User login
The Role of Computed Tomography in Evaluating Intra-Articular Distal Humerus Fractures
Elbow fractures constitute 7% of all adult fractures, and 30% of these fractures are distal humerus fractures.1,2 Of these, 96% involve disruption of the articular surface.3 Intra-articular distal humerus fracture patterns can be difficult to characterize on plain radiographs, and therefore computed tomography (CT) is often used. The surgeon’s understanding of the fracture pattern and the deforming forces affects choice of surgical approach. In particular, multiplanar fracture patterns, including coronal shear fractures of the capitellum or trochlea, are often difficult to recognize on plain radiographs. Identification of a multiplanar fracture pattern may require a change in approach or fixation. CT is useful for other intra-articular fractures, such as those of the proximal humerus,3-6 but involves increased radiation and cost.
We conducted a study to determine the effect of adding CT evaluation to plain radiographic evaluation on the classification of, and treatment plans for, intra-articular distal humerus fractures. We hypothesized that adding CT images to plain radiographs would change the classification and treatment of these fractures and would improve interobserver agreement on classification and treatment.
Materials and Methods
After obtaining University of Southern California Institutional Review Board approval, we retrospectively studied 30 consecutive cases of adult intra-articular distal humerus fractures treated by Dr. Itamura at a level I trauma center between 1995 and 2008. In each case, the injured elbow was imaged with plain radiography and CT. Multiple machines were used for CT, but all according to the radiology department’s standard protocol. The images were evaluated by 9 independent observers from the same institution: 3 orthopedic surgeons (1 fellowship-trained shoulder/elbow subspecialist, 1 fellowship-trained upper extremity subspecialist, 1 fellowship-trained orthopedic trauma surgeon), 3 shoulder/elbow fellows, and 3 senior residents pursuing upper extremity fellowships on graduation. No observer was involved in the care of any of the patients. All identifying details were removed from the patient information presented to the observers. For each set of images, the observer was asked to classify the fractures according to the Mehne and Matta classification system,7,8 which is the predominant system used at our institution.
Diagrams of this classification system were provided, but there was no formal observer training or calibration. Seven treatment options were presented: (1) open reduction and internal fixation (ORIF) using a posterior approach with olecranon osteotomy, (2) ORIF using a posterior approach, (3) ORIF using a lateral approach, (4) ORIF using a medial approach, (5) ORIF using an anterior/anterolateral approach, (6) total elbow arthroplasty, and (7) nonoperative management. The only clinical data provided were patient age and sex.
Images were evaluated in blinded fashion. Two rounds of evaluation were compared. In round 1, plain radiographs were evaluated; in round 2, the same radiographs plus corresponding 2-dimensional (2-D) CT images. A minimum of 1 month was required between viewing rounds.
Statistical Analysis
Statistical analysis was performed by the Statistical Consultation and Research Center at our institution. Cohen κ was calculated to estimate the reliability of the fracture classification and treatment plan made by different observers on the same occasion (interobserver reliability). Cramer V9 was calculated to estimate the reliability of the fracture classification and treatment plan made by the same observer on separate occasions (intraobserver reliability). It measures the association between the 2 ratings as a percentage of their total variation. The κ value and Cramer V value were also used to evaluate results based on the observers’ training levels. Both κ and Cramer V values are interpreted as follows: .00 to .20 indicates slight agreement; .21 to .40, fair agreement; .41-.60, moderate agreement; .61 to .80, substantial agreement; and ≥.81, almost perfect agreement. Zero represents no agreement, and 1.00 represents perfect agreement.
Results
Overall intraobserver reliability for classification was fair (.393). It was moderate for the treatment plan (.426) between viewing rounds. Residents had the highest Cramer V value at .60 (moderate) for classification reliability, and attending surgeons had the highest value at .52 (moderate) for treatment plan. All 3 groups (residents, fellows, attending surgeons) showed moderate intraobserver agreement for treatment plan (Table 1).
Interobserver reliability did not improve with the addition of CT in round 2. Reliability was fair at both viewing rounds for classification and for treatment. For classification, the overall κ value was .21 for the first round and .20 for the second round. For treatment plan, the overall κ value was .28 for the first round and .27 for the second round. Attending surgeons decreased in agreement with regard to treatment plan with the addition of CT (.46, moderate, to .32, fair). Fellows had only slight agreement for both rounds with regard to classification as well as treatment (Table 2).
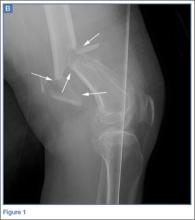
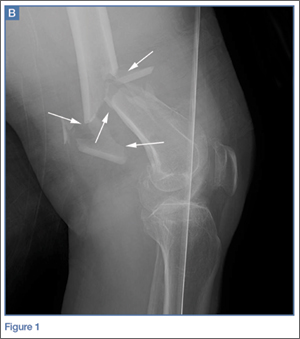
ORIF using a posterior approach with an olecranon osteotomy was the most common choice of treatment method overall at both time points (58.1% and 63.7%) and was still the most common choice when each group of observers (residents, fellows, faculty) was considered separately (Figure 1).
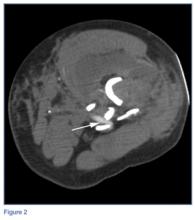
When classifying the fractures, attending surgeons chose the multiplanar fracture pattern 25.6% of the time when viewing radiographs only, and remained consistent in choosing this pattern 23.3% of the time when CT was added to radiographs. Fellows and residents chose this fracture pattern much less often (8.9% and 7.8%, respectively) when viewing radiographs only. Both fellows and residents increased their choice of the multiplanar fracture pattern by 10% (18.9% for fellows, 17.8% for residents) when CT was added (Figure 2).
Overall, the recognition of a multiplanar fracture pattern increased when CT was added. On 30 occasions, an answer was changed from another classification pattern to the multiplanar pattern when CT was added. Only 6 times did an observer change a multiplanar pattern selection at round 1 to another choice at round 2.
Adding CT in round 2 changed the treatment plan for multiplanar fractures. At round 1, 73.7% chose ORIF using a lateral approach for treatment of the multiplanar fracture versus 10.5% who chose ORIF using a posterior approach with an olecranon osteotomy. The choice of the posterior approach with olecranon osteotomy increased to 51.9% at round 2, using the technique we have previously described.5,10
Overall intraobserver reliability for classification was fair (.393). It was moderate for the treatment plan (.426) between viewing rounds. Residents had the highest Cramer V value at .60 (moderate) for classification reliability, and faculty had the highest value at .52 (moderate) for treatment plan. All 3 groups (residents, fellows, attending surgeons) showed moderate intraobserver agreement for treatment plan (Table 1).
Interobserver reliability did not improve with the addition of CT in round 2. Reliability for classification was fair for round 1 and slight for round 2. Reliability was fair at both viewing rounds for treatment. For classification, the overall κ value was .21 for round 1 and .20 for round 2. For treatment plan, the overall κ value was .28 for round 1 and .27 for round 2. Attending surgeons decreased in agreement with regard to treatment plan with the addition of CT (.46, moderate, to .32, fair). Fellows had only slight agreement for both rounds with regard to classification as well as treatment (Table 2).
Discussion
In this study, CT changed both classification and treatment when added to plain radiographs. Interestingly, interobserver reliability did not improve for classification or treatment with the addition of CT. This finding suggests substantial disagreement among qualified observers that is not resolved with more sophisticated imaging. We propose this disagreement is caused by differences in training and experience with specific fracture patterns and surgical approaches.
Our fair to moderate interobserver reliability using radiographs only is consistent with a study by Wainwright and colleagues,11 who demonstrated fair to moderate interobserver reliability with radiographs only using 3 different classification systems. CT did not improve interobserver reliability in the present study.
To our knowledge, the effect of adding CT to plain radiographs on classification and treatment plan has not been evaluated. Doornberg and colleagues2 evaluated the effect of adding 3-dimensional (3-D) CT to a combination of radiographs and 2-D CT. Using the AO (Arbeitsgemeinschaft für Osteosynthesefragen) classification12 and the classification system of Mehne and Matta, they found that 3-D CT improved intraobserver and interobserver reliability for classification but improved only intraobserver agreement for treatment. Interobserver agreement for treatment plan remained fair. In parallel with their study, fracture classification in our study was more often changed with CT than the treatment plan was. Training level appeared not to affect this finding. We found fair interobserver agreement for treatment choice as well, which was not improved by adding CT. Doornberg and colleagues2 concluded that the “relatively small added expense of three-dimensional computed tomography scans seems worthwhile.”
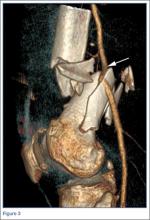
When evaluating specific fracture patterns in the Mehne and Matta classification system, we observed that less experienced surgeons (residents, fellows) were much more likely to identify multiplanar fracture patterns with the aid of CT. Use of CT did not change attending surgeons’ recognition of these multiplanar fractures, suggesting that the faculty were more capable of appreciating these fracture patterns with radiographs only (Figure 3). We also observed that adding CT changed the predominant treatment plan for multiplanar fractures from a lateral approach to a posterior approach with an olecranon osteotomy. Failure to appreciate this component of the fracture before surgery could lead to an increased intraoperative difficulty level. Failure to appreciate it during surgery could lead to unexpected postoperative displacement and ultimately poorer outcome.
There are limitations to our study. There is no gold standard for assessing the accuracy of classification decisions. Intraoperative classification could have served as a gold standard, but the fractures were not routinely assigned a classification during surgery. Brouwer and colleagues13 evaluated the diagnostic accuracy of CT (including 3-D CT) with intraoperative AO classification as a reference point and found improvement in intraobserver agreement but not interobserver agreement when describing fracture characteristics—and no significant effect on classification.
We used a single classification system, the one primarily used at our institution and by Dr. Itamura. There are many systems,7,12,14 all with their strengths and weaknesses, and no one system is used universally. Adding a system would have allowed us to compare results of more than one system. Our aim, however, was to keep our form simple for the sake of participation and completion of the viewings by each volunteer.
Only 2-D CT was used for this study, as 3-D images were not available for all patients. Although this is a potential weakness, it appears that, based on the study by Doornberg and colleagues,2 adding 3-D imaging resulted in only modest improvement in the reliability of classification and no significant improvement in agreement on treatment recommendation.
In addition, our results were likely biased by the fact that 8 of the 9 evaluators were trained by Dr. Itamura, who very often uses a posterior approach with an olecranon osteotomy for internal fixation of distal humerus intra-articular fractures, as previously described.8,10 Therefore, selection of this treatment option may have been overestimated in this study. Nevertheless, after reviewing the literature, Ljungquist and colleagues15 wrote, “There do not seem to be superior functional results associated with any one surgical approach to the distal humerus.”
We did not give the evaluators an indication of patients’ activity demands (only age and sex), which may have been relevant when considering total elbow arthroplasty.
Last, performing another round of evaluations with only plain radiographs, before introducing CT, would have provided intraobserver reliability results on plain radiograph evaluation, which could have been compared with intraobserver reliability when CT was added. Again, this was excluded to encourage participation and create the least cumbersome evaluation experience possible, which was thought appropriate, as this information is already in the literature.
Conclusion
Adding CT changed classifications and treatment plans. Raters were more likely to change their classifications than their treatment plans. The addition of CT did not increase agreement between observers. Despite the added radiation and cost, we recommend performing CT for all intra-articular distal humerus fractures because it improves understanding of the fracture pattern and affects treatment planning, especially for fractures with a coronal shear component, which is often not appreciated on plain radiographs.
1. Anglen J. Distal humerus. J Am Acad Orthop Surg. 2005;13(5):291-297.
2. Doornberg J, Lindenhovius A, Kloen P, van Dijk CN, Zurakowski D, Ring D. Two and three-dimensional computed tomography for the classification and management of distal humerus fractures. Evaluation of reliability and diagnostic accuracy. J Bone Joint Surg Am. 2006;88(8):1795-1801.
3. Pollock JW, Faber KJ, Athwal GS. Distal humerus fractures. Orthop Clin North Am. 2008;39(2):187-200.
4. Castagno AA, Shuman WP, Kilcoyne RF, Haynor DR, Morris ME, Matsen FA. Complex fractures of the proximal humerus: role of CT in treatment. Radiology. 1987;165(3):759-762.
5. Palvanen M, Kannus P, Niemi S, Parkkari J. Secular trends in the osteoporotic fractures of the distal humerus in elderly women. Eur J Epidemiol. 1998;14(2):159-164.
6. Siebenrock KA, Gerber C. The reproducibility of classification of fractures of the proximal end of the humerus. J Bone Joint Surg Am. 1993;75(12):1751-1755.
7. Jupiter JB, Mehne DK. Fractures of the distal humerus. Orthopedics. 1992;15(7):825-833.
8. Zalavras CG, McAllister ET, Singh A, Itamura JM. Operative treatment of intra-articular distal humerus fractures. Am J Orthop. 2007;36(12 suppl):8-12.
9. Cramer H. Mathematical Methods of Statistics. Princeton, NJ: Princeton University Press; 1946.
10. Panossian V, Zalavras C, Mirzayan R, Itamura JM. Intra-articular distal humerus fractures. In: Mirzayan R, Itamura JM, eds. Shoulder and Elbow Trauma. New York, NY: Thieme; 2004:67-78.
11. Wainwright AM, Williams JR, Carr AJ. Interobserver and intraobserver variation in classification systems for fractures of the distal humerus. J Bone Joint Surg Br. 2000;82(5):636-642.
12. Müller ME, Nazarian S, Koch P, Schatzker J. The Comprehensive Classification of Fractures in Long Bones. Berlin, Germany: Springer-Verlag; 1990.
13. Brouwer KM, Lindenhovius AL, Dyer GS, Zurakowski D, Mudgal C, Ring D. Diagnostic accuracy of 2- and 3-dimensional imaging and modeling of distal humerus fractures. J Shoulder Elbow Surg. 2012;21(6):772-776.
14. Riseborough EJ, Radin EL. Intercondylar T fractures of the humerus in the adult. A comparison of operative and non-operative treatment in 29 cases. J Bone Joint Surg Am. 1969;51(1):130-141.
15. Ljungquist KL, Beran MC, Awan H. Effects of surgical approach on functional outcomes of open reduction and internal fixation of intra-articular distal humeral fractures: a systematic review. J Shoulder Elbow Surg. 2012;21(1):126-135.
Elbow fractures constitute 7% of all adult fractures, and 30% of these fractures are distal humerus fractures.1,2 Of these, 96% involve disruption of the articular surface.3 Intra-articular distal humerus fracture patterns can be difficult to characterize on plain radiographs, and therefore computed tomography (CT) is often used. The surgeon’s understanding of the fracture pattern and the deforming forces affects choice of surgical approach. In particular, multiplanar fracture patterns, including coronal shear fractures of the capitellum or trochlea, are often difficult to recognize on plain radiographs. Identification of a multiplanar fracture pattern may require a change in approach or fixation. CT is useful for other intra-articular fractures, such as those of the proximal humerus,3-6 but involves increased radiation and cost.
We conducted a study to determine the effect of adding CT evaluation to plain radiographic evaluation on the classification of, and treatment plans for, intra-articular distal humerus fractures. We hypothesized that adding CT images to plain radiographs would change the classification and treatment of these fractures and would improve interobserver agreement on classification and treatment.
Materials and Methods
After obtaining University of Southern California Institutional Review Board approval, we retrospectively studied 30 consecutive cases of adult intra-articular distal humerus fractures treated by Dr. Itamura at a level I trauma center between 1995 and 2008. In each case, the injured elbow was imaged with plain radiography and CT. Multiple machines were used for CT, but all according to the radiology department’s standard protocol. The images were evaluated by 9 independent observers from the same institution: 3 orthopedic surgeons (1 fellowship-trained shoulder/elbow subspecialist, 1 fellowship-trained upper extremity subspecialist, 1 fellowship-trained orthopedic trauma surgeon), 3 shoulder/elbow fellows, and 3 senior residents pursuing upper extremity fellowships on graduation. No observer was involved in the care of any of the patients. All identifying details were removed from the patient information presented to the observers. For each set of images, the observer was asked to classify the fractures according to the Mehne and Matta classification system,7,8 which is the predominant system used at our institution.
Diagrams of this classification system were provided, but there was no formal observer training or calibration. Seven treatment options were presented: (1) open reduction and internal fixation (ORIF) using a posterior approach with olecranon osteotomy, (2) ORIF using a posterior approach, (3) ORIF using a lateral approach, (4) ORIF using a medial approach, (5) ORIF using an anterior/anterolateral approach, (6) total elbow arthroplasty, and (7) nonoperative management. The only clinical data provided were patient age and sex.
Images were evaluated in blinded fashion. Two rounds of evaluation were compared. In round 1, plain radiographs were evaluated; in round 2, the same radiographs plus corresponding 2-dimensional (2-D) CT images. A minimum of 1 month was required between viewing rounds.
Statistical Analysis
Statistical analysis was performed by the Statistical Consultation and Research Center at our institution. Cohen κ was calculated to estimate the reliability of the fracture classification and treatment plan made by different observers on the same occasion (interobserver reliability). Cramer V9 was calculated to estimate the reliability of the fracture classification and treatment plan made by the same observer on separate occasions (intraobserver reliability). It measures the association between the 2 ratings as a percentage of their total variation. The κ value and Cramer V value were also used to evaluate results based on the observers’ training levels. Both κ and Cramer V values are interpreted as follows: .00 to .20 indicates slight agreement; .21 to .40, fair agreement; .41-.60, moderate agreement; .61 to .80, substantial agreement; and ≥.81, almost perfect agreement. Zero represents no agreement, and 1.00 represents perfect agreement.
Results
Overall intraobserver reliability for classification was fair (.393). It was moderate for the treatment plan (.426) between viewing rounds. Residents had the highest Cramer V value at .60 (moderate) for classification reliability, and attending surgeons had the highest value at .52 (moderate) for treatment plan. All 3 groups (residents, fellows, attending surgeons) showed moderate intraobserver agreement for treatment plan (Table 1).
Interobserver reliability did not improve with the addition of CT in round 2. Reliability was fair at both viewing rounds for classification and for treatment. For classification, the overall κ value was .21 for the first round and .20 for the second round. For treatment plan, the overall κ value was .28 for the first round and .27 for the second round. Attending surgeons decreased in agreement with regard to treatment plan with the addition of CT (.46, moderate, to .32, fair). Fellows had only slight agreement for both rounds with regard to classification as well as treatment (Table 2).
ORIF using a posterior approach with an olecranon osteotomy was the most common choice of treatment method overall at both time points (58.1% and 63.7%) and was still the most common choice when each group of observers (residents, fellows, faculty) was considered separately (Figure 1).
When classifying the fractures, attending surgeons chose the multiplanar fracture pattern 25.6% of the time when viewing radiographs only, and remained consistent in choosing this pattern 23.3% of the time when CT was added to radiographs. Fellows and residents chose this fracture pattern much less often (8.9% and 7.8%, respectively) when viewing radiographs only. Both fellows and residents increased their choice of the multiplanar fracture pattern by 10% (18.9% for fellows, 17.8% for residents) when CT was added (Figure 2).
Overall, the recognition of a multiplanar fracture pattern increased when CT was added. On 30 occasions, an answer was changed from another classification pattern to the multiplanar pattern when CT was added. Only 6 times did an observer change a multiplanar pattern selection at round 1 to another choice at round 2.
Adding CT in round 2 changed the treatment plan for multiplanar fractures. At round 1, 73.7% chose ORIF using a lateral approach for treatment of the multiplanar fracture versus 10.5% who chose ORIF using a posterior approach with an olecranon osteotomy. The choice of the posterior approach with olecranon osteotomy increased to 51.9% at round 2, using the technique we have previously described.5,10
Overall intraobserver reliability for classification was fair (.393). It was moderate for the treatment plan (.426) between viewing rounds. Residents had the highest Cramer V value at .60 (moderate) for classification reliability, and faculty had the highest value at .52 (moderate) for treatment plan. All 3 groups (residents, fellows, attending surgeons) showed moderate intraobserver agreement for treatment plan (Table 1).
Interobserver reliability did not improve with the addition of CT in round 2. Reliability for classification was fair for round 1 and slight for round 2. Reliability was fair at both viewing rounds for treatment. For classification, the overall κ value was .21 for round 1 and .20 for round 2. For treatment plan, the overall κ value was .28 for round 1 and .27 for round 2. Attending surgeons decreased in agreement with regard to treatment plan with the addition of CT (.46, moderate, to .32, fair). Fellows had only slight agreement for both rounds with regard to classification as well as treatment (Table 2).
Discussion
In this study, CT changed both classification and treatment when added to plain radiographs. Interestingly, interobserver reliability did not improve for classification or treatment with the addition of CT. This finding suggests substantial disagreement among qualified observers that is not resolved with more sophisticated imaging. We propose this disagreement is caused by differences in training and experience with specific fracture patterns and surgical approaches.
Our fair to moderate interobserver reliability using radiographs only is consistent with a study by Wainwright and colleagues,11 who demonstrated fair to moderate interobserver reliability with radiographs only using 3 different classification systems. CT did not improve interobserver reliability in the present study.
To our knowledge, the effect of adding CT to plain radiographs on classification and treatment plan has not been evaluated. Doornberg and colleagues2 evaluated the effect of adding 3-dimensional (3-D) CT to a combination of radiographs and 2-D CT. Using the AO (Arbeitsgemeinschaft für Osteosynthesefragen) classification12 and the classification system of Mehne and Matta, they found that 3-D CT improved intraobserver and interobserver reliability for classification but improved only intraobserver agreement for treatment. Interobserver agreement for treatment plan remained fair. In parallel with their study, fracture classification in our study was more often changed with CT than the treatment plan was. Training level appeared not to affect this finding. We found fair interobserver agreement for treatment choice as well, which was not improved by adding CT. Doornberg and colleagues2 concluded that the “relatively small added expense of three-dimensional computed tomography scans seems worthwhile.”
When evaluating specific fracture patterns in the Mehne and Matta classification system, we observed that less experienced surgeons (residents, fellows) were much more likely to identify multiplanar fracture patterns with the aid of CT. Use of CT did not change attending surgeons’ recognition of these multiplanar fractures, suggesting that the faculty were more capable of appreciating these fracture patterns with radiographs only (Figure 3). We also observed that adding CT changed the predominant treatment plan for multiplanar fractures from a lateral approach to a posterior approach with an olecranon osteotomy. Failure to appreciate this component of the fracture before surgery could lead to an increased intraoperative difficulty level. Failure to appreciate it during surgery could lead to unexpected postoperative displacement and ultimately poorer outcome.
There are limitations to our study. There is no gold standard for assessing the accuracy of classification decisions. Intraoperative classification could have served as a gold standard, but the fractures were not routinely assigned a classification during surgery. Brouwer and colleagues13 evaluated the diagnostic accuracy of CT (including 3-D CT) with intraoperative AO classification as a reference point and found improvement in intraobserver agreement but not interobserver agreement when describing fracture characteristics—and no significant effect on classification.
We used a single classification system, the one primarily used at our institution and by Dr. Itamura. There are many systems,7,12,14 all with their strengths and weaknesses, and no one system is used universally. Adding a system would have allowed us to compare results of more than one system. Our aim, however, was to keep our form simple for the sake of participation and completion of the viewings by each volunteer.
Only 2-D CT was used for this study, as 3-D images were not available for all patients. Although this is a potential weakness, it appears that, based on the study by Doornberg and colleagues,2 adding 3-D imaging resulted in only modest improvement in the reliability of classification and no significant improvement in agreement on treatment recommendation.
In addition, our results were likely biased by the fact that 8 of the 9 evaluators were trained by Dr. Itamura, who very often uses a posterior approach with an olecranon osteotomy for internal fixation of distal humerus intra-articular fractures, as previously described.8,10 Therefore, selection of this treatment option may have been overestimated in this study. Nevertheless, after reviewing the literature, Ljungquist and colleagues15 wrote, “There do not seem to be superior functional results associated with any one surgical approach to the distal humerus.”
We did not give the evaluators an indication of patients’ activity demands (only age and sex), which may have been relevant when considering total elbow arthroplasty.
Last, performing another round of evaluations with only plain radiographs, before introducing CT, would have provided intraobserver reliability results on plain radiograph evaluation, which could have been compared with intraobserver reliability when CT was added. Again, this was excluded to encourage participation and create the least cumbersome evaluation experience possible, which was thought appropriate, as this information is already in the literature.
Conclusion
Adding CT changed classifications and treatment plans. Raters were more likely to change their classifications than their treatment plans. The addition of CT did not increase agreement between observers. Despite the added radiation and cost, we recommend performing CT for all intra-articular distal humerus fractures because it improves understanding of the fracture pattern and affects treatment planning, especially for fractures with a coronal shear component, which is often not appreciated on plain radiographs.
Elbow fractures constitute 7% of all adult fractures, and 30% of these fractures are distal humerus fractures.1,2 Of these, 96% involve disruption of the articular surface.3 Intra-articular distal humerus fracture patterns can be difficult to characterize on plain radiographs, and therefore computed tomography (CT) is often used. The surgeon’s understanding of the fracture pattern and the deforming forces affects choice of surgical approach. In particular, multiplanar fracture patterns, including coronal shear fractures of the capitellum or trochlea, are often difficult to recognize on plain radiographs. Identification of a multiplanar fracture pattern may require a change in approach or fixation. CT is useful for other intra-articular fractures, such as those of the proximal humerus,3-6 but involves increased radiation and cost.
We conducted a study to determine the effect of adding CT evaluation to plain radiographic evaluation on the classification of, and treatment plans for, intra-articular distal humerus fractures. We hypothesized that adding CT images to plain radiographs would change the classification and treatment of these fractures and would improve interobserver agreement on classification and treatment.
Materials and Methods
After obtaining University of Southern California Institutional Review Board approval, we retrospectively studied 30 consecutive cases of adult intra-articular distal humerus fractures treated by Dr. Itamura at a level I trauma center between 1995 and 2008. In each case, the injured elbow was imaged with plain radiography and CT. Multiple machines were used for CT, but all according to the radiology department’s standard protocol. The images were evaluated by 9 independent observers from the same institution: 3 orthopedic surgeons (1 fellowship-trained shoulder/elbow subspecialist, 1 fellowship-trained upper extremity subspecialist, 1 fellowship-trained orthopedic trauma surgeon), 3 shoulder/elbow fellows, and 3 senior residents pursuing upper extremity fellowships on graduation. No observer was involved in the care of any of the patients. All identifying details were removed from the patient information presented to the observers. For each set of images, the observer was asked to classify the fractures according to the Mehne and Matta classification system,7,8 which is the predominant system used at our institution.
Diagrams of this classification system were provided, but there was no formal observer training or calibration. Seven treatment options were presented: (1) open reduction and internal fixation (ORIF) using a posterior approach with olecranon osteotomy, (2) ORIF using a posterior approach, (3) ORIF using a lateral approach, (4) ORIF using a medial approach, (5) ORIF using an anterior/anterolateral approach, (6) total elbow arthroplasty, and (7) nonoperative management. The only clinical data provided were patient age and sex.
Images were evaluated in blinded fashion. Two rounds of evaluation were compared. In round 1, plain radiographs were evaluated; in round 2, the same radiographs plus corresponding 2-dimensional (2-D) CT images. A minimum of 1 month was required between viewing rounds.
Statistical Analysis
Statistical analysis was performed by the Statistical Consultation and Research Center at our institution. Cohen κ was calculated to estimate the reliability of the fracture classification and treatment plan made by different observers on the same occasion (interobserver reliability). Cramer V9 was calculated to estimate the reliability of the fracture classification and treatment plan made by the same observer on separate occasions (intraobserver reliability). It measures the association between the 2 ratings as a percentage of their total variation. The κ value and Cramer V value were also used to evaluate results based on the observers’ training levels. Both κ and Cramer V values are interpreted as follows: .00 to .20 indicates slight agreement; .21 to .40, fair agreement; .41-.60, moderate agreement; .61 to .80, substantial agreement; and ≥.81, almost perfect agreement. Zero represents no agreement, and 1.00 represents perfect agreement.
Results
Overall intraobserver reliability for classification was fair (.393). It was moderate for the treatment plan (.426) between viewing rounds. Residents had the highest Cramer V value at .60 (moderate) for classification reliability, and attending surgeons had the highest value at .52 (moderate) for treatment plan. All 3 groups (residents, fellows, attending surgeons) showed moderate intraobserver agreement for treatment plan (Table 1).
Interobserver reliability did not improve with the addition of CT in round 2. Reliability was fair at both viewing rounds for classification and for treatment. For classification, the overall κ value was .21 for the first round and .20 for the second round. For treatment plan, the overall κ value was .28 for the first round and .27 for the second round. Attending surgeons decreased in agreement with regard to treatment plan with the addition of CT (.46, moderate, to .32, fair). Fellows had only slight agreement for both rounds with regard to classification as well as treatment (Table 2).
ORIF using a posterior approach with an olecranon osteotomy was the most common choice of treatment method overall at both time points (58.1% and 63.7%) and was still the most common choice when each group of observers (residents, fellows, faculty) was considered separately (Figure 1).
When classifying the fractures, attending surgeons chose the multiplanar fracture pattern 25.6% of the time when viewing radiographs only, and remained consistent in choosing this pattern 23.3% of the time when CT was added to radiographs. Fellows and residents chose this fracture pattern much less often (8.9% and 7.8%, respectively) when viewing radiographs only. Both fellows and residents increased their choice of the multiplanar fracture pattern by 10% (18.9% for fellows, 17.8% for residents) when CT was added (Figure 2).
Overall, the recognition of a multiplanar fracture pattern increased when CT was added. On 30 occasions, an answer was changed from another classification pattern to the multiplanar pattern when CT was added. Only 6 times did an observer change a multiplanar pattern selection at round 1 to another choice at round 2.
Adding CT in round 2 changed the treatment plan for multiplanar fractures. At round 1, 73.7% chose ORIF using a lateral approach for treatment of the multiplanar fracture versus 10.5% who chose ORIF using a posterior approach with an olecranon osteotomy. The choice of the posterior approach with olecranon osteotomy increased to 51.9% at round 2, using the technique we have previously described.5,10
Overall intraobserver reliability for classification was fair (.393). It was moderate for the treatment plan (.426) between viewing rounds. Residents had the highest Cramer V value at .60 (moderate) for classification reliability, and faculty had the highest value at .52 (moderate) for treatment plan. All 3 groups (residents, fellows, attending surgeons) showed moderate intraobserver agreement for treatment plan (Table 1).
Interobserver reliability did not improve with the addition of CT in round 2. Reliability for classification was fair for round 1 and slight for round 2. Reliability was fair at both viewing rounds for treatment. For classification, the overall κ value was .21 for round 1 and .20 for round 2. For treatment plan, the overall κ value was .28 for round 1 and .27 for round 2. Attending surgeons decreased in agreement with regard to treatment plan with the addition of CT (.46, moderate, to .32, fair). Fellows had only slight agreement for both rounds with regard to classification as well as treatment (Table 2).
Discussion
In this study, CT changed both classification and treatment when added to plain radiographs. Interestingly, interobserver reliability did not improve for classification or treatment with the addition of CT. This finding suggests substantial disagreement among qualified observers that is not resolved with more sophisticated imaging. We propose this disagreement is caused by differences in training and experience with specific fracture patterns and surgical approaches.
Our fair to moderate interobserver reliability using radiographs only is consistent with a study by Wainwright and colleagues,11 who demonstrated fair to moderate interobserver reliability with radiographs only using 3 different classification systems. CT did not improve interobserver reliability in the present study.
To our knowledge, the effect of adding CT to plain radiographs on classification and treatment plan has not been evaluated. Doornberg and colleagues2 evaluated the effect of adding 3-dimensional (3-D) CT to a combination of radiographs and 2-D CT. Using the AO (Arbeitsgemeinschaft für Osteosynthesefragen) classification12 and the classification system of Mehne and Matta, they found that 3-D CT improved intraobserver and interobserver reliability for classification but improved only intraobserver agreement for treatment. Interobserver agreement for treatment plan remained fair. In parallel with their study, fracture classification in our study was more often changed with CT than the treatment plan was. Training level appeared not to affect this finding. We found fair interobserver agreement for treatment choice as well, which was not improved by adding CT. Doornberg and colleagues2 concluded that the “relatively small added expense of three-dimensional computed tomography scans seems worthwhile.”
When evaluating specific fracture patterns in the Mehne and Matta classification system, we observed that less experienced surgeons (residents, fellows) were much more likely to identify multiplanar fracture patterns with the aid of CT. Use of CT did not change attending surgeons’ recognition of these multiplanar fractures, suggesting that the faculty were more capable of appreciating these fracture patterns with radiographs only (Figure 3). We also observed that adding CT changed the predominant treatment plan for multiplanar fractures from a lateral approach to a posterior approach with an olecranon osteotomy. Failure to appreciate this component of the fracture before surgery could lead to an increased intraoperative difficulty level. Failure to appreciate it during surgery could lead to unexpected postoperative displacement and ultimately poorer outcome.
There are limitations to our study. There is no gold standard for assessing the accuracy of classification decisions. Intraoperative classification could have served as a gold standard, but the fractures were not routinely assigned a classification during surgery. Brouwer and colleagues13 evaluated the diagnostic accuracy of CT (including 3-D CT) with intraoperative AO classification as a reference point and found improvement in intraobserver agreement but not interobserver agreement when describing fracture characteristics—and no significant effect on classification.
We used a single classification system, the one primarily used at our institution and by Dr. Itamura. There are many systems,7,12,14 all with their strengths and weaknesses, and no one system is used universally. Adding a system would have allowed us to compare results of more than one system. Our aim, however, was to keep our form simple for the sake of participation and completion of the viewings by each volunteer.
Only 2-D CT was used for this study, as 3-D images were not available for all patients. Although this is a potential weakness, it appears that, based on the study by Doornberg and colleagues,2 adding 3-D imaging resulted in only modest improvement in the reliability of classification and no significant improvement in agreement on treatment recommendation.
In addition, our results were likely biased by the fact that 8 of the 9 evaluators were trained by Dr. Itamura, who very often uses a posterior approach with an olecranon osteotomy for internal fixation of distal humerus intra-articular fractures, as previously described.8,10 Therefore, selection of this treatment option may have been overestimated in this study. Nevertheless, after reviewing the literature, Ljungquist and colleagues15 wrote, “There do not seem to be superior functional results associated with any one surgical approach to the distal humerus.”
We did not give the evaluators an indication of patients’ activity demands (only age and sex), which may have been relevant when considering total elbow arthroplasty.
Last, performing another round of evaluations with only plain radiographs, before introducing CT, would have provided intraobserver reliability results on plain radiograph evaluation, which could have been compared with intraobserver reliability when CT was added. Again, this was excluded to encourage participation and create the least cumbersome evaluation experience possible, which was thought appropriate, as this information is already in the literature.
Conclusion
Adding CT changed classifications and treatment plans. Raters were more likely to change their classifications than their treatment plans. The addition of CT did not increase agreement between observers. Despite the added radiation and cost, we recommend performing CT for all intra-articular distal humerus fractures because it improves understanding of the fracture pattern and affects treatment planning, especially for fractures with a coronal shear component, which is often not appreciated on plain radiographs.
1. Anglen J. Distal humerus. J Am Acad Orthop Surg. 2005;13(5):291-297.
2. Doornberg J, Lindenhovius A, Kloen P, van Dijk CN, Zurakowski D, Ring D. Two and three-dimensional computed tomography for the classification and management of distal humerus fractures. Evaluation of reliability and diagnostic accuracy. J Bone Joint Surg Am. 2006;88(8):1795-1801.
3. Pollock JW, Faber KJ, Athwal GS. Distal humerus fractures. Orthop Clin North Am. 2008;39(2):187-200.
4. Castagno AA, Shuman WP, Kilcoyne RF, Haynor DR, Morris ME, Matsen FA. Complex fractures of the proximal humerus: role of CT in treatment. Radiology. 1987;165(3):759-762.
5. Palvanen M, Kannus P, Niemi S, Parkkari J. Secular trends in the osteoporotic fractures of the distal humerus in elderly women. Eur J Epidemiol. 1998;14(2):159-164.
6. Siebenrock KA, Gerber C. The reproducibility of classification of fractures of the proximal end of the humerus. J Bone Joint Surg Am. 1993;75(12):1751-1755.
7. Jupiter JB, Mehne DK. Fractures of the distal humerus. Orthopedics. 1992;15(7):825-833.
8. Zalavras CG, McAllister ET, Singh A, Itamura JM. Operative treatment of intra-articular distal humerus fractures. Am J Orthop. 2007;36(12 suppl):8-12.
9. Cramer H. Mathematical Methods of Statistics. Princeton, NJ: Princeton University Press; 1946.
10. Panossian V, Zalavras C, Mirzayan R, Itamura JM. Intra-articular distal humerus fractures. In: Mirzayan R, Itamura JM, eds. Shoulder and Elbow Trauma. New York, NY: Thieme; 2004:67-78.
11. Wainwright AM, Williams JR, Carr AJ. Interobserver and intraobserver variation in classification systems for fractures of the distal humerus. J Bone Joint Surg Br. 2000;82(5):636-642.
12. Müller ME, Nazarian S, Koch P, Schatzker J. The Comprehensive Classification of Fractures in Long Bones. Berlin, Germany: Springer-Verlag; 1990.
13. Brouwer KM, Lindenhovius AL, Dyer GS, Zurakowski D, Mudgal C, Ring D. Diagnostic accuracy of 2- and 3-dimensional imaging and modeling of distal humerus fractures. J Shoulder Elbow Surg. 2012;21(6):772-776.
14. Riseborough EJ, Radin EL. Intercondylar T fractures of the humerus in the adult. A comparison of operative and non-operative treatment in 29 cases. J Bone Joint Surg Am. 1969;51(1):130-141.
15. Ljungquist KL, Beran MC, Awan H. Effects of surgical approach on functional outcomes of open reduction and internal fixation of intra-articular distal humeral fractures: a systematic review. J Shoulder Elbow Surg. 2012;21(1):126-135.
1. Anglen J. Distal humerus. J Am Acad Orthop Surg. 2005;13(5):291-297.
2. Doornberg J, Lindenhovius A, Kloen P, van Dijk CN, Zurakowski D, Ring D. Two and three-dimensional computed tomography for the classification and management of distal humerus fractures. Evaluation of reliability and diagnostic accuracy. J Bone Joint Surg Am. 2006;88(8):1795-1801.
3. Pollock JW, Faber KJ, Athwal GS. Distal humerus fractures. Orthop Clin North Am. 2008;39(2):187-200.
4. Castagno AA, Shuman WP, Kilcoyne RF, Haynor DR, Morris ME, Matsen FA. Complex fractures of the proximal humerus: role of CT in treatment. Radiology. 1987;165(3):759-762.
5. Palvanen M, Kannus P, Niemi S, Parkkari J. Secular trends in the osteoporotic fractures of the distal humerus in elderly women. Eur J Epidemiol. 1998;14(2):159-164.
6. Siebenrock KA, Gerber C. The reproducibility of classification of fractures of the proximal end of the humerus. J Bone Joint Surg Am. 1993;75(12):1751-1755.
7. Jupiter JB, Mehne DK. Fractures of the distal humerus. Orthopedics. 1992;15(7):825-833.
8. Zalavras CG, McAllister ET, Singh A, Itamura JM. Operative treatment of intra-articular distal humerus fractures. Am J Orthop. 2007;36(12 suppl):8-12.
9. Cramer H. Mathematical Methods of Statistics. Princeton, NJ: Princeton University Press; 1946.
10. Panossian V, Zalavras C, Mirzayan R, Itamura JM. Intra-articular distal humerus fractures. In: Mirzayan R, Itamura JM, eds. Shoulder and Elbow Trauma. New York, NY: Thieme; 2004:67-78.
11. Wainwright AM, Williams JR, Carr AJ. Interobserver and intraobserver variation in classification systems for fractures of the distal humerus. J Bone Joint Surg Br. 2000;82(5):636-642.
12. Müller ME, Nazarian S, Koch P, Schatzker J. The Comprehensive Classification of Fractures in Long Bones. Berlin, Germany: Springer-Verlag; 1990.
13. Brouwer KM, Lindenhovius AL, Dyer GS, Zurakowski D, Mudgal C, Ring D. Diagnostic accuracy of 2- and 3-dimensional imaging and modeling of distal humerus fractures. J Shoulder Elbow Surg. 2012;21(6):772-776.
14. Riseborough EJ, Radin EL. Intercondylar T fractures of the humerus in the adult. A comparison of operative and non-operative treatment in 29 cases. J Bone Joint Surg Am. 1969;51(1):130-141.
15. Ljungquist KL, Beran MC, Awan H. Effects of surgical approach on functional outcomes of open reduction and internal fixation of intra-articular distal humeral fractures: a systematic review. J Shoulder Elbow Surg. 2012;21(1):126-135.
Safety of Tourniquet Use in Total Knee Arthroplasty in Patients With Radiographic Evidence of Vascular Calcifications
Tourniquets are often used in total knee arthroplasty (TKA) to improve visualization of structures, shorten operative time, reduce intraoperative bleeding, and improve cementing technique. Despite these advantages, controversy remains regarding the safety of tourniquet use. Tourniquets have been associated with nerve palsies, vascular injury, and muscle damage.1-5 Some have hypothesized they may cause venous stasis or direct endothelial damage that may develop into deep vein thrombosis (DVT). Abdel-Salam and Eyres6 found an increased incidence of postoperative wound complications and DVTs associated with tourniquet use.
Moreover, investigators have analyzed the role of tourniquets in populations at high risk for wound complications. DeLaurentis and colleagues7 performed a prospective and retrospective analysis of 1182 TKA patients, 24 (2%) of whom had preexisting peripheral vascular disease (PVD), defined as a history of arterial insufficiency, absent dorsalis pedis and/or absent posterior tibial pulsations, and arterial calcifications. A tourniquet was used in each case. Arterial complications occurred in 6 of the 24 patients with PVD. As expected, the authors found that a history of intermittent claudication, pain at rest, and arterial ulcers resulted in a high risk for vascular complications. Further studies have supported this finding and expanded the list of predisposing factors to include previous vascular surgery and absent and asymmetric pedal pulsations.7-11 Of particular concern to total joint arthroplasty surgeons was the finding by DeLaurentis and colleagues7 that patients with radiographic evidence of calcification of the distal superficial femoral artery and/or popliteal artery were at risk for arterial complications. This finding is also supported by other studies.8,11 In TKA, damage to arterial structures proximal to the surgical field could manifest as impaired postoperative wound healing or an ischemic limb. Wound healing depends on adequate blood flow to the healing tissue, and any damage to arterial or venous structures can theoretically compromise this process.
Added to vascular/wound complications as concerning complications in orthopedic surgery is venous thromboembolism (VTE). The role of tourniquets in the formation of VTEs is controversial. A tourniquet has the potential to increase the risk for DVT because of the stasis of venous blood in the lower limb or possible damage to calcified blood vessels. Callam and colleagues12 studied the connection between artery disease and chronic leg ulcers and found that half the patients diagnosed with peripheral artery disease also had stigmata of chronic venous insufficiency. Therefore, the entities can occur in tandem, and surgeons should keep this in mind.
Here we report on a study we conducted to determine whether tourniquet use in TKA in patients with preexisting radiographic evidence of vascular disease increases the risk for wound complications or VTE.
Patients and Methods
We retrospectively reviewed 461 consecutive primary TKAs (373 patients) performed between January 2007 and June 2012 by 2 attending orthopedic surgeons specializing in adult reconstruction. Medical records and operative reports of 583 patients were examined after receiving institutional review board approval. Of these patients, 373 (64%) had a minimum of 12-month follow-up data available. Twelve months was deemed long enough to discover wound complications or DVTs secondary to the index procedure. Most of these outcomes manifest within the first 3 months after surgery and certainly by 12 months. Follow-up longer than 12 months may become a confounder, as wound complications outside the acute to subacute postoperative window could be related to patients’ underlying PVD and not directly to tourniquet use during surgery. Patient demographics and comorbidities were recorded. Comorbidities were obtained from preoperative medical evaluations and surgeons’ preoperative evaluations. All patients had preoperative palpable dorsalis pedis and posterior tibialis arterial pulses. No patient required preoperative vascular studies based on preoperative examination or comorbidities. No patient had prior vascular bypass surgery or stenting.
TKA was performed in a nonlaminar flow, positive-pressure, high-efficiency particulate air-filtered room with sterile toga/surgical helmet systems. For all patients, a pneumatic thigh tourniquet was applied, and the patient was prepared and draped. After limb exsanguination using a rubber bandage, the limb was elevated and the tourniquet inflated to a pressure of 250 to 300 mm Hg. The tourniquet was released either just before closure or immediately after closure in all cases; it was always let down before placement of final bandages.
Prophylactic chemical anticoagulation consisting of warfarin, aspirin, or enoxaparin was used in all patients and continued for 4 to 6 weeks after surgery. All patients received mechanical DVT prophylaxis with sequential compression devices, and all were mobilized out of bed beginning either the day of surgery or the next day. All patients received perioperative intravenous antibiotics, with the preoperative dose given before tourniquet inflation and the last postoperative dose stopped within 24 hours of surgery.
All patients who had primary TKA underwent preoperative medical evaluation and optimization. The patient’s hospital course was monitored closely, and complications noted by the orthopedic team were documented. Follow-up documentation was retrospectively reviewed for evidence of wound complications or VTE. Wound complications were defined as cellulitis, delayed wound healing, wound dehiscence, and/or periprosthetic joint infection. In the case of VTE, physical examination findings were not sufficient for inclusion. Venous duplex ultrasonography demonstrating the clot was reviewed before inclusion.
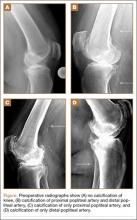
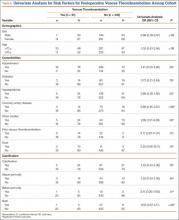
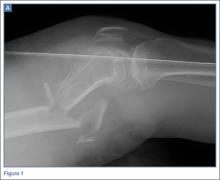
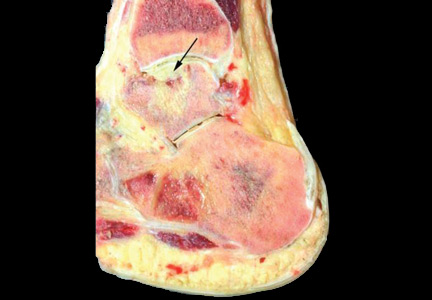
Preoperative radiographs were examined for arterial calcification (Figure). We refer to calcification seen above the knee joint as proximal calcification and to calcification observed below the joint as distal calcification. Patients exhibited calcification proximally only, distally only, or both proximally and distally. The 373 patients were placed into 2 groups based on whether they had preoperative arterial calcification on plain radiography of the knee. One group (285 patients with no radiographic evidence of preoperative knee arterial calcification) underwent 365 TKAs, and the other group (88 patients with radiographic evidence of preoperative knee arterial calcification) underwent 96 TKAs.
A sample size calculation was performed to determine how many patients were needed in each group with 80% power and an α of 0.05. With an estimated difference in VTE/wound complication rate between the calcification and no-calcification groups of 12%, we needed to review 316 TKAs total. This 12% difference was based on study findings of a 25% complication rate in PVD patients who underwent tourniquet-assisted TKA, and the rate of VTE/wound complication after TKA in patients overall, which can be up to 12%.7,13,14 We exceeded minimal enrollment and had 461 TKAs. Descriptive statistics were reported, with means and ranges provided where appropriate. Independent t test was used to evaluate the differences in continuous data (age) between the groups. Univariate analysis (using Pearson χ2 and Fisher exact tests) and multivariate logistic regression analysis were used to evaluate the effects of categorical variables (sex, comorbidity, calcification [presence, absence], and location of calcification [proximal only, distal only, both]) on wound complication and VTE rates. All tests were 2-tailed and performed with a type I error rate of 0.05. Data analysis was performed with SPSS Version 19.0 (SPSS).
