User login
Pulmonary infarction due to pulmonary embolism
A 76-year-old man whose history included abdominal aortic aneurysm repair, bilateral femoral artery bypass for popliteal artery aneurysm, hypertension, and peptic ulcer disease was admitted to a community hospital with pleuritic chest pain and shortness of breath. Two days earlier, he had undergone repair of a ventral hernia.
At the time of that admission, he reported no fever, chills, night sweats, cough, or history of heart or lung disease. His vital signs were normal, and physical examination had revealed no apparent respiratory distress, no jugular venous distention, normal heart sounds, and no pedal edema; however, decreased air entry was noted in the right lung base. Initial serum levels of troponin and N-terminal pro-B-type natriuretic peptide were normal.
At that time, computed tomographic angiography of the chest showed segmental pulmonary emboli in the left upper and right lower lobes of the lungs and right pleural effusion. Transthoracic echocardiography showed normal atrial and ventricular sizes with no right or left ventricular systolic dysfunction and a left ventricular ejection fraction of 59%.
Treatment with intravenous heparin was started, and the patient was transferred to our hospital.
PLEURAL EFFUSION AND PULMONARY EMBOLISM
1. Which of the following is true about pleural effusion?
- It is rarely, if ever, associated with pulmonary embolism
- Most patients with pleural effusion due to pulmonary embolism do not have pleuritic chest pain
- Pulmonary embolism should be excluded in all cases of pleural effusion without a clear cause
Pulmonary embolism should be excluded in all cases of pleural effusion that do not have a clear cause. As for the other answer choices:
- Pulmonary embolism is the fourth leading cause of pleural effusion in the United States, after heart failure, pneumonia, and malignancy.1
- About 75% of patients who develop pleural effusion in the setting of pulmonary embolism complain of pleuritic chest pain on the side of the effusion.2 Most effusions are unilateral, small, and usually exudative.3
EVALUATION BEGINS: RESULTS OF THORACENTESIS
Our patient continued to receive intravenous heparin.
He underwent thoracentesis on hospital day 3, and 1,000 mL of turbid sanguineous pleural fluid was removed. Analysis of the fluid showed pH 7.27, white blood cell count 3.797 × 109/L with 80% neutrophils, and lactate dehydrogenase (LDH) concentration 736 U/L (a ratio of pleural fluid LDH to a concurrent serum LDH > 0.6 is suggestive of an exudate); the fluid was also sent for culture and cytology. Thoracentesis was terminated early due to cough, and follow-up chest radiography showed a moderate-sized pneumothorax.
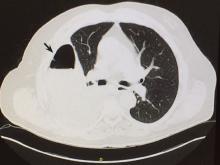
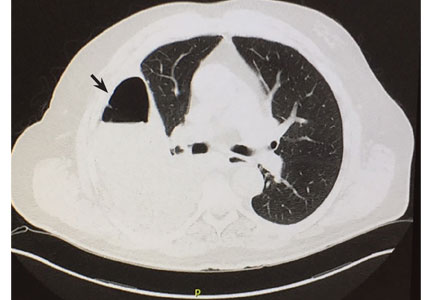
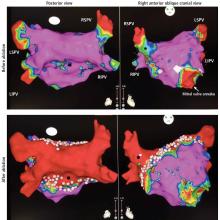
Computed tomography (CT) of the chest at this time showed a small wedge-shaped area of lung consolidation in the right lower lobe (also seen on CT done 1 day before admission to our hospital), with an intrinsic air-fluid level suggesting a focal infarct or lung abscess, now obscured by adjacent consolidation and atelectasis. In the interval since the previous CT, the multiloculated right pleural effusion had increased in size (Figure 1).
THE NEXT STEP
2. What is the most appropriate next step for this patient?
- Consult an interventional radiologist for chest tube placement
- Start empiric antibiotic therapy and ask an interventional radiologist to place a chest tube
- Start empiric antibiotic therapy, withhold anticoagulation, and consult a thoracic surgeon
- Start empiric antibiotic therapy and consult a thoracic surgeon while continuing anticoagulation
The most appropriate next step is to start empiric antibiotic therapy and consult a thoracic surgeon while continuing anticoagulation.
In this patient, it is appropriate to initiate antibiotics empirically on the basis of his significant pleural loculations, a wedge-shaped consolidation, and 80% neutrophils in the pleural fluid, all of which suggest infection. The unmasking of a wedge-shaped consolidation after thoracentesis, with a previously noted air-fluid level and an interval increase in multiloculated pleural fluid, raises suspicion of a necrotic infection that may have ruptured into the pleural space, a possible lung infarct, or a malignancy. Hence, simply placing a chest tube may not be enough.
Blood in the pleural fluid does not necessitate withholding anticoagulation unless the bleeding is heavy. A pleural fluid hematocrit greater than 50% of the peripheral blood hematocrit suggests hemothorax and is an indication to withhold anticoagulation.1 Our patient’s pleural fluid was qualitatively sanguineous but not frankly bloody, and therefore we judged that it was not necessary to stop his heparin.
HOW DOES PULMONARY INFARCTION PRESENT CLINICALLY?
3. Which of the following statements about pulmonary infarction is incorrect?
- Cavitation and infarction are more common with larger emboli
- Cavitation occurs in fewer than 10% of pulmonary infarctions
- Lung abscess develops in more than 50% of pulmonary infarctions
- Pulmonary thromboembolism is the most common cause of pulmonary infarction
Lung abscess develops in far fewer than 50% of cases of pulmonary infarction. The rest of the statements are correct.
Cavitation complicates about 4% to 7% of infarctions and is more common when the infarction is 4 cm or greater in diameter.4 These cavities are usually single and predominantly on the right side in the apical or posterior segment of the upper lobe or the apical segment of the right lower lobe, as in our patient.5–8 CT demonstrating scalloped inner margins and cross-cavity band shadows suggests a cavitary pulmonary infarction.9,10
Infection and abscess in pulmonary infarction are poorly understood but have been linked to larger infarctions, coexistent congestion or atelectasis, and dental or oropharyngeal infection. In an early series of 550 cases of pulmonary infarction, 23 patients (4.2%) developed lung abscess and 6 (1.1%) developed empyema.11 The mean time to cavitation for an infected pulmonary infarction has been reported to be 18 days.12
A reversed halo sign, generally described as a focal, rounded area of ground-glass opacity surrounded by a nearly complete ring of consolidation, has been reported to be more frequent with pulmonary infarction than with other diseases, especially when in the lower lobes.13
CASE CONTINUED: THORACOSCOPY
A cardiothoracic surgeon was consulted, intravenous heparin was discontinued, an inferior vena cava filter was placed, and the patient underwent video-assisted thoracoscopy.
Purulent fluid was noted on the lateral aspect of right lower lobe; this appeared to be the ruptured cavitary lesion functioning like an uncontrolled bronchopleural fistula. Two chest tubes, sizes 32F and 28F, were placed after decortication, resection of the lung abscess, and closure of the bronchopleural fistula. No significant air leak was noted after resection of this segment of lung.
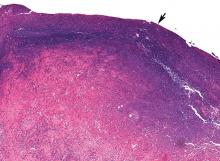
Pathologic study showed acute organizing pneumonia with abscess formation; no malignant cells or granulomas were seen (Figure 2). Pleural fluid cultures grew Streptococcus intermedius, while the tissue culture was negative for any growth, including acid-fast bacilli and fungi.
On 3 different occasions, both chest tubes were shortened, backed out 2 cm, and resecured with sutures and pins, and Heimlich valves were applied before the patient was discharged.
Intravenous piperacillin-tazobactam was started on the fifth hospital day. On discharge, the patient was advised to continue this treatment for 3 weeks at home.
The patient was receiving enoxaparin subcutaneously in prophylactic doses; 72 hours after the thorascopic procedure this was increased to therapeutic doses, continuing after discharge. Bridging to warfarin was not advised in view of his chest tubes.
Our patient appeared to have developed a right lower lobe infarction that cavitated and ruptured into the pleural space, causing a bronchopleural fistula with empyema after a recent pulmonary embolism. Other reported causes of pulmonary infarction in pulmonary embolism are malignancy and heavy clot burden,6 but these have not been confirmed in subsequent studies.5 Malignancy was ruled out by biopsy of the resected portion of the lung, and our patient did not have a history of heart failure. A clear cavity was not noted (because it ruptured into the pleura), but an air-fluid level was described in a wedge-shaped consolidation, suggesting infarction.
How common is pulmonary infarction after pulmonary embolism?
Pulmonary infarction occurs in few patients with pulmonary embolism.13 Since the lungs receive oxygen from the airways and have a dual blood supply from the pulmonary and bronchial arteries, they are not particularly vulnerable to ischemia. However, the reported incidence of pulmonary infarction in patients with pulmonary embolism has ranged from 10% to higher than 30%.5,14,15
The reasons behind pulmonary infarction with complications after pulmonary embolism have varied in different case series in different eras. CT, biopsy, or autopsy studies reveal pulmonary infarction after pulmonary embolism to be more common than suspected by clinical symptoms.
In a Mayo Clinic series of 43 cases of pulmonary infarction diagnosed over a 6-year period by surgical lung biopsy, 18 (42%) of the patients had underlying pulmonary thromboembolism, which was the most common cause.16
RISK FACTORS FOR PULMONARY INFARCTION
4. Which statement about risk factors for pulmonary infarction in pulmonary embolism is incorrect?
- Heart failure may be a risk factor for pulmonary infarction
- Pulmonary hemorrhage is a risk factor for pulmonary infarction
- Pulmonary infarction is more common with more proximal sites of pulmonary embolism
- Collateral circulation may protect against pulmonary infarction
Infarction is more common with emboli that are distal rather than proximal.
Dalen et al15 suggested that after pulmonary embolism, pulmonary hemorrhage is an important contributor to the development of pulmonary infarction independent of the presence or absence of associated cardiac or pulmonary disease, but that the effect depends on the site of obstruction.
This idea was first proposed in 1913, when Karsner and Ghoreyeb17 showed that when pulmonary arteries are completely obstructed, the bronchial arteries take over, except when the embolism is present in a small branch of the pulmonary artery. This is because the physiologic anastomosis between the pulmonary artery and the bronchial arteries is located at the precapillary level of the pulmonary artery, and the bronchial circulation does not take over until the pulmonary arterial pressure in the area of the embolism drops to zero.
Using CT data, Kirchner et al5 confirmed that the risk of pulmonary infarction is higher if the obstruction is peripheral, ie, distal.
Using autopsy data, Tsao et al18 reported a higher risk of pulmonary infarction in embolic occlusion of pulmonary vessels less than 3 mm in diameter.
Collateral circulation has been shown to protect against pulmonary infarction. For example, Miniati et al14 showed that healthy young patients with pulmonary embolism were more prone to develop pulmonary infarction, probably because they had less efficient collateral systems in the peripheral lung fields. In lung transplant recipients, it has been shown that the risk of infarction decreased with development of collateral circulation.19
Dalen et al,15 however, attributed delayed resolution of pulmonary hemorrhage (as measured by resolution of infiltrate on chest radiography) to higher underlying pulmonary venous pressure in patients with heart failure and consequent pulmonary infarction. In comparison, healthy patients without cardiac or pulmonary disease have faster resolution of pulmonary hemorrhage when present, and less likelihood of pulmonary infarction (and death in submassive pulmonary embolism).
Data on the management of infected pulmonary infarction are limited. Mortality rates have been as high as 41% with noninfected and 73% with infected cavitary infarctions.4 Some authors have advocated early surgical resection in view of high rates of failure of medical treatment due to lack of blood supply within the cavity and continued risk of infection.
KEY POINTS
In patients with a recently diagnosed pulmonary embolism and concurrent symptoms of bacterial pneumonia, a diagnosis of cavitary pulmonary infarction should be considered.
Consolidations that are pleural-based with sharp, rounded margins and with focal areas of central hyperlucencies representing hemorrhage on the mediastinal windows on CT are more likely to represent a pulmonary infarct.20
- Light RW. Pleural Diseases. 4th ed. Baltimore, MD: Lippincott, Williams & Wilkins; 2001.
- Stein PD, Terrin ML, Hales CA, et al. Clinical, laboratory, roentgenographic, and electrocardiographic findings in patients with acute pulmonary embolism and no pre-existing cardiac or pulmonary disease. Chest 1991; 100(3):598–603. pmid:1909617
- Light RW. Pleural effusion due to pulmonary emboli. Curr Opin Pulm Med 2001; 7(4):198–201. pmid:11470974
- Libby LS, King TE, LaForce FM, Schwarz MI. Pulmonary cavitation following pulmonary infarction. Medicine (Baltimore) 1985; 64(5):342–348. pmid:4033411
- Kirchner J, Obermann A, Stuckradt S, et al. Lung infarction following pulmonary embolism: a comparative study on clinical conditions and CT findings to identify predisposing factors. Rofo 2015; 187(6):440–444. doi:10.1055/s-0034-1399006
- He H, Stein MW, Zalta B, Haramati LB. Pulmonary infarction: spectrum of findings on multidetector helical CT. J Thorac Imaging 2006; 21(1):1–7. doi:10.1097/01.rti.0000187433.06762.fb
- Scharf J, Nahir AM, Munk J, Lichtig C. Aseptic cavitation in pulmonary infarction. Chest 1971; 59(4):456–458. pmid:5551596
- Wilson AG, Joseph AE, Butland RJ. The radiology of aseptic cavitation in pulmonary infarction. Clin Radiol 1986; 37(4):327–333. pmid:3731699
- Butler MD, Biscardi FH, Schain DC, Humphries JE, Blow O, Spotnitz WD. Pulmonary resection for treatment of cavitary pulmonary infarction. Ann Thorac Surg 1997; 63(3):849–850. pmid:9066420
- Koroscil MT, Hauser TR. Acute pulmonary embolism leading to cavitation and large pulmonary abscess: a rare complication of pulmonary infarction. Respir Med Case Rep 2016; 20:72–74. doi:10.1016/j.rmcr.2016.12.001
- Levin L, Kernohan JW, Moersch HJ. Pulmonary abscess secondary to bland pulmonary infarction. Dis Chest 1948; 14(2):218–232. pmid:18904835
- Marchiori E, Menna Barreto M, Pereira Freitas HM, et al. Morphological characteristics of the reversed halo sign that may strongly suggest pulmonary infarction. Clin Radiol 2018; 73(5):503.e7–503.e13. doi:10.1016/j.crad.2017.11.022
- Smith GT, Dexter L, Dammin GJ. Postmortem quantitative studies in pulmonary embolism. In: Sasahara AA, Stein M, eds. Pulmonary Embolic Disease. New York, NY: Grune & Stratton, Inc; 1965:120–126.
- Miniati M, Bottai M, Ciccotosto C, Roberto L, Monti S. Predictors of pulmonary infarction. Medicine (Baltimore) 2015; 94(41):e1488. doi:10.1097/MD.0000000000001488
- Dalen JE, Haffajee CI, Alpert JS, Howe JP, Ockene IS, Paraskos JA. Pulmonary embolism, pulmonary hemorrhage and pulmonary infarction. N Engl J Med 1977; 296(25):1431–1435. doi:10.1056/NEJM197706232962503
- Parambil JG, Savci CD, Tazelaar HD, Ryu JH. Causes and presenting features of pulmonary infarctions in 43 cases identified by surgical lung biopsy. Chest 2005; 127(4):1178–1183. doi:10.1378/chest.127.4.1178
- Karsner HT, Ghoreyeb AA. Studies in infarction: III. The circulation in experimental pulmonary embolism. J Exp Med 1913; 18(5):507–511. pmid:19867725
- Tsao MS, Schraufnagel D, Wang NS. Pathogenesis of pulmonary infarction. Am J Med 1982; 72(4):599–606. pmid:6462058
- Burns KE, Iacono AT. Incidence of clinically unsuspected pulmonary embolism in mechanically ventilated lung transplant recipients. Transplantation 2003; 76(6):964–968. doi:10.1097/01.TP.0000084523.58610.BA
- Yousem SA. The surgical pathology of pulmonary infarcts: diagnostic confusion with granulomatous disease, vasculitis, and neoplasia. Mod Pathol 2009; 22(5):679–685. doi:10.1038/modpathol.2009.20
A 76-year-old man whose history included abdominal aortic aneurysm repair, bilateral femoral artery bypass for popliteal artery aneurysm, hypertension, and peptic ulcer disease was admitted to a community hospital with pleuritic chest pain and shortness of breath. Two days earlier, he had undergone repair of a ventral hernia.
At the time of that admission, he reported no fever, chills, night sweats, cough, or history of heart or lung disease. His vital signs were normal, and physical examination had revealed no apparent respiratory distress, no jugular venous distention, normal heart sounds, and no pedal edema; however, decreased air entry was noted in the right lung base. Initial serum levels of troponin and N-terminal pro-B-type natriuretic peptide were normal.
At that time, computed tomographic angiography of the chest showed segmental pulmonary emboli in the left upper and right lower lobes of the lungs and right pleural effusion. Transthoracic echocardiography showed normal atrial and ventricular sizes with no right or left ventricular systolic dysfunction and a left ventricular ejection fraction of 59%.
Treatment with intravenous heparin was started, and the patient was transferred to our hospital.
PLEURAL EFFUSION AND PULMONARY EMBOLISM
1. Which of the following is true about pleural effusion?
- It is rarely, if ever, associated with pulmonary embolism
- Most patients with pleural effusion due to pulmonary embolism do not have pleuritic chest pain
- Pulmonary embolism should be excluded in all cases of pleural effusion without a clear cause
Pulmonary embolism should be excluded in all cases of pleural effusion that do not have a clear cause. As for the other answer choices:
- Pulmonary embolism is the fourth leading cause of pleural effusion in the United States, after heart failure, pneumonia, and malignancy.1
- About 75% of patients who develop pleural effusion in the setting of pulmonary embolism complain of pleuritic chest pain on the side of the effusion.2 Most effusions are unilateral, small, and usually exudative.3
EVALUATION BEGINS: RESULTS OF THORACENTESIS
Our patient continued to receive intravenous heparin.
He underwent thoracentesis on hospital day 3, and 1,000 mL of turbid sanguineous pleural fluid was removed. Analysis of the fluid showed pH 7.27, white blood cell count 3.797 × 109/L with 80% neutrophils, and lactate dehydrogenase (LDH) concentration 736 U/L (a ratio of pleural fluid LDH to a concurrent serum LDH > 0.6 is suggestive of an exudate); the fluid was also sent for culture and cytology. Thoracentesis was terminated early due to cough, and follow-up chest radiography showed a moderate-sized pneumothorax.
Computed tomography (CT) of the chest at this time showed a small wedge-shaped area of lung consolidation in the right lower lobe (also seen on CT done 1 day before admission to our hospital), with an intrinsic air-fluid level suggesting a focal infarct or lung abscess, now obscured by adjacent consolidation and atelectasis. In the interval since the previous CT, the multiloculated right pleural effusion had increased in size (Figure 1).
THE NEXT STEP
2. What is the most appropriate next step for this patient?
- Consult an interventional radiologist for chest tube placement
- Start empiric antibiotic therapy and ask an interventional radiologist to place a chest tube
- Start empiric antibiotic therapy, withhold anticoagulation, and consult a thoracic surgeon
- Start empiric antibiotic therapy and consult a thoracic surgeon while continuing anticoagulation
The most appropriate next step is to start empiric antibiotic therapy and consult a thoracic surgeon while continuing anticoagulation.
In this patient, it is appropriate to initiate antibiotics empirically on the basis of his significant pleural loculations, a wedge-shaped consolidation, and 80% neutrophils in the pleural fluid, all of which suggest infection. The unmasking of a wedge-shaped consolidation after thoracentesis, with a previously noted air-fluid level and an interval increase in multiloculated pleural fluid, raises suspicion of a necrotic infection that may have ruptured into the pleural space, a possible lung infarct, or a malignancy. Hence, simply placing a chest tube may not be enough.
Blood in the pleural fluid does not necessitate withholding anticoagulation unless the bleeding is heavy. A pleural fluid hematocrit greater than 50% of the peripheral blood hematocrit suggests hemothorax and is an indication to withhold anticoagulation.1 Our patient’s pleural fluid was qualitatively sanguineous but not frankly bloody, and therefore we judged that it was not necessary to stop his heparin.
HOW DOES PULMONARY INFARCTION PRESENT CLINICALLY?
3. Which of the following statements about pulmonary infarction is incorrect?
- Cavitation and infarction are more common with larger emboli
- Cavitation occurs in fewer than 10% of pulmonary infarctions
- Lung abscess develops in more than 50% of pulmonary infarctions
- Pulmonary thromboembolism is the most common cause of pulmonary infarction
Lung abscess develops in far fewer than 50% of cases of pulmonary infarction. The rest of the statements are correct.
Cavitation complicates about 4% to 7% of infarctions and is more common when the infarction is 4 cm or greater in diameter.4 These cavities are usually single and predominantly on the right side in the apical or posterior segment of the upper lobe or the apical segment of the right lower lobe, as in our patient.5–8 CT demonstrating scalloped inner margins and cross-cavity band shadows suggests a cavitary pulmonary infarction.9,10
Infection and abscess in pulmonary infarction are poorly understood but have been linked to larger infarctions, coexistent congestion or atelectasis, and dental or oropharyngeal infection. In an early series of 550 cases of pulmonary infarction, 23 patients (4.2%) developed lung abscess and 6 (1.1%) developed empyema.11 The mean time to cavitation for an infected pulmonary infarction has been reported to be 18 days.12
A reversed halo sign, generally described as a focal, rounded area of ground-glass opacity surrounded by a nearly complete ring of consolidation, has been reported to be more frequent with pulmonary infarction than with other diseases, especially when in the lower lobes.13
CASE CONTINUED: THORACOSCOPY
A cardiothoracic surgeon was consulted, intravenous heparin was discontinued, an inferior vena cava filter was placed, and the patient underwent video-assisted thoracoscopy.
Purulent fluid was noted on the lateral aspect of right lower lobe; this appeared to be the ruptured cavitary lesion functioning like an uncontrolled bronchopleural fistula. Two chest tubes, sizes 32F and 28F, were placed after decortication, resection of the lung abscess, and closure of the bronchopleural fistula. No significant air leak was noted after resection of this segment of lung.
Pathologic study showed acute organizing pneumonia with abscess formation; no malignant cells or granulomas were seen (Figure 2). Pleural fluid cultures grew Streptococcus intermedius, while the tissue culture was negative for any growth, including acid-fast bacilli and fungi.
On 3 different occasions, both chest tubes were shortened, backed out 2 cm, and resecured with sutures and pins, and Heimlich valves were applied before the patient was discharged.
Intravenous piperacillin-tazobactam was started on the fifth hospital day. On discharge, the patient was advised to continue this treatment for 3 weeks at home.
The patient was receiving enoxaparin subcutaneously in prophylactic doses; 72 hours after the thorascopic procedure this was increased to therapeutic doses, continuing after discharge. Bridging to warfarin was not advised in view of his chest tubes.
Our patient appeared to have developed a right lower lobe infarction that cavitated and ruptured into the pleural space, causing a bronchopleural fistula with empyema after a recent pulmonary embolism. Other reported causes of pulmonary infarction in pulmonary embolism are malignancy and heavy clot burden,6 but these have not been confirmed in subsequent studies.5 Malignancy was ruled out by biopsy of the resected portion of the lung, and our patient did not have a history of heart failure. A clear cavity was not noted (because it ruptured into the pleura), but an air-fluid level was described in a wedge-shaped consolidation, suggesting infarction.
How common is pulmonary infarction after pulmonary embolism?
Pulmonary infarction occurs in few patients with pulmonary embolism.13 Since the lungs receive oxygen from the airways and have a dual blood supply from the pulmonary and bronchial arteries, they are not particularly vulnerable to ischemia. However, the reported incidence of pulmonary infarction in patients with pulmonary embolism has ranged from 10% to higher than 30%.5,14,15
The reasons behind pulmonary infarction with complications after pulmonary embolism have varied in different case series in different eras. CT, biopsy, or autopsy studies reveal pulmonary infarction after pulmonary embolism to be more common than suspected by clinical symptoms.
In a Mayo Clinic series of 43 cases of pulmonary infarction diagnosed over a 6-year period by surgical lung biopsy, 18 (42%) of the patients had underlying pulmonary thromboembolism, which was the most common cause.16
RISK FACTORS FOR PULMONARY INFARCTION
4. Which statement about risk factors for pulmonary infarction in pulmonary embolism is incorrect?
- Heart failure may be a risk factor for pulmonary infarction
- Pulmonary hemorrhage is a risk factor for pulmonary infarction
- Pulmonary infarction is more common with more proximal sites of pulmonary embolism
- Collateral circulation may protect against pulmonary infarction
Infarction is more common with emboli that are distal rather than proximal.
Dalen et al15 suggested that after pulmonary embolism, pulmonary hemorrhage is an important contributor to the development of pulmonary infarction independent of the presence or absence of associated cardiac or pulmonary disease, but that the effect depends on the site of obstruction.
This idea was first proposed in 1913, when Karsner and Ghoreyeb17 showed that when pulmonary arteries are completely obstructed, the bronchial arteries take over, except when the embolism is present in a small branch of the pulmonary artery. This is because the physiologic anastomosis between the pulmonary artery and the bronchial arteries is located at the precapillary level of the pulmonary artery, and the bronchial circulation does not take over until the pulmonary arterial pressure in the area of the embolism drops to zero.
Using CT data, Kirchner et al5 confirmed that the risk of pulmonary infarction is higher if the obstruction is peripheral, ie, distal.
Using autopsy data, Tsao et al18 reported a higher risk of pulmonary infarction in embolic occlusion of pulmonary vessels less than 3 mm in diameter.
Collateral circulation has been shown to protect against pulmonary infarction. For example, Miniati et al14 showed that healthy young patients with pulmonary embolism were more prone to develop pulmonary infarction, probably because they had less efficient collateral systems in the peripheral lung fields. In lung transplant recipients, it has been shown that the risk of infarction decreased with development of collateral circulation.19
Dalen et al,15 however, attributed delayed resolution of pulmonary hemorrhage (as measured by resolution of infiltrate on chest radiography) to higher underlying pulmonary venous pressure in patients with heart failure and consequent pulmonary infarction. In comparison, healthy patients without cardiac or pulmonary disease have faster resolution of pulmonary hemorrhage when present, and less likelihood of pulmonary infarction (and death in submassive pulmonary embolism).
Data on the management of infected pulmonary infarction are limited. Mortality rates have been as high as 41% with noninfected and 73% with infected cavitary infarctions.4 Some authors have advocated early surgical resection in view of high rates of failure of medical treatment due to lack of blood supply within the cavity and continued risk of infection.
KEY POINTS
In patients with a recently diagnosed pulmonary embolism and concurrent symptoms of bacterial pneumonia, a diagnosis of cavitary pulmonary infarction should be considered.
Consolidations that are pleural-based with sharp, rounded margins and with focal areas of central hyperlucencies representing hemorrhage on the mediastinal windows on CT are more likely to represent a pulmonary infarct.20
A 76-year-old man whose history included abdominal aortic aneurysm repair, bilateral femoral artery bypass for popliteal artery aneurysm, hypertension, and peptic ulcer disease was admitted to a community hospital with pleuritic chest pain and shortness of breath. Two days earlier, he had undergone repair of a ventral hernia.
At the time of that admission, he reported no fever, chills, night sweats, cough, or history of heart or lung disease. His vital signs were normal, and physical examination had revealed no apparent respiratory distress, no jugular venous distention, normal heart sounds, and no pedal edema; however, decreased air entry was noted in the right lung base. Initial serum levels of troponin and N-terminal pro-B-type natriuretic peptide were normal.
At that time, computed tomographic angiography of the chest showed segmental pulmonary emboli in the left upper and right lower lobes of the lungs and right pleural effusion. Transthoracic echocardiography showed normal atrial and ventricular sizes with no right or left ventricular systolic dysfunction and a left ventricular ejection fraction of 59%.
Treatment with intravenous heparin was started, and the patient was transferred to our hospital.
PLEURAL EFFUSION AND PULMONARY EMBOLISM
1. Which of the following is true about pleural effusion?
- It is rarely, if ever, associated with pulmonary embolism
- Most patients with pleural effusion due to pulmonary embolism do not have pleuritic chest pain
- Pulmonary embolism should be excluded in all cases of pleural effusion without a clear cause
Pulmonary embolism should be excluded in all cases of pleural effusion that do not have a clear cause. As for the other answer choices:
- Pulmonary embolism is the fourth leading cause of pleural effusion in the United States, after heart failure, pneumonia, and malignancy.1
- About 75% of patients who develop pleural effusion in the setting of pulmonary embolism complain of pleuritic chest pain on the side of the effusion.2 Most effusions are unilateral, small, and usually exudative.3
EVALUATION BEGINS: RESULTS OF THORACENTESIS
Our patient continued to receive intravenous heparin.
He underwent thoracentesis on hospital day 3, and 1,000 mL of turbid sanguineous pleural fluid was removed. Analysis of the fluid showed pH 7.27, white blood cell count 3.797 × 109/L with 80% neutrophils, and lactate dehydrogenase (LDH) concentration 736 U/L (a ratio of pleural fluid LDH to a concurrent serum LDH > 0.6 is suggestive of an exudate); the fluid was also sent for culture and cytology. Thoracentesis was terminated early due to cough, and follow-up chest radiography showed a moderate-sized pneumothorax.
Computed tomography (CT) of the chest at this time showed a small wedge-shaped area of lung consolidation in the right lower lobe (also seen on CT done 1 day before admission to our hospital), with an intrinsic air-fluid level suggesting a focal infarct or lung abscess, now obscured by adjacent consolidation and atelectasis. In the interval since the previous CT, the multiloculated right pleural effusion had increased in size (Figure 1).
THE NEXT STEP
2. What is the most appropriate next step for this patient?
- Consult an interventional radiologist for chest tube placement
- Start empiric antibiotic therapy and ask an interventional radiologist to place a chest tube
- Start empiric antibiotic therapy, withhold anticoagulation, and consult a thoracic surgeon
- Start empiric antibiotic therapy and consult a thoracic surgeon while continuing anticoagulation
The most appropriate next step is to start empiric antibiotic therapy and consult a thoracic surgeon while continuing anticoagulation.
In this patient, it is appropriate to initiate antibiotics empirically on the basis of his significant pleural loculations, a wedge-shaped consolidation, and 80% neutrophils in the pleural fluid, all of which suggest infection. The unmasking of a wedge-shaped consolidation after thoracentesis, with a previously noted air-fluid level and an interval increase in multiloculated pleural fluid, raises suspicion of a necrotic infection that may have ruptured into the pleural space, a possible lung infarct, or a malignancy. Hence, simply placing a chest tube may not be enough.
Blood in the pleural fluid does not necessitate withholding anticoagulation unless the bleeding is heavy. A pleural fluid hematocrit greater than 50% of the peripheral blood hematocrit suggests hemothorax and is an indication to withhold anticoagulation.1 Our patient’s pleural fluid was qualitatively sanguineous but not frankly bloody, and therefore we judged that it was not necessary to stop his heparin.
HOW DOES PULMONARY INFARCTION PRESENT CLINICALLY?
3. Which of the following statements about pulmonary infarction is incorrect?
- Cavitation and infarction are more common with larger emboli
- Cavitation occurs in fewer than 10% of pulmonary infarctions
- Lung abscess develops in more than 50% of pulmonary infarctions
- Pulmonary thromboembolism is the most common cause of pulmonary infarction
Lung abscess develops in far fewer than 50% of cases of pulmonary infarction. The rest of the statements are correct.
Cavitation complicates about 4% to 7% of infarctions and is more common when the infarction is 4 cm or greater in diameter.4 These cavities are usually single and predominantly on the right side in the apical or posterior segment of the upper lobe or the apical segment of the right lower lobe, as in our patient.5–8 CT demonstrating scalloped inner margins and cross-cavity band shadows suggests a cavitary pulmonary infarction.9,10
Infection and abscess in pulmonary infarction are poorly understood but have been linked to larger infarctions, coexistent congestion or atelectasis, and dental or oropharyngeal infection. In an early series of 550 cases of pulmonary infarction, 23 patients (4.2%) developed lung abscess and 6 (1.1%) developed empyema.11 The mean time to cavitation for an infected pulmonary infarction has been reported to be 18 days.12
A reversed halo sign, generally described as a focal, rounded area of ground-glass opacity surrounded by a nearly complete ring of consolidation, has been reported to be more frequent with pulmonary infarction than with other diseases, especially when in the lower lobes.13
CASE CONTINUED: THORACOSCOPY
A cardiothoracic surgeon was consulted, intravenous heparin was discontinued, an inferior vena cava filter was placed, and the patient underwent video-assisted thoracoscopy.
Purulent fluid was noted on the lateral aspect of right lower lobe; this appeared to be the ruptured cavitary lesion functioning like an uncontrolled bronchopleural fistula. Two chest tubes, sizes 32F and 28F, were placed after decortication, resection of the lung abscess, and closure of the bronchopleural fistula. No significant air leak was noted after resection of this segment of lung.
Pathologic study showed acute organizing pneumonia with abscess formation; no malignant cells or granulomas were seen (Figure 2). Pleural fluid cultures grew Streptococcus intermedius, while the tissue culture was negative for any growth, including acid-fast bacilli and fungi.
On 3 different occasions, both chest tubes were shortened, backed out 2 cm, and resecured with sutures and pins, and Heimlich valves were applied before the patient was discharged.
Intravenous piperacillin-tazobactam was started on the fifth hospital day. On discharge, the patient was advised to continue this treatment for 3 weeks at home.
The patient was receiving enoxaparin subcutaneously in prophylactic doses; 72 hours after the thorascopic procedure this was increased to therapeutic doses, continuing after discharge. Bridging to warfarin was not advised in view of his chest tubes.
Our patient appeared to have developed a right lower lobe infarction that cavitated and ruptured into the pleural space, causing a bronchopleural fistula with empyema after a recent pulmonary embolism. Other reported causes of pulmonary infarction in pulmonary embolism are malignancy and heavy clot burden,6 but these have not been confirmed in subsequent studies.5 Malignancy was ruled out by biopsy of the resected portion of the lung, and our patient did not have a history of heart failure. A clear cavity was not noted (because it ruptured into the pleura), but an air-fluid level was described in a wedge-shaped consolidation, suggesting infarction.
How common is pulmonary infarction after pulmonary embolism?
Pulmonary infarction occurs in few patients with pulmonary embolism.13 Since the lungs receive oxygen from the airways and have a dual blood supply from the pulmonary and bronchial arteries, they are not particularly vulnerable to ischemia. However, the reported incidence of pulmonary infarction in patients with pulmonary embolism has ranged from 10% to higher than 30%.5,14,15
The reasons behind pulmonary infarction with complications after pulmonary embolism have varied in different case series in different eras. CT, biopsy, or autopsy studies reveal pulmonary infarction after pulmonary embolism to be more common than suspected by clinical symptoms.
In a Mayo Clinic series of 43 cases of pulmonary infarction diagnosed over a 6-year period by surgical lung biopsy, 18 (42%) of the patients had underlying pulmonary thromboembolism, which was the most common cause.16
RISK FACTORS FOR PULMONARY INFARCTION
4. Which statement about risk factors for pulmonary infarction in pulmonary embolism is incorrect?
- Heart failure may be a risk factor for pulmonary infarction
- Pulmonary hemorrhage is a risk factor for pulmonary infarction
- Pulmonary infarction is more common with more proximal sites of pulmonary embolism
- Collateral circulation may protect against pulmonary infarction
Infarction is more common with emboli that are distal rather than proximal.
Dalen et al15 suggested that after pulmonary embolism, pulmonary hemorrhage is an important contributor to the development of pulmonary infarction independent of the presence or absence of associated cardiac or pulmonary disease, but that the effect depends on the site of obstruction.
This idea was first proposed in 1913, when Karsner and Ghoreyeb17 showed that when pulmonary arteries are completely obstructed, the bronchial arteries take over, except when the embolism is present in a small branch of the pulmonary artery. This is because the physiologic anastomosis between the pulmonary artery and the bronchial arteries is located at the precapillary level of the pulmonary artery, and the bronchial circulation does not take over until the pulmonary arterial pressure in the area of the embolism drops to zero.
Using CT data, Kirchner et al5 confirmed that the risk of pulmonary infarction is higher if the obstruction is peripheral, ie, distal.
Using autopsy data, Tsao et al18 reported a higher risk of pulmonary infarction in embolic occlusion of pulmonary vessels less than 3 mm in diameter.
Collateral circulation has been shown to protect against pulmonary infarction. For example, Miniati et al14 showed that healthy young patients with pulmonary embolism were more prone to develop pulmonary infarction, probably because they had less efficient collateral systems in the peripheral lung fields. In lung transplant recipients, it has been shown that the risk of infarction decreased with development of collateral circulation.19
Dalen et al,15 however, attributed delayed resolution of pulmonary hemorrhage (as measured by resolution of infiltrate on chest radiography) to higher underlying pulmonary venous pressure in patients with heart failure and consequent pulmonary infarction. In comparison, healthy patients without cardiac or pulmonary disease have faster resolution of pulmonary hemorrhage when present, and less likelihood of pulmonary infarction (and death in submassive pulmonary embolism).
Data on the management of infected pulmonary infarction are limited. Mortality rates have been as high as 41% with noninfected and 73% with infected cavitary infarctions.4 Some authors have advocated early surgical resection in view of high rates of failure of medical treatment due to lack of blood supply within the cavity and continued risk of infection.
KEY POINTS
In patients with a recently diagnosed pulmonary embolism and concurrent symptoms of bacterial pneumonia, a diagnosis of cavitary pulmonary infarction should be considered.
Consolidations that are pleural-based with sharp, rounded margins and with focal areas of central hyperlucencies representing hemorrhage on the mediastinal windows on CT are more likely to represent a pulmonary infarct.20
- Light RW. Pleural Diseases. 4th ed. Baltimore, MD: Lippincott, Williams & Wilkins; 2001.
- Stein PD, Terrin ML, Hales CA, et al. Clinical, laboratory, roentgenographic, and electrocardiographic findings in patients with acute pulmonary embolism and no pre-existing cardiac or pulmonary disease. Chest 1991; 100(3):598–603. pmid:1909617
- Light RW. Pleural effusion due to pulmonary emboli. Curr Opin Pulm Med 2001; 7(4):198–201. pmid:11470974
- Libby LS, King TE, LaForce FM, Schwarz MI. Pulmonary cavitation following pulmonary infarction. Medicine (Baltimore) 1985; 64(5):342–348. pmid:4033411
- Kirchner J, Obermann A, Stuckradt S, et al. Lung infarction following pulmonary embolism: a comparative study on clinical conditions and CT findings to identify predisposing factors. Rofo 2015; 187(6):440–444. doi:10.1055/s-0034-1399006
- He H, Stein MW, Zalta B, Haramati LB. Pulmonary infarction: spectrum of findings on multidetector helical CT. J Thorac Imaging 2006; 21(1):1–7. doi:10.1097/01.rti.0000187433.06762.fb
- Scharf J, Nahir AM, Munk J, Lichtig C. Aseptic cavitation in pulmonary infarction. Chest 1971; 59(4):456–458. pmid:5551596
- Wilson AG, Joseph AE, Butland RJ. The radiology of aseptic cavitation in pulmonary infarction. Clin Radiol 1986; 37(4):327–333. pmid:3731699
- Butler MD, Biscardi FH, Schain DC, Humphries JE, Blow O, Spotnitz WD. Pulmonary resection for treatment of cavitary pulmonary infarction. Ann Thorac Surg 1997; 63(3):849–850. pmid:9066420
- Koroscil MT, Hauser TR. Acute pulmonary embolism leading to cavitation and large pulmonary abscess: a rare complication of pulmonary infarction. Respir Med Case Rep 2016; 20:72–74. doi:10.1016/j.rmcr.2016.12.001
- Levin L, Kernohan JW, Moersch HJ. Pulmonary abscess secondary to bland pulmonary infarction. Dis Chest 1948; 14(2):218–232. pmid:18904835
- Marchiori E, Menna Barreto M, Pereira Freitas HM, et al. Morphological characteristics of the reversed halo sign that may strongly suggest pulmonary infarction. Clin Radiol 2018; 73(5):503.e7–503.e13. doi:10.1016/j.crad.2017.11.022
- Smith GT, Dexter L, Dammin GJ. Postmortem quantitative studies in pulmonary embolism. In: Sasahara AA, Stein M, eds. Pulmonary Embolic Disease. New York, NY: Grune & Stratton, Inc; 1965:120–126.
- Miniati M, Bottai M, Ciccotosto C, Roberto L, Monti S. Predictors of pulmonary infarction. Medicine (Baltimore) 2015; 94(41):e1488. doi:10.1097/MD.0000000000001488
- Dalen JE, Haffajee CI, Alpert JS, Howe JP, Ockene IS, Paraskos JA. Pulmonary embolism, pulmonary hemorrhage and pulmonary infarction. N Engl J Med 1977; 296(25):1431–1435. doi:10.1056/NEJM197706232962503
- Parambil JG, Savci CD, Tazelaar HD, Ryu JH. Causes and presenting features of pulmonary infarctions in 43 cases identified by surgical lung biopsy. Chest 2005; 127(4):1178–1183. doi:10.1378/chest.127.4.1178
- Karsner HT, Ghoreyeb AA. Studies in infarction: III. The circulation in experimental pulmonary embolism. J Exp Med 1913; 18(5):507–511. pmid:19867725
- Tsao MS, Schraufnagel D, Wang NS. Pathogenesis of pulmonary infarction. Am J Med 1982; 72(4):599–606. pmid:6462058
- Burns KE, Iacono AT. Incidence of clinically unsuspected pulmonary embolism in mechanically ventilated lung transplant recipients. Transplantation 2003; 76(6):964–968. doi:10.1097/01.TP.0000084523.58610.BA
- Yousem SA. The surgical pathology of pulmonary infarcts: diagnostic confusion with granulomatous disease, vasculitis, and neoplasia. Mod Pathol 2009; 22(5):679–685. doi:10.1038/modpathol.2009.20
- Light RW. Pleural Diseases. 4th ed. Baltimore, MD: Lippincott, Williams & Wilkins; 2001.
- Stein PD, Terrin ML, Hales CA, et al. Clinical, laboratory, roentgenographic, and electrocardiographic findings in patients with acute pulmonary embolism and no pre-existing cardiac or pulmonary disease. Chest 1991; 100(3):598–603. pmid:1909617
- Light RW. Pleural effusion due to pulmonary emboli. Curr Opin Pulm Med 2001; 7(4):198–201. pmid:11470974
- Libby LS, King TE, LaForce FM, Schwarz MI. Pulmonary cavitation following pulmonary infarction. Medicine (Baltimore) 1985; 64(5):342–348. pmid:4033411
- Kirchner J, Obermann A, Stuckradt S, et al. Lung infarction following pulmonary embolism: a comparative study on clinical conditions and CT findings to identify predisposing factors. Rofo 2015; 187(6):440–444. doi:10.1055/s-0034-1399006
- He H, Stein MW, Zalta B, Haramati LB. Pulmonary infarction: spectrum of findings on multidetector helical CT. J Thorac Imaging 2006; 21(1):1–7. doi:10.1097/01.rti.0000187433.06762.fb
- Scharf J, Nahir AM, Munk J, Lichtig C. Aseptic cavitation in pulmonary infarction. Chest 1971; 59(4):456–458. pmid:5551596
- Wilson AG, Joseph AE, Butland RJ. The radiology of aseptic cavitation in pulmonary infarction. Clin Radiol 1986; 37(4):327–333. pmid:3731699
- Butler MD, Biscardi FH, Schain DC, Humphries JE, Blow O, Spotnitz WD. Pulmonary resection for treatment of cavitary pulmonary infarction. Ann Thorac Surg 1997; 63(3):849–850. pmid:9066420
- Koroscil MT, Hauser TR. Acute pulmonary embolism leading to cavitation and large pulmonary abscess: a rare complication of pulmonary infarction. Respir Med Case Rep 2016; 20:72–74. doi:10.1016/j.rmcr.2016.12.001
- Levin L, Kernohan JW, Moersch HJ. Pulmonary abscess secondary to bland pulmonary infarction. Dis Chest 1948; 14(2):218–232. pmid:18904835
- Marchiori E, Menna Barreto M, Pereira Freitas HM, et al. Morphological characteristics of the reversed halo sign that may strongly suggest pulmonary infarction. Clin Radiol 2018; 73(5):503.e7–503.e13. doi:10.1016/j.crad.2017.11.022
- Smith GT, Dexter L, Dammin GJ. Postmortem quantitative studies in pulmonary embolism. In: Sasahara AA, Stein M, eds. Pulmonary Embolic Disease. New York, NY: Grune & Stratton, Inc; 1965:120–126.
