User login
Computed tomography angiography after NCCT delays thrombectomy
BALTIMORE – Performing computed tomography angiography (CTA) following noncontrast computed tomography (NCCT) to obtain a high-resolution image of the large-vessel occlusion significantly delays the time to thrombectomy.
In clinical practice, omitting CTA in patients whose middle cerebral artery visualized on NCCT reveals the hyperdense sign may speed the time to thrombectomy and improve outcome. The findings of a retrospective cohort study of prospectively collected data were presented by Kunakorn Atchaneeyasakul, MD, of the University of Miami, as a poster and a brief oral presentation at the annual meeting of the American Neurological Association.
This study retrospectively compared the time from imaging to groin puncture, which is the first step in thrombectomy, in patients who received NCCT followed by CTA with those who received just NCCT for anterior circulation occlusion at the tertiary care University of Miami medical center. Of the 289 patients who received thrombectomy, 255 were excluded because of transfer from another hospital, occurrence of stroke while hospitalized, or use of other imaging prior to thrombectomy.
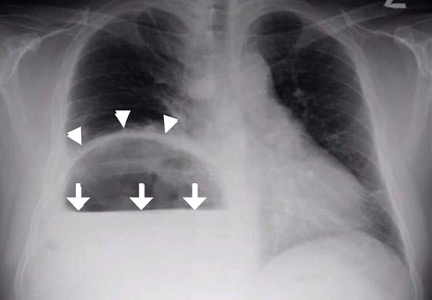
The remaining 34 patients were all evaluated with thin (0.625-mm) NCCT with automated image reconstruction. Fourteen received NCCT only, and 20 received CTA in addition to NCCT. The two groups were similar in mean age (64-71 years), gender (50% were female in each group), prevalence of hypertension (64% and 70% in the NCCT and NCCT + CTA group, respectively), and prevalence of diabetes, hyperlipidemia, atrial fibrillation, smoking, occlusion site, modified Rankin Scale score at discharge, and National Institutes of Health Stroke Scale scores at presentation and discharge. All 14 NCCT patients received intravenous tPA in contrast to 11 of the 20 (55%) NCCT + CTA patients (P = .003).
The middle cerebral artery was visualized on NCCT in about 85% of patients in each treatment group. Reperfusion was successful in 64% and 80% of patients receiving NCCT and NCCT + CTA, respectively (P = .31).
The total duration of imaging was 2 minutes (range, 1-6) in the NCCT group. The duration was significantly longer in the NCCT + CTA group (28 minutes; range, 23-65; P less than .001). The time from imaging to groin puncture was 68 minutes (range, 32-99) in the NCCT group. This was more than 30 minutes shorter than the NCCT + CTA group (104 minutes; range, 79-128; P = .030).
The times from emergency department admission to NCCT and from admission to groin puncture were similar in both groups.
“Avoiding advanced imaging in patients with anterior circulation large-vessel occlusion in whom thin-section NCCT with maximum-intensity projections reveals a hyperdense sign significantly shortens the imaging to groin puncture time,” concluded Dr. Atchaneeyasakul.
In the scenario, the detection of hyperdense middle cerebral artery would fast track the patient to the angiography suite, forgoing CTA. The result, according to Dr. Atchaneeyasakul, could alleviate a delay in thrombectomy, which could better preserve brain function.
Funding information was not provided.
BALTIMORE – Performing computed tomography angiography (CTA) following noncontrast computed tomography (NCCT) to obtain a high-resolution image of the large-vessel occlusion significantly delays the time to thrombectomy.
In clinical practice, omitting CTA in patients whose middle cerebral artery visualized on NCCT reveals the hyperdense sign may speed the time to thrombectomy and improve outcome. The findings of a retrospective cohort study of prospectively collected data were presented by Kunakorn Atchaneeyasakul, MD, of the University of Miami, as a poster and a brief oral presentation at the annual meeting of the American Neurological Association.
This study retrospectively compared the time from imaging to groin puncture, which is the first step in thrombectomy, in patients who received NCCT followed by CTA with those who received just NCCT for anterior circulation occlusion at the tertiary care University of Miami medical center. Of the 289 patients who received thrombectomy, 255 were excluded because of transfer from another hospital, occurrence of stroke while hospitalized, or use of other imaging prior to thrombectomy.
The remaining 34 patients were all evaluated with thin (0.625-mm) NCCT with automated image reconstruction. Fourteen received NCCT only, and 20 received CTA in addition to NCCT. The two groups were similar in mean age (64-71 years), gender (50% were female in each group), prevalence of hypertension (64% and 70% in the NCCT and NCCT + CTA group, respectively), and prevalence of diabetes, hyperlipidemia, atrial fibrillation, smoking, occlusion site, modified Rankin Scale score at discharge, and National Institutes of Health Stroke Scale scores at presentation and discharge. All 14 NCCT patients received intravenous tPA in contrast to 11 of the 20 (55%) NCCT + CTA patients (P = .003).
The middle cerebral artery was visualized on NCCT in about 85% of patients in each treatment group. Reperfusion was successful in 64% and 80% of patients receiving NCCT and NCCT + CTA, respectively (P = .31).
The total duration of imaging was 2 minutes (range, 1-6) in the NCCT group. The duration was significantly longer in the NCCT + CTA group (28 minutes; range, 23-65; P less than .001). The time from imaging to groin puncture was 68 minutes (range, 32-99) in the NCCT group. This was more than 30 minutes shorter than the NCCT + CTA group (104 minutes; range, 79-128; P = .030).
The times from emergency department admission to NCCT and from admission to groin puncture were similar in both groups.
“Avoiding advanced imaging in patients with anterior circulation large-vessel occlusion in whom thin-section NCCT with maximum-intensity projections reveals a hyperdense sign significantly shortens the imaging to groin puncture time,” concluded Dr. Atchaneeyasakul.
In the scenario, the detection of hyperdense middle cerebral artery would fast track the patient to the angiography suite, forgoing CTA. The result, according to Dr. Atchaneeyasakul, could alleviate a delay in thrombectomy, which could better preserve brain function.
Funding information was not provided.
BALTIMORE – Performing computed tomography angiography (CTA) following noncontrast computed tomography (NCCT) to obtain a high-resolution image of the large-vessel occlusion significantly delays the time to thrombectomy.
In clinical practice, omitting CTA in patients whose middle cerebral artery visualized on NCCT reveals the hyperdense sign may speed the time to thrombectomy and improve outcome. The findings of a retrospective cohort study of prospectively collected data were presented by Kunakorn Atchaneeyasakul, MD, of the University of Miami, as a poster and a brief oral presentation at the annual meeting of the American Neurological Association.
This study retrospectively compared the time from imaging to groin puncture, which is the first step in thrombectomy, in patients who received NCCT followed by CTA with those who received just NCCT for anterior circulation occlusion at the tertiary care University of Miami medical center. Of the 289 patients who received thrombectomy, 255 were excluded because of transfer from another hospital, occurrence of stroke while hospitalized, or use of other imaging prior to thrombectomy.
The remaining 34 patients were all evaluated with thin (0.625-mm) NCCT with automated image reconstruction. Fourteen received NCCT only, and 20 received CTA in addition to NCCT. The two groups were similar in mean age (64-71 years), gender (50% were female in each group), prevalence of hypertension (64% and 70% in the NCCT and NCCT + CTA group, respectively), and prevalence of diabetes, hyperlipidemia, atrial fibrillation, smoking, occlusion site, modified Rankin Scale score at discharge, and National Institutes of Health Stroke Scale scores at presentation and discharge. All 14 NCCT patients received intravenous tPA in contrast to 11 of the 20 (55%) NCCT + CTA patients (P = .003).
The middle cerebral artery was visualized on NCCT in about 85% of patients in each treatment group. Reperfusion was successful in 64% and 80% of patients receiving NCCT and NCCT + CTA, respectively (P = .31).
The total duration of imaging was 2 minutes (range, 1-6) in the NCCT group. The duration was significantly longer in the NCCT + CTA group (28 minutes; range, 23-65; P less than .001). The time from imaging to groin puncture was 68 minutes (range, 32-99) in the NCCT group. This was more than 30 minutes shorter than the NCCT + CTA group (104 minutes; range, 79-128; P = .030).
The times from emergency department admission to NCCT and from admission to groin puncture were similar in both groups.
“Avoiding advanced imaging in patients with anterior circulation large-vessel occlusion in whom thin-section NCCT with maximum-intensity projections reveals a hyperdense sign significantly shortens the imaging to groin puncture time,” concluded Dr. Atchaneeyasakul.
In the scenario, the detection of hyperdense middle cerebral artery would fast track the patient to the angiography suite, forgoing CTA. The result, according to Dr. Atchaneeyasakul, could alleviate a delay in thrombectomy, which could better preserve brain function.
Funding information was not provided.
AT ANA 2016
Key clinical point:
Major finding: Time from imaging to groin puncture was 68 minutes for NCCT vs. 104 minutes for NCCT + CTA.
Data source: Retrospective cohort study of prospectively collected data.
Disclosures: Dr. Atchaneeyasakul had no disclosures.
Emergency Imaging: Acute abdominal pain
An 89-year-old woman with a history of coronary artery disease, diabetes mellitus, hypertension, chronic constipation, and glaucoma presented to the ED for evaluation of chest pain and headache. Upon arrival at the ED, the patient also began to experience unrelenting abdominal pain. Abdominal examination showed mild tenderness in the right lower quadrant upon palpation. An abdominal radiograph and a computed tomography (CT) scan were ordered; representative images are presented above (Figure 1a-1d).
What is the diagnosis? What is the preferred management for this patient?
Answer
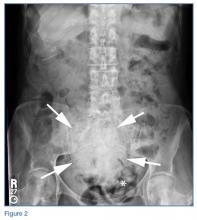
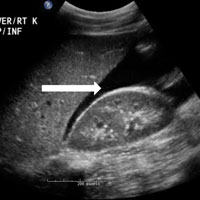
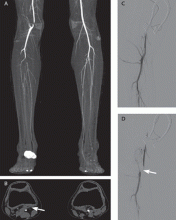
The abdominal radiograph showed no evidence of bowel obstruction. There was, however, a round area of increased density in the pelvis, suggesting the presence of a soft-tissue mass (white arrows, Figure 2) directly adjacent to the sigmoid colon (white asterisk, Figure 2).
Giant Colonic Diverticula
Giant colonic diverticula (GCD) are diverticula larger than 4 cm. This is a rare manifestation of diverticular disease of the bowel and most commonly occurs within the sigmoid colon. The majority of patients who develop GCD are older than age 60 years.1
The clinical presentation of GCD is nonspecific but can include abdominal pain, vomiting, nausea, and fever in the acute setting.2 Chronic presentations of GCD include intermittent abdominal pain, bloating, and constipation. In two-thirds of patients, a palpable abdominal mass is found on physical examination.3
Diagnosis
Due to the nonspecific presentation of GCD, imaging studies are typically required for diagnosis. Although radiographs may show a dilated air-filled structure in the abdomen, differentiation from a normal air-filled bowel may be difficult. Computed tomography is the imaging modality of choice based on its ability to demonstrate the presence of a smooth-walled gas-containing structure that communicates with the bowel lumen. In addition, CT has the ability to visualize the fluid and stool that are often present within the diverticulum. In cases of acute inflammation, diverticular wall thickening also may be present on CT.
Though no longer routinely used, barium enema is another option for diagnosing GCD because it can also demonstrate communication between the giant diverticula and the bowel lumen. However, barium enema is not often used in the emergency setting due to an increased risk of perforation and peritonitis.1
Management
Complications caused by GCD occur in 15% to 35% of cases and most commonly include perforation with associated peritonitis and abscess formation.4 Due to associated morbidity, the preferred treatment is surgical management—even when GCD is found incidentally in asymptomatic patients. In uncomplicated cases, surgical resection of the diverticulum and adjacent colon is performed with primary colic anastomosis. In some cases, a diverting ileostomy is created. In the presence of perforation and/or abscess, percutaneous catheter drainage and two-stage colectomy with colostomy typically is performed.5
1. Zeina AR, Mahamid A, Nachtigal A, Ashkenazi I, Shapira-Rootman M. Giant colonic diverticulum: radiographic and MDCT characteristics. Insights Imaging. 2015;6(6):659-664. doi: 10.1007/s13244-015-0433-x.
2. Custer TJ, Blevins DV, Vara TM. Giant colonic diverticulum: a rare manifestation of a common disease. J Gastrointest Surg. 1999;3(5):543-548.
3. de Oliveira NC, Welch JP. Giant diverticula of the colon: a clinical assessment. Am J Gastroenterol. 1997;92(7):1092-1096.
4. Majeski J, Durst G Jr. Obstructing giant colonic diverticulum. South Med J. 2000;93(8):797-799.
5. Nigri G, Petrucciani N, Giannini G, et al. Giant colonic diverticulum: clinical presentation, diagnosis and treatment: systematic review of 166 cases. World J Gastroenterol. 2015;21(1):360-368. doi: 10.3748/wjg.v21.i1.360.
An 89-year-old woman with a history of coronary artery disease, diabetes mellitus, hypertension, chronic constipation, and glaucoma presented to the ED for evaluation of chest pain and headache. Upon arrival at the ED, the patient also began to experience unrelenting abdominal pain. Abdominal examination showed mild tenderness in the right lower quadrant upon palpation. An abdominal radiograph and a computed tomography (CT) scan were ordered; representative images are presented above (Figure 1a-1d).
What is the diagnosis? What is the preferred management for this patient?
Answer
The abdominal radiograph showed no evidence of bowel obstruction. There was, however, a round area of increased density in the pelvis, suggesting the presence of a soft-tissue mass (white arrows, Figure 2) directly adjacent to the sigmoid colon (white asterisk, Figure 2).
Giant Colonic Diverticula
Giant colonic diverticula (GCD) are diverticula larger than 4 cm. This is a rare manifestation of diverticular disease of the bowel and most commonly occurs within the sigmoid colon. The majority of patients who develop GCD are older than age 60 years.1
The clinical presentation of GCD is nonspecific but can include abdominal pain, vomiting, nausea, and fever in the acute setting.2 Chronic presentations of GCD include intermittent abdominal pain, bloating, and constipation. In two-thirds of patients, a palpable abdominal mass is found on physical examination.3
Diagnosis
Due to the nonspecific presentation of GCD, imaging studies are typically required for diagnosis. Although radiographs may show a dilated air-filled structure in the abdomen, differentiation from a normal air-filled bowel may be difficult. Computed tomography is the imaging modality of choice based on its ability to demonstrate the presence of a smooth-walled gas-containing structure that communicates with the bowel lumen. In addition, CT has the ability to visualize the fluid and stool that are often present within the diverticulum. In cases of acute inflammation, diverticular wall thickening also may be present on CT.
Though no longer routinely used, barium enema is another option for diagnosing GCD because it can also demonstrate communication between the giant diverticula and the bowel lumen. However, barium enema is not often used in the emergency setting due to an increased risk of perforation and peritonitis.1
Management
Complications caused by GCD occur in 15% to 35% of cases and most commonly include perforation with associated peritonitis and abscess formation.4 Due to associated morbidity, the preferred treatment is surgical management—even when GCD is found incidentally in asymptomatic patients. In uncomplicated cases, surgical resection of the diverticulum and adjacent colon is performed with primary colic anastomosis. In some cases, a diverting ileostomy is created. In the presence of perforation and/or abscess, percutaneous catheter drainage and two-stage colectomy with colostomy typically is performed.5
An 89-year-old woman with a history of coronary artery disease, diabetes mellitus, hypertension, chronic constipation, and glaucoma presented to the ED for evaluation of chest pain and headache. Upon arrival at the ED, the patient also began to experience unrelenting abdominal pain. Abdominal examination showed mild tenderness in the right lower quadrant upon palpation. An abdominal radiograph and a computed tomography (CT) scan were ordered; representative images are presented above (Figure 1a-1d).
What is the diagnosis? What is the preferred management for this patient?
Answer
The abdominal radiograph showed no evidence of bowel obstruction. There was, however, a round area of increased density in the pelvis, suggesting the presence of a soft-tissue mass (white arrows, Figure 2) directly adjacent to the sigmoid colon (white asterisk, Figure 2).
Giant Colonic Diverticula
Giant colonic diverticula (GCD) are diverticula larger than 4 cm. This is a rare manifestation of diverticular disease of the bowel and most commonly occurs within the sigmoid colon. The majority of patients who develop GCD are older than age 60 years.1
The clinical presentation of GCD is nonspecific but can include abdominal pain, vomiting, nausea, and fever in the acute setting.2 Chronic presentations of GCD include intermittent abdominal pain, bloating, and constipation. In two-thirds of patients, a palpable abdominal mass is found on physical examination.3
Diagnosis
Due to the nonspecific presentation of GCD, imaging studies are typically required for diagnosis. Although radiographs may show a dilated air-filled structure in the abdomen, differentiation from a normal air-filled bowel may be difficult. Computed tomography is the imaging modality of choice based on its ability to demonstrate the presence of a smooth-walled gas-containing structure that communicates with the bowel lumen. In addition, CT has the ability to visualize the fluid and stool that are often present within the diverticulum. In cases of acute inflammation, diverticular wall thickening also may be present on CT.
Though no longer routinely used, barium enema is another option for diagnosing GCD because it can also demonstrate communication between the giant diverticula and the bowel lumen. However, barium enema is not often used in the emergency setting due to an increased risk of perforation and peritonitis.1
Management
Complications caused by GCD occur in 15% to 35% of cases and most commonly include perforation with associated peritonitis and abscess formation.4 Due to associated morbidity, the preferred treatment is surgical management—even when GCD is found incidentally in asymptomatic patients. In uncomplicated cases, surgical resection of the diverticulum and adjacent colon is performed with primary colic anastomosis. In some cases, a diverting ileostomy is created. In the presence of perforation and/or abscess, percutaneous catheter drainage and two-stage colectomy with colostomy typically is performed.5
1. Zeina AR, Mahamid A, Nachtigal A, Ashkenazi I, Shapira-Rootman M. Giant colonic diverticulum: radiographic and MDCT characteristics. Insights Imaging. 2015;6(6):659-664. doi: 10.1007/s13244-015-0433-x.
2. Custer TJ, Blevins DV, Vara TM. Giant colonic diverticulum: a rare manifestation of a common disease. J Gastrointest Surg. 1999;3(5):543-548.
3. de Oliveira NC, Welch JP. Giant diverticula of the colon: a clinical assessment. Am J Gastroenterol. 1997;92(7):1092-1096.
4. Majeski J, Durst G Jr. Obstructing giant colonic diverticulum. South Med J. 2000;93(8):797-799.
5. Nigri G, Petrucciani N, Giannini G, et al. Giant colonic diverticulum: clinical presentation, diagnosis and treatment: systematic review of 166 cases. World J Gastroenterol. 2015;21(1):360-368. doi: 10.3748/wjg.v21.i1.360.
1. Zeina AR, Mahamid A, Nachtigal A, Ashkenazi I, Shapira-Rootman M. Giant colonic diverticulum: radiographic and MDCT characteristics. Insights Imaging. 2015;6(6):659-664. doi: 10.1007/s13244-015-0433-x.
2. Custer TJ, Blevins DV, Vara TM. Giant colonic diverticulum: a rare manifestation of a common disease. J Gastrointest Surg. 1999;3(5):543-548.
3. de Oliveira NC, Welch JP. Giant diverticula of the colon: a clinical assessment. Am J Gastroenterol. 1997;92(7):1092-1096.
4. Majeski J, Durst G Jr. Obstructing giant colonic diverticulum. South Med J. 2000;93(8):797-799.
5. Nigri G, Petrucciani N, Giannini G, et al. Giant colonic diverticulum: clinical presentation, diagnosis and treatment: systematic review of 166 cases. World J Gastroenterol. 2015;21(1):360-368. doi: 10.3748/wjg.v21.i1.360.
Nontraumatic Splenic Rupture
Case
A 25-year-old college student presented to the ED following a near-syncopal episode. The patient stated he had felt lightheaded and had fallen to his knees immediately after taking a shower earlier that morning, but did not experience any loss of consciousness or injury. He denied a history of syncope or any recent trauma or fatigue. A review of the patient’s systems was negative. His medical history was remarkable for irritable bowel syndrome; he had no surgical history. Regarding his social history, he admitted to occasional alcohol use but denied any tobacco or illicit drug use. He was not on any current prescription or over-the-counter medications and denied any allergies.
The patient’s initial vital signs at presentation were: blood pressure, 112/58 mm Hg; heart rate, 86 beats/min; temperature, 97.9°F; and respiratory rate, 18 breaths/min. Oxygen saturation was 100% on room air. The patient reported pain in his left shoulder, epigastric region, and right flank. He rated his pain as a “4” on a 0-to-10 pain scale.
On physical examination, the patient was alert and oriented; he was thin and had mild pallor. His head, eyes, ears, nose, and throat; cardiac; pulmonary; and neurological examinations were normal. The abdominal examination revealed a soft, minimally tender epigastrium but with normal bowel sounds. Initial laboratory studies were remarkable for low hemoglobin (Hgb; 12.0 g/dL) and elevated aspartate transaminase (105 U/L), alanine aminotransferase (168 U/L), total bilirubin (1.6 mg/dL), and glucose (179 mg/dL) levels. The patient’s troponin I and lipase levels were within normal range. An electrocardiogram was unremarkable.
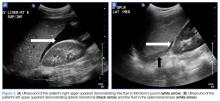
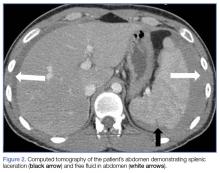
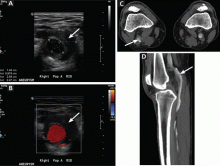
Given the patient’s elevated hepatic enzymes, right upper quadrant ultrasound was obtained, which demonstrated a normal gallbladder, a moderate amount of complicated free fluid (with hyper-echoic densities suggestive of coagulated blood) in all four quadrants, and splenomegaly measuring 13.7 cm (Figure 1a and 1b).
The patient’s status, including his vital signs, remained stable throughout his entire ED course. However, repeat laboratory studies taken 4 hours aft
Positive:
- Epstein-Barr virus (EBV)
- Viral capsid antigen (VCA) immunoglobulin G
- VCA immunoglobulin M
Negative:
- Mononuclear spot test
- Human immunodeficiency virus
- Hepatitis B and C
- Antinuclear antibodies
- Venereal disease research laboratory test
The rest of the patient’s recovery was uneventful, and he was discharged home in stable condition on hospital day 3.
Discussion
Although the spleen is the most common intra-abdominal organ that can rupture with blunt abdominal trauma, splenic rupture in the absence of trauma is very rare. Nontraumatic splenic rupture (NSR) has been associated with pathological and nonpathological spleens.1,2 A systemic review of NSRs showed that 7% of the 845 patients in the review had completely normal spleens; the remaining 93% had some form of splenic pathology.1
Etiology
The top three causes of splenic enlargement associated with NSR include hematologic malignancies, viral infections, and inflammation.1,2 Although viruses, such as EBV and cytomegalovirus, represent almost 15% of the pathological causes of NSR, it is not uncommon for a patient to have multiple pathological processes present.1 Our patient’s enlarged spleen was due to acute infectious mononucleosis.
Signs and Symptoms
Diagnosing NSR can be challenging and it is often missed or discovered incidentally during evaluation (as was initially the case with our patient).3 Several signs and symptoms present in our patient were red herrings that warranted closer analysis. The patient’s complaint of left shoulder pain suggested left hemidiaphragm irritation from the NSR. Furthermore, our patient’s near-syncopal episode was possibly due to acute vagal simulation from the initial contact of blood with the peritoneal cavity.4 The maximal vagal stimulus was likely transient, as our patient returned to baseline after a brief near-syncopal episode.
As illustrated in our case, though tachycardia is common in splenic rupture, not all patients present with this sign. The absence of tachycardia in our patient can be explained by the elevation of his baseline enteric vagal tone due to the continued presence of blood in the peritoneum.5 There are also other factors associated with the absence of tachycardia. For example, a well-conditioned athlete presenting with states of shock due to splenic rupture may not show signs of tachycardia.6
San Francisco Syncope Rule
The San Francisco Syncope Rule (SFSR) is a clinical decision-making risk-stratification tool used to determine outcomes and disposition of ED patients presenting with syncope.7 It is important to note that if we had used a straightforward application of the SFSR upon our patient’s initial presentation, the results would have been negative, suggesting he was not at risk for short-term serious outcomes.7
Imaging Studies
As demonstrated in our patient, a quick point-of-care (POC) bedside ultrasound scan can reveal the presence of free fluid in the abdomen to help with the diagnosis. On ultrasound, the presence of free fluid in the right upper quadrant is more commonly found in the hepatorenal recess, whereas in the left upper quadrant free fluid is seen sub-diaphragmatic/suprasplenic first before fluid is seen in the splenorenal recess. Bedside ultrasound can accurately detect as little as 100 mL of free fluid in the abdominal cavity, with a 90% sensitivity and 99% specificity.8
An ultrasound is highly sensitive as a preliminary screening tool to identify the presence of free intraperitoneal fluid and has some limited utility in identifying any disruption in the splenic echotexture that may suggest a laceration or hematoma. Ultrasound, however, has poor specificity in identifying solid organ injuries.9
Computed tomography scanning is the imaging modality of choice for assessing splenic injuries, and should be obtained to confirm the presence of a solid organ injury, as well as to grade the degree of injury and thereby determine the need for surgical intervention.10 It is worth noting that in a hemodynamically unstable patient, exploratory laparotomy may be embarked upon without a CT scan and positive free fluid on ultrasound.
Splenic Injury Scale
Splenic injury is classified on a scale of 1 (mild injury) to 5 (severe injury) (Table).11
Conclusion
This case illustrates an uncommon presentation of NSR and underscores the importance of considering NSR in the differential diagnoses of patients presenting with abdominal pain—a sign with such a broad differential that NSR could easily be missed during evaluation. Based on its high sensitivity and specificity in detecting the presence of free fluid in the abdominal cavity, POC ultrasound imaging should be used to evaluate patients presenting with abdominal pain and syncopal or near-syncopal symptoms. This case further demonstrates that the absence of tachycardia or signs of shock should not rule out NSR.
1. Renzulli P, Hostettler A, Schoepfer AM, Gloor B, Candinas D. Systematic review of atraumatic splenic rupture. Br J Surg. 2009;96(10):1114-1121. doi: 10.1002/bjs.6737.
2. Aubrey-Bassler FK, Sowers N. 613 cases of splenic rupture without risk factors or previously diagnosed disease: a systematic review. BMC Emerg Med. 2012;12:11. doi: 10.1186/1471-227X-12-11.
3. Schattner A, Meital A, Mavor E. Red-flag syncope: spontaneous splenic rupture. Am J Med. 2014;127(6):501-502. doi: 10.1016/j.amjmed.2014.02.024.
4. Moya A, Sutton R, Ammirati F, et al; Task Force for the Diagnosis and Management of Syncope; European Society of Cardiology (ESC); European Heart Rhythm Association (EHRA); Heart Failure Association (HFA); Heart Rhythm Society (HRS). Guidelines for the diagnosis and management of syncope (version 2009). Eur Heart J. 2009;30(21):2631-2671. doi: 10.1093/eurheartj/ehp298.
