User login
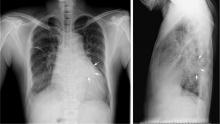
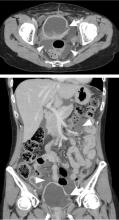
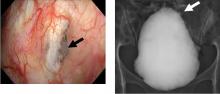
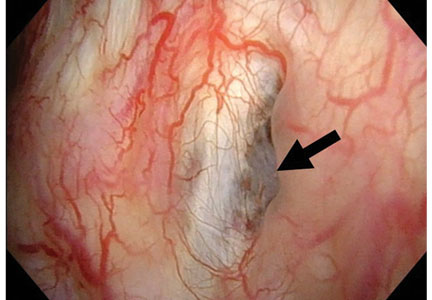
Hidden lesion easily missed on chest radiography
A 46-year-old man with poorly controlled hypertension presented with the sudden onset of chest pain and shortness of breath. His blood pressure was 158/96 mm Hg and his left ventricular ejection fraction was less than 20%. He was admitted to the hospital for newly diagnosed heart failure.
SACCULAR AORTIC ANEURYSMS
This case shows the value of carefully examining the chest radiograph, especially behind the heart.
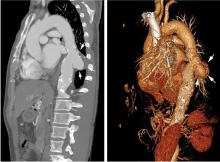
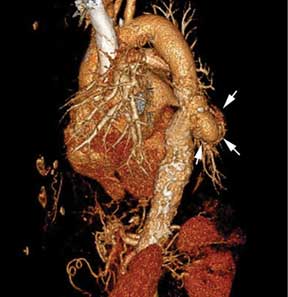
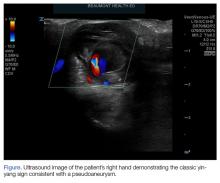
Saccular aneurysms of the descending thoracic aorta are rare and can be easily missed if asymptomatic. They are less common than fusiform aneurysms and may be more prone to rupture. Shang et al1 identified atherosclerosis as the most frequent cause of saccular aneurysms of the thoracic aorta. However, they can be caused by other inflammatory conditions and infections.1
Without surgical repair, thoracic aortic aneurysms larger than 6 cm have higher rates of expansion and rupture (a 20% 6-year cumulative risk) than smaller ones.2 Mid-descending aortic aneurysms expand faster than those of the ascending aorta.3
Indications for surgery include rupture, severe chest pain, compressive symptoms, large size (eg, ≥ 5.5 cm for asymptomatic descending thoracic aneurysms), and rapid growth rate (≥ 10 mm per year), all of which are associated with a higher mortality rate.4
Endovascular grafting should be strongly considered in patients who have significant comorbidities, but this approach may have poorer long-term outcomes compared with open surgery. Blood pressure should be lowered as far as the patient can tolerate without adverse effects, usually with a beta-blocker along with either an angiotensin-converting enzyme inhibitor or an angiotensin receptor blocker.4
Statin therapy to lower the low-density lipoprotein cholesterol level to less than 70 mg/dL is also needed to reduce the risk of complications and cardiovascular disease.
All patients with saccular aortic aneurysm should be followed closely and be evaluated for surgery.
- Shang EK, Nathan DP, Boonn WW, et al. A modern experience with saccular aortic aneurysms. J Vasc Surg 2013; 57(1):84–88. doi:10.1016/j.jvs.2012.07.002
- Dapunt OE, Galla JD, Sadeghi AM, et al. The natural history of thoracic aortic aneurysms. J Thorac Cardiovasc Surg 1994; 107(5):1323–1333.
- Bonser RS, Pagano D, Lewis ME, et al. Clinical and patho-anatomical factors affecting expansion of thoracic aortic aneurysms. Heart 2000; 84(3):277–283.
- Hiratzka LF, Bakris GL, Beckman JA, et al. 2010 ACCF/AHA/AATS/ACR/ASA/SCA/SCAI/SIR/STS/SVM guidelines for the diagnosis and management of patients with thoracic aortic disease. Circulation 2010; 121(13):e266–e369. doi:10.1161/CIR.0b013e3181d4739e
Jessica Stempe
A 46-year-old man with poorly controlled hypertension presented with the sudden onset of chest pain and shortness of breath. His blood pressure was 158/96 mm Hg and his left ventricular ejection fraction was less than 20%. He was admitted to the hospital for newly diagnosed heart failure.
SACCULAR AORTIC ANEURYSMS
This case shows the value of carefully examining the chest radiograph, especially behind the heart.
Saccular aneurysms of the descending thoracic aorta are rare and can be easily missed if asymptomatic. They are less common than fusiform aneurysms and may be more prone to rupture. Shang et al1 identified atherosclerosis as the most frequent cause of saccular aneurysms of the thoracic aorta. However, they can be caused by other inflammatory conditions and infections.1
Without surgical repair, thoracic aortic aneurysms larger than 6 cm have higher rates of expansion and rupture (a 20% 6-year cumulative risk) than smaller ones.2 Mid-descending aortic aneurysms expand faster than those of the ascending aorta.3
Indications for surgery include rupture, severe chest pain, compressive symptoms, large size (eg, ≥ 5.5 cm for asymptomatic descending thoracic aneurysms), and rapid growth rate (≥ 10 mm per year), all of which are associated with a higher mortality rate.4
Endovascular grafting should be strongly considered in patients who have significant comorbidities, but this approach may have poorer long-term outcomes compared with open surgery. Blood pressure should be lowered as far as the patient can tolerate without adverse effects, usually with a beta-blocker along with either an angiotensin-converting enzyme inhibitor or an angiotensin receptor blocker.4
Statin therapy to lower the low-density lipoprotein cholesterol level to less than 70 mg/dL is also needed to reduce the risk of complications and cardiovascular disease.
All patients with saccular aortic aneurysm should be followed closely and be evaluated for surgery.
A 46-year-old man with poorly controlled hypertension presented with the sudden onset of chest pain and shortness of breath. His blood pressure was 158/96 mm Hg and his left ventricular ejection fraction was less than 20%. He was admitted to the hospital for newly diagnosed heart failure.
SACCULAR AORTIC ANEURYSMS
This case shows the value of carefully examining the chest radiograph, especially behind the heart.
Saccular aneurysms of the descending thoracic aorta are rare and can be easily missed if asymptomatic. They are less common than fusiform aneurysms and may be more prone to rupture. Shang et al1 identified atherosclerosis as the most frequent cause of saccular aneurysms of the thoracic aorta. However, they can be caused by other inflammatory conditions and infections.1
Without surgical repair, thoracic aortic aneurysms larger than 6 cm have higher rates of expansion and rupture (a 20% 6-year cumulative risk) than smaller ones.2 Mid-descending aortic aneurysms expand faster than those of the ascending aorta.3
Indications for surgery include rupture, severe chest pain, compressive symptoms, large size (eg, ≥ 5.5 cm for asymptomatic descending thoracic aneurysms), and rapid growth rate (≥ 10 mm per year), all of which are associated with a higher mortality rate.4
Endovascular grafting should be strongly considered in patients who have significant comorbidities, but this approach may have poorer long-term outcomes compared with open surgery. Blood pressure should be lowered as far as the patient can tolerate without adverse effects, usually with a beta-blocker along with either an angiotensin-converting enzyme inhibitor or an angiotensin receptor blocker.4
Statin therapy to lower the low-density lipoprotein cholesterol level to less than 70 mg/dL is also needed to reduce the risk of complications and cardiovascular disease.
All patients with saccular aortic aneurysm should be followed closely and be evaluated for surgery.
- Shang EK, Nathan DP, Boonn WW, et al. A modern experience with saccular aortic aneurysms. J Vasc Surg 2013; 57(1):84–88. doi:10.1016/j.jvs.2012.07.002
- Dapunt OE, Galla JD, Sadeghi AM, et al. The natural history of thoracic aortic aneurysms. J Thorac Cardiovasc Surg 1994; 107(5):1323–1333.
- Bonser RS, Pagano D, Lewis ME, et al. Clinical and patho-anatomical factors affecting expansion of thoracic aortic aneurysms. Heart 2000; 84(3):277–283.
- Hiratzka LF, Bakris GL, Beckman JA, et al. 2010 ACCF/AHA/AATS/ACR/ASA/SCA/SCAI/SIR/STS/SVM guidelines for the diagnosis and management of patients with thoracic aortic disease. Circulation 2010; 121(13):e266–e369. doi:10.1161/CIR.0b013e3181d4739e
- Shang EK, Nathan DP, Boonn WW, et al. A modern experience with saccular aortic aneurysms. J Vasc Surg 2013; 57(1):84–88. doi:10.1016/j.jvs.2012.07.002
- Dapunt OE, Galla JD, Sadeghi AM, et al. The natural history of thoracic aortic aneurysms. J Thorac Cardiovasc Surg 1994; 107(5):1323–1333.
- Bonser RS, Pagano D, Lewis ME, et al. Clinical and patho-anatomical factors affecting expansion of thoracic aortic aneurysms. Heart 2000; 84(3):277–283.
- Hiratzka LF, Bakris GL, Beckman JA, et al. 2010 ACCF/AHA/AATS/ACR/ASA/SCA/SCAI/SIR/STS/SVM guidelines for the diagnosis and management of patients with thoracic aortic disease. Circulation 2010; 121(13):e266–e369. doi:10.1161/CIR.0b013e3181d4739e
Jessica Stempe
Jessica Stempe
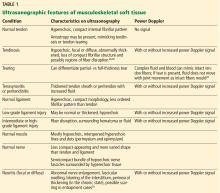
Musculoskeletal ultrasonography basics
Ultrasonography has been used to evaluate musculoskeletal problems for decades but has only recently become more widely available in the United States. Advances in technology and physician familiarity are increasing its role in orthopedic imaging.
No single imaging method can yield all musculoskeletal diagnoses. Like any imaging technique, ultrasonography has strengths and weaknesses specific to orthopedics. Radiography, computed tomography (CT), and magnetic resonance imaging (MRI) play important roles for investigating musculoskeletal problems and are complementary to each other and to ultrasonography.
To help clinicians make informed decisions about ordering musculoskeletal ultrasonography, this article reviews the basic physics underlying ultrasonography, its advantages and disadvantages compared with other imaging methods, and common clinical applications.
CLASSIC TECHNOLOGY MAKING A RESURGENCE
The first reports of the use of musculoskeletal ultrasonography appeared in the 1970s for investigating the rotator cuff,1–3 actually preceding reports of its use in obstetrics and gynecology.4 In the 1980s, reports emerged for evaluating the Achilles tendon.5,6 After that, its popularity in the United States plateaued, likely because of the advent of MRI, lower reimbursement and greater variability in interpretation compared with MRI, as well as a lack of physicians and sonographers trained in its use.7,8
Musculoskeletal ultrasonography is currently experiencing a resurgence. Although it remains a specialized service more commonly available in large hospitals, its use is increasing rapidly, and it will likely become more widely available.
SPECIAL TRAINING REQUIRED
Musculoskeletal ultrasonography is simply an ultrasonographic examination of part of the musculoskeletal system. But because not all ultrasonographic transducers offer sufficient resolution for musculoskeletal evaluation and not all sonographers and imaging physicians are familiar with the specialized techniques, musculoskeletal ultrasonography often has a separate designation (eg, “MSKUS,” “MSUS”). At Cleveland Clinic, it is offered through the department of musculoskeletal imaging by subspecialty-trained musculoskeletal radiologists and specially trained musculoskeletal ultrasonographers with 4 to 5 years of training in the technique.
Musculoskeletal ultrasonography is also performed by physician groups with specialized training, including sports medicine physicians, rheumatologists, physiatrists, neurologists, and orthopedic surgeons. The American Institute of Ultrasound in Medicine offers voluntary accreditation for practice groups using musculoskeletal ultrasonography. Certification in musculoskeletal radiology is offered to sonographers through the American Registry for Diagnostic Medical Sonography.
SONOGRAPHY HAS UNIQUE QUALITIES
Ultrasonography uses high-frequency sound waves to generate images. The transducer (or probe) emits sound from the many piezoelectric elements at its surface, and the sound waves travel through and react with tissues. Sound reflected by tissues is detected by the transducer and converted to an image. Objects that reflect sound appear hyperechoic (brighter), whereas tissues that reflect little or no sound appear hypoechoic.
High-resolution imaging of superficial structures
(B, arrow).
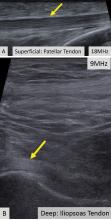
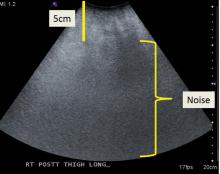
Ultrasonography involves a fundamental trade-off between image resolution and imaging depth. Higher-frequency sound waves do not penetrate far into tissues but generate a higher-resolution image; lower-frequency sound waves can penetrate much further but yield a lower-resolution image. Although high-resolution imaging of deep structures with ultrasonography is not possible (Figure 1), many musculoskeletal structures are located superficially and are amenable to ultrasonographic evaluation.
Be aware of artifacts
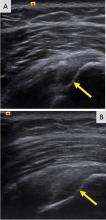
Some materials attenuate sound very little, such as simple fluid. Low attenuation results in artifacts on ultrasonography, making tissues behind the simple fluid appear brighter than neighboring tissues. These artifacts may be reported as “increased through transmission” or “posterior acoustic enhancement.” Conversely, metal and bone reflect all sound waves that reach them, rendering any structures beyond them invisible. This “shadowing” creates a problem for imaging of structures in or near bone. Subcutaneous fat also attenuates sound waves, limiting the use of ultrasonography for patients with obesity (Figure 2).
High-frequency linear transducer sharpens images
High-frequency linear transducers reduce anisotropy because their flat surface keeps sound waves more uniformly perpendicular to the structure of interest.4,7 Their development has allowed imaging of superficial structures that is superior to that of MRI. A high-frequency linear transducer offers more than twice the spatial resolution of a typical 1.5T MRI examination of superficial tissue.12,13
Operator experience is critical
Ultrasonography examinations, more than other imaging tests, are dependent on operator experience. A solid understanding of musculoskeletal anatomy is imperative. Because the probe images only a thin section of tissue (about the thickness of a credit card), referencing adjacent structures for orientation is more difficult with ultrasonography than with CT or MRI.
The accuracy of ultrasonography is highly dependent on acquiring and interpreting images, whereas the accuracy of MRI is dependent primarily on image interpretation.7 Interpreting physicians must check that sonographers capture relevant targets.
STRENGTHS OF MUSCULOSKELETAL ULTRASONOGRAPHY
Ultrasonography has multiple advantages:
No ionizing radiation exposure.
Portability. Unlike CT or MRI, ultrasonography equipment is portable.
Increased patient comfort. Patient positioning for an ultrasonography examination is more flexible than for MRI or CT,14 and the examination does not induce claustrophobia.8
High-resolution imaging. Ultrasonography provides very-high-resolution imaging of superficial soft tissues—in some cases, higher than MRI or CT.
Real-time dynamic examinations are possible with ultrasonography, unlike with CT or MRI, and may increase test sensitivity.4,15–18
Implanted hardware is less of a problem. Although ultrasonography cannot image beyond implanted orthopedic metallic hardware, the hardware does not obscure surrounding soft tissues as it does on CT and MRI.6,19,20 Also, ultrasonography is safe for patients with a pacemaker.8
WEAKNESSES
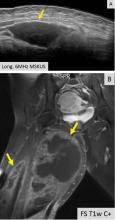
The main disadvantages of musculoskeletal ultrasonography are inherent to its limited field of view, making it inappropriate for a survey examination (eg, for ankle pain, knee pain, hip pain).4 Unlike CT and MRI, ultrasonography does not provide a “bird’s-eye view,” and important abnormalities can be missed during evaluation of large areas (Figure 4).
Ultrasonography also cannot evaluate bone or intra-articular structures such as cartilage, bone marrow, labrum, and intra-articular ligaments; MRI is the standard for evaluating these structures.21
Ultrasonography is time-consuming. To perform a detailed examination of the anterior, posterior, medial, and lateral aspects of the hip, knee, or ankle would require 1.5 to 2 hours of scanning time and an additional 10 to 25 minutes of image checking and interpretation.
CURRENT CLINICAL INDICATIONS
Musculoskeletal ultrasonography is best used for clinical questions regarding limited, superficial musculoskeletal problems.
Fluid collections
Ultrasonography can help evaluate small fluid collections in soft tissue. As is true for a lung opacity on chest radiography, soft-tissue fluid detected on ultrasonography is nonspecific, and results must be correlated with the clinical picture to narrow the differential diagnosis.
Fluid collections can be classified as loculated or nonloculated.
Nonloculated fluid involves more fluid than is simply interposed between tissue planes and has no wall or defined margins. It can be simple or complex in appearance: simple fluid is anechoic, and complex fluid appears more heterogeneous and may contain septations or debris.
Subcutaneous edema, which may occur postoperatively or from trauma, venous insufficiency, or inflammatory or infectious processes, appears on ultrasonography as nonloculated fluid interspersed between subcutaneous fat lobules.
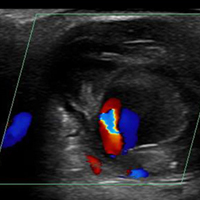
Loculated fluid collections have well-defined margins or a discrete wall that does not follow normal tissue planes. They can also be simple or complex and can be caused by hematoma, abscess, or ganglion. Less commonly, neoplasms can mimic a loculated fluid collection (Figure 4).
A ganglion is a specific type of loculated fluid collection containing synovial fluid arising from a joint or tendon sheath. It tends to occur in specific locations, most commonly around the wrist, most often arising from the dorsal scapholunate ligament and volar wrist between the radial artery and flexor carpi radialis.22 On MRI, it can be difficult to distinguish between small vascular structures and a small ganglion, especially in the hands and feet.23
Ultrasonography can also help identify a Baker cyst, a specific fluid collection arising from the semimembranosus bursa between the medial head of the gastrocnemius tendon and the semimembranosus tendon. Ultrasonography can also detect inflammation, rupture, or leaking associated with a Baker cyst.24
Power Doppler is an ultrasonographic examination that can detect increased blood flow surrounding a fluid collection and determine the likelihood of an acute inflammatory or infectious cause.25
Joint effusion and synovitis
Musculoskeletal ultrasonography can help evaluate joints for effusion and synovitis. It is highly sensitive (94%) and specific (95%) for synovitis, making it superior to contrast-enhanced MRI.26,27 The area of concern should be limited to 1 quadrant of a joint (anterior, posterior, medial, or lateral); for problems beyond that, MRI should be considered.
A joint effusion appears as a distended joint capsule containing hypoechoic (complex) or anechoic (simple) joint fluid.
Complex joint fluid may contain debris and occurs with hemarthrosis, infection, and inflammation.23 Hypertrophied synovium is hypoechoic and can mimic complex joint fluid.
Power Doppler evaluation can help distinguish synovitis from joint fluid by demonstrating blood flow, a feature of synovitis but not of simple joint fluid. Power Doppler is the most sensitive means of detecting blood flow, although it does not show direction of flow.28
Using ultrasonography can help to improve disease control and minimize disabling changes by monitoring synovitis therapy. In addition, subclinical synovitis and enthesitis (inflammation of insertion sites of tendons or ligaments into bone) detected by ultrasonography may predict future disease and disease flares.29–31
Ultrasonographic guidance for a wide range of procedures is increasing rapidly.32–36 Multiple studies have shown the advantage of ultrasonography-guided aspiration and injection compared with techniques without imaging guidance.37,38
Soft-tissue masses
Accurately diagnosing soft-tissue masses can be difficult. A mass may remain indeterminate even after multiple imaging studies, requiring biopsy or surgical referral. However, for a few specific masses, ultrasonography is highly accurate and can eliminate the need for further imaging.
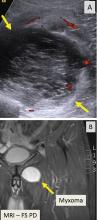
Ultrasonography can help evaluate soft- tissue masses no larger than 5 cm in diameter and no deeper than superficial muscular fascia. If the mass is larger or deeper than that, ultrasonography is less reliable for showing the margins of the mass and its relationship to adjacent structures (Figure 5). Further imaging by MRI may be recommended in such cases.
Fortunately, many of the most common soft-tissue masses can be accurately diagnosed with ultrasonography, including lipomas, ganglion cysts, foreign bodies, and simple fluid collections.4,39 Nerve-sheath tumors can also be diagnosed with ultrasonography if the lesion clearly arises from a nerve. Other soft-tissue masses are likely to be indeterminate with ultrasonography, requiring follow-up with MRI with contrast.
Tendons
Musculoskeletal ultrasonography can be effective for evaluating tendons around joints, especially 1 or a small number of nearby superficial tendons. Tendons particularly well suited for ultrasonographic examination include:
- Upper-extremity tendons located in the rotator cuff or around the elbow, and flexor and extensor tendons of the hands; ultrasonographic evaluation of the rotator cuff is highly accurate, equivalent to that of MRI for partial-thickness and full-thickness tearing40–43
- Lower-extremity tendons of the extensor mechanism of the knee, distal hamstring tendons, tendons around the ankle,44–46 and flexor and extensor tendons of the foot.
Ultrasonography can help diagnose a variety of tendon abnormalities (Table 1),48,49 including tearing, for which a dynamic examination can be performed.
Many tendons have a tendon sheath containing tenosynovium, while others have surrounding peritenon only; either can become thickened and inflamed. Tenosynovitis is a nonspecific finding and may be inflammatory, infectious, or posttraumatic. The presence of tendon sheath fluid alone on ultrasonography can be a normal finding, and some tendon sheaths that communicate with adjacent joints (eg, the long head biceps tendon, the flexor hallucis longus tendon) commonly contain simple fluid.6 A dynamic examination with ultrasonography can help diagnose snapping related to abnormal tendon movement, for example, in the case of intra-sheath and extra-sheath subluxation of the peroneal tendons.45,50,51
Ligaments
Ultrasonography can detect abnormalities in many superficial ligaments (Table 1).
Ankle. Ankle ligaments are superficial and can be clearly visualized. The diagnostic accuracy of ultrasonography for tearing of the anterior talofibular ligament may be as high as 100%.50,52,53
Elbow and thumb. The larger of the collateral ligaments of the elbow, especially the ulnar collateral ligament, and the ulnar collateral ligament of the thumb can be effectively evaluated with ultrasonography.54,55
Knee. The collateral ligaments of the knee can be seen with ultrasonography, but injuries of the external ligaments of the knee are often associated with intrinsic derangements that cannot be evaluated with ultrasonography.56,57 Intra-articular ligaments such as the anterior cruciate ligament are also not amenable to ultrasonography.
Dynamic examination of a ligament with ultrasonography can help determine the grade of the injury.
Deeply located ligaments (eg, around the hip) and ligaments surrounded by bone, such as the Lisfranc ligament, cannot be completely seen on ultrasonography.
Muscle
Musculoskeletal ultrasonography is useful for small areas of concern within a muscle (Table 1). It can detect muscle strains and tears, intramuscular collections or lesions, and fascial scarring or fascial injuries such as superficial muscle herniation. Although ultrasonography may yield a definitive diagnosis for a muscle problem, further imaging may be needed.
Nerves
Ultrasonography is useful for peripheral nerve investigation but requires a steep learning curve for sonographers and interpreting physicians.58,59 It is best suited for directed questions regarding focal abnormal nerve findings on physical examination.
Ultrasonography can help identify areas of nerve entrapment caused by a mass or dynamic compression. It can detect neuritis (Table 1), lesions of peripheral nerves (eg, nerve-sheath tumors), and neuromas (eg, Morton neuroma of the intermetatarsal space). In a large meta-analysis, ultrasonography and MRI were found to be equally accurate for detecting Morton neuroma.60 Even for nerve-sheath tumors located deep to the muscular fascia, ultrasonography can confirm the diagnosis because of the characteristic appearance of the nerves. Ultrasonography can also demonstrate a large extent of the course of superficial peripheral nerves while keeping the imaging plane appropriately oriented to the nerves.
Acknowledgment: We would like to sincerely thank Megan Griffiths, MA, for her help in the preparation and submission of this manuscript.
- Hamilton JV, Flinn G Jr, Haynie CC, Cefalo RC. Diagnosis of rectus sheath hematoma by B-mode ultrasound: a case report. Am J Obstet Gynecol 1976; 125(4):562–565. doi:10.1016/0002-9378(76)90379-3
- Zweymüller VK, Kratochwil A. Ultrasound diagnosis of bone and soft tissue tumours. Wien Klin Wochenschr 1975; 87(12):397–398. German.
- Mayer V. Ultrasonography of the rotator cuff. J Ultrasound Med 1985; 4(11):608, 607. doi:10.7863/jum.1985.4.11.608
- McNally EG. The development and clinical applications of musculoskeletal ultrasound. Skeletal Radiol 2011; 40(9):1223–1231. doi:10.1007/s00256-011-1220-5
- Ignashin NS, Girshin SG, Tsypin IS. Ultrasonic scanning in subcutaneous rupture of the Achilles tendon. Vestn Khir Im I I Grek 1981; 127(9):82–85. Russian.
