User login
Radiographically Silent Loosening of the Acetabular Component in Hip Arthroplasty
Total hip arthroplasty (THA) is an excellent option for the treatment of osteoarthritis of the hip. In numerous studies, modern implants have shown survivorship of more than 90% at 10 years.1,2 Polyethylene wear and subsequent osteolysis are major obstacles to the long-term success of THA.3-5 Polyethylene wear particles are phagocytized by macrophages, inducing an inflammatory response that can ultimately lead to osteolysis of the bony architecture surrounding the bone–implant interface.6,7 As modern implants often rely on direct implant-to-bone ingrowth to maintain fixation, wear at this junction can lead to aseptic component loosening and ultimately require revision surgery.8-10 Osteolysis can be diagnosed with plain radiography or computed tomography (CT).11 CT is more sensitive than plain radiography for the diagnosis of osteolysis and is better able to determine the size and location of osteolytic lesions.12,13
Although diagnosis of osteolysis is well defined in the literature, what is more challenging is radiographic diagnosis of a loose acetabular component.11 The most commonly described criteria for loosening are presence of a complete radiolucent line of more than 2 mm in width at the bone–implant interface and any progressive tilting or migration of the component.14,15 CT-based criteria for component loosening remain largely undefined, though Egawa and colleagues16 showed that acetabular osteolysis involving less than 40% of the total cup surface is not associated with component loosening. Although a patient may show signs of osteolysis on postoperative imaging, this finding does not necessitate immediate revision surgery.17 Osteolysis may be monitored clinically and followed radiographically to determine when intervention is necessary.13
The goals of revision surgery are to eliminate the wear generator and bone-graft lytic lesions where needed to help maintain the bone–implant interface.17 The timing of such surgery is important, as the surgeon must balance the risk for gross component migration against the morbidity and mortality associated with acetabular component revision.18 This is in contrast to the settings of infection, periprosthetic fracture, recurrent instability, and component fracture/loosening, in which revision is urgently indicated and the case cannot be managed conservatively.
We conducted a study to determine the incidence of loose acetabular components without radiographic or clinical findings that would necessitate urgent revision THA. Radiographically silent loosening (RSL) was defined as an acetabular component that was loose at time of revision surgery but that did not show frank signs of loosening on either plain radiography or CT. Although these patients make up a small minority of the revision population, knowing the incidence of RSL can help raise surgeon awareness of this potentially dangerous situation. We further sought to determine whether patients with RSL present with different demographic characteristics or clinical symptoms than patients with stable acetabular components.
Materials and Methods
In this retrospective, case–control, institutional review board–approved study, we evaluated patients who had undergone revision THA and had preoperative plain radiographs and CT images. We identified patients by International Classification of Diseases, Ninth Revision (ICD‑9) codes (00.70, 00.71, 00.72, 00.73, 80.05, 81.53, 84.56, 84.57) and searched for those cases treated between 2000 and 2012.
Inclusion criteria were confirmed revision THA and confirmed plain radiography and CT of the THA performed before revision. When osteolysis was diagnosed by plain radiography, CT was ordered to determine the extent of bony lesions or to evaluate for eccentric head position or component malposition. Last, all patients included in the study had a detailed operative report clearly indicating acetabular component stability at time of revision. Acetabular component stability at time of surgery was determined according to the criteria defined by Berger and colleagues.19 Cups were evaluated for gross motion during both hip dislocation and during edge loading of the component after thorough scar and capsular débridement.
Patients who did not have CT performed before revision surgery were excluded from the study. These patients had been diagnosed by clinical history and/or plain radiography. Cases revised for periprosthetic infection or periprosthetic fracture were also excluded. Patients with metal-on-metal bearings were excluded, as were any cases revised from hemiarthroplasty to THA, as well as cases revised for recurrent dislocations or component malposition.
All plain radiographs and CT images were evaluated by the orthopedic surgeon who performed the revision and by a radiologist. Images were inspected for signs of gross component migration, tilting, and concentric lucency of the bone–implant interface. Patients with imaging that showed signs of component movement or migration (as seen by the attending surgeon or the radiologist) were excluded. Patients with radiographic evidence of femoral stem loosening were also excluded, as they had an indication to undergo revision arthroplasty. The remaining patients were then stratified into 2 groups: those with stable acetabular components at time of revision and those with loose acetabular components. Stable acetabular shells showed no gross motion of the implant with dislocation, edge loading with an impactor, or pulling with a Kocher clamp after débridement and screw removal.15,19 The 2 groups were then compared with respect to age, sex, and most common presenting symptoms and diagnoses. Fischer exact test and Student t test were used to statistically compare the groups.
Results
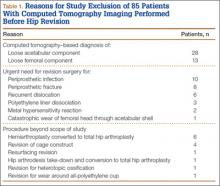
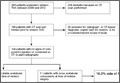
Overall, 393 patients underwent revision arthroplasty for the diagnoses (ICD-9 codes) indicated (Figure). One hundred eighty-nine patients (48.1%) had CT performed before revision. Of these 189 patients, 85 were excluded for diagnoses that were evident on either plain radiography or CT, that necessitated urgent revision, or for procedures beyond the scope of the study (Table 1). CT showed a loose cup in 28 patients; 6 of these cups were also seen on CT. Thirteen patients were diagnosed with a loose femoral stem, 10 with a periprosthetic infection, and 8 with a periprosthetic fracture.
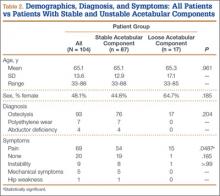
One hundred four patients (54 men, 50 women) met the study inclusion criteria. Mean age was 65.1 years. Of these 104 patients, 87 (83.7%) had a stable acetabular shell at time of revision surgery; the other 17 (16.3%) were diagnosed with RSL of the acetabular shell. Osteolysis was the most common diagnosis (89.4%) in the overall population, and pain was the most common complaint at time of presentation (66.6%). Lack of symptoms was the second most common presentation at time of revision (19.2%) (Table 2). Patients without symptoms underwent revision surgery because of concern about impending compromise of the bone–implant interface and progressive osteolysis.
The 2 groups (stable vs unstable acetabular shells) were not significantly different with respect to age (P = .961) or sex distribution (P = .185). All patients in the RSL group were diagnosed with osteolysis radiographically, and 15 (88%) of 17 patients presented with pain as the primary complaint, compared with only 54 (62%) of 87 patients in the group with stable shells. Patients with RSL were significantly more likely to present with pain as the primary complaint (P = .0487). Nineteen patients in the stable implant group and only 1 patient in the RSL group were asymptomatic, but this was not statistically significant (P = .185) when compared against all other diagnoses.
Discussion
We defined RSL as an acetabular component that was loose at time of revision surgery but that did not show frank signs of loosening on either plain radiography or CT. Patients with RSL and the surgeons who treat them are in a difficult position. In the setting of osteolysis, patients can be treated with serial radiographic imaging and clinical monitoring to determine if and when revision arthroplasty should be performed.17 However, given the complexity and risks associated with revision THA, surgeons should be aware that the acetabular shell may necessitate revision even if it does not appear to be frankly unstable on radiographic imaging.18
Of the 393 patients who underwent revision THA at our institution, 48.1% were evaluated with CT. Eighty-five of the 189 patients who underwent CT were diagnosed with radiographic loosening, or were diagnosed as needing urgent revision THA in the setting of loose components, periprosthetic infection, periprosthetic fracture, or catastrophic implant failure. Of the remaining 104 patients, 17 (16.3%) met the diagnosis of RSL of the acetabular component. The most common complaint was pain, and the most common diagnoses were osteolysis and polyethylene wear. Age and sex were not associated with increased likelihood of RSL.
Our study has several limitations. We defined the radiographic diagnosis of loose acetabular components as components showing frank migration, tilting, or a 2-mm concentric lucency on plain radiography or CT. Although these are common definitions of loose acetabular components, more sensitive radiographic measures have been described.16 We also excluded patients with recurrent dislocations and metal-on-metal prostheses, as these cases increase the metal artifact on CT and limit the ability to evaluate the bone–implant interface. Metal artifact remains an ongoing challenge to use of CT for post-THA imaging. However, tailored imaging protocols are helping to eliminate metal artifact. Bone scan was not used to evaluate for possible component loosening. Although sensitivity and specificity are about 67% and 76%, respectively,20 Temmerman and colleagues21 also found poor intraobserver reliability (0.53) for bone scans in the setting of uncemented acetabular components. Last, our study did not evaluate the bony ingrowth patterns that corresponded to stable or unstable fixation and did not evaluate the volumetric size or anatomical location of the osteolytic lesions on CT. Careful assessment of these variables is clinically relevant when trying to determine if revision arthroplasty is warranted.
Although we used relatively simple radiographic criteria to define loose components, more sensitive and specific techniques have been described for both plain radiography and CT. Moore and colleagues22 described 5 radiographic signs of bony ingrowth; when 3 or more were present, sensitivity was 89.6% and specificity 76.9%. Mehin and colleagues23 suggested that osteolysis involving more than 50% of the circumference of the shell on a standard pelvic radiograph might require revision arthroplasty. However, more recent studies have found that anteroposterior and lateral radiographs are less able to evaluate the anterior and posterior rims of the bone–implant interface, and it is this ingrowth area that may be the most crucial for stable osseointegration.12,16
CT has expanded our ability to evaluate the bone–implant interface in 3 dimensions. Egawa and colleagues16 described using CT to evaluate the surface area involved with osteolysis and found that less than 40% involvement of the surface area generally corresponded to well-fixed components. Furthermore, they found that osteolysis generally creates lesions inferior and superior to the acetabular component and less often involves the anterior and posterior rims, which may be more important for stable fixation. The authors noted that volumetric analysis and CT were not as cost-effective as plain radiography and were more time- and skill-intensive.
Osteolysis itself remains a common indication for revision THA. However, the most appropriate procedure remains controversial. Mallory and colleagues24 recommended explanting all acetabular shells in the setting of revision arthroplasty. They indicated that full assessment of the bony pelvis and any lytic defects was possible only with the wide exposure gained by acetabular component removal. More recent studies have begun to justify isolated component revision in the setting of well-fixed acetabular shells. Studies by Maloney and colleagues,10 Park and colleagues,15 and Beaulé and colleagues25 have shown excellent outcomes with retention of well-fixed acetabular shells and removal of the wear generator in the setting of osteolysis. Haidukewych17 wrote that the goals in addressing osteolysis in revision THA are to eliminate the wear generator, débride osteolytic lesions, and restore bone stock. Surgeons should weigh the benefits of component retention and isolated liner exchange against the morbidity associated with explantation and preparation for implanting a new component. Good outcomes have been achieved with isolated component exchange, but surgeons should be aware that instability remains the most common complication after isolated liner exchange.8
The majority of our patients with RSL presented with complaints of pain and the diagnosis of osteolysis. One patient who had the diagnosis but was clinically asymptomatic was found to have a loose acetabular shell at time of revision surgery. Given the increased morbidity associated with acetabular component revision, this patient’s condition represents a dangerous combination of RSL and clinically asymptomatic component loosening. By raising awareness about RSL and its incidence, we should be able to increase our ability to detect RSL. A surgeon who detects RSL before gross migration or movement of the acetabular component may be better able to plan for revision arthroplasty before a catastrophic event that may necessitate a larger, more complex procedure. With the number of patients who require revision THA continuing to rise, surgeons should be aware of the incidence of RSL and the potential of RSL to affect patient care and potential surgical options.
1. Milošev I, Kovač S, Trebše R, Levašič V, Pišot V. Comparison of ten-year survivorship of hip prostheses with use of conventional polyethylene, metal-on-metal, or ceramic-on-ceramic bearings. J Bone Joint Surg Am. 2012;94(19):1756-1763.
2. D’Antonio JA, Capello WN, Naughton M. Ceramic bearings for total hip arthroplasty have high survivorship at 10 years. Clin Orthop Relat Res. 2012;470(2):373-381.
3. Dowd JE, Sychterz CJ, Young AM, Engh CA. Characterization of long-term femoral-head-penetration rates. Association with and prediction of osteolysis. J Bone Joint Surg Am. 2000;82(8):1102-1107.
4. Orishimo KF, Claus AM, Sychterz CJ, Engh CA. Relationship between polyethylene wear and osteolysis in hips with a second-generation porous-coated cementless cup after seven years of follow-up. J Bone Joint Surg Am. 2003;85(6):1095-1099.
5. Harris WH. Wear and periprosthetic osteolysis: the problem. Clin Orthop Relat Res. 2001;(393):66-70.
6. Holt G, Murnaghan C, Reilly J, Meek RM. The biology of aseptic osteolysis. Clin Orthop Relat Res. 2007;(460):240-252.
7. Catelas I, Jacobs JJ. Biologic activity of wear particles. Instr Course Lect. 2010;59:3-16.
8. Paprosky WG, Nourbash P, Gill P. Treatment of progressive periacetabular osteolysis: cup revision versus liner exchange and bone grafting. Paper presented at: Annual Meeting of the American Academy of Orthopaedic Surgeons; February 4-8, 1999; Anaheim, CA.
9. Engh CA Jr, Claus AM, Hopper RH Jr, Engh CA. Long-term results using the anatomic medullary locking hip prosthesis. Clin Orthop Relat Res. 2001;(393):137-146.
10. Maloney WJ, Peters P, Engh CA, Chandler H. Severe osteolysis of the pelvic in association with acetabular replacement without cement. J Bone Joint Surg Am. 1993;75(11):1627-1635.
11. Claus AM, Engh CA Jr, Sychterz CJ, Xenos JS, Orishimo KF, Engh CA Sr. Radiographic definition of pelvic osteolysis following total hip arthroplasty. J Bone Joint Surg Am. 2003;85(8):1519-1526.
12. Puri L, Wixson RL, Stern SH, Kohli J, Hendrix RW, Stulberg SD. Use of helical computed tomography for the assessment of acetabular osteolysis after total hip arthroplasty. J Bone Joint Surg Am. 2002;84(4):609-614.
13. Stulberg SD, Wixson RL, Adams AD, Hendrix RW, Bernfield JB. Monitoring pelvic osteolysis following total hip replacement surgery: an algorithm for surveillance. J Bone Joint Surg Am. 2002;84(suppl 2):116-122.
14. Massin P, Schmidt L, Engh CA. Evaluation of cementless acetabular component migration. An experimental study. J Arthroplasty. 1989;4(3):245-251.
15. Park KS, Yoon TR, Song EK, Lee KB. Results of isolated femoral component revision with well-fixed acetabular implant retention. J Arthroplasty. 2010;25(8):1188-1195.
16. Egawa H, Ho H, Hopper RH Jr, Engh CA Jr, Engh CA. Computed tomography assessment of pelvic osteolysis and cup–lesion interface involvement with a press-fit porous-coated acetabular cup. J Arthroplasty. 2009;24(2):233-239.
17. Haidukewych GJ. Osteolysis in the well-fixed socket: cup retention or revision? J Bone Joint Surg Br. 2012;94(12):65-69.
18. Stulberg BN, Della Valle AG. What are the guidelines for the surgical and nonsurgical treatment of periprosthetic osteolysis? J Am Acad Orthop Surg. 2008;16(suppl 1):S20-S25.
19. Berger RA, Quigley LR, Jacobs JJ, Sheinkop MB, Rosenberg AG, Galante JO. The fate of stable cemented acetabular components retained during revision of a femoral component of a total hip arthroplasty. J Bone Joint Surg Am. 1999;81(12):1682-1691.
20. Temmerman OP, Raijmakers PG, Deville WL, Berkhof J, Hooft L, Heyligers IC. The use of plain radiography, subtraction arthrography, nuclear arthrography, and bone scintigraphy in the diagnosis of a loose acetabular component of a total hip prosthesis: a systematic review. J Arthroplasty. 2007;22(6):818-827.
21. Temmerman OP, Raijmakers PG, David EF, et al. A comparison of radiographic and scintigraphic techniques to assess aseptic loosening of the acetabular component in a total hip replacement. J Bone Joint Surg Am. 2004;86(11):2456-2463.
22. Moore MS, McAuley JP, Young AM, Engh CA Sr. Radiographic signs of osseointegration in porous-coated acetabular components. Clin Orthop Relat Res. 2006;(444):176-183.
23. Mehin R, Yuan X, Haydon C, et al. Retroacetabular osteolysis: when to operate? Clin Orthop Relat Res. 2004;(428):247-255.
24. Mallory TH, Lombardi AV Jr, Fada RA, Adams JB, Kefauver CA, Eberle RW. Noncemented acetabular component removal in the presence of osteolysis: the affirmative. Clin Orthop Relat Res. 2000;(381):120-128.
25. Beaulé PE, Le Duff MJ, Dorey FJ, Amstutz HC. Fate of cementless acetabular components retained during revision total hip arthroplasty. J Bone Joint Surg Am. 2003;85(12):2288-2293.
Total hip arthroplasty (THA) is an excellent option for the treatment of osteoarthritis of the hip. In numerous studies, modern implants have shown survivorship of more than 90% at 10 years.1,2 Polyethylene wear and subsequent osteolysis are major obstacles to the long-term success of THA.3-5 Polyethylene wear particles are phagocytized by macrophages, inducing an inflammatory response that can ultimately lead to osteolysis of the bony architecture surrounding the bone–implant interface.6,7 As modern implants often rely on direct implant-to-bone ingrowth to maintain fixation, wear at this junction can lead to aseptic component loosening and ultimately require revision surgery.8-10 Osteolysis can be diagnosed with plain radiography or computed tomography (CT).11 CT is more sensitive than plain radiography for the diagnosis of osteolysis and is better able to determine the size and location of osteolytic lesions.12,13
Although diagnosis of osteolysis is well defined in the literature, what is more challenging is radiographic diagnosis of a loose acetabular component.11 The most commonly described criteria for loosening are presence of a complete radiolucent line of more than 2 mm in width at the bone–implant interface and any progressive tilting or migration of the component.14,15 CT-based criteria for component loosening remain largely undefined, though Egawa and colleagues16 showed that acetabular osteolysis involving less than 40% of the total cup surface is not associated with component loosening. Although a patient may show signs of osteolysis on postoperative imaging, this finding does not necessitate immediate revision surgery.17 Osteolysis may be monitored clinically and followed radiographically to determine when intervention is necessary.13
The goals of revision surgery are to eliminate the wear generator and bone-graft lytic lesions where needed to help maintain the bone–implant interface.17 The timing of such surgery is important, as the surgeon must balance the risk for gross component migration against the morbidity and mortality associated with acetabular component revision.18 This is in contrast to the settings of infection, periprosthetic fracture, recurrent instability, and component fracture/loosening, in which revision is urgently indicated and the case cannot be managed conservatively.
We conducted a study to determine the incidence of loose acetabular components without radiographic or clinical findings that would necessitate urgent revision THA. Radiographically silent loosening (RSL) was defined as an acetabular component that was loose at time of revision surgery but that did not show frank signs of loosening on either plain radiography or CT. Although these patients make up a small minority of the revision population, knowing the incidence of RSL can help raise surgeon awareness of this potentially dangerous situation. We further sought to determine whether patients with RSL present with different demographic characteristics or clinical symptoms than patients with stable acetabular components.
Materials and Methods
In this retrospective, case–control, institutional review board–approved study, we evaluated patients who had undergone revision THA and had preoperative plain radiographs and CT images. We identified patients by International Classification of Diseases, Ninth Revision (ICD‑9) codes (00.70, 00.71, 00.72, 00.73, 80.05, 81.53, 84.56, 84.57) and searched for those cases treated between 2000 and 2012.
Inclusion criteria were confirmed revision THA and confirmed plain radiography and CT of the THA performed before revision. When osteolysis was diagnosed by plain radiography, CT was ordered to determine the extent of bony lesions or to evaluate for eccentric head position or component malposition. Last, all patients included in the study had a detailed operative report clearly indicating acetabular component stability at time of revision. Acetabular component stability at time of surgery was determined according to the criteria defined by Berger and colleagues.19 Cups were evaluated for gross motion during both hip dislocation and during edge loading of the component after thorough scar and capsular débridement.
Patients who did not have CT performed before revision surgery were excluded from the study. These patients had been diagnosed by clinical history and/or plain radiography. Cases revised for periprosthetic infection or periprosthetic fracture were also excluded. Patients with metal-on-metal bearings were excluded, as were any cases revised from hemiarthroplasty to THA, as well as cases revised for recurrent dislocations or component malposition.
All plain radiographs and CT images were evaluated by the orthopedic surgeon who performed the revision and by a radiologist. Images were inspected for signs of gross component migration, tilting, and concentric lucency of the bone–implant interface. Patients with imaging that showed signs of component movement or migration (as seen by the attending surgeon or the radiologist) were excluded. Patients with radiographic evidence of femoral stem loosening were also excluded, as they had an indication to undergo revision arthroplasty. The remaining patients were then stratified into 2 groups: those with stable acetabular components at time of revision and those with loose acetabular components. Stable acetabular shells showed no gross motion of the implant with dislocation, edge loading with an impactor, or pulling with a Kocher clamp after débridement and screw removal.15,19 The 2 groups were then compared with respect to age, sex, and most common presenting symptoms and diagnoses. Fischer exact test and Student t test were used to statistically compare the groups.
Results
Overall, 393 patients underwent revision arthroplasty for the diagnoses (ICD-9 codes) indicated (Figure). One hundred eighty-nine patients (48.1%) had CT performed before revision. Of these 189 patients, 85 were excluded for diagnoses that were evident on either plain radiography or CT, that necessitated urgent revision, or for procedures beyond the scope of the study (Table 1). CT showed a loose cup in 28 patients; 6 of these cups were also seen on CT. Thirteen patients were diagnosed with a loose femoral stem, 10 with a periprosthetic infection, and 8 with a periprosthetic fracture.
One hundred four patients (54 men, 50 women) met the study inclusion criteria. Mean age was 65.1 years. Of these 104 patients, 87 (83.7%) had a stable acetabular shell at time of revision surgery; the other 17 (16.3%) were diagnosed with RSL of the acetabular shell. Osteolysis was the most common diagnosis (89.4%) in the overall population, and pain was the most common complaint at time of presentation (66.6%). Lack of symptoms was the second most common presentation at time of revision (19.2%) (Table 2). Patients without symptoms underwent revision surgery because of concern about impending compromise of the bone–implant interface and progressive osteolysis.
The 2 groups (stable vs unstable acetabular shells) were not significantly different with respect to age (P = .961) or sex distribution (P = .185). All patients in the RSL group were diagnosed with osteolysis radiographically, and 15 (88%) of 17 patients presented with pain as the primary complaint, compared with only 54 (62%) of 87 patients in the group with stable shells. Patients with RSL were significantly more likely to present with pain as the primary complaint (P = .0487). Nineteen patients in the stable implant group and only 1 patient in the RSL group were asymptomatic, but this was not statistically significant (P = .185) when compared against all other diagnoses.
Discussion
We defined RSL as an acetabular component that was loose at time of revision surgery but that did not show frank signs of loosening on either plain radiography or CT. Patients with RSL and the surgeons who treat them are in a difficult position. In the setting of osteolysis, patients can be treated with serial radiographic imaging and clinical monitoring to determine if and when revision arthroplasty should be performed.17 However, given the complexity and risks associated with revision THA, surgeons should be aware that the acetabular shell may necessitate revision even if it does not appear to be frankly unstable on radiographic imaging.18
Of the 393 patients who underwent revision THA at our institution, 48.1% were evaluated with CT. Eighty-five of the 189 patients who underwent CT were diagnosed with radiographic loosening, or were diagnosed as needing urgent revision THA in the setting of loose components, periprosthetic infection, periprosthetic fracture, or catastrophic implant failure. Of the remaining 104 patients, 17 (16.3%) met the diagnosis of RSL of the acetabular component. The most common complaint was pain, and the most common diagnoses were osteolysis and polyethylene wear. Age and sex were not associated with increased likelihood of RSL.
Our study has several limitations. We defined the radiographic diagnosis of loose acetabular components as components showing frank migration, tilting, or a 2-mm concentric lucency on plain radiography or CT. Although these are common definitions of loose acetabular components, more sensitive radiographic measures have been described.16 We also excluded patients with recurrent dislocations and metal-on-metal prostheses, as these cases increase the metal artifact on CT and limit the ability to evaluate the bone–implant interface. Metal artifact remains an ongoing challenge to use of CT for post-THA imaging. However, tailored imaging protocols are helping to eliminate metal artifact. Bone scan was not used to evaluate for possible component loosening. Although sensitivity and specificity are about 67% and 76%, respectively,20 Temmerman and colleagues21 also found poor intraobserver reliability (0.53) for bone scans in the setting of uncemented acetabular components. Last, our study did not evaluate the bony ingrowth patterns that corresponded to stable or unstable fixation and did not evaluate the volumetric size or anatomical location of the osteolytic lesions on CT. Careful assessment of these variables is clinically relevant when trying to determine if revision arthroplasty is warranted.
Although we used relatively simple radiographic criteria to define loose components, more sensitive and specific techniques have been described for both plain radiography and CT. Moore and colleagues22 described 5 radiographic signs of bony ingrowth; when 3 or more were present, sensitivity was 89.6% and specificity 76.9%. Mehin and colleagues23 suggested that osteolysis involving more than 50% of the circumference of the shell on a standard pelvic radiograph might require revision arthroplasty. However, more recent studies have found that anteroposterior and lateral radiographs are less able to evaluate the anterior and posterior rims of the bone–implant interface, and it is this ingrowth area that may be the most crucial for stable osseointegration.12,16
CT has expanded our ability to evaluate the bone–implant interface in 3 dimensions. Egawa and colleagues16 described using CT to evaluate the surface area involved with osteolysis and found that less than 40% involvement of the surface area generally corresponded to well-fixed components. Furthermore, they found that osteolysis generally creates lesions inferior and superior to the acetabular component and less often involves the anterior and posterior rims, which may be more important for stable fixation. The authors noted that volumetric analysis and CT were not as cost-effective as plain radiography and were more time- and skill-intensive.
Osteolysis itself remains a common indication for revision THA. However, the most appropriate procedure remains controversial. Mallory and colleagues24 recommended explanting all acetabular shells in the setting of revision arthroplasty. They indicated that full assessment of the bony pelvis and any lytic defects was possible only with the wide exposure gained by acetabular component removal. More recent studies have begun to justify isolated component revision in the setting of well-fixed acetabular shells. Studies by Maloney and colleagues,10 Park and colleagues,15 and Beaulé and colleagues25 have shown excellent outcomes with retention of well-fixed acetabular shells and removal of the wear generator in the setting of osteolysis. Haidukewych17 wrote that the goals in addressing osteolysis in revision THA are to eliminate the wear generator, débride osteolytic lesions, and restore bone stock. Surgeons should weigh the benefits of component retention and isolated liner exchange against the morbidity associated with explantation and preparation for implanting a new component. Good outcomes have been achieved with isolated component exchange, but surgeons should be aware that instability remains the most common complication after isolated liner exchange.8
The majority of our patients with RSL presented with complaints of pain and the diagnosis of osteolysis. One patient who had the diagnosis but was clinically asymptomatic was found to have a loose acetabular shell at time of revision surgery. Given the increased morbidity associated with acetabular component revision, this patient’s condition represents a dangerous combination of RSL and clinically asymptomatic component loosening. By raising awareness about RSL and its incidence, we should be able to increase our ability to detect RSL. A surgeon who detects RSL before gross migration or movement of the acetabular component may be better able to plan for revision arthroplasty before a catastrophic event that may necessitate a larger, more complex procedure. With the number of patients who require revision THA continuing to rise, surgeons should be aware of the incidence of RSL and the potential of RSL to affect patient care and potential surgical options.
Total hip arthroplasty (THA) is an excellent option for the treatment of osteoarthritis of the hip. In numerous studies, modern implants have shown survivorship of more than 90% at 10 years.1,2 Polyethylene wear and subsequent osteolysis are major obstacles to the long-term success of THA.3-5 Polyethylene wear particles are phagocytized by macrophages, inducing an inflammatory response that can ultimately lead to osteolysis of the bony architecture surrounding the bone–implant interface.6,7 As modern implants often rely on direct implant-to-bone ingrowth to maintain fixation, wear at this junction can lead to aseptic component loosening and ultimately require revision surgery.8-10 Osteolysis can be diagnosed with plain radiography or computed tomography (CT).11 CT is more sensitive than plain radiography for the diagnosis of osteolysis and is better able to determine the size and location of osteolytic lesions.12,13
Although diagnosis of osteolysis is well defined in the literature, what is more challenging is radiographic diagnosis of a loose acetabular component.11 The most commonly described criteria for loosening are presence of a complete radiolucent line of more than 2 mm in width at the bone–implant interface and any progressive tilting or migration of the component.14,15 CT-based criteria for component loosening remain largely undefined, though Egawa and colleagues16 showed that acetabular osteolysis involving less than 40% of the total cup surface is not associated with component loosening. Although a patient may show signs of osteolysis on postoperative imaging, this finding does not necessitate immediate revision surgery.17 Osteolysis may be monitored clinically and followed radiographically to determine when intervention is necessary.13
The goals of revision surgery are to eliminate the wear generator and bone-graft lytic lesions where needed to help maintain the bone–implant interface.17 The timing of such surgery is important, as the surgeon must balance the risk for gross component migration against the morbidity and mortality associated with acetabular component revision.18 This is in contrast to the settings of infection, periprosthetic fracture, recurrent instability, and component fracture/loosening, in which revision is urgently indicated and the case cannot be managed conservatively.
We conducted a study to determine the incidence of loose acetabular components without radiographic or clinical findings that would necessitate urgent revision THA. Radiographically silent loosening (RSL) was defined as an acetabular component that was loose at time of revision surgery but that did not show frank signs of loosening on either plain radiography or CT. Although these patients make up a small minority of the revision population, knowing the incidence of RSL can help raise surgeon awareness of this potentially dangerous situation. We further sought to determine whether patients with RSL present with different demographic characteristics or clinical symptoms than patients with stable acetabular components.
Materials and Methods
In this retrospective, case–control, institutional review board–approved study, we evaluated patients who had undergone revision THA and had preoperative plain radiographs and CT images. We identified patients by International Classification of Diseases, Ninth Revision (ICD‑9) codes (00.70, 00.71, 00.72, 00.73, 80.05, 81.53, 84.56, 84.57) and searched for those cases treated between 2000 and 2012.
Inclusion criteria were confirmed revision THA and confirmed plain radiography and CT of the THA performed before revision. When osteolysis was diagnosed by plain radiography, CT was ordered to determine the extent of bony lesions or to evaluate for eccentric head position or component malposition. Last, all patients included in the study had a detailed operative report clearly indicating acetabular component stability at time of revision. Acetabular component stability at time of surgery was determined according to the criteria defined by Berger and colleagues.19 Cups were evaluated for gross motion during both hip dislocation and during edge loading of the component after thorough scar and capsular débridement.
Patients who did not have CT performed before revision surgery were excluded from the study. These patients had been diagnosed by clinical history and/or plain radiography. Cases revised for periprosthetic infection or periprosthetic fracture were also excluded. Patients with metal-on-metal bearings were excluded, as were any cases revised from hemiarthroplasty to THA, as well as cases revised for recurrent dislocations or component malposition.
All plain radiographs and CT images were evaluated by the orthopedic surgeon who performed the revision and by a radiologist. Images were inspected for signs of gross component migration, tilting, and concentric lucency of the bone–implant interface. Patients with imaging that showed signs of component movement or migration (as seen by the attending surgeon or the radiologist) were excluded. Patients with radiographic evidence of femoral stem loosening were also excluded, as they had an indication to undergo revision arthroplasty. The remaining patients were then stratified into 2 groups: those with stable acetabular components at time of revision and those with loose acetabular components. Stable acetabular shells showed no gross motion of the implant with dislocation, edge loading with an impactor, or pulling with a Kocher clamp after débridement and screw removal.15,19 The 2 groups were then compared with respect to age, sex, and most common presenting symptoms and diagnoses. Fischer exact test and Student t test were used to statistically compare the groups.
Results
Overall, 393 patients underwent revision arthroplasty for the diagnoses (ICD-9 codes) indicated (Figure). One hundred eighty-nine patients (48.1%) had CT performed before revision. Of these 189 patients, 85 were excluded for diagnoses that were evident on either plain radiography or CT, that necessitated urgent revision, or for procedures beyond the scope of the study (Table 1). CT showed a loose cup in 28 patients; 6 of these cups were also seen on CT. Thirteen patients were diagnosed with a loose femoral stem, 10 with a periprosthetic infection, and 8 with a periprosthetic fracture.
One hundred four patients (54 men, 50 women) met the study inclusion criteria. Mean age was 65.1 years. Of these 104 patients, 87 (83.7%) had a stable acetabular shell at time of revision surgery; the other 17 (16.3%) were diagnosed with RSL of the acetabular shell. Osteolysis was the most common diagnosis (89.4%) in the overall population, and pain was the most common complaint at time of presentation (66.6%). Lack of symptoms was the second most common presentation at time of revision (19.2%) (Table 2). Patients without symptoms underwent revision surgery because of concern about impending compromise of the bone–implant interface and progressive osteolysis.
The 2 groups (stable vs unstable acetabular shells) were not significantly different with respect to age (P = .961) or sex distribution (P = .185). All patients in the RSL group were diagnosed with osteolysis radiographically, and 15 (88%) of 17 patients presented with pain as the primary complaint, compared with only 54 (62%) of 87 patients in the group with stable shells. Patients with RSL were significantly more likely to present with pain as the primary complaint (P = .0487). Nineteen patients in the stable implant group and only 1 patient in the RSL group were asymptomatic, but this was not statistically significant (P = .185) when compared against all other diagnoses.
Discussion
We defined RSL as an acetabular component that was loose at time of revision surgery but that did not show frank signs of loosening on either plain radiography or CT. Patients with RSL and the surgeons who treat them are in a difficult position. In the setting of osteolysis, patients can be treated with serial radiographic imaging and clinical monitoring to determine if and when revision arthroplasty should be performed.17 However, given the complexity and risks associated with revision THA, surgeons should be aware that the acetabular shell may necessitate revision even if it does not appear to be frankly unstable on radiographic imaging.18
Of the 393 patients who underwent revision THA at our institution, 48.1% were evaluated with CT. Eighty-five of the 189 patients who underwent CT were diagnosed with radiographic loosening, or were diagnosed as needing urgent revision THA in the setting of loose components, periprosthetic infection, periprosthetic fracture, or catastrophic implant failure. Of the remaining 104 patients, 17 (16.3%) met the diagnosis of RSL of the acetabular component. The most common complaint was pain, and the most common diagnoses were osteolysis and polyethylene wear. Age and sex were not associated with increased likelihood of RSL.
Our study has several limitations. We defined the radiographic diagnosis of loose acetabular components as components showing frank migration, tilting, or a 2-mm concentric lucency on plain radiography or CT. Although these are common definitions of loose acetabular components, more sensitive radiographic measures have been described.16 We also excluded patients with recurrent dislocations and metal-on-metal prostheses, as these cases increase the metal artifact on CT and limit the ability to evaluate the bone–implant interface. Metal artifact remains an ongoing challenge to use of CT for post-THA imaging. However, tailored imaging protocols are helping to eliminate metal artifact. Bone scan was not used to evaluate for possible component loosening. Although sensitivity and specificity are about 67% and 76%, respectively,20 Temmerman and colleagues21 also found poor intraobserver reliability (0.53) for bone scans in the setting of uncemented acetabular components. Last, our study did not evaluate the bony ingrowth patterns that corresponded to stable or unstable fixation and did not evaluate the volumetric size or anatomical location of the osteolytic lesions on CT. Careful assessment of these variables is clinically relevant when trying to determine if revision arthroplasty is warranted.
Although we used relatively simple radiographic criteria to define loose components, more sensitive and specific techniques have been described for both plain radiography and CT. Moore and colleagues22 described 5 radiographic signs of bony ingrowth; when 3 or more were present, sensitivity was 89.6% and specificity 76.9%. Mehin and colleagues23 suggested that osteolysis involving more than 50% of the circumference of the shell on a standard pelvic radiograph might require revision arthroplasty. However, more recent studies have found that anteroposterior and lateral radiographs are less able to evaluate the anterior and posterior rims of the bone–implant interface, and it is this ingrowth area that may be the most crucial for stable osseointegration.12,16
CT has expanded our ability to evaluate the bone–implant interface in 3 dimensions. Egawa and colleagues16 described using CT to evaluate the surface area involved with osteolysis and found that less than 40% involvement of the surface area generally corresponded to well-fixed components. Furthermore, they found that osteolysis generally creates lesions inferior and superior to the acetabular component and less often involves the anterior and posterior rims, which may be more important for stable fixation. The authors noted that volumetric analysis and CT were not as cost-effective as plain radiography and were more time- and skill-intensive.
Osteolysis itself remains a common indication for revision THA. However, the most appropriate procedure remains controversial. Mallory and colleagues24 recommended explanting all acetabular shells in the setting of revision arthroplasty. They indicated that full assessment of the bony pelvis and any lytic defects was possible only with the wide exposure gained by acetabular component removal. More recent studies have begun to justify isolated component revision in the setting of well-fixed acetabular shells. Studies by Maloney and colleagues,10 Park and colleagues,15 and Beaulé and colleagues25 have shown excellent outcomes with retention of well-fixed acetabular shells and removal of the wear generator in the setting of osteolysis. Haidukewych17 wrote that the goals in addressing osteolysis in revision THA are to eliminate the wear generator, débride osteolytic lesions, and restore bone stock. Surgeons should weigh the benefits of component retention and isolated liner exchange against the morbidity associated with explantation and preparation for implanting a new component. Good outcomes have been achieved with isolated component exchange, but surgeons should be aware that instability remains the most common complication after isolated liner exchange.8
The majority of our patients with RSL presented with complaints of pain and the diagnosis of osteolysis. One patient who had the diagnosis but was clinically asymptomatic was found to have a loose acetabular shell at time of revision surgery. Given the increased morbidity associated with acetabular component revision, this patient’s condition represents a dangerous combination of RSL and clinically asymptomatic component loosening. By raising awareness about RSL and its incidence, we should be able to increase our ability to detect RSL. A surgeon who detects RSL before gross migration or movement of the acetabular component may be better able to plan for revision arthroplasty before a catastrophic event that may necessitate a larger, more complex procedure. With the number of patients who require revision THA continuing to rise, surgeons should be aware of the incidence of RSL and the potential of RSL to affect patient care and potential surgical options.
1. Milošev I, Kovač S, Trebše R, Levašič V, Pišot V. Comparison of ten-year survivorship of hip prostheses with use of conventional polyethylene, metal-on-metal, or ceramic-on-ceramic bearings. J Bone Joint Surg Am. 2012;94(19):1756-1763.
2. D’Antonio JA, Capello WN, Naughton M. Ceramic bearings for total hip arthroplasty have high survivorship at 10 years. Clin Orthop Relat Res. 2012;470(2):373-381.
3. Dowd JE, Sychterz CJ, Young AM, Engh CA. Characterization of long-term femoral-head-penetration rates. Association with and prediction of osteolysis. J Bone Joint Surg Am. 2000;82(8):1102-1107.
4. Orishimo KF, Claus AM, Sychterz CJ, Engh CA. Relationship between polyethylene wear and osteolysis in hips with a second-generation porous-coated cementless cup after seven years of follow-up. J Bone Joint Surg Am. 2003;85(6):1095-1099.
5. Harris WH. Wear and periprosthetic osteolysis: the problem. Clin Orthop Relat Res. 2001;(393):66-70.
6. Holt G, Murnaghan C, Reilly J, Meek RM. The biology of aseptic osteolysis. Clin Orthop Relat Res. 2007;(460):240-252.
7. Catelas I, Jacobs JJ. Biologic activity of wear particles. Instr Course Lect. 2010;59:3-16.
8. Paprosky WG, Nourbash P, Gill P. Treatment of progressive periacetabular osteolysis: cup revision versus liner exchange and bone grafting. Paper presented at: Annual Meeting of the American Academy of Orthopaedic Surgeons; February 4-8, 1999; Anaheim, CA.
