User login
When to screen asymptomatic diabetics for CAD
SNOWMASS, COLO. – The use of coronary artery calcium screening in the subset of asymptomatic diabetes patients at higher clinical risk of CAD appears to offer a practical strategy for identifying a subgroup in whom costlier stress cardiac imaging may be justified, Marcelo F. di Carli, MD, said at the Annual Cardiovascular Conference at Snowmass.
The ultimate goal is to reliably identify those patients who have asymptomatic diabetes with significant CAD warranting revascularization or maximal medical therapy for primary cardiovascular prevention.
“Coronary artery calcium is a simple test that’s accessible and inexpensive and can give us a quick read on the extent of atherosclerosis in the coronary arteries,” said Dr. di Carli, professor of radiology and medicine at Harvard University in Boston. “There’s good data that in diabetic patients there’s a gradation of risk across the spectrum of calcium scores. Risk increases exponentially from a coronary artery calcium score of 0 to more than 400. The calcium score can also provide a snapshot of which patients are more likely to have flow-limiting coronary disease.”
Atherosclerotic cardiovascular disease is the biggest contributor to the direct and indirect costs of diabetes, and diabetes experts are eager to avoid jacking up those costs further by routinely ordering stress nuclear imaging, stress echocardiography, cardiac magnetic resonance, and other expensive noninvasive imaging methods unless they can be shown to lead to improved outcomes. There is general agreement on the value of noninvasive imaging in diabetic patients with CAD symptoms. However, the routine use of such testing in asymptomatic diabetic patients has been controversial.
Indeed, according to the 2017 American Diabetes Association Standards of Medical Care in Diabetes: “In asymptomatic patients, routine screening for coronary artery disease is not recommended as it does not improve outcomes as long as atherosclerotic cardiovascular disease risk factors are treated (Diabetes Care. 2017 Jan;40[Suppl. 1]:S75-87). That’s a Level A recommendation.
But Dr. di Carli is among many cardiologists who believe this statement paints with too broad a brush. He considers it an overgeneralization that’s based on the negative results of two randomized trials of routine screening in asymptomatic diabetics: DIAD, which utilized stress single-photon emission CT (SPECT) imaging (JAMA. 2009 Apr 15;301[15]:1547-55), and FACTOR-64, which relied upon coronary CT angiography (JAMA. 2014 Dec 3;312[21]: 2234-43). Both studies found relatively low yields of severe CAD and showed no survival benefit for screening. And of course, these are also costly and inconvenient tests.
The problem in generalizing from DIAD and FACTOR-64 to the overall population of asymptomatic diabetic patients is that both studies were conducted in asymptomatic patients at the lower end of the cardiovascular risk spectrum. They were young, with an average age of 60 years. They had a history of diabetes of less than 10 years, and their diabetes was reasonably well controlled. They had normal ECGs and preserved renal function. Peripheral artery disease (PAD) was present in only 9% of the DIAD population and no one in FACTOR-64. So this would not be expected to be a high-risk/high-yield population, according to Dr. di Carli, executive director of the cardiovascular imaging program at Brigham and Women’s Hospital, Boston.
An earlier study from the Mayo Clinic identified the clinical factors that can potentially be used to identify a higher-risk cohort of asymptomatic diabetic patients in whom high-tech noninvasive testing for significant CAD may be justified, he continued. This was a nonrandomized study of 1,427 asymptomatic diabetic patients without known CAD who underwent SPECT imaging. Compared with the study populations in DIAD and FACTOR-64, the Mayo Clinic patients had a longer duration of diabetes and substantially higher rates of poor diabetes control, renal dysfunction, hypertension, and dyslipidemia. One-third of them had PAD.
Fifty-eight percent of the 1,427 patients in the Mayo cohort proved to have an abnormal SPECT imaging scan, and 18% had a high-risk scan. In a multivariate analysis, the investigators identified several factors independently associated with a high-risk scan. Q waves were present on the ECGs of 9% of the asymptomatic diabetes patients, and 43% of that subgroup had a high-risk scan. Thirty-eight percent of patients had other ECG abnormalities, and 28% of them had a high-risk scan. Age greater than 65 was associated with an increased likelihood of a high-risk SPECT result. And 28% of patients with PAD had a high-risk scan.
On the other hand, the likelihood of a high-risk scan in the 69% of subjects without PAD was 14% (J Am Coll Cardiol. 2005 Jan 4;45[1]:43-9).
The 2017 ADA guidelines acknowledge this and similar evidence by providing as a relatively weak Level E recommendation: “Consider screening for CAD in the presence of any of the following: atypical cardiac symptoms (e.g., unexplained dyspnea, chest discomfort); signs of symptoms of associated vascular disease including carotid bruits, transient ischemic attack, stroke, claudication, or PAD; or electrogram abnormalities (e.g., Q waves).”
Dr. di Carli would add to that list age older than 65, diabetes duration of greater than 10 years, poor diabetes control, and a high burden of standard cardiovascular risk factors. And he proposed the coronary artery calcium (CAC) score as a sensible gateway to selective use of further screening tests, citing as support a report from the National Institutes of Health–sponsored Multi-Ethnic Study of Atherosclerosis (MESA).
The MESA investigators assessed CAC in 6,603 persons aged 45-84 free of known CAD at baseline, including 881 with diabetes. Participants were subsequently followed prospectively for an average of 6.4 years. Compared with diabetes patients who had a baseline CAC score of 0, those with a score of 1-99 were at a risk factor– and ethnicity-adjusted 2.9-fold increased risk for developing coronary heart disease during the follow-up period. The CHD risk climbed stepwise with an increasing CAC score such that subjects with a score of 400 or higher were at 9.5-fold increased risk (Diabetes Care. 2011 Oct;34[10]L2285-90).
Using CAC measurement in this way as a screening tool in asymptomatic diabetes patients with clinical factors placing them at higher risk of significant CAD is consistent with appropriate use criteria for the detection and risk assessment of stable ischemic heart disease. The criteria were provided in a 2014 joint report by the American College of Cardiology, American Heart Association, American Society of Echocardiography, American Society of Nuclear Cardiology, Heart Failure Society of America, Heart Rhythm Society, Society for Cardiovascular Angiography and Interventions, Society of Cardiovascular Computed Tomography, Society for Cardiovascular Magnetic Resonance, and Society of Thoracic Surgeons.
The report rates CAC testing as “May Be Appropriate” for asymptomatic patients of intermediate or high global risk. As such, CAC “can be an option for further evaluation of potential SIHD [stable ischemic heart disease] in an individual patient when deemed reasonable by the patient’s physician,” according to the appropriate use criteria guidance, which was created with the express purpose of developing standards to avoid overuse of costly cardiovascular testing (J Am Coll Cardiol. 2014 Feb 4;63[4]:380-406).
Dr. di Carli reported having no financial conflicts.
SNOWMASS, COLO. – The use of coronary artery calcium screening in the subset of asymptomatic diabetes patients at higher clinical risk of CAD appears to offer a practical strategy for identifying a subgroup in whom costlier stress cardiac imaging may be justified, Marcelo F. di Carli, MD, said at the Annual Cardiovascular Conference at Snowmass.
The ultimate goal is to reliably identify those patients who have asymptomatic diabetes with significant CAD warranting revascularization or maximal medical therapy for primary cardiovascular prevention.
“Coronary artery calcium is a simple test that’s accessible and inexpensive and can give us a quick read on the extent of atherosclerosis in the coronary arteries,” said Dr. di Carli, professor of radiology and medicine at Harvard University in Boston. “There’s good data that in diabetic patients there’s a gradation of risk across the spectrum of calcium scores. Risk increases exponentially from a coronary artery calcium score of 0 to more than 400. The calcium score can also provide a snapshot of which patients are more likely to have flow-limiting coronary disease.”
Atherosclerotic cardiovascular disease is the biggest contributor to the direct and indirect costs of diabetes, and diabetes experts are eager to avoid jacking up those costs further by routinely ordering stress nuclear imaging, stress echocardiography, cardiac magnetic resonance, and other expensive noninvasive imaging methods unless they can be shown to lead to improved outcomes. There is general agreement on the value of noninvasive imaging in diabetic patients with CAD symptoms. However, the routine use of such testing in asymptomatic diabetic patients has been controversial.
Indeed, according to the 2017 American Diabetes Association Standards of Medical Care in Diabetes: “In asymptomatic patients, routine screening for coronary artery disease is not recommended as it does not improve outcomes as long as atherosclerotic cardiovascular disease risk factors are treated (Diabetes Care. 2017 Jan;40[Suppl. 1]:S75-87). That’s a Level A recommendation.
But Dr. di Carli is among many cardiologists who believe this statement paints with too broad a brush. He considers it an overgeneralization that’s based on the negative results of two randomized trials of routine screening in asymptomatic diabetics: DIAD, which utilized stress single-photon emission CT (SPECT) imaging (JAMA. 2009 Apr 15;301[15]:1547-55), and FACTOR-64, which relied upon coronary CT angiography (JAMA. 2014 Dec 3;312[21]: 2234-43). Both studies found relatively low yields of severe CAD and showed no survival benefit for screening. And of course, these are also costly and inconvenient tests.
The problem in generalizing from DIAD and FACTOR-64 to the overall population of asymptomatic diabetic patients is that both studies were conducted in asymptomatic patients at the lower end of the cardiovascular risk spectrum. They were young, with an average age of 60 years. They had a history of diabetes of less than 10 years, and their diabetes was reasonably well controlled. They had normal ECGs and preserved renal function. Peripheral artery disease (PAD) was present in only 9% of the DIAD population and no one in FACTOR-64. So this would not be expected to be a high-risk/high-yield population, according to Dr. di Carli, executive director of the cardiovascular imaging program at Brigham and Women’s Hospital, Boston.
An earlier study from the Mayo Clinic identified the clinical factors that can potentially be used to identify a higher-risk cohort of asymptomatic diabetic patients in whom high-tech noninvasive testing for significant CAD may be justified, he continued. This was a nonrandomized study of 1,427 asymptomatic diabetic patients without known CAD who underwent SPECT imaging. Compared with the study populations in DIAD and FACTOR-64, the Mayo Clinic patients had a longer duration of diabetes and substantially higher rates of poor diabetes control, renal dysfunction, hypertension, and dyslipidemia. One-third of them had PAD.
Fifty-eight percent of the 1,427 patients in the Mayo cohort proved to have an abnormal SPECT imaging scan, and 18% had a high-risk scan. In a multivariate analysis, the investigators identified several factors independently associated with a high-risk scan. Q waves were present on the ECGs of 9% of the asymptomatic diabetes patients, and 43% of that subgroup had a high-risk scan. Thirty-eight percent of patients had other ECG abnormalities, and 28% of them had a high-risk scan. Age greater than 65 was associated with an increased likelihood of a high-risk SPECT result. And 28% of patients with PAD had a high-risk scan.
On the other hand, the likelihood of a high-risk scan in the 69% of subjects without PAD was 14% (J Am Coll Cardiol. 2005 Jan 4;45[1]:43-9).
The 2017 ADA guidelines acknowledge this and similar evidence by providing as a relatively weak Level E recommendation: “Consider screening for CAD in the presence of any of the following: atypical cardiac symptoms (e.g., unexplained dyspnea, chest discomfort); signs of symptoms of associated vascular disease including carotid bruits, transient ischemic attack, stroke, claudication, or PAD; or electrogram abnormalities (e.g., Q waves).”
Dr. di Carli would add to that list age older than 65, diabetes duration of greater than 10 years, poor diabetes control, and a high burden of standard cardiovascular risk factors. And he proposed the coronary artery calcium (CAC) score as a sensible gateway to selective use of further screening tests, citing as support a report from the National Institutes of Health–sponsored Multi-Ethnic Study of Atherosclerosis (MESA).
The MESA investigators assessed CAC in 6,603 persons aged 45-84 free of known CAD at baseline, including 881 with diabetes. Participants were subsequently followed prospectively for an average of 6.4 years. Compared with diabetes patients who had a baseline CAC score of 0, those with a score of 1-99 were at a risk factor– and ethnicity-adjusted 2.9-fold increased risk for developing coronary heart disease during the follow-up period. The CHD risk climbed stepwise with an increasing CAC score such that subjects with a score of 400 or higher were at 9.5-fold increased risk (Diabetes Care. 2011 Oct;34[10]L2285-90).
Using CAC measurement in this way as a screening tool in asymptomatic diabetes patients with clinical factors placing them at higher risk of significant CAD is consistent with appropriate use criteria for the detection and risk assessment of stable ischemic heart disease. The criteria were provided in a 2014 joint report by the American College of Cardiology, American Heart Association, American Society of Echocardiography, American Society of Nuclear Cardiology, Heart Failure Society of America, Heart Rhythm Society, Society for Cardiovascular Angiography and Interventions, Society of Cardiovascular Computed Tomography, Society for Cardiovascular Magnetic Resonance, and Society of Thoracic Surgeons.
The report rates CAC testing as “May Be Appropriate” for asymptomatic patients of intermediate or high global risk. As such, CAC “can be an option for further evaluation of potential SIHD [stable ischemic heart disease] in an individual patient when deemed reasonable by the patient’s physician,” according to the appropriate use criteria guidance, which was created with the express purpose of developing standards to avoid overuse of costly cardiovascular testing (J Am Coll Cardiol. 2014 Feb 4;63[4]:380-406).
Dr. di Carli reported having no financial conflicts.
SNOWMASS, COLO. – The use of coronary artery calcium screening in the subset of asymptomatic diabetes patients at higher clinical risk of CAD appears to offer a practical strategy for identifying a subgroup in whom costlier stress cardiac imaging may be justified, Marcelo F. di Carli, MD, said at the Annual Cardiovascular Conference at Snowmass.
The ultimate goal is to reliably identify those patients who have asymptomatic diabetes with significant CAD warranting revascularization or maximal medical therapy for primary cardiovascular prevention.
“Coronary artery calcium is a simple test that’s accessible and inexpensive and can give us a quick read on the extent of atherosclerosis in the coronary arteries,” said Dr. di Carli, professor of radiology and medicine at Harvard University in Boston. “There’s good data that in diabetic patients there’s a gradation of risk across the spectrum of calcium scores. Risk increases exponentially from a coronary artery calcium score of 0 to more than 400. The calcium score can also provide a snapshot of which patients are more likely to have flow-limiting coronary disease.”
Atherosclerotic cardiovascular disease is the biggest contributor to the direct and indirect costs of diabetes, and diabetes experts are eager to avoid jacking up those costs further by routinely ordering stress nuclear imaging, stress echocardiography, cardiac magnetic resonance, and other expensive noninvasive imaging methods unless they can be shown to lead to improved outcomes. There is general agreement on the value of noninvasive imaging in diabetic patients with CAD symptoms. However, the routine use of such testing in asymptomatic diabetic patients has been controversial.
Indeed, according to the 2017 American Diabetes Association Standards of Medical Care in Diabetes: “In asymptomatic patients, routine screening for coronary artery disease is not recommended as it does not improve outcomes as long as atherosclerotic cardiovascular disease risk factors are treated (Diabetes Care. 2017 Jan;40[Suppl. 1]:S75-87). That’s a Level A recommendation.
But Dr. di Carli is among many cardiologists who believe this statement paints with too broad a brush. He considers it an overgeneralization that’s based on the negative results of two randomized trials of routine screening in asymptomatic diabetics: DIAD, which utilized stress single-photon emission CT (SPECT) imaging (JAMA. 2009 Apr 15;301[15]:1547-55), and FACTOR-64, which relied upon coronary CT angiography (JAMA. 2014 Dec 3;312[21]: 2234-43). Both studies found relatively low yields of severe CAD and showed no survival benefit for screening. And of course, these are also costly and inconvenient tests.
The problem in generalizing from DIAD and FACTOR-64 to the overall population of asymptomatic diabetic patients is that both studies were conducted in asymptomatic patients at the lower end of the cardiovascular risk spectrum. They were young, with an average age of 60 years. They had a history of diabetes of less than 10 years, and their diabetes was reasonably well controlled. They had normal ECGs and preserved renal function. Peripheral artery disease (PAD) was present in only 9% of the DIAD population and no one in FACTOR-64. So this would not be expected to be a high-risk/high-yield population, according to Dr. di Carli, executive director of the cardiovascular imaging program at Brigham and Women’s Hospital, Boston.
An earlier study from the Mayo Clinic identified the clinical factors that can potentially be used to identify a higher-risk cohort of asymptomatic diabetic patients in whom high-tech noninvasive testing for significant CAD may be justified, he continued. This was a nonrandomized study of 1,427 asymptomatic diabetic patients without known CAD who underwent SPECT imaging. Compared with the study populations in DIAD and FACTOR-64, the Mayo Clinic patients had a longer duration of diabetes and substantially higher rates of poor diabetes control, renal dysfunction, hypertension, and dyslipidemia. One-third of them had PAD.
Fifty-eight percent of the 1,427 patients in the Mayo cohort proved to have an abnormal SPECT imaging scan, and 18% had a high-risk scan. In a multivariate analysis, the investigators identified several factors independently associated with a high-risk scan. Q waves were present on the ECGs of 9% of the asymptomatic diabetes patients, and 43% of that subgroup had a high-risk scan. Thirty-eight percent of patients had other ECG abnormalities, and 28% of them had a high-risk scan. Age greater than 65 was associated with an increased likelihood of a high-risk SPECT result. And 28% of patients with PAD had a high-risk scan.
On the other hand, the likelihood of a high-risk scan in the 69% of subjects without PAD was 14% (J Am Coll Cardiol. 2005 Jan 4;45[1]:43-9).
The 2017 ADA guidelines acknowledge this and similar evidence by providing as a relatively weak Level E recommendation: “Consider screening for CAD in the presence of any of the following: atypical cardiac symptoms (e.g., unexplained dyspnea, chest discomfort); signs of symptoms of associated vascular disease including carotid bruits, transient ischemic attack, stroke, claudication, or PAD; or electrogram abnormalities (e.g., Q waves).”
Dr. di Carli would add to that list age older than 65, diabetes duration of greater than 10 years, poor diabetes control, and a high burden of standard cardiovascular risk factors. And he proposed the coronary artery calcium (CAC) score as a sensible gateway to selective use of further screening tests, citing as support a report from the National Institutes of Health–sponsored Multi-Ethnic Study of Atherosclerosis (MESA).
The MESA investigators assessed CAC in 6,603 persons aged 45-84 free of known CAD at baseline, including 881 with diabetes. Participants were subsequently followed prospectively for an average of 6.4 years. Compared with diabetes patients who had a baseline CAC score of 0, those with a score of 1-99 were at a risk factor– and ethnicity-adjusted 2.9-fold increased risk for developing coronary heart disease during the follow-up period. The CHD risk climbed stepwise with an increasing CAC score such that subjects with a score of 400 or higher were at 9.5-fold increased risk (Diabetes Care. 2011 Oct;34[10]L2285-90).
Using CAC measurement in this way as a screening tool in asymptomatic diabetes patients with clinical factors placing them at higher risk of significant CAD is consistent with appropriate use criteria for the detection and risk assessment of stable ischemic heart disease. The criteria were provided in a 2014 joint report by the American College of Cardiology, American Heart Association, American Society of Echocardiography, American Society of Nuclear Cardiology, Heart Failure Society of America, Heart Rhythm Society, Society for Cardiovascular Angiography and Interventions, Society of Cardiovascular Computed Tomography, Society for Cardiovascular Magnetic Resonance, and Society of Thoracic Surgeons.
The report rates CAC testing as “May Be Appropriate” for asymptomatic patients of intermediate or high global risk. As such, CAC “can be an option for further evaluation of potential SIHD [stable ischemic heart disease] in an individual patient when deemed reasonable by the patient’s physician,” according to the appropriate use criteria guidance, which was created with the express purpose of developing standards to avoid overuse of costly cardiovascular testing (J Am Coll Cardiol. 2014 Feb 4;63[4]:380-406).
Dr. di Carli reported having no financial conflicts.
EXPERT ANALYSIS FROM THE CARDIOVASCULAR CONFERENCE AT SNOWMASS
Diagnosis of Severe Acute Lower Gastrointestinal Bleeding with CTA
Case
A 31-year-old white man presented to the ED with abdominal and rectal pain accompanied by multiple episodes of bloody diarrhea. He stated he had mild rectal pain the previous night but was pain-free and in his usual state of health the morning of his presentation. Approximately 2 hours before presenting to the ED, however, he began experiencing mild stomach pain, then bloody diarrhea which he described as bright red and “filling the toilet bowl with blood.” He had no history of inflammatory bowel disease or other gastrointestinal (GI) disorder, no recent travel, no complaints of nausea or vomiting, and no infectious symptoms. He described a remote history of external hemorrhoids, and review of his family history was significant for multiple paternal relatives with aortic aneurysms. He was not taking any medications and was a nonsmoker with a normal body mass index (24.3 kg/m2).
Upon arrival at the ED, the patient’s vital signs were: heart rate, 112 beats/min; and blood pressure, 139/102 mm Hg; respiratory rate and temperature were normal, as was the patient’s oxygen saturation on room air. Physical examination was notable for no subjective or objective findings of orthostatic hypotension; increased bowel sounds and diffuse mild abdominal tenderness; and no external hemorrhoids, fissures, or rectal tenderness. Laboratory evaluation was significant for hemoglobin (Hgb), 15.0 g/dL; blood urea nitrogen (BUN)-to-creatinine (Cr) ratio, 11.6; and anion gap, 17 mEq/L.
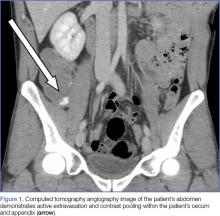
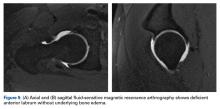
Upon initial presentation, there was some concern for an infection. However, as the patient continued to have bowel movements consisting almost entirely of frank blood and did not have any infectious signs, a vascular etiology was more strongly considered. Given the patient’s relatively stable vital signs, BUN-to-Cr ratio of less than 20, and lack of orthostatic hypotension, there was low concern for an upper GI etiology, and endoscopy was not obtained emergently. The patient instead underwent abdominal computed tomography angiography (CTA), which identified active extravasation and contrast pooling within the cecum and appendix (Figure 1).
Shortly after the patient returned from imaging, repeat laboratory studies were performed, demonstrating an Hgb drop from 15.0 g/dL to 12.3 g/dL, and surgical services was emergently consulted. The surgeon recommended that embolization first be attempted, with surgery as the option of last resort given the poor localization of the bleed on CTA and the long-term consequences of colonic resection in a young, otherwise healthy man.
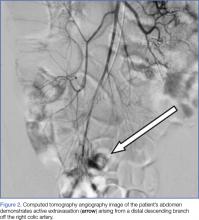
Interventional radiology was consulted, and the patient was brought immediately to the angiography suite, where he was found to have “active extravasation arising from a distal descending branch off the right colic artery” (Figure 2). Coil embolization resulted in complete resolution of the hemorrhage.
Later that evening, the patient’s Hgb continued to drop, reaching nadir at 7.3 g/dL, and he continued to have severe hematochezia. His falling Hgb was thought to be indicative of the degree of hemorrhage he had sustained prior to embolization, and the clearance of such blood as the source of his ongoing hematochezia. Following transfusion of 2 U of packed red blood cells (PRBCs), the patient’s Hgb improved to 12.0 g/dL, and he did not experience any significant bleeding for the remainder of his hospital stay.
The following morning, the patient underwent an extensive colonoscopy (extending 25 cm into the terminal ileum), which was unable to detect any signs of arteriovenous malformations, angiodysplasia, or any other possible source of bleeding. After 24 hours with stable vital signs and Hgb levels, the patient was discharged home with close surgical and gastroenterological follow-up, with possible genetic testing for connective tissue diseases. The diagnosis at discharge was spontaneous mesenteric hemorrhage of unknown etiology.
Discussion
Acute lower GI bleeding has an estimated annual hospitalization rate of 36 patients per 100,000, or about half the rate for upper GI bleeding.1,2 The majority of patients (>80%) will have spontaneous resolution and can be worked up nonemergently.
Etiology and Work-Up
Assessment of the etiology of hematochezia begins with ruling out an upper GI source of the bleed; 10% to 15% of patients presenting with hematochezia without hematemesis are ultimately diagnosed with an upper GI etiology. 4,5
BUN-to-Cr Ratio. In a study of patients presenting with hematochezia but no hematemesis or renal failure, Srygley et al6 found a BUN-to-Cr ratio greater than 93% to be sensitive for an upper GI source, with a likelihood ratio of 7.5. The proposed etiology is some combination of absorption of digested blood products and prerenal azotemia due to hypovolemia.
Tachycardia and Orthostatic Hypotension. There have been discussions in the literature about other findings to rule in/out upper GI bleeding. While some studies have found statistically significant results between upper and lower GI bleeding for tachycardia and orthostatic hypotension (increased percentage of both in upper GI bleeding), there is disagreement about whether these findings are clinically significant.7-9
Nasogastric Lavage. Although nasogastric (NG) lavage is no longer the standard of care in the ED due to poor sensitivity and marked discomfort to the patient, most current gastroenterology guidelines still recommend its use; therefore, NG may be requested by the GI consultant.10-12
Diagnosis
Once an upper GI source has been ruled out, identification of the lesion is the next step. The differential diagnosis includes common sources such as diverticular disease, angiodysplasia, colitis, anorectal sources, and neoplasm.5 Less common, but associated with a high risk of mortality, is aortoenteric fistula (100% mortality without surgical intervention).5
Colonoscopy. Emergent colonoscopy can be used for both diagnosis and (potential) therapeutic intervention and is therefore the first option of choice.1,3,4,9 However, as seen in our case, some patients experience such profound hemorrhage that visualization of the colon may be difficult or impossible; patients may also be too unstable to await bowel preparation or undergo a procedure.
Computed Tomography Angiography. For patients in whom colonoscopy is contraindicated, CTA is the imaging modality of choice, and has a 91% to 92% sensitivity in identifying active bleeding (>0.35 mL/min).13-16
Computed tomography of the abdomen and pelvis with contrast alone, as opposed to CTA, is insufficient for detecting GI bleeding, as it is timed so that imaging is obtained when the contrast is in the portal venous capillary beds, rather than in the arteries or arterioles. By protocol, though, many institutions require abdominal and pelvic CTA to include both arterial phase and venous phase images, allowing for assessment of both active arterial bleeding and alternative lower GI sources of hematochezia (eg, mesenteric ischemia).
When ordering a CT study, an awareness of local practice is important in understanding the information that will be obtained from the study. Protocols for lower GI bleed that include CTA have reported accuracy and efficiency without worsening of renal function, despite the increased contrast load.17
Triphasic CT Enterography. Another CT modality to consider is triphasic CT enterography, which uses IV and oral contrast. In a preliminary trial, this modality achieved a specificity of 100% (sensitivity 42%) in detecting GI bleeding.18
Red Blood Cell Scintigraphy. An additional imaging modality that has been the subject of much debate in the GI literature is tagged RBC scintigraphy with Technetium-99m. Various studies have found bleeding-site confirmation in 24% to 97% of patients, and correct localization in 41% to 100% of patients. Given the extensive variability within the literature on selection criteria, localization, site confirmation, and other variables, as well as evidence from one prospective trial by Zink et al19 that found a significant disagreement between CTA and scintigraphy, RBC scintigraphy is not recommended as an alternative imaging modality for the rapid diagnosis of an acute lower GI bleed.
Conclusion
Severe hematochezia is a potential surgical emergency with a broad differential diagnosis. While emergent colonoscopy is an excellent first option, in patients with severe hematochezia, there may be too much blood in the colon to obtain adequate visual images; additionally, depending on practice setting, emergency colonoscopy may not be immediately available. In either case, CTA—a readily available, noninvasive, rapid, and repeatable diagnostic tool—should be considered as an alternate to colonoscopy, particularly in patients with brisk hematochezia.
If a patient with severe hematochezia presents to the ED, the emergency physician (EP) must recognize that the degree of hemorrhage may not correlate with the patient’s vital signs or initial laboratory values. For this reason, the EP must have a high index of suspicion, and consider CTA to allow for a rapid definitive diagnosis and prompt discussion between surgical, interventional radiology, and/or gastroenterology teams to improve clinical outcomes and decrease morbidity and mortality.20
1. Ghassemi K, Jensen D. Lower GI bleeding: epidemiology and management. Curr Gastroenterol Rep. 2013;15(7):333. doi:10.1007/s11894-013-0333-5.
2. Strate LL, Ayanian JZ, Kotler G, Syngal S. Risk factors for mortality in lower intestinal bleeding. Clin Gastroenterol Hepatol. 2008;6(9):1004-1010. doi:10.1016/j.cgh.2008.03.021.
3. Qayad E, Dagar G, Nanchal R. Lower gastrointestinal hemorrhage. Crit Care Clin. 2016;32(2):241-254. doi:10.1016/j.ccc.2015.12.004.
