User login
Venuous Thromboembolism in Cancer Patients
Case
A 62-year-old man with known adenocarcinoma of the lung with metastasis to the liver and bones presented to the ED complaining of nonproductive cough and progressively worsening shortness of breath for 2 weeks. He was started on crizotinib a week prior to presentation, and had also received multiple courses of oral antibiotics and steroids for presumed exacerbation of chronic obstructive pulmonary disease (COPD). In addition to COPD, the patient had a history of degenerative joint disease, and was previously a chronic smoker. His current medications included tiotropium, inhaled daily; albuterol, two inhalations as needed; and hydrocodone/acetaminophen as needed for pain.
His vital signs on physical examination were: heart rate, 98 beats/minute; blood pressure 138/89 mm Hg; respiratory rate, 20 breaths/minute; temperature, 99.3°F. Oxygen saturation was 84% on room air, which improved to 92% with 2 L of oxygen. He had mild rhonchi on chest auscultation, but normal heart sounds and normal neurological, gastrointestinal, and extremity examination. Laboratory data revealed persistent elevation of leukocytes ranging between 12 and 15 x 109/L; hemoglobin, 9.2 g/dL; and platelet count, 220 x 109/L. A basic metabolic panel was within the normal range. The chest radiograph showed no evidence of pulmonary or cardiac abnormalities.
The patient was given nebulizer therapy with albuterol, along with oral prednisone. He improved with treatment and was discharged with oral prednisone daily for 4 days and oral doxycycline daily for 7 days. A week later, however, the patient had a cardiorespiratory arrest at home and could not be revived. A postmortem examination confirmed bilateral massive pulmonary embolism (PE).
Cancer Prevalence
According to the Cancer Facts & Figures 2014 published by the American Cancer Society, approximately 13.7 million Americans with a history of cancer were alive on January 1, 2012.1 In 2014, there will be an estimated 1,665,540 new cancer diagnoses and 585,720 cancer deaths in the United States.1 Cancer remains the second most common cause of death in the United States, accounting for nearly one out of every four deaths.
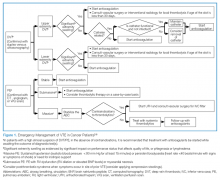
As seen in the above case presentation, cancer patients are at an increased risk for developing venous thromboembolism (VTE), the symptoms of which may not always be overt. Thus, with the increasing incidence and prevalence of cancer, enhanced vision and emphasis on education of emergency physicians—especially in relation to comorbidities associated with the disease—will improve care for this unique population. (See Figure 1 for an algorithm outlining the appropriate emergency management of VTE in cancer patients.)
Venous Thromboembolism
Symptoms
Classic clinical symptoms of acute VTE may not be seen in all patients. Therefore, a high level of clinical suspicion should be made on cancer patients presenting with any clinically overt signs or symptoms that could represent acute VTE. Symptoms of DVT include pain or tenderness and swelling of one or more extremities. On physical examination, increased warmth, edema, and erythema are often found. The most common signs and symptoms of PE include dyspnea, tachypnea, and pleuritic chest pain. Tachycardia, hypoxia, cough, and syncope are not present in all cases of acute PE.2
Deep Vein Thrombosis and Pulmonary Embolism
Deep vein thrombosis and PE are manifestations of a single disease entity, namely, VTE. The association between VTE and cancer has been well established since it was first recognized by the French internist Armand Trousseau more than 150 years ago.
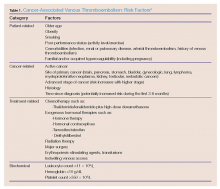
Deep vein thrombosis is characterized by a thrombus, usually in one of the deep venous conduits that return blood to the heart from the lower extremities, proximal to and including the popliteal veins. However, emboli can also originate from the pelvic veins, the inferior vena cava, and the upper extremities. Pulmonary embolism is characterized by a fully or partially occluding thrombus in one or more arteries of the lungs. Patients with cancer carry an increased risk of developing VTE because of tumor- and treatment-mediated hypercoagulability.6-8 Table 1 provides a summary of the risk factors associated with cancer-related VTE.
Venous thromboembolism is the second leading noncancer-related cause of death in oncology patients, with infection being the first.9-11 Mortality from an acute thrombotic event is four to eight times greater in patients with cancer than in those without cancer.12-14 Based on its high prevalence (in part due to prothrombotic effects of oncologic therapies) and high mortality rate, awareness of VTE diagnostic approaches and treatment is particularly important in the ED patient with cancer.
Epidemiology
Patients with advanced metastatic disease of the brain, lung, stomach, pancreas, kidneys, uterus, and bladder have the highest 1-year VTE incidence rate, and are at a four to 13 times higher risk of developing VTE compared to those with localized disease.15 Approximately 15% of patients with a malignancy present with VTE at some point during the course of their illness16 compared to 2.5% of the general population.17 Furthermore, an increasing frequency of VTE among cancer patients has been observed over the years due to improved survival.18 The strongest predictor for recurrent VTE is a previous diagnosis of the condition.19 The prognosis of VTE depends on the aggressiveness of the underlying malignancy and the presence of other cancer-associated complications.20
In the postoperative setting, the risk of lower extremity DVT is about twice that of noncancer patients.21 While commonly noted, there is limited evidence to support the relationship between DVT location and cancer. It is reasonable, however, to propose that localized anatomical compression by the tumor of nearby veins causes stasis and subsequent DVT. Bilateral DVT of the lower extremities may be seen with pelvic malignancies, and DVT of the upper extremities is associated with central venous catheters, axillary lymphomas, mediastinal tumor masses, and intravenous (IV) chemotherapy. Idiopathic DVT is a significant risk factor for underlying malignancy, found in approximately 10% of cases.22 In a prospective study of patients with newly diagnosed DVT, Hettiarachchi, et al23 report routine clinical evaluations are sufficient to find underlying malignancies and that extensive screening is unwarranted.
Risk Assessment
The common risk factors of VTE in the general population are older age, female gender, race, prolonged immobilization, hospitalization, previous history of VTE, obesity, heart disease (ie, cardiomyopathy, atrial fibrillation), smoking, poor performance status, familial or acquired hypercoagulability (eg, pregnancy, hormone therapy, indwelling venous catheters). The cancer population, however, is unique and has other risk factors placing patients at risk for developing a VTE. Such risk factors can be grouped into four different categories: patient-related, cancer-related, treatment-related, and biochemical-related.
Patient-Related Risk Factors. These include age, obesity, smoking, decreased mobility, history of previous VTE, comorbidities, and the presence of prothrombotic mutations.
Cancer-Related Risk Factors. Risk factors for VTE depend on the site, stage, and histological type and time from diagnosis of the cancer. Brain tumors and stomach and pancreatic cancers are associated with a high risk of VTE.9,24 Timp, et al24 also studied a strong association between the 1-year relative mortality of a cancer type and its associated thrombogenic potential. In hematologic malignancies, patients with high-grade lymphoma and acute promyelocytic leukemia are at higher risk than other forms of lymphoma or leukemia.25
Treatment-Related Risk Factors. These include anticancer drugs (chemotherapy and hormonal therapy), radiation therapy, recent major surgery, and the presence of a central venous access device (CVAD).
Patients who received chemotherapy with thalidomide, lenalidomide, cisplatin, and platinum were found to be at high risk of developing VTE.9 Hormonal therapies including tamoxifen, raloxifene, oral contraceptives, and diethylstilbestrol phosphate (used in combination with doxorubicin) also increase the risk of VTE in cancer patients.26-28
The incidence of symptomatic catheter-associated thrombosis is shown to be as high as 28.3%.29 A recent meta-analysis of 11 studies showed that peripherally inserted central catheters are associated with a higher risk of DVT than are central venous catheters.30 Mural thrombus extending from the catheter into the lumen of a vessel and leading to partial or total occlusion with or without clinical symptoms is defined as CVAD-associated thrombosis.
Biochemical-Related Risk Factors. The patient’s hemoglobin level, leukocyte, and platelet counts are associated with an increased VTE risk. Patients with a hemoglobin level <10 g/dL and a leukocyte count >11 x 109/L had an almost 2-fold increased risk of developing a VTE in a study conducted by Khorana, et al.31 The Awareness of Neutropenia in Chemotherapy Group Registry Study reported that a platelet count ≥350 x 109/L prior to chemotherapy is associated with an increased risk of VTE.32
Risk Assessment Tools
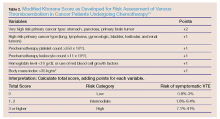
The modified Korana score is a well-validated risk-assessment model incorporating clinical parameters and laboratory biomarkers to stratify cancer patients according to their propensity to develop VTE, and represents a practical approach for clinical practice.31 The multivariate model identified the five most predictive variables: cancer site, pretreatment platelet count and leukocyte count, hemoglobin level, and body mass index (Table 2). For example, in the case presented, considering this patient’s age, primary site of the cancer, and his laboratory results, he was at a high-risk (up to 40%) for developing VTE.
Diagnosis
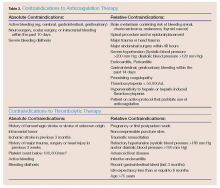
In patients with a high clinical suspicion of VTE and without contraindications to anticoagulation (Table 3), early initiation of anticoagulants should be considered while awaiting results from laboratory and imaging studies.5 The diagnostic accuracy for VTE improves with the following tests and scores: complete blood count, platelet count, D-dimer, Wells criteria, Modified Khorana Score, duplex ultrasonography, computed tomography angiography (CTA); and ventilation perfusion (V/Q) scan.
Complete Blood Count and Platelet Count. As previously discussed, a prechemotherapy platelet count ≥350 x 109/L, hemoglobin level <10g/dL, or prechemotherapy leukocyte count >11 x 109/L are good predictive biomarkers to consider VTE in a cancer patient.
D-Dimer. In view of the lack of specificity of D-dimer testing (most cancer patients have elevated D-dimer levels) and the high prevalence of VTE in cancer patients, D-dimer testing is less useful in this setting. The number of false positive D-dimer assays was 3-fold higher in cancer patients compared with noncancer patients.33 Hence, D-dimer testing is not recommended for the diagnosis of VTE in cancer patients.
Wells Criteria. Clinical prediction models such as the Wells criteria for VTE have proven useful in predicting the diagnosis of VTE: 62.5% of the 40 patients who were categorized as high-risk had positive studies; 16.1% of the 492 patients who were classified as moderate-risk and 10.2% of the 219 patients who were classified as low-risk had positive results.34 However, since cancer patients comprised a minority of the subjects in this study, it is unclear whether Wells criteria is as predictive for VTE in this patient population.
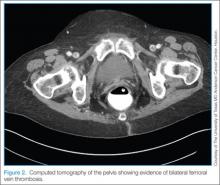
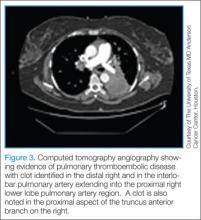
Computed Tomography/Computed Tomography Angiography. Computed tomography angiography is the preferred imaging technique for the initial diagnosis of PE in most patients.5 Along with accurate imaging of mediastinal and parenchymal structures and visualization of emboli in the pulmonary vasculature, CTA is also useful to detect signs of right heart enlargement, which can be used in assessing the patient’s risk for adverse clinical outcomes.36 (See Figures 2 and 3 for examples of CT and CTA, demonstrating venous thrombosis and pulmonary thromboembolism.)
Ventilation Perfusion Scan. As it is associated with less fetal radiation exposure than CTA, the V/Q scan is useful for assessing pregnant patients and those with renal insufficiency or untreatable contrast allergies in whom IV contrast is not feasible. A normal V/Q scan essentially rules out PE.38
Chest X-Ray and Electrocardiogram. Both a chest X-ray and electrocardiogram (ECG) facilitate the diagnosis of comorbidities and conditions with clinically similar presentations and can also assist in evaluating existing cardiac conditions. Findings on chest X-ray suspicious for PE include pleural effusion, localized infiltrate, Hamptons hump, and Westermark sign. In ECG, the most common finding in PE is nonspecific ST-T wave changes; a new right heart strain or an S1Q3T3 pattern is suspicious for PE.
Transthoracic Echocardiogram. More than 80% of patients with a documented PE will have some type of right heart abnormality, most commonly direct visualization of the thrombus, right ventricular dilatation, right ventricular hypokinesia with apical sparing, tricuspid regurgitation, abnormal interventricular septal motion, and pulmonary artery dilatation.39
Miniati et al40 have shown that using PE pretest probabilities of 10%, 50%, and 90%, the posttest probabilities with a positive echocardiogram (echo) were 38%, 85%, and 98%. With a negative echo, posttest probabilities were 5%, 33%, and 81%, respectively. The value of transthoracic echo in the diagnosis of PE results were taken from this prospective study in unselected patients.40 Because of the poor sensitivity, echocardiography should not be used as a routine test to screen patients for suspected PE.
Magnetic Resonance Imaging. In pregnant women and in patients with kidney disease, MRI has been used to detect PE. However, the PIOPED III study found that many of the participating centers had difficulty obtaining adequate quality MR angiography (MRA) for suspected PE. The study therefore determined that this modality should be ordered only when there is appropriate expertise in both performing and reading the images, and in patients who have contraindications to standard tests.4
Considering the high risk of developing VTE in the cancer population, there should be a low threshold for diagnostic imaging (ie, CTA and duplex ultrasonography). The patient in this case would have benefited from a CTA to rule-out PE before he was discharged.
Treatment
The National Comprehensive Cancer Network2 (NCCN), American Society of Clinical Oncology3 (ASCO), European Society for Medical Oncology4 (ESMO), International Society on Thrombosis and Haemostasis42 and American College of Chest Physicians5 all have recently published evidence-based clinical practice guidelines for the treatment of established VTE in cancer patients. Once a diagnosis of VTE has been established, immediate treatment with a parenteral anticoagulant should be initiated. For the purpose of this article we shall discuss only the initial treatment of VTE in the emergency setting. The available treatment options are low molecular weight heparin (LMWH), unfractionated heparin (UFH), fondaparinux, warfarin, apixaban and rivaroxaban, and thrombolytic agents.