Results
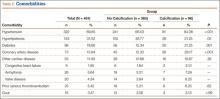
Patient characteristics are summarized in Table 1. Of the 373 patients, 285 lacked calcification, and 88 had calcification. Mean age was 67.73 years (range, 24-92 years) for all patients, 65.99 years (range, 24-89 years) for the no-calcification group, and 74.32 years (range, 54-92 years) for the calcification group; the calcification group demonstrated a trend toward older age, but the difference was not significantly different (P = .07). Of the 373 patients, 156 (41.82%) were male: 110 in the no-calcification group (38.60%) and 46 in the calcification group (52.27%); sex was significantly (P = .002) different between groups, with more males in the calcification group.
Data on total preoperative comorbidities are summarized in Table 2. Hypertension, hyperlipidemia, diabetes, and coronary artery disease (CAD) were the most common comorbidities, and they were all significantly (P ≤ .05) increased in the calcification group.
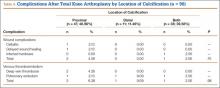
No patients had reported arterial complications, such as arterial bleeding, aneurysm, intimal tears, or loss of distal pulses. Wound complication after TKA was detected in 3.04% of all cases (Table 3). Rate of DVT after TKA was 2.60% of all cases, and rate of pulmonary embolism after TKA was 2.17% of all cases. Of the 96 TKAs with preoperative radiographic evidence of calcification, 47 (48.96%) had proximal calcification only, 11 (11.46%) had distal calcification only, and 38 (39.58%) had both proximal and distal calcification (Table 4). There was no significant difference between the rate of wound complication or VTE based on location of vascular calcification.
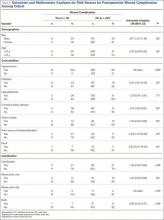
Univariate analysis demonstrated that presence of arterial knee calcification did not increase the risk for postoperative wound complication (odds ratio [OR], 1.04; 95% confidence interval [CI], 0.28-3.80; P > .05) (Table 5). Location of arterial knee calcification also did not increase the risk for postoperative wound complication. In addition, univariate analysis demonstrated that presence of arterial knee calcification did not increase the risk for postoperative VTE (OR, 1.20; 95% CI, 0.43-3.36; P > .05 (Table 6).
Of the 14 wound complications, the most common infections were cellulitis (5/14 cases; 35.71%) and infected hardware that required component revision (5/14 cases; 35.71%). Mean time from TKA to infection was 137.93 days (range, 5-783 days). The most common organism grown in culture from the wound was Staphylococcus (5/14 cases; 35.71%).
Additional univariate statistical analysis revealed that presence of diabetes, hypertension, prior VTE, CAD, and male sex was linked to higher incidence of wound complication (P < .05) (Table 5). When multivariate analysis was performed, hypertension, prior VTE, and male sex remained significant (P < .05) (Table 5).
Discussion
TKA is a safe and effective procedure used to treat osteoarthritis of the knee and improve patients’ quality of life.15 About 700,000 TKAs are performed annually in the United States.16 Because of improvements in preventive medicine and medical technology, life expectancy is increasing, and TKAs are now being performed in higher numbers and in an older patient population. Over the next few decades, these developments will lead to more postoperative complications. It is projected that, by 2030, the need for TKAs in the United States will increase by 673% to 3.48 million.17 Postoperative complications are rare but unfortunately often lead to poor outcomes or even mortality.18 To help minimize the number of postoperative complications, we must understand the safety of tourniquet use in TKA. Other investigators have concluded that tourniquet use is unsafe in patients with preoperative vascular calcifications on plain radiographs.7,8,11 The present study, designed to elucidate whether preoperative evidence of knee arterial calcification may predispose TKA patients to postoperative wound complication or VTE, had some important findings.
In our study, wound complication and VTE occurred in a considerable number of patients after TKA. Despite exceeding the number of patients calculated by the power analysis, our population may have been inadequate to fully detect statistical significance. Thus, our conclusion of failing to reject the null hypothesis may have been because of sample size, a type II error. We found that, after primary TKA, 3.04% of patients developed wound complications and 4.77% VTE. According to the literature, the incidence of infection after primary TKA is between 0.5% and 12%, and that of VTE reported within 3 months after TKA is 1.3% to 10%.13,14 Although we had 100% VTE prophylaxis, meeting the standard of care, VTE after TKA remains a postoperative complication.19 This study also found that a considerable percentage of primary TKA patients (23.59%) had preoperative calcification of the knee arteries. To our knowledge, this study was the first to quantify the incidence of knee arterial calcification in patients who underwent TKA.
Preoperative calcification of the knee arteries in patients who underwent TKA did not increase the risk for wound complication, VTE, or arterial damage. These calcifications, however, do pose an increased systemic vascular risk.20 Calcification of the vascular wall predicts increased cardiovascular risk, independent of classical cardiovascular risk factors.3,18,21-24 Clinically, patients who have both diabetes and calcifications are at significant excess risk for total mortality, stroke mortality, and cardiovascular mortality, compared with patients with diabetes but without such calcifications. They also had a significantly higher incidence of coronary heart disease events, stroke events, and lower extremity amputations.25,26
All our patients underwent tourniquet-assisted TKA. Although previous studies have indicated that tourniquet use may increase arterial complications and wound complications or even limb loss in patients with calcified arteries, we did not find this link.7,27 Our population had no reported arterial complications related to tourniquet use. Other, smaller studies have had similar findings. Vandenbussche and colleagues28 prospectively studied 80 TKA cases randomized to tourniquet use or no tourniquet use and found no postoperative nerve palsies, wound infections, wound healing problems, or hematomas. Our study is also in accord with studies that have reported tourniquet use did not increase risk for DVT.29 Therefore, unlike earlier data, our data demonstrated that tourniquet use in patients with knee arterial calcification was safe.7,27,30,31
Patients with calcification were more likely to have the medical comorbidities of hypertension, diabetes, hyperlipidemia, and CAD. All these comorbidities are linked to the development of arterial calcification, or atherosclerotic occlusive disease.32,33 As life expectancy and the need for TKA increase, it is likely that a larger percentage of TKA patients will have preoperative radiographic evidence of knee arterial calcification. Although current dogma is that tourniquet-assisted TKA is contraindicated for patients with preoperative radiographic evidence of femoral-popliteal calcification, our study results showed that this calcification should not affect preoperative TKA planning for these patients.
We divided our patients into 3 categories: those with proximal calcification (above the joint line), those with distal calcification (below the joint line), and those with both proximal and distal calcification. Location of arterial calcification did not have an effect on their rates of postoperative wound complication or VTE. We hypothesized that patients with proximal calcification would be at increased risk for direct arterial injury and subsequent wound complication because the tourniquet is placed proximally. Previous research has indicated that arterial occlusion and subsequent wound complication can occur because of low blood flow stemming from tourniquet use.7 Further, intraoperative manipulation (flexing) of a knee with calcified vessels causes arterial complications after TKA because these vessels are less elastic than nonatheromatous vessels.31 However, we found no such effect. At the same time, having arterial calcification might also be an indication of venous disease in this location,12 which may be especially important for proximal calcifications. Proximal DVT more likely is a precursor to pulmonary embolic events than distal DVT is.31,34 However, we found no difference in VTE rates among the 3 arterial location groups, which is supported by studies that have found that tourniquet use does not increase DVT incidence.29,35-40
Risk for wound complications was higher in male patients and in patients with diabetes, prior VTE, hypertension, or CAD. This finding is important because, with the increasing age of patients who undergo TKA, those with serious medical comorbidities will continue to need and have this surgery.17 Diabetes may increase the rate of wound complication because patients with diabetes have poor microcirculation, poor collagen synthesis, and reduced wound strength.41 Malinzak and colleagues42 demonstrated that, compared with patients without diabetes, those with diabetes had a significantly higher risk for infection after TKA. Prior VTE, specifically DVT, may increase the rate of wound complication because after DVT the deep veins may be damaged and exhibit valvular dysfunction. Labropoulos and colleagues43 showed that DVT history was strongly associated with ulcer nonhealing. Perhaps hypertension has been overlooked as a risk factor for wound complication in TKA. No previous studies have assessed the link between hypertension and wound complications after TKA. However, a study of wound healing after total hip arthroplasty found that, compared with normotensive patients, hypertensive patients had delayed wound healing, putting them at higher risk for infection.44 In addition, we found that patients with CAD were at increased risk for wound complications—an unexpected finding, as CAD traditionally is not a risk factor for infection or poor wound healing. Recently, however, CAD was identified as an independent risk factor for surgical site infections in posterior lumbar–instrumented arthrodesis.45 The etiology of this association is unknown. Also, male patients were at increased risk for wound complication. Male sex has been implicated as an independent risk factor for development of surgical site infections and has been established as an important predisposing factor for periprosthetic joint infections.46
It is possible that patients who present with diabetes, VTE, hypertension, or CAD before TKA should have a consultation with a vascular surgeon or should have TKA performed without a tourniquet, but this conclusion cannot be considered definitive without a large prospective randomized trial or possibly registry data. Our data indicate that patients with these comorbidities have higher rates of wound complications irrespective of preoperative radiographic calcifications. On the basis of our study results, however, we certainly recommend that patients with these risk factors have preoperative medical optimization. Orthopedic surgeons should take a thorough history and perform a meticulous physical examination on these patients to look for evidence of PVD. We recommend that, if vascular claudication is elicited in the history, or if there is evidence of peripheral arterial disease—such as hair loss, skin discoloration, dystrophic nail changes, or absent or unequal peripheral pulses—the ankle-brachial index test should be performed. If the index value is less than 0.9, then a preoperative vascular surgery consultation should be obtained.
This study had some weaknesses. First, it was retrospective, so it is possible that some wound or VTE complications were not reported and thus not found in the paper charts or electronic medical records. Some patients may have had VTE diagnostic scans at other hospitals, and their results may not have been recorded across databases. Moreover, some patients may have seen wound specialists for wound infections or wound healing problems, and these may not have been reported to the orthopedic surgeons. Second, though our patient population was not small, it may not have been of adequate size to fully detect statistical significance. We met our enrollment numbers based on our sample size calculations from an a priori power analysis; however, we still draw conclusions with the possibility of committing a type II error in mind by failing to reject the null hypothesis when in reality a statistically significant difference does exist. Third, none of our consecutive patients carried the preoperative diagnosis of PVD, and none had preoperative vascular surgery. Therefore, though calcifications were noted on radiographs, clinically our patients were asymptomatic with respect to vascular health. Last, the 2 groups were not randomized. All patients underwent tourniquet-assisted TKA.
Conclusion
To our knowledge, this is the largest study to examine the effect of preoperative knee arterial calcification on wound complication and VTE after tourniquet-assisted TKA. Contrary to previously published recommendations, we conclude that TKA can be safely performed with a tourniquet in the presence of preoperative radiographic evidence of such calcification. However, we recommend that patients with diabetes, hypertension, CAD, or prior VTE undergo an appropriate physical examination to elicit any signs or symptoms of vascular disease. If before surgery there is any question of vascular competence, a vascular surgeon should be consulted.
1. Guanche CA. Tourniquet-induced tibial nerve palsy complicating anterior cruciate ligament reconstruction. Arthroscopy. 1995;11(5):620-622.
2. Irvine GB, Chan RN. Arterial calcification and tourniquets. Lancet. 1986;2(8517):1217.
3. Patterson S, Klenerman L. The effect of pneumatic tourniquets on the ultrastructure of skeletal muscle. J Bone Joint Surg Br. 1979;61(2):178-183.
4. Rorabeck CH, Kennedy JC. Tourniquet-induced nerve ischemia complicating knee ligament surgery. Am J Sports Med. 1980;8(2):98-102.
5. Shenton DW, Spitzer SA, Mulrennan BM. Tourniquet-induced rhabdomyolysis. A case report. J Bone Joint Surg Am. 1990;72(9):1405-1406.
6. Abdel-Salam A, Eyres KS. Effects of tourniquet during total knee arthroplasty. A prospective randomised study. J Bone Joint Surg Br. 1995;77(2):250-253.
7. DeLaurentis DA, Levitsky KA, Booth RE, et al. Arterial and ischemic aspects of total knee arthroplasty. Am J Surg. 1992;164(3):237-240.
8. Holmberg A, Milbrink J, Bergqvist D. Arterial complications and knee arthroplasty. Acta Orthop Scand. 1996;67(1):75-8.
9. Hozack WJ, Cole PA, Gardner R, Corces A. Popliteal aneurysm after total knee arthroplasty. Case reports and review of the literature. J Arthroplasty. 1990;5(4):301-305.
10. Kumar SN, Chapman JA, Rawlins I. Vascular injuries after total knee arthroplasty: a review of the problem with special reference to the possible effects of the tourniquet. J Arthroplasty. 1998;13(2):211-216.
11. Rush JH, Vidovich JD, Johanson MA. Arterial complications and total knee arthroplasty. The Australian experience. J Bone Joint Surg Br. 1987;69(3):400-402.
12. Callam MJ, Harper DR, Dale JJ, Ruckley CV. Arterial disease in chronic leg ulceration: an underestimated hazard? Lothian and Forth Valley Leg Ulcer Study. Br Med J (Clin Res Ed). 1987;294(6577):929-931.
13. Blom AW, Brown J, Taylor AH, Pattison G, Whitehouse S, Bannister GC. Infection after total knee arthroplasty. J Bone Joint Surg Br. 2004;86(5):688-691.
14. Geerts WH, Bergqvist D, Pinco G, et al. Prevention of venous thromboembolism. Chest. 2008;133(6 suppl):381S-453S.
15. Pulido L, Parvizi J, Macgibeny M, et al. In hospital complications after total joint arthroplasty. J Arthroplasty. 2008;23(6 Suppl 1):139-145.
16. Arthritis: data and statistics. Centers for Disease Control and Prevention website. http://www.cdc.gov/arthritis/data_statistics.htm. Updated March 11, 2015. Accessed July 27, 2015.
17. Kurtz S, Ong K, Lau E, Mowat F, Halpern M. Projections of primary and revision hip and knee arthroplasty in the United States from 2005 to 2030. J Bone Joint Surg Am. 2007;89(4):780-785.
18. Pulido L, Ghanem E, Joshi A, Purtill JJ, Parvizi J. Periprosthetic joint infection: the incidence, timing, and predisposing factors. Clin Orthop Relat Res. 2008;466(7):1710-1715.
19. Warwick D. Prevention of venous thromboembolism in total knee and hip replacement. Circulation. 2012;125(17):2151-2155.
20. Rennenberg RJ, Kessels AG, Schurgers LJ, van Engelshoven JM, de Leeuw PW, Kroon AA. Vascular calcifications as a marker of increased cardiovascular risk: a meta-analysis. Vasc Health Risk Manag. 2009;5(1):185-197.
21. Arad Y, Goodman KJ, Roth M, Newstein D, Guerci AD. Coronary calcification, coronary disease risk factors, C-reactive protein, and atherosclerotic cardiovascular disease events: the St. Francis Heart Study. J Am Coll Cardiol. 2005;46(1):158-165.
22. Iribarren C, Sidney S, Sternfeld B, Browner WS. Calcification of the aortic arch: risk factors and association with coronary heart disease, stroke, and peripheral vascular disease. JAMA. 2000;283(21):2810-2815.
23. Shaw LJ, Raggi P, Schisterman E, Berman DS, Callister TQ. Prognostic value of cardiac risk factors and coronary artery calcium screening for all-cause mortality. Radiology. 2003;228(3):826-833.
24. Taylor AJ, Bindeman J, Feuerstein I, Cao F, Brazaitis M, O’Malley PG. Coronary calcium independently predicts incident premature coronary heart disease over measured cardiovascular risk factors: mean three-year outcomes in the Prospective Army Coronary Calcium (PACC) project. J Am Coll Cardiol. 2005;46(5):807-814.
25. Lehto S, Niskanen L, Suhonen M, Rönnemaa T, Laakso M. Medial artery calcification. A neglected harbinger of cardiovascular complications in non-insulin-dependent diabetes mellitus. Arterioscler Thromb Vasc Biol. 1996;16(8):978-983.
26. Niskanen L, Siitonen O, Suhonen M, Uusitupa MI. Medial artery calcification predicts cardiovascular mortality in patients with NIDDM. Diabetes Care. 1994;17(11):1252-1256.
27. Smith DE, McGraw RW, Taylor DC, et al. Arterial complications and total knee arthroplasty. J Am Acad Orthop Surg. 2001;9(4):253-257.
28. Vandenbussche E, Duranthon L, Couturier M, Pidhorz L, Augereau B. The effect of tourniquet use in total knee arthroplasty. Int Orthop. 2002;26(5):306-309.
29. Fukunda A, Hasegawa M, Kato K, Shi D, Sudo A, Uchida A. Effect of tourniquet application on deep vein thrombosis after total knee thrombosis. Arch Orthop Trauma Surg. 2007;127(8):671-675.
30. Butt U, Samuel R, Sahu A, Butt IS, Johnson DS, Turner PG. Arterial injury in total knee arthroplasty. J Arthroplasty. 2010;25(8):1311-1318.
31. Langkamer VG. Local vascular complications after knee replacement: a review with illustrative case reports. Knee. 2001;8(4):259-264.
32. Hussein A, Uno K, Wolski K, et al. Peripheral arterial disease and progression of coronary atherosclerosis. J Am Coll Cardiol. 2011;57(10):1220-1225.
33. Ouriel K. Peripheral arterial disease. Lancet. 2001;358(9289):1257-1264.
34. Monreal M, Rufz J, Olazabal A, Arias A, Roca J. Deep venous thrombosis and the risk of pulmonary embolism. Chest. 1992;102(3):677-681.
35. Angus PD, Nakielny R, Goodrum DT. The pneumatic tourniquet and deep venous thrombosis. J Bone Joint Surg Br. 1983;65(3):336-339.
36. Fahmy NR, Patel DG. Hemostatic changes and postoperative deep-vein thrombosis associated with use of a pneumatic tourniquet. J Bone Joint Surg Am. 1981;63(3):461-465.
37. Harvey EJ, Leclerc J, Brooks CE, Burke DL. Effect of tourniquet use on blood loss and incidence of deep vein thrombosis in total knee arthroplasty. J Arthroplasty. 1997;12(3):291-296.
38. Simon MA, Mass DP, Zarins CK, Bidani N, Gudas CJ, Metz CE. The effect of a thigh tourniquet on the incidence of deep venous thrombosis after operations on the fore part of the foot. J Bone Joint Surg Am. 1982;64(2):188-191.
39. Stulberg BN, Insall JN, Williams GW, Ghelman B. Deep-vein thrombosis following total knee replacement. An analysis of six hundred and thirty-eight arthroplasties. J Bone Joint Surg Am. 1984;66(2):194-201.
40. Wakankar HM, Nicholl JE, Koka R, D’Arcy JC. The tourniquet in total knee arthroplasty. A prospective, randomized study. J Bone Joint Surg Br. 1999;81(1):30-33.
41. Vince K, Chivas D, Droll K. Wound complications after total knee arthroplasty. J Arthroplasty. 2007;22(4 Suppl 1):39-44.
42. Malinzak RA, Ritter MA, Berend ME, Meding JB, Olberding EM, Davis KE. Morbidly obese, diabetic, younger, and unilateral joint arthroplasty patients have elevated total joint arthroplasty infection rates. J Arthroplasty. 2009;24(6 Suppl):84-88.
43. Labropoulos N, Wang E, Lanier S, Khan SU. Factors associated with poor healing and recurrence of venous ulceration. Plast Reconstr Surg. 2011;129(1):179-186.
44. Ahmed AA, Mooar PA, Kleiner M, Torg JS, Miyamoto CT. Hypertensive patients show delayed wound healing following total hip arthroplasty. PLoS One. 2011;6(8):e23224.
45. Koutsoumbelis S, Hughes AP, Girardi FP, et al. Risk factors for postoperative infection following posterior lumbar instrumented arthrodesis. J Bone Joint Surg Am. 2001;93(17):1627-1633.
46. Poultsides LA, Ma Y, Della Valle AG, Chiu YL, Sculco TP, Memtsoudis SG. In-hospital surgical site infections after primary hip and knee arthroplasty—incidence and risk factors. J Arthroplasty. 2013;28(3):385-389.
Tourniquets are often used in total knee arthroplasty (TKA) to improve visualization of structures, shorten operative time, reduce intraoperative bleeding, and improve cementing technique. Despite these advantages, controversy remains regarding the safety of tourniquet use. Tourniquets have been associated with nerve palsies, vascular injury, and muscle damage.1-5 Some have hypothesized they may cause venous stasis or direct endothelial damage that may develop into deep vein thrombosis (DVT). Abdel-Salam and Eyres6 found an increased incidence of postoperative wound complications and DVTs associated with tourniquet use.
Moreover, investigators have analyzed the role of tourniquets in populations at high risk for wound complications. DeLaurentis and colleagues7 performed a prospective and retrospective analysis of 1182 TKA patients, 24 (2%) of whom had preexisting peripheral vascular disease (PVD), defined as a history of arterial insufficiency, absent dorsalis pedis and/or absent posterior tibial pulsations, and arterial calcifications. A tourniquet was used in each case. Arterial complications occurred in 6 of the 24 patients with PVD. As expected, the authors found that a history of intermittent claudication, pain at rest, and arterial ulcers resulted in a high risk for vascular complications. Further studies have supported this finding and expanded the list of predisposing factors to include previous vascular surgery and absent and asymmetric pedal pulsations.7-11 Of particular concern to total joint arthroplasty surgeons was the finding by DeLaurentis and colleagues7 that patients with radiographic evidence of calcification of the distal superficial femoral artery and/or popliteal artery were at risk for arterial complications. This finding is also supported by other studies.8,11 In TKA, damage to arterial structures proximal to the surgical field could manifest as impaired postoperative wound healing or an ischemic limb. Wound healing depends on adequate blood flow to the healing tissue, and any damage to arterial or venous structures can theoretically compromise this process.
Added to vascular/wound complications as concerning complications in orthopedic surgery is venous thromboembolism (VTE). The role of tourniquets in the formation of VTEs is controversial. A tourniquet has the potential to increase the risk for DVT because of the stasis of venous blood in the lower limb or possible damage to calcified blood vessels. Callam and colleagues12 studied the connection between artery disease and chronic leg ulcers and found that half the patients diagnosed with peripheral artery disease also had stigmata of chronic venous insufficiency. Therefore, the entities can occur in tandem, and surgeons should keep this in mind.
Here we report on a study we conducted to determine whether tourniquet use in TKA in patients with preexisting radiographic evidence of vascular disease increases the risk for wound complications or VTE.
Patients and Methods
We retrospectively reviewed 461 consecutive primary TKAs (373 patients) performed between January 2007 and June 2012 by 2 attending orthopedic surgeons specializing in adult reconstruction. Medical records and operative reports of 583 patients were examined after receiving institutional review board approval. Of these patients, 373 (64%) had a minimum of 12-month follow-up data available. Twelve months was deemed long enough to discover wound complications or DVTs secondary to the index procedure. Most of these outcomes manifest within the first 3 months after surgery and certainly by 12 months. Follow-up longer than 12 months may become a confounder, as wound complications outside the acute to subacute postoperative window could be related to patients’ underlying PVD and not directly to tourniquet use during surgery. Patient demographics and comorbidities were recorded. Comorbidities were obtained from preoperative medical evaluations and surgeons’ preoperative evaluations. All patients had preoperative palpable dorsalis pedis and posterior tibialis arterial pulses. No patient required preoperative vascular studies based on preoperative examination or comorbidities. No patient had prior vascular bypass surgery or stenting.
TKA was performed in a nonlaminar flow, positive-pressure, high-efficiency particulate air-filtered room with sterile toga/surgical helmet systems. For all patients, a pneumatic thigh tourniquet was applied, and the patient was prepared and draped. After limb exsanguination using a rubber bandage, the limb was elevated and the tourniquet inflated to a pressure of 250 to 300 mm Hg. The tourniquet was released either just before closure or immediately after closure in all cases; it was always let down before placement of final bandages.
Prophylactic chemical anticoagulation consisting of warfarin, aspirin, or enoxaparin was used in all patients and continued for 4 to 6 weeks after surgery. All patients received mechanical DVT prophylaxis with sequential compression devices, and all were mobilized out of bed beginning either the day of surgery or the next day. All patients received perioperative intravenous antibiotics, with the preoperative dose given before tourniquet inflation and the last postoperative dose stopped within 24 hours of surgery.
All patients who had primary TKA underwent preoperative medical evaluation and optimization. The patient’s hospital course was monitored closely, and complications noted by the orthopedic team were documented. Follow-up documentation was retrospectively reviewed for evidence of wound complications or VTE. Wound complications were defined as cellulitis, delayed wound healing, wound dehiscence, and/or periprosthetic joint infection. In the case of VTE, physical examination findings were not sufficient for inclusion. Venous duplex ultrasonography demonstrating the clot was reviewed before inclusion.
Preoperative radiographs were examined for arterial calcification (Figure). We refer to calcification seen above the knee joint as proximal calcification and to calcification observed below the joint as distal calcification. Patients exhibited calcification proximally only, distally only, or both proximally and distally. The 373 patients were placed into 2 groups based on whether they had preoperative arterial calcification on plain radiography of the knee. One group (285 patients with no radiographic evidence of preoperative knee arterial calcification) underwent 365 TKAs, and the other group (88 patients with radiographic evidence of preoperative knee arterial calcification) underwent 96 TKAs.
A sample size calculation was performed to determine how many patients were needed in each group with 80% power and an α of 0.05. With an estimated difference in VTE/wound complication rate between the calcification and no-calcification groups of 12%, we needed to review 316 TKAs total. This 12% difference was based on study findings of a 25% complication rate in PVD patients who underwent tourniquet-assisted TKA, and the rate of VTE/wound complication after TKA in patients overall, which can be up to 12%.7,13,14 We exceeded minimal enrollment and had 461 TKAs. Descriptive statistics were reported, with means and ranges provided where appropriate. Independent t test was used to evaluate the differences in continuous data (age) between the groups. Univariate analysis (using Pearson χ2 and Fisher exact tests) and multivariate logistic regression analysis were used to evaluate the effects of categorical variables (sex, comorbidity, calcification [presence, absence], and location of calcification [proximal only, distal only, both]) on wound complication and VTE rates. All tests were 2-tailed and performed with a type I error rate of 0.05. Data analysis was performed with SPSS Version 19.0 (SPSS).
Results
Patient characteristics are summarized in Table 1. Of the 373 patients, 285 lacked calcification, and 88 had calcification. Mean age was 67.73 years (range, 24-92 years) for all patients, 65.99 years (range, 24-89 years) for the no-calcification group, and 74.32 years (range, 54-92 years) for the calcification group; the calcification group demonstrated a trend toward older age, but the difference was not significantly different (P = .07). Of the 373 patients, 156 (41.82%) were male: 110 in the no-calcification group (38.60%) and 46 in the calcification group (52.27%); sex was significantly (P = .002) different between groups, with more males in the calcification group.
Data on total preoperative comorbidities are summarized in Table 2. Hypertension, hyperlipidemia, diabetes, and coronary artery disease (CAD) were the most common comorbidities, and they were all significantly (P ≤ .05) increased in the calcification group.
No patients had reported arterial complications, such as arterial bleeding, aneurysm, intimal tears, or loss of distal pulses. Wound complication after TKA was detected in 3.04% of all cases (Table 3). Rate of DVT after TKA was 2.60% of all cases, and rate of pulmonary embolism after TKA was 2.17% of all cases. Of the 96 TKAs with preoperative radiographic evidence of calcification, 47 (48.96%) had proximal calcification only, 11 (11.46%) had distal calcification only, and 38 (39.58%) had both proximal and distal calcification (Table 4). There was no significant difference between the rate of wound complication or VTE based on location of vascular calcification.
Univariate analysis demonstrated that presence of arterial knee calcification did not increase the risk for postoperative wound complication (odds ratio [OR], 1.04; 95% confidence interval [CI], 0.28-3.80; P > .05) (Table 5). Location of arterial knee calcification also did not increase the risk for postoperative wound complication. In addition, univariate analysis demonstrated that presence of arterial knee calcification did not increase the risk for postoperative VTE (OR, 1.20; 95% CI, 0.43-3.36; P > .05 (Table 6).
Of the 14 wound complications, the most common infections were cellulitis (5/14 cases; 35.71%) and infected hardware that required component revision (5/14 cases; 35.71%). Mean time from TKA to infection was 137.93 days (range, 5-783 days). The most common organism grown in culture from the wound was Staphylococcus (5/14 cases; 35.71%).
Additional univariate statistical analysis revealed that presence of diabetes, hypertension, prior VTE, CAD, and male sex was linked to higher incidence of wound complication (P < .05) (Table 5). When multivariate analysis was performed, hypertension, prior VTE, and male sex remained significant (P < .05) (Table 5).
Discussion
TKA is a safe and effective procedure used to treat osteoarthritis of the knee and improve patients’ quality of life.15 About 700,000 TKAs are performed annually in the United States.16 Because of improvements in preventive medicine and medical technology, life expectancy is increasing, and TKAs are now being performed in higher numbers and in an older patient population. Over the next few decades, these developments will lead to more postoperative complications. It is projected that, by 2030, the need for TKAs in the United States will increase by 673% to 3.48 million.17 Postoperative complications are rare but unfortunately often lead to poor outcomes or even mortality.18 To help minimize the number of postoperative complications, we must understand the safety of tourniquet use in TKA. Other investigators have concluded that tourniquet use is unsafe in patients with preoperative vascular calcifications on plain radiographs.7,8,11 The present study, designed to elucidate whether preoperative evidence of knee arterial calcification may predispose TKA patients to postoperative wound complication or VTE, had some important findings.
In our study, wound complication and VTE occurred in a considerable number of patients after TKA. Despite exceeding the number of patients calculated by the power analysis, our population may have been inadequate to fully detect statistical significance. Thus, our conclusion of failing to reject the null hypothesis may have been because of sample size, a type II error. We found that, after primary TKA, 3.04% of patients developed wound complications and 4.77% VTE. According to the literature, the incidence of infection after primary TKA is between 0.5% and 12%, and that of VTE reported within 3 months after TKA is 1.3% to 10%.13,14 Although we had 100% VTE prophylaxis, meeting the standard of care, VTE after TKA remains a postoperative complication.19 This study also found that a considerable percentage of primary TKA patients (23.59%) had preoperative calcification of the knee arteries. To our knowledge, this study was the first to quantify the incidence of knee arterial calcification in patients who underwent TKA.
Preoperative calcification of the knee arteries in patients who underwent TKA did not increase the risk for wound complication, VTE, or arterial damage. These calcifications, however, do pose an increased systemic vascular risk.20 Calcification of the vascular wall predicts increased cardiovascular risk, independent of classical cardiovascular risk factors.3,18,21-24 Clinically, patients who have both diabetes and calcifications are at significant excess risk for total mortality, stroke mortality, and cardiovascular mortality, compared with patients with diabetes but without such calcifications. They also had a significantly higher incidence of coronary heart disease events, stroke events, and lower extremity amputations.25,26
All our patients underwent tourniquet-assisted TKA. Although previous studies have indicated that tourniquet use may increase arterial complications and wound complications or even limb loss in patients with calcified arteries, we did not find this link.7,27 Our population had no reported arterial complications related to tourniquet use. Other, smaller studies have had similar findings. Vandenbussche and colleagues28 prospectively studied 80 TKA cases randomized to tourniquet use or no tourniquet use and found no postoperative nerve palsies, wound infections, wound healing problems, or hematomas. Our study is also in accord with studies that have reported tourniquet use did not increase risk for DVT.29 Therefore, unlike earlier data, our data demonstrated that tourniquet use in patients with knee arterial calcification was safe.7,27,30,31
Patients with calcification were more likely to have the medical comorbidities of hypertension, diabetes, hyperlipidemia, and CAD. All these comorbidities are linked to the development of arterial calcification, or atherosclerotic occlusive disease.32,33 As life expectancy and the need for TKA increase, it is likely that a larger percentage of TKA patients will have preoperative radiographic evidence of knee arterial calcification. Although current dogma is that tourniquet-assisted TKA is contraindicated for patients with preoperative radiographic evidence of femoral-popliteal calcification, our study results showed that this calcification should not affect preoperative TKA planning for these patients.
We divided our patients into 3 categories: those with proximal calcification (above the joint line), those with distal calcification (below the joint line), and those with both proximal and distal calcification. Location of arterial calcification did not have an effect on their rates of postoperative wound complication or VTE. We hypothesized that patients with proximal calcification would be at increased risk for direct arterial injury and subsequent wound complication because the tourniquet is placed proximally. Previous research has indicated that arterial occlusion and subsequent wound complication can occur because of low blood flow stemming from tourniquet use.7 Further, intraoperative manipulation (flexing) of a knee with calcified vessels causes arterial complications after TKA because these vessels are less elastic than nonatheromatous vessels.31 However, we found no such effect. At the same time, having arterial calcification might also be an indication of venous disease in this location,12 which may be especially important for proximal calcifications. Proximal DVT more likely is a precursor to pulmonary embolic events than distal DVT is.31,34 However, we found no difference in VTE rates among the 3 arterial location groups, which is supported by studies that have found that tourniquet use does not increase DVT incidence.29,35-40
Risk for wound complications was higher in male patients and in patients with diabetes, prior VTE, hypertension, or CAD. This finding is important because, with the increasing age of patients who undergo TKA, those with serious medical comorbidities will continue to need and have this surgery.17 Diabetes may increase the rate of wound complication because patients with diabetes have poor microcirculation, poor collagen synthesis, and reduced wound strength.41 Malinzak and colleagues42 demonstrated that, compared with patients without diabetes, those with diabetes had a significantly higher risk for infection after TKA. Prior VTE, specifically DVT, may increase the rate of wound complication because after DVT the deep veins may be damaged and exhibit valvular dysfunction. Labropoulos and colleagues43 showed that DVT history was strongly associated with ulcer nonhealing. Perhaps hypertension has been overlooked as a risk factor for wound complication in TKA. No previous studies have assessed the link between hypertension and wound complications after TKA. However, a study of wound healing after total hip arthroplasty found that, compared with normotensive patients, hypertensive patients had delayed wound healing, putting them at higher risk for infection.44 In addition, we found that patients with CAD were at increased risk for wound complications—an unexpected finding, as CAD traditionally is not a risk factor for infection or poor wound healing. Recently, however, CAD was identified as an independent risk factor for surgical site infections in posterior lumbar–instrumented arthrodesis.45 The etiology of this association is unknown. Also, male patients were at increased risk for wound complication. Male sex has been implicated as an independent risk factor for development of surgical site infections and has been established as an important predisposing factor for periprosthetic joint infections.46
It is possible that patients who present with diabetes, VTE, hypertension, or CAD before TKA should have a consultation with a vascular surgeon or should have TKA performed without a tourniquet, but this conclusion cannot be considered definitive without a large prospective randomized trial or possibly registry data. Our data indicate that patients with these comorbidities have higher rates of wound complications irrespective of preoperative radiographic calcifications. On the basis of our study results, however, we certainly recommend that patients with these risk factors have preoperative medical optimization. Orthopedic surgeons should take a thorough history and perform a meticulous physical examination on these patients to look for evidence of PVD. We recommend that, if vascular claudication is elicited in the history, or if there is evidence of peripheral arterial disease—such as hair loss, skin discoloration, dystrophic nail changes, or absent or unequal peripheral pulses—the ankle-brachial index test should be performed. If the index value is less than 0.9, then a preoperative vascular surgery consultation should be obtained.
This study had some weaknesses. First, it was retrospective, so it is possible that some wound or VTE complications were not reported and thus not found in the paper charts or electronic medical records. Some patients may have had VTE diagnostic scans at other hospitals, and their results may not have been recorded across databases. Moreover, some patients may have seen wound specialists for wound infections or wound healing problems, and these may not have been reported to the orthopedic surgeons. Second, though our patient population was not small, it may not have been of adequate size to fully detect statistical significance. We met our enrollment numbers based on our sample size calculations from an a priori power analysis; however, we still draw conclusions with the possibility of committing a type II error in mind by failing to reject the null hypothesis when in reality a statistically significant difference does exist. Third, none of our consecutive patients carried the preoperative diagnosis of PVD, and none had preoperative vascular surgery. Therefore, though calcifications were noted on radiographs, clinically our patients were asymptomatic with respect to vascular health. Last, the 2 groups were not randomized. All patients underwent tourniquet-assisted TKA.
Conclusion
To our knowledge, this is the largest study to examine the effect of preoperative knee arterial calcification on wound complication and VTE after tourniquet-assisted TKA. Contrary to previously published recommendations, we conclude that TKA can be safely performed with a tourniquet in the presence of preoperative radiographic evidence of such calcification. However, we recommend that patients with diabetes, hypertension, CAD, or prior VTE undergo an appropriate physical examination to elicit any signs or symptoms of vascular disease. If before surgery there is any question of vascular competence, a vascular surgeon should be consulted.
Tourniquets are often used in total knee arthroplasty (TKA) to improve visualization of structures, shorten operative time, reduce intraoperative bleeding, and improve cementing technique. Despite these advantages, controversy remains regarding the safety of tourniquet use. Tourniquets have been associated with nerve palsies, vascular injury, and muscle damage.1-5 Some have hypothesized they may cause venous stasis or direct endothelial damage that may develop into deep vein thrombosis (DVT). Abdel-Salam and Eyres6 found an increased incidence of postoperative wound complications and DVTs associated with tourniquet use.
Moreover, investigators have analyzed the role of tourniquets in populations at high risk for wound complications. DeLaurentis and colleagues7 performed a prospective and retrospective analysis of 1182 TKA patients, 24 (2%) of whom had preexisting peripheral vascular disease (PVD), defined as a history of arterial insufficiency, absent dorsalis pedis and/or absent posterior tibial pulsations, and arterial calcifications. A tourniquet was used in each case. Arterial complications occurred in 6 of the 24 patients with PVD. As expected, the authors found that a history of intermittent claudication, pain at rest, and arterial ulcers resulted in a high risk for vascular complications. Further studies have supported this finding and expanded the list of predisposing factors to include previous vascular surgery and absent and asymmetric pedal pulsations.7-11 Of particular concern to total joint arthroplasty surgeons was the finding by DeLaurentis and colleagues7 that patients with radiographic evidence of calcification of the distal superficial femoral artery and/or popliteal artery were at risk for arterial complications. This finding is also supported by other studies.8,11 In TKA, damage to arterial structures proximal to the surgical field could manifest as impaired postoperative wound healing or an ischemic limb. Wound healing depends on adequate blood flow to the healing tissue, and any damage to arterial or venous structures can theoretically compromise this process.
Added to vascular/wound complications as concerning complications in orthopedic surgery is venous thromboembolism (VTE). The role of tourniquets in the formation of VTEs is controversial. A tourniquet has the potential to increase the risk for DVT because of the stasis of venous blood in the lower limb or possible damage to calcified blood vessels. Callam and colleagues12 studied the connection between artery disease and chronic leg ulcers and found that half the patients diagnosed with peripheral artery disease also had stigmata of chronic venous insufficiency. Therefore, the entities can occur in tandem, and surgeons should keep this in mind.
Here we report on a study we conducted to determine whether tourniquet use in TKA in patients with preexisting radiographic evidence of vascular disease increases the risk for wound complications or VTE.
Patients and Methods
We retrospectively reviewed 461 consecutive primary TKAs (373 patients) performed between January 2007 and June 2012 by 2 attending orthopedic surgeons specializing in adult reconstruction. Medical records and operative reports of 583 patients were examined after receiving institutional review board approval. Of these patients, 373 (64%) had a minimum of 12-month follow-up data available. Twelve months was deemed long enough to discover wound complications or DVTs secondary to the index procedure. Most of these outcomes manifest within the first 3 months after surgery and certainly by 12 months. Follow-up longer than 12 months may become a confounder, as wound complications outside the acute to subacute postoperative window could be related to patients’ underlying PVD and not directly to tourniquet use during surgery. Patient demographics and comorbidities were recorded. Comorbidities were obtained from preoperative medical evaluations and surgeons’ preoperative evaluations. All patients had preoperative palpable dorsalis pedis and posterior tibialis arterial pulses. No patient required preoperative vascular studies based on preoperative examination or comorbidities. No patient had prior vascular bypass surgery or stenting.
TKA was performed in a nonlaminar flow, positive-pressure, high-efficiency particulate air-filtered room with sterile toga/surgical helmet systems. For all patients, a pneumatic thigh tourniquet was applied, and the patient was prepared and draped. After limb exsanguination using a rubber bandage, the limb was elevated and the tourniquet inflated to a pressure of 250 to 300 mm Hg. The tourniquet was released either just before closure or immediately after closure in all cases; it was always let down before placement of final bandages.
Prophylactic chemical anticoagulation consisting of warfarin, aspirin, or enoxaparin was used in all patients and continued for 4 to 6 weeks after surgery. All patients received mechanical DVT prophylaxis with sequential compression devices, and all were mobilized out of bed beginning either the day of surgery or the next day. All patients received perioperative intravenous antibiotics, with the preoperative dose given before tourniquet inflation and the last postoperative dose stopped within 24 hours of surgery.
All patients who had primary TKA underwent preoperative medical evaluation and optimization. The patient’s hospital course was monitored closely, and complications noted by the orthopedic team were documented. Follow-up documentation was retrospectively reviewed for evidence of wound complications or VTE. Wound complications were defined as cellulitis, delayed wound healing, wound dehiscence, and/or periprosthetic joint infection. In the case of VTE, physical examination findings were not sufficient for inclusion. Venous duplex ultrasonography demonstrating the clot was reviewed before inclusion.
Preoperative radiographs were examined for arterial calcification (Figure). We refer to calcification seen above the knee joint as proximal calcification and to calcification observed below the joint as distal calcification. Patients exhibited calcification proximally only, distally only, or both proximally and distally. The 373 patients were placed into 2 groups based on whether they had preoperative arterial calcification on plain radiography of the knee. One group (285 patients with no radiographic evidence of preoperative knee arterial calcification) underwent 365 TKAs, and the other group (88 patients with radiographic evidence of preoperative knee arterial calcification) underwent 96 TKAs.
A sample size calculation was performed to determine how many patients were needed in each group with 80% power and an α of 0.05. With an estimated difference in VTE/wound complication rate between the calcification and no-calcification groups of 12%, we needed to review 316 TKAs total. This 12% difference was based on study findings of a 25% complication rate in PVD patients who underwent tourniquet-assisted TKA, and the rate of VTE/wound complication after TKA in patients overall, which can be up to 12%.7,13,14 We exceeded minimal enrollment and had 461 TKAs. Descriptive statistics were reported, with means and ranges provided where appropriate. Independent t test was used to evaluate the differences in continuous data (age) between the groups. Univariate analysis (using Pearson χ2 and Fisher exact tests) and multivariate logistic regression analysis were used to evaluate the effects of categorical variables (sex, comorbidity, calcification [presence, absence], and location of calcification [proximal only, distal only, both]) on wound complication and VTE rates. All tests were 2-tailed and performed with a type I error rate of 0.05. Data analysis was performed with SPSS Version 19.0 (SPSS).
Results
Patient characteristics are summarized in Table 1. Of the 373 patients, 285 lacked calcification, and 88 had calcification. Mean age was 67.73 years (range, 24-92 years) for all patients, 65.99 years (range, 24-89 years) for the no-calcification group, and 74.32 years (range, 54-92 years) for the calcification group; the calcification group demonstrated a trend toward older age, but the difference was not significantly different (P = .07). Of the 373 patients, 156 (41.82%) were male: 110 in the no-calcification group (38.60%) and 46 in the calcification group (52.27%); sex was significantly (P = .002) different between groups, with more males in the calcification group.
Data on total preoperative comorbidities are summarized in Table 2. Hypertension, hyperlipidemia, diabetes, and coronary artery disease (CAD) were the most common comorbidities, and they were all significantly (P ≤ .05) increased in the calcification group.
No patients had reported arterial complications, such as arterial bleeding, aneurysm, intimal tears, or loss of distal pulses. Wound complication after TKA was detected in 3.04% of all cases (Table 3). Rate of DVT after TKA was 2.60% of all cases, and rate of pulmonary embolism after TKA was 2.17% of all cases. Of the 96 TKAs with preoperative radiographic evidence of calcification, 47 (48.96%) had proximal calcification only, 11 (11.46%) had distal calcification only, and 38 (39.58%) had both proximal and distal calcification (Table 4). There was no significant difference between the rate of wound complication or VTE based on location of vascular calcification.
Univariate analysis demonstrated that presence of arterial knee calcification did not increase the risk for postoperative wound complication (odds ratio [OR], 1.04; 95% confidence interval [CI], 0.28-3.80; P > .05) (Table 5). Location of arterial knee calcification also did not increase the risk for postoperative wound complication. In addition, univariate analysis demonstrated that presence of arterial knee calcification did not increase the risk for postoperative VTE (OR, 1.20; 95% CI, 0.43-3.36; P > .05 (Table 6).
Of the 14 wound complications, the most common infections were cellulitis (5/14 cases; 35.71%) and infected hardware that required component revision (5/14 cases; 35.71%). Mean time from TKA to infection was 137.93 days (range, 5-783 days). The most common organism grown in culture from the wound was Staphylococcus (5/14 cases; 35.71%).
Additional univariate statistical analysis revealed that presence of diabetes, hypertension, prior VTE, CAD, and male sex was linked to higher incidence of wound complication (P < .05) (Table 5). When multivariate analysis was performed, hypertension, prior VTE, and male sex remained significant (P < .05) (Table 5).
Discussion
TKA is a safe and effective procedure used to treat osteoarthritis of the knee and improve patients’ quality of life.15 About 700,000 TKAs are performed annually in the United States.16 Because of improvements in preventive medicine and medical technology, life expectancy is increasing, and TKAs are now being performed in higher numbers and in an older patient population. Over the next few decades, these developments will lead to more postoperative complications. It is projected that, by 2030, the need for TKAs in the United States will increase by 673% to 3.48 million.17 Postoperative complications are rare but unfortunately often lead to poor outcomes or even mortality.18 To help minimize the number of postoperative complications, we must understand the safety of tourniquet use in TKA. Other investigators have concluded that tourniquet use is unsafe in patients with preoperative vascular calcifications on plain radiographs.7,8,11 The present study, designed to elucidate whether preoperative evidence of knee arterial calcification may predispose TKA patients to postoperative wound complication or VTE, had some important findings.
In our study, wound complication and VTE occurred in a considerable number of patients after TKA. Despite exceeding the number of patients calculated by the power analysis, our population may have been inadequate to fully detect statistical significance. Thus, our conclusion of failing to reject the null hypothesis may have been because of sample size, a type II error. We found that, after primary TKA, 3.04% of patients developed wound complications and 4.77% VTE. According to the literature, the incidence of infection after primary TKA is between 0.5% and 12%, and that of VTE reported within 3 months after TKA is 1.3% to 10%.13,14 Although we had 100% VTE prophylaxis, meeting the standard of care, VTE after TKA remains a postoperative complication.19 This study also found that a considerable percentage of primary TKA patients (23.59%) had preoperative calcification of the knee arteries. To our knowledge, this study was the first to quantify the incidence of knee arterial calcification in patients who underwent TKA.