5. Rana MS, Khalid U, Law S. Paradoxical bradycardia in a patient with haemorrhagic shock secondary to blunt abdominal trauma. BMJ Case Rep. 2010;2010. doi: 10.1136/bcr.04.2010.2872.
6. Kiss O, Sydó N, Vargha P, et al. Prevalence of physiological and pathological electrocardiographic findings in Hungarian athletes. Acta Physiol Hung. 2015;102(2):228-237. doi: 10.1556/036.102.2015.2.13.
7. Quinn JV, Stiell IG, McDermott DA, Sellers KL, Kohn MA, Wells GA. Derivation of the San Francisco Syncope Rule to predict patients with short-term serious outcomes. Ann Emerg Med. 2004;43(2):224-232.
8. Ma OJ, Mateer JR, Ogata M, Kefer MP, Wittmann D, Aprahamian C. Prospective analysis of a rapid trauma ultrasound examination performed by emergency physicians. J Trauma. 1995;38(6):879-885.
9. Kendall JL, Faragher J, Hewitt GJ, Burcham G, Haukoos JS. Emergency Department Ultrasound Is not a Sensitive Detector of Solid Organ Injury. West J Emerg Med. 2009;10(1):1-5.
10. Hassan R, Abd Aziz A, Md Ralib AR, Saat A. Computed tomography of blunt spleen injury: a pictorial review. Malays J Med Sci. 2011;18(1):60-67.
11. Moore EE, Cogbill TH, Jurkovich GJ, Shackford SR, Malangoni MA, Champion HR. Organ injury scaling: spleen and liver (1994 revision). J Trauma. 1995;38(3):323-324.
12. Cirocchi R, Boselli C, Corsi A, et al. Is non-operative management safe and effective for all splenic blunt trauma? A systematic review. Crit Care. 2013;17(5):R185. doi: 10.1186/cc12868.
Case
A 25-year-old college student presented to the ED following a near-syncopal episode. The patient stated he had felt lightheaded and had fallen to his knees immediately after taking a shower earlier that morning, but did not experience any loss of consciousness or injury. He denied a history of syncope or any recent trauma or fatigue. A review of the patient’s systems was negative. His medical history was remarkable for irritable bowel syndrome; he had no surgical history. Regarding his social history, he admitted to occasional alcohol use but denied any tobacco or illicit drug use. He was not on any current prescription or over-the-counter medications and denied any allergies.
The patient’s initial vital signs at presentation were: blood pressure, 112/58 mm Hg; heart rate, 86 beats/min; temperature, 97.9°F; and respiratory rate, 18 breaths/min. Oxygen saturation was 100% on room air. The patient reported pain in his left shoulder, epigastric region, and right flank. He rated his pain as a “4” on a 0-to-10 pain scale.
On physical examination, the patient was alert and oriented; he was thin and had mild pallor. His head, eyes, ears, nose, and throat; cardiac; pulmonary; and neurological examinations were normal. The abdominal examination revealed a soft, minimally tender epigastrium but with normal bowel sounds. Initial laboratory studies were remarkable for low hemoglobin (Hgb; 12.0 g/dL) and elevated aspartate transaminase (105 U/L), alanine aminotransferase (168 U/L), total bilirubin (1.6 mg/dL), and glucose (179 mg/dL) levels. The patient’s troponin I and lipase levels were within normal range. An electrocardiogram was unremarkable.
Given the patient’s elevated hepatic enzymes, right upper quadrant ultrasound was obtained, which demonstrated a normal gallbladder, a moderate amount of complicated free fluid (with hyper-echoic densities suggestive of coagulated blood) in all four quadrants, and splenomegaly measuring 13.7 cm (Figure 1a and 1b).
The patient’s status, including his vital signs, remained stable throughout his entire ED course. However, repeat laboratory studies taken 4 hours aft
Positive:
- Epstein-Barr virus (EBV)
- Viral capsid antigen (VCA) immunoglobulin G
- VCA immunoglobulin M
Negative:
- Mononuclear spot test
- Human immunodeficiency virus
- Hepatitis B and C
- Antinuclear antibodies
- Venereal disease research laboratory test
The rest of the patient’s recovery was uneventful, and he was discharged home in stable condition on hospital day 3.
Discussion
Although the spleen is the most common intra-abdominal organ that can rupture with blunt abdominal trauma, splenic rupture in the absence of trauma is very rare. Nontraumatic splenic rupture (NSR) has been associated with pathological and nonpathological spleens.1,2 A systemic review of NSRs showed that 7% of the 845 patients in the review had completely normal spleens; the remaining 93% had some form of splenic pathology.1
Etiology
The top three causes of splenic enlargement associated with NSR include hematologic malignancies, viral infections, and inflammation.1,2 Although viruses, such as EBV and cytomegalovirus, represent almost 15% of the pathological causes of NSR, it is not uncommon for a patient to have multiple pathological processes present.1 Our patient’s enlarged spleen was due to acute infectious mononucleosis.
Signs and Symptoms
Diagnosing NSR can be challenging and it is often missed or discovered incidentally during evaluation (as was initially the case with our patient).3 Several signs and symptoms present in our patient were red herrings that warranted closer analysis. The patient’s complaint of left shoulder pain suggested left hemidiaphragm irritation from the NSR. Furthermore, our patient’s near-syncopal episode was possibly due to acute vagal simulation from the initial contact of blood with the peritoneal cavity.4 The maximal vagal stimulus was likely transient, as our patient returned to baseline after a brief near-syncopal episode.
As illustrated in our case, though tachycardia is common in splenic rupture, not all patients present with this sign. The absence of tachycardia in our patient can be explained by the elevation of his baseline enteric vagal tone due to the continued presence of blood in the peritoneum.5 There are also other factors associated with the absence of tachycardia. For example, a well-conditioned athlete presenting with states of shock due to splenic rupture may not show signs of tachycardia.6
San Francisco Syncope Rule
The San Francisco Syncope Rule (SFSR) is a clinical decision-making risk-stratification tool used to determine outcomes and disposition of ED patients presenting with syncope.7 It is important to note that if we had used a straightforward application of the SFSR upon our patient’s initial presentation, the results would have been negative, suggesting he was not at risk for short-term serious outcomes.7
Imaging Studies
As demonstrated in our patient, a quick point-of-care (POC) bedside ultrasound scan can reveal the presence of free fluid in the abdomen to help with the diagnosis. On ultrasound, the presence of free fluid in the right upper quadrant is more commonly found in the hepatorenal recess, whereas in the left upper quadrant free fluid is seen sub-diaphragmatic/suprasplenic first before fluid is seen in the splenorenal recess. Bedside ultrasound can accurately detect as little as 100 mL of free fluid in the abdominal cavity, with a 90% sensitivity and 99% specificity.8
An ultrasound is highly sensitive as a preliminary screening tool to identify the presence of free intraperitoneal fluid and has some limited utility in identifying any disruption in the splenic echotexture that may suggest a laceration or hematoma. Ultrasound, however, has poor specificity in identifying solid organ injuries.9
Computed tomography scanning is the imaging modality of choice for assessing splenic injuries, and should be obtained to confirm the presence of a solid organ injury, as well as to grade the degree of injury and thereby determine the need for surgical intervention.10 It is worth noting that in a hemodynamically unstable patient, exploratory laparotomy may be embarked upon without a CT scan and positive free fluid on ultrasound.
Splenic Injury Scale
Splenic injury is classified on a scale of 1 (mild injury) to 5 (severe injury) (Table).11
Conclusion
This case illustrates an uncommon presentation of NSR and underscores the importance of considering NSR in the differential diagnoses of patients presenting with abdominal pain—a sign with such a broad differential that NSR could easily be missed during evaluation. Based on its high sensitivity and specificity in detecting the presence of free fluid in the abdominal cavity, POC ultrasound imaging should be used to evaluate patients presenting with abdominal pain and syncopal or near-syncopal symptoms. This case further demonstrates that the absence of tachycardia or signs of shock should not rule out NSR.
Case
A 25-year-old college student presented to the ED following a near-syncopal episode. The patient stated he had felt lightheaded and had fallen to his knees immediately after taking a shower earlier that morning, but did not experience any loss of consciousness or injury. He denied a history of syncope or any recent trauma or fatigue. A review of the patient’s systems was negative. His medical history was remarkable for irritable bowel syndrome; he had no surgical history. Regarding his social history, he admitted to occasional alcohol use but denied any tobacco or illicit drug use. He was not on any current prescription or over-the-counter medications and denied any allergies.
The patient’s initial vital signs at presentation were: blood pressure, 112/58 mm Hg; heart rate, 86 beats/min; temperature, 97.9°F; and respiratory rate, 18 breaths/min. Oxygen saturation was 100% on room air. The patient reported pain in his left shoulder, epigastric region, and right flank. He rated his pain as a “4” on a 0-to-10 pain scale.
On physical examination, the patient was alert and oriented; he was thin and had mild pallor. His head, eyes, ears, nose, and throat; cardiac; pulmonary; and neurological examinations were normal. The abdominal examination revealed a soft, minimally tender epigastrium but with normal bowel sounds. Initial laboratory studies were remarkable for low hemoglobin (Hgb; 12.0 g/dL) and elevated aspartate transaminase (105 U/L), alanine aminotransferase (168 U/L), total bilirubin (1.6 mg/dL), and glucose (179 mg/dL) levels. The patient’s troponin I and lipase levels were within normal range. An electrocardiogram was unremarkable.
Given the patient’s elevated hepatic enzymes, right upper quadrant ultrasound was obtained, which demonstrated a normal gallbladder, a moderate amount of complicated free fluid (with hyper-echoic densities suggestive of coagulated blood) in all four quadrants, and splenomegaly measuring 13.7 cm (Figure 1a and 1b).
The patient’s status, including his vital signs, remained stable throughout his entire ED course. However, repeat laboratory studies taken 4 hours aft
Positive:
- Epstein-Barr virus (EBV)
- Viral capsid antigen (VCA) immunoglobulin G
- VCA immunoglobulin M
Negative:
- Mononuclear spot test
- Human immunodeficiency virus
- Hepatitis B and C
- Antinuclear antibodies
- Venereal disease research laboratory test
The rest of the patient’s recovery was uneventful, and he was discharged home in stable condition on hospital day 3.
Discussion
Although the spleen is the most common intra-abdominal organ that can rupture with blunt abdominal trauma, splenic rupture in the absence of trauma is very rare. Nontraumatic splenic rupture (NSR) has been associated with pathological and nonpathological spleens.1,2 A systemic review of NSRs showed that 7% of the 845 patients in the review had completely normal spleens; the remaining 93% had some form of splenic pathology.1
Etiology
The top three causes of splenic enlargement associated with NSR include hematologic malignancies, viral infections, and inflammation.1,2 Although viruses, such as EBV and cytomegalovirus, represent almost 15% of the pathological causes of NSR, it is not uncommon for a patient to have multiple pathological processes present.1 Our patient’s enlarged spleen was due to acute infectious mononucleosis.
Signs and Symptoms
Diagnosing NSR can be challenging and it is often missed or discovered incidentally during evaluation (as was initially the case with our patient).3 Several signs and symptoms present in our patient were red herrings that warranted closer analysis. The patient’s complaint of left shoulder pain suggested left hemidiaphragm irritation from the NSR. Furthermore, our patient’s near-syncopal episode was possibly due to acute vagal simulation from the initial contact of blood with the peritoneal cavity.4 The maximal vagal stimulus was likely transient, as our patient returned to baseline after a brief near-syncopal episode.
As illustrated in our case, though tachycardia is common in splenic rupture, not all patients present with this sign. The absence of tachycardia in our patient can be explained by the elevation of his baseline enteric vagal tone due to the continued presence of blood in the peritoneum.5 There are also other factors associated with the absence of tachycardia. For example, a well-conditioned athlete presenting with states of shock due to splenic rupture may not show signs of tachycardia.6
San Francisco Syncope Rule
The San Francisco Syncope Rule (SFSR) is a clinical decision-making risk-stratification tool used to determine outcomes and disposition of ED patients presenting with syncope.7 It is important to note that if we had used a straightforward application of the SFSR upon our patient’s initial presentation, the results would have been negative, suggesting he was not at risk for short-term serious outcomes.7
Imaging Studies
As demonstrated in our patient, a quick point-of-care (POC) bedside ultrasound scan can reveal the presence of free fluid in the abdomen to help with the diagnosis. On ultrasound, the presence of free fluid in the right upper quadrant is more commonly found in the hepatorenal recess, whereas in the left upper quadrant free fluid is seen sub-diaphragmatic/suprasplenic first before fluid is seen in the splenorenal recess. Bedside ultrasound can accurately detect as little as 100 mL of free fluid in the abdominal cavity, with a 90% sensitivity and 99% specificity.8
An ultrasound is highly sensitive as a preliminary screening tool to identify the presence of free intraperitoneal fluid and has some limited utility in identifying any disruption in the splenic echotexture that may suggest a laceration or hematoma. Ultrasound, however, has poor specificity in identifying solid organ injuries.9
Computed tomography scanning is the imaging modality of choice for assessing splenic injuries, and should be obtained to confirm the presence of a solid organ injury, as well as to grade the degree of injury and thereby determine the need for surgical intervention.10 It is worth noting that in a hemodynamically unstable patient, exploratory laparotomy may be embarked upon without a CT scan and positive free fluid on ultrasound.
Splenic Injury Scale
Splenic injury is classified on a scale of 1 (mild injury) to 5 (severe injury) (Table).11
Conclusion
This case illustrates an uncommon presentation of NSR and underscores the importance of considering NSR in the differential diagnoses of patients presenting with abdominal pain—a sign with such a broad differential that NSR could easily be missed during evaluation. Based on its high sensitivity and specificity in detecting the presence of free fluid in the abdominal cavity, POC ultrasound imaging should be used to evaluate patients presenting with abdominal pain and syncopal or near-syncopal symptoms. This case further demonstrates that the absence of tachycardia or signs of shock should not rule out NSR.
1. Renzulli P, Hostettler A, Schoepfer AM, Gloor B, Candinas D. Systematic review of atraumatic splenic rupture. Br J Surg. 2009;96(10):1114-1121. doi: 10.1002/bjs.6737.
2. Aubrey-Bassler FK, Sowers N. 613 cases of splenic rupture without risk factors or previously diagnosed disease: a systematic review. BMC Emerg Med. 2012;12:11. doi: 10.1186/1471-227X-12-11.
3. Schattner A, Meital A, Mavor E. Red-flag syncope: spontaneous splenic rupture. Am J Med. 2014;127(6):501-502. doi: 10.1016/j.amjmed.2014.02.024.
4. Moya A, Sutton R, Ammirati F, et al; Task Force for the Diagnosis and Management of Syncope; European Society of Cardiology (ESC); European Heart Rhythm Association (EHRA); Heart Failure Association (HFA); Heart Rhythm Society (HRS). Guidelines for the diagnosis and management of syncope (version 2009). Eur Heart J. 2009;30(21):2631-2671. doi: 10.1093/eurheartj/ehp298.
5. Rana MS, Khalid U, Law S. Paradoxical bradycardia in a patient with haemorrhagic shock secondary to blunt abdominal trauma. BMJ Case Rep. 2010;2010. doi: 10.1136/bcr.04.2010.2872.
6. Kiss O, Sydó N, Vargha P, et al. Prevalence of physiological and pathological electrocardiographic findings in Hungarian athletes. Acta Physiol Hung. 2015;102(2):228-237. doi: 10.1556/036.102.2015.2.13.
7. Quinn JV, Stiell IG, McDermott DA, Sellers KL, Kohn MA, Wells GA. Derivation of the San Francisco Syncope Rule to predict patients with short-term serious outcomes. Ann Emerg Med. 2004;43(2):224-232.
8. Ma OJ, Mateer JR, Ogata M, Kefer MP, Wittmann D, Aprahamian C. Prospective analysis of a rapid trauma ultrasound examination performed by emergency physicians. J Trauma. 1995;38(6):879-885.
9. Kendall JL, Faragher J, Hewitt GJ, Burcham G, Haukoos JS. Emergency Department Ultrasound Is not a Sensitive Detector of Solid Organ Injury. West J Emerg Med. 2009;10(1):1-5.
10. Hassan R, Abd Aziz A, Md Ralib AR, Saat A. Computed tomography of blunt spleen injury: a pictorial review. Malays J Med Sci. 2011;18(1):60-67.
11. Moore EE, Cogbill TH, Jurkovich GJ, Shackford SR, Malangoni MA, Champion HR. Organ injury scaling: spleen and liver (1994 revision). J Trauma. 1995;38(3):323-324.
12. Cirocchi R, Boselli C, Corsi A, et al. Is non-operative management safe and effective for all splenic blunt trauma? A systematic review. Crit Care. 2013;17(5):R185. doi: 10.1186/cc12868.
1. Renzulli P, Hostettler A, Schoepfer AM, Gloor B, Candinas D. Systematic review of atraumatic splenic rupture. Br J Surg. 2009;96(10):1114-1121. doi: 10.1002/bjs.6737.
2. Aubrey-Bassler FK, Sowers N. 613 cases of splenic rupture without risk factors or previously diagnosed disease: a systematic review. BMC Emerg Med. 2012;12:11. doi: 10.1186/1471-227X-12-11.
3. Schattner A, Meital A, Mavor E. Red-flag syncope: spontaneous splenic rupture. Am J Med. 2014;127(6):501-502. doi: 10.1016/j.amjmed.2014.02.024.
4. Moya A, Sutton R, Ammirati F, et al; Task Force for the Diagnosis and Management of Syncope; European Society of Cardiology (ESC); European Heart Rhythm Association (EHRA); Heart Failure Association (HFA); Heart Rhythm Society (HRS). Guidelines for the diagnosis and management of syncope (version 2009). Eur Heart J. 2009;30(21):2631-2671. doi: 10.1093/eurheartj/ehp298.
5. Rana MS, Khalid U, Law S. Paradoxical bradycardia in a patient with haemorrhagic shock secondary to blunt abdominal trauma. BMJ Case Rep. 2010;2010. doi: 10.1136/bcr.04.2010.2872.
6. Kiss O, Sydó N, Vargha P, et al. Prevalence of physiological and pathological electrocardiographic findings in Hungarian athletes. Acta Physiol Hung. 2015;102(2):228-237. doi: 10.1556/036.102.2015.2.13.
7. Quinn JV, Stiell IG, McDermott DA, Sellers KL, Kohn MA, Wells GA. Derivation of the San Francisco Syncope Rule to predict patients with short-term serious outcomes. Ann Emerg Med. 2004;43(2):224-232.
8. Ma OJ, Mateer JR, Ogata M, Kefer MP, Wittmann D, Aprahamian C. Prospective analysis of a rapid trauma ultrasound examination performed by emergency physicians. J Trauma. 1995;38(6):879-885.
9. Kendall JL, Faragher J, Hewitt GJ, Burcham G, Haukoos JS. Emergency Department Ultrasound Is not a Sensitive Detector of Solid Organ Injury. West J Emerg Med. 2009;10(1):1-5.
10. Hassan R, Abd Aziz A, Md Ralib AR, Saat A. Computed tomography of blunt spleen injury: a pictorial review. Malays J Med Sci. 2011;18(1):60-67.
11. Moore EE, Cogbill TH, Jurkovich GJ, Shackford SR, Malangoni MA, Champion HR. Organ injury scaling: spleen and liver (1994 revision). J Trauma. 1995;38(3):323-324.
12. Cirocchi R, Boselli C, Corsi A, et al. Is non-operative management safe and effective for all splenic blunt trauma? A systematic review. Crit Care. 2013;17(5):R185. doi: 10.1186/cc12868.
Algorithm for suspected pulmonary embolism safely cut CT rate
ROME – A newly validated, simplified algorithm for the management of patients with suspected acute pulmonary embolism enables physicians to safely exclude the disorder in roughly half of patients without resorting to CT pulmonary angiography, Tom van der Hulle, MD, reported at the annual congress of the European Society of Cardiology.
“This is the largest study ever performed in the diagnostic management of suspected pulmonary embolism. Based on our results, I think the YEARS algorithm is ready to be used in daily clinical practice,” declared Dr. van der Hulle of the department of thrombosis and hemostasis at Leiden (the Netherlands) University Medical Center.
Using the YEARS algorithm, PE was reliably ruled out without need for CT pulmonary angiography – considered the standard in the diagnosis of PE – in 48% of patients. In contrast, adherence to the Wells rule would have meant that 62% of patients would have gotten a CT scan to rule out PE with a comparably high degree of accuracy.
But that 62% figure underestimates the actual CT rate in clinical practice. The reality is that although the guideline-recommended Wells rule and revised Geneva score have been shown to be safe and accurate, they are so complex, cumbersome, and out of sync with the flow of routine clinical practice that many physicians skip the algorithms and go straight to CT, Dr. van der Hulle said. This approach results in many unnecessary CTs, needlessly exposing patients to the risks of radiation and intravenous contrast material while driving up health care costs, he added.
Using the Wells rule or revised Geneva score, the patient evaluation begins with an assessment of the clinical probability of PE based upon a risk score involving seven or eight factors. Only patients with a low or intermediate clinical probability of PE get a D-dimer test; those with a high clinical probability go straight to CT.
The YEARS algorithm is much simpler than that, Dr. van der Hulle explained. Everyone who presents with suspected acute PE gets a D-dimer test while the physician simultaneously applies a brief, three-item clinical prediction rule. These three items were selected by the Dutch investigators because they were the three strongest predictors of PE out of the original seven in the Wells rule. They are hemoptysis, clinical signs of deep vein thrombosis such as leg swelling or hyperpigmentation, and the clinician’s global impression of PE as being the most likely diagnosis.
In the YEARS algorithm, the threshold for a positive D-dimer test warranting CT pulmonary angiography depends upon whether any of the three clinical predictors is present. If none is present, the threshold is 1,000 ng/mL or above; if one or more is present, the threshold for a positive D-dimer test drops to 500 ng/mL.
Using these criteria, PE was excluded without resort to CT in 1,306 patients with none of the three YEARS items and a D-dimer test result below 1,000 ng/mL, as well as in another 327 patients with one or more YEARS items present but a D-dimer below 500 ng/mL. Those two groups were left untreated and followed prospectively for 3 months.
The 964 patients with one or more YEARS predictors present and a D-dimer score of at least 500 ng/mL underwent CT imaging, as did the 352 with no YEARS items and a D-dimer of at least 1,000 ng/mL.
The prevalence of CT-confirmed PE in the study was 13.2%. Affected patients were treated with anticoagulants.
The primary study endpoint was the total rate of deep vein thrombosis during 3 months of follow-up after PE had been excluded. The rate was 0.61%, including a fatal PE rate of 0.20%. The rate in patients managed without CT was 0.43%, including a 0.12% rate of fatal PE. In patients managed with diagnostic CT, the deep vein thrombosis rate was 0.84%, with a fatal PE rate of 0.30%.
“I think these results are completely comparable to those in previous studies using the standard algorithms,” Dr. van der Hulle commented.
The study’s main limitation is that it wasn’t a randomized, controlled trial. But given the tiny event rates, detecting any small differences between management strategies would require an unrealistically huge sample size, he added.
Asked if he thinks physicians will actually use the new tool, Dr. van der Hulle replied that some physicians feel driven to be 100% sure that a patient doesn’t have PE, and they will probably keep overordering CT scans. But others will embrace the YEARS algorithm because it reduces wasted resources and minimizes radiation exposure, a particularly compelling consideration in young female patients.
Discussant Marion Delcroix, MD, had reservations. She said she appreciated the appeal of a simple algorithm, but she asked, “Couldn’t we do better with a bit more sophistication, perhaps by adjusting the D-dimer cutoff for age and also adding some other items, like oxygen saturation and estrogen use?
“My concern is about the applicability. The age of the study cohort is relatively young, at a mean of 53 years. The peak age of PE in a very large contemporary German database is 70-80 years. We don’t know if the YEARS score is any good in this older population,” asserted Dr. Delcroix, professor of medicine and respiratory physiology and head of the center for pulmonary vascular diseases at University Hospital in Leuven, Belgium.
“If the aim is to decrease the number of CT pulmonary angiograms for safety reasons, why not reintroduce compression ultrasound of the lower limbs in the diagnostic algorithm?” she continued. “It has been shown to effectively reduce the need for further imaging.”
Dr. Delcroix predicted that the YEARS algorithm study will prove “too optimistic” regarding the number of CT scans avoided, particularly in elderly patients.
The YEARS study was funded by the trial’s 12 participating Dutch hospitals. Dr. van der Hulle reported having no financial conflicts of interest.
ROME – A newly validated, simplified algorithm for the management of patients with suspected acute pulmonary embolism enables physicians to safely exclude the disorder in roughly half of patients without resorting to CT pulmonary angiography, Tom van der Hulle, MD, reported at the annual congress of the European Society of Cardiology.
“This is the largest study ever performed in the diagnostic management of suspected pulmonary embolism. Based on our results, I think the YEARS algorithm is ready to be used in daily clinical practice,” declared Dr. van der Hulle of the department of thrombosis and hemostasis at Leiden (the Netherlands) University Medical Center.
Using the YEARS algorithm, PE was reliably ruled out without need for CT pulmonary angiography – considered the standard in the diagnosis of PE – in 48% of patients. In contrast, adherence to the Wells rule would have meant that 62% of patients would have gotten a CT scan to rule out PE with a comparably high degree of accuracy.
But that 62% figure underestimates the actual CT rate in clinical practice. The reality is that although the guideline-recommended Wells rule and revised Geneva score have been shown to be safe and accurate, they are so complex, cumbersome, and out of sync with the flow of routine clinical practice that many physicians skip the algorithms and go straight to CT, Dr. van der Hulle said. This approach results in many unnecessary CTs, needlessly exposing patients to the risks of radiation and intravenous contrast material while driving up health care costs, he added.
Using the Wells rule or revised Geneva score, the patient evaluation begins with an assessment of the clinical probability of PE based upon a risk score involving seven or eight factors. Only patients with a low or intermediate clinical probability of PE get a D-dimer test; those with a high clinical probability go straight to CT.
The YEARS algorithm is much simpler than that, Dr. van der Hulle explained. Everyone who presents with suspected acute PE gets a D-dimer test while the physician simultaneously applies a brief, three-item clinical prediction rule. These three items were selected by the Dutch investigators because they were the three strongest predictors of PE out of the original seven in the Wells rule. They are hemoptysis, clinical signs of deep vein thrombosis such as leg swelling or hyperpigmentation, and the clinician’s global impression of PE as being the most likely diagnosis.
In the YEARS algorithm, the threshold for a positive D-dimer test warranting CT pulmonary angiography depends upon whether any of the three clinical predictors is present. If none is present, the threshold is 1,000 ng/mL or above; if one or more is present, the threshold for a positive D-dimer test drops to 500 ng/mL.
Using these criteria, PE was excluded without resort to CT in 1,306 patients with none of the three YEARS items and a D-dimer test result below 1,000 ng/mL, as well as in another 327 patients with one or more YEARS items present but a D-dimer below 500 ng/mL. Those two groups were left untreated and followed prospectively for 3 months.
The 964 patients with one or more YEARS predictors present and a D-dimer score of at least 500 ng/mL underwent CT imaging, as did the 352 with no YEARS items and a D-dimer of at least 1,000 ng/mL.