4. Strate LL. Lower GI bleeding: epidemiology and diagnosis. Gastroenterol Clin North Am. 2005;34(4):643-664.
5. Goralnick E, Meguerdichian D. Gastrointestinal bleeding. In: Marx J, Hockberger R, Walls R. (Eds.). Rosen’s Emergency Medicine, 8th Edition. Philadelphia, PA: Saunders, 2014;248-253.
6. Srygley FD, Gerando CJ, Tran T, Fisher DA. Does this patient have a severe upper gastrointestinal bleed? JAMA. 2012;307(10):1072-1079. doi:10.1001/jama.2012.253.
7. Whelen C, Chen C, Kaboli P, Siddique J, Prochaska M, Meltzer DO. Upper versus lower gastrointestinal bleeding: a direct comparison of clinical presentation, outcomes, and resource utilization. J Hosp Med. 2010;5(3):141-147. doi:10.1002/jhm.606.
8. Sittichanbunch Y, Senasu S, Thongkrau T, Keeratiksikorn C, Sawanyawisuth K. How to differentiate sites of gastrointestinal bleeding in patients with hematochezia by using clinical factors? Gastroenterol Res Pract. 2013;2013:265076. doi:10.1155/2013/265076.
9. Velayos F, Williamson A, Sousa KH, et al. Early predictors of severe lower gastrointestinal bleeding and adverse outcomes: a prospective study. Clin Gastroenterol Hepatol. 2004;2(6):485-490.
10. Palamidessi N, Sinert R, Falzon L, Zehtabchi S. Nasogastric aspiration and lavage in emergency department patients with hematochezia or melena without hematemesis. Acad Emerg Med. 2010;17(2):126-132. doi:10.1111/j.1553-2712.2009.00609.x.
11. Singer AJ, Richman PB, Kowalska A, Thode HC Jr. Comparison of patient and practitioner assessments of pain from commonly performed emergency department procedures. Ann Emerg Med. 1999;33(6):652-658.
12. Strate L, Gralnek I. ACG clinical guideline: management of patients with acute lower gastrointestinal bleeding. Am J Gastroenterol. 2016;111(4):459-474. doi:10.1038/ajg.2016.41.
13. Wu LM, Xu JR, Yin Y, Qu XH. Usefulness of CT angiography in diagnosing acute gastrointestinal bleeding: a meta-analysis. World J Gastroenterol. 2010;16(31):3957-3963.
14. Geffroy Y, Rodallec MH, Boulay-Coletta I, Julles MC, Ridereau-Zins C, Zins M. Multidetector CT angiography in acute gastrointestinal bleeding: why, when, and how. Radiographics. 2011;31(3):E35-E46.
15. Reis F, Cardia P, D’Ippolito G. Computed tomography angiography in patients with active gastrointestinal bleeding. Radiol Bras. 2015;48(6):381-390. doi:10.1590/0100-3984.2014.0014.
16. Chan V, Tse D, Dixon S, et al. Outcome following a negative CT angiogram for gastrointestinal hemorrhage. Cardiovasc Intervent Radiol. 2015;38(2):329-335. doi:10.1007/s00270-014-0928-8.
17. Jacovides T, Nadolski G, Allen S, et al. Arteriography for lower gastrointestinal hemorrhage: role of preceding abdominal computed tomographic angiogram in diagnosis and localization. JAMA Surgery. 2015;150(7):650-656. doi:10.1001/jamasurg.2015.97.
18. Hara AK, Walker FB, Silva AC, Leighton JA. Preliminary estimate of triphasic CT enterography performance in hemodynamically stable patients with suspected gastrointestinal bleeding. AJR Am J Roentgenol. 2009;193(5):1252-1260. doi:10.2214/AJR.08.1494.
19. Zink SI, Ohki SK, Stein B, et al. Noninvasive evaluation of active lower gastrointestinal bleeding: comparison between contrast-enhanced MDCT and 99mTc-labeled RBC scintigraphy. AJR Am J Roentgenol. 2008;91(4):1107-1114. doi:10.2214/AJR.07.3642.
20. Nable J, Graham A. Gastrointestinal bleeding. Emerg Med Clin N Am. 2016;34(2):309-325. doi:10.1016/j.emc.2015.12.001.
Case
A 31-year-old white man presented to the ED with abdominal and rectal pain accompanied by multiple episodes of bloody diarrhea. He stated he had mild rectal pain the previous night but was pain-free and in his usual state of health the morning of his presentation. Approximately 2 hours before presenting to the ED, however, he began experiencing mild stomach pain, then bloody diarrhea which he described as bright red and “filling the toilet bowl with blood.” He had no history of inflammatory bowel disease or other gastrointestinal (GI) disorder, no recent travel, no complaints of nausea or vomiting, and no infectious symptoms. He described a remote history of external hemorrhoids, and review of his family history was significant for multiple paternal relatives with aortic aneurysms. He was not taking any medications and was a nonsmoker with a normal body mass index (24.3 kg/m2).
Upon arrival at the ED, the patient’s vital signs were: heart rate, 112 beats/min; and blood pressure, 139/102 mm Hg; respiratory rate and temperature were normal, as was the patient’s oxygen saturation on room air. Physical examination was notable for no subjective or objective findings of orthostatic hypotension; increased bowel sounds and diffuse mild abdominal tenderness; and no external hemorrhoids, fissures, or rectal tenderness. Laboratory evaluation was significant for hemoglobin (Hgb), 15.0 g/dL; blood urea nitrogen (BUN)-to-creatinine (Cr) ratio, 11.6; and anion gap, 17 mEq/L.
Upon initial presentation, there was some concern for an infection. However, as the patient continued to have bowel movements consisting almost entirely of frank blood and did not have any infectious signs, a vascular etiology was more strongly considered. Given the patient’s relatively stable vital signs, BUN-to-Cr ratio of less than 20, and lack of orthostatic hypotension, there was low concern for an upper GI etiology, and endoscopy was not obtained emergently. The patient instead underwent abdominal computed tomography angiography (CTA), which identified active extravasation and contrast pooling within the cecum and appendix (Figure 1).
Shortly after the patient returned from imaging, repeat laboratory studies were performed, demonstrating an Hgb drop from 15.0 g/dL to 12.3 g/dL, and surgical services was emergently consulted. The surgeon recommended that embolization first be attempted, with surgery as the option of last resort given the poor localization of the bleed on CTA and the long-term consequences of colonic resection in a young, otherwise healthy man.
Interventional radiology was consulted, and the patient was brought immediately to the angiography suite, where he was found to have “active extravasation arising from a distal descending branch off the right colic artery” (Figure 2). Coil embolization resulted in complete resolution of the hemorrhage.
Later that evening, the patient’s Hgb continued to drop, reaching nadir at 7.3 g/dL, and he continued to have severe hematochezia. His falling Hgb was thought to be indicative of the degree of hemorrhage he had sustained prior to embolization, and the clearance of such blood as the source of his ongoing hematochezia. Following transfusion of 2 U of packed red blood cells (PRBCs), the patient’s Hgb improved to 12.0 g/dL, and he did not experience any significant bleeding for the remainder of his hospital stay.
The following morning, the patient underwent an extensive colonoscopy (extending 25 cm into the terminal ileum), which was unable to detect any signs of arteriovenous malformations, angiodysplasia, or any other possible source of bleeding. After 24 hours with stable vital signs and Hgb levels, the patient was discharged home with close surgical and gastroenterological follow-up, with possible genetic testing for connective tissue diseases. The diagnosis at discharge was spontaneous mesenteric hemorrhage of unknown etiology.
Discussion
Acute lower GI bleeding has an estimated annual hospitalization rate of 36 patients per 100,000, or about half the rate for upper GI bleeding.1,2 The majority of patients (>80%) will have spontaneous resolution and can be worked up nonemergently.
Etiology and Work-Up
Assessment of the etiology of hematochezia begins with ruling out an upper GI source of the bleed; 10% to 15% of patients presenting with hematochezia without hematemesis are ultimately diagnosed with an upper GI etiology. 4,5
BUN-to-Cr Ratio. In a study of patients presenting with hematochezia but no hematemesis or renal failure, Srygley et al6 found a BUN-to-Cr ratio greater than 93% to be sensitive for an upper GI source, with a likelihood ratio of 7.5. The proposed etiology is some combination of absorption of digested blood products and prerenal azotemia due to hypovolemia.
Tachycardia and Orthostatic Hypotension. There have been discussions in the literature about other findings to rule in/out upper GI bleeding. While some studies have found statistically significant results between upper and lower GI bleeding for tachycardia and orthostatic hypotension (increased percentage of both in upper GI bleeding), there is disagreement about whether these findings are clinically significant.7-9
Nasogastric Lavage. Although nasogastric (NG) lavage is no longer the standard of care in the ED due to poor sensitivity and marked discomfort to the patient, most current gastroenterology guidelines still recommend its use; therefore, NG may be requested by the GI consultant.10-12
Diagnosis
Once an upper GI source has been ruled out, identification of the lesion is the next step. The differential diagnosis includes common sources such as diverticular disease, angiodysplasia, colitis, anorectal sources, and neoplasm.5 Less common, but associated with a high risk of mortality, is aortoenteric fistula (100% mortality without surgical intervention).5
Colonoscopy. Emergent colonoscopy can be used for both diagnosis and (potential) therapeutic intervention and is therefore the first option of choice.1,3,4,9 However, as seen in our case, some patients experience such profound hemorrhage that visualization of the colon may be difficult or impossible; patients may also be too unstable to await bowel preparation or undergo a procedure.
Computed Tomography Angiography. For patients in whom colonoscopy is contraindicated, CTA is the imaging modality of choice, and has a 91% to 92% sensitivity in identifying active bleeding (>0.35 mL/min).13-16
Computed tomography of the abdomen and pelvis with contrast alone, as opposed to CTA, is insufficient for detecting GI bleeding, as it is timed so that imaging is obtained when the contrast is in the portal venous capillary beds, rather than in the arteries or arterioles. By protocol, though, many institutions require abdominal and pelvic CTA to include both arterial phase and venous phase images, allowing for assessment of both active arterial bleeding and alternative lower GI sources of hematochezia (eg, mesenteric ischemia).
When ordering a CT study, an awareness of local practice is important in understanding the information that will be obtained from the study. Protocols for lower GI bleed that include CTA have reported accuracy and efficiency without worsening of renal function, despite the increased contrast load.17
Triphasic CT Enterography. Another CT modality to consider is triphasic CT enterography, which uses IV and oral contrast. In a preliminary trial, this modality achieved a specificity of 100% (sensitivity 42%) in detecting GI bleeding.18
Red Blood Cell Scintigraphy. An additional imaging modality that has been the subject of much debate in the GI literature is tagged RBC scintigraphy with Technetium-99m. Various studies have found bleeding-site confirmation in 24% to 97% of patients, and correct localization in 41% to 100% of patients. Given the extensive variability within the literature on selection criteria, localization, site confirmation, and other variables, as well as evidence from one prospective trial by Zink et al19 that found a significant disagreement between CTA and scintigraphy, RBC scintigraphy is not recommended as an alternative imaging modality for the rapid diagnosis of an acute lower GI bleed.
Conclusion
Severe hematochezia is a potential surgical emergency with a broad differential diagnosis. While emergent colonoscopy is an excellent first option, in patients with severe hematochezia, there may be too much blood in the colon to obtain adequate visual images; additionally, depending on practice setting, emergency colonoscopy may not be immediately available. In either case, CTA—a readily available, noninvasive, rapid, and repeatable diagnostic tool—should be considered as an alternate to colonoscopy, particularly in patients with brisk hematochezia.
If a patient with severe hematochezia presents to the ED, the emergency physician (EP) must recognize that the degree of hemorrhage may not correlate with the patient’s vital signs or initial laboratory values. For this reason, the EP must have a high index of suspicion, and consider CTA to allow for a rapid definitive diagnosis and prompt discussion between surgical, interventional radiology, and/or gastroenterology teams to improve clinical outcomes and decrease morbidity and mortality.20
Case
A 31-year-old white man presented to the ED with abdominal and rectal pain accompanied by multiple episodes of bloody diarrhea. He stated he had mild rectal pain the previous night but was pain-free and in his usual state of health the morning of his presentation. Approximately 2 hours before presenting to the ED, however, he began experiencing mild stomach pain, then bloody diarrhea which he described as bright red and “filling the toilet bowl with blood.” He had no history of inflammatory bowel disease or other gastrointestinal (GI) disorder, no recent travel, no complaints of nausea or vomiting, and no infectious symptoms. He described a remote history of external hemorrhoids, and review of his family history was significant for multiple paternal relatives with aortic aneurysms. He was not taking any medications and was a nonsmoker with a normal body mass index (24.3 kg/m2).
Upon arrival at the ED, the patient’s vital signs were: heart rate, 112 beats/min; and blood pressure, 139/102 mm Hg; respiratory rate and temperature were normal, as was the patient’s oxygen saturation on room air. Physical examination was notable for no subjective or objective findings of orthostatic hypotension; increased bowel sounds and diffuse mild abdominal tenderness; and no external hemorrhoids, fissures, or rectal tenderness. Laboratory evaluation was significant for hemoglobin (Hgb), 15.0 g/dL; blood urea nitrogen (BUN)-to-creatinine (Cr) ratio, 11.6; and anion gap, 17 mEq/L.
Upon initial presentation, there was some concern for an infection. However, as the patient continued to have bowel movements consisting almost entirely of frank blood and did not have any infectious signs, a vascular etiology was more strongly considered. Given the patient’s relatively stable vital signs, BUN-to-Cr ratio of less than 20, and lack of orthostatic hypotension, there was low concern for an upper GI etiology, and endoscopy was not obtained emergently. The patient instead underwent abdominal computed tomography angiography (CTA), which identified active extravasation and contrast pooling within the cecum and appendix (Figure 1).
Shortly after the patient returned from imaging, repeat laboratory studies were performed, demonstrating an Hgb drop from 15.0 g/dL to 12.3 g/dL, and surgical services was emergently consulted. The surgeon recommended that embolization first be attempted, with surgery as the option of last resort given the poor localization of the bleed on CTA and the long-term consequences of colonic resection in a young, otherwise healthy man.
Interventional radiology was consulted, and the patient was brought immediately to the angiography suite, where he was found to have “active extravasation arising from a distal descending branch off the right colic artery” (Figure 2). Coil embolization resulted in complete resolution of the hemorrhage.
Later that evening, the patient’s Hgb continued to drop, reaching nadir at 7.3 g/dL, and he continued to have severe hematochezia. His falling Hgb was thought to be indicative of the degree of hemorrhage he had sustained prior to embolization, and the clearance of such blood as the source of his ongoing hematochezia. Following transfusion of 2 U of packed red blood cells (PRBCs), the patient’s Hgb improved to 12.0 g/dL, and he did not experience any significant bleeding for the remainder of his hospital stay.
The following morning, the patient underwent an extensive colonoscopy (extending 25 cm into the terminal ileum), which was unable to detect any signs of arteriovenous malformations, angiodysplasia, or any other possible source of bleeding. After 24 hours with stable vital signs and Hgb levels, the patient was discharged home with close surgical and gastroenterological follow-up, with possible genetic testing for connective tissue diseases. The diagnosis at discharge was spontaneous mesenteric hemorrhage of unknown etiology.
Discussion
Acute lower GI bleeding has an estimated annual hospitalization rate of 36 patients per 100,000, or about half the rate for upper GI bleeding.1,2 The majority of patients (>80%) will have spontaneous resolution and can be worked up nonemergently.
Etiology and Work-Up
Assessment of the etiology of hematochezia begins with ruling out an upper GI source of the bleed; 10% to 15% of patients presenting with hematochezia without hematemesis are ultimately diagnosed with an upper GI etiology. 4,5
BUN-to-Cr Ratio. In a study of patients presenting with hematochezia but no hematemesis or renal failure, Srygley et al6 found a BUN-to-Cr ratio greater than 93% to be sensitive for an upper GI source, with a likelihood ratio of 7.5. The proposed etiology is some combination of absorption of digested blood products and prerenal azotemia due to hypovolemia.
Tachycardia and Orthostatic Hypotension. There have been discussions in the literature about other findings to rule in/out upper GI bleeding. While some studies have found statistically significant results between upper and lower GI bleeding for tachycardia and orthostatic hypotension (increased percentage of both in upper GI bleeding), there is disagreement about whether these findings are clinically significant.7-9
Nasogastric Lavage. Although nasogastric (NG) lavage is no longer the standard of care in the ED due to poor sensitivity and marked discomfort to the patient, most current gastroenterology guidelines still recommend its use; therefore, NG may be requested by the GI consultant.10-12
Diagnosis
Once an upper GI source has been ruled out, identification of the lesion is the next step. The differential diagnosis includes common sources such as diverticular disease, angiodysplasia, colitis, anorectal sources, and neoplasm.5 Less common, but associated with a high risk of mortality, is aortoenteric fistula (100% mortality without surgical intervention).5
Colonoscopy. Emergent colonoscopy can be used for both diagnosis and (potential) therapeutic intervention and is therefore the first option of choice.1,3,4,9 However, as seen in our case, some patients experience such profound hemorrhage that visualization of the colon may be difficult or impossible; patients may also be too unstable to await bowel preparation or undergo a procedure.
Computed Tomography Angiography. For patients in whom colonoscopy is contraindicated, CTA is the imaging modality of choice, and has a 91% to 92% sensitivity in identifying active bleeding (>0.35 mL/min).13-16
Computed tomography of the abdomen and pelvis with contrast alone, as opposed to CTA, is insufficient for detecting GI bleeding, as it is timed so that imaging is obtained when the contrast is in the portal venous capillary beds, rather than in the arteries or arterioles. By protocol, though, many institutions require abdominal and pelvic CTA to include both arterial phase and venous phase images, allowing for assessment of both active arterial bleeding and alternative lower GI sources of hematochezia (eg, mesenteric ischemia).
When ordering a CT study, an awareness of local practice is important in understanding the information that will be obtained from the study. Protocols for lower GI bleed that include CTA have reported accuracy and efficiency without worsening of renal function, despite the increased contrast load.17
Triphasic CT Enterography. Another CT modality to consider is triphasic CT enterography, which uses IV and oral contrast. In a preliminary trial, this modality achieved a specificity of 100% (sensitivity 42%) in detecting GI bleeding.18
Red Blood Cell Scintigraphy. An additional imaging modality that has been the subject of much debate in the GI literature is tagged RBC scintigraphy with Technetium-99m. Various studies have found bleeding-site confirmation in 24% to 97% of patients, and correct localization in 41% to 100% of patients. Given the extensive variability within the literature on selection criteria, localization, site confirmation, and other variables, as well as evidence from one prospective trial by Zink et al19 that found a significant disagreement between CTA and scintigraphy, RBC scintigraphy is not recommended as an alternative imaging modality for the rapid diagnosis of an acute lower GI bleed.
Conclusion
Severe hematochezia is a potential surgical emergency with a broad differential diagnosis. While emergent colonoscopy is an excellent first option, in patients with severe hematochezia, there may be too much blood in the colon to obtain adequate visual images; additionally, depending on practice setting, emergency colonoscopy may not be immediately available. In either case, CTA—a readily available, noninvasive, rapid, and repeatable diagnostic tool—should be considered as an alternate to colonoscopy, particularly in patients with brisk hematochezia.
If a patient with severe hematochezia presents to the ED, the emergency physician (EP) must recognize that the degree of hemorrhage may not correlate with the patient’s vital signs or initial laboratory values. For this reason, the EP must have a high index of suspicion, and consider CTA to allow for a rapid definitive diagnosis and prompt discussion between surgical, interventional radiology, and/or gastroenterology teams to improve clinical outcomes and decrease morbidity and mortality.20
1. Ghassemi K, Jensen D. Lower GI bleeding: epidemiology and management. Curr Gastroenterol Rep. 2013;15(7):333. doi:10.1007/s11894-013-0333-5.
2. Strate LL, Ayanian JZ, Kotler G, Syngal S. Risk factors for mortality in lower intestinal bleeding. Clin Gastroenterol Hepatol. 2008;6(9):1004-1010. doi:10.1016/j.cgh.2008.03.021.
3. Qayad E, Dagar G, Nanchal R. Lower gastrointestinal hemorrhage. Crit Care Clin. 2016;32(2):241-254. doi:10.1016/j.ccc.2015.12.004.
4. Strate LL. Lower GI bleeding: epidemiology and diagnosis. Gastroenterol Clin North Am. 2005;34(4):643-664.
5. Goralnick E, Meguerdichian D. Gastrointestinal bleeding. In: Marx J, Hockberger R, Walls R. (Eds.). Rosen’s Emergency Medicine, 8th Edition. Philadelphia, PA: Saunders, 2014;248-253.
6. Srygley FD, Gerando CJ, Tran T, Fisher DA. Does this patient have a severe upper gastrointestinal bleed? JAMA. 2012;307(10):1072-1079. doi:10.1001/jama.2012.253.
7. Whelen C, Chen C, Kaboli P, Siddique J, Prochaska M, Meltzer DO. Upper versus lower gastrointestinal bleeding: a direct comparison of clinical presentation, outcomes, and resource utilization. J Hosp Med. 2010;5(3):141-147. doi:10.1002/jhm.606.
8. Sittichanbunch Y, Senasu S, Thongkrau T, Keeratiksikorn C, Sawanyawisuth K. How to differentiate sites of gastrointestinal bleeding in patients with hematochezia by using clinical factors? Gastroenterol Res Pract. 2013;2013:265076. doi:10.1155/2013/265076.
9. Velayos F, Williamson A, Sousa KH, et al. Early predictors of severe lower gastrointestinal bleeding and adverse outcomes: a prospective study. Clin Gastroenterol Hepatol. 2004;2(6):485-490.
10. Palamidessi N, Sinert R, Falzon L, Zehtabchi S. Nasogastric aspiration and lavage in emergency department patients with hematochezia or melena without hematemesis. Acad Emerg Med. 2010;17(2):126-132. doi:10.1111/j.1553-2712.2009.00609.x.
11. Singer AJ, Richman PB, Kowalska A, Thode HC Jr. Comparison of patient and practitioner assessments of pain from commonly performed emergency department procedures. Ann Emerg Med. 1999;33(6):652-658.
12. Strate L, Gralnek I. ACG clinical guideline: management of patients with acute lower gastrointestinal bleeding. Am J Gastroenterol. 2016;111(4):459-474. doi:10.1038/ajg.2016.41.
13. Wu LM, Xu JR, Yin Y, Qu XH. Usefulness of CT angiography in diagnosing acute gastrointestinal bleeding: a meta-analysis. World J Gastroenterol. 2010;16(31):3957-3963.
14. Geffroy Y, Rodallec MH, Boulay-Coletta I, Julles MC, Ridereau-Zins C, Zins M. Multidetector CT angiography in acute gastrointestinal bleeding: why, when, and how. Radiographics. 2011;31(3):E35-E46.
15. Reis F, Cardia P, D’Ippolito G. Computed tomography angiography in patients with active gastrointestinal bleeding. Radiol Bras. 2015;48(6):381-390. doi:10.1590/0100-3984.2014.0014.
16. Chan V, Tse D, Dixon S, et al. Outcome following a negative CT angiogram for gastrointestinal hemorrhage. Cardiovasc Intervent Radiol. 2015;38(2):329-335. doi:10.1007/s00270-014-0928-8.
17. Jacovides T, Nadolski G, Allen S, et al. Arteriography for lower gastrointestinal hemorrhage: role of preceding abdominal computed tomographic angiogram in diagnosis and localization. JAMA Surgery. 2015;150(7):650-656. doi:10.1001/jamasurg.2015.97.
18. Hara AK, Walker FB, Silva AC, Leighton JA. Preliminary estimate of triphasic CT enterography performance in hemodynamically stable patients with suspected gastrointestinal bleeding. AJR Am J Roentgenol. 2009;193(5):1252-1260. doi:10.2214/AJR.08.1494.
19. Zink SI, Ohki SK, Stein B, et al. Noninvasive evaluation of active lower gastrointestinal bleeding: comparison between contrast-enhanced MDCT and 99mTc-labeled RBC scintigraphy. AJR Am J Roentgenol. 2008;91(4):1107-1114. doi:10.2214/AJR.07.3642.
20. Nable J, Graham A. Gastrointestinal bleeding. Emerg Med Clin N Am. 2016;34(2):309-325. doi:10.1016/j.emc.2015.12.001.
1. Ghassemi K, Jensen D. Lower GI bleeding: epidemiology and management. Curr Gastroenterol Rep. 2013;15(7):333. doi:10.1007/s11894-013-0333-5.
2. Strate LL, Ayanian JZ, Kotler G, Syngal S. Risk factors for mortality in lower intestinal bleeding. Clin Gastroenterol Hepatol. 2008;6(9):1004-1010. doi:10.1016/j.cgh.2008.03.021.
3. Qayad E, Dagar G, Nanchal R. Lower gastrointestinal hemorrhage. Crit Care Clin. 2016;32(2):241-254. doi:10.1016/j.ccc.2015.12.004.
4. Strate LL. Lower GI bleeding: epidemiology and diagnosis. Gastroenterol Clin North Am. 2005;34(4):643-664.
5. Goralnick E, Meguerdichian D. Gastrointestinal bleeding. In: Marx J, Hockberger R, Walls R. (Eds.). Rosen’s Emergency Medicine, 8th Edition. Philadelphia, PA: Saunders, 2014;248-253.
6. Srygley FD, Gerando CJ, Tran T, Fisher DA. Does this patient have a severe upper gastrointestinal bleed? JAMA. 2012;307(10):1072-1079. doi:10.1001/jama.2012.253.
7. Whelen C, Chen C, Kaboli P, Siddique J, Prochaska M, Meltzer DO. Upper versus lower gastrointestinal bleeding: a direct comparison of clinical presentation, outcomes, and resource utilization. J Hosp Med. 2010;5(3):141-147. doi:10.1002/jhm.606.
8. Sittichanbunch Y, Senasu S, Thongkrau T, Keeratiksikorn C, Sawanyawisuth K. How to differentiate sites of gastrointestinal bleeding in patients with hematochezia by using clinical factors? Gastroenterol Res Pract. 2013;2013:265076. doi:10.1155/2013/265076.
9. Velayos F, Williamson A, Sousa KH, et al. Early predictors of severe lower gastrointestinal bleeding and adverse outcomes: a prospective study. Clin Gastroenterol Hepatol. 2004;2(6):485-490.
10. Palamidessi N, Sinert R, Falzon L, Zehtabchi S. Nasogastric aspiration and lavage in emergency department patients with hematochezia or melena without hematemesis. Acad Emerg Med. 2010;17(2):126-132. doi:10.1111/j.1553-2712.2009.00609.x.
11. Singer AJ, Richman PB, Kowalska A, Thode HC Jr. Comparison of patient and practitioner assessments of pain from commonly performed emergency department procedures. Ann Emerg Med. 1999;33(6):652-658.
12. Strate L, Gralnek I. ACG clinical guideline: management of patients with acute lower gastrointestinal bleeding. Am J Gastroenterol. 2016;111(4):459-474. doi:10.1038/ajg.2016.41.
13. Wu LM, Xu JR, Yin Y, Qu XH. Usefulness of CT angiography in diagnosing acute gastrointestinal bleeding: a meta-analysis. World J Gastroenterol. 2010;16(31):3957-3963.
14. Geffroy Y, Rodallec MH, Boulay-Coletta I, Julles MC, Ridereau-Zins C, Zins M. Multidetector CT angiography in acute gastrointestinal bleeding: why, when, and how. Radiographics. 2011;31(3):E35-E46.
15. Reis F, Cardia P, D’Ippolito G. Computed tomography angiography in patients with active gastrointestinal bleeding. Radiol Bras. 2015;48(6):381-390. doi:10.1590/0100-3984.2014.0014.
16. Chan V, Tse D, Dixon S, et al. Outcome following a negative CT angiogram for gastrointestinal hemorrhage. Cardiovasc Intervent Radiol. 2015;38(2):329-335. doi:10.1007/s00270-014-0928-8.
17. Jacovides T, Nadolski G, Allen S, et al. Arteriography for lower gastrointestinal hemorrhage: role of preceding abdominal computed tomographic angiogram in diagnosis and localization. JAMA Surgery. 2015;150(7):650-656. doi:10.1001/jamasurg.2015.97.
18. Hara AK, Walker FB, Silva AC, Leighton JA. Preliminary estimate of triphasic CT enterography performance in hemodynamically stable patients with suspected gastrointestinal bleeding. AJR Am J Roentgenol. 2009;193(5):1252-1260. doi:10.2214/AJR.08.1494.
19. Zink SI, Ohki SK, Stein B, et al. Noninvasive evaluation of active lower gastrointestinal bleeding: comparison between contrast-enhanced MDCT and 99mTc-labeled RBC scintigraphy. AJR Am J Roentgenol. 2008;91(4):1107-1114. doi:10.2214/AJR.07.3642.
