, after provisional fixation using 2.0-mm Kirschner wires. The screws were countersunk, and the graft was contoured by hand to match the previous reaming. A 3-pegged 52-mm glenoid component was then cemented into position with excellent stability.
Case 5
A 64-year-old man presented to our clinic with left hip pain 40 years after THA. The original procedure was performed for resolved proximal femoral osteomyelitis. Plain radiographs showed a loose cemented McKee-Farrar hip arthroplasty (Figure 5). Because of the elevated position of the acetabular component relative to the native hip center, CT was used to determine the amount of femoral bone loss.
We used our 3-D printer to create a model and tried to recreate the native hip center with conventional off-the-shelf implants. A 50-mm hemispherical socket trial was placed in the appropriate location, along with a trabecular metal augment trial to provide extended coverage over the superolateral portion of the socket. Noted between the socket and the augment was a large gap; a substantial amount of cement would have been needed to unitize the construct. We thought a custom acetabular component would avoid the need for cement. In addition, given the patient’s small stature, the conventional acetabular component would allow a head only 32 mm in diameter. With a custom implant, the head could be enlarged to 36 mm, providing improved ROM and stability.
The patient underwent revision left hip arthroplasty using a custom acetabular component. A 3-D model available at time of surgery was used to aid implant placement.
Case 6
A 23-year-old man with multiple hereditary exostoses presented to our clinic with a painful mass in the left calf. Plain radiographs showed extensive osteochondromatosis involving the left proximal tibiofibular joint (Figure 6). The exostosis extended posteromedially, displacing the arterial trifurcation. MRI showed a small cartilage cap without evidence of malignant transformation.
CT angiogram allowed the vasculature to be modeled along with the deformity. A 3-D model was fabricated. The model included the entire proximal tibiofibular joint, as well as the anterior tibial, peroneal, and posterior tibial arteries. Cautious intralesional resection was recommended because of the proximity to all 3 vessels.
The patient underwent tumor resection through a longitudinal posterior approach. The interval between the medial and lateral heads of the gastrocnemius muscles was developed to expose the underlying soleus muscle. The soleus was split longitudinally from its hiatus to the inferior portion of the exostosis. This allowed for identification of the trifurcation and the tibial nerve, which were protected. Osteotomes were used to resect the mass at its base, the edges were carefully trimmed, and bone wax was placed over the defect. Anterior and lateral to this mass was another large mass (under the soleus muscle), which was also transected using an osteotome. The gastrocnemius and soleus muscles were then reflected off the fibula in order to remove 2 other exostoses, beneath the neck and head of the fibula.
Case 7
A 71-year-old man with a history of idiopathic lymphedema and peripheral neuropathy presented to our clinic with a left cavovarus foot deformity and a history of recurrent neuropathic foot ulcers (Figure 7). Physical examination revealed a callus over the lateral aspect of the base of the fifth metatarsal. Preoperative radiograph showed evidence of prior triple arthrodesis with a cavovarus foot deformity. CT scan was used to create a 3-D model of the foot. The model was then used to identify an appropriate location for lateral midtarsal and calcaneal closing-wedge osteotomies.
The patient underwent midfoot and hindfoot surgical correction. At surgery, the lateral closing-wedge osteotomies were performed according to the preoperative model. Radiographs 1 year after surgery showed correction of the forefoot varus.
Discussion
Three-dimensional printing for medical applications of anatomical modeling is not a new concept.1,3,4 Its use has been reported for a variety of applications in orthopedic surgery, including the printing of porous and metallic surfaces5 and bone-tissue engineering.6-9 Rapid prototyping for medical application was first reported in 1990 when a CT-based model was used to create a cranial bone.10 Reports of using the technique are becoming more widespread, particularly in the dental and maxillofacial literature, which includes reports on a variety of applications, including patient-specific drill guides, splints, and implants.11-14 The ability to perform mock surgery in advance of an actual procedure provides an invaluable opportunity to anticipate potential intraoperative problems, reduce operative time, and improve the accuracy of reconstruction.
Office-based rapid prototyping that uses an in-house 3-D printer is a novel application of this technology. It allows for creation of a patient-specific model for preoperative planning purposes. We are unaware of any other reports demonstrating the breadth and utility of office-based rapid prototyping in orthopedic surgery. For general reference, a printer similar to ours requires an initial investment of $52,000 to $56,000. This cost generally covers the printer, printer base cabinet, installation, training, and printer software (different from the 3-D modeling software), plus a 1-year warranty. A service agreement costs about $4000 annually. Printer and model supply expenses depend on the material used for the model (eg, ABS [acrylonitrile butadiene styrene]) and on the size and complexity of the 3-D models created. Average time to generate an appropriately formatted 3-D printing file is about 1 hour, though times can vary largely, according to amount of metal artifact subtraction necessary and the experience of the software user. For the rare, extremely complex deformities that require a significant amount of metal artifact subtraction, file preparation times can exceed 3 or 4 hours. We think these preparation times will decrease as communication between radiology file export format and modeling software ultimately allows for metal artifact subtraction images to function within the modeling software environment. Once an appropriately formatted file has been created, typical printing times vary according to the size of the to-be-modeled bone. For a hemipelvis, printing time is 30 to 40 hours; printing that is started on a Friday afternoon will be complete by Monday morning.
There are few reports of rapid prototyping in orthopedic surgery. In 2003, Minns and colleagues15 used a 3-D model in the planning of a tibial resection for TKA. They found the model to be accurate at time of surgery, resulting in appropriate tibial coverage by a conventional meniscal-bearing implant. Munjal and colleagues16 reported on 10 complex failed hip arthroplasty cases in which patients had revision surgery after preoperative planning using 3-D modeling techniques. The authors found that, in 8 of the 10 cases, conventional classification systems of bone loss were inaccurate in comparison with the prototype. Four cases required reconstruction with a custom triflange when conventional implants were not deemed reasonable based on the pelvic model. Tam and colleagues17 reported using a 3-D prototype as an aid in surgical planning for resection of a scapular osteochondroma in a 6-year-old patient. They found the rapid prototype to be useful at time of resection—similar to what we found with 1 patient (case 6). Adding contrast media to our patient’s scan allowed for 3-D visualization of the lesion and the encased vasculature. Fu and colleagues18 reported using a patient-specific drill template to insert anterior transpedicular screws. They constructed 24 prototypes of a formalin-preserved cervical vertebra to create a patient-specific biocompatible drill template for use in correcting multilevel cervical instability. They found the technique to be highly reproducible and accurate. Zein and colleagues19 used a rapid prototype of 3 consecutive human livers to preoperatively identify the vascular and biliary tract anatomy. They reported a high degree of accuracy—mean dimensional errors of less than 4 mm for the entire model and 1.3 mm for the vascular diameter.
The models created by implant manufacturers in this series were used to perform “mock” surgery before the actual procedures. Working with these models prompted us to buy our own 3-D printer. The learning curve can be steep, but commercially available 3-D printers allow for prompt in-office production of high-quality realistic prototypes at relatively low per-case cost (Figure 8). Three-dimensional modeling allows surgeons to assess the accuracy of their original surgical plans and, if necessary, correct them before surgery. Although computer-aided design models are useful, the ability to “perform surgery preoperatively” adds another element to surgeons’ understanding of the potential issues that may arise. Also, an in-office printer can help improve surgeons’ understanding and control over the process by which images are translated from radiographic file to 3-D model. Disadvantages of an in-office system include start-up and maintenance costs, office space requirements, and a significant learning curve for software and hardware applications. In addition, creation of 3-D models requires close interaction with radiologists who can provide appropriately formatted DICOM images, as metal artifact subtraction can be challenging. We think that, as image formatting and software capabilities are continually refined, this technology will become an invaluable part of multiple subspecialties across orthopedic surgery, with potentially infinite clinical, educational, and research applications.
Three-dimensional (3-D) printing is a rapidly evolving technology with both medical and nonmedical applications.1,2 Rapid prototyping involves creating a physical model of human tissue from a 3-D computer-generated rendering.3 The method relies on export of Digital Imaging and Communications in Medicine (DICOM)–based computed tomography (CT) or magnetic resonance imaging (MRI) data into standard triangular language (STL) format. Reducing CT or MRI slice thickness increases resolution of the final model.2 Five types of rapid prototyping exist: STL, selective laser sintering, fused deposition modeling, multijet modeling, and 3-D printing.
Most implant manufacturers can produce a 3-D model based on surgeon-provided DICOM images. The ability to produce anatomical models in an office-based setting is a more recent development. Three-dimensional modeling may allow for more accurate and extensive preoperative planning than radiographic examination alone does, and may even allow surgeons to perform procedures as part of preoperative preparation. This can allow for early recognition of unanticipated intraoperative problems or of the need for special techniques and implants that would not have been otherwise available, all of which may ultimately reduce operative time.
The breadth of applications for office-based 3-D prototyping is not well described in the orthopedic surgery literature. In this article, we describe 7 cases of complex orthopedic disorders that were surgically treated after preoperative planning in which use of a 3-D printer allowed for “mock” surgery before the actual procedures. In 3 of the cases, the models were made by the implant manufacturers. Working with these models prompted us to buy a 3-D printer (Fortus 250; Stratasys, Eden Prairie, Minnesota) for in-office use. In the other 4 cases, we used this printer to create our own models. As indicated in the manufacturer’s literature, the printer uses fused deposition modeling, which builds a model layer by layer by heating thermoplastic material to a semi-liquid state and extruding it according to computer-controlled pathways.
We present preoperative images, preoperative 3-D modeling, and intraoperative and postoperative images along with brief case descriptions (Table). The patients provided written informed consent for print and electronic publication of these case reports.
Case Reports
Case 1
A 28-year-old woman with a history of spondyloepiphyseal dysplasia presented to our clinic with bilateral hip pain. About 8 years earlier, she had undergone bilateral proximal and distal femoral osteotomies. Her function had initially improved, but over the 2 to 3 years before presentation she began having more pain and stiffness with activity. At time of initial evaluation, she was able to walk only 1 to 2 blocks and had difficulty getting in and out of a car and up out of a seated position.
On physical examination, the patient was 3 feet 10 inches tall and weighed 77 pounds. She ambulated with decreased stance phase on both lower extremities and had developed a significant amount of increased forward pelvic inclination and increased lumbar lordosis. Both hips and thighs had multiple healed scars from prior surgeries and pin tracts. Range of motion (ROM) on both sides was restricted to 85° of flexion, 10° of internal rotation, 15° of external rotation, and 15° of abduction.
Plain radiographs showed advanced degenerative joint disease (DJD) of both hips with dysplastic acetabuli and evidence of healed osteotomies (Figure 1). Femoral deformities, noted bilaterally, consisted of marked valgus proximally and varus distally. Preoperative CT was used to create a 3-D model of the pelvis and femur. The model was created by the same implant manufacturer that produced the final components (Depuy, Warsaw, Indiana). Corrective femoral osteotomy was performed on the model to allow for design and use of a custom implant, while the modeled pelvis confirmed the ability to reproduce the normal hip center with a 44-mm conventional hemispherical socket.
After surgery, the patient was able to ambulate without a limp and return to work. Her hip ROM was pain-free passively and actively with flexion to 100°, internal rotation to 35°, external rotation to 20°, and abduction to 30°.
Case 2
A 48-year-old woman with a history of Crowe IV hip dysplasia presented to our clinic with a chronically dislocated right total hip arthroplasty (THA) (Figure 2). Her initial THA was revised 1 year later because of acetabular component failure. Two years later, she was diagnosed with a deep periprosthetic infection, which was ultimately treated with 2-stage reimplantation. She subsequently dislocated and underwent re-revision of the S-ROM body and stem (DePuy Synthes, Warsaw, Indiana). At a visit after that revision, she was noted to be chronically dislocated, and was sent to our clinic for further management.
Preoperative radiographs showed a right uncemented THA with the femoral head dislocated toward the false acetabulum, retained hardware, and an old ununited trochanteric fragment. Both the femoral and acetabular components appeared well-fixed, though the acetabular component was positioned inferior, toward the obturator foramen.
Preoperative CT with metal artifact subtraction was used to create a 3-D model of the residual bony pelvis. The model was made by an implant manufacturer (Zimmer, Warsaw, Indiana). The shape of the superior defect was amenable to reconstruction using a modified revision trabecular metal socket. The pelvic model was reamed to accept a conventional hemispherical socket. The defect was reamed to accept a modified revision trabecular metal socket. The real implant was fashioned before surgery and was sterilized to avoid the need for intraoperative modification. Use of the preoperative model significantly reduced the time that would have been needed to modify the implant during actual surgery.
The patient’s right THA was revised. At time of surgery, the modified revision trabecular metal acetabular component was noted to seat appropriately in the superior defect. The true acetabulum was reestablished, and a hemispherical socket was placed with multiple screws. The 2 components were then unitized using cement in the same manner as would be done with an off-the-shelf augment.
Case 3
A 57-year-old man presented with a 10-year history of right knee pain. About 30 years before presentation at our clinic, he was treated for an open right tibia fracture sustained in a motorcycle accident. He had been treated nonsurgically, with injections, but they failed to provide sustained relief.
Preoperative radiographs showed severe advanced DJD in conjunction with an extra-articular posttraumatic varus tibial shaft deformity (Figure 3). An implant manufacturer (Zimmer) used a CT scan to create a model of the deformity. The resultant center of rotation angle was calculated using preoperative images and conventional techniques for deformity correction, and a lateral closing-wedge osteotomy was performed on the CT-based model. The initial attempt at deformity correction was slightly excessive, and the amount of resected bone slightly thicker than the calculated wedge, resulting in a valgus deformity. This error was noted, and the decision was made to recut a new model with a slight amount of residual varus that could be corrected during the final knee arthroplasty procedure.
Corrective osteotomy was performed with a lateral plate. Six months later, the patient had no residual pain, and CT confirmed union at the osteotomy site and a slight amount of residual varus. The patient then underwent routine total knee arthroplasty (TKA) using an abbreviated keel to avoid the need for removal of the previously placed hardware. The varus deformity was completely corrected.
Case 4
A 73-year-old man had a history of shoulder pain dating back to his childhood. Despite treatment with nonsteroidal anti-inflammatory drugs, physical therapy, and injections, his debilitating pain persisted. Physical examination revealed limited ROM and an intact rotator cuff.
Plain radiographs showed severe DJD of the glenohumeral joint (Figure 4). Severe erosions of the glenoid were noted, prompting further workup with CT, which showed significant bone loss, particularly along the posterior margin of the glenoid. We used our 3-D printer to create a model of the scapula from CT images. The model was then reamed in the usual fashion to accept a 3-pegged glenoid component. On placement of a trial implant, a large deficiency was seen posteriorly. We thought the size and location of the defect made it amenable to grafting using the patient’s humeral head.
The patient elected to undergo right total shoulder arthroplasty. During the procedure, the glenoid defect was found to be identical to what was encountered with the model before surgery. A portion of the patient’s humeral head was then fashioned to fit the defect, and was secured with three 2.7-mm screws, after provisional fixation using 2.0-mm Kirschner wires. The screws were countersunk, and the graft was contoured by hand to match the previous reaming. A 3-pegged 52-mm glenoid component was then cemented into position with excellent stability.
Case 5
A 64-year-old man presented to our clinic with left hip pain 40 years after THA. The original procedure was performed for resolved proximal femoral osteomyelitis. Plain radiographs showed a loose cemented McKee-Farrar hip arthroplasty (Figure 5). Because of the elevated position of the acetabular component relative to the native hip center, CT was used to determine the amount of femoral bone loss.
We used our 3-D printer to create a model and tried to recreate the native hip center with conventional off-the-shelf implants. A 50-mm hemispherical socket trial was placed in the appropriate location, along with a trabecular metal augment trial to provide extended coverage over the superolateral portion of the socket. Noted between the socket and the augment was a large gap; a substantial amount of cement would have been needed to unitize the construct. We thought a custom acetabular component would avoid the need for cement. In addition, given the patient’s small stature, the conventional acetabular component would allow a head only 32 mm in diameter. With a custom implant, the head could be enlarged to 36 mm, providing improved ROM and stability.
The patient underwent revision left hip arthroplasty using a custom acetabular component. A 3-D model available at time of surgery was used to aid implant placement.
Case 6
A 23-year-old man with multiple hereditary exostoses presented to our clinic with a painful mass in the left calf. Plain radiographs showed extensive osteochondromatosis involving the left proximal tibiofibular joint (Figure 6). The exostosis extended posteromedially, displacing the arterial trifurcation. MRI showed a small cartilage cap without evidence of malignant transformation.
CT angiogram allowed the vasculature to be modeled along with the deformity. A 3-D model was fabricated. The model included the entire proximal tibiofibular joint, as well as the anterior tibial, peroneal, and posterior tibial arteries. Cautious intralesional resection was recommended because of the proximity to all 3 vessels.
The patient underwent tumor resection through a longitudinal posterior approach. The interval between the medial and lateral heads of the gastrocnemius muscles was developed to expose the underlying soleus muscle. The soleus was split longitudinally from its hiatus to the inferior portion of the exostosis. This allowed for identification of the trifurcation and the tibial nerve, which were protected. Osteotomes were used to resect the mass at its base, the edges were carefully trimmed, and bone wax was placed over the defect. Anterior and lateral to this mass was another large mass (under the soleus muscle), which was also transected using an osteotome. The gastrocnemius and soleus muscles were then reflected off the fibula in order to remove 2 other exostoses, beneath the neck and head of the fibula.
Case 7
A 71-year-old man with a history of idiopathic lymphedema and peripheral neuropathy presented to our clinic with a left cavovarus foot deformity and a history of recurrent neuropathic foot ulcers (Figure 7). Physical examination revealed a callus over the lateral aspect of the base of the fifth metatarsal. Preoperative radiograph showed evidence of prior triple arthrodesis with a cavovarus foot deformity. CT scan was used to create a 3-D model of the foot. The model was then used to identify an appropriate location for lateral midtarsal and calcaneal closing-wedge osteotomies.
The patient underwent midfoot and hindfoot surgical correction. At surgery, the lateral closing-wedge osteotomies were performed according to the preoperative model. Radiographs 1 year after surgery showed correction of the forefoot varus.
Discussion
Three-dimensional printing for medical applications of anatomical modeling is not a new concept.1,3,4 Its use has been reported for a variety of applications in orthopedic surgery, including the printing of porous and metallic surfaces5 and bone-tissue engineering.6-9 Rapid prototyping for medical application was first reported in 1990 when a CT-based model was used to create a cranial bone.10 Reports of using the technique are becoming more widespread, particularly in the dental and maxillofacial literature, which includes reports on a variety of applications, including patient-specific drill guides, splints, and implants.11-14 The ability to perform mock surgery in advance of an actual procedure provides an invaluable opportunity to anticipate potential intraoperative problems, reduce operative time, and improve the accuracy of reconstruction.
Office-based rapid prototyping that uses an in-house 3-D printer is a novel application of this technology. It allows for creation of a patient-specific model for preoperative planning purposes. We are unaware of any other reports demonstrating the breadth and utility of office-based rapid prototyping in orthopedic surgery. For general reference, a printer similar to ours requires an initial investment of $52,000 to $56,000. This cost generally covers the printer, printer base cabinet, installation, training, and printer software (different from the 3-D modeling software), plus a 1-year warranty. A service agreement costs about $4000 annually. Printer and model supply expenses depend on the material used for the model (eg, ABS [acrylonitrile butadiene styrene]) and on the size and complexity of the 3-D models created. Average time to generate an appropriately formatted 3-D printing file is about 1 hour, though times can vary largely, according to amount of metal artifact subtraction necessary and the experience of the software user. For the rare, extremely complex deformities that require a significant amount of metal artifact subtraction, file preparation times can exceed 3 or 4 hours. We think these preparation times will decrease as communication between radiology file export format and modeling software ultimately allows for metal artifact subtraction images to function within the modeling software environment. Once an appropriately formatted file has been created, typical printing times vary according to the size of the to-be-modeled bone. For a hemipelvis, printing time is 30 to 40 hours; printing that is started on a Friday afternoon will be complete by Monday morning.
There are few reports of rapid prototyping in orthopedic surgery. In 2003, Minns and colleagues15 used a 3-D model in the planning of a tibial resection for TKA. They found the model to be accurate at time of surgery, resulting in appropriate tibial coverage by a conventional meniscal-bearing implant. Munjal and colleagues16 reported on 10 complex failed hip arthroplasty cases in which patients had revision surgery after preoperative planning using 3-D modeling techniques. The authors found that, in 8 of the 10 cases, conventional classification systems of bone loss were inaccurate in comparison with the prototype. Four cases required reconstruction with a custom triflange when conventional implants were not deemed reasonable based on the pelvic model. Tam and colleagues17 reported using a 3-D prototype as an aid in surgical planning for resection of a scapular osteochondroma in a 6-year-old patient. They found the rapid prototype to be useful at time of resection—similar to what we found with 1 patient (case 6). Adding contrast media to our patient’s scan allowed for 3-D visualization of the lesion and the encased vasculature. Fu and colleagues18 reported using a patient-specific drill template to insert anterior transpedicular screws. They constructed 24 prototypes of a formalin-preserved cervical vertebra to create a patient-specific biocompatible drill template for use in correcting multilevel cervical instability. They found the technique to be highly reproducible and accurate. Zein and colleagues19 used a rapid prototype of 3 consecutive human livers to preoperatively identify the vascular and biliary tract anatomy. They reported a high degree of accuracy—mean dimensional errors of less than 4 mm for the entire model and 1.3 mm for the vascular diameter.
The models created by implant manufacturers in this series were used to perform “mock” surgery before the actual procedures. Working with these models prompted us to buy our own 3-D printer. The learning curve can be steep, but commercially available 3-D printers allow for prompt in-office production of high-quality realistic prototypes at relatively low per-case cost (Figure 8). Three-dimensional modeling allows surgeons to assess the accuracy of their original surgical plans and, if necessary, correct them before surgery. Although computer-aided design models are useful, the ability to “perform surgery preoperatively” adds another element to surgeons’ understanding of the potential issues that may arise. Also, an in-office printer can help improve surgeons’ understanding and control over the process by which images are translated from radiographic file to 3-D model. Disadvantages of an in-office system include start-up and maintenance costs, office space requirements, and a significant learning curve for software and hardware applications. In addition, creation of 3-D models requires close interaction with radiologists who can provide appropriately formatted DICOM images, as metal artifact subtraction can be challenging. We think that, as image formatting and software capabilities are continually refined, this technology will become an invaluable part of multiple subspecialties across orthopedic surgery, with potentially infinite clinical, educational, and research applications.
1. McGurk M, Amis AA, Potamianos P, Goodger NM. Rapid prototyping techniques for anatomical modelling in medicine. Ann R Coll Surg Engl. 1997;79(3):169-174.
2. Webb PA. A review of rapid prototyping (RP) techniques in the medical and biomedical sector. J Med Eng Technol. 2000;24(4):149-153.
3. Esses SJ, Berman P, Bloom AI, Sosna J. Clinical applications of physical 3D models derived from MDCT data and created by rapid prototyping. AJR Am J Roentgenol. 2011;196(6):W683-W688.
4. Torres K, Staśkiewicz G, Śnieżyński M, Drop A, Maciejewski R. Application of rapid prototyping techniques for modelling of anatomical structures in medical training and education. Folia Morphol. 2011;70(1):1-4.
5. Melican MC, Zimmerman MC, Dhillon MS, Ponnambalam AR, Curodeau A, Parsons JR. Three-dimensional printing and porous metallic surfaces: a new orthopedic application. J Biomed Mater Res. 2001;55(2):194-202.
6. Butscher A, Bohner M, Hofmann S, Gauckler L, Müller R. Structural and material approaches to bone tissue engineering in powder-based three-dimensional printing. Acta Biomater. 2011;7(3):907-920.
7. Ciocca L, De Crescenzio F, Fantini M, Scotti R. CAD/CAM and rapid prototyped scaffold construction for bone regenerative medicine and surgical transfer of virtual planning: a pilot study. Comput Med Imaging Graph. 2009;33(1):58-62.
8. Leukers B, Gülkan H, Irsen SH, et al. Hydroxyapatite scaffolds for bone tissue engineering made by 3D printing. J Mater Sci Mater Med. 2005;16(12):1121-1124.
9. Seitz H, Rieder W, Irsen S, Leukers B, Tille C. Three-dimensional printing of porous ceramic scaffolds for bone tissue engineering. J Biomed Mater Res B Appl Biomater. 2005;74(2):782-788.
10. Mankovich NJ, Cheeseman AM, Stoker NG. The display of three-dimensional anatomy with stereolithographic models. J Digit Imaging. 1990;3(3):200-203.
11. Flügge TV, Nelson K, Schmelzeisen R, Metzger MC. Three-dimensional plotting and printing of an implant drilling guide: simplifying guided implant surgery. J Oral Maxillofac Surg. 2013;71(8):1340-1346.
12. Goiato MC, Santos MR, Pesqueira AA, Moreno A, dos Santos DM, Haddad MF. Prototyping for surgical and prosthetic treatment. J Craniofac Surg. 2011;22(3):914-917.
13. Metzger MC, Hohlweg-Majert B, Schwarz U, Teschner M, Hammer B, Schmelzeisen R. Manufacturing splints for orthognathic surgery using a three-dimensional printer. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2008;105(2):e1-e7.
14. Robiony M, Salvo I, Costa F, et al. Virtual reality surgical planning for maxillofacial distraction osteogenesis: the role of reverse engineering rapid prototyping and cooperative work. J Oral Maxillofac Surg. 2007;65(6):1198-1208.
15. Minns RJ, Bibb R, Banks R, Sutton RA. The use of a reconstructed three-dimensional solid model from CT to aid the surgical management of a total knee arthroplasty: a case study. Med Eng Phys. 2003;25(6):523-526.
