User login
Emergency Imaging: Facial Trauma After a Fall
An 89-year-old man presented to the ED with facial trauma due to a mechanical fall after losing his balance on uneven pavement and hitting the right side of his face. Physical examination revealed an ecchymosis inferior to the right eye and tenderness to palpation at the right maxilla and bilateral nasolabial folds. Maxillofacial computed tomography (CT) was ordered for further evaluation; representative images are presented above (Figure 1a and 1b).

What is the diagnosis?
Answer
A noncontrast CT of the maxillofacial bones demonstrated acute fractures through the bilateral pterygoid plates (white arrows, Figure 2a). The fractures extended through the medial and lateral walls of the bilateral maxillary sinuses (red arrows, Figure 2a), and propagated to the frontal processes of the maxilla (red arrows, Figure 2b), extending toward the alveolar process, indicating involvement of the anterolateral margin of the nasal fossa. The full extent of the fracture is best seen on a 3D-reconstructed image (red arrows, Figure 3). Additional images (not presented here) confirmed no fracture involvement of the orbital floors, nasal bones, or zygomatic arches. Expected posttraumatic hemorrhage was appreciated within the maxillary sinuses (white asterisks, Figure 2a).
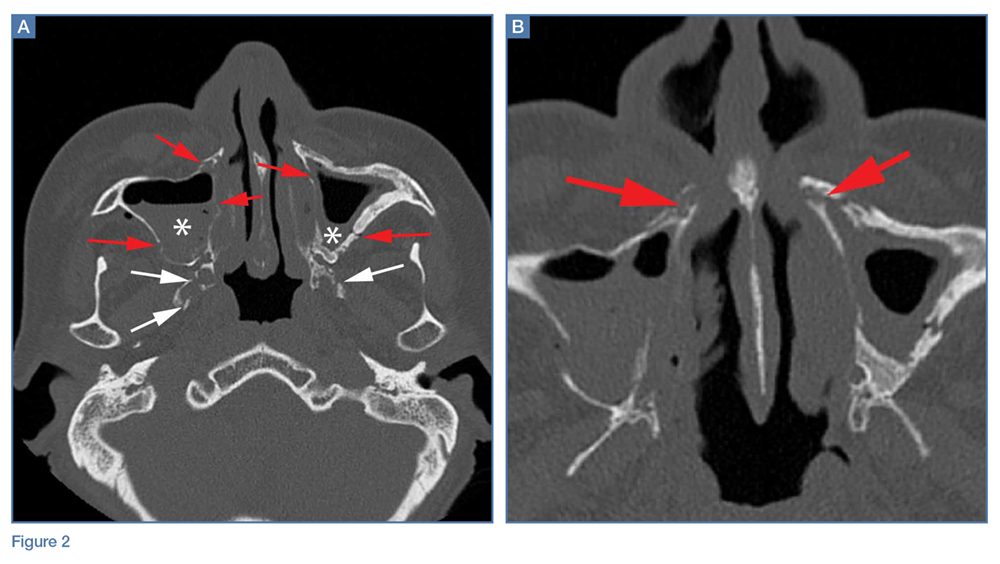
Le Fort Fractures
The findings described above are characteristic of a Le Fort I fracture pattern. Initially described in 1901 by René Le Fort, a French surgeon, the Le Fort classification system details somewhat predictable midface fracture patterns resulting in various degrees of craniofacial disassociation.1 Using weights that were dropped on cadaveric heads, Le Fort discovered that the pterygoid plates must be disrupted in order for the midface facial bones to separate from the skull base. As such, when diagnosing a Le Fort fracture, fracture of the pterygoid plate must be present, regardless of the fracture type (Le Fort I, II, and III).2
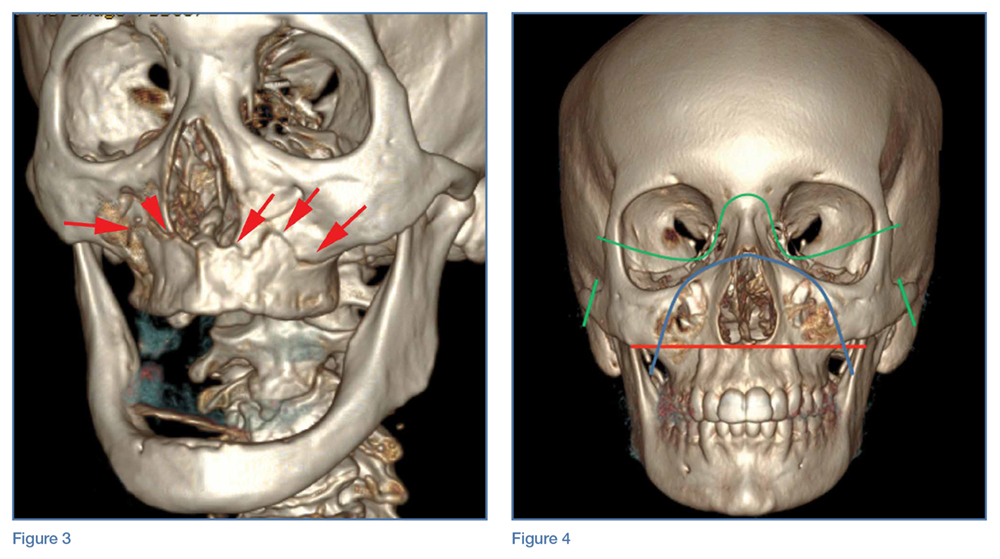
Le Fort I Fracture. This fracture pattern (red line, Figure 4) is referred to as a “floating palate” and involves separation of the hard palate from the skull base via fracture extension from the pterygoid plates into the maxillary sinus walls, as demonstrated in this case. The key distinguisher of the Le Fort I pattern is involvement of the anterolateral margin of the nasal fossa.2
Le Fort II Fracture. This fracture pattern (blue line, Figure 4) describes a “floating maxilla” wherein the pterygoid plate fractures are met with a pyramidal-type fracture pattern of the midface. The maxillary teeth form the base of the pyramid, and the fracture extends superiorly through the infraorbital rims bilaterally and toward the nasofrontal suture.2,3 Le Fort II fractures result in the maxilla floating freely from the rest of the midface and skull base.
Le Fort III Fracture. This fracture pattern (green lines, Figure 4) describes a “floating face” with complete craniofacial disjunction resulting from fracture of the pterygoid plates, nasofrontal suture, maxillofrontal suture, orbital wall, and zygomatic arch/zygomaticofrontal suture.2,3
It is important to note that midface trauma represents a complex spectrum of injuries, and Le Fort fractures only account for a small percentage of facial bone fractures that present through Level 1 trauma centers.2 Le Fort fracture patterns can coexist with other fracture patterns and also can be seen in combination with each other. For example, one side of the face may demonstrate a Le Fort II pattern while the other side concurrently demonstrates a Le Fort III pattern. Though not robust enough for complete description of and surgical planning for facial fractures, this classification system is a succinct and well-accepted means of describing major fracture planes.
1. Le Fort R. Etude experimentale sur les fractures de la machoire superieure. Rev Chir. 1901;23:208-227, 360-379, 479-507.
2. Rhea JT, Novelline RA. How to simplify the CT diagnosis of Le Fort fractures. AJR Am J Roentgenol. 2005;184(5):1700-1705.
3. Hopper RA, Salemy S, Sze RW. Diagnosis of midface fractures with CT: what the surgeon needs to know. Radiographics. 2006;26(3):783-793.
An 89-year-old man presented to the ED with facial trauma due to a mechanical fall after losing his balance on uneven pavement and hitting the right side of his face. Physical examination revealed an ecchymosis inferior to the right eye and tenderness to palpation at the right maxilla and bilateral nasolabial folds. Maxillofacial computed tomography (CT) was ordered for further evaluation; representative images are presented above (Figure 1a and 1b).

What is the diagnosis?
Answer
A noncontrast CT of the maxillofacial bones demonstrated acute fractures through the bilateral pterygoid plates (white arrows, Figure 2a). The fractures extended through the medial and lateral walls of the bilateral maxillary sinuses (red arrows, Figure 2a), and propagated to the frontal processes of the maxilla (red arrows, Figure 2b), extending toward the alveolar process, indicating involvement of the anterolateral margin of the nasal fossa. The full extent of the fracture is best seen on a 3D-reconstructed image (red arrows, Figure 3). Additional images (not presented here) confirmed no fracture involvement of the orbital floors, nasal bones, or zygomatic arches. Expected posttraumatic hemorrhage was appreciated within the maxillary sinuses (white asterisks, Figure 2a).


Le Fort Fractures
The findings described above are characteristic of a Le Fort I fracture pattern. Initially described in 1901 by René Le Fort, a French surgeon, the Le Fort classification system details somewhat predictable midface fracture patterns resulting in various degrees of craniofacial disassociation.1 Using weights that were dropped on cadaveric heads, Le Fort discovered that the pterygoid plates must be disrupted in order for the midface facial bones to separate from the skull base. As such, when diagnosing a Le Fort fracture, fracture of the pterygoid plate must be present, regardless of the fracture type (Le Fort I, II, and III).2
Le Fort I Fracture. This fracture pattern (red line, Figure 4) is referred to as a “floating palate” and involves separation of the hard palate from the skull base via fracture extension from the pterygoid plates into the maxillary sinus walls, as demonstrated in this case. The key distinguisher of the Le Fort I pattern is involvement of the anterolateral margin of the nasal fossa.2
Le Fort II Fracture. This fracture pattern (blue line, Figure 4) describes a “floating maxilla” wherein the pterygoid plate fractures are met with a pyramidal-type fracture pattern of the midface. The maxillary teeth form the base of the pyramid, and the fracture extends superiorly through the infraorbital rims bilaterally and toward the nasofrontal suture.2,3 Le Fort II fractures result in the maxilla floating freely from the rest of the midface and skull base.
Le Fort III Fracture. This fracture pattern (green lines, Figure 4) describes a “floating face” with complete craniofacial disjunction resulting from fracture of the pterygoid plates, nasofrontal suture, maxillofrontal suture, orbital wall, and zygomatic arch/zygomaticofrontal suture.2,3
It is important to note that midface trauma represents a complex spectrum of injuries, and Le Fort fractures only account for a small percentage of facial bone fractures that present through Level 1 trauma centers.2 Le Fort fracture patterns can coexist with other fracture patterns and also can be seen in combination with each other. For example, one side of the face may demonstrate a Le Fort II pattern while the other side concurrently demonstrates a Le Fort III pattern. Though not robust enough for complete description of and surgical planning for facial fractures, this classification system is a succinct and well-accepted means of describing major fracture planes.
An 89-year-old man presented to the ED with facial trauma due to a mechanical fall after losing his balance on uneven pavement and hitting the right side of his face. Physical examination revealed an ecchymosis inferior to the right eye and tenderness to palpation at the right maxilla and bilateral nasolabial folds. Maxillofacial computed tomography (CT) was ordered for further evaluation; representative images are presented above (Figure 1a and 1b).

What is the diagnosis?
Answer
A noncontrast CT of the maxillofacial bones demonstrated acute fractures through the bilateral pterygoid plates (white arrows, Figure 2a). The fractures extended through the medial and lateral walls of the bilateral maxillary sinuses (red arrows, Figure 2a), and propagated to the frontal processes of the maxilla (red arrows, Figure 2b), extending toward the alveolar process, indicating involvement of the anterolateral margin of the nasal fossa. The full extent of the fracture is best seen on a 3D-reconstructed image (red arrows, Figure 3). Additional images (not presented here) confirmed no fracture involvement of the orbital floors, nasal bones, or zygomatic arches. Expected posttraumatic hemorrhage was appreciated within the maxillary sinuses (white asterisks, Figure 2a).


Le Fort Fractures
The findings described above are characteristic of a Le Fort I fracture pattern. Initially described in 1901 by René Le Fort, a French surgeon, the Le Fort classification system details somewhat predictable midface fracture patterns resulting in various degrees of craniofacial disassociation.1 Using weights that were dropped on cadaveric heads, Le Fort discovered that the pterygoid plates must be disrupted in order for the midface facial bones to separate from the skull base. As such, when diagnosing a Le Fort fracture, fracture of the pterygoid plate must be present, regardless of the fracture type (Le Fort I, II, and III).2
Le Fort I Fracture. This fracture pattern (red line, Figure 4) is referred to as a “floating palate” and involves separation of the hard palate from the skull base via fracture extension from the pterygoid plates into the maxillary sinus walls, as demonstrated in this case. The key distinguisher of the Le Fort I pattern is involvement of the anterolateral margin of the nasal fossa.2
Le Fort II Fracture. This fracture pattern (blue line, Figure 4) describes a “floating maxilla” wherein the pterygoid plate fractures are met with a pyramidal-type fracture pattern of the midface. The maxillary teeth form the base of the pyramid, and the fracture extends superiorly through the infraorbital rims bilaterally and toward the nasofrontal suture.2,3 Le Fort II fractures result in the maxilla floating freely from the rest of the midface and skull base.
Le Fort III Fracture. This fracture pattern (green lines, Figure 4) describes a “floating face” with complete craniofacial disjunction resulting from fracture of the pterygoid plates, nasofrontal suture, maxillofrontal suture, orbital wall, and zygomatic arch/zygomaticofrontal suture.2,3
It is important to note that midface trauma represents a complex spectrum of injuries, and Le Fort fractures only account for a small percentage of facial bone fractures that present through Level 1 trauma centers.2 Le Fort fracture patterns can coexist with other fracture patterns and also can be seen in combination with each other. For example, one side of the face may demonstrate a Le Fort II pattern while the other side concurrently demonstrates a Le Fort III pattern. Though not robust enough for complete description of and surgical planning for facial fractures, this classification system is a succinct and well-accepted means of describing major fracture planes.
1. Le Fort R. Etude experimentale sur les fractures de la machoire superieure. Rev Chir. 1901;23:208-227, 360-379, 479-507.
2. Rhea JT, Novelline RA. How to simplify the CT diagnosis of Le Fort fractures. AJR Am J Roentgenol. 2005;184(5):1700-1705.
3. Hopper RA, Salemy S, Sze RW. Diagnosis of midface fractures with CT: what the surgeon needs to know. Radiographics. 2006;26(3):783-793.
1. Le Fort R. Etude experimentale sur les fractures de la machoire superieure. Rev Chir. 1901;23:208-227, 360-379, 479-507.
2. Rhea JT, Novelline RA. How to simplify the CT diagnosis of Le Fort fractures. AJR Am J Roentgenol. 2005;184(5):1700-1705.
3. Hopper RA, Salemy S, Sze RW. Diagnosis of midface fractures with CT: what the surgeon needs to know. Radiographics. 2006;26(3):783-793.
Phlegmasia cerulea dolens from radiation-induced venous stenosis
A 77-year-old man presented with a 5-day history of painful swelling of his right leg. He reported no trauma, no recent surgery, no history of thrombophilic disorder, and no prolonged immobilization. However, he had a history of prostate cancer, treated 10 years earlier with pelvic radiation.
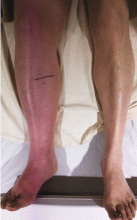
Examination revealed massive right leg swelling extending from the thigh to the ankle, along with bluish-red skin discoloration (Figure 1). Doppler ultrasonography demonstrated acute thrombosis involving the right iliofemoral veins. These findings were consistent with phlegmasia cerulea dolens.
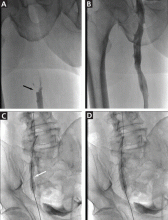
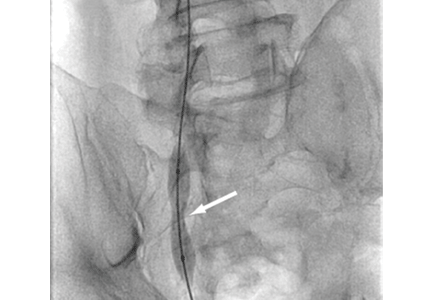
Urgent percutaneous catheter-directed thrombolysis was performed. Venography revealed extensive thrombosis of the femoral vein (Figure 2A) extending into the right external iliac vein. This was treated with catheter-directed pharmacomechanical thrombectomy.
Venography after this procedure showed significant improvement in venous blood flow (Figure 2B). However, stenosis of the right external iliac vein was also noted (Figure 2C) and was treated with balloon angioplasty (Figure 2D) followed by placement of a stent (14 × 40 mm).
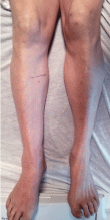
In the immediate postprocedural period, there was marked reduction in swelling and normalization of skin color (Figure 3). The patient did not experience significant bleeding during or after the procedure. Treatment with intravenous unfractionated heparin was continued during the hospital stay, and he was discharged on warfarin with a therapeutic international normalized ratio. At a follow-up visit 3 months later, he was asymptomatic.
A RARE BUT SEVERE TYPE OF ACUTE DEEP VEIN THROMBOSIS
Phlegmasia cerulea dolens (painful cyanotic swollen leg) is a rare and severe form of acute deep vein thrombosis (DVT) characterized by marked limb pain, swelling, and blue discoloration.1 DVT is the most common cause of acute-onset unilateral leg pain, swelling, and skin discoloration.2
The differential diagnosis
The differential diagnosis includes infection (cellulitis, necrotizing fasciitis), compartment syndrome from limb injury, musculoskeletal conditions such as ruptured Baker cyst, venous stasis due to external compression (May-Thurner syndrome, iliac vein compression syndrome, pelvic tumor), acute limb ischemia from arterial obstruction, and complex regional pain syndrome (reflex sympathetic dystrophy).
Management recommendations
As in most cases of DVT, initial treatment of phlegmasia cerulea dolens involves systemic anticoagulation with heparin, elevation of the affected extremity, and fluid resuscitation if the patient is hypotensive. However, phlegmasia cerulea dolens is a major indication for catheter-directed thrombolysis,3,4 so an urgent vascular surgery or interventional cardiology consultation is also required. The American College of Chest Physicians recommends catheter-directed thrombolysis for acute DVT of the iliofemoral veins in patients with symptoms for less than 14 days, good functional capacity, and a life expectancy beyond 1 year.5 This intervention results in reduced incidence of postthrombotic syndrome and improved quality of life5,6 compared with anticoagulation therapy alone.
Who is at risk?
Risk factors for phlegmasia cerulea dolens include a history of malignancy, inherited or acquired thrombophilia, surgery, radiation therapy, trauma, placement of an inferior vena cava filter, and pregnancy. In our patient, the iliac vein stenosis most likely was the result of the radiation therapy he had undergone for prostate cancer.
Arterial stenosis is a well-known complication of radiation therapy and is associated with an increased risk of cardiovascular events.7,8 Radiation induces endothelial damage followed by proliferation of smooth muscle cells, resulting in luminal stenosis and thrombosis. At the cellular level, radiation leads to an acute increase in pro-inflammatory cytokines and endothelial adhesion molecules, causing the recruitment of inflammatory cells to radiation-exposed vessels and chronic activation of transcription factor NF-kappa B, leading to long-term inflammation and angiogenesis.9
Carotid, coronary, and iliac artery stenosis are known to occur around 10 years after radiation therapy to the head, neck, breast, and pelvis. Radiation-induced iliac vein stenosis is rare and can manifest as acute proximal DVT.
- Mumoli N, Invernizzi C, Luschi R, Carmignani G, Camaiti A, Cei M. Phlegmasia cerulea dolens. Circulation 2012; 125:1056–1057.
- Ely JW, Osheroff JA, Chambliss ML, Ebell MH. Approach to leg edema of unclear etiology. J Am Board Fam Med 2006; 19:148–160.
- Casey ET, Murad MH, Zumaeta-Garcia M, et al. Treatment of acute iliofemoral deep vein thrombosis. J Vasc Surg. 2012; 55:1463–1473.
- Chinsakchai K, Ten Duis K, Moll FL, de Borst GJ. Trends in management of phlegmasia cerulea dolens. Vasc Endovascular Surg 2011; 45:5–14.
- Kearon C, Kahn SR, Agnelli G, Goldhaber S, Raskob GE, Comerota AJ; American College of Chest Physicians. Antithrombotic therapy for venous thromboembolic disease: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines (8th Edition). Chest 2008; 133(suppl 6):454S–545S.
- Enden T, Haig Y, Kløw NE, et al; CaVenT Study Group. Long-term outcome after additional catheter-directed thrombolysis versus standard treatment for acute iliofemoral deep vein thrombosis (the CaVenT study): a randomised controlled trial. Lancet 2012; 379:31–38.
- Hooning MJ, Botma A, Aleman BM, et al. Long-term risk of cardiovascular disease in 10-year survivors of breast cancer. J Natl Cancer Inst 2007; 99:365–375.
- Weintraub NL, Jones WK, Manka D. Understanding radiation-induced vascular disease. J Am Coll Cardiol 2010; 55:1237–1239.
- Halle M, Gabrielsen A, Paulsson-Berne G, et al. Sustained inflammation due to nuclear factor-kappa B activation in irradiated human arteries. J Am Coll Cardiol 2010; 55:1227–1236.
A 77-year-old man presented with a 5-day history of painful swelling of his right leg. He reported no trauma, no recent surgery, no history of thrombophilic disorder, and no prolonged immobilization. However, he had a history of prostate cancer, treated 10 years earlier with pelvic radiation.
Examination revealed massive right leg swelling extending from the thigh to the ankle, along with bluish-red skin discoloration (Figure 1). Doppler ultrasonography demonstrated acute thrombosis involving the right iliofemoral veins. These findings were consistent with phlegmasia cerulea dolens.
Urgent percutaneous catheter-directed thrombolysis was performed. Venography revealed extensive thrombosis of the femoral vein (Figure 2A) extending into the right external iliac vein. This was treated with catheter-directed pharmacomechanical thrombectomy.
Venography after this procedure showed significant improvement in venous blood flow (Figure 2B). However, stenosis of the right external iliac vein was also noted (Figure 2C) and was treated with balloon angioplasty (Figure 2D) followed by placement of a stent (14 × 40 mm).
In the immediate postprocedural period, there was marked reduction in swelling and normalization of skin color (Figure 3). The patient did not experience significant bleeding during or after the procedure. Treatment with intravenous unfractionated heparin was continued during the hospital stay, and he was discharged on warfarin with a therapeutic international normalized ratio. At a follow-up visit 3 months later, he was asymptomatic.
A RARE BUT SEVERE TYPE OF ACUTE DEEP VEIN THROMBOSIS
Phlegmasia cerulea dolens (painful cyanotic swollen leg) is a rare and severe form of acute deep vein thrombosis (DVT) characterized by marked limb pain, swelling, and blue discoloration.1 DVT is the most common cause of acute-onset unilateral leg pain, swelling, and skin discoloration.2
The differential diagnosis
The differential diagnosis includes infection (cellulitis, necrotizing fasciitis), compartment syndrome from limb injury, musculoskeletal conditions such as ruptured Baker cyst, venous stasis due to external compression (May-Thurner syndrome, iliac vein compression syndrome, pelvic tumor), acute limb ischemia from arterial obstruction, and complex regional pain syndrome (reflex sympathetic dystrophy).
Management recommendations
As in most cases of DVT, initial treatment of phlegmasia cerulea dolens involves systemic anticoagulation with heparin, elevation of the affected extremity, and fluid resuscitation if the patient is hypotensive. However, phlegmasia cerulea dolens is a major indication for catheter-directed thrombolysis,3,4 so an urgent vascular surgery or interventional cardiology consultation is also required. The American College of Chest Physicians recommends catheter-directed thrombolysis for acute DVT of the iliofemoral veins in patients with symptoms for less than 14 days, good functional capacity, and a life expectancy beyond 1 year.5 This intervention results in reduced incidence of postthrombotic syndrome and improved quality of life5,6 compared with anticoagulation therapy alone.
Who is at risk?
Risk factors for phlegmasia cerulea dolens include a history of malignancy, inherited or acquired thrombophilia, surgery, radiation therapy, trauma, placement of an inferior vena cava filter, and pregnancy. In our patient, the iliac vein stenosis most likely was the result of the radiation therapy he had undergone for prostate cancer.
Arterial stenosis is a well-known complication of radiation therapy and is associated with an increased risk of cardiovascular events.7,8 Radiation induces endothelial damage followed by proliferation of smooth muscle cells, resulting in luminal stenosis and thrombosis. At the cellular level, radiation leads to an acute increase in pro-inflammatory cytokines and endothelial adhesion molecules, causing the recruitment of inflammatory cells to radiation-exposed vessels and chronic activation of transcription factor NF-kappa B, leading to long-term inflammation and angiogenesis.9
Carotid, coronary, and iliac artery stenosis are known to occur around 10 years after radiation therapy to the head, neck, breast, and pelvis. Radiation-induced iliac vein stenosis is rare and can manifest as acute proximal DVT.
A 77-year-old man presented with a 5-day history of painful swelling of his right leg. He reported no trauma, no recent surgery, no history of thrombophilic disorder, and no prolonged immobilization. However, he had a history of prostate cancer, treated 10 years earlier with pelvic radiation.
Examination revealed massive right leg swelling extending from the thigh to the ankle, along with bluish-red skin discoloration (Figure 1). Doppler ultrasonography demonstrated acute thrombosis involving the right iliofemoral veins. These findings were consistent with phlegmasia cerulea dolens.
Urgent percutaneous catheter-directed thrombolysis was performed. Venography revealed extensive thrombosis of the femoral vein (Figure 2A) extending into the right external iliac vein. This was treated with catheter-directed pharmacomechanical thrombectomy.
Venography after this procedure showed significant improvement in venous blood flow (Figure 2B). However, stenosis of the right external iliac vein was also noted (Figure 2C) and was treated with balloon angioplasty (Figure 2D) followed by placement of a stent (14 × 40 mm).
In the immediate postprocedural period, there was marked reduction in swelling and normalization of skin color (Figure 3). The patient did not experience significant bleeding during or after the procedure. Treatment with intravenous unfractionated heparin was continued during the hospital stay, and he was discharged on warfarin with a therapeutic international normalized ratio. At a follow-up visit 3 months later, he was asymptomatic.
A RARE BUT SEVERE TYPE OF ACUTE DEEP VEIN THROMBOSIS
Phlegmasia cerulea dolens (painful cyanotic swollen leg) is a rare and severe form of acute deep vein thrombosis (DVT) characterized by marked limb pain, swelling, and blue discoloration.1 DVT is the most common cause of acute-onset unilateral leg pain, swelling, and skin discoloration.2
The differential diagnosis
The differential diagnosis includes infection (cellulitis, necrotizing fasciitis), compartment syndrome from limb injury, musculoskeletal conditions such as ruptured Baker cyst, venous stasis due to external compression (May-Thurner syndrome, iliac vein compression syndrome, pelvic tumor), acute limb ischemia from arterial obstruction, and complex regional pain syndrome (reflex sympathetic dystrophy).
Management recommendations
As in most cases of DVT, initial treatment of phlegmasia cerulea dolens involves systemic anticoagulation with heparin, elevation of the affected extremity, and fluid resuscitation if the patient is hypotensive. However, phlegmasia cerulea dolens is a major indication for catheter-directed thrombolysis,3,4 so an urgent vascular surgery or interventional cardiology consultation is also required. The American College of Chest Physicians recommends catheter-directed thrombolysis for acute DVT of the iliofemoral veins in patients with symptoms for less than 14 days, good functional capacity, and a life expectancy beyond 1 year.5 This intervention results in reduced incidence of postthrombotic syndrome and improved quality of life5,6 compared with anticoagulation therapy alone.
Who is at risk?
Risk factors for phlegmasia cerulea dolens include a history of malignancy, inherited or acquired thrombophilia, surgery, radiation therapy, trauma, placement of an inferior vena cava filter, and pregnancy. In our patient, the iliac vein stenosis most likely was the result of the radiation therapy he had undergone for prostate cancer.
Arterial stenosis is a well-known complication of radiation therapy and is associated with an increased risk of cardiovascular events.7,8 Radiation induces endothelial damage followed by proliferation of smooth muscle cells, resulting in luminal stenosis and thrombosis. At the cellular level, radiation leads to an acute increase in pro-inflammatory cytokines and endothelial adhesion molecules, causing the recruitment of inflammatory cells to radiation-exposed vessels and chronic activation of transcription factor NF-kappa B, leading to long-term inflammation and angiogenesis.9
Carotid, coronary, and iliac artery stenosis are known to occur around 10 years after radiation therapy to the head, neck, breast, and pelvis. Radiation-induced iliac vein stenosis is rare and can manifest as acute proximal DVT.
- Mumoli N, Invernizzi C, Luschi R, Carmignani G, Camaiti A, Cei M. Phlegmasia cerulea dolens. Circulation 2012; 125:1056–1057.
- Ely JW, Osheroff JA, Chambliss ML, Ebell MH. Approach to leg edema of unclear etiology. J Am Board Fam Med 2006; 19:148–160.
- Casey ET, Murad MH, Zumaeta-Garcia M, et al. Treatment of acute iliofemoral deep vein thrombosis. J Vasc Surg. 2012; 55:1463–1473.
- Chinsakchai K, Ten Duis K, Moll FL, de Borst GJ. Trends in management of phlegmasia cerulea dolens. Vasc Endovascular Surg 2011; 45:5–14.
- Kearon C, Kahn SR, Agnelli G, Goldhaber S, Raskob GE, Comerota AJ; American College of Chest Physicians. Antithrombotic therapy for venous thromboembolic disease: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines (8th Edition). Chest 2008; 133(suppl 6):454S–545S.
- Enden T, Haig Y, Kløw NE, et al; CaVenT Study Group. Long-term outcome after additional catheter-directed thrombolysis versus standard treatment for acute iliofemoral deep vein thrombosis (the CaVenT study): a randomised controlled trial. Lancet 2012; 379:31–38.
- Hooning MJ, Botma A, Aleman BM, et al. Long-term risk of cardiovascular disease in 10-year survivors of breast cancer. J Natl Cancer Inst 2007; 99:365–375.
- Weintraub NL, Jones WK, Manka D. Understanding radiation-induced vascular disease. J Am Coll Cardiol 2010; 55:1237–1239.
- Halle M, Gabrielsen A, Paulsson-Berne G, et al. Sustained inflammation due to nuclear factor-kappa B activation in irradiated human arteries. J Am Coll Cardiol 2010; 55:1227–1236.
- Mumoli N, Invernizzi C, Luschi R, Carmignani G, Camaiti A, Cei M. Phlegmasia cerulea dolens. Circulation 2012; 125:1056–1057.
- Ely JW, Osheroff JA, Chambliss ML, Ebell MH. Approach to leg edema of unclear etiology. J Am Board Fam Med 2006; 19:148–160.
- Casey ET, Murad MH, Zumaeta-Garcia M, et al. Treatment of acute iliofemoral deep vein thrombosis. J Vasc Surg. 2012; 55:1463–1473.
- Chinsakchai K, Ten Duis K, Moll FL, de Borst GJ. Trends in management of phlegmasia cerulea dolens. Vasc Endovascular Surg 2011; 45:5–14.
- Kearon C, Kahn SR, Agnelli G, Goldhaber S, Raskob GE, Comerota AJ; American College of Chest Physicians. Antithrombotic therapy for venous thromboembolic disease: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines (8th Edition). Chest 2008; 133(suppl 6):454S–545S.
- Enden T, Haig Y, Kløw NE, et al; CaVenT Study Group. Long-term outcome after additional catheter-directed thrombolysis versus standard treatment for acute iliofemoral deep vein thrombosis (the CaVenT study): a randomised controlled trial. Lancet 2012; 379:31–38.
- Hooning MJ, Botma A, Aleman BM, et al. Long-term risk of cardiovascular disease in 10-year survivors of breast cancer. J Natl Cancer Inst 2007; 99:365–375.
- Weintraub NL, Jones WK, Manka D. Understanding radiation-induced vascular disease. J Am Coll Cardiol 2010; 55:1237–1239.
- Halle M, Gabrielsen A, Paulsson-Berne G, et al. Sustained inflammation due to nuclear factor-kappa B activation in irradiated human arteries. J Am Coll Cardiol 2010; 55:1227–1236.
Abdominal pain under immunosuppressive conditions
A 69-year-old diabetic woman with stage 4 non–small-cell lung cancer presented with a 3-day history of abdominal pain and loss of appetite. She was being treated with corticosteroids for a brain metastasis.
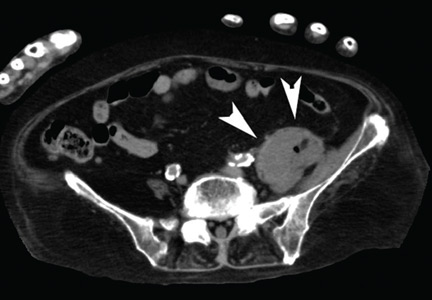
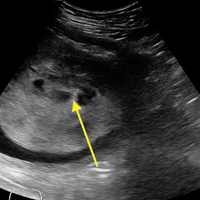
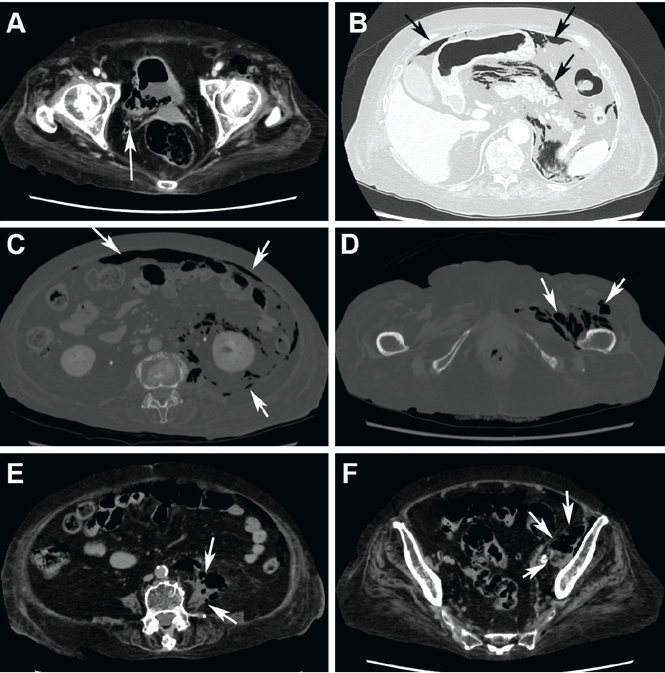
Computed tomography (CT) (Figure 1) revealed air within the bladder wall and lumen; diffuse air in the intraperitoneum and retroperitoneum; air distributed from the left iliopsoas muscle to the left femur that spread around the obturator muscle; air in the left ureter; and an abscess in the psoas major muscle extending to the ala of the ilium. A diagnosis of emphysematous cystitis complicated by extensive abdominal emphysema and abscess was made.
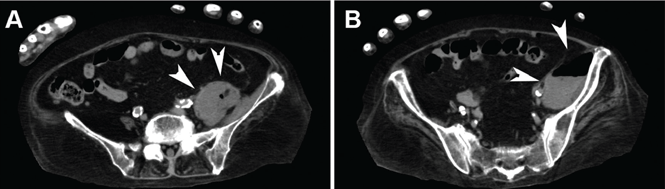
Blood cultures were negative, but urine cultures grew extended-spectrum beta-lactamase-producing Escherichia coli, which was sensitive to meropenem. Meropenem was given intravenously for 24 days and was stopped when levels of inflammatory markers improved and urine cultures were negative. However, on day 29, the patient developed a fever. Follow-up CT showed that the abscess in the psoas muscle had enlarged (Figure 2). We chose not to surgically drain the abscess because the patient had terminal lung cancer. The patient expired 6 days later, 35 days after her hospital admission.
EMPHYSEMATOUS CYSTITIS ASSOCIATED WITH A PSOAS MUSCLE ABSCESS
Emphysematous cystitis is an uncommon urinary tract infection characterized by air within the bladder wall and lumen that is caused by gas-producing pathogens.1,2 The disease is often found in elderly diabetic women. Treatment of emphysematous cystitis typically includes intravenous antibiotics, adequate bladder drainage, and, for diabetic patients, appropriate glycemic control.
Psoas muscle abscess is a collection of pus in the retroperitoneal space.3 It can be primary, caused by hematogenous spread from the site of an occult infection, or secondary, caused by contiguous spread from adjacent infected organs, including those of the urinary tract. Psoas muscle abscess associated with emphysematous cystitis, as in our patient, is rare. We have seen only one other report in the medical literature.4
TREATMENT
The treatment of psoas muscle abscess involves the use of broad-spectrum antibiotics and drainage.5 Small abscesses (less than 3.5 cm) can be controlled with antibiotics alone. Image-guided percutaneous drainage is a safe, minimally invasive option. Surgery is indicated for unsuccessful percutaneous drainage, loculated abscesses, and abscesses difficult to approach percutaneously, or when the underlying disease requires definitive surgical management.
As in our patient, the presence of additional comorbid immunosuppressive conditions2 such as lung cancer and treatment with corticosteroids can allow the infection to become widespread and life-threatening.
- Thomas AA, Lane BR, Thomas AZ, Remer EM, Campbell SC, Shoskes DA. Emphysematous cystitis: a review of 135 cases. BJU Int 2007; 100:17–20.
- Grupper M, Kravtsov A, Potasman I. Emphysematous cystitis: illustrative case report and review of the literature. Medicine (Baltimore) 2007; 86:47–53.
- Mallick IH, Thoufeeq MH, Rajendran TP. Iliopsoas abscesses. Postgrad Med J 2004; 80:459–462.
- Choi JK, Kwon JC. Bilateral psoas muscle abscess associated with emphysematous cystitis. Case Rep Med 2015; 2015:285652.
- Tabrizian P, Nguyen SQ, Greenstein A, Rajhbeharrysingh U, Divino CM. Management and treatment of iliopsoas abscess. Arch Surg 2009; 144:946–949.
A 69-year-old diabetic woman with stage 4 non–small-cell lung cancer presented with a 3-day history of abdominal pain and loss of appetite. She was being treated with corticosteroids for a brain metastasis.
Computed tomography (CT) (Figure 1) revealed air within the bladder wall and lumen; diffuse air in the intraperitoneum and retroperitoneum; air distributed from the left iliopsoas muscle to the left femur that spread around the obturator muscle; air in the left ureter; and an abscess in the psoas major muscle extending to the ala of the ilium. A diagnosis of emphysematous cystitis complicated by extensive abdominal emphysema and abscess was made.