The prevalence of CT-confirmed PE in the study was 13.2%. Affected patients were treated with anticoagulants.
The primary study endpoint was the total rate of deep vein thrombosis during 3 months of follow-up after PE had been excluded. The rate was 0.61%, including a fatal PE rate of 0.20%. The rate in patients managed without CT was 0.43%, including a 0.12% rate of fatal PE. In patients managed with diagnostic CT, the deep vein thrombosis rate was 0.84%, with a fatal PE rate of 0.30%.
“I think these results are completely comparable to those in previous studies using the standard algorithms,” Dr. van der Hulle commented.
The study’s main limitation is that it wasn’t a randomized, controlled trial. But given the tiny event rates, detecting any small differences between management strategies would require an unrealistically huge sample size, he added.
Asked if he thinks physicians will actually use the new tool, Dr. van der Hulle replied that some physicians feel driven to be 100% sure that a patient doesn’t have PE, and they will probably keep overordering CT scans. But others will embrace the YEARS algorithm because it reduces wasted resources and minimizes radiation exposure, a particularly compelling consideration in young female patients.
Discussant Marion Delcroix, MD, had reservations. She said she appreciated the appeal of a simple algorithm, but she asked, “Couldn’t we do better with a bit more sophistication, perhaps by adjusting the D-dimer cutoff for age and also adding some other items, like oxygen saturation and estrogen use?
“My concern is about the applicability. The age of the study cohort is relatively young, at a mean of 53 years. The peak age of PE in a very large contemporary German database is 70-80 years. We don’t know if the YEARS score is any good in this older population,” asserted Dr. Delcroix, professor of medicine and respiratory physiology and head of the center for pulmonary vascular diseases at University Hospital in Leuven, Belgium.
“If the aim is to decrease the number of CT pulmonary angiograms for safety reasons, why not reintroduce compression ultrasound of the lower limbs in the diagnostic algorithm?” she continued. “It has been shown to effectively reduce the need for further imaging.”
Dr. Delcroix predicted that the YEARS algorithm study will prove “too optimistic” regarding the number of CT scans avoided, particularly in elderly patients.
The YEARS study was funded by the trial’s 12 participating Dutch hospitals. Dr. van der Hulle reported having no financial conflicts of interest.
ROME – A newly validated, simplified algorithm for the management of patients with suspected acute pulmonary embolism enables physicians to safely exclude the disorder in roughly half of patients without resorting to CT pulmonary angiography, Tom van der Hulle, MD, reported at the annual congress of the European Society of Cardiology.
“This is the largest study ever performed in the diagnostic management of suspected pulmonary embolism. Based on our results, I think the YEARS algorithm is ready to be used in daily clinical practice,” declared Dr. van der Hulle of the department of thrombosis and hemostasis at Leiden (the Netherlands) University Medical Center.
Using the YEARS algorithm, PE was reliably ruled out without need for CT pulmonary angiography – considered the standard in the diagnosis of PE – in 48% of patients. In contrast, adherence to the Wells rule would have meant that 62% of patients would have gotten a CT scan to rule out PE with a comparably high degree of accuracy.
But that 62% figure underestimates the actual CT rate in clinical practice. The reality is that although the guideline-recommended Wells rule and revised Geneva score have been shown to be safe and accurate, they are so complex, cumbersome, and out of sync with the flow of routine clinical practice that many physicians skip the algorithms and go straight to CT, Dr. van der Hulle said. This approach results in many unnecessary CTs, needlessly exposing patients to the risks of radiation and intravenous contrast material while driving up health care costs, he added.
Using the Wells rule or revised Geneva score, the patient evaluation begins with an assessment of the clinical probability of PE based upon a risk score involving seven or eight factors. Only patients with a low or intermediate clinical probability of PE get a D-dimer test; those with a high clinical probability go straight to CT.
The YEARS algorithm is much simpler than that, Dr. van der Hulle explained. Everyone who presents with suspected acute PE gets a D-dimer test while the physician simultaneously applies a brief, three-item clinical prediction rule. These three items were selected by the Dutch investigators because they were the three strongest predictors of PE out of the original seven in the Wells rule. They are hemoptysis, clinical signs of deep vein thrombosis such as leg swelling or hyperpigmentation, and the clinician’s global impression of PE as being the most likely diagnosis.
In the YEARS algorithm, the threshold for a positive D-dimer test warranting CT pulmonary angiography depends upon whether any of the three clinical predictors is present. If none is present, the threshold is 1,000 ng/mL or above; if one or more is present, the threshold for a positive D-dimer test drops to 500 ng/mL.
Using these criteria, PE was excluded without resort to CT in 1,306 patients with none of the three YEARS items and a D-dimer test result below 1,000 ng/mL, as well as in another 327 patients with one or more YEARS items present but a D-dimer below 500 ng/mL. Those two groups were left untreated and followed prospectively for 3 months.
The 964 patients with one or more YEARS predictors present and a D-dimer score of at least 500 ng/mL underwent CT imaging, as did the 352 with no YEARS items and a D-dimer of at least 1,000 ng/mL.
The prevalence of CT-confirmed PE in the study was 13.2%. Affected patients were treated with anticoagulants.
The primary study endpoint was the total rate of deep vein thrombosis during 3 months of follow-up after PE had been excluded. The rate was 0.61%, including a fatal PE rate of 0.20%. The rate in patients managed without CT was 0.43%, including a 0.12% rate of fatal PE. In patients managed with diagnostic CT, the deep vein thrombosis rate was 0.84%, with a fatal PE rate of 0.30%.
“I think these results are completely comparable to those in previous studies using the standard algorithms,” Dr. van der Hulle commented.
The study’s main limitation is that it wasn’t a randomized, controlled trial. But given the tiny event rates, detecting any small differences between management strategies would require an unrealistically huge sample size, he added.
Asked if he thinks physicians will actually use the new tool, Dr. van der Hulle replied that some physicians feel driven to be 100% sure that a patient doesn’t have PE, and they will probably keep overordering CT scans. But others will embrace the YEARS algorithm because it reduces wasted resources and minimizes radiation exposure, a particularly compelling consideration in young female patients.
Discussant Marion Delcroix, MD, had reservations. She said she appreciated the appeal of a simple algorithm, but she asked, “Couldn’t we do better with a bit more sophistication, perhaps by adjusting the D-dimer cutoff for age and also adding some other items, like oxygen saturation and estrogen use?
“My concern is about the applicability. The age of the study cohort is relatively young, at a mean of 53 years. The peak age of PE in a very large contemporary German database is 70-80 years. We don’t know if the YEARS score is any good in this older population,” asserted Dr. Delcroix, professor of medicine and respiratory physiology and head of the center for pulmonary vascular diseases at University Hospital in Leuven, Belgium.
“If the aim is to decrease the number of CT pulmonary angiograms for safety reasons, why not reintroduce compression ultrasound of the lower limbs in the diagnostic algorithm?” she continued. “It has been shown to effectively reduce the need for further imaging.”
Dr. Delcroix predicted that the YEARS algorithm study will prove “too optimistic” regarding the number of CT scans avoided, particularly in elderly patients.
The YEARS study was funded by the trial’s 12 participating Dutch hospitals. Dr. van der Hulle reported having no financial conflicts of interest.
Key clinical point:
Major finding: Applying the YEARS algorithm to a large population of patients with suspected PE, the 3-month incidence of deep vein thrombosis after PE had been excluded was 0.61%.
Data source: This was a prospective study of clinical outcomes in nearly 3,000 consecutive Dutch patients who presented with suspected acute PE and were managed in accord with the YEARS algorithm.
Disclosures: The YEARS algorithm validation study was funded by the trial’s 12 participating Dutch hospitals. The study presenter reported having no financial conflicts of interest.
Air leakage in multiple compartments after endoscopy
A 68-year-old man with metastatic periampullary adenocarcinoma presented to his usual clinic for a scheduled biliary stent exchange by endoscopic retrograde cholangiopancreatography (ERCP). The stent had been placed 5 months before, and no complications had been reported during that procedure.
During the stent exchange procedure, the endoscopist advanced the scope to the second part of the duodenum, where a large, ulcerated, friable mass was visualized surrounding the ampulla, consistent with patient’s known periampullary cancer. The biliary stent was removed without much difficulty. However, several attempts to cannulate the common bile duct with a preloaded guidewire failed because of extensive edema and tissue friability, and to avoid further discomfort to the patient, the procedure was aborted. No perforation was visualized during or at the end of the procedure.
During the first hour after the procedure was stopped, the patient suddenly developed abdominal pain and distention and crepitus of the right chest wall. Supine abdominal radiography showed extensive pneumoperitoneum and subcutaneous emphysema in the chest. A nasogastric tube was placed for decompression, and the patient was transferred to the surgical intensive care unit at our hospital.
EVIDENCE OF PERFORATION NOTED
On arrival, the patient’s oxygen saturation was 99% while receiving oxygen at 2 L/minute by nasal cannula. The physical examination revealed neck swelling, abdominal distention, and crepitus in the neck, abdomen, scrotum, and right lower extremity.
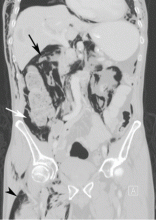
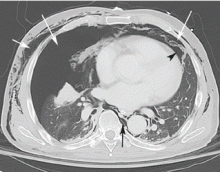
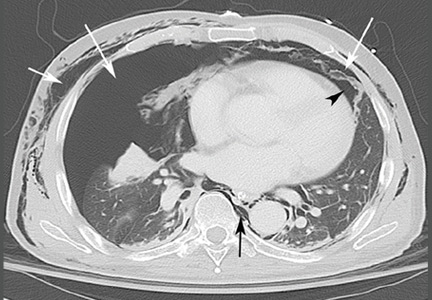
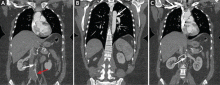
Computed tomography (CT) of the abdomen and pelvis with oral and intravenous contrast revealed widespread pneumoretroperitoneum, pneumoperitoneum, and air along the intermuscular planes in the right lower extremity, with no evidence of extravasation of oral contrast (Figure 1). Also noted were bilateral pneumothorax, pneumomediastinum, pneumopericardium, and extensive subcutaneous emphysema (Figure 2).
Despite these impressive findings, the patient remained hemodynamically stable and was managed conservatively with broad-spectrum antibiotics, gastric decompression, and bowel rest. But repeat chest radiography 5 hours after admission to the hospital revealed an enlarging right pneumothorax, which was treated with placement of a pigtail catheter. The patient continued to improve with conservative management and was discharged on the 6th day of hospitalization.
PERFORATION DURING ERCP: INCIDENCE AND COMPLICATIONS
Although perforation is an uncommon complication of ERCP, with an incidence of 1%, mortality rates as high as 18% have been reported.1 Older age, longer procedural time, anatomic variations, and diseases of the duodenum and common bile duct can increase the risk of perforation.2
Types of perforation
Stapfer et al1 classified perforation during ERCP into four types, based on etiology and site of perforation. Type 1 is perforation of the lateral or medial duodenal wall caused by excessive pressure from the endoscope or its acute angulation. Type 2 is periampullary injury, often associated with sphincterotomy or difficulty accessing the biliary tree. Type 3 is injury to the common bile duct or pancreatic duct caused by instrumentation. Type 4 is the presence of retroperitoneal free air with no evidence of actual perforation; this is usually an incidental finding and is of little or no clinical consequence.1
In 2015, a review of 18 studies described the distribution of ERCP perforation according to the Stapfer classification: 25% were type 1, 46% were type 2, and 22% were type 3.3
Effects of air insufflation
ERCP requires air insufflation for optimal visualization. During difficult or prolonged procedures, a larger amount of air may be insufflated to maintain bowel lumen visibility. Depending on the site and size of the defect, a variable amount of air can leak under pressure once the perforation occurs. A rapid retroperitoneal air leak can spread to multiple body compartments, including the mediastinum, pleura, neck, subcutaneous tissues, scrotum, and musculature by tracking through various fascial planes. Rarely, rapid ingress of air in these areas can lead to compartment syndrome.4
Small perforations tend to close spontaneously and may remain clinically silent, but large or persistent perforations are known to cause subcutaneous emphysema, sepsis, and respiratory failure.5
Our patient’s type 2 perforation
We presumed that our patient had a type 2 perforation, given the finding of retroperitoneal air. Difficulty cannulating the biliary tree via the friable malignant tissue at the site of the major papilla likely caused punctate perforations, resulting in air leakage into the retroperitoneum. Punctate perforations typically do not allow contrast extravasation, explaining the absence of oral contrast leakage on CT.
TREATMENT OF ENDOSCOPY-RELATED PERFORATION
Conventional supine and upright abdominal radiography is an appropriate initial imaging modality to confirm the diagnosis. However, CT is more sensitive and accurate, especially when air leakage is confined to the retroperitoneum. Intravenous or oral contrast is not necessary but may help localize the perforation and better delineate fluid collections and abscesses.2
Once perforation is suspected, treatment with a broad-spectrum antibiotic, bowel rest, and stomach decompression is imperative.6 Further management depends on the type of perforation and the overall clinical picture. Type 1 perforations usually require immediate surgical intervention. Type 2 perforations often seal spontaneously within 2 to 3 days and thus are managed conservatively (ie, a broad-spectrum antibiotic, gastric decompression, and bowel rest), unless there is a persistent leak or a large fluid collection. Type 3 perforations rarely require surgery since most are very small and close spontaneously, and so they are managed conservatively. Type 4 perforations are the least serious. They result in retroperitoneal free air that is thought be related to the use of compressed air for lumen patency. They require only conservative measures.1
- Stapfer M, Selby RR, Stain SC, et al. Management of duodenal perforation after endoscopic retrograde cholangiopancreatography and sphincterotomy. Ann Surg 2000; 232:191–198.
- Enns M, Eloubeidi K, Mergener P, et al. ERCP-related perforations: risk factors and management. Endoscopy 2002; 34:293–298.
- Vezakis A, Fragulidis G, Polydorou A. Endoscopic retrograde cholangiopancreatography-related perforations: diagnosis and management. World J Gastrointest Endosc 2015; 7:1135–1341.
- Frias Vilaca A, Reis AM, Vidal IM. The anatomical compartments and their connections as demonstrated by ectopic air. Insights Imaging 2013; 4:759–772.
- Machado N. Management of duodenal perforation post-endoscopic retrograde cholangiopancreatography. When and whom to operate and what factors determine the outcome? A review article. JOP (Online) 2012; 13:18–25.
- Dubecz A, Ottmann J, Schweigert M, et al. Management of ERCP-related small bowel perforations: the pivotal role of physical investigation. Can J Surg 2012; 55:99–104.
A 68-year-old man with metastatic periampullary adenocarcinoma presented to his usual clinic for a scheduled biliary stent exchange by endoscopic retrograde cholangiopancreatography (ERCP). The stent had been placed 5 months before, and no complications had been reported during that procedure.
During the stent exchange procedure, the endoscopist advanced the scope to the second part of the duodenum, where a large, ulcerated, friable mass was visualized surrounding the ampulla, consistent with patient’s known periampullary cancer. The biliary stent was removed without much difficulty. However, several attempts to cannulate the common bile duct with a preloaded guidewire failed because of extensive edema and tissue friability, and to avoid further discomfort to the patient, the procedure was aborted. No perforation was visualized during or at the end of the procedure.
During the first hour after the procedure was stopped, the patient suddenly developed abdominal pain and distention and crepitus of the right chest wall. Supine abdominal radiography showed extensive pneumoperitoneum and subcutaneous emphysema in the chest. A nasogastric tube was placed for decompression, and the patient was transferred to the surgical intensive care unit at our hospital.
EVIDENCE OF PERFORATION NOTED
On arrival, the patient’s oxygen saturation was 99% while receiving oxygen at 2 L/minute by nasal cannula. The physical examination revealed neck swelling, abdominal distention, and crepitus in the neck, abdomen, scrotum, and right lower extremity.
Computed tomography (CT) of the abdomen and pelvis with oral and intravenous contrast revealed widespread pneumoretroperitoneum, pneumoperitoneum, and air along the intermuscular planes in the right lower extremity, with no evidence of extravasation of oral contrast (Figure 1). Also noted were bilateral pneumothorax, pneumomediastinum, pneumopericardium, and extensive subcutaneous emphysema (Figure 2).
Despite these impressive findings, the patient remained hemodynamically stable and was managed conservatively with broad-spectrum antibiotics, gastric decompression, and bowel rest. But repeat chest radiography 5 hours after admission to the hospital revealed an enlarging right pneumothorax, which was treated with placement of a pigtail catheter. The patient continued to improve with conservative management and was discharged on the 6th day of hospitalization.
PERFORATION DURING ERCP: INCIDENCE AND COMPLICATIONS
Although perforation is an uncommon complication of ERCP, with an incidence of 1%, mortality rates as high as 18% have been reported.1 Older age, longer procedural time, anatomic variations, and diseases of the duodenum and common bile duct can increase the risk of perforation.2
Types of perforation
Stapfer et al1 classified perforation during ERCP into four types, based on etiology and site of perforation. Type 1 is perforation of the lateral or medial duodenal wall caused by excessive pressure from the endoscope or its acute angulation. Type 2 is periampullary injury, often associated with sphincterotomy or difficulty accessing the biliary tree. Type 3 is injury to the common bile duct or pancreatic duct caused by instrumentation. Type 4 is the presence of retroperitoneal free air with no evidence of actual perforation; this is usually an incidental finding and is of little or no clinical consequence.1
In 2015, a review of 18 studies described the distribution of ERCP perforation according to the Stapfer classification: 25% were type 1, 46% were type 2, and 22% were type 3.3
Effects of air insufflation
ERCP requires air insufflation for optimal visualization. During difficult or prolonged procedures, a larger amount of air may be insufflated to maintain bowel lumen visibility. Depending on the site and size of the defect, a variable amount of air can leak under pressure once the perforation occurs. A rapid retroperitoneal air leak can spread to multiple body compartments, including the mediastinum, pleura, neck, subcutaneous tissues, scrotum, and musculature by tracking through various fascial planes. Rarely, rapid ingress of air in these areas can lead to compartment syndrome.4
Small perforations tend to close spontaneously and may remain clinically silent, but large or persistent perforations are known to cause subcutaneous emphysema, sepsis, and respiratory failure.5
Our patient’s type 2 perforation
We presumed that our patient had a type 2 perforation, given the finding of retroperitoneal air. Difficulty cannulating the biliary tree via the friable malignant tissue at the site of the major papilla likely caused punctate perforations, resulting in air leakage into the retroperitoneum. Punctate perforations typically do not allow contrast extravasation, explaining the absence of oral contrast leakage on CT.
TREATMENT OF ENDOSCOPY-RELATED PERFORATION
Conventional supine and upright abdominal radiography is an appropriate initial imaging modality to confirm the diagnosis. However, CT is more sensitive and accurate, especially when air leakage is confined to the retroperitoneum. Intravenous or oral contrast is not necessary but may help localize the perforation and better delineate fluid collections and abscesses.2
Once perforation is suspected, treatment with a broad-spectrum antibiotic, bowel rest, and stomach decompression is imperative.6 Further management depends on the type of perforation and the overall clinical picture. Type 1 perforations usually require immediate surgical intervention. Type 2 perforations often seal spontaneously within 2 to 3 days and thus are managed conservatively (ie, a broad-spectrum antibiotic, gastric decompression, and bowel rest), unless there is a persistent leak or a large fluid collection. Type 3 perforations rarely require surgery since most are very small and close spontaneously, and so they are managed conservatively. Type 4 perforations are the least serious. They result in retroperitoneal free air that is thought be related to the use of compressed air for lumen patency. They require only conservative measures.1
A 68-year-old man with metastatic periampullary adenocarcinoma presented to his usual clinic for a scheduled biliary stent exchange by endoscopic retrograde cholangiopancreatography (ERCP). The stent had been placed 5 months before, and no complications had been reported during that procedure.
During the stent exchange procedure, the endoscopist advanced the scope to the second part of the duodenum, where a large, ulcerated, friable mass was visualized surrounding the ampulla, consistent with patient’s known periampullary cancer. The biliary stent was removed without much difficulty. However, several attempts to cannulate the common bile duct with a preloaded guidewire failed because of extensive edema and tissue friability, and to avoid further discomfort to the patient, the procedure was aborted. No perforation was visualized during or at the end of the procedure.
During the first hour after the procedure was stopped, the patient suddenly developed abdominal pain and distention and crepitus of the right chest wall. Supine abdominal radiography showed extensive pneumoperitoneum and subcutaneous emphysema in the chest. A nasogastric tube was placed for decompression, and the patient was transferred to the surgical intensive care unit at our hospital.
EVIDENCE OF PERFORATION NOTED
On arrival, the patient’s oxygen saturation was 99% while receiving oxygen at 2 L/minute by nasal cannula. The physical examination revealed neck swelling, abdominal distention, and crepitus in the neck, abdomen, scrotum, and right lower extremity.
Computed tomography (CT) of the abdomen and pelvis with oral and intravenous contrast revealed widespread pneumoretroperitoneum, pneumoperitoneum, and air along the intermuscular planes in the right lower extremity, with no evidence of extravasation of oral contrast (Figure 1). Also noted were bilateral pneumothorax, pneumomediastinum, pneumopericardium, and extensive subcutaneous emphysema (Figure 2).
Despite these impressive findings, the patient remained hemodynamically stable and was managed conservatively with broad-spectrum antibiotics, gastric decompression, and bowel rest. But repeat chest radiography 5 hours after admission to the hospital revealed an enlarging right pneumothorax, which was treated with placement of a pigtail catheter. The patient continued to improve with conservative management and was discharged on the 6th day of hospitalization.
PERFORATION DURING ERCP: INCIDENCE AND COMPLICATIONS
Although perforation is an uncommon complication of ERCP, with an incidence of 1%, mortality rates as high as 18% have been reported.1 Older age, longer procedural time, anatomic variations, and diseases of the duodenum and common bile duct can increase the risk of perforation.2
Types of perforation
Stapfer et al1 classified perforation during ERCP into four types, based on etiology and site of perforation. Type 1 is perforation of the lateral or medial duodenal wall caused by excessive pressure from the endoscope or its acute angulation. Type 2 is periampullary injury, often associated with sphincterotomy or difficulty accessing the biliary tree. Type 3 is injury to the common bile duct or pancreatic duct caused by instrumentation. Type 4 is the presence of retroperitoneal free air with no evidence of actual perforation; this is usually an incidental finding and is of little or no clinical consequence.1
In 2015, a review of 18 studies described the distribution of ERCP perforation according to the Stapfer classification: 25% were type 1, 46% were type 2, and 22% were type 3.3
Effects of air insufflation
ERCP requires air insufflation for optimal visualization. During difficult or prolonged procedures, a larger amount of air may be insufflated to maintain bowel lumen visibility. Depending on the site and size of the defect, a variable amount of air can leak under pressure once the perforation occurs. A rapid retroperitoneal air leak can spread to multiple body compartments, including the mediastinum, pleura, neck, subcutaneous tissues, scrotum, and musculature by tracking through various fascial planes. Rarely, rapid ingress of air in these areas can lead to compartment syndrome.4
Small perforations tend to close spontaneously and may remain clinically silent, but large or persistent perforations are known to cause subcutaneous emphysema, sepsis, and respiratory failure.5
Our patient’s type 2 perforation
We presumed that our patient had a type 2 perforation, given the finding of retroperitoneal air. Difficulty cannulating the biliary tree via the friable malignant tissue at the site of the major papilla likely caused punctate perforations, resulting in air leakage into the retroperitoneum. Punctate perforations typically do not allow contrast extravasation, explaining the absence of oral contrast leakage on CT.
TREATMENT OF ENDOSCOPY-RELATED PERFORATION
Conventional supine and upright abdominal radiography is an appropriate initial imaging modality to confirm the diagnosis. However, CT is more sensitive and accurate, especially when air leakage is confined to the retroperitoneum. Intravenous or oral contrast is not necessary but may help localize the perforation and better delineate fluid collections and abscesses.2
Once perforation is suspected, treatment with a broad-spectrum antibiotic, bowel rest, and stomach decompression is imperative.6 Further management depends on the type of perforation and the overall clinical picture. Type 1 perforations usually require immediate surgical intervention. Type 2 perforations often seal spontaneously within 2 to 3 days and thus are managed conservatively (ie, a broad-spectrum antibiotic, gastric decompression, and bowel rest), unless there is a persistent leak or a large fluid collection. Type 3 perforations rarely require surgery since most are very small and close spontaneously, and so they are managed conservatively. Type 4 perforations are the least serious. They result in retroperitoneal free air that is thought be related to the use of compressed air for lumen patency. They require only conservative measures.1
- Stapfer M, Selby RR, Stain SC, et al. Management of duodenal perforation after endoscopic retrograde cholangiopancreatography and sphincterotomy. Ann Surg 2000; 232:191–198.
- Enns M, Eloubeidi K, Mergener P, et al. ERCP-related perforations: risk factors and management. Endoscopy 2002; 34:293–298.
- Vezakis A, Fragulidis G, Polydorou A. Endoscopic retrograde cholangiopancreatography-related perforations: diagnosis and management. World J Gastrointest Endosc 2015; 7:1135–1341.
- Frias Vilaca A, Reis AM, Vidal IM. The anatomical compartments and their connections as demonstrated by ectopic air. Insights Imaging 2013; 4:759–772.
- Machado N. Management of duodenal perforation post-endoscopic retrograde cholangiopancreatography. When and whom to operate and what factors determine the outcome? A review article. JOP (Online) 2012; 13:18–25.
- Dubecz A, Ottmann J, Schweigert M, et al. Management of ERCP-related small bowel perforations: the pivotal role of physical investigation. Can J Surg 2012; 55:99–104.
- Stapfer M, Selby RR, Stain SC, et al. Management of duodenal perforation after endoscopic retrograde cholangiopancreatography and sphincterotomy. Ann Surg 2000; 232:191–198.
- Enns M, Eloubeidi K, Mergener P, et al. ERCP-related perforations: risk factors and management. Endoscopy 2002; 34:293–298.
- Vezakis A, Fragulidis G, Polydorou A. Endoscopic retrograde cholangiopancreatography-related perforations: diagnosis and management. World J Gastrointest Endosc 2015; 7:1135–1341.
- Frias Vilaca A, Reis AM, Vidal IM. The anatomical compartments and their connections as demonstrated by ectopic air. Insights Imaging 2013; 4:759–772.
- Machado N. Management of duodenal perforation post-endoscopic retrograde cholangiopancreatography. When and whom to operate and what factors determine the outcome? A review article. JOP (Online) 2012; 13:18–25.
- Dubecz A, Ottmann J, Schweigert M, et al. Management of ERCP-related small bowel perforations: the pivotal role of physical investigation. Can J Surg 2012; 55:99–104.
Nonatherosclerotic limb ischemia: Prompt evaluation and diagnosis
Timely diagnosis of limb ischemia is critical to limb health and limb salvage. The cause in most cases is related to atherosclerosis, and patients with limb ischemia are usually older and have risk factors for atherosclerosis, such as smoking, diabetes, hypertension, hyperlipidemia, and coronary artery disease. When younger patients develop limb ischemia, the diagnosis is often delayed since the index of suspicion is quite low in the absence of the usual risk factors.