16. Munjal S, Leopold SS, Kornreich D, Shott S, Finn HA. CT-generated 3-dimensional models for complex acetabular reconstruction. J Arthroplasty. 2000;15(5):644-653.
17. Tam MD, Laycock SD, Bell D, Chojnowski A. 3-D printout of a DICOM file to aid surgical planning in a 6 year old patient with a large scapular osteochondroma complicating congenital diaphyseal aclasia. J Radiol Case Rep. 2012;6(1):31-37.
18. Fu M, Lin L, Kong X, et al. Construction and accuracy assessment of patient-specific biocompatible drill template for cervical anterior transpedicular screw (ATPS) insertion: an in vitro study. PLoS One. 2013;8(1):e53580.
19. Zein NN, Hanouneh IA, Bishop PD, et al. Three-dimensional print of a liver for preoperative planning in living donor liver transplantation. Liver Transpl. 2013;19(12):1304-1310.
1. McGurk M, Amis AA, Potamianos P, Goodger NM. Rapid prototyping techniques for anatomical modelling in medicine. Ann R Coll Surg Engl. 1997;79(3):169-174.
2. Webb PA. A review of rapid prototyping (RP) techniques in the medical and biomedical sector. J Med Eng Technol. 2000;24(4):149-153.
3. Esses SJ, Berman P, Bloom AI, Sosna J. Clinical applications of physical 3D models derived from MDCT data and created by rapid prototyping. AJR Am J Roentgenol. 2011;196(6):W683-W688.
4. Torres K, Staśkiewicz G, Śnieżyński M, Drop A, Maciejewski R. Application of rapid prototyping techniques for modelling of anatomical structures in medical training and education. Folia Morphol. 2011;70(1):1-4.
5. Melican MC, Zimmerman MC, Dhillon MS, Ponnambalam AR, Curodeau A, Parsons JR. Three-dimensional printing and porous metallic surfaces: a new orthopedic application. J Biomed Mater Res. 2001;55(2):194-202.
6. Butscher A, Bohner M, Hofmann S, Gauckler L, Müller R. Structural and material approaches to bone tissue engineering in powder-based three-dimensional printing. Acta Biomater. 2011;7(3):907-920.
7. Ciocca L, De Crescenzio F, Fantini M, Scotti R. CAD/CAM and rapid prototyped scaffold construction for bone regenerative medicine and surgical transfer of virtual planning: a pilot study. Comput Med Imaging Graph. 2009;33(1):58-62.
8. Leukers B, Gülkan H, Irsen SH, et al. Hydroxyapatite scaffolds for bone tissue engineering made by 3D printing. J Mater Sci Mater Med. 2005;16(12):1121-1124.
9. Seitz H, Rieder W, Irsen S, Leukers B, Tille C. Three-dimensional printing of porous ceramic scaffolds for bone tissue engineering. J Biomed Mater Res B Appl Biomater. 2005;74(2):782-788.
10. Mankovich NJ, Cheeseman AM, Stoker NG. The display of three-dimensional anatomy with stereolithographic models. J Digit Imaging. 1990;3(3):200-203.
11. Flügge TV, Nelson K, Schmelzeisen R, Metzger MC. Three-dimensional plotting and printing of an implant drilling guide: simplifying guided implant surgery. J Oral Maxillofac Surg. 2013;71(8):1340-1346.
12. Goiato MC, Santos MR, Pesqueira AA, Moreno A, dos Santos DM, Haddad MF. Prototyping for surgical and prosthetic treatment. J Craniofac Surg. 2011;22(3):914-917.
13. Metzger MC, Hohlweg-Majert B, Schwarz U, Teschner M, Hammer B, Schmelzeisen R. Manufacturing splints for orthognathic surgery using a three-dimensional printer. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2008;105(2):e1-e7.
14. Robiony M, Salvo I, Costa F, et al. Virtual reality surgical planning for maxillofacial distraction osteogenesis: the role of reverse engineering rapid prototyping and cooperative work. J Oral Maxillofac Surg. 2007;65(6):1198-1208.
15. Minns RJ, Bibb R, Banks R, Sutton RA. The use of a reconstructed three-dimensional solid model from CT to aid the surgical management of a total knee arthroplasty: a case study. Med Eng Phys. 2003;25(6):523-526.
16. Munjal S, Leopold SS, Kornreich D, Shott S, Finn HA. CT-generated 3-dimensional models for complex acetabular reconstruction. J Arthroplasty. 2000;15(5):644-653.
17. Tam MD, Laycock SD, Bell D, Chojnowski A. 3-D printout of a DICOM file to aid surgical planning in a 6 year old patient with a large scapular osteochondroma complicating congenital diaphyseal aclasia. J Radiol Case Rep. 2012;6(1):31-37.
18. Fu M, Lin L, Kong X, et al. Construction and accuracy assessment of patient-specific biocompatible drill template for cervical anterior transpedicular screw (ATPS) insertion: an in vitro study. PLoS One. 2013;8(1):e53580.
19. Zein NN, Hanouneh IA, Bishop PD, et al. Three-dimensional print of a liver for preoperative planning in living donor liver transplantation. Liver Transpl. 2013;19(12):1304-1310.
VIDEO: Focused cardiac ultrasound aids acute heart failure patients
VIENNA – Bedside echocardiography has become a key part of quickly assessing patients with acute heart failure to decide the best management strategy.
But bedside echo images often are challenging to interpret, so physicians performing an initial work-up of an acute heart failure patient need training in a basic echocardiography examination, Dr. Nuno Cardim said in an interview during the annual meeting of the European Association of Cardiovascular Imaging.
Known as Focused Cardiac Ultrasound (FoCUS), this triage-level exam is distinct from a comprehensive echocardiography assessment. Instead, FoCUS addresses some basic, yes-or-no, present-or-absent questions regarding systolic function, hypovolemia, tamponade, pleural effusion, and pulmonary embolus.
In a position paper and then in practice recommendations issued in 2014, the European Association of Cardiovascular Imaging acknowledged that use of FoCUS in acute heart failure patients was “irreversible,” said Dr. Cardim, professor and director of echocardiography and cardiac imaging at Hospital da Luz in Lisbon.
Cardiologists and echocardiography specialists need to make sure that FoCUS training is available to all physicians who might see suspected acute heart failure patients in emergency medicine settings, Dr. Cardim said.
Dr. Cardim had no disclosures.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
On Twitter @mitchelzoler
VIENNA – Bedside echocardiography has become a key part of quickly assessing patients with acute heart failure to decide the best management strategy.
But bedside echo images often are challenging to interpret, so physicians performing an initial work-up of an acute heart failure patient need training in a basic echocardiography examination, Dr. Nuno Cardim said in an interview during the annual meeting of the European Association of Cardiovascular Imaging.
Known as Focused Cardiac Ultrasound (FoCUS), this triage-level exam is distinct from a comprehensive echocardiography assessment. Instead, FoCUS addresses some basic, yes-or-no, present-or-absent questions regarding systolic function, hypovolemia, tamponade, pleural effusion, and pulmonary embolus.
In a position paper and then in practice recommendations issued in 2014, the European Association of Cardiovascular Imaging acknowledged that use of FoCUS in acute heart failure patients was “irreversible,” said Dr. Cardim, professor and director of echocardiography and cardiac imaging at Hospital da Luz in Lisbon.
Cardiologists and echocardiography specialists need to make sure that FoCUS training is available to all physicians who might see suspected acute heart failure patients in emergency medicine settings, Dr. Cardim said.
Dr. Cardim had no disclosures.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
On Twitter @mitchelzoler
VIENNA – Bedside echocardiography has become a key part of quickly assessing patients with acute heart failure to decide the best management strategy.
But bedside echo images often are challenging to interpret, so physicians performing an initial work-up of an acute heart failure patient need training in a basic echocardiography examination, Dr. Nuno Cardim said in an interview during the annual meeting of the European Association of Cardiovascular Imaging.
Known as Focused Cardiac Ultrasound (FoCUS), this triage-level exam is distinct from a comprehensive echocardiography assessment. Instead, FoCUS addresses some basic, yes-or-no, present-or-absent questions regarding systolic function, hypovolemia, tamponade, pleural effusion, and pulmonary embolus.
In a position paper and then in practice recommendations issued in 2014, the European Association of Cardiovascular Imaging acknowledged that use of FoCUS in acute heart failure patients was “irreversible,” said Dr. Cardim, professor and director of echocardiography and cardiac imaging at Hospital da Luz in Lisbon.
Cardiologists and echocardiography specialists need to make sure that FoCUS training is available to all physicians who might see suspected acute heart failure patients in emergency medicine settings, Dr. Cardim said.
Dr. Cardim had no disclosures.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
On Twitter @mitchelzoler
AT EUROECHO-IMAGING 2014
Intra-Articular Injections of Mesenchymal Stem Cells for Knee Osteoarthritis
Knee osteoarthritis (KOA), a common disabling disease with a high impact on quality of life, has a large societal cost. Yet no procedure halts progressive degeneration of the osteoarthritic knee joint.1,2
According to Barry,3 mesenchymal stem cells (MSCs) differentiate into many different connective tissue cells, including cartilage. MSCs can be isolated from bone marrow, skeletal muscle, fat, and synovium. MSCs are multipotent cells with the capacity for self-renewal. Therefore, adult MSCs may regenerate tissues damaged by disease. In OA, the proliferative capacity and ability to differentiate are reduced in MSCs. Intra-articular injections of MSCs (MSC therapy) could repair progressively degenerated knee cartilage.
This review article summarizes the knowledge on the role of intra-articular injections of MSCs in the treatment of KOA, based on studies published in PubMed and the Cochrane Library. The article also reviews the methodology and results of the animal and clinical studies published so far on the topic.
Materials and Methods
PubMed (Medline) and the Cochrane Library were searched for literature on the role of MSC therapy in treating KOA. The key words used were stem cells and knee osteoarthritis. The period searched was from when these search engines began until January 31, 2014. One hundred thirty-five articles (including negative studies) were found, but only the 25 deeply focused on the topic were reviewed. The Figure shows the flow diagram of this study.
Results
Several experimental models of KOA have shown that MSC therapy can delay progressive degeneration of the knee joint (Appendix 1).4-15 Using a rabbit massive meniscal defect model, Hatsushika and colleagues13 found that a single intra-articular injection of synovial MSCs into the knee adhered around the meniscal defect and promoted meniscal regeneration. Park and colleagues14 conducted an experimental study in dogs—the first demonstrating regional and systemic safety and systemic immunomodulatory effects of repeated local delivery of allogeneic MSCs in vivo. Regarding the observed systemic immunomodulatory effects, clinical and pathologic examinations revealed no severe consequences of repeated MSC transplantations. Results of mixed leukocyte reactions demonstrated suppression of T-cell proliferation after MSC transplantations.
Of the human studies published so far, only 3 were prospective randomized trials (level II evidence) included in the Cochrane Library (Appendix 2).16-18 Varma and colleagues16 found that intra-articular injections of MSCs considerably improved overall KOA outcome scores. Fifty patients with mild to moderate KOA were divided into 2 groups. Group A underwent arthroscopic débridement, and group B had buffy coat (MSC concentrate) injection and arthroscopic débridement. Patients were assessed on the basis of their visual analog scale (VAS) pain scores and osteoarthritis outcome scores.
Wong and colleagues17 analyzed 56 knees in 56 patients (mean age, 51 years) with unicompartmental KOA and genu varum. Patients were randomly assigned to 2 groups, MSC and control. All patients underwent high tibial osteotomy (HTO) and microfracture. Patients in the MSC group received intra-articular injection of cultured MSCs with hyaluronic acid (HA) 3 weeks after surgery. Patients in the control group received only HA. The primary outcome measure was International Knee Documentation Committee (IKDC) score 6 months, 1 year, and 2 years after surgery. Secondary outcome measures were Tegner and Lysholm clinical scores and 1-year postoperative Magnetic Resonance Observation of Cartilage Repair Tissue (MOCART) scores. Both treatment arms achieved improvements in Tegner, Lysholm, and IKDC scores. After adjustment for age, baseline scores, and time of evaluation, the MSC group had significantly better scores. One year after surgery, magnetic resonance imaging (MRI) scans showed significantly better MOCART scores for the MSC group. Intra-articular injection of MSCs appeared to be effective in improving short-term clinical and MOCART outcomes in patients who underwent HTO and microfracture for varus knees with cartilage defects.
Saw and colleagues18 compared histologic and MRI evaluation of articular cartilage regeneration in patients with chondral lesions treated by arthroscopic subchondral drilling followed by postoperative intra-articular injections of HA with and without peripheral blood stem cells (PBSCs). Fifty patients (ages, 18-50 years) with International Cartilage Repair Society grades 3 and 4 lesions of the knee joint underwent arthroscopic subchondral drilling; 25 patients were randomized to the intervention group (HA + PBSC) and 25 to the control group (HA). Both groups received 5 weekly injections starting 1 week after surgery. Three additional injections of either HA + PBSC or HA only were given at weekly intervals 6 months after surgery. After arthroscopic subchondral drilling into grades 3 and 4 chondral lesions, postoperative intra-articular injections of autologous PBSC combined with HA resulted in improved quality of articular cartilage repair over the same treatment without PBSC.
The other human studies analyzed had a low level of evidence (grade IV, case series) but found that intra-articular injections of MSCs reduced pain and improved function in patients with KOA over the short term, 1 year (Appendix 3).19-25
Discussion
This review aimed to define the role of MSC therapy in the treatment of KOA. MSC therapy has yielded encouraging outcomes in experimental models of KOA.4-15 These experimental studies have suggested that MSCs can halt cartilage degeneration in KOA. So far, however, only 3 human studies with grade II evidence (randomized prospective trials) have been reported on the role of MSCs in KOA, but results of these studies have suggested that MSCs can reduce pain and improve function.16-18
Previous reviews of the literature1,2 have analyzed the role of MSC therapy in KOA. Barry and Murphy1 reported that several early-stage clinical trials, initiated or under way in 2013, were testing MSC delivery as an intra-articular injection into the knee, but optimal dose and vehicle were yet to be established. Filardo and colleagues2 reported that, despite growing interest in this biological approach to cartilage regeneration, knowledge on the topic is still preliminary, as shown by the prevalence of preclinical studies and the presence of low-quality clinical studies.
Study design weakness prevents effective comparison of the efficacy of MSC therapy with that of other treatments for relief of pain and other outcomes in KOA. The consistency of evidence of the clinical studies is low because of many uncontrolled variables.1-3
Conclusion
The results of MSC therapy in KOA are encouraging. However, optimal dose and vehicle are yet to be established.1 Knowledge on this topic is still preliminary. Many aspects have to be optimized, and further randomized controlled trials are needed to support the potential of this biological treatment for cartilage repair and to evaluate advantages and disadvantages with respect to the available treatments. The relative short duration of these studies is also a limitation for the technique at present.
1. Barry F, Murphy M. Mesenchymal stem cells in joint disease and repair. Nat Rev Rheumatol. 2013;9(10):584-594.
2. Filardo G, Madry H, Jelic M, Roffi A, Cucchiarini M, Kon E. Mesenchymal stem cells for the treatment of cartilage lesions: from preclinical findings to clinical application in orthopaedics. Knee Surg Sports Traumatol Arthrosc. 2013;21(8):1717-1729.
3. Barry FP. Mesenchymal stem cell therapy in joint disease. Novartis Found Symp. 2003;249:86-96.
4. Murphy JM, Fink DJ, Hunziker EB, Barry FP. Stem cell therapy in a caprine model of osteoarthritis. Arthritis Rheum. 2003;48(12):3464-3474.
5. Al Faqeh H, Norhamdan MY, Chua KH, Chen HC, Aminuddin BS, Ruszymah BH. Cell based therapy for osteoarthritis in a sheep model: gross and histological assessment. Med J Malaysia. 2008;63(suppl A):37-38.
6. Grigolo B, Lisignoli G, Desando G, et al. Osteoarthritis treated with mesenchymal stem cells on hyaluronan-based scaffold in rabbit. Tissue Eng Part C Methods. 2009;15(4):647-658.
7. Toghraie FS, Chenari N, Gholipour MA, et al. Treatment of osteoarthritis with infrapatellar fat pad derived mesenchymal stem cells in rabbit. Knee. 2011;18(2):71-75.
8. Sato M, Uchida K, Nakajima H, et al. Direct transplantation of mesenchymal stem cells into the knee joints of Hartley strain guinea pigs with spontaneous osteoarthritis. Arthritis Res Ther. 2012;14(1):R31.
9. Suhaeb AM, Naveen S, Mansor A, Kamarul T. Hyaluronic acid with or without bone marrow derived-mesenchymal stem cells improves osteoarthritic knee changes in rat model: a preliminary report. Indian J Exp Biol. 2012;50(6):383-390.
10. Al Faqeh H, Nor Hamdan BM, Chen HC, Aminuddin BS, Ruszymah BH. The potential of intra-articular injection of chondrogenic-induced bone marrow stem cells to retard the progression of osteoarthritis in a sheep model. Exp Gerontol. 2012;47(6):458-464.
11. Toghraie F, Razmkhah M, Gholipour MA, et al. Scaffold-free adipose-derived stem cells (ASCs) improve experimentally induced osteoarthritis in rabbits. Arch Iran Med. 2012;15(8):495-499.
12. ter Huurne M, Schelbergen R, Blattes R, et al. Antiinflammatory and chondroprotective effects of intraarticular injection of adipose-derived stem cells in experimental osteoarthritis. Arthritis Rheum. 2012;64(11):3604-3613.
13. Hatsushika D, Muneta T, Horie M, Koga H, Tsuji K, Sekiya I. Intraarticular injection of synovial stem cells promotes meniscal regeneration in a rabbit massive meniscal defect model. J Orthop Res. 2013;31(9):1354-1359.
14. Park SA, Reilly CM, Wood JA, et al. Safety and immunomodulatory effects of allogeneic canine adipose-derived mesenchymal stromal cells transplanted into the region of the lacrimal gland, the gland of the third eyelid and the knee joint. Cytotherapy. 2013;15(12):1498-1510.
15. Nam H, Karunanithi P, Loo WC, et al. The effects of staged intra-articular injection of cultured autologous mesenchymal stromal cells on the repair of damaged cartilage: a pilot study in caprine model. Arthritis Res Ther. 2013;15(5):R129.
16. Varma HS, Dadarya B, Vidyarthi A. The new avenues in the management of osteo-arthritis of knee—stem cells. J Indian Med Assoc. 2010;108(9):583-585.
17. Wong KL, Lee KB, Tai BC, Law P, Lee EH, Hui JH. Injectable cultured bone marrow–derived mesenchymal stem cells in varus knees with cartilage defects undergoing high tibial osteotomy: a prospective, randomized controlled clinical trial with 2 years’ follow-up. Arthroscopy. 2013;29(12):2020-2028.
18. Saw KY, Anz A, Siew-Yoke Jee C, et al. Articular cartilage regeneration with autologous peripheral blood stem cells versus hyaluronic acid: a randomized controlled trial. Arthroscopy. 2013;29(4):684-694.
19. Davatchi F, Abdollahi BS, Mohyeddin M, Shahram F, Nikbin B. Mesenchymal stem cell therapy for knee osteoarthritis. Preliminary report of four patients. Int J Rheum Dis. 2011;14(2):211-215.
20. Koh YG, Choi YJ. Infrapatellar fat pad–derived mesenchymal stem cell therapy for knee osteoarthritis. Knee. 2012;19(4):902-907.
21. Orozco L, Munar A, Soler R, et al. Treatment of knee osteoarthritis with autologous mesenchymal stem cells: a pilot study. Transplantation. 2013;95(12):1535-1541.
22. Koh YG, Jo SB, Kwon OR, et al. Mesenchymal stem cell injections improve symptoms of knee osteoarthritis. Arthroscopy. 2013;29(4):748-755.
23. Koh YG, Choi YJ, Kwon SK, Kim YS, Yeo JE. Clinical results and second-look arthroscopic findings after treatment with adipose-derived stem cells for knee osteoarthritis. Knee Surg Sports Traumatol Arthrosc. 2013 Dec 11. [Epub ahead of print].
24. Jo CH, Lee YG, Shin WH, et al. Intra-articular injection of mesenchymal stem cells for the treatment of osteoarthritis of the knee: a proof-of-concept clinical trial. Stem Cells. 2014;32(5):1254-1266.
25. Gobbi A, Karnatzikos G, Sankineani SR. One-step surgery with multipotent stem cells for the treatment of large full-thickness chondral defects of the knee. Am J Sports Med. 2014;42(3):648-657.
Knee osteoarthritis (KOA), a common disabling disease with a high impact on quality of life, has a large societal cost. Yet no procedure halts progressive degeneration of the osteoarthritic knee joint.1,2
According to Barry,3 mesenchymal stem cells (MSCs) differentiate into many different connective tissue cells, including cartilage. MSCs can be isolated from bone marrow, skeletal muscle, fat, and synovium. MSCs are multipotent cells with the capacity for self-renewal. Therefore, adult MSCs may regenerate tissues damaged by disease. In OA, the proliferative capacity and ability to differentiate are reduced in MSCs. Intra-articular injections of MSCs (MSC therapy) could repair progressively degenerated knee cartilage.
This review article summarizes the knowledge on the role of intra-articular injections of MSCs in the treatment of KOA, based on studies published in PubMed and the Cochrane Library. The article also reviews the methodology and results of the animal and clinical studies published so far on the topic.
Materials and Methods
PubMed (Medline) and the Cochrane Library were searched for literature on the role of MSC therapy in treating KOA. The key words used were stem cells and knee osteoarthritis. The period searched was from when these search engines began until January 31, 2014. One hundred thirty-five articles (including negative studies) were found, but only the 25 deeply focused on the topic were reviewed. The Figure shows the flow diagram of this study.
Results
Several experimental models of KOA have shown that MSC therapy can delay progressive degeneration of the knee joint (Appendix 1).4-15 Using a rabbit massive meniscal defect model, Hatsushika and colleagues13 found that a single intra-articular injection of synovial MSCs into the knee adhered around the meniscal defect and promoted meniscal regeneration. Park and colleagues14 conducted an experimental study in dogs—the first demonstrating regional and systemic safety and systemic immunomodulatory effects of repeated local delivery of allogeneic MSCs in vivo. Regarding the observed systemic immunomodulatory effects, clinical and pathologic examinations revealed no severe consequences of repeated MSC transplantations. Results of mixed leukocyte reactions demonstrated suppression of T-cell proliferation after MSC transplantations.
Of the human studies published so far, only 3 were prospective randomized trials (level II evidence) included in the Cochrane Library (Appendix 2).16-18 Varma and colleagues16 found that intra-articular injections of MSCs considerably improved overall KOA outcome scores. Fifty patients with mild to moderate KOA were divided into 2 groups. Group A underwent arthroscopic débridement, and group B had buffy coat (MSC concentrate) injection and arthroscopic débridement. Patients were assessed on the basis of their visual analog scale (VAS) pain scores and osteoarthritis outcome scores.
Wong and colleagues17 analyzed 56 knees in 56 patients (mean age, 51 years) with unicompartmental KOA and genu varum. Patients were randomly assigned to 2 groups, MSC and control. All patients underwent high tibial osteotomy (HTO) and microfracture. Patients in the MSC group received intra-articular injection of cultured MSCs with hyaluronic acid (HA) 3 weeks after surgery. Patients in the control group received only HA. The primary outcome measure was International Knee Documentation Committee (IKDC) score 6 months, 1 year, and 2 years after surgery. Secondary outcome measures were Tegner and Lysholm clinical scores and 1-year postoperative Magnetic Resonance Observation of Cartilage Repair Tissue (MOCART) scores. Both treatment arms achieved improvements in Tegner, Lysholm, and IKDC scores. After adjustment for age, baseline scores, and time of evaluation, the MSC group had significantly better scores. One year after surgery, magnetic resonance imaging (MRI) scans showed significantly better MOCART scores for the MSC group. Intra-articular injection of MSCs appeared to be effective in improving short-term clinical and MOCART outcomes in patients who underwent HTO and microfracture for varus knees with cartilage defects.
Saw and colleagues18 compared histologic and MRI evaluation of articular cartilage regeneration in patients with chondral lesions treated by arthroscopic subchondral drilling followed by postoperative intra-articular injections of HA with and without peripheral blood stem cells (PBSCs). Fifty patients (ages, 18-50 years) with International Cartilage Repair Society grades 3 and 4 lesions of the knee joint underwent arthroscopic subchondral drilling; 25 patients were randomized to the intervention group (HA + PBSC) and 25 to the control group (HA). Both groups received 5 weekly injections starting 1 week after surgery. Three additional injections of either HA + PBSC or HA only were given at weekly intervals 6 months after surgery. After arthroscopic subchondral drilling into grades 3 and 4 chondral lesions, postoperative intra-articular injections of autologous PBSC combined with HA resulted in improved quality of articular cartilage repair over the same treatment without PBSC.
The other human studies analyzed had a low level of evidence (grade IV, case series) but found that intra-articular injections of MSCs reduced pain and improved function in patients with KOA over the short term, 1 year (Appendix 3).19-25
Discussion
This review aimed to define the role of MSC therapy in the treatment of KOA. MSC therapy has yielded encouraging outcomes in experimental models of KOA.4-15 These experimental studies have suggested that MSCs can halt cartilage degeneration in KOA. So far, however, only 3 human studies with grade II evidence (randomized prospective trials) have been reported on the role of MSCs in KOA, but results of these studies have suggested that MSCs can reduce pain and improve function.16-18
Previous reviews of the literature1,2 have analyzed the role of MSC therapy in KOA. Barry and Murphy1 reported that several early-stage clinical trials, initiated or under way in 2013, were testing MSC delivery as an intra-articular injection into the knee, but optimal dose and vehicle were yet to be established. Filardo and colleagues2 reported that, despite growing interest in this biological approach to cartilage regeneration, knowledge on the topic is still preliminary, as shown by the prevalence of preclinical studies and the presence of low-quality clinical studies.