9. Engh CA Jr, Claus AM, Hopper RH Jr, Engh CA. Long-term results using the anatomic medullary locking hip prosthesis. Clin Orthop Relat Res. 2001;(393):137-146.
10. Maloney WJ, Peters P, Engh CA, Chandler H. Severe osteolysis of the pelvic in association with acetabular replacement without cement. J Bone Joint Surg Am. 1993;75(11):1627-1635.
11. Claus AM, Engh CA Jr, Sychterz CJ, Xenos JS, Orishimo KF, Engh CA Sr. Radiographic definition of pelvic osteolysis following total hip arthroplasty. J Bone Joint Surg Am. 2003;85(8):1519-1526.
12. Puri L, Wixson RL, Stern SH, Kohli J, Hendrix RW, Stulberg SD. Use of helical computed tomography for the assessment of acetabular osteolysis after total hip arthroplasty. J Bone Joint Surg Am. 2002;84(4):609-614.
13. Stulberg SD, Wixson RL, Adams AD, Hendrix RW, Bernfield JB. Monitoring pelvic osteolysis following total hip replacement surgery: an algorithm for surveillance. J Bone Joint Surg Am. 2002;84(suppl 2):116-122.
14. Massin P, Schmidt L, Engh CA. Evaluation of cementless acetabular component migration. An experimental study. J Arthroplasty. 1989;4(3):245-251.
15. Park KS, Yoon TR, Song EK, Lee KB. Results of isolated femoral component revision with well-fixed acetabular implant retention. J Arthroplasty. 2010;25(8):1188-1195.
16. Egawa H, Ho H, Hopper RH Jr, Engh CA Jr, Engh CA. Computed tomography assessment of pelvic osteolysis and cup–lesion interface involvement with a press-fit porous-coated acetabular cup. J Arthroplasty. 2009;24(2):233-239.
17. Haidukewych GJ. Osteolysis in the well-fixed socket: cup retention or revision? J Bone Joint Surg Br. 2012;94(12):65-69.
18. Stulberg BN, Della Valle AG. What are the guidelines for the surgical and nonsurgical treatment of periprosthetic osteolysis? J Am Acad Orthop Surg. 2008;16(suppl 1):S20-S25.
19. Berger RA, Quigley LR, Jacobs JJ, Sheinkop MB, Rosenberg AG, Galante JO. The fate of stable cemented acetabular components retained during revision of a femoral component of a total hip arthroplasty. J Bone Joint Surg Am. 1999;81(12):1682-1691.
20. Temmerman OP, Raijmakers PG, Deville WL, Berkhof J, Hooft L, Heyligers IC. The use of plain radiography, subtraction arthrography, nuclear arthrography, and bone scintigraphy in the diagnosis of a loose acetabular component of a total hip prosthesis: a systematic review. J Arthroplasty. 2007;22(6):818-827.
21. Temmerman OP, Raijmakers PG, David EF, et al. A comparison of radiographic and scintigraphic techniques to assess aseptic loosening of the acetabular component in a total hip replacement. J Bone Joint Surg Am. 2004;86(11):2456-2463.
22. Moore MS, McAuley JP, Young AM, Engh CA Sr. Radiographic signs of osseointegration in porous-coated acetabular components. Clin Orthop Relat Res. 2006;(444):176-183.
23. Mehin R, Yuan X, Haydon C, et al. Retroacetabular osteolysis: when to operate? Clin Orthop Relat Res. 2004;(428):247-255.
24. Mallory TH, Lombardi AV Jr, Fada RA, Adams JB, Kefauver CA, Eberle RW. Noncemented acetabular component removal in the presence of osteolysis: the affirmative. Clin Orthop Relat Res. 2000;(381):120-128.
25. Beaulé PE, Le Duff MJ, Dorey FJ, Amstutz HC. Fate of cementless acetabular components retained during revision total hip arthroplasty. J Bone Joint Surg Am. 2003;85(12):2288-2293.
1. Milošev I, Kovač S, Trebše R, Levašič V, Pišot V. Comparison of ten-year survivorship of hip prostheses with use of conventional polyethylene, metal-on-metal, or ceramic-on-ceramic bearings. J Bone Joint Surg Am. 2012;94(19):1756-1763.
2. D’Antonio JA, Capello WN, Naughton M. Ceramic bearings for total hip arthroplasty have high survivorship at 10 years. Clin Orthop Relat Res. 2012;470(2):373-381.
3. Dowd JE, Sychterz CJ, Young AM, Engh CA. Characterization of long-term femoral-head-penetration rates. Association with and prediction of osteolysis. J Bone Joint Surg Am. 2000;82(8):1102-1107.
4. Orishimo KF, Claus AM, Sychterz CJ, Engh CA. Relationship between polyethylene wear and osteolysis in hips with a second-generation porous-coated cementless cup after seven years of follow-up. J Bone Joint Surg Am. 2003;85(6):1095-1099.
5. Harris WH. Wear and periprosthetic osteolysis: the problem. Clin Orthop Relat Res. 2001;(393):66-70.
6. Holt G, Murnaghan C, Reilly J, Meek RM. The biology of aseptic osteolysis. Clin Orthop Relat Res. 2007;(460):240-252.
7. Catelas I, Jacobs JJ. Biologic activity of wear particles. Instr Course Lect. 2010;59:3-16.
8. Paprosky WG, Nourbash P, Gill P. Treatment of progressive periacetabular osteolysis: cup revision versus liner exchange and bone grafting. Paper presented at: Annual Meeting of the American Academy of Orthopaedic Surgeons; February 4-8, 1999; Anaheim, CA.
9. Engh CA Jr, Claus AM, Hopper RH Jr, Engh CA. Long-term results using the anatomic medullary locking hip prosthesis. Clin Orthop Relat Res. 2001;(393):137-146.
10. Maloney WJ, Peters P, Engh CA, Chandler H. Severe osteolysis of the pelvic in association with acetabular replacement without cement. J Bone Joint Surg Am. 1993;75(11):1627-1635.
11. Claus AM, Engh CA Jr, Sychterz CJ, Xenos JS, Orishimo KF, Engh CA Sr. Radiographic definition of pelvic osteolysis following total hip arthroplasty. J Bone Joint Surg Am. 2003;85(8):1519-1526.
12. Puri L, Wixson RL, Stern SH, Kohli J, Hendrix RW, Stulberg SD. Use of helical computed tomography for the assessment of acetabular osteolysis after total hip arthroplasty. J Bone Joint Surg Am. 2002;84(4):609-614.
13. Stulberg SD, Wixson RL, Adams AD, Hendrix RW, Bernfield JB. Monitoring pelvic osteolysis following total hip replacement surgery: an algorithm for surveillance. J Bone Joint Surg Am. 2002;84(suppl 2):116-122.
14. Massin P, Schmidt L, Engh CA. Evaluation of cementless acetabular component migration. An experimental study. J Arthroplasty. 1989;4(3):245-251.
15. Park KS, Yoon TR, Song EK, Lee KB. Results of isolated femoral component revision with well-fixed acetabular implant retention. J Arthroplasty. 2010;25(8):1188-1195.
16. Egawa H, Ho H, Hopper RH Jr, Engh CA Jr, Engh CA. Computed tomography assessment of pelvic osteolysis and cup–lesion interface involvement with a press-fit porous-coated acetabular cup. J Arthroplasty. 2009;24(2):233-239.
17. Haidukewych GJ. Osteolysis in the well-fixed socket: cup retention or revision? J Bone Joint Surg Br. 2012;94(12):65-69.
18. Stulberg BN, Della Valle AG. What are the guidelines for the surgical and nonsurgical treatment of periprosthetic osteolysis? J Am Acad Orthop Surg. 2008;16(suppl 1):S20-S25.
19. Berger RA, Quigley LR, Jacobs JJ, Sheinkop MB, Rosenberg AG, Galante JO. The fate of stable cemented acetabular components retained during revision of a femoral component of a total hip arthroplasty. J Bone Joint Surg Am. 1999;81(12):1682-1691.
20. Temmerman OP, Raijmakers PG, Deville WL, Berkhof J, Hooft L, Heyligers IC. The use of plain radiography, subtraction arthrography, nuclear arthrography, and bone scintigraphy in the diagnosis of a loose acetabular component of a total hip prosthesis: a systematic review. J Arthroplasty. 2007;22(6):818-827.
21. Temmerman OP, Raijmakers PG, David EF, et al. A comparison of radiographic and scintigraphic techniques to assess aseptic loosening of the acetabular component in a total hip replacement. J Bone Joint Surg Am. 2004;86(11):2456-2463.
22. Moore MS, McAuley JP, Young AM, Engh CA Sr. Radiographic signs of osseointegration in porous-coated acetabular components. Clin Orthop Relat Res. 2006;(444):176-183.
23. Mehin R, Yuan X, Haydon C, et al. Retroacetabular osteolysis: when to operate? Clin Orthop Relat Res. 2004;(428):247-255.
24. Mallory TH, Lombardi AV Jr, Fada RA, Adams JB, Kefauver CA, Eberle RW. Noncemented acetabular component removal in the presence of osteolysis: the affirmative. Clin Orthop Relat Res. 2000;(381):120-128.
25. Beaulé PE, Le Duff MJ, Dorey FJ, Amstutz HC. Fate of cementless acetabular components retained during revision total hip arthroplasty. J Bone Joint Surg Am. 2003;85(12):2288-2293.
CT scan utilization down in children’s hospitals
The use of computed tomography in children’s hospitals appears to be on the decline across the top 10 all-patient-refined, diagnosis-related groups (APR-DRGs), with alternate imaging processes increasing in utilization for 8 of those 10 groups.
New research examining the use of CT scans on children admitted to 33 pediatric tertiary-care hospitals participating in the Pediatric Health Information System between Jan. 1, 2004, and Dec. 31, 2012, reveals the decrease (Pediatrics 2015 Aug 24.[doi: 10.1542/peds.2015-0995]).
“This decrease occurred with a concomitant increase in alternative imaging modalities in 8 studied diagnostic groups, supporting the hypothesis that previously noted declines in CT utilization are associated with shifts to alternate modalities,” Dr. Michelle W. Parker of Children’s Hospital Medical Center in Cincinnati and her colleagues wrote.
The 10 APR-DRGs studies are:
• seizure.
• ventricular shunt procedures.
• appendectomy.
• craniotomy except for trauma.
• concussion, closed skull fracture, uncomplicated intracranial injury, coma less than 1 hour or no coma (concussion).
• head trauma with coma greater than 1 hour or hemorrhage (severe head trauma).
• infections of upper respiratory tract.
• nonbacterial gastroenteritis with nausea and vomiting (gastroenteritis).
• abdominal pain.
• other ear, nose, mouth, throat, and craniofacial diagnoses.
“The decrease was most noted for patients with an APR-DRG of seizure where CT utilization decreased almost 50%, and MRI utilization decreased by greater than 10%,” the authors wrote.
Severe head trauma had the largest reduction in CT utilization. “MRI utilization could not be evaluated for appendectomy, gastroenteritis, or infections of the upper respiratory tract because of limited study volume. Statistically significant changes in MRI use were noted across the remaining APR-DRGs,with marked increases for ventricular shunt procedures,” Dr. Parker and her colleagues reported.
“There were no ultrasound studies meeting inclusion criteria for seizures, concussion, or severe head trauma. Ultrasound use increased during the study period for all other APR-DRGs, with the greatest increase noted in appendectomy and gastroenteritis,” they said.
Dr. Parker and her colleagues suggested a number of reasons for the decrease, including a 2006 effort by the Alliance for Radiation Safety in Pediatric Imaging, an initiative within the Society for Pediatric Radiology that raised awareness on the number of pediatric CT scans and the use of adult dose protocols in pediatric imaging. They also mentioned other technological advances, such as electronic health records and the sharing of existing images, which may have contributed to the lower utilization of CT scans.
The authors note that the decrease does have public health implications, as lower CT utilization decreases exposure to hazardous ionizing radiation.“Therefore, the substitution of an imaging modality that does not confer ionizing radiation may affect lifetime cancer risk in children who receive diagnostic imaging.”
Dr. Parker and her colleagues reported no conflicts of interest or external funding sources for the research.
The use of computed tomography in children’s hospitals appears to be on the decline across the top 10 all-patient-refined, diagnosis-related groups (APR-DRGs), with alternate imaging processes increasing in utilization for 8 of those 10 groups.
New research examining the use of CT scans on children admitted to 33 pediatric tertiary-care hospitals participating in the Pediatric Health Information System between Jan. 1, 2004, and Dec. 31, 2012, reveals the decrease (Pediatrics 2015 Aug 24.[doi: 10.1542/peds.2015-0995]).
“This decrease occurred with a concomitant increase in alternative imaging modalities in 8 studied diagnostic groups, supporting the hypothesis that previously noted declines in CT utilization are associated with shifts to alternate modalities,” Dr. Michelle W. Parker of Children’s Hospital Medical Center in Cincinnati and her colleagues wrote.
The 10 APR-DRGs studies are:
• seizure.
• ventricular shunt procedures.
• appendectomy.
• craniotomy except for trauma.
• concussion, closed skull fracture, uncomplicated intracranial injury, coma less than 1 hour or no coma (concussion).
• head trauma with coma greater than 1 hour or hemorrhage (severe head trauma).
• infections of upper respiratory tract.
• nonbacterial gastroenteritis with nausea and vomiting (gastroenteritis).
• abdominal pain.
• other ear, nose, mouth, throat, and craniofacial diagnoses.
“The decrease was most noted for patients with an APR-DRG of seizure where CT utilization decreased almost 50%, and MRI utilization decreased by greater than 10%,” the authors wrote.
Severe head trauma had the largest reduction in CT utilization. “MRI utilization could not be evaluated for appendectomy, gastroenteritis, or infections of the upper respiratory tract because of limited study volume. Statistically significant changes in MRI use were noted across the remaining APR-DRGs,with marked increases for ventricular shunt procedures,” Dr. Parker and her colleagues reported.
“There were no ultrasound studies meeting inclusion criteria for seizures, concussion, or severe head trauma. Ultrasound use increased during the study period for all other APR-DRGs, with the greatest increase noted in appendectomy and gastroenteritis,” they said.
Dr. Parker and her colleagues suggested a number of reasons for the decrease, including a 2006 effort by the Alliance for Radiation Safety in Pediatric Imaging, an initiative within the Society for Pediatric Radiology that raised awareness on the number of pediatric CT scans and the use of adult dose protocols in pediatric imaging. They also mentioned other technological advances, such as electronic health records and the sharing of existing images, which may have contributed to the lower utilization of CT scans.
The authors note that the decrease does have public health implications, as lower CT utilization decreases exposure to hazardous ionizing radiation.“Therefore, the substitution of an imaging modality that does not confer ionizing radiation may affect lifetime cancer risk in children who receive diagnostic imaging.”
Dr. Parker and her colleagues reported no conflicts of interest or external funding sources for the research.
The use of computed tomography in children’s hospitals appears to be on the decline across the top 10 all-patient-refined, diagnosis-related groups (APR-DRGs), with alternate imaging processes increasing in utilization for 8 of those 10 groups.
New research examining the use of CT scans on children admitted to 33 pediatric tertiary-care hospitals participating in the Pediatric Health Information System between Jan. 1, 2004, and Dec. 31, 2012, reveals the decrease (Pediatrics 2015 Aug 24.[doi: 10.1542/peds.2015-0995]).
“This decrease occurred with a concomitant increase in alternative imaging modalities in 8 studied diagnostic groups, supporting the hypothesis that previously noted declines in CT utilization are associated with shifts to alternate modalities,” Dr. Michelle W. Parker of Children’s Hospital Medical Center in Cincinnati and her colleagues wrote.
The 10 APR-DRGs studies are:
• seizure.
• ventricular shunt procedures.
• appendectomy.
• craniotomy except for trauma.
• concussion, closed skull fracture, uncomplicated intracranial injury, coma less than 1 hour or no coma (concussion).
• head trauma with coma greater than 1 hour or hemorrhage (severe head trauma).
• infections of upper respiratory tract.
• nonbacterial gastroenteritis with nausea and vomiting (gastroenteritis).
• abdominal pain.
• other ear, nose, mouth, throat, and craniofacial diagnoses.
“The decrease was most noted for patients with an APR-DRG of seizure where CT utilization decreased almost 50%, and MRI utilization decreased by greater than 10%,” the authors wrote.
Severe head trauma had the largest reduction in CT utilization. “MRI utilization could not be evaluated for appendectomy, gastroenteritis, or infections of the upper respiratory tract because of limited study volume. Statistically significant changes in MRI use were noted across the remaining APR-DRGs,with marked increases for ventricular shunt procedures,” Dr. Parker and her colleagues reported.
“There were no ultrasound studies meeting inclusion criteria for seizures, concussion, or severe head trauma. Ultrasound use increased during the study period for all other APR-DRGs, with the greatest increase noted in appendectomy and gastroenteritis,” they said.
Dr. Parker and her colleagues suggested a number of reasons for the decrease, including a 2006 effort by the Alliance for Radiation Safety in Pediatric Imaging, an initiative within the Society for Pediatric Radiology that raised awareness on the number of pediatric CT scans and the use of adult dose protocols in pediatric imaging. They also mentioned other technological advances, such as electronic health records and the sharing of existing images, which may have contributed to the lower utilization of CT scans.
The authors note that the decrease does have public health implications, as lower CT utilization decreases exposure to hazardous ionizing radiation.“Therefore, the substitution of an imaging modality that does not confer ionizing radiation may affect lifetime cancer risk in children who receive diagnostic imaging.”
Dr. Parker and her colleagues reported no conflicts of interest or external funding sources for the research.
FROM PEDIATRICS
Key clinical point: The use of CT scans in children’s hospitals declined in the top 10 APR-DRGs.
Major finding: The decrease was greatest for patients with an APR-DRG of seizure with an almost 50% decrease in CT scans; MRI use decreased by greater than 10%.
Data source: Utilization of CT scans on children admitted to 33 pediatric tertiary-care hospitals participating in the Pediatric Health Information System between Jan.1, 2004, and Dec. 31, 2012.
Disclosures: Dr. Parker and her colleagues reported no conflicts of interest or external funding sources for the research.
FDA investigating risk of gadolinium contrast agent brain deposits
The Food and Drug Administration is investigating the risk of brain deposits after recurring use of gadolinium-based contrast agents for MRI, the agency announced in a statement.
Studies suggest that gadolinium-based contrast agent (GBCA) deposits may stay in the brains of patients who have four or more contrast MRI scans, though it is unknown whether these deposits cause adverse effects, the FDA said.
GBCAs are usually expelled through the kidneys, but may remain in the brain after repeated exposure. FDA’s National Center for Toxicological
Research will further investigate safety risks in consultation with researchers and industry, the statement said.
The FDA is not requiring manufacturers to change the labels of GBCA products until more information is known. The agency is, however, recommending that clinicians limit GBCA use to situations in which it would be necessary for patient care.
“Health care professionals are also urged to reassess the necessity of repetitive GBCA MRIs in established treatment protocols,” the FDA said.
Patients may report side effects and adverse events to the FDA’s MedWatch Safety Information and Adverse Event Reporting Program.
The Food and Drug Administration is investigating the risk of brain deposits after recurring use of gadolinium-based contrast agents for MRI, the agency announced in a statement.
Studies suggest that gadolinium-based contrast agent (GBCA) deposits may stay in the brains of patients who have four or more contrast MRI scans, though it is unknown whether these deposits cause adverse effects, the FDA said.
GBCAs are usually expelled through the kidneys, but may remain in the brain after repeated exposure. FDA’s National Center for Toxicological
Research will further investigate safety risks in consultation with researchers and industry, the statement said.
The FDA is not requiring manufacturers to change the labels of GBCA products until more information is known. The agency is, however, recommending that clinicians limit GBCA use to situations in which it would be necessary for patient care.
“Health care professionals are also urged to reassess the necessity of repetitive GBCA MRIs in established treatment protocols,” the FDA said.
Patients may report side effects and adverse events to the FDA’s MedWatch Safety Information and Adverse Event Reporting Program.
The Food and Drug Administration is investigating the risk of brain deposits after recurring use of gadolinium-based contrast agents for MRI, the agency announced in a statement.
Studies suggest that gadolinium-based contrast agent (GBCA) deposits may stay in the brains of patients who have four or more contrast MRI scans, though it is unknown whether these deposits cause adverse effects, the FDA said.
GBCAs are usually expelled through the kidneys, but may remain in the brain after repeated exposure. FDA’s National Center for Toxicological
Research will further investigate safety risks in consultation with researchers and industry, the statement said.
The FDA is not requiring manufacturers to change the labels of GBCA products until more information is known. The agency is, however, recommending that clinicians limit GBCA use to situations in which it would be necessary for patient care.
“Health care professionals are also urged to reassess the necessity of repetitive GBCA MRIs in established treatment protocols,” the FDA said.
Patients may report side effects and adverse events to the FDA’s MedWatch Safety Information and Adverse Event Reporting Program.
Giant Solitary Synovial Chondromatosis Mimicking Chondrosarcoma: Report of a Rare Histologic Presentation and Literature Review
Synovial chondromatosis (SCM) is a relatively rare benign lesion of the synovium.1 Its pathogenesis has been thought to be a chondral metaplasia of the subintimal layer of the intra- or extra-articular synovium.2 However, evidence supporting a neoplastic cause of the disease is emerging.3 When intra-articular, any joint can be affected, though large joints are more prone to the disease; the knee, hip, and elbow are the most common locations.4 The synovial layer of tendons or bursae can be the origin of extra-articular SCM.5
Synovial chondrosarcoma (SCS), an even rarer pathology, can be caused by malignant transformation of SCM or can appear de novo on a synovial background.6 Histologic differentiation from SCM might be difficult because of the high incidence of hypercellularity, cellular atypia, and binucleated cells.6 Some features, such as presence of a very large mass or erosion of the surrounding bones, have been indicated as possible signs of malignancy.3 An unusual presentation of SCM, giant solitary synovial chondromatosis (GSSCM), can be hard to distinguish from SCS because of the large volume and possible aggressive radiologic findings.7 Some histologic features, such as presence of necrosis and mitotic cells, have been suggested as distinctive criteria for malignancy.8
In this article, we present a case of benign GSSCM with a histologic feature that has not been considered typical for benign SCM. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
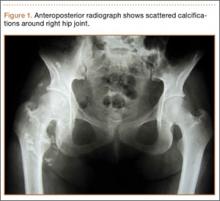
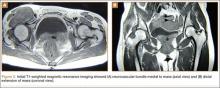
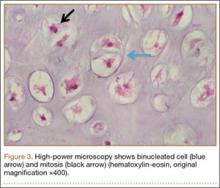
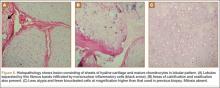
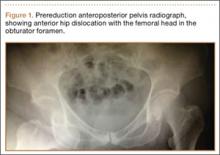
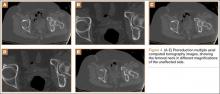
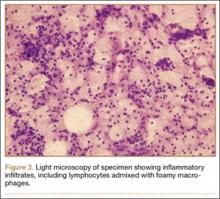
An 18-year-old woman presented with a large mass over the right hip. The mass had been growing slowly for 2 years. One year before presentation, a radiograph showed a large hip mass with fluffy calcification (Figure 1), and magnetic resonance imaging (MRI) showed a large nonhomogeneous mass anterior to the hip capsule and extending into the hip joint back to the posterior part of the joint (Figures 2A, 2B). Open incisional biopsy was performed in a local hospital at the time, and the histologic analysis revealed presence of atypical binucleated cells and pleomorphism, in addition to some mitotic activity (0 to 1 per high-power field) (Figure 3). These findings suggested malignancy. The patient declined surgery up until the time she presented to our hospital, 1 year later.
Clinical examination findings on admission to our hospital were striking. The patient had a large mass in the groin region. It was fairly tender and firm to palpation, immobile, and close to the skin. Hip motion was mildly painful but obviously restricted.
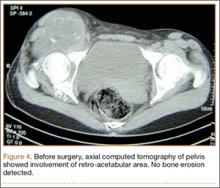
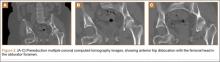
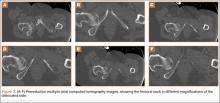
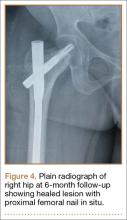
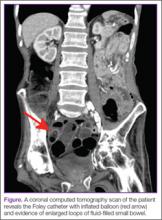
The mass was restaged. New radiographs and MRI did not show any significant changes since the previous year, computed tomography (CT) did not show any bone erosion (Figure 4), and chest radiograph, CT, and whole-body bone scan did not demonstrate any signs of metastasis.
Given the clinical presentation and previous histopathologic findings, a diagnosis of GSSCM with possible malignant transformation was made. The patient was scheduled for surgery. During surgery, the tumor was exposed through the Smith-Petersen approach. The mass was extruding under the fascia between the femoral neurovascular bundle medially and iliopsoas muscle laterally. There was no adhesion of the surrounding structures, including the femoral neurovascular bundle, to the mass. The muscle was sitting on the anterolateral surface of the mass, which was considered located in the iliopsoas bursa but extending to the joint. In the vertical plane, the mass extended down to the subtrochanteric area. The entire solid extra-articular mass was excised en bloc, and hip capsulotomy was performed inferior to the area of emergence of the mass. The joint was occupied by a single solid cartilaginous mass molding around the femoral neck, filling the piriformis fossa and propagating to the posterior joint space. Obtaining enough exposure to the back of the joint required surgical hip dislocation. The visualized acetabular fossa revealed chondral fragments, which were excised. Bone erosion or significant osteoarthritis was not detected in any part of the joint. A nearly total synovectomy was performed, leaving the ascending retinacular vessels intact. Meticulous technique was used to avoid contaminating the extra-articular tissues. The wound was closed in the routine way after hip relocation.
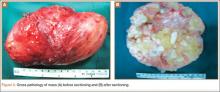
The 16×9.5×9-cm mass (Figure 5A) had a conglomerated internal structure (Figure 5B). Multiple specimens from the intra- and extra-articular portions of the mass were sent for histopathologic analysis, which revealed clusters of mature chondrocytes arranged in a lobular pattern and separated by thin fibrous bands. Areas of calcification and ossification were appreciated as well (Figures 6A-6C). No necrosis, mitosis, or bone permeation was detected. These findings were compatible with typical SCM. Given these pathologic findings and the lack of clinical deterioration over the previous year, a diagnosis of GSSCM with extension along the iliopsoas and obturator externus bursae was made. The already-performed marginal excision was deemed sufficient treatment. At most recent follow-up, 38 months after surgery, the patient was pain-free and had good hip range of motion and no indication of recurrence.
Discussion
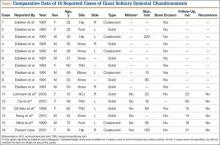
SCM is a benign disorder emerging from the synovium as a result of proliferative changes in the synovial membrane of the joints, tendon sheaths, or bursae, leading to the formation of numerous cartilaginous nodules, usually a few millimeters in diameter.8 In a rare presentation of the disease, the nodules may coalesce to form a large mass, or a single cartilaginous nodule may enlarge to form a mass. Edeiken and colleagues7 named this previously unrecognized SCM feature as GSSCM when there was a major single mass larger than 1 cm in diameter. There have been other SCM cases with multiple giant masses.9,10 In the English-language literature, we found 15 GSSCM cases, which include the first reported, by Edeiken and colleagues7 (Table). However, earlier SCM cases would be reclassified GSSCM according to their definition.11
The present case brings the total to 16. Nine of the 16 patients were male. Mean age at presentation was 41 years (range, 10-80 years). The knee was the most common GSSCM site (6 cases), followed by the temporomandibular and hip joints (3 each). Regarding gross pathology, 10 lesions were solid, and 6 (including the present one) were formed by conglomeration of the chondromatosis nodules. Lesions varied in size (16-200 mm), and 2 were primarily extra-articular (foot). One common issue with most of the cases was the initial diagnosis of chondrosarcoma. The exact surgical technique used was described for 6 cases (cases 11-16); the technique was marginal excision. In no case was recurrence 14 to 60 months after surgery reported.
This chondroproliferative process is potentially a diagnostic challenge, as distinguishing it from a chondrosarcoma, a more common lesion, could be difficult based on clinical and imaging findings, and, as is true for other chondral lesions, even histologic differentiation of the conditions might not be conclusive.12,13 Confusion in diagnosis was almost universal in this series of patients.
One important differentiating feature of benign and malignant skeletal lesions is the time course of the disease. Malignant tumors are expected to demonstrate rapid enlargement and local or systemic spread. Unfortunately, often SCS cannot be distinguished by this characteristic, as grade I or II chondrosarcoma is usually a slow-growing tumor and does not metastasize early.14 Although lack of recurrence is assuring, recurrence is not necessarily a sign of malignancy, as a considerable percentage of benign chondromatosis lesions recur.8
Radiologic differentiation between SCM and SCS is another challenge. Although bone erosion caused by a lesion not originating from bone is usually considered a sign of malignancy, GSSCM was reported as causing bone erosion in 5 of the 16 cases in our literature review.7,15 Our patient did not experience any bone erosion. However, lack of bone erosion is not a reliable criterion for excluding SCS, and bone erosion was noted in only 3 of the 9 SCS cases in the series reported by Bertoni and colleagues.6 Moreover, tumor size and propagation of tumor to surrounding tissue could be surprising in GSSCM. Large size (up to 20 cm) and extra-articular spread of a lesion originating in a joint are common findings.6,16 Our case was an obvious extension of a hip GSSCM to the iliopsoas and obturator externus bursa, which is the most common pattern of extracapsular spread of hip SCM.17 An interesting feature of the present case, however, was the relatively superficial location of the mass immediately under the fascia.
Calcified matrix is key in diagnosing a chondral lesion on imaging studies, but, in some cases, SCM does not demonstrate any radiographically detectable calcification at time of diagnosis.18 However, all the GSSCM cases reported to date had obvious calcified matrix.
The hypercellularity, cellular atypia, binucleated cells, and pleomorphism in the histologic examination of the present case are not features of malignancy in SCM.8 On the contrary, several other characteristics, including qualitative differences in the arrangement of chondrocytes (sheets rather than clusters), myxoid matrix, hypercellularity with crowding and spindling of the nuclei at the periphery, necrosis, and, most important, permeation of the trabecular bone with the filling up of marrow spaces, have been assumed to be indicative of malignancy.8 Furthermore, Davis and colleagues8 found no mitotic activity in the histopathologic investigation of 53 SCM cases. Even in 3 cases that developed malignant transformation to SCS, mitosis was not found in the initial biopsy specimens before transformation. This was compatible with the common opinion that SCM is not a neoplastic, but a metaplastic, process. Histopathologic data were available for only 8 of the previous 15 GSSCM cases. There were no reports of mitosis, and necrosis was found in only 1 case.16 In our patient’s case, however, the first biopsy did show remarkable mitotic activity. This was not the case for the second biopsy, when mature chondrocytes associated with marked calcification and ossification were prominent features (Figures 6A, 6B). We presume that, within a limited period during earlier stages of tissue maturation in SCM, mitotic activity might be a possible finding. Of note, none of the other aforementioned histologic criteria for malignancy was seen in the first or second biopsy in the present case (Figures 3, 6C).
The original idea that SCM originates from a metaplasia in the subintimal layer of the synovium, where the synovium is in direct contact with the articular cartilage, has been challenged. The high incidence of hypercellularity, binucleated cells, and cellular atypia was always an argument against a metaplastic origin for the disease. Evidence of clonal chromosomal changes, like translocation of chromosome 1218 and chromosome 5 and 6 abnormalities,19,20 in addition to other alterations,19,21 provide some evidence supporting a neoplastic rather than a metaplastic origin for SCM. Given the presence of mitosis in the present case, the lack of mitotic activity in SCM, as stated by other authors,22 is not a universal feature and cannot be used as an argument against a neoplastic origin for SCM.
Although mitotic activity is uncommon in SCM, the present case illustrates the possible presence of mitotic activity in GSSCM. The simple presence of mitotic activity, a common finding in some other chondral tumors,23,24 does not preclude the diagnosis of benign SCM, as suggested before,8 and correlation of the clinical and radiologic manifestations with histopathologic findings is crucial for a correct diagnosis.
1. Milgram JW. Synovial osteochondromatosis: a histopathological study of thirty cases. J Bone Joint Surg Am. 1977;59(6):792-801.
2. Trias A, Quintana O. Synovial chondrometaplasia: review of world literature and a study of 18 Canadian cases. Can J Surg. 1976;19(2):151-158.
3. Murphey MD, Vidal JA, Fanburg-Smith JC, Gajewski DA. Imaging of synovial chondromatosis with radiologic-pathologic correlation. Radiographics. 2007;27(5):1465-1488.
4. Milgram JW. Synovial osteochondromatosis in association with Legg-Calve-Perthes disease. Clin Orthop Relat Res. 1979;(145):179-182.
5. Sim FH, Dahlin DC, Ivins JC. Extra-articular synovial chondromatosis. J Bone Joint Surg Am. 1977;59(4):492-495.
6. Bertoni F, Unni KK, Beabout JW, Sim FH. Chondrosarcomas of the synovium. Cancer. 1991;67(1):155-162.
7. Edeiken J, Edeiken BS, Ayala AG, Raymond AK, Murray JA, Guo SQ. Giant solitary synovial chondromatosis. Skeletal Radiol. 1994;23(1):23-29.
8. Davis RI, Hamilton A, Biggart JD. Primary synovial chondromatosis: a clinicopathologic review and assessment of malignant potential. Hum Pathol. 1998;29(7):683-688.
9. Goel A, Cullen C, Paul AS, Freemont AJ. Multiple giant synovial chondromatosis of the knee. Knee. 2001;8(3):243-245.
10. Dogan A, Harman M, Uslu M, Bayram I, Akpinar F. Rocky form giant synovial chondromatosis: a case report. Knee Surg Sports Traumatol Arthrosc. 2006;14(5):465-468.
11. Eisenberg KS, Johnston JO. Synovial chondromatosis of the hip joint presenting as an intrapelvic mass: a case report. J Bone Joint Surg Am. 1972;54(1):176-178.
12. Lohmann CH, Köster G, Klinger HM, Kunze E. Giant synovial osteochondromatosis of the acromio-clavicular joint in a child. A case report and review of the literature. J Pediatr Orthop B. 2005;14(2):126-128.
13. Cai XY, Yang C, Chen MJ, Jiang B, Wang BL. Arthroscopically guided removal of large solitary synovial chondromatosis from the temporomandibular joint. Int J Oral Maxillofac Surg. 2010;39(12):1236-1239.
14. Gil-Salu JL, Lazaro R, Aldasoro J, Gonzalez-Darder JM. Giant solitary synovial chondromatosis of the temporomandibular joint with intracranial extension. Skull Base Surg. 1998;8(2):99-104.
15. Kang CH, Park JH, Lee DH, Kim CH, Park JM, Lee WS. Giant synovial chondromatosis of the knee mimicking a parosteal osteosarcoma: a case report. J Korean Bone Joint Tumor Soc. 2010;16(2):95-98.
16. Nihal A, Read CJ, Henderson DC, Malcolm AJ. Extra-articular giant solitary synovial chondromatosis of the foot: a case report and literature review. Foot Ankle Surg. 1999;5(1):29-32.
17. Robinson P, White LM, Kandel R, Bell RS, Wunder JS. Primary synovial osteochondromatosis of the hip: extracapsular patterns of spread. Skeletal Radiol. 2004;33(4):210-215.
18. Tallini G, Dorfman H, Brys P, et al. Correlation between clinicopathological features and karyotype in 100 cartilaginous and chordoid tumours. A report from the Chromosomes and Morphology (CHAMP) Collaborative Study Group. J Pathol. 2002;196(2):194-203.
19. Sah AP, Geller DS, Mankin HJ, et al. Malignant transformation of synovial chondromatosis of the shoulder to chondrosarcoma. A case report. J Bone Joint Surg Am. 2007;89(6):1321-1328.
20. Buddingh EP, Krallman P, Neff JR, Nelson M, Liu J, Bridge JA. Chromosome 6 abnormalities are recurrent in synovial chondromatosis. Cancer Genet Cytogenet. 2003;140(1):18-22.
21. Rizzo M, Ghert MA, Harrelson JM, Scully SP. Chondrosarcoma of bone: analysis of 108 cases and evaluation for predictors of outcome. Clin Orthop Relat Res. 2001;(391):224-233.
22. Davis RI, Foster H, Arthur K, Trewin S, Hamilton PW, Biggart DJ. Cell proliferation studies in primary synovial chondromatosis. J Pathol. 1998;184(1):18-23.
23. Ishikawa E, Tsuboi K, Onizawa K, et al. Chondroblastoma of the temporal base with high mitotic activity. Neurol Med Chir (Tokyo). 2002;42(11):516-520.
24. Kirin I, Jurisic D, Mokrovic H, Stanec Z, Stalekar H. Chondromyxoid fibroma of the second metacarpal bone—a case report. Coll Antropol. 2011;35(3):929-931.
Synovial chondromatosis (SCM) is a relatively rare benign lesion of the synovium.1 Its pathogenesis has been thought to be a chondral metaplasia of the subintimal layer of the intra- or extra-articular synovium.2 However, evidence supporting a neoplastic cause of the disease is emerging.3 When intra-articular, any joint can be affected, though large joints are more prone to the disease; the knee, hip, and elbow are the most common locations.4 The synovial layer of tendons or bursae can be the origin of extra-articular SCM.5
Synovial chondrosarcoma (SCS), an even rarer pathology, can be caused by malignant transformation of SCM or can appear de novo on a synovial background.6 Histologic differentiation from SCM might be difficult because of the high incidence of hypercellularity, cellular atypia, and binucleated cells.6 Some features, such as presence of a very large mass or erosion of the surrounding bones, have been indicated as possible signs of malignancy.3 An unusual presentation of SCM, giant solitary synovial chondromatosis (GSSCM), can be hard to distinguish from SCS because of the large volume and possible aggressive radiologic findings.7 Some histologic features, such as presence of necrosis and mitotic cells, have been suggested as distinctive criteria for malignancy.8
In this article, we present a case of benign GSSCM with a histologic feature that has not been considered typical for benign SCM. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
An 18-year-old woman presented with a large mass over the right hip. The mass had been growing slowly for 2 years. One year before presentation, a radiograph showed a large hip mass with fluffy calcification (Figure 1), and magnetic resonance imaging (MRI) showed a large nonhomogeneous mass anterior to the hip capsule and extending into the hip joint back to the posterior part of the joint (Figures 2A, 2B). Open incisional biopsy was performed in a local hospital at the time, and the histologic analysis revealed presence of atypical binucleated cells and pleomorphism, in addition to some mitotic activity (0 to 1 per high-power field) (Figure 3). These findings suggested malignancy. The patient declined surgery up until the time she presented to our hospital, 1 year later.
Clinical examination findings on admission to our hospital were striking. The patient had a large mass in the groin region. It was fairly tender and firm to palpation, immobile, and close to the skin. Hip motion was mildly painful but obviously restricted.
The mass was restaged. New radiographs and MRI did not show any significant changes since the previous year, computed tomography (CT) did not show any bone erosion (Figure 4), and chest radiograph, CT, and whole-body bone scan did not demonstrate any signs of metastasis.
Given the clinical presentation and previous histopathologic findings, a diagnosis of GSSCM with possible malignant transformation was made. The patient was scheduled for surgery. During surgery, the tumor was exposed through the Smith-Petersen approach. The mass was extruding under the fascia between the femoral neurovascular bundle medially and iliopsoas muscle laterally. There was no adhesion of the surrounding structures, including the femoral neurovascular bundle, to the mass. The muscle was sitting on the anterolateral surface of the mass, which was considered located in the iliopsoas bursa but extending to the joint. In the vertical plane, the mass extended down to the subtrochanteric area. The entire solid extra-articular mass was excised en bloc, and hip capsulotomy was performed inferior to the area of emergence of the mass. The joint was occupied by a single solid cartilaginous mass molding around the femoral neck, filling the piriformis fossa and propagating to the posterior joint space. Obtaining enough exposure to the back of the joint required surgical hip dislocation. The visualized acetabular fossa revealed chondral fragments, which were excised. Bone erosion or significant osteoarthritis was not detected in any part of the joint. A nearly total synovectomy was performed, leaving the ascending retinacular vessels intact. Meticulous technique was used to avoid contaminating the extra-articular tissues. The wound was closed in the routine way after hip relocation.