- Robinson P. Sonography of common tendon injuries. AJR Am J Roentgenol 2009; 193(3):607–618. doi:10.2214/AJR.09.2808
- Jacobson JA. Musculoskeletal ultrasound: focused impact on MRI. AJR Am J Roentgenol 2009; 193(3):619–627. doi:10.2214/AJR.09.2841
- Nazarian LN. The top 10 reasons musculoskeletal sonography is an important complementary or alternative technique to MRI. AJR Am J Roentgenol 2008; 190(6):1621–1626. doi:10.2214/AJR.07.3385
- AIUM technical bulletin. Transducer manipulation. American Institute of Ultrasound in Medicine. J Ultrasound Med 1999; 18(2):169–175. doi:10.7863/jum.1999.18.2.169
- Connolly DJ, Berman L, McNally EG. The use of beam angulation to overcome anisotropy when viewing human tendon with high frequency linear array ultrasound. Br J Radiol 2001; 74 (878):183–185. doi:10.1259/bjr.74.878.740183
- Crass JR, van de Vegte GL, Harkavy LA. Tendon echogenicity: ex vivo study. Radiology 1988; 167(2):499–501. doi:10.1148/radiology.167.2.3282264
- Erickson SJ. High-resolution imaging of the musculoskeletal system. Radiology 1997; 205(3):593–618. doi:10.1148/radiology.205.3.9393511
- Link TM, Majumdar S, Peterfy C, et al. High resolution MRI of small joints: impact of spatial resolution on diagnostic performance and SNR. Magn Reson Imaging 1998; 16(2):147–155. doi:10.1016/S0730-725X(97)00244-0
- Middleton WD, Payne WT, Teefey SA, Hildebolt CF, Rubin DA, Yamaguchi K. Sonography and MRI of the shoulder: comparison of patient satisfaction. AJR Am J Roentgenol 2004; 183(5):1449–1452. doi:10.2214/ajr.183.5.1831449
- Khoury V, Cardinal E, Bureau NJ. Musculoskeletal sonography: a dynamic tool for usual and unusual disorders. AJR Am J Roentgenol 2007; 188(1):W63–W73. doi:10.2214/AJR.06.0579
- Farin PU, Jaroma H, Harju A, Soimakallio S. Medial displacement of the biceps brachii tendon: evaluation with dynamic sonography during maximal external shoulder rotation. Radiology 1995; 195(3):845–848. doi:10.1148/radiology.195.3.7754019
- Miller TT, Adler RS, Friedman L. Sonography of injury of the ulnar collateral ligament of the elbow-initial experience. Skeletal Radiol 2004; 33(7):386–391. doi:10.1007/s00256-004-0788-4
- Nazarian LN, McShane JM, Ciccotti MG, O’Kane PL, Harwood MI. Dynamic US of the anterior band of the ulnar collateral ligament of the elbow in asymptomatic major league baseball pitchers. Radiology 2003; 227(1):149–154. doi:10.1148/radiol.2271020288
- Jacobson JA, Lax MJ. Musculoskeletal sonography of the postoperative orthopedic patient. Semin Musculoskelet Radiol 2002; 6(1):67–77. doi:10.1055/s-2002-23165
- Sofka CM, Adler RS. Original report. Sonographic evaluation of shoulder arthroplasty. AJR Am J Roentgenol 2003; 180(4):1117–1120. doi:10.2214/ajr.180.4.1801117
- Silvestri E, Martinoli C, Derchi LE, Bertolotto M, Chiaramondia M, Rosenberg I. Echotexture of peripheral nerves: correlation between US and histologic findings and criteria to differentiate tendons. Radiology 1995; 197(1):291–296. doi:10.1148/radiology.197.1.7568840
- Cardinal E, Buckwalter KA, Braunstein EM, Mih AD. Occult dorsal carpal ganglion: comparison of US and MR imaging. Radiology 1994; 193(1):259–262. doi:10.1148/radiology.193.1.8090903
- Jacobson JA. Musculoskeletal ultrasound and MRI: which do I choose? Semin Musculoskelet Radiol 2005; 9(2):135–149. doi:10.1055/s-2005-872339
- Ward EE, Jacobson JA, Fessell DP, Hayes CW, van Holsbeeck M. Sonographic detection of Baker’s cysts: comparison with MR imaging. AJR Am J Roentgenol 2001; 176(2):373–380. doi:10.2214/ajr.176.2.1760373
- Bhasin S, Cheung PP. The role of power Doppler ultrasonography as disease activity marker in rheumatoid arthritis. Dis Markers 2015; 2015:325909. doi:10.1155/2015/325909
- Fukuba E, Yoshizako T, Kitagaki H, Murakawa Y, Kondo M, Uchida N. Power Doppler ultrasonography for assessment of rheumatoid synovitis: comparison with dynamic magnetic resonance imaging. Clin Imaging 2013; 37(1):134–137. doi:10.1016/j.clinimag.2012.02.008
- Takase-Minegishi K, Horita N, Kobayashi K, et al. Diagnostic test accuracy of ultrasound for synovitis in rheumatoid arthritis: systematic review and meta-analysis. Rheumatology (Oxford) 2018; 57(1):49–58. doi:10.1093/rheumatology/kex036
- Klareskog L, Catrina AI, Paget S. Rheumatoid arthritis. Lancet 2009; 373(9664):659–672. doi:10.1016/S0140-6736(09)60008-8
- Ash ZR, Tinazzi I, Gallego CC, et al. Psoriasis patients with nail disease have a greater magnitude of underlying systemic subclinical enthesopathy than those with normal nails. Ann Rheum Dis 2012; 71(4):553–556. doi:10.1136/annrheumdis-2011-200478
- Han J, Geng Y, Deng X, Zhang Z. Subclinical synovitis assessed by ultrasound predicts flare and progressive bone erosion in rheumatoid arthritis patients with clinical remission: a systematic review and metaanalysis. J Rheumatol 2016; 43(11):2010–2018. doi.org/10.3899/jrheum.160193
- Iagnocco A, Finucci A, Ceccarelli F, Perricone C, Iorgoveanu V, Valesini G. Power Doppler ultrasound monitoring of response to anti-tumour necrosis factor alpha treatment in patients with rheumatoid arthritis. Rheumatology (Oxford) 2015; 54(10):1890–1896. doi:10.1093/rheumatology/kev211
- Henning PT. Ultrasound-guided foot and ankle procedures. Phys Med Rehabil Clin N Am 2016; 27(3):649–671. doi:10.1016/j.pmr.2016.04.005
- Lueders DR, Smith J, Sellon JL. Ultrasound-guided knee procedures. Phys Med Rehabil Clin North Am 2016; 27(3):631–648. doi:10.1016/j.pmr.2016.04.010
- Payne JM. Ultrasound-guided hip procedures. Phys Med Rehabil Clin North Am 2016; 27(3):607–629. doi:10.1016/j.pmr.2016.04.004
- Strakowski JA. Ultrasound-guided peripheral nerve procedures. Phys Med Rehabil Clin North Am 2016; 27(3):687–715. doi:10.1016/j.pmr.2016.04.006
- Sussman WI, Williams CJ, Mautner K. Ultrasound-guided elbow procedures. Phys Med Rehabil Clin North Am 2016; 27(3):573–587. doi:10.1016/j.pmr.2016.04.002
- Finnoff JT. The evolution of diagnostic and interventional ultrasound in sports medicine. PM R 2016; 8(suppl 3):S133–S138. doi:10.1016/j.pmrj.2015.09.022
- Wu T, Dong Y, Song H, Fu Y, Li JH. Ultrasound-guided versus landmark in knee arthrocentesis: a systematic review. Semin Arthritis Rheum 2016; 45(5):627–632. doi:10.1016/j.semarthrit.2015.10.011
- Failla JM, van Holsbeeck M, Vanderschueren G. Detection of a 0.5-mm-thick thorn using ultrasound: a case report. J Hand Surg Am 1995; 20(3):456–457.
- Teefey SA, Hasan SA, Middleton WD, Patel M, Wright RW, Yamaguchi K. Ultrasonography of the rotator cuff. A comparison of ultrasonographic and arthroscopic findings in one hundred consecutive cases. J Bone Joint Surg Am 2000; 82(4):498–504.
- van Holsbeeck MT, Kolowich PA, Eyler WR, et al. US depiction of partial-thickness tear of the rotator cuff. Radiology 1995; 197(2):443–446. doi:10.1148/radiology.197.2.7480690
- Balich SM, Sheley RC, Brown TR, Sauser DD, Quinn SF. MR imaging of the rotator cuff tendon: interobserver agreement and analysis of interpretive errors. Radiology 1997; 204(1):191–194. doi:10.1148/radiology.204.1.9205245
- Dinnes J, Loveman E, McIntyre L, Waugh N. The effectiveness of diagnostic tests for the assessment of shoulder pain due to soft tissue disorders: a systematic review. Health Technol Assess 2003; 7(29):1–166. doi:10.3310/hta7290
- Rockett MS, Waitches G, Sudakoff G, Brage M. Use of ultrasonography versus magnetic resonance imaging for tendon abnormalities around the ankle. Foot Ankle Int 1998; 19(9):604–612.
- Grant TH, Kelikian AS, Jereb SE, McCarthy RJ. Ultrasound diagnosis of peroneal tendon tears. A surgical correlation. J Bone Joint Surg Am 2005; 87(8):1788–1794. doi:10.2106/JBJS.D.02450
- Hartgerink P, Fessell DP, Jacobson JA, van Holsbeeck MT. Full- versus partial-thickness Achilles tendon tears: sonographic accuracy and characterization in 26 cases with surgical correlation. Radiology 2001; 220(2):406–412. doi:10.1148/radiology.220.2.r01au41406
- Cho KH, Park BH, Yeon KM. Ultrasound of the adult hip. Semin Ultrasound CT MR 2000; 21(3):214–230.
- Adler RS, Finzel KC. The complementary roles of MR imaging and ultrasound of tendons. Radiol Clin North Am 2005; 43(4):771–807. doi:10.1016/j.rcl.2005.02.011
- Martinoli C, Bianchi S, Derchi LE. Tendon and nerve sonography. Radiol Clin North Am 1999; 37(4):691–711. doi:10.1016/S0033-8389(05)70124-X
- Fessell DP, Vanderschueren GM, Jacobson JA, et al. US of the ankle: technique, anatomy, and diagnosis of pathologic conditions. Radiographics 1998; 18(2):325–340. doi:10.1148/radiographics.18.2.9536481
- Neustadter J, Raikin SM, Nazarian LN. Dynamic sonographic evaluation of peroneal tendon subluxation. AJR Am J Roentgenol 2004; 183(4):985–988. doi:10.2214/ajr.183.4.1830985
- Verhaven EF, Shahabpour M, Handelberg FW, Vaes PH, Opdecam PJ. The accuracy of three-dimensional magnetic resonance imaging in the diagnosis of ruptures of the lateral ligaments of the ankle. Am J Sports Med 1991; 19(6):583–587. doi:10.1177/036354659101900605
- Milz P, Milz S, Steinborn M, Mittlmeier T, Putz R, Reiser M. Lateral ankle ligaments and tibiofibular syndesmosis. 13-MHz high-frequency sonography and MRI compared in 20 patients. Acta Orthop Scand 1998; 69(1):51–55.
- De Smet AA, Winter TC, Best TM, Bernhardt DT. Dynamic sonography with valgus stress to assess elbow ulnar collateral ligament injury in baseball pitchers. Skeletal Radiol 2002; 31(11):671–676. doi:10.1007/s00256-002-0558-0
- Melville DM, Jacobson JA, Fessell DP. Ultrasound of the thumb ulnar collateral ligament: technique and pathology. AJR Am J Roentgenol 2014; 202(2):W168. doi:10.2214/AJR.13.11335
- Court-Payen M. Sonography of the knee: intra-articular pathology. J Clin Ultrasound 2004; 32(9):481–490. doi:10.1002/jcu.20069
- Azzoni R, Cabitza P. Is there a role for sonography in the diagnosis of tears of the knee menisci? J Clin Ultrasound 2002; 30(8):472–476. doi:10.1002/jcu.10106
- Jacobson JA, Wilson TJ, Yang LJ. Sonography of common peripheral nerve disorders with clinical correlation. J Ultrasound Med 2016; 35(4):683–693. doi:10.7863/ultra.15.05061
- Ali ZS, Pisapia JM, Ma TS, Zager EL, Heuer GG, Khoury V. Ultrasonographic evaluation of peripheral nerves. World Neurosurg 2016; 85(1):333–339. doi:10.1016/j.wneu.2015.10.005
- Bignotti B, Signori A, Sormani MP, Molfetta L, Martinoli C, Tagliafico A. Ultrasound versus magnetic resonance imaging for Morton neuroma: systematic review and meta-analysis. Eur Radiol 2015; 25(8):2254–2262. doi:10.1007/s00330-015-3633-3
Ultrasonography has been used to evaluate musculoskeletal problems for decades but has only recently become more widely available in the United States. Advances in technology and physician familiarity are increasing its role in orthopedic imaging.
No single imaging method can yield all musculoskeletal diagnoses. Like any imaging technique, ultrasonography has strengths and weaknesses specific to orthopedics. Radiography, computed tomography (CT), and magnetic resonance imaging (MRI) play important roles for investigating musculoskeletal problems and are complementary to each other and to ultrasonography.
To help clinicians make informed decisions about ordering musculoskeletal ultrasonography, this article reviews the basic physics underlying ultrasonography, its advantages and disadvantages compared with other imaging methods, and common clinical applications.
CLASSIC TECHNOLOGY MAKING A RESURGENCE
The first reports of the use of musculoskeletal ultrasonography appeared in the 1970s for investigating the rotator cuff,1–3 actually preceding reports of its use in obstetrics and gynecology.4 In the 1980s, reports emerged for evaluating the Achilles tendon.5,6 After that, its popularity in the United States plateaued, likely because of the advent of MRI, lower reimbursement and greater variability in interpretation compared with MRI, as well as a lack of physicians and sonographers trained in its use.7,8
Musculoskeletal ultrasonography is currently experiencing a resurgence. Although it remains a specialized service more commonly available in large hospitals, its use is increasing rapidly, and it will likely become more widely available.
SPECIAL TRAINING REQUIRED
Musculoskeletal ultrasonography is simply an ultrasonographic examination of part of the musculoskeletal system. But because not all ultrasonographic transducers offer sufficient resolution for musculoskeletal evaluation and not all sonographers and imaging physicians are familiar with the specialized techniques, musculoskeletal ultrasonography often has a separate designation (eg, “MSKUS,” “MSUS”). At Cleveland Clinic, it is offered through the department of musculoskeletal imaging by subspecialty-trained musculoskeletal radiologists and specially trained musculoskeletal ultrasonographers with 4 to 5 years of training in the technique.
Musculoskeletal ultrasonography is also performed by physician groups with specialized training, including sports medicine physicians, rheumatologists, physiatrists, neurologists, and orthopedic surgeons. The American Institute of Ultrasound in Medicine offers voluntary accreditation for practice groups using musculoskeletal ultrasonography. Certification in musculoskeletal radiology is offered to sonographers through the American Registry for Diagnostic Medical Sonography.
SONOGRAPHY HAS UNIQUE QUALITIES
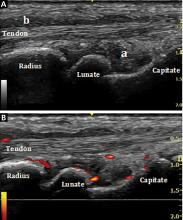
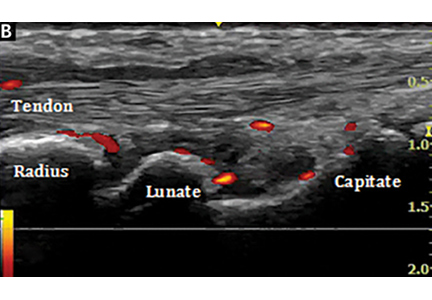
Ultrasonography uses high-frequency sound waves to generate images. The transducer (or probe) emits sound from the many piezoelectric elements at its surface, and the sound waves travel through and react with tissues. Sound reflected by tissues is detected by the transducer and converted to an image. Objects that reflect sound appear hyperechoic (brighter), whereas tissues that reflect little or no sound appear hypoechoic.
High-resolution imaging of superficial structures
(B, arrow).
Ultrasonography involves a fundamental trade-off between image resolution and imaging depth. Higher-frequency sound waves do not penetrate far into tissues but generate a higher-resolution image; lower-frequency sound waves can penetrate much further but yield a lower-resolution image. Although high-resolution imaging of deep structures with ultrasonography is not possible (Figure 1), many musculoskeletal structures are located superficially and are amenable to ultrasonographic evaluation.
Be aware of artifacts
Some materials attenuate sound very little, such as simple fluid. Low attenuation results in artifacts on ultrasonography, making tissues behind the simple fluid appear brighter than neighboring tissues. These artifacts may be reported as “increased through transmission” or “posterior acoustic enhancement.” Conversely, metal and bone reflect all sound waves that reach them, rendering any structures beyond them invisible. This “shadowing” creates a problem for imaging of structures in or near bone. Subcutaneous fat also attenuates sound waves, limiting the use of ultrasonography for patients with obesity (Figure 2).
High-frequency linear transducer sharpens images
High-frequency linear transducers reduce anisotropy because their flat surface keeps sound waves more uniformly perpendicular to the structure of interest.4,7 Their development has allowed imaging of superficial structures that is superior to that of MRI. A high-frequency linear transducer offers more than twice the spatial resolution of a typical 1.5T MRI examination of superficial tissue.12,13
Operator experience is critical
Ultrasonography examinations, more than other imaging tests, are dependent on operator experience. A solid understanding of musculoskeletal anatomy is imperative. Because the probe images only a thin section of tissue (about the thickness of a credit card), referencing adjacent structures for orientation is more difficult with ultrasonography than with CT or MRI.
The accuracy of ultrasonography is highly dependent on acquiring and interpreting images, whereas the accuracy of MRI is dependent primarily on image interpretation.7 Interpreting physicians must check that sonographers capture relevant targets.
STRENGTHS OF MUSCULOSKELETAL ULTRASONOGRAPHY
Ultrasonography has multiple advantages:
No ionizing radiation exposure.
Portability. Unlike CT or MRI, ultrasonography equipment is portable.
Increased patient comfort. Patient positioning for an ultrasonography examination is more flexible than for MRI or CT,14 and the examination does not induce claustrophobia.8
High-resolution imaging. Ultrasonography provides very-high-resolution imaging of superficial soft tissues—in some cases, higher than MRI or CT.
Real-time dynamic examinations are possible with ultrasonography, unlike with CT or MRI, and may increase test sensitivity.4,15–18
Implanted hardware is less of a problem. Although ultrasonography cannot image beyond implanted orthopedic metallic hardware, the hardware does not obscure surrounding soft tissues as it does on CT and MRI.6,19,20 Also, ultrasonography is safe for patients with a pacemaker.8
WEAKNESSES
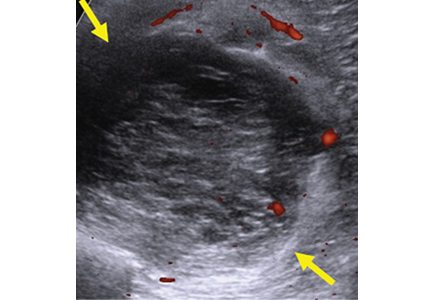
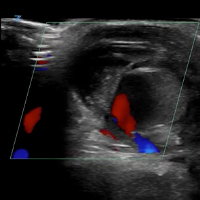
The main disadvantages of musculoskeletal ultrasonography are inherent to its limited field of view, making it inappropriate for a survey examination (eg, for ankle pain, knee pain, hip pain).4 Unlike CT and MRI, ultrasonography does not provide a “bird’s-eye view,” and important abnormalities can be missed during evaluation of large areas (Figure 4).
Ultrasonography also cannot evaluate bone or intra-articular structures such as cartilage, bone marrow, labrum, and intra-articular ligaments; MRI is the standard for evaluating these structures.21
Ultrasonography is time-consuming. To perform a detailed examination of the anterior, posterior, medial, and lateral aspects of the hip, knee, or ankle would require 1.5 to 2 hours of scanning time and an additional 10 to 25 minutes of image checking and interpretation.
CURRENT CLINICAL INDICATIONS
Musculoskeletal ultrasonography is best used for clinical questions regarding limited, superficial musculoskeletal problems.
Fluid collections
Ultrasonography can help evaluate small fluid collections in soft tissue. As is true for a lung opacity on chest radiography, soft-tissue fluid detected on ultrasonography is nonspecific, and results must be correlated with the clinical picture to narrow the differential diagnosis.
Fluid collections can be classified as loculated or nonloculated.
Nonloculated fluid involves more fluid than is simply interposed between tissue planes and has no wall or defined margins. It can be simple or complex in appearance: simple fluid is anechoic, and complex fluid appears more heterogeneous and may contain septations or debris.
Subcutaneous edema, which may occur postoperatively or from trauma, venous insufficiency, or inflammatory or infectious processes, appears on ultrasonography as nonloculated fluid interspersed between subcutaneous fat lobules.
Loculated fluid collections have well-defined margins or a discrete wall that does not follow normal tissue planes. They can also be simple or complex and can be caused by hematoma, abscess, or ganglion. Less commonly, neoplasms can mimic a loculated fluid collection (Figure 4).
A ganglion is a specific type of loculated fluid collection containing synovial fluid arising from a joint or tendon sheath. It tends to occur in specific locations, most commonly around the wrist, most often arising from the dorsal scapholunate ligament and volar wrist between the radial artery and flexor carpi radialis.22 On MRI, it can be difficult to distinguish between small vascular structures and a small ganglion, especially in the hands and feet.23
Ultrasonography can also help identify a Baker cyst, a specific fluid collection arising from the semimembranosus bursa between the medial head of the gastrocnemius tendon and the semimembranosus tendon. Ultrasonography can also detect inflammation, rupture, or leaking associated with a Baker cyst.24
Power Doppler is an ultrasonographic examination that can detect increased blood flow surrounding a fluid collection and determine the likelihood of an acute inflammatory or infectious cause.25
Joint effusion and synovitis
Musculoskeletal ultrasonography can help evaluate joints for effusion and synovitis. It is highly sensitive (94%) and specific (95%) for synovitis, making it superior to contrast-enhanced MRI.26,27 The area of concern should be limited to 1 quadrant of a joint (anterior, posterior, medial, or lateral); for problems beyond that, MRI should be considered.
A joint effusion appears as a distended joint capsule containing hypoechoic (complex) or anechoic (simple) joint fluid.
Complex joint fluid may contain debris and occurs with hemarthrosis, infection, and inflammation.23 Hypertrophied synovium is hypoechoic and can mimic complex joint fluid.
Power Doppler evaluation can help distinguish synovitis from joint fluid by demonstrating blood flow, a feature of synovitis but not of simple joint fluid. Power Doppler is the most sensitive means of detecting blood flow, although it does not show direction of flow.28
Using ultrasonography can help to improve disease control and minimize disabling changes by monitoring synovitis therapy. In addition, subclinical synovitis and enthesitis (inflammation of insertion sites of tendons or ligaments into bone) detected by ultrasonography may predict future disease and disease flares.29–31
Ultrasonographic guidance for a wide range of procedures is increasing rapidly.32–36 Multiple studies have shown the advantage of ultrasonography-guided aspiration and injection compared with techniques without imaging guidance.37,38
Soft-tissue masses
Accurately diagnosing soft-tissue masses can be difficult. A mass may remain indeterminate even after multiple imaging studies, requiring biopsy or surgical referral. However, for a few specific masses, ultrasonography is highly accurate and can eliminate the need for further imaging.
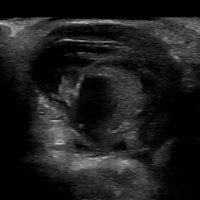
Ultrasonography can help evaluate soft- tissue masses no larger than 5 cm in diameter and no deeper than superficial muscular fascia. If the mass is larger or deeper than that, ultrasonography is less reliable for showing the margins of the mass and its relationship to adjacent structures (Figure 5). Further imaging by MRI may be recommended in such cases.
Fortunately, many of the most common soft-tissue masses can be accurately diagnosed with ultrasonography, including lipomas, ganglion cysts, foreign bodies, and simple fluid collections.4,39 Nerve-sheath tumors can also be diagnosed with ultrasonography if the lesion clearly arises from a nerve. Other soft-tissue masses are likely to be indeterminate with ultrasonography, requiring follow-up with MRI with contrast.
Tendons
Musculoskeletal ultrasonography can be effective for evaluating tendons around joints, especially 1 or a small number of nearby superficial tendons. Tendons particularly well suited for ultrasonographic examination include:
- Upper-extremity tendons located in the rotator cuff or around the elbow, and flexor and extensor tendons of the hands; ultrasonographic evaluation of the rotator cuff is highly accurate, equivalent to that of MRI for partial-thickness and full-thickness tearing40–43
- Lower-extremity tendons of the extensor mechanism of the knee, distal hamstring tendons, tendons around the ankle,44–46 and flexor and extensor tendons of the foot.
Ultrasonography can help diagnose a variety of tendon abnormalities (Table 1),48,49 including tearing, for which a dynamic examination can be performed.
Many tendons have a tendon sheath containing tenosynovium, while others have surrounding peritenon only; either can become thickened and inflamed. Tenosynovitis is a nonspecific finding and may be inflammatory, infectious, or posttraumatic. The presence of tendon sheath fluid alone on ultrasonography can be a normal finding, and some tendon sheaths that communicate with adjacent joints (eg, the long head biceps tendon, the flexor hallucis longus tendon) commonly contain simple fluid.6 A dynamic examination with ultrasonography can help diagnose snapping related to abnormal tendon movement, for example, in the case of intra-sheath and extra-sheath subluxation of the peroneal tendons.45,50,51
Ligaments
Ultrasonography can detect abnormalities in many superficial ligaments (Table 1).
Ankle. Ankle ligaments are superficial and can be clearly visualized. The diagnostic accuracy of ultrasonography for tearing of the anterior talofibular ligament may be as high as 100%.50,52,53
Elbow and thumb. The larger of the collateral ligaments of the elbow, especially the ulnar collateral ligament, and the ulnar collateral ligament of the thumb can be effectively evaluated with ultrasonography.54,55
Knee. The collateral ligaments of the knee can be seen with ultrasonography, but injuries of the external ligaments of the knee are often associated with intrinsic derangements that cannot be evaluated with ultrasonography.56,57 Intra-articular ligaments such as the anterior cruciate ligament are also not amenable to ultrasonography.
Dynamic examination of a ligament with ultrasonography can help determine the grade of the injury.
Deeply located ligaments (eg, around the hip) and ligaments surrounded by bone, such as the Lisfranc ligament, cannot be completely seen on ultrasonography.
Muscle
Musculoskeletal ultrasonography is useful for small areas of concern within a muscle (Table 1). It can detect muscle strains and tears, intramuscular collections or lesions, and fascial scarring or fascial injuries such as superficial muscle herniation. Although ultrasonography may yield a definitive diagnosis for a muscle problem, further imaging may be needed.
Nerves
Ultrasonography is useful for peripheral nerve investigation but requires a steep learning curve for sonographers and interpreting physicians.58,59 It is best suited for directed questions regarding focal abnormal nerve findings on physical examination.
Ultrasonography can help identify areas of nerve entrapment caused by a mass or dynamic compression. It can detect neuritis (Table 1), lesions of peripheral nerves (eg, nerve-sheath tumors), and neuromas (eg, Morton neuroma of the intermetatarsal space). In a large meta-analysis, ultrasonography and MRI were found to be equally accurate for detecting Morton neuroma.60 Even for nerve-sheath tumors located deep to the muscular fascia, ultrasonography can confirm the diagnosis because of the characteristic appearance of the nerves. Ultrasonography can also demonstrate a large extent of the course of superficial peripheral nerves while keeping the imaging plane appropriately oriented to the nerves.
Acknowledgment: We would like to sincerely thank Megan Griffiths, MA, for her help in the preparation and submission of this manuscript.
Ultrasonography has been used to evaluate musculoskeletal problems for decades but has only recently become more widely available in the United States. Advances in technology and physician familiarity are increasing its role in orthopedic imaging.
No single imaging method can yield all musculoskeletal diagnoses. Like any imaging technique, ultrasonography has strengths and weaknesses specific to orthopedics. Radiography, computed tomography (CT), and magnetic resonance imaging (MRI) play important roles for investigating musculoskeletal problems and are complementary to each other and to ultrasonography.
To help clinicians make informed decisions about ordering musculoskeletal ultrasonography, this article reviews the basic physics underlying ultrasonography, its advantages and disadvantages compared with other imaging methods, and common clinical applications.
CLASSIC TECHNOLOGY MAKING A RESURGENCE
The first reports of the use of musculoskeletal ultrasonography appeared in the 1970s for investigating the rotator cuff,1–3 actually preceding reports of its use in obstetrics and gynecology.4 In the 1980s, reports emerged for evaluating the Achilles tendon.5,6 After that, its popularity in the United States plateaued, likely because of the advent of MRI, lower reimbursement and greater variability in interpretation compared with MRI, as well as a lack of physicians and sonographers trained in its use.7,8
Musculoskeletal ultrasonography is currently experiencing a resurgence. Although it remains a specialized service more commonly available in large hospitals, its use is increasing rapidly, and it will likely become more widely available.
SPECIAL TRAINING REQUIRED
Musculoskeletal ultrasonography is simply an ultrasonographic examination of part of the musculoskeletal system. But because not all ultrasonographic transducers offer sufficient resolution for musculoskeletal evaluation and not all sonographers and imaging physicians are familiar with the specialized techniques, musculoskeletal ultrasonography often has a separate designation (eg, “MSKUS,” “MSUS”). At Cleveland Clinic, it is offered through the department of musculoskeletal imaging by subspecialty-trained musculoskeletal radiologists and specially trained musculoskeletal ultrasonographers with 4 to 5 years of training in the technique.
Musculoskeletal ultrasonography is also performed by physician groups with specialized training, including sports medicine physicians, rheumatologists, physiatrists, neurologists, and orthopedic surgeons. The American Institute of Ultrasound in Medicine offers voluntary accreditation for practice groups using musculoskeletal ultrasonography. Certification in musculoskeletal radiology is offered to sonographers through the American Registry for Diagnostic Medical Sonography.
SONOGRAPHY HAS UNIQUE QUALITIES
Ultrasonography uses high-frequency sound waves to generate images. The transducer (or probe) emits sound from the many piezoelectric elements at its surface, and the sound waves travel through and react with tissues. Sound reflected by tissues is detected by the transducer and converted to an image. Objects that reflect sound appear hyperechoic (brighter), whereas tissues that reflect little or no sound appear hypoechoic.