Study design weakness prevents effective comparison of the efficacy of MSC therapy with that of other treatments for relief of pain and other outcomes in KOA. The consistency of evidence of the clinical studies is low because of many uncontrolled variables.1-3
Conclusion
The results of MSC therapy in KOA are encouraging. However, optimal dose and vehicle are yet to be established.1 Knowledge on this topic is still preliminary. Many aspects have to be optimized, and further randomized controlled trials are needed to support the potential of this biological treatment for cartilage repair and to evaluate advantages and disadvantages with respect to the available treatments. The relative short duration of these studies is also a limitation for the technique at present.
Knee osteoarthritis (KOA), a common disabling disease with a high impact on quality of life, has a large societal cost. Yet no procedure halts progressive degeneration of the osteoarthritic knee joint.1,2
According to Barry,3 mesenchymal stem cells (MSCs) differentiate into many different connective tissue cells, including cartilage. MSCs can be isolated from bone marrow, skeletal muscle, fat, and synovium. MSCs are multipotent cells with the capacity for self-renewal. Therefore, adult MSCs may regenerate tissues damaged by disease. In OA, the proliferative capacity and ability to differentiate are reduced in MSCs. Intra-articular injections of MSCs (MSC therapy) could repair progressively degenerated knee cartilage.
This review article summarizes the knowledge on the role of intra-articular injections of MSCs in the treatment of KOA, based on studies published in PubMed and the Cochrane Library. The article also reviews the methodology and results of the animal and clinical studies published so far on the topic.
Materials and Methods
PubMed (Medline) and the Cochrane Library were searched for literature on the role of MSC therapy in treating KOA. The key words used were stem cells and knee osteoarthritis. The period searched was from when these search engines began until January 31, 2014. One hundred thirty-five articles (including negative studies) were found, but only the 25 deeply focused on the topic were reviewed. The Figure shows the flow diagram of this study.
Results
Several experimental models of KOA have shown that MSC therapy can delay progressive degeneration of the knee joint (Appendix 1).4-15 Using a rabbit massive meniscal defect model, Hatsushika and colleagues13 found that a single intra-articular injection of synovial MSCs into the knee adhered around the meniscal defect and promoted meniscal regeneration. Park and colleagues14 conducted an experimental study in dogs—the first demonstrating regional and systemic safety and systemic immunomodulatory effects of repeated local delivery of allogeneic MSCs in vivo. Regarding the observed systemic immunomodulatory effects, clinical and pathologic examinations revealed no severe consequences of repeated MSC transplantations. Results of mixed leukocyte reactions demonstrated suppression of T-cell proliferation after MSC transplantations.
Of the human studies published so far, only 3 were prospective randomized trials (level II evidence) included in the Cochrane Library (Appendix 2).16-18 Varma and colleagues16 found that intra-articular injections of MSCs considerably improved overall KOA outcome scores. Fifty patients with mild to moderate KOA were divided into 2 groups. Group A underwent arthroscopic débridement, and group B had buffy coat (MSC concentrate) injection and arthroscopic débridement. Patients were assessed on the basis of their visual analog scale (VAS) pain scores and osteoarthritis outcome scores.
Wong and colleagues17 analyzed 56 knees in 56 patients (mean age, 51 years) with unicompartmental KOA and genu varum. Patients were randomly assigned to 2 groups, MSC and control. All patients underwent high tibial osteotomy (HTO) and microfracture. Patients in the MSC group received intra-articular injection of cultured MSCs with hyaluronic acid (HA) 3 weeks after surgery. Patients in the control group received only HA. The primary outcome measure was International Knee Documentation Committee (IKDC) score 6 months, 1 year, and 2 years after surgery. Secondary outcome measures were Tegner and Lysholm clinical scores and 1-year postoperative Magnetic Resonance Observation of Cartilage Repair Tissue (MOCART) scores. Both treatment arms achieved improvements in Tegner, Lysholm, and IKDC scores. After adjustment for age, baseline scores, and time of evaluation, the MSC group had significantly better scores. One year after surgery, magnetic resonance imaging (MRI) scans showed significantly better MOCART scores for the MSC group. Intra-articular injection of MSCs appeared to be effective in improving short-term clinical and MOCART outcomes in patients who underwent HTO and microfracture for varus knees with cartilage defects.
Saw and colleagues18 compared histologic and MRI evaluation of articular cartilage regeneration in patients with chondral lesions treated by arthroscopic subchondral drilling followed by postoperative intra-articular injections of HA with and without peripheral blood stem cells (PBSCs). Fifty patients (ages, 18-50 years) with International Cartilage Repair Society grades 3 and 4 lesions of the knee joint underwent arthroscopic subchondral drilling; 25 patients were randomized to the intervention group (HA + PBSC) and 25 to the control group (HA). Both groups received 5 weekly injections starting 1 week after surgery. Three additional injections of either HA + PBSC or HA only were given at weekly intervals 6 months after surgery. After arthroscopic subchondral drilling into grades 3 and 4 chondral lesions, postoperative intra-articular injections of autologous PBSC combined with HA resulted in improved quality of articular cartilage repair over the same treatment without PBSC.
The other human studies analyzed had a low level of evidence (grade IV, case series) but found that intra-articular injections of MSCs reduced pain and improved function in patients with KOA over the short term, 1 year (Appendix 3).19-25
Discussion
This review aimed to define the role of MSC therapy in the treatment of KOA. MSC therapy has yielded encouraging outcomes in experimental models of KOA.4-15 These experimental studies have suggested that MSCs can halt cartilage degeneration in KOA. So far, however, only 3 human studies with grade II evidence (randomized prospective trials) have been reported on the role of MSCs in KOA, but results of these studies have suggested that MSCs can reduce pain and improve function.16-18
Previous reviews of the literature1,2 have analyzed the role of MSC therapy in KOA. Barry and Murphy1 reported that several early-stage clinical trials, initiated or under way in 2013, were testing MSC delivery as an intra-articular injection into the knee, but optimal dose and vehicle were yet to be established. Filardo and colleagues2 reported that, despite growing interest in this biological approach to cartilage regeneration, knowledge on the topic is still preliminary, as shown by the prevalence of preclinical studies and the presence of low-quality clinical studies.
Study design weakness prevents effective comparison of the efficacy of MSC therapy with that of other treatments for relief of pain and other outcomes in KOA. The consistency of evidence of the clinical studies is low because of many uncontrolled variables.1-3
Conclusion
The results of MSC therapy in KOA are encouraging. However, optimal dose and vehicle are yet to be established.1 Knowledge on this topic is still preliminary. Many aspects have to be optimized, and further randomized controlled trials are needed to support the potential of this biological treatment for cartilage repair and to evaluate advantages and disadvantages with respect to the available treatments. The relative short duration of these studies is also a limitation for the technique at present.
1. Barry F, Murphy M. Mesenchymal stem cells in joint disease and repair. Nat Rev Rheumatol. 2013;9(10):584-594.
2. Filardo G, Madry H, Jelic M, Roffi A, Cucchiarini M, Kon E. Mesenchymal stem cells for the treatment of cartilage lesions: from preclinical findings to clinical application in orthopaedics. Knee Surg Sports Traumatol Arthrosc. 2013;21(8):1717-1729.
3. Barry FP. Mesenchymal stem cell therapy in joint disease. Novartis Found Symp. 2003;249:86-96.
4. Murphy JM, Fink DJ, Hunziker EB, Barry FP. Stem cell therapy in a caprine model of osteoarthritis. Arthritis Rheum. 2003;48(12):3464-3474.
5. Al Faqeh H, Norhamdan MY, Chua KH, Chen HC, Aminuddin BS, Ruszymah BH. Cell based therapy for osteoarthritis in a sheep model: gross and histological assessment. Med J Malaysia. 2008;63(suppl A):37-38.
6. Grigolo B, Lisignoli G, Desando G, et al. Osteoarthritis treated with mesenchymal stem cells on hyaluronan-based scaffold in rabbit. Tissue Eng Part C Methods. 2009;15(4):647-658.
7. Toghraie FS, Chenari N, Gholipour MA, et al. Treatment of osteoarthritis with infrapatellar fat pad derived mesenchymal stem cells in rabbit. Knee. 2011;18(2):71-75.
8. Sato M, Uchida K, Nakajima H, et al. Direct transplantation of mesenchymal stem cells into the knee joints of Hartley strain guinea pigs with spontaneous osteoarthritis. Arthritis Res Ther. 2012;14(1):R31.
9. Suhaeb AM, Naveen S, Mansor A, Kamarul T. Hyaluronic acid with or without bone marrow derived-mesenchymal stem cells improves osteoarthritic knee changes in rat model: a preliminary report. Indian J Exp Biol. 2012;50(6):383-390.
10. Al Faqeh H, Nor Hamdan BM, Chen HC, Aminuddin BS, Ruszymah BH. The potential of intra-articular injection of chondrogenic-induced bone marrow stem cells to retard the progression of osteoarthritis in a sheep model. Exp Gerontol. 2012;47(6):458-464.
11. Toghraie F, Razmkhah M, Gholipour MA, et al. Scaffold-free adipose-derived stem cells (ASCs) improve experimentally induced osteoarthritis in rabbits. Arch Iran Med. 2012;15(8):495-499.
12. ter Huurne M, Schelbergen R, Blattes R, et al. Antiinflammatory and chondroprotective effects of intraarticular injection of adipose-derived stem cells in experimental osteoarthritis. Arthritis Rheum. 2012;64(11):3604-3613.
13. Hatsushika D, Muneta T, Horie M, Koga H, Tsuji K, Sekiya I. Intraarticular injection of synovial stem cells promotes meniscal regeneration in a rabbit massive meniscal defect model. J Orthop Res. 2013;31(9):1354-1359.
14. Park SA, Reilly CM, Wood JA, et al. Safety and immunomodulatory effects of allogeneic canine adipose-derived mesenchymal stromal cells transplanted into the region of the lacrimal gland, the gland of the third eyelid and the knee joint. Cytotherapy. 2013;15(12):1498-1510.
15. Nam H, Karunanithi P, Loo WC, et al. The effects of staged intra-articular injection of cultured autologous mesenchymal stromal cells on the repair of damaged cartilage: a pilot study in caprine model. Arthritis Res Ther. 2013;15(5):R129.
16. Varma HS, Dadarya B, Vidyarthi A. The new avenues in the management of osteo-arthritis of knee—stem cells. J Indian Med Assoc. 2010;108(9):583-585.
17. Wong KL, Lee KB, Tai BC, Law P, Lee EH, Hui JH. Injectable cultured bone marrow–derived mesenchymal stem cells in varus knees with cartilage defects undergoing high tibial osteotomy: a prospective, randomized controlled clinical trial with 2 years’ follow-up. Arthroscopy. 2013;29(12):2020-2028.
18. Saw KY, Anz A, Siew-Yoke Jee C, et al. Articular cartilage regeneration with autologous peripheral blood stem cells versus hyaluronic acid: a randomized controlled trial. Arthroscopy. 2013;29(4):684-694.
19. Davatchi F, Abdollahi BS, Mohyeddin M, Shahram F, Nikbin B. Mesenchymal stem cell therapy for knee osteoarthritis. Preliminary report of four patients. Int J Rheum Dis. 2011;14(2):211-215.
20. Koh YG, Choi YJ. Infrapatellar fat pad–derived mesenchymal stem cell therapy for knee osteoarthritis. Knee. 2012;19(4):902-907.
21. Orozco L, Munar A, Soler R, et al. Treatment of knee osteoarthritis with autologous mesenchymal stem cells: a pilot study. Transplantation. 2013;95(12):1535-1541.
22. Koh YG, Jo SB, Kwon OR, et al. Mesenchymal stem cell injections improve symptoms of knee osteoarthritis. Arthroscopy. 2013;29(4):748-755.
23. Koh YG, Choi YJ, Kwon SK, Kim YS, Yeo JE. Clinical results and second-look arthroscopic findings after treatment with adipose-derived stem cells for knee osteoarthritis. Knee Surg Sports Traumatol Arthrosc. 2013 Dec 11. [Epub ahead of print].
24. Jo CH, Lee YG, Shin WH, et al. Intra-articular injection of mesenchymal stem cells for the treatment of osteoarthritis of the knee: a proof-of-concept clinical trial. Stem Cells. 2014;32(5):1254-1266.
25. Gobbi A, Karnatzikos G, Sankineani SR. One-step surgery with multipotent stem cells for the treatment of large full-thickness chondral defects of the knee. Am J Sports Med. 2014;42(3):648-657.
1. Barry F, Murphy M. Mesenchymal stem cells in joint disease and repair. Nat Rev Rheumatol. 2013;9(10):584-594.
2. Filardo G, Madry H, Jelic M, Roffi A, Cucchiarini M, Kon E. Mesenchymal stem cells for the treatment of cartilage lesions: from preclinical findings to clinical application in orthopaedics. Knee Surg Sports Traumatol Arthrosc. 2013;21(8):1717-1729.
3. Barry FP. Mesenchymal stem cell therapy in joint disease. Novartis Found Symp. 2003;249:86-96.
4. Murphy JM, Fink DJ, Hunziker EB, Barry FP. Stem cell therapy in a caprine model of osteoarthritis. Arthritis Rheum. 2003;48(12):3464-3474.
5. Al Faqeh H, Norhamdan MY, Chua KH, Chen HC, Aminuddin BS, Ruszymah BH. Cell based therapy for osteoarthritis in a sheep model: gross and histological assessment. Med J Malaysia. 2008;63(suppl A):37-38.
6. Grigolo B, Lisignoli G, Desando G, et al. Osteoarthritis treated with mesenchymal stem cells on hyaluronan-based scaffold in rabbit. Tissue Eng Part C Methods. 2009;15(4):647-658.
7. Toghraie FS, Chenari N, Gholipour MA, et al. Treatment of osteoarthritis with infrapatellar fat pad derived mesenchymal stem cells in rabbit. Knee. 2011;18(2):71-75.
8. Sato M, Uchida K, Nakajima H, et al. Direct transplantation of mesenchymal stem cells into the knee joints of Hartley strain guinea pigs with spontaneous osteoarthritis. Arthritis Res Ther. 2012;14(1):R31.
9. Suhaeb AM, Naveen S, Mansor A, Kamarul T. Hyaluronic acid with or without bone marrow derived-mesenchymal stem cells improves osteoarthritic knee changes in rat model: a preliminary report. Indian J Exp Biol. 2012;50(6):383-390.
10. Al Faqeh H, Nor Hamdan BM, Chen HC, Aminuddin BS, Ruszymah BH. The potential of intra-articular injection of chondrogenic-induced bone marrow stem cells to retard the progression of osteoarthritis in a sheep model. Exp Gerontol. 2012;47(6):458-464.
11. Toghraie F, Razmkhah M, Gholipour MA, et al. Scaffold-free adipose-derived stem cells (ASCs) improve experimentally induced osteoarthritis in rabbits. Arch Iran Med. 2012;15(8):495-499.
12. ter Huurne M, Schelbergen R, Blattes R, et al. Antiinflammatory and chondroprotective effects of intraarticular injection of adipose-derived stem cells in experimental osteoarthritis. Arthritis Rheum. 2012;64(11):3604-3613.
13. Hatsushika D, Muneta T, Horie M, Koga H, Tsuji K, Sekiya I. Intraarticular injection of synovial stem cells promotes meniscal regeneration in a rabbit massive meniscal defect model. J Orthop Res. 2013;31(9):1354-1359.
14. Park SA, Reilly CM, Wood JA, et al. Safety and immunomodulatory effects of allogeneic canine adipose-derived mesenchymal stromal cells transplanted into the region of the lacrimal gland, the gland of the third eyelid and the knee joint. Cytotherapy. 2013;15(12):1498-1510.
15. Nam H, Karunanithi P, Loo WC, et al. The effects of staged intra-articular injection of cultured autologous mesenchymal stromal cells on the repair of damaged cartilage: a pilot study in caprine model. Arthritis Res Ther. 2013;15(5):R129.
16. Varma HS, Dadarya B, Vidyarthi A. The new avenues in the management of osteo-arthritis of knee—stem cells. J Indian Med Assoc. 2010;108(9):583-585.
17. Wong KL, Lee KB, Tai BC, Law P, Lee EH, Hui JH. Injectable cultured bone marrow–derived mesenchymal stem cells in varus knees with cartilage defects undergoing high tibial osteotomy: a prospective, randomized controlled clinical trial with 2 years’ follow-up. Arthroscopy. 2013;29(12):2020-2028.
18. Saw KY, Anz A, Siew-Yoke Jee C, et al. Articular cartilage regeneration with autologous peripheral blood stem cells versus hyaluronic acid: a randomized controlled trial. Arthroscopy. 2013;29(4):684-694.
19. Davatchi F, Abdollahi BS, Mohyeddin M, Shahram F, Nikbin B. Mesenchymal stem cell therapy for knee osteoarthritis. Preliminary report of four patients. Int J Rheum Dis. 2011;14(2):211-215.
20. Koh YG, Choi YJ. Infrapatellar fat pad–derived mesenchymal stem cell therapy for knee osteoarthritis. Knee. 2012;19(4):902-907.
21. Orozco L, Munar A, Soler R, et al. Treatment of knee osteoarthritis with autologous mesenchymal stem cells: a pilot study. Transplantation. 2013;95(12):1535-1541.
22. Koh YG, Jo SB, Kwon OR, et al. Mesenchymal stem cell injections improve symptoms of knee osteoarthritis. Arthroscopy. 2013;29(4):748-755.
23. Koh YG, Choi YJ, Kwon SK, Kim YS, Yeo JE. Clinical results and second-look arthroscopic findings after treatment with adipose-derived stem cells for knee osteoarthritis. Knee Surg Sports Traumatol Arthrosc. 2013 Dec 11. [Epub ahead of print].
24. Jo CH, Lee YG, Shin WH, et al. Intra-articular injection of mesenchymal stem cells for the treatment of osteoarthritis of the knee: a proof-of-concept clinical trial. Stem Cells. 2014;32(5):1254-1266.
25. Gobbi A, Karnatzikos G, Sankineani SR. One-step surgery with multipotent stem cells for the treatment of large full-thickness chondral defects of the knee. Am J Sports Med. 2014;42(3):648-657.
VIDEO: CT and 3D-echo imaging boost TAVR performance
VIENNA – Transcatheter aortic valve replacement has benefited enormously from the recent introduction of CT and three-dimensional echocardiography imaging into routine preprocedural planning and periprocedural guidance.
These two imaging methods have allowed clinicians to better select the right valve size for each patient, and 3D echo performed during transcatheter aortic valve replacement (TAVR) has also refined valve placement. The result has been TAVR procedures that go faster and have more accurate results while producing fewer complications, Dr. Rebecca Hahn said during an interview at the annual meeting of the European Association of Cardiovascular Imaging.
Perhaps the biggest impact of better valve-size selection and more detailed procedural guidance has been a reduced incidence and severity of paravalvular regurgitation once TAVR is complete and the replacement valve deployed. In recent months, Dr. Hahn and her associates at Columbia University Medical Center in New York have had no patients develop moderate or severe paravalvular regurgitation following TAVR, and the incidence of mild paravalvular leaks has dropped by about half, compared with when the procedure was first performed about 5 years ago, said Dr. Hahn, director of invasive and valvular echocardiography at Columbia.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
On Twitter@mitchelzoler
VIENNA – Transcatheter aortic valve replacement has benefited enormously from the recent introduction of CT and three-dimensional echocardiography imaging into routine preprocedural planning and periprocedural guidance.
These two imaging methods have allowed clinicians to better select the right valve size for each patient, and 3D echo performed during transcatheter aortic valve replacement (TAVR) has also refined valve placement. The result has been TAVR procedures that go faster and have more accurate results while producing fewer complications, Dr. Rebecca Hahn said during an interview at the annual meeting of the European Association of Cardiovascular Imaging.
Perhaps the biggest impact of better valve-size selection and more detailed procedural guidance has been a reduced incidence and severity of paravalvular regurgitation once TAVR is complete and the replacement valve deployed. In recent months, Dr. Hahn and her associates at Columbia University Medical Center in New York have had no patients develop moderate or severe paravalvular regurgitation following TAVR, and the incidence of mild paravalvular leaks has dropped by about half, compared with when the procedure was first performed about 5 years ago, said Dr. Hahn, director of invasive and valvular echocardiography at Columbia.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
On Twitter@mitchelzoler
VIENNA – Transcatheter aortic valve replacement has benefited enormously from the recent introduction of CT and three-dimensional echocardiography imaging into routine preprocedural planning and periprocedural guidance.
These two imaging methods have allowed clinicians to better select the right valve size for each patient, and 3D echo performed during transcatheter aortic valve replacement (TAVR) has also refined valve placement. The result has been TAVR procedures that go faster and have more accurate results while producing fewer complications, Dr. Rebecca Hahn said during an interview at the annual meeting of the European Association of Cardiovascular Imaging.
Perhaps the biggest impact of better valve-size selection and more detailed procedural guidance has been a reduced incidence and severity of paravalvular regurgitation once TAVR is complete and the replacement valve deployed. In recent months, Dr. Hahn and her associates at Columbia University Medical Center in New York have had no patients develop moderate or severe paravalvular regurgitation following TAVR, and the incidence of mild paravalvular leaks has dropped by about half, compared with when the procedure was first performed about 5 years ago, said Dr. Hahn, director of invasive and valvular echocardiography at Columbia.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
On Twitter@mitchelzoler
AT EUROECHO-IMAGING 2014
Emergency Imaging
A 48-year-old man presented to the ED via emergency medical services after experiencing two episodes of syncope following the acute onset of right lower quadrant pain. He was unresponsive at the time of presentation. His vital signs were notable for tachycardia with a heart rate of 130 beats/minute. Although normotensive at initial presentation, the patient’s blood pressure began to fall rapidly. Laboratory values revealed a decreased hemoglobin and hematocrit of 5 g/dL and 18.1%, respectively.
Following his stabilization, a computed tomography (CT) scan of the abdomen and pelvis was performed. Figures 1a and 1b represent selected noncontrast and postcontrast axial images obtained through the lower abdomen/upper pelvis.
What is the diagnosis?
Answer
The precontrast image (Figure 1c) demonstrates a large and irregular high-density collection within the right upper pelvis (white arrows) and high density free fluid along the left lateral abdominal wall (black arrows). The right psoas muscle is obscured (white asterisk, Figure 1c) when compared to the normal psoas muscle on the contralateral side (black asterisk, Figure 1c). The postcontrast image (Figure 1d) shows the same findings and also reveals an enlarged right common iliac artery (red asterisk) and contrast actively extravasating from the artery (red arrow). These findings indicate the presence of a ruptured common iliac artery aneurysm. Both the enlarged common iliac artery aneurysm (red arrow, Figure 1d) and the extravasated contrast (red asterisk, Figure 1d) were confirmed on the three-dimensional reformats of the CT scan (Figure 1e).
An isolated aneurysm of the common iliac artery is uncommon, occurring in only 1% to 2% of the population—the same frequency as aortic aneurysm.1 However, the risk of rupture of this type of aneurysm is high.2 Patients with common iliac artery aneurysm are typically asymptomatic prior to rupture. However, some patients have reportedly presented with claudication, tenesmus/constipation, sciatica, and lower extremity paresis due to nerve compression, as well as urinary obstruction caused by ureteral obstruction.1,3 Once the iliac artery aneurysm has ruptured, patients typically present with acute abdominal, groin, and/or thigh pain, although isolated testicular pain has been described in the literature.1,4
Treatment for iliac artery aneurysm includes open and endovascular repair, depending on patient presentation and the adjacent structures involved. While mortality is low for patients with a nonruptured common iliac aneurysm, acute rupture is present in approximately one out of three cases, and the surgical mortality rate is as high as 55%.3
The patient presented in this case was taken to the operating room where an angiogram of the right common iliac artery (red arrow, Figure 1f) revealed continued acute extravasation of contrast (red asterisk, Figure 1f). Surgical repair was attempted but the patient did not survive due to complications of hypotension and cardiac arrest.
2. Reber PU, Brunner K, Hakki H, Stirnemann P, Kniemeyer HW. Incidence, classification and therapy of isolated pelvic artery aneurysm. Chirurg. 2001;72(4):419-424.
3. Bacharach JM, Slovut DP. State of the art: management of iliac artery aneurysmal disease. Catheter Cardiovasc Interv. 2008;71(5):708-714.
4. Dolan RD, Zino S. A ruptured left common iliac aneurysm presenting as testicular pain in a 56-year-old man. BMJ Case Rep. 2014. doi:10.1136/bcr-2012-006568.
A 48-year-old man presented to the ED via emergency medical services after experiencing two episodes of syncope following the acute onset of right lower quadrant pain. He was unresponsive at the time of presentation. His vital signs were notable for tachycardia with a heart rate of 130 beats/minute. Although normotensive at initial presentation, the patient’s blood pressure began to fall rapidly. Laboratory values revealed a decreased hemoglobin and hematocrit of 5 g/dL and 18.1%, respectively.
Following his stabilization, a computed tomography (CT) scan of the abdomen and pelvis was performed. Figures 1a and 1b represent selected noncontrast and postcontrast axial images obtained through the lower abdomen/upper pelvis.
What is the diagnosis?
Answer
The precontrast image (Figure 1c) demonstrates a large and irregular high-density collection within the right upper pelvis (white arrows) and high density free fluid along the left lateral abdominal wall (black arrows). The right psoas muscle is obscured (white asterisk, Figure 1c) when compared to the normal psoas muscle on the contralateral side (black asterisk, Figure 1c). The postcontrast image (Figure 1d) shows the same findings and also reveals an enlarged right common iliac artery (red asterisk) and contrast actively extravasating from the artery (red arrow). These findings indicate the presence of a ruptured common iliac artery aneurysm. Both the enlarged common iliac artery aneurysm (red arrow, Figure 1d) and the extravasated contrast (red asterisk, Figure 1d) were confirmed on the three-dimensional reformats of the CT scan (Figure 1e).