The 16×9.5×9-cm mass (Figure 5A) had a conglomerated internal structure (Figure 5B). Multiple specimens from the intra- and extra-articular portions of the mass were sent for histopathologic analysis, which revealed clusters of mature chondrocytes arranged in a lobular pattern and separated by thin fibrous bands. Areas of calcification and ossification were appreciated as well (Figures 6A-6C). No necrosis, mitosis, or bone permeation was detected. These findings were compatible with typical SCM. Given these pathologic findings and the lack of clinical deterioration over the previous year, a diagnosis of GSSCM with extension along the iliopsoas and obturator externus bursae was made. The already-performed marginal excision was deemed sufficient treatment. At most recent follow-up, 38 months after surgery, the patient was pain-free and had good hip range of motion and no indication of recurrence.
Discussion
SCM is a benign disorder emerging from the synovium as a result of proliferative changes in the synovial membrane of the joints, tendon sheaths, or bursae, leading to the formation of numerous cartilaginous nodules, usually a few millimeters in diameter.8 In a rare presentation of the disease, the nodules may coalesce to form a large mass, or a single cartilaginous nodule may enlarge to form a mass. Edeiken and colleagues7 named this previously unrecognized SCM feature as GSSCM when there was a major single mass larger than 1 cm in diameter. There have been other SCM cases with multiple giant masses.9,10 In the English-language literature, we found 15 GSSCM cases, which include the first reported, by Edeiken and colleagues7 (Table). However, earlier SCM cases would be reclassified GSSCM according to their definition.11
The present case brings the total to 16. Nine of the 16 patients were male. Mean age at presentation was 41 years (range, 10-80 years). The knee was the most common GSSCM site (6 cases), followed by the temporomandibular and hip joints (3 each). Regarding gross pathology, 10 lesions were solid, and 6 (including the present one) were formed by conglomeration of the chondromatosis nodules. Lesions varied in size (16-200 mm), and 2 were primarily extra-articular (foot). One common issue with most of the cases was the initial diagnosis of chondrosarcoma. The exact surgical technique used was described for 6 cases (cases 11-16); the technique was marginal excision. In no case was recurrence 14 to 60 months after surgery reported.
This chondroproliferative process is potentially a diagnostic challenge, as distinguishing it from a chondrosarcoma, a more common lesion, could be difficult based on clinical and imaging findings, and, as is true for other chondral lesions, even histologic differentiation of the conditions might not be conclusive.12,13 Confusion in diagnosis was almost universal in this series of patients.
One important differentiating feature of benign and malignant skeletal lesions is the time course of the disease. Malignant tumors are expected to demonstrate rapid enlargement and local or systemic spread. Unfortunately, often SCS cannot be distinguished by this characteristic, as grade I or II chondrosarcoma is usually a slow-growing tumor and does not metastasize early.14 Although lack of recurrence is assuring, recurrence is not necessarily a sign of malignancy, as a considerable percentage of benign chondromatosis lesions recur.8
Radiologic differentiation between SCM and SCS is another challenge. Although bone erosion caused by a lesion not originating from bone is usually considered a sign of malignancy, GSSCM was reported as causing bone erosion in 5 of the 16 cases in our literature review.7,15 Our patient did not experience any bone erosion. However, lack of bone erosion is not a reliable criterion for excluding SCS, and bone erosion was noted in only 3 of the 9 SCS cases in the series reported by Bertoni and colleagues.6 Moreover, tumor size and propagation of tumor to surrounding tissue could be surprising in GSSCM. Large size (up to 20 cm) and extra-articular spread of a lesion originating in a joint are common findings.6,16 Our case was an obvious extension of a hip GSSCM to the iliopsoas and obturator externus bursa, which is the most common pattern of extracapsular spread of hip SCM.17 An interesting feature of the present case, however, was the relatively superficial location of the mass immediately under the fascia.
Calcified matrix is key in diagnosing a chondral lesion on imaging studies, but, in some cases, SCM does not demonstrate any radiographically detectable calcification at time of diagnosis.18 However, all the GSSCM cases reported to date had obvious calcified matrix.
The hypercellularity, cellular atypia, binucleated cells, and pleomorphism in the histologic examination of the present case are not features of malignancy in SCM.8 On the contrary, several other characteristics, including qualitative differences in the arrangement of chondrocytes (sheets rather than clusters), myxoid matrix, hypercellularity with crowding and spindling of the nuclei at the periphery, necrosis, and, most important, permeation of the trabecular bone with the filling up of marrow spaces, have been assumed to be indicative of malignancy.8 Furthermore, Davis and colleagues8 found no mitotic activity in the histopathologic investigation of 53 SCM cases. Even in 3 cases that developed malignant transformation to SCS, mitosis was not found in the initial biopsy specimens before transformation. This was compatible with the common opinion that SCM is not a neoplastic, but a metaplastic, process. Histopathologic data were available for only 8 of the previous 15 GSSCM cases. There were no reports of mitosis, and necrosis was found in only 1 case.16 In our patient’s case, however, the first biopsy did show remarkable mitotic activity. This was not the case for the second biopsy, when mature chondrocytes associated with marked calcification and ossification were prominent features (Figures 6A, 6B). We presume that, within a limited period during earlier stages of tissue maturation in SCM, mitotic activity might be a possible finding. Of note, none of the other aforementioned histologic criteria for malignancy was seen in the first or second biopsy in the present case (Figures 3, 6C).
The original idea that SCM originates from a metaplasia in the subintimal layer of the synovium, where the synovium is in direct contact with the articular cartilage, has been challenged. The high incidence of hypercellularity, binucleated cells, and cellular atypia was always an argument against a metaplastic origin for the disease. Evidence of clonal chromosomal changes, like translocation of chromosome 1218 and chromosome 5 and 6 abnormalities,19,20 in addition to other alterations,19,21 provide some evidence supporting a neoplastic rather than a metaplastic origin for SCM. Given the presence of mitosis in the present case, the lack of mitotic activity in SCM, as stated by other authors,22 is not a universal feature and cannot be used as an argument against a neoplastic origin for SCM.
Although mitotic activity is uncommon in SCM, the present case illustrates the possible presence of mitotic activity in GSSCM. The simple presence of mitotic activity, a common finding in some other chondral tumors,23,24 does not preclude the diagnosis of benign SCM, as suggested before,8 and correlation of the clinical and radiologic manifestations with histopathologic findings is crucial for a correct diagnosis.
Synovial chondromatosis (SCM) is a relatively rare benign lesion of the synovium.1 Its pathogenesis has been thought to be a chondral metaplasia of the subintimal layer of the intra- or extra-articular synovium.2 However, evidence supporting a neoplastic cause of the disease is emerging.3 When intra-articular, any joint can be affected, though large joints are more prone to the disease; the knee, hip, and elbow are the most common locations.4 The synovial layer of tendons or bursae can be the origin of extra-articular SCM.5
Synovial chondrosarcoma (SCS), an even rarer pathology, can be caused by malignant transformation of SCM or can appear de novo on a synovial background.6 Histologic differentiation from SCM might be difficult because of the high incidence of hypercellularity, cellular atypia, and binucleated cells.6 Some features, such as presence of a very large mass or erosion of the surrounding bones, have been indicated as possible signs of malignancy.3 An unusual presentation of SCM, giant solitary synovial chondromatosis (GSSCM), can be hard to distinguish from SCS because of the large volume and possible aggressive radiologic findings.7 Some histologic features, such as presence of necrosis and mitotic cells, have been suggested as distinctive criteria for malignancy.8
In this article, we present a case of benign GSSCM with a histologic feature that has not been considered typical for benign SCM. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
An 18-year-old woman presented with a large mass over the right hip. The mass had been growing slowly for 2 years. One year before presentation, a radiograph showed a large hip mass with fluffy calcification (Figure 1), and magnetic resonance imaging (MRI) showed a large nonhomogeneous mass anterior to the hip capsule and extending into the hip joint back to the posterior part of the joint (Figures 2A, 2B). Open incisional biopsy was performed in a local hospital at the time, and the histologic analysis revealed presence of atypical binucleated cells and pleomorphism, in addition to some mitotic activity (0 to 1 per high-power field) (Figure 3). These findings suggested malignancy. The patient declined surgery up until the time she presented to our hospital, 1 year later.
Clinical examination findings on admission to our hospital were striking. The patient had a large mass in the groin region. It was fairly tender and firm to palpation, immobile, and close to the skin. Hip motion was mildly painful but obviously restricted.
The mass was restaged. New radiographs and MRI did not show any significant changes since the previous year, computed tomography (CT) did not show any bone erosion (Figure 4), and chest radiograph, CT, and whole-body bone scan did not demonstrate any signs of metastasis.
Given the clinical presentation and previous histopathologic findings, a diagnosis of GSSCM with possible malignant transformation was made. The patient was scheduled for surgery. During surgery, the tumor was exposed through the Smith-Petersen approach. The mass was extruding under the fascia between the femoral neurovascular bundle medially and iliopsoas muscle laterally. There was no adhesion of the surrounding structures, including the femoral neurovascular bundle, to the mass. The muscle was sitting on the anterolateral surface of the mass, which was considered located in the iliopsoas bursa but extending to the joint. In the vertical plane, the mass extended down to the subtrochanteric area. The entire solid extra-articular mass was excised en bloc, and hip capsulotomy was performed inferior to the area of emergence of the mass. The joint was occupied by a single solid cartilaginous mass molding around the femoral neck, filling the piriformis fossa and propagating to the posterior joint space. Obtaining enough exposure to the back of the joint required surgical hip dislocation. The visualized acetabular fossa revealed chondral fragments, which were excised. Bone erosion or significant osteoarthritis was not detected in any part of the joint. A nearly total synovectomy was performed, leaving the ascending retinacular vessels intact. Meticulous technique was used to avoid contaminating the extra-articular tissues. The wound was closed in the routine way after hip relocation.
The 16×9.5×9-cm mass (Figure 5A) had a conglomerated internal structure (Figure 5B). Multiple specimens from the intra- and extra-articular portions of the mass were sent for histopathologic analysis, which revealed clusters of mature chondrocytes arranged in a lobular pattern and separated by thin fibrous bands. Areas of calcification and ossification were appreciated as well (Figures 6A-6C). No necrosis, mitosis, or bone permeation was detected. These findings were compatible with typical SCM. Given these pathologic findings and the lack of clinical deterioration over the previous year, a diagnosis of GSSCM with extension along the iliopsoas and obturator externus bursae was made. The already-performed marginal excision was deemed sufficient treatment. At most recent follow-up, 38 months after surgery, the patient was pain-free and had good hip range of motion and no indication of recurrence.
Discussion
SCM is a benign disorder emerging from the synovium as a result of proliferative changes in the synovial membrane of the joints, tendon sheaths, or bursae, leading to the formation of numerous cartilaginous nodules, usually a few millimeters in diameter.8 In a rare presentation of the disease, the nodules may coalesce to form a large mass, or a single cartilaginous nodule may enlarge to form a mass. Edeiken and colleagues7 named this previously unrecognized SCM feature as GSSCM when there was a major single mass larger than 1 cm in diameter. There have been other SCM cases with multiple giant masses.9,10 In the English-language literature, we found 15 GSSCM cases, which include the first reported, by Edeiken and colleagues7 (Table). However, earlier SCM cases would be reclassified GSSCM according to their definition.11
The present case brings the total to 16. Nine of the 16 patients were male. Mean age at presentation was 41 years (range, 10-80 years). The knee was the most common GSSCM site (6 cases), followed by the temporomandibular and hip joints (3 each). Regarding gross pathology, 10 lesions were solid, and 6 (including the present one) were formed by conglomeration of the chondromatosis nodules. Lesions varied in size (16-200 mm), and 2 were primarily extra-articular (foot). One common issue with most of the cases was the initial diagnosis of chondrosarcoma. The exact surgical technique used was described for 6 cases (cases 11-16); the technique was marginal excision. In no case was recurrence 14 to 60 months after surgery reported.
This chondroproliferative process is potentially a diagnostic challenge, as distinguishing it from a chondrosarcoma, a more common lesion, could be difficult based on clinical and imaging findings, and, as is true for other chondral lesions, even histologic differentiation of the conditions might not be conclusive.12,13 Confusion in diagnosis was almost universal in this series of patients.
One important differentiating feature of benign and malignant skeletal lesions is the time course of the disease. Malignant tumors are expected to demonstrate rapid enlargement and local or systemic spread. Unfortunately, often SCS cannot be distinguished by this characteristic, as grade I or II chondrosarcoma is usually a slow-growing tumor and does not metastasize early.14 Although lack of recurrence is assuring, recurrence is not necessarily a sign of malignancy, as a considerable percentage of benign chondromatosis lesions recur.8
Radiologic differentiation between SCM and SCS is another challenge. Although bone erosion caused by a lesion not originating from bone is usually considered a sign of malignancy, GSSCM was reported as causing bone erosion in 5 of the 16 cases in our literature review.7,15 Our patient did not experience any bone erosion. However, lack of bone erosion is not a reliable criterion for excluding SCS, and bone erosion was noted in only 3 of the 9 SCS cases in the series reported by Bertoni and colleagues.6 Moreover, tumor size and propagation of tumor to surrounding tissue could be surprising in GSSCM. Large size (up to 20 cm) and extra-articular spread of a lesion originating in a joint are common findings.6,16 Our case was an obvious extension of a hip GSSCM to the iliopsoas and obturator externus bursa, which is the most common pattern of extracapsular spread of hip SCM.17 An interesting feature of the present case, however, was the relatively superficial location of the mass immediately under the fascia.
Calcified matrix is key in diagnosing a chondral lesion on imaging studies, but, in some cases, SCM does not demonstrate any radiographically detectable calcification at time of diagnosis.18 However, all the GSSCM cases reported to date had obvious calcified matrix.
The hypercellularity, cellular atypia, binucleated cells, and pleomorphism in the histologic examination of the present case are not features of malignancy in SCM.8 On the contrary, several other characteristics, including qualitative differences in the arrangement of chondrocytes (sheets rather than clusters), myxoid matrix, hypercellularity with crowding and spindling of the nuclei at the periphery, necrosis, and, most important, permeation of the trabecular bone with the filling up of marrow spaces, have been assumed to be indicative of malignancy.8 Furthermore, Davis and colleagues8 found no mitotic activity in the histopathologic investigation of 53 SCM cases. Even in 3 cases that developed malignant transformation to SCS, mitosis was not found in the initial biopsy specimens before transformation. This was compatible with the common opinion that SCM is not a neoplastic, but a metaplastic, process. Histopathologic data were available for only 8 of the previous 15 GSSCM cases. There were no reports of mitosis, and necrosis was found in only 1 case.16 In our patient’s case, however, the first biopsy did show remarkable mitotic activity. This was not the case for the second biopsy, when mature chondrocytes associated with marked calcification and ossification were prominent features (Figures 6A, 6B). We presume that, within a limited period during earlier stages of tissue maturation in SCM, mitotic activity might be a possible finding. Of note, none of the other aforementioned histologic criteria for malignancy was seen in the first or second biopsy in the present case (Figures 3, 6C).
The original idea that SCM originates from a metaplasia in the subintimal layer of the synovium, where the synovium is in direct contact with the articular cartilage, has been challenged. The high incidence of hypercellularity, binucleated cells, and cellular atypia was always an argument against a metaplastic origin for the disease. Evidence of clonal chromosomal changes, like translocation of chromosome 1218 and chromosome 5 and 6 abnormalities,19,20 in addition to other alterations,19,21 provide some evidence supporting a neoplastic rather than a metaplastic origin for SCM. Given the presence of mitosis in the present case, the lack of mitotic activity in SCM, as stated by other authors,22 is not a universal feature and cannot be used as an argument against a neoplastic origin for SCM.
Although mitotic activity is uncommon in SCM, the present case illustrates the possible presence of mitotic activity in GSSCM. The simple presence of mitotic activity, a common finding in some other chondral tumors,23,24 does not preclude the diagnosis of benign SCM, as suggested before,8 and correlation of the clinical and radiologic manifestations with histopathologic findings is crucial for a correct diagnosis.
1. Milgram JW. Synovial osteochondromatosis: a histopathological study of thirty cases. J Bone Joint Surg Am. 1977;59(6):792-801.
2. Trias A, Quintana O. Synovial chondrometaplasia: review of world literature and a study of 18 Canadian cases. Can J Surg. 1976;19(2):151-158.
3. Murphey MD, Vidal JA, Fanburg-Smith JC, Gajewski DA. Imaging of synovial chondromatosis with radiologic-pathologic correlation. Radiographics. 2007;27(5):1465-1488.
4. Milgram JW. Synovial osteochondromatosis in association with Legg-Calve-Perthes disease. Clin Orthop Relat Res. 1979;(145):179-182.
5. Sim FH, Dahlin DC, Ivins JC. Extra-articular synovial chondromatosis. J Bone Joint Surg Am. 1977;59(4):492-495.
6. Bertoni F, Unni KK, Beabout JW, Sim FH. Chondrosarcomas of the synovium. Cancer. 1991;67(1):155-162.
7. Edeiken J, Edeiken BS, Ayala AG, Raymond AK, Murray JA, Guo SQ. Giant solitary synovial chondromatosis. Skeletal Radiol. 1994;23(1):23-29.
8. Davis RI, Hamilton A, Biggart JD. Primary synovial chondromatosis: a clinicopathologic review and assessment of malignant potential. Hum Pathol. 1998;29(7):683-688.
9. Goel A, Cullen C, Paul AS, Freemont AJ. Multiple giant synovial chondromatosis of the knee. Knee. 2001;8(3):243-245.
10. Dogan A, Harman M, Uslu M, Bayram I, Akpinar F. Rocky form giant synovial chondromatosis: a case report. Knee Surg Sports Traumatol Arthrosc. 2006;14(5):465-468.
11. Eisenberg KS, Johnston JO. Synovial chondromatosis of the hip joint presenting as an intrapelvic mass: a case report. J Bone Joint Surg Am. 1972;54(1):176-178.
12. Lohmann CH, Köster G, Klinger HM, Kunze E. Giant synovial osteochondromatosis of the acromio-clavicular joint in a child. A case report and review of the literature. J Pediatr Orthop B. 2005;14(2):126-128.
13. Cai XY, Yang C, Chen MJ, Jiang B, Wang BL. Arthroscopically guided removal of large solitary synovial chondromatosis from the temporomandibular joint. Int J Oral Maxillofac Surg. 2010;39(12):1236-1239.
14. Gil-Salu JL, Lazaro R, Aldasoro J, Gonzalez-Darder JM. Giant solitary synovial chondromatosis of the temporomandibular joint with intracranial extension. Skull Base Surg. 1998;8(2):99-104.
15. Kang CH, Park JH, Lee DH, Kim CH, Park JM, Lee WS. Giant synovial chondromatosis of the knee mimicking a parosteal osteosarcoma: a case report. J Korean Bone Joint Tumor Soc. 2010;16(2):95-98.
16. Nihal A, Read CJ, Henderson DC, Malcolm AJ. Extra-articular giant solitary synovial chondromatosis of the foot: a case report and literature review. Foot Ankle Surg. 1999;5(1):29-32.
17. Robinson P, White LM, Kandel R, Bell RS, Wunder JS. Primary synovial osteochondromatosis of the hip: extracapsular patterns of spread. Skeletal Radiol. 2004;33(4):210-215.
18. Tallini G, Dorfman H, Brys P, et al. Correlation between clinicopathological features and karyotype in 100 cartilaginous and chordoid tumours. A report from the Chromosomes and Morphology (CHAMP) Collaborative Study Group. J Pathol. 2002;196(2):194-203.
19. Sah AP, Geller DS, Mankin HJ, et al. Malignant transformation of synovial chondromatosis of the shoulder to chondrosarcoma. A case report. J Bone Joint Surg Am. 2007;89(6):1321-1328.
20. Buddingh EP, Krallman P, Neff JR, Nelson M, Liu J, Bridge JA. Chromosome 6 abnormalities are recurrent in synovial chondromatosis. Cancer Genet Cytogenet. 2003;140(1):18-22.
21. Rizzo M, Ghert MA, Harrelson JM, Scully SP. Chondrosarcoma of bone: analysis of 108 cases and evaluation for predictors of outcome. Clin Orthop Relat Res. 2001;(391):224-233.
22. Davis RI, Foster H, Arthur K, Trewin S, Hamilton PW, Biggart DJ. Cell proliferation studies in primary synovial chondromatosis. J Pathol. 1998;184(1):18-23.
23. Ishikawa E, Tsuboi K, Onizawa K, et al. Chondroblastoma of the temporal base with high mitotic activity. Neurol Med Chir (Tokyo). 2002;42(11):516-520.
24. Kirin I, Jurisic D, Mokrovic H, Stanec Z, Stalekar H. Chondromyxoid fibroma of the second metacarpal bone—a case report. Coll Antropol. 2011;35(3):929-931.
1. Milgram JW. Synovial osteochondromatosis: a histopathological study of thirty cases. J Bone Joint Surg Am. 1977;59(6):792-801.
2. Trias A, Quintana O. Synovial chondrometaplasia: review of world literature and a study of 18 Canadian cases. Can J Surg. 1976;19(2):151-158.
3. Murphey MD, Vidal JA, Fanburg-Smith JC, Gajewski DA. Imaging of synovial chondromatosis with radiologic-pathologic correlation. Radiographics. 2007;27(5):1465-1488.
4. Milgram JW. Synovial osteochondromatosis in association with Legg-Calve-Perthes disease. Clin Orthop Relat Res. 1979;(145):179-182.
5. Sim FH, Dahlin DC, Ivins JC. Extra-articular synovial chondromatosis. J Bone Joint Surg Am. 1977;59(4):492-495.
6. Bertoni F, Unni KK, Beabout JW, Sim FH. Chondrosarcomas of the synovium. Cancer. 1991;67(1):155-162.
7. Edeiken J, Edeiken BS, Ayala AG, Raymond AK, Murray JA, Guo SQ. Giant solitary synovial chondromatosis. Skeletal Radiol. 1994;23(1):23-29.
8. Davis RI, Hamilton A, Biggart JD. Primary synovial chondromatosis: a clinicopathologic review and assessment of malignant potential. Hum Pathol. 1998;29(7):683-688.
9. Goel A, Cullen C, Paul AS, Freemont AJ. Multiple giant synovial chondromatosis of the knee. Knee. 2001;8(3):243-245.
10. Dogan A, Harman M, Uslu M, Bayram I, Akpinar F. Rocky form giant synovial chondromatosis: a case report. Knee Surg Sports Traumatol Arthrosc. 2006;14(5):465-468.
11. Eisenberg KS, Johnston JO. Synovial chondromatosis of the hip joint presenting as an intrapelvic mass: a case report. J Bone Joint Surg Am. 1972;54(1):176-178.
12. Lohmann CH, Köster G, Klinger HM, Kunze E. Giant synovial osteochondromatosis of the acromio-clavicular joint in a child. A case report and review of the literature. J Pediatr Orthop B. 2005;14(2):126-128.
13. Cai XY, Yang C, Chen MJ, Jiang B, Wang BL. Arthroscopically guided removal of large solitary synovial chondromatosis from the temporomandibular joint. Int J Oral Maxillofac Surg. 2010;39(12):1236-1239.
14. Gil-Salu JL, Lazaro R, Aldasoro J, Gonzalez-Darder JM. Giant solitary synovial chondromatosis of the temporomandibular joint with intracranial extension. Skull Base Surg. 1998;8(2):99-104.
15. Kang CH, Park JH, Lee DH, Kim CH, Park JM, Lee WS. Giant synovial chondromatosis of the knee mimicking a parosteal osteosarcoma: a case report. J Korean Bone Joint Tumor Soc. 2010;16(2):95-98.
16. Nihal A, Read CJ, Henderson DC, Malcolm AJ. Extra-articular giant solitary synovial chondromatosis of the foot: a case report and literature review. Foot Ankle Surg. 1999;5(1):29-32.
17. Robinson P, White LM, Kandel R, Bell RS, Wunder JS. Primary synovial osteochondromatosis of the hip: extracapsular patterns of spread. Skeletal Radiol. 2004;33(4):210-215.
18. Tallini G, Dorfman H, Brys P, et al. Correlation between clinicopathological features and karyotype in 100 cartilaginous and chordoid tumours. A report from the Chromosomes and Morphology (CHAMP) Collaborative Study Group. J Pathol. 2002;196(2):194-203.
19. Sah AP, Geller DS, Mankin HJ, et al. Malignant transformation of synovial chondromatosis of the shoulder to chondrosarcoma. A case report. J Bone Joint Surg Am. 2007;89(6):1321-1328.
20. Buddingh EP, Krallman P, Neff JR, Nelson M, Liu J, Bridge JA. Chromosome 6 abnormalities are recurrent in synovial chondromatosis. Cancer Genet Cytogenet. 2003;140(1):18-22.
21. Rizzo M, Ghert MA, Harrelson JM, Scully SP. Chondrosarcoma of bone: analysis of 108 cases and evaluation for predictors of outcome. Clin Orthop Relat Res. 2001;(391):224-233.
22. Davis RI, Foster H, Arthur K, Trewin S, Hamilton PW, Biggart DJ. Cell proliferation studies in primary synovial chondromatosis. J Pathol. 1998;184(1):18-23.
23. Ishikawa E, Tsuboi K, Onizawa K, et al. Chondroblastoma of the temporal base with high mitotic activity. Neurol Med Chir (Tokyo). 2002;42(11):516-520.
24. Kirin I, Jurisic D, Mokrovic H, Stanec Z, Stalekar H. Chondromyxoid fibroma of the second metacarpal bone—a case report. Coll Antropol. 2011;35(3):929-931.
Iatrogenic Femoral Neck Fracture After Closed Reduction of Anterior Hip Dislocation in the Emergency Department
Anterior hip dislocations have been reported to account for approximately 5% to 10% of all hip dislocations.1 Epstein and Wiss2 originally divided anterior hip dislocations into superior (type I, including pubic or subspinous) and inferior (type II, including obturator and perineal) dislocations. This classification was further subdivided based on the presence of either no associated fracture (type A), fracture of the femoral head or neck (FNF; type B), or fracture of the acetabulum (type C).3 Of all anterior hip dislocations, it has been reported that the inferior or obturator type of dislocation is more common, constituting approximately 70% of all anterior dislocations.4 In 1943, Pringle5 described the mechanism of obturator dislocation as simultaneous abduction, flexion, and external rotation of the hip. Our literature search found only 2 case reports in non-English-language journals of a complete FNF associated with an attempted reduction of an anterior hip dislocation.6,7 Indentation fractures of the femoral head have been more commonly reported than FNFs, with a reported incidence of 35% to 55% after anterior dislocation.4,8 DeLee and colleagues8 also found that those patients with indentation fractures were at a higher risk for developing avascular necrosis of the femoral head in addition to being more likely to report poor or fair function of the hip 2 years after reduction.
There have been a number of different reduction maneuvers for anterior dislocation of hips published in the literature. Epstein and Harvey9 advocated reduction by traction in the line of the femur with the hip flexed and in gentle internal rotation and abduction while the patient was under general anesthesia. Toms and Williams,10 however, recommended adduction with gradual release of the longitudinal traction. Polesky and Polesky11 described a reduction method involving sharp internal rotation, which was found to be associated with FNF. The patient provided written informed consent for print and electronic publication of this case report, and approval was obtained from the Emory University Institutional Review Board.
Case Report
The patient was a 73-year-old woman, an independent ambulator with minimal antecedent hip pain, who, as a pedestrian, was struck by a heavy-duty pickup truck at low velocity. She was flown to our level I trauma center from an outside hospital. The patient arrived hemodynamically stable, with a Glasgow Coma Scale score of 15 and with major complaints of right shoulder and right hip pain. She had a positive Focused Assessment with Sonography for Trauma (FAST), and underwent a subsequent urgent chest, abdomen, and pelvis computed tomography (CT) scan for further investigation. CT showed a grade 1 liver laceration. Her anteroposterior (AP) pelvic radiograph and pelvic CT scan showed an anterior hip dislocation with the femoral head located adjacent to the obturator foramen (Figures 1, 2). The AP pelvic radiograph and pelvic CT scan were scrutinized extensively before reduction to rule out a possible FNF. Comparing the right and left femoral necks through multiple axial CT images showed no obvious differences between the 2 sides (Figures 3, 4). Her only other orthopedic injury was an inferior shoulder dislocation. It is not routine for the general surgery trauma team to obtain a pelvic CT scan prior to involvement of the orthopedic service and prompt reduction of a hip dislocation. Upon initial examination of her right hip, it was fixed in slight flexion and external rotation; she was neurovascularly intact.
After being cleared by the trauma service, the patient provided informed consent for closed reduction of the hip and shoulder under conscious sedation, performed by the emergency department (ED) staff. She received intravenous fentanyl and midazolam, and the reduction was attempted. The reduction maneuver was performed with gentle inline traction, adduction, and internal rotation and extension. There was an audible clunk, and the hip was thought to be reduced and stable. The right leg lower extremity was placed into a knee immobilizer and she remained neurovascularly intact. The shoulder was reduced. After the procedure, the patient had an episode of hypoxia requiring oxygenation via a bag valve mask by the ED staff. Postreduction radiographs confirmed reduction of the right shoulder; however, they also showed a FNF with the femoral head retained near the obturator foramen (Figures 5, 6). The patient and her family were informed of the fracture, and a total hip arthroplasty (THA) was recommended, given her pre-injury mild symptomatic osteoarthritis in the hip and her age. The patient was admitted to the intensive care unit for cardiopulmonary monitoring and was found to have a troponin leak on hospital day 1. She was evaluated by the cardiology service; serial electrocardiograms and troponins ruled out acute myocardial infarction. The patient was cleared for surgery on hospital day 4.
On hospital day 5, she underwent a right THA via a Kocher-Langenbeck approach. The patient’s femoral head was found to be anterior and laterally adjacent to her ischial tuberosity with an indentation fracture. The sciatic nerve was identified and found to be intact. A metal-on-polyethylene Stryker Accolade femoral component and Trident acetabular shell were implanted, and a posterior capsular repair was performed (Figure 7).
The patient tolerated the procedure well, and her postoperative course was uneventful. She was discharged to a subacute rehabilitation facility on postoperative day 3. The patient returned for her 2-week postoperative visit ambulating without assistance. At her last follow-up visit, approximately 6 weeks after surgery, she was a functionally independent community ambulator. Phone conversations with her private orthopedist at 6 months confirmed continued ambulation without problems.
Discussion
This case report of a complication that occurred in our institution has resulted in a change in our protocol for treatment of geriatric anterior hip dislocations. Our institution is a level I trauma center, and traumatic hip dislocations are relatively common, occurring usually in young patients with high-energy trauma. Although somewhat controversial, it is generally assumed that the incidence of avascular necrosis of the femoral head after dislocation of the hip is correlated with the time interval from dislocation to reduction of the hip. Therefore, our protocol for hip dislocations of the hip in young trauma patients is urgent reduction in the ED under appropriate analgesia and muscle relaxation.
In this case report, the patient was older than 65 years with radiographic evidence of possible impingement and postsurgical evidence of impingement of the femoral head in the obturator foremen (Figures 1, 2, 8). In addition, the patient was significantly osteopenic radiographically. An attempted reduction in the ED resulted in FNF requiring THA (Figures 5, 6, 9). After discussion of this complication in our institution’s morbidity and mortality conference, we have developed a protocol for the geriatric patient (older than 65 years) with a traumatic hip dislocation. These patients will undergo attempted reduction under controlled analgesia and muscle relaxation in the operating room (OR) with an attending surgeon present, ideally, an attending surgeon comfortable with arthroplasty in a terminally cleaned OR room. Our institution’s surgical site infection rate after total joint arthroplasty has significantly decreased with improved patient selection and the use of terminally cleaned OR rooms. Because our policy is to perform closed reduction of dislocated hips in an urgent manner, if there is not a terminally clean room or an arthroplasty-trained attending orthopedic surgeon available, then informed consent with discussion of the possibility of fracture requiring a subsequent arthroplasty should be obtained from the patient before the attempted reduction.
After review of the available literature, we believe that this case highlights some of the important treatment principles when treating anterior hip dislocations in the ED. The relatively high incidence of indentation fractures of the femoral head with obturator dislocations puts these fractures at higher risk for possible impingement around the obturator ring. This impingement, coupled with preexisting osteopenia, can predispose these dislocations to FNF, if appropriate analgesia and sedation are not obtained and gentle reduction is not performed. In addition, while it may not be time- or cost-effective to perform closed reduction on every hip dislocation, we bring geriatric patients with radiographic osteopenia to the OR for more controlled reductions. In the informed consent discussion, the possibility of FNF is mentioned, and the patient and family are told that an elective total hip replacement will be performed if this complication occurs.
We consider the following to be risk factors for closed reductions of anterior hip dislocations: (1) preexisting osteopenia on plain films, (2) age greater than 65 years, and (3) radiographic femoral head impingement on the surrounding bony pelvis. We continue to consider closed reduction of both anterior and posterior hip dislocations as urgent (within 6 hours from time of dislocation). This case adds to the existing literature on the risk of FNF with closed reduction of obturator hip dislocations, and we hope that it will encourage further study into the safest and most cost-effective reduction protocol.
1. Amihood, S. Anterior dislocation of the hip. Injury. 1975;7(2):107-110.
2. Epstein HC, Wiss DA. Traumatic anterior dislocation of the hip. Orthopedics. 1985;8(1):130, 132-134.
3. Epstein HC. Traumatic dislocations of the hip. Clin Orthop Relat Res. 1973(92):116-142.
4. Erb RE, Steele JR, Nance EP Jr, Edwards JR. Traumatic anterior dislocation of the hip: spectrum of plain film and CT findings. AJR Am J Roentgenol. 1995;165(5):1215-1219.
5. Pringle JH. Traumatic dislocation at the hip joint. An experimental study in the cadaver. Glasgow Med J. 1943;21:25-40.
6. Esenkaya I, Görgeç M. Traumatic anterior dislocation of the hip associated with ipsilateral femoral neck fracture: a case report. Acta Orthop Traumatol Turc. 2002;36(4):366-368.
7. Sadler AH, DiStefano M. Anterior dislocation of the hip with ipsilateral basicervical fracture. A case report. J Bone Joint Surg Am. 1985;67(2):326-329.
8. DeLee JC, Evans JA, Thomas J. Anterior dislocation of the hip and associated femoral-head fractures. J Bone Joint Surg Am. 1980;62(6):960-964.
9. Epstein HC, Harvey JP Jr. Traumatic anterior dislocations of the hip: management and results. An analysis of fifty-five cases. J Bone Joint Surg Am. 1972;54(7):1561-1562.
10. Toms AD, Williams S, White SH. Obturator dislocation of the hip. J Bone Joint Surg Br. 2001;83(1):113-115.
11. Polesky RE, Polesky FA. Intrapelvic dislocation of the femoral head following anterior dislocation of the hip. A case report. J Bone Joint Surg Am. 1972;54(5):1097-1098.
Anterior hip dislocations have been reported to account for approximately 5% to 10% of all hip dislocations.1 Epstein and Wiss2 originally divided anterior hip dislocations into superior (type I, including pubic or subspinous) and inferior (type II, including obturator and perineal) dislocations. This classification was further subdivided based on the presence of either no associated fracture (type A), fracture of the femoral head or neck (FNF; type B), or fracture of the acetabulum (type C).3 Of all anterior hip dislocations, it has been reported that the inferior or obturator type of dislocation is more common, constituting approximately 70% of all anterior dislocations.4 In 1943, Pringle5 described the mechanism of obturator dislocation as simultaneous abduction, flexion, and external rotation of the hip. Our literature search found only 2 case reports in non-English-language journals of a complete FNF associated with an attempted reduction of an anterior hip dislocation.6,7 Indentation fractures of the femoral head have been more commonly reported than FNFs, with a reported incidence of 35% to 55% after anterior dislocation.4,8 DeLee and colleagues8 also found that those patients with indentation fractures were at a higher risk for developing avascular necrosis of the femoral head in addition to being more likely to report poor or fair function of the hip 2 years after reduction.
There have been a number of different reduction maneuvers for anterior dislocation of hips published in the literature. Epstein and Harvey9 advocated reduction by traction in the line of the femur with the hip flexed and in gentle internal rotation and abduction while the patient was under general anesthesia. Toms and Williams,10 however, recommended adduction with gradual release of the longitudinal traction. Polesky and Polesky11 described a reduction method involving sharp internal rotation, which was found to be associated with FNF. The patient provided written informed consent for print and electronic publication of this case report, and approval was obtained from the Emory University Institutional Review Board.
Case Report
The patient was a 73-year-old woman, an independent ambulator with minimal antecedent hip pain, who, as a pedestrian, was struck by a heavy-duty pickup truck at low velocity. She was flown to our level I trauma center from an outside hospital. The patient arrived hemodynamically stable, with a Glasgow Coma Scale score of 15 and with major complaints of right shoulder and right hip pain. She had a positive Focused Assessment with Sonography for Trauma (FAST), and underwent a subsequent urgent chest, abdomen, and pelvis computed tomography (CT) scan for further investigation. CT showed a grade 1 liver laceration. Her anteroposterior (AP) pelvic radiograph and pelvic CT scan showed an anterior hip dislocation with the femoral head located adjacent to the obturator foramen (Figures 1, 2). The AP pelvic radiograph and pelvic CT scan were scrutinized extensively before reduction to rule out a possible FNF. Comparing the right and left femoral necks through multiple axial CT images showed no obvious differences between the 2 sides (Figures 3, 4). Her only other orthopedic injury was an inferior shoulder dislocation. It is not routine for the general surgery trauma team to obtain a pelvic CT scan prior to involvement of the orthopedic service and prompt reduction of a hip dislocation. Upon initial examination of her right hip, it was fixed in slight flexion and external rotation; she was neurovascularly intact.
After being cleared by the trauma service, the patient provided informed consent for closed reduction of the hip and shoulder under conscious sedation, performed by the emergency department (ED) staff. She received intravenous fentanyl and midazolam, and the reduction was attempted. The reduction maneuver was performed with gentle inline traction, adduction, and internal rotation and extension. There was an audible clunk, and the hip was thought to be reduced and stable. The right leg lower extremity was placed into a knee immobilizer and she remained neurovascularly intact. The shoulder was reduced. After the procedure, the patient had an episode of hypoxia requiring oxygenation via a bag valve mask by the ED staff. Postreduction radiographs confirmed reduction of the right shoulder; however, they also showed a FNF with the femoral head retained near the obturator foramen (Figures 5, 6). The patient and her family were informed of the fracture, and a total hip arthroplasty (THA) was recommended, given her pre-injury mild symptomatic osteoarthritis in the hip and her age. The patient was admitted to the intensive care unit for cardiopulmonary monitoring and was found to have a troponin leak on hospital day 1. She was evaluated by the cardiology service; serial electrocardiograms and troponins ruled out acute myocardial infarction. The patient was cleared for surgery on hospital day 4.
On hospital day 5, she underwent a right THA via a Kocher-Langenbeck approach. The patient’s femoral head was found to be anterior and laterally adjacent to her ischial tuberosity with an indentation fracture. The sciatic nerve was identified and found to be intact. A metal-on-polyethylene Stryker Accolade femoral component and Trident acetabular shell were implanted, and a posterior capsular repair was performed (Figure 7).
The patient tolerated the procedure well, and her postoperative course was uneventful. She was discharged to a subacute rehabilitation facility on postoperative day 3. The patient returned for her 2-week postoperative visit ambulating without assistance. At her last follow-up visit, approximately 6 weeks after surgery, she was a functionally independent community ambulator. Phone conversations with her private orthopedist at 6 months confirmed continued ambulation without problems.