20. Nable J, Graham A. Gastrointestinal bleeding. Emerg Med Clin N Am. 2016;34(2):309-325. doi:10.1016/j.emc.2015.12.001.
Emergency Imaging: Abdominal Pain 6 Months After Cesarean Delivery
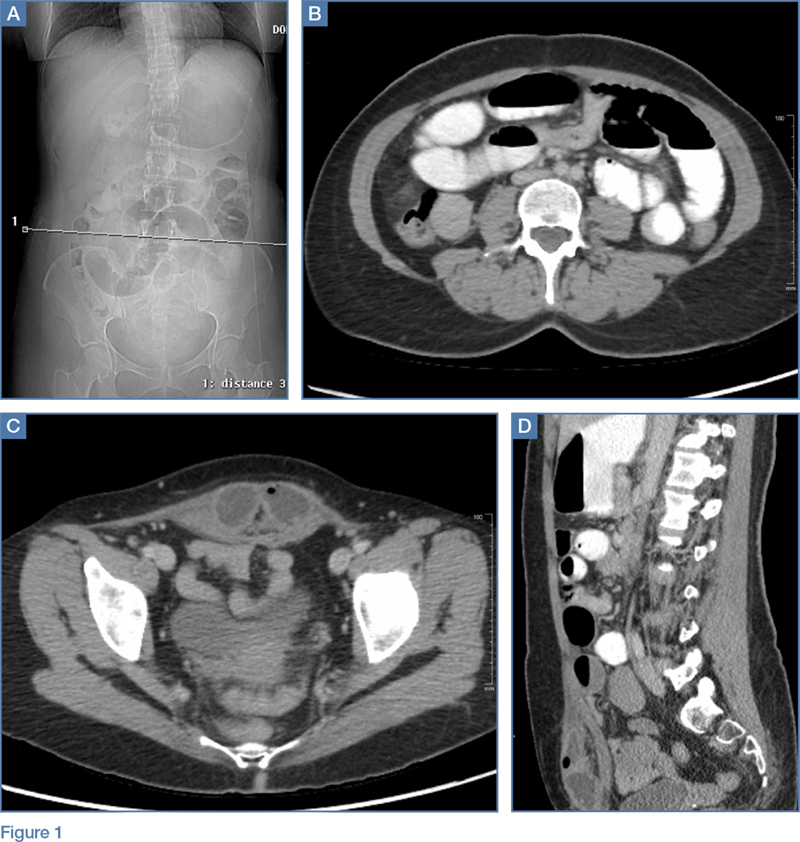
A 45-year-old woman with a history of polycystic ovary syndrome presented to the ED for evaluation of acute abdominal pain. The patient’s surgical history was significant for a cesarean delivery 6 months prior to presentation. Abdominal examination revealed a well-healed suprapubic cesarean incision scar, which was tender upon palpation. A computed tomography (CT) scan of the abdomen and pelvis with contrast were ordered; representative images are shown above (Figure 1a-1d).
What is the diagnosis? What are the associated complications and preferred management for this entity?
Answer
The scout image from the CT scan shows multiple dilated loops of small bowel (white arrows, Figure 2a) and only a small amount of air within a decompressed colon (red arrow, Figure 2a). The multiplanar CT image confirmed multiple dilated small bowel loops (white arrows, Figure 2b) and the decompressed large bowel (red arrows, Figure 2b), indicating the presence of a small bowel obstruction. A distal small bowel loop (white arrows, Figure 2c and 2d) was identified in a hernia sac within the walls of the rectus abdominis muscle (red arrows, Figure 2c and 2d). Mesenteric stranding within the hernia sac was suggestive of incarceration (black arrow, Figure 2d). No signs of intestinal ischemia, such as pneumatosis or wall thickening, were present.
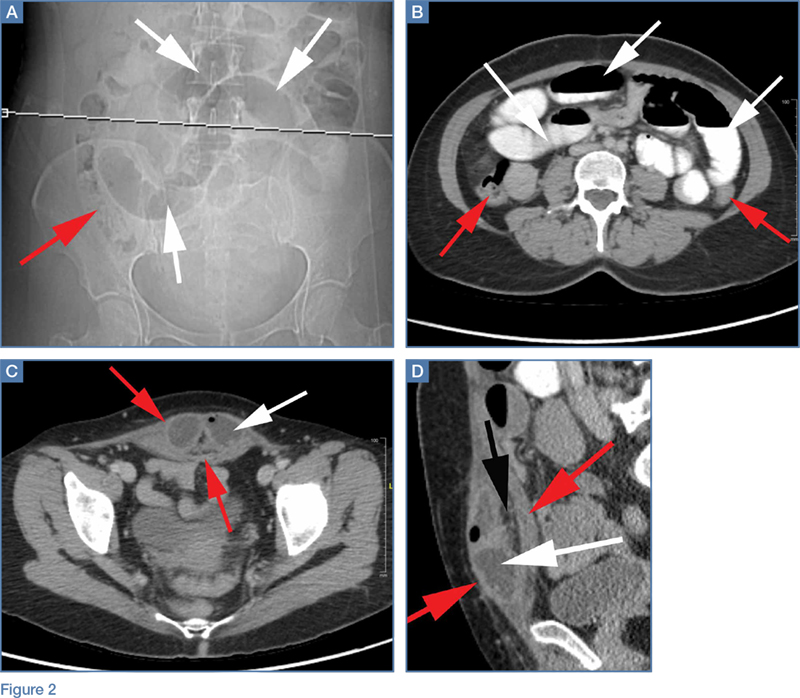
An exploratory laparotomy was emergently performed, which confirmed the presence of incarcerated small bowel within the posterior rectus sheath defect without evidence of strangulation. Reduction of small bowel and primary closure of the hernia defect was subsequently performed without complication.
Abdominal Wall Hernias
Abdominal wall hernias are common in the United States, with more than 1 million abdominal wall hernia repairs performed annually.1 A posterior rectus sheath hernia is a rare type of abdominal wall hernia; the majority are postsurgical (as seen in this patient) or posttraumatic, with only a few reported congenital cases.2
Anatomy
The rectus sheath encloses the rectus abdominis muscle and is composed of the aponeuroses of the transversus abdominis, external oblique, and internal oblique muscles. The aponeuroses form an anterior and posterior sheath, which together serve as a strong barrier against the herniation of abdominal contents, accounting for the rarity of a spontaneous rectus sheath hernia. However, inferior to the umbilicus (below the arcuate line), the posterior rectus sheath is composed primarily of transversalis fascia, which may make this region more susceptible to herniation.3 Additional predisposing factors to herniation include increased muscle weakness and elevated intra-abdominal pressure, such as that which occurs during pregnancy or from ascites.4
Clinical Presentation
Like other abdominal wall hernias, the clinical presentation of posterior rectus sheath hernias is nonspecific. Patients may be asymptomatic or may develop abdominal pain, distension, and vomiting as a result of acute complications that necessitate emergent surgery. During history-taking, inquiry into a patient’s surgical history is crucial because it may raise clinical suspicion for an abdominal wall hernia, as was the case in our patient, who recently had a cesarean delivery.
Diagnosis
Because prompt and accurate diagnosis of acute complications of abdominal wall hernias is essential, imaging studies are typically required for diagnosis. Computed tomography is the modality of choice based on its ability to provide superior anatomic detail of the abdominal wall, permitting identification of hernias and differentiating them from other abdominal masses, such as hematomas, abscesses, or tumors. Additionally, CT is able to detect early signs of hernia sac complications, including bowel obstruction, incarceration, and strangulation.5
Treatment
Treatment for a posterior rectus sheath hernia is surgical with primary closure being the preferred method. Prosthetic repair may also be performed, particularly when the hernia defect is large, but it has been shown to be associated with an increased risk of intestinal strangulation.3
1. Rutkow IM. Demographic and socioeconomic aspects of hernia repair in the United States in 2003. Surg Clin North Am. 2003;83(5):1045-1051, v-vi. doi:10.1016/S0039-6109(03)00132-4.
2. Lenobel S, Lenobel R, Yu J. Posterior rectus sheath hernia causing intermittent small bowel obstruction. J Radiol Case Rep J. 2014;8(9):25-29. doi:10.3941/jrcr.v8i9.2081.
3. Losanoff JE, Basson MD, Gruber SA. Spontaneous hernia through the posterior rectus abdominis sheath: case report and review of the published literature 1937-2008. Hernia. 2009;13(5):555-558. doi:10.1007/s10029-009-0481-6.
4. Bentzon N, Adamsen S. Hernia of the posterior rectus sheath: a new entity? Eur J Surg. 1995;161(3):215-216.
5. Aguirre DA, Santosa AC, Casola G, Sirlin CB. Abdominal wall hernias: imaging features, complications, and diagnostic pitfalls at mutli-detector row CT. Radiographics. 2005;25(6):1501-1520. doi:10.1148/rg.256055018.
A 45-year-old woman with a history of polycystic ovary syndrome presented to the ED for evaluation of acute abdominal pain. The patient’s surgical history was significant for a cesarean delivery 6 months prior to presentation. Abdominal examination revealed a well-healed suprapubic cesarean incision scar, which was tender upon palpation. A computed tomography (CT) scan of the abdomen and pelvis with contrast were ordered; representative images are shown above (Figure 1a-1d).

What is the diagnosis? What are the associated complications and preferred management for this entity?
Answer
The scout image from the CT scan shows multiple dilated loops of small bowel (white arrows, Figure 2a) and only a small amount of air within a decompressed colon (red arrow, Figure 2a). The multiplanar CT image confirmed multiple dilated small bowel loops (white arrows, Figure 2b) and the decompressed large bowel (red arrows, Figure 2b), indicating the presence of a small bowel obstruction. A distal small bowel loop (white arrows, Figure 2c and 2d) was identified in a hernia sac within the walls of the rectus abdominis muscle (red arrows, Figure 2c and 2d). Mesenteric stranding within the hernia sac was suggestive of incarceration (black arrow, Figure 2d). No signs of intestinal ischemia, such as pneumatosis or wall thickening, were present.

An exploratory laparotomy was emergently performed, which confirmed the presence of incarcerated small bowel within the posterior rectus sheath defect without evidence of strangulation. Reduction of small bowel and primary closure of the hernia defect was subsequently performed without complication.
Abdominal Wall Hernias
Abdominal wall hernias are common in the United States, with more than 1 million abdominal wall hernia repairs performed annually.1 A posterior rectus sheath hernia is a rare type of abdominal wall hernia; the majority are postsurgical (as seen in this patient) or posttraumatic, with only a few reported congenital cases.2
Anatomy
The rectus sheath encloses the rectus abdominis muscle and is composed of the aponeuroses of the transversus abdominis, external oblique, and internal oblique muscles. The aponeuroses form an anterior and posterior sheath, which together serve as a strong barrier against the herniation of abdominal contents, accounting for the rarity of a spontaneous rectus sheath hernia. However, inferior to the umbilicus (below the arcuate line), the posterior rectus sheath is composed primarily of transversalis fascia, which may make this region more susceptible to herniation.3 Additional predisposing factors to herniation include increased muscle weakness and elevated intra-abdominal pressure, such as that which occurs during pregnancy or from ascites.4
Clinical Presentation
Like other abdominal wall hernias, the clinical presentation of posterior rectus sheath hernias is nonspecific. Patients may be asymptomatic or may develop abdominal pain, distension, and vomiting as a result of acute complications that necessitate emergent surgery. During history-taking, inquiry into a patient’s surgical history is crucial because it may raise clinical suspicion for an abdominal wall hernia, as was the case in our patient, who recently had a cesarean delivery.
Diagnosis
Because prompt and accurate diagnosis of acute complications of abdominal wall hernias is essential, imaging studies are typically required for diagnosis. Computed tomography is the modality of choice based on its ability to provide superior anatomic detail of the abdominal wall, permitting identification of hernias and differentiating them from other abdominal masses, such as hematomas, abscesses, or tumors. Additionally, CT is able to detect early signs of hernia sac complications, including bowel obstruction, incarceration, and strangulation.5
Treatment
Treatment for a posterior rectus sheath hernia is surgical with primary closure being the preferred method. Prosthetic repair may also be performed, particularly when the hernia defect is large, but it has been shown to be associated with an increased risk of intestinal strangulation.3
A 45-year-old woman with a history of polycystic ovary syndrome presented to the ED for evaluation of acute abdominal pain. The patient’s surgical history was significant for a cesarean delivery 6 months prior to presentation. Abdominal examination revealed a well-healed suprapubic cesarean incision scar, which was tender upon palpation. A computed tomography (CT) scan of the abdomen and pelvis with contrast were ordered; representative images are shown above (Figure 1a-1d).

What is the diagnosis? What are the associated complications and preferred management for this entity?
Answer
The scout image from the CT scan shows multiple dilated loops of small bowel (white arrows, Figure 2a) and only a small amount of air within a decompressed colon (red arrow, Figure 2a). The multiplanar CT image confirmed multiple dilated small bowel loops (white arrows, Figure 2b) and the decompressed large bowel (red arrows, Figure 2b), indicating the presence of a small bowel obstruction. A distal small bowel loop (white arrows, Figure 2c and 2d) was identified in a hernia sac within the walls of the rectus abdominis muscle (red arrows, Figure 2c and 2d). Mesenteric stranding within the hernia sac was suggestive of incarceration (black arrow, Figure 2d). No signs of intestinal ischemia, such as pneumatosis or wall thickening, were present.

An exploratory laparotomy was emergently performed, which confirmed the presence of incarcerated small bowel within the posterior rectus sheath defect without evidence of strangulation. Reduction of small bowel and primary closure of the hernia defect was subsequently performed without complication.
Abdominal Wall Hernias
Abdominal wall hernias are common in the United States, with more than 1 million abdominal wall hernia repairs performed annually.1 A posterior rectus sheath hernia is a rare type of abdominal wall hernia; the majority are postsurgical (as seen in this patient) or posttraumatic, with only a few reported congenital cases.2
Anatomy
The rectus sheath encloses the rectus abdominis muscle and is composed of the aponeuroses of the transversus abdominis, external oblique, and internal oblique muscles. The aponeuroses form an anterior and posterior sheath, which together serve as a strong barrier against the herniation of abdominal contents, accounting for the rarity of a spontaneous rectus sheath hernia. However, inferior to the umbilicus (below the arcuate line), the posterior rectus sheath is composed primarily of transversalis fascia, which may make this region more susceptible to herniation.3 Additional predisposing factors to herniation include increased muscle weakness and elevated intra-abdominal pressure, such as that which occurs during pregnancy or from ascites.4
Clinical Presentation
Like other abdominal wall hernias, the clinical presentation of posterior rectus sheath hernias is nonspecific. Patients may be asymptomatic or may develop abdominal pain, distension, and vomiting as a result of acute complications that necessitate emergent surgery. During history-taking, inquiry into a patient’s surgical history is crucial because it may raise clinical suspicion for an abdominal wall hernia, as was the case in our patient, who recently had a cesarean delivery.
Diagnosis
Because prompt and accurate diagnosis of acute complications of abdominal wall hernias is essential, imaging studies are typically required for diagnosis. Computed tomography is the modality of choice based on its ability to provide superior anatomic detail of the abdominal wall, permitting identification of hernias and differentiating them from other abdominal masses, such as hematomas, abscesses, or tumors. Additionally, CT is able to detect early signs of hernia sac complications, including bowel obstruction, incarceration, and strangulation.5
Treatment
Treatment for a posterior rectus sheath hernia is surgical with primary closure being the preferred method. Prosthetic repair may also be performed, particularly when the hernia defect is large, but it has been shown to be associated with an increased risk of intestinal strangulation.3
1. Rutkow IM. Demographic and socioeconomic aspects of hernia repair in the United States in 2003. Surg Clin North Am. 2003;83(5):1045-1051, v-vi. doi:10.1016/S0039-6109(03)00132-4.
2. Lenobel S, Lenobel R, Yu J. Posterior rectus sheath hernia causing intermittent small bowel obstruction. J Radiol Case Rep J. 2014;8(9):25-29. doi:10.3941/jrcr.v8i9.2081.
3. Losanoff JE, Basson MD, Gruber SA. Spontaneous hernia through the posterior rectus abdominis sheath: case report and review of the published literature 1937-2008. Hernia. 2009;13(5):555-558. doi:10.1007/s10029-009-0481-6.
4. Bentzon N, Adamsen S. Hernia of the posterior rectus sheath: a new entity? Eur J Surg. 1995;161(3):215-216.
5. Aguirre DA, Santosa AC, Casola G, Sirlin CB. Abdominal wall hernias: imaging features, complications, and diagnostic pitfalls at mutli-detector row CT. Radiographics. 2005;25(6):1501-1520. doi:10.1148/rg.256055018.
1. Rutkow IM. Demographic and socioeconomic aspects of hernia repair in the United States in 2003. Surg Clin North Am. 2003;83(5):1045-1051, v-vi. doi:10.1016/S0039-6109(03)00132-4.
2. Lenobel S, Lenobel R, Yu J. Posterior rectus sheath hernia causing intermittent small bowel obstruction. J Radiol Case Rep J. 2014;8(9):25-29. doi:10.3941/jrcr.v8i9.2081.
3. Losanoff JE, Basson MD, Gruber SA. Spontaneous hernia through the posterior rectus abdominis sheath: case report and review of the published literature 1937-2008. Hernia. 2009;13(5):555-558. doi:10.1007/s10029-009-0481-6.
4. Bentzon N, Adamsen S. Hernia of the posterior rectus sheath: a new entity? Eur J Surg. 1995;161(3):215-216.
5. Aguirre DA, Santosa AC, Casola G, Sirlin CB. Abdominal wall hernias: imaging features, complications, and diagnostic pitfalls at mutli-detector row CT. Radiographics. 2005;25(6):1501-1520. doi:10.1148/rg.256055018.
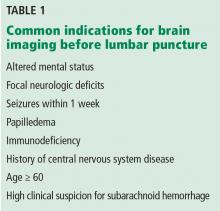
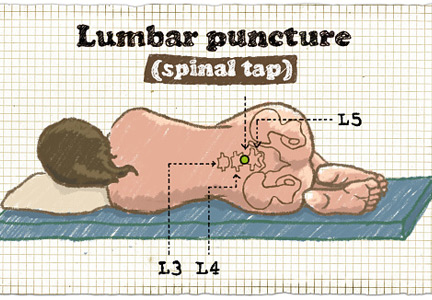
When should brain imaging precede lumbar puncture in cases of suspected bacterial meningitis?
Brain imaging should precede lumbar puncture in patients with focal neurologic deficits or immunodeficiency, or with altered mental status or seizures during the previous week. However, lumbar puncture can be safely done in most patients without first obtaining brain imaging. Empiric antibiotic and corticosteroid therapy must not be delayed; they should be started immediately after the lumber puncture is done, without waiting for the results. If the lumbar puncture is going to be delayed, these treatments should be started immediately after obtaining blood samples for culture.
A MEDICAL EMERGENCY
Bacterial meningitis is a medical emergency and requires prompt recognition and treatment. It is associated with a nearly 15% death rate as well as neurologic effects such as deafness, seizures, and cognitive decline in about the same percentage of patients.1 Microbiologic information from lumbar puncture and cerebrospinal fluid analysis is an essential part of the initial workup, whenever possible. Lumbar puncture can be done safely at the bedside in most patients and so should not be delayed unless certain contraindications exist, as discussed below.2
INDICATIONS FOR BRAIN IMAGING BEFORE LUMBAR PUNCTURE
Table 1 lists common indications for brain imaging before lumbar puncture. However, there is a lack of good evidence to support them.
Current guidelines on acute bacterial meningitis from the Infectious Diseases Society of America recommend computed tomography (CT) of the brain before lumbar puncture in patients presenting with:
- Altered mental status
- A new focal neurologic deficit (eg, cranial nerve palsy, extremity weakness or drift, dysarthria, aphasia)
- Papilledema
- Seizure within the past week
- History of central nervous system disease (eg, stroke, tumor)
- Age 60 or older (likely because of the association with previous central nervous system disease)
- Immunocompromised state (due to human immunodeficiency virus infection, chemotherapy, or immunosuppressive drugs for transplant or rheumatologic disease)
- A high clinical suspicion for subarachnoid hemorrhage.3–5
However, a normal result on head CT does not rule out the possibility of increased intracranial pressure and the risk of brain herniation. Actually, patients with acute bacterial meningitis are inherently at higher risk of spontaneous brain herniation even without lumbar puncture, and some cases of brain herniation after lumbar puncture could have represented the natural course of disease. Importantly, lumbar puncture may not be independently associated with the risk of brain herniation in patients with altered mental status (Glasgow Coma Scale score ≤ 8).6 A prospective randomized study is needed to better understand when to order brain imaging before lumbar puncture and when it is safe to proceed directly to lumbar puncture.
CONTRAINDICATIONS TO LUMBAR PUNCTURE
General contraindications to lumbar puncture are listed in Table 2.
Gopal et al3 analyzed clinical and radiographic data for 113 adults requiring urgent lumbar puncture and reported that altered mental status (likelihood ratio [LR] 2.2), focal neurologic deficit (LR 4.3), papilledema (LR 11.1), and clinical impression (LR 18.8) were associated with abnormalities on CT.
Hasbun et al4 prospectively analyzed whether clinical variables correlated with abnormal results of head CT that would preclude lumbar puncture in 301 patients requiring urgent lumbar puncture. They found that age 60 and older, immunodeficiency, a history of central nervous system disease, recent seizure (within 1 week), and neurologic deficits were associated with abnormal findings on head CT (eg, lesion with mass effect, midline shift). Importantly, absence of these characteristics had a 97% negative predictive value for abnormal findings on head CT. However, neither a normal head CT nor a normal clinical neurologic examination rules out increased intracranial pressure.4,7
CHIEF CONCERNS ABOUT LUMBAR PUNCTURE
Lumbar puncture is generally well tolerated. Major complications are rare2 and can be prevented by checking for contraindications and by using appropriate procedural hygiene and technique. Complications include pain at the puncture site, postprocedural headache, epidural hematoma, meningitis, osteomyelitis or discitis, bleeding, epidermoid tumor, and, most worrisome, brain herniation.
Brain herniation
Concern about causing brain herniation is the reason imaging may be ordered before lumbar puncture. Cerebral edema and increased intracranial pressure are common in patients with bacterial meningitis, as well as in other conditions such as bleeding, tumor, and abscess.1 If intracranial pressure is elevated, lumbar puncture can cause cerebral herniation with further neurologic compromise and possibly death. Herniation is believed to be due to a sudden decrease in pressure in the spinal cord caused by removal of cerebrospinal fluid. However, the only information we have about this complication comes from case reports and case series, so we don’t really know how often it happens.
On the other hand, ordering ancillary tests before lumbar puncture and starting empiric antibiotics in patients with suspected bacterial meningitis may delay treatment and lead to worse clinical outcomes and thus should be discouraged.8
Also important to note is the lack of good data regarding the safety of lumbar puncture in patients with potential hemostatic problems (thrombocytopenia, coagulopathy). The recommendation not to do lumbar puncture in these situations (Table 1) is taken from neuraxial anesthesia guidelines.9 Further, a small retrospective study of thrombocytopenic oncology patients requiring lumbar puncture did not demonstrate an increased risk of complications.10
ADDITIONAL CONSIDERATIONS
In a retrospective study in 2015, Glimåker et al6 demonstrated that lumbar puncture without prior brain CT was safe in patients with suspected acute bacterial meningitis with moderate to severe impairment of mental status, and that it led to a shorter “door-to-antibiotic time.” Lumbar puncture before imaging was also associated with a concomitant decrease in the risk of death, with no increase in the rate of complications.6
If brain imaging is to be done before lumbar puncture, then blood cultures (and cultures of other fluids, whenever appropriate) should be collected and the patient should be started on empiric management for central nervous system infection first. CT evidence of diffuse cerebral edema, focal lesions with mass effect, and ventriculomegaly should be viewed as further contraindications to lumbar puncture.1
Antibiotic therapy
When contraindications to lumbar puncture exist, the choice of antibiotic and the duration of therapy should be based on the patient’s history, demographics, risk factors, and microbiologic data from blood culture, urine culture, sputum culture, and detection of microbiological antigens.1 The choice of antibiotic is beyond the scope of this article. However, empiric antibiotic therapy with a third-generation cephalosporin (eg, ceftriaxone) and vancomycin and anti-inflammatory therapy (dexamethasone) should in most cases be started immediately after collecting samples for blood culture and must not be delayed by neuroimaging and lumbar puncture with cerebrospinal fluid sampling, given the high rates of mortality and morbidity if treatment is delayed.5,8
Consultation with the neurosurgery service regarding alternative brain ventricular fluid sampling should be considered.11
- Thigpen MC, Whitney CG, Messonnier NE, et al; Emerging Infections Programs Network. Bacterial meningitis in the United States, 1998–2007. N Engl J Med 2011; 364:2016–2025.
- Ellenby MS, Tegtmeyer K, Lai S, Braner DA. Videos in clinical medicine. Lumbar puncture. N Engl J Med 2006; 355: e12.
- Gopal AK, Whitehouse JD, Simel DL, Corey GR. Cranial computed tomography before lumbar puncture: a prospective clinical evaluation. Arch Intern Med 1999; 159:2681–2685.
- Hasbun R, Abrahams J, Jekel J, Quagliarello VJ. Computed tomography of the head before lumbar puncture in adults with suspected meningitis. N Engl J Med 2001; 345:1727–1733.
- Tunkel AR, Hartman BJ, Kaplan SL, et al. Practice guidelines for the management of bacterial meningitis. Clin Infect Dis 2004; 39:1267–1284.
- Glimåker M, Johansson B, Grindborg Ö, Bottai M, Lindquist L, Sjölin J. Adult bacterial meningitis: earlier treatment and improved outcome following guideline revision promoting prompt lumbar puncture. Clin Infect Dis 2015; 60:1162–1169.
- Baraff LJ, Byyny RL, Probst MA, Salamon N, Linetsky M, Mower WR. Prevalence of herniation and intracranial shift on cranial tomography in patients with subarachnoid hemorrhage and a normal neurologic examination. Acad Emerg Med 2010; 17:423–428.
- Proulx N, Fréchette D, Toye B, Chan J, Kravcik S. Delays in the administration of antibiotics are associated with mortality from adult acute bacterial meningitis. QJM 2005; 98:291–298.
- Horlocker TT, Wedel DJ, Rowlingson JC, et al. Regional anesthesia in the patient receiving antithrombotic or thrombolytic therapy: American Society of Regional Anesthesia and Pain Medicine Evidence-Based Guidelines (Third Edition). Reg Anesth Pain Med 2010; 35:64–101.
- Ning S, Kerbel B, Callum J, Lin Y. Safety of lumbar punctures in patients with thrombocytopenia. Vox Sang 2016; 110:393–400.
- Joffe AR. Lumbar puncture and brain herniation in acute bacterial meningitis: a review. J Intensive Care Med 2007; 22:194–207.
Brain imaging should precede lumbar puncture in patients with focal neurologic deficits or immunodeficiency, or with altered mental status or seizures during the previous week. However, lumbar puncture can be safely done in most patients without first obtaining brain imaging. Empiric antibiotic and corticosteroid therapy must not be delayed; they should be started immediately after the lumber puncture is done, without waiting for the results. If the lumbar puncture is going to be delayed, these treatments should be started immediately after obtaining blood samples for culture.
A MEDICAL EMERGENCY
Bacterial meningitis is a medical emergency and requires prompt recognition and treatment. It is associated with a nearly 15% death rate as well as neurologic effects such as deafness, seizures, and cognitive decline in about the same percentage of patients.1 Microbiologic information from lumbar puncture and cerebrospinal fluid analysis is an essential part of the initial workup, whenever possible. Lumbar puncture can be done safely at the bedside in most patients and so should not be delayed unless certain contraindications exist, as discussed below.2
INDICATIONS FOR BRAIN IMAGING BEFORE LUMBAR PUNCTURE
Table 1 lists common indications for brain imaging before lumbar puncture. However, there is a lack of good evidence to support them.
Current guidelines on acute bacterial meningitis from the Infectious Diseases Society of America recommend computed tomography (CT) of the brain before lumbar puncture in patients presenting with:
- Altered mental status
- A new focal neurologic deficit (eg, cranial nerve palsy, extremity weakness or drift, dysarthria, aphasia)
- Papilledema
- Seizure within the past week
- History of central nervous system disease (eg, stroke, tumor)
- Age 60 or older (likely because of the association with previous central nervous system disease)
- Immunocompromised state (due to human immunodeficiency virus infection, chemotherapy, or immunosuppressive drugs for transplant or rheumatologic disease)
- A high clinical suspicion for subarachnoid hemorrhage.3–5
However, a normal result on head CT does not rule out the possibility of increased intracranial pressure and the risk of brain herniation. Actually, patients with acute bacterial meningitis are inherently at higher risk of spontaneous brain herniation even without lumbar puncture, and some cases of brain herniation after lumbar puncture could have represented the natural course of disease. Importantly, lumbar puncture may not be independently associated with the risk of brain herniation in patients with altered mental status (Glasgow Coma Scale score ≤ 8).6 A prospective randomized study is needed to better understand when to order brain imaging before lumbar puncture and when it is safe to proceed directly to lumbar puncture.