An isolated aneurysm of the common iliac artery is uncommon, occurring in only 1% to 2% of the population—the same frequency as aortic aneurysm.1 However, the risk of rupture of this type of aneurysm is high.2 Patients with common iliac artery aneurysm are typically asymptomatic prior to rupture. However, some patients have reportedly presented with claudication, tenesmus/constipation, sciatica, and lower extremity paresis due to nerve compression, as well as urinary obstruction caused by ureteral obstruction.1,3 Once the iliac artery aneurysm has ruptured, patients typically present with acute abdominal, groin, and/or thigh pain, although isolated testicular pain has been described in the literature.1,4
Treatment for iliac artery aneurysm includes open and endovascular repair, depending on patient presentation and the adjacent structures involved. While mortality is low for patients with a nonruptured common iliac aneurysm, acute rupture is present in approximately one out of three cases, and the surgical mortality rate is as high as 55%.3
The patient presented in this case was taken to the operating room where an angiogram of the right common iliac artery (red arrow, Figure 1f) revealed continued acute extravasation of contrast (red asterisk, Figure 1f). Surgical repair was attempted but the patient did not survive due to complications of hypotension and cardiac arrest.
A 48-year-old man presented to the ED via emergency medical services after experiencing two episodes of syncope following the acute onset of right lower quadrant pain. He was unresponsive at the time of presentation. His vital signs were notable for tachycardia with a heart rate of 130 beats/minute. Although normotensive at initial presentation, the patient’s blood pressure began to fall rapidly. Laboratory values revealed a decreased hemoglobin and hematocrit of 5 g/dL and 18.1%, respectively.
Following his stabilization, a computed tomography (CT) scan of the abdomen and pelvis was performed. Figures 1a and 1b represent selected noncontrast and postcontrast axial images obtained through the lower abdomen/upper pelvis.
What is the diagnosis?
Answer
The precontrast image (Figure 1c) demonstrates a large and irregular high-density collection within the right upper pelvis (white arrows) and high density free fluid along the left lateral abdominal wall (black arrows). The right psoas muscle is obscured (white asterisk, Figure 1c) when compared to the normal psoas muscle on the contralateral side (black asterisk, Figure 1c). The postcontrast image (Figure 1d) shows the same findings and also reveals an enlarged right common iliac artery (red asterisk) and contrast actively extravasating from the artery (red arrow). These findings indicate the presence of a ruptured common iliac artery aneurysm. Both the enlarged common iliac artery aneurysm (red arrow, Figure 1d) and the extravasated contrast (red asterisk, Figure 1d) were confirmed on the three-dimensional reformats of the CT scan (Figure 1e).
An isolated aneurysm of the common iliac artery is uncommon, occurring in only 1% to 2% of the population—the same frequency as aortic aneurysm.1 However, the risk of rupture of this type of aneurysm is high.2 Patients with common iliac artery aneurysm are typically asymptomatic prior to rupture. However, some patients have reportedly presented with claudication, tenesmus/constipation, sciatica, and lower extremity paresis due to nerve compression, as well as urinary obstruction caused by ureteral obstruction.1,3 Once the iliac artery aneurysm has ruptured, patients typically present with acute abdominal, groin, and/or thigh pain, although isolated testicular pain has been described in the literature.1,4
Treatment for iliac artery aneurysm includes open and endovascular repair, depending on patient presentation and the adjacent structures involved. While mortality is low for patients with a nonruptured common iliac aneurysm, acute rupture is present in approximately one out of three cases, and the surgical mortality rate is as high as 55%.3
The patient presented in this case was taken to the operating room where an angiogram of the right common iliac artery (red arrow, Figure 1f) revealed continued acute extravasation of contrast (red asterisk, Figure 1f). Surgical repair was attempted but the patient did not survive due to complications of hypotension and cardiac arrest.
2. Reber PU, Brunner K, Hakki H, Stirnemann P, Kniemeyer HW. Incidence, classification and therapy of isolated pelvic artery aneurysm. Chirurg. 2001;72(4):419-424.
3. Bacharach JM, Slovut DP. State of the art: management of iliac artery aneurysmal disease. Catheter Cardiovasc Interv. 2008;71(5):708-714.
4. Dolan RD, Zino S. A ruptured left common iliac aneurysm presenting as testicular pain in a 56-year-old man. BMJ Case Rep. 2014. doi:10.1136/bcr-2012-006568.
2. Reber PU, Brunner K, Hakki H, Stirnemann P, Kniemeyer HW. Incidence, classification and therapy of isolated pelvic artery aneurysm. Chirurg. 2001;72(4):419-424.
3. Bacharach JM, Slovut DP. State of the art: management of iliac artery aneurysmal disease. Catheter Cardiovasc Interv. 2008;71(5):708-714.
4. Dolan RD, Zino S. A ruptured left common iliac aneurysm presenting as testicular pain in a 56-year-old man. BMJ Case Rep. 2014. doi:10.1136/bcr-2012-006568.
Venuous Thromboembolism in Cancer Patients
Case
A 62-year-old man with known adenocarcinoma of the lung with metastasis to the liver and bones presented to the ED complaining of nonproductive cough and progressively worsening shortness of breath for 2 weeks. He was started on crizotinib a week prior to presentation, and had also received multiple courses of oral antibiotics and steroids for presumed exacerbation of chronic obstructive pulmonary disease (COPD). In addition to COPD, the patient had a history of degenerative joint disease, and was previously a chronic smoker. His current medications included tiotropium, inhaled daily; albuterol, two inhalations as needed; and hydrocodone/acetaminophen as needed for pain.
His vital signs on physical examination were: heart rate, 98 beats/minute; blood pressure 138/89 mm Hg; respiratory rate, 20 breaths/minute; temperature, 99.3°F. Oxygen saturation was 84% on room air, which improved to 92% with 2 L of oxygen. He had mild rhonchi on chest auscultation, but normal heart sounds and normal neurological, gastrointestinal, and extremity examination. Laboratory data revealed persistent elevation of leukocytes ranging between 12 and 15 x 109/L; hemoglobin, 9.2 g/dL; and platelet count, 220 x 109/L. A basic metabolic panel was within the normal range. The chest radiograph showed no evidence of pulmonary or cardiac abnormalities.
The patient was given nebulizer therapy with albuterol, along with oral prednisone. He improved with treatment and was discharged with oral prednisone daily for 4 days and oral doxycycline daily for 7 days. A week later, however, the patient had a cardiorespiratory arrest at home and could not be revived. A postmortem examination confirmed bilateral massive pulmonary embolism (PE).
Cancer Prevalence
According to the Cancer Facts & Figures 2014 published by the American Cancer Society, approximately 13.7 million Americans with a history of cancer were alive on January 1, 2012.1 In 2014, there will be an estimated 1,665,540 new cancer diagnoses and 585,720 cancer deaths in the United States.1 Cancer remains the second most common cause of death in the United States, accounting for nearly one out of every four deaths.
As seen in the above case presentation, cancer patients are at an increased risk for developing venous thromboembolism (VTE), the symptoms of which may not always be overt. Thus, with the increasing incidence and prevalence of cancer, enhanced vision and emphasis on education of emergency physicians—especially in relation to comorbidities associated with the disease—will improve care for this unique population. (See Figure 1 for an algorithm outlining the appropriate emergency management of VTE in cancer patients.)
Venous Thromboembolism
Symptoms
Classic clinical symptoms of acute VTE may not be seen in all patients. Therefore, a high level of clinical suspicion should be made on cancer patients presenting with any clinically overt signs or symptoms that could represent acute VTE. Symptoms of DVT include pain or tenderness and swelling of one or more extremities. On physical examination, increased warmth, edema, and erythema are often found. The most common signs and symptoms of PE include dyspnea, tachypnea, and pleuritic chest pain. Tachycardia, hypoxia, cough, and syncope are not present in all cases of acute PE.2
Deep Vein Thrombosis and Pulmonary Embolism
Deep vein thrombosis and PE are manifestations of a single disease entity, namely, VTE. The association between VTE and cancer has been well established since it was first recognized by the French internist Armand Trousseau more than 150 years ago.
Deep vein thrombosis is characterized by a thrombus, usually in one of the deep venous conduits that return blood to the heart from the lower extremities, proximal to and including the popliteal veins. However, emboli can also originate from the pelvic veins, the inferior vena cava, and the upper extremities. Pulmonary embolism is characterized by a fully or partially occluding thrombus in one or more arteries of the lungs. Patients with cancer carry an increased risk of developing VTE because of tumor- and treatment-mediated hypercoagulability.6-8 Table 1 provides a summary of the risk factors associated with cancer-related VTE.
Venous thromboembolism is the second leading noncancer-related cause of death in oncology patients, with infection being the first.9-11 Mortality from an acute thrombotic event is four to eight times greater in patients with cancer than in those without cancer.12-14 Based on its high prevalence (in part due to prothrombotic effects of oncologic therapies) and high mortality rate, awareness of VTE diagnostic approaches and treatment is particularly important in the ED patient with cancer.
Epidemiology
Patients with advanced metastatic disease of the brain, lung, stomach, pancreas, kidneys, uterus, and bladder have the highest 1-year VTE incidence rate, and are at a four to 13 times higher risk of developing VTE compared to those with localized disease.15 Approximately 15% of patients with a malignancy present with VTE at some point during the course of their illness16 compared to 2.5% of the general population.17 Furthermore, an increasing frequency of VTE among cancer patients has been observed over the years due to improved survival.18 The strongest predictor for recurrent VTE is a previous diagnosis of the condition.19 The prognosis of VTE depends on the aggressiveness of the underlying malignancy and the presence of other cancer-associated complications.20
In the postoperative setting, the risk of lower extremity DVT is about twice that of noncancer patients.21 While commonly noted, there is limited evidence to support the relationship between DVT location and cancer. It is reasonable, however, to propose that localized anatomical compression by the tumor of nearby veins causes stasis and subsequent DVT. Bilateral DVT of the lower extremities may be seen with pelvic malignancies, and DVT of the upper extremities is associated with central venous catheters, axillary lymphomas, mediastinal tumor masses, and intravenous (IV) chemotherapy. Idiopathic DVT is a significant risk factor for underlying malignancy, found in approximately 10% of cases.22 In a prospective study of patients with newly diagnosed DVT, Hettiarachchi, et al23 report routine clinical evaluations are sufficient to find underlying malignancies and that extensive screening is unwarranted.
Risk Assessment
The common risk factors of VTE in the general population are older age, female gender, race, prolonged immobilization, hospitalization, previous history of VTE, obesity, heart disease (ie, cardiomyopathy, atrial fibrillation), smoking, poor performance status, familial or acquired hypercoagulability (eg, pregnancy, hormone therapy, indwelling venous catheters). The cancer population, however, is unique and has other risk factors placing patients at risk for developing a VTE. Such risk factors can be grouped into four different categories: patient-related, cancer-related, treatment-related, and biochemical-related.
Patient-Related Risk Factors. These include age, obesity, smoking, decreased mobility, history of previous VTE, comorbidities, and the presence of prothrombotic mutations.
Cancer-Related Risk Factors. Risk factors for VTE depend on the site, stage, and histological type and time from diagnosis of the cancer. Brain tumors and stomach and pancreatic cancers are associated with a high risk of VTE.9,24 Timp, et al24 also studied a strong association between the 1-year relative mortality of a cancer type and its associated thrombogenic potential. In hematologic malignancies, patients with high-grade lymphoma and acute promyelocytic leukemia are at higher risk than other forms of lymphoma or leukemia.25
Treatment-Related Risk Factors. These include anticancer drugs (chemotherapy and hormonal therapy), radiation therapy, recent major surgery, and the presence of a central venous access device (CVAD).
Patients who received chemotherapy with thalidomide, lenalidomide, cisplatin, and platinum were found to be at high risk of developing VTE.9 Hormonal therapies including tamoxifen, raloxifene, oral contraceptives, and diethylstilbestrol phosphate (used in combination with doxorubicin) also increase the risk of VTE in cancer patients.26-28
The incidence of symptomatic catheter-associated thrombosis is shown to be as high as 28.3%.29 A recent meta-analysis of 11 studies showed that peripherally inserted central catheters are associated with a higher risk of DVT than are central venous catheters.30 Mural thrombus extending from the catheter into the lumen of a vessel and leading to partial or total occlusion with or without clinical symptoms is defined as CVAD-associated thrombosis.
Biochemical-Related Risk Factors. The patient’s hemoglobin level, leukocyte, and platelet counts are associated with an increased VTE risk. Patients with a hemoglobin level <10 g/dL and a leukocyte count >11 x 109/L had an almost 2-fold increased risk of developing a VTE in a study conducted by Khorana, et al.31 The Awareness of Neutropenia in Chemotherapy Group Registry Study reported that a platelet count ≥350 x 109/L prior to chemotherapy is associated with an increased risk of VTE.32
Risk Assessment Tools
The modified Korana score is a well-validated risk-assessment model incorporating clinical parameters and laboratory biomarkers to stratify cancer patients according to their propensity to develop VTE, and represents a practical approach for clinical practice.31 The multivariate model identified the five most predictive variables: cancer site, pretreatment platelet count and leukocyte count, hemoglobin level, and body mass index (Table 2). For example, in the case presented, considering this patient’s age, primary site of the cancer, and his laboratory results, he was at a high-risk (up to 40%) for developing VTE.
Diagnosis
In patients with a high clinical suspicion of VTE and without contraindications to anticoagulation (Table 3), early initiation of anticoagulants should be considered while awaiting results from laboratory and imaging studies.5 The diagnostic accuracy for VTE improves with the following tests and scores: complete blood count, platelet count, D-dimer, Wells criteria, Modified Khorana Score, duplex ultrasonography, computed tomography angiography (CTA); and ventilation perfusion (V/Q) scan.
Complete Blood Count and Platelet Count. As previously discussed, a prechemotherapy platelet count ≥350 x 109/L, hemoglobin level <10g/dL, or prechemotherapy leukocyte count >11 x 109/L are good predictive biomarkers to consider VTE in a cancer patient.
D-Dimer. In view of the lack of specificity of D-dimer testing (most cancer patients have elevated D-dimer levels) and the high prevalence of VTE in cancer patients, D-dimer testing is less useful in this setting. The number of false positive D-dimer assays was 3-fold higher in cancer patients compared with noncancer patients.33 Hence, D-dimer testing is not recommended for the diagnosis of VTE in cancer patients.
Wells Criteria. Clinical prediction models such as the Wells criteria for VTE have proven useful in predicting the diagnosis of VTE: 62.5% of the 40 patients who were categorized as high-risk had positive studies; 16.1% of the 492 patients who were classified as moderate-risk and 10.2% of the 219 patients who were classified as low-risk had positive results.34 However, since cancer patients comprised a minority of the subjects in this study, it is unclear whether Wells criteria is as predictive for VTE in this patient population.
Computed Tomography/Computed Tomography Angiography. Computed tomography angiography is the preferred imaging technique for the initial diagnosis of PE in most patients.5 Along with accurate imaging of mediastinal and parenchymal structures and visualization of emboli in the pulmonary vasculature, CTA is also useful to detect signs of right heart enlargement, which can be used in assessing the patient’s risk for adverse clinical outcomes.36 (See Figures 2 and 3 for examples of CT and CTA, demonstrating venous thrombosis and pulmonary thromboembolism.)
Ventilation Perfusion Scan. As it is associated with less fetal radiation exposure than CTA, the V/Q scan is useful for assessing pregnant patients and those with renal insufficiency or untreatable contrast allergies in whom IV contrast is not feasible. A normal V/Q scan essentially rules out PE.38
Chest X-Ray and Electrocardiogram. Both a chest X-ray and electrocardiogram (ECG) facilitate the diagnosis of comorbidities and conditions with clinically similar presentations and can also assist in evaluating existing cardiac conditions. Findings on chest X-ray suspicious for PE include pleural effusion, localized infiltrate, Hamptons hump, and Westermark sign. In ECG, the most common finding in PE is nonspecific ST-T wave changes; a new right heart strain or an S1Q3T3 pattern is suspicious for PE.
Transthoracic Echocardiogram. More than 80% of patients with a documented PE will have some type of right heart abnormality, most commonly direct visualization of the thrombus, right ventricular dilatation, right ventricular hypokinesia with apical sparing, tricuspid regurgitation, abnormal interventricular septal motion, and pulmonary artery dilatation.39
Miniati et al40 have shown that using PE pretest probabilities of 10%, 50%, and 90%, the posttest probabilities with a positive echocardiogram (echo) were 38%, 85%, and 98%. With a negative echo, posttest probabilities were 5%, 33%, and 81%, respectively. The value of transthoracic echo in the diagnosis of PE results were taken from this prospective study in unselected patients.40 Because of the poor sensitivity, echocardiography should not be used as a routine test to screen patients for suspected PE.
Magnetic Resonance Imaging. In pregnant women and in patients with kidney disease, MRI has been used to detect PE. However, the PIOPED III study found that many of the participating centers had difficulty obtaining adequate quality MR angiography (MRA) for suspected PE. The study therefore determined that this modality should be ordered only when there is appropriate expertise in both performing and reading the images, and in patients who have contraindications to standard tests.4
Considering the high risk of developing VTE in the cancer population, there should be a low threshold for diagnostic imaging (ie, CTA and duplex ultrasonography). The patient in this case would have benefited from a CTA to rule-out PE before he was discharged.
Treatment
The National Comprehensive Cancer Network2 (NCCN), American Society of Clinical Oncology3 (ASCO), European Society for Medical Oncology4 (ESMO), International Society on Thrombosis and Haemostasis42 and American College of Chest Physicians5 all have recently published evidence-based clinical practice guidelines for the treatment of established VTE in cancer patients. Once a diagnosis of VTE has been established, immediate treatment with a parenteral anticoagulant should be initiated. For the purpose of this article we shall discuss only the initial treatment of VTE in the emergency setting. The available treatment options are low molecular weight heparin (LMWH), unfractionated heparin (UFH), fondaparinux, warfarin, apixaban and rivaroxaban, and thrombolytic agents.
Low Molecular Weight Heparin. As recommended by the ASCO, NCCN, and ESMO guidelines, the preferred initial treatment for VTE in cancer patients is LMWH. This recommendation stems from the Comparison of Low-Molecular-Weight Heparin versus Oral Anticoagulant Therapy for the Prevention of Recurrent Venous Thromboembolism in Patients with Cancer (CLOT) trial,43 which showed a decreased incidence of recurrent VTE in patients treated with a LMWH versus a coumarin derivative. Recurrent VTE occurred in 17% of patients who received warfarin versus 9% of patients who received dalteparin after 6 months. Numerous other studies evaluating LMWH versus warfarin have shown superior efficacy with LMWH for the treatment of VTE in cancer patients.
Options for LMWH currently available include enoxaparin, dalteparin, and tinzaparin. Enoxaparin is dosed at 1 mg/kg subcutaneously every 12 hours or 1.5 mg/kg subcutaneously every 24 hours. A subgroup analysis of cancer patients with PE showed a higher VTE recurrence rate in those who received once-daily versus twice-daily dosing of enoxaparin in a trial conducted by Merli and colleagues.44 Therefore, it is the authors’ recommendation to utilize the twice-daily regimen of enoxaparin for the treatment of PE in cancer patients during the acute phase of treatment.
Dalteparin is dosed at 200 IU/kg subcutaneously every 24 hours for the first month, and then 150 IU/kg subcutaneously every 24 hours thereafter. Tinzaparin is dosed at 175 IU/kg subcutaneously every 24 hours.
Since the LMWH drugs are renally eliminated, they can accumulate in patients with renal impairment. Patients with renal impairment should be considered for dose reductions if applicable, anti-Xa monitoring, and carefully observed for bleeding. Limited data with both dalteparin and tinzaparin, though, suggest less accumulation in patients with severe renal impairment compared to enoxaparin.
Unfractionated Heparin. The UFH drugs are administered via an IV bolus dose of 80 units/kg, followed by a continuous infusion of 18 units/kg/h, titrated to a goal activated partial thromboplastin time. Although LMWHs are preferred, UFH should be considered in certain settings, such as in patients with renal failure, patients with questionable absorption via the subcutaneous route, or patients who are under consideration for systemic thrombolytic therapy.5 Hospitalized patients who are at high risk for bleeding or patients who may undergo invasive procedures are good candidates for UFH over LMWHs. This is due to the fact that UFH can be promptly discontinued and is reversible with protamine sulfate (versus the partial reversibility and longer half-life of LMWH).
Fondaparinux. Fondaparinux is an indirect factor Xa (FXa) inhibitor given subcutaneously. Advantages of fondaparinux include the lack of monitoring required and the ability to use it in patients with a history of heparin-induced thrombocytopenia. Fondaparinux is contraindicated in patients with a creatinine clearance <30 mL/minute. Van Doormaal et al42 found the rate of VTE recurrence was lower with fondaparinux than UFH in the treatment of PE. In another study conducted by Akl et al,46 the rate of VTE recurrence was higher with fondaparinux than LMWH in the treatment of DVT.
Warfarin. Warfarin is very problematic in cancer patients due to the need for frequent laboratory monitoring, numerous drug-drug and drug-nutrient interactions, slow onset of action, and long duration of action. Although it has shown inferiority in cancer patients when compared to LMWH, it may still be an option for long-term treatment, especially for patients with financial barriers, adversity to injections, or renal failure. Due to the slow onset of action of warfarin (steady state is not achieved for 5-7 days), it is important to bridge with a parenteral anticoagulant until therapeutic International normalized ratio is achieved.
Apixaban and Rivaroxaban. Both of these agents are direct FXa inhibitors and can be classified as new oral anticoagulants. Although initial evidence is promising and these agents may be attractive due to feasibility of oral administration without required monitoring, they are currently not recommended for initial therapy in cancer patients. Current published trials have included very few cancer patients, thus limiting the authors’ ability to extrapolate results to the cancer population. Also, these anticoagulants lack reliable agents for reversal in cases of life-threatening bleeding complications.
Thrombolytics. These agents promote rapid clot lysis, reduce venous outflow obstruction, and prevent venous valvular dysfunction, which may help to limit long-term complications such as postthrombotic syndrome. No significant differences in reduction of recurrent PE, death, or major bleeding were found in a recent meta-analysis comparing heparin and thrombolytic agents in patients with acute PE.47 However, thrombolytic therapy is recommended for massive PE (ie, defined as PE with a systolic blood pressure <90 mm Hg).48 There is also growing evidence to support the use of thrombolytics in certain populations for submassive or moderate PE.2,5,48 Alteplase 100 mg via IV infusion over 2 hours can be given for thrombolysis in these settings. Absolute contraindications to thrombolysis include, but are not limited to severe, uncontrolled hypertension; known intracranial (IC) neoplasm or aneurysm; internal bleeding; or recent IC surgery or trauma (Table 3).
Nonpharmacological Treatment
Inferior Vena Cava Filters. In patients with contraindication to therapeutic anticoagulation, an inferior vena cava (IVC) filter can be inserted for prevention of PE.49 Insertion of an IVC filter should be assessed on a case-by-case basis after evaluation of the risk-benefit ratio. If contraindications to anticoagulant therapy are no longer present, anticoagulant therapy should be initiated.
Other treatment strategies such as catheter-directed thrombolysis and surgical thrombectomy can be considered, especially in patients presenting with severe, gangrenous limbs due to obstructive VTE. Until recently, thrombolytics were delivered systemically through an IV catheter, the main disadvantage of which was increased likelihood of bleeding and reduced efficacy of the therapy. Catheter-directed delivery of thrombolytic agents directly into the clot has allowed more localized targeting of therapy and, in combination with mechanical thrombectomy, results in a significant higher rate of complete clot dissolution than systemic anticoagulation.50
Treatment Recommendation
Per the guidelines outlined above, the preferred therapy in cancer patients with VTE is LMWH. Depending on other factors such as risk of bleeding, renal function, anticipated procedures, and financial barriers, other anticoagulant options are available but treatment should be assessed on a case-by-case basis.
Challenging Cases
Incidental VTE. Several studies have suggested that approximately half of cancer-associated VTE events are detected incidentally on routine CT scans performed for diagnosis, staging, or follow-up.51 A meta-analysis of 12 studies including more than 10,000 patients, had a weighted mean incidental PE prevalence of 3.1% (95% CI, 2.2-4.1%).52 Incidental VTE found on routine CT scans should be treated similarly to patients with symptomatic VTE, as many have subtle clinical symptoms of active disease on further evaluation.