Discussion
This case report of a complication that occurred in our institution has resulted in a change in our protocol for treatment of geriatric anterior hip dislocations. Our institution is a level I trauma center, and traumatic hip dislocations are relatively common, occurring usually in young patients with high-energy trauma. Although somewhat controversial, it is generally assumed that the incidence of avascular necrosis of the femoral head after dislocation of the hip is correlated with the time interval from dislocation to reduction of the hip. Therefore, our protocol for hip dislocations of the hip in young trauma patients is urgent reduction in the ED under appropriate analgesia and muscle relaxation.
In this case report, the patient was older than 65 years with radiographic evidence of possible impingement and postsurgical evidence of impingement of the femoral head in the obturator foremen (Figures 1, 2, 8). In addition, the patient was significantly osteopenic radiographically. An attempted reduction in the ED resulted in FNF requiring THA (Figures 5, 6, 9). After discussion of this complication in our institution’s morbidity and mortality conference, we have developed a protocol for the geriatric patient (older than 65 years) with a traumatic hip dislocation. These patients will undergo attempted reduction under controlled analgesia and muscle relaxation in the operating room (OR) with an attending surgeon present, ideally, an attending surgeon comfortable with arthroplasty in a terminally cleaned OR room. Our institution’s surgical site infection rate after total joint arthroplasty has significantly decreased with improved patient selection and the use of terminally cleaned OR rooms. Because our policy is to perform closed reduction of dislocated hips in an urgent manner, if there is not a terminally clean room or an arthroplasty-trained attending orthopedic surgeon available, then informed consent with discussion of the possibility of fracture requiring a subsequent arthroplasty should be obtained from the patient before the attempted reduction.
After review of the available literature, we believe that this case highlights some of the important treatment principles when treating anterior hip dislocations in the ED. The relatively high incidence of indentation fractures of the femoral head with obturator dislocations puts these fractures at higher risk for possible impingement around the obturator ring. This impingement, coupled with preexisting osteopenia, can predispose these dislocations to FNF, if appropriate analgesia and sedation are not obtained and gentle reduction is not performed. In addition, while it may not be time- or cost-effective to perform closed reduction on every hip dislocation, we bring geriatric patients with radiographic osteopenia to the OR for more controlled reductions. In the informed consent discussion, the possibility of FNF is mentioned, and the patient and family are told that an elective total hip replacement will be performed if this complication occurs.
We consider the following to be risk factors for closed reductions of anterior hip dislocations: (1) preexisting osteopenia on plain films, (2) age greater than 65 years, and (3) radiographic femoral head impingement on the surrounding bony pelvis. We continue to consider closed reduction of both anterior and posterior hip dislocations as urgent (within 6 hours from time of dislocation). This case adds to the existing literature on the risk of FNF with closed reduction of obturator hip dislocations, and we hope that it will encourage further study into the safest and most cost-effective reduction protocol.
Anterior hip dislocations have been reported to account for approximately 5% to 10% of all hip dislocations.1 Epstein and Wiss2 originally divided anterior hip dislocations into superior (type I, including pubic or subspinous) and inferior (type II, including obturator and perineal) dislocations. This classification was further subdivided based on the presence of either no associated fracture (type A), fracture of the femoral head or neck (FNF; type B), or fracture of the acetabulum (type C).3 Of all anterior hip dislocations, it has been reported that the inferior or obturator type of dislocation is more common, constituting approximately 70% of all anterior dislocations.4 In 1943, Pringle5 described the mechanism of obturator dislocation as simultaneous abduction, flexion, and external rotation of the hip. Our literature search found only 2 case reports in non-English-language journals of a complete FNF associated with an attempted reduction of an anterior hip dislocation.6,7 Indentation fractures of the femoral head have been more commonly reported than FNFs, with a reported incidence of 35% to 55% after anterior dislocation.4,8 DeLee and colleagues8 also found that those patients with indentation fractures were at a higher risk for developing avascular necrosis of the femoral head in addition to being more likely to report poor or fair function of the hip 2 years after reduction.
There have been a number of different reduction maneuvers for anterior dislocation of hips published in the literature. Epstein and Harvey9 advocated reduction by traction in the line of the femur with the hip flexed and in gentle internal rotation and abduction while the patient was under general anesthesia. Toms and Williams,10 however, recommended adduction with gradual release of the longitudinal traction. Polesky and Polesky11 described a reduction method involving sharp internal rotation, which was found to be associated with FNF. The patient provided written informed consent for print and electronic publication of this case report, and approval was obtained from the Emory University Institutional Review Board.
Case Report
The patient was a 73-year-old woman, an independent ambulator with minimal antecedent hip pain, who, as a pedestrian, was struck by a heavy-duty pickup truck at low velocity. She was flown to our level I trauma center from an outside hospital. The patient arrived hemodynamically stable, with a Glasgow Coma Scale score of 15 and with major complaints of right shoulder and right hip pain. She had a positive Focused Assessment with Sonography for Trauma (FAST), and underwent a subsequent urgent chest, abdomen, and pelvis computed tomography (CT) scan for further investigation. CT showed a grade 1 liver laceration. Her anteroposterior (AP) pelvic radiograph and pelvic CT scan showed an anterior hip dislocation with the femoral head located adjacent to the obturator foramen (Figures 1, 2). The AP pelvic radiograph and pelvic CT scan were scrutinized extensively before reduction to rule out a possible FNF. Comparing the right and left femoral necks through multiple axial CT images showed no obvious differences between the 2 sides (Figures 3, 4). Her only other orthopedic injury was an inferior shoulder dislocation. It is not routine for the general surgery trauma team to obtain a pelvic CT scan prior to involvement of the orthopedic service and prompt reduction of a hip dislocation. Upon initial examination of her right hip, it was fixed in slight flexion and external rotation; she was neurovascularly intact.
After being cleared by the trauma service, the patient provided informed consent for closed reduction of the hip and shoulder under conscious sedation, performed by the emergency department (ED) staff. She received intravenous fentanyl and midazolam, and the reduction was attempted. The reduction maneuver was performed with gentle inline traction, adduction, and internal rotation and extension. There was an audible clunk, and the hip was thought to be reduced and stable. The right leg lower extremity was placed into a knee immobilizer and she remained neurovascularly intact. The shoulder was reduced. After the procedure, the patient had an episode of hypoxia requiring oxygenation via a bag valve mask by the ED staff. Postreduction radiographs confirmed reduction of the right shoulder; however, they also showed a FNF with the femoral head retained near the obturator foramen (Figures 5, 6). The patient and her family were informed of the fracture, and a total hip arthroplasty (THA) was recommended, given her pre-injury mild symptomatic osteoarthritis in the hip and her age. The patient was admitted to the intensive care unit for cardiopulmonary monitoring and was found to have a troponin leak on hospital day 1. She was evaluated by the cardiology service; serial electrocardiograms and troponins ruled out acute myocardial infarction. The patient was cleared for surgery on hospital day 4.
On hospital day 5, she underwent a right THA via a Kocher-Langenbeck approach. The patient’s femoral head was found to be anterior and laterally adjacent to her ischial tuberosity with an indentation fracture. The sciatic nerve was identified and found to be intact. A metal-on-polyethylene Stryker Accolade femoral component and Trident acetabular shell were implanted, and a posterior capsular repair was performed (Figure 7).
The patient tolerated the procedure well, and her postoperative course was uneventful. She was discharged to a subacute rehabilitation facility on postoperative day 3. The patient returned for her 2-week postoperative visit ambulating without assistance. At her last follow-up visit, approximately 6 weeks after surgery, she was a functionally independent community ambulator. Phone conversations with her private orthopedist at 6 months confirmed continued ambulation without problems.
Discussion
This case report of a complication that occurred in our institution has resulted in a change in our protocol for treatment of geriatric anterior hip dislocations. Our institution is a level I trauma center, and traumatic hip dislocations are relatively common, occurring usually in young patients with high-energy trauma. Although somewhat controversial, it is generally assumed that the incidence of avascular necrosis of the femoral head after dislocation of the hip is correlated with the time interval from dislocation to reduction of the hip. Therefore, our protocol for hip dislocations of the hip in young trauma patients is urgent reduction in the ED under appropriate analgesia and muscle relaxation.
In this case report, the patient was older than 65 years with radiographic evidence of possible impingement and postsurgical evidence of impingement of the femoral head in the obturator foremen (Figures 1, 2, 8). In addition, the patient was significantly osteopenic radiographically. An attempted reduction in the ED resulted in FNF requiring THA (Figures 5, 6, 9). After discussion of this complication in our institution’s morbidity and mortality conference, we have developed a protocol for the geriatric patient (older than 65 years) with a traumatic hip dislocation. These patients will undergo attempted reduction under controlled analgesia and muscle relaxation in the operating room (OR) with an attending surgeon present, ideally, an attending surgeon comfortable with arthroplasty in a terminally cleaned OR room. Our institution’s surgical site infection rate after total joint arthroplasty has significantly decreased with improved patient selection and the use of terminally cleaned OR rooms. Because our policy is to perform closed reduction of dislocated hips in an urgent manner, if there is not a terminally clean room or an arthroplasty-trained attending orthopedic surgeon available, then informed consent with discussion of the possibility of fracture requiring a subsequent arthroplasty should be obtained from the patient before the attempted reduction.
After review of the available literature, we believe that this case highlights some of the important treatment principles when treating anterior hip dislocations in the ED. The relatively high incidence of indentation fractures of the femoral head with obturator dislocations puts these fractures at higher risk for possible impingement around the obturator ring. This impingement, coupled with preexisting osteopenia, can predispose these dislocations to FNF, if appropriate analgesia and sedation are not obtained and gentle reduction is not performed. In addition, while it may not be time- or cost-effective to perform closed reduction on every hip dislocation, we bring geriatric patients with radiographic osteopenia to the OR for more controlled reductions. In the informed consent discussion, the possibility of FNF is mentioned, and the patient and family are told that an elective total hip replacement will be performed if this complication occurs.
We consider the following to be risk factors for closed reductions of anterior hip dislocations: (1) preexisting osteopenia on plain films, (2) age greater than 65 years, and (3) radiographic femoral head impingement on the surrounding bony pelvis. We continue to consider closed reduction of both anterior and posterior hip dislocations as urgent (within 6 hours from time of dislocation). This case adds to the existing literature on the risk of FNF with closed reduction of obturator hip dislocations, and we hope that it will encourage further study into the safest and most cost-effective reduction protocol.
1. Amihood, S. Anterior dislocation of the hip. Injury. 1975;7(2):107-110.
2. Epstein HC, Wiss DA. Traumatic anterior dislocation of the hip. Orthopedics. 1985;8(1):130, 132-134.
3. Epstein HC. Traumatic dislocations of the hip. Clin Orthop Relat Res. 1973(92):116-142.
4. Erb RE, Steele JR, Nance EP Jr, Edwards JR. Traumatic anterior dislocation of the hip: spectrum of plain film and CT findings. AJR Am J Roentgenol. 1995;165(5):1215-1219.
5. Pringle JH. Traumatic dislocation at the hip joint. An experimental study in the cadaver. Glasgow Med J. 1943;21:25-40.
6. Esenkaya I, Görgeç M. Traumatic anterior dislocation of the hip associated with ipsilateral femoral neck fracture: a case report. Acta Orthop Traumatol Turc. 2002;36(4):366-368.
7. Sadler AH, DiStefano M. Anterior dislocation of the hip with ipsilateral basicervical fracture. A case report. J Bone Joint Surg Am. 1985;67(2):326-329.
8. DeLee JC, Evans JA, Thomas J. Anterior dislocation of the hip and associated femoral-head fractures. J Bone Joint Surg Am. 1980;62(6):960-964.
9. Epstein HC, Harvey JP Jr. Traumatic anterior dislocations of the hip: management and results. An analysis of fifty-five cases. J Bone Joint Surg Am. 1972;54(7):1561-1562.
10. Toms AD, Williams S, White SH. Obturator dislocation of the hip. J Bone Joint Surg Br. 2001;83(1):113-115.
11. Polesky RE, Polesky FA. Intrapelvic dislocation of the femoral head following anterior dislocation of the hip. A case report. J Bone Joint Surg Am. 1972;54(5):1097-1098.
1. Amihood, S. Anterior dislocation of the hip. Injury. 1975;7(2):107-110.
2. Epstein HC, Wiss DA. Traumatic anterior dislocation of the hip. Orthopedics. 1985;8(1):130, 132-134.
3. Epstein HC. Traumatic dislocations of the hip. Clin Orthop Relat Res. 1973(92):116-142.
4. Erb RE, Steele JR, Nance EP Jr, Edwards JR. Traumatic anterior dislocation of the hip: spectrum of plain film and CT findings. AJR Am J Roentgenol. 1995;165(5):1215-1219.
5. Pringle JH. Traumatic dislocation at the hip joint. An experimental study in the cadaver. Glasgow Med J. 1943;21:25-40.
6. Esenkaya I, Görgeç M. Traumatic anterior dislocation of the hip associated with ipsilateral femoral neck fracture: a case report. Acta Orthop Traumatol Turc. 2002;36(4):366-368.
7. Sadler AH, DiStefano M. Anterior dislocation of the hip with ipsilateral basicervical fracture. A case report. J Bone Joint Surg Am. 1985;67(2):326-329.
8. DeLee JC, Evans JA, Thomas J. Anterior dislocation of the hip and associated femoral-head fractures. J Bone Joint Surg Am. 1980;62(6):960-964.
9. Epstein HC, Harvey JP Jr. Traumatic anterior dislocations of the hip: management and results. An analysis of fifty-five cases. J Bone Joint Surg Am. 1972;54(7):1561-1562.
10. Toms AD, Williams S, White SH. Obturator dislocation of the hip. J Bone Joint Surg Br. 2001;83(1):113-115.
11. Polesky RE, Polesky FA. Intrapelvic dislocation of the femoral head following anterior dislocation of the hip. A case report. J Bone Joint Surg Am. 1972;54(5):1097-1098.
Bilateral Superior Labrum Anterior to Posterior (SLAP) Tears With Abnormal Anatomy of Biceps Tendon
The biceps brachii derives its name from the 2 heads of the muscle. The short head originates from the coracoid apex, with the coracobrachialis muscle. The long head of the biceps tendon (LHBT) starts within the capsule of the shoulder joint, running from the supraglenoid tubercle or labrum.1 The tendon typically runs free along its intra-articular course, but it is also extrasynovial and ensheathed by a continuation of the synovial lining of the articular capsule that extends to the inferior-most extent of the bicipital groove.2 Congenital anomalies of the LHBT are uncommon, although several atypical forms have been described. A literature search for anomalous LHBT identified several variations in anatomic descriptions, including Y-shaped variant, complete absence of tendon, extra-articular attachment, and a variety of intracapsular attachments. In all, 8 case reports of aberrant intracapsular attachment of LHBT3-12 were identified. These cases presented with a variety of clinical manifestations and pathologic changes. Often, these anatomic variations are considered innocuous, yet some present with pathologic findings.
We present the clinical, magnetic resonance imaging (MRI), and arthroscopic findings of a relatively young athletic patient who was experiencing symptoms of bilateral superior labrum anterior to posterior (SLAP) tears that were unresponsive to conservative management. A unique anatomic variant of the LHBT that involved confluence of the LHBT with the undersurface of the anterosuperior capsule at the rotator interval, as well as a Buford complex anteriorly, was identified and treated. We believe that the tethering of the biceps tendon to the capsule combined with the Buford complex created increased stress on the superior labrum and biceps anchor variant, leading to the development of bilateral symptomatic type II SLAP tears. Knowledge of this variant, though perhaps rare, may be relevant for diagnostic recognition of young athletic patients who present with recalcitrant shoulder symptoms. The patient and the patient’s parents provided written informed consent for print and electronic publication of this case report.
Case Report
A 15-year-old healthy and active athletic boy presented with pain in the right shoulder without history of trauma. He was active in both swimming and baseball. He complained of pain that was present with activities, such as lifting weights, swimming, and throwing. His treatment prior to the office visit consisted of nonsteroidal anti-inflammatory medication, rest, and a therapy program initiated by his high school athletic trainer.
Physical examination demonstrated tenderness to palpation over the posterior capsule and biceps. Motion was full, cuff strength was normal, and SLAP signs (O’Brien, Speed, and Jobe relocation) were positive. A radiograph showed no sign of fracture or dislocation, and no evidence of bony abnormality.
The patient was sent for an MRI arthrogram, which showed a SLAP tear extending from 1 o’clock anteriorly to 10 o’clock posteriorly without intra-articular displacement. No rotator cuff tear was noted. The biceps tendon was noted to be unremarkable and located within the bicipital groove, although retrospective review of the MRI showed that the intra-articular biceps tendon was somewhat confluent with the adjacent tissues.
The patient underwent right shoulder arthroscopy. The shoulder was stable to ligamentous examination under anesthesia. Arthroscopic evaluation revealed that there was a type II SLAP tear extending from the 11-o’clock to the 2-o’clock positions. The superior glenohumeral ligament was identified as it arose from the upper pole of the glenoid labrum and then ran parallel and inferior to the tendon of the biceps towards the lesser tubercle. Surprisingly, there was a very unusual attachment of the intracapsular LHBT to the undersurface of the rotator interval, which restricted biceps excursion in relation to the rotator cuff. Additionally, there was a thick cord-like middle glenohumeral ligament anteriorly that lacked the normal glenoid attachments, thus representing a Buford complex. Interestingly, the labral tear could not only be displaced with a probe, but placing the shoulder through a range of motion also led to increased displacement of the labrum from the glenoid, likely because the biceps tendon was tethered to the undersurface of the capsule.
At the time of arthroscopy, the LHBT was released from its attachment to the capsule at the rotator interval with a radiofrequency wand and shaver. A labral repair was performed using three 2.9-mm bioabsorbable suture anchors, placing 2 posterior and 1 anterior to the biceps tendon. The integrity of the labral repair was observed while placing the shoulder through range of motion.
Postoperatively, the patient was kept in a sling for 5 weeks. Home exercises were initiated at 2 weeks, and outpatient physical therapy was implemented at 4 weeks. The patient resumed swimming, throwing, and other activities—with minimal discomfort—at 6 months postoperatively.
Three years after his initial visit, the patient returned to the office with a similar complaint of pain and limitation of function in his left shoulder after returning to full athletic competition. Once again, there was no history of injury, and history, physical examination, and MRI arthrogram (Figures 1A, 1B) evaluation proved to be very similar to this young athlete’s right shoulder work-up.
The patient once again underwent shoulder arthroscopy and treatment. Although this was now the left shoulder, the findings were essentially identical to the right shoulder. Once again, the labrum was detached from the 11-o’clock to 2-o’clock positions, and a Buford complex was present anteriorly (Figure 2A). The labral tear was easily displaceable from the glenoid with a probe, and placing the shoulder through a range of motion led to increased displacement of the labrum from the glenoid. There was also confluence of the intra-articular LHBT with the undersurface of the capsule within the rotator interval (Figure 2B). A radiofrequency wand, shaver, and elevator were used to define the biceps tendon and separate it from the undersurface of the capsule. The SLAP repair was performed using three 2.9-mm absorbable suture anchors with 2 posterior and 1 anterior to the biceps tendon insertion. The labral repair was observed while placing the shoulder through range of motion and the shoulder was seen to be free of any undue tension on the labrum.
Postoperatively, the patient’s sling and rehabilitation protocol was identical to that of the right shoulder. The patient progressed well, was released to full activity at 6 months, and has not returned with any further complaints of left or right shoulder pain. Approximately 3 years after treatment the patient was contacted via phone and asked about symptoms, pain, and activity. He denies current symptoms of clicking or instability and has no pain that he can identify as being related to previous pathology or treatment. Since the surgery, he has ceased competitive sports and weight lifting, which he attributes to deconditioning associated with postsurgical immobilization and lack of motivation.
Discussion
Of the 8 case reports in the literature that identified variable intra-articular biceps insertional anatomy, only 2 reports represented confluence of the biceps within the rotator interval.7 Interestingly, of the cases identified, the single case that presented a patient with similar pathology of a type II SLAP lesion had an almost identical anatomical variant presentation consisting of both the anomalous insertion of the LHBT into the undersurface of the rotator interval and a Buford variant of the anterosuperior glenohumeral ligament complex. To our knowledge, our bilateral case of an altered intra-articular biceps insertion and a concomitant SLAP tear supports the theory that this pattern of anomalous insertion may very well have altered the biomechanics of the tendon, resulting in acquired pathology to the superior labrum.
The literature reviewed showed the prevalence of anatomic variations of the LHBT ranged from 1.9% to 7.4%.13,14 These variations are generally considered benign; however, in some cases—as in the cases of the young athletes presented by Wahl and MacGillivray7 and in this report—anatomic variation may play an important role in pathogenesis of different injury patterns. The primary function of the LHBT is the stabilization of the glenohumeral joint during abduction and external rotation.15 When the insertion diverges from normal (eg, when the tendon is tethered to the undersurface of the rotator cuff), the biomechanical stresses on the tendon likely change. As a result of the anomalous position of the LHBT origin, there may be a change in the shoulder joint’s biomechanics, with increased strain on the glenohumeral ligament and its attachment onto the glenoid.16
This case report differs from publications on variable superior glenohumeral ligament attachments because a discrete superior glenohumeral ligament structure was isolated from the biceps tendon. Although a larger case series or patient cohort, as well as more involved biomechanical analysis, would certainly be necessary to prove our hypothesis, we believe that this case suggests certain anatomic LHBT and labral variations can contribute to the develop of SLAP tears in younger individuals.
1. Vangsness CT Jr, Jorgenson SS, Watson T, Johnson DL. The origin of the long head of the biceps from the scapula and glenoid labrum. An anatomical study of 100 shoulders. J Bone Joint Surg Br. 1994;76(6):951-954.
2. Burkhead WZ Jr. The biceps tendon. In: Rockwood CA Jr, Matsen FA III, eds. The Shoulder. Vol. 2. Philadelphia: WB Saunders; 1990:791-836.
3. Parikh SN, Bonnaig N, Zbojniewicz A. Intracapsular origin of the long head biceps tendon with glenoid avulsion of the glenohumeral ligaments. Orthopedics. 2011;34(11):781-784.
4. Gaskin CM, Golish SR, Blount KJ, Diduch DR. Anomalies of the long head of the biceps brachii tendon: clinical significance, MR arthrographic findings, and arthroscopic correlation in two patients. Skeletal Radiol. 2007;36(8):785-789.
5. Yeh L, Pedowitz R, Kwak S, et al. Intracapsular origin of the long head of the biceps tendon. Skeletal Radiol. 1999;28(3):178-181.
6. Richards DP, Schwartz M. Anomalous intraarticular origin of the long head of the biceps brachii. Clin J Sport Med. 2003;13(2):122-124.
7. Wahl CJ, MacGillivray JD. Three congenital variations in the long head of the biceps tendon: a review of the pathoanatomic considerations and case reports. J Shoulder Elbow Surg. 2007;16(6):e25-e30.I
8. Egea JM, Melguizo C, Prados J, Aránega A. Capsular origin of the long head of the biceps tendon: a clinical case. Rom J Morphol Embryol. 2010;51(2):375-377.
9. Hyman JL, Warren RF. Extra-articular origin of biceps brachii. Arthroscopy. 2001;17(7): E29.
10. Enad JG. Bifurcate origin of the long head of the biceps tendon. Arthroscopy. 2004;20(10):1081-1083.
11. Mariani PP, Bellelli A, Botticella C. Arthroscopic absence of the long head of the biceps tendon. Arthroscopy. 1997;13(4):499-501.
12. Koplas MC, Winalski CS, Ulmer WH Jr, Recht M. Bilateral congenital absence of the long head of the biceps tendon. Skeletal Radiol. 2009;38(7):715-719.
13. Kanatli U, Ozturk BY, Eisen E, Bolukbasi S. Intra-articular variations of the long head of the biceps tendon. Knee Surg Sports Traumatol Arthrosc. 2011;19(9):1576-1581.
14. Dierickx C, Ceccarelli E, Conti M, Vanlommel J, Castagna A. Variations of the intra-articular portion of the long head of the biceps tendon: a classification of embryologically explained variations. J Shoulder Elbow Surg. 2009;18(4):556-565.
15. Rodosky MW, Harner CD, Fu FH. The role of the long head of the biceps muscle and superior glenoid labrum in anterior stability of the shoulder. Am J Sports Med. 1994;22(1):121-130.
16. Bigliani LU, Kelkar R, Flatow EL, Pollock RG, Mow VC. Glenohumeral stability. Biomechanical properties of passive and active stabilizers. Clin Orthop Relat Res. 1996;(330):13-30.
The biceps brachii derives its name from the 2 heads of the muscle. The short head originates from the coracoid apex, with the coracobrachialis muscle. The long head of the biceps tendon (LHBT) starts within the capsule of the shoulder joint, running from the supraglenoid tubercle or labrum.1 The tendon typically runs free along its intra-articular course, but it is also extrasynovial and ensheathed by a continuation of the synovial lining of the articular capsule that extends to the inferior-most extent of the bicipital groove.2 Congenital anomalies of the LHBT are uncommon, although several atypical forms have been described. A literature search for anomalous LHBT identified several variations in anatomic descriptions, including Y-shaped variant, complete absence of tendon, extra-articular attachment, and a variety of intracapsular attachments. In all, 8 case reports of aberrant intracapsular attachment of LHBT3-12 were identified. These cases presented with a variety of clinical manifestations and pathologic changes. Often, these anatomic variations are considered innocuous, yet some present with pathologic findings.
We present the clinical, magnetic resonance imaging (MRI), and arthroscopic findings of a relatively young athletic patient who was experiencing symptoms of bilateral superior labrum anterior to posterior (SLAP) tears that were unresponsive to conservative management. A unique anatomic variant of the LHBT that involved confluence of the LHBT with the undersurface of the anterosuperior capsule at the rotator interval, as well as a Buford complex anteriorly, was identified and treated. We believe that the tethering of the biceps tendon to the capsule combined with the Buford complex created increased stress on the superior labrum and biceps anchor variant, leading to the development of bilateral symptomatic type II SLAP tears. Knowledge of this variant, though perhaps rare, may be relevant for diagnostic recognition of young athletic patients who present with recalcitrant shoulder symptoms. The patient and the patient’s parents provided written informed consent for print and electronic publication of this case report.
Case Report
A 15-year-old healthy and active athletic boy presented with pain in the right shoulder without history of trauma. He was active in both swimming and baseball. He complained of pain that was present with activities, such as lifting weights, swimming, and throwing. His treatment prior to the office visit consisted of nonsteroidal anti-inflammatory medication, rest, and a therapy program initiated by his high school athletic trainer.
Physical examination demonstrated tenderness to palpation over the posterior capsule and biceps. Motion was full, cuff strength was normal, and SLAP signs (O’Brien, Speed, and Jobe relocation) were positive. A radiograph showed no sign of fracture or dislocation, and no evidence of bony abnormality.
The patient was sent for an MRI arthrogram, which showed a SLAP tear extending from 1 o’clock anteriorly to 10 o’clock posteriorly without intra-articular displacement. No rotator cuff tear was noted. The biceps tendon was noted to be unremarkable and located within the bicipital groove, although retrospective review of the MRI showed that the intra-articular biceps tendon was somewhat confluent with the adjacent tissues.
The patient underwent right shoulder arthroscopy. The shoulder was stable to ligamentous examination under anesthesia. Arthroscopic evaluation revealed that there was a type II SLAP tear extending from the 11-o’clock to the 2-o’clock positions. The superior glenohumeral ligament was identified as it arose from the upper pole of the glenoid labrum and then ran parallel and inferior to the tendon of the biceps towards the lesser tubercle. Surprisingly, there was a very unusual attachment of the intracapsular LHBT to the undersurface of the rotator interval, which restricted biceps excursion in relation to the rotator cuff. Additionally, there was a thick cord-like middle glenohumeral ligament anteriorly that lacked the normal glenoid attachments, thus representing a Buford complex. Interestingly, the labral tear could not only be displaced with a probe, but placing the shoulder through a range of motion also led to increased displacement of the labrum from the glenoid, likely because the biceps tendon was tethered to the undersurface of the capsule.
At the time of arthroscopy, the LHBT was released from its attachment to the capsule at the rotator interval with a radiofrequency wand and shaver. A labral repair was performed using three 2.9-mm bioabsorbable suture anchors, placing 2 posterior and 1 anterior to the biceps tendon. The integrity of the labral repair was observed while placing the shoulder through range of motion.
Postoperatively, the patient was kept in a sling for 5 weeks. Home exercises were initiated at 2 weeks, and outpatient physical therapy was implemented at 4 weeks. The patient resumed swimming, throwing, and other activities—with minimal discomfort—at 6 months postoperatively.
Three years after his initial visit, the patient returned to the office with a similar complaint of pain and limitation of function in his left shoulder after returning to full athletic competition. Once again, there was no history of injury, and history, physical examination, and MRI arthrogram (Figures 1A, 1B) evaluation proved to be very similar to this young athlete’s right shoulder work-up.
The patient once again underwent shoulder arthroscopy and treatment. Although this was now the left shoulder, the findings were essentially identical to the right shoulder. Once again, the labrum was detached from the 11-o’clock to 2-o’clock positions, and a Buford complex was present anteriorly (Figure 2A). The labral tear was easily displaceable from the glenoid with a probe, and placing the shoulder through a range of motion led to increased displacement of the labrum from the glenoid. There was also confluence of the intra-articular LHBT with the undersurface of the capsule within the rotator interval (Figure 2B). A radiofrequency wand, shaver, and elevator were used to define the biceps tendon and separate it from the undersurface of the capsule. The SLAP repair was performed using three 2.9-mm absorbable suture anchors with 2 posterior and 1 anterior to the biceps tendon insertion. The labral repair was observed while placing the shoulder through range of motion and the shoulder was seen to be free of any undue tension on the labrum.
Postoperatively, the patient’s sling and rehabilitation protocol was identical to that of the right shoulder. The patient progressed well, was released to full activity at 6 months, and has not returned with any further complaints of left or right shoulder pain. Approximately 3 years after treatment the patient was contacted via phone and asked about symptoms, pain, and activity. He denies current symptoms of clicking or instability and has no pain that he can identify as being related to previous pathology or treatment. Since the surgery, he has ceased competitive sports and weight lifting, which he attributes to deconditioning associated with postsurgical immobilization and lack of motivation.
Discussion
Of the 8 case reports in the literature that identified variable intra-articular biceps insertional anatomy, only 2 reports represented confluence of the biceps within the rotator interval.7 Interestingly, of the cases identified, the single case that presented a patient with similar pathology of a type II SLAP lesion had an almost identical anatomical variant presentation consisting of both the anomalous insertion of the LHBT into the undersurface of the rotator interval and a Buford variant of the anterosuperior glenohumeral ligament complex. To our knowledge, our bilateral case of an altered intra-articular biceps insertion and a concomitant SLAP tear supports the theory that this pattern of anomalous insertion may very well have altered the biomechanics of the tendon, resulting in acquired pathology to the superior labrum.
The literature reviewed showed the prevalence of anatomic variations of the LHBT ranged from 1.9% to 7.4%.13,14 These variations are generally considered benign; however, in some cases—as in the cases of the young athletes presented by Wahl and MacGillivray7 and in this report—anatomic variation may play an important role in pathogenesis of different injury patterns. The primary function of the LHBT is the stabilization of the glenohumeral joint during abduction and external rotation.15 When the insertion diverges from normal (eg, when the tendon is tethered to the undersurface of the rotator cuff), the biomechanical stresses on the tendon likely change. As a result of the anomalous position of the LHBT origin, there may be a change in the shoulder joint’s biomechanics, with increased strain on the glenohumeral ligament and its attachment onto the glenoid.16
This case report differs from publications on variable superior glenohumeral ligament attachments because a discrete superior glenohumeral ligament structure was isolated from the biceps tendon. Although a larger case series or patient cohort, as well as more involved biomechanical analysis, would certainly be necessary to prove our hypothesis, we believe that this case suggests certain anatomic LHBT and labral variations can contribute to the develop of SLAP tears in younger individuals.
The biceps brachii derives its name from the 2 heads of the muscle. The short head originates from the coracoid apex, with the coracobrachialis muscle. The long head of the biceps tendon (LHBT) starts within the capsule of the shoulder joint, running from the supraglenoid tubercle or labrum.1 The tendon typically runs free along its intra-articular course, but it is also extrasynovial and ensheathed by a continuation of the synovial lining of the articular capsule that extends to the inferior-most extent of the bicipital groove.2 Congenital anomalies of the LHBT are uncommon, although several atypical forms have been described. A literature search for anomalous LHBT identified several variations in anatomic descriptions, including Y-shaped variant, complete absence of tendon, extra-articular attachment, and a variety of intracapsular attachments. In all, 8 case reports of aberrant intracapsular attachment of LHBT3-12 were identified. These cases presented with a variety of clinical manifestations and pathologic changes. Often, these anatomic variations are considered innocuous, yet some present with pathologic findings.
We present the clinical, magnetic resonance imaging (MRI), and arthroscopic findings of a relatively young athletic patient who was experiencing symptoms of bilateral superior labrum anterior to posterior (SLAP) tears that were unresponsive to conservative management. A unique anatomic variant of the LHBT that involved confluence of the LHBT with the undersurface of the anterosuperior capsule at the rotator interval, as well as a Buford complex anteriorly, was identified and treated. We believe that the tethering of the biceps tendon to the capsule combined with the Buford complex created increased stress on the superior labrum and biceps anchor variant, leading to the development of bilateral symptomatic type II SLAP tears. Knowledge of this variant, though perhaps rare, may be relevant for diagnostic recognition of young athletic patients who present with recalcitrant shoulder symptoms. The patient and the patient’s parents provided written informed consent for print and electronic publication of this case report.
Case Report
A 15-year-old healthy and active athletic boy presented with pain in the right shoulder without history of trauma. He was active in both swimming and baseball. He complained of pain that was present with activities, such as lifting weights, swimming, and throwing. His treatment prior to the office visit consisted of nonsteroidal anti-inflammatory medication, rest, and a therapy program initiated by his high school athletic trainer.
Physical examination demonstrated tenderness to palpation over the posterior capsule and biceps. Motion was full, cuff strength was normal, and SLAP signs (O’Brien, Speed, and Jobe relocation) were positive. A radiograph showed no sign of fracture or dislocation, and no evidence of bony abnormality.
The patient was sent for an MRI arthrogram, which showed a SLAP tear extending from 1 o’clock anteriorly to 10 o’clock posteriorly without intra-articular displacement. No rotator cuff tear was noted. The biceps tendon was noted to be unremarkable and located within the bicipital groove, although retrospective review of the MRI showed that the intra-articular biceps tendon was somewhat confluent with the adjacent tissues.
The patient underwent right shoulder arthroscopy. The shoulder was stable to ligamentous examination under anesthesia. Arthroscopic evaluation revealed that there was a type II SLAP tear extending from the 11-o’clock to the 2-o’clock positions. The superior glenohumeral ligament was identified as it arose from the upper pole of the glenoid labrum and then ran parallel and inferior to the tendon of the biceps towards the lesser tubercle. Surprisingly, there was a very unusual attachment of the intracapsular LHBT to the undersurface of the rotator interval, which restricted biceps excursion in relation to the rotator cuff. Additionally, there was a thick cord-like middle glenohumeral ligament anteriorly that lacked the normal glenoid attachments, thus representing a Buford complex. Interestingly, the labral tear could not only be displaced with a probe, but placing the shoulder through a range of motion also led to increased displacement of the labrum from the glenoid, likely because the biceps tendon was tethered to the undersurface of the capsule.
At the time of arthroscopy, the LHBT was released from its attachment to the capsule at the rotator interval with a radiofrequency wand and shaver. A labral repair was performed using three 2.9-mm bioabsorbable suture anchors, placing 2 posterior and 1 anterior to the biceps tendon. The integrity of the labral repair was observed while placing the shoulder through range of motion.
Postoperatively, the patient was kept in a sling for 5 weeks. Home exercises were initiated at 2 weeks, and outpatient physical therapy was implemented at 4 weeks. The patient resumed swimming, throwing, and other activities—with minimal discomfort—at 6 months postoperatively.
Three years after his initial visit, the patient returned to the office with a similar complaint of pain and limitation of function in his left shoulder after returning to full athletic competition. Once again, there was no history of injury, and history, physical examination, and MRI arthrogram (Figures 1A, 1B) evaluation proved to be very similar to this young athlete’s right shoulder work-up.
The patient once again underwent shoulder arthroscopy and treatment. Although this was now the left shoulder, the findings were essentially identical to the right shoulder. Once again, the labrum was detached from the 11-o’clock to 2-o’clock positions, and a Buford complex was present anteriorly (Figure 2A). The labral tear was easily displaceable from the glenoid with a probe, and placing the shoulder through a range of motion led to increased displacement of the labrum from the glenoid. There was also confluence of the intra-articular LHBT with the undersurface of the capsule within the rotator interval (Figure 2B). A radiofrequency wand, shaver, and elevator were used to define the biceps tendon and separate it from the undersurface of the capsule. The SLAP repair was performed using three 2.9-mm absorbable suture anchors with 2 posterior and 1 anterior to the biceps tendon insertion. The labral repair was observed while placing the shoulder through range of motion and the shoulder was seen to be free of any undue tension on the labrum.
Postoperatively, the patient’s sling and rehabilitation protocol was identical to that of the right shoulder. The patient progressed well, was released to full activity at 6 months, and has not returned with any further complaints of left or right shoulder pain. Approximately 3 years after treatment the patient was contacted via phone and asked about symptoms, pain, and activity. He denies current symptoms of clicking or instability and has no pain that he can identify as being related to previous pathology or treatment. Since the surgery, he has ceased competitive sports and weight lifting, which he attributes to deconditioning associated with postsurgical immobilization and lack of motivation.
Discussion
Of the 8 case reports in the literature that identified variable intra-articular biceps insertional anatomy, only 2 reports represented confluence of the biceps within the rotator interval.7 Interestingly, of the cases identified, the single case that presented a patient with similar pathology of a type II SLAP lesion had an almost identical anatomical variant presentation consisting of both the anomalous insertion of the LHBT into the undersurface of the rotator interval and a Buford variant of the anterosuperior glenohumeral ligament complex. To our knowledge, our bilateral case of an altered intra-articular biceps insertion and a concomitant SLAP tear supports the theory that this pattern of anomalous insertion may very well have altered the biomechanics of the tendon, resulting in acquired pathology to the superior labrum.
The literature reviewed showed the prevalence of anatomic variations of the LHBT ranged from 1.9% to 7.4%.13,14 These variations are generally considered benign; however, in some cases—as in the cases of the young athletes presented by Wahl and MacGillivray7 and in this report—anatomic variation may play an important role in pathogenesis of different injury patterns. The primary function of the LHBT is the stabilization of the glenohumeral joint during abduction and external rotation.15 When the insertion diverges from normal (eg, when the tendon is tethered to the undersurface of the rotator cuff), the biomechanical stresses on the tendon likely change. As a result of the anomalous position of the LHBT origin, there may be a change in the shoulder joint’s biomechanics, with increased strain on the glenohumeral ligament and its attachment onto the glenoid.16
This case report differs from publications on variable superior glenohumeral ligament attachments because a discrete superior glenohumeral ligament structure was isolated from the biceps tendon. Although a larger case series or patient cohort, as well as more involved biomechanical analysis, would certainly be necessary to prove our hypothesis, we believe that this case suggests certain anatomic LHBT and labral variations can contribute to the develop of SLAP tears in younger individuals.
1. Vangsness CT Jr, Jorgenson SS, Watson T, Johnson DL. The origin of the long head of the biceps from the scapula and glenoid labrum. An anatomical study of 100 shoulders. J Bone Joint Surg Br. 1994;76(6):951-954.
2. Burkhead WZ Jr. The biceps tendon. In: Rockwood CA Jr, Matsen FA III, eds. The Shoulder. Vol. 2. Philadelphia: WB Saunders; 1990:791-836.
3. Parikh SN, Bonnaig N, Zbojniewicz A. Intracapsular origin of the long head biceps tendon with glenoid avulsion of the glenohumeral ligaments. Orthopedics. 2011;34(11):781-784.