CONTRAINDICATIONS TO LUMBAR PUNCTURE
General contraindications to lumbar puncture are listed in Table 2.
Gopal et al3 analyzed clinical and radiographic data for 113 adults requiring urgent lumbar puncture and reported that altered mental status (likelihood ratio [LR] 2.2), focal neurologic deficit (LR 4.3), papilledema (LR 11.1), and clinical impression (LR 18.8) were associated with abnormalities on CT.
Hasbun et al4 prospectively analyzed whether clinical variables correlated with abnormal results of head CT that would preclude lumbar puncture in 301 patients requiring urgent lumbar puncture. They found that age 60 and older, immunodeficiency, a history of central nervous system disease, recent seizure (within 1 week), and neurologic deficits were associated with abnormal findings on head CT (eg, lesion with mass effect, midline shift). Importantly, absence of these characteristics had a 97% negative predictive value for abnormal findings on head CT. However, neither a normal head CT nor a normal clinical neurologic examination rules out increased intracranial pressure.4,7
CHIEF CONCERNS ABOUT LUMBAR PUNCTURE
Lumbar puncture is generally well tolerated. Major complications are rare2 and can be prevented by checking for contraindications and by using appropriate procedural hygiene and technique. Complications include pain at the puncture site, postprocedural headache, epidural hematoma, meningitis, osteomyelitis or discitis, bleeding, epidermoid tumor, and, most worrisome, brain herniation.
Brain herniation
Concern about causing brain herniation is the reason imaging may be ordered before lumbar puncture. Cerebral edema and increased intracranial pressure are common in patients with bacterial meningitis, as well as in other conditions such as bleeding, tumor, and abscess.1 If intracranial pressure is elevated, lumbar puncture can cause cerebral herniation with further neurologic compromise and possibly death. Herniation is believed to be due to a sudden decrease in pressure in the spinal cord caused by removal of cerebrospinal fluid. However, the only information we have about this complication comes from case reports and case series, so we don’t really know how often it happens.
On the other hand, ordering ancillary tests before lumbar puncture and starting empiric antibiotics in patients with suspected bacterial meningitis may delay treatment and lead to worse clinical outcomes and thus should be discouraged.8
Also important to note is the lack of good data regarding the safety of lumbar puncture in patients with potential hemostatic problems (thrombocytopenia, coagulopathy). The recommendation not to do lumbar puncture in these situations (Table 1) is taken from neuraxial anesthesia guidelines.9 Further, a small retrospective study of thrombocytopenic oncology patients requiring lumbar puncture did not demonstrate an increased risk of complications.10
ADDITIONAL CONSIDERATIONS
In a retrospective study in 2015, Glimåker et al6 demonstrated that lumbar puncture without prior brain CT was safe in patients with suspected acute bacterial meningitis with moderate to severe impairment of mental status, and that it led to a shorter “door-to-antibiotic time.” Lumbar puncture before imaging was also associated with a concomitant decrease in the risk of death, with no increase in the rate of complications.6
If brain imaging is to be done before lumbar puncture, then blood cultures (and cultures of other fluids, whenever appropriate) should be collected and the patient should be started on empiric management for central nervous system infection first. CT evidence of diffuse cerebral edema, focal lesions with mass effect, and ventriculomegaly should be viewed as further contraindications to lumbar puncture.1
Antibiotic therapy
When contraindications to lumbar puncture exist, the choice of antibiotic and the duration of therapy should be based on the patient’s history, demographics, risk factors, and microbiologic data from blood culture, urine culture, sputum culture, and detection of microbiological antigens.1 The choice of antibiotic is beyond the scope of this article. However, empiric antibiotic therapy with a third-generation cephalosporin (eg, ceftriaxone) and vancomycin and anti-inflammatory therapy (dexamethasone) should in most cases be started immediately after collecting samples for blood culture and must not be delayed by neuroimaging and lumbar puncture with cerebrospinal fluid sampling, given the high rates of mortality and morbidity if treatment is delayed.5,8
Consultation with the neurosurgery service regarding alternative brain ventricular fluid sampling should be considered.11
Brain imaging should precede lumbar puncture in patients with focal neurologic deficits or immunodeficiency, or with altered mental status or seizures during the previous week. However, lumbar puncture can be safely done in most patients without first obtaining brain imaging. Empiric antibiotic and corticosteroid therapy must not be delayed; they should be started immediately after the lumber puncture is done, without waiting for the results. If the lumbar puncture is going to be delayed, these treatments should be started immediately after obtaining blood samples for culture.
A MEDICAL EMERGENCY
Bacterial meningitis is a medical emergency and requires prompt recognition and treatment. It is associated with a nearly 15% death rate as well as neurologic effects such as deafness, seizures, and cognitive decline in about the same percentage of patients.1 Microbiologic information from lumbar puncture and cerebrospinal fluid analysis is an essential part of the initial workup, whenever possible. Lumbar puncture can be done safely at the bedside in most patients and so should not be delayed unless certain contraindications exist, as discussed below.2
INDICATIONS FOR BRAIN IMAGING BEFORE LUMBAR PUNCTURE
Table 1 lists common indications for brain imaging before lumbar puncture. However, there is a lack of good evidence to support them.
Current guidelines on acute bacterial meningitis from the Infectious Diseases Society of America recommend computed tomography (CT) of the brain before lumbar puncture in patients presenting with:
- Altered mental status
- A new focal neurologic deficit (eg, cranial nerve palsy, extremity weakness or drift, dysarthria, aphasia)
- Papilledema
- Seizure within the past week
- History of central nervous system disease (eg, stroke, tumor)
- Age 60 or older (likely because of the association with previous central nervous system disease)
- Immunocompromised state (due to human immunodeficiency virus infection, chemotherapy, or immunosuppressive drugs for transplant or rheumatologic disease)
- A high clinical suspicion for subarachnoid hemorrhage.3–5
However, a normal result on head CT does not rule out the possibility of increased intracranial pressure and the risk of brain herniation. Actually, patients with acute bacterial meningitis are inherently at higher risk of spontaneous brain herniation even without lumbar puncture, and some cases of brain herniation after lumbar puncture could have represented the natural course of disease. Importantly, lumbar puncture may not be independently associated with the risk of brain herniation in patients with altered mental status (Glasgow Coma Scale score ≤ 8).6 A prospective randomized study is needed to better understand when to order brain imaging before lumbar puncture and when it is safe to proceed directly to lumbar puncture.
CONTRAINDICATIONS TO LUMBAR PUNCTURE
General contraindications to lumbar puncture are listed in Table 2.
Gopal et al3 analyzed clinical and radiographic data for 113 adults requiring urgent lumbar puncture and reported that altered mental status (likelihood ratio [LR] 2.2), focal neurologic deficit (LR 4.3), papilledema (LR 11.1), and clinical impression (LR 18.8) were associated with abnormalities on CT.
Hasbun et al4 prospectively analyzed whether clinical variables correlated with abnormal results of head CT that would preclude lumbar puncture in 301 patients requiring urgent lumbar puncture. They found that age 60 and older, immunodeficiency, a history of central nervous system disease, recent seizure (within 1 week), and neurologic deficits were associated with abnormal findings on head CT (eg, lesion with mass effect, midline shift). Importantly, absence of these characteristics had a 97% negative predictive value for abnormal findings on head CT. However, neither a normal head CT nor a normal clinical neurologic examination rules out increased intracranial pressure.4,7
CHIEF CONCERNS ABOUT LUMBAR PUNCTURE
Lumbar puncture is generally well tolerated. Major complications are rare2 and can be prevented by checking for contraindications and by using appropriate procedural hygiene and technique. Complications include pain at the puncture site, postprocedural headache, epidural hematoma, meningitis, osteomyelitis or discitis, bleeding, epidermoid tumor, and, most worrisome, brain herniation.
Brain herniation
Concern about causing brain herniation is the reason imaging may be ordered before lumbar puncture. Cerebral edema and increased intracranial pressure are common in patients with bacterial meningitis, as well as in other conditions such as bleeding, tumor, and abscess.1 If intracranial pressure is elevated, lumbar puncture can cause cerebral herniation with further neurologic compromise and possibly death. Herniation is believed to be due to a sudden decrease in pressure in the spinal cord caused by removal of cerebrospinal fluid. However, the only information we have about this complication comes from case reports and case series, so we don’t really know how often it happens.
On the other hand, ordering ancillary tests before lumbar puncture and starting empiric antibiotics in patients with suspected bacterial meningitis may delay treatment and lead to worse clinical outcomes and thus should be discouraged.8
Also important to note is the lack of good data regarding the safety of lumbar puncture in patients with potential hemostatic problems (thrombocytopenia, coagulopathy). The recommendation not to do lumbar puncture in these situations (Table 1) is taken from neuraxial anesthesia guidelines.9 Further, a small retrospective study of thrombocytopenic oncology patients requiring lumbar puncture did not demonstrate an increased risk of complications.10
ADDITIONAL CONSIDERATIONS
In a retrospective study in 2015, Glimåker et al6 demonstrated that lumbar puncture without prior brain CT was safe in patients with suspected acute bacterial meningitis with moderate to severe impairment of mental status, and that it led to a shorter “door-to-antibiotic time.” Lumbar puncture before imaging was also associated with a concomitant decrease in the risk of death, with no increase in the rate of complications.6
If brain imaging is to be done before lumbar puncture, then blood cultures (and cultures of other fluids, whenever appropriate) should be collected and the patient should be started on empiric management for central nervous system infection first. CT evidence of diffuse cerebral edema, focal lesions with mass effect, and ventriculomegaly should be viewed as further contraindications to lumbar puncture.1
Antibiotic therapy
When contraindications to lumbar puncture exist, the choice of antibiotic and the duration of therapy should be based on the patient’s history, demographics, risk factors, and microbiologic data from blood culture, urine culture, sputum culture, and detection of microbiological antigens.1 The choice of antibiotic is beyond the scope of this article. However, empiric antibiotic therapy with a third-generation cephalosporin (eg, ceftriaxone) and vancomycin and anti-inflammatory therapy (dexamethasone) should in most cases be started immediately after collecting samples for blood culture and must not be delayed by neuroimaging and lumbar puncture with cerebrospinal fluid sampling, given the high rates of mortality and morbidity if treatment is delayed.5,8
Consultation with the neurosurgery service regarding alternative brain ventricular fluid sampling should be considered.11
- Thigpen MC, Whitney CG, Messonnier NE, et al; Emerging Infections Programs Network. Bacterial meningitis in the United States, 1998–2007. N Engl J Med 2011; 364:2016–2025.
- Ellenby MS, Tegtmeyer K, Lai S, Braner DA. Videos in clinical medicine. Lumbar puncture. N Engl J Med 2006; 355: e12.
- Gopal AK, Whitehouse JD, Simel DL, Corey GR. Cranial computed tomography before lumbar puncture: a prospective clinical evaluation. Arch Intern Med 1999; 159:2681–2685.
- Hasbun R, Abrahams J, Jekel J, Quagliarello VJ. Computed tomography of the head before lumbar puncture in adults with suspected meningitis. N Engl J Med 2001; 345:1727–1733.
- Tunkel AR, Hartman BJ, Kaplan SL, et al. Practice guidelines for the management of bacterial meningitis. Clin Infect Dis 2004; 39:1267–1284.
- Glimåker M, Johansson B, Grindborg Ö, Bottai M, Lindquist L, Sjölin J. Adult bacterial meningitis: earlier treatment and improved outcome following guideline revision promoting prompt lumbar puncture. Clin Infect Dis 2015; 60:1162–1169.
- Baraff LJ, Byyny RL, Probst MA, Salamon N, Linetsky M, Mower WR. Prevalence of herniation and intracranial shift on cranial tomography in patients with subarachnoid hemorrhage and a normal neurologic examination. Acad Emerg Med 2010; 17:423–428.
- Proulx N, Fréchette D, Toye B, Chan J, Kravcik S. Delays in the administration of antibiotics are associated with mortality from adult acute bacterial meningitis. QJM 2005; 98:291–298.
- Horlocker TT, Wedel DJ, Rowlingson JC, et al. Regional anesthesia in the patient receiving antithrombotic or thrombolytic therapy: American Society of Regional Anesthesia and Pain Medicine Evidence-Based Guidelines (Third Edition). Reg Anesth Pain Med 2010; 35:64–101.
- Ning S, Kerbel B, Callum J, Lin Y. Safety of lumbar punctures in patients with thrombocytopenia. Vox Sang 2016; 110:393–400.
- Joffe AR. Lumbar puncture and brain herniation in acute bacterial meningitis: a review. J Intensive Care Med 2007; 22:194–207.
- Thigpen MC, Whitney CG, Messonnier NE, et al; Emerging Infections Programs Network. Bacterial meningitis in the United States, 1998–2007. N Engl J Med 2011; 364:2016–2025.
- Ellenby MS, Tegtmeyer K, Lai S, Braner DA. Videos in clinical medicine. Lumbar puncture. N Engl J Med 2006; 355: e12.
- Gopal AK, Whitehouse JD, Simel DL, Corey GR. Cranial computed tomography before lumbar puncture: a prospective clinical evaluation. Arch Intern Med 1999; 159:2681–2685.
- Hasbun R, Abrahams J, Jekel J, Quagliarello VJ. Computed tomography of the head before lumbar puncture in adults with suspected meningitis. N Engl J Med 2001; 345:1727–1733.
- Tunkel AR, Hartman BJ, Kaplan SL, et al. Practice guidelines for the management of bacterial meningitis. Clin Infect Dis 2004; 39:1267–1284.
- Glimåker M, Johansson B, Grindborg Ö, Bottai M, Lindquist L, Sjölin J. Adult bacterial meningitis: earlier treatment and improved outcome following guideline revision promoting prompt lumbar puncture. Clin Infect Dis 2015; 60:1162–1169.
- Baraff LJ, Byyny RL, Probst MA, Salamon N, Linetsky M, Mower WR. Prevalence of herniation and intracranial shift on cranial tomography in patients with subarachnoid hemorrhage and a normal neurologic examination. Acad Emerg Med 2010; 17:423–428.
- Proulx N, Fréchette D, Toye B, Chan J, Kravcik S. Delays in the administration of antibiotics are associated with mortality from adult acute bacterial meningitis. QJM 2005; 98:291–298.
- Horlocker TT, Wedel DJ, Rowlingson JC, et al. Regional anesthesia in the patient receiving antithrombotic or thrombolytic therapy: American Society of Regional Anesthesia and Pain Medicine Evidence-Based Guidelines (Third Edition). Reg Anesth Pain Med 2010; 35:64–101.
- Ning S, Kerbel B, Callum J, Lin Y. Safety of lumbar punctures in patients with thrombocytopenia. Vox Sang 2016; 110:393–400.
- Joffe AR. Lumbar puncture and brain herniation in acute bacterial meningitis: a review. J Intensive Care Med 2007; 22:194–207.
Ring-enhancing cerebral lesions
A 39-year-old woman with a history of human immunodeficiency virus (HIV) and hepatitis B virus infection was brought to the emergency department for evaluation of seizures, which had started a few days earlier. She was born and raised in a state bordering the Ohio River, an area where Histoplasma capsulatum is endemic. She denied any recent travel.
Her vital signs and neurologic examination were normal. Computed tomography of the head showed two areas of increased attenuation anterior to the frontal horns. To better characterize those lesions, magnetic resonance imaging (MRI) with contrast was done, which showed about a dozen 1-cm ring-enhancing lesions in the right cerebellum and both cerebral hemispheres (Figure 1).
Results of a complete blood cell count, metabolic profile, and chest radiography were normal. Her CD4 count was 428/μL (reference range 533–1,674) and 20% (60%–89%); her HIV viral load was 326,000 copies/mL.
She was initially treated empirically with sulfadiazine, pyrimethamine, and leukovorin for possible toxoplasmosis, which is the most common cause of ring-enhancing brain lesions in HIV patients. In the meantime, cerebrospinal fluid, blood, and urine were sent for a detailed workup for fungi, including Histoplasma. Results of the Histoplasma antibody and antigen studies of the serum, urine, and cerebrospinal fluid were positive, while cerebrospinal fluid testing for Toxoplasma by polymerase chain reaction testing was negative. Empirical treatment for toxoplasmosis was stopped and amphotericin B was started to treat disseminated histoplasmosis.
During her hospital course, she underwent brain biopsy via right frontotemporal craniotomy with resection of right frontal lesions. Pathologic study showed partially organizing abscesses with central necrosis (Figure 2), microscopy with Grocott-Gomori methenamine silver stain was positive for budding yeast forms consistent with H capsulatum (Figure 3), and special stain for acid-fast bacilli was negative for mycobacteria. Cultures of the brain biopsy specimen, blood, and cerebrospinal fluid for fungi, acid-fast bacilli, and bacteria did not reveal any growth after 28 days.
The patient was discharged home with instructions to take amphotericin B for a total of 6 weeks and then itraconazole. About 1 year later, she remained free of symptoms, although repeat MRI did not show any significant change in the size or number of histoplasmomas.
She did not comply well with her HIV treatment, and her immune status did not improve, so we decided to continue her itraconazole treatment for more than 1 year.
CEREBRAL HISTOPLASMOMA
The term “histoplasmoma” was introduced by Shapiro et al1 in 1955, when they first described numerous focal areas of softening, up to 1 cm in diameter, scattered throughout the brain at autopsy in a 41-year-old man who had died of disseminated histoplasmosis. They coined the word to describe these discrete areas of necrosis that might resemble tumors on the basis of their size, location, and capability of causing increased intracranial pressure.
Central nervous system involvement can either be a manifestation of disseminated disease or present as an isolated illness.2 It occurs in 5% to 10% of cases of disseminated histoplasmosis.3 Histoplasmosis of the central nervous system can have different manifestations; the most common presentation is chronic meningitis.4
Laboratory diagnosis is based on detecting H capsulatum antigen and antibody in the urine, blood, and cerebrospinal fluid. Tissue biopsy (histopathology) as well as cultures of tissue samples or body fluids may also establish the diagnosis.4
Toxoplasmosis and primary central nervous system lymphoma are the most common causes of brain ring-enhancing lesions in HIV patients in developed countries, while in the developing world neurocysticercosis and tuberculomas are more common.5,6 Much less common causes include brain abscesses secondary to bacterial infections (pyogenic abscess),7 cryptococcomas,8 syphilitic cerebral gummata,9 primary brain tumors (gliomas), and metastases.10
Compared with other forms of the disease, histoplasmosis of the central nervous system has higher rates of treatment failure and relapse, so treatment should be prolonged and aggressive.2,3 The cure rate with amphotericin B ranges from 33% to 61%, and higher doses produce better response rates.3
Current treatment recommendations are based on 2007 guidelines of the Infectious Diseases Society of America.11 Liposomal amphotericin B is the drug of choice because it achieves higher concentrations in the central nervous system than other drugs and is less toxic. It is given for 4 to 6 weeks, followed by itraconazole for at least 1 year and until the cerebrospinal fluid Histoplasma antigen test is negative and other cerebrospinal fluid abnormalities are resolved.
In patients who have primary disseminated histoplasmosis that includes the central nervous system, itraconazole can be given for more than 1 year or until immune recovery is achieved—or lifelong if necessary.2,12 Long-term suppressive antifungal therapy also should be considered in patients for whom appropriate initial therapy fails.2
Nephrotoxicity (acute kidney injury, hypokalemia, and hypomagnesemia), infusion-related drug reactions, and rash are among the well-described side effects of amphotericin B. Maintenance of intravascular volume and replacement of electrolytes should be an integral part of the amphotericin B treatment regimen.13
TAKE-AWAY POINTS
- Histoplasmomas should be considered in the differential diagnosis of ring-enhancing lesions of the central nervous system, along with toxoplasmosis and primary central nervous system lymphoma. This will allow timely initiation of the diagnostic workup, avoiding unnecessary and potentially risky interventions and delays in starting targeted antifungal therapy.
- There is no single gold standard test for central nervous system histoplasmosis. Rather, the final diagnosis is based on the combination of clinical, laboratory, and radiologic findings.
Acknowledgment: Library research assistance provided by HSHS St. John’s Hospital Health Sciences Library staff.
- Shapiro JL, Lux JJ, Sprofkin BE. Histoplasmosis of the central nervous system. Am J Pathol 1955; 31:319–335.
- Wheat LJ, Musial CE, Jenny-Avital E. Diagnosis and management of central nervous system histoplasmosis. Clin Infect Dis 2005; 40:844–852.
- Wheat LJ, Batteiger BE, Sathapatayavongs B. Histoplasma capsulatum infections of the central nervous system: a clinical review. Medicine (Baltimore) 1990; 69:244–260.
- Kauffman CA. Histoplasmosis: a clinical and laboratory update. Clin Microbiol Rev 2007; 20:115–132.
- Modi M, Mochan A, Modi G. Management of HIV-associated focal brain lesions in developing countries. QJM 2004; 97:413–421.
- Miller RF, Hall-Craggs MA, Costa DC, et al. Magnetic resonance imaging, thallium-201 SPET scanning, and laboratory analyses for discrimination of cerebral lymphoma and toxoplasmosis in AIDS. Sex Transm Infect 1998; 74:258–264.
- Cohen WA. Intracranial bacterial infections in patients with AIDS. Neuroimaging Clin N Am 1997; 7:223–229.
- Troncoso A, Fumagalli J, Shinzato R, Gulotta H, Toller M, Bava J. CNS cryptococcoma in an HIV-positive patient. J Int Assoc Physicians AIDS Care (Chic) 2002; 1:131–133.
- Land AM, Nelson GA, Bell SG, Denby KJ, Estrada CA, Willett LL. Widening the differential for brain masses in human immunodeficiency virus-positive patients: syphilitic cerebral gummata. Am J Med Sci 2013; 346:253–255.
- Balsys R, Janousek JE, Batnitzky S, Templeton AW. Peripheral enhancement in computerized cranial tomography: a non-specific finding. Surg Neurol 1979; 11:207–216.
- Wheat LJ, Freifeld AG, Kleiman MB, et al; Infectious Diseases Society of America. Clinical practice guidelines for the management of patients with histoplasmosis: 2007 update by the Infectious Diseases Society of America. Clin Infect Dis 2007; 45:807–825.
- Wheat J, Hafner R, Wulfsohn M, et al; National Institute of Allergy and Infectious Diseases Clinical Trials and Mycoses Study Group Collaborators. Prevention of relapse of histoplasmosis with itraconazole in patients with the acquired immunodeficiency syndrome. Ann Intern Med 1993; 118:610–616.
- Saccente M. Central nervous system histoplasmosis. Curr Treat Options Neurol 2008; 10:161–167.
A 39-year-old woman with a history of human immunodeficiency virus (HIV) and hepatitis B virus infection was brought to the emergency department for evaluation of seizures, which had started a few days earlier. She was born and raised in a state bordering the Ohio River, an area where Histoplasma capsulatum is endemic. She denied any recent travel.
Her vital signs and neurologic examination were normal. Computed tomography of the head showed two areas of increased attenuation anterior to the frontal horns. To better characterize those lesions, magnetic resonance imaging (MRI) with contrast was done, which showed about a dozen 1-cm ring-enhancing lesions in the right cerebellum and both cerebral hemispheres (Figure 1).
Results of a complete blood cell count, metabolic profile, and chest radiography were normal. Her CD4 count was 428/μL (reference range 533–1,674) and 20% (60%–89%); her HIV viral load was 326,000 copies/mL.
She was initially treated empirically with sulfadiazine, pyrimethamine, and leukovorin for possible toxoplasmosis, which is the most common cause of ring-enhancing brain lesions in HIV patients. In the meantime, cerebrospinal fluid, blood, and urine were sent for a detailed workup for fungi, including Histoplasma. Results of the Histoplasma antibody and antigen studies of the serum, urine, and cerebrospinal fluid were positive, while cerebrospinal fluid testing for Toxoplasma by polymerase chain reaction testing was negative. Empirical treatment for toxoplasmosis was stopped and amphotericin B was started to treat disseminated histoplasmosis.
During her hospital course, she underwent brain biopsy via right frontotemporal craniotomy with resection of right frontal lesions. Pathologic study showed partially organizing abscesses with central necrosis (Figure 2), microscopy with Grocott-Gomori methenamine silver stain was positive for budding yeast forms consistent with H capsulatum (Figure 3), and special stain for acid-fast bacilli was negative for mycobacteria. Cultures of the brain biopsy specimen, blood, and cerebrospinal fluid for fungi, acid-fast bacilli, and bacteria did not reveal any growth after 28 days.
The patient was discharged home with instructions to take amphotericin B for a total of 6 weeks and then itraconazole. About 1 year later, she remained free of symptoms, although repeat MRI did not show any significant change in the size or number of histoplasmomas.
She did not comply well with her HIV treatment, and her immune status did not improve, so we decided to continue her itraconazole treatment for more than 1 year.
CEREBRAL HISTOPLASMOMA
The term “histoplasmoma” was introduced by Shapiro et al1 in 1955, when they first described numerous focal areas of softening, up to 1 cm in diameter, scattered throughout the brain at autopsy in a 41-year-old man who had died of disseminated histoplasmosis. They coined the word to describe these discrete areas of necrosis that might resemble tumors on the basis of their size, location, and capability of causing increased intracranial pressure.
Central nervous system involvement can either be a manifestation of disseminated disease or present as an isolated illness.2 It occurs in 5% to 10% of cases of disseminated histoplasmosis.3 Histoplasmosis of the central nervous system can have different manifestations; the most common presentation is chronic meningitis.4
Laboratory diagnosis is based on detecting H capsulatum antigen and antibody in the urine, blood, and cerebrospinal fluid. Tissue biopsy (histopathology) as well as cultures of tissue samples or body fluids may also establish the diagnosis.4
Toxoplasmosis and primary central nervous system lymphoma are the most common causes of brain ring-enhancing lesions in HIV patients in developed countries, while in the developing world neurocysticercosis and tuberculomas are more common.5,6 Much less common causes include brain abscesses secondary to bacterial infections (pyogenic abscess),7 cryptococcomas,8 syphilitic cerebral gummata,9 primary brain tumors (gliomas), and metastases.10
Compared with other forms of the disease, histoplasmosis of the central nervous system has higher rates of treatment failure and relapse, so treatment should be prolonged and aggressive.2,3 The cure rate with amphotericin B ranges from 33% to 61%, and higher doses produce better response rates.3
Current treatment recommendations are based on 2007 guidelines of the Infectious Diseases Society of America.11 Liposomal amphotericin B is the drug of choice because it achieves higher concentrations in the central nervous system than other drugs and is less toxic. It is given for 4 to 6 weeks, followed by itraconazole for at least 1 year and until the cerebrospinal fluid Histoplasma antigen test is negative and other cerebrospinal fluid abnormalities are resolved.
In patients who have primary disseminated histoplasmosis that includes the central nervous system, itraconazole can be given for more than 1 year or until immune recovery is achieved—or lifelong if necessary.2,12 Long-term suppressive antifungal therapy also should be considered in patients for whom appropriate initial therapy fails.2
Nephrotoxicity (acute kidney injury, hypokalemia, and hypomagnesemia), infusion-related drug reactions, and rash are among the well-described side effects of amphotericin B. Maintenance of intravascular volume and replacement of electrolytes should be an integral part of the amphotericin B treatment regimen.13
TAKE-AWAY POINTS
- Histoplasmomas should be considered in the differential diagnosis of ring-enhancing lesions of the central nervous system, along with toxoplasmosis and primary central nervous system lymphoma. This will allow timely initiation of the diagnostic workup, avoiding unnecessary and potentially risky interventions and delays in starting targeted antifungal therapy.
- There is no single gold standard test for central nervous system histoplasmosis. Rather, the final diagnosis is based on the combination of clinical, laboratory, and radiologic findings.
Acknowledgment: Library research assistance provided by HSHS St. John’s Hospital Health Sciences Library staff.
A 39-year-old woman with a history of human immunodeficiency virus (HIV) and hepatitis B virus infection was brought to the emergency department for evaluation of seizures, which had started a few days earlier. She was born and raised in a state bordering the Ohio River, an area where Histoplasma capsulatum is endemic. She denied any recent travel.
Her vital signs and neurologic examination were normal. Computed tomography of the head showed two areas of increased attenuation anterior to the frontal horns. To better characterize those lesions, magnetic resonance imaging (MRI) with contrast was done, which showed about a dozen 1-cm ring-enhancing lesions in the right cerebellum and both cerebral hemispheres (Figure 1).