Patients With Thrombocytopenia. The subcommittee on hemostasis and malignancy for the Scientific and Standardization Committee of the International Society on Thrombosis and haemostasis has recently recommended that anticoagulation be administered if the platelet count can be maintained above 50 x 109/L.53 For platelet counts between 20 and 50 x 109/L, half-dose LMWH can be administered with close monitoring for possible bleeding. If the platelet count is less than 20 x 109/L therapeutic doses of anticoagulation should be held. In these cases, the placement of an IVC filter is recommended to prevent PE. Once thrombocytopenia has resolved, it is recommended to initiate or resume therapeutic anticoagulation.2-4,42
Patients With Bleeding. In view of life-threatening bleeding complications, all patients should be assessed on a case-by-case basis and careful monitoring of additional bleeding risk is recommended. In patients with minor bleeding, anticoagulation may be continued as long as close follow-up is available; whereas, in patients with absolute contraindications to anticoagulation, the risk of bleeding likely outweighs the benefit of treatment, and anticoagulants should be withheld.3 The use of IVC filters are also recommended in this population—at least until the bleeding risk subsides and anticoagulation can be initiated.2-4,42
Patients With Brain Tumors. As per the national and international guidelines,3,42 a brain tumor per se is not a contraindication for anticoagulation. Anticoagulation is more effective and safer than filters when maintained in the therapeutic range in patients with brain metastases and VTE.54 Although LMWH is the preferred drug of choice in this group of patients, a decision should be made based on individual clinical assessment. Consideration should also be made for the primary tumor type since brain metastases from certain malignancies, such as melanoma, have higher tendencies to bleed.55
Patients With Renal Failure. Since LMWHs and fondaparinux are dependent on significant renal clearance, these agents should be avoided in patients with a creatinine clearance ≤30 mL/minute. Unfractionated heparin is the drug of choice in this setting and vitamin K antagonists can be started as early as on day 1.42 Fixed-dose or adjusted-dose subcutaneous UFH is also an option of outpatient management of VTE in patients with renal failure.5
Patients With Recurrent VTE on Anticoagulants. Extension of or a new DVT or PE despite being on anticoagulation therapy is defined as anticoagulation failure. Recurrent VTE is common in cancer patients, as seen in 9% of patients from the CLOT trial. Management strategies for recurrent VTE include optimizing or increasing the dose, switching agents, or placing an IVC filter. If patients are currently on once-daily enoxaparin regimens, this should be switched to the twice-daily regimen. Patients on agents other than LMWH should be switched to LMWH if possible, and dosed accurately based on the most recent total body weight and renal function. Although not useful in the emergency setting, an anti-Xa level should be drawn for patients who present with recurrent VTE on LMWH to assess whether patients are in therapeutic range. Peak anti-Xa levels should be checked 4 to 6 hours after the last dose. Lastly, dosing of LMWH could be increased by 20% to 25%, based on safety and efficacy data from a retrospective cohort study.56
Patients With Symptomatic Catheter-Related Thrombosis. For the treatment of symptomatic catheter-related thrombosis in cancer patients, the international clinical practice guidelines recommend use of anticoagulants for a minimum of 3 months.42 The CHEST guidelines suggest that the catheter not be removed if it is functional, not infected, and there is an ongoing need for the catheter.5 Little data exist in this subgroup of patients, but LMWHs are suggested.
Factors one should consider before commencing anticoagulation treatment include patient refusal, lack of therapeutic benefit, quality of life and life expectancy, bleeding risk, and whether anticoagulation is associated with an unreasonable burden.
Outpatient Treatment Criteria. In the absence of renal impairment, high risk of bleeding, or poor social circumstances, patients with DVT can be treated as an outpatient.57 Although recent studies support the safety and efficacy of outpatient PE management in noncancer patients,58,59 there is no study to support this in cancer patients. Several prognostic tools exist to aid the clinician in deciding whether cancer patients with PE can be treated as outpatients or require hospitalization. These prediction tools identify patients at a low in-hospital mortality in which outpatient management is reasonable. A few examples include the Geneva Pulmonary Embolism Prognostic Index, Pulmonary Embolism Severity Index, and the Aujesky and Murugappan prediction tools.57
The Decision Not to Treat
Acute intervention is not the only option in this unique population. Factors one should consider before commencing anticoagulation treatment include patient refusal, lack of therapeutic benefit, quality of life and life expectancy, bleeding risk, and whether anticoagulation is associated with an unreasonable burden.
Conclusion
In view of an increased risk of VTE in cancer patients, an increased level of clinical suspicion should be maintained. Risk factors related to patient, cancer type, treatment, and biochemical markers should be assessed, and the clinician should also consider using the modified Khorana score. Diagnostic imaging studies should also be performed in cases in which there is a high clinical suspicion of VTE.
Regarding treatment, the preferred drug of choice in the acute phase of treatment is LMWH—unless contraindications exist. In cancer patients with VTE who have severe renal failure, thrombocytopenia, brain tumors, or catheter related thrombosis, recommendations are based on best clinical practice, and the clinician should balance the desirable and undesirable effects depending on the bleeding risk versus VTE risk.
Dr Banala is a clinical fellow in the department of emergency medicine, University of Texas MD Anderson Cancer Center, Houston. Dr Wattana is an instructor in the department of emergency medicine, University of Texas MD Anderson Cancer Center, Houston. Ms Ma is a clinical pharmacy specialist in the division of pharmacy, University of Texas MD Anderson Cancer Center, Houston. Dr Todd is professor and chair of the department of emergency medicine, University of Texas MD Anderson Cancer Center. He is also a member of the EMERGENCY MEDICINE editorial board.
- American Cancer Society. Cancer Facts & Figures 2014. http://www.cancer.org/acs/groups/content/@research/documents/webcontent/acspc-042151.pdf. Accessed December 3, 2014. PAGES 1 AND 2
- National Comprehensive Cancer Network (NCCN) clinical practice guidelines - version1.2014, Cancer-Associated Venous Thromboembolic Disease. Available at www.nccn.org.
- Lyman GH, Khorana AA, Kuderer NM, et al; American Society of Clinical Oncology Clinical Practice. Venous thromboembolism prophylaxis and treatment in patients with cancer: American Society of Clinical Oncology clinical practice guideline update. Clin Oncol. 2013;31(17):2189-2204.
- Mandalà M, Falanga A, Roila F; ESMO Guidelines Working Group. Management of venous thromboembolism (VTE) in cancer patients: ESMO Clinical Practice Guidelines. Ann Oncol. 2011;22(Suppl 6):vi85-vi92.
- Kearon C, Akl EA, Comerota AJ, et al; American College of Chest Physicians. Antithrombotic therapy for VTE disease: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012;141(2 Suppl):e419S-e494S.
- Khorana AA. Venous thromboembolism and prognosis in cancer. Thromb Res. 2010;125(6):490-493.
- Khorana AA, Francis CW, eds. Cancer-Associated Thrombosis: New Findings in Translational Science, Prevention, and Treatment. New York, NY: Informa Healthcare; 2007: 17–34.
- Horsted F, West J, Grainge MJ. Risk of venous thromboembolism in patients with cancer: a systematic review and meta-analysis. PLoS Med. 2012;9(7):e1001275).
- Khorana AA, Connolly GC. Assessing risk of venous thromboembolism in the patient with cancer. J Clin Oncol. 2009;27(29):4839-4847.
- Khorana AA. Risk assessment and prophylaxis for vte in cancer patients. J Natl Compr Canc Netw. 2011;9(7):789-797.
- Khorana AA, Kuderer NM, Culakova E, Lyman GH, Francis CW. Development and validation of a predictive model for chemotherapy-associated thrombosis. Blood. 2008;111(10):4902-4907.
- Lee AY, Levine MN. Venous thromboembolism and cancer: risks and outcomes. Circulation. 2003;107(23 Suppl 1):I17-121.
- Carson JL, Kelley MA, Duff A, et al. The clinical course of pulmonary embolism. N Engl J Med. 1992;326(19):1240-1245.
- Sorensen HT, Mellemkjaer L, Olsen JH, Baron JA. Prognosis of cancers associated with venous thromboembolism. N Engl J Med. 2000;343(25):1846-1850.
- Chew HK, Wun T, Harvey D, Zhou H,White RH. Incidence of venous thromboembolism and its effect on survival among patients with common cancers. Arch Intern Med. 2006;166(4);458-464.
- Green KB. Silverstein RL. Hypercoagulability in cancer. Hematol Oncol Clin North Am. 1996;10(2):499-530.
- Hansson PO, Welin L, Tibblin G, Eriksson H. Deep vein thrombosis and pulmonary embolism in the general population. ‘The Study of Men Born in 1913.’ Arch Intern Med. 1997;157(15):1665-1670.
- Walker AJ, Card TR, West J, Crooks C, Grainge MJ. Incidence of venous thromboembolism in patients with cancer – a cohort study using linked United Kingdom databases. Eur J Cancer. 2013;49(6):1404-1413.
- Shea-Budgell MA, Wu CM, Easaw JC. Evidence-based guidance on venous thromboembolism in patients with solid tumours. Curr Oncol. 2014;21(3):e504-e514.
- Mandalà M, Reni M, Cascinu S, et al. Venous thromboembolism predicts poor prognosis in irresectable pancreatic cancer patients. Ann Oncol. 2007;18(10):1660-1665.
- Prandoni P. Antithrombotic strategies in patients with cancer. Thromb Haemost. 1997;78(1):141-144.
- Piccioli A, Lensing AW, Prins MH, et al; SOMIT Investigators Group. Extensive screening for occult malignant disease in idiopathic venous thromboembolism: a prospective randomized clinical trial. J Thromb Haemost. 2004;2(6):884-889.
- Hettiarachchi RJ, Lok J, Prins MH, Büller HR, Prandoni P. Undiagnosed malignancy in patients with deep vein thrombosis: incidence, risk indicators, and diagnosis. Cancer. 1998;83(1):180-185.
- Timp JF, Braekkan SK, Versteeg HH, Cannegiester SC. Epidemiology of cancer-associated venous thrombosis. Blood. 2013;122(10):1712-1723.
- Falanga A, Marchetti M. Venous thromboembolism in the hematologic malignancies. J Clin Oncol. 2009;27(29):4848-4857.
- Fisher B, Costantino JP, Wickerham DL, et al. Tamoxifen for the prevention of breast cancer: current status of the National Surgical Adjuvant Breast and Bowel Project P-1 study. J Natl Cancer Inst. 2005;97(22):1652-1662.
- Manzoli L, De Vito C, Marzuillo C, Boccia A, Villari P. Oral contraceptives and venous thromboembolism: A systematic review and meta-analysis. Drug Saf. 2012;35(3):191-205.
- Leaf AN, Propert K, Corcoran C, et al. Phase III study of combined chemohormonal therapy in metastatic prostate cancer (ECOG 3882): An Eastern Cooperative Oncology Group study. Med Oncol. 2003;20(2):137-146.
- Verso M, Agnelli G. Venous thromboembolism associated with long-term use of central venous catheters in cancer patients. J Clin Oncol. 2003;21(19):3665-3675.
- Chopra V, Anand S, Hickner A, et al. Risk of venous thromboembolism associated with peripherally inserted central catheters: a systematic review and meta-analysis. Lancet. 2013;382(9889):311-325.
- Khorana AA, Kuderer NM, Culakova E, Lyman GH, Francis CW. Development and validation of a predictive model for chemotherapy-associated thrombosis. Blood. 2008; 111(10):4902-4907.
- Khorana AA, Francis CW, Culakowa E, Lyman GH. Risk factors for chemotherapy-associated venous thromboembolism in a prospective observational study. Cancer. 2005;104(12):2822-2829.
- Sohne M, Kruip MJ, Nijkeuter M, et al; Christoper Study Group. Accuracy of clinical decision rule, D-dimer and spiral computed tomography in patients with malignancy, previous venous thromboembolism, COPD or heart failure and in older patients with suspected pulmonary embolism. J Thromb Haemost. 2006;4(5):1042-1046.
- Wells PS, Anderson DR, Rodger M, et al. Excluding pulmonary embolism at the bedside without diagnostic imaging: management of patients with suspected pulmonary embolism presenting to the emergency department by using a simple clinical model and D-dimer. Ann Intern Med. 2001;135(2):98-107.
- Zierler BK. Ultrasonography and diagnosis of venous thromboembolism. Circulation. 2004;109(12 Suppl 1):I-9-I-4.
- Schoepf UJ, Kucher N, Kipfmueller F, Quiroz R, Costello P, Goldhaber SZ. Right ventricular enlargement on chest computed tomography: a predictor of early death in acute pulmonary embolism. Circulation. 2004;110(20):3276-3280.
- Stein PD, Woodard PK, Weg JG, et al; PIOPED II investigators. Diagnostic pathways in acute pulmonary embolism: recommendations of the PIOPED II investigators. Am J Med. 2006;119(12):1048-1055.
- Anderson DR, Kahn SR, Rodger MA, et al. Computed tomographic pulmonary angiography vs ventilation-perfusion lung scanning in patients with suspected pulmonary embolism: a randomized controlled trial. JAMA. 2007;298(23):2743-2753.
- Goldhaber SZ. Pulmonary embolism. In: Braunwald E, Zipes DP, Libby P, eds. Braunwald’s Heart Disease: A Textbook of Cardiovascular Medicine. 6th ed. WB Saunders: Philadelphia, PA; 2006:1894-1895.
- Miniati M, Monti S, Pratali L, et al. Value of transthoracic echocardiography in the diagnosis of pulmonary embolism: results of a prospective study in unselected patients. Am J Med. 2001;110(7):528-535.
- Stein PD, Gottschalk A, Sostman HD, et al. Methods of Prospective Investigation of Pulmonary Embolism Diagnosis III (PIOPED III). Semin Nucl Med. 2008;38(6):462-470.
- Farge D, Debourdeau P, Beckers M, et al. International clinical practice guidelines for the treatment and prophylaxis of venous thromboembolism in patients with cancer. J Thromb Haemost. 2013;11(1):56-70.
- Lee AY, Levine MN, Baker RI, et al; Randomized Comparison of Low-Molecular-Weight Heparin versus Oral Anticoagulant Therapy for the Prevention of Recurrent Venous Thromboembolism in Patients with Cancer (CLOT) Investigators. Low-molecular-weight heparin versus a coumarin for the prevention of recurrent venous thromboembolism in patients with cancer. N Engl J Med. 2003;349(2):146-153.
- Merli G, Spiro TE, Olsson CG, et al; Enoxaparin Clinical Trial Group. Subcutaneous enoxaparin once or twice daily compared with intravenous unfractionated heparin for treatment of venous thromboembolic disease. Ann Intern Med. 2001;134(3):191-202.
- Van Doormaal FF, Raskob GE, Davidson BL, et al. Treatment of venous thromboembolism in patients with cancer: subgroup analysis of the Matisse clinical trials. Thromb Haemost. 2009;101(4):762-769.
- Akl EA, Vasireddi SR, Gunukula S, et al. Anticoagulation for the initial treatment of venous thromboembolism in patients with cancer. Cochrane Database Syst Rev. 2011;15(6)CD006649.
- Wan S, Quinlan DJ, Agnelli G, Eikelboom JW. Thrombolysis compared with heparin for the initial treatment of pulmonary embolism: a meta-analysis of the randomized controlled trials. Circulation. 2004;110(6):744-749.
- Sharifi M, Bay C, Skrocki L, Rahimi F, Mehdipour M; “MOPETT” Investigators. Moderate Pulmonary Embolism treated with thrombolysis (from the MOPETT trial). Am J Cardiol. 2013;111(2):273-277
- Docousus H; PREPIC (Prevention du Risque d’Embolie Pulmonaire par Interruption Cave) Study Group. Eight-year follow-up of patients with permanent vena cava filters in the prevention of pulmonary embolism: the PREPIC (Prevention du Risque d’Embolie Pulmonaire par Interruption Cave) randomized study. Circulation. 2005;112(3):416-422.
- Mewissen MW, Seabrook GR, Meissner MH, Cynamon J, Labropoulos N, Haughton SH. Catheter-directed thrombolysis for lower extremity deep venous thrombosis: report of a national multicenter registry. Radiology. 1999;211(1):39-49.
- Di Nisio M, Ferrante N, De Tursi M, et al. Incidental venous thromboembolism inn ambulatory cancer patients receiving chemotherapy. Thromb Haemost. 2010;104(5):1049-1054.
- Dentali F, Ageno W, Becattini C, et al. Prevalence and clinical history of incidental, asymptomatic pulmonary embolism: A meta-analysis. Thromb Res. 2010;125(6):518-522.
- Lee AY, Carrier M. Treatment of cancer-associated thrombosis: perspectives on the use of novel oral anticoagulants. Thromb Res. 2014;133(Suppl 2):S167-S171.
- Schiff D, DeAngelis LM. Therapy of venous thromboembolism in patients with brain metastases. Cancer. 1994;73(2):493-498.
- Kondziolka D, Bernstein M, Resch L, et al. Significance of hemorrhage into brain tumors: clinicopathological study. J Neurosurg. 1987;67(6):852-857.
- Carrier M, Le Gal G, Cho R, Tierney S, Rodger M, Lee AY. Dose escalation of low molecular weight heparin to manage recurrent venous thromboembolic events despite systemic anticoagulation in cancer patients. J Thromb Haemost. 2009;7(5):760-765.
- Wells PS, Forgie MA, Rodger MA. Treatment of venous thromboembolism. JAMA. 2014;311(7):717-728.
- Erkens PM, Gandara E, Wells PS, et al. Safety of outpatient treatment in acute pulmonary embolism. J Thromb Haemost. 2010;8(11):2412-2417.
- Aujesky D, Roy PM, Verschuren F, et al. Outpatient vs inpatient treatment for patients with acute pulmonary embolism: an international, open-label, randomised, non-inferiority trial. Lancet. 2011;378(9785):41-48.
Case
A 62-year-old man with known adenocarcinoma of the lung with metastasis to the liver and bones presented to the ED complaining of nonproductive cough and progressively worsening shortness of breath for 2 weeks. He was started on crizotinib a week prior to presentation, and had also received multiple courses of oral antibiotics and steroids for presumed exacerbation of chronic obstructive pulmonary disease (COPD). In addition to COPD, the patient had a history of degenerative joint disease, and was previously a chronic smoker. His current medications included tiotropium, inhaled daily; albuterol, two inhalations as needed; and hydrocodone/acetaminophen as needed for pain.
His vital signs on physical examination were: heart rate, 98 beats/minute; blood pressure 138/89 mm Hg; respiratory rate, 20 breaths/minute; temperature, 99.3°F. Oxygen saturation was 84% on room air, which improved to 92% with 2 L of oxygen. He had mild rhonchi on chest auscultation, but normal heart sounds and normal neurological, gastrointestinal, and extremity examination. Laboratory data revealed persistent elevation of leukocytes ranging between 12 and 15 x 109/L; hemoglobin, 9.2 g/dL; and platelet count, 220 x 109/L. A basic metabolic panel was within the normal range. The chest radiograph showed no evidence of pulmonary or cardiac abnormalities.
The patient was given nebulizer therapy with albuterol, along with oral prednisone. He improved with treatment and was discharged with oral prednisone daily for 4 days and oral doxycycline daily for 7 days. A week later, however, the patient had a cardiorespiratory arrest at home and could not be revived. A postmortem examination confirmed bilateral massive pulmonary embolism (PE).
Cancer Prevalence
According to the Cancer Facts & Figures 2014 published by the American Cancer Society, approximately 13.7 million Americans with a history of cancer were alive on January 1, 2012.1 In 2014, there will be an estimated 1,665,540 new cancer diagnoses and 585,720 cancer deaths in the United States.1 Cancer remains the second most common cause of death in the United States, accounting for nearly one out of every four deaths.
As seen in the above case presentation, cancer patients are at an increased risk for developing venous thromboembolism (VTE), the symptoms of which may not always be overt. Thus, with the increasing incidence and prevalence of cancer, enhanced vision and emphasis on education of emergency physicians—especially in relation to comorbidities associated with the disease—will improve care for this unique population. (See Figure 1 for an algorithm outlining the appropriate emergency management of VTE in cancer patients.)
Venous Thromboembolism
Symptoms
Classic clinical symptoms of acute VTE may not be seen in all patients. Therefore, a high level of clinical suspicion should be made on cancer patients presenting with any clinically overt signs or symptoms that could represent acute VTE. Symptoms of DVT include pain or tenderness and swelling of one or more extremities. On physical examination, increased warmth, edema, and erythema are often found. The most common signs and symptoms of PE include dyspnea, tachypnea, and pleuritic chest pain. Tachycardia, hypoxia, cough, and syncope are not present in all cases of acute PE.2
Deep Vein Thrombosis and Pulmonary Embolism
Deep vein thrombosis and PE are manifestations of a single disease entity, namely, VTE. The association between VTE and cancer has been well established since it was first recognized by the French internist Armand Trousseau more than 150 years ago.
Deep vein thrombosis is characterized by a thrombus, usually in one of the deep venous conduits that return blood to the heart from the lower extremities, proximal to and including the popliteal veins. However, emboli can also originate from the pelvic veins, the inferior vena cava, and the upper extremities. Pulmonary embolism is characterized by a fully or partially occluding thrombus in one or more arteries of the lungs. Patients with cancer carry an increased risk of developing VTE because of tumor- and treatment-mediated hypercoagulability.6-8 Table 1 provides a summary of the risk factors associated with cancer-related VTE.
Venous thromboembolism is the second leading noncancer-related cause of death in oncology patients, with infection being the first.9-11 Mortality from an acute thrombotic event is four to eight times greater in patients with cancer than in those without cancer.12-14 Based on its high prevalence (in part due to prothrombotic effects of oncologic therapies) and high mortality rate, awareness of VTE diagnostic approaches and treatment is particularly important in the ED patient with cancer.
Epidemiology
Patients with advanced metastatic disease of the brain, lung, stomach, pancreas, kidneys, uterus, and bladder have the highest 1-year VTE incidence rate, and are at a four to 13 times higher risk of developing VTE compared to those with localized disease.15 Approximately 15% of patients with a malignancy present with VTE at some point during the course of their illness16 compared to 2.5% of the general population.17 Furthermore, an increasing frequency of VTE among cancer patients has been observed over the years due to improved survival.18 The strongest predictor for recurrent VTE is a previous diagnosis of the condition.19 The prognosis of VTE depends on the aggressiveness of the underlying malignancy and the presence of other cancer-associated complications.20
In the postoperative setting, the risk of lower extremity DVT is about twice that of noncancer patients.21 While commonly noted, there is limited evidence to support the relationship between DVT location and cancer. It is reasonable, however, to propose that localized anatomical compression by the tumor of nearby veins causes stasis and subsequent DVT. Bilateral DVT of the lower extremities may be seen with pelvic malignancies, and DVT of the upper extremities is associated with central venous catheters, axillary lymphomas, mediastinal tumor masses, and intravenous (IV) chemotherapy. Idiopathic DVT is a significant risk factor for underlying malignancy, found in approximately 10% of cases.22 In a prospective study of patients with newly diagnosed DVT, Hettiarachchi, et al23 report routine clinical evaluations are sufficient to find underlying malignancies and that extensive screening is unwarranted.
Risk Assessment
The common risk factors of VTE in the general population are older age, female gender, race, prolonged immobilization, hospitalization, previous history of VTE, obesity, heart disease (ie, cardiomyopathy, atrial fibrillation), smoking, poor performance status, familial or acquired hypercoagulability (eg, pregnancy, hormone therapy, indwelling venous catheters). The cancer population, however, is unique and has other risk factors placing patients at risk for developing a VTE. Such risk factors can be grouped into four different categories: patient-related, cancer-related, treatment-related, and biochemical-related.
Patient-Related Risk Factors. These include age, obesity, smoking, decreased mobility, history of previous VTE, comorbidities, and the presence of prothrombotic mutations.
Cancer-Related Risk Factors. Risk factors for VTE depend on the site, stage, and histological type and time from diagnosis of the cancer. Brain tumors and stomach and pancreatic cancers are associated with a high risk of VTE.9,24 Timp, et al24 also studied a strong association between the 1-year relative mortality of a cancer type and its associated thrombogenic potential. In hematologic malignancies, patients with high-grade lymphoma and acute promyelocytic leukemia are at higher risk than other forms of lymphoma or leukemia.25
Treatment-Related Risk Factors. These include anticancer drugs (chemotherapy and hormonal therapy), radiation therapy, recent major surgery, and the presence of a central venous access device (CVAD).
Patients who received chemotherapy with thalidomide, lenalidomide, cisplatin, and platinum were found to be at high risk of developing VTE.9 Hormonal therapies including tamoxifen, raloxifene, oral contraceptives, and diethylstilbestrol phosphate (used in combination with doxorubicin) also increase the risk of VTE in cancer patients.26-28
The incidence of symptomatic catheter-associated thrombosis is shown to be as high as 28.3%.29 A recent meta-analysis of 11 studies showed that peripherally inserted central catheters are associated with a higher risk of DVT than are central venous catheters.30 Mural thrombus extending from the catheter into the lumen of a vessel and leading to partial or total occlusion with or without clinical symptoms is defined as CVAD-associated thrombosis.
Biochemical-Related Risk Factors. The patient’s hemoglobin level, leukocyte, and platelet counts are associated with an increased VTE risk. Patients with a hemoglobin level <10 g/dL and a leukocyte count >11 x 109/L had an almost 2-fold increased risk of developing a VTE in a study conducted by Khorana, et al.31 The Awareness of Neutropenia in Chemotherapy Group Registry Study reported that a platelet count ≥350 x 109/L prior to chemotherapy is associated with an increased risk of VTE.32
Risk Assessment Tools
The modified Korana score is a well-validated risk-assessment model incorporating clinical parameters and laboratory biomarkers to stratify cancer patients according to their propensity to develop VTE, and represents a practical approach for clinical practice.31 The multivariate model identified the five most predictive variables: cancer site, pretreatment platelet count and leukocyte count, hemoglobin level, and body mass index (Table 2). For example, in the case presented, considering this patient’s age, primary site of the cancer, and his laboratory results, he was at a high-risk (up to 40%) for developing VTE.
Diagnosis
In patients with a high clinical suspicion of VTE and without contraindications to anticoagulation (Table 3), early initiation of anticoagulants should be considered while awaiting results from laboratory and imaging studies.5 The diagnostic accuracy for VTE improves with the following tests and scores: complete blood count, platelet count, D-dimer, Wells criteria, Modified Khorana Score, duplex ultrasonography, computed tomography angiography (CTA); and ventilation perfusion (V/Q) scan.
Complete Blood Count and Platelet Count. As previously discussed, a prechemotherapy platelet count ≥350 x 109/L, hemoglobin level <10g/dL, or prechemotherapy leukocyte count >11 x 109/L are good predictive biomarkers to consider VTE in a cancer patient.