Blood cultures were negative, but urine cultures grew extended-spectrum beta-lactamase-producing Escherichia coli, which was sensitive to meropenem. Meropenem was given intravenously for 24 days and was stopped when levels of inflammatory markers improved and urine cultures were negative. However, on day 29, the patient developed a fever. Follow-up CT showed that the abscess in the psoas muscle had enlarged (Figure 2). We chose not to surgically drain the abscess because the patient had terminal lung cancer. The patient expired 6 days later, 35 days after her hospital admission.

EMPHYSEMATOUS CYSTITIS ASSOCIATED WITH A PSOAS MUSCLE ABSCESS
Emphysematous cystitis is an uncommon urinary tract infection characterized by air within the bladder wall and lumen that is caused by gas-producing pathogens.1,2 The disease is often found in elderly diabetic women. Treatment of emphysematous cystitis typically includes intravenous antibiotics, adequate bladder drainage, and, for diabetic patients, appropriate glycemic control.
Psoas muscle abscess is a collection of pus in the retroperitoneal space.3 It can be primary, caused by hematogenous spread from the site of an occult infection, or secondary, caused by contiguous spread from adjacent infected organs, including those of the urinary tract. Psoas muscle abscess associated with emphysematous cystitis, as in our patient, is rare. We have seen only one other report in the medical literature.4
TREATMENT
The treatment of psoas muscle abscess involves the use of broad-spectrum antibiotics and drainage.5 Small abscesses (less than 3.5 cm) can be controlled with antibiotics alone. Image-guided percutaneous drainage is a safe, minimally invasive option. Surgery is indicated for unsuccessful percutaneous drainage, loculated abscesses, and abscesses difficult to approach percutaneously, or when the underlying disease requires definitive surgical management.
As in our patient, the presence of additional comorbid immunosuppressive conditions2 such as lung cancer and treatment with corticosteroids can allow the infection to become widespread and life-threatening.
A 69-year-old diabetic woman with stage 4 non–small-cell lung cancer presented with a 3-day history of abdominal pain and loss of appetite. She was being treated with corticosteroids for a brain metastasis.
Computed tomography (CT) (Figure 1) revealed air within the bladder wall and lumen; diffuse air in the intraperitoneum and retroperitoneum; air distributed from the left iliopsoas muscle to the left femur that spread around the obturator muscle; air in the left ureter; and an abscess in the psoas major muscle extending to the ala of the ilium. A diagnosis of emphysematous cystitis complicated by extensive abdominal emphysema and abscess was made.

Blood cultures were negative, but urine cultures grew extended-spectrum beta-lactamase-producing Escherichia coli, which was sensitive to meropenem. Meropenem was given intravenously for 24 days and was stopped when levels of inflammatory markers improved and urine cultures were negative. However, on day 29, the patient developed a fever. Follow-up CT showed that the abscess in the psoas muscle had enlarged (Figure 2). We chose not to surgically drain the abscess because the patient had terminal lung cancer. The patient expired 6 days later, 35 days after her hospital admission.

