User login
When stroke runs in the family
A 54-year-old man presented to our hospital with acute-onset left-sided weakness and right facial droop. Three days earlier he had also had migraine-like headaches, which he had never experienced before. He also reported a change in behavior during the past week, which his family had described as inappropriate laughter.
He had no history of hypertension, diabetes, or dyslipidemia. He did not smoke or drink alcohol. However, he had an extensive family history of stroke. His mother had a stroke at age 50, his brother a stroke at age 57, and his sister had been admitted for a stroke 1 month earlier at the age of 52.
On examination, he had weakness of the left arm and leg, right facial droop, and hyperactive reflexes on the left side. He had no sensory or cerebellar deficits. He had episodes of laughter during the examination.
We learned that the patient’s sister had undergone a workup showing mutations in the NOTCH3 gene and a skin biopsy study consistent with CADASIL.
Our patient was started on antiplatelet and high-intensity statin therapy. His symptoms improved, and he was discharged to an acute inpatient rehabilitation facility. He was referred to a CADASIL registry.
STROKE AND HEREDITY
CADASIL is a rare hereditary vascular disorder inherited in an autosomal dominant manner. It is the most common inherited form of small-vessel disease and results from a mutation in the NOTCH3 gene that leads to degeneration of smooth muscle in cerebral blood vessels. It can manifest as migraine with aura, vascular dementia, cognitive impairment, or ischemic stroke.
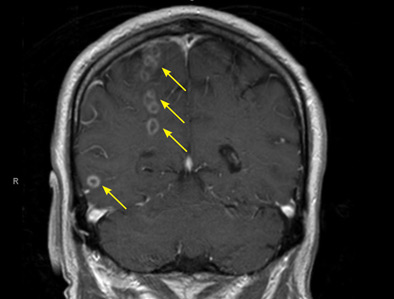
The diagnosis is based on a clinical picture that typically includes stroke at a young age (age 40 to 50) in the absence of stroke risk factors, or frequent lacunar infarction episodes that can manifest as migraine, lacunar infarct, or dementia.1 Some patients, such as ours, may have subtle nonspecific behavioral changes such as inappropriate laughter, which may herald the development of an infarct.
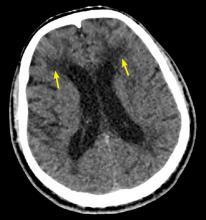
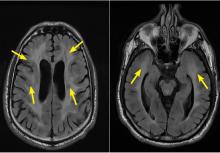
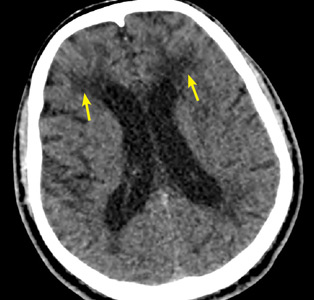
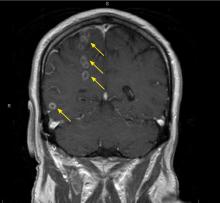
Characteristic findings on MRI are white matter hyperintensities that tend to be bilateral and symmetrical in the periventricular areas. Symmetrical involvement in the temporal lobes has high sensitivity and specificity for CADASIL.2 Biopsy study of the skin, muscle, or sural nerve shows small-vessel changes that include thickening of the media, granular material positive on periodic acid-Schiff staining, and narrowing of the lumen. However, the gold standard for diagnosis is confirmation of the NOTCH3 mutation on chromosome 19.1,2
There is no known treatment for CADASIL.
- Davous P. CADASIL: a review with proposed diagnostic criteria. Eur J Neurol 1998; 5(3):219–233. pmid:10210836
- Stojanov D, Vojinovic S, Aracki-Trenkic A, et al. Imaging characteristics of cerebral autosomal dominant arteriopathy with subcortical infarcts and leucoencephalopathy (CADASIL). Bosn J Basic Med Sci 2015; 15(1):1–8. doi:10.17305/bjbms.2015.247
A 54-year-old man presented to our hospital with acute-onset left-sided weakness and right facial droop. Three days earlier he had also had migraine-like headaches, which he had never experienced before. He also reported a change in behavior during the past week, which his family had described as inappropriate laughter.
He had no history of hypertension, diabetes, or dyslipidemia. He did not smoke or drink alcohol. However, he had an extensive family history of stroke. His mother had a stroke at age 50, his brother a stroke at age 57, and his sister had been admitted for a stroke 1 month earlier at the age of 52.
On examination, he had weakness of the left arm and leg, right facial droop, and hyperactive reflexes on the left side. He had no sensory or cerebellar deficits. He had episodes of laughter during the examination.
We learned that the patient’s sister had undergone a workup showing mutations in the NOTCH3 gene and a skin biopsy study consistent with CADASIL.
Our patient was started on antiplatelet and high-intensity statin therapy. His symptoms improved, and he was discharged to an acute inpatient rehabilitation facility. He was referred to a CADASIL registry.
STROKE AND HEREDITY
CADASIL is a rare hereditary vascular disorder inherited in an autosomal dominant manner. It is the most common inherited form of small-vessel disease and results from a mutation in the NOTCH3 gene that leads to degeneration of smooth muscle in cerebral blood vessels. It can manifest as migraine with aura, vascular dementia, cognitive impairment, or ischemic stroke.
The diagnosis is based on a clinical picture that typically includes stroke at a young age (age 40 to 50) in the absence of stroke risk factors, or frequent lacunar infarction episodes that can manifest as migraine, lacunar infarct, or dementia.1 Some patients, such as ours, may have subtle nonspecific behavioral changes such as inappropriate laughter, which may herald the development of an infarct.
Characteristic findings on MRI are white matter hyperintensities that tend to be bilateral and symmetrical in the periventricular areas. Symmetrical involvement in the temporal lobes has high sensitivity and specificity for CADASIL.2 Biopsy study of the skin, muscle, or sural nerve shows small-vessel changes that include thickening of the media, granular material positive on periodic acid-Schiff staining, and narrowing of the lumen. However, the gold standard for diagnosis is confirmation of the NOTCH3 mutation on chromosome 19.1,2
There is no known treatment for CADASIL.
A 54-year-old man presented to our hospital with acute-onset left-sided weakness and right facial droop. Three days earlier he had also had migraine-like headaches, which he had never experienced before. He also reported a change in behavior during the past week, which his family had described as inappropriate laughter.
He had no history of hypertension, diabetes, or dyslipidemia. He did not smoke or drink alcohol. However, he had an extensive family history of stroke. His mother had a stroke at age 50, his brother a stroke at age 57, and his sister had been admitted for a stroke 1 month earlier at the age of 52.
On examination, he had weakness of the left arm and leg, right facial droop, and hyperactive reflexes on the left side. He had no sensory or cerebellar deficits. He had episodes of laughter during the examination.
We learned that the patient’s sister had undergone a workup showing mutations in the NOTCH3 gene and a skin biopsy study consistent with CADASIL.
Our patient was started on antiplatelet and high-intensity statin therapy. His symptoms improved, and he was discharged to an acute inpatient rehabilitation facility. He was referred to a CADASIL registry.
STROKE AND HEREDITY
CADASIL is a rare hereditary vascular disorder inherited in an autosomal dominant manner. It is the most common inherited form of small-vessel disease and results from a mutation in the NOTCH3 gene that leads to degeneration of smooth muscle in cerebral blood vessels. It can manifest as migraine with aura, vascular dementia, cognitive impairment, or ischemic stroke.
The diagnosis is based on a clinical picture that typically includes stroke at a young age (age 40 to 50) in the absence of stroke risk factors, or frequent lacunar infarction episodes that can manifest as migraine, lacunar infarct, or dementia.1 Some patients, such as ours, may have subtle nonspecific behavioral changes such as inappropriate laughter, which may herald the development of an infarct.
Characteristic findings on MRI are white matter hyperintensities that tend to be bilateral and symmetrical in the periventricular areas. Symmetrical involvement in the temporal lobes has high sensitivity and specificity for CADASIL.2 Biopsy study of the skin, muscle, or sural nerve shows small-vessel changes that include thickening of the media, granular material positive on periodic acid-Schiff staining, and narrowing of the lumen. However, the gold standard for diagnosis is confirmation of the NOTCH3 mutation on chromosome 19.1,2
There is no known treatment for CADASIL.
- Davous P. CADASIL: a review with proposed diagnostic criteria. Eur J Neurol 1998; 5(3):219–233. pmid:10210836
- Stojanov D, Vojinovic S, Aracki-Trenkic A, et al. Imaging characteristics of cerebral autosomal dominant arteriopathy with subcortical infarcts and leucoencephalopathy (CADASIL). Bosn J Basic Med Sci 2015; 15(1):1–8. doi:10.17305/bjbms.2015.247
- Davous P. CADASIL: a review with proposed diagnostic criteria. Eur J Neurol 1998; 5(3):219–233. pmid:10210836
- Stojanov D, Vojinovic S, Aracki-Trenkic A, et al. Imaging characteristics of cerebral autosomal dominant arteriopathy with subcortical infarcts and leucoencephalopathy (CADASIL). Bosn J Basic Med Sci 2015; 15(1):1–8. doi:10.17305/bjbms.2015.247
How long should we follow simple ovarian cysts with pelvic ultrasonography?
A 54-year-old postmenopausal woman presents with a 3-day history of left lower quadrant pain. Abdominal and pelvic computed tomography confirm the diagnosis of acute diverticulitis, and a left ovarian cyst is incidentally noted. Her abdominal discomfort resolves with antibiotics.
Transvaginal ultrasonography confirms the presence of a 4.5-cm simple left ovarian cyst. The radiologist recommends follow-up ultrasonography in 3 months “if clinically indicated.” The patient feels well and is anxious about having additional testing. What do you recommend?
HOW USEFUL IS ULTRASONOGRAPHY FOR OVARIAN CYSTS?
Ovarian cysts are common and may affect up to 20% of women at some time during their life.1 In a prospective study of almost 40,000 women enrolled in an ovarian cancer screening program, the prevalence of ovarian cysts was 15.3% in premenopausal women and 8.2% in postmenopausal women.2
Pelvic ultrasonography is the most effective way to evaluate incidentally noted cysts, and the transvaginal approach is preferred.3 The International Ovarian Tumor Analysis group has outlined morphologic features, referred to as “simple rules,” for predicting if a cyst is malignant or benign.4 In a prospective validation study, these simple rules were applied in 76% of cases, with a sensitivity of 95% and a specificity of 91%.4 However, it should be noted that these rules apply to examinations done by experienced gynecologic ultrasonographers, as accuracy of ultrasonography is both machine- and operator-dependent.
WHAT IS THE MALIGNANCY POTENTIAL OF A SIMPLE OVARIAN CYST?
A simple ovarian cyst is defined as an anechoic round or oval lesion, different from a unilocular cyst, which may contain septations, solid wall irregularities, or internal echoes.5 Overall, simple ovarian cysts have a very low likelihood of malignancy. In the large, multi-site Prostate, Lung, Colorectal, and Ovarian Cancer Screening Trial, simple cysts were observed in 14% of postmenopausal women,6 but no cyst was associated with the development of ovarian cancer over 4 years of follow-up.
HOW OFTEN SHOULD IMAGING BE REPEATED?
In premenopausal women, most simple (thin-walled) ovarian cysts less than 5 cm in maximum diameter resolve in 2 to 3 menstrual cycles and do not require further intervention.3 Larger cysts (5–7 cm in diameter) should be followed with ultrasonography yearly. Cysts larger than 7 cm require advanced imaging or surgical intervention, and the patient should be referred to a gynecologist.3
In postmenopausal women, serum markers are combined with ultrasonography results to determine the risk of malignancy. Markers studied include cancer antigen 125 (CA-125), human epididymis protein 4, lactate dehydrogenase, alpha fetoprotein, and beta human chorionic gonadotropin (beta hCG).7
CA-125, the most studied marker, is elevated in more than 90% of advanced-stage ovarian cancers, but in only 50% of patients wth early-stage cancer.1,8 However, CA-125 may be elevated in a variety of other settings, including benign gynecologic disorders (pelvic infection, fibroids, endometriosis, adenomyosis) and nongynecologic disorders (liver disease, pancreatitis, and diverticulitis). Thus, it is unreliable for distinguishing benign from malignant ovarian masses in premenopausal women.1,3
Current guidelines recommend routine measurement of CA-125 in the initial evaluation of all postmenopausal women with an ovarian mass.7,8
Using a cutoff of 30 IU/mL, CA-125 has a sensitivity of 81% and a specificity of 75% for ovarian malignancy. However, serial measurements may be more useful for assessing ovarian cancer risk, especially in the setting of rapidly rising values.1,3
The Risk for Malignancy Index (RMI), which categorizes a cyst’s risk for malignancy, can be calculated based on the patient’s menopausal status, ultrasonographic characteristics (1 point each for multilocular cyst, solid area, metastasis, ascites, and bilateral lesions), and serum CA-125 level. The RMI has a sensitivity of 78% and a specificity of 87% for predicting ovarian cancer.8
Postmenopausal women with an asymptomatic small cyst (< 5 cm), a normal CA-125 level, and an RMI < 200 can be followed conservatively, with repeat ultrasonography in 4 to 6 months. At that time, if the cyst has not grown and the CA-125 level is normal, expectant management can continue, with reassessment in 4 to 6 months. If imaging remains unchanged and the CA-125 is persistently normal, the patient may be discharged from follow-up.8
If at any time during the evaluation the calculated RMI is greater than 200, there is an increased risk for malignancy, and the patient should be referred to a gynecologic oncologist for advanced imaging.
An algorithm from the Royal College of Obstetricians and Gynaecologists for managing ovarian cysts in postmenopausal women is available at www.rcog.org.uk/globalassets/documents/guidelines/green-top-guidelines/gtg_34.pdf.8
CURRENT GUIDELINES ON REPEAT IMAGING
The American College of Radiology (ACR) has created a “Choosing Wisely” guideline to clarify when repeat imaging for ovarian cysts is indicated, to reduce both patient anxiety and healthcare costs.9 These guidelines highlight the distress women may experience from repeat testing due to concerns about cancer.
The guidelines also note that testing is often done during varying times of the menstrual cycle, thereby detecting new cysts, as opposed to monitoring previously detected cysts. Repeat ultrasonography may lead to surgical interventions that are not evidence-based, such as cystectomy or oophorectomy, in patients without radiologic features of malignancy or associated pelvic pain. And while ultrasonography is less expensive than other imaging tests, unnecessary imaging can mean additional costs to the patient, such as copayments, and possibly large payments for patients without insurance.
The American College of Obstetricians and Gynecologists (ACOG) and the ACR guidelines recommend against unnecessary repeat imaging for ovarian cysts.7,10 The ACOG Practice Bulletin on the Evaluation and Management of Adnexal Masses states, “Simple cysts up to 10 cm in diameter on transvaginal ultrasonography performed by experienced ultrasonographers are likely benign and may be safely monitored using repeat imaging without surgical intervention, even in postmenopausal patients.”7
The ideal frequency for repeat testing is yet to be determined. In postmenopausal women with a simple cyst smaller than 5 cm, ACOG guidelines recommend an interval of 4 to 6 months for initial repeat imaging. ACR guidelines recommend no follow-up imaging for simple cysts smaller than 5 cm detected by high-quality ultrasonography in asymptomatic women of reproductive age or for simple cysts smaller than 1 cm in postmenopausal women.10
THE CLINICAL BOTTOM LINE
Simple ovarian cysts can develop as part of the normal menstrual cycle, and although they are more common in premenopausal women, they have been detected in 1 out of 5 postmenopausal women.9 Simple ovarian cysts are typically not cancerous in women of any age. Therefore, most simple ovarian cysts in asymptomatic women either require no follow-up imaging or can be safely monitored with limited repeat ultrasonography for a defined length of time.
Our 54-year-old postmenopausal patient has a simple cyst smaller than 5 cm. Based on current guidelines, the CA-125 level should be measured, with subsequent calculation of the RMI. Assuming a normal CA-125 and RMI, she should be reassured that the risk of progression to malignancy is extremely low. Repeating ultrasonography 4 to 6 months after the initial imaging is warranted. At that time, if no change in cyst size or composition is detected, ultrasonography can be repeated at 1 year after initial detection. After that, assuming no changes of the cyst on repeat imaging, the patient does not require additional follow-up.
- van Nagell JR Jr, Miiler RW. Evaluation and management of ultrasonographically detected ovarian tumors in asymptomatic women. Obstet Gynecol 2016; 127(5):848–858. doi:10.1097/AOG.0000000000001384
- Pavlik EJ, Ueland FR, Miller RW, et al. Frequency and disposition of ovarian abnormalities followed with serial transvaginal ultrasonography. Obstet Gynecol 2013; 122(2 pt 1):210–217. doi:10.1097/AOG.0b013e318298def5
- Royal College of Obstetricians and Gynaecologists. Management of suspected ovarian masses in premenopausal women. Green-top guideline 2011; 62:1–14. www.rcog.org.uk/globalassets/documents/guidelines/gtg_62.pdf. Accessed August 16, 2018.
- Timmerman D, Testa AC, Bourne T, et al. Simple ultrasound-based rules for the diagnosis of ovarian cancer. Ultrasound Obstet Gynecol 2008; 31(6):681–690. doi:10.1002/uog.5365
- Glanc P, Benacerraf B, Bourne T, et al. First international consensus report on adnexal masses: management recommendations. J Ultrasound Med 2017; 36(5):849–863. doi:10.1002/jum.14197
- Greenlee RT, Kessel B, Williams CR, et al. Prevalence, incidence, and natural history of simple ovarian cysts among women > 55 years old in a large cancer screening trial. Am J Obstet Gynecol 2010; 202(4):373.e1–e9. doi:10.1016/j.ajog.2009.11.029
- American College of Obstetricians and Gynecologists’ Committee on Practice Bulletins—Gynecology. Practice Bulletin No. 174: Evaluation and Management of Adnexal Masses. Obstet Gynecol 2016; 128(5):e210-226. doi:10.1097/AOG.0000000000001768
- Royal College of Obstetricians and Gynaecologists. The management of ovarian cysts in postmenopausal women. Green-top guideline 2016; 34:1–31. www.rcog.org.uk/globalassets/documents/guidelines/green-top-guidelines/gtg_34.pdf. Accessed August 16, 2018.
- American College of Radiology Choosing Wisely. Imaging tests for ovarian cysts. When you need an ultrasound—and when you don’t. www.choosingwisely.org/wp-content/uploads/2012/09/ChoosingWiselyOvarianCystsACR-ER_Update.pdf. Accessed August 16, 2018.
- Levine D, Brown DL, Andreotti RF, et al. Management of asymptomatic ovarian and other adnexal cysts imaged at US: Society of Radiologists in Ultrasound Consensus Conference Statement. Radiology 2010; 256(3):943–954. doi:10.1148/radiol.10100213
A 54-year-old postmenopausal woman presents with a 3-day history of left lower quadrant pain. Abdominal and pelvic computed tomography confirm the diagnosis of acute diverticulitis, and a left ovarian cyst is incidentally noted. Her abdominal discomfort resolves with antibiotics.
Transvaginal ultrasonography confirms the presence of a 4.5-cm simple left ovarian cyst. The radiologist recommends follow-up ultrasonography in 3 months “if clinically indicated.” The patient feels well and is anxious about having additional testing. What do you recommend?
HOW USEFUL IS ULTRASONOGRAPHY FOR OVARIAN CYSTS?
Ovarian cysts are common and may affect up to 20% of women at some time during their life.1 In a prospective study of almost 40,000 women enrolled in an ovarian cancer screening program, the prevalence of ovarian cysts was 15.3% in premenopausal women and 8.2% in postmenopausal women.2
Pelvic ultrasonography is the most effective way to evaluate incidentally noted cysts, and the transvaginal approach is preferred.3 The International Ovarian Tumor Analysis group has outlined morphologic features, referred to as “simple rules,” for predicting if a cyst is malignant or benign.4 In a prospective validation study, these simple rules were applied in 76% of cases, with a sensitivity of 95% and a specificity of 91%.4 However, it should be noted that these rules apply to examinations done by experienced gynecologic ultrasonographers, as accuracy of ultrasonography is both machine- and operator-dependent.
WHAT IS THE MALIGNANCY POTENTIAL OF A SIMPLE OVARIAN CYST?
A simple ovarian cyst is defined as an anechoic round or oval lesion, different from a unilocular cyst, which may contain septations, solid wall irregularities, or internal echoes.5 Overall, simple ovarian cysts have a very low likelihood of malignancy. In the large, multi-site Prostate, Lung, Colorectal, and Ovarian Cancer Screening Trial, simple cysts were observed in 14% of postmenopausal women,6 but no cyst was associated with the development of ovarian cancer over 4 years of follow-up.
HOW OFTEN SHOULD IMAGING BE REPEATED?
In premenopausal women, most simple (thin-walled) ovarian cysts less than 5 cm in maximum diameter resolve in 2 to 3 menstrual cycles and do not require further intervention.3 Larger cysts (5–7 cm in diameter) should be followed with ultrasonography yearly. Cysts larger than 7 cm require advanced imaging or surgical intervention, and the patient should be referred to a gynecologist.3
In postmenopausal women, serum markers are combined with ultrasonography results to determine the risk of malignancy. Markers studied include cancer antigen 125 (CA-125), human epididymis protein 4, lactate dehydrogenase, alpha fetoprotein, and beta human chorionic gonadotropin (beta hCG).7
CA-125, the most studied marker, is elevated in more than 90% of advanced-stage ovarian cancers, but in only 50% of patients wth early-stage cancer.1,8 However, CA-125 may be elevated in a variety of other settings, including benign gynecologic disorders (pelvic infection, fibroids, endometriosis, adenomyosis) and nongynecologic disorders (liver disease, pancreatitis, and diverticulitis). Thus, it is unreliable for distinguishing benign from malignant ovarian masses in premenopausal women.1,3
Current guidelines recommend routine measurement of CA-125 in the initial evaluation of all postmenopausal women with an ovarian mass.7,8
Using a cutoff of 30 IU/mL, CA-125 has a sensitivity of 81% and a specificity of 75% for ovarian malignancy. However, serial measurements may be more useful for assessing ovarian cancer risk, especially in the setting of rapidly rising values.1,3
The Risk for Malignancy Index (RMI), which categorizes a cyst’s risk for malignancy, can be calculated based on the patient’s menopausal status, ultrasonographic characteristics (1 point each for multilocular cyst, solid area, metastasis, ascites, and bilateral lesions), and serum CA-125 level. The RMI has a sensitivity of 78% and a specificity of 87% for predicting ovarian cancer.8
Postmenopausal women with an asymptomatic small cyst (< 5 cm), a normal CA-125 level, and an RMI < 200 can be followed conservatively, with repeat ultrasonography in 4 to 6 months. At that time, if the cyst has not grown and the CA-125 level is normal, expectant management can continue, with reassessment in 4 to 6 months. If imaging remains unchanged and the CA-125 is persistently normal, the patient may be discharged from follow-up.8
If at any time during the evaluation the calculated RMI is greater than 200, there is an increased risk for malignancy, and the patient should be referred to a gynecologic oncologist for advanced imaging.
An algorithm from the Royal College of Obstetricians and Gynaecologists for managing ovarian cysts in postmenopausal women is available at www.rcog.org.uk/globalassets/documents/guidelines/green-top-guidelines/gtg_34.pdf.8
CURRENT GUIDELINES ON REPEAT IMAGING
The American College of Radiology (ACR) has created a “Choosing Wisely” guideline to clarify when repeat imaging for ovarian cysts is indicated, to reduce both patient anxiety and healthcare costs.9 These guidelines highlight the distress women may experience from repeat testing due to concerns about cancer.
The guidelines also note that testing is often done during varying times of the menstrual cycle, thereby detecting new cysts, as opposed to monitoring previously detected cysts. Repeat ultrasonography may lead to surgical interventions that are not evidence-based, such as cystectomy or oophorectomy, in patients without radiologic features of malignancy or associated pelvic pain. And while ultrasonography is less expensive than other imaging tests, unnecessary imaging can mean additional costs to the patient, such as copayments, and possibly large payments for patients without insurance.
The American College of Obstetricians and Gynecologists (ACOG) and the ACR guidelines recommend against unnecessary repeat imaging for ovarian cysts.7,10 The ACOG Practice Bulletin on the Evaluation and Management of Adnexal Masses states, “Simple cysts up to 10 cm in diameter on transvaginal ultrasonography performed by experienced ultrasonographers are likely benign and may be safely monitored using repeat imaging without surgical intervention, even in postmenopausal patients.”7
The ideal frequency for repeat testing is yet to be determined. In postmenopausal women with a simple cyst smaller than 5 cm, ACOG guidelines recommend an interval of 4 to 6 months for initial repeat imaging. ACR guidelines recommend no follow-up imaging for simple cysts smaller than 5 cm detected by high-quality ultrasonography in asymptomatic women of reproductive age or for simple cysts smaller than 1 cm in postmenopausal women.10
THE CLINICAL BOTTOM LINE
Simple ovarian cysts can develop as part of the normal menstrual cycle, and although they are more common in premenopausal women, they have been detected in 1 out of 5 postmenopausal women.9 Simple ovarian cysts are typically not cancerous in women of any age. Therefore, most simple ovarian cysts in asymptomatic women either require no follow-up imaging or can be safely monitored with limited repeat ultrasonography for a defined length of time.
Our 54-year-old postmenopausal patient has a simple cyst smaller than 5 cm. Based on current guidelines, the CA-125 level should be measured, with subsequent calculation of the RMI. Assuming a normal CA-125 and RMI, she should be reassured that the risk of progression to malignancy is extremely low. Repeating ultrasonography 4 to 6 months after the initial imaging is warranted. At that time, if no change in cyst size or composition is detected, ultrasonography can be repeated at 1 year after initial detection. After that, assuming no changes of the cyst on repeat imaging, the patient does not require additional follow-up.
A 54-year-old postmenopausal woman presents with a 3-day history of left lower quadrant pain. Abdominal and pelvic computed tomography confirm the diagnosis of acute diverticulitis, and a left ovarian cyst is incidentally noted. Her abdominal discomfort resolves with antibiotics.
Transvaginal ultrasonography confirms the presence of a 4.5-cm simple left ovarian cyst. The radiologist recommends follow-up ultrasonography in 3 months “if clinically indicated.” The patient feels well and is anxious about having additional testing. What do you recommend?
HOW USEFUL IS ULTRASONOGRAPHY FOR OVARIAN CYSTS?
Ovarian cysts are common and may affect up to 20% of women at some time during their life.1 In a prospective study of almost 40,000 women enrolled in an ovarian cancer screening program, the prevalence of ovarian cysts was 15.3% in premenopausal women and 8.2% in postmenopausal women.2
Pelvic ultrasonography is the most effective way to evaluate incidentally noted cysts, and the transvaginal approach is preferred.3 The International Ovarian Tumor Analysis group has outlined morphologic features, referred to as “simple rules,” for predicting if a cyst is malignant or benign.4 In a prospective validation study, these simple rules were applied in 76% of cases, with a sensitivity of 95% and a specificity of 91%.4 However, it should be noted that these rules apply to examinations done by experienced gynecologic ultrasonographers, as accuracy of ultrasonography is both machine- and operator-dependent.
WHAT IS THE MALIGNANCY POTENTIAL OF A SIMPLE OVARIAN CYST?
A simple ovarian cyst is defined as an anechoic round or oval lesion, different from a unilocular cyst, which may contain septations, solid wall irregularities, or internal echoes.5 Overall, simple ovarian cysts have a very low likelihood of malignancy. In the large, multi-site Prostate, Lung, Colorectal, and Ovarian Cancer Screening Trial, simple cysts were observed in 14% of postmenopausal women,6 but no cyst was associated with the development of ovarian cancer over 4 years of follow-up.
HOW OFTEN SHOULD IMAGING BE REPEATED?
In premenopausal women, most simple (thin-walled) ovarian cysts less than 5 cm in maximum diameter resolve in 2 to 3 menstrual cycles and do not require further intervention.3 Larger cysts (5–7 cm in diameter) should be followed with ultrasonography yearly. Cysts larger than 7 cm require advanced imaging or surgical intervention, and the patient should be referred to a gynecologist.3
In postmenopausal women, serum markers are combined with ultrasonography results to determine the risk of malignancy. Markers studied include cancer antigen 125 (CA-125), human epididymis protein 4, lactate dehydrogenase, alpha fetoprotein, and beta human chorionic gonadotropin (beta hCG).7
CA-125, the most studied marker, is elevated in more than 90% of advanced-stage ovarian cancers, but in only 50% of patients wth early-stage cancer.1,8 However, CA-125 may be elevated in a variety of other settings, including benign gynecologic disorders (pelvic infection, fibroids, endometriosis, adenomyosis) and nongynecologic disorders (liver disease, pancreatitis, and diverticulitis). Thus, it is unreliable for distinguishing benign from malignant ovarian masses in premenopausal women.1,3
Current guidelines recommend routine measurement of CA-125 in the initial evaluation of all postmenopausal women with an ovarian mass.7,8
Using a cutoff of 30 IU/mL, CA-125 has a sensitivity of 81% and a specificity of 75% for ovarian malignancy. However, serial measurements may be more useful for assessing ovarian cancer risk, especially in the setting of rapidly rising values.1,3
The Risk for Malignancy Index (RMI), which categorizes a cyst’s risk for malignancy, can be calculated based on the patient’s menopausal status, ultrasonographic characteristics (1 point each for multilocular cyst, solid area, metastasis, ascites, and bilateral lesions), and serum CA-125 level. The RMI has a sensitivity of 78% and a specificity of 87% for predicting ovarian cancer.8
Postmenopausal women with an asymptomatic small cyst (< 5 cm), a normal CA-125 level, and an RMI < 200 can be followed conservatively, with repeat ultrasonography in 4 to 6 months. At that time, if the cyst has not grown and the CA-125 level is normal, expectant management can continue, with reassessment in 4 to 6 months. If imaging remains unchanged and the CA-125 is persistently normal, the patient may be discharged from follow-up.8
If at any time during the evaluation the calculated RMI is greater than 200, there is an increased risk for malignancy, and the patient should be referred to a gynecologic oncologist for advanced imaging.
An algorithm from the Royal College of Obstetricians and Gynaecologists for managing ovarian cysts in postmenopausal women is available at www.rcog.org.uk/globalassets/documents/guidelines/green-top-guidelines/gtg_34.pdf.8
CURRENT GUIDELINES ON REPEAT IMAGING
The American College of Radiology (ACR) has created a “Choosing Wisely” guideline to clarify when repeat imaging for ovarian cysts is indicated, to reduce both patient anxiety and healthcare costs.9 These guidelines highlight the distress women may experience from repeat testing due to concerns about cancer.
The guidelines also note that testing is often done during varying times of the menstrual cycle, thereby detecting new cysts, as opposed to monitoring previously detected cysts. Repeat ultrasonography may lead to surgical interventions that are not evidence-based, such as cystectomy or oophorectomy, in patients without radiologic features of malignancy or associated pelvic pain. And while ultrasonography is less expensive than other imaging tests, unnecessary imaging can mean additional costs to the patient, such as copayments, and possibly large payments for patients without insurance.
The American College of Obstetricians and Gynecologists (ACOG) and the ACR guidelines recommend against unnecessary repeat imaging for ovarian cysts.7,10 The ACOG Practice Bulletin on the Evaluation and Management of Adnexal Masses states, “Simple cysts up to 10 cm in diameter on transvaginal ultrasonography performed by experienced ultrasonographers are likely benign and may be safely monitored using repeat imaging without surgical intervention, even in postmenopausal patients.”7
The ideal frequency for repeat testing is yet to be determined. In postmenopausal women with a simple cyst smaller than 5 cm, ACOG guidelines recommend an interval of 4 to 6 months for initial repeat imaging. ACR guidelines recommend no follow-up imaging for simple cysts smaller than 5 cm detected by high-quality ultrasonography in asymptomatic women of reproductive age or for simple cysts smaller than 1 cm in postmenopausal women.10
THE CLINICAL BOTTOM LINE
Simple ovarian cysts can develop as part of the normal menstrual cycle, and although they are more common in premenopausal women, they have been detected in 1 out of 5 postmenopausal women.9 Simple ovarian cysts are typically not cancerous in women of any age. Therefore, most simple ovarian cysts in asymptomatic women either require no follow-up imaging or can be safely monitored with limited repeat ultrasonography for a defined length of time.
Our 54-year-old postmenopausal patient has a simple cyst smaller than 5 cm. Based on current guidelines, the CA-125 level should be measured, with subsequent calculation of the RMI. Assuming a normal CA-125 and RMI, she should be reassured that the risk of progression to malignancy is extremely low. Repeating ultrasonography 4 to 6 months after the initial imaging is warranted. At that time, if no change in cyst size or composition is detected, ultrasonography can be repeated at 1 year after initial detection. After that, assuming no changes of the cyst on repeat imaging, the patient does not require additional follow-up.
- van Nagell JR Jr, Miiler RW. Evaluation and management of ultrasonographically detected ovarian tumors in asymptomatic women. Obstet Gynecol 2016; 127(5):848–858. doi:10.1097/AOG.0000000000001384
- Pavlik EJ, Ueland FR, Miller RW, et al. Frequency and disposition of ovarian abnormalities followed with serial transvaginal ultrasonography. Obstet Gynecol 2013; 122(2 pt 1):210–217. doi:10.1097/AOG.0b013e318298def5
- Royal College of Obstetricians and Gynaecologists. Management of suspected ovarian masses in premenopausal women. Green-top guideline 2011; 62:1–14. www.rcog.org.uk/globalassets/documents/guidelines/gtg_62.pdf. Accessed August 16, 2018.
- Timmerman D, Testa AC, Bourne T, et al. Simple ultrasound-based rules for the diagnosis of ovarian cancer. Ultrasound Obstet Gynecol 2008; 31(6):681–690. doi:10.1002/uog.5365
- Glanc P, Benacerraf B, Bourne T, et al. First international consensus report on adnexal masses: management recommendations. J Ultrasound Med 2017; 36(5):849–863. doi:10.1002/jum.14197
- Greenlee RT, Kessel B, Williams CR, et al. Prevalence, incidence, and natural history of simple ovarian cysts among women > 55 years old in a large cancer screening trial. Am J Obstet Gynecol 2010; 202(4):373.e1–e9. doi:10.1016/j.ajog.2009.11.029
- American College of Obstetricians and Gynecologists’ Committee on Practice Bulletins—Gynecology. Practice Bulletin No. 174: Evaluation and Management of Adnexal Masses. Obstet Gynecol 2016; 128(5):e210-226. doi:10.1097/AOG.0000000000001768
- Royal College of Obstetricians and Gynaecologists. The management of ovarian cysts in postmenopausal women. Green-top guideline 2016; 34:1–31. www.rcog.org.uk/globalassets/documents/guidelines/green-top-guidelines/gtg_34.pdf. Accessed August 16, 2018.
- American College of Radiology Choosing Wisely. Imaging tests for ovarian cysts. When you need an ultrasound—and when you don’t. www.choosingwisely.org/wp-content/uploads/2012/09/ChoosingWiselyOvarianCystsACR-ER_Update.pdf. Accessed August 16, 2018.
- Levine D, Brown DL, Andreotti RF, et al. Management of asymptomatic ovarian and other adnexal cysts imaged at US: Society of Radiologists in Ultrasound Consensus Conference Statement. Radiology 2010; 256(3):943–954. doi:10.1148/radiol.10100213
- van Nagell JR Jr, Miiler RW. Evaluation and management of ultrasonographically detected ovarian tumors in asymptomatic women. Obstet Gynecol 2016; 127(5):848–858. doi:10.1097/AOG.0000000000001384
- Pavlik EJ, Ueland FR, Miller RW, et al. Frequency and disposition of ovarian abnormalities followed with serial transvaginal ultrasonography. Obstet Gynecol 2013; 122(2 pt 1):210–217. doi:10.1097/AOG.0b013e318298def5
- Royal College of Obstetricians and Gynaecologists. Management of suspected ovarian masses in premenopausal women. Green-top guideline 2011; 62:1–14. www.rcog.org.uk/globalassets/documents/guidelines/gtg_62.pdf. Accessed August 16, 2018.