Low Molecular Weight Heparin. As recommended by the ASCO, NCCN, and ESMO guidelines, the preferred initial treatment for VTE in cancer patients is LMWH. This recommendation stems from the Comparison of Low-Molecular-Weight Heparin versus Oral Anticoagulant Therapy for the Prevention of Recurrent Venous Thromboembolism in Patients with Cancer (CLOT) trial,43 which showed a decreased incidence of recurrent VTE in patients treated with a LMWH versus a coumarin derivative. Recurrent VTE occurred in 17% of patients who received warfarin versus 9% of patients who received dalteparin after 6 months. Numerous other studies evaluating LMWH versus warfarin have shown superior efficacy with LMWH for the treatment of VTE in cancer patients.
Options for LMWH currently available include enoxaparin, dalteparin, and tinzaparin. Enoxaparin is dosed at 1 mg/kg subcutaneously every 12 hours or 1.5 mg/kg subcutaneously every 24 hours. A subgroup analysis of cancer patients with PE showed a higher VTE recurrence rate in those who received once-daily versus twice-daily dosing of enoxaparin in a trial conducted by Merli and colleagues.44 Therefore, it is the authors’ recommendation to utilize the twice-daily regimen of enoxaparin for the treatment of PE in cancer patients during the acute phase of treatment.
Dalteparin is dosed at 200 IU/kg subcutaneously every 24 hours for the first month, and then 150 IU/kg subcutaneously every 24 hours thereafter. Tinzaparin is dosed at 175 IU/kg subcutaneously every 24 hours.
Since the LMWH drugs are renally eliminated, they can accumulate in patients with renal impairment. Patients with renal impairment should be considered for dose reductions if applicable, anti-Xa monitoring, and carefully observed for bleeding. Limited data with both dalteparin and tinzaparin, though, suggest less accumulation in patients with severe renal impairment compared to enoxaparin.
Unfractionated Heparin. The UFH drugs are administered via an IV bolus dose of 80 units/kg, followed by a continuous infusion of 18 units/kg/h, titrated to a goal activated partial thromboplastin time. Although LMWHs are preferred, UFH should be considered in certain settings, such as in patients with renal failure, patients with questionable absorption via the subcutaneous route, or patients who are under consideration for systemic thrombolytic therapy.5 Hospitalized patients who are at high risk for bleeding or patients who may undergo invasive procedures are good candidates for UFH over LMWHs. This is due to the fact that UFH can be promptly discontinued and is reversible with protamine sulfate (versus the partial reversibility and longer half-life of LMWH).
Fondaparinux. Fondaparinux is an indirect factor Xa (FXa) inhibitor given subcutaneously. Advantages of fondaparinux include the lack of monitoring required and the ability to use it in patients with a history of heparin-induced thrombocytopenia. Fondaparinux is contraindicated in patients with a creatinine clearance <30 mL/minute. Van Doormaal et al42 found the rate of VTE recurrence was lower with fondaparinux than UFH in the treatment of PE. In another study conducted by Akl et al,46 the rate of VTE recurrence was higher with fondaparinux than LMWH in the treatment of DVT.
Warfarin. Warfarin is very problematic in cancer patients due to the need for frequent laboratory monitoring, numerous drug-drug and drug-nutrient interactions, slow onset of action, and long duration of action. Although it has shown inferiority in cancer patients when compared to LMWH, it may still be an option for long-term treatment, especially for patients with financial barriers, adversity to injections, or renal failure. Due to the slow onset of action of warfarin (steady state is not achieved for 5-7 days), it is important to bridge with a parenteral anticoagulant until therapeutic International normalized ratio is achieved.
Apixaban and Rivaroxaban. Both of these agents are direct FXa inhibitors and can be classified as new oral anticoagulants. Although initial evidence is promising and these agents may be attractive due to feasibility of oral administration without required monitoring, they are currently not recommended for initial therapy in cancer patients. Current published trials have included very few cancer patients, thus limiting the authors’ ability to extrapolate results to the cancer population. Also, these anticoagulants lack reliable agents for reversal in cases of life-threatening bleeding complications.
Thrombolytics. These agents promote rapid clot lysis, reduce venous outflow obstruction, and prevent venous valvular dysfunction, which may help to limit long-term complications such as postthrombotic syndrome. No significant differences in reduction of recurrent PE, death, or major bleeding were found in a recent meta-analysis comparing heparin and thrombolytic agents in patients with acute PE.47 However, thrombolytic therapy is recommended for massive PE (ie, defined as PE with a systolic blood pressure <90 mm Hg).48 There is also growing evidence to support the use of thrombolytics in certain populations for submassive or moderate PE.2,5,48 Alteplase 100 mg via IV infusion over 2 hours can be given for thrombolysis in these settings. Absolute contraindications to thrombolysis include, but are not limited to severe, uncontrolled hypertension; known intracranial (IC) neoplasm or aneurysm; internal bleeding; or recent IC surgery or trauma (Table 3).
Nonpharmacological Treatment
Inferior Vena Cava Filters. In patients with contraindication to therapeutic anticoagulation, an inferior vena cava (IVC) filter can be inserted for prevention of PE.49 Insertion of an IVC filter should be assessed on a case-by-case basis after evaluation of the risk-benefit ratio. If contraindications to anticoagulant therapy are no longer present, anticoagulant therapy should be initiated.
Other treatment strategies such as catheter-directed thrombolysis and surgical thrombectomy can be considered, especially in patients presenting with severe, gangrenous limbs due to obstructive VTE. Until recently, thrombolytics were delivered systemically through an IV catheter, the main disadvantage of which was increased likelihood of bleeding and reduced efficacy of the therapy. Catheter-directed delivery of thrombolytic agents directly into the clot has allowed more localized targeting of therapy and, in combination with mechanical thrombectomy, results in a significant higher rate of complete clot dissolution than systemic anticoagulation.50
Treatment Recommendation
Per the guidelines outlined above, the preferred therapy in cancer patients with VTE is LMWH. Depending on other factors such as risk of bleeding, renal function, anticipated procedures, and financial barriers, other anticoagulant options are available but treatment should be assessed on a case-by-case basis.
Challenging Cases
Incidental VTE. Several studies have suggested that approximately half of cancer-associated VTE events are detected incidentally on routine CT scans performed for diagnosis, staging, or follow-up.51 A meta-analysis of 12 studies including more than 10,000 patients, had a weighted mean incidental PE prevalence of 3.1% (95% CI, 2.2-4.1%).52 Incidental VTE found on routine CT scans should be treated similarly to patients with symptomatic VTE, as many have subtle clinical symptoms of active disease on further evaluation.
Patients With Thrombocytopenia. The subcommittee on hemostasis and malignancy for the Scientific and Standardization Committee of the International Society on Thrombosis and haemostasis has recently recommended that anticoagulation be administered if the platelet count can be maintained above 50 x 109/L.53 For platelet counts between 20 and 50 x 109/L, half-dose LMWH can be administered with close monitoring for possible bleeding. If the platelet count is less than 20 x 109/L therapeutic doses of anticoagulation should be held. In these cases, the placement of an IVC filter is recommended to prevent PE. Once thrombocytopenia has resolved, it is recommended to initiate or resume therapeutic anticoagulation.2-4,42
Patients With Bleeding. In view of life-threatening bleeding complications, all patients should be assessed on a case-by-case basis and careful monitoring of additional bleeding risk is recommended. In patients with minor bleeding, anticoagulation may be continued as long as close follow-up is available; whereas, in patients with absolute contraindications to anticoagulation, the risk of bleeding likely outweighs the benefit of treatment, and anticoagulants should be withheld.3 The use of IVC filters are also recommended in this population—at least until the bleeding risk subsides and anticoagulation can be initiated.2-4,42
Patients With Brain Tumors. As per the national and international guidelines,3,42 a brain tumor per se is not a contraindication for anticoagulation. Anticoagulation is more effective and safer than filters when maintained in the therapeutic range in patients with brain metastases and VTE.54 Although LMWH is the preferred drug of choice in this group of patients, a decision should be made based on individual clinical assessment. Consideration should also be made for the primary tumor type since brain metastases from certain malignancies, such as melanoma, have higher tendencies to bleed.55
Patients With Renal Failure. Since LMWHs and fondaparinux are dependent on significant renal clearance, these agents should be avoided in patients with a creatinine clearance ≤30 mL/minute. Unfractionated heparin is the drug of choice in this setting and vitamin K antagonists can be started as early as on day 1.42 Fixed-dose or adjusted-dose subcutaneous UFH is also an option of outpatient management of VTE in patients with renal failure.5
Patients With Recurrent VTE on Anticoagulants. Extension of or a new DVT or PE despite being on anticoagulation therapy is defined as anticoagulation failure. Recurrent VTE is common in cancer patients, as seen in 9% of patients from the CLOT trial. Management strategies for recurrent VTE include optimizing or increasing the dose, switching agents, or placing an IVC filter. If patients are currently on once-daily enoxaparin regimens, this should be switched to the twice-daily regimen. Patients on agents other than LMWH should be switched to LMWH if possible, and dosed accurately based on the most recent total body weight and renal function. Although not useful in the emergency setting, an anti-Xa level should be drawn for patients who present with recurrent VTE on LMWH to assess whether patients are in therapeutic range. Peak anti-Xa levels should be checked 4 to 6 hours after the last dose. Lastly, dosing of LMWH could be increased by 20% to 25%, based on safety and efficacy data from a retrospective cohort study.56
Patients With Symptomatic Catheter-Related Thrombosis. For the treatment of symptomatic catheter-related thrombosis in cancer patients, the international clinical practice guidelines recommend use of anticoagulants for a minimum of 3 months.42 The CHEST guidelines suggest that the catheter not be removed if it is functional, not infected, and there is an ongoing need for the catheter.5 Little data exist in this subgroup of patients, but LMWHs are suggested.
Factors one should consider before commencing anticoagulation treatment include patient refusal, lack of therapeutic benefit, quality of life and life expectancy, bleeding risk, and whether anticoagulation is associated with an unreasonable burden.
Outpatient Treatment Criteria. In the absence of renal impairment, high risk of bleeding, or poor social circumstances, patients with DVT can be treated as an outpatient.57 Although recent studies support the safety and efficacy of outpatient PE management in noncancer patients,58,59 there is no study to support this in cancer patients. Several prognostic tools exist to aid the clinician in deciding whether cancer patients with PE can be treated as outpatients or require hospitalization. These prediction tools identify patients at a low in-hospital mortality in which outpatient management is reasonable. A few examples include the Geneva Pulmonary Embolism Prognostic Index, Pulmonary Embolism Severity Index, and the Aujesky and Murugappan prediction tools.57
The Decision Not to Treat
Acute intervention is not the only option in this unique population. Factors one should consider before commencing anticoagulation treatment include patient refusal, lack of therapeutic benefit, quality of life and life expectancy, bleeding risk, and whether anticoagulation is associated with an unreasonable burden.
Conclusion
In view of an increased risk of VTE in cancer patients, an increased level of clinical suspicion should be maintained. Risk factors related to patient, cancer type, treatment, and biochemical markers should be assessed, and the clinician should also consider using the modified Khorana score. Diagnostic imaging studies should also be performed in cases in which there is a high clinical suspicion of VTE.
Regarding treatment, the preferred drug of choice in the acute phase of treatment is LMWH—unless contraindications exist. In cancer patients with VTE who have severe renal failure, thrombocytopenia, brain tumors, or catheter related thrombosis, recommendations are based on best clinical practice, and the clinician should balance the desirable and undesirable effects depending on the bleeding risk versus VTE risk.
Dr Banala is a clinical fellow in the department of emergency medicine, University of Texas MD Anderson Cancer Center, Houston. Dr Wattana is an instructor in the department of emergency medicine, University of Texas MD Anderson Cancer Center, Houston. Ms Ma is a clinical pharmacy specialist in the division of pharmacy, University of Texas MD Anderson Cancer Center, Houston. Dr Todd is professor and chair of the department of emergency medicine, University of Texas MD Anderson Cancer Center. He is also a member of the EMERGENCY MEDICINE editorial board.
- American Cancer Society. Cancer Facts & Figures 2014. http://www.cancer.org/acs/groups/content/@research/documents/webcontent/acspc-042151.pdf. Accessed December 3, 2014. PAGES 1 AND 2
- National Comprehensive Cancer Network (NCCN) clinical practice guidelines - version1.2014, Cancer-Associated Venous Thromboembolic Disease. Available at www.nccn.org.
- Lyman GH, Khorana AA, Kuderer NM, et al; American Society of Clinical Oncology Clinical Practice. Venous thromboembolism prophylaxis and treatment in patients with cancer: American Society of Clinical Oncology clinical practice guideline update. Clin Oncol. 2013;31(17):2189-2204.
- Mandalà M, Falanga A, Roila F; ESMO Guidelines Working Group. Management of venous thromboembolism (VTE) in cancer patients: ESMO Clinical Practice Guidelines. Ann Oncol. 2011;22(Suppl 6):vi85-vi92.
- Kearon C, Akl EA, Comerota AJ, et al; American College of Chest Physicians. Antithrombotic therapy for VTE disease: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012;141(2 Suppl):e419S-e494S.
- Khorana AA. Venous thromboembolism and prognosis in cancer. Thromb Res. 2010;125(6):490-493.
- Khorana AA, Francis CW, eds. Cancer-Associated Thrombosis: New Findings in Translational Science, Prevention, and Treatment. New York, NY: Informa Healthcare; 2007: 17–34.
- Horsted F, West J, Grainge MJ. Risk of venous thromboembolism in patients with cancer: a systematic review and meta-analysis. PLoS Med. 2012;9(7):e1001275).
- Khorana AA, Connolly GC. Assessing risk of venous thromboembolism in the patient with cancer. J Clin Oncol. 2009;27(29):4839-4847.
- Khorana AA. Risk assessment and prophylaxis for vte in cancer patients. J Natl Compr Canc Netw. 2011;9(7):789-797.
- Khorana AA, Kuderer NM, Culakova E, Lyman GH, Francis CW. Development and validation of a predictive model for chemotherapy-associated thrombosis. Blood. 2008;111(10):4902-4907.
- Lee AY, Levine MN. Venous thromboembolism and cancer: risks and outcomes. Circulation. 2003;107(23 Suppl 1):I17-121.
- Carson JL, Kelley MA, Duff A, et al. The clinical course of pulmonary embolism. N Engl J Med. 1992;326(19):1240-1245.