EMPHYSEMATOUS CYSTITIS ASSOCIATED WITH A PSOAS MUSCLE ABSCESS
Emphysematous cystitis is an uncommon urinary tract infection characterized by air within the bladder wall and lumen that is caused by gas-producing pathogens.1,2 The disease is often found in elderly diabetic women. Treatment of emphysematous cystitis typically includes intravenous antibiotics, adequate bladder drainage, and, for diabetic patients, appropriate glycemic control.
Psoas muscle abscess is a collection of pus in the retroperitoneal space.3 It can be primary, caused by hematogenous spread from the site of an occult infection, or secondary, caused by contiguous spread from adjacent infected organs, including those of the urinary tract. Psoas muscle abscess associated with emphysematous cystitis, as in our patient, is rare. We have seen only one other report in the medical literature.4
TREATMENT
The treatment of psoas muscle abscess involves the use of broad-spectrum antibiotics and drainage.5 Small abscesses (less than 3.5 cm) can be controlled with antibiotics alone. Image-guided percutaneous drainage is a safe, minimally invasive option. Surgery is indicated for unsuccessful percutaneous drainage, loculated abscesses, and abscesses difficult to approach percutaneously, or when the underlying disease requires definitive surgical management.
As in our patient, the presence of additional comorbid immunosuppressive conditions2 such as lung cancer and treatment with corticosteroids can allow the infection to become widespread and life-threatening.
- Thomas AA, Lane BR, Thomas AZ, Remer EM, Campbell SC, Shoskes DA. Emphysematous cystitis: a review of 135 cases. BJU Int 2007; 100:17–20.
- Grupper M, Kravtsov A, Potasman I. Emphysematous cystitis: illustrative case report and review of the literature. Medicine (Baltimore) 2007; 86:47–53.
- Mallick IH, Thoufeeq MH, Rajendran TP. Iliopsoas abscesses. Postgrad Med J 2004; 80:459–462.
- Choi JK, Kwon JC. Bilateral psoas muscle abscess associated with emphysematous cystitis. Case Rep Med 2015; 2015:285652.
- Tabrizian P, Nguyen SQ, Greenstein A, Rajhbeharrysingh U, Divino CM. Management and treatment of iliopsoas abscess. Arch Surg 2009; 144:946–949.
- Thomas AA, Lane BR, Thomas AZ, Remer EM, Campbell SC, Shoskes DA. Emphysematous cystitis: a review of 135 cases. BJU Int 2007; 100:17–20.
- Grupper M, Kravtsov A, Potasman I. Emphysematous cystitis: illustrative case report and review of the literature. Medicine (Baltimore) 2007; 86:47–53.
- Mallick IH, Thoufeeq MH, Rajendran TP. Iliopsoas abscesses. Postgrad Med J 2004; 80:459–462.
- Choi JK, Kwon JC. Bilateral psoas muscle abscess associated with emphysematous cystitis. Case Rep Med 2015; 2015:285652.
- Tabrizian P, Nguyen SQ, Greenstein A, Rajhbeharrysingh U, Divino CM. Management and treatment of iliopsoas abscess. Arch Surg 2009; 144:946–949.
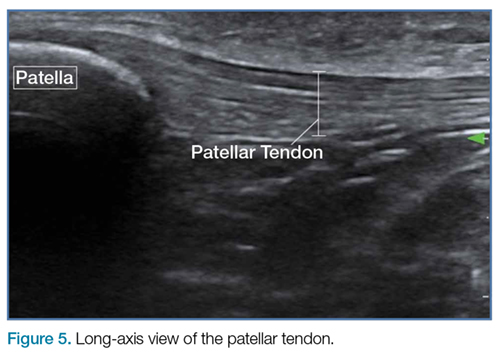
A Guide to Ultrasound of the Shoulder, Part 3: Interventional and Procedural Uses
Ultrasound has classically been marketed and used as a diagnostic tool. Radiologists, emergency physicians, and sports physicians used ultrasound units to rapidly and appropriately diagnose numerous injuries and disorders, in a timely and cost effective manner. Part 11 and Part 22 of this series showed how to use ultrasound in the shoulder for diagnosis and how to code and get reimbursed for its use.Ultrasound can also be used to help guide procedures and interventions performed to treat patients. Currently, more physicians are beginning to recognize the utility of this modality as an aid to interventional procedures.
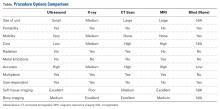
First-generation procedures use ultrasound to improve accuracy of joint, bursal, tendon, and muscular injections.3 Recent studies have shown a significant improvement in accuracy, outcomes, and patient satisfaction using ultrasound guidance for injections.3-12 Within the limitation of using a needle, second-generation procedures—hydrodissection of peripherally entrapped nerves, capsular distention, mechanical disruption of neovascularization, and needle fenestration or barbotage in chronic tendinopathy—try to simulate surgical objectives while minimizing tissue burden and other complications of surgery.3 More advanced procedures include needle fenestration/release of the carpal ligament in carpal tunnel syndrome and A1 pulley needle release in the setting of trigger finger.3 Innovative third-generation procedures involve the use of surgical tools such as hook blades under ultrasound guidance to perform surgical procedures. Surgeons are now improving already established percutaneous, arthroscopic, and open surgical procedures with ultrasound assistance.3 Aside from better guidance, reducing cost and improving surgeon comfort may be additional benefits of ultrasound assisted surgery.
Image-Guided Treatment Options
Prior to image guidance, palpation of surface anatomy helped physicians determine the anatomic placement of injections, incisions, or portals. Joints and bursas that do not have any inflammation or fluid can sometimes be difficult to identify or locate by palpation alone. Palpation-guided joint injections often miss their target and cause significant pain when the therapeutic agent is injected into a muscle, tendon, ligament, fat, or other tissue. Ultrasound-guided injections have proven to be more accurate and have better patient satisfaction when compared to blind injections.3-12
X-ray fluoroscopy has been the primary option for surgeons to assist in surgery. This is a natural modality for orthopedic surgeons; their primary use is for bone to help with fracture reduction and fixation as the bone, instrumentation, and fixation methods are usually radio-opaque. With the advancement in technology, many orthopedic surgeons are regularly using radiolucent fixation devices and working with soft tissue as opposed to bone. Fixation of tendons, ligaments, and muscles would be done using a large incision, palpation of the anatomy, then fixation or repair. Many surgeons began looking for ways to minimize the incisions. Turning to fluoroscopy, a traditional and well-used modality, was a natural progression. Guides and methods were developed to isolate insertions and drill placements. However, fluoroscopy is limited by its difficulty in changing planes and the large equipment required. Also, it is limited in its ability to image soft tissue.
Computed tomography (CT) scans and magnetic resonance imaging (MRI) are far better at imaging soft tissue but cannot be taken for use into the office or surgical suite. These modalities are also far more expensive and take up significant space.
Ultrasound Procedural Basics
Appropriate use of ultrasound still remains highly technician-dependent. Unlike other imaging modalities, ultrasound requires a higher skill level by the physician to implement the use of ultrasound and identification of pathology to treat these disease processes. However, this is no different from the use of arthroscopy or fluoroscopy to treat patients. Training is required, as well as an understanding of the ultrasound machine, anatomy, and sono-anatomy—identification of anatomy and pathology as shown by the ultrasound machine.2
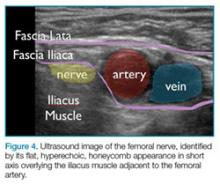
In ultrasound, the long axis refers to looking at a structure along its length, as in longitudinal. The short axis refers to evaluating a structure in cross-section, transverse, or along its shortest length. “In plane” refers to performing a procedure where the needle or object being used enters the ultrasound field along the plane of the transducer, allowing visualization of the majority of the needle as it crosses tissue planes. “Out of plane” has the needle entering perpendicular to the plane of the transducer, showing the needle on the monitor as a bright, hyperechoic dot. Some studies have suggested that novice ultrasonographers should start in a long axis view and use the in plane technique when injecting, as doing so may decrease time to identify the target and improve mean imaging quality during needle advancement.13
Anisotropy is the property of being directionally dependent. The ultrasound beam needs to be perpendicular to the structure being imaged to give the optimal image. When the beam hits a longitudinal structure like a needle at an angle <90°, the linear structure might reflect most of the beam away from the transducer. So when using a needle to localize or inject a specific area, maintaining the probe as close to perpendicular as possible with the needle will give a better image. New technology exists to better visualize needles even at high acuity angles by using a multi-beam processing algorithm, which can significantly aid the physician without the need for specialized needles.
Despite better technology, advance planning is key to a successful procedure. Positioning the patient and ultrasound machine in a manner that is comfortable and makes the desired target accessible while being able to visualize the ultrasound monitor comes first. Identifying the target, mapping the needle trajectory using depth markings, and scanning for nerves, vessels, and other structures that may be damaged along the needle path comes next. Using the in plane ultrasound technique with color Doppler and the nerve contrast setting can ensure that the physician has placed the therapeutic agent to the proper location while avoiding any nerves, arteries, or veins. Marking the borders of the ultrasound probe and needle entry site can be helpful to return to the same area after sterile preparation is done. As in any procedure, sterile technique is paramount. Sterile technique considerations may include using sterile gloves and a probe cover with sterile gel, cleaning the area thoroughly, planning the needle entry point 3 cm to 5 cm away from the probe, and maintaining a dry and gel-free needle entry.14-15 The probe should be sterilized between patients to avoid cross-contamination; note that certain solutions like alcohol or ethyl chloride can damage the transducer.14-15 However, simple injections do not require such stringent standards when simple sterile technique is observed by cleaning and then never touching the cleaned area again except with the needle to avoid contamination. Also, ethyl chloride has been found to not contaminate a sterile site and can be used safely to anesthetize the skin.
Ultrasound-Guided Procedures
Many injectable therapeutic options exist as interventions. Cortisone, hyaluronic acid, platelet-rich plasma (PRP), stem cells/bone marrow concentrate (BMC), amniotic fluid, prolotherapy, and saline are now commonly used.16-17 A meta-analysis of the literature assessing the accuracy of ultrasound-guided shoulder girdle injections vs a landmark-guided injection was done in 2015.18 It showed that for the acromioclavicular joint, accuracy was 93.6% vs 68.2% (P < .0001), based on single studies. The accuracy of ultrasound vs a landmark-guided injection was 65% vs 70% for the subacromial space (P > .05); 86.7% vs 26.7% for the biceps tendon sheath (P < .05); and 92.5% vs 72.5% for the glenohumeral joint (P = .025).18
With cortisone, injecting into muscle, ligament, or tendons could potentially harm the tissue or cause worsening of the disease process.19-20 With the advent of orthobiologics, injecting into these structures is now desirable, instead of a potential complication.19-20 Ultrasound has become even more important to the accurate delivery of these therapies to the disease locations. Multiple studies using leukocyte-poor PRP for osteoarthritis show significant differences in pain scores.21-23 Peerbooms and colleagues24,25 also showed that PRP reduced pain and increased function compared to cortisone injections for lateral epicondylitis in 1- and 2-year double-blind randomized controlled trials. Centeno and colleagues26 performed a prospective, multi-site registry study on 102 patients with symptomatic osteoarthritis and/or rotator cuff tears that were injected with bone marrow concentrate. There was a statistically significant improvement in Disabilities of the Arm, Shoulder and Hand (DASH) scores from 36.1 to 17.1 (P < .001) and numeric pain scores improved from 4.3 to 2.4 (P < .001).
By being able to see the pathology, like a hypoechoic region in a tendon, ligament, or muscle, the physician can reliably place the therapeutic agent into the precise location. Also, adjacent para-tendon or para-ligament injections allow for in-season athletes to get some relief from symptoms while allowing to return to play quickly; injections into muscle, ligament, or tendon can damage the structure and require days or weeks of rest, while para-tendon and para-ligament injections are far less painful.
Second-generation techniques have provided patients with great options that can help avoid surgery. Calcific tendonitis appears brightly hyperechoic on ultrasound and is easily identified. The physician can attempt to break up the calcium by fenestration or barbotage of the calcium. The same can be accomplished by injecting the density with PRP or stem cells. If the calcium is soft or “toothpaste-like,” the negative pressure will make it easy to aspirate it into the syringe. A 2-year, longitudinal prospective study of 121 patients demonstrated that visual analog score (VAS) pain scores and size of calcium significantly decreased with ultrasound-guided percutaneous needle lavage; 89% of patients were pain-free at 1-year follow-up.27 Moreover, a randomized controlled trial of 48 patients comparing needle lavage vs subacromial steroid injection showed statistically significant radiographic and clinically better outcomes with the needle lavage group at the 1-year mark.28
The Tenex procedure is a novel technique that uses ultrasonic energy to fenestrate diseased tendon tissue. It also can be used to break up calcific deposits. After the Tenex probe is guided to the diseased tendon/calcium, the TX-1 tip oscillates at the speed of sound, fenestrating/cutting through the tendon or calcium while lavaging the tendon with saline. Multiple prospective, noncontrolled studies done in common extensor, patellar, and rotator cuff tendinopathy have demonstrated good to excellent improvements in pain scores with the Tenex procedure.29-31
Ultrasound is extremely useful in the treatment of adhesive capsulitis.32 The posterior glenohumeral capsule can be distended using a large volume (60 cc) of saline to loosen adhesions in preparation for manipulation. Because the manipulation can be an extremely painful procedure, ultrasound can be used to perform an inter-scalene block for regional anesthesia prior to the procedure. In 2014, Park and colleagues33 performed a randomized prospective trial that showed that capsular distension followed by manipulation was more effective than cortisone injection alone for the treatment of adhesive capsulitis.Ultrasound guidance was found to be just as efficacious as fluoroscopy in a randomized controlled trial in 2014; the authors noted that ultrasound does not expose the patient or clinician to radiation and can be done in office.34
Currently, techniques to perform ultrasound-guided percutaneous tenotomies of the long head of the biceps tendon using hook blades are being studied.35
Ultrasound-Assisted Surgery
Ultrasound has been a boon to surgeons who perform minimally invasive procedures. It is far less cumbersome than classic fluoroscopy. Fluoroscopy requires the use of heavy lead aprons by the surgeons. Combining this with the impervious gowns and hot lights, the surgeons’ comfort level is severely sacrificed. When having to do many long surgeries in a row, this situation can take a toll on the surgeons’ endurance and strength. Improving the comfort of the surgeon is not the primary goal of surgery, but can significantly help our ability to do a better job.
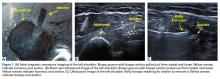
Ultrasound allows the surgeon to localize any superficial foreign objects, especially with radiolucent objects like fragments of glass. Small glass fragments or pieces of wood have always been extremely difficult to remove. X-rays cannot localize these objects, so getting a proper orientation is difficult. MRI and CT scans easily identify these types of foreign objects, but cannot be used intraoperatively (Figure 1A). Often, these objects cannot be felt and therefore require a large dissection. The objects may encapsulate and be easily confused with other soft tissues.
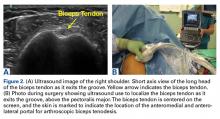
By using the ultrasound intraoperatively, the surgeon can identify the exact position of the biceps tendon (medial/lateral) and where it lies just below the groove and above the pectoralis major (superior/inferior) (Figure 2A).
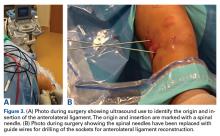
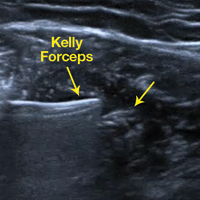
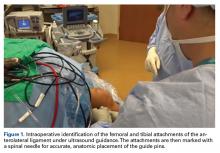
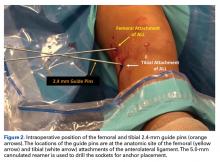
Reconstruction of ligaments is another ideal use of ultrasound. Surface anatomy cannot always tell the exact location of a ligament or tendon insertion. The best example of this is the anterolateral ligament (ALL). Identification of the lateral epicondyle of the femur and anatomic insertion of the ALL can be difficult in some patients. Ultrasound can be used to identify the origin and insertion of the ALL during surgery under sterile conditions (see page 418). A spinal needle can be placed under direct vision with an in-plane ultrasound guidance over the bony insertion (Figure 3A). A percutaneous incision is made.
This technique is also used by the senior author (AMH) to repair, reconstruct, or internally brace the medial collateral ligament, medial patellofemoral ligament, and lateral collateral ligament. This technique is ideally suited to superficial ligament and tendon reattachment, reconstruction, or internal bracing. The knee, ankle, and elbow superficial ligaments are especially amenable to this easy, percutaneous technique.
Conclusion
Ultrasound is quickly becoming a popular imaging modality due to its simplicity, portability, and cost efficiency. Its use as a diagnostic tool is widely known. As an adjunct for procedures and interventions, its advantages over larger, more expensive modalities such as fluoroscopy, CT, or MRI make it stand out. Ultrasound is not the perfect solution to all problems, but it is clearly a technology that is gaining traction. Ultrasound is another imaging modality and tool that physicians and surgeons can use to improve their patients’ treatment.
1. Hirahara AM, Panero AJ. A guide to ultrasound of the shoulder, part 1: coding and reimbursement. Am J Orthop. 2016;45(3):176-182.
2. Panero AJ, Hirahara AM. A guide to ultrasound of the shoulder, part 2: the diagnostic evaluation. Am J Orthop. 2016; 45(4):233-238.
3. Finnoff JT, Hall MM, Adams E, et al. American Medical Society for Sports Medicine (AMSSM) position statement: Interventional musculoskeletal ultrasound in sports medicine. Br J Sports Med. 2015;49(3):145-150.
4. Sivan M, Brown J, Brennan S, Bhakta B. A one-stop approach to the management of soft tissue and degenerative musculoskeletal conditions using clinic-based ultrasonography. Musculoskeletal Care. 2011;9(2):63-68.
5. Eustace J, Brophy D, Gibney R, Bresnihan B, FitzGerald O. Comparison of the accuracy of steroid placement with clinical outcome in patients with shoulder symptoms. Ann Rheum Dis. 1997;56(1):59-63.
6. Partington P, Broome G. Diagnostic injection around the shoulder: Hit and miss? A cadaveric study of injection accuracy. J Shoulder Elbow Surg. 1998;7(2):147-150.
7. Rutten M, Maresch B, Jager G, de Waal Malefijt M. Injection of the subacromial-subdeltoid bursa: Blind or ultrasound-guided? Acta Orthop. 2007;78(2):254-257.
8. Kang M, Rizio L, Prybicien M, Middlemas D, Blacksin M. The accuracy of subacromial corticosteroid injections: A comparison of multiple methods. J Shoulder Elbow Surg. 2008;17(1 Suppl):61S-66S.
9. Yamakado K. The targeting accuracy of subacromial injection to the shoulder: An arthrographic evaluation. Arthroscopy. 2002;19(8):887-891.
10. Henkus HE, Cobben M, Coerkamp E, Nelissen R, van Arkel E. The accuracy of subacromial injections: A prospective randomized magnetic resonance imaging study. Arthroscopy. 2006;22(3):277-282.
11. Sethi P, El Attrache N. Accuracy of intra-articular injection of the glenohumeral joint: A cadaveric study. Orthopedics. 2006;29(2):149-152.
12. Naredo E, Cabero F, Beneyto P, et al. A randomized comparative study of short term response to blind injection versus sonographic-guided injection of local corticosteroids in patients with painful shoulder. J Rheumatol. 2004;31(2):308-314.
13. Speer M, McLennan N, Nixon C. Novice learner in-plane ultrasound imaging: which visualization technique? Reg Anesth Pain Med. 2013;38(4):350-352.
14. Marhofer P, Schebesta K, Marhofer D. [Hygiene aspects in ultrasound-guided regional anesthesia]. Anaesthesist. 2016;65(7):492-498.
15. Sherman T, Ferguson J, Davis W, Russo M, Argintar E. Does the use of ultrasound affect contamination of musculoskeletal injection sites? Clin Orthop Relat Res. 2015;473(1):351-357.
16. Bashir J, Panero AJ, Sherman AL. The emerging use of platelet-rich plasma in musculoskeletal medicine. J Am Osteopath Assoc. 2015;115(1):23-31.
17. Royall NA, Farrin E, Bahner DP, Stanislaw PA. Ultrasound-assisted musculoskeletal procedures: A practical overview of current literature. World J Orthop. 2011;2(7):57-66.
18. Aly AR, Rajasekaran S, Ashworth N. Ultrasound-guided shoulder girdle injections are more accurate and more effective than landmark-guided injections: a systematic review and meta-analysis. Br J Sports Med. 2015;49(16):1042-1049.
19. Maman E, Yehuda C, Pritsch T, et al. Detrimental effect of repeated and single subacromial corticosteroid injections on the intact and injured rotator cuff: A biomechanical and imaging study in rats. Am J Sports Med. 2016;44(1):177-182.
20. Gautam VK, Verma S, Batra S, Bhatnagar N, Arora S. Platelet-rich plasma versus corticosteroid injection for recalcitrant lateral epicondylitis: clinical and ultrasonographic evaluation. J Orthop Surg (Hong Kong). 2015;23(1):1-5.
21. Patel S, Dhillon MS, Aggarwal S, Marwaha N, Jain A. Treatment with platelet-rich plasma is more effective than placebo for knee osteoarthritis: a prospective, double-blind, randomized trial. Am J Sports Med. 2013;41(2):356-364.
22. Cerza F, Carni S, Carcangiu A, et al. Comparison between hyaluronic acid and platelet-rich plasma, intra-articular infiltration in the treatment of gonarthrosis. Am J Sports Med. 2012;40(12):2822-2827.
23. Spakova T, Rosocha J, Lacko M, Harvanova D, Gharaibeh A. Treatment of knee joint osteoarthritis with autologous platelet-rich plasma in comparison with hyaluronic acid. Am J Phys Med Rehabil. 2012;91(5):411-417.
24. Peerbooms JC, Sluimer J, Brujin DJ, Gosens T. Positive effects of an autologous platelet concentrate in lateral epicondylitis in a double-blind randomized controlled trial: platelet-rich plasma versus corticosteroid injection with a 1-year follow-up. Am J Sports Med. 2010;38(2):255-262.
25. Gosens T, Peerbooms JC, van Laar W, den Oudsten BL. Ongoing positive effects of platelet-rich plasma versus corticosteroid injection in lateral epicondylitis: a double-blind randomized controlled trial with a 2-year follow-up. Am J Sports Med. 2011;39(6):1200-1208.
26. Centeno CJ, Al-Sayegh H, Bashir J, Goodyear S, Freeman MD. A prospective multi-site registry study of a specific protocol of autologous bone marrow concentrate for the treatment of shoulder rotator cuff tears and osteoarthritis. J Pain Res. 2015;8:269-276.
27. Del Castillo-Gonzalez F, Ramos-Alvarez JJ, Rodriguez-Fabian G, Gonzalez-Perez J, Calderon-Montero J. Treatment of the calcific tendinopathy of the rotator cuff by ultrasound-guided percutaneous needle lavage. Two years prospective study. Muscles Ligaments Tendons J. 2015;4(4):407-412.
28. De Witte PB, Selten JW, Navas A, et al. Calcific tendinitis of the rotator cuff: a randomized controlled trial of ultrasound-guided needling and lavage versus subacromial corticosteroids. Am J Sports Med. 2013;41(7):1665-1673.
29. Koh J, Mohan P, Morrey B, et al. Fasciotomy and surgical tenotomy for recalcitrant lateral elbow tendinopathy: early clinical experience with a novel device for minimally invasive percutaneous microresection. Am J Sports Med. 2013;41(3):636-644.
30. Elattrache N, Morrey B. Percutaneous ultrasonic tenotomy as a treatment for chronic patellar tendinopathy–Jumper’s knee. Oper Tech Orthop. 2013;23(2):98-103
31. Patel MM. A novel treatment for refractory plantar fasciitis. Am J Orthop. 2015;444(3):107-110.
32. Harris G, Bou-Haidar P, Harris C. Adhesive capsulitis: Review of imaging and treatment. J Med Imaging Radiat Oncol. 2013;57:633-643.
33. Park SW, Lee HS, Kim JH. The effectiveness of intensive mobilization techniques combined with capsular distention for adhesive capsulitis of the shoulder. J Phys Ther Sci. 2014;26(11):1776-1770.
34. Bae JH, Park YS, Chang HJ, et al. Randomized controlled trial for efficacy of capsular distension for adhesive capsulitis: Fluoroscopy-guided anterior versus ultrasonography-guided posterolateral approach. Ann Rehabil Med. 2014;38(3):360-368.
35. Aly AR, Rajasekaran S, Mohamed A, Beavis C, Obaid H. Feasibility of ultrasound-guided percutaneous tenotomy of long head of the biceps tendon–A pilot cadaveric study. J Clin Ultrasound. 2015;43(6):361-366.
Ultrasound has classically been marketed and used as a diagnostic tool. Radiologists, emergency physicians, and sports physicians used ultrasound units to rapidly and appropriately diagnose numerous injuries and disorders, in a timely and cost effective manner. Part 11 and Part 22 of this series showed how to use ultrasound in the shoulder for diagnosis and how to code and get reimbursed for its use.Ultrasound can also be used to help guide procedures and interventions performed to treat patients. Currently, more physicians are beginning to recognize the utility of this modality as an aid to interventional procedures.
First-generation procedures use ultrasound to improve accuracy of joint, bursal, tendon, and muscular injections.3 Recent studies have shown a significant improvement in accuracy, outcomes, and patient satisfaction using ultrasound guidance for injections.3-12 Within the limitation of using a needle, second-generation procedures—hydrodissection of peripherally entrapped nerves, capsular distention, mechanical disruption of neovascularization, and needle fenestration or barbotage in chronic tendinopathy—try to simulate surgical objectives while minimizing tissue burden and other complications of surgery.3 More advanced procedures include needle fenestration/release of the carpal ligament in carpal tunnel syndrome and A1 pulley needle release in the setting of trigger finger.3 Innovative third-generation procedures involve the use of surgical tools such as hook blades under ultrasound guidance to perform surgical procedures. Surgeons are now improving already established percutaneous, arthroscopic, and open surgical procedures with ultrasound assistance.3 Aside from better guidance, reducing cost and improving surgeon comfort may be additional benefits of ultrasound assisted surgery.
Image-Guided Treatment Options
Prior to image guidance, palpation of surface anatomy helped physicians determine the anatomic placement of injections, incisions, or portals. Joints and bursas that do not have any inflammation or fluid can sometimes be difficult to identify or locate by palpation alone. Palpation-guided joint injections often miss their target and cause significant pain when the therapeutic agent is injected into a muscle, tendon, ligament, fat, or other tissue. Ultrasound-guided injections have proven to be more accurate and have better patient satisfaction when compared to blind injections.3-12
X-ray fluoroscopy has been the primary option for surgeons to assist in surgery. This is a natural modality for orthopedic surgeons; their primary use is for bone to help with fracture reduction and fixation as the bone, instrumentation, and fixation methods are usually radio-opaque. With the advancement in technology, many orthopedic surgeons are regularly using radiolucent fixation devices and working with soft tissue as opposed to bone. Fixation of tendons, ligaments, and muscles would be done using a large incision, palpation of the anatomy, then fixation or repair. Many surgeons began looking for ways to minimize the incisions. Turning to fluoroscopy, a traditional and well-used modality, was a natural progression. Guides and methods were developed to isolate insertions and drill placements. However, fluoroscopy is limited by its difficulty in changing planes and the large equipment required. Also, it is limited in its ability to image soft tissue.
Computed tomography (CT) scans and magnetic resonance imaging (MRI) are far better at imaging soft tissue but cannot be taken for use into the office or surgical suite. These modalities are also far more expensive and take up significant space.
Ultrasound Procedural Basics
Appropriate use of ultrasound still remains highly technician-dependent. Unlike other imaging modalities, ultrasound requires a higher skill level by the physician to implement the use of ultrasound and identification of pathology to treat these disease processes. However, this is no different from the use of arthroscopy or fluoroscopy to treat patients. Training is required, as well as an understanding of the ultrasound machine, anatomy, and sono-anatomy—identification of anatomy and pathology as shown by the ultrasound machine.2
In ultrasound, the long axis refers to looking at a structure along its length, as in longitudinal. The short axis refers to evaluating a structure in cross-section, transverse, or along its shortest length. “In plane” refers to performing a procedure where the needle or object being used enters the ultrasound field along the plane of the transducer, allowing visualization of the majority of the needle as it crosses tissue planes. “Out of plane” has the needle entering perpendicular to the plane of the transducer, showing the needle on the monitor as a bright, hyperechoic dot. Some studies have suggested that novice ultrasonographers should start in a long axis view and use the in plane technique when injecting, as doing so may decrease time to identify the target and improve mean imaging quality during needle advancement.13
Anisotropy is the property of being directionally dependent. The ultrasound beam needs to be perpendicular to the structure being imaged to give the optimal image. When the beam hits a longitudinal structure like a needle at an angle <90°, the linear structure might reflect most of the beam away from the transducer. So when using a needle to localize or inject a specific area, maintaining the probe as close to perpendicular as possible with the needle will give a better image. New technology exists to better visualize needles even at high acuity angles by using a multi-beam processing algorithm, which can significantly aid the physician without the need for specialized needles.
Despite better technology, advance planning is key to a successful procedure. Positioning the patient and ultrasound machine in a manner that is comfortable and makes the desired target accessible while being able to visualize the ultrasound monitor comes first. Identifying the target, mapping the needle trajectory using depth markings, and scanning for nerves, vessels, and other structures that may be damaged along the needle path comes next. Using the in plane ultrasound technique with color Doppler and the nerve contrast setting can ensure that the physician has placed the therapeutic agent to the proper location while avoiding any nerves, arteries, or veins. Marking the borders of the ultrasound probe and needle entry site can be helpful to return to the same area after sterile preparation is done. As in any procedure, sterile technique is paramount. Sterile technique considerations may include using sterile gloves and a probe cover with sterile gel, cleaning the area thoroughly, planning the needle entry point 3 cm to 5 cm away from the probe, and maintaining a dry and gel-free needle entry.14-15 The probe should be sterilized between patients to avoid cross-contamination; note that certain solutions like alcohol or ethyl chloride can damage the transducer.14-15 However, simple injections do not require such stringent standards when simple sterile technique is observed by cleaning and then never touching the cleaned area again except with the needle to avoid contamination. Also, ethyl chloride has been found to not contaminate a sterile site and can be used safely to anesthetize the skin.
Ultrasound-Guided Procedures
Many injectable therapeutic options exist as interventions. Cortisone, hyaluronic acid, platelet-rich plasma (PRP), stem cells/bone marrow concentrate (BMC), amniotic fluid, prolotherapy, and saline are now commonly used.16-17 A meta-analysis of the literature assessing the accuracy of ultrasound-guided shoulder girdle injections vs a landmark-guided injection was done in 2015.18 It showed that for the acromioclavicular joint, accuracy was 93.6% vs 68.2% (P < .0001), based on single studies. The accuracy of ultrasound vs a landmark-guided injection was 65% vs 70% for the subacromial space (P > .05); 86.7% vs 26.7% for the biceps tendon sheath (P < .05); and 92.5% vs 72.5% for the glenohumeral joint (P = .025).18
With cortisone, injecting into muscle, ligament, or tendons could potentially harm the tissue or cause worsening of the disease process.19-20 With the advent of orthobiologics, injecting into these structures is now desirable, instead of a potential complication.19-20 Ultrasound has become even more important to the accurate delivery of these therapies to the disease locations. Multiple studies using leukocyte-poor PRP for osteoarthritis show significant differences in pain scores.21-23 Peerbooms and colleagues24,25 also showed that PRP reduced pain and increased function compared to cortisone injections for lateral epicondylitis in 1- and 2-year double-blind randomized controlled trials. Centeno and colleagues26 performed a prospective, multi-site registry study on 102 patients with symptomatic osteoarthritis and/or rotator cuff tears that were injected with bone marrow concentrate. There was a statistically significant improvement in Disabilities of the Arm, Shoulder and Hand (DASH) scores from 36.1 to 17.1 (P < .001) and numeric pain scores improved from 4.3 to 2.4 (P < .001).
By being able to see the pathology, like a hypoechoic region in a tendon, ligament, or muscle, the physician can reliably place the therapeutic agent into the precise location. Also, adjacent para-tendon or para-ligament injections allow for in-season athletes to get some relief from symptoms while allowing to return to play quickly; injections into muscle, ligament, or tendon can damage the structure and require days or weeks of rest, while para-tendon and para-ligament injections are far less painful.
Second-generation techniques have provided patients with great options that can help avoid surgery. Calcific tendonitis appears brightly hyperechoic on ultrasound and is easily identified. The physician can attempt to break up the calcium by fenestration or barbotage of the calcium. The same can be accomplished by injecting the density with PRP or stem cells. If the calcium is soft or “toothpaste-like,” the negative pressure will make it easy to aspirate it into the syringe. A 2-year, longitudinal prospective study of 121 patients demonstrated that visual analog score (VAS) pain scores and size of calcium significantly decreased with ultrasound-guided percutaneous needle lavage; 89% of patients were pain-free at 1-year follow-up.27 Moreover, a randomized controlled trial of 48 patients comparing needle lavage vs subacromial steroid injection showed statistically significant radiographic and clinically better outcomes with the needle lavage group at the 1-year mark.28
The Tenex procedure is a novel technique that uses ultrasonic energy to fenestrate diseased tendon tissue. It also can be used to break up calcific deposits. After the Tenex probe is guided to the diseased tendon/calcium, the TX-1 tip oscillates at the speed of sound, fenestrating/cutting through the tendon or calcium while lavaging the tendon with saline. Multiple prospective, noncontrolled studies done in common extensor, patellar, and rotator cuff tendinopathy have demonstrated good to excellent improvements in pain scores with the Tenex procedure.29-31
Ultrasound is extremely useful in the treatment of adhesive capsulitis.32 The posterior glenohumeral capsule can be distended using a large volume (60 cc) of saline to loosen adhesions in preparation for manipulation. Because the manipulation can be an extremely painful procedure, ultrasound can be used to perform an inter-scalene block for regional anesthesia prior to the procedure. In 2014, Park and colleagues33 performed a randomized prospective trial that showed that capsular distension followed by manipulation was more effective than cortisone injection alone for the treatment of adhesive capsulitis.Ultrasound guidance was found to be just as efficacious as fluoroscopy in a randomized controlled trial in 2014; the authors noted that ultrasound does not expose the patient or clinician to radiation and can be done in office.34
Currently, techniques to perform ultrasound-guided percutaneous tenotomies of the long head of the biceps tendon using hook blades are being studied.35
Ultrasound-Assisted Surgery
Ultrasound has been a boon to surgeons who perform minimally invasive procedures. It is far less cumbersome than classic fluoroscopy. Fluoroscopy requires the use of heavy lead aprons by the surgeons. Combining this with the impervious gowns and hot lights, the surgeons’ comfort level is severely sacrificed. When having to do many long surgeries in a row, this situation can take a toll on the surgeons’ endurance and strength. Improving the comfort of the surgeon is not the primary goal of surgery, but can significantly help our ability to do a better job.
Ultrasound allows the surgeon to localize any superficial foreign objects, especially with radiolucent objects like fragments of glass. Small glass fragments or pieces of wood have always been extremely difficult to remove. X-rays cannot localize these objects, so getting a proper orientation is difficult. MRI and CT scans easily identify these types of foreign objects, but cannot be used intraoperatively (Figure 1A). Often, these objects cannot be felt and therefore require a large dissection. The objects may encapsulate and be easily confused with other soft tissues.
By using the ultrasound intraoperatively, the surgeon can identify the exact position of the biceps tendon (medial/lateral) and where it lies just below the groove and above the pectoralis major (superior/inferior) (Figure 2A).
Reconstruction of ligaments is another ideal use of ultrasound. Surface anatomy cannot always tell the exact location of a ligament or tendon insertion. The best example of this is the anterolateral ligament (ALL). Identification of the lateral epicondyle of the femur and anatomic insertion of the ALL can be difficult in some patients. Ultrasound can be used to identify the origin and insertion of the ALL during surgery under sterile conditions (see page 418). A spinal needle can be placed under direct vision with an in-plane ultrasound guidance over the bony insertion (Figure 3A). A percutaneous incision is made.
This technique is also used by the senior author (AMH) to repair, reconstruct, or internally brace the medial collateral ligament, medial patellofemoral ligament, and lateral collateral ligament. This technique is ideally suited to superficial ligament and tendon reattachment, reconstruction, or internal bracing. The knee, ankle, and elbow superficial ligaments are especially amenable to this easy, percutaneous technique.
Conclusion
Ultrasound is quickly becoming a popular imaging modality due to its simplicity, portability, and cost efficiency. Its use as a diagnostic tool is widely known. As an adjunct for procedures and interventions, its advantages over larger, more expensive modalities such as fluoroscopy, CT, or MRI make it stand out. Ultrasound is not the perfect solution to all problems, but it is clearly a technology that is gaining traction. Ultrasound is another imaging modality and tool that physicians and surgeons can use to improve their patients’ treatment.
Ultrasound has classically been marketed and used as a diagnostic tool. Radiologists, emergency physicians, and sports physicians used ultrasound units to rapidly and appropriately diagnose numerous injuries and disorders, in a timely and cost effective manner. Part 11 and Part 22 of this series showed how to use ultrasound in the shoulder for diagnosis and how to code and get reimbursed for its use.Ultrasound can also be used to help guide procedures and interventions performed to treat patients. Currently, more physicians are beginning to recognize the utility of this modality as an aid to interventional procedures.
First-generation procedures use ultrasound to improve accuracy of joint, bursal, tendon, and muscular injections.3 Recent studies have shown a significant improvement in accuracy, outcomes, and patient satisfaction using ultrasound guidance for injections.3-12 Within the limitation of using a needle, second-generation procedures—hydrodissection of peripherally entrapped nerves, capsular distention, mechanical disruption of neovascularization, and needle fenestration or barbotage in chronic tendinopathy—try to simulate surgical objectives while minimizing tissue burden and other complications of surgery.3 More advanced procedures include needle fenestration/release of the carpal ligament in carpal tunnel syndrome and A1 pulley needle release in the setting of trigger finger.3 Innovative third-generation procedures involve the use of surgical tools such as hook blades under ultrasound guidance to perform surgical procedures. Surgeons are now improving already established percutaneous, arthroscopic, and open surgical procedures with ultrasound assistance.3 Aside from better guidance, reducing cost and improving surgeon comfort may be additional benefits of ultrasound assisted surgery.
Image-Guided Treatment Options
Prior to image guidance, palpation of surface anatomy helped physicians determine the anatomic placement of injections, incisions, or portals. Joints and bursas that do not have any inflammation or fluid can sometimes be difficult to identify or locate by palpation alone. Palpation-guided joint injections often miss their target and cause significant pain when the therapeutic agent is injected into a muscle, tendon, ligament, fat, or other tissue. Ultrasound-guided injections have proven to be more accurate and have better patient satisfaction when compared to blind injections.3-12
X-ray fluoroscopy has been the primary option for surgeons to assist in surgery. This is a natural modality for orthopedic surgeons; their primary use is for bone to help with fracture reduction and fixation as the bone, instrumentation, and fixation methods are usually radio-opaque. With the advancement in technology, many orthopedic surgeons are regularly using radiolucent fixation devices and working with soft tissue as opposed to bone. Fixation of tendons, ligaments, and muscles would be done using a large incision, palpation of the anatomy, then fixation or repair. Many surgeons began looking for ways to minimize the incisions. Turning to fluoroscopy, a traditional and well-used modality, was a natural progression. Guides and methods were developed to isolate insertions and drill placements. However, fluoroscopy is limited by its difficulty in changing planes and the large equipment required. Also, it is limited in its ability to image soft tissue.
Computed tomography (CT) scans and magnetic resonance imaging (MRI) are far better at imaging soft tissue but cannot be taken for use into the office or surgical suite. These modalities are also far more expensive and take up significant space.
Ultrasound Procedural Basics
Appropriate use of ultrasound still remains highly technician-dependent. Unlike other imaging modalities, ultrasound requires a higher skill level by the physician to implement the use of ultrasound and identification of pathology to treat these disease processes. However, this is no different from the use of arthroscopy or fluoroscopy to treat patients. Training is required, as well as an understanding of the ultrasound machine, anatomy, and sono-anatomy—identification of anatomy and pathology as shown by the ultrasound machine.2
In ultrasound, the long axis refers to looking at a structure along its length, as in longitudinal. The short axis refers to evaluating a structure in cross-section, transverse, or along its shortest length. “In plane” refers to performing a procedure where the needle or object being used enters the ultrasound field along the plane of the transducer, allowing visualization of the majority of the needle as it crosses tissue planes. “Out of plane” has the needle entering perpendicular to the plane of the transducer, showing the needle on the monitor as a bright, hyperechoic dot. Some studies have suggested that novice ultrasonographers should start in a long axis view and use the in plane technique when injecting, as doing so may decrease time to identify the target and improve mean imaging quality during needle advancement.13
Anisotropy is the property of being directionally dependent. The ultrasound beam needs to be perpendicular to the structure being imaged to give the optimal image. When the beam hits a longitudinal structure like a needle at an angle <90°, the linear structure might reflect most of the beam away from the transducer. So when using a needle to localize or inject a specific area, maintaining the probe as close to perpendicular as possible with the needle will give a better image. New technology exists to better visualize needles even at high acuity angles by using a multi-beam processing algorithm, which can significantly aid the physician without the need for specialized needles.
Despite better technology, advance planning is key to a successful procedure. Positioning the patient and ultrasound machine in a manner that is comfortable and makes the desired target accessible while being able to visualize the ultrasound monitor comes first. Identifying the target, mapping the needle trajectory using depth markings, and scanning for nerves, vessels, and other structures that may be damaged along the needle path comes next. Using the in plane ultrasound technique with color Doppler and the nerve contrast setting can ensure that the physician has placed the therapeutic agent to the proper location while avoiding any nerves, arteries, or veins. Marking the borders of the ultrasound probe and needle entry site can be helpful to return to the same area after sterile preparation is done. As in any procedure, sterile technique is paramount. Sterile technique considerations may include using sterile gloves and a probe cover with sterile gel, cleaning the area thoroughly, planning the needle entry point 3 cm to 5 cm away from the probe, and maintaining a dry and gel-free needle entry.14-15 The probe should be sterilized between patients to avoid cross-contamination; note that certain solutions like alcohol or ethyl chloride can damage the transducer.14-15 However, simple injections do not require such stringent standards when simple sterile technique is observed by cleaning and then never touching the cleaned area again except with the needle to avoid contamination. Also, ethyl chloride has been found to not contaminate a sterile site and can be used safely to anesthetize the skin.
Ultrasound-Guided Procedures
Many injectable therapeutic options exist as interventions. Cortisone, hyaluronic acid, platelet-rich plasma (PRP), stem cells/bone marrow concentrate (BMC), amniotic fluid, prolotherapy, and saline are now commonly used.16-17 A meta-analysis of the literature assessing the accuracy of ultrasound-guided shoulder girdle injections vs a landmark-guided injection was done in 2015.18 It showed that for the acromioclavicular joint, accuracy was 93.6% vs 68.2% (P < .0001), based on single studies. The accuracy of ultrasound vs a landmark-guided injection was 65% vs 70% for the subacromial space (P > .05); 86.7% vs 26.7% for the biceps tendon sheath (P < .05); and 92.5% vs 72.5% for the glenohumeral joint (P = .025).18
With cortisone, injecting into muscle, ligament, or tendons could potentially harm the tissue or cause worsening of the disease process.19-20 With the advent of orthobiologics, injecting into these structures is now desirable, instead of a potential complication.19-20 Ultrasound has become even more important to the accurate delivery of these therapies to the disease locations. Multiple studies using leukocyte-poor PRP for osteoarthritis show significant differences in pain scores.21-23 Peerbooms and colleagues24,25 also showed that PRP reduced pain and increased function compared to cortisone injections for lateral epicondylitis in 1- and 2-year double-blind randomized controlled trials. Centeno and colleagues26 performed a prospective, multi-site registry study on 102 patients with symptomatic osteoarthritis and/or rotator cuff tears that were injected with bone marrow concentrate. There was a statistically significant improvement in Disabilities of the Arm, Shoulder and Hand (DASH) scores from 36.1 to 17.1 (P < .001) and numeric pain scores improved from 4.3 to 2.4 (P < .001).
By being able to see the pathology, like a hypoechoic region in a tendon, ligament, or muscle, the physician can reliably place the therapeutic agent into the precise location. Also, adjacent para-tendon or para-ligament injections allow for in-season athletes to get some relief from symptoms while allowing to return to play quickly; injections into muscle, ligament, or tendon can damage the structure and require days or weeks of rest, while para-tendon and para-ligament injections are far less painful.
Second-generation techniques have provided patients with great options that can help avoid surgery. Calcific tendonitis appears brightly hyperechoic on ultrasound and is easily identified. The physician can attempt to break up the calcium by fenestration or barbotage of the calcium. The same can be accomplished by injecting the density with PRP or stem cells. If the calcium is soft or “toothpaste-like,” the negative pressure will make it easy to aspirate it into the syringe. A 2-year, longitudinal prospective study of 121 patients demonstrated that visual analog score (VAS) pain scores and size of calcium significantly decreased with ultrasound-guided percutaneous needle lavage; 89% of patients were pain-free at 1-year follow-up.27 Moreover, a randomized controlled trial of 48 patients comparing needle lavage vs subacromial steroid injection showed statistically significant radiographic and clinically better outcomes with the needle lavage group at the 1-year mark.28
The Tenex procedure is a novel technique that uses ultrasonic energy to fenestrate diseased tendon tissue. It also can be used to break up calcific deposits. After the Tenex probe is guided to the diseased tendon/calcium, the TX-1 tip oscillates at the speed of sound, fenestrating/cutting through the tendon or calcium while lavaging the tendon with saline. Multiple prospective, noncontrolled studies done in common extensor, patellar, and rotator cuff tendinopathy have demonstrated good to excellent improvements in pain scores with the Tenex procedure.29-31
Ultrasound is extremely useful in the treatment of adhesive capsulitis.32 The posterior glenohumeral capsule can be distended using a large volume (60 cc) of saline to loosen adhesions in preparation for manipulation. Because the manipulation can be an extremely painful procedure, ultrasound can be used to perform an inter-scalene block for regional anesthesia prior to the procedure. In 2014, Park and colleagues33 performed a randomized prospective trial that showed that capsular distension followed by manipulation was more effective than cortisone injection alone for the treatment of adhesive capsulitis.Ultrasound guidance was found to be just as efficacious as fluoroscopy in a randomized controlled trial in 2014; the authors noted that ultrasound does not expose the patient or clinician to radiation and can be done in office.34
Currently, techniques to perform ultrasound-guided percutaneous tenotomies of the long head of the biceps tendon using hook blades are being studied.35
Ultrasound-Assisted Surgery
Ultrasound has been a boon to surgeons who perform minimally invasive procedures. It is far less cumbersome than classic fluoroscopy. Fluoroscopy requires the use of heavy lead aprons by the surgeons. Combining this with the impervious gowns and hot lights, the surgeons’ comfort level is severely sacrificed. When having to do many long surgeries in a row, this situation can take a toll on the surgeons’ endurance and strength. Improving the comfort of the surgeon is not the primary goal of surgery, but can significantly help our ability to do a better job.
Ultrasound allows the surgeon to localize any superficial foreign objects, especially with radiolucent objects like fragments of glass. Small glass fragments or pieces of wood have always been extremely difficult to remove. X-rays cannot localize these objects, so getting a proper orientation is difficult. MRI and CT scans easily identify these types of foreign objects, but cannot be used intraoperatively (Figure 1A). Often, these objects cannot be felt and therefore require a large dissection. The objects may encapsulate and be easily confused with other soft tissues.
By using the ultrasound intraoperatively, the surgeon can identify the exact position of the biceps tendon (medial/lateral) and where it lies just below the groove and above the pectoralis major (superior/inferior) (Figure 2A).
Reconstruction of ligaments is another ideal use of ultrasound. Surface anatomy cannot always tell the exact location of a ligament or tendon insertion. The best example of this is the anterolateral ligament (ALL). Identification of the lateral epicondyle of the femur and anatomic insertion of the ALL can be difficult in some patients. Ultrasound can be used to identify the origin and insertion of the ALL during surgery under sterile conditions (see page 418). A spinal needle can be placed under direct vision with an in-plane ultrasound guidance over the bony insertion (Figure 3A). A percutaneous incision is made.
This technique is also used by the senior author (AMH) to repair, reconstruct, or internally brace the medial collateral ligament, medial patellofemoral ligament, and lateral collateral ligament. This technique is ideally suited to superficial ligament and tendon reattachment, reconstruction, or internal bracing. The knee, ankle, and elbow superficial ligaments are especially amenable to this easy, percutaneous technique.
Conclusion
Ultrasound is quickly becoming a popular imaging modality due to its simplicity, portability, and cost efficiency. Its use as a diagnostic tool is widely known. As an adjunct for procedures and interventions, its advantages over larger, more expensive modalities such as fluoroscopy, CT, or MRI make it stand out. Ultrasound is not the perfect solution to all problems, but it is clearly a technology that is gaining traction. Ultrasound is another imaging modality and tool that physicians and surgeons can use to improve their patients’ treatment.
1. Hirahara AM, Panero AJ. A guide to ultrasound of the shoulder, part 1: coding and reimbursement. Am J Orthop. 2016;45(3):176-182.
2. Panero AJ, Hirahara AM. A guide to ultrasound of the shoulder, part 2: the diagnostic evaluation. Am J Orthop. 2016; 45(4):233-238.
3. Finnoff JT, Hall MM, Adams E, et al. American Medical Society for Sports Medicine (AMSSM) position statement: Interventional musculoskeletal ultrasound in sports medicine. Br J Sports Med. 2015;49(3):145-150.
4. Sivan M, Brown J, Brennan S, Bhakta B. A one-stop approach to the management of soft tissue and degenerative musculoskeletal conditions using clinic-based ultrasonography. Musculoskeletal Care. 2011;9(2):63-68.
5. Eustace J, Brophy D, Gibney R, Bresnihan B, FitzGerald O. Comparison of the accuracy of steroid placement with clinical outcome in patients with shoulder symptoms. Ann Rheum Dis. 1997;56(1):59-63.
6. Partington P, Broome G. Diagnostic injection around the shoulder: Hit and miss? A cadaveric study of injection accuracy. J Shoulder Elbow Surg. 1998;7(2):147-150.
7. Rutten M, Maresch B, Jager G, de Waal Malefijt M. Injection of the subacromial-subdeltoid bursa: Blind or ultrasound-guided? Acta Orthop. 2007;78(2):254-257.
8. Kang M, Rizio L, Prybicien M, Middlemas D, Blacksin M. The accuracy of subacromial corticosteroid injections: A comparison of multiple methods. J Shoulder Elbow Surg. 2008;17(1 Suppl):61S-66S.
9. Yamakado K. The targeting accuracy of subacromial injection to the shoulder: An arthrographic evaluation. Arthroscopy. 2002;19(8):887-891.
10. Henkus HE, Cobben M, Coerkamp E, Nelissen R, van Arkel E. The accuracy of subacromial injections: A prospective randomized magnetic resonance imaging study. Arthroscopy. 2006;22(3):277-282.
11. Sethi P, El Attrache N. Accuracy of intra-articular injection of the glenohumeral joint: A cadaveric study. Orthopedics. 2006;29(2):149-152.
12. Naredo E, Cabero F, Beneyto P, et al. A randomized comparative study of short term response to blind injection versus sonographic-guided injection of local corticosteroids in patients with painful shoulder. J Rheumatol. 2004;31(2):308-314.
13. Speer M, McLennan N, Nixon C. Novice learner in-plane ultrasound imaging: which visualization technique? Reg Anesth Pain Med. 2013;38(4):350-352.
14. Marhofer P, Schebesta K, Marhofer D. [Hygiene aspects in ultrasound-guided regional anesthesia]. Anaesthesist. 2016;65(7):492-498.
15. Sherman T, Ferguson J, Davis W, Russo M, Argintar E. Does the use of ultrasound affect contamination of musculoskeletal injection sites? Clin Orthop Relat Res. 2015;473(1):351-357.
16. Bashir J, Panero AJ, Sherman AL. The emerging use of platelet-rich plasma in musculoskeletal medicine. J Am Osteopath Assoc. 2015;115(1):23-31.
17. Royall NA, Farrin E, Bahner DP, Stanislaw PA. Ultrasound-assisted musculoskeletal procedures: A practical overview of current literature. World J Orthop. 2011;2(7):57-66.
18. Aly AR, Rajasekaran S, Ashworth N. Ultrasound-guided shoulder girdle injections are more accurate and more effective than landmark-guided injections: a systematic review and meta-analysis. Br J Sports Med. 2015;49(16):1042-1049.
19. Maman E, Yehuda C, Pritsch T, et al. Detrimental effect of repeated and single subacromial corticosteroid injections on the intact and injured rotator cuff: A biomechanical and imaging study in rats. Am J Sports Med. 2016;44(1):177-182.
20. Gautam VK, Verma S, Batra S, Bhatnagar N, Arora S. Platelet-rich plasma versus corticosteroid injection for recalcitrant lateral epicondylitis: clinical and ultrasonographic evaluation. J Orthop Surg (Hong Kong). 2015;23(1):1-5.
21. Patel S, Dhillon MS, Aggarwal S, Marwaha N, Jain A. Treatment with platelet-rich plasma is more effective than placebo for knee osteoarthritis: a prospective, double-blind, randomized trial. Am J Sports Med. 2013;41(2):356-364.
22. Cerza F, Carni S, Carcangiu A, et al. Comparison between hyaluronic acid and platelet-rich plasma, intra-articular infiltration in the treatment of gonarthrosis. Am J Sports Med. 2012;40(12):2822-2827.
23. Spakova T, Rosocha J, Lacko M, Harvanova D, Gharaibeh A. Treatment of knee joint osteoarthritis with autologous platelet-rich plasma in comparison with hyaluronic acid. Am J Phys Med Rehabil. 2012;91(5):411-417.
24. Peerbooms JC, Sluimer J, Brujin DJ, Gosens T. Positive effects of an autologous platelet concentrate in lateral epicondylitis in a double-blind randomized controlled trial: platelet-rich plasma versus corticosteroid injection with a 1-year follow-up. Am J Sports Med. 2010;38(2):255-262.
25. Gosens T, Peerbooms JC, van Laar W, den Oudsten BL. Ongoing positive effects of platelet-rich plasma versus corticosteroid injection in lateral epicondylitis: a double-blind randomized controlled trial with a 2-year follow-up. Am J Sports Med. 2011;39(6):1200-1208.
26. Centeno CJ, Al-Sayegh H, Bashir J, Goodyear S, Freeman MD. A prospective multi-site registry study of a specific protocol of autologous bone marrow concentrate for the treatment of shoulder rotator cuff tears and osteoarthritis. J Pain Res. 2015;8:269-276.
27. Del Castillo-Gonzalez F, Ramos-Alvarez JJ, Rodriguez-Fabian G, Gonzalez-Perez J, Calderon-Montero J. Treatment of the calcific tendinopathy of the rotator cuff by ultrasound-guided percutaneous needle lavage. Two years prospective study. Muscles Ligaments Tendons J. 2015;4(4):407-412.
28. De Witte PB, Selten JW, Navas A, et al. Calcific tendinitis of the rotator cuff: a randomized controlled trial of ultrasound-guided needling and lavage versus subacromial corticosteroids. Am J Sports Med. 2013;41(7):1665-1673.
29. Koh J, Mohan P, Morrey B, et al. Fasciotomy and surgical tenotomy for recalcitrant lateral elbow tendinopathy: early clinical experience with a novel device for minimally invasive percutaneous microresection. Am J Sports Med. 2013;41(3):636-644.
30. Elattrache N, Morrey B. Percutaneous ultrasonic tenotomy as a treatment for chronic patellar tendinopathy–Jumper’s knee. Oper Tech Orthop. 2013;23(2):98-103
31. Patel MM. A novel treatment for refractory plantar fasciitis. Am J Orthop. 2015;444(3):107-110.
32. Harris G, Bou-Haidar P, Harris C. Adhesive capsulitis: Review of imaging and treatment. J Med Imaging Radiat Oncol. 2013;57:633-643.
33. Park SW, Lee HS, Kim JH. The effectiveness of intensive mobilization techniques combined with capsular distention for adhesive capsulitis of the shoulder. J Phys Ther Sci. 2014;26(11):1776-1770.
34. Bae JH, Park YS, Chang HJ, et al. Randomized controlled trial for efficacy of capsular distension for adhesive capsulitis: Fluoroscopy-guided anterior versus ultrasonography-guided posterolateral approach. Ann Rehabil Med. 2014;38(3):360-368.
35. Aly AR, Rajasekaran S, Mohamed A, Beavis C, Obaid H. Feasibility of ultrasound-guided percutaneous tenotomy of long head of the biceps tendon–A pilot cadaveric study. J Clin Ultrasound. 2015;43(6):361-366.
1. Hirahara AM, Panero AJ. A guide to ultrasound of the shoulder, part 1: coding and reimbursement. Am J Orthop. 2016;45(3):176-182.
2. Panero AJ, Hirahara AM. A guide to ultrasound of the shoulder, part 2: the diagnostic evaluation. Am J Orthop. 2016; 45(4):233-238.
3. Finnoff JT, Hall MM, Adams E, et al. American Medical Society for Sports Medicine (AMSSM) position statement: Interventional musculoskeletal ultrasound in sports medicine. Br J Sports Med. 2015;49(3):145-150.
4. Sivan M, Brown J, Brennan S, Bhakta B. A one-stop approach to the management of soft tissue and degenerative musculoskeletal conditions using clinic-based ultrasonography. Musculoskeletal Care. 2011;9(2):63-68.
5. Eustace J, Brophy D, Gibney R, Bresnihan B, FitzGerald O. Comparison of the accuracy of steroid placement with clinical outcome in patients with shoulder symptoms. Ann Rheum Dis. 1997;56(1):59-63.
6. Partington P, Broome G. Diagnostic injection around the shoulder: Hit and miss? A cadaveric study of injection accuracy. J Shoulder Elbow Surg. 1998;7(2):147-150.
7. Rutten M, Maresch B, Jager G, de Waal Malefijt M. Injection of the subacromial-subdeltoid bursa: Blind or ultrasound-guided? Acta Orthop. 2007;78(2):254-257.
8. Kang M, Rizio L, Prybicien M, Middlemas D, Blacksin M. The accuracy of subacromial corticosteroid injections: A comparison of multiple methods. J Shoulder Elbow Surg. 2008;17(1 Suppl):61S-66S.
9. Yamakado K. The targeting accuracy of subacromial injection to the shoulder: An arthrographic evaluation. Arthroscopy. 2002;19(8):887-891.
10. Henkus HE, Cobben M, Coerkamp E, Nelissen R, van Arkel E. The accuracy of subacromial injections: A prospective randomized magnetic resonance imaging study. Arthroscopy. 2006;22(3):277-282.
11. Sethi P, El Attrache N. Accuracy of intra-articular injection of the glenohumeral joint: A cadaveric study. Orthopedics. 2006;29(2):149-152.
12. Naredo E, Cabero F, Beneyto P, et al. A randomized comparative study of short term response to blind injection versus sonographic-guided injection of local corticosteroids in patients with painful shoulder. J Rheumatol. 2004;31(2):308-314.
13. Speer M, McLennan N, Nixon C. Novice learner in-plane ultrasound imaging: which visualization technique? Reg Anesth Pain Med. 2013;38(4):350-352.
14. Marhofer P, Schebesta K, Marhofer D. [Hygiene aspects in ultrasound-guided regional anesthesia]. Anaesthesist. 2016;65(7):492-498.
15. Sherman T, Ferguson J, Davis W, Russo M, Argintar E. Does the use of ultrasound affect contamination of musculoskeletal injection sites? Clin Orthop Relat Res. 2015;473(1):351-357.
16. Bashir J, Panero AJ, Sherman AL. The emerging use of platelet-rich plasma in musculoskeletal medicine. J Am Osteopath Assoc. 2015;115(1):23-31.
17. Royall NA, Farrin E, Bahner DP, Stanislaw PA. Ultrasound-assisted musculoskeletal procedures: A practical overview of current literature. World J Orthop. 2011;2(7):57-66.
18. Aly AR, Rajasekaran S, Ashworth N. Ultrasound-guided shoulder girdle injections are more accurate and more effective than landmark-guided injections: a systematic review and meta-analysis. Br J Sports Med. 2015;49(16):1042-1049.
19. Maman E, Yehuda C, Pritsch T, et al. Detrimental effect of repeated and single subacromial corticosteroid injections on the intact and injured rotator cuff: A biomechanical and imaging study in rats. Am J Sports Med. 2016;44(1):177-182.
20. Gautam VK, Verma S, Batra S, Bhatnagar N, Arora S. Platelet-rich plasma versus corticosteroid injection for recalcitrant lateral epicondylitis: clinical and ultrasonographic evaluation. J Orthop Surg (Hong Kong). 2015;23(1):1-5.
21. Patel S, Dhillon MS, Aggarwal S, Marwaha N, Jain A. Treatment with platelet-rich plasma is more effective than placebo for knee osteoarthritis: a prospective, double-blind, randomized trial. Am J Sports Med. 2013;41(2):356-364.
22. Cerza F, Carni S, Carcangiu A, et al. Comparison between hyaluronic acid and platelet-rich plasma, intra-articular infiltration in the treatment of gonarthrosis. Am J Sports Med. 2012;40(12):2822-2827.
23. Spakova T, Rosocha J, Lacko M, Harvanova D, Gharaibeh A. Treatment of knee joint osteoarthritis with autologous platelet-rich plasma in comparison with hyaluronic acid. Am J Phys Med Rehabil. 2012;91(5):411-417.
24. Peerbooms JC, Sluimer J, Brujin DJ, Gosens T. Positive effects of an autologous platelet concentrate in lateral epicondylitis in a double-blind randomized controlled trial: platelet-rich plasma versus corticosteroid injection with a 1-year follow-up. Am J Sports Med. 2010;38(2):255-262.
25. Gosens T, Peerbooms JC, van Laar W, den Oudsten BL. Ongoing positive effects of platelet-rich plasma versus corticosteroid injection in lateral epicondylitis: a double-blind randomized controlled trial with a 2-year follow-up. Am J Sports Med. 2011;39(6):1200-1208.
26. Centeno CJ, Al-Sayegh H, Bashir J, Goodyear S, Freeman MD. A prospective multi-site registry study of a specific protocol of autologous bone marrow concentrate for the treatment of shoulder rotator cuff tears and osteoarthritis. J Pain Res. 2015;8:269-276.
27. Del Castillo-Gonzalez F, Ramos-Alvarez JJ, Rodriguez-Fabian G, Gonzalez-Perez J, Calderon-Montero J. Treatment of the calcific tendinopathy of the rotator cuff by ultrasound-guided percutaneous needle lavage. Two years prospective study. Muscles Ligaments Tendons J. 2015;4(4):407-412.
28. De Witte PB, Selten JW, Navas A, et al. Calcific tendinitis of the rotator cuff: a randomized controlled trial of ultrasound-guided needling and lavage versus subacromial corticosteroids. Am J Sports Med. 2013;41(7):1665-1673.
29. Koh J, Mohan P, Morrey B, et al. Fasciotomy and surgical tenotomy for recalcitrant lateral elbow tendinopathy: early clinical experience with a novel device for minimally invasive percutaneous microresection. Am J Sports Med. 2013;41(3):636-644.
30. Elattrache N, Morrey B. Percutaneous ultrasonic tenotomy as a treatment for chronic patellar tendinopathy–Jumper’s knee. Oper Tech Orthop. 2013;23(2):98-103
31. Patel MM. A novel treatment for refractory plantar fasciitis. Am J Orthop. 2015;444(3):107-110.
32. Harris G, Bou-Haidar P, Harris C. Adhesive capsulitis: Review of imaging and treatment. J Med Imaging Radiat Oncol. 2013;57:633-643.
33. Park SW, Lee HS, Kim JH. The effectiveness of intensive mobilization techniques combined with capsular distention for adhesive capsulitis of the shoulder. J Phys Ther Sci. 2014;26(11):1776-1770.
34. Bae JH, Park YS, Chang HJ, et al. Randomized controlled trial for efficacy of capsular distension for adhesive capsulitis: Fluoroscopy-guided anterior versus ultrasonography-guided posterolateral approach. Ann Rehabil Med. 2014;38(3):360-368.
35. Aly AR, Rajasekaran S, Mohamed A, Beavis C, Obaid H. Feasibility of ultrasound-guided percutaneous tenotomy of long head of the biceps tendon–A pilot cadaveric study. J Clin Ultrasound. 2015;43(6):361-366.
Ultrasound-Guided Percutaneous Reconstruction of the Anterolateral Ligament: Surgical Technique and Case Report
Restoring native kinematics of the knee has been a primary goal of anterior cruciate ligament (ACL) procedures. Double-bundle ACL reconstruction, compared to single-bundle, has been hypothesized to more effectively re-establish rotational stability by re-creating the anatomic ACL, but has not yet proven to result in better clinical outcomes.1
In 1879, Dr. Paul Segond described a “fibrous, pearly band” at the lateral aspect of the knee that avulsed off the anterolateral proximal tibia during many ACL injuries.2 The role of the lateral tissues in knee stability and their relationship with ACL pathology has attracted noteworthy attention in recent time. There have been multiple studies presenting an anatomical description of a structure at the anterolateral portion of the knee with definitive femoral, meniscal, and tibial attachments, which helps control internal rotational forces.3-7 Claes and colleagues4 later found that band of tissue to be the anterolateral ligament (ALL) and determined its injury to be pathognomonic with ACL ruptures.
The ALL is a vital static stabilizer of the tibio-femoral joint, especially during internal tibial rotation.8-10 In their report on ALL and ACL reconstruction, Helito and colleagues11 acknowledge the necessity of accurate assessment of the lateral structures through imaging to determine the presence of extra-articular injury. Musculoskeletal diagnostic ultrasound has been established as an appropriate means to identify the ALL.12
Ultrasound can accurately determine the exact anatomic location of the origin and insertion of the ALL. Reconstruction of the ALL could yield better patient outcomes for those who experience concurrent ACL/ALL injury. Here we present an innovative technique for an ultrasound-guided percutaneous method for reconstruction of the ALL and report on a patient who had underwent ALL reconstruction.
Surgical Indications
All patients undergo an ultrasound evaluation preoperatively to determine if the ALL is intact or injured. Our experience has shown that when ultrasound evaluation reveals an intact ALL, the pivot shift has never been a grade III.
Surgical Technique
For a demonstration of this technique, see the video that accompanies this article.
The pivot shift test is conducted under anesthesia to determine whether an ALL reconstruction is required. The patient is placed in a supine position with the knee flexed at 30o, at neutral rotation, and without any varus or valgus stress.
A No. 15 blade is used to make a small incision centered on each spinal needle. The spinal needle is replaced with a 2.4-mm drill pin (Figure 2).
The graft and FiberTape are then passed under the IT band to the distal incision. Using the length of the BioComposite SwiveLock anchor as a guide, a mark is made on the graft after tensioning the construct in line with the leg, distal to the tibial drill pin (Table 2, Figure 4).
Rehabilitation
Rehabilitation following an ALL procedure is similar to traditional ACL rehabilitation with an added emphasis on minimizing rotational torque of the tibia in the early stages.
Case Report
In January 2013, a 17-year-old male soccer player suffered an ACL rupture of his right knee. Later that spring, he had an ACL reconstruction with an allograft. Twelve months postoperatively, the patient returned, saying that he felt much better; however, anytime he tried to plant his foot and rotate over that fixed foot, his knee felt unstable. The physical examination revealed both negative Lachman and anterior drawer tests but a I+ pivot shift test. A magnetic resonance imaging (MRI) examination revealed an intact ACL graft. A diagnostic ultrasound evaluation revealed a distal ALL injury. After discussing the risks, benefits, and goals with the patient, we opted for a diagnostic arthroscopy and a percutaneous, ultrasound-guided reconstruction of the ALL.
Postoperatively, the patient did very well. One week after surgery, he returned, saying he felt completely stable and demonstrated by repeating the rotation of his knee. The patient continued to have no issues until he returned 13 months post-ALL surgery, complaining of a recent injury that had caused the return of his feelings of instability. An MRI evaluation showed an intact ACL graft and the possibility of a ruptured ALL. Fifteen months after the initial ALL reconstruction, we proceeded with surgery. At arthroscopy, the patient was found to have a pivot shift of I+ and an intact ACL graft. The ALL was reconstructed again using an allograft, internal brace, and bone marrow concentrate. At 13 months post-ALL reconstruction revision, the patient had no complaints.
Discussion
Reconstruction of the ALL is aimed to restore anatomic rotational kinematics. Sonnery-Cottet and colleagues14 have reported promising initial results in their 2-year follow-up study of combined ACL and ALL reconstruction outcomes. This surgical technique includes use of an internal brace, which negates the necessity for external support devices and allows for earlier mobilization of the joint. A reconstruction of the ALL, performed concurrently with the ACL, does not add recovery time, but could prevent postsurgical complications and improve rehabilitation by eliminating rotational instability that presents in some ACL-reconstructed patients.
Sonnery-Cottet and colleagues15 state that their arthroscopic identification of the ALL can help to cultivate a “less invasive and more anatomic” reconstruction. The use of musculoskeletal ultrasound allows our technique to utilize a completely noninvasive imaging tool that allows proper establishment of ALL anatomy prior to the procedure. The entirety of the ALL is easily identifiable,4,12 which has proven to be shortcoming of MRI evaluation.15-17 Accurate preoperative assessment of the lateral structures is necessary in ACL-deficient individuals.11,15 Sonography also provides a means of accurate guidance and socket creation, without generating large incisions.
If the ALL is responsible for internal rotatory stability as asserted, the structure should exhibit biomechanical properties during movement. In their study on the function of the ligament, Parsons and colleagues9 established the inverse relationship between the ALL and ACL during internal rotation. As their cadaveric knees were subjected to an internal rotatory force through increasing angles of flexion, the contribution of the ALL towards stability significantly increased while the ACL declined. Helito and colleagues8 and Zens and colleagues10 have demonstrated length changes of the ligament through varying degrees of flexion and internal rotation. Their reports indicate greater tension during knee movements, coinciding with the description of increasing ALL stability contribution by Parsons and colleagues.9 Kennedy and colleagues7 conducted a pull-to-failure test on the ALL. The average failure load was 175 N with a stiffness of 20 N/mm, illustrating the structure is a candidate for most traditional soft tissue grafts. The biomechanical evidence of the structural properties of the ALL confirms its importance in knee function and the necessity for its reconstruction.
With the understanding that ACL contributes to rotatory stability to some extent, the notion begs the question of how a centrally located ligament is able to prevent excessive rotation in a structure with a large relative radius. Biomechanically, with such a small moment arm, the ACL would experience tremendous stress when a rotatory force is applied. The same torque applied to a more superficial structure, with a greater moment, would sustain a large reduction in the applied force. The concept of a wheel and an axle should be considered. The equation is F1 × R1 = F2 × R2. We measured on a cadaveric knee the distance from the center of rotation to the ACL and the ALL, finding the radii were 5 mm and 30 mm, respectively. Taking these measurements, we would then expect the force experienced on the axle (ACL) to be 6 times greater than what would be experienced on the periphery of the wheel (ALL). The ALL (wheel) has a significant biomechanical advantage over the ACL (axle) in controlling and enduring internal rotatory forces of the knee. This would imply that if the ALL were damaged and not re-established, the ACL would experience a 6 times greater force trying to control internal rotation, which would result in a significantly increased chance of failure and rupture.
While there is a degree of dissent on the presence of the ALL, a number of studies have classified the tissue as an independent ligamentous structure.3-7 While there is disagreement on the precise location of the femoral attachment, there is a consensus on the location of the tibial and meniscal attachments. Claes and colleagues4 originally outlined the femoral attachment as anterior and distal to the origin of the fibular collateral ligament (FCL), which is the description this technique follows. Since Claes and colleagues’4 report, many have investigated the ligament’s femoral origin with delineations ranging from posterior and proximal3,5,7 to anterior and distal.6,16-18
The accurate, noninvasive nature of the musculoskeletal ultrasound prior to any incisions being made makes this technique innovative and superior to other open surgical techniques or those that require fluoroscopy.
Conclusion
The ALL has been determined to play an integral role in the rotational stability of the knee. In the setting of instability and insufficiency, reconstruction will lead to better patient outcomes for concurrent ACL/ALL injuries and postsurgical rotatory instability following ACL procedures. This innovative technique utilizes ultrasound to ascertain the precise anatomical attachments of the ALL prior to the operation. The novel nature of this ultrasound-guided reconstruction has the potential to be applicable in many other surgical procedures.
1. Suomalainen P, Järvelä T, Paakkala A, Kannus P, Järvinen M. Double-bundle versus single-bundle anterior cruciate ligament reconstruction: A prospective randomized study with 5-year results. Am J Sports Med. 2012;40(7):1511-1518.
2. Segond P. Recherches cliniques et expérimentales sur les épanchements sanguins du genou par entorse. Progrés Médical. 1879;6(6):1-85. French.
3. Caterine S, Litchfield R, Johnson M, Chronik B, Getgood A. A cadaveric study of the anterolateral ligament: re-introducing the lateral capsular ligament. Knee Surg Sports Traumatol Athrosc. 2015;23(11):3186-3195.
4. Claes S, Vereecke E, Maes M, Victor J, Verdonk P, Bellemans J. Anatomy of the anterolateral ligament of the knee. J Anat. 2013;223(4):321-328.
5. Dodds AL, Halewood C, Gupte CM, Williams A, Amis AA. The anterolateral ligament: Anatomy, length changes and association with the segond fracture. Bone Joint J. 2014;96-B(3):325-331.
6. Helito CP, Demange MK, Bonadio MB, et al. Anatomy and histology of the knee anterolateral ligament. Orthop J Sports Med. 2013;1(7):2325967113513546.
7. Kennedy MI, Claes S, Fuso FA, et al. The anterolateral ligament: An anatomic, radiographic, and biomechanical analysis. Am J Sports Med. 2015;43(7):1606-1615.
8. Helito CP, Helito PV, Bonadio MB, et al. Evaluation of the length and isometric pattern of the anterolateral ligament with serial computer tomography. Orthop J Sports Med. 2014;2(12):2325967114562205.
9. Parsons EM, Gee AO, Spiekerman C, Cavanagh PR. The biomechanical function of the anterolateral ligament of the knee. Am J Sports Med. 2015;43(3):669-674.
10. Zens M, Niemeyer P, Ruhhamer J, et al. Length changes of the anterolateral ligament during passive knee motion: A human cadaveric study. Am J Sports Med. 2015;43(10):2545-2552.
11. Helito CP, Bonadio MB, Gobbi RG, et al. Combined intra- and extra-articular reconstruction of the anterior cruciate ligament: the reconstruction of the knee anterolateral ligament. Arthrosc Tech. 2015;4(3):e239-e244.
12. Cianca J, John J, Pandit S, Chiou-Tan FY. Musculoskeletal ultrasound imaging of the recently described anterolateral ligament of the knee. Am J Phys Med Rehabil. 2014;93(2):186
13. Adams JE, Zobitz ME, Reach JS, et al. Rotator cuff repair using an acellular dermal matrix graft: An in vivo study in a canine model. Arthroscopy. 2006;22(7):700-709.
14. Sonnery-Cottet B, Thaunat M, Freychet B, Pupim BHB, Murphy CG, Claes S. Outcome of a combined anterior cruciate ligament and anterolateral ligament reconstruction technique with a minimum 2-year follow-up. Am J Sports Med. 2015;43(7):1598-1605.
15. Sonnery-Cottet B, Archbold P, Rezende FC, Neto AM, Fayard JM, Thaunat M. Arthroscopic identification of the anterolateral ligament of the knee. Arthrosc Tech. 2014;3(3):e389-e392.
16. Helito CP, Helito PV, Costa HP, et al. MRI evaluation of the anterolateral ligament of the knee: assessment in routine 1.5-T scans. Skeletal Radiol. 2014;43(10):1421-1427.
17. Helito CP, Demange MK, Helito PV, et al. Evaluation of the anterolateral ligament of the knee by means of magnetic resonance examination. Rev Bras Orthop. 2015;50(2):214-219.
18. Helito CP, Demange MK, Bonadio MB, et al. Radiographic landmarks for locating the femoral origin and tibial insertion of the knee anterolateral ligament. Am J Sports Med. 2014;42(10):2356-2362.
Restoring native kinematics of the knee has been a primary goal of anterior cruciate ligament (ACL) procedures. Double-bundle ACL reconstruction, compared to single-bundle, has been hypothesized to more effectively re-establish rotational stability by re-creating the anatomic ACL, but has not yet proven to result in better clinical outcomes.1
In 1879, Dr. Paul Segond described a “fibrous, pearly band” at the lateral aspect of the knee that avulsed off the anterolateral proximal tibia during many ACL injuries.2 The role of the lateral tissues in knee stability and their relationship with ACL pathology has attracted noteworthy attention in recent time. There have been multiple studies presenting an anatomical description of a structure at the anterolateral portion of the knee with definitive femoral, meniscal, and tibial attachments, which helps control internal rotational forces.3-7 Claes and colleagues4 later found that band of tissue to be the anterolateral ligament (ALL) and determined its injury to be pathognomonic with ACL ruptures.
The ALL is a vital static stabilizer of the tibio-femoral joint, especially during internal tibial rotation.8-10 In their report on ALL and ACL reconstruction, Helito and colleagues11 acknowledge the necessity of accurate assessment of the lateral structures through imaging to determine the presence of extra-articular injury. Musculoskeletal diagnostic ultrasound has been established as an appropriate means to identify the ALL.12
Ultrasound can accurately determine the exact anatomic location of the origin and insertion of the ALL. Reconstruction of the ALL could yield better patient outcomes for those who experience concurrent ACL/ALL injury. Here we present an innovative technique for an ultrasound-guided percutaneous method for reconstruction of the ALL and report on a patient who had underwent ALL reconstruction.
Surgical Indications
All patients undergo an ultrasound evaluation preoperatively to determine if the ALL is intact or injured. Our experience has shown that when ultrasound evaluation reveals an intact ALL, the pivot shift has never been a grade III.
Surgical Technique
For a demonstration of this technique, see the video that accompanies this article.
The pivot shift test is conducted under anesthesia to determine whether an ALL reconstruction is required. The patient is placed in a supine position with the knee flexed at 30o, at neutral rotation, and without any varus or valgus stress.
A No. 15 blade is used to make a small incision centered on each spinal needle. The spinal needle is replaced with a 2.4-mm drill pin (Figure 2).
The graft and FiberTape are then passed under the IT band to the distal incision. Using the length of the BioComposite SwiveLock anchor as a guide, a mark is made on the graft after tensioning the construct in line with the leg, distal to the tibial drill pin (Table 2, Figure 4).
Rehabilitation
Rehabilitation following an ALL procedure is similar to traditional ACL rehabilitation with an added emphasis on minimizing rotational torque of the tibia in the early stages.
Case Report
In January 2013, a 17-year-old male soccer player suffered an ACL rupture of his right knee. Later that spring, he had an ACL reconstruction with an allograft. Twelve months postoperatively, the patient returned, saying that he felt much better; however, anytime he tried to plant his foot and rotate over that fixed foot, his knee felt unstable. The physical examination revealed both negative Lachman and anterior drawer tests but a I+ pivot shift test. A magnetic resonance imaging (MRI) examination revealed an intact ACL graft. A diagnostic ultrasound evaluation revealed a distal ALL injury. After discussing the risks, benefits, and goals with the patient, we opted for a diagnostic arthroscopy and a percutaneous, ultrasound-guided reconstruction of the ALL.
Postoperatively, the patient did very well. One week after surgery, he returned, saying he felt completely stable and demonstrated by repeating the rotation of his knee. The patient continued to have no issues until he returned 13 months post-ALL surgery, complaining of a recent injury that had caused the return of his feelings of instability. An MRI evaluation showed an intact ACL graft and the possibility of a ruptured ALL. Fifteen months after the initial ALL reconstruction, we proceeded with surgery. At arthroscopy, the patient was found to have a pivot shift of I+ and an intact ACL graft. The ALL was reconstructed again using an allograft, internal brace, and bone marrow concentrate. At 13 months post-ALL reconstruction revision, the patient had no complaints.
Discussion
Reconstruction of the ALL is aimed to restore anatomic rotational kinematics. Sonnery-Cottet and colleagues14 have reported promising initial results in their 2-year follow-up study of combined ACL and ALL reconstruction outcomes. This surgical technique includes use of an internal brace, which negates the necessity for external support devices and allows for earlier mobilization of the joint. A reconstruction of the ALL, performed concurrently with the ACL, does not add recovery time, but could prevent postsurgical complications and improve rehabilitation by eliminating rotational instability that presents in some ACL-reconstructed patients.
Sonnery-Cottet and colleagues15 state that their arthroscopic identification of the ALL can help to cultivate a “less invasive and more anatomic” reconstruction. The use of musculoskeletal ultrasound allows our technique to utilize a completely noninvasive imaging tool that allows proper establishment of ALL anatomy prior to the procedure. The entirety of the ALL is easily identifiable,4,12 which has proven to be shortcoming of MRI evaluation.15-17 Accurate preoperative assessment of the lateral structures is necessary in ACL-deficient individuals.11,15 Sonography also provides a means of accurate guidance and socket creation, without generating large incisions.
If the ALL is responsible for internal rotatory stability as asserted, the structure should exhibit biomechanical properties during movement. In their study on the function of the ligament, Parsons and colleagues9 established the inverse relationship between the ALL and ACL during internal rotation. As their cadaveric knees were subjected to an internal rotatory force through increasing angles of flexion, the contribution of the ALL towards stability significantly increased while the ACL declined. Helito and colleagues8 and Zens and colleagues10 have demonstrated length changes of the ligament through varying degrees of flexion and internal rotation. Their reports indicate greater tension during knee movements, coinciding with the description of increasing ALL stability contribution by Parsons and colleagues.9 Kennedy and colleagues7 conducted a pull-to-failure test on the ALL. The average failure load was 175 N with a stiffness of 20 N/mm, illustrating the structure is a candidate for most traditional soft tissue grafts. The biomechanical evidence of the structural properties of the ALL confirms its importance in knee function and the necessity for its reconstruction.
With the understanding that ACL contributes to rotatory stability to some extent, the notion begs the question of how a centrally located ligament is able to prevent excessive rotation in a structure with a large relative radius. Biomechanically, with such a small moment arm, the ACL would experience tremendous stress when a rotatory force is applied. The same torque applied to a more superficial structure, with a greater moment, would sustain a large reduction in the applied force. The concept of a wheel and an axle should be considered. The equation is F1 × R1 = F2 × R2. We measured on a cadaveric knee the distance from the center of rotation to the ACL and the ALL, finding the radii were 5 mm and 30 mm, respectively. Taking these measurements, we would then expect the force experienced on the axle (ACL) to be 6 times greater than what would be experienced on the periphery of the wheel (ALL). The ALL (wheel) has a significant biomechanical advantage over the ACL (axle) in controlling and enduring internal rotatory forces of the knee. This would imply that if the ALL were damaged and not re-established, the ACL would experience a 6 times greater force trying to control internal rotation, which would result in a significantly increased chance of failure and rupture.
While there is a degree of dissent on the presence of the ALL, a number of studies have classified the tissue as an independent ligamentous structure.3-7 While there is disagreement on the precise location of the femoral attachment, there is a consensus on the location of the tibial and meniscal attachments. Claes and colleagues4 originally outlined the femoral attachment as anterior and distal to the origin of the fibular collateral ligament (FCL), which is the description this technique follows. Since Claes and colleagues’4 report, many have investigated the ligament’s femoral origin with delineations ranging from posterior and proximal3,5,7 to anterior and distal.6,16-18
The accurate, noninvasive nature of the musculoskeletal ultrasound prior to any incisions being made makes this technique innovative and superior to other open surgical techniques or those that require fluoroscopy.
Conclusion
The ALL has been determined to play an integral role in the rotational stability of the knee. In the setting of instability and insufficiency, reconstruction will lead to better patient outcomes for concurrent ACL/ALL injuries and postsurgical rotatory instability following ACL procedures. This innovative technique utilizes ultrasound to ascertain the precise anatomical attachments of the ALL prior to the operation. The novel nature of this ultrasound-guided reconstruction has the potential to be applicable in many other surgical procedures.
Restoring native kinematics of the knee has been a primary goal of anterior cruciate ligament (ACL) procedures. Double-bundle ACL reconstruction, compared to single-bundle, has been hypothesized to more effectively re-establish rotational stability by re-creating the anatomic ACL, but has not yet proven to result in better clinical outcomes.1
In 1879, Dr. Paul Segond described a “fibrous, pearly band” at the lateral aspect of the knee that avulsed off the anterolateral proximal tibia during many ACL injuries.2 The role of the lateral tissues in knee stability and their relationship with ACL pathology has attracted noteworthy attention in recent time. There have been multiple studies presenting an anatomical description of a structure at the anterolateral portion of the knee with definitive femoral, meniscal, and tibial attachments, which helps control internal rotational forces.3-7 Claes and colleagues4 later found that band of tissue to be the anterolateral ligament (ALL) and determined its injury to be pathognomonic with ACL ruptures.
The ALL is a vital static stabilizer of the tibio-femoral joint, especially during internal tibial rotation.8-10 In their report on ALL and ACL reconstruction, Helito and colleagues11 acknowledge the necessity of accurate assessment of the lateral structures through imaging to determine the presence of extra-articular injury. Musculoskeletal diagnostic ultrasound has been established as an appropriate means to identify the ALL.12
Ultrasound can accurately determine the exact anatomic location of the origin and insertion of the ALL. Reconstruction of the ALL could yield better patient outcomes for those who experience concurrent ACL/ALL injury. Here we present an innovative technique for an ultrasound-guided percutaneous method for reconstruction of the ALL and report on a patient who had underwent ALL reconstruction.
Surgical Indications
All patients undergo an ultrasound evaluation preoperatively to determine if the ALL is intact or injured. Our experience has shown that when ultrasound evaluation reveals an intact ALL, the pivot shift has never been a grade III.
Surgical Technique
For a demonstration of this technique, see the video that accompanies this article.
The pivot shift test is conducted under anesthesia to determine whether an ALL reconstruction is required. The patient is placed in a supine position with the knee flexed at 30o, at neutral rotation, and without any varus or valgus stress.
A No. 15 blade is used to make a small incision centered on each spinal needle. The spinal needle is replaced with a 2.4-mm drill pin (Figure 2).
The graft and FiberTape are then passed under the IT band to the distal incision. Using the length of the BioComposite SwiveLock anchor as a guide, a mark is made on the graft after tensioning the construct in line with the leg, distal to the tibial drill pin (Table 2, Figure 4).
Rehabilitation
Rehabilitation following an ALL procedure is similar to traditional ACL rehabilitation with an added emphasis on minimizing rotational torque of the tibia in the early stages.
Case Report
In January 2013, a 17-year-old male soccer player suffered an ACL rupture of his right knee. Later that spring, he had an ACL reconstruction with an allograft. Twelve months postoperatively, the patient returned, saying that he felt much better; however, anytime he tried to plant his foot and rotate over that fixed foot, his knee felt unstable. The physical examination revealed both negative Lachman and anterior drawer tests but a I+ pivot shift test. A magnetic resonance imaging (MRI) examination revealed an intact ACL graft. A diagnostic ultrasound evaluation revealed a distal ALL injury. After discussing the risks, benefits, and goals with the patient, we opted for a diagnostic arthroscopy and a percutaneous, ultrasound-guided reconstruction of the ALL.
Postoperatively, the patient did very well. One week after surgery, he returned, saying he felt completely stable and demonstrated by repeating the rotation of his knee. The patient continued to have no issues until he returned 13 months post-ALL surgery, complaining of a recent injury that had caused the return of his feelings of instability. An MRI evaluation showed an intact ACL graft and the possibility of a ruptured ALL. Fifteen months after the initial ALL reconstruction, we proceeded with surgery. At arthroscopy, the patient was found to have a pivot shift of I+ and an intact ACL graft. The ALL was reconstructed again using an allograft, internal brace, and bone marrow concentrate. At 13 months post-ALL reconstruction revision, the patient had no complaints.
Discussion
Reconstruction of the ALL is aimed to restore anatomic rotational kinematics. Sonnery-Cottet and colleagues14 have reported promising initial results in their 2-year follow-up study of combined ACL and ALL reconstruction outcomes. This surgical technique includes use of an internal brace, which negates the necessity for external support devices and allows for earlier mobilization of the joint. A reconstruction of the ALL, performed concurrently with the ACL, does not add recovery time, but could prevent postsurgical complications and improve rehabilitation by eliminating rotational instability that presents in some ACL-reconstructed patients.
Sonnery-Cottet and colleagues15 state that their arthroscopic identification of the ALL can help to cultivate a “less invasive and more anatomic” reconstruction. The use of musculoskeletal ultrasound allows our technique to utilize a completely noninvasive imaging tool that allows proper establishment of ALL anatomy prior to the procedure. The entirety of the ALL is easily identifiable,4,12 which has proven to be shortcoming of MRI evaluation.15-17 Accurate preoperative assessment of the lateral structures is necessary in ACL-deficient individuals.11,15 Sonography also provides a means of accurate guidance and socket creation, without generating large incisions.
If the ALL is responsible for internal rotatory stability as asserted, the structure should exhibit biomechanical properties during movement. In their study on the function of the ligament, Parsons and colleagues9 established the inverse relationship between the ALL and ACL during internal rotation. As their cadaveric knees were subjected to an internal rotatory force through increasing angles of flexion, the contribution of the ALL towards stability significantly increased while the ACL declined. Helito and colleagues8 and Zens and colleagues10 have demonstrated length changes of the ligament through varying degrees of flexion and internal rotation. Their reports indicate greater tension during knee movements, coinciding with the description of increasing ALL stability contribution by Parsons and colleagues.9 Kennedy and colleagues7 conducted a pull-to-failure test on the ALL. The average failure load was 175 N with a stiffness of 20 N/mm, illustrating the structure is a candidate for most traditional soft tissue grafts. The biomechanical evidence of the structural properties of the ALL confirms its importance in knee function and the necessity for its reconstruction.
With the understanding that ACL contributes to rotatory stability to some extent, the notion begs the question of how a centrally located ligament is able to prevent excessive rotation in a structure with a large relative radius. Biomechanically, with such a small moment arm, the ACL would experience tremendous stress when a rotatory force is applied. The same torque applied to a more superficial structure, with a greater moment, would sustain a large reduction in the applied force. The concept of a wheel and an axle should be considered. The equation is F1 × R1 = F2 × R2. We measured on a cadaveric knee the distance from the center of rotation to the ACL and the ALL, finding the radii were 5 mm and 30 mm, respectively. Taking these measurements, we would then expect the force experienced on the axle (ACL) to be 6 times greater than what would be experienced on the periphery of the wheel (ALL). The ALL (wheel) has a significant biomechanical advantage over the ACL (axle) in controlling and enduring internal rotatory forces of the knee. This would imply that if the ALL were damaged and not re-established, the ACL would experience a 6 times greater force trying to control internal rotation, which would result in a significantly increased chance of failure and rupture.
While there is a degree of dissent on the presence of the ALL, a number of studies have classified the tissue as an independent ligamentous structure.3-7 While there is disagreement on the precise location of the femoral attachment, there is a consensus on the location of the tibial and meniscal attachments. Claes and colleagues4 originally outlined the femoral attachment as anterior and distal to the origin of the fibular collateral ligament (FCL), which is the description this technique follows. Since Claes and colleagues’4 report, many have investigated the ligament’s femoral origin with delineations ranging from posterior and proximal3,5,7 to anterior and distal.6,16-18
The accurate, noninvasive nature of the musculoskeletal ultrasound prior to any incisions being made makes this technique innovative and superior to other open surgical techniques or those that require fluoroscopy.
Conclusion
The ALL has been determined to play an integral role in the rotational stability of the knee. In the setting of instability and insufficiency, reconstruction will lead to better patient outcomes for concurrent ACL/ALL injuries and postsurgical rotatory instability following ACL procedures. This innovative technique utilizes ultrasound to ascertain the precise anatomical attachments of the ALL prior to the operation. The novel nature of this ultrasound-guided reconstruction has the potential to be applicable in many other surgical procedures.
1. Suomalainen P, Järvelä T, Paakkala A, Kannus P, Järvinen M. Double-bundle versus single-bundle anterior cruciate ligament reconstruction: A prospective randomized study with 5-year results. Am J Sports Med. 2012;40(7):1511-1518.
2. Segond P. Recherches cliniques et expérimentales sur les épanchements sanguins du genou par entorse. Progrés Médical. 1879;6(6):1-85. French.
3. Caterine S, Litchfield R, Johnson M, Chronik B, Getgood A. A cadaveric study of the anterolateral ligament: re-introducing the lateral capsular ligament. Knee Surg Sports Traumatol Athrosc. 2015;23(11):3186-3195.
4. Claes S, Vereecke E, Maes M, Victor J, Verdonk P, Bellemans J. Anatomy of the anterolateral ligament of the knee. J Anat. 2013;223(4):321-328.
5. Dodds AL, Halewood C, Gupte CM, Williams A, Amis AA. The anterolateral ligament: Anatomy, length changes and association with the segond fracture. Bone Joint J. 2014;96-B(3):325-331.
6. Helito CP, Demange MK, Bonadio MB, et al. Anatomy and histology of the knee anterolateral ligament. Orthop J Sports Med. 2013;1(7):2325967113513546.
7. Kennedy MI, Claes S, Fuso FA, et al. The anterolateral ligament: An anatomic, radiographic, and biomechanical analysis. Am J Sports Med. 2015;43(7):1606-1615.
8. Helito CP, Helito PV, Bonadio MB, et al. Evaluation of the length and isometric pattern of the anterolateral ligament with serial computer tomography. Orthop J Sports Med. 2014;2(12):2325967114562205.
9. Parsons EM, Gee AO, Spiekerman C, Cavanagh PR. The biomechanical function of the anterolateral ligament of the knee. Am J Sports Med. 2015;43(3):669-674.
10. Zens M, Niemeyer P, Ruhhamer J, et al. Length changes of the anterolateral ligament during passive knee motion: A human cadaveric study. Am J Sports Med. 2015;43(10):2545-2552.
11. Helito CP, Bonadio MB, Gobbi RG, et al. Combined intra- and extra-articular reconstruction of the anterior cruciate ligament: the reconstruction of the knee anterolateral ligament. Arthrosc Tech. 2015;4(3):e239-e244.
12. Cianca J, John J, Pandit S, Chiou-Tan FY. Musculoskeletal ultrasound imaging of the recently described anterolateral ligament of the knee. Am J Phys Med Rehabil. 2014;93(2):186
13. Adams JE, Zobitz ME, Reach JS, et al. Rotator cuff repair using an acellular dermal matrix graft: An in vivo study in a canine model. Arthroscopy. 2006;22(7):700-709.
14. Sonnery-Cottet B, Thaunat M, Freychet B, Pupim BHB, Murphy CG, Claes S. Outcome of a combined anterior cruciate ligament and anterolateral ligament reconstruction technique with a minimum 2-year follow-up. Am J Sports Med. 2015;43(7):1598-1605.
15. Sonnery-Cottet B, Archbold P, Rezende FC, Neto AM, Fayard JM, Thaunat M. Arthroscopic identification of the anterolateral ligament of the knee. Arthrosc Tech. 2014;3(3):e389-e392.
16. Helito CP, Helito PV, Costa HP, et al. MRI evaluation of the anterolateral ligament of the knee: assessment in routine 1.5-T scans. Skeletal Radiol. 2014;43(10):1421-1427.
17. Helito CP, Demange MK, Helito PV, et al. Evaluation of the anterolateral ligament of the knee by means of magnetic resonance examination. Rev Bras Orthop. 2015;50(2):214-219.
18. Helito CP, Demange MK, Bonadio MB, et al. Radiographic landmarks for locating the femoral origin and tibial insertion of the knee anterolateral ligament. Am J Sports Med. 2014;42(10):2356-2362.
1. Suomalainen P, Järvelä T, Paakkala A, Kannus P, Järvinen M. Double-bundle versus single-bundle anterior cruciate ligament reconstruction: A prospective randomized study with 5-year results. Am J Sports Med. 2012;40(7):1511-1518.
2. Segond P. Recherches cliniques et expérimentales sur les épanchements sanguins du genou par entorse. Progrés Médical. 1879;6(6):1-85. French.
3. Caterine S, Litchfield R, Johnson M, Chronik B, Getgood A. A cadaveric study of the anterolateral ligament: re-introducing the lateral capsular ligament. Knee Surg Sports Traumatol Athrosc. 2015;23(11):3186-3195.
4. Claes S, Vereecke E, Maes M, Victor J, Verdonk P, Bellemans J. Anatomy of the anterolateral ligament of the knee. J Anat. 2013;223(4):321-328.
5. Dodds AL, Halewood C, Gupte CM, Williams A, Amis AA. The anterolateral ligament: Anatomy, length changes and association with the segond fracture. Bone Joint J. 2014;96-B(3):325-331.
6. Helito CP, Demange MK, Bonadio MB, et al. Anatomy and histology of the knee anterolateral ligament. Orthop J Sports Med. 2013;1(7):2325967113513546.
7. Kennedy MI, Claes S, Fuso FA, et al. The anterolateral ligament: An anatomic, radiographic, and biomechanical analysis. Am J Sports Med. 2015;43(7):1606-1615.
8. Helito CP, Helito PV, Bonadio MB, et al. Evaluation of the length and isometric pattern of the anterolateral ligament with serial computer tomography. Orthop J Sports Med. 2014;2(12):2325967114562205.
9. Parsons EM, Gee AO, Spiekerman C, Cavanagh PR. The biomechanical function of the anterolateral ligament of the knee. Am J Sports Med. 2015;43(3):669-674.
10. Zens M, Niemeyer P, Ruhhamer J, et al. Length changes of the anterolateral ligament during passive knee motion: A human cadaveric study. Am J Sports Med. 2015;43(10):2545-2552.
11. Helito CP, Bonadio MB, Gobbi RG, et al. Combined intra- and extra-articular reconstruction of the anterior cruciate ligament: the reconstruction of the knee anterolateral ligament. Arthrosc Tech. 2015;4(3):e239-e244.
12. Cianca J, John J, Pandit S, Chiou-Tan FY. Musculoskeletal ultrasound imaging of the recently described anterolateral ligament of the knee. Am J Phys Med Rehabil. 2014;93(2):186
13. Adams JE, Zobitz ME, Reach JS, et al. Rotator cuff repair using an acellular dermal matrix graft: An in vivo study in a canine model. Arthroscopy. 2006;22(7):700-709.
14. Sonnery-Cottet B, Thaunat M, Freychet B, Pupim BHB, Murphy CG, Claes S. Outcome of a combined anterior cruciate ligament and anterolateral ligament reconstruction technique with a minimum 2-year follow-up. Am J Sports Med. 2015;43(7):1598-1605.
15. Sonnery-Cottet B, Archbold P, Rezende FC, Neto AM, Fayard JM, Thaunat M. Arthroscopic identification of the anterolateral ligament of the knee. Arthrosc Tech. 2014;3(3):e389-e392.
16. Helito CP, Helito PV, Costa HP, et al. MRI evaluation of the anterolateral ligament of the knee: assessment in routine 1.5-T scans. Skeletal Radiol. 2014;43(10):1421-1427.
17. Helito CP, Demange MK, Helito PV, et al. Evaluation of the anterolateral ligament of the knee by means of magnetic resonance examination. Rev Bras Orthop. 2015;50(2):214-219.
18. Helito CP, Demange MK, Bonadio MB, et al. Radiographic landmarks for locating the femoral origin and tibial insertion of the knee anterolateral ligament. Am J Sports Med. 2014;42(10):2356-2362.
Malpractice Counsel: Missed Nodule
Case
A 48-year-old man presented to the ED with a 2-day history of cough and congestion. He described the cough as gradual in onset and, though initially nonproductive, it was now productive of green sputum. He denied fevers or chills, chest pain, nausea, vomiting, or diarrhea, and complained of only mild shortness of breath. His medical history was significant for hypertension, which was well managed with daily lisinopril-hydrochlorothiazide. He admitted to smoking one pack of cigarettes per day for the past 25 years, but denied alcohol or illicit drug use.
On physical examination, the patient’s vital signs were: blood pressure, 112/64 mm Hg; heart rate, 84 beats/min; respiratory rate, 20 breaths/min; and temperature, 98oF. Oxygen saturation was 97% on room air. The head, eyes, ears, nose, and throat examination was normal. Auscultation of the lungs revealed bilateral breath sounds with scattered, faint expiratory wheezing; the heart had a regular rate and rhythm, without murmurs, rubs, or gallops.
The emergency physician (EP) ordered posteroanterior and lateral chest X-rays (CXR), which he interpreted as normal. He also ordered an albuterol handheld nebulizer treatment for the patient. After the albuterol treatment, the patient felt he was breathing more easily. The frequency of his cough had also decreased following treatment and, on re-examination, he exhibited no wheezing and was given azithromycin 500 mg orally in the ED. The EP diagnosed the patient with acute bronchitis and discharged him home with an albuterol metered dose inhaler with a spacer, and a 4-day course of azithromycin. He also encouraged the patient to quit smoking.
The next day the radiologist’s official reading of the patient’s radiographs included the finding of a very small pulmonary nodule, which was seen only on the lateral X-ray. The radiologist recommended a repeat CXR or a computed tomography (CT) scan of the chest in 6 months.
Unfortunately, the EP never saw this information, and the patient was not contacted regarding the abnormal radiology finding and the need for follow-up. Approximately 20 months later, the patient was diagnosed with lung cancer with metastasis to the thoracic spine and liver. Despite chemotherapy and radiation treatment, he died from the cancer.
The patient’s family brought a malpractice suit against the EP, stating that the cancer could have been successfully treated prior to any metastasis if the patient had been informed of the abnormal radiology findings at his ED visit 20 months prior. The EP argued that he never saw the official radiology report, and therefore had no knowledge of the need for follow-up. At trial, a jury verdict was returned in favor of the defendant.
Discussion
Unfortunately, some version of this scenario occurs on a frequent basis. While imaging studies account for the majority of such cases, the same situation can occur with abnormal laboratory results, body-fluid cultures, or pathology reports in which an abnormality is identified (eg, positive blood culture, missed fracture) but, for a myriad of reasons, the critical information does not get related to the patient.
Because of the episodic nature of the practice of emergency medicine (EM), a process must be in place to ensure any “positive” test results or findings discovered after patient discharge are reviewed and compared to the ED diagnosis, and that any “misses” result in notifying the patient and/or his or her primary care physician and arranging follow-up. In cases such as the one presented here, a system issue existed—one that was not due to any fault or oversight of the EP. Ideally, EM leadership should work closely with leadership from radiology and laboratory services and hospital risk management to develop such a process—one that will be effective every day, including weekends and holidays.
Missed fractures on radiographs are a common cause of malpractice litigation against EPs. In one review by Kachalia et al1 examining malpractice claims involving EPs, missed fractures on radiographs accounted for 19% (the most common) of the 79 missed diagnoses identified in their study.In a similar study by Karcz et al,2 missed fractures ranked second in frequency and dollars lost in malpractice cases against EPs in Massachusetts.
While missed lesions on CXR do not occur with the same frequency as missed fractures, the results are much more devastating when the lesion turns out to be malignant. Three common areas where such lesions are missed on CXR include: the apex of the lung, obscured by overlying clavicle and ribs; the retrocardiac region (as in the patient in this case); and the lung bases obscured by the diaphragm.
Emergency physicians are neither trained nor expected to identify every single abnormality—especially subtle radiographic abnormalities. This is why there are radiology overreads, and a system or process must be in place to ensure patients are informed of any positive findings and to arrange proper follow-up.
1. Kachalia A, Gandhi TK, Puopolo AL, et al. Missed and delayed diagnoses in the emergency department: a study of closed malpractice claims from 4 liability insurers. Ann Emerg Med. 2007;49(2):196-205.
2. Karcz A, Korn R, Burke MC, et al. Malpractice claims against emergency physicians in Massachusetts: 1975-1993. Am J Emerg Med. 1996;14(4):341-345.
Case
A 48-year-old man presented to the ED with a 2-day history of cough and congestion. He described the cough as gradual in onset and, though initially nonproductive, it was now productive of green sputum. He denied fevers or chills, chest pain, nausea, vomiting, or diarrhea, and complained of only mild shortness of breath. His medical history was significant for hypertension, which was well managed with daily lisinopril-hydrochlorothiazide. He admitted to smoking one pack of cigarettes per day for the past 25 years, but denied alcohol or illicit drug use.
On physical examination, the patient’s vital signs were: blood pressure, 112/64 mm Hg; heart rate, 84 beats/min; respiratory rate, 20 breaths/min; and temperature, 98oF. Oxygen saturation was 97% on room air. The head, eyes, ears, nose, and throat examination was normal. Auscultation of the lungs revealed bilateral breath sounds with scattered, faint expiratory wheezing; the heart had a regular rate and rhythm, without murmurs, rubs, or gallops.
The emergency physician (EP) ordered posteroanterior and lateral chest X-rays (CXR), which he interpreted as normal. He also ordered an albuterol handheld nebulizer treatment for the patient. After the albuterol treatment, the patient felt he was breathing more easily. The frequency of his cough had also decreased following treatment and, on re-examination, he exhibited no wheezing and was given azithromycin 500 mg orally in the ED. The EP diagnosed the patient with acute bronchitis and discharged him home with an albuterol metered dose inhaler with a spacer, and a 4-day course of azithromycin. He also encouraged the patient to quit smoking.
The next day the radiologist’s official reading of the patient’s radiographs included the finding of a very small pulmonary nodule, which was seen only on the lateral X-ray. The radiologist recommended a repeat CXR or a computed tomography (CT) scan of the chest in 6 months.
Unfortunately, the EP never saw this information, and the patient was not contacted regarding the abnormal radiology finding and the need for follow-up. Approximately 20 months later, the patient was diagnosed with lung cancer with metastasis to the thoracic spine and liver. Despite chemotherapy and radiation treatment, he died from the cancer.
The patient’s family brought a malpractice suit against the EP, stating that the cancer could have been successfully treated prior to any metastasis if the patient had been informed of the abnormal radiology findings at his ED visit 20 months prior. The EP argued that he never saw the official radiology report, and therefore had no knowledge of the need for follow-up. At trial, a jury verdict was returned in favor of the defendant.
Discussion
Unfortunately, some version of this scenario occurs on a frequent basis. While imaging studies account for the majority of such cases, the same situation can occur with abnormal laboratory results, body-fluid cultures, or pathology reports in which an abnormality is identified (eg, positive blood culture, missed fracture) but, for a myriad of reasons, the critical information does not get related to the patient.
Because of the episodic nature of the practice of emergency medicine (EM), a process must be in place to ensure any “positive” test results or findings discovered after patient discharge are reviewed and compared to the ED diagnosis, and that any “misses” result in notifying the patient and/or his or her primary care physician and arranging follow-up. In cases such as the one presented here, a system issue existed—one that was not due to any fault or oversight of the EP. Ideally, EM leadership should work closely with leadership from radiology and laboratory services and hospital risk management to develop such a process—one that will be effective every day, including weekends and holidays.
Missed fractures on radiographs are a common cause of malpractice litigation against EPs. In one review by Kachalia et al1 examining malpractice claims involving EPs, missed fractures on radiographs accounted for 19% (the most common) of the 79 missed diagnoses identified in their study.In a similar study by Karcz et al,2 missed fractures ranked second in frequency and dollars lost in malpractice cases against EPs in Massachusetts.
While missed lesions on CXR do not occur with the same frequency as missed fractures, the results are much more devastating when the lesion turns out to be malignant. Three common areas where such lesions are missed on CXR include: the apex of the lung, obscured by overlying clavicle and ribs; the retrocardiac region (as in the patient in this case); and the lung bases obscured by the diaphragm.
Emergency physicians are neither trained nor expected to identify every single abnormality—especially subtle radiographic abnormalities. This is why there are radiology overreads, and a system or process must be in place to ensure patients are informed of any positive findings and to arrange proper follow-up.
Case
A 48-year-old man presented to the ED with a 2-day history of cough and congestion. He described the cough as gradual in onset and, though initially nonproductive, it was now productive of green sputum. He denied fevers or chills, chest pain, nausea, vomiting, or diarrhea, and complained of only mild shortness of breath. His medical history was significant for hypertension, which was well managed with daily lisinopril-hydrochlorothiazide. He admitted to smoking one pack of cigarettes per day for the past 25 years, but denied alcohol or illicit drug use.
On physical examination, the patient’s vital signs were: blood pressure, 112/64 mm Hg; heart rate, 84 beats/min; respiratory rate, 20 breaths/min; and temperature, 98oF. Oxygen saturation was 97% on room air. The head, eyes, ears, nose, and throat examination was normal. Auscultation of the lungs revealed bilateral breath sounds with scattered, faint expiratory wheezing; the heart had a regular rate and rhythm, without murmurs, rubs, or gallops.
The emergency physician (EP) ordered posteroanterior and lateral chest X-rays (CXR), which he interpreted as normal. He also ordered an albuterol handheld nebulizer treatment for the patient. After the albuterol treatment, the patient felt he was breathing more easily. The frequency of his cough had also decreased following treatment and, on re-examination, he exhibited no wheezing and was given azithromycin 500 mg orally in the ED. The EP diagnosed the patient with acute bronchitis and discharged him home with an albuterol metered dose inhaler with a spacer, and a 4-day course of azithromycin. He also encouraged the patient to quit smoking.
The next day the radiologist’s official reading of the patient’s radiographs included the finding of a very small pulmonary nodule, which was seen only on the lateral X-ray. The radiologist recommended a repeat CXR or a computed tomography (CT) scan of the chest in 6 months.
Unfortunately, the EP never saw this information, and the patient was not contacted regarding the abnormal radiology finding and the need for follow-up. Approximately 20 months later, the patient was diagnosed with lung cancer with metastasis to the thoracic spine and liver. Despite chemotherapy and radiation treatment, he died from the cancer.
The patient’s family brought a malpractice suit against the EP, stating that the cancer could have been successfully treated prior to any metastasis if the patient had been informed of the abnormal radiology findings at his ED visit 20 months prior. The EP argued that he never saw the official radiology report, and therefore had no knowledge of the need for follow-up. At trial, a jury verdict was returned in favor of the defendant.
Discussion
Unfortunately, some version of this scenario occurs on a frequent basis. While imaging studies account for the majority of such cases, the same situation can occur with abnormal laboratory results, body-fluid cultures, or pathology reports in which an abnormality is identified (eg, positive blood culture, missed fracture) but, for a myriad of reasons, the critical information does not get related to the patient.
Because of the episodic nature of the practice of emergency medicine (EM), a process must be in place to ensure any “positive” test results or findings discovered after patient discharge are reviewed and compared to the ED diagnosis, and that any “misses” result in notifying the patient and/or his or her primary care physician and arranging follow-up. In cases such as the one presented here, a system issue existed—one that was not due to any fault or oversight of the EP. Ideally, EM leadership should work closely with leadership from radiology and laboratory services and hospital risk management to develop such a process—one that will be effective every day, including weekends and holidays.
Missed fractures on radiographs are a common cause of malpractice litigation against EPs. In one review by Kachalia et al1 examining malpractice claims involving EPs, missed fractures on radiographs accounted for 19% (the most common) of the 79 missed diagnoses identified in their study.In a similar study by Karcz et al,2 missed fractures ranked second in frequency and dollars lost in malpractice cases against EPs in Massachusetts.
While missed lesions on CXR do not occur with the same frequency as missed fractures, the results are much more devastating when the lesion turns out to be malignant. Three common areas where such lesions are missed on CXR include: the apex of the lung, obscured by overlying clavicle and ribs; the retrocardiac region (as in the patient in this case); and the lung bases obscured by the diaphragm.
Emergency physicians are neither trained nor expected to identify every single abnormality—especially subtle radiographic abnormalities. This is why there are radiology overreads, and a system or process must be in place to ensure patients are informed of any positive findings and to arrange proper follow-up.
1. Kachalia A, Gandhi TK, Puopolo AL, et al. Missed and delayed diagnoses in the emergency department: a study of closed malpractice claims from 4 liability insurers. Ann Emerg Med. 2007;49(2):196-205.
2. Karcz A, Korn R, Burke MC, et al. Malpractice claims against emergency physicians in Massachusetts: 1975-1993. Am J Emerg Med. 1996;14(4):341-345.
1. Kachalia A, Gandhi TK, Puopolo AL, et al. Missed and delayed diagnoses in the emergency department: a study of closed malpractice claims from 4 liability insurers. Ann Emerg Med. 2007;49(2):196-205.
2. Karcz A, Korn R, Burke MC, et al. Malpractice claims against emergency physicians in Massachusetts: 1975-1993. Am J Emerg Med. 1996;14(4):341-345.
Emergency Ultrasound: Ultrasound-Guided Femoral Nerve Block
Case Scenario
A young man presented to the ED for evaluation of a large laceration to the anterior thigh that resulted from an industrial accident (Figure 1).
Femoral nerve blocks are useful in a variety of clinical scenarios, including fractures of the femur or hip1 and laceration repairs (Figure 2).
Identifying the Femoral Nerve on Ultrasound
To perform this nerve block, one must recall the anatomy of femoral central-line placement. The femoral nerve lies lateral to the femoral artery and vein. The high-frequency probe should be placed over the femoral crease (Figure 3).
Performing the Block
An ultrasound-guided femoral nerve block can be performed using a 22-gauge blunt tip spinal needle, and an in-plane or out-of-plane technique can be employed. We prefer using an in-plane technique because the entire shaft of the needle can be visualized as it approaches the nerve. Anatomically, the femoral nerve lies in a separate fascial plane from the artery and vein, beneath the fascia iliaca (Figure 4). You can use this anatomic location of the femoral nerve to your advantage when performing the block. The needle can be advanced to a target slightly lateral to the nerve until it pops beneath the fascia iliaca. On the ultrasound, you can monitor the spread of anesthetic as it is injected. If the needle is in the right location, the hypoechoic fluid will spread medially toward the nerve, but will not track around the artery or vein. At least 15 cc to 20 cc of local anesthetic is typically required.2,3 If you prefer, the anesthetic can be diluted in normal saline, in a 1:1 ratio, to achieve adequate volume.
If you do not see the anesthetic spread during the injection, you should stop and check the needle placement, as it may be intravascular. Using a more lateral approach, targeting the injection at the fascial plane, rather than the nerve, helps to avoid direct intraneural injection or contact with the nerve—and it keeps the needle far away from the femoral vascular bundle.
Safety Considerations
As with any technique, prior to the procedure, aseptic measures should be taken, including the use of a sterile probe cover and sterile gloves. All patients undergoing ultrasound-guided nerve blocks proximal to the wrist or ankle should be placed on a cardiac monitor. In addition, intralipid emulsion should be readily available for administration in the unlikely event there is inadvertent intravascular injection of local anesthetic and cardiovascular collapse occurs.
Summary
With practice, ultrasound guidance can improve the procedural success of femoral nerve blocks and decrease the risk of nerve injury compared to blind nerve blocks
1. Dickman E, Pushkar I, Likourezos A, et al. Ultrasound-guided nerve blocks for intracapsular and extracapsular hip fractures. Am J Emerg Med. 2016;34(3):586-589.
2. Femoral nerve block. In: Hadzic A, Carrera A, Clark T, et al, eds. Hadzic’s Peripheral Nerve Blocks: An Anatomy for Ultrasound-Guided Regional Anesthesia. 2nd ed. New York, NY: The McGraw Hill Companies, Inc; 2012:267-279.
3. Ultrasound-guided femoral nerve block. In: Hadzic A, Carrera A, Clark T, et al, eds. Hadzic’s Peripheral Nerve Blocks: An Anatomy for Ultrasound-Guided Regional Anesthesia. 2nd ed. New York, NY: The McGraw Hill Companies, Inc; 2012:397-404.
Case Scenario
A young man presented to the ED for evaluation of a large laceration to the anterior thigh that resulted from an industrial accident (Figure 1).
Femoral nerve blocks are useful in a variety of clinical scenarios, including fractures of the femur or hip1 and laceration repairs (Figure 2).
Identifying the Femoral Nerve on Ultrasound
To perform this nerve block, one must recall the anatomy of femoral central-line placement. The femoral nerve lies lateral to the femoral artery and vein. The high-frequency probe should be placed over the femoral crease (Figure 3).
Performing the Block
An ultrasound-guided femoral nerve block can be performed using a 22-gauge blunt tip spinal needle, and an in-plane or out-of-plane technique can be employed. We prefer using an in-plane technique because the entire shaft of the needle can be visualized as it approaches the nerve. Anatomically, the femoral nerve lies in a separate fascial plane from the artery and vein, beneath the fascia iliaca (Figure 4). You can use this anatomic location of the femoral nerve to your advantage when performing the block. The needle can be advanced to a target slightly lateral to the nerve until it pops beneath the fascia iliaca. On the ultrasound, you can monitor the spread of anesthetic as it is injected. If the needle is in the right location, the hypoechoic fluid will spread medially toward the nerve, but will not track around the artery or vein. At least 15 cc to 20 cc of local anesthetic is typically required.2,3 If you prefer, the anesthetic can be diluted in normal saline, in a 1:1 ratio, to achieve adequate volume.
If you do not see the anesthetic spread during the injection, you should stop and check the needle placement, as it may be intravascular. Using a more lateral approach, targeting the injection at the fascial plane, rather than the nerve, helps to avoid direct intraneural injection or contact with the nerve—and it keeps the needle far away from the femoral vascular bundle.
Safety Considerations
As with any technique, prior to the procedure, aseptic measures should be taken, including the use of a sterile probe cover and sterile gloves. All patients undergoing ultrasound-guided nerve blocks proximal to the wrist or ankle should be placed on a cardiac monitor. In addition, intralipid emulsion should be readily available for administration in the unlikely event there is inadvertent intravascular injection of local anesthetic and cardiovascular collapse occurs.
Summary
With practice, ultrasound guidance can improve the procedural success of femoral nerve blocks and decrease the risk of nerve injury compared to blind nerve blocks
Case Scenario
A young man presented to the ED for evaluation of a large laceration to the anterior thigh that resulted from an industrial accident (Figure 1).
Femoral nerve blocks are useful in a variety of clinical scenarios, including fractures of the femur or hip1 and laceration repairs (Figure 2).
Identifying the Femoral Nerve on Ultrasound
To perform this nerve block, one must recall the anatomy of femoral central-line placement. The femoral nerve lies lateral to the femoral artery and vein. The high-frequency probe should be placed over the femoral crease (Figure 3).
Performing the Block
An ultrasound-guided femoral nerve block can be performed using a 22-gauge blunt tip spinal needle, and an in-plane or out-of-plane technique can be employed. We prefer using an in-plane technique because the entire shaft of the needle can be visualized as it approaches the nerve. Anatomically, the femoral nerve lies in a separate fascial plane from the artery and vein, beneath the fascia iliaca (Figure 4). You can use this anatomic location of the femoral nerve to your advantage when performing the block. The needle can be advanced to a target slightly lateral to the nerve until it pops beneath the fascia iliaca. On the ultrasound, you can monitor the spread of anesthetic as it is injected. If the needle is in the right location, the hypoechoic fluid will spread medially toward the nerve, but will not track around the artery or vein. At least 15 cc to 20 cc of local anesthetic is typically required.2,3 If you prefer, the anesthetic can be diluted in normal saline, in a 1:1 ratio, to achieve adequate volume.
If you do not see the anesthetic spread during the injection, you should stop and check the needle placement, as it may be intravascular. Using a more lateral approach, targeting the injection at the fascial plane, rather than the nerve, helps to avoid direct intraneural injection or contact with the nerve—and it keeps the needle far away from the femoral vascular bundle.
Safety Considerations
As with any technique, prior to the procedure, aseptic measures should be taken, including the use of a sterile probe cover and sterile gloves. All patients undergoing ultrasound-guided nerve blocks proximal to the wrist or ankle should be placed on a cardiac monitor. In addition, intralipid emulsion should be readily available for administration in the unlikely event there is inadvertent intravascular injection of local anesthetic and cardiovascular collapse occurs.
Summary
With practice, ultrasound guidance can improve the procedural success of femoral nerve blocks and decrease the risk of nerve injury compared to blind nerve blocks
1. Dickman E, Pushkar I, Likourezos A, et al. Ultrasound-guided nerve blocks for intracapsular and extracapsular hip fractures. Am J Emerg Med. 2016;34(3):586-589.
2. Femoral nerve block. In: Hadzic A, Carrera A, Clark T, et al, eds. Hadzic’s Peripheral Nerve Blocks: An Anatomy for Ultrasound-Guided Regional Anesthesia. 2nd ed. New York, NY: The McGraw Hill Companies, Inc; 2012:267-279.
3. Ultrasound-guided femoral nerve block. In: Hadzic A, Carrera A, Clark T, et al, eds. Hadzic’s Peripheral Nerve Blocks: An Anatomy for Ultrasound-Guided Regional Anesthesia. 2nd ed. New York, NY: The McGraw Hill Companies, Inc; 2012:397-404.
1. Dickman E, Pushkar I, Likourezos A, et al. Ultrasound-guided nerve blocks for intracapsular and extracapsular hip fractures. Am J Emerg Med. 2016;34(3):586-589.
2. Femoral nerve block. In: Hadzic A, Carrera A, Clark T, et al, eds. Hadzic’s Peripheral Nerve Blocks: An Anatomy for Ultrasound-Guided Regional Anesthesia. 2nd ed. New York, NY: The McGraw Hill Companies, Inc; 2012:267-279.
3. Ultrasound-guided femoral nerve block. In: Hadzic A, Carrera A, Clark T, et al, eds. Hadzic’s Peripheral Nerve Blocks: An Anatomy for Ultrasound-Guided Regional Anesthesia. 2nd ed. New York, NY: The McGraw Hill Companies, Inc; 2012:397-404.
Delayed bleeding possible with EBUS-TBNA on antiplatelets
LOS ANGELES – There might be a slight increase in delayed bleeding when patients have endobronchial ultrasound with transbronchial needle aspiration within 5 days of taking oral antiplatelets, according to a review of 404 patients at Riverside Methodist Hospital in Columbus, Ohio.
This study is unusual in that it looked at the 48 hour mark. Previous studies have tended to focus on immediate bleeding events that require the procedure to be stopped; only some of that research has found an increased bleeding risk with antiplatelet therapy.
In the study at Riverside Methodist, none of the 20 patients on dual antiplatelet therapy – clopidogrel (Plavix) plus aspirin – bled during the procedure, but one (5%) had a hemoglobin drop of more than 2 g within 48 hours and another was readmitted to the hospital within 48 hours for procedure-related hemoptysis. Overall, the delayed bleeding event rate for patients using the dual antiplatelet therapy was 10%. Additionally, one of the 13 patients (7.7%) on clopidogrel alone experienced a greater than 2 g drop in hemoglobin.
Among the 270 patients not exposed to antiplatelets, the overall bleeding event rate was 2.6%, and the event rate for delayed bleeding was 1.1%. Four patients (1.5%) bled during the procedure, two (0.7%) had hemoglobin drops greater than 2 g within 48 hours, and one (0.4%) was readmitted for hemoptysis.
There were no bleeding events in the 101 patients who only took aspirin.
“There was a trend toward delayed bleeding events in patients” on clopidogrel or dual antiplatelets. “It’s worth considering a thoughtful pause in decision making. Maybe with the bleeding events we’re seeing, it would be worthwhile, if possible, to defer” endobronchial ultrasound with transbronchial needle aspiration “until after the antiplatelet therapy,” said Kevin Swiatek, DO, a medicine resident at Riverside.
Patients were excluded from the study if they had histories of bleeding or clotting disorders; low platelet counts; or if they were on anticoagulation. Subjects on antiplatelets were about 10 years older, on average, than those who were not (about 68 versus 59 years old), and more likely to have had a heart attack or stroke, and to be hypertensive.
There was no industry funding for the work, and the investigators had no disclosures.
LOS ANGELES – There might be a slight increase in delayed bleeding when patients have endobronchial ultrasound with transbronchial needle aspiration within 5 days of taking oral antiplatelets, according to a review of 404 patients at Riverside Methodist Hospital in Columbus, Ohio.
This study is unusual in that it looked at the 48 hour mark. Previous studies have tended to focus on immediate bleeding events that require the procedure to be stopped; only some of that research has found an increased bleeding risk with antiplatelet therapy.
In the study at Riverside Methodist, none of the 20 patients on dual antiplatelet therapy – clopidogrel (Plavix) plus aspirin – bled during the procedure, but one (5%) had a hemoglobin drop of more than 2 g within 48 hours and another was readmitted to the hospital within 48 hours for procedure-related hemoptysis. Overall, the delayed bleeding event rate for patients using the dual antiplatelet therapy was 10%. Additionally, one of the 13 patients (7.7%) on clopidogrel alone experienced a greater than 2 g drop in hemoglobin.
Among the 270 patients not exposed to antiplatelets, the overall bleeding event rate was 2.6%, and the event rate for delayed bleeding was 1.1%. Four patients (1.5%) bled during the procedure, two (0.7%) had hemoglobin drops greater than 2 g within 48 hours, and one (0.4%) was readmitted for hemoptysis.
There were no bleeding events in the 101 patients who only took aspirin.
“There was a trend toward delayed bleeding events in patients” on clopidogrel or dual antiplatelets. “It’s worth considering a thoughtful pause in decision making. Maybe with the bleeding events we’re seeing, it would be worthwhile, if possible, to defer” endobronchial ultrasound with transbronchial needle aspiration “until after the antiplatelet therapy,” said Kevin Swiatek, DO, a medicine resident at Riverside.
Patients were excluded from the study if they had histories of bleeding or clotting disorders; low platelet counts; or if they were on anticoagulation. Subjects on antiplatelets were about 10 years older, on average, than those who were not (about 68 versus 59 years old), and more likely to have had a heart attack or stroke, and to be hypertensive.
There was no industry funding for the work, and the investigators had no disclosures.
LOS ANGELES – There might be a slight increase in delayed bleeding when patients have endobronchial ultrasound with transbronchial needle aspiration within 5 days of taking oral antiplatelets, according to a review of 404 patients at Riverside Methodist Hospital in Columbus, Ohio.
This study is unusual in that it looked at the 48 hour mark. Previous studies have tended to focus on immediate bleeding events that require the procedure to be stopped; only some of that research has found an increased bleeding risk with antiplatelet therapy.
In the study at Riverside Methodist, none of the 20 patients on dual antiplatelet therapy – clopidogrel (Plavix) plus aspirin – bled during the procedure, but one (5%) had a hemoglobin drop of more than 2 g within 48 hours and another was readmitted to the hospital within 48 hours for procedure-related hemoptysis. Overall, the delayed bleeding event rate for patients using the dual antiplatelet therapy was 10%. Additionally, one of the 13 patients (7.7%) on clopidogrel alone experienced a greater than 2 g drop in hemoglobin.
Among the 270 patients not exposed to antiplatelets, the overall bleeding event rate was 2.6%, and the event rate for delayed bleeding was 1.1%. Four patients (1.5%) bled during the procedure, two (0.7%) had hemoglobin drops greater than 2 g within 48 hours, and one (0.4%) was readmitted for hemoptysis.
There were no bleeding events in the 101 patients who only took aspirin.
“There was a trend toward delayed bleeding events in patients” on clopidogrel or dual antiplatelets. “It’s worth considering a thoughtful pause in decision making. Maybe with the bleeding events we’re seeing, it would be worthwhile, if possible, to defer” endobronchial ultrasound with transbronchial needle aspiration “until after the antiplatelet therapy,” said Kevin Swiatek, DO, a medicine resident at Riverside.
Patients were excluded from the study if they had histories of bleeding or clotting disorders; low platelet counts; or if they were on anticoagulation. Subjects on antiplatelets were about 10 years older, on average, than those who were not (about 68 versus 59 years old), and more likely to have had a heart attack or stroke, and to be hypertensive.
There was no industry funding for the work, and the investigators had no disclosures.
AT CHEST 2016
Key clinical point:
Major finding: Ten percent of patients on dual antiplatelet therapy bled within 48 hours, versus 1.1% of those not on antiplatelet therapy.
Data source: Single-center review of 404 patients.
Disclosures: There was no industry funding for the work, and the investigators had no disclosures.
ReACT: No benefit from routine coronary angiography after PCI
Routine follow-up coronary angiography after percutaneous coronary intervention leads to increased rates of coronary revascularization but without any significant benefits for outcomes, according to a study presented at the Transcatheter Cardiovascular Therapeutics annual meeting and published simultaneously on Nov. 1 in the Journal of the American College of Cardiology: Cardiovascular Interventions.
Hiroki Shiomi, MD, from Kyoto University, and his coauthors reported on ReACT, a prospective, open-label randomized controlled trial of routine follow-up coronary angiography in 700 patients who underwent successful percutaneous coronary intervention (PCI).
Among the 349 patients randomized to follow-up coronary angiography (FUCAG), 12.8% underwent any coronary revascularization within the first year after PCI, compared with 3.8% of the 351 patients randomized to standard clinical follow-up. The routine angiography group also had a higher incidence of target lesion revascularization in the first year after the index PCI (7.0% vs. 1.7%).
In both these cases, the cumulative 5-year incidence of coronary or target lesion revascularization was not significantly different between the routine angiography and control groups. However researchers saw no significant benefit from routine FUCAG in terms of the cumulative 5-year incidence of all-cause death, myocardial infarction, stroke, or emergency hospitalizations for acute coronary syndrome or heart failure, compared with clinical follow-up (22.4% vs. 24.7%; P = 0.70).
Nor were there any significant differences between the two groups in these individual components, or in the cumulative 5-year incidence of major bleeding (JACC Cardiovasc Interv. 2016 Nov 1.)
The authors commented that several previous studies have shown that routine FUCAG does not improve clinical outcomes, although it is still commonly performed in Japan after PCI.
“However, previous studies in the drug-eluting stents (DES) era were conducted in the context of pivotal randomized trials of DES and there have been no randomized clinical trials evaluating long-term clinical impact of routine FUCAG after PCI in the real world clinical practice including high-risk patients for cardiovascular events risk such as complex coronary artery disease and acute myocardial infarction (AMI) presentation,” the authors wrote.
Overall, 85.4% of patients in the routine angiography group and 12% of those in the clinical care group underwent coronary angiography in the first year, including for clinical reasons.
In the clinical follow-up group, coronary angiography was performed because of acute coronary syndrome (14%), recurrence of angina (60%), other clinical reasons (14%), or no clinical reason (12%). The control group also had more noninvasive physiological stress testing such as treadmill exercise test and stress nuclear study.
“Considering the invasive nature of coronary angiography and increased medical expenses, routine FUCAG after PCI would not be allowed as the usual clinical practice, unless patients have recurrent symptoms or objective evidence of ischemia,” the authors wrote.
“On the other hand, there was no excess of adverse clinical events with routine angiographic follow-up strategy except for the increased rate of 1-year repeat coronary revascularization.”
Given this, they suggested that scheduled angiographic follow-up might still be considered acceptable for early in vivo or significant coronary device trials.
While the authors said the trial ended up being underpowered because of a reduced final sample size and lower-than-anticipated event rate, it did warrant further larger-scale studies. In particular, they highlighted the question of what impact routine follow-up angiography might have in higher-risk patients, such as those with left main or multivessel coronary artery disease.
“Finally, because patient demographics, practice patterns including the indication of coronary revascularization, and clinical outcomes in Japan may be different from those outside Japan, generalizing the present study results to populations outside Japan should be done with caution.”
This study was supported by an educational grant from the Research Institute for Production Development (Kyoto). One author declared honoraria for education consulting from Boston Scientific Corporation.
Routine follow-up coronary angiography after percutaneous coronary intervention leads to increased rates of coronary revascularization but without any significant benefits for outcomes, according to a study presented at the Transcatheter Cardiovascular Therapeutics annual meeting and published simultaneously on Nov. 1 in the Journal of the American College of Cardiology: Cardiovascular Interventions.
Hiroki Shiomi, MD, from Kyoto University, and his coauthors reported on ReACT, a prospective, open-label randomized controlled trial of routine follow-up coronary angiography in 700 patients who underwent successful percutaneous coronary intervention (PCI).
Among the 349 patients randomized to follow-up coronary angiography (FUCAG), 12.8% underwent any coronary revascularization within the first year after PCI, compared with 3.8% of the 351 patients randomized to standard clinical follow-up. The routine angiography group also had a higher incidence of target lesion revascularization in the first year after the index PCI (7.0% vs. 1.7%).
In both these cases, the cumulative 5-year incidence of coronary or target lesion revascularization was not significantly different between the routine angiography and control groups. However researchers saw no significant benefit from routine FUCAG in terms of the cumulative 5-year incidence of all-cause death, myocardial infarction, stroke, or emergency hospitalizations for acute coronary syndrome or heart failure, compared with clinical follow-up (22.4% vs. 24.7%; P = 0.70).
Nor were there any significant differences between the two groups in these individual components, or in the cumulative 5-year incidence of major bleeding (JACC Cardiovasc Interv. 2016 Nov 1.)
The authors commented that several previous studies have shown that routine FUCAG does not improve clinical outcomes, although it is still commonly performed in Japan after PCI.
“However, previous studies in the drug-eluting stents (DES) era were conducted in the context of pivotal randomized trials of DES and there have been no randomized clinical trials evaluating long-term clinical impact of routine FUCAG after PCI in the real world clinical practice including high-risk patients for cardiovascular events risk such as complex coronary artery disease and acute myocardial infarction (AMI) presentation,” the authors wrote.
Overall, 85.4% of patients in the routine angiography group and 12% of those in the clinical care group underwent coronary angiography in the first year, including for clinical reasons.
In the clinical follow-up group, coronary angiography was performed because of acute coronary syndrome (14%), recurrence of angina (60%), other clinical reasons (14%), or no clinical reason (12%). The control group also had more noninvasive physiological stress testing such as treadmill exercise test and stress nuclear study.
“Considering the invasive nature of coronary angiography and increased medical expenses, routine FUCAG after PCI would not be allowed as the usual clinical practice, unless patients have recurrent symptoms or objective evidence of ischemia,” the authors wrote.
“On the other hand, there was no excess of adverse clinical events with routine angiographic follow-up strategy except for the increased rate of 1-year repeat coronary revascularization.”
Given this, they suggested that scheduled angiographic follow-up might still be considered acceptable for early in vivo or significant coronary device trials.
While the authors said the trial ended up being underpowered because of a reduced final sample size and lower-than-anticipated event rate, it did warrant further larger-scale studies. In particular, they highlighted the question of what impact routine follow-up angiography might have in higher-risk patients, such as those with left main or multivessel coronary artery disease.
“Finally, because patient demographics, practice patterns including the indication of coronary revascularization, and clinical outcomes in Japan may be different from those outside Japan, generalizing the present study results to populations outside Japan should be done with caution.”
This study was supported by an educational grant from the Research Institute for Production Development (Kyoto). One author declared honoraria for education consulting from Boston Scientific Corporation.
Routine follow-up coronary angiography after percutaneous coronary intervention leads to increased rates of coronary revascularization but without any significant benefits for outcomes, according to a study presented at the Transcatheter Cardiovascular Therapeutics annual meeting and published simultaneously on Nov. 1 in the Journal of the American College of Cardiology: Cardiovascular Interventions.
Hiroki Shiomi, MD, from Kyoto University, and his coauthors reported on ReACT, a prospective, open-label randomized controlled trial of routine follow-up coronary angiography in 700 patients who underwent successful percutaneous coronary intervention (PCI).
Among the 349 patients randomized to follow-up coronary angiography (FUCAG), 12.8% underwent any coronary revascularization within the first year after PCI, compared with 3.8% of the 351 patients randomized to standard clinical follow-up. The routine angiography group also had a higher incidence of target lesion revascularization in the first year after the index PCI (7.0% vs. 1.7%).
In both these cases, the cumulative 5-year incidence of coronary or target lesion revascularization was not significantly different between the routine angiography and control groups. However researchers saw no significant benefit from routine FUCAG in terms of the cumulative 5-year incidence of all-cause death, myocardial infarction, stroke, or emergency hospitalizations for acute coronary syndrome or heart failure, compared with clinical follow-up (22.4% vs. 24.7%; P = 0.70).
Nor were there any significant differences between the two groups in these individual components, or in the cumulative 5-year incidence of major bleeding (JACC Cardiovasc Interv. 2016 Nov 1.)
The authors commented that several previous studies have shown that routine FUCAG does not improve clinical outcomes, although it is still commonly performed in Japan after PCI.
“However, previous studies in the drug-eluting stents (DES) era were conducted in the context of pivotal randomized trials of DES and there have been no randomized clinical trials evaluating long-term clinical impact of routine FUCAG after PCI in the real world clinical practice including high-risk patients for cardiovascular events risk such as complex coronary artery disease and acute myocardial infarction (AMI) presentation,” the authors wrote.
Overall, 85.4% of patients in the routine angiography group and 12% of those in the clinical care group underwent coronary angiography in the first year, including for clinical reasons.
In the clinical follow-up group, coronary angiography was performed because of acute coronary syndrome (14%), recurrence of angina (60%), other clinical reasons (14%), or no clinical reason (12%). The control group also had more noninvasive physiological stress testing such as treadmill exercise test and stress nuclear study.
“Considering the invasive nature of coronary angiography and increased medical expenses, routine FUCAG after PCI would not be allowed as the usual clinical practice, unless patients have recurrent symptoms or objective evidence of ischemia,” the authors wrote.
“On the other hand, there was no excess of adverse clinical events with routine angiographic follow-up strategy except for the increased rate of 1-year repeat coronary revascularization.”
Given this, they suggested that scheduled angiographic follow-up might still be considered acceptable for early in vivo or significant coronary device trials.
While the authors said the trial ended up being underpowered because of a reduced final sample size and lower-than-anticipated event rate, it did warrant further larger-scale studies. In particular, they highlighted the question of what impact routine follow-up angiography might have in higher-risk patients, such as those with left main or multivessel coronary artery disease.
“Finally, because patient demographics, practice patterns including the indication of coronary revascularization, and clinical outcomes in Japan may be different from those outside Japan, generalizing the present study results to populations outside Japan should be done with caution.”
This study was supported by an educational grant from the Research Institute for Production Development (Kyoto). One author declared honoraria for education consulting from Boston Scientific Corporation.
Key clinical point: Routine follow-up coronary angiography after percutaneous coronary intervention increases rates of coronary revascularization but does not improve outcomes.
Major finding: Patients who underwent routine angiographic follow-up had a similar cumulative 5-year incidence of all-cause death, myocardial infarction, stroke, or emergency hospitalizations for acute coronary syndrome or heart failure as those who had standard clinical follow-up.
Data source: ReACT: a prospective, open-label randomized controlled trial in 700 patients after percutaneous coronary intervention.
Disclosures: This study was supported by an educational grant from the Research Institute for Production Development (Kyoto). One author declared honoraria for education consulting from Boston Scientific Corporation.
Left ventricular thrombosis can still complicate acute myocardial infarction
A 62-year-old man with hypertension, type 2 diabetes mellitus, and hypercholesterolemia presented to the emergency department with substernal chest pain that started about 15 hours earlier while he was at rest watching television.
On examination, his pulse was 92 beats per minute and regular, his blood pressure was 160/88 mm Hg, and he had no evidence of jugular venous distention or pedal edema. Lung examination was positive for bibasilar crackles.
Electrocardiography revealed Q waves with ST elevation in leads I, aVL, V4, V5, and V6 with reciprocal ST depression in leads II, III, and aVF.
His troponin T level on presentation was markedly elevated.