- Timmerman D, Testa AC, Bourne T, et al. Simple ultrasound-based rules for the diagnosis of ovarian cancer. Ultrasound Obstet Gynecol 2008; 31(6):681–690. doi:10.1002/uog.5365
- Glanc P, Benacerraf B, Bourne T, et al. First international consensus report on adnexal masses: management recommendations. J Ultrasound Med 2017; 36(5):849–863. doi:10.1002/jum.14197
- Greenlee RT, Kessel B, Williams CR, et al. Prevalence, incidence, and natural history of simple ovarian cysts among women > 55 years old in a large cancer screening trial. Am J Obstet Gynecol 2010; 202(4):373.e1–e9. doi:10.1016/j.ajog.2009.11.029
- American College of Obstetricians and Gynecologists’ Committee on Practice Bulletins—Gynecology. Practice Bulletin No. 174: Evaluation and Management of Adnexal Masses. Obstet Gynecol 2016; 128(5):e210-226. doi:10.1097/AOG.0000000000001768
- Royal College of Obstetricians and Gynaecologists. The management of ovarian cysts in postmenopausal women. Green-top guideline 2016; 34:1–31. www.rcog.org.uk/globalassets/documents/guidelines/green-top-guidelines/gtg_34.pdf. Accessed August 16, 2018.
- American College of Radiology Choosing Wisely. Imaging tests for ovarian cysts. When you need an ultrasound—and when you don’t. www.choosingwisely.org/wp-content/uploads/2012/09/ChoosingWiselyOvarianCystsACR-ER_Update.pdf. Accessed August 16, 2018.
- Levine D, Brown DL, Andreotti RF, et al. Management of asymptomatic ovarian and other adnexal cysts imaged at US: Society of Radiologists in Ultrasound Consensus Conference Statement. Radiology 2010; 256(3):943–954. doi:10.1148/radiol.10100213
Coronary CT FFR sharpens patient assessment in two studies
MUNICH – Noninvasive assessment of fractional flow reserve (FFR) within coronary arteries using data collected by CT angiography again has been shown to provide important additional diagnostic information that better guides patient management.
“The value of FFRCT is to reduce the number of patients who go to the cath lab. For patients with a stenosis of 60% that is not likely to have functional significance we can avoid catheterization and treat the patient medically. FFRCT is a valuable technology, but my concern is that currently it costs about $1,400 for this test,” commented Todd C. Villines, MD, a cardiologist at Georgetown University in Washington who was a discussant for the study. “Given the cost, we need to better define the patients on whom we use FFRCT and integrate it into clinical decision making,” Dr. Villines said in an interview.
Perhaps the best demonstration of the potential role for FFRCT came from a single-center study at Aarhus (Denmark) University with 3,674 patients with stable chest pain who underwent CCTA as their initial assessment for suspected coronary artery disease between May 2014 and December 2016. More than two-thirds of these patients had coronary stenoses of less than 30% and had no further assessment or treatment, and 11% had at least one coronary stenosis of at least 70% on CCTA and then had follow-up testing by either conventional angiography or myocardial perfusion imaging. The report at the congress focused on the 697 patients with an inconclusive result based on CCTA alone and at least one stenosis of 30%-69% who underwent FFRCT analysis, and focused specifically on 677 patients with a useful FFRCT result.
Of these patients, 410 (61% of this subgroup) had no coronary lesion that created a FFRCT of 0.8 or less. All received treatment with optimal medical therapy only, and after a median follow-up had a 3.9% incidence of the primary endpoint, the combined rate of all-cause death, nonfatal MI, hospitalization for unstable angina, or unplanned revascularization. This 3.9% rate was not significantly different from the 2.8% rate seen during follow-up of the patients with no coronary stenosis of 30% or greater.
The remaining 267 patients (39% of the subgroup) with a FFRCT that showed 80% or less flow reserve either received optimal medical therapy (112 patients, 42% of this group) or angiography by coronary catheterization (155 patients, 58% of this group).
The second report used data collected from 5,083 patients entered into a multinational registry, ADVANCE, with symptoms suggestive of coronary artery disease and results from CCTA that suggested coronary stenosis. The collaborating researchers then used the CCTA results to generate a FFR analysis for 4,893 (96%) of the patients, and the analysis was usable for 4,737 of them. The FFRCT results led to reclassification of the management strategy for 67% of the patients, the primary endpoint for this analysis, reported Timothy A. Fairbairn, MD, a cardiologist at the Liverpool (England) Heart and Chest Hospital.
One limitation of this study was the relatively brief, 90-day follow-up, but it is the first real-world, multicenter assessment of the utility and safety of FFRCT.
These findings highlight what a “disruptive technology” FFRCT represents, commented Dr. Villines. He also noted that the reclassifications triggered by the FFRCT analysis led to fewer patients undergoing invasive angiography, a good outcome from a cost-effectiveness perspective.
Concurrently with Dr. Fairbairn’s report the results from ADVANCE also appeared in an article published online (Euro Heart J. 2018 Aug 25. doi: 10.1093/eurheartj/ehy530).
A third FFRCT study reported at the session, the Computed Tomographic Evaluation of Atherosclerotic Determinants of Myocardial Ischemia (CREDENCE) study, enrolled 612 patients with suspected coronary artery disease who had been referred for and underwent invasive coronary angiography with FFR evaluation at 13 international centers, including several in the United States. All 612 patients also had assessment by CCTA and FFRCT, and also some type of functional myocardial perfusion assessment using positron emission tomography, single-photon emission CT, or coronary MR.
The Aarhus University study received no commercial funding. Dr. Nørgaard has received research funding from Edwards; Siemens; and HeartFlow, the company that markets FFR analysis for coronary CT angiography data. The ADVANCE registry was sponsored by HeartFlow. Dr. Fairbairn has been a speaker for Heartflow. Dr. Stuijfzand and Dr. Villines had no relevant disclosures.
MUNICH – Noninvasive assessment of fractional flow reserve (FFR) within coronary arteries using data collected by CT angiography again has been shown to provide important additional diagnostic information that better guides patient management.
“The value of FFRCT is to reduce the number of patients who go to the cath lab. For patients with a stenosis of 60% that is not likely to have functional significance we can avoid catheterization and treat the patient medically. FFRCT is a valuable technology, but my concern is that currently it costs about $1,400 for this test,” commented Todd C. Villines, MD, a cardiologist at Georgetown University in Washington who was a discussant for the study. “Given the cost, we need to better define the patients on whom we use FFRCT and integrate it into clinical decision making,” Dr. Villines said in an interview.
Perhaps the best demonstration of the potential role for FFRCT came from a single-center study at Aarhus (Denmark) University with 3,674 patients with stable chest pain who underwent CCTA as their initial assessment for suspected coronary artery disease between May 2014 and December 2016. More than two-thirds of these patients had coronary stenoses of less than 30% and had no further assessment or treatment, and 11% had at least one coronary stenosis of at least 70% on CCTA and then had follow-up testing by either conventional angiography or myocardial perfusion imaging. The report at the congress focused on the 697 patients with an inconclusive result based on CCTA alone and at least one stenosis of 30%-69% who underwent FFRCT analysis, and focused specifically on 677 patients with a useful FFRCT result.
Of these patients, 410 (61% of this subgroup) had no coronary lesion that created a FFRCT of 0.8 or less. All received treatment with optimal medical therapy only, and after a median follow-up had a 3.9% incidence of the primary endpoint, the combined rate of all-cause death, nonfatal MI, hospitalization for unstable angina, or unplanned revascularization. This 3.9% rate was not significantly different from the 2.8% rate seen during follow-up of the patients with no coronary stenosis of 30% or greater.
The remaining 267 patients (39% of the subgroup) with a FFRCT that showed 80% or less flow reserve either received optimal medical therapy (112 patients, 42% of this group) or angiography by coronary catheterization (155 patients, 58% of this group).
The second report used data collected from 5,083 patients entered into a multinational registry, ADVANCE, with symptoms suggestive of coronary artery disease and results from CCTA that suggested coronary stenosis. The collaborating researchers then used the CCTA results to generate a FFR analysis for 4,893 (96%) of the patients, and the analysis was usable for 4,737 of them. The FFRCT results led to reclassification of the management strategy for 67% of the patients, the primary endpoint for this analysis, reported Timothy A. Fairbairn, MD, a cardiologist at the Liverpool (England) Heart and Chest Hospital.
One limitation of this study was the relatively brief, 90-day follow-up, but it is the first real-world, multicenter assessment of the utility and safety of FFRCT.
These findings highlight what a “disruptive technology” FFRCT represents, commented Dr. Villines. He also noted that the reclassifications triggered by the FFRCT analysis led to fewer patients undergoing invasive angiography, a good outcome from a cost-effectiveness perspective.
Concurrently with Dr. Fairbairn’s report the results from ADVANCE also appeared in an article published online (Euro Heart J. 2018 Aug 25. doi: 10.1093/eurheartj/ehy530).
A third FFRCT study reported at the session, the Computed Tomographic Evaluation of Atherosclerotic Determinants of Myocardial Ischemia (CREDENCE) study, enrolled 612 patients with suspected coronary artery disease who had been referred for and underwent invasive coronary angiography with FFR evaluation at 13 international centers, including several in the United States. All 612 patients also had assessment by CCTA and FFRCT, and also some type of functional myocardial perfusion assessment using positron emission tomography, single-photon emission CT, or coronary MR.
The Aarhus University study received no commercial funding. Dr. Nørgaard has received research funding from Edwards; Siemens; and HeartFlow, the company that markets FFR analysis for coronary CT angiography data. The ADVANCE registry was sponsored by HeartFlow. Dr. Fairbairn has been a speaker for Heartflow. Dr. Stuijfzand and Dr. Villines had no relevant disclosures.
MUNICH – Noninvasive assessment of fractional flow reserve (FFR) within coronary arteries using data collected by CT angiography again has been shown to provide important additional diagnostic information that better guides patient management.
“The value of FFRCT is to reduce the number of patients who go to the cath lab. For patients with a stenosis of 60% that is not likely to have functional significance we can avoid catheterization and treat the patient medically. FFRCT is a valuable technology, but my concern is that currently it costs about $1,400 for this test,” commented Todd C. Villines, MD, a cardiologist at Georgetown University in Washington who was a discussant for the study. “Given the cost, we need to better define the patients on whom we use FFRCT and integrate it into clinical decision making,” Dr. Villines said in an interview.
Perhaps the best demonstration of the potential role for FFRCT came from a single-center study at Aarhus (Denmark) University with 3,674 patients with stable chest pain who underwent CCTA as their initial assessment for suspected coronary artery disease between May 2014 and December 2016. More than two-thirds of these patients had coronary stenoses of less than 30% and had no further assessment or treatment, and 11% had at least one coronary stenosis of at least 70% on CCTA and then had follow-up testing by either conventional angiography or myocardial perfusion imaging. The report at the congress focused on the 697 patients with an inconclusive result based on CCTA alone and at least one stenosis of 30%-69% who underwent FFRCT analysis, and focused specifically on 677 patients with a useful FFRCT result.
Of these patients, 410 (61% of this subgroup) had no coronary lesion that created a FFRCT of 0.8 or less. All received treatment with optimal medical therapy only, and after a median follow-up had a 3.9% incidence of the primary endpoint, the combined rate of all-cause death, nonfatal MI, hospitalization for unstable angina, or unplanned revascularization. This 3.9% rate was not significantly different from the 2.8% rate seen during follow-up of the patients with no coronary stenosis of 30% or greater.
The remaining 267 patients (39% of the subgroup) with a FFRCT that showed 80% or less flow reserve either received optimal medical therapy (112 patients, 42% of this group) or angiography by coronary catheterization (155 patients, 58% of this group).
The second report used data collected from 5,083 patients entered into a multinational registry, ADVANCE, with symptoms suggestive of coronary artery disease and results from CCTA that suggested coronary stenosis. The collaborating researchers then used the CCTA results to generate a FFR analysis for 4,893 (96%) of the patients, and the analysis was usable for 4,737 of them. The FFRCT results led to reclassification of the management strategy for 67% of the patients, the primary endpoint for this analysis, reported Timothy A. Fairbairn, MD, a cardiologist at the Liverpool (England) Heart and Chest Hospital.
One limitation of this study was the relatively brief, 90-day follow-up, but it is the first real-world, multicenter assessment of the utility and safety of FFRCT.
These findings highlight what a “disruptive technology” FFRCT represents, commented Dr. Villines. He also noted that the reclassifications triggered by the FFRCT analysis led to fewer patients undergoing invasive angiography, a good outcome from a cost-effectiveness perspective.
Concurrently with Dr. Fairbairn’s report the results from ADVANCE also appeared in an article published online (Euro Heart J. 2018 Aug 25. doi: 10.1093/eurheartj/ehy530).
A third FFRCT study reported at the session, the Computed Tomographic Evaluation of Atherosclerotic Determinants of Myocardial Ischemia (CREDENCE) study, enrolled 612 patients with suspected coronary artery disease who had been referred for and underwent invasive coronary angiography with FFR evaluation at 13 international centers, including several in the United States. All 612 patients also had assessment by CCTA and FFRCT, and also some type of functional myocardial perfusion assessment using positron emission tomography, single-photon emission CT, or coronary MR.
The Aarhus University study received no commercial funding. Dr. Nørgaard has received research funding from Edwards; Siemens; and HeartFlow, the company that markets FFR analysis for coronary CT angiography data. The ADVANCE registry was sponsored by HeartFlow. Dr. Fairbairn has been a speaker for Heartflow. Dr. Stuijfzand and Dr. Villines had no relevant disclosures.
REPORTING FROM THE ESC CONGRESS 2018
Coronary CT angiography radiation dose fell 78% from 2007-2017
MUNICH – The median radiation dosage received by patients worldwide undergoing coronary CT angiography fell by 78% from 2007 to 2017, according to a prospective study with more than 4,500 patients.
This substantial drop in radiation occurred with a steady rate of nondiagnostic CT scans, less than 2% in both 2007 and 2017.
“Given the high diagnostic accuracy and the low radiation dose, coronary CT angiography should be considered as a first-line diagnostic test,” Jörg Hausleiter, MD, said at the annual congress of the European Society of Cardiology.
The results also showed a huge disparity in the range of radiation doses used worldwide, with a 37-fold intersite variability in the median dose. This finding “underlines the need for further site-specific training and adaptation of contemporary cardiac scan protocols,” said Dr. Hausleiter, professor of medicine at the University of Munich Clinic. He suggested updated imaging guidelines on radiation levels, more educational sessions on how to perform coronary CT angiography, and actions by vendors to adjust their standard imaging protocols.
The Prospective Multicenter Registry on Radiation Dose Estimates of Cardiac CT Angiography in Daily Practice in 2017 (PROTECTION-VI) study included 4,502 patients from a total of 61 sites in 32 countries. At each participating site, investigators enrolled consecutive adults during a randomly selected month in 2017, with a median of 51 patients enrolled at each site undergoing diagnostic coronary CT angiography. Comparison data for 2007 came from a similar study run by Dr. Hausleiter and his associates at that time, with 1,965 patients undergoing coronary CT angiography (JAMA. 2009 Feb 4;301[5]:500-7). In 2007, the median dose-length product of radiation for each scan was 885 mGy x cm, which corresponds to a radiation dose of about 12.4 mSv. In 2017, the median dose-length product was 195 mGy x cm, corresponding to a dose of about 2.7 mSv. By both measures the median dose dropped by roughly 78%.
A multivariate analysis identified three changes in the way clinicians obtained most of the CT scans during the two studied time periods that seemed to explain the drop in radiation dose. First, more scan protocols in 2017 used low tube potential; second, more protocols in 2017 used prospectively ECG-triggered axial high-pitch scans; and third, 2017 had increased use of iterative image reconstruction, Dr. Hausleiter said. Patient variables that had modest but significant links with increased radiation doses were higher body weight, higher heart rate, and no sinus rhythm.
Concurrently with Dr. Hausleiter’s talk at the congress, the results appeared in an article online (Euro Heart J. 2018 Aug 25. doi: 10.1093/eurheartj/ehy546).
The results from the PROTECTION VI study show that the radiation doses used today for coronary CT angiography are very low. But the study is limited by looking only at the median doses used at 61 sites worldwide. I hope that the dose level seen in the study is what is now used at community hospitals across the United States, but for the time being we can’t be sure.
With today’s CT technology, as long as the dose-length product a patient receives is less than 200 mGy x cm, the facility is doing a good job of minimizing radiation exposure. As CT technology continues to improve, we can expect the median dose to fall even more in the future.
Todd C. Villines, MD , a cardiologist at Georgetown University in Washington and immediate past president of the Society of Cardiovascular CT, made these comments in an interview. He had no relevant disclosures.
The results from the PROTECTION VI study show that the radiation doses used today for coronary CT angiography are very low. But the study is limited by looking only at the median doses used at 61 sites worldwide. I hope that the dose level seen in the study is what is now used at community hospitals across the United States, but for the time being we can’t be sure.
With today’s CT technology, as long as the dose-length product a patient receives is less than 200 mGy x cm, the facility is doing a good job of minimizing radiation exposure. As CT technology continues to improve, we can expect the median dose to fall even more in the future.
Todd C. Villines, MD , a cardiologist at Georgetown University in Washington and immediate past president of the Society of Cardiovascular CT, made these comments in an interview. He had no relevant disclosures.
The results from the PROTECTION VI study show that the radiation doses used today for coronary CT angiography are very low. But the study is limited by looking only at the median doses used at 61 sites worldwide. I hope that the dose level seen in the study is what is now used at community hospitals across the United States, but for the time being we can’t be sure.
With today’s CT technology, as long as the dose-length product a patient receives is less than 200 mGy x cm, the facility is doing a good job of minimizing radiation exposure. As CT technology continues to improve, we can expect the median dose to fall even more in the future.
Todd C. Villines, MD , a cardiologist at Georgetown University in Washington and immediate past president of the Society of Cardiovascular CT, made these comments in an interview. He had no relevant disclosures.
MUNICH – The median radiation dosage received by patients worldwide undergoing coronary CT angiography fell by 78% from 2007 to 2017, according to a prospective study with more than 4,500 patients.
This substantial drop in radiation occurred with a steady rate of nondiagnostic CT scans, less than 2% in both 2007 and 2017.
“Given the high diagnostic accuracy and the low radiation dose, coronary CT angiography should be considered as a first-line diagnostic test,” Jörg Hausleiter, MD, said at the annual congress of the European Society of Cardiology.
The results also showed a huge disparity in the range of radiation doses used worldwide, with a 37-fold intersite variability in the median dose. This finding “underlines the need for further site-specific training and adaptation of contemporary cardiac scan protocols,” said Dr. Hausleiter, professor of medicine at the University of Munich Clinic. He suggested updated imaging guidelines on radiation levels, more educational sessions on how to perform coronary CT angiography, and actions by vendors to adjust their standard imaging protocols.
The Prospective Multicenter Registry on Radiation Dose Estimates of Cardiac CT Angiography in Daily Practice in 2017 (PROTECTION-VI) study included 4,502 patients from a total of 61 sites in 32 countries. At each participating site, investigators enrolled consecutive adults during a randomly selected month in 2017, with a median of 51 patients enrolled at each site undergoing diagnostic coronary CT angiography. Comparison data for 2007 came from a similar study run by Dr. Hausleiter and his associates at that time, with 1,965 patients undergoing coronary CT angiography (JAMA. 2009 Feb 4;301[5]:500-7). In 2007, the median dose-length product of radiation for each scan was 885 mGy x cm, which corresponds to a radiation dose of about 12.4 mSv. In 2017, the median dose-length product was 195 mGy x cm, corresponding to a dose of about 2.7 mSv. By both measures the median dose dropped by roughly 78%.
A multivariate analysis identified three changes in the way clinicians obtained most of the CT scans during the two studied time periods that seemed to explain the drop in radiation dose. First, more scan protocols in 2017 used low tube potential; second, more protocols in 2017 used prospectively ECG-triggered axial high-pitch scans; and third, 2017 had increased use of iterative image reconstruction, Dr. Hausleiter said. Patient variables that had modest but significant links with increased radiation doses were higher body weight, higher heart rate, and no sinus rhythm.
Concurrently with Dr. Hausleiter’s talk at the congress, the results appeared in an article online (Euro Heart J. 2018 Aug 25. doi: 10.1093/eurheartj/ehy546).
MUNICH – The median radiation dosage received by patients worldwide undergoing coronary CT angiography fell by 78% from 2007 to 2017, according to a prospective study with more than 4,500 patients.
This substantial drop in radiation occurred with a steady rate of nondiagnostic CT scans, less than 2% in both 2007 and 2017.
“Given the high diagnostic accuracy and the low radiation dose, coronary CT angiography should be considered as a first-line diagnostic test,” Jörg Hausleiter, MD, said at the annual congress of the European Society of Cardiology.
The results also showed a huge disparity in the range of radiation doses used worldwide, with a 37-fold intersite variability in the median dose. This finding “underlines the need for further site-specific training and adaptation of contemporary cardiac scan protocols,” said Dr. Hausleiter, professor of medicine at the University of Munich Clinic. He suggested updated imaging guidelines on radiation levels, more educational sessions on how to perform coronary CT angiography, and actions by vendors to adjust their standard imaging protocols.
The Prospective Multicenter Registry on Radiation Dose Estimates of Cardiac CT Angiography in Daily Practice in 2017 (PROTECTION-VI) study included 4,502 patients from a total of 61 sites in 32 countries. At each participating site, investigators enrolled consecutive adults during a randomly selected month in 2017, with a median of 51 patients enrolled at each site undergoing diagnostic coronary CT angiography. Comparison data for 2007 came from a similar study run by Dr. Hausleiter and his associates at that time, with 1,965 patients undergoing coronary CT angiography (JAMA. 2009 Feb 4;301[5]:500-7). In 2007, the median dose-length product of radiation for each scan was 885 mGy x cm, which corresponds to a radiation dose of about 12.4 mSv. In 2017, the median dose-length product was 195 mGy x cm, corresponding to a dose of about 2.7 mSv. By both measures the median dose dropped by roughly 78%.
A multivariate analysis identified three changes in the way clinicians obtained most of the CT scans during the two studied time periods that seemed to explain the drop in radiation dose. First, more scan protocols in 2017 used low tube potential; second, more protocols in 2017 used prospectively ECG-triggered axial high-pitch scans; and third, 2017 had increased use of iterative image reconstruction, Dr. Hausleiter said. Patient variables that had modest but significant links with increased radiation doses were higher body weight, higher heart rate, and no sinus rhythm.
Concurrently with Dr. Hausleiter’s talk at the congress, the results appeared in an article online (Euro Heart J. 2018 Aug 25. doi: 10.1093/eurheartj/ehy546).
REPORTING FROM THE ESC CONGRESS 2018
Key clinical point: The median radiation dose during coronary CT angiography fell from 2007 to 2017.
Major finding: The median dose-length product was 195 mGY x cm in 2017 and 885 mGy x cm in 2007.
Study details: PROTECTION VI, a prospective study run at 61 sites in 32 countries.
Disclosures: PROTECTION VI received no commercial funding. Dr. Hausleiter has received research funding from Abbott Vascular.
A Three-View Radiographic Approach to Femoroacetabular Impingement
ABSTRACT
Femoroacetabular impingement (FAI) is an abnormality of the hip joint that is increasingly being recognized as a cause of athletic disability and early degenerative hip disease. Despite significant advances in the knowledge of FAI, it remains a frequently unrecognized cause of hip pain in adolescents and young adults among orthopedic providers. The purpose of this article is to present a simple 3-view radiographic approach to young adults with hip pain. The radiographs include a standing anteroposterior view of the pelvis, a cross-table lateral view, and a false profile view. Good quality radiographs showing the common sites of potential impingement combined with a basic understanding of certain radiographic parameters may allow faster diagnosis, eliminate unnecessary studies, and allow earlier referral and management.
Continue to: The prevalence of femoroacetabular impingement...
The prevalence of femoroacetabular impingement (FAI) in the general population is estimated at 23.1%.1 While FAI is often bilateral,2 patients usually present with unilateral symptoms.3 Young, highly active individuals are most commonly affected.3 Despite significant improvement in our understanding of FAI in recent years, it remains a poorly recognized cause of hip pain among orthopedic providers. Clohisy and colleagues3 found that the average time to diagnosis was 3.1 years (range, 3-15 years) and the average number of providers seen before correct diagnosis was 4.2 (range, 1-16) with nearly half those providers being orthopedic specialists. This is likely attributed to limited training and lack of appropriate imaging. Multiple comprehensive radiographic approaches have been described, including plain films, computed tomography, and magnetic resonance imaging.2,4 The objective of this article is to present a simple 3-view plain film approach for young adults with hip pain. While history and physical examination remain key to FAI diagnosis, a basic knowledge of the common sites of impingement with appropriate radiographic views to visualize these sites may help eliminate unnecessary imaging and delayed diagnosis.
STANDING ANTEROPOSTERIOR VIEW OF THE PELVIS
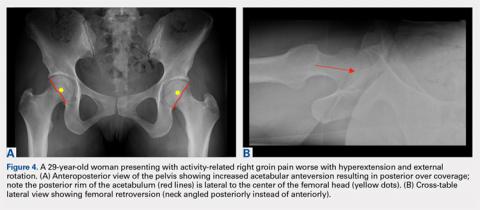
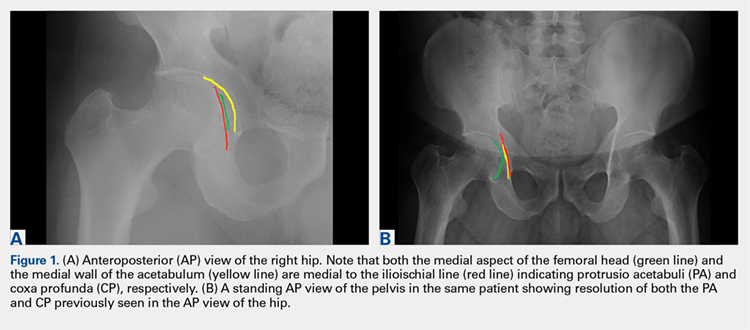
An anteroposterior (AP) view of the pelvis, as opposed to an AP view of the hip, is an important first radiograph in the evaluation of young patients presenting with hip pain. Not only does it permit visualization of the contralateral hip for comparison, but it also allows more accurate measurements of several radiographic parameters (Table). An AP view of the hip often gives the false impression of global over coverage, such as coxa profunda2 and protrusio acetabuli (Figures 1A, 1B), and may overestimate the amount of acetabular anteversion.2
Table. Summary of Common Radiographic Parameters When Assessing Young Adults with Hip Pain2,4
Sign | Best Radiographic View | Measurement | Quoted Normal Valuesa | Clinical Relevance of Abnormal Values |
Acetabular depth | AP pelvis | Medial wall of the acetabulum (MWA) relative to the ilioischial line (IIL) | MWA is lateral to IIL | Global overcoverage (ie, coxa profunda) |
Femoral depth | AP pelvis | Medial surface of the femoral head (MFH) relative to the IIL | MFH is lateral and within 10 mm of the IIL | >10 mm may indicate undercoverage (ie, dysplasia)
MFH medial to IIL may indicate overcoverage (ie, protrusio acetabuli) |
Tonnis angle | AP pelvis | Angle between the weight-bearing surface of the acetabulum and a line parallel to the horizontal axis of the pelvis (eg, inter-teardrop line) | 0°-10° | >10° may indicate undercoverage (ie, dysplasia)
<0° may indicate overcoverage (ie, pincer-type FAI) |
Lateral center edge angle | AP pelvis | Angle between a line perpendicular to the horizontal axis of the pelvis through the center of the femoral head and a line connecting the center of the femoral head to the lateral most edge of the acetabular weight-bearing surface | 25°-40° | >40° may indicate overcoverage (ie, pincer-type FAI)
<25° may indicate undercoverage (ie, dysplasia) |
Crossover sign | AP pelvis | Intersection between the anterior and posterior rims of the acetabulum | Crossover occurs at the lateral most aspect of the acetabular weight-bearing surface | Crossover occurring distal to the lateral most aspect of the acetabular weight-bearing surface may indicate acetabular retroversion |
Femoral neck-shaft angle | AP pelvis | Angle between the femoral shaft and the longitudinal axis of the neck | 135° ± 5° | >140° may indicate coxa valga
<130° may indicate coxa vara |
Alpha angle | Cross-table lateral | Angle between a line connecting the center of the femoral neck to the center of the femoral head and a line connecting the center of the head to a point on the anterolateral aspect of the head-neck junction where the head sphericity ends | >55° | Decreased head-neck offset (ie, cam-type impingement) |
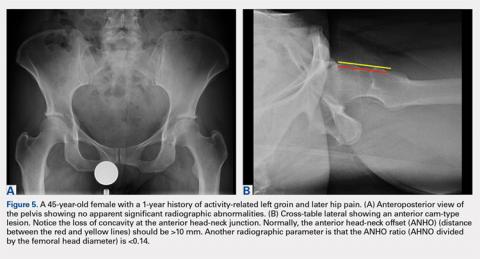
Anterior head-neck offset | Cross-table lateral | Distance between 2 lines parallel to the longitudinal axis of the femoral neck: 1 line tangent to the anterior most aspect of the neck and 1 line tangent to the anterior surface of the femoral head | >10 mm | Decreased head-neck offset (ie, cam-type impingement) |
Anterior head-neck offset ratio | Cross-table lateral | Anterior head-neck offset divided by the diameter of the femoral head | >0.14 | Decreased head-neck offset (ie, cam-type impingement) |
Femoral version | Cross-table lateral | Angle between the longitudinal axis of the femoral neck and the longitudinal axis of the femoral shaft | 15° ± 5° | Developmental disorders (eg, dysplasia, slipped capital femoral epiphysis) |
Anterior center edge angle | False profile view | Angle between a vertical line through the center of the femoral head and a line connecting the center of the femoral head to the anterior most edge of the acetabular weight-bearing surface | >20° | Undercoverage (ie, dysplasia) |
aNormal values are provided for reference only and should not be solely relied on for diagnosis.
Abbreviations: AP, anteroposterior; FAI, femoroacetabular impingement.
A good quality radiograph is important for accurate assessment. The X-ray beam should be perpendicular to the coronal plane of the pelvis. Neutral rotation of the pelvis is a prerequisite and can be confirmed by the presence of symmetric obturator foramina, iliac wings, and coccyx vertically in line with the pubic symphysis. Deviations from this configuration can significantly affect the ability to accurately assess the acetabular version. This is because the rotational profile of the acetabulum is sensitive to pelvic rotation.5,6
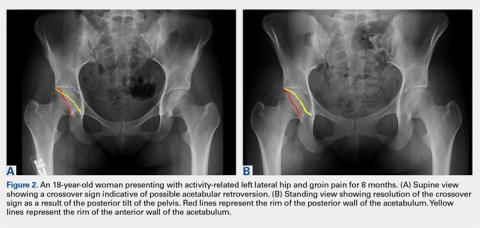
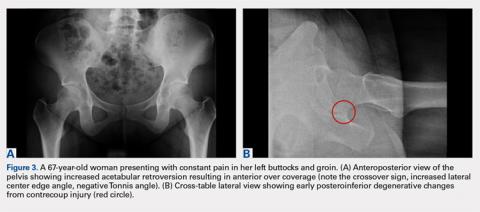
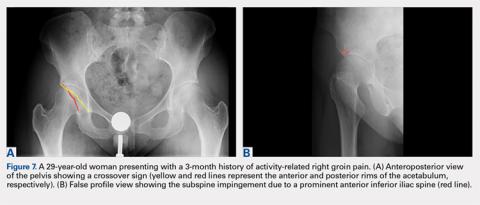
While the AP view of the pelvis can be obtained in either supine or standing positions, the standing position is recommended. A supine view tends to increase the likelihood of finding a crossover sign that often disappears in the standing position (Figures 2A, 2B). This is attributed to the posterior tilt of the pelvis in the sagittal plane with standing, which functionally increases acetabular anteversion, eliminating the crossover sign.5,6 In contrast, a crossover sign that persists in the standing position combined with other abnormal radiographic parameters, such as a negative Tonnis angle and/or increased lateral center edge angle, are concerning for pincer-type FAI (Figures 3A, 3B). An isolated crossover sign may be a normal variant in young asymptomatic patients7 and is not a reliable indicator of acetabular retroversion.5
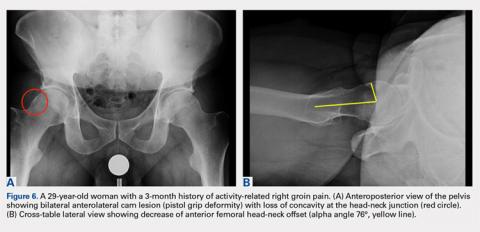
In addition to assessing the acetabular coverage and version (Figures 1A, 1B, 3A, 3B, and 4A, 4B), the AP view of the pelvis can provide valuable information regarding the proximal femur. One should pay attention to the sphericity of the head (pistol grip cam lesions are most obvious on this view), congruency between the femoral head and the acetabulum, femoral offset, and neck-shaft angle. While we tend to traditionally classify FAI into cam and pincer osseous bumps, alterations in hip dynamics (i.e., coxa vara and coxa breva) can result in functional impingement even in the absence of the osseous bumps.
Continue to: CROSS-TABLE LATERAL...
CROSS-TABLE LATERAL
A cross-table lateral of the affected hip is another important radiographic adjunct in the evaluation of hip pain in young patients. This view provides AP axial visualization of the hip joint identifying potential pathologies such as anterior cam lesions that may not be apparent on frog-leg lateral radiographs (Figures 5A, 5B and 6A, 6B). The cross-table lateral view can also show posterior impingement and/or joint space narrowing from countercoup lesions associated with pincer-type FAI (Figures 3A, 3B). In addition, the rotational profile of the proximal femur is best assessed in this view (Figure 4B). The challenge with a cross-table lateral, however, is that it is operator-dependent. In circumstances where a good quality cross-table lateral cannot be obtained, we default to a frog-leg lateral to avoid excess radiation exposure.
FALSE PROFILE VIEW
A false profile view provides a good visualization of the anterosuperior aspect of the acetabulum. It can show anterior acetabular over or under coverage. It may also show sub-spine impingement (Figures 7A, 7B). Sub-spine impingement is characterized by a prominent anterior inferior iliac spine (AIIS) that extends to the level of the anterosuperior acetabular rim. The prominent AIIS can impinge on the femoral head-neck junction during hip flexion. A prominent AIIS has also been shown to give the false impression of a crossover sign.8
CONCLUSION
Even to the trained eye, radiographic findings of FAI can be quite subtle and easily missed. A systematic approach when interpreting plain radiographs is important. Radiographic assessment starts with good quality X-rays with the pelvis in neutral rotation. Because of the young age of most patients, radiation exposure should be minimized. An understanding of the potential sites of impingement and the specific radiographs to visualize these sites minimizes radiation exposure and other unnecessary imaging. In our experience, the 3-view radiographic approach presented combined with supportive history and physical examination findings are highly sensitive to identify cases of FAI. Advanced imaging is reserved for patients who have failed conservative management or considering surgical intervention.
1. Fernquest S, Arnold C, Palmer A, et al. Osseous impingement occurs early in flexion in cam-type femoroacetabular impingement: a 4D CT model. Bone Joint J. 2017;99-B(4 Supple B):41-48. doi:10.1302/0301-620X.99B4.BJJ-2016-1274.R1.
2. Tannast M, Siebenrock KA, Anderson SE. Femoroacetabular impingement: radiographic diagnosis--what the radiologist should know. AJR Am J Roentgenol. 2007;188(6):1540-1552. doi:10.2214/AJR.06.0921.