Here, we discuss several nonatherosclerotic causes of limb ischemia: popliteal artery entrapment syndrome, popliteal artery aneurysm, cystic adventitial disease, persistent sciatic artery, phlegmasia cerulea dolens, Buerger disease, Takayasu arteritis, arterial thoracic outlet syndrome, and external iliac endofibrosis (Table 1). Our goal is to help clinicians make a timely diagnosis and ultimately save the patient’s limb.
POPLITEAL ARTERY ENTRAPMENT SYNDROME
Popliteal artery entrapment syndrome occurs when the popliteal artery becomes compressed in the popliteal fossa, particularly during exercise.1,2 The underlying problem may be that the popliteal artery has an aberrant course lateral to the medial head of the gastrocnemius muscle, or the medial head of the gastrocnemius may have an abnormal insertion, or there may be fibrous bands in the popliteal fossa, or a combination of these (Figure 1).1–3 Functional popliteal artery entrapment syndrome occurs when there is compression of the artery without an anatomic cause.1–3
The classic clinical presentation is a young athletic patient with calf or foot claudication (crampy pain with exercise, relieved with rest), but other symptoms can include coldness, paresthesias, and numbness. Pain at rest and tissue loss are rare on presentation but may develop if the diagnosis and treatment are delayed.3
Continued compression and microtrauma to the artery may lead to an intramural hematoma, thrombus formation, aneurysmal degeneration, dissection, or even acute thrombosis.2 If the diagnosis is delayed, the patient’s condition may progress from intermittent arterial compression with plantar flexion to complete arterial thrombosis and critical limb ischemia, putting the patient at risk of limb loss.
Diagnosing popliteal artery entrapment syndrome
The diagnostic workup includes a detailed history with a focus on the cause of pain (usually exercise), a comprehensive physical examination that includes looking for wounds, and a thorough pulse examination.
The workup should start with noninvasive imaging such as duplex arterial ultrasonography with and without provocative measures (plantar flexion), the ankle-brachial index with and without provocative measures, and exercise treadmill testing with ankle-brachial index measurement.1,2 Plantar flexion may be necessary to elicit arterial compression that is usually absent at rest.
Magnetic resonance imaging (MRI) and computed tomography (CT) of the lower extremity are useful to identify an arterial abnormality and aberrant muscle anatomy1,3; MRI is currently the gold standard for delineating the muscles of the popliteal fossa.4 If these studies do not shed light on the diagnosis, arterial angiography with and without provocative maneuvers is useful in identifying compression of the popliteal artery.1–3
Treating popliteal artery entrapment syndrome
Treatment depends on the level of arterial injury.
For patients with symptoms but no evidence of arterial injury, the most common procedure offered is popliteal fossa decompression.1–3 This involves surgical release of the medial head of the gastrocnemius muscle and other muscles compressing the popliteal artery.
For patients with evidence of arterial injury such as stenosis, dissection, or aneurysm, bypass grafting may be required.
For patients who present with acute limb ischemia, both surgical thrombectomy with possible bypass and intraarterial lysis have been described.1,2,5
POPLITEAL ARTERY ANEURYSM
Popliteal artery aneurysm (Figure 2) is the most common type of aneurysm of the peripheral arteries of the lower extremity and is present in about 1% of men over age 65. Fifty percent are bilateral, and 50% are associated with an abdominal aortic aneurysm.6,7 While up to 80% patients with this type of aneurysm have no symptoms at the time of diagnosis, symptoms develop at a rate of 14% per year, with acute limb ischemia occurring in up to one-third of cases.6,7
When popliteal artery aneurysm progresses to acute limb ischemia, the consequences are often deleterious, as the tibial arteries distal to the popliteal artery are often occluded, limiting treatment options.
Popliteal artery aneurysm is defined as a local dilation of the artery of 2 cm or greater or an increase in the diameter to 1.5 times normal.6
Acute thrombosis of the aneurysm with limb ischemia is the most common presenting symptom and occurs in 50% of symptomatic cases of popliteal artery aneurysm.7 Almost 25% of patients present with intermittent claudication secondary to thrombosis, partial thrombosis with distal embolization, or combined aneurysmal and atherosclerotic disease. Compression of the popliteal vein by the popliteal artery aneurysm can cause leg swelling with or without deep vein thrombosis in up to 5% of patients.6 Rupture is very rare, with a rate of 2% to 4%.6,7
Diagnosing popliteal artery aneurysm
The diagnosis can be made with arterial duplex ultrasonography, which is also useful for follow-up surveillance.6–8 In the acute setting, computed tomographic angiography (CTA) or magnetic resonance angiography (MRA) is useful not only to identify the popliteal aneurysm, but also to define the distal tibial outflow vessels.6,7
Treating popliteal artery aneurysm
Management of an acutely thrombosed popliteal artery aneurysm starts with systemic anticoagulation with intravenous heparin, followed initially by arterial angiography and lysis.8–11 This approach has been shown to be safe and effective even in the absence of arterial runoff distal to the thrombosed popliteal aneurysm. Conversion to open thrombectomy and bypass can be done if initial lytic therapy fails, if the patient develops complications of lytic therapy, or if the patient needs emergency revascularization because of motor and neurologic deficits in the affected extremity.8,10,11
How to manage the asymptomatic patient depends on the size of the aneurysm. Most studies recommend 2 cm or larger as the criterion for repair,6–8,12 while others suggest treating even smaller aneurysms if thrombus is detected.9 Preoperative imaging before elective treatment of an asymptomatic popliteal artery aneurysm includes either CTA or MRA,8,10 which allows the surgeon to visualize the full extent of the aneurysm to best plan the surgical approach. Diagnostic angiography can help determine the most suitable bypass target and can better characterize tibial outflow.
Asymptomatic popliteal artery aneurysm has traditionally been treated with surgical bypass with exclusion of the aneurysm,6–8,12 but more recently, endovascular approaches using self-expanding stent grafts have been described. Further study is needed to determine the long-term efficacy of the endovascular approach.8,10
CYSTIC ADVENTITIAL DISEASE
Cystic adventitial disease is a rare condition in which a blood vessel is narrowed due to mucin-containing cysts in the adventitia. More than 80% of cases occur in the popliteal artery, but it has been described in other peripheral arteries and veins.13,14 It is more common in men than in women and typically occurs in the 4th or 5th decade of life. Most patients present with the sudden onset of calf claudication without the usual risk factors for peripheral vascular disease.13
Diagnosing cystic adventitial disease
Noninvasive arterial or venous duplex ultrasonography can be a good screening tool, as the cysts appear hypoechoic, but results are operator-dependent. CTA and MRA are the imaging tests of choice, as they can detect the cystic lesions and define vessel anatomy for intervention. Diagnostic angiography does not show the cysts themselves but instead reveals a classic “hourglass” and “scimitar” pattern of arterial narrowing that suggests the underlying pathology.13,14
Treating cystic adventitial disease
Usual treatment is complete cyst resection and vessel reconstruction by surgical bypass. Other therapies include open surgical cyst evacuation and removal of the cyst wall, open surgical cyst aspiration, aspiration guided by ultrasonography or CT, and percutaneous angioplasty. However, these nonsurgical treatments have not been shown to be as effective and long-lasting as cyst excision and bypass.13,14
PERSISTENT SCIATIC ARTERY
Persistent sciatic artery is a rare developmental abnormality.15–17 Normally, as the femoral artery develops in the embryo, the sciatic artery involutes to form the inferior gluteal artery. But if the femoral system fails to mature, the sciatic artery, which is adjacent to the sciatic nerve posteriorly as it goes through the sciatic foramen, persists and functions as the major artery supplying the lower extremity, continuing to the posterior thigh and joining the popliteal artery (Figure 3).15,17
Persistent sciatic artery has an incidence of 2.5 to 4 per 10,000 per year15 and is bilateral in almost half of cases.16 Up to 40% of patients have no symptoms, but symptoms may develop by age 40 to 50. Because of repeated trauma to the vessel as it passes through the sciatic foramen,18 the persistent sciatic artery typically sustains accelerated atherosclerotic changes that make it susceptible to aneurysm formation,15 and up to 46% of patients present with aneurysmal degeneration.17
Classically, patients present with lower extremity ischemia from atherosclerotic changes in the persistent sciatic artery or aneurysmal degeneration and thromboembolism.15 Rarely, these aneurysms rupture.15,17 Other signs and symptoms include a pulsatile mass in the buttock, lower extremity numbness, motor weakness, and radicular pain along the sciatic nerve distribution from nerve compression.15–17
Physical findings vary but are distinguished by the lack of femoral pulses in the presence of pedal pulses. A pulsatile buttock mass with evidence of lower extremity nerve compression or limb ischemia or both is pathognomonic of a persistent sciatic artery aneurysm.16,18
Diagnosing persistent sciatic artery
Diagnostic angiography is the gold standard imaging test,15,19 although CTA is starting to replace it.16,18
Treating persistent sciatic artery
Persistent sciatic artery that is asymptomatic and is found incidentally does not require repair; however, it should be followed with duplex ultrasonography to look for evidence of aneurysm degeneration. Degeneration requires repair in most cases.15,16,18,19 When the persistent sciatic artery is the only blood supply to the distal extremity, open aneurysm excision and bypass is the treatment of choice.15,16,19 If collateral flow is adequate, endovascular coil embolization is an option.15 Endovascular stent graft placement has also been described.16,19
PHLEGMASIA CERULEA DOLENS
Phlegmasia cerulea dolens is a rare syndrome caused by extensive acute thrombosis of the ileofemoral vein.20–23 It is defined as total or near-total occlusion of the venous outflow of an extremity, causing massive swelling and congestion that impedes arterial inflow.20,22
Phlegmasia cerulea dolens is associated with four cardinal signs: edema, violaceous discoloration, pain, and severe venous outflow obstruction (Figure 4).22 Patients present with sudden onset of lower extremity pain, swelling, cyanosis, and arterial ischemia with or without loss of distal pulses.20,22
This syndrome can progress to gangrene and massive fluid sequestration leading to shock and death.21–23 From 25% to 40% of patients die, and of those who survive, 20% to 50% require amputation of the limb.20,23
Risk factors include malignancy, immobility, heart failure, heparin-induced thrombocytopenia, antiphospholipid syndrome, pregnancy, venous catheterization (eg, to insert an inferior vena cava filter), and surgery.20–22
Diagnosing phlegmasia cerulea dolens
The diagnosis is made on clinical suspicion with evidence of iliofemoral deep vein thrombosis. Most experts suggest venous duplex ultrasonography to identify the deep vein thrombosis,23 although CT or MR venography can be used to better delineate the proximal extent of the thrombus.20,23
Treating phlegmasia cerulea dolens
Initial management is aggressive fluid resuscitation, elevation of the affected limb, strict bed rest, and anticoagulation with intravenous heparin.20,23 Interventions are aimed at urgently restoring venous outflow to prevent progression to venous gangrene and limb loss.
Although conservative therapy can succeed by itself,23 if the condition does not improve or has already progressed to an advanced stage, the two mainstays of treatment are open venous thrombectomy and endovascular treatment.21–23 Endovascular treatment includes catheter-directed thrombolytic therapy (with or without percutaneous mechanical or pharmacomechanical thrombectomy) and stenting.20,23 The success rate for endovascular therapy can be as high as 90% with near-complete resolution of thrombosis.20 A disadvantage is that, compared with open surgical thrombectomy, more time is needed to achieve venous outflow.20,22
If endovascular therapy is ineffective, if lytic therapy is contraindicated, or if the disease has progressed to gangrene, open surgical thrombectomy with possible fasciotomy is the preferred option.20,21,23 Open surgery has the advantage of restoring venous outflow faster, but disadvantages include the inability to open the smaller veins of the extremity, blood loss, and risks associated with general anesthesia.20–22
BUERGER DISEASE
Buerger disease (thromboangiitis obliterans) is a nonatherosclerotic segmental inflammatory disease involving the small and medium-sized vessels of the arms and legs.24–27 It is differentiated from other vasculitides by its marked male predominance, its close association with smoking, the rarity of systemic signs and symptoms, and the absence of elevated inflammatory markers.26
The rate of major amputation is reported to be 11% at 5 years and 23% at 20 years.24
The classic patient is a young male smoker with symptoms of arterial disease before age 45.24,26 Patients can present with migratory thrombophlebitis or signs of arterial insufficiency in the upper or lower extremities. Two or more limbs are commonly involved. Arterial insufficiency can range from claudication and exertional discomfort of the extremity to ischemic pain at rest leading to ulceration of the distal fingers and toes. Physical findings are similar to those seen in peripheral vascular disease and arterial insufficiency, with decreased arterial brachial index, cool extremities, and wounds.
Diagnosing Buerger disease
- The Shionoya diagnostic criteria for Buerger disease are the following five clinical features24,27:
- History of smoking
- Onset before age 50
- Infrapopliteal arterial occlusive disease
- Upper-limb involvement or phlebitis migrans
- Absence of atherosclerotic risk factors other than heavy smoking.
Various other major and minor criteria have been described to make the diagnosis as well.24
There is no specific laboratory test to confirm the diagnosis of Buerger disease. A full panel of laboratory tests should be sent to rule out other causes of arterial insufficiency and vasculitides; these tests should include C-reactive protein, rheumatoid factor, erythrocyte sedimentation rate, antinuclear antibodies, antiphospholipid antibodies, anti-Scl-70 antibodies, anticentromere antibodies, complement level measurement, and hypercoagulability workup.
Imaging studies include arterial duplex ultrasonography with ankle-brachial indices and segmental pressures and CTA or MRA.26 Angiography can show a “corkscrew” pattern of occlusive disease and collateral formation, which is highly associated with Buerger disease.24
Treating Buerger disease
The only treatment shown to reduce the risk of amputation is complete abstention from tobacco and nicotine (smoking, secondhand smoke, and nicotine patches and gum).24,26
Symptoms of claudication can be managed with aspirin, clopidogrel, vasodilators, pentoxifylline, and cilostazol.26
Surgical bypass is rarely an option, as Buerger disease typically affects the distal blood vessels, thus precluding bypass, and the 5-year patency rate is only 49%.26 Other treatments including arterial thrombolysis, sympathectomy, stem cell injection, spinal cord stimulators, omental grafting, and immunomodulation have been described, but there are only limited data to offer guidance in choosing the appropriate one.24
TAKAYASU ARTERITIS
Takayasu arteritis is a form of vasculitis involving the aorta and its main branches (Figure 5).28 Although seen around the world, it has a higher incidence in young Asian women. Patients can present with systemic symptoms such as fever, fatigue, vague pain, and cardinal signs of limb ischemia associated with Takayasu arteritis, such as weak or absent pulses, differences between the arms in pulses and blood pressures, unobtainable blood pressure measurement in one or both arms, limb fatigability, and pain.28
Diagnosing Takayasu arteritis
Multiple diagnostic criteria have been proposed to define Takayasu arteritis.28 CTA, MRA, and positron emission tomography have replaced invasive angiography as the diagnostic imaging tests of choice.29
Treating Takayasu arteritis
Takayasu arteritis has an acute and chronic course. Interventions are typically reserved for severe cases, with indications that include uncontrollable hypertension from renal artery stenosis, severe coronary or cerebrovascular disease, severe aortic regurgitation or coarctation, stenotic or occlusive lesions resulting in critical limb ischemia, and aneurysm at risk of rupture.28–30
THORACIC OUTLET SYNDROME
Thoracic outlet syndrome is compression of the brachial plexus, subclavian vein, or subclavian artery as it exits the thoracic outlet through an area known as the scalene triangle, which is bordered by the anterior scalene, first rib, and clavicle.31 Presenting symptoms depend on the structure compressed.
By far the most common presentation32 is neurogenic thoracic outlet syndrome, accounting for more than 90% of cases, followed by venous thoracic outlet syndrome. Arterial thoracic outlet syndrome is the least frequent at less than 1%, but carries the greatest morbidity with potential for limb loss.31–33
The subclavian artery exits the thoracic outlet between the anterior and middle scalene muscles, and then travels over the first rib and underneath the clavicle.31 Repeated trauma from compression of the artery results in intimal injury leading to compression, stenosis, occlusion, or aneurysm formation.31,32
Symptoms of arterial thoracic outlet syndrome can start out as effort fatigue of the upper extremity secondary to compression. These symptoms are usually vague and difficult to define,31 as these patients typically are young and do not have atherosclerotic risk factors that would prompt suspicion of a vascular cause.
The most common presentation of arterial thoracic outlet syndrome is upper extremity embolization from a partially thrombosed aneurysm or area of stenosis with ischemia.32 Symptoms can range from ischemia of the fingers due to microembolization to acute limb ischemia due to complete thrombosis of the subclavian artery.31,32 Arterial thoracic outlet syndrome is most commonly associated with a bony abnormality (ie, cervical rib or anomalous first rib),31–33 and on physical examination the bony abnormality may be palpated in the supraclavicular fossa.31
Other physical findings include a bruit over the subclavian artery, a blood pressure difference of 20 mm Hg or more between the affected and unaffected arms, loss of brachial, radial, or ulnar pulses with arm abduction, and loss of the radial pulse with the head rotated to the affected side as the patient takes a deep breath (the Adson maneuver).31 While postural changes in the pulse examination hint at arterial thoracic outlet syndrome, extremity pulses may be reduced or even absent in up to 60% of normal patients.32
Diagnosing thoracic outlet syndrome
The workup should start with noninvasive imaging with pulse volume recording and wrist and finger systolic pressures, followed by arterial duplex ultrasonography.
Chest radiography may be able to identify bony abnormalities, and MRA or CTA with the patient in two positions—ie, arms down at the sides, and arms held above the head—can help identify arterial compression from bony or muscular structures in the thoracic outlet. Upper extremity angiography provides high-resolution imaging of the digital arteries and can help identify a subclavian artery aneurysm, which may be a subtle finding.31
It is important to have objective evidence of arterial or venous mechanical obstruction before deciding to remove the first rib.
Treating thoracic outlet syndrome
Treatment is determined by the severity and acuity of symptoms. If the patient presents with acute limb ischemia, prompt treatment with either open surgery or endovascular treatment is required.31,32,34 Once the acute phase has resolved or if the patient presents with chronic disease, open surgical repair is needed to remove the compression of the artery. If an arterial abnormality is identified (aneurysm or significant stenosis), an arterial reconstruction with bypass may be required.31
The standard treatment for thoracic outlet syndrome is resection of the first rib (and removal of the cervical rib if present).31,34 This can be by a transaxillary approach unless arterial reconstruction is needed, in which case a supraclavicular approach is used.31,34 When a patient without symptoms is found to have evidence of arterial compression, most experts would recommend resection of the first rib if there is evidence of an arterial abnormality, or follow-up with duplex imaging for patients with only subtle findings.31
EXTERNAL ILIAC ENDOFIBROSIS
External iliac endofibrosis is a rare cause of intermittent claudication, typically in high-performance athletes, resulting from thickening of the intima in the external iliac artery causing luminal narrowing and resultant ischemia.35–37 The estimated incidence is as high as 20% in elite competitive cyclists, and the condition has been described in other sports as well.37
External iliac endofibrosis typically presents as unilateral leg pain or cramping at near-maximal exercise with an associated feeling of swelling and numbness on the affected side.35,37 It is bilateral in up to 15% of cases.35 While claudication of the thigh is the predominant presenting symptom, dissection and thrombosis of the external iliac artery have been described, presenting with acute limb ischemia in up to 4% of patients.35,36
The condition has been attributed to factors such as physical position, psoas hypertrophy, tethering of the external iliac artery to the psoas muscle, kinking and tortuosity of the vessel, and high-flow states secondary to increased cardiac output and adaptive systolic hypertension.36,37
Diagnosing external iliac endofibrosis
The diagnosis is difficult, as symptoms typically manifest only during maximal exercise. Delays of 12 to 41 months between the onset of symptoms and diagnosis have been reported.37 Physical findings are nonspecific, and pulses and ankle-brachial indices are typically normal at rest. A careful history with a focus on location and duration of symptoms and a high index of suspicion have been shown to increase the sensitivity of diagnosis.36
Noninvasive vascular imaging with arterial duplex ultrasonography with physiologic studies (the ankle-brachial index) at rest and at maximal exertion should be obtained first.35,37 If findings on ultrasonography are positive, CTA or MRA can be used to identify a suspected stenosis.
Diagnostic angiography is still the gold standard for imaging, as real-time images of the artery with different leg positions can be obtained and pressure gradients can be measured with or without the use of a vasodilator to determine the hemodynamic significance of a lesion.35–37
Treating external iliac endofibrosis
Treatment should initially be conservative. Recreational athletes should consider changing to a sport that does not require hip flexion, and cyclists should be advised to reduce the amount of time spent cycling and to raise the handlebars or bring the saddle position forward to minimize hip flexion.37
Definitive treatment is open surgical repair. Surgical options include arterial release of the tethered artery, endofibrosectomy and vessel shortening, endofibrosectomy and patch angioplasty, and interposition bypass grafting.35–37
- Sinha S, Houghton J, Holt PJ, Thompson MM, Loftus IM, Hinchliffe RJ. Popliteal entrapment syndrome. J Vasc Surg 2012; 55:252–262.e30.
- Gokkus K, Sagtas E, Bakalim T, Taskaya E, Aydin AT. Popliteal entrapment syndrome. A systematic review of the literature and case presentation. Muscles Ligaments Tendons J 2014; 4:141–148.
- Pillai J. A current interpretation of popliteal vascular entrapment. J Vasc Surg 2008; 48(suppl 6):61S–65S.
- Liu Y, Sun Y, He X, et al. Imaging diagnosis and surgical treatment of popliteal artery entrapment syndrome: a single-center experience. Ann Vasc Surg 2014; 28:330–337.
- Kim SY, Min SK, Ahn S, Min SI, Ha J, Kim SJ. Long-term outcomes after revascularization for advanced popliteal artery entrapment syndrome with segmental arterial occlusion. J Vasc Surg 2012; 55:90–97.
- Galland RB. Popliteal aneurysms: from John Hunter to the 21st century. Ann R Coll Surg Engl 2007; 89:466–471.
- Dawson J, Fitridge R. Update on aneurysm disease: current insights and controversies: peripheral aneurysms: when to intervene—is rupture really a danger? Prog Cardiovasc Dis 2013; 56:26–35.
- Stone PA, Jagannath P, Thompson SN, et al. Evolving treatment of popliteal artery aneurysms. J Vasc Surg 2013; 57:1306–1310.
- Eslami MH, Rybin D, Doros G, Farber A. Open repair of asymptomatic popliteal artery aneurysm is associated with better outcomes than endovascular repair. J Vasc Surg 2015; 61:663–669.
- Serrano Hernando FJ, Martínez López I, Hernández Mateo MM, et al. Comparison of popliteal artery aneurysm therapies. J Vasc Surg 2015; 61:655–661.
- Marty B, Wicky S, Ris HB, et al. Success of thrombolysis as a predictor of outcome in acute thrombosis of popliteal aneurysms. J Vasc Surg 2002; 35:487–493.
- Hall HA, Minc S, Babrowski T. Peripheral artery aneurysm. Surg Clin North Am 2013; 93:911–923.
- Veraldi GF, Scudo G, Scorsone L, Mezzetto L, Castellani RL. Cystic adventitial disease of the popliteal artery: report of two cases and review of the literature. G Chir 2014; 35:229–234.
- Desy NM, Spinner RJ. The etiology and management of cystic adventitial disease. J Vasc Surg 2014; 60:235–245.e1–e11.
- Patel MV, Patel NH, Schneider JR, Kim S, Verta MJ. Persistent sciatic artery presenting with limb ischemia. J Vasc Surg 2013; 57:225–229.
- Kesri G, Mangtani J, Kumar G, Dangayach KK. Persistent sciatic artery aneurysm with lower limb ischemia. Case Rep Vasc Med 2014; 2014:183969.
- Nuño-Escobar C, Pérez-Durán MA, Ramos-López R, et al. Persistent sciatic artery aneurysm. Ann Vasc Surg 2013; 27:1182.e13–e16.
- Vaz C, Machado R, Rego D, Matos A, Almeida R. Hybrid approach in a case of persistent sciatic artery aneurysm. Ann Vasc Surg 2014; 28:1313.e5–e7.
- Abularrage CJ, Crawford RS, Patel VI, Conrad MF. Diagnostic strategies for the persistent sciatic artery. Vasc Endovascular Surg 2009; 43:485–489.
- Suwanabol PA, Tefera G, Schwarze ML. Syndromes associated with the deep veins: phlegmasia cerulea dolens, May-Thurner syndrome, and nutcracker syndrome. Perspect Vasc Surg Endovasc Ther 2010; 22:223–230.
- Vysetti S, Shinde S, Chaudhry S, Subramoney K. Phlegmasia cerulea dolens—a rare, life-threatening condition. ScientificWorldJournal 2009; 9:1105–1106.
- Mumoli N, Invernizzi C, Luschi R, Carmignani G, Camaiti A, Cei M. Phlegmasia cerulea dolens. Circulation 2012; 125:1056–1057.
- Chinsakchai K, Ten Duis K, Moll FL, de Borst GJ. Trends in management of phlegmasia cerulea dolens. Vasc Endovascular Surg 2011; 45:5–14.
- Dargon PT, Landry GJ. Buerger’s disease. Ann Vasc Surg 2012; 26:871–880.
- Faizer R, Forbes TL. Buerger’s disease. J Vasc Surg 2007; 46:812.
- Vijayakumar A, Tiwari R, Kumar Prabhuswamy V. Thromboangiitis obliterans (Buerger’s disease)—current practices. Int J Inflam 2013; 2013:156905.
- Ohta T, Ishibashi H, Sugimoto I, et al. The clinical course of Buerger’s disease. Ann Vasc Dis 2008; 1:85–90.
- de Souza AWS, de Carvalho JF. Diagnostic and classification criteria of Takayasu arteritis. J Autoimmun 2014; 48–49:79–83.
- Perera AH, Mason JC, Wolfe JH. Takayasu arteritis: criteria for surgical intervention should not be ignored. Int J Vasc Med 2013; 2013:618910.
- Keser G, Direskeneli H, Aksu K. Management of Takayasu arteritis: a systematic review. Rheumatology (Oxford) 2014; 53:793–801.
- Sanders RJ, Annest SJ. Thoracic outlet and pectoralis minor syndromes. Semin Vasc Surg 2014; 27:86–117.
- Criado E, Berguer R, Greenfield L. The spectrum of arterial compression at the thoracic outlet. J Vasc Surg 2010; 52:406–411.
- Povlsen B, Hansson T, Povlsen SD. Treatment for thoracic outlet syndrome. Cochrane Database Syst Rev 2014; 11:CD007218.
- Orlando MS, Likes KC, Mirza S, et al. A decade of excellent outcomes after surgical intervention in 538 patients with thoracic outlet syndrome. J Am Coll Surg 2015; 220:934–939.
- Bucci F, Ottaviani N, Plagnol P. Acute thrombosis of external iliac artery secondary to endofibrosis. Ann Vasc Surg 2011; 25:698.e5–e7.
- Willson TD, Revesz E, Podbielski FJ, Blecha MJ. External iliac artery dissection secondary to endofibrosis in a cyclist. J Vasc Surg 2010; 52:219–221.