High-resolution imaging of superficial structures
(B, arrow).
Ultrasonography involves a fundamental trade-off between image resolution and imaging depth. Higher-frequency sound waves do not penetrate far into tissues but generate a higher-resolution image; lower-frequency sound waves can penetrate much further but yield a lower-resolution image. Although high-resolution imaging of deep structures with ultrasonography is not possible (Figure 1), many musculoskeletal structures are located superficially and are amenable to ultrasonographic evaluation.
Be aware of artifacts
Some materials attenuate sound very little, such as simple fluid. Low attenuation results in artifacts on ultrasonography, making tissues behind the simple fluid appear brighter than neighboring tissues. These artifacts may be reported as “increased through transmission” or “posterior acoustic enhancement.” Conversely, metal and bone reflect all sound waves that reach them, rendering any structures beyond them invisible. This “shadowing” creates a problem for imaging of structures in or near bone. Subcutaneous fat also attenuates sound waves, limiting the use of ultrasonography for patients with obesity (Figure 2).
High-frequency linear transducer sharpens images
High-frequency linear transducers reduce anisotropy because their flat surface keeps sound waves more uniformly perpendicular to the structure of interest.4,7 Their development has allowed imaging of superficial structures that is superior to that of MRI. A high-frequency linear transducer offers more than twice the spatial resolution of a typical 1.5T MRI examination of superficial tissue.12,13
Operator experience is critical
Ultrasonography examinations, more than other imaging tests, are dependent on operator experience. A solid understanding of musculoskeletal anatomy is imperative. Because the probe images only a thin section of tissue (about the thickness of a credit card), referencing adjacent structures for orientation is more difficult with ultrasonography than with CT or MRI.
The accuracy of ultrasonography is highly dependent on acquiring and interpreting images, whereas the accuracy of MRI is dependent primarily on image interpretation.7 Interpreting physicians must check that sonographers capture relevant targets.
STRENGTHS OF MUSCULOSKELETAL ULTRASONOGRAPHY
Ultrasonography has multiple advantages:
No ionizing radiation exposure.
Portability. Unlike CT or MRI, ultrasonography equipment is portable.
Increased patient comfort. Patient positioning for an ultrasonography examination is more flexible than for MRI or CT,14 and the examination does not induce claustrophobia.8
High-resolution imaging. Ultrasonography provides very-high-resolution imaging of superficial soft tissues—in some cases, higher than MRI or CT.
Real-time dynamic examinations are possible with ultrasonography, unlike with CT or MRI, and may increase test sensitivity.4,15–18
Implanted hardware is less of a problem. Although ultrasonography cannot image beyond implanted orthopedic metallic hardware, the hardware does not obscure surrounding soft tissues as it does on CT and MRI.6,19,20 Also, ultrasonography is safe for patients with a pacemaker.8
WEAKNESSES
The main disadvantages of musculoskeletal ultrasonography are inherent to its limited field of view, making it inappropriate for a survey examination (eg, for ankle pain, knee pain, hip pain).4 Unlike CT and MRI, ultrasonography does not provide a “bird’s-eye view,” and important abnormalities can be missed during evaluation of large areas (Figure 4).
Ultrasonography also cannot evaluate bone or intra-articular structures such as cartilage, bone marrow, labrum, and intra-articular ligaments; MRI is the standard for evaluating these structures.21
Ultrasonography is time-consuming. To perform a detailed examination of the anterior, posterior, medial, and lateral aspects of the hip, knee, or ankle would require 1.5 to 2 hours of scanning time and an additional 10 to 25 minutes of image checking and interpretation.
CURRENT CLINICAL INDICATIONS
Musculoskeletal ultrasonography is best used for clinical questions regarding limited, superficial musculoskeletal problems.
Fluid collections
Ultrasonography can help evaluate small fluid collections in soft tissue. As is true for a lung opacity on chest radiography, soft-tissue fluid detected on ultrasonography is nonspecific, and results must be correlated with the clinical picture to narrow the differential diagnosis.
Fluid collections can be classified as loculated or nonloculated.
Nonloculated fluid involves more fluid than is simply interposed between tissue planes and has no wall or defined margins. It can be simple or complex in appearance: simple fluid is anechoic, and complex fluid appears more heterogeneous and may contain septations or debris.
Subcutaneous edema, which may occur postoperatively or from trauma, venous insufficiency, or inflammatory or infectious processes, appears on ultrasonography as nonloculated fluid interspersed between subcutaneous fat lobules.
Loculated fluid collections have well-defined margins or a discrete wall that does not follow normal tissue planes. They can also be simple or complex and can be caused by hematoma, abscess, or ganglion. Less commonly, neoplasms can mimic a loculated fluid collection (Figure 4).
A ganglion is a specific type of loculated fluid collection containing synovial fluid arising from a joint or tendon sheath. It tends to occur in specific locations, most commonly around the wrist, most often arising from the dorsal scapholunate ligament and volar wrist between the radial artery and flexor carpi radialis.22 On MRI, it can be difficult to distinguish between small vascular structures and a small ganglion, especially in the hands and feet.23
Ultrasonography can also help identify a Baker cyst, a specific fluid collection arising from the semimembranosus bursa between the medial head of the gastrocnemius tendon and the semimembranosus tendon. Ultrasonography can also detect inflammation, rupture, or leaking associated with a Baker cyst.24
Power Doppler is an ultrasonographic examination that can detect increased blood flow surrounding a fluid collection and determine the likelihood of an acute inflammatory or infectious cause.25
Joint effusion and synovitis
Musculoskeletal ultrasonography can help evaluate joints for effusion and synovitis. It is highly sensitive (94%) and specific (95%) for synovitis, making it superior to contrast-enhanced MRI.26,27 The area of concern should be limited to 1 quadrant of a joint (anterior, posterior, medial, or lateral); for problems beyond that, MRI should be considered.
A joint effusion appears as a distended joint capsule containing hypoechoic (complex) or anechoic (simple) joint fluid.
Complex joint fluid may contain debris and occurs with hemarthrosis, infection, and inflammation.23 Hypertrophied synovium is hypoechoic and can mimic complex joint fluid.
Power Doppler evaluation can help distinguish synovitis from joint fluid by demonstrating blood flow, a feature of synovitis but not of simple joint fluid. Power Doppler is the most sensitive means of detecting blood flow, although it does not show direction of flow.28
Using ultrasonography can help to improve disease control and minimize disabling changes by monitoring synovitis therapy. In addition, subclinical synovitis and enthesitis (inflammation of insertion sites of tendons or ligaments into bone) detected by ultrasonography may predict future disease and disease flares.29–31
Ultrasonographic guidance for a wide range of procedures is increasing rapidly.32–36 Multiple studies have shown the advantage of ultrasonography-guided aspiration and injection compared with techniques without imaging guidance.37,38
Soft-tissue masses
Accurately diagnosing soft-tissue masses can be difficult. A mass may remain indeterminate even after multiple imaging studies, requiring biopsy or surgical referral. However, for a few specific masses, ultrasonography is highly accurate and can eliminate the need for further imaging.
Ultrasonography can help evaluate soft- tissue masses no larger than 5 cm in diameter and no deeper than superficial muscular fascia. If the mass is larger or deeper than that, ultrasonography is less reliable for showing the margins of the mass and its relationship to adjacent structures (Figure 5). Further imaging by MRI may be recommended in such cases.
Fortunately, many of the most common soft-tissue masses can be accurately diagnosed with ultrasonography, including lipomas, ganglion cysts, foreign bodies, and simple fluid collections.4,39 Nerve-sheath tumors can also be diagnosed with ultrasonography if the lesion clearly arises from a nerve. Other soft-tissue masses are likely to be indeterminate with ultrasonography, requiring follow-up with MRI with contrast.
Tendons
Musculoskeletal ultrasonography can be effective for evaluating tendons around joints, especially 1 or a small number of nearby superficial tendons. Tendons particularly well suited for ultrasonographic examination include:
- Upper-extremity tendons located in the rotator cuff or around the elbow, and flexor and extensor tendons of the hands; ultrasonographic evaluation of the rotator cuff is highly accurate, equivalent to that of MRI for partial-thickness and full-thickness tearing40–43
- Lower-extremity tendons of the extensor mechanism of the knee, distal hamstring tendons, tendons around the ankle,44–46 and flexor and extensor tendons of the foot.
Ultrasonography can help diagnose a variety of tendon abnormalities (Table 1),48,49 including tearing, for which a dynamic examination can be performed.
Many tendons have a tendon sheath containing tenosynovium, while others have surrounding peritenon only; either can become thickened and inflamed. Tenosynovitis is a nonspecific finding and may be inflammatory, infectious, or posttraumatic. The presence of tendon sheath fluid alone on ultrasonography can be a normal finding, and some tendon sheaths that communicate with adjacent joints (eg, the long head biceps tendon, the flexor hallucis longus tendon) commonly contain simple fluid.6 A dynamic examination with ultrasonography can help diagnose snapping related to abnormal tendon movement, for example, in the case of intra-sheath and extra-sheath subluxation of the peroneal tendons.45,50,51
Ligaments
Ultrasonography can detect abnormalities in many superficial ligaments (Table 1).
Ankle. Ankle ligaments are superficial and can be clearly visualized. The diagnostic accuracy of ultrasonography for tearing of the anterior talofibular ligament may be as high as 100%.50,52,53
Elbow and thumb. The larger of the collateral ligaments of the elbow, especially the ulnar collateral ligament, and the ulnar collateral ligament of the thumb can be effectively evaluated with ultrasonography.54,55
Knee. The collateral ligaments of the knee can be seen with ultrasonography, but injuries of the external ligaments of the knee are often associated with intrinsic derangements that cannot be evaluated with ultrasonography.56,57 Intra-articular ligaments such as the anterior cruciate ligament are also not amenable to ultrasonography.
Dynamic examination of a ligament with ultrasonography can help determine the grade of the injury.
Deeply located ligaments (eg, around the hip) and ligaments surrounded by bone, such as the Lisfranc ligament, cannot be completely seen on ultrasonography.
Muscle
Musculoskeletal ultrasonography is useful for small areas of concern within a muscle (Table 1). It can detect muscle strains and tears, intramuscular collections or lesions, and fascial scarring or fascial injuries such as superficial muscle herniation. Although ultrasonography may yield a definitive diagnosis for a muscle problem, further imaging may be needed.
Nerves
Ultrasonography is useful for peripheral nerve investigation but requires a steep learning curve for sonographers and interpreting physicians.58,59 It is best suited for directed questions regarding focal abnormal nerve findings on physical examination.
Ultrasonography can help identify areas of nerve entrapment caused by a mass or dynamic compression. It can detect neuritis (Table 1), lesions of peripheral nerves (eg, nerve-sheath tumors), and neuromas (eg, Morton neuroma of the intermetatarsal space). In a large meta-analysis, ultrasonography and MRI were found to be equally accurate for detecting Morton neuroma.60 Even for nerve-sheath tumors located deep to the muscular fascia, ultrasonography can confirm the diagnosis because of the characteristic appearance of the nerves. Ultrasonography can also demonstrate a large extent of the course of superficial peripheral nerves while keeping the imaging plane appropriately oriented to the nerves.
Acknowledgment: We would like to sincerely thank Megan Griffiths, MA, for her help in the preparation and submission of this manuscript.
- Hamilton JV, Flinn G Jr, Haynie CC, Cefalo RC. Diagnosis of rectus sheath hematoma by B-mode ultrasound: a case report. Am J Obstet Gynecol 1976; 125(4):562–565. doi:10.1016/0002-9378(76)90379-3
- Zweymüller VK, Kratochwil A. Ultrasound diagnosis of bone and soft tissue tumours. Wien Klin Wochenschr 1975; 87(12):397–398. German.
- Mayer V. Ultrasonography of the rotator cuff. J Ultrasound Med 1985; 4(11):608, 607. doi:10.7863/jum.1985.4.11.608
- McNally EG. The development and clinical applications of musculoskeletal ultrasound. Skeletal Radiol 2011; 40(9):1223–1231. doi:10.1007/s00256-011-1220-5
- Ignashin NS, Girshin SG, Tsypin IS. Ultrasonic scanning in subcutaneous rupture of the Achilles tendon. Vestn Khir Im I I Grek 1981; 127(9):82–85. Russian.
- Robinson P. Sonography of common tendon injuries. AJR Am J Roentgenol 2009; 193(3):607–618. doi:10.2214/AJR.09.2808
- Jacobson JA. Musculoskeletal ultrasound: focused impact on MRI. AJR Am J Roentgenol 2009; 193(3):619–627. doi:10.2214/AJR.09.2841
- Nazarian LN. The top 10 reasons musculoskeletal sonography is an important complementary or alternative technique to MRI. AJR Am J Roentgenol 2008; 190(6):1621–1626. doi:10.2214/AJR.07.3385
- AIUM technical bulletin. Transducer manipulation. American Institute of Ultrasound in Medicine. J Ultrasound Med 1999; 18(2):169–175. doi:10.7863/jum.1999.18.2.169
- Connolly DJ, Berman L, McNally EG. The use of beam angulation to overcome anisotropy when viewing human tendon with high frequency linear array ultrasound. Br J Radiol 2001; 74 (878):183–185. doi:10.1259/bjr.74.878.740183
- Crass JR, van de Vegte GL, Harkavy LA. Tendon echogenicity: ex vivo study. Radiology 1988; 167(2):499–501. doi:10.1148/radiology.167.2.3282264
- Erickson SJ. High-resolution imaging of the musculoskeletal system. Radiology 1997; 205(3):593–618. doi:10.1148/radiology.205.3.9393511
- Link TM, Majumdar S, Peterfy C, et al. High resolution MRI of small joints: impact of spatial resolution on diagnostic performance and SNR. Magn Reson Imaging 1998; 16(2):147–155. doi:10.1016/S0730-725X(97)00244-0
- Middleton WD, Payne WT, Teefey SA, Hildebolt CF, Rubin DA, Yamaguchi K. Sonography and MRI of the shoulder: comparison of patient satisfaction. AJR Am J Roentgenol 2004; 183(5):1449–1452. doi:10.2214/ajr.183.5.1831449
- Khoury V, Cardinal E, Bureau NJ. Musculoskeletal sonography: a dynamic tool for usual and unusual disorders. AJR Am J Roentgenol 2007; 188(1):W63–W73. doi:10.2214/AJR.06.0579
- Farin PU, Jaroma H, Harju A, Soimakallio S. Medial displacement of the biceps brachii tendon: evaluation with dynamic sonography during maximal external shoulder rotation. Radiology 1995; 195(3):845–848. doi:10.1148/radiology.195.3.7754019
- Miller TT, Adler RS, Friedman L. Sonography of injury of the ulnar collateral ligament of the elbow-initial experience. Skeletal Radiol 2004; 33(7):386–391. doi:10.1007/s00256-004-0788-4
- Nazarian LN, McShane JM, Ciccotti MG, O’Kane PL, Harwood MI. Dynamic US of the anterior band of the ulnar collateral ligament of the elbow in asymptomatic major league baseball pitchers. Radiology 2003; 227(1):149–154. doi:10.1148/radiol.2271020288
- Jacobson JA, Lax MJ. Musculoskeletal sonography of the postoperative orthopedic patient. Semin Musculoskelet Radiol 2002; 6(1):67–77. doi:10.1055/s-2002-23165
- Sofka CM, Adler RS. Original report. Sonographic evaluation of shoulder arthroplasty. AJR Am J Roentgenol 2003; 180(4):1117–1120. doi:10.2214/ajr.180.4.1801117
- Silvestri E, Martinoli C, Derchi LE, Bertolotto M, Chiaramondia M, Rosenberg I. Echotexture of peripheral nerves: correlation between US and histologic findings and criteria to differentiate tendons. Radiology 1995; 197(1):291–296. doi:10.1148/radiology.197.1.7568840
- Cardinal E, Buckwalter KA, Braunstein EM, Mih AD. Occult dorsal carpal ganglion: comparison of US and MR imaging. Radiology 1994; 193(1):259–262. doi:10.1148/radiology.193.1.8090903
- Jacobson JA. Musculoskeletal ultrasound and MRI: which do I choose? Semin Musculoskelet Radiol 2005; 9(2):135–149. doi:10.1055/s-2005-872339
- Ward EE, Jacobson JA, Fessell DP, Hayes CW, van Holsbeeck M. Sonographic detection of Baker’s cysts: comparison with MR imaging. AJR Am J Roentgenol 2001; 176(2):373–380. doi:10.2214/ajr.176.2.1760373
- Bhasin S, Cheung PP. The role of power Doppler ultrasonography as disease activity marker in rheumatoid arthritis. Dis Markers 2015; 2015:325909. doi:10.1155/2015/325909
- Fukuba E, Yoshizako T, Kitagaki H, Murakawa Y, Kondo M, Uchida N. Power Doppler ultrasonography for assessment of rheumatoid synovitis: comparison with dynamic magnetic resonance imaging. Clin Imaging 2013; 37(1):134–137. doi:10.1016/j.clinimag.2012.02.008
- Takase-Minegishi K, Horita N, Kobayashi K, et al. Diagnostic test accuracy of ultrasound for synovitis in rheumatoid arthritis: systematic review and meta-analysis. Rheumatology (Oxford) 2018; 57(1):49–58. doi:10.1093/rheumatology/kex036
- Klareskog L, Catrina AI, Paget S. Rheumatoid arthritis. Lancet 2009; 373(9664):659–672. doi:10.1016/S0140-6736(09)60008-8
- Ash ZR, Tinazzi I, Gallego CC, et al. Psoriasis patients with nail disease have a greater magnitude of underlying systemic subclinical enthesopathy than those with normal nails. Ann Rheum Dis 2012; 71(4):553–556. doi:10.1136/annrheumdis-2011-200478
- Han J, Geng Y, Deng X, Zhang Z. Subclinical synovitis assessed by ultrasound predicts flare and progressive bone erosion in rheumatoid arthritis patients with clinical remission: a systematic review and metaanalysis. J Rheumatol 2016; 43(11):2010–2018. doi.org/10.3899/jrheum.160193
- Iagnocco A, Finucci A, Ceccarelli F, Perricone C, Iorgoveanu V, Valesini G. Power Doppler ultrasound monitoring of response to anti-tumour necrosis factor alpha treatment in patients with rheumatoid arthritis. Rheumatology (Oxford) 2015; 54(10):1890–1896. doi:10.1093/rheumatology/kev211
- Henning PT. Ultrasound-guided foot and ankle procedures. Phys Med Rehabil Clin N Am 2016; 27(3):649–671. doi:10.1016/j.pmr.2016.04.005
- Lueders DR, Smith J, Sellon JL. Ultrasound-guided knee procedures. Phys Med Rehabil Clin North Am 2016; 27(3):631–648. doi:10.1016/j.pmr.2016.04.010
- Payne JM. Ultrasound-guided hip procedures. Phys Med Rehabil Clin North Am 2016; 27(3):607–629. doi:10.1016/j.pmr.2016.04.004
- Strakowski JA. Ultrasound-guided peripheral nerve procedures. Phys Med Rehabil Clin North Am 2016; 27(3):687–715. doi:10.1016/j.pmr.2016.04.006
- Sussman WI, Williams CJ, Mautner K. Ultrasound-guided elbow procedures. Phys Med Rehabil Clin North Am 2016; 27(3):573–587. doi:10.1016/j.pmr.2016.04.002
- Finnoff JT. The evolution of diagnostic and interventional ultrasound in sports medicine. PM R 2016; 8(suppl 3):S133–S138. doi:10.1016/j.pmrj.2015.09.022
- Wu T, Dong Y, Song H, Fu Y, Li JH. Ultrasound-guided versus landmark in knee arthrocentesis: a systematic review. Semin Arthritis Rheum 2016; 45(5):627–632. doi:10.1016/j.semarthrit.2015.10.011
- Failla JM, van Holsbeeck M, Vanderschueren G. Detection of a 0.5-mm-thick thorn using ultrasound: a case report. J Hand Surg Am 1995; 20(3):456–457.
- Teefey SA, Hasan SA, Middleton WD, Patel M, Wright RW, Yamaguchi K. Ultrasonography of the rotator cuff. A comparison of ultrasonographic and arthroscopic findings in one hundred consecutive cases. J Bone Joint Surg Am 2000; 82(4):498–504.
- van Holsbeeck MT, Kolowich PA, Eyler WR, et al. US depiction of partial-thickness tear of the rotator cuff. Radiology 1995; 197(2):443–446. doi:10.1148/radiology.197.2.7480690
- Balich SM, Sheley RC, Brown TR, Sauser DD, Quinn SF. MR imaging of the rotator cuff tendon: interobserver agreement and analysis of interpretive errors. Radiology 1997; 204(1):191–194. doi:10.1148/radiology.204.1.9205245
- Dinnes J, Loveman E, McIntyre L, Waugh N. The effectiveness of diagnostic tests for the assessment of shoulder pain due to soft tissue disorders: a systematic review. Health Technol Assess 2003; 7(29):1–166. doi:10.3310/hta7290
- Rockett MS, Waitches G, Sudakoff G, Brage M. Use of ultrasonography versus magnetic resonance imaging for tendon abnormalities around the ankle. Foot Ankle Int 1998; 19(9):604–612.
- Grant TH, Kelikian AS, Jereb SE, McCarthy RJ. Ultrasound diagnosis of peroneal tendon tears. A surgical correlation. J Bone Joint Surg Am 2005; 87(8):1788–1794. doi:10.2106/JBJS.D.02450
- Hartgerink P, Fessell DP, Jacobson JA, van Holsbeeck MT. Full- versus partial-thickness Achilles tendon tears: sonographic accuracy and characterization in 26 cases with surgical correlation. Radiology 2001; 220(2):406–412. doi:10.1148/radiology.220.2.r01au41406
- Cho KH, Park BH, Yeon KM. Ultrasound of the adult hip. Semin Ultrasound CT MR 2000; 21(3):214–230.
- Adler RS, Finzel KC. The complementary roles of MR imaging and ultrasound of tendons. Radiol Clin North Am 2005; 43(4):771–807. doi:10.1016/j.rcl.2005.02.011
- Martinoli C, Bianchi S, Derchi LE. Tendon and nerve sonography. Radiol Clin North Am 1999; 37(4):691–711. doi:10.1016/S0033-8389(05)70124-X
- Fessell DP, Vanderschueren GM, Jacobson JA, et al. US of the ankle: technique, anatomy, and diagnosis of pathologic conditions. Radiographics 1998; 18(2):325–340. doi:10.1148/radiographics.18.2.9536481
- Neustadter J, Raikin SM, Nazarian LN. Dynamic sonographic evaluation of peroneal tendon subluxation. AJR Am J Roentgenol 2004; 183(4):985–988. doi:10.2214/ajr.183.4.1830985
- Verhaven EF, Shahabpour M, Handelberg FW, Vaes PH, Opdecam PJ. The accuracy of three-dimensional magnetic resonance imaging in the diagnosis of ruptures of the lateral ligaments of the ankle. Am J Sports Med 1991; 19(6):583–587. doi:10.1177/036354659101900605
- Milz P, Milz S, Steinborn M, Mittlmeier T, Putz R, Reiser M. Lateral ankle ligaments and tibiofibular syndesmosis. 13-MHz high-frequency sonography and MRI compared in 20 patients. Acta Orthop Scand 1998; 69(1):51–55.
- De Smet AA, Winter TC, Best TM, Bernhardt DT. Dynamic sonography with valgus stress to assess elbow ulnar collateral ligament injury in baseball pitchers. Skeletal Radiol 2002; 31(11):671–676. doi:10.1007/s00256-002-0558-0
- Melville DM, Jacobson JA, Fessell DP. Ultrasound of the thumb ulnar collateral ligament: technique and pathology. AJR Am J Roentgenol 2014; 202(2):W168. doi:10.2214/AJR.13.11335
- Court-Payen M. Sonography of the knee: intra-articular pathology. J Clin Ultrasound 2004; 32(9):481–490. doi:10.1002/jcu.20069
- Azzoni R, Cabitza P. Is there a role for sonography in the diagnosis of tears of the knee menisci? J Clin Ultrasound 2002; 30(8):472–476. doi:10.1002/jcu.10106
- Jacobson JA, Wilson TJ, Yang LJ. Sonography of common peripheral nerve disorders with clinical correlation. J Ultrasound Med 2016; 35(4):683–693. doi:10.7863/ultra.15.05061
- Ali ZS, Pisapia JM, Ma TS, Zager EL, Heuer GG, Khoury V. Ultrasonographic evaluation of peripheral nerves. World Neurosurg 2016; 85(1):333–339. doi:10.1016/j.wneu.2015.10.005
- Bignotti B, Signori A, Sormani MP, Molfetta L, Martinoli C, Tagliafico A. Ultrasound versus magnetic resonance imaging for Morton neuroma: systematic review and meta-analysis. Eur Radiol 2015; 25(8):2254–2262. doi:10.1007/s00330-015-3633-3
- Hamilton JV, Flinn G Jr, Haynie CC, Cefalo RC. Diagnosis of rectus sheath hematoma by B-mode ultrasound: a case report. Am J Obstet Gynecol 1976; 125(4):562–565. doi:10.1016/0002-9378(76)90379-3
- Zweymüller VK, Kratochwil A. Ultrasound diagnosis of bone and soft tissue tumours. Wien Klin Wochenschr 1975; 87(12):397–398. German.
- Mayer V. Ultrasonography of the rotator cuff. J Ultrasound Med 1985; 4(11):608, 607. doi:10.7863/jum.1985.4.11.608
- McNally EG. The development and clinical applications of musculoskeletal ultrasound. Skeletal Radiol 2011; 40(9):1223–1231. doi:10.1007/s00256-011-1220-5
- Ignashin NS, Girshin SG, Tsypin IS. Ultrasonic scanning in subcutaneous rupture of the Achilles tendon. Vestn Khir Im I I Grek 1981; 127(9):82–85. Russian.