- Miniati M, Bottai M, Ciccotosto C, Roberto L, Monti S. Predictors of pulmonary infarction. Medicine (Baltimore) 2015; 94(41):e1488. doi:10.1097/MD.0000000000001488
- Dalen JE, Haffajee CI, Alpert JS, Howe JP, Ockene IS, Paraskos JA. Pulmonary embolism, pulmonary hemorrhage and pulmonary infarction. N Engl J Med 1977; 296(25):1431–1435. doi:10.1056/NEJM197706232962503
- Parambil JG, Savci CD, Tazelaar HD, Ryu JH. Causes and presenting features of pulmonary infarctions in 43 cases identified by surgical lung biopsy. Chest 2005; 127(4):1178–1183. doi:10.1378/chest.127.4.1178
- Karsner HT, Ghoreyeb AA. Studies in infarction: III. The circulation in experimental pulmonary embolism. J Exp Med 1913; 18(5):507–511. pmid:19867725
- Tsao MS, Schraufnagel D, Wang NS. Pathogenesis of pulmonary infarction. Am J Med 1982; 72(4):599–606. pmid:6462058
- Burns KE, Iacono AT. Incidence of clinically unsuspected pulmonary embolism in mechanically ventilated lung transplant recipients. Transplantation 2003; 76(6):964–968. doi:10.1097/01.TP.0000084523.58610.BA
- Yousem SA. The surgical pathology of pulmonary infarcts: diagnostic confusion with granulomatous disease, vasculitis, and neoplasia. Mod Pathol 2009; 22(5):679–685. doi:10.1038/modpathol.2009.20
Blood test may obviate need for head CTs in brain trauma evaluation
SAN DIEGO – A biomarker test based on the presence of two proteins in the blood appears to be suitable for ruling out significant intracranial injuries in patients with a history of mild traumatic brain injury (TBI) without the need for a CT head scan, according to data presented at the annual meeting of the American College of Emergency Physicians.
according to Jeffrey J. Bazarian, MD, professor of emergency medicine, University of Rochester (New York).
In the ALERT-TBI study, which evaluated the biomarker test, 1,959 patients with suspected TBI at 22 participating EDs in the United States and Europe were enrolled and available for analysis. All had mild TBI as defined as a Glasgow Coma Scale (GCS) score of 13-15.
The treating ED physician’s decision to order a head CT scan was the major criterion for study entry. All enrolled patients had their blood drawn within 12 hours in order to quantify two biomarkers, C-terminal hydrolase-L1 (UCH-L1) and glial fibrillary acidic protein (GFAP).
The biomarker test for TBI was negative when the UCH-L1 value was less than 327 pg/mL and the GFAP was less than 22 pg/mL; the test was positive if either value was at this threshold or higher. To evaluate the sensitivity and specificity of this dual-biomarker test, results were correlated with head CT scans read by two neurologists blinded to the biomarker values.
The mean age of the study population was 48.8 years and slightly more than half were male. About half of the suspected TBI in these patients was attributed to falls and about one third to motor vehicle accidents.
Typical of TBI with GCS scores in the mild range, only 6% of the patients had a positive CT head scan. Of the 125 positive CT scans, the most common injury detected on CT scan was subarachnoid hemorrhage followed by subdural hematoma.
Of the 671 negative biomarker tests, 668 had normal head CT scans. Of the three false positives, one included a cavernous malformation that may have been present prior to the TBI. The others were a small subarachnoid hemorrhage and a small subdural hematoma. Overall the negative predictive value was 99.6% and the sensitivity was 97.6%.
Although the biomarker specificity was only 36% with an even-lower positive predictive value, the goal of the test was to rule out significant TBI to avoid the need for CT scan. On this basis, the biomarker test, which is being developed under the proprietary name Banyan BTI, appears to be promising. The data, according to Dr. Bazarian, have been submitted to the Food and Drug Administration.
“Head CT scans are the current standard for evaluating intracranial injuries after TBI, but they are overused, based on the high proportion that do not show an injury,” said Dr. Bazarian. Although he does not know the disposition of the FDA application, he said, based on these data, “I would definitely be using this test if it were available.”
SAN DIEGO – A biomarker test based on the presence of two proteins in the blood appears to be suitable for ruling out significant intracranial injuries in patients with a history of mild traumatic brain injury (TBI) without the need for a CT head scan, according to data presented at the annual meeting of the American College of Emergency Physicians.
according to Jeffrey J. Bazarian, MD, professor of emergency medicine, University of Rochester (New York).
In the ALERT-TBI study, which evaluated the biomarker test, 1,959 patients with suspected TBI at 22 participating EDs in the United States and Europe were enrolled and available for analysis. All had mild TBI as defined as a Glasgow Coma Scale (GCS) score of 13-15.
The treating ED physician’s decision to order a head CT scan was the major criterion for study entry. All enrolled patients had their blood drawn within 12 hours in order to quantify two biomarkers, C-terminal hydrolase-L1 (UCH-L1) and glial fibrillary acidic protein (GFAP).
The biomarker test for TBI was negative when the UCH-L1 value was less than 327 pg/mL and the GFAP was less than 22 pg/mL; the test was positive if either value was at this threshold or higher. To evaluate the sensitivity and specificity of this dual-biomarker test, results were correlated with head CT scans read by two neurologists blinded to the biomarker values.
The mean age of the study population was 48.8 years and slightly more than half were male. About half of the suspected TBI in these patients was attributed to falls and about one third to motor vehicle accidents.
Typical of TBI with GCS scores in the mild range, only 6% of the patients had a positive CT head scan. Of the 125 positive CT scans, the most common injury detected on CT scan was subarachnoid hemorrhage followed by subdural hematoma.
Of the 671 negative biomarker tests, 668 had normal head CT scans. Of the three false positives, one included a cavernous malformation that may have been present prior to the TBI. The others were a small subarachnoid hemorrhage and a small subdural hematoma. Overall the negative predictive value was 99.6% and the sensitivity was 97.6%.
Although the biomarker specificity was only 36% with an even-lower positive predictive value, the goal of the test was to rule out significant TBI to avoid the need for CT scan. On this basis, the biomarker test, which is being developed under the proprietary name Banyan BTI, appears to be promising. The data, according to Dr. Bazarian, have been submitted to the Food and Drug Administration.
“Head CT scans are the current standard for evaluating intracranial injuries after TBI, but they are overused, based on the high proportion that do not show an injury,” said Dr. Bazarian. Although he does not know the disposition of the FDA application, he said, based on these data, “I would definitely be using this test if it were available.”
SAN DIEGO – A biomarker test based on the presence of two proteins in the blood appears to be suitable for ruling out significant intracranial injuries in patients with a history of mild traumatic brain injury (TBI) without the need for a CT head scan, according to data presented at the annual meeting of the American College of Emergency Physicians.
according to Jeffrey J. Bazarian, MD, professor of emergency medicine, University of Rochester (New York).
In the ALERT-TBI study, which evaluated the biomarker test, 1,959 patients with suspected TBI at 22 participating EDs in the United States and Europe were enrolled and available for analysis. All had mild TBI as defined as a Glasgow Coma Scale (GCS) score of 13-15.
The treating ED physician’s decision to order a head CT scan was the major criterion for study entry. All enrolled patients had their blood drawn within 12 hours in order to quantify two biomarkers, C-terminal hydrolase-L1 (UCH-L1) and glial fibrillary acidic protein (GFAP).
The biomarker test for TBI was negative when the UCH-L1 value was less than 327 pg/mL and the GFAP was less than 22 pg/mL; the test was positive if either value was at this threshold or higher. To evaluate the sensitivity and specificity of this dual-biomarker test, results were correlated with head CT scans read by two neurologists blinded to the biomarker values.
The mean age of the study population was 48.8 years and slightly more than half were male. About half of the suspected TBI in these patients was attributed to falls and about one third to motor vehicle accidents.
Typical of TBI with GCS scores in the mild range, only 6% of the patients had a positive CT head scan. Of the 125 positive CT scans, the most common injury detected on CT scan was subarachnoid hemorrhage followed by subdural hematoma.
Of the 671 negative biomarker tests, 668 had normal head CT scans. Of the three false positives, one included a cavernous malformation that may have been present prior to the TBI. The others were a small subarachnoid hemorrhage and a small subdural hematoma. Overall the negative predictive value was 99.6% and the sensitivity was 97.6%.
Although the biomarker specificity was only 36% with an even-lower positive predictive value, the goal of the test was to rule out significant TBI to avoid the need for CT scan. On this basis, the biomarker test, which is being developed under the proprietary name Banyan BTI, appears to be promising. The data, according to Dr. Bazarian, have been submitted to the Food and Drug Administration.
“Head CT scans are the current standard for evaluating intracranial injuries after TBI, but they are overused, based on the high proportion that do not show an injury,” said Dr. Bazarian. Although he does not know the disposition of the FDA application, he said, based on these data, “I would definitely be using this test if it were available.”
FROM ACEP 2018
Key clinical point: In patients with mild head trauma, a simple blood test may eliminate need and cost for routine CT scans.
Major finding: In patients a history of head trauma, the biomarker test had a 99.6% negative predictive value in ruling out injury.
Study details: Prospective, controlled registration study.
Disclosures: Dr. Bazarian reported no financial relationships relevant to this study, which was in part funded by Banyan Biomarkers.
Fat attenuation index boosts coronary CT prognostication
MUNICH – The perivascular fat attenuation index, a new measure of coronary plaque inflammation using data collected by a conventional coronary CT scan, identified an elevated risk for future cardiovascular death that was independent of standard risk factors in both derivation and validation studies with prospective data from more than 3,900 patients.
Patients with a high fat attenuation index (FAI) in the perivascular fat surrounding their right coronary artery had a five- to ninefold higher risk of cardiac mortality during 4-6 years of follow-up than did those with lower scores, after adjustment for conventional risk factors and standard findings from the coronary CT, Charalambos Antoniades, MD, said at the annual congress of the European Society of Cardiology.
The FAI is a “powerful, novel technology for cardiovascular disease risk stratification. It has striking prognostic value for cardiac death and nonfatal MI over and above other risk scores and state-of-the-art interpretation of coronary CT angiography,” said Dr. Antoniades, professor of cardiovascular medicine at the University of Oxford (England). He also highlighted that the data he reported suggested a protective effect in patients with a high FAI who received treatment with aspirin or a statin, and he suggested that FAI might be a way to better target an anti-inflammatory agent such as canakinumab (Ilaris), which showed cardiovascular protective effects in the CANTOS trial (N Engl J Med. 2017 Sep 21;377[12]:1119-31).
Another key feature of the FAI analysis is that it uses data collected by “any standard” coronary CT angiogram, Dr. Antoniades said. He is a founder of, shareholder in, and chief scientific officer of Caristo Diagnostics, the company developing the software that uses coronary CT data to calculate the FAI.
Dr. Antoniades and his associates derived the FAI using data collected from 1,872 patients who underwent planned coronary CT angiography at a clinic in Erlangen, Germany, during 2005-2009. The researchers correlated FAI scores with cardiovascular disease outcomes during a median follow-up of 72 months. They then validated the FAI using data collected from 2,040 patients who underwent a planned coronary CT exam at the Cleveland Clinic during 2008-2016 and then had a median follow-up of 54 months. The researchers called this overall post-hoc analysis of prospectively collected CT and outcomes data the Cardiovascular Risk Prediction using Computed Tomography (CRISP-CT) study.
Using the derivation data, FAI measurements taken from fat around the proximal right coronary artery that met or exceeded the specified cutoff value of –70.1 Hounsfield units were tied to a 9.04-fold greater rate of cardiac death during follow-up, compared with patients with a lower FAI, and after adjustment for several demographic, clinical, and CT-angiography variables. When the researchers ran this analysis using data from the validation patients and the same cutoff value they found that an elevated FAI linked with a 5.6-fold higher rate of cardiac death. Concurrently with Dr. Antoniades’ talk the results appeared in an online article that was later published (Lancet. 2018 Sep 15;392[10151]:929-39).
Calculating a patient’s FAI offers the prospect for “better use of CT information,” Dr. Antoniades said during the discussion of his report. After a coronary CT scan and conventional data processing, about half the patients have no finding that warrants intervention, but about 10% of these patients actually have an inflamed coronary plaque that is at high risk for rupture that could trigger a cardiac event. The FAI provides a way to use coronary CT angiography to identify these at-risk patients, he explained.
MUNICH – The perivascular fat attenuation index, a new measure of coronary plaque inflammation using data collected by a conventional coronary CT scan, identified an elevated risk for future cardiovascular death that was independent of standard risk factors in both derivation and validation studies with prospective data from more than 3,900 patients.
Patients with a high fat attenuation index (FAI) in the perivascular fat surrounding their right coronary artery had a five- to ninefold higher risk of cardiac mortality during 4-6 years of follow-up than did those with lower scores, after adjustment for conventional risk factors and standard findings from the coronary CT, Charalambos Antoniades, MD, said at the annual congress of the European Society of Cardiology.
The FAI is a “powerful, novel technology for cardiovascular disease risk stratification. It has striking prognostic value for cardiac death and nonfatal MI over and above other risk scores and state-of-the-art interpretation of coronary CT angiography,” said Dr. Antoniades, professor of cardiovascular medicine at the University of Oxford (England). He also highlighted that the data he reported suggested a protective effect in patients with a high FAI who received treatment with aspirin or a statin, and he suggested that FAI might be a way to better target an anti-inflammatory agent such as canakinumab (Ilaris), which showed cardiovascular protective effects in the CANTOS trial (N Engl J Med. 2017 Sep 21;377[12]:1119-31).
Another key feature of the FAI analysis is that it uses data collected by “any standard” coronary CT angiogram, Dr. Antoniades said. He is a founder of, shareholder in, and chief scientific officer of Caristo Diagnostics, the company developing the software that uses coronary CT data to calculate the FAI.
Dr. Antoniades and his associates derived the FAI using data collected from 1,872 patients who underwent planned coronary CT angiography at a clinic in Erlangen, Germany, during 2005-2009. The researchers correlated FAI scores with cardiovascular disease outcomes during a median follow-up of 72 months. They then validated the FAI using data collected from 2,040 patients who underwent a planned coronary CT exam at the Cleveland Clinic during 2008-2016 and then had a median follow-up of 54 months. The researchers called this overall post-hoc analysis of prospectively collected CT and outcomes data the Cardiovascular Risk Prediction using Computed Tomography (CRISP-CT) study.
Using the derivation data, FAI measurements taken from fat around the proximal right coronary artery that met or exceeded the specified cutoff value of –70.1 Hounsfield units were tied to a 9.04-fold greater rate of cardiac death during follow-up, compared with patients with a lower FAI, and after adjustment for several demographic, clinical, and CT-angiography variables. When the researchers ran this analysis using data from the validation patients and the same cutoff value they found that an elevated FAI linked with a 5.6-fold higher rate of cardiac death. Concurrently with Dr. Antoniades’ talk the results appeared in an online article that was later published (Lancet. 2018 Sep 15;392[10151]:929-39).
Calculating a patient’s FAI offers the prospect for “better use of CT information,” Dr. Antoniades said during the discussion of his report. After a coronary CT scan and conventional data processing, about half the patients have no finding that warrants intervention, but about 10% of these patients actually have an inflamed coronary plaque that is at high risk for rupture that could trigger a cardiac event. The FAI provides a way to use coronary CT angiography to identify these at-risk patients, he explained.
MUNICH – The perivascular fat attenuation index, a new measure of coronary plaque inflammation using data collected by a conventional coronary CT scan, identified an elevated risk for future cardiovascular death that was independent of standard risk factors in both derivation and validation studies with prospective data from more than 3,900 patients.
Patients with a high fat attenuation index (FAI) in the perivascular fat surrounding their right coronary artery had a five- to ninefold higher risk of cardiac mortality during 4-6 years of follow-up than did those with lower scores, after adjustment for conventional risk factors and standard findings from the coronary CT, Charalambos Antoniades, MD, said at the annual congress of the European Society of Cardiology.
The FAI is a “powerful, novel technology for cardiovascular disease risk stratification. It has striking prognostic value for cardiac death and nonfatal MI over and above other risk scores and state-of-the-art interpretation of coronary CT angiography,” said Dr. Antoniades, professor of cardiovascular medicine at the University of Oxford (England). He also highlighted that the data he reported suggested a protective effect in patients with a high FAI who received treatment with aspirin or a statin, and he suggested that FAI might be a way to better target an anti-inflammatory agent such as canakinumab (Ilaris), which showed cardiovascular protective effects in the CANTOS trial (N Engl J Med. 2017 Sep 21;377[12]:1119-31).
Another key feature of the FAI analysis is that it uses data collected by “any standard” coronary CT angiogram, Dr. Antoniades said. He is a founder of, shareholder in, and chief scientific officer of Caristo Diagnostics, the company developing the software that uses coronary CT data to calculate the FAI.
Dr. Antoniades and his associates derived the FAI using data collected from 1,872 patients who underwent planned coronary CT angiography at a clinic in Erlangen, Germany, during 2005-2009. The researchers correlated FAI scores with cardiovascular disease outcomes during a median follow-up of 72 months. They then validated the FAI using data collected from 2,040 patients who underwent a planned coronary CT exam at the Cleveland Clinic during 2008-2016 and then had a median follow-up of 54 months. The researchers called this overall post-hoc analysis of prospectively collected CT and outcomes data the Cardiovascular Risk Prediction using Computed Tomography (CRISP-CT) study.
Using the derivation data, FAI measurements taken from fat around the proximal right coronary artery that met or exceeded the specified cutoff value of –70.1 Hounsfield units were tied to a 9.04-fold greater rate of cardiac death during follow-up, compared with patients with a lower FAI, and after adjustment for several demographic, clinical, and CT-angiography variables. When the researchers ran this analysis using data from the validation patients and the same cutoff value they found that an elevated FAI linked with a 5.6-fold higher rate of cardiac death. Concurrently with Dr. Antoniades’ talk the results appeared in an online article that was later published (Lancet. 2018 Sep 15;392[10151]:929-39).
Calculating a patient’s FAI offers the prospect for “better use of CT information,” Dr. Antoniades said during the discussion of his report. After a coronary CT scan and conventional data processing, about half the patients have no finding that warrants intervention, but about 10% of these patients actually have an inflamed coronary plaque that is at high risk for rupture that could trigger a cardiac event. The FAI provides a way to use coronary CT angiography to identify these at-risk patients, he explained.
REPORTING FROM THE ESC CONGRESS 2018
Key clinical point:
Major finding: Patients with a fat attenuation index at or above the selected cutoff had a five- to ninefold increased rate of cardiac death.
Study details: The CRISP-CT study, which ran a post-hoc analysis of CT angiography data from 3,912 patients.
Disclosures: Dr. Antoniades is a founder of, shareholder in, and chief scientific officer of Caristo Diagnostics, the company developing fat attenuation index software.
Use of Musculoskeletal Ultrasound and Regenerative Therapies in Soccer
ABSTRACT
Improvements in ultrasound technology have increased the popularity and use of ultrasound as a diagnostic and therapeutic modality for many soccer-related musculoskeletal (MSK) injuries. As a dynamic imaging modality, ultrasound offers increased accuracy and efficacy with minimally invasive procedures, such as guided injections, percutaneous tenotomy, and regenerative therapies, in the clinical setting. Emerging evidence indicates that regenerative therapies, such as platelet-rich-plasma (PRP), mesenchymal stem cells, and amniotic products, are a promising treatment for many MSK injuries and are gaining popularity among professional athletes. PRP is a safe treatment for a number of MSK conditions and has been included in the standard of care. However, conflicting evidence on return-to-play timeframes and efficacy in certain MSK conditions have led to inconsistent recommendations on indications for use, dose, and timing of treatment. Mesenchymal stem cell therapy, while promising, lacks high-level evidence of efficacy despite its increasing use among athletes. Currently, no data are available regarding the outcome of the use of amniotic products for the treatment of injuries in athletes. Furthermore, preparation of many regenerative therapies eclipses the concept of minimal manipulation and is subject to US Food and Drug Administration phase I to III trials. High-level research on regenerative medicine therapies should be continuously conducted to establish their clinical efficacy and safety data.
ULTRASOUND
Ultrasound (US) was first introduced for musculoskeletal (MSK) evaluation in 1957.1 Since then, US has gained increasing attention due to its ease of utilization in the clinical setting, repeatability, noninvasiveness, capability for contralateral comparison, lack of radiation exposure, and capability to provide real-time dynamic tissue assessment.1 Compared with magnetic resonance imaging or computed tomography, US presents limitations, including decreased resolution of certain tissues, limited field of view, limited penetration beyond osseous structures, incomplete evaluation of a joint or structure, and operator experience. However, advancements in technology, image resolution, and portability have improved the visualization of multiple anatomic structures and the accuracy of minimally invasive ultrasound-guided procedures at the point of care. The use of US for guided hip injections possibly decreases the cost relative to fluoroscopic guidance.2 Other studies have reported that US, as a result of its safety profile, has replaced fluoroscopy for certain procedures, such as barbotage of calcific tendinosis.3 US has been used for diagnostic purposes and guidance for therapeutic interventions, such as needle aspiration, diagnostic or therapeutic injection, needle tenotomy, tissue release, hydro-dissection, and biopsy.3 Given its expanding application, US has been increasingly used in the clinical setting, athletic training room, and sidelines of athletic events.
DIAGNOSTIC ULTRASOUND
An epidemiologic review of the National Collegiate Athletic Association (NCAA) men’s and women’s soccer injuries from 1988 to 2003 reported over 24,000 combined injuries. Over 70% of these injuries are MSK in nature and often affect the lower extremities.4,5 Ekstrand and colleagues6 also conducted an epidemiological review of muscle injuries among professional soccer players from 2001 to 2009. They found that 92% of all muscle injuries involved the lower extremities. The portability of US allows it to serve as an ideal modality for diagnostic evaluation of acute MSK injuries. Klauser and colleagues7 developed consensus based on the recommendations of the European Society of Musculoskeletal Radiology (ESSR) for the clinical indication of diagnostic ultrasound. A grading system was developed to describe the clinical utility of diagnostic US evaluation of MSK structures:
• Grade 0: Ultrasound is not indicated;
• Grade 1: Ultrasound is indicated if other imaging techniques are not appropriate;
• Grade 2: Ultrasound indication is equivalent to other imaging modalities;
• Grade 3: Ultrasound is the first-choice technique.
Henderson and colleagues8 conducted a review of 95 studies (12 systemic reviews and 83 diagnostic studies) that investigated the accuracy of diagnostic US imaging on soft tissue MSK injuries of the upper and lower extremities. They reported the sensitivity and specificity of the method for detection of over 40 hip, knee, ankle, and foot injuries and assigned corresponding grades based on diagnostic accuracy by using the same system developed by Klauser and colleagues.7,8 Common MSK injuries of the lower extremity and their corresponding ESSR grades are listed in the Table. This study demonstrated that diagnostic US is highly accurate for a number of soft tissue MSK injuries of the lower extremity and consistently matches the recommendation grades issued by Klauser and colleagues.7 In the hands of a skilled operator, US has become an increasingly popular and cost-effective modality for diagnosis and monitoring of acute muscle injuries and chronic tendinopathies among soccer athletes.
Table. Clinical Indication Grades for Diagnostic Ultrasound Evaluation of Common Lower Extremity Injuries7,8
Hip | Knee | Foot/Ankle |
Synovitis/Effusion: 3 | Quadricep tendinosis/tear: 3 | Anterior talofibular ligament injury: 3 |
Snapping hip (extra-articular): 3 | Patella tendinopathy: 3 | Calcaneofibular ligament injury: 3 |
Gluteal tendon tear: 3 | Pes anserine bursitis: 3 | Peroneal tendon tear/subluxation: 3 |
Meralgia paresthetica: 3 | Periarticular bursitis & ganglion: 3 | Posterior tibial tendinopathy: 3 |
Lateral femoral cutaneous nerve injury: 3 | Osgood-Schlatter & Sinding-Larsen: 3 | Plantaris tendon tear: 3 |
Femoral nerve injury: 3 | Synovitis/Effusion: 3 | Plantar fasciitis: 3 |
Sports hernia: 3 | Baker’s Cyst: 2-3 | Calcific tendonitis: 3 |
Morel-Lavallée lesions: 3 | MCL injury: 2 | Retrocalcaneal bursitis: 3 |
Muscle injury (high grade): 3 | IT band friction: 2 | Joint effusion: 3 |
Trochanteric bursitis: 2 | Medial patella plica syndrome: 2 | Ganglion cyst: 3 |
Proximal hamstring injury: 2 | Meniscal cyst: 2 | Retinacula pathology: 3 |
Sciatica: 1-2 | Common perineal neuropathy: 2 | Achilles tendinopathy: 2 |
Muscle injury (low grade): 1 | Distal hamstring tendon injury: 1-2 | Haglund disease: 2 |
Psoas tendon pathology: 1 | Intra-articular ganglion: 1 | Deltoid ligament injury: 2 |
Osteoarthritis: 0 | Hoffa’s fat pad syndrome: 1 | Plantar plate tear: 2 |
Labral tear: 0 | Loose bodies: 1 | Syndesmotic injury: 2 |
| LCL injury: 0-1 | Morton’s neuroma: 2 |
| Popliteal injury: 0-1 | Deltoid ligament injury: 1 |
| Plica syndrome: 0 | Spring ligament injury: 1 |
| Full/partial ACL tear: 0 | Anterolateral ankle impingement: 0 |
| PCL tear: 0 | Posterior talofibular ligament injury: 0 |
| Medial/lateral meniscus tear: 0 |
|
| Osteochondritis dissecans: 0 |
|
Abbreviations: ACL, anterior cruciate ligament; IT, iliotibial; LCL, lateral collateral ligament; MCL, medial collateral ligament; PCL, posterior cruciate ligament.
ULTRASOUND-GUIDED THERAPEUTIC PROCEDURES
The use of US at the point of care for needle guidance has led to its widespread application for therapeutic procedures, including injections and multiple regenerative therapies. Intra-articular US-guided injection and aspiration are common therapeutic interventions performed in the clinical setting. In a position statement of the American Medical Society for Sports Medicine, US-guided injections were found to be more accurate (SORT A evidence), effective (SORT B evidence), and cost effective (SORT B evidence) than landmark-guided injections.3 A recent meta-analysis conducted by Daniels and colleagues1 demonstrated the improved accuracy and efficacy of US-guided injections at the knee, ankle, and foot. Injections may serve a diagnostic purpose when anesthetics, such as lidocaine, are used in isolation, a therapeutic purpose, or both.
Continue to: Percutaneous tenotomy involve...
REGENERATIVE THERAPIES FOR MUSCULOSKELETAL CONDITIONS
PERCUTANEOUS TENOTOMY
Percutaneous tenotomy involves the introduction of a needle into damaged soft tissues, most often tendons (“needling”), in an effort to stimulate a healing response and resect the diseased tendon tissue. Although tenotomy was initially performed as an open or arthroscopic surgical technique, advances in US technology have led to improved sensitivity and specificity identifying areas of tendinous injury (hypervascularity, hypoechogenicity, and calcification); as such, the combination of these techniques has been used in the outpatient setting. New commercial models incorporate ultrasound guidance with needles or micro-resection probes for real-time débridement of damaged tissues. Percutaneous tenotomy has been described in the management of tendinopathy involving the rotator cuff, medial and lateral epicondyles, patellar and Achilles tendons, and plantar fascia.
Housner and colleagues9 evaluated the safety and short-term efficacy of US-guided needle tenotomy in 13 patients with chronic tendinosis of the patella, Achilles tendon, gluteus medius, iliotibial tract, hamstring, and rectus femoris. They reported no procedural complications and a significant decrease in pain scores at 4 and 12 weeks of follow-up.
Koh and colleagues10 conducted a prospective case series to evaluate the safety and efficacy of office-based, US-guided percutaneous tenotomy (using a commercial model) on 20 patients with chronic lateral epicondylitis. The authors reported no wound complications and significant improvement in pain scores at each follow-up period up to 1 year. Subsequent post-procedural US evaluation of injured tissues revealed evidence of healing (decreased tendon thickness, vascularity, and hypoechogenicity) in over half the cohort after 6 months compared with the baseline.11
Lee and colleagues12 evaluated the efficacy of US-guided needle tenotomy combined with platelet-rich plasma (PRP) injection on chronic recalcitrant gluteus medius tendinopathy. In this case series, 21 patients underwent PRP and “needling” through the hypoechoic regions of the injured tendon under direct US guidance. After a period of rest, all patients completed the structured rehabilitation protocol. After an average follow-up of 10 months, all patients displayed significant improvements in all outcome questionnaires and did not report any significant adverse events. The authors concluded that tenotomy combined with PRP is a safe and effective method for treatment for recalcitrant gluteus medius tendinopathy.
These studies indicate that US-guided percutaneous tenotomy, alone or in combination with regenerative therapies, such as PRP, is a safe and effective treatment option for various tendinopathies. However, while tenotomy appears safe with promising results and no reported major adverse events, the level of evidence remains low.
ORTHOBIOLOGICS
Orthobiologics are substances composed of biological materials that can be used to aid or even hasten the healing of bones, muscles, tendons, and ligaments. Orthobiologics may contain growth factors, which initiate or stimulate the body’s reparative process; matrix proteins, which serve as scaffolding for healing tissues; or stem cells, specifically adult stem cells, which are multipotent and can differentiate into several cell lines. Adult stem cells are categorized as hematopoietic, neural, epithelial, skin, and mesenchymal types. Mesenchymal stem cells (MSCs) are of particular interest in sports medicine applications because they secrete growth factors and cytokines with trophic, chemotactic, and immunosuppressive properties.13 MSCs are also multipotent and can differentiate into bones, muscles, cartilages, and tendons.14-17MSCs are readily isolated from many sources, including bone marrow, adipose tissues, synovial tissues, peripheral blood, skeletal muscles, umbilical cord blood, and placenta.13,14Several types of regenerative therapies used in orthopedic and sports medicine practice include PRP, stem cell therapy, and amniotic membrane/fluid preparations. While each therapy possesses the potential for promising results, the paucity of research and discrepancies among studies regarding the description of stem cell lines used limit the available evidence on the true clinical benefits of these regenerative therapies.
[HEAD 3] PLATELET-RICH PLASMA
PRP is an autologous product that has been used to stimulate biological factors and promote healing since the 1970s. Through the activation of platelets, PRP improves localized recruitment, proliferation, and differentiation of cells involved in tissue repair. Platelets, which are non-nucleated bodies located in peripheral blood, contain and release 3 groups of bioactive factors that enhance the healing process. Growth factors and cytokines released from alpha-granules play a role in cell proliferation, chemotaxis, cell differentiation, and angiogenesis. Bioactive factors, such as serotonin and histamine, released from dense granules, increase capillary permeability and improve cell recruitment and migration. Adhesion molecules also assist in cell migration and creation of an extracellular matrix, which acts as a scaffold for wound healing.18 Platelets are activated by mechanical trauma or contact with multiple activators, including Von Willebrand factor, collagen, thrombin, or calcium chloride. When activated, platelets release growth factors and cytokines, which create a pro-inflammatory environment that mediates the tissue repair process. After the procedure, the pro-inflammatory environment may result in patient discomfort, which can be managed with ice and acetaminophen. Use of nonsteroidal anti-inflammatory drugs may theoretically inhibit the inflammatory cascade induced by PRP, and they are avoided before and after the procedure, although evidence regarding necessary time frames is lacking.
Continue to: PRP consists of...
PRP consists of the fractionated liquid component of autologous whole blood, which contains increased concentrations of platelets and cytokines. Different methods and commercial preparations are available for collecting and preparing PRP. Variations in the amount of blood drawn, use of anticoagulants, presence or absence of an activating agent, number of centrifuge spins, and overall platelet and white blood cell concentrations lead to difficulty in evaluating and interpreting the available evidence regarding PRP therapy.
In vitro and animal studies demonstrated promising and safe results regarding the healing effect of PRP on injured soft tissues, such as tendons, ligaments, and muscles. In this regard, a number of studies have evaluated the effect of PRP on human MSK injuries. However, in addition to the above-mentioned variabilities in PRP, many of such studies lack standardization and randomization techniques and include a small number of patients only, thereby limiting the overall comparison and clinical application.
A landmark study conducted by Mishra and Pavelko19 concluded that PRP significantly reduced pain in patients with chronic elbow tendinosis. Similar findings were reported in high-level overhead athletes with ulnar collateral ligament insufficiency, which did not improve with conservative management.20 Fitzpatrick and colleagues21 found improvements in pain with the use of single PRP injection as treatment for chronic gluteal tendinopathy. PRP can effectively improve pain and recovery in chronic ligament and tendon injuries, such as lateral epicondylitis, patellar tendinopathy, and plantar fasciitis, when patients are unresponsive to traditional conservative management. The application of PRP to treat acute MSK injuries has produced mixed results. Hamid and colleagues22 conducted a level II randomized controlled trial to evaluate the effect of PRP combined with a rehabilitation program for treatment of grade 2 hamstring injuries on return-to-play compared with rehabilitation alone. Fourteen athletes were randomized into the study and control groups. Hamid and colleagues22 reported improved return-to-play in the study group compared with that in the control (26.7 and 42.5 days, respectively). This study also reported lower pain scores in the PRP group over time, but the difference was not statistically significant. Zanon and colleagues23 conducted a prospective study to evaluate return-to-play in professional soccer players with acute hamstring strains treated with PRP and a rehabilitation program. This study determined that athletes treated with PRP were “match fit,” meaning they would be available for match selection in an average of 36.8 days. However, Zanon and colleagues23 did not include a control group for comparison. Other studies reported that PRP treatment of acutely injured muscles and medial collateral ligaments of soccer and basketball players decreased their return-to-play interval.18 Reviews by Hamilton and colleagues24 and Pas and colleagues25 concluded that PRP treatment of acutely injured tissues with good blood supply (eg, hamstring muscles) did not improve pain or return-to-play compared with standardized rehabilitation protocols. Similarly, in a double-blinded placebo controlled trial, Reurink and colleagues26 evaluated return-to-play in 80 athletes with acute hamstring injuries treated with a rehabilitation program and either PRP or placebo. Reurink and colleagues26 found no difference in return-to-play (42 days for both groups), but the difference was not statistically significant. PRP has also been used intraoperatively and shows promising results in total knee arthroplasty, anterior cruciate ligament reconstruction, acute Achilles tendon repair, rotator cuff repair, and cartilage repair. However, many of these intraoperative studies are limited to animal models.
In 2009, the World Anti-Doping Agency (WADA) prohibited the use of PRP because it contains autologous growth factors and IGF-1, which could produce an anabolic effect. Recent studies have failed to demonstrate any athletic advantages of using PRP. WADA has since removed PRP from its prohibited list. PRP is also not prohibited by the US Anti-Doping Agency (USADA) and many major professional sporting leagues in the United States. However, care must be taken in reviewing the components of PRP because many commercially available products differ in PRP formulation. Since 2010, many team physicians have increasingly used PRP to treat a wide range of athletic injuries. A recent anonymous survey conducted by a team of physicians on PRP use in elite athletes revealed minimal complications but significant variability among physicians with regard to timing, belief in evidence, and formulation and dosing of PRP treatments. Many physicians did implicate athlete desire as the main indication for treatment.27
As an autologous treatment, PRP injection has no serious adverse effects beyond mild discomfort as a result of the procedure and pro-inflammatory state in the days following injection. Recent concerns regarding the potential of PRP treatment for heterotopic ossification have been reported, but published information is limited to case reports. PRP can improve pain and function in patients with chronic MSK injury. PRP appears to be a safe and effective alternative to surgery for patients with injury to poorly perfused tissue, which has not improved with conservative measures, such as rest, physical therapy, and anti-inflammatory medications. Care should be taken when treating athletes with PRP to establish regulations on doping by individual governing bodies.
Continue to: Use of stem...
STEM CELL THERAPY
Use of stem cell therapy is based on the properties of the proliferation and differentiation of multipoint MSC lines. These stem cells can theoretically regenerate injured tissues and influence repair through immunomodulation; paracrine activity through the release of bioactive agents, such as cytokines, trophic, and chemotactic molecules; and cell differentiation into various cell lineages.15,16,13,17 Orthopedic surgeons have used microfracture to recruit MSCs during cartilage repair procedures for over 20 years. This procedure draws multipotent MSCs to the injured site to induce chondrogenic proliferation and fibrocartilage repair.28
Adult MSCs provide a readily accessible autologous source of stem cells for regenerative therapies. MSCs can be isolated from a variety of tissues, including bone marrow, adipose tissues, synovia, human umbilical cord blood, and peripheral blood. The majority of stem cell therapies in the United States for sports medicine purposes are conducted using bone marrow aspirate concentrate (BMAC) and adipose tissues. The US Food and Drug Administration (FDA) allows the use of minimally manipulated autologous stem cells to be injected into the same patient on the same day. However, some studies reported that culturing stem cells or introducing products, such as collagenase to stem cells, can increase the stem cell concentration prior to injection. These processes constitute more than “minimal manipulation” and therefore would require drug trials prior to use in the United States.
Although MSCs can be readily obtained from a variety of tissue sources, the makeup of the cell concentrate differs. Bone marrow and adipose tissues are readily available sources of homogenous MSCs. Harvesting stem cells from adipose tissues provides a less invasive route of collection than from BMAC. Harvested BMAC and adipose tissues consist of heterogeneous cell populations that are composed of precursor and accessory cells, such as pericytes, endothelial cells, smooth muscle cells, fibroblasts, and macrophages in addition to MSCs.
Animal studies reported promising results when evaluating soft tissue lesions in small and large animal models.14,15 Although clinical and human evidence remains limited, the potential of MSCs for regenerative repair has led to a recent increase in the number of related clinical studies. Multiple systematic reviews have concluded that MSC therapy is safe for the treatment of osteoarthritis, cartilage lesions, and tendinopathies. Limited evidence is available regarding the safety of intramuscular use, and a theoretical concern arises on the development of heterotopic bone formation as a result of treatment.13,16 The efficacy of MSC therapy is difficult to determine due to the lack of standardization in stem cell populations, adjuvants (eg, PRP, hyaluronic acid, and scaffolding preparations), and delivery methods used.13,17
Similar to PRP, the increased use of MSC therapy among high-profile athletes has led to the promotion of these therapies as safe and effective despite limited evidence.29 Although MSC therapy is a promising and safe treatment option for patients with soft tissue injuries, the paucity in data and human studies limit its clinical use. Moreover, data of MSC efficacy is complicated because of the disparity between clinical studies regarding MSC collection method (many of which eclipse the “minimal manipulation” standard), description of isolated cell concentrates, dosage, method of delivery, use of adjuvants, and lack of randomization. Further studies using [standardized] methods are needed before establishing a true consensus on the safety and efficacy of MSC therapy.
AMNIOTIC MEMBRANE
The placenta is a source of MSCs, a collagen-rich extracellular matrix, and bioactive growth and regulatory factors. The capacity of the placenta to modulate biological activities and tissue formation is thought to provide a means of tissue repair and healing. The placenta consists of amniotic fluid, amniotic membrane (AM), chorionic membrane, and umbilical cord blood and tissues. Although MSCs have been isolated from each component of placental tissues, amniotic and chorionic membranes and umbilical cord tissues yield the highest concentration.
The majority of regenerative studies involving the placenta used AM alone or in combination with other placental tissues. AM is a metabolically active tissue that consists of an epithelial layer, a basement membrane, and a mesenchymal tissue layer. In addition to being a source of stem cells, AM synthesizes many growth factors, vasoactive peptides, and cytokines, which are capable of tissue regeneration. AM was initially used as a biological scaffold for the treatment of skin burns and wounds. Other intrinsic properties of AM include the provision of a matrix for cellular migration and proliferation, enhanced wound healing with reduced scar formation, antibacterial activity, and lastly, non-immunogenic and immunosuppressive properties. These inherent characteristics have spurred studies on the potential use of AM in sports medicine as a minimally invasive means to treat osteoarthritis and injuries of tendons, ligaments, muscles, fascia, and cartilages.
Continue to: Animal studies reported...
Animal studies reported positive results with the use of AM to treat osteoarthritis, cartilage defects, and tendon and ligament injuries. Few studies involving human participants also revealed favorable results with regard to the use of AM for the treatment of plantar fasciitis and osteoarthritis; however, these studies are industry-sponsored and employed small sample sizes. The unique mixture of a collagen-rich extracellular matrix, bioactive growth factors, and pluripotent stem cells may allow AM to become an effective treatment for MSK injuries. Although initial animal and human studies show promising results, variabilities regarding models (animal and human), pathologies, placental tissues, and methods of preparation, preservation, and delivery used limit the ability for comparison, analysis, and drawing of definitive conclusions. Thus far, no studies have evaluated the use of currently available AM products for the treatment of injuries sustained by soccer players.
Despite the current popularity of AM as regenerative therapy in academic research and potential use in clinical treatment in sports medicine, physicians should remain aware of the limited evidence available. Other barriers to research and use AM as a regenerative therapy include regulatory classifications based on the concept of “minimal manipulation” in biologic therapies. Minimally manipulated placental allografts are less regulated, less costly to study, and more easily commercialized. These products are not required to undergo FDA phase I to III trials prior to premarket approval. In 2000, the FDA position on all AM products falls into 2 categories. The first position states that AM that contains allogenic stem cells mixed with another drug that is micronized and/or cryopreserved is more than “minimally manipulated” and therefore categorized as “biologic” and would be subject to phase I to III trials. Dehydrated and decellularized AM, however, may meet the concept of minimal manipulation and is only approved by the FDA as a wound covering. Thus, any application of AM for the treatment of sports medicine pathology is not currently FDA-approved, considered off-label, not covered by insurance, and subject to out-of-pocket pay.30,31
CONCLUSION
With improvements in technology and portability, US has become an effective imaging modality for point-of-care evaluation, diagnosis, and continuous monitoring of many MSK injuries. Additionally, as a dynamic imaging modality, US allows for increased accuracy and efficacy when combined with minimally invasive procedures, such as diagnostic and therapeutic guided injections and percutaneous tenotomy, in the clinical setting; thereby decreasing the overall healthcare costs. PRP is proven to be a safe treatment for several MSK conditions, such as lateral epicondylitis, patellar tendonitis, and plantar fasciitis. Although PRP has been included in the standard of care in some areas, this technique may be predominantly athlete driven. Conflicting evidence with regard to return-to-play timeframes following PRP treatment for muscular injuries and poor evidence in conditions, such as Achilles tendonitis, have led to inconsistent indications for use, dose, and timing of treatment. Although early evidence of MSC therapy is promising, high-level evidence for MSC therapy is insufficient, despite its increased use among athletes. Thus far, no data are available regarding the outcomes of the use of amniotic products for the treatment of injuries among athletes. Furthermore, the preparation of amniotic products has many regulatory concerns. The authors advocate for continuous high-level research on regenerative medicine therapies to establish clinical efficacy and safety data.
1. Daniels E, Cole D, Jacobs B, Phillips S. Existing Evidence on ultrasound-guided injections in sports medicine. Orthop J Sports Med. 2018;6(2):2325967118756576. doi:10.1177/2325967118756576.