He underwent heart catheterization and was found to have 100% occlusion of the proximal left anterior descending artery. He underwent successful percutaneous coronary intervention with placement of a drug-eluting stent, and afterward had grade 3 flow on the Thrombolysis in Myocardial Infarction (TIMI) scale.
Echocardiography the next day revealed a mobile echo-dense mass in the left ventricular apex (Figure 1) and a left ventricular ejection fraction of 35%.
THE INCIDENCE OF LEFT VENTRICULAR THROMBOSIS IN ACUTE MI
1. What is the incidence of left ventricular thrombosis after acute myocardial infarction (MI), now that primary percutaneous coronary intervention is common?
- 0.1%
- 2%
- 20%
- 40%
Left ventricular thrombosis is a serious complication of acute MI that can cause systemic thromboembolism, including stroke.1 Before thrombolytic therapy was available, this complication occurred in 20% to 60% of patients with acute MI.2,3 But early reperfusion strategies, anticoagulation for the first 48 hours, and dual antiplatelet therapy have reduced the incidence of this complication significantly.
In the thrombolytic era, the incidence of left ventricular thrombosis was 5.1% in the Gruppo Italiano per lo Studio della Sopravvivenza nell’Infarto Miocardico (GISSI) 3 study, which had 8,326 patients. A subset of patients who had an anterior MI had almost double the incidence (11.5%).3