3. Clohisy JC, Knaus ER, Hunt DM, Lesher JM, Harris-Hayes M, Prather H. Clinical presentation of patients with symptomatic anterior hip impingement. Clin Orthop Relat Res. 2009;467(3):638-644. doi:10.1007/s11999-008-0680-y.
4. Clohisy JC, Carlisle JC, Beaule PE, et al. A systematic approach to the plain radiographic evaluation of the young adult hip. J Bone Joint Surg Am. 2008;90 Suppl 4:47-66. doi:10.2106/JBJS.H.00756.
5. Dandachli W, Islam SU, Liu M, Richards R, Hall-Craggs M, Witt J. Three-dimensional CT analysis to determine acetabular retroversion and the implications for the management of femoro-acetabular impingement. J Bone Joint Surg Br. 2009;91(8):1031-1036. doi:10.1302/0301-620X.91B8.22389.
6. Dandachli W, Kannan V, Richards R, Shah Z, Hall-Craggs M, Witt J. Analysis of cover of the femoral head in normal and dysplastic hips: new CT-based technique. J Bone Joint Surg Br. 2008;90(11):1428-1434. doi:10.1302/0301-620X.90B11.20073.
7. Larson CM, Moreau-Gaudry A, Kelly BT, et al. Are normal hips being labeled as pathologic? A CT-based method for defining normal acetabular coverage. Clin Orthop Relat Res. 2015;473(4):1247-1254. doi:10.1007/s11999-014-4055-2.
8. Zaltz I, Kelly BT, Hetsroni I, Bedi A. The crossover sign overestimates acetabular retroversion. Clin Orthop Relat Res. 2013;471(8):2463-2470. doi:10.1007/s11999-012-2689-5.
ABSTRACT
Femoroacetabular impingement (FAI) is an abnormality of the hip joint that is increasingly being recognized as a cause of athletic disability and early degenerative hip disease. Despite significant advances in the knowledge of FAI, it remains a frequently unrecognized cause of hip pain in adolescents and young adults among orthopedic providers. The purpose of this article is to present a simple 3-view radiographic approach to young adults with hip pain. The radiographs include a standing anteroposterior view of the pelvis, a cross-table lateral view, and a false profile view. Good quality radiographs showing the common sites of potential impingement combined with a basic understanding of certain radiographic parameters may allow faster diagnosis, eliminate unnecessary studies, and allow earlier referral and management.
Continue to: The prevalence of femoroacetabular impingement...
The prevalence of femoroacetabular impingement (FAI) in the general population is estimated at 23.1%.1 While FAI is often bilateral,2 patients usually present with unilateral symptoms.3 Young, highly active individuals are most commonly affected.3 Despite significant improvement in our understanding of FAI in recent years, it remains a poorly recognized cause of hip pain among orthopedic providers. Clohisy and colleagues3 found that the average time to diagnosis was 3.1 years (range, 3-15 years) and the average number of providers seen before correct diagnosis was 4.2 (range, 1-16) with nearly half those providers being orthopedic specialists. This is likely attributed to limited training and lack of appropriate imaging. Multiple comprehensive radiographic approaches have been described, including plain films, computed tomography, and magnetic resonance imaging.2,4 The objective of this article is to present a simple 3-view plain film approach for young adults with hip pain. While history and physical examination remain key to FAI diagnosis, a basic knowledge of the common sites of impingement with appropriate radiographic views to visualize these sites may help eliminate unnecessary imaging and delayed diagnosis.
STANDING ANTEROPOSTERIOR VIEW OF THE PELVIS
An anteroposterior (AP) view of the pelvis, as opposed to an AP view of the hip, is an important first radiograph in the evaluation of young patients presenting with hip pain. Not only does it permit visualization of the contralateral hip for comparison, but it also allows more accurate measurements of several radiographic parameters (Table). An AP view of the hip often gives the false impression of global over coverage, such as coxa profunda2 and protrusio acetabuli (Figures 1A, 1B), and may overestimate the amount of acetabular anteversion.2
Table. Summary of Common Radiographic Parameters When Assessing Young Adults with Hip Pain2,4
Sign | Best Radiographic View | Measurement | Quoted Normal Valuesa | Clinical Relevance of Abnormal Values |
Acetabular depth | AP pelvis | Medial wall of the acetabulum (MWA) relative to the ilioischial line (IIL) | MWA is lateral to IIL | Global overcoverage (ie, coxa profunda) |
Femoral depth | AP pelvis | Medial surface of the femoral head (MFH) relative to the IIL | MFH is lateral and within 10 mm of the IIL | >10 mm may indicate undercoverage (ie, dysplasia)
MFH medial to IIL may indicate overcoverage (ie, protrusio acetabuli) |
Tonnis angle | AP pelvis | Angle between the weight-bearing surface of the acetabulum and a line parallel to the horizontal axis of the pelvis (eg, inter-teardrop line) | 0°-10° | >10° may indicate undercoverage (ie, dysplasia)
<0° may indicate overcoverage (ie, pincer-type FAI) |
Lateral center edge angle | AP pelvis | Angle between a line perpendicular to the horizontal axis of the pelvis through the center of the femoral head and a line connecting the center of the femoral head to the lateral most edge of the acetabular weight-bearing surface | 25°-40° | >40° may indicate overcoverage (ie, pincer-type FAI)
<25° may indicate undercoverage (ie, dysplasia) |
Crossover sign | AP pelvis | Intersection between the anterior and posterior rims of the acetabulum | Crossover occurs at the lateral most aspect of the acetabular weight-bearing surface | Crossover occurring distal to the lateral most aspect of the acetabular weight-bearing surface may indicate acetabular retroversion |
Femoral neck-shaft angle | AP pelvis | Angle between the femoral shaft and the longitudinal axis of the neck | 135° ± 5° | >140° may indicate coxa valga
<130° may indicate coxa vara |
Alpha angle | Cross-table lateral | Angle between a line connecting the center of the femoral neck to the center of the femoral head and a line connecting the center of the head to a point on the anterolateral aspect of the head-neck junction where the head sphericity ends | >55° | Decreased head-neck offset (ie, cam-type impingement) |
Anterior head-neck offset | Cross-table lateral | Distance between 2 lines parallel to the longitudinal axis of the femoral neck: 1 line tangent to the anterior most aspect of the neck and 1 line tangent to the anterior surface of the femoral head | >10 mm | Decreased head-neck offset (ie, cam-type impingement) |
Anterior head-neck offset ratio | Cross-table lateral | Anterior head-neck offset divided by the diameter of the femoral head | >0.14 | Decreased head-neck offset (ie, cam-type impingement) |
Femoral version | Cross-table lateral | Angle between the longitudinal axis of the femoral neck and the longitudinal axis of the femoral shaft | 15° ± 5° | Developmental disorders (eg, dysplasia, slipped capital femoral epiphysis) |
Anterior center edge angle | False profile view | Angle between a vertical line through the center of the femoral head and a line connecting the center of the femoral head to the anterior most edge of the acetabular weight-bearing surface | >20° | Undercoverage (ie, dysplasia) |
aNormal values are provided for reference only and should not be solely relied on for diagnosis.
Abbreviations: AP, anteroposterior; FAI, femoroacetabular impingement.

A good quality radiograph is important for accurate assessment. The X-ray beam should be perpendicular to the coronal plane of the pelvis. Neutral rotation of the pelvis is a prerequisite and can be confirmed by the presence of symmetric obturator foramina, iliac wings, and coccyx vertically in line with the pubic symphysis. Deviations from this configuration can significantly affect the ability to accurately assess the acetabular version. This is because the rotational profile of the acetabulum is sensitive to pelvic rotation.5,6
While the AP view of the pelvis can be obtained in either supine or standing positions, the standing position is recommended. A supine view tends to increase the likelihood of finding a crossover sign that often disappears in the standing position (Figures 2A, 2B). This is attributed to the posterior tilt of the pelvis in the sagittal plane with standing, which functionally increases acetabular anteversion, eliminating the crossover sign.5,6 In contrast, a crossover sign that persists in the standing position combined with other abnormal radiographic parameters, such as a negative Tonnis angle and/or increased lateral center edge angle, are concerning for pincer-type FAI (Figures 3A, 3B). An isolated crossover sign may be a normal variant in young asymptomatic patients7 and is not a reliable indicator of acetabular retroversion.5
In addition to assessing the acetabular coverage and version (Figures 1A, 1B, 3A, 3B, and 4A, 4B), the AP view of the pelvis can provide valuable information regarding the proximal femur. One should pay attention to the sphericity of the head (pistol grip cam lesions are most obvious on this view), congruency between the femoral head and the acetabulum, femoral offset, and neck-shaft angle. While we tend to traditionally classify FAI into cam and pincer osseous bumps, alterations in hip dynamics (i.e., coxa vara and coxa breva) can result in functional impingement even in the absence of the osseous bumps.
Continue to: CROSS-TABLE LATERAL...
CROSS-TABLE LATERAL
A cross-table lateral of the affected hip is another important radiographic adjunct in the evaluation of hip pain in young patients. This view provides AP axial visualization of the hip joint identifying potential pathologies such as anterior cam lesions that may not be apparent on frog-leg lateral radiographs (Figures 5A, 5B and 6A, 6B). The cross-table lateral view can also show posterior impingement and/or joint space narrowing from countercoup lesions associated with pincer-type FAI (Figures 3A, 3B). In addition, the rotational profile of the proximal femur is best assessed in this view (Figure 4B). The challenge with a cross-table lateral, however, is that it is operator-dependent. In circumstances where a good quality cross-table lateral cannot be obtained, we default to a frog-leg lateral to avoid excess radiation exposure.
FALSE PROFILE VIEW
A false profile view provides a good visualization of the anterosuperior aspect of the acetabulum. It can show anterior acetabular over or under coverage. It may also show sub-spine impingement (Figures 7A, 7B). Sub-spine impingement is characterized by a prominent anterior inferior iliac spine (AIIS) that extends to the level of the anterosuperior acetabular rim. The prominent AIIS can impinge on the femoral head-neck junction during hip flexion. A prominent AIIS has also been shown to give the false impression of a crossover sign.8
CONCLUSION
Even to the trained eye, radiographic findings of FAI can be quite subtle and easily missed. A systematic approach when interpreting plain radiographs is important. Radiographic assessment starts with good quality X-rays with the pelvis in neutral rotation. Because of the young age of most patients, radiation exposure should be minimized. An understanding of the potential sites of impingement and the specific radiographs to visualize these sites minimizes radiation exposure and other unnecessary imaging. In our experience, the 3-view radiographic approach presented combined with supportive history and physical examination findings are highly sensitive to identify cases of FAI. Advanced imaging is reserved for patients who have failed conservative management or considering surgical intervention.
ABSTRACT
Femoroacetabular impingement (FAI) is an abnormality of the hip joint that is increasingly being recognized as a cause of athletic disability and early degenerative hip disease. Despite significant advances in the knowledge of FAI, it remains a frequently unrecognized cause of hip pain in adolescents and young adults among orthopedic providers. The purpose of this article is to present a simple 3-view radiographic approach to young adults with hip pain. The radiographs include a standing anteroposterior view of the pelvis, a cross-table lateral view, and a false profile view. Good quality radiographs showing the common sites of potential impingement combined with a basic understanding of certain radiographic parameters may allow faster diagnosis, eliminate unnecessary studies, and allow earlier referral and management.
Continue to: The prevalence of femoroacetabular impingement...
The prevalence of femoroacetabular impingement (FAI) in the general population is estimated at 23.1%.1 While FAI is often bilateral,2 patients usually present with unilateral symptoms.3 Young, highly active individuals are most commonly affected.3 Despite significant improvement in our understanding of FAI in recent years, it remains a poorly recognized cause of hip pain among orthopedic providers. Clohisy and colleagues3 found that the average time to diagnosis was 3.1 years (range, 3-15 years) and the average number of providers seen before correct diagnosis was 4.2 (range, 1-16) with nearly half those providers being orthopedic specialists. This is likely attributed to limited training and lack of appropriate imaging. Multiple comprehensive radiographic approaches have been described, including plain films, computed tomography, and magnetic resonance imaging.2,4 The objective of this article is to present a simple 3-view plain film approach for young adults with hip pain. While history and physical examination remain key to FAI diagnosis, a basic knowledge of the common sites of impingement with appropriate radiographic views to visualize these sites may help eliminate unnecessary imaging and delayed diagnosis.
STANDING ANTEROPOSTERIOR VIEW OF THE PELVIS
An anteroposterior (AP) view of the pelvis, as opposed to an AP view of the hip, is an important first radiograph in the evaluation of young patients presenting with hip pain. Not only does it permit visualization of the contralateral hip for comparison, but it also allows more accurate measurements of several radiographic parameters (Table). An AP view of the hip often gives the false impression of global over coverage, such as coxa profunda2 and protrusio acetabuli (Figures 1A, 1B), and may overestimate the amount of acetabular anteversion.2
Table. Summary of Common Radiographic Parameters When Assessing Young Adults with Hip Pain2,4
Sign | Best Radiographic View | Measurement | Quoted Normal Valuesa | Clinical Relevance of Abnormal Values |
Acetabular depth | AP pelvis | Medial wall of the acetabulum (MWA) relative to the ilioischial line (IIL) | MWA is lateral to IIL | Global overcoverage (ie, coxa profunda) |
Femoral depth | AP pelvis | Medial surface of the femoral head (MFH) relative to the IIL | MFH is lateral and within 10 mm of the IIL | >10 mm may indicate undercoverage (ie, dysplasia)
MFH medial to IIL may indicate overcoverage (ie, protrusio acetabuli) |
Tonnis angle | AP pelvis | Angle between the weight-bearing surface of the acetabulum and a line parallel to the horizontal axis of the pelvis (eg, inter-teardrop line) | 0°-10° | >10° may indicate undercoverage (ie, dysplasia)
<0° may indicate overcoverage (ie, pincer-type FAI) |
Lateral center edge angle | AP pelvis | Angle between a line perpendicular to the horizontal axis of the pelvis through the center of the femoral head and a line connecting the center of the femoral head to the lateral most edge of the acetabular weight-bearing surface | 25°-40° | >40° may indicate overcoverage (ie, pincer-type FAI)
<25° may indicate undercoverage (ie, dysplasia) |
Crossover sign | AP pelvis | Intersection between the anterior and posterior rims of the acetabulum | Crossover occurs at the lateral most aspect of the acetabular weight-bearing surface | Crossover occurring distal to the lateral most aspect of the acetabular weight-bearing surface may indicate acetabular retroversion |
Femoral neck-shaft angle | AP pelvis | Angle between the femoral shaft and the longitudinal axis of the neck | 135° ± 5° | >140° may indicate coxa valga
<130° may indicate coxa vara |
Alpha angle | Cross-table lateral | Angle between a line connecting the center of the femoral neck to the center of the femoral head and a line connecting the center of the head to a point on the anterolateral aspect of the head-neck junction where the head sphericity ends | >55° | Decreased head-neck offset (ie, cam-type impingement) |
Anterior head-neck offset | Cross-table lateral | Distance between 2 lines parallel to the longitudinal axis of the femoral neck: 1 line tangent to the anterior most aspect of the neck and 1 line tangent to the anterior surface of the femoral head | >10 mm | Decreased head-neck offset (ie, cam-type impingement) |
Anterior head-neck offset ratio | Cross-table lateral | Anterior head-neck offset divided by the diameter of the femoral head | >0.14 | Decreased head-neck offset (ie, cam-type impingement) |
Femoral version | Cross-table lateral | Angle between the longitudinal axis of the femoral neck and the longitudinal axis of the femoral shaft | 15° ± 5° | Developmental disorders (eg, dysplasia, slipped capital femoral epiphysis) |
Anterior center edge angle | False profile view | Angle between a vertical line through the center of the femoral head and a line connecting the center of the femoral head to the anterior most edge of the acetabular weight-bearing surface | >20° | Undercoverage (ie, dysplasia) |
aNormal values are provided for reference only and should not be solely relied on for diagnosis.
Abbreviations: AP, anteroposterior; FAI, femoroacetabular impingement.

A good quality radiograph is important for accurate assessment. The X-ray beam should be perpendicular to the coronal plane of the pelvis. Neutral rotation of the pelvis is a prerequisite and can be confirmed by the presence of symmetric obturator foramina, iliac wings, and coccyx vertically in line with the pubic symphysis. Deviations from this configuration can significantly affect the ability to accurately assess the acetabular version. This is because the rotational profile of the acetabulum is sensitive to pelvic rotation.5,6
While the AP view of the pelvis can be obtained in either supine or standing positions, the standing position is recommended. A supine view tends to increase the likelihood of finding a crossover sign that often disappears in the standing position (Figures 2A, 2B). This is attributed to the posterior tilt of the pelvis in the sagittal plane with standing, which functionally increases acetabular anteversion, eliminating the crossover sign.5,6 In contrast, a crossover sign that persists in the standing position combined with other abnormal radiographic parameters, such as a negative Tonnis angle and/or increased lateral center edge angle, are concerning for pincer-type FAI (Figures 3A, 3B). An isolated crossover sign may be a normal variant in young asymptomatic patients7 and is not a reliable indicator of acetabular retroversion.5
In addition to assessing the acetabular coverage and version (Figures 1A, 1B, 3A, 3B, and 4A, 4B), the AP view of the pelvis can provide valuable information regarding the proximal femur. One should pay attention to the sphericity of the head (pistol grip cam lesions are most obvious on this view), congruency between the femoral head and the acetabulum, femoral offset, and neck-shaft angle. While we tend to traditionally classify FAI into cam and pincer osseous bumps, alterations in hip dynamics (i.e., coxa vara and coxa breva) can result in functional impingement even in the absence of the osseous bumps.
Continue to: CROSS-TABLE LATERAL...
CROSS-TABLE LATERAL
A cross-table lateral of the affected hip is another important radiographic adjunct in the evaluation of hip pain in young patients. This view provides AP axial visualization of the hip joint identifying potential pathologies such as anterior cam lesions that may not be apparent on frog-leg lateral radiographs (Figures 5A, 5B and 6A, 6B). The cross-table lateral view can also show posterior impingement and/or joint space narrowing from countercoup lesions associated with pincer-type FAI (Figures 3A, 3B). In addition, the rotational profile of the proximal femur is best assessed in this view (Figure 4B). The challenge with a cross-table lateral, however, is that it is operator-dependent. In circumstances where a good quality cross-table lateral cannot be obtained, we default to a frog-leg lateral to avoid excess radiation exposure.
FALSE PROFILE VIEW
A false profile view provides a good visualization of the anterosuperior aspect of the acetabulum. It can show anterior acetabular over or under coverage. It may also show sub-spine impingement (Figures 7A, 7B). Sub-spine impingement is characterized by a prominent anterior inferior iliac spine (AIIS) that extends to the level of the anterosuperior acetabular rim. The prominent AIIS can impinge on the femoral head-neck junction during hip flexion. A prominent AIIS has also been shown to give the false impression of a crossover sign.8
CONCLUSION
Even to the trained eye, radiographic findings of FAI can be quite subtle and easily missed. A systematic approach when interpreting plain radiographs is important. Radiographic assessment starts with good quality X-rays with the pelvis in neutral rotation. Because of the young age of most patients, radiation exposure should be minimized. An understanding of the potential sites of impingement and the specific radiographs to visualize these sites minimizes radiation exposure and other unnecessary imaging. In our experience, the 3-view radiographic approach presented combined with supportive history and physical examination findings are highly sensitive to identify cases of FAI. Advanced imaging is reserved for patients who have failed conservative management or considering surgical intervention.
1. Fernquest S, Arnold C, Palmer A, et al. Osseous impingement occurs early in flexion in cam-type femoroacetabular impingement: a 4D CT model. Bone Joint J. 2017;99-B(4 Supple B):41-48. doi:10.1302/0301-620X.99B4.BJJ-2016-1274.R1.
2. Tannast M, Siebenrock KA, Anderson SE. Femoroacetabular impingement: radiographic diagnosis--what the radiologist should know. AJR Am J Roentgenol. 2007;188(6):1540-1552. doi:10.2214/AJR.06.0921.
3. Clohisy JC, Knaus ER, Hunt DM, Lesher JM, Harris-Hayes M, Prather H. Clinical presentation of patients with symptomatic anterior hip impingement. Clin Orthop Relat Res. 2009;467(3):638-644. doi:10.1007/s11999-008-0680-y.
4. Clohisy JC, Carlisle JC, Beaule PE, et al. A systematic approach to the plain radiographic evaluation of the young adult hip. J Bone Joint Surg Am. 2008;90 Suppl 4:47-66. doi:10.2106/JBJS.H.00756.
5. Dandachli W, Islam SU, Liu M, Richards R, Hall-Craggs M, Witt J. Three-dimensional CT analysis to determine acetabular retroversion and the implications for the management of femoro-acetabular impingement. J Bone Joint Surg Br. 2009;91(8):1031-1036. doi:10.1302/0301-620X.91B8.22389.
6. Dandachli W, Kannan V, Richards R, Shah Z, Hall-Craggs M, Witt J. Analysis of cover of the femoral head in normal and dysplastic hips: new CT-based technique. J Bone Joint Surg Br. 2008;90(11):1428-1434. doi:10.1302/0301-620X.90B11.20073.
7. Larson CM, Moreau-Gaudry A, Kelly BT, et al. Are normal hips being labeled as pathologic? A CT-based method for defining normal acetabular coverage. Clin Orthop Relat Res. 2015;473(4):1247-1254. doi:10.1007/s11999-014-4055-2.
8. Zaltz I, Kelly BT, Hetsroni I, Bedi A. The crossover sign overestimates acetabular retroversion. Clin Orthop Relat Res. 2013;471(8):2463-2470. doi:10.1007/s11999-012-2689-5.
1. Fernquest S, Arnold C, Palmer A, et al. Osseous impingement occurs early in flexion in cam-type femoroacetabular impingement: a 4D CT model. Bone Joint J. 2017;99-B(4 Supple B):41-48. doi:10.1302/0301-620X.99B4.BJJ-2016-1274.R1.
2. Tannast M, Siebenrock KA, Anderson SE. Femoroacetabular impingement: radiographic diagnosis--what the radiologist should know. AJR Am J Roentgenol. 2007;188(6):1540-1552. doi:10.2214/AJR.06.0921.
3. Clohisy JC, Knaus ER, Hunt DM, Lesher JM, Harris-Hayes M, Prather H. Clinical presentation of patients with symptomatic anterior hip impingement. Clin Orthop Relat Res. 2009;467(3):638-644. doi:10.1007/s11999-008-0680-y.
4. Clohisy JC, Carlisle JC, Beaule PE, et al. A systematic approach to the plain radiographic evaluation of the young adult hip. J Bone Joint Surg Am. 2008;90 Suppl 4:47-66. doi:10.2106/JBJS.H.00756.
5. Dandachli W, Islam SU, Liu M, Richards R, Hall-Craggs M, Witt J. Three-dimensional CT analysis to determine acetabular retroversion and the implications for the management of femoro-acetabular impingement. J Bone Joint Surg Br. 2009;91(8):1031-1036. doi:10.1302/0301-620X.91B8.22389.
6. Dandachli W, Kannan V, Richards R, Shah Z, Hall-Craggs M, Witt J. Analysis of cover of the femoral head in normal and dysplastic hips: new CT-based technique. J Bone Joint Surg Br. 2008;90(11):1428-1434. doi:10.1302/0301-620X.90B11.20073.
7. Larson CM, Moreau-Gaudry A, Kelly BT, et al. Are normal hips being labeled as pathologic? A CT-based method for defining normal acetabular coverage. Clin Orthop Relat Res. 2015;473(4):1247-1254. doi:10.1007/s11999-014-4055-2.
8. Zaltz I, Kelly BT, Hetsroni I, Bedi A. The crossover sign overestimates acetabular retroversion. Clin Orthop Relat Res. 2013;471(8):2463-2470. doi:10.1007/s11999-012-2689-5.
TAKE-HOME POINTS
- FAI is a frequently unrecognized cause of hip pain in adolescents and young adults.
- Understanding the potential sites of impingement and the specific radiographs to visualize these sites can help avoid unnecessary imaging and delayed diagnosis.
- A simple radiographic approach consisting of a standing AP view of the pelvis, a cross-table lateral view, and a false profile view is often a sufficient screening tool.
- While we tend to classify FAI into cam and pincer osseous bumps, alterations in hip dynamics can result in functional impingement even in the absence of the osseous bumps.
- Advanced imaging is reserved for patients who have failed conservative management or are considering surgical intervention.
ATTR-ACT shows treatment breakthrough in amyloid cardiomyopathy
MUNICH – The big news in the field of heart failure at the annual congress of the European Society of Cardiology concerned an obscure form of the disease traditionally considered rare: transthyretin amyloid cardiomyopathy (TAC).
It turns out that TAC is far more common than previously recognized; it can now be diagnosed and staged noninvasively; and – most important of all – there is for the first time an effective disease-modifying treatment in the form of a novel oral drug called tafamidis, as demonstrated in the Transthyretin Amyloidosis Cardiomyopathy Clinical Trial (ATTR-ACT) presented at the meeting.
“This is the first phase 3 trial that can offer a chance for people with a terrible, severe disease. And within the last year, while the trial was being conducted, it became clear that this disease is much more underdiagnosed than rare,” said Claudio Rapezzi, MD, ATTR-ACT principal investigator and director of the school of cardiovascular diseases at the University of Bologna, Italy.
ATTR-ACT participants randomized to tafamidis showed significant reductions in all-cause mortality and cardiovascular hospitalizations, compared with placebo-treated controls at 30 months follow-up. They also experienced significantly lesser declines in both quality of life as reflected in Kansas City Cardiomyopathy Questionnaire scores and in physical function as captured in 6-minute walk distance.
Designated discussant Jacob George, MD, was over the moon regarding the results.
“This is a pioneering, game-changing trial that is likely to transform the way we diagnose and treat patients with cardiac amyloidosis,” said Dr. George of Kaplan Medical Center in Rehovot, Israel.
“We’re now in an era that, to my opinion, any patient with nonischemic unexplained heart failure should be screened for the presence of amyloidosis because, first, we now know how to prognosticate these patients, and second, we can offer them a real disease-modifying agent,” he added.
An underdiagnosed disease
Transthyretin amyloid cardiomyopathy occurs when transthyretin, a transport protein, becomes destabilized and misfolds, promoting deposition of amyloid fibrils in the myocardium. This results in progressive ventricular wall thickening and stiffness, manifest as restrictive cardiomyopathy and progressive heart failure. The cause of transthyretin destabilization can be either autosomal dominant inheritance of any of more than 100 pathogenic mutations in the transthyretin gene identified to date or a spontaneous wild type protein.
Think of TAC as a sort of dementia of the heart. As Dr. George noted, the cardiac disease bears “remarkable similarities” to Alzheimer’s disease, with both conditions entailing extracellular deposition of amyloid.
In the heritable form of TAC, patients typically present with heart failure symptoms at about age 50-55, while the wild type form becomes symptomatic much later at a mean age of about 75. Average survival from time of diagnosis is only about 3 years.
Recent studies from multiple centers have reported that the prevalence of TAC was 16% in patients undergoing transcatheter aortic valve replacement for severe aortic stenosis, 13% among patients with heart failure with preserved ejection fraction, and 5% in patients who had been presumed to have hypertrophic cardiomyopathy: So, not a rare condition.
“In our clinic, vast and surprising numbers of patients with unexplained nonischemic heart failure are scan positive [for TAC],” according to Dr. George.
Breakthroughs in diagnosis and staging
The echocardiographic red flag for TAC in a patient with heart failure symptoms is symmetric hypertrophy with a normal end-diastolic volume and thickened ventricles. The end-diastolic interventricular septal wall thickness is typically about 15 mm. The left ventricular ejection fraction is typically in the normal range, “but the clue is not the preservation of the ejection fraction, it’s the [normal] quality of the volume,” Dr. Rapezzi said.
A clinical clue suggestive of TAC upon physical examination, even in the absence of heart failure symptoms, is development of bilateral carpal tunnel syndrome in an older man. That’s because the same disease process that results in TAC can involve deposition of amyloid fibrils in peripheral nerves. Indeed, tafamidis is already approved in Europe and Japan under the trade name Vyndaqel as a treatment for familial amyloid polyneuropathy. For TAC, however, tafamidis remains investigational with fast-track status provided by both the Food and Drug Administration and the European Medicines Agency.
When TAC is suspected, it’s no longer necessary to subject patients to an onerous myocardial biopsy. Total body scintigraphy with bone tracers has been shown to be nearly as sensitive and specific as biopsy for the diagnosis.
Staging can now be done noninvasively as well. Investigators at the U.K. National Amyloidosis Centre recently reported that patients with TAC can be accurately staged using two biomarkers: N-terminal pro-B-type natriuretic peptide (NT-proBNP) and estimated glomerular filtration rate (eGFR). In their series of 869 patients with TAC, median survival for those with stage I disease as defined by their protocol was 69 months, compared with 47 months for stage II disease and 24 months for those with stage III disease. This simple U.K. staging system was then validated in a separate French cohort of TAC patients (Eur Heart J. 2018 Aug 7;39[30]:2799-806).
The ATTR-ACT trial
Dr. Rapezzi reported on 441 patients with TAC who were randomized to oral tafamidis at either 20 mg or 80 mg per day or placebo and followed prospectively for 30 months in the 13-country, double-blind, phase 3 trial. At 30 months, all-cause mortality was 29.5% in patients who received tafamidis, compared with 42.9% in controls, for a 30% relative risk reduction. The rate of cardiovascular hospitalizations was 0.48 per year with tafamidis, compared with 0.70 per year with placebo, for a 38% relative risk reduction. The mortality benefit didn’t achieve significance until 15-18 months into the trial, as to be expected given tafamidis’ mechanism of action, which involves binding to transthyretin, gradually stabilizing it, and curbing amyloid fibril deposition.
Of note, the benefit was similar regardless of the dose used and whether patients had hereditary or wild type TAC.
Tafamidis proved safe and well tolerated, with a side-effect profile similar to placebo. While diarrhea and urinary tract infections have been an issue in tafamidis-treated patients with familial amyloid polyneuropathy, these adverse events were actually less common in TAC patients who received tafamidis than with placebo, according to Dr. Rapezzi.
A key point, the cardiologist emphasized, is that the benefits of active treatment were greatest in patients with earlier-stage disease. Therefore it’s vital that the diagnosis of TAC be made early, with prompt initiation of treatment to follow, in order to catch the disease at a more reversible stage. That could mean there will be a whole lot more bone scintigraphy being done in patients with unexplained nonischemic heart failure.
Dr. Rapezzi reported receiving research grants, speaker honoraria, and consulting fees from Pfizer, which sponsored the ATTR-ACT trial. Simultaneous with his presentation in Munich, the study results were published online at NEJM.org (doi: 10.1056/NEJMoa1805689). Dr. George reported no financial conflicts.
MUNICH – The big news in the field of heart failure at the annual congress of the European Society of Cardiology concerned an obscure form of the disease traditionally considered rare: transthyretin amyloid cardiomyopathy (TAC).
It turns out that TAC is far more common than previously recognized; it can now be diagnosed and staged noninvasively; and – most important of all – there is for the first time an effective disease-modifying treatment in the form of a novel oral drug called tafamidis, as demonstrated in the Transthyretin Amyloidosis Cardiomyopathy Clinical Trial (ATTR-ACT) presented at the meeting.
“This is the first phase 3 trial that can offer a chance for people with a terrible, severe disease. And within the last year, while the trial was being conducted, it became clear that this disease is much more underdiagnosed than rare,” said Claudio Rapezzi, MD, ATTR-ACT principal investigator and director of the school of cardiovascular diseases at the University of Bologna, Italy.
ATTR-ACT participants randomized to tafamidis showed significant reductions in all-cause mortality and cardiovascular hospitalizations, compared with placebo-treated controls at 30 months follow-up. They also experienced significantly lesser declines in both quality of life as reflected in Kansas City Cardiomyopathy Questionnaire scores and in physical function as captured in 6-minute walk distance.
Designated discussant Jacob George, MD, was over the moon regarding the results.
“This is a pioneering, game-changing trial that is likely to transform the way we diagnose and treat patients with cardiac amyloidosis,” said Dr. George of Kaplan Medical Center in Rehovot, Israel.
“We’re now in an era that, to my opinion, any patient with nonischemic unexplained heart failure should be screened for the presence of amyloidosis because, first, we now know how to prognosticate these patients, and second, we can offer them a real disease-modifying agent,” he added.
An underdiagnosed disease
Transthyretin amyloid cardiomyopathy occurs when transthyretin, a transport protein, becomes destabilized and misfolds, promoting deposition of amyloid fibrils in the myocardium. This results in progressive ventricular wall thickening and stiffness, manifest as restrictive cardiomyopathy and progressive heart failure. The cause of transthyretin destabilization can be either autosomal dominant inheritance of any of more than 100 pathogenic mutations in the transthyretin gene identified to date or a spontaneous wild type protein.
Think of TAC as a sort of dementia of the heart. As Dr. George noted, the cardiac disease bears “remarkable similarities” to Alzheimer’s disease, with both conditions entailing extracellular deposition of amyloid.
In the heritable form of TAC, patients typically present with heart failure symptoms at about age 50-55, while the wild type form becomes symptomatic much later at a mean age of about 75. Average survival from time of diagnosis is only about 3 years.
Recent studies from multiple centers have reported that the prevalence of TAC was 16% in patients undergoing transcatheter aortic valve replacement for severe aortic stenosis, 13% among patients with heart failure with preserved ejection fraction, and 5% in patients who had been presumed to have hypertrophic cardiomyopathy: So, not a rare condition.
“In our clinic, vast and surprising numbers of patients with unexplained nonischemic heart failure are scan positive [for TAC],” according to Dr. George.
Breakthroughs in diagnosis and staging
The echocardiographic red flag for TAC in a patient with heart failure symptoms is symmetric hypertrophy with a normal end-diastolic volume and thickened ventricles. The end-diastolic interventricular septal wall thickness is typically about 15 mm. The left ventricular ejection fraction is typically in the normal range, “but the clue is not the preservation of the ejection fraction, it’s the [normal] quality of the volume,” Dr. Rapezzi said.
A clinical clue suggestive of TAC upon physical examination, even in the absence of heart failure symptoms, is development of bilateral carpal tunnel syndrome in an older man. That’s because the same disease process that results in TAC can involve deposition of amyloid fibrils in peripheral nerves. Indeed, tafamidis is already approved in Europe and Japan under the trade name Vyndaqel as a treatment for familial amyloid polyneuropathy. For TAC, however, tafamidis remains investigational with fast-track status provided by both the Food and Drug Administration and the European Medicines Agency.
When TAC is suspected, it’s no longer necessary to subject patients to an onerous myocardial biopsy. Total body scintigraphy with bone tracers has been shown to be nearly as sensitive and specific as biopsy for the diagnosis.
Staging can now be done noninvasively as well. Investigators at the U.K. National Amyloidosis Centre recently reported that patients with TAC can be accurately staged using two biomarkers: N-terminal pro-B-type natriuretic peptide (NT-proBNP) and estimated glomerular filtration rate (eGFR). In their series of 869 patients with TAC, median survival for those with stage I disease as defined by their protocol was 69 months, compared with 47 months for stage II disease and 24 months for those with stage III disease. This simple U.K. staging system was then validated in a separate French cohort of TAC patients (Eur Heart J. 2018 Aug 7;39[30]:2799-806).
The ATTR-ACT trial
Dr. Rapezzi reported on 441 patients with TAC who were randomized to oral tafamidis at either 20 mg or 80 mg per day or placebo and followed prospectively for 30 months in the 13-country, double-blind, phase 3 trial. At 30 months, all-cause mortality was 29.5% in patients who received tafamidis, compared with 42.9% in controls, for a 30% relative risk reduction. The rate of cardiovascular hospitalizations was 0.48 per year with tafamidis, compared with 0.70 per year with placebo, for a 38% relative risk reduction. The mortality benefit didn’t achieve significance until 15-18 months into the trial, as to be expected given tafamidis’ mechanism of action, which involves binding to transthyretin, gradually stabilizing it, and curbing amyloid fibril deposition.