2. Henne M, Centurion A, Rosas S, Youmans H, Osbahr D. Trends in utilization of image-guided hip joint injections. Unpublished. 2018.
3. Finnoff JT, Hall MM, Adams E, et al. American Medical Society for Sports Medicine position statement: Interventional musculoskeletal ultrasound in sports medicine. Clin J Sport Med. 2015;25:6-22. doi:10.1097/JSM.0000000000000175.
4. Agel J, Evans TA, Dick R, Putukian M, Marshal S. Descriptive epidemiology of collegiate men’s soccer injuries: National Collegiate Athletic Association Injury Surveillance System, 1988-1989 through 2002-2003. J Athl Train. 2007;42(2):270-277.
5. Dick R, Putukian M, Agel J, Evans T, Marshall S. Descriptive epidemiology of collegiate women’s soccer injuries: National Collegiate Athletic Association Injury Surveillance System, 1988-1989 through 2002-2003. J Athl Train. 2007;42(2):278-285.
6. Ekstrand J, Hagglund M, Walden M. Epidemiology of muscle injuries in professional football (soccer). Am J Sports Med. 2011;39(6):1226-1232. doi:10.1177/0363546510395879.
7. Klauser A, Tagliafico A, Allen G, et al. Clinical indications for musculoskeletal ultrasound: A Delphi-based consensus paper of the European society of musculoskeletal radiology. Eur Radiol. 2012;22(5):1140-1148. doi:10.1007/s00330-011-2356-3.
8. Henderson R, Walker B, Young K. The accuracy of diagnostic ultrasound imaging for musculoskeletal soft tissue pathology of the extremities: a comprehensive review of the literature. Chiropr Man Therap. 2015;23(1):31. doi:10.1186/s12998-015-0076-5.
9. Housner JA, Jacobson JA, Misko R. Sonographically guided percutaneous needle tenotomy for the treatment of chronic tendinosis. J Ultrasound Med. 2009;28(9):1187-1192. doi:10.7863/jum.2009.28.9.1187.
10. Koh J, Mohan PC, Howe TS, et al. Fasciotomy and surgical tenotomy for recalcitrant lateral elbow tendinopathy: early clinical experience with a novel device for minimally invasive percutaneous microresection. Am J Sports Med. 2013;41(3):636-644. doi:10.1177/0363546512470625.
11. Seng C, Mohan PC, Koh J, et al. Ultrasonic percutaneous tenotomy for recalcitrant lateral elbow tendinopathy: sustainability and sonographic progression at 3 years. Am J Sports Med. 2015;44(2):504-510. doi:10.1177/0363546515612758.
12. Lee J, Harrison J, Boachie-Adjei K, Vargas E, Moley P. Platelet-rich plasma injections with needle tenotomy for gluteus medius tendinopathy: A registry study with prospective follow-up. Orthop J Sports Med. 2016;4(11):2325967116671692. doi:10.1177/2325967116671692.
13. Osborne H, Anderson L, Burt P, Young M, Gerrard D. Australasian College of Sports Physicians-Position statement: the place of mesenchymal stem/stromal cell therapies in sport and exercise medicine. Br J Sports Med. 2016;50:1237-1244. doi:10.1136/bjsports-2015-095711.
14. Anderson J, Little D, Toth A, et al. Stem cell therapies for knee cartilage repair. The current status of preclinical and clinical studies. Am J Sports Med. 2013;42(9)2253-2261. doi:10.1177/0363546513508744.
15. Lee S, Kwon B, Lee Kyoungbun, Son Y, Chung S. Therapeutic mechanisms of human adipose-derived mesenchymal stem cells in a rat tendon injury model. Am J Sports Med. 2017;45(6):1429-1439. doi:10.1177/0363546517689874.
16. McIntyre J, Jones I, Han B, Vangsness C. Intra-articular mesenchymal stem cell therapy for the human joint. A systematic review. Am J Sports Med. 2017;0363546517735844. doi:10.1177/0363546517735844.
17. Pas HIMFL, Moen M, Haisma J, Winters M. No evidence for the use of stem cell therapy for tendon disorders: a systematic review. Br J Sports Med. 2017;51:996-1002. doi:10.1136/bjsports-2016-096794.
18. Foster T, Puskas B, Mandelbaum B, Gerhardt M, Rodeo S. Platelet-rich plasma: from basic science to clinical applications. Am J Sports Med. 2009;37(11):2259-2272. doi:10.1177/0363546509349921.
19. Mishra A, Pavelko T. Treatment of chronic elbow tendinosis with buffered platelet-rich plasma. Am J Sports Med. 2006;34(11):1774-1778. doi:10.1177/0363546506288850.
20. Dines J, Williams P, ElAttrache N, et al. Platelet-rich plasma can be used to successfully treat elbow ulnar collateral ligament insufficiency in high-level throwers. Am J Orthop. 2016;45(4):296-300.
21. Fitzpatrick J, Bulsara M, O’Donnel J, McCrory P, Zheng M. The effectiveness of platelet-rich plasma injections in gluteal tendinopathy. A randomized, double-blind controlled trial comparing a single platelet-rich plasma injection with a single corticosteroid injection. Am J Sports Med. 2018;46(4)933-939. doi:10.1177/0363546517745525.
22. Hamid M, Ali M, Yusof A, George J, Lee L. Platelet-rich plasma injections for the treatment of hamstring injuries: A randomized controlled trial. Am J Sports Med. 2014;42(10):2410-2418. doi:10.1177/0363546514541540.
23. Zanon G, Combi F, Combi A, Perticarini L, Sammarchi L, Benazzo F. Platelet-rich plasma in the treatment of acute hamstring injuries in professional football players. Joints. 2016;4(1):17-23. doi:10.11138/jts/2016.4.1.017.
24. Hamilton B, Tol JL, Almusa E, et al. Platelet-rich plasma does not enhance return to play in hamstring injuries: a randomized controlled trial. Br J Sports Med. 2015;49:943-950. doi:10.1136/bjsports-2015-094603.
25. Pas HIMFL, Reurink G, Tol JL, Wier A, Winters M, Moen M. Efficacy of rehabilitation (lengthening) exercises, platelet-rich plasma injections, and other conservative interventions in acute hamstring injuries: an updated systematic review and meta-analysis. Br J Sports Med. 2015;49:1197-1205. doi:10.1136/bjsports-2015-094879.
26. Reurink G, Goudswaard G, Moen M, et al. Platelet-rich plasma injections in acute muscle injury. N Engl J Med. 2014;370:2546-2547. doi:10.1056/NEJMc1402340.
27. Kantrowitz D, Padaki A, Ahmad C, Lynch T. Defining platelet-rich plasma usage by team physicians in elite athletes. Orthop J Sports Med. 2018;6(4):2325967118767077. doi:10.1177/2325967118767077.
28. Mithoefer K, Peterson L, Zenobi-Wong M, Mandelbaum B. Cartilage issues in football-today’s problems and tomorrow’s solutions. Br J Sports Med. 2015;49(9):590-596. doi:1136/bjsports-2015-094772.
29. Matthews K, Cuchiara M. Regional regulatory insights: U.S. National Football League Athletes seeking unproven stem cell treatments. Stem Cells Dev. 2014;23(S1):60-64. doi:10.1089/scd.2014.0358.
30. McIntyre J, Jones I, Danilkovich A, Vangsness T. The placenta: applications in orthopaedic sports medicine. Am J Sports Med. 2018;46(1):234-247. doi:10.1177/0363546517697682.
31. Riboh J, Saltzman B, Yankee A, Cole BJ. Human amniotic membrane-derived products in sports medicine: Basic science, early results, and potential clinical applications. Am J Sports Med. 2015;44(9)2425-2434. doi:10.1177/0363546515612750.
ABSTRACT
Improvements in ultrasound technology have increased the popularity and use of ultrasound as a diagnostic and therapeutic modality for many soccer-related musculoskeletal (MSK) injuries. As a dynamic imaging modality, ultrasound offers increased accuracy and efficacy with minimally invasive procedures, such as guided injections, percutaneous tenotomy, and regenerative therapies, in the clinical setting. Emerging evidence indicates that regenerative therapies, such as platelet-rich-plasma (PRP), mesenchymal stem cells, and amniotic products, are a promising treatment for many MSK injuries and are gaining popularity among professional athletes. PRP is a safe treatment for a number of MSK conditions and has been included in the standard of care. However, conflicting evidence on return-to-play timeframes and efficacy in certain MSK conditions have led to inconsistent recommendations on indications for use, dose, and timing of treatment. Mesenchymal stem cell therapy, while promising, lacks high-level evidence of efficacy despite its increasing use among athletes. Currently, no data are available regarding the outcome of the use of amniotic products for the treatment of injuries in athletes. Furthermore, preparation of many regenerative therapies eclipses the concept of minimal manipulation and is subject to US Food and Drug Administration phase I to III trials. High-level research on regenerative medicine therapies should be continuously conducted to establish their clinical efficacy and safety data.
ULTRASOUND
Ultrasound (US) was first introduced for musculoskeletal (MSK) evaluation in 1957.1 Since then, US has gained increasing attention due to its ease of utilization in the clinical setting, repeatability, noninvasiveness, capability for contralateral comparison, lack of radiation exposure, and capability to provide real-time dynamic tissue assessment.1 Compared with magnetic resonance imaging or computed tomography, US presents limitations, including decreased resolution of certain tissues, limited field of view, limited penetration beyond osseous structures, incomplete evaluation of a joint or structure, and operator experience. However, advancements in technology, image resolution, and portability have improved the visualization of multiple anatomic structures and the accuracy of minimally invasive ultrasound-guided procedures at the point of care. The use of US for guided hip injections possibly decreases the cost relative to fluoroscopic guidance.2 Other studies have reported that US, as a result of its safety profile, has replaced fluoroscopy for certain procedures, such as barbotage of calcific tendinosis.3 US has been used for diagnostic purposes and guidance for therapeutic interventions, such as needle aspiration, diagnostic or therapeutic injection, needle tenotomy, tissue release, hydro-dissection, and biopsy.3 Given its expanding application, US has been increasingly used in the clinical setting, athletic training room, and sidelines of athletic events.
DIAGNOSTIC ULTRASOUND
An epidemiologic review of the National Collegiate Athletic Association (NCAA) men’s and women’s soccer injuries from 1988 to 2003 reported over 24,000 combined injuries. Over 70% of these injuries are MSK in nature and often affect the lower extremities.4,5 Ekstrand and colleagues6 also conducted an epidemiological review of muscle injuries among professional soccer players from 2001 to 2009. They found that 92% of all muscle injuries involved the lower extremities. The portability of US allows it to serve as an ideal modality for diagnostic evaluation of acute MSK injuries. Klauser and colleagues7 developed consensus based on the recommendations of the European Society of Musculoskeletal Radiology (ESSR) for the clinical indication of diagnostic ultrasound. A grading system was developed to describe the clinical utility of diagnostic US evaluation of MSK structures:
• Grade 0: Ultrasound is not indicated;
• Grade 1: Ultrasound is indicated if other imaging techniques are not appropriate;
• Grade 2: Ultrasound indication is equivalent to other imaging modalities;
• Grade 3: Ultrasound is the first-choice technique.
Henderson and colleagues8 conducted a review of 95 studies (12 systemic reviews and 83 diagnostic studies) that investigated the accuracy of diagnostic US imaging on soft tissue MSK injuries of the upper and lower extremities. They reported the sensitivity and specificity of the method for detection of over 40 hip, knee, ankle, and foot injuries and assigned corresponding grades based on diagnostic accuracy by using the same system developed by Klauser and colleagues.7,8 Common MSK injuries of the lower extremity and their corresponding ESSR grades are listed in the Table. This study demonstrated that diagnostic US is highly accurate for a number of soft tissue MSK injuries of the lower extremity and consistently matches the recommendation grades issued by Klauser and colleagues.7 In the hands of a skilled operator, US has become an increasingly popular and cost-effective modality for diagnosis and monitoring of acute muscle injuries and chronic tendinopathies among soccer athletes.
Table. Clinical Indication Grades for Diagnostic Ultrasound Evaluation of Common Lower Extremity Injuries7,8
Hip | Knee | Foot/Ankle |
Synovitis/Effusion: 3 | Quadricep tendinosis/tear: 3 | Anterior talofibular ligament injury: 3 |
Snapping hip (extra-articular): 3 | Patella tendinopathy: 3 | Calcaneofibular ligament injury: 3 |
Gluteal tendon tear: 3 | Pes anserine bursitis: 3 | Peroneal tendon tear/subluxation: 3 |
Meralgia paresthetica: 3 | Periarticular bursitis & ganglion: 3 | Posterior tibial tendinopathy: 3 |
Lateral femoral cutaneous nerve injury: 3 | Osgood-Schlatter & Sinding-Larsen: 3 | Plantaris tendon tear: 3 |
Femoral nerve injury: 3 | Synovitis/Effusion: 3 | Plantar fasciitis: 3 |
Sports hernia: 3 | Baker’s Cyst: 2-3 | Calcific tendonitis: 3 |
Morel-Lavallée lesions: 3 | MCL injury: 2 | Retrocalcaneal bursitis: 3 |
Muscle injury (high grade): 3 | IT band friction: 2 | Joint effusion: 3 |
Trochanteric bursitis: 2 | Medial patella plica syndrome: 2 | Ganglion cyst: 3 |
Proximal hamstring injury: 2 | Meniscal cyst: 2 | Retinacula pathology: 3 |
Sciatica: 1-2 | Common perineal neuropathy: 2 | Achilles tendinopathy: 2 |
Muscle injury (low grade): 1 | Distal hamstring tendon injury: 1-2 | Haglund disease: 2 |
Psoas tendon pathology: 1 | Intra-articular ganglion: 1 | Deltoid ligament injury: 2 |
Osteoarthritis: 0 | Hoffa’s fat pad syndrome: 1 | Plantar plate tear: 2 |
Labral tear: 0 | Loose bodies: 1 | Syndesmotic injury: 2 |
| LCL injury: 0-1 | Morton’s neuroma: 2 |
| Popliteal injury: 0-1 | Deltoid ligament injury: 1 |
| Plica syndrome: 0 | Spring ligament injury: 1 |
| Full/partial ACL tear: 0 | Anterolateral ankle impingement: 0 |
| PCL tear: 0 | Posterior talofibular ligament injury: 0 |
| Medial/lateral meniscus tear: 0 |
|
| Osteochondritis dissecans: 0 |
|
Abbreviations: ACL, anterior cruciate ligament; IT, iliotibial; LCL, lateral collateral ligament; MCL, medial collateral ligament; PCL, posterior cruciate ligament.
ULTRASOUND-GUIDED THERAPEUTIC PROCEDURES
The use of US at the point of care for needle guidance has led to its widespread application for therapeutic procedures, including injections and multiple regenerative therapies. Intra-articular US-guided injection and aspiration are common therapeutic interventions performed in the clinical setting. In a position statement of the American Medical Society for Sports Medicine, US-guided injections were found to be more accurate (SORT A evidence), effective (SORT B evidence), and cost effective (SORT B evidence) than landmark-guided injections.3 A recent meta-analysis conducted by Daniels and colleagues1 demonstrated the improved accuracy and efficacy of US-guided injections at the knee, ankle, and foot. Injections may serve a diagnostic purpose when anesthetics, such as lidocaine, are used in isolation, a therapeutic purpose, or both.
Continue to: Percutaneous tenotomy involve...
REGENERATIVE THERAPIES FOR MUSCULOSKELETAL CONDITIONS
PERCUTANEOUS TENOTOMY
Percutaneous tenotomy involves the introduction of a needle into damaged soft tissues, most often tendons (“needling”), in an effort to stimulate a healing response and resect the diseased tendon tissue. Although tenotomy was initially performed as an open or arthroscopic surgical technique, advances in US technology have led to improved sensitivity and specificity identifying areas of tendinous injury (hypervascularity, hypoechogenicity, and calcification); as such, the combination of these techniques has been used in the outpatient setting. New commercial models incorporate ultrasound guidance with needles or micro-resection probes for real-time débridement of damaged tissues. Percutaneous tenotomy has been described in the management of tendinopathy involving the rotator cuff, medial and lateral epicondyles, patellar and Achilles tendons, and plantar fascia.
Housner and colleagues9 evaluated the safety and short-term efficacy of US-guided needle tenotomy in 13 patients with chronic tendinosis of the patella, Achilles tendon, gluteus medius, iliotibial tract, hamstring, and rectus femoris. They reported no procedural complications and a significant decrease in pain scores at 4 and 12 weeks of follow-up.
Koh and colleagues10 conducted a prospective case series to evaluate the safety and efficacy of office-based, US-guided percutaneous tenotomy (using a commercial model) on 20 patients with chronic lateral epicondylitis. The authors reported no wound complications and significant improvement in pain scores at each follow-up period up to 1 year. Subsequent post-procedural US evaluation of injured tissues revealed evidence of healing (decreased tendon thickness, vascularity, and hypoechogenicity) in over half the cohort after 6 months compared with the baseline.11
Lee and colleagues12 evaluated the efficacy of US-guided needle tenotomy combined with platelet-rich plasma (PRP) injection on chronic recalcitrant gluteus medius tendinopathy. In this case series, 21 patients underwent PRP and “needling” through the hypoechoic regions of the injured tendon under direct US guidance. After a period of rest, all patients completed the structured rehabilitation protocol. After an average follow-up of 10 months, all patients displayed significant improvements in all outcome questionnaires and did not report any significant adverse events. The authors concluded that tenotomy combined with PRP is a safe and effective method for treatment for recalcitrant gluteus medius tendinopathy.
These studies indicate that US-guided percutaneous tenotomy, alone or in combination with regenerative therapies, such as PRP, is a safe and effective treatment option for various tendinopathies. However, while tenotomy appears safe with promising results and no reported major adverse events, the level of evidence remains low.
ORTHOBIOLOGICS
Orthobiologics are substances composed of biological materials that can be used to aid or even hasten the healing of bones, muscles, tendons, and ligaments. Orthobiologics may contain growth factors, which initiate or stimulate the body’s reparative process; matrix proteins, which serve as scaffolding for healing tissues; or stem cells, specifically adult stem cells, which are multipotent and can differentiate into several cell lines. Adult stem cells are categorized as hematopoietic, neural, epithelial, skin, and mesenchymal types. Mesenchymal stem cells (MSCs) are of particular interest in sports medicine applications because they secrete growth factors and cytokines with trophic, chemotactic, and immunosuppressive properties.13 MSCs are also multipotent and can differentiate into bones, muscles, cartilages, and tendons.14-17MSCs are readily isolated from many sources, including bone marrow, adipose tissues, synovial tissues, peripheral blood, skeletal muscles, umbilical cord blood, and placenta.13,14Several types of regenerative therapies used in orthopedic and sports medicine practice include PRP, stem cell therapy, and amniotic membrane/fluid preparations. While each therapy possesses the potential for promising results, the paucity of research and discrepancies among studies regarding the description of stem cell lines used limit the available evidence on the true clinical benefits of these regenerative therapies.
[HEAD 3] PLATELET-RICH PLASMA
PRP is an autologous product that has been used to stimulate biological factors and promote healing since the 1970s. Through the activation of platelets, PRP improves localized recruitment, proliferation, and differentiation of cells involved in tissue repair. Platelets, which are non-nucleated bodies located in peripheral blood, contain and release 3 groups of bioactive factors that enhance the healing process. Growth factors and cytokines released from alpha-granules play a role in cell proliferation, chemotaxis, cell differentiation, and angiogenesis. Bioactive factors, such as serotonin and histamine, released from dense granules, increase capillary permeability and improve cell recruitment and migration. Adhesion molecules also assist in cell migration and creation of an extracellular matrix, which acts as a scaffold for wound healing.18 Platelets are activated by mechanical trauma or contact with multiple activators, including Von Willebrand factor, collagen, thrombin, or calcium chloride. When activated, platelets release growth factors and cytokines, which create a pro-inflammatory environment that mediates the tissue repair process. After the procedure, the pro-inflammatory environment may result in patient discomfort, which can be managed with ice and acetaminophen. Use of nonsteroidal anti-inflammatory drugs may theoretically inhibit the inflammatory cascade induced by PRP, and they are avoided before and after the procedure, although evidence regarding necessary time frames is lacking.
Continue to: PRP consists of...
PRP consists of the fractionated liquid component of autologous whole blood, which contains increased concentrations of platelets and cytokines. Different methods and commercial preparations are available for collecting and preparing PRP. Variations in the amount of blood drawn, use of anticoagulants, presence or absence of an activating agent, number of centrifuge spins, and overall platelet and white blood cell concentrations lead to difficulty in evaluating and interpreting the available evidence regarding PRP therapy.
In vitro and animal studies demonstrated promising and safe results regarding the healing effect of PRP on injured soft tissues, such as tendons, ligaments, and muscles. In this regard, a number of studies have evaluated the effect of PRP on human MSK injuries. However, in addition to the above-mentioned variabilities in PRP, many of such studies lack standardization and randomization techniques and include a small number of patients only, thereby limiting the overall comparison and clinical application.
A landmark study conducted by Mishra and Pavelko19 concluded that PRP significantly reduced pain in patients with chronic elbow tendinosis. Similar findings were reported in high-level overhead athletes with ulnar collateral ligament insufficiency, which did not improve with conservative management.20 Fitzpatrick and colleagues21 found improvements in pain with the use of single PRP injection as treatment for chronic gluteal tendinopathy. PRP can effectively improve pain and recovery in chronic ligament and tendon injuries, such as lateral epicondylitis, patellar tendinopathy, and plantar fasciitis, when patients are unresponsive to traditional conservative management. The application of PRP to treat acute MSK injuries has produced mixed results. Hamid and colleagues22 conducted a level II randomized controlled trial to evaluate the effect of PRP combined with a rehabilitation program for treatment of grade 2 hamstring injuries on return-to-play compared with rehabilitation alone. Fourteen athletes were randomized into the study and control groups. Hamid and colleagues22 reported improved return-to-play in the study group compared with that in the control (26.7 and 42.5 days, respectively). This study also reported lower pain scores in the PRP group over time, but the difference was not statistically significant. Zanon and colleagues23 conducted a prospective study to evaluate return-to-play in professional soccer players with acute hamstring strains treated with PRP and a rehabilitation program. This study determined that athletes treated with PRP were “match fit,” meaning they would be available for match selection in an average of 36.8 days. However, Zanon and colleagues23 did not include a control group for comparison. Other studies reported that PRP treatment of acutely injured muscles and medial collateral ligaments of soccer and basketball players decreased their return-to-play interval.18 Reviews by Hamilton and colleagues24 and Pas and colleagues25 concluded that PRP treatment of acutely injured tissues with good blood supply (eg, hamstring muscles) did not improve pain or return-to-play compared with standardized rehabilitation protocols. Similarly, in a double-blinded placebo controlled trial, Reurink and colleagues26 evaluated return-to-play in 80 athletes with acute hamstring injuries treated with a rehabilitation program and either PRP or placebo. Reurink and colleagues26 found no difference in return-to-play (42 days for both groups), but the difference was not statistically significant. PRP has also been used intraoperatively and shows promising results in total knee arthroplasty, anterior cruciate ligament reconstruction, acute Achilles tendon repair, rotator cuff repair, and cartilage repair. However, many of these intraoperative studies are limited to animal models.
In 2009, the World Anti-Doping Agency (WADA) prohibited the use of PRP because it contains autologous growth factors and IGF-1, which could produce an anabolic effect. Recent studies have failed to demonstrate any athletic advantages of using PRP. WADA has since removed PRP from its prohibited list. PRP is also not prohibited by the US Anti-Doping Agency (USADA) and many major professional sporting leagues in the United States. However, care must be taken in reviewing the components of PRP because many commercially available products differ in PRP formulation. Since 2010, many team physicians have increasingly used PRP to treat a wide range of athletic injuries. A recent anonymous survey conducted by a team of physicians on PRP use in elite athletes revealed minimal complications but significant variability among physicians with regard to timing, belief in evidence, and formulation and dosing of PRP treatments. Many physicians did implicate athlete desire as the main indication for treatment.27
As an autologous treatment, PRP injection has no serious adverse effects beyond mild discomfort as a result of the procedure and pro-inflammatory state in the days following injection. Recent concerns regarding the potential of PRP treatment for heterotopic ossification have been reported, but published information is limited to case reports. PRP can improve pain and function in patients with chronic MSK injury. PRP appears to be a safe and effective alternative to surgery for patients with injury to poorly perfused tissue, which has not improved with conservative measures, such as rest, physical therapy, and anti-inflammatory medications. Care should be taken when treating athletes with PRP to establish regulations on doping by individual governing bodies.
Continue to: Use of stem...
STEM CELL THERAPY
Use of stem cell therapy is based on the properties of the proliferation and differentiation of multipoint MSC lines. These stem cells can theoretically regenerate injured tissues and influence repair through immunomodulation; paracrine activity through the release of bioactive agents, such as cytokines, trophic, and chemotactic molecules; and cell differentiation into various cell lineages.15,16,13,17 Orthopedic surgeons have used microfracture to recruit MSCs during cartilage repair procedures for over 20 years. This procedure draws multipotent MSCs to the injured site to induce chondrogenic proliferation and fibrocartilage repair.28
Adult MSCs provide a readily accessible autologous source of stem cells for regenerative therapies. MSCs can be isolated from a variety of tissues, including bone marrow, adipose tissues, synovia, human umbilical cord blood, and peripheral blood. The majority of stem cell therapies in the United States for sports medicine purposes are conducted using bone marrow aspirate concentrate (BMAC) and adipose tissues. The US Food and Drug Administration (FDA) allows the use of minimally manipulated autologous stem cells to be injected into the same patient on the same day. However, some studies reported that culturing stem cells or introducing products, such as collagenase to stem cells, can increase the stem cell concentration prior to injection. These processes constitute more than “minimal manipulation” and therefore would require drug trials prior to use in the United States.
Although MSCs can be readily obtained from a variety of tissue sources, the makeup of the cell concentrate differs. Bone marrow and adipose tissues are readily available sources of homogenous MSCs. Harvesting stem cells from adipose tissues provides a less invasive route of collection than from BMAC. Harvested BMAC and adipose tissues consist of heterogeneous cell populations that are composed of precursor and accessory cells, such as pericytes, endothelial cells, smooth muscle cells, fibroblasts, and macrophages in addition to MSCs.
Animal studies reported promising results when evaluating soft tissue lesions in small and large animal models.14,15 Although clinical and human evidence remains limited, the potential of MSCs for regenerative repair has led to a recent increase in the number of related clinical studies. Multiple systematic reviews have concluded that MSC therapy is safe for the treatment of osteoarthritis, cartilage lesions, and tendinopathies. Limited evidence is available regarding the safety of intramuscular use, and a theoretical concern arises on the development of heterotopic bone formation as a result of treatment.13,16 The efficacy of MSC therapy is difficult to determine due to the lack of standardization in stem cell populations, adjuvants (eg, PRP, hyaluronic acid, and scaffolding preparations), and delivery methods used.13,17
Similar to PRP, the increased use of MSC therapy among high-profile athletes has led to the promotion of these therapies as safe and effective despite limited evidence.29 Although MSC therapy is a promising and safe treatment option for patients with soft tissue injuries, the paucity in data and human studies limit its clinical use. Moreover, data of MSC efficacy is complicated because of the disparity between clinical studies regarding MSC collection method (many of which eclipse the “minimal manipulation” standard), description of isolated cell concentrates, dosage, method of delivery, use of adjuvants, and lack of randomization. Further studies using [standardized] methods are needed before establishing a true consensus on the safety and efficacy of MSC therapy.
AMNIOTIC MEMBRANE
The placenta is a source of MSCs, a collagen-rich extracellular matrix, and bioactive growth and regulatory factors. The capacity of the placenta to modulate biological activities and tissue formation is thought to provide a means of tissue repair and healing. The placenta consists of amniotic fluid, amniotic membrane (AM), chorionic membrane, and umbilical cord blood and tissues. Although MSCs have been isolated from each component of placental tissues, amniotic and chorionic membranes and umbilical cord tissues yield the highest concentration.
The majority of regenerative studies involving the placenta used AM alone or in combination with other placental tissues. AM is a metabolically active tissue that consists of an epithelial layer, a basement membrane, and a mesenchymal tissue layer. In addition to being a source of stem cells, AM synthesizes many growth factors, vasoactive peptides, and cytokines, which are capable of tissue regeneration. AM was initially used as a biological scaffold for the treatment of skin burns and wounds. Other intrinsic properties of AM include the provision of a matrix for cellular migration and proliferation, enhanced wound healing with reduced scar formation, antibacterial activity, and lastly, non-immunogenic and immunosuppressive properties. These inherent characteristics have spurred studies on the potential use of AM in sports medicine as a minimally invasive means to treat osteoarthritis and injuries of tendons, ligaments, muscles, fascia, and cartilages.
Continue to: Animal studies reported...
Animal studies reported positive results with the use of AM to treat osteoarthritis, cartilage defects, and tendon and ligament injuries. Few studies involving human participants also revealed favorable results with regard to the use of AM for the treatment of plantar fasciitis and osteoarthritis; however, these studies are industry-sponsored and employed small sample sizes. The unique mixture of a collagen-rich extracellular matrix, bioactive growth factors, and pluripotent stem cells may allow AM to become an effective treatment for MSK injuries. Although initial animal and human studies show promising results, variabilities regarding models (animal and human), pathologies, placental tissues, and methods of preparation, preservation, and delivery used limit the ability for comparison, analysis, and drawing of definitive conclusions. Thus far, no studies have evaluated the use of currently available AM products for the treatment of injuries sustained by soccer players.
Despite the current popularity of AM as regenerative therapy in academic research and potential use in clinical treatment in sports medicine, physicians should remain aware of the limited evidence available. Other barriers to research and use AM as a regenerative therapy include regulatory classifications based on the concept of “minimal manipulation” in biologic therapies. Minimally manipulated placental allografts are less regulated, less costly to study, and more easily commercialized. These products are not required to undergo FDA phase I to III trials prior to premarket approval. In 2000, the FDA position on all AM products falls into 2 categories. The first position states that AM that contains allogenic stem cells mixed with another drug that is micronized and/or cryopreserved is more than “minimally manipulated” and therefore categorized as “biologic” and would be subject to phase I to III trials. Dehydrated and decellularized AM, however, may meet the concept of minimal manipulation and is only approved by the FDA as a wound covering. Thus, any application of AM for the treatment of sports medicine pathology is not currently FDA-approved, considered off-label, not covered by insurance, and subject to out-of-pocket pay.30,31
CONCLUSION
With improvements in technology and portability, US has become an effective imaging modality for point-of-care evaluation, diagnosis, and continuous monitoring of many MSK injuries. Additionally, as a dynamic imaging modality, US allows for increased accuracy and efficacy when combined with minimally invasive procedures, such as diagnostic and therapeutic guided injections and percutaneous tenotomy, in the clinical setting; thereby decreasing the overall healthcare costs. PRP is proven to be a safe treatment for several MSK conditions, such as lateral epicondylitis, patellar tendonitis, and plantar fasciitis. Although PRP has been included in the standard of care in some areas, this technique may be predominantly athlete driven. Conflicting evidence with regard to return-to-play timeframes following PRP treatment for muscular injuries and poor evidence in conditions, such as Achilles tendonitis, have led to inconsistent indications for use, dose, and timing of treatment. Although early evidence of MSC therapy is promising, high-level evidence for MSC therapy is insufficient, despite its increased use among athletes. Thus far, no data are available regarding the outcomes of the use of amniotic products for the treatment of injuries among athletes. Furthermore, the preparation of amniotic products has many regulatory concerns. The authors advocate for continuous high-level research on regenerative medicine therapies to establish clinical efficacy and safety data.
ABSTRACT
Improvements in ultrasound technology have increased the popularity and use of ultrasound as a diagnostic and therapeutic modality for many soccer-related musculoskeletal (MSK) injuries. As a dynamic imaging modality, ultrasound offers increased accuracy and efficacy with minimally invasive procedures, such as guided injections, percutaneous tenotomy, and regenerative therapies, in the clinical setting. Emerging evidence indicates that regenerative therapies, such as platelet-rich-plasma (PRP), mesenchymal stem cells, and amniotic products, are a promising treatment for many MSK injuries and are gaining popularity among professional athletes. PRP is a safe treatment for a number of MSK conditions and has been included in the standard of care. However, conflicting evidence on return-to-play timeframes and efficacy in certain MSK conditions have led to inconsistent recommendations on indications for use, dose, and timing of treatment. Mesenchymal stem cell therapy, while promising, lacks high-level evidence of efficacy despite its increasing use among athletes. Currently, no data are available regarding the outcome of the use of amniotic products for the treatment of injuries in athletes. Furthermore, preparation of many regenerative therapies eclipses the concept of minimal manipulation and is subject to US Food and Drug Administration phase I to III trials. High-level research on regenerative medicine therapies should be continuously conducted to establish their clinical efficacy and safety data.
ULTRASOUND
Ultrasound (US) was first introduced for musculoskeletal (MSK) evaluation in 1957.1 Since then, US has gained increasing attention due to its ease of utilization in the clinical setting, repeatability, noninvasiveness, capability for contralateral comparison, lack of radiation exposure, and capability to provide real-time dynamic tissue assessment.1 Compared with magnetic resonance imaging or computed tomography, US presents limitations, including decreased resolution of certain tissues, limited field of view, limited penetration beyond osseous structures, incomplete evaluation of a joint or structure, and operator experience. However, advancements in technology, image resolution, and portability have improved the visualization of multiple anatomic structures and the accuracy of minimally invasive ultrasound-guided procedures at the point of care. The use of US for guided hip injections possibly decreases the cost relative to fluoroscopic guidance.2 Other studies have reported that US, as a result of its safety profile, has replaced fluoroscopy for certain procedures, such as barbotage of calcific tendinosis.3 US has been used for diagnostic purposes and guidance for therapeutic interventions, such as needle aspiration, diagnostic or therapeutic injection, needle tenotomy, tissue release, hydro-dissection, and biopsy.3 Given its expanding application, US has been increasingly used in the clinical setting, athletic training room, and sidelines of athletic events.
DIAGNOSTIC ULTRASOUND
An epidemiologic review of the National Collegiate Athletic Association (NCAA) men’s and women’s soccer injuries from 1988 to 2003 reported over 24,000 combined injuries. Over 70% of these injuries are MSK in nature and often affect the lower extremities.4,5 Ekstrand and colleagues6 also conducted an epidemiological review of muscle injuries among professional soccer players from 2001 to 2009. They found that 92% of all muscle injuries involved the lower extremities. The portability of US allows it to serve as an ideal modality for diagnostic evaluation of acute MSK injuries. Klauser and colleagues7 developed consensus based on the recommendations of the European Society of Musculoskeletal Radiology (ESSR) for the clinical indication of diagnostic ultrasound. A grading system was developed to describe the clinical utility of diagnostic US evaluation of MSK structures:
• Grade 0: Ultrasound is not indicated;
• Grade 1: Ultrasound is indicated if other imaging techniques are not appropriate;
• Grade 2: Ultrasound indication is equivalent to other imaging modalities;
• Grade 3: Ultrasound is the first-choice technique.
Henderson and colleagues8 conducted a review of 95 studies (12 systemic reviews and 83 diagnostic studies) that investigated the accuracy of diagnostic US imaging on soft tissue MSK injuries of the upper and lower extremities. They reported the sensitivity and specificity of the method for detection of over 40 hip, knee, ankle, and foot injuries and assigned corresponding grades based on diagnostic accuracy by using the same system developed by Klauser and colleagues.7,8 Common MSK injuries of the lower extremity and their corresponding ESSR grades are listed in the Table. This study demonstrated that diagnostic US is highly accurate for a number of soft tissue MSK injuries of the lower extremity and consistently matches the recommendation grades issued by Klauser and colleagues.7 In the hands of a skilled operator, US has become an increasingly popular and cost-effective modality for diagnosis and monitoring of acute muscle injuries and chronic tendinopathies among soccer athletes.
Table. Clinical Indication Grades for Diagnostic Ultrasound Evaluation of Common Lower Extremity Injuries7,8
Hip | Knee | Foot/Ankle |
Synovitis/Effusion: 3 | Quadricep tendinosis/tear: 3 | Anterior talofibular ligament injury: 3 |
Snapping hip (extra-articular): 3 | Patella tendinopathy: 3 | Calcaneofibular ligament injury: 3 |
Gluteal tendon tear: 3 | Pes anserine bursitis: 3 | Peroneal tendon tear/subluxation: 3 |
Meralgia paresthetica: 3 | Periarticular bursitis & ganglion: 3 | Posterior tibial tendinopathy: 3 |
Lateral femoral cutaneous nerve injury: 3 | Osgood-Schlatter & Sinding-Larsen: 3 | Plantaris tendon tear: 3 |
Femoral nerve injury: 3 | Synovitis/Effusion: 3 | Plantar fasciitis: 3 |
Sports hernia: 3 | Baker’s Cyst: 2-3 | Calcific tendonitis: 3 |
Morel-Lavallée lesions: 3 | MCL injury: 2 | Retrocalcaneal bursitis: 3 |
Muscle injury (high grade): 3 | IT band friction: 2 | Joint effusion: 3 |
Trochanteric bursitis: 2 | Medial patella plica syndrome: 2 | Ganglion cyst: 3 |
Proximal hamstring injury: 2 | Meniscal cyst: 2 | Retinacula pathology: 3 |
Sciatica: 1-2 | Common perineal neuropathy: 2 | Achilles tendinopathy: 2 |
Muscle injury (low grade): 1 | Distal hamstring tendon injury: 1-2 | Haglund disease: 2 |
Psoas tendon pathology: 1 | Intra-articular ganglion: 1 | Deltoid ligament injury: 2 |
Osteoarthritis: 0 | Hoffa’s fat pad syndrome: 1 | Plantar plate tear: 2 |
Labral tear: 0 | Loose bodies: 1 | Syndesmotic injury: 2 |
| LCL injury: 0-1 | Morton’s neuroma: 2 |
| Popliteal injury: 0-1 | Deltoid ligament injury: 1 |
| Plica syndrome: 0 | Spring ligament injury: 1 |
| Full/partial ACL tear: 0 | Anterolateral ankle impingement: 0 |
| PCL tear: 0 | Posterior talofibular ligament injury: 0 |
| Medial/lateral meniscus tear: 0 |
|
| Osteochondritis dissecans: 0 |
|
Abbreviations: ACL, anterior cruciate ligament; IT, iliotibial; LCL, lateral collateral ligament; MCL, medial collateral ligament; PCL, posterior cruciate ligament.
ULTRASOUND-GUIDED THERAPEUTIC PROCEDURES
The use of US at the point of care for needle guidance has led to its widespread application for therapeutic procedures, including injections and multiple regenerative therapies. Intra-articular US-guided injection and aspiration are common therapeutic interventions performed in the clinical setting. In a position statement of the American Medical Society for Sports Medicine, US-guided injections were found to be more accurate (SORT A evidence), effective (SORT B evidence), and cost effective (SORT B evidence) than landmark-guided injections.3 A recent meta-analysis conducted by Daniels and colleagues1 demonstrated the improved accuracy and efficacy of US-guided injections at the knee, ankle, and foot. Injections may serve a diagnostic purpose when anesthetics, such as lidocaine, are used in isolation, a therapeutic purpose, or both.
Continue to: Percutaneous tenotomy involve...
REGENERATIVE THERAPIES FOR MUSCULOSKELETAL CONDITIONS
PERCUTANEOUS TENOTOMY
Percutaneous tenotomy involves the introduction of a needle into damaged soft tissues, most often tendons (“needling”), in an effort to stimulate a healing response and resect the diseased tendon tissue. Although tenotomy was initially performed as an open or arthroscopic surgical technique, advances in US technology have led to improved sensitivity and specificity identifying areas of tendinous injury (hypervascularity, hypoechogenicity, and calcification); as such, the combination of these techniques has been used in the outpatient setting. New commercial models incorporate ultrasound guidance with needles or micro-resection probes for real-time débridement of damaged tissues. Percutaneous tenotomy has been described in the management of tendinopathy involving the rotator cuff, medial and lateral epicondyles, patellar and Achilles tendons, and plantar fascia.
Housner and colleagues9 evaluated the safety and short-term efficacy of US-guided needle tenotomy in 13 patients with chronic tendinosis of the patella, Achilles tendon, gluteus medius, iliotibial tract, hamstring, and rectus femoris. They reported no procedural complications and a significant decrease in pain scores at 4 and 12 weeks of follow-up.
Koh and colleagues10 conducted a prospective case series to evaluate the safety and efficacy of office-based, US-guided percutaneous tenotomy (using a commercial model) on 20 patients with chronic lateral epicondylitis. The authors reported no wound complications and significant improvement in pain scores at each follow-up period up to 1 year. Subsequent post-procedural US evaluation of injured tissues revealed evidence of healing (decreased tendon thickness, vascularity, and hypoechogenicity) in over half the cohort after 6 months compared with the baseline.11
Lee and colleagues12 evaluated the efficacy of US-guided needle tenotomy combined with platelet-rich plasma (PRP) injection on chronic recalcitrant gluteus medius tendinopathy. In this case series, 21 patients underwent PRP and “needling” through the hypoechoic regions of the injured tendon under direct US guidance. After a period of rest, all patients completed the structured rehabilitation protocol. After an average follow-up of 10 months, all patients displayed significant improvements in all outcome questionnaires and did not report any significant adverse events. The authors concluded that tenotomy combined with PRP is a safe and effective method for treatment for recalcitrant gluteus medius tendinopathy.
These studies indicate that US-guided percutaneous tenotomy, alone or in combination with regenerative therapies, such as PRP, is a safe and effective treatment option for various tendinopathies. However, while tenotomy appears safe with promising results and no reported major adverse events, the level of evidence remains low.
ORTHOBIOLOGICS
Orthobiologics are substances composed of biological materials that can be used to aid or even hasten the healing of bones, muscles, tendons, and ligaments. Orthobiologics may contain growth factors, which initiate or stimulate the body’s reparative process; matrix proteins, which serve as scaffolding for healing tissues; or stem cells, specifically adult stem cells, which are multipotent and can differentiate into several cell lines. Adult stem cells are categorized as hematopoietic, neural, epithelial, skin, and mesenchymal types. Mesenchymal stem cells (MSCs) are of particular interest in sports medicine applications because they secrete growth factors and cytokines with trophic, chemotactic, and immunosuppressive properties.13 MSCs are also multipotent and can differentiate into bones, muscles, cartilages, and tendons.14-17MSCs are readily isolated from many sources, including bone marrow, adipose tissues, synovial tissues, peripheral blood, skeletal muscles, umbilical cord blood, and placenta.13,14Several types of regenerative therapies used in orthopedic and sports medicine practice include PRP, stem cell therapy, and amniotic membrane/fluid preparations. While each therapy possesses the potential for promising results, the paucity of research and discrepancies among studies regarding the description of stem cell lines used limit the available evidence on the true clinical benefits of these regenerative therapies.
[HEAD 3] PLATELET-RICH PLASMA
PRP is an autologous product that has been used to stimulate biological factors and promote healing since the 1970s. Through the activation of platelets, PRP improves localized recruitment, proliferation, and differentiation of cells involved in tissue repair. Platelets, which are non-nucleated bodies located in peripheral blood, contain and release 3 groups of bioactive factors that enhance the healing process. Growth factors and cytokines released from alpha-granules play a role in cell proliferation, chemotaxis, cell differentiation, and angiogenesis. Bioactive factors, such as serotonin and histamine, released from dense granules, increase capillary permeability and improve cell recruitment and migration. Adhesion molecules also assist in cell migration and creation of an extracellular matrix, which acts as a scaffold for wound healing.18 Platelets are activated by mechanical trauma or contact with multiple activators, including Von Willebrand factor, collagen, thrombin, or calcium chloride. When activated, platelets release growth factors and cytokines, which create a pro-inflammatory environment that mediates the tissue repair process. After the procedure, the pro-inflammatory environment may result in patient discomfort, which can be managed with ice and acetaminophen. Use of nonsteroidal anti-inflammatory drugs may theoretically inhibit the inflammatory cascade induced by PRP, and they are avoided before and after the procedure, although evidence regarding necessary time frames is lacking.