The incidence has further declined with the advent of primary percutaneous coronary intervention, likely thanks to enhanced myocardial salvage, and now ranges from 2.5% to 15% (Table 1).4–11 The largest observational study, with 2,911 patients undergoing percutaneous coronary intervention, reported an incidence of 2.5% within 3 to 5 days of the MI.7 At our center, the incidence was found to be even lower, 1.8% in 1,700 patients presenting with ST-elevation MI undergoing primary percutaneous coronary intervention. Hence, of the answers to the question above, 2% would be closest.
Large infarct size with a low left ventricular ejection fraction (< 40%), anterior wall MI, hypertension, and delay in time from symptom onset to intervention were independent predictors of left ventricular thrombus formation in most studies.7,12 The risk is highest during the first 2 weeks after MI, and thrombosis almost never occurs more than 3 months after the index event.5,13–16
WHAT IS THE PATHOGENESIS OF LEFT VENTRICULAR THROMBOSIS?
A large transmural infarct results in loss of contractile function, which causes stagnation and pooling of blood adjacent to the infarcted ventricular segment. In addition, endocardial injury exposes tissue factor, which then initiates the coagulation cascade. To make matters worse, MI results in a hypercoagulable state through unclear mechanisms, which completes the Virchow triad for thrombus formation. Elevations of D-dimer, fibrinogen, anticardiolipin antibodies (IgM and IgG), and tissue factor have also been reported after acute MI.17
Thrombus formation begins with platelet aggregation at the site of endocardial damage, forming a platelet plug, followed by activation of clotting factors. These thrombi are referred to as “mural,” as they adhere to the chamber wall (endocardium). They are composed of fibrin and entrapped red and white blood cells (Figure 2).
The natural course of thrombus evolution is established but variable. A left ventricular thrombus may dislodge and embolize, resulting in stroke or other thromboembolic complications. Alternately, it can dissolve over time, aided by intrinsic fibrinolytic mechanisms. On other occasions, the thrombus may organize, a process characterized by ingrowth of smooth muscle cells, fibroblasts, and endothelium.
HOW IS LEFT VENTRICULAR THROMBOSIS DIAGNOSED?
2. What is the best imaging test for detecting a thrombus?
- Transesophageal echocardiography
- Transthoracic echocardiography
- Cardiac magnetic resonance imaging (MRI) without gadolinium contrast
- Cardiac MRI with gadolinium contrast
Evaluation of left ventricular function after acute MI carries a class I indication (ie, it should be performed).18
Echocardiography is commonly used, and it has a 60% sensitivity to detect a thrombus.19 In patients with poorer transthoracic echocardiographic windows, contrast can be used to better delineate the left ventricular cavity and show the thrombus. Transesophageal echocardiography is seldom useful, as the left ventricular apex is foreshortened and in the far field.
A left ventricular thrombus is confirmed if an echo-dense mass with well-demarcated margins distinct from the endocardium is seen throughout the cardiac cycle. It should be evident in at least two different views (apical and short-axis) and should be adjacent to a hypokinetic or akinetic left ventricular wall. False-positive findings can occur due to misidentified false tendons, papillary muscles, and trabeculae.
Cardiac MRI with late gadolinium enhancement is now the gold standard for diagnostic imaging, as it accurately characterizes the shape, size, and location of the thrombus (Figure 3). Gadolinium contrast increases the enhancement of the ventricular cavity, thus allowing easy detection of thrombus, which appears dark. Cardiac MRI with delayed enhancement has 88% to 91% sensitivity and 99% specificity to detect left ventricular thrombosis.20,21 However, compared with echocardiography, routine cardiac MRI is time-intensive, costly, and not routinely available. As a result, it should be performed only in patients with poor acoustic windows and a high clinical suspicion of left ventricular thrombosis.
Delayed-contrast cardiac computed tomography can be used to identify left ventricular thrombosis, using absence of contrast uptake. The need to use contrast is a disadvantage, but computed tomography can be an alternative in patients with contraindications to cardiac MRI.
WHAT COMPLICATIONS ARISE FROM LEFT VENTRICULAR THROMBOSIS?
The most feared complication of left ventricular thrombosis is thromboembolism. Cardioembolic stroke is generally severe, prone to early and long-term recurrence, and associated with a higher death rate than noncardioembolic ischemic stroke.22,23 Thrombi associated with thromboembolism are often acute and mobile rather than organized and immobile.24 They may embolize to the brain, spleen, kidneys, and bowel.25 In a meta-analysis of 11 studies, the pooled odds ratio for risk of embolization was 5.45 (95% confidence interval [CI] 3.02–9.83) with left ventricular thrombi vs without.26 Before systemic thrombolysis and antiplatelet therapy became available, stroke rates ranged from 1.5% to 10%.27–29
In a meta-analysis of 22 studies from 1978 to 2004, the incidence of ischemic stroke after MI during hospitalization was around 11.1 per 1,000 MIs.30 This study found that anterior MI was associated with a higher risk of stroke, but reported no difference in the incidence of stroke with percutaneous coronary intervention, systemic thrombolysis, or no reperfusion.
In a large prospective cohort study of 2,160 patients,31 259 (12%) had a stroke after MI. In multivariable analysis, age, diabetes, and previous stroke were predictors of stroke after MI. This study reported significantly fewer strokes in patients who underwent percutaneous coronary intervention than with other or no reperfusion therapies.31
ANTICOAGULATION TREATMENT
3. How would you treat a patient who has a drug-eluting stent in the left anterior descending artery and a new diagnosis of left ventricular thrombosis?
- Warfarin
- Aspirin and clopidogrel
- Aspirin, clopidogrel, and warfarin
- Aspirin and warfarin
The management of left ventricular thrombosis has been summarized in guidelines from the American College of Chest Physicians (ACCP) in 2012,32 and from the American College of Cardiology/American Heart Association in 2013,18 which recommend anticoagulation for at least 3 months, or indefinitely if bleeding risk is low, for all patients developing a left ventricular thrombus.
For patients with acute MI and left ventricular thrombosis, the ACCP guidelines recommend warfarin with a target international normalized ratio of 2.0 to 3.0 plus dual antiplatelet therapy (eg, aspirin plus clopidogrel) for 3 months, after which warfarin is discontinued but dual antiplatelet therapy is continued for up to 12 months.32
The European Society of Cardiology guidelines33 recommend 6 months of anticoagulation. However, if the patient is receiving dual antiplatelet therapy, they recommend repeated imaging of the left ventricle after 3 months of anticoagulation, which may allow for earlier discontinuation of anticoagulation if the thrombus has resolved and apical wall motion has recovered. Therefore, most experts recommend 3 months of anticoagulation when used in combination with dual antiplatelet therapy and repeating echocardiography at 3 months to safely discontinue anticoagulation. The best answer to the question posed here is aspirin, clopidogrel, and warfarin.
Decisions about antithrombotic therapy may also depend on stent type and the patient’s bleeding risk. With bare-metal stents, dual antiplatelet therapy along with anticoagulation should be used for 1 month, after which anticoagulation should be used with a single antiplatelet agent for another 2 months; after this, the anticoagulant can be discontinued and dual antiplatelet therapy can be resumed for a total of 12 months. Newer anticoagulants such as rivaroxaban, dabigatran, edoxaban, and apixaban may also have a role, but they have not yet been studied for this indication.
Surgical thrombectomy is rarely considered now, given the known efficacy of anticoagulants in dissolving the thrombus. It was done in the past for large, mobile, or protruding left ventricular thrombi, which have a higher potential for embolization.34 Currently, it can be done under very special circumstances, such as before placement of a left ventricular assist device or if the thrombus is large, to prevent embolism.35,36
BLEEDING COMPLICATIONS WITH TRIPLE ANTITHROMBOTIC THERAPY
After stent placement, almost all patients need to be on dual antiplatelet therapy for a specified duration depending on the type and generation of stent used. Such patients end up on “triple” antithrombotic therapy (two antiplatelet drugs plus an anticoagulant), which poses a high risk of bleeding.37 Consideration needs to be given to the risks of stroke, stent thrombosis, and major bleeding when selecting the antithrombotic regimen.38 Triple antithrombotic therapy has been associated with a risk of fatal and nonfatal bleeding of 4% to 16% when used for indications such as atrial fibrillation.39–41
Risks of triple antithrombotic therapy (aspirin 80–100 mg, clopidogrel 75 mg, and warfarin) were compared with those of clopidogrel plus warfarin in the What Is the Optimal Antiplatelet and Anticoagulant therapy in Patients With Oral Anticoagulation and Coronary Stenting Trial,37 which reported a significantly lower risk of major and minor bleeding with clopidogrel-plus-warfarin therapy than with triple antithrombotic therapy, 14.3% vs 31.7% (hazard ratio 0.40, 95% CI 0.28–0.58, P < .0001).
Additionally, the increased risk of major and minor bleeding associated with triple antithrombotic therapy has been confirmed in many observational studies; other studies found a trend toward lower risk with triple therapy, but this was not statistically significant (Table 2).38,40,42–55 A large multicenter European trial is being conducted to compare dual antiplatelet therapy vs triple antithrombotic therapy in patients with left ventricular thrombosis.
CASE FOLLOW-UP
Our patient was started on warfarin, clopidogrel 75 mg, and aspirin 75 mg at the time of discharge. He was continued on warfarin for 3 months, at which time a follow-up echocardiogram showed no thrombus in the left ventricle. Warfarin was discontinued, and he had no thromboembolic complications.
TAKE-HOME POINTS
Left ventricular thrombosis after an acute MI is very important to detect, as it can lead to serious complications through arterial embolism.
The incidence of left ventricular thrombosis has declined significantly with the use of percutaneous coronary intervention. However, it may still occur in a small number of patients with larger infarcts owing to delay in revascularization or proximal (left main or left anterior descending) occlusions with larger infarct size.
Echocardiography, which is routinely performed after acute MI to assess myocardial function, uncovers most left ventricular thrombi. In high-risk cases, MRI with late gadolinium enhancement can increase the diagnostic yield.
Anticoagulation with warfarin is recommended for at least 3 months. Post-MI patients undergoing stent implantation may need triple antithrombotic therapy, which, however, increases the bleeding risk significantly. Large randomized trials are needed to guide physicians in risk stratification of such patients.
- Lip GY, Piotrponikowski P, Andreotti F, et al; Heart Failure Association (EHFA) of the European Society of Cardiology (ESC) and the ESC Working Group on Thrombosis. Thromboembolism and antithrombotic therapy for heart failure in sinus rhythm: an executive summary of a joint consensus document from the ESC Heart Failure Association and the ESC Working Group on Thrombosis. Thromb Haemost 2012; 108:1009–1022.
- Turpie AG, Robinson JG, Doyle DJ, et al. Comparison of high-dose with low-dose subcutaneous heparin to prevent left ventricular mural thrombosis in patients with acute transmural anterior myocardial infarction. N Engl J Med 1989; 320:352–357.
- Chiarella F, Santoro E, Domenicucci S, Maggioni A, Vecchio C. Predischarge two-dimensional echocardiographic evaluation of left ventricular thrombosis after acute myocardial infarction in the GISSI-3 study. Am J Cardiol 1998; 81:822–827.
- Kalra A, Jang IK. Prevalence of early left ventricular thrombus after primary coronary intervention for acute myocardial infarction. J Thromb Thrombolysis 2000; 10:133–136.
- Nayak D, Aronow WS, Sukhija R, McClung JA, Monsen CE, Belkin RN. Comparison of frequency of left ventricular thrombi in patients with anterior wall versus non-anterior wall acute myocardial infarction treated with antithrombotic and antiplatelet therapy with or without coronary revascularization. Am J Cardiol 2004; 93:1529–1530.
- Rehan A, Kanwar M, Rosman H, et al. Incidence of post myocardial infarction left ventricular thrombus formation in the era of primary percutaneous intervention and glycoprotein IIb/IIIa inhibitors. A prospective observational study. Cardiovasc Ultrasound 2006;4:20.
- Zielinska M, Kaczmarek K, Tylkowski M. Predictors of left ventricular thrombus formation in acute myocardial infarction treated with successful primary angioplasty with stenting. Am J Med Sci 2008; 335:171–176.
- Osherov AB, Borovik-Raz M, Aronson D, et al. Incidence of early left ventricular thrombus after acute anterior wall myocardial infarction in the primary coronary intervention era. Am Heart J 2009; 157:1074–1080.
- Solheim S, Seljeflot I, Lunde K, et al. Frequency of left ventricular thrombus in patients with anterior wall acute myocardial infarction treated with percutaneous coronary intervention and dual antiplatelet therapy. Am J Cardiol 2010; 106:1197–1200.
- Shacham Y, Leshem-Rubinow E, Ben Assa E, et al. Comparison of C-reactive protein and fibrinogen levels in patients having anterior wall ST-segment elevation myocardial infarction with versus without left ventricular thrombus (from a primary percutaneous coronary intervention cohort). Am J Cardiol 2013; 112:57–60.
- Gianstefani S, Douiri A, Delithanasis I, et al. Incidence and predictors of early left ventricular thrombus after ST-elevation myocardial infarction in the contemporary era of primary percutaneous coronary intervention. Am J Cardiol 2014; 113:1111–1116.
- Shacham Y, Birati EY, Rogovski O, Cogan Y, Keren G, Roth A. Left ventricular thrombus formation and bleeding complications during continuous in-hospital anticoagulation for acute anterior myocardial infarction. Isr Med Assoc J 2012; 14:742–746.
- Asinger RW, Mikell FL, Elsperger J, Hodges M. Incidence of left-ventricular thrombosis after acute transmural myocardial infarction. Serial evaluation by two-dimensional echocardiography. N Engl J Med 1981; 305:297–302.
- Nihoyannopoulos P, Smith GC, Maseri A, Foale RA. The natural history of left ventricular thrombus in myocardial infarction: a rationale in support of masterly inactivity. J Am Coll Cardiol 1989; 14:903–911.
- Weinreich DJ, Burke JF, Pauletto FJ. Left ventricular mural thrombi complicating acute myocardial infarction. Long-term follow-up with serial echocardiography. Ann Intern Med 1984; 100:789–794.
- Greaves SC, Zhi G, Lee RT, et al. Incidence and natural history of left ventricular thrombus following anterior wall acute myocardial infarction. Am J Cardiol 1997; 80:442–448.
- Solheim S, Seljeflot I, Lunde K, et al. Prothrombotic markers in patients with acute myocardial infarction and left ventricular thrombus formation treated with pci and dual antiplatelet therapy. Thromb J 2013; 11:1.
- O’Gara PT, Kushner FG, Ascheim DD, et al; American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. 2013 ACCF/AHA guideline for the management of ST-elevation myocardial infarction: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation 2013; 127:e362–e425.
- Weinsaft JW, Kim HW, Crowley AL, et al. LV thrombus detection by routine echocardiography: insights into performance characteristics using delayed enhancement CMR. JACC Cardiovasc Imaging 2011; 4:702–712.
- Mollet NR, Dymarkowski S, Volders W, et al. Visualization of ventricular thrombi with contrast-enhanced magnetic resonance imaging in patients with ischemic heart disease. Circulation 2002; 106:2873–2876.
- Srichai MB, Junor C, Rodriguez LL, et al. Clinical, imaging, and pathological characteristics of left ventricular thrombus: a comparison of contrast-enhanced magnetic resonance imaging, transthoracic echocardiography, and transesophageal echocardiography with surgical or pathological validation. Am Heart J 2006; 152:75–84.
- Eriksson SE, Olsson JE. Survival and recurrent strokes in patients with different subtypes of stroke: a fourteen-year follow-up study. Cerebrovasc Dis 2001; 12:171–180.
- Grau AJ, Weimar C, Buggle F, et al. Risk factors, outcome, and treatment in subtypes of ischemic stroke: the German Stroke Data Bank. Stroke 2001; 32:2559–2566.
- Keren A, Goldberg S, Gottlieb S, et al. Natural history of left ventricular thrombi: their appearance and resolution in the posthospitalization period of acute myocardial infarction. J Am Coll Cardiol 1990; 15:790–800.
- Jordan RA, Miller RD, Edwards JE, Parker RL. Thrombo-embolism in acute and in healed myocardial infarction. I. Intracardiac mural thrombosis. Circulation 1952; 6:1–6.
- Vaitkus PT, Barnathan ES. Embolic potential, prevention and management of mural thrombus complicating anterior myocardial infarction: a meta-analysis. J Am Coll Cardiol 1993; 22:1004–1009.
- ISIS-2 (Second International Study of Infarct Survival) Collaborative Group. Randomised trial of intravenous streptokinase, oral aspirin, both, or neither among 17,187 cases of suspected acute myocardial infarction: ISIS-2. Lancet 1988; 2:349–360.
- Cabin HS, Roberts WC. Left ventricular aneurysm, intraaneurysmal thrombus and systemic embolus in coronary heart disease. Chest 1980; 77:586–590.
- Keating EC, Gross SA, Schlamowitz RA, et al. Mural thrombi in myocardial infarctions. Prospective evaluation by two-dimensional echocardiography. Am J Med 1983; 74:989–995.
- Witt BJ, Ballman KV, Brown RD Jr, Meverden RA, Jacobsen SJ, Roger VL. The incidence of stroke after myocardial infarction: a meta-analysis. Am J Med 2006; 119:354.e1–354.e9.
- Witt BJ, Brown RD Jr, Jacobsen SJ, Weston SA, Yawn BP, Roger VL. A community-based study of stroke incidence after myocardial infarction. Ann Intern Med 2005; 143:785–792.
- Vandvik PO, Lincoff AM, Gore JM, et al; American College of Chest Physicians. Primary and secondary prevention of cardiovascular disease: antithrombotic therapy and prevention of thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest 2012; 141(suppl):e637S–e68S.
- Steg G, James SK, Atar D, et al. ESC guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation. Eur Heart J 2012; 33:2569–2619.
- Nili M, Deviri E, Jortner R, Strasberg B, Levy MJ. Surgical removal of a mobile, pedunculated left ventricular thrombus: report of 4 cases. Ann Thorac Surg 1988; 46:396–400.
- Kanemitsu S, Miyake Y, Okabe M. Surgical removal of a left ventricular thrombus associated with cardiac sarcoidosis. Interact Cardiovasc Thorac Surg 2008; 7:333–335.
- Engin C, Yagdi T, Balcioglu O, et al. Left ventricular assist device implantation in heart failure patients with a left ventricular thrombus. Transplant Proc 2013; 45:1017–1019.
- Dewilde WJ, Oirbans T, Verheugt FW, et al; WOEST study investigators. Use of clopidogrel with or without aspirin in patients taking oral anticoagulant therapy and undergoing percutaneous coronary intervention: an open-label, randomised, controlled trial. Lancet 2013; 381:1107–1115.
- Faxon DP, Eikelboom JW, Berger PB, et al. Antithrombotic therapy in patients with atrial fibrillation undergoing coronary stenting: a North American perspective: executive summary. Circ Cardiovasc Interv 2011; 4:522–534.
- Hansen ML, Sorensen R, Clausen MT, et al. Risk of bleeding with single, dual, or triple therapy with warfarin, aspirin, and clopidogrel in patients with atrial fibrillation. Arch Intern Med 2010; 170:1433–1441.
- Karjalainen PP, Porela P, Ylitalo A, et al. Safety and efficacy of combined antiplatelet-warfarin therapy after coronary stenting. Eur Heart J 2007; 28:726–732.
- Doyle BJ, Rihal CS, Gastineau DA, Holmes DR Jr. Bleeding, blood transfusion, and increased mortality after percutaneous coronary intervention: implications for contemporary practice. J Am Coll Cardiol 2009; 53:2019–2027.
- Azoulay L, Dell’Aniello S, Simon T, Renoux C, Suissa S. The concurrent use of antithrombotic therapies and the risk of bleeding in patients with atrial fibrillation. Thromb Haemost 2013; 109:431–439.
- Deshmukh A, Hilleman DE, Del Core M, Nair CK. Antithrombotic regimens in patients with indication for long-term anticoagulation undergoing coronary interventions-systematic analysis, review of literature, and implications on management. Am J Ther 2013; 20:654–663.
- Fosbol EL, Wang TY, Li S, et al. Warfarin use among older atrial fibrillation patients with non-ST-segment elevation myocardial infarction managed with coronary stenting and dual antiplatelet therapy. Am Heart J 2013; 166:864–870.
- Gao F, Zhou YJ, Wang ZJ, et al. Meta-analysis of the combination of warfarin and dual antiplatelet therapy after coronary stenting in patients with indications for chronic oral anticoagulation. Int J Cardiol 2011; 148:96–101.
- Hansen ML, Sorensen R, Clausen MT, et al. Risk of bleeding with single, dual, or triple therapy with warfarin, aspirin, and clopidogrel in patients with atrial fibrillation. Arch Intern Med 2010; 170:1433–1441.
- Hermosillo AJ, Spinler SA. Aspirin, clopidogrel, and warfarin: is the combination appropriate and effective or inappropriate and too dangerous? Ann Pharmacother 2008; 42:790–805.
- Holmes DR Jr, Kereiakes DJ, Kleiman NS, Moliterno DJ, Patti G, Grines CL. Combining antiplatelet and anticoagulant therapies. J Am Coll Cardiol 2009; 54:95–109.
- Khurram Z, Chou E, Minutello R, et al. Combination therapy with aspirin, clopidogrel and warfarin following coronary stenting is associated with a significant risk of bleeding. J Invasive Cardiol 2006; 18:162–164.
- Orford JL, Fasseas P, Melby S, et al. Safety and efficacy of aspirin, clopidogrel, and warfarin after coronary stent placement in patients with an indication for anticoagulation. Am Heart J 2004; 147:463–467.
- Porter A, Konstantino Y, Iakobishvili Z, Shachar L, Battler A, Hasdai D. Short-term triple therapy with aspirin, warfarin, and a thienopyridine among patients undergoing percutaneous coronary intervention. Catheter Cardiovasc Interv 2006; 68:56–61.
- DeEugenio D, Kolman L, DeCaro M, et al. Risk of major bleeding with concomitant dual antiplatelet therapy after percutaneous coronary intervention in patients receiving long-term warfarin therapy. Pharmacotherapy 2007; 27:691–696.
- Ruiz-Nodar JM, Marin F, Hurtado JA, et al. Anticoagulant and antiplatelet therapy use in 426 patients with atrial fibrillation undergoing percutaneous coronary intervention and stent implantation implications for bleeding risk and prognosis. J Am Coll Cardiol 2008; 51:818–825.
- Sarafoff N, Ndrepepa G, Mehilli J, et al. Aspirin and clopidogrel with or without phenprocoumon after drug eluting coronary stent placement in patients on chronic oral anticoagulation. J Intern Med 2008; 264:472–480.
- Rossini R, Musumeci GF, Lettieri CF, et al. Long-term outcomes in patients undergoing coronary stenting on dual oral antiplatelet treatment requiring oral anticoagulant therapy. Am J Cardiol 2008; 102:1618–1623.
A 62-year-old man with hypertension, type 2 diabetes mellitus, and hypercholesterolemia presented to the emergency department with substernal chest pain that started about 15 hours earlier while he was at rest watching television.
On examination, his pulse was 92 beats per minute and regular, his blood pressure was 160/88 mm Hg, and he had no evidence of jugular venous distention or pedal edema. Lung examination was positive for bibasilar crackles.
Electrocardiography revealed Q waves with ST elevation in leads I, aVL, V4, V5, and V6 with reciprocal ST depression in leads II, III, and aVF.
His troponin T level on presentation was markedly elevated.