Preoperative calcification of the knee arteries in patients who underwent TKA did not increase the risk for wound complication, VTE, or arterial damage. These calcifications, however, do pose an increased systemic vascular risk.20 Calcification of the vascular wall predicts increased cardiovascular risk, independent of classical cardiovascular risk factors.3,18,21-24 Clinically, patients who have both diabetes and calcifications are at significant excess risk for total mortality, stroke mortality, and cardiovascular mortality, compared with patients with diabetes but without such calcifications. They also had a significantly higher incidence of coronary heart disease events, stroke events, and lower extremity amputations.25,26
All our patients underwent tourniquet-assisted TKA. Although previous studies have indicated that tourniquet use may increase arterial complications and wound complications or even limb loss in patients with calcified arteries, we did not find this link.7,27 Our population had no reported arterial complications related to tourniquet use. Other, smaller studies have had similar findings. Vandenbussche and colleagues28 prospectively studied 80 TKA cases randomized to tourniquet use or no tourniquet use and found no postoperative nerve palsies, wound infections, wound healing problems, or hematomas. Our study is also in accord with studies that have reported tourniquet use did not increase risk for DVT.29 Therefore, unlike earlier data, our data demonstrated that tourniquet use in patients with knee arterial calcification was safe.7,27,30,31
Patients with calcification were more likely to have the medical comorbidities of hypertension, diabetes, hyperlipidemia, and CAD. All these comorbidities are linked to the development of arterial calcification, or atherosclerotic occlusive disease.32,33 As life expectancy and the need for TKA increase, it is likely that a larger percentage of TKA patients will have preoperative radiographic evidence of knee arterial calcification. Although current dogma is that tourniquet-assisted TKA is contraindicated for patients with preoperative radiographic evidence of femoral-popliteal calcification, our study results showed that this calcification should not affect preoperative TKA planning for these patients.
We divided our patients into 3 categories: those with proximal calcification (above the joint line), those with distal calcification (below the joint line), and those with both proximal and distal calcification. Location of arterial calcification did not have an effect on their rates of postoperative wound complication or VTE. We hypothesized that patients with proximal calcification would be at increased risk for direct arterial injury and subsequent wound complication because the tourniquet is placed proximally. Previous research has indicated that arterial occlusion and subsequent wound complication can occur because of low blood flow stemming from tourniquet use.7 Further, intraoperative manipulation (flexing) of a knee with calcified vessels causes arterial complications after TKA because these vessels are less elastic than nonatheromatous vessels.31 However, we found no such effect. At the same time, having arterial calcification might also be an indication of venous disease in this location,12 which may be especially important for proximal calcifications. Proximal DVT more likely is a precursor to pulmonary embolic events than distal DVT is.31,34 However, we found no difference in VTE rates among the 3 arterial location groups, which is supported by studies that have found that tourniquet use does not increase DVT incidence.29,35-40
Risk for wound complications was higher in male patients and in patients with diabetes, prior VTE, hypertension, or CAD. This finding is important because, with the increasing age of patients who undergo TKA, those with serious medical comorbidities will continue to need and have this surgery.17 Diabetes may increase the rate of wound complication because patients with diabetes have poor microcirculation, poor collagen synthesis, and reduced wound strength.41 Malinzak and colleagues42 demonstrated that, compared with patients without diabetes, those with diabetes had a significantly higher risk for infection after TKA. Prior VTE, specifically DVT, may increase the rate of wound complication because after DVT the deep veins may be damaged and exhibit valvular dysfunction. Labropoulos and colleagues43 showed that DVT history was strongly associated with ulcer nonhealing. Perhaps hypertension has been overlooked as a risk factor for wound complication in TKA. No previous studies have assessed the link between hypertension and wound complications after TKA. However, a study of wound healing after total hip arthroplasty found that, compared with normotensive patients, hypertensive patients had delayed wound healing, putting them at higher risk for infection.44 In addition, we found that patients with CAD were at increased risk for wound complications—an unexpected finding, as CAD traditionally is not a risk factor for infection or poor wound healing. Recently, however, CAD was identified as an independent risk factor for surgical site infections in posterior lumbar–instrumented arthrodesis.45 The etiology of this association is unknown. Also, male patients were at increased risk for wound complication. Male sex has been implicated as an independent risk factor for development of surgical site infections and has been established as an important predisposing factor for periprosthetic joint infections.46
It is possible that patients who present with diabetes, VTE, hypertension, or CAD before TKA should have a consultation with a vascular surgeon or should have TKA performed without a tourniquet, but this conclusion cannot be considered definitive without a large prospective randomized trial or possibly registry data. Our data indicate that patients with these comorbidities have higher rates of wound complications irrespective of preoperative radiographic calcifications. On the basis of our study results, however, we certainly recommend that patients with these risk factors have preoperative medical optimization. Orthopedic surgeons should take a thorough history and perform a meticulous physical examination on these patients to look for evidence of PVD. We recommend that, if vascular claudication is elicited in the history, or if there is evidence of peripheral arterial disease—such as hair loss, skin discoloration, dystrophic nail changes, or absent or unequal peripheral pulses—the ankle-brachial index test should be performed. If the index value is less than 0.9, then a preoperative vascular surgery consultation should be obtained.
This study had some weaknesses. First, it was retrospective, so it is possible that some wound or VTE complications were not reported and thus not found in the paper charts or electronic medical records. Some patients may have had VTE diagnostic scans at other hospitals, and their results may not have been recorded across databases. Moreover, some patients may have seen wound specialists for wound infections or wound healing problems, and these may not have been reported to the orthopedic surgeons. Second, though our patient population was not small, it may not have been of adequate size to fully detect statistical significance. We met our enrollment numbers based on our sample size calculations from an a priori power analysis; however, we still draw conclusions with the possibility of committing a type II error in mind by failing to reject the null hypothesis when in reality a statistically significant difference does exist. Third, none of our consecutive patients carried the preoperative diagnosis of PVD, and none had preoperative vascular surgery. Therefore, though calcifications were noted on radiographs, clinically our patients were asymptomatic with respect to vascular health. Last, the 2 groups were not randomized. All patients underwent tourniquet-assisted TKA.
Conclusion
To our knowledge, this is the largest study to examine the effect of preoperative knee arterial calcification on wound complication and VTE after tourniquet-assisted TKA. Contrary to previously published recommendations, we conclude that TKA can be safely performed with a tourniquet in the presence of preoperative radiographic evidence of such calcification. However, we recommend that patients with diabetes, hypertension, CAD, or prior VTE undergo an appropriate physical examination to elicit any signs or symptoms of vascular disease. If before surgery there is any question of vascular competence, a vascular surgeon should be consulted.
1. Guanche CA. Tourniquet-induced tibial nerve palsy complicating anterior cruciate ligament reconstruction. Arthroscopy. 1995;11(5):620-622.
2. Irvine GB, Chan RN. Arterial calcification and tourniquets. Lancet. 1986;2(8517):1217.
3. Patterson S, Klenerman L. The effect of pneumatic tourniquets on the ultrastructure of skeletal muscle. J Bone Joint Surg Br. 1979;61(2):178-183.
4. Rorabeck CH, Kennedy JC. Tourniquet-induced nerve ischemia complicating knee ligament surgery. Am J Sports Med. 1980;8(2):98-102.
5. Shenton DW, Spitzer SA, Mulrennan BM. Tourniquet-induced rhabdomyolysis. A case report. J Bone Joint Surg Am. 1990;72(9):1405-1406.
6. Abdel-Salam A, Eyres KS. Effects of tourniquet during total knee arthroplasty. A prospective randomised study. J Bone Joint Surg Br. 1995;77(2):250-253.
7. DeLaurentis DA, Levitsky KA, Booth RE, et al. Arterial and ischemic aspects of total knee arthroplasty. Am J Surg. 1992;164(3):237-240.
8. Holmberg A, Milbrink J, Bergqvist D. Arterial complications and knee arthroplasty. Acta Orthop Scand. 1996;67(1):75-8.
9. Hozack WJ, Cole PA, Gardner R, Corces A. Popliteal aneurysm after total knee arthroplasty. Case reports and review of the literature. J Arthroplasty. 1990;5(4):301-305.
10. Kumar SN, Chapman JA, Rawlins I. Vascular injuries after total knee arthroplasty: a review of the problem with special reference to the possible effects of the tourniquet. J Arthroplasty. 1998;13(2):211-216.
11. Rush JH, Vidovich JD, Johanson MA. Arterial complications and total knee arthroplasty. The Australian experience. J Bone Joint Surg Br. 1987;69(3):400-402.
12. Callam MJ, Harper DR, Dale JJ, Ruckley CV. Arterial disease in chronic leg ulceration: an underestimated hazard? Lothian and Forth Valley Leg Ulcer Study. Br Med J (Clin Res Ed). 1987;294(6577):929-931.
13. Blom AW, Brown J, Taylor AH, Pattison G, Whitehouse S, Bannister GC. Infection after total knee arthroplasty. J Bone Joint Surg Br. 2004;86(5):688-691.
14. Geerts WH, Bergqvist D, Pinco G, et al. Prevention of venous thromboembolism. Chest. 2008;133(6 suppl):381S-453S.
15. Pulido L, Parvizi J, Macgibeny M, et al. In hospital complications after total joint arthroplasty. J Arthroplasty. 2008;23(6 Suppl 1):139-145.
16. Arthritis: data and statistics. Centers for Disease Control and Prevention website. http://www.cdc.gov/arthritis/data_statistics.htm. Updated March 11, 2015. Accessed July 27, 2015.
17. Kurtz S, Ong K, Lau E, Mowat F, Halpern M. Projections of primary and revision hip and knee arthroplasty in the United States from 2005 to 2030. J Bone Joint Surg Am. 2007;89(4):780-785.
18. Pulido L, Ghanem E, Joshi A, Purtill JJ, Parvizi J. Periprosthetic joint infection: the incidence, timing, and predisposing factors. Clin Orthop Relat Res. 2008;466(7):1710-1715.
19. Warwick D. Prevention of venous thromboembolism in total knee and hip replacement. Circulation. 2012;125(17):2151-2155.
20. Rennenberg RJ, Kessels AG, Schurgers LJ, van Engelshoven JM, de Leeuw PW, Kroon AA. Vascular calcifications as a marker of increased cardiovascular risk: a meta-analysis. Vasc Health Risk Manag. 2009;5(1):185-197.
21. Arad Y, Goodman KJ, Roth M, Newstein D, Guerci AD. Coronary calcification, coronary disease risk factors, C-reactive protein, and atherosclerotic cardiovascular disease events: the St. Francis Heart Study. J Am Coll Cardiol. 2005;46(1):158-165.
22. Iribarren C, Sidney S, Sternfeld B, Browner WS. Calcification of the aortic arch: risk factors and association with coronary heart disease, stroke, and peripheral vascular disease. JAMA. 2000;283(21):2810-2815.
23. Shaw LJ, Raggi P, Schisterman E, Berman DS, Callister TQ. Prognostic value of cardiac risk factors and coronary artery calcium screening for all-cause mortality. Radiology. 2003;228(3):826-833.
24. Taylor AJ, Bindeman J, Feuerstein I, Cao F, Brazaitis M, O’Malley PG. Coronary calcium independently predicts incident premature coronary heart disease over measured cardiovascular risk factors: mean three-year outcomes in the Prospective Army Coronary Calcium (PACC) project. J Am Coll Cardiol. 2005;46(5):807-814.
25. Lehto S, Niskanen L, Suhonen M, Rönnemaa T, Laakso M. Medial artery calcification. A neglected harbinger of cardiovascular complications in non-insulin-dependent diabetes mellitus. Arterioscler Thromb Vasc Biol. 1996;16(8):978-983.
26. Niskanen L, Siitonen O, Suhonen M, Uusitupa MI. Medial artery calcification predicts cardiovascular mortality in patients with NIDDM. Diabetes Care. 1994;17(11):1252-1256.
27. Smith DE, McGraw RW, Taylor DC, et al. Arterial complications and total knee arthroplasty. J Am Acad Orthop Surg. 2001;9(4):253-257.
28. Vandenbussche E, Duranthon L, Couturier M, Pidhorz L, Augereau B. The effect of tourniquet use in total knee arthroplasty. Int Orthop. 2002;26(5):306-309.
29. Fukunda A, Hasegawa M, Kato K, Shi D, Sudo A, Uchida A. Effect of tourniquet application on deep vein thrombosis after total knee thrombosis. Arch Orthop Trauma Surg. 2007;127(8):671-675.
30. Butt U, Samuel R, Sahu A, Butt IS, Johnson DS, Turner PG. Arterial injury in total knee arthroplasty. J Arthroplasty. 2010;25(8):1311-1318.
31. Langkamer VG. Local vascular complications after knee replacement: a review with illustrative case reports. Knee. 2001;8(4):259-264.
32. Hussein A, Uno K, Wolski K, et al. Peripheral arterial disease and progression of coronary atherosclerosis. J Am Coll Cardiol. 2011;57(10):1220-1225.
33. Ouriel K. Peripheral arterial disease. Lancet. 2001;358(9289):1257-1264.
34. Monreal M, Rufz J, Olazabal A, Arias A, Roca J. Deep venous thrombosis and the risk of pulmonary embolism. Chest. 1992;102(3):677-681.
35. Angus PD, Nakielny R, Goodrum DT. The pneumatic tourniquet and deep venous thrombosis. J Bone Joint Surg Br. 1983;65(3):336-339.
36. Fahmy NR, Patel DG. Hemostatic changes and postoperative deep-vein thrombosis associated with use of a pneumatic tourniquet. J Bone Joint Surg Am. 1981;63(3):461-465.
37. Harvey EJ, Leclerc J, Brooks CE, Burke DL. Effect of tourniquet use on blood loss and incidence of deep vein thrombosis in total knee arthroplasty. J Arthroplasty. 1997;12(3):291-296.
38. Simon MA, Mass DP, Zarins CK, Bidani N, Gudas CJ, Metz CE. The effect of a thigh tourniquet on the incidence of deep venous thrombosis after operations on the fore part of the foot. J Bone Joint Surg Am. 1982;64(2):188-191.
39. Stulberg BN, Insall JN, Williams GW, Ghelman B. Deep-vein thrombosis following total knee replacement. An analysis of six hundred and thirty-eight arthroplasties. J Bone Joint Surg Am. 1984;66(2):194-201.
40. Wakankar HM, Nicholl JE, Koka R, D’Arcy JC. The tourniquet in total knee arthroplasty. A prospective, randomized study. J Bone Joint Surg Br. 1999;81(1):30-33.
41. Vince K, Chivas D, Droll K. Wound complications after total knee arthroplasty. J Arthroplasty. 2007;22(4 Suppl 1):39-44.
42. Malinzak RA, Ritter MA, Berend ME, Meding JB, Olberding EM, Davis KE. Morbidly obese, diabetic, younger, and unilateral joint arthroplasty patients have elevated total joint arthroplasty infection rates. J Arthroplasty. 2009;24(6 Suppl):84-88.
43. Labropoulos N, Wang E, Lanier S, Khan SU. Factors associated with poor healing and recurrence of venous ulceration. Plast Reconstr Surg. 2011;129(1):179-186.
44. Ahmed AA, Mooar PA, Kleiner M, Torg JS, Miyamoto CT. Hypertensive patients show delayed wound healing following total hip arthroplasty. PLoS One. 2011;6(8):e23224.
45. Koutsoumbelis S, Hughes AP, Girardi FP, et al. Risk factors for postoperative infection following posterior lumbar instrumented arthrodesis. J Bone Joint Surg Am. 2001;93(17):1627-1633.
46. Poultsides LA, Ma Y, Della Valle AG, Chiu YL, Sculco TP, Memtsoudis SG. In-hospital surgical site infections after primary hip and knee arthroplasty—incidence and risk factors. J Arthroplasty. 2013;28(3):385-389.
1. Guanche CA. Tourniquet-induced tibial nerve palsy complicating anterior cruciate ligament reconstruction. Arthroscopy. 1995;11(5):620-622.
2. Irvine GB, Chan RN. Arterial calcification and tourniquets. Lancet. 1986;2(8517):1217.
3. Patterson S, Klenerman L. The effect of pneumatic tourniquets on the ultrastructure of skeletal muscle. J Bone Joint Surg Br. 1979;61(2):178-183.
4. Rorabeck CH, Kennedy JC. Tourniquet-induced nerve ischemia complicating knee ligament surgery. Am J Sports Med. 1980;8(2):98-102.
5. Shenton DW, Spitzer SA, Mulrennan BM. Tourniquet-induced rhabdomyolysis. A case report. J Bone Joint Surg Am. 1990;72(9):1405-1406.
6. Abdel-Salam A, Eyres KS. Effects of tourniquet during total knee arthroplasty. A prospective randomised study. J Bone Joint Surg Br. 1995;77(2):250-253.
7. DeLaurentis DA, Levitsky KA, Booth RE, et al. Arterial and ischemic aspects of total knee arthroplasty. Am J Surg. 1992;164(3):237-240.
8. Holmberg A, Milbrink J, Bergqvist D. Arterial complications and knee arthroplasty. Acta Orthop Scand. 1996;67(1):75-8.
9. Hozack WJ, Cole PA, Gardner R, Corces A. Popliteal aneurysm after total knee arthroplasty. Case reports and review of the literature. J Arthroplasty. 1990;5(4):301-305.
10. Kumar SN, Chapman JA, Rawlins I. Vascular injuries after total knee arthroplasty: a review of the problem with special reference to the possible effects of the tourniquet. J Arthroplasty. 1998;13(2):211-216.
11. Rush JH, Vidovich JD, Johanson MA. Arterial complications and total knee arthroplasty. The Australian experience. J Bone Joint Surg Br. 1987;69(3):400-402.
12. Callam MJ, Harper DR, Dale JJ, Ruckley CV. Arterial disease in chronic leg ulceration: an underestimated hazard? Lothian and Forth Valley Leg Ulcer Study. Br Med J (Clin Res Ed). 1987;294(6577):929-931.
13. Blom AW, Brown J, Taylor AH, Pattison G, Whitehouse S, Bannister GC. Infection after total knee arthroplasty. J Bone Joint Surg Br. 2004;86(5):688-691.
14. Geerts WH, Bergqvist D, Pinco G, et al. Prevention of venous thromboembolism. Chest. 2008;133(6 suppl):381S-453S.
15. Pulido L, Parvizi J, Macgibeny M, et al. In hospital complications after total joint arthroplasty. J Arthroplasty. 2008;23(6 Suppl 1):139-145.
16. Arthritis: data and statistics. Centers for Disease Control and Prevention website. http://www.cdc.gov/arthritis/data_statistics.htm. Updated March 11, 2015. Accessed July 27, 2015.
17. Kurtz S, Ong K, Lau E, Mowat F, Halpern M. Projections of primary and revision hip and knee arthroplasty in the United States from 2005 to 2030. J Bone Joint Surg Am. 2007;89(4):780-785.
18. Pulido L, Ghanem E, Joshi A, Purtill JJ, Parvizi J. Periprosthetic joint infection: the incidence, timing, and predisposing factors. Clin Orthop Relat Res. 2008;466(7):1710-1715.
19. Warwick D. Prevention of venous thromboembolism in total knee and hip replacement. Circulation. 2012;125(17):2151-2155.
20. Rennenberg RJ, Kessels AG, Schurgers LJ, van Engelshoven JM, de Leeuw PW, Kroon AA. Vascular calcifications as a marker of increased cardiovascular risk: a meta-analysis. Vasc Health Risk Manag. 2009;5(1):185-197.
21. Arad Y, Goodman KJ, Roth M, Newstein D, Guerci AD. Coronary calcification, coronary disease risk factors, C-reactive protein, and atherosclerotic cardiovascular disease events: the St. Francis Heart Study. J Am Coll Cardiol. 2005;46(1):158-165.
22. Iribarren C, Sidney S, Sternfeld B, Browner WS. Calcification of the aortic arch: risk factors and association with coronary heart disease, stroke, and peripheral vascular disease. JAMA. 2000;283(21):2810-2815.
23. Shaw LJ, Raggi P, Schisterman E, Berman DS, Callister TQ. Prognostic value of cardiac risk factors and coronary artery calcium screening for all-cause mortality. Radiology. 2003;228(3):826-833.
24. Taylor AJ, Bindeman J, Feuerstein I, Cao F, Brazaitis M, O’Malley PG. Coronary calcium independently predicts incident premature coronary heart disease over measured cardiovascular risk factors: mean three-year outcomes in the Prospective Army Coronary Calcium (PACC) project. J Am Coll Cardiol. 2005;46(5):807-814.
25. Lehto S, Niskanen L, Suhonen M, Rönnemaa T, Laakso M. Medial artery calcification. A neglected harbinger of cardiovascular complications in non-insulin-dependent diabetes mellitus. Arterioscler Thromb Vasc Biol. 1996;16(8):978-983.
26. Niskanen L, Siitonen O, Suhonen M, Uusitupa MI. Medial artery calcification predicts cardiovascular mortality in patients with NIDDM. Diabetes Care. 1994;17(11):1252-1256.
27. Smith DE, McGraw RW, Taylor DC, et al. Arterial complications and total knee arthroplasty. J Am Acad Orthop Surg. 2001;9(4):253-257.
28. Vandenbussche E, Duranthon L, Couturier M, Pidhorz L, Augereau B. The effect of tourniquet use in total knee arthroplasty. Int Orthop. 2002;26(5):306-309.
29. Fukunda A, Hasegawa M, Kato K, Shi D, Sudo A, Uchida A. Effect of tourniquet application on deep vein thrombosis after total knee thrombosis. Arch Orthop Trauma Surg. 2007;127(8):671-675.
30. Butt U, Samuel R, Sahu A, Butt IS, Johnson DS, Turner PG. Arterial injury in total knee arthroplasty. J Arthroplasty. 2010;25(8):1311-1318.
31. Langkamer VG. Local vascular complications after knee replacement: a review with illustrative case reports. Knee. 2001;8(4):259-264.
32. Hussein A, Uno K, Wolski K, et al. Peripheral arterial disease and progression of coronary atherosclerosis. J Am Coll Cardiol. 2011;57(10):1220-1225.
33. Ouriel K. Peripheral arterial disease. Lancet. 2001;358(9289):1257-1264.
34. Monreal M, Rufz J, Olazabal A, Arias A, Roca J. Deep venous thrombosis and the risk of pulmonary embolism. Chest. 1992;102(3):677-681.
35. Angus PD, Nakielny R, Goodrum DT. The pneumatic tourniquet and deep venous thrombosis. J Bone Joint Surg Br. 1983;65(3):336-339.
36. Fahmy NR, Patel DG. Hemostatic changes and postoperative deep-vein thrombosis associated with use of a pneumatic tourniquet. J Bone Joint Surg Am. 1981;63(3):461-465.
37. Harvey EJ, Leclerc J, Brooks CE, Burke DL. Effect of tourniquet use on blood loss and incidence of deep vein thrombosis in total knee arthroplasty. J Arthroplasty. 1997;12(3):291-296.
38. Simon MA, Mass DP, Zarins CK, Bidani N, Gudas CJ, Metz CE. The effect of a thigh tourniquet on the incidence of deep venous thrombosis after operations on the fore part of the foot. J Bone Joint Surg Am. 1982;64(2):188-191.
39. Stulberg BN, Insall JN, Williams GW, Ghelman B. Deep-vein thrombosis following total knee replacement. An analysis of six hundred and thirty-eight arthroplasties. J Bone Joint Surg Am. 1984;66(2):194-201.
40. Wakankar HM, Nicholl JE, Koka R, D’Arcy JC. The tourniquet in total knee arthroplasty. A prospective, randomized study. J Bone Joint Surg Br. 1999;81(1):30-33.
41. Vince K, Chivas D, Droll K. Wound complications after total knee arthroplasty. J Arthroplasty. 2007;22(4 Suppl 1):39-44.
42. Malinzak RA, Ritter MA, Berend ME, Meding JB, Olberding EM, Davis KE. Morbidly obese, diabetic, younger, and unilateral joint arthroplasty patients have elevated total joint arthroplasty infection rates. J Arthroplasty. 2009;24(6 Suppl):84-88.
43. Labropoulos N, Wang E, Lanier S, Khan SU. Factors associated with poor healing and recurrence of venous ulceration. Plast Reconstr Surg. 2011;129(1):179-186.
44. Ahmed AA, Mooar PA, Kleiner M, Torg JS, Miyamoto CT. Hypertensive patients show delayed wound healing following total hip arthroplasty. PLoS One. 2011;6(8):e23224.
45. Koutsoumbelis S, Hughes AP, Girardi FP, et al. Risk factors for postoperative infection following posterior lumbar instrumented arthrodesis. J Bone Joint Surg Am. 2001;93(17):1627-1633.
46. Poultsides LA, Ma Y, Della Valle AG, Chiu YL, Sculco TP, Memtsoudis SG. In-hospital surgical site infections after primary hip and knee arthroplasty—incidence and risk factors. J Arthroplasty. 2013;28(3):385-389.
Perilunate Injuries
Perilunate injuries typically stem from a high-energy insult to the carpus. Because of their relative infrequency and often subtle radiographic and physical examination findings, these injuries are often undetected in the emergency department setting.1 Early anatomic reduction of any carpal malalignment is essential. Even with optimal treatment, complications such as generalized wrist stiffness, diminished grip strength, and posttraumatic arthritis, commonly develop; however, recent studies suggest these issues are often well tolerated.1-5 In this article, the diagnosis, treatment, and outcomes after perilunate injuries are examined.
History and Physical Examination
Perilunate injuries result from high-energy trauma to the carpus. Patients with these injuries often present with vague wrist pain and loss of wrist motion. Their fingers are frequently held in slight flexion. The patient may complain of numbness and tingling in the median nerve distribution. An acute carpal tunnel syndrome can rapidly develop. The general belief is that acute carpal tunnel syndrome occurs more commonly in pure volar lunate dislocations than in dorsal perilunate dislocations. However, no studies compare the incidence of acute carpal tunnel syndrome in lunate versus perilunate dislocations.
Radiographic Evaluation
Standard radiographic evaluation of a potential perilunate injury includes posteroanterior (PA), lateral, and oblique views of the wrist (Figure 1). A scaphoid view (ie, PA view with the wrist in ulnar deviation) may also be helpful. The PA view is particularly helpful because it enables assessment of Gilula lines, which are imaginary lines drawn across the proximal and distal aspects of the proximal carpal row and the proximal aspect of the distal carpal row. These lines should appear as 3 smooth arcs running nearly parallel to each other.6 Any disruption in these lines suggests carpal incongruity. It may be possible to note a triangular-shaped lunate on the PA view, which is a sign of lunate dislocation.7
While the PA view is certainly useful, the lateral view is the most important in diagnosing a perilunate injury. The lateral view allows assessment of the collinearity of radius, lunate, and capitate. Any disruption in this collinearity strongly suggests a perilunate dislocation.7,8
Classification
Mayfield and colleagues9,10 described 4 stages of perilunate instability proceeding from a radial to an ulnar direction around the lunate. Stage I involves disruption of the scapholunate joint, while stage II involves both the scapholunate and capitolunate joints. In stage III, the scapholunate, capitolunate, and lunotriquetral ligaments are disrupted, and the result is a perilunate dislocation, usually dorsal. Finally, in stage IV, all the ligaments surrounding the lunate are disrupted and the lunate dislocates, most often volarly.
Lastly, perilunate injuries can be classified as greater-arc injuries if concomitant fracture of the carpus occurs, lesser-arc injuries if the injury is purely ligamentous, or inferior-arc injuries if there is an associated fracture of the volar rim of the distal radius.8
Treatment
Closed Reduction
All acute perilunate dislocations should be managed initially with an attempted closed reduction.11 If the injury is older than 72 hours, such an attempt may be futile. For any closed reduction performed in the emergency department setting, intravenous sedation is generally advised for muscle relaxation. Gentle traction with finger traps can also be used prior to the reduction attempt. For a dorsal perilunate dislocation, longitudinal traction followed by volar flexion of the wrist with volar pressure on the lunate and dorsal pressure on the capitate (ie, Tavernier’s maneuver) is required. Once reduction is complete, PA and lateral views of the wrist should be obtained to assess carpal alignment. If closed reduction is unsuccessful, an open reduction is required, although the timing of said procedure is an area of debate, which we will discuss later.1,3 Restoration of anatomic carpal alignment is essential to optimizing outcome, although it does not guarantee a good overall result.
Open Reduction
If successful closed reduction is achieved, the patient can be immobilized temporarily in a short-arm plaster splint. However, open reduction and either pinning or internal fixation will be required to maintain this alignment. The exact timing of open reduction and fixation is debatable and often dictated by the absence or presence of median nerve symptoms.1,3 If a patient with no median nerve symptoms undergoes a successful closed reduction, he or she may be stabilized surgically within 3 to 5 days (or longer) with either pins or headless screws. If closed reduction is unsuccessful, an open reduction should be done within 2 to 3 days. However, if the patient has progressive numbness in the median nerve distribution upon presentation that fails to improve or worsens despite a successful closed reduction, an urgent open reduction (within 24 hours) and carpal tunnel release should be performed to prevent permanent damage to the median nerve.
Once open reduction is undertaken, a dorsal, volar, and combined approach can be used.2-4 In most cases the dorsal approach is selected first. A longitudinal incision is made over the dorsum of the wrist, centered on the Lister tubercle. Dissection occurs between the third and fourth dorsal compartments. After the capsule is exposed, reduction of the lunate to the capitate is confirmed. If any fractures are present in the carpus (eg, scaphoid), they are internally fixed. The scapholunate articulation is then addressed. In general, the scapholunate ligament is not disrupted with a transscaphoid perilunate dislocation. However, if the scapholunate ligament is disrupted, the joint should be reduced and pinned. Repair or reconstruction of the scapholunate ligament is performed. Finally, the lunotriquetral articulation is reduced and stabilized with pins. There are no studies that specifically suggest direct repair of the lunotriquetral ligament versus pinning of the lunotriquetral articulation, but the lunotriquetral ligament could be repaired in similar fashion to the scapholunate ligament at the surgeon’s discretion.
As an alternative to percutaneous pinning, intercarpal screw fixation can be used to stabilize the carpus. A 2007 study by Souer and colleagues12 showed no substantial difference in outcome between the 2 methods of fixation. However, a second procedure is required to remove the screws.
The volar approach, if selected, is typically done second and performed via an extended carpal tunnel incision. It allows decompression of the carpal tunnel and enables repair of volar capsular ligaments (ie, long and short radiolunate ligaments, volar scapholunate ligament, and volar lunotriquetral ligament), which increases overall carpal stability. Currently, many surgeons favor a combined dorsal-volar approach for its efficacy.2,3 Some use a dorsal approach in all patients and perform a volar procedure only if the patient has median nerve symptoms.4 However, Başar and colleagues13 report use of only the volar approach for treatment of perilunate injuries. The authors repaired the long and short radiolunate ligaments, volar scapholunate ligament, and volar lunotriquetral ligament. They reported reasonably good outcomes, which are equivalent to those reported in similar studies using dorsal or combined dorsal-volar approaches. However, no studies in the literature directly compare any of the different approaches with each other.
Postoperatively, patients are placed in a long-arm thumb-spica cast for 4 weeks, and then in a short-arm cast for 4 to 8 weeks (Figure 2). If present, pins are removed in 3 to 12 weeks, with most authors recommending removal at 8 weeks.2,14
Lastly, carpal tunnel symptoms can develop late and even after a successful reduction and surgical stabilization. One theory is that a significant perilunate injury can create slightly higher baseline carpal tunnel pressures, which can compromise the blood flow to the median nerve and cause carpal tunnel symptoms. Additionally, it is possible that direct median nerve contusion and/or traction injury via a displaced lunate can also cause these symptoms. Whatever the inciting cause of median-nerve irritation, a delayed carpal tunnel release is sometimes required.
Conclusion
Outcomes after either perilunate or lunate dislocation are fair to good at best but can be optimized with prompt, appropriate treatment. Closed reduction and casting as definitive treatment has been abandoned because of frequent loss of reduction.12 Early open reduction (ie, less than 3 days after injury) has been shown to be beneficial.1,2 However, even those treated early and with anatomic restoration of carpal alignment can expect a loss of grip strength and a range of motion of approximately 70% compared with the contralateral side.2-5 A recent study has suggested that lesser-arc injures generally have a poorer overall outcome than their greater-arc counterparts.15
More than half of all patients with perilunate injuries will develop radiographic signs of osteoarthritis, and some will require additional salvage procedures.3-5 Kremer and colleagues4 showed that overall results after perilunate injuries deteriorate with time. However, according to a paper by Forli and colleagues5 in which patients were followed a minimum of 10 years after their injuries, the authors found that, despite radiographic progression of arthritis, most patients maintained reasonable hand function.
1. Herzberg G, Comtet JJ, Linscheid RL, Amadio PC, Cooney WP, Stalder J. Perilunate dislocations and fracture-dislocations: a multicenter study. J Hand Surg Am. 1993;18(5):768-779.
2. Sotereanos DG, Mitsionis GJ, Giannakopoulos PN, Tomaino MM, Herndon JH. Perilunate dislocation and fracture dislocation: a critical analysis of the volar-dorsal approach. J Hand Surg Am. 1997;22(1):49-56.
3. Hildebrand KA, Ross DC, Patterson SD, Roth JH, MacDermid JC, King GJ. Dorsal perilunate dislocations and fracture-dislocations: questionnaire, clinical, and radiographic evaluation. J Hand Surg Am. 2000;25(6):1069-1079.
4. Kremer T, Wendt M, Riedel K, Sauerbier M, Germann G, Bickert B. Open reduction for perilunate injuries--clinical outcome and patient satisfaction. J Hand Surg Am. 2010;35(10):1599-1606.
5. Forli A, Courvoisier A, Wimsey S, Corcella D, Moutet F. Perilunate dislocations and transscaphoid perilunate fracture-dislocations: a retrospective study with minimum ten-year follow-up. J Hand Surg Am. 2010;35(1):62-68.
6. Gilula LA. Carpal injuries: analytic approach and case exercises. AJR Am J Roentgenol. 1979;133(3):503-517.
7. Kozin SH. Perilunate injuries: diagnosis and treatment. J Am Acad Orthop Surg. 1998;6(2):114-120.
8. Graham TJ. The inferior arc injury: an addition to the family of complex carpal fracture-dislocation patterns. Am J Orthop. 2003;32(9 suppl):10-19.
9. Mayfield JK, Johnson RP, Kilcoyne RK. Carpal dislocations: pathomechanics and progressive perilunar instability. J Hand Surg Am. 1980;5(3):226-241.
10. Mayfield JK. Mechanism of carpal injuries. Clin Orthop Relat Res. 1980;149:45-54.
11. Adkison JW, Chapman MW. Treatment of acute lunate and perilunate dislocations. Clin Orthop Relat Res. 1982;164:199-207.
12. Souer JS, Rutgers M, Andermahr J, Jupiter JB, Ring D. Perilunate fracture-dislocations of the wrist: comparison of temporary screw versus K-wire fixation. J Hand Surg Am. 2007;32(3):318-325.
13. Başar H, Başar B, Erol B, Tetik C. Isolated volar surgical approach for the treatment of perilunate and lunate dislocations. Indian J Orthop. 2014;48(3):301-315.
14. Komurcu M, Kürklü M, Ozturan KE, Mahirogullari M, Basbozkurt M. Early and delayed treatment of dorsal transscaphoid perilunate fracture-dislocations. J Orthop Trauma. 2008;22:535-540.
15. Massoud AH, Naam NH. Functional outcome of open reduction of chronic perilunate injuries. J Hand Surg Am. 2012;37(9):1852-1860.
Perilunate injuries typically stem from a high-energy insult to the carpus. Because of their relative infrequency and often subtle radiographic and physical examination findings, these injuries are often undetected in the emergency department setting.1 Early anatomic reduction of any carpal malalignment is essential. Even with optimal treatment, complications such as generalized wrist stiffness, diminished grip strength, and posttraumatic arthritis, commonly develop; however, recent studies suggest these issues are often well tolerated.1-5 In this article, the diagnosis, treatment, and outcomes after perilunate injuries are examined.
History and Physical Examination
Perilunate injuries result from high-energy trauma to the carpus. Patients with these injuries often present with vague wrist pain and loss of wrist motion. Their fingers are frequently held in slight flexion. The patient may complain of numbness and tingling in the median nerve distribution. An acute carpal tunnel syndrome can rapidly develop. The general belief is that acute carpal tunnel syndrome occurs more commonly in pure volar lunate dislocations than in dorsal perilunate dislocations. However, no studies compare the incidence of acute carpal tunnel syndrome in lunate versus perilunate dislocations.
Radiographic Evaluation
Standard radiographic evaluation of a potential perilunate injury includes posteroanterior (PA), lateral, and oblique views of the wrist (Figure 1). A scaphoid view (ie, PA view with the wrist in ulnar deviation) may also be helpful. The PA view is particularly helpful because it enables assessment of Gilula lines, which are imaginary lines drawn across the proximal and distal aspects of the proximal carpal row and the proximal aspect of the distal carpal row. These lines should appear as 3 smooth arcs running nearly parallel to each other.6 Any disruption in these lines suggests carpal incongruity. It may be possible to note a triangular-shaped lunate on the PA view, which is a sign of lunate dislocation.7
While the PA view is certainly useful, the lateral view is the most important in diagnosing a perilunate injury. The lateral view allows assessment of the collinearity of radius, lunate, and capitate. Any disruption in this collinearity strongly suggests a perilunate dislocation.7,8
Classification
Mayfield and colleagues9,10 described 4 stages of perilunate instability proceeding from a radial to an ulnar direction around the lunate. Stage I involves disruption of the scapholunate joint, while stage II involves both the scapholunate and capitolunate joints. In stage III, the scapholunate, capitolunate, and lunotriquetral ligaments are disrupted, and the result is a perilunate dislocation, usually dorsal. Finally, in stage IV, all the ligaments surrounding the lunate are disrupted and the lunate dislocates, most often volarly.
Lastly, perilunate injuries can be classified as greater-arc injuries if concomitant fracture of the carpus occurs, lesser-arc injuries if the injury is purely ligamentous, or inferior-arc injuries if there is an associated fracture of the volar rim of the distal radius.8
Treatment
Closed Reduction
All acute perilunate dislocations should be managed initially with an attempted closed reduction.11 If the injury is older than 72 hours, such an attempt may be futile. For any closed reduction performed in the emergency department setting, intravenous sedation is generally advised for muscle relaxation. Gentle traction with finger traps can also be used prior to the reduction attempt. For a dorsal perilunate dislocation, longitudinal traction followed by volar flexion of the wrist with volar pressure on the lunate and dorsal pressure on the capitate (ie, Tavernier’s maneuver) is required. Once reduction is complete, PA and lateral views of the wrist should be obtained to assess carpal alignment. If closed reduction is unsuccessful, an open reduction is required, although the timing of said procedure is an area of debate, which we will discuss later.1,3 Restoration of anatomic carpal alignment is essential to optimizing outcome, although it does not guarantee a good overall result.
Open Reduction
If successful closed reduction is achieved, the patient can be immobilized temporarily in a short-arm plaster splint. However, open reduction and either pinning or internal fixation will be required to maintain this alignment. The exact timing of open reduction and fixation is debatable and often dictated by the absence or presence of median nerve symptoms.1,3 If a patient with no median nerve symptoms undergoes a successful closed reduction, he or she may be stabilized surgically within 3 to 5 days (or longer) with either pins or headless screws. If closed reduction is unsuccessful, an open reduction should be done within 2 to 3 days. However, if the patient has progressive numbness in the median nerve distribution upon presentation that fails to improve or worsens despite a successful closed reduction, an urgent open reduction (within 24 hours) and carpal tunnel release should be performed to prevent permanent damage to the median nerve.
Once open reduction is undertaken, a dorsal, volar, and combined approach can be used.2-4 In most cases the dorsal approach is selected first. A longitudinal incision is made over the dorsum of the wrist, centered on the Lister tubercle. Dissection occurs between the third and fourth dorsal compartments. After the capsule is exposed, reduction of the lunate to the capitate is confirmed. If any fractures are present in the carpus (eg, scaphoid), they are internally fixed. The scapholunate articulation is then addressed. In general, the scapholunate ligament is not disrupted with a transscaphoid perilunate dislocation. However, if the scapholunate ligament is disrupted, the joint should be reduced and pinned. Repair or reconstruction of the scapholunate ligament is performed. Finally, the lunotriquetral articulation is reduced and stabilized with pins. There are no studies that specifically suggest direct repair of the lunotriquetral ligament versus pinning of the lunotriquetral articulation, but the lunotriquetral ligament could be repaired in similar fashion to the scapholunate ligament at the surgeon’s discretion.
As an alternative to percutaneous pinning, intercarpal screw fixation can be used to stabilize the carpus. A 2007 study by Souer and colleagues12 showed no substantial difference in outcome between the 2 methods of fixation. However, a second procedure is required to remove the screws.
The volar approach, if selected, is typically done second and performed via an extended carpal tunnel incision. It allows decompression of the carpal tunnel and enables repair of volar capsular ligaments (ie, long and short radiolunate ligaments, volar scapholunate ligament, and volar lunotriquetral ligament), which increases overall carpal stability. Currently, many surgeons favor a combined dorsal-volar approach for its efficacy.2,3 Some use a dorsal approach in all patients and perform a volar procedure only if the patient has median nerve symptoms.4 However, Başar and colleagues13 report use of only the volar approach for treatment of perilunate injuries. The authors repaired the long and short radiolunate ligaments, volar scapholunate ligament, and volar lunotriquetral ligament. They reported reasonably good outcomes, which are equivalent to those reported in similar studies using dorsal or combined dorsal-volar approaches. However, no studies in the literature directly compare any of the different approaches with each other.
Postoperatively, patients are placed in a long-arm thumb-spica cast for 4 weeks, and then in a short-arm cast for 4 to 8 weeks (Figure 2). If present, pins are removed in 3 to 12 weeks, with most authors recommending removal at 8 weeks.2,14
Lastly, carpal tunnel symptoms can develop late and even after a successful reduction and surgical stabilization. One theory is that a significant perilunate injury can create slightly higher baseline carpal tunnel pressures, which can compromise the blood flow to the median nerve and cause carpal tunnel symptoms. Additionally, it is possible that direct median nerve contusion and/or traction injury via a displaced lunate can also cause these symptoms. Whatever the inciting cause of median-nerve irritation, a delayed carpal tunnel release is sometimes required.
Conclusion
Outcomes after either perilunate or lunate dislocation are fair to good at best but can be optimized with prompt, appropriate treatment. Closed reduction and casting as definitive treatment has been abandoned because of frequent loss of reduction.12 Early open reduction (ie, less than 3 days after injury) has been shown to be beneficial.1,2 However, even those treated early and with anatomic restoration of carpal alignment can expect a loss of grip strength and a range of motion of approximately 70% compared with the contralateral side.2-5 A recent study has suggested that lesser-arc injures generally have a poorer overall outcome than their greater-arc counterparts.15
More than half of all patients with perilunate injuries will develop radiographic signs of osteoarthritis, and some will require additional salvage procedures.3-5 Kremer and colleagues4 showed that overall results after perilunate injuries deteriorate with time. However, according to a paper by Forli and colleagues5 in which patients were followed a minimum of 10 years after their injuries, the authors found that, despite radiographic progression of arthritis, most patients maintained reasonable hand function.
Perilunate injuries typically stem from a high-energy insult to the carpus. Because of their relative infrequency and often subtle radiographic and physical examination findings, these injuries are often undetected in the emergency department setting.1 Early anatomic reduction of any carpal malalignment is essential. Even with optimal treatment, complications such as generalized wrist stiffness, diminished grip strength, and posttraumatic arthritis, commonly develop; however, recent studies suggest these issues are often well tolerated.1-5 In this article, the diagnosis, treatment, and outcomes after perilunate injuries are examined.
History and Physical Examination
Perilunate injuries result from high-energy trauma to the carpus. Patients with these injuries often present with vague wrist pain and loss of wrist motion. Their fingers are frequently held in slight flexion. The patient may complain of numbness and tingling in the median nerve distribution. An acute carpal tunnel syndrome can rapidly develop. The general belief is that acute carpal tunnel syndrome occurs more commonly in pure volar lunate dislocations than in dorsal perilunate dislocations. However, no studies compare the incidence of acute carpal tunnel syndrome in lunate versus perilunate dislocations.
Radiographic Evaluation
Standard radiographic evaluation of a potential perilunate injury includes posteroanterior (PA), lateral, and oblique views of the wrist (Figure 1). A scaphoid view (ie, PA view with the wrist in ulnar deviation) may also be helpful. The PA view is particularly helpful because it enables assessment of Gilula lines, which are imaginary lines drawn across the proximal and distal aspects of the proximal carpal row and the proximal aspect of the distal carpal row. These lines should appear as 3 smooth arcs running nearly parallel to each other.6 Any disruption in these lines suggests carpal incongruity. It may be possible to note a triangular-shaped lunate on the PA view, which is a sign of lunate dislocation.7
While the PA view is certainly useful, the lateral view is the most important in diagnosing a perilunate injury. The lateral view allows assessment of the collinearity of radius, lunate, and capitate. Any disruption in this collinearity strongly suggests a perilunate dislocation.7,8
Classification
Mayfield and colleagues9,10 described 4 stages of perilunate instability proceeding from a radial to an ulnar direction around the lunate. Stage I involves disruption of the scapholunate joint, while stage II involves both the scapholunate and capitolunate joints. In stage III, the scapholunate, capitolunate, and lunotriquetral ligaments are disrupted, and the result is a perilunate dislocation, usually dorsal. Finally, in stage IV, all the ligaments surrounding the lunate are disrupted and the lunate dislocates, most often volarly.
Lastly, perilunate injuries can be classified as greater-arc injuries if concomitant fracture of the carpus occurs, lesser-arc injuries if the injury is purely ligamentous, or inferior-arc injuries if there is an associated fracture of the volar rim of the distal radius.8
Treatment
Closed Reduction
All acute perilunate dislocations should be managed initially with an attempted closed reduction.11 If the injury is older than 72 hours, such an attempt may be futile. For any closed reduction performed in the emergency department setting, intravenous sedation is generally advised for muscle relaxation. Gentle traction with finger traps can also be used prior to the reduction attempt. For a dorsal perilunate dislocation, longitudinal traction followed by volar flexion of the wrist with volar pressure on the lunate and dorsal pressure on the capitate (ie, Tavernier’s maneuver) is required. Once reduction is complete, PA and lateral views of the wrist should be obtained to assess carpal alignment. If closed reduction is unsuccessful, an open reduction is required, although the timing of said procedure is an area of debate, which we will discuss later.1,3 Restoration of anatomic carpal alignment is essential to optimizing outcome, although it does not guarantee a good overall result.
Open Reduction
If successful closed reduction is achieved, the patient can be immobilized temporarily in a short-arm plaster splint. However, open reduction and either pinning or internal fixation will be required to maintain this alignment. The exact timing of open reduction and fixation is debatable and often dictated by the absence or presence of median nerve symptoms.1,3 If a patient with no median nerve symptoms undergoes a successful closed reduction, he or she may be stabilized surgically within 3 to 5 days (or longer) with either pins or headless screws. If closed reduction is unsuccessful, an open reduction should be done within 2 to 3 days. However, if the patient has progressive numbness in the median nerve distribution upon presentation that fails to improve or worsens despite a successful closed reduction, an urgent open reduction (within 24 hours) and carpal tunnel release should be performed to prevent permanent damage to the median nerve.
Once open reduction is undertaken, a dorsal, volar, and combined approach can be used.2-4 In most cases the dorsal approach is selected first. A longitudinal incision is made over the dorsum of the wrist, centered on the Lister tubercle. Dissection occurs between the third and fourth dorsal compartments. After the capsule is exposed, reduction of the lunate to the capitate is confirmed. If any fractures are present in the carpus (eg, scaphoid), they are internally fixed. The scapholunate articulation is then addressed. In general, the scapholunate ligament is not disrupted with a transscaphoid perilunate dislocation. However, if the scapholunate ligament is disrupted, the joint should be reduced and pinned. Repair or reconstruction of the scapholunate ligament is performed. Finally, the lunotriquetral articulation is reduced and stabilized with pins. There are no studies that specifically suggest direct repair of the lunotriquetral ligament versus pinning of the lunotriquetral articulation, but the lunotriquetral ligament could be repaired in similar fashion to the scapholunate ligament at the surgeon’s discretion.