- Robinson P. Sonography of common tendon injuries. AJR Am J Roentgenol 2009; 193(3):607–618. doi:10.2214/AJR.09.2808
- Jacobson JA. Musculoskeletal ultrasound: focused impact on MRI. AJR Am J Roentgenol 2009; 193(3):619–627. doi:10.2214/AJR.09.2841
- Nazarian LN. The top 10 reasons musculoskeletal sonography is an important complementary or alternative technique to MRI. AJR Am J Roentgenol 2008; 190(6):1621–1626. doi:10.2214/AJR.07.3385
- AIUM technical bulletin. Transducer manipulation. American Institute of Ultrasound in Medicine. J Ultrasound Med 1999; 18(2):169–175. doi:10.7863/jum.1999.18.2.169
- Connolly DJ, Berman L, McNally EG. The use of beam angulation to overcome anisotropy when viewing human tendon with high frequency linear array ultrasound. Br J Radiol 2001; 74 (878):183–185. doi:10.1259/bjr.74.878.740183
- Crass JR, van de Vegte GL, Harkavy LA. Tendon echogenicity: ex vivo study. Radiology 1988; 167(2):499–501. doi:10.1148/radiology.167.2.3282264
- Erickson SJ. High-resolution imaging of the musculoskeletal system. Radiology 1997; 205(3):593–618. doi:10.1148/radiology.205.3.9393511
- Link TM, Majumdar S, Peterfy C, et al. High resolution MRI of small joints: impact of spatial resolution on diagnostic performance and SNR. Magn Reson Imaging 1998; 16(2):147–155. doi:10.1016/S0730-725X(97)00244-0
- Middleton WD, Payne WT, Teefey SA, Hildebolt CF, Rubin DA, Yamaguchi K. Sonography and MRI of the shoulder: comparison of patient satisfaction. AJR Am J Roentgenol 2004; 183(5):1449–1452. doi:10.2214/ajr.183.5.1831449
- Khoury V, Cardinal E, Bureau NJ. Musculoskeletal sonography: a dynamic tool for usual and unusual disorders. AJR Am J Roentgenol 2007; 188(1):W63–W73. doi:10.2214/AJR.06.0579
- Farin PU, Jaroma H, Harju A, Soimakallio S. Medial displacement of the biceps brachii tendon: evaluation with dynamic sonography during maximal external shoulder rotation. Radiology 1995; 195(3):845–848. doi:10.1148/radiology.195.3.7754019
- Miller TT, Adler RS, Friedman L. Sonography of injury of the ulnar collateral ligament of the elbow-initial experience. Skeletal Radiol 2004; 33(7):386–391. doi:10.1007/s00256-004-0788-4
- Nazarian LN, McShane JM, Ciccotti MG, O’Kane PL, Harwood MI. Dynamic US of the anterior band of the ulnar collateral ligament of the elbow in asymptomatic major league baseball pitchers. Radiology 2003; 227(1):149–154. doi:10.1148/radiol.2271020288
- Jacobson JA, Lax MJ. Musculoskeletal sonography of the postoperative orthopedic patient. Semin Musculoskelet Radiol 2002; 6(1):67–77. doi:10.1055/s-2002-23165
- Sofka CM, Adler RS. Original report. Sonographic evaluation of shoulder arthroplasty. AJR Am J Roentgenol 2003; 180(4):1117–1120. doi:10.2214/ajr.180.4.1801117
- Silvestri E, Martinoli C, Derchi LE, Bertolotto M, Chiaramondia M, Rosenberg I. Echotexture of peripheral nerves: correlation between US and histologic findings and criteria to differentiate tendons. Radiology 1995; 197(1):291–296. doi:10.1148/radiology.197.1.7568840
- Cardinal E, Buckwalter KA, Braunstein EM, Mih AD. Occult dorsal carpal ganglion: comparison of US and MR imaging. Radiology 1994; 193(1):259–262. doi:10.1148/radiology.193.1.8090903
- Jacobson JA. Musculoskeletal ultrasound and MRI: which do I choose? Semin Musculoskelet Radiol 2005; 9(2):135–149. doi:10.1055/s-2005-872339
- Ward EE, Jacobson JA, Fessell DP, Hayes CW, van Holsbeeck M. Sonographic detection of Baker’s cysts: comparison with MR imaging. AJR Am J Roentgenol 2001; 176(2):373–380. doi:10.2214/ajr.176.2.1760373
- Bhasin S, Cheung PP. The role of power Doppler ultrasonography as disease activity marker in rheumatoid arthritis. Dis Markers 2015; 2015:325909. doi:10.1155/2015/325909
- Fukuba E, Yoshizako T, Kitagaki H, Murakawa Y, Kondo M, Uchida N. Power Doppler ultrasonography for assessment of rheumatoid synovitis: comparison with dynamic magnetic resonance imaging. Clin Imaging 2013; 37(1):134–137. doi:10.1016/j.clinimag.2012.02.008
- Takase-Minegishi K, Horita N, Kobayashi K, et al. Diagnostic test accuracy of ultrasound for synovitis in rheumatoid arthritis: systematic review and meta-analysis. Rheumatology (Oxford) 2018; 57(1):49–58. doi:10.1093/rheumatology/kex036
- Klareskog L, Catrina AI, Paget S. Rheumatoid arthritis. Lancet 2009; 373(9664):659–672. doi:10.1016/S0140-6736(09)60008-8
- Ash ZR, Tinazzi I, Gallego CC, et al. Psoriasis patients with nail disease have a greater magnitude of underlying systemic subclinical enthesopathy than those with normal nails. Ann Rheum Dis 2012; 71(4):553–556. doi:10.1136/annrheumdis-2011-200478
- Han J, Geng Y, Deng X, Zhang Z. Subclinical synovitis assessed by ultrasound predicts flare and progressive bone erosion in rheumatoid arthritis patients with clinical remission: a systematic review and metaanalysis. J Rheumatol 2016; 43(11):2010–2018. doi.org/10.3899/jrheum.160193
- Iagnocco A, Finucci A, Ceccarelli F, Perricone C, Iorgoveanu V, Valesini G. Power Doppler ultrasound monitoring of response to anti-tumour necrosis factor alpha treatment in patients with rheumatoid arthritis. Rheumatology (Oxford) 2015; 54(10):1890–1896. doi:10.1093/rheumatology/kev211
- Henning PT. Ultrasound-guided foot and ankle procedures. Phys Med Rehabil Clin N Am 2016; 27(3):649–671. doi:10.1016/j.pmr.2016.04.005
- Lueders DR, Smith J, Sellon JL. Ultrasound-guided knee procedures. Phys Med Rehabil Clin North Am 2016; 27(3):631–648. doi:10.1016/j.pmr.2016.04.010
- Payne JM. Ultrasound-guided hip procedures. Phys Med Rehabil Clin North Am 2016; 27(3):607–629. doi:10.1016/j.pmr.2016.04.004
- Strakowski JA. Ultrasound-guided peripheral nerve procedures. Phys Med Rehabil Clin North Am 2016; 27(3):687–715. doi:10.1016/j.pmr.2016.04.006
- Sussman WI, Williams CJ, Mautner K. Ultrasound-guided elbow procedures. Phys Med Rehabil Clin North Am 2016; 27(3):573–587. doi:10.1016/j.pmr.2016.04.002
- Finnoff JT. The evolution of diagnostic and interventional ultrasound in sports medicine. PM R 2016; 8(suppl 3):S133–S138. doi:10.1016/j.pmrj.2015.09.022
- Wu T, Dong Y, Song H, Fu Y, Li JH. Ultrasound-guided versus landmark in knee arthrocentesis: a systematic review. Semin Arthritis Rheum 2016; 45(5):627–632. doi:10.1016/j.semarthrit.2015.10.011
- Failla JM, van Holsbeeck M, Vanderschueren G. Detection of a 0.5-mm-thick thorn using ultrasound: a case report. J Hand Surg Am 1995; 20(3):456–457.
- Teefey SA, Hasan SA, Middleton WD, Patel M, Wright RW, Yamaguchi K. Ultrasonography of the rotator cuff. A comparison of ultrasonographic and arthroscopic findings in one hundred consecutive cases. J Bone Joint Surg Am 2000; 82(4):498–504.
- van Holsbeeck MT, Kolowich PA, Eyler WR, et al. US depiction of partial-thickness tear of the rotator cuff. Radiology 1995; 197(2):443–446. doi:10.1148/radiology.197.2.7480690
- Balich SM, Sheley RC, Brown TR, Sauser DD, Quinn SF. MR imaging of the rotator cuff tendon: interobserver agreement and analysis of interpretive errors. Radiology 1997; 204(1):191–194. doi:10.1148/radiology.204.1.9205245
- Dinnes J, Loveman E, McIntyre L, Waugh N. The effectiveness of diagnostic tests for the assessment of shoulder pain due to soft tissue disorders: a systematic review. Health Technol Assess 2003; 7(29):1–166. doi:10.3310/hta7290
- Rockett MS, Waitches G, Sudakoff G, Brage M. Use of ultrasonography versus magnetic resonance imaging for tendon abnormalities around the ankle. Foot Ankle Int 1998; 19(9):604–612.
- Grant TH, Kelikian AS, Jereb SE, McCarthy RJ. Ultrasound diagnosis of peroneal tendon tears. A surgical correlation. J Bone Joint Surg Am 2005; 87(8):1788–1794. doi:10.2106/JBJS.D.02450
- Hartgerink P, Fessell DP, Jacobson JA, van Holsbeeck MT. Full- versus partial-thickness Achilles tendon tears: sonographic accuracy and characterization in 26 cases with surgical correlation. Radiology 2001; 220(2):406–412. doi:10.1148/radiology.220.2.r01au41406
- Cho KH, Park BH, Yeon KM. Ultrasound of the adult hip. Semin Ultrasound CT MR 2000; 21(3):214–230.
- Adler RS, Finzel KC. The complementary roles of MR imaging and ultrasound of tendons. Radiol Clin North Am 2005; 43(4):771–807. doi:10.1016/j.rcl.2005.02.011
- Martinoli C, Bianchi S, Derchi LE. Tendon and nerve sonography. Radiol Clin North Am 1999; 37(4):691–711. doi:10.1016/S0033-8389(05)70124-X
- Fessell DP, Vanderschueren GM, Jacobson JA, et al. US of the ankle: technique, anatomy, and diagnosis of pathologic conditions. Radiographics 1998; 18(2):325–340. doi:10.1148/radiographics.18.2.9536481
- Neustadter J, Raikin SM, Nazarian LN. Dynamic sonographic evaluation of peroneal tendon subluxation. AJR Am J Roentgenol 2004; 183(4):985–988. doi:10.2214/ajr.183.4.1830985
- Verhaven EF, Shahabpour M, Handelberg FW, Vaes PH, Opdecam PJ. The accuracy of three-dimensional magnetic resonance imaging in the diagnosis of ruptures of the lateral ligaments of the ankle. Am J Sports Med 1991; 19(6):583–587. doi:10.1177/036354659101900605
- Milz P, Milz S, Steinborn M, Mittlmeier T, Putz R, Reiser M. Lateral ankle ligaments and tibiofibular syndesmosis. 13-MHz high-frequency sonography and MRI compared in 20 patients. Acta Orthop Scand 1998; 69(1):51–55.
- De Smet AA, Winter TC, Best TM, Bernhardt DT. Dynamic sonography with valgus stress to assess elbow ulnar collateral ligament injury in baseball pitchers. Skeletal Radiol 2002; 31(11):671–676. doi:10.1007/s00256-002-0558-0
- Melville DM, Jacobson JA, Fessell DP. Ultrasound of the thumb ulnar collateral ligament: technique and pathology. AJR Am J Roentgenol 2014; 202(2):W168. doi:10.2214/AJR.13.11335
- Court-Payen M. Sonography of the knee: intra-articular pathology. J Clin Ultrasound 2004; 32(9):481–490. doi:10.1002/jcu.20069
- Azzoni R, Cabitza P. Is there a role for sonography in the diagnosis of tears of the knee menisci? J Clin Ultrasound 2002; 30(8):472–476. doi:10.1002/jcu.10106
- Jacobson JA, Wilson TJ, Yang LJ. Sonography of common peripheral nerve disorders with clinical correlation. J Ultrasound Med 2016; 35(4):683–693. doi:10.7863/ultra.15.05061
- Ali ZS, Pisapia JM, Ma TS, Zager EL, Heuer GG, Khoury V. Ultrasonographic evaluation of peripheral nerves. World Neurosurg 2016; 85(1):333–339. doi:10.1016/j.wneu.2015.10.005
- Bignotti B, Signori A, Sormani MP, Molfetta L, Martinoli C, Tagliafico A. Ultrasound versus magnetic resonance imaging for Morton neuroma: systematic review and meta-analysis. Eur Radiol 2015; 25(8):2254–2262. doi:10.1007/s00330-015-3633-3
KEY POINTS
- Ultrasonography can be used to evaluate small fluid collections in soft tissue; joint effusions and synovitis; soft tissue masses (≤ 5 cm in diameter); tendon, ligament and muscle injuries; and peripheral nerve entrapment and lesions.
- Ultrasonography is not appropriate for survey examinations of vague or diffuse symptoms or for evaluating soft-tissue areas more than a few centimeters in diameter or more than a few centimeters deep.
- Musculoskeletal ultrasonography requires specially trained sonographers and interpreting physicians.
Musculoskeletal ultrasonography has arrived
A 50-year-old woman with hypertension presents with a history of polyarticular small-joint pain for the last 3 months. Her pain is worse in the morning, and it affects her metacarpal, proximal, and distal phalangeal joints. She describes intermittent swelling of her hands and morning stiffness lasting 15 to 30 minutes.
Her physical examination is unremarkable, with no evidence of active inflammation (synovitis), joint tenderness, restrictions in movement, or deformity. Her description of her symptoms raises suspicion for an inflammatory arthritis, but her physical examination does not support this diagnosis.
Bedside musculoskeletal ultrasonography of her wrists reveals synovial hypertrophy, and power Doppler shows active inflammation, findings consistent with synovitis (Figure 1).
This scenario illustrates how musculoskeletal ultrasonography can prevent delayed diagnosis, thus directing the ordering of appropriate laboratory studies and allowing treatment for pain relief to be started promptly.
ULTRASONOGRAPHY HAS GAINED A SOLID ROLE
Ultrasonography has gained a solid role in the care of patients with musculoskeletal conditions.
Using obtained images, as well as power Doppler to assess inflammation, the clinician can visualize superficial anatomic structures, including the skin, muscles, joints, nerves, and the cortical layer of bone. Combining the dynamic assessment with the clinical history and findings of the physical examination makes musculoskeletal ultrasonography a powerful tool for diagnosis and management.1
In this issue, Forney and Delzell2 review the clinical use of ultrasonography of the muscles and bones and its advantages and disadvantages compared with other imaging methods. They describe its gain in popularity over the last decade and its incorporation into clinical care in multiple medical subspecialties.
Musculoskeletal ultrasonography is performed and interpreted by specially trained sonographers. It should be viewed as a complementary procedure, not as a replacement for a thorough and systematic clinical examination.3
ADVANTAGES ARE MANY
A major advantage of musculoskeletal ultrasonography over other imaging techniques is its capacity to dynamically assess joint and tendon movements4 and to immediately interpret them in real time.
In rheumatology, where it has made the biggest impact, it can help evaluate inflammatory and noninflammatory rheumatic diseases, assess treatment response, and guide joint injections.1 It has been demonstrated to significantly improve timely diagnosis and management,5 decrease dependence on other imaging modalities, and reduce healthcare costs.6
With its easy portability, ultrasonography has also been integrated into orthopedics, podiatry, physical medicine and rehabilitation, sports medicine, and emergency medicine. Its role is expanding to include the assessment of the skin in systemic sclerosis, parotid and submandibular glands in Sjögren syndrome, nails in patients with psoriasis, and temporal arteries in giant cell arteritis.
A ROLE IN MEDICAL EDUCATION
Musculoskeletal ultrasonography has entered into medical education, with an increasing number of medical schools incorporating it into their curriculum over the last few years.7 It enhances student learning of anatomy, the physical examination, and pathologic findings of rheumatic diseases.7,8 Some internal medicine residency programs have added ultrasonography to help identify anatomic structures for invasive procedures, increasing patient safety and reducing procedural complications.9
It has been incorporated into the core curriculum in many rheumatology fellowship training programs.10 Rheumatologists can now also take additional courses to enhance their skills and become certified sonographers.
Musculoskeletal ultrasonography has proven to be a useful adjunct to the physical examination. With its many advantages, it has gained acceptance and is now a mainstay in many subspecialties.
- Cannella AC, Kissin EY, Torralba KD, Higgs JB, Kaeley GS. Evolution of musculoskeletal ultrasound in the United States: implementation and practice in rheumatology. Arthritis Care Res (Hoboken) 2014; 66(1):7–13. doi:10.1002/acr.22183
- Forney MC, Delzell PB. Musculoskeletal ultrasonography basics. Cleve Clin J Med 2018; 85(4):283–300. doi:10.3949/ccjm.85a.17014
- McAlindon T, Kissin E, Nazarian L, et al. American College of Rheumatology report on reasonable use of musculoskeletal ultrasonography in rheumatology clinical practice. Arthritis Care Res (Hoboken) 2012; 64(11):1625–1640. doi:10.1002/acr.21836
- Backhaus M, Burmester GR, Gerber T, et al; Working Group for Musculoskeletal Ultrasound in the EULAR Standing Committee on International Clinical Studies including Therapeutic Trials. Guidelines for musculoskeletal ultrasound in rheumatology. Ann Rheum Dis 2001; 60(7):641–649.
- Micu MC, Alcalde M, Saenz JI, et al. Impact of musculoskeletal ultrasound in an outpatient rheumatology clinic. Arthritis Care Res (Hoboken) 2013; 65(4):615–621. doi:10.1002/acr.21853
- Kay JC, Higgs JB, Battafarano DF. Utility of musculoskeletal ultrasound in a Department of Defense rheumatology practice: a four-year retrospective experience. Arthritis Care Res (Hoboken) 2014; 66(1):14–18. doi:10.1002/acr.22127
- Dinh VA, Fu JY, Lu S, Chiem A, Fox JC, Blaivas M. Integration of ultrasound in medical education at United States medical schools. J Ultrasound Med 2016; 35(2):413–419. doi:10.7863/ultra.15.05073
- Wright SA, Bell AL. Enhancement of undergraduate rheumatology teaching through the use of musculoskeletal ultrasound. Rheumatology (Oxford) 2008; 47(10):1564–1566. doi:10.1093/rheumatology/ken324
- Keddis MT, Cullen MW, Reed DA, et al. Effectiveness of an ultrasound training module for internal medicine residents. BMC Med Educ 2011; 11:75. doi:0.1186/1472-6920-11-75
- Torralba K, Cannella AC, Kissin EY, et al. Musculoskeletal ultrasound instruction in adult rheumatology fellowship programs. Arthritis Care Res (Hoboken) 2017. Epub ahead of print. doi:10.1002/acr.23336
A 50-year-old woman with hypertension presents with a history of polyarticular small-joint pain for the last 3 months. Her pain is worse in the morning, and it affects her metacarpal, proximal, and distal phalangeal joints. She describes intermittent swelling of her hands and morning stiffness lasting 15 to 30 minutes.
Her physical examination is unremarkable, with no evidence of active inflammation (synovitis), joint tenderness, restrictions in movement, or deformity. Her description of her symptoms raises suspicion for an inflammatory arthritis, but her physical examination does not support this diagnosis.
Bedside musculoskeletal ultrasonography of her wrists reveals synovial hypertrophy, and power Doppler shows active inflammation, findings consistent with synovitis (Figure 1).
This scenario illustrates how musculoskeletal ultrasonography can prevent delayed diagnosis, thus directing the ordering of appropriate laboratory studies and allowing treatment for pain relief to be started promptly.
ULTRASONOGRAPHY HAS GAINED A SOLID ROLE
Ultrasonography has gained a solid role in the care of patients with musculoskeletal conditions.
Using obtained images, as well as power Doppler to assess inflammation, the clinician can visualize superficial anatomic structures, including the skin, muscles, joints, nerves, and the cortical layer of bone. Combining the dynamic assessment with the clinical history and findings of the physical examination makes musculoskeletal ultrasonography a powerful tool for diagnosis and management.1
In this issue, Forney and Delzell2 review the clinical use of ultrasonography of the muscles and bones and its advantages and disadvantages compared with other imaging methods. They describe its gain in popularity over the last decade and its incorporation into clinical care in multiple medical subspecialties.
Musculoskeletal ultrasonography is performed and interpreted by specially trained sonographers. It should be viewed as a complementary procedure, not as a replacement for a thorough and systematic clinical examination.3
ADVANTAGES ARE MANY
A major advantage of musculoskeletal ultrasonography over other imaging techniques is its capacity to dynamically assess joint and tendon movements4 and to immediately interpret them in real time.
In rheumatology, where it has made the biggest impact, it can help evaluate inflammatory and noninflammatory rheumatic diseases, assess treatment response, and guide joint injections.1 It has been demonstrated to significantly improve timely diagnosis and management,5 decrease dependence on other imaging modalities, and reduce healthcare costs.6
With its easy portability, ultrasonography has also been integrated into orthopedics, podiatry, physical medicine and rehabilitation, sports medicine, and emergency medicine. Its role is expanding to include the assessment of the skin in systemic sclerosis, parotid and submandibular glands in Sjögren syndrome, nails in patients with psoriasis, and temporal arteries in giant cell arteritis.
A ROLE IN MEDICAL EDUCATION
Musculoskeletal ultrasonography has entered into medical education, with an increasing number of medical schools incorporating it into their curriculum over the last few years.7 It enhances student learning of anatomy, the physical examination, and pathologic findings of rheumatic diseases.7,8 Some internal medicine residency programs have added ultrasonography to help identify anatomic structures for invasive procedures, increasing patient safety and reducing procedural complications.9
It has been incorporated into the core curriculum in many rheumatology fellowship training programs.10 Rheumatologists can now also take additional courses to enhance their skills and become certified sonographers.
Musculoskeletal ultrasonography has proven to be a useful adjunct to the physical examination. With its many advantages, it has gained acceptance and is now a mainstay in many subspecialties.
A 50-year-old woman with hypertension presents with a history of polyarticular small-joint pain for the last 3 months. Her pain is worse in the morning, and it affects her metacarpal, proximal, and distal phalangeal joints. She describes intermittent swelling of her hands and morning stiffness lasting 15 to 30 minutes.
Her physical examination is unremarkable, with no evidence of active inflammation (synovitis), joint tenderness, restrictions in movement, or deformity. Her description of her symptoms raises suspicion for an inflammatory arthritis, but her physical examination does not support this diagnosis.
Bedside musculoskeletal ultrasonography of her wrists reveals synovial hypertrophy, and power Doppler shows active inflammation, findings consistent with synovitis (Figure 1).
This scenario illustrates how musculoskeletal ultrasonography can prevent delayed diagnosis, thus directing the ordering of appropriate laboratory studies and allowing treatment for pain relief to be started promptly.
ULTRASONOGRAPHY HAS GAINED A SOLID ROLE
Ultrasonography has gained a solid role in the care of patients with musculoskeletal conditions.
Using obtained images, as well as power Doppler to assess inflammation, the clinician can visualize superficial anatomic structures, including the skin, muscles, joints, nerves, and the cortical layer of bone. Combining the dynamic assessment with the clinical history and findings of the physical examination makes musculoskeletal ultrasonography a powerful tool for diagnosis and management.1
In this issue, Forney and Delzell2 review the clinical use of ultrasonography of the muscles and bones and its advantages and disadvantages compared with other imaging methods. They describe its gain in popularity over the last decade and its incorporation into clinical care in multiple medical subspecialties.
Musculoskeletal ultrasonography is performed and interpreted by specially trained sonographers. It should be viewed as a complementary procedure, not as a replacement for a thorough and systematic clinical examination.3
ADVANTAGES ARE MANY
A major advantage of musculoskeletal ultrasonography over other imaging techniques is its capacity to dynamically assess joint and tendon movements4 and to immediately interpret them in real time.
In rheumatology, where it has made the biggest impact, it can help evaluate inflammatory and noninflammatory rheumatic diseases, assess treatment response, and guide joint injections.1 It has been demonstrated to significantly improve timely diagnosis and management,5 decrease dependence on other imaging modalities, and reduce healthcare costs.6
With its easy portability, ultrasonography has also been integrated into orthopedics, podiatry, physical medicine and rehabilitation, sports medicine, and emergency medicine. Its role is expanding to include the assessment of the skin in systemic sclerosis, parotid and submandibular glands in Sjögren syndrome, nails in patients with psoriasis, and temporal arteries in giant cell arteritis.
A ROLE IN MEDICAL EDUCATION
Musculoskeletal ultrasonography has entered into medical education, with an increasing number of medical schools incorporating it into their curriculum over the last few years.7 It enhances student learning of anatomy, the physical examination, and pathologic findings of rheumatic diseases.7,8 Some internal medicine residency programs have added ultrasonography to help identify anatomic structures for invasive procedures, increasing patient safety and reducing procedural complications.9
It has been incorporated into the core curriculum in many rheumatology fellowship training programs.10 Rheumatologists can now also take additional courses to enhance their skills and become certified sonographers.
Musculoskeletal ultrasonography has proven to be a useful adjunct to the physical examination. With its many advantages, it has gained acceptance and is now a mainstay in many subspecialties.
- Cannella AC, Kissin EY, Torralba KD, Higgs JB, Kaeley GS. Evolution of musculoskeletal ultrasound in the United States: implementation and practice in rheumatology. Arthritis Care Res (Hoboken) 2014; 66(1):7–13. doi:10.1002/acr.22183
- Forney MC, Delzell PB. Musculoskeletal ultrasonography basics. Cleve Clin J Med 2018; 85(4):283–300. doi:10.3949/ccjm.85a.17014
- McAlindon T, Kissin E, Nazarian L, et al. American College of Rheumatology report on reasonable use of musculoskeletal ultrasonography in rheumatology clinical practice. Arthritis Care Res (Hoboken) 2012; 64(11):1625–1640. doi:10.1002/acr.21836
- Backhaus M, Burmester GR, Gerber T, et al; Working Group for Musculoskeletal Ultrasound in the EULAR Standing Committee on International Clinical Studies including Therapeutic Trials. Guidelines for musculoskeletal ultrasound in rheumatology. Ann Rheum Dis 2001; 60(7):641–649.
- Micu MC, Alcalde M, Saenz JI, et al. Impact of musculoskeletal ultrasound in an outpatient rheumatology clinic. Arthritis Care Res (Hoboken) 2013; 65(4):615–621. doi:10.1002/acr.21853
- Kay JC, Higgs JB, Battafarano DF. Utility of musculoskeletal ultrasound in a Department of Defense rheumatology practice: a four-year retrospective experience. Arthritis Care Res (Hoboken) 2014; 66(1):14–18. doi:10.1002/acr.22127
- Dinh VA, Fu JY, Lu S, Chiem A, Fox JC, Blaivas M. Integration of ultrasound in medical education at United States medical schools. J Ultrasound Med 2016; 35(2):413–419. doi:10.7863/ultra.15.05073
- Wright SA, Bell AL. Enhancement of undergraduate rheumatology teaching through the use of musculoskeletal ultrasound. Rheumatology (Oxford) 2008; 47(10):1564–1566. doi:10.1093/rheumatology/ken324
- Keddis MT, Cullen MW, Reed DA, et al. Effectiveness of an ultrasound training module for internal medicine residents. BMC Med Educ 2011; 11:75. doi:0.1186/1472-6920-11-75
- Torralba K, Cannella AC, Kissin EY, et al. Musculoskeletal ultrasound instruction in adult rheumatology fellowship programs. Arthritis Care Res (Hoboken) 2017. Epub ahead of print. doi:10.1002/acr.23336
- Cannella AC, Kissin EY, Torralba KD, Higgs JB, Kaeley GS. Evolution of musculoskeletal ultrasound in the United States: implementation and practice in rheumatology. Arthritis Care Res (Hoboken) 2014; 66(1):7–13. doi:10.1002/acr.22183
- Forney MC, Delzell PB. Musculoskeletal ultrasonography basics. Cleve Clin J Med 2018; 85(4):283–300. doi:10.3949/ccjm.85a.17014
- McAlindon T, Kissin E, Nazarian L, et al. American College of Rheumatology report on reasonable use of musculoskeletal ultrasonography in rheumatology clinical practice. Arthritis Care Res (Hoboken) 2012; 64(11):1625–1640. doi:10.1002/acr.21836
- Backhaus M, Burmester GR, Gerber T, et al; Working Group for Musculoskeletal Ultrasound in the EULAR Standing Committee on International Clinical Studies including Therapeutic Trials. Guidelines for musculoskeletal ultrasound in rheumatology. Ann Rheum Dis 2001; 60(7):641–649.