Continue to: PRP consists of...
PRP consists of the fractionated liquid component of autologous whole blood, which contains increased concentrations of platelets and cytokines. Different methods and commercial preparations are available for collecting and preparing PRP. Variations in the amount of blood drawn, use of anticoagulants, presence or absence of an activating agent, number of centrifuge spins, and overall platelet and white blood cell concentrations lead to difficulty in evaluating and interpreting the available evidence regarding PRP therapy.
In vitro and animal studies demonstrated promising and safe results regarding the healing effect of PRP on injured soft tissues, such as tendons, ligaments, and muscles. In this regard, a number of studies have evaluated the effect of PRP on human MSK injuries. However, in addition to the above-mentioned variabilities in PRP, many of such studies lack standardization and randomization techniques and include a small number of patients only, thereby limiting the overall comparison and clinical application.
A landmark study conducted by Mishra and Pavelko19 concluded that PRP significantly reduced pain in patients with chronic elbow tendinosis. Similar findings were reported in high-level overhead athletes with ulnar collateral ligament insufficiency, which did not improve with conservative management.20 Fitzpatrick and colleagues21 found improvements in pain with the use of single PRP injection as treatment for chronic gluteal tendinopathy. PRP can effectively improve pain and recovery in chronic ligament and tendon injuries, such as lateral epicondylitis, patellar tendinopathy, and plantar fasciitis, when patients are unresponsive to traditional conservative management. The application of PRP to treat acute MSK injuries has produced mixed results. Hamid and colleagues22 conducted a level II randomized controlled trial to evaluate the effect of PRP combined with a rehabilitation program for treatment of grade 2 hamstring injuries on return-to-play compared with rehabilitation alone. Fourteen athletes were randomized into the study and control groups. Hamid and colleagues22 reported improved return-to-play in the study group compared with that in the control (26.7 and 42.5 days, respectively). This study also reported lower pain scores in the PRP group over time, but the difference was not statistically significant. Zanon and colleagues23 conducted a prospective study to evaluate return-to-play in professional soccer players with acute hamstring strains treated with PRP and a rehabilitation program. This study determined that athletes treated with PRP were “match fit,” meaning they would be available for match selection in an average of 36.8 days. However, Zanon and colleagues23 did not include a control group for comparison. Other studies reported that PRP treatment of acutely injured muscles and medial collateral ligaments of soccer and basketball players decreased their return-to-play interval.18 Reviews by Hamilton and colleagues24 and Pas and colleagues25 concluded that PRP treatment of acutely injured tissues with good blood supply (eg, hamstring muscles) did not improve pain or return-to-play compared with standardized rehabilitation protocols. Similarly, in a double-blinded placebo controlled trial, Reurink and colleagues26 evaluated return-to-play in 80 athletes with acute hamstring injuries treated with a rehabilitation program and either PRP or placebo. Reurink and colleagues26 found no difference in return-to-play (42 days for both groups), but the difference was not statistically significant. PRP has also been used intraoperatively and shows promising results in total knee arthroplasty, anterior cruciate ligament reconstruction, acute Achilles tendon repair, rotator cuff repair, and cartilage repair. However, many of these intraoperative studies are limited to animal models.
In 2009, the World Anti-Doping Agency (WADA) prohibited the use of PRP because it contains autologous growth factors and IGF-1, which could produce an anabolic effect. Recent studies have failed to demonstrate any athletic advantages of using PRP. WADA has since removed PRP from its prohibited list. PRP is also not prohibited by the US Anti-Doping Agency (USADA) and many major professional sporting leagues in the United States. However, care must be taken in reviewing the components of PRP because many commercially available products differ in PRP formulation. Since 2010, many team physicians have increasingly used PRP to treat a wide range of athletic injuries. A recent anonymous survey conducted by a team of physicians on PRP use in elite athletes revealed minimal complications but significant variability among physicians with regard to timing, belief in evidence, and formulation and dosing of PRP treatments. Many physicians did implicate athlete desire as the main indication for treatment.27
As an autologous treatment, PRP injection has no serious adverse effects beyond mild discomfort as a result of the procedure and pro-inflammatory state in the days following injection. Recent concerns regarding the potential of PRP treatment for heterotopic ossification have been reported, but published information is limited to case reports. PRP can improve pain and function in patients with chronic MSK injury. PRP appears to be a safe and effective alternative to surgery for patients with injury to poorly perfused tissue, which has not improved with conservative measures, such as rest, physical therapy, and anti-inflammatory medications. Care should be taken when treating athletes with PRP to establish regulations on doping by individual governing bodies.
Continue to: Use of stem...
STEM CELL THERAPY
Use of stem cell therapy is based on the properties of the proliferation and differentiation of multipoint MSC lines. These stem cells can theoretically regenerate injured tissues and influence repair through immunomodulation; paracrine activity through the release of bioactive agents, such as cytokines, trophic, and chemotactic molecules; and cell differentiation into various cell lineages.15,16,13,17 Orthopedic surgeons have used microfracture to recruit MSCs during cartilage repair procedures for over 20 years. This procedure draws multipotent MSCs to the injured site to induce chondrogenic proliferation and fibrocartilage repair.28
Adult MSCs provide a readily accessible autologous source of stem cells for regenerative therapies. MSCs can be isolated from a variety of tissues, including bone marrow, adipose tissues, synovia, human umbilical cord blood, and peripheral blood. The majority of stem cell therapies in the United States for sports medicine purposes are conducted using bone marrow aspirate concentrate (BMAC) and adipose tissues. The US Food and Drug Administration (FDA) allows the use of minimally manipulated autologous stem cells to be injected into the same patient on the same day. However, some studies reported that culturing stem cells or introducing products, such as collagenase to stem cells, can increase the stem cell concentration prior to injection. These processes constitute more than “minimal manipulation” and therefore would require drug trials prior to use in the United States.
Although MSCs can be readily obtained from a variety of tissue sources, the makeup of the cell concentrate differs. Bone marrow and adipose tissues are readily available sources of homogenous MSCs. Harvesting stem cells from adipose tissues provides a less invasive route of collection than from BMAC. Harvested BMAC and adipose tissues consist of heterogeneous cell populations that are composed of precursor and accessory cells, such as pericytes, endothelial cells, smooth muscle cells, fibroblasts, and macrophages in addition to MSCs.
Animal studies reported promising results when evaluating soft tissue lesions in small and large animal models.14,15 Although clinical and human evidence remains limited, the potential of MSCs for regenerative repair has led to a recent increase in the number of related clinical studies. Multiple systematic reviews have concluded that MSC therapy is safe for the treatment of osteoarthritis, cartilage lesions, and tendinopathies. Limited evidence is available regarding the safety of intramuscular use, and a theoretical concern arises on the development of heterotopic bone formation as a result of treatment.13,16 The efficacy of MSC therapy is difficult to determine due to the lack of standardization in stem cell populations, adjuvants (eg, PRP, hyaluronic acid, and scaffolding preparations), and delivery methods used.13,17
Similar to PRP, the increased use of MSC therapy among high-profile athletes has led to the promotion of these therapies as safe and effective despite limited evidence.29 Although MSC therapy is a promising and safe treatment option for patients with soft tissue injuries, the paucity in data and human studies limit its clinical use. Moreover, data of MSC efficacy is complicated because of the disparity between clinical studies regarding MSC collection method (many of which eclipse the “minimal manipulation” standard), description of isolated cell concentrates, dosage, method of delivery, use of adjuvants, and lack of randomization. Further studies using [standardized] methods are needed before establishing a true consensus on the safety and efficacy of MSC therapy.
AMNIOTIC MEMBRANE
The placenta is a source of MSCs, a collagen-rich extracellular matrix, and bioactive growth and regulatory factors. The capacity of the placenta to modulate biological activities and tissue formation is thought to provide a means of tissue repair and healing. The placenta consists of amniotic fluid, amniotic membrane (AM), chorionic membrane, and umbilical cord blood and tissues. Although MSCs have been isolated from each component of placental tissues, amniotic and chorionic membranes and umbilical cord tissues yield the highest concentration.
The majority of regenerative studies involving the placenta used AM alone or in combination with other placental tissues. AM is a metabolically active tissue that consists of an epithelial layer, a basement membrane, and a mesenchymal tissue layer. In addition to being a source of stem cells, AM synthesizes many growth factors, vasoactive peptides, and cytokines, which are capable of tissue regeneration. AM was initially used as a biological scaffold for the treatment of skin burns and wounds. Other intrinsic properties of AM include the provision of a matrix for cellular migration and proliferation, enhanced wound healing with reduced scar formation, antibacterial activity, and lastly, non-immunogenic and immunosuppressive properties. These inherent characteristics have spurred studies on the potential use of AM in sports medicine as a minimally invasive means to treat osteoarthritis and injuries of tendons, ligaments, muscles, fascia, and cartilages.
Continue to: Animal studies reported...
Animal studies reported positive results with the use of AM to treat osteoarthritis, cartilage defects, and tendon and ligament injuries. Few studies involving human participants also revealed favorable results with regard to the use of AM for the treatment of plantar fasciitis and osteoarthritis; however, these studies are industry-sponsored and employed small sample sizes. The unique mixture of a collagen-rich extracellular matrix, bioactive growth factors, and pluripotent stem cells may allow AM to become an effective treatment for MSK injuries. Although initial animal and human studies show promising results, variabilities regarding models (animal and human), pathologies, placental tissues, and methods of preparation, preservation, and delivery used limit the ability for comparison, analysis, and drawing of definitive conclusions. Thus far, no studies have evaluated the use of currently available AM products for the treatment of injuries sustained by soccer players.
Despite the current popularity of AM as regenerative therapy in academic research and potential use in clinical treatment in sports medicine, physicians should remain aware of the limited evidence available. Other barriers to research and use AM as a regenerative therapy include regulatory classifications based on the concept of “minimal manipulation” in biologic therapies. Minimally manipulated placental allografts are less regulated, less costly to study, and more easily commercialized. These products are not required to undergo FDA phase I to III trials prior to premarket approval. In 2000, the FDA position on all AM products falls into 2 categories. The first position states that AM that contains allogenic stem cells mixed with another drug that is micronized and/or cryopreserved is more than “minimally manipulated” and therefore categorized as “biologic” and would be subject to phase I to III trials. Dehydrated and decellularized AM, however, may meet the concept of minimal manipulation and is only approved by the FDA as a wound covering. Thus, any application of AM for the treatment of sports medicine pathology is not currently FDA-approved, considered off-label, not covered by insurance, and subject to out-of-pocket pay.30,31
CONCLUSION
With improvements in technology and portability, US has become an effective imaging modality for point-of-care evaluation, diagnosis, and continuous monitoring of many MSK injuries. Additionally, as a dynamic imaging modality, US allows for increased accuracy and efficacy when combined with minimally invasive procedures, such as diagnostic and therapeutic guided injections and percutaneous tenotomy, in the clinical setting; thereby decreasing the overall healthcare costs. PRP is proven to be a safe treatment for several MSK conditions, such as lateral epicondylitis, patellar tendonitis, and plantar fasciitis. Although PRP has been included in the standard of care in some areas, this technique may be predominantly athlete driven. Conflicting evidence with regard to return-to-play timeframes following PRP treatment for muscular injuries and poor evidence in conditions, such as Achilles tendonitis, have led to inconsistent indications for use, dose, and timing of treatment. Although early evidence of MSC therapy is promising, high-level evidence for MSC therapy is insufficient, despite its increased use among athletes. Thus far, no data are available regarding the outcomes of the use of amniotic products for the treatment of injuries among athletes. Furthermore, the preparation of amniotic products has many regulatory concerns. The authors advocate for continuous high-level research on regenerative medicine therapies to establish clinical efficacy and safety data.
1. Daniels E, Cole D, Jacobs B, Phillips S. Existing Evidence on ultrasound-guided injections in sports medicine. Orthop J Sports Med. 2018;6(2):2325967118756576. doi:10.1177/2325967118756576.
2. Henne M, Centurion A, Rosas S, Youmans H, Osbahr D. Trends in utilization of image-guided hip joint injections. Unpublished. 2018.
3. Finnoff JT, Hall MM, Adams E, et al. American Medical Society for Sports Medicine position statement: Interventional musculoskeletal ultrasound in sports medicine. Clin J Sport Med. 2015;25:6-22. doi:10.1097/JSM.0000000000000175.
4. Agel J, Evans TA, Dick R, Putukian M, Marshal S. Descriptive epidemiology of collegiate men’s soccer injuries: National Collegiate Athletic Association Injury Surveillance System, 1988-1989 through 2002-2003. J Athl Train. 2007;42(2):270-277.
5. Dick R, Putukian M, Agel J, Evans T, Marshall S. Descriptive epidemiology of collegiate women’s soccer injuries: National Collegiate Athletic Association Injury Surveillance System, 1988-1989 through 2002-2003. J Athl Train. 2007;42(2):278-285.
6. Ekstrand J, Hagglund M, Walden M. Epidemiology of muscle injuries in professional football (soccer). Am J Sports Med. 2011;39(6):1226-1232. doi:10.1177/0363546510395879.
7. Klauser A, Tagliafico A, Allen G, et al. Clinical indications for musculoskeletal ultrasound: A Delphi-based consensus paper of the European society of musculoskeletal radiology. Eur Radiol. 2012;22(5):1140-1148. doi:10.1007/s00330-011-2356-3.
8. Henderson R, Walker B, Young K. The accuracy of diagnostic ultrasound imaging for musculoskeletal soft tissue pathology of the extremities: a comprehensive review of the literature. Chiropr Man Therap. 2015;23(1):31. doi:10.1186/s12998-015-0076-5.
9. Housner JA, Jacobson JA, Misko R. Sonographically guided percutaneous needle tenotomy for the treatment of chronic tendinosis. J Ultrasound Med. 2009;28(9):1187-1192. doi:10.7863/jum.2009.28.9.1187.
10. Koh J, Mohan PC, Howe TS, et al. Fasciotomy and surgical tenotomy for recalcitrant lateral elbow tendinopathy: early clinical experience with a novel device for minimally invasive percutaneous microresection. Am J Sports Med. 2013;41(3):636-644. doi:10.1177/0363546512470625.
11. Seng C, Mohan PC, Koh J, et al. Ultrasonic percutaneous tenotomy for recalcitrant lateral elbow tendinopathy: sustainability and sonographic progression at 3 years. Am J Sports Med. 2015;44(2):504-510. doi:10.1177/0363546515612758.
12. Lee J, Harrison J, Boachie-Adjei K, Vargas E, Moley P. Platelet-rich plasma injections with needle tenotomy for gluteus medius tendinopathy: A registry study with prospective follow-up. Orthop J Sports Med. 2016;4(11):2325967116671692. doi:10.1177/2325967116671692.
13. Osborne H, Anderson L, Burt P, Young M, Gerrard D. Australasian College of Sports Physicians-Position statement: the place of mesenchymal stem/stromal cell therapies in sport and exercise medicine. Br J Sports Med. 2016;50:1237-1244. doi:10.1136/bjsports-2015-095711.
14. Anderson J, Little D, Toth A, et al. Stem cell therapies for knee cartilage repair. The current status of preclinical and clinical studies. Am J Sports Med. 2013;42(9)2253-2261. doi:10.1177/0363546513508744.
15. Lee S, Kwon B, Lee Kyoungbun, Son Y, Chung S. Therapeutic mechanisms of human adipose-derived mesenchymal stem cells in a rat tendon injury model. Am J Sports Med. 2017;45(6):1429-1439. doi:10.1177/0363546517689874.
16. McIntyre J, Jones I, Han B, Vangsness C. Intra-articular mesenchymal stem cell therapy for the human joint. A systematic review. Am J Sports Med. 2017;0363546517735844. doi:10.1177/0363546517735844.
17. Pas HIMFL, Moen M, Haisma J, Winters M. No evidence for the use of stem cell therapy for tendon disorders: a systematic review. Br J Sports Med. 2017;51:996-1002. doi:10.1136/bjsports-2016-096794.
18. Foster T, Puskas B, Mandelbaum B, Gerhardt M, Rodeo S. Platelet-rich plasma: from basic science to clinical applications. Am J Sports Med. 2009;37(11):2259-2272. doi:10.1177/0363546509349921.
19. Mishra A, Pavelko T. Treatment of chronic elbow tendinosis with buffered platelet-rich plasma. Am J Sports Med. 2006;34(11):1774-1778. doi:10.1177/0363546506288850.
20. Dines J, Williams P, ElAttrache N, et al. Platelet-rich plasma can be used to successfully treat elbow ulnar collateral ligament insufficiency in high-level throwers. Am J Orthop. 2016;45(4):296-300.
21. Fitzpatrick J, Bulsara M, O’Donnel J, McCrory P, Zheng M. The effectiveness of platelet-rich plasma injections in gluteal tendinopathy. A randomized, double-blind controlled trial comparing a single platelet-rich plasma injection with a single corticosteroid injection. Am J Sports Med. 2018;46(4)933-939. doi:10.1177/0363546517745525.
22. Hamid M, Ali M, Yusof A, George J, Lee L. Platelet-rich plasma injections for the treatment of hamstring injuries: A randomized controlled trial. Am J Sports Med. 2014;42(10):2410-2418. doi:10.1177/0363546514541540.
23. Zanon G, Combi F, Combi A, Perticarini L, Sammarchi L, Benazzo F. Platelet-rich plasma in the treatment of acute hamstring injuries in professional football players. Joints. 2016;4(1):17-23. doi:10.11138/jts/2016.4.1.017.
24. Hamilton B, Tol JL, Almusa E, et al. Platelet-rich plasma does not enhance return to play in hamstring injuries: a randomized controlled trial. Br J Sports Med. 2015;49:943-950. doi:10.1136/bjsports-2015-094603.
25. Pas HIMFL, Reurink G, Tol JL, Wier A, Winters M, Moen M. Efficacy of rehabilitation (lengthening) exercises, platelet-rich plasma injections, and other conservative interventions in acute hamstring injuries: an updated systematic review and meta-analysis. Br J Sports Med. 2015;49:1197-1205. doi:10.1136/bjsports-2015-094879.
26. Reurink G, Goudswaard G, Moen M, et al. Platelet-rich plasma injections in acute muscle injury. N Engl J Med. 2014;370:2546-2547. doi:10.1056/NEJMc1402340.
27. Kantrowitz D, Padaki A, Ahmad C, Lynch T. Defining platelet-rich plasma usage by team physicians in elite athletes. Orthop J Sports Med. 2018;6(4):2325967118767077. doi:10.1177/2325967118767077.
28. Mithoefer K, Peterson L, Zenobi-Wong M, Mandelbaum B. Cartilage issues in football-today’s problems and tomorrow’s solutions. Br J Sports Med. 2015;49(9):590-596. doi:1136/bjsports-2015-094772.
29. Matthews K, Cuchiara M. Regional regulatory insights: U.S. National Football League Athletes seeking unproven stem cell treatments. Stem Cells Dev. 2014;23(S1):60-64. doi:10.1089/scd.2014.0358.
30. McIntyre J, Jones I, Danilkovich A, Vangsness T. The placenta: applications in orthopaedic sports medicine. Am J Sports Med. 2018;46(1):234-247. doi:10.1177/0363546517697682.
31. Riboh J, Saltzman B, Yankee A, Cole BJ. Human amniotic membrane-derived products in sports medicine: Basic science, early results, and potential clinical applications. Am J Sports Med. 2015;44(9)2425-2434. doi:10.1177/0363546515612750.
1. Daniels E, Cole D, Jacobs B, Phillips S. Existing Evidence on ultrasound-guided injections in sports medicine. Orthop J Sports Med. 2018;6(2):2325967118756576. doi:10.1177/2325967118756576.
2. Henne M, Centurion A, Rosas S, Youmans H, Osbahr D. Trends in utilization of image-guided hip joint injections. Unpublished. 2018.
3. Finnoff JT, Hall MM, Adams E, et al. American Medical Society for Sports Medicine position statement: Interventional musculoskeletal ultrasound in sports medicine. Clin J Sport Med. 2015;25:6-22. doi:10.1097/JSM.0000000000000175.
4. Agel J, Evans TA, Dick R, Putukian M, Marshal S. Descriptive epidemiology of collegiate men’s soccer injuries: National Collegiate Athletic Association Injury Surveillance System, 1988-1989 through 2002-2003. J Athl Train. 2007;42(2):270-277.
5. Dick R, Putukian M, Agel J, Evans T, Marshall S. Descriptive epidemiology of collegiate women’s soccer injuries: National Collegiate Athletic Association Injury Surveillance System, 1988-1989 through 2002-2003. J Athl Train. 2007;42(2):278-285.
6. Ekstrand J, Hagglund M, Walden M. Epidemiology of muscle injuries in professional football (soccer). Am J Sports Med. 2011;39(6):1226-1232. doi:10.1177/0363546510395879.
7. Klauser A, Tagliafico A, Allen G, et al. Clinical indications for musculoskeletal ultrasound: A Delphi-based consensus paper of the European society of musculoskeletal radiology. Eur Radiol. 2012;22(5):1140-1148. doi:10.1007/s00330-011-2356-3.
8. Henderson R, Walker B, Young K. The accuracy of diagnostic ultrasound imaging for musculoskeletal soft tissue pathology of the extremities: a comprehensive review of the literature. Chiropr Man Therap. 2015;23(1):31. doi:10.1186/s12998-015-0076-5.
9. Housner JA, Jacobson JA, Misko R. Sonographically guided percutaneous needle tenotomy for the treatment of chronic tendinosis. J Ultrasound Med. 2009;28(9):1187-1192. doi:10.7863/jum.2009.28.9.1187.
10. Koh J, Mohan PC, Howe TS, et al. Fasciotomy and surgical tenotomy for recalcitrant lateral elbow tendinopathy: early clinical experience with a novel device for minimally invasive percutaneous microresection. Am J Sports Med. 2013;41(3):636-644. doi:10.1177/0363546512470625.
11. Seng C, Mohan PC, Koh J, et al. Ultrasonic percutaneous tenotomy for recalcitrant lateral elbow tendinopathy: sustainability and sonographic progression at 3 years. Am J Sports Med. 2015;44(2):504-510. doi:10.1177/0363546515612758.
12. Lee J, Harrison J, Boachie-Adjei K, Vargas E, Moley P. Platelet-rich plasma injections with needle tenotomy for gluteus medius tendinopathy: A registry study with prospective follow-up. Orthop J Sports Med. 2016;4(11):2325967116671692. doi:10.1177/2325967116671692.
13. Osborne H, Anderson L, Burt P, Young M, Gerrard D. Australasian College of Sports Physicians-Position statement: the place of mesenchymal stem/stromal cell therapies in sport and exercise medicine. Br J Sports Med. 2016;50:1237-1244. doi:10.1136/bjsports-2015-095711.
14. Anderson J, Little D, Toth A, et al. Stem cell therapies for knee cartilage repair. The current status of preclinical and clinical studies. Am J Sports Med. 2013;42(9)2253-2261. doi:10.1177/0363546513508744.
15. Lee S, Kwon B, Lee Kyoungbun, Son Y, Chung S. Therapeutic mechanisms of human adipose-derived mesenchymal stem cells in a rat tendon injury model. Am J Sports Med. 2017;45(6):1429-1439. doi:10.1177/0363546517689874.
16. McIntyre J, Jones I, Han B, Vangsness C. Intra-articular mesenchymal stem cell therapy for the human joint. A systematic review. Am J Sports Med. 2017;0363546517735844. doi:10.1177/0363546517735844.
17. Pas HIMFL, Moen M, Haisma J, Winters M. No evidence for the use of stem cell therapy for tendon disorders: a systematic review. Br J Sports Med. 2017;51:996-1002. doi:10.1136/bjsports-2016-096794.
18. Foster T, Puskas B, Mandelbaum B, Gerhardt M, Rodeo S. Platelet-rich plasma: from basic science to clinical applications. Am J Sports Med. 2009;37(11):2259-2272. doi:10.1177/0363546509349921.
19. Mishra A, Pavelko T. Treatment of chronic elbow tendinosis with buffered platelet-rich plasma. Am J Sports Med. 2006;34(11):1774-1778. doi:10.1177/0363546506288850.
20. Dines J, Williams P, ElAttrache N, et al. Platelet-rich plasma can be used to successfully treat elbow ulnar collateral ligament insufficiency in high-level throwers. Am J Orthop. 2016;45(4):296-300.
21. Fitzpatrick J, Bulsara M, O’Donnel J, McCrory P, Zheng M. The effectiveness of platelet-rich plasma injections in gluteal tendinopathy. A randomized, double-blind controlled trial comparing a single platelet-rich plasma injection with a single corticosteroid injection. Am J Sports Med. 2018;46(4)933-939. doi:10.1177/0363546517745525.
22. Hamid M, Ali M, Yusof A, George J, Lee L. Platelet-rich plasma injections for the treatment of hamstring injuries: A randomized controlled trial. Am J Sports Med. 2014;42(10):2410-2418. doi:10.1177/0363546514541540.
23. Zanon G, Combi F, Combi A, Perticarini L, Sammarchi L, Benazzo F. Platelet-rich plasma in the treatment of acute hamstring injuries in professional football players. Joints. 2016;4(1):17-23. doi:10.11138/jts/2016.4.1.017.
24. Hamilton B, Tol JL, Almusa E, et al. Platelet-rich plasma does not enhance return to play in hamstring injuries: a randomized controlled trial. Br J Sports Med. 2015;49:943-950. doi:10.1136/bjsports-2015-094603.
25. Pas HIMFL, Reurink G, Tol JL, Wier A, Winters M, Moen M. Efficacy of rehabilitation (lengthening) exercises, platelet-rich plasma injections, and other conservative interventions in acute hamstring injuries: an updated systematic review and meta-analysis. Br J Sports Med. 2015;49:1197-1205. doi:10.1136/bjsports-2015-094879.
26. Reurink G, Goudswaard G, Moen M, et al. Platelet-rich plasma injections in acute muscle injury. N Engl J Med. 2014;370:2546-2547. doi:10.1056/NEJMc1402340.
27. Kantrowitz D, Padaki A, Ahmad C, Lynch T. Defining platelet-rich plasma usage by team physicians in elite athletes. Orthop J Sports Med. 2018;6(4):2325967118767077. doi:10.1177/2325967118767077.
28. Mithoefer K, Peterson L, Zenobi-Wong M, Mandelbaum B. Cartilage issues in football-today’s problems and tomorrow’s solutions. Br J Sports Med. 2015;49(9):590-596. doi:1136/bjsports-2015-094772.
29. Matthews K, Cuchiara M. Regional regulatory insights: U.S. National Football League Athletes seeking unproven stem cell treatments. Stem Cells Dev. 2014;23(S1):60-64. doi:10.1089/scd.2014.0358.
30. McIntyre J, Jones I, Danilkovich A, Vangsness T. The placenta: applications in orthopaedic sports medicine. Am J Sports Med. 2018;46(1):234-247. doi:10.1177/0363546517697682.
31. Riboh J, Saltzman B, Yankee A, Cole BJ. Human amniotic membrane-derived products in sports medicine: Basic science, early results, and potential clinical applications. Am J Sports Med. 2015;44(9)2425-2434. doi:10.1177/0363546515612750.
TAKE-HOME POINTS
- Improvements in ultrasound technology have increased its use as a therapeutic and diagnostic modality.
- Ultrasound offers increased accuracy and efficacy with minimally invasive procedures.
- PRP is a safe and effective treatment for many musculoskeletal injuries, however return-to-play time frames limit its efficacy.
- While stem cell and amniotic products offer promising results, the paucity in data limits overall use.
- Care should be taken when discussing regenerative therapy as many products eclipse the concept of “minimal manipulation” and therefore require USFDA trials to establish safety data.
Ultrasound can’t rule out pulmonary embolism in the ED
SAN DIEGO – In the ICU, ultrasound has been shown to reduce the need for CT to evaluate potential pulmonary embolism. But in the ED, this strategy hasn’t worked out so far, according to Joseph Brown, MD, of the department of emergency medicine at the University of California, San Francisco.

Based on the data so far, the ED patients were less likely than the ICU patients to have another etiology identified on ultrasound that explained their symptoms. Further, ultrasound alone missed small subsegmental pulmonary emboli that were detected on subsequent CT scans in 2 of 11 patients.
The study is continuing, and Dr. Brown explains in this interview how ultrasound might be combined with other risk stratification measures to safely achieve reductions in CT scans.
SAN DIEGO – In the ICU, ultrasound has been shown to reduce the need for CT to evaluate potential pulmonary embolism. But in the ED, this strategy hasn’t worked out so far, according to Joseph Brown, MD, of the department of emergency medicine at the University of California, San Francisco.

Based on the data so far, the ED patients were less likely than the ICU patients to have another etiology identified on ultrasound that explained their symptoms. Further, ultrasound alone missed small subsegmental pulmonary emboli that were detected on subsequent CT scans in 2 of 11 patients.
The study is continuing, and Dr. Brown explains in this interview how ultrasound might be combined with other risk stratification measures to safely achieve reductions in CT scans.
SAN DIEGO – In the ICU, ultrasound has been shown to reduce the need for CT to evaluate potential pulmonary embolism. But in the ED, this strategy hasn’t worked out so far, according to Joseph Brown, MD, of the department of emergency medicine at the University of California, San Francisco.

Based on the data so far, the ED patients were less likely than the ICU patients to have another etiology identified on ultrasound that explained their symptoms. Further, ultrasound alone missed small subsegmental pulmonary emboli that were detected on subsequent CT scans in 2 of 11 patients.
The study is continuing, and Dr. Brown explains in this interview how ultrasound might be combined with other risk stratification measures to safely achieve reductions in CT scans.
REPORTING FROM ACEP18
ULTIMATE: IVUS-guided stent placement bests angiography
SAN DIEGO – Clinical outcomes were better when stent placement was guided by intravascular ultrasound rather than angiography, based on the results of a trial conducted in China. Unlike previous comparative studies, which focused on patients with more complex lesions, this trial included all patients undergoing drug-eluting stent (DES) placement.
In the ULTIMATE trial, 1,448 all-comer patients receiving DES were randomized to either intravascular ultrasound (IVUS)–guided or angiography-guided implantation, reported Junjie Zhang, PhD, of Nanjing Medical University in China, and colleagues. The study excluded patients who had a life expectancy shorter than 12 months, who were intolerant of dual antiplatelet therapy, and who had severe calcification needing rotational atherectomy.
At 30 days after stent placement, the incidence of target vessel failure was 0.8% in the IVUS group and 1.9% in the angiography group, a nonsignificant trend. However, outcomes were significantly different at 1 year, with target vessel failure occurring in 2.9% of IVUS patients and 5.4% of angiography patients (hazard ratio, 0.530; 95% confidence interval, 0.312-0.901; P = .019).
The work was simultaneously published in the Journal of the American College of Cardiology (2018 Sept. doi: 10.1016/j.jacc.2018.09.013) .
Despite good results in this and prior studies, uptake of the IVUS procedure is not high in the United States or Europe, according to members of a panel that reviewed the results at the Transcatheter Cardiovascular Therapeutics annual meeting.
“How can people continue to ignore the importance of imaging-guided stent optimization? Even with second-generation DES, the results are consistent across the studies. This is just another piece of irrefutable evidence,” said Gary S. Mintz, MD, chief medical officer at the Cardiovascular Research Foundation, and a discussant at the meeting, sponsored by the Cardiovascular Research Foundation.
That sentiment was generally echoed by the rest of the panel. John M. Hodson, MD, a professor of medicine at MetroHealth Medical Center in Cleveland, pointed out that the study included a variety of cases, and angiography was performed to a high standard in that arm of the study. “It shows that, even with a good angiographic approach, IVUS still wins. I’m amazed that there’s still some resistance to [IVUS] image guidance,” said Dr. Hodgson.
The ULTIMATE study also found that the procedural time was longer (60.88 minutes vs. 45.49 minutes; P less than .001), and the contrast volume was higher (178.29 mL vs. 161.96 mL; P less than .001) in the IVUS than in the angiography group.
A postprocedure IVUS assessment was performed to determine whether the stent was optimally deployed. The criteria for optimal deployment included minimal lumen area in the stented segment of at least 5 mm2, or 90% of the minimal lumen area at distal reference segment meeting that criteria; a less than 50% plaque burden at the 5 mm of vessel proximal or distal to the stent edge; and no edge dissection involving media greater than 3 mm in length.
In the IVUS group, 53% of patients had optimal placement. The rate of target vessel failure was 1.6% of patients with optimal placement and 4.4% of patients who failed to achieve all optimal criteria (HR, 0.349; 95% CI, 0.135-0.898; P = 0.029). Compared with angiography guidance, IVUS guidance was of similar benefit for patients with either American College of Cardiology/American Heart Association–defined B2/C lesions or A/B1 lesions in terms of the composite endpoint. The significant reduction of clinically driven target lesion revascularization or definite stent thrombosis (HR, 0.407; 95% CI: 0.188-0.880; P = 0.018) based on lesion-level analysis by IVUS guidance was not achieved when patient-level analysis was performed.
“I’m particularly impressed by the analysis of the optimal versus nonoptimal group. If you don’t use IVUS correctly, you don’t get a benefit. The ones [in the IVUS group] who did not get optimal stenting were very similar to the angiographic group,” said Dr. Mintz.
The study was funded by the National Science Foundation of China, Six Talent Peaks Project, Nanjing Health and Family Planning Commission, Nanjing Health Youth Talent Training Project, and the Nanjing Municipal Commission of Science & Technology. None of the study authors had relevant financial disclosures. Dr. Mintz reported received research support from Abbott Vascular and Boston Scientific. He has been a consultant for Boston Scientific, Volcano, and Infraredx. Dr. Hodgson reported received research support and consulted for Volcano.
SAN DIEGO – Clinical outcomes were better when stent placement was guided by intravascular ultrasound rather than angiography, based on the results of a trial conducted in China. Unlike previous comparative studies, which focused on patients with more complex lesions, this trial included all patients undergoing drug-eluting stent (DES) placement.
In the ULTIMATE trial, 1,448 all-comer patients receiving DES were randomized to either intravascular ultrasound (IVUS)–guided or angiography-guided implantation, reported Junjie Zhang, PhD, of Nanjing Medical University in China, and colleagues. The study excluded patients who had a life expectancy shorter than 12 months, who were intolerant of dual antiplatelet therapy, and who had severe calcification needing rotational atherectomy.
At 30 days after stent placement, the incidence of target vessel failure was 0.8% in the IVUS group and 1.9% in the angiography group, a nonsignificant trend. However, outcomes were significantly different at 1 year, with target vessel failure occurring in 2.9% of IVUS patients and 5.4% of angiography patients (hazard ratio, 0.530; 95% confidence interval, 0.312-0.901; P = .019).
The work was simultaneously published in the Journal of the American College of Cardiology (2018 Sept. doi: 10.1016/j.jacc.2018.09.013) .
Despite good results in this and prior studies, uptake of the IVUS procedure is not high in the United States or Europe, according to members of a panel that reviewed the results at the Transcatheter Cardiovascular Therapeutics annual meeting.
“How can people continue to ignore the importance of imaging-guided stent optimization? Even with second-generation DES, the results are consistent across the studies. This is just another piece of irrefutable evidence,” said Gary S. Mintz, MD, chief medical officer at the Cardiovascular Research Foundation, and a discussant at the meeting, sponsored by the Cardiovascular Research Foundation.
That sentiment was generally echoed by the rest of the panel. John M. Hodson, MD, a professor of medicine at MetroHealth Medical Center in Cleveland, pointed out that the study included a variety of cases, and angiography was performed to a high standard in that arm of the study. “It shows that, even with a good angiographic approach, IVUS still wins. I’m amazed that there’s still some resistance to [IVUS] image guidance,” said Dr. Hodgson.
The ULTIMATE study also found that the procedural time was longer (60.88 minutes vs. 45.49 minutes; P less than .001), and the contrast volume was higher (178.29 mL vs. 161.96 mL; P less than .001) in the IVUS than in the angiography group.
A postprocedure IVUS assessment was performed to determine whether the stent was optimally deployed. The criteria for optimal deployment included minimal lumen area in the stented segment of at least 5 mm2, or 90% of the minimal lumen area at distal reference segment meeting that criteria; a less than 50% plaque burden at the 5 mm of vessel proximal or distal to the stent edge; and no edge dissection involving media greater than 3 mm in length.
In the IVUS group, 53% of patients had optimal placement. The rate of target vessel failure was 1.6% of patients with optimal placement and 4.4% of patients who failed to achieve all optimal criteria (HR, 0.349; 95% CI, 0.135-0.898; P = 0.029). Compared with angiography guidance, IVUS guidance was of similar benefit for patients with either American College of Cardiology/American Heart Association–defined B2/C lesions or A/B1 lesions in terms of the composite endpoint. The significant reduction of clinically driven target lesion revascularization or definite stent thrombosis (HR, 0.407; 95% CI: 0.188-0.880; P = 0.018) based on lesion-level analysis by IVUS guidance was not achieved when patient-level analysis was performed.
“I’m particularly impressed by the analysis of the optimal versus nonoptimal group. If you don’t use IVUS correctly, you don’t get a benefit. The ones [in the IVUS group] who did not get optimal stenting were very similar to the angiographic group,” said Dr. Mintz.
The study was funded by the National Science Foundation of China, Six Talent Peaks Project, Nanjing Health and Family Planning Commission, Nanjing Health Youth Talent Training Project, and the Nanjing Municipal Commission of Science & Technology. None of the study authors had relevant financial disclosures. Dr. Mintz reported received research support from Abbott Vascular and Boston Scientific. He has been a consultant for Boston Scientific, Volcano, and Infraredx. Dr. Hodgson reported received research support and consulted for Volcano.
SAN DIEGO – Clinical outcomes were better when stent placement was guided by intravascular ultrasound rather than angiography, based on the results of a trial conducted in China. Unlike previous comparative studies, which focused on patients with more complex lesions, this trial included all patients undergoing drug-eluting stent (DES) placement.
In the ULTIMATE trial, 1,448 all-comer patients receiving DES were randomized to either intravascular ultrasound (IVUS)–guided or angiography-guided implantation, reported Junjie Zhang, PhD, of Nanjing Medical University in China, and colleagues. The study excluded patients who had a life expectancy shorter than 12 months, who were intolerant of dual antiplatelet therapy, and who had severe calcification needing rotational atherectomy.
At 30 days after stent placement, the incidence of target vessel failure was 0.8% in the IVUS group and 1.9% in the angiography group, a nonsignificant trend. However, outcomes were significantly different at 1 year, with target vessel failure occurring in 2.9% of IVUS patients and 5.4% of angiography patients (hazard ratio, 0.530; 95% confidence interval, 0.312-0.901; P = .019).
The work was simultaneously published in the Journal of the American College of Cardiology (2018 Sept. doi: 10.1016/j.jacc.2018.09.013) .
Despite good results in this and prior studies, uptake of the IVUS procedure is not high in the United States or Europe, according to members of a panel that reviewed the results at the Transcatheter Cardiovascular Therapeutics annual meeting.
“How can people continue to ignore the importance of imaging-guided stent optimization? Even with second-generation DES, the results are consistent across the studies. This is just another piece of irrefutable evidence,” said Gary S. Mintz, MD, chief medical officer at the Cardiovascular Research Foundation, and a discussant at the meeting, sponsored by the Cardiovascular Research Foundation.
That sentiment was generally echoed by the rest of the panel. John M. Hodson, MD, a professor of medicine at MetroHealth Medical Center in Cleveland, pointed out that the study included a variety of cases, and angiography was performed to a high standard in that arm of the study. “It shows that, even with a good angiographic approach, IVUS still wins. I’m amazed that there’s still some resistance to [IVUS] image guidance,” said Dr. Hodgson.
The ULTIMATE study also found that the procedural time was longer (60.88 minutes vs. 45.49 minutes; P less than .001), and the contrast volume was higher (178.29 mL vs. 161.96 mL; P less than .001) in the IVUS than in the angiography group.
A postprocedure IVUS assessment was performed to determine whether the stent was optimally deployed. The criteria for optimal deployment included minimal lumen area in the stented segment of at least 5 mm2, or 90% of the minimal lumen area at distal reference segment meeting that criteria; a less than 50% plaque burden at the 5 mm of vessel proximal or distal to the stent edge; and no edge dissection involving media greater than 3 mm in length.
In the IVUS group, 53% of patients had optimal placement. The rate of target vessel failure was 1.6% of patients with optimal placement and 4.4% of patients who failed to achieve all optimal criteria (HR, 0.349; 95% CI, 0.135-0.898; P = 0.029). Compared with angiography guidance, IVUS guidance was of similar benefit for patients with either American College of Cardiology/American Heart Association–defined B2/C lesions or A/B1 lesions in terms of the composite endpoint. The significant reduction of clinically driven target lesion revascularization or definite stent thrombosis (HR, 0.407; 95% CI: 0.188-0.880; P = 0.018) based on lesion-level analysis by IVUS guidance was not achieved when patient-level analysis was performed.
“I’m particularly impressed by the analysis of the optimal versus nonoptimal group. If you don’t use IVUS correctly, you don’t get a benefit. The ones [in the IVUS group] who did not get optimal stenting were very similar to the angiographic group,” said Dr. Mintz.
The study was funded by the National Science Foundation of China, Six Talent Peaks Project, Nanjing Health and Family Planning Commission, Nanjing Health Youth Talent Training Project, and the Nanjing Municipal Commission of Science & Technology. None of the study authors had relevant financial disclosures. Dr. Mintz reported received research support from Abbott Vascular and Boston Scientific. He has been a consultant for Boston Scientific, Volcano, and Infraredx. Dr. Hodgson reported received research support and consulted for Volcano.
REPORTING FROM TCT 2018
Key clinical point: Intravascular ultrasound–guided placement of drug-eluting stents resulted in a lower target vessel failure rate than did angiography guidance.
Major finding: The 1-year target vessel failure rate was 2.9% in the intravascular ultrasound–guided group and 5.4% in the angiography group.
Study details: A randomized, controlled trial of 1,448 all-comer patients.
Disclosures: The study was funded by the National Science Foundation of China, Six Talent Peaks Project, Nanjing Health and Family Planning Commission, Nanjing Health Youth Talent Training Project, and the Nanjing Municipal Commission of Science & Technology. None of the study authors had relevant financial disclosures. Dr. Mintz reported receiving research support from Abbott Vascular and Boston Scientific. He has been a consultant for Boston Scientific, Volcano, and Infraredx. Dr. Hodgson reported receiving research support and consulted for Volcano.
CorMicA: Nonobstructive angina should trigger functional testing
SAN DIEGO – Don’t be satisfied with a diagnosis of angina with no obstructive coronary artery disease; push for acetylcholine testing, the findings from a trial in Scotland suggest.
Patients will have less angina and a better quality of life at 6 months, according to investigators from the University of Glasgow (Scotland).
Invasive angiography usually ends on both sides of the Atlantic when no occlusions are found. There are concerns about the safety of going further with acetylcholine challenges, and until now, there had been no grade A evidence from a randomized trial that it improves outcomes. The Glasgow team filled the evidence gap with their presentation of the Coronary Microvascular Angina (CorMicA) trial at the Transcatheter Cardiovascular Therapeutics (TCT) annual meeting, and there wasn’t a single serious adverse event (J Am Coll Cardiol. 2018 Sep 25. doi: 10.1016/j.jacc.2018.09.006).
“This was a proof-of-concept study, which we believe [should] substantiate a large, multicenter trial,” said senior investigator Colin Berry, PhD, a professor of cardiology and imaging at the university.