Of note, the benefit was similar regardless of the dose used and whether patients had hereditary or wild type TAC.
Tafamidis proved safe and well tolerated, with a side-effect profile similar to placebo. While diarrhea and urinary tract infections have been an issue in tafamidis-treated patients with familial amyloid polyneuropathy, these adverse events were actually less common in TAC patients who received tafamidis than with placebo, according to Dr. Rapezzi.
A key point, the cardiologist emphasized, is that the benefits of active treatment were greatest in patients with earlier-stage disease. Therefore it’s vital that the diagnosis of TAC be made early, with prompt initiation of treatment to follow, in order to catch the disease at a more reversible stage. That could mean there will be a whole lot more bone scintigraphy being done in patients with unexplained nonischemic heart failure.
Dr. Rapezzi reported receiving research grants, speaker honoraria, and consulting fees from Pfizer, which sponsored the ATTR-ACT trial. Simultaneous with his presentation in Munich, the study results were published online at NEJM.org (doi: 10.1056/NEJMoa1805689). Dr. George reported no financial conflicts.
MUNICH – The big news in the field of heart failure at the annual congress of the European Society of Cardiology concerned an obscure form of the disease traditionally considered rare: transthyretin amyloid cardiomyopathy (TAC).
It turns out that TAC is far more common than previously recognized; it can now be diagnosed and staged noninvasively; and – most important of all – there is for the first time an effective disease-modifying treatment in the form of a novel oral drug called tafamidis, as demonstrated in the Transthyretin Amyloidosis Cardiomyopathy Clinical Trial (ATTR-ACT) presented at the meeting.
“This is the first phase 3 trial that can offer a chance for people with a terrible, severe disease. And within the last year, while the trial was being conducted, it became clear that this disease is much more underdiagnosed than rare,” said Claudio Rapezzi, MD, ATTR-ACT principal investigator and director of the school of cardiovascular diseases at the University of Bologna, Italy.
ATTR-ACT participants randomized to tafamidis showed significant reductions in all-cause mortality and cardiovascular hospitalizations, compared with placebo-treated controls at 30 months follow-up. They also experienced significantly lesser declines in both quality of life as reflected in Kansas City Cardiomyopathy Questionnaire scores and in physical function as captured in 6-minute walk distance.
Designated discussant Jacob George, MD, was over the moon regarding the results.
“This is a pioneering, game-changing trial that is likely to transform the way we diagnose and treat patients with cardiac amyloidosis,” said Dr. George of Kaplan Medical Center in Rehovot, Israel.
“We’re now in an era that, to my opinion, any patient with nonischemic unexplained heart failure should be screened for the presence of amyloidosis because, first, we now know how to prognosticate these patients, and second, we can offer them a real disease-modifying agent,” he added.
An underdiagnosed disease
Transthyretin amyloid cardiomyopathy occurs when transthyretin, a transport protein, becomes destabilized and misfolds, promoting deposition of amyloid fibrils in the myocardium. This results in progressive ventricular wall thickening and stiffness, manifest as restrictive cardiomyopathy and progressive heart failure. The cause of transthyretin destabilization can be either autosomal dominant inheritance of any of more than 100 pathogenic mutations in the transthyretin gene identified to date or a spontaneous wild type protein.
Think of TAC as a sort of dementia of the heart. As Dr. George noted, the cardiac disease bears “remarkable similarities” to Alzheimer’s disease, with both conditions entailing extracellular deposition of amyloid.
In the heritable form of TAC, patients typically present with heart failure symptoms at about age 50-55, while the wild type form becomes symptomatic much later at a mean age of about 75. Average survival from time of diagnosis is only about 3 years.
Recent studies from multiple centers have reported that the prevalence of TAC was 16% in patients undergoing transcatheter aortic valve replacement for severe aortic stenosis, 13% among patients with heart failure with preserved ejection fraction, and 5% in patients who had been presumed to have hypertrophic cardiomyopathy: So, not a rare condition.
“In our clinic, vast and surprising numbers of patients with unexplained nonischemic heart failure are scan positive [for TAC],” according to Dr. George.
Breakthroughs in diagnosis and staging
The echocardiographic red flag for TAC in a patient with heart failure symptoms is symmetric hypertrophy with a normal end-diastolic volume and thickened ventricles. The end-diastolic interventricular septal wall thickness is typically about 15 mm. The left ventricular ejection fraction is typically in the normal range, “but the clue is not the preservation of the ejection fraction, it’s the [normal] quality of the volume,” Dr. Rapezzi said.
A clinical clue suggestive of TAC upon physical examination, even in the absence of heart failure symptoms, is development of bilateral carpal tunnel syndrome in an older man. That’s because the same disease process that results in TAC can involve deposition of amyloid fibrils in peripheral nerves. Indeed, tafamidis is already approved in Europe and Japan under the trade name Vyndaqel as a treatment for familial amyloid polyneuropathy. For TAC, however, tafamidis remains investigational with fast-track status provided by both the Food and Drug Administration and the European Medicines Agency.
When TAC is suspected, it’s no longer necessary to subject patients to an onerous myocardial biopsy. Total body scintigraphy with bone tracers has been shown to be nearly as sensitive and specific as biopsy for the diagnosis.
Staging can now be done noninvasively as well. Investigators at the U.K. National Amyloidosis Centre recently reported that patients with TAC can be accurately staged using two biomarkers: N-terminal pro-B-type natriuretic peptide (NT-proBNP) and estimated glomerular filtration rate (eGFR). In their series of 869 patients with TAC, median survival for those with stage I disease as defined by their protocol was 69 months, compared with 47 months for stage II disease and 24 months for those with stage III disease. This simple U.K. staging system was then validated in a separate French cohort of TAC patients (Eur Heart J. 2018 Aug 7;39[30]:2799-806).
The ATTR-ACT trial
Dr. Rapezzi reported on 441 patients with TAC who were randomized to oral tafamidis at either 20 mg or 80 mg per day or placebo and followed prospectively for 30 months in the 13-country, double-blind, phase 3 trial. At 30 months, all-cause mortality was 29.5% in patients who received tafamidis, compared with 42.9% in controls, for a 30% relative risk reduction. The rate of cardiovascular hospitalizations was 0.48 per year with tafamidis, compared with 0.70 per year with placebo, for a 38% relative risk reduction. The mortality benefit didn’t achieve significance until 15-18 months into the trial, as to be expected given tafamidis’ mechanism of action, which involves binding to transthyretin, gradually stabilizing it, and curbing amyloid fibril deposition.
Of note, the benefit was similar regardless of the dose used and whether patients had hereditary or wild type TAC.
Tafamidis proved safe and well tolerated, with a side-effect profile similar to placebo. While diarrhea and urinary tract infections have been an issue in tafamidis-treated patients with familial amyloid polyneuropathy, these adverse events were actually less common in TAC patients who received tafamidis than with placebo, according to Dr. Rapezzi.
A key point, the cardiologist emphasized, is that the benefits of active treatment were greatest in patients with earlier-stage disease. Therefore it’s vital that the diagnosis of TAC be made early, with prompt initiation of treatment to follow, in order to catch the disease at a more reversible stage. That could mean there will be a whole lot more bone scintigraphy being done in patients with unexplained nonischemic heart failure.
Dr. Rapezzi reported receiving research grants, speaker honoraria, and consulting fees from Pfizer, which sponsored the ATTR-ACT trial. Simultaneous with his presentation in Munich, the study results were published online at NEJM.org (doi: 10.1056/NEJMoa1805689). Dr. George reported no financial conflicts.
REPORTING FROM THE ESC CONGRESS 2018
Key clinical point: Tafamidis is the first-ever proven disease-modifying therapy for patients with a rapidly progressive form of cardiomyopathy.
Major finding: .
Study details: This 13-country, randomized, phase 3, double-blind trial included 441 patients with transthyretin amyloid cardiomyopathy.
Disclosures: The presenter reported receiving research grants, speaker honoraria, and consultant fees from Pfizer, which sponsored the ATTR-ACT trial.
Coronary artery calcium scoring: Its practicality and clinical utility in primary care
The United States has seen a decline in fatal myocardial infarctions, largely thanks to early detection of coronary artery disease. Current guidelines on assessment of cardiovascular risk still rely on the traditional 10-year risk model in clinical practice. However, the predictive value of this approach is only moderate, and many coronary events occur in people considered to be at low or intermediate risk.
Coronary artery calcium scoring has emerged as a means of risk stratification by direct measurement of disease. Primary care providers are either using it or are seeing it used by consulting physicians, and its relatively low cost and ease of performance have contributed to its widespread use. However, downstream costs, radiation exposure, and lack of randomized controlled trials have raised concerns.
This article reviews the usefulness and pitfalls of coronary artery calcium scoring, providing a better understanding of the test, its limitations, and the interpretation of results.
ATHEROSCLEROSIS AND CALCIUM
As the calcium deposits grow, they can be detected by imaging tests such as computed tomography (CT), and quantified to assess the extent of disease.4
CALCIFICATION AND CORONARY ARTERY DISEASE
Coronary calcification occurs almost exclusively in atherosclerosis. Several autopsy studies5,6 and histopathologic studies7 have shown a direct relationship between the extent of calcification and atherosclerotic disease.
Sangiorgi et al7 performed a histologic analysis of 723 coronary artery segments. The amount of calcium correlated well with the area of plaque:
- r = 0.89, P < .0001 in the left anterior descending artery
- r = 0.7, P < .001 in the left circumflex artery
- r = 0.89, P < .0001 in the right coronary artery.
Coronary artery calcium has also been associated with obstructive coronary artery disease in studies using intravascular ultrasonography and optical coherence tomography.8,9
TECHNICAL INFORMATION ABOUT THE TEST
First-generation CT scanners used for calcium scoring in the 1980s were electron-beam systems in which a stationary x-ray tube generated an oscillating electron beam, which was reflected around the patient table.10 A single, stationary detector ring captured the images.
These systems have been replaced by multidetector scanners, in which the x-ray tube and multiple rows of detectors are combined in a gantry that rotates at high speed around the patient.
Coronary calcium is measured by noncontrast CT of the heart. Thus, there is no risk of contrast-induced nephropathy or allergic reactions. Images are acquired while the patient holds his or her breath for 3 to 5 seconds. Electrocardiographic gating is used to reduce motion artifact.11,12 With modern scanners, the effective radiation dose associated with calcium testing is as low as 0.5 to 1.5 mSv,13,14 ie, about the same dose as that with mammography. The entire test takes 10 to 15 minutes.
The results fall into 4 categories, which correlate with the severity of coronary artery disease, ranging from no significant disease to severe disease (Table 1). Other scores, which are not commonly used, include the calcium volume score16 and the calcium mass score.17 Figure 2 shows a screenshot from a coronary artery calcium scoring program.
CALCIUM SCORING AS A DIAGNOSTIC TOOL
Early multicenter studies evaluated the utility of calcium scoring to predict coronary stenosis in patients who underwent both cardiac CT and coronary angiography. The sensitivity of calcium scoring for angiographically significant disease was high (95%), but its specificity was low (about 44%).18
Budoff et al,19 reviewing these and subsequent results, concluded that the value of calcium scoring is its high negative predictive value (about 98%); a negative score (no calcification) is strongly associated with the absence of obstructive coronary disease.
Blaha et al20 concluded that a score of 0 would indicate that the patient had a low risk of cardiovascular disease. A test with these characteristics is helpful in excluding cardiovascular disease or at least in determining that it is less likely to be present in a patient deemed to be at intermediate risk.
CALCIUM SCORING AS A PROGNOSTIC TOOL
Early on, investigators recognized the value of calcium scoring in predicting the risk of future cardiovascular events and death.21–25
Predicting cardiovascular events
Pletcher et al21 performed a meta-analysis of studies that measured calcification in asymptomatic patients with subsequent follow-up. The summary-adjusted relative risk of cardiac events such as myocardial infarction, coronary artery revascularization, and coronary heart disease-related death rose with the calcium score:
- 2.1 (95% confidence interval [CI] 1.6–2.9) with a score of 1 to 100
- 4.2 (95% CI 2.5–7.2) with scores of 101 to 400
- 7.2 (95% CI 3.9-13.0) with scores greater than 400.
The meta-analysis was limited in that it included only 4 studies, which were observational.
Kavousi et al,22 in a subsequent meta-analysis of 6,739 women at low risk of atherosclerotic cardiovascular disease based on the American College of Cardiology/American Heart Association (ACC/AHA) pooled cohort equation (10-year risk < 7.5%), found that 36.1% had calcium scores greater than 0. Compared with those whose score was 0, those with higher scores had a higher risk of atherosclerotic cardiovascular disease events. The incidence rates per 1,000 person-years were 1.41 vs 4.33 (relative risk 2.92, 95% CI 2.02–3.83; multivariable-adjusted hazard ratio 2.04, 95% CI 1.44–2.90). This study was limited because the population was mostly of European descent, making it less generalizable to non-European populations.
Calcium scoring has also been shown to be a strong predictor of incident cardiovascular events across different races beyond traditional risk factors such as hypertension, hyperlipidemia, and tobacco use.
Detrano et al,23 in a study of 6,722 patients with diverse ethnic backgrounds, found that the adjusted risk of a coronary event was increased by a factor of 7.73 for calcium scores between 101 and 300 and by a factor of 9.67 for scores above 300 (P < .001). A limitation of this study was that the patients and physicians were informed of the scores, which could have led to bias.
Carr et al24 found an association between calcium and coronary heart disease in a younger population (ages 32–46). In 12.5 years of follow-up, the hazard ratio for cardiovascular events increased exponentially with the calcium score:
- 2.6 (95% CI 1.0–5.7, P = .03) with calcium scores of 1 through 19
- 9.8 (95% CI 4.5–20.5, P < .001) with scores greater than 100.
Predicting mortality
Budoff et al,25 in an observational study of 25,253 patients, found coronary calcium to be an independent predictor of mortality in a multivariable model controlling for age, sex, ethnicity, and cardiac risk factors (model chi-square = 2,017, P < .0001). However, most of the patients were already known to have cardiac risk factors, making the study findings less generalizable to the general population.
Nasir et al26 found that mortality rates rose with the calcium score in a study with 44,052 participants. The annualized mortality rates per 1,000 person-years were:
- 0.87 (95% CI 0.72–1.06) with a score of 0
- 2.97 (95% CI 2.61–3.37) with scores of 1–100
- 6.90 (95% CI 6.02–7.90) with scores of 101–400
- 17.68 (95% CI 5.93–19.62) with scores higher than 400.
The mortality rate also rose with the number of traditional risk factors present, ie, current tobacco use, dyslipidemia, diabetes mellitus, hypertension, and family history of coronary artery disease. Interestingly, those with no risk factors but a calcium score greater than 400 had a higher mortality rate than those with no coronary calcium but more than 3 risk factors (16.89 per 1,000 person-years vs 2.72 per 1,000 person years). As in the previous study, the patient population that was analyzed was at high risk and therefore the findings are not generalizable.
Shaw et al27 found that patients without symptoms but with elevated coronary calcium scores had higher all-cause mortality rates at 15 years than those with a score of 0. The difference remained significant after Cox regression was performed, adjusting for traditional risk factors.
Coronary artery calcium scoring vs other risk-stratification methods
Current guidelines on assessing risk still rely on the traditional 10-year risk model in clinical practice.25 Patients are thus classified as being at low, intermediate, or high risk based on their probability of developing a cardiovascular event or cardiovascular disease-related death in the subsequent 10 years.
However, the predictive value of this approach is only moderate,28 and a significant number of cardiovascular events, including sudden cardiac death, occur in people who were believed to be at low or intermediate risk according to traditional risk factor-based predictions. Because risk scores are strongly influenced by age,29 they are least reliable in young adults.30
Akosah et al31 reviewed the records of 222 young adults (women age 55 or younger, men age 65 or younger) who presented with their first myocardial infarction, and found that only 25% would have qualified for primary prevention pharmacologic treatments according to the National Cholesterol Education Program III guidelines.32,33 Similar findings have been reported regarding previous versions of the risk scores.33
Thus, risk predictions based exclusively on traditional risk factors are not sensitive for detecting young individuals at increased risk, and lead to late treatment of young adults with atherosclerosis, which may be a less effective strategy.34
The reliance on age in risk algorithms also results in low specificity in elderly adults. Using risk scores, elderly adults are systematically stratified in higher risk categories, expanding the indication for statin therapy to almost all men age 65 or older regardless of their actual vascular health, according to current clinical practice guidelines.35,36
Risk scores are based on self-reported history and single-day measurements, since this kind of information is readily available to the physician in the clinic. Moreover, our knowledge about genetic and epigenetic factors associated with the development of atherosclerosis is still in its infancy, with current guidelines not supporting genetic testing as part of cardiovascular risk assessment.37 Thus, a reliable measure of an individual’s lifelong exposure to a number of environmental and genetic factors that may affect cardiovascular health appears unfeasible.
Atherosclerosis is a process in which interactions between genetic, epigenetic, environmental, and traditional risk factors result in subclinical inflammation that could develop into clinically significant disease. Therefore, subclinical coronary atherosclerosis has been shown to be a strong predictor of future incident cardiovascular disease events and death. Thus, alternative approaches that directly measure disease, such as calcium scoring, may help further refine risk stratification of cardiovascular disease.
The MESA trial (Multi-Ethnic Study of Atherosclerosis), for instance, in 6,814 participants, found coronary calcium to provide better discrimination and risk reclassification than the ankle-brachial index, high sensitivity C-reactive protein level, and family history.38 Coronary calcium also had the highest incremental improvement of the area under the receiver operating curve when added to the Framingham Risk Score (0.623 vs 0.784).
Reclassifying cardiovascular risk also has implications regarding whether to start therapies such as statins and aspirin.
For considering statin therapy
Nasir et al39 showed that, in patients eligible for statin therapy by the pooled cohort equation, the absence of coronary artery calcium reclassified approximately one-half of candidates as not eligible for statin therapy. The number needed to treat to prevent an atherosclerotic cardiovascular event in the population who were recommended a statin was 64 with a calcium score of 0, and 24 with a calcium score greater than 100. In the population for whom a statin was considered, the number needed to treat was 223 with a calcium score of 0 and 46 for those with a score greater than 100. Moreover, 57% of intermediate-risk patients and 41% of high-risk patients based on the Framingham Risk Score were found to have a calcium score of 0, implying that these patients may actually be at a lower risk.
The Society of Cardiovascular Computed Tomography guidelines40 say that statin therapy can be considered in patients who have a calcium score greater than 0.
For considering aspirin therapy
Miedema et al41 studied the role of coronary artery calcium in guiding aspirin therapy in 4,229 participants in the MESA trial who were not taking aspirin at baseline. Those with a calcium score higher than 100 had a number needed to treat of 173 in the group with a Framingham Risk Score less than 10% and 92 with a Framingham Risk Score of 10% or higher. The estimated number needed to harm for a major bleeding event was 442. For those who had a score of 0, the estimated number needed to treat was 2,036 for a Framingham Risk Score less than 10% and 808 for a Framingham Risk Score of 10% or higher, with an estimated number needed to harm of 442 for a major bleeding event.
The Society of Cardiovascular Computed Tomography guidelines40 recommend considering aspirin therapy for patients with a coronary calcium score of more than 100.
McClelland et al42 developed a MESA risk score to predict 10-year risk of coronary heart disease using the traditional risk factors along with coronary calcium. The score was validated externally with 2 separate longitudinal studies. Thus, this may serve as another tool to help providers further risk-stratify patients.
COST-EFFECTIVENESS OF THE TEST
As coronary calcium measurement began to be widely used, concerns were raised about the lack of data on its cost-effectiveness.
Cost-effectiveness depends not only on patient selection but also on the cost of therapy. For example, if the cost of a generic statin is $85 per year, then calcium scoring would not be beneficial. However, if the cost of a statin is more than $200, then calcium scoring would be much more cost-effective, offering a way to avoid treating some patients who do not need to be treated.43
Hong et al43 showed that coronary calcium testing was cost-effective when the patient and physician share decision-making about initiating statin therapy. This is especially important if the patient has financial limitations, is concerned about side effects, or wants to avoid taking unnecessary medications.
RISKS AND DOWNSIDES OF CALCIUM SCORING
According to some reports, $8.5 billion is spent annually for low-value care.44 Many of the 80 million CT scans performed annually in the United States are believed to be unnecessary and may lead to additional testing to investigate incidental findings.45
Growing use of coronary calcium measurement has raised similar concerns about radiation exposure, healthcare costs, and increased downstream testing triggered by the detection of incidental noncardiac findings. For instance, Onuma et al46 reported that, in 503 patients undergoing CT to evaluate coronary artery disease, noncardiac findings were seen in 58.1% of them, but only 22.7% of the 503 had clinically significant findings.
Some of these concerns have been addressed. Modern scanners can acquire images in only a few seconds, entailing lower radiation doses than in the past.13,14 The cost of the test is currently less than $100 in many US metropolitan areas.47 However, further studies are needed to adequately and cost-effectively guide follow-up imaging of incidental noncardiac findings.48
An important limitation of calcium scoring for risk assessment is that no randomized controlled trial has evaluated the impact of preventive interventions guided by calcium scores on hard event outcomes. It can be argued that there have been plenty of observational studies that have shown the benefit of coronary calcium scoring when judiciously done in the appropriate population.49 Similarly, no randomized controlled trial has tested the pooled cohort equation and the application of statins based on its use with the current guidelines. The feasibility and cost of a large randomized controlled trial to assess outcomes after coronary artery calcium measurement must also be considered.
Another limitation of coronary calcium scoring is that it cannot rule out the presence of noncalcified atherosclerotic plaque, which often is more unstable and prone to rupture.
In addition, calcification in the coronary vascular bed (even if severe) does not necessarily mean there is clinically relevant coronary stenosis. For instance, an asymptomatic patient could have a coronary artery calcium score higher than 100 and then get a coronary angiogram that reveals only a 30% lesion in the left anterior descending coronary artery. This is because accumulation of (calcified) plaque in the vessel wall is accommodated by expansion of vessel diameter, maintaining luminal dimensions (positive remodeling). By definition, this patient does have coronary artery disease but would be best served by medical management. This could have been determined without an invasive test in an otherwise asymptomatic patient. Thus, performing coronary angiography based on a coronary artery calcium score alone would not have changed this patient’s management and may have exposed the patient to risks of procedural complications, in addition to extra healthcare costs. Therefore, the presence or absence of symptoms should guide the clinician on whether to pursue stress testing for invasive coronary angiography based on the appropriate use criteria.50,51
WHO SHOULD BE TESTED?
In the ACC/AHA 2013 guidelines,37 coronary calcium scoring has a class IIB recommendation in scenarios where it may appear that the risk-based treatment decision is uncertain after formal risk estimation has been done. As discussed above, a score higher than 100 could be a rationale for starting aspirin therapy, and a score higher than 0 for statin therapy. The current guidelines also mention that the coronary calcium score is comparable to other predictors such as the C-reactive protein level and the ankle-brachial index.
Compared with the ACC/AHA guidelines, the 2016 Society of Cardiovascular Computed Tomography guidelines and expert consensus recently have added more specifics in terms of using this test for asymptomatic patients at intermediate risk (10-year risk of atherosclerotic cardiovascular disease 5%–20%) and in selected patients with a family history of premature coronary artery disease and 10-year risk less than 5%.40,52 The 2010 ACC/AHA guidelines were more specific, offering a class IIA recommendation for patients who were at intermediate risk (Framingham Risk Score 10%–20%).53
The ACC/AHA cited cost and radiation exposure as reasons they did not give coronary calcium measurement a stronger recommendation.37 However, as data continue to come in, the guidelines may change, especially since low-dose radiation tools are being used for cancer screening (lungs and breast) and since the cost has declined over the past decade.
OUR APPROACH
Given the negative predictive value of the coronary calcium score, our approach has been to use this test in asymptomatic patients who are found to be at intermediate risk of atherosclerotic cardiovascular disease based on the ACC/AHA risk calculation and are reluctant to start pharmacologic therapy, or who want a more personalized measure of coronary artery disease. This is preceded by a lengthy patient-physician discussion about the risks and benefits of the test.54
The patient’s risk can then be further clarified and possibly reclassified as either low or high if it doesn’t remain intermediate. A discussion can then take place on potentially starting pharmacologic therapy, intensive lifestyle modifications, or both.54,55 If an electronic medical record is available, CT results can be shown to the patient in the office to point out coronary calcifications. Seeing the lesions may serve an as additional motivating factor as patients embark on primary preventive efforts.56
Below, we describe cases of what we would consider appropriate and inappropriate use of coronary artery calcium scoring.
Example 1
A 55-year-old man presents for an annual physical and is found to have a 10-year risk of atherosclerotic cardiovascular disease of 7%, placing him in the intermediate-risk category. Despite an extensive conversation about lifestyle modifications and pharmacologic therapy, he is reluctant to initiate these measures. He is otherwise asymptomatic. Would calcium scoring be reasonable?
Yes, it would be reasonable to perform coronary artery calcium scoring in an otherwise asymptomatic man to help reclassify his risk for a coronary vascular event. The objective data provided by the test could motivate the patient to undertake primary prevention efforts or, if his score is 0, to show that he may not need drug therapy.
Example 2
A 55-year-old man who has a family history of coronary artery disease, is an active smoker, and has diabetes mellitus presents to the clinic with 2 months of exertional chest pain that resolves with rest. Would coronary artery calcium scoring be reasonable?
This patient is symptomatic and is at high risk of coronary artery disease. Statin therapy is already indicated in the AHA/ACC guidelines, since he has diabetes. Therefore, calcium scoring would not be helpful, as it would not change this patient’s management. Instead, he would be best served by stress testing or coronary angiography based on the stability of his symptoms and cardiac biomarkers.
Example 3
A 30-year-old woman with no medical history presents with on-and-off chest pain at both exertion and rest. Her electrocardiogram is unremarkable, and cardiac enzyme tests are negative. Would coronary calcium scoring be reasonable?
This young patient’s story is not typical for coronary artery disease. Therefore, she has a low pretest probability of obstructive coronary artery disease. Moreover, calcium scoring may not be helpful because at her young age there has not been enough time for calcification to develop (median age is the fifth decade of life). Thus, she would be exposed to radiation unnecessarily at a young age.
What to do with an elevated calcium score?
Coronary artery calcification is now being incidentally detected as patients undergo CT for other reasons such as screening for lung cancer based on the US Preventive Services Task Force guidelines. Patients may also get the test done on their own and then present to a provider with an elevated score.
It is important to consider the entire clinical scenario in such patients and not just the score. If a patient presents with an elevated calcium score but has no symptoms and falls in the intermediate-risk group, there is evidence to suggest that he or she should be started on statin or aspirin therapy or both.
As mentioned above, an abnormal test result does not mean that the patient should undergo more-invasive testing such as cardiac catheterization or even stress testing, especially if he or she has no symptoms. However, if the patient is symptomatic, then further cardiac evaluation would be recommended.
SUMMARY
Measuring coronary artery calcium has been found to be valuable in detecting coronary artery disease and in predicting cardiovascular events and death. The test is relatively easy to perform, with newer technology allowing for less radiation and cost. It serves as a more personalized measure of disease and can help facilitate patient-physician discussions about starting pharmacologic therapy, especially if a patient is reluctant.
Currently, coronary calcium scoring has a class IIB recommendation in scenarios in which the risk-based treatment decision is uncertain after formal risk estimation has been done according to the ACC/AHA guideline. The Society of Cardiovascular Computed Tomography guideline and expert consensus documents are more specific in recommending the test in asymptomatic patients in the intermediate-risk group.
Limitations of calcium scoring include the possibility of unnecessary cardiovascular testing such as cardiac catheterization or stress testing being driven by the calcium score alone, as well as the impact of incidental findings. With increased reporting of the coronary calcium score in patients undergoing CT for lung cancer screening, the score should be interpreted in view of the entire clinical scenario.
- Hansson GK. Inflammation, atherosclerosis and coronary artery disease. N Engl J Med 2005; 352(16):1685–1695. doi:10.1056/NEJM199408183310709
- Ross R. Atherosclerosis—an inflammatory disease. N Engl J Med 1999; 340(2):115–126. doi:10.1056/NEJM199901143400207
- Stary HC, Chandler AB, Dinsmore RE, et al. A definition of advanced types of atherosclerotic lesions and a histological classification of atherosclerosis: a report from the Committee on Vascular Lesions of the Council on Arteriosclerosis, American Heart Association. Arterioscler Thromb Vasc Biol 1995; 15(9):1512–1531. doi:10.1161/atvb.15.9.1512
- Maurovich-Horvat P, Ferencik M, Voros S, Merkely B, Hoffmann U. Comprehensive plaque assessment by coronary CT angiography. Nat Rev Cardiol 2014; 11(7):390–402. doi:10.1038/nrcardio.2014.60
- Eggen DA, Strong JP, McGill HC. Coronary calcification. Relationship to clinically significant coronary lesions and race, sex, and topographic distribution. Circulation 1965; 32(6):948–955. pmid:5845254
- Oliver MF, Samuel E, Morley P, Young GB, Kapur PL. Detection of coronary-artery calcification during life. Lancet 1964; 283(7339):891–895. doi:10.1016/S0140-6736(64)91625-3
- Sangiorgi G, Rumberger JA, Severson A, et al. Arterial calcification and not lumen stenosis is highly correlated with atherosclerotic plaque burden in humans: a histologic study of 723 coronary artery segments using nondecalcifying methodology. J Am Coll Cardiol 1998; 31(1):126–133. doi:10.1016/S0735-1097(97)00443-9
- Baumgart D, Schmermund A, Goerge G, et al. Comparison of electron beam computed tomography with intracoronary ultrasound and coronary angiography for detection of coronary atherosclerosis. J Am Coll Cardiol 1997; 30(1):57–64. pmid:9207621
- Krishnamoorthy P, Vengrenyuk Y, Ueda H, et al. Three-dimensional volumetric assessment of coronary artery calcification in patients with stable coronary artery disease by OCT. EuroIntervention 2017; 13(3):312–319. doi:10.4244/EIJ-D-16-00139
- Budoff MJ, Achenbach S, Blumenthal RS, et al. Assessment of coronary artery disease by cardiac computed tomography: a scientific statement from the American Heart Association Committee on Cardiovascular Imaging and Intervention, Council on Cardiovascular Radiology and Intervention, and Committee on C. Circulation 2006; 114(16):1761–1791. doi:10.1161/CIRCULATIONAHA.106.178458
- Schoepf UJ, Becker CR, Bruening RD, et al. Electrocardiographically gated thin-section CT of the lung. Radiology 1999; 212(3):649–654. doi:10.1148/radiology.212.3.r99se08649
- Abbara S, Arbab-Zadeh A, Callister TQ, et al. SCCT guidelines for performance of coronary computed tomographic angiography: a report of the Society of Cardiovascular Computed Tomography Guidelines Committee. J Cardiovasc Comput Tomogr 2009; 3(3):190–204. doi:10.1016/j.jcct.2009.03.004
- Nakazato R, Dey D, Gutstein A, et al. Coronary artery calcium scoring using a reduced tube voltage and radiation dose protocol with dual-source computed tomography. J Cardiovasc Comput Tomogr 2009; 3(6):394–400. doi:10.1016/j.jcct.2009.10.002
- Hecht HS, De Siqueira MEM, Cham M, et al. Low- vs. standard-dose coronary artery calcium scanning. Eur Heart J Cardiovasc Imaging 2015; 16(4):358–363. doi:10.1093/ehjci/jeu218
- Agatston AS, Janowitz WR, Hildner FJ, Zusmer NR, Viamonte M, Detrano R. Quantification of coronary artery calcium using ultrafast computed tomography. J Am Coll Cardiol 1990; 15(4):827–832. doi:10.1016/0735-1097(90)90282-T
- Nezarat N, Kim M, Budoff M. Role of coronary calcium for risk stratification and prognostication. Curr Treat Options Cardiovasc Med 2017; 19(2):8. doi:10.1007/s11936-017-0509-7
- Callister TQ, Cooil B, Raya SP, Lippolis NJ, Russo DJ, Raggi P. Coronary artery disease: improved reproducibility of calcium scoring with an electron-beam CT volumetric method. Radiology 1998; 208(3):807–814. doi:10.1148/radiology.208.3.9722864
- Budoff MJ, Georgiou D, Brody A, et al. Ultrafast computed tomography as a diagnostic modality in the detection of coronary artery disease: a multicenter study. Circulation 1996; 93(5):898–904. pmid:8598080
- Budoff MJ, Diamond GA, Raggi P, et al. Continuous probabilistic prediction of angiographically significant coronary artery disease using electron beam tomography. Circulation 2002; 105(15):1791–1796. doi:10.1161/01.CIR.0000014483.43921.8C
- Blaha MJ, Cainzos-Achirica M, Greenland P, et al. Role of coronary artery calcium score of zero and other negative risk markers for cardiovascular disease: the Multi-Ethnic Study of Atherosclerosis (MESA). Circulation 2016; 133(9):849–858. doi:10.1161/CIRCULATIONAHA.115.018524
- Pletcher MJ, Tice JA, Pignone M, Browner WS. Using the coronary artery calcium score to predict coronary heart disease events: a systematic review and meta-analysis. Arch Intern Med 2004; 164(12):1285–1292. doi:10.1001/archinte.164.12.1285
- Kavousi M, Desai CS, Ayers C, et al. Prevalence and prognostic implications of coronary artery calcification in low-risk women: a meta-analysis. JAMA 2016; 316(20):2126–2134. doi:10.1001/jama.2016.17020
- Detrano R, Guerci AD, Carr JJ, et al. Coronary calcium as a predictor of coronary events in four racial or ethnic groups. N Engl J Med 2008; 358(13):1336-1345. doi:10.1056/NEJMoa072100
- Carr JJ, Jacobs DR, Terry JG, et al. Association of coronary artery calcium in adults aged 32 to 46 years with incident coronary heart disease and death. JAMA Cardiol 2017; 2(4):391–399. doi:10.1001/jamacardio.2016.5493
- Budoff MJ, Shaw LJ, Liu ST, et al. Long-term prognosis associated with coronary calcification. Observations from a registry of 25,253 patients. J Am Coll Cardiol 2007; 49(18):1860–1870. doi:10.1016/j.jacc.2006.10.079
- Nasir K, Rubin J, Blaha MJ, et al. Interplay of coronary artery calcification and traditional risk factors for the prediction of all-cause mortality in asymptomatic individuals. Circ Cardiovasc Imaging 2012; 5(4):467–473. doi:10.1161/CIRCIMAGING.111.964528
- Shaw LJ, Giambrone AE, Blaha MJ, et al. Long-term prognosis after coronary artery calcification testing in asymptomatic patients: a cohort study. Ann Intern Med 2015; 163(1):14–21. doi:10.7326/M14-0612
- Jackson G, Nehra A, Miner M, et al. The assessment of vascular risk in men with erectile dysfunction: the role of the cardiologist and general physician. Int J Clin Pract 2013; 67(11):1163–1172. doi:10.1111/ijcp.12200
- Cook NR, Paynter NP, Eaton CB, et al. Comparison of the Framingham and Reynolds risk scores for global cardiovascular risk prediction in the multiethnic Women’s Health Initiative. Circulation 2012; 125(14):1748–1756. doi:10.1161/CIRCULATIONAHA.111.075929
- Ford ES, Giles WH, Mokdad AH. The distribution of 10-year risk for coronary heart disease among U.S. adults: findings from the National Health and Nutrition Examination Survey III. J Am Coll Cardiol 2004; 43(10):1791–1796. doi:10.1016/j.jacc.2003.11.061
- Akosah KO, Schaper A, Cogbill C, Schoenfeld P. Preventing myocardial infarction in the young adult in the first place: how do the National Cholesterol Education Panel III guidelines perform? J Am Coll Cardiol 2003;41(9):1475–1479. doi:10.1016/S0735-1097(03)00187-6
- Lloyd-Jones DM, Leip EP, Larson MG, et al. Prediction of lifetime risk for cardiovascular disease by risk factor burden at 50 years of age. Circulation 2006;113(6):791–798. doi:10.1161/CIRCULATIONAHA.105.548206
- Expert Panel on Detection and Treatment of High Blood Cholesterol in Adults. Executive summary of the third report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III). JAMA 2001; 285:24862497. pmid:11368702
- Akosah KO, Gower E, Groon L, Rooney BL, Schaper A. Mild hypercholesterolemia and premature heart disease: do the national criteria underestimate disease risk? J Am Coll Cardiol 2000; 35(5):1178–1184. doi:10.1016/S0735-1097(00)00556-8
- Steinberg D, Grundy SM. The case for treating hypercholesterolemia at an earlier age: moving toward consensus. J Am Coll Cardiol 2012; 60(25):2640–2641. doi:10.1016/j.jacc.2012.09.016
- Martin SS, Blaha MJ, Blankstein R, et al. Dyslipidemia, coronary artery calcium, and incident atherosclerotic cardiovascular disease: Implications for statin therapy from the multi-ethnic study of atherosclerosis. Circulation 2014; 129(1):77–86. doi:10.1161/CIRCULATIONAHA.113.003625
- Goff DC, Lloyd-Jones DM, Bennett G, et al. 2013 ACC/AHA guideline on the assessment of cardiovascular risk: a report of the American College of Cardiology/American Heart Association Task Force on Practice guidelines. Circulation 2014; 129(25 suppl 2):S49–S73. doi:10.1161/01.cir.0000437741.48606.98
- Yeboah J, McClelland RL, Polonsky TS, et al. Comparison of novel risk markers for improvement in cardiovascular risk assessment in intermediate-risk individuals. JAMA 2012; 308(8):788–795. doi:10.1001/jama.2012.9624
- Nasir K, Bittencourt MS, Blaha MJ, et al. Implications of coronary artery calcium testing among statin candidates according to American College of Cardiology/American Heart Association cholesterol management guidelines MESA (Multi-Ethnic Study of Atherosclerosis). J Am Coll Cardiol 2015; 66(15):1657–1668. doi:10.1016/j.jacc.2015.07.066
- Hecht H, Blaha MJ, Berman DS, et al. Clinical indications for coronary artery calcium scoring in asymptomatic patients: expert consensus statement from the Society of Cardiovascular Computed Tomography. J Cardiovasc Comput Tomogr 2017; 11(2):157–168. doi:10.1016/j.jcct.2017.02.010
- Miedema MD, Duprez DA, Misialek JR, et al. Use of coronary artery calcium testing to guide aspirin utilization for primary prevention: estimates from the multi-ethnic study of atherosclerosis. Circ Cardiovasc Qual Outcomes 2014; 7(3):453–460. doi:10.1161/CIRCOUTCOMES.113.000690
- McClelland RL, Jorgensen NW, Budoff M, et al. 10-year coronary heart disease risk prediction using coronary artery calcium and traditional risk factors. J Am Coll Cardiol 2015; 66(15):1643–1653. doi:10.1016/j.jacc.2015.08.035
- Hong JC, Blankstein R, Shaw LJ, et al. Implications of coronary artery calcium testing for treatment decisions among statin candidates according to the ACC/AHA cholesterol management guidelines: a cost-effectiveness analysis. JACC Cardiovasc Imaging 2017; 10(8):938–952. doi:10.1016/j.jcmg.2017.04.014
- Schwartz AL, Landon BE, Elshaug AG, Chernew ME, McWilliams JM. Measuring low-value care in Medicare. JAMA Intern Med 2014; 174(7):1067–1076. doi:10.1001/jamainternmed.2014.1541
- Lehnert BE, Bree RL. Analysis of appropriateness of outpatient CT and MRI referred from primary care clinics at an academic medical center: how critical is the need for improved decision support? J Am Coll Radiol 2010; 7(3):192–197. doi:10.1016/j.jacr.2009.11.010
- Onuma Y, Tanabe K, Nakazawa G, et al. Noncardiac findings in cardiac imaging with multidetector computed tomography. J Am Coll Cardiol 2006; 48(2):402–406. doi:10.1016/j.jacc.2006.04.071
- Hecht HS. Coronary artery calcium scanning: past, present, and future. JACC Cardiovasc Imaging 2015; 8(5):579–596. doi:10.1016/j.jcmg.2015.02.006
- MacHaalany J, Yam Y, Ruddy TD, et al. Potential clinical and economic consequences of noncardiac incidental findings on cardiac computed tomography. J Am Coll Cardiol 2009; 54(16):1533–1541. doi:10.1016/j.jacc.2009.06.026
- McEvoy JW, Martin SS, Blaha MJ, et al. The case for and against a coronary artery calcium trial: means, motive, and opportunity. JACC Cardiovasc Imaging 2016; 9(8):994–1002. doi:10.1016/j.jcmg.2016.03.012
- Patel MR, Bailey SR, Bonow RO, et al. ACCF/SCAI/AATS/AHA/ASE/ASNC/HFSA/HRS/SCCM/SCCT/SCMR/STS 2012 appropriate use criteria for diagnostic catheterization. J Thorac Cardiovasc Surg 2012; 144(1):39–71. doi:10.1016/j.jtcvs.2012.04.013
- Villines TC, Hulten EA, Shaw LJ, et al. Prevalence and severity of coronary artery disease and adverse events among symptomatic patients with coronary artery calcification scores of zero undergoing coronary computed tomography angiography. J Am Coll Cardiol 2011; 58(24):2533–2540. doi:10.1016/j.jacc.2011.10.851
- Hecht HS, Cronin P, Blaha MJ, et al. 2016 SCCT/STR guidelines for coronary artery calcium scoring of noncontrast noncardiac chest CT scans: a report of the Society of Cardiovascular Computed Tomography and Society of Thoracic Radiology. J Thorac Imaging 2017; 32(5):W54–W66. doi:10.1097/RTI.0000000000000287
- Greenland P, Alpert JS, Beller GA, et al. 2010 ACCF/AHA guideline for assessment of cardiovascular risk in asymptomatic adults: executive summary: a report of the American College of Cardiology foundation/American Heart Association task force on practice guidelines. Circulation 2010; 122(25):2748–2764. doi:10.1161/CIR.0b013e3182051bab
- Martin SS, Sperling LS, Blaha MJ, et al. Clinician-patient risk discussion for atherosclerotic cardiovascular disease prevention: importance to implementation of the 2013 ACC/AHA guidelines. J Am Coll Cardiol 2015; 65(13):1361–1368. doi:10.1016/j.jacc.2015.01.043
- Gupta A, Lau E, Varshney R, et al. The identification of calcified coronary plaque is associated with initiation and continuation of pharmacologic and lifestyle preventive therapies: a systematic review and meta-analysis. JACC Cardiovasc Imaging 2017; 10(8):833–842. doi:10.1016/j.jcmg.2017.01.030
- Rozanski A, Gransar H, Shaw LJ, et al. Impact of coronary artery calcium scanning on coronary risk factors and downstream testing: The EISNER (Early Identification of Subclinical Atherosclerosis by Noninvasive Imaging Research) prospective randomized trial. J Am Coll Cardiol 2011; 57(15):1622–1632. doi:10.1016/j.jacc.2011.01.019
The United States has seen a decline in fatal myocardial infarctions, largely thanks to early detection of coronary artery disease. Current guidelines on assessment of cardiovascular risk still rely on the traditional 10-year risk model in clinical practice. However, the predictive value of this approach is only moderate, and many coronary events occur in people considered to be at low or intermediate risk.