- Micu MC, Alcalde M, Saenz JI, et al. Impact of musculoskeletal ultrasound in an outpatient rheumatology clinic. Arthritis Care Res (Hoboken) 2013; 65(4):615–621. doi:10.1002/acr.21853
- Kay JC, Higgs JB, Battafarano DF. Utility of musculoskeletal ultrasound in a Department of Defense rheumatology practice: a four-year retrospective experience. Arthritis Care Res (Hoboken) 2014; 66(1):14–18. doi:10.1002/acr.22127
- Dinh VA, Fu JY, Lu S, Chiem A, Fox JC, Blaivas M. Integration of ultrasound in medical education at United States medical schools. J Ultrasound Med 2016; 35(2):413–419. doi:10.7863/ultra.15.05073
- Wright SA, Bell AL. Enhancement of undergraduate rheumatology teaching through the use of musculoskeletal ultrasound. Rheumatology (Oxford) 2008; 47(10):1564–1566. doi:10.1093/rheumatology/ken324
- Keddis MT, Cullen MW, Reed DA, et al. Effectiveness of an ultrasound training module for internal medicine residents. BMC Med Educ 2011; 11:75. doi:0.1186/1472-6920-11-75
- Torralba K, Cannella AC, Kissin EY, et al. Musculoskeletal ultrasound instruction in adult rheumatology fellowship programs. Arthritis Care Res (Hoboken) 2017. Epub ahead of print. doi:10.1002/acr.23336
Bedside Ultrasound for Pulsatile Hand Mass
Case
A 23-year-old man presented to an outside hospital’s ED for evaluation of a wound on his right hand, which he sustained after he accidentally stabbed himself with a steak knife. At presentation, the patient’s vital signs were: heart rate, 90 beats/min; respiratory rate, 16 breaths/min; blood pressure, 150/92 mm Hg; and temperature, 98.1°F. Oxygen saturation was 98% on room air. Examination revealed a laceration on the patient’s right hand measuring 2 cm in length. The emergency physician (EP) closed the wound using four nylon sutures and administered a Boostrix shot. The patient was discharged home with a prescription for cephalexin capsule 500 mg to be taken four times daily for 5 days. He was instructed to return in 10 days for suture removal, but failed to follow-up.
The patient presented to our ED two months after the initial injury for evaluation of a 1.5-cm round pulsatile mass on his right palm, at the base of the middle finger, from which exuded a small amount of sanguineous fluid. The patient complained of numbness and difficulty extending his right index and middle fingers.
Discussion
Palmar Pseudoaneurysms
A pseudoaneurysm, also referred to as a traumatic aneurysm, develops when a tear of the vessel wall and hemorrhage is contained by a thin-walled capsule, typically following traumatic perforation of the arterial wall. Unlike a true aneurysm, a pseudoaneurysm does not contain all three layers of intima, media, and adventitia. Thin walls lead to inevitable expansion over time; in some cases, a patient will present with a soft-tissue mass years after the initial injury. Compression of nearby structures can cause neuropathy, peripheral edema, venous thrombosis, arterial occlusion or emboli, and even bone erosion.1,2
Hand pseudoaneurysms are more likely to occur on the palmar surface, involving the superficial palmar arch,3 and are due to a penetrating injury or repetitive microtrauma. Hypothenar hammer syndrome occurs when repetitive microtrauma is applied to the ulnar artery as it passes under the hook of the hamate bone into the hand. This condition is also referred to as “hammer hand syndrome” because it frequently occurs in laborers such as mechanics, carpenters, and machinists as a result of repetitive palm trauma. Cases have also been reported in baseball players and cooks who also expose their hands to repetitive trauma.3 Likewise, elderly patients who use walking canes can also present with bilateral hammer hand syndrome,3 and patients who need crutches for a prolonged period of time may also develop axillary artery aneurysms.1,2
Although rare, there have also been cases of spontaneous hand pseudoaneurysms in patients on anticoagulation therapy;4,5 however, pseudoaneurysms are not an absolute contraindication to initiating or continuing use of anticoagulants.
Evaluation
Physical Examination. The patient’s mass in this case was clearly pulsatile on examination, but physical examination alone is not a reliable indicator of pseudoaneurysm, as patients may present only with soft-tissue swelling, pain, erythema, or neurological symptoms.3,6,7
Ultrasound Imaging. In the emergency setting, POC ultrasound should be performed to evaluate any soft-tissue hand mass, especially in the context of trauma or any neurovascular findings, since palmar pseudoaneurysms can easily be confused with an abscess, foreign body, cyst, or even a tendon tear.6 Ultrasound studies using the linear vascular probe should always be done before any attempt to incise and drain the mass.
Three ultrasound characteristics of pseudoaneurysms include expansile pulsatility, turbulent flow with a classic yin-yang sign on Doppler, and a hematoma with variable echogenicity. Variable echogenicity may represent separate episodes of bleeding and rebleeding.8 A “to-and-fro” spectral waveform is pathognomonic for palmar pseudoaneurysms.8
Computed Tomography and Magnetic Resonance Angiography. Definitive imaging for operative management includes computed tomography or magnetic resonance angiography to assess for the exact location and presence of collateral circulation.
Treatment
Treatment of pseudoaneurysms includes conservative compression therapy, surgical excision, or anastomosis, and more recently, ultrasound-guided thrombin injection (UGTI).
Compression Therapy. Compression therapy is often used for femoral artery pseudoaneurysms that develop after iatrogenic injury. However, this technique is time consuming, is uncomfortable for patients, is not effective in treating large pseudoaneurysms, and is contraindicated in patients on anticoagulation therapy. Compression therapy also has a high-failure rate of resolving pseudoaneurysms. Traditionally, surgical excision or anastomosis has been the definitive treatment for palmar pseudoaneurysms.
Ultrasound-Guided Thrombin Injection. A more recent treatment option is UGTI, which is usually performed by an interventional radiologist. Although there is no consensus on exact dose of thrombin for this procedure, the literature describes UGTI to treat both the radial and ulnar arteries.9,10 One study of 83 pseudoaneurysms demonstrated a relationship between the size of the palmar pseudoaneurysm and the number of thrombin injections required to resolve it. Depending on the size of the palmar pseudoaneurysm, the effective thrombin doses ranged from 200 to 2,500 U. Regarding adverse effects and events from treatment, this study reported one case of transient distal ischemia.11
Intravascular balloon occlusion of the pseudoaneurysm neck has also been recommended for UGTI in the femoral artery if the neck is greater than 1 mm, but there is currently nothing in the literature describing its use in palmar pseudoaneurysms.12
Complications
There are more descriptions of palmar, radial, and ulnar pseudoaneurysms in critical care patients due to the frequent, but necessary, use of invasive lines. Emergency physicians frequently place radial or femoral arterial lines for hemodynamic monitoring in critically ill patients. However, the incidence of pseudoaneurysms and its sequelae from these lines are not usually observed in the ED setting.
Radial arterial lines may cause thrombosis in 19% to 57% of cases, and local infection in 1% to 18% of cases.10 In a study of 12,500 patients with radial artery catheters, the rate of radial pseudoaneurysm was only 0.05%.11 Although this is a small complication rate, pseudoaneurysms can lead to significant loss of function. To decrease the number of attempts and penetrating injuries to the arteries, ultrasound guidance for these procedures in the ED is strongly recommended. In addition to decreasing the risk of developing a pseudoaneurysm, ultrasound-guidance decreases the discomfort level of the patient and reduces the risk of bleeding, hematoma formation, and infection. Arterial line placement in the ED using ultrasound guidance decreases the risk of developing pseudoaneurysms and their sequelae, such as distal embolization.
Case Conclusion
The patient in this case underwent an arterial duplex study, which found a partially thrombosed right superficial palmar arch pseudoaneurysm measuring 1.91 cm x 2.08 cm, with an active flow area measuring 0.58 cm x 0.68 cm. The flow to the index finger medial artery and middle finger lateral artery was also diminished. The patient was discharged home with a bulky soft dressing and underwent excision and repair by hand surgery 3 days later. At the 1-month postoperative follow-up visit, the patient had full sensation but mildly decreased range of motion in his fingers.
Summary
Hand pseudoaneurysms are often associated with penetrating injuries—as demonstrated in our case—or repetitive microtrauma. Hand pseudoaneurysms can present with minimal findings such as isolated soft-tissue swelling, pain, or neuropathy. The EP should consider vascular pathology in the differential for patients who present with posttraumatic neuropathy. Regarding imaging studies, ultrasound is the best imaging modality to assess for pseudoaneurysms, and EPs should have a low threshold for its use at bedside—especially prior to attempting any invasive procedure. Patients with a confirmed pseudoaneurysm should be referred to a hand or vascular surgeon for surgical repair, or to an interventional radiologist for UGTI.
1. Newton EJ, Arora S. Peripheral vascular injury. In: Marx JA, Hockberger RS, Walls RM, et al, eds. Rosen’s Emergency Medicine Concepts and Clinical Practice. Vol 1. 8th ed. Philadelphia, PA: Elsevier Saunders; 2014:502.
2. Aufderheide TP. Peripheral arteriovascular disease. In: Marx JA, Hockberger RS, Walls RM, et al, eds. Rosen’s Emergency Medicine Concepts and Clinical Practice. Vol 1. 8th ed. 2014:1147-1149.
3. Anderson SE, De Monaco D, Buechler U, et al. Imaging features of pseudoaneurysms of the hand in children and adults. AJR Am J Roentgenol. 2003;180(3):659-664. doi:10.2214/ajr.180.3.1800659.
4. Shah S, Powell-Brett S, Garnham A. Pseudoaneurysm: an unusual cause of post-traumatic hand swelling. BMJ Case Rep. 2015;2015. pii: bcr2014208750. doi:10.1136/bcr-2014-208750.
5. Kitamura A, Mukohara N. Spontaneous pseudoaneurysm of the hand. Ann Vasc Surg. 2014;28(3):739.e1-e3. doi:10.1016/j.avsg.2013.04.033.
6. Huang SW, Wei TS, Liu SY, Wang WT. Spontaneous totally thrombosed pseudoaneurysm mimicking a tendon tear of the wrist. Orthopedics. 2010;33(10):776. doi:10.3928/01477447-20100826-23.
7. Belyayev L, Rich NM, McKay P, Nesti L, Wind G. Traumatic ulnar artery pseudoaneurysm following a grenade blast: report of a case. Mil Med. 2015;180(6):e725-e727. doi:10.7205/MILMED-D-14-00400.
8. Pero T, Herrick J. Pseudoaneurysm of the radial artery diagnosed by bedside ultrasound. West J Emerg Med. 2009;10(2):89-91.
9. Bosman A, Veger HTC, Doornink F, Hedeman Joosten PPA. A pseudoaneurysm of the deep palmar arch after penetrating trauma to the hand: successful exclusion by ultrasound guided percutaneous thrombin injection. EJVES Short Rep. 2016;31:9-11. doi:10.1016/j.ejvssr.2016.03.002.
10. Komorowska-Timek E, Teruya TH, Abou-Zamzam AM Jr, Papa D, Ballard JL. Treatment of radial and ulnar artery pseudoaneurysms using percutaneous thrombin injection. J Hand Surg. 2004;29A(5):936-942. doi:10.1016/j.jhsa.2004.05.009.
11. Falk PS, Scuderi PE, Sherertz RJ, Motsinger SM. Infected radial artery pseudoaneurysms occurring after percutaneous cannulation. Chest. 1992;101(2):490-495.
12. Kang SS, Labropoulos N, Mansour MA, et al. Expanded indications for ultrasound-guided thrombin injection of pseudoaneurysms. J Vasc Surg. 2000;31(2):289-298.
Case
A 23-year-old man presented to an outside hospital’s ED for evaluation of a wound on his right hand, which he sustained after he accidentally stabbed himself with a steak knife. At presentation, the patient’s vital signs were: heart rate, 90 beats/min; respiratory rate, 16 breaths/min; blood pressure, 150/92 mm Hg; and temperature, 98.1°F. Oxygen saturation was 98% on room air. Examination revealed a laceration on the patient’s right hand measuring 2 cm in length. The emergency physician (EP) closed the wound using four nylon sutures and administered a Boostrix shot. The patient was discharged home with a prescription for cephalexin capsule 500 mg to be taken four times daily for 5 days. He was instructed to return in 10 days for suture removal, but failed to follow-up.
The patient presented to our ED two months after the initial injury for evaluation of a 1.5-cm round pulsatile mass on his right palm, at the base of the middle finger, from which exuded a small amount of sanguineous fluid. The patient complained of numbness and difficulty extending his right index and middle fingers.
Discussion
Palmar Pseudoaneurysms
A pseudoaneurysm, also referred to as a traumatic aneurysm, develops when a tear of the vessel wall and hemorrhage is contained by a thin-walled capsule, typically following traumatic perforation of the arterial wall. Unlike a true aneurysm, a pseudoaneurysm does not contain all three layers of intima, media, and adventitia. Thin walls lead to inevitable expansion over time; in some cases, a patient will present with a soft-tissue mass years after the initial injury. Compression of nearby structures can cause neuropathy, peripheral edema, venous thrombosis, arterial occlusion or emboli, and even bone erosion.1,2
Hand pseudoaneurysms are more likely to occur on the palmar surface, involving the superficial palmar arch,3 and are due to a penetrating injury or repetitive microtrauma. Hypothenar hammer syndrome occurs when repetitive microtrauma is applied to the ulnar artery as it passes under the hook of the hamate bone into the hand. This condition is also referred to as “hammer hand syndrome” because it frequently occurs in laborers such as mechanics, carpenters, and machinists as a result of repetitive palm trauma. Cases have also been reported in baseball players and cooks who also expose their hands to repetitive trauma.3 Likewise, elderly patients who use walking canes can also present with bilateral hammer hand syndrome,3 and patients who need crutches for a prolonged period of time may also develop axillary artery aneurysms.1,2
Although rare, there have also been cases of spontaneous hand pseudoaneurysms in patients on anticoagulation therapy;4,5 however, pseudoaneurysms are not an absolute contraindication to initiating or continuing use of anticoagulants.
Evaluation
Physical Examination. The patient’s mass in this case was clearly pulsatile on examination, but physical examination alone is not a reliable indicator of pseudoaneurysm, as patients may present only with soft-tissue swelling, pain, erythema, or neurological symptoms.3,6,7
Ultrasound Imaging. In the emergency setting, POC ultrasound should be performed to evaluate any soft-tissue hand mass, especially in the context of trauma or any neurovascular findings, since palmar pseudoaneurysms can easily be confused with an abscess, foreign body, cyst, or even a tendon tear.6 Ultrasound studies using the linear vascular probe should always be done before any attempt to incise and drain the mass.
Three ultrasound characteristics of pseudoaneurysms include expansile pulsatility, turbulent flow with a classic yin-yang sign on Doppler, and a hematoma with variable echogenicity. Variable echogenicity may represent separate episodes of bleeding and rebleeding.8 A “to-and-fro” spectral waveform is pathognomonic for palmar pseudoaneurysms.8
Computed Tomography and Magnetic Resonance Angiography. Definitive imaging for operative management includes computed tomography or magnetic resonance angiography to assess for the exact location and presence of collateral circulation.
Treatment
Treatment of pseudoaneurysms includes conservative compression therapy, surgical excision, or anastomosis, and more recently, ultrasound-guided thrombin injection (UGTI).
Compression Therapy. Compression therapy is often used for femoral artery pseudoaneurysms that develop after iatrogenic injury. However, this technique is time consuming, is uncomfortable for patients, is not effective in treating large pseudoaneurysms, and is contraindicated in patients on anticoagulation therapy. Compression therapy also has a high-failure rate of resolving pseudoaneurysms. Traditionally, surgical excision or anastomosis has been the definitive treatment for palmar pseudoaneurysms.
Ultrasound-Guided Thrombin Injection. A more recent treatment option is UGTI, which is usually performed by an interventional radiologist. Although there is no consensus on exact dose of thrombin for this procedure, the literature describes UGTI to treat both the radial and ulnar arteries.9,10 One study of 83 pseudoaneurysms demonstrated a relationship between the size of the palmar pseudoaneurysm and the number of thrombin injections required to resolve it. Depending on the size of the palmar pseudoaneurysm, the effective thrombin doses ranged from 200 to 2,500 U. Regarding adverse effects and events from treatment, this study reported one case of transient distal ischemia.11
Intravascular balloon occlusion of the pseudoaneurysm neck has also been recommended for UGTI in the femoral artery if the neck is greater than 1 mm, but there is currently nothing in the literature describing its use in palmar pseudoaneurysms.12
Complications
There are more descriptions of palmar, radial, and ulnar pseudoaneurysms in critical care patients due to the frequent, but necessary, use of invasive lines. Emergency physicians frequently place radial or femoral arterial lines for hemodynamic monitoring in critically ill patients. However, the incidence of pseudoaneurysms and its sequelae from these lines are not usually observed in the ED setting.
Radial arterial lines may cause thrombosis in 19% to 57% of cases, and local infection in 1% to 18% of cases.10 In a study of 12,500 patients with radial artery catheters, the rate of radial pseudoaneurysm was only 0.05%.11 Although this is a small complication rate, pseudoaneurysms can lead to significant loss of function. To decrease the number of attempts and penetrating injuries to the arteries, ultrasound guidance for these procedures in the ED is strongly recommended. In addition to decreasing the risk of developing a pseudoaneurysm, ultrasound-guidance decreases the discomfort level of the patient and reduces the risk of bleeding, hematoma formation, and infection. Arterial line placement in the ED using ultrasound guidance decreases the risk of developing pseudoaneurysms and their sequelae, such as distal embolization.
Case Conclusion
The patient in this case underwent an arterial duplex study, which found a partially thrombosed right superficial palmar arch pseudoaneurysm measuring 1.91 cm x 2.08 cm, with an active flow area measuring 0.58 cm x 0.68 cm. The flow to the index finger medial artery and middle finger lateral artery was also diminished. The patient was discharged home with a bulky soft dressing and underwent excision and repair by hand surgery 3 days later. At the 1-month postoperative follow-up visit, the patient had full sensation but mildly decreased range of motion in his fingers.
Summary
Hand pseudoaneurysms are often associated with penetrating injuries—as demonstrated in our case—or repetitive microtrauma. Hand pseudoaneurysms can present with minimal findings such as isolated soft-tissue swelling, pain, or neuropathy. The EP should consider vascular pathology in the differential for patients who present with posttraumatic neuropathy. Regarding imaging studies, ultrasound is the best imaging modality to assess for pseudoaneurysms, and EPs should have a low threshold for its use at bedside—especially prior to attempting any invasive procedure. Patients with a confirmed pseudoaneurysm should be referred to a hand or vascular surgeon for surgical repair, or to an interventional radiologist for UGTI.
Case
A 23-year-old man presented to an outside hospital’s ED for evaluation of a wound on his right hand, which he sustained after he accidentally stabbed himself with a steak knife. At presentation, the patient’s vital signs were: heart rate, 90 beats/min; respiratory rate, 16 breaths/min; blood pressure, 150/92 mm Hg; and temperature, 98.1°F. Oxygen saturation was 98% on room air. Examination revealed a laceration on the patient’s right hand measuring 2 cm in length. The emergency physician (EP) closed the wound using four nylon sutures and administered a Boostrix shot. The patient was discharged home with a prescription for cephalexin capsule 500 mg to be taken four times daily for 5 days. He was instructed to return in 10 days for suture removal, but failed to follow-up.
The patient presented to our ED two months after the initial injury for evaluation of a 1.5-cm round pulsatile mass on his right palm, at the base of the middle finger, from which exuded a small amount of sanguineous fluid. The patient complained of numbness and difficulty extending his right index and middle fingers.
Discussion
Palmar Pseudoaneurysms
A pseudoaneurysm, also referred to as a traumatic aneurysm, develops when a tear of the vessel wall and hemorrhage is contained by a thin-walled capsule, typically following traumatic perforation of the arterial wall. Unlike a true aneurysm, a pseudoaneurysm does not contain all three layers of intima, media, and adventitia. Thin walls lead to inevitable expansion over time; in some cases, a patient will present with a soft-tissue mass years after the initial injury. Compression of nearby structures can cause neuropathy, peripheral edema, venous thrombosis, arterial occlusion or emboli, and even bone erosion.1,2
Hand pseudoaneurysms are more likely to occur on the palmar surface, involving the superficial palmar arch,3 and are due to a penetrating injury or repetitive microtrauma. Hypothenar hammer syndrome occurs when repetitive microtrauma is applied to the ulnar artery as it passes under the hook of the hamate bone into the hand. This condition is also referred to as “hammer hand syndrome” because it frequently occurs in laborers such as mechanics, carpenters, and machinists as a result of repetitive palm trauma. Cases have also been reported in baseball players and cooks who also expose their hands to repetitive trauma.3 Likewise, elderly patients who use walking canes can also present with bilateral hammer hand syndrome,3 and patients who need crutches for a prolonged period of time may also develop axillary artery aneurysms.1,2
Although rare, there have also been cases of spontaneous hand pseudoaneurysms in patients on anticoagulation therapy;4,5 however, pseudoaneurysms are not an absolute contraindication to initiating or continuing use of anticoagulants.
Evaluation
Physical Examination. The patient’s mass in this case was clearly pulsatile on examination, but physical examination alone is not a reliable indicator of pseudoaneurysm, as patients may present only with soft-tissue swelling, pain, erythema, or neurological symptoms.3,6,7
Ultrasound Imaging. In the emergency setting, POC ultrasound should be performed to evaluate any soft-tissue hand mass, especially in the context of trauma or any neurovascular findings, since palmar pseudoaneurysms can easily be confused with an abscess, foreign body, cyst, or even a tendon tear.6 Ultrasound studies using the linear vascular probe should always be done before any attempt to incise and drain the mass.
Three ultrasound characteristics of pseudoaneurysms include expansile pulsatility, turbulent flow with a classic yin-yang sign on Doppler, and a hematoma with variable echogenicity. Variable echogenicity may represent separate episodes of bleeding and rebleeding.8 A “to-and-fro” spectral waveform is pathognomonic for palmar pseudoaneurysms.8
Computed Tomography and Magnetic Resonance Angiography. Definitive imaging for operative management includes computed tomography or magnetic resonance angiography to assess for the exact location and presence of collateral circulation.
Treatment
Treatment of pseudoaneurysms includes conservative compression therapy, surgical excision, or anastomosis, and more recently, ultrasound-guided thrombin injection (UGTI).
Compression Therapy. Compression therapy is often used for femoral artery pseudoaneurysms that develop after iatrogenic injury. However, this technique is time consuming, is uncomfortable for patients, is not effective in treating large pseudoaneurysms, and is contraindicated in patients on anticoagulation therapy. Compression therapy also has a high-failure rate of resolving pseudoaneurysms. Traditionally, surgical excision or anastomosis has been the definitive treatment for palmar pseudoaneurysms.
Ultrasound-Guided Thrombin Injection. A more recent treatment option is UGTI, which is usually performed by an interventional radiologist. Although there is no consensus on exact dose of thrombin for this procedure, the literature describes UGTI to treat both the radial and ulnar arteries.9,10 One study of 83 pseudoaneurysms demonstrated a relationship between the size of the palmar pseudoaneurysm and the number of thrombin injections required to resolve it. Depending on the size of the palmar pseudoaneurysm, the effective thrombin doses ranged from 200 to 2,500 U. Regarding adverse effects and events from treatment, this study reported one case of transient distal ischemia.11
Intravascular balloon occlusion of the pseudoaneurysm neck has also been recommended for UGTI in the femoral artery if the neck is greater than 1 mm, but there is currently nothing in the literature describing its use in palmar pseudoaneurysms.12
Complications
There are more descriptions of palmar, radial, and ulnar pseudoaneurysms in critical care patients due to the frequent, but necessary, use of invasive lines. Emergency physicians frequently place radial or femoral arterial lines for hemodynamic monitoring in critically ill patients. However, the incidence of pseudoaneurysms and its sequelae from these lines are not usually observed in the ED setting.
Radial arterial lines may cause thrombosis in 19% to 57% of cases, and local infection in 1% to 18% of cases.10 In a study of 12,500 patients with radial artery catheters, the rate of radial pseudoaneurysm was only 0.05%.11 Although this is a small complication rate, pseudoaneurysms can lead to significant loss of function. To decrease the number of attempts and penetrating injuries to the arteries, ultrasound guidance for these procedures in the ED is strongly recommended. In addition to decreasing the risk of developing a pseudoaneurysm, ultrasound-guidance decreases the discomfort level of the patient and reduces the risk of bleeding, hematoma formation, and infection. Arterial line placement in the ED using ultrasound guidance decreases the risk of developing pseudoaneurysms and their sequelae, such as distal embolization.
Case Conclusion
The patient in this case underwent an arterial duplex study, which found a partially thrombosed right superficial palmar arch pseudoaneurysm measuring 1.91 cm x 2.08 cm, with an active flow area measuring 0.58 cm x 0.68 cm. The flow to the index finger medial artery and middle finger lateral artery was also diminished. The patient was discharged home with a bulky soft dressing and underwent excision and repair by hand surgery 3 days later. At the 1-month postoperative follow-up visit, the patient had full sensation but mildly decreased range of motion in his fingers.
Summary
Hand pseudoaneurysms are often associated with penetrating injuries—as demonstrated in our case—or repetitive microtrauma. Hand pseudoaneurysms can present with minimal findings such as isolated soft-tissue swelling, pain, or neuropathy. The EP should consider vascular pathology in the differential for patients who present with posttraumatic neuropathy. Regarding imaging studies, ultrasound is the best imaging modality to assess for pseudoaneurysms, and EPs should have a low threshold for its use at bedside—especially prior to attempting any invasive procedure. Patients with a confirmed pseudoaneurysm should be referred to a hand or vascular surgeon for surgical repair, or to an interventional radiologist for UGTI.
1. Newton EJ, Arora S. Peripheral vascular injury. In: Marx JA, Hockberger RS, Walls RM, et al, eds. Rosen’s Emergency Medicine Concepts and Clinical Practice. Vol 1. 8th ed. Philadelphia, PA: Elsevier Saunders; 2014:502.
2. Aufderheide TP. Peripheral arteriovascular disease. In: Marx JA, Hockberger RS, Walls RM, et al, eds. Rosen’s Emergency Medicine Concepts and Clinical Practice. Vol 1. 8th ed. 2014:1147-1149.
3. Anderson SE, De Monaco D, Buechler U, et al. Imaging features of pseudoaneurysms of the hand in children and adults. AJR Am J Roentgenol. 2003;180(3):659-664. doi:10.2214/ajr.180.3.1800659.
4. Shah S, Powell-Brett S, Garnham A. Pseudoaneurysm: an unusual cause of post-traumatic hand swelling. BMJ Case Rep. 2015;2015. pii: bcr2014208750. doi:10.1136/bcr-2014-208750.
5. Kitamura A, Mukohara N. Spontaneous pseudoaneurysm of the hand. Ann Vasc Surg. 2014;28(3):739.e1-e3. doi:10.1016/j.avsg.2013.04.033.
6. Huang SW, Wei TS, Liu SY, Wang WT. Spontaneous totally thrombosed pseudoaneurysm mimicking a tendon tear of the wrist. Orthopedics. 2010;33(10):776. doi:10.3928/01477447-20100826-23.
7. Belyayev L, Rich NM, McKay P, Nesti L, Wind G. Traumatic ulnar artery pseudoaneurysm following a grenade blast: report of a case. Mil Med. 2015;180(6):e725-e727. doi:10.7205/MILMED-D-14-00400.
8. Pero T, Herrick J. Pseudoaneurysm of the radial artery diagnosed by bedside ultrasound. West J Emerg Med. 2009;10(2):89-91.
9. Bosman A, Veger HTC, Doornink F, Hedeman Joosten PPA. A pseudoaneurysm of the deep palmar arch after penetrating trauma to the hand: successful exclusion by ultrasound guided percutaneous thrombin injection. EJVES Short Rep. 2016;31:9-11. doi:10.1016/j.ejvssr.2016.03.002.
10. Komorowska-Timek E, Teruya TH, Abou-Zamzam AM Jr, Papa D, Ballard JL. Treatment of radial and ulnar artery pseudoaneurysms using percutaneous thrombin injection. J Hand Surg. 2004;29A(5):936-942. doi:10.1016/j.jhsa.2004.05.009.
11. Falk PS, Scuderi PE, Sherertz RJ, Motsinger SM. Infected radial artery pseudoaneurysms occurring after percutaneous cannulation. Chest. 1992;101(2):490-495.