Results of a complete blood cell count, metabolic profile, and chest radiography were normal. Her CD4 count was 428/μL (reference range 533–1,674) and 20% (60%–89%); her HIV viral load was 326,000 copies/mL.
She was initially treated empirically with sulfadiazine, pyrimethamine, and leukovorin for possible toxoplasmosis, which is the most common cause of ring-enhancing brain lesions in HIV patients. In the meantime, cerebrospinal fluid, blood, and urine were sent for a detailed workup for fungi, including Histoplasma. Results of the Histoplasma antibody and antigen studies of the serum, urine, and cerebrospinal fluid were positive, while cerebrospinal fluid testing for Toxoplasma by polymerase chain reaction testing was negative. Empirical treatment for toxoplasmosis was stopped and amphotericin B was started to treat disseminated histoplasmosis.
During her hospital course, she underwent brain biopsy via right frontotemporal craniotomy with resection of right frontal lesions. Pathologic study showed partially organizing abscesses with central necrosis (Figure 2), microscopy with Grocott-Gomori methenamine silver stain was positive for budding yeast forms consistent with H capsulatum (Figure 3), and special stain for acid-fast bacilli was negative for mycobacteria. Cultures of the brain biopsy specimen, blood, and cerebrospinal fluid for fungi, acid-fast bacilli, and bacteria did not reveal any growth after 28 days.
The patient was discharged home with instructions to take amphotericin B for a total of 6 weeks and then itraconazole. About 1 year later, she remained free of symptoms, although repeat MRI did not show any significant change in the size or number of histoplasmomas.
She did not comply well with her HIV treatment, and her immune status did not improve, so we decided to continue her itraconazole treatment for more than 1 year.
CEREBRAL HISTOPLASMOMA
The term “histoplasmoma” was introduced by Shapiro et al1 in 1955, when they first described numerous focal areas of softening, up to 1 cm in diameter, scattered throughout the brain at autopsy in a 41-year-old man who had died of disseminated histoplasmosis. They coined the word to describe these discrete areas of necrosis that might resemble tumors on the basis of their size, location, and capability of causing increased intracranial pressure.
Central nervous system involvement can either be a manifestation of disseminated disease or present as an isolated illness.2 It occurs in 5% to 10% of cases of disseminated histoplasmosis.3 Histoplasmosis of the central nervous system can have different manifestations; the most common presentation is chronic meningitis.4
Laboratory diagnosis is based on detecting H capsulatum antigen and antibody in the urine, blood, and cerebrospinal fluid. Tissue biopsy (histopathology) as well as cultures of tissue samples or body fluids may also establish the diagnosis.4
Toxoplasmosis and primary central nervous system lymphoma are the most common causes of brain ring-enhancing lesions in HIV patients in developed countries, while in the developing world neurocysticercosis and tuberculomas are more common.5,6 Much less common causes include brain abscesses secondary to bacterial infections (pyogenic abscess),7 cryptococcomas,8 syphilitic cerebral gummata,9 primary brain tumors (gliomas), and metastases.10
Compared with other forms of the disease, histoplasmosis of the central nervous system has higher rates of treatment failure and relapse, so treatment should be prolonged and aggressive.2,3 The cure rate with amphotericin B ranges from 33% to 61%, and higher doses produce better response rates.3
Current treatment recommendations are based on 2007 guidelines of the Infectious Diseases Society of America.11 Liposomal amphotericin B is the drug of choice because it achieves higher concentrations in the central nervous system than other drugs and is less toxic. It is given for 4 to 6 weeks, followed by itraconazole for at least 1 year and until the cerebrospinal fluid Histoplasma antigen test is negative and other cerebrospinal fluid abnormalities are resolved.
In patients who have primary disseminated histoplasmosis that includes the central nervous system, itraconazole can be given for more than 1 year or until immune recovery is achieved—or lifelong if necessary.2,12 Long-term suppressive antifungal therapy also should be considered in patients for whom appropriate initial therapy fails.2
Nephrotoxicity (acute kidney injury, hypokalemia, and hypomagnesemia), infusion-related drug reactions, and rash are among the well-described side effects of amphotericin B. Maintenance of intravascular volume and replacement of electrolytes should be an integral part of the amphotericin B treatment regimen.13
TAKE-AWAY POINTS
- Histoplasmomas should be considered in the differential diagnosis of ring-enhancing lesions of the central nervous system, along with toxoplasmosis and primary central nervous system lymphoma. This will allow timely initiation of the diagnostic workup, avoiding unnecessary and potentially risky interventions and delays in starting targeted antifungal therapy.
- There is no single gold standard test for central nervous system histoplasmosis. Rather, the final diagnosis is based on the combination of clinical, laboratory, and radiologic findings.
Acknowledgment: Library research assistance provided by HSHS St. John’s Hospital Health Sciences Library staff.
- Shapiro JL, Lux JJ, Sprofkin BE. Histoplasmosis of the central nervous system. Am J Pathol 1955; 31:319–335.
- Wheat LJ, Musial CE, Jenny-Avital E. Diagnosis and management of central nervous system histoplasmosis. Clin Infect Dis 2005; 40:844–852.
- Wheat LJ, Batteiger BE, Sathapatayavongs B. Histoplasma capsulatum infections of the central nervous system: a clinical review. Medicine (Baltimore) 1990; 69:244–260.
- Kauffman CA. Histoplasmosis: a clinical and laboratory update. Clin Microbiol Rev 2007; 20:115–132.
- Modi M, Mochan A, Modi G. Management of HIV-associated focal brain lesions in developing countries. QJM 2004; 97:413–421.
- Miller RF, Hall-Craggs MA, Costa DC, et al. Magnetic resonance imaging, thallium-201 SPET scanning, and laboratory analyses for discrimination of cerebral lymphoma and toxoplasmosis in AIDS. Sex Transm Infect 1998; 74:258–264.
- Cohen WA. Intracranial bacterial infections in patients with AIDS. Neuroimaging Clin N Am 1997; 7:223–229.
- Troncoso A, Fumagalli J, Shinzato R, Gulotta H, Toller M, Bava J. CNS cryptococcoma in an HIV-positive patient. J Int Assoc Physicians AIDS Care (Chic) 2002; 1:131–133.
- Land AM, Nelson GA, Bell SG, Denby KJ, Estrada CA, Willett LL. Widening the differential for brain masses in human immunodeficiency virus-positive patients: syphilitic cerebral gummata. Am J Med Sci 2013; 346:253–255.
- Balsys R, Janousek JE, Batnitzky S, Templeton AW. Peripheral enhancement in computerized cranial tomography: a non-specific finding. Surg Neurol 1979; 11:207–216.
- Wheat LJ, Freifeld AG, Kleiman MB, et al; Infectious Diseases Society of America. Clinical practice guidelines for the management of patients with histoplasmosis: 2007 update by the Infectious Diseases Society of America. Clin Infect Dis 2007; 45:807–825.
- Wheat J, Hafner R, Wulfsohn M, et al; National Institute of Allergy and Infectious Diseases Clinical Trials and Mycoses Study Group Collaborators. Prevention of relapse of histoplasmosis with itraconazole in patients with the acquired immunodeficiency syndrome. Ann Intern Med 1993; 118:610–616.
- Saccente M. Central nervous system histoplasmosis. Curr Treat Options Neurol 2008; 10:161–167.
- Shapiro JL, Lux JJ, Sprofkin BE. Histoplasmosis of the central nervous system. Am J Pathol 1955; 31:319–335.
- Wheat LJ, Musial CE, Jenny-Avital E. Diagnosis and management of central nervous system histoplasmosis. Clin Infect Dis 2005; 40:844–852.
- Wheat LJ, Batteiger BE, Sathapatayavongs B. Histoplasma capsulatum infections of the central nervous system: a clinical review. Medicine (Baltimore) 1990; 69:244–260.
- Kauffman CA. Histoplasmosis: a clinical and laboratory update. Clin Microbiol Rev 2007; 20:115–132.
- Modi M, Mochan A, Modi G. Management of HIV-associated focal brain lesions in developing countries. QJM 2004; 97:413–421.
- Miller RF, Hall-Craggs MA, Costa DC, et al. Magnetic resonance imaging, thallium-201 SPET scanning, and laboratory analyses for discrimination of cerebral lymphoma and toxoplasmosis in AIDS. Sex Transm Infect 1998; 74:258–264.
- Cohen WA. Intracranial bacterial infections in patients with AIDS. Neuroimaging Clin N Am 1997; 7:223–229.
- Troncoso A, Fumagalli J, Shinzato R, Gulotta H, Toller M, Bava J. CNS cryptococcoma in an HIV-positive patient. J Int Assoc Physicians AIDS Care (Chic) 2002; 1:131–133.
- Land AM, Nelson GA, Bell SG, Denby KJ, Estrada CA, Willett LL. Widening the differential for brain masses in human immunodeficiency virus-positive patients: syphilitic cerebral gummata. Am J Med Sci 2013; 346:253–255.
- Balsys R, Janousek JE, Batnitzky S, Templeton AW. Peripheral enhancement in computerized cranial tomography: a non-specific finding. Surg Neurol 1979; 11:207–216.
- Wheat LJ, Freifeld AG, Kleiman MB, et al; Infectious Diseases Society of America. Clinical practice guidelines for the management of patients with histoplasmosis: 2007 update by the Infectious Diseases Society of America. Clin Infect Dis 2007; 45:807–825.
- Wheat J, Hafner R, Wulfsohn M, et al; National Institute of Allergy and Infectious Diseases Clinical Trials and Mycoses Study Group Collaborators. Prevention of relapse of histoplasmosis with itraconazole in patients with the acquired immunodeficiency syndrome. Ann Intern Med 1993; 118:610–616.
- Saccente M. Central nervous system histoplasmosis. Curr Treat Options Neurol 2008; 10:161–167.
Evidence helps, but some decisions remain within the art of medicine
Despite advances in therapy, more than 10% of patients with acute bacterial meningitis still die of it, and more suffer significant morbidity, including cognitive dysfunction and deafness. Well-defined protocols that include empiric antibiotics and systemic corticosteroids have improved the outcomes of patients with meningitis. But, as with other closed-space infections such as septic arthritis, any delay in providing appropriate antibiotic treatment is associated with a worse prognosis. In the case of bacterial meningitis, a retrospective analysis concluded that each hour of delay in delivering antibiotics and a corticosteroid can be associated with a relative (not absolute) increase in mortality of 13%.1
The precise diagnosis of bacterial meningitis depends entirely on obtaining cerebrospinal fluid for analysis, including culture and antibiotic sensitivity testing. But that simple statement belies several current and historical complexities. From my experience, getting a prompt diagnostic lumbar puncture is not as simple as it once was.
Many hospitals have imposed patient safety initiatives, which overall have been beneficial but have had the effect that medical residents and probably even hospitalists in some medical centers are less frequently the ones doing interventional procedures. Some procedures, such as placement of pulmonary arterial catheters in the medical intensive care unit, have been shown to be less useful and to pose more risk than once believed. The tasks of placing other central lines and performing thoracenteses have been relegated to special procedure teams trained in using ultrasound guidance. Interventional radiologists now often do the visceral biopsies and lumbar punctures, and as a result, it is hoped that procedural complication rates will decline. On the other hand, these changes mean that medical residents and future staff are less experienced in performing these procedures, even though there are times that they are the only ones available to perform them. The result is a potential delay in performing a necessary lumbar puncture.
Another reason that a lumbar puncture may be delayed is concern over iatrogenic herniation if the procedure is done in a patient who has elevated intracranial pressure. We do not know precisely how often this occurs if there is an undiagnosed brain mass lesion such as an abscess, which can mimic bacterial meningitis, or a malignancy, and meningitis itself may be associated with herniation. Yet, for years physicians have hesitated to perform lumbar punctures in some patients without first ruling out a brain mass by computed tomography (CT), a diagnostic flow algorithm that often introduces at least an hour of delay in performing the procedure and in obtaining cultures before starting antibiotics.
When I was in training, we were perhaps more cavalier, appropriately or not. If the history and examination did not suggest a brain mass and the patient had retinal vein pulsations without papilledema, we did the lumbar puncture. It was a different time, and there was a different perspective on risks and benefits. More recently, the trend has been to obtain a CT scan before a lumbar puncture in several subsets of patients.
A 2015 analysis from Sweden1 showed that we can probably do a lumbar puncture for suspected bacterial meningitis without first doing a CT scan in most patients, even in patients with moderately impaired mentation. Perhaps some other concerns can also be assuaged if evaluated, but we don’t have data. Mirrakhimov et al, in this issue of the Journal, review the current evidence on when to do CT before a lumbar puncture, even if it may significantly delay the procedure and the timely delivery of antibiotics. A perfect algorithm that balances the risks of delaying treatment, initiating less-than-ideal empiric antibiotics potentially without definitive culture, and inducing complications from a procedure done promptly may well be impossible to develop. Evidence helps us refine the diagnostic approach, but with limited data, some important decisions unfortunately remain within the “art” rather than the science of medicine.
- Glimåker M, Johansson B, Grindborg Ö, Bottai M, Lindquist L, Sjölin J. Adult bacterial meningitis: earlier treatment and improved outcome following guideline revision promoting prompt lumbar puncture. Clin Infect Dis 2015; 60:1162–1169.
Despite advances in therapy, more than 10% of patients with acute bacterial meningitis still die of it, and more suffer significant morbidity, including cognitive dysfunction and deafness. Well-defined protocols that include empiric antibiotics and systemic corticosteroids have improved the outcomes of patients with meningitis. But, as with other closed-space infections such as septic arthritis, any delay in providing appropriate antibiotic treatment is associated with a worse prognosis. In the case of bacterial meningitis, a retrospective analysis concluded that each hour of delay in delivering antibiotics and a corticosteroid can be associated with a relative (not absolute) increase in mortality of 13%.1
The precise diagnosis of bacterial meningitis depends entirely on obtaining cerebrospinal fluid for analysis, including culture and antibiotic sensitivity testing. But that simple statement belies several current and historical complexities. From my experience, getting a prompt diagnostic lumbar puncture is not as simple as it once was.
Many hospitals have imposed patient safety initiatives, which overall have been beneficial but have had the effect that medical residents and probably even hospitalists in some medical centers are less frequently the ones doing interventional procedures. Some procedures, such as placement of pulmonary arterial catheters in the medical intensive care unit, have been shown to be less useful and to pose more risk than once believed. The tasks of placing other central lines and performing thoracenteses have been relegated to special procedure teams trained in using ultrasound guidance. Interventional radiologists now often do the visceral biopsies and lumbar punctures, and as a result, it is hoped that procedural complication rates will decline. On the other hand, these changes mean that medical residents and future staff are less experienced in performing these procedures, even though there are times that they are the only ones available to perform them. The result is a potential delay in performing a necessary lumbar puncture.
Another reason that a lumbar puncture may be delayed is concern over iatrogenic herniation if the procedure is done in a patient who has elevated intracranial pressure. We do not know precisely how often this occurs if there is an undiagnosed brain mass lesion such as an abscess, which can mimic bacterial meningitis, or a malignancy, and meningitis itself may be associated with herniation. Yet, for years physicians have hesitated to perform lumbar punctures in some patients without first ruling out a brain mass by computed tomography (CT), a diagnostic flow algorithm that often introduces at least an hour of delay in performing the procedure and in obtaining cultures before starting antibiotics.
When I was in training, we were perhaps more cavalier, appropriately or not. If the history and examination did not suggest a brain mass and the patient had retinal vein pulsations without papilledema, we did the lumbar puncture. It was a different time, and there was a different perspective on risks and benefits. More recently, the trend has been to obtain a CT scan before a lumbar puncture in several subsets of patients.
A 2015 analysis from Sweden1 showed that we can probably do a lumbar puncture for suspected bacterial meningitis without first doing a CT scan in most patients, even in patients with moderately impaired mentation. Perhaps some other concerns can also be assuaged if evaluated, but we don’t have data. Mirrakhimov et al, in this issue of the Journal, review the current evidence on when to do CT before a lumbar puncture, even if it may significantly delay the procedure and the timely delivery of antibiotics. A perfect algorithm that balances the risks of delaying treatment, initiating less-than-ideal empiric antibiotics potentially without definitive culture, and inducing complications from a procedure done promptly may well be impossible to develop. Evidence helps us refine the diagnostic approach, but with limited data, some important decisions unfortunately remain within the “art” rather than the science of medicine.
Despite advances in therapy, more than 10% of patients with acute bacterial meningitis still die of it, and more suffer significant morbidity, including cognitive dysfunction and deafness. Well-defined protocols that include empiric antibiotics and systemic corticosteroids have improved the outcomes of patients with meningitis. But, as with other closed-space infections such as septic arthritis, any delay in providing appropriate antibiotic treatment is associated with a worse prognosis. In the case of bacterial meningitis, a retrospective analysis concluded that each hour of delay in delivering antibiotics and a corticosteroid can be associated with a relative (not absolute) increase in mortality of 13%.1
The precise diagnosis of bacterial meningitis depends entirely on obtaining cerebrospinal fluid for analysis, including culture and antibiotic sensitivity testing. But that simple statement belies several current and historical complexities. From my experience, getting a prompt diagnostic lumbar puncture is not as simple as it once was.
Many hospitals have imposed patient safety initiatives, which overall have been beneficial but have had the effect that medical residents and probably even hospitalists in some medical centers are less frequently the ones doing interventional procedures. Some procedures, such as placement of pulmonary arterial catheters in the medical intensive care unit, have been shown to be less useful and to pose more risk than once believed. The tasks of placing other central lines and performing thoracenteses have been relegated to special procedure teams trained in using ultrasound guidance. Interventional radiologists now often do the visceral biopsies and lumbar punctures, and as a result, it is hoped that procedural complication rates will decline. On the other hand, these changes mean that medical residents and future staff are less experienced in performing these procedures, even though there are times that they are the only ones available to perform them. The result is a potential delay in performing a necessary lumbar puncture.
Another reason that a lumbar puncture may be delayed is concern over iatrogenic herniation if the procedure is done in a patient who has elevated intracranial pressure. We do not know precisely how often this occurs if there is an undiagnosed brain mass lesion such as an abscess, which can mimic bacterial meningitis, or a malignancy, and meningitis itself may be associated with herniation. Yet, for years physicians have hesitated to perform lumbar punctures in some patients without first ruling out a brain mass by computed tomography (CT), a diagnostic flow algorithm that often introduces at least an hour of delay in performing the procedure and in obtaining cultures before starting antibiotics.
When I was in training, we were perhaps more cavalier, appropriately or not. If the history and examination did not suggest a brain mass and the patient had retinal vein pulsations without papilledema, we did the lumbar puncture. It was a different time, and there was a different perspective on risks and benefits. More recently, the trend has been to obtain a CT scan before a lumbar puncture in several subsets of patients.
A 2015 analysis from Sweden1 showed that we can probably do a lumbar puncture for suspected bacterial meningitis without first doing a CT scan in most patients, even in patients with moderately impaired mentation. Perhaps some other concerns can also be assuaged if evaluated, but we don’t have data. Mirrakhimov et al, in this issue of the Journal, review the current evidence on when to do CT before a lumbar puncture, even if it may significantly delay the procedure and the timely delivery of antibiotics. A perfect algorithm that balances the risks of delaying treatment, initiating less-than-ideal empiric antibiotics potentially without definitive culture, and inducing complications from a procedure done promptly may well be impossible to develop. Evidence helps us refine the diagnostic approach, but with limited data, some important decisions unfortunately remain within the “art” rather than the science of medicine.
- Glimåker M, Johansson B, Grindborg Ö, Bottai M, Lindquist L, Sjölin J. Adult bacterial meningitis: earlier treatment and improved outcome following guideline revision promoting prompt lumbar puncture. Clin Infect Dis 2015; 60:1162–1169.
- Glimåker M, Johansson B, Grindborg Ö, Bottai M, Lindquist L, Sjölin J. Adult bacterial meningitis: earlier treatment and improved outcome following guideline revision promoting prompt lumbar puncture. Clin Infect Dis 2015; 60:1162–1169.
Bedside Cardiac Ultrasound to Aid in Diagnosing Takotsubo Cardiomyopathy
Cardiac ultrasound is among the many beneficial applications of point-of-care (POC) ultrasound in the ED. This modality can prove extremely beneficial in evaluating the critically ill patient. For example, POC cardiac ultrasound not only permits the emergency physician (EP) to diagnose a pericardial effusion and cardiac tamponade, but also perform a pericardiocentesis.1 The EP can also employ beside ultrasound to estimate an ejection fraction (EF) almost as well as cardiology services,2 look for signs of right-heart strain in patients with pulmonary embolism (PE),3 and guide fluid management in patients who have septic shock.4 In addition to only taking a few minutes to perform, POC cardiac ultrasound can also drastically change the course of management in some patients. Our case illustrates the use of POC ultrasound to diagnose Takotsubo cardiomyopathy in a 64-year-old patient and guide management when she became unstable prior to cardiac catheterization.
Case
A 64-year-old white woman with a medical history of diabetes, obesity, and nephrolithiasis presented to the ED with chest pain and shortness of breath, which she stated had begun earlier in the day. The patient’s chest pain did not intensify upon exertion, but the shortness of breath worsened when she was in the supine position.
Three months prior, the patient had also presented to our ED with chest pain. Evaluation during that visit included a negative stress echocardiogram with an EF of 55%. At this second visit, an electrocardiogram (ECG) showed new T-wave inversions in the anterior, lateral, and inferior leads. Vital signs at presentation were: blood pressure, 107/63 mm Hg; heart rate, 100 beats/min; respiratory rate, 18 breaths/min; and temperature, 97.9°F. Oxygen saturation was 97% on room air when patient was sitting upright, but decreased to 90% when she was supine. A chest X-ray showed left basilar atelectasis with a trace effusion. Laboratory evaluation was remarkable for the following: troponin I, 2.99 ng/mL; D-dimer, 294 ng/mL; and brain natriuretic peptide, 559 pg/mL.
Given the patient’s vital signs and positive troponin I level, a computed tomography (CT) scan was ordered to assess for a PE. This was done despite the patient’s negative D-dimer results, as it was felt that she was not low-risk for PE. At the same time the CT scan was ordered, a POC cardiac ultrasound was performed to assess for signs of right heart strain.
Based on the ultrasound findings and a normal EF 3 months prior, there was concern for Takotsubo cardiomyopathy. The patient was further questioned as to the events surrounding the onset of her chest pain. She informed the EP the pain started when she learned that she might be evicted from her home.
The CT scan was negative for PE. The consulting cardiologist was informed of the results of the ultrasound findings, and the patient was given aspirin, heparin, morphine, and furosemide, and was admitted to the cardiac progressive unit. She was also initially given morphine for pain management, but due to intolerance, she was switched to nitroglycerin.
During the first evening of her inpatient stay, the patient experienced acute changes in her chest pain that resulted in activating the rapid response team. Secondary to the information gathered in the ED, the patient was managed conservatively and was evaluated by a physician extender who repeated laboratory studies, provided supplemental potassium and magnesium, and ordered another ECG in consultation with the cardiologist (who was caring for the patient via telephone). In the morning, the patient continued to have chest pain, and a repeat ECG showed worsening of previous T-wave inversions. Based on these findings, the cardiologist ordered cardiac catheterization.
On hospital day 2, the cardiologist performed another echocardiogram, which confirmed the low EF of 20% with severe global hypokinesis with sparing of the basal segments. Cardiac catheterization showed no significant disease (20% lesion in the mid-left anterior descending artery) with the left ventriculogram showing an EF of 10%, cardiac output of 3.7, and cardiac index of 1.8, confirming the diagnosis of Takotsubo cardiomyopathy. The patient remained in the hospital for a total of 8 days while awaiting a life vest; however, a repeat echocardiogram on hospital day 8 showed an EF of 55%.
Discussion
Takotsubo cardiomyopathy is an acute, stress-induced cardiomyopathy that was first described in Japan in the early 1990s.5 It is thought to be due to catecholamine-induced dysfunction from a stressful event,6-8 such as the death of a loved one, which is why it is often referred to as “broken heart syndrome.” However there are case reports highlighting other causes of Takotsubo cardiomyopathy, such as cocaine use,9 scuba diving,10 and diabetic ketoacidosis combined with hypothermia.11
Patients with Takotsubo cardiomyopathy will frequently have ECG abnormalities, including ST-segment elevation or depression, or T-wave changes; troponin levels also may be elevated. The majority of patients (>80%) are postmenopausal women, typically aged 50 to 75 years.6,12 Echocardiogram findings in Takotsubo cardiomyopathy show significant left ventricular (LV) dysfunction or regional dysfunction that is not in one coronary artery distribution.12,13 There will often be apical dilation or ballooning with dyskinesia but more preserved function at the base and normal dimensions.14,15 A negative cardiac catheterization or catheterization in the absence of significant disease is required to confirm the diagnosis.16 The LV function usually returns to baseline in 1 to 4 weeks, but there can be recurrence in some patients.6,17 The condition is also associated with a large burden of morbidity and mortality.6,18 In a case series by Gopalakrishnan et al6 of 56 patients, there was an 8.9% in-hospital mortality rate and an additional 17.9% out-of-hospital mortality rate even in patients in whom LV function had returned to normal.
In a review by Gianni et al,19 4.2% of patients with Takotsubo cardiomyopathy present with or go into cardiogenic shock at some point during admission, and up to 2% of patients who present with acute myocardial infarction have Takotsubo cardiomyopathy. Patients can go into cardiogenic shock due to depressed EF or LV outflow tract obstruction from hyperkinesis of the basilar segments. Some of these patients may be sent directly to the catheter laboratory based on ST elevations on ECG, in which case the diagnosis is made there. Our patient, however, did not have ST elevation and later became unstable on the floor. Citro et al20 suggest that a patient with a predisposition for Takotsubo cardiomyopathy (eg, postmenopausal patients, those who experienced a trigger event), in the right clinical setting and without ST-segment elevation on ECG, could be managed more conservatively with delayed cardiac angiography or CT angiography (CTA) evaluation of the coronary arteries (sparing the patient an invasive procedure)—as long as ultrasound was consistent with typical Takotsubo cardiomyopathy findings. However, CTA is still needed to make the diagnosis.
At this time, Takotsubo cardiomyopathy should remain an important part of the differential diagnosis for emergency patients who have chest pain—especially for postmenopausal women with a history of significant stressor—as early recognition can lead to better patient care.
Conclusion
This case highlights the importance of POC ultrasound in the management of patients in the ED and after admission. The care of our patient was enhanced by the ability to take a real-time look at her EF and cardiac function at the time of admission through bedside ultrasound. This information guided her management and optimized stabilization.
1. Goodman A, Perera P, Mailhot T, Mandavia D. The role of bedside ultrasound in the diagnosis of pericardial effusion and cardiac tamponade. J Emerg Trauma Shock. 2012;5(1):72-75. doi:10.4103/0974-2700.93118.
2. Unlüer EE, Karagöz A, Akoğlu H, Bayata S. Visual estimation of bedside echocardiographic ejection fraction by emergency physicians. West J Emerg Med. 2014;15(2):221-226. doi:10.5811/westjem.2013.9.16185.
3. McConnell MV, Solomon SD, Rayan ME, Come PC, Goldhaber SZ, Lee RT. Regional right ventricular dysfunction detected by echocardiography in acute pulmonary embolism. Am J Cardiol. 1996;78(4):469-473.
4. Coen D, Cortellaro F, Pasini S, et al. Towards a less invasive approach to the early goal-directed treatment of septic shock in the ED. Am J Emerg Med. 2014;32(6):563-568. doi:10.1016/j.ajem.2014.02.011.
5. Dote K, Sato H, Tateishi H, Uchida T, Ishihara M. [Myocardial stunning due to simultaneous multivessel coronary spasms: a review of 5 cases.] J Cardiol. 1991;21(2):203-214.
6. Gopalakrishnan M, Hassan A, Villines D, Nasr S, Chandrasekaran M, Klein LW. Predictors of short- and long-term outcomes of Takotsubo cardiomyopathy. Am J Cardiol. 2015;116(10):1586-1590. doi:10.1016/j.amjcard.2015.08.024.
7. Paur H, Wright PT, Sikkel MB, et al. High levels of circulating epinephrine trigger apical cardiodepression in a β2-adrenergic receptor/Gi-dependent manner: a new model of Takotsubo cardiomyopathy. Circulation. 2012;126(6):697-706. doi:10.1161/CIRCULATIONAHA.112.111591.
8. Wittstein IS, Thiemann DR, Lima JA, et al. Neurohumoral features of myocardial stunning due to sudden emotional stress. N Engl J Med. 2005;352(6):539-548. doi:10.1056/NEJMoa043046.
9. Butterfield M, Riguzzi C, Frenkel O, Nagdev A. Stimulant-related Takotsubo cardiomyopathy. Am J Emerg Med. 2015;33(3):476.e1-e3. doi:10.1016/j.ajem.2014.08.058.