D-Dimer. In view of the lack of specificity of D-dimer testing (most cancer patients have elevated D-dimer levels) and the high prevalence of VTE in cancer patients, D-dimer testing is less useful in this setting. The number of false positive D-dimer assays was 3-fold higher in cancer patients compared with noncancer patients.33 Hence, D-dimer testing is not recommended for the diagnosis of VTE in cancer patients.
Wells Criteria. Clinical prediction models such as the Wells criteria for VTE have proven useful in predicting the diagnosis of VTE: 62.5% of the 40 patients who were categorized as high-risk had positive studies; 16.1% of the 492 patients who were classified as moderate-risk and 10.2% of the 219 patients who were classified as low-risk had positive results.34 However, since cancer patients comprised a minority of the subjects in this study, it is unclear whether Wells criteria is as predictive for VTE in this patient population.
Computed Tomography/Computed Tomography Angiography. Computed tomography angiography is the preferred imaging technique for the initial diagnosis of PE in most patients.5 Along with accurate imaging of mediastinal and parenchymal structures and visualization of emboli in the pulmonary vasculature, CTA is also useful to detect signs of right heart enlargement, which can be used in assessing the patient’s risk for adverse clinical outcomes.36 (See Figures 2 and 3 for examples of CT and CTA, demonstrating venous thrombosis and pulmonary thromboembolism.)
Ventilation Perfusion Scan. As it is associated with less fetal radiation exposure than CTA, the V/Q scan is useful for assessing pregnant patients and those with renal insufficiency or untreatable contrast allergies in whom IV contrast is not feasible. A normal V/Q scan essentially rules out PE.38
Chest X-Ray and Electrocardiogram. Both a chest X-ray and electrocardiogram (ECG) facilitate the diagnosis of comorbidities and conditions with clinically similar presentations and can also assist in evaluating existing cardiac conditions. Findings on chest X-ray suspicious for PE include pleural effusion, localized infiltrate, Hamptons hump, and Westermark sign. In ECG, the most common finding in PE is nonspecific ST-T wave changes; a new right heart strain or an S1Q3T3 pattern is suspicious for PE.
Transthoracic Echocardiogram. More than 80% of patients with a documented PE will have some type of right heart abnormality, most commonly direct visualization of the thrombus, right ventricular dilatation, right ventricular hypokinesia with apical sparing, tricuspid regurgitation, abnormal interventricular septal motion, and pulmonary artery dilatation.39
Miniati et al40 have shown that using PE pretest probabilities of 10%, 50%, and 90%, the posttest probabilities with a positive echocardiogram (echo) were 38%, 85%, and 98%. With a negative echo, posttest probabilities were 5%, 33%, and 81%, respectively. The value of transthoracic echo in the diagnosis of PE results were taken from this prospective study in unselected patients.40 Because of the poor sensitivity, echocardiography should not be used as a routine test to screen patients for suspected PE.
Magnetic Resonance Imaging. In pregnant women and in patients with kidney disease, MRI has been used to detect PE. However, the PIOPED III study found that many of the participating centers had difficulty obtaining adequate quality MR angiography (MRA) for suspected PE. The study therefore determined that this modality should be ordered only when there is appropriate expertise in both performing and reading the images, and in patients who have contraindications to standard tests.4
Considering the high risk of developing VTE in the cancer population, there should be a low threshold for diagnostic imaging (ie, CTA and duplex ultrasonography). The patient in this case would have benefited from a CTA to rule-out PE before he was discharged.
Treatment
The National Comprehensive Cancer Network2 (NCCN), American Society of Clinical Oncology3 (ASCO), European Society for Medical Oncology4 (ESMO), International Society on Thrombosis and Haemostasis42 and American College of Chest Physicians5 all have recently published evidence-based clinical practice guidelines for the treatment of established VTE in cancer patients. Once a diagnosis of VTE has been established, immediate treatment with a parenteral anticoagulant should be initiated. For the purpose of this article we shall discuss only the initial treatment of VTE in the emergency setting. The available treatment options are low molecular weight heparin (LMWH), unfractionated heparin (UFH), fondaparinux, warfarin, apixaban and rivaroxaban, and thrombolytic agents.
Low Molecular Weight Heparin. As recommended by the ASCO, NCCN, and ESMO guidelines, the preferred initial treatment for VTE in cancer patients is LMWH. This recommendation stems from the Comparison of Low-Molecular-Weight Heparin versus Oral Anticoagulant Therapy for the Prevention of Recurrent Venous Thromboembolism in Patients with Cancer (CLOT) trial,43 which showed a decreased incidence of recurrent VTE in patients treated with a LMWH versus a coumarin derivative. Recurrent VTE occurred in 17% of patients who received warfarin versus 9% of patients who received dalteparin after 6 months. Numerous other studies evaluating LMWH versus warfarin have shown superior efficacy with LMWH for the treatment of VTE in cancer patients.
Options for LMWH currently available include enoxaparin, dalteparin, and tinzaparin. Enoxaparin is dosed at 1 mg/kg subcutaneously every 12 hours or 1.5 mg/kg subcutaneously every 24 hours. A subgroup analysis of cancer patients with PE showed a higher VTE recurrence rate in those who received once-daily versus twice-daily dosing of enoxaparin in a trial conducted by Merli and colleagues.44 Therefore, it is the authors’ recommendation to utilize the twice-daily regimen of enoxaparin for the treatment of PE in cancer patients during the acute phase of treatment.
Dalteparin is dosed at 200 IU/kg subcutaneously every 24 hours for the first month, and then 150 IU/kg subcutaneously every 24 hours thereafter. Tinzaparin is dosed at 175 IU/kg subcutaneously every 24 hours.
Since the LMWH drugs are renally eliminated, they can accumulate in patients with renal impairment. Patients with renal impairment should be considered for dose reductions if applicable, anti-Xa monitoring, and carefully observed for bleeding. Limited data with both dalteparin and tinzaparin, though, suggest less accumulation in patients with severe renal impairment compared to enoxaparin.
Unfractionated Heparin. The UFH drugs are administered via an IV bolus dose of 80 units/kg, followed by a continuous infusion of 18 units/kg/h, titrated to a goal activated partial thromboplastin time. Although LMWHs are preferred, UFH should be considered in certain settings, such as in patients with renal failure, patients with questionable absorption via the subcutaneous route, or patients who are under consideration for systemic thrombolytic therapy.5 Hospitalized patients who are at high risk for bleeding or patients who may undergo invasive procedures are good candidates for UFH over LMWHs. This is due to the fact that UFH can be promptly discontinued and is reversible with protamine sulfate (versus the partial reversibility and longer half-life of LMWH).
Fondaparinux. Fondaparinux is an indirect factor Xa (FXa) inhibitor given subcutaneously. Advantages of fondaparinux include the lack of monitoring required and the ability to use it in patients with a history of heparin-induced thrombocytopenia. Fondaparinux is contraindicated in patients with a creatinine clearance <30 mL/minute. Van Doormaal et al42 found the rate of VTE recurrence was lower with fondaparinux than UFH in the treatment of PE. In another study conducted by Akl et al,46 the rate of VTE recurrence was higher with fondaparinux than LMWH in the treatment of DVT.
Warfarin. Warfarin is very problematic in cancer patients due to the need for frequent laboratory monitoring, numerous drug-drug and drug-nutrient interactions, slow onset of action, and long duration of action. Although it has shown inferiority in cancer patients when compared to LMWH, it may still be an option for long-term treatment, especially for patients with financial barriers, adversity to injections, or renal failure. Due to the slow onset of action of warfarin (steady state is not achieved for 5-7 days), it is important to bridge with a parenteral anticoagulant until therapeutic International normalized ratio is achieved.
Apixaban and Rivaroxaban. Both of these agents are direct FXa inhibitors and can be classified as new oral anticoagulants. Although initial evidence is promising and these agents may be attractive due to feasibility of oral administration without required monitoring, they are currently not recommended for initial therapy in cancer patients. Current published trials have included very few cancer patients, thus limiting the authors’ ability to extrapolate results to the cancer population. Also, these anticoagulants lack reliable agents for reversal in cases of life-threatening bleeding complications.
Thrombolytics. These agents promote rapid clot lysis, reduce venous outflow obstruction, and prevent venous valvular dysfunction, which may help to limit long-term complications such as postthrombotic syndrome. No significant differences in reduction of recurrent PE, death, or major bleeding were found in a recent meta-analysis comparing heparin and thrombolytic agents in patients with acute PE.47 However, thrombolytic therapy is recommended for massive PE (ie, defined as PE with a systolic blood pressure <90 mm Hg).48 There is also growing evidence to support the use of thrombolytics in certain populations for submassive or moderate PE.2,5,48 Alteplase 100 mg via IV infusion over 2 hours can be given for thrombolysis in these settings. Absolute contraindications to thrombolysis include, but are not limited to severe, uncontrolled hypertension; known intracranial (IC) neoplasm or aneurysm; internal bleeding; or recent IC surgery or trauma (Table 3).
Nonpharmacological Treatment
Inferior Vena Cava Filters. In patients with contraindication to therapeutic anticoagulation, an inferior vena cava (IVC) filter can be inserted for prevention of PE.49 Insertion of an IVC filter should be assessed on a case-by-case basis after evaluation of the risk-benefit ratio. If contraindications to anticoagulant therapy are no longer present, anticoagulant therapy should be initiated.
Other treatment strategies such as catheter-directed thrombolysis and surgical thrombectomy can be considered, especially in patients presenting with severe, gangrenous limbs due to obstructive VTE. Until recently, thrombolytics were delivered systemically through an IV catheter, the main disadvantage of which was increased likelihood of bleeding and reduced efficacy of the therapy. Catheter-directed delivery of thrombolytic agents directly into the clot has allowed more localized targeting of therapy and, in combination with mechanical thrombectomy, results in a significant higher rate of complete clot dissolution than systemic anticoagulation.50
Treatment Recommendation
Per the guidelines outlined above, the preferred therapy in cancer patients with VTE is LMWH. Depending on other factors such as risk of bleeding, renal function, anticipated procedures, and financial barriers, other anticoagulant options are available but treatment should be assessed on a case-by-case basis.
Challenging Cases
Incidental VTE. Several studies have suggested that approximately half of cancer-associated VTE events are detected incidentally on routine CT scans performed for diagnosis, staging, or follow-up.51 A meta-analysis of 12 studies including more than 10,000 patients, had a weighted mean incidental PE prevalence of 3.1% (95% CI, 2.2-4.1%).52 Incidental VTE found on routine CT scans should be treated similarly to patients with symptomatic VTE, as many have subtle clinical symptoms of active disease on further evaluation.
Patients With Thrombocytopenia. The subcommittee on hemostasis and malignancy for the Scientific and Standardization Committee of the International Society on Thrombosis and haemostasis has recently recommended that anticoagulation be administered if the platelet count can be maintained above 50 x 109/L.53 For platelet counts between 20 and 50 x 109/L, half-dose LMWH can be administered with close monitoring for possible bleeding. If the platelet count is less than 20 x 109/L therapeutic doses of anticoagulation should be held. In these cases, the placement of an IVC filter is recommended to prevent PE. Once thrombocytopenia has resolved, it is recommended to initiate or resume therapeutic anticoagulation.2-4,42
Patients With Bleeding. In view of life-threatening bleeding complications, all patients should be assessed on a case-by-case basis and careful monitoring of additional bleeding risk is recommended. In patients with minor bleeding, anticoagulation may be continued as long as close follow-up is available; whereas, in patients with absolute contraindications to anticoagulation, the risk of bleeding likely outweighs the benefit of treatment, and anticoagulants should be withheld.3 The use of IVC filters are also recommended in this population—at least until the bleeding risk subsides and anticoagulation can be initiated.2-4,42
Patients With Brain Tumors. As per the national and international guidelines,3,42 a brain tumor per se is not a contraindication for anticoagulation. Anticoagulation is more effective and safer than filters when maintained in the therapeutic range in patients with brain metastases and VTE.54 Although LMWH is the preferred drug of choice in this group of patients, a decision should be made based on individual clinical assessment. Consideration should also be made for the primary tumor type since brain metastases from certain malignancies, such as melanoma, have higher tendencies to bleed.55
Patients With Renal Failure. Since LMWHs and fondaparinux are dependent on significant renal clearance, these agents should be avoided in patients with a creatinine clearance ≤30 mL/minute. Unfractionated heparin is the drug of choice in this setting and vitamin K antagonists can be started as early as on day 1.42 Fixed-dose or adjusted-dose subcutaneous UFH is also an option of outpatient management of VTE in patients with renal failure.5
Patients With Recurrent VTE on Anticoagulants. Extension of or a new DVT or PE despite being on anticoagulation therapy is defined as anticoagulation failure. Recurrent VTE is common in cancer patients, as seen in 9% of patients from the CLOT trial. Management strategies for recurrent VTE include optimizing or increasing the dose, switching agents, or placing an IVC filter. If patients are currently on once-daily enoxaparin regimens, this should be switched to the twice-daily regimen. Patients on agents other than LMWH should be switched to LMWH if possible, and dosed accurately based on the most recent total body weight and renal function. Although not useful in the emergency setting, an anti-Xa level should be drawn for patients who present with recurrent VTE on LMWH to assess whether patients are in therapeutic range. Peak anti-Xa levels should be checked 4 to 6 hours after the last dose. Lastly, dosing of LMWH could be increased by 20% to 25%, based on safety and efficacy data from a retrospective cohort study.56
Patients With Symptomatic Catheter-Related Thrombosis. For the treatment of symptomatic catheter-related thrombosis in cancer patients, the international clinical practice guidelines recommend use of anticoagulants for a minimum of 3 months.42 The CHEST guidelines suggest that the catheter not be removed if it is functional, not infected, and there is an ongoing need for the catheter.5 Little data exist in this subgroup of patients, but LMWHs are suggested.
Factors one should consider before commencing anticoagulation treatment include patient refusal, lack of therapeutic benefit, quality of life and life expectancy, bleeding risk, and whether anticoagulation is associated with an unreasonable burden.
Outpatient Treatment Criteria. In the absence of renal impairment, high risk of bleeding, or poor social circumstances, patients with DVT can be treated as an outpatient.57 Although recent studies support the safety and efficacy of outpatient PE management in noncancer patients,58,59 there is no study to support this in cancer patients. Several prognostic tools exist to aid the clinician in deciding whether cancer patients with PE can be treated as outpatients or require hospitalization. These prediction tools identify patients at a low in-hospital mortality in which outpatient management is reasonable. A few examples include the Geneva Pulmonary Embolism Prognostic Index, Pulmonary Embolism Severity Index, and the Aujesky and Murugappan prediction tools.57
The Decision Not to Treat
Acute intervention is not the only option in this unique population. Factors one should consider before commencing anticoagulation treatment include patient refusal, lack of therapeutic benefit, quality of life and life expectancy, bleeding risk, and whether anticoagulation is associated with an unreasonable burden.
Conclusion
In view of an increased risk of VTE in cancer patients, an increased level of clinical suspicion should be maintained. Risk factors related to patient, cancer type, treatment, and biochemical markers should be assessed, and the clinician should also consider using the modified Khorana score. Diagnostic imaging studies should also be performed in cases in which there is a high clinical suspicion of VTE.
Regarding treatment, the preferred drug of choice in the acute phase of treatment is LMWH—unless contraindications exist. In cancer patients with VTE who have severe renal failure, thrombocytopenia, brain tumors, or catheter related thrombosis, recommendations are based on best clinical practice, and the clinician should balance the desirable and undesirable effects depending on the bleeding risk versus VTE risk.
Dr Banala is a clinical fellow in the department of emergency medicine, University of Texas MD Anderson Cancer Center, Houston. Dr Wattana is an instructor in the department of emergency medicine, University of Texas MD Anderson Cancer Center, Houston. Ms Ma is a clinical pharmacy specialist in the division of pharmacy, University of Texas MD Anderson Cancer Center, Houston. Dr Todd is professor and chair of the department of emergency medicine, University of Texas MD Anderson Cancer Center. He is also a member of the EMERGENCY MEDICINE editorial board.
Case
A 62-year-old man with known adenocarcinoma of the lung with metastasis to the liver and bones presented to the ED complaining of nonproductive cough and progressively worsening shortness of breath for 2 weeks. He was started on crizotinib a week prior to presentation, and had also received multiple courses of oral antibiotics and steroids for presumed exacerbation of chronic obstructive pulmonary disease (COPD). In addition to COPD, the patient had a history of degenerative joint disease, and was previously a chronic smoker. His current medications included tiotropium, inhaled daily; albuterol, two inhalations as needed; and hydrocodone/acetaminophen as needed for pain.
His vital signs on physical examination were: heart rate, 98 beats/minute; blood pressure 138/89 mm Hg; respiratory rate, 20 breaths/minute; temperature, 99.3°F. Oxygen saturation was 84% on room air, which improved to 92% with 2 L of oxygen. He had mild rhonchi on chest auscultation, but normal heart sounds and normal neurological, gastrointestinal, and extremity examination. Laboratory data revealed persistent elevation of leukocytes ranging between 12 and 15 x 109/L; hemoglobin, 9.2 g/dL; and platelet count, 220 x 109/L. A basic metabolic panel was within the normal range. The chest radiograph showed no evidence of pulmonary or cardiac abnormalities.
The patient was given nebulizer therapy with albuterol, along with oral prednisone. He improved with treatment and was discharged with oral prednisone daily for 4 days and oral doxycycline daily for 7 days. A week later, however, the patient had a cardiorespiratory arrest at home and could not be revived. A postmortem examination confirmed bilateral massive pulmonary embolism (PE).
Cancer Prevalence
According to the Cancer Facts & Figures 2014 published by the American Cancer Society, approximately 13.7 million Americans with a history of cancer were alive on January 1, 2012.1 In 2014, there will be an estimated 1,665,540 new cancer diagnoses and 585,720 cancer deaths in the United States.1 Cancer remains the second most common cause of death in the United States, accounting for nearly one out of every four deaths.
As seen in the above case presentation, cancer patients are at an increased risk for developing venous thromboembolism (VTE), the symptoms of which may not always be overt. Thus, with the increasing incidence and prevalence of cancer, enhanced vision and emphasis on education of emergency physicians—especially in relation to comorbidities associated with the disease—will improve care for this unique population. (See Figure 1 for an algorithm outlining the appropriate emergency management of VTE in cancer patients.)
Venous Thromboembolism
Symptoms
Classic clinical symptoms of acute VTE may not be seen in all patients. Therefore, a high level of clinical suspicion should be made on cancer patients presenting with any clinically overt signs or symptoms that could represent acute VTE. Symptoms of DVT include pain or tenderness and swelling of one or more extremities. On physical examination, increased warmth, edema, and erythema are often found. The most common signs and symptoms of PE include dyspnea, tachypnea, and pleuritic chest pain. Tachycardia, hypoxia, cough, and syncope are not present in all cases of acute PE.2
Deep Vein Thrombosis and Pulmonary Embolism
Deep vein thrombosis and PE are manifestations of a single disease entity, namely, VTE. The association between VTE and cancer has been well established since it was first recognized by the French internist Armand Trousseau more than 150 years ago.
Deep vein thrombosis is characterized by a thrombus, usually in one of the deep venous conduits that return blood to the heart from the lower extremities, proximal to and including the popliteal veins. However, emboli can also originate from the pelvic veins, the inferior vena cava, and the upper extremities. Pulmonary embolism is characterized by a fully or partially occluding thrombus in one or more arteries of the lungs. Patients with cancer carry an increased risk of developing VTE because of tumor- and treatment-mediated hypercoagulability.6-8 Table 1 provides a summary of the risk factors associated with cancer-related VTE.
Venous thromboembolism is the second leading noncancer-related cause of death in oncology patients, with infection being the first.9-11 Mortality from an acute thrombotic event is four to eight times greater in patients with cancer than in those without cancer.12-14 Based on its high prevalence (in part due to prothrombotic effects of oncologic therapies) and high mortality rate, awareness of VTE diagnostic approaches and treatment is particularly important in the ED patient with cancer.
Epidemiology
Patients with advanced metastatic disease of the brain, lung, stomach, pancreas, kidneys, uterus, and bladder have the highest 1-year VTE incidence rate, and are at a four to 13 times higher risk of developing VTE compared to those with localized disease.15 Approximately 15% of patients with a malignancy present with VTE at some point during the course of their illness16 compared to 2.5% of the general population.17 Furthermore, an increasing frequency of VTE among cancer patients has been observed over the years due to improved survival.18 The strongest predictor for recurrent VTE is a previous diagnosis of the condition.19 The prognosis of VTE depends on the aggressiveness of the underlying malignancy and the presence of other cancer-associated complications.20
In the postoperative setting, the risk of lower extremity DVT is about twice that of noncancer patients.21 While commonly noted, there is limited evidence to support the relationship between DVT location and cancer. It is reasonable, however, to propose that localized anatomical compression by the tumor of nearby veins causes stasis and subsequent DVT. Bilateral DVT of the lower extremities may be seen with pelvic malignancies, and DVT of the upper extremities is associated with central venous catheters, axillary lymphomas, mediastinal tumor masses, and intravenous (IV) chemotherapy. Idiopathic DVT is a significant risk factor for underlying malignancy, found in approximately 10% of cases.22 In a prospective study of patients with newly diagnosed DVT, Hettiarachchi, et al23 report routine clinical evaluations are sufficient to find underlying malignancies and that extensive screening is unwarranted.
Risk Assessment
The common risk factors of VTE in the general population are older age, female gender, race, prolonged immobilization, hospitalization, previous history of VTE, obesity, heart disease (ie, cardiomyopathy, atrial fibrillation), smoking, poor performance status, familial or acquired hypercoagulability (eg, pregnancy, hormone therapy, indwelling venous catheters). The cancer population, however, is unique and has other risk factors placing patients at risk for developing a VTE. Such risk factors can be grouped into four different categories: patient-related, cancer-related, treatment-related, and biochemical-related.
Patient-Related Risk Factors. These include age, obesity, smoking, decreased mobility, history of previous VTE, comorbidities, and the presence of prothrombotic mutations.
Cancer-Related Risk Factors. Risk factors for VTE depend on the site, stage, and histological type and time from diagnosis of the cancer. Brain tumors and stomach and pancreatic cancers are associated with a high risk of VTE.9,24 Timp, et al24 also studied a strong association between the 1-year relative mortality of a cancer type and its associated thrombogenic potential. In hematologic malignancies, patients with high-grade lymphoma and acute promyelocytic leukemia are at higher risk than other forms of lymphoma or leukemia.25
Treatment-Related Risk Factors. These include anticancer drugs (chemotherapy and hormonal therapy), radiation therapy, recent major surgery, and the presence of a central venous access device (CVAD).
Patients who received chemotherapy with thalidomide, lenalidomide, cisplatin, and platinum were found to be at high risk of developing VTE.9 Hormonal therapies including tamoxifen, raloxifene, oral contraceptives, and diethylstilbestrol phosphate (used in combination with doxorubicin) also increase the risk of VTE in cancer patients.26-28
The incidence of symptomatic catheter-associated thrombosis is shown to be as high as 28.3%.29 A recent meta-analysis of 11 studies showed that peripherally inserted central catheters are associated with a higher risk of DVT than are central venous catheters.30 Mural thrombus extending from the catheter into the lumen of a vessel and leading to partial or total occlusion with or without clinical symptoms is defined as CVAD-associated thrombosis.
Biochemical-Related Risk Factors. The patient’s hemoglobin level, leukocyte, and platelet counts are associated with an increased VTE risk. Patients with a hemoglobin level <10 g/dL and a leukocyte count >11 x 109/L had an almost 2-fold increased risk of developing a VTE in a study conducted by Khorana, et al.31 The Awareness of Neutropenia in Chemotherapy Group Registry Study reported that a platelet count ≥350 x 109/L prior to chemotherapy is associated with an increased risk of VTE.32
Risk Assessment Tools
The modified Korana score is a well-validated risk-assessment model incorporating clinical parameters and laboratory biomarkers to stratify cancer patients according to their propensity to develop VTE, and represents a practical approach for clinical practice.31 The multivariate model identified the five most predictive variables: cancer site, pretreatment platelet count and leukocyte count, hemoglobin level, and body mass index (Table 2). For example, in the case presented, considering this patient’s age, primary site of the cancer, and his laboratory results, he was at a high-risk (up to 40%) for developing VTE.
Diagnosis
In patients with a high clinical suspicion of VTE and without contraindications to anticoagulation (Table 3), early initiation of anticoagulants should be considered while awaiting results from laboratory and imaging studies.5 The diagnostic accuracy for VTE improves with the following tests and scores: complete blood count, platelet count, D-dimer, Wells criteria, Modified Khorana Score, duplex ultrasonography, computed tomography angiography (CTA); and ventilation perfusion (V/Q) scan.
Complete Blood Count and Platelet Count. As previously discussed, a prechemotherapy platelet count ≥350 x 109/L, hemoglobin level <10g/dL, or prechemotherapy leukocyte count >11 x 109/L are good predictive biomarkers to consider VTE in a cancer patient.
D-Dimer. In view of the lack of specificity of D-dimer testing (most cancer patients have elevated D-dimer levels) and the high prevalence of VTE in cancer patients, D-dimer testing is less useful in this setting. The number of false positive D-dimer assays was 3-fold higher in cancer patients compared with noncancer patients.33 Hence, D-dimer testing is not recommended for the diagnosis of VTE in cancer patients.
Wells Criteria. Clinical prediction models such as the Wells criteria for VTE have proven useful in predicting the diagnosis of VTE: 62.5% of the 40 patients who were categorized as high-risk had positive studies; 16.1% of the 492 patients who were classified as moderate-risk and 10.2% of the 219 patients who were classified as low-risk had positive results.34 However, since cancer patients comprised a minority of the subjects in this study, it is unclear whether Wells criteria is as predictive for VTE in this patient population.