- Sorensen HT, Mellemkjaer L, Olsen JH, Baron JA. Prognosis of cancers associated with venous thromboembolism. N Engl J Med. 2000;343(25):1846-1850.
- Chew HK, Wun T, Harvey D, Zhou H,White RH. Incidence of venous thromboembolism and its effect on survival among patients with common cancers. Arch Intern Med. 2006;166(4);458-464.
- Green KB. Silverstein RL. Hypercoagulability in cancer. Hematol Oncol Clin North Am. 1996;10(2):499-530.
- Hansson PO, Welin L, Tibblin G, Eriksson H. Deep vein thrombosis and pulmonary embolism in the general population. ‘The Study of Men Born in 1913.’ Arch Intern Med. 1997;157(15):1665-1670.
- Walker AJ, Card TR, West J, Crooks C, Grainge MJ. Incidence of venous thromboembolism in patients with cancer – a cohort study using linked United Kingdom databases. Eur J Cancer. 2013;49(6):1404-1413.
- Shea-Budgell MA, Wu CM, Easaw JC. Evidence-based guidance on venous thromboembolism in patients with solid tumours. Curr Oncol. 2014;21(3):e504-e514.
- Mandalà M, Reni M, Cascinu S, et al. Venous thromboembolism predicts poor prognosis in irresectable pancreatic cancer patients. Ann Oncol. 2007;18(10):1660-1665.
- Prandoni P. Antithrombotic strategies in patients with cancer. Thromb Haemost. 1997;78(1):141-144.
- Piccioli A, Lensing AW, Prins MH, et al; SOMIT Investigators Group. Extensive screening for occult malignant disease in idiopathic venous thromboembolism: a prospective randomized clinical trial. J Thromb Haemost. 2004;2(6):884-889.
- Hettiarachchi RJ, Lok J, Prins MH, Büller HR, Prandoni P. Undiagnosed malignancy in patients with deep vein thrombosis: incidence, risk indicators, and diagnosis. Cancer. 1998;83(1):180-185.
- Timp JF, Braekkan SK, Versteeg HH, Cannegiester SC. Epidemiology of cancer-associated venous thrombosis. Blood. 2013;122(10):1712-1723.
- Falanga A, Marchetti M. Venous thromboembolism in the hematologic malignancies. J Clin Oncol. 2009;27(29):4848-4857.
- Fisher B, Costantino JP, Wickerham DL, et al. Tamoxifen for the prevention of breast cancer: current status of the National Surgical Adjuvant Breast and Bowel Project P-1 study. J Natl Cancer Inst. 2005;97(22):1652-1662.
- Manzoli L, De Vito C, Marzuillo C, Boccia A, Villari P. Oral contraceptives and venous thromboembolism: A systematic review and meta-analysis. Drug Saf. 2012;35(3):191-205.
- Leaf AN, Propert K, Corcoran C, et al. Phase III study of combined chemohormonal therapy in metastatic prostate cancer (ECOG 3882): An Eastern Cooperative Oncology Group study. Med Oncol. 2003;20(2):137-146.
- Verso M, Agnelli G. Venous thromboembolism associated with long-term use of central venous catheters in cancer patients. J Clin Oncol. 2003;21(19):3665-3675.
- Chopra V, Anand S, Hickner A, et al. Risk of venous thromboembolism associated with peripherally inserted central catheters: a systematic review and meta-analysis. Lancet. 2013;382(9889):311-325.
- Khorana AA, Kuderer NM, Culakova E, Lyman GH, Francis CW. Development and validation of a predictive model for chemotherapy-associated thrombosis. Blood. 2008; 111(10):4902-4907.
- Khorana AA, Francis CW, Culakowa E, Lyman GH. Risk factors for chemotherapy-associated venous thromboembolism in a prospective observational study. Cancer. 2005;104(12):2822-2829.
- Sohne M, Kruip MJ, Nijkeuter M, et al; Christoper Study Group. Accuracy of clinical decision rule, D-dimer and spiral computed tomography in patients with malignancy, previous venous thromboembolism, COPD or heart failure and in older patients with suspected pulmonary embolism. J Thromb Haemost. 2006;4(5):1042-1046.
- Wells PS, Anderson DR, Rodger M, et al. Excluding pulmonary embolism at the bedside without diagnostic imaging: management of patients with suspected pulmonary embolism presenting to the emergency department by using a simple clinical model and D-dimer. Ann Intern Med. 2001;135(2):98-107.
- Zierler BK. Ultrasonography and diagnosis of venous thromboembolism. Circulation. 2004;109(12 Suppl 1):I-9-I-4.
- Schoepf UJ, Kucher N, Kipfmueller F, Quiroz R, Costello P, Goldhaber SZ. Right ventricular enlargement on chest computed tomography: a predictor of early death in acute pulmonary embolism. Circulation. 2004;110(20):3276-3280.
- Stein PD, Woodard PK, Weg JG, et al; PIOPED II investigators. Diagnostic pathways in acute pulmonary embolism: recommendations of the PIOPED II investigators. Am J Med. 2006;119(12):1048-1055.
- Anderson DR, Kahn SR, Rodger MA, et al. Computed tomographic pulmonary angiography vs ventilation-perfusion lung scanning in patients with suspected pulmonary embolism: a randomized controlled trial. JAMA. 2007;298(23):2743-2753.
- Goldhaber SZ. Pulmonary embolism. In: Braunwald E, Zipes DP, Libby P, eds. Braunwald’s Heart Disease: A Textbook of Cardiovascular Medicine. 6th ed. WB Saunders: Philadelphia, PA; 2006:1894-1895.
- Miniati M, Monti S, Pratali L, et al. Value of transthoracic echocardiography in the diagnosis of pulmonary embolism: results of a prospective study in unselected patients. Am J Med. 2001;110(7):528-535.
- Stein PD, Gottschalk A, Sostman HD, et al. Methods of Prospective Investigation of Pulmonary Embolism Diagnosis III (PIOPED III). Semin Nucl Med. 2008;38(6):462-470.
- Farge D, Debourdeau P, Beckers M, et al. International clinical practice guidelines for the treatment and prophylaxis of venous thromboembolism in patients with cancer. J Thromb Haemost. 2013;11(1):56-70.
- Lee AY, Levine MN, Baker RI, et al; Randomized Comparison of Low-Molecular-Weight Heparin versus Oral Anticoagulant Therapy for the Prevention of Recurrent Venous Thromboembolism in Patients with Cancer (CLOT) Investigators. Low-molecular-weight heparin versus a coumarin for the prevention of recurrent venous thromboembolism in patients with cancer. N Engl J Med. 2003;349(2):146-153.
- Merli G, Spiro TE, Olsson CG, et al; Enoxaparin Clinical Trial Group. Subcutaneous enoxaparin once or twice daily compared with intravenous unfractionated heparin for treatment of venous thromboembolic disease. Ann Intern Med. 2001;134(3):191-202.
- Van Doormaal FF, Raskob GE, Davidson BL, et al. Treatment of venous thromboembolism in patients with cancer: subgroup analysis of the Matisse clinical trials. Thromb Haemost. 2009;101(4):762-769.
- Akl EA, Vasireddi SR, Gunukula S, et al. Anticoagulation for the initial treatment of venous thromboembolism in patients with cancer. Cochrane Database Syst Rev. 2011;15(6)CD006649.
- Wan S, Quinlan DJ, Agnelli G, Eikelboom JW. Thrombolysis compared with heparin for the initial treatment of pulmonary embolism: a meta-analysis of the randomized controlled trials. Circulation. 2004;110(6):744-749.
- Sharifi M, Bay C, Skrocki L, Rahimi F, Mehdipour M; “MOPETT” Investigators. Moderate Pulmonary Embolism treated with thrombolysis (from the MOPETT trial). Am J Cardiol. 2013;111(2):273-277
- Docousus H; PREPIC (Prevention du Risque d’Embolie Pulmonaire par Interruption Cave) Study Group. Eight-year follow-up of patients with permanent vena cava filters in the prevention of pulmonary embolism: the PREPIC (Prevention du Risque d’Embolie Pulmonaire par Interruption Cave) randomized study. Circulation. 2005;112(3):416-422.
- Mewissen MW, Seabrook GR, Meissner MH, Cynamon J, Labropoulos N, Haughton SH. Catheter-directed thrombolysis for lower extremity deep venous thrombosis: report of a national multicenter registry. Radiology. 1999;211(1):39-49.
- Di Nisio M, Ferrante N, De Tursi M, et al. Incidental venous thromboembolism inn ambulatory cancer patients receiving chemotherapy. Thromb Haemost. 2010;104(5):1049-1054.
- Dentali F, Ageno W, Becattini C, et al. Prevalence and clinical history of incidental, asymptomatic pulmonary embolism: A meta-analysis. Thromb Res. 2010;125(6):518-522.
- Lee AY, Carrier M. Treatment of cancer-associated thrombosis: perspectives on the use of novel oral anticoagulants. Thromb Res. 2014;133(Suppl 2):S167-S171.
- Schiff D, DeAngelis LM. Therapy of venous thromboembolism in patients with brain metastases. Cancer. 1994;73(2):493-498.
- Kondziolka D, Bernstein M, Resch L, et al. Significance of hemorrhage into brain tumors: clinicopathological study. J Neurosurg. 1987;67(6):852-857.
- Carrier M, Le Gal G, Cho R, Tierney S, Rodger M, Lee AY. Dose escalation of low molecular weight heparin to manage recurrent venous thromboembolic events despite systemic anticoagulation in cancer patients. J Thromb Haemost. 2009;7(5):760-765.
- Wells PS, Forgie MA, Rodger MA. Treatment of venous thromboembolism. JAMA. 2014;311(7):717-728.
- Erkens PM, Gandara E, Wells PS, et al. Safety of outpatient treatment in acute pulmonary embolism. J Thromb Haemost. 2010;8(11):2412-2417.
- Aujesky D, Roy PM, Verschuren F, et al. Outpatient vs inpatient treatment for patients with acute pulmonary embolism: an international, open-label, randomised, non-inferiority trial. Lancet. 2011;378(9785):41-48.
Case
A 62-year-old man with known adenocarcinoma of the lung with metastasis to the liver and bones presented to the ED complaining of nonproductive cough and progressively worsening shortness of breath for 2 weeks. He was started on crizotinib a week prior to presentation, and had also received multiple courses of oral antibiotics and steroids for presumed exacerbation of chronic obstructive pulmonary disease (COPD). In addition to COPD, the patient had a history of degenerative joint disease, and was previously a chronic smoker. His current medications included tiotropium, inhaled daily; albuterol, two inhalations as needed; and hydrocodone/acetaminophen as needed for pain.
His vital signs on physical examination were: heart rate, 98 beats/minute; blood pressure 138/89 mm Hg; respiratory rate, 20 breaths/minute; temperature, 99.3°F. Oxygen saturation was 84% on room air, which improved to 92% with 2 L of oxygen. He had mild rhonchi on chest auscultation, but normal heart sounds and normal neurological, gastrointestinal, and extremity examination. Laboratory data revealed persistent elevation of leukocytes ranging between 12 and 15 x 109/L; hemoglobin, 9.2 g/dL; and platelet count, 220 x 109/L. A basic metabolic panel was within the normal range. The chest radiograph showed no evidence of pulmonary or cardiac abnormalities.
The patient was given nebulizer therapy with albuterol, along with oral prednisone. He improved with treatment and was discharged with oral prednisone daily for 4 days and oral doxycycline daily for 7 days. A week later, however, the patient had a cardiorespiratory arrest at home and could not be revived. A postmortem examination confirmed bilateral massive pulmonary embolism (PE).
Cancer Prevalence
According to the Cancer Facts & Figures 2014 published by the American Cancer Society, approximately 13.7 million Americans with a history of cancer were alive on January 1, 2012.1 In 2014, there will be an estimated 1,665,540 new cancer diagnoses and 585,720 cancer deaths in the United States.1 Cancer remains the second most common cause of death in the United States, accounting for nearly one out of every four deaths.
As seen in the above case presentation, cancer patients are at an increased risk for developing venous thromboembolism (VTE), the symptoms of which may not always be overt. Thus, with the increasing incidence and prevalence of cancer, enhanced vision and emphasis on education of emergency physicians—especially in relation to comorbidities associated with the disease—will improve care for this unique population. (See Figure 1 for an algorithm outlining the appropriate emergency management of VTE in cancer patients.)
Venous Thromboembolism
Symptoms
Classic clinical symptoms of acute VTE may not be seen in all patients. Therefore, a high level of clinical suspicion should be made on cancer patients presenting with any clinically overt signs or symptoms that could represent acute VTE. Symptoms of DVT include pain or tenderness and swelling of one or more extremities. On physical examination, increased warmth, edema, and erythema are often found. The most common signs and symptoms of PE include dyspnea, tachypnea, and pleuritic chest pain. Tachycardia, hypoxia, cough, and syncope are not present in all cases of acute PE.2
Deep Vein Thrombosis and Pulmonary Embolism
Deep vein thrombosis and PE are manifestations of a single disease entity, namely, VTE. The association between VTE and cancer has been well established since it was first recognized by the French internist Armand Trousseau more than 150 years ago.
Deep vein thrombosis is characterized by a thrombus, usually in one of the deep venous conduits that return blood to the heart from the lower extremities, proximal to and including the popliteal veins. However, emboli can also originate from the pelvic veins, the inferior vena cava, and the upper extremities. Pulmonary embolism is characterized by a fully or partially occluding thrombus in one or more arteries of the lungs. Patients with cancer carry an increased risk of developing VTE because of tumor- and treatment-mediated hypercoagulability.6-8 Table 1 provides a summary of the risk factors associated with cancer-related VTE.
Venous thromboembolism is the second leading noncancer-related cause of death in oncology patients, with infection being the first.9-11 Mortality from an acute thrombotic event is four to eight times greater in patients with cancer than in those without cancer.12-14 Based on its high prevalence (in part due to prothrombotic effects of oncologic therapies) and high mortality rate, awareness of VTE diagnostic approaches and treatment is particularly important in the ED patient with cancer.