- Peach G, Schep G, Palfreeman R, Beard JD, Thompson MM, Hinchliffe RJ. Endofibrosis and kinking of the Iliac arteries in athletes: a systematic review. Eur J Vasc Endovasc Surg 2012; 43:208–217.
Timely diagnosis of limb ischemia is critical to limb health and limb salvage. The cause in most cases is related to atherosclerosis, and patients with limb ischemia are usually older and have risk factors for atherosclerosis, such as smoking, diabetes, hypertension, hyperlipidemia, and coronary artery disease. When younger patients develop limb ischemia, the diagnosis is often delayed since the index of suspicion is quite low in the absence of the usual risk factors.
Here, we discuss several nonatherosclerotic causes of limb ischemia: popliteal artery entrapment syndrome, popliteal artery aneurysm, cystic adventitial disease, persistent sciatic artery, phlegmasia cerulea dolens, Buerger disease, Takayasu arteritis, arterial thoracic outlet syndrome, and external iliac endofibrosis (Table 1). Our goal is to help clinicians make a timely diagnosis and ultimately save the patient’s limb.
POPLITEAL ARTERY ENTRAPMENT SYNDROME
Popliteal artery entrapment syndrome occurs when the popliteal artery becomes compressed in the popliteal fossa, particularly during exercise.1,2 The underlying problem may be that the popliteal artery has an aberrant course lateral to the medial head of the gastrocnemius muscle, or the medial head of the gastrocnemius may have an abnormal insertion, or there may be fibrous bands in the popliteal fossa, or a combination of these (Figure 1).1–3 Functional popliteal artery entrapment syndrome occurs when there is compression of the artery without an anatomic cause.1–3
The classic clinical presentation is a young athletic patient with calf or foot claudication (crampy pain with exercise, relieved with rest), but other symptoms can include coldness, paresthesias, and numbness. Pain at rest and tissue loss are rare on presentation but may develop if the diagnosis and treatment are delayed.3
Continued compression and microtrauma to the artery may lead to an intramural hematoma, thrombus formation, aneurysmal degeneration, dissection, or even acute thrombosis.2 If the diagnosis is delayed, the patient’s condition may progress from intermittent arterial compression with plantar flexion to complete arterial thrombosis and critical limb ischemia, putting the patient at risk of limb loss.
Diagnosing popliteal artery entrapment syndrome
The diagnostic workup includes a detailed history with a focus on the cause of pain (usually exercise), a comprehensive physical examination that includes looking for wounds, and a thorough pulse examination.
The workup should start with noninvasive imaging such as duplex arterial ultrasonography with and without provocative measures (plantar flexion), the ankle-brachial index with and without provocative measures, and exercise treadmill testing with ankle-brachial index measurement.1,2 Plantar flexion may be necessary to elicit arterial compression that is usually absent at rest.
Magnetic resonance imaging (MRI) and computed tomography (CT) of the lower extremity are useful to identify an arterial abnormality and aberrant muscle anatomy1,3; MRI is currently the gold standard for delineating the muscles of the popliteal fossa.4 If these studies do not shed light on the diagnosis, arterial angiography with and without provocative maneuvers is useful in identifying compression of the popliteal artery.1–3
Treating popliteal artery entrapment syndrome
Treatment depends on the level of arterial injury.
For patients with symptoms but no evidence of arterial injury, the most common procedure offered is popliteal fossa decompression.1–3 This involves surgical release of the medial head of the gastrocnemius muscle and other muscles compressing the popliteal artery.
For patients with evidence of arterial injury such as stenosis, dissection, or aneurysm, bypass grafting may be required.
For patients who present with acute limb ischemia, both surgical thrombectomy with possible bypass and intraarterial lysis have been described.1,2,5
POPLITEAL ARTERY ANEURYSM
Popliteal artery aneurysm (Figure 2) is the most common type of aneurysm of the peripheral arteries of the lower extremity and is present in about 1% of men over age 65. Fifty percent are bilateral, and 50% are associated with an abdominal aortic aneurysm.6,7 While up to 80% patients with this type of aneurysm have no symptoms at the time of diagnosis, symptoms develop at a rate of 14% per year, with acute limb ischemia occurring in up to one-third of cases.6,7
When popliteal artery aneurysm progresses to acute limb ischemia, the consequences are often deleterious, as the tibial arteries distal to the popliteal artery are often occluded, limiting treatment options.
Popliteal artery aneurysm is defined as a local dilation of the artery of 2 cm or greater or an increase in the diameter to 1.5 times normal.6
Acute thrombosis of the aneurysm with limb ischemia is the most common presenting symptom and occurs in 50% of symptomatic cases of popliteal artery aneurysm.7 Almost 25% of patients present with intermittent claudication secondary to thrombosis, partial thrombosis with distal embolization, or combined aneurysmal and atherosclerotic disease. Compression of the popliteal vein by the popliteal artery aneurysm can cause leg swelling with or without deep vein thrombosis in up to 5% of patients.6 Rupture is very rare, with a rate of 2% to 4%.6,7
Diagnosing popliteal artery aneurysm
The diagnosis can be made with arterial duplex ultrasonography, which is also useful for follow-up surveillance.6–8 In the acute setting, computed tomographic angiography (CTA) or magnetic resonance angiography (MRA) is useful not only to identify the popliteal aneurysm, but also to define the distal tibial outflow vessels.6,7
Treating popliteal artery aneurysm
Management of an acutely thrombosed popliteal artery aneurysm starts with systemic anticoagulation with intravenous heparin, followed initially by arterial angiography and lysis.8–11 This approach has been shown to be safe and effective even in the absence of arterial runoff distal to the thrombosed popliteal aneurysm. Conversion to open thrombectomy and bypass can be done if initial lytic therapy fails, if the patient develops complications of lytic therapy, or if the patient needs emergency revascularization because of motor and neurologic deficits in the affected extremity.8,10,11
How to manage the asymptomatic patient depends on the size of the aneurysm. Most studies recommend 2 cm or larger as the criterion for repair,6–8,12 while others suggest treating even smaller aneurysms if thrombus is detected.9 Preoperative imaging before elective treatment of an asymptomatic popliteal artery aneurysm includes either CTA or MRA,8,10 which allows the surgeon to visualize the full extent of the aneurysm to best plan the surgical approach. Diagnostic angiography can help determine the most suitable bypass target and can better characterize tibial outflow.
Asymptomatic popliteal artery aneurysm has traditionally been treated with surgical bypass with exclusion of the aneurysm,6–8,12 but more recently, endovascular approaches using self-expanding stent grafts have been described. Further study is needed to determine the long-term efficacy of the endovascular approach.8,10
CYSTIC ADVENTITIAL DISEASE
Cystic adventitial disease is a rare condition in which a blood vessel is narrowed due to mucin-containing cysts in the adventitia. More than 80% of cases occur in the popliteal artery, but it has been described in other peripheral arteries and veins.13,14 It is more common in men than in women and typically occurs in the 4th or 5th decade of life. Most patients present with the sudden onset of calf claudication without the usual risk factors for peripheral vascular disease.13
Diagnosing cystic adventitial disease
Noninvasive arterial or venous duplex ultrasonography can be a good screening tool, as the cysts appear hypoechoic, but results are operator-dependent. CTA and MRA are the imaging tests of choice, as they can detect the cystic lesions and define vessel anatomy for intervention. Diagnostic angiography does not show the cysts themselves but instead reveals a classic “hourglass” and “scimitar” pattern of arterial narrowing that suggests the underlying pathology.13,14
Treating cystic adventitial disease
Usual treatment is complete cyst resection and vessel reconstruction by surgical bypass. Other therapies include open surgical cyst evacuation and removal of the cyst wall, open surgical cyst aspiration, aspiration guided by ultrasonography or CT, and percutaneous angioplasty. However, these nonsurgical treatments have not been shown to be as effective and long-lasting as cyst excision and bypass.13,14
PERSISTENT SCIATIC ARTERY
Persistent sciatic artery is a rare developmental abnormality.15–17 Normally, as the femoral artery develops in the embryo, the sciatic artery involutes to form the inferior gluteal artery. But if the femoral system fails to mature, the sciatic artery, which is adjacent to the sciatic nerve posteriorly as it goes through the sciatic foramen, persists and functions as the major artery supplying the lower extremity, continuing to the posterior thigh and joining the popliteal artery (Figure 3).15,17
Persistent sciatic artery has an incidence of 2.5 to 4 per 10,000 per year15 and is bilateral in almost half of cases.16 Up to 40% of patients have no symptoms, but symptoms may develop by age 40 to 50. Because of repeated trauma to the vessel as it passes through the sciatic foramen,18 the persistent sciatic artery typically sustains accelerated atherosclerotic changes that make it susceptible to aneurysm formation,15 and up to 46% of patients present with aneurysmal degeneration.17
Classically, patients present with lower extremity ischemia from atherosclerotic changes in the persistent sciatic artery or aneurysmal degeneration and thromboembolism.15 Rarely, these aneurysms rupture.15,17 Other signs and symptoms include a pulsatile mass in the buttock, lower extremity numbness, motor weakness, and radicular pain along the sciatic nerve distribution from nerve compression.15–17
Physical findings vary but are distinguished by the lack of femoral pulses in the presence of pedal pulses. A pulsatile buttock mass with evidence of lower extremity nerve compression or limb ischemia or both is pathognomonic of a persistent sciatic artery aneurysm.16,18
Diagnosing persistent sciatic artery
Diagnostic angiography is the gold standard imaging test,15,19 although CTA is starting to replace it.16,18
Treating persistent sciatic artery
Persistent sciatic artery that is asymptomatic and is found incidentally does not require repair; however, it should be followed with duplex ultrasonography to look for evidence of aneurysm degeneration. Degeneration requires repair in most cases.15,16,18,19 When the persistent sciatic artery is the only blood supply to the distal extremity, open aneurysm excision and bypass is the treatment of choice.15,16,19 If collateral flow is adequate, endovascular coil embolization is an option.15 Endovascular stent graft placement has also been described.16,19
PHLEGMASIA CERULEA DOLENS
Phlegmasia cerulea dolens is a rare syndrome caused by extensive acute thrombosis of the ileofemoral vein.20–23 It is defined as total or near-total occlusion of the venous outflow of an extremity, causing massive swelling and congestion that impedes arterial inflow.20,22
Phlegmasia cerulea dolens is associated with four cardinal signs: edema, violaceous discoloration, pain, and severe venous outflow obstruction (Figure 4).22 Patients present with sudden onset of lower extremity pain, swelling, cyanosis, and arterial ischemia with or without loss of distal pulses.20,22
This syndrome can progress to gangrene and massive fluid sequestration leading to shock and death.21–23 From 25% to 40% of patients die, and of those who survive, 20% to 50% require amputation of the limb.20,23
Risk factors include malignancy, immobility, heart failure, heparin-induced thrombocytopenia, antiphospholipid syndrome, pregnancy, venous catheterization (eg, to insert an inferior vena cava filter), and surgery.20–22
Diagnosing phlegmasia cerulea dolens
The diagnosis is made on clinical suspicion with evidence of iliofemoral deep vein thrombosis. Most experts suggest venous duplex ultrasonography to identify the deep vein thrombosis,23 although CT or MR venography can be used to better delineate the proximal extent of the thrombus.20,23
Treating phlegmasia cerulea dolens
Initial management is aggressive fluid resuscitation, elevation of the affected limb, strict bed rest, and anticoagulation with intravenous heparin.20,23 Interventions are aimed at urgently restoring venous outflow to prevent progression to venous gangrene and limb loss.
Although conservative therapy can succeed by itself,23 if the condition does not improve or has already progressed to an advanced stage, the two mainstays of treatment are open venous thrombectomy and endovascular treatment.21–23 Endovascular treatment includes catheter-directed thrombolytic therapy (with or without percutaneous mechanical or pharmacomechanical thrombectomy) and stenting.20,23 The success rate for endovascular therapy can be as high as 90% with near-complete resolution of thrombosis.20 A disadvantage is that, compared with open surgical thrombectomy, more time is needed to achieve venous outflow.20,22
If endovascular therapy is ineffective, if lytic therapy is contraindicated, or if the disease has progressed to gangrene, open surgical thrombectomy with possible fasciotomy is the preferred option.20,21,23 Open surgery has the advantage of restoring venous outflow faster, but disadvantages include the inability to open the smaller veins of the extremity, blood loss, and risks associated with general anesthesia.20–22
BUERGER DISEASE
Buerger disease (thromboangiitis obliterans) is a nonatherosclerotic segmental inflammatory disease involving the small and medium-sized vessels of the arms and legs.24–27 It is differentiated from other vasculitides by its marked male predominance, its close association with smoking, the rarity of systemic signs and symptoms, and the absence of elevated inflammatory markers.26
The rate of major amputation is reported to be 11% at 5 years and 23% at 20 years.24
The classic patient is a young male smoker with symptoms of arterial disease before age 45.24,26 Patients can present with migratory thrombophlebitis or signs of arterial insufficiency in the upper or lower extremities. Two or more limbs are commonly involved. Arterial insufficiency can range from claudication and exertional discomfort of the extremity to ischemic pain at rest leading to ulceration of the distal fingers and toes. Physical findings are similar to those seen in peripheral vascular disease and arterial insufficiency, with decreased arterial brachial index, cool extremities, and wounds.
Diagnosing Buerger disease
- The Shionoya diagnostic criteria for Buerger disease are the following five clinical features24,27:
- History of smoking
- Onset before age 50
- Infrapopliteal arterial occlusive disease
- Upper-limb involvement or phlebitis migrans
- Absence of atherosclerotic risk factors other than heavy smoking.
Various other major and minor criteria have been described to make the diagnosis as well.24
There is no specific laboratory test to confirm the diagnosis of Buerger disease. A full panel of laboratory tests should be sent to rule out other causes of arterial insufficiency and vasculitides; these tests should include C-reactive protein, rheumatoid factor, erythrocyte sedimentation rate, antinuclear antibodies, antiphospholipid antibodies, anti-Scl-70 antibodies, anticentromere antibodies, complement level measurement, and hypercoagulability workup.
Imaging studies include arterial duplex ultrasonography with ankle-brachial indices and segmental pressures and CTA or MRA.26 Angiography can show a “corkscrew” pattern of occlusive disease and collateral formation, which is highly associated with Buerger disease.24
Treating Buerger disease
The only treatment shown to reduce the risk of amputation is complete abstention from tobacco and nicotine (smoking, secondhand smoke, and nicotine patches and gum).24,26
Symptoms of claudication can be managed with aspirin, clopidogrel, vasodilators, pentoxifylline, and cilostazol.26
Surgical bypass is rarely an option, as Buerger disease typically affects the distal blood vessels, thus precluding bypass, and the 5-year patency rate is only 49%.26 Other treatments including arterial thrombolysis, sympathectomy, stem cell injection, spinal cord stimulators, omental grafting, and immunomodulation have been described, but there are only limited data to offer guidance in choosing the appropriate one.24
TAKAYASU ARTERITIS
Takayasu arteritis is a form of vasculitis involving the aorta and its main branches (Figure 5).28 Although seen around the world, it has a higher incidence in young Asian women. Patients can present with systemic symptoms such as fever, fatigue, vague pain, and cardinal signs of limb ischemia associated with Takayasu arteritis, such as weak or absent pulses, differences between the arms in pulses and blood pressures, unobtainable blood pressure measurement in one or both arms, limb fatigability, and pain.28
Diagnosing Takayasu arteritis
Multiple diagnostic criteria have been proposed to define Takayasu arteritis.28 CTA, MRA, and positron emission tomography have replaced invasive angiography as the diagnostic imaging tests of choice.29
Treating Takayasu arteritis
Takayasu arteritis has an acute and chronic course. Interventions are typically reserved for severe cases, with indications that include uncontrollable hypertension from renal artery stenosis, severe coronary or cerebrovascular disease, severe aortic regurgitation or coarctation, stenotic or occlusive lesions resulting in critical limb ischemia, and aneurysm at risk of rupture.28–30
THORACIC OUTLET SYNDROME
Thoracic outlet syndrome is compression of the brachial plexus, subclavian vein, or subclavian artery as it exits the thoracic outlet through an area known as the scalene triangle, which is bordered by the anterior scalene, first rib, and clavicle.31 Presenting symptoms depend on the structure compressed.
By far the most common presentation32 is neurogenic thoracic outlet syndrome, accounting for more than 90% of cases, followed by venous thoracic outlet syndrome. Arterial thoracic outlet syndrome is the least frequent at less than 1%, but carries the greatest morbidity with potential for limb loss.31–33
The subclavian artery exits the thoracic outlet between the anterior and middle scalene muscles, and then travels over the first rib and underneath the clavicle.31 Repeated trauma from compression of the artery results in intimal injury leading to compression, stenosis, occlusion, or aneurysm formation.31,32
Symptoms of arterial thoracic outlet syndrome can start out as effort fatigue of the upper extremity secondary to compression. These symptoms are usually vague and difficult to define,31 as these patients typically are young and do not have atherosclerotic risk factors that would prompt suspicion of a vascular cause.
The most common presentation of arterial thoracic outlet syndrome is upper extremity embolization from a partially thrombosed aneurysm or area of stenosis with ischemia.32 Symptoms can range from ischemia of the fingers due to microembolization to acute limb ischemia due to complete thrombosis of the subclavian artery.31,32 Arterial thoracic outlet syndrome is most commonly associated with a bony abnormality (ie, cervical rib or anomalous first rib),31–33 and on physical examination the bony abnormality may be palpated in the supraclavicular fossa.31
Other physical findings include a bruit over the subclavian artery, a blood pressure difference of 20 mm Hg or more between the affected and unaffected arms, loss of brachial, radial, or ulnar pulses with arm abduction, and loss of the radial pulse with the head rotated to the affected side as the patient takes a deep breath (the Adson maneuver).31 While postural changes in the pulse examination hint at arterial thoracic outlet syndrome, extremity pulses may be reduced or even absent in up to 60% of normal patients.32
Diagnosing thoracic outlet syndrome
The workup should start with noninvasive imaging with pulse volume recording and wrist and finger systolic pressures, followed by arterial duplex ultrasonography.
Chest radiography may be able to identify bony abnormalities, and MRA or CTA with the patient in two positions—ie, arms down at the sides, and arms held above the head—can help identify arterial compression from bony or muscular structures in the thoracic outlet. Upper extremity angiography provides high-resolution imaging of the digital arteries and can help identify a subclavian artery aneurysm, which may be a subtle finding.31
It is important to have objective evidence of arterial or venous mechanical obstruction before deciding to remove the first rib.
Treating thoracic outlet syndrome
Treatment is determined by the severity and acuity of symptoms. If the patient presents with acute limb ischemia, prompt treatment with either open surgery or endovascular treatment is required.31,32,34 Once the acute phase has resolved or if the patient presents with chronic disease, open surgical repair is needed to remove the compression of the artery. If an arterial abnormality is identified (aneurysm or significant stenosis), an arterial reconstruction with bypass may be required.31
The standard treatment for thoracic outlet syndrome is resection of the first rib (and removal of the cervical rib if present).31,34 This can be by a transaxillary approach unless arterial reconstruction is needed, in which case a supraclavicular approach is used.31,34 When a patient without symptoms is found to have evidence of arterial compression, most experts would recommend resection of the first rib if there is evidence of an arterial abnormality, or follow-up with duplex imaging for patients with only subtle findings.31
EXTERNAL ILIAC ENDOFIBROSIS
External iliac endofibrosis is a rare cause of intermittent claudication, typically in high-performance athletes, resulting from thickening of the intima in the external iliac artery causing luminal narrowing and resultant ischemia.35–37 The estimated incidence is as high as 20% in elite competitive cyclists, and the condition has been described in other sports as well.37
External iliac endofibrosis typically presents as unilateral leg pain or cramping at near-maximal exercise with an associated feeling of swelling and numbness on the affected side.35,37 It is bilateral in up to 15% of cases.35 While claudication of the thigh is the predominant presenting symptom, dissection and thrombosis of the external iliac artery have been described, presenting with acute limb ischemia in up to 4% of patients.35,36
The condition has been attributed to factors such as physical position, psoas hypertrophy, tethering of the external iliac artery to the psoas muscle, kinking and tortuosity of the vessel, and high-flow states secondary to increased cardiac output and adaptive systolic hypertension.36,37
Diagnosing external iliac endofibrosis
The diagnosis is difficult, as symptoms typically manifest only during maximal exercise. Delays of 12 to 41 months between the onset of symptoms and diagnosis have been reported.37 Physical findings are nonspecific, and pulses and ankle-brachial indices are typically normal at rest. A careful history with a focus on location and duration of symptoms and a high index of suspicion have been shown to increase the sensitivity of diagnosis.36
Noninvasive vascular imaging with arterial duplex ultrasonography with physiologic studies (the ankle-brachial index) at rest and at maximal exertion should be obtained first.35,37 If findings on ultrasonography are positive, CTA or MRA can be used to identify a suspected stenosis.
Diagnostic angiography is still the gold standard for imaging, as real-time images of the artery with different leg positions can be obtained and pressure gradients can be measured with or without the use of a vasodilator to determine the hemodynamic significance of a lesion.35–37
Treating external iliac endofibrosis
Treatment should initially be conservative. Recreational athletes should consider changing to a sport that does not require hip flexion, and cyclists should be advised to reduce the amount of time spent cycling and to raise the handlebars or bring the saddle position forward to minimize hip flexion.37
Definitive treatment is open surgical repair. Surgical options include arterial release of the tethered artery, endofibrosectomy and vessel shortening, endofibrosectomy and patch angioplasty, and interposition bypass grafting.35–37
Timely diagnosis of limb ischemia is critical to limb health and limb salvage. The cause in most cases is related to atherosclerosis, and patients with limb ischemia are usually older and have risk factors for atherosclerosis, such as smoking, diabetes, hypertension, hyperlipidemia, and coronary artery disease. When younger patients develop limb ischemia, the diagnosis is often delayed since the index of suspicion is quite low in the absence of the usual risk factors.
Here, we discuss several nonatherosclerotic causes of limb ischemia: popliteal artery entrapment syndrome, popliteal artery aneurysm, cystic adventitial disease, persistent sciatic artery, phlegmasia cerulea dolens, Buerger disease, Takayasu arteritis, arterial thoracic outlet syndrome, and external iliac endofibrosis (Table 1). Our goal is to help clinicians make a timely diagnosis and ultimately save the patient’s limb.
POPLITEAL ARTERY ENTRAPMENT SYNDROME
Popliteal artery entrapment syndrome occurs when the popliteal artery becomes compressed in the popliteal fossa, particularly during exercise.1,2 The underlying problem may be that the popliteal artery has an aberrant course lateral to the medial head of the gastrocnemius muscle, or the medial head of the gastrocnemius may have an abnormal insertion, or there may be fibrous bands in the popliteal fossa, or a combination of these (Figure 1).1–3 Functional popliteal artery entrapment syndrome occurs when there is compression of the artery without an anatomic cause.1–3
The classic clinical presentation is a young athletic patient with calf or foot claudication (crampy pain with exercise, relieved with rest), but other symptoms can include coldness, paresthesias, and numbness. Pain at rest and tissue loss are rare on presentation but may develop if the diagnosis and treatment are delayed.3
Continued compression and microtrauma to the artery may lead to an intramural hematoma, thrombus formation, aneurysmal degeneration, dissection, or even acute thrombosis.2 If the diagnosis is delayed, the patient’s condition may progress from intermittent arterial compression with plantar flexion to complete arterial thrombosis and critical limb ischemia, putting the patient at risk of limb loss.
Diagnosing popliteal artery entrapment syndrome
The diagnostic workup includes a detailed history with a focus on the cause of pain (usually exercise), a comprehensive physical examination that includes looking for wounds, and a thorough pulse examination.
The workup should start with noninvasive imaging such as duplex arterial ultrasonography with and without provocative measures (plantar flexion), the ankle-brachial index with and without provocative measures, and exercise treadmill testing with ankle-brachial index measurement.1,2 Plantar flexion may be necessary to elicit arterial compression that is usually absent at rest.
Magnetic resonance imaging (MRI) and computed tomography (CT) of the lower extremity are useful to identify an arterial abnormality and aberrant muscle anatomy1,3; MRI is currently the gold standard for delineating the muscles of the popliteal fossa.4 If these studies do not shed light on the diagnosis, arterial angiography with and without provocative maneuvers is useful in identifying compression of the popliteal artery.1–3
Treating popliteal artery entrapment syndrome
Treatment depends on the level of arterial injury.
For patients with symptoms but no evidence of arterial injury, the most common procedure offered is popliteal fossa decompression.1–3 This involves surgical release of the medial head of the gastrocnemius muscle and other muscles compressing the popliteal artery.
For patients with evidence of arterial injury such as stenosis, dissection, or aneurysm, bypass grafting may be required.
For patients who present with acute limb ischemia, both surgical thrombectomy with possible bypass and intraarterial lysis have been described.1,2,5
POPLITEAL ARTERY ANEURYSM
Popliteal artery aneurysm (Figure 2) is the most common type of aneurysm of the peripheral arteries of the lower extremity and is present in about 1% of men over age 65. Fifty percent are bilateral, and 50% are associated with an abdominal aortic aneurysm.6,7 While up to 80% patients with this type of aneurysm have no symptoms at the time of diagnosis, symptoms develop at a rate of 14% per year, with acute limb ischemia occurring in up to one-third of cases.6,7
When popliteal artery aneurysm progresses to acute limb ischemia, the consequences are often deleterious, as the tibial arteries distal to the popliteal artery are often occluded, limiting treatment options.
Popliteal artery aneurysm is defined as a local dilation of the artery of 2 cm or greater or an increase in the diameter to 1.5 times normal.6
Acute thrombosis of the aneurysm with limb ischemia is the most common presenting symptom and occurs in 50% of symptomatic cases of popliteal artery aneurysm.7 Almost 25% of patients present with intermittent claudication secondary to thrombosis, partial thrombosis with distal embolization, or combined aneurysmal and atherosclerotic disease. Compression of the popliteal vein by the popliteal artery aneurysm can cause leg swelling with or without deep vein thrombosis in up to 5% of patients.6 Rupture is very rare, with a rate of 2% to 4%.6,7
Diagnosing popliteal artery aneurysm
The diagnosis can be made with arterial duplex ultrasonography, which is also useful for follow-up surveillance.6–8 In the acute setting, computed tomographic angiography (CTA) or magnetic resonance angiography (MRA) is useful not only to identify the popliteal aneurysm, but also to define the distal tibial outflow vessels.6,7
Treating popliteal artery aneurysm
Management of an acutely thrombosed popliteal artery aneurysm starts with systemic anticoagulation with intravenous heparin, followed initially by arterial angiography and lysis.8–11 This approach has been shown to be safe and effective even in the absence of arterial runoff distal to the thrombosed popliteal aneurysm. Conversion to open thrombectomy and bypass can be done if initial lytic therapy fails, if the patient develops complications of lytic therapy, or if the patient needs emergency revascularization because of motor and neurologic deficits in the affected extremity.8,10,11
How to manage the asymptomatic patient depends on the size of the aneurysm. Most studies recommend 2 cm or larger as the criterion for repair,6–8,12 while others suggest treating even smaller aneurysms if thrombus is detected.9 Preoperative imaging before elective treatment of an asymptomatic popliteal artery aneurysm includes either CTA or MRA,8,10 which allows the surgeon to visualize the full extent of the aneurysm to best plan the surgical approach. Diagnostic angiography can help determine the most suitable bypass target and can better characterize tibial outflow.