He underwent heart catheterization and was found to have 100% occlusion of the proximal left anterior descending artery. He underwent successful percutaneous coronary intervention with placement of a drug-eluting stent, and afterward had grade 3 flow on the Thrombolysis in Myocardial Infarction (TIMI) scale.
Echocardiography the next day revealed a mobile echo-dense mass in the left ventricular apex (Figure 1) and a left ventricular ejection fraction of 35%.
THE INCIDENCE OF LEFT VENTRICULAR THROMBOSIS IN ACUTE MI
1. What is the incidence of left ventricular thrombosis after acute myocardial infarction (MI), now that primary percutaneous coronary intervention is common?
- 0.1%
- 2%
- 20%
- 40%
Left ventricular thrombosis is a serious complication of acute MI that can cause systemic thromboembolism, including stroke.1 Before thrombolytic therapy was available, this complication occurred in 20% to 60% of patients with acute MI.2,3 But early reperfusion strategies, anticoagulation for the first 48 hours, and dual antiplatelet therapy have reduced the incidence of this complication significantly.
In the thrombolytic era, the incidence of left ventricular thrombosis was 5.1% in the Gruppo Italiano per lo Studio della Sopravvivenza nell’Infarto Miocardico (GISSI) 3 study, which had 8,326 patients. A subset of patients who had an anterior MI had almost double the incidence (11.5%).3