12. Kang SS, Labropoulos N, Mansour MA, et al. Expanded indications for ultrasound-guided thrombin injection of pseudoaneurysms. J Vasc Surg. 2000;31(2):289-298.
1. Newton EJ, Arora S. Peripheral vascular injury. In: Marx JA, Hockberger RS, Walls RM, et al, eds. Rosen’s Emergency Medicine Concepts and Clinical Practice. Vol 1. 8th ed. Philadelphia, PA: Elsevier Saunders; 2014:502.
2. Aufderheide TP. Peripheral arteriovascular disease. In: Marx JA, Hockberger RS, Walls RM, et al, eds. Rosen’s Emergency Medicine Concepts and Clinical Practice. Vol 1. 8th ed. 2014:1147-1149.
3. Anderson SE, De Monaco D, Buechler U, et al. Imaging features of pseudoaneurysms of the hand in children and adults. AJR Am J Roentgenol. 2003;180(3):659-664. doi:10.2214/ajr.180.3.1800659.
4. Shah S, Powell-Brett S, Garnham A. Pseudoaneurysm: an unusual cause of post-traumatic hand swelling. BMJ Case Rep. 2015;2015. pii: bcr2014208750. doi:10.1136/bcr-2014-208750.
5. Kitamura A, Mukohara N. Spontaneous pseudoaneurysm of the hand. Ann Vasc Surg. 2014;28(3):739.e1-e3. doi:10.1016/j.avsg.2013.04.033.
6. Huang SW, Wei TS, Liu SY, Wang WT. Spontaneous totally thrombosed pseudoaneurysm mimicking a tendon tear of the wrist. Orthopedics. 2010;33(10):776. doi:10.3928/01477447-20100826-23.
7. Belyayev L, Rich NM, McKay P, Nesti L, Wind G. Traumatic ulnar artery pseudoaneurysm following a grenade blast: report of a case. Mil Med. 2015;180(6):e725-e727. doi:10.7205/MILMED-D-14-00400.
8. Pero T, Herrick J. Pseudoaneurysm of the radial artery diagnosed by bedside ultrasound. West J Emerg Med. 2009;10(2):89-91.
9. Bosman A, Veger HTC, Doornink F, Hedeman Joosten PPA. A pseudoaneurysm of the deep palmar arch after penetrating trauma to the hand: successful exclusion by ultrasound guided percutaneous thrombin injection. EJVES Short Rep. 2016;31:9-11. doi:10.1016/j.ejvssr.2016.03.002.
10. Komorowska-Timek E, Teruya TH, Abou-Zamzam AM Jr, Papa D, Ballard JL. Treatment of radial and ulnar artery pseudoaneurysms using percutaneous thrombin injection. J Hand Surg. 2004;29A(5):936-942. doi:10.1016/j.jhsa.2004.05.009.
11. Falk PS, Scuderi PE, Sherertz RJ, Motsinger SM. Infected radial artery pseudoaneurysms occurring after percutaneous cannulation. Chest. 1992;101(2):490-495.
12. Kang SS, Labropoulos N, Mansour MA, et al. Expanded indications for ultrasound-guided thrombin injection of pseudoaneurysms. J Vasc Surg. 2000;31(2):289-298.
VIDEO: Ultrasound with Doppler
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
VIDEO: Initial Bedside Ultrasound of Pulsatile Hand Mass



CT angiography boosts success in chronic total occlusion revascularization
WASHINGTON – When performed prior to revascularization, CT angiography almost doubles the likelihood of successful revascularization of chronic total occlusion relative to no CT angiography, according to a meta-analysis.
Because the meta-analysis relied primarily on retrospective data, the conclusion was characterized as hypothesis-generating. But the author, Wael Abuzeid, MD, an interventional cardiologist and assistant professor at Queen’s University, Kingston, Ont., suggested that there are several arguments to be made for pursuing a randomized trial.
This is not a new idea, according to results of a systematic review of the literature. Although only four articles met prespecified criteria for entry into the meta-analysis that was eventually conducted, Dr. Abuzeid and his coauthors found 424 articles on this subject in a search of three literature databases.
Only one of the four studies entered into the meta-analysis involved prospective data collection, but three of the four found a significant advantage for preprocedural CTA when compared with no CTA for procedural success, meaning restoration of blood flow in the target CTO. The fourth study also associated preprocedural CTA with improved procedural success, but the advantage did not reach statistical significance.
When the data were combined for a meta-analysis, the odds ratio for procedural success for conducting a preprocedural CTA relative to no preprocedural CTA was 1.89 with a significant 95% confidence interval (1.18-3.04; P less than .05).
The four studies were published during 2012-2015. Two of them used CTA with only a 64-slice capacity, raising the possibility that an even greater improvement in results would have been achieved with CTA using the greater resolutions now available.
There were several important limitations of the study, particularly the potential for selection bias from the nonrandomized designs in the studies evaluated, Dr. Abuzeid acknowledged. However, the most likely selection bias would be funneling of more challenging cases to preprocedural CTA, a potential disadvantage for CTA if this resulted in a patient population likely to have a poor outcome.
Currently, few centers employ preprocedural CTA in routine management of CTO, according to Dr. Abuzeid.
“CTA prior to revascularization in CTO is not a standard approach even in complex patients,” Dr. Abuzeid said. However, he believes an argument can already be made for preprocedural CTA in some types of challenging patients, such as those with particularly long target lesions. In such cases, CTA could provide advance information about negative distal remodeling and the likelihood that a retrograde approach might be needed.
“For the junior operator, I think the information provided by preprocedural CTA could be very useful in planning,” Dr. Abuzeid said.
The arguments against preprocedural CTA include higher radiation exposure and a delay in the time to procedure by adding the extra step of first conducting the imaging study. In addition to verifying that procedural success is achieved with preprocedural CTA, these issues are among those that deserve evaluation in a prospective study.
“The appropriate randomized trial would be conducted at high-volume centers with all comers, not just complex patients, randomized to undergo a preprocedural CTA or no preprocedural imaging,” Dr. Abuzeid suggested. He said the appropriate primary outcome is relative success at restoring Thrombolysis in Myocardial Infarction grade 2 flow, but suggested that other endpoints, such as procedure time and rate of complications, would be useful for determining the value of this approach.
Dr. Abuzeid reports having no financial relationships.
SOURCE: Abuzeid W. CRT 18.
WASHINGTON – When performed prior to revascularization, CT angiography almost doubles the likelihood of successful revascularization of chronic total occlusion relative to no CT angiography, according to a meta-analysis.
Because the meta-analysis relied primarily on retrospective data, the conclusion was characterized as hypothesis-generating. But the author, Wael Abuzeid, MD, an interventional cardiologist and assistant professor at Queen’s University, Kingston, Ont., suggested that there are several arguments to be made for pursuing a randomized trial.
This is not a new idea, according to results of a systematic review of the literature. Although only four articles met prespecified criteria for entry into the meta-analysis that was eventually conducted, Dr. Abuzeid and his coauthors found 424 articles on this subject in a search of three literature databases.
Only one of the four studies entered into the meta-analysis involved prospective data collection, but three of the four found a significant advantage for preprocedural CTA when compared with no CTA for procedural success, meaning restoration of blood flow in the target CTO. The fourth study also associated preprocedural CTA with improved procedural success, but the advantage did not reach statistical significance.
When the data were combined for a meta-analysis, the odds ratio for procedural success for conducting a preprocedural CTA relative to no preprocedural CTA was 1.89 with a significant 95% confidence interval (1.18-3.04; P less than .05).
The four studies were published during 2012-2015. Two of them used CTA with only a 64-slice capacity, raising the possibility that an even greater improvement in results would have been achieved with CTA using the greater resolutions now available.
There were several important limitations of the study, particularly the potential for selection bias from the nonrandomized designs in the studies evaluated, Dr. Abuzeid acknowledged. However, the most likely selection bias would be funneling of more challenging cases to preprocedural CTA, a potential disadvantage for CTA if this resulted in a patient population likely to have a poor outcome.
Currently, few centers employ preprocedural CTA in routine management of CTO, according to Dr. Abuzeid.
“CTA prior to revascularization in CTO is not a standard approach even in complex patients,” Dr. Abuzeid said. However, he believes an argument can already be made for preprocedural CTA in some types of challenging patients, such as those with particularly long target lesions. In such cases, CTA could provide advance information about negative distal remodeling and the likelihood that a retrograde approach might be needed.
“For the junior operator, I think the information provided by preprocedural CTA could be very useful in planning,” Dr. Abuzeid said.
The arguments against preprocedural CTA include higher radiation exposure and a delay in the time to procedure by adding the extra step of first conducting the imaging study. In addition to verifying that procedural success is achieved with preprocedural CTA, these issues are among those that deserve evaluation in a prospective study.
“The appropriate randomized trial would be conducted at high-volume centers with all comers, not just complex patients, randomized to undergo a preprocedural CTA or no preprocedural imaging,” Dr. Abuzeid suggested. He said the appropriate primary outcome is relative success at restoring Thrombolysis in Myocardial Infarction grade 2 flow, but suggested that other endpoints, such as procedure time and rate of complications, would be useful for determining the value of this approach.
Dr. Abuzeid reports having no financial relationships.
SOURCE: Abuzeid W. CRT 18.
WASHINGTON – When performed prior to revascularization, CT angiography almost doubles the likelihood of successful revascularization of chronic total occlusion relative to no CT angiography, according to a meta-analysis.
Because the meta-analysis relied primarily on retrospective data, the conclusion was characterized as hypothesis-generating. But the author, Wael Abuzeid, MD, an interventional cardiologist and assistant professor at Queen’s University, Kingston, Ont., suggested that there are several arguments to be made for pursuing a randomized trial.
This is not a new idea, according to results of a systematic review of the literature. Although only four articles met prespecified criteria for entry into the meta-analysis that was eventually conducted, Dr. Abuzeid and his coauthors found 424 articles on this subject in a search of three literature databases.
Only one of the four studies entered into the meta-analysis involved prospective data collection, but three of the four found a significant advantage for preprocedural CTA when compared with no CTA for procedural success, meaning restoration of blood flow in the target CTO. The fourth study also associated preprocedural CTA with improved procedural success, but the advantage did not reach statistical significance.
When the data were combined for a meta-analysis, the odds ratio for procedural success for conducting a preprocedural CTA relative to no preprocedural CTA was 1.89 with a significant 95% confidence interval (1.18-3.04; P less than .05).
The four studies were published during 2012-2015. Two of them used CTA with only a 64-slice capacity, raising the possibility that an even greater improvement in results would have been achieved with CTA using the greater resolutions now available.
There were several important limitations of the study, particularly the potential for selection bias from the nonrandomized designs in the studies evaluated, Dr. Abuzeid acknowledged. However, the most likely selection bias would be funneling of more challenging cases to preprocedural CTA, a potential disadvantage for CTA if this resulted in a patient population likely to have a poor outcome.
Currently, few centers employ preprocedural CTA in routine management of CTO, according to Dr. Abuzeid.
“CTA prior to revascularization in CTO is not a standard approach even in complex patients,” Dr. Abuzeid said. However, he believes an argument can already be made for preprocedural CTA in some types of challenging patients, such as those with particularly long target lesions. In such cases, CTA could provide advance information about negative distal remodeling and the likelihood that a retrograde approach might be needed.
“For the junior operator, I think the information provided by preprocedural CTA could be very useful in planning,” Dr. Abuzeid said.
The arguments against preprocedural CTA include higher radiation exposure and a delay in the time to procedure by adding the extra step of first conducting the imaging study. In addition to verifying that procedural success is achieved with preprocedural CTA, these issues are among those that deserve evaluation in a prospective study.
“The appropriate randomized trial would be conducted at high-volume centers with all comers, not just complex patients, randomized to undergo a preprocedural CTA or no preprocedural imaging,” Dr. Abuzeid suggested. He said the appropriate primary outcome is relative success at restoring Thrombolysis in Myocardial Infarction grade 2 flow, but suggested that other endpoints, such as procedure time and rate of complications, would be useful for determining the value of this approach.
Dr. Abuzeid reports having no financial relationships.
SOURCE: Abuzeid W. CRT 18.
REPORTING FROM CRT 2018
Key clinical point:
Major finding: In a meta-analysis of data from four studies, CT angiography almost doubled the likelihood of procedural success (OR 1.89; P less than .05).
Study details: A systematic review and meta-analysis of four studies.
Disclosures: Dr. Abuzeid reports having no financial relationships.
Source: Abuzaid W. CRT 18.
Which test for CAD should be used in patients with left bundle branch block?
A 62-year-old woman with hypertension and type 2 diabetes mellitus has been experiencing shortness of breath on exertion and chest discomfort for 2 months. Her hypertension has been suboptimally controlled, and her most recent hemoglobin A1c measurement was 7.0%. She has never smoked and has no family history of premature coronary artery disease (CAD). She is otherwise well and walks for 30 minutes 3 times per week. A 12-lead electrocardiogram demonstrated normal sinus rhythm with left bundle branch block. Her physician suspects she has CAD. What testing does this patient need?
LIMITED DATA, GUIDELINES
For clinicians investigating suspected obstructive CAD in patients with left bundle branch block on resting electrocardiography, the data and guidelines are limited regarding the optimal noninvasive tests and how to interpret them.
Here, we present a practical review of the diagnostic utility of exercise stress electrocardiography, exercise stress echocardiography, dobutamine stress echocardiography, nuclear myocardial perfusion imaging, and computed tomographic (CT) angiography for assessing suspected obstructive CAD in patients with resting left bundle branch block.
WHAT IS LEFT BUNDLE BRANCH BLOCK?
In left bundle branch block, as the name implies, electrical conduction along the left bundle branch is blocked or delayed. Ventricular activation therefore begins in the right ventricle and the right side of the interventricular septum.1 Transseptal activation from the right ventricle to the left ventricle is slow, because it is transmyocardial.1 Left ventricular basal and posterolateral wall segments become activated last.1 Due to delay in the onset of left ventricular contraction, ventricular contraction is dyssynchronous. Classically, interventricular septal motion during systole has been described as paradoxical, with anterior septal motion.2–4
On electrocardiography, the QRS duration is widened (≥ 120 ms), with a distinctive morphology as shown in Figure 1. Left bundle branch block makes it difficult to accurately assess for dynamic ST-segment changes with exercise, rendering exercise stress electrocardiography a suboptimal test for obstructive CAD if left bundle branch block is present.
LEFT BUNDLE BRANCH BLOCK AND RISK OF DEATH
Although left bundle branch block can be an isolated finding, it can also be associated with underlying obstructive CAD5 or cardiomyopathy.6 When it occurs at rest, the risk of death from a cardiovascular event is 3 to 4 times higher.7 However, the exact incidence of significant obstructive CAD in asymptomatic patients with incidentally detected left bundle branch block is unknown.
Acute left bundle branch block accompanying acute myocardial infarction is associated with a high risk of death. Hindman et al,8 in a 1978 multicenter study, described 432 patients with acute myocardial infarction and left or right bundle branch block. In the 163 patients who had left bundle branch block, the in-hospital mortality rate was 24% and the 1-year mortality rate was 32%.
Freedman et al9 in 1987 reviewed 15,609 patients with chronic CAD who underwent coronary angiography, of whom 522 had left or right bundle branch block. During a follow-up of nearly 5 years, 2,386 patients died. The actuarial probability of death at 2 years in patients with left bundle branch block was more than 5 times that of patients without it (P < .0001).
During 18 years of observation in the Framingham study,10 55 participants developed left bundle branch block, at a mean age at onset of 62. Twenty-six (48%) of these participants developed clinically significant CAD or heart failure coincident with or subsequent to the onset of left bundle branch block. Fifty percent of the participants who developed left bundle branch block died of cardiovascular disease within 10 years of its onset.
EXERCISE STRESS ELECTROCARDIOGRAPHY
Exercise stress electrocardiography, although valuable for assessing functional capacity, cannot be used to diagnose obstructive CAD in patients with left bundle branch block.11
EXERCISE STRESS ECHOCARDIOGRAPHY
Exercise stress echocardiography is proven and widely used for assessing myocardial ischemia in patients with suspected obstructive CAD. But the data are limited on its diagnostic utility in patients with left bundle branch block. Until recently, recommendations for its use in this situation were based on only 1 small study.12
Peteiro et al12 in 2000 described 35 patients who underwent exercise stress echocardiography and coronary angiography. Detection of wall-motion abnormalities had high sensitivity (76%), specificity (83%), and diagnostic accuracy (80%).
Of note, 8 (23%) of the patients could not achieve at least 85% of the maximum predicted heart rate, and for them, the study was not diagnostic for ischemia. (Technically, the study is said to be nondiagnostic when the patient fails to achieve the target heart rate of at least 85% of the maximum predicted heart rate.)
Additionally, 18 of the 35 patients—over half—had a decrease in left ventricular ejection fraction in response to exercise. These 18 patients included 12 of the 17 patients with obstructive CAD and 6 of the 18 patients without obstructive CAD.12 It is unclear whether a significant proportion of these 18 patients would have been otherwise categorized as having a globally abnormal left ventricular contractile response to exercise according to contemporary (2007) reporting standards.13
Xu et al14,15 in 2016 examined the diagnostic utility of exercise stress echocardiography in assessing suspected obstructive CAD in 191 patients with resting left bundle branch block; 17 patients who failed to achieve a heart rate of at least 85% of the age-predicted maximum heart rate were excluded. Of the remaining 174 patients, 82 demonstrated a normal left ventricular contractile response to exercise and 92 had an abnormal response. In the abnormal group, 70 patients had a globally abnormal response, and 22 patients had a regional ischemic response. Of those who had a globally abnormal left ventricular contractile response who subsequently underwent angiography, only 30% were found to have obstructive CAD.
Although the sensitivity of exercise stress echocardiography was high (94%), its specificity and diagnostic accuracy were poor (specificity 21%, diagnostic accuracy 52%).14,15 These results suggest that for patients with resting left bundle branch block undergoing exercise stress echocardiography, obstructive CAD cannot be reliably diagnosed in those who develop a globally abnormal left ventricular contractile response. Therefore, an alternative imaging strategy should be considered.
DOBUTAMINE STRESS ECHOCARDIOGRAPHY
The evidence base for dobutamine stress echocardiography in patients with left bundle branch block is more robust than that for exercise stress echocardiography.
Geleijnse et al1 studied 64 patients with left bundle branch block undergoing dobutamine stress echocardiography who also underwent coronary angiography. Dobutamine stress echocardiography was moderately sensitive for detecting anterior and posterior myocardial wall ischemia (60% and 67%, respectively). Its specificity and diagnostic accuracy were high, at 94% and 98%, respectively.
Yanik et al16 studied 30 patients with left bundle branch block undergoing both dobutamine stress echocardiography and coronary angiography. The sensitivity of dobutamine stress echocardiography for identifying ischemia in the left anterior descending territory was 82%, the specificity was 95%, and the diagnostic accuracy was 90%. For identifying ischemia in the circumflex and right coronary artery territories, the sensitivity was 88%, specificity 96%, and accuracy 93%.
Mairesse et al17 studied 24 patients with left bundle branch block undergoing dobutamine stress echocardiography, myocardial perfusion tomography, and coronary angiography. Dobutamine stress echocardiography performed well in detecting ischemia in the left anterior descending territory, with a sensitivity of 83%, specificity 92%, and diagnostic accuracy 87%.
Of note, the available data come from very small studies published more than 15 years ago, and pharmacologic stress testing cannot provide the very important prognostic information derived from treadmill testing.
NUCLEAR MYOCARDIAL PERFUSION IMAGING
Exercise nuclear single-photon emission computed tomography (SPECT) myocardial perfusion imaging in patients with left bundle branch block is challenging, due to the development of septal perfusion defects at rest and during exercise in the absence of obstructive disease in the left anterior descending artery (Figure 2).18,19 Asynchronous contraction of the septum, with resulting compression of the septal arteries, decreased flow demands to the septal region, and attenuation artifacts are possible explanations for this phenomenon.20
Pharmacologic stress has been reported to improve the diagnostic accuracy of SPECT myocardial perfusion imaging.21
Biagini et al,21 in a meta-analysis of noninvasive techniques for diagnosing CAD in patients with left bundle branch block, found 1,785 patients from 39 studies who underwent nuclear myocardial perfusion imaging (48.8% with exercise, 41.9% with pharmacologic stress). Overall, sensitivity was high for both exercise and pharmacologic stress (92.9% and 88.5%). However, the reported specificity with exercise stress was significantly lower than with pharmacologic stress (23.3% vs 74.2%, P < .01).
Nuclear positron-emission tomography (PET) may further improve the diagnostic utility of nuclear myocardial perfusion imaging in patients with left bundle branch block. In a study of 440 patients with left bundle branch block undergoing myocardial perfusion imaging, 67 underwent PET and 373 underwent SPECT.22 Possible septal perfusion artifacts were significantly less common with PET than with SPECT (1.5% vs 19.3%, P < .001).
CT ANGIOGRAPHY
CT angiography has a high sensitivity and specificity for detecting significant obstructive CAD.23,24 Machines with 320 detector rows have been reported to have a sensitivity of 94% and specificity of 87% for detecting significant CAD and are not affected by resting left bundle branch block.25
Of note, coronary artery calcification increases in older patients, especially those age 65 and older,26 and this confers a higher likelihood of “bystander” CAD. Significant coronary artery calcification limits the diagnostic accuracy of multidetector cardiac CT. Additionally, the detection of bystander CAD leads to positive findings of uncertain clinical significance.
CURRENT GUIDELINES
Exercise stress echocardiography
American College of Cardiology Foundation/American Heart Association guidelines for diagnosis and management of patients with stable ischemic heart disease recommend exercise stress echocardiography for patients with an intermediate to high pretest probability of ischemic heart disease who have an uninterpretable electrocardiogram and at least moderate physical functioning or no disabling comorbidity (class 1 indication, level of evidence B).11
Current American Society of Echocardiography guidelines also support exercise stress echocardiography as an appropriate test for suspected obstructive CAD in patients with resting left bundle branch block.27 However, this recommendation is based on limited data.
Pharmacologic stress nuclear myocardial perfusion imaging
American Society of Nuclear Cardiology guidelines endorse pharmacologic stress nuclear myocardial perfusion imaging using coronary vasodilators for evaluating suspected obstructive CAD in patients with resting left bundle branch block.28,29
THE POSSIBLE HARMS OF TESTING
Although current guidelines recommend it, recent data show that exercise stress echocardiography has poor specificity and diagnostic accuracy for significant obstructive CAD in patients with resting left bundle branch block. And performing this test in patients with left bundle branch block may result in further downstream investigations.
Based on limited data from a small number of studies published more than 15 years ago, dobutamine stress echocardiography has moderate sensitivity and specificity for significant CAD in patients with resting left bundle branch block. However, this test does not provide functional information about the patient’s exercise performance.
Pharmacologic stress nuclear myocardial perfusion imaging using coronary vasodilators is an appropriate investigation strategy. However, radiation exposure is a limitation.30
CT angiography can assess for significant obstructive CAD in patients with resting left bundle branch block. However, its diagnostic accuracy can be affected by coronary calcification in older patients. Additionally, each scan is associated with a small amount of radiation exposure,31 and a small number of patients will have a true contrast allergy.32
CLINICAL BOTTOM LINE
For patients with typical ischemic symptoms and new left bundle branch block on electrocardiography, specialist cardiology consultation should be sought, with consideration given to proceeding directly to coronary angiography. For stable outpatients, we propose the following diagnostic approach (Figure 3).
Exercise stress echocardiography is recommended by current guidelines, but it cannot reliably detect significant obstructive CAD in patients with resting left bundle branch block—its specificity and diagnostic accuracy are poor.14,15 Alternative imaging strategies include CT angiography, pharmacologic nuclear myocardial perfusion imaging using coronary vasodilators, and dobutamine stress echocardiography.
For investigating suspected obstructive CAD in patients with resting left bundle branch block, we propose CT angiography as the first-line imaging test for patients under age 65 and pharmacologic stress nuclear myocardial perfusion imaging using coronary vasodilators or dobutamine stress echocardiography for those age 65 and older. For patients who cannot tolerate contrast due to renal impairment or who have a true contrast allergy, pharmacologic nuclear myocardial perfusion imaging using coronary vasodilators and dobutamine stress echocardiography may be used as alternatives.
- Geleijnse ML, Vigna C, Kasprzak JD, et al. Usefulness and limitations of dobutamine-atropine stress echocardiography for the diagnosis of coronary artery disease in patients with left bundle branch block. A multicentre study. Eur Heart J 2000; 21:1666–1673.
- Dillon JC, Chang S, Feigenbaum H. Echocardiographic manifestations of left bundle branch block. Circulation 1974; 49:876–880.
- Abbasi AS, Eber LM, Macalpin RN, Kattus AA. Paradoxical motion of interventricular septum in left bundle branch block. Circulation 1974; 49:423–427.
- McDonald IG. Echocardiographic demonstration of abnormal motion of the interventricular septum in left bundle branch block. Circulation 1973; 48:272–280.
- Bouzas-Mosquera A, Peteiro J, Alvarez-García N, et al. Prognostic value of exercise echocardiography in patients with left bundle branch block. JACC Cardiovasc Imaging 2009; 2:251–259.
- Vaillant C, Martins RP, Donal E, et al. Resolution of left bundle branch block-induced cardiomyopathy by cardiac resynchronization therapy. J Am Coll Cardiol 2013; 61:1089–1095.
- Schneider JF, Thomas HE Jr, Sorlie P, Kreger BE, McNamara PM, Kannel WB. Comparative features of newly acquired left and right bundle branch block in the general population: the Framingham study. Am J Cardiol 1981; 47:931–940.
- Hindman MC, Wagner GS, JaRo M, et al. The clinical significance of bundle branch block complicating acute myocardial infarction. Circulation 1978; 58:689–699.
- Freedman RA, Alderman EL, Sheffield LT, Saporito M, Fisher LD. Bundle branch block in patients with chronic coronary artery disease: angiographic correlates and prognostic significance. J Am Coll Cardiol 1987; 10:73–80.
- Schneider JF, Thomas HE Jr, Kreger BE, McNamara PM, Kannel WB. Newly acquired left bundle-branch block: the Framingham study. Ann Intern Med 1979; 90:303–310.
- Fihn SD, Gardin JM, Abrams J, et al. 2012 ACCF/AHA/ACP/AATS/PCNA/SCAI/STS guideline for the diagnosis and management of patients with stable ischemic heart disease: executive summary. J Am Coll Cardiol 2012; 60:2564–2603.
- Peteiro J, Monserrat L, Martinez D, Castro-Beiras A. Accuracy of exercise echocardiography to detect coronary artery disease in left bundle branch block unassociated with either acute or healed myocardial infarction. Am J Cardiol 2000; 85:890–893, A9.
- Pellikka PA, Nagueh SF, Elhendy AA, Kuehl CA, Sawada SG; American Society of Echocardiography. American Society of Echocardiography recommendations for performance, interpretation, and application of stress echocardiography. J Am Soc Echocardiogr 2007; 20:1021–1041.
- Xu B, Dobson L, Mottram P, Moir S. Is exercise stress echocardiography useful in patients with suspected obstructive coronary artery disease who have resting left bundle branch block? J Am Coll Cardiol 2016; 67:1570.
- Xu B, Dobson L, Mottram P, Nasis A, Cameron J, Moir S. Is exercise stress echocardiography useful in patients with suspected obstructive coronary artery disease who have resting left bundle branch block? Clin Cardiol 2018; in press.
- Yanik A, Yetkin E, Senen K, et al. Value of dobutamine stress echocardiography for diagnosis of coronary artery disease in patients with left bundle branch. Coron Artery Dis 2000; 11:545–548.
- Mairesse GH, Marwick TH, Arnese M, et al. Improved identification of coronary artery disease in patients with left bundle branch block by use of dobutamine stress echocardiography and comparison with myocardial perfusion tomography. Am J Cardiol 1995; 76:321–325.