Asymptomatic popliteal artery aneurysm has traditionally been treated with surgical bypass with exclusion of the aneurysm,6–8,12 but more recently, endovascular approaches using self-expanding stent grafts have been described. Further study is needed to determine the long-term efficacy of the endovascular approach.8,10
CYSTIC ADVENTITIAL DISEASE
Cystic adventitial disease is a rare condition in which a blood vessel is narrowed due to mucin-containing cysts in the adventitia. More than 80% of cases occur in the popliteal artery, but it has been described in other peripheral arteries and veins.13,14 It is more common in men than in women and typically occurs in the 4th or 5th decade of life. Most patients present with the sudden onset of calf claudication without the usual risk factors for peripheral vascular disease.13
Diagnosing cystic adventitial disease
Noninvasive arterial or venous duplex ultrasonography can be a good screening tool, as the cysts appear hypoechoic, but results are operator-dependent. CTA and MRA are the imaging tests of choice, as they can detect the cystic lesions and define vessel anatomy for intervention. Diagnostic angiography does not show the cysts themselves but instead reveals a classic “hourglass” and “scimitar” pattern of arterial narrowing that suggests the underlying pathology.13,14
Treating cystic adventitial disease
Usual treatment is complete cyst resection and vessel reconstruction by surgical bypass. Other therapies include open surgical cyst evacuation and removal of the cyst wall, open surgical cyst aspiration, aspiration guided by ultrasonography or CT, and percutaneous angioplasty. However, these nonsurgical treatments have not been shown to be as effective and long-lasting as cyst excision and bypass.13,14
PERSISTENT SCIATIC ARTERY
Persistent sciatic artery is a rare developmental abnormality.15–17 Normally, as the femoral artery develops in the embryo, the sciatic artery involutes to form the inferior gluteal artery. But if the femoral system fails to mature, the sciatic artery, which is adjacent to the sciatic nerve posteriorly as it goes through the sciatic foramen, persists and functions as the major artery supplying the lower extremity, continuing to the posterior thigh and joining the popliteal artery (Figure 3).15,17
Persistent sciatic artery has an incidence of 2.5 to 4 per 10,000 per year15 and is bilateral in almost half of cases.16 Up to 40% of patients have no symptoms, but symptoms may develop by age 40 to 50. Because of repeated trauma to the vessel as it passes through the sciatic foramen,18 the persistent sciatic artery typically sustains accelerated atherosclerotic changes that make it susceptible to aneurysm formation,15 and up to 46% of patients present with aneurysmal degeneration.17
Classically, patients present with lower extremity ischemia from atherosclerotic changes in the persistent sciatic artery or aneurysmal degeneration and thromboembolism.15 Rarely, these aneurysms rupture.15,17 Other signs and symptoms include a pulsatile mass in the buttock, lower extremity numbness, motor weakness, and radicular pain along the sciatic nerve distribution from nerve compression.15–17
Physical findings vary but are distinguished by the lack of femoral pulses in the presence of pedal pulses. A pulsatile buttock mass with evidence of lower extremity nerve compression or limb ischemia or both is pathognomonic of a persistent sciatic artery aneurysm.16,18
Diagnosing persistent sciatic artery
Diagnostic angiography is the gold standard imaging test,15,19 although CTA is starting to replace it.16,18
Treating persistent sciatic artery
Persistent sciatic artery that is asymptomatic and is found incidentally does not require repair; however, it should be followed with duplex ultrasonography to look for evidence of aneurysm degeneration. Degeneration requires repair in most cases.15,16,18,19 When the persistent sciatic artery is the only blood supply to the distal extremity, open aneurysm excision and bypass is the treatment of choice.15,16,19 If collateral flow is adequate, endovascular coil embolization is an option.15 Endovascular stent graft placement has also been described.16,19
PHLEGMASIA CERULEA DOLENS
Phlegmasia cerulea dolens is a rare syndrome caused by extensive acute thrombosis of the ileofemoral vein.20–23 It is defined as total or near-total occlusion of the venous outflow of an extremity, causing massive swelling and congestion that impedes arterial inflow.20,22
Phlegmasia cerulea dolens is associated with four cardinal signs: edema, violaceous discoloration, pain, and severe venous outflow obstruction (Figure 4).22 Patients present with sudden onset of lower extremity pain, swelling, cyanosis, and arterial ischemia with or without loss of distal pulses.20,22
This syndrome can progress to gangrene and massive fluid sequestration leading to shock and death.21–23 From 25% to 40% of patients die, and of those who survive, 20% to 50% require amputation of the limb.20,23
Risk factors include malignancy, immobility, heart failure, heparin-induced thrombocytopenia, antiphospholipid syndrome, pregnancy, venous catheterization (eg, to insert an inferior vena cava filter), and surgery.20–22
Diagnosing phlegmasia cerulea dolens
The diagnosis is made on clinical suspicion with evidence of iliofemoral deep vein thrombosis. Most experts suggest venous duplex ultrasonography to identify the deep vein thrombosis,23 although CT or MR venography can be used to better delineate the proximal extent of the thrombus.20,23
Treating phlegmasia cerulea dolens
Initial management is aggressive fluid resuscitation, elevation of the affected limb, strict bed rest, and anticoagulation with intravenous heparin.20,23 Interventions are aimed at urgently restoring venous outflow to prevent progression to venous gangrene and limb loss.
Although conservative therapy can succeed by itself,23 if the condition does not improve or has already progressed to an advanced stage, the two mainstays of treatment are open venous thrombectomy and endovascular treatment.21–23 Endovascular treatment includes catheter-directed thrombolytic therapy (with or without percutaneous mechanical or pharmacomechanical thrombectomy) and stenting.20,23 The success rate for endovascular therapy can be as high as 90% with near-complete resolution of thrombosis.20 A disadvantage is that, compared with open surgical thrombectomy, more time is needed to achieve venous outflow.20,22
If endovascular therapy is ineffective, if lytic therapy is contraindicated, or if the disease has progressed to gangrene, open surgical thrombectomy with possible fasciotomy is the preferred option.20,21,23 Open surgery has the advantage of restoring venous outflow faster, but disadvantages include the inability to open the smaller veins of the extremity, blood loss, and risks associated with general anesthesia.20–22
BUERGER DISEASE
Buerger disease (thromboangiitis obliterans) is a nonatherosclerotic segmental inflammatory disease involving the small and medium-sized vessels of the arms and legs.24–27 It is differentiated from other vasculitides by its marked male predominance, its close association with smoking, the rarity of systemic signs and symptoms, and the absence of elevated inflammatory markers.26
The rate of major amputation is reported to be 11% at 5 years and 23% at 20 years.24
The classic patient is a young male smoker with symptoms of arterial disease before age 45.24,26 Patients can present with migratory thrombophlebitis or signs of arterial insufficiency in the upper or lower extremities. Two or more limbs are commonly involved. Arterial insufficiency can range from claudication and exertional discomfort of the extremity to ischemic pain at rest leading to ulceration of the distal fingers and toes. Physical findings are similar to those seen in peripheral vascular disease and arterial insufficiency, with decreased arterial brachial index, cool extremities, and wounds.
Diagnosing Buerger disease
- The Shionoya diagnostic criteria for Buerger disease are the following five clinical features24,27:
- History of smoking
- Onset before age 50
- Infrapopliteal arterial occlusive disease
- Upper-limb involvement or phlebitis migrans
- Absence of atherosclerotic risk factors other than heavy smoking.
Various other major and minor criteria have been described to make the diagnosis as well.24
There is no specific laboratory test to confirm the diagnosis of Buerger disease. A full panel of laboratory tests should be sent to rule out other causes of arterial insufficiency and vasculitides; these tests should include C-reactive protein, rheumatoid factor, erythrocyte sedimentation rate, antinuclear antibodies, antiphospholipid antibodies, anti-Scl-70 antibodies, anticentromere antibodies, complement level measurement, and hypercoagulability workup.
Imaging studies include arterial duplex ultrasonography with ankle-brachial indices and segmental pressures and CTA or MRA.26 Angiography can show a “corkscrew” pattern of occlusive disease and collateral formation, which is highly associated with Buerger disease.24
Treating Buerger disease
The only treatment shown to reduce the risk of amputation is complete abstention from tobacco and nicotine (smoking, secondhand smoke, and nicotine patches and gum).24,26
Symptoms of claudication can be managed with aspirin, clopidogrel, vasodilators, pentoxifylline, and cilostazol.26
Surgical bypass is rarely an option, as Buerger disease typically affects the distal blood vessels, thus precluding bypass, and the 5-year patency rate is only 49%.26 Other treatments including arterial thrombolysis, sympathectomy, stem cell injection, spinal cord stimulators, omental grafting, and immunomodulation have been described, but there are only limited data to offer guidance in choosing the appropriate one.24
TAKAYASU ARTERITIS
Takayasu arteritis is a form of vasculitis involving the aorta and its main branches (Figure 5).28 Although seen around the world, it has a higher incidence in young Asian women. Patients can present with systemic symptoms such as fever, fatigue, vague pain, and cardinal signs of limb ischemia associated with Takayasu arteritis, such as weak or absent pulses, differences between the arms in pulses and blood pressures, unobtainable blood pressure measurement in one or both arms, limb fatigability, and pain.28
Diagnosing Takayasu arteritis
Multiple diagnostic criteria have been proposed to define Takayasu arteritis.28 CTA, MRA, and positron emission tomography have replaced invasive angiography as the diagnostic imaging tests of choice.29
Treating Takayasu arteritis
Takayasu arteritis has an acute and chronic course. Interventions are typically reserved for severe cases, with indications that include uncontrollable hypertension from renal artery stenosis, severe coronary or cerebrovascular disease, severe aortic regurgitation or coarctation, stenotic or occlusive lesions resulting in critical limb ischemia, and aneurysm at risk of rupture.28–30
THORACIC OUTLET SYNDROME
Thoracic outlet syndrome is compression of the brachial plexus, subclavian vein, or subclavian artery as it exits the thoracic outlet through an area known as the scalene triangle, which is bordered by the anterior scalene, first rib, and clavicle.31 Presenting symptoms depend on the structure compressed.
By far the most common presentation32 is neurogenic thoracic outlet syndrome, accounting for more than 90% of cases, followed by venous thoracic outlet syndrome. Arterial thoracic outlet syndrome is the least frequent at less than 1%, but carries the greatest morbidity with potential for limb loss.31–33
The subclavian artery exits the thoracic outlet between the anterior and middle scalene muscles, and then travels over the first rib and underneath the clavicle.31 Repeated trauma from compression of the artery results in intimal injury leading to compression, stenosis, occlusion, or aneurysm formation.31,32
Symptoms of arterial thoracic outlet syndrome can start out as effort fatigue of the upper extremity secondary to compression. These symptoms are usually vague and difficult to define,31 as these patients typically are young and do not have atherosclerotic risk factors that would prompt suspicion of a vascular cause.
The most common presentation of arterial thoracic outlet syndrome is upper extremity embolization from a partially thrombosed aneurysm or area of stenosis with ischemia.32 Symptoms can range from ischemia of the fingers due to microembolization to acute limb ischemia due to complete thrombosis of the subclavian artery.31,32 Arterial thoracic outlet syndrome is most commonly associated with a bony abnormality (ie, cervical rib or anomalous first rib),31–33 and on physical examination the bony abnormality may be palpated in the supraclavicular fossa.31
Other physical findings include a bruit over the subclavian artery, a blood pressure difference of 20 mm Hg or more between the affected and unaffected arms, loss of brachial, radial, or ulnar pulses with arm abduction, and loss of the radial pulse with the head rotated to the affected side as the patient takes a deep breath (the Adson maneuver).31 While postural changes in the pulse examination hint at arterial thoracic outlet syndrome, extremity pulses may be reduced or even absent in up to 60% of normal patients.32
Diagnosing thoracic outlet syndrome
The workup should start with noninvasive imaging with pulse volume recording and wrist and finger systolic pressures, followed by arterial duplex ultrasonography.
Chest radiography may be able to identify bony abnormalities, and MRA or CTA with the patient in two positions—ie, arms down at the sides, and arms held above the head—can help identify arterial compression from bony or muscular structures in the thoracic outlet. Upper extremity angiography provides high-resolution imaging of the digital arteries and can help identify a subclavian artery aneurysm, which may be a subtle finding.31
It is important to have objective evidence of arterial or venous mechanical obstruction before deciding to remove the first rib.
Treating thoracic outlet syndrome
Treatment is determined by the severity and acuity of symptoms. If the patient presents with acute limb ischemia, prompt treatment with either open surgery or endovascular treatment is required.31,32,34 Once the acute phase has resolved or if the patient presents with chronic disease, open surgical repair is needed to remove the compression of the artery. If an arterial abnormality is identified (aneurysm or significant stenosis), an arterial reconstruction with bypass may be required.31
The standard treatment for thoracic outlet syndrome is resection of the first rib (and removal of the cervical rib if present).31,34 This can be by a transaxillary approach unless arterial reconstruction is needed, in which case a supraclavicular approach is used.31,34 When a patient without symptoms is found to have evidence of arterial compression, most experts would recommend resection of the first rib if there is evidence of an arterial abnormality, or follow-up with duplex imaging for patients with only subtle findings.31
EXTERNAL ILIAC ENDOFIBROSIS
External iliac endofibrosis is a rare cause of intermittent claudication, typically in high-performance athletes, resulting from thickening of the intima in the external iliac artery causing luminal narrowing and resultant ischemia.35–37 The estimated incidence is as high as 20% in elite competitive cyclists, and the condition has been described in other sports as well.37
External iliac endofibrosis typically presents as unilateral leg pain or cramping at near-maximal exercise with an associated feeling of swelling and numbness on the affected side.35,37 It is bilateral in up to 15% of cases.35 While claudication of the thigh is the predominant presenting symptom, dissection and thrombosis of the external iliac artery have been described, presenting with acute limb ischemia in up to 4% of patients.35,36
The condition has been attributed to factors such as physical position, psoas hypertrophy, tethering of the external iliac artery to the psoas muscle, kinking and tortuosity of the vessel, and high-flow states secondary to increased cardiac output and adaptive systolic hypertension.36,37
Diagnosing external iliac endofibrosis
The diagnosis is difficult, as symptoms typically manifest only during maximal exercise. Delays of 12 to 41 months between the onset of symptoms and diagnosis have been reported.37 Physical findings are nonspecific, and pulses and ankle-brachial indices are typically normal at rest. A careful history with a focus on location and duration of symptoms and a high index of suspicion have been shown to increase the sensitivity of diagnosis.36
Noninvasive vascular imaging with arterial duplex ultrasonography with physiologic studies (the ankle-brachial index) at rest and at maximal exertion should be obtained first.35,37 If findings on ultrasonography are positive, CTA or MRA can be used to identify a suspected stenosis.
Diagnostic angiography is still the gold standard for imaging, as real-time images of the artery with different leg positions can be obtained and pressure gradients can be measured with or without the use of a vasodilator to determine the hemodynamic significance of a lesion.35–37
Treating external iliac endofibrosis
Treatment should initially be conservative. Recreational athletes should consider changing to a sport that does not require hip flexion, and cyclists should be advised to reduce the amount of time spent cycling and to raise the handlebars or bring the saddle position forward to minimize hip flexion.37
Definitive treatment is open surgical repair. Surgical options include arterial release of the tethered artery, endofibrosectomy and vessel shortening, endofibrosectomy and patch angioplasty, and interposition bypass grafting.35–37
- Sinha S, Houghton J, Holt PJ, Thompson MM, Loftus IM, Hinchliffe RJ. Popliteal entrapment syndrome. J Vasc Surg 2012; 55:252–262.e30.
- Gokkus K, Sagtas E, Bakalim T, Taskaya E, Aydin AT. Popliteal entrapment syndrome. A systematic review of the literature and case presentation. Muscles Ligaments Tendons J 2014; 4:141–148.
- Pillai J. A current interpretation of popliteal vascular entrapment. J Vasc Surg 2008; 48(suppl 6):61S–65S.
- Liu Y, Sun Y, He X, et al. Imaging diagnosis and surgical treatment of popliteal artery entrapment syndrome: a single-center experience. Ann Vasc Surg 2014; 28:330–337.
- Kim SY, Min SK, Ahn S, Min SI, Ha J, Kim SJ. Long-term outcomes after revascularization for advanced popliteal artery entrapment syndrome with segmental arterial occlusion. J Vasc Surg 2012; 55:90–97.
- Galland RB. Popliteal aneurysms: from John Hunter to the 21st century. Ann R Coll Surg Engl 2007; 89:466–471.
- Dawson J, Fitridge R. Update on aneurysm disease: current insights and controversies: peripheral aneurysms: when to intervene—is rupture really a danger? Prog Cardiovasc Dis 2013; 56:26–35.
- Stone PA, Jagannath P, Thompson SN, et al. Evolving treatment of popliteal artery aneurysms. J Vasc Surg 2013; 57:1306–1310.
- Eslami MH, Rybin D, Doros G, Farber A. Open repair of asymptomatic popliteal artery aneurysm is associated with better outcomes than endovascular repair. J Vasc Surg 2015; 61:663–669.
- Serrano Hernando FJ, Martínez López I, Hernández Mateo MM, et al. Comparison of popliteal artery aneurysm therapies. J Vasc Surg 2015; 61:655–661.
- Marty B, Wicky S, Ris HB, et al. Success of thrombolysis as a predictor of outcome in acute thrombosis of popliteal aneurysms. J Vasc Surg 2002; 35:487–493.
- Hall HA, Minc S, Babrowski T. Peripheral artery aneurysm. Surg Clin North Am 2013; 93:911–923.
- Veraldi GF, Scudo G, Scorsone L, Mezzetto L, Castellani RL. Cystic adventitial disease of the popliteal artery: report of two cases and review of the literature. G Chir 2014; 35:229–234.
- Desy NM, Spinner RJ. The etiology and management of cystic adventitial disease. J Vasc Surg 2014; 60:235–245.e1–e11.
- Patel MV, Patel NH, Schneider JR, Kim S, Verta MJ. Persistent sciatic artery presenting with limb ischemia. J Vasc Surg 2013; 57:225–229.
- Kesri G, Mangtani J, Kumar G, Dangayach KK. Persistent sciatic artery aneurysm with lower limb ischemia. Case Rep Vasc Med 2014; 2014:183969.
- Nuño-Escobar C, Pérez-Durán MA, Ramos-López R, et al. Persistent sciatic artery aneurysm. Ann Vasc Surg 2013; 27:1182.e13–e16.
- Vaz C, Machado R, Rego D, Matos A, Almeida R. Hybrid approach in a case of persistent sciatic artery aneurysm. Ann Vasc Surg 2014; 28:1313.e5–e7.
- Abularrage CJ, Crawford RS, Patel VI, Conrad MF. Diagnostic strategies for the persistent sciatic artery. Vasc Endovascular Surg 2009; 43:485–489.
- Suwanabol PA, Tefera G, Schwarze ML. Syndromes associated with the deep veins: phlegmasia cerulea dolens, May-Thurner syndrome, and nutcracker syndrome. Perspect Vasc Surg Endovasc Ther 2010; 22:223–230.
- Vysetti S, Shinde S, Chaudhry S, Subramoney K. Phlegmasia cerulea dolens—a rare, life-threatening condition. ScientificWorldJournal 2009; 9:1105–1106.
- Mumoli N, Invernizzi C, Luschi R, Carmignani G, Camaiti A, Cei M. Phlegmasia cerulea dolens. Circulation 2012; 125:1056–1057.
- Chinsakchai K, Ten Duis K, Moll FL, de Borst GJ. Trends in management of phlegmasia cerulea dolens. Vasc Endovascular Surg 2011; 45:5–14.
- Dargon PT, Landry GJ. Buerger’s disease. Ann Vasc Surg 2012; 26:871–880.
- Faizer R, Forbes TL. Buerger’s disease. J Vasc Surg 2007; 46:812.
- Vijayakumar A, Tiwari R, Kumar Prabhuswamy V. Thromboangiitis obliterans (Buerger’s disease)—current practices. Int J Inflam 2013; 2013:156905.
- Ohta T, Ishibashi H, Sugimoto I, et al. The clinical course of Buerger’s disease. Ann Vasc Dis 2008; 1:85–90.
- de Souza AWS, de Carvalho JF. Diagnostic and classification criteria of Takayasu arteritis. J Autoimmun 2014; 48–49:79–83.
- Perera AH, Mason JC, Wolfe JH. Takayasu arteritis: criteria for surgical intervention should not be ignored. Int J Vasc Med 2013; 2013:618910.
- Keser G, Direskeneli H, Aksu K. Management of Takayasu arteritis: a systematic review. Rheumatology (Oxford) 2014; 53:793–801.
- Sanders RJ, Annest SJ. Thoracic outlet and pectoralis minor syndromes. Semin Vasc Surg 2014; 27:86–117.
- Criado E, Berguer R, Greenfield L. The spectrum of arterial compression at the thoracic outlet. J Vasc Surg 2010; 52:406–411.
- Povlsen B, Hansson T, Povlsen SD. Treatment for thoracic outlet syndrome. Cochrane Database Syst Rev 2014; 11:CD007218.
- Orlando MS, Likes KC, Mirza S, et al. A decade of excellent outcomes after surgical intervention in 538 patients with thoracic outlet syndrome. J Am Coll Surg 2015; 220:934–939.
- Bucci F, Ottaviani N, Plagnol P. Acute thrombosis of external iliac artery secondary to endofibrosis. Ann Vasc Surg 2011; 25:698.e5–e7.
- Willson TD, Revesz E, Podbielski FJ, Blecha MJ. External iliac artery dissection secondary to endofibrosis in a cyclist. J Vasc Surg 2010; 52:219–221.
- Peach G, Schep G, Palfreeman R, Beard JD, Thompson MM, Hinchliffe RJ. Endofibrosis and kinking of the Iliac arteries in athletes: a systematic review. Eur J Vasc Endovasc Surg 2012; 43:208–217.
- Sinha S, Houghton J, Holt PJ, Thompson MM, Loftus IM, Hinchliffe RJ. Popliteal entrapment syndrome. J Vasc Surg 2012; 55:252–262.e30.
- Gokkus K, Sagtas E, Bakalim T, Taskaya E, Aydin AT. Popliteal entrapment syndrome. A systematic review of the literature and case presentation. Muscles Ligaments Tendons J 2014; 4:141–148.
- Pillai J. A current interpretation of popliteal vascular entrapment. J Vasc Surg 2008; 48(suppl 6):61S–65S.
- Liu Y, Sun Y, He X, et al. Imaging diagnosis and surgical treatment of popliteal artery entrapment syndrome: a single-center experience. Ann Vasc Surg 2014; 28:330–337.
- Kim SY, Min SK, Ahn S, Min SI, Ha J, Kim SJ. Long-term outcomes after revascularization for advanced popliteal artery entrapment syndrome with segmental arterial occlusion. J Vasc Surg 2012; 55:90–97.
- Galland RB. Popliteal aneurysms: from John Hunter to the 21st century. Ann R Coll Surg Engl 2007; 89:466–471.
- Dawson J, Fitridge R. Update on aneurysm disease: current insights and controversies: peripheral aneurysms: when to intervene—is rupture really a danger? Prog Cardiovasc Dis 2013; 56:26–35.
- Stone PA, Jagannath P, Thompson SN, et al. Evolving treatment of popliteal artery aneurysms. J Vasc Surg 2013; 57:1306–1310.
- Eslami MH, Rybin D, Doros G, Farber A. Open repair of asymptomatic popliteal artery aneurysm is associated with better outcomes than endovascular repair. J Vasc Surg 2015; 61:663–669.
- Serrano Hernando FJ, Martínez López I, Hernández Mateo MM, et al. Comparison of popliteal artery aneurysm therapies. J Vasc Surg 2015; 61:655–661.
- Marty B, Wicky S, Ris HB, et al. Success of thrombolysis as a predictor of outcome in acute thrombosis of popliteal aneurysms. J Vasc Surg 2002; 35:487–493.
- Hall HA, Minc S, Babrowski T. Peripheral artery aneurysm. Surg Clin North Am 2013; 93:911–923.
- Veraldi GF, Scudo G, Scorsone L, Mezzetto L, Castellani RL. Cystic adventitial disease of the popliteal artery: report of two cases and review of the literature. G Chir 2014; 35:229–234.
- Desy NM, Spinner RJ. The etiology and management of cystic adventitial disease. J Vasc Surg 2014; 60:235–245.e1–e11.
- Patel MV, Patel NH, Schneider JR, Kim S, Verta MJ. Persistent sciatic artery presenting with limb ischemia. J Vasc Surg 2013; 57:225–229.
- Kesri G, Mangtani J, Kumar G, Dangayach KK. Persistent sciatic artery aneurysm with lower limb ischemia. Case Rep Vasc Med 2014; 2014:183969.
- Nuño-Escobar C, Pérez-Durán MA, Ramos-López R, et al. Persistent sciatic artery aneurysm. Ann Vasc Surg 2013; 27:1182.e13–e16.
- Vaz C, Machado R, Rego D, Matos A, Almeida R. Hybrid approach in a case of persistent sciatic artery aneurysm. Ann Vasc Surg 2014; 28:1313.e5–e7.
- Abularrage CJ, Crawford RS, Patel VI, Conrad MF. Diagnostic strategies for the persistent sciatic artery. Vasc Endovascular Surg 2009; 43:485–489.
- Suwanabol PA, Tefera G, Schwarze ML. Syndromes associated with the deep veins: phlegmasia cerulea dolens, May-Thurner syndrome, and nutcracker syndrome. Perspect Vasc Surg Endovasc Ther 2010; 22:223–230.
- Vysetti S, Shinde S, Chaudhry S, Subramoney K. Phlegmasia cerulea dolens—a rare, life-threatening condition. ScientificWorldJournal 2009; 9:1105–1106.
- Mumoli N, Invernizzi C, Luschi R, Carmignani G, Camaiti A, Cei M. Phlegmasia cerulea dolens. Circulation 2012; 125:1056–1057.
- Chinsakchai K, Ten Duis K, Moll FL, de Borst GJ. Trends in management of phlegmasia cerulea dolens. Vasc Endovascular Surg 2011; 45:5–14.
- Dargon PT, Landry GJ. Buerger’s disease. Ann Vasc Surg 2012; 26:871–880.
- Faizer R, Forbes TL. Buerger’s disease. J Vasc Surg 2007; 46:812.
- Vijayakumar A, Tiwari R, Kumar Prabhuswamy V. Thromboangiitis obliterans (Buerger’s disease)—current practices. Int J Inflam 2013; 2013:156905.
- Ohta T, Ishibashi H, Sugimoto I, et al. The clinical course of Buerger’s disease. Ann Vasc Dis 2008; 1:85–90.
- de Souza AWS, de Carvalho JF. Diagnostic and classification criteria of Takayasu arteritis. J Autoimmun 2014; 48–49:79–83.