Epidemiology
Patients with advanced metastatic disease of the brain, lung, stomach, pancreas, kidneys, uterus, and bladder have the highest 1-year VTE incidence rate, and are at a four to 13 times higher risk of developing VTE compared to those with localized disease.15 Approximately 15% of patients with a malignancy present with VTE at some point during the course of their illness16 compared to 2.5% of the general population.17 Furthermore, an increasing frequency of VTE among cancer patients has been observed over the years due to improved survival.18 The strongest predictor for recurrent VTE is a previous diagnosis of the condition.19 The prognosis of VTE depends on the aggressiveness of the underlying malignancy and the presence of other cancer-associated complications.20
In the postoperative setting, the risk of lower extremity DVT is about twice that of noncancer patients.21 While commonly noted, there is limited evidence to support the relationship between DVT location and cancer. It is reasonable, however, to propose that localized anatomical compression by the tumor of nearby veins causes stasis and subsequent DVT. Bilateral DVT of the lower extremities may be seen with pelvic malignancies, and DVT of the upper extremities is associated with central venous catheters, axillary lymphomas, mediastinal tumor masses, and intravenous (IV) chemotherapy. Idiopathic DVT is a significant risk factor for underlying malignancy, found in approximately 10% of cases.22 In a prospective study of patients with newly diagnosed DVT, Hettiarachchi, et al23 report routine clinical evaluations are sufficient to find underlying malignancies and that extensive screening is unwarranted.
Risk Assessment
The common risk factors of VTE in the general population are older age, female gender, race, prolonged immobilization, hospitalization, previous history of VTE, obesity, heart disease (ie, cardiomyopathy, atrial fibrillation), smoking, poor performance status, familial or acquired hypercoagulability (eg, pregnancy, hormone therapy, indwelling venous catheters). The cancer population, however, is unique and has other risk factors placing patients at risk for developing a VTE. Such risk factors can be grouped into four different categories: patient-related, cancer-related, treatment-related, and biochemical-related.
Patient-Related Risk Factors. These include age, obesity, smoking, decreased mobility, history of previous VTE, comorbidities, and the presence of prothrombotic mutations.
Cancer-Related Risk Factors. Risk factors for VTE depend on the site, stage, and histological type and time from diagnosis of the cancer. Brain tumors and stomach and pancreatic cancers are associated with a high risk of VTE.9,24 Timp, et al24 also studied a strong association between the 1-year relative mortality of a cancer type and its associated thrombogenic potential. In hematologic malignancies, patients with high-grade lymphoma and acute promyelocytic leukemia are at higher risk than other forms of lymphoma or leukemia.25
Treatment-Related Risk Factors. These include anticancer drugs (chemotherapy and hormonal therapy), radiation therapy, recent major surgery, and the presence of a central venous access device (CVAD).
Patients who received chemotherapy with thalidomide, lenalidomide, cisplatin, and platinum were found to be at high risk of developing VTE.9 Hormonal therapies including tamoxifen, raloxifene, oral contraceptives, and diethylstilbestrol phosphate (used in combination with doxorubicin) also increase the risk of VTE in cancer patients.26-28
The incidence of symptomatic catheter-associated thrombosis is shown to be as high as 28.3%.29 A recent meta-analysis of 11 studies showed that peripherally inserted central catheters are associated with a higher risk of DVT than are central venous catheters.30 Mural thrombus extending from the catheter into the lumen of a vessel and leading to partial or total occlusion with or without clinical symptoms is defined as CVAD-associated thrombosis.
Biochemical-Related Risk Factors. The patient’s hemoglobin level, leukocyte, and platelet counts are associated with an increased VTE risk. Patients with a hemoglobin level <10 g/dL and a leukocyte count >11 x 109/L had an almost 2-fold increased risk of developing a VTE in a study conducted by Khorana, et al.31 The Awareness of Neutropenia in Chemotherapy Group Registry Study reported that a platelet count ≥350 x 109/L prior to chemotherapy is associated with an increased risk of VTE.32
Risk Assessment Tools
The modified Korana score is a well-validated risk-assessment model incorporating clinical parameters and laboratory biomarkers to stratify cancer patients according to their propensity to develop VTE, and represents a practical approach for clinical practice.31 The multivariate model identified the five most predictive variables: cancer site, pretreatment platelet count and leukocyte count, hemoglobin level, and body mass index (Table 2). For example, in the case presented, considering this patient’s age, primary site of the cancer, and his laboratory results, he was at a high-risk (up to 40%) for developing VTE.
Diagnosis
In patients with a high clinical suspicion of VTE and without contraindications to anticoagulation (Table 3), early initiation of anticoagulants should be considered while awaiting results from laboratory and imaging studies.5 The diagnostic accuracy for VTE improves with the following tests and scores: complete blood count, platelet count, D-dimer, Wells criteria, Modified Khorana Score, duplex ultrasonography, computed tomography angiography (CTA); and ventilation perfusion (V/Q) scan.
Complete Blood Count and Platelet Count. As previously discussed, a prechemotherapy platelet count ≥350 x 109/L, hemoglobin level <10g/dL, or prechemotherapy leukocyte count >11 x 109/L are good predictive biomarkers to consider VTE in a cancer patient.
D-Dimer. In view of the lack of specificity of D-dimer testing (most cancer patients have elevated D-dimer levels) and the high prevalence of VTE in cancer patients, D-dimer testing is less useful in this setting. The number of false positive D-dimer assays was 3-fold higher in cancer patients compared with noncancer patients.33 Hence, D-dimer testing is not recommended for the diagnosis of VTE in cancer patients.
Wells Criteria. Clinical prediction models such as the Wells criteria for VTE have proven useful in predicting the diagnosis of VTE: 62.5% of the 40 patients who were categorized as high-risk had positive studies; 16.1% of the 492 patients who were classified as moderate-risk and 10.2% of the 219 patients who were classified as low-risk had positive results.34 However, since cancer patients comprised a minority of the subjects in this study, it is unclear whether Wells criteria is as predictive for VTE in this patient population.
Computed Tomography/Computed Tomography Angiography. Computed tomography angiography is the preferred imaging technique for the initial diagnosis of PE in most patients.5 Along with accurate imaging of mediastinal and parenchymal structures and visualization of emboli in the pulmonary vasculature, CTA is also useful to detect signs of right heart enlargement, which can be used in assessing the patient’s risk for adverse clinical outcomes.36 (See Figures 2 and 3 for examples of CT and CTA, demonstrating venous thrombosis and pulmonary thromboembolism.)
Ventilation Perfusion Scan. As it is associated with less fetal radiation exposure than CTA, the V/Q scan is useful for assessing pregnant patients and those with renal insufficiency or untreatable contrast allergies in whom IV contrast is not feasible. A normal V/Q scan essentially rules out PE.38
Chest X-Ray and Electrocardiogram. Both a chest X-ray and electrocardiogram (ECG) facilitate the diagnosis of comorbidities and conditions with clinically similar presentations and can also assist in evaluating existing cardiac conditions. Findings on chest X-ray suspicious for PE include pleural effusion, localized infiltrate, Hamptons hump, and Westermark sign. In ECG, the most common finding in PE is nonspecific ST-T wave changes; a new right heart strain or an S1Q3T3 pattern is suspicious for PE.
Transthoracic Echocardiogram. More than 80% of patients with a documented PE will have some type of right heart abnormality, most commonly direct visualization of the thrombus, right ventricular dilatation, right ventricular hypokinesia with apical sparing, tricuspid regurgitation, abnormal interventricular septal motion, and pulmonary artery dilatation.39
Miniati et al40 have shown that using PE pretest probabilities of 10%, 50%, and 90%, the posttest probabilities with a positive echocardiogram (echo) were 38%, 85%, and 98%. With a negative echo, posttest probabilities were 5%, 33%, and 81%, respectively. The value of transthoracic echo in the diagnosis of PE results were taken from this prospective study in unselected patients.40 Because of the poor sensitivity, echocardiography should not be used as a routine test to screen patients for suspected PE.
Magnetic Resonance Imaging. In pregnant women and in patients with kidney disease, MRI has been used to detect PE. However, the PIOPED III study found that many of the participating centers had difficulty obtaining adequate quality MR angiography (MRA) for suspected PE. The study therefore determined that this modality should be ordered only when there is appropriate expertise in both performing and reading the images, and in patients who have contraindications to standard tests.4
Considering the high risk of developing VTE in the cancer population, there should be a low threshold for diagnostic imaging (ie, CTA and duplex ultrasonography). The patient in this case would have benefited from a CTA to rule-out PE before he was discharged.
Treatment
The National Comprehensive Cancer Network2 (NCCN), American Society of Clinical Oncology3 (ASCO), European Society for Medical Oncology4 (ESMO), International Society on Thrombosis and Haemostasis42 and American College of Chest Physicians5 all have recently published evidence-based clinical practice guidelines for the treatment of established VTE in cancer patients. Once a diagnosis of VTE has been established, immediate treatment with a parenteral anticoagulant should be initiated. For the purpose of this article we shall discuss only the initial treatment of VTE in the emergency setting. The available treatment options are low molecular weight heparin (LMWH), unfractionated heparin (UFH), fondaparinux, warfarin, apixaban and rivaroxaban, and thrombolytic agents.
Low Molecular Weight Heparin. As recommended by the ASCO, NCCN, and ESMO guidelines, the preferred initial treatment for VTE in cancer patients is LMWH. This recommendation stems from the Comparison of Low-Molecular-Weight Heparin versus Oral Anticoagulant Therapy for the Prevention of Recurrent Venous Thromboembolism in Patients with Cancer (CLOT) trial,43 which showed a decreased incidence of recurrent VTE in patients treated with a LMWH versus a coumarin derivative. Recurrent VTE occurred in 17% of patients who received warfarin versus 9% of patients who received dalteparin after 6 months. Numerous other studies evaluating LMWH versus warfarin have shown superior efficacy with LMWH for the treatment of VTE in cancer patients.
Options for LMWH currently available include enoxaparin, dalteparin, and tinzaparin. Enoxaparin is dosed at 1 mg/kg subcutaneously every 12 hours or 1.5 mg/kg subcutaneously every 24 hours. A subgroup analysis of cancer patients with PE showed a higher VTE recurrence rate in those who received once-daily versus twice-daily dosing of enoxaparin in a trial conducted by Merli and colleagues.44 Therefore, it is the authors’ recommendation to utilize the twice-daily regimen of enoxaparin for the treatment of PE in cancer patients during the acute phase of treatment.
Dalteparin is dosed at 200 IU/kg subcutaneously every 24 hours for the first month, and then 150 IU/kg subcutaneously every 24 hours thereafter. Tinzaparin is dosed at 175 IU/kg subcutaneously every 24 hours.
Since the LMWH drugs are renally eliminated, they can accumulate in patients with renal impairment. Patients with renal impairment should be considered for dose reductions if applicable, anti-Xa monitoring, and carefully observed for bleeding. Limited data with both dalteparin and tinzaparin, though, suggest less accumulation in patients with severe renal impairment compared to enoxaparin.
Unfractionated Heparin. The UFH drugs are administered via an IV bolus dose of 80 units/kg, followed by a continuous infusion of 18 units/kg/h, titrated to a goal activated partial thromboplastin time. Although LMWHs are preferred, UFH should be considered in certain settings, such as in patients with renal failure, patients with questionable absorption via the subcutaneous route, or patients who are under consideration for systemic thrombolytic therapy.5 Hospitalized patients who are at high risk for bleeding or patients who may undergo invasive procedures are good candidates for UFH over LMWHs. This is due to the fact that UFH can be promptly discontinued and is reversible with protamine sulfate (versus the partial reversibility and longer half-life of LMWH).
Fondaparinux. Fondaparinux is an indirect factor Xa (FXa) inhibitor given subcutaneously. Advantages of fondaparinux include the lack of monitoring required and the ability to use it in patients with a history of heparin-induced thrombocytopenia. Fondaparinux is contraindicated in patients with a creatinine clearance <30 mL/minute. Van Doormaal et al42 found the rate of VTE recurrence was lower with fondaparinux than UFH in the treatment of PE. In another study conducted by Akl et al,46 the rate of VTE recurrence was higher with fondaparinux than LMWH in the treatment of DVT.
Warfarin. Warfarin is very problematic in cancer patients due to the need for frequent laboratory monitoring, numerous drug-drug and drug-nutrient interactions, slow onset of action, and long duration of action. Although it has shown inferiority in cancer patients when compared to LMWH, it may still be an option for long-term treatment, especially for patients with financial barriers, adversity to injections, or renal failure. Due to the slow onset of action of warfarin (steady state is not achieved for 5-7 days), it is important to bridge with a parenteral anticoagulant until therapeutic International normalized ratio is achieved.
Apixaban and Rivaroxaban. Both of these agents are direct FXa inhibitors and can be classified as new oral anticoagulants. Although initial evidence is promising and these agents may be attractive due to feasibility of oral administration without required monitoring, they are currently not recommended for initial therapy in cancer patients. Current published trials have included very few cancer patients, thus limiting the authors’ ability to extrapolate results to the cancer population. Also, these anticoagulants lack reliable agents for reversal in cases of life-threatening bleeding complications.
Thrombolytics. These agents promote rapid clot lysis, reduce venous outflow obstruction, and prevent venous valvular dysfunction, which may help to limit long-term complications such as postthrombotic syndrome. No significant differences in reduction of recurrent PE, death, or major bleeding were found in a recent meta-analysis comparing heparin and thrombolytic agents in patients with acute PE.47 However, thrombolytic therapy is recommended for massive PE (ie, defined as PE with a systolic blood pressure <90 mm Hg).48 There is also growing evidence to support the use of thrombolytics in certain populations for submassive or moderate PE.2,5,48 Alteplase 100 mg via IV infusion over 2 hours can be given for thrombolysis in these settings. Absolute contraindications to thrombolysis include, but are not limited to severe, uncontrolled hypertension; known intracranial (IC) neoplasm or aneurysm; internal bleeding; or recent IC surgery or trauma (Table 3).
Nonpharmacological Treatment
Inferior Vena Cava Filters. In patients with contraindication to therapeutic anticoagulation, an inferior vena cava (IVC) filter can be inserted for prevention of PE.49 Insertion of an IVC filter should be assessed on a case-by-case basis after evaluation of the risk-benefit ratio. If contraindications to anticoagulant therapy are no longer present, anticoagulant therapy should be initiated.