The incidence has further declined with the advent of primary percutaneous coronary intervention, likely thanks to enhanced myocardial salvage, and now ranges from 2.5% to 15% (Table 1).4–11 The largest observational study, with 2,911 patients undergoing percutaneous coronary intervention, reported an incidence of 2.5% within 3 to 5 days of the MI.7 At our center, the incidence was found to be even lower, 1.8% in 1,700 patients presenting with ST-elevation MI undergoing primary percutaneous coronary intervention. Hence, of the answers to the question above, 2% would be closest.
Large infarct size with a low left ventricular ejection fraction (< 40%), anterior wall MI, hypertension, and delay in time from symptom onset to intervention were independent predictors of left ventricular thrombus formation in most studies.7,12 The risk is highest during the first 2 weeks after MI, and thrombosis almost never occurs more than 3 months after the index event.5,13–16
WHAT IS THE PATHOGENESIS OF LEFT VENTRICULAR THROMBOSIS?
A large transmural infarct results in loss of contractile function, which causes stagnation and pooling of blood adjacent to the infarcted ventricular segment. In addition, endocardial injury exposes tissue factor, which then initiates the coagulation cascade. To make matters worse, MI results in a hypercoagulable state through unclear mechanisms, which completes the Virchow triad for thrombus formation. Elevations of D-dimer, fibrinogen, anticardiolipin antibodies (IgM and IgG), and tissue factor have also been reported after acute MI.17
Thrombus formation begins with platelet aggregation at the site of endocardial damage, forming a platelet plug, followed by activation of clotting factors. These thrombi are referred to as “mural,” as they adhere to the chamber wall (endocardium). They are composed of fibrin and entrapped red and white blood cells (Figure 2).
The natural course of thrombus evolution is established but variable. A left ventricular thrombus may dislodge and embolize, resulting in stroke or other thromboembolic complications. Alternately, it can dissolve over time, aided by intrinsic fibrinolytic mechanisms. On other occasions, the thrombus may organize, a process characterized by ingrowth of smooth muscle cells, fibroblasts, and endothelium.
HOW IS LEFT VENTRICULAR THROMBOSIS DIAGNOSED?
2. What is the best imaging test for detecting a thrombus?
- Transesophageal echocardiography
- Transthoracic echocardiography
- Cardiac magnetic resonance imaging (MRI) without gadolinium contrast
- Cardiac MRI with gadolinium contrast
Evaluation of left ventricular function after acute MI carries a class I indication (ie, it should be performed).18
Echocardiography is commonly used, and it has a 60% sensitivity to detect a thrombus.19 In patients with poorer transthoracic echocardiographic windows, contrast can be used to better delineate the left ventricular cavity and show the thrombus. Transesophageal echocardiography is seldom useful, as the left ventricular apex is foreshortened and in the far field.
A left ventricular thrombus is confirmed if an echo-dense mass with well-demarcated margins distinct from the endocardium is seen throughout the cardiac cycle. It should be evident in at least two different views (apical and short-axis) and should be adjacent to a hypokinetic or akinetic left ventricular wall. False-positive findings can occur due to misidentified false tendons, papillary muscles, and trabeculae.
Cardiac MRI with late gadolinium enhancement is now the gold standard for diagnostic imaging, as it accurately characterizes the shape, size, and location of the thrombus (Figure 3). Gadolinium contrast increases the enhancement of the ventricular cavity, thus allowing easy detection of thrombus, which appears dark. Cardiac MRI with delayed enhancement has 88% to 91% sensitivity and 99% specificity to detect left ventricular thrombosis.20,21 However, compared with echocardiography, routine cardiac MRI is time-intensive, costly, and not routinely available. As a result, it should be performed only in patients with poor acoustic windows and a high clinical suspicion of left ventricular thrombosis.
Delayed-contrast cardiac computed tomography can be used to identify left ventricular thrombosis, using absence of contrast uptake. The need to use contrast is a disadvantage, but computed tomography can be an alternative in patients with contraindications to cardiac MRI.
WHAT COMPLICATIONS ARISE FROM LEFT VENTRICULAR THROMBOSIS?
The most feared complication of left ventricular thrombosis is thromboembolism. Cardioembolic stroke is generally severe, prone to early and long-term recurrence, and associated with a higher death rate than noncardioembolic ischemic stroke.22,23 Thrombi associated with thromboembolism are often acute and mobile rather than organized and immobile.24 They may embolize to the brain, spleen, kidneys, and bowel.25 In a meta-analysis of 11 studies, the pooled odds ratio for risk of embolization was 5.45 (95% confidence interval [CI] 3.02–9.83) with left ventricular thrombi vs without.26 Before systemic thrombolysis and antiplatelet therapy became available, stroke rates ranged from 1.5% to 10%.27–29
In a meta-analysis of 22 studies from 1978 to 2004, the incidence of ischemic stroke after MI during hospitalization was around 11.1 per 1,000 MIs.30 This study found that anterior MI was associated with a higher risk of stroke, but reported no difference in the incidence of stroke with percutaneous coronary intervention, systemic thrombolysis, or no reperfusion.
In a large prospective cohort study of 2,160 patients,31 259 (12%) had a stroke after MI. In multivariable analysis, age, diabetes, and previous stroke were predictors of stroke after MI. This study reported significantly fewer strokes in patients who underwent percutaneous coronary intervention than with other or no reperfusion therapies.31
ANTICOAGULATION TREATMENT
3. How would you treat a patient who has a drug-eluting stent in the left anterior descending artery and a new diagnosis of left ventricular thrombosis?
- Warfarin
- Aspirin and clopidogrel
- Aspirin, clopidogrel, and warfarin
- Aspirin and warfarin
The management of left ventricular thrombosis has been summarized in guidelines from the American College of Chest Physicians (ACCP) in 2012,32 and from the American College of Cardiology/American Heart Association in 2013,18 which recommend anticoagulation for at least 3 months, or indefinitely if bleeding risk is low, for all patients developing a left ventricular thrombus.
For patients with acute MI and left ventricular thrombosis, the ACCP guidelines recommend warfarin with a target international normalized ratio of 2.0 to 3.0 plus dual antiplatelet therapy (eg, aspirin plus clopidogrel) for 3 months, after which warfarin is discontinued but dual antiplatelet therapy is continued for up to 12 months.32
The European Society of Cardiology guidelines33 recommend 6 months of anticoagulation. However, if the patient is receiving dual antiplatelet therapy, they recommend repeated imaging of the left ventricle after 3 months of anticoagulation, which may allow for earlier discontinuation of anticoagulation if the thrombus has resolved and apical wall motion has recovered. Therefore, most experts recommend 3 months of anticoagulation when used in combination with dual antiplatelet therapy and repeating echocardiography at 3 months to safely discontinue anticoagulation. The best answer to the question posed here is aspirin, clopidogrel, and warfarin.
Decisions about antithrombotic therapy may also depend on stent type and the patient’s bleeding risk. With bare-metal stents, dual antiplatelet therapy along with anticoagulation should be used for 1 month, after which anticoagulation should be used with a single antiplatelet agent for another 2 months; after this, the anticoagulant can be discontinued and dual antiplatelet therapy can be resumed for a total of 12 months. Newer anticoagulants such as rivaroxaban, dabigatran, edoxaban, and apixaban may also have a role, but they have not yet been studied for this indication.
Surgical thrombectomy is rarely considered now, given the known efficacy of anticoagulants in dissolving the thrombus. It was done in the past for large, mobile, or protruding left ventricular thrombi, which have a higher potential for embolization.34 Currently, it can be done under very special circumstances, such as before placement of a left ventricular assist device or if the thrombus is large, to prevent embolism.35,36
BLEEDING COMPLICATIONS WITH TRIPLE ANTITHROMBOTIC THERAPY
After stent placement, almost all patients need to be on dual antiplatelet therapy for a specified duration depending on the type and generation of stent used. Such patients end up on “triple” antithrombotic therapy (two antiplatelet drugs plus an anticoagulant), which poses a high risk of bleeding.37 Consideration needs to be given to the risks of stroke, stent thrombosis, and major bleeding when selecting the antithrombotic regimen.38 Triple antithrombotic therapy has been associated with a risk of fatal and nonfatal bleeding of 4% to 16% when used for indications such as atrial fibrillation.39–41
Risks of triple antithrombotic therapy (aspirin 80–100 mg, clopidogrel 75 mg, and warfarin) were compared with those of clopidogrel plus warfarin in the What Is the Optimal Antiplatelet and Anticoagulant therapy in Patients With Oral Anticoagulation and Coronary Stenting Trial,37 which reported a significantly lower risk of major and minor bleeding with clopidogrel-plus-warfarin therapy than with triple antithrombotic therapy, 14.3% vs 31.7% (hazard ratio 0.40, 95% CI 0.28–0.58, P < .0001).
Additionally, the increased risk of major and minor bleeding associated with triple antithrombotic therapy has been confirmed in many observational studies; other studies found a trend toward lower risk with triple therapy, but this was not statistically significant (Table 2).38,40,42–55 A large multicenter European trial is being conducted to compare dual antiplatelet therapy vs triple antithrombotic therapy in patients with left ventricular thrombosis.
CASE FOLLOW-UP
Our patient was started on warfarin, clopidogrel 75 mg, and aspirin 75 mg at the time of discharge. He was continued on warfarin for 3 months, at which time a follow-up echocardiogram showed no thrombus in the left ventricle. Warfarin was discontinued, and he had no thromboembolic complications.
TAKE-HOME POINTS
Left ventricular thrombosis after an acute MI is very important to detect, as it can lead to serious complications through arterial embolism.
The incidence of left ventricular thrombosis has declined significantly with the use of percutaneous coronary intervention. However, it may still occur in a small number of patients with larger infarcts owing to delay in revascularization or proximal (left main or left anterior descending) occlusions with larger infarct size.
Echocardiography, which is routinely performed after acute MI to assess myocardial function, uncovers most left ventricular thrombi. In high-risk cases, MRI with late gadolinium enhancement can increase the diagnostic yield.
Anticoagulation with warfarin is recommended for at least 3 months. Post-MI patients undergoing stent implantation may need triple antithrombotic therapy, which, however, increases the bleeding risk significantly. Large randomized trials are needed to guide physicians in risk stratification of such patients.
A 62-year-old man with hypertension, type 2 diabetes mellitus, and hypercholesterolemia presented to the emergency department with substernal chest pain that started about 15 hours earlier while he was at rest watching television.
On examination, his pulse was 92 beats per minute and regular, his blood pressure was 160/88 mm Hg, and he had no evidence of jugular venous distention or pedal edema. Lung examination was positive for bibasilar crackles.
Electrocardiography revealed Q waves with ST elevation in leads I, aVL, V4, V5, and V6 with reciprocal ST depression in leads II, III, and aVF.
His troponin T level on presentation was markedly elevated.

He underwent heart catheterization and was found to have 100% occlusion of the proximal left anterior descending artery. He underwent successful percutaneous coronary intervention with placement of a drug-eluting stent, and afterward had grade 3 flow on the Thrombolysis in Myocardial Infarction (TIMI) scale.
Echocardiography the next day revealed a mobile echo-dense mass in the left ventricular apex (Figure 1) and a left ventricular ejection fraction of 35%.
THE INCIDENCE OF LEFT VENTRICULAR THROMBOSIS IN ACUTE MI
1. What is the incidence of left ventricular thrombosis after acute myocardial infarction (MI), now that primary percutaneous coronary intervention is common?
- 0.1%
- 2%
- 20%
- 40%
Left ventricular thrombosis is a serious complication of acute MI that can cause systemic thromboembolism, including stroke.1 Before thrombolytic therapy was available, this complication occurred in 20% to 60% of patients with acute MI.2,3 But early reperfusion strategies, anticoagulation for the first 48 hours, and dual antiplatelet therapy have reduced the incidence of this complication significantly.
In the thrombolytic era, the incidence of left ventricular thrombosis was 5.1% in the Gruppo Italiano per lo Studio della Sopravvivenza nell’Infarto Miocardico (GISSI) 3 study, which had 8,326 patients. A subset of patients who had an anterior MI had almost double the incidence (11.5%).3