Coronary artery calcium scoring has emerged as a means of risk stratification by direct measurement of disease. Primary care providers are either using it or are seeing it used by consulting physicians, and its relatively low cost and ease of performance have contributed to its widespread use. However, downstream costs, radiation exposure, and lack of randomized controlled trials have raised concerns.
This article reviews the usefulness and pitfalls of coronary artery calcium scoring, providing a better understanding of the test, its limitations, and the interpretation of results.
ATHEROSCLEROSIS AND CALCIUM
As the calcium deposits grow, they can be detected by imaging tests such as computed tomography (CT), and quantified to assess the extent of disease.4
CALCIFICATION AND CORONARY ARTERY DISEASE
Coronary calcification occurs almost exclusively in atherosclerosis. Several autopsy studies5,6 and histopathologic studies7 have shown a direct relationship between the extent of calcification and atherosclerotic disease.
Sangiorgi et al7 performed a histologic analysis of 723 coronary artery segments. The amount of calcium correlated well with the area of plaque:
- r = 0.89, P < .0001 in the left anterior descending artery
- r = 0.7, P < .001 in the left circumflex artery
- r = 0.89, P < .0001 in the right coronary artery.
Coronary artery calcium has also been associated with obstructive coronary artery disease in studies using intravascular ultrasonography and optical coherence tomography.8,9
TECHNICAL INFORMATION ABOUT THE TEST
First-generation CT scanners used for calcium scoring in the 1980s were electron-beam systems in which a stationary x-ray tube generated an oscillating electron beam, which was reflected around the patient table.10 A single, stationary detector ring captured the images.
These systems have been replaced by multidetector scanners, in which the x-ray tube and multiple rows of detectors are combined in a gantry that rotates at high speed around the patient.
Coronary calcium is measured by noncontrast CT of the heart. Thus, there is no risk of contrast-induced nephropathy or allergic reactions. Images are acquired while the patient holds his or her breath for 3 to 5 seconds. Electrocardiographic gating is used to reduce motion artifact.11,12 With modern scanners, the effective radiation dose associated with calcium testing is as low as 0.5 to 1.5 mSv,13,14 ie, about the same dose as that with mammography. The entire test takes 10 to 15 minutes.
The results fall into 4 categories, which correlate with the severity of coronary artery disease, ranging from no significant disease to severe disease (Table 1). Other scores, which are not commonly used, include the calcium volume score16 and the calcium mass score.17 Figure 2 shows a screenshot from a coronary artery calcium scoring program.
CALCIUM SCORING AS A DIAGNOSTIC TOOL
Early multicenter studies evaluated the utility of calcium scoring to predict coronary stenosis in patients who underwent both cardiac CT and coronary angiography. The sensitivity of calcium scoring for angiographically significant disease was high (95%), but its specificity was low (about 44%).18
Budoff et al,19 reviewing these and subsequent results, concluded that the value of calcium scoring is its high negative predictive value (about 98%); a negative score (no calcification) is strongly associated with the absence of obstructive coronary disease.
Blaha et al20 concluded that a score of 0 would indicate that the patient had a low risk of cardiovascular disease. A test with these characteristics is helpful in excluding cardiovascular disease or at least in determining that it is less likely to be present in a patient deemed to be at intermediate risk.
CALCIUM SCORING AS A PROGNOSTIC TOOL
Early on, investigators recognized the value of calcium scoring in predicting the risk of future cardiovascular events and death.21–25
Predicting cardiovascular events
Pletcher et al21 performed a meta-analysis of studies that measured calcification in asymptomatic patients with subsequent follow-up. The summary-adjusted relative risk of cardiac events such as myocardial infarction, coronary artery revascularization, and coronary heart disease-related death rose with the calcium score:
- 2.1 (95% confidence interval [CI] 1.6–2.9) with a score of 1 to 100
- 4.2 (95% CI 2.5–7.2) with scores of 101 to 400
- 7.2 (95% CI 3.9-13.0) with scores greater than 400.
The meta-analysis was limited in that it included only 4 studies, which were observational.
Kavousi et al,22 in a subsequent meta-analysis of 6,739 women at low risk of atherosclerotic cardiovascular disease based on the American College of Cardiology/American Heart Association (ACC/AHA) pooled cohort equation (10-year risk < 7.5%), found that 36.1% had calcium scores greater than 0. Compared with those whose score was 0, those with higher scores had a higher risk of atherosclerotic cardiovascular disease events. The incidence rates per 1,000 person-years were 1.41 vs 4.33 (relative risk 2.92, 95% CI 2.02–3.83; multivariable-adjusted hazard ratio 2.04, 95% CI 1.44–2.90). This study was limited because the population was mostly of European descent, making it less generalizable to non-European populations.
Calcium scoring has also been shown to be a strong predictor of incident cardiovascular events across different races beyond traditional risk factors such as hypertension, hyperlipidemia, and tobacco use.
Detrano et al,23 in a study of 6,722 patients with diverse ethnic backgrounds, found that the adjusted risk of a coronary event was increased by a factor of 7.73 for calcium scores between 101 and 300 and by a factor of 9.67 for scores above 300 (P < .001). A limitation of this study was that the patients and physicians were informed of the scores, which could have led to bias.
Carr et al24 found an association between calcium and coronary heart disease in a younger population (ages 32–46). In 12.5 years of follow-up, the hazard ratio for cardiovascular events increased exponentially with the calcium score:
- 2.6 (95% CI 1.0–5.7, P = .03) with calcium scores of 1 through 19
- 9.8 (95% CI 4.5–20.5, P < .001) with scores greater than 100.
Predicting mortality
Budoff et al,25 in an observational study of 25,253 patients, found coronary calcium to be an independent predictor of mortality in a multivariable model controlling for age, sex, ethnicity, and cardiac risk factors (model chi-square = 2,017, P < .0001). However, most of the patients were already known to have cardiac risk factors, making the study findings less generalizable to the general population.
Nasir et al26 found that mortality rates rose with the calcium score in a study with 44,052 participants. The annualized mortality rates per 1,000 person-years were:
- 0.87 (95% CI 0.72–1.06) with a score of 0
- 2.97 (95% CI 2.61–3.37) with scores of 1–100
- 6.90 (95% CI 6.02–7.90) with scores of 101–400
- 17.68 (95% CI 5.93–19.62) with scores higher than 400.
The mortality rate also rose with the number of traditional risk factors present, ie, current tobacco use, dyslipidemia, diabetes mellitus, hypertension, and family history of coronary artery disease. Interestingly, those with no risk factors but a calcium score greater than 400 had a higher mortality rate than those with no coronary calcium but more than 3 risk factors (16.89 per 1,000 person-years vs 2.72 per 1,000 person years). As in the previous study, the patient population that was analyzed was at high risk and therefore the findings are not generalizable.
Shaw et al27 found that patients without symptoms but with elevated coronary calcium scores had higher all-cause mortality rates at 15 years than those with a score of 0. The difference remained significant after Cox regression was performed, adjusting for traditional risk factors.
Coronary artery calcium scoring vs other risk-stratification methods
Current guidelines on assessing risk still rely on the traditional 10-year risk model in clinical practice.25 Patients are thus classified as being at low, intermediate, or high risk based on their probability of developing a cardiovascular event or cardiovascular disease-related death in the subsequent 10 years.
However, the predictive value of this approach is only moderate,28 and a significant number of cardiovascular events, including sudden cardiac death, occur in people who were believed to be at low or intermediate risk according to traditional risk factor-based predictions. Because risk scores are strongly influenced by age,29 they are least reliable in young adults.30
Akosah et al31 reviewed the records of 222 young adults (women age 55 or younger, men age 65 or younger) who presented with their first myocardial infarction, and found that only 25% would have qualified for primary prevention pharmacologic treatments according to the National Cholesterol Education Program III guidelines.32,33 Similar findings have been reported regarding previous versions of the risk scores.33
Thus, risk predictions based exclusively on traditional risk factors are not sensitive for detecting young individuals at increased risk, and lead to late treatment of young adults with atherosclerosis, which may be a less effective strategy.34
The reliance on age in risk algorithms also results in low specificity in elderly adults. Using risk scores, elderly adults are systematically stratified in higher risk categories, expanding the indication for statin therapy to almost all men age 65 or older regardless of their actual vascular health, according to current clinical practice guidelines.35,36
Risk scores are based on self-reported history and single-day measurements, since this kind of information is readily available to the physician in the clinic. Moreover, our knowledge about genetic and epigenetic factors associated with the development of atherosclerosis is still in its infancy, with current guidelines not supporting genetic testing as part of cardiovascular risk assessment.37 Thus, a reliable measure of an individual’s lifelong exposure to a number of environmental and genetic factors that may affect cardiovascular health appears unfeasible.
Atherosclerosis is a process in which interactions between genetic, epigenetic, environmental, and traditional risk factors result in subclinical inflammation that could develop into clinically significant disease. Therefore, subclinical coronary atherosclerosis has been shown to be a strong predictor of future incident cardiovascular disease events and death. Thus, alternative approaches that directly measure disease, such as calcium scoring, may help further refine risk stratification of cardiovascular disease.
The MESA trial (Multi-Ethnic Study of Atherosclerosis), for instance, in 6,814 participants, found coronary calcium to provide better discrimination and risk reclassification than the ankle-brachial index, high sensitivity C-reactive protein level, and family history.38 Coronary calcium also had the highest incremental improvement of the area under the receiver operating curve when added to the Framingham Risk Score (0.623 vs 0.784).
Reclassifying cardiovascular risk also has implications regarding whether to start therapies such as statins and aspirin.
For considering statin therapy
Nasir et al39 showed that, in patients eligible for statin therapy by the pooled cohort equation, the absence of coronary artery calcium reclassified approximately one-half of candidates as not eligible for statin therapy. The number needed to treat to prevent an atherosclerotic cardiovascular event in the population who were recommended a statin was 64 with a calcium score of 0, and 24 with a calcium score greater than 100. In the population for whom a statin was considered, the number needed to treat was 223 with a calcium score of 0 and 46 for those with a score greater than 100. Moreover, 57% of intermediate-risk patients and 41% of high-risk patients based on the Framingham Risk Score were found to have a calcium score of 0, implying that these patients may actually be at a lower risk.
The Society of Cardiovascular Computed Tomography guidelines40 say that statin therapy can be considered in patients who have a calcium score greater than 0.
For considering aspirin therapy
Miedema et al41 studied the role of coronary artery calcium in guiding aspirin therapy in 4,229 participants in the MESA trial who were not taking aspirin at baseline. Those with a calcium score higher than 100 had a number needed to treat of 173 in the group with a Framingham Risk Score less than 10% and 92 with a Framingham Risk Score of 10% or higher. The estimated number needed to harm for a major bleeding event was 442. For those who had a score of 0, the estimated number needed to treat was 2,036 for a Framingham Risk Score less than 10% and 808 for a Framingham Risk Score of 10% or higher, with an estimated number needed to harm of 442 for a major bleeding event.
The Society of Cardiovascular Computed Tomography guidelines40 recommend considering aspirin therapy for patients with a coronary calcium score of more than 100.
McClelland et al42 developed a MESA risk score to predict 10-year risk of coronary heart disease using the traditional risk factors along with coronary calcium. The score was validated externally with 2 separate longitudinal studies. Thus, this may serve as another tool to help providers further risk-stratify patients.
COST-EFFECTIVENESS OF THE TEST
As coronary calcium measurement began to be widely used, concerns were raised about the lack of data on its cost-effectiveness.
Cost-effectiveness depends not only on patient selection but also on the cost of therapy. For example, if the cost of a generic statin is $85 per year, then calcium scoring would not be beneficial. However, if the cost of a statin is more than $200, then calcium scoring would be much more cost-effective, offering a way to avoid treating some patients who do not need to be treated.43
Hong et al43 showed that coronary calcium testing was cost-effective when the patient and physician share decision-making about initiating statin therapy. This is especially important if the patient has financial limitations, is concerned about side effects, or wants to avoid taking unnecessary medications.
RISKS AND DOWNSIDES OF CALCIUM SCORING
According to some reports, $8.5 billion is spent annually for low-value care.44 Many of the 80 million CT scans performed annually in the United States are believed to be unnecessary and may lead to additional testing to investigate incidental findings.45
Growing use of coronary calcium measurement has raised similar concerns about radiation exposure, healthcare costs, and increased downstream testing triggered by the detection of incidental noncardiac findings. For instance, Onuma et al46 reported that, in 503 patients undergoing CT to evaluate coronary artery disease, noncardiac findings were seen in 58.1% of them, but only 22.7% of the 503 had clinically significant findings.
Some of these concerns have been addressed. Modern scanners can acquire images in only a few seconds, entailing lower radiation doses than in the past.13,14 The cost of the test is currently less than $100 in many US metropolitan areas.47 However, further studies are needed to adequately and cost-effectively guide follow-up imaging of incidental noncardiac findings.48
An important limitation of calcium scoring for risk assessment is that no randomized controlled trial has evaluated the impact of preventive interventions guided by calcium scores on hard event outcomes. It can be argued that there have been plenty of observational studies that have shown the benefit of coronary calcium scoring when judiciously done in the appropriate population.49 Similarly, no randomized controlled trial has tested the pooled cohort equation and the application of statins based on its use with the current guidelines. The feasibility and cost of a large randomized controlled trial to assess outcomes after coronary artery calcium measurement must also be considered.
Another limitation of coronary calcium scoring is that it cannot rule out the presence of noncalcified atherosclerotic plaque, which often is more unstable and prone to rupture.
In addition, calcification in the coronary vascular bed (even if severe) does not necessarily mean there is clinically relevant coronary stenosis. For instance, an asymptomatic patient could have a coronary artery calcium score higher than 100 and then get a coronary angiogram that reveals only a 30% lesion in the left anterior descending coronary artery. This is because accumulation of (calcified) plaque in the vessel wall is accommodated by expansion of vessel diameter, maintaining luminal dimensions (positive remodeling). By definition, this patient does have coronary artery disease but would be best served by medical management. This could have been determined without an invasive test in an otherwise asymptomatic patient. Thus, performing coronary angiography based on a coronary artery calcium score alone would not have changed this patient’s management and may have exposed the patient to risks of procedural complications, in addition to extra healthcare costs. Therefore, the presence or absence of symptoms should guide the clinician on whether to pursue stress testing for invasive coronary angiography based on the appropriate use criteria.50,51
WHO SHOULD BE TESTED?
In the ACC/AHA 2013 guidelines,37 coronary calcium scoring has a class IIB recommendation in scenarios where it may appear that the risk-based treatment decision is uncertain after formal risk estimation has been done. As discussed above, a score higher than 100 could be a rationale for starting aspirin therapy, and a score higher than 0 for statin therapy. The current guidelines also mention that the coronary calcium score is comparable to other predictors such as the C-reactive protein level and the ankle-brachial index.
Compared with the ACC/AHA guidelines, the 2016 Society of Cardiovascular Computed Tomography guidelines and expert consensus recently have added more specifics in terms of using this test for asymptomatic patients at intermediate risk (10-year risk of atherosclerotic cardiovascular disease 5%–20%) and in selected patients with a family history of premature coronary artery disease and 10-year risk less than 5%.40,52 The 2010 ACC/AHA guidelines were more specific, offering a class IIA recommendation for patients who were at intermediate risk (Framingham Risk Score 10%–20%).53
The ACC/AHA cited cost and radiation exposure as reasons they did not give coronary calcium measurement a stronger recommendation.37 However, as data continue to come in, the guidelines may change, especially since low-dose radiation tools are being used for cancer screening (lungs and breast) and since the cost has declined over the past decade.
OUR APPROACH
Given the negative predictive value of the coronary calcium score, our approach has been to use this test in asymptomatic patients who are found to be at intermediate risk of atherosclerotic cardiovascular disease based on the ACC/AHA risk calculation and are reluctant to start pharmacologic therapy, or who want a more personalized measure of coronary artery disease. This is preceded by a lengthy patient-physician discussion about the risks and benefits of the test.54
The patient’s risk can then be further clarified and possibly reclassified as either low or high if it doesn’t remain intermediate. A discussion can then take place on potentially starting pharmacologic therapy, intensive lifestyle modifications, or both.54,55 If an electronic medical record is available, CT results can be shown to the patient in the office to point out coronary calcifications. Seeing the lesions may serve an as additional motivating factor as patients embark on primary preventive efforts.56
Below, we describe cases of what we would consider appropriate and inappropriate use of coronary artery calcium scoring.
Example 1
A 55-year-old man presents for an annual physical and is found to have a 10-year risk of atherosclerotic cardiovascular disease of 7%, placing him in the intermediate-risk category. Despite an extensive conversation about lifestyle modifications and pharmacologic therapy, he is reluctant to initiate these measures. He is otherwise asymptomatic. Would calcium scoring be reasonable?
Yes, it would be reasonable to perform coronary artery calcium scoring in an otherwise asymptomatic man to help reclassify his risk for a coronary vascular event. The objective data provided by the test could motivate the patient to undertake primary prevention efforts or, if his score is 0, to show that he may not need drug therapy.
Example 2
A 55-year-old man who has a family history of coronary artery disease, is an active smoker, and has diabetes mellitus presents to the clinic with 2 months of exertional chest pain that resolves with rest. Would coronary artery calcium scoring be reasonable?
This patient is symptomatic and is at high risk of coronary artery disease. Statin therapy is already indicated in the AHA/ACC guidelines, since he has diabetes. Therefore, calcium scoring would not be helpful, as it would not change this patient’s management. Instead, he would be best served by stress testing or coronary angiography based on the stability of his symptoms and cardiac biomarkers.
Example 3
A 30-year-old woman with no medical history presents with on-and-off chest pain at both exertion and rest. Her electrocardiogram is unremarkable, and cardiac enzyme tests are negative. Would coronary calcium scoring be reasonable?
This young patient’s story is not typical for coronary artery disease. Therefore, she has a low pretest probability of obstructive coronary artery disease. Moreover, calcium scoring may not be helpful because at her young age there has not been enough time for calcification to develop (median age is the fifth decade of life). Thus, she would be exposed to radiation unnecessarily at a young age.
What to do with an elevated calcium score?
Coronary artery calcification is now being incidentally detected as patients undergo CT for other reasons such as screening for lung cancer based on the US Preventive Services Task Force guidelines. Patients may also get the test done on their own and then present to a provider with an elevated score.
It is important to consider the entire clinical scenario in such patients and not just the score. If a patient presents with an elevated calcium score but has no symptoms and falls in the intermediate-risk group, there is evidence to suggest that he or she should be started on statin or aspirin therapy or both.
As mentioned above, an abnormal test result does not mean that the patient should undergo more-invasive testing such as cardiac catheterization or even stress testing, especially if he or she has no symptoms. However, if the patient is symptomatic, then further cardiac evaluation would be recommended.
SUMMARY
Measuring coronary artery calcium has been found to be valuable in detecting coronary artery disease and in predicting cardiovascular events and death. The test is relatively easy to perform, with newer technology allowing for less radiation and cost. It serves as a more personalized measure of disease and can help facilitate patient-physician discussions about starting pharmacologic therapy, especially if a patient is reluctant.
Currently, coronary calcium scoring has a class IIB recommendation in scenarios in which the risk-based treatment decision is uncertain after formal risk estimation has been done according to the ACC/AHA guideline. The Society of Cardiovascular Computed Tomography guideline and expert consensus documents are more specific in recommending the test in asymptomatic patients in the intermediate-risk group.
Limitations of calcium scoring include the possibility of unnecessary cardiovascular testing such as cardiac catheterization or stress testing being driven by the calcium score alone, as well as the impact of incidental findings. With increased reporting of the coronary calcium score in patients undergoing CT for lung cancer screening, the score should be interpreted in view of the entire clinical scenario.
The United States has seen a decline in fatal myocardial infarctions, largely thanks to early detection of coronary artery disease. Current guidelines on assessment of cardiovascular risk still rely on the traditional 10-year risk model in clinical practice. However, the predictive value of this approach is only moderate, and many coronary events occur in people considered to be at low or intermediate risk.
Coronary artery calcium scoring has emerged as a means of risk stratification by direct measurement of disease. Primary care providers are either using it or are seeing it used by consulting physicians, and its relatively low cost and ease of performance have contributed to its widespread use. However, downstream costs, radiation exposure, and lack of randomized controlled trials have raised concerns.
This article reviews the usefulness and pitfalls of coronary artery calcium scoring, providing a better understanding of the test, its limitations, and the interpretation of results.
ATHEROSCLEROSIS AND CALCIUM
As the calcium deposits grow, they can be detected by imaging tests such as computed tomography (CT), and quantified to assess the extent of disease.4
CALCIFICATION AND CORONARY ARTERY DISEASE
Coronary calcification occurs almost exclusively in atherosclerosis. Several autopsy studies5,6 and histopathologic studies7 have shown a direct relationship between the extent of calcification and atherosclerotic disease.
Sangiorgi et al7 performed a histologic analysis of 723 coronary artery segments. The amount of calcium correlated well with the area of plaque:
- r = 0.89, P < .0001 in the left anterior descending artery
- r = 0.7, P < .001 in the left circumflex artery
- r = 0.89, P < .0001 in the right coronary artery.
Coronary artery calcium has also been associated with obstructive coronary artery disease in studies using intravascular ultrasonography and optical coherence tomography.8,9
TECHNICAL INFORMATION ABOUT THE TEST
First-generation CT scanners used for calcium scoring in the 1980s were electron-beam systems in which a stationary x-ray tube generated an oscillating electron beam, which was reflected around the patient table.10 A single, stationary detector ring captured the images.
These systems have been replaced by multidetector scanners, in which the x-ray tube and multiple rows of detectors are combined in a gantry that rotates at high speed around the patient.
Coronary calcium is measured by noncontrast CT of the heart. Thus, there is no risk of contrast-induced nephropathy or allergic reactions. Images are acquired while the patient holds his or her breath for 3 to 5 seconds. Electrocardiographic gating is used to reduce motion artifact.11,12 With modern scanners, the effective radiation dose associated with calcium testing is as low as 0.5 to 1.5 mSv,13,14 ie, about the same dose as that with mammography. The entire test takes 10 to 15 minutes.
The results fall into 4 categories, which correlate with the severity of coronary artery disease, ranging from no significant disease to severe disease (Table 1). Other scores, which are not commonly used, include the calcium volume score16 and the calcium mass score.17 Figure 2 shows a screenshot from a coronary artery calcium scoring program.
CALCIUM SCORING AS A DIAGNOSTIC TOOL
Early multicenter studies evaluated the utility of calcium scoring to predict coronary stenosis in patients who underwent both cardiac CT and coronary angiography. The sensitivity of calcium scoring for angiographically significant disease was high (95%), but its specificity was low (about 44%).18
Budoff et al,19 reviewing these and subsequent results, concluded that the value of calcium scoring is its high negative predictive value (about 98%); a negative score (no calcification) is strongly associated with the absence of obstructive coronary disease.
Blaha et al20 concluded that a score of 0 would indicate that the patient had a low risk of cardiovascular disease. A test with these characteristics is helpful in excluding cardiovascular disease or at least in determining that it is less likely to be present in a patient deemed to be at intermediate risk.
CALCIUM SCORING AS A PROGNOSTIC TOOL
Early on, investigators recognized the value of calcium scoring in predicting the risk of future cardiovascular events and death.21–25
Predicting cardiovascular events
Pletcher et al21 performed a meta-analysis of studies that measured calcification in asymptomatic patients with subsequent follow-up. The summary-adjusted relative risk of cardiac events such as myocardial infarction, coronary artery revascularization, and coronary heart disease-related death rose with the calcium score:
- 2.1 (95% confidence interval [CI] 1.6–2.9) with a score of 1 to 100
- 4.2 (95% CI 2.5–7.2) with scores of 101 to 400
- 7.2 (95% CI 3.9-13.0) with scores greater than 400.
The meta-analysis was limited in that it included only 4 studies, which were observational.
Kavousi et al,22 in a subsequent meta-analysis of 6,739 women at low risk of atherosclerotic cardiovascular disease based on the American College of Cardiology/American Heart Association (ACC/AHA) pooled cohort equation (10-year risk < 7.5%), found that 36.1% had calcium scores greater than 0. Compared with those whose score was 0, those with higher scores had a higher risk of atherosclerotic cardiovascular disease events. The incidence rates per 1,000 person-years were 1.41 vs 4.33 (relative risk 2.92, 95% CI 2.02–3.83; multivariable-adjusted hazard ratio 2.04, 95% CI 1.44–2.90). This study was limited because the population was mostly of European descent, making it less generalizable to non-European populations.
Calcium scoring has also been shown to be a strong predictor of incident cardiovascular events across different races beyond traditional risk factors such as hypertension, hyperlipidemia, and tobacco use.
Detrano et al,23 in a study of 6,722 patients with diverse ethnic backgrounds, found that the adjusted risk of a coronary event was increased by a factor of 7.73 for calcium scores between 101 and 300 and by a factor of 9.67 for scores above 300 (P < .001). A limitation of this study was that the patients and physicians were informed of the scores, which could have led to bias.
Carr et al24 found an association between calcium and coronary heart disease in a younger population (ages 32–46). In 12.5 years of follow-up, the hazard ratio for cardiovascular events increased exponentially with the calcium score:
- 2.6 (95% CI 1.0–5.7, P = .03) with calcium scores of 1 through 19
- 9.8 (95% CI 4.5–20.5, P < .001) with scores greater than 100.
Predicting mortality
Budoff et al,25 in an observational study of 25,253 patients, found coronary calcium to be an independent predictor of mortality in a multivariable model controlling for age, sex, ethnicity, and cardiac risk factors (model chi-square = 2,017, P < .0001). However, most of the patients were already known to have cardiac risk factors, making the study findings less generalizable to the general population.
Nasir et al26 found that mortality rates rose with the calcium score in a study with 44,052 participants. The annualized mortality rates per 1,000 person-years were:
- 0.87 (95% CI 0.72–1.06) with a score of 0
- 2.97 (95% CI 2.61–3.37) with scores of 1–100
- 6.90 (95% CI 6.02–7.90) with scores of 101–400
- 17.68 (95% CI 5.93–19.62) with scores higher than 400.
The mortality rate also rose with the number of traditional risk factors present, ie, current tobacco use, dyslipidemia, diabetes mellitus, hypertension, and family history of coronary artery disease. Interestingly, those with no risk factors but a calcium score greater than 400 had a higher mortality rate than those with no coronary calcium but more than 3 risk factors (16.89 per 1,000 person-years vs 2.72 per 1,000 person years). As in the previous study, the patient population that was analyzed was at high risk and therefore the findings are not generalizable.
Shaw et al27 found that patients without symptoms but with elevated coronary calcium scores had higher all-cause mortality rates at 15 years than those with a score of 0. The difference remained significant after Cox regression was performed, adjusting for traditional risk factors.
Coronary artery calcium scoring vs other risk-stratification methods
Current guidelines on assessing risk still rely on the traditional 10-year risk model in clinical practice.25 Patients are thus classified as being at low, intermediate, or high risk based on their probability of developing a cardiovascular event or cardiovascular disease-related death in the subsequent 10 years.
However, the predictive value of this approach is only moderate,28 and a significant number of cardiovascular events, including sudden cardiac death, occur in people who were believed to be at low or intermediate risk according to traditional risk factor-based predictions. Because risk scores are strongly influenced by age,29 they are least reliable in young adults.30
Akosah et al31 reviewed the records of 222 young adults (women age 55 or younger, men age 65 or younger) who presented with their first myocardial infarction, and found that only 25% would have qualified for primary prevention pharmacologic treatments according to the National Cholesterol Education Program III guidelines.32,33 Similar findings have been reported regarding previous versions of the risk scores.33
Thus, risk predictions based exclusively on traditional risk factors are not sensitive for detecting young individuals at increased risk, and lead to late treatment of young adults with atherosclerosis, which may be a less effective strategy.34
The reliance on age in risk algorithms also results in low specificity in elderly adults. Using risk scores, elderly adults are systematically stratified in higher risk categories, expanding the indication for statin therapy to almost all men age 65 or older regardless of their actual vascular health, according to current clinical practice guidelines.35,36
Risk scores are based on self-reported history and single-day measurements, since this kind of information is readily available to the physician in the clinic. Moreover, our knowledge about genetic and epigenetic factors associated with the development of atherosclerosis is still in its infancy, with current guidelines not supporting genetic testing as part of cardiovascular risk assessment.37 Thus, a reliable measure of an individual’s lifelong exposure to a number of environmental and genetic factors that may affect cardiovascular health appears unfeasible.