10. Baber A, Nair SU, Duggal S, Bhatti S, Sundlof DW. Stress cardiomyopathy caused by diving: case report and review of the literature. J Emerg Med. 2016;50(2):277-280. doi:10.1016/j.jemermed.2015.09.045.
11. Katayama Y, Hifumi T, Inoue J, Koido Y. A case of Takotsubo cardiomyopathy induced by accidental hypothermia and diabetic ketoacidosis. BMJ Case Rep. 2013;2013:1-3. doi:10.1136/bcr-2012-008143.
12. Bybee KA, Kara T, Prasad A, et al. Systematic review: transient left ventricular apical ballooning: a syndrome that mimics ST-segment elevation myocardial infarction. Ann Intern Med. 2004;141(11):858-865.
13. Virani SS, Khan AN, Mendoza CE, Ferreira AC, de Marchena E. Takotsubo cardiomyopathy, or brokenheart syndrome. Tex Heart Inst J. 2007;34(1):76-79.
14. Okura H. Echocardiographic assessment of takotsubo cardiomyopathy: beyond apical ballooning. J Echocardiogr. 2016;14(1):13-20. doi:10.1007/s12574-015-0271-3.
15. Naser N, Buksa M, Kusljugic Z, Terzic I, Sokolovic S, Hodzic E. The role of echocardiography in diagnosis and follow up of patients with takotsubo cardiomyopathy or acute ballooning syndrome. Med Arh. 2011;65(5):287-290.
16. Ono R, Falcão LM. Takotsubo cardiomyopathy systematic review: Pathophysiologic process, clinical presentation and diagnostic approach to Takotsubo cardiomyopathy. Int J Cardiol. 2016;209:196-205. doi:10.1016/j.ijcard.2016.02.012.
17. Opolski G, Budnik M, Kochanowski J, Kowalik R, Piatkowski R, Kochman J. Four episodes of takotsubo cardiomyopathy in one patient. Int J Cardiol. 2016;203:53-54. doi:10.1016/j.ijcard.2015.10.048.
18. Templin C, Ghadri JR, Diekmann J, et al. Clinical features and outcomes of Takotsubo (stress) cardiomyopathy. N Engl J Med. 2015;373(10):929-938.
19. Gianni M, Dentali F, Grandi AM, Sumner G, Hiralal R, Lonn E. Apical ballooning syndrome or takotsubo cardiomyopathy: a systematic review. Eur Heart J. 2006;27(13):1523-1529. doi:10.1093/eurheartj/ehl032.
20. Citro R, Lyon AR, Meimoun P, et al. Standard and advanced echocardiography in Takotsubo (stress) cardiomyopathy: clinical and prognostic implications. J Am Soc Echocardiogr. 2015;28(1):57-74. doi:10.1016/j.echo.2014.08.020.
Cardiac ultrasound is among the many beneficial applications of point-of-care (POC) ultrasound in the ED. This modality can prove extremely beneficial in evaluating the critically ill patient. For example, POC cardiac ultrasound not only permits the emergency physician (EP) to diagnose a pericardial effusion and cardiac tamponade, but also perform a pericardiocentesis.1 The EP can also employ beside ultrasound to estimate an ejection fraction (EF) almost as well as cardiology services,2 look for signs of right-heart strain in patients with pulmonary embolism (PE),3 and guide fluid management in patients who have septic shock.4 In addition to only taking a few minutes to perform, POC cardiac ultrasound can also drastically change the course of management in some patients. Our case illustrates the use of POC ultrasound to diagnose Takotsubo cardiomyopathy in a 64-year-old patient and guide management when she became unstable prior to cardiac catheterization.
Case
A 64-year-old white woman with a medical history of diabetes, obesity, and nephrolithiasis presented to the ED with chest pain and shortness of breath, which she stated had begun earlier in the day. The patient’s chest pain did not intensify upon exertion, but the shortness of breath worsened when she was in the supine position.
Three months prior, the patient had also presented to our ED with chest pain. Evaluation during that visit included a negative stress echocardiogram with an EF of 55%. At this second visit, an electrocardiogram (ECG) showed new T-wave inversions in the anterior, lateral, and inferior leads. Vital signs at presentation were: blood pressure, 107/63 mm Hg; heart rate, 100 beats/min; respiratory rate, 18 breaths/min; and temperature, 97.9°F. Oxygen saturation was 97% on room air when patient was sitting upright, but decreased to 90% when she was supine. A chest X-ray showed left basilar atelectasis with a trace effusion. Laboratory evaluation was remarkable for the following: troponin I, 2.99 ng/mL; D-dimer, 294 ng/mL; and brain natriuretic peptide, 559 pg/mL.
Given the patient’s vital signs and positive troponin I level, a computed tomography (CT) scan was ordered to assess for a PE. This was done despite the patient’s negative D-dimer results, as it was felt that she was not low-risk for PE. At the same time the CT scan was ordered, a POC cardiac ultrasound was performed to assess for signs of right heart strain.
Based on the ultrasound findings and a normal EF 3 months prior, there was concern for Takotsubo cardiomyopathy. The patient was further questioned as to the events surrounding the onset of her chest pain. She informed the EP the pain started when she learned that she might be evicted from her home.
The CT scan was negative for PE. The consulting cardiologist was informed of the results of the ultrasound findings, and the patient was given aspirin, heparin, morphine, and furosemide, and was admitted to the cardiac progressive unit. She was also initially given morphine for pain management, but due to intolerance, she was switched to nitroglycerin.
During the first evening of her inpatient stay, the patient experienced acute changes in her chest pain that resulted in activating the rapid response team. Secondary to the information gathered in the ED, the patient was managed conservatively and was evaluated by a physician extender who repeated laboratory studies, provided supplemental potassium and magnesium, and ordered another ECG in consultation with the cardiologist (who was caring for the patient via telephone). In the morning, the patient continued to have chest pain, and a repeat ECG showed worsening of previous T-wave inversions. Based on these findings, the cardiologist ordered cardiac catheterization.
On hospital day 2, the cardiologist performed another echocardiogram, which confirmed the low EF of 20% with severe global hypokinesis with sparing of the basal segments. Cardiac catheterization showed no significant disease (20% lesion in the mid-left anterior descending artery) with the left ventriculogram showing an EF of 10%, cardiac output of 3.7, and cardiac index of 1.8, confirming the diagnosis of Takotsubo cardiomyopathy. The patient remained in the hospital for a total of 8 days while awaiting a life vest; however, a repeat echocardiogram on hospital day 8 showed an EF of 55%.
Discussion
Takotsubo cardiomyopathy is an acute, stress-induced cardiomyopathy that was first described in Japan in the early 1990s.5 It is thought to be due to catecholamine-induced dysfunction from a stressful event,6-8 such as the death of a loved one, which is why it is often referred to as “broken heart syndrome.” However there are case reports highlighting other causes of Takotsubo cardiomyopathy, such as cocaine use,9 scuba diving,10 and diabetic ketoacidosis combined with hypothermia.11
Patients with Takotsubo cardiomyopathy will frequently have ECG abnormalities, including ST-segment elevation or depression, or T-wave changes; troponin levels also may be elevated. The majority of patients (>80%) are postmenopausal women, typically aged 50 to 75 years.6,12 Echocardiogram findings in Takotsubo cardiomyopathy show significant left ventricular (LV) dysfunction or regional dysfunction that is not in one coronary artery distribution.12,13 There will often be apical dilation or ballooning with dyskinesia but more preserved function at the base and normal dimensions.14,15 A negative cardiac catheterization or catheterization in the absence of significant disease is required to confirm the diagnosis.16 The LV function usually returns to baseline in 1 to 4 weeks, but there can be recurrence in some patients.6,17 The condition is also associated with a large burden of morbidity and mortality.6,18 In a case series by Gopalakrishnan et al6 of 56 patients, there was an 8.9% in-hospital mortality rate and an additional 17.9% out-of-hospital mortality rate even in patients in whom LV function had returned to normal.
In a review by Gianni et al,19 4.2% of patients with Takotsubo cardiomyopathy present with or go into cardiogenic shock at some point during admission, and up to 2% of patients who present with acute myocardial infarction have Takotsubo cardiomyopathy. Patients can go into cardiogenic shock due to depressed EF or LV outflow tract obstruction from hyperkinesis of the basilar segments. Some of these patients may be sent directly to the catheter laboratory based on ST elevations on ECG, in which case the diagnosis is made there. Our patient, however, did not have ST elevation and later became unstable on the floor. Citro et al20 suggest that a patient with a predisposition for Takotsubo cardiomyopathy (eg, postmenopausal patients, those who experienced a trigger event), in the right clinical setting and without ST-segment elevation on ECG, could be managed more conservatively with delayed cardiac angiography or CT angiography (CTA) evaluation of the coronary arteries (sparing the patient an invasive procedure)—as long as ultrasound was consistent with typical Takotsubo cardiomyopathy findings. However, CTA is still needed to make the diagnosis.
At this time, Takotsubo cardiomyopathy should remain an important part of the differential diagnosis for emergency patients who have chest pain—especially for postmenopausal women with a history of significant stressor—as early recognition can lead to better patient care.
Conclusion
This case highlights the importance of POC ultrasound in the management of patients in the ED and after admission. The care of our patient was enhanced by the ability to take a real-time look at her EF and cardiac function at the time of admission through bedside ultrasound. This information guided her management and optimized stabilization.
Cardiac ultrasound is among the many beneficial applications of point-of-care (POC) ultrasound in the ED. This modality can prove extremely beneficial in evaluating the critically ill patient. For example, POC cardiac ultrasound not only permits the emergency physician (EP) to diagnose a pericardial effusion and cardiac tamponade, but also perform a pericardiocentesis.1 The EP can also employ beside ultrasound to estimate an ejection fraction (EF) almost as well as cardiology services,2 look for signs of right-heart strain in patients with pulmonary embolism (PE),3 and guide fluid management in patients who have septic shock.4 In addition to only taking a few minutes to perform, POC cardiac ultrasound can also drastically change the course of management in some patients. Our case illustrates the use of POC ultrasound to diagnose Takotsubo cardiomyopathy in a 64-year-old patient and guide management when she became unstable prior to cardiac catheterization.
Case
A 64-year-old white woman with a medical history of diabetes, obesity, and nephrolithiasis presented to the ED with chest pain and shortness of breath, which she stated had begun earlier in the day. The patient’s chest pain did not intensify upon exertion, but the shortness of breath worsened when she was in the supine position.
Three months prior, the patient had also presented to our ED with chest pain. Evaluation during that visit included a negative stress echocardiogram with an EF of 55%. At this second visit, an electrocardiogram (ECG) showed new T-wave inversions in the anterior, lateral, and inferior leads. Vital signs at presentation were: blood pressure, 107/63 mm Hg; heart rate, 100 beats/min; respiratory rate, 18 breaths/min; and temperature, 97.9°F. Oxygen saturation was 97% on room air when patient was sitting upright, but decreased to 90% when she was supine. A chest X-ray showed left basilar atelectasis with a trace effusion. Laboratory evaluation was remarkable for the following: troponin I, 2.99 ng/mL; D-dimer, 294 ng/mL; and brain natriuretic peptide, 559 pg/mL.
Given the patient’s vital signs and positive troponin I level, a computed tomography (CT) scan was ordered to assess for a PE. This was done despite the patient’s negative D-dimer results, as it was felt that she was not low-risk for PE. At the same time the CT scan was ordered, a POC cardiac ultrasound was performed to assess for signs of right heart strain.
Based on the ultrasound findings and a normal EF 3 months prior, there was concern for Takotsubo cardiomyopathy. The patient was further questioned as to the events surrounding the onset of her chest pain. She informed the EP the pain started when she learned that she might be evicted from her home.
The CT scan was negative for PE. The consulting cardiologist was informed of the results of the ultrasound findings, and the patient was given aspirin, heparin, morphine, and furosemide, and was admitted to the cardiac progressive unit. She was also initially given morphine for pain management, but due to intolerance, she was switched to nitroglycerin.
During the first evening of her inpatient stay, the patient experienced acute changes in her chest pain that resulted in activating the rapid response team. Secondary to the information gathered in the ED, the patient was managed conservatively and was evaluated by a physician extender who repeated laboratory studies, provided supplemental potassium and magnesium, and ordered another ECG in consultation with the cardiologist (who was caring for the patient via telephone). In the morning, the patient continued to have chest pain, and a repeat ECG showed worsening of previous T-wave inversions. Based on these findings, the cardiologist ordered cardiac catheterization.
On hospital day 2, the cardiologist performed another echocardiogram, which confirmed the low EF of 20% with severe global hypokinesis with sparing of the basal segments. Cardiac catheterization showed no significant disease (20% lesion in the mid-left anterior descending artery) with the left ventriculogram showing an EF of 10%, cardiac output of 3.7, and cardiac index of 1.8, confirming the diagnosis of Takotsubo cardiomyopathy. The patient remained in the hospital for a total of 8 days while awaiting a life vest; however, a repeat echocardiogram on hospital day 8 showed an EF of 55%.
Discussion
Takotsubo cardiomyopathy is an acute, stress-induced cardiomyopathy that was first described in Japan in the early 1990s.5 It is thought to be due to catecholamine-induced dysfunction from a stressful event,6-8 such as the death of a loved one, which is why it is often referred to as “broken heart syndrome.” However there are case reports highlighting other causes of Takotsubo cardiomyopathy, such as cocaine use,9 scuba diving,10 and diabetic ketoacidosis combined with hypothermia.11
Patients with Takotsubo cardiomyopathy will frequently have ECG abnormalities, including ST-segment elevation or depression, or T-wave changes; troponin levels also may be elevated. The majority of patients (>80%) are postmenopausal women, typically aged 50 to 75 years.6,12 Echocardiogram findings in Takotsubo cardiomyopathy show significant left ventricular (LV) dysfunction or regional dysfunction that is not in one coronary artery distribution.12,13 There will often be apical dilation or ballooning with dyskinesia but more preserved function at the base and normal dimensions.14,15 A negative cardiac catheterization or catheterization in the absence of significant disease is required to confirm the diagnosis.16 The LV function usually returns to baseline in 1 to 4 weeks, but there can be recurrence in some patients.6,17 The condition is also associated with a large burden of morbidity and mortality.6,18 In a case series by Gopalakrishnan et al6 of 56 patients, there was an 8.9% in-hospital mortality rate and an additional 17.9% out-of-hospital mortality rate even in patients in whom LV function had returned to normal.
In a review by Gianni et al,19 4.2% of patients with Takotsubo cardiomyopathy present with or go into cardiogenic shock at some point during admission, and up to 2% of patients who present with acute myocardial infarction have Takotsubo cardiomyopathy. Patients can go into cardiogenic shock due to depressed EF or LV outflow tract obstruction from hyperkinesis of the basilar segments. Some of these patients may be sent directly to the catheter laboratory based on ST elevations on ECG, in which case the diagnosis is made there. Our patient, however, did not have ST elevation and later became unstable on the floor. Citro et al20 suggest that a patient with a predisposition for Takotsubo cardiomyopathy (eg, postmenopausal patients, those who experienced a trigger event), in the right clinical setting and without ST-segment elevation on ECG, could be managed more conservatively with delayed cardiac angiography or CT angiography (CTA) evaluation of the coronary arteries (sparing the patient an invasive procedure)—as long as ultrasound was consistent with typical Takotsubo cardiomyopathy findings. However, CTA is still needed to make the diagnosis.
At this time, Takotsubo cardiomyopathy should remain an important part of the differential diagnosis for emergency patients who have chest pain—especially for postmenopausal women with a history of significant stressor—as early recognition can lead to better patient care.
Conclusion
This case highlights the importance of POC ultrasound in the management of patients in the ED and after admission. The care of our patient was enhanced by the ability to take a real-time look at her EF and cardiac function at the time of admission through bedside ultrasound. This information guided her management and optimized stabilization.
1. Goodman A, Perera P, Mailhot T, Mandavia D. The role of bedside ultrasound in the diagnosis of pericardial effusion and cardiac tamponade. J Emerg Trauma Shock. 2012;5(1):72-75. doi:10.4103/0974-2700.93118.
2. Unlüer EE, Karagöz A, Akoğlu H, Bayata S. Visual estimation of bedside echocardiographic ejection fraction by emergency physicians. West J Emerg Med. 2014;15(2):221-226. doi:10.5811/westjem.2013.9.16185.
3. McConnell MV, Solomon SD, Rayan ME, Come PC, Goldhaber SZ, Lee RT. Regional right ventricular dysfunction detected by echocardiography in acute pulmonary embolism. Am J Cardiol. 1996;78(4):469-473.
4. Coen D, Cortellaro F, Pasini S, et al. Towards a less invasive approach to the early goal-directed treatment of septic shock in the ED. Am J Emerg Med. 2014;32(6):563-568. doi:10.1016/j.ajem.2014.02.011.
5. Dote K, Sato H, Tateishi H, Uchida T, Ishihara M. [Myocardial stunning due to simultaneous multivessel coronary spasms: a review of 5 cases.] J Cardiol. 1991;21(2):203-214.
6. Gopalakrishnan M, Hassan A, Villines D, Nasr S, Chandrasekaran M, Klein LW. Predictors of short- and long-term outcomes of Takotsubo cardiomyopathy. Am J Cardiol. 2015;116(10):1586-1590. doi:10.1016/j.amjcard.2015.08.024.
7. Paur H, Wright PT, Sikkel MB, et al. High levels of circulating epinephrine trigger apical cardiodepression in a β2-adrenergic receptor/Gi-dependent manner: a new model of Takotsubo cardiomyopathy. Circulation. 2012;126(6):697-706. doi:10.1161/CIRCULATIONAHA.112.111591.
8. Wittstein IS, Thiemann DR, Lima JA, et al. Neurohumoral features of myocardial stunning due to sudden emotional stress. N Engl J Med. 2005;352(6):539-548. doi:10.1056/NEJMoa043046.
9. Butterfield M, Riguzzi C, Frenkel O, Nagdev A. Stimulant-related Takotsubo cardiomyopathy. Am J Emerg Med. 2015;33(3):476.e1-e3. doi:10.1016/j.ajem.2014.08.058.
10. Baber A, Nair SU, Duggal S, Bhatti S, Sundlof DW. Stress cardiomyopathy caused by diving: case report and review of the literature. J Emerg Med. 2016;50(2):277-280. doi:10.1016/j.jemermed.2015.09.045.
11. Katayama Y, Hifumi T, Inoue J, Koido Y. A case of Takotsubo cardiomyopathy induced by accidental hypothermia and diabetic ketoacidosis. BMJ Case Rep. 2013;2013:1-3. doi:10.1136/bcr-2012-008143.
12. Bybee KA, Kara T, Prasad A, et al. Systematic review: transient left ventricular apical ballooning: a syndrome that mimics ST-segment elevation myocardial infarction. Ann Intern Med. 2004;141(11):858-865.
13. Virani SS, Khan AN, Mendoza CE, Ferreira AC, de Marchena E. Takotsubo cardiomyopathy, or brokenheart syndrome. Tex Heart Inst J. 2007;34(1):76-79.
14. Okura H. Echocardiographic assessment of takotsubo cardiomyopathy: beyond apical ballooning. J Echocardiogr. 2016;14(1):13-20. doi:10.1007/s12574-015-0271-3.
15. Naser N, Buksa M, Kusljugic Z, Terzic I, Sokolovic S, Hodzic E. The role of echocardiography in diagnosis and follow up of patients with takotsubo cardiomyopathy or acute ballooning syndrome. Med Arh. 2011;65(5):287-290.
16. Ono R, Falcão LM. Takotsubo cardiomyopathy systematic review: Pathophysiologic process, clinical presentation and diagnostic approach to Takotsubo cardiomyopathy. Int J Cardiol. 2016;209:196-205. doi:10.1016/j.ijcard.2016.02.012.
17. Opolski G, Budnik M, Kochanowski J, Kowalik R, Piatkowski R, Kochman J. Four episodes of takotsubo cardiomyopathy in one patient. Int J Cardiol. 2016;203:53-54. doi:10.1016/j.ijcard.2015.10.048.
18. Templin C, Ghadri JR, Diekmann J, et al. Clinical features and outcomes of Takotsubo (stress) cardiomyopathy. N Engl J Med. 2015;373(10):929-938.
19. Gianni M, Dentali F, Grandi AM, Sumner G, Hiralal R, Lonn E. Apical ballooning syndrome or takotsubo cardiomyopathy: a systematic review. Eur Heart J. 2006;27(13):1523-1529. doi:10.1093/eurheartj/ehl032.
20. Citro R, Lyon AR, Meimoun P, et al. Standard and advanced echocardiography in Takotsubo (stress) cardiomyopathy: clinical and prognostic implications. J Am Soc Echocardiogr. 2015;28(1):57-74. doi:10.1016/j.echo.2014.08.020.
1. Goodman A, Perera P, Mailhot T, Mandavia D. The role of bedside ultrasound in the diagnosis of pericardial effusion and cardiac tamponade. J Emerg Trauma Shock. 2012;5(1):72-75. doi:10.4103/0974-2700.93118.
2. Unlüer EE, Karagöz A, Akoğlu H, Bayata S. Visual estimation of bedside echocardiographic ejection fraction by emergency physicians. West J Emerg Med. 2014;15(2):221-226. doi:10.5811/westjem.2013.9.16185.
3. McConnell MV, Solomon SD, Rayan ME, Come PC, Goldhaber SZ, Lee RT. Regional right ventricular dysfunction detected by echocardiography in acute pulmonary embolism. Am J Cardiol. 1996;78(4):469-473.
4. Coen D, Cortellaro F, Pasini S, et al. Towards a less invasive approach to the early goal-directed treatment of septic shock in the ED. Am J Emerg Med. 2014;32(6):563-568. doi:10.1016/j.ajem.2014.02.011.
5. Dote K, Sato H, Tateishi H, Uchida T, Ishihara M. [Myocardial stunning due to simultaneous multivessel coronary spasms: a review of 5 cases.] J Cardiol. 1991;21(2):203-214.
6. Gopalakrishnan M, Hassan A, Villines D, Nasr S, Chandrasekaran M, Klein LW. Predictors of short- and long-term outcomes of Takotsubo cardiomyopathy. Am J Cardiol. 2015;116(10):1586-1590. doi:10.1016/j.amjcard.2015.08.024.
7. Paur H, Wright PT, Sikkel MB, et al. High levels of circulating epinephrine trigger apical cardiodepression in a β2-adrenergic receptor/Gi-dependent manner: a new model of Takotsubo cardiomyopathy. Circulation. 2012;126(6):697-706. doi:10.1161/CIRCULATIONAHA.112.111591.
8. Wittstein IS, Thiemann DR, Lima JA, et al. Neurohumoral features of myocardial stunning due to sudden emotional stress. N Engl J Med. 2005;352(6):539-548. doi:10.1056/NEJMoa043046.
9. Butterfield M, Riguzzi C, Frenkel O, Nagdev A. Stimulant-related Takotsubo cardiomyopathy. Am J Emerg Med. 2015;33(3):476.e1-e3. doi:10.1016/j.ajem.2014.08.058.
10. Baber A, Nair SU, Duggal S, Bhatti S, Sundlof DW. Stress cardiomyopathy caused by diving: case report and review of the literature. J Emerg Med. 2016;50(2):277-280. doi:10.1016/j.jemermed.2015.09.045.
11. Katayama Y, Hifumi T, Inoue J, Koido Y. A case of Takotsubo cardiomyopathy induced by accidental hypothermia and diabetic ketoacidosis. BMJ Case Rep. 2013;2013:1-3. doi:10.1136/bcr-2012-008143.
12. Bybee KA, Kara T, Prasad A, et al. Systematic review: transient left ventricular apical ballooning: a syndrome that mimics ST-segment elevation myocardial infarction. Ann Intern Med. 2004;141(11):858-865.
13. Virani SS, Khan AN, Mendoza CE, Ferreira AC, de Marchena E. Takotsubo cardiomyopathy, or brokenheart syndrome. Tex Heart Inst J. 2007;34(1):76-79.
14. Okura H. Echocardiographic assessment of takotsubo cardiomyopathy: beyond apical ballooning. J Echocardiogr. 2016;14(1):13-20. doi:10.1007/s12574-015-0271-3.
15. Naser N, Buksa M, Kusljugic Z, Terzic I, Sokolovic S, Hodzic E. The role of echocardiography in diagnosis and follow up of patients with takotsubo cardiomyopathy or acute ballooning syndrome. Med Arh. 2011;65(5):287-290.
16. Ono R, Falcão LM. Takotsubo cardiomyopathy systematic review: Pathophysiologic process, clinical presentation and diagnostic approach to Takotsubo cardiomyopathy. Int J Cardiol. 2016;209:196-205. doi:10.1016/j.ijcard.2016.02.012.
17. Opolski G, Budnik M, Kochanowski J, Kowalik R, Piatkowski R, Kochman J. Four episodes of takotsubo cardiomyopathy in one patient. Int J Cardiol. 2016;203:53-54. doi:10.1016/j.ijcard.2015.10.048.
18. Templin C, Ghadri JR, Diekmann J, et al. Clinical features and outcomes of Takotsubo (stress) cardiomyopathy. N Engl J Med. 2015;373(10):929-938.
19. Gianni M, Dentali F, Grandi AM, Sumner G, Hiralal R, Lonn E. Apical ballooning syndrome or takotsubo cardiomyopathy: a systematic review. Eur Heart J. 2006;27(13):1523-1529. doi:10.1093/eurheartj/ehl032.
20. Citro R, Lyon AR, Meimoun P, et al. Standard and advanced echocardiography in Takotsubo (stress) cardiomyopathy: clinical and prognostic implications. J Am Soc Echocardiogr. 2015;28(1):57-74. doi:10.1016/j.echo.2014.08.020.
Emergency Ultrasound: Ultrasound-Guided Hip Arthrocentesis
Hip ultrasound has long been considered an effective diagnostic and interventional tool to identify hip effusions and perform guided arthrocentesis in patients with suspected septic arthritis. Although imaging and interventional techniques are typically performed by interventional radiologists, several case reports support the use of these techniques by the emergency physician (EP) in both pediatric and adult patients presenting with hip pain.1,2
Hip ultrasound permits rapid visualization of the joint space to assess the presence of a hip effusion, and provides the opportunity for the clinician to quickly perform hip arthrocentesis and to obtain synovial fluid for analysis—the current gold standard of diagnosis. The current literature shows treatment of effusion in the adult hip via ultrasound-guided interventional methods to be more convenient and less painful than traditional fluoroscopic-guided techniques, and to have the same procedural success rate.3 With the increasing utilization of point-of-care (POC) ultrasound in the ED, ultrasound-guided hip arthrocentesis has become a powerful tool in the EP’s armamentarium to aid in evaluating and treating patients in the ED presenting with hip pain.
Imaging Technique
To perform an ultrasound-guided arthrocentesis, the patient should be placed in the supine position, with both knees bent and the hips externally rotated in the frog leg position (Figure 1).
Arthrocentesis
When an effusion is present, arthrocentesis is warranted. To perform this procedure, the femoral vessels should be identified inferior to the inguinal ligament and avoided laterally. The hip should be prepared in a sterile fashion and a lubricated probe should be placed in a sterile dressing with a cord cover. The effusion should be visualized again, and the area should be anesthetized superficially and deeply with local anesthetic, aspirating prior to infusing at the deeper levels. An 18-gauge spinal needle affixed to a 20-mL syringe should be introduced and advanced while aspirating under direct visualization through the capsule of the hip into the effusion. The fluid is then aspirated and sent for laboratory analysis.
Summary
A delayed diagnosis of hip effusion and failure to initiate prompt treatment are the most common causes of late complications of septic arthritis.4 Point-of-care diagnostic and interventional ultrasound of the hip permit instant visualization and implementation of immediate diagnostic and therapeutic measures, which decrease morbidity in adult patients with septic arthritis. Hip arthrocentesis with subsequent synovial fluid analysis, the gold standard of diagnosis, has traditionally been performed by radiology services. Recent literature, however, has shown performance of these ultrasound-guided techniques by EPs to be safe and efficient, facilitating time to treatment.
1. Freeman K, Dewitz A, Baker WE. Ultrasound-guided hip arthrocentesis in the ED. Am J Emerg Med. 2007;25(1):80-86. doi:10.1016/j.ajem.2006.08.002.
2. Minardi JJ, Lander OM. Septic hip arthritis: diagnosis and arthrocentesis using bedside ultrasound. J Emerg Med. 2012;43(2):316-318. doi:10.1016/j.jemermed.2011.09.029.
3. Byrd JW, Potts EA, Allison RK, Jones KS. Ultrasound-guided hip injections: a comparative study with fluoroscopy-guided injections. Arthroscopy. 2014;30(1):42-46. doi:10.1016/j.arthro.2013.09.083.