- Vaduganathan P, He ZX, Raghavan C, Mahmarian JJ, Verani MS. Detection of left anterior descending coronary artery stenosis in patients with left bundle branch block: exercise, adenosine or dobutamine imaging? J Am Coll Cardiol 1996; 28:543–550.
- Jazmati B, Sadaniantz A, Emaus SP, Heller GV. Exercise thallium-201 imaging in complete left bundle branch block and the prevalence of septal perfusion defects. Am J Cardiol 1991; 67:46–49.
- Hasegawa S, Sakata Y, Ishikura F, et al. Mechanism for abnormal thallium-201 myocardial scintigraphy in patients with left bundle branch block in the absence of angiographic coronary artery disease. Ann Nucl Med 1999; 13:253–259.
- Biagini E, Shaw LJ, Poldermans D, et al. Accuracy of non-invasive techniques for diagnosis of coronary artery disease and prediction of cardiac events in patients with left bundle branch block: a meta-analysis. Eur J Nucl Med Mol Imaging 2006; 33:1442–1451.
- Cremer P, Brunken R, Menon V, Cerqueira M, Jaber W. Septal perfusion abnormalities are common in regadenoson SPECT myocardial perfusion imaging (MPI) but not PET MPI in patients with left bundle branch block (LBBB). J Am Coll Cardiol 2015; 65:A1148.
- Arbab-Zadeh A, Miller JM, Rochitte CE, et al. Diagnostic accuracy of computed tomography coronary angiography according to pre-test probability of coronary artery disease and severity of coronary arterial calcification. The CORE-64 (Coronary Artery Evaluation Using 64-Row Multidetector Computed Tomography Angiography) International Multicenter Study. J Am Coll Cardiol 2012; 59:379–387.
- Chow BJ, Abraham A, Wells GA, et al. Diagnostic accuracy and impact of computed tomographic coronary angiography on utilization of invasive coronary angiography. Circ Cardiovasc Imaging 2009; 2:16–23.
- Nasis A, Leung MC, Antonis PR, et al. Diagnostic accuracy of noninvasive coronary angiography with 320-detector row computed tomography. Am J Cardiol 2010; 106:1429–1435.
- Whelton SP, Silverman MG, McEvoy JW, et al. Predictors of long-term healthy arterial aging: coronary artery calcium nondevelopment in the MESA study. JACC Cardiovasc Imaging 2015; 8:1393–1400.
- Douglas PS, Garcia MJ, Haines DE, et al. ACCF/ASE/AHA/ASNC/HFSA/HRS/SCAI/SCCM/SCCT/SCMR 2011 appropriate use criteria for echocardiography. J Am Soc Echocardiogr 2011; 24:229–267.
- Henzlova MJ, Duvall WL, Einstein AJ, Travin MI, Verberne HJ. ASNC imaging guidelines for SPECT nuclear cardiology procedures: Stress, protocols, and tracers. J Nucl Cardiol 2016; 23:606–639.
- Wolk MJ, Bailey SR, Doherty JU, et al. ACCF/AHA/ASE/ASNC/HFSA/HRS/SCAI/SCCT/SCMR/STS 2013 multimodality appropriate use criteria for the detection and risk assessment of stable ischemic heart disease. J Am Coll Cardiol 2014; 63:380–406.
- Cerqueira MD, Allman KC, Ficaro EP, et al. Recommendations for reducing radiation exposure in myocardial perfusion imaging. J Nucl Cardiol 2010; 17:709–718.
- Halliburton SS, Abbara S, Chen MY, et al; Society of Cardiovascular Computed Tomography. SCCT guidelines on radiation dose and dose-optimization strategies in cardiovascular CT. J Cardiovasc Comput Tomogr 2011; 5:198–224.
- Wang CL, Cohan RH, Ellis JH, Caoili EM, Wang G, Francis IR. Frequency, outcome, and appropriateness of treatment of nonionic iodinated contrast media reactions. AJR Am J Roentgenol 2008; 191:409–415.
A 62-year-old woman with hypertension and type 2 diabetes mellitus has been experiencing shortness of breath on exertion and chest discomfort for 2 months. Her hypertension has been suboptimally controlled, and her most recent hemoglobin A1c measurement was 7.0%. She has never smoked and has no family history of premature coronary artery disease (CAD). She is otherwise well and walks for 30 minutes 3 times per week. A 12-lead electrocardiogram demonstrated normal sinus rhythm with left bundle branch block. Her physician suspects she has CAD. What testing does this patient need?
LIMITED DATA, GUIDELINES
For clinicians investigating suspected obstructive CAD in patients with left bundle branch block on resting electrocardiography, the data and guidelines are limited regarding the optimal noninvasive tests and how to interpret them.
Here, we present a practical review of the diagnostic utility of exercise stress electrocardiography, exercise stress echocardiography, dobutamine stress echocardiography, nuclear myocardial perfusion imaging, and computed tomographic (CT) angiography for assessing suspected obstructive CAD in patients with resting left bundle branch block.
WHAT IS LEFT BUNDLE BRANCH BLOCK?
In left bundle branch block, as the name implies, electrical conduction along the left bundle branch is blocked or delayed. Ventricular activation therefore begins in the right ventricle and the right side of the interventricular septum.1 Transseptal activation from the right ventricle to the left ventricle is slow, because it is transmyocardial.1 Left ventricular basal and posterolateral wall segments become activated last.1 Due to delay in the onset of left ventricular contraction, ventricular contraction is dyssynchronous. Classically, interventricular septal motion during systole has been described as paradoxical, with anterior septal motion.2–4
On electrocardiography, the QRS duration is widened (≥ 120 ms), with a distinctive morphology as shown in Figure 1. Left bundle branch block makes it difficult to accurately assess for dynamic ST-segment changes with exercise, rendering exercise stress electrocardiography a suboptimal test for obstructive CAD if left bundle branch block is present.
LEFT BUNDLE BRANCH BLOCK AND RISK OF DEATH
Although left bundle branch block can be an isolated finding, it can also be associated with underlying obstructive CAD5 or cardiomyopathy.6 When it occurs at rest, the risk of death from a cardiovascular event is 3 to 4 times higher.7 However, the exact incidence of significant obstructive CAD in asymptomatic patients with incidentally detected left bundle branch block is unknown.
Acute left bundle branch block accompanying acute myocardial infarction is associated with a high risk of death. Hindman et al,8 in a 1978 multicenter study, described 432 patients with acute myocardial infarction and left or right bundle branch block. In the 163 patients who had left bundle branch block, the in-hospital mortality rate was 24% and the 1-year mortality rate was 32%.
Freedman et al9 in 1987 reviewed 15,609 patients with chronic CAD who underwent coronary angiography, of whom 522 had left or right bundle branch block. During a follow-up of nearly 5 years, 2,386 patients died. The actuarial probability of death at 2 years in patients with left bundle branch block was more than 5 times that of patients without it (P < .0001).
During 18 years of observation in the Framingham study,10 55 participants developed left bundle branch block, at a mean age at onset of 62. Twenty-six (48%) of these participants developed clinically significant CAD or heart failure coincident with or subsequent to the onset of left bundle branch block. Fifty percent of the participants who developed left bundle branch block died of cardiovascular disease within 10 years of its onset.
EXERCISE STRESS ELECTROCARDIOGRAPHY
Exercise stress electrocardiography, although valuable for assessing functional capacity, cannot be used to diagnose obstructive CAD in patients with left bundle branch block.11
EXERCISE STRESS ECHOCARDIOGRAPHY
Exercise stress echocardiography is proven and widely used for assessing myocardial ischemia in patients with suspected obstructive CAD. But the data are limited on its diagnostic utility in patients with left bundle branch block. Until recently, recommendations for its use in this situation were based on only 1 small study.12
Peteiro et al12 in 2000 described 35 patients who underwent exercise stress echocardiography and coronary angiography. Detection of wall-motion abnormalities had high sensitivity (76%), specificity (83%), and diagnostic accuracy (80%).
Of note, 8 (23%) of the patients could not achieve at least 85% of the maximum predicted heart rate, and for them, the study was not diagnostic for ischemia. (Technically, the study is said to be nondiagnostic when the patient fails to achieve the target heart rate of at least 85% of the maximum predicted heart rate.)
Additionally, 18 of the 35 patients—over half—had a decrease in left ventricular ejection fraction in response to exercise. These 18 patients included 12 of the 17 patients with obstructive CAD and 6 of the 18 patients without obstructive CAD.12 It is unclear whether a significant proportion of these 18 patients would have been otherwise categorized as having a globally abnormal left ventricular contractile response to exercise according to contemporary (2007) reporting standards.13
Xu et al14,15 in 2016 examined the diagnostic utility of exercise stress echocardiography in assessing suspected obstructive CAD in 191 patients with resting left bundle branch block; 17 patients who failed to achieve a heart rate of at least 85% of the age-predicted maximum heart rate were excluded. Of the remaining 174 patients, 82 demonstrated a normal left ventricular contractile response to exercise and 92 had an abnormal response. In the abnormal group, 70 patients had a globally abnormal response, and 22 patients had a regional ischemic response. Of those who had a globally abnormal left ventricular contractile response who subsequently underwent angiography, only 30% were found to have obstructive CAD.
Although the sensitivity of exercise stress echocardiography was high (94%), its specificity and diagnostic accuracy were poor (specificity 21%, diagnostic accuracy 52%).14,15 These results suggest that for patients with resting left bundle branch block undergoing exercise stress echocardiography, obstructive CAD cannot be reliably diagnosed in those who develop a globally abnormal left ventricular contractile response. Therefore, an alternative imaging strategy should be considered.
DOBUTAMINE STRESS ECHOCARDIOGRAPHY
The evidence base for dobutamine stress echocardiography in patients with left bundle branch block is more robust than that for exercise stress echocardiography.
Geleijnse et al1 studied 64 patients with left bundle branch block undergoing dobutamine stress echocardiography who also underwent coronary angiography. Dobutamine stress echocardiography was moderately sensitive for detecting anterior and posterior myocardial wall ischemia (60% and 67%, respectively). Its specificity and diagnostic accuracy were high, at 94% and 98%, respectively.
Yanik et al16 studied 30 patients with left bundle branch block undergoing both dobutamine stress echocardiography and coronary angiography. The sensitivity of dobutamine stress echocardiography for identifying ischemia in the left anterior descending territory was 82%, the specificity was 95%, and the diagnostic accuracy was 90%. For identifying ischemia in the circumflex and right coronary artery territories, the sensitivity was 88%, specificity 96%, and accuracy 93%.
Mairesse et al17 studied 24 patients with left bundle branch block undergoing dobutamine stress echocardiography, myocardial perfusion tomography, and coronary angiography. Dobutamine stress echocardiography performed well in detecting ischemia in the left anterior descending territory, with a sensitivity of 83%, specificity 92%, and diagnostic accuracy 87%.
Of note, the available data come from very small studies published more than 15 years ago, and pharmacologic stress testing cannot provide the very important prognostic information derived from treadmill testing.
NUCLEAR MYOCARDIAL PERFUSION IMAGING
Exercise nuclear single-photon emission computed tomography (SPECT) myocardial perfusion imaging in patients with left bundle branch block is challenging, due to the development of septal perfusion defects at rest and during exercise in the absence of obstructive disease in the left anterior descending artery (Figure 2).18,19 Asynchronous contraction of the septum, with resulting compression of the septal arteries, decreased flow demands to the septal region, and attenuation artifacts are possible explanations for this phenomenon.20
Pharmacologic stress has been reported to improve the diagnostic accuracy of SPECT myocardial perfusion imaging.21
Biagini et al,21 in a meta-analysis of noninvasive techniques for diagnosing CAD in patients with left bundle branch block, found 1,785 patients from 39 studies who underwent nuclear myocardial perfusion imaging (48.8% with exercise, 41.9% with pharmacologic stress). Overall, sensitivity was high for both exercise and pharmacologic stress (92.9% and 88.5%). However, the reported specificity with exercise stress was significantly lower than with pharmacologic stress (23.3% vs 74.2%, P < .01).
Nuclear positron-emission tomography (PET) may further improve the diagnostic utility of nuclear myocardial perfusion imaging in patients with left bundle branch block. In a study of 440 patients with left bundle branch block undergoing myocardial perfusion imaging, 67 underwent PET and 373 underwent SPECT.22 Possible septal perfusion artifacts were significantly less common with PET than with SPECT (1.5% vs 19.3%, P < .001).
CT ANGIOGRAPHY
CT angiography has a high sensitivity and specificity for detecting significant obstructive CAD.23,24 Machines with 320 detector rows have been reported to have a sensitivity of 94% and specificity of 87% for detecting significant CAD and are not affected by resting left bundle branch block.25
Of note, coronary artery calcification increases in older patients, especially those age 65 and older,26 and this confers a higher likelihood of “bystander” CAD. Significant coronary artery calcification limits the diagnostic accuracy of multidetector cardiac CT. Additionally, the detection of bystander CAD leads to positive findings of uncertain clinical significance.
CURRENT GUIDELINES
Exercise stress echocardiography
American College of Cardiology Foundation/American Heart Association guidelines for diagnosis and management of patients with stable ischemic heart disease recommend exercise stress echocardiography for patients with an intermediate to high pretest probability of ischemic heart disease who have an uninterpretable electrocardiogram and at least moderate physical functioning or no disabling comorbidity (class 1 indication, level of evidence B).11
Current American Society of Echocardiography guidelines also support exercise stress echocardiography as an appropriate test for suspected obstructive CAD in patients with resting left bundle branch block.27 However, this recommendation is based on limited data.
Pharmacologic stress nuclear myocardial perfusion imaging
American Society of Nuclear Cardiology guidelines endorse pharmacologic stress nuclear myocardial perfusion imaging using coronary vasodilators for evaluating suspected obstructive CAD in patients with resting left bundle branch block.28,29
THE POSSIBLE HARMS OF TESTING
Although current guidelines recommend it, recent data show that exercise stress echocardiography has poor specificity and diagnostic accuracy for significant obstructive CAD in patients with resting left bundle branch block. And performing this test in patients with left bundle branch block may result in further downstream investigations.
Based on limited data from a small number of studies published more than 15 years ago, dobutamine stress echocardiography has moderate sensitivity and specificity for significant CAD in patients with resting left bundle branch block. However, this test does not provide functional information about the patient’s exercise performance.
Pharmacologic stress nuclear myocardial perfusion imaging using coronary vasodilators is an appropriate investigation strategy. However, radiation exposure is a limitation.30
CT angiography can assess for significant obstructive CAD in patients with resting left bundle branch block. However, its diagnostic accuracy can be affected by coronary calcification in older patients. Additionally, each scan is associated with a small amount of radiation exposure,31 and a small number of patients will have a true contrast allergy.32
CLINICAL BOTTOM LINE
For patients with typical ischemic symptoms and new left bundle branch block on electrocardiography, specialist cardiology consultation should be sought, with consideration given to proceeding directly to coronary angiography. For stable outpatients, we propose the following diagnostic approach (Figure 3).
Exercise stress echocardiography is recommended by current guidelines, but it cannot reliably detect significant obstructive CAD in patients with resting left bundle branch block—its specificity and diagnostic accuracy are poor.14,15 Alternative imaging strategies include CT angiography, pharmacologic nuclear myocardial perfusion imaging using coronary vasodilators, and dobutamine stress echocardiography.
For investigating suspected obstructive CAD in patients with resting left bundle branch block, we propose CT angiography as the first-line imaging test for patients under age 65 and pharmacologic stress nuclear myocardial perfusion imaging using coronary vasodilators or dobutamine stress echocardiography for those age 65 and older. For patients who cannot tolerate contrast due to renal impairment or who have a true contrast allergy, pharmacologic nuclear myocardial perfusion imaging using coronary vasodilators and dobutamine stress echocardiography may be used as alternatives.
A 62-year-old woman with hypertension and type 2 diabetes mellitus has been experiencing shortness of breath on exertion and chest discomfort for 2 months. Her hypertension has been suboptimally controlled, and her most recent hemoglobin A1c measurement was 7.0%. She has never smoked and has no family history of premature coronary artery disease (CAD). She is otherwise well and walks for 30 minutes 3 times per week. A 12-lead electrocardiogram demonstrated normal sinus rhythm with left bundle branch block. Her physician suspects she has CAD. What testing does this patient need?
LIMITED DATA, GUIDELINES
For clinicians investigating suspected obstructive CAD in patients with left bundle branch block on resting electrocardiography, the data and guidelines are limited regarding the optimal noninvasive tests and how to interpret them.
Here, we present a practical review of the diagnostic utility of exercise stress electrocardiography, exercise stress echocardiography, dobutamine stress echocardiography, nuclear myocardial perfusion imaging, and computed tomographic (CT) angiography for assessing suspected obstructive CAD in patients with resting left bundle branch block.
WHAT IS LEFT BUNDLE BRANCH BLOCK?
In left bundle branch block, as the name implies, electrical conduction along the left bundle branch is blocked or delayed. Ventricular activation therefore begins in the right ventricle and the right side of the interventricular septum.1 Transseptal activation from the right ventricle to the left ventricle is slow, because it is transmyocardial.1 Left ventricular basal and posterolateral wall segments become activated last.1 Due to delay in the onset of left ventricular contraction, ventricular contraction is dyssynchronous. Classically, interventricular septal motion during systole has been described as paradoxical, with anterior septal motion.2–4
On electrocardiography, the QRS duration is widened (≥ 120 ms), with a distinctive morphology as shown in Figure 1. Left bundle branch block makes it difficult to accurately assess for dynamic ST-segment changes with exercise, rendering exercise stress electrocardiography a suboptimal test for obstructive CAD if left bundle branch block is present.
LEFT BUNDLE BRANCH BLOCK AND RISK OF DEATH
Although left bundle branch block can be an isolated finding, it can also be associated with underlying obstructive CAD5 or cardiomyopathy.6 When it occurs at rest, the risk of death from a cardiovascular event is 3 to 4 times higher.7 However, the exact incidence of significant obstructive CAD in asymptomatic patients with incidentally detected left bundle branch block is unknown.
Acute left bundle branch block accompanying acute myocardial infarction is associated with a high risk of death. Hindman et al,8 in a 1978 multicenter study, described 432 patients with acute myocardial infarction and left or right bundle branch block. In the 163 patients who had left bundle branch block, the in-hospital mortality rate was 24% and the 1-year mortality rate was 32%.
Freedman et al9 in 1987 reviewed 15,609 patients with chronic CAD who underwent coronary angiography, of whom 522 had left or right bundle branch block. During a follow-up of nearly 5 years, 2,386 patients died. The actuarial probability of death at 2 years in patients with left bundle branch block was more than 5 times that of patients without it (P < .0001).
During 18 years of observation in the Framingham study,10 55 participants developed left bundle branch block, at a mean age at onset of 62. Twenty-six (48%) of these participants developed clinically significant CAD or heart failure coincident with or subsequent to the onset of left bundle branch block. Fifty percent of the participants who developed left bundle branch block died of cardiovascular disease within 10 years of its onset.
EXERCISE STRESS ELECTROCARDIOGRAPHY
Exercise stress electrocardiography, although valuable for assessing functional capacity, cannot be used to diagnose obstructive CAD in patients with left bundle branch block.11
EXERCISE STRESS ECHOCARDIOGRAPHY
Exercise stress echocardiography is proven and widely used for assessing myocardial ischemia in patients with suspected obstructive CAD. But the data are limited on its diagnostic utility in patients with left bundle branch block. Until recently, recommendations for its use in this situation were based on only 1 small study.12
Peteiro et al12 in 2000 described 35 patients who underwent exercise stress echocardiography and coronary angiography. Detection of wall-motion abnormalities had high sensitivity (76%), specificity (83%), and diagnostic accuracy (80%).
Of note, 8 (23%) of the patients could not achieve at least 85% of the maximum predicted heart rate, and for them, the study was not diagnostic for ischemia. (Technically, the study is said to be nondiagnostic when the patient fails to achieve the target heart rate of at least 85% of the maximum predicted heart rate.)
Additionally, 18 of the 35 patients—over half—had a decrease in left ventricular ejection fraction in response to exercise. These 18 patients included 12 of the 17 patients with obstructive CAD and 6 of the 18 patients without obstructive CAD.12 It is unclear whether a significant proportion of these 18 patients would have been otherwise categorized as having a globally abnormal left ventricular contractile response to exercise according to contemporary (2007) reporting standards.13
Xu et al14,15 in 2016 examined the diagnostic utility of exercise stress echocardiography in assessing suspected obstructive CAD in 191 patients with resting left bundle branch block; 17 patients who failed to achieve a heart rate of at least 85% of the age-predicted maximum heart rate were excluded. Of the remaining 174 patients, 82 demonstrated a normal left ventricular contractile response to exercise and 92 had an abnormal response. In the abnormal group, 70 patients had a globally abnormal response, and 22 patients had a regional ischemic response. Of those who had a globally abnormal left ventricular contractile response who subsequently underwent angiography, only 30% were found to have obstructive CAD.
Although the sensitivity of exercise stress echocardiography was high (94%), its specificity and diagnostic accuracy were poor (specificity 21%, diagnostic accuracy 52%).14,15 These results suggest that for patients with resting left bundle branch block undergoing exercise stress echocardiography, obstructive CAD cannot be reliably diagnosed in those who develop a globally abnormal left ventricular contractile response. Therefore, an alternative imaging strategy should be considered.
DOBUTAMINE STRESS ECHOCARDIOGRAPHY
The evidence base for dobutamine stress echocardiography in patients with left bundle branch block is more robust than that for exercise stress echocardiography.
Geleijnse et al1 studied 64 patients with left bundle branch block undergoing dobutamine stress echocardiography who also underwent coronary angiography. Dobutamine stress echocardiography was moderately sensitive for detecting anterior and posterior myocardial wall ischemia (60% and 67%, respectively). Its specificity and diagnostic accuracy were high, at 94% and 98%, respectively.
Yanik et al16 studied 30 patients with left bundle branch block undergoing both dobutamine stress echocardiography and coronary angiography. The sensitivity of dobutamine stress echocardiography for identifying ischemia in the left anterior descending territory was 82%, the specificity was 95%, and the diagnostic accuracy was 90%. For identifying ischemia in the circumflex and right coronary artery territories, the sensitivity was 88%, specificity 96%, and accuracy 93%.
Mairesse et al17 studied 24 patients with left bundle branch block undergoing dobutamine stress echocardiography, myocardial perfusion tomography, and coronary angiography. Dobutamine stress echocardiography performed well in detecting ischemia in the left anterior descending territory, with a sensitivity of 83%, specificity 92%, and diagnostic accuracy 87%.
Of note, the available data come from very small studies published more than 15 years ago, and pharmacologic stress testing cannot provide the very important prognostic information derived from treadmill testing.
NUCLEAR MYOCARDIAL PERFUSION IMAGING
Exercise nuclear single-photon emission computed tomography (SPECT) myocardial perfusion imaging in patients with left bundle branch block is challenging, due to the development of septal perfusion defects at rest and during exercise in the absence of obstructive disease in the left anterior descending artery (Figure 2).18,19 Asynchronous contraction of the septum, with resulting compression of the septal arteries, decreased flow demands to the septal region, and attenuation artifacts are possible explanations for this phenomenon.20
Pharmacologic stress has been reported to improve the diagnostic accuracy of SPECT myocardial perfusion imaging.21
Biagini et al,21 in a meta-analysis of noninvasive techniques for diagnosing CAD in patients with left bundle branch block, found 1,785 patients from 39 studies who underwent nuclear myocardial perfusion imaging (48.8% with exercise, 41.9% with pharmacologic stress). Overall, sensitivity was high for both exercise and pharmacologic stress (92.9% and 88.5%). However, the reported specificity with exercise stress was significantly lower than with pharmacologic stress (23.3% vs 74.2%, P < .01).
Nuclear positron-emission tomography (PET) may further improve the diagnostic utility of nuclear myocardial perfusion imaging in patients with left bundle branch block. In a study of 440 patients with left bundle branch block undergoing myocardial perfusion imaging, 67 underwent PET and 373 underwent SPECT.22 Possible septal perfusion artifacts were significantly less common with PET than with SPECT (1.5% vs 19.3%, P < .001).
CT ANGIOGRAPHY
CT angiography has a high sensitivity and specificity for detecting significant obstructive CAD.23,24 Machines with 320 detector rows have been reported to have a sensitivity of 94% and specificity of 87% for detecting significant CAD and are not affected by resting left bundle branch block.25
Of note, coronary artery calcification increases in older patients, especially those age 65 and older,26 and this confers a higher likelihood of “bystander” CAD. Significant coronary artery calcification limits the diagnostic accuracy of multidetector cardiac CT. Additionally, the detection of bystander CAD leads to positive findings of uncertain clinical significance.
CURRENT GUIDELINES
Exercise stress echocardiography
American College of Cardiology Foundation/American Heart Association guidelines for diagnosis and management of patients with stable ischemic heart disease recommend exercise stress echocardiography for patients with an intermediate to high pretest probability of ischemic heart disease who have an uninterpretable electrocardiogram and at least moderate physical functioning or no disabling comorbidity (class 1 indication, level of evidence B).11
Current American Society of Echocardiography guidelines also support exercise stress echocardiography as an appropriate test for suspected obstructive CAD in patients with resting left bundle branch block.27 However, this recommendation is based on limited data.
Pharmacologic stress nuclear myocardial perfusion imaging
American Society of Nuclear Cardiology guidelines endorse pharmacologic stress nuclear myocardial perfusion imaging using coronary vasodilators for evaluating suspected obstructive CAD in patients with resting left bundle branch block.28,29
THE POSSIBLE HARMS OF TESTING
Although current guidelines recommend it, recent data show that exercise stress echocardiography has poor specificity and diagnostic accuracy for significant obstructive CAD in patients with resting left bundle branch block. And performing this test in patients with left bundle branch block may result in further downstream investigations.
Based on limited data from a small number of studies published more than 15 years ago, dobutamine stress echocardiography has moderate sensitivity and specificity for significant CAD in patients with resting left bundle branch block. However, this test does not provide functional information about the patient’s exercise performance.
Pharmacologic stress nuclear myocardial perfusion imaging using coronary vasodilators is an appropriate investigation strategy. However, radiation exposure is a limitation.30
CT angiography can assess for significant obstructive CAD in patients with resting left bundle branch block. However, its diagnostic accuracy can be affected by coronary calcification in older patients. Additionally, each scan is associated with a small amount of radiation exposure,31 and a small number of patients will have a true contrast allergy.32
CLINICAL BOTTOM LINE
For patients with typical ischemic symptoms and new left bundle branch block on electrocardiography, specialist cardiology consultation should be sought, with consideration given to proceeding directly to coronary angiography. For stable outpatients, we propose the following diagnostic approach (Figure 3).
Exercise stress echocardiography is recommended by current guidelines, but it cannot reliably detect significant obstructive CAD in patients with resting left bundle branch block—its specificity and diagnostic accuracy are poor.14,15 Alternative imaging strategies include CT angiography, pharmacologic nuclear myocardial perfusion imaging using coronary vasodilators, and dobutamine stress echocardiography.
For investigating suspected obstructive CAD in patients with resting left bundle branch block, we propose CT angiography as the first-line imaging test for patients under age 65 and pharmacologic stress nuclear myocardial perfusion imaging using coronary vasodilators or dobutamine stress echocardiography for those age 65 and older. For patients who cannot tolerate contrast due to renal impairment or who have a true contrast allergy, pharmacologic nuclear myocardial perfusion imaging using coronary vasodilators and dobutamine stress echocardiography may be used as alternatives.
- Geleijnse ML, Vigna C, Kasprzak JD, et al. Usefulness and limitations of dobutamine-atropine stress echocardiography for the diagnosis of coronary artery disease in patients with left bundle branch block. A multicentre study. Eur Heart J 2000; 21:1666–1673.