Acetylcholine was infused down the pressure wire in 151 subjects diagnosed with angina with no obstructive coronary artery diseases before they left the catheter lab. Of these patients, 76 were randomized to have their results shared with their treating cardiologist, and 75 were randomized to not have their results shared. Coronary functional testing is hardly ever done, so the no-share group was considered the standard-of-care control arm. The idea was to see whether it made a difference when treating physicians knew what was causing chest pain when their patients didn’t have occlusive disease.
It turned out to make a huge difference. The diagnosis of “chest pain of noncardiac origin” almost fell off the map. Once cardiologists knew what was going on, they switched up treatment according to European Society of Cardiology guidelines for functional heart pain. Patients with microvascular angina were given beta-blockers and switched off nitrates because these drugs make angina worse in microvascular disease. Subjects with vasospasms were shifted to calcium channel blockers and long-acting nitrates and away from beta-blockers because beta-blockers make vasospasms worse.
Cardiologists who didn’t know the results kept muddling along with what patients came in on at baseline – beta-blockers in two-thirds, long-acting nitrates in half, and calcium channel blockers in a third.
Subjects who got the right treatment because of acetylcholine testing outpaced the standard care group by almost 12 points on the Seattle Angina Questionnaire at 6 months; they could walk farther and didn’t have crushing angina almost every day (P = .001). They reported a statistically significant improvement in quality of life, and they were much happier with their doctors.
“This is the first randomized, sham-controlled trial in this space”; functional testing “was routinely safe and feasible. Therapy guided by the results of physiologic testing improved outcomes” and “treatment satisfaction,” said University of Glasgow interventional cardiologist Tom Ford, MD.
Acetylcholine was infused down the pressure wire into the radial artery, with the left anterior descending coronary artery as the target vessel. A final bolus of less than 100 mcg checked for coronary artery spasms; a symptomatic constriction of greater than 90% was considered positive. Glyceryl trinitrate was used to reverse the effects.
Three-quarters of the subjects were women, which Dr. Ford noted is unusual in an angina study. The mean age was 61 years, and subjects had about a 20% chance of a heart attack within 10 years. The whole procedure, including the angiogram, randomization, and functional testing, took a median of about 60 minutes.
There were no differences in major adverse cardiac events at 6 months, at 2.6% in both groups.
One patient developed persistent atrial fibrillation with acetylcholine testing that was converted to sinus rhythm with intravenous amiodarone, without a night in the hospital.
The work was funded by the British Heart Foundation. No companies were involved. The investigators didn’t have any relevant disclosures. The TCT meeting is sponsored by the Cardiovascular Research Foundation.
[email protected]
SOURCE: Ford TG et al. TCT 2018, Late-Breaking Trial.
SAN DIEGO – Don’t be satisfied with a diagnosis of angina with no obstructive coronary artery disease; push for acetylcholine testing, the findings from a trial in Scotland suggest.
Patients will have less angina and a better quality of life at 6 months, according to investigators from the University of Glasgow (Scotland).
Invasive angiography usually ends on both sides of the Atlantic when no occlusions are found. There are concerns about the safety of going further with acetylcholine challenges, and until now, there had been no grade A evidence from a randomized trial that it improves outcomes. The Glasgow team filled the evidence gap with their presentation of the Coronary Microvascular Angina (CorMicA) trial at the Transcatheter Cardiovascular Therapeutics (TCT) annual meeting, and there wasn’t a single serious adverse event (J Am Coll Cardiol. 2018 Sep 25. doi: 10.1016/j.jacc.2018.09.006).
“This was a proof-of-concept study, which we believe [should] substantiate a large, multicenter trial,” said senior investigator Colin Berry, PhD, a professor of cardiology and imaging at the university.
Acetylcholine was infused down the pressure wire in 151 subjects diagnosed with angina with no obstructive coronary artery diseases before they left the catheter lab. Of these patients, 76 were randomized to have their results shared with their treating cardiologist, and 75 were randomized to not have their results shared. Coronary functional testing is hardly ever done, so the no-share group was considered the standard-of-care control arm. The idea was to see whether it made a difference when treating physicians knew what was causing chest pain when their patients didn’t have occlusive disease.
It turned out to make a huge difference. The diagnosis of “chest pain of noncardiac origin” almost fell off the map. Once cardiologists knew what was going on, they switched up treatment according to European Society of Cardiology guidelines for functional heart pain. Patients with microvascular angina were given beta-blockers and switched off nitrates because these drugs make angina worse in microvascular disease. Subjects with vasospasms were shifted to calcium channel blockers and long-acting nitrates and away from beta-blockers because beta-blockers make vasospasms worse.
Cardiologists who didn’t know the results kept muddling along with what patients came in on at baseline – beta-blockers in two-thirds, long-acting nitrates in half, and calcium channel blockers in a third.
Subjects who got the right treatment because of acetylcholine testing outpaced the standard care group by almost 12 points on the Seattle Angina Questionnaire at 6 months; they could walk farther and didn’t have crushing angina almost every day (P = .001). They reported a statistically significant improvement in quality of life, and they were much happier with their doctors.
“This is the first randomized, sham-controlled trial in this space”; functional testing “was routinely safe and feasible. Therapy guided by the results of physiologic testing improved outcomes” and “treatment satisfaction,” said University of Glasgow interventional cardiologist Tom Ford, MD.
Acetylcholine was infused down the pressure wire into the radial artery, with the left anterior descending coronary artery as the target vessel. A final bolus of less than 100 mcg checked for coronary artery spasms; a symptomatic constriction of greater than 90% was considered positive. Glyceryl trinitrate was used to reverse the effects.
Three-quarters of the subjects were women, which Dr. Ford noted is unusual in an angina study. The mean age was 61 years, and subjects had about a 20% chance of a heart attack within 10 years. The whole procedure, including the angiogram, randomization, and functional testing, took a median of about 60 minutes.
There were no differences in major adverse cardiac events at 6 months, at 2.6% in both groups.
One patient developed persistent atrial fibrillation with acetylcholine testing that was converted to sinus rhythm with intravenous amiodarone, without a night in the hospital.
The work was funded by the British Heart Foundation. No companies were involved. The investigators didn’t have any relevant disclosures. The TCT meeting is sponsored by the Cardiovascular Research Foundation.
[email protected]
SOURCE: Ford TG et al. TCT 2018, Late-Breaking Trial.
SAN DIEGO – Don’t be satisfied with a diagnosis of angina with no obstructive coronary artery disease; push for acetylcholine testing, the findings from a trial in Scotland suggest.
Patients will have less angina and a better quality of life at 6 months, according to investigators from the University of Glasgow (Scotland).
Invasive angiography usually ends on both sides of the Atlantic when no occlusions are found. There are concerns about the safety of going further with acetylcholine challenges, and until now, there had been no grade A evidence from a randomized trial that it improves outcomes. The Glasgow team filled the evidence gap with their presentation of the Coronary Microvascular Angina (CorMicA) trial at the Transcatheter Cardiovascular Therapeutics (TCT) annual meeting, and there wasn’t a single serious adverse event (J Am Coll Cardiol. 2018 Sep 25. doi: 10.1016/j.jacc.2018.09.006).
“This was a proof-of-concept study, which we believe [should] substantiate a large, multicenter trial,” said senior investigator Colin Berry, PhD, a professor of cardiology and imaging at the university.
Acetylcholine was infused down the pressure wire in 151 subjects diagnosed with angina with no obstructive coronary artery diseases before they left the catheter lab. Of these patients, 76 were randomized to have their results shared with their treating cardiologist, and 75 were randomized to not have their results shared. Coronary functional testing is hardly ever done, so the no-share group was considered the standard-of-care control arm. The idea was to see whether it made a difference when treating physicians knew what was causing chest pain when their patients didn’t have occlusive disease.
It turned out to make a huge difference. The diagnosis of “chest pain of noncardiac origin” almost fell off the map. Once cardiologists knew what was going on, they switched up treatment according to European Society of Cardiology guidelines for functional heart pain. Patients with microvascular angina were given beta-blockers and switched off nitrates because these drugs make angina worse in microvascular disease. Subjects with vasospasms were shifted to calcium channel blockers and long-acting nitrates and away from beta-blockers because beta-blockers make vasospasms worse.
Cardiologists who didn’t know the results kept muddling along with what patients came in on at baseline – beta-blockers in two-thirds, long-acting nitrates in half, and calcium channel blockers in a third.
Subjects who got the right treatment because of acetylcholine testing outpaced the standard care group by almost 12 points on the Seattle Angina Questionnaire at 6 months; they could walk farther and didn’t have crushing angina almost every day (P = .001). They reported a statistically significant improvement in quality of life, and they were much happier with their doctors.
“This is the first randomized, sham-controlled trial in this space”; functional testing “was routinely safe and feasible. Therapy guided by the results of physiologic testing improved outcomes” and “treatment satisfaction,” said University of Glasgow interventional cardiologist Tom Ford, MD.
Acetylcholine was infused down the pressure wire into the radial artery, with the left anterior descending coronary artery as the target vessel. A final bolus of less than 100 mcg checked for coronary artery spasms; a symptomatic constriction of greater than 90% was considered positive. Glyceryl trinitrate was used to reverse the effects.
Three-quarters of the subjects were women, which Dr. Ford noted is unusual in an angina study. The mean age was 61 years, and subjects had about a 20% chance of a heart attack within 10 years. The whole procedure, including the angiogram, randomization, and functional testing, took a median of about 60 minutes.
There were no differences in major adverse cardiac events at 6 months, at 2.6% in both groups.
One patient developed persistent atrial fibrillation with acetylcholine testing that was converted to sinus rhythm with intravenous amiodarone, without a night in the hospital.
The work was funded by the British Heart Foundation. No companies were involved. The investigators didn’t have any relevant disclosures. The TCT meeting is sponsored by the Cardiovascular Research Foundation.
[email protected]
SOURCE: Ford TG et al. TCT 2018, Late-Breaking Trial.
REPORTING FROM TCT 2018
Key clinical point: A diagnosis of angina with no obstructive coronary artery disease is insufficient; acetylcholine testing should be considered.
Major finding: The diagnosis of “chest pain of noncardiac origin” almost fell off the map. At 6 months, patients could walk farther and didn’t have crushing angina almost every day (P = .001). They reported a statistically significant improvement in quality of life, and they were much happier with their doctors.
Study details: Randomized trial with 151 people who had chest pain but no coronary occlusions on angiography.
Disclosures: There was no industry funding, and the investigators had no relevant industry disclosures.
Source: Ford TG et al. TCT 2018, Late-Breaking Trial.
Bicuspid aortic valve: Basics and beyond
Bicuspid aortic valve may initially be asymptomatic, but it is associated with progressive valvular and aortic abnormalities that can lead to chronic heart failure and sudden death. Regular monitoring is required with an eye toward surgery when indicated.
This article reviews inheritance patterns and conditions associated with bicuspid aortic valve. We discuss diagnosis, management, and monitoring, and offer surgical recommendations. Special guidance for dental procedures, pregnancy, and athletes is also provided.
A YOUNG MAN WITH PALPITATIONS AND A MURMUR
A 34-year-old man presented to an outpatient clinic with occasional palpitations over the past several months. He reported that he had been diagnosed with a murmur as a child but had received no further testing.
Physical examination at this time revealed a faint systolic crescendo-decrescendo murmur along the right sternal border without radiation to the carotid arteries or to the apex. Transthoracic echocardiography (TTE) showed a bicuspid aortic valve with fusion of the right and left coronary cusps, with no aortic valve stenosis or insufficiency. There was mild dilation of the aortic root, but the mid-ascending aorta could not be evaluated because of limited acoustic windows.
Is further diagnostic testing needed, and if so, what? May he participate in exertional physical activity? Does his newborn son need evaluation?
ABNORMALITIES OCCUR DURING EMBRYOGENESIS
Bicuspid aortic valve develops because of abnormal valvulogenesis. Adjacent cusps fail to separate from each other, resulting in only 2 cusps, with 1 usually larger than the other. Morphology varies according to which commissures are fused.1
Bicuspid aortic valve is associated with abnormalities in the coronary artery anatomy in about 2% of patients, including anomalous origins of the coronary arteries and upwardly displaced coronary ostia.2 Such features need to be considered before surgical intervention.
Bicuspid aortic valve can be found in 1% to 2% of the general population, with a male-to-female predominance of 3:1.1,3,4 It is one of the most common congenital cardiac malformations and is the leading congenital cause of aortic valve stenosis.1,3 However, routine screening of newborns for the condition is not recommended, and most cases are diagnosed incidentally.
GENETIC FACTORS PROMINENT
Bicuspid aortic valve is thought to be primarily inherited in an autosomal-dominant pattern, but there is evidence of genetic heterogeneity, and the pattern may be variable.5,6
No single gene responsible for bicuspid aortic valve has been identified. The condition may occur as a component of different pleiotropic genetic syndromes such as Loeys-Dietz, DiGeorge, and Marfan syndromes,7,8 as well as in patients with Turner syndrome and Williams syndrome.8–11 It also commonly coexists with other congenital heart diseases, including ventricular septal defect, isolated aortic arch obstruction, and patent ductus arteriosus.9
Studies have found a 15% rate of familial clustering.6,12 In a study of 142 patients with bicuspid aortic valve, 20% of first-degree relatives had some cardiac abnormality found by screening, of whom 68% had bicuspid aortic valve. Of these, 71% were newly detected abnormalities.13
CHARACTERISTIC CLICK AND MURMUR
Physical examination findings of a functionally normal bicuspid aortic valve include a systolic ejection click followed by an early peaking systolic murmur at the apex or left lower sternal border. With progression of aortic stenosis, the ejection murmur has a harsher sound, with later peaking, and the S2 sound diminishes or becomes inaudible.14 If aortic regurgitation is present, a diastolic decrescendo murmur is heard best at the left lower sternal border.
DISEASE PROGRESSION
Although bicuspid aortic valve is typically asymptomatic at first, it is commonly associated with progressive valvulopathy and thoracic aortic disease.1,3,4,15 It can lead to chronic heart failure and increase the risk of acute aortic syndromes and sudden cardiac death.15
Michelena et al16 studied 212 cases of asymptomatic bicuspid aortic valve. Although the survival rate 20 years after diagnosis was the same as for an age-matched cohort in the general population, the frequency of adverse cardiovascular events and surgical interventions was higher.
Aortic stenosis progresses rapidly
Aortic stenosis associated with a bicuspid aortic valve tends to affect younger patients and progress more rapidly than when associated with a tricuspid valve.17
In a study of 542 patients with congenital bicuspid aortic valve undergoing aortic valve replacement,3 75% had isolated aortic stenosis, 10% had aortic stenosis with some degree of aortic insufficiency, and 13% had isolated aortic insufficiency. Given the tendency of aortic stenosis to progress rapidly, early surgery is often pursued.17,18
Aneurysmal disease is common
The thoracic aorta is at increased risk of aneurysmal disease, coarctation, and dissection in patients with a bicuspid aortic valve.1,6,15
Michelena et al16 reported that in patients without an aneurysm at the time of bicuspid aortic valve diagnosis, the 25-year risk of aneurysm formation was approximately 26%. In patients with an aneurysm at the time of diagnosis, the 15-year risk of aortic surgery after the diagnosis of aneurysm was about 46% and the risk of aortic dissection after aneurysm diagnosis was 7%.15 Compared with the general population, the age-adjusted relative risk of aortic aneurysm in patients with bicuspid aortic valve was 86.2, and that of aortic dissection was 8.4. Although the absolute incidence of dissection is low in these patients, it is markedly higher than in the general population, particularly in older patients (age > 50) and those with an aneurysm at the time of diagnosis.15
The risk of infective endocarditis
Patients with bicuspid aortic valve are highly prone to infective endocarditis for reasons that remain poorly understood. The pathogens in most cases are staphylococci or viridans streptococci.19 Patients with infective endocarditis typically require emergency surgery. Complications including valvular abscess, myocardial abscess, and overt heart failure are common.19
Lamas and Eykyn20 studied 408 cases of native valve endocarditis; in 12.3%, the patient had a bicuspid aortic valve. In this subset, all were male, the mean age was 39 at diagnosis, 82% needed surgery, and the death rate was 14%.
Patients with bicuspid aortic valve do not routinely need antibiotics before dental and surgical procedures, but if they have had endocarditis in the past, they need antibiotics to prevent a recurrence.21
REGULAR MONITORING NEEDED
Because complications may be life-threatening, early detection of progressive disease by regular screening is critical. Echocardiographic evaluation of valvular function, ventricular dimensions and function, and diameter of the aortic root and ascending aorta should be performed in every patient with bicuspid aortic valve. If initial imaging is normal and there is no aortic dilation, imaging should be repeated every 5 to 10 years. If any abnormality is found, repeat imaging is needed every year.22
Magnetic resonance imaging (MRI) or computed tomographic (CT) angiography may be required to better assess the aorta for patients requiring a surgical intervention, or when aortic dimensions are not clearly visualized on TTE. MRI has 2 advantages over CT angiography: it poses no radiation risk, and it provides more information on left ventricular function and dimensions, in addition to valve assessment.23,24
No published study has compared MRI or CT angiography and transesophageal echocardiography (TEE), but in a study of 174 patients with dilated aortic root, combined TTE and TEE detected aortic valve morphology accurately in 98% of cases. As TEE is more invasive, it is not recommended for regular surveillance (Figures 1 and 2).25
FAMILY SCREENING RECOMMENDED
Close relatives should be evaluated for aortic valve and thoracic aortic disease.12,13,23,26
The American College of Cardiology (ACC) and the American Heart Association (AHA), backed by radiologic and cardiovascular associations, concur in recommending echocardiographic screening and routine screening of the thoracic aorta for aortic root dilation in first-degree relatives (ie, siblings, parents, and children) of patients with bicuspid aortic valve (class I recommendation).22,27,28
A comprehensive physical examination is recommended for family members in addition to TTE, with careful assessment of the aortic valve in short and long axes, and of the aortic root.14 If the aorta cannot be adequately evaluated with TTE, further assessment should be pursued with CT angiography or MRI.
EXERCISE RESTRICTIONS
The 2015 ACC/AHA guidelines for competitive athletes with cardiovascular abnormalities recommend annual screening with TTE or MRI angiography for athletes with bicuspid aortic valve and coexisting dilation of the ascending aorta (aortic diameter 40–42 mm in men and 36–39 mm in women) (class I recommendation, level of evidence C).29
Athletes with a bicuspid aortic valve and a normal aortic root and ascending aorta may participate in all competitive activities.29 However, those with a dilated aorta should avoid strenuous activities because of the increased risk of rupture.30 The ACC/AHA recommendations29 depend on the diameter of the ascending aorta and the nature of the sport:
- For an aortic diameter 40 to 42 mm in men or 36 to 39 mm in women, and no features of connective tissue disease or familial thoracic ascending aortic syndrome, low- and moderate-intensity sports with a low likelihood of significant body contact may be considered; consider avoiding intense weight training (class IIb, level of evidence C)
- For an aortic diameter 43 to 45 mm, low-intensity sports with a low likelihood of body contact may be considered (class IIb, level of evidence C)
- For an aortic diameter greater than 43 mm in men or greater than 40 mm in women, sports involving body collision should be avoided (class III, level of evidence C)
- For an aortic diameter greater than 45 mm, sports activities should be avoided (class III, level of evidence C).
PREGNANCY CONSIDERATIONS
Bicuspid aortic valve is associated with aortic dissection, mainly in the third trimester.31 Patients should ideally undergo echocardiographic screening before conception. The 2010 ACC/AHA guidelines for managing thoracic aortic disease recommend monthly or bimonthly echocardiography until delivery in pregnant women with a dilated thoracic aorta.22
Patients with bicuspid aortic valve and aortic root enlargement of more than 40 mm should have preconception counseling about surgery for aortic root replacement before becoming pregnant. If the diagnosis of enlarged aortic root is made during pregnancy, echocardiographic surveillance at 4- to 6-week intervals is indicated.32
SURGICAL MANAGEMENT
In the past, beta-blockers and angiotensin-converting enzyme inhibitors were recommended to minimize shear stress, with the goal of slowing progression of aortic dilation. However, evidence to support their use is inadequate.33,34
The only definitive treatment is surgery, with various procedures that lower the risk of death or dissection.24,35
The dimensions of the aortic root or ascending aorta should be examined vigilantly, according to the 2014 ACC/AHA guidelines27:
- Repairing the aortic sinuses or replacing the ascending aorta is indicated if the diameter of the aortic sinuses or ascending aorta is greater than 5.5 cm (class I, level of evidence B)
- Repairing the aortic sinuses or replacing the ascending aorta is reasonable if the diameter of the aortic sinuses or ascending aorta is greater than 5.0 cm and the patient has a risk factor for dissection such as a family history of aortic dissection or an increase in diameter of 0.5 cm or greater per year (class IIa, level of evidence C)
- Replacement of the ascending aorta is reasonable if the diameter of the ascending aorta is greater than 4.5 cm and the patient is undergoing aortic valve surgery for severe aortic stenosis or regurgitation.
Valve repair or replacement
Aortic valve repair or replacement is sometimes done separately from aortic root repair.
The value of aortic valve repair is debatable, but a series of 728 patients at Cleveland Clinic showed a very low mortality rate (0.41%) and an annual reoperation rate of 2.6% during up to 15 years of follow-up.36
Aortic valve replacement is usually considered for patients with severe valve dysfunction, abnormal left ventricular dimensions, or symptoms. It is important to determine if the patient is a good surgical candidate and to refer early for surgical evaluation to avoid the higher risk of death associated with emergency surgery.36
Transcatheter aortic valve replacement has been studied in patients deemed to be at too high a risk for surgical replacement. Short- and intermediate-term outcomes have been good in these patients, but long-term data are lacking.37
Surveillance after surgery
The type of operation determines postoperative surveillance.
After isolated aortic valve repair or replacement, patients should continue with surveillance at least annually to monitor for progressive aortopathy, as they remain at increased risk of dissection or rupture after isolated valve surgery, especially if they had aortic insufficiency preoperatively.38
After definitive surgery with replacement or repair of the ascending aorta, no clear recommendations have been established for continued surveillance. However, it is reasonable to image these patients with either MRI or CT angiography 3 to 5 years after their surgery to monitor for anastomotic complications.
CASE QUESTIONS ANSWERED
Our patient should undergo repeat TTE in 1 year. He should also undergo CT angiography of the ascending aorta if it is not seen by TTE. He can participate in low-intensity sports but should avoid intense weight training. His parents, siblings, and children should be screened for bicuspid aortic valve or associated aortopathies.
- Roberts WC. The congenitally bicuspid aortic valve. A study of 85 autopsy cases. Am J Cardiol 1970; 26(1):72–83. pmid:5427836
- Michalowska IM, Hryniewiecki T, Kwiatek P, Stoklosa P, Swoboda-Rydz U, Szymanski P. Coronary artery variants and anomalies in patients with bicuspid aortic valve. J Thorac Imaging 2016; 31(3):156–162. doi:10.1097/RTI.0000000000000205
- Sabet HY, Edwards WD, Tazelaar HD, Daly RC. Congenitally bicuspid aortic valves: a surgical pathology study of 542 cases (1991 through 1996) and a literature review of 2,715 additional cases. Mayo Clin Proc 1999; 74(1):14–26. doi:10.4065/74.1.14
- Tutar E, Ekici F, Atalay S, Nacar N. The prevalence of bicuspid aortic valve in newborns by echocardiographic screening. Am Heart J 2005; 150(3):513–515. doi:10.1016/j.ahj.2004.10.036
- Benson DW. The genetics of congenital heart disease: a point in the revolution. Cardiol Clin 2002; 20(3):385–394. pmid:12371007
- Emanuel R, Withers R, O’Brien K, Ross P, Feizi O. Congenitally bicuspid aortic valves. Clinicogenetic study of 41 families. Br Heart J 1978; 40(12):1402–1407. pmid:737099
- Giusti B, Sticchi E, De Cario R, Magi A, Nistri S, Pepe G. Genetic bases of bicuspid aortic valve: the contribution of traditional and high-throughput sequencing approaches on research and diagnosis. Front Physiol 2017; 8:612. doi:10.3389/fphys.2017.00612
- Sachdev V, Matura LA, Sidenko S, et al. Aortic valve disease in Turner syndrome. J Am Coll Cardiol 2008; 51(19):1904–1909. doi:10.1016/j.jacc.2008.02.035
- Duran AC, Frescura C, Sans-Coma V, Angelini A, Basso C, Thiene G. Bicuspid aortic valves in hearts with other congenital heart disease. J Heart Valve Dis 1995; 4(6):581–590. pmid:8611973
- De Rubens Figueroa J, Rodríguez LM, Hach JL, Del Castillo Ruíz V, Martínez HO. Cardiovascular spectrum in Williams-Beuren syndrome: the Mexican experience in 40 patients. Tex Heart Inst J 2008; 35(3):279–285. pmid:18941598
- Yuan SM, Jing H. The bicuspid aortic valve and related disorders. Sao Paulo Med J 2010; 128(5):296–301. pmid:21181071
- Cripe L, Andelfinger G, Martin LJ, Shooner K, Benson DW. Bicuspid aortic valve is heritable. J Am Coll Cardiol 2004; 44(1):138–143. doi:10.1016/j.jacc.2004.03.050
- Kerstjens-Frederikse WS, Sarvaas GJ, Ruiter JS, et al. Left ventricular outflow tract obstruction: should cardiac screening be offered to first-degree relatives? Heart 2011; 97(15):1228–1232. doi:10.1136/hrt.2010.211433
- Siu SC, Silversides CK. Bicuspid aortic valve disease. J Am Coll Cardiol 2010; 55(25):2789–2800. doi:10.1016/j.jacc.2009.12.068
- Michelena HI, Khanna AD, Mahoney D, et al. Incidence of aortic complications in patients with bicuspid aortic valves. JAMA 2011; 306(10):1104–1112.
- Michelena HI, Desjardins VA, Avierinos JF, et al. Natural history of asymptomatic patients with normally functioning or minimally dysfunctional bicuspid aortic valve in the community. Circulation 2008; 117(21):2776–2784. doi:10.1161/CIRCULATIONAHA.107.740878
- Beppu S, Suzuki S, Matsuda H, Ohmori F, Nagata S, Miyatake K. Rapidity of progression of aortic stenosis in patients with congenital bicuspid aortic valves. Am J Cardiol 1993; 71(4):322–327. pmid:8427176
- Roberts WC, Ko JM. Frequency by decades of unicuspid, bicuspid, and tricuspid aortic valves in adults having isolated aortic valve replacement for aortic stenosis, with or without associated aortic regurgitation. Circulation 2005; 111(7):920–925. doi:10.1161/01.CIR.0000155623.48408.C5
- Yener N, Oktar GL, Erer D, Yardimci MM, Yener A. Bicuspid aortic valve. Ann Thorac Cardiovasc Surg 2002; 8(5):264–267. pmid:12472407
- Lamas CC, Eykyn SJ. Bicuspid aortic valve—a silent danger: analysis of 50 cases of infective endocarditis. Clin Infect Dis 2000; 30(2):336–341. doi:10.1086/313646
- Wilson W, Taubert KA, Gewitz M, et al. Prevention of infective endocarditis: guidelines from the American Heart Association. Circulation 2007; 116(15):1736–1754. doi:10.1161/CIRCULATIONAHA.106.183095
- Hiratzka L, Bakris G, Beckman JA, et al. 2010 ACCF/AHA/AATS/ACR/ASA/SCA/SCAI/SIR/STS/SVM guidelines for the diagnosis and management of patients with thoracic aortic disease. Circulation 2010; 121(13):e266–e369. doi:10.1161/CIR.0b013e3181d4739e
- Chun EJ, Choi SI, Lim C, et al. Aortic stenosis: evaluation with multidetector CT angiography and MR imaging. Korean J Radiol 2008; 9(5):439–448. doi:10.3348/kjr.2008.9.5.439
- Kiefer TL, Wang A, Hughes GC, Bashore TM. Management of patients with bicuspid aortic valve disease. Curr Treat Options Cardiovasc Med 2011; 13(6):489–505. doi:10.1007/s11936-011-0152-7
- Alegret JM, Palazon O, Duran I, Vernis JM. Aortic valve morphology definition with transthoracic combined with transesophageal echocardiography in a population with high prevalence of bicuspid aortic valve. Int J Cardiovasc Imaging 2005; 21(2-3):213–217. doi:10.1007/s10554-004-3901-9
- Biner S, Rafique AM, Ray I, Cuk O, Siegel RJ, Tolstrup K. Aortopathy is prevalent in relatives of bicuspid aortic valve patients. J Am Coll Cardiol 2009; 53(24):2288–2295. doi:10.1016/j.jacc.2009.03.027
- Nishimura RA, Otto CM, Bonow RO, et al. 2014 AHA/ACC guideline for the management of patients with valvular heart disease. J Thorac Cardiovasc Surg 2014; 148(1):e1-e132. doi:10.1016/j.jtcvs.2014.05.014
- Warnes CA, Williams RG, Bashore TM, et al. ACC/AHA 2008 guidelines for the management of adults with congenital heart disease. J Am Coll Cardiol 2008; 52(23):e143–e263. doi:10.1016/j.jacc.2008.10.001
- Braverman AC, Harris KM, Kovacs RJ, Maron BJ. Eligibility and disqualification recommendations for competitive athletes with cardiovascular abnormalities: Task Force 7: aortic diseases, including Marfan syndrome. Circulation 2015; 132(22):e303–e309. doi:10.1161/CIR.0000000000000243
- De Mozzi P, Longo UG, Galanti G, Maffulli N. Bicuspid aortic valve: a literature review and its impact on sport activity. Br Med Bull 2008; 85:63–85. doi:10.1093/bmb/ldn002
- Thorne SA. Pregnancy in heart disease. Heart 2004; 90(4):450–456. pmid:15020530
- Immer FF, Bansi AG, Immer-Bansi AS, et al. Aortic dissection in pregnancy: analysis of risk factors and outcome. Ann Thorac Surg 2003; 76(1):309–314. pmid:12842575
- Allen BD, Markl M, Barker AJ, et al. Influence of beta-blocker therapy on aortic blood flow in patients with bicuspid aortic valve. Int J Cardiovasc Imaging 2016; 32(4):621–628. doi:10.1007/s10554-015-0819-3
- Ohnemus D, Oster ME, Gatlin S, Jokhadar M, Mahle WT. The effect of angiotensin-converting enzyme inhibitors on the rate of ascending aorta dilation in patients with bicuspid aortic valve. Congenit Heart Dis 2015; 10(1):E1–E5. doi:10.1111/chd.12184
- Masri A, Kalahasti V, Alkharabsheh S, et al. Characteristics and long-term outcomes of contemporary patients with bicuspid aortic valves. J Thorac Cardiovasc Surg 2016; 151(6):1650–1659.e1. doi:10.1016/j.jtcvs.2015.12.019
- Svensson LG, Al Kindi AH, Vivacqua A, et al. Long-term durability of bicuspid aortic valve repair. Ann Thorac Surg 2014; 97(5):1539–1548. doi:10.1016/j.athoracsur.2013.11.036
- Mylotte D, Lefevre T, Sondergaard L, et al. Transcatheter aortic valve replacement in bicuspid aortic valve disease. J Am Coll Cardiol 2014; 64(22):2330–2339. doi:10.1016/j.jacc.2014.09.039
- Girdauskas E, Disha K, Raisin HH, Secknus MA, Borger MA, Kuntze T. Risk of late aortic events after an isolated aortic valve replacement for bicuspid aortic valve stenosis with concomitant ascending aortic dilation. Eur J Cardiothorac Surg 2012; 42(5):832–838. doi:10.1093/ejcts/ezs137
Bicuspid aortic valve may initially be asymptomatic, but it is associated with progressive valvular and aortic abnormalities that can lead to chronic heart failure and sudden death. Regular monitoring is required with an eye toward surgery when indicated.
This article reviews inheritance patterns and conditions associated with bicuspid aortic valve. We discuss diagnosis, management, and monitoring, and offer surgical recommendations. Special guidance for dental procedures, pregnancy, and athletes is also provided.
A YOUNG MAN WITH PALPITATIONS AND A MURMUR
A 34-year-old man presented to an outpatient clinic with occasional palpitations over the past several months. He reported that he had been diagnosed with a murmur as a child but had received no further testing.
Physical examination at this time revealed a faint systolic crescendo-decrescendo murmur along the right sternal border without radiation to the carotid arteries or to the apex. Transthoracic echocardiography (TTE) showed a bicuspid aortic valve with fusion of the right and left coronary cusps, with no aortic valve stenosis or insufficiency. There was mild dilation of the aortic root, but the mid-ascending aorta could not be evaluated because of limited acoustic windows.
Is further diagnostic testing needed, and if so, what? May he participate in exertional physical activity? Does his newborn son need evaluation?
ABNORMALITIES OCCUR DURING EMBRYOGENESIS
Bicuspid aortic valve develops because of abnormal valvulogenesis. Adjacent cusps fail to separate from each other, resulting in only 2 cusps, with 1 usually larger than the other. Morphology varies according to which commissures are fused.1
Bicuspid aortic valve is associated with abnormalities in the coronary artery anatomy in about 2% of patients, including anomalous origins of the coronary arteries and upwardly displaced coronary ostia.2 Such features need to be considered before surgical intervention.
Bicuspid aortic valve can be found in 1% to 2% of the general population, with a male-to-female predominance of 3:1.1,3,4 It is one of the most common congenital cardiac malformations and is the leading congenital cause of aortic valve stenosis.1,3 However, routine screening of newborns for the condition is not recommended, and most cases are diagnosed incidentally.
GENETIC FACTORS PROMINENT
Bicuspid aortic valve is thought to be primarily inherited in an autosomal-dominant pattern, but there is evidence of genetic heterogeneity, and the pattern may be variable.5,6
No single gene responsible for bicuspid aortic valve has been identified. The condition may occur as a component of different pleiotropic genetic syndromes such as Loeys-Dietz, DiGeorge, and Marfan syndromes,7,8 as well as in patients with Turner syndrome and Williams syndrome.8–11 It also commonly coexists with other congenital heart diseases, including ventricular septal defect, isolated aortic arch obstruction, and patent ductus arteriosus.9
Studies have found a 15% rate of familial clustering.6,12 In a study of 142 patients with bicuspid aortic valve, 20% of first-degree relatives had some cardiac abnormality found by screening, of whom 68% had bicuspid aortic valve. Of these, 71% were newly detected abnormalities.13
CHARACTERISTIC CLICK AND MURMUR
Physical examination findings of a functionally normal bicuspid aortic valve include a systolic ejection click followed by an early peaking systolic murmur at the apex or left lower sternal border. With progression of aortic stenosis, the ejection murmur has a harsher sound, with later peaking, and the S2 sound diminishes or becomes inaudible.14 If aortic regurgitation is present, a diastolic decrescendo murmur is heard best at the left lower sternal border.
DISEASE PROGRESSION
Although bicuspid aortic valve is typically asymptomatic at first, it is commonly associated with progressive valvulopathy and thoracic aortic disease.1,3,4,15 It can lead to chronic heart failure and increase the risk of acute aortic syndromes and sudden cardiac death.15
Michelena et al16 studied 212 cases of asymptomatic bicuspid aortic valve. Although the survival rate 20 years after diagnosis was the same as for an age-matched cohort in the general population, the frequency of adverse cardiovascular events and surgical interventions was higher.
Aortic stenosis progresses rapidly
Aortic stenosis associated with a bicuspid aortic valve tends to affect younger patients and progress more rapidly than when associated with a tricuspid valve.17
In a study of 542 patients with congenital bicuspid aortic valve undergoing aortic valve replacement,3 75% had isolated aortic stenosis, 10% had aortic stenosis with some degree of aortic insufficiency, and 13% had isolated aortic insufficiency. Given the tendency of aortic stenosis to progress rapidly, early surgery is often pursued.17,18
Aneurysmal disease is common
The thoracic aorta is at increased risk of aneurysmal disease, coarctation, and dissection in patients with a bicuspid aortic valve.1,6,15
Michelena et al16 reported that in patients without an aneurysm at the time of bicuspid aortic valve diagnosis, the 25-year risk of aneurysm formation was approximately 26%. In patients with an aneurysm at the time of diagnosis, the 15-year risk of aortic surgery after the diagnosis of aneurysm was about 46% and the risk of aortic dissection after aneurysm diagnosis was 7%.15 Compared with the general population, the age-adjusted relative risk of aortic aneurysm in patients with bicuspid aortic valve was 86.2, and that of aortic dissection was 8.4. Although the absolute incidence of dissection is low in these patients, it is markedly higher than in the general population, particularly in older patients (age > 50) and those with an aneurysm at the time of diagnosis.15
The risk of infective endocarditis
Patients with bicuspid aortic valve are highly prone to infective endocarditis for reasons that remain poorly understood. The pathogens in most cases are staphylococci or viridans streptococci.19 Patients with infective endocarditis typically require emergency surgery. Complications including valvular abscess, myocardial abscess, and overt heart failure are common.19
Lamas and Eykyn20 studied 408 cases of native valve endocarditis; in 12.3%, the patient had a bicuspid aortic valve. In this subset, all were male, the mean age was 39 at diagnosis, 82% needed surgery, and the death rate was 14%.
Patients with bicuspid aortic valve do not routinely need antibiotics before dental and surgical procedures, but if they have had endocarditis in the past, they need antibiotics to prevent a recurrence.21
REGULAR MONITORING NEEDED
Because complications may be life-threatening, early detection of progressive disease by regular screening is critical. Echocardiographic evaluation of valvular function, ventricular dimensions and function, and diameter of the aortic root and ascending aorta should be performed in every patient with bicuspid aortic valve. If initial imaging is normal and there is no aortic dilation, imaging should be repeated every 5 to 10 years. If any abnormality is found, repeat imaging is needed every year.22
Magnetic resonance imaging (MRI) or computed tomographic (CT) angiography may be required to better assess the aorta for patients requiring a surgical intervention, or when aortic dimensions are not clearly visualized on TTE. MRI has 2 advantages over CT angiography: it poses no radiation risk, and it provides more information on left ventricular function and dimensions, in addition to valve assessment.23,24
No published study has compared MRI or CT angiography and transesophageal echocardiography (TEE), but in a study of 174 patients with dilated aortic root, combined TTE and TEE detected aortic valve morphology accurately in 98% of cases. As TEE is more invasive, it is not recommended for regular surveillance (Figures 1 and 2).25
FAMILY SCREENING RECOMMENDED
Close relatives should be evaluated for aortic valve and thoracic aortic disease.12,13,23,26
The American College of Cardiology (ACC) and the American Heart Association (AHA), backed by radiologic and cardiovascular associations, concur in recommending echocardiographic screening and routine screening of the thoracic aorta for aortic root dilation in first-degree relatives (ie, siblings, parents, and children) of patients with bicuspid aortic valve (class I recommendation).22,27,28
A comprehensive physical examination is recommended for family members in addition to TTE, with careful assessment of the aortic valve in short and long axes, and of the aortic root.14 If the aorta cannot be adequately evaluated with TTE, further assessment should be pursued with CT angiography or MRI.
EXERCISE RESTRICTIONS
The 2015 ACC/AHA guidelines for competitive athletes with cardiovascular abnormalities recommend annual screening with TTE or MRI angiography for athletes with bicuspid aortic valve and coexisting dilation of the ascending aorta (aortic diameter 40–42 mm in men and 36–39 mm in women) (class I recommendation, level of evidence C).29
Athletes with a bicuspid aortic valve and a normal aortic root and ascending aorta may participate in all competitive activities.29 However, those with a dilated aorta should avoid strenuous activities because of the increased risk of rupture.30 The ACC/AHA recommendations29 depend on the diameter of the ascending aorta and the nature of the sport:
- For an aortic diameter 40 to 42 mm in men or 36 to 39 mm in women, and no features of connective tissue disease or familial thoracic ascending aortic syndrome, low- and moderate-intensity sports with a low likelihood of significant body contact may be considered; consider avoiding intense weight training (class IIb, level of evidence C)
- For an aortic diameter 43 to 45 mm, low-intensity sports with a low likelihood of body contact may be considered (class IIb, level of evidence C)
- For an aortic diameter greater than 43 mm in men or greater than 40 mm in women, sports involving body collision should be avoided (class III, level of evidence C)
- For an aortic diameter greater than 45 mm, sports activities should be avoided (class III, level of evidence C).
PREGNANCY CONSIDERATIONS
Bicuspid aortic valve is associated with aortic dissection, mainly in the third trimester.31 Patients should ideally undergo echocardiographic screening before conception. The 2010 ACC/AHA guidelines for managing thoracic aortic disease recommend monthly or bimonthly echocardiography until delivery in pregnant women with a dilated thoracic aorta.22
Patients with bicuspid aortic valve and aortic root enlargement of more than 40 mm should have preconception counseling about surgery for aortic root replacement before becoming pregnant. If the diagnosis of enlarged aortic root is made during pregnancy, echocardiographic surveillance at 4- to 6-week intervals is indicated.32
SURGICAL MANAGEMENT
In the past, beta-blockers and angiotensin-converting enzyme inhibitors were recommended to minimize shear stress, with the goal of slowing progression of aortic dilation. However, evidence to support their use is inadequate.33,34
The only definitive treatment is surgery, with various procedures that lower the risk of death or dissection.24,35
The dimensions of the aortic root or ascending aorta should be examined vigilantly, according to the 2014 ACC/AHA guidelines27:
- Repairing the aortic sinuses or replacing the ascending aorta is indicated if the diameter of the aortic sinuses or ascending aorta is greater than 5.5 cm (class I, level of evidence B)
- Repairing the aortic sinuses or replacing the ascending aorta is reasonable if the diameter of the aortic sinuses or ascending aorta is greater than 5.0 cm and the patient has a risk factor for dissection such as a family history of aortic dissection or an increase in diameter of 0.5 cm or greater per year (class IIa, level of evidence C)
- Replacement of the ascending aorta is reasonable if the diameter of the ascending aorta is greater than 4.5 cm and the patient is undergoing aortic valve surgery for severe aortic stenosis or regurgitation.
Valve repair or replacement
Aortic valve repair or replacement is sometimes done separately from aortic root repair.
The value of aortic valve repair is debatable, but a series of 728 patients at Cleveland Clinic showed a very low mortality rate (0.41%) and an annual reoperation rate of 2.6% during up to 15 years of follow-up.36
Aortic valve replacement is usually considered for patients with severe valve dysfunction, abnormal left ventricular dimensions, or symptoms. It is important to determine if the patient is a good surgical candidate and to refer early for surgical evaluation to avoid the higher risk of death associated with emergency surgery.36
Transcatheter aortic valve replacement has been studied in patients deemed to be at too high a risk for surgical replacement. Short- and intermediate-term outcomes have been good in these patients, but long-term data are lacking.37
Surveillance after surgery
The type of operation determines postoperative surveillance.
After isolated aortic valve repair or replacement, patients should continue with surveillance at least annually to monitor for progressive aortopathy, as they remain at increased risk of dissection or rupture after isolated valve surgery, especially if they had aortic insufficiency preoperatively.38
After definitive surgery with replacement or repair of the ascending aorta, no clear recommendations have been established for continued surveillance. However, it is reasonable to image these patients with either MRI or CT angiography 3 to 5 years after their surgery to monitor for anastomotic complications.
CASE QUESTIONS ANSWERED
Our patient should undergo repeat TTE in 1 year. He should also undergo CT angiography of the ascending aorta if it is not seen by TTE. He can participate in low-intensity sports but should avoid intense weight training. His parents, siblings, and children should be screened for bicuspid aortic valve or associated aortopathies.
Bicuspid aortic valve may initially be asymptomatic, but it is associated with progressive valvular and aortic abnormalities that can lead to chronic heart failure and sudden death. Regular monitoring is required with an eye toward surgery when indicated.
This article reviews inheritance patterns and conditions associated with bicuspid aortic valve. We discuss diagnosis, management, and monitoring, and offer surgical recommendations. Special guidance for dental procedures, pregnancy, and athletes is also provided.
A YOUNG MAN WITH PALPITATIONS AND A MURMUR
A 34-year-old man presented to an outpatient clinic with occasional palpitations over the past several months. He reported that he had been diagnosed with a murmur as a child but had received no further testing.