4. Mascioli AA, Park AL. Infectious arthritis. In: Canale ST, Beaty JH eds. Campbell’s Operative Orthopaedics. Vol 1. 13th ed. Philadelphia, PA: Elsevier Mosby; 2013:749-772.
Hip ultrasound has long been considered an effective diagnostic and interventional tool to identify hip effusions and perform guided arthrocentesis in patients with suspected septic arthritis. Although imaging and interventional techniques are typically performed by interventional radiologists, several case reports support the use of these techniques by the emergency physician (EP) in both pediatric and adult patients presenting with hip pain.1,2
Hip ultrasound permits rapid visualization of the joint space to assess the presence of a hip effusion, and provides the opportunity for the clinician to quickly perform hip arthrocentesis and to obtain synovial fluid for analysis—the current gold standard of diagnosis. The current literature shows treatment of effusion in the adult hip via ultrasound-guided interventional methods to be more convenient and less painful than traditional fluoroscopic-guided techniques, and to have the same procedural success rate.3 With the increasing utilization of point-of-care (POC) ultrasound in the ED, ultrasound-guided hip arthrocentesis has become a powerful tool in the EP’s armamentarium to aid in evaluating and treating patients in the ED presenting with hip pain.
Imaging Technique
To perform an ultrasound-guided arthrocentesis, the patient should be placed in the supine position, with both knees bent and the hips externally rotated in the frog leg position (Figure 1).
Arthrocentesis
When an effusion is present, arthrocentesis is warranted. To perform this procedure, the femoral vessels should be identified inferior to the inguinal ligament and avoided laterally. The hip should be prepared in a sterile fashion and a lubricated probe should be placed in a sterile dressing with a cord cover. The effusion should be visualized again, and the area should be anesthetized superficially and deeply with local anesthetic, aspirating prior to infusing at the deeper levels. An 18-gauge spinal needle affixed to a 20-mL syringe should be introduced and advanced while aspirating under direct visualization through the capsule of the hip into the effusion. The fluid is then aspirated and sent for laboratory analysis.
Summary
A delayed diagnosis of hip effusion and failure to initiate prompt treatment are the most common causes of late complications of septic arthritis.4 Point-of-care diagnostic and interventional ultrasound of the hip permit instant visualization and implementation of immediate diagnostic and therapeutic measures, which decrease morbidity in adult patients with septic arthritis. Hip arthrocentesis with subsequent synovial fluid analysis, the gold standard of diagnosis, has traditionally been performed by radiology services. Recent literature, however, has shown performance of these ultrasound-guided techniques by EPs to be safe and efficient, facilitating time to treatment.
Hip ultrasound has long been considered an effective diagnostic and interventional tool to identify hip effusions and perform guided arthrocentesis in patients with suspected septic arthritis. Although imaging and interventional techniques are typically performed by interventional radiologists, several case reports support the use of these techniques by the emergency physician (EP) in both pediatric and adult patients presenting with hip pain.1,2
Hip ultrasound permits rapid visualization of the joint space to assess the presence of a hip effusion, and provides the opportunity for the clinician to quickly perform hip arthrocentesis and to obtain synovial fluid for analysis—the current gold standard of diagnosis. The current literature shows treatment of effusion in the adult hip via ultrasound-guided interventional methods to be more convenient and less painful than traditional fluoroscopic-guided techniques, and to have the same procedural success rate.3 With the increasing utilization of point-of-care (POC) ultrasound in the ED, ultrasound-guided hip arthrocentesis has become a powerful tool in the EP’s armamentarium to aid in evaluating and treating patients in the ED presenting with hip pain.
Imaging Technique
To perform an ultrasound-guided arthrocentesis, the patient should be placed in the supine position, with both knees bent and the hips externally rotated in the frog leg position (Figure 1).
Arthrocentesis
When an effusion is present, arthrocentesis is warranted. To perform this procedure, the femoral vessels should be identified inferior to the inguinal ligament and avoided laterally. The hip should be prepared in a sterile fashion and a lubricated probe should be placed in a sterile dressing with a cord cover. The effusion should be visualized again, and the area should be anesthetized superficially and deeply with local anesthetic, aspirating prior to infusing at the deeper levels. An 18-gauge spinal needle affixed to a 20-mL syringe should be introduced and advanced while aspirating under direct visualization through the capsule of the hip into the effusion. The fluid is then aspirated and sent for laboratory analysis.
Summary
A delayed diagnosis of hip effusion and failure to initiate prompt treatment are the most common causes of late complications of septic arthritis.4 Point-of-care diagnostic and interventional ultrasound of the hip permit instant visualization and implementation of immediate diagnostic and therapeutic measures, which decrease morbidity in adult patients with septic arthritis. Hip arthrocentesis with subsequent synovial fluid analysis, the gold standard of diagnosis, has traditionally been performed by radiology services. Recent literature, however, has shown performance of these ultrasound-guided techniques by EPs to be safe and efficient, facilitating time to treatment.
1. Freeman K, Dewitz A, Baker WE. Ultrasound-guided hip arthrocentesis in the ED. Am J Emerg Med. 2007;25(1):80-86. doi:10.1016/j.ajem.2006.08.002.
2. Minardi JJ, Lander OM. Septic hip arthritis: diagnosis and arthrocentesis using bedside ultrasound. J Emerg Med. 2012;43(2):316-318. doi:10.1016/j.jemermed.2011.09.029.
3. Byrd JW, Potts EA, Allison RK, Jones KS. Ultrasound-guided hip injections: a comparative study with fluoroscopy-guided injections. Arthroscopy. 2014;30(1):42-46. doi:10.1016/j.arthro.2013.09.083.
4. Mascioli AA, Park AL. Infectious arthritis. In: Canale ST, Beaty JH eds. Campbell’s Operative Orthopaedics. Vol 1. 13th ed. Philadelphia, PA: Elsevier Mosby; 2013:749-772.
1. Freeman K, Dewitz A, Baker WE. Ultrasound-guided hip arthrocentesis in the ED. Am J Emerg Med. 2007;25(1):80-86. doi:10.1016/j.ajem.2006.08.002.
2. Minardi JJ, Lander OM. Septic hip arthritis: diagnosis and arthrocentesis using bedside ultrasound. J Emerg Med. 2012;43(2):316-318. doi:10.1016/j.jemermed.2011.09.029.
3. Byrd JW, Potts EA, Allison RK, Jones KS. Ultrasound-guided hip injections: a comparative study with fluoroscopy-guided injections. Arthroscopy. 2014;30(1):42-46. doi:10.1016/j.arthro.2013.09.083.
4. Mascioli AA, Park AL. Infectious arthritis. In: Canale ST, Beaty JH eds. Campbell’s Operative Orthopaedics. Vol 1. 13th ed. Philadelphia, PA: Elsevier Mosby; 2013:749-772.
Imaging for Nonarthritic Hip Pathology
Take-Home Points
- Be sure to have a well centered AP pelvis without rotation.
- Get at least 3 plain radiographs—AP pelvis, false profile, and lateral hip view.
- Ensure that there is sufficient acetabular coverage, LCEA >20° on AP pelvis and ACEA >20° on false profile view.
- CT scans are helpful for precise hip pathomorphology but must be weighed against risk of radiation exposure.
- MRI or MRA can be helpful to diagnose intra-articular as well as extra-articular hip and pelvis abnormalities.
In the work-up for nonarthritic hip pain, the value of diagnostic imaging is in objective findings, which can support or weaken the leading diagnoses based on subjective complaints, recalled history, and, in some cases, elusive physical examination findings. Morphologic changes alone, however, do not always indicate pathology.1,2 At presentation and at each step in the work-up, it is imperative to evaluate the entire clinical picture. The prudent clinician uses both clinical and radiographic findings to make the diagnosis and direct treatment.
Radiography
The first step in diagnostic imaging is radiography. Although use of plain radiographs is routine, their value cannot be understated. Standard hip radiographs—an anteroposterior (AP) radiograph of the pelvis and AP and frog-leg (cross-table lateral) radiographs of the hip—provide a wealth of information.3-6
Evaluated first is the radiograph itself. For example, the ideal AP radiograph of the pelvis (Figure 1) is centered on the lower sacrum, and the patient is not rotated.
AP radiographs allow for evaluation of fractures, intraosseous sclerosis, acetabular depth, inclination and version, acetabular overcoverage, joint-space narrowing, femoroacetabular congruency, femoral head sphericity, and femoral head–neck offset.7,8,10 Inspection for labral calcification is important, as it can indicate repetitive damage at the extremes of range of motion.
On AP pelvis radiographs, it is important to distinguish coxa profunda from acetabular protrusion. These entities are on the same pathomorphologic spectrum and are similar but distinctively different. Coxa profunda refers to the depth of the acetabulum relative to the ilioischial line, and acetabular protrusion refers to the depth (or medial position) of the femoral head relative to the ilioischial line. Each condition suggests—but is not diagnostic for—pincer-type femoroacetabular impingement (FAI).11Acetabular rotation is another important entity that can be evaluated on well-centered, nontilted AP pelvic radiographs. Acetabular rotation refers to the opening direction of the acetabulum. It may be anterior (anteverted), neutral, or posterior (retroverted). Anteversion is present when the anterior acetabular rim does not traverse the posterior rim shadow4; in other words, the ring formed by the acetabulum is not twisted. When the walls overlap but do not intersect, the cup has neutral version. Retroversion is qualitatively determined by the crossover (figure-of-8) and posterior wall signs12 and is associated with pincer-type FAI and the development of hip osteoarthritis.12Dunn lateral radiographs (Figure 2A), taken with 90° hip flexion, were originally used to measure femoral neck anteversion.13
False-profile radiographs (Figure 6), valuable in evaluating anterior acetabular coverage and femoral head–neck junction morphology,14,15 allow characterization of both cam-type and pincer-type FAI.
Quantitative measures warrant specific consideration (Table). Femoroacetabular morphology is quantitatively measured by α angle, Tönnis angle (acetabular inclination angle), and lateral center-edge angle (LCEA).7,8,10 The α angle (Figure 4) detects the loss of normal anterosuperior femoral head–neck junction concavity caused by a convex osseous prominence. An α angle >50° represents a cam deformity.16 In a cohort study of 338 patients, Nepple and colleagues17 qualitatively associated increased α angle with severe intra-articular hip disease. Murphy and colleagues18 found a Tönnis angle >15° to be a poor prognostic factor in untreated hip dysplasia. LCEA quantifies superolateral femoral head coverage,19 and its normal range is 20° to 40°.20 LCEA <20° indicates dysplasia of the femoroacetabular joint, and LCEA >40° indicates overcoverage and pincer-type FAI. As with any quantitative radiographic measurement, results should be interpreted within the presenting clinical context.
Radiographic findings, even findings based on these special radiographs, may underestimate the pathologic process.
Computed Tomography
The benefits of computed tomography (CT) outweigh the risk of radiation exposure. CT is most useful in characterizing osseous morphology.21 In FAI cases, CT can distinguish acetabular version abnormalities from femoral torsion (Figures 7A-7C), entities with very different treatment approaches.21
Magnetic Resonance Imaging
MRI is becoming essential in the work-up for nonarthritic hip pain.11,22 It is used for assessment of osseous, chondral, and musculotendinous soft tissues. Further, it affords appreciation of outside-the-hip-joint pathology that may mimic joint-centered pathology.
MRI techniques range from noncontrast to indirect and direct magnetic resonance arthrography (MRA).22 Indirect MRA is performed with contrast medium administered through an intravenous line. Direct MRA has contrast administered intra-articularly and is more sensitive and specific for labral tears and ligamentous injury.23 Excellent detection of intra-articular pathology on noncontrast studies questions the need for MRA.24 Nevertheless, direct MRA can also be used as a therapeutic procedure when lidocaine is included in the injected gadolinium.
Labral tears, focal chondral defects, and stress or insufficiency fractures are important differentials in the work-up for nonarthritic hip pain. Over the dysplasia-to-FAI spectrum, MRI distinguishes symptomatic pathoanatomy from asymptomatic anatomical variants by revealing underlying bone edema. Capsule findings should also be considered.21The most practical classification of labral tears, proposed by Blankenbaker and colleagues,25 is based on tear type (frayed, unstable, flap), location, and extent. More than half of labral tears occur in the anterosuperior quadrant of the labrum.25
Chondral damage is identified much as labral tears are. With chondral injury, the normal intermediate signal is interrupted by a fluid-intense signal extending to the subchondral bone. A fat-saturated T2or short-tau inversion recovery (STIR) sequence is useful in emphasizing this finding.27
MRI detects osseous pathology from surrounding soft-tissue edema and bone remodeling to stress and fragility fractures. In athletes, the most common fractures are pubic rami, sacral, and apophyseal avulsion fractures.28 In all patients, attention should be given to the lower spine and the proximal femurs. Aside from MRI, nuclear medicine bone scan might also identify active osseous reaction representative of a fracture.
Conclusion
The work-up for nonarthritic hip pain substantiates differential diagnoses. A case’s complexity determines the course of diagnostic imaging. At presentation and at each step in the work-up, it is imperative to evaluate the entire clinical picture. The prudent clinician uses both clinical and radiographic findings to make the diagnosis and direct treatment.
Am J Orthop . 2017;46(1):17-22. Copyright Frontline Medical Communications Inc. 2017. All rights reserved.
1. McCall DA, Safran MR. MRI and arthroscopy correlations of the hip: a case-based approach. Instr Course Lect . 2012;61:327-344.
2. Register B, Pennock AT, Ho CP, Strickland CD, Lawand A, Philippon MJ. Prevalence of abnormal hip findings in asymptomatic participants: a prospective, blinded study. Am J Sports Med . 2012;40(12):2720-2724.
3. Campbell SE. Radiography of the hip: lines, signs, and patterns of disease. Semin Roentgenol . 2005;40(3):290-319.
4. Clohisy JC, Carlisle JC, Beaulé PE, et al. A systematic approach to the plain radiographic evaluation of the young adult hip. J Bone Joint Surg Am . 2008;90(suppl 4):47-66.
5. Malviya A, Raza A, Witt JD. Reliability in the diagnosis of femoroacetabular impingement and dysplasia among hip surgeons: role of surgeon volume and experience. Hip Int . 2016;26(3):284-289.
6. Nepple JJ, Martel JM, Kim YJ, Zaltz I, Clohisy JC, Group AS. Do plain radiographs correlate with CT for imaging of cam-type femoroacetabular impingement? Clin Orthop Relat Res . 2012;470(12):3313-3320.
7. Kosuge D, Cordier T, Solomon LB, Howie DW. Dilemmas in imaging for peri-acetabular osteotomy: the influence of patient position and imaging technique on the radiological features of hip dysplasia. Bone Joint J . 2014;96(9):1155-1160.
8. Tannast M, Fritsch S, Zheng G, Siebenrock KA, Steppacher SD. Which radiographic hip parameters do not have to be corrected for pelvic rotation and tilt? Clin Orthop Relat Res . 2015;473(4):1255-1266.
9. Siebenrock KA, Kalbermatten DF, Ganz R. Effect of pelvic tilt on acetabular retroversion: a study of pelves from cadavers. Clin Orthop Relat Res . 2003;(407):241-248.
10. Griffin JW, Weber AE, Kuhns B, Lewis P, Nho SJ. Imaging in hip arthroscopy for femoroacetabular impingement: a comprehensive approach. Clin Sports Med . 2016;35(3):331-344.
11. Nepple JJ, Lehmann CL, Ross JR, Schoenecker PL, Clohisy JC. Coxa profunda is not a useful radiographic parameter for diagnosing pincer-type femoroacetabular impingement. J Bone Joint Surg Am . 2013;95(5):417-423.
12. Reynolds D, Lucas J, Klaue K. Retroversion of the acetabulum. A cause of hip pain. J Bone Joint Surg Br . 1999;81(2):281-288.
13. Dunn DM. Anteversion of the neck of the femur; a method of measurement. J Bone Joint Surg Br . 1952;34(2):181-186.
14. Meyer DC, Beck M, Ellis T, Ganz R, Leunig M. Comparison of six radiographic projections to assess femoral head/neck asphericity. Clin Orthop Relat Res . 2006;(445):181-185.
15. Hellman MD, Mascarenhas R, Gupta A, et al. The false-profile view may be used to identify cam morphology. Arthroscopy . 2015;31(9):1728-1732.
16. Barton C, Salineros MJ, Rakhra KS, Beaulé PE. Validity of the alpha angle measurement on plain radiographs in the evaluation of cam-type femoroacetabular impingement. Clin Orthop Relat Res . 2011;469(2):464-469.
17. Nepple JJ, Carlisle JC, Nunley RM, Clohisy JC. Clinical and radiographic predictors of intra-articular hip disease in arthroscopy. Am J Sports Med . 2011;39(2):296-303.
18. Murphy SB, Ganz R, Muller ME. The prognosis in untreated dysplasia of the hip. A study of radiographic factors that predict the outcome. J Bone Joint Surg Am . 1995;77(7):985-989.
19. Mast NH, Impellizzeri F, Keller S, Leunig M. Reliability and agreement of measures used in radiographic evaluation of the adult hip. Clin Orthop Relat Res . 2011;469(1):188-199.
20. Monazzam S, Bomar JD, Cidambi K, Kruk P, Hosalkar H. Lateral center-edge angle on conventional radiography and computed tomography. Clin Orthop Relat Res . 2013;471(7):2233-2237.
21. Weber AE, Jacobson JA, Bedi A. A review of imaging modalities for the hip. Curr Rev Musculoskelet Med . 2013;6(3):226-234.
22. Bencardino JT, Palmer WE. Imaging of hip disorders in athletes. Radiol Clin North Am . 2002;40(2):267-287, vi-vii.
23. Byrd JW, Jones KS. Diagnostic accuracy of clinical assessment, magnetic resonance imaging, magnetic resonance arthrography, and intra-articular injection in hip arthroscopy patients. Am J Sports Med . 2004;32(7):1668-1674.
24. Mintz DN, Hooper T, Connell D, Buly R, Padgett DE, Potter HG. Magnetic resonance imaging of the hip: detection of labral and chondral abnormalities using noncontrast imaging. Arthroscopy . 2005;21(4):385-393.
25. Blankenbaker DG, De Smet AA, Keene JS, Fine JP. Classification and localization of acetabular labral tears. Skeletal Radiol . 2007;36(5):391-397.
26. Aydingöz U, Oztürk MH. MR imaging of the acetabular labrum: a comparative study of both hips in 180 asymptomatic volunteers. Eur Radiol . 2001;11(4):567-574.
27. Gold GE, Chen CA, Koo S, Hargreaves BA, Bangerter NK. Recent advances in MRI of articular cartilage. AJR Am J Roentgenol . 2009;193(3):628-638.
28. Liong SY, Whitehouse RW. Lower extremity and pelvic stress fractures in athletes. Br J Radiol . 2012;85(1016):1148-1156.
Take-Home Points
- Be sure to have a well centered AP pelvis without rotation.
- Get at least 3 plain radiographs—AP pelvis, false profile, and lateral hip view.
- Ensure that there is sufficient acetabular coverage, LCEA >20° on AP pelvis and ACEA >20° on false profile view.
- CT scans are helpful for precise hip pathomorphology but must be weighed against risk of radiation exposure.
- MRI or MRA can be helpful to diagnose intra-articular as well as extra-articular hip and pelvis abnormalities.
In the work-up for nonarthritic hip pain, the value of diagnostic imaging is in objective findings, which can support or weaken the leading diagnoses based on subjective complaints, recalled history, and, in some cases, elusive physical examination findings. Morphologic changes alone, however, do not always indicate pathology.1,2 At presentation and at each step in the work-up, it is imperative to evaluate the entire clinical picture. The prudent clinician uses both clinical and radiographic findings to make the diagnosis and direct treatment.
Radiography
The first step in diagnostic imaging is radiography. Although use of plain radiographs is routine, their value cannot be understated. Standard hip radiographs—an anteroposterior (AP) radiograph of the pelvis and AP and frog-leg (cross-table lateral) radiographs of the hip—provide a wealth of information.3-6
Evaluated first is the radiograph itself. For example, the ideal AP radiograph of the pelvis (Figure 1) is centered on the lower sacrum, and the patient is not rotated.
AP radiographs allow for evaluation of fractures, intraosseous sclerosis, acetabular depth, inclination and version, acetabular overcoverage, joint-space narrowing, femoroacetabular congruency, femoral head sphericity, and femoral head–neck offset.7,8,10 Inspection for labral calcification is important, as it can indicate repetitive damage at the extremes of range of motion.
On AP pelvis radiographs, it is important to distinguish coxa profunda from acetabular protrusion. These entities are on the same pathomorphologic spectrum and are similar but distinctively different. Coxa profunda refers to the depth of the acetabulum relative to the ilioischial line, and acetabular protrusion refers to the depth (or medial position) of the femoral head relative to the ilioischial line. Each condition suggests—but is not diagnostic for—pincer-type femoroacetabular impingement (FAI).11Acetabular rotation is another important entity that can be evaluated on well-centered, nontilted AP pelvic radiographs. Acetabular rotation refers to the opening direction of the acetabulum. It may be anterior (anteverted), neutral, or posterior (retroverted). Anteversion is present when the anterior acetabular rim does not traverse the posterior rim shadow4; in other words, the ring formed by the acetabulum is not twisted. When the walls overlap but do not intersect, the cup has neutral version. Retroversion is qualitatively determined by the crossover (figure-of-8) and posterior wall signs12 and is associated with pincer-type FAI and the development of hip osteoarthritis.12Dunn lateral radiographs (Figure 2A), taken with 90° hip flexion, were originally used to measure femoral neck anteversion.13
False-profile radiographs (Figure 6), valuable in evaluating anterior acetabular coverage and femoral head–neck junction morphology,14,15 allow characterization of both cam-type and pincer-type FAI.
Quantitative measures warrant specific consideration (Table). Femoroacetabular morphology is quantitatively measured by α angle, Tönnis angle (acetabular inclination angle), and lateral center-edge angle (LCEA).7,8,10 The α angle (Figure 4) detects the loss of normal anterosuperior femoral head–neck junction concavity caused by a convex osseous prominence. An α angle >50° represents a cam deformity.16 In a cohort study of 338 patients, Nepple and colleagues17 qualitatively associated increased α angle with severe intra-articular hip disease. Murphy and colleagues18 found a Tönnis angle >15° to be a poor prognostic factor in untreated hip dysplasia. LCEA quantifies superolateral femoral head coverage,19 and its normal range is 20° to 40°.20 LCEA <20° indicates dysplasia of the femoroacetabular joint, and LCEA >40° indicates overcoverage and pincer-type FAI. As with any quantitative radiographic measurement, results should be interpreted within the presenting clinical context.
Radiographic findings, even findings based on these special radiographs, may underestimate the pathologic process.
Computed Tomography
The benefits of computed tomography (CT) outweigh the risk of radiation exposure. CT is most useful in characterizing osseous morphology.21 In FAI cases, CT can distinguish acetabular version abnormalities from femoral torsion (Figures 7A-7C), entities with very different treatment approaches.21
Magnetic Resonance Imaging
MRI is becoming essential in the work-up for nonarthritic hip pain.11,22 It is used for assessment of osseous, chondral, and musculotendinous soft tissues. Further, it affords appreciation of outside-the-hip-joint pathology that may mimic joint-centered pathology.
MRI techniques range from noncontrast to indirect and direct magnetic resonance arthrography (MRA).22 Indirect MRA is performed with contrast medium administered through an intravenous line. Direct MRA has contrast administered intra-articularly and is more sensitive and specific for labral tears and ligamentous injury.23 Excellent detection of intra-articular pathology on noncontrast studies questions the need for MRA.24 Nevertheless, direct MRA can also be used as a therapeutic procedure when lidocaine is included in the injected gadolinium.
Labral tears, focal chondral defects, and stress or insufficiency fractures are important differentials in the work-up for nonarthritic hip pain. Over the dysplasia-to-FAI spectrum, MRI distinguishes symptomatic pathoanatomy from asymptomatic anatomical variants by revealing underlying bone edema. Capsule findings should also be considered.21The most practical classification of labral tears, proposed by Blankenbaker and colleagues,25 is based on tear type (frayed, unstable, flap), location, and extent. More than half of labral tears occur in the anterosuperior quadrant of the labrum.25
Chondral damage is identified much as labral tears are. With chondral injury, the normal intermediate signal is interrupted by a fluid-intense signal extending to the subchondral bone. A fat-saturated T2or short-tau inversion recovery (STIR) sequence is useful in emphasizing this finding.27
MRI detects osseous pathology from surrounding soft-tissue edema and bone remodeling to stress and fragility fractures. In athletes, the most common fractures are pubic rami, sacral, and apophyseal avulsion fractures.28 In all patients, attention should be given to the lower spine and the proximal femurs. Aside from MRI, nuclear medicine bone scan might also identify active osseous reaction representative of a fracture.
Conclusion
The work-up for nonarthritic hip pain substantiates differential diagnoses. A case’s complexity determines the course of diagnostic imaging. At presentation and at each step in the work-up, it is imperative to evaluate the entire clinical picture. The prudent clinician uses both clinical and radiographic findings to make the diagnosis and direct treatment.
Am J Orthop . 2017;46(1):17-22. Copyright Frontline Medical Communications Inc. 2017. All rights reserved.
Take-Home Points
- Be sure to have a well centered AP pelvis without rotation.
- Get at least 3 plain radiographs—AP pelvis, false profile, and lateral hip view.
- Ensure that there is sufficient acetabular coverage, LCEA >20° on AP pelvis and ACEA >20° on false profile view.
- CT scans are helpful for precise hip pathomorphology but must be weighed against risk of radiation exposure.
- MRI or MRA can be helpful to diagnose intra-articular as well as extra-articular hip and pelvis abnormalities.
In the work-up for nonarthritic hip pain, the value of diagnostic imaging is in objective findings, which can support or weaken the leading diagnoses based on subjective complaints, recalled history, and, in some cases, elusive physical examination findings. Morphologic changes alone, however, do not always indicate pathology.1,2 At presentation and at each step in the work-up, it is imperative to evaluate the entire clinical picture. The prudent clinician uses both clinical and radiographic findings to make the diagnosis and direct treatment.
Radiography
The first step in diagnostic imaging is radiography. Although use of plain radiographs is routine, their value cannot be understated. Standard hip radiographs—an anteroposterior (AP) radiograph of the pelvis and AP and frog-leg (cross-table lateral) radiographs of the hip—provide a wealth of information.3-6
Evaluated first is the radiograph itself. For example, the ideal AP radiograph of the pelvis (Figure 1) is centered on the lower sacrum, and the patient is not rotated.
AP radiographs allow for evaluation of fractures, intraosseous sclerosis, acetabular depth, inclination and version, acetabular overcoverage, joint-space narrowing, femoroacetabular congruency, femoral head sphericity, and femoral head–neck offset.7,8,10 Inspection for labral calcification is important, as it can indicate repetitive damage at the extremes of range of motion.
On AP pelvis radiographs, it is important to distinguish coxa profunda from acetabular protrusion. These entities are on the same pathomorphologic spectrum and are similar but distinctively different. Coxa profunda refers to the depth of the acetabulum relative to the ilioischial line, and acetabular protrusion refers to the depth (or medial position) of the femoral head relative to the ilioischial line. Each condition suggests—but is not diagnostic for—pincer-type femoroacetabular impingement (FAI).11Acetabular rotation is another important entity that can be evaluated on well-centered, nontilted AP pelvic radiographs. Acetabular rotation refers to the opening direction of the acetabulum. It may be anterior (anteverted), neutral, or posterior (retroverted). Anteversion is present when the anterior acetabular rim does not traverse the posterior rim shadow4; in other words, the ring formed by the acetabulum is not twisted. When the walls overlap but do not intersect, the cup has neutral version. Retroversion is qualitatively determined by the crossover (figure-of-8) and posterior wall signs12 and is associated with pincer-type FAI and the development of hip osteoarthritis.12Dunn lateral radiographs (Figure 2A), taken with 90° hip flexion, were originally used to measure femoral neck anteversion.13
False-profile radiographs (Figure 6), valuable in evaluating anterior acetabular coverage and femoral head–neck junction morphology,14,15 allow characterization of both cam-type and pincer-type FAI.
Quantitative measures warrant specific consideration (Table). Femoroacetabular morphology is quantitatively measured by α angle, Tönnis angle (acetabular inclination angle), and lateral center-edge angle (LCEA).7,8,10 The α angle (Figure 4) detects the loss of normal anterosuperior femoral head–neck junction concavity caused by a convex osseous prominence. An α angle >50° represents a cam deformity.16 In a cohort study of 338 patients, Nepple and colleagues17 qualitatively associated increased α angle with severe intra-articular hip disease. Murphy and colleagues18 found a Tönnis angle >15° to be a poor prognostic factor in untreated hip dysplasia. LCEA quantifies superolateral femoral head coverage,19 and its normal range is 20° to 40°.20 LCEA <20° indicates dysplasia of the femoroacetabular joint, and LCEA >40° indicates overcoverage and pincer-type FAI. As with any quantitative radiographic measurement, results should be interpreted within the presenting clinical context.