4. Gaskin CM, Golish SR, Blount KJ, Diduch DR. Anomalies of the long head of the biceps brachii tendon: clinical significance, MR arthrographic findings, and arthroscopic correlation in two patients. Skeletal Radiol. 2007;36(8):785-789.
5. Yeh L, Pedowitz R, Kwak S, et al. Intracapsular origin of the long head of the biceps tendon. Skeletal Radiol. 1999;28(3):178-181.
6. Richards DP, Schwartz M. Anomalous intraarticular origin of the long head of the biceps brachii. Clin J Sport Med. 2003;13(2):122-124.
7. Wahl CJ, MacGillivray JD. Three congenital variations in the long head of the biceps tendon: a review of the pathoanatomic considerations and case reports. J Shoulder Elbow Surg. 2007;16(6):e25-e30.I
8. Egea JM, Melguizo C, Prados J, Aránega A. Capsular origin of the long head of the biceps tendon: a clinical case. Rom J Morphol Embryol. 2010;51(2):375-377.
9. Hyman JL, Warren RF. Extra-articular origin of biceps brachii. Arthroscopy. 2001;17(7): E29.
10. Enad JG. Bifurcate origin of the long head of the biceps tendon. Arthroscopy. 2004;20(10):1081-1083.
11. Mariani PP, Bellelli A, Botticella C. Arthroscopic absence of the long head of the biceps tendon. Arthroscopy. 1997;13(4):499-501.
12. Koplas MC, Winalski CS, Ulmer WH Jr, Recht M. Bilateral congenital absence of the long head of the biceps tendon. Skeletal Radiol. 2009;38(7):715-719.
13. Kanatli U, Ozturk BY, Eisen E, Bolukbasi S. Intra-articular variations of the long head of the biceps tendon. Knee Surg Sports Traumatol Arthrosc. 2011;19(9):1576-1581.
14. Dierickx C, Ceccarelli E, Conti M, Vanlommel J, Castagna A. Variations of the intra-articular portion of the long head of the biceps tendon: a classification of embryologically explained variations. J Shoulder Elbow Surg. 2009;18(4):556-565.
15. Rodosky MW, Harner CD, Fu FH. The role of the long head of the biceps muscle and superior glenoid labrum in anterior stability of the shoulder. Am J Sports Med. 1994;22(1):121-130.
16. Bigliani LU, Kelkar R, Flatow EL, Pollock RG, Mow VC. Glenohumeral stability. Biomechanical properties of passive and active stabilizers. Clin Orthop Relat Res. 1996;(330):13-30.
1. Vangsness CT Jr, Jorgenson SS, Watson T, Johnson DL. The origin of the long head of the biceps from the scapula and glenoid labrum. An anatomical study of 100 shoulders. J Bone Joint Surg Br. 1994;76(6):951-954.
2. Burkhead WZ Jr. The biceps tendon. In: Rockwood CA Jr, Matsen FA III, eds. The Shoulder. Vol. 2. Philadelphia: WB Saunders; 1990:791-836.
3. Parikh SN, Bonnaig N, Zbojniewicz A. Intracapsular origin of the long head biceps tendon with glenoid avulsion of the glenohumeral ligaments. Orthopedics. 2011;34(11):781-784.
4. Gaskin CM, Golish SR, Blount KJ, Diduch DR. Anomalies of the long head of the biceps brachii tendon: clinical significance, MR arthrographic findings, and arthroscopic correlation in two patients. Skeletal Radiol. 2007;36(8):785-789.
5. Yeh L, Pedowitz R, Kwak S, et al. Intracapsular origin of the long head of the biceps tendon. Skeletal Radiol. 1999;28(3):178-181.
6. Richards DP, Schwartz M. Anomalous intraarticular origin of the long head of the biceps brachii. Clin J Sport Med. 2003;13(2):122-124.
7. Wahl CJ, MacGillivray JD. Three congenital variations in the long head of the biceps tendon: a review of the pathoanatomic considerations and case reports. J Shoulder Elbow Surg. 2007;16(6):e25-e30.I
8. Egea JM, Melguizo C, Prados J, Aránega A. Capsular origin of the long head of the biceps tendon: a clinical case. Rom J Morphol Embryol. 2010;51(2):375-377.
9. Hyman JL, Warren RF. Extra-articular origin of biceps brachii. Arthroscopy. 2001;17(7): E29.
10. Enad JG. Bifurcate origin of the long head of the biceps tendon. Arthroscopy. 2004;20(10):1081-1083.
11. Mariani PP, Bellelli A, Botticella C. Arthroscopic absence of the long head of the biceps tendon. Arthroscopy. 1997;13(4):499-501.
12. Koplas MC, Winalski CS, Ulmer WH Jr, Recht M. Bilateral congenital absence of the long head of the biceps tendon. Skeletal Radiol. 2009;38(7):715-719.
13. Kanatli U, Ozturk BY, Eisen E, Bolukbasi S. Intra-articular variations of the long head of the biceps tendon. Knee Surg Sports Traumatol Arthrosc. 2011;19(9):1576-1581.
14. Dierickx C, Ceccarelli E, Conti M, Vanlommel J, Castagna A. Variations of the intra-articular portion of the long head of the biceps tendon: a classification of embryologically explained variations. J Shoulder Elbow Surg. 2009;18(4):556-565.
15. Rodosky MW, Harner CD, Fu FH. The role of the long head of the biceps muscle and superior glenoid labrum in anterior stability of the shoulder. Am J Sports Med. 1994;22(1):121-130.
16. Bigliani LU, Kelkar R, Flatow EL, Pollock RG, Mow VC. Glenohumeral stability. Biomechanical properties of passive and active stabilizers. Clin Orthop Relat Res. 1996;(330):13-30.
Xanthogranulomatous Osteomyelitis of Proximal Femur Masquerading as Benign Bone Tumor
Xanthogranulomatous osteomyelitis (XO) is a type of chronic inflammatory process that is characterized by the collection of foamy macrophages along with mononuclear cells in the tissue.1 Xanthogranulomatous osteomyelitis is characterized by the presence of granular, eosinophilic, periodic acid–Schiff–positive histiocytes in the initial stages, followed by the mixture of foamy macrophages and activated plasma cells and, last, by the presence of suppurative foci and hemorrhage. This is an uncommon process best known to occur in the gallbladder, kidney, urinary bladder, fallopian tube, ovary, vagina, prostate, testis, epididymis, colon, and appendix.2-4 Very rarely, it can affect lungs, brain, or bone. Only 5 cases of XO have been reported in the literature.5-8
We report XO of the proximal femur in a 65-year-old woman who initially had a clinical and radiologic diagnosis of aneurysmal bone cyst; however, histopathologic examination confirmed the diagnosis of XO. Xanthogranulomatous osteomyelitis mimics a neoplastic pathology in gallbladder, kidney, and prostrate on gross clinical and radiologic examination.9 The pathogenesis of XO is best characterized by a delayed type of hypersensitivity reaction.10 The differential diagnosis includes chronic recurrent multifocal osteomyelitis, xanthoma, infiltrative storage disorder, malakoplakia, Langerhans cell histiocytosis, fibrohistiocytic tumor, Erdheim-Chester disease, and metastatic renal cell carcinoma.11-14 The patient provided written informed consent for print and electronic publication of this case report.
Case Report
A 65-year-old hypertensive woman presented with complaints of pain in the right hip for a duration of 6 months. Pain was radiating from the right hip region to the anteromedial aspect of the knee and progressively increasing, with a history of pain at rest suggestive of a nonmechanical pathology in the hip. There was no history of fever, weight loss, loss of appetite, pain in any other joint, or morning stiffness. The patient was mobile without support and was able to squat and sit cross-legged; however, the stance phase on the right side was less than on the left side, suggestive of an antalgic component in the gait.
On examining the patient, there was anterior hip joint tenderness with no local sign of any infective or inflammatory pathology. Trochanteric tenderness was present, but there was no irregularity, broadening, or thickening of the trochanter. There was no restriction in the range of motion, and no coronal or sagittal plane deformity in the right hip. There was no limb-length discrepancy. However, the patient was not able to raise her leg actively, probably because of pain in the right hip.
On plain radiographs of the pelvis with bilateral hips, a well-defined nonexpansile uniloculated lytic lesion with sclerotic margins was present in the neck of the right femur, extending to the intertrochanteric area (Figure 1). Ground-glass appearance was also noted. Considering the benign nature of the lesion radiologically and clinically, a differential diagnosis of hyperparathyroidism, renal osteodystrophy, multiple myeloma, and fibrous dysplasia was considered. Hematologic investigations, skeletal survey, and magnetic resonance imaging (MRI) of the bilateral hips were performed to rule out the differential diagnosis.
The patient’s hemoglobin level was 11.8 g/dL with total white blood cell count of 10,300/µL. Renal and hepatic functions were within normal limit. Serum erythrocyte sedimentation rate (ESR) was 12 mm/h and C-reactive protein level was normal. Serum parathyroid level was 32 pg/mL, which was within normal limits, with an alkaline phosphatase level of 101 U/L. The skeletal survey showed no other bony lesion in the body. T1-weighted MRI of both hips showed a well-defined hypointense lesion in the neck and intertrochanteric area of the right hip, which was hyperintense on T2-weighted MRI, suggestive of aneurysmal bone cyst (Figure 2).
Normal ESR, hemoglobin, alkaline phosphatase, and serum parathyroid levels and normal skeletal survey almost ruled out multiple myeloma and hyperparathyroidism. Normal renal profile ruled out renal osteodystrophy and the osteitis fibrosa cystica lesion associated with it. We planned for prophylactic internal fixation of the lesion to prevent a pathologic fracture. According to Mirels,15 if there is a lytic lesion covering more than two-thirds of the circumference of the bone in the peritrochanteric area, the chances of a pathologic fracture are high and such fractures should be fixed.
We planned for curettage of the lesion with bone grafting and in situ intramedullary fixation of the lesion. Curettage was done according to the plan and the sample was sent for histopathologic examination. In situ internal fixation and bone grafting were performed by using a proximal femoral intramedullary nail. To our surprise, the biopsy sample was reported as xanthogranuloma, with multiple foamy macrophages mixed with inflammatory cells and aggregates of lymphocytes (Figure 3). Mycobacterial and routine bacterial cultures were reported as negative. The patient was kept on oral antibiotics (cefixime and moxifloxacin) for 6 weeks, and she made an uneventful recovery. At 6-month follow-up, a radiograph of the right hip showed a healed lesion with proximal femoral nail in situ (Figure 4).
Discussion
To the best of our knowledge, a total of 5 cases of XO have been reported in the literature. The earliest of these reports were by Cozzutto and Carbone,1 who reported 2 cases of XO of the first rib and of the epiphysis of the tibia, respectively. The importance of these lesions to diagnosis is their confusion with a neoplastic disease, as XO is itself a benign disorder. These lesions can mimic a neoplastic lesion in clinical and radiologic presentation and the only way to differentiate the lesion from a neoplastic disease is by histopathologic examination of the tissue. Hypothetically, xanthogranulomatous disorders can be related to trauma or infection.
In 2007, Vankalakunti and colleagues6 reported XO of the ulna in a 50-year-old postmenopausal woman. In that case, progressive swelling was present on the extensor aspect of her right forearm for a period of 2 years, for which curettage and bone grafting were performed, using autograft from the ipsilateral iliac crest. The tissue culture was sterile, and XO was diagnosed as a result of the histopathologic examination. In 2009, Cennimo and colleagues7 reported XO of the index finger and wrist of a man complaining of pain and swelling for 1 year, which was unresponsive to antibiotics. The diagnosis of XO was confirmed histopathologically, when the culture of the same tissue grew Mycobacterium marinum. Radical synovectomy of the lesion was performed, after which minocycline, clarithromycin, and ethambutol were administered. In 2012, Borjian and colleagues8 reported a case of XO of the proximal humerus and proximal fibula in a 14-year-old child. The child, who presented with fever, pain, and restriction of shoulder movements, was started on oral antibiotics as the tissue culture grew Staphylococcus aureus; the patient did not complete the course of treatment in the hospital. No surgical intervention was done in this case. The diagnosis of XO was confirmed by microscopic examination of the tissue.
An association between bacterial infection and xanthogranulomatous inflammation has existed in several organs, such as the kidneys, and in the gastrointestinal system, but such an association of the 2 is yet to be determined for bone.5,10,16-19 Because of the paucity of literature on the disease, a management protocol for XO of bone has not been defined, and decisions have to be made considering the natural history of the disease in other organs. We present this case primarily because of its rarity, curability, and its close resemblance to bone tumors. While XO is benign, it can mimic a neoplastic bone lesion in its imaging and clinical manifestations, and appropriate differentiation is crucial. Currently, histopathologic examination of lesions is the most specific and is the gold standard for diagnosis.
Conclusion
Xanthogranulomatous osteomyelitis is a very rare entity, and only a few cases have been reported in the English-language literature. Though rare, XO warrants greater emphasis than it receives in the literature. It is a chronic inflammatory disease having a close resemblance to bone tumors. A high index of suspicion must be practiced to differentiate XO from tumors. Histopathologic examination is mandatory to establish definitive diagnosis and correct treatment.
1. Cozzutto C, Carbone A. The xanthogranulomatous process. Xanthogranulomatous inflammation. Pathol Res Pract. 1988;183(4):395-402.
2. Ladefoged C, Lorentzen M. Xanthogranulomatous cholecystitis. A clinicopathological study of 20 cases and review of the literature. APMIS. 1993;101(11):869-875.
3. Nistal M, Gonzalez-Peramato P, Serrano A, Regadera J. Xanthogranulomatous funiculitis and orchiepididymitis: report of 2 cases with immunohistochemical study and literature review. Arch Pathol Lab Med. 2004;128(8):911-914.
4. Oh YH, Seong SS, Jang KS, et al. Xanthogranulomatous inflammation presenting as a submucosal mass of the sigmoid colon. Pathol Int. 2005;55(7):440-444.
5. Cozzutto C. Xanthogranulomatous osteomyelitis. Arch Pathol Lab Med. 1984;108(12):973-6.
6. Vankalakunti M, Saikia UN, Mathew M, Kang M. Xanthogranulomatous osteomyelitis of ulna mimicking neoplasm. World J Surg Oncol. 2007;30(5):46.
7. Cennimo DJ, Agag R, Fleegler E, et al. Mycobacterium marinum hand infection in a “sushi chef.” Eplasty. 2009;14(9):e43.
8. Borjian A, Rezaei F, Eshaghi MA, Shemshaki H. Xanthogranulomatous osteomyelitis. J Orthop Traumatol. 2012;13(4):217-220.
9. Rafique M, Yaqoob N. Xanthogranulomatous prostatitis: a mimic of carcinoma of prostate. World J Surg Oncol. 2006;4:30.
10. Nakashiro H, Haraoka S, Fujiwara K, Harada S, Hisatsugu T, Watanabe T. Xanthogranulomatous cholecystis. Cell composition and a possible pathogenetic role of cell-mediated immunity. Pathol Res Pract. 1995;191(11):1078-1086.
11. Hamada T, Ito H, Araki Y, Fujii K, Inoue M, Ishida O. Benign fibrous histiocytoma of the femur: review of three cases. Skeletal Radiol. 1996;25(1):25-29.
12. Kossard S, Chow E, Wilkinson B, Killingsworth M. Lipid and giant cell poor necrobiotic xanthogranuloma. J Cutan Pathol. 2000;27(7):374-378.
13. Girschick HJ, Huppertz HI, Harmsen D, Krauspe R, Müller-Hermelink HK, Papadopoulos T. Chronic recurrent multifocal osteomyelitis in children: diagnostic value of histopathology and microbial testing. Hum Pathol. 1999;30(1):59-65.
14. Kayser R, Mahlfeld K, Grasshoff H. Vertebral Langerhans-cell histiocytosis in childhood – a differential diagnosis of spinal osteomyelitis. Klin Padiatr. 1999;211(5):399-402.
15. Mirels H. Metastatic disease in long bones. A proposed scoring system for diagnosing impending pathologic fractures. Clin Orthop Relat Res. 1989;249:256-264.
16. Machiz S, Gordon J, Block N, Politano VA. Salmonella typhosa urinary tract infection and xanthogranulomatous pyelonephritis. Case report and review of literature. J Fla Med Assoc. 1974;61(9):703-705.
17. Gauperaa T, Stalsberg H. Renal endometriosis. A case report. Scand J Urol Nephrol. 1977;11(2):189-191.
18. Goodman M, Curry T, Russell T. Xanthogranulomatous pyelonephritis (XGP): a local disease with systemic manifestations. Report of 23 patients and review of the literature. Medicine. 1979;58(2):171-181.
19. Guarino M, Reale D, Micoli G, Tricomi P, Cristofori E. Xanthogranulomatous gastritis: association with xanthogranulomatous cholecystitis. J Clin Pathol. 1993;46(1):88-90.
Xanthogranulomatous osteomyelitis (XO) is a type of chronic inflammatory process that is characterized by the collection of foamy macrophages along with mononuclear cells in the tissue.1 Xanthogranulomatous osteomyelitis is characterized by the presence of granular, eosinophilic, periodic acid–Schiff–positive histiocytes in the initial stages, followed by the mixture of foamy macrophages and activated plasma cells and, last, by the presence of suppurative foci and hemorrhage. This is an uncommon process best known to occur in the gallbladder, kidney, urinary bladder, fallopian tube, ovary, vagina, prostate, testis, epididymis, colon, and appendix.2-4 Very rarely, it can affect lungs, brain, or bone. Only 5 cases of XO have been reported in the literature.5-8
We report XO of the proximal femur in a 65-year-old woman who initially had a clinical and radiologic diagnosis of aneurysmal bone cyst; however, histopathologic examination confirmed the diagnosis of XO. Xanthogranulomatous osteomyelitis mimics a neoplastic pathology in gallbladder, kidney, and prostrate on gross clinical and radiologic examination.9 The pathogenesis of XO is best characterized by a delayed type of hypersensitivity reaction.10 The differential diagnosis includes chronic recurrent multifocal osteomyelitis, xanthoma, infiltrative storage disorder, malakoplakia, Langerhans cell histiocytosis, fibrohistiocytic tumor, Erdheim-Chester disease, and metastatic renal cell carcinoma.11-14 The patient provided written informed consent for print and electronic publication of this case report.
Case Report
A 65-year-old hypertensive woman presented with complaints of pain in the right hip for a duration of 6 months. Pain was radiating from the right hip region to the anteromedial aspect of the knee and progressively increasing, with a history of pain at rest suggestive of a nonmechanical pathology in the hip. There was no history of fever, weight loss, loss of appetite, pain in any other joint, or morning stiffness. The patient was mobile without support and was able to squat and sit cross-legged; however, the stance phase on the right side was less than on the left side, suggestive of an antalgic component in the gait.
On examining the patient, there was anterior hip joint tenderness with no local sign of any infective or inflammatory pathology. Trochanteric tenderness was present, but there was no irregularity, broadening, or thickening of the trochanter. There was no restriction in the range of motion, and no coronal or sagittal plane deformity in the right hip. There was no limb-length discrepancy. However, the patient was not able to raise her leg actively, probably because of pain in the right hip.
On plain radiographs of the pelvis with bilateral hips, a well-defined nonexpansile uniloculated lytic lesion with sclerotic margins was present in the neck of the right femur, extending to the intertrochanteric area (Figure 1). Ground-glass appearance was also noted. Considering the benign nature of the lesion radiologically and clinically, a differential diagnosis of hyperparathyroidism, renal osteodystrophy, multiple myeloma, and fibrous dysplasia was considered. Hematologic investigations, skeletal survey, and magnetic resonance imaging (MRI) of the bilateral hips were performed to rule out the differential diagnosis.
The patient’s hemoglobin level was 11.8 g/dL with total white blood cell count of 10,300/µL. Renal and hepatic functions were within normal limit. Serum erythrocyte sedimentation rate (ESR) was 12 mm/h and C-reactive protein level was normal. Serum parathyroid level was 32 pg/mL, which was within normal limits, with an alkaline phosphatase level of 101 U/L. The skeletal survey showed no other bony lesion in the body. T1-weighted MRI of both hips showed a well-defined hypointense lesion in the neck and intertrochanteric area of the right hip, which was hyperintense on T2-weighted MRI, suggestive of aneurysmal bone cyst (Figure 2).
Normal ESR, hemoglobin, alkaline phosphatase, and serum parathyroid levels and normal skeletal survey almost ruled out multiple myeloma and hyperparathyroidism. Normal renal profile ruled out renal osteodystrophy and the osteitis fibrosa cystica lesion associated with it. We planned for prophylactic internal fixation of the lesion to prevent a pathologic fracture. According to Mirels,15 if there is a lytic lesion covering more than two-thirds of the circumference of the bone in the peritrochanteric area, the chances of a pathologic fracture are high and such fractures should be fixed.
We planned for curettage of the lesion with bone grafting and in situ intramedullary fixation of the lesion. Curettage was done according to the plan and the sample was sent for histopathologic examination. In situ internal fixation and bone grafting were performed by using a proximal femoral intramedullary nail. To our surprise, the biopsy sample was reported as xanthogranuloma, with multiple foamy macrophages mixed with inflammatory cells and aggregates of lymphocytes (Figure 3). Mycobacterial and routine bacterial cultures were reported as negative. The patient was kept on oral antibiotics (cefixime and moxifloxacin) for 6 weeks, and she made an uneventful recovery. At 6-month follow-up, a radiograph of the right hip showed a healed lesion with proximal femoral nail in situ (Figure 4).
Discussion
To the best of our knowledge, a total of 5 cases of XO have been reported in the literature. The earliest of these reports were by Cozzutto and Carbone,1 who reported 2 cases of XO of the first rib and of the epiphysis of the tibia, respectively. The importance of these lesions to diagnosis is their confusion with a neoplastic disease, as XO is itself a benign disorder. These lesions can mimic a neoplastic lesion in clinical and radiologic presentation and the only way to differentiate the lesion from a neoplastic disease is by histopathologic examination of the tissue. Hypothetically, xanthogranulomatous disorders can be related to trauma or infection.
In 2007, Vankalakunti and colleagues6 reported XO of the ulna in a 50-year-old postmenopausal woman. In that case, progressive swelling was present on the extensor aspect of her right forearm for a period of 2 years, for which curettage and bone grafting were performed, using autograft from the ipsilateral iliac crest. The tissue culture was sterile, and XO was diagnosed as a result of the histopathologic examination. In 2009, Cennimo and colleagues7 reported XO of the index finger and wrist of a man complaining of pain and swelling for 1 year, which was unresponsive to antibiotics. The diagnosis of XO was confirmed histopathologically, when the culture of the same tissue grew Mycobacterium marinum. Radical synovectomy of the lesion was performed, after which minocycline, clarithromycin, and ethambutol were administered. In 2012, Borjian and colleagues8 reported a case of XO of the proximal humerus and proximal fibula in a 14-year-old child. The child, who presented with fever, pain, and restriction of shoulder movements, was started on oral antibiotics as the tissue culture grew Staphylococcus aureus; the patient did not complete the course of treatment in the hospital. No surgical intervention was done in this case. The diagnosis of XO was confirmed by microscopic examination of the tissue.
An association between bacterial infection and xanthogranulomatous inflammation has existed in several organs, such as the kidneys, and in the gastrointestinal system, but such an association of the 2 is yet to be determined for bone.5,10,16-19 Because of the paucity of literature on the disease, a management protocol for XO of bone has not been defined, and decisions have to be made considering the natural history of the disease in other organs. We present this case primarily because of its rarity, curability, and its close resemblance to bone tumors. While XO is benign, it can mimic a neoplastic bone lesion in its imaging and clinical manifestations, and appropriate differentiation is crucial. Currently, histopathologic examination of lesions is the most specific and is the gold standard for diagnosis.
Conclusion
Xanthogranulomatous osteomyelitis is a very rare entity, and only a few cases have been reported in the English-language literature. Though rare, XO warrants greater emphasis than it receives in the literature. It is a chronic inflammatory disease having a close resemblance to bone tumors. A high index of suspicion must be practiced to differentiate XO from tumors. Histopathologic examination is mandatory to establish definitive diagnosis and correct treatment.
Xanthogranulomatous osteomyelitis (XO) is a type of chronic inflammatory process that is characterized by the collection of foamy macrophages along with mononuclear cells in the tissue.1 Xanthogranulomatous osteomyelitis is characterized by the presence of granular, eosinophilic, periodic acid–Schiff–positive histiocytes in the initial stages, followed by the mixture of foamy macrophages and activated plasma cells and, last, by the presence of suppurative foci and hemorrhage. This is an uncommon process best known to occur in the gallbladder, kidney, urinary bladder, fallopian tube, ovary, vagina, prostate, testis, epididymis, colon, and appendix.2-4 Very rarely, it can affect lungs, brain, or bone. Only 5 cases of XO have been reported in the literature.5-8
We report XO of the proximal femur in a 65-year-old woman who initially had a clinical and radiologic diagnosis of aneurysmal bone cyst; however, histopathologic examination confirmed the diagnosis of XO. Xanthogranulomatous osteomyelitis mimics a neoplastic pathology in gallbladder, kidney, and prostrate on gross clinical and radiologic examination.9 The pathogenesis of XO is best characterized by a delayed type of hypersensitivity reaction.10 The differential diagnosis includes chronic recurrent multifocal osteomyelitis, xanthoma, infiltrative storage disorder, malakoplakia, Langerhans cell histiocytosis, fibrohistiocytic tumor, Erdheim-Chester disease, and metastatic renal cell carcinoma.11-14 The patient provided written informed consent for print and electronic publication of this case report.
Case Report
A 65-year-old hypertensive woman presented with complaints of pain in the right hip for a duration of 6 months. Pain was radiating from the right hip region to the anteromedial aspect of the knee and progressively increasing, with a history of pain at rest suggestive of a nonmechanical pathology in the hip. There was no history of fever, weight loss, loss of appetite, pain in any other joint, or morning stiffness. The patient was mobile without support and was able to squat and sit cross-legged; however, the stance phase on the right side was less than on the left side, suggestive of an antalgic component in the gait.
On examining the patient, there was anterior hip joint tenderness with no local sign of any infective or inflammatory pathology. Trochanteric tenderness was present, but there was no irregularity, broadening, or thickening of the trochanter. There was no restriction in the range of motion, and no coronal or sagittal plane deformity in the right hip. There was no limb-length discrepancy. However, the patient was not able to raise her leg actively, probably because of pain in the right hip.
On plain radiographs of the pelvis with bilateral hips, a well-defined nonexpansile uniloculated lytic lesion with sclerotic margins was present in the neck of the right femur, extending to the intertrochanteric area (Figure 1). Ground-glass appearance was also noted. Considering the benign nature of the lesion radiologically and clinically, a differential diagnosis of hyperparathyroidism, renal osteodystrophy, multiple myeloma, and fibrous dysplasia was considered. Hematologic investigations, skeletal survey, and magnetic resonance imaging (MRI) of the bilateral hips were performed to rule out the differential diagnosis.
The patient’s hemoglobin level was 11.8 g/dL with total white blood cell count of 10,300/µL. Renal and hepatic functions were within normal limit. Serum erythrocyte sedimentation rate (ESR) was 12 mm/h and C-reactive protein level was normal. Serum parathyroid level was 32 pg/mL, which was within normal limits, with an alkaline phosphatase level of 101 U/L. The skeletal survey showed no other bony lesion in the body. T1-weighted MRI of both hips showed a well-defined hypointense lesion in the neck and intertrochanteric area of the right hip, which was hyperintense on T2-weighted MRI, suggestive of aneurysmal bone cyst (Figure 2).
Normal ESR, hemoglobin, alkaline phosphatase, and serum parathyroid levels and normal skeletal survey almost ruled out multiple myeloma and hyperparathyroidism. Normal renal profile ruled out renal osteodystrophy and the osteitis fibrosa cystica lesion associated with it. We planned for prophylactic internal fixation of the lesion to prevent a pathologic fracture. According to Mirels,15 if there is a lytic lesion covering more than two-thirds of the circumference of the bone in the peritrochanteric area, the chances of a pathologic fracture are high and such fractures should be fixed.
We planned for curettage of the lesion with bone grafting and in situ intramedullary fixation of the lesion. Curettage was done according to the plan and the sample was sent for histopathologic examination. In situ internal fixation and bone grafting were performed by using a proximal femoral intramedullary nail. To our surprise, the biopsy sample was reported as xanthogranuloma, with multiple foamy macrophages mixed with inflammatory cells and aggregates of lymphocytes (Figure 3). Mycobacterial and routine bacterial cultures were reported as negative. The patient was kept on oral antibiotics (cefixime and moxifloxacin) for 6 weeks, and she made an uneventful recovery. At 6-month follow-up, a radiograph of the right hip showed a healed lesion with proximal femoral nail in situ (Figure 4).
Discussion
To the best of our knowledge, a total of 5 cases of XO have been reported in the literature. The earliest of these reports were by Cozzutto and Carbone,1 who reported 2 cases of XO of the first rib and of the epiphysis of the tibia, respectively. The importance of these lesions to diagnosis is their confusion with a neoplastic disease, as XO is itself a benign disorder. These lesions can mimic a neoplastic lesion in clinical and radiologic presentation and the only way to differentiate the lesion from a neoplastic disease is by histopathologic examination of the tissue. Hypothetically, xanthogranulomatous disorders can be related to trauma or infection.
In 2007, Vankalakunti and colleagues6 reported XO of the ulna in a 50-year-old postmenopausal woman. In that case, progressive swelling was present on the extensor aspect of her right forearm for a period of 2 years, for which curettage and bone grafting were performed, using autograft from the ipsilateral iliac crest. The tissue culture was sterile, and XO was diagnosed as a result of the histopathologic examination. In 2009, Cennimo and colleagues7 reported XO of the index finger and wrist of a man complaining of pain and swelling for 1 year, which was unresponsive to antibiotics. The diagnosis of XO was confirmed histopathologically, when the culture of the same tissue grew Mycobacterium marinum. Radical synovectomy of the lesion was performed, after which minocycline, clarithromycin, and ethambutol were administered. In 2012, Borjian and colleagues8 reported a case of XO of the proximal humerus and proximal fibula in a 14-year-old child. The child, who presented with fever, pain, and restriction of shoulder movements, was started on oral antibiotics as the tissue culture grew Staphylococcus aureus; the patient did not complete the course of treatment in the hospital. No surgical intervention was done in this case. The diagnosis of XO was confirmed by microscopic examination of the tissue.
An association between bacterial infection and xanthogranulomatous inflammation has existed in several organs, such as the kidneys, and in the gastrointestinal system, but such an association of the 2 is yet to be determined for bone.5,10,16-19 Because of the paucity of literature on the disease, a management protocol for XO of bone has not been defined, and decisions have to be made considering the natural history of the disease in other organs. We present this case primarily because of its rarity, curability, and its close resemblance to bone tumors. While XO is benign, it can mimic a neoplastic bone lesion in its imaging and clinical manifestations, and appropriate differentiation is crucial. Currently, histopathologic examination of lesions is the most specific and is the gold standard for diagnosis.
Conclusion
Xanthogranulomatous osteomyelitis is a very rare entity, and only a few cases have been reported in the English-language literature. Though rare, XO warrants greater emphasis than it receives in the literature. It is a chronic inflammatory disease having a close resemblance to bone tumors. A high index of suspicion must be practiced to differentiate XO from tumors. Histopathologic examination is mandatory to establish definitive diagnosis and correct treatment.
1. Cozzutto C, Carbone A. The xanthogranulomatous process. Xanthogranulomatous inflammation. Pathol Res Pract. 1988;183(4):395-402.
2. Ladefoged C, Lorentzen M. Xanthogranulomatous cholecystitis. A clinicopathological study of 20 cases and review of the literature. APMIS. 1993;101(11):869-875.
3. Nistal M, Gonzalez-Peramato P, Serrano A, Regadera J. Xanthogranulomatous funiculitis and orchiepididymitis: report of 2 cases with immunohistochemical study and literature review. Arch Pathol Lab Med. 2004;128(8):911-914.
4. Oh YH, Seong SS, Jang KS, et al. Xanthogranulomatous inflammation presenting as a submucosal mass of the sigmoid colon. Pathol Int. 2005;55(7):440-444.
5. Cozzutto C. Xanthogranulomatous osteomyelitis. Arch Pathol Lab Med. 1984;108(12):973-6.
6. Vankalakunti M, Saikia UN, Mathew M, Kang M. Xanthogranulomatous osteomyelitis of ulna mimicking neoplasm. World J Surg Oncol. 2007;30(5):46.
7. Cennimo DJ, Agag R, Fleegler E, et al. Mycobacterium marinum hand infection in a “sushi chef.” Eplasty. 2009;14(9):e43.
8. Borjian A, Rezaei F, Eshaghi MA, Shemshaki H. Xanthogranulomatous osteomyelitis. J Orthop Traumatol. 2012;13(4):217-220.
9. Rafique M, Yaqoob N. Xanthogranulomatous prostatitis: a mimic of carcinoma of prostate. World J Surg Oncol. 2006;4:30.
10. Nakashiro H, Haraoka S, Fujiwara K, Harada S, Hisatsugu T, Watanabe T. Xanthogranulomatous cholecystis. Cell composition and a possible pathogenetic role of cell-mediated immunity. Pathol Res Pract. 1995;191(11):1078-1086.
11. Hamada T, Ito H, Araki Y, Fujii K, Inoue M, Ishida O. Benign fibrous histiocytoma of the femur: review of three cases. Skeletal Radiol. 1996;25(1):25-29.
12. Kossard S, Chow E, Wilkinson B, Killingsworth M. Lipid and giant cell poor necrobiotic xanthogranuloma. J Cutan Pathol. 2000;27(7):374-378.
13. Girschick HJ, Huppertz HI, Harmsen D, Krauspe R, Müller-Hermelink HK, Papadopoulos T. Chronic recurrent multifocal osteomyelitis in children: diagnostic value of histopathology and microbial testing. Hum Pathol. 1999;30(1):59-65.
14. Kayser R, Mahlfeld K, Grasshoff H. Vertebral Langerhans-cell histiocytosis in childhood – a differential diagnosis of spinal osteomyelitis. Klin Padiatr. 1999;211(5):399-402.
15. Mirels H. Metastatic disease in long bones. A proposed scoring system for diagnosing impending pathologic fractures. Clin Orthop Relat Res. 1989;249:256-264.
16. Machiz S, Gordon J, Block N, Politano VA. Salmonella typhosa urinary tract infection and xanthogranulomatous pyelonephritis. Case report and review of literature. J Fla Med Assoc. 1974;61(9):703-705.
17. Gauperaa T, Stalsberg H. Renal endometriosis. A case report. Scand J Urol Nephrol. 1977;11(2):189-191.
18. Goodman M, Curry T, Russell T. Xanthogranulomatous pyelonephritis (XGP): a local disease with systemic manifestations. Report of 23 patients and review of the literature. Medicine. 1979;58(2):171-181.
19. Guarino M, Reale D, Micoli G, Tricomi P, Cristofori E. Xanthogranulomatous gastritis: association with xanthogranulomatous cholecystitis. J Clin Pathol. 1993;46(1):88-90.
1. Cozzutto C, Carbone A. The xanthogranulomatous process. Xanthogranulomatous inflammation. Pathol Res Pract. 1988;183(4):395-402.
2. Ladefoged C, Lorentzen M. Xanthogranulomatous cholecystitis. A clinicopathological study of 20 cases and review of the literature. APMIS. 1993;101(11):869-875.
3. Nistal M, Gonzalez-Peramato P, Serrano A, Regadera J. Xanthogranulomatous funiculitis and orchiepididymitis: report of 2 cases with immunohistochemical study and literature review. Arch Pathol Lab Med. 2004;128(8):911-914.
4. Oh YH, Seong SS, Jang KS, et al. Xanthogranulomatous inflammation presenting as a submucosal mass of the sigmoid colon. Pathol Int. 2005;55(7):440-444.
5. Cozzutto C. Xanthogranulomatous osteomyelitis. Arch Pathol Lab Med. 1984;108(12):973-6.
6. Vankalakunti M, Saikia UN, Mathew M, Kang M. Xanthogranulomatous osteomyelitis of ulna mimicking neoplasm. World J Surg Oncol. 2007;30(5):46.
7. Cennimo DJ, Agag R, Fleegler E, et al. Mycobacterium marinum hand infection in a “sushi chef.” Eplasty. 2009;14(9):e43.
8. Borjian A, Rezaei F, Eshaghi MA, Shemshaki H. Xanthogranulomatous osteomyelitis. J Orthop Traumatol. 2012;13(4):217-220.
9. Rafique M, Yaqoob N. Xanthogranulomatous prostatitis: a mimic of carcinoma of prostate. World J Surg Oncol. 2006;4:30.
10. Nakashiro H, Haraoka S, Fujiwara K, Harada S, Hisatsugu T, Watanabe T. Xanthogranulomatous cholecystis. Cell composition and a possible pathogenetic role of cell-mediated immunity. Pathol Res Pract. 1995;191(11):1078-1086.
11. Hamada T, Ito H, Araki Y, Fujii K, Inoue M, Ishida O. Benign fibrous histiocytoma of the femur: review of three cases. Skeletal Radiol. 1996;25(1):25-29.
12. Kossard S, Chow E, Wilkinson B, Killingsworth M. Lipid and giant cell poor necrobiotic xanthogranuloma. J Cutan Pathol. 2000;27(7):374-378.
13. Girschick HJ, Huppertz HI, Harmsen D, Krauspe R, Müller-Hermelink HK, Papadopoulos T. Chronic recurrent multifocal osteomyelitis in children: diagnostic value of histopathology and microbial testing. Hum Pathol. 1999;30(1):59-65.
14. Kayser R, Mahlfeld K, Grasshoff H. Vertebral Langerhans-cell histiocytosis in childhood – a differential diagnosis of spinal osteomyelitis. Klin Padiatr. 1999;211(5):399-402.
15. Mirels H. Metastatic disease in long bones. A proposed scoring system for diagnosing impending pathologic fractures. Clin Orthop Relat Res. 1989;249:256-264.
16. Machiz S, Gordon J, Block N, Politano VA. Salmonella typhosa urinary tract infection and xanthogranulomatous pyelonephritis. Case report and review of literature. J Fla Med Assoc. 1974;61(9):703-705.
17. Gauperaa T, Stalsberg H. Renal endometriosis. A case report. Scand J Urol Nephrol. 1977;11(2):189-191.
18. Goodman M, Curry T, Russell T. Xanthogranulomatous pyelonephritis (XGP): a local disease with systemic manifestations. Report of 23 patients and review of the literature. Medicine. 1979;58(2):171-181.
19. Guarino M, Reale D, Micoli G, Tricomi P, Cristofori E. Xanthogranulomatous gastritis: association with xanthogranulomatous cholecystitis. J Clin Pathol. 1993;46(1):88-90.
Case Report: The Hungry, Hungry Haustra: The Case of a Missing Feeding Tube
Introduction
Percutaneous endoscopic gastrostomy (PEG) tubes are a common method employed for long-term feeding in patients who are unable to tolerate oral feedings.1 Though PEG-tube placement is a common, safe, and well-studied practice, there are known complications, including wound infection, dislodgement, and peritonitis.2 Dislodgement and recurrent ED visits are increasingly becoming a burden on both patients and healthcare providers, as up to 12.8% of patients will require ED replacement of a dislodged tube, totaling an estimated $1,200 per visit.3
Newer techniques include Roux-en-Y feeding jejunostomy tubes, which are anticipated to reduce long-term complications.4,5 However, dislodgement, sinus tracts, and superimposed infections still occur, also leading to ED visits.6 Foley catheters are a readily available and low-cost alternative to replace commercial feeding-tubes in the ED, and are commonly used when the original feeding-tube is not suitable for reuse.7 In the following presentation, the authors describe a previously unseen case of a fully intussuscepted Foley catheter though a Roux-en-Y jejunostomy.
Case Report
A 69-year-old man, recently diagnosed with invasive squamous cell carcinoma of the distal esophagus, presented to the ED with a chief complaint of “J-tube fell out.” One month prior to presentation, the patient had undergone a laparoscopic Janeway Roux-en-Y nipple jejunostomy. He had been previously evaluated several times in the ED for a displaced J-tube, and his commercial feeding tube had been replaced with a Foley catheter without incident.