Physical examination at this time revealed a faint systolic crescendo-decrescendo murmur along the right sternal border without radiation to the carotid arteries or to the apex. Transthoracic echocardiography (TTE) showed a bicuspid aortic valve with fusion of the right and left coronary cusps, with no aortic valve stenosis or insufficiency. There was mild dilation of the aortic root, but the mid-ascending aorta could not be evaluated because of limited acoustic windows.
Is further diagnostic testing needed, and if so, what? May he participate in exertional physical activity? Does his newborn son need evaluation?
ABNORMALITIES OCCUR DURING EMBRYOGENESIS
Bicuspid aortic valve develops because of abnormal valvulogenesis. Adjacent cusps fail to separate from each other, resulting in only 2 cusps, with 1 usually larger than the other. Morphology varies according to which commissures are fused.1
Bicuspid aortic valve is associated with abnormalities in the coronary artery anatomy in about 2% of patients, including anomalous origins of the coronary arteries and upwardly displaced coronary ostia.2 Such features need to be considered before surgical intervention.
Bicuspid aortic valve can be found in 1% to 2% of the general population, with a male-to-female predominance of 3:1.1,3,4 It is one of the most common congenital cardiac malformations and is the leading congenital cause of aortic valve stenosis.1,3 However, routine screening of newborns for the condition is not recommended, and most cases are diagnosed incidentally.
GENETIC FACTORS PROMINENT
Bicuspid aortic valve is thought to be primarily inherited in an autosomal-dominant pattern, but there is evidence of genetic heterogeneity, and the pattern may be variable.5,6
No single gene responsible for bicuspid aortic valve has been identified. The condition may occur as a component of different pleiotropic genetic syndromes such as Loeys-Dietz, DiGeorge, and Marfan syndromes,7,8 as well as in patients with Turner syndrome and Williams syndrome.8–11 It also commonly coexists with other congenital heart diseases, including ventricular septal defect, isolated aortic arch obstruction, and patent ductus arteriosus.9
Studies have found a 15% rate of familial clustering.6,12 In a study of 142 patients with bicuspid aortic valve, 20% of first-degree relatives had some cardiac abnormality found by screening, of whom 68% had bicuspid aortic valve. Of these, 71% were newly detected abnormalities.13
CHARACTERISTIC CLICK AND MURMUR
Physical examination findings of a functionally normal bicuspid aortic valve include a systolic ejection click followed by an early peaking systolic murmur at the apex or left lower sternal border. With progression of aortic stenosis, the ejection murmur has a harsher sound, with later peaking, and the S2 sound diminishes or becomes inaudible.14 If aortic regurgitation is present, a diastolic decrescendo murmur is heard best at the left lower sternal border.
DISEASE PROGRESSION
Although bicuspid aortic valve is typically asymptomatic at first, it is commonly associated with progressive valvulopathy and thoracic aortic disease.1,3,4,15 It can lead to chronic heart failure and increase the risk of acute aortic syndromes and sudden cardiac death.15
Michelena et al16 studied 212 cases of asymptomatic bicuspid aortic valve. Although the survival rate 20 years after diagnosis was the same as for an age-matched cohort in the general population, the frequency of adverse cardiovascular events and surgical interventions was higher.
Aortic stenosis progresses rapidly
Aortic stenosis associated with a bicuspid aortic valve tends to affect younger patients and progress more rapidly than when associated with a tricuspid valve.17
In a study of 542 patients with congenital bicuspid aortic valve undergoing aortic valve replacement,3 75% had isolated aortic stenosis, 10% had aortic stenosis with some degree of aortic insufficiency, and 13% had isolated aortic insufficiency. Given the tendency of aortic stenosis to progress rapidly, early surgery is often pursued.17,18
Aneurysmal disease is common
The thoracic aorta is at increased risk of aneurysmal disease, coarctation, and dissection in patients with a bicuspid aortic valve.1,6,15
Michelena et al16 reported that in patients without an aneurysm at the time of bicuspid aortic valve diagnosis, the 25-year risk of aneurysm formation was approximately 26%. In patients with an aneurysm at the time of diagnosis, the 15-year risk of aortic surgery after the diagnosis of aneurysm was about 46% and the risk of aortic dissection after aneurysm diagnosis was 7%.15 Compared with the general population, the age-adjusted relative risk of aortic aneurysm in patients with bicuspid aortic valve was 86.2, and that of aortic dissection was 8.4. Although the absolute incidence of dissection is low in these patients, it is markedly higher than in the general population, particularly in older patients (age > 50) and those with an aneurysm at the time of diagnosis.15
The risk of infective endocarditis
Patients with bicuspid aortic valve are highly prone to infective endocarditis for reasons that remain poorly understood. The pathogens in most cases are staphylococci or viridans streptococci.19 Patients with infective endocarditis typically require emergency surgery. Complications including valvular abscess, myocardial abscess, and overt heart failure are common.19
Lamas and Eykyn20 studied 408 cases of native valve endocarditis; in 12.3%, the patient had a bicuspid aortic valve. In this subset, all were male, the mean age was 39 at diagnosis, 82% needed surgery, and the death rate was 14%.
Patients with bicuspid aortic valve do not routinely need antibiotics before dental and surgical procedures, but if they have had endocarditis in the past, they need antibiotics to prevent a recurrence.21
REGULAR MONITORING NEEDED
Because complications may be life-threatening, early detection of progressive disease by regular screening is critical. Echocardiographic evaluation of valvular function, ventricular dimensions and function, and diameter of the aortic root and ascending aorta should be performed in every patient with bicuspid aortic valve. If initial imaging is normal and there is no aortic dilation, imaging should be repeated every 5 to 10 years. If any abnormality is found, repeat imaging is needed every year.22
Magnetic resonance imaging (MRI) or computed tomographic (CT) angiography may be required to better assess the aorta for patients requiring a surgical intervention, or when aortic dimensions are not clearly visualized on TTE. MRI has 2 advantages over CT angiography: it poses no radiation risk, and it provides more information on left ventricular function and dimensions, in addition to valve assessment.23,24
No published study has compared MRI or CT angiography and transesophageal echocardiography (TEE), but in a study of 174 patients with dilated aortic root, combined TTE and TEE detected aortic valve morphology accurately in 98% of cases. As TEE is more invasive, it is not recommended for regular surveillance (Figures 1 and 2).25
FAMILY SCREENING RECOMMENDED
Close relatives should be evaluated for aortic valve and thoracic aortic disease.12,13,23,26
The American College of Cardiology (ACC) and the American Heart Association (AHA), backed by radiologic and cardiovascular associations, concur in recommending echocardiographic screening and routine screening of the thoracic aorta for aortic root dilation in first-degree relatives (ie, siblings, parents, and children) of patients with bicuspid aortic valve (class I recommendation).22,27,28
A comprehensive physical examination is recommended for family members in addition to TTE, with careful assessment of the aortic valve in short and long axes, and of the aortic root.14 If the aorta cannot be adequately evaluated with TTE, further assessment should be pursued with CT angiography or MRI.
EXERCISE RESTRICTIONS
The 2015 ACC/AHA guidelines for competitive athletes with cardiovascular abnormalities recommend annual screening with TTE or MRI angiography for athletes with bicuspid aortic valve and coexisting dilation of the ascending aorta (aortic diameter 40–42 mm in men and 36–39 mm in women) (class I recommendation, level of evidence C).29
Athletes with a bicuspid aortic valve and a normal aortic root and ascending aorta may participate in all competitive activities.29 However, those with a dilated aorta should avoid strenuous activities because of the increased risk of rupture.30 The ACC/AHA recommendations29 depend on the diameter of the ascending aorta and the nature of the sport:
- For an aortic diameter 40 to 42 mm in men or 36 to 39 mm in women, and no features of connective tissue disease or familial thoracic ascending aortic syndrome, low- and moderate-intensity sports with a low likelihood of significant body contact may be considered; consider avoiding intense weight training (class IIb, level of evidence C)
- For an aortic diameter 43 to 45 mm, low-intensity sports with a low likelihood of body contact may be considered (class IIb, level of evidence C)
- For an aortic diameter greater than 43 mm in men or greater than 40 mm in women, sports involving body collision should be avoided (class III, level of evidence C)
- For an aortic diameter greater than 45 mm, sports activities should be avoided (class III, level of evidence C).
PREGNANCY CONSIDERATIONS
Bicuspid aortic valve is associated with aortic dissection, mainly in the third trimester.31 Patients should ideally undergo echocardiographic screening before conception. The 2010 ACC/AHA guidelines for managing thoracic aortic disease recommend monthly or bimonthly echocardiography until delivery in pregnant women with a dilated thoracic aorta.22
Patients with bicuspid aortic valve and aortic root enlargement of more than 40 mm should have preconception counseling about surgery for aortic root replacement before becoming pregnant. If the diagnosis of enlarged aortic root is made during pregnancy, echocardiographic surveillance at 4- to 6-week intervals is indicated.32
SURGICAL MANAGEMENT
In the past, beta-blockers and angiotensin-converting enzyme inhibitors were recommended to minimize shear stress, with the goal of slowing progression of aortic dilation. However, evidence to support their use is inadequate.33,34
The only definitive treatment is surgery, with various procedures that lower the risk of death or dissection.24,35
The dimensions of the aortic root or ascending aorta should be examined vigilantly, according to the 2014 ACC/AHA guidelines27:
- Repairing the aortic sinuses or replacing the ascending aorta is indicated if the diameter of the aortic sinuses or ascending aorta is greater than 5.5 cm (class I, level of evidence B)
- Repairing the aortic sinuses or replacing the ascending aorta is reasonable if the diameter of the aortic sinuses or ascending aorta is greater than 5.0 cm and the patient has a risk factor for dissection such as a family history of aortic dissection or an increase in diameter of 0.5 cm or greater per year (class IIa, level of evidence C)
- Replacement of the ascending aorta is reasonable if the diameter of the ascending aorta is greater than 4.5 cm and the patient is undergoing aortic valve surgery for severe aortic stenosis or regurgitation.
Valve repair or replacement
Aortic valve repair or replacement is sometimes done separately from aortic root repair.
The value of aortic valve repair is debatable, but a series of 728 patients at Cleveland Clinic showed a very low mortality rate (0.41%) and an annual reoperation rate of 2.6% during up to 15 years of follow-up.36
Aortic valve replacement is usually considered for patients with severe valve dysfunction, abnormal left ventricular dimensions, or symptoms. It is important to determine if the patient is a good surgical candidate and to refer early for surgical evaluation to avoid the higher risk of death associated with emergency surgery.36
Transcatheter aortic valve replacement has been studied in patients deemed to be at too high a risk for surgical replacement. Short- and intermediate-term outcomes have been good in these patients, but long-term data are lacking.37
Surveillance after surgery
The type of operation determines postoperative surveillance.
After isolated aortic valve repair or replacement, patients should continue with surveillance at least annually to monitor for progressive aortopathy, as they remain at increased risk of dissection or rupture after isolated valve surgery, especially if they had aortic insufficiency preoperatively.38
After definitive surgery with replacement or repair of the ascending aorta, no clear recommendations have been established for continued surveillance. However, it is reasonable to image these patients with either MRI or CT angiography 3 to 5 years after their surgery to monitor for anastomotic complications.
CASE QUESTIONS ANSWERED
Our patient should undergo repeat TTE in 1 year. He should also undergo CT angiography of the ascending aorta if it is not seen by TTE. He can participate in low-intensity sports but should avoid intense weight training. His parents, siblings, and children should be screened for bicuspid aortic valve or associated aortopathies.
- Roberts WC. The congenitally bicuspid aortic valve. A study of 85 autopsy cases. Am J Cardiol 1970; 26(1):72–83. pmid:5427836
- Michalowska IM, Hryniewiecki T, Kwiatek P, Stoklosa P, Swoboda-Rydz U, Szymanski P. Coronary artery variants and anomalies in patients with bicuspid aortic valve. J Thorac Imaging 2016; 31(3):156–162. doi:10.1097/RTI.0000000000000205
- Sabet HY, Edwards WD, Tazelaar HD, Daly RC. Congenitally bicuspid aortic valves: a surgical pathology study of 542 cases (1991 through 1996) and a literature review of 2,715 additional cases. Mayo Clin Proc 1999; 74(1):14–26. doi:10.4065/74.1.14
- Tutar E, Ekici F, Atalay S, Nacar N. The prevalence of bicuspid aortic valve in newborns by echocardiographic screening. Am Heart J 2005; 150(3):513–515. doi:10.1016/j.ahj.2004.10.036
- Benson DW. The genetics of congenital heart disease: a point in the revolution. Cardiol Clin 2002; 20(3):385–394. pmid:12371007
- Emanuel R, Withers R, O’Brien K, Ross P, Feizi O. Congenitally bicuspid aortic valves. Clinicogenetic study of 41 families. Br Heart J 1978; 40(12):1402–1407. pmid:737099
- Giusti B, Sticchi E, De Cario R, Magi A, Nistri S, Pepe G. Genetic bases of bicuspid aortic valve: the contribution of traditional and high-throughput sequencing approaches on research and diagnosis. Front Physiol 2017; 8:612. doi:10.3389/fphys.2017.00612
- Sachdev V, Matura LA, Sidenko S, et al. Aortic valve disease in Turner syndrome. J Am Coll Cardiol 2008; 51(19):1904–1909. doi:10.1016/j.jacc.2008.02.035
- Duran AC, Frescura C, Sans-Coma V, Angelini A, Basso C, Thiene G. Bicuspid aortic valves in hearts with other congenital heart disease. J Heart Valve Dis 1995; 4(6):581–590. pmid:8611973
- De Rubens Figueroa J, Rodríguez LM, Hach JL, Del Castillo Ruíz V, Martínez HO. Cardiovascular spectrum in Williams-Beuren syndrome: the Mexican experience in 40 patients. Tex Heart Inst J 2008; 35(3):279–285. pmid:18941598
- Yuan SM, Jing H. The bicuspid aortic valve and related disorders. Sao Paulo Med J 2010; 128(5):296–301. pmid:21181071
- Cripe L, Andelfinger G, Martin LJ, Shooner K, Benson DW. Bicuspid aortic valve is heritable. J Am Coll Cardiol 2004; 44(1):138–143. doi:10.1016/j.jacc.2004.03.050
- Kerstjens-Frederikse WS, Sarvaas GJ, Ruiter JS, et al. Left ventricular outflow tract obstruction: should cardiac screening be offered to first-degree relatives? Heart 2011; 97(15):1228–1232. doi:10.1136/hrt.2010.211433
- Siu SC, Silversides CK. Bicuspid aortic valve disease. J Am Coll Cardiol 2010; 55(25):2789–2800. doi:10.1016/j.jacc.2009.12.068
- Michelena HI, Khanna AD, Mahoney D, et al. Incidence of aortic complications in patients with bicuspid aortic valves. JAMA 2011; 306(10):1104–1112.
- Michelena HI, Desjardins VA, Avierinos JF, et al. Natural history of asymptomatic patients with normally functioning or minimally dysfunctional bicuspid aortic valve in the community. Circulation 2008; 117(21):2776–2784. doi:10.1161/CIRCULATIONAHA.107.740878
- Beppu S, Suzuki S, Matsuda H, Ohmori F, Nagata S, Miyatake K. Rapidity of progression of aortic stenosis in patients with congenital bicuspid aortic valves. Am J Cardiol 1993; 71(4):322–327. pmid:8427176
- Roberts WC, Ko JM. Frequency by decades of unicuspid, bicuspid, and tricuspid aortic valves in adults having isolated aortic valve replacement for aortic stenosis, with or without associated aortic regurgitation. Circulation 2005; 111(7):920–925. doi:10.1161/01.CIR.0000155623.48408.C5
- Yener N, Oktar GL, Erer D, Yardimci MM, Yener A. Bicuspid aortic valve. Ann Thorac Cardiovasc Surg 2002; 8(5):264–267. pmid:12472407
- Lamas CC, Eykyn SJ. Bicuspid aortic valve—a silent danger: analysis of 50 cases of infective endocarditis. Clin Infect Dis 2000; 30(2):336–341. doi:10.1086/313646
- Wilson W, Taubert KA, Gewitz M, et al. Prevention of infective endocarditis: guidelines from the American Heart Association. Circulation 2007; 116(15):1736–1754. doi:10.1161/CIRCULATIONAHA.106.183095
- Hiratzka L, Bakris G, Beckman JA, et al. 2010 ACCF/AHA/AATS/ACR/ASA/SCA/SCAI/SIR/STS/SVM guidelines for the diagnosis and management of patients with thoracic aortic disease. Circulation 2010; 121(13):e266–e369. doi:10.1161/CIR.0b013e3181d4739e
- Chun EJ, Choi SI, Lim C, et al. Aortic stenosis: evaluation with multidetector CT angiography and MR imaging. Korean J Radiol 2008; 9(5):439–448. doi:10.3348/kjr.2008.9.5.439
- Kiefer TL, Wang A, Hughes GC, Bashore TM. Management of patients with bicuspid aortic valve disease. Curr Treat Options Cardiovasc Med 2011; 13(6):489–505. doi:10.1007/s11936-011-0152-7
- Alegret JM, Palazon O, Duran I, Vernis JM. Aortic valve morphology definition with transthoracic combined with transesophageal echocardiography in a population with high prevalence of bicuspid aortic valve. Int J Cardiovasc Imaging 2005; 21(2-3):213–217. doi:10.1007/s10554-004-3901-9
- Biner S, Rafique AM, Ray I, Cuk O, Siegel RJ, Tolstrup K. Aortopathy is prevalent in relatives of bicuspid aortic valve patients. J Am Coll Cardiol 2009; 53(24):2288–2295. doi:10.1016/j.jacc.2009.03.027
- Nishimura RA, Otto CM, Bonow RO, et al. 2014 AHA/ACC guideline for the management of patients with valvular heart disease. J Thorac Cardiovasc Surg 2014; 148(1):e1-e132. doi:10.1016/j.jtcvs.2014.05.014
- Warnes CA, Williams RG, Bashore TM, et al. ACC/AHA 2008 guidelines for the management of adults with congenital heart disease. J Am Coll Cardiol 2008; 52(23):e143–e263. doi:10.1016/j.jacc.2008.10.001
- Braverman AC, Harris KM, Kovacs RJ, Maron BJ. Eligibility and disqualification recommendations for competitive athletes with cardiovascular abnormalities: Task Force 7: aortic diseases, including Marfan syndrome. Circulation 2015; 132(22):e303–e309. doi:10.1161/CIR.0000000000000243
- De Mozzi P, Longo UG, Galanti G, Maffulli N. Bicuspid aortic valve: a literature review and its impact on sport activity. Br Med Bull 2008; 85:63–85. doi:10.1093/bmb/ldn002
- Thorne SA. Pregnancy in heart disease. Heart 2004; 90(4):450–456. pmid:15020530
- Immer FF, Bansi AG, Immer-Bansi AS, et al. Aortic dissection in pregnancy: analysis of risk factors and outcome. Ann Thorac Surg 2003; 76(1):309–314. pmid:12842575
- Allen BD, Markl M, Barker AJ, et al. Influence of beta-blocker therapy on aortic blood flow in patients with bicuspid aortic valve. Int J Cardiovasc Imaging 2016; 32(4):621–628. doi:10.1007/s10554-015-0819-3
- Ohnemus D, Oster ME, Gatlin S, Jokhadar M, Mahle WT. The effect of angiotensin-converting enzyme inhibitors on the rate of ascending aorta dilation in patients with bicuspid aortic valve. Congenit Heart Dis 2015; 10(1):E1–E5. doi:10.1111/chd.12184
- Masri A, Kalahasti V, Alkharabsheh S, et al. Characteristics and long-term outcomes of contemporary patients with bicuspid aortic valves. J Thorac Cardiovasc Surg 2016; 151(6):1650–1659.e1. doi:10.1016/j.jtcvs.2015.12.019
- Svensson LG, Al Kindi AH, Vivacqua A, et al. Long-term durability of bicuspid aortic valve repair. Ann Thorac Surg 2014; 97(5):1539–1548. doi:10.1016/j.athoracsur.2013.11.036
- Mylotte D, Lefevre T, Sondergaard L, et al. Transcatheter aortic valve replacement in bicuspid aortic valve disease. J Am Coll Cardiol 2014; 64(22):2330–2339. doi:10.1016/j.jacc.2014.09.039
- Girdauskas E, Disha K, Raisin HH, Secknus MA, Borger MA, Kuntze T. Risk of late aortic events after an isolated aortic valve replacement for bicuspid aortic valve stenosis with concomitant ascending aortic dilation. Eur J Cardiothorac Surg 2012; 42(5):832–838. doi:10.1093/ejcts/ezs137
- Roberts WC. The congenitally bicuspid aortic valve. A study of 85 autopsy cases. Am J Cardiol 1970; 26(1):72–83. pmid:5427836
- Michalowska IM, Hryniewiecki T, Kwiatek P, Stoklosa P, Swoboda-Rydz U, Szymanski P. Coronary artery variants and anomalies in patients with bicuspid aortic valve. J Thorac Imaging 2016; 31(3):156–162. doi:10.1097/RTI.0000000000000205
- Sabet HY, Edwards WD, Tazelaar HD, Daly RC. Congenitally bicuspid aortic valves: a surgical pathology study of 542 cases (1991 through 1996) and a literature review of 2,715 additional cases. Mayo Clin Proc 1999; 74(1):14–26. doi:10.4065/74.1.14
- Tutar E, Ekici F, Atalay S, Nacar N. The prevalence of bicuspid aortic valve in newborns by echocardiographic screening. Am Heart J 2005; 150(3):513–515. doi:10.1016/j.ahj.2004.10.036
- Benson DW. The genetics of congenital heart disease: a point in the revolution. Cardiol Clin 2002; 20(3):385–394. pmid:12371007
- Emanuel R, Withers R, O’Brien K, Ross P, Feizi O. Congenitally bicuspid aortic valves. Clinicogenetic study of 41 families. Br Heart J 1978; 40(12):1402–1407. pmid:737099
- Giusti B, Sticchi E, De Cario R, Magi A, Nistri S, Pepe G. Genetic bases of bicuspid aortic valve: the contribution of traditional and high-throughput sequencing approaches on research and diagnosis. Front Physiol 2017; 8:612. doi:10.3389/fphys.2017.00612
- Sachdev V, Matura LA, Sidenko S, et al. Aortic valve disease in Turner syndrome. J Am Coll Cardiol 2008; 51(19):1904–1909. doi:10.1016/j.jacc.2008.02.035
- Duran AC, Frescura C, Sans-Coma V, Angelini A, Basso C, Thiene G. Bicuspid aortic valves in hearts with other congenital heart disease. J Heart Valve Dis 1995; 4(6):581–590. pmid:8611973
- De Rubens Figueroa J, Rodríguez LM, Hach JL, Del Castillo Ruíz V, Martínez HO. Cardiovascular spectrum in Williams-Beuren syndrome: the Mexican experience in 40 patients. Tex Heart Inst J 2008; 35(3):279–285. pmid:18941598
- Yuan SM, Jing H. The bicuspid aortic valve and related disorders. Sao Paulo Med J 2010; 128(5):296–301. pmid:21181071
- Cripe L, Andelfinger G, Martin LJ, Shooner K, Benson DW. Bicuspid aortic valve is heritable. J Am Coll Cardiol 2004; 44(1):138–143. doi:10.1016/j.jacc.2004.03.050
- Kerstjens-Frederikse WS, Sarvaas GJ, Ruiter JS, et al. Left ventricular outflow tract obstruction: should cardiac screening be offered to first-degree relatives? Heart 2011; 97(15):1228–1232. doi:10.1136/hrt.2010.211433
- Siu SC, Silversides CK. Bicuspid aortic valve disease. J Am Coll Cardiol 2010; 55(25):2789–2800. doi:10.1016/j.jacc.2009.12.068
- Michelena HI, Khanna AD, Mahoney D, et al. Incidence of aortic complications in patients with bicuspid aortic valves. JAMA 2011; 306(10):1104–1112.
- Michelena HI, Desjardins VA, Avierinos JF, et al. Natural history of asymptomatic patients with normally functioning or minimally dysfunctional bicuspid aortic valve in the community. Circulation 2008; 117(21):2776–2784. doi:10.1161/CIRCULATIONAHA.107.740878
- Beppu S, Suzuki S, Matsuda H, Ohmori F, Nagata S, Miyatake K. Rapidity of progression of aortic stenosis in patients with congenital bicuspid aortic valves. Am J Cardiol 1993; 71(4):322–327. pmid:8427176
- Roberts WC, Ko JM. Frequency by decades of unicuspid, bicuspid, and tricuspid aortic valves in adults having isolated aortic valve replacement for aortic stenosis, with or without associated aortic regurgitation. Circulation 2005; 111(7):920–925. doi:10.1161/01.CIR.0000155623.48408.C5
- Yener N, Oktar GL, Erer D, Yardimci MM, Yener A. Bicuspid aortic valve. Ann Thorac Cardiovasc Surg 2002; 8(5):264–267. pmid:12472407
- Lamas CC, Eykyn SJ. Bicuspid aortic valve—a silent danger: analysis of 50 cases of infective endocarditis. Clin Infect Dis 2000; 30(2):336–341. doi:10.1086/313646
- Wilson W, Taubert KA, Gewitz M, et al. Prevention of infective endocarditis: guidelines from the American Heart Association. Circulation 2007; 116(15):1736–1754. doi:10.1161/CIRCULATIONAHA.106.183095
- Hiratzka L, Bakris G, Beckman JA, et al. 2010 ACCF/AHA/AATS/ACR/ASA/SCA/SCAI/SIR/STS/SVM guidelines for the diagnosis and management of patients with thoracic aortic disease. Circulation 2010; 121(13):e266–e369. doi:10.1161/CIR.0b013e3181d4739e
- Chun EJ, Choi SI, Lim C, et al. Aortic stenosis: evaluation with multidetector CT angiography and MR imaging. Korean J Radiol 2008; 9(5):439–448. doi:10.3348/kjr.2008.9.5.439
- Kiefer TL, Wang A, Hughes GC, Bashore TM. Management of patients with bicuspid aortic valve disease. Curr Treat Options Cardiovasc Med 2011; 13(6):489–505. doi:10.1007/s11936-011-0152-7
- Alegret JM, Palazon O, Duran I, Vernis JM. Aortic valve morphology definition with transthoracic combined with transesophageal echocardiography in a population with high prevalence of bicuspid aortic valve. Int J Cardiovasc Imaging 2005; 21(2-3):213–217. doi:10.1007/s10554-004-3901-9
- Biner S, Rafique AM, Ray I, Cuk O, Siegel RJ, Tolstrup K. Aortopathy is prevalent in relatives of bicuspid aortic valve patients. J Am Coll Cardiol 2009; 53(24):2288–2295. doi:10.1016/j.jacc.2009.03.027
- Nishimura RA, Otto CM, Bonow RO, et al. 2014 AHA/ACC guideline for the management of patients with valvular heart disease. J Thorac Cardiovasc Surg 2014; 148(1):e1-e132. doi:10.1016/j.jtcvs.2014.05.014
- Warnes CA, Williams RG, Bashore TM, et al. ACC/AHA 2008 guidelines for the management of adults with congenital heart disease. J Am Coll Cardiol 2008; 52(23):e143–e263. doi:10.1016/j.jacc.2008.10.001
- Braverman AC, Harris KM, Kovacs RJ, Maron BJ. Eligibility and disqualification recommendations for competitive athletes with cardiovascular abnormalities: Task Force 7: aortic diseases, including Marfan syndrome. Circulation 2015; 132(22):e303–e309. doi:10.1161/CIR.0000000000000243
- De Mozzi P, Longo UG, Galanti G, Maffulli N. Bicuspid aortic valve: a literature review and its impact on sport activity. Br Med Bull 2008; 85:63–85. doi:10.1093/bmb/ldn002
- Thorne SA. Pregnancy in heart disease. Heart 2004; 90(4):450–456. pmid:15020530
- Immer FF, Bansi AG, Immer-Bansi AS, et al. Aortic dissection in pregnancy: analysis of risk factors and outcome. Ann Thorac Surg 2003; 76(1):309–314. pmid:12842575
- Allen BD, Markl M, Barker AJ, et al. Influence of beta-blocker therapy on aortic blood flow in patients with bicuspid aortic valve. Int J Cardiovasc Imaging 2016; 32(4):621–628. doi:10.1007/s10554-015-0819-3
- Ohnemus D, Oster ME, Gatlin S, Jokhadar M, Mahle WT. The effect of angiotensin-converting enzyme inhibitors on the rate of ascending aorta dilation in patients with bicuspid aortic valve. Congenit Heart Dis 2015; 10(1):E1–E5. doi:10.1111/chd.12184
- Masri A, Kalahasti V, Alkharabsheh S, et al. Characteristics and long-term outcomes of contemporary patients with bicuspid aortic valves. J Thorac Cardiovasc Surg 2016; 151(6):1650–1659.e1. doi:10.1016/j.jtcvs.2015.12.019
- Svensson LG, Al Kindi AH, Vivacqua A, et al. Long-term durability of bicuspid aortic valve repair. Ann Thorac Surg 2014; 97(5):1539–1548. doi:10.1016/j.athoracsur.2013.11.036
- Mylotte D, Lefevre T, Sondergaard L, et al. Transcatheter aortic valve replacement in bicuspid aortic valve disease. J Am Coll Cardiol 2014; 64(22):2330–2339. doi:10.1016/j.jacc.2014.09.039
- Girdauskas E, Disha K, Raisin HH, Secknus MA, Borger MA, Kuntze T. Risk of late aortic events after an isolated aortic valve replacement for bicuspid aortic valve stenosis with concomitant ascending aortic dilation. Eur J Cardiothorac Surg 2012; 42(5):832–838. doi:10.1093/ejcts/ezs137
KEY POINTS
- Associated aortopathies such as aortic root dilation, aneurysm, dissection, and coarctation may initially be asymptomatic.
- Regular surveillance with transthoracic echocardiography (TTE) is required.
- Transesophageal echocardiography should be performed if TTE does not clearly show the aorta and aortic root. Magnetic resonance imaging or computed tomographic angiography may also be needed to measure the aortic root and ascending thoracic aorta.
- If initial imaging is normal and there is no aortic dilation, repeat imaging should be done every 5 to 10 years. If any abnormality is found, annual surveillance is needed.
- Women with a bicuspid aortic valve who are contemplating pregnancy should undergo echocardiography first, and some may need to undergo surgery.
Ablation of atrial fibrillation: Facts for the referring physician
A 64-year-old man with hypertension but without known structural heart disease presents for a second opinion on management of his atrial fibrillation. The condition was first diagnosed at age 38, when he experienced palpitations and shortness of breath on exertion; at times he also experienced decreased endurance and fatigue without overt palpitations. At first, these episodes occurred about twice a year, and the patient was managed with a beta-blocker for rate control and an oral anticoagulant.
Over the past 10 years, the episodes have become more frequent and longer-lasting and have required frequent cardioversions. He was given flecainide for rhythm control but continued to have frequent episodes, and so about 1 year ago he was switched to amiodarone, which controlled his rhythm better. However, after reading about side effects of amiodarone, he decided to seek a second opinion.
He was evaluated by our team and eventually underwent radiofrequency ablation. During the procedure, he was noted to have diffuse scarring and fibrosis of his left atrium, and afterward he continued to require antiarrhythmic drugs to maintain sinus rhythm.
Should he have been referred sooner? What factors should primary care physicians consider when referring a patient with atrial fibrillation for ablation?
THE EPIDEMIC OF ATRIAL FIBRILLATION
Atrial fibrillation is a large and growing public health problem. In 2010, it was estimated to affect 2.7 to 6.1 million people in the United States, and with the rapid aging of our population, its prevalence is expected to rise to between 5.6 and 12 million by 2050.1–3 It is associated with significant morbidity, poor quality of life, and increased risk of death, heart failure, stroke, and cognitive impairment.
The number of new cases per year has increased over the years despite research and preventive measures, which may reflect aging of the population and increased survival rates in patients with cardiovascular or comorbid conditions.1,4
Thus, atrial fibrillation is one of the most common cardiovascular conditions encountered by primary care physicians and cardiologists, putting them at the forefront of its management. Proper treatment in its early stages and referral to a specialist for advanced management may alter its natural history and improve clinical outcomes.
HOW DOES ATRIAL FIBRILLATION ARISE AND PERSIST?
Much is still unknown about the pathogenesis of atrial fibrillation, but considerable progress has been made in the past few decades, opening the door for clinical ablative strategies.
Multiple wavelet hypothesis
Until the late 1980s, the most widely accepted conceptual mechanism of atrial fibrillation was the multiple wavelet hypothesis developed by Moe et al.5 According to this hypothesis, atrial fibrillation begins with multiple independent wavelets occurring simultaneously and spreading randomly throughout both atria, and it persists if there are a minimum number of coexisting wavelets, increased atrial mass, and heterogeneous conduction delays across the atrial tissue.
The surgical maze procedure, in which a series of incisions arranged in a maze-like pattern is created in the left atrium, was predicated on this model. The theory was that these surgical lesions would compartmentalize the atria into discrete electrical segments and thereby reduce the number of circulating random wavelets.6,7
However, experimental and clinical studies suggest that although randomly propagating wavelets can contribute to maintaining atrial fibrillation, focal triggers are noted in most cases.
Focal triggers
In 1997, Jaïs et al8 observed that atrial fibrillation is often triggered by a rapidly firing ectopic focus and that ablation of that focus can eliminate it. These ectopic foci are often found at or near the ostia of the pulmonary veins or near the superior vena cava.8,9 It is now well established that ectopic foci in the pulmonary veins are crucial triggers that initiate atrial fibrillation.
Trigger-and-substrate theory
The substrate for maintaining atrial fibrillation consists of an abnormal left atrium with heterogeneous fibrosis (scarring) and conduction delays. Any heart disease that increases left atrial pressure could lead to atrial dilation and remodeling, which could be substrates for atrial fibrillation. Extensive atrial remodeling and scarring are associated with progression and persistence of atrial fibrillation and make rhythm control more challenging.
Atrial fibrillation begets atrial fibrillation
As shown in the case above, over time, paroxysmal atrial fibrillation often progresses to persistent and long-standing atrial fibrillation if not aggressively managed initially.
In 1972, Davies and Pomerance10 performed 100 autopsies and found that the people who had had atrial fibrillation for longer than 1 month had lost muscle mass in the sinus node and internodal tract, and their atria were dilated. The study introduced the concept that atrial fibrillation itself causes pathologic changes in the atrium.
Wijffels et al,11 in an experiment in goats, showed that atrial fibrillation produced by rapid bursts of atrial pacing was initially paroxysmal. However, as they continued to induce atrial fibrillation over and over again, it lasted progressively longer until it would persist for more than 24 hours. Thus, in a relatively short time, the atria went from supporting paroxysmal fibrillation to supporting persistent fibrillation.
Atrial fibrillation leads to electrophysiologic and anatomic remodeling in the atrium, which leads to a shorter action potential duration and a shorter refractory period. This in turn makes it easier for atrial fibrillation to persist.12
Because atrial fibrillation tends to progress, intervening early may improve its outcomes. Early ablation has been shown to improve the chances of staying in sinus rhythm in both paroxysmal and persistent atrial fibrillation.13–15
CATHETER ABLATION OF ATRIAL FIBRILLATION
The goal of ablation is to prevent atrial fibrillation by eliminating the trigger that initiates it, altering the arrhythmogenic substrate, or both.
Pulmonary vein isolation
The most common ablation strategy is to electrically isolate the pulmonary veins by creating circumferential lesions around their antra. This creates a nonconducting rim of scar tissue, electrically disconnecting the pulmonary veins from the atrium.
Ablation outside of the pulmonary veins
Because recurrence rates are high in patients with persistent atrial fibrillation who undergo pulmonary vein ablation alone, the search continues for adjunctive strategies to improve outcomes. Although these strategies have a sound rationale based on experimental data and anecdotal evidence in humans, they have not yet been convincingly shown to be helpful in large clinical studies. Nonetheless, it is possible that more extensive substrate ablation—atrial “debulking”—could improve outcomes by reducing the amount of tissue that can fibrillate.
Linear ablation. Creating lines of ablation (as in the maze procedure) isolates different segments of the left atrium. Often, these lines are created along the roof of the left atrium between the right and left upper pulmonary veins and from the mitral valve to the left inferior pulmonary vein. The benefit of linear ablation has not been proven, and gaps in such lines may introduce atrial flutter.
Triggers not in the pulmonary veins. Common sites of nonpulmonary vein triggers include the posterior wall of the left atrium, the superior vena cava, the coronary sinus, and along the ligament of Marshall. Provocative maneuvers such as isoproterenol infusion can help find those triggers, which can then be ablated. A limitation is that there is no protocol proven to reproducibly elicit triggers.
Complex fractionated atrial electrograms are areas in the atrium with highly fractionated, low voltage potentials. They may be critical sites of substrate for atrial fibrillation, and many electrophysiologists target them in patients with persistent atrial fibrillation. But despite initial enthusiasm, doing so has not resulted in better outcomes in persistent atrial fibrillation.
Rotors. Animal studies have shown that atrial fibrillation can be triggered or maintained by localized sources of organized reentrant circuits (rotors) or focal impulses. Recent studies have shown that these electrical rotors and focal sources could potentially be mapped and ablated in humans. But positive results in initial reports have not been reproduced, and this remains an area of controversy.
Our practice. We isolate the pulmonary veins with antral ablations, ablate the posterior wall, and extend the ablation toward the septum and inferior to the right pulmonary veins, with good long-term outcomes.14 The rationale behind ablating the posterior wall is that it shares embryologic origins with the pulmonary veins and may be a common source of triggers in atrial fibrillation.
We do not routinely create empiric ablation lines in the left or right atrium unless the patient has atrial flutter. Empiric ablation lines have not been convincingly shown to provide additional benefit compared with our extensive ablation approach, which involves the posterior wall. Empiric ablation of the appendage or coronary sinus is typically reserved for repeat ablation in patients with recurrent persistent atrial fibrillation.
RATIONALE FOR TREATING ATRIAL FIBRILLATION WITH ABLATION
To control symptoms
At this time, the primary aim of atrial fibrillation ablation is to reduce symptoms and improve quality of life. In theory, ablation could also decrease the risk of stroke, heart failure, and death. However, these outcomes have not been systematically evaluated in any large randomized controlled trial.
To control rhythm and improve survival
Randomized controlled trials of rhythm vs rate control of atrial fibrillation16–18 have failed to demonstrate that restoring sinus rhythm is associated with better survival. All of these trials used antiarrhythmic drugs for rhythm control. However, nonrandomized studies19,20 showed that maintaining sinus rhythm is associated with a significant reduction in mortality rates, whereas the use of antiarrhythmic drugs increased mortality risk.
This suggests that the beneficial effect of restoring sinus rhythm may be offset by adverse effects of antiarrhythmic drugs, and if rhythm control could be achieved by a method other than antiarrhythmic drug therapy, it may be superior to rate control. On the other hand, these data may be affected by residual confounding. This topic deserves further research, but maintaining sinus rhythm is typically preferred whenever possible.
Discontinuing anticoagulation is not a goal at this time
Retrospective studies have reported a low risk of stroke in patients who discontinue anticoagulation several months after undergoing atrial fibrillation ablation.21–23 However, atrial fibrillation can recur, and risk of stroke increases with age.
Therefore, guidelines24 still recommend continuing anticoagulation after ablation. Generally, we do not offer ablation with a goal of discontinuing anticoagulation. That said, stopping anticoagulation may be considered after long-term suppression of paroxysmal atrial fibrillation on a case-by-case basis in patients deemed to be at low risk. Left atrial appendage closure devices may eventually allow concomitant atrial fibrillation ablation and closure of the appendage, so that anticoagulation could then be stopped. This remains a topic of investigation.
Who should be considered for ablation?
There are no absolute age or comorbidity contraindications to ablation. Everyone who has atrial fibrillation deserves, in our opinion, a referral to the electrophysiology clinic.
PROCEDURAL CONSIDERATIONS
Atrial fibrillation ablation is most often performed by electrophysiologists using a minimally invasive endovascular approach. The patient can be under either moderate sedation or general anesthesia; we prefer general anesthesia for patient comfort, safety, and efficacy.
We use an electrogram-based technique to target and eliminate electrical potentials and ensure continuity of ablation sets, with additional guidance by 3-dimensional cardiac mapping systems and intracardiac echocardiography. We also use contact force-sensing catheters to ensure catheter-tissue contact during ablation and to avoid excessive contact, which may enhance the safety of the procedure.
Energy: Hot or cold
Two types of energy can be used for ablation:
Radiofrequency energy (low voltage, high frequency—30 kHz to 1.5 mHz) is delivered to the endocardial surface via a point-source catheter. The radiofrequency energy produces controlled, focal thermal ablation.
In a randomized trial,25 these ablation technologies were shown to be equivalent for preventing recurrences of atrial fibrillation. We use both in our practice. The choice depends primarily on the planned ablation set, given that balloon cryoablation can achieve antral isolation of the pulmonary veins but allows little or no substrate modification.
Improved ablation technology
Contact force-sensing catheters. Radiofrequency ablation catheters are now equipped with a pressure sensor at the tip that measures how hard the catheter is pressing on the heart wall.26,27 In our experience, this has improved the outcomes of ablation procedures, primarily in persistent atrial fibrillation.28
Complications of ablation
Although catheter ablation for atrial fibrillation is safe, it is still one of the most complex electrophysiologic procedures. Improvements in technology and techniques and accumulated experience over the past 15 years have made ablation safer, especially in tertiary care centers. But adverse outcomes are more frequent in low-volume centers.29
Minor procedural complications include pericarditis, complications at the site of vascular access, and anesthesia-related complications. While they do not affect the long-term outcome for the patient, they may increase hospital length of stay and cause temporary inconvenience.
Major complications include cardiac perforation and tamponade, periprocedural stroke, pulmonary vein stenosis, atrioesophageal fistula, phrenic nerve paralysis, major bleeding, myocardial infarction, and death. In a worldwide survey published in 2005, when atrial fibrillation ablation was still novel, the rate of major complications was 6%.30 By 2010, this had declined to 4.5%,31 and the rates of major complications may be significantly lower in more experienced centers.29 In our practice, in 2015, the rate of major complications was 1.3% (unpublished data).
Outcomes of catheter ablation
Clinical outcomes depend on many factors including the type of atrial fibrillation (paroxysmal vs nonparoxysmal), overall health of the atria (atrial size and scarring), patient age and comorbidities, and most importantly, the center’s and operator’s experience.
In randomized controlled trials comparing ablation and antiarrhythmic drug therapy, the efficacy of ablation in maintaining sinus rhythm has been in the range of 66% to 86% vs 16% to 22% for drug therapy,32,33 but these trials have been predominantly in middle-aged white men with paroxysmal atrial fibrillation. These trials also showed that catheter ablation reduced symptoms and improved quality of life. Ablation is less effective in persistent than in paroxysmal atrial fibrillation.34
In a long-term study from our group,14 660 (79.4%) of 831 patients who underwent ablation in 2005 were arrhythmia-free and not on antiarrhythmic drug therapy after a total of 1,019 ablations (an average of 1.2 ablations per patient) at a median of 55 months; 125 patients (15%, 41 with more than 1 ablation) continued to have atrial arrhythmia, controlled with drugs in 87 patients (69.6%). Only 38 patients (4.6%) continued to have drug-resistant atrial fibrillation and were treated with rate control with negative dromotropic agents.
Recent evidence
The largest randomized controlled trial of catheter ablation vs drug therapy for atrial fibrillation (Catheter Ablation Versus Antiarrhythmic Drug Therapy for Atrial Fibrillation [CABANA]) was completed recently, and the results were presented at a national meeting, although they have not yet been published in a peer-reviewed journal.35
A total of 2,204 patients with atrial fibrillation (42.4% paroxysmal, 47.3% persistent, and 10.3% long-standing persistent) were randomized to either ablation or drug therapy. Median follow-up was 4 years. The crossover rate was high—9.2% of those randomized to ablation did not undergo it, and 27.5% of those randomized to drug therapy underwent ablation.