As an alternative to percutaneous pinning, intercarpal screw fixation can be used to stabilize the carpus. A 2007 study by Souer and colleagues12 showed no substantial difference in outcome between the 2 methods of fixation. However, a second procedure is required to remove the screws.
The volar approach, if selected, is typically done second and performed via an extended carpal tunnel incision. It allows decompression of the carpal tunnel and enables repair of volar capsular ligaments (ie, long and short radiolunate ligaments, volar scapholunate ligament, and volar lunotriquetral ligament), which increases overall carpal stability. Currently, many surgeons favor a combined dorsal-volar approach for its efficacy.2,3 Some use a dorsal approach in all patients and perform a volar procedure only if the patient has median nerve symptoms.4 However, Başar and colleagues13 report use of only the volar approach for treatment of perilunate injuries. The authors repaired the long and short radiolunate ligaments, volar scapholunate ligament, and volar lunotriquetral ligament. They reported reasonably good outcomes, which are equivalent to those reported in similar studies using dorsal or combined dorsal-volar approaches. However, no studies in the literature directly compare any of the different approaches with each other.
Postoperatively, patients are placed in a long-arm thumb-spica cast for 4 weeks, and then in a short-arm cast for 4 to 8 weeks (Figure 2). If present, pins are removed in 3 to 12 weeks, with most authors recommending removal at 8 weeks.2,14
Lastly, carpal tunnel symptoms can develop late and even after a successful reduction and surgical stabilization. One theory is that a significant perilunate injury can create slightly higher baseline carpal tunnel pressures, which can compromise the blood flow to the median nerve and cause carpal tunnel symptoms. Additionally, it is possible that direct median nerve contusion and/or traction injury via a displaced lunate can also cause these symptoms. Whatever the inciting cause of median-nerve irritation, a delayed carpal tunnel release is sometimes required.
Conclusion
Outcomes after either perilunate or lunate dislocation are fair to good at best but can be optimized with prompt, appropriate treatment. Closed reduction and casting as definitive treatment has been abandoned because of frequent loss of reduction.12 Early open reduction (ie, less than 3 days after injury) has been shown to be beneficial.1,2 However, even those treated early and with anatomic restoration of carpal alignment can expect a loss of grip strength and a range of motion of approximately 70% compared with the contralateral side.2-5 A recent study has suggested that lesser-arc injures generally have a poorer overall outcome than their greater-arc counterparts.15
More than half of all patients with perilunate injuries will develop radiographic signs of osteoarthritis, and some will require additional salvage procedures.3-5 Kremer and colleagues4 showed that overall results after perilunate injuries deteriorate with time. However, according to a paper by Forli and colleagues5 in which patients were followed a minimum of 10 years after their injuries, the authors found that, despite radiographic progression of arthritis, most patients maintained reasonable hand function.
1. Herzberg G, Comtet JJ, Linscheid RL, Amadio PC, Cooney WP, Stalder J. Perilunate dislocations and fracture-dislocations: a multicenter study. J Hand Surg Am. 1993;18(5):768-779.
2. Sotereanos DG, Mitsionis GJ, Giannakopoulos PN, Tomaino MM, Herndon JH. Perilunate dislocation and fracture dislocation: a critical analysis of the volar-dorsal approach. J Hand Surg Am. 1997;22(1):49-56.
3. Hildebrand KA, Ross DC, Patterson SD, Roth JH, MacDermid JC, King GJ. Dorsal perilunate dislocations and fracture-dislocations: questionnaire, clinical, and radiographic evaluation. J Hand Surg Am. 2000;25(6):1069-1079.
4. Kremer T, Wendt M, Riedel K, Sauerbier M, Germann G, Bickert B. Open reduction for perilunate injuries--clinical outcome and patient satisfaction. J Hand Surg Am. 2010;35(10):1599-1606.
5. Forli A, Courvoisier A, Wimsey S, Corcella D, Moutet F. Perilunate dislocations and transscaphoid perilunate fracture-dislocations: a retrospective study with minimum ten-year follow-up. J Hand Surg Am. 2010;35(1):62-68.
6. Gilula LA. Carpal injuries: analytic approach and case exercises. AJR Am J Roentgenol. 1979;133(3):503-517.
7. Kozin SH. Perilunate injuries: diagnosis and treatment. J Am Acad Orthop Surg. 1998;6(2):114-120.
8. Graham TJ. The inferior arc injury: an addition to the family of complex carpal fracture-dislocation patterns. Am J Orthop. 2003;32(9 suppl):10-19.
9. Mayfield JK, Johnson RP, Kilcoyne RK. Carpal dislocations: pathomechanics and progressive perilunar instability. J Hand Surg Am. 1980;5(3):226-241.
10. Mayfield JK. Mechanism of carpal injuries. Clin Orthop Relat Res. 1980;149:45-54.
11. Adkison JW, Chapman MW. Treatment of acute lunate and perilunate dislocations. Clin Orthop Relat Res. 1982;164:199-207.
12. Souer JS, Rutgers M, Andermahr J, Jupiter JB, Ring D. Perilunate fracture-dislocations of the wrist: comparison of temporary screw versus K-wire fixation. J Hand Surg Am. 2007;32(3):318-325.
13. Başar H, Başar B, Erol B, Tetik C. Isolated volar surgical approach for the treatment of perilunate and lunate dislocations. Indian J Orthop. 2014;48(3):301-315.
14. Komurcu M, Kürklü M, Ozturan KE, Mahirogullari M, Basbozkurt M. Early and delayed treatment of dorsal transscaphoid perilunate fracture-dislocations. J Orthop Trauma. 2008;22:535-540.
15. Massoud AH, Naam NH. Functional outcome of open reduction of chronic perilunate injuries. J Hand Surg Am. 2012;37(9):1852-1860.
1. Herzberg G, Comtet JJ, Linscheid RL, Amadio PC, Cooney WP, Stalder J. Perilunate dislocations and fracture-dislocations: a multicenter study. J Hand Surg Am. 1993;18(5):768-779.
2. Sotereanos DG, Mitsionis GJ, Giannakopoulos PN, Tomaino MM, Herndon JH. Perilunate dislocation and fracture dislocation: a critical analysis of the volar-dorsal approach. J Hand Surg Am. 1997;22(1):49-56.
3. Hildebrand KA, Ross DC, Patterson SD, Roth JH, MacDermid JC, King GJ. Dorsal perilunate dislocations and fracture-dislocations: questionnaire, clinical, and radiographic evaluation. J Hand Surg Am. 2000;25(6):1069-1079.
4. Kremer T, Wendt M, Riedel K, Sauerbier M, Germann G, Bickert B. Open reduction for perilunate injuries--clinical outcome and patient satisfaction. J Hand Surg Am. 2010;35(10):1599-1606.
5. Forli A, Courvoisier A, Wimsey S, Corcella D, Moutet F. Perilunate dislocations and transscaphoid perilunate fracture-dislocations: a retrospective study with minimum ten-year follow-up. J Hand Surg Am. 2010;35(1):62-68.
6. Gilula LA. Carpal injuries: analytic approach and case exercises. AJR Am J Roentgenol. 1979;133(3):503-517.
7. Kozin SH. Perilunate injuries: diagnosis and treatment. J Am Acad Orthop Surg. 1998;6(2):114-120.
8. Graham TJ. The inferior arc injury: an addition to the family of complex carpal fracture-dislocation patterns. Am J Orthop. 2003;32(9 suppl):10-19.
9. Mayfield JK, Johnson RP, Kilcoyne RK. Carpal dislocations: pathomechanics and progressive perilunar instability. J Hand Surg Am. 1980;5(3):226-241.
10. Mayfield JK. Mechanism of carpal injuries. Clin Orthop Relat Res. 1980;149:45-54.
11. Adkison JW, Chapman MW. Treatment of acute lunate and perilunate dislocations. Clin Orthop Relat Res. 1982;164:199-207.
12. Souer JS, Rutgers M, Andermahr J, Jupiter JB, Ring D. Perilunate fracture-dislocations of the wrist: comparison of temporary screw versus K-wire fixation. J Hand Surg Am. 2007;32(3):318-325.
13. Başar H, Başar B, Erol B, Tetik C. Isolated volar surgical approach for the treatment of perilunate and lunate dislocations. Indian J Orthop. 2014;48(3):301-315.
14. Komurcu M, Kürklü M, Ozturan KE, Mahirogullari M, Basbozkurt M. Early and delayed treatment of dorsal transscaphoid perilunate fracture-dislocations. J Orthop Trauma. 2008;22:535-540.
15. Massoud AH, Naam NH. Functional outcome of open reduction of chronic perilunate injuries. J Hand Surg Am. 2012;37(9):1852-1860.
Case Report: A Bittersweet Death
Case
A 32-year-old Hispanic man presented to the ED with complications associated with diabetes mellitus (DM), the symptoms of which started approximately 3 days prior to arrival. The patient reported feelings of fatigue, dry mouth, increased thirst, and frequent urination. He denied sweating, nausea, chest pain, shortness of breath, diarrhea, or blood in his urine; he also denied blurry vision or dizziness.
During history intake, the patient informed the emergency physician (EP) that he had been diagnosed with DM and hyperglycemia earlier that day by his primary care physician, who had immediately referred the patient to the ED for urgent management. The patient’s own medical history was noncontributory; however, his father’s history was notable for DM and chronic renal failure. The patient further stated that he was not on any medications. Regarding his social history, he denied cigarette smoking and noted only occasional alcohol consumption.
The patient’s vital signs on presentation were: blood pressure (BP), 116/74 mm Hg; heart rate, 113 beats/minute; respiratory rate, 26 breaths/minute; and temperature, 97.8°F. Oxygen saturation was 97% on room air. On physical examination, the patient was severely anxious, with tachycardia and respiratory distress. He was obese, with a body mass index of 30.9 kg/m2 (height, 5 feet, 4 inches; weight, 180 lb).
The patient was started on an intravenous (IV) bolus of 0.9% normal saline (2 L at 20 mL/kg). After a consultation with endocrinology, he was then given a maintenance dose of normal saline IV at 250 cc/h and an IV insulin drip at 0.1 U/kg/h following a bolus of 8 units of insulin IV. His glucose levels were carefully monitored via hourly finger-stick glucose testing.
Although the patient’s condition stabilized, he collapsed while walking to the bathroom. He had agonal respirations and no pulse. Resuscitation efforts were started with bag-valve-mask ventilation, along with emergent advanced cardiac life support (ACLS) treatment, the protocol of which included epinephrine administration (x2) IV push 5 minutes apart, 2 ampules of sodium bicarbonate (50 mEq each) IV push, and calcium gluconate 10% (x1) 10 mL (1 g) IV push. A pulse was re-established, and the patient was intubated.
The patient was diagnosed with diabetic ketoacidosis (DKA) and admitted to the intensive care unit where repeat laboratory evaluation was ordered. Additional pharmacological management included IV administration of dopamine, norepinephrine, phenylephrine, vasopressin, antibiotics (azithromycin, meropenem, and vancomycin), pantoprazole, and subcutaneous heparin.
During treatment, the patient coded a second time and was revived according to ACLS protocols. Shortly thereafter, he coded a third time, but resuscitation efforts failed. Pathology reported no biological cause of death, and the coroner closed the case as death due to DM-related complications.
Diabetic Ketoacidosis
Diabetic ketoacidosis is a major complication of DM.4 Although the condition usually occurs in type 1 DM, it can also develop in type 2 DM. Diabetic ketoacidosis may be an inciting event leading to the eventual diagnosis of DM, but can also develop during a concurrent illness such as a urinary tract infection or an eating disorder.5 Risk factors for DKA include patients with type 1 or type 2 DM, a family history of DM, obesity, and nonwhite patients whose ethnic background places them at increased risk.6 Hispanic, black, and African American patients are at a greater risk of developing DKA and are more likely to develop “ketosis-prone” type 2 DM.7
Patients who do not fit into the definitive categories of type 1 or 2 DM can be classified under ketosis-prone DM.7,8 Diabetic ketoacidosis acts as the inciting event for the disease and evolves into severe β-cell dysfunction, hence blurring the lines between the archetypal DM categories. Fifty percent of ketosis-prone DM patients are A-β+ (absent autoantibodies, present β-cell function), which indicates that the dysfunction can be partially reversed. Reversal of the condition is largely based on long-term β-cell reserves, which are dependent on tight glycemic control and insulin dependence. Higher incidences of the A-β+ variant of ketosis-prone diabetes are seen in the male population and are often unprovoked.9-11
Diabetic ketoacidosis is the result of either a decrease or absence of insulin in the body (Table 2).4 Without insulin modulating exogenous glucose intake and endogenous glucose production (via glucagon, glycogenolysis, and gluconeogenesis), high levels of glucose are found in the circulation, leading to prominent hyperglycemia (>250 mg/dL or >13.8 mmol/L).6 This environment causes the body to switch from carbohydrate metabolism to fatty acid metabolism. As a result, acidic ketone bodies such β-hydroxybutyrate and acetoacetate are produced. These physiological changes in the body cause the signs and symptoms typically found in DKA.
Signs and Symptoms
Over a period of 24 hours, symptoms such as nausea, vomiting, increased thirst, and polyuria develop due to dehydration caused by osmotic diuresis and glucosuria.5 Patients may also present with hypotension and tachycardia. Confusion, deep gasping breaths or Kussmaul respirations, and metabolic acidosis result from hyperventilation and failure to compensate for the increased serum concentration of ketone bodies. Ketone production leads to a fruit-like odor in the patient’s breath and ketonuria in the urinalysis. In DKA, laboratory values will indicate metabolic acidosis and abnormal serum electrolytes. In both DM and DKA, increased urea and creatinine due to dehydration, increased ketones, and the presence of diabetic nephropathy are useful indicators of impaired kidney function.12
Management and Treatment
Diabetic ketoacidosis can be managed and reversed, especially when recognized and treated early.6,13 Dehydration in DKA can be corrected with IV fluid replacement. Normal saline (0.9%) can be started at 15 to 20 mL/kg/h or 1 L/h. As the patient’s vital signs stabilize, IV fluids can be titrated to a lower dose of 250 to 500 mL/h. Monitoring BP and electrolytes are key at this point as alterations in sodium levels and glucose levels may require switching to half-normal saline and/or dextrose.
The hyperglycemic state of patients with DKA is managed by IV insulin. An initial bolus of 0.1 U/kg/h can be given, but should only be administered when potassium levels are greater than 3.3 mmol/L.14 If adequate perfusion can be maintained, then 0.14 U/kg/h can be used instead of a bolus. Glucose levels must be monitored; once the levels decrease to approximately 200 mg/dL, the infusion rate of insulin should be titrated down to 0.05 to 0.1 U/kg/h. Dextrose is then added to maintain glucose levels at approximately 150 to 200 mg/dL.
Electrolytes, especially potassium, must be monitored closely in patients with DKA. Insulin leads to the shift of potassium into cells. The lack of insulin keeps potassium in the extracellular space. Due to osmotic diuresis, potassium is lost in the urine, leading to hypokalemia. Potassium levels in patients with DKA should be maintained at a level between 4 to 5 mmol/L. Patients with potassium levels between 3.3 to 5.2 mmol/L can be started on IV potassium between 20 to 30 mmol/h. If the patient is severely hypokalemic (<3.3 mmol/L), insulin should be withheld, and only IV potassium should be given at a rate of 20 to 30 mmol/h.
Bicarbonate levels can also be managed as acidosis can lead to both neurological and cardiac complications. If the patient’s pH is less than 6.9, the American Diabetes Association recommends starting 100 mmol of sodium bicarbonate in 400 mL sterile water (in addition to potassium chloride at 200 mL/h) for 2 hours. Dosing should be repeated every 2 hours until the patient’s pH is greater than 6.9.
In uncomplicated cases of DKA, the condition is resolved when a patient’s pH is greater than 7.3; glucose level is less than 200 mg/dL; and bicarbonate level is greater than or equal to 18 mmol/L. After patients become hemodynamically stable, they can be discharged and managed at home with a combination of intermediate- or long-acting insulin as well as short- or rapid-acting insulin.
Complications and Mortality
Diabetic ketoacidosis can cause sudden and fluctuating changes in the body. Therefore, it is very important to monitor a patient’s laboratory values very carefully and frequently to avoid any pitfalls. Since patients can present with hyponatremia due to the osmotic draw of glucose in the blood,13 sodium levels may have to be corrected. The corrected serum sodium can be calculated by adding 1.6 mmol/L for every 100 mg/dL of glucose (when finger-stick readings are above 200 mg/dL).15 Patients with DKA can also present with leukocytosis (even in the absence of infection) and hypertriglyceridemia (due to impaired lipoprotein lipase).15 Serum creatine may be elevated due to blood acetoacetate levels.15
Interestingly, there are other acute conditions that can mimic DKA.15 For example, chronic ethanol abuse can lead to ketoacidosis. Unlike DKA, however, alcoholic ketoacidosis does not have profound hyperglycemia, which can help differentiate the two during initial assessment.
Complications due to DKA can arise comprising the patient’s health, including hypoglycemia, hypokalemia, rhabdomyolysis, acute renal failure, pulmonary edema, and shock.16 Cerebral edema is seen in up to 1% of DKA patients,15 the cause of which may be due to the severity of the acidosis, high glucose levels, and rapid hydration. Even when cerebral edema is reduced, patients are often neurologically impaired. Mortality rates from DKA deaths due to cerebral edema can be as high as 24%.13 In the United States, over 100,000 patients with DM per year are admitted to the hospital for DKA, and 9% of patients with DM suffer from DKA-related complications postdischarge.15 With current treatment protocols, mortality rates for DKA-associated deaths are now down to 1%.6,15
Diabetes ketoacidosis-related deaths are usually the result of the following: a triad of DKA symptoms (hyperglycemia, hyperketonemia, and metabolic acidosis), another underlying comorbid condition (eg, myocardial infarction, sepsis, acute respiratory distress syndrome), or the release of biological markers (ie, catecholamines).14,15,17 Thus, as previously stated, the management of potassium levels is important as both hyperkalemia and hypokalemia can lead to fatal arrthythmias.15
Direct mortality from DKA has dropped significantly over the past 20 years, from 8% to less than 1%.6 The US Centers for Disease Control and Prevention has observed a downward trend in death and estimates that 2,417 patients died in 2009 due to DKA,18 and recent postmortem studies have revealed new insights into DKA-related deaths.19 Blood and vitreous acetone concentrations are strong indicators for predeath hyperglycemia and ketosis (if there are no underlying comorbid and/or pharmacological provocations). Blood acetone levels greater than 0.01 g/dL antemortem are suggestive of DKA. It is recommended that these tests should be performed in sudden deaths which have no biological or anatomical cause of death. Postmortem diagnosis of DKA is made with the following criteria: history of DM, increased vitreous glucose concentrations, and elevated blood/vitreous/urine acetone concentrations (>200 mg/dL). If results of the abovementioned parameters are inconclusive, measurement of lactic acid postmortem is thought to further support a diagnosis of DKA.19
Patient Counseling and Education
Approximately 33% of patients whose death was associated with DKA had no personal history of DM.19 This statistic emphasizes the importance of taking a thorough history, physical examination, blood glucose evaluation, and educating patients about the signs and symptoms of DM and DKA.
Patient counseling and education are important, especially in patients whose racial/ethnic background places them at increased risk of developing DM (eg, patients of black or African American, American Indian, Alaskan Native, Asian American, Hispanic, Native Hawaiian, or Pacific Islander descent).20,21 Strategies for preventive management include advocating regular glucose monitoring as well as dietary and lifestyle modifications. In patients with DM, successful management of the condition and its comorbidities can help prevent DKA and associated mortality.
Conclusion
As this case demonstrates, despite prompt diagnosis and management, patients with DKA—especially those with uncontrolled, undiagnosed, or advanced DM—are associated with fatal outcomes. In many cases, however, DKA can be successfully managed and reversed, especially when the condition is recognized early. Management includes not only IV therapy to adjust fluid and insulin levels, but also restoring electrolyte balance (especially potassium and bicarbonate). Frequent and careful evaluation of laboratory values is vital to the successful treatment of DKA, as there are numerous pitfalls and complications that the emergency physician can encounter. Patients who either have or are at an increased risk of developing DM or DKA may benefit from preventive measures, including regular glucose monitoring and appropriate diet and lifestyle modifications.
Mr Hassan-Ali is a fourth-year medical student at Windsor University School of Medicine, St Kitts, West Indies. Dr Raziuddin is an internist and an emergency medicine physician at Weiss Memorial, Thorek Memorial, and Westlake Hospitals, Chicago, Illinois.
- Kitabchi AH, Umpierrez GE, Murphy MB, et al. Management of hyperglycemic crises in patients with diabetes. Diabetes Care. 2001;24(1):131-153.
- Farinda A. Lab values, normal adult: laboratory reference ranges in healthy adults. 2015. Medscape Web site. http://emedicine.medscape.com/article/2172316-overview. Updated May 14, 2014. Accessed August 14, 2015.
- Young D. Implementation of SI units for clinical laboratory data. Ann Intern Med. 1987;106(1):114-129.
- Maitra A. The endocrine system. In: Kumar V, Abbas AK, Aster JC. Robbins and Cotran Pathologic Basis of Disease. 9th ed. New York, NY: Elsevier Saunders; 2015:1105-1120.
- Powers AC. Diabetes mellitus: management and therapies. In: Kasper DL, Fauci AS, Longo DL, Hauser SL, Jameson JL, Loscalzo J. Harrison’s Principles of Internal Medicine. 19th ed. New York, NY; McGraw-Hill Medical Publishing Division; 2015:2407-2422.
- Kitabchi AE, Umpierrez GE, Miles JM, Fisher JN. Hyperglycemic crises in adult patients with diabetes. Diabetes Care. 2009;32(7):1335-1343.
- Umpierrez GE, Smiley D, Kitabchi AE. Narrative review: ketosis-prone type 2 diabetes mellitus. Ann Intern Med. 2006;144(5):350-357.
- Umpierrez G, Smiley D, Gosmanov A, Thomason D. Ketosis-prone type 2 diabetes: effect of hyperglycemia on beta-cell function and skeletal muscle insulin signaling. Endocr Pract. 2007;13(3):283-290.
- Mauvais-Jarvis F, Sobngwi E, Porcher R, et al. Ketosis-prone type 2 diabetes in patients of sub-Saharan African origin: clinical pathophysiology and natural history of beta-cell dysfunction and insulin resistance. Diabetes. 2004;53(3):645-653.
- Umpierrez GE, Casals MM, Gebhart SP, Mixon PS, Clark WS, Phillips LS. Diabetic ketoacidosis in obese African-Americans. Diabetes. 1995;44(7):790-795.
- Piñero-Piloña A, Raskin P. Idiopathic type 1 diabetes. J Diabetes Complications. 2001;15(6):328-335.
- Kemperman FA, Weber JA, Gorgels J, van Zanten AP, Krediet RT, Arisz L. The influence of ketoacids on plasma creatinine assays in diabetic ketoacidosis. J Intern Med. 2000;248(6):511-517.
- Westerberg DP. Diabetic ketoacidosis: evaluation and treatment. Am Fam Physician. 2013;87(5):337-346.
- Trachtenbarg DE. Diabetic ketoacidosis. Am Fam Physician. 2005;71(9):1705-1714.
- Umpierrez GE, Murphy MB, Kitabchi AE. Diabetic ketoacidosis and hyperglycemic hyperosmolar ayndrome. Diabetes Spectrum. 2002;15(1):28-36.
- Wolfsdorf J, Glaser N, Sperling MA; American Diabetes Association. Diabetic ketoacidosis in infants, children, and adolescents: A consensus statement from the American Diabetes Association. Diabetes Care. 2006;29(5):1150-1159.
- Rosenbloom AL. Sudden death of a young woman attributed to diabetic ketoacidosis. J Forensic Leg Med. 2013;20(8):1063-1065.
- Centers for Disease Control and Prevention. Number of deaths for hyperglycemic crises as underlying cause, United States, 1980-2009. http://www.cdc.gov/diabetes/statistics/mortalitydka/fnumberofdka.htm. Updated November 19, 2013. Accessed August 14, 2015.
- Ali Z, Levine B, Ripple M, Fowler DR. Diabetic ketoacidosis: a silent death. Am J Forensic Med Pathol. 2012;33(3):189-193.
- US Department of Health and Human Services Office of Minority Health. Diabetes and Hispanic Americans. http://minorityhealth.hhs.gov/omh/browse.aspx?lvl=4&lvlid=63. Updated June 15, 2013. Accessed August 14, 2015.
- US Department of Health and Human Services Office of Minority Health. Profile: Native Hawaiian/Other Pacific Islanders. http://minorityhealth.hhs.gov/omh/browse.aspx?lvl=3&lvlid=65. Updated January 15, 2015. Accessed August 14, 2015.
Case
A 32-year-old Hispanic man presented to the ED with complications associated with diabetes mellitus (DM), the symptoms of which started approximately 3 days prior to arrival. The patient reported feelings of fatigue, dry mouth, increased thirst, and frequent urination. He denied sweating, nausea, chest pain, shortness of breath, diarrhea, or blood in his urine; he also denied blurry vision or dizziness.
During history intake, the patient informed the emergency physician (EP) that he had been diagnosed with DM and hyperglycemia earlier that day by his primary care physician, who had immediately referred the patient to the ED for urgent management. The patient’s own medical history was noncontributory; however, his father’s history was notable for DM and chronic renal failure. The patient further stated that he was not on any medications. Regarding his social history, he denied cigarette smoking and noted only occasional alcohol consumption.
The patient’s vital signs on presentation were: blood pressure (BP), 116/74 mm Hg; heart rate, 113 beats/minute; respiratory rate, 26 breaths/minute; and temperature, 97.8°F. Oxygen saturation was 97% on room air. On physical examination, the patient was severely anxious, with tachycardia and respiratory distress. He was obese, with a body mass index of 30.9 kg/m2 (height, 5 feet, 4 inches; weight, 180 lb).
The patient was started on an intravenous (IV) bolus of 0.9% normal saline (2 L at 20 mL/kg). After a consultation with endocrinology, he was then given a maintenance dose of normal saline IV at 250 cc/h and an IV insulin drip at 0.1 U/kg/h following a bolus of 8 units of insulin IV. His glucose levels were carefully monitored via hourly finger-stick glucose testing.
Although the patient’s condition stabilized, he collapsed while walking to the bathroom. He had agonal respirations and no pulse. Resuscitation efforts were started with bag-valve-mask ventilation, along with emergent advanced cardiac life support (ACLS) treatment, the protocol of which included epinephrine administration (x2) IV push 5 minutes apart, 2 ampules of sodium bicarbonate (50 mEq each) IV push, and calcium gluconate 10% (x1) 10 mL (1 g) IV push. A pulse was re-established, and the patient was intubated.
The patient was diagnosed with diabetic ketoacidosis (DKA) and admitted to the intensive care unit where repeat laboratory evaluation was ordered. Additional pharmacological management included IV administration of dopamine, norepinephrine, phenylephrine, vasopressin, antibiotics (azithromycin, meropenem, and vancomycin), pantoprazole, and subcutaneous heparin.
During treatment, the patient coded a second time and was revived according to ACLS protocols. Shortly thereafter, he coded a third time, but resuscitation efforts failed. Pathology reported no biological cause of death, and the coroner closed the case as death due to DM-related complications.
Diabetic Ketoacidosis
Diabetic ketoacidosis is a major complication of DM.4 Although the condition usually occurs in type 1 DM, it can also develop in type 2 DM. Diabetic ketoacidosis may be an inciting event leading to the eventual diagnosis of DM, but can also develop during a concurrent illness such as a urinary tract infection or an eating disorder.5 Risk factors for DKA include patients with type 1 or type 2 DM, a family history of DM, obesity, and nonwhite patients whose ethnic background places them at increased risk.6 Hispanic, black, and African American patients are at a greater risk of developing DKA and are more likely to develop “ketosis-prone” type 2 DM.7
Patients who do not fit into the definitive categories of type 1 or 2 DM can be classified under ketosis-prone DM.7,8 Diabetic ketoacidosis acts as the inciting event for the disease and evolves into severe β-cell dysfunction, hence blurring the lines between the archetypal DM categories. Fifty percent of ketosis-prone DM patients are A-β+ (absent autoantibodies, present β-cell function), which indicates that the dysfunction can be partially reversed. Reversal of the condition is largely based on long-term β-cell reserves, which are dependent on tight glycemic control and insulin dependence. Higher incidences of the A-β+ variant of ketosis-prone diabetes are seen in the male population and are often unprovoked.9-11
Diabetic ketoacidosis is the result of either a decrease or absence of insulin in the body (Table 2).4 Without insulin modulating exogenous glucose intake and endogenous glucose production (via glucagon, glycogenolysis, and gluconeogenesis), high levels of glucose are found in the circulation, leading to prominent hyperglycemia (>250 mg/dL or >13.8 mmol/L).6 This environment causes the body to switch from carbohydrate metabolism to fatty acid metabolism. As a result, acidic ketone bodies such β-hydroxybutyrate and acetoacetate are produced. These physiological changes in the body cause the signs and symptoms typically found in DKA.
Signs and Symptoms
Over a period of 24 hours, symptoms such as nausea, vomiting, increased thirst, and polyuria develop due to dehydration caused by osmotic diuresis and glucosuria.5 Patients may also present with hypotension and tachycardia. Confusion, deep gasping breaths or Kussmaul respirations, and metabolic acidosis result from hyperventilation and failure to compensate for the increased serum concentration of ketone bodies. Ketone production leads to a fruit-like odor in the patient’s breath and ketonuria in the urinalysis. In DKA, laboratory values will indicate metabolic acidosis and abnormal serum electrolytes. In both DM and DKA, increased urea and creatinine due to dehydration, increased ketones, and the presence of diabetic nephropathy are useful indicators of impaired kidney function.12
Management and Treatment
Diabetic ketoacidosis can be managed and reversed, especially when recognized and treated early.6,13 Dehydration in DKA can be corrected with IV fluid replacement. Normal saline (0.9%) can be started at 15 to 20 mL/kg/h or 1 L/h. As the patient’s vital signs stabilize, IV fluids can be titrated to a lower dose of 250 to 500 mL/h. Monitoring BP and electrolytes are key at this point as alterations in sodium levels and glucose levels may require switching to half-normal saline and/or dextrose.
The hyperglycemic state of patients with DKA is managed by IV insulin. An initial bolus of 0.1 U/kg/h can be given, but should only be administered when potassium levels are greater than 3.3 mmol/L.14 If adequate perfusion can be maintained, then 0.14 U/kg/h can be used instead of a bolus. Glucose levels must be monitored; once the levels decrease to approximately 200 mg/dL, the infusion rate of insulin should be titrated down to 0.05 to 0.1 U/kg/h. Dextrose is then added to maintain glucose levels at approximately 150 to 200 mg/dL.
Electrolytes, especially potassium, must be monitored closely in patients with DKA. Insulin leads to the shift of potassium into cells. The lack of insulin keeps potassium in the extracellular space. Due to osmotic diuresis, potassium is lost in the urine, leading to hypokalemia. Potassium levels in patients with DKA should be maintained at a level between 4 to 5 mmol/L. Patients with potassium levels between 3.3 to 5.2 mmol/L can be started on IV potassium between 20 to 30 mmol/h. If the patient is severely hypokalemic (<3.3 mmol/L), insulin should be withheld, and only IV potassium should be given at a rate of 20 to 30 mmol/h.
Bicarbonate levels can also be managed as acidosis can lead to both neurological and cardiac complications. If the patient’s pH is less than 6.9, the American Diabetes Association recommends starting 100 mmol of sodium bicarbonate in 400 mL sterile water (in addition to potassium chloride at 200 mL/h) for 2 hours. Dosing should be repeated every 2 hours until the patient’s pH is greater than 6.9.
In uncomplicated cases of DKA, the condition is resolved when a patient’s pH is greater than 7.3; glucose level is less than 200 mg/dL; and bicarbonate level is greater than or equal to 18 mmol/L. After patients become hemodynamically stable, they can be discharged and managed at home with a combination of intermediate- or long-acting insulin as well as short- or rapid-acting insulin.
Complications and Mortality
Diabetic ketoacidosis can cause sudden and fluctuating changes in the body. Therefore, it is very important to monitor a patient’s laboratory values very carefully and frequently to avoid any pitfalls. Since patients can present with hyponatremia due to the osmotic draw of glucose in the blood,13 sodium levels may have to be corrected. The corrected serum sodium can be calculated by adding 1.6 mmol/L for every 100 mg/dL of glucose (when finger-stick readings are above 200 mg/dL).15 Patients with DKA can also present with leukocytosis (even in the absence of infection) and hypertriglyceridemia (due to impaired lipoprotein lipase).15 Serum creatine may be elevated due to blood acetoacetate levels.15
Interestingly, there are other acute conditions that can mimic DKA.15 For example, chronic ethanol abuse can lead to ketoacidosis. Unlike DKA, however, alcoholic ketoacidosis does not have profound hyperglycemia, which can help differentiate the two during initial assessment.
Complications due to DKA can arise comprising the patient’s health, including hypoglycemia, hypokalemia, rhabdomyolysis, acute renal failure, pulmonary edema, and shock.16 Cerebral edema is seen in up to 1% of DKA patients,15 the cause of which may be due to the severity of the acidosis, high glucose levels, and rapid hydration. Even when cerebral edema is reduced, patients are often neurologically impaired. Mortality rates from DKA deaths due to cerebral edema can be as high as 24%.13 In the United States, over 100,000 patients with DM per year are admitted to the hospital for DKA, and 9% of patients with DM suffer from DKA-related complications postdischarge.15 With current treatment protocols, mortality rates for DKA-associated deaths are now down to 1%.6,15
Diabetes ketoacidosis-related deaths are usually the result of the following: a triad of DKA symptoms (hyperglycemia, hyperketonemia, and metabolic acidosis), another underlying comorbid condition (eg, myocardial infarction, sepsis, acute respiratory distress syndrome), or the release of biological markers (ie, catecholamines).14,15,17 Thus, as previously stated, the management of potassium levels is important as both hyperkalemia and hypokalemia can lead to fatal arrthythmias.15
Direct mortality from DKA has dropped significantly over the past 20 years, from 8% to less than 1%.6 The US Centers for Disease Control and Prevention has observed a downward trend in death and estimates that 2,417 patients died in 2009 due to DKA,18 and recent postmortem studies have revealed new insights into DKA-related deaths.19 Blood and vitreous acetone concentrations are strong indicators for predeath hyperglycemia and ketosis (if there are no underlying comorbid and/or pharmacological provocations). Blood acetone levels greater than 0.01 g/dL antemortem are suggestive of DKA. It is recommended that these tests should be performed in sudden deaths which have no biological or anatomical cause of death. Postmortem diagnosis of DKA is made with the following criteria: history of DM, increased vitreous glucose concentrations, and elevated blood/vitreous/urine acetone concentrations (>200 mg/dL). If results of the abovementioned parameters are inconclusive, measurement of lactic acid postmortem is thought to further support a diagnosis of DKA.19
Patient Counseling and Education
Approximately 33% of patients whose death was associated with DKA had no personal history of DM.19 This statistic emphasizes the importance of taking a thorough history, physical examination, blood glucose evaluation, and educating patients about the signs and symptoms of DM and DKA.
Patient counseling and education are important, especially in patients whose racial/ethnic background places them at increased risk of developing DM (eg, patients of black or African American, American Indian, Alaskan Native, Asian American, Hispanic, Native Hawaiian, or Pacific Islander descent).20,21 Strategies for preventive management include advocating regular glucose monitoring as well as dietary and lifestyle modifications. In patients with DM, successful management of the condition and its comorbidities can help prevent DKA and associated mortality.
Conclusion
As this case demonstrates, despite prompt diagnosis and management, patients with DKA—especially those with uncontrolled, undiagnosed, or advanced DM—are associated with fatal outcomes. In many cases, however, DKA can be successfully managed and reversed, especially when the condition is recognized early. Management includes not only IV therapy to adjust fluid and insulin levels, but also restoring electrolyte balance (especially potassium and bicarbonate). Frequent and careful evaluation of laboratory values is vital to the successful treatment of DKA, as there are numerous pitfalls and complications that the emergency physician can encounter. Patients who either have or are at an increased risk of developing DM or DKA may benefit from preventive measures, including regular glucose monitoring and appropriate diet and lifestyle modifications.
Mr Hassan-Ali is a fourth-year medical student at Windsor University School of Medicine, St Kitts, West Indies. Dr Raziuddin is an internist and an emergency medicine physician at Weiss Memorial, Thorek Memorial, and Westlake Hospitals, Chicago, Illinois.
Case
A 32-year-old Hispanic man presented to the ED with complications associated with diabetes mellitus (DM), the symptoms of which started approximately 3 days prior to arrival. The patient reported feelings of fatigue, dry mouth, increased thirst, and frequent urination. He denied sweating, nausea, chest pain, shortness of breath, diarrhea, or blood in his urine; he also denied blurry vision or dizziness.
During history intake, the patient informed the emergency physician (EP) that he had been diagnosed with DM and hyperglycemia earlier that day by his primary care physician, who had immediately referred the patient to the ED for urgent management. The patient’s own medical history was noncontributory; however, his father’s history was notable for DM and chronic renal failure. The patient further stated that he was not on any medications. Regarding his social history, he denied cigarette smoking and noted only occasional alcohol consumption.
The patient’s vital signs on presentation were: blood pressure (BP), 116/74 mm Hg; heart rate, 113 beats/minute; respiratory rate, 26 breaths/minute; and temperature, 97.8°F. Oxygen saturation was 97% on room air. On physical examination, the patient was severely anxious, with tachycardia and respiratory distress. He was obese, with a body mass index of 30.9 kg/m2 (height, 5 feet, 4 inches; weight, 180 lb).
The patient was started on an intravenous (IV) bolus of 0.9% normal saline (2 L at 20 mL/kg). After a consultation with endocrinology, he was then given a maintenance dose of normal saline IV at 250 cc/h and an IV insulin drip at 0.1 U/kg/h following a bolus of 8 units of insulin IV. His glucose levels were carefully monitored via hourly finger-stick glucose testing.
Although the patient’s condition stabilized, he collapsed while walking to the bathroom. He had agonal respirations and no pulse. Resuscitation efforts were started with bag-valve-mask ventilation, along with emergent advanced cardiac life support (ACLS) treatment, the protocol of which included epinephrine administration (x2) IV push 5 minutes apart, 2 ampules of sodium bicarbonate (50 mEq each) IV push, and calcium gluconate 10% (x1) 10 mL (1 g) IV push. A pulse was re-established, and the patient was intubated.
The patient was diagnosed with diabetic ketoacidosis (DKA) and admitted to the intensive care unit where repeat laboratory evaluation was ordered. Additional pharmacological management included IV administration of dopamine, norepinephrine, phenylephrine, vasopressin, antibiotics (azithromycin, meropenem, and vancomycin), pantoprazole, and subcutaneous heparin.
During treatment, the patient coded a second time and was revived according to ACLS protocols. Shortly thereafter, he coded a third time, but resuscitation efforts failed. Pathology reported no biological cause of death, and the coroner closed the case as death due to DM-related complications.
Diabetic Ketoacidosis
Diabetic ketoacidosis is a major complication of DM.4 Although the condition usually occurs in type 1 DM, it can also develop in type 2 DM. Diabetic ketoacidosis may be an inciting event leading to the eventual diagnosis of DM, but can also develop during a concurrent illness such as a urinary tract infection or an eating disorder.5 Risk factors for DKA include patients with type 1 or type 2 DM, a family history of DM, obesity, and nonwhite patients whose ethnic background places them at increased risk.6 Hispanic, black, and African American patients are at a greater risk of developing DKA and are more likely to develop “ketosis-prone” type 2 DM.7
Patients who do not fit into the definitive categories of type 1 or 2 DM can be classified under ketosis-prone DM.7,8 Diabetic ketoacidosis acts as the inciting event for the disease and evolves into severe β-cell dysfunction, hence blurring the lines between the archetypal DM categories. Fifty percent of ketosis-prone DM patients are A-β+ (absent autoantibodies, present β-cell function), which indicates that the dysfunction can be partially reversed. Reversal of the condition is largely based on long-term β-cell reserves, which are dependent on tight glycemic control and insulin dependence. Higher incidences of the A-β+ variant of ketosis-prone diabetes are seen in the male population and are often unprovoked.9-11
Diabetic ketoacidosis is the result of either a decrease or absence of insulin in the body (Table 2).4 Without insulin modulating exogenous glucose intake and endogenous glucose production (via glucagon, glycogenolysis, and gluconeogenesis), high levels of glucose are found in the circulation, leading to prominent hyperglycemia (>250 mg/dL or >13.8 mmol/L).6 This environment causes the body to switch from carbohydrate metabolism to fatty acid metabolism. As a result, acidic ketone bodies such β-hydroxybutyrate and acetoacetate are produced. These physiological changes in the body cause the signs and symptoms typically found in DKA.
Signs and Symptoms
Over a period of 24 hours, symptoms such as nausea, vomiting, increased thirst, and polyuria develop due to dehydration caused by osmotic diuresis and glucosuria.5 Patients may also present with hypotension and tachycardia. Confusion, deep gasping breaths or Kussmaul respirations, and metabolic acidosis result from hyperventilation and failure to compensate for the increased serum concentration of ketone bodies. Ketone production leads to a fruit-like odor in the patient’s breath and ketonuria in the urinalysis. In DKA, laboratory values will indicate metabolic acidosis and abnormal serum electrolytes. In both DM and DKA, increased urea and creatinine due to dehydration, increased ketones, and the presence of diabetic nephropathy are useful indicators of impaired kidney function.12
Management and Treatment
Diabetic ketoacidosis can be managed and reversed, especially when recognized and treated early.6,13 Dehydration in DKA can be corrected with IV fluid replacement. Normal saline (0.9%) can be started at 15 to 20 mL/kg/h or 1 L/h. As the patient’s vital signs stabilize, IV fluids can be titrated to a lower dose of 250 to 500 mL/h. Monitoring BP and electrolytes are key at this point as alterations in sodium levels and glucose levels may require switching to half-normal saline and/or dextrose.
The hyperglycemic state of patients with DKA is managed by IV insulin. An initial bolus of 0.1 U/kg/h can be given, but should only be administered when potassium levels are greater than 3.3 mmol/L.14 If adequate perfusion can be maintained, then 0.14 U/kg/h can be used instead of a bolus. Glucose levels must be monitored; once the levels decrease to approximately 200 mg/dL, the infusion rate of insulin should be titrated down to 0.05 to 0.1 U/kg/h. Dextrose is then added to maintain glucose levels at approximately 150 to 200 mg/dL.
Electrolytes, especially potassium, must be monitored closely in patients with DKA. Insulin leads to the shift of potassium into cells. The lack of insulin keeps potassium in the extracellular space. Due to osmotic diuresis, potassium is lost in the urine, leading to hypokalemia. Potassium levels in patients with DKA should be maintained at a level between 4 to 5 mmol/L. Patients with potassium levels between 3.3 to 5.2 mmol/L can be started on IV potassium between 20 to 30 mmol/h. If the patient is severely hypokalemic (<3.3 mmol/L), insulin should be withheld, and only IV potassium should be given at a rate of 20 to 30 mmol/h.
Bicarbonate levels can also be managed as acidosis can lead to both neurological and cardiac complications. If the patient’s pH is less than 6.9, the American Diabetes Association recommends starting 100 mmol of sodium bicarbonate in 400 mL sterile water (in addition to potassium chloride at 200 mL/h) for 2 hours. Dosing should be repeated every 2 hours until the patient’s pH is greater than 6.9.
In uncomplicated cases of DKA, the condition is resolved when a patient’s pH is greater than 7.3; glucose level is less than 200 mg/dL; and bicarbonate level is greater than or equal to 18 mmol/L. After patients become hemodynamically stable, they can be discharged and managed at home with a combination of intermediate- or long-acting insulin as well as short- or rapid-acting insulin.
Complications and Mortality
Diabetic ketoacidosis can cause sudden and fluctuating changes in the body. Therefore, it is very important to monitor a patient’s laboratory values very carefully and frequently to avoid any pitfalls. Since patients can present with hyponatremia due to the osmotic draw of glucose in the blood,13 sodium levels may have to be corrected. The corrected serum sodium can be calculated by adding 1.6 mmol/L for every 100 mg/dL of glucose (when finger-stick readings are above 200 mg/dL).15 Patients with DKA can also present with leukocytosis (even in the absence of infection) and hypertriglyceridemia (due to impaired lipoprotein lipase).15 Serum creatine may be elevated due to blood acetoacetate levels.15
Interestingly, there are other acute conditions that can mimic DKA.15 For example, chronic ethanol abuse can lead to ketoacidosis. Unlike DKA, however, alcoholic ketoacidosis does not have profound hyperglycemia, which can help differentiate the two during initial assessment.
Complications due to DKA can arise comprising the patient’s health, including hypoglycemia, hypokalemia, rhabdomyolysis, acute renal failure, pulmonary edema, and shock.16 Cerebral edema is seen in up to 1% of DKA patients,15 the cause of which may be due to the severity of the acidosis, high glucose levels, and rapid hydration. Even when cerebral edema is reduced, patients are often neurologically impaired. Mortality rates from DKA deaths due to cerebral edema can be as high as 24%.13 In the United States, over 100,000 patients with DM per year are admitted to the hospital for DKA, and 9% of patients with DM suffer from DKA-related complications postdischarge.15 With current treatment protocols, mortality rates for DKA-associated deaths are now down to 1%.6,15
Diabetes ketoacidosis-related deaths are usually the result of the following: a triad of DKA symptoms (hyperglycemia, hyperketonemia, and metabolic acidosis), another underlying comorbid condition (eg, myocardial infarction, sepsis, acute respiratory distress syndrome), or the release of biological markers (ie, catecholamines).14,15,17 Thus, as previously stated, the management of potassium levels is important as both hyperkalemia and hypokalemia can lead to fatal arrthythmias.15
Direct mortality from DKA has dropped significantly over the past 20 years, from 8% to less than 1%.6 The US Centers for Disease Control and Prevention has observed a downward trend in death and estimates that 2,417 patients died in 2009 due to DKA,18 and recent postmortem studies have revealed new insights into DKA-related deaths.19 Blood and vitreous acetone concentrations are strong indicators for predeath hyperglycemia and ketosis (if there are no underlying comorbid and/or pharmacological provocations). Blood acetone levels greater than 0.01 g/dL antemortem are suggestive of DKA. It is recommended that these tests should be performed in sudden deaths which have no biological or anatomical cause of death. Postmortem diagnosis of DKA is made with the following criteria: history of DM, increased vitreous glucose concentrations, and elevated blood/vitreous/urine acetone concentrations (>200 mg/dL). If results of the abovementioned parameters are inconclusive, measurement of lactic acid postmortem is thought to further support a diagnosis of DKA.19
Patient Counseling and Education
Approximately 33% of patients whose death was associated with DKA had no personal history of DM.19 This statistic emphasizes the importance of taking a thorough history, physical examination, blood glucose evaluation, and educating patients about the signs and symptoms of DM and DKA.
Patient counseling and education are important, especially in patients whose racial/ethnic background places them at increased risk of developing DM (eg, patients of black or African American, American Indian, Alaskan Native, Asian American, Hispanic, Native Hawaiian, or Pacific Islander descent).20,21 Strategies for preventive management include advocating regular glucose monitoring as well as dietary and lifestyle modifications. In patients with DM, successful management of the condition and its comorbidities can help prevent DKA and associated mortality.
Conclusion
As this case demonstrates, despite prompt diagnosis and management, patients with DKA—especially those with uncontrolled, undiagnosed, or advanced DM—are associated with fatal outcomes. In many cases, however, DKA can be successfully managed and reversed, especially when the condition is recognized early. Management includes not only IV therapy to adjust fluid and insulin levels, but also restoring electrolyte balance (especially potassium and bicarbonate). Frequent and careful evaluation of laboratory values is vital to the successful treatment of DKA, as there are numerous pitfalls and complications that the emergency physician can encounter. Patients who either have or are at an increased risk of developing DM or DKA may benefit from preventive measures, including regular glucose monitoring and appropriate diet and lifestyle modifications.