On this visit, the patient’s wife reported that the Foley catheter had become displaced 3 days prior to presentation, and she assumed that the patient had accidentally pulled it out. According to the patient’s wife, he had attempted oral feedings, but had difficulty swallowing as well as coughing episodes.
Upon initial evaluation, the patient complained of diffuse abdominal pain and cramping. He denied any nausea or vomiting, and reported normal bowel movements. The physical examination was remarkable for the following: hypotension (blood pressure, 64/46 mm Hg); heart rate, 94 beats/minute; temperature, 96.4°F; cachexia; a diffusely tender abdomen; and viable stoma on the anterior abdominal wall. Purulent and malodorous drainage was noted at the stoma site. There was no Foley catheter or J-tube in place, and neither the patient nor his wife had brought the dislodged tube to the ED.
A computed tomography scan of the patient’s abdomen and pelvis were ordered with IV and oral contrast. The imaging studies revealed multiple dilated, fluid-filled loops of small bowel, and a Foley catheter proximal to the ileocecal valve, with the balloon still inflated (Figure).
The emergency physician notified the original surgical team of the patient’s status. The surgical team placed a new, 14 French (Fr)-Foley catheter through the stoma, sutured it in place, and admitted the patient to their service. The patient was maintained on IV antibiotics and fluids. As he continued to pass flatus and stool, a diet was advanced through the replacement Foley catheter. The intussuscepted Foley was subsequently passed naturally on day 4 of his hospital admission. The patient unfortunately died several days later of hypoxic respiratory failure, which was not thought to be related to the ingested catheter.
Discussion
Percutaneous Foley catheters, either pre- or postpyloric, have been used for decades as permanent feeding tubes for patients unable to tolerate oral feedings. These catheters are well-known to be inexpensive and safe replacements for commercial gastrostomy tubes.7 However, a number of complications unique to Foley feeding tubes have been described in case reports, including mechanical obstruction leading to pancreatitis, duodenal obstruction, bowel ischemia secondary to balloon overfilling, pyloric obstruction, bowel infection, as well as broken and digested catheters.8-10
Interestingly, despite multiple case reports demonstrating tube migration, prospective studies have shown this to be a relatively uncommon complication.11 In 2012, a patient in Israel ingested a Foley catheter via the gastrostomy stoma, resulting in small bowel obstruction relieved only by enterotomy and removal of the catheter. There have been no previous documented reports of ingested tubes via jejunostomy stoma.12 Significant forces exerted on Foley catheters have been described, resulting in skin necrosis at the hub and stretching of the catheter from the proximal small bowel to the terminal ileum. In this case presentation, bowel peristalsis was able to advance the entire tube through the skin.13
Management of feeding-Foley-catheter complications typically involves deflation of the balloon and removal and replacement of the offending catheter—usually with a smaller sized Foley catheter (eg, 12Fr, 14Fr, 16Fr). Complicated cases with catheter malfunction have been successfully managed endoscopically.14 The patient in this case was likely at higher risk of complication given the abnormally large wound surrounding the stoma and skin breakdown secondary to superimposed infection.
Conclusion
This case highlights the potent peristaltic forces that are exerted upon a feeding Foley catheter and reinforces the importance of proper tube anchorage. Although this patient did well with direct skin suturing of the replacement catheter, previous studies recommend using a plastic retention ring. Placing a mark on the outside of the catheter as a means to continuously visualize its proper anchorage and placement has also been suggested in the literature. Additionally, whenever a patient presents with a displaced feeding tube (Foley catheter or commercial tube), providers should not assume that the tube has been displaced externally and should maintain a low-threshold for advanced imaging and/or endoscopy if the tube cannot otherwise be located.
Dr Lefkove is an attending physician in the department of emergency medicine, DeKalb Medical Center, Atlanta, Georgia. Dr Meloy is an assistant professor of emergency medicine at Emory University School of Medicine, Atlanta, Georgia.
- Vanis N, Saray A, Gornjakovic S, et al. Percutaneous endoscopic gastrostomy (PEG): retrospective analysis of a 7-year clinical experience. Acta Inform Med. 2012;20(4):235-237.
- Schapiro GD, Edmundowicz SA. Complications of percutaneous endoscopic gastrostomy. Gastrointest Endosc Clin N Am. 1996;6(2):409-422.
- Rosenberger LH, Newhook T, Schirmer B, Sawyer RG. Late accidental dislodgement of a percutaneous endoscopic gastrostomy tube: an underestimated burden on patients and the health care system. Surg Endosc. 2011;25(10):3307-3311.
- Neuman HB, Phillips JD. Laparoscopic Roux-en-Y feeding jejunostomy: a new minimally invasive surgical procedure for permanent feeding access in children with gastric dysfunction. J Laparoendosc Adv Surg Tech A. 2005;15(1):71-74.
- Arnal E, Voiglio EJ, Robert M, Schreiber V, Ceruze P, Caillot JL. Laparoscopic Janeway gastrostomy: an advantageous solution for self-sufficient enteral feeding. Ann Chir. 2005;130(10):613-617.
- Maple JT, Petersen BT, Baron TH, Gostout CJ, Wong Kee Song LM, Buttar NS. Direct percutaneous endoscopic jejunostomy: outcomes in 307 consecutive attempts. Am J Gastroenterol. 2005;100(12):2681-2688.
- Kadakia SC, Cassaday M, Shaffer RT. Comparison of Foley catheter as a replacement gastrostomy tube with commercial replacement gastrostomy tube: a prospective randomized trial. Gastrointest Endosc. 1994;40(2 Pt 1):188-193.
- Brauner E, Kluger Y. Gastrostomy tube dislodgment acute pancreatitis. World J Emerg Surg. 2014;9(1):23.
- Hopens T, Schwesinger WH. Complications of tube gastrostomy: radiologic manifestations. South Med J. 1983;76(1):9-11.
- Martel G, Lingas RI, Gutauskas A, Clark HD. Complication of a percutaneous endoscopic gastrostomy tube causing duodenal ischemia. Surg Laparosc Endosc Percutan Tech. 2006;16(6):445-446.
- Kadakia SC, Cassaday M, Shaffer RT. Prospective evaluation of Foley catheter as a replacement gastrostomy tube. Am J Gastroenterol. 1992;87(11):1594-1597.
- Netz U, Perry ZH, Mizrahi S. The lost foley catheter. Am Surg. 2012;78(9):E407-E408.
- Date RS, Das N, Bateson PG. Unusual complications of ballooned feeding tubes. Ir Med J. 2002;95(6):181-182.
- O’Keefe KP, Dula DJ, Varano V. Duodenal obstruction by a nondeflating Foley catheter gastrostomy tube. Ann Emerg Med. 1990;19(12):1454-1457.
Introduction
Percutaneous endoscopic gastrostomy (PEG) tubes are a common method employed for long-term feeding in patients who are unable to tolerate oral feedings.1 Though PEG-tube placement is a common, safe, and well-studied practice, there are known complications, including wound infection, dislodgement, and peritonitis.2 Dislodgement and recurrent ED visits are increasingly becoming a burden on both patients and healthcare providers, as up to 12.8% of patients will require ED replacement of a dislodged tube, totaling an estimated $1,200 per visit.3
Newer techniques include Roux-en-Y feeding jejunostomy tubes, which are anticipated to reduce long-term complications.4,5 However, dislodgement, sinus tracts, and superimposed infections still occur, also leading to ED visits.6 Foley catheters are a readily available and low-cost alternative to replace commercial feeding-tubes in the ED, and are commonly used when the original feeding-tube is not suitable for reuse.7 In the following presentation, the authors describe a previously unseen case of a fully intussuscepted Foley catheter though a Roux-en-Y jejunostomy.
Case Report
A 69-year-old man, recently diagnosed with invasive squamous cell carcinoma of the distal esophagus, presented to the ED with a chief complaint of “J-tube fell out.” One month prior to presentation, the patient had undergone a laparoscopic Janeway Roux-en-Y nipple jejunostomy. He had been previously evaluated several times in the ED for a displaced J-tube, and his commercial feeding tube had been replaced with a Foley catheter without incident.
On this visit, the patient’s wife reported that the Foley catheter had become displaced 3 days prior to presentation, and she assumed that the patient had accidentally pulled it out. According to the patient’s wife, he had attempted oral feedings, but had difficulty swallowing as well as coughing episodes.
Upon initial evaluation, the patient complained of diffuse abdominal pain and cramping. He denied any nausea or vomiting, and reported normal bowel movements. The physical examination was remarkable for the following: hypotension (blood pressure, 64/46 mm Hg); heart rate, 94 beats/minute; temperature, 96.4°F; cachexia; a diffusely tender abdomen; and viable stoma on the anterior abdominal wall. Purulent and malodorous drainage was noted at the stoma site. There was no Foley catheter or J-tube in place, and neither the patient nor his wife had brought the dislodged tube to the ED.
A computed tomography scan of the patient’s abdomen and pelvis were ordered with IV and oral contrast. The imaging studies revealed multiple dilated, fluid-filled loops of small bowel, and a Foley catheter proximal to the ileocecal valve, with the balloon still inflated (Figure).
The emergency physician notified the original surgical team of the patient’s status. The surgical team placed a new, 14 French (Fr)-Foley catheter through the stoma, sutured it in place, and admitted the patient to their service. The patient was maintained on IV antibiotics and fluids. As he continued to pass flatus and stool, a diet was advanced through the replacement Foley catheter. The intussuscepted Foley was subsequently passed naturally on day 4 of his hospital admission. The patient unfortunately died several days later of hypoxic respiratory failure, which was not thought to be related to the ingested catheter.
Discussion
Percutaneous Foley catheters, either pre- or postpyloric, have been used for decades as permanent feeding tubes for patients unable to tolerate oral feedings. These catheters are well-known to be inexpensive and safe replacements for commercial gastrostomy tubes.7 However, a number of complications unique to Foley feeding tubes have been described in case reports, including mechanical obstruction leading to pancreatitis, duodenal obstruction, bowel ischemia secondary to balloon overfilling, pyloric obstruction, bowel infection, as well as broken and digested catheters.8-10
Interestingly, despite multiple case reports demonstrating tube migration, prospective studies have shown this to be a relatively uncommon complication.11 In 2012, a patient in Israel ingested a Foley catheter via the gastrostomy stoma, resulting in small bowel obstruction relieved only by enterotomy and removal of the catheter. There have been no previous documented reports of ingested tubes via jejunostomy stoma.12 Significant forces exerted on Foley catheters have been described, resulting in skin necrosis at the hub and stretching of the catheter from the proximal small bowel to the terminal ileum. In this case presentation, bowel peristalsis was able to advance the entire tube through the skin.13
Management of feeding-Foley-catheter complications typically involves deflation of the balloon and removal and replacement of the offending catheter—usually with a smaller sized Foley catheter (eg, 12Fr, 14Fr, 16Fr). Complicated cases with catheter malfunction have been successfully managed endoscopically.14 The patient in this case was likely at higher risk of complication given the abnormally large wound surrounding the stoma and skin breakdown secondary to superimposed infection.
Conclusion
This case highlights the potent peristaltic forces that are exerted upon a feeding Foley catheter and reinforces the importance of proper tube anchorage. Although this patient did well with direct skin suturing of the replacement catheter, previous studies recommend using a plastic retention ring. Placing a mark on the outside of the catheter as a means to continuously visualize its proper anchorage and placement has also been suggested in the literature. Additionally, whenever a patient presents with a displaced feeding tube (Foley catheter or commercial tube), providers should not assume that the tube has been displaced externally and should maintain a low-threshold for advanced imaging and/or endoscopy if the tube cannot otherwise be located.
Dr Lefkove is an attending physician in the department of emergency medicine, DeKalb Medical Center, Atlanta, Georgia. Dr Meloy is an assistant professor of emergency medicine at Emory University School of Medicine, Atlanta, Georgia.
Introduction
Percutaneous endoscopic gastrostomy (PEG) tubes are a common method employed for long-term feeding in patients who are unable to tolerate oral feedings.1 Though PEG-tube placement is a common, safe, and well-studied practice, there are known complications, including wound infection, dislodgement, and peritonitis.2 Dislodgement and recurrent ED visits are increasingly becoming a burden on both patients and healthcare providers, as up to 12.8% of patients will require ED replacement of a dislodged tube, totaling an estimated $1,200 per visit.3
Newer techniques include Roux-en-Y feeding jejunostomy tubes, which are anticipated to reduce long-term complications.4,5 However, dislodgement, sinus tracts, and superimposed infections still occur, also leading to ED visits.6 Foley catheters are a readily available and low-cost alternative to replace commercial feeding-tubes in the ED, and are commonly used when the original feeding-tube is not suitable for reuse.7 In the following presentation, the authors describe a previously unseen case of a fully intussuscepted Foley catheter though a Roux-en-Y jejunostomy.
Case Report
A 69-year-old man, recently diagnosed with invasive squamous cell carcinoma of the distal esophagus, presented to the ED with a chief complaint of “J-tube fell out.” One month prior to presentation, the patient had undergone a laparoscopic Janeway Roux-en-Y nipple jejunostomy. He had been previously evaluated several times in the ED for a displaced J-tube, and his commercial feeding tube had been replaced with a Foley catheter without incident.
On this visit, the patient’s wife reported that the Foley catheter had become displaced 3 days prior to presentation, and she assumed that the patient had accidentally pulled it out. According to the patient’s wife, he had attempted oral feedings, but had difficulty swallowing as well as coughing episodes.
Upon initial evaluation, the patient complained of diffuse abdominal pain and cramping. He denied any nausea or vomiting, and reported normal bowel movements. The physical examination was remarkable for the following: hypotension (blood pressure, 64/46 mm Hg); heart rate, 94 beats/minute; temperature, 96.4°F; cachexia; a diffusely tender abdomen; and viable stoma on the anterior abdominal wall. Purulent and malodorous drainage was noted at the stoma site. There was no Foley catheter or J-tube in place, and neither the patient nor his wife had brought the dislodged tube to the ED.
A computed tomography scan of the patient’s abdomen and pelvis were ordered with IV and oral contrast. The imaging studies revealed multiple dilated, fluid-filled loops of small bowel, and a Foley catheter proximal to the ileocecal valve, with the balloon still inflated (Figure).
The emergency physician notified the original surgical team of the patient’s status. The surgical team placed a new, 14 French (Fr)-Foley catheter through the stoma, sutured it in place, and admitted the patient to their service. The patient was maintained on IV antibiotics and fluids. As he continued to pass flatus and stool, a diet was advanced through the replacement Foley catheter. The intussuscepted Foley was subsequently passed naturally on day 4 of his hospital admission. The patient unfortunately died several days later of hypoxic respiratory failure, which was not thought to be related to the ingested catheter.
Discussion
Percutaneous Foley catheters, either pre- or postpyloric, have been used for decades as permanent feeding tubes for patients unable to tolerate oral feedings. These catheters are well-known to be inexpensive and safe replacements for commercial gastrostomy tubes.7 However, a number of complications unique to Foley feeding tubes have been described in case reports, including mechanical obstruction leading to pancreatitis, duodenal obstruction, bowel ischemia secondary to balloon overfilling, pyloric obstruction, bowel infection, as well as broken and digested catheters.8-10
Interestingly, despite multiple case reports demonstrating tube migration, prospective studies have shown this to be a relatively uncommon complication.11 In 2012, a patient in Israel ingested a Foley catheter via the gastrostomy stoma, resulting in small bowel obstruction relieved only by enterotomy and removal of the catheter. There have been no previous documented reports of ingested tubes via jejunostomy stoma.12 Significant forces exerted on Foley catheters have been described, resulting in skin necrosis at the hub and stretching of the catheter from the proximal small bowel to the terminal ileum. In this case presentation, bowel peristalsis was able to advance the entire tube through the skin.13
Management of feeding-Foley-catheter complications typically involves deflation of the balloon and removal and replacement of the offending catheter—usually with a smaller sized Foley catheter (eg, 12Fr, 14Fr, 16Fr). Complicated cases with catheter malfunction have been successfully managed endoscopically.14 The patient in this case was likely at higher risk of complication given the abnormally large wound surrounding the stoma and skin breakdown secondary to superimposed infection.
Conclusion
This case highlights the potent peristaltic forces that are exerted upon a feeding Foley catheter and reinforces the importance of proper tube anchorage. Although this patient did well with direct skin suturing of the replacement catheter, previous studies recommend using a plastic retention ring. Placing a mark on the outside of the catheter as a means to continuously visualize its proper anchorage and placement has also been suggested in the literature. Additionally, whenever a patient presents with a displaced feeding tube (Foley catheter or commercial tube), providers should not assume that the tube has been displaced externally and should maintain a low-threshold for advanced imaging and/or endoscopy if the tube cannot otherwise be located.
Dr Lefkove is an attending physician in the department of emergency medicine, DeKalb Medical Center, Atlanta, Georgia. Dr Meloy is an assistant professor of emergency medicine at Emory University School of Medicine, Atlanta, Georgia.
- Vanis N, Saray A, Gornjakovic S, et al. Percutaneous endoscopic gastrostomy (PEG): retrospective analysis of a 7-year clinical experience. Acta Inform Med. 2012;20(4):235-237.
- Schapiro GD, Edmundowicz SA. Complications of percutaneous endoscopic gastrostomy. Gastrointest Endosc Clin N Am. 1996;6(2):409-422.
- Rosenberger LH, Newhook T, Schirmer B, Sawyer RG. Late accidental dislodgement of a percutaneous endoscopic gastrostomy tube: an underestimated burden on patients and the health care system. Surg Endosc. 2011;25(10):3307-3311.
- Neuman HB, Phillips JD. Laparoscopic Roux-en-Y feeding jejunostomy: a new minimally invasive surgical procedure for permanent feeding access in children with gastric dysfunction. J Laparoendosc Adv Surg Tech A. 2005;15(1):71-74.
- Arnal E, Voiglio EJ, Robert M, Schreiber V, Ceruze P, Caillot JL. Laparoscopic Janeway gastrostomy: an advantageous solution for self-sufficient enteral feeding. Ann Chir. 2005;130(10):613-617.
- Maple JT, Petersen BT, Baron TH, Gostout CJ, Wong Kee Song LM, Buttar NS. Direct percutaneous endoscopic jejunostomy: outcomes in 307 consecutive attempts. Am J Gastroenterol. 2005;100(12):2681-2688.
- Kadakia SC, Cassaday M, Shaffer RT. Comparison of Foley catheter as a replacement gastrostomy tube with commercial replacement gastrostomy tube: a prospective randomized trial. Gastrointest Endosc. 1994;40(2 Pt 1):188-193.
- Brauner E, Kluger Y. Gastrostomy tube dislodgment acute pancreatitis. World J Emerg Surg. 2014;9(1):23.
- Hopens T, Schwesinger WH. Complications of tube gastrostomy: radiologic manifestations. South Med J. 1983;76(1):9-11.
- Martel G, Lingas RI, Gutauskas A, Clark HD. Complication of a percutaneous endoscopic gastrostomy tube causing duodenal ischemia. Surg Laparosc Endosc Percutan Tech. 2006;16(6):445-446.
- Kadakia SC, Cassaday M, Shaffer RT. Prospective evaluation of Foley catheter as a replacement gastrostomy tube. Am J Gastroenterol. 1992;87(11):1594-1597.
- Netz U, Perry ZH, Mizrahi S. The lost foley catheter. Am Surg. 2012;78(9):E407-E408.
- Date RS, Das N, Bateson PG. Unusual complications of ballooned feeding tubes. Ir Med J. 2002;95(6):181-182.
- O’Keefe KP, Dula DJ, Varano V. Duodenal obstruction by a nondeflating Foley catheter gastrostomy tube. Ann Emerg Med. 1990;19(12):1454-1457.
- Vanis N, Saray A, Gornjakovic S, et al. Percutaneous endoscopic gastrostomy (PEG): retrospective analysis of a 7-year clinical experience. Acta Inform Med. 2012;20(4):235-237.
- Schapiro GD, Edmundowicz SA. Complications of percutaneous endoscopic gastrostomy. Gastrointest Endosc Clin N Am. 1996;6(2):409-422.
- Rosenberger LH, Newhook T, Schirmer B, Sawyer RG. Late accidental dislodgement of a percutaneous endoscopic gastrostomy tube: an underestimated burden on patients and the health care system. Surg Endosc. 2011;25(10):3307-3311.
- Neuman HB, Phillips JD. Laparoscopic Roux-en-Y feeding jejunostomy: a new minimally invasive surgical procedure for permanent feeding access in children with gastric dysfunction. J Laparoendosc Adv Surg Tech A. 2005;15(1):71-74.
- Arnal E, Voiglio EJ, Robert M, Schreiber V, Ceruze P, Caillot JL. Laparoscopic Janeway gastrostomy: an advantageous solution for self-sufficient enteral feeding. Ann Chir. 2005;130(10):613-617.
- Maple JT, Petersen BT, Baron TH, Gostout CJ, Wong Kee Song LM, Buttar NS. Direct percutaneous endoscopic jejunostomy: outcomes in 307 consecutive attempts. Am J Gastroenterol. 2005;100(12):2681-2688.
- Kadakia SC, Cassaday M, Shaffer RT. Comparison of Foley catheter as a replacement gastrostomy tube with commercial replacement gastrostomy tube: a prospective randomized trial. Gastrointest Endosc. 1994;40(2 Pt 1):188-193.
- Brauner E, Kluger Y. Gastrostomy tube dislodgment acute pancreatitis. World J Emerg Surg. 2014;9(1):23.
- Hopens T, Schwesinger WH. Complications of tube gastrostomy: radiologic manifestations. South Med J. 1983;76(1):9-11.
- Martel G, Lingas RI, Gutauskas A, Clark HD. Complication of a percutaneous endoscopic gastrostomy tube causing duodenal ischemia. Surg Laparosc Endosc Percutan Tech. 2006;16(6):445-446.
- Kadakia SC, Cassaday M, Shaffer RT. Prospective evaluation of Foley catheter as a replacement gastrostomy tube. Am J Gastroenterol. 1992;87(11):1594-1597.
- Netz U, Perry ZH, Mizrahi S. The lost foley catheter. Am Surg. 2012;78(9):E407-E408.
- Date RS, Das N, Bateson PG. Unusual complications of ballooned feeding tubes. Ir Med J. 2002;95(6):181-182.
- O’Keefe KP, Dula DJ, Varano V. Duodenal obstruction by a nondeflating Foley catheter gastrostomy tube. Ann Emerg Med. 1990;19(12):1454-1457.
Emergency Ultrasound: Abdominal Aortic Aneurysm
Background
Abdominal aortic aneurysm (AAA) is a life-threatening condition, with a high mortality rate in undiagnosed cases. Several studies have shown that emergency physicians (EPs) can accurately diagnose an AAA through bedside ultrasound. This examination, which takes approximately 1 to 2 minutes, is easy to learn, perform, and interpret. In cases of a ruptured aneurysm, where minutes matter, this modality can significantly decrease time to diagnosis and expedite surgical consultation and repair.
To perform the scan, the clinician should use a curvilinear (abdominal) probe with the marker pointed toward the patient’s right side. The probe should be placed just caudal to the xiphoid process in a transverse orientation. To locate the aorta, the “spine shadow” should first be identified for orientation. Vertebral bodies will have a bright rounded white cortex anterior to a dark shadow (Figure 1). The aorta is circular in shape, pulsatile, and lies just anterior to the spine. Care should be taken not to confuse the aorta with the inferior vena cava (IVC), which can also be seen in this view. The IVC is located to the right of the spine (in this orientation, it will be seen on the left side of the clinician’s image). The IVC has a thinner wall and is typically more oval in shape than the aorta.
Once the aorta is visualized, the clinician should slide the probe caudally while keeping the aorta in the center of the screen. The celiac trunk will be initially visualized as the probe is moved, and the superior mesenteric artery will be superior caudal to the celiac trunk. The clinician should then continue to move the probe down caudally until the bifurcation, which is typically found just above the level of the umbilicus, is visualized. It is especially important to visualize the distal (infrarenal) aorta just above the bifurcation since this is where most AAAs occur.
A normal aorta caliber will measure less than 3 cm in diameter, and the common iliac vessels should measure half of that diameter (1.5 cm in adult women, 1.85 cm in adult men). Since the entire diameter of aorta that predicts the risk of rupture, it is critical that measurements include any thrombus present (Figure 2).
Once the entire aorta and bifurcation is seen in cross-section, the probe should be rotated clockwise 90 degrees so that the indicator marker is pointed toward the patient’s head; this will allow a longitudinal view of the aorta (Figure 3). When scanning longitudinally, it is important to place the probe over the center of the aorta as any lateral movement will cause the aorta to appear smaller than its actual size.
Points and Tips to Remember
To ensure the scan is performed correctly, it is essential to begin at the xiphoid process and then proceed caudally—without lifting the transducer off the patient. This will ensure the entire aorta is visualized, which is necessary to rule out an AAA.
During evaluation, bowel gas can obscure the image. If this occurs, the clinician should first try increasing the transducer pressure gradually or moving the probe back and forth while applying pressure in an attempt to displace the bowel gas. If this technique is unsuccessful, scanning off the midline or angling the probe may capture the aorta. Alternatively, a coronal image can be obtained by placing the probe on the patient’s right side in the midaxillary line with the indicator marker pointing toward the head.
Limitations
Despite the high sensitivity of ultrasound in detecting AAAs, there are some limitations to its use. Since the majority of AAAs rupture into the retroperitoneum, a rupture or leak is difficult to visualize on ultrasound. The EP should therefore recognize that the presence of an aneurysm alone in the setting of a convincing clinical history is sufficient to make the diagnosis.
Conclusion
Prompt evaluation and management of patients presenting with a suspected AAA are essential to avoid rupture, a catastrophic event with an extremely high-mortality rate. When the proper techniques for visualization are employed, including proper measurement of the aorta, bedside ultrasound is highly sensitive and specific for detecting an AAA.
Dr Taylor is an assistant professor and director of postgraduate medical education, department of emergency medicine, Emory University School of Medicine, Atlanta, Georgia. Dr Beck is an assistant professor, department of emergency medicine, Emory University School of Medicine, Atlanta, Georgia. Dr Meer is an assistant professor and director of emergency ultrasound, department of emergency medicine, Emory University School of Medicine, Atlanta, Georgia.
Background
Abdominal aortic aneurysm (AAA) is a life-threatening condition, with a high mortality rate in undiagnosed cases. Several studies have shown that emergency physicians (EPs) can accurately diagnose an AAA through bedside ultrasound. This examination, which takes approximately 1 to 2 minutes, is easy to learn, perform, and interpret. In cases of a ruptured aneurysm, where minutes matter, this modality can significantly decrease time to diagnosis and expedite surgical consultation and repair.
To perform the scan, the clinician should use a curvilinear (abdominal) probe with the marker pointed toward the patient’s right side. The probe should be placed just caudal to the xiphoid process in a transverse orientation. To locate the aorta, the “spine shadow” should first be identified for orientation. Vertebral bodies will have a bright rounded white cortex anterior to a dark shadow (Figure 1). The aorta is circular in shape, pulsatile, and lies just anterior to the spine. Care should be taken not to confuse the aorta with the inferior vena cava (IVC), which can also be seen in this view. The IVC is located to the right of the spine (in this orientation, it will be seen on the left side of the clinician’s image). The IVC has a thinner wall and is typically more oval in shape than the aorta.
Once the aorta is visualized, the clinician should slide the probe caudally while keeping the aorta in the center of the screen. The celiac trunk will be initially visualized as the probe is moved, and the superior mesenteric artery will be superior caudal to the celiac trunk. The clinician should then continue to move the probe down caudally until the bifurcation, which is typically found just above the level of the umbilicus, is visualized. It is especially important to visualize the distal (infrarenal) aorta just above the bifurcation since this is where most AAAs occur.
A normal aorta caliber will measure less than 3 cm in diameter, and the common iliac vessels should measure half of that diameter (1.5 cm in adult women, 1.85 cm in adult men). Since the entire diameter of aorta that predicts the risk of rupture, it is critical that measurements include any thrombus present (Figure 2).
Once the entire aorta and bifurcation is seen in cross-section, the probe should be rotated clockwise 90 degrees so that the indicator marker is pointed toward the patient’s head; this will allow a longitudinal view of the aorta (Figure 3). When scanning longitudinally, it is important to place the probe over the center of the aorta as any lateral movement will cause the aorta to appear smaller than its actual size.
Points and Tips to Remember
To ensure the scan is performed correctly, it is essential to begin at the xiphoid process and then proceed caudally—without lifting the transducer off the patient. This will ensure the entire aorta is visualized, which is necessary to rule out an AAA.
During evaluation, bowel gas can obscure the image. If this occurs, the clinician should first try increasing the transducer pressure gradually or moving the probe back and forth while applying pressure in an attempt to displace the bowel gas. If this technique is unsuccessful, scanning off the midline or angling the probe may capture the aorta. Alternatively, a coronal image can be obtained by placing the probe on the patient’s right side in the midaxillary line with the indicator marker pointing toward the head.
Limitations
Despite the high sensitivity of ultrasound in detecting AAAs, there are some limitations to its use. Since the majority of AAAs rupture into the retroperitoneum, a rupture or leak is difficult to visualize on ultrasound. The EP should therefore recognize that the presence of an aneurysm alone in the setting of a convincing clinical history is sufficient to make the diagnosis.
Conclusion
Prompt evaluation and management of patients presenting with a suspected AAA are essential to avoid rupture, a catastrophic event with an extremely high-mortality rate. When the proper techniques for visualization are employed, including proper measurement of the aorta, bedside ultrasound is highly sensitive and specific for detecting an AAA.
Dr Taylor is an assistant professor and director of postgraduate medical education, department of emergency medicine, Emory University School of Medicine, Atlanta, Georgia. Dr Beck is an assistant professor, department of emergency medicine, Emory University School of Medicine, Atlanta, Georgia. Dr Meer is an assistant professor and director of emergency ultrasound, department of emergency medicine, Emory University School of Medicine, Atlanta, Georgia.
Background
Abdominal aortic aneurysm (AAA) is a life-threatening condition, with a high mortality rate in undiagnosed cases. Several studies have shown that emergency physicians (EPs) can accurately diagnose an AAA through bedside ultrasound. This examination, which takes approximately 1 to 2 minutes, is easy to learn, perform, and interpret. In cases of a ruptured aneurysm, where minutes matter, this modality can significantly decrease time to diagnosis and expedite surgical consultation and repair.
To perform the scan, the clinician should use a curvilinear (abdominal) probe with the marker pointed toward the patient’s right side. The probe should be placed just caudal to the xiphoid process in a transverse orientation. To locate the aorta, the “spine shadow” should first be identified for orientation. Vertebral bodies will have a bright rounded white cortex anterior to a dark shadow (Figure 1). The aorta is circular in shape, pulsatile, and lies just anterior to the spine. Care should be taken not to confuse the aorta with the inferior vena cava (IVC), which can also be seen in this view. The IVC is located to the right of the spine (in this orientation, it will be seen on the left side of the clinician’s image). The IVC has a thinner wall and is typically more oval in shape than the aorta.
Once the aorta is visualized, the clinician should slide the probe caudally while keeping the aorta in the center of the screen. The celiac trunk will be initially visualized as the probe is moved, and the superior mesenteric artery will be superior caudal to the celiac trunk. The clinician should then continue to move the probe down caudally until the bifurcation, which is typically found just above the level of the umbilicus, is visualized. It is especially important to visualize the distal (infrarenal) aorta just above the bifurcation since this is where most AAAs occur.
A normal aorta caliber will measure less than 3 cm in diameter, and the common iliac vessels should measure half of that diameter (1.5 cm in adult women, 1.85 cm in adult men). Since the entire diameter of aorta that predicts the risk of rupture, it is critical that measurements include any thrombus present (Figure 2).
Once the entire aorta and bifurcation is seen in cross-section, the probe should be rotated clockwise 90 degrees so that the indicator marker is pointed toward the patient’s head; this will allow a longitudinal view of the aorta (Figure 3). When scanning longitudinally, it is important to place the probe over the center of the aorta as any lateral movement will cause the aorta to appear smaller than its actual size.
Points and Tips to Remember
To ensure the scan is performed correctly, it is essential to begin at the xiphoid process and then proceed caudally—without lifting the transducer off the patient. This will ensure the entire aorta is visualized, which is necessary to rule out an AAA.
During evaluation, bowel gas can obscure the image. If this occurs, the clinician should first try increasing the transducer pressure gradually or moving the probe back and forth while applying pressure in an attempt to displace the bowel gas. If this technique is unsuccessful, scanning off the midline or angling the probe may capture the aorta. Alternatively, a coronal image can be obtained by placing the probe on the patient’s right side in the midaxillary line with the indicator marker pointing toward the head.
Limitations
Despite the high sensitivity of ultrasound in detecting AAAs, there are some limitations to its use. Since the majority of AAAs rupture into the retroperitoneum, a rupture or leak is difficult to visualize on ultrasound. The EP should therefore recognize that the presence of an aneurysm alone in the setting of a convincing clinical history is sufficient to make the diagnosis.
Conclusion
Prompt evaluation and management of patients presenting with a suspected AAA are essential to avoid rupture, a catastrophic event with an extremely high-mortality rate. When the proper techniques for visualization are employed, including proper measurement of the aorta, bedside ultrasound is highly sensitive and specific for detecting an AAA.
Dr Taylor is an assistant professor and director of postgraduate medical education, department of emergency medicine, Emory University School of Medicine, Atlanta, Georgia. Dr Beck is an assistant professor, department of emergency medicine, Emory University School of Medicine, Atlanta, Georgia. Dr Meer is an assistant professor and director of emergency ultrasound, department of emergency medicine, Emory University School of Medicine, Atlanta, Georgia.
Thoracic Outlet Syndrome: Current Concepts, Imaging Features, and Therapeutic Strategies
Thoracic outlet syndrome (TOS) was first described by Coot in 1861,1,2 and the term was coined by Peet and colleagues3 in 1956 to cover a spectrum of conditions caused by dynamic compression of the brachial plexus (neurogenic), subclavian artery (arterial), or subclavian vein (venous). The estimated incidence of TOS is 10 in 100,000.4 However, cadaveric studies have suggested that up to 90% of the population may have what is considered abnormal anatomy of the thoracic outlet,5 which in turn suggests a multifactorial etiology for symptomatic disease. TOS is most commonly diagnosed in patients 20 to 40 years of age, with females affected in a 4:1 ratio.6 Although historically TOS is a clinical diagnosis, advanced imaging is often helpful in determining the nature and location of the structure undergoing compression and the structure producing compression, which help guide management. Computed tomography angiography (CTA) and magnetic resonance imaging (MRI) performed in association with postural maneuvers aid in the diagnosis in patients with dynamically acquired compression.7
Pathophysiology
The pathophysiology of TOS is attributable to the unique anatomy of the thoracic outlet. Compromise of the neurovascular structures can occur through congenital or acquired narrowing in 3 distinct compartments: the interscalene triangle, the costoclavicular space, and the retropectoralis minor space. The interscalene triangle is the most medial of the compartments. Containing the subclavian artery and the 3 trunks of the brachial plexus, it is bordered anteriorly by the anterior scalene muscle, posteriorly by the middle and posterior scalene muscles, and inferiorly by the first rib. The interscalene triangle is the most frequent site of neurologic compression.8 The middle compartment is the costoclavicular space, which is bordered superiorly by the clavicle, anteriorly by the subclavius muscle, and posteriorly by the first rib and the middle scalene muscle. The costoclavicular space is the most frequent site of arterial compression,8 where the artery lies directly anterior to the subclavian vein and is surrounded by the 3 cords of the brachial plexus. The most lateral compartment is the retropectoralis minor space, which is bordered anteriorly by the pectoralis minor muscle, superiorly by the subscapularis muscle, and inferiorly by the anterior chest wall. Sources of neurovascular compression within any of the spaces include cervical ribs9; elongated C7 transverse processes; hypertrophy of the anterior or middle scalene, subclavius, or pectoralis minor muscles10; anomalous scalenus minimus muscle; repetitive overhead arm movements (pitching, swimming)11; anomalous fascial bands; degenerative spine disease; bone destruction from primary or secondary neoplasms (Pancoast tumor); hyperextension/flexion injury of the neck12; and malunion of clavicle fractures, among others.13
Classification
Three distinct TOSs have been described, individually or combined, depending on the injured component: neurogenic from brachial plexus compression, arterial from subclavian artery compression, and venous from subclavian or axillary vein compression.14,15
Neurogenic TOS has 2 reported types: true (classic) and disputed. True neurogenic TOS is rare, with an estimated incidence of 1 in 1 million.16 First described in 1970 as a lower trunk plexopathy involving slowly progressive unilateral weakness of the intrinsic hand muscles and sensory abnormalities in the ulnar and medial antebrachial cutaneous nerve distributions, true neurogenic TOS was originally called Gilliatt-Sumner hand syndrome.17 A congenital band extending between the first rib and an elongated C7 transverse process was thought to be the location of brachial plexus injury in true neurogenic TOS. Conversely, disputed neurogenic TOS is the most common form of TOS, occurring in 3 to 80 per 100018 and accounting for 90% to 95% of all TOS cases.13,19 In contrast to true neurogenic TOS, in which anatomical and electrodiagnostic evidence supports the diagnosis, objective clinical findings are often lacking in the disputed form.18 Patients with disputed neurogenic TOS present with a diverse array of symptoms, including pain, numbness, and weakness affecting the neck, shoulder, and arm, exacerbated by activities requiring elevation or sustained use of the extremity.20
Arterial TOS accounts for 1% to 5% of all TOS cases.21 Arterial TOS typically affects patients who perform repetitive movements of the upper extremities with their arms above their shoulders, resulting in compression of the subclavian artery. Symptoms of arterial TOS include pain, weakness, coolness, pallor, and paresthesia.18,22 In severe cases of compression, subclavian artery damage can result in thrombosis with distal embolization, poststenotic aneurysm, or even retrograde extension causing stroke.22,23
Last, representing 2% to 3% of all TOS cases, venous TOS results from compression of the subclavian or axillary vein.18,24 Two mechanisms for vascular compromise have been described. The first involves compression of the vein between the clavicle and the first rib with overhead activities.18 Patients often experience intermittent “heaviness” of the extremity with repeated overhead use. The second mechanism involves repeated stress between the clavicle and vein, causing an intravascular thrombosis.18 Patients may experience pain, edema, cyanosis, venous distention, and even spontaneous venous thrombosis, referred to as Paget-Schroetter syndrome, which can lead to pulmonary embolism.6,25,26
Clinical Features
In cases of suspected TOS, clinicians should take a thorough history and perform a thorough physical examination. The differential diagnosis for unilateral, upper limb pain, numbness, tingling, and/or weakness exacerbated by movement includes shoulder and rotator cuff pathology, cervical spine injury, cervical radiculitis, distal compressive neuropathies (carpal or cubital tunnel syndrome), and neuralgic amyotrophy (Parsonage-Turner syndrome/acute brachial radiculitis).27,28 The clinician should pursue a history of trauma to the shoulder or neck as well as any occupational or recreational activities involving elevation of the upper extremity for extended periods.29 Physical examination must include an evaluation of the contralateral side and may begin with visual inspection to assess for muscle asymmetry, atrophy, color changes, edema, or deformities.18 Next, palpation should be used to assess for any tenderness, texture changes, masses, or vascular pulsations. Attention should be directed at examination of the cervical spine as well as neurologic and vascular assessments of the bilateral upper extremities, including range of motion and strength testing,18 to rule out alternative etiologies.