Atherosclerosis is a process in which interactions between genetic, epigenetic, environmental, and traditional risk factors result in subclinical inflammation that could develop into clinically significant disease. Therefore, subclinical coronary atherosclerosis has been shown to be a strong predictor of future incident cardiovascular disease events and death. Thus, alternative approaches that directly measure disease, such as calcium scoring, may help further refine risk stratification of cardiovascular disease.
The MESA trial (Multi-Ethnic Study of Atherosclerosis), for instance, in 6,814 participants, found coronary calcium to provide better discrimination and risk reclassification than the ankle-brachial index, high sensitivity C-reactive protein level, and family history.38 Coronary calcium also had the highest incremental improvement of the area under the receiver operating curve when added to the Framingham Risk Score (0.623 vs 0.784).
Reclassifying cardiovascular risk also has implications regarding whether to start therapies such as statins and aspirin.
For considering statin therapy
Nasir et al39 showed that, in patients eligible for statin therapy by the pooled cohort equation, the absence of coronary artery calcium reclassified approximately one-half of candidates as not eligible for statin therapy. The number needed to treat to prevent an atherosclerotic cardiovascular event in the population who were recommended a statin was 64 with a calcium score of 0, and 24 with a calcium score greater than 100. In the population for whom a statin was considered, the number needed to treat was 223 with a calcium score of 0 and 46 for those with a score greater than 100. Moreover, 57% of intermediate-risk patients and 41% of high-risk patients based on the Framingham Risk Score were found to have a calcium score of 0, implying that these patients may actually be at a lower risk.
The Society of Cardiovascular Computed Tomography guidelines40 say that statin therapy can be considered in patients who have a calcium score greater than 0.
For considering aspirin therapy
Miedema et al41 studied the role of coronary artery calcium in guiding aspirin therapy in 4,229 participants in the MESA trial who were not taking aspirin at baseline. Those with a calcium score higher than 100 had a number needed to treat of 173 in the group with a Framingham Risk Score less than 10% and 92 with a Framingham Risk Score of 10% or higher. The estimated number needed to harm for a major bleeding event was 442. For those who had a score of 0, the estimated number needed to treat was 2,036 for a Framingham Risk Score less than 10% and 808 for a Framingham Risk Score of 10% or higher, with an estimated number needed to harm of 442 for a major bleeding event.
The Society of Cardiovascular Computed Tomography guidelines40 recommend considering aspirin therapy for patients with a coronary calcium score of more than 100.
McClelland et al42 developed a MESA risk score to predict 10-year risk of coronary heart disease using the traditional risk factors along with coronary calcium. The score was validated externally with 2 separate longitudinal studies. Thus, this may serve as another tool to help providers further risk-stratify patients.
COST-EFFECTIVENESS OF THE TEST
As coronary calcium measurement began to be widely used, concerns were raised about the lack of data on its cost-effectiveness.
Cost-effectiveness depends not only on patient selection but also on the cost of therapy. For example, if the cost of a generic statin is $85 per year, then calcium scoring would not be beneficial. However, if the cost of a statin is more than $200, then calcium scoring would be much more cost-effective, offering a way to avoid treating some patients who do not need to be treated.43
Hong et al43 showed that coronary calcium testing was cost-effective when the patient and physician share decision-making about initiating statin therapy. This is especially important if the patient has financial limitations, is concerned about side effects, or wants to avoid taking unnecessary medications.
RISKS AND DOWNSIDES OF CALCIUM SCORING
According to some reports, $8.5 billion is spent annually for low-value care.44 Many of the 80 million CT scans performed annually in the United States are believed to be unnecessary and may lead to additional testing to investigate incidental findings.45
Growing use of coronary calcium measurement has raised similar concerns about radiation exposure, healthcare costs, and increased downstream testing triggered by the detection of incidental noncardiac findings. For instance, Onuma et al46 reported that, in 503 patients undergoing CT to evaluate coronary artery disease, noncardiac findings were seen in 58.1% of them, but only 22.7% of the 503 had clinically significant findings.
Some of these concerns have been addressed. Modern scanners can acquire images in only a few seconds, entailing lower radiation doses than in the past.13,14 The cost of the test is currently less than $100 in many US metropolitan areas.47 However, further studies are needed to adequately and cost-effectively guide follow-up imaging of incidental noncardiac findings.48
An important limitation of calcium scoring for risk assessment is that no randomized controlled trial has evaluated the impact of preventive interventions guided by calcium scores on hard event outcomes. It can be argued that there have been plenty of observational studies that have shown the benefit of coronary calcium scoring when judiciously done in the appropriate population.49 Similarly, no randomized controlled trial has tested the pooled cohort equation and the application of statins based on its use with the current guidelines. The feasibility and cost of a large randomized controlled trial to assess outcomes after coronary artery calcium measurement must also be considered.
Another limitation of coronary calcium scoring is that it cannot rule out the presence of noncalcified atherosclerotic plaque, which often is more unstable and prone to rupture.
In addition, calcification in the coronary vascular bed (even if severe) does not necessarily mean there is clinically relevant coronary stenosis. For instance, an asymptomatic patient could have a coronary artery calcium score higher than 100 and then get a coronary angiogram that reveals only a 30% lesion in the left anterior descending coronary artery. This is because accumulation of (calcified) plaque in the vessel wall is accommodated by expansion of vessel diameter, maintaining luminal dimensions (positive remodeling). By definition, this patient does have coronary artery disease but would be best served by medical management. This could have been determined without an invasive test in an otherwise asymptomatic patient. Thus, performing coronary angiography based on a coronary artery calcium score alone would not have changed this patient’s management and may have exposed the patient to risks of procedural complications, in addition to extra healthcare costs. Therefore, the presence or absence of symptoms should guide the clinician on whether to pursue stress testing for invasive coronary angiography based on the appropriate use criteria.50,51
WHO SHOULD BE TESTED?
In the ACC/AHA 2013 guidelines,37 coronary calcium scoring has a class IIB recommendation in scenarios where it may appear that the risk-based treatment decision is uncertain after formal risk estimation has been done. As discussed above, a score higher than 100 could be a rationale for starting aspirin therapy, and a score higher than 0 for statin therapy. The current guidelines also mention that the coronary calcium score is comparable to other predictors such as the C-reactive protein level and the ankle-brachial index.
Compared with the ACC/AHA guidelines, the 2016 Society of Cardiovascular Computed Tomography guidelines and expert consensus recently have added more specifics in terms of using this test for asymptomatic patients at intermediate risk (10-year risk of atherosclerotic cardiovascular disease 5%–20%) and in selected patients with a family history of premature coronary artery disease and 10-year risk less than 5%.40,52 The 2010 ACC/AHA guidelines were more specific, offering a class IIA recommendation for patients who were at intermediate risk (Framingham Risk Score 10%–20%).53
The ACC/AHA cited cost and radiation exposure as reasons they did not give coronary calcium measurement a stronger recommendation.37 However, as data continue to come in, the guidelines may change, especially since low-dose radiation tools are being used for cancer screening (lungs and breast) and since the cost has declined over the past decade.
OUR APPROACH
Given the negative predictive value of the coronary calcium score, our approach has been to use this test in asymptomatic patients who are found to be at intermediate risk of atherosclerotic cardiovascular disease based on the ACC/AHA risk calculation and are reluctant to start pharmacologic therapy, or who want a more personalized measure of coronary artery disease. This is preceded by a lengthy patient-physician discussion about the risks and benefits of the test.54
The patient’s risk can then be further clarified and possibly reclassified as either low or high if it doesn’t remain intermediate. A discussion can then take place on potentially starting pharmacologic therapy, intensive lifestyle modifications, or both.54,55 If an electronic medical record is available, CT results can be shown to the patient in the office to point out coronary calcifications. Seeing the lesions may serve an as additional motivating factor as patients embark on primary preventive efforts.56
Below, we describe cases of what we would consider appropriate and inappropriate use of coronary artery calcium scoring.
Example 1
A 55-year-old man presents for an annual physical and is found to have a 10-year risk of atherosclerotic cardiovascular disease of 7%, placing him in the intermediate-risk category. Despite an extensive conversation about lifestyle modifications and pharmacologic therapy, he is reluctant to initiate these measures. He is otherwise asymptomatic. Would calcium scoring be reasonable?
Yes, it would be reasonable to perform coronary artery calcium scoring in an otherwise asymptomatic man to help reclassify his risk for a coronary vascular event. The objective data provided by the test could motivate the patient to undertake primary prevention efforts or, if his score is 0, to show that he may not need drug therapy.
Example 2
A 55-year-old man who has a family history of coronary artery disease, is an active smoker, and has diabetes mellitus presents to the clinic with 2 months of exertional chest pain that resolves with rest. Would coronary artery calcium scoring be reasonable?
This patient is symptomatic and is at high risk of coronary artery disease. Statin therapy is already indicated in the AHA/ACC guidelines, since he has diabetes. Therefore, calcium scoring would not be helpful, as it would not change this patient’s management. Instead, he would be best served by stress testing or coronary angiography based on the stability of his symptoms and cardiac biomarkers.
Example 3
A 30-year-old woman with no medical history presents with on-and-off chest pain at both exertion and rest. Her electrocardiogram is unremarkable, and cardiac enzyme tests are negative. Would coronary calcium scoring be reasonable?
This young patient’s story is not typical for coronary artery disease. Therefore, she has a low pretest probability of obstructive coronary artery disease. Moreover, calcium scoring may not be helpful because at her young age there has not been enough time for calcification to develop (median age is the fifth decade of life). Thus, she would be exposed to radiation unnecessarily at a young age.
What to do with an elevated calcium score?
Coronary artery calcification is now being incidentally detected as patients undergo CT for other reasons such as screening for lung cancer based on the US Preventive Services Task Force guidelines. Patients may also get the test done on their own and then present to a provider with an elevated score.
It is important to consider the entire clinical scenario in such patients and not just the score. If a patient presents with an elevated calcium score but has no symptoms and falls in the intermediate-risk group, there is evidence to suggest that he or she should be started on statin or aspirin therapy or both.
As mentioned above, an abnormal test result does not mean that the patient should undergo more-invasive testing such as cardiac catheterization or even stress testing, especially if he or she has no symptoms. However, if the patient is symptomatic, then further cardiac evaluation would be recommended.
SUMMARY
Measuring coronary artery calcium has been found to be valuable in detecting coronary artery disease and in predicting cardiovascular events and death. The test is relatively easy to perform, with newer technology allowing for less radiation and cost. It serves as a more personalized measure of disease and can help facilitate patient-physician discussions about starting pharmacologic therapy, especially if a patient is reluctant.
Currently, coronary calcium scoring has a class IIB recommendation in scenarios in which the risk-based treatment decision is uncertain after formal risk estimation has been done according to the ACC/AHA guideline. The Society of Cardiovascular Computed Tomography guideline and expert consensus documents are more specific in recommending the test in asymptomatic patients in the intermediate-risk group.
Limitations of calcium scoring include the possibility of unnecessary cardiovascular testing such as cardiac catheterization or stress testing being driven by the calcium score alone, as well as the impact of incidental findings. With increased reporting of the coronary calcium score in patients undergoing CT for lung cancer screening, the score should be interpreted in view of the entire clinical scenario.
- Hansson GK. Inflammation, atherosclerosis and coronary artery disease. N Engl J Med 2005; 352(16):1685–1695. doi:10.1056/NEJM199408183310709
- Ross R. Atherosclerosis—an inflammatory disease. N Engl J Med 1999; 340(2):115–126. doi:10.1056/NEJM199901143400207
- Stary HC, Chandler AB, Dinsmore RE, et al. A definition of advanced types of atherosclerotic lesions and a histological classification of atherosclerosis: a report from the Committee on Vascular Lesions of the Council on Arteriosclerosis, American Heart Association. Arterioscler Thromb Vasc Biol 1995; 15(9):1512–1531. doi:10.1161/atvb.15.9.1512
- Maurovich-Horvat P, Ferencik M, Voros S, Merkely B, Hoffmann U. Comprehensive plaque assessment by coronary CT angiography. Nat Rev Cardiol 2014; 11(7):390–402. doi:10.1038/nrcardio.2014.60
- Eggen DA, Strong JP, McGill HC. Coronary calcification. Relationship to clinically significant coronary lesions and race, sex, and topographic distribution. Circulation 1965; 32(6):948–955. pmid:5845254
- Oliver MF, Samuel E, Morley P, Young GB, Kapur PL. Detection of coronary-artery calcification during life. Lancet 1964; 283(7339):891–895. doi:10.1016/S0140-6736(64)91625-3
- Sangiorgi G, Rumberger JA, Severson A, et al. Arterial calcification and not lumen stenosis is highly correlated with atherosclerotic plaque burden in humans: a histologic study of 723 coronary artery segments using nondecalcifying methodology. J Am Coll Cardiol 1998; 31(1):126–133. doi:10.1016/S0735-1097(97)00443-9
- Baumgart D, Schmermund A, Goerge G, et al. Comparison of electron beam computed tomography with intracoronary ultrasound and coronary angiography for detection of coronary atherosclerosis. J Am Coll Cardiol 1997; 30(1):57–64. pmid:9207621
- Krishnamoorthy P, Vengrenyuk Y, Ueda H, et al. Three-dimensional volumetric assessment of coronary artery calcification in patients with stable coronary artery disease by OCT. EuroIntervention 2017; 13(3):312–319. doi:10.4244/EIJ-D-16-00139
- Budoff MJ, Achenbach S, Blumenthal RS, et al. Assessment of coronary artery disease by cardiac computed tomography: a scientific statement from the American Heart Association Committee on Cardiovascular Imaging and Intervention, Council on Cardiovascular Radiology and Intervention, and Committee on C. Circulation 2006; 114(16):1761–1791. doi:10.1161/CIRCULATIONAHA.106.178458
- Schoepf UJ, Becker CR, Bruening RD, et al. Electrocardiographically gated thin-section CT of the lung. Radiology 1999; 212(3):649–654. doi:10.1148/radiology.212.3.r99se08649
- Abbara S, Arbab-Zadeh A, Callister TQ, et al. SCCT guidelines for performance of coronary computed tomographic angiography: a report of the Society of Cardiovascular Computed Tomography Guidelines Committee. J Cardiovasc Comput Tomogr 2009; 3(3):190–204. doi:10.1016/j.jcct.2009.03.004
- Nakazato R, Dey D, Gutstein A, et al. Coronary artery calcium scoring using a reduced tube voltage and radiation dose protocol with dual-source computed tomography. J Cardiovasc Comput Tomogr 2009; 3(6):394–400. doi:10.1016/j.jcct.2009.10.002
- Hecht HS, De Siqueira MEM, Cham M, et al. Low- vs. standard-dose coronary artery calcium scanning. Eur Heart J Cardiovasc Imaging 2015; 16(4):358–363. doi:10.1093/ehjci/jeu218
- Agatston AS, Janowitz WR, Hildner FJ, Zusmer NR, Viamonte M, Detrano R. Quantification of coronary artery calcium using ultrafast computed tomography. J Am Coll Cardiol 1990; 15(4):827–832. doi:10.1016/0735-1097(90)90282-T
- Nezarat N, Kim M, Budoff M. Role of coronary calcium for risk stratification and prognostication. Curr Treat Options Cardiovasc Med 2017; 19(2):8. doi:10.1007/s11936-017-0509-7
- Callister TQ, Cooil B, Raya SP, Lippolis NJ, Russo DJ, Raggi P. Coronary artery disease: improved reproducibility of calcium scoring with an electron-beam CT volumetric method. Radiology 1998; 208(3):807–814. doi:10.1148/radiology.208.3.9722864
- Budoff MJ, Georgiou D, Brody A, et al. Ultrafast computed tomography as a diagnostic modality in the detection of coronary artery disease: a multicenter study. Circulation 1996; 93(5):898–904. pmid:8598080
- Budoff MJ, Diamond GA, Raggi P, et al. Continuous probabilistic prediction of angiographically significant coronary artery disease using electron beam tomography. Circulation 2002; 105(15):1791–1796. doi:10.1161/01.CIR.0000014483.43921.8C
- Blaha MJ, Cainzos-Achirica M, Greenland P, et al. Role of coronary artery calcium score of zero and other negative risk markers for cardiovascular disease: the Multi-Ethnic Study of Atherosclerosis (MESA). Circulation 2016; 133(9):849–858. doi:10.1161/CIRCULATIONAHA.115.018524
- Pletcher MJ, Tice JA, Pignone M, Browner WS. Using the coronary artery calcium score to predict coronary heart disease events: a systematic review and meta-analysis. Arch Intern Med 2004; 164(12):1285–1292. doi:10.1001/archinte.164.12.1285
- Kavousi M, Desai CS, Ayers C, et al. Prevalence and prognostic implications of coronary artery calcification in low-risk women: a meta-analysis. JAMA 2016; 316(20):2126–2134. doi:10.1001/jama.2016.17020
- Detrano R, Guerci AD, Carr JJ, et al. Coronary calcium as a predictor of coronary events in four racial or ethnic groups. N Engl J Med 2008; 358(13):1336-1345. doi:10.1056/NEJMoa072100
- Carr JJ, Jacobs DR, Terry JG, et al. Association of coronary artery calcium in adults aged 32 to 46 years with incident coronary heart disease and death. JAMA Cardiol 2017; 2(4):391–399. doi:10.1001/jamacardio.2016.5493
- Budoff MJ, Shaw LJ, Liu ST, et al. Long-term prognosis associated with coronary calcification. Observations from a registry of 25,253 patients. J Am Coll Cardiol 2007; 49(18):1860–1870. doi:10.1016/j.jacc.2006.10.079
- Nasir K, Rubin J, Blaha MJ, et al. Interplay of coronary artery calcification and traditional risk factors for the prediction of all-cause mortality in asymptomatic individuals. Circ Cardiovasc Imaging 2012; 5(4):467–473. doi:10.1161/CIRCIMAGING.111.964528
- Shaw LJ, Giambrone AE, Blaha MJ, et al. Long-term prognosis after coronary artery calcification testing in asymptomatic patients: a cohort study. Ann Intern Med 2015; 163(1):14–21. doi:10.7326/M14-0612
- Jackson G, Nehra A, Miner M, et al. The assessment of vascular risk in men with erectile dysfunction: the role of the cardiologist and general physician. Int J Clin Pract 2013; 67(11):1163–1172. doi:10.1111/ijcp.12200
- Cook NR, Paynter NP, Eaton CB, et al. Comparison of the Framingham and Reynolds risk scores for global cardiovascular risk prediction in the multiethnic Women’s Health Initiative. Circulation 2012; 125(14):1748–1756. doi:10.1161/CIRCULATIONAHA.111.075929
- Ford ES, Giles WH, Mokdad AH. The distribution of 10-year risk for coronary heart disease among U.S. adults: findings from the National Health and Nutrition Examination Survey III. J Am Coll Cardiol 2004; 43(10):1791–1796. doi:10.1016/j.jacc.2003.11.061
- Akosah KO, Schaper A, Cogbill C, Schoenfeld P. Preventing myocardial infarction in the young adult in the first place: how do the National Cholesterol Education Panel III guidelines perform? J Am Coll Cardiol 2003;41(9):1475–1479. doi:10.1016/S0735-1097(03)00187-6
- Lloyd-Jones DM, Leip EP, Larson MG, et al. Prediction of lifetime risk for cardiovascular disease by risk factor burden at 50 years of age. Circulation 2006;113(6):791–798. doi:10.1161/CIRCULATIONAHA.105.548206
- Expert Panel on Detection and Treatment of High Blood Cholesterol in Adults. Executive summary of the third report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III). JAMA 2001; 285:24862497. pmid:11368702
- Akosah KO, Gower E, Groon L, Rooney BL, Schaper A. Mild hypercholesterolemia and premature heart disease: do the national criteria underestimate disease risk? J Am Coll Cardiol 2000; 35(5):1178–1184. doi:10.1016/S0735-1097(00)00556-8
- Steinberg D, Grundy SM. The case for treating hypercholesterolemia at an earlier age: moving toward consensus. J Am Coll Cardiol 2012; 60(25):2640–2641. doi:10.1016/j.jacc.2012.09.016
- Martin SS, Blaha MJ, Blankstein R, et al. Dyslipidemia, coronary artery calcium, and incident atherosclerotic cardiovascular disease: Implications for statin therapy from the multi-ethnic study of atherosclerosis. Circulation 2014; 129(1):77–86. doi:10.1161/CIRCULATIONAHA.113.003625
- Goff DC, Lloyd-Jones DM, Bennett G, et al. 2013 ACC/AHA guideline on the assessment of cardiovascular risk: a report of the American College of Cardiology/American Heart Association Task Force on Practice guidelines. Circulation 2014; 129(25 suppl 2):S49–S73. doi:10.1161/01.cir.0000437741.48606.98
- Yeboah J, McClelland RL, Polonsky TS, et al. Comparison of novel risk markers for improvement in cardiovascular risk assessment in intermediate-risk individuals. JAMA 2012; 308(8):788–795. doi:10.1001/jama.2012.9624
- Nasir K, Bittencourt MS, Blaha MJ, et al. Implications of coronary artery calcium testing among statin candidates according to American College of Cardiology/American Heart Association cholesterol management guidelines MESA (Multi-Ethnic Study of Atherosclerosis). J Am Coll Cardiol 2015; 66(15):1657–1668. doi:10.1016/j.jacc.2015.07.066
- Hecht H, Blaha MJ, Berman DS, et al. Clinical indications for coronary artery calcium scoring in asymptomatic patients: expert consensus statement from the Society of Cardiovascular Computed Tomography. J Cardiovasc Comput Tomogr 2017; 11(2):157–168. doi:10.1016/j.jcct.2017.02.010
- Miedema MD, Duprez DA, Misialek JR, et al. Use of coronary artery calcium testing to guide aspirin utilization for primary prevention: estimates from the multi-ethnic study of atherosclerosis. Circ Cardiovasc Qual Outcomes 2014; 7(3):453–460. doi:10.1161/CIRCOUTCOMES.113.000690
- McClelland RL, Jorgensen NW, Budoff M, et al. 10-year coronary heart disease risk prediction using coronary artery calcium and traditional risk factors. J Am Coll Cardiol 2015; 66(15):1643–1653. doi:10.1016/j.jacc.2015.08.035
- Hong JC, Blankstein R, Shaw LJ, et al. Implications of coronary artery calcium testing for treatment decisions among statin candidates according to the ACC/AHA cholesterol management guidelines: a cost-effectiveness analysis. JACC Cardiovasc Imaging 2017; 10(8):938–952. doi:10.1016/j.jcmg.2017.04.014
- Schwartz AL, Landon BE, Elshaug AG, Chernew ME, McWilliams JM. Measuring low-value care in Medicare. JAMA Intern Med 2014; 174(7):1067–1076. doi:10.1001/jamainternmed.2014.1541
- Lehnert BE, Bree RL. Analysis of appropriateness of outpatient CT and MRI referred from primary care clinics at an academic medical center: how critical is the need for improved decision support? J Am Coll Radiol 2010; 7(3):192–197. doi:10.1016/j.jacr.2009.11.010
- Onuma Y, Tanabe K, Nakazawa G, et al. Noncardiac findings in cardiac imaging with multidetector computed tomography. J Am Coll Cardiol 2006; 48(2):402–406. doi:10.1016/j.jacc.2006.04.071
- Hecht HS. Coronary artery calcium scanning: past, present, and future. JACC Cardiovasc Imaging 2015; 8(5):579–596. doi:10.1016/j.jcmg.2015.02.006
- MacHaalany J, Yam Y, Ruddy TD, et al. Potential clinical and economic consequences of noncardiac incidental findings on cardiac computed tomography. J Am Coll Cardiol 2009; 54(16):1533–1541. doi:10.1016/j.jacc.2009.06.026
- McEvoy JW, Martin SS, Blaha MJ, et al. The case for and against a coronary artery calcium trial: means, motive, and opportunity. JACC Cardiovasc Imaging 2016; 9(8):994–1002. doi:10.1016/j.jcmg.2016.03.012
- Patel MR, Bailey SR, Bonow RO, et al. ACCF/SCAI/AATS/AHA/ASE/ASNC/HFSA/HRS/SCCM/SCCT/SCMR/STS 2012 appropriate use criteria for diagnostic catheterization. J Thorac Cardiovasc Surg 2012; 144(1):39–71. doi:10.1016/j.jtcvs.2012.04.013
- Villines TC, Hulten EA, Shaw LJ, et al. Prevalence and severity of coronary artery disease and adverse events among symptomatic patients with coronary artery calcification scores of zero undergoing coronary computed tomography angiography. J Am Coll Cardiol 2011; 58(24):2533–2540. doi:10.1016/j.jacc.2011.10.851
- Hecht HS, Cronin P, Blaha MJ, et al. 2016 SCCT/STR guidelines for coronary artery calcium scoring of noncontrast noncardiac chest CT scans: a report of the Society of Cardiovascular Computed Tomography and Society of Thoracic Radiology. J Thorac Imaging 2017; 32(5):W54–W66. doi:10.1097/RTI.0000000000000287
- Greenland P, Alpert JS, Beller GA, et al. 2010 ACCF/AHA guideline for assessment of cardiovascular risk in asymptomatic adults: executive summary: a report of the American College of Cardiology foundation/American Heart Association task force on practice guidelines. Circulation 2010; 122(25):2748–2764. doi:10.1161/CIR.0b013e3182051bab
- Martin SS, Sperling LS, Blaha MJ, et al. Clinician-patient risk discussion for atherosclerotic cardiovascular disease prevention: importance to implementation of the 2013 ACC/AHA guidelines. J Am Coll Cardiol 2015; 65(13):1361–1368. doi:10.1016/j.jacc.2015.01.043
- Gupta A, Lau E, Varshney R, et al. The identification of calcified coronary plaque is associated with initiation and continuation of pharmacologic and lifestyle preventive therapies: a systematic review and meta-analysis. JACC Cardiovasc Imaging 2017; 10(8):833–842. doi:10.1016/j.jcmg.2017.01.030
- Rozanski A, Gransar H, Shaw LJ, et al. Impact of coronary artery calcium scanning on coronary risk factors and downstream testing: The EISNER (Early Identification of Subclinical Atherosclerosis by Noninvasive Imaging Research) prospective randomized trial. J Am Coll Cardiol 2011; 57(15):1622–1632. doi:10.1016/j.jacc.2011.01.019
- Hansson GK. Inflammation, atherosclerosis and coronary artery disease. N Engl J Med 2005; 352(16):1685–1695. doi:10.1056/NEJM199408183310709
- Ross R. Atherosclerosis—an inflammatory disease. N Engl J Med 1999; 340(2):115–126. doi:10.1056/NEJM199901143400207
- Stary HC, Chandler AB, Dinsmore RE, et al. A definition of advanced types of atherosclerotic lesions and a histological classification of atherosclerosis: a report from the Committee on Vascular Lesions of the Council on Arteriosclerosis, American Heart Association. Arterioscler Thromb Vasc Biol 1995; 15(9):1512–1531. doi:10.1161/atvb.15.9.1512
- Maurovich-Horvat P, Ferencik M, Voros S, Merkely B, Hoffmann U. Comprehensive plaque assessment by coronary CT angiography. Nat Rev Cardiol 2014; 11(7):390–402. doi:10.1038/nrcardio.2014.60
- Eggen DA, Strong JP, McGill HC. Coronary calcification. Relationship to clinically significant coronary lesions and race, sex, and topographic distribution. Circulation 1965; 32(6):948–955. pmid:5845254
- Oliver MF, Samuel E, Morley P, Young GB, Kapur PL. Detection of coronary-artery calcification during life. Lancet 1964; 283(7339):891–895. doi:10.1016/S0140-6736(64)91625-3
- Sangiorgi G, Rumberger JA, Severson A, et al. Arterial calcification and not lumen stenosis is highly correlated with atherosclerotic plaque burden in humans: a histologic study of 723 coronary artery segments using nondecalcifying methodology. J Am Coll Cardiol 1998; 31(1):126–133. doi:10.1016/S0735-1097(97)00443-9
- Baumgart D, Schmermund A, Goerge G, et al. Comparison of electron beam computed tomography with intracoronary ultrasound and coronary angiography for detection of coronary atherosclerosis. J Am Coll Cardiol 1997; 30(1):57–64. pmid:9207621
- Krishnamoorthy P, Vengrenyuk Y, Ueda H, et al. Three-dimensional volumetric assessment of coronary artery calcification in patients with stable coronary artery disease by OCT. EuroIntervention 2017; 13(3):312–319. doi:10.4244/EIJ-D-16-00139
- Budoff MJ, Achenbach S, Blumenthal RS, et al. Assessment of coronary artery disease by cardiac computed tomography: a scientific statement from the American Heart Association Committee on Cardiovascular Imaging and Intervention, Council on Cardiovascular Radiology and Intervention, and Committee on C. Circulation 2006; 114(16):1761–1791. doi:10.1161/CIRCULATIONAHA.106.178458
- Schoepf UJ, Becker CR, Bruening RD, et al. Electrocardiographically gated thin-section CT of the lung. Radiology 1999; 212(3):649–654. doi:10.1148/radiology.212.3.r99se08649
- Abbara S, Arbab-Zadeh A, Callister TQ, et al. SCCT guidelines for performance of coronary computed tomographic angiography: a report of the Society of Cardiovascular Computed Tomography Guidelines Committee. J Cardiovasc Comput Tomogr 2009; 3(3):190–204. doi:10.1016/j.jcct.2009.03.004
- Nakazato R, Dey D, Gutstein A, et al. Coronary artery calcium scoring using a reduced tube voltage and radiation dose protocol with dual-source computed tomography. J Cardiovasc Comput Tomogr 2009; 3(6):394–400. doi:10.1016/j.jcct.2009.10.002
- Hecht HS, De Siqueira MEM, Cham M, et al. Low- vs. standard-dose coronary artery calcium scanning. Eur Heart J Cardiovasc Imaging 2015; 16(4):358–363. doi:10.1093/ehjci/jeu218
- Agatston AS, Janowitz WR, Hildner FJ, Zusmer NR, Viamonte M, Detrano R. Quantification of coronary artery calcium using ultrafast computed tomography. J Am Coll Cardiol 1990; 15(4):827–832. doi:10.1016/0735-1097(90)90282-T
- Nezarat N, Kim M, Budoff M. Role of coronary calcium for risk stratification and prognostication. Curr Treat Options Cardiovasc Med 2017; 19(2):8. doi:10.1007/s11936-017-0509-7
- Callister TQ, Cooil B, Raya SP, Lippolis NJ, Russo DJ, Raggi P. Coronary artery disease: improved reproducibility of calcium scoring with an electron-beam CT volumetric method. Radiology 1998; 208(3):807–814. doi:10.1148/radiology.208.3.9722864
- Budoff MJ, Georgiou D, Brody A, et al. Ultrafast computed tomography as a diagnostic modality in the detection of coronary artery disease: a multicenter study. Circulation 1996; 93(5):898–904. pmid:8598080
- Budoff MJ, Diamond GA, Raggi P, et al. Continuous probabilistic prediction of angiographically significant coronary artery disease using electron beam tomography. Circulation 2002; 105(15):1791–1796. doi:10.1161/01.CIR.0000014483.43921.8C
- Blaha MJ, Cainzos-Achirica M, Greenland P, et al. Role of coronary artery calcium score of zero and other negative risk markers for cardiovascular disease: the Multi-Ethnic Study of Atherosclerosis (MESA). Circulation 2016; 133(9):849–858. doi:10.1161/CIRCULATIONAHA.115.018524
- Pletcher MJ, Tice JA, Pignone M, Browner WS. Using the coronary artery calcium score to predict coronary heart disease events: a systematic review and meta-analysis. Arch Intern Med 2004; 164(12):1285–1292. doi:10.1001/archinte.164.12.1285
- Kavousi M, Desai CS, Ayers C, et al. Prevalence and prognostic implications of coronary artery calcification in low-risk women: a meta-analysis. JAMA 2016; 316(20):2126–2134. doi:10.1001/jama.2016.17020
- Detrano R, Guerci AD, Carr JJ, et al. Coronary calcium as a predictor of coronary events in four racial or ethnic groups. N Engl J Med 2008; 358(13):1336-1345. doi:10.1056/NEJMoa072100
- Carr JJ, Jacobs DR, Terry JG, et al. Association of coronary artery calcium in adults aged 32 to 46 years with incident coronary heart disease and death. JAMA Cardiol 2017; 2(4):391–399. doi:10.1001/jamacardio.2016.5493
- Budoff MJ, Shaw LJ, Liu ST, et al. Long-term prognosis associated with coronary calcification. Observations from a registry of 25,253 patients. J Am Coll Cardiol 2007; 49(18):1860–1870. doi:10.1016/j.jacc.2006.10.079
- Nasir K, Rubin J, Blaha MJ, et al. Interplay of coronary artery calcification and traditional risk factors for the prediction of all-cause mortality in asymptomatic individuals. Circ Cardiovasc Imaging 2012; 5(4):467–473. doi:10.1161/CIRCIMAGING.111.964528
- Shaw LJ, Giambrone AE, Blaha MJ, et al. Long-term prognosis after coronary artery calcification testing in asymptomatic patients: a cohort study. Ann Intern Med 2015; 163(1):14–21. doi:10.7326/M14-0612
- Jackson G, Nehra A, Miner M, et al. The assessment of vascular risk in men with erectile dysfunction: the role of the cardiologist and general physician. Int J Clin Pract 2013; 67(11):1163–1172. doi:10.1111/ijcp.12200
- Cook NR, Paynter NP, Eaton CB, et al. Comparison of the Framingham and Reynolds risk scores for global cardiovascular risk prediction in the multiethnic Women’s Health Initiative. Circulation 2012; 125(14):1748–1756. doi:10.1161/CIRCULATIONAHA.111.075929
- Ford ES, Giles WH, Mokdad AH. The distribution of 10-year risk for coronary heart disease among U.S. adults: findings from the National Health and Nutrition Examination Survey III. J Am Coll Cardiol 2004; 43(10):1791–1796. doi:10.1016/j.jacc.2003.11.061
- Akosah KO, Schaper A, Cogbill C, Schoenfeld P. Preventing myocardial infarction in the young adult in the first place: how do the National Cholesterol Education Panel III guidelines perform? J Am Coll Cardiol 2003;41(9):1475–1479. doi:10.1016/S0735-1097(03)00187-6
- Lloyd-Jones DM, Leip EP, Larson MG, et al. Prediction of lifetime risk for cardiovascular disease by risk factor burden at 50 years of age. Circulation 2006;113(6):791–798. doi:10.1161/CIRCULATIONAHA.105.548206
- Expert Panel on Detection and Treatment of High Blood Cholesterol in Adults. Executive summary of the third report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III). JAMA 2001; 285:24862497. pmid:11368702
- Akosah KO, Gower E, Groon L, Rooney BL, Schaper A. Mild hypercholesterolemia and premature heart disease: do the national criteria underestimate disease risk? J Am Coll Cardiol 2000; 35(5):1178–1184. doi:10.1016/S0735-1097(00)00556-8
- Steinberg D, Grundy SM. The case for treating hypercholesterolemia at an earlier age: moving toward consensus. J Am Coll Cardiol 2012; 60(25):2640–2641. doi:10.1016/j.jacc.2012.09.016
- Martin SS, Blaha MJ, Blankstein R, et al. Dyslipidemia, coronary artery calcium, and incident atherosclerotic cardiovascular disease: Implications for statin therapy from the multi-ethnic study of atherosclerosis. Circulation 2014; 129(1):77–86. doi:10.1161/CIRCULATIONAHA.113.003625
- Goff DC, Lloyd-Jones DM, Bennett G, et al. 2013 ACC/AHA guideline on the assessment of cardiovascular risk: a report of the American College of Cardiology/American Heart Association Task Force on Practice guidelines. Circulation 2014; 129(25 suppl 2):S49–S73. doi:10.1161/01.cir.0000437741.48606.98
- Yeboah J, McClelland RL, Polonsky TS, et al. Comparison of novel risk markers for improvement in cardiovascular risk assessment in intermediate-risk individuals. JAMA 2012; 308(8):788–795. doi:10.1001/jama.2012.9624
- Nasir K, Bittencourt MS, Blaha MJ, et al. Implications of coronary artery calcium testing among statin candidates according to American College of Cardiology/American Heart Association cholesterol management guidelines MESA (Multi-Ethnic Study of Atherosclerosis). J Am Coll Cardiol 2015; 66(15):1657–1668. doi:10.1016/j.jacc.2015.07.066
- Hecht H, Blaha MJ, Berman DS, et al. Clinical indications for coronary artery calcium scoring in asymptomatic patients: expert consensus statement from the Society of Cardiovascular Computed Tomography. J Cardiovasc Comput Tomogr 2017; 11(2):157–168. doi:10.1016/j.jcct.2017.02.010
- Miedema MD, Duprez DA, Misialek JR, et al. Use of coronary artery calcium testing to guide aspirin utilization for primary prevention: estimates from the multi-ethnic study of atherosclerosis. Circ Cardiovasc Qual Outcomes 2014; 7(3):453–460. doi:10.1161/CIRCOUTCOMES.113.000690
- McClelland RL, Jorgensen NW, Budoff M, et al. 10-year coronary heart disease risk prediction using coronary artery calcium and traditional risk factors. J Am Coll Cardiol 2015; 66(15):1643–1653. doi:10.1016/j.jacc.2015.08.035
- Hong JC, Blankstein R, Shaw LJ, et al. Implications of coronary artery calcium testing for treatment decisions among statin candidates according to the ACC/AHA cholesterol management guidelines: a cost-effectiveness analysis. JACC Cardiovasc Imaging 2017; 10(8):938–952. doi:10.1016/j.jcmg.2017.04.014
- Schwartz AL, Landon BE, Elshaug AG, Chernew ME, McWilliams JM. Measuring low-value care in Medicare. JAMA Intern Med 2014; 174(7):1067–1076. doi:10.1001/jamainternmed.2014.1541
- Lehnert BE, Bree RL. Analysis of appropriateness of outpatient CT and MRI referred from primary care clinics at an academic medical center: how critical is the need for improved decision support? J Am Coll Radiol 2010; 7(3):192–197. doi:10.1016/j.jacr.2009.11.010
- Onuma Y, Tanabe K, Nakazawa G, et al. Noncardiac findings in cardiac imaging with multidetector computed tomography. J Am Coll Cardiol 2006; 48(2):402–406. doi:10.1016/j.jacc.2006.04.071
- Hecht HS. Coronary artery calcium scanning: past, present, and future. JACC Cardiovasc Imaging 2015; 8(5):579–596. doi:10.1016/j.jcmg.2015.02.006
- MacHaalany J, Yam Y, Ruddy TD, et al. Potential clinical and economic consequences of noncardiac incidental findings on cardiac computed tomography. J Am Coll Cardiol 2009; 54(16):1533–1541. doi:10.1016/j.jacc.2009.06.026
- McEvoy JW, Martin SS, Blaha MJ, et al. The case for and against a coronary artery calcium trial: means, motive, and opportunity. JACC Cardiovasc Imaging 2016; 9(8):994–1002. doi:10.1016/j.jcmg.2016.03.012
- Patel MR, Bailey SR, Bonow RO, et al. ACCF/SCAI/AATS/AHA/ASE/ASNC/HFSA/HRS/SCCM/SCCT/SCMR/STS 2012 appropriate use criteria for diagnostic catheterization. J Thorac Cardiovasc Surg 2012; 144(1):39–71. doi:10.1016/j.jtcvs.2012.04.013
- Villines TC, Hulten EA, Shaw LJ, et al. Prevalence and severity of coronary artery disease and adverse events among symptomatic patients with coronary artery calcification scores of zero undergoing coronary computed tomography angiography. J Am Coll Cardiol 2011; 58(24):2533–2540. doi:10.1016/j.jacc.2011.10.851
- Hecht HS, Cronin P, Blaha MJ, et al. 2016 SCCT/STR guidelines for coronary artery calcium scoring of noncontrast noncardiac chest CT scans: a report of the Society of Cardiovascular Computed Tomography and Society of Thoracic Radiology. J Thorac Imaging 2017; 32(5):W54–W66. doi:10.1097/RTI.0000000000000287
- Greenland P, Alpert JS, Beller GA, et al. 2010 ACCF/AHA guideline for assessment of cardiovascular risk in asymptomatic adults: executive summary: a report of the American College of Cardiology foundation/American Heart Association task force on practice guidelines. Circulation 2010; 122(25):2748–2764. doi:10.1161/CIR.0b013e3182051bab
- Martin SS, Sperling LS, Blaha MJ, et al. Clinician-patient risk discussion for atherosclerotic cardiovascular disease prevention: importance to implementation of the 2013 ACC/AHA guidelines. J Am Coll Cardiol 2015; 65(13):1361–1368. doi:10.1016/j.jacc.2015.01.043
- Gupta A, Lau E, Varshney R, et al. The identification of calcified coronary plaque is associated with initiation and continuation of pharmacologic and lifestyle preventive therapies: a systematic review and meta-analysis. JACC Cardiovasc Imaging 2017; 10(8):833–842. doi:10.1016/j.jcmg.2017.01.030
- Rozanski A, Gransar H, Shaw LJ, et al. Impact of coronary artery calcium scanning on coronary risk factors and downstream testing: The EISNER (Early Identification of Subclinical Atherosclerosis by Noninvasive Imaging Research) prospective randomized trial. J Am Coll Cardiol 2011; 57(15):1622–1632. doi:10.1016/j.jacc.2011.01.019
KEY POINTS
- Coronary artery calcium testing is useful in diagnosing subclinical coronary artery disease and in predicting the risk of future cardiovascular events and death.