- Perera AH, Mason JC, Wolfe JH. Takayasu arteritis: criteria for surgical intervention should not be ignored. Int J Vasc Med 2013; 2013:618910.
- Keser G, Direskeneli H, Aksu K. Management of Takayasu arteritis: a systematic review. Rheumatology (Oxford) 2014; 53:793–801.
- Sanders RJ, Annest SJ. Thoracic outlet and pectoralis minor syndromes. Semin Vasc Surg 2014; 27:86–117.
- Criado E, Berguer R, Greenfield L. The spectrum of arterial compression at the thoracic outlet. J Vasc Surg 2010; 52:406–411.
- Povlsen B, Hansson T, Povlsen SD. Treatment for thoracic outlet syndrome. Cochrane Database Syst Rev 2014; 11:CD007218.
- Orlando MS, Likes KC, Mirza S, et al. A decade of excellent outcomes after surgical intervention in 538 patients with thoracic outlet syndrome. J Am Coll Surg 2015; 220:934–939.
- Bucci F, Ottaviani N, Plagnol P. Acute thrombosis of external iliac artery secondary to endofibrosis. Ann Vasc Surg 2011; 25:698.e5–e7.
- Willson TD, Revesz E, Podbielski FJ, Blecha MJ. External iliac artery dissection secondary to endofibrosis in a cyclist. J Vasc Surg 2010; 52:219–221.
- Peach G, Schep G, Palfreeman R, Beard JD, Thompson MM, Hinchliffe RJ. Endofibrosis and kinking of the Iliac arteries in athletes: a systematic review. Eur J Vasc Endovasc Surg 2012; 43:208–217.
KEY POINTS
- A high index of suspicion should be maintained to recognize symptoms consistent with limb ischemia in a younger patient in the absence of the usual atherosclerosis risk factors.
- A workup for most conditions includes noninvasive vascular ultrasonography to detect and quantify limb ischemia.
- Prompt referral for surgical or endovascular treatment is necessary for optimal limb salvage.
Smoking thickens LV wall, worsens function
Current smoking, as well as higher levels of cumulative cigarette exposure from past smoking, were both associated with higher left ventricular mass, a higher LV mass-to-volume ratio, and worse diastolic function in an elderly community-based population with no overt indications of coronary artery disease or heart failure, according to a report published online Sept. 13 in Circulation: Cardiovascular Imaging.
“These findings suggest that smoking is associated with subtle alterations in LV structure and function, which might help explain the higher risk of heart failure [HF] reported for smokers, independent of coronary artery disease [CAD],” said Wilson Nadruz Jr., MD, of the cardiovascular division, Brigham and Women’s Hospital, Boston, and his associates.
They analyzed links between smoking and echocardiographic features using data from the Atherosclerosis Risk in Communities (ARIC) study, an ongoing prospective observational study involving community-dwelling adults who were aged 45-64 years at baseline in 1987-1989. For their study, Dr. Nadruz and his colleagues assessed echocardiographic images taken for 4,580 ARIC participants at follow-up roughly 25 years later. None of these adults had any indication of CAD or HF; 287 (6.3%) were current smokers, 2,316 (50.5%) were former smokers, and 1,977 (43.2%) never smoked.
Compared with never smokers, current smokers showed a greater LV mass index (80.4 vs. 76.7), a greater LV mass-to-volume ratio (1.93 vs. 1.83), and a higher prevalence of LV hypertrophy (15% vs. 9%), as well as a higher prevalence of concentric LV hypertrophy and worse LV diastolic function. The same association was found between never smokers and former smokers who had higher levels of cumulative cigarette exposure, the investigators said (Circ Cardiovasc Imag. 2016 Sep 13. doi: 10.1161/circimaging.116.004950).
This association between smoking and altered LV structure and function remained robust after the data were adjusted to account for numerous cardiac risk factors such as older age, higher BMI, diabetes, hypertension, greater alcohol consumption, and higher heart rate. It also didn’t vary by patient sex, race, or income level. In contrast, there was no association between smoking and right ventricular structure or function.
“These data suggest that smoking can independently lead to thickening of the heart and worsening of heart function, which may lead to a higher risk for heart failure, even in people who don’t have heart attacks,” Dr. Nadruz said in a statement.
Looking at the results in a more positive light, senior author Scott D. Solomon, MD, professor of medicine at Harvard University, Boston, said “The good news is that former smokers had similar heart structure and function, compared with never smokers,” suggesting that “the potential effects of tobacco on the myocardium might be reversible after smoking cessation.”
Current smoking, as well as higher levels of cumulative cigarette exposure from past smoking, were both associated with higher left ventricular mass, a higher LV mass-to-volume ratio, and worse diastolic function in an elderly community-based population with no overt indications of coronary artery disease or heart failure, according to a report published online Sept. 13 in Circulation: Cardiovascular Imaging.
“These findings suggest that smoking is associated with subtle alterations in LV structure and function, which might help explain the higher risk of heart failure [HF] reported for smokers, independent of coronary artery disease [CAD],” said Wilson Nadruz Jr., MD, of the cardiovascular division, Brigham and Women’s Hospital, Boston, and his associates.
They analyzed links between smoking and echocardiographic features using data from the Atherosclerosis Risk in Communities (ARIC) study, an ongoing prospective observational study involving community-dwelling adults who were aged 45-64 years at baseline in 1987-1989. For their study, Dr. Nadruz and his colleagues assessed echocardiographic images taken for 4,580 ARIC participants at follow-up roughly 25 years later. None of these adults had any indication of CAD or HF; 287 (6.3%) were current smokers, 2,316 (50.5%) were former smokers, and 1,977 (43.2%) never smoked.
Compared with never smokers, current smokers showed a greater LV mass index (80.4 vs. 76.7), a greater LV mass-to-volume ratio (1.93 vs. 1.83), and a higher prevalence of LV hypertrophy (15% vs. 9%), as well as a higher prevalence of concentric LV hypertrophy and worse LV diastolic function. The same association was found between never smokers and former smokers who had higher levels of cumulative cigarette exposure, the investigators said (Circ Cardiovasc Imag. 2016 Sep 13. doi: 10.1161/circimaging.116.004950).
This association between smoking and altered LV structure and function remained robust after the data were adjusted to account for numerous cardiac risk factors such as older age, higher BMI, diabetes, hypertension, greater alcohol consumption, and higher heart rate. It also didn’t vary by patient sex, race, or income level. In contrast, there was no association between smoking and right ventricular structure or function.
“These data suggest that smoking can independently lead to thickening of the heart and worsening of heart function, which may lead to a higher risk for heart failure, even in people who don’t have heart attacks,” Dr. Nadruz said in a statement.
Looking at the results in a more positive light, senior author Scott D. Solomon, MD, professor of medicine at Harvard University, Boston, said “The good news is that former smokers had similar heart structure and function, compared with never smokers,” suggesting that “the potential effects of tobacco on the myocardium might be reversible after smoking cessation.”
Current smoking, as well as higher levels of cumulative cigarette exposure from past smoking, were both associated with higher left ventricular mass, a higher LV mass-to-volume ratio, and worse diastolic function in an elderly community-based population with no overt indications of coronary artery disease or heart failure, according to a report published online Sept. 13 in Circulation: Cardiovascular Imaging.
“These findings suggest that smoking is associated with subtle alterations in LV structure and function, which might help explain the higher risk of heart failure [HF] reported for smokers, independent of coronary artery disease [CAD],” said Wilson Nadruz Jr., MD, of the cardiovascular division, Brigham and Women’s Hospital, Boston, and his associates.
They analyzed links between smoking and echocardiographic features using data from the Atherosclerosis Risk in Communities (ARIC) study, an ongoing prospective observational study involving community-dwelling adults who were aged 45-64 years at baseline in 1987-1989. For their study, Dr. Nadruz and his colleagues assessed echocardiographic images taken for 4,580 ARIC participants at follow-up roughly 25 years later. None of these adults had any indication of CAD or HF; 287 (6.3%) were current smokers, 2,316 (50.5%) were former smokers, and 1,977 (43.2%) never smoked.
Compared with never smokers, current smokers showed a greater LV mass index (80.4 vs. 76.7), a greater LV mass-to-volume ratio (1.93 vs. 1.83), and a higher prevalence of LV hypertrophy (15% vs. 9%), as well as a higher prevalence of concentric LV hypertrophy and worse LV diastolic function. The same association was found between never smokers and former smokers who had higher levels of cumulative cigarette exposure, the investigators said (Circ Cardiovasc Imag. 2016 Sep 13. doi: 10.1161/circimaging.116.004950).
This association between smoking and altered LV structure and function remained robust after the data were adjusted to account for numerous cardiac risk factors such as older age, higher BMI, diabetes, hypertension, greater alcohol consumption, and higher heart rate. It also didn’t vary by patient sex, race, or income level. In contrast, there was no association between smoking and right ventricular structure or function.
“These data suggest that smoking can independently lead to thickening of the heart and worsening of heart function, which may lead to a higher risk for heart failure, even in people who don’t have heart attacks,” Dr. Nadruz said in a statement.
Looking at the results in a more positive light, senior author Scott D. Solomon, MD, professor of medicine at Harvard University, Boston, said “The good news is that former smokers had similar heart structure and function, compared with never smokers,” suggesting that “the potential effects of tobacco on the myocardium might be reversible after smoking cessation.”
FROM CIRCULATION: CARDIOVASCULAR IMAGING
Key clinical point: Current smoking was associated with higher left ventricular mass, a higher LV mass-to-volume ratio, and worse diastolic function.
Major finding: Compared with never smokers, current smokers showed a greater LV mass index (80.4 vs. 76.7), a greater LV mass-to-volume ratio (1.93 vs. 1.83), and a higher prevalence of LV hypertrophy (15% vs. 9%).
Data source: A secondary analysis of data for 4,580 elderly participants in ARIC, a large community-based cohort.
Disclosures: This study was supported by Brigham and Women’s Hospital, Boston. Dr. Nadruz and his associates reported having no relevant financial disclosures.
Emergency Ultrasound: Tips and Tricks for Imaging Digits
Clinicians familiar with point-of-care (POC) ultrasound know that structures such as the hands and feet require the use of the linear high-frequency transducer to obtain quality images. In reality, however, employing the standard technique (ie, applying gel to the probe surface and scanning the structure) can be challenging due to the uneven surfaces of the fingers and toes; therefore, obtaining good contact with the transducer is harder than it may seem at first glance. Additionally, since these structures are superficial, they are usually seen on the top half of the ultrasound display, while the focal zone of most ultrasound machines is located in the middle of the display and is nonadjustable.
We describe two simple adjuncts to POC ultrasound that can assist in visualizing digital structures with greater ease and improved image resolution: the water bath1,2 and standoff pad techniques.
Water Bath Technique
In the water bath technique, one fills a small basin with lukewarm water to a depth point where the extremity being studied (ie, hand or foot) is mostly—but not completely—submerged in the water bath. After the extremity is submerged, the high-frequency probe is then placed into the water bath (Figure 1). When employing this technique, the transducer does not need to make contact with the patient’s skin. Since the water acts as an excellent conduction medium for sound waves, no ultrasound gel is required. For a video demonstrating the use of the water bath technique to evaluate the distal tip of the finger, see below.
Standoff Pad
Another technique that enhances POC imaging of the digits involves a standoff pad. A variety of commercially available standoff pads can be used for this technique. Alternatively, the clinician can easily create a standoff pad using supplies that are readily available in the ED. One such method is to fill a latex glove with water, tie off the filled glove, and place it on top of the extremity to be imaged (Figure 2). The water in the glove will facilitate sound-wave transmission.
Pathology
The water bath and standoff pad techniques can facilitate visualization of several pathologies, including felons (Figure 3), flexor tenosynovitis, phalangeal and metacarpal/metatarsal fractures, and interphalangeal, metacarpophalangeal, and metatarsophalangeal joint effusions (Figure 4). In addition, these techniques also assist in visualizing digit tendons to evaluate for tears in these structures (Figure 5).
Summary
Point-of-care ultrasound imaging to evaluate superficial body parts such as hands or feet can be challenging due to the irregular shape and uneven surface of these structures. The employment of adjuncts such as the water bath or standoff pad techniques can mitigate these challenges, facilitating the acquisition of high-resolution images and providing easier identification of pathology.
1. Krishnamurthy R, Yoo JH, Thapa M, Callahan MJ. Water-bath method for sonographic evaluation of superficial structures of the extremities in children. Pediatr Radiol. 2013;4(Suppl 1):S41-S47. DOI:10.1007/s00247-012-2592-y.
2. Jeong HY, Krishnamurthy R. 1012: Water-bath method for sonographic evaluation of superficial structures of the extremities. Ultrasound Med Biol. 2009;35(8):S101-S102. doi:10.1016/j.ultrasmedbio.2009.06.394.
Clinicians familiar with point-of-care (POC) ultrasound know that structures such as the hands and feet require the use of the linear high-frequency transducer to obtain quality images. In reality, however, employing the standard technique (ie, applying gel to the probe surface and scanning the structure) can be challenging due to the uneven surfaces of the fingers and toes; therefore, obtaining good contact with the transducer is harder than it may seem at first glance. Additionally, since these structures are superficial, they are usually seen on the top half of the ultrasound display, while the focal zone of most ultrasound machines is located in the middle of the display and is nonadjustable.
We describe two simple adjuncts to POC ultrasound that can assist in visualizing digital structures with greater ease and improved image resolution: the water bath1,2 and standoff pad techniques.
Water Bath Technique
In the water bath technique, one fills a small basin with lukewarm water to a depth point where the extremity being studied (ie, hand or foot) is mostly—but not completely—submerged in the water bath. After the extremity is submerged, the high-frequency probe is then placed into the water bath (Figure 1). When employing this technique, the transducer does not need to make contact with the patient’s skin. Since the water acts as an excellent conduction medium for sound waves, no ultrasound gel is required. For a video demonstrating the use of the water bath technique to evaluate the distal tip of the finger, see below.
Standoff Pad
Another technique that enhances POC imaging of the digits involves a standoff pad. A variety of commercially available standoff pads can be used for this technique. Alternatively, the clinician can easily create a standoff pad using supplies that are readily available in the ED. One such method is to fill a latex glove with water, tie off the filled glove, and place it on top of the extremity to be imaged (Figure 2). The water in the glove will facilitate sound-wave transmission.
Pathology
The water bath and standoff pad techniques can facilitate visualization of several pathologies, including felons (Figure 3), flexor tenosynovitis, phalangeal and metacarpal/metatarsal fractures, and interphalangeal, metacarpophalangeal, and metatarsophalangeal joint effusions (Figure 4). In addition, these techniques also assist in visualizing digit tendons to evaluate for tears in these structures (Figure 5).
Summary
Point-of-care ultrasound imaging to evaluate superficial body parts such as hands or feet can be challenging due to the irregular shape and uneven surface of these structures. The employment of adjuncts such as the water bath or standoff pad techniques can mitigate these challenges, facilitating the acquisition of high-resolution images and providing easier identification of pathology.
Clinicians familiar with point-of-care (POC) ultrasound know that structures such as the hands and feet require the use of the linear high-frequency transducer to obtain quality images. In reality, however, employing the standard technique (ie, applying gel to the probe surface and scanning the structure) can be challenging due to the uneven surfaces of the fingers and toes; therefore, obtaining good contact with the transducer is harder than it may seem at first glance. Additionally, since these structures are superficial, they are usually seen on the top half of the ultrasound display, while the focal zone of most ultrasound machines is located in the middle of the display and is nonadjustable.
We describe two simple adjuncts to POC ultrasound that can assist in visualizing digital structures with greater ease and improved image resolution: the water bath1,2 and standoff pad techniques.
Water Bath Technique
In the water bath technique, one fills a small basin with lukewarm water to a depth point where the extremity being studied (ie, hand or foot) is mostly—but not completely—submerged in the water bath. After the extremity is submerged, the high-frequency probe is then placed into the water bath (Figure 1). When employing this technique, the transducer does not need to make contact with the patient’s skin. Since the water acts as an excellent conduction medium for sound waves, no ultrasound gel is required. For a video demonstrating the use of the water bath technique to evaluate the distal tip of the finger, see below.
Standoff Pad
Another technique that enhances POC imaging of the digits involves a standoff pad. A variety of commercially available standoff pads can be used for this technique. Alternatively, the clinician can easily create a standoff pad using supplies that are readily available in the ED. One such method is to fill a latex glove with water, tie off the filled glove, and place it on top of the extremity to be imaged (Figure 2). The water in the glove will facilitate sound-wave transmission.
Pathology
The water bath and standoff pad techniques can facilitate visualization of several pathologies, including felons (Figure 3), flexor tenosynovitis, phalangeal and metacarpal/metatarsal fractures, and interphalangeal, metacarpophalangeal, and metatarsophalangeal joint effusions (Figure 4). In addition, these techniques also assist in visualizing digit tendons to evaluate for tears in these structures (Figure 5).
Summary
Point-of-care ultrasound imaging to evaluate superficial body parts such as hands or feet can be challenging due to the irregular shape and uneven surface of these structures. The employment of adjuncts such as the water bath or standoff pad techniques can mitigate these challenges, facilitating the acquisition of high-resolution images and providing easier identification of pathology.
1. Krishnamurthy R, Yoo JH, Thapa M, Callahan MJ. Water-bath method for sonographic evaluation of superficial structures of the extremities in children. Pediatr Radiol. 2013;4(Suppl 1):S41-S47. DOI:10.1007/s00247-012-2592-y.
2. Jeong HY, Krishnamurthy R. 1012: Water-bath method for sonographic evaluation of superficial structures of the extremities. Ultrasound Med Biol. 2009;35(8):S101-S102. doi:10.1016/j.ultrasmedbio.2009.06.394.
1. Krishnamurthy R, Yoo JH, Thapa M, Callahan MJ. Water-bath method for sonographic evaluation of superficial structures of the extremities in children. Pediatr Radiol. 2013;4(Suppl 1):S41-S47. DOI:10.1007/s00247-012-2592-y.
2. Jeong HY, Krishnamurthy R. 1012: Water-bath method for sonographic evaluation of superficial structures of the extremities. Ultrasound Med Biol. 2009;35(8):S101-S102. doi:10.1016/j.ultrasmedbio.2009.06.394.
‘Air-raising’: An air-fluid level in the right subphrenic region
A 39-year-old Filipino man presented with nausea, vomiting, and abdominal pain of 2 weeks’ duration. He did not report trauma, and he had no history of medical illness or surgery.
On arrival, his blood pressure was 123/83 mm Hg, pulse 122 beats per minute, respiratory rate 18 breaths per minute, and temperature 100.7°F (38.1°C). On physical examination, he exhibited marked tenderness of the right upper quadrant on palpation. The abdomen was otherwise soft with no guarding or rebound tenderness.
Results of initial laboratory testing were as follows:
- Leukocyte count 17.0 × 109/L (reference range 4.5–11.0)
- Serum glucose 558 mg/dL without ketoacidosis
- Aspartate aminotransferase 109 U/L (2–40)
- Alanine aminotranferase 28 U/L (2–50)
- Total serum bilirubin 4.0 mg/dL (0.0–1.5).
Plain chest radiography showed dramatic elevation of the right hemidiaphragm with a large subphrenic air-fluid level (Figure 1). Abdominal computed tomography (CT) demonstrated a multiloculated hepatic abscess 18 × 13.5 cm subjacent to the diaphragm (Figure 2). Cultures of blood and the abscess yielded Klebsiella pneumoniae. The patient recovered after percutaneous drainage and a course of ceftriaxone.
PRIMARY KLEBSIELLA LIVER ABSCESS
K pneumoniae, a gram-negative aerobic encapsulated bacillus of the normal human intestinal flora, is closely related to Escherichia coli, historically the most frequent bacterial cause of pyogenic liver abscess.1 Over the last 30 years, K pneumoniae has eclipsed E coli as the most common causative agent, with the epicenter of this trend being located in Taiwan and South Korea, perhaps because rates of fecal Klebsiella carriage in that region are particularly high.1,2
Concurrently, there has been increasing recognition—initially across Asia, but lately in Europe and the Western Hemisphere—of the so-called invasive Klebsiella liver abscess (KLA) syndrome, virtually unique to the hypervirulent K1 and K2 capsular serotypes of K pneumoniae prevalent in Asia.3–6 This community-acquired syndrome is characterized by hematogenous deposition of the organism at distant sites, such as the lung, soft tissues, central nervous system, and eyes. Impairment of phagocytic function, as occurs in diabetes mellitus, and the resistance to phagocytosis conferred by the K1 and K2 serotypes have been identified as predisposing factors for dissemination.7,8 The mucoid phenotype of K pneumoniae, very common in Asian isolates of the K1 and K2 serotypes, is also associated with hypervirulence and extrahepatic spread, presumably through evasion of phagocytosis and complement-mediated opsonization.2,9
Our patient’s risk factors for KLA were his Asian origin and uncontrolled diabetes. No evidence of remote infection was detected during his hospitalization.
HEMIDIAPHRAGM ELEVATION
Acquired hemidiaphragm elevation is most commonly unilateral and typically represents an incidental radiologic finding attributable to paralysis of the corresponding diaphragm after phrenic nerve injury caused by trauma, surgery, or infection. Unilateral diaphragmatic paralysis is classically confirmed by performing a fluoroscopic sniff test, which is positive if the affected hemidiaphragm is observed in real time to paradoxically move upward during forced inhalation.10 This condition is usually asymptomatic at rest but could cause exertional dyspnea and contribute to ventilatory failure when pulmonary disease coexists.11
Occasionally, as in our patient, hemidiaphragm elevation is part of the presentation of active abdominal pathology that displaces the corresponding hemidiaphragm cephalad by mass effect. Examples of such space-occupying abdominal lesions include infections, malignancy, hepatosplenomegaly, and pneumoperitoneum from a ruptured viscus. Pneumoperitoneum is suggested by the presence of an air crescent immediately subjacent to the affected hemidiaphragm on an upright radiograph accompanied by peritoneal signs.
Although there was subphrenic air on this patient’s initial chest radiograph, it was actually part of an air-fluid level without associated peritoneal signs. An air-fluid level is characterized by a sharp horizontal demarcation between the lighter gas component floating at the top and the heavier fluid component settling on the bottom (Figure 1). The subsequent CT excluded free intra-abdominal air while identifying a large hepatic abscess as the cause of hemidiaphragm elevation. In trauma victims, CT is also helpful in ruling out diaphragmatic rupture, which can have a similar radiographic appearance.12
Our patient’s presentation was a reminder that an elevated hemidiaphragm may reflect abdominal pathology and that subphrenic air in this context need not be either “free” or a surgical emergency. Drainage of the abscess restored the normal position of our patient’s right hemidiaphragm (Figure 3).
- Huang CJ, Pitt HA, Lipsett PA, et al. Pyogenic hepatic abscess: changing trends over 42 years. Ann Surg 1996; 223:600–607.
- Lin YT, Siu LK, Lin JC, et al. Seroepidemiology of Klebsiella pneumoniae colonizing the intestinal tract of healthy Chinese and overseas Chinese adults in Asian countries. BMC Microbiol 2012; 12:13.
- Wang JH, Liu YC, Lee SS, et al. Primary liver abscess due to Klebsiella pneumoniae in Taiwan. Clin Infect Dis 1998; 26:1434–1438.
- Pastagia M, Arumugam V. Klebsiella pneumoniae liver abscesses in a public hospital in Queens, New York. Travel Med Infect Dis 2008; 6:228–233.
- Rahimian J, Wilson T, Oram V, Holzman RS. Pyogenic liver abscess: recent trends in etiology and mortality. Clin Infect Dis 2004; 39:1654–1659.
- Moore R, O’Shea D, Geoghegan T, Mallon PW, Sheehan G. Community-acquired Klebsiella pneumoniae liver abscess: an emerging infection in Ireland and Europe. Infection 2013; 41:681–686.
- Lecube A, Pachón G, Petriz J, Hernández C, Simó R. Phagocytic activity is impaired in type 2 diabetes mellitus and increases after metabolic improvement. PLoS One 2011; 6:e23366.
- Lin JC, Siu LK, Fung CP, et al. Impaired phagocytosis of capsular serotypes K1 or K2 Klebsiella pneumoniae in type 2 diabetes mellitus patients with poor glycemic control. J Clin Endocrinol Metab 2006; 91:3084–3087.
- Siu LK, Yeh KM, Lin JC, Fung CP, Chang FY. Klebsiella pneumoniae liver abscess: a new invasive syndrome. Lancet Infect Dis 2012; 12:881–887.
- Gierada DS, Slone RM, Fleishman MJ. Imaging evaluation of the diaphragm. Chest Surg Clin North Am 1998; 8:237–280.
- Qureshi A. Diaphragm paralysis. Semin Respir Crit Care Med 2009; 30:315–320.
- Havens JM, Kelly E, Patel V. A 78-year-old man with an elevated hemidiaphragm following trauma. Chest 2008; 134:1336–1339.
A 39-year-old Filipino man presented with nausea, vomiting, and abdominal pain of 2 weeks’ duration. He did not report trauma, and he had no history of medical illness or surgery.
On arrival, his blood pressure was 123/83 mm Hg, pulse 122 beats per minute, respiratory rate 18 breaths per minute, and temperature 100.7°F (38.1°C). On physical examination, he exhibited marked tenderness of the right upper quadrant on palpation. The abdomen was otherwise soft with no guarding or rebound tenderness.
Results of initial laboratory testing were as follows:
- Leukocyte count 17.0 × 109/L (reference range 4.5–11.0)
- Serum glucose 558 mg/dL without ketoacidosis
- Aspartate aminotransferase 109 U/L (2–40)
- Alanine aminotranferase 28 U/L (2–50)
- Total serum bilirubin 4.0 mg/dL (0.0–1.5).
Plain chest radiography showed dramatic elevation of the right hemidiaphragm with a large subphrenic air-fluid level (Figure 1). Abdominal computed tomography (CT) demonstrated a multiloculated hepatic abscess 18 × 13.5 cm subjacent to the diaphragm (Figure 2). Cultures of blood and the abscess yielded Klebsiella pneumoniae. The patient recovered after percutaneous drainage and a course of ceftriaxone.
PRIMARY KLEBSIELLA LIVER ABSCESS
K pneumoniae, a gram-negative aerobic encapsulated bacillus of the normal human intestinal flora, is closely related to Escherichia coli, historically the most frequent bacterial cause of pyogenic liver abscess.1 Over the last 30 years, K pneumoniae has eclipsed E coli as the most common causative agent, with the epicenter of this trend being located in Taiwan and South Korea, perhaps because rates of fecal Klebsiella carriage in that region are particularly high.1,2
Concurrently, there has been increasing recognition—initially across Asia, but lately in Europe and the Western Hemisphere—of the so-called invasive Klebsiella liver abscess (KLA) syndrome, virtually unique to the hypervirulent K1 and K2 capsular serotypes of K pneumoniae prevalent in Asia.3–6 This community-acquired syndrome is characterized by hematogenous deposition of the organism at distant sites, such as the lung, soft tissues, central nervous system, and eyes. Impairment of phagocytic function, as occurs in diabetes mellitus, and the resistance to phagocytosis conferred by the K1 and K2 serotypes have been identified as predisposing factors for dissemination.7,8 The mucoid phenotype of K pneumoniae, very common in Asian isolates of the K1 and K2 serotypes, is also associated with hypervirulence and extrahepatic spread, presumably through evasion of phagocytosis and complement-mediated opsonization.2,9
Our patient’s risk factors for KLA were his Asian origin and uncontrolled diabetes. No evidence of remote infection was detected during his hospitalization.