Four basic maneuvers—the Roos test,30 Adson test,31 Wright test,32 and costoclavicular test—traditionally have been used to diagnose TOS. A positive Roos test involves symptom reproduction with the patient slowly opening and closing the hand for 3 minutes with the arm externally rotated and abducted to 90°.33 However, the false-positive rate of the Roos test is as high as 77% in patients with carpal tunnel syndrome and up to 47% in normal subjects.34 The Adson test is performed by having the patient inhale deeply while the arm is kept in the anatomical position with the head extended and turned toward the involved extremity. The examiner monitors the radial pulse; an absent or diminished radial pulse suggests compression of the subclavian artery. The Adson test is not very reliable, however, because the pulse diminishes even in normal subjects,6,26 with a reported false-positive rate of 13.5%.35 A positive costoclavicular compression test occurs when depressing a patient’s shoulder reproduces symptoms. In one study, the false-positive rate of the costoclavicular compression test was 48% in patients with carpal tunnel syndrome and 16% in normal subjects.34 Last, the Wright test is performed by hyperabducting and externally rotating the affected shoulder. It is positive with a diminished pulse or reproduction of symptoms. One study found that the Wright test had 70% to 90% sensitivity and 29% to 53% specificity.36
Clinically distinguishing between the various forms of TOS may be difficult, and occasionally multiple types exist in a single patient, exacerbating one another and adding to the diagnostic difficulty. For example, arterial insufficiency may lead to disruption of the neural microcirculation, leading to concurrent arterial and neurogenic TOS. Because most cases present with nonspecific symptoms, advanced imaging modalities are often required to establish a definitive diagnosis and to target therapy to the appropriate site of compression.
Imaging Features
Plain Radiography
First, cervical spine and chest radiographs should be obtained to assess for bone abnormalities, including cervical ribs, long transverse processes, rib/clavicle fracture callus, rib anomalies, degenerative spine disease, and neoplasm (Pancoast/apical tumor) (Figure 1).18,25
Ultrasonography
Ultrasonography is useful in evaluating arterial or venous TOS because of its low cost, noninvasive nature, and high specificity for vessel occlusion.37,38 In arterial TOS, ultrasound may demonstrate increased flow velocity through a stenosis or an aneurysmal degeneration distal to the stenosis.7 In venous TOS, duplex ultrasound can identify stasis and thrombus.7 Obtaining duplex ultrasound with the upper extremity in multiple positions allows clinicians to correlate dynamically induced symptoms with ultrasonographic findings of altered blood flow.39-41 Despite the purported benefits of ultrasound, its drawback is that it is operator-dependent,42 with some studies reporting a high false-positive rate24 for diagnosis of venous TOS.
Electrodiagnostic Testing
Ruling out etiologies such as cervical radiculitis (Parsonage-Turner syndrome), cervical radiculopathies, brachial plexus lesions, and other distal compressive neuropathies requires nerve conduction studies and electromyography.18,43-46 In true neurogenic TOS, a combination of decreased sensory nerve action potentials in the ulnar and medial antebrachial cutaneous nerves and decreased compound motor action potentials in the median nerve is often found.18 Specifically, an abnormal ulnar sensory nerve action potential suggests the lesion is situated away from the intraspinal canal, which argues against a diagnosis of radiculopathy or myelopathy.43,44 In the disputed form of neurogenic TOS, the role of electrodiagnostic testing is less clear.18
Conventional Arteriography and Venography
Although CTA has superseded conventional arteriography and venography in most treatment centers, it may still be used in patients with acute symptoms requiring immediate thrombolytic therapy. Catheter angiography and venography with postural maneuvers are often the first invasive treatment modality in cases of thoracic outlet vascular compression.22,24 Presence of intraluminal thrombus, vessel dilatation, and collateral vessels is readily demonstrated (Figure 2A). Recanalization of occluded vessels can be attempted using balloon angioplasty and venoplasty (Figure 2B), but it is usually only temporarily successful if the cause of extrinsic compression is not corrected (Figures 2C, 2D). CTA or conventional angiography, used if sophisticated CTA with 3-dimensional (3-D) reconstruction is unavailable, is the gold standard in diagnosis of TOS.
CTA and Venography
Computed tomography (CT) is a valuable modality because it can be performed rapidly and effectively to depict the relationship of vascular structures to surrounding bone and muscle.47 In addition, CTA and venography provide high-quality representations of the vasculature, and 3-D reconstruction reliably identifies areas of neurovascular compression in patients with TOS.47,48 Furthermore, CT may be performed in a dynamic fashion, with the upper extremity in various positions to reproduce dynamic compression of the neurovascular structures (Figure 3A). Comparison of the images with the upper extremities in the anatomical position and elevated allows the physician to evaluate narrowing of the compartments and dynamic compression of neurovascular structures.8 CT is particularly valuable in arterial and venous TOS. In arterial TOS, the cross-sectional area or diameter of the artery can be measured to calculate the degree of stenosis.8,47 In venous TOS, dynamic narrowing of the vein can be visualized and may be associated with venous thrombosis or collateral circulation (Figure 3B). Although a variety of maneuvers is possible during CTA, the size of the CT tunnel as well as mandatory supine positioning of the patient may limit the series. Drawbacks of CT for diagnosing TOS include difficulties in analyzing the brachial plexus because of limited contrast resolution. In addition, the risks of CT (ionizing radiation, administration of iodinated contrast medium) must be considered before image acquisition.
MRI
MRI is a noninvasive and nonionizing technique that offers good resolution of the anatomical components of the thoracic outlet8 and that, because of its superior soft-tissue contrast, is the modality of choice for imaging brachial plexus nerve compression in TOS (Figure 4). Neurologic compression is identified with MRI when the fat surrounding the brachial plexus disappears.8 MRI reliably identifies the source of compression, which may include bony structures, muscle hypertrophy (scalenus, scalenus minimus, subclavius, pectoralis minor), and fibrous bands.49 Because of their craniocaudal direction, the sagittal plane is often most useful in demonstrating neurovascular compression.42 Analyzing the caliber of the vessel along its course may evaluate vascular compression, and magnetic resonance (MR) angiography and venography (Figures 5A, 5B) can often complement the findings.50 Specifically, in arterial TOS, poststenotic aneurysmal dilatation may be seen, whereas thrombosis and collateral circulation can be visualized in cases of venous TOS.50 Limitations of MRI in the diagnosis of TOS historically were similar to those of CT, and included supine positioning as well as restricted upper extremity maneuvers because of the size of the tunnel and the presence of surface coils.42 However, newer higher channel surface coils and wider bores allow for imaging in a wider range of motion, including arm hyperabduction (Figures 5C, 5D), which is often necessary to elicit pathology.
Management
Generally, therapeutic options for TOS are aimed at relieving the source of neurovascular compression. It is important that treatment be directed only toward symptomatic patients, as many patients have anatomy consistent with TOS and remain asymptomatic.5 Treatment of TOS is predominately conservative and involves a combination of patient education, activity modification, medication, and rehabilitation to promote appropriate body mechanics and posture.18
Physical Therapy
Physical therapy should be aimed at decreasing pressure on the neurovascular structures of the thoracic outlet by relaxing the scalene muscles, strengthening the shoulder muscles, and working on postural exercises to help the patient sit and stand straighter.51 The scalene muscles are the primary targets for TOS rehabilitation, but focus should also be given to the upper trapezius, levator scapulae, sternocleidomastoid, pectoral, and suboccipital muscles.18 Physical therapy is often combined with hydrotherapy, massage, nonsteroidal anti-inflammatory drugs, and muscle relaxants for maximal symptomatic relief. Some patients have found relief with selective anesthetic or botulinum toxin A injections in the scalene muscles.18 A minimum of 4 to 6 weeks (often 4-6 months) of physical therapy and conservative treatment should be attempted before consideration of any invasive intervention.13,18
Anticoagulation
In venous TOS with evidence of thrombus but no obstructive clot, conservative management is typically sufficient. In rare cases, however, intimal damage secondary to vascular compression in arterial and venous TOS leads to thrombus formation, impairing upper extremity perfusion and producing symptoms. Treatment guidelines for venous TOS involve catheter-directed thrombolysis within 2 weeks of symptom onset.15 Thrombolysis replaced the prior recommendation of systemic anticoagulation combined with extremity rest and elevation because anticoagulation and rest alone result in up to 75% morbidity,52,53 whereas thrombolysis reestablishes vessel patency in nearly all patients.54 After thrombolysis, patients should receive intravenous heparin, and conversion to oral anticoagulation should occur as soon as manageable. In patients with arterial TOS, the goal of treatment is revascularization to prevent or decrease ischemia. In mild arterial ischemia, catheter-directed thrombolysis can be attempted. However, the threshold for surgical thromboembolectomy must remain low, as acute upper extremity ischemia may result in compartment syndrome and permanent loss of function.13 Fixed arterial lesions, whether occlusive or aneurysmal, are an absolute indication for thromboembolectomy with possible thoracic outlet decompression.13
Thoracic Outlet Decompression
Indications for surgical decompression are controversial. They include symptomatic patients who have vascular (arterial or venous) TOS and are not at high risk for surgery, patients with true neurologic TOS and acute progressive neurologic weakness or disabling pain,55 and patients who have disputed neurologic TOS and have failed conservative management—keeping in mind that high recurrence rates and iatrogenic brachial plexopathy have been reported in this population.56 In general, surgical procedures are aimed at reducing soft-tissue compression (scalene release or neurolysis) or bony compression (cervical or first thoracic rib excision). Three surgical approaches (transaxillary, supraclavicular, infraclavicular) are commonly used for decompression, and surgeons choose one over another depending on the anatomical abnormality causing the compression. The transaxillary approach requires limited dissection but still allows for adequate visualization of the rib during resection.57 In this approach, a transverse incision along the inferior border of the axilla extends from the pectoralis major to the latissimus dorsi. After dissection of the axillary vessels and the first thoracic nerve root, the first rib is identified and can be removed, when indicated. In contrast, the supraclavicular approach provides a wide exposure, and the site of compression is directly visualized, allowing for arterial reconstruction.58 Through this approach, the anterior and middle scalene muscles can be resected, and neurolysis of the brachial plexus can be performed. Last, the infraclavicular approach allows for exposure of the central veins through extension of the incision medially, which allows for venous reconstruction. Some patients with neurogenic or arterial TOS present with symptoms of sympathetic overactivity, in which case cervical sympathectomy can be used with decompression.
Outcomes of surgical decompression for TOS depend on the clinical type but are generally good. For instance, in cases of disputed neurogenic TOS, symptom resolution after decompression is reportedly between 80% and 90%.59 However, major depression, work-related injuries,60 and diffuse preoperative arm symptoms61 all influence long-term results. In true neurogenic TOS, postoperative pain relief is often substantial, though recovery of strength can be slow because of the axonal injury.55 In arterial TOS, outcomes are influenced by time to surgical intervention, with early surgery demonstrating better outcomes than later surgery.62 In one study, Cormier and colleagues14 evaluated 47 patients who underwent correction of subclavian-axillary artery compression; 91% were asymptomatic a mean of 5.7 months after decompression. Last, outcomes of successful thrombolysis and decompression for venous TOS demonstrated patency rates higher than 95% at 5-year follow-up.54,63
Conclusions
TOS is a spectrum of disorders caused by compression of the brachial plexus, subclavian artery, or subclavian vein. Early recognition of TOS is imperative, as diagnostic or treatment delays may be associated with significant morbidity. Clinical examination alone is often inadequate for determining the compression site and the structure causing compression. CTA and MRI performed in association with postural maneuvers may demonstrate dynamic compression of the neurovascular structures in the thoracic outlet. These imaging modalities reliably identify the structures causing compression and can be crucial for effective management.
1. Urschel HC Jr. The history of surgery for thoracic outlet syndrome. Chest Surg Clin North Am. 2000;10(1):183-188, x-xi.
2. Atasoy E. History of thoracic outlet syndrome. Hand Clin. 2004;20(1):15-16, v.
3. Peet RM, Henriksen JD, Anderson TP, Martin GM. Thoracic-outlet syndrome: evaluation of a therapeutic exercise program. Proc Staff Meet Mayo Clin. 1956;31(9):281-287.
4. Edwards DP, Mulkern E, Raja AN, Barker P. Trans-axillary first rib excision for thoracic outlet syndrome. J R Coll Surg Edinb. 1999;44(6):362-365.
5. Juvonen T, Satta J, Laitala P, Luukkonen K, Nissinen J. Anomalies at the thoracic outlet are frequent in the general population. Am J Surg. 1995;170(1):33-37.
6. Atasoy E. Thoracic outlet compression syndrome. Orthop Clin North Am. 1996;27(2):265-303.
7. Demondion X, Herbinet P, Van Sint Jan S, Boutry N, Chantelot C, Cotten A. Imaging assessment of thoracic outlet syndrome. Radiographics. 2006;26(6):1735-1750.
8. Demondion X, Bacqueville E, Paul C, Duquesnoy B, Hachulla E, Cotten A. Thoracic outlet: assessment with MR imaging in asymptomatic and symptomatic populations. Radiology. 2003;227(2):461-468.
9. Makhoul RG, Machleder HI. Developmental anomalies at the thoracic outlet: an analysis of 200 consecutive cases. J Vasc Surg. 1992;16(4):534-542.
10. Sanders RJ, Jackson CG, Banchero N, Pearce WH. Scalene muscle abnormalities in traumatic thoracic outlet syndrome. Am J Surg. 1990;159(2):231-236.
11. Katirji B, Hardy RW Jr. Classic neurogenic thoracic outlet syndrome in a competitive swimmer: a true scalenus anticus syndrome. Muscle Nerve. 1995;18(2):229-233.
12. Casbas L, Chauffour X, Cau J, et al. Post-traumatic thoracic outlet syndromes. Ann Vasc Surg. 2005;19(1):25-28.
13. Povlsen B, Belzberg A, Hansson T, Dorsi M. Treatment for thoracic outlet syndrome. Cochrane Database Syst Rev. 2010;(1):CD007218.
14. Cormier JM, Amrane M, Ward A, Laurian C, Gigou F. Arterial complications of the thoracic outlet syndrome: fifty-five operative cases. J Vasc Surg. 1989;9(6):778-787.
15. Hood DB, Kuehne J, Yellin AE, Weaver FA. Vascular complications of thoracic outlet syndrome. Am Surg. 1997;63(10):913-917.
16. Ferrante MA. Brachial plexopathies: classification, causes, and consequences. Muscle Nerve. 2004;30(5):547-568.
17. Gilliatt RW, Le Quesne PM, Logue V, Sumner AJ. Wasting of the hand associated with a cervical rib or band. J Neurol Neurosurg Psychiatry. 1970;33(5):615-624.
18. Ozoa G, Alves D, Fish DE. Thoracic outlet syndrome. Phys Med Rehabil Clin North Am. 2011;22(3):473-483, viii-ix.
19. Schwartzman RJ. Brachial plexus traction injuries. Hand Clin. 1991;7(3):547-556.
20. Christo PJ, McGreevy K. Updated perspectives on neurogenic thoracic outlet syndrome. Curr Pain Headache Rep. 2011;15(1):14-21.
21. Vanti C, Natalini L, Romeo A, Tosarelli D, Pillastrini P. Conservative treatment of thoracic outlet syndrome. A review of the literature. Eura Medicophys. 2007;43(1):55-70.22. Patton GM. Arterial thoracic outlet syndrome. Hand Clin. 2004;20(1):107-111, viii.
23. Lee TS, Hines GL. Cerebral embolic stroke and arm ischemia in a teenager with arterial thoracic outlet syndrome: a case report. Vasc Endovasc Surg. 2007;41(3):254-257.
24. Sanders RJ, Hammond SL. Venous thoracic outlet syndrome. Hand Clin. 2004;20(1):113-118, viii.
25. Sanders RJ, Hammond SL, Rao NM. Diagnosis of thoracic outlet syndrome. J Vasc Surg. 2007;46(3):601-604.
26. Luoma A, Nelems B. Thoracic outlet syndrome. Thoracic surgery perspective. Neurosurg Clin North Am. 1991;2(1):187-226.
27. Cup EH, Ijspeert J, Janssen RJ, et al. Residual complaints after neuralgic amyotrophy. Arch Phys Med Rehabil. 2013;94(1):67-73.
28. van Alfen N, van Engelen BG. The clinical spectrum of neuralgic amyotrophy in 246 cases. Brain. 2006;129(pt 2):438-450.
29. Nichols AW. The thoracic outlet syndrome in athletes. J Am Board Fam Pract. 1996;9(5):346-355.
30. Roos DB, Owens JC. Thoracic outlet syndrome. Arch Surg. 1966;93(1):71-74.
31. Adson AW, Coffey JR. Cervical rib: a method of anterior approach for relief of symptoms by division of the scalenus anticus. Ann Surg. 1927;85(6):839-857.
32. Wright IS. The neurovascular syndrome produced by hyperabduction of the arms. Am Heart J. 1945;29:1-19.
33. Rayan GM, Jensen C. Thoracic outlet syndrome: provocative examination maneuvers in a typical population. J Shoulder Elbow Surg. 1995;4(2):113-117.
34. Nord KM, Kapoor P, Fisher J, et al. False positive rate of thoracic outlet syndrome diagnostic maneuvers. Electromyogr Clin Neurophysiol. 2008;48(2):67-74.
35. Novak CB. Thoracic outlet syndrome. Clin Plast Surg. 2003;30(2):175-188.
36. Gillard J, Pérez-Cousin M, Hachulla E, et al. Diagnosing thoracic outlet syndrome: contribution of provocative tests, ultrasonography, electrophysiology, and helical computed tomography in 48 patients. Joint Bone Spine. 2001;68(5):416-424.
37. Baxter GM, Kincaid W, Jeffrey RF, Millar GM, Porteous C, Morley P. Comparison of colour Doppler ultrasound with venography in the diagnosis of axillary and subclavian vein thrombosis. Br J Radiol. 1991;64(765):777-781.
38. Passman MA, Criado E, Farber MA, et al. Efficacy of color flow duplex imaging for proximal upper extremity venous outflow obstruction in hemodialysis patients. J Vasc Surg. 1998;28(5):869-875.
39. Wadhwani R, Chaubal N, Sukthankar R, Shroff M, Agarwala S. Color Doppler and duplex sonography in 5 patients with thoracic outlet syndrome. J Ultrasound Med. 2001;20(7):795-801.
40. Napoli V, Vignali C, Braccini G, et al. Echography and echo-Doppler in the study of thoracic outlet syndrome. Correlation with angiographic data [in Italian]. Radiol Med. 1993;85(6):733-740.
41. Longley DG, Yedlicka JW, Molina EJ, Schwabacher S, Hunter DW, Letourneau JG. Thoracic outlet syndrome: evaluation of the subclavian vessels by color duplex sonography. AJR Am J Roentgenol. 1992;158(3):623-630.
42. Demondion X, Herbinet P, Boutry N, Fontaine C, Francke JP, Cotten A. Sonographic mapping of the normal brachial plexus. AJNR Am J Neuroradiol. 2003;24(7):1303-1309.
43. Cruz-Martinez A, Arpa J. Electrophysiological assessment in neurogenic thoracic outlet syndrome. Electromyogr Clin Neurophysiol. 2001;41(4):253-256.
44. Ferrante MA, Wilbourn AJ. The utility of various sensory nerve conduction responses in assessing brachial plexopathies. Muscle Nerve. 1995;18(8):879-889.
45. Aminoff MJ, Olney RK, Parry GJ, Raskin NH. Relative utility of different electrophysiologic techniques in the evaluation of brachial plexopathies. Neurology. 1988;38(4):546-550.
46. Komanetsky RM, Novak CB, Mackinnon SE, Russo MH, Padberg AM, Louis S. Somatosensory evoked potentials fail to diagnose thoracic outlet syndrome. J Hand Surg Am. 1996;21(4):662-666.
47. Remy-Jardin M, Remy J, Masson P, et al. Helical CT angiography of thoracic outlet syndrome: functional anatomy. AJR Am J Roentgenol. 2000;174(6):1667-1674.
48. Matsumura JS, Rilling WS, Pearce WH, Nemcek AA Jr, Vogelzang RL, Yao JS. Helical computed tomography of the normal thoracic outlet. J Vasc Surg. 1997;26(5):776-783.
49. Dymarkowski S, Bosmans H, Marchal G, Bogaert J. Three-dimensional MR angiography in the evaluation of thoracic outlet syndrome. AJR Am J Roentgenol. 1999;173(4):1005-1008.
50. Charon JP, Milne W, Sheppard DG, Houston JG. Evaluation of MR angiographic technique in the assessment of thoracic outlet syndrome. Clin Radiol. 2004;59(7):588-595.
51. Cuetter AC, Bartoszek DM. The thoracic outlet syndrome: controversies, overdiagnosis, overtreatment, and recommendations for management. Muscle Nerve. 1989;12(5):410-419.
52. Urschel HC Jr, Razzuk MA. Paget-Schroetter syndrome: what is the best management? Ann Thorac Surg. 2000;69(6):1663-1668.
53. Lee JT, Karwowski JK, Harris EJ, Haukoos JS, Olcott C 4th. Long-term thrombotic recurrence after nonoperative management of Paget-Schroetter syndrome. J Vasc Surg. 2006;43(6):1236-1243.
54. Molina JE, Hunter DW, Dietz CA. Paget-Schroetter syndrome treated with thrombolytics and immediate surgery. J Vasc Surg. 2007;45(2):328-334.
55. Le Forestier N, Mouton P, Maisonobe T, et al. True neurological thoracic outlet syndrome [in French]. Rev Neurol (Paris). 2000;156(1):34-40.
56. Wilbourn AJ. Thoracic outlet syndrome surgery causing severe brachial plexopathy. Muscle Nerve. 1988;11(1):66-74.
57. Likes K, Dapash T, Rochlin DH, Freischlag JA. Remaining or residual first ribs are the cause of recurrent thoracic outlet syndrome. Ann Vasc Surg. 2014;28(4):939-945.
58. Aljabri B, Al-Omran M. Surgical management of vascular thoracic outlet syndrome: a teaching hospital experience. Ann Vasc Dis. 2013;6(1):74-79.
59. Sanders RJ, Pearce WH. The treatment of thoracic outlet syndrome: a comparison of different operations. J Vasc Surg. 1989;10(6):626-634.
60. Franklin GM, Fulton-Kehoe D, Bradley C, Smith-Weller T. Outcome of surgery for thoracic outlet syndrome in Washington state workers’ compensation. Neurology. 2000;54(6):1252-1257.
61. Axelrod DA, Proctor MC, Geisser ME, Roth RS, Greenfield LJ. Outcomes after surgery for thoracic outlet syndrome. J Vasc Surg. 2001;33(6):1220-1225.
62. Taylor JM, Telford RJ, Kinsella DC, Watkinson AF, Thompson JF. Long-term clinical and functional outcome following treatment for Paget-Schroetter syndrome. Br J Surg. 2013;100(11):1459-1464.
63. Schneider DB, Dimuzio PJ, Martin ND, et al. Combination treatment of venous thoracic outlet syndrome: open surgical decompression and intraoperative angioplasty. J Vasc Surg. 2004;40(4):599-603.
Thoracic outlet syndrome (TOS) was first described by Coot in 1861,1,2 and the term was coined by Peet and colleagues3 in 1956 to cover a spectrum of conditions caused by dynamic compression of the brachial plexus (neurogenic), subclavian artery (arterial), or subclavian vein (venous). The estimated incidence of TOS is 10 in 100,000.4 However, cadaveric studies have suggested that up to 90% of the population may have what is considered abnormal anatomy of the thoracic outlet,5 which in turn suggests a multifactorial etiology for symptomatic disease. TOS is most commonly diagnosed in patients 20 to 40 years of age, with females affected in a 4:1 ratio.6 Although historically TOS is a clinical diagnosis, advanced imaging is often helpful in determining the nature and location of the structure undergoing compression and the structure producing compression, which help guide management. Computed tomography angiography (CTA) and magnetic resonance imaging (MRI) performed in association with postural maneuvers aid in the diagnosis in patients with dynamically acquired compression.7
Pathophysiology
The pathophysiology of TOS is attributable to the unique anatomy of the thoracic outlet. Compromise of the neurovascular structures can occur through congenital or acquired narrowing in 3 distinct compartments: the interscalene triangle, the costoclavicular space, and the retropectoralis minor space. The interscalene triangle is the most medial of the compartments. Containing the subclavian artery and the 3 trunks of the brachial plexus, it is bordered anteriorly by the anterior scalene muscle, posteriorly by the middle and posterior scalene muscles, and inferiorly by the first rib. The interscalene triangle is the most frequent site of neurologic compression.8 The middle compartment is the costoclavicular space, which is bordered superiorly by the clavicle, anteriorly by the subclavius muscle, and posteriorly by the first rib and the middle scalene muscle. The costoclavicular space is the most frequent site of arterial compression,8 where the artery lies directly anterior to the subclavian vein and is surrounded by the 3 cords of the brachial plexus. The most lateral compartment is the retropectoralis minor space, which is bordered anteriorly by the pectoralis minor muscle, superiorly by the subscapularis muscle, and inferiorly by the anterior chest wall. Sources of neurovascular compression within any of the spaces include cervical ribs9; elongated C7 transverse processes; hypertrophy of the anterior or middle scalene, subclavius, or pectoralis minor muscles10; anomalous scalenus minimus muscle; repetitive overhead arm movements (pitching, swimming)11; anomalous fascial bands; degenerative spine disease; bone destruction from primary or secondary neoplasms (Pancoast tumor); hyperextension/flexion injury of the neck12; and malunion of clavicle fractures, among others.13
Classification
Three distinct TOSs have been described, individually or combined, depending on the injured component: neurogenic from brachial plexus compression, arterial from subclavian artery compression, and venous from subclavian or axillary vein compression.14,15
Neurogenic TOS has 2 reported types: true (classic) and disputed. True neurogenic TOS is rare, with an estimated incidence of 1 in 1 million.16 First described in 1970 as a lower trunk plexopathy involving slowly progressive unilateral weakness of the intrinsic hand muscles and sensory abnormalities in the ulnar and medial antebrachial cutaneous nerve distributions, true neurogenic TOS was originally called Gilliatt-Sumner hand syndrome.17 A congenital band extending between the first rib and an elongated C7 transverse process was thought to be the location of brachial plexus injury in true neurogenic TOS. Conversely, disputed neurogenic TOS is the most common form of TOS, occurring in 3 to 80 per 100018 and accounting for 90% to 95% of all TOS cases.13,19 In contrast to true neurogenic TOS, in which anatomical and electrodiagnostic evidence supports the diagnosis, objective clinical findings are often lacking in the disputed form.18 Patients with disputed neurogenic TOS present with a diverse array of symptoms, including pain, numbness, and weakness affecting the neck, shoulder, and arm, exacerbated by activities requiring elevation or sustained use of the extremity.20
Arterial TOS accounts for 1% to 5% of all TOS cases.21 Arterial TOS typically affects patients who perform repetitive movements of the upper extremities with their arms above their shoulders, resulting in compression of the subclavian artery. Symptoms of arterial TOS include pain, weakness, coolness, pallor, and paresthesia.18,22 In severe cases of compression, subclavian artery damage can result in thrombosis with distal embolization, poststenotic aneurysm, or even retrograde extension causing stroke.22,23
Last, representing 2% to 3% of all TOS cases, venous TOS results from compression of the subclavian or axillary vein.18,24 Two mechanisms for vascular compromise have been described. The first involves compression of the vein between the clavicle and the first rib with overhead activities.18 Patients often experience intermittent “heaviness” of the extremity with repeated overhead use. The second mechanism involves repeated stress between the clavicle and vein, causing an intravascular thrombosis.18 Patients may experience pain, edema, cyanosis, venous distention, and even spontaneous venous thrombosis, referred to as Paget-Schroetter syndrome, which can lead to pulmonary embolism.6,25,26
Clinical Features
In cases of suspected TOS, clinicians should take a thorough history and perform a thorough physical examination. The differential diagnosis for unilateral, upper limb pain, numbness, tingling, and/or weakness exacerbated by movement includes shoulder and rotator cuff pathology, cervical spine injury, cervical radiculitis, distal compressive neuropathies (carpal or cubital tunnel syndrome), and neuralgic amyotrophy (Parsonage-Turner syndrome/acute brachial radiculitis).27,28 The clinician should pursue a history of trauma to the shoulder or neck as well as any occupational or recreational activities involving elevation of the upper extremity for extended periods.29 Physical examination must include an evaluation of the contralateral side and may begin with visual inspection to assess for muscle asymmetry, atrophy, color changes, edema, or deformities.18 Next, palpation should be used to assess for any tenderness, texture changes, masses, or vascular pulsations. Attention should be directed at examination of the cervical spine as well as neurologic and vascular assessments of the bilateral upper extremities, including range of motion and strength testing,18 to rule out alternative etiologies.
Four basic maneuvers—the Roos test,30 Adson test,31 Wright test,32 and costoclavicular test—traditionally have been used to diagnose TOS. A positive Roos test involves symptom reproduction with the patient slowly opening and closing the hand for 3 minutes with the arm externally rotated and abducted to 90°.33 However, the false-positive rate of the Roos test is as high as 77% in patients with carpal tunnel syndrome and up to 47% in normal subjects.34 The Adson test is performed by having the patient inhale deeply while the arm is kept in the anatomical position with the head extended and turned toward the involved extremity. The examiner monitors the radial pulse; an absent or diminished radial pulse suggests compression of the subclavian artery. The Adson test is not very reliable, however, because the pulse diminishes even in normal subjects,6,26 with a reported false-positive rate of 13.5%.35 A positive costoclavicular compression test occurs when depressing a patient’s shoulder reproduces symptoms. In one study, the false-positive rate of the costoclavicular compression test was 48% in patients with carpal tunnel syndrome and 16% in normal subjects.34 Last, the Wright test is performed by hyperabducting and externally rotating the affected shoulder. It is positive with a diminished pulse or reproduction of symptoms. One study found that the Wright test had 70% to 90% sensitivity and 29% to 53% specificity.36
Clinically distinguishing between the various forms of TOS may be difficult, and occasionally multiple types exist in a single patient, exacerbating one another and adding to the diagnostic difficulty. For example, arterial insufficiency may lead to disruption of the neural microcirculation, leading to concurrent arterial and neurogenic TOS. Because most cases present with nonspecific symptoms, advanced imaging modalities are often required to establish a definitive diagnosis and to target therapy to the appropriate site of compression.
Imaging Features
Plain Radiography
First, cervical spine and chest radiographs should be obtained to assess for bone abnormalities, including cervical ribs, long transverse processes, rib/clavicle fracture callus, rib anomalies, degenerative spine disease, and neoplasm (Pancoast/apical tumor) (Figure 1).18,25
Ultrasonography
Ultrasonography is useful in evaluating arterial or venous TOS because of its low cost, noninvasive nature, and high specificity for vessel occlusion.37,38 In arterial TOS, ultrasound may demonstrate increased flow velocity through a stenosis or an aneurysmal degeneration distal to the stenosis.7 In venous TOS, duplex ultrasound can identify stasis and thrombus.7 Obtaining duplex ultrasound with the upper extremity in multiple positions allows clinicians to correlate dynamically induced symptoms with ultrasonographic findings of altered blood flow.39-41 Despite the purported benefits of ultrasound, its drawback is that it is operator-dependent,42 with some studies reporting a high false-positive rate24 for diagnosis of venous TOS.
Electrodiagnostic Testing
Ruling out etiologies such as cervical radiculitis (Parsonage-Turner syndrome), cervical radiculopathies, brachial plexus lesions, and other distal compressive neuropathies requires nerve conduction studies and electromyography.18,43-46 In true neurogenic TOS, a combination of decreased sensory nerve action potentials in the ulnar and medial antebrachial cutaneous nerves and decreased compound motor action potentials in the median nerve is often found.18 Specifically, an abnormal ulnar sensory nerve action potential suggests the lesion is situated away from the intraspinal canal, which argues against a diagnosis of radiculopathy or myelopathy.43,44 In the disputed form of neurogenic TOS, the role of electrodiagnostic testing is less clear.18
Conventional Arteriography and Venography
Although CTA has superseded conventional arteriography and venography in most treatment centers, it may still be used in patients with acute symptoms requiring immediate thrombolytic therapy. Catheter angiography and venography with postural maneuvers are often the first invasive treatment modality in cases of thoracic outlet vascular compression.22,24 Presence of intraluminal thrombus, vessel dilatation, and collateral vessels is readily demonstrated (Figure 2A). Recanalization of occluded vessels can be attempted using balloon angioplasty and venoplasty (Figure 2B), but it is usually only temporarily successful if the cause of extrinsic compression is not corrected (Figures 2C, 2D). CTA or conventional angiography, used if sophisticated CTA with 3-dimensional (3-D) reconstruction is unavailable, is the gold standard in diagnosis of TOS.
CTA and Venography
Computed tomography (CT) is a valuable modality because it can be performed rapidly and effectively to depict the relationship of vascular structures to surrounding bone and muscle.47 In addition, CTA and venography provide high-quality representations of the vasculature, and 3-D reconstruction reliably identifies areas of neurovascular compression in patients with TOS.47,48 Furthermore, CT may be performed in a dynamic fashion, with the upper extremity in various positions to reproduce dynamic compression of the neurovascular structures (Figure 3A). Comparison of the images with the upper extremities in the anatomical position and elevated allows the physician to evaluate narrowing of the compartments and dynamic compression of neurovascular structures.8 CT is particularly valuable in arterial and venous TOS. In arterial TOS, the cross-sectional area or diameter of the artery can be measured to calculate the degree of stenosis.8,47 In venous TOS, dynamic narrowing of the vein can be visualized and may be associated with venous thrombosis or collateral circulation (Figure 3B). Although a variety of maneuvers is possible during CTA, the size of the CT tunnel as well as mandatory supine positioning of the patient may limit the series. Drawbacks of CT for diagnosing TOS include difficulties in analyzing the brachial plexus because of limited contrast resolution. In addition, the risks of CT (ionizing radiation, administration of iodinated contrast medium) must be considered before image acquisition.
MRI
MRI is a noninvasive and nonionizing technique that offers good resolution of the anatomical components of the thoracic outlet8 and that, because of its superior soft-tissue contrast, is the modality of choice for imaging brachial plexus nerve compression in TOS (Figure 4). Neurologic compression is identified with MRI when the fat surrounding the brachial plexus disappears.8 MRI reliably identifies the source of compression, which may include bony structures, muscle hypertrophy (scalenus, scalenus minimus, subclavius, pectoralis minor), and fibrous bands.49 Because of their craniocaudal direction, the sagittal plane is often most useful in demonstrating neurovascular compression.42 Analyzing the caliber of the vessel along its course may evaluate vascular compression, and magnetic resonance (MR) angiography and venography (Figures 5A, 5B) can often complement the findings.50 Specifically, in arterial TOS, poststenotic aneurysmal dilatation may be seen, whereas thrombosis and collateral circulation can be visualized in cases of venous TOS.50 Limitations of MRI in the diagnosis of TOS historically were similar to those of CT, and included supine positioning as well as restricted upper extremity maneuvers because of the size of the tunnel and the presence of surface coils.42 However, newer higher channel surface coils and wider bores allow for imaging in a wider range of motion, including arm hyperabduction (Figures 5C, 5D), which is often necessary to elicit pathology.
Management
Generally, therapeutic options for TOS are aimed at relieving the source of neurovascular compression. It is important that treatment be directed only toward symptomatic patients, as many patients have anatomy consistent with TOS and remain asymptomatic.5 Treatment of TOS is predominately conservative and involves a combination of patient education, activity modification, medication, and rehabilitation to promote appropriate body mechanics and posture.18
Physical Therapy
Physical therapy should be aimed at decreasing pressure on the neurovascular structures of the thoracic outlet by relaxing the scalene muscles, strengthening the shoulder muscles, and working on postural exercises to help the patient sit and stand straighter.51 The scalene muscles are the primary targets for TOS rehabilitation, but focus should also be given to the upper trapezius, levator scapulae, sternocleidomastoid, pectoral, and suboccipital muscles.18 Physical therapy is often combined with hydrotherapy, massage, nonsteroidal anti-inflammatory drugs, and muscle relaxants for maximal symptomatic relief. Some patients have found relief with selective anesthetic or botulinum toxin A injections in the scalene muscles.18 A minimum of 4 to 6 weeks (often 4-6 months) of physical therapy and conservative treatment should be attempted before consideration of any invasive intervention.13,18
Anticoagulation
In venous TOS with evidence of thrombus but no obstructive clot, conservative management is typically sufficient. In rare cases, however, intimal damage secondary to vascular compression in arterial and venous TOS leads to thrombus formation, impairing upper extremity perfusion and producing symptoms. Treatment guidelines for venous TOS involve catheter-directed thrombolysis within 2 weeks of symptom onset.15 Thrombolysis replaced the prior recommendation of systemic anticoagulation combined with extremity rest and elevation because anticoagulation and rest alone result in up to 75% morbidity,52,53 whereas thrombolysis reestablishes vessel patency in nearly all patients.54 After thrombolysis, patients should receive intravenous heparin, and conversion to oral anticoagulation should occur as soon as manageable. In patients with arterial TOS, the goal of treatment is revascularization to prevent or decrease ischemia. In mild arterial ischemia, catheter-directed thrombolysis can be attempted. However, the threshold for surgical thromboembolectomy must remain low, as acute upper extremity ischemia may result in compartment syndrome and permanent loss of function.13 Fixed arterial lesions, whether occlusive or aneurysmal, are an absolute indication for thromboembolectomy with possible thoracic outlet decompression.13
Thoracic Outlet Decompression
Indications for surgical decompression are controversial. They include symptomatic patients who have vascular (arterial or venous) TOS and are not at high risk for surgery, patients with true neurologic TOS and acute progressive neurologic weakness or disabling pain,55 and patients who have disputed neurologic TOS and have failed conservative management—keeping in mind that high recurrence rates and iatrogenic brachial plexopathy have been reported in this population.56 In general, surgical procedures are aimed at reducing soft-tissue compression (scalene release or neurolysis) or bony compression (cervical or first thoracic rib excision). Three surgical approaches (transaxillary, supraclavicular, infraclavicular) are commonly used for decompression, and surgeons choose one over another depending on the anatomical abnormality causing the compression. The transaxillary approach requires limited dissection but still allows for adequate visualization of the rib during resection.57 In this approach, a transverse incision along the inferior border of the axilla extends from the pectoralis major to the latissimus dorsi. After dissection of the axillary vessels and the first thoracic nerve root, the first rib is identified and can be removed, when indicated. In contrast, the supraclavicular approach provides a wide exposure, and the site of compression is directly visualized, allowing for arterial reconstruction.58 Through this approach, the anterior and middle scalene muscles can be resected, and neurolysis of the brachial plexus can be performed. Last, the infraclavicular approach allows for exposure of the central veins through extension of the incision medially, which allows for venous reconstruction. Some patients with neurogenic or arterial TOS present with symptoms of sympathetic overactivity, in which case cervical sympathectomy can be used with decompression.
Outcomes of surgical decompression for TOS depend on the clinical type but are generally good. For instance, in cases of disputed neurogenic TOS, symptom resolution after decompression is reportedly between 80% and 90%.59 However, major depression, work-related injuries,60 and diffuse preoperative arm symptoms61 all influence long-term results. In true neurogenic TOS, postoperative pain relief is often substantial, though recovery of strength can be slow because of the axonal injury.55 In arterial TOS, outcomes are influenced by time to surgical intervention, with early surgery demonstrating better outcomes than later surgery.62 In one study, Cormier and colleagues14 evaluated 47 patients who underwent correction of subclavian-axillary artery compression; 91% were asymptomatic a mean of 5.7 months after decompression. Last, outcomes of successful thrombolysis and decompression for venous TOS demonstrated patency rates higher than 95% at 5-year follow-up.54,63
Conclusions
TOS is a spectrum of disorders caused by compression of the brachial plexus, subclavian artery, or subclavian vein. Early recognition of TOS is imperative, as diagnostic or treatment delays may be associated with significant morbidity. Clinical examination alone is often inadequate for determining the compression site and the structure causing compression. CTA and MRI performed in association with postural maneuvers may demonstrate dynamic compression of the neurovascular structures in the thoracic outlet. These imaging modalities reliably identify the structures causing compression and can be crucial for effective management.