The incidence of the primary end point (a composite of death, disabling stroke, serious bleeding, and cardiac arrest) was not significantly different between the 2 groups in the intention-to-treat analysis; however, given the high crossover rates, the as-treated and per-protocol analyses become important, and as-treated and per-protocol analyses revealed a significant benefit of ablation compared with drug therapy. The hazard ratio (HR) for the primary composite outcome was 0.67 (P = .006) on as-treated analysis and 0.73 (P = .05) on per-protocol analysis. The HR for all-cause mortality was 0.60 (P = .005) on as-treated analysis.
PERIPROCEDURAL CONSIDERATIONS
Periprocedural anticoagulation
The risk of thromboembolism is increased during, immediately following, and for several weeks to months after atrial fibrillation ablation.36,37
During the procedure, the risk is related to transseptal sheath placement, electrode catheters in the left atrium, and char formation on ablation catheters. These risks are mitigated with proper and careful sheath and catheter manipulation, maintenance of bubble-free irrigation through lines and sheaths, use of irrigated catheters, and initiation of heparin before transseptal access. Heparin is also infused during the procedure, with close monitoring of activated clotting time.
Postprocedurally, the transiently increased clotting risk could be due to damaged endothelium from the ablation itself and stunning of atrial tissue, which results in impaired contraction. Damaged endothelium improves as the tissue heals, and the stunning resolves by electrical reverse remodeling with sinus rhythm maintenance.
In view of these risks, the referring physician and electrophysiologist must pay careful attention to anticoagulation before and after ablation.
Before the procedure. It is safe to continue anticoagulation uninterrupted through the procedure.38,39 If the patient is on warfarin, we want the international normalized ratio to be in the therapeutic range when we perform atrial fibrillation ablation, and the patient takes his or her usual dose on the day of the procedure. If taking a direct oral anticoagulant, patients typically skip a dose the day before ablation and again on the morning of the procedure, and resume taking it immediately afterward while in the anesthesia recovery room.
During the procedure, we start heparin before transseptal puncture, adjust it to achieve an activated clotting time of 300 to 400 seconds, and keep it in this range as long as there are sheaths or catheters in the left atrium.
After the procedure. The current guidelines24 recommend that oral anticoagulation be continued without interruption for at least 2 months after the procedure, and in most cases indefinitely, depending on age and comorbidities. The decision to stop anticoagulation after 2 months is typically based on the stroke risk as assessed by the CHA2DS2-VASc score (www.chadsvasc.org) and not on the success of the ablation procedure.
ANTIARRHYTHMIC DRUGS AFTER THE PROCEDURE
Some patients actually experience more atrial fibrillation in the first weeks to months after the procedure. The mechanism in this setting may be different from that causing the arrhythmia in the first place. The causes of early recurrence of atrial arrhythmias include postablation inflammation, temporary autonomic imbalance, and delay of atrial radiofrequency lesion formation.40,41 These arrhythmias may completely resolve as the ablation lesions heal and scars mature.
It has been hypothesized that short-term use of antiarrhythmic drugs after atrial fibrillation ablation is effective in preventing arrhythmias because it alters atrial electrophysiologic characteristics induced by the above transient factors. A recent systematic review of 6 clinical trials showed that short-term use of antiarrhythmic drugs reduces the risk of early arrhythmia recurrence but does not reduce recurrence in the long term.42
In terms of outcomes, any arrhythmias that occur in the first 3 months do not necessarily affect long-term success. This is referred to as the “blanking period.” However, generally speaking, it is preferable to maintain sinus rhythm during that time to avoid further anatomic or electrical left atrial adverse remodeling. In many situations, patients continue taking the same antiarrhythmic agent or start on antiarrhythmic therapy in the first few months after ablation.43,44
The mechanisms of late recurrence of atrial arrhythmias after ablation are thought to be different from those in early recurrence. Late recurrence has been ascribed to incomplete pulmonary vein isolation, recovery of pulmonary vein-left atrium connections, or recovery of any other lines of ablation created in the procedure.45,46 For late recurrence of atrial arrhythmia, studies and guidelines suggest that repeat ablation may be an option.24,47
PRACTICAL CONSIDERATIONS FOR PROCEDURAL PLANNING
Before the procedure, some electrophysiologists use cardiac computed tomography or magnetic resonance imaging to evaluate the pulmonary vein anatomy. This helps in planning and in selecting the appropriate tools for the procedure.
The patient is asked to fast on the day of the procedure. The procedure can take 3 to 6 hours, depending on the patient’s anatomy and the operator’s technique and experience. It can be performed with the patient under general anesthesia or conscious sedation. Currently, we use general anesthesia most of the time to maximize patient comfort.
After the procedure, our patients must stay in bed for 4 hours and stay overnight for observation. If no complications arise, they are discharged the next day.
- Go AS. The epidemiology of atrial fibrillation in elderly persons: the tip of the iceberg. Am J Geriatr Cardiol 2005; 14(2):56–61. pmid:15785146
- Go AS, Hylek EM, Phillips KA, et al. Prevalence of diagnosed atrial fibrillation in adults: national implications for rhythm management and stroke prevention: the AnTicoagulation and Risk Factors in Atrial Fibrillation (ATRIA) Study. JAMA 2001; 285(18):2370–2375. pmid:11343485
- Miyasaka Y, Barnes ME, Gersh BJ, et al. Secular trends in incidence of atrial fibrillation in Olmsted County, Minnesota, 1980 to 2000, and implications on the projections for future prevalence. Circulation 2006; 114(2):119–125. doi:10.1161/CIRCULATIONAHA.105.595140
- Piccini JP, Hammill BG, Sinner MF, et al. Incidence and prevalence of atrial fibrillation and associated mortality among Medicare beneficiaries, 1993–2007. Circ Cardiovasc Qual Outcomes 2012; 5(1):85–93. doi:10.1161/CIRCOUTCOMES.111.962688
- Moe GK, Rheinboldt WC, Abildskov JA. A computer model of atrial fibrillation. Am Heart J 1964; 67:200–220. pmid:14118488
- Cox JL, Schuessler RB, Boineau JP. The surgical treatment of atrial fibrillation. I. Summary of the current concepts of the mechanisms of atrial flutter and atrial fibrillation. J Thorac Cardiovasc Surg 1991; 101(3):402–405. pmid:1999933
- Cox JL, Schuessler RB, D’Agostino HJ Jr, et al. The surgical treatment of atrial fibrillation. III. Development of a definitive surgical procedure. J Thorac Cardiovasc Surg 1991; 101(4):569–583. pmid:2008095
- Jaïs P, Haïssaguerre M, Shah DC, et al. A focal source of atrial fibrillation treated by discrete radiofrequency ablation. Circulation 1997; 95(3):572–576. pmid:9024141
- Haïssaguerre M, Jaïs P, Shah DC, et al. Spontaneous initiation of atrial fibrillation by ectopic beats originating in the pulmonary veins. N Engl J Med 1998; 339(10):659–666. doi:10.1056/NEJM199809033391003
- Davies MJ, Pomerance A. Pathology of atrial fibrillation in man. Br Heart J 1972; 34(5):520–525. pmid:5031645
- Wijffels MC, Kirchhof CJ, Dorland R, Allessie MA. Atrial fibrillation begets atrial fibrillation. A study in awake chronically instrumented goats. Circulation 1995; 92(7):1954–1968. pmid:7671380
- Nattel S. New ideas about atrial fibrillation 50 years on. Nature 2002; 415(6868):219–226. doi:10.1038/415219a
- Medi C, Sparks PB, Morton JB, et al. Pulmonary vein antral isolation for paroxysmal atrial fibrillation: results from long-term follow-up. J Cardiovasc Electrophysiol 2011; 22(2):137–141. doi:10.1111/j.1540-8167.2010.01885.x
- Hussein AA, Saliba WI, Martin DO, et al. Natural history and long-term outcomes of ablated atrial fibrillation. Circ Arrhythm Electrophysiol 2011; 4(3):271–278. doi:10.1161/CIRCEP.111.962100
- Hussein AA, Saliba WI, Barakat A, et al. Radiofrequency ablation of persistent atrial fibrillation: diagnosis-to-ablation time, markers of pathways of atrial remodeling, and outcomes. Circ Arrhythm Electrophysiol 2016; 9(1):e003669. doi:10.1161/CIRCEP.115.003669
- Carlsson J, Miketic S, Windeler J, et al. Randomized trial of rate-control versus rhythm-control in persistent atrial fibrillation: the Strategies of Treatment of Atrial Fibrillation (STAF) study. J Am Coll Cardiol 2003; 41(10):1690–1696. pmid:12767648
- Van Gelder IC, Hagens VE, Bosker HA, et al; Rate Control versus Electrical Cardioversion for Persistent Atrial Fibrillation Study Group. A comparison of rate control and rhythm control in patients with recurrent persistent atrial fibrillation. N Engl J Med 2002; 347(23):1834–1840. doi:10.1056/NEJMoa021375
- Wyse DG, Waldo AL, DiMarco JP, et al; Atrial Fibrillation Follow-up Investigation of Rhythm Management (AFFIRM) Investigators. A comparison of rate control and rhythm control in patients with atrial fibrillation. N Engl J Med 2002; 347(23):1825–1833. doi:10.1056/NEJMoa021328
- Hagens VE, Crijns HJ, Van Veldhuisen DJ, et al; RAte Control versus Electrical cardioversion for persistent atrial fibrillation study group. Rate control versus rhythm control for patients with persistent atrial fibrillation with mild to moderate heart failure: results from the RAte Control versus Electrical cardioversion (RACE) study. Am Heart J 2005; 149(6):1106–111. doi:10.1016/j.ahj.2004.11.030
- Pedersen OD, Bagger H, Keller N, Marchant B, Køber L, Torp-Pedersen C. Efficacy of dofetilide in the treatment of atrial fibrillation-flutter in patients with reduced left ventricular function: a Danish investigations of arrhythmia and mortality on dofetilide (diamond) substudy. Circulation 2001; 104(3):292–296. pmid:11457747
- Guiot A, Jongnarangsin K, Chugh A, et al. Anticoagulant therapy and risk of cerebrovascular events after catheter ablation of atrial fibrillation in the elderly. J Cardiovasc Electrophysiol 2012; 23(1):36–43. doi:10.1111/j.1540-8167.2011.02141.x
- Oral H, Chugh A, Ozaydin M, et al. Risk of thromboembolic events after percutaneous left atrial radiofrequency ablation of atrial fibrillation. Circulation 2006; 114(8):759–765. doi:10.1161/CIRCULATIONAHA.106.641225
- Themistoclakis S, Corrado A, Marchlinski FE, et al. The risk of thromboembolism and need for oral anticoagulation after successful atrial fibrillation ablation. J Am Coll Cardiol 2010; 55(8):735–743. doi:10.1016/j.jacc.2009.11.039
- Calkins H, Hindricks G, Cappato R, et al. 2017 HRS/EHRA/ECAS/APHRS/SOLAECE expert consensus statement on catheter and surgical ablation of atrial fibrillation: executive summary. J Arrhythm 2017; 33(5):369–409. doi:10.1016/j.joa.2017.08.001
- Kuck KH, Brugada J, Fürnkranz A, et al; FIRE AND ICE Investigators. Cryoballoon or radiofrequency ablation for paroxysmal atrial fibrillation. N Engl J Med 2016; 374(23):2235–2245. doi:10.1056/NEJMoa1602014
- Reddy VY, Dukkipati SR, Neuzil P, et al. Randomized, controlled trial of the safety and effectiveness of a contact force-sensing irrigated catheter for ablation of paroxysmal atrial fibrillation: results of the TactiCath Contact Force Ablation Catheter Study for Atrial Fibrillation (TOCCASTAR) Study. Circulation 2015; 132(10):907–915. doi:10.1161/CIRCULATIONAHA.114.014092
- Natale A, Reddy VY, Monir G, et al. Paroxysmal AF catheter ablation with a contact force sensing catheter: results of the prospective, multicenter SMART-AF trial. J Am Coll Cardiol 2014; 64(7):647–656. doi:10.1016/j.jacc.2014.04.072
- Hussein AA, Barakat AF, Saliba WI, et al. Persistent atrial fibrillation ablation with or without contact force sensing. J Cardiovasc Electrophysiol 2017; 28(5):483–488. doi:10.1111/jce.13179
- Deshmukh A, Patel NJ, Pant I, et al. In-hospital complications associated with catheter ablation of atrial fibrillation in the United States between 2000 and 2010: analysis of 93,801 procedures. Circulation 2013; 128(19):2104–2112. doi:10.1161/CIRCULATIONAHA.113.003862
- Cappato R, Calkins H, Chen SA, et al. Worldwide survey on the methods, efficacy, and safety of catheter ablation for human atrial fibrillation. Circulation 2005; 111(9):1100–1105. doi:10.1161/01.CIR.0000157153.30978.67
- Cappato R, Calkins H, Chen SA, et al. Updated worldwide survey on the methods, efficacy, and safety of catheter ablation for human atrial fibrillation. Circ Arrhythm Electrophysiol 2010; 3(1):32–38. doi:10.1161/CIRCEP.109.859116
- Wazni OM, Marrouche NF, Martin DO, et al. Radiofrequency ablation vs antiarrhythmic drugs as first-line treatment of symptomatic atrial fibrillation: a randomized trial. JAMA 2005; 293(21):2634–2640. doi:10.1001/jama.293.21.2634
- Jaïs P, Cauchemez B, Macle L, et al. Catheter ablation versus antiarrhythmic drugs for atrial fibrillation: the A4 study. Circulation 2008; 118(24):2498–2505. doi:10.1161/CIRCULATIONAHA.108.772582
- Brooks AG, Stiles MK, Laborderie J, et al. Outcomes of long-standing persistent atrial fibrillation ablation: a systematic review. Heart Rhythm 2010; 7(6):835–846. doi:10.1016/j.hrthm.2010.01.017
- Packer DL, Lee KL, Mark DB, Robb RA. Catheter Ablation versus Antiarrhythmic Drug Therapy for Atrial Fibrillation Trial, CABANA. http://cabanatrial.org/. Accessed September 10, 2018.
- Scherr D, Sharma K, Dalal D, et al. Incidence and predictors of periprocedural cerebrovascular accident in patients undergoing catheter ablation of atrial fibrillation. J Cardiovasc Electrophysiol 2009; 20(12):1357–1363. doi:10.1111/j.1540-8167.2009.01540.x
- Wazni OM, Rossillo A, Marrouche NF, et al. Embolic events and char formation during pulmonary vein isolation in patients with atrial fibrillation: impact of different anticoagulation regimens and importance of intracardiac echo imaging. J Cardiovasc Electrophysiol 2005; 16(6):576–581. doi:10.1111/j.1540-8167.2005.40480.x
- Hussein AA, Martin DO, Saliba W, et al. Radiofrequency ablation of atrial fibrillation under therapeutic international normalized ratio: a safe and efficacious periprocedural anticoagulation strategy. Heart Rhythm 2009; 6(10):1425–1429. doi:10.1016/j.hrthm.2009.07.007
- Bassiouny M, Saliba W, Rickard J, et al. Use of dabigatran for periprocedural anticoagulation in patients undergoing catheter ablation for atrial fibrillation. Circ Arrhythm Electrophysiol 2013; 6(3):460–466. doi:10.1161/CIRCEP.113.000320
- Koyama T, Tada H, Sekiguchi Y, et al. Prevention of atrial fibrillation recurrence with corticosteroids after radiofrequency catheter ablation: a randomized controlled trial. J Am Coll Cardiol 2010; 56(18):1463–1472. doi:10.1016/j.jacc.2010.04.057
- Oral H, Knight BP, Ozaydin M, et al. Clinical significance of early recurrences of atrial fibrillation after pulmonary vein isolation. J Am Coll Cardiol 2002; 40(1):100–104. pmid:12103262
- Chen W, Liu H, Ling Z, et al. Efficacy of short-term antiarrhythmic drugs use after catheter ablation of atrial fibrillation—a systematic review with meta-analyses and trial sequential analyses of randomized controlled trials. PLoS One 2016; 11(5):e0156121. doi:10.1371/journal.pone.0156121
- Leong-Sit P, Roux JF, Zado E, et al. Antiarrhythmics after ablation of atrial fibrillation (5A Study): six-month follow-up study. Circ Arrhythm Electrophysiol 2011; 4(1):11–14. doi:10.1161/CIRCEP.110.955393
- Roux JF, Zado E, Callans DJ, et al. Antiarrhythmics after ablation of atrial fibrillation (5A Study). Circulation 2009; 120(12):1036–1040. doi:10.1161/CIRCULATIONAHA.108.839639
- Sotomi Y, Inoue K, Ito N, et al. Cause of very late recurrence of atrial fibrillation or flutter after catheter ablation for atrial fibrillation. Am J Cardiol 2013; 111(4):552–556. doi:10.1016/j.amjcard.2012.10.040
- Lee SH, Tai CT, Hsieh MH, et al. Predictors of early and late recurrence of atrial fibrillation after catheter ablation of paroxysmal atrial fibrillation. J Interv Card Electrophysiol. 2004 Jun;10(3):221-6. doi:10.1023/B:JICE.0000026915.02503.92
- Zhang XD, Gu J, Jiang WF, et al. Optimal rhythm-control strategy for recurrent atrial tachycardia after catheter ablation of persistent atrial fibrillation: a randomized clinical trial. Eur Heart J 2014; 35(20):1327–1334. doi:10.1093/eurheartj/ehu017
A 64-year-old man with hypertension but without known structural heart disease presents for a second opinion on management of his atrial fibrillation. The condition was first diagnosed at age 38, when he experienced palpitations and shortness of breath on exertion; at times he also experienced decreased endurance and fatigue without overt palpitations. At first, these episodes occurred about twice a year, and the patient was managed with a beta-blocker for rate control and an oral anticoagulant.
Over the past 10 years, the episodes have become more frequent and longer-lasting and have required frequent cardioversions. He was given flecainide for rhythm control but continued to have frequent episodes, and so about 1 year ago he was switched to amiodarone, which controlled his rhythm better. However, after reading about side effects of amiodarone, he decided to seek a second opinion.
He was evaluated by our team and eventually underwent radiofrequency ablation. During the procedure, he was noted to have diffuse scarring and fibrosis of his left atrium, and afterward he continued to require antiarrhythmic drugs to maintain sinus rhythm.
Should he have been referred sooner? What factors should primary care physicians consider when referring a patient with atrial fibrillation for ablation?
THE EPIDEMIC OF ATRIAL FIBRILLATION
Atrial fibrillation is a large and growing public health problem. In 2010, it was estimated to affect 2.7 to 6.1 million people in the United States, and with the rapid aging of our population, its prevalence is expected to rise to between 5.6 and 12 million by 2050.1–3 It is associated with significant morbidity, poor quality of life, and increased risk of death, heart failure, stroke, and cognitive impairment.
The number of new cases per year has increased over the years despite research and preventive measures, which may reflect aging of the population and increased survival rates in patients with cardiovascular or comorbid conditions.1,4
Thus, atrial fibrillation is one of the most common cardiovascular conditions encountered by primary care physicians and cardiologists, putting them at the forefront of its management. Proper treatment in its early stages and referral to a specialist for advanced management may alter its natural history and improve clinical outcomes.
HOW DOES ATRIAL FIBRILLATION ARISE AND PERSIST?
Much is still unknown about the pathogenesis of atrial fibrillation, but considerable progress has been made in the past few decades, opening the door for clinical ablative strategies.
Multiple wavelet hypothesis
Until the late 1980s, the most widely accepted conceptual mechanism of atrial fibrillation was the multiple wavelet hypothesis developed by Moe et al.5 According to this hypothesis, atrial fibrillation begins with multiple independent wavelets occurring simultaneously and spreading randomly throughout both atria, and it persists if there are a minimum number of coexisting wavelets, increased atrial mass, and heterogeneous conduction delays across the atrial tissue.
The surgical maze procedure, in which a series of incisions arranged in a maze-like pattern is created in the left atrium, was predicated on this model. The theory was that these surgical lesions would compartmentalize the atria into discrete electrical segments and thereby reduce the number of circulating random wavelets.6,7
However, experimental and clinical studies suggest that although randomly propagating wavelets can contribute to maintaining atrial fibrillation, focal triggers are noted in most cases.
Focal triggers
In 1997, Jaïs et al8 observed that atrial fibrillation is often triggered by a rapidly firing ectopic focus and that ablation of that focus can eliminate it. These ectopic foci are often found at or near the ostia of the pulmonary veins or near the superior vena cava.8,9 It is now well established that ectopic foci in the pulmonary veins are crucial triggers that initiate atrial fibrillation.
Trigger-and-substrate theory
The substrate for maintaining atrial fibrillation consists of an abnormal left atrium with heterogeneous fibrosis (scarring) and conduction delays. Any heart disease that increases left atrial pressure could lead to atrial dilation and remodeling, which could be substrates for atrial fibrillation. Extensive atrial remodeling and scarring are associated with progression and persistence of atrial fibrillation and make rhythm control more challenging.
Atrial fibrillation begets atrial fibrillation
As shown in the case above, over time, paroxysmal atrial fibrillation often progresses to persistent and long-standing atrial fibrillation if not aggressively managed initially.
In 1972, Davies and Pomerance10 performed 100 autopsies and found that the people who had had atrial fibrillation for longer than 1 month had lost muscle mass in the sinus node and internodal tract, and their atria were dilated. The study introduced the concept that atrial fibrillation itself causes pathologic changes in the atrium.
Wijffels et al,11 in an experiment in goats, showed that atrial fibrillation produced by rapid bursts of atrial pacing was initially paroxysmal. However, as they continued to induce atrial fibrillation over and over again, it lasted progressively longer until it would persist for more than 24 hours. Thus, in a relatively short time, the atria went from supporting paroxysmal fibrillation to supporting persistent fibrillation.
Atrial fibrillation leads to electrophysiologic and anatomic remodeling in the atrium, which leads to a shorter action potential duration and a shorter refractory period. This in turn makes it easier for atrial fibrillation to persist.12
Because atrial fibrillation tends to progress, intervening early may improve its outcomes. Early ablation has been shown to improve the chances of staying in sinus rhythm in both paroxysmal and persistent atrial fibrillation.13–15
CATHETER ABLATION OF ATRIAL FIBRILLATION
The goal of ablation is to prevent atrial fibrillation by eliminating the trigger that initiates it, altering the arrhythmogenic substrate, or both.
Pulmonary vein isolation
The most common ablation strategy is to electrically isolate the pulmonary veins by creating circumferential lesions around their antra. This creates a nonconducting rim of scar tissue, electrically disconnecting the pulmonary veins from the atrium.
Ablation outside of the pulmonary veins
Because recurrence rates are high in patients with persistent atrial fibrillation who undergo pulmonary vein ablation alone, the search continues for adjunctive strategies to improve outcomes. Although these strategies have a sound rationale based on experimental data and anecdotal evidence in humans, they have not yet been convincingly shown to be helpful in large clinical studies. Nonetheless, it is possible that more extensive substrate ablation—atrial “debulking”—could improve outcomes by reducing the amount of tissue that can fibrillate.
Linear ablation. Creating lines of ablation (as in the maze procedure) isolates different segments of the left atrium. Often, these lines are created along the roof of the left atrium between the right and left upper pulmonary veins and from the mitral valve to the left inferior pulmonary vein. The benefit of linear ablation has not been proven, and gaps in such lines may introduce atrial flutter.
Triggers not in the pulmonary veins. Common sites of nonpulmonary vein triggers include the posterior wall of the left atrium, the superior vena cava, the coronary sinus, and along the ligament of Marshall. Provocative maneuvers such as isoproterenol infusion can help find those triggers, which can then be ablated. A limitation is that there is no protocol proven to reproducibly elicit triggers.
Complex fractionated atrial electrograms are areas in the atrium with highly fractionated, low voltage potentials. They may be critical sites of substrate for atrial fibrillation, and many electrophysiologists target them in patients with persistent atrial fibrillation. But despite initial enthusiasm, doing so has not resulted in better outcomes in persistent atrial fibrillation.
Rotors. Animal studies have shown that atrial fibrillation can be triggered or maintained by localized sources of organized reentrant circuits (rotors) or focal impulses. Recent studies have shown that these electrical rotors and focal sources could potentially be mapped and ablated in humans. But positive results in initial reports have not been reproduced, and this remains an area of controversy.
Our practice. We isolate the pulmonary veins with antral ablations, ablate the posterior wall, and extend the ablation toward the septum and inferior to the right pulmonary veins, with good long-term outcomes.14 The rationale behind ablating the posterior wall is that it shares embryologic origins with the pulmonary veins and may be a common source of triggers in atrial fibrillation.
We do not routinely create empiric ablation lines in the left or right atrium unless the patient has atrial flutter. Empiric ablation lines have not been convincingly shown to provide additional benefit compared with our extensive ablation approach, which involves the posterior wall. Empiric ablation of the appendage or coronary sinus is typically reserved for repeat ablation in patients with recurrent persistent atrial fibrillation.
RATIONALE FOR TREATING ATRIAL FIBRILLATION WITH ABLATION
To control symptoms
At this time, the primary aim of atrial fibrillation ablation is to reduce symptoms and improve quality of life. In theory, ablation could also decrease the risk of stroke, heart failure, and death. However, these outcomes have not been systematically evaluated in any large randomized controlled trial.
To control rhythm and improve survival
Randomized controlled trials of rhythm vs rate control of atrial fibrillation16–18 have failed to demonstrate that restoring sinus rhythm is associated with better survival. All of these trials used antiarrhythmic drugs for rhythm control. However, nonrandomized studies19,20 showed that maintaining sinus rhythm is associated with a significant reduction in mortality rates, whereas the use of antiarrhythmic drugs increased mortality risk.
This suggests that the beneficial effect of restoring sinus rhythm may be offset by adverse effects of antiarrhythmic drugs, and if rhythm control could be achieved by a method other than antiarrhythmic drug therapy, it may be superior to rate control. On the other hand, these data may be affected by residual confounding. This topic deserves further research, but maintaining sinus rhythm is typically preferred whenever possible.
Discontinuing anticoagulation is not a goal at this time
Retrospective studies have reported a low risk of stroke in patients who discontinue anticoagulation several months after undergoing atrial fibrillation ablation.21–23 However, atrial fibrillation can recur, and risk of stroke increases with age.
Therefore, guidelines24 still recommend continuing anticoagulation after ablation. Generally, we do not offer ablation with a goal of discontinuing anticoagulation. That said, stopping anticoagulation may be considered after long-term suppression of paroxysmal atrial fibrillation on a case-by-case basis in patients deemed to be at low risk. Left atrial appendage closure devices may eventually allow concomitant atrial fibrillation ablation and closure of the appendage, so that anticoagulation could then be stopped. This remains a topic of investigation.
Who should be considered for ablation?
There are no absolute age or comorbidity contraindications to ablation. Everyone who has atrial fibrillation deserves, in our opinion, a referral to the electrophysiology clinic.
PROCEDURAL CONSIDERATIONS
Atrial fibrillation ablation is most often performed by electrophysiologists using a minimally invasive endovascular approach. The patient can be under either moderate sedation or general anesthesia; we prefer general anesthesia for patient comfort, safety, and efficacy.
We use an electrogram-based technique to target and eliminate electrical potentials and ensure continuity of ablation sets, with additional guidance by 3-dimensional cardiac mapping systems and intracardiac echocardiography. We also use contact force-sensing catheters to ensure catheter-tissue contact during ablation and to avoid excessive contact, which may enhance the safety of the procedure.
Energy: Hot or cold
Two types of energy can be used for ablation:
Radiofrequency energy (low voltage, high frequency—30 kHz to 1.5 mHz) is delivered to the endocardial surface via a point-source catheter. The radiofrequency energy produces controlled, focal thermal ablation.
In a randomized trial,25 these ablation technologies were shown to be equivalent for preventing recurrences of atrial fibrillation. We use both in our practice. The choice depends primarily on the planned ablation set, given that balloon cryoablation can achieve antral isolation of the pulmonary veins but allows little or no substrate modification.
Improved ablation technology
Contact force-sensing catheters. Radiofrequency ablation catheters are now equipped with a pressure sensor at the tip that measures how hard the catheter is pressing on the heart wall.26,27 In our experience, this has improved the outcomes of ablation procedures, primarily in persistent atrial fibrillation.28
Complications of ablation
Although catheter ablation for atrial fibrillation is safe, it is still one of the most complex electrophysiologic procedures. Improvements in technology and techniques and accumulated experience over the past 15 years have made ablation safer, especially in tertiary care centers. But adverse outcomes are more frequent in low-volume centers.29
Minor procedural complications include pericarditis, complications at the site of vascular access, and anesthesia-related complications. While they do not affect the long-term outcome for the patient, they may increase hospital length of stay and cause temporary inconvenience.
Major complications include cardiac perforation and tamponade, periprocedural stroke, pulmonary vein stenosis, atrioesophageal fistula, phrenic nerve paralysis, major bleeding, myocardial infarction, and death. In a worldwide survey published in 2005, when atrial fibrillation ablation was still novel, the rate of major complications was 6%.30 By 2010, this had declined to 4.5%,31 and the rates of major complications may be significantly lower in more experienced centers.29 In our practice, in 2015, the rate of major complications was 1.3% (unpublished data).
Outcomes of catheter ablation
Clinical outcomes depend on many factors including the type of atrial fibrillation (paroxysmal vs nonparoxysmal), overall health of the atria (atrial size and scarring), patient age and comorbidities, and most importantly, the center’s and operator’s experience.
In randomized controlled trials comparing ablation and antiarrhythmic drug therapy, the efficacy of ablation in maintaining sinus rhythm has been in the range of 66% to 86% vs 16% to 22% for drug therapy,32,33 but these trials have been predominantly in middle-aged white men with paroxysmal atrial fibrillation. These trials also showed that catheter ablation reduced symptoms and improved quality of life. Ablation is less effective in persistent than in paroxysmal atrial fibrillation.34
In a long-term study from our group,14 660 (79.4%) of 831 patients who underwent ablation in 2005 were arrhythmia-free and not on antiarrhythmic drug therapy after a total of 1,019 ablations (an average of 1.2 ablations per patient) at a median of 55 months; 125 patients (15%, 41 with more than 1 ablation) continued to have atrial arrhythmia, controlled with drugs in 87 patients (69.6%). Only 38 patients (4.6%) continued to have drug-resistant atrial fibrillation and were treated with rate control with negative dromotropic agents.
Recent evidence
The largest randomized controlled trial of catheter ablation vs drug therapy for atrial fibrillation (Catheter Ablation Versus Antiarrhythmic Drug Therapy for Atrial Fibrillation [CABANA]) was completed recently, and the results were presented at a national meeting, although they have not yet been published in a peer-reviewed journal.35
A total of 2,204 patients with atrial fibrillation (42.4% paroxysmal, 47.3% persistent, and 10.3% long-standing persistent) were randomized to either ablation or drug therapy. Median follow-up was 4 years. The crossover rate was high—9.2% of those randomized to ablation did not undergo it, and 27.5% of those randomized to drug therapy underwent ablation.
The incidence of the primary end point (a composite of death, disabling stroke, serious bleeding, and cardiac arrest) was not significantly different between the 2 groups in the intention-to-treat analysis; however, given the high crossover rates, the as-treated and per-protocol analyses become important, and as-treated and per-protocol analyses revealed a significant benefit of ablation compared with drug therapy. The hazard ratio (HR) for the primary composite outcome was 0.67 (P = .006) on as-treated analysis and 0.73 (P = .05) on per-protocol analysis. The HR for all-cause mortality was 0.60 (P = .005) on as-treated analysis.
PERIPROCEDURAL CONSIDERATIONS
Periprocedural anticoagulation
The risk of thromboembolism is increased during, immediately following, and for several weeks to months after atrial fibrillation ablation.36,37
During the procedure, the risk is related to transseptal sheath placement, electrode catheters in the left atrium, and char formation on ablation catheters. These risks are mitigated with proper and careful sheath and catheter manipulation, maintenance of bubble-free irrigation through lines and sheaths, use of irrigated catheters, and initiation of heparin before transseptal access. Heparin is also infused during the procedure, with close monitoring of activated clotting time.
Postprocedurally, the transiently increased clotting risk could be due to damaged endothelium from the ablation itself and stunning of atrial tissue, which results in impaired contraction. Damaged endothelium improves as the tissue heals, and the stunning resolves by electrical reverse remodeling with sinus rhythm maintenance.
In view of these risks, the referring physician and electrophysiologist must pay careful attention to anticoagulation before and after ablation.
Before the procedure. It is safe to continue anticoagulation uninterrupted through the procedure.38,39 If the patient is on warfarin, we want the international normalized ratio to be in the therapeutic range when we perform atrial fibrillation ablation, and the patient takes his or her usual dose on the day of the procedure. If taking a direct oral anticoagulant, patients typically skip a dose the day before ablation and again on the morning of the procedure, and resume taking it immediately afterward while in the anesthesia recovery room.
During the procedure, we start heparin before transseptal puncture, adjust it to achieve an activated clotting time of 300 to 400 seconds, and keep it in this range as long as there are sheaths or catheters in the left atrium.
After the procedure. The current guidelines24 recommend that oral anticoagulation be continued without interruption for at least 2 months after the procedure, and in most cases indefinitely, depending on age and comorbidities. The decision to stop anticoagulation after 2 months is typically based on the stroke risk as assessed by the CHA2DS2-VASc score (www.chadsvasc.org) and not on the success of the ablation procedure.
ANTIARRHYTHMIC DRUGS AFTER THE PROCEDURE
Some patients actually experience more atrial fibrillation in the first weeks to months after the procedure. The mechanism in this setting may be different from that causing the arrhythmia in the first place. The causes of early recurrence of atrial arrhythmias include postablation inflammation, temporary autonomic imbalance, and delay of atrial radiofrequency lesion formation.40,41 These arrhythmias may completely resolve as the ablation lesions heal and scars mature.
It has been hypothesized that short-term use of antiarrhythmic drugs after atrial fibrillation ablation is effective in preventing arrhythmias because it alters atrial electrophysiologic characteristics induced by the above transient factors. A recent systematic review of 6 clinical trials showed that short-term use of antiarrhythmic drugs reduces the risk of early arrhythmia recurrence but does not reduce recurrence in the long term.42
In terms of outcomes, any arrhythmias that occur in the first 3 months do not necessarily affect long-term success. This is referred to as the “blanking period.” However, generally speaking, it is preferable to maintain sinus rhythm during that time to avoid further anatomic or electrical left atrial adverse remodeling. In many situations, patients continue taking the same antiarrhythmic agent or start on antiarrhythmic therapy in the first few months after ablation.43,44
The mechanisms of late recurrence of atrial arrhythmias after ablation are thought to be different from those in early recurrence. Late recurrence has been ascribed to incomplete pulmonary vein isolation, recovery of pulmonary vein-left atrium connections, or recovery of any other lines of ablation created in the procedure.45,46 For late recurrence of atrial arrhythmia, studies and guidelines suggest that repeat ablation may be an option.24,47
PRACTICAL CONSIDERATIONS FOR PROCEDURAL PLANNING
Before the procedure, some electrophysiologists use cardiac computed tomography or magnetic resonance imaging to evaluate the pulmonary vein anatomy. This helps in planning and in selecting the appropriate tools for the procedure.
The patient is asked to fast on the day of the procedure. The procedure can take 3 to 6 hours, depending on the patient’s anatomy and the operator’s technique and experience. It can be performed with the patient under general anesthesia or conscious sedation. Currently, we use general anesthesia most of the time to maximize patient comfort.
After the procedure, our patients must stay in bed for 4 hours and stay overnight for observation. If no complications arise, they are discharged the next day.
A 64-year-old man with hypertension but without known structural heart disease presents for a second opinion on management of his atrial fibrillation. The condition was first diagnosed at age 38, when he experienced palpitations and shortness of breath on exertion; at times he also experienced decreased endurance and fatigue without overt palpitations. At first, these episodes occurred about twice a year, and the patient was managed with a beta-blocker for rate control and an oral anticoagulant.
Over the past 10 years, the episodes have become more frequent and longer-lasting and have required frequent cardioversions. He was given flecainide for rhythm control but continued to have frequent episodes, and so about 1 year ago he was switched to amiodarone, which controlled his rhythm better. However, after reading about side effects of amiodarone, he decided to seek a second opinion.
He was evaluated by our team and eventually underwent radiofrequency ablation. During the procedure, he was noted to have diffuse scarring and fibrosis of his left atrium, and afterward he continued to require antiarrhythmic drugs to maintain sinus rhythm.
Should he have been referred sooner? What factors should primary care physicians consider when referring a patient with atrial fibrillation for ablation?
THE EPIDEMIC OF ATRIAL FIBRILLATION
Atrial fibrillation is a large and growing public health problem. In 2010, it was estimated to affect 2.7 to 6.1 million people in the United States, and with the rapid aging of our population, its prevalence is expected to rise to between 5.6 and 12 million by 2050.1–3 It is associated with significant morbidity, poor quality of life, and increased risk of death, heart failure, stroke, and cognitive impairment.
The number of new cases per year has increased over the years despite research and preventive measures, which may reflect aging of the population and increased survival rates in patients with cardiovascular or comorbid conditions.1,4
Thus, atrial fibrillation is one of the most common cardiovascular conditions encountered by primary care physicians and cardiologists, putting them at the forefront of its management. Proper treatment in its early stages and referral to a specialist for advanced management may alter its natural history and improve clinical outcomes.
HOW DOES ATRIAL FIBRILLATION ARISE AND PERSIST?
Much is still unknown about the pathogenesis of atrial fibrillation, but considerable progress has been made in the past few decades, opening the door for clinical ablative strategies.
Multiple wavelet hypothesis
Until the late 1980s, the most widely accepted conceptual mechanism of atrial fibrillation was the multiple wavelet hypothesis developed by Moe et al.5 According to this hypothesis, atrial fibrillation begins with multiple independent wavelets occurring simultaneously and spreading randomly throughout both atria, and it persists if there are a minimum number of coexisting wavelets, increased atrial mass, and heterogeneous conduction delays across the atrial tissue.
The surgical maze procedure, in which a series of incisions arranged in a maze-like pattern is created in the left atrium, was predicated on this model. The theory was that these surgical lesions would compartmentalize the atria into discrete electrical segments and thereby reduce the number of circulating random wavelets.6,7
However, experimental and clinical studies suggest that although randomly propagating wavelets can contribute to maintaining atrial fibrillation, focal triggers are noted in most cases.
Focal triggers
In 1997, Jaïs et al8 observed that atrial fibrillation is often triggered by a rapidly firing ectopic focus and that ablation of that focus can eliminate it. These ectopic foci are often found at or near the ostia of the pulmonary veins or near the superior vena cava.8,9 It is now well established that ectopic foci in the pulmonary veins are crucial triggers that initiate atrial fibrillation.
Trigger-and-substrate theory
The substrate for maintaining atrial fibrillation consists of an abnormal left atrium with heterogeneous fibrosis (scarring) and conduction delays. Any heart disease that increases left atrial pressure could lead to atrial dilation and remodeling, which could be substrates for atrial fibrillation. Extensive atrial remodeling and scarring are associated with progression and persistence of atrial fibrillation and make rhythm control more challenging.
Atrial fibrillation begets atrial fibrillation
As shown in the case above, over time, paroxysmal atrial fibrillation often progresses to persistent and long-standing atrial fibrillation if not aggressively managed initially.
In 1972, Davies and Pomerance10 performed 100 autopsies and found that the people who had had atrial fibrillation for longer than 1 month had lost muscle mass in the sinus node and internodal tract, and their atria were dilated. The study introduced the concept that atrial fibrillation itself causes pathologic changes in the atrium.
Wijffels et al,11 in an experiment in goats, showed that atrial fibrillation produced by rapid bursts of atrial pacing was initially paroxysmal. However, as they continued to induce atrial fibrillation over and over again, it lasted progressively longer until it would persist for more than 24 hours. Thus, in a relatively short time, the atria went from supporting paroxysmal fibrillation to supporting persistent fibrillation.
Atrial fibrillation leads to electrophysiologic and anatomic remodeling in the atrium, which leads to a shorter action potential duration and a shorter refractory period. This in turn makes it easier for atrial fibrillation to persist.12
Because atrial fibrillation tends to progress, intervening early may improve its outcomes. Early ablation has been shown to improve the chances of staying in sinus rhythm in both paroxysmal and persistent atrial fibrillation.13–15
CATHETER ABLATION OF ATRIAL FIBRILLATION
The goal of ablation is to prevent atrial fibrillation by eliminating the trigger that initiates it, altering the arrhythmogenic substrate, or both.
Pulmonary vein isolation
The most common ablation strategy is to electrically isolate the pulmonary veins by creating circumferential lesions around their antra. This creates a nonconducting rim of scar tissue, electrically disconnecting the pulmonary veins from the atrium.
Ablation outside of the pulmonary veins
Because recurrence rates are high in patients with persistent atrial fibrillation who undergo pulmonary vein ablation alone, the search continues for adjunctive strategies to improve outcomes. Although these strategies have a sound rationale based on experimental data and anecdotal evidence in humans, they have not yet been convincingly shown to be helpful in large clinical studies. Nonetheless, it is possible that more extensive substrate ablation—atrial “debulking”—could improve outcomes by reducing the amount of tissue that can fibrillate.
Linear ablation. Creating lines of ablation (as in the maze procedure) isolates different segments of the left atrium. Often, these lines are created along the roof of the left atrium between the right and left upper pulmonary veins and from the mitral valve to the left inferior pulmonary vein. The benefit of linear ablation has not been proven, and gaps in such lines may introduce atrial flutter.
Triggers not in the pulmonary veins. Common sites of nonpulmonary vein triggers include the posterior wall of the left atrium, the superior vena cava, the coronary sinus, and along the ligament of Marshall. Provocative maneuvers such as isoproterenol infusion can help find those triggers, which can then be ablated. A limitation is that there is no protocol proven to reproducibly elicit triggers.
Complex fractionated atrial electrograms are areas in the atrium with highly fractionated, low voltage potentials. They may be critical sites of substrate for atrial fibrillation, and many electrophysiologists target them in patients with persistent atrial fibrillation. But despite initial enthusiasm, doing so has not resulted in better outcomes in persistent atrial fibrillation.
Rotors. Animal studies have shown that atrial fibrillation can be triggered or maintained by localized sources of organized reentrant circuits (rotors) or focal impulses. Recent studies have shown that these electrical rotors and focal sources could potentially be mapped and ablated in humans. But positive results in initial reports have not been reproduced, and this remains an area of controversy.
Our practice. We isolate the pulmonary veins with antral ablations, ablate the posterior wall, and extend the ablation toward the septum and inferior to the right pulmonary veins, with good long-term outcomes.14 The rationale behind ablating the posterior wall is that it shares embryologic origins with the pulmonary veins and may be a common source of triggers in atrial fibrillation.
We do not routinely create empiric ablation lines in the left or right atrium unless the patient has atrial flutter. Empiric ablation lines have not been convincingly shown to provide additional benefit compared with our extensive ablation approach, which involves the posterior wall. Empiric ablation of the appendage or coronary sinus is typically reserved for repeat ablation in patients with recurrent persistent atrial fibrillation.
RATIONALE FOR TREATING ATRIAL FIBRILLATION WITH ABLATION
To control symptoms
At this time, the primary aim of atrial fibrillation ablation is to reduce symptoms and improve quality of life. In theory, ablation could also decrease the risk of stroke, heart failure, and death. However, these outcomes have not been systematically evaluated in any large randomized controlled trial.
To control rhythm and improve survival
Randomized controlled trials of rhythm vs rate control of atrial fibrillation16–18 have failed to demonstrate that restoring sinus rhythm is associated with better survival. All of these trials used antiarrhythmic drugs for rhythm control. However, nonrandomized studies19,20 showed that maintaining sinus rhythm is associated with a significant reduction in mortality rates, whereas the use of antiarrhythmic drugs increased mortality risk.