The incidence has further declined with the advent of primary percutaneous coronary intervention, likely thanks to enhanced myocardial salvage, and now ranges from 2.5% to 15% (Table 1).4–11 The largest observational study, with 2,911 patients undergoing percutaneous coronary intervention, reported an incidence of 2.5% within 3 to 5 days of the MI.7 At our center, the incidence was found to be even lower, 1.8% in 1,700 patients presenting with ST-elevation MI undergoing primary percutaneous coronary intervention. Hence, of the answers to the question above, 2% would be closest.
Large infarct size with a low left ventricular ejection fraction (< 40%), anterior wall MI, hypertension, and delay in time from symptom onset to intervention were independent predictors of left ventricular thrombus formation in most studies.7,12 The risk is highest during the first 2 weeks after MI, and thrombosis almost never occurs more than 3 months after the index event.5,13–16
WHAT IS THE PATHOGENESIS OF LEFT VENTRICULAR THROMBOSIS?
A large transmural infarct results in loss of contractile function, which causes stagnation and pooling of blood adjacent to the infarcted ventricular segment. In addition, endocardial injury exposes tissue factor, which then initiates the coagulation cascade. To make matters worse, MI results in a hypercoagulable state through unclear mechanisms, which completes the Virchow triad for thrombus formation. Elevations of D-dimer, fibrinogen, anticardiolipin antibodies (IgM and IgG), and tissue factor have also been reported after acute MI.17
Thrombus formation begins with platelet aggregation at the site of endocardial damage, forming a platelet plug, followed by activation of clotting factors. These thrombi are referred to as “mural,” as they adhere to the chamber wall (endocardium). They are composed of fibrin and entrapped red and white blood cells (Figure 2).
The natural course of thrombus evolution is established but variable. A left ventricular thrombus may dislodge and embolize, resulting in stroke or other thromboembolic complications. Alternately, it can dissolve over time, aided by intrinsic fibrinolytic mechanisms. On other occasions, the thrombus may organize, a process characterized by ingrowth of smooth muscle cells, fibroblasts, and endothelium.
HOW IS LEFT VENTRICULAR THROMBOSIS DIAGNOSED?
2. What is the best imaging test for detecting a thrombus?
- Transesophageal echocardiography
- Transthoracic echocardiography
- Cardiac magnetic resonance imaging (MRI) without gadolinium contrast
- Cardiac MRI with gadolinium contrast
Evaluation of left ventricular function after acute MI carries a class I indication (ie, it should be performed).18
Echocardiography is commonly used, and it has a 60% sensitivity to detect a thrombus.19 In patients with poorer transthoracic echocardiographic windows, contrast can be used to better delineate the left ventricular cavity and show the thrombus. Transesophageal echocardiography is seldom useful, as the left ventricular apex is foreshortened and in the far field.
A left ventricular thrombus is confirmed if an echo-dense mass with well-demarcated margins distinct from the endocardium is seen throughout the cardiac cycle. It should be evident in at least two different views (apical and short-axis) and should be adjacent to a hypokinetic or akinetic left ventricular wall. False-positive findings can occur due to misidentified false tendons, papillary muscles, and trabeculae.
Cardiac MRI with late gadolinium enhancement is now the gold standard for diagnostic imaging, as it accurately characterizes the shape, size, and location of the thrombus (Figure 3). Gadolinium contrast increases the enhancement of the ventricular cavity, thus allowing easy detection of thrombus, which appears dark. Cardiac MRI with delayed enhancement has 88% to 91% sensitivity and 99% specificity to detect left ventricular thrombosis.20,21 However, compared with echocardiography, routine cardiac MRI is time-intensive, costly, and not routinely available. As a result, it should be performed only in patients with poor acoustic windows and a high clinical suspicion of left ventricular thrombosis.
Delayed-contrast cardiac computed tomography can be used to identify left ventricular thrombosis, using absence of contrast uptake. The need to use contrast is a disadvantage, but computed tomography can be an alternative in patients with contraindications to cardiac MRI.
WHAT COMPLICATIONS ARISE FROM LEFT VENTRICULAR THROMBOSIS?
The most feared complication of left ventricular thrombosis is thromboembolism. Cardioembolic stroke is generally severe, prone to early and long-term recurrence, and associated with a higher death rate than noncardioembolic ischemic stroke.22,23 Thrombi associated with thromboembolism are often acute and mobile rather than organized and immobile.24 They may embolize to the brain, spleen, kidneys, and bowel.25 In a meta-analysis of 11 studies, the pooled odds ratio for risk of embolization was 5.45 (95% confidence interval [CI] 3.02–9.83) with left ventricular thrombi vs without.26 Before systemic thrombolysis and antiplatelet therapy became available, stroke rates ranged from 1.5% to 10%.27–29
In a meta-analysis of 22 studies from 1978 to 2004, the incidence of ischemic stroke after MI during hospitalization was around 11.1 per 1,000 MIs.30 This study found that anterior MI was associated with a higher risk of stroke, but reported no difference in the incidence of stroke with percutaneous coronary intervention, systemic thrombolysis, or no reperfusion.
In a large prospective cohort study of 2,160 patients,31 259 (12%) had a stroke after MI. In multivariable analysis, age, diabetes, and previous stroke were predictors of stroke after MI. This study reported significantly fewer strokes in patients who underwent percutaneous coronary intervention than with other or no reperfusion therapies.31
ANTICOAGULATION TREATMENT
3. How would you treat a patient who has a drug-eluting stent in the left anterior descending artery and a new diagnosis of left ventricular thrombosis?
- Warfarin
- Aspirin and clopidogrel
- Aspirin, clopidogrel, and warfarin
- Aspirin and warfarin
The management of left ventricular thrombosis has been summarized in guidelines from the American College of Chest Physicians (ACCP) in 2012,32 and from the American College of Cardiology/American Heart Association in 2013,18 which recommend anticoagulation for at least 3 months, or indefinitely if bleeding risk is low, for all patients developing a left ventricular thrombus.
For patients with acute MI and left ventricular thrombosis, the ACCP guidelines recommend warfarin with a target international normalized ratio of 2.0 to 3.0 plus dual antiplatelet therapy (eg, aspirin plus clopidogrel) for 3 months, after which warfarin is discontinued but dual antiplatelet therapy is continued for up to 12 months.32
The European Society of Cardiology guidelines33 recommend 6 months of anticoagulation. However, if the patient is receiving dual antiplatelet therapy, they recommend repeated imaging of the left ventricle after 3 months of anticoagulation, which may allow for earlier discontinuation of anticoagulation if the thrombus has resolved and apical wall motion has recovered. Therefore, most experts recommend 3 months of anticoagulation when used in combination with dual antiplatelet therapy and repeating echocardiography at 3 months to safely discontinue anticoagulation. The best answer to the question posed here is aspirin, clopidogrel, and warfarin.
Decisions about antithrombotic therapy may also depend on stent type and the patient’s bleeding risk. With bare-metal stents, dual antiplatelet therapy along with anticoagulation should be used for 1 month, after which anticoagulation should be used with a single antiplatelet agent for another 2 months; after this, the anticoagulant can be discontinued and dual antiplatelet therapy can be resumed for a total of 12 months. Newer anticoagulants such as rivaroxaban, dabigatran, edoxaban, and apixaban may also have a role, but they have not yet been studied for this indication.
Surgical thrombectomy is rarely considered now, given the known efficacy of anticoagulants in dissolving the thrombus. It was done in the past for large, mobile, or protruding left ventricular thrombi, which have a higher potential for embolization.34 Currently, it can be done under very special circumstances, such as before placement of a left ventricular assist device or if the thrombus is large, to prevent embolism.35,36
BLEEDING COMPLICATIONS WITH TRIPLE ANTITHROMBOTIC THERAPY
After stent placement, almost all patients need to be on dual antiplatelet therapy for a specified duration depending on the type and generation of stent used. Such patients end up on “triple” antithrombotic therapy (two antiplatelet drugs plus an anticoagulant), which poses a high risk of bleeding.37 Consideration needs to be given to the risks of stroke, stent thrombosis, and major bleeding when selecting the antithrombotic regimen.38 Triple antithrombotic therapy has been associated with a risk of fatal and nonfatal bleeding of 4% to 16% when used for indications such as atrial fibrillation.39–41
Risks of triple antithrombotic therapy (aspirin 80–100 mg, clopidogrel 75 mg, and warfarin) were compared with those of clopidogrel plus warfarin in the What Is the Optimal Antiplatelet and Anticoagulant therapy in Patients With Oral Anticoagulation and Coronary Stenting Trial,37 which reported a significantly lower risk of major and minor bleeding with clopidogrel-plus-warfarin therapy than with triple antithrombotic therapy, 14.3% vs 31.7% (hazard ratio 0.40, 95% CI 0.28–0.58, P < .0001).
Additionally, the increased risk of major and minor bleeding associated with triple antithrombotic therapy has been confirmed in many observational studies; other studies found a trend toward lower risk with triple therapy, but this was not statistically significant (Table 2).38,40,42–55 A large multicenter European trial is being conducted to compare dual antiplatelet therapy vs triple antithrombotic therapy in patients with left ventricular thrombosis.
CASE FOLLOW-UP
Our patient was started on warfarin, clopidogrel 75 mg, and aspirin 75 mg at the time of discharge. He was continued on warfarin for 3 months, at which time a follow-up echocardiogram showed no thrombus in the left ventricle. Warfarin was discontinued, and he had no thromboembolic complications.
TAKE-HOME POINTS
Left ventricular thrombosis after an acute MI is very important to detect, as it can lead to serious complications through arterial embolism.
The incidence of left ventricular thrombosis has declined significantly with the use of percutaneous coronary intervention. However, it may still occur in a small number of patients with larger infarcts owing to delay in revascularization or proximal (left main or left anterior descending) occlusions with larger infarct size.
Echocardiography, which is routinely performed after acute MI to assess myocardial function, uncovers most left ventricular thrombi. In high-risk cases, MRI with late gadolinium enhancement can increase the diagnostic yield.
Anticoagulation with warfarin is recommended for at least 3 months. Post-MI patients undergoing stent implantation may need triple antithrombotic therapy, which, however, increases the bleeding risk significantly. Large randomized trials are needed to guide physicians in risk stratification of such patients.
- Lip GY, Piotrponikowski P, Andreotti F, et al; Heart Failure Association (EHFA) of the European Society of Cardiology (ESC) and the ESC Working Group on Thrombosis. Thromboembolism and antithrombotic therapy for heart failure in sinus rhythm: an executive summary of a joint consensus document from the ESC Heart Failure Association and the ESC Working Group on Thrombosis. Thromb Haemost 2012; 108:1009–1022.
- Turpie AG, Robinson JG, Doyle DJ, et al. Comparison of high-dose with low-dose subcutaneous heparin to prevent left ventricular mural thrombosis in patients with acute transmural anterior myocardial infarction. N Engl J Med 1989; 320:352–357.
- Chiarella F, Santoro E, Domenicucci S, Maggioni A, Vecchio C. Predischarge two-dimensional echocardiographic evaluation of left ventricular thrombosis after acute myocardial infarction in the GISSI-3 study. Am J Cardiol 1998; 81:822–827.
- Kalra A, Jang IK. Prevalence of early left ventricular thrombus after primary coronary intervention for acute myocardial infarction. J Thromb Thrombolysis 2000; 10:133–136.
- Nayak D, Aronow WS, Sukhija R, McClung JA, Monsen CE, Belkin RN. Comparison of frequency of left ventricular thrombi in patients with anterior wall versus non-anterior wall acute myocardial infarction treated with antithrombotic and antiplatelet therapy with or without coronary revascularization. Am J Cardiol 2004; 93:1529–1530.
- Rehan A, Kanwar M, Rosman H, et al. Incidence of post myocardial infarction left ventricular thrombus formation in the era of primary percutaneous intervention and glycoprotein IIb/IIIa inhibitors. A prospective observational study. Cardiovasc Ultrasound 2006;4:20.
- Zielinska M, Kaczmarek K, Tylkowski M. Predictors of left ventricular thrombus formation in acute myocardial infarction treated with successful primary angioplasty with stenting. Am J Med Sci 2008; 335:171–176.
- Osherov AB, Borovik-Raz M, Aronson D, et al. Incidence of early left ventricular thrombus after acute anterior wall myocardial infarction in the primary coronary intervention era. Am Heart J 2009; 157:1074–1080.
- Solheim S, Seljeflot I, Lunde K, et al. Frequency of left ventricular thrombus in patients with anterior wall acute myocardial infarction treated with percutaneous coronary intervention and dual antiplatelet therapy. Am J Cardiol 2010; 106:1197–1200.
- Shacham Y, Leshem-Rubinow E, Ben Assa E, et al. Comparison of C-reactive protein and fibrinogen levels in patients having anterior wall ST-segment elevation myocardial infarction with versus without left ventricular thrombus (from a primary percutaneous coronary intervention cohort). Am J Cardiol 2013; 112:57–60.
- Gianstefani S, Douiri A, Delithanasis I, et al. Incidence and predictors of early left ventricular thrombus after ST-elevation myocardial infarction in the contemporary era of primary percutaneous coronary intervention. Am J Cardiol 2014; 113:1111–1116.
- Shacham Y, Birati EY, Rogovski O, Cogan Y, Keren G, Roth A. Left ventricular thrombus formation and bleeding complications during continuous in-hospital anticoagulation for acute anterior myocardial infarction. Isr Med Assoc J 2012; 14:742–746.
- Asinger RW, Mikell FL, Elsperger J, Hodges M. Incidence of left-ventricular thrombosis after acute transmural myocardial infarction. Serial evaluation by two-dimensional echocardiography. N Engl J Med 1981; 305:297–302.
- Nihoyannopoulos P, Smith GC, Maseri A, Foale RA. The natural history of left ventricular thrombus in myocardial infarction: a rationale in support of masterly inactivity. J Am Coll Cardiol 1989; 14:903–911.
- Weinreich DJ, Burke JF, Pauletto FJ. Left ventricular mural thrombi complicating acute myocardial infarction. Long-term follow-up with serial echocardiography. Ann Intern Med 1984; 100:789–794.
- Greaves SC, Zhi G, Lee RT, et al. Incidence and natural history of left ventricular thrombus following anterior wall acute myocardial infarction. Am J Cardiol 1997; 80:442–448.
- Solheim S, Seljeflot I, Lunde K, et al. Prothrombotic markers in patients with acute myocardial infarction and left ventricular thrombus formation treated with pci and dual antiplatelet therapy. Thromb J 2013; 11:1.
- O’Gara PT, Kushner FG, Ascheim DD, et al; American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. 2013 ACCF/AHA guideline for the management of ST-elevation myocardial infarction: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation 2013; 127:e362–e425.
- Weinsaft JW, Kim HW, Crowley AL, et al. LV thrombus detection by routine echocardiography: insights into performance characteristics using delayed enhancement CMR. JACC Cardiovasc Imaging 2011; 4:702–712.
- Mollet NR, Dymarkowski S, Volders W, et al. Visualization of ventricular thrombi with contrast-enhanced magnetic resonance imaging in patients with ischemic heart disease. Circulation 2002; 106:2873–2876.
- Srichai MB, Junor C, Rodriguez LL, et al. Clinical, imaging, and pathological characteristics of left ventricular thrombus: a comparison of contrast-enhanced magnetic resonance imaging, transthoracic echocardiography, and transesophageal echocardiography with surgical or pathological validation. Am Heart J 2006; 152:75–84.
- Eriksson SE, Olsson JE. Survival and recurrent strokes in patients with different subtypes of stroke: a fourteen-year follow-up study. Cerebrovasc Dis 2001; 12:171–180.
- Grau AJ, Weimar C, Buggle F, et al. Risk factors, outcome, and treatment in subtypes of ischemic stroke: the German Stroke Data Bank. Stroke 2001; 32:2559–2566.
- Keren A, Goldberg S, Gottlieb S, et al. Natural history of left ventricular thrombi: their appearance and resolution in the posthospitalization period of acute myocardial infarction. J Am Coll Cardiol 1990; 15:790–800.
- Jordan RA, Miller RD, Edwards JE, Parker RL. Thrombo-embolism in acute and in healed myocardial infarction. I. Intracardiac mural thrombosis. Circulation 1952; 6:1–6.
- Vaitkus PT, Barnathan ES. Embolic potential, prevention and management of mural thrombus complicating anterior myocardial infarction: a meta-analysis. J Am Coll Cardiol 1993; 22:1004–1009.
- ISIS-2 (Second International Study of Infarct Survival) Collaborative Group. Randomised trial of intravenous streptokinase, oral aspirin, both, or neither among 17,187 cases of suspected acute myocardial infarction: ISIS-2. Lancet 1988; 2:349–360.
- Cabin HS, Roberts WC. Left ventricular aneurysm, intraaneurysmal thrombus and systemic embolus in coronary heart disease. Chest 1980; 77:586–590.
- Keating EC, Gross SA, Schlamowitz RA, et al. Mural thrombi in myocardial infarctions. Prospective evaluation by two-dimensional echocardiography. Am J Med 1983; 74:989–995.
- Witt BJ, Ballman KV, Brown RD Jr, Meverden RA, Jacobsen SJ, Roger VL. The incidence of stroke after myocardial infarction: a meta-analysis. Am J Med 2006; 119:354.e1–354.e9.
- Witt BJ, Brown RD Jr, Jacobsen SJ, Weston SA, Yawn BP, Roger VL. A community-based study of stroke incidence after myocardial infarction. Ann Intern Med 2005; 143:785–792.
- Vandvik PO, Lincoff AM, Gore JM, et al; American College of Chest Physicians. Primary and secondary prevention of cardiovascular disease: antithrombotic therapy and prevention of thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest 2012; 141(suppl):e637S–e68S.
- Steg G, James SK, Atar D, et al. ESC guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation. Eur Heart J 2012; 33:2569–2619.
- Nili M, Deviri E, Jortner R, Strasberg B, Levy MJ. Surgical removal of a mobile, pedunculated left ventricular thrombus: report of 4 cases. Ann Thorac Surg 1988; 46:396–400.
- Kanemitsu S, Miyake Y, Okabe M. Surgical removal of a left ventricular thrombus associated with cardiac sarcoidosis. Interact Cardiovasc Thorac Surg 2008; 7:333–335.
- Engin C, Yagdi T, Balcioglu O, et al. Left ventricular assist device implantation in heart failure patients with a left ventricular thrombus. Transplant Proc 2013; 45:1017–1019.
- Dewilde WJ, Oirbans T, Verheugt FW, et al; WOEST study investigators. Use of clopidogrel with or without aspirin in patients taking oral anticoagulant therapy and undergoing percutaneous coronary intervention: an open-label, randomised, controlled trial. Lancet 2013; 381:1107–1115.
- Faxon DP, Eikelboom JW, Berger PB, et al. Antithrombotic therapy in patients with atrial fibrillation undergoing coronary stenting: a North American perspective: executive summary. Circ Cardiovasc Interv 2011; 4:522–534.
- Hansen ML, Sorensen R, Clausen MT, et al. Risk of bleeding with single, dual, or triple therapy with warfarin, aspirin, and clopidogrel in patients with atrial fibrillation. Arch Intern Med 2010; 170:1433–1441.
- Karjalainen PP, Porela P, Ylitalo A, et al. Safety and efficacy of combined antiplatelet-warfarin therapy after coronary stenting. Eur Heart J 2007; 28:726–732.
- Doyle BJ, Rihal CS, Gastineau DA, Holmes DR Jr. Bleeding, blood transfusion, and increased mortality after percutaneous coronary intervention: implications for contemporary practice. J Am Coll Cardiol 2009; 53:2019–2027.
- Azoulay L, Dell’Aniello S, Simon T, Renoux C, Suissa S. The concurrent use of antithrombotic therapies and the risk of bleeding in patients with atrial fibrillation. Thromb Haemost 2013; 109:431–439.
- Deshmukh A, Hilleman DE, Del Core M, Nair CK. Antithrombotic regimens in patients with indication for long-term anticoagulation undergoing coronary interventions-systematic analysis, review of literature, and implications on management. Am J Ther 2013; 20:654–663.
- Fosbol EL, Wang TY, Li S, et al. Warfarin use among older atrial fibrillation patients with non-ST-segment elevation myocardial infarction managed with coronary stenting and dual antiplatelet therapy. Am Heart J 2013; 166:864–870.
- Gao F, Zhou YJ, Wang ZJ, et al. Meta-analysis of the combination of warfarin and dual antiplatelet therapy after coronary stenting in patients with indications for chronic oral anticoagulation. Int J Cardiol 2011; 148:96–101.
- Hansen ML, Sorensen R, Clausen MT, et al. Risk of bleeding with single, dual, or triple therapy with warfarin, aspirin, and clopidogrel in patients with atrial fibrillation. Arch Intern Med 2010; 170:1433–1441.
- Hermosillo AJ, Spinler SA. Aspirin, clopidogrel, and warfarin: is the combination appropriate and effective or inappropriate and too dangerous? Ann Pharmacother 2008; 42:790–805.
- Holmes DR Jr, Kereiakes DJ, Kleiman NS, Moliterno DJ, Patti G, Grines CL. Combining antiplatelet and anticoagulant therapies. J Am Coll Cardiol 2009; 54:95–109.
- Khurram Z, Chou E, Minutello R, et al. Combination therapy with aspirin, clopidogrel and warfarin following coronary stenting is associated with a significant risk of bleeding. J Invasive Cardiol 2006; 18:162–164.
- Orford JL, Fasseas P, Melby S, et al. Safety and efficacy of aspirin, clopidogrel, and warfarin after coronary stent placement in patients with an indication for anticoagulation. Am Heart J 2004; 147:463–467.
- Porter A, Konstantino Y, Iakobishvili Z, Shachar L, Battler A, Hasdai D. Short-term triple therapy with aspirin, warfarin, and a thienopyridine among patients undergoing percutaneous coronary intervention. Catheter Cardiovasc Interv 2006; 68:56–61.
- DeEugenio D, Kolman L, DeCaro M, et al. Risk of major bleeding with concomitant dual antiplatelet therapy after percutaneous coronary intervention in patients receiving long-term warfarin therapy. Pharmacotherapy 2007; 27:691–696.
- Ruiz-Nodar JM, Marin F, Hurtado JA, et al. Anticoagulant and antiplatelet therapy use in 426 patients with atrial fibrillation undergoing percutaneous coronary intervention and stent implantation implications for bleeding risk and prognosis. J Am Coll Cardiol 2008; 51:818–825.
- Sarafoff N, Ndrepepa G, Mehilli J, et al. Aspirin and clopidogrel with or without phenprocoumon after drug eluting coronary stent placement in patients on chronic oral anticoagulation. J Intern Med 2008; 264:472–480.
- Rossini R, Musumeci GF, Lettieri CF, et al. Long-term outcomes in patients undergoing coronary stenting on dual oral antiplatelet treatment requiring oral anticoagulant therapy. Am J Cardiol 2008; 102:1618–1623.
- Lip GY, Piotrponikowski P, Andreotti F, et al; Heart Failure Association (EHFA) of the European Society of Cardiology (ESC) and the ESC Working Group on Thrombosis. Thromboembolism and antithrombotic therapy for heart failure in sinus rhythm: an executive summary of a joint consensus document from the ESC Heart Failure Association and the ESC Working Group on Thrombosis. Thromb Haemost 2012; 108:1009–1022.
- Turpie AG, Robinson JG, Doyle DJ, et al. Comparison of high-dose with low-dose subcutaneous heparin to prevent left ventricular mural thrombosis in patients with acute transmural anterior myocardial infarction. N Engl J Med 1989; 320:352–357.
- Chiarella F, Santoro E, Domenicucci S, Maggioni A, Vecchio C. Predischarge two-dimensional echocardiographic evaluation of left ventricular thrombosis after acute myocardial infarction in the GISSI-3 study. Am J Cardiol 1998; 81:822–827.
- Kalra A, Jang IK. Prevalence of early left ventricular thrombus after primary coronary intervention for acute myocardial infarction. J Thromb Thrombolysis 2000; 10:133–136.
- Nayak D, Aronow WS, Sukhija R, McClung JA, Monsen CE, Belkin RN. Comparison of frequency of left ventricular thrombi in patients with anterior wall versus non-anterior wall acute myocardial infarction treated with antithrombotic and antiplatelet therapy with or without coronary revascularization. Am J Cardiol 2004; 93:1529–1530.
- Rehan A, Kanwar M, Rosman H, et al. Incidence of post myocardial infarction left ventricular thrombus formation in the era of primary percutaneous intervention and glycoprotein IIb/IIIa inhibitors. A prospective observational study. Cardiovasc Ultrasound 2006;4:20.
- Zielinska M, Kaczmarek K, Tylkowski M. Predictors of left ventricular thrombus formation in acute myocardial infarction treated with successful primary angioplasty with stenting. Am J Med Sci 2008; 335:171–176.
- Osherov AB, Borovik-Raz M, Aronson D, et al. Incidence of early left ventricular thrombus after acute anterior wall myocardial infarction in the primary coronary intervention era. Am Heart J 2009; 157:1074–1080.
- Solheim S, Seljeflot I, Lunde K, et al. Frequency of left ventricular thrombus in patients with anterior wall acute myocardial infarction treated with percutaneous coronary intervention and dual antiplatelet therapy. Am J Cardiol 2010; 106:1197–1200.
- Shacham Y, Leshem-Rubinow E, Ben Assa E, et al. Comparison of C-reactive protein and fibrinogen levels in patients having anterior wall ST-segment elevation myocardial infarction with versus without left ventricular thrombus (from a primary percutaneous coronary intervention cohort). Am J Cardiol 2013; 112:57–60.
- Gianstefani S, Douiri A, Delithanasis I, et al. Incidence and predictors of early left ventricular thrombus after ST-elevation myocardial infarction in the contemporary era of primary percutaneous coronary intervention. Am J Cardiol 2014; 113:1111–1116.
- Shacham Y, Birati EY, Rogovski O, Cogan Y, Keren G, Roth A. Left ventricular thrombus formation and bleeding complications during continuous in-hospital anticoagulation for acute anterior myocardial infarction. Isr Med Assoc J 2012; 14:742–746.
- Asinger RW, Mikell FL, Elsperger J, Hodges M. Incidence of left-ventricular thrombosis after acute transmural myocardial infarction. Serial evaluation by two-dimensional echocardiography. N Engl J Med 1981; 305:297–302.
- Nihoyannopoulos P, Smith GC, Maseri A, Foale RA. The natural history of left ventricular thrombus in myocardial infarction: a rationale in support of masterly inactivity. J Am Coll Cardiol 1989; 14:903–911.
- Weinreich DJ, Burke JF, Pauletto FJ. Left ventricular mural thrombi complicating acute myocardial infarction. Long-term follow-up with serial echocardiography. Ann Intern Med 1984; 100:789–794.
- Greaves SC, Zhi G, Lee RT, et al. Incidence and natural history of left ventricular thrombus following anterior wall acute myocardial infarction. Am J Cardiol 1997; 80:442–448.
- Solheim S, Seljeflot I, Lunde K, et al. Prothrombotic markers in patients with acute myocardial infarction and left ventricular thrombus formation treated with pci and dual antiplatelet therapy. Thromb J 2013; 11:1.
- O’Gara PT, Kushner FG, Ascheim DD, et al; American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. 2013 ACCF/AHA guideline for the management of ST-elevation myocardial infarction: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation 2013; 127:e362–e425.
- Weinsaft JW, Kim HW, Crowley AL, et al. LV thrombus detection by routine echocardiography: insights into performance characteristics using delayed enhancement CMR. JACC Cardiovasc Imaging 2011; 4:702–712.
- Mollet NR, Dymarkowski S, Volders W, et al. Visualization of ventricular thrombi with contrast-enhanced magnetic resonance imaging in patients with ischemic heart disease. Circulation 2002; 106:2873–2876.
- Srichai MB, Junor C, Rodriguez LL, et al. Clinical, imaging, and pathological characteristics of left ventricular thrombus: a comparison of contrast-enhanced magnetic resonance imaging, transthoracic echocardiography, and transesophageal echocardiography with surgical or pathological validation. Am Heart J 2006; 152:75–84.
- Eriksson SE, Olsson JE. Survival and recurrent strokes in patients with different subtypes of stroke: a fourteen-year follow-up study. Cerebrovasc Dis 2001; 12:171–180.
- Grau AJ, Weimar C, Buggle F, et al. Risk factors, outcome, and treatment in subtypes of ischemic stroke: the German Stroke Data Bank. Stroke 2001; 32:2559–2566.
- Keren A, Goldberg S, Gottlieb S, et al. Natural history of left ventricular thrombi: their appearance and resolution in the posthospitalization period of acute myocardial infarction. J Am Coll Cardiol 1990; 15:790–800.
- Jordan RA, Miller RD, Edwards JE, Parker RL. Thrombo-embolism in acute and in healed myocardial infarction. I. Intracardiac mural thrombosis. Circulation 1952; 6:1–6.
- Vaitkus PT, Barnathan ES. Embolic potential, prevention and management of mural thrombus complicating anterior myocardial infarction: a meta-analysis. J Am Coll Cardiol 1993; 22:1004–1009.
- ISIS-2 (Second International Study of Infarct Survival) Collaborative Group. Randomised trial of intravenous streptokinase, oral aspirin, both, or neither among 17,187 cases of suspected acute myocardial infarction: ISIS-2. Lancet 1988; 2:349–360.
- Cabin HS, Roberts WC. Left ventricular aneurysm, intraaneurysmal thrombus and systemic embolus in coronary heart disease. Chest 1980; 77:586–590.
- Keating EC, Gross SA, Schlamowitz RA, et al. Mural thrombi in myocardial infarctions. Prospective evaluation by two-dimensional echocardiography. Am J Med 1983; 74:989–995.
- Witt BJ, Ballman KV, Brown RD Jr, Meverden RA, Jacobsen SJ, Roger VL. The incidence of stroke after myocardial infarction: a meta-analysis. Am J Med 2006; 119:354.e1–354.e9.
- Witt BJ, Brown RD Jr, Jacobsen SJ, Weston SA, Yawn BP, Roger VL. A community-based study of stroke incidence after myocardial infarction. Ann Intern Med 2005; 143:785–792.
- Vandvik PO, Lincoff AM, Gore JM, et al; American College of Chest Physicians. Primary and secondary prevention of cardiovascular disease: antithrombotic therapy and prevention of thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest 2012; 141(suppl):e637S–e68S.
- Steg G, James SK, Atar D, et al. ESC guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation. Eur Heart J 2012; 33:2569–2619.
- Nili M, Deviri E, Jortner R, Strasberg B, Levy MJ. Surgical removal of a mobile, pedunculated left ventricular thrombus: report of 4 cases. Ann Thorac Surg 1988; 46:396–400.
- Kanemitsu S, Miyake Y, Okabe M. Surgical removal of a left ventricular thrombus associated with cardiac sarcoidosis. Interact Cardiovasc Thorac Surg 2008; 7:333–335.
- Engin C, Yagdi T, Balcioglu O, et al. Left ventricular assist device implantation in heart failure patients with a left ventricular thrombus. Transplant Proc 2013; 45:1017–1019.
- Dewilde WJ, Oirbans T, Verheugt FW, et al; WOEST study investigators. Use of clopidogrel with or without aspirin in patients taking oral anticoagulant therapy and undergoing percutaneous coronary intervention: an open-label, randomised, controlled trial. Lancet 2013; 381:1107–1115.
- Faxon DP, Eikelboom JW, Berger PB, et al. Antithrombotic therapy in patients with atrial fibrillation undergoing coronary stenting: a North American perspective: executive summary. Circ Cardiovasc Interv 2011; 4:522–534.
- Hansen ML, Sorensen R, Clausen MT, et al. Risk of bleeding with single, dual, or triple therapy with warfarin, aspirin, and clopidogrel in patients with atrial fibrillation. Arch Intern Med 2010; 170:1433–1441.
- Karjalainen PP, Porela P, Ylitalo A, et al. Safety and efficacy of combined antiplatelet-warfarin therapy after coronary stenting. Eur Heart J 2007; 28:726–732.
- Doyle BJ, Rihal CS, Gastineau DA, Holmes DR Jr. Bleeding, blood transfusion, and increased mortality after percutaneous coronary intervention: implications for contemporary practice. J Am Coll Cardiol 2009; 53:2019–2027.
- Azoulay L, Dell’Aniello S, Simon T, Renoux C, Suissa S. The concurrent use of antithrombotic therapies and the risk of bleeding in patients with atrial fibrillation. Thromb Haemost 2013; 109:431–439.
- Deshmukh A, Hilleman DE, Del Core M, Nair CK. Antithrombotic regimens in patients with indication for long-term anticoagulation undergoing coronary interventions-systematic analysis, review of literature, and implications on management. Am J Ther 2013; 20:654–663.
- Fosbol EL, Wang TY, Li S, et al. Warfarin use among older atrial fibrillation patients with non-ST-segment elevation myocardial infarction managed with coronary stenting and dual antiplatelet therapy. Am Heart J 2013; 166:864–870.
- Gao F, Zhou YJ, Wang ZJ, et al. Meta-analysis of the combination of warfarin and dual antiplatelet therapy after coronary stenting in patients with indications for chronic oral anticoagulation. Int J Cardiol 2011; 148:96–101.
- Hansen ML, Sorensen R, Clausen MT, et al. Risk of bleeding with single, dual, or triple therapy with warfarin, aspirin, and clopidogrel in patients with atrial fibrillation. Arch Intern Med 2010; 170:1433–1441.
- Hermosillo AJ, Spinler SA. Aspirin, clopidogrel, and warfarin: is the combination appropriate and effective or inappropriate and too dangerous? Ann Pharmacother 2008; 42:790–805.
- Holmes DR Jr, Kereiakes DJ, Kleiman NS, Moliterno DJ, Patti G, Grines CL. Combining antiplatelet and anticoagulant therapies. J Am Coll Cardiol 2009; 54:95–109.
- Khurram Z, Chou E, Minutello R, et al. Combination therapy with aspirin, clopidogrel and warfarin following coronary stenting is associated with a significant risk of bleeding. J Invasive Cardiol 2006; 18:162–164.
- Orford JL, Fasseas P, Melby S, et al. Safety and efficacy of aspirin, clopidogrel, and warfarin after coronary stent placement in patients with an indication for anticoagulation. Am Heart J 2004; 147:463–467.
- Porter A, Konstantino Y, Iakobishvili Z, Shachar L, Battler A, Hasdai D. Short-term triple therapy with aspirin, warfarin, and a thienopyridine among patients undergoing percutaneous coronary intervention. Catheter Cardiovasc Interv 2006; 68:56–61.
- DeEugenio D, Kolman L, DeCaro M, et al. Risk of major bleeding with concomitant dual antiplatelet therapy after percutaneous coronary intervention in patients receiving long-term warfarin therapy. Pharmacotherapy 2007; 27:691–696.
- Ruiz-Nodar JM, Marin F, Hurtado JA, et al. Anticoagulant and antiplatelet therapy use in 426 patients with atrial fibrillation undergoing percutaneous coronary intervention and stent implantation implications for bleeding risk and prognosis. J Am Coll Cardiol 2008; 51:818–825.
- Sarafoff N, Ndrepepa G, Mehilli J, et al. Aspirin and clopidogrel with or without phenprocoumon after drug eluting coronary stent placement in patients on chronic oral anticoagulation. J Intern Med 2008; 264:472–480.
- Rossini R, Musumeci GF, Lettieri CF, et al. Long-term outcomes in patients undergoing coronary stenting on dual oral antiplatelet treatment requiring oral anticoagulant therapy. Am J Cardiol 2008; 102:1618–1623.