Thoracic outlet syndrome (TOS) was first described by Coot in 1861,1,2 and the term was coined by Peet and colleagues3 in 1956 to cover a spectrum of conditions caused by dynamic compression of the brachial plexus (neurogenic), subclavian artery (arterial), or subclavian vein (venous). The estimated incidence of TOS is 10 in 100,000.4 However, cadaveric studies have suggested that up to 90% of the population may have what is considered abnormal anatomy of the thoracic outlet,5 which in turn suggests a multifactorial etiology for symptomatic disease. TOS is most commonly diagnosed in patients 20 to 40 years of age, with females affected in a 4:1 ratio.6 Although historically TOS is a clinical diagnosis, advanced imaging is often helpful in determining the nature and location of the structure undergoing compression and the structure producing compression, which help guide management. Computed tomography angiography (CTA) and magnetic resonance imaging (MRI) performed in association with postural maneuvers aid in the diagnosis in patients with dynamically acquired compression.7
Pathophysiology
The pathophysiology of TOS is attributable to the unique anatomy of the thoracic outlet. Compromise of the neurovascular structures can occur through congenital or acquired narrowing in 3 distinct compartments: the interscalene triangle, the costoclavicular space, and the retropectoralis minor space. The interscalene triangle is the most medial of the compartments. Containing the subclavian artery and the 3 trunks of the brachial plexus, it is bordered anteriorly by the anterior scalene muscle, posteriorly by the middle and posterior scalene muscles, and inferiorly by the first rib. The interscalene triangle is the most frequent site of neurologic compression.8 The middle compartment is the costoclavicular space, which is bordered superiorly by the clavicle, anteriorly by the subclavius muscle, and posteriorly by the first rib and the middle scalene muscle. The costoclavicular space is the most frequent site of arterial compression,8 where the artery lies directly anterior to the subclavian vein and is surrounded by the 3 cords of the brachial plexus. The most lateral compartment is the retropectoralis minor space, which is bordered anteriorly by the pectoralis minor muscle, superiorly by the subscapularis muscle, and inferiorly by the anterior chest wall. Sources of neurovascular compression within any of the spaces include cervical ribs9; elongated C7 transverse processes; hypertrophy of the anterior or middle scalene, subclavius, or pectoralis minor muscles10; anomalous scalenus minimus muscle; repetitive overhead arm movements (pitching, swimming)11; anomalous fascial bands; degenerative spine disease; bone destruction from primary or secondary neoplasms (Pancoast tumor); hyperextension/flexion injury of the neck12; and malunion of clavicle fractures, among others.13
Classification
Three distinct TOSs have been described, individually or combined, depending on the injured component: neurogenic from brachial plexus compression, arterial from subclavian artery compression, and venous from subclavian or axillary vein compression.14,15
Neurogenic TOS has 2 reported types: true (classic) and disputed. True neurogenic TOS is rare, with an estimated incidence of 1 in 1 million.16 First described in 1970 as a lower trunk plexopathy involving slowly progressive unilateral weakness of the intrinsic hand muscles and sensory abnormalities in the ulnar and medial antebrachial cutaneous nerve distributions, true neurogenic TOS was originally called Gilliatt-Sumner hand syndrome.17 A congenital band extending between the first rib and an elongated C7 transverse process was thought to be the location of brachial plexus injury in true neurogenic TOS. Conversely, disputed neurogenic TOS is the most common form of TOS, occurring in 3 to 80 per 100018 and accounting for 90% to 95% of all TOS cases.13,19 In contrast to true neurogenic TOS, in which anatomical and electrodiagnostic evidence supports the diagnosis, objective clinical findings are often lacking in the disputed form.18 Patients with disputed neurogenic TOS present with a diverse array of symptoms, including pain, numbness, and weakness affecting the neck, shoulder, and arm, exacerbated by activities requiring elevation or sustained use of the extremity.20
Arterial TOS accounts for 1% to 5% of all TOS cases.21 Arterial TOS typically affects patients who perform repetitive movements of the upper extremities with their arms above their shoulders, resulting in compression of the subclavian artery. Symptoms of arterial TOS include pain, weakness, coolness, pallor, and paresthesia.18,22 In severe cases of compression, subclavian artery damage can result in thrombosis with distal embolization, poststenotic aneurysm, or even retrograde extension causing stroke.22,23
Last, representing 2% to 3% of all TOS cases, venous TOS results from compression of the subclavian or axillary vein.18,24 Two mechanisms for vascular compromise have been described. The first involves compression of the vein between the clavicle and the first rib with overhead activities.18 Patients often experience intermittent “heaviness” of the extremity with repeated overhead use. The second mechanism involves repeated stress between the clavicle and vein, causing an intravascular thrombosis.18 Patients may experience pain, edema, cyanosis, venous distention, and even spontaneous venous thrombosis, referred to as Paget-Schroetter syndrome, which can lead to pulmonary embolism.6,25,26
Clinical Features
In cases of suspected TOS, clinicians should take a thorough history and perform a thorough physical examination. The differential diagnosis for unilateral, upper limb pain, numbness, tingling, and/or weakness exacerbated by movement includes shoulder and rotator cuff pathology, cervical spine injury, cervical radiculitis, distal compressive neuropathies (carpal or cubital tunnel syndrome), and neuralgic amyotrophy (Parsonage-Turner syndrome/acute brachial radiculitis).27,28 The clinician should pursue a history of trauma to the shoulder or neck as well as any occupational or recreational activities involving elevation of the upper extremity for extended periods.29 Physical examination must include an evaluation of the contralateral side and may begin with visual inspection to assess for muscle asymmetry, atrophy, color changes, edema, or deformities.18 Next, palpation should be used to assess for any tenderness, texture changes, masses, or vascular pulsations. Attention should be directed at examination of the cervical spine as well as neurologic and vascular assessments of the bilateral upper extremities, including range of motion and strength testing,18 to rule out alternative etiologies.
Four basic maneuvers—the Roos test,30 Adson test,31 Wright test,32 and costoclavicular test—traditionally have been used to diagnose TOS. A positive Roos test involves symptom reproduction with the patient slowly opening and closing the hand for 3 minutes with the arm externally rotated and abducted to 90°.33 However, the false-positive rate of the Roos test is as high as 77% in patients with carpal tunnel syndrome and up to 47% in normal subjects.34 The Adson test is performed by having the patient inhale deeply while the arm is kept in the anatomical position with the head extended and turned toward the involved extremity. The examiner monitors the radial pulse; an absent or diminished radial pulse suggests compression of the subclavian artery. The Adson test is not very reliable, however, because the pulse diminishes even in normal subjects,6,26 with a reported false-positive rate of 13.5%.35 A positive costoclavicular compression test occurs when depressing a patient’s shoulder reproduces symptoms. In one study, the false-positive rate of the costoclavicular compression test was 48% in patients with carpal tunnel syndrome and 16% in normal subjects.34 Last, the Wright test is performed by hyperabducting and externally rotating the affected shoulder. It is positive with a diminished pulse or reproduction of symptoms. One study found that the Wright test had 70% to 90% sensitivity and 29% to 53% specificity.36
Clinically distinguishing between the various forms of TOS may be difficult, and occasionally multiple types exist in a single patient, exacerbating one another and adding to the diagnostic difficulty. For example, arterial insufficiency may lead to disruption of the neural microcirculation, leading to concurrent arterial and neurogenic TOS. Because most cases present with nonspecific symptoms, advanced imaging modalities are often required to establish a definitive diagnosis and to target therapy to the appropriate site of compression.
Imaging Features
Plain Radiography
First, cervical spine and chest radiographs should be obtained to assess for bone abnormalities, including cervical ribs, long transverse processes, rib/clavicle fracture callus, rib anomalies, degenerative spine disease, and neoplasm (Pancoast/apical tumor) (Figure 1).18,25
Ultrasonography
Ultrasonography is useful in evaluating arterial or venous TOS because of its low cost, noninvasive nature, and high specificity for vessel occlusion.37,38 In arterial TOS, ultrasound may demonstrate increased flow velocity through a stenosis or an aneurysmal degeneration distal to the stenosis.7 In venous TOS, duplex ultrasound can identify stasis and thrombus.7 Obtaining duplex ultrasound with the upper extremity in multiple positions allows clinicians to correlate dynamically induced symptoms with ultrasonographic findings of altered blood flow.39-41 Despite the purported benefits of ultrasound, its drawback is that it is operator-dependent,42 with some studies reporting a high false-positive rate24 for diagnosis of venous TOS.
Electrodiagnostic Testing
Ruling out etiologies such as cervical radiculitis (Parsonage-Turner syndrome), cervical radiculopathies, brachial plexus lesions, and other distal compressive neuropathies requires nerve conduction studies and electromyography.18,43-46 In true neurogenic TOS, a combination of decreased sensory nerve action potentials in the ulnar and medial antebrachial cutaneous nerves and decreased compound motor action potentials in the median nerve is often found.18 Specifically, an abnormal ulnar sensory nerve action potential suggests the lesion is situated away from the intraspinal canal, which argues against a diagnosis of radiculopathy or myelopathy.43,44 In the disputed form of neurogenic TOS, the role of electrodiagnostic testing is less clear.18
Conventional Arteriography and Venography
Although CTA has superseded conventional arteriography and venography in most treatment centers, it may still be used in patients with acute symptoms requiring immediate thrombolytic therapy. Catheter angiography and venography with postural maneuvers are often the first invasive treatment modality in cases of thoracic outlet vascular compression.22,24 Presence of intraluminal thrombus, vessel dilatation, and collateral vessels is readily demonstrated (Figure 2A). Recanalization of occluded vessels can be attempted using balloon angioplasty and venoplasty (Figure 2B), but it is usually only temporarily successful if the cause of extrinsic compression is not corrected (Figures 2C, 2D). CTA or conventional angiography, used if sophisticated CTA with 3-dimensional (3-D) reconstruction is unavailable, is the gold standard in diagnosis of TOS.
CTA and Venography
Computed tomography (CT) is a valuable modality because it can be performed rapidly and effectively to depict the relationship of vascular structures to surrounding bone and muscle.47 In addition, CTA and venography provide high-quality representations of the vasculature, and 3-D reconstruction reliably identifies areas of neurovascular compression in patients with TOS.47,48 Furthermore, CT may be performed in a dynamic fashion, with the upper extremity in various positions to reproduce dynamic compression of the neurovascular structures (Figure 3A). Comparison of the images with the upper extremities in the anatomical position and elevated allows the physician to evaluate narrowing of the compartments and dynamic compression of neurovascular structures.8 CT is particularly valuable in arterial and venous TOS. In arterial TOS, the cross-sectional area or diameter of the artery can be measured to calculate the degree of stenosis.8,47 In venous TOS, dynamic narrowing of the vein can be visualized and may be associated with venous thrombosis or collateral circulation (Figure 3B). Although a variety of maneuvers is possible during CTA, the size of the CT tunnel as well as mandatory supine positioning of the patient may limit the series. Drawbacks of CT for diagnosing TOS include difficulties in analyzing the brachial plexus because of limited contrast resolution. In addition, the risks of CT (ionizing radiation, administration of iodinated contrast medium) must be considered before image acquisition.
MRI
MRI is a noninvasive and nonionizing technique that offers good resolution of the anatomical components of the thoracic outlet8 and that, because of its superior soft-tissue contrast, is the modality of choice for imaging brachial plexus nerve compression in TOS (Figure 4). Neurologic compression is identified with MRI when the fat surrounding the brachial plexus disappears.8 MRI reliably identifies the source of compression, which may include bony structures, muscle hypertrophy (scalenus, scalenus minimus, subclavius, pectoralis minor), and fibrous bands.49 Because of their craniocaudal direction, the sagittal plane is often most useful in demonstrating neurovascular compression.42 Analyzing the caliber of the vessel along its course may evaluate vascular compression, and magnetic resonance (MR) angiography and venography (Figures 5A, 5B) can often complement the findings.50 Specifically, in arterial TOS, poststenotic aneurysmal dilatation may be seen, whereas thrombosis and collateral circulation can be visualized in cases of venous TOS.50 Limitations of MRI in the diagnosis of TOS historically were similar to those of CT, and included supine positioning as well as restricted upper extremity maneuvers because of the size of the tunnel and the presence of surface coils.42 However, newer higher channel surface coils and wider bores allow for imaging in a wider range of motion, including arm hyperabduction (Figures 5C, 5D), which is often necessary to elicit pathology.
Management
Generally, therapeutic options for TOS are aimed at relieving the source of neurovascular compression. It is important that treatment be directed only toward symptomatic patients, as many patients have anatomy consistent with TOS and remain asymptomatic.5 Treatment of TOS is predominately conservative and involves a combination of patient education, activity modification, medication, and rehabilitation to promote appropriate body mechanics and posture.18
Physical Therapy
Physical therapy should be aimed at decreasing pressure on the neurovascular structures of the thoracic outlet by relaxing the scalene muscles, strengthening the shoulder muscles, and working on postural exercises to help the patient sit and stand straighter.51 The scalene muscles are the primary targets for TOS rehabilitation, but focus should also be given to the upper trapezius, levator scapulae, sternocleidomastoid, pectoral, and suboccipital muscles.18 Physical therapy is often combined with hydrotherapy, massage, nonsteroidal anti-inflammatory drugs, and muscle relaxants for maximal symptomatic relief. Some patients have found relief with selective anesthetic or botulinum toxin A injections in the scalene muscles.18 A minimum of 4 to 6 weeks (often 4-6 months) of physical therapy and conservative treatment should be attempted before consideration of any invasive intervention.13,18
Anticoagulation
In venous TOS with evidence of thrombus but no obstructive clot, conservative management is typically sufficient. In rare cases, however, intimal damage secondary to vascular compression in arterial and venous TOS leads to thrombus formation, impairing upper extremity perfusion and producing symptoms. Treatment guidelines for venous TOS involve catheter-directed thrombolysis within 2 weeks of symptom onset.15 Thrombolysis replaced the prior recommendation of systemic anticoagulation combined with extremity rest and elevation because anticoagulation and rest alone result in up to 75% morbidity,52,53 whereas thrombolysis reestablishes vessel patency in nearly all patients.54 After thrombolysis, patients should receive intravenous heparin, and conversion to oral anticoagulation should occur as soon as manageable. In patients with arterial TOS, the goal of treatment is revascularization to prevent or decrease ischemia. In mild arterial ischemia, catheter-directed thrombolysis can be attempted. However, the threshold for surgical thromboembolectomy must remain low, as acute upper extremity ischemia may result in compartment syndrome and permanent loss of function.13 Fixed arterial lesions, whether occlusive or aneurysmal, are an absolute indication for thromboembolectomy with possible thoracic outlet decompression.13
Thoracic Outlet Decompression
Indications for surgical decompression are controversial. They include symptomatic patients who have vascular (arterial or venous) TOS and are not at high risk for surgery, patients with true neurologic TOS and acute progressive neurologic weakness or disabling pain,55 and patients who have disputed neurologic TOS and have failed conservative management—keeping in mind that high recurrence rates and iatrogenic brachial plexopathy have been reported in this population.56 In general, surgical procedures are aimed at reducing soft-tissue compression (scalene release or neurolysis) or bony compression (cervical or first thoracic rib excision). Three surgical approaches (transaxillary, supraclavicular, infraclavicular) are commonly used for decompression, and surgeons choose one over another depending on the anatomical abnormality causing the compression. The transaxillary approach requires limited dissection but still allows for adequate visualization of the rib during resection.57 In this approach, a transverse incision along the inferior border of the axilla extends from the pectoralis major to the latissimus dorsi. After dissection of the axillary vessels and the first thoracic nerve root, the first rib is identified and can be removed, when indicated. In contrast, the supraclavicular approach provides a wide exposure, and the site of compression is directly visualized, allowing for arterial reconstruction.58 Through this approach, the anterior and middle scalene muscles can be resected, and neurolysis of the brachial plexus can be performed. Last, the infraclavicular approach allows for exposure of the central veins through extension of the incision medially, which allows for venous reconstruction. Some patients with neurogenic or arterial TOS present with symptoms of sympathetic overactivity, in which case cervical sympathectomy can be used with decompression.
Outcomes of surgical decompression for TOS depend on the clinical type but are generally good. For instance, in cases of disputed neurogenic TOS, symptom resolution after decompression is reportedly between 80% and 90%.59 However, major depression, work-related injuries,60 and diffuse preoperative arm symptoms61 all influence long-term results. In true neurogenic TOS, postoperative pain relief is often substantial, though recovery of strength can be slow because of the axonal injury.55 In arterial TOS, outcomes are influenced by time to surgical intervention, with early surgery demonstrating better outcomes than later surgery.62 In one study, Cormier and colleagues14 evaluated 47 patients who underwent correction of subclavian-axillary artery compression; 91% were asymptomatic a mean of 5.7 months after decompression. Last, outcomes of successful thrombolysis and decompression for venous TOS demonstrated patency rates higher than 95% at 5-year follow-up.54,63
Conclusions
TOS is a spectrum of disorders caused by compression of the brachial plexus, subclavian artery, or subclavian vein. Early recognition of TOS is imperative, as diagnostic or treatment delays may be associated with significant morbidity. Clinical examination alone is often inadequate for determining the compression site and the structure causing compression. CTA and MRI performed in association with postural maneuvers may demonstrate dynamic compression of the neurovascular structures in the thoracic outlet. These imaging modalities reliably identify the structures causing compression and can be crucial for effective management.
1. Urschel HC Jr. The history of surgery for thoracic outlet syndrome. Chest Surg Clin North Am. 2000;10(1):183-188, x-xi.
2. Atasoy E. History of thoracic outlet syndrome. Hand Clin. 2004;20(1):15-16, v.
3. Peet RM, Henriksen JD, Anderson TP, Martin GM. Thoracic-outlet syndrome: evaluation of a therapeutic exercise program. Proc Staff Meet Mayo Clin. 1956;31(9):281-287.
4. Edwards DP, Mulkern E, Raja AN, Barker P. Trans-axillary first rib excision for thoracic outlet syndrome. J R Coll Surg Edinb. 1999;44(6):362-365.
5. Juvonen T, Satta J, Laitala P, Luukkonen K, Nissinen J. Anomalies at the thoracic outlet are frequent in the general population. Am J Surg. 1995;170(1):33-37.
6. Atasoy E. Thoracic outlet compression syndrome. Orthop Clin North Am. 1996;27(2):265-303.
7. Demondion X, Herbinet P, Van Sint Jan S, Boutry N, Chantelot C, Cotten A. Imaging assessment of thoracic outlet syndrome. Radiographics. 2006;26(6):1735-1750.
8. Demondion X, Bacqueville E, Paul C, Duquesnoy B, Hachulla E, Cotten A. Thoracic outlet: assessment with MR imaging in asymptomatic and symptomatic populations. Radiology. 2003;227(2):461-468.
9. Makhoul RG, Machleder HI. Developmental anomalies at the thoracic outlet: an analysis of 200 consecutive cases. J Vasc Surg. 1992;16(4):534-542.
10. Sanders RJ, Jackson CG, Banchero N, Pearce WH. Scalene muscle abnormalities in traumatic thoracic outlet syndrome. Am J Surg. 1990;159(2):231-236.
11. Katirji B, Hardy RW Jr. Classic neurogenic thoracic outlet syndrome in a competitive swimmer: a true scalenus anticus syndrome. Muscle Nerve. 1995;18(2):229-233.
12. Casbas L, Chauffour X, Cau J, et al. Post-traumatic thoracic outlet syndromes. Ann Vasc Surg. 2005;19(1):25-28.
13. Povlsen B, Belzberg A, Hansson T, Dorsi M. Treatment for thoracic outlet syndrome. Cochrane Database Syst Rev. 2010;(1):CD007218.
14. Cormier JM, Amrane M, Ward A, Laurian C, Gigou F. Arterial complications of the thoracic outlet syndrome: fifty-five operative cases. J Vasc Surg. 1989;9(6):778-787.
15. Hood DB, Kuehne J, Yellin AE, Weaver FA. Vascular complications of thoracic outlet syndrome. Am Surg. 1997;63(10):913-917.
16. Ferrante MA. Brachial plexopathies: classification, causes, and consequences. Muscle Nerve. 2004;30(5):547-568.
17. Gilliatt RW, Le Quesne PM, Logue V, Sumner AJ. Wasting of the hand associated with a cervical rib or band. J Neurol Neurosurg Psychiatry. 1970;33(5):615-624.
18. Ozoa G, Alves D, Fish DE. Thoracic outlet syndrome. Phys Med Rehabil Clin North Am. 2011;22(3):473-483, viii-ix.
19. Schwartzman RJ. Brachial plexus traction injuries. Hand Clin. 1991;7(3):547-556.
20. Christo PJ, McGreevy K. Updated perspectives on neurogenic thoracic outlet syndrome. Curr Pain Headache Rep. 2011;15(1):14-21.
21. Vanti C, Natalini L, Romeo A, Tosarelli D, Pillastrini P. Conservative treatment of thoracic outlet syndrome. A review of the literature. Eura Medicophys. 2007;43(1):55-70.22. Patton GM. Arterial thoracic outlet syndrome. Hand Clin. 2004;20(1):107-111, viii.
23. Lee TS, Hines GL. Cerebral embolic stroke and arm ischemia in a teenager with arterial thoracic outlet syndrome: a case report. Vasc Endovasc Surg. 2007;41(3):254-257.
24. Sanders RJ, Hammond SL. Venous thoracic outlet syndrome. Hand Clin. 2004;20(1):113-118, viii.
25. Sanders RJ, Hammond SL, Rao NM. Diagnosis of thoracic outlet syndrome. J Vasc Surg. 2007;46(3):601-604.
26. Luoma A, Nelems B. Thoracic outlet syndrome. Thoracic surgery perspective. Neurosurg Clin North Am. 1991;2(1):187-226.
27. Cup EH, Ijspeert J, Janssen RJ, et al. Residual complaints after neuralgic amyotrophy. Arch Phys Med Rehabil. 2013;94(1):67-73.
28. van Alfen N, van Engelen BG. The clinical spectrum of neuralgic amyotrophy in 246 cases. Brain. 2006;129(pt 2):438-450.
29. Nichols AW. The thoracic outlet syndrome in athletes. J Am Board Fam Pract. 1996;9(5):346-355.
30. Roos DB, Owens JC. Thoracic outlet syndrome. Arch Surg. 1966;93(1):71-74.
31. Adson AW, Coffey JR. Cervical rib: a method of anterior approach for relief of symptoms by division of the scalenus anticus. Ann Surg. 1927;85(6):839-857.
32. Wright IS. The neurovascular syndrome produced by hyperabduction of the arms. Am Heart J. 1945;29:1-19.
33. Rayan GM, Jensen C. Thoracic outlet syndrome: provocative examination maneuvers in a typical population. J Shoulder Elbow Surg. 1995;4(2):113-117.
34. Nord KM, Kapoor P, Fisher J, et al. False positive rate of thoracic outlet syndrome diagnostic maneuvers. Electromyogr Clin Neurophysiol. 2008;48(2):67-74.
35. Novak CB. Thoracic outlet syndrome. Clin Plast Surg. 2003;30(2):175-188.
36. Gillard J, Pérez-Cousin M, Hachulla E, et al. Diagnosing thoracic outlet syndrome: contribution of provocative tests, ultrasonography, electrophysiology, and helical computed tomography in 48 patients. Joint Bone Spine. 2001;68(5):416-424.
37. Baxter GM, Kincaid W, Jeffrey RF, Millar GM, Porteous C, Morley P. Comparison of colour Doppler ultrasound with venography in the diagnosis of axillary and subclavian vein thrombosis. Br J Radiol. 1991;64(765):777-781.
38. Passman MA, Criado E, Farber MA, et al. Efficacy of color flow duplex imaging for proximal upper extremity venous outflow obstruction in hemodialysis patients. J Vasc Surg. 1998;28(5):869-875.
39. Wadhwani R, Chaubal N, Sukthankar R, Shroff M, Agarwala S. Color Doppler and duplex sonography in 5 patients with thoracic outlet syndrome. J Ultrasound Med. 2001;20(7):795-801.
40. Napoli V, Vignali C, Braccini G, et al. Echography and echo-Doppler in the study of thoracic outlet syndrome. Correlation with angiographic data [in Italian]. Radiol Med. 1993;85(6):733-740.
41. Longley DG, Yedlicka JW, Molina EJ, Schwabacher S, Hunter DW, Letourneau JG. Thoracic outlet syndrome: evaluation of the subclavian vessels by color duplex sonography. AJR Am J Roentgenol. 1992;158(3):623-630.
42. Demondion X, Herbinet P, Boutry N, Fontaine C, Francke JP, Cotten A. Sonographic mapping of the normal brachial plexus. AJNR Am J Neuroradiol. 2003;24(7):1303-1309.
43. Cruz-Martinez A, Arpa J. Electrophysiological assessment in neurogenic thoracic outlet syndrome. Electromyogr Clin Neurophysiol. 2001;41(4):253-256.
44. Ferrante MA, Wilbourn AJ. The utility of various sensory nerve conduction responses in assessing brachial plexopathies. Muscle Nerve. 1995;18(8):879-889.
45. Aminoff MJ, Olney RK, Parry GJ, Raskin NH. Relative utility of different electrophysiologic techniques in the evaluation of brachial plexopathies. Neurology. 1988;38(4):546-550.
46. Komanetsky RM, Novak CB, Mackinnon SE, Russo MH, Padberg AM, Louis S. Somatosensory evoked potentials fail to diagnose thoracic outlet syndrome. J Hand Surg Am. 1996;21(4):662-666.
47. Remy-Jardin M, Remy J, Masson P, et al. Helical CT angiography of thoracic outlet syndrome: functional anatomy. AJR Am J Roentgenol. 2000;174(6):1667-1674.
48. Matsumura JS, Rilling WS, Pearce WH, Nemcek AA Jr, Vogelzang RL, Yao JS. Helical computed tomography of the normal thoracic outlet. J Vasc Surg. 1997;26(5):776-783.
49. Dymarkowski S, Bosmans H, Marchal G, Bogaert J. Three-dimensional MR angiography in the evaluation of thoracic outlet syndrome. AJR Am J Roentgenol. 1999;173(4):1005-1008.
50. Charon JP, Milne W, Sheppard DG, Houston JG. Evaluation of MR angiographic technique in the assessment of thoracic outlet syndrome. Clin Radiol. 2004;59(7):588-595.
51. Cuetter AC, Bartoszek DM. The thoracic outlet syndrome: controversies, overdiagnosis, overtreatment, and recommendations for management. Muscle Nerve. 1989;12(5):410-419.
52. Urschel HC Jr, Razzuk MA. Paget-Schroetter syndrome: what is the best management? Ann Thorac Surg. 2000;69(6):1663-1668.
53. Lee JT, Karwowski JK, Harris EJ, Haukoos JS, Olcott C 4th. Long-term thrombotic recurrence after nonoperative management of Paget-Schroetter syndrome. J Vasc Surg. 2006;43(6):1236-1243.
54. Molina JE, Hunter DW, Dietz CA. Paget-Schroetter syndrome treated with thrombolytics and immediate surgery. J Vasc Surg. 2007;45(2):328-334.
55. Le Forestier N, Mouton P, Maisonobe T, et al. True neurological thoracic outlet syndrome [in French]. Rev Neurol (Paris). 2000;156(1):34-40.
56. Wilbourn AJ. Thoracic outlet syndrome surgery causing severe brachial plexopathy. Muscle Nerve. 1988;11(1):66-74.
57. Likes K, Dapash T, Rochlin DH, Freischlag JA. Remaining or residual first ribs are the cause of recurrent thoracic outlet syndrome. Ann Vasc Surg. 2014;28(4):939-945.
58. Aljabri B, Al-Omran M. Surgical management of vascular thoracic outlet syndrome: a teaching hospital experience. Ann Vasc Dis. 2013;6(1):74-79.
59. Sanders RJ, Pearce WH. The treatment of thoracic outlet syndrome: a comparison of different operations. J Vasc Surg. 1989;10(6):626-634.
60. Franklin GM, Fulton-Kehoe D, Bradley C, Smith-Weller T. Outcome of surgery for thoracic outlet syndrome in Washington state workers’ compensation. Neurology. 2000;54(6):1252-1257.
61. Axelrod DA, Proctor MC, Geisser ME, Roth RS, Greenfield LJ. Outcomes after surgery for thoracic outlet syndrome. J Vasc Surg. 2001;33(6):1220-1225.
62. Taylor JM, Telford RJ, Kinsella DC, Watkinson AF, Thompson JF. Long-term clinical and functional outcome following treatment for Paget-Schroetter syndrome. Br J Surg. 2013;100(11):1459-1464.
63. Schneider DB, Dimuzio PJ, Martin ND, et al. Combination treatment of venous thoracic outlet syndrome: open surgical decompression and intraoperative angioplasty. J Vasc Surg. 2004;40(4):599-603.
1. Urschel HC Jr. The history of surgery for thoracic outlet syndrome. Chest Surg Clin North Am. 2000;10(1):183-188, x-xi.
2. Atasoy E. History of thoracic outlet syndrome. Hand Clin. 2004;20(1):15-16, v.
3. Peet RM, Henriksen JD, Anderson TP, Martin GM. Thoracic-outlet syndrome: evaluation of a therapeutic exercise program. Proc Staff Meet Mayo Clin. 1956;31(9):281-287.
4. Edwards DP, Mulkern E, Raja AN, Barker P. Trans-axillary first rib excision for thoracic outlet syndrome. J R Coll Surg Edinb. 1999;44(6):362-365.
5. Juvonen T, Satta J, Laitala P, Luukkonen K, Nissinen J. Anomalies at the thoracic outlet are frequent in the general population. Am J Surg. 1995;170(1):33-37.
6. Atasoy E. Thoracic outlet compression syndrome. Orthop Clin North Am. 1996;27(2):265-303.
7. Demondion X, Herbinet P, Van Sint Jan S, Boutry N, Chantelot C, Cotten A. Imaging assessment of thoracic outlet syndrome. Radiographics. 2006;26(6):1735-1750.
8. Demondion X, Bacqueville E, Paul C, Duquesnoy B, Hachulla E, Cotten A. Thoracic outlet: assessment with MR imaging in asymptomatic and symptomatic populations. Radiology. 2003;227(2):461-468.
9. Makhoul RG, Machleder HI. Developmental anomalies at the thoracic outlet: an analysis of 200 consecutive cases. J Vasc Surg. 1992;16(4):534-542.
10. Sanders RJ, Jackson CG, Banchero N, Pearce WH. Scalene muscle abnormalities in traumatic thoracic outlet syndrome. Am J Surg. 1990;159(2):231-236.
11. Katirji B, Hardy RW Jr. Classic neurogenic thoracic outlet syndrome in a competitive swimmer: a true scalenus anticus syndrome. Muscle Nerve. 1995;18(2):229-233.
12. Casbas L, Chauffour X, Cau J, et al. Post-traumatic thoracic outlet syndromes. Ann Vasc Surg. 2005;19(1):25-28.
13. Povlsen B, Belzberg A, Hansson T, Dorsi M. Treatment for thoracic outlet syndrome. Cochrane Database Syst Rev. 2010;(1):CD007218.
14. Cormier JM, Amrane M, Ward A, Laurian C, Gigou F. Arterial complications of the thoracic outlet syndrome: fifty-five operative cases. J Vasc Surg. 1989;9(6):778-787.
15. Hood DB, Kuehne J, Yellin AE, Weaver FA. Vascular complications of thoracic outlet syndrome. Am Surg. 1997;63(10):913-917.
16. Ferrante MA. Brachial plexopathies: classification, causes, and consequences. Muscle Nerve. 2004;30(5):547-568.
17. Gilliatt RW, Le Quesne PM, Logue V, Sumner AJ. Wasting of the hand associated with a cervical rib or band. J Neurol Neurosurg Psychiatry. 1970;33(5):615-624.
18. Ozoa G, Alves D, Fish DE. Thoracic outlet syndrome. Phys Med Rehabil Clin North Am. 2011;22(3):473-483, viii-ix.
19. Schwartzman RJ. Brachial plexus traction injuries. Hand Clin. 1991;7(3):547-556.
20. Christo PJ, McGreevy K. Updated perspectives on neurogenic thoracic outlet syndrome. Curr Pain Headache Rep. 2011;15(1):14-21.
21. Vanti C, Natalini L, Romeo A, Tosarelli D, Pillastrini P. Conservative treatment of thoracic outlet syndrome. A review of the literature. Eura Medicophys. 2007;43(1):55-70.22. Patton GM. Arterial thoracic outlet syndrome. Hand Clin. 2004;20(1):107-111, viii.
23. Lee TS, Hines GL. Cerebral embolic stroke and arm ischemia in a teenager with arterial thoracic outlet syndrome: a case report. Vasc Endovasc Surg. 2007;41(3):254-257.
24. Sanders RJ, Hammond SL. Venous thoracic outlet syndrome. Hand Clin. 2004;20(1):113-118, viii.
25. Sanders RJ, Hammond SL, Rao NM. Diagnosis of thoracic outlet syndrome. J Vasc Surg. 2007;46(3):601-604.
26. Luoma A, Nelems B. Thoracic outlet syndrome. Thoracic surgery perspective. Neurosurg Clin North Am. 1991;2(1):187-226.
27. Cup EH, Ijspeert J, Janssen RJ, et al. Residual complaints after neuralgic amyotrophy. Arch Phys Med Rehabil. 2013;94(1):67-73.
28. van Alfen N, van Engelen BG. The clinical spectrum of neuralgic amyotrophy in 246 cases. Brain. 2006;129(pt 2):438-450.
29. Nichols AW. The thoracic outlet syndrome in athletes. J Am Board Fam Pract. 1996;9(5):346-355.
30. Roos DB, Owens JC. Thoracic outlet syndrome. Arch Surg. 1966;93(1):71-74.
31. Adson AW, Coffey JR. Cervical rib: a method of anterior approach for relief of symptoms by division of the scalenus anticus. Ann Surg. 1927;85(6):839-857.
32. Wright IS. The neurovascular syndrome produced by hyperabduction of the arms. Am Heart J. 1945;29:1-19.
33. Rayan GM, Jensen C. Thoracic outlet syndrome: provocative examination maneuvers in a typical population. J Shoulder Elbow Surg. 1995;4(2):113-117.
34. Nord KM, Kapoor P, Fisher J, et al. False positive rate of thoracic outlet syndrome diagnostic maneuvers. Electromyogr Clin Neurophysiol. 2008;48(2):67-74.
35. Novak CB. Thoracic outlet syndrome. Clin Plast Surg. 2003;30(2):175-188.
36. Gillard J, Pérez-Cousin M, Hachulla E, et al. Diagnosing thoracic outlet syndrome: contribution of provocative tests, ultrasonography, electrophysiology, and helical computed tomography in 48 patients. Joint Bone Spine. 2001;68(5):416-424.
37. Baxter GM, Kincaid W, Jeffrey RF, Millar GM, Porteous C, Morley P. Comparison of colour Doppler ultrasound with venography in the diagnosis of axillary and subclavian vein thrombosis. Br J Radiol. 1991;64(765):777-781.
38. Passman MA, Criado E, Farber MA, et al. Efficacy of color flow duplex imaging for proximal upper extremity venous outflow obstruction in hemodialysis patients. J Vasc Surg. 1998;28(5):869-875.
39. Wadhwani R, Chaubal N, Sukthankar R, Shroff M, Agarwala S. Color Doppler and duplex sonography in 5 patients with thoracic outlet syndrome. J Ultrasound Med. 2001;20(7):795-801.
40. Napoli V, Vignali C, Braccini G, et al. Echography and echo-Doppler in the study of thoracic outlet syndrome. Correlation with angiographic data [in Italian]. Radiol Med. 1993;85(6):733-740.
41. Longley DG, Yedlicka JW, Molina EJ, Schwabacher S, Hunter DW, Letourneau JG. Thoracic outlet syndrome: evaluation of the subclavian vessels by color duplex sonography. AJR Am J Roentgenol. 1992;158(3):623-630.
42. Demondion X, Herbinet P, Boutry N, Fontaine C, Francke JP, Cotten A. Sonographic mapping of the normal brachial plexus. AJNR Am J Neuroradiol. 2003;24(7):1303-1309.
43. Cruz-Martinez A, Arpa J. Electrophysiological assessment in neurogenic thoracic outlet syndrome. Electromyogr Clin Neurophysiol. 2001;41(4):253-256.
44. Ferrante MA, Wilbourn AJ. The utility of various sensory nerve conduction responses in assessing brachial plexopathies. Muscle Nerve. 1995;18(8):879-889.
45. Aminoff MJ, Olney RK, Parry GJ, Raskin NH. Relative utility of different electrophysiologic techniques in the evaluation of brachial plexopathies. Neurology. 1988;38(4):546-550.
46. Komanetsky RM, Novak CB, Mackinnon SE, Russo MH, Padberg AM, Louis S. Somatosensory evoked potentials fail to diagnose thoracic outlet syndrome. J Hand Surg Am. 1996;21(4):662-666.
47. Remy-Jardin M, Remy J, Masson P, et al. Helical CT angiography of thoracic outlet syndrome: functional anatomy. AJR Am J Roentgenol. 2000;174(6):1667-1674.
48. Matsumura JS, Rilling WS, Pearce WH, Nemcek AA Jr, Vogelzang RL, Yao JS. Helical computed tomography of the normal thoracic outlet. J Vasc Surg. 1997;26(5):776-783.
49. Dymarkowski S, Bosmans H, Marchal G, Bogaert J. Three-dimensional MR angiography in the evaluation of thoracic outlet syndrome. AJR Am J Roentgenol. 1999;173(4):1005-1008.
50. Charon JP, Milne W, Sheppard DG, Houston JG. Evaluation of MR angiographic technique in the assessment of thoracic outlet syndrome. Clin Radiol. 2004;59(7):588-595.
51. Cuetter AC, Bartoszek DM. The thoracic outlet syndrome: controversies, overdiagnosis, overtreatment, and recommendations for management. Muscle Nerve. 1989;12(5):410-419.
52. Urschel HC Jr, Razzuk MA. Paget-Schroetter syndrome: what is the best management? Ann Thorac Surg. 2000;69(6):1663-1668.
53. Lee JT, Karwowski JK, Harris EJ, Haukoos JS, Olcott C 4th. Long-term thrombotic recurrence after nonoperative management of Paget-Schroetter syndrome. J Vasc Surg. 2006;43(6):1236-1243.
54. Molina JE, Hunter DW, Dietz CA. Paget-Schroetter syndrome treated with thrombolytics and immediate surgery. J Vasc Surg. 2007;45(2):328-334.
55. Le Forestier N, Mouton P, Maisonobe T, et al. True neurological thoracic outlet syndrome [in French]. Rev Neurol (Paris). 2000;156(1):34-40.
56. Wilbourn AJ. Thoracic outlet syndrome surgery causing severe brachial plexopathy. Muscle Nerve. 1988;11(1):66-74.
57. Likes K, Dapash T, Rochlin DH, Freischlag JA. Remaining or residual first ribs are the cause of recurrent thoracic outlet syndrome. Ann Vasc Surg. 2014;28(4):939-945.
58. Aljabri B, Al-Omran M. Surgical management of vascular thoracic outlet syndrome: a teaching hospital experience. Ann Vasc Dis. 2013;6(1):74-79.
59. Sanders RJ, Pearce WH. The treatment of thoracic outlet syndrome: a comparison of different operations. J Vasc Surg. 1989;10(6):626-634.
60. Franklin GM, Fulton-Kehoe D, Bradley C, Smith-Weller T. Outcome of surgery for thoracic outlet syndrome in Washington state workers’ compensation. Neurology. 2000;54(6):1252-1257.
61. Axelrod DA, Proctor MC, Geisser ME, Roth RS, Greenfield LJ. Outcomes after surgery for thoracic outlet syndrome. J Vasc Surg. 2001;33(6):1220-1225.
62. Taylor JM, Telford RJ, Kinsella DC, Watkinson AF, Thompson JF. Long-term clinical and functional outcome following treatment for Paget-Schroetter syndrome. Br J Surg. 2013;100(11):1459-1464.
63. Schneider DB, Dimuzio PJ, Martin ND, et al. Combination treatment of venous thoracic outlet syndrome: open surgical decompression and intraoperative angioplasty. J Vasc Surg. 2004;40(4):599-603.