- Dillon JC, Chang S, Feigenbaum H. Echocardiographic manifestations of left bundle branch block. Circulation 1974; 49:876–880.
- Abbasi AS, Eber LM, Macalpin RN, Kattus AA. Paradoxical motion of interventricular septum in left bundle branch block. Circulation 1974; 49:423–427.
- McDonald IG. Echocardiographic demonstration of abnormal motion of the interventricular septum in left bundle branch block. Circulation 1973; 48:272–280.
- Bouzas-Mosquera A, Peteiro J, Alvarez-García N, et al. Prognostic value of exercise echocardiography in patients with left bundle branch block. JACC Cardiovasc Imaging 2009; 2:251–259.
- Vaillant C, Martins RP, Donal E, et al. Resolution of left bundle branch block-induced cardiomyopathy by cardiac resynchronization therapy. J Am Coll Cardiol 2013; 61:1089–1095.
- Schneider JF, Thomas HE Jr, Sorlie P, Kreger BE, McNamara PM, Kannel WB. Comparative features of newly acquired left and right bundle branch block in the general population: the Framingham study. Am J Cardiol 1981; 47:931–940.
- Hindman MC, Wagner GS, JaRo M, et al. The clinical significance of bundle branch block complicating acute myocardial infarction. Circulation 1978; 58:689–699.
- Freedman RA, Alderman EL, Sheffield LT, Saporito M, Fisher LD. Bundle branch block in patients with chronic coronary artery disease: angiographic correlates and prognostic significance. J Am Coll Cardiol 1987; 10:73–80.
- Schneider JF, Thomas HE Jr, Kreger BE, McNamara PM, Kannel WB. Newly acquired left bundle-branch block: the Framingham study. Ann Intern Med 1979; 90:303–310.
- Fihn SD, Gardin JM, Abrams J, et al. 2012 ACCF/AHA/ACP/AATS/PCNA/SCAI/STS guideline for the diagnosis and management of patients with stable ischemic heart disease: executive summary. J Am Coll Cardiol 2012; 60:2564–2603.
- Peteiro J, Monserrat L, Martinez D, Castro-Beiras A. Accuracy of exercise echocardiography to detect coronary artery disease in left bundle branch block unassociated with either acute or healed myocardial infarction. Am J Cardiol 2000; 85:890–893, A9.
- Pellikka PA, Nagueh SF, Elhendy AA, Kuehl CA, Sawada SG; American Society of Echocardiography. American Society of Echocardiography recommendations for performance, interpretation, and application of stress echocardiography. J Am Soc Echocardiogr 2007; 20:1021–1041.
- Xu B, Dobson L, Mottram P, Moir S. Is exercise stress echocardiography useful in patients with suspected obstructive coronary artery disease who have resting left bundle branch block? J Am Coll Cardiol 2016; 67:1570.
- Xu B, Dobson L, Mottram P, Nasis A, Cameron J, Moir S. Is exercise stress echocardiography useful in patients with suspected obstructive coronary artery disease who have resting left bundle branch block? Clin Cardiol 2018; in press.
- Yanik A, Yetkin E, Senen K, et al. Value of dobutamine stress echocardiography for diagnosis of coronary artery disease in patients with left bundle branch. Coron Artery Dis 2000; 11:545–548.
- Mairesse GH, Marwick TH, Arnese M, et al. Improved identification of coronary artery disease in patients with left bundle branch block by use of dobutamine stress echocardiography and comparison with myocardial perfusion tomography. Am J Cardiol 1995; 76:321–325.
- Vaduganathan P, He ZX, Raghavan C, Mahmarian JJ, Verani MS. Detection of left anterior descending coronary artery stenosis in patients with left bundle branch block: exercise, adenosine or dobutamine imaging? J Am Coll Cardiol 1996; 28:543–550.
- Jazmati B, Sadaniantz A, Emaus SP, Heller GV. Exercise thallium-201 imaging in complete left bundle branch block and the prevalence of septal perfusion defects. Am J Cardiol 1991; 67:46–49.
- Hasegawa S, Sakata Y, Ishikura F, et al. Mechanism for abnormal thallium-201 myocardial scintigraphy in patients with left bundle branch block in the absence of angiographic coronary artery disease. Ann Nucl Med 1999; 13:253–259.
- Biagini E, Shaw LJ, Poldermans D, et al. Accuracy of non-invasive techniques for diagnosis of coronary artery disease and prediction of cardiac events in patients with left bundle branch block: a meta-analysis. Eur J Nucl Med Mol Imaging 2006; 33:1442–1451.
- Cremer P, Brunken R, Menon V, Cerqueira M, Jaber W. Septal perfusion abnormalities are common in regadenoson SPECT myocardial perfusion imaging (MPI) but not PET MPI in patients with left bundle branch block (LBBB). J Am Coll Cardiol 2015; 65:A1148.
- Arbab-Zadeh A, Miller JM, Rochitte CE, et al. Diagnostic accuracy of computed tomography coronary angiography according to pre-test probability of coronary artery disease and severity of coronary arterial calcification. The CORE-64 (Coronary Artery Evaluation Using 64-Row Multidetector Computed Tomography Angiography) International Multicenter Study. J Am Coll Cardiol 2012; 59:379–387.
- Chow BJ, Abraham A, Wells GA, et al. Diagnostic accuracy and impact of computed tomographic coronary angiography on utilization of invasive coronary angiography. Circ Cardiovasc Imaging 2009; 2:16–23.
- Nasis A, Leung MC, Antonis PR, et al. Diagnostic accuracy of noninvasive coronary angiography with 320-detector row computed tomography. Am J Cardiol 2010; 106:1429–1435.
- Whelton SP, Silverman MG, McEvoy JW, et al. Predictors of long-term healthy arterial aging: coronary artery calcium nondevelopment in the MESA study. JACC Cardiovasc Imaging 2015; 8:1393–1400.
- Douglas PS, Garcia MJ, Haines DE, et al. ACCF/ASE/AHA/ASNC/HFSA/HRS/SCAI/SCCM/SCCT/SCMR 2011 appropriate use criteria for echocardiography. J Am Soc Echocardiogr 2011; 24:229–267.
- Henzlova MJ, Duvall WL, Einstein AJ, Travin MI, Verberne HJ. ASNC imaging guidelines for SPECT nuclear cardiology procedures: Stress, protocols, and tracers. J Nucl Cardiol 2016; 23:606–639.
- Wolk MJ, Bailey SR, Doherty JU, et al. ACCF/AHA/ASE/ASNC/HFSA/HRS/SCAI/SCCT/SCMR/STS 2013 multimodality appropriate use criteria for the detection and risk assessment of stable ischemic heart disease. J Am Coll Cardiol 2014; 63:380–406.
- Cerqueira MD, Allman KC, Ficaro EP, et al. Recommendations for reducing radiation exposure in myocardial perfusion imaging. J Nucl Cardiol 2010; 17:709–718.
- Halliburton SS, Abbara S, Chen MY, et al; Society of Cardiovascular Computed Tomography. SCCT guidelines on radiation dose and dose-optimization strategies in cardiovascular CT. J Cardiovasc Comput Tomogr 2011; 5:198–224.
- Wang CL, Cohan RH, Ellis JH, Caoili EM, Wang G, Francis IR. Frequency, outcome, and appropriateness of treatment of nonionic iodinated contrast media reactions. AJR Am J Roentgenol 2008; 191:409–415.
- Geleijnse ML, Vigna C, Kasprzak JD, et al. Usefulness and limitations of dobutamine-atropine stress echocardiography for the diagnosis of coronary artery disease in patients with left bundle branch block. A multicentre study. Eur Heart J 2000; 21:1666–1673.
- Dillon JC, Chang S, Feigenbaum H. Echocardiographic manifestations of left bundle branch block. Circulation 1974; 49:876–880.
- Abbasi AS, Eber LM, Macalpin RN, Kattus AA. Paradoxical motion of interventricular septum in left bundle branch block. Circulation 1974; 49:423–427.
- McDonald IG. Echocardiographic demonstration of abnormal motion of the interventricular septum in left bundle branch block. Circulation 1973; 48:272–280.
- Bouzas-Mosquera A, Peteiro J, Alvarez-García N, et al. Prognostic value of exercise echocardiography in patients with left bundle branch block. JACC Cardiovasc Imaging 2009; 2:251–259.
- Vaillant C, Martins RP, Donal E, et al. Resolution of left bundle branch block-induced cardiomyopathy by cardiac resynchronization therapy. J Am Coll Cardiol 2013; 61:1089–1095.
- Schneider JF, Thomas HE Jr, Sorlie P, Kreger BE, McNamara PM, Kannel WB. Comparative features of newly acquired left and right bundle branch block in the general population: the Framingham study. Am J Cardiol 1981; 47:931–940.
- Hindman MC, Wagner GS, JaRo M, et al. The clinical significance of bundle branch block complicating acute myocardial infarction. Circulation 1978; 58:689–699.
- Freedman RA, Alderman EL, Sheffield LT, Saporito M, Fisher LD. Bundle branch block in patients with chronic coronary artery disease: angiographic correlates and prognostic significance. J Am Coll Cardiol 1987; 10:73–80.
- Schneider JF, Thomas HE Jr, Kreger BE, McNamara PM, Kannel WB. Newly acquired left bundle-branch block: the Framingham study. Ann Intern Med 1979; 90:303–310.
- Fihn SD, Gardin JM, Abrams J, et al. 2012 ACCF/AHA/ACP/AATS/PCNA/SCAI/STS guideline for the diagnosis and management of patients with stable ischemic heart disease: executive summary. J Am Coll Cardiol 2012; 60:2564–2603.
- Peteiro J, Monserrat L, Martinez D, Castro-Beiras A. Accuracy of exercise echocardiography to detect coronary artery disease in left bundle branch block unassociated with either acute or healed myocardial infarction. Am J Cardiol 2000; 85:890–893, A9.
- Pellikka PA, Nagueh SF, Elhendy AA, Kuehl CA, Sawada SG; American Society of Echocardiography. American Society of Echocardiography recommendations for performance, interpretation, and application of stress echocardiography. J Am Soc Echocardiogr 2007; 20:1021–1041.
- Xu B, Dobson L, Mottram P, Moir S. Is exercise stress echocardiography useful in patients with suspected obstructive coronary artery disease who have resting left bundle branch block? J Am Coll Cardiol 2016; 67:1570.
- Xu B, Dobson L, Mottram P, Nasis A, Cameron J, Moir S. Is exercise stress echocardiography useful in patients with suspected obstructive coronary artery disease who have resting left bundle branch block? Clin Cardiol 2018; in press.
- Yanik A, Yetkin E, Senen K, et al. Value of dobutamine stress echocardiography for diagnosis of coronary artery disease in patients with left bundle branch. Coron Artery Dis 2000; 11:545–548.
- Mairesse GH, Marwick TH, Arnese M, et al. Improved identification of coronary artery disease in patients with left bundle branch block by use of dobutamine stress echocardiography and comparison with myocardial perfusion tomography. Am J Cardiol 1995; 76:321–325.
- Vaduganathan P, He ZX, Raghavan C, Mahmarian JJ, Verani MS. Detection of left anterior descending coronary artery stenosis in patients with left bundle branch block: exercise, adenosine or dobutamine imaging? J Am Coll Cardiol 1996; 28:543–550.
- Jazmati B, Sadaniantz A, Emaus SP, Heller GV. Exercise thallium-201 imaging in complete left bundle branch block and the prevalence of septal perfusion defects. Am J Cardiol 1991; 67:46–49.
- Hasegawa S, Sakata Y, Ishikura F, et al. Mechanism for abnormal thallium-201 myocardial scintigraphy in patients with left bundle branch block in the absence of angiographic coronary artery disease. Ann Nucl Med 1999; 13:253–259.
- Biagini E, Shaw LJ, Poldermans D, et al. Accuracy of non-invasive techniques for diagnosis of coronary artery disease and prediction of cardiac events in patients with left bundle branch block: a meta-analysis. Eur J Nucl Med Mol Imaging 2006; 33:1442–1451.
- Cremer P, Brunken R, Menon V, Cerqueira M, Jaber W. Septal perfusion abnormalities are common in regadenoson SPECT myocardial perfusion imaging (MPI) but not PET MPI in patients with left bundle branch block (LBBB). J Am Coll Cardiol 2015; 65:A1148.
- Arbab-Zadeh A, Miller JM, Rochitte CE, et al. Diagnostic accuracy of computed tomography coronary angiography according to pre-test probability of coronary artery disease and severity of coronary arterial calcification. The CORE-64 (Coronary Artery Evaluation Using 64-Row Multidetector Computed Tomography Angiography) International Multicenter Study. J Am Coll Cardiol 2012; 59:379–387.
- Chow BJ, Abraham A, Wells GA, et al. Diagnostic accuracy and impact of computed tomographic coronary angiography on utilization of invasive coronary angiography. Circ Cardiovasc Imaging 2009; 2:16–23.
- Nasis A, Leung MC, Antonis PR, et al. Diagnostic accuracy of noninvasive coronary angiography with 320-detector row computed tomography. Am J Cardiol 2010; 106:1429–1435.
- Whelton SP, Silverman MG, McEvoy JW, et al. Predictors of long-term healthy arterial aging: coronary artery calcium nondevelopment in the MESA study. JACC Cardiovasc Imaging 2015; 8:1393–1400.
- Douglas PS, Garcia MJ, Haines DE, et al. ACCF/ASE/AHA/ASNC/HFSA/HRS/SCAI/SCCM/SCCT/SCMR 2011 appropriate use criteria for echocardiography. J Am Soc Echocardiogr 2011; 24:229–267.
- Henzlova MJ, Duvall WL, Einstein AJ, Travin MI, Verberne HJ. ASNC imaging guidelines for SPECT nuclear cardiology procedures: Stress, protocols, and tracers. J Nucl Cardiol 2016; 23:606–639.
- Wolk MJ, Bailey SR, Doherty JU, et al. ACCF/AHA/ASE/ASNC/HFSA/HRS/SCAI/SCCT/SCMR/STS 2013 multimodality appropriate use criteria for the detection and risk assessment of stable ischemic heart disease. J Am Coll Cardiol 2014; 63:380–406.
- Cerqueira MD, Allman KC, Ficaro EP, et al. Recommendations for reducing radiation exposure in myocardial perfusion imaging. J Nucl Cardiol 2010; 17:709–718.
- Halliburton SS, Abbara S, Chen MY, et al; Society of Cardiovascular Computed Tomography. SCCT guidelines on radiation dose and dose-optimization strategies in cardiovascular CT. J Cardiovasc Comput Tomogr 2011; 5:198–224.
- Wang CL, Cohan RH, Ellis JH, Caoili EM, Wang G, Francis IR. Frequency, outcome, and appropriateness of treatment of nonionic iodinated contrast media reactions. AJR Am J Roentgenol 2008; 191:409–415.
KEY POINTS
- Although current guidelines recommend exercise stress echocardiography, it cannot reliably detect significant obstructive CAD in patients who have left bundle branch block at rest.
- CT angiography is the first-line imaging test for these patients if they are under age 65. For those 65 and older, the first-line test is either pharmacologic stress nuclear myocardial perfusion imaging with coronary vasodilators or dobutamine stress echocardiography.
- For patients who cannot tolerate CT contrast due to renal impairment or who have a true contrast allergy, pharmacologic nuclear myocardial perfusion imaging using coronary vasodilators and dobutamine stress echocardiography can be alternatives.
Ascites from intraperitoneal urine leakage after pelvic radiation
A 44-year-old woman was admitted to the hospital for the second time in 2 months with acute onset of severe abdominal pain. She had a history of cervical cancer treated with total hysterectomy with bilateral salpingo-oophorectomy, chemotherapy, and radiotherapy at age 38.
LONG-TERM EFFECTS OF RADIATION ON THE BLADDER
Urinary ascites from intraperitoneal urine leakage is a rare but clinically important sequel to bladder fistula or bladder wall rupture. Fistula or rupture can be caused by pelvic irradiation, blunt trauma, or surgical procedures, but may also be spontaneous.2
When the total radiation dose to the bladder exceeds 60 Gy, radiation cystitis may occur, leading to bladder fistula.3 Effects of radiation on the bladder are usually seen within 2 to 4 years3 but may occur long after the completion of radiation therapy—10 years2 or even 30 to 40 years later.4 Therefore, ascites of unknown origin in a patient with a history of pelvic radiation therapy should lead to an evaluation for late radiation cystitis and urinary ascites from bladder rupture.
- Ramcharan K, Poon-King TM, Indar R. Spontaneous intraperitoneal rupture of a neurogenic bladder; the importance of ascitic fluid urea and electrolytes in diagnosis. Postgrad Med J 1987; 63:999–1000.
- Matsumura M, Ando N, Kumabe A, Dhaliwal G. Pseudo-renal failure: bladder rupture with urinary ascites. BMJ Case Rep 2015; pii:bcr2015212671.
- Shi F, Wang T, Wang J, et al. Peritoneal bladder fistula following radiotherapy for cervical cancer: a case report. Oncol Lett 2016; 12:2008–2010.
- Hayashi W, Nishino T, Namie S, Obata Y, Furukawa M, Kohno S. Spontaneous bladder rupture diagnosis based on urinary appearance of mesothelial cells: a case report. J Med Case Rep 2014; 8:46.
A 44-year-old woman was admitted to the hospital for the second time in 2 months with acute onset of severe abdominal pain. She had a history of cervical cancer treated with total hysterectomy with bilateral salpingo-oophorectomy, chemotherapy, and radiotherapy at age 38.
LONG-TERM EFFECTS OF RADIATION ON THE BLADDER
Urinary ascites from intraperitoneal urine leakage is a rare but clinically important sequel to bladder fistula or bladder wall rupture. Fistula or rupture can be caused by pelvic irradiation, blunt trauma, or surgical procedures, but may also be spontaneous.2
When the total radiation dose to the bladder exceeds 60 Gy, radiation cystitis may occur, leading to bladder fistula.3 Effects of radiation on the bladder are usually seen within 2 to 4 years3 but may occur long after the completion of radiation therapy—10 years2 or even 30 to 40 years later.4 Therefore, ascites of unknown origin in a patient with a history of pelvic radiation therapy should lead to an evaluation for late radiation cystitis and urinary ascites from bladder rupture.
A 44-year-old woman was admitted to the hospital for the second time in 2 months with acute onset of severe abdominal pain. She had a history of cervical cancer treated with total hysterectomy with bilateral salpingo-oophorectomy, chemotherapy, and radiotherapy at age 38.
LONG-TERM EFFECTS OF RADIATION ON THE BLADDER
Urinary ascites from intraperitoneal urine leakage is a rare but clinically important sequel to bladder fistula or bladder wall rupture. Fistula or rupture can be caused by pelvic irradiation, blunt trauma, or surgical procedures, but may also be spontaneous.2
When the total radiation dose to the bladder exceeds 60 Gy, radiation cystitis may occur, leading to bladder fistula.3 Effects of radiation on the bladder are usually seen within 2 to 4 years3 but may occur long after the completion of radiation therapy—10 years2 or even 30 to 40 years later.4 Therefore, ascites of unknown origin in a patient with a history of pelvic radiation therapy should lead to an evaluation for late radiation cystitis and urinary ascites from bladder rupture.
- Ramcharan K, Poon-King TM, Indar R. Spontaneous intraperitoneal rupture of a neurogenic bladder; the importance of ascitic fluid urea and electrolytes in diagnosis. Postgrad Med J 1987; 63:999–1000.
- Matsumura M, Ando N, Kumabe A, Dhaliwal G. Pseudo-renal failure: bladder rupture with urinary ascites. BMJ Case Rep 2015; pii:bcr2015212671.
- Shi F, Wang T, Wang J, et al. Peritoneal bladder fistula following radiotherapy for cervical cancer: a case report. Oncol Lett 2016; 12:2008–2010.
- Hayashi W, Nishino T, Namie S, Obata Y, Furukawa M, Kohno S. Spontaneous bladder rupture diagnosis based on urinary appearance of mesothelial cells: a case report. J Med Case Rep 2014; 8:46.
- Ramcharan K, Poon-King TM, Indar R. Spontaneous intraperitoneal rupture of a neurogenic bladder; the importance of ascitic fluid urea and electrolytes in diagnosis. Postgrad Med J 1987; 63:999–1000.
- Matsumura M, Ando N, Kumabe A, Dhaliwal G. Pseudo-renal failure: bladder rupture with urinary ascites. BMJ Case Rep 2015; pii:bcr2015212671.
- Shi F, Wang T, Wang J, et al. Peritoneal bladder fistula following radiotherapy for cervical cancer: a case report. Oncol Lett 2016; 12:2008–2010.
- Hayashi W, Nishino T, Namie S, Obata Y, Furukawa M, Kohno S. Spontaneous bladder rupture diagnosis based on urinary appearance of mesothelial cells: a case report. J Med Case Rep 2014; 8:46.
Lung scan often not requested for new SSc patients
Only half of American general rheumatologists and two-thirds of global systemic sclerosis experts routinely request high-resolution CT chest scans for all their newly diagnosed systemic sclerosis patients despite their increased risk of interstitial lung disease, according to survey data from approximately 200 clinicians.
The researchers, led by Elana J. Bernstein, MD, of Columbia University, New York, conducted the survey because of a lack of data on how often rheumatologists order high-resolution CT for their newly diagnosed patients and the absence of clinical practice guidelines that recommend screening for interstitial lung disease (ILD) in systemic sclerosis (SSc).
In a study published in Arthritis & Rheumatology, the researchers surveyed 676 American College of Rheumatology members and 356 global experts on systemic sclerosis; of these, 76 ACR general rheumatologists and 135 SSc experts responded. The use of high-resolution CT varied widely by country or region: 0 of 5 respondents from Australia, 2 of 6 from Canada, 28 of 47 from the United States, 45 of 57 from Europe, 4 of 5 from Asia, and 7 of 7 from Latin America.
The researchers also found little consensus on indications for high-resolution CT in SSc patients. Among the SSc experts who do not routinely obtain screening high-resolution CTs in their SSc patients, 81% said they would request one for dyspnea on exertion, 74% would request one for an abnormal forced vital capacity less than 80% of predicted, and 52% would request one for an abnormal diffusion capacity for carbon monoxide less than 80% predicted.
A significant limitation of the study was the low response rate, and more research is needed on the clinical impact of high-resolution CT screening for ILD in SSc patients, the researchers noted. However, the results highlight the need for a clinical practice guideline to create a more consistent approach to identifying ILD in these patients, they said.
The researchers had no financial conflicts to disclose. Dr. Bernstein was supported by a Rheumatology Research Foundation Scientist Development Award, and two of her colleagues were funded in part by the National Institute of Arthritis and Musculoskeletal and Skin Diseases and the National Heart, Lung, and Blood Institute.
SOURCE: Bernstein E et al. Arthritis Rheumatol. 2018 Feb 9. doi: 10.1002/art.40441.
Only half of American general rheumatologists and two-thirds of global systemic sclerosis experts routinely request high-resolution CT chest scans for all their newly diagnosed systemic sclerosis patients despite their increased risk of interstitial lung disease, according to survey data from approximately 200 clinicians.
The researchers, led by Elana J. Bernstein, MD, of Columbia University, New York, conducted the survey because of a lack of data on how often rheumatologists order high-resolution CT for their newly diagnosed patients and the absence of clinical practice guidelines that recommend screening for interstitial lung disease (ILD) in systemic sclerosis (SSc).
In a study published in Arthritis & Rheumatology, the researchers surveyed 676 American College of Rheumatology members and 356 global experts on systemic sclerosis; of these, 76 ACR general rheumatologists and 135 SSc experts responded. The use of high-resolution CT varied widely by country or region: 0 of 5 respondents from Australia, 2 of 6 from Canada, 28 of 47 from the United States, 45 of 57 from Europe, 4 of 5 from Asia, and 7 of 7 from Latin America.
The researchers also found little consensus on indications for high-resolution CT in SSc patients. Among the SSc experts who do not routinely obtain screening high-resolution CTs in their SSc patients, 81% said they would request one for dyspnea on exertion, 74% would request one for an abnormal forced vital capacity less than 80% of predicted, and 52% would request one for an abnormal diffusion capacity for carbon monoxide less than 80% predicted.
A significant limitation of the study was the low response rate, and more research is needed on the clinical impact of high-resolution CT screening for ILD in SSc patients, the researchers noted. However, the results highlight the need for a clinical practice guideline to create a more consistent approach to identifying ILD in these patients, they said.
The researchers had no financial conflicts to disclose. Dr. Bernstein was supported by a Rheumatology Research Foundation Scientist Development Award, and two of her colleagues were funded in part by the National Institute of Arthritis and Musculoskeletal and Skin Diseases and the National Heart, Lung, and Blood Institute.
SOURCE: Bernstein E et al. Arthritis Rheumatol. 2018 Feb 9. doi: 10.1002/art.40441.
Only half of American general rheumatologists and two-thirds of global systemic sclerosis experts routinely request high-resolution CT chest scans for all their newly diagnosed systemic sclerosis patients despite their increased risk of interstitial lung disease, according to survey data from approximately 200 clinicians.
The researchers, led by Elana J. Bernstein, MD, of Columbia University, New York, conducted the survey because of a lack of data on how often rheumatologists order high-resolution CT for their newly diagnosed patients and the absence of clinical practice guidelines that recommend screening for interstitial lung disease (ILD) in systemic sclerosis (SSc).
In a study published in Arthritis & Rheumatology, the researchers surveyed 676 American College of Rheumatology members and 356 global experts on systemic sclerosis; of these, 76 ACR general rheumatologists and 135 SSc experts responded. The use of high-resolution CT varied widely by country or region: 0 of 5 respondents from Australia, 2 of 6 from Canada, 28 of 47 from the United States, 45 of 57 from Europe, 4 of 5 from Asia, and 7 of 7 from Latin America.
The researchers also found little consensus on indications for high-resolution CT in SSc patients. Among the SSc experts who do not routinely obtain screening high-resolution CTs in their SSc patients, 81% said they would request one for dyspnea on exertion, 74% would request one for an abnormal forced vital capacity less than 80% of predicted, and 52% would request one for an abnormal diffusion capacity for carbon monoxide less than 80% predicted.
A significant limitation of the study was the low response rate, and more research is needed on the clinical impact of high-resolution CT screening for ILD in SSc patients, the researchers noted. However, the results highlight the need for a clinical practice guideline to create a more consistent approach to identifying ILD in these patients, they said.
The researchers had no financial conflicts to disclose. Dr. Bernstein was supported by a Rheumatology Research Foundation Scientist Development Award, and two of her colleagues were funded in part by the National Institute of Arthritis and Musculoskeletal and Skin Diseases and the National Heart, Lung, and Blood Institute.
SOURCE: Bernstein E et al. Arthritis Rheumatol. 2018 Feb 9. doi: 10.1002/art.40441.
FROM ARTHRITIS & RHEUMATOLOGY
Key clinical point: Despite the risk of interstitial lung disease in systemic sclerosis patients, the use of high-resolution CT scans of the chest is inconsistent.
Major finding: Overall, 51% of ACR general rheumatologists and 66% of global systemic sclerosis experts ordered high-resolution CTs for new SSc patients.
Study details: The data come from surveys completed by 76 ACR general rheumatologists and 135 SSc experts worldwide.
Disclosures: The researchers had no financial conflicts to disclose. Dr. Bernstein was supported by a Rheumatology Research Foundation Scientist Development Award, and two of her colleagues were funded in part by the National Institute of Arthritis and Musculoskeletal and Skin Diseases and the National Heart, Lung, and Blood Institute.
Source: Bernstein E et al. Arthritis Rheumatol. 2018 Feb 9. doi: 10.1002/art.40441.