Other treatment strategies such as catheter-directed thrombolysis and surgical thrombectomy can be considered, especially in patients presenting with severe, gangrenous limbs due to obstructive VTE. Until recently, thrombolytics were delivered systemically through an IV catheter, the main disadvantage of which was increased likelihood of bleeding and reduced efficacy of the therapy. Catheter-directed delivery of thrombolytic agents directly into the clot has allowed more localized targeting of therapy and, in combination with mechanical thrombectomy, results in a significant higher rate of complete clot dissolution than systemic anticoagulation.50
Treatment Recommendation
Per the guidelines outlined above, the preferred therapy in cancer patients with VTE is LMWH. Depending on other factors such as risk of bleeding, renal function, anticipated procedures, and financial barriers, other anticoagulant options are available but treatment should be assessed on a case-by-case basis.
Challenging Cases
Incidental VTE. Several studies have suggested that approximately half of cancer-associated VTE events are detected incidentally on routine CT scans performed for diagnosis, staging, or follow-up.51 A meta-analysis of 12 studies including more than 10,000 patients, had a weighted mean incidental PE prevalence of 3.1% (95% CI, 2.2-4.1%).52 Incidental VTE found on routine CT scans should be treated similarly to patients with symptomatic VTE, as many have subtle clinical symptoms of active disease on further evaluation.
Patients With Thrombocytopenia. The subcommittee on hemostasis and malignancy for the Scientific and Standardization Committee of the International Society on Thrombosis and haemostasis has recently recommended that anticoagulation be administered if the platelet count can be maintained above 50 x 109/L.53 For platelet counts between 20 and 50 x 109/L, half-dose LMWH can be administered with close monitoring for possible bleeding. If the platelet count is less than 20 x 109/L therapeutic doses of anticoagulation should be held. In these cases, the placement of an IVC filter is recommended to prevent PE. Once thrombocytopenia has resolved, it is recommended to initiate or resume therapeutic anticoagulation.2-4,42
Patients With Bleeding. In view of life-threatening bleeding complications, all patients should be assessed on a case-by-case basis and careful monitoring of additional bleeding risk is recommended. In patients with minor bleeding, anticoagulation may be continued as long as close follow-up is available; whereas, in patients with absolute contraindications to anticoagulation, the risk of bleeding likely outweighs the benefit of treatment, and anticoagulants should be withheld.3 The use of IVC filters are also recommended in this population—at least until the bleeding risk subsides and anticoagulation can be initiated.2-4,42
Patients With Brain Tumors. As per the national and international guidelines,3,42 a brain tumor per se is not a contraindication for anticoagulation. Anticoagulation is more effective and safer than filters when maintained in the therapeutic range in patients with brain metastases and VTE.54 Although LMWH is the preferred drug of choice in this group of patients, a decision should be made based on individual clinical assessment. Consideration should also be made for the primary tumor type since brain metastases from certain malignancies, such as melanoma, have higher tendencies to bleed.55
Patients With Renal Failure. Since LMWHs and fondaparinux are dependent on significant renal clearance, these agents should be avoided in patients with a creatinine clearance ≤30 mL/minute. Unfractionated heparin is the drug of choice in this setting and vitamin K antagonists can be started as early as on day 1.42 Fixed-dose or adjusted-dose subcutaneous UFH is also an option of outpatient management of VTE in patients with renal failure.5
Patients With Recurrent VTE on Anticoagulants. Extension of or a new DVT or PE despite being on anticoagulation therapy is defined as anticoagulation failure. Recurrent VTE is common in cancer patients, as seen in 9% of patients from the CLOT trial. Management strategies for recurrent VTE include optimizing or increasing the dose, switching agents, or placing an IVC filter. If patients are currently on once-daily enoxaparin regimens, this should be switched to the twice-daily regimen. Patients on agents other than LMWH should be switched to LMWH if possible, and dosed accurately based on the most recent total body weight and renal function. Although not useful in the emergency setting, an anti-Xa level should be drawn for patients who present with recurrent VTE on LMWH to assess whether patients are in therapeutic range. Peak anti-Xa levels should be checked 4 to 6 hours after the last dose. Lastly, dosing of LMWH could be increased by 20% to 25%, based on safety and efficacy data from a retrospective cohort study.56
Patients With Symptomatic Catheter-Related Thrombosis. For the treatment of symptomatic catheter-related thrombosis in cancer patients, the international clinical practice guidelines recommend use of anticoagulants for a minimum of 3 months.42 The CHEST guidelines suggest that the catheter not be removed if it is functional, not infected, and there is an ongoing need for the catheter.5 Little data exist in this subgroup of patients, but LMWHs are suggested.
Factors one should consider before commencing anticoagulation treatment include patient refusal, lack of therapeutic benefit, quality of life and life expectancy, bleeding risk, and whether anticoagulation is associated with an unreasonable burden.
Outpatient Treatment Criteria. In the absence of renal impairment, high risk of bleeding, or poor social circumstances, patients with DVT can be treated as an outpatient.57 Although recent studies support the safety and efficacy of outpatient PE management in noncancer patients,58,59 there is no study to support this in cancer patients. Several prognostic tools exist to aid the clinician in deciding whether cancer patients with PE can be treated as outpatients or require hospitalization. These prediction tools identify patients at a low in-hospital mortality in which outpatient management is reasonable. A few examples include the Geneva Pulmonary Embolism Prognostic Index, Pulmonary Embolism Severity Index, and the Aujesky and Murugappan prediction tools.57
The Decision Not to Treat
Acute intervention is not the only option in this unique population. Factors one should consider before commencing anticoagulation treatment include patient refusal, lack of therapeutic benefit, quality of life and life expectancy, bleeding risk, and whether anticoagulation is associated with an unreasonable burden.
Conclusion
In view of an increased risk of VTE in cancer patients, an increased level of clinical suspicion should be maintained. Risk factors related to patient, cancer type, treatment, and biochemical markers should be assessed, and the clinician should also consider using the modified Khorana score. Diagnostic imaging studies should also be performed in cases in which there is a high clinical suspicion of VTE.
Regarding treatment, the preferred drug of choice in the acute phase of treatment is LMWH—unless contraindications exist. In cancer patients with VTE who have severe renal failure, thrombocytopenia, brain tumors, or catheter related thrombosis, recommendations are based on best clinical practice, and the clinician should balance the desirable and undesirable effects depending on the bleeding risk versus VTE risk.
Dr Banala is a clinical fellow in the department of emergency medicine, University of Texas MD Anderson Cancer Center, Houston. Dr Wattana is an instructor in the department of emergency medicine, University of Texas MD Anderson Cancer Center, Houston. Ms Ma is a clinical pharmacy specialist in the division of pharmacy, University of Texas MD Anderson Cancer Center, Houston. Dr Todd is professor and chair of the department of emergency medicine, University of Texas MD Anderson Cancer Center. He is also a member of the EMERGENCY MEDICINE editorial board.
Case
A 62-year-old man with known adenocarcinoma of the lung with metastasis to the liver and bones presented to the ED complaining of nonproductive cough and progressively worsening shortness of breath for 2 weeks. He was started on crizotinib a week prior to presentation, and had also received multiple courses of oral antibiotics and steroids for presumed exacerbation of chronic obstructive pulmonary disease (COPD). In addition to COPD, the patient had a history of degenerative joint disease, and was previously a chronic smoker. His current medications included tiotropium, inhaled daily; albuterol, two inhalations as needed; and hydrocodone/acetaminophen as needed for pain.
His vital signs on physical examination were: heart rate, 98 beats/minute; blood pressure 138/89 mm Hg; respiratory rate, 20 breaths/minute; temperature, 99.3°F. Oxygen saturation was 84% on room air, which improved to 92% with 2 L of oxygen. He had mild rhonchi on chest auscultation, but normal heart sounds and normal neurological, gastrointestinal, and extremity examination. Laboratory data revealed persistent elevation of leukocytes ranging between 12 and 15 x 109/L; hemoglobin, 9.2 g/dL; and platelet count, 220 x 109/L. A basic metabolic panel was within the normal range. The chest radiograph showed no evidence of pulmonary or cardiac abnormalities.
The patient was given nebulizer therapy with albuterol, along with oral prednisone. He improved with treatment and was discharged with oral prednisone daily for 4 days and oral doxycycline daily for 7 days. A week later, however, the patient had a cardiorespiratory arrest at home and could not be revived. A postmortem examination confirmed bilateral massive pulmonary embolism (PE).
Cancer Prevalence
According to the Cancer Facts & Figures 2014 published by the American Cancer Society, approximately 13.7 million Americans with a history of cancer were alive on January 1, 2012.1 In 2014, there will be an estimated 1,665,540 new cancer diagnoses and 585,720 cancer deaths in the United States.1 Cancer remains the second most common cause of death in the United States, accounting for nearly one out of every four deaths.
As seen in the above case presentation, cancer patients are at an increased risk for developing venous thromboembolism (VTE), the symptoms of which may not always be overt. Thus, with the increasing incidence and prevalence of cancer, enhanced vision and emphasis on education of emergency physicians—especially in relation to comorbidities associated with the disease—will improve care for this unique population. (See Figure 1 for an algorithm outlining the appropriate emergency management of VTE in cancer patients.)
Venous Thromboembolism
Symptoms
Classic clinical symptoms of acute VTE may not be seen in all patients. Therefore, a high level of clinical suspicion should be made on cancer patients presenting with any clinically overt signs or symptoms that could represent acute VTE. Symptoms of DVT include pain or tenderness and swelling of one or more extremities. On physical examination, increased warmth, edema, and erythema are often found. The most common signs and symptoms of PE include dyspnea, tachypnea, and pleuritic chest pain. Tachycardia, hypoxia, cough, and syncope are not present in all cases of acute PE.2
Deep Vein Thrombosis and Pulmonary Embolism
Deep vein thrombosis and PE are manifestations of a single disease entity, namely, VTE. The association between VTE and cancer has been well established since it was first recognized by the French internist Armand Trousseau more than 150 years ago.
Deep vein thrombosis is characterized by a thrombus, usually in one of the deep venous conduits that return blood to the heart from the lower extremities, proximal to and including the popliteal veins. However, emboli can also originate from the pelvic veins, the inferior vena cava, and the upper extremities. Pulmonary embolism is characterized by a fully or partially occluding thrombus in one or more arteries of the lungs. Patients with cancer carry an increased risk of developing VTE because of tumor- and treatment-mediated hypercoagulability.6-8 Table 1 provides a summary of the risk factors associated with cancer-related VTE.
Venous thromboembolism is the second leading noncancer-related cause of death in oncology patients, with infection being the first.9-11 Mortality from an acute thrombotic event is four to eight times greater in patients with cancer than in those without cancer.12-14 Based on its high prevalence (in part due to prothrombotic effects of oncologic therapies) and high mortality rate, awareness of VTE diagnostic approaches and treatment is particularly important in the ED patient with cancer.
Epidemiology
Patients with advanced metastatic disease of the brain, lung, stomach, pancreas, kidneys, uterus, and bladder have the highest 1-year VTE incidence rate, and are at a four to 13 times higher risk of developing VTE compared to those with localized disease.15 Approximately 15% of patients with a malignancy present with VTE at some point during the course of their illness16 compared to 2.5% of the general population.17 Furthermore, an increasing frequency of VTE among cancer patients has been observed over the years due to improved survival.18 The strongest predictor for recurrent VTE is a previous diagnosis of the condition.19 The prognosis of VTE depends on the aggressiveness of the underlying malignancy and the presence of other cancer-associated complications.20
In the postoperative setting, the risk of lower extremity DVT is about twice that of noncancer patients.21 While commonly noted, there is limited evidence to support the relationship between DVT location and cancer. It is reasonable, however, to propose that localized anatomical compression by the tumor of nearby veins causes stasis and subsequent DVT. Bilateral DVT of the lower extremities may be seen with pelvic malignancies, and DVT of the upper extremities is associated with central venous catheters, axillary lymphomas, mediastinal tumor masses, and intravenous (IV) chemotherapy. Idiopathic DVT is a significant risk factor for underlying malignancy, found in approximately 10% of cases.22 In a prospective study of patients with newly diagnosed DVT, Hettiarachchi, et al23 report routine clinical evaluations are sufficient to find underlying malignancies and that extensive screening is unwarranted.
Risk Assessment
The common risk factors of VTE in the general population are older age, female gender, race, prolonged immobilization, hospitalization, previous history of VTE, obesity, heart disease (ie, cardiomyopathy, atrial fibrillation), smoking, poor performance status, familial or acquired hypercoagulability (eg, pregnancy, hormone therapy, indwelling venous catheters). The cancer population, however, is unique and has other risk factors placing patients at risk for developing a VTE. Such risk factors can be grouped into four different categories: patient-related, cancer-related, treatment-related, and biochemical-related.
Patient-Related Risk Factors. These include age, obesity, smoking, decreased mobility, history of previous VTE, comorbidities, and the presence of prothrombotic mutations.
Cancer-Related Risk Factors. Risk factors for VTE depend on the site, stage, and histological type and time from diagnosis of the cancer. Brain tumors and stomach and pancreatic cancers are associated with a high risk of VTE.9,24 Timp, et al24 also studied a strong association between the 1-year relative mortality of a cancer type and its associated thrombogenic potential. In hematologic malignancies, patients with high-grade lymphoma and acute promyelocytic leukemia are at higher risk than other forms of lymphoma or leukemia.25
Treatment-Related Risk Factors. These include anticancer drugs (chemotherapy and hormonal therapy), radiation therapy, recent major surgery, and the presence of a central venous access device (CVAD).
Patients who received chemotherapy with thalidomide, lenalidomide, cisplatin, and platinum were found to be at high risk of developing VTE.9 Hormonal therapies including tamoxifen, raloxifene, oral contraceptives, and diethylstilbestrol phosphate (used in combination with doxorubicin) also increase the risk of VTE in cancer patients.26-28
The incidence of symptomatic catheter-associated thrombosis is shown to be as high as 28.3%.29 A recent meta-analysis of 11 studies showed that peripherally inserted central catheters are associated with a higher risk of DVT than are central venous catheters.30 Mural thrombus extending from the catheter into the lumen of a vessel and leading to partial or total occlusion with or without clinical symptoms is defined as CVAD-associated thrombosis.