- Given the high negative predictive value of the test, it can also serve to reclassify risk in patients beyond traditional risk factors.
- Along with shared decision-making, elevated calcium scores can guide the initiation of statin or aspirin therapy.
- A high score in an asymptomatic patient should not trigger further testing without a comprehensive discussion of the risks and benefits.
Brain abscesses in a 60-year-old man
A 60-year-old man with hypertension and persistent atrial fibrillation refractory to radiofrequency ablation was brought to the hospital in status epilepticus requiring intubation. His wife said that during the past month he had experienced a number of episodic seizures, but due to his busy work schedule he had not sought medical attention. He had also been hospitalized 3 times during the past week for chills, tremors, and fevers with temperatures up to 101°F (38.3°C), and his symptoms had been ascribed to the amiodarone he had been taking for the past 11 days for atrial fibrillation. The amiodarone dose had been decreased to half a tablet after the first 7 days, but his symptoms had continued.
When the patient was able to speak, he denied intravenous drug abuse and claimed to be up to date with vaccinations. Colonoscopy 10 years earlier had been negative. He has no pets, but says that there are stray cats around his home and that he has had contact with cat feces while gardening. He works as a diesel mechanic and is exposed to motor oil and diesel fuel, but denies any direct exposure to carcinogenic chemicals.
On admission, his temperature was 37.7°C (99.9°F), blood pressure 92/69 mm Hg, heart rate 96 beats per minute, respiratory rate 21 per minute, and oxygen saturation 95% on room air and 100% on oxygen at 2 L per minute.
Decerebrate posturing and forced left visual gaze deviation was observed. Oral examination revealed severe decay of multiple teeth, with some teeth broken down to the level of the gingiva, and moderate generalized periodontal disease with heavy plaque and calculi in the gingiva.
The differential diagnosis for intracranial ring-enhancing lesions includes metastasis, abscess, infection in an immunocompromised state (eg, toxoplasmosis), glioblastoma, subacute infarct, neurocysticercosis, lymphoma, demyelination, and resolving hematoma. In our patient, further testing to narrow the differential included lumbar puncture, with results within normal limits, and transthoracic echocardiography, which was negative for endocarditis. A biopsy obtained by craniotomy confirmed the diagnosis of abscess surrounded by reactive glioses.
During his hospitalization, the patient’s antiseizure regimen was lorazepam 1 to 2 mg as needed, levetiracetam 1,500 mg twice daily, and fosphenytoin infusion at 100 mg phenytoin sodium equivalents per minute. Initial antibiotic therapy included ampicillin 2 g intravenously (IV) 4 times daily.
Because of persistent nocturnal fevers with temperatures ranging from 37.8°C (100°F) to 41.2°C (106.2°F), antibiotic coverage was broadened to meropenem 2 g IV every 8 hours. Testing for Toxoplasma gondii, human immunodeficiency virus, and JC polyomavirus was negative. Cerebrospinal fluid culture and abscess cultures were also negative. Blood cultures were eventually positive for Peptostreptococcus micros and Streptococcus constellatus. Based on review of culture results, antibiotic therapy was switched to ceftriaxone 2 g IV twice daily and metronidazole 500 mg IV 3 times daily.
For the dental infection, the patient underwent surgical irrigation and debridement with full dental extraction for multiple dental abscesses.
His regimen for seizure control was changed to phenytoin and valproic acid, and he was discharged in stable condition on the following drug regimen: ceftriaxone 2 g IV twice daily, metronidazole 500 mg IV 3 times daily for 6 weeks, levetiracetam 1,500 mg twice daily, and valproic acid 750 mg 3 times daily.
At a 3-month follow-up visit, he reported no seizure-like activity but demonstrated persistent neurologic deficits (dysdiadochokinesia and mild ataxia).
A LESS COMMON CAUSE OF BRAIN ABSCESS
In the United States, 1,500 to 2,000 cases of brain abscess are diagnosed every year, and this condition is responsible for an estimated 1 in 10,000 hospitalizations. Most patients hospitalized are men over age 60 or children. Most patients with hematogenous or embolic spread of infection from a primary infection source are immunocompromised.
However, the lesions in our patient were not from compromised immunity, but rather from septic hematogenous spread of an odontogenic infection. Odontogenic bacteria are a common cause of pyogenic orofascial infection, including periapical abscess and infection of adjoining fascial spaces of the head and neck.1
P micros and S constellatus have been commonly found in many types of odontogenic infection, including dentoalveolar infection, periodontitis, and pericoronitis.2 Our patient was found to have several periodontal abscesses with bacteremia and spread to the brain. Although transthoracic echocardiography was negative for vegetations or patent foramen ovale, the quality and location of the brain abscesses suggested embolic spread of infection. Most of the suspected septic emboli were in the right hemisphere, consistent with patterns seen with cardioembolic phenomena, and a number of lesions appeared to be within the distribution of the right anterior cerebral artery and the middle cerebral artery.
EMPIRIC AND SPECIFIC THERAPIES
Empiric antibiotic therapy for local odontogenic infection includes amoxicillin with clavulanic acid and metronidazole.1 Our patient’s treatment with ceftriaxone and metronidazole was based on the species and sensitivities of the bacteria in blood cultures.
Surgical irrigation with debridement is considered first-line therapy for local dental infection, with antimicrobials as adjunctive therapy. Initiation of antibiotic therapy before surgery has been associated with a shortened duration of infection and a reduced risk of bacteremia.3
First-line therapy for cerebral abscess is typically antibiotics, specifically ceftriaxone and metronidazole as in our patient. Ceftriaxone is selected for coverage against streptococci, enterobacteriacae, and most common anaerobes, whereas metronidazole is chosen for its efficacy against Bacteroides fragilis.
Computed tomography-guided stereotactic aspiration and open drainage are viable options for solitary and surgically accessible abscesses—typically those greater than 2 cm. Our patient had multiple small septic emboli in the right hemisphere, with the largest lesion measuring 1.5 cm, thus limiting the effectiveness of surgical intervention.
Some patients with mass effect or other evidence of increased intracranial pressure may benefit from high doses of a corticosteroid such as dexamethasone. However, since our patient had no clinical or diagnostic findings suggesting elevated intracranial pressure, we opted for nonsurgical management of the brain abscesses, with 6 weeks of intravenous antibiotics, an antiseizure regimen, and plans for repeat imaging in the outpatient setting.
- Bahl R, Sandhu S, Singh K, Sahai N, Gupta M. Odontogenic infections: microbiology and management. Contemp Clin Dent 2014; 5(3):307–311. doi:10.4103/0976-237X.137921
- Kuriyama T, Karasawa T, Nakagawa K, Yamamoto E, Nakamura S. Bacteriology and antimicrobial susceptibility of gram-positive cocci isolated from pus specimens of orofacial odontogenic infections. Oral Microbiol Immunol 2002; 17(2):132–135. pmid:11929563
- Peedikayil FC. Antibiotics in odontogenic infections—an update. J Antimicro 2016; 2:(2)117. doi:10.4172/2472-1212.1000117
A 60-year-old man with hypertension and persistent atrial fibrillation refractory to radiofrequency ablation was brought to the hospital in status epilepticus requiring intubation. His wife said that during the past month he had experienced a number of episodic seizures, but due to his busy work schedule he had not sought medical attention. He had also been hospitalized 3 times during the past week for chills, tremors, and fevers with temperatures up to 101°F (38.3°C), and his symptoms had been ascribed to the amiodarone he had been taking for the past 11 days for atrial fibrillation. The amiodarone dose had been decreased to half a tablet after the first 7 days, but his symptoms had continued.
When the patient was able to speak, he denied intravenous drug abuse and claimed to be up to date with vaccinations. Colonoscopy 10 years earlier had been negative. He has no pets, but says that there are stray cats around his home and that he has had contact with cat feces while gardening. He works as a diesel mechanic and is exposed to motor oil and diesel fuel, but denies any direct exposure to carcinogenic chemicals.
On admission, his temperature was 37.7°C (99.9°F), blood pressure 92/69 mm Hg, heart rate 96 beats per minute, respiratory rate 21 per minute, and oxygen saturation 95% on room air and 100% on oxygen at 2 L per minute.
Decerebrate posturing and forced left visual gaze deviation was observed. Oral examination revealed severe decay of multiple teeth, with some teeth broken down to the level of the gingiva, and moderate generalized periodontal disease with heavy plaque and calculi in the gingiva.
The differential diagnosis for intracranial ring-enhancing lesions includes metastasis, abscess, infection in an immunocompromised state (eg, toxoplasmosis), glioblastoma, subacute infarct, neurocysticercosis, lymphoma, demyelination, and resolving hematoma. In our patient, further testing to narrow the differential included lumbar puncture, with results within normal limits, and transthoracic echocardiography, which was negative for endocarditis. A biopsy obtained by craniotomy confirmed the diagnosis of abscess surrounded by reactive glioses.
During his hospitalization, the patient’s antiseizure regimen was lorazepam 1 to 2 mg as needed, levetiracetam 1,500 mg twice daily, and fosphenytoin infusion at 100 mg phenytoin sodium equivalents per minute. Initial antibiotic therapy included ampicillin 2 g intravenously (IV) 4 times daily.
Because of persistent nocturnal fevers with temperatures ranging from 37.8°C (100°F) to 41.2°C (106.2°F), antibiotic coverage was broadened to meropenem 2 g IV every 8 hours. Testing for Toxoplasma gondii, human immunodeficiency virus, and JC polyomavirus was negative. Cerebrospinal fluid culture and abscess cultures were also negative. Blood cultures were eventually positive for Peptostreptococcus micros and Streptococcus constellatus. Based on review of culture results, antibiotic therapy was switched to ceftriaxone 2 g IV twice daily and metronidazole 500 mg IV 3 times daily.
For the dental infection, the patient underwent surgical irrigation and debridement with full dental extraction for multiple dental abscesses.
His regimen for seizure control was changed to phenytoin and valproic acid, and he was discharged in stable condition on the following drug regimen: ceftriaxone 2 g IV twice daily, metronidazole 500 mg IV 3 times daily for 6 weeks, levetiracetam 1,500 mg twice daily, and valproic acid 750 mg 3 times daily.
At a 3-month follow-up visit, he reported no seizure-like activity but demonstrated persistent neurologic deficits (dysdiadochokinesia and mild ataxia).
A LESS COMMON CAUSE OF BRAIN ABSCESS
In the United States, 1,500 to 2,000 cases of brain abscess are diagnosed every year, and this condition is responsible for an estimated 1 in 10,000 hospitalizations. Most patients hospitalized are men over age 60 or children. Most patients with hematogenous or embolic spread of infection from a primary infection source are immunocompromised.
However, the lesions in our patient were not from compromised immunity, but rather from septic hematogenous spread of an odontogenic infection. Odontogenic bacteria are a common cause of pyogenic orofascial infection, including periapical abscess and infection of adjoining fascial spaces of the head and neck.1
P micros and S constellatus have been commonly found in many types of odontogenic infection, including dentoalveolar infection, periodontitis, and pericoronitis.2 Our patient was found to have several periodontal abscesses with bacteremia and spread to the brain. Although transthoracic echocardiography was negative for vegetations or patent foramen ovale, the quality and location of the brain abscesses suggested embolic spread of infection. Most of the suspected septic emboli were in the right hemisphere, consistent with patterns seen with cardioembolic phenomena, and a number of lesions appeared to be within the distribution of the right anterior cerebral artery and the middle cerebral artery.
EMPIRIC AND SPECIFIC THERAPIES
Empiric antibiotic therapy for local odontogenic infection includes amoxicillin with clavulanic acid and metronidazole.1 Our patient’s treatment with ceftriaxone and metronidazole was based on the species and sensitivities of the bacteria in blood cultures.
Surgical irrigation with debridement is considered first-line therapy for local dental infection, with antimicrobials as adjunctive therapy. Initiation of antibiotic therapy before surgery has been associated with a shortened duration of infection and a reduced risk of bacteremia.3
First-line therapy for cerebral abscess is typically antibiotics, specifically ceftriaxone and metronidazole as in our patient. Ceftriaxone is selected for coverage against streptococci, enterobacteriacae, and most common anaerobes, whereas metronidazole is chosen for its efficacy against Bacteroides fragilis.
Computed tomography-guided stereotactic aspiration and open drainage are viable options for solitary and surgically accessible abscesses—typically those greater than 2 cm. Our patient had multiple small septic emboli in the right hemisphere, with the largest lesion measuring 1.5 cm, thus limiting the effectiveness of surgical intervention.
Some patients with mass effect or other evidence of increased intracranial pressure may benefit from high doses of a corticosteroid such as dexamethasone. However, since our patient had no clinical or diagnostic findings suggesting elevated intracranial pressure, we opted for nonsurgical management of the brain abscesses, with 6 weeks of intravenous antibiotics, an antiseizure regimen, and plans for repeat imaging in the outpatient setting.
A 60-year-old man with hypertension and persistent atrial fibrillation refractory to radiofrequency ablation was brought to the hospital in status epilepticus requiring intubation. His wife said that during the past month he had experienced a number of episodic seizures, but due to his busy work schedule he had not sought medical attention. He had also been hospitalized 3 times during the past week for chills, tremors, and fevers with temperatures up to 101°F (38.3°C), and his symptoms had been ascribed to the amiodarone he had been taking for the past 11 days for atrial fibrillation. The amiodarone dose had been decreased to half a tablet after the first 7 days, but his symptoms had continued.
When the patient was able to speak, he denied intravenous drug abuse and claimed to be up to date with vaccinations. Colonoscopy 10 years earlier had been negative. He has no pets, but says that there are stray cats around his home and that he has had contact with cat feces while gardening. He works as a diesel mechanic and is exposed to motor oil and diesel fuel, but denies any direct exposure to carcinogenic chemicals.
On admission, his temperature was 37.7°C (99.9°F), blood pressure 92/69 mm Hg, heart rate 96 beats per minute, respiratory rate 21 per minute, and oxygen saturation 95% on room air and 100% on oxygen at 2 L per minute.
Decerebrate posturing and forced left visual gaze deviation was observed. Oral examination revealed severe decay of multiple teeth, with some teeth broken down to the level of the gingiva, and moderate generalized periodontal disease with heavy plaque and calculi in the gingiva.
The differential diagnosis for intracranial ring-enhancing lesions includes metastasis, abscess, infection in an immunocompromised state (eg, toxoplasmosis), glioblastoma, subacute infarct, neurocysticercosis, lymphoma, demyelination, and resolving hematoma. In our patient, further testing to narrow the differential included lumbar puncture, with results within normal limits, and transthoracic echocardiography, which was negative for endocarditis. A biopsy obtained by craniotomy confirmed the diagnosis of abscess surrounded by reactive glioses.
During his hospitalization, the patient’s antiseizure regimen was lorazepam 1 to 2 mg as needed, levetiracetam 1,500 mg twice daily, and fosphenytoin infusion at 100 mg phenytoin sodium equivalents per minute. Initial antibiotic therapy included ampicillin 2 g intravenously (IV) 4 times daily.
Because of persistent nocturnal fevers with temperatures ranging from 37.8°C (100°F) to 41.2°C (106.2°F), antibiotic coverage was broadened to meropenem 2 g IV every 8 hours. Testing for Toxoplasma gondii, human immunodeficiency virus, and JC polyomavirus was negative. Cerebrospinal fluid culture and abscess cultures were also negative. Blood cultures were eventually positive for Peptostreptococcus micros and Streptococcus constellatus. Based on review of culture results, antibiotic therapy was switched to ceftriaxone 2 g IV twice daily and metronidazole 500 mg IV 3 times daily.
For the dental infection, the patient underwent surgical irrigation and debridement with full dental extraction for multiple dental abscesses.
His regimen for seizure control was changed to phenytoin and valproic acid, and he was discharged in stable condition on the following drug regimen: ceftriaxone 2 g IV twice daily, metronidazole 500 mg IV 3 times daily for 6 weeks, levetiracetam 1,500 mg twice daily, and valproic acid 750 mg 3 times daily.
At a 3-month follow-up visit, he reported no seizure-like activity but demonstrated persistent neurologic deficits (dysdiadochokinesia and mild ataxia).
A LESS COMMON CAUSE OF BRAIN ABSCESS
In the United States, 1,500 to 2,000 cases of brain abscess are diagnosed every year, and this condition is responsible for an estimated 1 in 10,000 hospitalizations. Most patients hospitalized are men over age 60 or children. Most patients with hematogenous or embolic spread of infection from a primary infection source are immunocompromised.
However, the lesions in our patient were not from compromised immunity, but rather from septic hematogenous spread of an odontogenic infection. Odontogenic bacteria are a common cause of pyogenic orofascial infection, including periapical abscess and infection of adjoining fascial spaces of the head and neck.1
P micros and S constellatus have been commonly found in many types of odontogenic infection, including dentoalveolar infection, periodontitis, and pericoronitis.2 Our patient was found to have several periodontal abscesses with bacteremia and spread to the brain. Although transthoracic echocardiography was negative for vegetations or patent foramen ovale, the quality and location of the brain abscesses suggested embolic spread of infection. Most of the suspected septic emboli were in the right hemisphere, consistent with patterns seen with cardioembolic phenomena, and a number of lesions appeared to be within the distribution of the right anterior cerebral artery and the middle cerebral artery.
EMPIRIC AND SPECIFIC THERAPIES
Empiric antibiotic therapy for local odontogenic infection includes amoxicillin with clavulanic acid and metronidazole.1 Our patient’s treatment with ceftriaxone and metronidazole was based on the species and sensitivities of the bacteria in blood cultures.
Surgical irrigation with debridement is considered first-line therapy for local dental infection, with antimicrobials as adjunctive therapy. Initiation of antibiotic therapy before surgery has been associated with a shortened duration of infection and a reduced risk of bacteremia.3
First-line therapy for cerebral abscess is typically antibiotics, specifically ceftriaxone and metronidazole as in our patient. Ceftriaxone is selected for coverage against streptococci, enterobacteriacae, and most common anaerobes, whereas metronidazole is chosen for its efficacy against Bacteroides fragilis.
Computed tomography-guided stereotactic aspiration and open drainage are viable options for solitary and surgically accessible abscesses—typically those greater than 2 cm. Our patient had multiple small septic emboli in the right hemisphere, with the largest lesion measuring 1.5 cm, thus limiting the effectiveness of surgical intervention.
Some patients with mass effect or other evidence of increased intracranial pressure may benefit from high doses of a corticosteroid such as dexamethasone. However, since our patient had no clinical or diagnostic findings suggesting elevated intracranial pressure, we opted for nonsurgical management of the brain abscesses, with 6 weeks of intravenous antibiotics, an antiseizure regimen, and plans for repeat imaging in the outpatient setting.
- Bahl R, Sandhu S, Singh K, Sahai N, Gupta M. Odontogenic infections: microbiology and management. Contemp Clin Dent 2014; 5(3):307–311. doi:10.4103/0976-237X.137921
- Kuriyama T, Karasawa T, Nakagawa K, Yamamoto E, Nakamura S. Bacteriology and antimicrobial susceptibility of gram-positive cocci isolated from pus specimens of orofacial odontogenic infections. Oral Microbiol Immunol 2002; 17(2):132–135. pmid:11929563
- Peedikayil FC. Antibiotics in odontogenic infections—an update. J Antimicro 2016; 2:(2)117. doi:10.4172/2472-1212.1000117
- Bahl R, Sandhu S, Singh K, Sahai N, Gupta M. Odontogenic infections: microbiology and management. Contemp Clin Dent 2014; 5(3):307–311. doi:10.4103/0976-237X.137921
- Kuriyama T, Karasawa T, Nakagawa K, Yamamoto E, Nakamura S. Bacteriology and antimicrobial susceptibility of gram-positive cocci isolated from pus specimens of orofacial odontogenic infections. Oral Microbiol Immunol 2002; 17(2):132–135. pmid:11929563
- Peedikayil FC. Antibiotics in odontogenic infections—an update. J Antimicro 2016; 2:(2)117. doi:10.4172/2472-1212.1000117
Coronary artery calcium scoring: A valuable tool in primary care
In 1984, Jim Fixx, who wrote The Complete Book of Running,1 went out for his daily run and died of a massive heart attack. He was 48. Unbeknownst to him, he had 3-vessel coronary artery disease.
His case illustrates the difficulty of diagnosing coronary artery disease in patients who have no symptoms of it. For many, the initial presentation is myocardial infarction or death. Until recently, there was no reliable way to diagnose subclinical coronary artery disease other than angiography, and there is still no way to rule it out. As a result, physicians have concentrated less on diagnosing subclinical disease and more on assessing the risk of myocardial infarction.
ASSESSING RISK
The risk factors for coronary artery disease (age, male sex, smoking, hypertension, and cholesterol) have been well known for half a century. By combining risk factors with the appropriate weighting, it is possible to predict an individual’s risk of a myocardial infarction.
In 2013, the American College of Cardiology/American Heart Association (ACC/AHA) guidelines applied this risk-based approach to prescribing statins for primary prevention.2 Instead of focusing on low-density lipoprotein cholesterol concentration, which by itself is a poor predictor of myocardial infarction, they recommended using the Pooled Cohort Equation3 to determine the risk of a cardiovascular event within 10 years. For patients at high risk (> 7.5%), the benefits of a statin generally outweigh the harms. For those at low risk (< 5%), the opposite is true. For patients in between, there is room for shared decision-making.
Debate has focused on the predictive accuracy of the equation, the threshold for treatment, and the fact that almost all men over 60 qualify for treatment.4 These objections stem from the focus on risk rather than on diagnosis of the underlying disease.
Because one-third of “high-risk” patients never develop cardiovascular disease,5 the risk-based approach necessitates overtreatment. Those without disease cannot benefit from treatment but nonetheless suffer its side effects, cost, and inconvenience. Raising treatment thresholds (eg, treating only patients whose 10-year risk exceeds 10%) improves the ratio of patients with disease to those without but also misses diseased patients who have few risk factors. “Low risk” is not “no risk.”
TESTING FOR DISEASE IN THOSE AT INTERMEDIATE RISK
Diagnostic testing is preferred if such testing is safe and inexpensive.
In this issue of Cleveland Clinic Journal of Medicine, Parikh and colleagues6 review coronary artery calcium scoring, a diagnostic test for coronary artery disease. They conclude that calcium scoring is strongly predictive but should be reserved for patients at intermediate risk to help them decide about treatment. This is clearly the right approach, but the authors leave the term “intermediate” undefined, and their clinical examples offer little guidance as to where the borders lie.
The ACC/AHA guidelines specify a narrow intermediate range (5.0%–7.4%). For these patients, calcium scoring could reclassify most as being at high or low risk, helping to clarify whether statins are indicated.
However, only 12% of patients fall into this category.7 What about patients at higher risk? Could they be reclassified as being at low risk if their calcium score was 0?8 Conversely, could some low-risk patients discover that they are at high risk and perhaps take action?
The ACC/AHA guidelines recommend against calcium scoring in these circumstances. One concern was that calcium scoring had not been tested with the Pooled Cohort Equation. Another concern related to cost and radiation exposure, but as Parikh et al point out, the cost has now fallen to less than $100, and radiation exposure is similar to that with mammography.
SHOULD WE TEST PATIENTS AT HIGH OR LOW RISK?
Who, then, should we test? For patients at high or low risk according to the Pooled Cohort Equation, 2 questions determine whether calcium scoring is warranted: how much would an extremely high or low score (ie, 0 or > 400) change the risk of an event, and how likely is an extreme score?
The first question relates to the usefulness of the test, the second to its cost-effectiveness. If even an extreme score cannot move a patient’s risk into or out of the treatment range, then testing is unwarranted. At the same time, if few patients have an extreme score, then cost per test that changes practice will be high.
Because calcium scoring is a direct test for disease, it is extremely predictive. When added to risk-factor models, it substantially improves discrimination9 and exhibits excellent calibration.10 This is true whether the outcome is a major cardiovascular event or death from any cause.
But the calcium score is not strong enough to override all other risk factors. A patient with a predicted 10-year risk of 18% according to the Pooled Cohort Equation and a calcium score of 0 could be reclassified as being at low risk, but a patient with a 10-year predicted risk of 35% could not. The same is true for patients at low risk. A patient with a 4% risk and a calcium score higher than 400 would be reclassified as being at high risk, but not a patient with a 1% risk.
Extreme calcium scores are common, especially in patients at high risk. In the Multi-Ethnic Study of Atherosclerosis (MESA) cohort, 45% of patients with a 10-year predicted risk of 7.5% to 20% had a calcium score of 0, reclassifying them into the low-risk category.11 Even if the predicted risk was greater than 20%, 1 in 4 patients had a score of 0. In contrast, if the 10-year predicted risk was below 5%, one-fifth of patients had a calcium score greater than 0, but only 4% had a score greater than 100.
Nevertheless, patients in the low-risk category whose baseline risk is close to 5% may wish to undergo calcium scoring, because a positive test opens the door to a potentially lifesaving treatment. In general, the closer patients are to the treatment threshold, the more likely they are to be reclassified by calcium scoring.
The Society for Cardiovascular Computed Tomography currently recommends coronary artery calcium scoring for patients whose 10-year risk is between 5% and 20%.12 These numbers are easy to remember and a reasonable approximation of the number of patients likely to benefit from testing.
COMBINING CALCIUM SCORING WITH TRADITIONAL RISK FACTORS
Primary care physicians interested in more exact personalized medicine can use a risk calculator derived from the MESA cohort.13 Based on 10-year outcomes for 6,814 participants, Blaha et al8 derived and validated this risk-prediction tool incorporating all the elements of the Pooled Cohort Equation in addition to family history, race, and calcium score.
The tool offered good discrimination and calibration when validated against 2 external cohorts (the Heinz Nixdorf Recall Study and the Dallas Heart Study).10 The C statistics were 0.78 and 0.82, with 10-year risk predicted by the tool within half a percent of the observed event rate in each cohort.
The online calculator displays the 10-year risk based on risk factors alone or including a calcium score, allowing the clinician to gauge the value of testing. For example, a 70-year-old nonsmoking white man with a total cholesterol level of 240 mg/dL, high-density lipoprotein cholesterol 40 mg/dL, and systolic blood pressure 130 mm Hg on amlodipine has a 15.2% 10-year risk (well above the 7.5% threshold for statin therapy). However, if his calcium score is 0, his risk falls to 4.3% (well below the threshold). Sharing such information with patients could help them to decide whether to undergo coronary artery calcium scoring.
Ultimately, the decision to take a statin for primary prevention of coronary artery disease is a personal one. It involves weighing risks, benefits, and preferences. Physicians can facilitate the process by providing information and guidance. Patients are best served by having the most accurate information. In many cases, that information should include calcium scoring.
- Fixx JF. The Complete Book of Running. New York: Random House, 1977.
- Goff DC, Jr, Lloyd-Jones DM, Bennett G, et al. 2013 ACC/AHA guideline on the assessment of cardiovascular risk: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation 2014; 129(25 suppl 2):S49–S73. doi:10.1161/01.cir.0000437741.48606.98
- American Heart Association, American College of Cardiology. 2013 Prevention guidelines tools. CV risk calculator. ASCVD risk calculator. https://professional.heart.org/professional/GuidelinesStatements/PreventionGuidelines/UCM_457698_ASCVD-Risk-Calculator.jsp. Accessed August 17, 2018.
- Pencina MJ, Navar-Boggan AM, D’Agostino RB, Sr, et al. Application of new cholesterol guidelines to a population-based sample. N Engl J Med 2014; 370(15):1422–1431. doi:10.1056/NEJMoa1315665
- Wilkins JT, Ning H, Berry J, Zhao L, Dyer AR, Lloyd-Jones DM. Lifetime risk and years lived free of total cardiovascular disease. JAMA 2012; 308(17):1795–1801. doi:10.1001/jama.2012.14312
- Parikh P, Shah N, Ahmed H, Schoenhagen P, Fares M. Coronary artery calcium scoring: its practicality and clinical utility in primary care. Cleve Clin J Med 2018; 85(9):707–716. doi:10.3949/ccjm.85a.17097
- Blaha MJ, Dardari ZA, Blumenthal RS, Martin SS, Nasir K, Al-Mallah MH. The new “intermediate risk” group: a comparative analysis of the new 2013 ACC/AHA risk assessment guidelines versus prior guidelines in men. Atherosclerosis 2014; 237(1):1–4. doi:10.1016/j.atherosclerosis.2014.08.024
- Blaha MJ, Cainzos-Achirica M, Greenland P, et al. Role of coronary artery calcium score of zero and other negative risk markers for cardiovascular disease: the Multi-Ethnic Study of Atherosclerosis (MESA). Circulation 2016; 133(9):849–858. doi:10.1161/CIRCULATIONAHA.115.018524
- Peters SAE, den Ruijter HM, Bots ML, Moons KGM. Improvements in risk stratification for the occurrence of cardiovascular disease by imaging subclinical atherosclerosis: a systematic review. Heart 2012; 98(3):177–184. doi:10.1136/heartjnl-2011-300747
- McClelland RL, Jorgensen NW, Budoff M, et al. Ten-year coronary heart disease risk prediction using coronary artery calcium and traditional risk factors: derivation in the Multi-Ethnic Study of Atherosclerosis with validation in the Heinz Nixdorf Recall Study and the Dallas Heart Study. J Am Coll Cardiol 2015; 66(15):1643–1653. doi:10.1016/j.jacc.2015.08.035
- Nasir K, Bittencourt MS, Blaha MJ, et al. Implications of coronary artery calcium testing among statin candidates according to American College of Cardiology/American Heart Association cholesterol management guidelines: MESA (Multi-Ethnic Study of Atherosclerosis). J Am Coll Cardiol 2015; 66(15):1657–1668. doi:10.1016/j.jacc.2015.07.066
- Hecht H, Blaha MJ, Berman DS, et al. Clinical indications for coronary artery calcium scoring in asymptomatic patients: expert consensus statement from the Society of Cardiovascular Computed Tomography. J Cardiovasc Comput Tomogr 2017; 11(2):157–168. doi:10.1016/j.jcct.2017.02.010
- MESA. The Multi-Ethnic Study of Atherosclerosis. MESA 10-year CHD risk with coronary artery calcification. www.mesa-nhlbi.org/MESACHDRisk/MesaRiskScore/RiskScore.aspx. Accessed August 17, 2018.