Radiographic findings, even findings based on these special radiographs, may underestimate the pathologic process.
Computed Tomography
The benefits of computed tomography (CT) outweigh the risk of radiation exposure. CT is most useful in characterizing osseous morphology.21 In FAI cases, CT can distinguish acetabular version abnormalities from femoral torsion (Figures 7A-7C), entities with very different treatment approaches.21
Magnetic Resonance Imaging
MRI is becoming essential in the work-up for nonarthritic hip pain.11,22 It is used for assessment of osseous, chondral, and musculotendinous soft tissues. Further, it affords appreciation of outside-the-hip-joint pathology that may mimic joint-centered pathology.
MRI techniques range from noncontrast to indirect and direct magnetic resonance arthrography (MRA).22 Indirect MRA is performed with contrast medium administered through an intravenous line. Direct MRA has contrast administered intra-articularly and is more sensitive and specific for labral tears and ligamentous injury.23 Excellent detection of intra-articular pathology on noncontrast studies questions the need for MRA.24 Nevertheless, direct MRA can also be used as a therapeutic procedure when lidocaine is included in the injected gadolinium.
Labral tears, focal chondral defects, and stress or insufficiency fractures are important differentials in the work-up for nonarthritic hip pain. Over the dysplasia-to-FAI spectrum, MRI distinguishes symptomatic pathoanatomy from asymptomatic anatomical variants by revealing underlying bone edema. Capsule findings should also be considered.21The most practical classification of labral tears, proposed by Blankenbaker and colleagues,25 is based on tear type (frayed, unstable, flap), location, and extent. More than half of labral tears occur in the anterosuperior quadrant of the labrum.25
Chondral damage is identified much as labral tears are. With chondral injury, the normal intermediate signal is interrupted by a fluid-intense signal extending to the subchondral bone. A fat-saturated T2or short-tau inversion recovery (STIR) sequence is useful in emphasizing this finding.27
MRI detects osseous pathology from surrounding soft-tissue edema and bone remodeling to stress and fragility fractures. In athletes, the most common fractures are pubic rami, sacral, and apophyseal avulsion fractures.28 In all patients, attention should be given to the lower spine and the proximal femurs. Aside from MRI, nuclear medicine bone scan might also identify active osseous reaction representative of a fracture.
Conclusion
The work-up for nonarthritic hip pain substantiates differential diagnoses. A case’s complexity determines the course of diagnostic imaging. At presentation and at each step in the work-up, it is imperative to evaluate the entire clinical picture. The prudent clinician uses both clinical and radiographic findings to make the diagnosis and direct treatment.
Am J Orthop . 2017;46(1):17-22. Copyright Frontline Medical Communications Inc. 2017. All rights reserved.
1. McCall DA, Safran MR. MRI and arthroscopy correlations of the hip: a case-based approach. Instr Course Lect . 2012;61:327-344.
2. Register B, Pennock AT, Ho CP, Strickland CD, Lawand A, Philippon MJ. Prevalence of abnormal hip findings in asymptomatic participants: a prospective, blinded study. Am J Sports Med . 2012;40(12):2720-2724.
3. Campbell SE. Radiography of the hip: lines, signs, and patterns of disease. Semin Roentgenol . 2005;40(3):290-319.
4. Clohisy JC, Carlisle JC, Beaulé PE, et al. A systematic approach to the plain radiographic evaluation of the young adult hip. J Bone Joint Surg Am . 2008;90(suppl 4):47-66.
5. Malviya A, Raza A, Witt JD. Reliability in the diagnosis of femoroacetabular impingement and dysplasia among hip surgeons: role of surgeon volume and experience. Hip Int . 2016;26(3):284-289.
6. Nepple JJ, Martel JM, Kim YJ, Zaltz I, Clohisy JC, Group AS. Do plain radiographs correlate with CT for imaging of cam-type femoroacetabular impingement? Clin Orthop Relat Res . 2012;470(12):3313-3320.
7. Kosuge D, Cordier T, Solomon LB, Howie DW. Dilemmas in imaging for peri-acetabular osteotomy: the influence of patient position and imaging technique on the radiological features of hip dysplasia. Bone Joint J . 2014;96(9):1155-1160.
8. Tannast M, Fritsch S, Zheng G, Siebenrock KA, Steppacher SD. Which radiographic hip parameters do not have to be corrected for pelvic rotation and tilt? Clin Orthop Relat Res . 2015;473(4):1255-1266.
9. Siebenrock KA, Kalbermatten DF, Ganz R. Effect of pelvic tilt on acetabular retroversion: a study of pelves from cadavers. Clin Orthop Relat Res . 2003;(407):241-248.
10. Griffin JW, Weber AE, Kuhns B, Lewis P, Nho SJ. Imaging in hip arthroscopy for femoroacetabular impingement: a comprehensive approach. Clin Sports Med . 2016;35(3):331-344.
11. Nepple JJ, Lehmann CL, Ross JR, Schoenecker PL, Clohisy JC. Coxa profunda is not a useful radiographic parameter for diagnosing pincer-type femoroacetabular impingement. J Bone Joint Surg Am . 2013;95(5):417-423.
12. Reynolds D, Lucas J, Klaue K. Retroversion of the acetabulum. A cause of hip pain. J Bone Joint Surg Br . 1999;81(2):281-288.
13. Dunn DM. Anteversion of the neck of the femur; a method of measurement. J Bone Joint Surg Br . 1952;34(2):181-186.
14. Meyer DC, Beck M, Ellis T, Ganz R, Leunig M. Comparison of six radiographic projections to assess femoral head/neck asphericity. Clin Orthop Relat Res . 2006;(445):181-185.
15. Hellman MD, Mascarenhas R, Gupta A, et al. The false-profile view may be used to identify cam morphology. Arthroscopy . 2015;31(9):1728-1732.
16. Barton C, Salineros MJ, Rakhra KS, Beaulé PE. Validity of the alpha angle measurement on plain radiographs in the evaluation of cam-type femoroacetabular impingement. Clin Orthop Relat Res . 2011;469(2):464-469.
17. Nepple JJ, Carlisle JC, Nunley RM, Clohisy JC. Clinical and radiographic predictors of intra-articular hip disease in arthroscopy. Am J Sports Med . 2011;39(2):296-303.
18. Murphy SB, Ganz R, Muller ME. The prognosis in untreated dysplasia of the hip. A study of radiographic factors that predict the outcome. J Bone Joint Surg Am . 1995;77(7):985-989.
19. Mast NH, Impellizzeri F, Keller S, Leunig M. Reliability and agreement of measures used in radiographic evaluation of the adult hip. Clin Orthop Relat Res . 2011;469(1):188-199.
20. Monazzam S, Bomar JD, Cidambi K, Kruk P, Hosalkar H. Lateral center-edge angle on conventional radiography and computed tomography. Clin Orthop Relat Res . 2013;471(7):2233-2237.
21. Weber AE, Jacobson JA, Bedi A. A review of imaging modalities for the hip. Curr Rev Musculoskelet Med . 2013;6(3):226-234.
22. Bencardino JT, Palmer WE. Imaging of hip disorders in athletes. Radiol Clin North Am . 2002;40(2):267-287, vi-vii.
23. Byrd JW, Jones KS. Diagnostic accuracy of clinical assessment, magnetic resonance imaging, magnetic resonance arthrography, and intra-articular injection in hip arthroscopy patients. Am J Sports Med . 2004;32(7):1668-1674.
24. Mintz DN, Hooper T, Connell D, Buly R, Padgett DE, Potter HG. Magnetic resonance imaging of the hip: detection of labral and chondral abnormalities using noncontrast imaging. Arthroscopy . 2005;21(4):385-393.
25. Blankenbaker DG, De Smet AA, Keene JS, Fine JP. Classification and localization of acetabular labral tears. Skeletal Radiol . 2007;36(5):391-397.
26. Aydingöz U, Oztürk MH. MR imaging of the acetabular labrum: a comparative study of both hips in 180 asymptomatic volunteers. Eur Radiol . 2001;11(4):567-574.
27. Gold GE, Chen CA, Koo S, Hargreaves BA, Bangerter NK. Recent advances in MRI of articular cartilage. AJR Am J Roentgenol . 2009;193(3):628-638.
28. Liong SY, Whitehouse RW. Lower extremity and pelvic stress fractures in athletes. Br J Radiol . 2012;85(1016):1148-1156.
1. McCall DA, Safran MR. MRI and arthroscopy correlations of the hip: a case-based approach. Instr Course Lect . 2012;61:327-344.
2. Register B, Pennock AT, Ho CP, Strickland CD, Lawand A, Philippon MJ. Prevalence of abnormal hip findings in asymptomatic participants: a prospective, blinded study. Am J Sports Med . 2012;40(12):2720-2724.
3. Campbell SE. Radiography of the hip: lines, signs, and patterns of disease. Semin Roentgenol . 2005;40(3):290-319.
4. Clohisy JC, Carlisle JC, Beaulé PE, et al. A systematic approach to the plain radiographic evaluation of the young adult hip. J Bone Joint Surg Am . 2008;90(suppl 4):47-66.
5. Malviya A, Raza A, Witt JD. Reliability in the diagnosis of femoroacetabular impingement and dysplasia among hip surgeons: role of surgeon volume and experience. Hip Int . 2016;26(3):284-289.
6. Nepple JJ, Martel JM, Kim YJ, Zaltz I, Clohisy JC, Group AS. Do plain radiographs correlate with CT for imaging of cam-type femoroacetabular impingement? Clin Orthop Relat Res . 2012;470(12):3313-3320.
7. Kosuge D, Cordier T, Solomon LB, Howie DW. Dilemmas in imaging for peri-acetabular osteotomy: the influence of patient position and imaging technique on the radiological features of hip dysplasia. Bone Joint J . 2014;96(9):1155-1160.
8. Tannast M, Fritsch S, Zheng G, Siebenrock KA, Steppacher SD. Which radiographic hip parameters do not have to be corrected for pelvic rotation and tilt? Clin Orthop Relat Res . 2015;473(4):1255-1266.
9. Siebenrock KA, Kalbermatten DF, Ganz R. Effect of pelvic tilt on acetabular retroversion: a study of pelves from cadavers. Clin Orthop Relat Res . 2003;(407):241-248.
10. Griffin JW, Weber AE, Kuhns B, Lewis P, Nho SJ. Imaging in hip arthroscopy for femoroacetabular impingement: a comprehensive approach. Clin Sports Med . 2016;35(3):331-344.
11. Nepple JJ, Lehmann CL, Ross JR, Schoenecker PL, Clohisy JC. Coxa profunda is not a useful radiographic parameter for diagnosing pincer-type femoroacetabular impingement. J Bone Joint Surg Am . 2013;95(5):417-423.
12. Reynolds D, Lucas J, Klaue K. Retroversion of the acetabulum. A cause of hip pain. J Bone Joint Surg Br . 1999;81(2):281-288.
13. Dunn DM. Anteversion of the neck of the femur; a method of measurement. J Bone Joint Surg Br . 1952;34(2):181-186.
14. Meyer DC, Beck M, Ellis T, Ganz R, Leunig M. Comparison of six radiographic projections to assess femoral head/neck asphericity. Clin Orthop Relat Res . 2006;(445):181-185.
15. Hellman MD, Mascarenhas R, Gupta A, et al. The false-profile view may be used to identify cam morphology. Arthroscopy . 2015;31(9):1728-1732.
16. Barton C, Salineros MJ, Rakhra KS, Beaulé PE. Validity of the alpha angle measurement on plain radiographs in the evaluation of cam-type femoroacetabular impingement. Clin Orthop Relat Res . 2011;469(2):464-469.
17. Nepple JJ, Carlisle JC, Nunley RM, Clohisy JC. Clinical and radiographic predictors of intra-articular hip disease in arthroscopy. Am J Sports Med . 2011;39(2):296-303.
18. Murphy SB, Ganz R, Muller ME. The prognosis in untreated dysplasia of the hip. A study of radiographic factors that predict the outcome. J Bone Joint Surg Am . 1995;77(7):985-989.
19. Mast NH, Impellizzeri F, Keller S, Leunig M. Reliability and agreement of measures used in radiographic evaluation of the adult hip. Clin Orthop Relat Res . 2011;469(1):188-199.
20. Monazzam S, Bomar JD, Cidambi K, Kruk P, Hosalkar H. Lateral center-edge angle on conventional radiography and computed tomography. Clin Orthop Relat Res . 2013;471(7):2233-2237.
21. Weber AE, Jacobson JA, Bedi A. A review of imaging modalities for the hip. Curr Rev Musculoskelet Med . 2013;6(3):226-234.
22. Bencardino JT, Palmer WE. Imaging of hip disorders in athletes. Radiol Clin North Am . 2002;40(2):267-287, vi-vii.
23. Byrd JW, Jones KS. Diagnostic accuracy of clinical assessment, magnetic resonance imaging, magnetic resonance arthrography, and intra-articular injection in hip arthroscopy patients. Am J Sports Med . 2004;32(7):1668-1674.
24. Mintz DN, Hooper T, Connell D, Buly R, Padgett DE, Potter HG. Magnetic resonance imaging of the hip: detection of labral and chondral abnormalities using noncontrast imaging. Arthroscopy . 2005;21(4):385-393.
25. Blankenbaker DG, De Smet AA, Keene JS, Fine JP. Classification and localization of acetabular labral tears. Skeletal Radiol . 2007;36(5):391-397.
26. Aydingöz U, Oztürk MH. MR imaging of the acetabular labrum: a comparative study of both hips in 180 asymptomatic volunteers. Eur Radiol . 2001;11(4):567-574.
27. Gold GE, Chen CA, Koo S, Hargreaves BA, Bangerter NK. Recent advances in MRI of articular cartilage. AJR Am J Roentgenol . 2009;193(3):628-638.
28. Liong SY, Whitehouse RW. Lower extremity and pelvic stress fractures in athletes. Br J Radiol . 2012;85(1016):1148-1156.
Imaging suggestive, but symptoms atypical
A 66-year-old man with chronic obstructive pulmonary disease (COPD) was brought to the emergency department with a 2-week history of progressive dyspnea followed by altered mental status. He had no history of diabetes mellitus, hypertension, or drug abuse.
On physical examination, he was stuporous. He had no fever or hypotension, but his pulse and breathing were rapid, and he had central cyanosis, bilateral conjunctival congestion, a puffy face, generalized wheezing, basilar crackles in both lungs, and leg edema.
Laboratory testing showed hypoxia and severe hypercarbia. His hematocrit was 65% (reference range 39–51) and his hemoglobin level was 21.5 g/dL (13–17).
The patient was diagnosed with an exacerbation of COPD. He was intubated, placed on mechanical ventilation, and admitted to the intensive care unit.
Computed tomography (CT) performed because of his decreased level of consciousness (Figure 1) showed increased attenuation in the ambient cistern and the lateral aspect of the lateral cerebral fissure, suggesting subarachnoid hemorrhage. The attenuation value in these areas was 89 Hounsfield units (typical values for brain tissue are in the 20s to 30s, and for blood in the 30s to 40s). To further evaluate for subarachnoid hemorrhage, lumbar puncture was performed, but analysis of the fluid sample showed normal protein and glucose levels and no cells.
Based on the results of cerebrospinal fluid evaluation and on the CT attenuation value, a diagnosis of pseudosubarachnoid hemorrhage due to polycythemia was made.
SUBARACHNOID VS PSEUDOSUBARACHNOID HEMORRHAGE
Subarachnoid hemorrhage typically begins with a “thunder-clap” headache (beginning suddenly and described by patients as “the worst headache ever.”) While not all patients have this presentation, if imaging suggests subarachnoid hemorrhage but the patient has atypical signs and symptoms (eg, other than headache), then pseudosubarachnoid hemorrhage should be considered.
Brain CT is one of the most reliable tools for diagnosing subarachnoid hemorrhage in the emergency department. Done within 6 hours of symptom onset, it has a sensitivity of 98.7% and a specificity of 99.9%.1 Magnetic resonance imaging can also visualize subarachnoid hemorrhage within the first 12 hours, typically as a hyperintensity in the subarachnoid space on fluid-attenuated inversion-recovery sequences2 and on susceptibility-weighted sequences.
Lumbar puncture is also an important diagnostic tool but carries a risk of brain herniation in patients with brain edema.
Pseudosubarachnoid hemorrhage is an artifact of CT imaging. It is rare, and its prevalence is unknown.3 However, it may be seen in up to 20% of patients after resuscitation, as a result of diffuse cerebral edema that lowers the attenuation of brain tissue on CT, making the vessels relatively conspicuous. It can also be seen in purulent meningitis (due to proteinaceous influx after blood-brain barrier disruption),4 in meningeal leukemia (due to increased cellularity in the leptomeninges), and in severe polycythemia (from a higher concentration of blood and hemoglobin in the vessels).3,5–7
Although the level of attenuation on CT may help distinguish subarachnoid from pseudosubarachnoid hemorrhage, its accuracy has not been defined. Inspecting the CT images may clarify whether areas with high attenuation look like blood vessels vs subarachnoid hemorrhage.
Our patient recovered and had an uneventful follow-up. The cause of his elevated hematocrit was likely chronic hypoxia from COPD.
Acknowledgment: We thank Dr. Saeide Khanbagi and Dr. Azade Nasr-lari for their cooperation.
- Dubosh NM, Bellolio MF, Rabinstein AA, Edlow JA. Sensitivity of early brain computed tomography to exclude aneurysmal subarachnoid hemorrhage: a systematic review and meta-analysis. Stroke 2016; 47:750–755.
- Sohn CH, Baik SK, Lee HJ, et al. MR imaging of hyperacute subarachnoid and intraventricular hemorrhage at 3T: a preliminary report of gradient echo T2*-weighted sequences. AJNR Am J Neuroradiol 2005; 26:662–665.
- Yuzawa H, Higano S, Mugikura S, et al. Pseudo-subarachnoid hemorrhage found in patients with postresuscitation encephalopathy: characteristics of CT findings and clinical importance. AJNR Am J Neuroradiol 2008; 29:1544–1549.
- Given CA 2nd, Burdette JH, Elster AD, Williams DW 3rd. Pseudo-subarachnoid hemorrhage: a potential imaging pitfall associated with diffuse cerebral edema. AJNR Am J Neuroradiol 2003; 24:254–256.
- Avrahami E, Katz R, Rabin A, Friedman V. CT diagnosis of non-traumatic subarachnoid haemorrhage in patients with brain edema. Eur J Radiol 1998; 28:222–225.
- Ben Salem D, Osseby GV, Rezaizadeh-Bourdariat K, et al. Spontaneous hyperdense intracranial vessels seen on CT scan in polycythemia cases. J Radiol 2003; 84:605–608. French.
- Hsieh SW, Khor GT, Chen CN, Huang P. Pseudo subarachnoid hemorrhage in meningeal leukemia. J Emerg Med 2012; 42:e109–e111.
A 66-year-old man with chronic obstructive pulmonary disease (COPD) was brought to the emergency department with a 2-week history of progressive dyspnea followed by altered mental status. He had no history of diabetes mellitus, hypertension, or drug abuse.
On physical examination, he was stuporous. He had no fever or hypotension, but his pulse and breathing were rapid, and he had central cyanosis, bilateral conjunctival congestion, a puffy face, generalized wheezing, basilar crackles in both lungs, and leg edema.
Laboratory testing showed hypoxia and severe hypercarbia. His hematocrit was 65% (reference range 39–51) and his hemoglobin level was 21.5 g/dL (13–17).
The patient was diagnosed with an exacerbation of COPD. He was intubated, placed on mechanical ventilation, and admitted to the intensive care unit.
Computed tomography (CT) performed because of his decreased level of consciousness (Figure 1) showed increased attenuation in the ambient cistern and the lateral aspect of the lateral cerebral fissure, suggesting subarachnoid hemorrhage. The attenuation value in these areas was 89 Hounsfield units (typical values for brain tissue are in the 20s to 30s, and for blood in the 30s to 40s). To further evaluate for subarachnoid hemorrhage, lumbar puncture was performed, but analysis of the fluid sample showed normal protein and glucose levels and no cells.
Based on the results of cerebrospinal fluid evaluation and on the CT attenuation value, a diagnosis of pseudosubarachnoid hemorrhage due to polycythemia was made.
SUBARACHNOID VS PSEUDOSUBARACHNOID HEMORRHAGE
Subarachnoid hemorrhage typically begins with a “thunder-clap” headache (beginning suddenly and described by patients as “the worst headache ever.”) While not all patients have this presentation, if imaging suggests subarachnoid hemorrhage but the patient has atypical signs and symptoms (eg, other than headache), then pseudosubarachnoid hemorrhage should be considered.
Brain CT is one of the most reliable tools for diagnosing subarachnoid hemorrhage in the emergency department. Done within 6 hours of symptom onset, it has a sensitivity of 98.7% and a specificity of 99.9%.1 Magnetic resonance imaging can also visualize subarachnoid hemorrhage within the first 12 hours, typically as a hyperintensity in the subarachnoid space on fluid-attenuated inversion-recovery sequences2 and on susceptibility-weighted sequences.
Lumbar puncture is also an important diagnostic tool but carries a risk of brain herniation in patients with brain edema.
Pseudosubarachnoid hemorrhage is an artifact of CT imaging. It is rare, and its prevalence is unknown.3 However, it may be seen in up to 20% of patients after resuscitation, as a result of diffuse cerebral edema that lowers the attenuation of brain tissue on CT, making the vessels relatively conspicuous. It can also be seen in purulent meningitis (due to proteinaceous influx after blood-brain barrier disruption),4 in meningeal leukemia (due to increased cellularity in the leptomeninges), and in severe polycythemia (from a higher concentration of blood and hemoglobin in the vessels).3,5–7
Although the level of attenuation on CT may help distinguish subarachnoid from pseudosubarachnoid hemorrhage, its accuracy has not been defined. Inspecting the CT images may clarify whether areas with high attenuation look like blood vessels vs subarachnoid hemorrhage.
Our patient recovered and had an uneventful follow-up. The cause of his elevated hematocrit was likely chronic hypoxia from COPD.
Acknowledgment: We thank Dr. Saeide Khanbagi and Dr. Azade Nasr-lari for their cooperation.
A 66-year-old man with chronic obstructive pulmonary disease (COPD) was brought to the emergency department with a 2-week history of progressive dyspnea followed by altered mental status. He had no history of diabetes mellitus, hypertension, or drug abuse.
On physical examination, he was stuporous. He had no fever or hypotension, but his pulse and breathing were rapid, and he had central cyanosis, bilateral conjunctival congestion, a puffy face, generalized wheezing, basilar crackles in both lungs, and leg edema.
Laboratory testing showed hypoxia and severe hypercarbia. His hematocrit was 65% (reference range 39–51) and his hemoglobin level was 21.5 g/dL (13–17).
The patient was diagnosed with an exacerbation of COPD. He was intubated, placed on mechanical ventilation, and admitted to the intensive care unit.
Computed tomography (CT) performed because of his decreased level of consciousness (Figure 1) showed increased attenuation in the ambient cistern and the lateral aspect of the lateral cerebral fissure, suggesting subarachnoid hemorrhage. The attenuation value in these areas was 89 Hounsfield units (typical values for brain tissue are in the 20s to 30s, and for blood in the 30s to 40s). To further evaluate for subarachnoid hemorrhage, lumbar puncture was performed, but analysis of the fluid sample showed normal protein and glucose levels and no cells.
Based on the results of cerebrospinal fluid evaluation and on the CT attenuation value, a diagnosis of pseudosubarachnoid hemorrhage due to polycythemia was made.
SUBARACHNOID VS PSEUDOSUBARACHNOID HEMORRHAGE
Subarachnoid hemorrhage typically begins with a “thunder-clap” headache (beginning suddenly and described by patients as “the worst headache ever.”) While not all patients have this presentation, if imaging suggests subarachnoid hemorrhage but the patient has atypical signs and symptoms (eg, other than headache), then pseudosubarachnoid hemorrhage should be considered.
Brain CT is one of the most reliable tools for diagnosing subarachnoid hemorrhage in the emergency department. Done within 6 hours of symptom onset, it has a sensitivity of 98.7% and a specificity of 99.9%.1 Magnetic resonance imaging can also visualize subarachnoid hemorrhage within the first 12 hours, typically as a hyperintensity in the subarachnoid space on fluid-attenuated inversion-recovery sequences2 and on susceptibility-weighted sequences.
Lumbar puncture is also an important diagnostic tool but carries a risk of brain herniation in patients with brain edema.
Pseudosubarachnoid hemorrhage is an artifact of CT imaging. It is rare, and its prevalence is unknown.3 However, it may be seen in up to 20% of patients after resuscitation, as a result of diffuse cerebral edema that lowers the attenuation of brain tissue on CT, making the vessels relatively conspicuous. It can also be seen in purulent meningitis (due to proteinaceous influx after blood-brain barrier disruption),4 in meningeal leukemia (due to increased cellularity in the leptomeninges), and in severe polycythemia (from a higher concentration of blood and hemoglobin in the vessels).3,5–7
Although the level of attenuation on CT may help distinguish subarachnoid from pseudosubarachnoid hemorrhage, its accuracy has not been defined. Inspecting the CT images may clarify whether areas with high attenuation look like blood vessels vs subarachnoid hemorrhage.
Our patient recovered and had an uneventful follow-up. The cause of his elevated hematocrit was likely chronic hypoxia from COPD.
Acknowledgment: We thank Dr. Saeide Khanbagi and Dr. Azade Nasr-lari for their cooperation.
- Dubosh NM, Bellolio MF, Rabinstein AA, Edlow JA. Sensitivity of early brain computed tomography to exclude aneurysmal subarachnoid hemorrhage: a systematic review and meta-analysis. Stroke 2016; 47:750–755.
- Sohn CH, Baik SK, Lee HJ, et al. MR imaging of hyperacute subarachnoid and intraventricular hemorrhage at 3T: a preliminary report of gradient echo T2*-weighted sequences. AJNR Am J Neuroradiol 2005; 26:662–665.
- Yuzawa H, Higano S, Mugikura S, et al. Pseudo-subarachnoid hemorrhage found in patients with postresuscitation encephalopathy: characteristics of CT findings and clinical importance. AJNR Am J Neuroradiol 2008; 29:1544–1549.
- Given CA 2nd, Burdette JH, Elster AD, Williams DW 3rd. Pseudo-subarachnoid hemorrhage: a potential imaging pitfall associated with diffuse cerebral edema. AJNR Am J Neuroradiol 2003; 24:254–256.
- Avrahami E, Katz R, Rabin A, Friedman V. CT diagnosis of non-traumatic subarachnoid haemorrhage in patients with brain edema. Eur J Radiol 1998; 28:222–225.
- Ben Salem D, Osseby GV, Rezaizadeh-Bourdariat K, et al. Spontaneous hyperdense intracranial vessels seen on CT scan in polycythemia cases. J Radiol 2003; 84:605–608. French.
- Hsieh SW, Khor GT, Chen CN, Huang P. Pseudo subarachnoid hemorrhage in meningeal leukemia. J Emerg Med 2012; 42:e109–e111.
- Dubosh NM, Bellolio MF, Rabinstein AA, Edlow JA. Sensitivity of early brain computed tomography to exclude aneurysmal subarachnoid hemorrhage: a systematic review and meta-analysis. Stroke 2016; 47:750–755.
- Sohn CH, Baik SK, Lee HJ, et al. MR imaging of hyperacute subarachnoid and intraventricular hemorrhage at 3T: a preliminary report of gradient echo T2*-weighted sequences. AJNR Am J Neuroradiol 2005; 26:662–665.
- Yuzawa H, Higano S, Mugikura S, et al. Pseudo-subarachnoid hemorrhage found in patients with postresuscitation encephalopathy: characteristics of CT findings and clinical importance. AJNR Am J Neuroradiol 2008; 29:1544–1549.
- Given CA 2nd, Burdette JH, Elster AD, Williams DW 3rd. Pseudo-subarachnoid hemorrhage: a potential imaging pitfall associated with diffuse cerebral edema. AJNR Am J Neuroradiol 2003; 24:254–256.
- Avrahami E, Katz R, Rabin A, Friedman V. CT diagnosis of non-traumatic subarachnoid haemorrhage in patients with brain edema. Eur J Radiol 1998; 28:222–225.
- Ben Salem D, Osseby GV, Rezaizadeh-Bourdariat K, et al. Spontaneous hyperdense intracranial vessels seen on CT scan in polycythemia cases. J Radiol 2003; 84:605–608. French.
- Hsieh SW, Khor GT, Chen CN, Huang P. Pseudo subarachnoid hemorrhage in meningeal leukemia. J Emerg Med 2012; 42:e109–e111.