HEMIDIAPHRAGM ELEVATION
Acquired hemidiaphragm elevation is most commonly unilateral and typically represents an incidental radiologic finding attributable to paralysis of the corresponding diaphragm after phrenic nerve injury caused by trauma, surgery, or infection. Unilateral diaphragmatic paralysis is classically confirmed by performing a fluoroscopic sniff test, which is positive if the affected hemidiaphragm is observed in real time to paradoxically move upward during forced inhalation.10 This condition is usually asymptomatic at rest but could cause exertional dyspnea and contribute to ventilatory failure when pulmonary disease coexists.11
Occasionally, as in our patient, hemidiaphragm elevation is part of the presentation of active abdominal pathology that displaces the corresponding hemidiaphragm cephalad by mass effect. Examples of such space-occupying abdominal lesions include infections, malignancy, hepatosplenomegaly, and pneumoperitoneum from a ruptured viscus. Pneumoperitoneum is suggested by the presence of an air crescent immediately subjacent to the affected hemidiaphragm on an upright radiograph accompanied by peritoneal signs.
Although there was subphrenic air on this patient’s initial chest radiograph, it was actually part of an air-fluid level without associated peritoneal signs. An air-fluid level is characterized by a sharp horizontal demarcation between the lighter gas component floating at the top and the heavier fluid component settling on the bottom (Figure 1). The subsequent CT excluded free intra-abdominal air while identifying a large hepatic abscess as the cause of hemidiaphragm elevation. In trauma victims, CT is also helpful in ruling out diaphragmatic rupture, which can have a similar radiographic appearance.12
Our patient’s presentation was a reminder that an elevated hemidiaphragm may reflect abdominal pathology and that subphrenic air in this context need not be either “free” or a surgical emergency. Drainage of the abscess restored the normal position of our patient’s right hemidiaphragm (Figure 3).
A 39-year-old Filipino man presented with nausea, vomiting, and abdominal pain of 2 weeks’ duration. He did not report trauma, and he had no history of medical illness or surgery.
On arrival, his blood pressure was 123/83 mm Hg, pulse 122 beats per minute, respiratory rate 18 breaths per minute, and temperature 100.7°F (38.1°C). On physical examination, he exhibited marked tenderness of the right upper quadrant on palpation. The abdomen was otherwise soft with no guarding or rebound tenderness.
Results of initial laboratory testing were as follows:
- Leukocyte count 17.0 × 109/L (reference range 4.5–11.0)
- Serum glucose 558 mg/dL without ketoacidosis
- Aspartate aminotransferase 109 U/L (2–40)
- Alanine aminotranferase 28 U/L (2–50)
- Total serum bilirubin 4.0 mg/dL (0.0–1.5).
Plain chest radiography showed dramatic elevation of the right hemidiaphragm with a large subphrenic air-fluid level (Figure 1). Abdominal computed tomography (CT) demonstrated a multiloculated hepatic abscess 18 × 13.5 cm subjacent to the diaphragm (Figure 2). Cultures of blood and the abscess yielded Klebsiella pneumoniae. The patient recovered after percutaneous drainage and a course of ceftriaxone.
PRIMARY KLEBSIELLA LIVER ABSCESS
K pneumoniae, a gram-negative aerobic encapsulated bacillus of the normal human intestinal flora, is closely related to Escherichia coli, historically the most frequent bacterial cause of pyogenic liver abscess.1 Over the last 30 years, K pneumoniae has eclipsed E coli as the most common causative agent, with the epicenter of this trend being located in Taiwan and South Korea, perhaps because rates of fecal Klebsiella carriage in that region are particularly high.1,2
Concurrently, there has been increasing recognition—initially across Asia, but lately in Europe and the Western Hemisphere—of the so-called invasive Klebsiella liver abscess (KLA) syndrome, virtually unique to the hypervirulent K1 and K2 capsular serotypes of K pneumoniae prevalent in Asia.3–6 This community-acquired syndrome is characterized by hematogenous deposition of the organism at distant sites, such as the lung, soft tissues, central nervous system, and eyes. Impairment of phagocytic function, as occurs in diabetes mellitus, and the resistance to phagocytosis conferred by the K1 and K2 serotypes have been identified as predisposing factors for dissemination.7,8 The mucoid phenotype of K pneumoniae, very common in Asian isolates of the K1 and K2 serotypes, is also associated with hypervirulence and extrahepatic spread, presumably through evasion of phagocytosis and complement-mediated opsonization.2,9
Our patient’s risk factors for KLA were his Asian origin and uncontrolled diabetes. No evidence of remote infection was detected during his hospitalization.
HEMIDIAPHRAGM ELEVATION
Acquired hemidiaphragm elevation is most commonly unilateral and typically represents an incidental radiologic finding attributable to paralysis of the corresponding diaphragm after phrenic nerve injury caused by trauma, surgery, or infection. Unilateral diaphragmatic paralysis is classically confirmed by performing a fluoroscopic sniff test, which is positive if the affected hemidiaphragm is observed in real time to paradoxically move upward during forced inhalation.10 This condition is usually asymptomatic at rest but could cause exertional dyspnea and contribute to ventilatory failure when pulmonary disease coexists.11
Occasionally, as in our patient, hemidiaphragm elevation is part of the presentation of active abdominal pathology that displaces the corresponding hemidiaphragm cephalad by mass effect. Examples of such space-occupying abdominal lesions include infections, malignancy, hepatosplenomegaly, and pneumoperitoneum from a ruptured viscus. Pneumoperitoneum is suggested by the presence of an air crescent immediately subjacent to the affected hemidiaphragm on an upright radiograph accompanied by peritoneal signs.
Although there was subphrenic air on this patient’s initial chest radiograph, it was actually part of an air-fluid level without associated peritoneal signs. An air-fluid level is characterized by a sharp horizontal demarcation between the lighter gas component floating at the top and the heavier fluid component settling on the bottom (Figure 1). The subsequent CT excluded free intra-abdominal air while identifying a large hepatic abscess as the cause of hemidiaphragm elevation. In trauma victims, CT is also helpful in ruling out diaphragmatic rupture, which can have a similar radiographic appearance.12
Our patient’s presentation was a reminder that an elevated hemidiaphragm may reflect abdominal pathology and that subphrenic air in this context need not be either “free” or a surgical emergency. Drainage of the abscess restored the normal position of our patient’s right hemidiaphragm (Figure 3).
- Huang CJ, Pitt HA, Lipsett PA, et al. Pyogenic hepatic abscess: changing trends over 42 years. Ann Surg 1996; 223:600–607.
- Lin YT, Siu LK, Lin JC, et al. Seroepidemiology of Klebsiella pneumoniae colonizing the intestinal tract of healthy Chinese and overseas Chinese adults in Asian countries. BMC Microbiol 2012; 12:13.
- Wang JH, Liu YC, Lee SS, et al. Primary liver abscess due to Klebsiella pneumoniae in Taiwan. Clin Infect Dis 1998; 26:1434–1438.
- Pastagia M, Arumugam V. Klebsiella pneumoniae liver abscesses in a public hospital in Queens, New York. Travel Med Infect Dis 2008; 6:228–233.
- Rahimian J, Wilson T, Oram V, Holzman RS. Pyogenic liver abscess: recent trends in etiology and mortality. Clin Infect Dis 2004; 39:1654–1659.
- Moore R, O’Shea D, Geoghegan T, Mallon PW, Sheehan G. Community-acquired Klebsiella pneumoniae liver abscess: an emerging infection in Ireland and Europe. Infection 2013; 41:681–686.
- Lecube A, Pachón G, Petriz J, Hernández C, Simó R. Phagocytic activity is impaired in type 2 diabetes mellitus and increases after metabolic improvement. PLoS One 2011; 6:e23366.
- Lin JC, Siu LK, Fung CP, et al. Impaired phagocytosis of capsular serotypes K1 or K2 Klebsiella pneumoniae in type 2 diabetes mellitus patients with poor glycemic control. J Clin Endocrinol Metab 2006; 91:3084–3087.
- Siu LK, Yeh KM, Lin JC, Fung CP, Chang FY. Klebsiella pneumoniae liver abscess: a new invasive syndrome. Lancet Infect Dis 2012; 12:881–887.
- Gierada DS, Slone RM, Fleishman MJ. Imaging evaluation of the diaphragm. Chest Surg Clin North Am 1998; 8:237–280.
- Qureshi A. Diaphragm paralysis. Semin Respir Crit Care Med 2009; 30:315–320.
- Havens JM, Kelly E, Patel V. A 78-year-old man with an elevated hemidiaphragm following trauma. Chest 2008; 134:1336–1339.
- Huang CJ, Pitt HA, Lipsett PA, et al. Pyogenic hepatic abscess: changing trends over 42 years. Ann Surg 1996; 223:600–607.
- Lin YT, Siu LK, Lin JC, et al. Seroepidemiology of Klebsiella pneumoniae colonizing the intestinal tract of healthy Chinese and overseas Chinese adults in Asian countries. BMC Microbiol 2012; 12:13.
- Wang JH, Liu YC, Lee SS, et al. Primary liver abscess due to Klebsiella pneumoniae in Taiwan. Clin Infect Dis 1998; 26:1434–1438.
- Pastagia M, Arumugam V. Klebsiella pneumoniae liver abscesses in a public hospital in Queens, New York. Travel Med Infect Dis 2008; 6:228–233.
- Rahimian J, Wilson T, Oram V, Holzman RS. Pyogenic liver abscess: recent trends in etiology and mortality. Clin Infect Dis 2004; 39:1654–1659.
- Moore R, O’Shea D, Geoghegan T, Mallon PW, Sheehan G. Community-acquired Klebsiella pneumoniae liver abscess: an emerging infection in Ireland and Europe. Infection 2013; 41:681–686.
- Lecube A, Pachón G, Petriz J, Hernández C, Simó R. Phagocytic activity is impaired in type 2 diabetes mellitus and increases after metabolic improvement. PLoS One 2011; 6:e23366.
- Lin JC, Siu LK, Fung CP, et al. Impaired phagocytosis of capsular serotypes K1 or K2 Klebsiella pneumoniae in type 2 diabetes mellitus patients with poor glycemic control. J Clin Endocrinol Metab 2006; 91:3084–3087.
- Siu LK, Yeh KM, Lin JC, Fung CP, Chang FY. Klebsiella pneumoniae liver abscess: a new invasive syndrome. Lancet Infect Dis 2012; 12:881–887.
- Gierada DS, Slone RM, Fleishman MJ. Imaging evaluation of the diaphragm. Chest Surg Clin North Am 1998; 8:237–280.
- Qureshi A. Diaphragm paralysis. Semin Respir Crit Care Med 2009; 30:315–320.
- Havens JM, Kelly E, Patel V. A 78-year-old man with an elevated hemidiaphragm following trauma. Chest 2008; 134:1336–1339.
VIDEO: Functional noninvasive imaging cuts unnecessary angiography
ROME – Functional, noninvasive cardiac imaging using cardiovascular MR or myocardial perfusion scintigraphy was significantly better than was a current and well regarded guideline-based approach to identifying patients with chest pain and suspected coronary artery disease who could safely avoid angiography, thereby cutting the rate of unnecessary angiography by about 75%.
Following the guideline formula adopted by the British National Institute for Health and Care Excellence (NICE) resulted in a 29% rate of unnecessary angiography compared with rates of 7.5% using cardiovascular MR (CMR) and 7.1% using myocardial perfusion scintigraphy (MPS) in a multicenter randomized trial with 1,202 patients, John P. Greenwood, MBChB, said at the annual congress of the European Society of Cardiology.
This universal use of a functional, noninvasive imaging strategy to guide angiography resulted in no significant penalty of missed coronary disease or subsequent coronary events. The rate of positive angiography findings was 12% among the 240 patients managed according to the NICE guidelines, 10% among 481 patients screened by CMR, and 9% among the 481 patients screened using MPS, reported Dr. Greenwood, professor of cardiology at the University of Leeds (England). The rate of major adverse coronary events after 12 months of follow-up were 3% following the NICE protocol and 4% when screening by CMR or with MPS.
Concurrently with Dr. Greenwood’s report, the findings from the Clinical Evaluation of Magnetic Resonance Imaging in Coronary Heart Disease 2 (CE-MARC2) study appeared in an article online (JAMA. 2016 Aug 29. doi: 10.1001/jama.2016.12680).
“We showed that a functional test with CMR or MPS can reduce the rate of unnecessary coronary angiography. Cutting unnecessary angiography is really important to patients, and it may also cost effective,” he said, but cautioned that a formal cost analysis of the options tested in this study is still being run.
The NICE guidelines manage patients with chest pain that could be angina by their pretest probability of having coronary artery disease (CAD), and at the time the study was designed the NICE guidelines, issued in 2010, provided the most up-to-date expert guidance on how to triage these patients. The study enrolled patients with a pretest probability for CAD of 10%-90%; collectively their average probability was 50%. The patients participated in the study at one of six U.K. centers during November 2012 to March 2015. The average age was 56 years.
MPS is “probably the noninvasive imaging approach most commonly used worldwide to detect coronary ischemia,” Dr. Greenwood said. But he led an earlier study that showed that CMR, using a gadolinium-based tracing agent, works even better than MPS (in this study single photon emission CT) to predict a patient’s risk for major cardiac events. He said this superiority is probably because of the greater spatial resolution with CMR.
“The higher spatial resolution of CMR, about 5- to 10-fold greater that MPS, is less likely to produce false negative results,” he said in an interview. “We showed that CMR has higher diagnostic accuracy, is a better prognosticator, and is more cost effective” than MPS. Dr. Greenwood attributed the similar performance of CMR and MPS in CE-MARC2 to the study’s design, which led to fewer patients undergoing each of the two imaging methods and made CE-MARC2 underpowered to discern a difference in specificity. In his earlier study, which included 752 patients who underwent examination with both CMR and MPS, the negative predictive value of CMR was 91% compared with 79% with MPS.
CMR uses conventional MR machines, is now widely available, and is being widely used today as a first-line test in the United Kingdom and Europe, he added.
Dr. Greenwood believes that in his new study functional imaging outperformed the NICE guidelines because the pretest models used in the guidelines “tend to overestimate risk,” the factor that produces angiography overuse.
His report included two additional analyses that assessed the impact of CMR and MPS in the subgroup of patients with a high pretest probability for CAD, 61%-90%, and in the subgroup with a low pretest probability, 10%-29%. Among the patients with a high likelihood for CAD the two functional imaging methods cut the rate of unnecessary angiography by 95%, a statistically significant difference. Among those with a low likelihood functional imaging cut the rate 56%, a difference that did not reach statistical significance.
[email protected]
On Twitter @mitchelzoler
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
The results from CE-MARC2 very nicely showed that imaging-guided angiography is as safe as compulsory angiography in the highest-risk subgroup of the enrolled patients, those with a pretest probability of 61%-90% for having coronary artery disease. Findings from the economic analysis of this study that remains pending will be crucial for eventually recommending one strategy over the other in this setting.

|
| Mitchel L. Zoler/Frontline Medical News Dr. Udo Sechtem |
The 12-month rate of the hardest clinical endpoints measured in this study, cardiovascular deaths and MIs, was very low in this study: 1.3% in the patients managed with NICE guidance, 1% in those who first underwent cardiovascular MR, and 0.8% in the patients who first underwent myocardial perfusion scintigraphy. Despite this low risk, the patients in each of the three arms of the study underwent roughly 500 test procedures.
We should therefore consider a totally different approach. Instead of immediately performing a noninvasive test or the tests called for by the NICE guidelines, what about no testing at all. Instead, patients would first undergo optimal preventive and symptomatic medical treatments. If patients failed this strategy they then could be considered for revascularization. I propose a study that would compare imaging-guided conditional angiography, as tested in CE-MARC2, with symptom-guided conditional angiography. Functional, noninvasive testing for all needs to be compared against optimal management and symptom driven interventions.
Udo Sechtem, Dr Med, is head of cardiology at the Robert-Bosch-Hospital in Stuttgart, Germany. He made these comments as the designated discussant for the study. He had no disclosures.
The results from CE-MARC2 very nicely showed that imaging-guided angiography is as safe as compulsory angiography in the highest-risk subgroup of the enrolled patients, those with a pretest probability of 61%-90% for having coronary artery disease. Findings from the economic analysis of this study that remains pending will be crucial for eventually recommending one strategy over the other in this setting.

|
| Mitchel L. Zoler/Frontline Medical News Dr. Udo Sechtem |
The 12-month rate of the hardest clinical endpoints measured in this study, cardiovascular deaths and MIs, was very low in this study: 1.3% in the patients managed with NICE guidance, 1% in those who first underwent cardiovascular MR, and 0.8% in the patients who first underwent myocardial perfusion scintigraphy. Despite this low risk, the patients in each of the three arms of the study underwent roughly 500 test procedures.
We should therefore consider a totally different approach. Instead of immediately performing a noninvasive test or the tests called for by the NICE guidelines, what about no testing at all. Instead, patients would first undergo optimal preventive and symptomatic medical treatments. If patients failed this strategy they then could be considered for revascularization. I propose a study that would compare imaging-guided conditional angiography, as tested in CE-MARC2, with symptom-guided conditional angiography. Functional, noninvasive testing for all needs to be compared against optimal management and symptom driven interventions.
Udo Sechtem, Dr Med, is head of cardiology at the Robert-Bosch-Hospital in Stuttgart, Germany. He made these comments as the designated discussant for the study. He had no disclosures.
The results from CE-MARC2 very nicely showed that imaging-guided angiography is as safe as compulsory angiography in the highest-risk subgroup of the enrolled patients, those with a pretest probability of 61%-90% for having coronary artery disease. Findings from the economic analysis of this study that remains pending will be crucial for eventually recommending one strategy over the other in this setting.

|
| Mitchel L. Zoler/Frontline Medical News Dr. Udo Sechtem |
The 12-month rate of the hardest clinical endpoints measured in this study, cardiovascular deaths and MIs, was very low in this study: 1.3% in the patients managed with NICE guidance, 1% in those who first underwent cardiovascular MR, and 0.8% in the patients who first underwent myocardial perfusion scintigraphy. Despite this low risk, the patients in each of the three arms of the study underwent roughly 500 test procedures.
We should therefore consider a totally different approach. Instead of immediately performing a noninvasive test or the tests called for by the NICE guidelines, what about no testing at all. Instead, patients would first undergo optimal preventive and symptomatic medical treatments. If patients failed this strategy they then could be considered for revascularization. I propose a study that would compare imaging-guided conditional angiography, as tested in CE-MARC2, with symptom-guided conditional angiography. Functional, noninvasive testing for all needs to be compared against optimal management and symptom driven interventions.
Udo Sechtem, Dr Med, is head of cardiology at the Robert-Bosch-Hospital in Stuttgart, Germany. He made these comments as the designated discussant for the study. He had no disclosures.
ROME – Functional, noninvasive cardiac imaging using cardiovascular MR or myocardial perfusion scintigraphy was significantly better than was a current and well regarded guideline-based approach to identifying patients with chest pain and suspected coronary artery disease who could safely avoid angiography, thereby cutting the rate of unnecessary angiography by about 75%.
Following the guideline formula adopted by the British National Institute for Health and Care Excellence (NICE) resulted in a 29% rate of unnecessary angiography compared with rates of 7.5% using cardiovascular MR (CMR) and 7.1% using myocardial perfusion scintigraphy (MPS) in a multicenter randomized trial with 1,202 patients, John P. Greenwood, MBChB, said at the annual congress of the European Society of Cardiology.
This universal use of a functional, noninvasive imaging strategy to guide angiography resulted in no significant penalty of missed coronary disease or subsequent coronary events. The rate of positive angiography findings was 12% among the 240 patients managed according to the NICE guidelines, 10% among 481 patients screened by CMR, and 9% among the 481 patients screened using MPS, reported Dr. Greenwood, professor of cardiology at the University of Leeds (England). The rate of major adverse coronary events after 12 months of follow-up were 3% following the NICE protocol and 4% when screening by CMR or with MPS.
Concurrently with Dr. Greenwood’s report, the findings from the Clinical Evaluation of Magnetic Resonance Imaging in Coronary Heart Disease 2 (CE-MARC2) study appeared in an article online (JAMA. 2016 Aug 29. doi: 10.1001/jama.2016.12680).
“We showed that a functional test with CMR or MPS can reduce the rate of unnecessary coronary angiography. Cutting unnecessary angiography is really important to patients, and it may also cost effective,” he said, but cautioned that a formal cost analysis of the options tested in this study is still being run.
The NICE guidelines manage patients with chest pain that could be angina by their pretest probability of having coronary artery disease (CAD), and at the time the study was designed the NICE guidelines, issued in 2010, provided the most up-to-date expert guidance on how to triage these patients. The study enrolled patients with a pretest probability for CAD of 10%-90%; collectively their average probability was 50%. The patients participated in the study at one of six U.K. centers during November 2012 to March 2015. The average age was 56 years.
MPS is “probably the noninvasive imaging approach most commonly used worldwide to detect coronary ischemia,” Dr. Greenwood said. But he led an earlier study that showed that CMR, using a gadolinium-based tracing agent, works even better than MPS (in this study single photon emission CT) to predict a patient’s risk for major cardiac events. He said this superiority is probably because of the greater spatial resolution with CMR.
“The higher spatial resolution of CMR, about 5- to 10-fold greater that MPS, is less likely to produce false negative results,” he said in an interview. “We showed that CMR has higher diagnostic accuracy, is a better prognosticator, and is more cost effective” than MPS. Dr. Greenwood attributed the similar performance of CMR and MPS in CE-MARC2 to the study’s design, which led to fewer patients undergoing each of the two imaging methods and made CE-MARC2 underpowered to discern a difference in specificity. In his earlier study, which included 752 patients who underwent examination with both CMR and MPS, the negative predictive value of CMR was 91% compared with 79% with MPS.
CMR uses conventional MR machines, is now widely available, and is being widely used today as a first-line test in the United Kingdom and Europe, he added.
Dr. Greenwood believes that in his new study functional imaging outperformed the NICE guidelines because the pretest models used in the guidelines “tend to overestimate risk,” the factor that produces angiography overuse.
His report included two additional analyses that assessed the impact of CMR and MPS in the subgroup of patients with a high pretest probability for CAD, 61%-90%, and in the subgroup with a low pretest probability, 10%-29%. Among the patients with a high likelihood for CAD the two functional imaging methods cut the rate of unnecessary angiography by 95%, a statistically significant difference. Among those with a low likelihood functional imaging cut the rate 56%, a difference that did not reach statistical significance.
[email protected]
On Twitter @mitchelzoler
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
ROME – Functional, noninvasive cardiac imaging using cardiovascular MR or myocardial perfusion scintigraphy was significantly better than was a current and well regarded guideline-based approach to identifying patients with chest pain and suspected coronary artery disease who could safely avoid angiography, thereby cutting the rate of unnecessary angiography by about 75%.
Following the guideline formula adopted by the British National Institute for Health and Care Excellence (NICE) resulted in a 29% rate of unnecessary angiography compared with rates of 7.5% using cardiovascular MR (CMR) and 7.1% using myocardial perfusion scintigraphy (MPS) in a multicenter randomized trial with 1,202 patients, John P. Greenwood, MBChB, said at the annual congress of the European Society of Cardiology.
This universal use of a functional, noninvasive imaging strategy to guide angiography resulted in no significant penalty of missed coronary disease or subsequent coronary events. The rate of positive angiography findings was 12% among the 240 patients managed according to the NICE guidelines, 10% among 481 patients screened by CMR, and 9% among the 481 patients screened using MPS, reported Dr. Greenwood, professor of cardiology at the University of Leeds (England). The rate of major adverse coronary events after 12 months of follow-up were 3% following the NICE protocol and 4% when screening by CMR or with MPS.
Concurrently with Dr. Greenwood’s report, the findings from the Clinical Evaluation of Magnetic Resonance Imaging in Coronary Heart Disease 2 (CE-MARC2) study appeared in an article online (JAMA. 2016 Aug 29. doi: 10.1001/jama.2016.12680).
“We showed that a functional test with CMR or MPS can reduce the rate of unnecessary coronary angiography. Cutting unnecessary angiography is really important to patients, and it may also cost effective,” he said, but cautioned that a formal cost analysis of the options tested in this study is still being run.
The NICE guidelines manage patients with chest pain that could be angina by their pretest probability of having coronary artery disease (CAD), and at the time the study was designed the NICE guidelines, issued in 2010, provided the most up-to-date expert guidance on how to triage these patients. The study enrolled patients with a pretest probability for CAD of 10%-90%; collectively their average probability was 50%. The patients participated in the study at one of six U.K. centers during November 2012 to March 2015. The average age was 56 years.
MPS is “probably the noninvasive imaging approach most commonly used worldwide to detect coronary ischemia,” Dr. Greenwood said. But he led an earlier study that showed that CMR, using a gadolinium-based tracing agent, works even better than MPS (in this study single photon emission CT) to predict a patient’s risk for major cardiac events. He said this superiority is probably because of the greater spatial resolution with CMR.
“The higher spatial resolution of CMR, about 5- to 10-fold greater that MPS, is less likely to produce false negative results,” he said in an interview. “We showed that CMR has higher diagnostic accuracy, is a better prognosticator, and is more cost effective” than MPS. Dr. Greenwood attributed the similar performance of CMR and MPS in CE-MARC2 to the study’s design, which led to fewer patients undergoing each of the two imaging methods and made CE-MARC2 underpowered to discern a difference in specificity. In his earlier study, which included 752 patients who underwent examination with both CMR and MPS, the negative predictive value of CMR was 91% compared with 79% with MPS.
CMR uses conventional MR machines, is now widely available, and is being widely used today as a first-line test in the United Kingdom and Europe, he added.
Dr. Greenwood believes that in his new study functional imaging outperformed the NICE guidelines because the pretest models used in the guidelines “tend to overestimate risk,” the factor that produces angiography overuse.
His report included two additional analyses that assessed the impact of CMR and MPS in the subgroup of patients with a high pretest probability for CAD, 61%-90%, and in the subgroup with a low pretest probability, 10%-29%. Among the patients with a high likelihood for CAD the two functional imaging methods cut the rate of unnecessary angiography by 95%, a statistically significant difference. Among those with a low likelihood functional imaging cut the rate 56%, a difference that did not reach statistical significance.
[email protected]
On Twitter @mitchelzoler
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
AT THE ESC CONGRESS 2016
Key clinical point: Screening patients with suspected angina via cardiovascular MR or myocardial perfusion imaging substantially reduced the rate of unnecessary angiography compared with the screening algorithm currently endorsed by British national guidelines.
Major finding: The unnecessary angiography rate was 29% with the guideline algorithm, 7.5% with cardiovascular MR, and 7.1% with myocardial perfusion scintigraphy.
Data source: CE MARC2, a multicenter, randomized trial with 1,202 patients.
Disclosures: Dr. Greenwood had no disclosures.