In 1984, Jim Fixx, who wrote The Complete Book of Running,1 went out for his daily run and died of a massive heart attack. He was 48. Unbeknownst to him, he had 3-vessel coronary artery disease.
His case illustrates the difficulty of diagnosing coronary artery disease in patients who have no symptoms of it. For many, the initial presentation is myocardial infarction or death. Until recently, there was no reliable way to diagnose subclinical coronary artery disease other than angiography, and there is still no way to rule it out. As a result, physicians have concentrated less on diagnosing subclinical disease and more on assessing the risk of myocardial infarction.
ASSESSING RISK
The risk factors for coronary artery disease (age, male sex, smoking, hypertension, and cholesterol) have been well known for half a century. By combining risk factors with the appropriate weighting, it is possible to predict an individual’s risk of a myocardial infarction.
In 2013, the American College of Cardiology/American Heart Association (ACC/AHA) guidelines applied this risk-based approach to prescribing statins for primary prevention.2 Instead of focusing on low-density lipoprotein cholesterol concentration, which by itself is a poor predictor of myocardial infarction, they recommended using the Pooled Cohort Equation3 to determine the risk of a cardiovascular event within 10 years. For patients at high risk (> 7.5%), the benefits of a statin generally outweigh the harms. For those at low risk (< 5%), the opposite is true. For patients in between, there is room for shared decision-making.
Debate has focused on the predictive accuracy of the equation, the threshold for treatment, and the fact that almost all men over 60 qualify for treatment.4 These objections stem from the focus on risk rather than on diagnosis of the underlying disease.
Because one-third of “high-risk” patients never develop cardiovascular disease,5 the risk-based approach necessitates overtreatment. Those without disease cannot benefit from treatment but nonetheless suffer its side effects, cost, and inconvenience. Raising treatment thresholds (eg, treating only patients whose 10-year risk exceeds 10%) improves the ratio of patients with disease to those without but also misses diseased patients who have few risk factors. “Low risk” is not “no risk.”
TESTING FOR DISEASE IN THOSE AT INTERMEDIATE RISK
Diagnostic testing is preferred if such testing is safe and inexpensive.
In this issue of Cleveland Clinic Journal of Medicine, Parikh and colleagues6 review coronary artery calcium scoring, a diagnostic test for coronary artery disease. They conclude that calcium scoring is strongly predictive but should be reserved for patients at intermediate risk to help them decide about treatment. This is clearly the right approach, but the authors leave the term “intermediate” undefined, and their clinical examples offer little guidance as to where the borders lie.
The ACC/AHA guidelines specify a narrow intermediate range (5.0%–7.4%). For these patients, calcium scoring could reclassify most as being at high or low risk, helping to clarify whether statins are indicated.
However, only 12% of patients fall into this category.7 What about patients at higher risk? Could they be reclassified as being at low risk if their calcium score was 0?8 Conversely, could some low-risk patients discover that they are at high risk and perhaps take action?
The ACC/AHA guidelines recommend against calcium scoring in these circumstances. One concern was that calcium scoring had not been tested with the Pooled Cohort Equation. Another concern related to cost and radiation exposure, but as Parikh et al point out, the cost has now fallen to less than $100, and radiation exposure is similar to that with mammography.
SHOULD WE TEST PATIENTS AT HIGH OR LOW RISK?
Who, then, should we test? For patients at high or low risk according to the Pooled Cohort Equation, 2 questions determine whether calcium scoring is warranted: how much would an extremely high or low score (ie, 0 or > 400) change the risk of an event, and how likely is an extreme score?
The first question relates to the usefulness of the test, the second to its cost-effectiveness. If even an extreme score cannot move a patient’s risk into or out of the treatment range, then testing is unwarranted. At the same time, if few patients have an extreme score, then cost per test that changes practice will be high.
Because calcium scoring is a direct test for disease, it is extremely predictive. When added to risk-factor models, it substantially improves discrimination9 and exhibits excellent calibration.10 This is true whether the outcome is a major cardiovascular event or death from any cause.
But the calcium score is not strong enough to override all other risk factors. A patient with a predicted 10-year risk of 18% according to the Pooled Cohort Equation and a calcium score of 0 could be reclassified as being at low risk, but a patient with a 10-year predicted risk of 35% could not. The same is true for patients at low risk. A patient with a 4% risk and a calcium score higher than 400 would be reclassified as being at high risk, but not a patient with a 1% risk.
Extreme calcium scores are common, especially in patients at high risk. In the Multi-Ethnic Study of Atherosclerosis (MESA) cohort, 45% of patients with a 10-year predicted risk of 7.5% to 20% had a calcium score of 0, reclassifying them into the low-risk category.11 Even if the predicted risk was greater than 20%, 1 in 4 patients had a score of 0. In contrast, if the 10-year predicted risk was below 5%, one-fifth of patients had a calcium score greater than 0, but only 4% had a score greater than 100.
Nevertheless, patients in the low-risk category whose baseline risk is close to 5% may wish to undergo calcium scoring, because a positive test opens the door to a potentially lifesaving treatment. In general, the closer patients are to the treatment threshold, the more likely they are to be reclassified by calcium scoring.
The Society for Cardiovascular Computed Tomography currently recommends coronary artery calcium scoring for patients whose 10-year risk is between 5% and 20%.12 These numbers are easy to remember and a reasonable approximation of the number of patients likely to benefit from testing.
COMBINING CALCIUM SCORING WITH TRADITIONAL RISK FACTORS
Primary care physicians interested in more exact personalized medicine can use a risk calculator derived from the MESA cohort.13 Based on 10-year outcomes for 6,814 participants, Blaha et al8 derived and validated this risk-prediction tool incorporating all the elements of the Pooled Cohort Equation in addition to family history, race, and calcium score.
The tool offered good discrimination and calibration when validated against 2 external cohorts (the Heinz Nixdorf Recall Study and the Dallas Heart Study).10 The C statistics were 0.78 and 0.82, with 10-year risk predicted by the tool within half a percent of the observed event rate in each cohort.
The online calculator displays the 10-year risk based on risk factors alone or including a calcium score, allowing the clinician to gauge the value of testing. For example, a 70-year-old nonsmoking white man with a total cholesterol level of 240 mg/dL, high-density lipoprotein cholesterol 40 mg/dL, and systolic blood pressure 130 mm Hg on amlodipine has a 15.2% 10-year risk (well above the 7.5% threshold for statin therapy). However, if his calcium score is 0, his risk falls to 4.3% (well below the threshold). Sharing such information with patients could help them to decide whether to undergo coronary artery calcium scoring.
Ultimately, the decision to take a statin for primary prevention of coronary artery disease is a personal one. It involves weighing risks, benefits, and preferences. Physicians can facilitate the process by providing information and guidance. Patients are best served by having the most accurate information. In many cases, that information should include calcium scoring.
In 1984, Jim Fixx, who wrote The Complete Book of Running,1 went out for his daily run and died of a massive heart attack. He was 48. Unbeknownst to him, he had 3-vessel coronary artery disease.
His case illustrates the difficulty of diagnosing coronary artery disease in patients who have no symptoms of it. For many, the initial presentation is myocardial infarction or death. Until recently, there was no reliable way to diagnose subclinical coronary artery disease other than angiography, and there is still no way to rule it out. As a result, physicians have concentrated less on diagnosing subclinical disease and more on assessing the risk of myocardial infarction.
ASSESSING RISK
The risk factors for coronary artery disease (age, male sex, smoking, hypertension, and cholesterol) have been well known for half a century. By combining risk factors with the appropriate weighting, it is possible to predict an individual’s risk of a myocardial infarction.
In 2013, the American College of Cardiology/American Heart Association (ACC/AHA) guidelines applied this risk-based approach to prescribing statins for primary prevention.2 Instead of focusing on low-density lipoprotein cholesterol concentration, which by itself is a poor predictor of myocardial infarction, they recommended using the Pooled Cohort Equation3 to determine the risk of a cardiovascular event within 10 years. For patients at high risk (> 7.5%), the benefits of a statin generally outweigh the harms. For those at low risk (< 5%), the opposite is true. For patients in between, there is room for shared decision-making.
Debate has focused on the predictive accuracy of the equation, the threshold for treatment, and the fact that almost all men over 60 qualify for treatment.4 These objections stem from the focus on risk rather than on diagnosis of the underlying disease.
Because one-third of “high-risk” patients never develop cardiovascular disease,5 the risk-based approach necessitates overtreatment. Those without disease cannot benefit from treatment but nonetheless suffer its side effects, cost, and inconvenience. Raising treatment thresholds (eg, treating only patients whose 10-year risk exceeds 10%) improves the ratio of patients with disease to those without but also misses diseased patients who have few risk factors. “Low risk” is not “no risk.”
TESTING FOR DISEASE IN THOSE AT INTERMEDIATE RISK
Diagnostic testing is preferred if such testing is safe and inexpensive.
In this issue of Cleveland Clinic Journal of Medicine, Parikh and colleagues6 review coronary artery calcium scoring, a diagnostic test for coronary artery disease. They conclude that calcium scoring is strongly predictive but should be reserved for patients at intermediate risk to help them decide about treatment. This is clearly the right approach, but the authors leave the term “intermediate” undefined, and their clinical examples offer little guidance as to where the borders lie.
The ACC/AHA guidelines specify a narrow intermediate range (5.0%–7.4%). For these patients, calcium scoring could reclassify most as being at high or low risk, helping to clarify whether statins are indicated.
However, only 12% of patients fall into this category.7 What about patients at higher risk? Could they be reclassified as being at low risk if their calcium score was 0?8 Conversely, could some low-risk patients discover that they are at high risk and perhaps take action?
The ACC/AHA guidelines recommend against calcium scoring in these circumstances. One concern was that calcium scoring had not been tested with the Pooled Cohort Equation. Another concern related to cost and radiation exposure, but as Parikh et al point out, the cost has now fallen to less than $100, and radiation exposure is similar to that with mammography.
SHOULD WE TEST PATIENTS AT HIGH OR LOW RISK?
Who, then, should we test? For patients at high or low risk according to the Pooled Cohort Equation, 2 questions determine whether calcium scoring is warranted: how much would an extremely high or low score (ie, 0 or > 400) change the risk of an event, and how likely is an extreme score?
The first question relates to the usefulness of the test, the second to its cost-effectiveness. If even an extreme score cannot move a patient’s risk into or out of the treatment range, then testing is unwarranted. At the same time, if few patients have an extreme score, then cost per test that changes practice will be high.
Because calcium scoring is a direct test for disease, it is extremely predictive. When added to risk-factor models, it substantially improves discrimination9 and exhibits excellent calibration.10 This is true whether the outcome is a major cardiovascular event or death from any cause.
But the calcium score is not strong enough to override all other risk factors. A patient with a predicted 10-year risk of 18% according to the Pooled Cohort Equation and a calcium score of 0 could be reclassified as being at low risk, but a patient with a 10-year predicted risk of 35% could not. The same is true for patients at low risk. A patient with a 4% risk and a calcium score higher than 400 would be reclassified as being at high risk, but not a patient with a 1% risk.
Extreme calcium scores are common, especially in patients at high risk. In the Multi-Ethnic Study of Atherosclerosis (MESA) cohort, 45% of patients with a 10-year predicted risk of 7.5% to 20% had a calcium score of 0, reclassifying them into the low-risk category.11 Even if the predicted risk was greater than 20%, 1 in 4 patients had a score of 0. In contrast, if the 10-year predicted risk was below 5%, one-fifth of patients had a calcium score greater than 0, but only 4% had a score greater than 100.
Nevertheless, patients in the low-risk category whose baseline risk is close to 5% may wish to undergo calcium scoring, because a positive test opens the door to a potentially lifesaving treatment. In general, the closer patients are to the treatment threshold, the more likely they are to be reclassified by calcium scoring.
The Society for Cardiovascular Computed Tomography currently recommends coronary artery calcium scoring for patients whose 10-year risk is between 5% and 20%.12 These numbers are easy to remember and a reasonable approximation of the number of patients likely to benefit from testing.
COMBINING CALCIUM SCORING WITH TRADITIONAL RISK FACTORS
Primary care physicians interested in more exact personalized medicine can use a risk calculator derived from the MESA cohort.13 Based on 10-year outcomes for 6,814 participants, Blaha et al8 derived and validated this risk-prediction tool incorporating all the elements of the Pooled Cohort Equation in addition to family history, race, and calcium score.
The tool offered good discrimination and calibration when validated against 2 external cohorts (the Heinz Nixdorf Recall Study and the Dallas Heart Study).10 The C statistics were 0.78 and 0.82, with 10-year risk predicted by the tool within half a percent of the observed event rate in each cohort.
The online calculator displays the 10-year risk based on risk factors alone or including a calcium score, allowing the clinician to gauge the value of testing. For example, a 70-year-old nonsmoking white man with a total cholesterol level of 240 mg/dL, high-density lipoprotein cholesterol 40 mg/dL, and systolic blood pressure 130 mm Hg on amlodipine has a 15.2% 10-year risk (well above the 7.5% threshold for statin therapy). However, if his calcium score is 0, his risk falls to 4.3% (well below the threshold). Sharing such information with patients could help them to decide whether to undergo coronary artery calcium scoring.
Ultimately, the decision to take a statin for primary prevention of coronary artery disease is a personal one. It involves weighing risks, benefits, and preferences. Physicians can facilitate the process by providing information and guidance. Patients are best served by having the most accurate information. In many cases, that information should include calcium scoring.
- Fixx JF. The Complete Book of Running. New York: Random House, 1977.
- Goff DC, Jr, Lloyd-Jones DM, Bennett G, et al. 2013 ACC/AHA guideline on the assessment of cardiovascular risk: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation 2014; 129(25 suppl 2):S49–S73. doi:10.1161/01.cir.0000437741.48606.98
- American Heart Association, American College of Cardiology. 2013 Prevention guidelines tools. CV risk calculator. ASCVD risk calculator. https://professional.heart.org/professional/GuidelinesStatements/PreventionGuidelines/UCM_457698_ASCVD-Risk-Calculator.jsp. Accessed August 17, 2018.
- Pencina MJ, Navar-Boggan AM, D’Agostino RB, Sr, et al. Application of new cholesterol guidelines to a population-based sample. N Engl J Med 2014; 370(15):1422–1431. doi:10.1056/NEJMoa1315665
- Wilkins JT, Ning H, Berry J, Zhao L, Dyer AR, Lloyd-Jones DM. Lifetime risk and years lived free of total cardiovascular disease. JAMA 2012; 308(17):1795–1801. doi:10.1001/jama.2012.14312
- Parikh P, Shah N, Ahmed H, Schoenhagen P, Fares M. Coronary artery calcium scoring: its practicality and clinical utility in primary care. Cleve Clin J Med 2018; 85(9):707–716. doi:10.3949/ccjm.85a.17097
- Blaha MJ, Dardari ZA, Blumenthal RS, Martin SS, Nasir K, Al-Mallah MH. The new “intermediate risk” group: a comparative analysis of the new 2013 ACC/AHA risk assessment guidelines versus prior guidelines in men. Atherosclerosis 2014; 237(1):1–4. doi:10.1016/j.atherosclerosis.2014.08.024
- Blaha MJ, Cainzos-Achirica M, Greenland P, et al. Role of coronary artery calcium score of zero and other negative risk markers for cardiovascular disease: the Multi-Ethnic Study of Atherosclerosis (MESA). Circulation 2016; 133(9):849–858. doi:10.1161/CIRCULATIONAHA.115.018524
- Peters SAE, den Ruijter HM, Bots ML, Moons KGM. Improvements in risk stratification for the occurrence of cardiovascular disease by imaging subclinical atherosclerosis: a systematic review. Heart 2012; 98(3):177–184. doi:10.1136/heartjnl-2011-300747
- McClelland RL, Jorgensen NW, Budoff M, et al. Ten-year coronary heart disease risk prediction using coronary artery calcium and traditional risk factors: derivation in the Multi-Ethnic Study of Atherosclerosis with validation in the Heinz Nixdorf Recall Study and the Dallas Heart Study. J Am Coll Cardiol 2015; 66(15):1643–1653. doi:10.1016/j.jacc.2015.08.035
- Nasir K, Bittencourt MS, Blaha MJ, et al. Implications of coronary artery calcium testing among statin candidates according to American College of Cardiology/American Heart Association cholesterol management guidelines: MESA (Multi-Ethnic Study of Atherosclerosis). J Am Coll Cardiol 2015; 66(15):1657–1668. doi:10.1016/j.jacc.2015.07.066
- Hecht H, Blaha MJ, Berman DS, et al. Clinical indications for coronary artery calcium scoring in asymptomatic patients: expert consensus statement from the Society of Cardiovascular Computed Tomography. J Cardiovasc Comput Tomogr 2017; 11(2):157–168. doi:10.1016/j.jcct.2017.02.010
- MESA. The Multi-Ethnic Study of Atherosclerosis. MESA 10-year CHD risk with coronary artery calcification. www.mesa-nhlbi.org/MESACHDRisk/MesaRiskScore/RiskScore.aspx. Accessed August 17, 2018.
- Fixx JF. The Complete Book of Running. New York: Random House, 1977.
- Goff DC, Jr, Lloyd-Jones DM, Bennett G, et al. 2013 ACC/AHA guideline on the assessment of cardiovascular risk: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation 2014; 129(25 suppl 2):S49–S73. doi:10.1161/01.cir.0000437741.48606.98
- American Heart Association, American College of Cardiology. 2013 Prevention guidelines tools. CV risk calculator. ASCVD risk calculator. https://professional.heart.org/professional/GuidelinesStatements/PreventionGuidelines/UCM_457698_ASCVD-Risk-Calculator.jsp. Accessed August 17, 2018.
- Pencina MJ, Navar-Boggan AM, D’Agostino RB, Sr, et al. Application of new cholesterol guidelines to a population-based sample. N Engl J Med 2014; 370(15):1422–1431. doi:10.1056/NEJMoa1315665
- Wilkins JT, Ning H, Berry J, Zhao L, Dyer AR, Lloyd-Jones DM. Lifetime risk and years lived free of total cardiovascular disease. JAMA 2012; 308(17):1795–1801. doi:10.1001/jama.2012.14312
- Parikh P, Shah N, Ahmed H, Schoenhagen P, Fares M. Coronary artery calcium scoring: its practicality and clinical utility in primary care. Cleve Clin J Med 2018; 85(9):707–716. doi:10.3949/ccjm.85a.17097
- Blaha MJ, Dardari ZA, Blumenthal RS, Martin SS, Nasir K, Al-Mallah MH. The new “intermediate risk” group: a comparative analysis of the new 2013 ACC/AHA risk assessment guidelines versus prior guidelines in men. Atherosclerosis 2014; 237(1):1–4. doi:10.1016/j.atherosclerosis.2014.08.024
- Blaha MJ, Cainzos-Achirica M, Greenland P, et al. Role of coronary artery calcium score of zero and other negative risk markers for cardiovascular disease: the Multi-Ethnic Study of Atherosclerosis (MESA). Circulation 2016; 133(9):849–858. doi:10.1161/CIRCULATIONAHA.115.018524
- Peters SAE, den Ruijter HM, Bots ML, Moons KGM. Improvements in risk stratification for the occurrence of cardiovascular disease by imaging subclinical atherosclerosis: a systematic review. Heart 2012; 98(3):177–184. doi:10.1136/heartjnl-2011-300747
- McClelland RL, Jorgensen NW, Budoff M, et al. Ten-year coronary heart disease risk prediction using coronary artery calcium and traditional risk factors: derivation in the Multi-Ethnic Study of Atherosclerosis with validation in the Heinz Nixdorf Recall Study and the Dallas Heart Study. J Am Coll Cardiol 2015; 66(15):1643–1653. doi:10.1016/j.jacc.2015.08.035
- Nasir K, Bittencourt MS, Blaha MJ, et al. Implications of coronary artery calcium testing among statin candidates according to American College of Cardiology/American Heart Association cholesterol management guidelines: MESA (Multi-Ethnic Study of Atherosclerosis). J Am Coll Cardiol 2015; 66(15):1657–1668. doi:10.1016/j.jacc.2015.07.066
- Hecht H, Blaha MJ, Berman DS, et al. Clinical indications for coronary artery calcium scoring in asymptomatic patients: expert consensus statement from the Society of Cardiovascular Computed Tomography. J Cardiovasc Comput Tomogr 2017; 11(2):157–168. doi:10.1016/j.jcct.2017.02.010
- MESA. The Multi-Ethnic Study of Atherosclerosis. MESA 10-year CHD risk with coronary artery calcification. www.mesa-nhlbi.org/MESACHDRisk/MesaRiskScore/RiskScore.aspx. Accessed August 17, 2018.
New MI definition aims to better distinguish infarction from injury
MUNICH – The worldwide cardiology community’s newly revised universal definition of an MI refines the way that cardiologists distinguish between myocardial infarction and myocardial injury, said Joseph S. Alpert, MD, one of the two chairs of the definition-writing panel.
“We had three previous definitions, but there is still a lot of confusion [about distinguishing] between injury and infarction. We definitely hope that this fourth definition will further help people distinguish the two and help people determine whether or not a patient has an MI,” said Dr. Alpert following a session at the annual congress of the European Society of Cardiology that introduced some of the key elements of the new revision.
Days before the ESC congress, a task force formed by the European Society of Cardiology, the American College of Cardiology, the American Heart Association, and the World Heart Federation released the Fourth Universal Definition of Myocardial Infarction (2018) (J Am Coll Cardiol. 2018 Aug 24. doi: 10.1016/j.jacc.2018.08.1038), which follows the series of three prior MI definitions that these groups have issued since the first iteration came out in 2007 (J Am Coll Cardiol. 2007;50[22]:2173-95).
The new revision includes 5 “new concepts,” 14 updated concepts, and 6 new sections since the third universal definition from 2012. The change that topped Dr. Alpert’s list of key messages was the need to determine whether a rise in cardiac troponin, a key biomarker of cardiac damage, resulted from infarction or injury.
These two alternative diagnoses mean “a very different outlook for patients. Treatment is different, and their prognosis is different. It’s important to make the distinction,” said Dr. Alpert, professor of medicine at the University of Arizona in Tucson.
The new changes to making an MI diagnosis will likely help drive a couple of important changes in the way U.S. patients with suspected myocardial injury or infarction get assessed, he said in an interview. The first change will be wide uptake of high sensitivity cardiac troponin (hscTn) assays over the next 5 years or so, as the ability to measure this key diagnostic biomarker progresses from its initial Food and Drug Administration approval for the U.S. market in 2017 to “close to 100% of U.S. hospitals using it,” he predicted. A big issue that is currently slowing even quicker adoption of hscTn is that many hospitals, including the one where Dr. Alpert practices, still have laboratory contracts in place that tether them to older troponin-testing technologies and make it economically unfeasible to change until their contracts expire. The contract in place where Dr. Alpert practices runs out in 2019, and soon after that happens he expects to gain the ability to order a hscTn test.
The new, fourth definition says that hscTn is “recommended for routine clinical use,” but routine U.S. use “won’t be immediate because many hospitals will put in hscTn only when their old contract runs out,” he said.
Another practice-changing impact from the fourth definition may be expanded U.S. availability and use of MR imaging, which the fourth definition identified as the most informative and versatile of the several imaging options used to confirm or rule out an MI.
Cardiac MR “provides both functional and tissue characterization. It’s the technique with the most potential,” able to noninvasively identify “both the nature and extent of myocardial damage,” explained Chiara Bucciarelli-Ducci, MD, a cardiologist and imaging specialist at the Bristol (England) Heart Institute. A single cardiac MR scan “gives many answers,” said Dr. Bucciarelli-Ducci, who also served on the fourth definition task force and spoke at the session about the document’s revised imaging recommendations.
“In the setting of acute MI, cardiac MR can also be used to assess the presence and extent of myocardium at risk (myocardial edema), myocardial salvage, microvascular obstruction, intramyocardial hemorrhage, and infarct size, all markers of myocardial injury that have prognostic value,” according to the fourth definition. “In patients with possible acute MI but unobstructed coronary arteries, cardiac MR can help to diagnose alternative conditions such as myocarditis, Takotsubo syndrome, embolic infarction, or MI with spontaneous recanalization.”
“What’s turning out is that, a large number of patients with chest pain have an infection and not an MI, and cardiac MR can distinguish inflammation and myocarditis from infarction. We’re now doing a lot more MRs,” Dr. Alpert said. Although MR capability is not as widely available today as other imaging methods, like echocardiography and CT, over the next 5 years that will likely change, he said. But Dr. Alpert cautioned that not every patient with a suspected MI needs MR assessment. It’s best focused for selected patients with an uncertain diagnosis based on the core indicators of disease: history, ECG, changes in hscTn levels over time, and a chest x-ray. “MR is for when there are questions,” he said. When patients present with classic MI signs and symptoms the diagnosis can depend just on the basics, perhaps supplemented with a more widely available imaging method like echocardiography to look for wall motion abnormalities, he said. “If echo shows good left ventricular function you probably don’t need MR.” he said.
CT coronary angiography (CTCA) is another useful diagnostic tool, and right now is more widely available than MR. CTCA “may be used to diagnose coronary artery disease in patients with an acute coronary syndrome event in the emergency department or chest pain unit, particularly in low- to intermediate-risk patients with normal hscTn at presentation,” said the fourth definition. But Dr. Alpert cited the radiation dose from CT as a limiting factor. “We have patients who get repeat CT scans, and we know that increases their cancer risk. There is no such thing as a totally safe dose of radiation.” Lack of radiation exposure is another feature that makes MR imaging attractive.
Dr. Alpert had no disclosures. Dr. Bucciarelli-Ducci has had a financial relationship with Circle Cardiovascular Imaging.
MUNICH – The worldwide cardiology community’s newly revised universal definition of an MI refines the way that cardiologists distinguish between myocardial infarction and myocardial injury, said Joseph S. Alpert, MD, one of the two chairs of the definition-writing panel.
“We had three previous definitions, but there is still a lot of confusion [about distinguishing] between injury and infarction. We definitely hope that this fourth definition will further help people distinguish the two and help people determine whether or not a patient has an MI,” said Dr. Alpert following a session at the annual congress of the European Society of Cardiology that introduced some of the key elements of the new revision.
Days before the ESC congress, a task force formed by the European Society of Cardiology, the American College of Cardiology, the American Heart Association, and the World Heart Federation released the Fourth Universal Definition of Myocardial Infarction (2018) (J Am Coll Cardiol. 2018 Aug 24. doi: 10.1016/j.jacc.2018.08.1038), which follows the series of three prior MI definitions that these groups have issued since the first iteration came out in 2007 (J Am Coll Cardiol. 2007;50[22]:2173-95).
The new revision includes 5 “new concepts,” 14 updated concepts, and 6 new sections since the third universal definition from 2012. The change that topped Dr. Alpert’s list of key messages was the need to determine whether a rise in cardiac troponin, a key biomarker of cardiac damage, resulted from infarction or injury.
These two alternative diagnoses mean “a very different outlook for patients. Treatment is different, and their prognosis is different. It’s important to make the distinction,” said Dr. Alpert, professor of medicine at the University of Arizona in Tucson.
The new changes to making an MI diagnosis will likely help drive a couple of important changes in the way U.S. patients with suspected myocardial injury or infarction get assessed, he said in an interview. The first change will be wide uptake of high sensitivity cardiac troponin (hscTn) assays over the next 5 years or so, as the ability to measure this key diagnostic biomarker progresses from its initial Food and Drug Administration approval for the U.S. market in 2017 to “close to 100% of U.S. hospitals using it,” he predicted. A big issue that is currently slowing even quicker adoption of hscTn is that many hospitals, including the one where Dr. Alpert practices, still have laboratory contracts in place that tether them to older troponin-testing technologies and make it economically unfeasible to change until their contracts expire. The contract in place where Dr. Alpert practices runs out in 2019, and soon after that happens he expects to gain the ability to order a hscTn test.
The new, fourth definition says that hscTn is “recommended for routine clinical use,” but routine U.S. use “won’t be immediate because many hospitals will put in hscTn only when their old contract runs out,” he said.
Another practice-changing impact from the fourth definition may be expanded U.S. availability and use of MR imaging, which the fourth definition identified as the most informative and versatile of the several imaging options used to confirm or rule out an MI.
Cardiac MR “provides both functional and tissue characterization. It’s the technique with the most potential,” able to noninvasively identify “both the nature and extent of myocardial damage,” explained Chiara Bucciarelli-Ducci, MD, a cardiologist and imaging specialist at the Bristol (England) Heart Institute. A single cardiac MR scan “gives many answers,” said Dr. Bucciarelli-Ducci, who also served on the fourth definition task force and spoke at the session about the document’s revised imaging recommendations.
“In the setting of acute MI, cardiac MR can also be used to assess the presence and extent of myocardium at risk (myocardial edema), myocardial salvage, microvascular obstruction, intramyocardial hemorrhage, and infarct size, all markers of myocardial injury that have prognostic value,” according to the fourth definition. “In patients with possible acute MI but unobstructed coronary arteries, cardiac MR can help to diagnose alternative conditions such as myocarditis, Takotsubo syndrome, embolic infarction, or MI with spontaneous recanalization.”
“What’s turning out is that, a large number of patients with chest pain have an infection and not an MI, and cardiac MR can distinguish inflammation and myocarditis from infarction. We’re now doing a lot more MRs,” Dr. Alpert said. Although MR capability is not as widely available today as other imaging methods, like echocardiography and CT, over the next 5 years that will likely change, he said. But Dr. Alpert cautioned that not every patient with a suspected MI needs MR assessment. It’s best focused for selected patients with an uncertain diagnosis based on the core indicators of disease: history, ECG, changes in hscTn levels over time, and a chest x-ray. “MR is for when there are questions,” he said. When patients present with classic MI signs and symptoms the diagnosis can depend just on the basics, perhaps supplemented with a more widely available imaging method like echocardiography to look for wall motion abnormalities, he said. “If echo shows good left ventricular function you probably don’t need MR.” he said.
CT coronary angiography (CTCA) is another useful diagnostic tool, and right now is more widely available than MR. CTCA “may be used to diagnose coronary artery disease in patients with an acute coronary syndrome event in the emergency department or chest pain unit, particularly in low- to intermediate-risk patients with normal hscTn at presentation,” said the fourth definition. But Dr. Alpert cited the radiation dose from CT as a limiting factor. “We have patients who get repeat CT scans, and we know that increases their cancer risk. There is no such thing as a totally safe dose of radiation.” Lack of radiation exposure is another feature that makes MR imaging attractive.
Dr. Alpert had no disclosures. Dr. Bucciarelli-Ducci has had a financial relationship with Circle Cardiovascular Imaging.
MUNICH – The worldwide cardiology community’s newly revised universal definition of an MI refines the way that cardiologists distinguish between myocardial infarction and myocardial injury, said Joseph S. Alpert, MD, one of the two chairs of the definition-writing panel.
“We had three previous definitions, but there is still a lot of confusion [about distinguishing] between injury and infarction. We definitely hope that this fourth definition will further help people distinguish the two and help people determine whether or not a patient has an MI,” said Dr. Alpert following a session at the annual congress of the European Society of Cardiology that introduced some of the key elements of the new revision.
Days before the ESC congress, a task force formed by the European Society of Cardiology, the American College of Cardiology, the American Heart Association, and the World Heart Federation released the Fourth Universal Definition of Myocardial Infarction (2018) (J Am Coll Cardiol. 2018 Aug 24. doi: 10.1016/j.jacc.2018.08.1038), which follows the series of three prior MI definitions that these groups have issued since the first iteration came out in 2007 (J Am Coll Cardiol. 2007;50[22]:2173-95).
The new revision includes 5 “new concepts,” 14 updated concepts, and 6 new sections since the third universal definition from 2012. The change that topped Dr. Alpert’s list of key messages was the need to determine whether a rise in cardiac troponin, a key biomarker of cardiac damage, resulted from infarction or injury.
These two alternative diagnoses mean “a very different outlook for patients. Treatment is different, and their prognosis is different. It’s important to make the distinction,” said Dr. Alpert, professor of medicine at the University of Arizona in Tucson.
The new changes to making an MI diagnosis will likely help drive a couple of important changes in the way U.S. patients with suspected myocardial injury or infarction get assessed, he said in an interview. The first change will be wide uptake of high sensitivity cardiac troponin (hscTn) assays over the next 5 years or so, as the ability to measure this key diagnostic biomarker progresses from its initial Food and Drug Administration approval for the U.S. market in 2017 to “close to 100% of U.S. hospitals using it,” he predicted. A big issue that is currently slowing even quicker adoption of hscTn is that many hospitals, including the one where Dr. Alpert practices, still have laboratory contracts in place that tether them to older troponin-testing technologies and make it economically unfeasible to change until their contracts expire. The contract in place where Dr. Alpert practices runs out in 2019, and soon after that happens he expects to gain the ability to order a hscTn test.
The new, fourth definition says that hscTn is “recommended for routine clinical use,” but routine U.S. use “won’t be immediate because many hospitals will put in hscTn only when their old contract runs out,” he said.
Another practice-changing impact from the fourth definition may be expanded U.S. availability and use of MR imaging, which the fourth definition identified as the most informative and versatile of the several imaging options used to confirm or rule out an MI.
Cardiac MR “provides both functional and tissue characterization. It’s the technique with the most potential,” able to noninvasively identify “both the nature and extent of myocardial damage,” explained Chiara Bucciarelli-Ducci, MD, a cardiologist and imaging specialist at the Bristol (England) Heart Institute. A single cardiac MR scan “gives many answers,” said Dr. Bucciarelli-Ducci, who also served on the fourth definition task force and spoke at the session about the document’s revised imaging recommendations.
“In the setting of acute MI, cardiac MR can also be used to assess the presence and extent of myocardium at risk (myocardial edema), myocardial salvage, microvascular obstruction, intramyocardial hemorrhage, and infarct size, all markers of myocardial injury that have prognostic value,” according to the fourth definition. “In patients with possible acute MI but unobstructed coronary arteries, cardiac MR can help to diagnose alternative conditions such as myocarditis, Takotsubo syndrome, embolic infarction, or MI with spontaneous recanalization.”
“What’s turning out is that, a large number of patients with chest pain have an infection and not an MI, and cardiac MR can distinguish inflammation and myocarditis from infarction. We’re now doing a lot more MRs,” Dr. Alpert said. Although MR capability is not as widely available today as other imaging methods, like echocardiography and CT, over the next 5 years that will likely change, he said. But Dr. Alpert cautioned that not every patient with a suspected MI needs MR assessment. It’s best focused for selected patients with an uncertain diagnosis based on the core indicators of disease: history, ECG, changes in hscTn levels over time, and a chest x-ray. “MR is for when there are questions,” he said. When patients present with classic MI signs and symptoms the diagnosis can depend just on the basics, perhaps supplemented with a more widely available imaging method like echocardiography to look for wall motion abnormalities, he said. “If echo shows good left ventricular function you probably don’t need MR.” he said.
CT coronary angiography (CTCA) is another useful diagnostic tool, and right now is more widely available than MR. CTCA “may be used to diagnose coronary artery disease in patients with an acute coronary syndrome event in the emergency department or chest pain unit, particularly in low- to intermediate-risk patients with normal hscTn at presentation,” said the fourth definition. But Dr. Alpert cited the radiation dose from CT as a limiting factor. “We have patients who get repeat CT scans, and we know that increases their cancer risk. There is no such thing as a totally safe dose of radiation.” Lack of radiation exposure is another feature that makes MR imaging attractive.
Dr. Alpert had no disclosures. Dr. Bucciarelli-Ducci has had a financial relationship with Circle Cardiovascular Imaging.
REPORTING FROM THE ESC CONGRESS 2018