Biochemical-Related Risk Factors. The patient’s hemoglobin level, leukocyte, and platelet counts are associated with an increased VTE risk. Patients with a hemoglobin level <10 g/dL and a leukocyte count >11 x 109/L had an almost 2-fold increased risk of developing a VTE in a study conducted by Khorana, et al.31 The Awareness of Neutropenia in Chemotherapy Group Registry Study reported that a platelet count ≥350 x 109/L prior to chemotherapy is associated with an increased risk of VTE.32
Risk Assessment Tools
The modified Korana score is a well-validated risk-assessment model incorporating clinical parameters and laboratory biomarkers to stratify cancer patients according to their propensity to develop VTE, and represents a practical approach for clinical practice.31 The multivariate model identified the five most predictive variables: cancer site, pretreatment platelet count and leukocyte count, hemoglobin level, and body mass index (Table 2). For example, in the case presented, considering this patient’s age, primary site of the cancer, and his laboratory results, he was at a high-risk (up to 40%) for developing VTE.
Diagnosis
In patients with a high clinical suspicion of VTE and without contraindications to anticoagulation (Table 3), early initiation of anticoagulants should be considered while awaiting results from laboratory and imaging studies.5 The diagnostic accuracy for VTE improves with the following tests and scores: complete blood count, platelet count, D-dimer, Wells criteria, Modified Khorana Score, duplex ultrasonography, computed tomography angiography (CTA); and ventilation perfusion (V/Q) scan.
Complete Blood Count and Platelet Count. As previously discussed, a prechemotherapy platelet count ≥350 x 109/L, hemoglobin level <10g/dL, or prechemotherapy leukocyte count >11 x 109/L are good predictive biomarkers to consider VTE in a cancer patient.
D-Dimer. In view of the lack of specificity of D-dimer testing (most cancer patients have elevated D-dimer levels) and the high prevalence of VTE in cancer patients, D-dimer testing is less useful in this setting. The number of false positive D-dimer assays was 3-fold higher in cancer patients compared with noncancer patients.33 Hence, D-dimer testing is not recommended for the diagnosis of VTE in cancer patients.
Wells Criteria. Clinical prediction models such as the Wells criteria for VTE have proven useful in predicting the diagnosis of VTE: 62.5% of the 40 patients who were categorized as high-risk had positive studies; 16.1% of the 492 patients who were classified as moderate-risk and 10.2% of the 219 patients who were classified as low-risk had positive results.34 However, since cancer patients comprised a minority of the subjects in this study, it is unclear whether Wells criteria is as predictive for VTE in this patient population.
Computed Tomography/Computed Tomography Angiography. Computed tomography angiography is the preferred imaging technique for the initial diagnosis of PE in most patients.5 Along with accurate imaging of mediastinal and parenchymal structures and visualization of emboli in the pulmonary vasculature, CTA is also useful to detect signs of right heart enlargement, which can be used in assessing the patient’s risk for adverse clinical outcomes.36 (See Figures 2 and 3 for examples of CT and CTA, demonstrating venous thrombosis and pulmonary thromboembolism.)
Ventilation Perfusion Scan. As it is associated with less fetal radiation exposure than CTA, the V/Q scan is useful for assessing pregnant patients and those with renal insufficiency or untreatable contrast allergies in whom IV contrast is not feasible. A normal V/Q scan essentially rules out PE.38
Chest X-Ray and Electrocardiogram. Both a chest X-ray and electrocardiogram (ECG) facilitate the diagnosis of comorbidities and conditions with clinically similar presentations and can also assist in evaluating existing cardiac conditions. Findings on chest X-ray suspicious for PE include pleural effusion, localized infiltrate, Hamptons hump, and Westermark sign. In ECG, the most common finding in PE is nonspecific ST-T wave changes; a new right heart strain or an S1Q3T3 pattern is suspicious for PE.
Transthoracic Echocardiogram. More than 80% of patients with a documented PE will have some type of right heart abnormality, most commonly direct visualization of the thrombus, right ventricular dilatation, right ventricular hypokinesia with apical sparing, tricuspid regurgitation, abnormal interventricular septal motion, and pulmonary artery dilatation.39
Miniati et al40 have shown that using PE pretest probabilities of 10%, 50%, and 90%, the posttest probabilities with a positive echocardiogram (echo) were 38%, 85%, and 98%. With a negative echo, posttest probabilities were 5%, 33%, and 81%, respectively. The value of transthoracic echo in the diagnosis of PE results were taken from this prospective study in unselected patients.40 Because of the poor sensitivity, echocardiography should not be used as a routine test to screen patients for suspected PE.
Magnetic Resonance Imaging. In pregnant women and in patients with kidney disease, MRI has been used to detect PE. However, the PIOPED III study found that many of the participating centers had difficulty obtaining adequate quality MR angiography (MRA) for suspected PE. The study therefore determined that this modality should be ordered only when there is appropriate expertise in both performing and reading the images, and in patients who have contraindications to standard tests.4
Considering the high risk of developing VTE in the cancer population, there should be a low threshold for diagnostic imaging (ie, CTA and duplex ultrasonography). The patient in this case would have benefited from a CTA to rule-out PE before he was discharged.
Treatment
The National Comprehensive Cancer Network2 (NCCN), American Society of Clinical Oncology3 (ASCO), European Society for Medical Oncology4 (ESMO), International Society on Thrombosis and Haemostasis42 and American College of Chest Physicians5 all have recently published evidence-based clinical practice guidelines for the treatment of established VTE in cancer patients. Once a diagnosis of VTE has been established, immediate treatment with a parenteral anticoagulant should be initiated. For the purpose of this article we shall discuss only the initial treatment of VTE in the emergency setting. The available treatment options are low molecular weight heparin (LMWH), unfractionated heparin (UFH), fondaparinux, warfarin, apixaban and rivaroxaban, and thrombolytic agents.
Low Molecular Weight Heparin. As recommended by the ASCO, NCCN, and ESMO guidelines, the preferred initial treatment for VTE in cancer patients is LMWH. This recommendation stems from the Comparison of Low-Molecular-Weight Heparin versus Oral Anticoagulant Therapy for the Prevention of Recurrent Venous Thromboembolism in Patients with Cancer (CLOT) trial,43 which showed a decreased incidence of recurrent VTE in patients treated with a LMWH versus a coumarin derivative. Recurrent VTE occurred in 17% of patients who received warfarin versus 9% of patients who received dalteparin after 6 months. Numerous other studies evaluating LMWH versus warfarin have shown superior efficacy with LMWH for the treatment of VTE in cancer patients.
Options for LMWH currently available include enoxaparin, dalteparin, and tinzaparin. Enoxaparin is dosed at 1 mg/kg subcutaneously every 12 hours or 1.5 mg/kg subcutaneously every 24 hours. A subgroup analysis of cancer patients with PE showed a higher VTE recurrence rate in those who received once-daily versus twice-daily dosing of enoxaparin in a trial conducted by Merli and colleagues.44 Therefore, it is the authors’ recommendation to utilize the twice-daily regimen of enoxaparin for the treatment of PE in cancer patients during the acute phase of treatment.
Dalteparin is dosed at 200 IU/kg subcutaneously every 24 hours for the first month, and then 150 IU/kg subcutaneously every 24 hours thereafter. Tinzaparin is dosed at 175 IU/kg subcutaneously every 24 hours.
Since the LMWH drugs are renally eliminated, they can accumulate in patients with renal impairment. Patients with renal impairment should be considered for dose reductions if applicable, anti-Xa monitoring, and carefully observed for bleeding. Limited data with both dalteparin and tinzaparin, though, suggest less accumulation in patients with severe renal impairment compared to enoxaparin.
Unfractionated Heparin. The UFH drugs are administered via an IV bolus dose of 80 units/kg, followed by a continuous infusion of 18 units/kg/h, titrated to a goal activated partial thromboplastin time. Although LMWHs are preferred, UFH should be considered in certain settings, such as in patients with renal failure, patients with questionable absorption via the subcutaneous route, or patients who are under consideration for systemic thrombolytic therapy.5 Hospitalized patients who are at high risk for bleeding or patients who may undergo invasive procedures are good candidates for UFH over LMWHs. This is due to the fact that UFH can be promptly discontinued and is reversible with protamine sulfate (versus the partial reversibility and longer half-life of LMWH).
Fondaparinux. Fondaparinux is an indirect factor Xa (FXa) inhibitor given subcutaneously. Advantages of fondaparinux include the lack of monitoring required and the ability to use it in patients with a history of heparin-induced thrombocytopenia. Fondaparinux is contraindicated in patients with a creatinine clearance <30 mL/minute. Van Doormaal et al42 found the rate of VTE recurrence was lower with fondaparinux than UFH in the treatment of PE. In another study conducted by Akl et al,46 the rate of VTE recurrence was higher with fondaparinux than LMWH in the treatment of DVT.
Warfarin. Warfarin is very problematic in cancer patients due to the need for frequent laboratory monitoring, numerous drug-drug and drug-nutrient interactions, slow onset of action, and long duration of action. Although it has shown inferiority in cancer patients when compared to LMWH, it may still be an option for long-term treatment, especially for patients with financial barriers, adversity to injections, or renal failure. Due to the slow onset of action of warfarin (steady state is not achieved for 5-7 days), it is important to bridge with a parenteral anticoagulant until therapeutic International normalized ratio is achieved.
Apixaban and Rivaroxaban. Both of these agents are direct FXa inhibitors and can be classified as new oral anticoagulants. Although initial evidence is promising and these agents may be attractive due to feasibility of oral administration without required monitoring, they are currently not recommended for initial therapy in cancer patients. Current published trials have included very few cancer patients, thus limiting the authors’ ability to extrapolate results to the cancer population. Also, these anticoagulants lack reliable agents for reversal in cases of life-threatening bleeding complications.
Thrombolytics. These agents promote rapid clot lysis, reduce venous outflow obstruction, and prevent venous valvular dysfunction, which may help to limit long-term complications such as postthrombotic syndrome. No significant differences in reduction of recurrent PE, death, or major bleeding were found in a recent meta-analysis comparing heparin and thrombolytic agents in patients with acute PE.47 However, thrombolytic therapy is recommended for massive PE (ie, defined as PE with a systolic blood pressure <90 mm Hg).48 There is also growing evidence to support the use of thrombolytics in certain populations for submassive or moderate PE.2,5,48 Alteplase 100 mg via IV infusion over 2 hours can be given for thrombolysis in these settings. Absolute contraindications to thrombolysis include, but are not limited to severe, uncontrolled hypertension; known intracranial (IC) neoplasm or aneurysm; internal bleeding; or recent IC surgery or trauma (Table 3).
Nonpharmacological Treatment
Inferior Vena Cava Filters. In patients with contraindication to therapeutic anticoagulation, an inferior vena cava (IVC) filter can be inserted for prevention of PE.49 Insertion of an IVC filter should be assessed on a case-by-case basis after evaluation of the risk-benefit ratio. If contraindications to anticoagulant therapy are no longer present, anticoagulant therapy should be initiated.
Other treatment strategies such as catheter-directed thrombolysis and surgical thrombectomy can be considered, especially in patients presenting with severe, gangrenous limbs due to obstructive VTE. Until recently, thrombolytics were delivered systemically through an IV catheter, the main disadvantage of which was increased likelihood of bleeding and reduced efficacy of the therapy. Catheter-directed delivery of thrombolytic agents directly into the clot has allowed more localized targeting of therapy and, in combination with mechanical thrombectomy, results in a significant higher rate of complete clot dissolution than systemic anticoagulation.50
Treatment Recommendation
Per the guidelines outlined above, the preferred therapy in cancer patients with VTE is LMWH. Depending on other factors such as risk of bleeding, renal function, anticipated procedures, and financial barriers, other anticoagulant options are available but treatment should be assessed on a case-by-case basis.
Challenging Cases
Incidental VTE. Several studies have suggested that approximately half of cancer-associated VTE events are detected incidentally on routine CT scans performed for diagnosis, staging, or follow-up.51 A meta-analysis of 12 studies including more than 10,000 patients, had a weighted mean incidental PE prevalence of 3.1% (95% CI, 2.2-4.1%).52 Incidental VTE found on routine CT scans should be treated similarly to patients with symptomatic VTE, as many have subtle clinical symptoms of active disease on further evaluation.
Patients With Thrombocytopenia. The subcommittee on hemostasis and malignancy for the Scientific and Standardization Committee of the International Society on Thrombosis and haemostasis has recently recommended that anticoagulation be administered if the platelet count can be maintained above 50 x 109/L.53 For platelet counts between 20 and 50 x 109/L, half-dose LMWH can be administered with close monitoring for possible bleeding. If the platelet count is less than 20 x 109/L therapeutic doses of anticoagulation should be held. In these cases, the placement of an IVC filter is recommended to prevent PE. Once thrombocytopenia has resolved, it is recommended to initiate or resume therapeutic anticoagulation.2-4,42
Patients With Bleeding. In view of life-threatening bleeding complications, all patients should be assessed on a case-by-case basis and careful monitoring of additional bleeding risk is recommended. In patients with minor bleeding, anticoagulation may be continued as long as close follow-up is available; whereas, in patients with absolute contraindications to anticoagulation, the risk of bleeding likely outweighs the benefit of treatment, and anticoagulants should be withheld.3 The use of IVC filters are also recommended in this population—at least until the bleeding risk subsides and anticoagulation can be initiated.2-4,42
Patients With Brain Tumors. As per the national and international guidelines,3,42 a brain tumor per se is not a contraindication for anticoagulation. Anticoagulation is more effective and safer than filters when maintained in the therapeutic range in patients with brain metastases and VTE.54 Although LMWH is the preferred drug of choice in this group of patients, a decision should be made based on individual clinical assessment. Consideration should also be made for the primary tumor type since brain metastases from certain malignancies, such as melanoma, have higher tendencies to bleed.55
Patients With Renal Failure. Since LMWHs and fondaparinux are dependent on significant renal clearance, these agents should be avoided in patients with a creatinine clearance ≤30 mL/minute. Unfractionated heparin is the drug of choice in this setting and vitamin K antagonists can be started as early as on day 1.42 Fixed-dose or adjusted-dose subcutaneous UFH is also an option of outpatient management of VTE in patients with renal failure.5
Patients With Recurrent VTE on Anticoagulants. Extension of or a new DVT or PE despite being on anticoagulation therapy is defined as anticoagulation failure. Recurrent VTE is common in cancer patients, as seen in 9% of patients from the CLOT trial. Management strategies for recurrent VTE include optimizing or increasing the dose, switching agents, or placing an IVC filter. If patients are currently on once-daily enoxaparin regimens, this should be switched to the twice-daily regimen. Patients on agents other than LMWH should be switched to LMWH if possible, and dosed accurately based on the most recent total body weight and renal function. Although not useful in the emergency setting, an anti-Xa level should be drawn for patients who present with recurrent VTE on LMWH to assess whether patients are in therapeutic range. Peak anti-Xa levels should be checked 4 to 6 hours after the last dose. Lastly, dosing of LMWH could be increased by 20% to 25%, based on safety and efficacy data from a retrospective cohort study.56
Patients With Symptomatic Catheter-Related Thrombosis. For the treatment of symptomatic catheter-related thrombosis in cancer patients, the international clinical practice guidelines recommend use of anticoagulants for a minimum of 3 months.42 The CHEST guidelines suggest that the catheter not be removed if it is functional, not infected, and there is an ongoing need for the catheter.5 Little data exist in this subgroup of patients, but LMWHs are suggested.