Mr Hassan-Ali is a fourth-year medical student at Windsor University School of Medicine, St Kitts, West Indies. Dr Raziuddin is an internist and an emergency medicine physician at Weiss Memorial, Thorek Memorial, and Westlake Hospitals, Chicago, Illinois.
- Kitabchi AH, Umpierrez GE, Murphy MB, et al. Management of hyperglycemic crises in patients with diabetes. Diabetes Care. 2001;24(1):131-153.
- Farinda A. Lab values, normal adult: laboratory reference ranges in healthy adults. 2015. Medscape Web site. http://emedicine.medscape.com/article/2172316-overview. Updated May 14, 2014. Accessed August 14, 2015.
- Young D. Implementation of SI units for clinical laboratory data. Ann Intern Med. 1987;106(1):114-129.
- Maitra A. The endocrine system. In: Kumar V, Abbas AK, Aster JC. Robbins and Cotran Pathologic Basis of Disease. 9th ed. New York, NY: Elsevier Saunders; 2015:1105-1120.
- Powers AC. Diabetes mellitus: management and therapies. In: Kasper DL, Fauci AS, Longo DL, Hauser SL, Jameson JL, Loscalzo J. Harrison’s Principles of Internal Medicine. 19th ed. New York, NY; McGraw-Hill Medical Publishing Division; 2015:2407-2422.
- Kitabchi AE, Umpierrez GE, Miles JM, Fisher JN. Hyperglycemic crises in adult patients with diabetes. Diabetes Care. 2009;32(7):1335-1343.
- Umpierrez GE, Smiley D, Kitabchi AE. Narrative review: ketosis-prone type 2 diabetes mellitus. Ann Intern Med. 2006;144(5):350-357.
- Umpierrez G, Smiley D, Gosmanov A, Thomason D. Ketosis-prone type 2 diabetes: effect of hyperglycemia on beta-cell function and skeletal muscle insulin signaling. Endocr Pract. 2007;13(3):283-290.
- Mauvais-Jarvis F, Sobngwi E, Porcher R, et al. Ketosis-prone type 2 diabetes in patients of sub-Saharan African origin: clinical pathophysiology and natural history of beta-cell dysfunction and insulin resistance. Diabetes. 2004;53(3):645-653.
- Umpierrez GE, Casals MM, Gebhart SP, Mixon PS, Clark WS, Phillips LS. Diabetic ketoacidosis in obese African-Americans. Diabetes. 1995;44(7):790-795.
- Piñero-Piloña A, Raskin P. Idiopathic type 1 diabetes. J Diabetes Complications. 2001;15(6):328-335.
- Kemperman FA, Weber JA, Gorgels J, van Zanten AP, Krediet RT, Arisz L. The influence of ketoacids on plasma creatinine assays in diabetic ketoacidosis. J Intern Med. 2000;248(6):511-517.
- Westerberg DP. Diabetic ketoacidosis: evaluation and treatment. Am Fam Physician. 2013;87(5):337-346.
- Trachtenbarg DE. Diabetic ketoacidosis. Am Fam Physician. 2005;71(9):1705-1714.
- Umpierrez GE, Murphy MB, Kitabchi AE. Diabetic ketoacidosis and hyperglycemic hyperosmolar ayndrome. Diabetes Spectrum. 2002;15(1):28-36.
- Wolfsdorf J, Glaser N, Sperling MA; American Diabetes Association. Diabetic ketoacidosis in infants, children, and adolescents: A consensus statement from the American Diabetes Association. Diabetes Care. 2006;29(5):1150-1159.
- Rosenbloom AL. Sudden death of a young woman attributed to diabetic ketoacidosis. J Forensic Leg Med. 2013;20(8):1063-1065.
- Centers for Disease Control and Prevention. Number of deaths for hyperglycemic crises as underlying cause, United States, 1980-2009. http://www.cdc.gov/diabetes/statistics/mortalitydka/fnumberofdka.htm. Updated November 19, 2013. Accessed August 14, 2015.
- Ali Z, Levine B, Ripple M, Fowler DR. Diabetic ketoacidosis: a silent death. Am J Forensic Med Pathol. 2012;33(3):189-193.
- US Department of Health and Human Services Office of Minority Health. Diabetes and Hispanic Americans. http://minorityhealth.hhs.gov/omh/browse.aspx?lvl=4&lvlid=63. Updated June 15, 2013. Accessed August 14, 2015.
- US Department of Health and Human Services Office of Minority Health. Profile: Native Hawaiian/Other Pacific Islanders. http://minorityhealth.hhs.gov/omh/browse.aspx?lvl=3&lvlid=65. Updated January 15, 2015. Accessed August 14, 2015.
- Kitabchi AH, Umpierrez GE, Murphy MB, et al. Management of hyperglycemic crises in patients with diabetes. Diabetes Care. 2001;24(1):131-153.
- Farinda A. Lab values, normal adult: laboratory reference ranges in healthy adults. 2015. Medscape Web site. http://emedicine.medscape.com/article/2172316-overview. Updated May 14, 2014. Accessed August 14, 2015.
- Young D. Implementation of SI units for clinical laboratory data. Ann Intern Med. 1987;106(1):114-129.
- Maitra A. The endocrine system. In: Kumar V, Abbas AK, Aster JC. Robbins and Cotran Pathologic Basis of Disease. 9th ed. New York, NY: Elsevier Saunders; 2015:1105-1120.
- Powers AC. Diabetes mellitus: management and therapies. In: Kasper DL, Fauci AS, Longo DL, Hauser SL, Jameson JL, Loscalzo J. Harrison’s Principles of Internal Medicine. 19th ed. New York, NY; McGraw-Hill Medical Publishing Division; 2015:2407-2422.
- Kitabchi AE, Umpierrez GE, Miles JM, Fisher JN. Hyperglycemic crises in adult patients with diabetes. Diabetes Care. 2009;32(7):1335-1343.
- Umpierrez GE, Smiley D, Kitabchi AE. Narrative review: ketosis-prone type 2 diabetes mellitus. Ann Intern Med. 2006;144(5):350-357.
- Umpierrez G, Smiley D, Gosmanov A, Thomason D. Ketosis-prone type 2 diabetes: effect of hyperglycemia on beta-cell function and skeletal muscle insulin signaling. Endocr Pract. 2007;13(3):283-290.
- Mauvais-Jarvis F, Sobngwi E, Porcher R, et al. Ketosis-prone type 2 diabetes in patients of sub-Saharan African origin: clinical pathophysiology and natural history of beta-cell dysfunction and insulin resistance. Diabetes. 2004;53(3):645-653.
- Umpierrez GE, Casals MM, Gebhart SP, Mixon PS, Clark WS, Phillips LS. Diabetic ketoacidosis in obese African-Americans. Diabetes. 1995;44(7):790-795.
- Piñero-Piloña A, Raskin P. Idiopathic type 1 diabetes. J Diabetes Complications. 2001;15(6):328-335.
- Kemperman FA, Weber JA, Gorgels J, van Zanten AP, Krediet RT, Arisz L. The influence of ketoacids on plasma creatinine assays in diabetic ketoacidosis. J Intern Med. 2000;248(6):511-517.
- Westerberg DP. Diabetic ketoacidosis: evaluation and treatment. Am Fam Physician. 2013;87(5):337-346.
- Trachtenbarg DE. Diabetic ketoacidosis. Am Fam Physician. 2005;71(9):1705-1714.
- Umpierrez GE, Murphy MB, Kitabchi AE. Diabetic ketoacidosis and hyperglycemic hyperosmolar ayndrome. Diabetes Spectrum. 2002;15(1):28-36.
- Wolfsdorf J, Glaser N, Sperling MA; American Diabetes Association. Diabetic ketoacidosis in infants, children, and adolescents: A consensus statement from the American Diabetes Association. Diabetes Care. 2006;29(5):1150-1159.
- Rosenbloom AL. Sudden death of a young woman attributed to diabetic ketoacidosis. J Forensic Leg Med. 2013;20(8):1063-1065.
- Centers for Disease Control and Prevention. Number of deaths for hyperglycemic crises as underlying cause, United States, 1980-2009. http://www.cdc.gov/diabetes/statistics/mortalitydka/fnumberofdka.htm. Updated November 19, 2013. Accessed August 14, 2015.
- Ali Z, Levine B, Ripple M, Fowler DR. Diabetic ketoacidosis: a silent death. Am J Forensic Med Pathol. 2012;33(3):189-193.
- US Department of Health and Human Services Office of Minority Health. Diabetes and Hispanic Americans. http://minorityhealth.hhs.gov/omh/browse.aspx?lvl=4&lvlid=63. Updated June 15, 2013. Accessed August 14, 2015.
- US Department of Health and Human Services Office of Minority Health. Profile: Native Hawaiian/Other Pacific Islanders. http://minorityhealth.hhs.gov/omh/browse.aspx?lvl=3&lvlid=65. Updated January 15, 2015. Accessed August 14, 2015.
Emergency Imaging
A 45-year-old man presented to the ED with severe left leg pain after he was struck by a sports utility vehicle while driving his moped. A radiograph of the affected extremity was taken in the trauma bay (Figure 1a). During the initial evaluation, it was noted that the patient had no pulses in his left foot.
What is the suspected diagnosis?
What would be the next imaging test (if any) that should be performed?
The radiograph of the knee demonstrates acute comminuted fracture (arrows, Figure 1b) of the distal metadiaphysis of the left femur with angulation and marked posterior displacement of several of the fracture fragments. In a patient with evidence of vascular compromise, an injury to the femoral or popliteal arteries should be considered.
Since this patient required computed tomography (CT) of the abdomen and pelvis, a CT angiogram (CTA) of the left leg was also ordered. The axial CTA image demonstrated acute angulation/compression of the femoral artery (white arrow, Figure 2) at the level of the adductor canal due to adjacent angulated/displaced fracture fragments. Three-dimensional reconstructed image from the CTA further delineated this acute arterial angulation/compression of the femoral artery (white arrow, Figure 3) There was no evidence of contrast extravasation to indicate vessel rupture or pseudoaneurysm formation.
The femoral artery is well protected by musculature as it travels caudally down the leg. It enters the adductor canal at the femoral triangle just inferior to the inguinal ligament. It stays medial to the femoral shaft and exits the adductor canal at the adductor hiatus within the adductor magnus muscle. Upon exiting the adductor canal at the level of the femoral metadiaphysis, the artery travels into the popliteal fossa posterior to the knee, where it becomes the popliteal artery. It is within the popliteal fossa that the artery is most susceptible to injury. The risk of arterial injury increases significantly if the knee is dislocated posteriorly.3 The patient in this case likely avoided serious arterial injury due to the fact that his knee joint remained aligned.
Regarding treatment, the patient was taken to the operating room for external fixation to stabilize the fracture. The pulses returned with reduction of the fracture. The extent of arterial and soft-tissue injury was investigated and no significant arterial damage was noted.
In most fractures of the distal femur, a CT scan of the leg is recommended to exclude intra-articular involvement. If there is clinical suspicion of vascular injury, the diagnostic options include CTA, catheter angiography, or surgical exploration. In most centers, CTA is the fastest, the most cost-effective option, though imaging studies should not delay the surgical intervention of a frankly ischemic limb. Vascular imaging should always be performed in patients who suffer a dislocation of the knee joint, as these patients have a 30% to 40% risk of arterial injury.4
Dr Daniels is a postgraduate year 2 resident in diagnostic radiology at New York-Presbyterian Hospital/Weill Cornell Medical Center, New York City. Dr Bartolotta is an assistant professor of radiology at Weill Cornell Medical College in New York City and assistant attending radiologist at New York-Presbyterian Hospital/Weill Cornell Medical Center. Dr Sy is an assistant professor of clinical radiology at Weill Cornell Medical College, and an assistant attending radiologist at New York-Presbyterian Hospital-Weill Cornell Campus, New York City. Dr Hentel is an associate professor of clinical radiology at Weill Cornell Medical College in New York City. He is also chief of emergency/musculoskeletal imaging and executive vice-chairman for the department of radiology at New York-Presbyterian Hospital/Weill Cornell Medical Center; and associate editor, imaging, of the EMERGENCY MEDICINE editorial board.
- Court-Brown CM, Caesar B. Epidemiology of adult fracture: a review. Injury. 2006;37(8):691-697.
- Arneson TJ, Melton LJ 3rd, Lewallen DG, O’Fallon WM. Epidemiology of diaphyseal and distal femoral fractures in Rochester, Minnesota, 1965-1984. Clin Orthop Relat Res.1988;(234):188-194.
- Meyers MH, Moore TM, Harvey JP Jr. Traumatic dislocation of the knee joint. J Bone Joint Surg Am. 1975;57(3):430-433.
- Kaufman SL, Martin LG. Arterial injuries associated with complete dislocation of the knee. Radiology. 1992;184(1):153-155.
A 45-year-old man presented to the ED with severe left leg pain after he was struck by a sports utility vehicle while driving his moped. A radiograph of the affected extremity was taken in the trauma bay (Figure 1a). During the initial evaluation, it was noted that the patient had no pulses in his left foot.
What is the suspected diagnosis?
What would be the next imaging test (if any) that should be performed?
The radiograph of the knee demonstrates acute comminuted fracture (arrows, Figure 1b) of the distal metadiaphysis of the left femur with angulation and marked posterior displacement of several of the fracture fragments. In a patient with evidence of vascular compromise, an injury to the femoral or popliteal arteries should be considered.
Since this patient required computed tomography (CT) of the abdomen and pelvis, a CT angiogram (CTA) of the left leg was also ordered. The axial CTA image demonstrated acute angulation/compression of the femoral artery (white arrow, Figure 2) at the level of the adductor canal due to adjacent angulated/displaced fracture fragments. Three-dimensional reconstructed image from the CTA further delineated this acute arterial angulation/compression of the femoral artery (white arrow, Figure 3) There was no evidence of contrast extravasation to indicate vessel rupture or pseudoaneurysm formation.
The femoral artery is well protected by musculature as it travels caudally down the leg. It enters the adductor canal at the femoral triangle just inferior to the inguinal ligament. It stays medial to the femoral shaft and exits the adductor canal at the adductor hiatus within the adductor magnus muscle. Upon exiting the adductor canal at the level of the femoral metadiaphysis, the artery travels into the popliteal fossa posterior to the knee, where it becomes the popliteal artery. It is within the popliteal fossa that the artery is most susceptible to injury. The risk of arterial injury increases significantly if the knee is dislocated posteriorly.3 The patient in this case likely avoided serious arterial injury due to the fact that his knee joint remained aligned.
Regarding treatment, the patient was taken to the operating room for external fixation to stabilize the fracture. The pulses returned with reduction of the fracture. The extent of arterial and soft-tissue injury was investigated and no significant arterial damage was noted.
In most fractures of the distal femur, a CT scan of the leg is recommended to exclude intra-articular involvement. If there is clinical suspicion of vascular injury, the diagnostic options include CTA, catheter angiography, or surgical exploration. In most centers, CTA is the fastest, the most cost-effective option, though imaging studies should not delay the surgical intervention of a frankly ischemic limb. Vascular imaging should always be performed in patients who suffer a dislocation of the knee joint, as these patients have a 30% to 40% risk of arterial injury.4
Dr Daniels is a postgraduate year 2 resident in diagnostic radiology at New York-Presbyterian Hospital/Weill Cornell Medical Center, New York City. Dr Bartolotta is an assistant professor of radiology at Weill Cornell Medical College in New York City and assistant attending radiologist at New York-Presbyterian Hospital/Weill Cornell Medical Center. Dr Sy is an assistant professor of clinical radiology at Weill Cornell Medical College, and an assistant attending radiologist at New York-Presbyterian Hospital-Weill Cornell Campus, New York City. Dr Hentel is an associate professor of clinical radiology at Weill Cornell Medical College in New York City. He is also chief of emergency/musculoskeletal imaging and executive vice-chairman for the department of radiology at New York-Presbyterian Hospital/Weill Cornell Medical Center; and associate editor, imaging, of the EMERGENCY MEDICINE editorial board.
A 45-year-old man presented to the ED with severe left leg pain after he was struck by a sports utility vehicle while driving his moped. A radiograph of the affected extremity was taken in the trauma bay (Figure 1a). During the initial evaluation, it was noted that the patient had no pulses in his left foot.
What is the suspected diagnosis?
What would be the next imaging test (if any) that should be performed?
The radiograph of the knee demonstrates acute comminuted fracture (arrows, Figure 1b) of the distal metadiaphysis of the left femur with angulation and marked posterior displacement of several of the fracture fragments. In a patient with evidence of vascular compromise, an injury to the femoral or popliteal arteries should be considered.
Since this patient required computed tomography (CT) of the abdomen and pelvis, a CT angiogram (CTA) of the left leg was also ordered. The axial CTA image demonstrated acute angulation/compression of the femoral artery (white arrow, Figure 2) at the level of the adductor canal due to adjacent angulated/displaced fracture fragments. Three-dimensional reconstructed image from the CTA further delineated this acute arterial angulation/compression of the femoral artery (white arrow, Figure 3) There was no evidence of contrast extravasation to indicate vessel rupture or pseudoaneurysm formation.
The femoral artery is well protected by musculature as it travels caudally down the leg. It enters the adductor canal at the femoral triangle just inferior to the inguinal ligament. It stays medial to the femoral shaft and exits the adductor canal at the adductor hiatus within the adductor magnus muscle. Upon exiting the adductor canal at the level of the femoral metadiaphysis, the artery travels into the popliteal fossa posterior to the knee, where it becomes the popliteal artery. It is within the popliteal fossa that the artery is most susceptible to injury. The risk of arterial injury increases significantly if the knee is dislocated posteriorly.3 The patient in this case likely avoided serious arterial injury due to the fact that his knee joint remained aligned.
Regarding treatment, the patient was taken to the operating room for external fixation to stabilize the fracture. The pulses returned with reduction of the fracture. The extent of arterial and soft-tissue injury was investigated and no significant arterial damage was noted.
In most fractures of the distal femur, a CT scan of the leg is recommended to exclude intra-articular involvement. If there is clinical suspicion of vascular injury, the diagnostic options include CTA, catheter angiography, or surgical exploration. In most centers, CTA is the fastest, the most cost-effective option, though imaging studies should not delay the surgical intervention of a frankly ischemic limb. Vascular imaging should always be performed in patients who suffer a dislocation of the knee joint, as these patients have a 30% to 40% risk of arterial injury.4
Dr Daniels is a postgraduate year 2 resident in diagnostic radiology at New York-Presbyterian Hospital/Weill Cornell Medical Center, New York City. Dr Bartolotta is an assistant professor of radiology at Weill Cornell Medical College in New York City and assistant attending radiologist at New York-Presbyterian Hospital/Weill Cornell Medical Center. Dr Sy is an assistant professor of clinical radiology at Weill Cornell Medical College, and an assistant attending radiologist at New York-Presbyterian Hospital-Weill Cornell Campus, New York City. Dr Hentel is an associate professor of clinical radiology at Weill Cornell Medical College in New York City. He is also chief of emergency/musculoskeletal imaging and executive vice-chairman for the department of radiology at New York-Presbyterian Hospital/Weill Cornell Medical Center; and associate editor, imaging, of the EMERGENCY MEDICINE editorial board.
- Court-Brown CM, Caesar B. Epidemiology of adult fracture: a review. Injury. 2006;37(8):691-697.
- Arneson TJ, Melton LJ 3rd, Lewallen DG, O’Fallon WM. Epidemiology of diaphyseal and distal femoral fractures in Rochester, Minnesota, 1965-1984. Clin Orthop Relat Res.1988;(234):188-194.
- Meyers MH, Moore TM, Harvey JP Jr. Traumatic dislocation of the knee joint. J Bone Joint Surg Am. 1975;57(3):430-433.
- Kaufman SL, Martin LG. Arterial injuries associated with complete dislocation of the knee. Radiology. 1992;184(1):153-155.
- Court-Brown CM, Caesar B. Epidemiology of adult fracture: a review. Injury. 2006;37(8):691-697.
- Arneson TJ, Melton LJ 3rd, Lewallen DG, O’Fallon WM. Epidemiology of diaphyseal and distal femoral fractures in Rochester, Minnesota, 1965-1984. Clin Orthop Relat Res.1988;(234):188-194.
- Meyers MH, Moore TM, Harvey JP Jr. Traumatic dislocation of the knee joint. J Bone Joint Surg Am. 1975;57(3):430-433.
- Kaufman SL, Martin LG. Arterial injuries associated with complete dislocation of the knee. Radiology. 1992;184(1):153-155.
Ankle pain in a young woman with Gaucher disease
A 20-year-old woman with Gaucher disease presents with pain in her right ankle and in her back. She has had the ankle pain for the past 12 months and the back pain for the past 2 years. She describes the ankle pain as stabbing and moderately severe. It is constant, present both at rest and during physical activity, but aggravated by walking and twisting movements. She has noticed grinding and clicking sounds as she moves her ankle. The ankle pain has worsened over the past several months.
She says her back pain is similar to her ankle pain but less severe. She also reports generalized mild aches and bone pain. No other joints are involved. She has no history of fever, chills, or trauma.
A COMPLICATED MEDICAL HISTORY
Her Gaucher disease was diagnosed at age 4 when she presented with failure to thrive and with thrombocytopenia and splenomegaly. She and was found to have an N370S/IVS2+1 mutation of the GBA gene. She underwent removal of 90% of her spleen at the time of diagnosis and was on enzyme replacement therapy with imiglucerase until 3 years ago, when the treatment was stopped because the drug had become unavailable (because of a temporary closure of the manufacturing facility), and because she had developed neutralizing antibodies to it. Despite a dosage as high as 120 U/kg every 2 weeks (the recommended range is 2.5 U/kg three times a week up to 60 U/kg every 2 weeks), her anemia and thrombocytopenia worsened to the point that she became dependent on transfusion of red blood cells and platelets. She has also taken glucocorticoids at various times in the past as a premedication before enzyme replacement therapy.
About 3 years ago, she developed dryness of the skin, pruritus, shiny skin, hardening of the skin, and decreased oral aperture, which was diagnosed as scleroderma.
During the past 5 years, she has had multiple episodes of pale coloration of her skin on exposure to cold, suggestive of Raynaud phenomenon. And for the past 5 months, she has noticed a burning sensation in her throat and retrosternal pain, suggestive of gastroesophageal reflux disease.
She is a college student, with no history of smoking or use of alcohol or recreational drugs. She is sexually active, with no history of sexually transmitted disease, and she uses condoms and oral contraceptives for contraception.
Her father and mother are both carriers of Gaucher disease. She is not of Ashkenazi Jewish descent.
FINDINGS ON PHYSICAL EXAMINATION
On physical examination, her temperature, blood pressure, pulse, and respiratory rate are within normal limits. She has extensive tattooing on her upper chest to hide scarring from previous cannulation ports. The right ankle joint is moderately swollen but shows no other signs of inflammation; its range of motion is limited by severe pain. She has tenderness of the spinous processes and paraspinal area, in addition to multiple tender points in the thoracolumbar area. Palpation of the right hip reveals tenderness of the groin and trochanteric bursa.
No lymphadenopathy, hepatomegaly, splenomegaly, or abdominal masses are noted. Neurologic examination is essentially nonfocal.
Her current medications include omeprazole, ergocalciferol, calcium carbonate, gabapentin, citalopram, and celecoxib. She also takes a multivitamin daily.
1. Which is the most likely underlying cause of her ankle pain?
- Rheumatoid arthritis
- Gaucher disease
- Septic arthritis
- Avascular necrosis secondary to steroid use
Rheumatoid arthritis varies in its presentation. It is usually insidious in onset, migratory, and intermittent, with polyarticular or even monoarticular involvement, and it presents with pain, stiffness, and swelling of the joint.1 Most often affected are the metacarpophalangeal, proximal interphalangeal, wrist, and metatarsophalangeal joints. Involvement of large joints of the upper and lower limbs is also common.2 This is not the most likely cause of this patient’s symptoms, based on the history and the current presentation.
Gaucher disease is a lipidosis caused by accumulation of cellular glycolipids, especially glucocerebrosides, due to deficiency of the enzyme beta-glucosidase. Clinical manifestations include hepatomegaly, splenomegaly, and bone marrow disease presenting as anemia, thrombocytopenia, or skeletal disease.3 Skeletal involvement in Gaucher disease includes bone pain, bone infarcts, and lytic lesions.
Whether splenectomy predisposes the patient to bone manifestations is controversial. Some believe that splenectomy decreases the total body reservoir for the storage of glycolipids and predisposes to their deposition in bone, which in turn results in cortical thinning, impaired remodeling, and decreased intraosseous blood flow, leading to osteonecrosis and fractures.4 This is more common in patients with type 1 Gaucher disease who have undergone splenectomy. (Types 2 and 3 are much rarer, occurring mainly in children; central nervous system involvement is a key feature. A discussion of these types is beyond the focus of this paper.) However, some studies suggest that the increase in bone manifestations after splenectomy may be simply because of severe disease.5 It should be noted that, since the advent of enzyme replacement therapy for Gaucher disease, splenectomy is now rarely performed.6
Anemia is also considered an independent risk factor for the development of avascular necrosis in type 1 Gaucher disease.7 Osteonecrosis due to Gaucher disease is relatively common in the femur, tibia, and humerus and uncommon in the ankle joints.8
Septic arthritis is unlikely in this patient in the absence of fever or signs of inflammation of the joint. Her long-standing history of ankle pain would also be unusual for infection, but a superimposed infectious process should always be suspected in an arthritic joint.
Avascular necrosis secondary to steroid use. Glucocorticoids are notorious for their adverse effects on bone. They induce osteocyte apoptosis and a decrease in bone remodeling, potentially predisposing to osteonecrosis.9 There is a high incidence of osteoporosis, osteonecrosis, and fracture risk with glucocorticoid therapy, and the incidence is dose-dependent. Discontinuation of the drug only partially restores fracture risk to baseline levels.10,11
A meta-analysis of cohort studies with a total sample size of about 42,000 reported an increased risk of fracture at all ages with the use of glucocorticoids.12 Because the minimum dosage and duration of therapy to prevent glucocorticoid-induced osteoporosis are not known, the only recommendation is to keep the dosage as low as possible.13
Glucocorticoid therapy is the most common cause of nontraumatic avascular necrosis. The risk of osteonecrosis in patients on long-term glucocorticoid therapy may be as high as 40%.14 The risk is increased with prolonged treatment and with high doses, but it can also occur with short-term exposure to high doses. The increased risk has been shown to persist for as long as 2 years after the drugs are discontinued.15 Glucocorticoid-induced bone disease commonly affects the hip and vertebrae.
At this stage of the workup, we cannot completely rule out glucocorticoid use as the cause. However, after considering this patient’s presentation and the key features of the other diagnoses, her ankle pain and back pain are more likely caused by her preexisting Gaucher disease.
CONTINUED EVALUATION
Initial laboratory tests (Table 1) reveal severe anemia and thrombocytopenia. Bone marrow biopsy of the iliac crest done as part of the workup for these conditions shows extensive bone marrow space replacement by histiocytic infiltrate, consistent with Gaucher disease. No other marrow process is observed.
Radiography of the ankle (Figure 1) shows a subtle lucency in the talar dome with minimal subarticular collapse seen on the lateral view, suggestive of avascular necrosis and diffuse osteopenia. Joint spaces are maintained.
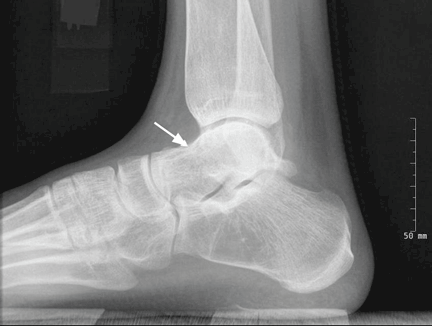
Magnetic resonance imaging (MRI) of the ankle shows numerous bone infarcts with an approximately 15-mm region of mild articular surface collapse in the central and lateral aspect of the talar dome.
MRI of the back shows extensive abnormal bone marrow signal intensity throughout the spine, compatible with a marrow replacement process. Patchy nonexpansile T2/stir hyperintensity with serpiginous enhancement within the T9, T11, T12, L2, and L3 vertebral bodies as well as throughout the entire sacrum is consistent with bone infarct.
2. Based on the results of radiographic studies, which is most likely the immediate cause of her ankle pain?
- Talar avascular necrosis secondary to rheumatoid arthritis
- Talar avascular necrosis secondary to Gaucher disease
- Trauma-induced fracture of the talus
- Plantar fasciitis
Of the bones of the feet, the talus is unique. It is the second largest of the tarsal bones and does not have muscular or tendinous attachments. Sixty percent of the talus bone is covered by articular cartilage,16 so only a limited area is available for penetration of blood vessels. Also, small nutrient vessels and variations of intraosseous anastomoses with a lack of collateral circulation predispose the talus to osteonecrosis when the vascular supply is compromised.16
Radiographic evidence of avascular necrosis is the presence of bone that is more radiopaque than normal bone; this is necrotic bone surrounded by osteopenic bone. Avascular necrosis causes hyperemia and resorption of bone. The resorption does not take place in necrotic bone because of the lack of a vascular supply, and so it appears radiopaque, whereas the bone surrounding the necrotic bone becomes osteopenic and radiolucent.
The sclerotic rim of a bone infarct is also enhanced by an attempted healing process in which new bone forms on the surface of necrotic trabeculae, a process known as “creeping substitution.” This gives a typical sclerotic picture of the talus.
MRI is the most sensitive technique for detecting osteonecrosis. A characteristic radiographic pattern is seen with osteonecrosis of the talus starting with talar dome opacity, followed by deformity and, in severe cases, articular collapse and bone fragmentation.17
The radiograph in our patient’s case is not consistent with features of rheumatoid arthritis or traumatic fracture of the talus. In plantar fasciitis, radiographs are used to rule out other pathologies of the foot, and the only finding may be a bone spur seen at the site of pain. The bone spur is not the cause of pain in plantar fasciitis but may be a result of the plantar fasciitis itself.
Therefore, avascular necrosis secondary to Gaucher disease is most likely the immediate cause of her ankle pain.
THE COURSE OF TREATMENT
The patient is started on enzyme replacement therapy with taliglucerase alfa (see discussion of enzyme replacement below). For the ankle pain, conservative management is prescribed, with application of a splint and a boot.
After 4 months of conservative management, radiography (Figure 2) and magnetic resonance imaging (Figure 3) show progressive deterioration of the talus body, and her ankle pain has worsened. A 6-week trial of an ankle brace also proves futile. Her pain continues to worsen and is not controllable with high doses of pain medication. She requests below-the-knee amputation.
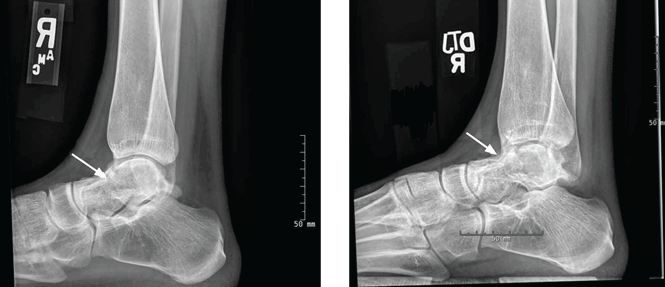
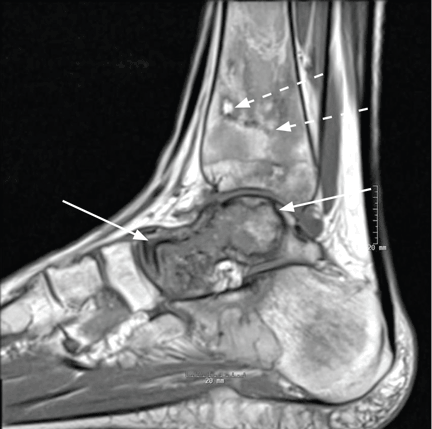
Given the complexity of this patient’s medical condition, fusion of the ankle and hindfoot—which in some patients is preferable to amputation—is not considered because of her extensive bone involvement and ongoing thrombocytopenia, which would impede healing after the procedure. Below-the-knee amputation is performed without complications.
Study of the specimen after amputation reveals talar bone necrosis and bone marrow infiltration by foamy macrophages, consistent with Gaucher disease (Figures 4–6).
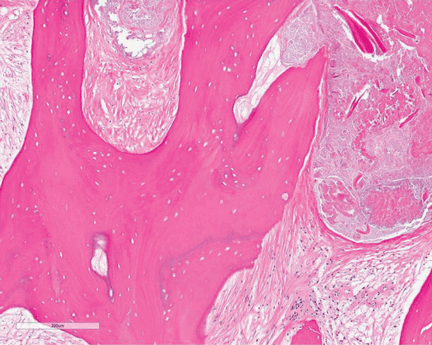
GAUCHER DISEASE
Pharmacologic treatments, effective only for type 1 Gaucher disease, target hepatosplenomegaly, cytopenia, and bone manifestations. Two approaches are enzyme replacement therapy—ie, to replace the defective enzyme—and substrate reduction therapy—ie, to reduce the production and thus the accumulation of glucocerebroside. Enzyme replacement is the first choice of therapy; substrate reduction is reserved for patients unable to tolerate enzyme replacement therapy.
Enzyme replacement
Current drugs for enzyme replacement therapy are imiglucerase, taliglucerase alfa, and velaglucerase alfa. The drugs are given by intravenous infusion over 1 to 2 hours in an outpatient clinic or office every 2 weeks.
These drugs are extremely expensive. Currently, the estimated cost of therapy for 1 year would be $432,978 for imiglucerase, $324,870 for taliglucerase alfa, and $368,550 for velaglucerase alfa. (The estimated costs are for 1 year of treatment for a 70-kg patient at 60 U/kg every 2 weeks.)18 Taliglucerase alfa is less expensive than the other two because it is plant-derived and thus can be more readily produced on a large scale.19
Substrate reduction
Current drugs for substrate reduction therapy are eliglustat and miglustat. They are given orally. Eliglustat is the first oral drug approved as a first-line treatment for Gaucher disease.20 Miglustat is approved only for mild to moderate disease when enzyme replacement fails or is not tolerated.
Patients can develop antibodies to any of the enzyme replacement drugs. It is not known whether this antibody response differs among the three drugs.21
Avascular necrosis of bone can occur in many clinical settings especially after a fracture, particularly of the head of the femur, which leads to interruption of blood supply to the area. Patients with sickle cell disease, those on corticosteroids or bisphosphonates (the latter causing osteonecrosis of the jaw), and those who have pancreatitis or human immunodeficiency virus infection are more prone to this bone complication.
In Gaucher disease, osteonecrosis is associated with splenectomy and severe disease and tends to occur at a younger age than in patients with other diagnoses.8 The plasma chitotriosidase activity and pulmonary and activation-regulated chemokines (PARC/CCL18), which are 10 to 40 times higher than normal in symptomatic patients with Gaucher disease, can be used as a biomarker of disease activity.8 Only plasma chitotriosidase is clinically available and used on a routine basis.
Bone involvement is seen in approximately 75% of the patients with type 1 Gaucher disease,22 and osteonecrosis is a severe form of bone involvement. Monitoring of patients for bone involvement is recommended. Enzyme replacement therapy for Gaucher disease needs to be started even if visceral disease is absent if the patient has evidence of bone involvement in the form of avascular necrosis.7 Prospective studies have shown that enzyme replacement therapy reduces the incidence of osteonecrosis.23
FOLLOW-UP MANAGEMENT OF OUR PATIENT
Avascular necrosis in Gaucher disease more typically involves the hips and shoulders. In the case of our patient, the talus was the most affected bone. Other contributing factors may have been the use of steroids as a premedication (often unnecessary) for her enzyme replacement therapy, as well as the coexistent scleroderma.24
The decision to switch from imiglucerase, to which she developed antibodies, to taliglucerase was made in the hope that the antibodies would not cross-react. After she started taliglucerase, her complete blood count values improved steadily. She did not require transfusions for more than 1 year. Her platelet count rose to 90 × 109/L, and her hemoglobin to 12 g/dL.
A multidisciplinary approach with regular monitoring and appropriate initiation of therapy is necessary to prevent disastrous complications in patients with Gaucher disease.
- Lee DM, Weinblatt ME. Rheumatoid arthritis. Lancet 2001; 358:903–911.
- Fleming A, Crown JM, Corbett M. Early rheumatoid disease. I. Onset. Ann Rheum Dis 1976; 35:357–360.
- Grabowski GA, Andria G, Baldellou A, et al. Pediatric non-neuronopathic Gaucher disease: presentation, diagnosis, and assessment. Consensus statements. Eur J Pediatr 2004; 163:58–66.
- Rodrigue SW, Rosenthal DI, Barton NW, Zurakowski D, Mankin HJ. Risk factors for osteonecrosis in patients with type 1 Gaucher’s disease. Clin Orthop Relat Res 1999; May (362):201–207.
- Lee RE. The pathology of Gaucher disease. Prog Clin Biol Res 1982; 95:177–217.
- Cox TM, Aerts JM, Belmatoug N, et al. Management of non-neuronopathic Gaucher disease with special reference to pregnancy, splenectomy, bisphosphonate therapy, use of biomarkers and bone disease monitoring. J Inherit Metab Dis 2008; 31:319–336.
- Khan A, Hangartner T, Weinreb NJ, Taylor JS, Mistry PK. Risk factors for fractures and avascular osteonecrosis in type 1 Gaucher disease: a study from the International Collaborative Gaucher Group (ICGG) Gaucher Registry. J Bone Miner Res 2012; 27:1839–1848.
- Deegan PB, Pavlova E, Tindall J, et al. Osseous manifestations of adult Gaucher disease in the era of enzyme replacement therapy. Medicine (Baltimore) 2011; 90:52–60.
- Weinstein RS. Glucocorticoid-induced osteonecrosis. Endocrine 2012; 41:183–190.
- Compston J. Management of glucocorticoid-induced osteoporosis. Nat Rev Rheumatol 2010; 6:82–88.
- Van Staa TP, Laan RF, Barton IP, Cohen S, Reid DM, Cooper C. Bone density threshold and other predictors of vertebral fracture in patients receiving oral glucocorticoid therapy. Arthritis Rheum 2003; 48:3224–3229.
- Kanis JA, Johnell O, Oden A, et al. The risk and burden of vertebral fractures in Sweden. Osteoporos Int 2004; 15:20–26.
- Seguro LP, Rosario C, Shoenfeld Y. Long-term complications of past glucocorticoid use. Autoimmun Rev 2013; 12:629–632.
- Weinstein RS. Glucocorticoid-induced osteoporosis and osteonecrosis. Endocrinol Metab Clin North Am 2012; 41:595–611.
- Cooper C, Steinbuch M, Stevenson R, Miday R, Watts NB. The epidemiology of osteonecrosis: findings from the GPRD and THIN databases in the UK. Osteoporos Int 2010; 21:569–577.
- Mulfinger GL, Trueta J. The blood supply of the talus. J Bone Joint Surg Br 1970; 52:160–167.
- Pearce DH, Mongiardi CN, Fornasier VL, Daniels TR. Avascular necrosis of the talus: a pictoral essay. Radiographics 2005; 25:399–410.
- In brief: Taliglucerase (Elelyso) for Gaucher disease. Med Lett Drugs Ther 2012 Jul 9; 54(1394):56.
- Hollak CE. An evidence-based review of the potential benefits of taliglucerase alfa in the treatment of patients with Gaucher disease. Core Evid 2012; 7:15–20.
- Poole RM. Eliglustat: first global approval. Drugs 2014; 74:1829–1836.
- Bennett LL, Mohan D. Gaucher disease and its treatment options. Ann Pharmacother 2013; 47:1182–1193.
- Germain DP. Gaucher’s disease: a paradigm for interventional genetics. Clin Genet 2004; 65:77–86.
- Sims KB, Pastores GM, Weinreb NJ, et al. Improvement of bone disease by imiglucerase (Cerezyme) therapy in patients with skeletal manifestations of type 1 Gaucher disease: results of a 48-month longitudinal cohort study. Clin Genet 2008; 73:430–440.
- Rennie C, Britton J, Prouse P. Bilateral avascular necrosis of the lunate in a patient with severe Raynaud’s phenomenon and scleroderma. J Clin Rheumatol 1999; 5:165–168.
A 20-year-old woman with Gaucher disease presents with pain in her right ankle and in her back. She has had the ankle pain for the past 12 months and the back pain for the past 2 years. She describes the ankle pain as stabbing and moderately severe. It is constant, present both at rest and during physical activity, but aggravated by walking and twisting movements. She has noticed grinding and clicking sounds as she moves her ankle. The ankle pain has worsened over the past several months.
She says her back pain is similar to her ankle pain but less severe. She also reports generalized mild aches and bone pain. No other joints are involved. She has no history of fever, chills, or trauma.
A COMPLICATED MEDICAL HISTORY
Her Gaucher disease was diagnosed at age 4 when she presented with failure to thrive and with thrombocytopenia and splenomegaly. She and was found to have an N370S/IVS2+1 mutation of the GBA gene. She underwent removal of 90% of her spleen at the time of diagnosis and was on enzyme replacement therapy with imiglucerase until 3 years ago, when the treatment was stopped because the drug had become unavailable (because of a temporary closure of the manufacturing facility), and because she had developed neutralizing antibodies to it. Despite a dosage as high as 120 U/kg every 2 weeks (the recommended range is 2.5 U/kg three times a week up to 60 U/kg every 2 weeks), her anemia and thrombocytopenia worsened to the point that she became dependent on transfusion of red blood cells and platelets. She has also taken glucocorticoids at various times in the past as a premedication before enzyme replacement therapy.
About 3 years ago, she developed dryness of the skin, pruritus, shiny skin, hardening of the skin, and decreased oral aperture, which was diagnosed as scleroderma.
During the past 5 years, she has had multiple episodes of pale coloration of her skin on exposure to cold, suggestive of Raynaud phenomenon. And for the past 5 months, she has noticed a burning sensation in her throat and retrosternal pain, suggestive of gastroesophageal reflux disease.
She is a college student, with no history of smoking or use of alcohol or recreational drugs. She is sexually active, with no history of sexually transmitted disease, and she uses condoms and oral contraceptives for contraception.
Her father and mother are both carriers of Gaucher disease. She is not of Ashkenazi Jewish descent.
FINDINGS ON PHYSICAL EXAMINATION
On physical examination, her temperature, blood pressure, pulse, and respiratory rate are within normal limits. She has extensive tattooing on her upper chest to hide scarring from previous cannulation ports. The right ankle joint is moderately swollen but shows no other signs of inflammation; its range of motion is limited by severe pain. She has tenderness of the spinous processes and paraspinal area, in addition to multiple tender points in the thoracolumbar area. Palpation of the right hip reveals tenderness of the groin and trochanteric bursa.
No lymphadenopathy, hepatomegaly, splenomegaly, or abdominal masses are noted. Neurologic examination is essentially nonfocal.
Her current medications include omeprazole, ergocalciferol, calcium carbonate, gabapentin, citalopram, and celecoxib. She also takes a multivitamin daily.
1. Which is the most likely underlying cause of her ankle pain?
- Rheumatoid arthritis
- Gaucher disease
- Septic arthritis
- Avascular necrosis secondary to steroid use
Rheumatoid arthritis varies in its presentation. It is usually insidious in onset, migratory, and intermittent, with polyarticular or even monoarticular involvement, and it presents with pain, stiffness, and swelling of the joint.1 Most often affected are the metacarpophalangeal, proximal interphalangeal, wrist, and metatarsophalangeal joints. Involvement of large joints of the upper and lower limbs is also common.2 This is not the most likely cause of this patient’s symptoms, based on the history and the current presentation.
Gaucher disease is a lipidosis caused by accumulation of cellular glycolipids, especially glucocerebrosides, due to deficiency of the enzyme beta-glucosidase. Clinical manifestations include hepatomegaly, splenomegaly, and bone marrow disease presenting as anemia, thrombocytopenia, or skeletal disease.3 Skeletal involvement in Gaucher disease includes bone pain, bone infarcts, and lytic lesions.
Whether splenectomy predisposes the patient to bone manifestations is controversial. Some believe that splenectomy decreases the total body reservoir for the storage of glycolipids and predisposes to their deposition in bone, which in turn results in cortical thinning, impaired remodeling, and decreased intraosseous blood flow, leading to osteonecrosis and fractures.4 This is more common in patients with type 1 Gaucher disease who have undergone splenectomy. (Types 2 and 3 are much rarer, occurring mainly in children; central nervous system involvement is a key feature. A discussion of these types is beyond the focus of this paper.) However, some studies suggest that the increase in bone manifestations after splenectomy may be simply because of severe disease.5 It should be noted that, since the advent of enzyme replacement therapy for Gaucher disease, splenectomy is now rarely performed.6
Anemia is also considered an independent risk factor for the development of avascular necrosis in type 1 Gaucher disease.7 Osteonecrosis due to Gaucher disease is relatively common in the femur, tibia, and humerus and uncommon in the ankle joints.8
Septic arthritis is unlikely in this patient in the absence of fever or signs of inflammation of the joint. Her long-standing history of ankle pain would also be unusual for infection, but a superimposed infectious process should always be suspected in an arthritic joint.
Avascular necrosis secondary to steroid use. Glucocorticoids are notorious for their adverse effects on bone. They induce osteocyte apoptosis and a decrease in bone remodeling, potentially predisposing to osteonecrosis.9 There is a high incidence of osteoporosis, osteonecrosis, and fracture risk with glucocorticoid therapy, and the incidence is dose-dependent. Discontinuation of the drug only partially restores fracture risk to baseline levels.10,11
A meta-analysis of cohort studies with a total sample size of about 42,000 reported an increased risk of fracture at all ages with the use of glucocorticoids.12 Because the minimum dosage and duration of therapy to prevent glucocorticoid-induced osteoporosis are not known, the only recommendation is to keep the dosage as low as possible.13
Glucocorticoid therapy is the most common cause of nontraumatic avascular necrosis. The risk of osteonecrosis in patients on long-term glucocorticoid therapy may be as high as 40%.14 The risk is increased with prolonged treatment and with high doses, but it can also occur with short-term exposure to high doses. The increased risk has been shown to persist for as long as 2 years after the drugs are discontinued.15 Glucocorticoid-induced bone disease commonly affects the hip and vertebrae.
At this stage of the workup, we cannot completely rule out glucocorticoid use as the cause. However, after considering this patient’s presentation and the key features of the other diagnoses, her ankle pain and back pain are more likely caused by her preexisting Gaucher disease.
CONTINUED EVALUATION
Initial laboratory tests (Table 1) reveal severe anemia and thrombocytopenia. Bone marrow biopsy of the iliac crest done as part of the workup for these conditions shows extensive bone marrow space replacement by histiocytic infiltrate, consistent with Gaucher disease. No other marrow process is observed.
Radiography of the ankle (Figure 1) shows a subtle lucency in the talar dome with minimal subarticular collapse seen on the lateral view, suggestive of avascular necrosis and diffuse osteopenia. Joint spaces are maintained.

Magnetic resonance imaging (MRI) of the ankle shows numerous bone infarcts with an approximately 15-mm region of mild articular surface collapse in the central and lateral aspect of the talar dome.
MRI of the back shows extensive abnormal bone marrow signal intensity throughout the spine, compatible with a marrow replacement process. Patchy nonexpansile T2/stir hyperintensity with serpiginous enhancement within the T9, T11, T12, L2, and L3 vertebral bodies as well as throughout the entire sacrum is consistent with bone infarct.