Computed Tomography/Computed Tomography Angiography. Computed tomography angiography is the preferred imaging technique for the initial diagnosis of PE in most patients.5 Along with accurate imaging of mediastinal and parenchymal structures and visualization of emboli in the pulmonary vasculature, CTA is also useful to detect signs of right heart enlargement, which can be used in assessing the patient’s risk for adverse clinical outcomes.36 (See Figures 2 and 3 for examples of CT and CTA, demonstrating venous thrombosis and pulmonary thromboembolism.)
Ventilation Perfusion Scan. As it is associated with less fetal radiation exposure than CTA, the V/Q scan is useful for assessing pregnant patients and those with renal insufficiency or untreatable contrast allergies in whom IV contrast is not feasible. A normal V/Q scan essentially rules out PE.38
Chest X-Ray and Electrocardiogram. Both a chest X-ray and electrocardiogram (ECG) facilitate the diagnosis of comorbidities and conditions with clinically similar presentations and can also assist in evaluating existing cardiac conditions. Findings on chest X-ray suspicious for PE include pleural effusion, localized infiltrate, Hamptons hump, and Westermark sign. In ECG, the most common finding in PE is nonspecific ST-T wave changes; a new right heart strain or an S1Q3T3 pattern is suspicious for PE.
Transthoracic Echocardiogram. More than 80% of patients with a documented PE will have some type of right heart abnormality, most commonly direct visualization of the thrombus, right ventricular dilatation, right ventricular hypokinesia with apical sparing, tricuspid regurgitation, abnormal interventricular septal motion, and pulmonary artery dilatation.39
Miniati et al40 have shown that using PE pretest probabilities of 10%, 50%, and 90%, the posttest probabilities with a positive echocardiogram (echo) were 38%, 85%, and 98%. With a negative echo, posttest probabilities were 5%, 33%, and 81%, respectively. The value of transthoracic echo in the diagnosis of PE results were taken from this prospective study in unselected patients.40 Because of the poor sensitivity, echocardiography should not be used as a routine test to screen patients for suspected PE.
Magnetic Resonance Imaging. In pregnant women and in patients with kidney disease, MRI has been used to detect PE. However, the PIOPED III study found that many of the participating centers had difficulty obtaining adequate quality MR angiography (MRA) for suspected PE. The study therefore determined that this modality should be ordered only when there is appropriate expertise in both performing and reading the images, and in patients who have contraindications to standard tests.4
Considering the high risk of developing VTE in the cancer population, there should be a low threshold for diagnostic imaging (ie, CTA and duplex ultrasonography). The patient in this case would have benefited from a CTA to rule-out PE before he was discharged.
Treatment
The National Comprehensive Cancer Network2 (NCCN), American Society of Clinical Oncology3 (ASCO), European Society for Medical Oncology4 (ESMO), International Society on Thrombosis and Haemostasis42 and American College of Chest Physicians5 all have recently published evidence-based clinical practice guidelines for the treatment of established VTE in cancer patients. Once a diagnosis of VTE has been established, immediate treatment with a parenteral anticoagulant should be initiated. For the purpose of this article we shall discuss only the initial treatment of VTE in the emergency setting. The available treatment options are low molecular weight heparin (LMWH), unfractionated heparin (UFH), fondaparinux, warfarin, apixaban and rivaroxaban, and thrombolytic agents.
Low Molecular Weight Heparin. As recommended by the ASCO, NCCN, and ESMO guidelines, the preferred initial treatment for VTE in cancer patients is LMWH. This recommendation stems from the Comparison of Low-Molecular-Weight Heparin versus Oral Anticoagulant Therapy for the Prevention of Recurrent Venous Thromboembolism in Patients with Cancer (CLOT) trial,43 which showed a decreased incidence of recurrent VTE in patients treated with a LMWH versus a coumarin derivative. Recurrent VTE occurred in 17% of patients who received warfarin versus 9% of patients who received dalteparin after 6 months. Numerous other studies evaluating LMWH versus warfarin have shown superior efficacy with LMWH for the treatment of VTE in cancer patients.
Options for LMWH currently available include enoxaparin, dalteparin, and tinzaparin. Enoxaparin is dosed at 1 mg/kg subcutaneously every 12 hours or 1.5 mg/kg subcutaneously every 24 hours. A subgroup analysis of cancer patients with PE showed a higher VTE recurrence rate in those who received once-daily versus twice-daily dosing of enoxaparin in a trial conducted by Merli and colleagues.44 Therefore, it is the authors’ recommendation to utilize the twice-daily regimen of enoxaparin for the treatment of PE in cancer patients during the acute phase of treatment.
Dalteparin is dosed at 200 IU/kg subcutaneously every 24 hours for the first month, and then 150 IU/kg subcutaneously every 24 hours thereafter. Tinzaparin is dosed at 175 IU/kg subcutaneously every 24 hours.
Since the LMWH drugs are renally eliminated, they can accumulate in patients with renal impairment. Patients with renal impairment should be considered for dose reductions if applicable, anti-Xa monitoring, and carefully observed for bleeding. Limited data with both dalteparin and tinzaparin, though, suggest less accumulation in patients with severe renal impairment compared to enoxaparin.
Unfractionated Heparin. The UFH drugs are administered via an IV bolus dose of 80 units/kg, followed by a continuous infusion of 18 units/kg/h, titrated to a goal activated partial thromboplastin time. Although LMWHs are preferred, UFH should be considered in certain settings, such as in patients with renal failure, patients with questionable absorption via the subcutaneous route, or patients who are under consideration for systemic thrombolytic therapy.5 Hospitalized patients who are at high risk for bleeding or patients who may undergo invasive procedures are good candidates for UFH over LMWHs. This is due to the fact that UFH can be promptly discontinued and is reversible with protamine sulfate (versus the partial reversibility and longer half-life of LMWH).
Fondaparinux. Fondaparinux is an indirect factor Xa (FXa) inhibitor given subcutaneously. Advantages of fondaparinux include the lack of monitoring required and the ability to use it in patients with a history of heparin-induced thrombocytopenia. Fondaparinux is contraindicated in patients with a creatinine clearance <30 mL/minute. Van Doormaal et al42 found the rate of VTE recurrence was lower with fondaparinux than UFH in the treatment of PE. In another study conducted by Akl et al,46 the rate of VTE recurrence was higher with fondaparinux than LMWH in the treatment of DVT.
Warfarin. Warfarin is very problematic in cancer patients due to the need for frequent laboratory monitoring, numerous drug-drug and drug-nutrient interactions, slow onset of action, and long duration of action. Although it has shown inferiority in cancer patients when compared to LMWH, it may still be an option for long-term treatment, especially for patients with financial barriers, adversity to injections, or renal failure. Due to the slow onset of action of warfarin (steady state is not achieved for 5-7 days), it is important to bridge with a parenteral anticoagulant until therapeutic International normalized ratio is achieved.
Apixaban and Rivaroxaban. Both of these agents are direct FXa inhibitors and can be classified as new oral anticoagulants. Although initial evidence is promising and these agents may be attractive due to feasibility of oral administration without required monitoring, they are currently not recommended for initial therapy in cancer patients. Current published trials have included very few cancer patients, thus limiting the authors’ ability to extrapolate results to the cancer population. Also, these anticoagulants lack reliable agents for reversal in cases of life-threatening bleeding complications.
Thrombolytics. These agents promote rapid clot lysis, reduce venous outflow obstruction, and prevent venous valvular dysfunction, which may help to limit long-term complications such as postthrombotic syndrome. No significant differences in reduction of recurrent PE, death, or major bleeding were found in a recent meta-analysis comparing heparin and thrombolytic agents in patients with acute PE.47 However, thrombolytic therapy is recommended for massive PE (ie, defined as PE with a systolic blood pressure <90 mm Hg).48 There is also growing evidence to support the use of thrombolytics in certain populations for submassive or moderate PE.2,5,48 Alteplase 100 mg via IV infusion over 2 hours can be given for thrombolysis in these settings. Absolute contraindications to thrombolysis include, but are not limited to severe, uncontrolled hypertension; known intracranial (IC) neoplasm or aneurysm; internal bleeding; or recent IC surgery or trauma (Table 3).
Nonpharmacological Treatment
Inferior Vena Cava Filters. In patients with contraindication to therapeutic anticoagulation, an inferior vena cava (IVC) filter can be inserted for prevention of PE.49 Insertion of an IVC filter should be assessed on a case-by-case basis after evaluation of the risk-benefit ratio. If contraindications to anticoagulant therapy are no longer present, anticoagulant therapy should be initiated.
Other treatment strategies such as catheter-directed thrombolysis and surgical thrombectomy can be considered, especially in patients presenting with severe, gangrenous limbs due to obstructive VTE. Until recently, thrombolytics were delivered systemically through an IV catheter, the main disadvantage of which was increased likelihood of bleeding and reduced efficacy of the therapy. Catheter-directed delivery of thrombolytic agents directly into the clot has allowed more localized targeting of therapy and, in combination with mechanical thrombectomy, results in a significant higher rate of complete clot dissolution than systemic anticoagulation.50
Treatment Recommendation
Per the guidelines outlined above, the preferred therapy in cancer patients with VTE is LMWH. Depending on other factors such as risk of bleeding, renal function, anticipated procedures, and financial barriers, other anticoagulant options are available but treatment should be assessed on a case-by-case basis.
Challenging Cases
Incidental VTE. Several studies have suggested that approximately half of cancer-associated VTE events are detected incidentally on routine CT scans performed for diagnosis, staging, or follow-up.51 A meta-analysis of 12 studies including more than 10,000 patients, had a weighted mean incidental PE prevalence of 3.1% (95% CI, 2.2-4.1%).52 Incidental VTE found on routine CT scans should be treated similarly to patients with symptomatic VTE, as many have subtle clinical symptoms of active disease on further evaluation.
Patients With Thrombocytopenia. The subcommittee on hemostasis and malignancy for the Scientific and Standardization Committee of the International Society on Thrombosis and haemostasis has recently recommended that anticoagulation be administered if the platelet count can be maintained above 50 x 109/L.53 For platelet counts between 20 and 50 x 109/L, half-dose LMWH can be administered with close monitoring for possible bleeding. If the platelet count is less than 20 x 109/L therapeutic doses of anticoagulation should be held. In these cases, the placement of an IVC filter is recommended to prevent PE. Once thrombocytopenia has resolved, it is recommended to initiate or resume therapeutic anticoagulation.2-4,42
Patients With Bleeding. In view of life-threatening bleeding complications, all patients should be assessed on a case-by-case basis and careful monitoring of additional bleeding risk is recommended. In patients with minor bleeding, anticoagulation may be continued as long as close follow-up is available; whereas, in patients with absolute contraindications to anticoagulation, the risk of bleeding likely outweighs the benefit of treatment, and anticoagulants should be withheld.3 The use of IVC filters are also recommended in this population—at least until the bleeding risk subsides and anticoagulation can be initiated.2-4,42
Patients With Brain Tumors. As per the national and international guidelines,3,42 a brain tumor per se is not a contraindication for anticoagulation. Anticoagulation is more effective and safer than filters when maintained in the therapeutic range in patients with brain metastases and VTE.54 Although LMWH is the preferred drug of choice in this group of patients, a decision should be made based on individual clinical assessment. Consideration should also be made for the primary tumor type since brain metastases from certain malignancies, such as melanoma, have higher tendencies to bleed.55
Patients With Renal Failure. Since LMWHs and fondaparinux are dependent on significant renal clearance, these agents should be avoided in patients with a creatinine clearance ≤30 mL/minute. Unfractionated heparin is the drug of choice in this setting and vitamin K antagonists can be started as early as on day 1.42 Fixed-dose or adjusted-dose subcutaneous UFH is also an option of outpatient management of VTE in patients with renal failure.5
Patients With Recurrent VTE on Anticoagulants. Extension of or a new DVT or PE despite being on anticoagulation therapy is defined as anticoagulation failure. Recurrent VTE is common in cancer patients, as seen in 9% of patients from the CLOT trial. Management strategies for recurrent VTE include optimizing or increasing the dose, switching agents, or placing an IVC filter. If patients are currently on once-daily enoxaparin regimens, this should be switched to the twice-daily regimen. Patients on agents other than LMWH should be switched to LMWH if possible, and dosed accurately based on the most recent total body weight and renal function. Although not useful in the emergency setting, an anti-Xa level should be drawn for patients who present with recurrent VTE on LMWH to assess whether patients are in therapeutic range. Peak anti-Xa levels should be checked 4 to 6 hours after the last dose. Lastly, dosing of LMWH could be increased by 20% to 25%, based on safety and efficacy data from a retrospective cohort study.56
Patients With Symptomatic Catheter-Related Thrombosis. For the treatment of symptomatic catheter-related thrombosis in cancer patients, the international clinical practice guidelines recommend use of anticoagulants for a minimum of 3 months.42 The CHEST guidelines suggest that the catheter not be removed if it is functional, not infected, and there is an ongoing need for the catheter.5 Little data exist in this subgroup of patients, but LMWHs are suggested.
Factors one should consider before commencing anticoagulation treatment include patient refusal, lack of therapeutic benefit, quality of life and life expectancy, bleeding risk, and whether anticoagulation is associated with an unreasonable burden.
Outpatient Treatment Criteria. In the absence of renal impairment, high risk of bleeding, or poor social circumstances, patients with DVT can be treated as an outpatient.57 Although recent studies support the safety and efficacy of outpatient PE management in noncancer patients,58,59 there is no study to support this in cancer patients. Several prognostic tools exist to aid the clinician in deciding whether cancer patients with PE can be treated as outpatients or require hospitalization. These prediction tools identify patients at a low in-hospital mortality in which outpatient management is reasonable. A few examples include the Geneva Pulmonary Embolism Prognostic Index, Pulmonary Embolism Severity Index, and the Aujesky and Murugappan prediction tools.57
The Decision Not to Treat
Acute intervention is not the only option in this unique population. Factors one should consider before commencing anticoagulation treatment include patient refusal, lack of therapeutic benefit, quality of life and life expectancy, bleeding risk, and whether anticoagulation is associated with an unreasonable burden.
Conclusion
In view of an increased risk of VTE in cancer patients, an increased level of clinical suspicion should be maintained. Risk factors related to patient, cancer type, treatment, and biochemical markers should be assessed, and the clinician should also consider using the modified Khorana score. Diagnostic imaging studies should also be performed in cases in which there is a high clinical suspicion of VTE.
Regarding treatment, the preferred drug of choice in the acute phase of treatment is LMWH—unless contraindications exist. In cancer patients with VTE who have severe renal failure, thrombocytopenia, brain tumors, or catheter related thrombosis, recommendations are based on best clinical practice, and the clinician should balance the desirable and undesirable effects depending on the bleeding risk versus VTE risk.
Dr Banala is a clinical fellow in the department of emergency medicine, University of Texas MD Anderson Cancer Center, Houston. Dr Wattana is an instructor in the department of emergency medicine, University of Texas MD Anderson Cancer Center, Houston. Ms Ma is a clinical pharmacy specialist in the division of pharmacy, University of Texas MD Anderson Cancer Center, Houston. Dr Todd is professor and chair of the department of emergency medicine, University of Texas MD Anderson Cancer Center. He is also a member of the EMERGENCY MEDICINE editorial board.
- American Cancer Society. Cancer Facts & Figures 2014. http://www.cancer.org/acs/groups/content/@research/documents/webcontent/acspc-042151.pdf. Accessed December 3, 2014. PAGES 1 AND 2
- National Comprehensive Cancer Network (NCCN) clinical practice guidelines - version1.2014, Cancer-Associated Venous Thromboembolic Disease. Available at www.nccn.org.
- Lyman GH, Khorana AA, Kuderer NM, et al; American Society of Clinical Oncology Clinical Practice. Venous thromboembolism prophylaxis and treatment in patients with cancer: American Society of Clinical Oncology clinical practice guideline update. Clin Oncol. 2013;31(17):2189-2204.
- Mandalà M, Falanga A, Roila F; ESMO Guidelines Working Group. Management of venous thromboembolism (VTE) in cancer patients: ESMO Clinical Practice Guidelines. Ann Oncol. 2011;22(Suppl 6):vi85-vi92.
- Kearon C, Akl EA, Comerota AJ, et al; American College of Chest Physicians. Antithrombotic therapy for VTE disease: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012;141(2 Suppl):e419S-e494S.
- Khorana AA. Venous thromboembolism and prognosis in cancer. Thromb Res. 2010;125(6):490-493.
- Khorana AA, Francis CW, eds. Cancer-Associated Thrombosis: New Findings in Translational Science, Prevention, and Treatment. New York, NY: Informa Healthcare; 2007: 17–34.
- Horsted F, West J, Grainge MJ. Risk of venous thromboembolism in patients with cancer: a systematic review and meta-analysis. PLoS Med. 2012;9(7):e1001275).
- Khorana AA, Connolly GC. Assessing risk of venous thromboembolism in the patient with cancer. J Clin Oncol. 2009;27(29):4839-4847.
- Khorana AA. Risk assessment and prophylaxis for vte in cancer patients. J Natl Compr Canc Netw. 2011;9(7):789-797.
- Khorana AA, Kuderer NM, Culakova E, Lyman GH, Francis CW. Development and validation of a predictive model for chemotherapy-associated thrombosis. Blood. 2008;111(10):4902-4907.
- Lee AY, Levine MN. Venous thromboembolism and cancer: risks and outcomes. Circulation. 2003;107(23 Suppl 1):I17-121.
- Carson JL, Kelley MA, Duff A, et al. The clinical course of pulmonary embolism. N Engl J Med. 1992;326(19):1240-1245.
- Sorensen HT, Mellemkjaer L, Olsen JH, Baron JA. Prognosis of cancers associated with venous thromboembolism. N Engl J Med. 2000;343(25):1846-1850.
- Chew HK, Wun T, Harvey D, Zhou H,White RH. Incidence of venous thromboembolism and its effect on survival among patients with common cancers. Arch Intern Med. 2006;166(4);458-464.
- Green KB. Silverstein RL. Hypercoagulability in cancer. Hematol Oncol Clin North Am. 1996;10(2):499-530.
- Hansson PO, Welin L, Tibblin G, Eriksson H. Deep vein thrombosis and pulmonary embolism in the general population. ‘The Study of Men Born in 1913.’ Arch Intern Med. 1997;157(15):1665-1670.
- Walker AJ, Card TR, West J, Crooks C, Grainge MJ. Incidence of venous thromboembolism in patients with cancer – a cohort study using linked United Kingdom databases. Eur J Cancer. 2013;49(6):1404-1413.
- Shea-Budgell MA, Wu CM, Easaw JC. Evidence-based guidance on venous thromboembolism in patients with solid tumours. Curr Oncol. 2014;21(3):e504-e514.
- Mandalà M, Reni M, Cascinu S, et al. Venous thromboembolism predicts poor prognosis in irresectable pancreatic cancer patients. Ann Oncol. 2007;18(10):1660-1665.
- Prandoni P. Antithrombotic strategies in patients with cancer. Thromb Haemost. 1997;78(1):141-144.
- Piccioli A, Lensing AW, Prins MH, et al; SOMIT Investigators Group. Extensive screening for occult malignant disease in idiopathic venous thromboembolism: a prospective randomized clinical trial. J Thromb Haemost. 2004;2(6):884-889.
- Hettiarachchi RJ, Lok J, Prins MH, Büller HR, Prandoni P. Undiagnosed malignancy in patients with deep vein thrombosis: incidence, risk indicators, and diagnosis. Cancer. 1998;83(1):180-185.
- Timp JF, Braekkan SK, Versteeg HH, Cannegiester SC. Epidemiology of cancer-associated venous thrombosis. Blood. 2013;122(10):1712-1723.
- Falanga A, Marchetti M. Venous thromboembolism in the hematologic malignancies. J Clin Oncol. 2009;27(29):4848-4857.
- Fisher B, Costantino JP, Wickerham DL, et al. Tamoxifen for the prevention of breast cancer: current status of the National Surgical Adjuvant Breast and Bowel Project P-1 study. J Natl Cancer Inst. 2005;97(22):1652-1662.
- Manzoli L, De Vito C, Marzuillo C, Boccia A, Villari P. Oral contraceptives and venous thromboembolism: A systematic review and meta-analysis. Drug Saf. 2012;35(3):191-205.
- Leaf AN, Propert K, Corcoran C, et al. Phase III study of combined chemohormonal therapy in metastatic prostate cancer (ECOG 3882): An Eastern Cooperative Oncology Group study. Med Oncol. 2003;20(2):137-146.
- Verso M, Agnelli G. Venous thromboembolism associated with long-term use of central venous catheters in cancer patients. J Clin Oncol. 2003;21(19):3665-3675.
- Chopra V, Anand S, Hickner A, et al. Risk of venous thromboembolism associated with peripherally inserted central catheters: a systematic review and meta-analysis. Lancet. 2013;382(9889):311-325.
- Khorana AA, Kuderer NM, Culakova E, Lyman GH, Francis CW. Development and validation of a predictive model for chemotherapy-associated thrombosis. Blood. 2008; 111(10):4902-4907.
- Khorana AA, Francis CW, Culakowa E, Lyman GH. Risk factors for chemotherapy-associated venous thromboembolism in a prospective observational study. Cancer. 2005;104(12):2822-2829.
- Sohne M, Kruip MJ, Nijkeuter M, et al; Christoper Study Group. Accuracy of clinical decision rule, D-dimer and spiral computed tomography in patients with malignancy, previous venous thromboembolism, COPD or heart failure and in older patients with suspected pulmonary embolism. J Thromb Haemost. 2006;4(5):1042-1046.
- Wells PS, Anderson DR, Rodger M, et al. Excluding pulmonary embolism at the bedside without diagnostic imaging: management of patients with suspected pulmonary embolism presenting to the emergency department by using a simple clinical model and D-dimer. Ann Intern Med. 2001;135(2):98-107.
- Zierler BK. Ultrasonography and diagnosis of venous thromboembolism. Circulation. 2004;109(12 Suppl 1):I-9-I-4.
- Schoepf UJ, Kucher N, Kipfmueller F, Quiroz R, Costello P, Goldhaber SZ. Right ventricular enlargement on chest computed tomography: a predictor of early death in acute pulmonary embolism. Circulation. 2004;110(20):3276-3280.
- Stein PD, Woodard PK, Weg JG, et al; PIOPED II investigators. Diagnostic pathways in acute pulmonary embolism: recommendations of the PIOPED II investigators. Am J Med. 2006;119(12):1048-1055.
- Anderson DR, Kahn SR, Rodger MA, et al. Computed tomographic pulmonary angiography vs ventilation-perfusion lung scanning in patients with suspected pulmonary embolism: a randomized controlled trial. JAMA. 2007;298(23):2743-2753.
- Goldhaber SZ. Pulmonary embolism. In: Braunwald E, Zipes DP, Libby P, eds. Braunwald’s Heart Disease: A Textbook of Cardiovascular Medicine. 6th ed. WB Saunders: Philadelphia, PA; 2006:1894-1895.
- Miniati M, Monti S, Pratali L, et al. Value of transthoracic echocardiography in the diagnosis of pulmonary embolism: results of a prospective study in unselected patients. Am J Med. 2001;110(7):528-535.
- Stein PD, Gottschalk A, Sostman HD, et al. Methods of Prospective Investigation of Pulmonary Embolism Diagnosis III (PIOPED III). Semin Nucl Med. 2008;38(6):462-470.
- Farge D, Debourdeau P, Beckers M, et al. International clinical practice guidelines for the treatment and prophylaxis of venous thromboembolism in patients with cancer. J Thromb Haemost. 2013;11(1):56-70.
- Lee AY, Levine MN, Baker RI, et al; Randomized Comparison of Low-Molecular-Weight Heparin versus Oral Anticoagulant Therapy for the Prevention of Recurrent Venous Thromboembolism in Patients with Cancer (CLOT) Investigators. Low-molecular-weight heparin versus a coumarin for the prevention of recurrent venous thromboembolism in patients with cancer. N Engl J Med. 2003;349(2):146-153.
- Merli G, Spiro TE, Olsson CG, et al; Enoxaparin Clinical Trial Group. Subcutaneous enoxaparin once or twice daily compared with intravenous unfractionated heparin for treatment of venous thromboembolic disease. Ann Intern Med. 2001;134(3):191-202.
- Van Doormaal FF, Raskob GE, Davidson BL, et al. Treatment of venous thromboembolism in patients with cancer: subgroup analysis of the Matisse clinical trials. Thromb Haemost. 2009;101(4):762-769.
- Akl EA, Vasireddi SR, Gunukula S, et al. Anticoagulation for the initial treatment of venous thromboembolism in patients with cancer. Cochrane Database Syst Rev. 2011;15(6)CD006649.
- Wan S, Quinlan DJ, Agnelli G, Eikelboom JW. Thrombolysis compared with heparin for the initial treatment of pulmonary embolism: a meta-analysis of the randomized controlled trials. Circulation. 2004;110(6):744-749.
- Sharifi M, Bay C, Skrocki L, Rahimi F, Mehdipour M; “MOPETT” Investigators. Moderate Pulmonary Embolism treated with thrombolysis (from the MOPETT trial). Am J Cardiol. 2013;111(2):273-277
- Docousus H; PREPIC (Prevention du Risque d’Embolie Pulmonaire par Interruption Cave) Study Group. Eight-year follow-up of patients with permanent vena cava filters in the prevention of pulmonary embolism: the PREPIC (Prevention du Risque d’Embolie Pulmonaire par Interruption Cave) randomized study. Circulation. 2005;112(3):416-422.
- Mewissen MW, Seabrook GR, Meissner MH, Cynamon J, Labropoulos N, Haughton SH. Catheter-directed thrombolysis for lower extremity deep venous thrombosis: report of a national multicenter registry. Radiology. 1999;211(1):39-49.