Factors one should consider before commencing anticoagulation treatment include patient refusal, lack of therapeutic benefit, quality of life and life expectancy, bleeding risk, and whether anticoagulation is associated with an unreasonable burden.
Outpatient Treatment Criteria. In the absence of renal impairment, high risk of bleeding, or poor social circumstances, patients with DVT can be treated as an outpatient.57 Although recent studies support the safety and efficacy of outpatient PE management in noncancer patients,58,59 there is no study to support this in cancer patients. Several prognostic tools exist to aid the clinician in deciding whether cancer patients with PE can be treated as outpatients or require hospitalization. These prediction tools identify patients at a low in-hospital mortality in which outpatient management is reasonable. A few examples include the Geneva Pulmonary Embolism Prognostic Index, Pulmonary Embolism Severity Index, and the Aujesky and Murugappan prediction tools.57
The Decision Not to Treat
Acute intervention is not the only option in this unique population. Factors one should consider before commencing anticoagulation treatment include patient refusal, lack of therapeutic benefit, quality of life and life expectancy, bleeding risk, and whether anticoagulation is associated with an unreasonable burden.
Conclusion
In view of an increased risk of VTE in cancer patients, an increased level of clinical suspicion should be maintained. Risk factors related to patient, cancer type, treatment, and biochemical markers should be assessed, and the clinician should also consider using the modified Khorana score. Diagnostic imaging studies should also be performed in cases in which there is a high clinical suspicion of VTE.
Regarding treatment, the preferred drug of choice in the acute phase of treatment is LMWH—unless contraindications exist. In cancer patients with VTE who have severe renal failure, thrombocytopenia, brain tumors, or catheter related thrombosis, recommendations are based on best clinical practice, and the clinician should balance the desirable and undesirable effects depending on the bleeding risk versus VTE risk.
Dr Banala is a clinical fellow in the department of emergency medicine, University of Texas MD Anderson Cancer Center, Houston. Dr Wattana is an instructor in the department of emergency medicine, University of Texas MD Anderson Cancer Center, Houston. Ms Ma is a clinical pharmacy specialist in the division of pharmacy, University of Texas MD Anderson Cancer Center, Houston. Dr Todd is professor and chair of the department of emergency medicine, University of Texas MD Anderson Cancer Center. He is also a member of the EMERGENCY MEDICINE editorial board.
- American Cancer Society. Cancer Facts & Figures 2014. http://www.cancer.org/acs/groups/content/@research/documents/webcontent/acspc-042151.pdf. Accessed December 3, 2014. PAGES 1 AND 2
- National Comprehensive Cancer Network (NCCN) clinical practice guidelines - version1.2014, Cancer-Associated Venous Thromboembolic Disease. Available at www.nccn.org.
- Lyman GH, Khorana AA, Kuderer NM, et al; American Society of Clinical Oncology Clinical Practice. Venous thromboembolism prophylaxis and treatment in patients with cancer: American Society of Clinical Oncology clinical practice guideline update. Clin Oncol. 2013;31(17):2189-2204.
- Mandalà M, Falanga A, Roila F; ESMO Guidelines Working Group. Management of venous thromboembolism (VTE) in cancer patients: ESMO Clinical Practice Guidelines. Ann Oncol. 2011;22(Suppl 6):vi85-vi92.
- Kearon C, Akl EA, Comerota AJ, et al; American College of Chest Physicians. Antithrombotic therapy for VTE disease: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012;141(2 Suppl):e419S-e494S.
- Khorana AA. Venous thromboembolism and prognosis in cancer. Thromb Res. 2010;125(6):490-493.
- Khorana AA, Francis CW, eds. Cancer-Associated Thrombosis: New Findings in Translational Science, Prevention, and Treatment. New York, NY: Informa Healthcare; 2007: 17–34.
- Horsted F, West J, Grainge MJ. Risk of venous thromboembolism in patients with cancer: a systematic review and meta-analysis. PLoS Med. 2012;9(7):e1001275).
- Khorana AA, Connolly GC. Assessing risk of venous thromboembolism in the patient with cancer. J Clin Oncol. 2009;27(29):4839-4847.
- Khorana AA. Risk assessment and prophylaxis for vte in cancer patients. J Natl Compr Canc Netw. 2011;9(7):789-797.
- Khorana AA, Kuderer NM, Culakova E, Lyman GH, Francis CW. Development and validation of a predictive model for chemotherapy-associated thrombosis. Blood. 2008;111(10):4902-4907.
- Lee AY, Levine MN. Venous thromboembolism and cancer: risks and outcomes. Circulation. 2003;107(23 Suppl 1):I17-121.
- Carson JL, Kelley MA, Duff A, et al. The clinical course of pulmonary embolism. N Engl J Med. 1992;326(19):1240-1245.
- Sorensen HT, Mellemkjaer L, Olsen JH, Baron JA. Prognosis of cancers associated with venous thromboembolism. N Engl J Med. 2000;343(25):1846-1850.
- Chew HK, Wun T, Harvey D, Zhou H,White RH. Incidence of venous thromboembolism and its effect on survival among patients with common cancers. Arch Intern Med. 2006;166(4);458-464.
- Green KB. Silverstein RL. Hypercoagulability in cancer. Hematol Oncol Clin North Am. 1996;10(2):499-530.
- Hansson PO, Welin L, Tibblin G, Eriksson H. Deep vein thrombosis and pulmonary embolism in the general population. ‘The Study of Men Born in 1913.’ Arch Intern Med. 1997;157(15):1665-1670.
- Walker AJ, Card TR, West J, Crooks C, Grainge MJ. Incidence of venous thromboembolism in patients with cancer – a cohort study using linked United Kingdom databases. Eur J Cancer. 2013;49(6):1404-1413.
- Shea-Budgell MA, Wu CM, Easaw JC. Evidence-based guidance on venous thromboembolism in patients with solid tumours. Curr Oncol. 2014;21(3):e504-e514.
- Mandalà M, Reni M, Cascinu S, et al. Venous thromboembolism predicts poor prognosis in irresectable pancreatic cancer patients. Ann Oncol. 2007;18(10):1660-1665.
- Prandoni P. Antithrombotic strategies in patients with cancer. Thromb Haemost. 1997;78(1):141-144.
- Piccioli A, Lensing AW, Prins MH, et al; SOMIT Investigators Group. Extensive screening for occult malignant disease in idiopathic venous thromboembolism: a prospective randomized clinical trial. J Thromb Haemost. 2004;2(6):884-889.
- Hettiarachchi RJ, Lok J, Prins MH, Büller HR, Prandoni P. Undiagnosed malignancy in patients with deep vein thrombosis: incidence, risk indicators, and diagnosis. Cancer. 1998;83(1):180-185.
- Timp JF, Braekkan SK, Versteeg HH, Cannegiester SC. Epidemiology of cancer-associated venous thrombosis. Blood. 2013;122(10):1712-1723.
- Falanga A, Marchetti M. Venous thromboembolism in the hematologic malignancies. J Clin Oncol. 2009;27(29):4848-4857.
- Fisher B, Costantino JP, Wickerham DL, et al. Tamoxifen for the prevention of breast cancer: current status of the National Surgical Adjuvant Breast and Bowel Project P-1 study. J Natl Cancer Inst. 2005;97(22):1652-1662.
- Manzoli L, De Vito C, Marzuillo C, Boccia A, Villari P. Oral contraceptives and venous thromboembolism: A systematic review and meta-analysis. Drug Saf. 2012;35(3):191-205.
- Leaf AN, Propert K, Corcoran C, et al. Phase III study of combined chemohormonal therapy in metastatic prostate cancer (ECOG 3882): An Eastern Cooperative Oncology Group study. Med Oncol. 2003;20(2):137-146.
- Verso M, Agnelli G. Venous thromboembolism associated with long-term use of central venous catheters in cancer patients. J Clin Oncol. 2003;21(19):3665-3675.
- Chopra V, Anand S, Hickner A, et al. Risk of venous thromboembolism associated with peripherally inserted central catheters: a systematic review and meta-analysis. Lancet. 2013;382(9889):311-325.
- Khorana AA, Kuderer NM, Culakova E, Lyman GH, Francis CW. Development and validation of a predictive model for chemotherapy-associated thrombosis. Blood. 2008; 111(10):4902-4907.
- Khorana AA, Francis CW, Culakowa E, Lyman GH. Risk factors for chemotherapy-associated venous thromboembolism in a prospective observational study. Cancer. 2005;104(12):2822-2829.
- Sohne M, Kruip MJ, Nijkeuter M, et al; Christoper Study Group. Accuracy of clinical decision rule, D-dimer and spiral computed tomography in patients with malignancy, previous venous thromboembolism, COPD or heart failure and in older patients with suspected pulmonary embolism. J Thromb Haemost. 2006;4(5):1042-1046.
- Wells PS, Anderson DR, Rodger M, et al. Excluding pulmonary embolism at the bedside without diagnostic imaging: management of patients with suspected pulmonary embolism presenting to the emergency department by using a simple clinical model and D-dimer. Ann Intern Med. 2001;135(2):98-107.
- Zierler BK. Ultrasonography and diagnosis of venous thromboembolism. Circulation. 2004;109(12 Suppl 1):I-9-I-4.
- Schoepf UJ, Kucher N, Kipfmueller F, Quiroz R, Costello P, Goldhaber SZ. Right ventricular enlargement on chest computed tomography: a predictor of early death in acute pulmonary embolism. Circulation. 2004;110(20):3276-3280.
- Stein PD, Woodard PK, Weg JG, et al; PIOPED II investigators. Diagnostic pathways in acute pulmonary embolism: recommendations of the PIOPED II investigators. Am J Med. 2006;119(12):1048-1055.
- Anderson DR, Kahn SR, Rodger MA, et al. Computed tomographic pulmonary angiography vs ventilation-perfusion lung scanning in patients with suspected pulmonary embolism: a randomized controlled trial. JAMA. 2007;298(23):2743-2753.
- Goldhaber SZ. Pulmonary embolism. In: Braunwald E, Zipes DP, Libby P, eds. Braunwald’s Heart Disease: A Textbook of Cardiovascular Medicine. 6th ed. WB Saunders: Philadelphia, PA; 2006:1894-1895.
- Miniati M, Monti S, Pratali L, et al. Value of transthoracic echocardiography in the diagnosis of pulmonary embolism: results of a prospective study in unselected patients. Am J Med. 2001;110(7):528-535.
- Stein PD, Gottschalk A, Sostman HD, et al. Methods of Prospective Investigation of Pulmonary Embolism Diagnosis III (PIOPED III). Semin Nucl Med. 2008;38(6):462-470.
- Farge D, Debourdeau P, Beckers M, et al. International clinical practice guidelines for the treatment and prophylaxis of venous thromboembolism in patients with cancer. J Thromb Haemost. 2013;11(1):56-70.
- Lee AY, Levine MN, Baker RI, et al; Randomized Comparison of Low-Molecular-Weight Heparin versus Oral Anticoagulant Therapy for the Prevention of Recurrent Venous Thromboembolism in Patients with Cancer (CLOT) Investigators. Low-molecular-weight heparin versus a coumarin for the prevention of recurrent venous thromboembolism in patients with cancer. N Engl J Med. 2003;349(2):146-153.
- Merli G, Spiro TE, Olsson CG, et al; Enoxaparin Clinical Trial Group. Subcutaneous enoxaparin once or twice daily compared with intravenous unfractionated heparin for treatment of venous thromboembolic disease. Ann Intern Med. 2001;134(3):191-202.
- Van Doormaal FF, Raskob GE, Davidson BL, et al. Treatment of venous thromboembolism in patients with cancer: subgroup analysis of the Matisse clinical trials. Thromb Haemost. 2009;101(4):762-769.
- Akl EA, Vasireddi SR, Gunukula S, et al. Anticoagulation for the initial treatment of venous thromboembolism in patients with cancer. Cochrane Database Syst Rev. 2011;15(6)CD006649.
- Wan S, Quinlan DJ, Agnelli G, Eikelboom JW. Thrombolysis compared with heparin for the initial treatment of pulmonary embolism: a meta-analysis of the randomized controlled trials. Circulation. 2004;110(6):744-749.
- Sharifi M, Bay C, Skrocki L, Rahimi F, Mehdipour M; “MOPETT” Investigators. Moderate Pulmonary Embolism treated with thrombolysis (from the MOPETT trial). Am J Cardiol. 2013;111(2):273-277
- Docousus H; PREPIC (Prevention du Risque d’Embolie Pulmonaire par Interruption Cave) Study Group. Eight-year follow-up of patients with permanent vena cava filters in the prevention of pulmonary embolism: the PREPIC (Prevention du Risque d’Embolie Pulmonaire par Interruption Cave) randomized study. Circulation. 2005;112(3):416-422.
- Mewissen MW, Seabrook GR, Meissner MH, Cynamon J, Labropoulos N, Haughton SH. Catheter-directed thrombolysis for lower extremity deep venous thrombosis: report of a national multicenter registry. Radiology. 1999;211(1):39-49.