2. Based on the results of radiographic studies, which is most likely the immediate cause of her ankle pain?
- Talar avascular necrosis secondary to rheumatoid arthritis
- Talar avascular necrosis secondary to Gaucher disease
- Trauma-induced fracture of the talus
- Plantar fasciitis
Of the bones of the feet, the talus is unique. It is the second largest of the tarsal bones and does not have muscular or tendinous attachments. Sixty percent of the talus bone is covered by articular cartilage,16 so only a limited area is available for penetration of blood vessels. Also, small nutrient vessels and variations of intraosseous anastomoses with a lack of collateral circulation predispose the talus to osteonecrosis when the vascular supply is compromised.16
Radiographic evidence of avascular necrosis is the presence of bone that is more radiopaque than normal bone; this is necrotic bone surrounded by osteopenic bone. Avascular necrosis causes hyperemia and resorption of bone. The resorption does not take place in necrotic bone because of the lack of a vascular supply, and so it appears radiopaque, whereas the bone surrounding the necrotic bone becomes osteopenic and radiolucent.
The sclerotic rim of a bone infarct is also enhanced by an attempted healing process in which new bone forms on the surface of necrotic trabeculae, a process known as “creeping substitution.” This gives a typical sclerotic picture of the talus.
MRI is the most sensitive technique for detecting osteonecrosis. A characteristic radiographic pattern is seen with osteonecrosis of the talus starting with talar dome opacity, followed by deformity and, in severe cases, articular collapse and bone fragmentation.17
The radiograph in our patient’s case is not consistent with features of rheumatoid arthritis or traumatic fracture of the talus. In plantar fasciitis, radiographs are used to rule out other pathologies of the foot, and the only finding may be a bone spur seen at the site of pain. The bone spur is not the cause of pain in plantar fasciitis but may be a result of the plantar fasciitis itself.
Therefore, avascular necrosis secondary to Gaucher disease is most likely the immediate cause of her ankle pain.
THE COURSE OF TREATMENT
The patient is started on enzyme replacement therapy with taliglucerase alfa (see discussion of enzyme replacement below). For the ankle pain, conservative management is prescribed, with application of a splint and a boot.
After 4 months of conservative management, radiography (Figure 2) and magnetic resonance imaging (Figure 3) show progressive deterioration of the talus body, and her ankle pain has worsened. A 6-week trial of an ankle brace also proves futile. Her pain continues to worsen and is not controllable with high doses of pain medication. She requests below-the-knee amputation.


Given the complexity of this patient’s medical condition, fusion of the ankle and hindfoot—which in some patients is preferable to amputation—is not considered because of her extensive bone involvement and ongoing thrombocytopenia, which would impede healing after the procedure. Below-the-knee amputation is performed without complications.
Study of the specimen after amputation reveals talar bone necrosis and bone marrow infiltration by foamy macrophages, consistent with Gaucher disease (Figures 4–6).



GAUCHER DISEASE
Pharmacologic treatments, effective only for type 1 Gaucher disease, target hepatosplenomegaly, cytopenia, and bone manifestations. Two approaches are enzyme replacement therapy—ie, to replace the defective enzyme—and substrate reduction therapy—ie, to reduce the production and thus the accumulation of glucocerebroside. Enzyme replacement is the first choice of therapy; substrate reduction is reserved for patients unable to tolerate enzyme replacement therapy.
Enzyme replacement
Current drugs for enzyme replacement therapy are imiglucerase, taliglucerase alfa, and velaglucerase alfa. The drugs are given by intravenous infusion over 1 to 2 hours in an outpatient clinic or office every 2 weeks.
These drugs are extremely expensive. Currently, the estimated cost of therapy for 1 year would be $432,978 for imiglucerase, $324,870 for taliglucerase alfa, and $368,550 for velaglucerase alfa. (The estimated costs are for 1 year of treatment for a 70-kg patient at 60 U/kg every 2 weeks.)18 Taliglucerase alfa is less expensive than the other two because it is plant-derived and thus can be more readily produced on a large scale.19
Substrate reduction
Current drugs for substrate reduction therapy are eliglustat and miglustat. They are given orally. Eliglustat is the first oral drug approved as a first-line treatment for Gaucher disease.20 Miglustat is approved only for mild to moderate disease when enzyme replacement fails or is not tolerated.
Patients can develop antibodies to any of the enzyme replacement drugs. It is not known whether this antibody response differs among the three drugs.21
Avascular necrosis of bone can occur in many clinical settings especially after a fracture, particularly of the head of the femur, which leads to interruption of blood supply to the area. Patients with sickle cell disease, those on corticosteroids or bisphosphonates (the latter causing osteonecrosis of the jaw), and those who have pancreatitis or human immunodeficiency virus infection are more prone to this bone complication.
In Gaucher disease, osteonecrosis is associated with splenectomy and severe disease and tends to occur at a younger age than in patients with other diagnoses.8 The plasma chitotriosidase activity and pulmonary and activation-regulated chemokines (PARC/CCL18), which are 10 to 40 times higher than normal in symptomatic patients with Gaucher disease, can be used as a biomarker of disease activity.8 Only plasma chitotriosidase is clinically available and used on a routine basis.
Bone involvement is seen in approximately 75% of the patients with type 1 Gaucher disease,22 and osteonecrosis is a severe form of bone involvement. Monitoring of patients for bone involvement is recommended. Enzyme replacement therapy for Gaucher disease needs to be started even if visceral disease is absent if the patient has evidence of bone involvement in the form of avascular necrosis.7 Prospective studies have shown that enzyme replacement therapy reduces the incidence of osteonecrosis.23
FOLLOW-UP MANAGEMENT OF OUR PATIENT
Avascular necrosis in Gaucher disease more typically involves the hips and shoulders. In the case of our patient, the talus was the most affected bone. Other contributing factors may have been the use of steroids as a premedication (often unnecessary) for her enzyme replacement therapy, as well as the coexistent scleroderma.24
The decision to switch from imiglucerase, to which she developed antibodies, to taliglucerase was made in the hope that the antibodies would not cross-react. After she started taliglucerase, her complete blood count values improved steadily. She did not require transfusions for more than 1 year. Her platelet count rose to 90 × 109/L, and her hemoglobin to 12 g/dL.
A multidisciplinary approach with regular monitoring and appropriate initiation of therapy is necessary to prevent disastrous complications in patients with Gaucher disease.
A 20-year-old woman with Gaucher disease presents with pain in her right ankle and in her back. She has had the ankle pain for the past 12 months and the back pain for the past 2 years. She describes the ankle pain as stabbing and moderately severe. It is constant, present both at rest and during physical activity, but aggravated by walking and twisting movements. She has noticed grinding and clicking sounds as she moves her ankle. The ankle pain has worsened over the past several months.
She says her back pain is similar to her ankle pain but less severe. She also reports generalized mild aches and bone pain. No other joints are involved. She has no history of fever, chills, or trauma.
A COMPLICATED MEDICAL HISTORY
Her Gaucher disease was diagnosed at age 4 when she presented with failure to thrive and with thrombocytopenia and splenomegaly. She and was found to have an N370S/IVS2+1 mutation of the GBA gene. She underwent removal of 90% of her spleen at the time of diagnosis and was on enzyme replacement therapy with imiglucerase until 3 years ago, when the treatment was stopped because the drug had become unavailable (because of a temporary closure of the manufacturing facility), and because she had developed neutralizing antibodies to it. Despite a dosage as high as 120 U/kg every 2 weeks (the recommended range is 2.5 U/kg three times a week up to 60 U/kg every 2 weeks), her anemia and thrombocytopenia worsened to the point that she became dependent on transfusion of red blood cells and platelets. She has also taken glucocorticoids at various times in the past as a premedication before enzyme replacement therapy.
About 3 years ago, she developed dryness of the skin, pruritus, shiny skin, hardening of the skin, and decreased oral aperture, which was diagnosed as scleroderma.
During the past 5 years, she has had multiple episodes of pale coloration of her skin on exposure to cold, suggestive of Raynaud phenomenon. And for the past 5 months, she has noticed a burning sensation in her throat and retrosternal pain, suggestive of gastroesophageal reflux disease.
She is a college student, with no history of smoking or use of alcohol or recreational drugs. She is sexually active, with no history of sexually transmitted disease, and she uses condoms and oral contraceptives for contraception.
Her father and mother are both carriers of Gaucher disease. She is not of Ashkenazi Jewish descent.
FINDINGS ON PHYSICAL EXAMINATION
On physical examination, her temperature, blood pressure, pulse, and respiratory rate are within normal limits. She has extensive tattooing on her upper chest to hide scarring from previous cannulation ports. The right ankle joint is moderately swollen but shows no other signs of inflammation; its range of motion is limited by severe pain. She has tenderness of the spinous processes and paraspinal area, in addition to multiple tender points in the thoracolumbar area. Palpation of the right hip reveals tenderness of the groin and trochanteric bursa.
No lymphadenopathy, hepatomegaly, splenomegaly, or abdominal masses are noted. Neurologic examination is essentially nonfocal.
Her current medications include omeprazole, ergocalciferol, calcium carbonate, gabapentin, citalopram, and celecoxib. She also takes a multivitamin daily.
1. Which is the most likely underlying cause of her ankle pain?
- Rheumatoid arthritis
- Gaucher disease
- Septic arthritis
- Avascular necrosis secondary to steroid use
Rheumatoid arthritis varies in its presentation. It is usually insidious in onset, migratory, and intermittent, with polyarticular or even monoarticular involvement, and it presents with pain, stiffness, and swelling of the joint.1 Most often affected are the metacarpophalangeal, proximal interphalangeal, wrist, and metatarsophalangeal joints. Involvement of large joints of the upper and lower limbs is also common.2 This is not the most likely cause of this patient’s symptoms, based on the history and the current presentation.
Gaucher disease is a lipidosis caused by accumulation of cellular glycolipids, especially glucocerebrosides, due to deficiency of the enzyme beta-glucosidase. Clinical manifestations include hepatomegaly, splenomegaly, and bone marrow disease presenting as anemia, thrombocytopenia, or skeletal disease.3 Skeletal involvement in Gaucher disease includes bone pain, bone infarcts, and lytic lesions.
Whether splenectomy predisposes the patient to bone manifestations is controversial. Some believe that splenectomy decreases the total body reservoir for the storage of glycolipids and predisposes to their deposition in bone, which in turn results in cortical thinning, impaired remodeling, and decreased intraosseous blood flow, leading to osteonecrosis and fractures.4 This is more common in patients with type 1 Gaucher disease who have undergone splenectomy. (Types 2 and 3 are much rarer, occurring mainly in children; central nervous system involvement is a key feature. A discussion of these types is beyond the focus of this paper.) However, some studies suggest that the increase in bone manifestations after splenectomy may be simply because of severe disease.5 It should be noted that, since the advent of enzyme replacement therapy for Gaucher disease, splenectomy is now rarely performed.6
Anemia is also considered an independent risk factor for the development of avascular necrosis in type 1 Gaucher disease.7 Osteonecrosis due to Gaucher disease is relatively common in the femur, tibia, and humerus and uncommon in the ankle joints.8
Septic arthritis is unlikely in this patient in the absence of fever or signs of inflammation of the joint. Her long-standing history of ankle pain would also be unusual for infection, but a superimposed infectious process should always be suspected in an arthritic joint.
Avascular necrosis secondary to steroid use. Glucocorticoids are notorious for their adverse effects on bone. They induce osteocyte apoptosis and a decrease in bone remodeling, potentially predisposing to osteonecrosis.9 There is a high incidence of osteoporosis, osteonecrosis, and fracture risk with glucocorticoid therapy, and the incidence is dose-dependent. Discontinuation of the drug only partially restores fracture risk to baseline levels.10,11
A meta-analysis of cohort studies with a total sample size of about 42,000 reported an increased risk of fracture at all ages with the use of glucocorticoids.12 Because the minimum dosage and duration of therapy to prevent glucocorticoid-induced osteoporosis are not known, the only recommendation is to keep the dosage as low as possible.13
Glucocorticoid therapy is the most common cause of nontraumatic avascular necrosis. The risk of osteonecrosis in patients on long-term glucocorticoid therapy may be as high as 40%.14 The risk is increased with prolonged treatment and with high doses, but it can also occur with short-term exposure to high doses. The increased risk has been shown to persist for as long as 2 years after the drugs are discontinued.15 Glucocorticoid-induced bone disease commonly affects the hip and vertebrae.
At this stage of the workup, we cannot completely rule out glucocorticoid use as the cause. However, after considering this patient’s presentation and the key features of the other diagnoses, her ankle pain and back pain are more likely caused by her preexisting Gaucher disease.
CONTINUED EVALUATION
Initial laboratory tests (Table 1) reveal severe anemia and thrombocytopenia. Bone marrow biopsy of the iliac crest done as part of the workup for these conditions shows extensive bone marrow space replacement by histiocytic infiltrate, consistent with Gaucher disease. No other marrow process is observed.
Radiography of the ankle (Figure 1) shows a subtle lucency in the talar dome with minimal subarticular collapse seen on the lateral view, suggestive of avascular necrosis and diffuse osteopenia. Joint spaces are maintained.

Magnetic resonance imaging (MRI) of the ankle shows numerous bone infarcts with an approximately 15-mm region of mild articular surface collapse in the central and lateral aspect of the talar dome.
MRI of the back shows extensive abnormal bone marrow signal intensity throughout the spine, compatible with a marrow replacement process. Patchy nonexpansile T2/stir hyperintensity with serpiginous enhancement within the T9, T11, T12, L2, and L3 vertebral bodies as well as throughout the entire sacrum is consistent with bone infarct.
2. Based on the results of radiographic studies, which is most likely the immediate cause of her ankle pain?
- Talar avascular necrosis secondary to rheumatoid arthritis
- Talar avascular necrosis secondary to Gaucher disease
- Trauma-induced fracture of the talus
- Plantar fasciitis
Of the bones of the feet, the talus is unique. It is the second largest of the tarsal bones and does not have muscular or tendinous attachments. Sixty percent of the talus bone is covered by articular cartilage,16 so only a limited area is available for penetration of blood vessels. Also, small nutrient vessels and variations of intraosseous anastomoses with a lack of collateral circulation predispose the talus to osteonecrosis when the vascular supply is compromised.16
Radiographic evidence of avascular necrosis is the presence of bone that is more radiopaque than normal bone; this is necrotic bone surrounded by osteopenic bone. Avascular necrosis causes hyperemia and resorption of bone. The resorption does not take place in necrotic bone because of the lack of a vascular supply, and so it appears radiopaque, whereas the bone surrounding the necrotic bone becomes osteopenic and radiolucent.
The sclerotic rim of a bone infarct is also enhanced by an attempted healing process in which new bone forms on the surface of necrotic trabeculae, a process known as “creeping substitution.” This gives a typical sclerotic picture of the talus.
MRI is the most sensitive technique for detecting osteonecrosis. A characteristic radiographic pattern is seen with osteonecrosis of the talus starting with talar dome opacity, followed by deformity and, in severe cases, articular collapse and bone fragmentation.17
The radiograph in our patient’s case is not consistent with features of rheumatoid arthritis or traumatic fracture of the talus. In plantar fasciitis, radiographs are used to rule out other pathologies of the foot, and the only finding may be a bone spur seen at the site of pain. The bone spur is not the cause of pain in plantar fasciitis but may be a result of the plantar fasciitis itself.
Therefore, avascular necrosis secondary to Gaucher disease is most likely the immediate cause of her ankle pain.
THE COURSE OF TREATMENT
The patient is started on enzyme replacement therapy with taliglucerase alfa (see discussion of enzyme replacement below). For the ankle pain, conservative management is prescribed, with application of a splint and a boot.
After 4 months of conservative management, radiography (Figure 2) and magnetic resonance imaging (Figure 3) show progressive deterioration of the talus body, and her ankle pain has worsened. A 6-week trial of an ankle brace also proves futile. Her pain continues to worsen and is not controllable with high doses of pain medication. She requests below-the-knee amputation.


Given the complexity of this patient’s medical condition, fusion of the ankle and hindfoot—which in some patients is preferable to amputation—is not considered because of her extensive bone involvement and ongoing thrombocytopenia, which would impede healing after the procedure. Below-the-knee amputation is performed without complications.
Study of the specimen after amputation reveals talar bone necrosis and bone marrow infiltration by foamy macrophages, consistent with Gaucher disease (Figures 4–6).



GAUCHER DISEASE
Pharmacologic treatments, effective only for type 1 Gaucher disease, target hepatosplenomegaly, cytopenia, and bone manifestations. Two approaches are enzyme replacement therapy—ie, to replace the defective enzyme—and substrate reduction therapy—ie, to reduce the production and thus the accumulation of glucocerebroside. Enzyme replacement is the first choice of therapy; substrate reduction is reserved for patients unable to tolerate enzyme replacement therapy.
Enzyme replacement
Current drugs for enzyme replacement therapy are imiglucerase, taliglucerase alfa, and velaglucerase alfa. The drugs are given by intravenous infusion over 1 to 2 hours in an outpatient clinic or office every 2 weeks.
These drugs are extremely expensive. Currently, the estimated cost of therapy for 1 year would be $432,978 for imiglucerase, $324,870 for taliglucerase alfa, and $368,550 for velaglucerase alfa. (The estimated costs are for 1 year of treatment for a 70-kg patient at 60 U/kg every 2 weeks.)18 Taliglucerase alfa is less expensive than the other two because it is plant-derived and thus can be more readily produced on a large scale.19
Substrate reduction
Current drugs for substrate reduction therapy are eliglustat and miglustat. They are given orally. Eliglustat is the first oral drug approved as a first-line treatment for Gaucher disease.20 Miglustat is approved only for mild to moderate disease when enzyme replacement fails or is not tolerated.
Patients can develop antibodies to any of the enzyme replacement drugs. It is not known whether this antibody response differs among the three drugs.21
Avascular necrosis of bone can occur in many clinical settings especially after a fracture, particularly of the head of the femur, which leads to interruption of blood supply to the area. Patients with sickle cell disease, those on corticosteroids or bisphosphonates (the latter causing osteonecrosis of the jaw), and those who have pancreatitis or human immunodeficiency virus infection are more prone to this bone complication.
In Gaucher disease, osteonecrosis is associated with splenectomy and severe disease and tends to occur at a younger age than in patients with other diagnoses.8 The plasma chitotriosidase activity and pulmonary and activation-regulated chemokines (PARC/CCL18), which are 10 to 40 times higher than normal in symptomatic patients with Gaucher disease, can be used as a biomarker of disease activity.8 Only plasma chitotriosidase is clinically available and used on a routine basis.
Bone involvement is seen in approximately 75% of the patients with type 1 Gaucher disease,22 and osteonecrosis is a severe form of bone involvement. Monitoring of patients for bone involvement is recommended. Enzyme replacement therapy for Gaucher disease needs to be started even if visceral disease is absent if the patient has evidence of bone involvement in the form of avascular necrosis.7 Prospective studies have shown that enzyme replacement therapy reduces the incidence of osteonecrosis.23
FOLLOW-UP MANAGEMENT OF OUR PATIENT
Avascular necrosis in Gaucher disease more typically involves the hips and shoulders. In the case of our patient, the talus was the most affected bone. Other contributing factors may have been the use of steroids as a premedication (often unnecessary) for her enzyme replacement therapy, as well as the coexistent scleroderma.24
The decision to switch from imiglucerase, to which she developed antibodies, to taliglucerase was made in the hope that the antibodies would not cross-react. After she started taliglucerase, her complete blood count values improved steadily. She did not require transfusions for more than 1 year. Her platelet count rose to 90 × 109/L, and her hemoglobin to 12 g/dL.
A multidisciplinary approach with regular monitoring and appropriate initiation of therapy is necessary to prevent disastrous complications in patients with Gaucher disease.
- Lee DM, Weinblatt ME. Rheumatoid arthritis. Lancet 2001; 358:903–911.
- Fleming A, Crown JM, Corbett M. Early rheumatoid disease. I. Onset. Ann Rheum Dis 1976; 35:357–360.
- Grabowski GA, Andria G, Baldellou A, et al. Pediatric non-neuronopathic Gaucher disease: presentation, diagnosis, and assessment. Consensus statements. Eur J Pediatr 2004; 163:58–66.
- Rodrigue SW, Rosenthal DI, Barton NW, Zurakowski D, Mankin HJ. Risk factors for osteonecrosis in patients with type 1 Gaucher’s disease. Clin Orthop Relat Res 1999; May (362):201–207.
- Lee RE. The pathology of Gaucher disease. Prog Clin Biol Res 1982; 95:177–217.
- Cox TM, Aerts JM, Belmatoug N, et al. Management of non-neuronopathic Gaucher disease with special reference to pregnancy, splenectomy, bisphosphonate therapy, use of biomarkers and bone disease monitoring. J Inherit Metab Dis 2008; 31:319–336.
- Khan A, Hangartner T, Weinreb NJ, Taylor JS, Mistry PK. Risk factors for fractures and avascular osteonecrosis in type 1 Gaucher disease: a study from the International Collaborative Gaucher Group (ICGG) Gaucher Registry. J Bone Miner Res 2012; 27:1839–1848.
- Deegan PB, Pavlova E, Tindall J, et al. Osseous manifestations of adult Gaucher disease in the era of enzyme replacement therapy. Medicine (Baltimore) 2011; 90:52–60.
- Weinstein RS. Glucocorticoid-induced osteonecrosis. Endocrine 2012; 41:183–190.
- Compston J. Management of glucocorticoid-induced osteoporosis. Nat Rev Rheumatol 2010; 6:82–88.
- Van Staa TP, Laan RF, Barton IP, Cohen S, Reid DM, Cooper C. Bone density threshold and other predictors of vertebral fracture in patients receiving oral glucocorticoid therapy. Arthritis Rheum 2003; 48:3224–3229.
- Kanis JA, Johnell O, Oden A, et al. The risk and burden of vertebral fractures in Sweden. Osteoporos Int 2004; 15:20–26.
- Seguro LP, Rosario C, Shoenfeld Y. Long-term complications of past glucocorticoid use. Autoimmun Rev 2013; 12:629–632.
- Weinstein RS. Glucocorticoid-induced osteoporosis and osteonecrosis. Endocrinol Metab Clin North Am 2012; 41:595–611.
- Cooper C, Steinbuch M, Stevenson R, Miday R, Watts NB. The epidemiology of osteonecrosis: findings from the GPRD and THIN databases in the UK. Osteoporos Int 2010; 21:569–577.
- Mulfinger GL, Trueta J. The blood supply of the talus. J Bone Joint Surg Br 1970; 52:160–167.
- Pearce DH, Mongiardi CN, Fornasier VL, Daniels TR. Avascular necrosis of the talus: a pictoral essay. Radiographics 2005; 25:399–410.
- In brief: Taliglucerase (Elelyso) for Gaucher disease. Med Lett Drugs Ther 2012 Jul 9; 54(1394):56.
- Hollak CE. An evidence-based review of the potential benefits of taliglucerase alfa in the treatment of patients with Gaucher disease. Core Evid 2012; 7:15–20.
- Poole RM. Eliglustat: first global approval. Drugs 2014; 74:1829–1836.
- Bennett LL, Mohan D. Gaucher disease and its treatment options. Ann Pharmacother 2013; 47:1182–1193.
- Germain DP. Gaucher’s disease: a paradigm for interventional genetics. Clin Genet 2004; 65:77–86.
- Sims KB, Pastores GM, Weinreb NJ, et al. Improvement of bone disease by imiglucerase (Cerezyme) therapy in patients with skeletal manifestations of type 1 Gaucher disease: results of a 48-month longitudinal cohort study. Clin Genet 2008; 73:430–440.
- Rennie C, Britton J, Prouse P. Bilateral avascular necrosis of the lunate in a patient with severe Raynaud’s phenomenon and scleroderma. J Clin Rheumatol 1999; 5:165–168.
- Lee DM, Weinblatt ME. Rheumatoid arthritis. Lancet 2001; 358:903–911.
- Fleming A, Crown JM, Corbett M. Early rheumatoid disease. I. Onset. Ann Rheum Dis 1976; 35:357–360.
- Grabowski GA, Andria G, Baldellou A, et al. Pediatric non-neuronopathic Gaucher disease: presentation, diagnosis, and assessment. Consensus statements. Eur J Pediatr 2004; 163:58–66.
- Rodrigue SW, Rosenthal DI, Barton NW, Zurakowski D, Mankin HJ. Risk factors for osteonecrosis in patients with type 1 Gaucher’s disease. Clin Orthop Relat Res 1999; May (362):201–207.
- Lee RE. The pathology of Gaucher disease. Prog Clin Biol Res 1982; 95:177–217.
- Cox TM, Aerts JM, Belmatoug N, et al. Management of non-neuronopathic Gaucher disease with special reference to pregnancy, splenectomy, bisphosphonate therapy, use of biomarkers and bone disease monitoring. J Inherit Metab Dis 2008; 31:319–336.
- Khan A, Hangartner T, Weinreb NJ, Taylor JS, Mistry PK. Risk factors for fractures and avascular osteonecrosis in type 1 Gaucher disease: a study from the International Collaborative Gaucher Group (ICGG) Gaucher Registry. J Bone Miner Res 2012; 27:1839–1848.
- Deegan PB, Pavlova E, Tindall J, et al. Osseous manifestations of adult Gaucher disease in the era of enzyme replacement therapy. Medicine (Baltimore) 2011; 90:52–60.
- Weinstein RS. Glucocorticoid-induced osteonecrosis. Endocrine 2012; 41:183–190.
- Compston J. Management of glucocorticoid-induced osteoporosis. Nat Rev Rheumatol 2010; 6:82–88.
- Van Staa TP, Laan RF, Barton IP, Cohen S, Reid DM, Cooper C. Bone density threshold and other predictors of vertebral fracture in patients receiving oral glucocorticoid therapy. Arthritis Rheum 2003; 48:3224–3229.
- Kanis JA, Johnell O, Oden A, et al. The risk and burden of vertebral fractures in Sweden. Osteoporos Int 2004; 15:20–26.
- Seguro LP, Rosario C, Shoenfeld Y. Long-term complications of past glucocorticoid use. Autoimmun Rev 2013; 12:629–632.
- Weinstein RS. Glucocorticoid-induced osteoporosis and osteonecrosis. Endocrinol Metab Clin North Am 2012; 41:595–611.
- Cooper C, Steinbuch M, Stevenson R, Miday R, Watts NB. The epidemiology of osteonecrosis: findings from the GPRD and THIN databases in the UK. Osteoporos Int 2010; 21:569–577.
- Mulfinger GL, Trueta J. The blood supply of the talus. J Bone Joint Surg Br 1970; 52:160–167.
- Pearce DH, Mongiardi CN, Fornasier VL, Daniels TR. Avascular necrosis of the talus: a pictoral essay. Radiographics 2005; 25:399–410.
- In brief: Taliglucerase (Elelyso) for Gaucher disease. Med Lett Drugs Ther 2012 Jul 9; 54(1394):56.
- Hollak CE. An evidence-based review of the potential benefits of taliglucerase alfa in the treatment of patients with Gaucher disease. Core Evid 2012; 7:15–20.
- Poole RM. Eliglustat: first global approval. Drugs 2014; 74:1829–1836.
- Bennett LL, Mohan D. Gaucher disease and its treatment options. Ann Pharmacother 2013; 47:1182–1193.
- Germain DP. Gaucher’s disease: a paradigm for interventional genetics. Clin Genet 2004; 65:77–86.
- Sims KB, Pastores GM, Weinreb NJ, et al. Improvement of bone disease by imiglucerase (Cerezyme) therapy in patients with skeletal manifestations of type 1 Gaucher disease: results of a 48-month longitudinal cohort study. Clin Genet 2008; 73:430–440.
- Rennie C, Britton J, Prouse P. Bilateral avascular necrosis of the lunate in a patient with severe Raynaud’s phenomenon and scleroderma. J Clin Rheumatol 1999; 5:165–168.
2015 Update on Parkinson disease
This has been a boom year for Parkinson disease, with the US Food and Drug Administration (FDA) approving two new therapies, and with others in the pipeline.
This article details clinical signs of Parkinson disease, discusses functional imaging, provides an update on current thinking on disease pathogenesis, and gives an overview of managing parkinsonian symptoms and dyskinesias.
DIAGNOSIS REMAINS CLINICAL
Although a better understanding of Parkinson disease has been gained in recent years, with the recognition of several premotor features and potential biomarkers, its diagnosis is still primarily based on clinical motor findings. The four cardinal motor features have the mnemonic TRAP:
- Tremor at rest can be subtle, involving just the thumb, best observed when the patient is sitting with the hand resting on the lap; or it can be obvious, involving the entire hand, arm, feet, lips, and chin.
- Rigidity can be felt rather than seen, by slowly passively rotating the patient’s wrist or elbow and feeling resistance. The right and left sides often differ.
- Akinesia or bradykinesia (slowness or lack of movement) can be observed by having the patient walk down a hallway. One may observe reduced arm swing and hesitation in initiating movement.
- Postural instability usually develops later rather than sooner in the disease progression. The patient may need to hold onto someone to maintain balance when getting up or walking.
At least two features must be present to make the diagnosis of parkinsonism. One feature must be tremor or rigidity.
Although the criteria for parkinsonism appear simple, the diagnosis of Parkinson disease is not always clear-cut. For example, shaking can be secondary to a dopamine receptor-blocking medication, to anxiety, or to essential tremor; rigidity and slowness may be due to arthritis; and postural instability can result from a neuropathy. Moreover, other neurodegenerative parkinsonian disorders may respond to levodopa (at least initially) and may present with levodopa-induced dyskinesias. Robust response to levodopa and the occurrence of dyskinesias are two additional features that strongly suggest the diagnosis of Parkinson disease.
Supporting parkinsonian features include stooped posture, masked facies, micrographia (small handwriting), drooling, speech changes (eg, hypophonia or soft speech, stuttering, slurring, monotonic speech), and a shuffling, festinating gait (quick short steps as if falling forward).
PARKINSON MIMICS
Parkinsonism is a broader term than Parkinson disease or idiopathic Parkinson disease. It is characterized by akinetic rigidity and impaired motor activity that leads to reduced function and falls; behavioral changes also may occur.
In the United States, Parkinson disease is the most common cause of parkinsonism. Other nonneurodegenerative causes are drug-induced parkinsonism (due to dopamine receptor antagonists such as antipsychotic or antiemetic drugs), stroke (in the basal ganglia or frontal lobe), and normal-pressure hydrocephalus (causing lower-body parkinsonism). Mimics of parkinsonism include essential tremor and psychogenic parkinsonism.
Parkinsonism can also be caused by Parkinson-plus disorders, ie, neurodegenerative conditions characterized by parkinsonism along with additional signs and symptoms, as listed below. Parkinson-plus disorders include progressive supranuclear palsy, multiple system atrophy, corticobasal degeneration, and Lewy body disease.
Clinical features that suggest a diagnosis other than Parkinson disease include1:
- Poor response to adequate dosages of levodopa
- Early onset of postural instability and falls
- Axial rigidity (eg, stiff neck) more than appendicular rigidity
- Early dementia
- Supranuclear gaze palsy
- Unusual movements besides tremor, eg, limb dystonia, myoclonus, limb levitation or alien limb syndrome
- Profound autonomic dysfunction
- Psychotic symptoms before taking levodopa or dopaminergic medication.
The precise diagnosis of Parkinson-plus disorders is not critical, as the treatment is generally the same for all of them: ie, levodopa (if it shows some efficacy and is well tolerated), with additional symptomatic treatment for features such as depression, cognitive impairment, and autonomic dysfunction, and supportive therapy including physical, occupational, speech, and swallowing therapy.
IMAGING MAY ASSIST IN THE DIAGNOSIS
Dopamine transporter single-photon emission computed tomography (SPECT) is a functional imaging technique that supposedly reflects dopamine uptake by surviving presynaptic dopaminergic neurons in the striate bodies of the basal ganglia. Normal uptake shows distinct cashew-shaped enhancement bilaterally. In Parkinson disease, the enhanced areas are smaller and asymmetric, first with diminution of the tail (representing the putamen), then later involving the head (representing the caudate) along with the other striate bodies (Figure 1).
Dopamine transporter SPECT does not distinguish one neurodegenerative parkinsonian disorder from another. Therefore, it should not be used to distinguish Parkinson disease from other Parkinson-plus syndromes. But it does distinguish neurodegenerative parkinsonian disorders from nonneurodegenerative conditions and mimics, which have a normal result on dopamine transporter SPECT (Table 1).
SLOWING DISEASE PROGRESSION
Current treatments for Parkinson disease can significantly improve symptoms but, unfortunately, do not cure the disease or slow its progression. Testing whether agents modify the disease course is particularly difficult with Parkinson disease, because it affects individuals differently, has a wide spectrum of symptoms, has a long time course, and lacks definitive markers to monitor progression. Some agents have shown promise:
Caffeine. People who drink coffee are less likely to develop Parkinson disease, with the risk declining with the number of cups per day.2 For those who have the disease, drinking coffee is associated with reduced symptoms.
Exercise improves Parkinson disease and may prevent it, and some studies suggest that it can delay its progression.3 Exercise has been shown in an animal model to reduce the vulnerability of dopamine neurons to the toxic agent 6-hydroxydopamine.4 Functional magnetic resonance imaging studies have shown blood flow patterns before and after exercise that are similar to those seen in patients with and without Parkinson medication.3
Rasagiline, a monoamine oxidase B (MAO-B) inhibitor used for symptomatic treatment of Parkinson disease, had conflicting results in a neuroprotective clinical trial. Patients who received rasagiline 1 mg daily—but not those who received 2 mg daily—at the beginning of the trial had better Parkinson motor scores compared with patients who received rasagiline 9 months later.5
Inosine is a urate precursor that elevates urate levels in serum and the central nervous system. For unknown reasons, patients with Parkinson disease tend to have a low uric acid level, and higher levels are associated with milder disease. It is hoped that raising the uric acid level to a “pre-gout level” may slow the progression of Parkinson disease.
Isradipine, a calcium channel blocker, was found in an epidemiologic study of elderly patients to be associated with reduced likelihood of developing Parkinson disease.6 The drug is now undergoing clinical trials.
Smoking. Although cigarette smokers have long been recognized as having a very low risk of developing Parkinson disease, smoking is not recommended.
Agents found ineffective. Agents that have been tested and found ineffective in modifying the course of Parkinson disease include vitamin E, coenzyme Q10, riluzole, GPI-1485, pramipexole, cogane, CEP-1347, TCH-346, and creatine.
NOT JUST DOPAMINE—OR TREMORS
Dopamine deficiency is central to the current understanding of the pathogenesis of Parkinson disease and the focus of treatment efforts, but if dopamine deficiency were the only problem, replacing it should completely ameliorate all parkinsonian features. Other neurotransmitters also play roles: norepinephrine is implicated in orthostatic symptoms and apathy, acetylcholine in cognitive behaviors, glutamate in dyskinesias, and serotonin in depression, anxiety, and sleep abnormalities.
The most recognized area of involvement in the brain has traditionally been the substantia nigra in the midbrain. However, current thinking is that the disease starts lower in the caudal area of the brainstem (along with the olfactory tubercle), moves through the pons to the midbrain, then spreads across the cerebrum with extensive neocortical involvement.
Early premotor indicators are now recognized to occur 15 to 20 years before a tremor appears. The first signs are often hyposmia (diminished sense of smell, reflecting involvement of the olfactory tubercle) and constipation (reflecting involvement of the medulla and the vagus nucleus). With pons involvement, the patient can develop rapid eye movement sleep behavior disorder, depression, or anxiety. Only then does the disease spread to the midbrain and cause resting tremor, rigidity, and bradykinesia.7
Identifying the preclinical stages and starting disease-modifying treatments before the onset of motor symptoms may one day prove important, but at this point, the premotor symptoms (anosmia, constipation, depression) are too nonspecific to be useful, and such treatments have not yet been identified.
TREATMENT: LEVODOPA STILL PRIMARY
When to start drug treatment depends primarily on how much the symptoms bother the patient. Regardless of the clinician’s (or patient’s) belief in the benefits of delaying symptomatic treatment, it is universally considered necessary to start medication when gait problems develop because of the danger of a fall and resulting disability.
Carbidopa-levodopa combination therapy remains the most effective treatment; if it is not effective, another diagnosis may need to be considered. Carbidopa-levodopa improves tremor, rigidity, and bradykinesia, particularly in the early stages of Parkinson disease. It is well tolerated, has rapid onset, reduces the risk of death, and is the least expensive of the medications for Parkinson disease.
Immediate-release and continued-release formulations are available, as well as one that dissolves rapidly on the tongue and can be taken without water. An oral extended-release carbidopa-levodopa formulation (Rytary) was approved by the FDA in January 2015. Tablets are filled with drug-containing microbeads that dissolve at different rates to achieve therapeutic levodopa levels as quickly as the immediate-release formulation and maintain them for an extended time.8
The development of dyskinesias is the major psychological drawback of levodopa, occurring in 80% of patients after 5 to 10 years of treatment. Although many patients fear this side effect, most patients who develop it find it preferable to the rigidity and bradykinesia of Parkinson disease. In most cases, bothersome dyskinesias can be controlled by adjusting medications.9,10
Dopamine agonists include pramipexole, ropinirole, and rotigotine. They are available in generic form as three-times-daily dosing; once-daily dosing is also available, but not as a generic formulation. Dopamine agonists have the advantage of potentially improving depression and delaying the onset of dyskinesias.
However, dopamine agonists have a number of disadvantages compared with levodopa: they have a longer titration period, are less effective, and are less well tolerated, especially in the elderly. Side effects occur more frequently than with levodopa and include general and peripheral edema, hallucinations, nausea, lightheadedness, and sleepiness.11,12 These drugs are also associated with “sleep attacks” (sudden falling asleep while active, such as while driving or eating) and with compulsive and impulsive behaviors such as hypersexuality, buying, binge eating, and gambling. Although these behaviors occur in fewer than 10% of patients, they can be devastating, leading to marital, financial, and legal problems. A bothersome clinical state termed dopamine agonist withdrawal syndrome is characterized by anxiety, depression, jitteriness, and palpitations when dopamine agonists are tapered or discontinued because of a side effect.13
MAO-B inhibitors delay the breakdown of dopamine, allowing it to “stay” in the brain for a longer period of time. Rasagiline for early monotherapy has the advantages of once-daily dosing, no titration, and excellent tolerability, even in the elderly. Potential drug interactions should be considered when using this drug. Early warnings about interactions with tyramine-rich foods were lifted after trials showed that this was not a problem.14
Amantadine is an N-methyl-d-aspartate (NMDA) receptor antagonist often used in early Parkinson disease and for treatment of dyskinesias and fatigue. It is the only drug that is intrinsically antidyskinetic and also improves Parkinson symptoms.15 Side effects include leg swelling, livedo reticularis, and neuropsychiatric and anticholinergic effects.
Anticholinergic agents (eg, trihexyphenidyl) improve tremor but are not as useful for bradykinesia or rigidity, and often have anticholinergic effects such as mental dullness, dry mouth, dry eye, and urinary hesitancy, especially in the elderly, so they have a limited role in Parkinson treatment.
MOTOR COMPLICATIONS: FLUCTUATIONS AND DYSKINESIAS
Motor fluctuations are changes between the akinetic and mobile phases of Parkinson disease, or the off-periods and on-periods of drug treatment. A patient who is “off” is generally rigid and feels that the medication is not working. A patient who is “on” feels loose and mobile and that the medication is working. Variants of motor fluctuations include:
- End-of-dose deterioration
- Delayed onset of response (more than half an hour after taking medication)
- Drug-resistant offs—medication has become ineffective
- Random oscillation—on-off phenomenon
- Freezing—unpredictable inability to start or finish a movement.
Dyskinesias are abnormal involuntary movements such as writhing and twisting. They are associated with dopaminergic therapy at peak dose, when the drug starts to turn on or wear off (termed diphasic dyskinesias).16
The storage hypothesis provides a plausible explanation for the development of motor complications as the disease progresses. Although the half-life of levodopa is only 60 to 90 minutes, it is effective in early disease when given three times a day. It is believed that at this stage of the disease, enough dopaminergic neurons survive to “store” dopamine and release it as needed. As the disease progresses and dopaminergic neurons die, storage capacity diminishes, and the clinical effect slowly starts to approximate the pharmacokinetic profile of the drug. Upon taking the medication, the patient gets a surge of drug, causing dyskinesias, followed later by rigidity as the effect wears off since there are fewer surviving dopaminergic cells to store dopamine.
MANAGING DYSKINESIAS
Patients with dyskinesias should first be asked if they are bothered by them; not all patients are troubled by dyskinesias. If the movements only bother others (eg, family members), then education is often the only treatment needed. If the patient is uncomfortable, the following measures can be tried:
- Taking lower, more frequent doses of levodopa (however, risk of wearing off becomes a problem)
- Adding a dopamine agonist or MAO-B inhibitor while lowering the levodopa dose (however, MAO-B inhibitors pose a risk of side effects in elderly patients)
- Adding clozapine (periodic laboratory testing is required to monitor blood levels and liver and kidney function)
- Adding amantadine (however, this poses a risk of cognitive side effects).
Deep-brain-stimulation surgery is appropriate for select patients who are generally physically healthy, cognitively intact, and emotionally stable, with a strong family support system, but who are bothered by symptoms of parkinsonism (such as tremors), motor fluctuations, or dyskinesias.17
Infusion pump. In January 2015, the FDA approved a new system that continuously delivers levodopa-carbidopa in a 4:1 ratio in gel suspension for 16 hours directly into the small intestine, minimizing motor fluctuations. The patient changes the cartridge daily and turns it off at bedtime.
*Dr. Fernandez has received research support from AbbVie, Acadia, Auspex, Biotie Therapies, Civitas, Kyowa/ProStrakan, Michael J. Fox Foundation, Movement Disorders Society, NIH/NINDS, Parkinson Study Group, Rhythm, Synosia, and Teva. He also has received honoraria from Carling Communications, International Parkinson and Movement Disorders Society, The Ohio State University, and PRIME Education, Inc as a speaker in CME events. He has received honoraria from Biogen, GE Health Care, Lundbeck, Merz Pharmaceuticals, and Pfizer as a consultant. He has received royalty payments from Demos Publishing for serving as a book author/editor. Cleveland Clinic has contracts with AbbVie and Merz Pharmaceuticals for Dr. Fernandez’s role as a member of the Global Steering Committee for LCIG studies and as a consultant or speaker, and as Head Principal Investigator for the Xeomin Registry Study. Dr. Fernandez has received a stipend from International Parkinson and Movement Disorders Society for serving as medical editor of the Movement Disorders Society website.
- Wenning GK, Ben-Shlomo Y, Hughes A, Daniel SE, Lees A, Quinn NP. What clinical features are most useful to distinguish definite multiple system atrophy from Parkinson’s disease? J Neurol Neurosurg Psychiatry 2000; 68:434–440.
- Hernán MA, Takkouche B, Caamaño-Isoma F, et al. A meta-analysis of coffee drinking, cigarette smoking, and risk of Parkinson’s disease. Ann Neurol 2002; 52:276–84.
- Ridgel A, Thota A, Vitek JL, Alberts JL. Forced, not voluntary, exercise improves motor function in Parkinson’s disease patients. Neurorehabil Neural Repair 2009; 23:600–608.
- Smith AD, Zigmond MJ. Can the brain be protected through exercise? Lessons from an animal model of parkinsonism. Exp Neurol 2003; 184:31–39.
- Olanow CW, Rascol O, Hauser R, et al, for the ADAGIO Study Investigators. A double-blind, delayed-start trial of rasagiline in Parkinson’s disease. N Engl J Med 2009; 361:1268–1278.
- Pasternak B, Svanström H, Nielsen NM, Fugger L, Melbye M, Hviid A. Use of calcium channel blockers and Parkinson’s disease. Am J Epidemiol 2012; 175:627-635.
- Braak H, Del Tredici K, Rüb U, de Vos RAI, Jansen Steur ENH, Braak E. Staging of brain pathology related to sporadic Parkinson’s disease. Neurobiol Aging 2003; 24:197–211.
- Hauser RA, Ellenbogen AL, Metman LV, et al. Crossover comparison of IPX066 and a standard levodopa formulation in advanced Parkinson’s disease. Mov Disord 2011; 26:2246–2252.
- Hely MA, Morris JG, Reid WG, Trafficante R. Sydney Multicenter Study of Parkinson’s disease: non-L-dopa-responsive problems dominate at 15 years. Mov Disord 2005; 20:190–199.
- Hung SW, Adeli GM, Arenovich T, Fox SH, Lang AE. Patient perception of dyskinesia in Parkinson’s disease. J Neurol Neurosurg Psychiatry 2010; 81:1112–1115.
- Rascol O, Brooks DJ, Korczyn AD, De Deyn PP, Clarke CE, Lang AE. A five-year study of the incidence of dyskinesia in patients with early Parkinson’s disease who were treated with ropinirole or levodopa. N Engl J Med 2000; 342:1484–1491.
- Parkinson Study Group. Pramipexole vs levodopa as initial treatment for Parkinson disease: a randomized controlled trial. Parkinson Study Group. JAMA 2000; 284:1931–1938.
- Nirenberg MJ. Dopamine agonist withdrawal syndrome: implications for patient care. Drugs Aging 2013; 30:587–592.
- Teva Neuroscience, Inc. Azilect prescribing information. https://www.azilect.com/Content/pdf/azi-40850-azilect-electronic-pi.pdf. Accessed June 29, 2015.
- Snow BJ, Macdonald L, Mcauley D, Wallis W. The effect of amantadine on levodopa-induced dyskinesias in Parkinson’s disease: a double-blind, placebo-controlled study. Clin Neuropharmacol 2000; 23:82–85.
- Adler CH, Ahlskog JE, eds. Parkinson’s Disease and Movement Disorders: Diagnosis and Treatment Guidelines for the Practicing Physician. Totowa, NJ: Humana Press; 2000.
- Machado A, Fernandez HH, Deogaonkar M. Deep brain stimulation: what can patients expect from it? Cleve Clin J Med 2012; 79:113–120.
This has been a boom year for Parkinson disease, with the US Food and Drug Administration (FDA) approving two new therapies, and with others in the pipeline.
This article details clinical signs of Parkinson disease, discusses functional imaging, provides an update on current thinking on disease pathogenesis, and gives an overview of managing parkinsonian symptoms and dyskinesias.
DIAGNOSIS REMAINS CLINICAL
Although a better understanding of Parkinson disease has been gained in recent years, with the recognition of several premotor features and potential biomarkers, its diagnosis is still primarily based on clinical motor findings. The four cardinal motor features have the mnemonic TRAP:
- Tremor at rest can be subtle, involving just the thumb, best observed when the patient is sitting with the hand resting on the lap; or it can be obvious, involving the entire hand, arm, feet, lips, and chin.
- Rigidity can be felt rather than seen, by slowly passively rotating the patient’s wrist or elbow and feeling resistance. The right and left sides often differ.
- Akinesia or bradykinesia (slowness or lack of movement) can be observed by having the patient walk down a hallway. One may observe reduced arm swing and hesitation in initiating movement.
- Postural instability usually develops later rather than sooner in the disease progression. The patient may need to hold onto someone to maintain balance when getting up or walking.
At least two features must be present to make the diagnosis of parkinsonism. One feature must be tremor or rigidity.
Although the criteria for parkinsonism appear simple, the diagnosis of Parkinson disease is not always clear-cut. For example, shaking can be secondary to a dopamine receptor-blocking medication, to anxiety, or to essential tremor; rigidity and slowness may be due to arthritis; and postural instability can result from a neuropathy. Moreover, other neurodegenerative parkinsonian disorders may respond to levodopa (at least initially) and may present with levodopa-induced dyskinesias. Robust response to levodopa and the occurrence of dyskinesias are two additional features that strongly suggest the diagnosis of Parkinson disease.
Supporting parkinsonian features include stooped posture, masked facies, micrographia (small handwriting), drooling, speech changes (eg, hypophonia or soft speech, stuttering, slurring, monotonic speech), and a shuffling, festinating gait (quick short steps as if falling forward).