- Di Nisio M, Ferrante N, De Tursi M, et al. Incidental venous thromboembolism inn ambulatory cancer patients receiving chemotherapy. Thromb Haemost. 2010;104(5):1049-1054.
- Dentali F, Ageno W, Becattini C, et al. Prevalence and clinical history of incidental, asymptomatic pulmonary embolism: A meta-analysis. Thromb Res. 2010;125(6):518-522.
- Lee AY, Carrier M. Treatment of cancer-associated thrombosis: perspectives on the use of novel oral anticoagulants. Thromb Res. 2014;133(Suppl 2):S167-S171.
- Schiff D, DeAngelis LM. Therapy of venous thromboembolism in patients with brain metastases. Cancer. 1994;73(2):493-498.
- Kondziolka D, Bernstein M, Resch L, et al. Significance of hemorrhage into brain tumors: clinicopathological study. J Neurosurg. 1987;67(6):852-857.
- Carrier M, Le Gal G, Cho R, Tierney S, Rodger M, Lee AY. Dose escalation of low molecular weight heparin to manage recurrent venous thromboembolic events despite systemic anticoagulation in cancer patients. J Thromb Haemost. 2009;7(5):760-765.
- Wells PS, Forgie MA, Rodger MA. Treatment of venous thromboembolism. JAMA. 2014;311(7):717-728.
- Erkens PM, Gandara E, Wells PS, et al. Safety of outpatient treatment in acute pulmonary embolism. J Thromb Haemost. 2010;8(11):2412-2417.
- Aujesky D, Roy PM, Verschuren F, et al. Outpatient vs inpatient treatment for patients with acute pulmonary embolism: an international, open-label, randomised, non-inferiority trial. Lancet. 2011;378(9785):41-48.
- American Cancer Society. Cancer Facts & Figures 2014. http://www.cancer.org/acs/groups/content/@research/documents/webcontent/acspc-042151.pdf. Accessed December 3, 2014. PAGES 1 AND 2
- National Comprehensive Cancer Network (NCCN) clinical practice guidelines - version1.2014, Cancer-Associated Venous Thromboembolic Disease. Available at www.nccn.org.
- Lyman GH, Khorana AA, Kuderer NM, et al; American Society of Clinical Oncology Clinical Practice. Venous thromboembolism prophylaxis and treatment in patients with cancer: American Society of Clinical Oncology clinical practice guideline update. Clin Oncol. 2013;31(17):2189-2204.
- Mandalà M, Falanga A, Roila F; ESMO Guidelines Working Group. Management of venous thromboembolism (VTE) in cancer patients: ESMO Clinical Practice Guidelines. Ann Oncol. 2011;22(Suppl 6):vi85-vi92.
- Kearon C, Akl EA, Comerota AJ, et al; American College of Chest Physicians. Antithrombotic therapy for VTE disease: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012;141(2 Suppl):e419S-e494S.
- Khorana AA. Venous thromboembolism and prognosis in cancer. Thromb Res. 2010;125(6):490-493.
- Khorana AA, Francis CW, eds. Cancer-Associated Thrombosis: New Findings in Translational Science, Prevention, and Treatment. New York, NY: Informa Healthcare; 2007: 17–34.
- Horsted F, West J, Grainge MJ. Risk of venous thromboembolism in patients with cancer: a systematic review and meta-analysis. PLoS Med. 2012;9(7):e1001275).
- Khorana AA, Connolly GC. Assessing risk of venous thromboembolism in the patient with cancer. J Clin Oncol. 2009;27(29):4839-4847.
- Khorana AA. Risk assessment and prophylaxis for vte in cancer patients. J Natl Compr Canc Netw. 2011;9(7):789-797.
- Khorana AA, Kuderer NM, Culakova E, Lyman GH, Francis CW. Development and validation of a predictive model for chemotherapy-associated thrombosis. Blood. 2008;111(10):4902-4907.
- Lee AY, Levine MN. Venous thromboembolism and cancer: risks and outcomes. Circulation. 2003;107(23 Suppl 1):I17-121.
- Carson JL, Kelley MA, Duff A, et al. The clinical course of pulmonary embolism. N Engl J Med. 1992;326(19):1240-1245.
- Sorensen HT, Mellemkjaer L, Olsen JH, Baron JA. Prognosis of cancers associated with venous thromboembolism. N Engl J Med. 2000;343(25):1846-1850.
- Chew HK, Wun T, Harvey D, Zhou H,White RH. Incidence of venous thromboembolism and its effect on survival among patients with common cancers. Arch Intern Med. 2006;166(4);458-464.
- Green KB. Silverstein RL. Hypercoagulability in cancer. Hematol Oncol Clin North Am. 1996;10(2):499-530.
- Hansson PO, Welin L, Tibblin G, Eriksson H. Deep vein thrombosis and pulmonary embolism in the general population. ‘The Study of Men Born in 1913.’ Arch Intern Med. 1997;157(15):1665-1670.
- Walker AJ, Card TR, West J, Crooks C, Grainge MJ. Incidence of venous thromboembolism in patients with cancer – a cohort study using linked United Kingdom databases. Eur J Cancer. 2013;49(6):1404-1413.
- Shea-Budgell MA, Wu CM, Easaw JC. Evidence-based guidance on venous thromboembolism in patients with solid tumours. Curr Oncol. 2014;21(3):e504-e514.
- Mandalà M, Reni M, Cascinu S, et al. Venous thromboembolism predicts poor prognosis in irresectable pancreatic cancer patients. Ann Oncol. 2007;18(10):1660-1665.
- Prandoni P. Antithrombotic strategies in patients with cancer. Thromb Haemost. 1997;78(1):141-144.
- Piccioli A, Lensing AW, Prins MH, et al; SOMIT Investigators Group. Extensive screening for occult malignant disease in idiopathic venous thromboembolism: a prospective randomized clinical trial. J Thromb Haemost. 2004;2(6):884-889.
- Hettiarachchi RJ, Lok J, Prins MH, Büller HR, Prandoni P. Undiagnosed malignancy in patients with deep vein thrombosis: incidence, risk indicators, and diagnosis. Cancer. 1998;83(1):180-185.
- Timp JF, Braekkan SK, Versteeg HH, Cannegiester SC. Epidemiology of cancer-associated venous thrombosis. Blood. 2013;122(10):1712-1723.
- Falanga A, Marchetti M. Venous thromboembolism in the hematologic malignancies. J Clin Oncol. 2009;27(29):4848-4857.
- Fisher B, Costantino JP, Wickerham DL, et al. Tamoxifen for the prevention of breast cancer: current status of the National Surgical Adjuvant Breast and Bowel Project P-1 study. J Natl Cancer Inst. 2005;97(22):1652-1662.
- Manzoli L, De Vito C, Marzuillo C, Boccia A, Villari P. Oral contraceptives and venous thromboembolism: A systematic review and meta-analysis. Drug Saf. 2012;35(3):191-205.
- Leaf AN, Propert K, Corcoran C, et al. Phase III study of combined chemohormonal therapy in metastatic prostate cancer (ECOG 3882): An Eastern Cooperative Oncology Group study. Med Oncol. 2003;20(2):137-146.
- Verso M, Agnelli G. Venous thromboembolism associated with long-term use of central venous catheters in cancer patients. J Clin Oncol. 2003;21(19):3665-3675.
- Chopra V, Anand S, Hickner A, et al. Risk of venous thromboembolism associated with peripherally inserted central catheters: a systematic review and meta-analysis. Lancet. 2013;382(9889):311-325.
- Khorana AA, Kuderer NM, Culakova E, Lyman GH, Francis CW. Development and validation of a predictive model for chemotherapy-associated thrombosis. Blood. 2008; 111(10):4902-4907.
- Khorana AA, Francis CW, Culakowa E, Lyman GH. Risk factors for chemotherapy-associated venous thromboembolism in a prospective observational study. Cancer. 2005;104(12):2822-2829.
- Sohne M, Kruip MJ, Nijkeuter M, et al; Christoper Study Group. Accuracy of clinical decision rule, D-dimer and spiral computed tomography in patients with malignancy, previous venous thromboembolism, COPD or heart failure and in older patients with suspected pulmonary embolism. J Thromb Haemost. 2006;4(5):1042-1046.
- Wells PS, Anderson DR, Rodger M, et al. Excluding pulmonary embolism at the bedside without diagnostic imaging: management of patients with suspected pulmonary embolism presenting to the emergency department by using a simple clinical model and D-dimer. Ann Intern Med. 2001;135(2):98-107.
- Zierler BK. Ultrasonography and diagnosis of venous thromboembolism. Circulation. 2004;109(12 Suppl 1):I-9-I-4.
- Schoepf UJ, Kucher N, Kipfmueller F, Quiroz R, Costello P, Goldhaber SZ. Right ventricular enlargement on chest computed tomography: a predictor of early death in acute pulmonary embolism. Circulation. 2004;110(20):3276-3280.
- Stein PD, Woodard PK, Weg JG, et al; PIOPED II investigators. Diagnostic pathways in acute pulmonary embolism: recommendations of the PIOPED II investigators. Am J Med. 2006;119(12):1048-1055.
- Anderson DR, Kahn SR, Rodger MA, et al. Computed tomographic pulmonary angiography vs ventilation-perfusion lung scanning in patients with suspected pulmonary embolism: a randomized controlled trial. JAMA. 2007;298(23):2743-2753.
- Goldhaber SZ. Pulmonary embolism. In: Braunwald E, Zipes DP, Libby P, eds. Braunwald’s Heart Disease: A Textbook of Cardiovascular Medicine. 6th ed. WB Saunders: Philadelphia, PA; 2006:1894-1895.
- Miniati M, Monti S, Pratali L, et al. Value of transthoracic echocardiography in the diagnosis of pulmonary embolism: results of a prospective study in unselected patients. Am J Med. 2001;110(7):528-535.
- Stein PD, Gottschalk A, Sostman HD, et al. Methods of Prospective Investigation of Pulmonary Embolism Diagnosis III (PIOPED III). Semin Nucl Med. 2008;38(6):462-470.
- Farge D, Debourdeau P, Beckers M, et al. International clinical practice guidelines for the treatment and prophylaxis of venous thromboembolism in patients with cancer. J Thromb Haemost. 2013;11(1):56-70.
- Lee AY, Levine MN, Baker RI, et al; Randomized Comparison of Low-Molecular-Weight Heparin versus Oral Anticoagulant Therapy for the Prevention of Recurrent Venous Thromboembolism in Patients with Cancer (CLOT) Investigators. Low-molecular-weight heparin versus a coumarin for the prevention of recurrent venous thromboembolism in patients with cancer. N Engl J Med. 2003;349(2):146-153.
- Merli G, Spiro TE, Olsson CG, et al; Enoxaparin Clinical Trial Group. Subcutaneous enoxaparin once or twice daily compared with intravenous unfractionated heparin for treatment of venous thromboembolic disease. Ann Intern Med. 2001;134(3):191-202.
- Van Doormaal FF, Raskob GE, Davidson BL, et al. Treatment of venous thromboembolism in patients with cancer: subgroup analysis of the Matisse clinical trials. Thromb Haemost. 2009;101(4):762-769.
- Akl EA, Vasireddi SR, Gunukula S, et al. Anticoagulation for the initial treatment of venous thromboembolism in patients with cancer. Cochrane Database Syst Rev. 2011;15(6)CD006649.
- Wan S, Quinlan DJ, Agnelli G, Eikelboom JW. Thrombolysis compared with heparin for the initial treatment of pulmonary embolism: a meta-analysis of the randomized controlled trials. Circulation. 2004;110(6):744-749.
- Sharifi M, Bay C, Skrocki L, Rahimi F, Mehdipour M; “MOPETT” Investigators. Moderate Pulmonary Embolism treated with thrombolysis (from the MOPETT trial). Am J Cardiol. 2013;111(2):273-277
- Docousus H; PREPIC (Prevention du Risque d’Embolie Pulmonaire par Interruption Cave) Study Group. Eight-year follow-up of patients with permanent vena cava filters in the prevention of pulmonary embolism: the PREPIC (Prevention du Risque d’Embolie Pulmonaire par Interruption Cave) randomized study. Circulation. 2005;112(3):416-422.
- Mewissen MW, Seabrook GR, Meissner MH, Cynamon J, Labropoulos N, Haughton SH. Catheter-directed thrombolysis for lower extremity deep venous thrombosis: report of a national multicenter registry. Radiology. 1999;211(1):39-49.
- Di Nisio M, Ferrante N, De Tursi M, et al. Incidental venous thromboembolism inn ambulatory cancer patients receiving chemotherapy. Thromb Haemost. 2010;104(5):1049-1054.
- Dentali F, Ageno W, Becattini C, et al. Prevalence and clinical history of incidental, asymptomatic pulmonary embolism: A meta-analysis. Thromb Res. 2010;125(6):518-522.
- Lee AY, Carrier M. Treatment of cancer-associated thrombosis: perspectives on the use of novel oral anticoagulants. Thromb Res. 2014;133(Suppl 2):S167-S171.
- Schiff D, DeAngelis LM. Therapy of venous thromboembolism in patients with brain metastases. Cancer. 1994;73(2):493-498.
- Kondziolka D, Bernstein M, Resch L, et al. Significance of hemorrhage into brain tumors: clinicopathological study. J Neurosurg. 1987;67(6):852-857.
- Carrier M, Le Gal G, Cho R, Tierney S, Rodger M, Lee AY. Dose escalation of low molecular weight heparin to manage recurrent venous thromboembolic events despite systemic anticoagulation in cancer patients. J Thromb Haemost. 2009;7(5):760-765.
- Wells PS, Forgie MA, Rodger MA. Treatment of venous thromboembolism. JAMA. 2014;311(7):717-728.
- Erkens PM, Gandara E, Wells PS, et al. Safety of outpatient treatment in acute pulmonary embolism. J Thromb Haemost. 2010;8(11):2412-2417.
- Aujesky D, Roy PM, Verschuren F, et al. Outpatient vs inpatient treatment for patients with acute pulmonary embolism: an international, open-label, randomised, non-inferiority trial. Lancet. 2011;378(9785):41-48.
FDA clears noninvasive method of obtaining FFR measurements
Software that provides an estimate of a patient’s fractional flow reserve using data from a coronary CT scan has been cleared for marketing, the Food and Drug Administration announced Nov. 26.
The software, HeartFlow FFRCT, “is a computer modeling program that provides a functional assessment of blood flow in the coronary arteries from detailed anatomical data,” Dr. William Maisel, deputy director for science and chief scientist in the FDA’s Center for Devices and Radiological Health, said in the FDA statement. He described it as a noninvasive method that “is an additional tool for clinicians who are considering the risks and benefits of invasive coronary procedures.”
The healthcare professional transmits a patient’s coronary CT scan data to the headquarters of the manufacturer, HeartFlow, where an analyst creates 3-D models of the patient’s heart and runs a blood flow simulator program on the models. The clinician is then sent a report with the estimated fractional flow reserve (FFR)-CT values “displayed as color images of the patient’s heart,” according to the FDA statement.
The FDA cleared the device based on data that compared FFR-CT measurements to those obtained with cardiac catheterization in patients with suspected coronary artery disease. The FFR-CT measurements correctly identified 84% of the significant blockages that FFR identified as requiring intervention, and 86% of blockages that FFR identified as not requiring intervention, according to the FDA.
Software that provides an estimate of a patient’s fractional flow reserve using data from a coronary CT scan has been cleared for marketing, the Food and Drug Administration announced Nov. 26.
The software, HeartFlow FFRCT, “is a computer modeling program that provides a functional assessment of blood flow in the coronary arteries from detailed anatomical data,” Dr. William Maisel, deputy director for science and chief scientist in the FDA’s Center for Devices and Radiological Health, said in the FDA statement. He described it as a noninvasive method that “is an additional tool for clinicians who are considering the risks and benefits of invasive coronary procedures.”
The healthcare professional transmits a patient’s coronary CT scan data to the headquarters of the manufacturer, HeartFlow, where an analyst creates 3-D models of the patient’s heart and runs a blood flow simulator program on the models. The clinician is then sent a report with the estimated fractional flow reserve (FFR)-CT values “displayed as color images of the patient’s heart,” according to the FDA statement.
The FDA cleared the device based on data that compared FFR-CT measurements to those obtained with cardiac catheterization in patients with suspected coronary artery disease. The FFR-CT measurements correctly identified 84% of the significant blockages that FFR identified as requiring intervention, and 86% of blockages that FFR identified as not requiring intervention, according to the FDA.
Software that provides an estimate of a patient’s fractional flow reserve using data from a coronary CT scan has been cleared for marketing, the Food and Drug Administration announced Nov. 26.
The software, HeartFlow FFRCT, “is a computer modeling program that provides a functional assessment of blood flow in the coronary arteries from detailed anatomical data,” Dr. William Maisel, deputy director for science and chief scientist in the FDA’s Center for Devices and Radiological Health, said in the FDA statement. He described it as a noninvasive method that “is an additional tool for clinicians who are considering the risks and benefits of invasive coronary procedures.”
The healthcare professional transmits a patient’s coronary CT scan data to the headquarters of the manufacturer, HeartFlow, where an analyst creates 3-D models of the patient’s heart and runs a blood flow simulator program on the models. The clinician is then sent a report with the estimated fractional flow reserve (FFR)-CT values “displayed as color images of the patient’s heart,” according to the FDA statement.
The FDA cleared the device based on data that compared FFR-CT measurements to those obtained with cardiac catheterization in patients with suspected coronary artery disease. The FFR-CT measurements correctly identified 84% of the significant blockages that FFR identified as requiring intervention, and 86% of blockages that FFR identified as not requiring intervention, according to the FDA.
CT overutilized to diagnose appendicitis
SAN FRANCISCO – At least 25% of CT scans to diagnose appendicitis were unnecessary, potentially resulting in $1.8 million in costs at one institution and up to four new cancers from the radiation exposure, a retrospective study suggests.
The review of 1,054 patients who underwent appendectomy at the University of California, Davis, in 2005-2010 focused on costs for the patients who had high Alvarado scores, a clinical scoring system used to diagnose appendicitis, before they underwent appendectomy. CT scans to help diagnose appendicitis were performed on 77% of all patients.
Records showed that 26% of patients had an Alvarado score of 8-10, meaning that appendicitis was highly likely. CT was performed on 70% of patients with an Alvarado score of 8 and 77% of patients with a score of 9-10, comprising nearly 25% of all CT scans. That resulted in an estimated $1,813,399 in unnecessary costs for imaging, Dr. Adam Dougherty and his associates reported at the annual clinical congress of the American College of Surgeons.
This “overutilization” of CT scans delivered more than 4,009 mSv in unnecessary radiation exposure, averaging 19.75 mSV per scan, which is 20 times the annual limit suggested for safety, said Dr. Dougherty of the university. That excess radiation could be expected to produce up to four new cancers down the line, resulting in additional costs, he said.
The investigators also looked at the 9% of patients with low Alvarado scores, meaning that appendicitis was unlikely. CT scans were performed in 75% of patients with a score of 0-3 and 80% of patients with an Alvarado score of 4. In this subgroup, 24% showed normal/early pathology on appendectomy, which “argues against imaging and surgical treatment,” Dr. Dougherty said. The 44 CT scans in this subgroup resulted in an estimated $393,052 in unnecessary costs, he said.
That doesn’t include additional costs that could be expected from imaging, such as wait time, appendectomy and its sequelae, and potential workups of incidentalomas in the low-risk group, he added.
Previous studies have shown that a comprehensive clinical exam is as accurate as CT in diagnosing appendicitis, and that clinical assessment unaided by CT can reliably diagnose acute appendicitis, Dr. Dougherty said.
With a 72% increase in abdominal CT scans documented in other U.S. data from 2000 to 2005, he called for a “necessary, fundamental culture change” to restrain resource utilization “in order to maximize the value of the health care dollar while doing what is best for the patient.”
The investigators proposed a clinical pathway for the workup of suspected appendicitis that places greater emphasis on ultrasound imaging and conservative pathways, such as 23-hour admission for observation and next-day follow-up.
In the study, ultrasonography was underutilized across all subgroups as a viable alternative to CT scans, he said.
Dr. Dougherty reported having no financial disclosures.
On Twitter @sherryboschert
SAN FRANCISCO – At least 25% of CT scans to diagnose appendicitis were unnecessary, potentially resulting in $1.8 million in costs at one institution and up to four new cancers from the radiation exposure, a retrospective study suggests.
The review of 1,054 patients who underwent appendectomy at the University of California, Davis, in 2005-2010 focused on costs for the patients who had high Alvarado scores, a clinical scoring system used to diagnose appendicitis, before they underwent appendectomy. CT scans to help diagnose appendicitis were performed on 77% of all patients.
Records showed that 26% of patients had an Alvarado score of 8-10, meaning that appendicitis was highly likely. CT was performed on 70% of patients with an Alvarado score of 8 and 77% of patients with a score of 9-10, comprising nearly 25% of all CT scans. That resulted in an estimated $1,813,399 in unnecessary costs for imaging, Dr. Adam Dougherty and his associates reported at the annual clinical congress of the American College of Surgeons.
This “overutilization” of CT scans delivered more than 4,009 mSv in unnecessary radiation exposure, averaging 19.75 mSV per scan, which is 20 times the annual limit suggested for safety, said Dr. Dougherty of the university. That excess radiation could be expected to produce up to four new cancers down the line, resulting in additional costs, he said.
The investigators also looked at the 9% of patients with low Alvarado scores, meaning that appendicitis was unlikely. CT scans were performed in 75% of patients with a score of 0-3 and 80% of patients with an Alvarado score of 4. In this subgroup, 24% showed normal/early pathology on appendectomy, which “argues against imaging and surgical treatment,” Dr. Dougherty said. The 44 CT scans in this subgroup resulted in an estimated $393,052 in unnecessary costs, he said.
That doesn’t include additional costs that could be expected from imaging, such as wait time, appendectomy and its sequelae, and potential workups of incidentalomas in the low-risk group, he added.
Previous studies have shown that a comprehensive clinical exam is as accurate as CT in diagnosing appendicitis, and that clinical assessment unaided by CT can reliably diagnose acute appendicitis, Dr. Dougherty said.
With a 72% increase in abdominal CT scans documented in other U.S. data from 2000 to 2005, he called for a “necessary, fundamental culture change” to restrain resource utilization “in order to maximize the value of the health care dollar while doing what is best for the patient.”
The investigators proposed a clinical pathway for the workup of suspected appendicitis that places greater emphasis on ultrasound imaging and conservative pathways, such as 23-hour admission for observation and next-day follow-up.
In the study, ultrasonography was underutilized across all subgroups as a viable alternative to CT scans, he said.
Dr. Dougherty reported having no financial disclosures.
On Twitter @sherryboschert
SAN FRANCISCO – At least 25% of CT scans to diagnose appendicitis were unnecessary, potentially resulting in $1.8 million in costs at one institution and up to four new cancers from the radiation exposure, a retrospective study suggests.
The review of 1,054 patients who underwent appendectomy at the University of California, Davis, in 2005-2010 focused on costs for the patients who had high Alvarado scores, a clinical scoring system used to diagnose appendicitis, before they underwent appendectomy. CT scans to help diagnose appendicitis were performed on 77% of all patients.
Records showed that 26% of patients had an Alvarado score of 8-10, meaning that appendicitis was highly likely. CT was performed on 70% of patients with an Alvarado score of 8 and 77% of patients with a score of 9-10, comprising nearly 25% of all CT scans. That resulted in an estimated $1,813,399 in unnecessary costs for imaging, Dr. Adam Dougherty and his associates reported at the annual clinical congress of the American College of Surgeons.
This “overutilization” of CT scans delivered more than 4,009 mSv in unnecessary radiation exposure, averaging 19.75 mSV per scan, which is 20 times the annual limit suggested for safety, said Dr. Dougherty of the university. That excess radiation could be expected to produce up to four new cancers down the line, resulting in additional costs, he said.
The investigators also looked at the 9% of patients with low Alvarado scores, meaning that appendicitis was unlikely. CT scans were performed in 75% of patients with a score of 0-3 and 80% of patients with an Alvarado score of 4. In this subgroup, 24% showed normal/early pathology on appendectomy, which “argues against imaging and surgical treatment,” Dr. Dougherty said. The 44 CT scans in this subgroup resulted in an estimated $393,052 in unnecessary costs, he said.
That doesn’t include additional costs that could be expected from imaging, such as wait time, appendectomy and its sequelae, and potential workups of incidentalomas in the low-risk group, he added.
Previous studies have shown that a comprehensive clinical exam is as accurate as CT in diagnosing appendicitis, and that clinical assessment unaided by CT can reliably diagnose acute appendicitis, Dr. Dougherty said.
With a 72% increase in abdominal CT scans documented in other U.S. data from 2000 to 2005, he called for a “necessary, fundamental culture change” to restrain resource utilization “in order to maximize the value of the health care dollar while doing what is best for the patient.”
The investigators proposed a clinical pathway for the workup of suspected appendicitis that places greater emphasis on ultrasound imaging and conservative pathways, such as 23-hour admission for observation and next-day follow-up.
In the study, ultrasonography was underutilized across all subgroups as a viable alternative to CT scans, he said.
Dr. Dougherty reported having no financial disclosures.
On Twitter @sherryboschert
AT THE ACS CLINICAL CONGRESS
Key clinical point: Records showed that 26% of patients who had CT scans for suspect appendicitis had an Alvarado score of 8-10.
Major finding: A quarter of CT scans were on patients with likely appendicitis by Alvarado score, producing $1.8 million in unnecessary costs.
Data source: A retrospective study of 1,054 patients undergoing appendectomy in 2005-2010 at one institution.
Disclosures: Dr. Dougherty reported having no financial disclosures.