This suggests that the beneficial effect of restoring sinus rhythm may be offset by adverse effects of antiarrhythmic drugs, and if rhythm control could be achieved by a method other than antiarrhythmic drug therapy, it may be superior to rate control. On the other hand, these data may be affected by residual confounding. This topic deserves further research, but maintaining sinus rhythm is typically preferred whenever possible.
Discontinuing anticoagulation is not a goal at this time
Retrospective studies have reported a low risk of stroke in patients who discontinue anticoagulation several months after undergoing atrial fibrillation ablation.21–23 However, atrial fibrillation can recur, and risk of stroke increases with age.
Therefore, guidelines24 still recommend continuing anticoagulation after ablation. Generally, we do not offer ablation with a goal of discontinuing anticoagulation. That said, stopping anticoagulation may be considered after long-term suppression of paroxysmal atrial fibrillation on a case-by-case basis in patients deemed to be at low risk. Left atrial appendage closure devices may eventually allow concomitant atrial fibrillation ablation and closure of the appendage, so that anticoagulation could then be stopped. This remains a topic of investigation.
Who should be considered for ablation?
There are no absolute age or comorbidity contraindications to ablation. Everyone who has atrial fibrillation deserves, in our opinion, a referral to the electrophysiology clinic.
PROCEDURAL CONSIDERATIONS
Atrial fibrillation ablation is most often performed by electrophysiologists using a minimally invasive endovascular approach. The patient can be under either moderate sedation or general anesthesia; we prefer general anesthesia for patient comfort, safety, and efficacy.
We use an electrogram-based technique to target and eliminate electrical potentials and ensure continuity of ablation sets, with additional guidance by 3-dimensional cardiac mapping systems and intracardiac echocardiography. We also use contact force-sensing catheters to ensure catheter-tissue contact during ablation and to avoid excessive contact, which may enhance the safety of the procedure.
Energy: Hot or cold
Two types of energy can be used for ablation:
Radiofrequency energy (low voltage, high frequency—30 kHz to 1.5 mHz) is delivered to the endocardial surface via a point-source catheter. The radiofrequency energy produces controlled, focal thermal ablation.
In a randomized trial,25 these ablation technologies were shown to be equivalent for preventing recurrences of atrial fibrillation. We use both in our practice. The choice depends primarily on the planned ablation set, given that balloon cryoablation can achieve antral isolation of the pulmonary veins but allows little or no substrate modification.
Improved ablation technology
Contact force-sensing catheters. Radiofrequency ablation catheters are now equipped with a pressure sensor at the tip that measures how hard the catheter is pressing on the heart wall.26,27 In our experience, this has improved the outcomes of ablation procedures, primarily in persistent atrial fibrillation.28
Complications of ablation
Although catheter ablation for atrial fibrillation is safe, it is still one of the most complex electrophysiologic procedures. Improvements in technology and techniques and accumulated experience over the past 15 years have made ablation safer, especially in tertiary care centers. But adverse outcomes are more frequent in low-volume centers.29
Minor procedural complications include pericarditis, complications at the site of vascular access, and anesthesia-related complications. While they do not affect the long-term outcome for the patient, they may increase hospital length of stay and cause temporary inconvenience.
Major complications include cardiac perforation and tamponade, periprocedural stroke, pulmonary vein stenosis, atrioesophageal fistula, phrenic nerve paralysis, major bleeding, myocardial infarction, and death. In a worldwide survey published in 2005, when atrial fibrillation ablation was still novel, the rate of major complications was 6%.30 By 2010, this had declined to 4.5%,31 and the rates of major complications may be significantly lower in more experienced centers.29 In our practice, in 2015, the rate of major complications was 1.3% (unpublished data).
Outcomes of catheter ablation
Clinical outcomes depend on many factors including the type of atrial fibrillation (paroxysmal vs nonparoxysmal), overall health of the atria (atrial size and scarring), patient age and comorbidities, and most importantly, the center’s and operator’s experience.
In randomized controlled trials comparing ablation and antiarrhythmic drug therapy, the efficacy of ablation in maintaining sinus rhythm has been in the range of 66% to 86% vs 16% to 22% for drug therapy,32,33 but these trials have been predominantly in middle-aged white men with paroxysmal atrial fibrillation. These trials also showed that catheter ablation reduced symptoms and improved quality of life. Ablation is less effective in persistent than in paroxysmal atrial fibrillation.34
In a long-term study from our group,14 660 (79.4%) of 831 patients who underwent ablation in 2005 were arrhythmia-free and not on antiarrhythmic drug therapy after a total of 1,019 ablations (an average of 1.2 ablations per patient) at a median of 55 months; 125 patients (15%, 41 with more than 1 ablation) continued to have atrial arrhythmia, controlled with drugs in 87 patients (69.6%). Only 38 patients (4.6%) continued to have drug-resistant atrial fibrillation and were treated with rate control with negative dromotropic agents.
Recent evidence
The largest randomized controlled trial of catheter ablation vs drug therapy for atrial fibrillation (Catheter Ablation Versus Antiarrhythmic Drug Therapy for Atrial Fibrillation [CABANA]) was completed recently, and the results were presented at a national meeting, although they have not yet been published in a peer-reviewed journal.35
A total of 2,204 patients with atrial fibrillation (42.4% paroxysmal, 47.3% persistent, and 10.3% long-standing persistent) were randomized to either ablation or drug therapy. Median follow-up was 4 years. The crossover rate was high—9.2% of those randomized to ablation did not undergo it, and 27.5% of those randomized to drug therapy underwent ablation.
The incidence of the primary end point (a composite of death, disabling stroke, serious bleeding, and cardiac arrest) was not significantly different between the 2 groups in the intention-to-treat analysis; however, given the high crossover rates, the as-treated and per-protocol analyses become important, and as-treated and per-protocol analyses revealed a significant benefit of ablation compared with drug therapy. The hazard ratio (HR) for the primary composite outcome was 0.67 (P = .006) on as-treated analysis and 0.73 (P = .05) on per-protocol analysis. The HR for all-cause mortality was 0.60 (P = .005) on as-treated analysis.
PERIPROCEDURAL CONSIDERATIONS
Periprocedural anticoagulation
The risk of thromboembolism is increased during, immediately following, and for several weeks to months after atrial fibrillation ablation.36,37
During the procedure, the risk is related to transseptal sheath placement, electrode catheters in the left atrium, and char formation on ablation catheters. These risks are mitigated with proper and careful sheath and catheter manipulation, maintenance of bubble-free irrigation through lines and sheaths, use of irrigated catheters, and initiation of heparin before transseptal access. Heparin is also infused during the procedure, with close monitoring of activated clotting time.
Postprocedurally, the transiently increased clotting risk could be due to damaged endothelium from the ablation itself and stunning of atrial tissue, which results in impaired contraction. Damaged endothelium improves as the tissue heals, and the stunning resolves by electrical reverse remodeling with sinus rhythm maintenance.
In view of these risks, the referring physician and electrophysiologist must pay careful attention to anticoagulation before and after ablation.
Before the procedure. It is safe to continue anticoagulation uninterrupted through the procedure.38,39 If the patient is on warfarin, we want the international normalized ratio to be in the therapeutic range when we perform atrial fibrillation ablation, and the patient takes his or her usual dose on the day of the procedure. If taking a direct oral anticoagulant, patients typically skip a dose the day before ablation and again on the morning of the procedure, and resume taking it immediately afterward while in the anesthesia recovery room.
During the procedure, we start heparin before transseptal puncture, adjust it to achieve an activated clotting time of 300 to 400 seconds, and keep it in this range as long as there are sheaths or catheters in the left atrium.
After the procedure. The current guidelines24 recommend that oral anticoagulation be continued without interruption for at least 2 months after the procedure, and in most cases indefinitely, depending on age and comorbidities. The decision to stop anticoagulation after 2 months is typically based on the stroke risk as assessed by the CHA2DS2-VASc score (www.chadsvasc.org) and not on the success of the ablation procedure.
ANTIARRHYTHMIC DRUGS AFTER THE PROCEDURE
Some patients actually experience more atrial fibrillation in the first weeks to months after the procedure. The mechanism in this setting may be different from that causing the arrhythmia in the first place. The causes of early recurrence of atrial arrhythmias include postablation inflammation, temporary autonomic imbalance, and delay of atrial radiofrequency lesion formation.40,41 These arrhythmias may completely resolve as the ablation lesions heal and scars mature.
It has been hypothesized that short-term use of antiarrhythmic drugs after atrial fibrillation ablation is effective in preventing arrhythmias because it alters atrial electrophysiologic characteristics induced by the above transient factors. A recent systematic review of 6 clinical trials showed that short-term use of antiarrhythmic drugs reduces the risk of early arrhythmia recurrence but does not reduce recurrence in the long term.42
In terms of outcomes, any arrhythmias that occur in the first 3 months do not necessarily affect long-term success. This is referred to as the “blanking period.” However, generally speaking, it is preferable to maintain sinus rhythm during that time to avoid further anatomic or electrical left atrial adverse remodeling. In many situations, patients continue taking the same antiarrhythmic agent or start on antiarrhythmic therapy in the first few months after ablation.43,44
The mechanisms of late recurrence of atrial arrhythmias after ablation are thought to be different from those in early recurrence. Late recurrence has been ascribed to incomplete pulmonary vein isolation, recovery of pulmonary vein-left atrium connections, or recovery of any other lines of ablation created in the procedure.45,46 For late recurrence of atrial arrhythmia, studies and guidelines suggest that repeat ablation may be an option.24,47
PRACTICAL CONSIDERATIONS FOR PROCEDURAL PLANNING
Before the procedure, some electrophysiologists use cardiac computed tomography or magnetic resonance imaging to evaluate the pulmonary vein anatomy. This helps in planning and in selecting the appropriate tools for the procedure.
The patient is asked to fast on the day of the procedure. The procedure can take 3 to 6 hours, depending on the patient’s anatomy and the operator’s technique and experience. It can be performed with the patient under general anesthesia or conscious sedation. Currently, we use general anesthesia most of the time to maximize patient comfort.
After the procedure, our patients must stay in bed for 4 hours and stay overnight for observation. If no complications arise, they are discharged the next day.
- Go AS. The epidemiology of atrial fibrillation in elderly persons: the tip of the iceberg. Am J Geriatr Cardiol 2005; 14(2):56–61. pmid:15785146
- Go AS, Hylek EM, Phillips KA, et al. Prevalence of diagnosed atrial fibrillation in adults: national implications for rhythm management and stroke prevention: the AnTicoagulation and Risk Factors in Atrial Fibrillation (ATRIA) Study. JAMA 2001; 285(18):2370–2375. pmid:11343485
- Miyasaka Y, Barnes ME, Gersh BJ, et al. Secular trends in incidence of atrial fibrillation in Olmsted County, Minnesota, 1980 to 2000, and implications on the projections for future prevalence. Circulation 2006; 114(2):119–125. doi:10.1161/CIRCULATIONAHA.105.595140
- Piccini JP, Hammill BG, Sinner MF, et al. Incidence and prevalence of atrial fibrillation and associated mortality among Medicare beneficiaries, 1993–2007. Circ Cardiovasc Qual Outcomes 2012; 5(1):85–93. doi:10.1161/CIRCOUTCOMES.111.962688
- Moe GK, Rheinboldt WC, Abildskov JA. A computer model of atrial fibrillation. Am Heart J 1964; 67:200–220. pmid:14118488
- Cox JL, Schuessler RB, Boineau JP. The surgical treatment of atrial fibrillation. I. Summary of the current concepts of the mechanisms of atrial flutter and atrial fibrillation. J Thorac Cardiovasc Surg 1991; 101(3):402–405. pmid:1999933
- Cox JL, Schuessler RB, D’Agostino HJ Jr, et al. The surgical treatment of atrial fibrillation. III. Development of a definitive surgical procedure. J Thorac Cardiovasc Surg 1991; 101(4):569–583. pmid:2008095
- Jaïs P, Haïssaguerre M, Shah DC, et al. A focal source of atrial fibrillation treated by discrete radiofrequency ablation. Circulation 1997; 95(3):572–576. pmid:9024141
- Haïssaguerre M, Jaïs P, Shah DC, et al. Spontaneous initiation of atrial fibrillation by ectopic beats originating in the pulmonary veins. N Engl J Med 1998; 339(10):659–666. doi:10.1056/NEJM199809033391003
- Davies MJ, Pomerance A. Pathology of atrial fibrillation in man. Br Heart J 1972; 34(5):520–525. pmid:5031645
- Wijffels MC, Kirchhof CJ, Dorland R, Allessie MA. Atrial fibrillation begets atrial fibrillation. A study in awake chronically instrumented goats. Circulation 1995; 92(7):1954–1968. pmid:7671380
- Nattel S. New ideas about atrial fibrillation 50 years on. Nature 2002; 415(6868):219–226. doi:10.1038/415219a
- Medi C, Sparks PB, Morton JB, et al. Pulmonary vein antral isolation for paroxysmal atrial fibrillation: results from long-term follow-up. J Cardiovasc Electrophysiol 2011; 22(2):137–141. doi:10.1111/j.1540-8167.2010.01885.x
- Hussein AA, Saliba WI, Martin DO, et al. Natural history and long-term outcomes of ablated atrial fibrillation. Circ Arrhythm Electrophysiol 2011; 4(3):271–278. doi:10.1161/CIRCEP.111.962100
- Hussein AA, Saliba WI, Barakat A, et al. Radiofrequency ablation of persistent atrial fibrillation: diagnosis-to-ablation time, markers of pathways of atrial remodeling, and outcomes. Circ Arrhythm Electrophysiol 2016; 9(1):e003669. doi:10.1161/CIRCEP.115.003669
- Carlsson J, Miketic S, Windeler J, et al. Randomized trial of rate-control versus rhythm-control in persistent atrial fibrillation: the Strategies of Treatment of Atrial Fibrillation (STAF) study. J Am Coll Cardiol 2003; 41(10):1690–1696. pmid:12767648
- Van Gelder IC, Hagens VE, Bosker HA, et al; Rate Control versus Electrical Cardioversion for Persistent Atrial Fibrillation Study Group. A comparison of rate control and rhythm control in patients with recurrent persistent atrial fibrillation. N Engl J Med 2002; 347(23):1834–1840. doi:10.1056/NEJMoa021375
- Wyse DG, Waldo AL, DiMarco JP, et al; Atrial Fibrillation Follow-up Investigation of Rhythm Management (AFFIRM) Investigators. A comparison of rate control and rhythm control in patients with atrial fibrillation. N Engl J Med 2002; 347(23):1825–1833. doi:10.1056/NEJMoa021328
- Hagens VE, Crijns HJ, Van Veldhuisen DJ, et al; RAte Control versus Electrical cardioversion for persistent atrial fibrillation study group. Rate control versus rhythm control for patients with persistent atrial fibrillation with mild to moderate heart failure: results from the RAte Control versus Electrical cardioversion (RACE) study. Am Heart J 2005; 149(6):1106–111. doi:10.1016/j.ahj.2004.11.030
- Pedersen OD, Bagger H, Keller N, Marchant B, Køber L, Torp-Pedersen C. Efficacy of dofetilide in the treatment of atrial fibrillation-flutter in patients with reduced left ventricular function: a Danish investigations of arrhythmia and mortality on dofetilide (diamond) substudy. Circulation 2001; 104(3):292–296. pmid:11457747
- Guiot A, Jongnarangsin K, Chugh A, et al. Anticoagulant therapy and risk of cerebrovascular events after catheter ablation of atrial fibrillation in the elderly. J Cardiovasc Electrophysiol 2012; 23(1):36–43. doi:10.1111/j.1540-8167.2011.02141.x
- Oral H, Chugh A, Ozaydin M, et al. Risk of thromboembolic events after percutaneous left atrial radiofrequency ablation of atrial fibrillation. Circulation 2006; 114(8):759–765. doi:10.1161/CIRCULATIONAHA.106.641225
- Themistoclakis S, Corrado A, Marchlinski FE, et al. The risk of thromboembolism and need for oral anticoagulation after successful atrial fibrillation ablation. J Am Coll Cardiol 2010; 55(8):735–743. doi:10.1016/j.jacc.2009.11.039
- Calkins H, Hindricks G, Cappato R, et al. 2017 HRS/EHRA/ECAS/APHRS/SOLAECE expert consensus statement on catheter and surgical ablation of atrial fibrillation: executive summary. J Arrhythm 2017; 33(5):369–409. doi:10.1016/j.joa.2017.08.001
- Kuck KH, Brugada J, Fürnkranz A, et al; FIRE AND ICE Investigators. Cryoballoon or radiofrequency ablation for paroxysmal atrial fibrillation. N Engl J Med 2016; 374(23):2235–2245. doi:10.1056/NEJMoa1602014
- Reddy VY, Dukkipati SR, Neuzil P, et al. Randomized, controlled trial of the safety and effectiveness of a contact force-sensing irrigated catheter for ablation of paroxysmal atrial fibrillation: results of the TactiCath Contact Force Ablation Catheter Study for Atrial Fibrillation (TOCCASTAR) Study. Circulation 2015; 132(10):907–915. doi:10.1161/CIRCULATIONAHA.114.014092
- Natale A, Reddy VY, Monir G, et al. Paroxysmal AF catheter ablation with a contact force sensing catheter: results of the prospective, multicenter SMART-AF trial. J Am Coll Cardiol 2014; 64(7):647–656. doi:10.1016/j.jacc.2014.04.072
- Hussein AA, Barakat AF, Saliba WI, et al. Persistent atrial fibrillation ablation with or without contact force sensing. J Cardiovasc Electrophysiol 2017; 28(5):483–488. doi:10.1111/jce.13179
- Deshmukh A, Patel NJ, Pant I, et al. In-hospital complications associated with catheter ablation of atrial fibrillation in the United States between 2000 and 2010: analysis of 93,801 procedures. Circulation 2013; 128(19):2104–2112. doi:10.1161/CIRCULATIONAHA.113.003862
- Cappato R, Calkins H, Chen SA, et al. Worldwide survey on the methods, efficacy, and safety of catheter ablation for human atrial fibrillation. Circulation 2005; 111(9):1100–1105. doi:10.1161/01.CIR.0000157153.30978.67
- Cappato R, Calkins H, Chen SA, et al. Updated worldwide survey on the methods, efficacy, and safety of catheter ablation for human atrial fibrillation. Circ Arrhythm Electrophysiol 2010; 3(1):32–38. doi:10.1161/CIRCEP.109.859116
- Wazni OM, Marrouche NF, Martin DO, et al. Radiofrequency ablation vs antiarrhythmic drugs as first-line treatment of symptomatic atrial fibrillation: a randomized trial. JAMA 2005; 293(21):2634–2640. doi:10.1001/jama.293.21.2634
- Jaïs P, Cauchemez B, Macle L, et al. Catheter ablation versus antiarrhythmic drugs for atrial fibrillation: the A4 study. Circulation 2008; 118(24):2498–2505. doi:10.1161/CIRCULATIONAHA.108.772582
- Brooks AG, Stiles MK, Laborderie J, et al. Outcomes of long-standing persistent atrial fibrillation ablation: a systematic review. Heart Rhythm 2010; 7(6):835–846. doi:10.1016/j.hrthm.2010.01.017
- Packer DL, Lee KL, Mark DB, Robb RA. Catheter Ablation versus Antiarrhythmic Drug Therapy for Atrial Fibrillation Trial, CABANA. http://cabanatrial.org/. Accessed September 10, 2018.
- Scherr D, Sharma K, Dalal D, et al. Incidence and predictors of periprocedural cerebrovascular accident in patients undergoing catheter ablation of atrial fibrillation. J Cardiovasc Electrophysiol 2009; 20(12):1357–1363. doi:10.1111/j.1540-8167.2009.01540.x
- Wazni OM, Rossillo A, Marrouche NF, et al. Embolic events and char formation during pulmonary vein isolation in patients with atrial fibrillation: impact of different anticoagulation regimens and importance of intracardiac echo imaging. J Cardiovasc Electrophysiol 2005; 16(6):576–581. doi:10.1111/j.1540-8167.2005.40480.x
- Hussein AA, Martin DO, Saliba W, et al. Radiofrequency ablation of atrial fibrillation under therapeutic international normalized ratio: a safe and efficacious periprocedural anticoagulation strategy. Heart Rhythm 2009; 6(10):1425–1429. doi:10.1016/j.hrthm.2009.07.007
- Bassiouny M, Saliba W, Rickard J, et al. Use of dabigatran for periprocedural anticoagulation in patients undergoing catheter ablation for atrial fibrillation. Circ Arrhythm Electrophysiol 2013; 6(3):460–466. doi:10.1161/CIRCEP.113.000320
- Koyama T, Tada H, Sekiguchi Y, et al. Prevention of atrial fibrillation recurrence with corticosteroids after radiofrequency catheter ablation: a randomized controlled trial. J Am Coll Cardiol 2010; 56(18):1463–1472. doi:10.1016/j.jacc.2010.04.057
- Oral H, Knight BP, Ozaydin M, et al. Clinical significance of early recurrences of atrial fibrillation after pulmonary vein isolation. J Am Coll Cardiol 2002; 40(1):100–104. pmid:12103262
- Chen W, Liu H, Ling Z, et al. Efficacy of short-term antiarrhythmic drugs use after catheter ablation of atrial fibrillation—a systematic review with meta-analyses and trial sequential analyses of randomized controlled trials. PLoS One 2016; 11(5):e0156121. doi:10.1371/journal.pone.0156121
- Leong-Sit P, Roux JF, Zado E, et al. Antiarrhythmics after ablation of atrial fibrillation (5A Study): six-month follow-up study. Circ Arrhythm Electrophysiol 2011; 4(1):11–14. doi:10.1161/CIRCEP.110.955393
- Roux JF, Zado E, Callans DJ, et al. Antiarrhythmics after ablation of atrial fibrillation (5A Study). Circulation 2009; 120(12):1036–1040. doi:10.1161/CIRCULATIONAHA.108.839639
- Sotomi Y, Inoue K, Ito N, et al. Cause of very late recurrence of atrial fibrillation or flutter after catheter ablation for atrial fibrillation. Am J Cardiol 2013; 111(4):552–556. doi:10.1016/j.amjcard.2012.10.040
- Lee SH, Tai CT, Hsieh MH, et al. Predictors of early and late recurrence of atrial fibrillation after catheter ablation of paroxysmal atrial fibrillation. J Interv Card Electrophysiol. 2004 Jun;10(3):221-6. doi:10.1023/B:JICE.0000026915.02503.92
- Zhang XD, Gu J, Jiang WF, et al. Optimal rhythm-control strategy for recurrent atrial tachycardia after catheter ablation of persistent atrial fibrillation: a randomized clinical trial. Eur Heart J 2014; 35(20):1327–1334. doi:10.1093/eurheartj/ehu017
- Go AS. The epidemiology of atrial fibrillation in elderly persons: the tip of the iceberg. Am J Geriatr Cardiol 2005; 14(2):56–61. pmid:15785146
- Go AS, Hylek EM, Phillips KA, et al. Prevalence of diagnosed atrial fibrillation in adults: national implications for rhythm management and stroke prevention: the AnTicoagulation and Risk Factors in Atrial Fibrillation (ATRIA) Study. JAMA 2001; 285(18):2370–2375. pmid:11343485
- Miyasaka Y, Barnes ME, Gersh BJ, et al. Secular trends in incidence of atrial fibrillation in Olmsted County, Minnesota, 1980 to 2000, and implications on the projections for future prevalence. Circulation 2006; 114(2):119–125. doi:10.1161/CIRCULATIONAHA.105.595140
- Piccini JP, Hammill BG, Sinner MF, et al. Incidence and prevalence of atrial fibrillation and associated mortality among Medicare beneficiaries, 1993–2007. Circ Cardiovasc Qual Outcomes 2012; 5(1):85–93. doi:10.1161/CIRCOUTCOMES.111.962688
- Moe GK, Rheinboldt WC, Abildskov JA. A computer model of atrial fibrillation. Am Heart J 1964; 67:200–220. pmid:14118488
- Cox JL, Schuessler RB, Boineau JP. The surgical treatment of atrial fibrillation. I. Summary of the current concepts of the mechanisms of atrial flutter and atrial fibrillation. J Thorac Cardiovasc Surg 1991; 101(3):402–405. pmid:1999933
- Cox JL, Schuessler RB, D’Agostino HJ Jr, et al. The surgical treatment of atrial fibrillation. III. Development of a definitive surgical procedure. J Thorac Cardiovasc Surg 1991; 101(4):569–583. pmid:2008095
- Jaïs P, Haïssaguerre M, Shah DC, et al. A focal source of atrial fibrillation treated by discrete radiofrequency ablation. Circulation 1997; 95(3):572–576. pmid:9024141
- Haïssaguerre M, Jaïs P, Shah DC, et al. Spontaneous initiation of atrial fibrillation by ectopic beats originating in the pulmonary veins. N Engl J Med 1998; 339(10):659–666. doi:10.1056/NEJM199809033391003
- Davies MJ, Pomerance A. Pathology of atrial fibrillation in man. Br Heart J 1972; 34(5):520–525. pmid:5031645
- Wijffels MC, Kirchhof CJ, Dorland R, Allessie MA. Atrial fibrillation begets atrial fibrillation. A study in awake chronically instrumented goats. Circulation 1995; 92(7):1954–1968. pmid:7671380
- Nattel S. New ideas about atrial fibrillation 50 years on. Nature 2002; 415(6868):219–226. doi:10.1038/415219a
- Medi C, Sparks PB, Morton JB, et al. Pulmonary vein antral isolation for paroxysmal atrial fibrillation: results from long-term follow-up. J Cardiovasc Electrophysiol 2011; 22(2):137–141. doi:10.1111/j.1540-8167.2010.01885.x
- Hussein AA, Saliba WI, Martin DO, et al. Natural history and long-term outcomes of ablated atrial fibrillation. Circ Arrhythm Electrophysiol 2011; 4(3):271–278. doi:10.1161/CIRCEP.111.962100
- Hussein AA, Saliba WI, Barakat A, et al. Radiofrequency ablation of persistent atrial fibrillation: diagnosis-to-ablation time, markers of pathways of atrial remodeling, and outcomes. Circ Arrhythm Electrophysiol 2016; 9(1):e003669. doi:10.1161/CIRCEP.115.003669
- Carlsson J, Miketic S, Windeler J, et al. Randomized trial of rate-control versus rhythm-control in persistent atrial fibrillation: the Strategies of Treatment of Atrial Fibrillation (STAF) study. J Am Coll Cardiol 2003; 41(10):1690–1696. pmid:12767648
- Van Gelder IC, Hagens VE, Bosker HA, et al; Rate Control versus Electrical Cardioversion for Persistent Atrial Fibrillation Study Group. A comparison of rate control and rhythm control in patients with recurrent persistent atrial fibrillation. N Engl J Med 2002; 347(23):1834–1840. doi:10.1056/NEJMoa021375
- Wyse DG, Waldo AL, DiMarco JP, et al; Atrial Fibrillation Follow-up Investigation of Rhythm Management (AFFIRM) Investigators. A comparison of rate control and rhythm control in patients with atrial fibrillation. N Engl J Med 2002; 347(23):1825–1833. doi:10.1056/NEJMoa021328
- Hagens VE, Crijns HJ, Van Veldhuisen DJ, et al; RAte Control versus Electrical cardioversion for persistent atrial fibrillation study group. Rate control versus rhythm control for patients with persistent atrial fibrillation with mild to moderate heart failure: results from the RAte Control versus Electrical cardioversion (RACE) study. Am Heart J 2005; 149(6):1106–111. doi:10.1016/j.ahj.2004.11.030
- Pedersen OD, Bagger H, Keller N, Marchant B, Køber L, Torp-Pedersen C. Efficacy of dofetilide in the treatment of atrial fibrillation-flutter in patients with reduced left ventricular function: a Danish investigations of arrhythmia and mortality on dofetilide (diamond) substudy. Circulation 2001; 104(3):292–296. pmid:11457747
- Guiot A, Jongnarangsin K, Chugh A, et al. Anticoagulant therapy and risk of cerebrovascular events after catheter ablation of atrial fibrillation in the elderly. J Cardiovasc Electrophysiol 2012; 23(1):36–43. doi:10.1111/j.1540-8167.2011.02141.x
- Oral H, Chugh A, Ozaydin M, et al. Risk of thromboembolic events after percutaneous left atrial radiofrequency ablation of atrial fibrillation. Circulation 2006; 114(8):759–765. doi:10.1161/CIRCULATIONAHA.106.641225
- Themistoclakis S, Corrado A, Marchlinski FE, et al. The risk of thromboembolism and need for oral anticoagulation after successful atrial fibrillation ablation. J Am Coll Cardiol 2010; 55(8):735–743. doi:10.1016/j.jacc.2009.11.039
- Calkins H, Hindricks G, Cappato R, et al. 2017 HRS/EHRA/ECAS/APHRS/SOLAECE expert consensus statement on catheter and surgical ablation of atrial fibrillation: executive summary. J Arrhythm 2017; 33(5):369–409. doi:10.1016/j.joa.2017.08.001
- Kuck KH, Brugada J, Fürnkranz A, et al; FIRE AND ICE Investigators. Cryoballoon or radiofrequency ablation for paroxysmal atrial fibrillation. N Engl J Med 2016; 374(23):2235–2245. doi:10.1056/NEJMoa1602014
- Reddy VY, Dukkipati SR, Neuzil P, et al. Randomized, controlled trial of the safety and effectiveness of a contact force-sensing irrigated catheter for ablation of paroxysmal atrial fibrillation: results of the TactiCath Contact Force Ablation Catheter Study for Atrial Fibrillation (TOCCASTAR) Study. Circulation 2015; 132(10):907–915. doi:10.1161/CIRCULATIONAHA.114.014092
- Natale A, Reddy VY, Monir G, et al. Paroxysmal AF catheter ablation with a contact force sensing catheter: results of the prospective, multicenter SMART-AF trial. J Am Coll Cardiol 2014; 64(7):647–656. doi:10.1016/j.jacc.2014.04.072
- Hussein AA, Barakat AF, Saliba WI, et al. Persistent atrial fibrillation ablation with or without contact force sensing. J Cardiovasc Electrophysiol 2017; 28(5):483–488. doi:10.1111/jce.13179
- Deshmukh A, Patel NJ, Pant I, et al. In-hospital complications associated with catheter ablation of atrial fibrillation in the United States between 2000 and 2010: analysis of 93,801 procedures. Circulation 2013; 128(19):2104–2112. doi:10.1161/CIRCULATIONAHA.113.003862
- Cappato R, Calkins H, Chen SA, et al. Worldwide survey on the methods, efficacy, and safety of catheter ablation for human atrial fibrillation. Circulation 2005; 111(9):1100–1105. doi:10.1161/01.CIR.0000157153.30978.67
- Cappato R, Calkins H, Chen SA, et al. Updated worldwide survey on the methods, efficacy, and safety of catheter ablation for human atrial fibrillation. Circ Arrhythm Electrophysiol 2010; 3(1):32–38. doi:10.1161/CIRCEP.109.859116
- Wazni OM, Marrouche NF, Martin DO, et al. Radiofrequency ablation vs antiarrhythmic drugs as first-line treatment of symptomatic atrial fibrillation: a randomized trial. JAMA 2005; 293(21):2634–2640. doi:10.1001/jama.293.21.2634
- Jaïs P, Cauchemez B, Macle L, et al. Catheter ablation versus antiarrhythmic drugs for atrial fibrillation: the A4 study. Circulation 2008; 118(24):2498–2505. doi:10.1161/CIRCULATIONAHA.108.772582
- Brooks AG, Stiles MK, Laborderie J, et al. Outcomes of long-standing persistent atrial fibrillation ablation: a systematic review. Heart Rhythm 2010; 7(6):835–846. doi:10.1016/j.hrthm.2010.01.017
- Packer DL, Lee KL, Mark DB, Robb RA. Catheter Ablation versus Antiarrhythmic Drug Therapy for Atrial Fibrillation Trial, CABANA. http://cabanatrial.org/. Accessed September 10, 2018.
- Scherr D, Sharma K, Dalal D, et al. Incidence and predictors of periprocedural cerebrovascular accident in patients undergoing catheter ablation of atrial fibrillation. J Cardiovasc Electrophysiol 2009; 20(12):1357–1363. doi:10.1111/j.1540-8167.2009.01540.x
- Wazni OM, Rossillo A, Marrouche NF, et al. Embolic events and char formation during pulmonary vein isolation in patients with atrial fibrillation: impact of different anticoagulation regimens and importance of intracardiac echo imaging. J Cardiovasc Electrophysiol 2005; 16(6):576–581. doi:10.1111/j.1540-8167.2005.40480.x
- Hussein AA, Martin DO, Saliba W, et al. Radiofrequency ablation of atrial fibrillation under therapeutic international normalized ratio: a safe and efficacious periprocedural anticoagulation strategy. Heart Rhythm 2009; 6(10):1425–1429. doi:10.1016/j.hrthm.2009.07.007
- Bassiouny M, Saliba W, Rickard J, et al. Use of dabigatran for periprocedural anticoagulation in patients undergoing catheter ablation for atrial fibrillation. Circ Arrhythm Electrophysiol 2013; 6(3):460–466. doi:10.1161/CIRCEP.113.000320
- Koyama T, Tada H, Sekiguchi Y, et al. Prevention of atrial fibrillation recurrence with corticosteroids after radiofrequency catheter ablation: a randomized controlled trial. J Am Coll Cardiol 2010; 56(18):1463–1472. doi:10.1016/j.jacc.2010.04.057
- Oral H, Knight BP, Ozaydin M, et al. Clinical significance of early recurrences of atrial fibrillation after pulmonary vein isolation. J Am Coll Cardiol 2002; 40(1):100–104. pmid:12103262
- Chen W, Liu H, Ling Z, et al. Efficacy of short-term antiarrhythmic drugs use after catheter ablation of atrial fibrillation—a systematic review with meta-analyses and trial sequential analyses of randomized controlled trials. PLoS One 2016; 11(5):e0156121. doi:10.1371/journal.pone.0156121
- Leong-Sit P, Roux JF, Zado E, et al. Antiarrhythmics after ablation of atrial fibrillation (5A Study): six-month follow-up study. Circ Arrhythm Electrophysiol 2011; 4(1):11–14. doi:10.1161/CIRCEP.110.955393
- Roux JF, Zado E, Callans DJ, et al. Antiarrhythmics after ablation of atrial fibrillation (5A Study). Circulation 2009; 120(12):1036–1040. doi:10.1161/CIRCULATIONAHA.108.839639
- Sotomi Y, Inoue K, Ito N, et al. Cause of very late recurrence of atrial fibrillation or flutter after catheter ablation for atrial fibrillation. Am J Cardiol 2013; 111(4):552–556. doi:10.1016/j.amjcard.2012.10.040
- Lee SH, Tai CT, Hsieh MH, et al. Predictors of early and late recurrence of atrial fibrillation after catheter ablation of paroxysmal atrial fibrillation. J Interv Card Electrophysiol. 2004 Jun;10(3):221-6. doi:10.1023/B:JICE.0000026915.02503.92
- Zhang XD, Gu J, Jiang WF, et al. Optimal rhythm-control strategy for recurrent atrial tachycardia after catheter ablation of persistent atrial fibrillation: a randomized clinical trial. Eur Heart J 2014; 35(20):1327–1334. doi:10.1093/eurheartj/ehu017
KEY POINTS
- Atrial fibrillation is increasing in prevalence with the aging of the US population and is associated with worsening quality of life and increased risk of stroke, heart failure, and death.
- Atrial fibrillation results in adverse atrial remodeling and fibrosis, eventually leading to persistence of the arrhythmia and making rhythm control difficult.
- Catheter ablation has evolved to be a safe procedure with technologic advancements, especially in experienced tertiary care centers.
- The primary aim of atrial fibrillation ablation is to reduce symptoms and improve quality of life. In theory, it could also decrease the risk of stroke, heart failure, and death, but these outcomes have not been systematically evaluated in a large randomized controlled trial.
When stroke runs in the family
A 54-year-old man presented to our hospital with acute-onset left-sided weakness and right facial droop. Three days earlier he had also had migraine-like headaches, which he had never experienced before. He also reported a change in behavior during the past week, which his family had described as inappropriate laughter.
He had no history of hypertension, diabetes, or dyslipidemia. He did not smoke or drink alcohol. However, he had an extensive family history of stroke. His mother had a stroke at age 50, his brother a stroke at age 57, and his sister had been admitted for a stroke 1 month earlier at the age of 52.
On examination, he had weakness of the left arm and leg, right facial droop, and hyperactive reflexes on the left side. He had no sensory or cerebellar deficits. He had episodes of laughter during the examination.
We learned that the patient’s sister had undergone a workup showing mutations in the NOTCH3 gene and a skin biopsy study consistent with CADASIL.
Our patient was started on antiplatelet and high-intensity statin therapy. His symptoms improved, and he was discharged to an acute inpatient rehabilitation facility. He was referred to a CADASIL registry.
STROKE AND HEREDITY
CADASIL is a rare hereditary vascular disorder inherited in an autosomal dominant manner. It is the most common inherited form of small-vessel disease and results from a mutation in the NOTCH3 gene that leads to degeneration of smooth muscle in cerebral blood vessels. It can manifest as migraine with aura, vascular dementia, cognitive impairment, or ischemic stroke.
The diagnosis is based on a clinical picture that typically includes stroke at a young age (age 40 to 50) in the absence of stroke risk factors, or frequent lacunar infarction episodes that can manifest as migraine, lacunar infarct, or dementia.1 Some patients, such as ours, may have subtle nonspecific behavioral changes such as inappropriate laughter, which may herald the development of an infarct.
Characteristic findings on MRI are white matter hyperintensities that tend to be bilateral and symmetrical in the periventricular areas. Symmetrical involvement in the temporal lobes has high sensitivity and specificity for CADASIL.2 Biopsy study of the skin, muscle, or sural nerve shows small-vessel changes that include thickening of the media, granular material positive on periodic acid-Schiff staining, and narrowing of the lumen. However, the gold standard for diagnosis is confirmation of the NOTCH3 mutation on chromosome 19.1,2
There is no known treatment for CADASIL.
- Davous P. CADASIL: a review with proposed diagnostic criteria. Eur J Neurol 1998; 5(3):219–233. pmid:10210836
- Stojanov D, Vojinovic S, Aracki-Trenkic A, et al. Imaging characteristics of cerebral autosomal dominant arteriopathy with subcortical infarcts and leucoencephalopathy (CADASIL). Bosn J Basic Med Sci 2015; 15(1):1–8. doi:10.17305/bjbms.2015.247
A 54-year-old man presented to our hospital with acute-onset left-sided weakness and right facial droop. Three days earlier he had also had migraine-like headaches, which he had never experienced before. He also reported a change in behavior during the past week, which his family had described as inappropriate laughter.
He had no history of hypertension, diabetes, or dyslipidemia. He did not smoke or drink alcohol. However, he had an extensive family history of stroke. His mother had a stroke at age 50, his brother a stroke at age 57, and his sister had been admitted for a stroke 1 month earlier at the age of 52.
On examination, he had weakness of the left arm and leg, right facial droop, and hyperactive reflexes on the left side. He had no sensory or cerebellar deficits. He had episodes of laughter during the examination.
We learned that the patient’s sister had undergone a workup showing mutations in the NOTCH3 gene and a skin biopsy study consistent with CADASIL.
Our patient was started on antiplatelet and high-intensity statin therapy. His symptoms improved, and he was discharged to an acute inpatient rehabilitation facility. He was referred to a CADASIL registry.
STROKE AND HEREDITY
CADASIL is a rare hereditary vascular disorder inherited in an autosomal dominant manner. It is the most common inherited form of small-vessel disease and results from a mutation in the NOTCH3 gene that leads to degeneration of smooth muscle in cerebral blood vessels. It can manifest as migraine with aura, vascular dementia, cognitive impairment, or ischemic stroke.
The diagnosis is based on a clinical picture that typically includes stroke at a young age (age 40 to 50) in the absence of stroke risk factors, or frequent lacunar infarction episodes that can manifest as migraine, lacunar infarct, or dementia.1 Some patients, such as ours, may have subtle nonspecific behavioral changes such as inappropriate laughter, which may herald the development of an infarct.
Characteristic findings on MRI are white matter hyperintensities that tend to be bilateral and symmetrical in the periventricular areas. Symmetrical involvement in the temporal lobes has high sensitivity and specificity for CADASIL.2 Biopsy study of the skin, muscle, or sural nerve shows small-vessel changes that include thickening of the media, granular material positive on periodic acid-Schiff staining, and narrowing of the lumen. However, the gold standard for diagnosis is confirmation of the NOTCH3 mutation on chromosome 19.1,2
There is no known treatment for CADASIL.
A 54-year-old man presented to our hospital with acute-onset left-sided weakness and right facial droop. Three days earlier he had also had migraine-like headaches, which he had never experienced before. He also reported a change in behavior during the past week, which his family had described as inappropriate laughter.
He had no history of hypertension, diabetes, or dyslipidemia. He did not smoke or drink alcohol. However, he had an extensive family history of stroke. His mother had a stroke at age 50, his brother a stroke at age 57, and his sister had been admitted for a stroke 1 month earlier at the age of 52.
On examination, he had weakness of the left arm and leg, right facial droop, and hyperactive reflexes on the left side. He had no sensory or cerebellar deficits. He had episodes of laughter during the examination.
We learned that the patient’s sister had undergone a workup showing mutations in the NOTCH3 gene and a skin biopsy study consistent with CADASIL.
Our patient was started on antiplatelet and high-intensity statin therapy. His symptoms improved, and he was discharged to an acute inpatient rehabilitation facility. He was referred to a CADASIL registry.
STROKE AND HEREDITY
CADASIL is a rare hereditary vascular disorder inherited in an autosomal dominant manner. It is the most common inherited form of small-vessel disease and results from a mutation in the NOTCH3 gene that leads to degeneration of smooth muscle in cerebral blood vessels. It can manifest as migraine with aura, vascular dementia, cognitive impairment, or ischemic stroke.
The diagnosis is based on a clinical picture that typically includes stroke at a young age (age 40 to 50) in the absence of stroke risk factors, or frequent lacunar infarction episodes that can manifest as migraine, lacunar infarct, or dementia.1 Some patients, such as ours, may have subtle nonspecific behavioral changes such as inappropriate laughter, which may herald the development of an infarct.
Characteristic findings on MRI are white matter hyperintensities that tend to be bilateral and symmetrical in the periventricular areas. Symmetrical involvement in the temporal lobes has high sensitivity and specificity for CADASIL.2 Biopsy study of the skin, muscle, or sural nerve shows small-vessel changes that include thickening of the media, granular material positive on periodic acid-Schiff staining, and narrowing of the lumen. However, the gold standard for diagnosis is confirmation of the NOTCH3 mutation on chromosome 19.1,2
There is no known treatment for CADASIL.
- Davous P. CADASIL: a review with proposed diagnostic criteria. Eur J Neurol 1998; 5(3):219–233. pmid:10210836
- Stojanov D, Vojinovic S, Aracki-Trenkic A, et al. Imaging characteristics of cerebral autosomal dominant arteriopathy with subcortical infarcts and leucoencephalopathy (CADASIL). Bosn J Basic Med Sci 2015; 15(1):1–8. doi:10.17305/bjbms.2015.247
- Davous P. CADASIL: a review with proposed diagnostic criteria. Eur J Neurol 1998; 5(3):219–233. pmid:10210836
- Stojanov D, Vojinovic S, Aracki-Trenkic A, et al. Imaging characteristics of cerebral autosomal dominant arteriopathy with subcortical infarcts and leucoencephalopathy (CADASIL). Bosn J Basic Med Sci 2015; 15(1):1–8. doi:10.17305/bjbms.2015.247