PARKINSON MIMICS
Parkinsonism is a broader term than Parkinson disease or idiopathic Parkinson disease. It is characterized by akinetic rigidity and impaired motor activity that leads to reduced function and falls; behavioral changes also may occur.
In the United States, Parkinson disease is the most common cause of parkinsonism. Other nonneurodegenerative causes are drug-induced parkinsonism (due to dopamine receptor antagonists such as antipsychotic or antiemetic drugs), stroke (in the basal ganglia or frontal lobe), and normal-pressure hydrocephalus (causing lower-body parkinsonism). Mimics of parkinsonism include essential tremor and psychogenic parkinsonism.
Parkinsonism can also be caused by Parkinson-plus disorders, ie, neurodegenerative conditions characterized by parkinsonism along with additional signs and symptoms, as listed below. Parkinson-plus disorders include progressive supranuclear palsy, multiple system atrophy, corticobasal degeneration, and Lewy body disease.
Clinical features that suggest a diagnosis other than Parkinson disease include1:
- Poor response to adequate dosages of levodopa
- Early onset of postural instability and falls
- Axial rigidity (eg, stiff neck) more than appendicular rigidity
- Early dementia
- Supranuclear gaze palsy
- Unusual movements besides tremor, eg, limb dystonia, myoclonus, limb levitation or alien limb syndrome
- Profound autonomic dysfunction
- Psychotic symptoms before taking levodopa or dopaminergic medication.
The precise diagnosis of Parkinson-plus disorders is not critical, as the treatment is generally the same for all of them: ie, levodopa (if it shows some efficacy and is well tolerated), with additional symptomatic treatment for features such as depression, cognitive impairment, and autonomic dysfunction, and supportive therapy including physical, occupational, speech, and swallowing therapy.
IMAGING MAY ASSIST IN THE DIAGNOSIS
Dopamine transporter single-photon emission computed tomography (SPECT) is a functional imaging technique that supposedly reflects dopamine uptake by surviving presynaptic dopaminergic neurons in the striate bodies of the basal ganglia. Normal uptake shows distinct cashew-shaped enhancement bilaterally. In Parkinson disease, the enhanced areas are smaller and asymmetric, first with diminution of the tail (representing the putamen), then later involving the head (representing the caudate) along with the other striate bodies (Figure 1).
Dopamine transporter SPECT does not distinguish one neurodegenerative parkinsonian disorder from another. Therefore, it should not be used to distinguish Parkinson disease from other Parkinson-plus syndromes. But it does distinguish neurodegenerative parkinsonian disorders from nonneurodegenerative conditions and mimics, which have a normal result on dopamine transporter SPECT (Table 1).
SLOWING DISEASE PROGRESSION
Current treatments for Parkinson disease can significantly improve symptoms but, unfortunately, do not cure the disease or slow its progression. Testing whether agents modify the disease course is particularly difficult with Parkinson disease, because it affects individuals differently, has a wide spectrum of symptoms, has a long time course, and lacks definitive markers to monitor progression. Some agents have shown promise:
Caffeine. People who drink coffee are less likely to develop Parkinson disease, with the risk declining with the number of cups per day.2 For those who have the disease, drinking coffee is associated with reduced symptoms.
Exercise improves Parkinson disease and may prevent it, and some studies suggest that it can delay its progression.3 Exercise has been shown in an animal model to reduce the vulnerability of dopamine neurons to the toxic agent 6-hydroxydopamine.4 Functional magnetic resonance imaging studies have shown blood flow patterns before and after exercise that are similar to those seen in patients with and without Parkinson medication.3
Rasagiline, a monoamine oxidase B (MAO-B) inhibitor used for symptomatic treatment of Parkinson disease, had conflicting results in a neuroprotective clinical trial. Patients who received rasagiline 1 mg daily—but not those who received 2 mg daily—at the beginning of the trial had better Parkinson motor scores compared with patients who received rasagiline 9 months later.5
Inosine is a urate precursor that elevates urate levels in serum and the central nervous system. For unknown reasons, patients with Parkinson disease tend to have a low uric acid level, and higher levels are associated with milder disease. It is hoped that raising the uric acid level to a “pre-gout level” may slow the progression of Parkinson disease.
Isradipine, a calcium channel blocker, was found in an epidemiologic study of elderly patients to be associated with reduced likelihood of developing Parkinson disease.6 The drug is now undergoing clinical trials.
Smoking. Although cigarette smokers have long been recognized as having a very low risk of developing Parkinson disease, smoking is not recommended.
Agents found ineffective. Agents that have been tested and found ineffective in modifying the course of Parkinson disease include vitamin E, coenzyme Q10, riluzole, GPI-1485, pramipexole, cogane, CEP-1347, TCH-346, and creatine.
NOT JUST DOPAMINE—OR TREMORS
Dopamine deficiency is central to the current understanding of the pathogenesis of Parkinson disease and the focus of treatment efforts, but if dopamine deficiency were the only problem, replacing it should completely ameliorate all parkinsonian features. Other neurotransmitters also play roles: norepinephrine is implicated in orthostatic symptoms and apathy, acetylcholine in cognitive behaviors, glutamate in dyskinesias, and serotonin in depression, anxiety, and sleep abnormalities.
The most recognized area of involvement in the brain has traditionally been the substantia nigra in the midbrain. However, current thinking is that the disease starts lower in the caudal area of the brainstem (along with the olfactory tubercle), moves through the pons to the midbrain, then spreads across the cerebrum with extensive neocortical involvement.
Early premotor indicators are now recognized to occur 15 to 20 years before a tremor appears. The first signs are often hyposmia (diminished sense of smell, reflecting involvement of the olfactory tubercle) and constipation (reflecting involvement of the medulla and the vagus nucleus). With pons involvement, the patient can develop rapid eye movement sleep behavior disorder, depression, or anxiety. Only then does the disease spread to the midbrain and cause resting tremor, rigidity, and bradykinesia.7
Identifying the preclinical stages and starting disease-modifying treatments before the onset of motor symptoms may one day prove important, but at this point, the premotor symptoms (anosmia, constipation, depression) are too nonspecific to be useful, and such treatments have not yet been identified.
TREATMENT: LEVODOPA STILL PRIMARY
When to start drug treatment depends primarily on how much the symptoms bother the patient. Regardless of the clinician’s (or patient’s) belief in the benefits of delaying symptomatic treatment, it is universally considered necessary to start medication when gait problems develop because of the danger of a fall and resulting disability.
Carbidopa-levodopa combination therapy remains the most effective treatment; if it is not effective, another diagnosis may need to be considered. Carbidopa-levodopa improves tremor, rigidity, and bradykinesia, particularly in the early stages of Parkinson disease. It is well tolerated, has rapid onset, reduces the risk of death, and is the least expensive of the medications for Parkinson disease.
Immediate-release and continued-release formulations are available, as well as one that dissolves rapidly on the tongue and can be taken without water. An oral extended-release carbidopa-levodopa formulation (Rytary) was approved by the FDA in January 2015. Tablets are filled with drug-containing microbeads that dissolve at different rates to achieve therapeutic levodopa levels as quickly as the immediate-release formulation and maintain them for an extended time.8
The development of dyskinesias is the major psychological drawback of levodopa, occurring in 80% of patients after 5 to 10 years of treatment. Although many patients fear this side effect, most patients who develop it find it preferable to the rigidity and bradykinesia of Parkinson disease. In most cases, bothersome dyskinesias can be controlled by adjusting medications.9,10
Dopamine agonists include pramipexole, ropinirole, and rotigotine. They are available in generic form as three-times-daily dosing; once-daily dosing is also available, but not as a generic formulation. Dopamine agonists have the advantage of potentially improving depression and delaying the onset of dyskinesias.
However, dopamine agonists have a number of disadvantages compared with levodopa: they have a longer titration period, are less effective, and are less well tolerated, especially in the elderly. Side effects occur more frequently than with levodopa and include general and peripheral edema, hallucinations, nausea, lightheadedness, and sleepiness.11,12 These drugs are also associated with “sleep attacks” (sudden falling asleep while active, such as while driving or eating) and with compulsive and impulsive behaviors such as hypersexuality, buying, binge eating, and gambling. Although these behaviors occur in fewer than 10% of patients, they can be devastating, leading to marital, financial, and legal problems. A bothersome clinical state termed dopamine agonist withdrawal syndrome is characterized by anxiety, depression, jitteriness, and palpitations when dopamine agonists are tapered or discontinued because of a side effect.13
MAO-B inhibitors delay the breakdown of dopamine, allowing it to “stay” in the brain for a longer period of time. Rasagiline for early monotherapy has the advantages of once-daily dosing, no titration, and excellent tolerability, even in the elderly. Potential drug interactions should be considered when using this drug. Early warnings about interactions with tyramine-rich foods were lifted after trials showed that this was not a problem.14
Amantadine is an N-methyl-d-aspartate (NMDA) receptor antagonist often used in early Parkinson disease and for treatment of dyskinesias and fatigue. It is the only drug that is intrinsically antidyskinetic and also improves Parkinson symptoms.15 Side effects include leg swelling, livedo reticularis, and neuropsychiatric and anticholinergic effects.
Anticholinergic agents (eg, trihexyphenidyl) improve tremor but are not as useful for bradykinesia or rigidity, and often have anticholinergic effects such as mental dullness, dry mouth, dry eye, and urinary hesitancy, especially in the elderly, so they have a limited role in Parkinson treatment.
MOTOR COMPLICATIONS: FLUCTUATIONS AND DYSKINESIAS
Motor fluctuations are changes between the akinetic and mobile phases of Parkinson disease, or the off-periods and on-periods of drug treatment. A patient who is “off” is generally rigid and feels that the medication is not working. A patient who is “on” feels loose and mobile and that the medication is working. Variants of motor fluctuations include:
- End-of-dose deterioration
- Delayed onset of response (more than half an hour after taking medication)
- Drug-resistant offs—medication has become ineffective
- Random oscillation—on-off phenomenon
- Freezing—unpredictable inability to start or finish a movement.
Dyskinesias are abnormal involuntary movements such as writhing and twisting. They are associated with dopaminergic therapy at peak dose, when the drug starts to turn on or wear off (termed diphasic dyskinesias).16
The storage hypothesis provides a plausible explanation for the development of motor complications as the disease progresses. Although the half-life of levodopa is only 60 to 90 minutes, it is effective in early disease when given three times a day. It is believed that at this stage of the disease, enough dopaminergic neurons survive to “store” dopamine and release it as needed. As the disease progresses and dopaminergic neurons die, storage capacity diminishes, and the clinical effect slowly starts to approximate the pharmacokinetic profile of the drug. Upon taking the medication, the patient gets a surge of drug, causing dyskinesias, followed later by rigidity as the effect wears off since there are fewer surviving dopaminergic cells to store dopamine.
MANAGING DYSKINESIAS
Patients with dyskinesias should first be asked if they are bothered by them; not all patients are troubled by dyskinesias. If the movements only bother others (eg, family members), then education is often the only treatment needed. If the patient is uncomfortable, the following measures can be tried:
- Taking lower, more frequent doses of levodopa (however, risk of wearing off becomes a problem)
- Adding a dopamine agonist or MAO-B inhibitor while lowering the levodopa dose (however, MAO-B inhibitors pose a risk of side effects in elderly patients)
- Adding clozapine (periodic laboratory testing is required to monitor blood levels and liver and kidney function)
- Adding amantadine (however, this poses a risk of cognitive side effects).
Deep-brain-stimulation surgery is appropriate for select patients who are generally physically healthy, cognitively intact, and emotionally stable, with a strong family support system, but who are bothered by symptoms of parkinsonism (such as tremors), motor fluctuations, or dyskinesias.17
Infusion pump. In January 2015, the FDA approved a new system that continuously delivers levodopa-carbidopa in a 4:1 ratio in gel suspension for 16 hours directly into the small intestine, minimizing motor fluctuations. The patient changes the cartridge daily and turns it off at bedtime.
*Dr. Fernandez has received research support from AbbVie, Acadia, Auspex, Biotie Therapies, Civitas, Kyowa/ProStrakan, Michael J. Fox Foundation, Movement Disorders Society, NIH/NINDS, Parkinson Study Group, Rhythm, Synosia, and Teva. He also has received honoraria from Carling Communications, International Parkinson and Movement Disorders Society, The Ohio State University, and PRIME Education, Inc as a speaker in CME events. He has received honoraria from Biogen, GE Health Care, Lundbeck, Merz Pharmaceuticals, and Pfizer as a consultant. He has received royalty payments from Demos Publishing for serving as a book author/editor. Cleveland Clinic has contracts with AbbVie and Merz Pharmaceuticals for Dr. Fernandez’s role as a member of the Global Steering Committee for LCIG studies and as a consultant or speaker, and as Head Principal Investigator for the Xeomin Registry Study. Dr. Fernandez has received a stipend from International Parkinson and Movement Disorders Society for serving as medical editor of the Movement Disorders Society website.
This has been a boom year for Parkinson disease, with the US Food and Drug Administration (FDA) approving two new therapies, and with others in the pipeline.
This article details clinical signs of Parkinson disease, discusses functional imaging, provides an update on current thinking on disease pathogenesis, and gives an overview of managing parkinsonian symptoms and dyskinesias.
DIAGNOSIS REMAINS CLINICAL
Although a better understanding of Parkinson disease has been gained in recent years, with the recognition of several premotor features and potential biomarkers, its diagnosis is still primarily based on clinical motor findings. The four cardinal motor features have the mnemonic TRAP:
- Tremor at rest can be subtle, involving just the thumb, best observed when the patient is sitting with the hand resting on the lap; or it can be obvious, involving the entire hand, arm, feet, lips, and chin.
- Rigidity can be felt rather than seen, by slowly passively rotating the patient’s wrist or elbow and feeling resistance. The right and left sides often differ.
- Akinesia or bradykinesia (slowness or lack of movement) can be observed by having the patient walk down a hallway. One may observe reduced arm swing and hesitation in initiating movement.
- Postural instability usually develops later rather than sooner in the disease progression. The patient may need to hold onto someone to maintain balance when getting up or walking.
At least two features must be present to make the diagnosis of parkinsonism. One feature must be tremor or rigidity.
Although the criteria for parkinsonism appear simple, the diagnosis of Parkinson disease is not always clear-cut. For example, shaking can be secondary to a dopamine receptor-blocking medication, to anxiety, or to essential tremor; rigidity and slowness may be due to arthritis; and postural instability can result from a neuropathy. Moreover, other neurodegenerative parkinsonian disorders may respond to levodopa (at least initially) and may present with levodopa-induced dyskinesias. Robust response to levodopa and the occurrence of dyskinesias are two additional features that strongly suggest the diagnosis of Parkinson disease.
Supporting parkinsonian features include stooped posture, masked facies, micrographia (small handwriting), drooling, speech changes (eg, hypophonia or soft speech, stuttering, slurring, monotonic speech), and a shuffling, festinating gait (quick short steps as if falling forward).
PARKINSON MIMICS
Parkinsonism is a broader term than Parkinson disease or idiopathic Parkinson disease. It is characterized by akinetic rigidity and impaired motor activity that leads to reduced function and falls; behavioral changes also may occur.
In the United States, Parkinson disease is the most common cause of parkinsonism. Other nonneurodegenerative causes are drug-induced parkinsonism (due to dopamine receptor antagonists such as antipsychotic or antiemetic drugs), stroke (in the basal ganglia or frontal lobe), and normal-pressure hydrocephalus (causing lower-body parkinsonism). Mimics of parkinsonism include essential tremor and psychogenic parkinsonism.
Parkinsonism can also be caused by Parkinson-plus disorders, ie, neurodegenerative conditions characterized by parkinsonism along with additional signs and symptoms, as listed below. Parkinson-plus disorders include progressive supranuclear palsy, multiple system atrophy, corticobasal degeneration, and Lewy body disease.
Clinical features that suggest a diagnosis other than Parkinson disease include1:
- Poor response to adequate dosages of levodopa
- Early onset of postural instability and falls
- Axial rigidity (eg, stiff neck) more than appendicular rigidity
- Early dementia
- Supranuclear gaze palsy
- Unusual movements besides tremor, eg, limb dystonia, myoclonus, limb levitation or alien limb syndrome
- Profound autonomic dysfunction
- Psychotic symptoms before taking levodopa or dopaminergic medication.
The precise diagnosis of Parkinson-plus disorders is not critical, as the treatment is generally the same for all of them: ie, levodopa (if it shows some efficacy and is well tolerated), with additional symptomatic treatment for features such as depression, cognitive impairment, and autonomic dysfunction, and supportive therapy including physical, occupational, speech, and swallowing therapy.
IMAGING MAY ASSIST IN THE DIAGNOSIS
Dopamine transporter single-photon emission computed tomography (SPECT) is a functional imaging technique that supposedly reflects dopamine uptake by surviving presynaptic dopaminergic neurons in the striate bodies of the basal ganglia. Normal uptake shows distinct cashew-shaped enhancement bilaterally. In Parkinson disease, the enhanced areas are smaller and asymmetric, first with diminution of the tail (representing the putamen), then later involving the head (representing the caudate) along with the other striate bodies (Figure 1).
Dopamine transporter SPECT does not distinguish one neurodegenerative parkinsonian disorder from another. Therefore, it should not be used to distinguish Parkinson disease from other Parkinson-plus syndromes. But it does distinguish neurodegenerative parkinsonian disorders from nonneurodegenerative conditions and mimics, which have a normal result on dopamine transporter SPECT (Table 1).
SLOWING DISEASE PROGRESSION
Current treatments for Parkinson disease can significantly improve symptoms but, unfortunately, do not cure the disease or slow its progression. Testing whether agents modify the disease course is particularly difficult with Parkinson disease, because it affects individuals differently, has a wide spectrum of symptoms, has a long time course, and lacks definitive markers to monitor progression. Some agents have shown promise:
Caffeine. People who drink coffee are less likely to develop Parkinson disease, with the risk declining with the number of cups per day.2 For those who have the disease, drinking coffee is associated with reduced symptoms.
Exercise improves Parkinson disease and may prevent it, and some studies suggest that it can delay its progression.3 Exercise has been shown in an animal model to reduce the vulnerability of dopamine neurons to the toxic agent 6-hydroxydopamine.4 Functional magnetic resonance imaging studies have shown blood flow patterns before and after exercise that are similar to those seen in patients with and without Parkinson medication.3
Rasagiline, a monoamine oxidase B (MAO-B) inhibitor used for symptomatic treatment of Parkinson disease, had conflicting results in a neuroprotective clinical trial. Patients who received rasagiline 1 mg daily—but not those who received 2 mg daily—at the beginning of the trial had better Parkinson motor scores compared with patients who received rasagiline 9 months later.5
Inosine is a urate precursor that elevates urate levels in serum and the central nervous system. For unknown reasons, patients with Parkinson disease tend to have a low uric acid level, and higher levels are associated with milder disease. It is hoped that raising the uric acid level to a “pre-gout level” may slow the progression of Parkinson disease.
Isradipine, a calcium channel blocker, was found in an epidemiologic study of elderly patients to be associated with reduced likelihood of developing Parkinson disease.6 The drug is now undergoing clinical trials.
Smoking. Although cigarette smokers have long been recognized as having a very low risk of developing Parkinson disease, smoking is not recommended.
Agents found ineffective. Agents that have been tested and found ineffective in modifying the course of Parkinson disease include vitamin E, coenzyme Q10, riluzole, GPI-1485, pramipexole, cogane, CEP-1347, TCH-346, and creatine.
NOT JUST DOPAMINE—OR TREMORS
Dopamine deficiency is central to the current understanding of the pathogenesis of Parkinson disease and the focus of treatment efforts, but if dopamine deficiency were the only problem, replacing it should completely ameliorate all parkinsonian features. Other neurotransmitters also play roles: norepinephrine is implicated in orthostatic symptoms and apathy, acetylcholine in cognitive behaviors, glutamate in dyskinesias, and serotonin in depression, anxiety, and sleep abnormalities.
The most recognized area of involvement in the brain has traditionally been the substantia nigra in the midbrain. However, current thinking is that the disease starts lower in the caudal area of the brainstem (along with the olfactory tubercle), moves through the pons to the midbrain, then spreads across the cerebrum with extensive neocortical involvement.
Early premotor indicators are now recognized to occur 15 to 20 years before a tremor appears. The first signs are often hyposmia (diminished sense of smell, reflecting involvement of the olfactory tubercle) and constipation (reflecting involvement of the medulla and the vagus nucleus). With pons involvement, the patient can develop rapid eye movement sleep behavior disorder, depression, or anxiety. Only then does the disease spread to the midbrain and cause resting tremor, rigidity, and bradykinesia.7
Identifying the preclinical stages and starting disease-modifying treatments before the onset of motor symptoms may one day prove important, but at this point, the premotor symptoms (anosmia, constipation, depression) are too nonspecific to be useful, and such treatments have not yet been identified.
TREATMENT: LEVODOPA STILL PRIMARY
When to start drug treatment depends primarily on how much the symptoms bother the patient. Regardless of the clinician’s (or patient’s) belief in the benefits of delaying symptomatic treatment, it is universally considered necessary to start medication when gait problems develop because of the danger of a fall and resulting disability.
Carbidopa-levodopa combination therapy remains the most effective treatment; if it is not effective, another diagnosis may need to be considered. Carbidopa-levodopa improves tremor, rigidity, and bradykinesia, particularly in the early stages of Parkinson disease. It is well tolerated, has rapid onset, reduces the risk of death, and is the least expensive of the medications for Parkinson disease.
Immediate-release and continued-release formulations are available, as well as one that dissolves rapidly on the tongue and can be taken without water. An oral extended-release carbidopa-levodopa formulation (Rytary) was approved by the FDA in January 2015. Tablets are filled with drug-containing microbeads that dissolve at different rates to achieve therapeutic levodopa levels as quickly as the immediate-release formulation and maintain them for an extended time.8
The development of dyskinesias is the major psychological drawback of levodopa, occurring in 80% of patients after 5 to 10 years of treatment. Although many patients fear this side effect, most patients who develop it find it preferable to the rigidity and bradykinesia of Parkinson disease. In most cases, bothersome dyskinesias can be controlled by adjusting medications.9,10
Dopamine agonists include pramipexole, ropinirole, and rotigotine. They are available in generic form as three-times-daily dosing; once-daily dosing is also available, but not as a generic formulation. Dopamine agonists have the advantage of potentially improving depression and delaying the onset of dyskinesias.
However, dopamine agonists have a number of disadvantages compared with levodopa: they have a longer titration period, are less effective, and are less well tolerated, especially in the elderly. Side effects occur more frequently than with levodopa and include general and peripheral edema, hallucinations, nausea, lightheadedness, and sleepiness.11,12 These drugs are also associated with “sleep attacks” (sudden falling asleep while active, such as while driving or eating) and with compulsive and impulsive behaviors such as hypersexuality, buying, binge eating, and gambling. Although these behaviors occur in fewer than 10% of patients, they can be devastating, leading to marital, financial, and legal problems. A bothersome clinical state termed dopamine agonist withdrawal syndrome is characterized by anxiety, depression, jitteriness, and palpitations when dopamine agonists are tapered or discontinued because of a side effect.13
MAO-B inhibitors delay the breakdown of dopamine, allowing it to “stay” in the brain for a longer period of time. Rasagiline for early monotherapy has the advantages of once-daily dosing, no titration, and excellent tolerability, even in the elderly. Potential drug interactions should be considered when using this drug. Early warnings about interactions with tyramine-rich foods were lifted after trials showed that this was not a problem.14
Amantadine is an N-methyl-d-aspartate (NMDA) receptor antagonist often used in early Parkinson disease and for treatment of dyskinesias and fatigue. It is the only drug that is intrinsically antidyskinetic and also improves Parkinson symptoms.15 Side effects include leg swelling, livedo reticularis, and neuropsychiatric and anticholinergic effects.
Anticholinergic agents (eg, trihexyphenidyl) improve tremor but are not as useful for bradykinesia or rigidity, and often have anticholinergic effects such as mental dullness, dry mouth, dry eye, and urinary hesitancy, especially in the elderly, so they have a limited role in Parkinson treatment.
MOTOR COMPLICATIONS: FLUCTUATIONS AND DYSKINESIAS
Motor fluctuations are changes between the akinetic and mobile phases of Parkinson disease, or the off-periods and on-periods of drug treatment. A patient who is “off” is generally rigid and feels that the medication is not working. A patient who is “on” feels loose and mobile and that the medication is working. Variants of motor fluctuations include:
- End-of-dose deterioration
- Delayed onset of response (more than half an hour after taking medication)
- Drug-resistant offs—medication has become ineffective
- Random oscillation—on-off phenomenon
- Freezing—unpredictable inability to start or finish a movement.
Dyskinesias are abnormal involuntary movements such as writhing and twisting. They are associated with dopaminergic therapy at peak dose, when the drug starts to turn on or wear off (termed diphasic dyskinesias).16
The storage hypothesis provides a plausible explanation for the development of motor complications as the disease progresses. Although the half-life of levodopa is only 60 to 90 minutes, it is effective in early disease when given three times a day. It is believed that at this stage of the disease, enough dopaminergic neurons survive to “store” dopamine and release it as needed. As the disease progresses and dopaminergic neurons die, storage capacity diminishes, and the clinical effect slowly starts to approximate the pharmacokinetic profile of the drug. Upon taking the medication, the patient gets a surge of drug, causing dyskinesias, followed later by rigidity as the effect wears off since there are fewer surviving dopaminergic cells to store dopamine.
MANAGING DYSKINESIAS
Patients with dyskinesias should first be asked if they are bothered by them; not all patients are troubled by dyskinesias. If the movements only bother others (eg, family members), then education is often the only treatment needed. If the patient is uncomfortable, the following measures can be tried:
- Taking lower, more frequent doses of levodopa (however, risk of wearing off becomes a problem)
- Adding a dopamine agonist or MAO-B inhibitor while lowering the levodopa dose (however, MAO-B inhibitors pose a risk of side effects in elderly patients)
- Adding clozapine (periodic laboratory testing is required to monitor blood levels and liver and kidney function)
- Adding amantadine (however, this poses a risk of cognitive side effects).
Deep-brain-stimulation surgery is appropriate for select patients who are generally physically healthy, cognitively intact, and emotionally stable, with a strong family support system, but who are bothered by symptoms of parkinsonism (such as tremors), motor fluctuations, or dyskinesias.17
Infusion pump. In January 2015, the FDA approved a new system that continuously delivers levodopa-carbidopa in a 4:1 ratio in gel suspension for 16 hours directly into the small intestine, minimizing motor fluctuations. The patient changes the cartridge daily and turns it off at bedtime.
*Dr. Fernandez has received research support from AbbVie, Acadia, Auspex, Biotie Therapies, Civitas, Kyowa/ProStrakan, Michael J. Fox Foundation, Movement Disorders Society, NIH/NINDS, Parkinson Study Group, Rhythm, Synosia, and Teva. He also has received honoraria from Carling Communications, International Parkinson and Movement Disorders Society, The Ohio State University, and PRIME Education, Inc as a speaker in CME events. He has received honoraria from Biogen, GE Health Care, Lundbeck, Merz Pharmaceuticals, and Pfizer as a consultant. He has received royalty payments from Demos Publishing for serving as a book author/editor. Cleveland Clinic has contracts with AbbVie and Merz Pharmaceuticals for Dr. Fernandez’s role as a member of the Global Steering Committee for LCIG studies and as a consultant or speaker, and as Head Principal Investigator for the Xeomin Registry Study. Dr. Fernandez has received a stipend from International Parkinson and Movement Disorders Society for serving as medical editor of the Movement Disorders Society website.
- Wenning GK, Ben-Shlomo Y, Hughes A, Daniel SE, Lees A, Quinn NP. What clinical features are most useful to distinguish definite multiple system atrophy from Parkinson’s disease? J Neurol Neurosurg Psychiatry 2000; 68:434–440.
- Hernán MA, Takkouche B, Caamaño-Isoma F, et al. A meta-analysis of coffee drinking, cigarette smoking, and risk of Parkinson’s disease. Ann Neurol 2002; 52:276–84.
- Ridgel A, Thota A, Vitek JL, Alberts JL. Forced, not voluntary, exercise improves motor function in Parkinson’s disease patients. Neurorehabil Neural Repair 2009; 23:600–608.
- Smith AD, Zigmond MJ. Can the brain be protected through exercise? Lessons from an animal model of parkinsonism. Exp Neurol 2003; 184:31–39.
- Olanow CW, Rascol O, Hauser R, et al, for the ADAGIO Study Investigators. A double-blind, delayed-start trial of rasagiline in Parkinson’s disease. N Engl J Med 2009; 361:1268–1278.
- Pasternak B, Svanström H, Nielsen NM, Fugger L, Melbye M, Hviid A. Use of calcium channel blockers and Parkinson’s disease. Am J Epidemiol 2012; 175:627-635.
- Braak H, Del Tredici K, Rüb U, de Vos RAI, Jansen Steur ENH, Braak E. Staging of brain pathology related to sporadic Parkinson’s disease. Neurobiol Aging 2003; 24:197–211.
- Hauser RA, Ellenbogen AL, Metman LV, et al. Crossover comparison of IPX066 and a standard levodopa formulation in advanced Parkinson’s disease. Mov Disord 2011; 26:2246–2252.
- Hely MA, Morris JG, Reid WG, Trafficante R. Sydney Multicenter Study of Parkinson’s disease: non-L-dopa-responsive problems dominate at 15 years. Mov Disord 2005; 20:190–199.
- Hung SW, Adeli GM, Arenovich T, Fox SH, Lang AE. Patient perception of dyskinesia in Parkinson’s disease. J Neurol Neurosurg Psychiatry 2010; 81:1112–1115.
- Rascol O, Brooks DJ, Korczyn AD, De Deyn PP, Clarke CE, Lang AE. A five-year study of the incidence of dyskinesia in patients with early Parkinson’s disease who were treated with ropinirole or levodopa. N Engl J Med 2000; 342:1484–1491.
- Parkinson Study Group. Pramipexole vs levodopa as initial treatment for Parkinson disease: a randomized controlled trial. Parkinson Study Group. JAMA 2000; 284:1931–1938.
- Nirenberg MJ. Dopamine agonist withdrawal syndrome: implications for patient care. Drugs Aging 2013; 30:587–592.
- Teva Neuroscience, Inc. Azilect prescribing information. https://www.azilect.com/Content/pdf/azi-40850-azilect-electronic-pi.pdf. Accessed June 29, 2015.
- Snow BJ, Macdonald L, Mcauley D, Wallis W. The effect of amantadine on levodopa-induced dyskinesias in Parkinson’s disease: a double-blind, placebo-controlled study. Clin Neuropharmacol 2000; 23:82–85.
- Adler CH, Ahlskog JE, eds. Parkinson’s Disease and Movement Disorders: Diagnosis and Treatment Guidelines for the Practicing Physician. Totowa, NJ: Humana Press; 2000.
- Machado A, Fernandez HH, Deogaonkar M. Deep brain stimulation: what can patients expect from it? Cleve Clin J Med 2012; 79:113–120.
- Wenning GK, Ben-Shlomo Y, Hughes A, Daniel SE, Lees A, Quinn NP. What clinical features are most useful to distinguish definite multiple system atrophy from Parkinson’s disease? J Neurol Neurosurg Psychiatry 2000; 68:434–440.
- Hernán MA, Takkouche B, Caamaño-Isoma F, et al. A meta-analysis of coffee drinking, cigarette smoking, and risk of Parkinson’s disease. Ann Neurol 2002; 52:276–84.
- Ridgel A, Thota A, Vitek JL, Alberts JL. Forced, not voluntary, exercise improves motor function in Parkinson’s disease patients. Neurorehabil Neural Repair 2009; 23:600–608.
- Smith AD, Zigmond MJ. Can the brain be protected through exercise? Lessons from an animal model of parkinsonism. Exp Neurol 2003; 184:31–39.
- Olanow CW, Rascol O, Hauser R, et al, for the ADAGIO Study Investigators. A double-blind, delayed-start trial of rasagiline in Parkinson’s disease. N Engl J Med 2009; 361:1268–1278.
- Pasternak B, Svanström H, Nielsen NM, Fugger L, Melbye M, Hviid A. Use of calcium channel blockers and Parkinson’s disease. Am J Epidemiol 2012; 175:627-635.
- Braak H, Del Tredici K, Rüb U, de Vos RAI, Jansen Steur ENH, Braak E. Staging of brain pathology related to sporadic Parkinson’s disease. Neurobiol Aging 2003; 24:197–211.
- Hauser RA, Ellenbogen AL, Metman LV, et al. Crossover comparison of IPX066 and a standard levodopa formulation in advanced Parkinson’s disease. Mov Disord 2011; 26:2246–2252.
- Hely MA, Morris JG, Reid WG, Trafficante R. Sydney Multicenter Study of Parkinson’s disease: non-L-dopa-responsive problems dominate at 15 years. Mov Disord 2005; 20:190–199.
- Hung SW, Adeli GM, Arenovich T, Fox SH, Lang AE. Patient perception of dyskinesia in Parkinson’s disease. J Neurol Neurosurg Psychiatry 2010; 81:1112–1115.
- Rascol O, Brooks DJ, Korczyn AD, De Deyn PP, Clarke CE, Lang AE. A five-year study of the incidence of dyskinesia in patients with early Parkinson’s disease who were treated with ropinirole or levodopa. N Engl J Med 2000; 342:1484–1491.
- Parkinson Study Group. Pramipexole vs levodopa as initial treatment for Parkinson disease: a randomized controlled trial. Parkinson Study Group. JAMA 2000; 284:1931–1938.
- Nirenberg MJ. Dopamine agonist withdrawal syndrome: implications for patient care. Drugs Aging 2013; 30:587–592.
- Teva Neuroscience, Inc. Azilect prescribing information. https://www.azilect.com/Content/pdf/azi-40850-azilect-electronic-pi.pdf. Accessed June 29, 2015.
- Snow BJ, Macdonald L, Mcauley D, Wallis W. The effect of amantadine on levodopa-induced dyskinesias in Parkinson’s disease: a double-blind, placebo-controlled study. Clin Neuropharmacol 2000; 23:82–85.
- Adler CH, Ahlskog JE, eds. Parkinson’s Disease and Movement Disorders: Diagnosis and Treatment Guidelines for the Practicing Physician. Totowa, NJ: Humana Press; 2000.
- Machado A, Fernandez HH, Deogaonkar M. Deep brain stimulation: what can patients expect from it? Cleve Clin J Med 2012; 79:113–120.
KEY POINTS
- Parkinson disease is diagnosed by clinical signs with the mnemonic TRAP: Tremor at rest, Rigidity, Akinesia or bradykinesia, and Postural/gait instability.
- A dopamine transporter functional scan can distinguish neurodegenerative parkinsonian disorders from nonneurodegenerative etiologies such as drug-induced parkinsonism and vascular parkinsonism, and from mimics such as psychogenic parkinsonism and essential tremor.
- Coffee consumption and exercise may benefit patients with Parkinson disease.
- Carbidopa-levodopa combination therapy is still the most effective treatment, but most patients develop dyskinesia after 5 to 10 years of treatment.
- Dyskinesias can be managed by adjusting or changing medications, switching to the new levodopa infusion pump system, or with deep-brain-stimulation surgery.
Radiographically Silent Loosening of the Acetabular Component in Hip Arthroplasty
Total hip arthroplasty (THA) is an excellent option for the treatment of osteoarthritis of the hip. In numerous studies, modern implants have shown survivorship of more than 90% at 10 years.1,2 Polyethylene wear and subsequent osteolysis are major obstacles to the long-term success of THA.3-5 Polyethylene wear particles are phagocytized by macrophages, inducing an inflammatory response that can ultimately lead to osteolysis of the bony architecture surrounding the bone–implant interface.6,7 As modern implants often rely on direct implant-to-bone ingrowth to maintain fixation, wear at this junction can lead to aseptic component loosening and ultimately require revision surgery.8-10 Osteolysis can be diagnosed with plain radiography or computed tomography (CT).11 CT is more sensitive than plain radiography for the diagnosis of osteolysis and is better able to determine the size and location of osteolytic lesions.12,13
Although diagnosis of osteolysis is well defined in the literature, what is more challenging is radiographic diagnosis of a loose acetabular component.11 The most commonly described criteria for loosening are presence of a complete radiolucent line of more than 2 mm in width at the bone–implant interface and any progressive tilting or migration of the component.14,15 CT-based criteria for component loosening remain largely undefined, though Egawa and colleagues16 showed that acetabular osteolysis involving less than 40% of the total cup surface is not associated with component loosening. Although a patient may show signs of osteolysis on postoperative imaging, this finding does not necessitate immediate revision surgery.17 Osteolysis may be monitored clinically and followed radiographically to determine when intervention is necessary.13
The goals of revision surgery are to eliminate the wear generator and bone-graft lytic lesions where needed to help maintain the bone–implant interface.17 The timing of such surgery is important, as the surgeon must balance the risk for gross component migration against the morbidity and mortality associated with acetabular component revision.18 This is in contrast to the settings of infection, periprosthetic fracture, recurrent instability, and component fracture/loosening, in which revision is urgently indicated and the case cannot be managed conservatively.
We conducted a study to determine the incidence of loose acetabular components without radiographic or clinical findings that would necessitate urgent revision THA. Radiographically silent loosening (RSL) was defined as an acetabular component that was loose at time of revision surgery but that did not show frank signs of loosening on either plain radiography or CT. Although these patients make up a small minority of the revision population, knowing the incidence of RSL can help raise surgeon awareness of this potentially dangerous situation. We further sought to determine whether patients with RSL present with different demographic characteristics or clinical symptoms than patients with stable acetabular components.
Materials and Methods
In this retrospective, case–control, institutional review board–approved study, we evaluated patients who had undergone revision THA and had preoperative plain radiographs and CT images. We identified patients by International Classification of Diseases, Ninth Revision (ICD‑9) codes (00.70, 00.71, 00.72, 00.73, 80.05, 81.53, 84.56, 84.57) and searched for those cases treated between 2000 and 2012.
Inclusion criteria were confirmed revision THA and confirmed plain radiography and CT of the THA performed before revision. When osteolysis was diagnosed by plain radiography, CT was ordered to determine the extent of bony lesions or to evaluate for eccentric head position or component malposition. Last, all patients included in the study had a detailed operative report clearly indicating acetabular component stability at time of revision. Acetabular component stability at time of surgery was determined according to the criteria defined by Berger and colleagues.19 Cups were evaluated for gross motion during both hip dislocation and during edge loading of the component after thorough scar and capsular débridement.
Patients who did not have CT performed before revision surgery were excluded from the study. These patients had been diagnosed by clinical history and/or plain radiography. Cases revised for periprosthetic infection or periprosthetic fracture were also excluded. Patients with metal-on-metal bearings were excluded, as were any cases revised from hemiarthroplasty to THA, as well as cases revised for recurrent dislocations or component malposition.
All plain radiographs and CT images were evaluated by the orthopedic surgeon who performed the revision and by a radiologist. Images were inspected for signs of gross component migration, tilting, and concentric lucency of the bone–implant interface. Patients with imaging that showed signs of component movement or migration (as seen by the attending surgeon or the radiologist) were excluded. Patients with radiographic evidence of femoral stem loosening were also excluded, as they had an indication to undergo revision arthroplasty. The remaining patients were then stratified into 2 groups: those with stable acetabular components at time of revision and those with loose acetabular components. Stable acetabular shells showed no gross motion of the implant with dislocation, edge loading with an impactor, or pulling with a Kocher clamp after débridement and screw removal.15,19 The 2 groups were then compared with respect to age, sex, and most common presenting symptoms and diagnoses. Fischer exact test and Student t test were used to statistically compare the groups.
Results
Overall, 393 patients underwent revision arthroplasty for the diagnoses (ICD-9 codes) indicated (Figure). One hundred eighty-nine patients (48.1%) had CT performed before revision. Of these 189 patients, 85 were excluded for diagnoses that were evident on either plain radiography or CT, that necessitated urgent revision, or for procedures beyond the scope of the study (Table 1). CT showed a loose cup in 28 patients; 6 of these cups were also seen on CT. Thirteen patients were diagnosed with a loose femoral stem, 10 with a periprosthetic infection, and 8 with a periprosthetic fracture.
One hundred four patients (54 men, 50 women) met the study inclusion criteria. Mean age was 65.1 years. Of these 104 patients, 87 (83.7%) had a stable acetabular shell at time of revision surgery; the other 17 (16.3%) were diagnosed with RSL of the acetabular shell. Osteolysis was the most common diagnosis (89.4%) in the overall population, and pain was the most common complaint at time of presentation (66.6%). Lack of symptoms was the second most common presentation at time of revision (19.2%) (Table 2). Patients without symptoms underwent revision surgery because of concern about impending compromise of the bone–implant interface and progressive osteolysis.
The 2 groups (stable vs unstable acetabular shells) were not significantly different with respect to age (P = .961) or sex distribution (P = .185). All patients in the RSL group were diagnosed with osteolysis radiographically, and 15 (88%) of 17 patients presented with pain as the primary complaint, compared with only 54 (62%) of 87 patients in the group with stable shells. Patients with RSL were significantly more likely to present with pain as the primary complaint (P = .0487). Nineteen patients in the stable implant group and only 1 patient in the RSL group were asymptomatic, but this was not statistically significant (P = .185) when compared against all other diagnoses.
Discussion
We defined RSL as an acetabular component that was loose at time of revision surgery but that did not show frank signs of loosening on either plain radiography or CT. Patients with RSL and the surgeons who treat them are in a difficult position. In the setting of osteolysis, patients can be treated with serial radiographic imaging and clinical monitoring to determine if and when revision arthroplasty should be performed.17 However, given the complexity and risks associated with revision THA, surgeons should be aware that the acetabular shell may necessitate revision even if it does not appear to be frankly unstable on radiographic imaging.18
Of the 393 patients who underwent revision THA at our institution, 48.1% were evaluated with CT. Eighty-five of the 189 patients who underwent CT were diagnosed with radiographic loosening, or were diagnosed as needing urgent revision THA in the setting of loose components, periprosthetic infection, periprosthetic fracture, or catastrophic implant failure. Of the remaining 104 patients, 17 (16.3%) met the diagnosis of RSL of the acetabular component. The most common complaint was pain, and the most common diagnoses were osteolysis and polyethylene wear. Age and sex were not associated with increased likelihood of RSL.
Our study has several limitations. We defined the radiographic diagnosis of loose acetabular components as components showing frank migration, tilting, or a 2-mm concentric lucency on plain radiography or CT. Although these are common definitions of loose acetabular components, more sensitive radiographic measures have been described.16 We also excluded patients with recurrent dislocations and metal-on-metal prostheses, as these cases increase the metal artifact on CT and limit the ability to evaluate the bone–implant interface. Metal artifact remains an ongoing challenge to use of CT for post-THA imaging. However, tailored imaging protocols are helping to eliminate metal artifact. Bone scan was not used to evaluate for possible component loosening. Although sensitivity and specificity are about 67% and 76%, respectively,20 Temmerman and colleagues21 also found poor intraobserver reliability (0.53) for bone scans in the setting of uncemented acetabular components. Last, our study did not evaluate the bony ingrowth patterns that corresponded to stable or unstable fixation and did not evaluate the volumetric size or anatomical location of the osteolytic lesions on CT. Careful assessment of these variables is clinically relevant when trying to determine if revision arthroplasty is warranted.
Although we used relatively simple radiographic criteria to define loose components, more sensitive and specific techniques have been described for both plain radiography and CT. Moore and colleagues22 described 5 radiographic signs of bony ingrowth; when 3 or more were present, sensitivity was 89.6% and specificity 76.9%. Mehin and colleagues23 suggested that osteolysis involving more than 50% of the circumference of the shell on a standard pelvic radiograph might require revision arthroplasty. However, more recent studies have found that anteroposterior and lateral radiographs are less able to evaluate the anterior and posterior rims of the bone–implant interface, and it is this ingrowth area that may be the most crucial for stable osseointegration.12,16
CT has expanded our ability to evaluate the bone–implant interface in 3 dimensions. Egawa and colleagues16 described using CT to evaluate the surface area involved with osteolysis and found that less than 40% involvement of the surface area generally corresponded to well-fixed components. Furthermore, they found that osteolysis generally creates lesions inferior and superior to the acetabular component and less often involves the anterior and posterior rims, which may be more important for stable fixation. The authors noted that volumetric analysis and CT were not as cost-effective as plain radiography and were more time- and skill-intensive.
Osteolysis itself remains a common indication for revision THA. However, the most appropriate procedure remains controversial. Mallory and colleagues24 recommended explanting all acetabular shells in the setting of revision arthroplasty. They indicated that full assessment of the bony pelvis and any lytic defects was possible only with the wide exposure gained by acetabular component removal. More recent studies have begun to justify isolated component revision in the setting of well-fixed acetabular shells. Studies by Maloney and colleagues,10 Park and colleagues,15 and Beaulé and colleagues25 have shown excellent outcomes with retention of well-fixed acetabular shells and removal of the wear generator in the setting of osteolysis. Haidukewych17 wrote that the goals in addressing osteolysis in revision THA are to eliminate the wear generator, débride osteolytic lesions, and restore bone stock. Surgeons should weigh the benefits of component retention and isolated liner exchange against the morbidity associated with explantation and preparation for implanting a new component. Good outcomes have been achieved with isolated component exchange, but surgeons should be aware that instability remains the most common complication after isolated liner exchange.8
The majority of our patients with RSL presented with complaints of pain and the diagnosis of osteolysis. One patient who had the diagnosis but was clinically asymptomatic was found to have a loose acetabular shell at time of revision surgery. Given the increased morbidity associated with acetabular component revision, this patient’s condition represents a dangerous combination of RSL and clinically asymptomatic component loosening. By raising awareness about RSL and its incidence, we should be able to increase our ability to detect RSL. A surgeon who detects RSL before gross migration or movement of the acetabular component may be better able to plan for revision arthroplasty before a catastrophic event that may necessitate a larger, more complex procedure. With the number of patients who require revision THA continuing to rise, surgeons should be aware of the incidence of RSL and the potential of RSL to affect patient care and potential surgical options.
1. Milošev I, Kovač S, Trebše R, Levašič V, Pišot V. Comparison of ten-year survivorship of hip prostheses with use of conventional polyethylene, metal-on-metal, or ceramic-on-ceramic bearings. J Bone Joint Surg Am. 2012;94(19):1756-1763.
2. D’Antonio JA, Capello WN, Naughton M. Ceramic bearings for total hip arthroplasty have high survivorship at 10 years. Clin Orthop Relat Res. 2012;470(2):373-381.
3. Dowd JE, Sychterz CJ, Young AM, Engh CA. Characterization of long-term femoral-head-penetration rates. Association with and prediction of osteolysis. J Bone Joint Surg Am. 2000;82(8):1102-1107.
4. Orishimo KF, Claus AM, Sychterz CJ, Engh CA. Relationship between polyethylene wear and osteolysis in hips with a second-generation porous-coated cementless cup after seven years of follow-up. J Bone Joint Surg Am. 2003;85(6):1095-1099.
5. Harris WH. Wear and periprosthetic osteolysis: the problem. Clin Orthop Relat Res. 2001;(393):66-70.
6. Holt G, Murnaghan C, Reilly J, Meek RM. The biology of aseptic osteolysis. Clin Orthop Relat Res. 2007;(460):240-252.
7. Catelas I, Jacobs JJ. Biologic activity of wear particles. Instr Course Lect. 2010;59:3-16.
8. Paprosky WG, Nourbash P, Gill P. Treatment of progressive periacetabular osteolysis: cup revision versus liner exchange and bone grafting. Paper presented at: Annual Meeting of the American Academy of Orthopaedic Surgeons; February 4-8, 1999; Anaheim, CA.