- Di Nisio M, Ferrante N, De Tursi M, et al. Incidental venous thromboembolism inn ambulatory cancer patients receiving chemotherapy. Thromb Haemost. 2010;104(5):1049-1054.
- Dentali F, Ageno W, Becattini C, et al. Prevalence and clinical history of incidental, asymptomatic pulmonary embolism: A meta-analysis. Thromb Res. 2010;125(6):518-522.
- Lee AY, Carrier M. Treatment of cancer-associated thrombosis: perspectives on the use of novel oral anticoagulants. Thromb Res. 2014;133(Suppl 2):S167-S171.
- Schiff D, DeAngelis LM. Therapy of venous thromboembolism in patients with brain metastases. Cancer. 1994;73(2):493-498.
- Kondziolka D, Bernstein M, Resch L, et al. Significance of hemorrhage into brain tumors: clinicopathological study. J Neurosurg. 1987;67(6):852-857.
- Carrier M, Le Gal G, Cho R, Tierney S, Rodger M, Lee AY. Dose escalation of low molecular weight heparin to manage recurrent venous thromboembolic events despite systemic anticoagulation in cancer patients. J Thromb Haemost. 2009;7(5):760-765.
- Wells PS, Forgie MA, Rodger MA. Treatment of venous thromboembolism. JAMA. 2014;311(7):717-728.
- Erkens PM, Gandara E, Wells PS, et al. Safety of outpatient treatment in acute pulmonary embolism. J Thromb Haemost. 2010;8(11):2412-2417.
- Aujesky D, Roy PM, Verschuren F, et al. Outpatient vs inpatient treatment for patients with acute pulmonary embolism: an international, open-label, randomised, non-inferiority trial. Lancet. 2011;378(9785):41-48.
- American Cancer Society. Cancer Facts & Figures 2014. http://www.cancer.org/acs/groups/content/@research/documents/webcontent/acspc-042151.pdf. Accessed December 3, 2014. PAGES 1 AND 2
- National Comprehensive Cancer Network (NCCN) clinical practice guidelines - version1.2014, Cancer-Associated Venous Thromboembolic Disease. Available at www.nccn.org.
- Lyman GH, Khorana AA, Kuderer NM, et al; American Society of Clinical Oncology Clinical Practice. Venous thromboembolism prophylaxis and treatment in patients with cancer: American Society of Clinical Oncology clinical practice guideline update. Clin Oncol. 2013;31(17):2189-2204.
- Mandalà M, Falanga A, Roila F; ESMO Guidelines Working Group. Management of venous thromboembolism (VTE) in cancer patients: ESMO Clinical Practice Guidelines. Ann Oncol. 2011;22(Suppl 6):vi85-vi92.
- Kearon C, Akl EA, Comerota AJ, et al; American College of Chest Physicians. Antithrombotic therapy for VTE disease: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012;141(2 Suppl):e419S-e494S.
- Khorana AA. Venous thromboembolism and prognosis in cancer. Thromb Res. 2010;125(6):490-493.
- Khorana AA, Francis CW, eds. Cancer-Associated Thrombosis: New Findings in Translational Science, Prevention, and Treatment. New York, NY: Informa Healthcare; 2007: 17–34.
- Horsted F, West J, Grainge MJ. Risk of venous thromboembolism in patients with cancer: a systematic review and meta-analysis. PLoS Med. 2012;9(7):e1001275).
- Khorana AA, Connolly GC. Assessing risk of venous thromboembolism in the patient with cancer. J Clin Oncol. 2009;27(29):4839-4847.
- Khorana AA. Risk assessment and prophylaxis for vte in cancer patients. J Natl Compr Canc Netw. 2011;9(7):789-797.
- Khorana AA, Kuderer NM, Culakova E, Lyman GH, Francis CW. Development and validation of a predictive model for chemotherapy-associated thrombosis. Blood. 2008;111(10):4902-4907.
- Lee AY, Levine MN. Venous thromboembolism and cancer: risks and outcomes. Circulation. 2003;107(23 Suppl 1):I17-121.
- Carson JL, Kelley MA, Duff A, et al. The clinical course of pulmonary embolism. N Engl J Med. 1992;326(19):1240-1245.
- Sorensen HT, Mellemkjaer L, Olsen JH, Baron JA. Prognosis of cancers associated with venous thromboembolism. N Engl J Med. 2000;343(25):1846-1850.
- Chew HK, Wun T, Harvey D, Zhou H,White RH. Incidence of venous thromboembolism and its effect on survival among patients with common cancers. Arch Intern Med. 2006;166(4);458-464.
- Green KB. Silverstein RL. Hypercoagulability in cancer. Hematol Oncol Clin North Am. 1996;10(2):499-530.
- Hansson PO, Welin L, Tibblin G, Eriksson H. Deep vein thrombosis and pulmonary embolism in the general population. ‘The Study of Men Born in 1913.’ Arch Intern Med. 1997;157(15):1665-1670.
- Walker AJ, Card TR, West J, Crooks C, Grainge MJ. Incidence of venous thromboembolism in patients with cancer – a cohort study using linked United Kingdom databases. Eur J Cancer. 2013;49(6):1404-1413.
- Shea-Budgell MA, Wu CM, Easaw JC. Evidence-based guidance on venous thromboembolism in patients with solid tumours. Curr Oncol. 2014;21(3):e504-e514.
- Mandalà M, Reni M, Cascinu S, et al. Venous thromboembolism predicts poor prognosis in irresectable pancreatic cancer patients. Ann Oncol. 2007;18(10):1660-1665.
- Prandoni P. Antithrombotic strategies in patients with cancer. Thromb Haemost. 1997;78(1):141-144.
- Piccioli A, Lensing AW, Prins MH, et al; SOMIT Investigators Group. Extensive screening for occult malignant disease in idiopathic venous thromboembolism: a prospective randomized clinical trial. J Thromb Haemost. 2004;2(6):884-889.
- Hettiarachchi RJ, Lok J, Prins MH, Büller HR, Prandoni P. Undiagnosed malignancy in patients with deep vein thrombosis: incidence, risk indicators, and diagnosis. Cancer. 1998;83(1):180-185.
- Timp JF, Braekkan SK, Versteeg HH, Cannegiester SC. Epidemiology of cancer-associated venous thrombosis. Blood. 2013;122(10):1712-1723.
- Falanga A, Marchetti M. Venous thromboembolism in the hematologic malignancies. J Clin Oncol. 2009;27(29):4848-4857.
- Fisher B, Costantino JP, Wickerham DL, et al. Tamoxifen for the prevention of breast cancer: current status of the National Surgical Adjuvant Breast and Bowel Project P-1 study. J Natl Cancer Inst. 2005;97(22):1652-1662.
- Manzoli L, De Vito C, Marzuillo C, Boccia A, Villari P. Oral contraceptives and venous thromboembolism: A systematic review and meta-analysis. Drug Saf. 2012;35(3):191-205.
- Leaf AN, Propert K, Corcoran C, et al. Phase III study of combined chemohormonal therapy in metastatic prostate cancer (ECOG 3882): An Eastern Cooperative Oncology Group study. Med Oncol. 2003;20(2):137-146.
- Verso M, Agnelli G. Venous thromboembolism associated with long-term use of central venous catheters in cancer patients. J Clin Oncol. 2003;21(19):3665-3675.
- Chopra V, Anand S, Hickner A, et al. Risk of venous thromboembolism associated with peripherally inserted central catheters: a systematic review and meta-analysis. Lancet. 2013;382(9889):311-325.
- Khorana AA, Kuderer NM, Culakova E, Lyman GH, Francis CW. Development and validation of a predictive model for chemotherapy-associated thrombosis. Blood. 2008; 111(10):4902-4907.
- Khorana AA, Francis CW, Culakowa E, Lyman GH. Risk factors for chemotherapy-associated venous thromboembolism in a prospective observational study. Cancer. 2005;104(12):2822-2829.
- Sohne M, Kruip MJ, Nijkeuter M, et al; Christoper Study Group. Accuracy of clinical decision rule, D-dimer and spiral computed tomography in patients with malignancy, previous venous thromboembolism, COPD or heart failure and in older patients with suspected pulmonary embolism. J Thromb Haemost. 2006;4(5):1042-1046.
- Wells PS, Anderson DR, Rodger M, et al. Excluding pulmonary embolism at the bedside without diagnostic imaging: management of patients with suspected pulmonary embolism presenting to the emergency department by using a simple clinical model and D-dimer. Ann Intern Med. 2001;135(2):98-107.
- Zierler BK. Ultrasonography and diagnosis of venous thromboembolism. Circulation. 2004;109(12 Suppl 1):I-9-I-4.
- Schoepf UJ, Kucher N, Kipfmueller F, Quiroz R, Costello P, Goldhaber SZ. Right ventricular enlargement on chest computed tomography: a predictor of early death in acute pulmonary embolism. Circulation. 2004;110(20):3276-3280.
- Stein PD, Woodard PK, Weg JG, et al; PIOPED II investigators. Diagnostic pathways in acute pulmonary embolism: recommendations of the PIOPED II investigators. Am J Med. 2006;119(12):1048-1055.
- Anderson DR, Kahn SR, Rodger MA, et al. Computed tomographic pulmonary angiography vs ventilation-perfusion lung scanning in patients with suspected pulmonary embolism: a randomized controlled trial. JAMA. 2007;298(23):2743-2753.
- Goldhaber SZ. Pulmonary embolism. In: Braunwald E, Zipes DP, Libby P, eds. Braunwald’s Heart Disease: A Textbook of Cardiovascular Medicine. 6th ed. WB Saunders: Philadelphia, PA; 2006:1894-1895.
- Miniati M, Monti S, Pratali L, et al. Value of transthoracic echocardiography in the diagnosis of pulmonary embolism: results of a prospective study in unselected patients. Am J Med. 2001;110(7):528-535.
- Stein PD, Gottschalk A, Sostman HD, et al. Methods of Prospective Investigation of Pulmonary Embolism Diagnosis III (PIOPED III). Semin Nucl Med. 2008;38(6):462-470.
- Farge D, Debourdeau P, Beckers M, et al. International clinical practice guidelines for the treatment and prophylaxis of venous thromboembolism in patients with cancer. J Thromb Haemost. 2013;11(1):56-70.
- Lee AY, Levine MN, Baker RI, et al; Randomized Comparison of Low-Molecular-Weight Heparin versus Oral Anticoagulant Therapy for the Prevention of Recurrent Venous Thromboembolism in Patients with Cancer (CLOT) Investigators. Low-molecular-weight heparin versus a coumarin for the prevention of recurrent venous thromboembolism in patients with cancer. N Engl J Med. 2003;349(2):146-153.
- Merli G, Spiro TE, Olsson CG, et al; Enoxaparin Clinical Trial Group. Subcutaneous enoxaparin once or twice daily compared with intravenous unfractionated heparin for treatment of venous thromboembolic disease. Ann Intern Med. 2001;134(3):191-202.
- Van Doormaal FF, Raskob GE, Davidson BL, et al. Treatment of venous thromboembolism in patients with cancer: subgroup analysis of the Matisse clinical trials. Thromb Haemost. 2009;101(4):762-769.
- Akl EA, Vasireddi SR, Gunukula S, et al. Anticoagulation for the initial treatment of venous thromboembolism in patients with cancer. Cochrane Database Syst Rev. 2011;15(6)CD006649.
- Wan S, Quinlan DJ, Agnelli G, Eikelboom JW. Thrombolysis compared with heparin for the initial treatment of pulmonary embolism: a meta-analysis of the randomized controlled trials. Circulation. 2004;110(6):744-749.
- Sharifi M, Bay C, Skrocki L, Rahimi F, Mehdipour M; “MOPETT” Investigators. Moderate Pulmonary Embolism treated with thrombolysis (from the MOPETT trial). Am J Cardiol. 2013;111(2):273-277
- Docousus H; PREPIC (Prevention du Risque d’Embolie Pulmonaire par Interruption Cave) Study Group. Eight-year follow-up of patients with permanent vena cava filters in the prevention of pulmonary embolism: the PREPIC (Prevention du Risque d’Embolie Pulmonaire par Interruption Cave) randomized study. Circulation. 2005;112(3):416-422.
- Mewissen MW, Seabrook GR, Meissner MH, Cynamon J, Labropoulos N, Haughton SH. Catheter-directed thrombolysis for lower extremity deep venous thrombosis: report of a national multicenter registry. Radiology. 1999;211(1):39-49.
- Di Nisio M, Ferrante N, De Tursi M, et al. Incidental venous thromboembolism inn ambulatory cancer patients receiving chemotherapy. Thromb Haemost. 2010;104(5):1049-1054.
- Dentali F, Ageno W, Becattini C, et al. Prevalence and clinical history of incidental, asymptomatic pulmonary embolism: A meta-analysis. Thromb Res. 2010;125(6):518-522.
- Lee AY, Carrier M. Treatment of cancer-associated thrombosis: perspectives on the use of novel oral anticoagulants. Thromb Res. 2014;133(Suppl 2):S167-S171.
- Schiff D, DeAngelis LM. Therapy of venous thromboembolism in patients with brain metastases. Cancer. 1994;73(2):493-498.
- Kondziolka D, Bernstein M, Resch L, et al. Significance of hemorrhage into brain tumors: clinicopathological study. J Neurosurg. 1987;67(6):852-857.
- Carrier M, Le Gal G, Cho R, Tierney S, Rodger M, Lee AY. Dose escalation of low molecular weight heparin to manage recurrent venous thromboembolic events despite systemic anticoagulation in cancer patients. J Thromb Haemost. 2009;7(5):760-765.
- Wells PS, Forgie MA, Rodger MA. Treatment of venous thromboembolism. JAMA. 2014;311(7):717-728.
- Erkens PM, Gandara E, Wells PS, et al. Safety of outpatient treatment in acute pulmonary embolism. J Thromb Haemost. 2010;8(11):2412-2417.
- Aujesky D, Roy PM, Verschuren F, et al. Outpatient vs inpatient treatment for patients with acute pulmonary embolism: an international, open-label, randomised, non-inferiority trial. Lancet. 2011;378(9785):41-48.