9. Engh CA Jr, Claus AM, Hopper RH Jr, Engh CA. Long-term results using the anatomic medullary locking hip prosthesis. Clin Orthop Relat Res. 2001;(393):137-146.
10. Maloney WJ, Peters P, Engh CA, Chandler H. Severe osteolysis of the pelvic in association with acetabular replacement without cement. J Bone Joint Surg Am. 1993;75(11):1627-1635.
11. Claus AM, Engh CA Jr, Sychterz CJ, Xenos JS, Orishimo KF, Engh CA Sr. Radiographic definition of pelvic osteolysis following total hip arthroplasty. J Bone Joint Surg Am. 2003;85(8):1519-1526.
12. Puri L, Wixson RL, Stern SH, Kohli J, Hendrix RW, Stulberg SD. Use of helical computed tomography for the assessment of acetabular osteolysis after total hip arthroplasty. J Bone Joint Surg Am. 2002;84(4):609-614.
13. Stulberg SD, Wixson RL, Adams AD, Hendrix RW, Bernfield JB. Monitoring pelvic osteolysis following total hip replacement surgery: an algorithm for surveillance. J Bone Joint Surg Am. 2002;84(suppl 2):116-122.
14. Massin P, Schmidt L, Engh CA. Evaluation of cementless acetabular component migration. An experimental study. J Arthroplasty. 1989;4(3):245-251.
15. Park KS, Yoon TR, Song EK, Lee KB. Results of isolated femoral component revision with well-fixed acetabular implant retention. J Arthroplasty. 2010;25(8):1188-1195.
16. Egawa H, Ho H, Hopper RH Jr, Engh CA Jr, Engh CA. Computed tomography assessment of pelvic osteolysis and cup–lesion interface involvement with a press-fit porous-coated acetabular cup. J Arthroplasty. 2009;24(2):233-239.
17. Haidukewych GJ. Osteolysis in the well-fixed socket: cup retention or revision? J Bone Joint Surg Br. 2012;94(12):65-69.
18. Stulberg BN, Della Valle AG. What are the guidelines for the surgical and nonsurgical treatment of periprosthetic osteolysis? J Am Acad Orthop Surg. 2008;16(suppl 1):S20-S25.
19. Berger RA, Quigley LR, Jacobs JJ, Sheinkop MB, Rosenberg AG, Galante JO. The fate of stable cemented acetabular components retained during revision of a femoral component of a total hip arthroplasty. J Bone Joint Surg Am. 1999;81(12):1682-1691.
20. Temmerman OP, Raijmakers PG, Deville WL, Berkhof J, Hooft L, Heyligers IC. The use of plain radiography, subtraction arthrography, nuclear arthrography, and bone scintigraphy in the diagnosis of a loose acetabular component of a total hip prosthesis: a systematic review. J Arthroplasty. 2007;22(6):818-827.
21. Temmerman OP, Raijmakers PG, David EF, et al. A comparison of radiographic and scintigraphic techniques to assess aseptic loosening of the acetabular component in a total hip replacement. J Bone Joint Surg Am. 2004;86(11):2456-2463.
22. Moore MS, McAuley JP, Young AM, Engh CA Sr. Radiographic signs of osseointegration in porous-coated acetabular components. Clin Orthop Relat Res. 2006;(444):176-183.
23. Mehin R, Yuan X, Haydon C, et al. Retroacetabular osteolysis: when to operate? Clin Orthop Relat Res. 2004;(428):247-255.
24. Mallory TH, Lombardi AV Jr, Fada RA, Adams JB, Kefauver CA, Eberle RW. Noncemented acetabular component removal in the presence of osteolysis: the affirmative. Clin Orthop Relat Res. 2000;(381):120-128.
25. Beaulé PE, Le Duff MJ, Dorey FJ, Amstutz HC. Fate of cementless acetabular components retained during revision total hip arthroplasty. J Bone Joint Surg Am. 2003;85(12):2288-2293.
Total hip arthroplasty (THA) is an excellent option for the treatment of osteoarthritis of the hip. In numerous studies, modern implants have shown survivorship of more than 90% at 10 years.1,2 Polyethylene wear and subsequent osteolysis are major obstacles to the long-term success of THA.3-5 Polyethylene wear particles are phagocytized by macrophages, inducing an inflammatory response that can ultimately lead to osteolysis of the bony architecture surrounding the bone–implant interface.6,7 As modern implants often rely on direct implant-to-bone ingrowth to maintain fixation, wear at this junction can lead to aseptic component loosening and ultimately require revision surgery.8-10 Osteolysis can be diagnosed with plain radiography or computed tomography (CT).11 CT is more sensitive than plain radiography for the diagnosis of osteolysis and is better able to determine the size and location of osteolytic lesions.12,13
Although diagnosis of osteolysis is well defined in the literature, what is more challenging is radiographic diagnosis of a loose acetabular component.11 The most commonly described criteria for loosening are presence of a complete radiolucent line of more than 2 mm in width at the bone–implant interface and any progressive tilting or migration of the component.14,15 CT-based criteria for component loosening remain largely undefined, though Egawa and colleagues16 showed that acetabular osteolysis involving less than 40% of the total cup surface is not associated with component loosening. Although a patient may show signs of osteolysis on postoperative imaging, this finding does not necessitate immediate revision surgery.17 Osteolysis may be monitored clinically and followed radiographically to determine when intervention is necessary.13
The goals of revision surgery are to eliminate the wear generator and bone-graft lytic lesions where needed to help maintain the bone–implant interface.17 The timing of such surgery is important, as the surgeon must balance the risk for gross component migration against the morbidity and mortality associated with acetabular component revision.18 This is in contrast to the settings of infection, periprosthetic fracture, recurrent instability, and component fracture/loosening, in which revision is urgently indicated and the case cannot be managed conservatively.
We conducted a study to determine the incidence of loose acetabular components without radiographic or clinical findings that would necessitate urgent revision THA. Radiographically silent loosening (RSL) was defined as an acetabular component that was loose at time of revision surgery but that did not show frank signs of loosening on either plain radiography or CT. Although these patients make up a small minority of the revision population, knowing the incidence of RSL can help raise surgeon awareness of this potentially dangerous situation. We further sought to determine whether patients with RSL present with different demographic characteristics or clinical symptoms than patients with stable acetabular components.
Materials and Methods
In this retrospective, case–control, institutional review board–approved study, we evaluated patients who had undergone revision THA and had preoperative plain radiographs and CT images. We identified patients by International Classification of Diseases, Ninth Revision (ICD‑9) codes (00.70, 00.71, 00.72, 00.73, 80.05, 81.53, 84.56, 84.57) and searched for those cases treated between 2000 and 2012.
Inclusion criteria were confirmed revision THA and confirmed plain radiography and CT of the THA performed before revision. When osteolysis was diagnosed by plain radiography, CT was ordered to determine the extent of bony lesions or to evaluate for eccentric head position or component malposition. Last, all patients included in the study had a detailed operative report clearly indicating acetabular component stability at time of revision. Acetabular component stability at time of surgery was determined according to the criteria defined by Berger and colleagues.19 Cups were evaluated for gross motion during both hip dislocation and during edge loading of the component after thorough scar and capsular débridement.
Patients who did not have CT performed before revision surgery were excluded from the study. These patients had been diagnosed by clinical history and/or plain radiography. Cases revised for periprosthetic infection or periprosthetic fracture were also excluded. Patients with metal-on-metal bearings were excluded, as were any cases revised from hemiarthroplasty to THA, as well as cases revised for recurrent dislocations or component malposition.
All plain radiographs and CT images were evaluated by the orthopedic surgeon who performed the revision and by a radiologist. Images were inspected for signs of gross component migration, tilting, and concentric lucency of the bone–implant interface. Patients with imaging that showed signs of component movement or migration (as seen by the attending surgeon or the radiologist) were excluded. Patients with radiographic evidence of femoral stem loosening were also excluded, as they had an indication to undergo revision arthroplasty. The remaining patients were then stratified into 2 groups: those with stable acetabular components at time of revision and those with loose acetabular components. Stable acetabular shells showed no gross motion of the implant with dislocation, edge loading with an impactor, or pulling with a Kocher clamp after débridement and screw removal.15,19 The 2 groups were then compared with respect to age, sex, and most common presenting symptoms and diagnoses. Fischer exact test and Student t test were used to statistically compare the groups.
Results
Overall, 393 patients underwent revision arthroplasty for the diagnoses (ICD-9 codes) indicated (Figure). One hundred eighty-nine patients (48.1%) had CT performed before revision. Of these 189 patients, 85 were excluded for diagnoses that were evident on either plain radiography or CT, that necessitated urgent revision, or for procedures beyond the scope of the study (Table 1). CT showed a loose cup in 28 patients; 6 of these cups were also seen on CT. Thirteen patients were diagnosed with a loose femoral stem, 10 with a periprosthetic infection, and 8 with a periprosthetic fracture.
One hundred four patients (54 men, 50 women) met the study inclusion criteria. Mean age was 65.1 years. Of these 104 patients, 87 (83.7%) had a stable acetabular shell at time of revision surgery; the other 17 (16.3%) were diagnosed with RSL of the acetabular shell. Osteolysis was the most common diagnosis (89.4%) in the overall population, and pain was the most common complaint at time of presentation (66.6%). Lack of symptoms was the second most common presentation at time of revision (19.2%) (Table 2). Patients without symptoms underwent revision surgery because of concern about impending compromise of the bone–implant interface and progressive osteolysis.
The 2 groups (stable vs unstable acetabular shells) were not significantly different with respect to age (P = .961) or sex distribution (P = .185). All patients in the RSL group were diagnosed with osteolysis radiographically, and 15 (88%) of 17 patients presented with pain as the primary complaint, compared with only 54 (62%) of 87 patients in the group with stable shells. Patients with RSL were significantly more likely to present with pain as the primary complaint (P = .0487). Nineteen patients in the stable implant group and only 1 patient in the RSL group were asymptomatic, but this was not statistically significant (P = .185) when compared against all other diagnoses.
Discussion
We defined RSL as an acetabular component that was loose at time of revision surgery but that did not show frank signs of loosening on either plain radiography or CT. Patients with RSL and the surgeons who treat them are in a difficult position. In the setting of osteolysis, patients can be treated with serial radiographic imaging and clinical monitoring to determine if and when revision arthroplasty should be performed.17 However, given the complexity and risks associated with revision THA, surgeons should be aware that the acetabular shell may necessitate revision even if it does not appear to be frankly unstable on radiographic imaging.18
Of the 393 patients who underwent revision THA at our institution, 48.1% were evaluated with CT. Eighty-five of the 189 patients who underwent CT were diagnosed with radiographic loosening, or were diagnosed as needing urgent revision THA in the setting of loose components, periprosthetic infection, periprosthetic fracture, or catastrophic implant failure. Of the remaining 104 patients, 17 (16.3%) met the diagnosis of RSL of the acetabular component. The most common complaint was pain, and the most common diagnoses were osteolysis and polyethylene wear. Age and sex were not associated with increased likelihood of RSL.
Our study has several limitations. We defined the radiographic diagnosis of loose acetabular components as components showing frank migration, tilting, or a 2-mm concentric lucency on plain radiography or CT. Although these are common definitions of loose acetabular components, more sensitive radiographic measures have been described.16 We also excluded patients with recurrent dislocations and metal-on-metal prostheses, as these cases increase the metal artifact on CT and limit the ability to evaluate the bone–implant interface. Metal artifact remains an ongoing challenge to use of CT for post-THA imaging. However, tailored imaging protocols are helping to eliminate metal artifact. Bone scan was not used to evaluate for possible component loosening. Although sensitivity and specificity are about 67% and 76%, respectively,20 Temmerman and colleagues21 also found poor intraobserver reliability (0.53) for bone scans in the setting of uncemented acetabular components. Last, our study did not evaluate the bony ingrowth patterns that corresponded to stable or unstable fixation and did not evaluate the volumetric size or anatomical location of the osteolytic lesions on CT. Careful assessment of these variables is clinically relevant when trying to determine if revision arthroplasty is warranted.
Although we used relatively simple radiographic criteria to define loose components, more sensitive and specific techniques have been described for both plain radiography and CT. Moore and colleagues22 described 5 radiographic signs of bony ingrowth; when 3 or more were present, sensitivity was 89.6% and specificity 76.9%. Mehin and colleagues23 suggested that osteolysis involving more than 50% of the circumference of the shell on a standard pelvic radiograph might require revision arthroplasty. However, more recent studies have found that anteroposterior and lateral radiographs are less able to evaluate the anterior and posterior rims of the bone–implant interface, and it is this ingrowth area that may be the most crucial for stable osseointegration.12,16
CT has expanded our ability to evaluate the bone–implant interface in 3 dimensions. Egawa and colleagues16 described using CT to evaluate the surface area involved with osteolysis and found that less than 40% involvement of the surface area generally corresponded to well-fixed components. Furthermore, they found that osteolysis generally creates lesions inferior and superior to the acetabular component and less often involves the anterior and posterior rims, which may be more important for stable fixation. The authors noted that volumetric analysis and CT were not as cost-effective as plain radiography and were more time- and skill-intensive.
Osteolysis itself remains a common indication for revision THA. However, the most appropriate procedure remains controversial. Mallory and colleagues24 recommended explanting all acetabular shells in the setting of revision arthroplasty. They indicated that full assessment of the bony pelvis and any lytic defects was possible only with the wide exposure gained by acetabular component removal. More recent studies have begun to justify isolated component revision in the setting of well-fixed acetabular shells. Studies by Maloney and colleagues,10 Park and colleagues,15 and Beaulé and colleagues25 have shown excellent outcomes with retention of well-fixed acetabular shells and removal of the wear generator in the setting of osteolysis. Haidukewych17 wrote that the goals in addressing osteolysis in revision THA are to eliminate the wear generator, débride osteolytic lesions, and restore bone stock. Surgeons should weigh the benefits of component retention and isolated liner exchange against the morbidity associated with explantation and preparation for implanting a new component. Good outcomes have been achieved with isolated component exchange, but surgeons should be aware that instability remains the most common complication after isolated liner exchange.8
The majority of our patients with RSL presented with complaints of pain and the diagnosis of osteolysis. One patient who had the diagnosis but was clinically asymptomatic was found to have a loose acetabular shell at time of revision surgery. Given the increased morbidity associated with acetabular component revision, this patient’s condition represents a dangerous combination of RSL and clinically asymptomatic component loosening. By raising awareness about RSL and its incidence, we should be able to increase our ability to detect RSL. A surgeon who detects RSL before gross migration or movement of the acetabular component may be better able to plan for revision arthroplasty before a catastrophic event that may necessitate a larger, more complex procedure. With the number of patients who require revision THA continuing to rise, surgeons should be aware of the incidence of RSL and the potential of RSL to affect patient care and potential surgical options.
Total hip arthroplasty (THA) is an excellent option for the treatment of osteoarthritis of the hip. In numerous studies, modern implants have shown survivorship of more than 90% at 10 years.1,2 Polyethylene wear and subsequent osteolysis are major obstacles to the long-term success of THA.3-5 Polyethylene wear particles are phagocytized by macrophages, inducing an inflammatory response that can ultimately lead to osteolysis of the bony architecture surrounding the bone–implant interface.6,7 As modern implants often rely on direct implant-to-bone ingrowth to maintain fixation, wear at this junction can lead to aseptic component loosening and ultimately require revision surgery.8-10 Osteolysis can be diagnosed with plain radiography or computed tomography (CT).11 CT is more sensitive than plain radiography for the diagnosis of osteolysis and is better able to determine the size and location of osteolytic lesions.12,13
Although diagnosis of osteolysis is well defined in the literature, what is more challenging is radiographic diagnosis of a loose acetabular component.11 The most commonly described criteria for loosening are presence of a complete radiolucent line of more than 2 mm in width at the bone–implant interface and any progressive tilting or migration of the component.14,15 CT-based criteria for component loosening remain largely undefined, though Egawa and colleagues16 showed that acetabular osteolysis involving less than 40% of the total cup surface is not associated with component loosening. Although a patient may show signs of osteolysis on postoperative imaging, this finding does not necessitate immediate revision surgery.17 Osteolysis may be monitored clinically and followed radiographically to determine when intervention is necessary.13
The goals of revision surgery are to eliminate the wear generator and bone-graft lytic lesions where needed to help maintain the bone–implant interface.17 The timing of such surgery is important, as the surgeon must balance the risk for gross component migration against the morbidity and mortality associated with acetabular component revision.18 This is in contrast to the settings of infection, periprosthetic fracture, recurrent instability, and component fracture/loosening, in which revision is urgently indicated and the case cannot be managed conservatively.
We conducted a study to determine the incidence of loose acetabular components without radiographic or clinical findings that would necessitate urgent revision THA. Radiographically silent loosening (RSL) was defined as an acetabular component that was loose at time of revision surgery but that did not show frank signs of loosening on either plain radiography or CT. Although these patients make up a small minority of the revision population, knowing the incidence of RSL can help raise surgeon awareness of this potentially dangerous situation. We further sought to determine whether patients with RSL present with different demographic characteristics or clinical symptoms than patients with stable acetabular components.
Materials and Methods
In this retrospective, case–control, institutional review board–approved study, we evaluated patients who had undergone revision THA and had preoperative plain radiographs and CT images. We identified patients by International Classification of Diseases, Ninth Revision (ICD‑9) codes (00.70, 00.71, 00.72, 00.73, 80.05, 81.53, 84.56, 84.57) and searched for those cases treated between 2000 and 2012.
Inclusion criteria were confirmed revision THA and confirmed plain radiography and CT of the THA performed before revision. When osteolysis was diagnosed by plain radiography, CT was ordered to determine the extent of bony lesions or to evaluate for eccentric head position or component malposition. Last, all patients included in the study had a detailed operative report clearly indicating acetabular component stability at time of revision. Acetabular component stability at time of surgery was determined according to the criteria defined by Berger and colleagues.19 Cups were evaluated for gross motion during both hip dislocation and during edge loading of the component after thorough scar and capsular débridement.
Patients who did not have CT performed before revision surgery were excluded from the study. These patients had been diagnosed by clinical history and/or plain radiography. Cases revised for periprosthetic infection or periprosthetic fracture were also excluded. Patients with metal-on-metal bearings were excluded, as were any cases revised from hemiarthroplasty to THA, as well as cases revised for recurrent dislocations or component malposition.
All plain radiographs and CT images were evaluated by the orthopedic surgeon who performed the revision and by a radiologist. Images were inspected for signs of gross component migration, tilting, and concentric lucency of the bone–implant interface. Patients with imaging that showed signs of component movement or migration (as seen by the attending surgeon or the radiologist) were excluded. Patients with radiographic evidence of femoral stem loosening were also excluded, as they had an indication to undergo revision arthroplasty. The remaining patients were then stratified into 2 groups: those with stable acetabular components at time of revision and those with loose acetabular components. Stable acetabular shells showed no gross motion of the implant with dislocation, edge loading with an impactor, or pulling with a Kocher clamp after débridement and screw removal.15,19 The 2 groups were then compared with respect to age, sex, and most common presenting symptoms and diagnoses. Fischer exact test and Student t test were used to statistically compare the groups.
Results
Overall, 393 patients underwent revision arthroplasty for the diagnoses (ICD-9 codes) indicated (Figure). One hundred eighty-nine patients (48.1%) had CT performed before revision. Of these 189 patients, 85 were excluded for diagnoses that were evident on either plain radiography or CT, that necessitated urgent revision, or for procedures beyond the scope of the study (Table 1). CT showed a loose cup in 28 patients; 6 of these cups were also seen on CT. Thirteen patients were diagnosed with a loose femoral stem, 10 with a periprosthetic infection, and 8 with a periprosthetic fracture.
One hundred four patients (54 men, 50 women) met the study inclusion criteria. Mean age was 65.1 years. Of these 104 patients, 87 (83.7%) had a stable acetabular shell at time of revision surgery; the other 17 (16.3%) were diagnosed with RSL of the acetabular shell. Osteolysis was the most common diagnosis (89.4%) in the overall population, and pain was the most common complaint at time of presentation (66.6%). Lack of symptoms was the second most common presentation at time of revision (19.2%) (Table 2). Patients without symptoms underwent revision surgery because of concern about impending compromise of the bone–implant interface and progressive osteolysis.
The 2 groups (stable vs unstable acetabular shells) were not significantly different with respect to age (P = .961) or sex distribution (P = .185). All patients in the RSL group were diagnosed with osteolysis radiographically, and 15 (88%) of 17 patients presented with pain as the primary complaint, compared with only 54 (62%) of 87 patients in the group with stable shells. Patients with RSL were significantly more likely to present with pain as the primary complaint (P = .0487). Nineteen patients in the stable implant group and only 1 patient in the RSL group were asymptomatic, but this was not statistically significant (P = .185) when compared against all other diagnoses.
Discussion
We defined RSL as an acetabular component that was loose at time of revision surgery but that did not show frank signs of loosening on either plain radiography or CT. Patients with RSL and the surgeons who treat them are in a difficult position. In the setting of osteolysis, patients can be treated with serial radiographic imaging and clinical monitoring to determine if and when revision arthroplasty should be performed.17 However, given the complexity and risks associated with revision THA, surgeons should be aware that the acetabular shell may necessitate revision even if it does not appear to be frankly unstable on radiographic imaging.18
Of the 393 patients who underwent revision THA at our institution, 48.1% were evaluated with CT. Eighty-five of the 189 patients who underwent CT were diagnosed with radiographic loosening, or were diagnosed as needing urgent revision THA in the setting of loose components, periprosthetic infection, periprosthetic fracture, or catastrophic implant failure. Of the remaining 104 patients, 17 (16.3%) met the diagnosis of RSL of the acetabular component. The most common complaint was pain, and the most common diagnoses were osteolysis and polyethylene wear. Age and sex were not associated with increased likelihood of RSL.
Our study has several limitations. We defined the radiographic diagnosis of loose acetabular components as components showing frank migration, tilting, or a 2-mm concentric lucency on plain radiography or CT. Although these are common definitions of loose acetabular components, more sensitive radiographic measures have been described.16 We also excluded patients with recurrent dislocations and metal-on-metal prostheses, as these cases increase the metal artifact on CT and limit the ability to evaluate the bone–implant interface. Metal artifact remains an ongoing challenge to use of CT for post-THA imaging. However, tailored imaging protocols are helping to eliminate metal artifact. Bone scan was not used to evaluate for possible component loosening. Although sensitivity and specificity are about 67% and 76%, respectively,20 Temmerman and colleagues21 also found poor intraobserver reliability (0.53) for bone scans in the setting of uncemented acetabular components. Last, our study did not evaluate the bony ingrowth patterns that corresponded to stable or unstable fixation and did not evaluate the volumetric size or anatomical location of the osteolytic lesions on CT. Careful assessment of these variables is clinically relevant when trying to determine if revision arthroplasty is warranted.
Although we used relatively simple radiographic criteria to define loose components, more sensitive and specific techniques have been described for both plain radiography and CT. Moore and colleagues22 described 5 radiographic signs of bony ingrowth; when 3 or more were present, sensitivity was 89.6% and specificity 76.9%. Mehin and colleagues23 suggested that osteolysis involving more than 50% of the circumference of the shell on a standard pelvic radiograph might require revision arthroplasty. However, more recent studies have found that anteroposterior and lateral radiographs are less able to evaluate the anterior and posterior rims of the bone–implant interface, and it is this ingrowth area that may be the most crucial for stable osseointegration.12,16
CT has expanded our ability to evaluate the bone–implant interface in 3 dimensions. Egawa and colleagues16 described using CT to evaluate the surface area involved with osteolysis and found that less than 40% involvement of the surface area generally corresponded to well-fixed components. Furthermore, they found that osteolysis generally creates lesions inferior and superior to the acetabular component and less often involves the anterior and posterior rims, which may be more important for stable fixation. The authors noted that volumetric analysis and CT were not as cost-effective as plain radiography and were more time- and skill-intensive.
Osteolysis itself remains a common indication for revision THA. However, the most appropriate procedure remains controversial. Mallory and colleagues24 recommended explanting all acetabular shells in the setting of revision arthroplasty. They indicated that full assessment of the bony pelvis and any lytic defects was possible only with the wide exposure gained by acetabular component removal. More recent studies have begun to justify isolated component revision in the setting of well-fixed acetabular shells. Studies by Maloney and colleagues,10 Park and colleagues,15 and Beaulé and colleagues25 have shown excellent outcomes with retention of well-fixed acetabular shells and removal of the wear generator in the setting of osteolysis. Haidukewych17 wrote that the goals in addressing osteolysis in revision THA are to eliminate the wear generator, débride osteolytic lesions, and restore bone stock. Surgeons should weigh the benefits of component retention and isolated liner exchange against the morbidity associated with explantation and preparation for implanting a new component. Good outcomes have been achieved with isolated component exchange, but surgeons should be aware that instability remains the most common complication after isolated liner exchange.8
The majority of our patients with RSL presented with complaints of pain and the diagnosis of osteolysis. One patient who had the diagnosis but was clinically asymptomatic was found to have a loose acetabular shell at time of revision surgery. Given the increased morbidity associated with acetabular component revision, this patient’s condition represents a dangerous combination of RSL and clinically asymptomatic component loosening. By raising awareness about RSL and its incidence, we should be able to increase our ability to detect RSL. A surgeon who detects RSL before gross migration or movement of the acetabular component may be better able to plan for revision arthroplasty before a catastrophic event that may necessitate a larger, more complex procedure. With the number of patients who require revision THA continuing to rise, surgeons should be aware of the incidence of RSL and the potential of RSL to affect patient care and potential surgical options.
1. Milošev I, Kovač S, Trebše R, Levašič V, Pišot V. Comparison of ten-year survivorship of hip prostheses with use of conventional polyethylene, metal-on-metal, or ceramic-on-ceramic bearings. J Bone Joint Surg Am. 2012;94(19):1756-1763.
2. D’Antonio JA, Capello WN, Naughton M. Ceramic bearings for total hip arthroplasty have high survivorship at 10 years. Clin Orthop Relat Res. 2012;470(2):373-381.
3. Dowd JE, Sychterz CJ, Young AM, Engh CA. Characterization of long-term femoral-head-penetration rates. Association with and prediction of osteolysis. J Bone Joint Surg Am. 2000;82(8):1102-1107.
4. Orishimo KF, Claus AM, Sychterz CJ, Engh CA. Relationship between polyethylene wear and osteolysis in hips with a second-generation porous-coated cementless cup after seven years of follow-up. J Bone Joint Surg Am. 2003;85(6):1095-1099.
5. Harris WH. Wear and periprosthetic osteolysis: the problem. Clin Orthop Relat Res. 2001;(393):66-70.
6. Holt G, Murnaghan C, Reilly J, Meek RM. The biology of aseptic osteolysis. Clin Orthop Relat Res. 2007;(460):240-252.
7. Catelas I, Jacobs JJ. Biologic activity of wear particles. Instr Course Lect. 2010;59:3-16.
8. Paprosky WG, Nourbash P, Gill P. Treatment of progressive periacetabular osteolysis: cup revision versus liner exchange and bone grafting. Paper presented at: Annual Meeting of the American Academy of Orthopaedic Surgeons; February 4-8, 1999; Anaheim, CA.
9. Engh CA Jr, Claus AM, Hopper RH Jr, Engh CA. Long-term results using the anatomic medullary locking hip prosthesis. Clin Orthop Relat Res. 2001;(393):137-146.
10. Maloney WJ, Peters P, Engh CA, Chandler H. Severe osteolysis of the pelvic in association with acetabular replacement without cement. J Bone Joint Surg Am. 1993;75(11):1627-1635.
11. Claus AM, Engh CA Jr, Sychterz CJ, Xenos JS, Orishimo KF, Engh CA Sr. Radiographic definition of pelvic osteolysis following total hip arthroplasty. J Bone Joint Surg Am. 2003;85(8):1519-1526.
12. Puri L, Wixson RL, Stern SH, Kohli J, Hendrix RW, Stulberg SD. Use of helical computed tomography for the assessment of acetabular osteolysis after total hip arthroplasty. J Bone Joint Surg Am. 2002;84(4):609-614.
13. Stulberg SD, Wixson RL, Adams AD, Hendrix RW, Bernfield JB. Monitoring pelvic osteolysis following total hip replacement surgery: an algorithm for surveillance. J Bone Joint Surg Am. 2002;84(suppl 2):116-122.
14. Massin P, Schmidt L, Engh CA. Evaluation of cementless acetabular component migration. An experimental study. J Arthroplasty. 1989;4(3):245-251.
15. Park KS, Yoon TR, Song EK, Lee KB. Results of isolated femoral component revision with well-fixed acetabular implant retention. J Arthroplasty. 2010;25(8):1188-1195.
16. Egawa H, Ho H, Hopper RH Jr, Engh CA Jr, Engh CA. Computed tomography assessment of pelvic osteolysis and cup–lesion interface involvement with a press-fit porous-coated acetabular cup. J Arthroplasty. 2009;24(2):233-239.
17. Haidukewych GJ. Osteolysis in the well-fixed socket: cup retention or revision? J Bone Joint Surg Br. 2012;94(12):65-69.
18. Stulberg BN, Della Valle AG. What are the guidelines for the surgical and nonsurgical treatment of periprosthetic osteolysis? J Am Acad Orthop Surg. 2008;16(suppl 1):S20-S25.
19. Berger RA, Quigley LR, Jacobs JJ, Sheinkop MB, Rosenberg AG, Galante JO. The fate of stable cemented acetabular components retained during revision of a femoral component of a total hip arthroplasty. J Bone Joint Surg Am. 1999;81(12):1682-1691.
20. Temmerman OP, Raijmakers PG, Deville WL, Berkhof J, Hooft L, Heyligers IC. The use of plain radiography, subtraction arthrography, nuclear arthrography, and bone scintigraphy in the diagnosis of a loose acetabular component of a total hip prosthesis: a systematic review. J Arthroplasty. 2007;22(6):818-827.
21. Temmerman OP, Raijmakers PG, David EF, et al. A comparison of radiographic and scintigraphic techniques to assess aseptic loosening of the acetabular component in a total hip replacement. J Bone Joint Surg Am. 2004;86(11):2456-2463.
22. Moore MS, McAuley JP, Young AM, Engh CA Sr. Radiographic signs of osseointegration in porous-coated acetabular components. Clin Orthop Relat Res. 2006;(444):176-183.
23. Mehin R, Yuan X, Haydon C, et al. Retroacetabular osteolysis: when to operate? Clin Orthop Relat Res. 2004;(428):247-255.
24. Mallory TH, Lombardi AV Jr, Fada RA, Adams JB, Kefauver CA, Eberle RW. Noncemented acetabular component removal in the presence of osteolysis: the affirmative. Clin Orthop Relat Res. 2000;(381):120-128.
25. Beaulé PE, Le Duff MJ, Dorey FJ, Amstutz HC. Fate of cementless acetabular components retained during revision total hip arthroplasty. J Bone Joint Surg Am. 2003;85(12):2288-2293.
1. Milošev I, Kovač S, Trebše R, Levašič V, Pišot V. Comparison of ten-year survivorship of hip prostheses with use of conventional polyethylene, metal-on-metal, or ceramic-on-ceramic bearings. J Bone Joint Surg Am. 2012;94(19):1756-1763.
2. D’Antonio JA, Capello WN, Naughton M. Ceramic bearings for total hip arthroplasty have high survivorship at 10 years. Clin Orthop Relat Res. 2012;470(2):373-381.
3. Dowd JE, Sychterz CJ, Young AM, Engh CA. Characterization of long-term femoral-head-penetration rates. Association with and prediction of osteolysis. J Bone Joint Surg Am. 2000;82(8):1102-1107.
4. Orishimo KF, Claus AM, Sychterz CJ, Engh CA. Relationship between polyethylene wear and osteolysis in hips with a second-generation porous-coated cementless cup after seven years of follow-up. J Bone Joint Surg Am. 2003;85(6):1095-1099.
5. Harris WH. Wear and periprosthetic osteolysis: the problem. Clin Orthop Relat Res. 2001;(393):66-70.
6. Holt G, Murnaghan C, Reilly J, Meek RM. The biology of aseptic osteolysis. Clin Orthop Relat Res. 2007;(460):240-252.
7. Catelas I, Jacobs JJ. Biologic activity of wear particles. Instr Course Lect. 2010;59:3-16.
8. Paprosky WG, Nourbash P, Gill P. Treatment of progressive periacetabular osteolysis: cup revision versus liner exchange and bone grafting. Paper presented at: Annual Meeting of the American Academy of Orthopaedic Surgeons; February 4-8, 1999; Anaheim, CA.
9. Engh CA Jr, Claus AM, Hopper RH Jr, Engh CA. Long-term results using the anatomic medullary locking hip prosthesis. Clin Orthop Relat Res. 2001;(393):137-146.
10. Maloney WJ, Peters P, Engh CA, Chandler H. Severe osteolysis of the pelvic in association with acetabular replacement without cement. J Bone Joint Surg Am. 1993;75(11):1627-1635.
11. Claus AM, Engh CA Jr, Sychterz CJ, Xenos JS, Orishimo KF, Engh CA Sr. Radiographic definition of pelvic osteolysis following total hip arthroplasty. J Bone Joint Surg Am. 2003;85(8):1519-1526.
12. Puri L, Wixson RL, Stern SH, Kohli J, Hendrix RW, Stulberg SD. Use of helical computed tomography for the assessment of acetabular osteolysis after total hip arthroplasty. J Bone Joint Surg Am. 2002;84(4):609-614.
13. Stulberg SD, Wixson RL, Adams AD, Hendrix RW, Bernfield JB. Monitoring pelvic osteolysis following total hip replacement surgery: an algorithm for surveillance. J Bone Joint Surg Am. 2002;84(suppl 2):116-122.
14. Massin P, Schmidt L, Engh CA. Evaluation of cementless acetabular component migration. An experimental study. J Arthroplasty. 1989;4(3):245-251.
15. Park KS, Yoon TR, Song EK, Lee KB. Results of isolated femoral component revision with well-fixed acetabular implant retention. J Arthroplasty. 2010;25(8):1188-1195.
16. Egawa H, Ho H, Hopper RH Jr, Engh CA Jr, Engh CA. Computed tomography assessment of pelvic osteolysis and cup–lesion interface involvement with a press-fit porous-coated acetabular cup. J Arthroplasty. 2009;24(2):233-239.
17. Haidukewych GJ. Osteolysis in the well-fixed socket: cup retention or revision? J Bone Joint Surg Br. 2012;94(12):65-69.
18. Stulberg BN, Della Valle AG. What are the guidelines for the surgical and nonsurgical treatment of periprosthetic osteolysis? J Am Acad Orthop Surg. 2008;16(suppl 1):S20-S25.
19. Berger RA, Quigley LR, Jacobs JJ, Sheinkop MB, Rosenberg AG, Galante JO. The fate of stable cemented acetabular components retained during revision of a femoral component of a total hip arthroplasty. J Bone Joint Surg Am. 1999;81(12):1682-1691.
20. Temmerman OP, Raijmakers PG, Deville WL, Berkhof J, Hooft L, Heyligers IC. The use of plain radiography, subtraction arthrography, nuclear arthrography, and bone scintigraphy in the diagnosis of a loose acetabular component of a total hip prosthesis: a systematic review. J Arthroplasty. 2007;22(6):818-827.
21. Temmerman OP, Raijmakers PG, David EF, et al. A comparison of radiographic and scintigraphic techniques to assess aseptic loosening of the acetabular component in a total hip replacement. J Bone Joint Surg Am. 2004;86(11):2456-2463.
22. Moore MS, McAuley JP, Young AM, Engh CA Sr. Radiographic signs of osseointegration in porous-coated acetabular components. Clin Orthop Relat Res. 2006;(444):176-183.
23. Mehin R, Yuan X, Haydon C, et al. Retroacetabular osteolysis: when to operate? Clin Orthop Relat Res. 2004;(428):247-255.
24. Mallory TH, Lombardi AV Jr, Fada RA, Adams JB, Kefauver CA, Eberle RW. Noncemented acetabular component removal in the presence of osteolysis: the affirmative. Clin Orthop Relat Res. 2000;(381):120-128.
25. Beaulé PE, Le Duff MJ, Dorey FJ, Amstutz HC. Fate of cementless acetabular components retained during revision total hip arthroplasty. J Bone Joint Surg Am. 2003;85(12):2288-2293.
CT scan utilization down in children’s hospitals
The use of computed tomography in children’s hospitals appears to be on the decline across the top 10 all-patient-refined, diagnosis-related groups (APR-DRGs), with alternate imaging processes increasing in utilization for 8 of those 10 groups.
New research examining the use of CT scans on children admitted to 33 pediatric tertiary-care hospitals participating in the Pediatric Health Information System between Jan. 1, 2004, and Dec. 31, 2012, reveals the decrease (Pediatrics 2015 Aug 24.[doi: 10.1542/peds.2015-0995]).
“This decrease occurred with a concomitant increase in alternative imaging modalities in 8 studied diagnostic groups, supporting the hypothesis that previously noted declines in CT utilization are associated with shifts to alternate modalities,” Dr. Michelle W. Parker of Children’s Hospital Medical Center in Cincinnati and her colleagues wrote.
The 10 APR-DRGs studies are:
• seizure.
• ventricular shunt procedures.
• appendectomy.
• craniotomy except for trauma.
• concussion, closed skull fracture, uncomplicated intracranial injury, coma less than 1 hour or no coma (concussion).
• head trauma with coma greater than 1 hour or hemorrhage (severe head trauma).
• infections of upper respiratory tract.
• nonbacterial gastroenteritis with nausea and vomiting (gastroenteritis).
• abdominal pain.
• other ear, nose, mouth, throat, and craniofacial diagnoses.
“The decrease was most noted for patients with an APR-DRG of seizure where CT utilization decreased almost 50%, and MRI utilization decreased by greater than 10%,” the authors wrote.
Severe head trauma had the largest reduction in CT utilization. “MRI utilization could not be evaluated for appendectomy, gastroenteritis, or infections of the upper respiratory tract because of limited study volume. Statistically significant changes in MRI use were noted across the remaining APR-DRGs,with marked increases for ventricular shunt procedures,” Dr. Parker and her colleagues reported.
“There were no ultrasound studies meeting inclusion criteria for seizures, concussion, or severe head trauma. Ultrasound use increased during the study period for all other APR-DRGs, with the greatest increase noted in appendectomy and gastroenteritis,” they said.
Dr. Parker and her colleagues suggested a number of reasons for the decrease, including a 2006 effort by the Alliance for Radiation Safety in Pediatric Imaging, an initiative within the Society for Pediatric Radiology that raised awareness on the number of pediatric CT scans and the use of adult dose protocols in pediatric imaging. They also mentioned other technological advances, such as electronic health records and the sharing of existing images, which may have contributed to the lower utilization of CT scans.
The authors note that the decrease does have public health implications, as lower CT utilization decreases exposure to hazardous ionizing radiation.“Therefore, the substitution of an imaging modality that does not confer ionizing radiation may affect lifetime cancer risk in children who receive diagnostic imaging.”
Dr. Parker and her colleagues reported no conflicts of interest or external funding sources for the research.
The use of computed tomography in children’s hospitals appears to be on the decline across the top 10 all-patient-refined, diagnosis-related groups (APR-DRGs), with alternate imaging processes increasing in utilization for 8 of those 10 groups.
New research examining the use of CT scans on children admitted to 33 pediatric tertiary-care hospitals participating in the Pediatric Health Information System between Jan. 1, 2004, and Dec. 31, 2012, reveals the decrease (Pediatrics 2015 Aug 24.[doi: 10.1542/peds.2015-0995]).
“This decrease occurred with a concomitant increase in alternative imaging modalities in 8 studied diagnostic groups, supporting the hypothesis that previously noted declines in CT utilization are associated with shifts to alternate modalities,” Dr. Michelle W. Parker of Children’s Hospital Medical Center in Cincinnati and her colleagues wrote.
The 10 APR-DRGs studies are:
• seizure.
• ventricular shunt procedures.
• appendectomy.
• craniotomy except for trauma.
• concussion, closed skull fracture, uncomplicated intracranial injury, coma less than 1 hour or no coma (concussion).
• head trauma with coma greater than 1 hour or hemorrhage (severe head trauma).
• infections of upper respiratory tract.
• nonbacterial gastroenteritis with nausea and vomiting (gastroenteritis).
• abdominal pain.
• other ear, nose, mouth, throat, and craniofacial diagnoses.
“The decrease was most noted for patients with an APR-DRG of seizure where CT utilization decreased almost 50%, and MRI utilization decreased by greater than 10%,” the authors wrote.
Severe head trauma had the largest reduction in CT utilization. “MRI utilization could not be evaluated for appendectomy, gastroenteritis, or infections of the upper respiratory tract because of limited study volume. Statistically significant changes in MRI use were noted across the remaining APR-DRGs,with marked increases for ventricular shunt procedures,” Dr. Parker and her colleagues reported.
“There were no ultrasound studies meeting inclusion criteria for seizures, concussion, or severe head trauma. Ultrasound use increased during the study period for all other APR-DRGs, with the greatest increase noted in appendectomy and gastroenteritis,” they said.
Dr. Parker and her colleagues suggested a number of reasons for the decrease, including a 2006 effort by the Alliance for Radiation Safety in Pediatric Imaging, an initiative within the Society for Pediatric Radiology that raised awareness on the number of pediatric CT scans and the use of adult dose protocols in pediatric imaging. They also mentioned other technological advances, such as electronic health records and the sharing of existing images, which may have contributed to the lower utilization of CT scans.
The authors note that the decrease does have public health implications, as lower CT utilization decreases exposure to hazardous ionizing radiation.“Therefore, the substitution of an imaging modality that does not confer ionizing radiation may affect lifetime cancer risk in children who receive diagnostic imaging.”
Dr. Parker and her colleagues reported no conflicts of interest or external funding sources for the research.
The use of computed tomography in children’s hospitals appears to be on the decline across the top 10 all-patient-refined, diagnosis-related groups (APR-DRGs), with alternate imaging processes increasing in utilization for 8 of those 10 groups.
New research examining the use of CT scans on children admitted to 33 pediatric tertiary-care hospitals participating in the Pediatric Health Information System between Jan. 1, 2004, and Dec. 31, 2012, reveals the decrease (Pediatrics 2015 Aug 24.[doi: 10.1542/peds.2015-0995]).
“This decrease occurred with a concomitant increase in alternative imaging modalities in 8 studied diagnostic groups, supporting the hypothesis that previously noted declines in CT utilization are associated with shifts to alternate modalities,” Dr. Michelle W. Parker of Children’s Hospital Medical Center in Cincinnati and her colleagues wrote.
The 10 APR-DRGs studies are:
• seizure.
• ventricular shunt procedures.
• appendectomy.
• craniotomy except for trauma.
• concussion, closed skull fracture, uncomplicated intracranial injury, coma less than 1 hour or no coma (concussion).
• head trauma with coma greater than 1 hour or hemorrhage (severe head trauma).
• infections of upper respiratory tract.
• nonbacterial gastroenteritis with nausea and vomiting (gastroenteritis).
• abdominal pain.
• other ear, nose, mouth, throat, and craniofacial diagnoses.
“The decrease was most noted for patients with an APR-DRG of seizure where CT utilization decreased almost 50%, and MRI utilization decreased by greater than 10%,” the authors wrote.
Severe head trauma had the largest reduction in CT utilization. “MRI utilization could not be evaluated for appendectomy, gastroenteritis, or infections of the upper respiratory tract because of limited study volume. Statistically significant changes in MRI use were noted across the remaining APR-DRGs,with marked increases for ventricular shunt procedures,” Dr. Parker and her colleagues reported.
“There were no ultrasound studies meeting inclusion criteria for seizures, concussion, or severe head trauma. Ultrasound use increased during the study period for all other APR-DRGs, with the greatest increase noted in appendectomy and gastroenteritis,” they said.
Dr. Parker and her colleagues suggested a number of reasons for the decrease, including a 2006 effort by the Alliance for Radiation Safety in Pediatric Imaging, an initiative within the Society for Pediatric Radiology that raised awareness on the number of pediatric CT scans and the use of adult dose protocols in pediatric imaging. They also mentioned other technological advances, such as electronic health records and the sharing of existing images, which may have contributed to the lower utilization of CT scans.
The authors note that the decrease does have public health implications, as lower CT utilization decreases exposure to hazardous ionizing radiation.“Therefore, the substitution of an imaging modality that does not confer ionizing radiation may affect lifetime cancer risk in children who receive diagnostic imaging.”
Dr. Parker and her colleagues reported no conflicts of interest or external funding sources for the research.
FROM PEDIATRICS
Key clinical point: The use of CT scans in children’s hospitals declined in the top 10 APR-DRGs.
Major finding: The decrease was greatest for patients with an APR-DRG of seizure with an almost 50% decrease in CT scans; MRI use decreased by greater than 10%.
Data source: Utilization of CT scans on children admitted to 33 pediatric tertiary-care hospitals participating in the Pediatric Health Information System between Jan.1, 2004, and Dec. 31, 2012.
Disclosures: Dr. Parker and her colleagues reported no conflicts of interest or external funding sources for the research.
FDA investigating risk of gadolinium contrast agent brain deposits
The Food and Drug Administration is investigating the risk of brain deposits after recurring use of gadolinium-based contrast agents for MRI, the agency announced in a statement.
Studies suggest that gadolinium-based contrast agent (GBCA) deposits may stay in the brains of patients who have four or more contrast MRI scans, though it is unknown whether these deposits cause adverse effects, the FDA said.
GBCAs are usually expelled through the kidneys, but may remain in the brain after repeated exposure. FDA’s National Center for Toxicological
Research will further investigate safety risks in consultation with researchers and industry, the statement said.
The FDA is not requiring manufacturers to change the labels of GBCA products until more information is known. The agency is, however, recommending that clinicians limit GBCA use to situations in which it would be necessary for patient care.
“Health care professionals are also urged to reassess the necessity of repetitive GBCA MRIs in established treatment protocols,” the FDA said.
Patients may report side effects and adverse events to the FDA’s MedWatch Safety Information and Adverse Event Reporting Program.
The Food and Drug Administration is investigating the risk of brain deposits after recurring use of gadolinium-based contrast agents for MRI, the agency announced in a statement.
Studies suggest that gadolinium-based contrast agent (GBCA) deposits may stay in the brains of patients who have four or more contrast MRI scans, though it is unknown whether these deposits cause adverse effects, the FDA said.
GBCAs are usually expelled through the kidneys, but may remain in the brain after repeated exposure. FDA’s National Center for Toxicological
Research will further investigate safety risks in consultation with researchers and industry, the statement said.
The FDA is not requiring manufacturers to change the labels of GBCA products until more information is known. The agency is, however, recommending that clinicians limit GBCA use to situations in which it would be necessary for patient care.
“Health care professionals are also urged to reassess the necessity of repetitive GBCA MRIs in established treatment protocols,” the FDA said.
Patients may report side effects and adverse events to the FDA’s MedWatch Safety Information and Adverse Event Reporting Program.
The Food and Drug Administration is investigating the risk of brain deposits after recurring use of gadolinium-based contrast agents for MRI, the agency announced in a statement.
Studies suggest that gadolinium-based contrast agent (GBCA) deposits may stay in the brains of patients who have four or more contrast MRI scans, though it is unknown whether these deposits cause adverse effects, the FDA said.
GBCAs are usually expelled through the kidneys, but may remain in the brain after repeated exposure. FDA’s National Center for Toxicological
Research will further investigate safety risks in consultation with researchers and industry, the statement said.
The FDA is not requiring manufacturers to change the labels of GBCA products until more information is known. The agency is, however, recommending that clinicians limit GBCA use to situations in which it would be necessary for patient care.
“Health care professionals are also urged to reassess the necessity of repetitive GBCA MRIs in established treatment protocols,” the FDA said.
Patients may report side effects and adverse events to the FDA’s MedWatch Safety Information and Adverse Event Reporting Program.